UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
(Mark One)
| |
☑ | |
☑
| ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31 2017, 2023
OR
| |
☐ | |
◻
| TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number 001-37813
SYROS PHARMACEUTICALS, INC.
(Exact Name of Registrant as Specified in Its Charter)
| | |
Delaware |
| 45-3772460 |
Delaware
| | 45-3772460
|
(State or other jurisdiction of
incorporation or organization) |
| (I.R.S. Employer
Identification No.) |
620 Memorial
35 CambridgePark Drive Suite 300, 4th Floor
Cambridge, Massachusetts |
| 0213902140
|
(Address of principal executive offices) |
| (Zip code) |
(617) (617) 744-1340
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| | | | |
Title of Class |
| Trading Symbol |
| Name of Exchange on Which Registered |
Common Stock, $0.001 par value |
| SYRS |
| Nasdaq Global Select Market |
Securities registered pursuant to Section 12(g) of the Act:
None.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ◻ ☐No☑
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ◻ ☐No☑
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes☑ No ◻☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes☑ No ◻☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of the registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☑
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer”filer,” “smaller reporting company,” and “smaller reporting“emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one):
| | | | | | |
Large accelerated filer | ☐ | ◻
|
| Accelerated filer
| Accelerated filer | ☑☐
|
Non-accelerated filer | ☑ | ◻
|
|
| Smaller reporting company | | ◻☑
|
(Do not check if a smaller reporting company)
|
|
|
|
| Emerging growth company | | ☑☐
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.☑☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant's executive officers during the relevant recovery period pursuant to § 240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ◻☐ No☑
As of June 30, 2017,2023, the last business day of the registrant’s most recently completed second fiscal quarter, the aggregate market value of the registrant’s common stock held by non-affiliates was $289,542,157,$58,560,692 based on the last reported sale price of such stock on the Nasdaq Global Select Market as of such date.
Portions of the registrant’s definitive proxy statement for its 20182024 Annual Meeting of Stockholders, which the registrant intends to file pursuant to Regulation 14A with the Securities and Exchange Commission not later than 120 days after the registrant’s fiscal year ended December 31, 2017,2023, are incorporated by reference into Items 10, 11, 12, 13 and 14 of Part III of this Annual Report on Form 10-K.As10-K. As of February 28, 2018,March 6, 2024, the registrant had 32,193,96126,453,891 shares of Common Stock, $0.001 par value per share, outstanding.
Cautionary Note Regarding Forward-Looking Statements and Industry Data
This Annual Report on Form 10-K, or Annual Report, contains forward‑looking statements that involve substantial risks and uncertainties. The forward‑looking statements are contained principally in the sections of this Annual Report entitled “Business,” “Risk Factors,” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” but are also contained in other sections of this Annual Report. All statements, other than statements of historical facts, contained in this Annual Report, including statements regarding our strategy, future operations, future financial position, future revenue, projected costs, prospects, plans and objectives of management and expected market growth are forward‑looking statements. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “would” and similar expressions are intended to identify forward‑looking statements, although not all forward‑looking statements contain these identifying words. In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. The forward‑looking statements and opinions contained in this Annual Report are based upon information available to us as of the date of this Annual Report and, while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information.
These forward‑looking statements include, among other things, statements about:
| ·
| | our plans to initiate, expand and/or report data from our clinical trials for SY‑1425 and SY‑1365;
|
•our plans to initiate and expand clinical trials of tamibarotene and our expectations for the timing, quantity and quality of information to be reported from our clinical trials of tamibarotene;
| ·
| | planned clinical trials for our product candidates, whether conducted by us or by any future collaborators, including the timing of these trials and of the anticipated results;
|
•our planned clinical trials for tamibarotene or for any other product candidates, whether conducted by us or by any collaborators, including the timing of these trials and of the anticipated results;
| ·
| | our plans to research, develop, manufacture and commercialize our current and future product candidates;
|
•our ability to replicate in any clinical trial of one of our product candidates the results we observed in preclinical or earlier clinical studies of such product candidate;
| ·
| | our plans to develop and seek approval of companion diagnostic tests for use in identifying patients who may benefit from treatment with our products and product candidates;
|
•our ability to replicate in the final results of any clinical trial of one of our product candidates the results we observed in interim results of such clinical trial;
| ·
| | our expectations regarding the potential benefits of our gene control platform and our approach;
|
•our plans to research, develop, seek approval for, manufacture and commercialize tamibarotene or any future product candidates;
| ·
| | our ability to enter into, and the terms and timing of, any collaborations, license agreements, or other arrangements;
|
•our plans to develop and seek approval of companion diagnostic tests for use in identifying patients who may benefit from treatment with tamibarotene or any future product candidates;
| ·
| | whether our collaboration with Incyte Corporation, or Incyte, will yield any validated targets, whether Incyte will exercise any of its options to exclusively license intellectual property directed to such targets, and whether and when any of the target validation fees, option exercise fees, milestone payments or royalties under the Incyte collaboration will ever be paid;
|
•our ability to enter into, and the terms and timing of, any collaborations, license agreements, or other arrangements;
| ·
| | the potential benefits of any future collaboration;
|
•our plans related to the potential to further develop SY-2101 in the future subject to additional capital availability;
| ·
| | the timing of and our ability to obtain and maintain regulatory approvals for our product candidates;
|
•the potential benefits of any collaboration;
| ·
| | the rate and degree of market acceptance and clinical utility of any products for which we receive marketing approval;
|
•developments relating to our competitors and our industry;
| ·
| | our commercialization, marketing and manufacturing capabilities and strategy;
|
•the impact of government laws and regulations;
| ·
| | our intellectual property position and strategy;
|
•the timing of and our ability to file new drug applications and obtain and maintain regulatory approvals for tamibarotene or any future product candidates;
| ·
| | • the rate and degree of market acceptance and clinical utility of any products for which we receive marketing approval; •our commercialization, marketing and manufacturing capabilities and strategy; •our intellectual property position and strategy; •our ability to identify additional products or product candidates with significant commercial potential; |
| ·
| | our expectations related to the use of our current cash, cash equivalents and marketable securities and the period of time in which such capital will be sufficient to fund our planned operations;
|
| ·
| | our estimates regarding expenses, future revenue, capital requirements and need for additional financing;
|
| ·
| | developments relating to our competitors and our industry; and
|
•our expectations related to the use of our current cash and cash equivalents and the period of time in which such capital will be sufficient to fund our planned operations;
| ·
| | the impact of government laws and regulations.
|
•our estimates regarding expenses, future revenue, capital requirements and need for additional financing; and
•general economic conditions, including inflation, recession risk and increasing interest rates.
We may not actually achieve the plans, intentions or expectations disclosed in our forward‑looking statements, and you should not place undue reliance on our forward‑looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward‑looking statements we make. New risks and uncertainties emerge from time to time, and it is not possible for us to predict all risks and uncertainties that could have an impact on the forward‑looking statements contained in this Annual Report.
We have included important factors in the cautionary statements included in this Annual Report particularly in the “Risk Factors” section, that could cause actual results or events to differ materially from the forward‑looking statements that we make. make, particularly the factors discussed below under the heading “Risk Factor Summary,” and the risk factors detailed further in Item 1A, “Risk Factors” of Part I of this Annual Report and in our Securities and Exchange Commission reports filed after this Annual Report.
Our forward‑looking statements also do not reflect the potential impact of any future acquisitions, mergers, dispositions, collaborations, joint ventures or investments that we may make or enter into.
This report also includes statistical and other industry and market data that we obtained from industry publications and research, surveys, and studies conducted by third parties as well as our own estimates. All of the market data used in this report involve a number of assumptions and limitations, and you are cautioned not to give undue weight to such data. Industry publications and third-party research, surveys, and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. Our estimates of the potential market opportunities for tamibarotene or any future product candidate include several key assumptions based on our industry knowledge, industry publications, third-party research, and other surveys, which may be based on a small sample size and may fail to accurately reflect market opportunities. While we believe that our internal assumptions are reasonable, no independent source has verified such assumptions.
You should read this Annual Report and the documents that we have filed as exhibits to this Annual Report completely and with the understanding that our actual future results may be materially different from what we expect. We do not assume any obligation to update any forward‑looking statements, whether as a result of new information, future events or otherwise, except as required by law.
Risk Factor Summary
Investment in our securities involves risk. You should carefully consider the following summary of what we believe to be the principal risks facing our business, in addition to the risks described more fully in Item 1A, “Risk Factors” of Part I of this Annual Report also includes statistical and other industry and market data that we obtained from industry publications and research, surveys and studies conducted by third parties. All of the market data usedinformation included in this Annual Report involvesReport. The risks and uncertainties described below are not the only risks and uncertainties we face. Additional risks and uncertainties not presently known to us or that we presently deem less significant may also impair our business operations.
If any of the following risks occurs, our business, financial condition, and results of operations and future growth prospects could be materially and adversely affected, and the actual outcomes of matters as to which forward-looking statements are made in this report could be materially different from those anticipated in such forward-looking statements.
•We have incurred significant losses since inception, expect to incur significant losses for at least the next several years, and may never achieve or maintain profitability.
•We will need substantial additional funding to execute our operating plan, and if we are unable to raise capital, we could be forced to delay, reduce or eliminate our product development programs or commercialization efforts.
•Unstable market and economic conditions may have serious adverse consequences on our business, financial condition and stock price.
•In the near term, we are dependent on the success of tamibarotene. If we are unable to complete the clinical development of, obtain marketing approval for, or successfully commercialize tamibarotene, either alone or with a numbercollaborator, or if we experience significant delays in doing so, our business will be substantially harmed.
•Adverse events or undesirable side effects caused by, or other unexpected properties of, assumptionsproduct candidates that we develop may be identified during development and limitations,could delay or prevent their marketing approval or limit their use.
•Failure to successfully develop, validate, obtain regulatory approval for, and you are cautionedcommercialize companion diagnostics could harm our commercialization strategy.
•If we, or any collaborators, experience delays or difficulties in the enrollment of patients in clinical trials, our or their receipt of necessary regulatory approvals could be delayed or prevented.
•We rely on third parties to conduct our clinical trials and certain aspects of our research, and those third parties may not perform satisfactorily, including by failing to give undue weight tomeet deadlines for the completion of such data. We believe thattrials or research.
•Even if we complete the informationnecessary clinical trials, the regulatory approval process is expensive, time consuming and uncertain and may prevent us or any collaborators from these industry publications, surveysobtaining approvals for the commercialization of tamibarotene or any future product candidates. As a result, we cannot predict when or if, and studies is reliable. The industry in which territories, we, operateor any collaborators, will obtain marketing approval to commercialize a product candidate.
•If we fail to comply with our obligations under our existing and any future intellectual property licenses with third parties, we could lose license rights that are important to our business.
•If we are unable to obtain and maintain sufficient patent protection for tamibarotene or any future product candidates, or if the scope of the patent protection is subjectnot sufficiently broad, our competitors could develop and commercialize products similar or identical to a high degree of uncertaintyours, and risk dueour ability to a variety of important factors, including those described in the “Risk Factors” section. These and other factors could cause results to differ materially from those expressed in the estimates made by the independent parties and by us.successfully commercialize such product candidates may be adversely affected.
Syros® is a registered trademark of Syros Pharmaceuticals, Inc. All rights reserved. All other trademarks used in this Annual Report are the property of their respective owners.
PART I
Item 1. BUSINESS
Overview
We are a biopharmaceutical company pioneering an understandingcommitted to developing new standards of care for the non-coding regulatory regionfrontline treatment of patients with hematologic malignancies. Driven by the genome controlling the activation and repression of genes. Our goal ismotivation to advancehelp patients with blood disorders that have largely eluded other targeted approaches, we are advancing tamibarotene, a new wave of medicines to control the expression of genes. We have builtselective retinoic acid receptor alpha, or RARα, agonist for which we are conducting SELECT-MDS-1, a proprietary gene control platform designed to systematically and efficiently analyze this unexploited region of DNAPhase 3 clinical trial evaluating tamibarotene in human disease tissue to identify novel targets linked tocombination with azacitidine in a genomically defined patient populationssubset of patients with higher-risk myelodysplastic syndrome, or HR-MDS, and to develop drugs against those targets based on our expertise in transcriptional chemistry. Because gene expression is fundamental to the function of all cells,for which we believe that our gene control platform has broad potential to create medicines that achieve profound and durable benefit across therapeutic areas andare conducting SELECT-AML-1, a range of diseases. We are currently focused on developing treatments for cancer and diseases resulting from modifications of a single gene, also known as monogenic diseases, and are building a pipeline of gene control medicines.
Our lead product candidates are:
| ·
| | SY-1425, a selective retinoic acid receptor alpha, or RARα, agonist that is being evaluated in combination with azacitidine, a hypomethylating agent frequently used to treat acute myeloid leukemia, or AML, and myelodysplastic syndrome, or MDS, patients, and with daratumumab, an anti-CD38 therapeutic antibody approved to treat multiple myeloma, in a Phase 2 clinical trial in genomically defined subsets of patients with AML and MDS; and
|
| ·
| | SY-1365, a selective inhibitor of cyclin-dependent kinase 7, or CDK7, in a Phase 1 clinical trial in patients with advanced solid tumors for which expansions in ovarian and breast cancer are planned.
|
We also have multiple programs in earlier stages of research and development in oncology, including immuno-oncology, and monogenic diseases. Our goal is to build a fully integrated biopharmaceutical company based on our leadership position in gene control.
At the 59th American Society of Hematology Annual Meeting and Exposition in December 2017, or ASH 2017, we presented clinical data from ourrandomized Phase 2 clinical trial evaluating SY-1425 astamibarotene in combination with venetoclax and azacitidine in a single agent ingenomically defined subsetssubset of AML and MDSnewly diagnosed patients with our proprietary RARAacute myeloid leukemia, or AML, who are not suitable candidates for standard intensive chemotherapy.
Tamibarotene
Overview
Tamibarotene is an oral, potent and IRF8 biomarkers. In the trial, we observed that chronic daily dosing of SY-1425 administered at 6 mg/m2 orally divided in two doses was generally well-tolerated and that clinical and biological activity was observed in patients enrolled in the trial. Specifically, clinical activity was observed in ten of 23 (43%) evaluable patients with relapsed or refractory AML and higher-risk MDS, including improvement in blood counts, reduction in leukemic blasts and one bone marrow complete response. Thirteenselective agonist of the 23 (57%) evaluable relapsed or refractorytranscription factor RARα. In cells overexpressing RARA, the gene that codes for RARα, tamibarotene has been observed to bind to RARα, saturating unliganded RARα receptors to trigger a transcriptional activation switch to restore myeloid differentiation, inhibit blast proliferation, and promote blast cell clearance. A growing body of data from multiple clinical trials supports our development strategy for tamibarotene in HR-MDS and AML patients who overexpress RARA. HR-MDS and higher-risk MDS patients had stable disease. Myeloid differentiation was also observedAML are hematologic malignancies that exist on the same disease continuum, with the two diseases diagnostically distinguished largely by the percentage blasts in the bone marrow, consistent with the underlying mechanism of action of SY-1425 as a differentiating agent. Induction of CD38, a marker of cell differentiation, was observed after one 28-day cycle of treatment in 11 of 13 (85%) patients with pre- and post-treatment immunophenotyping samples.
marrow. We believe that theseapproximately 50% of HR-MDS patients and approximately 30% of AML patients are positive for RARA overexpression.
Informed by feedback from the U.S. Food and Drug Administration, or FDA, we are enrolling newly diagnosed HR-MDS patients who overexpress RARA in SELECT-MDS-1, a double-blind placebo-controlled randomized Phase 3 clinical data, when combined with preclinical data showing the tumor-killing activity of SY-1425trial, to receive tamibarotene in combination with azacitidine, and with daratumumab, support the ongoing development of SY-1425 as a combination agent. SY-1425 has shown synergistic tumor-killing activityor placebo in combination with azacitidine, respectively. The primary efficacy endpoint is based on 190 patients to provide over 90% power to detect a difference in the rates of complete response, or CR, between the experimental and control arms, with a one-sided alpha of 0.025. The FDA has expressed that the CR rate is an acceptable efficacy endpoint for either full or accelerated approval for treatment of newly diagnosed HR-MDS with supporting data on durability of remission. Informed by feedback from the FDA, we amended the SELECT-MDS-1 clinical trial protocol in March 2023 to include a total of approximately 550 patients to enable us to assess overall survival, or OS, as wella key secondary endpoint, which could allow the trial to serve as a confirmatory study if needed to convert an accelerated approval to a full approval in the future. The amended clinical trial protocol is designed with daratumumab80% power to detect a difference in preclinical modelsOS rates for the key secondary endpoint between the experimental and control arms, also with a one-sided alpha of RARA biomarker-positive AML.0.025. In January 2023, the FDA granted Fast Track Designation to tamibarotene in combination with azacitidine SY-1425 demonstratedfor the treatment of adults with newly diagnosed HR-MDS who are positive for RARA overexpression. In the first quarter of 2024, we completed enrollment of the 190 patients necessary to support the CR primary endpoint analysis, and we expect to report pivotal CR data from the SELECT-MDS-1 trial by the middle of the fourth quarter of 2024.
In addition, we are advancing tamibarotene in combination with venetoclax and azacitidine in newly diagnosed unfit AML patients who are positive for RARA overexpression. Our ongoing Phase 2 clinical trial, known as SELECT-AML-1, included a single-arm safety lead-in to confirm the dosing regimen of the triplet to be used in the randomized portion of the trial, which is evaluating the safety and efficacy of tamibarotene in combination with venetoclax and azacitidine compared to venetoclax and azacitidine in approximately 80 patients randomized 1:1. The trial is also evaluating the triplet as a salvage strategy for patients in the control arm who do not respond to venetoclax and azacitidine. The primary endpoint of the trial is the CR/CRi rate (as defined below) and the trial is powered at 80% to detect a difference between the experimental and control arms. We reported initial data from the randomized portion of the SELECT-AML-1 trial in early December 2023. As of a November 13, 2023 data cut-off, seven of the nine response evaluable patients (78%) treated with the combination of tamibarotene, venetoclax and azacitidine achieved CR and two patients (22%) achieved complete response with incomplete blood count recovery, or CRi, for an aggregate CR/CRi rate of 100%. Three of the ten response evaluable patients (30%) treated with venetoclax and azacitidine achieved a CR and four patients (40%) achieved a CRi, for an aggregate CR/CRi rate of 70% in the control arm. We expect to report additional data from the SELECT-AML-1 trial in 2024. There can be no guarantee that this additional data will replicate the results of the initial data that was reported in December 2023.
We have entered into an agreement with a third-party commercial provider to provide a validated laboratory test under Clinical Laboratory Improvement Amendment guidelines using a diagnostic platform and approach that is being used to prospectively enroll patients with RARA gene overexpression in our clinical trials. In March 2022, we entered into a Master Collaboration Agreement and associated project work plan with Qiagen Manchester Limited, or Qiagen, pursuant to which Qiagen has agreed to develop and commercialize a companion diagnostic for this biomarker.
Tamibarotene in Combination with Azacitidine
In October 2018, we published preclinical data in Haematologica, a peer-reviewed journal of the European Hematology Association, supporting the rationale for combining tamibarotene with hypomethylating agents such as azacitidine in AML patients with RARA overexpression. These data showed that tamibarotene in combination with azacitidine resulted in synergistic anti-proliferative effects supported by evidence of DNA damage and apoptosis and, in patient-derived xenograft models of AML with RARA overexpression, tamibarotene in combination with azacitidine showed both greater clearance of tumor cells in bone marrow and other tissues and greater depth and duration of tumor response, in preclinical models, compared to either azacitidine or SY-1425tamibarotene alone.
In combination with daratumumab, SY-1425 induced robust immune cell-mediated tumor death in vitro. Notably, AML cells do not normally express high levelsan earlier cohort of CD38. We have shown that by inducing CD38 expression, SY-1425 sensitizes biomarker-positive AML models to the tumor-killing effects of daratumumab.
Our ongoingour Phase 2 clinical trial is assessingevaluating tamibarotene as a single agent, hematologic response was observed in 60% (3/5) of evaluable patients with relapsed or refractory HR-MDS, including one patient with a marrow CR. The evaluation of tamibarotene as a single agent was followed by evaluation of tamibarotene plus azacitidine in newly diagnosed unfit patients with AML in this same Phase 2 trial. We presented data at the 62nd American Society of Hematology Annual Meeting and Exposition held in December 2020, which was later published in Blood Advances in 2022, from our fully enrolled Phase 2 clinical trial evaluating the safety and efficacy of SY-1425tamibarotene in combination with azacitidine in approximately 25 newly diagnosed AML patients who are not suitable candidates for standard chemotherapy,chemotherapy. Fifty-one newly diagnosed unfit AML patients, including those with and without RARA gene overexpression, were eligible for a safety analysis. Eighteen patients who overexpress RARA were evaluable for clinical response. In those patients, the data showed that:
•Nine patients (50%) achieved CR, and two patients (11%) achieved CRi, for an aggregate CR/CRi rate of 61% (11/18).
o89% (8/9) of CRs were deep molecular or cytogenetic CRs.
oIn those with low-blast count AML with a blast percentage of ≤30%, CR was observed in 67% (4/6) of patients.
oResponses were seen across AML risk groups, including patients with mutations that are typically associated with poor outcomes.
•The median time to initial composite CR was 1.2 months.
•The median duration of response was 10.8 months, and median OS among patients who achieved a CR or CRi was 18.0 months.
•72% (13/18) of patients achieved or maintained transfusion independence.
For the 28 patients who were negative for RARA overexpression and who were evaluable for clinical response, the data showed that the overall response rate was 43% (12/28), with a composite complete response rate of 32% (9/28), including seven patients (25%) achieving CR and two patients (7%) achieving CRi. The median time to response was 3.0 months, and the median duration of response was 10.3 months.
Tamibarotene in combination with daratumumabazacitidine was generally well-tolerated with no evidence of increased toxicity relative to either as a single agent, including rates of myelosuppression that were comparable to single agent azacitidine.
Tamibarotene in approximately 12 relapsed or refractoryCombination with Venetoclax and Azacitidine
At the 64th American Society of Hematology Annual Meeting held in December 2022, we presented data from the safety lead-in portion of our ongoing SELECT-AML-1 Phase 2 trial evaluating tamibarotene in combination with venetoclax and azacitidine in newly diagnosed, unfit patients with AML and higher-risk MDS patients. AllRARA gene overexpression. As of October 13, 2022, eight newly diagnosed, unfit patients enrolled or to bewho were positive for RARA overexpression had been enrolled in the trial, have been or will be prospectively selected using our
proprietary RARA or IRF8 biomarkers. InOn December 2017,6, 2023, we entered into a clinical supply agreement with Janssen Research and Development, LLC, or Janssen, pursuant to which Janssen agreed to supply us daratumumabannounced initial data from the randomized portion of SELECT-AML-1. As of November 13, 2023, 23 newly diagnosed unfit AML patients positive for useRARA overexpression had enrolled in the trial. We are no longer enrolling patients in the cohortsrandomized portion of the trial, in which SY-1425including 19 who were evaluable for response. The CR/CRi rate was being evaluated as a single agent. We expect to report initial clinical data from100% among response evaluable patients (nine of nine) treated with the combination cohortsof tamibarotene, venetoclax and azacitidine, as compared to 70% of patients (seven of ten) treated with the control arm of venetoclax and azacitidine. Seven of the trial innine response evaluable patients (78%) treated with the fourth quartercombination of 2018.
We are continuing to dosetamibarotene, venetoclax and azacitidine achieved a CR and two patients in the dose-escalation phase of our ongoing Phase 1 clinical trial of SY-1365 and expect to report data from this phase(22%) achieved a CRi. Three of the trial in the fourth quarter of 2018. Once a maximum tolerated dose is reached in the dose escalation phase of the Phase 1 clinical trial and the recommended dosing schedule is identified, we intend to open expansion cohorts evaluating SY-1365 in multiple ovarian cancer populations as a single agent as well as in combination with carboplatin, a chemotherapeutic agent. The ovarian cancer populations include a 24-patient cohort evaluating SY-1365 as a single agent inten response evaluable patients who have relapsed after three or more prior therapies, a 24-patient cohort evaluating SY-1365 in combination with carboplatin in patients who relapsed after one or more prior therapies but who may still benefit from additional platinum-based treatment, and a 12-patient pilot cohort evaluating SY-1365 as a single agent in primary platinum-refractory disease. We also plan to evaluate SY-1365 in combination with fulvestrant, a hormonal medicine, in 12 patients with hormone-receptor positive, or HR+, HER-2-negative metastatic breast cancer who have progressed after treatment with a CDK4/6 inhibitor plus an aromatase inhibitor. In addition, we plan to evaluate the mechanism of action of SY-1365 in ten patients with any solid tumor accessible for biopsies. We expect that the expansion cohorts in our Phase 1 clinical trial will be open to enrollment in mid-2018.
We currently have five programs in our preclinical and discovery pipeline, including preclinical programs directed to the development of a novel CDK7 inhibitor that can be administered orally, inhibitors of cyclin-dependent kinase 12/13, and inhibitors of an immuno-oncology target, as well as discovery programs related to a gene control target to treat sickle cell disease and in the field of cancer. We plan to nominate a development candidate from one of our preclinical programs during 2018 that we can advance into studies to support a potential investigational new drug application, or IND, filing in 2019. We have and are continuing to use our gene control platform in collaboration with third parties to identify and validate targets in diseases beyond our current areas of focus. To this end, we entered a target discovery, research collaboration and option agreement with Incyte Corporation, or Incyte, in January 2018 under which we will use our platform to identify novel therapeutic targets with a focus on myeloproliferative neoplasms. See “—License and Collaboration Agreements – Incyte” below.
Our Focus—Gene Control Medicines
There are approximately 200 different cell types in the human body. Despite having identical genomes, each of these cell types has a different function. For example, a skin cell functions differently than a muscle cell despite sharing the exact same DNA. What determines cell type and function is the specific set of genes that is expressed, or turned “on” or “off,” in that given cell. This coordinated activation and repression of genes, known as the cell’s gene expression program, is controlled in part by a number of cellular components acting on non-coding regions of the genome. These components include transcription factors, transcriptional kinases, other transcriptional and regulatory proteins, and RNA. These transcriptional and regulatory proteins bind with specific regions of non‑coding DNA, including a specific category called enhancers, to control the rate and magnitude of gene transcription.
In some diseases, alterations in the physical state or function of the non‑coding regions of the genome can change a cell’s gene expression program, altering the type and function of that cell. Because the altered gene expression program is implemented by transcription factors, transcriptional kinases, other transcriptional and regulatory proteins, and RNA, these proteins and RNA can be important points for therapeutic intervention. Because this biology is fundamental to the function of all cells, it applies across diseases, whether the cause is genetic, environmental, bacterial, viral or multi‑factorial.
Although researchers have long believed that alterations in non‑coding regions of DNA, which account for 98% of the genome, play a key role in driving disease, the scientific community has lacked the tools to study these regions of the genome, rendering them poorly understood. As a result, the discovery and development of targeted therapies to date has focused almost exclusively on abnormal proteins resulting from genetic alterations found in regions of DNA that encode for proteins, which represent less than 2% of the entire genome.
While targeted therapies, in which the right drug is matched to the right patient, have dramatically improved the ability to treat certain cancers and other serious diseases, the opportunity to identify new drug targets by sequencing coding regions of DNA is limited, particularly in cancer. Moreover, in cancer, the clinical benefit from inhibiting
abnormal proteins resulting from single genetic alterations is often short-lived due to drug resistance. Furthermore, many serious diseases continue to go unaddressed due to the limitations of current drug discovery approaches. Taken together, these factors underscore the need for fundamentally new approaches to drug discovery and development.
Gene control medicines are intended to modulate the cell’s underlying gene expression program, influencing the expression of the crucial set of genes that are associated with disease. The relatively few gene control medicines available today are among the most important targeted therapies and are widely used for their approved indications. Drugs that target transcription factors, such as those which target the estrogen receptor in breast cancer, androgen receptor in prostate cancer and glucocorticoid receptor in inflammation, are important examples of gene control medicines that have produced transformative patient benefits. For example, tamoxifen, a gene control medicine targeting a transcription factor, revolutionized the treatment of certain breast cancers, illustrating the significant therapeutic potential of gene control medicines. However, the difficulty in studying non‑coding regions of the genome historically prevented a systematic approach to identifying these critical points of intervention, making gene control a largely untapped field for targeted drug discovery and development.
Based on the work of Syros’ scientific founders Richard A. Young, Ph.D., James Bradner, M.D. and Nathanael Gray, Ph.D., and other scientists, there is now a rapidly growing scientific understanding of how alterations in non‑coding regions of the genome drive disease and how to modulate gene control targets. One of the seminal discoveries that pushed the field forward came out of Dr. Young’s laboratory at the Whitehead Institute for Biomedical Research, or Whitehead. He discovered that a very small unique subset of enhancers, called super‑enhancers, are central to orchestrating gene expression programs. These highly specialized regulatory regions of non‑coding DNA bring together the cellular components needed for gene expression, assembling large amounts of transcription factors, transcriptional kinases and other transcriptional and regulatory proteins to drive increased expression of genes crucial to a given cell’s type and function.
Super‑enhancers exist in both normal and diseased cells. In many different diseases, super‑enhancers are associated with, and drive the expression of, disease‑causing genes. For example, multiple well‑known genes that are implicated in cancer, such as MYC, are associated with super‑enhancers. Notably, analysis of super‑enhancers and their associated genes allows us to rapidly and systematically elucidate gene expression programs, pinpointing the genes crucial to the function of a given cell and providing critical insights into changes in gene expression programs that contribute to disease.
These and other discoveries from our scientific founders, coupled with technological advancements, have enabled our pioneering approach to therapeutic gene control. We believe we have built the first proprietary platform designed to systematically and efficiently analyze non‑coding regions of the genome in healthy and diseased cells taken from patient tissues to identify alterations in gene expression programs that represent optimal points of therapeutic intervention and develop drugs to control the expression of disease‑driving genes. By doing so, we believe our gene control platform will allow us to (i) identify a wide array of potential new drug targets across a range of diseases, (ii) provide a new lens for diagnosing and segmenting patients, including those with complex, multi‑factorial diseases that have eluded segmentation with other genomic‑based approaches, and (iii) advance a new wave of medicines that have the potential to influence multiple drivers of disease through a single target, making them less susceptible to drug resistance and providing patients with a more profound and durable benefit than many of today’s targeted therapies.
Our Gene Control Platform
Our proprietary gene control platform consists of two fundamental pillars:
| ·
| | Identifying gene control targets that, when modulated with a drug, may provide a therapeutic benefit to a defined patient population. We analyze gene expression programs and the non-coding regions of the genome associated with those expression programs in diseased and healthy cells taken from patient tissues to identify points of therapeutic intervention and associated biomarkers in specific patient populations.
|
| ·
| | Drugging gene control targets. We develop product candidates to modulate gene control targets through:
|
| ·
| | internal drug discovery efforts focused on creating small molecule drugs targeting transcription factors, transcriptional kinases and other transcriptional and regulatory proteins; and
|
| ·
| | externally focused efforts to link existing drugs to specific patient populations identified through our platform. These externally focused efforts could enable us to identify drugs that we may seek to in‑license or acquire or use as starting points for our own drug discovery programs to accelerate our development path, as we did with SY-1425.
|
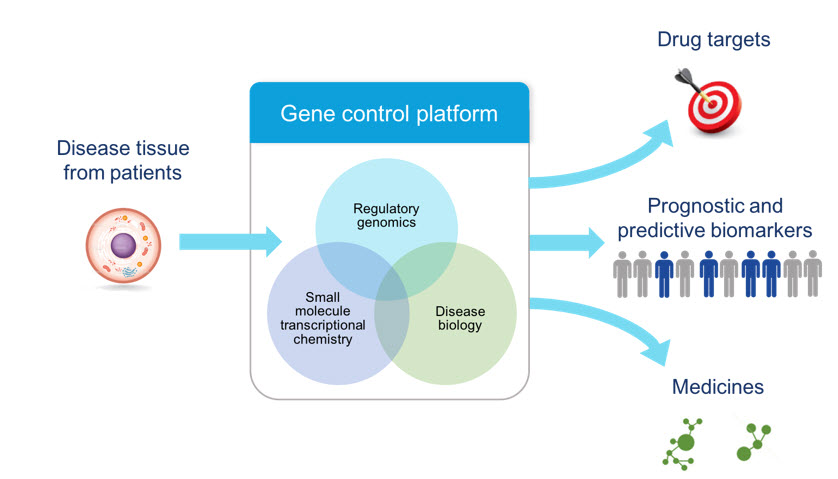
Identifying Novel Gene Control Targets
Our long‑term goal is to analyze gene expression programs in serious diseases where we believe currently underserved patients can benefit from gene control medicines. We have invested significant resources in building capabilities to discover novel gene control targets and associated biomarkers. Our approach is disease‑focused. Our platform consists of technologies and capabilities to analyze gene expression programs directly from patient tissue samples. We do this by employing our expertise and technologies in computational, gene control and cellular biology. We have in‑licensed intellectual property from the laboratories of our scientific founders at Whitehead and the Dana‑Farber Cancer Institute, Inc., or Dana-Farber. We have significantly improved this licensed technology, including computational algorithms and tissue processing systems, which have produced a highly efficient, scalable approach to analyze gene expression programs using small amounts of patient tissue. These advancements have enabled us to generate a substantial dataset of gene expression programs and to identify targets across many diseases and cell types. Through those efforts, we have identified drug targets in oncology, immuno‑oncology, immunology and monogenic diseases, and have validated several of these targets using biological methods to ablate, or knock out, the target gene or chemical methods to modulate the target’s activity. The discovery and validation of these targets has led to the identification of our product candidates SY‑1425 and SY-1365 as well as additional novel product candidates in earlier stages of research and preclinical development. We plan to analyze gene expression programs in other cancers, and to collaborate with third parties such as Incyte to identify and validate targets in diseases beyond our current areas of focus.
In some diseases, particularly monogenic diseases, research has revealed that a therapeutic benefit might be possible from modulating expression of a single gene. Another application of our platform is to characterize the regulatory region associated with such a gene and identify protein or RNA components that could be modulated to alter the expression of that single gene. We are applying this approach to sickle cell disease based on the hypothesis that increased expression of the fetal hemoglobin gene in individuals with sickle cell disease could be therapeutically beneficial.
Validation of Our Approach
We have validated our approach by successfully linking known targets of successful, marketed drugs to super‑enhancers in human disease tissue. Additionally, using our platform, we have identified super‑enhancers associated with genes linked to the hallmarks of a number of serious diseases.
During 2017, we presented data generated by our scientists in collaboration with researchers at Whitehead in which we analyzed regulatory regions of the genome in cancer stem cell enriched triple-negative breast cancer, or TNBC, cell lines. Cancer stem cells, or CSCs, are known to be involved in resistance to chemotherapies, relapse of disease and development of metastasis. The analysis revealed key genes that may be involved in driving disease relapse and metastasis, with implications for the discovery and development of novel therapies for TNBC. Notably, TP73, a gene that encodes a DNA-binding transcription factor called p73, was found to be a core driver of transcriptional circuitry in CSCs, controlling super-enhancer associated genes involved in cell migration, signal transduction and developmental processes. The set of TP73-controlled genes provide new leads for drug discovery and development with potential to yield much-needed new therapies for TNBC patients.
We also presented data during 2017 from our collaboration(30%) treated with the University of California, San Diego, in which our platformcontrol achieved a CR and four patients (40%) achieved a CRi. The median time to CR/CRi response was used to analyze and compare super-enhancers in cells21 days (ranging from pancreatic cancer patient tumors to those in cells from normal pancreatic tissues. These data showed that the leukemia inhibitory factor, or LIF, gene demonstrated one of the most significant changes in enhancer size from pancreatic tumors in comparison to normal pancreatic tissue. In preclinical mouse models, LIF enhanced the anti-tumor activity of chemotherapy and produced a survival benefit when inhibited using a monoclonal antibody.
Finally, we presented data in the last year showing alterations in regulatory regions of the genome in T cells from14-28) among patients treated with systemic lupus erythematosus, or SLE, revealing genes critical for activating T cells and driving disease. Specifically, we found that genes regulated by activation of the SYK kinase and IRF4 transcription factor are significantly enriched in SLE naïve and memory T cells, suggesting that they are key drivers of T cell activation in SLE and a core part of the transcriptional regulatory circuitry driving the disease. These findings provide biological insights into the autoimmune response in lupus that could lead to the identification of novel drug targets and therapeutic approaches to treat SLE.
Drugging Gene Control Targets
We develop product candidates against gene control targets through:
| ·
| | internal drug discovery efforts focused on creating small molecule drugs targeting transcription factors, transcriptional kinases and other transcriptional and regulatory proteins; and
|
| ·
| | linking existing drugs, which we could in‑license, to specific patient populations identified through our platform—a strategy we implemented by in-licensing SY-1425 to accelerate our clinical development path.
|
We have developed significant core internal capabilities in small molecule chemistry, biochemistry and structural biology to characterize the structure and function of transcription factors such as nuclear hormone receptors, transcriptional kinases, chromatin regulators, and other transcriptional and regulatory proteins in order to generate novel chemical matter. We have also developed a sophisticated suite of proprietary assays, which are internally developed tests to measure the biochemical, biophysical, cellular and genomic activity of known and novel compounds against gene control targets.
While our platform is designed to identify drug targets across a broad range of therapeutic areas and therapeutic modalities, our drug discovery and development efforts are focused on small molecule drugs to target specialized proteins responsible for gene expression, including transcription factors, transcriptional kinases and other transcriptional and regulatory proteins. Because these specialized proteins play a central role in implementing gene expression
programs, they are among the most promising and high potential gene control targets for therapeutic intervention. The graphic below illustrates our areas of focus for development of product candidates.
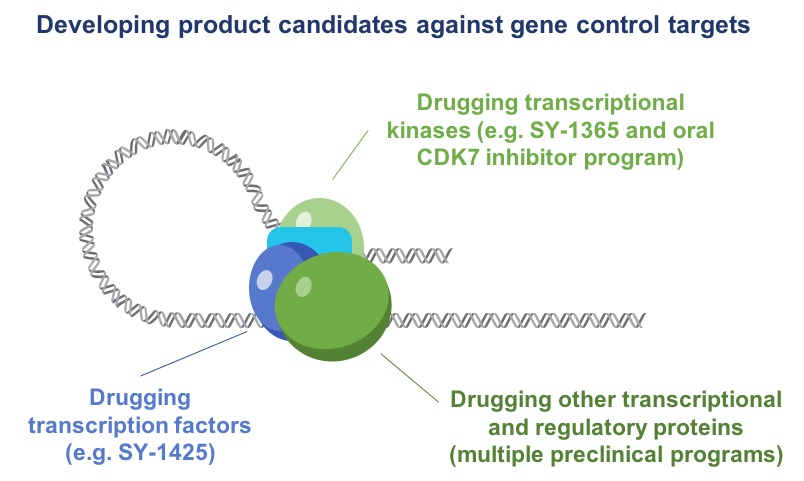
Drugging Transcriptional Kinases
SY‑1365 demonstrates our ability to identify tumors with transcriptional dependencies and to selectively drug transcriptional kinases. Using our core capabilities in gene control biology and biochemistry, we believe that we have the most advanced inhibitor of CDK7 in clinical development.
Drugging Transcription Factors
Leveraging our expertise in biology, biochemistry and chemistry, we have developed a suite of proprietary screens and assays to demonstrate direct binding of novel transcription factor inhibitors and to directly assess transcription factor inhibition in cells. We are using our capabilities and expertise in structure-based drug design and medicinal chemistry to identify small molecules that that interact with transcription factors.
Linking Existing Drugs to Novel Patient Populations
SY‑1425 demonstrates our ability to link existing drugs to novel genomically defined patient populations identified through our platform. We have established a process to systematically screen existing compounds for relationships between drug sensitivity and super‑enhancers that we identify in human disease tissue. To date, we have identified multiple drug and enhancer relationships, the most advanced leading to the identification of SY‑1425. This program exemplifies the general approach of using our platform to identify compounds that could accelerate our clinical development path.
Our Clinical Programs
SY‑1425
Overview
SY‑1425 (tamibarotene) is an oral, potent and selective agonist of the transcription factor RARα. In September 2015, we in‑licensed from TMRC Co., Ltd., or TMRC, the exclusive right to use intellectual property and data rights controlled by TMRC to develop and commercialize SY‑1425 for oncology indications in North America and Europe. We are currently conducting a Phase 2 clinical trial assessing the safety and efficacy of SY-1425 in combination with azacitidine, a hypomethylating agent frequently used to treat patients with AML and MDS, in approximately 25 newly diagnosed AML patients who are not suitable candidates for standard chemotherapy, and in combination with daratumumab, an anti-CD38 therapeutic antibody approved to treat multiple myeloma, in approximately 12 relapsed or refractory AML and higher-risk MDS patients. All patients enrolled or to be enrolled in the trial have been or will be prospectively selected using our proprietary RARA or IRF8 biomarkers. We expect to report initial clinical data from the combination cohorts of the trial in the fourth quarter of 2018.
Linking SY‑1425tamibarotene, venetoclax and azacitidine, as compared to Novel Patient Populations
We leveraged our platform to analyze gene expression programs in primary AML and breast cancer patient tissue samples. We discovered that RARA, the gene that codes for RARα, was associated with a super‑enhancer in some patients’ tumors but not in others. A super-enhancer is a highly specialized region of non-coding DNA central to orchestrating gene expression programs and driving increased expression of genes crucial to the function of a given cell. The function of RARα differs depending on whether it is bound to its ligand. In the absence of a ligand, RARα represses differentiation. We believe that in tumors25 days (ranging from 17-56) among patients treated with the RARA‑associated super‑enhancer, there is an abundance of unliganded RARα, resulting in the repression of differentiation, thereby locking the cell in an immature, proliferative and undifferentiated state. Introducing a RARα agonist, such as SY‑1425, simulates the activity of a ligand, activating differentiation.
In collaboration with researchers at Stanford University School of Medicine, we published data in the journal Cancer Discovery during 2017 where we used our gene control, platform to analyze 66 AML patients’ tumor samples and identified six distinct patient subsets based on super-enhancer profiles. The data showed that:
| ·
| | super-enhancer profiles were strongly associated with survival outcomes, often independent of known genetic mutations in AML;
|
| ·
| | the RARA super-enhancer was associated with high expression of the RARA gene, which codes for the RARα transcription factor;
|
| ·
| | RARA pathway activation was predictive of response to SY-1425 as demonstrated by reduced proliferation and increased differentiation in AML cells with high RARA expression. Moreover, SY-1425 decreased tumor burden and prolonged survival in mouse models in which the mice are implanted with human tumors, which are referred to as patient‑derived xenograft models, or PDX models, of AML with high RARA expression, while no effect was found on AML cells or PDX models with low RARA expression;
|
| ·
| | all trans retinoic acid, or ATRA, a less potent and non-selective retinoid, produced no survival benefit in PDX models with high RARA expression; and
|
| ·
| | SY-1425 induced significant transcriptional changes promoting cell differentiation in AML cells with high RARA expression, but little to no transcriptional changes in AML cells with low RARA expression.
|
SY‑1425 Development Plan
We plan to develop SY‑1425 in North America and Europe for treatment of AML and MDS in genomically defined subsets of patients with either the RARA or IRF8 biomarker, or both. In September 2016, we initiated a multi-center, open-label Phase 2 clinical trial enrolling genomically-defined subsets of patients with AML and MDS pursuant to an IND accepted by the U.S. Food and Drug Administration, or FDA, in May 2016. In the fourth quarter of 2016, an investigational device exemption for the assay being used to select patients with the RARA and IRF8 biomarkers for inclusion in this trial was approvedCR/CRi being reached by the FDA. In this trial, we have explored the safety and efficacy of SY-1425 as a single agent, and are continuing to explore the safety and efficacy of SY-1425 in combination with other approved and investigational agents, in the following patient cohorts:
| ·
| | Single-agent SY-1425 in approximately 25 patients with relapsed or refractory AML or relapsed higher‑risk MDS;
|
| ·
| | Single-agent SY-1425 in approximately 25 newly-diagnosed AML patients who are not suitable candidates for standard chemotherapy;
|
| ·
| | Single-agent SY-1425 in approximately 25 patients with lower-risk, transfusion-dependent MDS;
|
| ·
| | SY-1425 in combination with azacitidine in approximately 25 newly-diagnosed AML patients who are not suitable candidates for standard chemotherapy; and
|
| ·
| | SY-1425 in combination with daratumumab in approximately 12 patients with relapsed or refractory AML or relapsed higher‑risk MDS.
|
All patients enrolled or to be enrolled in the trial are prospectively selected using our proprietary RARA or IRF8 biomarkers. At the European School of Hematology’s 4th International Conference on AML in October 2017, or ESH, we presented data from 201 evaluable patients screened in our ongoing Phase 2 clinical trial demonstrating that approximately 40% of patients were positive for either the RARA or IRF8 biomarker, or both, and that approximately one-third of the relapsed or refractory AML and higher-risk MDS patients tested were biomarker positive.
Tamibarotene, the active pharmaceutical ingredient of SY‑1425, has been extensively studied and has a well‑established safety profile. In our Phase 2 clinical trial, we are using the same dosage used in the treatment of acute promyelocytic leukemia, or APL, in Japan. This same dosage for SY‑1425 was previously used in a U.S. trial in relapsed and refractory APL, for which an IND was opened. We have exclusively in‑licensed from TMRC certain intellectual property rights controlled by TMRC and the preclinical data package that was used for approval in Japan and the IND filing in the United States for use in all cancer indications in North America and Europe.
At ASH 2017, we presented clinical data from our Phase 2 clinical trial evaluating SY-1425 as a single agent in 29 patients with relapsed or refractory AML or relapsed higher-risk MDS, and in 29 patients with lower-risk transfusion-dependent MDS. The median age100% of patients in the relapsed or refractory and higher-risk AML cohort was 72,triplet arm by the end of cycle one, compared with more than half of the patients having poor risk cytogenetics and 45% having two or more prior therapies. The median age60% of patients in the lower-risk MDS cohort was 76. Indoublet control arm. Consistent with prior clinical experience from the trial, we observed that chronic daily dosingsafety lead-in portion of SY-1425this study, tamibarotene administered at 6 mg/m2 orally divided in twocombination with approved doses of venetoclax and azacitidine was generally well-tolerated,well tolerated, and the overall safety profile demonstrated no additive toxicities or new safety signals, or evidence of increased myelosuppression compared to treatment with a median treatment durationthe doublet combination of 80 daysvenetoclax and patients treated up to eight months and remaining on study.azacitidine. The majority of non-hematologic adverse events observed in the trial were low grade, with the most commonly-reportedlow-grade and reversible, and rates of serious adverse events across all grades and causality being elevated triglycerides (36%), fatigue (31%), and dermatologic effects (28%). The most common Grade 3 or 4 adverse event was elevated triglycerides (16%).
Atwere comparable between the timestudy arms. As of the data cut-off, there was comparable exposure across the treatment arms, consisting of 66 days (ranging from 8-188) among patients treated with the combination of tamibarotene, venetoclax and azacitidine, and 75 days (ranging from 7-227) for presenting data at ASH 2017, 48 patients were evaluabletreated with the control. Patients will be followed for duration of response, assessment, including 23minimal residual disease-negative response, and survival. We continue to enroll patients in the relapsed or refractory AML or relapsed higher-risk MDS cohortSELECT-AML-1 and 25 patients in the lower-risk transfusion-dependent MDS cohort. Clinical activity was observed in ten of 23 (43%) evaluable patients with relapsed or refractory AML and higher-risk MDS, and two of the 25 (8%) evaluable lower-risk MDS patients, including:
| ·
| | Nine patients with improvements in hematologic parameters, four of whom achieved hematologic improvement lasting at least eight weeks, as defined by the Revised International Working Group, or IWG, criteria;
|
| ·
| | Five patients with reductions in bone marrow blasts, with one relapsed or refractory higher-risk MDS patient achieving a marrow complete response as defined by IWG criteria;
|
| ·
| | Thirteen of the 23 (57%) evaluable relapsed or refractory AML and higher-risk MDS patients had stable disease; and
|
| ·
| | No patients with lower-risk MDS achieved transfusion independence.
|
In addition, myeloid differentiation was observed in the bone marrow, consistent with the underlying mechanism of action of SY-1425 as a differentiating agent. The graphic below depicts myeloid differentiation in one relapsed or refractory AML patient starting after one cycle, with marrow blast reduction of greater than 25% beginning after two cycles and continuing to the start of the fourth cycle:
In addition to morphologic evidence of differentiation, immunophenotyping of bone marrow samples demonstrated induction of CD38, a marker of cell differentiation, after one 28-day cycle of treatment in 11 of 13 (85%) patients with pre- and post-treatment immunophenotyping samples.
We are no longer enrolling patients in the cohorts ofanticipate reporting additional data from the trial in which SY-14252024. There can be no guarantee that this additional data will replicate the results of the initial data that was being evaluated as a single agent. reported in December 2023.
Tamibarotene Market Opportunity
We believe that these clinical data, when combined with preclinical data showing the tumor-killing activity of SY-1425 in combination with azacitidine and with daratumumab, support the ongoing development of SY-1425 as a combination agent. SY-1425 has shown synergistic tumor-killing activity in combination with azacitidine as well as with daratumumab in preclinical models of RARA biomarker-positive AML. In combination with azacitidine, SY-1425 demonstrated greater cell death in vitro as well as greater clearance of tumor cells in bone marrow and other tissues and greater depth and duration of tumor response in PDX models, compared to either azacitidine or SY-1425 alone:
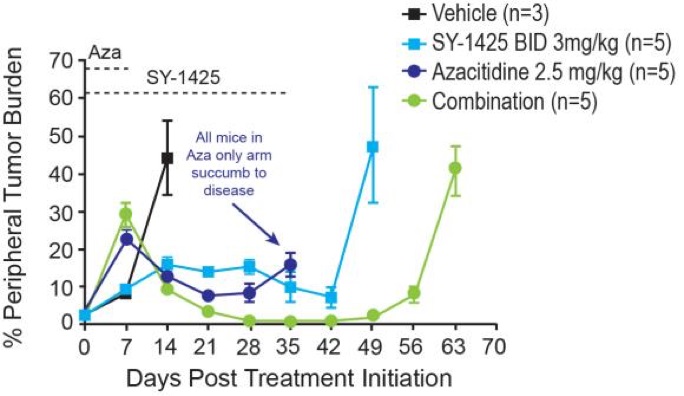
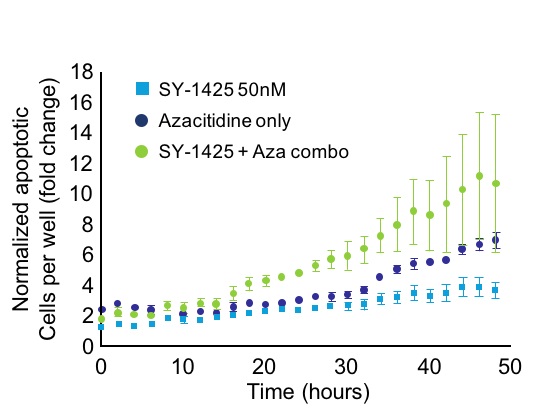
In combination with daratumumab, SY-1425 induced robust immune cell-mediated tumor death in vitro. Notably, AML cells do not normally express high levels of CD38. We have shown that by inducing CD38 expression, SY-1425 sensitizes biomarker-positive AML models to the tumor-killing effects of daratumumab. At the European Hematology Association 22nd Congress held in June 2017, we presented data showing that, by inducing CD38 expression, SY-1425 sensitizes biomarker-positive AML models to the tumor-killing effects of daratumumab. The preclinical studies showed that SY-1425 induced levels of CD38 expression in RARA-high AML cells comparable to those in multiple myeloma cells that are known to be responsive to daratumumab, and led to robust cell-mediated tumor
cell death in RARA-high AML cells when combined with daratumumab, as shown below:
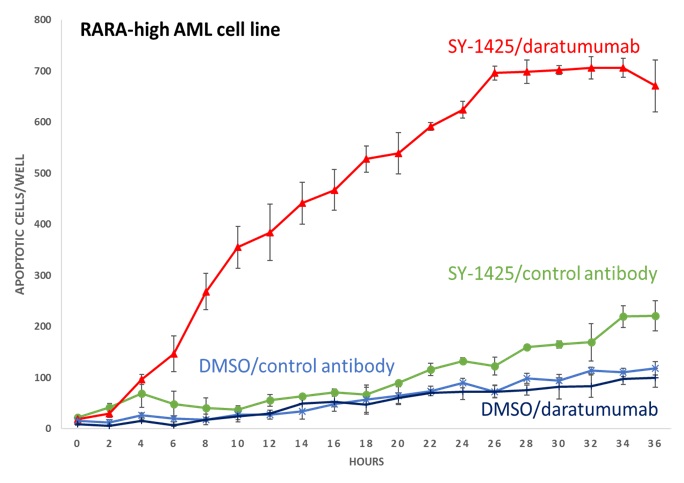
Our ongoing Phase 2 clinical trial is assessing the safety and efficacy of SY-1425 in combination with azacitidine in approximately 25 newly diagnosed AML patients who are not suitable candidates for standard chemotherapy and in combination with daratumumab in approximately 12 relapsed or refractory AML and higher-risk MDS patients. All patients enrolled or to be enrolled in the trial have been or will be prospectively selected using our proprietary RARA or IRF8 biomarkers. The primary endpoints for each of these cohorts of the trial are overall response rate, as determined by IWG criteria, as well as safety and tolerability. Secondary endpoints include duration of response, hematologic improvement and, for the daratumumab combination cohort, CD38 induction. In December 2017, we entered into a clinical supply agreement with Janssen pursuant to which Janssen agreed to supply us daratumumab for use in the trial. We expect to report initial clinical data from the combination cohorts of the trial in the fourth quarter of 2018.
We have entered into an agreement with a third party commercial provider to provide a validated laboratory test under Clinical Laboratory Improvement Amendment, or CLIA, guidelines using a well‑established diagnostic platform and approach that is being used to prospectively enroll RARA and IRF8 biomarker‑positive patients in our clinical trial. This CLIA laboratory test could become the basis for a commercial companion diagnostic. We are evaluating commercial providers to lead the development of a potential commercial companion diagnostic for these biomarkers, but have not yet selected a platform for development of a companion diagnostic test or entered in to an agreement with a third party for this work. We expect to do so in 2018.
We chose AML and MDS for our initial indications due to high levels of observed efficacy of SY‑1425 in our preclinical models, the significant unmet medical need of these patients and the potential for accelerated development. We intend to pursue additional indications, including breast cancer, upon establishing proof‑of‑concept in AML and MDS. Our preclinical data in breast cancer supports the development of SY‑1425 in genomically defined subsets of patients with breast cancers with our RARA biomarker. We also believe there are subsets of patients with other tumor types with our RARA biomarker and continue to research the role of the super‑enhancer associated with RARA in additional cancers.
Preclinical Data in Breast Cancer
Additionally, in our preclinical studies in breast cancer, many of which were presented at the San Antonio Breast Cancer Symposium in December 2016, we observed a strong link between sensitivity to treatment with SY‑1425 and breast cancer tumors with the RARA biomarker. As shown below, SY‑1425 was observed to result in significant tumor growth inhibition in PDX models derived from tumors with the RARA biomarker but was observed to have no effect in RARA biomarker-negative PDX models. Our in vitro studies have also shown that SY-1425 increased the anti-
tumor effects of standard-of-care therapies, including tamoxifen and palbociclib, in ER-positive breast cancer cells with high RARA expression and lapatinib in HER2-positive breast cancer cells with high RARA expression.

Prior Clinical Data
Tamibarotene, the active pharmaceutical ingredient of SY‑1425, is approved and marketed in Japan under the brand name Amnolake® for treatment of acute recurrent or intractable APL. Given the demonstrated efficacy of the drug in acute recurrent or intractable APL, we may evaluate SY‑1425 for treatment of APL in North America and Europe, but we do not have any current plans to do so. Extensive clinical work had been conducted on tamibarotene prior to us in‑licensing it from TMRC. The effectiveness of tamibarotene has been evaluated in patients with APL, including for relapsed patients and as maintenance therapy for newly diagnosed patients.
| ·
| | In a Phase 2 clinical trial of tamibarotene as a single agent in patients who relapsed following treatment with ATRA, 58% achieved a complete response. The majority of these patients went on to receive a bone marrow transplant or chemotherapy after treatment with tamibarotene and maintained a complete response for at least 14 months.
|
| ·
| | In a Phase 3 clinical trial comparing tamibarotene as an add‑on therapy to arsenic trioxide, or ATO, a standard of care treatment for APL, versus ATRA as an add‑on therapy to ATO in relapsed patients, patients in the tamibarotene‑treated group demonstrated:
|
| ·
| | An overall complete response rate of 80%, compared to 54% in the ATRA‑treated group (p=0.022); and
|
| ·
| | A complete molecular remission rate of 23%, compared to 3% in the ATRA‑treated group (p=0.0275). Complete molecular remission is achieved when there is no evidence of disease in the patient’s blood cells as detected by DNA‑based tests.
|
| ·
| | In a different Phase 3 clinical trial comparing tamibarotene to ATRA as maintenance therapy in newly diagnosed APL patients, the seven‑year relapse‑free survival rate in high‑risk patients treated with tamibarotene was 89%, compared to 62% in high‑risk patients treated with ATRA (p=0.034). In all patients, the seven-year relapse-free survival rate was 93% in the tamibarotene arm and was 84% in the ATRA arm (p=0.031).
|
In all these studies, tamibarotene was generally well tolerated. Adverse effects included mild or moderate dry skin, skin rash, headache and bone pain, as well as retinoic acid syndrome and elevated levels of cholesterol, lipids, liver function enzymes and white blood cells, which were severe in certain cases. One such adverse effect, retinoic acid syndrome, is referenced on the drug’s label and was infrequently observed clinically. Retinoic acid syndrome is a side effect associated with retinoids and arsenic trioxide and can be mitigated by regular monitoring of clinical parameters,
including white blood cell counts. A summary of four published clinical studies of tamibarotene use in APL is provided below.
| | | | | | | |
CRm
|
23%
| |
3%
|
Design | | Number
of
Patients | | Patient Population | | Tamibarotene
Treatment | | Efficacy / Duration |
Phase 2 in relapsed APL1 | | 25 | | Relapse after ATRA‑induced CR | | 6 mg/m2 daily, discontinued at CR | | CR = 58%
(14/24 evaluable)
(≥ 14 months duration in 5 patients in conjunction with BMT, 7 patients in conjunction with CT) |
Phase 3 tamibarotene vs. ATRA as APL maintenance2 | | 134 | | Front‑line following ATRA‑induced CR and consolidation | | Tamibarotene 6 mg/m2 vs. ATRA 45 mg/m2 14 days every 3 months for 2 years | | Overall 7‑year RFS: 93% vs. 84%;
7‑year RFS in high risk: 89% vs. 62% (tamibarotene vs. ATRA) |
Phase 2 in relapsed/refractory APL after ATRA and ATO3 | | 14 | | Patients with prior lines of treatment (9 with 2 prior lines, 3 with 3 prior lines and 2 with 5 prior lines) | | 6 mg/m2 daily for 56-day induction period then every other month as consolidation for up to one year | | CR = 36%
CRi = 29%
mEFS = 3.5 months
mOS = 9.5 months |
Phase 3, tamibarotene vs. ATRA as add-on to ATO in relapsed APL4 | | 71 | | | | 6 mg/m2/day tamibarotene, 25 mg/ m2/day ATRA add‑on to 0.15 mg/kg/day ATO for 56 days | | CR
CRm | Tamibarotene +ATO 80%
23% | | ATRA + ATO 54%
3% |
Table legend:
CR = complete remission
| CRm = complete molecular remission
| CRi = complete remission with incomplete blood count recovery
|
RFS = relapse‑free survival
| mEFS = median event‑free survival
| mOS = median overall survival
|
BMT = bone marrow transplant
| CT = chemotherapy
| |
| 1.
| | Tobita, et al. Blood, August 1997.
|
| 2.
| | Takeshita, et al. American Society of Hematology presentation, December 2017.
|
| 3.
| | Sanford D, et al. British Journal of Haematology, July 2015.
|
| 4.
| | Wang et al, American Society of Hematology presentation, December 2015.
|
Tamibarotene was also studied in a Phase 2 clinical trial for the treatment of unselected late‑stage non‑small cell lung cancer under a previous license between TMRC and a third party. The trial evaluated the efficacy and safety of adding tamibarotene or placebo to paclitaxel and carboplatin in patients with stage IIIb (plus pleural effusion) or IV non‑small cell lung cancer. This trial was terminated when interim data suggested that a primary endpoint of progression‑free survival for 18 months after starting therapy would not be reached. Interim data also showed that tamibarotene combined with paclitaxel and carboplatin chemotherapy was associated with increased toxicity in this non‑selected non‑small cell lung cancer patient population.
SY‑1425 Market Opportunity
We believe that SY‑1425 has the potential to address significant unmet medical need across a range of blood cancersfor HR-MDS and solid tumors. At ESH, we presentedunfit AML patients with RARA gene overexpression. Based on data from 201 evaluableour clinical trials, we believe approximately 50% of HR-MDS patients screened in our ongoing Phase 2 clinical trial demonstratingand approximately 30% of AML patients are positive for RARA overexpression.
We believe that approximately 40% of18,500 patients were positive for either the RARA or IRF8 biomarker, or both, and that approximately one-third of the relapsed or refractory AML and higher-risk MDS patients tested were biomarker positive.
There are an estimated 33,000 new AML diagnosesdiagnosed with HR-MDS in the United States Canada and Europe annually and we expect the five largest European countries each year. While several new drugstotal global market for the treatmentmyelodysplastic syndrome, or MDS, patients of AMLall risk groups to grow to approximately $4.7 billion by 2028. HR-MDS is progressive in nature and has a poor prognosis. Disease-related cytopenias result in significant morbidity and mortality, with more than half of HR-MDS patients progressing to AML. There have been approvedno new drug approvals for HR-MDS since 2006 other than hypomethylating agents, or HMAs. Azacitidine is an HMA that represents the current standard of care but offers a low CR rate of 17%, with a median overall survival of approximately 18.6 months.
In addition, we believe that approximately 25,000 patients are diagnosed with unfit AML in the last year,United States and Europe annually and we expect the overall total global market for all AML remains an areapatients to grow to approximately $7.5 billion by 2028. Despite a significant number of new product approvals in AML since 2018, there continues to be a significant unmet medical need. According to the American Cancer Society, newly diagnosed AML patients in the United States have a 27% five‑year survival rate. MoreIt is estimated that more than half of newly diagnosed AML patients are elderly or unfit for treatment with intensive therapies, underscoring the need for well-tolerated therapies that can be used in combination. Venetoclax with azacitidine is the standard therapies, leaving this groupof care, with limiteda 66% CR/CRi rate, a 37% CR rate and median OS of 14.7 months. Approximately one-third of patients do not respond, and nearly all relapse with a very poor prognosis, with median OS of only 2.4 months.
Other Assets
SY-2101
SY-2101 was previously in development for the treatment options and an average survival of less than one year. Despite initial responses to therapy in select patients, the majorityacute promyelocytic leukemia, or APL, a subtype of AML defined by a fusion of the RARA and promyelocytic leukemia genes. APL represents approximately 10% of all AML cases, and approximately 2,000 patients relapse or become refractory to current treatment options. There are an estimated 10,000 cases of relapsed or refractory AML each yeardiagnosed with APL in the United States Canada and the five largest European countries each year. In the absence of adequate therapies, these relapsed or refractory patients may be put into clinical trials for new and emerging therapies, and average survival of these patients is estimated to be less than six months.
Europe annually. An intravenously
There are approximately 32,000 new MDS diagnosesarsenic trioxide, or ATO, is approved for use in the countries listed above each year,combination with All-Trans-Retinoic-Acid, or ATRA, in patients with newly diagnosed lower-risk APL and, while curative in more than 80% of patients, its administration requires up to one‑third140 two- to four-hour infusions over the typical course of these newly diagnosed patients estimatedinduction and consolidation treatment. We believe SY-2101 has the potential to be likely to progress to AML. Of these patients, approximately 7,500 MDS patients have newly-diagnosed, higher‑risk MDS. Inbecome the United States, newly-diagnosed, higher‑risk MDS patients are expected to survivestandard-of-care frontline therapy for 0.8-1.6 years, and relapsed or refractory higher-risk AML patients have an average survival of less than six months. As with AML, treatment options for these patients are limited, with no new drugs having been approved forAPL by providing a substantially more convenient option that reduces the treatment relapsed or refractory, higher-risk MDS in overburden on patients, improving access, and lowering costs to the healthcare system. In a decade.
There are an estimated 2,500 new APL cases diagnosed in the United States, Canada and the five largest European countries each year. Despite advances in treating APL, approximately 20-30% of APL patients relapse and require salvage therapy.
SY‑1365
Overview
SY‑1365 is a highly potent and selective small molecule CDK7 inhibitor. CDK7, a member of the cyclin‑dependent kinase, or CDK, family, is a transcriptional kinase that plays a central role in the expression of key tumor-driving genes, transcription factors, and anti-apoptotic proteins. CDK7 activity has been implicated in various solid tumors with transcriptional dependencies. Inhibiting CDK7 preferentially lowers the expression of disease‑driving transcription factors controlled by super‑enhancers, and results in the preferential killing of cancer cells over non‑cancerous cells. Using our platform, we have generated several potent and selective small molecule CDK7 inhibitors, including SY‑1365.
SY-1365 is currently in aprior Phase 1 clinical trial, SY-2101 demonstrated bioavailability, pharmacokinetic exposures similar to IV ATO, and a generally well-tolerated safety profile. Based on an interim analysis from our Phase 1b dose confirmation study of SY-2101 in adult APL patients undergoing consolidation treatment with IV ATO plus ATRA, administration of SY-2101 has demonstrated comparable exposures to IV ATO at the approved dose of 0.15 mg/kg, high oral bioavailability, and a well-tolerated safety profile, consistent with the prior Phase 1 study. In October 2023, we announced that we would stop further development in SY-2101 in order to prioritize the ongoing development of tamibarotene. We may in the future pursue further development of SY-2101 subject to additional capital availability.
SY‑5609
We are currently seeking out-licensing opportunities for the further development of SY-5609, our highly selective and potent inhibitor of cyclin dependent kinase 7, or CDK7. At the American Society for Clinical Oncology Annual Meeting held in June 2023, or ASCO 2023, we presented data from the completed Phase 1/1b clinical trial evaluating SY-5609 in patients with advancedrelapsed/refractory pancreatic ductal adenocarcinoma, or PDAC, hormone receptor positive, or HR+, breast cancer and other solid tumors. We believe that SY-1365 is the most advanced selective CDK7 inhibitor in clinical development. SY-1365, alone and in combination with other therapeutic agents, has shown significant anti-proliferative and pro-apoptotic activity in multiple in vitro and in vivo modelsThe Phase 1/1b trial of difficult-to-treat solid tumors, including ovarian and breast cancers. SY-1365 has induced anti-tumor activity in both cell line-derived xenograft and patient-derived xenograft models, including tumor regressions atSY-5609 included a twice weekly dosing regimen consistent with the initial regimen being evaluated in our Phase 1 clinical trial. SY-1365 has also shown to anti-tumor activity in models of blood cancers such as acute leukemias, as evidenced by in vitro cell death or complete tumor growth inhibition in cell-derived xenografts. Finally, SY-1365 has been shown to lower the expression of oncogenic transcription factors, including MYC, a mechanistic effect consistent with inhibition of CDK7.
SY‑1365 Clinical Development Plan
In April 2017, the FDA accepted our investigational new drug, or IND, application to advance SY-1365 into a Phase 1 clinical trialdose escalation study evaluating single agent SY-5609 in patients with select advanced solid tumor malignancies for whom standard curative or palliative measures do not exist or are no longer effective. This trial began enrolling patients in the second quarter of 2017. The trial is testing the safety and tolerability of escalating doses of SY-1365 with the goal of establishing a maximum tolerated dose and a recommended Phase 2 dose and schedule. Additional study objectives include assessing pharmacodynamic changes and early signs of biological activity using biomarkers and clinical efficacy as measured by response rate using radiographic measures. We are currently exploring both a twice-weekly and a once-weekly dosing regimen, and we anticipate that approximately 35 patients will be enrolled in the dose escalation phase of the trial.
Once a maximum tolerated dose is reached in the dose escalation phase of the Phase 1 clinical trial and the recommended dosing schedule is identified, we intend to open expansion cohorts evaluating SY-1365 in multiple ovarian cancer populations as a single agent as well as in combination with carboplatin, a chemotherapeutic agent. The ovarian cancer populations include a 24-patient cohort evaluating SY-1365 as a single agent in patients who have relapsed after three or more prior therapies, a 24-patient cohort evaluating SY-1365 in combination with carboplatin in patients who relapsed after one or more prior therapies but who may still benefit from additional platinum-based treatment, and a 12-patient pilot cohort evaluating SY-1365 as a single agent in primary platinum-refractory disease. We also plan to evaluate SY-1365 in combination with fulvestrant, a hormonal medicine, in 12 patients with HR+, HER-2-negative metastatic breast cancer who have progressed after treatment with a CDK4/6 inhibitor and aromatase inhibitor.
In addition, we plan to evaluate the mechanism of action of SY-1365 in ten patients with any solid tumor accessible for biopsies. A schematic of our Phase 1 clinical trial of SY-1365 is below:
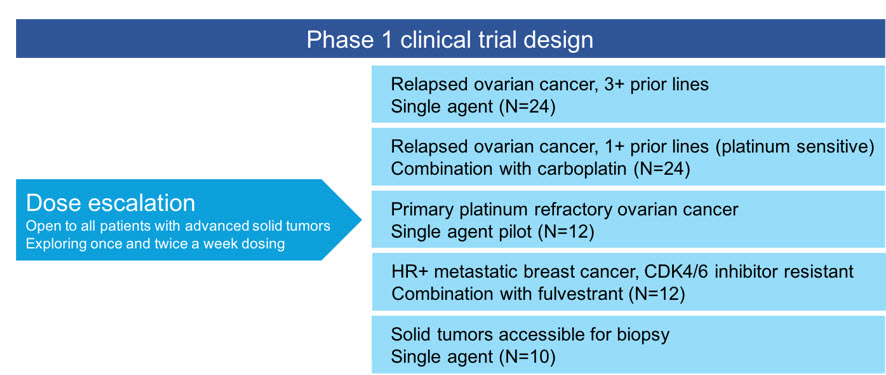
We expect that the expansion cohorts in our Phase 1 clinical trial will be open to enrollment in mid-2018 and that we will report clinical data from the dose-escalation phase of our Phase 1 trial, including safety and pharmacokinetic/pharmacodynamic data, in the fourth quarter of 2018.
Our Preclinical Data
Our clinical development program for SY-1365 is based on a robust preclinical development program showing significant anti-proliferative activity of SY-1365 in multiple in vitro and in vivo models of difficult-to-treat solid tumors and blood cancers, including ovarian cancer, breast cancer and AML.
To evaluate the in vivo activity of SY-1365 in ovarian cancer, we evaluated SY-1365 in a number of patient-derived xenograft models derived from heavily-pretreated ovarian cancer patients and compared tumor growth in mice administered SY-1365 twice-weekly at the maximum tolerated dose in that species to untreated mice. Significant tumor growth inhibition was observed in many of these models following administration of SY-1365, with data from a representative model set forth in the graphic below:
We have also shown the synergistic effect of treatment with SY-1365 and platinum-based chemotherapy in several ovarian cancer cell lines, as exemplified in the graphic below:
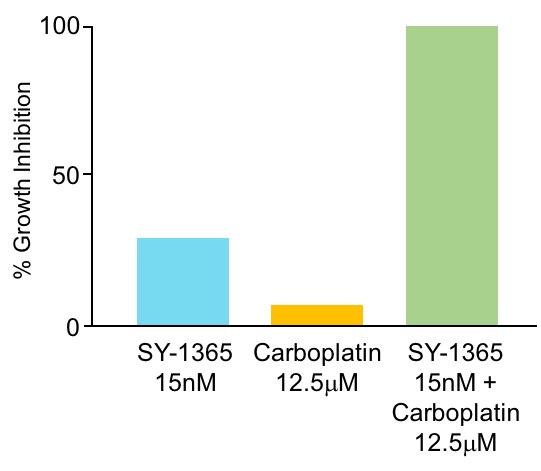
The inhibitory activity of SY-1365 was evaluated in vitro in many breast cancer cell lines, representing different subgroups of breast cancer. SY-1365 showed anti-tumor activity in all subgroups, inducing cell death in 15 of 19 TNBC cell lines and 17 of 21 HR+ and HER2-positive cell lines. At the San Antonio Breast Cancer Conference held in December 2017, we presented the results of our analysis of the anti-tumor activity of SY-1365 in xenograft models of TNBC. In the graphic below, we showed a complete response in mice administered SY-1365 twice weekly at the maximum tolerated dose in that species as compared to untreated mice.
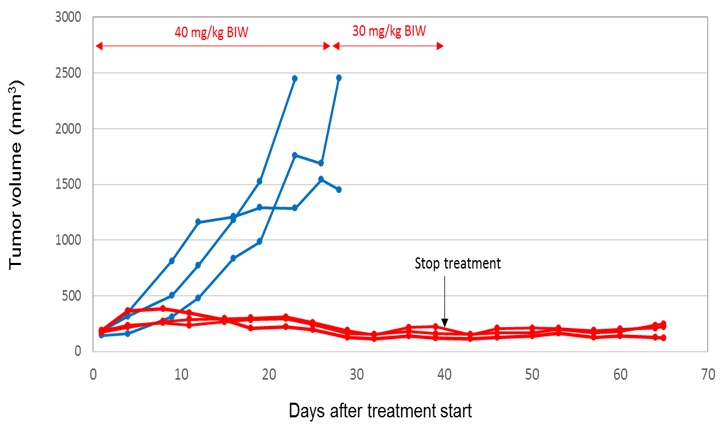
We, together with several of our academic collaborators, have demonstrated that SY-1365 could inhibit many HR+ cancer cells, including those with a form of acquired resistance to a class of hormonal treatments known as aromatase inhibitors, and that SY-1365 was able to inhibit breast cancer cell lines in vitro that no longer respond to inhibitors of CDK4/6. In addition, SY-1365 showed in vitro synergy with fulvestrant in several HR+ breast cancer cell lines. SY-1365 also showed a combination effect with fulvestrant in an in vivo model of HR+ breast cancer, as shown in the graphic below. We believe that these data, taken together, provide a mechanistic rationale for evaluating SY-1365 in combination with fulvestrant in HR+ breast cancer, and a combination safety lead-in designed to inform a dose expansion study evaluating the doublet regimen of SY-5609 and gemcitabine and the triplet regimen of SY-5609, gemcitabine and nab-paclitaxel in patients with HR+ metastatic breast cancer who have progressed following treatment with a CDK4/6 inhibitor plus an aromatase inhibitor.PDAC in their second or third line of treatment.
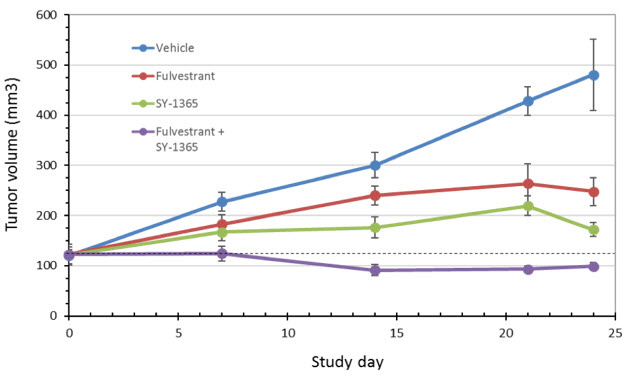
We also believe that SY-1365 has potential in blood cancers as well as in other solid tumor malignancies. The preclinical rationale for potential development of SY-1365 in blood cancers includes data we presented at ASH 2017 demonstrating that SY-1365:
| ·
| | inhibited proliferation in vitro in leukemia and lymphoma cells, as well as leukemia cells from primary patient cultures;
|
| ·
| | induced cell death in the majority of AML, leukemia and lymphoma cell lines tested; and
|
| ·
| | inhibited tumor growth, including inducing tumor regression, using bi-weekly dosing in preclinical mouse models of AML.
|
In addition, weASCO 2023 demonstrated that SY-1365 synergizedthe maximum tolerated dose, or MTD, of SY-5609 as a single agent was 10 mg using a 7 day on/7 day off dosing schedule. For the doublet regimen, the MTD was 5 mg SY-5609 plus 1000 mg gemcitabine. A MTD was not established using the triplet cohort of SY-5609, gemcitabine and nab-paclitaxel. Each of the single agent, doublet and the triplet regimens were generally well-tolerated with venetoclax, an investigational product being developedmostly low-grade events. Encouraging clinical activity was observed with SY-5609 both as a single agent (10 mg) and in AML, in AML cell lines in vitro, and that, as showncombination (4 or 5 mg plus gemcitabine). Among the three response evaluable patients with select solid tumors in the graphic below,10 mg single agent cohort, data demonstrated a 100% disease control rate, or DCR. This included one patient with PDAC who experienced a 10% tumor reduction. Of the administrationnine response-evaluable patients treated with 4 or 5 mg of SY-1365 plus venetoclaxSY-5609 in combination with gemcitabine, the data demonstrated a 44% DCR (four patients). The 10 mg single agent DCR of 100% is superior to results observed with lower doses of SY-5609 on the 7 day on/7 day off schedule and the doublet DCR of 44% is comparable to current second-line benchmarks.
Patients enrolled in the fulvestrant cohort of the study presented with advanced disease: 78.6% (11 of 14 patients) had liver metastases and were heavily pre-treated, 78.6% (11 of 14 patients) had received five or more prior therapies, 100% (14 of 14 patients) had progressed on CDK4/6 therapy, and 85.7% (12 of 14 patients) had received prior fulvestrant. The combination of SY-5609 and fulvestrant demonstrated an in vivo model resulted in greater tumor growth inhibition than eitheracceptable safety profile across a variety of dosing schedules. The adverse event profile of the combination was generally consistent with the safety profile of single agent alone.
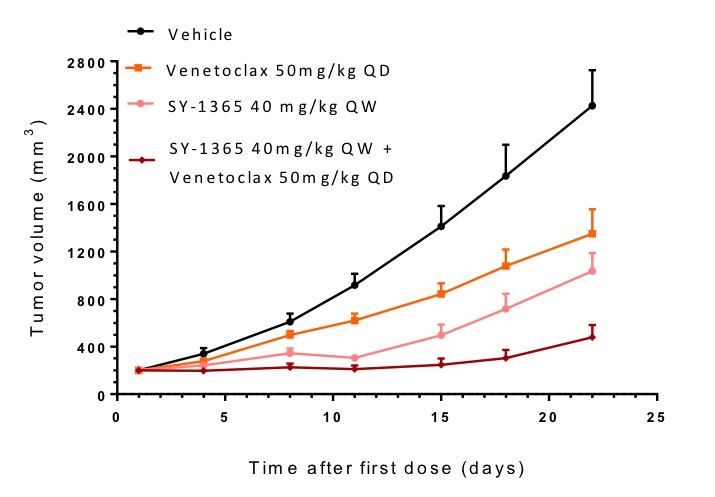
We also presented dataSY-5609 or fulvestrant, with no new safety signals emerging from the combination at the 2017 AACR-NCI-EORTC Molecular Targetsevaluated doses and Cancer Therapeutics conference showing the relationship between the pharmacokinetic, pharmacodynamic and anti-tumor activity of SY-1365 in multiple preclinical models of TNBC and AML,dosing schedules. An MTD was not established. Twelve patients were evaluable for response across a range of doses and regimens from daily to weekly dosing. These data showeddosing schedules. Five of 12 achieved stable disease, or SD, for a prolonged pharmacodynamic effect, as measured by CDK7 target occupancy, with a half-lifeDCR of approximately42%; three days, which supports the intermittent dosing regimen being evaluated in our ongoing Phase 1 clinical trial. We are using an assay measuring target occupancy as a target engagement marker in this trial. These data also showed a dose-dependent relationship between CDK7 target occupancy and anti-tumor activity in a preclinical model of AML, and sustained tumor regressions in multiple in vivo models using a twice-weekly dosing regimen.
SY‑1365 Market Opportunity
With SY‑1365, we believe we have the opportunity to address significant unmet medical needs across a range of cancers. The initial disease-specific focus of our clinical development program for SY‑1365 is expected to be in ovarian cancer and HR+ breast cancer.
Ovarian cancer is the fifth most common cause of cancer death in women. There are an estimated 59,000 ovarian cancer diagnoses each year in what we refer to as the developed pharmaceutical markets – the United States, Canada, Japan and the five largest European countries by population, which are Germany, the United Kingdom, France Spain and Italy. Of these, approximately 70% have high-grade serous ovarian cancer and present with advanced disease at initial diagnosis. The standard of care treatment for these patients is platinum-based chemotherapy. It is estimated that 10-15% of these five patients are “platinum-refractory,” which means that they progress during, or in lessachieved target lesion regression. Three patients remained on treatment with SD for greater than one month of completion of, platinum-based treatment. There are limited treatment options for platinum-refractory patients. Approximately 30% of these patients are “platinum-resistant,” which means that they progress within six months, of completing platinum-based treatment. We believe that subsequent treatment options for theseincluding patients atwith the present time have limited activity and significant toxicities. Approximately 55-60% of these patients are “platinum-sensitive,” which means that they initially respond to platinum-based treatment, but progress after six months TP53 mutation, prior fulvestrant exposure and/or more. The majority of these patients relapse within three to five years. The planned expansion cohorts of our Phase 1 clinical trial of SY-1365 include patients who have progressed after platinum-based treatments.liver disease.
Breast cancer is the most common tumor type in women and is the leading cause of cancer death in women worldwide. According to the American Cancer Society, approximately 266,000 new breast cancer cases will be diagnosed in the United States in 2018, with approximately 41,000 deaths from the disease. Approximately 80% of breast cancers express estrogen receptors, progesterone receptors, or both. Hormone-based therapies are the cornerstone for these HR+ cancers. In metastatic breast cancer, AIs are frequently used as a first-line treatment option. Recent approvals of a class of drugs that inhibit CDK4/6, such as palbociclib and ribociclib, have resulted in their use in combination with AIs for the treatment of HR+, HER2-negative, breast cancers. Patients who relapse following treatment with an AI in combination with a CDK4/6 inhibitor currently have few effective treatment options.
Other Programs
We currently have five programs in our preclinical and discovery pipeline, including preclinical programs directed to the development of a CDK7 inhibitor that can be administered orally, inhibitors of cyclin-dependent kinase 12/13, and inhibitors of an immuno-oncology target, as well as discovery programs related to a gene control target to treat sickle cell disease and in the field of cancer. We plan to nominate a development candidate from one of our preclinical programs during 2018 that we can advance into studies to support a potential IND filing in 2019, consistent with our objective of filing, on average, an IND every other year.
We are using our platform to analyze gene expression programs in tumors and immune cells across various cancers and monogenic diseases to identify optimal points of therapeutic intervention in specific subsets of patients and to create a pipeline of novel product candidates targeting transcriptional and regulatory proteins. We are also using our platform in collaboration with third parties to identify and validate targets in diseases beyond our current areas of focus. To this end, we entered a target discovery, research collaboration and option agreement with Incyte in January 2018 under which we will use our platform to identify novel therapeutic targets with a focus on myeloproliferative neoplasms. See “—License and Collaboration Agreements–Incyte” below.
Research and Development Expense
During the years ended December 31, 2017, 2016 and 2015, our research and development expenses were $41.9 million, $37.8 million and $24.4 million, respectively. For additional information regarding our research and development expense as well as our revenues, operating loss, and total assets, see the sections of this report entitled, “Financial Statements” in Part II, Item 8 and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in Part II, Item 7.
Intellectual Property
We file patent applications directed to our gene control platform, proprietary compositionvarious compositions of matter, formulations and methods related to our product candidates and compounds in an effort to establish intellectual property positions regarding all aspectsearlier stages of our business, including new chemical entities, or NCEs,development and uses of these NCEs in the treatment of diseases.other commercially relevant inventions. As of February 28, 2018,December 31, 2023, we own or co-own 25 issued U.S. patents and 16 pending U.S. utility patent applications, excluding patents owned by Tyme Inc., our wholly owned subsidiary. We are pursuing or maintaining 131 corresponding patent applications that are pending or granted in various jurisdictions outside the United States, including Europe, Japan, Australia, Canada and China, and we own two issued U.S. patents, 12 pending U.S. provisional patent applications twelve U.S. pending patent applications, 41 foreign applicationsthat are pending in a number of jurisdictions, including Europe, Australia, Japan, China, and Canada, and four pendingaccordance with the Patent Cooperation Treaty, or PCT, patent applications. In addition, as of February 28, 2018, we have exclusively licensed six issued U.S. patents, three U.S. pending patent applications, five issued foreign patents and 12 foreign patent applications pending in a number of jurisdictions, including Australia, Canada, China, Europe, and Japan.Treaty. A significant portion of our ownedthe patents and licensed pending patent applications we own pertain to our product candidates that are in clinical development,
to methods of using them in the treatment of disease, and associated biomarkers, key discovery and preclinical programs, specifically our CDK7 inhibitor program, and transcription factor modulators, and our gene control platform.to methods of selecting patients for treatment based on biomarker expression.
Our intellectual property portfolio as of February 28, 2018December 31, 2023 is summarizedfurther described below. For some of our pending patent applications, prosecution has yet to commence. ProsecutionProsecuting patent applications to allowance is often a lengthy process, during which the scope of the claims initially submitted for examination by the U.S. Patent and Trademark Office, or USPTO,various patent offices is often significantly narrowed, byand some claims may never be granted. It is possible that we will amend the time they issue, if they issue at all. We expect this to be the case with respect toclaims of our pending patent applications referred to below. In addition, welimit their scope. We may also elect to abandon prosecution of some of our pending patent applications, particularly those pending outside of the United States, if we determine that these applications do not have strategic significance to our programs or platform.programs.
SY‑1425
Our owned intellectual propertyThe patent portfolio we own for SY‑1425tamibarotene contains twoseven issued U.S. patents, twofour pending U.S. pendingutility patent applications, 11 foreignand 66 applications pending or granted in a number of jurisdictions,countries other than the United States, including Europe, Japan, Australia, Japan,Canada, China, Russia, Israel and Canada,Mexico. Some of these patents and three pending PCT patent applications directeddisclose methods of identifying and treating patients who are sensitive to patient stratification methodsRARα agonists, including tamibarotene, based on the expression of certain biomarkers, combinations andincluding RARA. Some of the applications disclose methods of use for agoniststreating selected patients with tamibarotene alone or with a combination of RARα, including SY-1425.tamibarotene and a second agent, such as azacitidine. One of our issued patents, U.S. Patent No. 9,845,508, covers a methodmethods of diagnosing and treating human patients suffering from non-APL AML by administering tamibarotene; the patients by determining whether they have elevated levelsare diagnosed based on the level of RARA messenger RNA, or mRNA, and, if they do, administering SY-1425.previously determined to be present in a sample of diseased cells from the subject. The granted claims of a second patent, U.S. Patent No. 10,167,518, cover methods of treating human subjects suffering from MDS. Selection of subjects for treatment is again based on the level of RARA mRNA expression, and subjects with RARA overexpression are treated with tamibarotene. A third patent, U.S. Patent No. 9,868,994, covers a methodmethods of treating non-APL AML andor MDS by administering SY-1425tamibarotene to patients knowna patient when a defined sample obtained from the patient is determined to have an elevated level of IRF8 mRNA or elevated levels of both IRF8 and RARA mRNA. For purposesA fourth patent, U.S. Patent No. 10,240,210, covers methods of these patents,treating non-APL AML does not include acute promyelocytic leukemia, or APL.MDS with a combination of tamibarotene and azacitidine when a defined sample from the subject has been determined to have an elevated RARA mRNA level or an elevated IRF8 mRNA level. A fifth patent, U.S. Patent No. 10,697,025, covers methods of treating subjects who have non-APL AML with tamibarotene; treatment proceeds when a sample of diseased cells from the subject was determined to have a super enhancer associated with a RARA gene or a level of primary RNA transcripts from the RARA gene that is equal to or above a pre-determined threshold. A sixth patent, U.S. Patent No. 11,053,552, covers methods of treating non-APL AML or MDS by administering a combination of tamibarotene and a second therapeutic agent, with further specification of the analysis of an IRF8 biomarker and/or a RARA biomarker. A seventh patent, U.S. Patent No. 11,447,831, covers methods of treating subjects who have MDS with tamibarotene; treatment proceeds when a sample of diseased cells from the subject is determined to have a super enhancer associated with a RARA gene or a level of primary RNA transcripts from the RARA gene that is equal to or above a pre-determined threshold. We believe that our currently-issuedthese seven U.S. patents related to our SY-1425 program are eligible for listing in the U.S. Food and Drug Administration’s OrangeFDA’s “Orange Book. The U.S.” These patents, and pending applications andas well as any U.S. or non‑U.S.additional patents that may grant from applications claiming priority to these pending applications, if issued,the benefit of the same filing date as the currently granted patents, have statutory expiration dates no earlier than March 2036. Patent term extensions could result in later expiration dates.
In addition, we have an exclusive license from TMRC Co. Ltd., or TMRC, to practice the inventions claimed in one U.S. patent and five patents or applications granted or pending in the United States or other jurisdictions, including Canada and Europe. The claims of the U.S. patent are exclusively licenseddirected to a tamibarotene capsule preparation. We do not have composition of matter patent protection with respect to tamibarotene.
The patent portfolio we own for SY‑2101 contains patents that have granted in North Americathe United States, Europe, Japan, China, Australia, Taiwan, and Mexico, and patent applications that are pending in the United States, Europe under two issued U.S.and other major pharmaceutical markets. Generally, these patents and five issued foreignapplications disclose methods of making lyophilized compositions comprising arsenic and formulations containing the lyophilized arsenic that can be orally administered to patients having APL and other hematological malignancies. These patents, as well as any additional patents that may grant from applications claiming the benefit of the same filing date as the currently granted patents, have statutory expiration dates no earlier than February 2036. Patent term extensions could result in later expiration dates.
The patent portfolio we own for SY-5609 contains patents that have granted in the United States, Europe, Japan and several additional countries, and patent applications that are pending in Canadathe United States, Europe and Europe,other major pharmaceutical markets generally directed to the compound SY-5609 and related CDK7 inhibitors, pharmaceutical kitsformulations containing them, and drug combinations comprising tamibarotenemethods of making and certain other chemotherapeutic agents, certain formulationsusing them. Any patent that has issued or will issue and that
claims the benefit of tamibarotene, and crystal formsthe priority date of tamibarotene and their preparation. One licensed issued U.S.one or more of these patents or patent covering formulations hasapplications will have a statutory expiration date of April 2028. The other licensed issued U.S. patent covering crystals has a statutory expiration date of August 2021.ranging from October 2034 to November 2042. Patent term adjustments or patent term extensions could result in later expiration dates for each of these patents. We do not have composition of matter patent protection with respect to SY‑1425.dates.
SY‑1365
The intellectual property portfolio for SY‑1365 and our other CDK7 inhibitors contains patent applications directed to compositions of matter for our compounds and analogs, compositions of matter for CDK7 inhibitors having different structural features (i.e., different compound families), as well as methods of use, biomarkers, and formulations for these novel compounds. As of February 28, 2018, we own six pending U.S. patent applications, 29 pending foreign applications in a number of jurisdictions, including Europe, Canada, China, Japan and Australia and one pending PCT patent application and seven pending U.S. provisional applications, directed to this program. Any U.S. or non‑U.S. patents issuing from these pending applications or applications claiming priority to the pending applications covering our compounds and related methods of use will have a statutory expiration date ranging from October 2034 to January 2039. Patent term adjustments or patent term extensions could result in later expiration dates.
We are also exclusively licensed under one U.S. patent, two pending U.S. patent applications and 11 pending foreign patent applications in a number of jurisdictions, including Australia, Canada, Europe, and Japan, directed to this program.
Other Programs
The intellectual property portfolio for our other programs contains patents and patent applications directed to compositions of matter for inhibiting transcription factors and immuno-oncology targets in multiple compound families, and methods of treating various diseases, including cancer and immunological diseases, through inhibition of specific transcription factor(s) or gene products. As of February 28, 2018, we own five U.S. provisional patent applications and three U.S. patent applications and are exclusively licensed to two issued U.S. patents directed to our other programs. The licensed U.S. patents have statutory expiration dates of July 2032 and November 2033. Any U.S. or non‑U.S. patents issuing from the pending applications or applications claiming priority to the pending applications covering transcription factor inhibitors, immuno-oncology target inhibitors or methods of treating disease by inhibition of transcription factors or gene products will have statutory expiration dates ranging from February 2031 to December 2038.
Platform
The intellectual property portfolio directed to our platform includes patent applications and patents directed to super‑enhancers and their detection and uses thereof to detect novel disease targets. As of February 28, 2018, we own one pending U.S. patent application and one pending patent application in Europe directed to these technologies which, if issued, will have a statutory expiration date of March 2034. In addition, we have an exclusive license to one issued U.S. patent, one U.S. pending patent application and one pending foreign patent application in Europe, directed to these technologies. The U.S. and foreign patent applications that we own are directed to the identification of new super‑enhancer components and methods of treating diseases by targeting those novel components, and if issued, will have a statutory expiration date no earlier than March 2034. The licensed U.S. patent has a statutory expiration date of October 2033 and the licensed pending applications directed to super‑enhancers and their detection and uses thereof to detect novel disease targets, if issued, will have a statutory expiration date no earlier than October 2033.
The term of individual patents depends upon the legal term for patents in the countries in which they are obtained. In most countries, including the United States, thea patent term isexpires 20 years from theits earliest effective filing date of a non‑provisional patent application.date. In the United States, a patent’s term may be lengthened by patent term adjustment, which compensates a patenteeto compensate for administrative delays by the U.S. Patent and Trademark Office, or USPTO, in examining and granting a patent or may be shortened if a patent is terminally disclaimed over an earlier filed patent. The term of aA patent that covers a drug or biological producttherapeutic agent may also be eligible for patent term extension when FDA approval is granted, provided statutory and regulatory requirements are met. See “—Government Regulation and Product Approvals—Marketing Authorization and Exclusivity”Authorization” below for additional information on such exclusivity. In the future, ifIf and when our product candidatesproducts receive approval by the FDA or foreign regulatory authorities,agencies in other countries, we expect to apply for a patent term extensionsextension on an issued patentspatent covering those drugs,a given product, depending upon the length of the clinical trials for each drugproduct and other factors. There can be no assurance that any of our pending patent applications will issue or that we will benefit from any patent term extension or favorable adjustment to the termterms of any of our patents.
As with other biotechnology and pharmaceutical companies, our ability to maintain and solidify our proprietary and intellectual property position forexclude others from making, using, or selling our product candidates and technologiesother inventions will depend on our success in obtaining effectivevalid patent claims and enforcing those claims, if granted. Ourclaims. One or more of our pending patent applications, and any patent applications that we may in the future file or license from third parties in the future may not, however, result in the issuance of patents.proceed to grant as an issued patent. We also cannot predict the breadth of claims that may be allowed or enforced in our patents. Any issued patents that we may receive in the futurepatent may be challenged, invalidated or circumvented. For example, we cannot be certain of the priority of inventions covered by pending third‑party patent applications. If third parties prepare and file patent applications in the United States that also claim technology or therapeutics to which we have rights, we may have to participate in interference proceedings in the USPTO to determine priority of invention, which could result in substantial costs to us, even if the eventual outcome is favorable to us, which is highly unpredictable. In addition, because of the extensive time required for clinical development and regulatory review of a product candidate we may develop, it is possible that, before any of our product candidates can be commercialized, any related patent may expire or remain in force for only a short period following commercialization, thereby limiting protection such patent would afford the respective product and any competitive advantage such patent may provide.
In addition to patents and pending patent applications, we rely upon unpatented trade secrets andunpatentable know‑how and continuing technological innovation to develop and maintain our competitive position. We seek to protect our proprietary information, in part, by executing confidentiality, agreements with our collaboratorsinvention assignment, and scientific advisors, and non‑competition, non‑solicitation, confidentiality, and invention assignmentnon-solicitation agreements with our employees, and consultants. We have also executed agreements requiring assignment of inventions with selectedcollaborators, scientific advisors and collaborators.consultants as appropriate. The confidentiality agreements we enter into are designed to protect our proprietary information and the agreements or clauses requiring assignment of inventions to us are designed to grant us ownership of technologies that are developed through our relationship with the respective counterparty. We cannot guarantee, however, that these agreements will afford us adequate protection of our intellectual property and proprietary information rights.
License and Collaboration Agreements
We are a party to global target discovery collaboration with Incyte under whichcollaborations that we believe will contribute to our ability to advance development and ultimately commercialize our product candidates. We expect to enter into additional collaborations in the future. For instance, we intend to identifyseek to enter into collaborations where we believe that realizing the full commercial value of our development programs will require access to broader geographic markets or the pursuit of broader patient populations or indications. Our existing collaborations impose, and validate novel therapeutic targets with a focus on myeloproliferative neoplasms. By enteringany collaborations we may enter into this collaboration, we aimin the future are likely to use our platform to benefit patients with diseases beyond our current areas of focus, although we do not have any rights to commercialize any products arising from this collaboration. These collaborations impose, certain performance obligations on us. We may enter into agreements similar to this one in the future.
In addition, we are a party to a number of license agreements under which we license patents, patent applications and other intellectual property from third parties. We enter into these agreements to augment our proprietary intellectual property portfolio. TheThis licensed intellectual property covers some of the compounds that we are researching and developing and some of the scientific processes that we use. These licenses impose various diligence and financial payment obligations on us. We expect to continue to enter into these types of license agreements in the future.
Incyte Corporation
In January 2018, we entered into a target discovery, research collaboration and option agreement with Incyte. Under this agreement, we will use our gene control platform to identify novel therapeutic targets with a focus on myeloproliferative neoplasms, and Incyte will receive options to obtain exclusive worldwide rights to intellectual property resulting from the collaboration for the development and commercialization of therapeutic products directed to up to seven validated targets. Incyte will have exclusive worldwide rights to develop and commercialize any therapies under the collaboration that modulate those validated targets.
Under the terms of the collaboration agreement, Incyte paid us $10.0 million in up-front consideration, consisting of $2.5 million in cash and $7.5 million in pre-paid research funding. Our activities under this agreement are subject to a joint research plan and, subject to certain exceptions, Incyte will be responsible for funding our activities under the research plan, including amounts in excess of the pre-paid research funding amount. We are eligible to receive target selection milestone payments and option exercise fees of up to an aggregate of $54.0 million if Incyte selects the maximum number of targets for validation and exercises its option to obtain exclusive rights to collaboration intellectual property for therapeutic products directed to all seven validated targets. Should any therapeutic product be developed by Incyte against a target as to which Incyte has exercised its option to obtain exclusive rights to collaboration intellectual property, we will be eligible to receive milestone payments and, if approved and commercialized, royalty payments from Incyte. For each of the seven validated targets, we would become eligible to receive from Incyte a total of up to $50.0 million in development and regulatory milestone payments. If products arising from the collaboration are approved, we would become eligible to receive from Incyte, for each validated target, a total of up to $65.0 million in commercial milestone payments. Upon approval and commercialization of any therapeutic product resulting from the collaboration, we would become eligible to receive low single-digit royalties on net sales of such product.
The term of the collaboration agreement with Incyte will, unless terminated by a party early, expire when all royalty obligations for products arising from the collaboration expire. The agreement may be terminated by Incyte for convenience on sixty (60) days’ prior written notice to us, or by us on thirty (30) days’ written notice in the event Incyte or one of its affiliates or sublicensees challenges the validity or enforceability of certain patent rights controlled by us. The agreement may also be terminated by either of the parties on thirty (30) days’ prior written notice in the event of an uncured material breach of the agreement by the other party or immediately in the case of certain bankruptcy events. Incyte’s right to terminate for convenience and each party’s right to terminate for uncured material breach may be exercised either with respect to the agreement in its entirety or, as applicable, in relation to the relevant validated target and associated therapeutic products.
TMRC
In connection with the collaboration agreement, we sold 793,021 shares of our common stock to Incyte for an aggregate purchase price of $10.0 million in cash, or $12.61 per share, in a private placement. In addition, from the closing of this sale until the earlier of the second anniversary of such closing or the expiration or termination of the collaboration agreement, we have granted to Incyte the right to purchase up to its pro rata share of the securities offered in certain subsequent offerings of our common stock or common stock equivalents, subject to the terms and conditions set forth in the stock purchase agreement. In February 2018, we sold 144,505 additional shares of our common stock to Incyte at a price of $9.55 per share, resulting in proceeds to us of $1.4 million.
We are obligated to make a payment to Whitehead representing a percentage of the up-front cash consideration and equity premium received from Incyte.
TMRC
In September 2015 we entered into, and in April 2016 we amended and restated, a license agreement with TMRC, which we refer to as the TMRC license agreement, pursuant to which TMRC granted us an exclusive license, with the right to sublicense, under TMRC patent rights, data, regulatory filings and other intellectual property for the North American and European development and commercialization of SY‑1425 (tamibarotene)tamibarotene products for the treatment of human cancer indications. In January 2021, we further amended the TMRC license agreement to expand the territory under which we are licensed to include Central and South America, Australia, Israel, and Russia. Under the TMRC license agreement, we have agreed to pay TMRC single‑digit royalties based on net sales if TMRC’s patents cover our product and low single‑digit royalties based on net sales with respect to know‑how licensed by TMRC during a predefined royalty term, and to make payments to TMRC upon meeting specified clinical and regulatory milestones in an aggregate amount of approximately $13.0 million per indication, of which $1.0 million was paid in the third quarter of 2016 upon successful dosing of the first patient in our Phase 2 clinical trial of SY-1425.tamibarotene. Under the TMRC license agreement, we must use commercially reasonable efforts to, among other things, commence development activities within one year, to develop SY‑1425tamibarotene in at least one cancer indication, and, following marketing approval, to market the product. The license agreement expires on the expiration of the subject patent rights or 15 years after the date of first commercial sale of product, whichever is later. The TMRC license agreement may be terminated by either party if the other party is in breach and the breach is not cured within a required amount of time or if the other party is in bankruptcy. If we have reason to do so, we may also terminate the agreement after one year from the original effective date at our sole discretion.
In connection with the TMRC license agreement, in April 2016 we entered into a supply management agreement with TMRC. Pursuant to the supply management agreement, we and TMRC have agreed to establish a joint manufacturing committee to discuss strategy for supply of SY‑1425.tamibarotene. In addition, we have agreed to pay TMRC a fee for each kilogram of SY‑1425 active pharmaceutical ingredienttamibarotene we procure for clinical trial or commercial use. The supply management agreement terminates on the expiration or termination of the TMRC license agreement, and our obligation to pay these fees survives the termination of the supply management agreement. In April 2016, we also entered into a standby license with TMRC and Toko Pharmaceuticals Ind. Co. Ltd., or Toko, the owner of the patent rights licensed to TMRC from which our license agreement with TMRC derives its rights, pursuant to which we obtain a standby license from Toko if Toko’s license with TMRC is terminated.
Dana‑Farber Cancer Institute, Inc.
We have developed our own patent portfolio related to tamibarotene, which generally discloses methods of identifying and treating patients who are sensitive to RARα agonists, including tamibarotene, based on the expression of certain biomarkers, including RARA. In February 2013,January 2021, we entered into a license agreement with Dana‑Farber pursuant toTMRC, which we wererefer to as the biomarker license agreement, under which we granted TMRC an exclusive worldwide, sublicensable license, with the right to grant sublicenses, under specified patents relatingthese patent rights and certain know-how that it controls related to CDK7 inhibitorsthe RARA biomarker for the development and JNK inhibitors ownedcommercialization of tamibarotene for human cancer indications in Japan, China, South Korea, India and Taiwan. Under the biomarker license agreement, TMRC will be obligated to pay us a low single-digit royalty on net sales of tamibarotene in these territories during a pre-specified royalty term to the extent the manufacture, use or controlled by Dana‑Farber. Thesale of tamibarotene infringes a valid claim of the patent rights or is developed using know-how licensed to TMRC under the biomarker license agreement.
Qiagen
In March 2022, we entered into a master collaboration agreement and a project schedule with Qiagen. Pursuant to this agreement, Qiagen has agreed to develop and commercialize an assay as a companion diagnostic test to determine the RARA gene expression levels for use with tamibarotene in newly diagnosed higher-risk MDS patients.
Under the agreement, Qiagen is responsible for all fields of usedeveloping, and obtaining and maintaining regulatory approvals for the companion diagnostic test in the United States and, at our request and subject to certain rights retained by Dana‑Farber for internal non‑commercial research, academic/teaching and government purposes. Subject to certain restrictions, Dana‑Farber granted us an option to obtain an exclusive commercial license to certain improvements created by Dana‑Farber during the first three yearsnegotiation of mutually agreed payments, in the following additional markets: Canada, the United Kingdom, the member states of the agreement, which would be negotiated in good faithEuropean Economic Area, Switzerland, Mexico, Australia, Russia, Israel and incorporated into this agreement. In connection with the agreement, we paid Dana‑Farber an upfront licensing fee and a milestone payment based on our first round of funding, such payments totaling $175,000, in addition to past patent expenses. We are obligated to pay Dana‑Farber annual license maintenance fees that are creditable against other payments and royalties due under the agreement and to make milestone payments of up to an aggregate of $3.4 million for each of the first two licensed products in connection with the achievement of certain clinical and regulatory milestones.Brazil. In addition, we are required to pay Dana‑Farber a tiered royalty on net sale of licensed products by us, our affiliates and sublicensees ranging from low single digit to mid‑single digit percentages, subject to certain adjustments, as well as a tiered mid‑single digit to low double-digit percentage of sublicense income. Our royalty and sublicensing income payment obligations are further subject to apportionment between designated agreements existing among us, Dana‑Farber and
Whitehead. We are required to meet certain diligence milestones andQiagen has agreed to use commercially reasonable efforts to among other things, developmanufacture the companion diagnostic test and, commercialize one or more licensed products or processes andupon negotiation of mutually agreed terms, to make one or more licensed products or processes reasonablythe companion diagnostic test commercially available in the United States, the additional markets described above, and such other countries as the parties may mutually agree. Qiagen has agreed to undertake specified actions to minimize the risk of an inability of supply occurring for the manufacture of the companion diagnostic test.
Subject to the public. The agreement contains specified diligence activities that if met on an annual basis would be deemed to satisfy someterms of our diligence obligations for the particular year. The agreement, unless earlier terminated by us or Dana‑Farber, will remain in effect until the expiration or abandonment of the licensed patent rights. We have the right to terminate the agreement for any reason provided that we provide Dana‑Farber the required notice and we pay all undisputed amounts due to Dana‑Farber at the time of termination. Dana‑Farber has the right to terminate the agreement if we cease doing business, or if we do not pay them the amounts owed under the agreement and fail to cure, or ifupon achievement of specified technical and development milestones, we commit and fail to cure a material breach under the agreement, or if our successor does not agree in writing to be bound by this agreement within the designated period following assignment.
Whitehead Institute for Biomedical Research and Dana‑Farber Cancer Institute, Inc.
In April 2013, we entered into a license agreement with Whitehead and Dana‑Farber, pursuant to which we were granted an exclusive, sublicensable with certain restrictions, license under specified patents relating to MYC modulators owned or controlled by Whitehead and Dana‑Farber, to make, have made, use, sell, offer for sale and import products and to perform and have performed licensed processes, in each case, in the applicable field. We were granted a non‑exclusive license to certain materials for the practice of our exclusive licenses. The licenses are subject to certain rights retained by Dana‑Farber and Whitehead for internal non‑commercial research, academic/teaching and government purposes. Commencing five years after the effective date and subject to certain terms and conditions, the agreement requires us to negotiate and potentially issue mandatory sublicenses under the patent rights outside of human health and therapeutics for fields and products that are not directly competitive with products in active development or commercialization by us, our affiliates or sublicensees.
In connection with the agreement, we paid Whitehead an upfront licensing fee, and a milestone payment based on our first round of funding, such payments totaling $100,000, in addition to past patent expenses. We are obligated to pay Whitehead annual license maintenance feesQiagen up to a high single-digit million dollar payment in the agreement over the term of the initial project schedule in connection with developing and obtaining and maintaining regulatory approval for the companion
diagnostic in the United States. In addition, we must reimburse Qiagen for certain pass-through costs. These amounts are subject to adjustment if the parties determine that changes in the scope of the development program are creditable against other payments and royalties duerequired. In addition, Qiagen will retain all proceeds from the commercialization of the companion diagnostic test. We have no financial obligations to Qiagen under the agreement and to make milestone paymentson the commercialization of up to an aggregate of $3.4 million in connection with the achievement of certain clinical and regulatory milestones. In addition, we are required to pay Whitehead a royalty on net salestamibarotene.
The initial term of the various products by us, our affiliates and sublicensees ranging from low single digitagreement expires on the later to mid‑single digit percentages, subject to certain adjustments, including a lower royalty on products identified throughoccur of (i) the usefifth anniversary of certain licensed products or processes. In addition, we are required to pay a tiered mid‑single digit to low double-digit percentage of our and our affiliates’ sublicense income and income we receive from the performance of licensed processes. Our royalty, sublicensing and licensed process income payment obligations are further subject to apportionment between designated agreements existing among us, Dana‑Farber and Whitehead. In connection with the agreement we also issued an aggregateand (ii) the expiration or termination of 98,099 sharesall project schedules executed under the agreement. Thereafter, the agreement automatically renews for additional periods of our common stockone year. We may terminate the agreement or a project schedule executed under the agreement for convenience upon 90 day’s prior written notice to Whitehead. We are required to achieve certain diligence milestones withinQiagen. Either party may terminate the specified timeframes, and failure to do so may result in our licenseagreement or any project schedule executed under certain patent rights being converted to non‑exclusive or otherwise be deemedthe agreement, as applicable, upon a material breach of the other party that is not cured within 30 days after written notice of such breach, immediately upon the bankruptcy or insolvency of the other party, or in certain other circumstances described in the agreement. The agreement further requiresIn the event that we use commercially reasonable efforts to, among other things, develop and commercialize one or more licensed products or processes and to make one or more licensed products or processes reasonably available to the public. The agreement contains specified diligence activities that if met on an annual basis would be deemed to satisfy some of our diligence obligations for the particular year. The agreement, unless earlier terminated by us, Whitehead or Dana‑Farber, will remain in effect until the expiration or abandonment of the licensed patent rights. We have the right to terminate the agreement for any reason, provided that we provide Dana‑Farber and Whitehead the required notice and we pay all undisputed amounts due to Whitehead and Dana‑Farber at the time of termination. Whitehead and Dana‑Farber have the right to terminate the agreement if we cease doing business, or if we do not pay them the amounts owed under the agreement and fail to cure, or if we commit and fail to cure areasons other than Qiagen’s material breach under the agreement, or if our successor does not agree in writing tobankruptcy, we will be bound by this agreement within the designated period following assignment.
Whitehead Institute for Biomedical Research
In April 2013, we entered into a license agreement with Whitehead, which we refer to as the Whitehead license agreement, pursuant to which we were granted a worldwide license under specified patents relating to super‑enhancers owned or controlled by Whitehead. This license was exclusive in all fields until April 2016, and can remain exclusive thereafter, on a field‑by‑field basis, as long as we can demonstrate that we are using commercially reasonable efforts in the applicable field, and if we are not using such commercially reasonable efforts in such applicable field, our license rights would become non‑exclusive with respect to such field. As of February 28, 2018, our license continued to be
exclusive in all fields. We were also granted a non‑exclusive license to use certain Whitehead materials in connection with the practice of the licensed Whitehead patents. In connection with the Whitehead license agreement, we paid Whitehead an upfront licensing fee of $30,000. In connection with the agreement, we also issued an aggregate of 73,575 shares of our common stock to Whitehead. We are obligated to pay Whitehead annual license maintenance fees that are creditable againstQiagen wind-down and other paymentscosts and royalties due under the agreement. In addition, we are required to pay Whitehead a tiered royalty on our net sales ranging from low single digit to mid‑single digit percentages, a lower royalty on products identified through the use of licensed products or processes, and a tiered mid‑single digit to low double digit percentage of sublicense income, which steps down depending on time, development stage of the products or processes and payments made to Whitehead, and patent expenses of Whitehead in connection with the licensed patents. We are required to use commercially reasonable efforts to, among other things, develop and commercialize one or more licensed products or processes and to make one or more licensed products or products reasonably available to the public. The Whitehead license agreement, unless earlier terminated by us or Whitehead, will remain in effect until the expiration or abandonment of the licensed patent rights. We have the right to terminate the Whitehead license agreement for any reason upon three months’ notice to Whitehead, provided that we pay all undisputed amounts due to Whitehead at the time of termination. Whitehead has the right to terminate the Whitehead license agreement immediately if we cease doing business, or if we do not pay Whitehead the amounts owed under the agreement or commit a material breach under the agreement, Whitehead has the right to terminate after we have had an opportunity to cure the breach.final payments.
Competition
The pharmaceutical and biotechnology industries are characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary products. While we believe that our technology, development experience, and scientific knowledge provide us with competitive advantages, we face potential competition from many different sources, including major pharmaceutical, specialty pharmaceutical and biotechnology companies, academic institutions and governmental agencies and public and private research institutions. Any product candidates that we successfully develop and commercialize will compete with existing therapies and new therapies that may become available in the future.
We compete in the segments of the pharmaceutical, biotechnology and other related markets that address gene control and cancer. There are other companies working to develop therapies intherapy for the fieldstreatment of gene control and cancer. These companies includecancer, including divisions of large pharmaceutical companies and biotechnology companies of various sizes.
The most common methods of treating patients with cancer are surgery, radiation and drug therapy, including chemotherapy, hormone therapy and targeted drug therapy. There are a variety of available drug therapies marketed for cancer. In many cases, these drugs are administered in combination to enhance efficacy. While our product candidates may compete with many existing drug and other therapies, they may also be used in combination with or as an adjunct to these therapies. Some of the currently approved drug therapies are branded and subject to patent protection, and others are available on a generic basis. Many of these approved drugs are well‑well established therapies and are widely accepted by physicians, patients and third‑partythird-party payors. In general, although there has been considerable progress over the past few decades in the treatment of cancer and the currently marketed therapies provide benefits to many patients, these therapies all are limited to some extent in their efficacy and frequency of adverse events. As a result, the level of morbidity and mortality from cancer remains high.
In addition to currently marketed therapies, there are also a number of medicines in late stagelate-stage clinical development to treat cancer. These medicines in development may provide efficacy, safety, convenience and other benefits that are not provided by currently marketed therapies. As a result, they may provide significant competition for any of our product candidates for which we obtain market approval.
Many of our competitors may have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved medicines than we do. Mergers and acquisitions in the pharmaceutical, biotechnology and diagnostic industries may result in even more resources being concentrated among a smaller number of our competitors. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs. Smaller or early stageearly-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies.
The key competitive factors affecting the success of all of our product candidates, if approved, are likely to be their efficacy, safety, convenience, price, the effectiveness of and ease of access to companion diagnostics in guiding the use of related therapeutics, the level of generic competition and the availability of reimbursement from government and other third‑partythird-party payors.
Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize medicines that are safer, more effective, have fewer or less severe side effects, are more convenient, have greater ease of access, or are less expensive than any medicines that we may develop. Our competitors also may obtain FDA or other regulatory approval for their medicines more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market. In addition, our ability to compete may be affected in many cases by insurers or other third‑partythird-party payors seeking to encourage the use of generic medicines. There are many generic medicines currently on the market for the indications that we are pursuing, and additional medicines are expected to become available on a generic basis over the coming years. If our therapeutic product candidates are approved, we expect that they will be priced at a significant premium over competitive generic medicines.
If the product candidates of our priority programsWe are approved for the indications for which we are currently planning clinical trials, they will compete with the drugs discussed below and will likely compete with other drugs currently in development.
SY‑1425
We plan to initially develop SY‑1425,developing tamibarotene, our RARα agonist, for patients with AML orand MDS. We will selectare selecting patients for our clinical trials based on high‑high levels of RARα as measured by our proprietary RARA and IRF8 biomarkers.gene expression. We are aware of fourseveral new drugs approved by the FDA during 2017since 2018 for the treatment of newly diagnosed unfit AML or patient subsets within AML: midostaurin, enasidanib, daunorubicin + cytarabine liposome,newly diagnosed unfit AML (including ivosidenib, venetoclax, and gemtuzumab. SY-1425glasdegib), and one new drug approved by the FDA in 2020 for the treatment of MDS or patient subsets within MDS (decitabine/cedazuridine). Tamibarotene may also face competition from other investigational productsagents currently in clinical development for AML and MDS, including venetoclax, which is currently being evaluated by AbbVie, Inc.those in two randomized pivotal studies in patients with AML, as well as investigational products inlate-stage development from Takeda Pharmaceutical Co. Ltd., Daiichi Sankyo Company, Limited, Agios Pharmaceuticals,Abbvie Inc., NovartisRoche Holding AG, Bristol-Myers Squibb Co., Eli Lilly & Co., EisaiTaiho Oncology, Inc., Celgene Corporation,and Pfizer Inc., Incyte Corporation and FORMA Therapeutics, LLC. We are not aware of only one otherany selective RARα program, a compound in development from Io Therapeutics, Inc. which, according to a government-sponsored website, is in an investigator initiated Phase 1/2 study in a non-selective patient group in relapsed and refractory AML, high-risk MDS and chronic myelomonocytic leukemia.
SY‑1365
We are conducting a Phase 1 clinical trial of SY-1365 in patients with advanced solid tumors and plan to open expansion cohorts in various ovarian and breast cancer populations once we establish the maximum tolerated dose during the dose escalation phase of the trial. We believe that SY‑1365 is the most advanced selective CDK7 inhibitor in clinical development. We are aware of an oral CDK7 inhibitor being developed by Carrick Therapeutics Ltd. that has recently entered Phase 1 clinical development, and are aware of several other selective CDK7 inhibitoragonist programs that we believe are in preclinical development, including programs from Aurigene Discovery Technologies Ltd., Ube Industries Ltd., Qurient Co. Ltd., and Beta Pharma, Inc. SY-1365 may face competition from these selective CDK7 inhibitors.active clinical development.
Government Regulation and Product Approvals
Government authorities in the United States, at the federal, state and local level, and in other countries and jurisdictions includingsuch as the European Union, or EU, extensively regulate, among other things, the research, development, testing, manufacture, pricing, quality control, approval, packaging, storage, recordkeeping, labeling, advertising, promotion, distribution, marketing, post-approval monitoring and reporting, and import and export of biopharmaceutical products. The processes for obtaining marketing approvals in the United States and in foreign countries and jurisdictions, along with compliance with applicable statutes and regulations and other regulatory authorities, require the expenditure of substantial time and financial resources.
Approval and Regulation of Drugs in the United States
In the United States, drug products are approved and regulated under the Federal Food, Drug and Cosmetic Act, or FDCA, and applicable implementing regulations and guidance. A company, institution or organization which takes responsibility for the initiation and management of a clinical development program for such products, and for their regulatory approval, is typically referred to as a sponsor. The failure of an applicanta sponsor to comply with the applicable regulatory requirements at any time during the product development process including non-clinical testing, clinical testing, the approval process or post-approval process, may result in delays to the conduct of a study, regulatory review and approval and/or administrative or judicial sanctions. These sanctions may include, but are not limited to, the FDA’s refusal to allow an applicant to proceed with clinical trials, refusal to approve pending applications, license suspension or revocation, withdrawal of an approval, warning letters, adverse publicity, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines and civil or criminal investigations and penalties brought by the FDA or Department of Justice, or DOJ, or other government entities, including state agencies.
An applicantSpecifically, a sponsor seeking approval to market and distribute a new drug in the United States generally must satisfactorily complete each of the following steps before the product candidate will be licensedapproved by the FDA:
| ·
| | preclinical testing including laboratory tests, animal studies and formulation studies, which must be performed in accordance with the FDA’s good laboratory practice, or GLP, regulations and standards;
|
•preclinical testing including laboratory tests, animal studies and formulation studies, which must be performed in accordance with the FDA’s good laboratory practice, or GLP, regulations and standards;
| ·
| | submission to the FDA of an IND for human clinical testing, which must become effective before human clinical trials may begin;
|
•design of a clinical protocol and submission to the FDA of an investigational new drug application, or IND, for human clinical testing, which must become effective before human clinical trials may begin;
| ·
| | approval by an independent institutional review board, or IRB, representing each clinical site before each clinical trial may be initiated;
|
•approval by an independent institutional review board, or IRB, representing each clinical site before each clinical trial may be initiated;
| ·
| | performance of adequate and well-controlled human clinical trials to establish the safety, potency and purity of the product candidate for each proposed indication, in accordance with current good clinical practices, or GCP;
|
•performance of adequate and well-controlled human clinical trials to establish the safety and efficacy of the drug product candidate for each proposed indication, in accordance with current good clinical practices, or GCP;
| ·
| | preparation and submission to the FDA of a new drug application, or NDA, for a drug product which includes not only the results of the clinical trials, but also, detailed information on the chemistry, manufacture and quality controls for the product candidate and proposed labelling for one or more proposed indication(s);
|
•preparation and submission to the FDA of a New Drug Application, or NDA, for a drug product which includes not only the results of the clinical trials, but also, detailed information on the chemistry, manufacture and quality controls for the product candidate and proposed labelling for one or more proposed indication(s);
12
| ·
| | review of the product candidate by an FDA advisory committee, where appropriate or if applicable;
|
| ·
| | satisfactory completion of an FDA inspection of the manufacturing facility or facilities, including those of third parties, at which the product candidate or components thereof are manufactured to assess compliance with current good manufacturing practices, or cGMP, requirements and to assure that the facilities, methods and controls are adequate to preserve the product’s identity, strength, quality and purity;
|
•review of the product candidate by an FDA advisory committee, where appropriate or if applicable;
| ·
| | satisfactory completion of any FDA audits of the non-clinical and clinical trial sites to assure compliance with GCP and the integrity of clinical data in support of the NDA;
|
•satisfactory completion of an FDA inspection of the manufacturing facility or facilities, including those of third parties, at which the product candidate or components thereof are manufactured to assess compliance with current good manufacturing practices, or cGMP, requirements and to assure that the facilities, methods and controls are adequate to preserve the product’s identity, strength, quality and purity;
| ·
| | payment of applicable user fees and securing FDA approval of the NDA to allow marketing of the new drug product; and
|
•satisfactory completion of any FDA audits of the non-clinical and clinical trial sites to assure compliance with GCP and the integrity of clinical data in support of the NDA;
| ·
| | compliance with any post-approval requirements, including the potential requirement to implement a Risk Evaluation and Mitigation Strategy, or REMS, and the potential requirement to conduct any post-approval studies required by the FDA.
|
•payment of user fees and securing FDA approval of the NDA to allow marketing of the new drug product; and
•compliance with any post-approval requirements, including the potential requirement to implement a Risk Evaluation and Mitigation Strategy, or REMS, and the potential requirement to conduct any post-approval studies required by the FDA.
Preclinical Studies
Before an applicanta sponsor begins testing a product candidate with potential therapeutic value in humans, the product candidate enters the preclinical testing stage. Preclinical tests include laboratory evaluations of product chemistry, formulation and stability, as well as other studies to evaluate, among other things, the toxicityThe results of the product candidate.preclinical tests, together with manufacturing information and analytical data, are submitted to the FDA as part of an IND and are generally referred to as IND-enabling studies. The conduct of the preclinical tests and formulation of the compounds for testing must comply with federal regulations and requirements, including GLP regulations and standards. The resultsstandards and the United States Department of the preclinical tests, together with manufacturing information and analytical data, are submitted to the FDA as part of an IND.Agriculture’s Animal Welfare Act, if applicable. Some long-term preclinical
testing, such as animal tests of reproductive adverse events and carcinogenicity, and long-term toxicity studies, may continue after the IND is submitted.
The IND and IRB Processes
An IND is an exemption from the FDCA that allows an unapproved product candidate to be shipped in interstate commerce for use in an investigational clinical trial and a request for FDA authorization to administer such investigational product to humans. Such authorization must be secured prior to interstate shipment and administration of any product candidate that is not the subject of an approved NDA. In support of a request for an IND, applicantssponsors must submit a protocol for each clinical trial and any subsequent protocol amendments must be submitted to the FDA as part of the IND. In addition, the results of the preclinical tests, together with manufacturing information, analytical data, any available clinical data or literature and plans for clinical trials, among other things,, must be submitted to the FDA as part of an IND. The FDA requires a 30-day waiting period after the filing of each IND before clinical trials may begin. This waiting period is designed to allow the FDA to review the IND to assure the safety and rights of patients and to determine whether human research subjectsthat the quality of the investigation will be exposedadequate to unreasonable health risks.permit an evaluation of the proposed drug’s effectiveness and safety. At any time during this 30-day period, or thereafter, the FDA may raise concerns or questions about the conduct of the trials as outlined in the IND and impose a clinical hold or partial clinical hold. In this case, the IND sponsor and the FDA must resolve any outstanding concerns before clinical trials can begin.
Following commencement of a clinical trial under an IND, the FDA may also place a clinical hold or partial clinical hold on that trial. A clinical hold is an order issued by the FDA to the sponsor to delay a proposed clinical investigation or to suspend an ongoing investigation. A partial clinical hold is a delay or suspension of only part of the clinical work requested under the IND. For example, a specific protocol or part of a protocol is not allowed to proceed, while other protocols may do so. No more than 30 days after imposition of a clinical hold or partial clinical hold, the FDA will provide the sponsor a written explanation of the basis for the hold. Following issuance of a clinical hold or partial clinical hold, an investigation may only resume after the FDA has notified the sponsor that the investigation may proceed. The FDA will base that determination on information provided by the sponsor correcting the deficiencies previously cited or otherwise satisfying the FDA that the investigation can proceed.
A sponsor may choose, but is not required, to conduct a foreign clinical study under an IND. When a foreign clinical study is conducted under an IND, all FDA IND requirements must be met unless waived. When a foreign clinical study is not conducted under an IND, the sponsor must ensure that the study complies with certain regulatory requirements of the FDA in order to use the study as support for an IND or application for marketing approval. Specifically, on April 28, 2008, the FDA amended its regulations governing the acceptance of foreign clinical studies not conducted under an investigational new drug application as support for an IND or a new drug application. The final rule provides that such studies must be conducted in accordance with good clinical practice, or GCP, including review and approval by an independent ethics committee, or IEC, and informed consent from subjects. The GCP requirements in the final rule encompass both ethical and data integrity standards for clinical studies. The FDA’s regulations are intended to help ensure the protection of human subjects enrolled in non-IND foreign clinical studies, as well as the quality and integrity of the resulting data. They further help ensure that non-IND foreign studies are conducted in a manner comparable to that required for IND studies.
In addition to the foregoing IND requirements, an IRB representing each institution participating in the clinical trial must review and approve the plan for any clinical trial before it commences at that institution, and the IRB must conduct continuing review and reapprove the study at least annually. The IRB must review and approve, among other things, the study protocol and informed consent information to be provided to study subjects. An IRB must operate in compliance with FDA regulations. An IRB can suspend or terminate approval of a clinical trial at its institution, or an institution it represents, if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the product candidate has been associated with unexpected serious harm to patients.
Additionally, some trials are overseen by an independent group of qualified experts organized by the trial sponsor, known as a data safety monitoring board or committee, or DSMB.committee. This group provides a recommendation as to whether or not a trial may move forward at designated check points based on access that only the group maintains to available data from the study. Suspension or termination of development during any phase of clinical trials can occur if it is determined that the participants or patients are being exposed to an unacceptable health risk. Other reasons for suspension or termination may be made by us based on evolving business objectives and/or competitive climate.
Information aboutClinical Studies Outside the United States in Support of FDA Approval
Sponsors frequently conduct clinical trials at sites outside the United States. When a foreign clinical study is conducted under an IND, all IND requirements must be submitted within specific timeframesmet unless waived. When a foreign clinical study is not conducted under an IND, the sponsor must ensure that the study complies with certain regulatory requirements of the FDA in order to use the study as support for an IND or application for marketing approval. Specifically, the studies must be conducted in accordance with GCP, including undergoing review and receiving approval by an independent ethics committee and seeking and receiving informed consent from subjects. GCP requirements encompass both ethical and data integrity standards for clinical studies. The FDA’s regulations are intended to help ensure the protection of human subjects enrolled in non-IND foreign clinical studies, as well as the quality and integrity of the resulting data. They further help ensure that non-IND foreign studies are conducted in a manner comparable to that required for IND studies.
The acceptance by the FDA of study data from clinical trials conducted outside the United States in support of U.S. approval may be subject to certain conditions or may not be accepted at all. In cases where data from foreign clinical trials are intended to serve as the sole basis for marketing approval in the U.S., the FDA will generally not approve the application on the basis of foreign data alone unless (i) the data are applicable to the National InstitutesU.S. population and U.S. medical practice; (ii) the trials were performed by clinical investigators of Health,recognized competence and pursuant to GCP regulations; and (iii) the data may be considered valid without the need for an on-site inspection by the FDA, or NIH,if the FDA considers such inspection to be necessary, the FDA is able to validate the data through an on-site inspection or other appropriate means.
In addition, even where the foreign study data are not intended to serve as the sole basis for public disseminationapproval, the FDA will not accept the data as support for an application for marketing approval unless the study is well-designed and well-conducted in accordance with GCP requirements and the FDA is able to validate the data from the study through an onsite inspection if deemed necessary. Many foreign regulatory authorities have similar approval requirements. In addition, such foreign trials are subject to the applicable local laws of the foreign jurisdictions where the trials are conducted.
Expanded Access to an Investigational Drug for Treatment Use
Expanded access, sometimes called “compassionate use,” is the use of investigational new drug products outside of clinical trials to treat patients with serious or immediately life-threatening diseases or conditions when there are no comparable or satisfactory alternative treatment options. The rules and regulations related to expanded access are intended to improve access to investigational drugs for patients who may benefit from investigational therapies. FDA regulations allow access to investigational drugs under an IND by the company or the treating physician for treatment purposes on a case-by-case basis for: individual patients (single-patient IND applications for treatment in emergency settings and non-emergency settings); intermediate-size patient populations; and larger populations for use of the drug under a treatment protocol or Treatment IND Application.
When considering an IND application for expanded access to an investigational product with the purpose of treating a patient or a group of patients, the sponsor and treating physicians or investigators will determine suitability when all of the following criteria apply: patient(s) have a serious or immediately life-threatening disease or condition, and there is no comparable or satisfactory alternative therapy to diagnose, monitor, or treat the disease or condition; the potential patient benefit justifies the potential risks of the treatment and the potential risks are not unreasonable in the context or condition to be treated; and the expanded use of the investigational drug for the requested treatment will not interfere with the initiation, conduct, or completion of clinical investigations that could support marketing approval of the product or otherwise compromise the potential development of the product.
There is no obligation for a sponsor to make its ClinicalTrials.gov website.investigational products available for expanded access. Sponsors of one or more investigational drugs for the treatment of a serious disease(s) or condition(s) must, however, make publicly available their policy for evaluating and responding to requests for expanded access for individual patients.
In addition, on May 30, 2018, the Right to Try Act, was signed into law. The law, among other things, provides a federal framework for certain patients to access certain investigational new drug products that have completed a Phase 1 clinical trial and that are undergoing investigation for FDA approval. Under certain circumstances, eligible patients can seek
the Right to Try Act, but the manufacturer must develop an internal policy and respond to patient requests according to that policy.
Human Clinical Trials in Support of an NDA
Clinical trials involve the administration of the investigational product candidate to human subjects under the supervision of a qualified investigator in accordance with GCP requirements which include, among other things, the requirement that all research subjects provide their informed consent in writing before their participation in any clinical trial. Clinical trials are conducted under written clinical trial protocols detailing, among other things, the objectives of the study, inclusion and exclusion criteria, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated.
Human clinical trials are typically conducted in three sequential phases, but the phases may overlap or be combined. Additional studies may also be required after approval.
Phase 1 clinical trials are initially conducted in a limited population to test the product candidate for safety, including adverse effects, dose tolerance, absorption, metabolism, distribution, excretion and pharmacodynamics in healthy humans or in patients. During Phase 1 clinical trials, information about the investigational drug product’s pharmacokinetics and pharmacological effects may be obtained to permit the design of well-controlled and scientifically valid Phase 2 clinical trials.
Phase 2 clinical trials are generally conducted in a limited patient population to identify possible adverse effects and safety risks, evaluate the efficacy of the product candidate for specific targeted indications and determine dose tolerance and optimal dosage and dosage schedule. Multiple Phase 2 clinical trials may be conducted by the sponsor to obtain information prior to beginning larger and more costly Phase 3 clinical trials. Phase 2 clinical trials are well controlled, closely monitored and conducted in a limited patient population.
Phase 3 clinical trials proceed if the Phase 2 clinical trials demonstrate that a dose range of the product candidate is potentially effective and has an acceptable safety profile. Phase 3 clinical trials are undertaken within an expanded patient population to further evaluate dosage, provide substantial evidence of clinical efficacy and further test for safety in an expanded and diverse patient population at multiple, geographically dispersed clinical trial sites.sites. A well-controlled, statistically robust Phase 3 clinical trial may be designed to deliver the data that regulatory authorities will use to decide whether or not to approve, and, if approved, how to appropriately label a drug: such Phase 3 studies are referred to as “pivotal.”
In some cases, the FDA may approve an NDA for a product candidate but require the sponsor to conduct additional clinical trials to further assess the product candidate’s safety and effectiveness after approval. Such post-approval trials are typically referred to as Phase 4 clinical trials. These studies are used to gain additional experience from the treatment of a larger number of patients in the intended treatment group and to further document a clinical benefit in the case of drugs approved under accelerated approval regulations. Failure to exhibit due diligence with regard to conducting Phase 4 clinical trials could result in withdrawal of approval for products.
In March 2022, the FDA finalized guidance entitled “Expansion Cohorts: Use in First-In-Human Clinical Trials to Expedite Development of Oncology Drugs and Biologics,” which outlines how sponsors can utilize an adaptive trial design in the early stages of oncology product development (i.e., the first-in-human clinical trial) to compress the traditional three phases of trials into one continuous trial called an expansion cohort trial. Information to support the design of individual expansion cohorts are included in IND applications and assessed by the FDA. Expansion cohort trials can potentially bring efficiency to product development and reduce developmental costs and time.Progress reports detailing the status and a brief description of available results of the clinical trials must be submitted at least annually to the FDA. In addition, IND safety reports must be submitted to the FDA for any of the following: serious and unexpected suspected adverse reactions; findings from other studies or animal or in vitro testing that suggest a significant risk in humans exposed to the product; and any clinically important increase in the case of a serious suspected adverse reaction over that listed in the protocol or investigator brochure. Phase 1, Phase 2 and Phase 3 clinical trials may not be completed successfully within any specified period, or completed at all. Furthermore, the FDA or the sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research subjects are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution, or an institution it represents, if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the product has been associated with unexpected serious harm to patients. The FDA will typically inspect one or more clinical sites to assure compliance with GCP and the integrity of the clinical data submitted.
In December 2022, with the passage of the Food and Drug Omnibus Reform Act, or FDORA, Congress required sponsors to develop and submit a diversity action plan for each Phase 3 clinical trial or any other “pivotal study” of a new drug or biological product. These plans are meant to encourage the enrollment of more diverse patient populations in late-stage clinical trials of FDA-regulated products. Specifically, action plans must include the sponsor’s goals for enrollment, the
underlying rationale for those goals, and an explanation of how the sponsor intends to meet them. In addition to these requirements, the legislation directs the FDA to issue new guidance on diversity action plans.
In June 2023, the FDA issued draft guidance with updated recommendations for GCPs aimed at modernizing the design and conduct of clinical trials. The updates are intended to help pave the way for more efficient clinical trials to facilitate the development of medical products. The draft guidance is adopted from the International Council for Harmonisation’s recently updated E6(R3) draft guideline that was developed to enable the incorporation of rapidly developing technological and methodological innovations into the clinical trial enterprise. In addition, the FDA issued draft guidance outlining recommendations for the implementation of decentralized clinical trials.
Sponsors of clinical trials are required to register and disclose certain clinical trial information on a public registry (clinicaltrials.gov) maintained by the U.S. National Institutes of Health. In particular, information related to the product, patient population, phase of investigation, study sites and investigators and other aspects of the clinical trial are made public as part of the registration of the clinical trial. The failure to submit clinical trial information to clinicaltrials.gov, as required, is a prohibited act under the FDCA with violations subject to potential civil monetary penalties of up to $10,000 for each day the violation continues. Although the FDA has historically not enforced these reporting requirements due to the long delay in issuing final implementing regulations by the Department of Health and Human Services, or HHS, the FDA has issued pre-notices for voluntary corrective action and several notices of non-compliance during the past two years. These notices of non-compliance did not result in civil monetary penalties.
Concurrent with clinical trials, companies often complete additional animal studies and must also develop additional information about the chemistry and physical characteristics of the investigational drug as well as finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the product candidate and, among other things, must develop methods for testing the identity, strength, quality, purity, and potency of the final drug. Additionally, appropriate
packaging must be selected and tested and stability studies must be conducted to demonstrate that the product candidate does not undergo unacceptable deterioration over its shelf life.
Interactions with FDA During the Clinical Development Program
Following the clearance of an IND and the commencement of clinical trials, the sponsor will continue to have interactions with the FDA. Progress reports detailing the results of the clinical trials must be submitted at least annually to the FDA and more frequently if serious adverse events occur. In addition, IND safety reports must be submitted to the FDA for any of the following: serious and unexpected suspected adverse reactions; findings from other studies or animal or in vitro testing that suggest a significant risk in humans exposed to the drug; and any clinically important increase in the case of a serious suspected adverse reaction over that listed in the protocol or investigator brochure. Phase 1, Phase 2 and Phase 3 clinical trials may not be completed successfully within any specified period, or at all.
In addition, sponsors are given opportunities to meet with the FDA at certain points in the clinical development program. There are five types of meetings that occur between sponsors and the FDA. Type A meetings are those that are necessary for an otherwise stalled product development program to proceed or to address an important safety issue. Type B meetings include pre-IND and pre-NDA meetings, as well as end of phase meetings such as EOP2 meetings. A Type C meeting is any meeting other than a Type A or Type B meeting regarding the development and review of a product, including for example meetings to facilitate early consultations on the use of a biomarker as a new surrogate endpoint that has never been previously used as the primary basis for product approval in the proposed context of use. A Type D meeting is focused on a narrow set of issues (should be limited to no more than 2 focused topics) and should not require input from more than 3 disciplines or divisions. Finally, INTERACT meetings are intended for novel products and development programs that present unique challenges in the early development of an investigational product.
These meetings provide an opportunity for the sponsor to share information about the data gathered to date with the FDA and for the FDA to provide advice on the next phase of development. The FDA has indicated that its responses, as conveyed in meeting minutes and advice letters, only constitute mere recommendations and/or advice made to a sponsor and, as such, sponsors are not bound by such recommendations and/or advice. Nonetheless, from a practical perspective, a sponsor’s failure to follow the FDA’s recommendations for design of a clinical program may put the program at significant risk of failure.
Review and Approval of an NDA
In order to obtain approval to market a drug product in the United States, a marketing application must be submitted to the FDA that provides sufficient data establishing the safety, purity and potency of the proposed drug product for its intended indication. The application includes all relevant data available from pertinent preclinical and clinical trials, together with detailed information relating to the product’s chemistry, manufacturing, controls and proposed labeling, among other things. Data can come from company-sponsored clinical trials intended to test the safety and effectiveness of a use of a product, or from a number of alternative sources, including studies initiated by investigators. To support marketing approval, the data submitted must be sufficient in quality and quantity to establish the safety, purity and potency of the drug product to the satisfaction of the FDA.
The NDA is a vehicle through which applicantssponsors formally propose that the FDA approve a new product for marketing and sale in the United States for one or more indications. Every new drug product candidate must be the subject of an approved NDA before it may be commercialized in the United States. Under federal law, the submission of most NDAs is subject to an application user fee, which for federal fiscal year 20182024 is $2,421,495$4,048,695 for an application requiring clinical data. The sponsor of an approved NDA is also subject to an annual program fee, which for federal fiscal year 20182024 is $304,162.$416,734. Certain exceptions and waivers are available for some of these fees, such as an exception from the application fee for products with orphan designation and a waiver for certain small businesses.businesses.
Following submission of an NDA, the FDA conducts a preliminary review of the application generally within 60 calendar days of its receipt and strives tomust inform the sponsor by the 74th day after the FDA’s receipt of the submissionthat time whether the application is sufficiently complete to permit substantive review. If not, the FDA will issue a Refuse to File determination to the sponsor. The FDA may request additional information rather than accept the application for filing. In this event, the application must be resubmitted with the additional information. The resubmitted application is also subject to review before the FDA accepts it for filing. Once the submission is accepted for filing, the FDA begins an in-depth substantive review. The FDA has agreed to specified performance goals in the review process of NDAs. Under that agreement, 90% of applications seeking approval of New Molecular Entities, or NMEs, are meant to be reviewed within ten months fromNDAs, but the date on which the FDA accepts the application for filing, and 90% of applications for NMEs that have been designated for “priority review” are meant to be reviewed within six months of the filing date. For applications seeking approval of products that are not NMEs, the ten-month and six-month review periods run from the date that the FDA receives the application. The review process and the Prescription Drug User Fee Act, or PDUFA, goal date may be extended by the FDA for three additional months to consider new information or clarification provided by the applicantsponsor to address an outstanding deficiency identified by the FDA following the original submission.
Before approvingIn connection with its review of an application, the FDA typically will inspect the facility or facilities where the product is or will be manufactured. These pre-approval inspections may cover all facilities associated with an NDA submission, including component manufacturing, finished product manufacturing and control testing laboratories. The FDA will not approve an application unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications.
Additionally, before approving an NDA, the FDA will typically inspect one or more clinical sites to assure compliance with GCP. Under With the passage of FDORA, Congress clarified the FDA’s authority to conduct inspections by expressly permitting inspection of facilities involved in the preparation, conduct, or analysis of clinical and non-clinical studies submitted to the FDA, Reauthorization Act of 2017, the FDA must implement a protocol to expedite review of responses to inspection reports pertaining to certain applications, including applications for products in shortageas well as other persons holding study records or those for which approval is dependent on remediation of conditions identifiedinvolved in the inspection report.study process.
In addition, as a condition of approval, the FDA may require an applicanta sponsor to develop a REMS. REMS use risk minimization strategies beyond the professional labeling to ensure that the benefits of the product outweigh the potential risks. To determine whether a REMS is needed, the FDA will consider the size of the population likely to use the product, seriousness of the disease, expected benefit of the product, expected duration of treatment, seriousness of known or potential adverse events and whether the product is a new molecular entity.
The FDA may refer an application for a novel product to an advisory committee or explain why such referral was not made. Typically, an advisory committee is a panel of independent experts, including clinicians and other scientific experts, that reviews, evaluates and provides a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
Fast Track, Breakthrough Therapy and Priority Review
The FDA is authorized to designate certain products for expedited review if they are intended to address an unmet medical need in the treatment of a serious or life‑threatening disease or condition. These programs, as applicable to our business, are referred to as fast track designation, breakthrough therapy designation and priority review designation. None of these programs changes the standards for approval but each may help expedite the development or approval process governing product candidates.
Specifically, the FDA may designate a product for Fast Track review if it is intended, whether alone or in combination with one or more other products, for the treatment of a serious or life‑threatening disease or condition, and it demonstrates the potential to address unmet medical needs for such a disease or condition. For Fast Track products, sponsors may have greater interactions with the FDA and the FDA may initiate review of sections of a Fast Track product’s application before the application is complete. This rolling review may be available if the FDA determines, after preliminary evaluation of clinical data submitted by the sponsor, that a Fast Track product may be effective. The sponsor must also provide, and the FDA must approve, a schedule for the submission of the remaining information and the sponsor must pay applicable user fees. However, the FDA’s time period goal for reviewing a Fast Track application does not begin until the last section of the application is submitted. In addition, the Fast Track designation may be withdrawn by the FDA if the FDA believes that the designation is no longer supported by data emerging in the clinical trial process.
Second, a product may be designated as a Breakthrough Therapy if it is intended, either alone or in combination with one or more other products, to treat a serious or life‑threatening disease or condition and preliminary clinical evidence indicates that the product may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. The FDA may take certain actions with respect to Breakthrough Therapies, including holding meetings with the sponsor throughout the development process; providing timely advice to the product sponsor regarding development and approval; involving more senior staff in the review process; assigning a cross‑disciplinary project lead for the review team; and taking other steps to design the clinical trials in an efficient manner.
Third, the FDA may designate a product for priority review if it is a product that treats a serious condition and, if approved, would provide a significant improvement in safety or effectiveness. The FDA determines, on a case‑by‑case basis, whether the proposed product represents a significant improvement when compared with other available therapies. Significant improvement may be illustrated by evidence of increased effectiveness in the treatment of a condition, elimination or substantial reduction of a treatment‑limiting product reaction, documented enhancement of patient compliance that may lead to improvement in serious outcomes, and evidence of safety and effectiveness in a new subpopulation. A priority designation is intended to direct overall attention and resources to the evaluation of such applications, and to shorten the FDA’s goal for taking action on a marketing application from ten months to six months.
Accelerated Approval Pathway
The FDA may grant accelerated approval to a product for a serious or life‑threatening condition that provides meaningful therapeutic advantage to patients over existing treatments based upon a determination that the product has an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit. The FDA may also grant accelerated approval for such a condition when the product has an effect on an intermediate clinical endpoint that can be measured earlier than an effect on irreversible morbidity or mortality, or IMM, and that is reasonably likely to predict an effect on irreversible morbidity or mortalityIMM or other clinical benefit, taking into account the severity, rarity or prevalence of the condition and the availability or lack of alternative treatments. Products granted accelerated approval must meet the same statutory standards for safety and effectiveness as those granted traditional approval.
For the purposes of accelerated approval, a surrogate endpoint is a marker, such as a laboratory measurement, radiographic image, physical sign or other measure that is thought to predict clinical benefit but is not itself a measure of clinical benefit. Surrogate endpoints can often be measured more easily or more rapidly than clinical endpoints. An intermediate clinical endpoint is a measurement of a therapeutic effect that is considered reasonably likely to predict the clinical benefit of a drug, such as an effect on IMM. The FDA has limited experience with accelerated approvals based on intermediate clinical endpoints but has indicated that such endpoints generally may support accelerated approval where the therapeutic effect measured by the endpoint is not itself a clinical benefit and basis for traditional approval, if there is a basis for concluding that the therapeutic effect is reasonably likely to predict the ultimate clinical benefit of a product.
The accelerated approval pathway is most often used in settings in which the course of a disease is long, and an extended period of time is required to measure the intended clinical benefit of a product, even if the effect on the surrogate or intermediate clinical endpoint occurs rapidly. Thus, accelerated approval has been used extensively in the development and approval of products for treatment of a variety of cancers in which the goal of therapy is generally to improve survival or decrease morbidity and the duration of the typical disease course requires lengthy and sometimes large trials to demonstrate a clinical or survival benefit. Thus, the benefit of accelerated approval derives from the potential to receive approval based on surrogate endpoints sooner than possible for trials with clinical or survival endpoints, rather than deriving from any explicit shortening of the FDA approval timeline, as is the case with priority review.
The accelerated approval pathway is usually contingent on a sponsor’s agreement to conduct, in a diligent manner, additional post‑approval confirmatory studies to verify and describe the product’s clinical benefit. As a result, a product candidate approved on this basis is subject to rigorous post‑marketing compliance requirements, including the completion of Phase 4 or post‑approval clinical trials to confirm the effect on the clinical endpoint. Failure to conduct required post‑approval studies, or confirm a clinical benefit during post‑marketing studies, would allow the FDA to initiate expedited proceedings to withdraw approval of the product. All promotional materials for product candidates approved under accelerated regulations are subject to prior review by the FDA.
With the passage of FDORA, in December 2022, Congress modified certain provisions governing accelerated approval of drug and biologic products. Specifically, the new legislation authorized the FDA to require a sponsor to have its confirmatory clinical trial underway before accelerated approval is awarded; to require a sponsor of a product granted accelerated approval to submit progress reports on its post-approval studies to the FDA every six months until the study is completed; and to use expedited procedures to withdraw accelerated approval of an NDA or BLA after the confirmatory trial fails to verify the product’s clinical benefit. Further, FDORA requires the agency to publish on its website “the rationale for why a post-approval study is not appropriate or necessary” whenever it decides not to require such a study upon granting accelerated approval.
In March 2023, the FDA issued draft guidance that outlines its current thinking and approach to accelerated approval. The agency indicated that the accelerated approval pathway is commonly used for approval of oncology drugs due to the serious and life-threatening nature of cancer. Although single-arm trials have been commonly used to support accelerated approval, a randomized controlled trial is the preferred approach as it provides a more robust efficacy and safety assessment and allows for direct comparisons to an available therapy. To that end, the FDA outlined considerations for designing, conducting, and analyzing data for trials intended to support accelerated approvals of oncology therapeutics. While this guidance is currently only in draft form and will ultimately not be legally binding even when finalized, sponsors typically observe FDA’s guidance closely to ensure that their investigational products qualify for accelerated approval.
The FDA’s Decision on an NDA
On the basis of the FDA’s evaluation of the application and accompanying information, including the results of the inspection of the manufacturing facilities, the FDA may issue an approval letter or a complete response letter. An approval letter, authorizes commercial marketingor CRL. To reach this determination, the FDA must determine that the drug is effective and that its expected benefits outweigh its potential risks to patients. This “benefit-risk” assessment is informed by the extensive body of evidence about the product’s safety and efficacy in the NDA. This assessment is also informed by other factors, including: the severity of the underlying condition and how well patients’ medical needs are addressed by currently available therapies; uncertainty about how the premarket clinical trial evidence will extrapolate to real-world use of the product within the post-market setting; and whether risk management tools are necessary to manage specific prescribing information for specific indications. risks.
A complete response letterCRL generally outlines the deficiencies in the submission and may require substantial additional testing or information in order for the FDA to reconsider the application. If and when those deficiencies have been addressed to the FDA’s satisfaction in a resubmission of the NDA, the FDA will issue an approval letter. The FDA has committed to reviewing such resubmissions in two or six months depending on the type of information included. Even with submission of this additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval.
An approval letter authorizes commercial marketing of the product with specific prescribing information for each indication. If the FDA approves a new product, it may limit the approved indications for use of the product. The agency may also require testing and surveillance programs to monitor the product after commercialization, or impose other conditions, including distribution restrictions or other risk management mechanisms, including REMS, to help ensure that the safe usebenefits of the product.product outweigh the potential risks. REMS can include medication guides, communication plans for health care professionals, and elements to assure safe use, or ETASU. ETASU can include, but are not limited to, special training or certification for prescribing or dispensing, dispensing only under certain circumstances, special monitoring and the use of patent registries. The FDA may prevent or limit further marketing of a product based on the results of post-market studies or surveillance programs. After approval, many types of changes to the approved product, such as adding new indications, manufacturing changes and additional labeling claims, are subject to further testing requirements and FDA review and approval.
Post-Approval Regulation
If regulatory approval for marketing of a product or new indication for an existing product is obtained, the sponsor will be required to comply with all regular post-approval regulatory requirements as well as any post-approval requirements that the FDA may have imposed as part of the approval process. The sponsor will be required to report, among other things, certain adverse reactions and manufacturing problems to the FDA, provide updated safety and efficacy information and comply with requirements concerning advertising and promotional labeling requirements. Manufacturers and certain of their subcontractors are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with ongoing regulatory requirements, including cGMP regulations, which impose certain procedural and documentation requirements upon manufacturers. Accordingly,The PREVENT Pandemics Act, which was enacted in December 2022, clarifies that foreign drug manufacturing establishments are subject to registration and listing requirements even if a drug undergoes further manufacture, preparation, propagation, compounding, or processing at a separate establishment outside the sponsor and its third-party manufacturers must continueUnited States prior to expend time, money and effort inbeing imported or offered for import into the areas of production and quality control to maintain compliance with cGMP regulations and other regulatory requirements.United States.
A product may also be subject to official lot release, meaning that the manufacturer is required to perform certain tests on each lot of the product before it is released for distribution. If the product is subject to official release, the manufacturer must submit samples of each lot, together with a release protocol showing a summary of the history of
manufacture of the lot and the results of all of the manufacturer’s tests performed on the lot, to the FDA. The FDA may in addition perform certain confirmatory tests on lots of some products before releasing the lots for distribution. Finally, the FDA will conduct laboratory research related to the safety, purity, potency and effectiveness of pharmaceutical products.
Once an approval is granted, the FDA may withdraw the approval if compliance with regulatory requirements is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in revisions to the approved labeling to add new safety information; imposition of post-market studies or clinical trials to assess safety risks; or imposition of distribution or other restrictions under a REMS program. Other potential consequences include, among other things:
| ·
| | restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls;
|
•restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls;
| ·
| | fines, warning letters or holds on post-approval clinical trials;
|
•fines, warning letters or holds on post-approval clinical trials;
| ·
| | refusal of the FDA to approve pending applications or supplements to approved applications, or suspension or revocation of product license approvals;
|
•refusal of the FDA to approve pending applications or supplements to approved applications, or suspension or revocation of product license approvals;
| ·
| | product seizure or detention, or refusal to permit the import or export of products; or
|
•product seizure or detention, or refusal to permit the import or export of products; or
| ·
| | injunctions or the imposition of civil or criminal penalties.
|
•injunctions or the imposition of civil or criminal penalties.
The FDA strictly regulates the marketing, labeling, advertising and promotion of prescription drug products placed on the market. This regulation includes, among other things, standards and regulations for direct-to-consumer advertising, communications regarding unapproved uses, industry-sponsored scientific and educational activities, and promotional activities involving the Internet and social media. Promotional claims about a drug’s safety or effectiveness are prohibited before the drug is approved. After approval, a drug product generally may not be promoted for uses that are not approved by the FDA, as reflected in the product’s prescribing information. In September 2021, the FDA published final regulations which describe the types of evidence that the agency will consider in determining the intended use of a drug product.
In the United States, health care professionals are generally permitted to prescribe drugs for such uses not described in the drug’s labeling, known as off-label uses, because the FDA does not regulate the practice of medicine. However, FDA regulations impose rigorous restrictions on manufacturers’ communications, prohibiting the promotion of off-label uses.uses. It may be permissible, under very specific, narrow conditions, for a manufacturer to engage in nonpromotional, non-misleading communication regarding off-label information, such as distributing scientific or medical journal information.
Further, with passage of the Pre-Approval Information Exchange Act in December 2022, sponsors of products that have not been approved may proactively communicate to payors certain information about products in development to help expedite patient access upon product approval. Previously, such communications were permitted under FDA guidance, but the new legislation explicitly provides protection to sponsors who convey certain information about products in development to payors, including unapproved uses of approved products. In addition, in October 2023, the FDA published draft guidance
outlining the agency’s non-binding policies governing the distribution of scientific information on unapproved uses to healthcare providers. This draft guidance calls for such communications to be truthful, non-misleading, factual, and unbiased and include all information necessary for healthcare providers to interpret the strengths and weaknesses and validity and utility of the information about the unapproved use.
If a company is found to have promoted off-label uses,, it may become subject to adverse public relations and administrative and judicial enforcement by the FDA, the DOJ,Department of Justice, or the Office of the Inspector General of the Department of Health and Human Services, as well as state authorities. This could subject a company to a range of penalties that could have a significant commercial impact, including civil and criminal fines and agreements that materially restrict the manner in which a company promotes or distributes drug products. The federal government has levied large civil and criminal fines against companies for alleged improper promotion, and has also requested that companies enter into consent decrees or permanent injunctions under which specified promotional conduct is changed or curtailed.
In addition, the distribution of prescription pharmaceutical products is subject to the Prescription Drug Marketing Act, or PDMA, and its implementing regulations, as well as the Drug Supply Chain Security Act, or DSCA, which regulate the distribution and tracing of prescription drug samples at the federal level, and set minimum standards for the regulation of distributors by the states. The PDMA, its implementing regulations and state laws limit the distribution of prescription pharmaceutical product samples, and the DSCA imposes requirements to ensure accountability in distribution and to identify and remove counterfeit and other illegitimate products from the market.
Pediatric Studies and Exclusivity
Under the Pediatric Research Equity Act of 2003,, or PREA, an NDA or supplement thereto must contain data that are adequate to assess the safety and effectiveness of the product for the claimed indications in all relevant pediatric subpopulations, and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. Sponsors must also submit pediatric study plans prior to the assessment data. Those plans must contain an outline of the proposed pediatric study or studies the applicantsponsor plans to conduct, including study objectives and design,
any deferral or waiver requests and other information required by regulation. The applicant,sponsor, the FDA, and the FDA’s internal review committee must then review the information submitted, consult with each other and agree upon a final plan. The FDA or the applicantsponsor may request an amendment to the plan at any time.
For drugs intended to treat a serious or life-threatening disease or condition, the FDA must, upon the request of an applicant, meet to discuss preparation of the initial pediatric study plan or to discuss deferral or waiver of pediatric assessments. In addition, FDA will meet early in the development process to discuss pediatric study plans with sponsors and FDA must meet with sponsors by no later than the end-of-phase 1 meeting for serious or life-threatening diseases and by no later than ninety (90) days after the FDA’s receipt of the study plan.
The FDA may, on its own initiative or at the request of the applicant, grant deferrals for submission of some or all pediatric data until after approval of the product for use in adults, or full or partial waivers from the pediatric data requirements. Additional requirements and procedures relating to deferral requests and requests for extension of deferrals are contained in FDASIA. Unless otherwise required by regulation, the pediatric data requirements do not apply to products with orphan designation.
The FDA Reauthorization Act of 2017 established new requirements to govern certain molecularly targeted cancer indications. Any company that submits an NDA three years after the date of enactment of that statute must submit pediatric assessments with the NDA if the drug is intended for the treatment of an adult cancer and is directed at a molecular target that the FDA determines to be substantially relevant to the growth or progression of a pediatric cancer. The investigation must be designed to yield clinically meaningful pediatric study data regarding the dosing, safety and preliminary efficacy to inform pediatric labeling for the product.
The FDA is required to send a PREA Non-Compliance letter to sponsors who have failed to submit their pediatric assessments required under PREA, have failed to seek or obtain a deferral or deferral extension or have failed to request approval for a required pediatric formulation. Unless otherwise required by regulation, the pediatric data requirements do not apply to products with orphan designation, although the FDA has recently taken steps to limit what it considers abuse of this statutory exemption in PREA by announcing that it does not intend to grant any additional orphan drug designations for rare pediatric subpopulations of what is otherwise a common disease. In May 2023, the FDA issued new draft guidance that further describes the pediatric study requirements under PREA.
Orphan Drug Designation and Exclusivity
Under the Orphan Drug Act, the FDA may designate a drug product as an “orphan drug” if it is intended to treat a rare disease or condition, generally meaning that it affects fewer than 200,000 individuals in the United States, or more in cases in which there is no reasonable expectation that the cost of developing and making a product available in the United States for treatment of the disease or condition will be recovered from sales of the product. A company must seek orphan drug designation before submitting an NDA for the product candidate. If the request is granted, the FDA will disclose the identity of the therapeutic agent and its potential use. Orphan drug designation does not shorten the PDUFA goal dates for the regulatory review and approval process, although it does convey certain advantages such as tax benefits and exemption from the PDUFA application fee.
If a product with orphan designation receives the first FDA approval for the disease or condition for which it has such designation or for a select indication or use within the rare disease or condition for which it was designated, the product generally will receive orphan drug exclusivity. Orphan drug exclusivity means that the FDA may not approve another sponsor’s marketing application for the same drug for the same condition for seven years, except in certain limited circumstances. Orphan exclusivity does not block the approval of a different product for the same rare disease or condition, nor does it block the approval of the same product for different conditions. If a drug designated as an orphan drug ultimately receives marketing approval for an indication broader than what was designated in its orphan drug application, it may not be entitled to exclusivity.
Orphan drug exclusivity will not bar approval of another product under certain circumstances, including if a subsequent product with the same drug for the same condition is shown to be clinically superior to the approved product on the basis of greater efficacy or safety, or providing a major contribution to patient care, or if the company with orphan drug exclusivity is not able to meet market demand. Under Omnibus legislation signed by former President Trump on December 27, 2020, the requirement for a product to show clinical superiority applies to drug products that received orphan drug designation before enactment of amendments to the FDCA in 2017 but have not yet been approved by the FDA.
In September 2021, the Court of Appeals for the 11th Circuit held that, for the purpose of determining the scope of market exclusivity, the term “same disease or condition” in the statute means the designated “rare disease or condition” and could not be interpreted by the FDA to mean the “indication or use.” Although there have been legislative proposals to overrule this decision, they have not been enacted into law. On January 23, 2023, the FDA announced that, in matters beyond the scope of that court order, the FDA will continue to apply its existing regulations tying orphan-drug exclusivity to the uses or indications for which the orphan drug was approved.
Pediatric Exclusivity
Pediatric exclusivity is another type of non-patent marketing exclusivity in the United States and, if granted, provides for the attachment of an additional six months of marketing protectionregulatory exclusivity to the term of any existing patent or other regulatory exclusivity, including the non-patent and orphan exclusivity.exclusivity, for drug products. For biologic products, only non-patent exclusivity is extended. This six-month exclusivity may be granted if an NDA sponsor submits pediatric data that fairly respond to a written request from the FDA for such data. The data do not need to show the product to be effective in the pediatric population studied; rather, if the clinical trial is deemed to fairly respond to the FDA’s request, the additional protection is granted. If reports of requested pediatric studies are submitted to and accepted by the FDA within the statutory time limits, whatever statutory or regulatory periods of exclusivity or patent protection cover the product are extended by six months. This is not a patent term extension, but it effectively extends the regulatory period during which the FDA cannot approve another application.
Orphan Drug Designation and Exclusivity
Under the Orphan Drug Act, the FDA may designate a drug product as an “orphan drug” if it is intended to treat a rare disease or condition, generally meaning that it affects fewer than 200,000 individuals in the United States, or more in cases in which there is no reasonable expectation that the cost of developing and making a product available in the United States for treatment of the disease or condition will be recovered from sales of the product. A company must seek orphan drug designation before submitting an NDA for the candidate product. If the request is granted, the FDA will disclose the identity of the therapeutic agent and its potential use. Orphan drug designation does not shorten the PDUFA goal dates for the regulatory review and approval process, although it does convey certain advantages such as tax benefits and exemption from the PDUFA application fee.
If a product with orphan designation receives the first FDA approval for the disease or condition for which it has such designation or for a select indication or use within the rare disease or condition for which it was designated, the product generally will receive orphan drug exclusivity. Orphan drug exclusivity means that the FDA may not approve another sponsor’s marketing application for the same drug for the same condition for seven years, except in certain limited circumstances. Orphan exclusivity does not block the approval of a different product for the same rare disease or condition, nor does it block the approval of the same product for different conditions. If a drug designated as an orphan drug ultimately receives marketing approval for an indication broader than what was designated in its orphan drug application, it may not be entitled to exclusivity.
Orphan drug exclusivity will not bar approval of another product under certain circumstances, including if a subsequent product with the same drug for the same condition is shown to be clinically superior to the approved product on the basis of greater efficacy or safety, or providing a major contribution to patient care, or if the company with orphan drug exclusivity is not able to meet market demand. This is the case despite an earlier court opinion holding that the
Orphan Drug Act unambiguously required the FDA to recognize orphan exclusivity regardless of a showing of clinical superiority.
Section 505(b)(2) NDAs
NDAs for most new drug products are based on well controlledtwo full clinical studies that providemust contain substantial evidence of the safety and efficacy of the proposed new product for the proposed use.use. These applications are submitted under Section 505(b)(1) of the FDCA. The FDA is, however, authorized to approve an alternative type of NDA under Section 505(b)(2) of the FDCA. This type of application allows the applicantsponsor to rely, in part, on the FDA’s previous findings of safety and efficacy for a similar product, or published literature. Specifically, Section 505(b)(2) applies to NDAs for a drug for which the investigations made to show whether or not the drug is safe for use and effective in use and relied upon by the applicantsponsor for approval of the application “were not conducted by or for the applicantsponsor and for which the applicantsponsor has not obtained a right of reference or use from the person by or for whom the investigations were conducted.”
Thus, Section 505(b)(2) authorizes the FDA to approve an NDA based on safety and effectiveness data that were not developed by the applicant.sponsor. NDAs filed under Section 505(b)(2) may provide an alternate and potentially more expeditious pathway to FDA approval for new or improved formulations or new uses of previously approved products. If the 505(b)(2) applicant can establish that reliance on the FDA’s previous approval is scientifically appropriate, the applicant may eliminate the need to conduct certain preclinical or clinical studies of the new product. The FDA may also require companies to perform additional studies or measurements to support the change from the approved product.product. The FDA may then approve the new product candidate for all or some of the label indications for which the referenced productdrug has been approved, as well as for any new indication sought by the Section 505(b)(2) applicant.
Abbreviated New Drug Applications for Generic Drugs
In 1984, with passage of the Hatch-Waxman Amendments to the FDCA, Congress established an abbreviated regulatory scheme authorizing the FDA to approve generic drugs that are shown to contain the same active ingredients as, and to be bioequivalent to, drugs previously approved by the FDA pursuant to NDAs. To obtain approval of a generic drug, an applicant must submit
Specifically, in order for an abbreviated new drug application, or ANDA, to the agency. An ANDA is a comprehensive submission that contains, among other things, data and information pertaining to the active pharmaceutical ingredient, bioequivalence, drug product formulation, specifications and stability of the generic drug, as well as analytical methods, manufacturing process validation data and quality control procedures. ANDAs are “abbreviated” because they generally do not include preclinical and clinical data to demonstrate safety and effectiveness. Instead, in support of such applications, a generic manufacturer may rely on the preclinical and clinical testing previously conducted for a drug product previously approved under an NDA, known as the reference-listed drug, or RLD.
Specifically, in order for an ANDA, to be approved, the FDA must find that the generic version is identical to the reference listed drug, or RLD, with respect to the active ingredients, the route of administration, the dosage form, the strength of the drug and the conditions of use of the drug. At the same time, the FDA must also determine that the generic drug is “bioequivalent” to the innovator drug. Under the statute, a generic drug is bioequivalent to a RLD if “the rate and extent of absorption of the drug do not show a significant difference from the rate and extent of absorption of the listed drug.” Upon approval of an ANDA, the FDA indicates whether the generic product is “therapeutically equivalent” to the RLD in its publication “Approved Drug Products with Therapeutic Equivalence Evaluations,” also referred to as the “Orange Book.” Physicians and pharmacists consider a therapeutic equivalent generic drug to be fully substitutable for the RLD. In addition, by operation of certain state laws and numerous health insurance programs, the FDA’s designation of therapeutic equivalence often results in substitution of the generic drug without the knowledge or consent of either the prescribing physician or patient.
Under the Hatch-Waxman Amendments, the FDA may not approve an ANDA until any applicable period of non-patent exclusivity for the RLD has expired. The FDCA provides a period of five years of non-patent data exclusivity for a
new drug containing a new chemical entity. For the purposes of this provision, a new chemical entity, or NCE, is a drug that contains no active moiety that has previously been approved by the FDA in any other NDA. An active moiety is the molecule or ion responsible for the physiological or pharmacological action of the drug substance. This interpretation of the FDCA by the FDA was confirmed with enactment of the Ensuring Innovation Act in April 2021. In cases where such NCE exclusivity has been granted, an ANDA may not be filed with the FDA until the expiration of five years unless the submission is accompanied by a Paragraph IV certification, in which case the applicant may submit its application four years following the original product approval.
The FDCA also provides for a period of three years of exclusivity if the NDA includes reports of one or more new clinical investigations, other than bioavailability or bioequivalence studies, that were conducted by or for the applicant and are essential to the approval of the application. This three-year exclusivity period often protects changes to a previously approved drug product, such as a new dosage form, route of administration, combination or indication. Three-year exclusivity would be available for a drug product that contains a previously approved active moiety, provided the statutory requirement for a new clinical investigation is satisfied. Unlike five-year NCE exclusivity, an award of three-year exclusivity does not block the FDA from accepting ANDAs seeking approval for generic versions of the drug as of the date of approval of the original drug product. The FDA typically makes decisions about awards of data exclusivity shortly before a product is approved.
The FDA must establish a priority review track for certain generic drugs, requiring the FDA to review a drug application within eight months for a drug that has three or fewer approved drugs listed in the Orange Book and is no longer protected by any patent or regulatory exclusivities, or is on the FDA’s drug shortage list. The new legislation also authorizes FDA to expedite review of ‘‘competitor generic therapies’’ or drugs with inadequate generic competition, including holding meetings with or providing advice to the drug sponsor prior to submission of the application.
Hatch-Waxman Patent Certification and the 30-Month Stay
Upon approval of an NDA or a supplement thereto, NDA sponsors are required to list with the FDA each patent with claims that cover the applicant’s product or an approved method of using the product. Each of the patents listed by the NDA sponsor is published in the Orange Book. The FDA’s regulations governing patent listings were largely codified into law with enactment of the Orange Book Modernization Act in January 2021. When an ANDA or 505(b)(2) applicant files its application with the FDA, the applicant is required to certify to the FDA concerning any patents listed for the reference product in the Orange Book, except for patents covering methods of use for which the ANDA applicant is not seeking approval. To the extent that the Section 505(b)(2) applicant is relying on studies conducted for an already approved product, the applicant is required to certify to the FDA concerning any patents listed for the approved product in the Orange Book to the same extent that an ANDA applicant would.
Specifically, the applicant must certify with respect to each patent that:
| ·
| | the required patent information has not been filed;
|
•the required patent information has not been filed;
| ·
| | the listed patent has expired;
|
•the listed patent has expired;
| ·
| | the listed patent has not expired, but will expire on a particular date and approval is sought after patent expiration; or
|
•the listed patent has not expired, but will expire on a particular date and approval is sought after patent expiration; or
| ·
| | the listed patent is invalid, unenforceable or will not be infringed by the new product.
|
•the listed patent is invalid, unenforceable or will not be infringed by the new product.
A certification that the new product will not infringe the already approved product’s listed patents or that such patents are invalid or unenforceable is called a Paragraph IV certification. If the applicant does not challenge the listed patents or indicates that it is not seeking approval of a patented method of use, the application will not be approved until all the listed patents claiming the referenced product have expired (other than method of use patents involving indications for which the applicant is not seeking approval).
If the ANDA applicant has provided a Paragraph IV certification to the FDA, the applicant must also send notice of the Paragraph IV certification to the NDA and patent holders once the ANDA has been accepted for filing by the FDA. The NDA and patent holders may then initiate a patent infringement lawsuit in response to the notice of the Paragraph IV certification. The filing of a patent infringement lawsuit within 45 days after the receipt of a Paragraph IV certification automatically prevents the FDA from approving the ANDA until the earlier of 30 months after the receipt of the Paragraph IV notice, expiration of the patent, or a decision in the infringement case that is favorable to the ANDA applicant.
To the extent that the Section 505(b)(2) applicant is relying on studies conducted for an already approved product, the applicant is required to certify to the FDA concerning any patents listed for the approved product in the Orange Book to the same extent that an ANDA applicant would. As a result, approval of a Section 505(b)(2) NDA can be stalled until all the listed patents claiming the referenced product have expired, until any non-patent exclusivity, such as exclusivity for obtaining approval of a new chemical entity,an NCE, listed in the Orange Book for the referenced product has expired, and, in the case of a Paragraph IV certification and subsequent patent infringement suit, until the earlier of 30
months, settlement of the lawsuit or a decision in the infringement case that is favorable to the Section 505(b)(2) applicant.
Patent Term Restoration and Extension
A patent claiming a new drug product may be eligible for a limited patent term extension under the Hatch-Waxman Act, which permits a patent restoration of up to five years for patent term lost during product development and the FDA regulatory review. The restoration period granted on a patent covering a product is typically one-half the time between the effective date of a clinical investigation involving human beings is begunthe IND and the submission date of an application, plus the time between the submission date of an application and the ultimate approval date. Patent term restoration cannot be used to extend the remaining term of a patent past a total of 14 years from the product’s approval date. Only one patent applicable to an approved product is eligible for the extension, and the application for the extension must be submitted prior to the expiration of the patent in question. A patent that covers multiple products for which approval is sought can only be extended in connection with one of the approvals. The United States Patent and Trademark Office reviews and approves the application for any patent term extension or restoration in consultation with the FDA.
23
The 21st Century Cures Act
On December 13, 2016, President Obama signed the 21st Century Cures Act, or Cures Act, into law. The Cures Act is designed to modernize and personalize healthcare, spur innovation and research, and streamline the discovery and development of new therapies through increased federal funding of particular programs. It authorizes increased funding for the FDA to spend on innovation projects. The new law also amends the Public Health Service Act, or PHSA, to reauthorize and expand funding for the NIH. The Cures Act establishes the NIH Innovation Fund to pay for the cost of development and implementation of a strategic plan, early stage investigators and research. It also charges NIH with leading and coordinating expanded pediatric research. Further, the Cures Act directs the Centers for Disease Control and Prevention to expand surveillance of neurological diseases.
With amendments to the FDCA and the PHSA, Title III of the Cures Act seeks to accelerate the discovery, development, and delivery of new medicines and medical technologies. To that end, and among other provisions, the Cures Act reauthorizes the existing priority review voucher program for certain drugs intended to treat rare pediatric diseases until 2020; creates a new priority review voucher program for drug applications determined to be material national security threat medical countermeasure applications; revises the FDCA to streamline review of combination product applications; requires FDA to evaluate the potential use of “real world evidence” to help support approval of new indications for approved drugs; provides a new “limited population” approval pathway for antibiotic and antifungal drugs intended to treat serious or life-threatening infections; and authorizes the FDA to designate a drug as a “regenerative advanced therapy,” thereby making it eligible for certain expedited review and approval designations.
FDA Approval and Regulation of Companion Diagnostics
If safe and effective use of a therapeutic depends on an in vitro diagnostic, then the FDA generally will require approval or clearance of that diagnostic, known as a companion diagnostic, at the same time that the FDA approves the therapeutic product. In August 2014, the FDA issued final guidance clarifying the requirements that will apply to approval of therapeutic products and in vitro companion diagnostics. According to the guidance, if the FDA determines that a companion diagnostic device is essential to the safe and effective use of a novel therapeutic product or indication, the FDA generally will not approve the therapeutic product or new therapeutic product indication if the companion diagnostic device is not approved or cleared for that indication. Approval or clearance of the companion diagnostic device will ensure that the device has been adequately evaluated and has adequate performance characteristics in the intended population. The review of in vitro companion diagnostics in conjunction with the review of our therapeutic treatments for cancer will, therefore, likely involve coordination of review by the FDA’s Center for Drug Evaluation and Research and the FDA’s Center for Devices and Radiological Health Office of In Vitro Diagnostics Device Evaluation and Safety.
Under the FDCA, in vitro diagnostics, including companion diagnostics, are regulated as medical devices. In the United States, the FDCA and its implementing regulations, and other federal and state statutes and regulations govern, among other things, medical device design and development, preclinical and clinical testing, premarket clearance or approval, registration and listing, manufacturing, labeling, storage, advertising and promotion, sales and distribution, export and import, and post‑market surveillance. Unless an exemption applies, diagnostic tests require
marketing clearance or approval from the FDA prior to commercial distribution. The two primary types of FDA marketing authorization applicable to a medical device are premarket notification, also called 510(k) clearance, and premarket approval, or PMA.
The FDA previously has required in vitro companion diagnostics intended to select the patients who will respond to the product candidate to obtain PMA approval.
simultaneously with approval of the therapeutic product candidate. The PMA process, including the gathering of clinical and preclinical data and the submission to and review by the FDA, can take several years or longer. It involves a rigorous premarket review during which the applicantsponsor must prepare and provide the FDA with reasonable assurance of the device’s safety and effectiveness and information about the device and its components regarding, among other things, device design, manufacturing and labeling. PMA applications are subject to an application fee. For federal fiscal year 2024, the standard fee which exceeds $250,000 for most PMAs. In addition, PMAs for certain devices must generally include the results from extensive preclinical and adequate and well‑controlled clinical trials to establish the safety and effectiveness of the device for each indication for which FDA approval is sought. In particular, for a diagnostic, a PMA application typically requires data regarding analytical and clinical validation studies. As part of the PMA review, the FDA will typically inspect the manufacturer’s facilities for compliance with the Quality System Regulation, or QSR, which imposes elaborate testing, control, documentation and other quality assurance requirements.
PMA approval is not guaranteed,$483,560 and the FDA may ultimately respond to a PMA submission with a not approvable determination based on deficiencies in the application and require additional clinical trial or other data that may be expensive and time‑consuming to generate and that can substantially delay approval. If the FDA’s evaluation of the PMA applicationsmall business fee is favorable, the FDA typically issues an approvable letter requiring the applicant’s agreement to specific conditions, such as changes in labeling, or specific additional information, such as submission of final labeling, in order to secure final approval of the PMA. If the FDA’s evaluation of the PMA or manufacturing facilities is not favorable, the FDA will deny approval of the PMA or issue a not approvable letter. A not approvable letter will outline the deficiencies in the application and, where practical, will identify what is necessary to make the PMA approvable. The FDA may also determine that additional clinical trials are necessary, in which case the PMA approval may be delayed for several months or years while the trials are conducted and then the data submitted in an amendment to the PMA. If the FDA concludes that the applicable criteria have been met, the FDA will issue a PMA for the approved indications, which can be more limited than those originally sought by the applicant. The PMA can include post‑approval conditions that the FDA believes necessary to ensure the safety and effectiveness of the device, including, among other things, restrictions on labeling, promotion, sale and distribution. Once granted, PMA approval may be withdrawn by the FDA if compliance with post approval requirements, conditions of approval or other regulatory standards are not maintained or problems are identified following initial marketing.$120,890.
After a device is placed on the market, it remains subject to significant regulatory requirements. Medical devices may be marketed only for the uses and indications for which they are cleared or approved. Device manufacturers must also establish registration and device listings with the FDA. A medical device manufacturer’s manufacturing processes and those of its suppliers are required to comply with the applicable portions of the QSR,Quality System Regulation, which covercovers the methods and documentation of the design, testing, production, processes, controls, quality assurance, labeling, packaging and shipping of medical devices. Domestic facility records and manufacturing processes are subject to periodic unscheduled inspections by the FDA. The FDA also may inspect foreign facilities that export products to the United States.
Health careHealthcare Law and Regulation
Health care providers and third-party payors play a primary role in the recommendation and prescription of drug products that are granted marketing approval. Arrangements with providers, consultants, third-party payors and customers are subject to broadly applicable fraud and abuse, anti-kickback, false claims laws, patient privacy laws and regulations and other health care laws and regulations that may constrain business and/or financial arrangements. Restrictions under applicable federal and state health care laws and regulations, include the following:
| ·
| | •the federal Anti-Kickback Statute, which prohibits, among other things, persons and entities from knowingly and willfully soliciting, offering, paying, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made, in whole or in part, under a federal health care program such as Medicare and Medicaid; •the federal civil and criminal false claims laws, including the civil False Claims Act, and civil monetary penalties laws, which prohibit individuals or entities from, among other things, knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false, fictitious or fraudulent or knowingly making, using or causing to made or used a false record or statement to avoid, decrease or conceal an obligation to pay money to the federal government; •the Foreign Corrupt Practices Act, which prohibits companies and their intermediaries from making, or offering or promising to make, improper payments to non-U.S. officials for the purpose of obtaining or retaining business or otherwise seeking favorable treatment; and entities from knowingly and willfully soliciting, offering, paying, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made, in whole or in part, under a federal health care program such as Medicare and Medicaid; |
| ·
| | the federal civil and criminal false claims laws, including the civil False Claims Act, and civil monetary penalties laws, which prohibit individuals or entities from, among other things, knowingly presenting, or
|
causing to be presented, to the federal government, claims for payment that are false, fictitious or fraudulent or knowingly making, using or causing to made or used a false record or statement to avoid, decrease or conceal an obligation to pay money to the federal government.
|
| ·
| | the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created additional federal criminal laws that prohibit, among other things, knowingly and willfully executing, or attempting to execute, a scheme to defraud any health care benefit program or making false statements relating to health care matters;
|
| ·
| | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, and their respective implementing regulations, including the Final Omnibus Rule published in January 2013, which impose obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information;
|
| ·
| | the federal false statements statute, which prohibits knowingly and willfully falsifying, concealing ·or covering up a material fact or making any materially false statement in connection with the delivery of or payment for health care benefits, items or services;
|
| ·
| | the federal transparency requirements known as the federal Physician Payments Sunshine Act, under the Patient Protection and Affordable Care Act, as amended by the Health Care Education Reconciliation Act, or the ACA, which requires certain manufacturers of drugs, devices, biologics and medical supplies to report annually to the Centers for Medicare & Medicaid Services, or CMS, within the United States Department of Health and Human Services, information related to payments and other transfers of value made by that entity to physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members; and
|
| ·
| | analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, which may apply to health care items or services that are reimbursed by non-government third-party payors, including private insurers.
|
SomeFurther, some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government in addition to requiring manufacturers to report information related to payments to physicians and other health care providers or marketing expenditures. StateAdditionally, some state and foreignlocal laws also governrequire the privacy and securityregistration of health informationpharmaceutical sales representatives in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.the jurisdiction.
Pharmaceutical Insurance Coverage and Health CareHealthcare Reform
In the United States and markets in other countries, patients who are prescribed treatments for their conditions and providers performing the prescribed services generally rely on third-party payors to reimburse all or part of the associated health care costs. Significant uncertainty exists as to the coverage and reimbursement status of products approved by the FDA and other government authorities. Thus, even if a product candidate is approved, sales of the product will depend, in part, on the extent to which third-party payors, including government health programs in the United States such as Medicare and Medicaid, commercial health insurers and managed care organizations, provide coverage and establish adequate reimbursement levels for, the product.product. The process for determining whether a payor will provide coverage for a product may be separate from the process for setting the price or reimbursement rate that the payor will pay for the product once coverage is approved. Third-party payors are increasingly challenging the prices charged, examining the medical necessity and reviewing the cost-effectiveness of medical products and services and imposing controls to manage costs. Third-party payors may limit coverage to specific products on an approved list, also known as a formulary, which might not include all of the approved products for a particular indication.
In order to secure coverage and reimbursement for any product that might be approved for sale, a company may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost-effectiveness of the product, in addition to the costs required to obtain FDA or other comparable marketing approvals. Nonetheless, product candidates may not be considered medically necessary or cost effective. A decision by a third-party payor not to cover a product could reduce physician utilization once the product is approved and have a material adverse effect on sales, results of operations and financial condition. Additionally, a payor’s decision to provide coverage for a
product does not imply that an adequate reimbursement rate will be approved. Further, one payor’s determination to provide coverage for a product does not assure that other payors will also provide coverage and reimbursement for the product, and the level of coverage and reimbursement can differ significantly from payor to payor.
The containment of health care costs also has become a priority of federal, state and foreign governments and the prices of products have been a focus in this effort. Governments have shown significant interest in implementing cost-containment programs, including price controls, restrictions on reimbursement and requirements for substitution of generic products. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit a company’s revenue generated from the sale of any approved products. Coverage policies and third-party reimbursement rates may change at any time. Even if favorable coverage and reimbursement status is attained for one or more products for which a company or its collaborators receive marketing approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
There have been a number of federal and state proposals during the last few years regarding the pricing of pharmaceutical and biopharmaceutical products, limiting coverage and reimbursement for drugs and biologics and other medical products, government control and other changes to the health care system in the United States. In March 2010, President Obama signed into law the ACA was enacted, which includes measures that have significantly changed health care financingPatient Protection and Affordable Care Act, as amended by both governmentalthe Health Care and private insurers. The provisions ofEducation Affordability Reconciliation Act, or collectively the ACA of importance to the pharmaceutical and biotechnology industry are, among others, the following:
| ·
| | an annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drug agents or biologic agents, which is apportioned among these entities according to their market share in certain government health care programs;
|
| ·
| | an increase in the rebates a manufacturer must pay under the Medicaid Drug Rebate Program to 23.1% and 13% of the average manufacturer price for branded and generic drugs, respectively;
|
| ·
| | a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% point-of-sale discounts to negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer's outpatient drugs to be covered under Medicare Part D;
|
| ·
| | extension of manufacturers' Medicaid rebate liability to covered drugs dispensed to individuals who are enrolled in Medicaid managed care organizations, unless the drug is subject to discounts under the 340B drug discount program;
|
| ·
| | a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted or injected;
|
| ·
| | expansion of eligibility criteria for Medicaid programs by, amongACA. In addition, other things, allowing states to offer Medicaid coverage to additional individuals and by adding new mandatory eligibility categories for certain individuals with income at or below 133% of the federal poverty level, thereby potentially increasing manufacturers' Medicaid rebate liability;
|
| ·
| | expansion of the entities eligible for discounts under the Public Health Service pharmaceutical pricing program;
|
| ·
| | new requirements under the federal Physician Payments Sunshine Act for drug manufacturers to report information related to payments and other transfers of value made to physicians and teaching hospitals as well as ownership or investment interests held by physicians and their immediate family members;
|
| ·
| | a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research;
|
| ·
| | creation of the Independent Payment Advisory Board, which, if and when impaneled, will have authority to recommend certain changes to the Medicare program that could result in reduced payments for prescription drugs; and
|
| ·
| | establishment of a Center for Medicare and Medicaid Innovation at CMS to test innovative payment and service delivery models to lower Medicare and Medicaid spending, potentially including prescription drug spending.
|
Other legislative changes have been proposed and adopted since the ACA was enacted. These changes includeIn August 2011, the Budget Control Act of 2011, which, among other things, ledcreated measures for spending reductions by Congress. A Joint Select Committee on Deficit Reduction, tasked with recommending a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, was unable to reach required goals, thereby triggering the legislation’s automatic reduction to several government programs. These changes included aggregate reductions to Medicare payments to providers of up to 2% per fiscal year, that startedwhich went into effect in April
2013 and will stayremain in effect through 2024 unless additional Congressional action is taken,2031 under the Coronavirus Aid, Relief, and Economic Security Act, or the CARES Act.
The American Taxpayer Relief Act of 2012, which, among other things, reduced Medicare payments to several types of providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. These new laws may result in additional reductions in Medicare and other healthcare funding and otherwise affect the prices we may obtain for any of our product candidates for which we may obtain regulatory approval or the frequency with which any such product candidate is prescribed or used. Further, there have been several recent U.S. congressional inquiries and proposed state and federal
Under current legislation, designed to, among other things, bring more transparency to drug pricing, review the relationship between pricing and manufacturer patient programs, reduce the costs of drugs under Medicare and reform government program reimbursement methodologies for drug products.
These healthcare reforms, as well as other healthcare reform measures that may be adopted in the future, may result in additionalactual reductions in Medicare payments may vary up to 4%. The Consolidated Appropriations Act, which was signed into law by President Biden in December 2022, made several changes to sequestration of the Medicare program. Section 1001 of the Consolidated Appropriations Act delays the 4% Statutory Pay-As-You-Go Act of 2010 (PAYGO) sequester for two years, through the end of calendar year 2024. Triggered by enactment of the American Rescue Plan Act of 2021, the 4% cut to the Medicare program would have taken effect in January 2023. The Consolidated Appropriations Act’s health care offset title includes Section 4163, which extends the 2% Budget Control Act of 2011 Medicare sequester for six months into fiscal year 2032 and other healthcare funding, more rigorous coverage criteria, newlowers the payment methodologiesreduction percentages in fiscal years 2030 and additional downward pressure on the price for any approved product and/or the level of reimbursement physicians receive for administering any approved product. Reductions in reimbursement levels may negatively impact the prices or the frequency with which products are prescribed or administered. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors. 2031.
Since enactment of the ACA, there have been, and continue to be, numerous legal challenges and Congressional actions to repeal and replace provisions of the law. In MayFor example, with enactment of the Tax Cuts and Jobs Act of 2017, which was signed by President Trump on December 22, 2017, Congress repealed the “individual mandate.” The repeal of this provision, which requires most Americans to carry a minimal level of health insurance, became effective in 2019. On June 17, 2021, the U.S. House of Representatives passed legislation known asSupreme Court dismissed the American Health Care Act of 2017. Thereafter, the Senate Republicans introduced and then updated a billmost recent judicial challenge to replace the ACA known asbrought by several states without specifically ruling on the Better Care Reconciliation Actconstitutionality of 2017. The Senate Republicans also introducedthe ACA. Litigation and legislation to repealover the ACA without companion legislationare likely to replace it,continue, with unpredictable and a “skinny” version of the Better Care Reconciliation Act of 2017. In addition, the Senate considered proposed healthcare reform legislation known as the Graham-Cassidy bill. None of these measures was passed by the U.S. Senate.uncertain results.
The Trump Administration has also takentook executive actions to undermine or delay implementation of the ACA. In January 2017, President Trump signed an Executive OrderACA, including directing federal agencies with authorities and responsibilities under the ACA to waive, defer, grant exemptions from, or delay the implementation of any provision of the ACA that would impose a fiscal or regulatory burden on states, individuals, healthcare providers, health insurers, or manufacturers of pharmaceuticals or medical devices. In October 2017, theOn January 28, 2021, however, President signedBiden issued a secondnew Executive Order allowingwhich directs federal agencies to reconsider rules and other policies that limit Americans’ access to health care and consider actions that will protect and strengthen that access. Under this Order, federal agencies are directed to re-examine: policies that undermine protections for the use of association health planspeople with pre-existing conditions, including complications related to COVID-19; demonstrations and short-term health insurance, which may provide fewer health benefits than the plans sold throughwaivers under Medicaid and the ACA exchanges. Atthat may reduce coverage or undermine the same time,programs, including work requirements; policies that undermine the Administration announcedHealth Insurance Marketplace or other markets for health insurance; policies that make it will discontinuemore difficult to enroll in Medicaid and the paymentACA; and policies that reduce affordability of cost-sharing reduction (CSR) payments to insurance companies until Congress approvescoverage or financial assistance, including for dependents.
The prices of prescription pharmaceuticals have also been the appropriationsubject of funds for such CSR payments. The loss of the CSR payments is expected to increase premiums on certain policies issued by qualified health plans under the ACA. A bipartisan bill to appropriate funds for CSR payments was introducedconsiderable discussion in the Senate, but the future of that bill is uncertain.
More recently, with enactment of the Tax Cuts and Jobs Act of 2017, which was signed by the President on December 22, 2017, Congress repealed the “individual mandate.” The repeal of this provision, which requires most Americans to carry a minimal level of health insurance, will become effective in 2019. According to the Congressional Budget Office, the repeal of the individual mandate will cause 13 million fewer Americans to be insured in 2027 and premiums in insurance markets may rise. Additionally, on January 22, 2018, President Trump signed a continuing resolution on appropriations for fiscal year 2018 that delayed the implementation of certain ACA-mandated fees, including the so-called “Cadillac” tax on certain high cost employer-sponsored insurance plans, the annual fee imposed on certain health insurance providers based on market share, and the medical device excise tax on non-exempt medical devices. Congress will likely consider other legislation to replace elements of the ACA, during the next Congressional session.
Further, thereUnited States. There have been several recent U.S. congressional inquiries, and proposed federal andas well as proposed and enacted state and federal legislation designed to, among other things, bring more transparency to drugpharmaceutical pricing, review the relationship between pricing and manufacturer patient programs, and reduce the costs of drugspharmaceuticals under Medicare and reform government program reimbursement methodologiesMedicaid. In 2020, President Trump issued several executive orders intended to lower the costs of prescription products and certain provisions in these orders have been incorporated into regulations. These regulations include an interim final rule implementing a most favored nation model for drug products. Atprices that would tie Medicare Part B payments for certain physician-administered pharmaceuticals to the federal level, Congresslowest price paid in other economically advanced countries, effective January 1, 2021. That rule, however, has been subject to a nationwide preliminary injunction and, the Trump
administration have each indicatedthis rule, CMS stated that it will explore all options to incorporate value into payments for Medicare Part B pharmaceuticals and improve beneficiaries' access to evidence-based care.
In addition, in October 2020, HHS and the FDA published a final rule allowing states and other entities to develop a Section 804 Importation Program to import certain prescription drugs from Canada into the United States. That regulation was challenged in a lawsuit by the Pharmaceutical Research and Manufacturers of America, or PhRMA, but the case was dismissed by a federal district court in February 2023 after the court found that PhRMA did not have standing to sue HHS. Nine states (Colorado, Florida, Maine, New Hampshire, New Mexico, North Dakota, Texas, Vermont and Wisconsin) have passed laws allowing for the importation of drugs from Canada. Certain of these states have submitted Section 804
Importation Program proposals and are awaiting FDA approval. On January 5, 2024, the FDA approved Florida’s plan for Canadian drug importation.
Further, on November 20, 2020, HHS finalized a regulation removing safe harbor protection for price reductions from pharmaceutical manufacturers to plan sponsors under Part D, either directly or through pharmacy benefit managers, unless the price reduction is required by law. The rule also creates a new safe harbor for price reductions reflected at the point-of-sale, as well as a safe harbor for certain fixed fee arrangements between pharmacy benefit managers and manufacturers. Pursuant to court order, the removal and addition of the aforementioned safe harbors were delayed and recent legislation imposed a moratorium on implementation of the rule until January 1, 2026. The Inflation Reduction Act of 2022, or IRA, further delayed implementation of this rule to January 1, 2032.
On August 16, 2022, the IRA was signed into law by President Biden. The new legislation has implications for Medicare Part D, which is a program available to individuals who are entitled to Medicare Part A or enrolled in Medicare Part B to give them the option of paying a monthly premium for outpatient prescription drug coverage. Among other things, the IRA requires manufacturers of certain drugs to engage in price negotiations with Medicare (beginning in 2026), with prices that can be negotiated subject to a cap; imposes rebates under Medicare Part B and Medicare Part D to penalize price increases that outpace inflation (first due in 2023); and replaces the Part D coverage gap discount program with a new discounting program (beginning in 2025). The IRA permits the Secretary of HHS to implement many of these provisions through guidance, as opposed to regulation, for the initial years.
Specifically, with respect to price negotiations, Congress authorized Medicare to negotiate lower prices for certain costly single-source drug and biologic products that do not have competing generics or biosimilars and are reimbursed under Medicare Part B and Part D. CMS may negotiate prices for ten high-cost drugs paid for by Medicare Part D starting in 2026, followed by 15 Part D drugs in 2027, 15 Part B or Part D drugs in 2028, and 20 Part B or Part D drugs in 2029 and beyond. This provision applies to drug products that have been approved for at least 9 years and biologics that have been licensed for 13 years, but it does not apply to drugs and biologics that have been approved for a single rare disease or condition. Further, the legislation subjects drug manufacturers to civil monetary penalties and a potential excise tax for failing to comply with the legislation by offering a price that is not equal to or less than the negotiated “maximum fair price” under the law or for taking price increases that exceed inflation. The legislation also requires manufacturers to pay rebates for drugs in Medicare Part D whose price increases exceed inflation. The new law also caps Medicare out-of-pocket drug costs at an estimated $4,000 a year in 2024 and, thereafter beginning in 2025, at $2,000 a year.
On June 6, 2023, Merck & Co. filed a lawsuit against HHS and CMS asserting that, among other things, the IRA’s Drug Price Negotiation Program for Medicare constitutes an uncompensated taking in violation of the Fifth Amendment of the Constitution. Subsequently, a number of other parties, including the U.S. Chamber of Commerce, Bristol Myers Squibb Company, PhRMA, Astellas, Novo Nordisk, Janssen Pharmaceuticals, Novartis, AstraZeneca and Boehringer Ingelheim, also filed lawsuits in various courts with similar constitutional claims against HHS and CMS. We expect that litigation involving these and other provisions of the IRA will continue, to seek new legislative and/or administrative measures to control drug costs. with unpredictable and uncertain results.
At the state level, individual stateslegislatures are increasinglyaggressive in passing legislation and implementing regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. In addition, regional health care authorities and individual hospitals are increasingly using bidding procedures to determine what pharmaceutical products and which suppliers will be included in their prescription drug and other health care programs. These measures could reduce the ultimate demand for our products, once approved, or put pressure on our product pricing. We expect that additional state and federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, which could result in reduced demand for our product candidates or additional pricing pressures.
ReviewFederal and State Data Privacy Laws
There are multiple privacy and data security laws that may impact our business activities, in the United States and in other countries where we conduct trials or where we may do business in the future. These laws are evolving and may increase both our obligations and our regulatory risks in the future. In the health care industry generally, under the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, HHS has issued regulations to protect the privacy and security of protected health information used or disclosed by covered entities including certain healthcare providers, health plans, and healthcare clearinghouses. HIPAA also regulates standardization of data content, codes and formats used in healthcare transactions and standardization of identifiers for health plans and providers. HIPAA also imposes certain
obligations on the business associates of covered entities that obtain protected health information in providing services to or on behalf of covered entities. HIPAA may apply to us in certain circumstances and may also apply to our business partners in ways that may impact our relationships with them. Our clinical trials are regulated by the Common Rule, which also includes specific privacy-related provisions. In addition to federal privacy regulations, there are a number of state laws governing confidentiality and security of health information that may be applicable to our business. In addition to possible federal civil and criminal penalties for HIPAA violations, state attorneys general are authorized to file civil actions for damages or injunctions in federal courts to enforce HIPAA and seek attorney’s fees and costs associated with pursuing federal civil actions. In addition, state attorneys general (along with private plaintiffs) have brought civil actions seeking injunctions and damages resulting from alleged violations of HIPAA’s privacy and security rules. State attorneys general also have authority to enforce state privacy and security laws. New laws and regulations governing privacy and security may be adopted in the future as well.
In 2018, California passed into law the California Consumer Privacy Act, or the CCPA, which took effect on January 1, 2020, and imposed many requirements on businesses that process the personal information of California residents. Many of the CCPA’s requirements are similar to those found in the General Data Protection Regulation, or the GDPR, including requiring businesses to provide notice to data subjects regarding the information collected about them and how such information is used and shared, and providing data subjects the right to request access to such personal information and, in certain cases, request the erasure of such personal information. The CCPA also affords California residents the right to opt-out of “sales” of their personal information. The CCPA contains significant penalties for companies that violate its requirements. In November 2020, California voters passed a ballot initiative for the California Privacy Rights Act, or the CPRA, which went into effect on January 1, 2023, and significantly expanded the CCPA to incorporate additional GDPR-like provisions including requiring that the use, retention, and sharing of personal information of California residents be reasonably necessary and proportionate to the purposes of collection or processing, granting additional protections for sensitive personal information, and requiring greater disclosures related to notice to residents regarding retention of information. The CPRA also created a new enforcement agency—the California Privacy Protection Agency—whose sole responsibility is to enforce the CPRA, which will further increase compliance risk. The provisions in the CPRA may apply to some of our business activities.
In addition to California, eleven other states have passed comprehensive privacy laws similar to the CCPA and CPRA. These laws are either in effect or will go into effect sometime before the end of 2026. Like the CCPA and CPRA, these laws create obligations related to the processing of personal information, as well as special obligations for the processing of “sensitive” data, which includes health data in some cases. Some of the provisions of these laws may apply to our business activities. There are also states that are strongly considering or have already passed comprehensive privacy laws during the 2024 legislative sessions that will go into effect in 2025 and beyond. Other states will be considering similar laws in the future, and Congress has also been debating passing a federal privacy law. There are also states that are specifically regulating health information that may affect our business. For example, the State of Washington passed the My Health My Data Act in 2023 which specifically regulated health information that is not otherwise regulated by the HIPAA rules, and the law also has a private right of action, which further increases the relevant compliance risk. Connecticut and Nevada have also passed similar laws regulating consumer health data, and more states are considering such legislation in 2024. These laws may impact our business activities, including our identification of research subjects, relationships with business partners and ultimately the marketing and distribution of our products.
Because of the breadth of these laws and the narrowness of the statutory exceptions and regulatory safe harbors available under such laws, it is possible that some of our current or future business activities, including certain clinical research, sales and marketing practices, and the provision of certain items and services to our customers, could be subject to challenge under one or more of such privacy and data security laws. The heightening compliance environment and the need to build and maintain robust and secure systems to comply with different privacy compliance and/or reporting requirements in multiple jurisdictions could increase the possibility that a healthcare company may fail to comply fully with one or more of these requirements. If our operations are found to be in violation of any of the privacy or data security laws or regulations described above that are applicable to us, or any other laws that apply to us, we may be subject to penalties, including potentially significant criminal, civil, and administrative penalties, damages, fines, contractual damages, reputational harm, diminished profits and future earnings, additional reporting requirements and/or oversight if we become subject to a consent decree or similar agreement to resolve allegations of non-compliance with these laws, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations. To the
extent that any product candidates we may develop, once approved, are sold in a foreign country, we may be subject to similar foreign laws.
Regulations and Procedures Governing Approval of Medicinal Products in the European Union
In order to market any product outside of the United States, a company must also comply with numerous and varying regulatory requirements of other countries and jurisdictions regarding quality, safety and efficacy and governing, among other things, clinical trials, marketing authorization, commercial sales and distribution of products. Whether or not it obtains FDA approval for a product, an applicanta sponsor will need to obtain the necessary approvals by the comparable non-U.S. regulatory authorities before it can commence clinical trials or marketing of the product in those countries or jurisdictions. Specifically, the process governing approval of medicinal products in the European Union, or EU, generally follows the same lines as in the United States. It entails satisfactory completion of preclinical studies and adequate and well-controlled clinical trials to establish the safety and efficacy of the product for each proposed indication. It also requires the submission to the relevant competent authorities of a marketing authorization application, or MAA, and granting of a marketing authorization by these authorities before the product can be marketed and sold in the EU.
Clinical Trial ApprovalNon-Clinical Studies
The Clinical TrialsNon-clinical studies are performed to demonstrate the health or environmental safety of new chemical or biological substances. Non-clinical (pharmaco-toxicological) studies must be conducted in compliance with the principles of GLP as set forth in EU Directive 2001/20/2004/10/EC (unless otherwise justified for certain particular medicinal products—e.g., radio-pharmaceutical precursors for radio-labeling purposes). In particular, non-clinical studies, both in vitro and in vivo, must be planned, performed, monitored, recorded, reported and archived in accordance with the Directive 2005/28/EC on Good Clinical Practice, or GCP,GLP principles, which define a set of rules and the related national implementing provisions of the individual EU Member States govern thecriteria for a quality system for the approval of clinical trials inorganizational process and the EU. Under this system, an applicant must obtain prior approval fromconditions for non-clinical studies. These GLP standards reflect the competent national authority of the EU Member States in which the clinical trial is to be conducted. Furthermore, the applicant may only start a clinical trial at a specific study site after the competent ethics committee has issued a favorable opinion. The clinical trial application must be accompanied by, among other documents, an investigational medicinal product dossier (the Common Technical Document) with supporting information prescribed by Directive 2001/20/EC, Directive 2005/28/EC, where relevant the implementing national provisions of the individual EU Member StatesOrganization for Economic Co-operation and further detailed in applicable guidance documents.Development requirements.
In April 2014,Clinical Trial Approval
On January 31, 2022, the new Clinical Trials Regulation (EU) No 536/2014 or Clinical Trials Regulation, was adopted. The Regulation was published on June 16, 2014 but is not expected to apply until 2019. The Clinical Trials Regulation will be directly applicablebecame effective in all the EU Member States, repealingEuropean Union and replaced the currentprior Clinical Trials Directive 2001/20/ECEC. The new regulation aims at simplifying and replacing any national legislation that was put in place to implementstreamlining the Directive. Conductauthorization, conduct and transparency of all clinical trials performed in the EU will continue to be bound by currently applicable provisions untilEuropean Union. Under the new Clinical Trials Regulation becomes applicable. The extent to which on-going clinical trials will be governed by the Clinical Trials Regulation will depend on when the Clinical Trials Regulation becomes applicable and on the duration of the individual clinical trial. If a clinical trial continuescoordinated procedure for more than three years from the day on which the Clinical Trials Regulation becomes applicable the Clinical Trials Regulation will at that time begin to apply to the clinical trial.
The new Clinical Trials Regulation aims to simplify and streamline the approval of clinical trials, the sponsor of a clinical trial to be conducted in more than one Member State of the EU. European Union, or EU Member State, is required to submit a single application for approval. The submission will be made through the Clinical Trials Information System, a new clinical trials portal overseen by the European Medicines Agency, or EMA, and available to clinical trial sponsors, competent authorities of the EU Member States and the public.
The main characteristics of the new regulation include: a streamlined application procedure via a single entry point, the “EU Portal and Database”;include a single set of documents to be prepared and submitted for the application, as well as simplified reporting procedures for clinical trial sponsors;sponsors, and a harmonized procedure for the assessment of applications for clinical trials, which is divided in two parts. Part I is assessed by the appointed reporting Member State, whose assessment report is submitted for review by the sponsor and all other competent authorities of all EU Member States in which an application for authorization of a clinical trial has been submitted (Concerned(EU Member States)States concerned). Part II is assessed separately by each Concerned Member State. concerned. Strict deadlines have been established for the assessment of clinical trial applications. The role of the relevant ethics committees in the assessment procedure will continue to be governed by the national law of the Concernedconcerned EU Member State. However, overall related timelines will be defined by the Clinical Trials Regulation.
the EU Member State in which the clinical trial is to be conducted. If the clinical trial is conducted in different EU Member States, the competent authorities in each of these EU Member States must provide their approval for the conduct of the clinical trial. Furthermore, the sponsor may only start a clinical trial at a specific study site after the applicable ethics committee has issued a favorable opinion.
Parties conducting certain clinical trials must, as in the United States, post clinical trial information in the EU at the EU Clinical Trials Register.
PRIME Designation in the EU
In March 2016, the European Medicines Agency, or EMA launched an initiative to facilitate development of product candidates in indications, often rare, for which few or no therapies currently exist. The PRIority MEdicines, or PRIME, scheme is intended to encourage
drug development in areas of unmet medical need and provides accelerated assessment of products representing substantial innovation reviewed under the centralized procedure. Products from small- and medium-sized enterprises may qualify for earlier entry into the PRIME scheme than larger companies. Many benefits accrue to sponsors of product candidates with PRIME designation, including but not limited to, early and proactive regulatory dialogue with the EMA, frequent discussions on clinical trial designs and other development program elements, and accelerated marketing authorization application assessment once a dossier has been submitted. Importantly, a dedicated Agency contact and rapporteur from the Committee for Human Medicinal Products, or CHMP, or Committee for Advanced Therapies are appointed early in PRIME scheme facilitating increased understanding of the product at EMA’s Committee level. A kick-off meeting initiates these relationships and includes a team of multidisciplinary experts at the EMA to provide guidance on the overall development and regulatory strategies.
Marketing Authorization
To obtain a marketing authorization for a product under EU regulatory systems, an applicanta sponsor must submit an MAA either under a centralized procedure administered by the EMA, or one of the procedures administered by competent authorities in the EU Member States (decentralized procedure, national procedure or mutual recognition procedure). A marketing authorization may be granted only to an applicanta sponsor established in the EU. Regulation (EC) No 1901/2006 provides that prior to obtaining a marketing authorization in the EU, applicants have tosponsors must demonstrate compliance with all measures included in an EMA-approved Paediatric Investigation Plan, or PIP, covering all subsets of the pediatric population, unless the EMA has granted (1)(i) a product-specific waiver, (2)(ii) a class waiver or (3)(iii) a deferral for one or more of the measures included in the PIP.
The centralized procedure provides for the grant of a single marketing authorization by the European Commission that is valid across the European Economic Area (i.e., the EU as well as Iceland, Liechtenstein and Norway). Pursuant to Regulation (EC) No 726/2004, the centralized procedure is compulsory for specific products, including for medicines produced by certain biotechnological processes, products designated as orphan medicinal products, advanced therapy medicinal products, and products with a new active substance indicated for the treatment of certain diseases, including products for the treatment of cancer. For products with a new active substance indicated for the treatment of other diseases and products that are highly innovative or for which a centralized process is in the interest of patients, the centralized procedure may be optional. The centralized procedure may at the request of the applicantsponsor also be used in certain other cases. We anticipate that the centralized procedure will be mandatory for the product candidates we are developing.
Under the centralized procedure, the Committee for Medicinal Products for Human Use, or CHMP, is also responsible for several post-authorization and maintenance activities, such as the assessment of modifications or extensions to an existing marketing authorization. Under the centralized procedure in the EU, the maximum timeframe for the evaluation of an MAA is 210 days, excluding clock stops, when additional information or written or oral explanation is to be provided by the applicantsponsor in response to questions of the CHMP. Accelerated evaluation might be granted by the CHMP in exceptional cases, when a medicinal product is of major interest from the point of view of public health and in particular from the viewpoint of therapeutic innovation. If the CHMP accepts such request, the time limit of 210 days will be reduced to 150 days, but it is possible that the CHMP can revert to the standard time limit for the centralized procedure if it considers that it is no longer appropriate to conduct an accelerated assessment. At the end of this period, the CHMP provides a scientific opinion on whether or not a marketing authorization should be granted in relation to a medicinal product. Within 15 calendar days of receipt of a final opinion from the CHMP, the European Commission must prepare a draft decision concerning an application for marketing authorization. This draft decision must take the opinion and any relevant provisions of EU law into account. Before arriving at a final decision on an application for centralized authorization of a medicinal product the European Commission must consult the Standing Committee on Medicinal Products for Human Use. The Standing Committee is composed of representatives of the EU Member States and chaired by a non-voting European Commission representative. The European Parliament also has a related “droit de regard”.regard.” The European Parliament's role is to ensure that the European Commission has not exceeded its powers in deciding to grant or refuse to grant a marketing authorization.
The decentralized procedure is available to sponsors who wish to market a product in various EU Member States where such product has not received marketing approval in any EU Member States before. The decentralized procedure provides for approval by one or more other, or concerned, member states of an assessment of an application performed by one member state designated by the sponsor, known as the reference member state. Under this procedure, a sponsor submits an application based on identical dossiers and related materials, including a draft summary of product characteristics, and draft labeling and package leaflet, to the reference member state and concerned member states. The reference member state prepares a draft assessment report and drafts of the related materials within 210 days after receipt of a valid application. Within 90 days of receiving the reference member state’s assessment report and related materials, each concerned member state must decide whether to approve the assessment report and related materials.
If a member state cannot approve the assessment report and related materials on the grounds of potential serious risk to public health, the disputed points are subject to a dispute resolution mechanism and may eventually be referred to the European Commission, may grant a so-called “marketing authorization under exceptional circumstances”. Such authorizationwhose decision is intended for products for which the applicant can demonstrate that it is unable to provide
binding on all member states.
comprehensive dataIn particular circumstances, European Union legislation (Article 14–a Regulation (EC) No 726/2004 (as amended by Regulation (EU) 2019/5 and Regulation (EC) No 507/2006 on the efficacy and safety under normal conditions of use, because the indicationsConditional Marketing Authorizations for which the product in question is intended are encountered so rarely that the applicant cannot reasonably be expectedMedicinal Products for Human Use) enables sponsors to provide comprehensive evidence, or in the present state of scientific knowledge, comprehensive information cannot be provided, or it would be contrary to generally accepted principles of medical ethics to collect such information. Consequently,obtain a conditional marketing authorization under exceptional circumstances may be granted subject to certain specific obligations, which may include the following:
| ·
| | the applicant must complete an identified program of studies within a time period specified by the competent authority, the results of which form the basis of a reassessment of the benefit/risk profile;
|
| ·
| | the medicinal product in question may be supplied on medical prescription only and may in certain cases be administered only under strict medical supervision, possibly in a hospital and in the case of a radiopharmaceutical, by an authorized person; and
|
| ·
| | the package leaflet and any medical information must draw the attention of the medical practitioner to the fact that the particulars available concerning the medicinal product in question are as yet inadequate in certain specified respects.
|
A marketing authorization under exceptional circumstances is subject to annual review to reassess the risk-benefit balance in an annual reassessment procedure. Continuation of the authorization is linked to the annual reassessment and a negative assessment could potentially result in the marketing authorization being suspended or revoked. The renewal of a marketing authorization of a medicinal product under exceptional circumstances, however, follows the same rules as a “normal” marketing authorization. Thus, a marketing authorization under exceptional circumstances is granted for an initial five years, after which the authorization will become valid indefinitely, unless the EMA decides that safety grounds merit one additional five-year renewal.
The European Commission may also grant a so-called “conditional marketing authorization” prior to obtaining the comprehensive clinical data required for an application for a full marketing authorization.authorization. Such conditional marketing authorizationsapprovals may be granted for product candidates (including medicines designated as orphan medicinal products), if (i) the risk-benefit balance of(1) the product candidate is positive, (ii) itintended for the treatment, prevention or medical diagnosis of seriously debilitating or life-threatening diseases; (2) the product candidate is likelyintended to meet unmet medical needs of patients; (3) a marketing authorization may be granted prior to submission of comprehensive clinical data provided that the applicant will be in a position to provide the required comprehensive clinical trial data, (iii) the product fulfills an unmet medical need and (iv) the benefit to public health of the immediate availability on the market of the medicinal product concerned outweighs the risk inherent in the fact that additional data are still required.required; (4) the risk-benefit balance of the product candidate is positive, and (5) it is likely that the sponsor will be in a position to provide the required comprehensive clinical trial data. A conditional marketing authorization may contain specific obligations to be fulfilled by the marketing authorization holder, including obligations with respect to the completion of ongoing or new studies and with respect to the collection of pharmacovigilance data. Conditional marketing authorizations are valid for one year, and may be renewed annually, if the risk-benefit balance remains positive, and after an assessment of the need for additional or modified conditions and/or specific obligations. The timelines for the centralized procedure described above also apply with respect to the review by the CHMP of applications for a conditional marketing authorization.authorization, but applicants can also request EMA to conduct an accelerated assessment, for instance in cases of unmet medical needs.
The EU medicines rules expressly permitExceptional Circumstances
A marketing authorization may also be granted “under exceptional circumstances” when the EU Member Statesapplicant can show that it is unable to adopt national legislation prohibitingprovide comprehensive data on the efficacy and safety under normal conditions of use even after the product has been authorized and subject to specific procedures being introduced. This may arise in particular when the intended indications are very rare and, in the present state of scientific knowledge, it is not possible to provide comprehensive information, or restrictingwhen generating data may be contrary to generally accepted ethical principles. This marketing authorization is close to the sale, supplyconditional marketing authorization as it is reserved to medicinal products to be approved for severe diseases or useunmet medical needs and the applicant does not hold the complete data set legally required for the grant of anya marketing authorization. However, unlike the conditional marketing authorization, the applicant does not, and will not in the future, have to provide the missing data. Although the marketing authorization “under exceptional circumstances” is granted definitively, the risk-benefit balance of the medicinal product containing, consisting of or derived from a specific type of human or animal cell, such as embryonic stem cells. Whileis reviewed annually and the products we have in development do not make use of embryonic stem cells, it is possible that the national laws in certain EU Member States may prohibit or restrict us from commercializing our products, even if they have been granted an EU marketing authorization.
Unlike the centralized authorization procedure, the decentralized marketing authorization procedure requires a separate application to, and leads to separate approval by,is withdrawn in case the competent authorities of each EU Member State in whichrisk-benefit ratio is no longer favorable. Under these procedures, before granting the product is to be marketed. This application is identical to the application that would be submitted tomarketing authorization, the EMA for authorization through the centralized procedure. The reference EU Member State prepares a draft assessment and drafts of the related materials within 120 days after receipt of a valid application. The resulting assessment report is submitted to the concerned EU Member States who, within 90 days of receipt, must decide whether to approve the assessment report and related materials. If a concerned EU Member State cannot approve the assessment report and related materials due to concerns relating to a potential serious risk to public health, disputed elements may be referred to the European Commission, whose decision is binding on all EU Member States.
The mutual recognition procedure similarly is based on the acceptance byor the competent authorities of the EU Member Statesmember states make an assessment of the risk-benefit balance of the product on the basis of scientific criteria concerning its quality, safety, and efficacy. Except conditional marketing authorizations, marketing authorizations have an initial duration of five years. After these five years, the authorization may be renewed on the basis of a medicinal product byreevaluation of the competent authorities of other EU Member
States. The holder of a national marketing authorization may submit an application to the competent authority of an EU Member State requesting that this authority recognize the marketing authorization delivered by the competent authority of another EU Member State.risk-benefit balance.
Regulatory Data Protection in the EU
In the EU, innovative medicinal products approved on the basis of a complete independent data package qualify for eight years of data exclusivity upon marketing authorization and an additional two years of market exclusivity pursuant to Directive 2001/83/EC. Regulation (EC) No 726/2004 repeats this entitlement for medicinal products authorized in accordance the centralized authorization procedure. Data exclusivity prevents applicantssponsors for authorization of generics of these innovative products from referencing the innovator’s data to assess a generic (abridged) application for a period of eight years. During an additional two-year period of market exclusivity,, a generic marketing authorization application can be submitted and authorized, and the innovator’s data may be referenced, but no generic medicinal product can be placed on the EU market until the expiration of the market exclusivity. The overall ten-year period will be extended to a maximum of 11 years if, during the first eight years of those ten years, the marketing authorization holder obtains an authorization for one or more new therapeutic indications which, during the scientific evaluation prior to their authorization, are held to bring a significant clinical benefit in comparison with existing therapies. Even if a compound is considered to be a new chemical entity so that the innovator gains the prescribed period of data exclusivity, another company nevertheless could also market another version of the product if such company obtained marketing authorization based on an MAA with a complete independent data package of pharmaceutical tests, preclinical tests and clinical trials.
Periods of Authorization and Renewals
A marketing authorization has an initial validity for five years in principle. The marketing authorization may be renewed after five years on the basis of a re-evaluation of the risk-benefit balance by the EMA or by the competent authority of the EU Member State. To this end, the marketing authorization holder must provide the EMA or the competent authority with a consolidated version of the file in respect of quality, safety and efficacy, including all variations introduced since the marketing authorization was granted, at least six months before the marketing authorization ceases to be valid. The European Commission or the competent authorities of the EU Member States may decide on justified grounds relating to pharmacovigilance, to proceed with one further five-year period of marketing authorization. Once subsequently definitively renewed, the marketing authorization shall be valid for an unlimited period. Any authorization which is not followed by the actual placing of the medicinal product on the EU n market (in case of centralized procedure) or on the market of the authorizing EU Member State within three years after authorization ceases to be valid. valid.
Orphan Drug Designation and Exclusivity
Regulation (EC) No. 141/2000, as implemented by Regulation (EC) No. 847/2000 provides that a drug can be designated as an orphan drug by the European Commission if its sponsor can establish: that the product is intended for the diagnosis, prevention or treatment of (1)(i) a life-threatening or chronically debilitating condition affecting not more than five in ten thousand persons in the EU when the application is made, or (2)(ii) a life-threatening, seriously debilitating or serious and chronic condition in the EU and that without incentives it is unlikely that the marketing of the drug in the EU would generate sufficient return to justify the necessary investment. For either of these conditions, the applicantsponsor must demonstrate that there exists no satisfactory method of diagnosis, prevention or treatment of the condition in question that has been authorized in the EU or, if such method exists, the drug will be of significant benefit to those affected by that condition.
Once authorized, orphan medicinal products are entitled to ten years of market exclusivity in all EU Member States and a range of other benefits during the development and regulatory review process including scientific assistance for study protocols, authorization through the centralized marketing authorization procedure covering all member countries and a reduction or elimination of registration and marketing authorization fees. However, marketing authorization may be granted to a similar medicinal product with the same orphan indication during the ten-year period with the consent of the marketing authorization holder for the original orphan medicinal product or if the manufacturer of the original orphan medicinal product is unable to supply sufficient quantities. Marketing authorization may also be granted to a similar medicinal product with the same orphan indication if this product is safer, more effective or otherwise clinically superior to the original orphan medicinal product. The period of market exclusivity may, in addition,
be reduced to six years if it can be demonstrated based on the basis of available evidence that the original orphan medicinal product is sufficiently profitable not to justify maintenance of market exclusivity.
Regulatory Requirements after a Marketing Authorization has been Obtained
In case an authorization for a medicinal product in the EU is obtained, the holder of the marketing authorization is required to comply with a range of requirements applicable to the manufacturing, marketing, promotion and sale of medicinal products. These include:
| ·
| | Compliance with the EU’s stringent pharmacovigilance or safety reporting rules must be ensured. These rules can impose post-authorization studies and additional monitoring obligations.
|
| ·
| | The manufacturing of authorized medicinal products, for which a separate manufacturer’s license is mandatory, must also be conducted in strict compliance with the applicable EU laws, regulations and guidance, including Directive 2001/83/EC, Directive 2003/94/EC, Regulation (EC) No 726/2004 and the European Commission Guidelines for Good Manufacturing Practice. These requirements include compliance with EU cGMP standards when manufacturing medicinal products and active pharmaceutical ingredients, including the manufacture of active pharmaceutical ingredients outside of the EU with the intention to import the active pharmaceutical ingredients into the EU.
|
| ·
| | The marketing and promotion of authorized drugs, including industry-sponsored continuing medical education and advertising directed toward the prescribers of drugs and/or the general public, are strictly regulated in the EU notably under Directive 2001/83EC, as amended, and EU Member State laws. Direct-to-consumer advertising of prescription medicines is prohibited across the EU.
|
Brexitinclude compliance with the EU’s stringent pharmacovigilance or safety reporting rules must be ensured, and the Regulatory Frameworkmanufacturing of authorized medicinal products, for which a separate manufacturer’s license is mandatory, must also be conducted in strict compliance with the applicable EU laws, regulations and guidance. These requirements include compliance with EU cGMP standards when manufacturing medicinal products and active pharmaceutical ingredients, including the manufacture of active pharmaceutical ingredients outside of the EU with the intention to import the active pharmaceutical ingredients into the EU, and the marketing and promotion of authorized drugs, including industry-sponsored continuing medical education and advertising directed toward the prescribers of drugs and/or the general public, which are strictly regulated in the United KingdomEU notably under Directive 2001/83EC, as amended, and EU Member State laws. Direct-to-consumer advertising of prescription medicines is prohibited across the EU.
On June 23, 2016, the electorate in the United Kingdom voted in favor of leaving the EU, which is commonly referred to as “Brexit”. Thereafter, on March 29, 2017, the country formally notified the EU of its intention to withdraw pursuant to Article 50 of the Lisbon Treaty. The withdrawal of the United Kingdom from the EU will take effect either on the effective date of the withdrawal agreement or, in the absence of agreement, two years after the United Kingdom provides a notice of withdrawal pursuant to the EU Treaty. Since the regulatory framework for pharmaceutical products in the United Kingdom. covering quality, safety and efficacy of pharmaceutical products, clinical trials, marketing authorization, commercial sales and distribution of pharmaceutical products is derived from EU directives and regulations, Brexit could materially impact the future regulatory regime which applies to products and the approval of product candidates in the United Kingdom. It remains to be seen how, if at all, Brexit will impact regulatory requirements for product candidates and products in the United Kingdom.
Pricing Decisions for Approved Products
In the EU, pricing and reimbursement schemes vary widely from country to country. Some countries provide that products may be marketed only after a reimbursement price has been agreed. Some countries may require the completion of additional studies that compare the cost-effectiveness of a particular product candidate to currently available therapies or so-called health technology assessments, in order to obtain reimbursement or pricing approval. For example, the EU provides options for its Member States to restrict the range of products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. Member States may approve a specific price for a product, or it may instead adopt a system of direct or indirect controls on the profitability of the company placing the product on the market. Other Member States allow companies to fix their own prices for products but monitor and control prescription volumes and issue guidance to physicians to limit prescriptions. Recently, many countries in the EU have increased the amount of discounts required on pharmaceuticals and these efforts could continue as countries attempt to manage health care expenditures, especially in light of the severe fiscal and debt crises experienced by many countries in the EU. The downward pressure on health care costs in general, particularly prescription products, has become intense. As a result, increasingly high barriers are being erected to the entry of new products. Political, economic and regulatory developments may further complicate pricing negotiations, and pricing negotiations may continue after reimbursement has been obtained. Reference pricing used by various Member States, and parallel trade, i.e., arbitrage between low-priced and high-priced Member States, can further reduce prices. There can be no assurance that any country that has price controls or reimbursement limitations for
pharmaceutical products will allow favorable reimbursement and pricing arrangements for any products, if approved in those countries.
Approval of Companion Diagnostic Devices
In the European Union, medical devices such as companion diagnostics must comply with the General Safety and Performance Requirements, or SPRs, detailed in Annex I of the EU Medical Devices Regulation (Regulation (EU) 2017/745), or MDR which came into force on May 26, 2021 and replaced the previously applicable EU Medical Devices Directive (Council Directive 93/42/EEC). Compliance with SPRs and additional requirements applicable to companion medical devices are prerequisites to be able to affix the CE Mark of Conformity to medical devices, without which they cannot be marketed or sold. To demonstrate compliance with the SPRs, a manufacturer must undergo a conformity assessment procedure, which varies according to the type of medical device and its classification. The MDR is meant to establish a uniform, transparent, predictable, and sustainable regulatory framework across the EU for medical devices.
Separately, the regulatory authorities in the EU also adopted a new In Vitro Diagnostic Regulation (EU) 2017/746, which became effective in May 2022. The new regulation replaces the In Vitro Diagnostics Directive (IVDD) 98/79/EC. Manufacturers wishing to apply to a notified body for a conformity assessment of their in vitro diagnostic medical device had until May 2022 to update their Technical Documentation to meet the requirements and comply with the new, more stringent Regulation. The regulation, among other things, strengthens the rules on placing devices on the market and reinforces surveillance once they are available; establishes explicit provisions on manufacturers’ responsibilities for the follow-up of the quality, performance, and safety of devices placed on the market; improves the traceability of medical devices throughout the supply chain to the end-user or patient through a unique identification number; sets up a central database to provide patients, healthcare professionals and the public with comprehensive information on products available in the European Union; and strengthens rules for the assessment of certain high-risk devices, such as implants, which may have to undergo an additional check by experts before they are placed on the market.
General Data Protection Regulation
There are significant privacy and data security laws that apply in Europe and other countries. The collection, use, disclosure, transfer, or other processing of personal data, including personal health data, regarding individuals who are located in the European Economic Area, or the EEA, and the processing of personal data that takes place in the EEA, is subject to the GDPR, which became effective on May 25, 2018. The GDPR is wide-ranging in scope and imposes numerous requirements on companies that process personal data, and it imposes heightened requirements on companies that process health and other sensitive data, such as requiring in many situations that a company obtain the consent of the individuals to whom the sensitive personal data relate before processing such data. Examples of obligations imposed by the GDPR on companies processing personal data that fall within the scope of the GDPR include providing information to individuals regarding data processing activities, implementing safeguards to protect the security and confidentiality of personal data, appointing a data protection officer, providing notification of data breaches, and taking certain measures when engaging third-party processors. The GDPR also imposes strict rules on the transfer of personal data to countries outside the EEA, including the U.S., and permits data protection authorities to impose large penalties for violations of the GDPR, including potential fines of up to €20 million or 4% of annual global revenues, whichever is greater. The GDPR also confers a private right of action on data subjects and consumer associations to lodge complaints with supervisory authorities, seek judicial remedies, and obtain compensation for damages resulting from violations of the GDPR. Compliance with the GDPR is a rigorous and time-intensive process that may increase the cost of doing business or require companies to change their business practices to ensure full compliance.
Following the Court of Justice of the European Union decision, in October 2022, President Biden signed an executive order to implement the EU-U.S. Data Privacy Framework, which would serve as a replacement to the EU-US Privacy Shield. The European Union initiated the process to adopt an adequacy decision for the EU-U.S. Data Privacy Framework in December 2022, and the European Commission adopted the adequacy decision in July 2023. The adequacy decision permits U.S. companies who self-certify to the EU-U.S. Data Privacy Framework to rely on it as a valid data transfer mechanism for data transfers from the European Union to the United States. However, some privacy advocacy groups have already suggested that they will be challenging the EU-U.S. Data Privacy Framework. If these challenges are successful, they may not only impact the EU-U.S. Data Privacy Framework, but also further limit the viability of the standard contractual clauses and other data transfer mechanisms. The uncertainty around this issue has the potential to impact our business.
Beyond the GDPR, there are privacy and data security laws in a growing number of countries around the world. While many loosely follow the GDPR as a model, other laws contain different or conflicting provisions. These laws will impact our ability to conduct our business activities, including both our clinical trials and any eventual sale and distribution of commercial products.
Brexit and the Regulatory Framework in the United Kingdom
The U.K.’s withdrawal from the EU took place on January 31, 2020. The EU and the U.K. reached an agreement on their new partnership in the Trade and Cooperation Agreement, or the Trade Agreement, which was applied provisionally beginning on January 1, 2021, and which entered into force on May 1, 2021. The Trade Agreement focuses primarily on free trade by ensuring no tariffs or quotas on trade in goods, including healthcare products such as medicinal products. Thereafter, the EU and the U.K. will form two separate markets governed by two distinct regulatory and legal regimes, except that Northern Ireland will continue to broadly follow EU laws as further described below. As such, the Trade Agreement seeks to minimize barriers to trade in goods while accepting that border checks will become inevitable as a consequence that the U.K. is no longer part of the single market. As of January 1, 2021, the Medicines and Healthcare products Regulatory Agency, or MHRA, became responsible for supervising medicines and medical devices in Great Britain, comprising England, Scotland, and Wales under domestic law whereas Northern Ireland continues to be subject to EU rules under the Northern Ireland Protocol.
On February 27, 2023, the U.K. government and the European Commission announced a political agreement in principle to replace the Northern Ireland Protocol with a new set of arrangements, known as the “Windsor Framework.” This new framework fundamentally changes the existing system under the Northern Ireland Protocol, including with respect to the regulation of medicinal products in the U.K. In particular, the MHRA will be responsible for approving all medicinal products destined for the U.K. market (i.e., Great Britain and Northern Ireland), and the EMA will no longer have any role in approving medicinal products destined for Northern Ireland. A single U.K.-wide market authorization will be granted by the MHRA for all medicinal products to be sold in the U.K., enabling products to be sold in a single pack and under a single authorization throughout the U.K. The Windsor Framework was approved by the EU-U.K. Joint Committee on March 24, 2023, so the U.K. government and the EU will enact legislative measures to bring it into law. On June 9, 2023, the MHRA announced that the medicines aspects of the Windsor Framework will apply from January 1, 2025. The Human Medicines Regulations 2012 (SI 2012/1916) (as amended), or HMR, is the primary legal instrument for the regulation of medicines in the U.K. The HMR has incorporated into the domestic law the body of EU law instruments governing medicinal products that pre-existed prior to the U.K.’s withdrawal from the EU.
EU laws which have been transposed into U.K. law through secondary legislation continue to be applicable as “retained EU law.” However, new legislation such as the (EU) Clinical Trials Regulation will not be applicable in Great Britain. Since a significant proportion of the regulatory framework for pharmaceutical products in the U.K. covering the quality, safety and efficacy of pharmaceutical products, clinical trials, MAs, commercial sales and distribution of pharmaceutical products is derived from EU directives and regulations, Brexit may have a material impact upon the regulatory regime with respect to the development, manufacture, importation, approval and commercialization of our product candidates in the U.K. For example, the U.K. is no longer covered by the centralized procedures for obtaining EU-wide marketing authorizations from the EMA, and a separate market authorization will be required to market our product candidates in the U.K. A new international recognition framework has been in place since January 1, 2024, whereby the MHRA will have regard to decisions on the approval of marketing authorizations made by the EMA and certain other regulators when determining an application for a new Great Britain marketing authorization.
As with other issues related to Brexit, there are open questions about how personal data will be protected in the U.K. and whether personal information can transfer from the EU to the U.K. Following the withdrawal of the U.K. from the EU, the U.K. Data Protection Act 2018 applies to the processing of personal data that takes place in the U.K. and includes parallel obligations to those set forth by GDPR. While the Data Protection Act of 2018 in the U.K. that “implements” and complements the GDPR has achieved Royal Assent on May 23, 2018 and is now effective in the U.K., it is unclear whether transfer of data from the EEA to the U.K. will remain lawful under the GDPR, although these transfers currently are permitted by an adequacy decision from the European Commission. The U.K. government has already determined that it considers all European Union 27 and EEA member states to be adequate for the purposes of data protection, ensuring that data flows from the U.K. to the EU/EEA remain unaffected. In addition, a recent decision from the European Commission appears to deem the U.K. as being “essentially adequate” for purposes of data transfer from the EU to the U.K., although this decision may be re-evaluated in the future. The U.K. and the U.S. have also agreed to a U.S.-U.K. “Data Bridge,” which functions similarly to the EU-U.S. Data Privacy Framework and provides an additional legal mechanism for companies to transfer data from the U.K. to the United States. In addition to the U.K., Switzerland is also in the process of approving an adequacy decision in relation to the Swiss-U.S. Data Privacy Framework (which would function similarly to the EU-U.S. Data Privacy Framework and the U.S.-U.K. Data Bridge in relation to data transfers from Switzerland to the United States). Any changes or updates to these developments have the potential to impact our business.
Sales and Marketing
We hold North American, European, Central and EuropeanSouth American, Australian, Israeli and Russian commercialization rights to SY‑1425tamibarotene for all cancer indications, and worldwide rights to SY‑1365SY-2101 and all of our other preclinical programsSY-5609 for all potential indications. Subject
We are planning our distribution, dispensing, commercial operations, and sales infrastructure strategy for a commercial launch of tamibarotene in the United States, subject to receiving marketing approval, weapproval. We intend to build a focused sales and marketingspecialized sales organization in the United States and potentially in Europe to sell our products.tamibarotene. We believe that such an organization will be able to address the community of physicians who are key specialists in treating the HR-MDS and unfit AML patient populations for which our product candidatestamibarotene is being developed. We are being developed.
We also plan to buildbuilding a marketing and sales managementcommercial organization to create and implement marketing strategies for any products that we market through our own sales organization and to oversee and support our sales force.the anticipated commercial launch of tamibarotene. The responsibilities of the marketing organization would include developing educational initiatives with respect to any approved products and, establishing relationshipsupon marketing approval of tamibarotene, engaging with institutional leaders, researchers and practitioners in relevant fields of medicine.
Outside of the United States and potentially Europe, whereWhere appropriate, we may elect in the future to utilize strategic partners, distributors or contract sales forces to assist in the commercialization of our products. In certain instances,To that end, we may consider building our own commercial infrastructure.intend to seek a partner to commercialize tamibarotene in the territories outside the United States.
Manufacturing
We do not currently own or operate manufacturing facilities for the production of clinical or commercial quantities of our product candidates. Although we intend to rely on third‑party contract manufacturers to produce our product candidates and any products we may develop in the future, we have recruited personnel with experience to manage these third‑party contract manufacturers.
Employees
As of December 31, 20172023, we had 56 full‑time68 full-time employees, including 2925 employees with M.D., Ph.D. or Ph.D.Pharm.D. degrees. Of these full‑timefull-time employees, 4446 employees are engaged in research and development activities and 1222 employees are engaged in general and administrative activities. During the year ended December 31, 2023, we hired 13 new employees, of whom 10 are engaged in research and development activities and three are engaged in general and administrative activities. None of our employees isare represented by a labor union or covered by a collective bargaining agreement. We conduct an employee engagement survey every year as well as regular pulse surveys, and based on the results of the surveys, we consider our relationship with our employees to be good. We focus on employee retention and attrition rates and our progress against equity, diversity and inclusion goals as key human capital measures in managing our business.
Corporate Information
We were incorporated under the laws of the State of Delaware on November 9, 2011 under the name LS22, Inc. We changed our name to Syros Pharmaceuticals, Inc. on August 15, 2012. Our principal executive office is located at 620 Memorial Drive, Suite 300, Cambridge, Massachusetts 02139, and our telephone number is (617) 744-1340.
Information Available on the Internet
Our Internet website address is www.syros.com. The information contained on, or that can be accessed through, our website is not a part of or incorporated by reference in this Annual Report on Form 10-K.Report. We have included our website address in this in this Annual Report on Form 10-K solely as an inactive textual reference. We make available free of charge through our website our Annual Report, on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to those reports filed or furnished pursuant to Sections 13(a) and 15(d) of the Exchange Act. We make these reports available through the “SEC Filings” section of our website as soon as reasonably practicable after we electronically file such reports with, or furnish such reports to, the SEC. We also make available, free of charge on our website, the reports filed with the U.S. Securities and Exchange Commission, or SEC, by our executive officers, directors and 10% stockholders pursuant to Section 16 under the Exchange Act as soon as reasonably practicable after copies of those filings are provided to us by those persons. You can find, copy and inspect information we file at the SEC’s public reference room, which is located at 100 F Street, N.E., Room 1580, Washington, DC 20549. Please call the SEC at 1-800-SEC-0330 for more information about the operation of the SEC’s public reference room. You can review our electronically filed reports and other information that we file with the SEC on the SEC’s website at http://www.sec.gov.
ITEM 1A. RISK FACTORS
ITEM 1A.RISK FACTORS
We operate in a dynamic and rapidly changing business environment that involves multiple risks and substantial uncertainty. The following discussion addresses risks and uncertainties that could cause, or contribute to causing, actual results to differ from expectations in material ways. In evaluating our business, investors should pay particular attentioncarefully consider the risks described below in addition to the risks and uncertainties described below andother information set forth in other sections of this Annual Report on Form 10-K, or Annual Report, including the Management’s Discussion and Analysis of Financial Condition and Results of Operations section and the consolidated financial statements and related notes. These risks, some of which have occurred and any of which may occur in the future, can have a material adverse effect on our subsequent filings withbusiness, financial condition, results of operations or the SEC. Theseprice of our publicly traded securities. The risks described below are not the only risks we face. Additional risks and uncertainties or other events that we do not currently anticipateknown to us, or that we currently deem to be immaterial, also may occur or become material in the future and adversely affect our business, reputation, financial condition, results of operations cash flows and financial condition. The tradingor the price of our common stock could also decline due topublicly traded securities. Therefore, historical operating results, financial and business performance, events and trends are often not a reliable indicator of future operating results, financial and business performance, events or trends. If any of thesethe following risks occurs, our business, financial condition, and youresults of operations and future growth prospects could lose all or part of your investment.be materially and adversely affected.
Risks Related to Our Financial Position and Need for Additional Capital
We have incurred significant losses since inception, expect to incur significant and increasing losses for at least the next several years, and may never achieve or maintain profitability.
We have incurred significant annual net operating losses in every year since our inception. We expect to continue to incur significant and increasing net operating losses for at least the next several years. Our net losses were $29.8 million, $47.7$164.6 million and $54.0$94.7 million for the years ended December 31, 2015, 20162023 and 2017,2022, respectively. As of December 31, 2017,2023, we had an accumulated deficit of $155.3$722.8 million. We have not generated any revenues from product sales, have not completed the development of any product candidate and may never have a product candidate approved for commercialization. We have financed our operations to date primarily through the saleissuance of equity securities.securities, through license and collaboration agreements, and through our credit facility with Oxford Finance LLC, or Oxford. We have devoted substantially all of our financial resources and efforts to research and development and general and administrative expense to support such research and development. Our net losses may fluctuate significantly from quarter to quarter and year to year. Net losses and negative cash flows have had, and will continue to have, an adverse effect on our stockholders' equity (deficit) and working capital.
We anticipate that our expensesfuture funding requirements, both short-term and long-term, will depend on many factors and will increase substantially if and as we:
| ·
| | continue our planned clinical development activities with respect to SY-1425, a selective retinoic acid receptor alpha, or RARα, agonist that is currently being evaluated in combination with azacitidine, a hypomethylating agent, and with daratumumab, an anti-CD38 antibody, in a Phase 2 clinical trial, and SY-1365, a selective inhibitor of cyclin-dependent kinase 7, or CDK7, that is currently in the dose-escalation portion of a Phase 1 clinical trial in patients with advanced solid tumors;
|
•continue our planned clinical development activities with respect to tamibarotene;
| ·
| | develop and seek approval of companion diagnostic tests for use in identifying patients who may benefit from treatment with our products and product candidates;
|
•develop and seek approval of companion diagnostic tests for use in identifying patients who may benefit from treatment with tamibarotene or any future product candidates;
| ·
| | initiate and continue research, preclinical and clinical development efforts for our research and preclinical programs;
|
•seek to identify and develop additional product candidates, which may involve entering into collaborations, licensing agreements or other arrangements;
| ·
| | further develop our gene control platform;
|
•acquire or in-license other product candidates or technologies;
| ·
| | seek to identify and develop additional product candidates;
|
•seek regulatory and marketing approvals for tamibarotene or any future product candidates that successfully complete clinical trials, if any;
| ·
| | acquire or in-license other product candidates or technologies;
|
•establish sales, marketing, distribution and other commercial infrastructure to commercialize tamibarotene or other products for which we may obtain marketing approval, if any;
| ·
| | seek regulatory and marketing approvals for our product candidates that successfully complete clinical trials, if any;
|
•become obligated to make milestone payments upon the successful completion of specified development and commercialization activities;
| ·
| | establish sales, marketing, distribution and other commercial infrastructure in the future to commercialize various products for which we may obtain marketing approval, if any;
|
| ·
| | require the manufacture of larger quantities of product candidates for clinical development and, potentially, commercialization; |
| ·
| | maintain, expand and protect our intellectual property portfolio;
|
| ·
| | hire and retain additional personnel and add operational, financial and management information systems, including personnel and systems to support our product development and commercialization efforts and help us comply with our obligations as a public company; and
|
•maintain, expand and protect our intellectual property portfolio; and
| ·
| | add equipment and physical infrastructure to support our research and development programs.
|
•hire and retain additional personnel and add operational, financial and management information systems, including personnel and systems to support our product development and commercialization efforts.
Our ability to become and remain profitable depends on our ability to generate revenue. We do not expect to generate significant revenue unless and until we are, or any future collaborator is, able to obtain marketing approval for, and successfully commercialize, onetamibarotene or more of ourany future product candidates. Successful commercialization will require achievement of key milestones, including initiating and successfully completing clinical trials of our product candidates, obtaining marketing approval for these product candidates, manufacturing, marketing and selling those products for which we, or any of our future collaborators, may obtain marketing approval has been obtained, satisfying any post-marketing requirements and obtaining reimbursement for our products from private insurance or government payors. Because of the uncertainties and risks associated with these activities, we are unable to accurately predict the timing and amount of revenues, and if or when we might achieve profitability. We and any future collaborators may never succeed in these activities and, even if we do, or any future collaborators do, we may never generate revenues that are large enough for us to achieve profitability. Even ifIf we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our failure to become and remain profitable would decrease the value of our company and could impair our ability to raise capital, expand our business, maintain our research and development efforts, diversify our pipeline of product candidates or continue our operations and cause a decline in the value of our common stock.
We have a limited operating history, no products approved for sale and no history of commercializing pharmaceutical products, which may make it difficult to evaluate the prospects for our future viability.
We commenced operations in 2011. Our operations to date have been limited to financing and staffing our company, developing our gene control platform and conducting preclinical and early clinical research. We have not yet demonstrated an ability to advance a program into a late-stage clinical trial, obtain marketing approvals, manufacture a product at commercial scale or arrange for a third party to do so on our behalf, develop companion diagnostic tests or arrange for a third party to do so on our behalf, or conduct sales and marketing activities necessary for successful product commercialization. Accordingly, you should consider our prospects in light of the costs, uncertainties, delays and difficulties frequently encountered by companies in the early stages of development, especially clinical stage biopharmaceutical companies such as ours. Any predictions you make about our future success or viability may not be as accurate as they could be if we had a longer operating history or a history of successfully developing and commercializing pharmaceutical products.
We may encounter unforeseen expenses, difficulties, complications, delays and other known or unknown factors in achieving our business objectives. We will eventually need to transition from a company with a development focus to a company capable of supporting commercial activities. We may not be successful in such a transition.
We expect our financial condition and operating results to continue to fluctuate significantly from quarter to quarter and year to year due to a variety of factors, many of which are beyond our control. Accordingly, you should not rely upon the results of any quarterly or annual periods as indications of future operating performance.
We will need substantial additional funding to execute our operating plan, and if we are unable to raise capital, when needed, we could be forced to delay, reduce or eliminate our product development programs or commercialization efforts.
Developing pharmaceutical products, including conducting preclinical studies and clinical trials, is a very time consuming, expensive and uncertain process that takes years to complete. We expect our expenses to increase in connection with our ongoing activities, particularly with respect to our ongoing Phase 2 clinical trial of SY-1425 in combination with azacitidine and daratumumab, as we develop and seek approval of companion diagnostic tests for use in identifying patients who may benefit from treatment with SY-1425, advance the clinical development of SY-1365 into planned Phase 1 expansion cohorts in ovarian and breast cancers, initiate new research and preclinical development efforts and seek marketing approval for any product candidates that we successfully develop. Moreover, under license agreements with various licensors, we are obligated to make milestone payments upon the successful completion of specified development and commercialization activities for products or product candidates covered by licensed
intellectual property rights. In addition, if we obtain marketing approval for any product candidate that we may successfully develop, we may incur significant commercialization expenses related to product sales, marketing, manufacturing and distribution to the extent that such sales, marketing, manufacturing and distribution are not the responsibility of a future collaborator. Furthermore, we expect to incur significant additional costs associated with operating as a public company.process. Accordingly, we will need to obtain substantial additional funding in connection with our continuing operations. If we are unable to raise capital when needed or on attractive terms, we may be forced to delay, reduce or eliminate our research and development programs or any future commercialization efforts.
We will be required to expend significant funds in order to advance the development of SY-1425 and SY-1365, as well as our other research and preclinical programs. In addition, while we may seek one or more collaborators for future development of our current product candidates or any future product candidates that we may develop for one or more indications, we may not be able to enter into a collaboration for any of our product candidates for such indications on suitable terms, on a timely basis, or at all. In any event, our existing cash, cash equivalents and marketable securities will not be sufficient to fund all of the efforts that we plan to undertake or to fund the completion of development of our product candidates or our other preclinical programs. Accordingly, we will be required to obtain further funding through public or private equity offerings, debt financings, collaborations and licensing arrangements or other sources. We do not have any committed external source of funds to support our internal research and development efforts. Adequate additional financing may not be available to us on acceptable terms, or at all. Our failure to raise capital as and when needed would have a negative impact on our financial condition and our ability to pursue our business strategy.
We believe that our existing cash and cash equivalents and marketable securities as of December 31, 2017, together with amounts received from Incyte Corporation, or Incyte, in connection with our collaboration and option agreement executed in January 2018 and the net proceeds from the underwritten public offering of our common stock and concurrent private placement of our common stock to Incyte that closed in February 2018,2023 will enable us to fund our planned operating expense and capital expenditure requirements into 2020.the second quarter of 2025. Our estimate as to how long we expect our existing cash and cash equivalents and marketable securities to be able to continue to fund our operations is based on assumptions that may prove to be wrong, and we could use our available capital resources sooner than we currently expect. Further, changing circumstances, some of which may be beyond our control, could cause us to consume capital significantly faster than we currently anticipate, and we may need to seek additional funds sooner than planned. In any event, our existing cash and cash equivalents will not be sufficient to fund all of the efforts that we plan to undertake or to fund the completion of development of all of our product candidates.
Our future funding requirements both short-term and long-term, will depend on many factors, including:including those discussed above under “We have incurred significant losses since inception, expect to incur significant losses for at least the next several years, and may never achieve or maintain profitability.” Our future funding requirements may also depend on:
| ·
| | the scope, progress, timing, costs and results of clinical trials of SY-1425 and SY-1365 and any associated companion diagnostic tests;
|
•the costs of precommercial activities related to tamibarotene and any future product candidates, including any physician education programs relating to selecting and treating genomically defined patient populations;
| ·
| | research and preclinical development efforts for any future product candidates that we may develop;
|
•the timing and amount of milestone and other payments due to TMRC Co. Ltd., or TMRC, associated with the development, manufacture and commercialization of tamibarotene;
| ·
| | the number of future product candidates that we pursue and their development requirements;
|
•the timing and amount of milestone payments due to Qiagen Manchester Limited, or Qiagen, associated with the development and commercialization of a companion diagnostic test for use with tamibarotene; and
| ·
| | our ability to enter into, and the terms and timing of, any collaborations, licensing agreements or other arrangements;
|
•the timing and amount of milestone payments due to Orsenix, LLC, or Orsenix, associated with any potential further development and commercialization of SY-2101 in the future.
| ·
| | whether our target discovery collaboration with Incyte Corporation, or Incyte, will yield any validated targets, whether Incyte will exercise any of its options to exclusively license intellectual property directed to such targets, and whether and when any of the target validation fees, option exercise fees, milestone payments or royalties under the collaboration agreement with Incyte will ever be paid;
|
| ·
| | the outcome, timing and costs of seeking regulatory approvals;
|
| ·
| | the costs of commercialization activities for any of our product candidates that receive marketing approval to the extent such costs are not the responsibility of any future collaborators, including the costs and timing of establishing product sales, marketing, distribution and manufacturing capabilities;
|
| ·
| | the costs of acquiring potential new product candidates or technology;
|
| ·
| | the costs of any physician education programs relating to selecting and treating genomically defined patient populations;
|
Unstable market and economic conditions may have serious adverse consequences on our business, financial condition and stock price.
Global credit and financial markets have experienced extreme volatility and disruptions in the past several years, including severely diminished liquidity and credit availability, declines in consumer confidence, declines in economic growth, increases in unemployment rates and uncertainty about economic stability, including most recently in connection with the impacts of COVID-19, disruptions impacting global supply, the conflict between Russia and Ukraine and related
| ·
| | the timing and amount of milestone and other payments due to licensors for patent and technology rights used in our development platform;
|
| ·
| | revenue received from commercial sales, if any, of our current and future product candidates;
|
| ·
| | our headcount growth and associated costs as we expand our research and development, operate as a public company, and establish a commercial infrastructure;
|
| ·
| | the costs of preparing, filing and prosecuting patent applications, maintaining and protecting our intellectual property rights and defending against intellectual property related claims; and
|
| ·
| | the costs of operating as a public company.
|
Raising additional capital may cause dilution to our stockholders, restrict our operations or require us to relinquish rights to our technologies or product candidates.
We expect our expenses to increaseremain high in connection with our planned operations. To the extent that we raise additional capital through the sale of common stock, convertible securities or other equity securities, as we did through a public offeringunderwritten offerings of our common stock that closed in February 2018,December 2023 and January 2021 and in a private placement of our securities in September 2022, the ownership interests of our existing stockholders may be substantially diluted, and the terms of these securities could include liquidation or other preferences and anti-dilution protections that could adversely affect our stockholders’ rights. In addition, debt financing, if available, would result insuch as our term loan facility with Oxford, has created fixed payment obligations and may involve agreements that includeimposed restrictive covenants that limit our ability to take specific actions, such as incurring additional debt, making capital expenditures, creating liens, redeeming stock or declaring dividends, that could adversely impact our ability to conduct our business. In addition, securing financing could require a substantial amount of time and attention from our management and may divert a disproportionate amount of their attention away from day-to-day activities, which may adversely affect our management'smanagement’s ability to oversee the development of our product candidates.
If we raise additional funds through collaborations or marketing, distribution or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams or product candidates or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves. In this regard, we are seeking out-licensing opportunities for the clinical development of SY-5609. However, we cannot provide assurance that such a transaction will be consummated, or that sufficient additional capital to support further development can be obtained or will be obtained on favorable terms.
The terms of our Loan and Security Agreement place restrictions on our operating and financial flexibility.
In February 2020, we entered into a Loan and Security Agreement with Oxford, which is secured by substantially all of our currently owned or later acquired personal property other than our intellectual property (but including the right to payments and proceeds of intellectual property), which is subject to a negative pledge. We refer to the Loan and Security Agreement with Oxford as the Loan Agreement. We borrowed $20.0 million upon execution of the Loan Agreement and borrowed an additional $20.0 million term loan advance in December 2020. One additional term loan advance of $20.0 million remains available under the Loan Agreement, subject to certain terms and conditions, including the achievement of certain milestones.
On July 3, 2022, we entered into an amendment to the Loan Agreement, or the Loan Amendment, pursuant to which Oxford, in its capacity as lender and agent, has agreed to modify the Loan Agreement in order to, among other things, (i) extend the interest only period from March 1, 2023 to March 1, 2024 and extend the maturity date from February 1, 2025 to February 1, 2026, and (ii) upon the achievement of certain milestones and subject to the payment of certain fees, further extend the interest only period to September 1, 2024 and maturity date to August 1, 2026.
The Loan Agreement, as amended by the Loan Amendment, contains representations and warranties, affirmative and negative covenants applicable to us and our subsidiaries and events of default, as more fully described in the Loan Agreement, as amended. In particular, the Loan Agreement also includes events of default, the occurrence and during the continuation of which provide Oxford, as collateral agent, with the right to exercise remedies against us and the collateral securing the loans under the Loan Agreement, including foreclosure against our property securing the Loan Agreement, including our cash, potentially requiring us to renegotiate our agreement on terms less favorable to us, or to immediately cease operations.
Further, if we are liquidated, the lenders’ right to repayment would be senior to the rights of the holders of our common stock to receive any proceeds from the liquidation. Oxford could declare a default upon the occurrence of any event that they interpret as a material adverse change as defined under the Loan Agreement, thereby requiring us to repay the loan
immediately or to attempt to reverse the declaration of default through negotiation or litigation. Any declaration by Oxford of an event of default could significantly harm our business and prospects and could cause the price of our common stock to decline.
Risks Related to the Discovery, Development and Commercialization of Product Candidates
Our approach to the discovery and development of product candidates based on our gene control platform is novel and unproven, and we do not know whether we will be able to develop any products of commercial value.
We are focused on discovering and developing medicines for the treatment of cancer and other diseases based upon our gene control platform. We are leveraging our platform to create a pipeline of gene control product candidates for genomically defined patients whose diseases have not been adequately addressed to date by other genomics approaches and to design and conduct efficient clinical trials with a higher likelihood of success. While we believe that applying our gene control platform to create medicines for genomically defined patient populations may potentially enable drug research and clinical development that is more efficient than conventional small molecule drug research and development, our approach is both novel and unproven. Because our approach is both novel and unproven, the cost and time needed to develop our product candidates is difficult to predict, and our efforts may not result in the discovery and development of commercially viable medicines. We may also be incorrect about the effects of our product candidates on the diseases of genomically defined patient populations, which may limit the utility of our approach or the perception of the utility of our approach. Furthermore, our estimates of genomically defined patient populations available for study and treatment may be lower than expected, which could adversely affect our ability to conduct clinical trials and may also adversely affect the size of any market for medicines we may successfully commercialize.
We have not yet succeeded and may never succeed in demonstrating efficacy and safety for our current or any future product candidates in a late-stage clinical trial or in obtaining marketing approval thereafter. For example, although we have discovered and evaluated compounds using our novel gene control platform, we have not yet
demonstrated sufficient safety or efficacy of any of our product candidates in clinical trials to warrant further development in the patient population studied.
Our gene control platform may fail to help us discover and develop additional potential product candidates.
A significant portion of the research that we are conducting involves identifying novel targets and points of intervention and developing new compounds using our gene control platform. The drug discovery that we are conducting using our gene control platform may not be successful in identifying compounds that have commercial value or therapeutic utility. Our gene control platform may initially show promise in identifying potential product candidates, yet fail to yield viable product candidates for clinical development or commercialization for a number of reasons, including:
| ·
| | insights regarding disease targets that are obtained through the use of our gene control platform may be generated independently through alternative approaches or be published by third parties;
|
| ·
| | compounds created through our gene control platform may not demonstrate efficacy, safety or tolerability;
|
| ·
| | potential product candidates may, on further study, be shown to have harmful side effects or other characteristics that indicate that they are unlikely to receive marketing approval and achieve market acceptance;
|
| ·
| | competitors may develop alternative therapies that render our potential product candidates non-competitive or less attractive; or
|
| ·
| | a potential product candidate may not be capable of being produced at an acceptable cost.
|
Our research programs to identify new product candidates will require substantial technical, financial and human resources, and we may be unsuccessful in our efforts to identify new product candidates. If we are unable to identify suitable additional compounds for preclinical and clinical development, our ability to develop product candidates and obtain product revenues in future periods could be compromised, which could result in significant harm to our financial position and adversely impact our stock price.
In the near term, we are dependent on the success of SY-1425 and SY-1365.tamibarotene. If we are unable to initiate or complete the clinical development of, obtain marketing approval for, or successfully commercialize SY-1425 or SY-1365,tamibarotene, either alone or with a collaborator, or if we experience significant delays in doing so, our business couldwill be substantially harmed.
We currently have no products approved for sale and are investing a significant portion offocusing our efforts and financial resources intowards the development of SY-1425 and SY-1365.tamibarotene. Our prospects are substantially dependent on our ability or that of any future collaborator, to develop, obtain marketing approval for and successfully commercializegenerate product candidates in one or more disease indications.
The success of SY-1425 and SY-1365revenue will depend heavily on several factors, including the following:
| ·
| | successful initiation, enrollment and completion of clinical trials;
|
| ·
| | a safety, tolerability and efficacy profile, alone or in combination with other approved or investigational products, that is satisfactory to the U.S. Food and Drug Administration, or FDA, or any comparable foreign regulatory authority for marketing approval;
|
| ·
| | timely receipt of marketing approvals from applicable regulatory authorities;
|
| ·
| | the successful development and approval of companion diagnostic tests for use in identifying patients who may benefit from treatment with SY-1425 or SY-1365; |
| ·
| | the performance of our future collaborators, if any;
|
| ·
| | the extent of any required post-marketing approval commitments to applicable regulatory authorities;
|
| ·
| | establishment of supply arrangements with third-party suppliers of raw materials and drug substance and drug product manufacturers;
|
| ·
| | establishment of arrangements with third-party manufacturers to obtain finished drug product that is appropriately packaged for sale;
|
| ·
| | adequate ongoing availability of raw materials and drug product for clinical development and any commercial sales;
|
| ·
| | obtaining and maintaining patent, trade secret protection and regulatory exclusivity, both in the United States and internationally, including our ability to maintain our license agreement with TMRC Co. Ltd., or TMRC;
|
| ·
| | protection of our rights in our intellectual property portfolio;
|
| ·
| | successful launch of commercial sales following any marketing approval;
|
| ·
| | a continued acceptable safety profile following any marketing approval;
|
| ·
| | commercial acceptance by patients, the medical community and third-party payors;
|
| ·
| | successful identification of biomarkers for patient selection;
|
| ·
| | continued availability of appropriate tissue samples to enable the identification of novel targets in genomically defined subsets of patients; and
|
| ·
| | our ability to compete with other therapies.
|
Many of these factors are beyond our control, including clinical development the regulatory submission process, potential threats to our intellectual property rights and the manufacturing, marketing and sales efforts of any future collaborator. If we are unable to develop, receive marketing approval for and successfully commercialize SY-1425 or SY-1365, on our own or with any future collaborator, or experience delays as a result of any of these factors or otherwise, our business could be substantially harmed.
If clinical trials of any product candidates that we, or any future collaborators, may develop fail to satisfactorily demonstrate safety and efficacy to the FDA and other regulators, we, or any future collaborators, may incur additional costs or experience delays in completing, or ultimately be unable to complete, the development andeventual commercialization of these product candidates.tamibarotene.
We, and any future collaborators, are not permitted to commercialize, market, promote or sell any product candidate in the United States without obtaining marketing approval from the FDA. Foreign regulatory authorities, such as the European Medicines Agency, or the EMA, impose similar requirements. We, andrequirements in foreign jurisdictions. Before obtaining marketing approval from regulatory authorities for the sale of tamibarotene or any future collaborators,product candidates, we must completeconduct extensive preclinical development and clinical trials to demonstrate the safety and efficacy of our product candidates in humans before we will be able to obtain these approvals.humans.
We are conductingClinical trials of a Phase 2product candidate require the activation of clinical trial sites and the enrollment of SY-1425 in combination with azacitidine in genomically defined subsetsa sufficient number of patients, with newly-diagnosed acute myeloid leukemia, or AML,including patients who are not suitable candidates for standard chemotherapy,suffering from the disease the product candidate is intended to treat and in combination with daratumumab in genomically defined patients with relapsed or refractory AML or higher-risk myelodysplastic syndrome, or MDS, identified using our biomarkers. We anticipate reporting clinical data from this trial in the fourth quarter of 2018. We are collaborating with a third party with respect to the clinical trial assay being used to select patients with our RARA and IRF8 biomarkers for inclusion in the trial.who meet other eligibility criteria. Our anticipated time to data in this trialour clinical trials and the quantity of data to be presented from these trials is and will continue to be subject to our continued ability to initiateactivate clinical trial sites, and recruit eligible patients, the performance of the clinical trial assay and the prevalence of patients with these biomarkers, and the satisfaction by biomarker-positive
patients of other eligibility criteria for participation in the trial. In the case of tamibarotene, our time to data is also dependent on the prevalence of patients who overexpress the RARA biomarker and the impact of new product approvals in the AML and MDS fields. The rate of site activations and patient enrollment in the trial is difficult to predict. As a result, there can be no assurance thatpredict, and we will enroll or have data fromexperienced slower-than-anticipated site activations in our SELECT-MDS-1 trial as we expanded the trial when we anticipate.
We are also conducting a Phase 1 clinical trial of SY-1365 in patients with advanced solid tumors. We expect to report initial clinical data from the dose escalation portion of this trial in the fourth quarter of 2018. Our anticipated time to data in this trial is subject to our ability to recruit eligible patients and the number of dose cohorts that will need to be enrolled prior to observing pharmacokinetic activity, if achieved at all. Our assumption as to the activity of SY-1365 at particular dose levels may prove to be incorrect.study global footprint. There can be no assurance that we will enroll or have data from the trialour clinical trials when we anticipate.
Further, we may experience delays in initiating or completing clinical trials and preparing for regulatory submissions, particularly if there are changes in or the enactment of additional statutes, promulgation of regulations or issuance of guidance during preclinical or clinical development. For example, in December 2022, with the passage of Food and Drug Omnibus Reform Act, Congress required sponsors to develop and submit a diversity action plan for each Phase 3 clinical trial or any other “pivotal study” of a new drug or biological product. These plans are meant to encourage the enrollment of more diverse patient populations in late-stage clinical trials of FDA regulated products.
Clinical testing is expensive, is difficult to design and implement, can take many years to complete and is inherently uncertain as to outcome. We cannot guarantee that any clinical trials will be conducted as planned or completed on schedule, if at all. The clinical development of our product candidates is susceptible to the risk of failure inherent at any stage of product development, including failuredevelopment. Moreover, we, or any collaborators, may experience any of a number of possible unforeseen adverse events in connection with clinical trials, many of which are beyond our control, including:
•we, or our collaborators, may fail to demonstrate efficacy in a clinical trial or across a broad population of patients, the occurrence of adverse events that are severe or medically or commercially unacceptable, failure to comply with protocols or applicable regulatory requirements and determination by the FDA or any comparable foreign regulatory authority that a product candidate may not continue development orpatients;
•it is not approvable. It is also possible that, even if one or more of our product candidates has a beneficial effect, that effect will not be detected during clinical evaluation as a result of one or more of a variety of factors, including the size, duration, design, measurements, conduct or analysis of our clinical trials. For example, in December 2017 we reported data from our Phase 2 clinical trial evaluating SY-1425 as a single agent in genomically defined subsets of patients with relapsed or refractory AML and higher risk MDS. While biological and clinical activity was observed in certain patients enrolled in the trial, the data were not sufficiently robust to warrant further development of SY-1425 as a single agent in these patient populations and we elected to stop enrollment in the portions of our Phase 2 clinical trial evaluating SY-1425 as a single agent. We face a similar risk of failure in our ongoing evaluation of SY-1425 in combination with azacitidine and daratumumab. Conversely, as a result of the same factors, our clinical trials may indicate an apparent positive effect of a product candidate that is greater than the actual positive effect, if any. Similarly,For example, many compounds that initially showed promise in our clinical trials we may failearlier stage testing have later been found to detect toxicitycause side effects that prevented further development of or intolerability caused by the compound;
•our product candidates may have undesirable side effects or mistakenly believeother unexpected characteristics or otherwise expose participants to unacceptable health risks, causing us, our collaborators or our investigators, regulators or institutional review boards or the data safety monitoring board for such trial to halt, delay, interrupt, suspend or terminate the trials or cause us, or any collaborators, to abandon development or limit
development of that product candidate to certain uses or subpopulations in which the undesirable side effects or other characteristics are less prevalent, less severe or more acceptable from a risk-benefit perspective;
•if our product candidates
are toxic or not well tolerated when that is not in fact the case.Any inability to successfully complete preclinical and clinical developmenthave undesirable side effects, it could result in additional costs to us,a more restrictive label, or any future collaborators,it could result in the delay or denial of marketing approval by the FDA or comparable foreign regulatory authorities;
•clinical trials of our product candidates may produce negative or inconclusive results, and impair our ability to generate revenues from product sales, regulatory and commercialization milestones and royalties. Moreover, if we, or any futureour collaborators, are requiredmay decide, or regulators may require us, to conduct additional clinical trials, including testing in more subjects, or other testingabandon product development programs;
•regulators or institutional review boards may not authorize us, our collaborators or our investigators to commence a clinical trial or conduct a clinical trial at a prospective trial site;
•we or our collaborators may have delays in reaching or fail to reach agreement on acceptable clinical trial contracts or clinical trial protocols with prospective trial sites;
•the number of patients required for clinical trials of our product candidates beyondmay be larger than we anticipate;
•enrollment in these clinical trials, which may be particularly challenging for some of the diseases we target, may be slower than we anticipate;
•or participants may drop out of these clinical trials at a higher rate than we anticipate;
•third-party contractors used by us or our collaborators may fail to comply with regulatory requirements or meet their contractual obligations in a timely manner, or at all;
•significant clinical trial delays could shorten any periods during which we, or any collaborators, may have the exclusive right to commercialize our product candidates or allow our competitors, or the competitors of any collaborators, to bring products to market before we, or any collaborators, do;
•the cost of clinical trials of our product candidates may be greater than anticipated; and
•the supply or quality of our product candidates or other materials necessary to conduct clinical trials of our product candidates may be insufficient or inadequate.
In addition, we are conducting our SELECT-MDS-1 and testingSELECT-AML-1 clinical trials in foreign countries and may conduct other clinical trials outside the United States in the future. We do not have employees or significant operational capabilities located outside of the United States, and we rely on third parties, such as contract research organizations, or CROs, to conduct our clinical trials in foreign countries. Conducting clinical trials in foreign countries presents additional risks that may delay completion of our clinical trials. These risks include the failure of enrolled patients in foreign countries to adhere to clinical protocols as a result of differences in healthcare services or cultural customs, managing additional administrative burdens associated with foreign regulatory schemes, as well as political and economic risks relevant to such foreign countries.
In addition, conducting clinical trials in non-U.S. countries, as we may do for our product candidates, may present additional risks that may delay completion of our clinical trials. For example, on January 31, 2022, the new Clinical Trials Regulation (EU) No 536/2014 became applicable in the European Union, or EU, and replaced the prior Clinical Trials Directive 2001/20/EC. The new regulation aims at simplifying and streamlining the authorization, conduct and transparency of clinical trials in the EU. Under the new coordinated procedure for the approval of clinical trials, the sponsor of a clinical trial to be conducted in more than one Member State of the European Union, or EU Member State, will only be required to submit a single application for approval. The submission will be made through the Clinical Trials Information System, a new clinical trials portal overseen by the EMA and available to clinical trial sponsors, competent authorities of the EU Member States and the public. We have not previously secured authorization to conduct clinical studies in the EU pursuant to this new regulation and, accordingly, there is a risk that we or they contemplate, if we, or they, are unablemay be delayed in commencing such studies.
Our failure to successfully complete clinical trials of our product candidates or other testing, or the results of these trials or tests are unfavorable, uncertain or are only modestly favorable, or there are unacceptable safety concerns associated with our product candidates, we,tamibarotene or any future collaborators, may:
| ·
| | incur additional unplanned costs;
|
| ·
| | be delayed in obtaining marketing approval for our product candidates;
|
| ·
| | not obtain marketing approval at all;
|
| ·
| | obtain approval for indications or patient populations that are not as broad as intended or desired;
|
| ·
| | obtain approval with labeling that includes significant use or distribution restrictions or significant safety warnings, including boxed warnings;
|
| ·
| | be subject to additional post-marketing testing or other requirements; or
|
| ·
| | be required to remove the product from the market after obtaining marketing approval.
|
Our failure to successfully initiate and complete clinical trials of our product candidates, and to demonstrate the efficacy and safety necessary to obtain regulatory approval to market any of oursuch product candidates, could result in additional costs to us, or any collaborators, would impair our ability to generate revenue from product sales, regulatory and commercialization milestones and royalties and would significantly harm our business.
tamibarotene is novel and unproven, and we do not know whether we will be able to recognize commercial value from tamibarotene.
We are currently focused on the development of tamibarotene, which is being evaluated in genomically defined patients whose diseases have not been adequately addressed to date by existing approaches. While we believe that targeting the patient population that overexpresses RARA may potentially lead to a higher likelihood of clinical success, our approach is both novel and unproven, and our efforts may not result in the development of a commercially viable medicine. We may also be incorrect about the effects of tamibarotene on the diseases of patients that overexpress RARA, which may limit the utility of our approach or the perception of the utility of our approach. For example, we have not yet succeeded and may never succeed in demonstrating efficacy and safety for tamibarotene or any other product candidates in a pivotal clinical trial or in obtaining marketing approval thereafter. Furthermore, our estimates of patient populations with RARA overexpression available for study and treatment may be lower than expected, which could adversely affect our ability to conduct our clinical trials of tamibarotene and may also adversely affect the size of any market for tamibarotene that we may successfully commercialize.
Adverse events or undesirable side effects caused by, or other unexpected properties of, product candidates that we develop may be identified during development and could delay or prevent their marketing approval or limit their use.
Adverse events or undesirable sideSide effects caused by, or other unexpected properties of, SY-1425, SY-1365 or any futurefrom product candidates that we may develop could cause us, any future collaborators, an institutional review board or regulatory authorities to interrupt, delay or haltundergoing clinical trials of one or more of our product candidates and could result in a more restrictive label or the delay or denial of marketing approval by the FDA or comparable foreign regulatory authorities. Because gene control techniques are relatively new, side effects from gene control approachesevaluation may be unpredictable. Tamibarotene the active ingredient in SY-1425, has been observed to be associated with adverse events, such as mild or moderate dry skin, skin rash, headache and bone pain, as well as retinoic acid syndrome and elevated levels of cholesterol, lipids, liver function enzymes and white blood cells, which were severe in certain cases. Furthermore, retinoids such as SY-1425tamibarotene may cause birth defects and therefore may carry a warning on their label. Other examples of retinoids, a class of chemical compounds that are related to vitamin A, include all trans retinoic acid (also known as ATRA), Retin-A, retinol (found in over-the-counter skin creams), isotretinoin and bexarotene. In addition, our experience administering SY-1365 to humans has been limited to date, so the safety profile that SY-1365 will demonstrate in human clinical trials is unknown. We are and expect to continue evaluating the administration of tamibarotene in combination with other biological and pharmaceutical products, including azacitidine and daratumumab, in patients with AML, MDS and other hematologic malignancies, and we plan to evaluate SY-1365 in combination with a chemotherapeutic agent in patients with ovarian cancer.
We cannot predict at this time whether the combination of ourtamibarotene or any future product candidates with another product, or with any premedication administered to mitigate potential side effects, will be well tolerated by patients in clinical studies or that any unexpected adverse events or undesirable side effects will not occur. If any of our product candidates is associated with adverse events or undesirable side effects or has properties that are unexpected, we, or any future collaborators, may need to abandon development or limit development of that product candidate to certain uses or subpopulations in which the undesirable side effects or other characteristics are less prevalent, less severe or more acceptable from a risk-benefit perspective. Many compounds
Failure to successfully develop, validate, obtain regulatory approval for, and commercialize companion diagnostics could harm our commercialization strategy.
If we are to succeed in obtaining regulatory approval for a companion diagnostic to identify genomically defined subsets of patients with AML or MDS using our RARA biomarker, we will need to demonstrate to regulatory authorities that initially showed promiseRARA biomarker selection is associated with a response to tamibarotene. We do not develop companion diagnostics internally and thus we will be dependent on the sustained cooperation and effort of third-party collaborators in clinicaldeveloping, obtaining approval for, and commercializing a companion diagnostic to identify patients with RARA overexpression. In March 2022, we entered into a Master Collaboration Agreement and associated project work plan with Qiagen, pursuant to which Qiagen has agreed to develop and commercialize a companion diagnostic for this biomarker. Any delay or earlier stage testing have later been found to cause undesirable or unexpected side effects that prevented further development of the compound.
If we,failure by us, Qiagen, or any future collaborators experience any of a number of possible unforeseen events in connection with clinical trials of our current product candidate or any future product candidates that we, or any future collaborators, mayto develop, potential clinical development, marketing approval or commercialization of our product candidates could be delayed or prevented.
We, or any future collaborators, may experience numerous unforeseen events during, or as a result of, clinical trials that could delay or prevent clinical development, marketing approval or commercialization of our current product candidate or any future product candidates that we, or any future collaborators, may develop, including:
| ·
| | regulators or institutional review boards may not authorize us, any future collaborators or our or their investigators to commence a clinical trial or conduct a clinical trial at a prospective trial site;
|
| ·
| | we, or any future collaborators, may have delays in reaching or fail to reach agreement on acceptable clinical trial contracts or clinical trial protocols with prospective trial sites;
|
| ·
| | clinical trials of our product candidates may produce unfavorable or inconclusive results;
|
| ·
| | we, or any future collaborators, may decide, or regulators may require us or them, to conduct additional clinical trials or abandon product development programs;
|
| ·
| | the number of patients required for clinical trials of our product candidates may be larger than we, or any future collaborators, anticipate, patient enrollment in these clinical trials may be slower than we, or any future collaborators, anticipate or participants may drop out of these clinical trials at a higher rate than we, or any future collaborators, anticipate;
|
| ·
| | our estimates of the genomically defined patient populations available for study may be higher than actual patient numbers and result in our inability to sufficiently enroll our trials;
|
| ·
| | the cost of planned clinical trials of our product candidates may be greater than we anticipate;
|
| ·
| | our third-party contractors or those of any future collaborators, including those manufacturing our product candidates or components or ingredients thereof or conducting clinical trials on our behalf or on behalf of any future collaborators, may fail to comply with regulatory requirements or meet their contractual obligations to us or any future collaborators in a timely manner or at all;
|
| ·
| | patients that enroll in a clinical trial may misrepresent their eligibility to do so or may otherwise not comply with the clinical trial protocol, resulting in the need to drop the patients from the clinical trial, increase the needed enrollment size for the clinical trial or extend the clinical trial's duration;
|
| ·
| | we, or any future collaborators, may have to delay, suspend or terminate clinical trials of our product candidates for various reasons, including a finding that the participants are being exposed to unacceptable health risks, undesirable side effects or other unexpected characteristics of the product candidate;
|
| ·
| | regulators or institutional review boards may require that we, or any future collaborators, or our or their investigators suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements or their standards of conduct, a finding that the participants are being exposed to unacceptable health risks, undesirable side effects or other unexpected characteristics of the product candidate or findings of undesirable effects caused by a chemically or mechanistically similar product or product candidate;
|
| ·
| | the FDA or comparable foreign regulatory authorities may disagree with our, or any future collaborators', clinical trial designs or our or their interpretation of data from preclinical studies and clinical trials;
|
| ·
| | the FDA or comparable foreign regulatory authorities may fail to approve or subsequently find fault with the manufacturing processes or facilities of third-party manufacturers with which we, or any future collaborators, enter into agreements for clinical and commercial supplies;
|
| ·
| | the supply or quality of raw materials or manufactured product candidates or other materials necessary to conduct clinical trials of our product candidates may be insufficient, inadequate or not available at an acceptable cost, or we may experience interruptions in supply; and
|
| ·
| | the approval policies or regulations of the FDA or comparable foreign regulatory authorities may significantly change in a manner rendering our clinical data insufficient to obtain marketing approval.
|
Product development costs for us, or any future collaborators, will increase if we, or they, experience delays in testing or pursuing marketing approvals and we, or they, may be required to obtain additional funds to complete clinical trials and prepare for possible commercialization of our product candidates. We do not know whether any preclinical tests or clinical trials will begin as planned, will need to be restructured, or will be completed on schedule or at all. Significant preclinical study or clinical trial delays also could shorten any periods during which we, or any future collaborators, may have the exclusive right to commercialize our product candidates or allow our competitors, or the competitors of any future collaborators, to bring products to market before we, or any future collaborators, do and impair our ability, or the ability of any future collaborators, to successfully commercialize our product candidates and may harm our business and results of operations. In addition, many of the factors that lead to clinical trial delays may ultimately lead to the denial of marketing approval of any of our product candidates.
Failure to successfully validate, develop and obtain regulatory approval for, or commercialize companion diagnostics could harm our drug development strategy.strategy by delaying or preventing approval of tamibarotene or any future product candidates, delaying the commercialization of such product candidates, or diminishing the likelihood of achieving the commercial potential for such product candidates.
As oneWe and our collaborators may encounter difficulties in developing and validating companion diagnostics, including issues relating to selectivity/specificity, analytical validation, reproducibility, or clinical validation. In addition, Qiagen or any future collaborators may encounter production difficulties that could constrain the supply of the key elements of our development strategy,companion diagnostics. In addition, Qiagen or any future collaborators may decide to discontinue selling or manufacturing the companion diagnostic
that we seek to identify genomically defined subsets of patients within a disease category who may derive benefit from the product candidates we are developing. In collaboration with partners, we plan to develop companion diagnostics to help us to more accurately identify patients within a particular subset, both during our clinical trials andanticipate using in connection with tamibarotene or any future product candidates due to lack of commercial viability, or our relationship with such collaborator may otherwise terminate. We may not be able to enter into arrangements with another provider to obtain supplies of an alternative diagnostic test for use in connection with such product candidates or do so on commercially reasonable terms. Any of these challenges could adversely affect and/or delay the development and commercialization of ourthe companion diagnostic and tamibarotene or any future product candidates that we are developing or may in the future develop. Companioncandidate.
In addition, companion diagnostics are subject to regulation by the FDA and comparable foreign regulatory authorities as medical devices and require separate regulatory approval prior to commercialization. We do not developWhile tamibarotene and any future drug candidates will have their marketing applications reviewed by FDA’s Center for Drug Evaluation and Research, companion diagnostics internallyrequire separate marketing applications under the primary jurisdiction of FDA’s Center for Devices and thus weRadiological Health. This parallel jurisdiction and separate marketing applications could result in coordination issues, require additional time and effort, or result in delays or failure to obtain marketing approval for either the companion diagnostic or related drug indications. Foreign regulatory authorities may also require clinical trials to demonstrate the safety and efficacy of companion diagnostics, which would require separate regulatory clearance or approval prior to commercialization in those countries.
The companion diagnostic approval process can impact the therapeutic product approval process in several ways. According to FDA guidance, if the FDA determines that a companion diagnostic device is essential to ensuring the safe and effective use of a novel therapeutic product or new indication, the FDA generally will not approve the therapeutic product or new therapeutic product indication if the companion diagnostic is not also approved or cleared for that indication. Additional regulations apply to companion diagnostics that are used to make critical treatment decisions. For example, the FDA has stated that a companion diagnostic used to determine patient selection will be dependent onconsidered a significant risk device requiring an investigational device exemption. If any companion diagnostic that we develop, whether alone or with a collaborator such as Qiagen, does not comply with these requirements, we would not be able to proceed with our planned trial of the sustained cooperationapplicable product candidate in these patient populations. Further, under the Federal Food, Drug, and effortCosmetic Act, or FDCA, the FDA has generally required companion diagnostics intended to select the patients who will respond to cancer treatment to obtain premarket approval, or a PMA. Consequently, certain of our third-party collaborators in developing and obtaining approval for these companion
diagnostics. We and our collaborators may encounter difficulties in developing and obtaining approval for the companion diagnostics including issues relating to selectivity/specificity, analytical validation, reproducibility,may require us or clinical validation. Any delay or failure by our collaborators to developobtain a PMA. The PMA process, including the gathering of clinical and preclinical data and the submission to and review by the FDA, involves a rigorous premarket review during which the sponsor must prepare and provide the FDA with reasonable assurance of the device’s safety and effectiveness and information about the device and its components regarding, among other things, device design, manufacturing and labeling. PMA is not guaranteed and may take considerable time, and the FDA may ultimately respond to a PMA submission with a not approvable determination based on deficiencies in the application and require additional clinical trial or other data that may be expensive and time-consuming to generate and that can substantially delay approval. As a result, if we or our collaborators are required by the FDA to obtain approval of a companion diagnostic for a candidate therapeutic product, and we or our collaborators do not obtain, or there are delays in obtaining, FDA approval of a diagnostic device, we may not be able to effectively commercialize the product candidate and our ability to generate revenue will be materially impaired.
We may also face challenges related to the commercialization of companion diagnostics. Any delays or failures related to the development, validation or regulatory approval of the companion diagnosticsprocesses described above could delay the commercialization of tamibarotene or prevent approval of ourany future product candidates.candidate. In addition, ourwhile we believe that the adoption of screening and treatment into clinical practice guidelines is important for payer access, reimbursement, utilization in medical practice and commercial success, we and Qiagen or any future collaborators may encounter production difficulties that could constrain the supply of the companion diagnostics, and both they and we may have difficulties gaining acceptance of the use of the companion diagnosticsdiagnostic in the clinical community. If such companion diagnostics fail to gain market acceptance, it would have an adverse effect on our ability to derive revenuesrevenue from sales, if any, of our products. In addition, the diagnostic company with whom we contract may decide to discontinue sellingtamibarotene or manufacturing the companion diagnostic that we anticipate using in connection with development and commercialization of ourany future product candidates orthat are approved for commercial sale. As a result, our relationship with such diagnostic company may otherwise terminate. We may notbusiness, results of operations and financial condition could be able to enter into arrangements with another diagnostic company to obtain supplies of an alternative diagnostic test for use in connection with the development and commercialization of our product candidates or do so on commercially reasonable terms, which could adversely affect and/or delay the development or commercialization of our product candidates.materially harmed.
If we, or any future collaborators, experience delays or difficulties in the enrollment of patients in clinical trials, our or their receipt of necessary regulatory approvals could be delayed or prevented.
We, or any future collaborators, may not be able to initiate or continue clinical trials for our current product candidatestamibarotene or any future product candidates that we, or any future collaborators, may develop if we, or they, are unable to locate and enroll a sufficient number of eligible patients to participate in clinical trials. Patient enrollment is a significant factor in the timing of clinical trials, and is affected by many factors, including:including the size and nature of the patient population, the severity of the disease under investigation, and the availability of approved or investigational therapeutics for the relevant disease, the proximity of patients to clinical sites, the eligibility criteria for and design of the trial, efforts to facilitate timely enrollment, competing clinical trials, clinicians’ and patients’ perceptions as to the potential advantages and risks of the drug being studied in
42
| ·
| | the size and nature of the patient population;
|
| ·
| | the severity of the disease under investigation;
|
| ·
| | the availability of approved therapeutics for the relevant disease;
|
| ·
| | the proximity of patients to clinical sites;
|
| ·
| | the eligibility criteria for the trial;
|
| ·
| | the design of the clinical trial;
|
| ·
| | efforts to facilitate timely enrollment;
|
| ·
| | competing clinical trials; and
|
| ·
| | clinicians' and patients' perceptions as to the potential advantages and risks of the drug being studied in relation to other available therapies, including any new drugs that may be approved for the indications we are investigating.
|
relation to other available therapies, and actual or threatened public health emergencies and outbreaks of disease (including, for example, the COVID-19 pandemic). In addition, patients that enroll may subsequently be dropped from the clinical trial due to having misrepresented their eligibility to participate or due to non-compliance with clinical trial protocol, resulting in the need to increase the enrollment size for the clinical trial or extend the clinical trial’s duration.
In particular, we intend to enrich our clinical trials of tamibarotene are enrolling patients with RARA overexpression, which patients we believe are most likely to respond to our gene control therapies. Genomically defined diseasestamibarotene. Our estimates as to the prevalence of RARA overexpression in the patient populations we are targeting may however, haveprove to be incorrect, and the relatively low prevalence andof RARA overexpression may make it may be difficult for us or third parties with whom we collaborate to identify patients for our trials, which may lead to delays in enrollment for our trials. We intend to develop, or engage third parties to develop, companion diagnostics for use in our clinical trials, but we or such third parties may not be successful in developing such companion diagnostics, furthering the difficulty in identifying genomically defined subsets of patients for our clinical trials. Moreover, in light of the recent approval of new products for the treatment of AML, there is substantial competition for patients to be enrolled in clinical trials for this disease. Our inability, or the inability of any future collaborators, to enroll a sufficient number of patients for our, or their, clinical trials could result in significant delays or may require us or them to abandon one or more clinical trials altogether. Enrollment delays in our, or their, clinical trials may result in increased development costs for ourtamibarotene or any future product candidates, delay or halt the development of and approval processes for oursuch product candidates and jeopardize our, or any future collaborators’, ability to commence sales of and generate revenues from oursuch product candidates, which could cause the value of our company to decline and limit our ability to obtain additional financing, if needed.
Results of preclinical studies and early clinical trials may not be predictive of results of future or late-stage clinical trials.
The data supporting our clinical development strategies for SY-1425 and SY-1365 have been derived entirely from preclinical studies. We cannot assure you that we will be able to replicate in human clinical trials the results we observed in animal models.earlier studies. Moreover, the outcome of preclinical studies and early clinical trials may not be predictive of the success of later or late-stage clinical trials, and interim results of clinical trials do not necessarily predict success in future clinical trials. Many companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in late-stage clinical trials after achieving positive results in earlier development, and we could face similar setbacks. The design of a clinical trial can determine whether its results will support approval of a product and flawssetbacks in the design of a clinical trial may not become apparent until the clinical trial is well advanced. We have limited experience in designingour clinical trials and may be unable to design and execute a clinical trial to support marketing approval. In addition, preclinical and clinical data are often susceptible to varying interpretations and analyses. Many companies that believed their product candidates performed satisfactorilyinvolving tamibarotene or in preclinical studies andany future clinical trials have nonetheless failed to obtain marketing approval for theinvolving other product candidates. Even if we, or any future collaborators, believe that the results of clinical trials for our product candidates warrant marketing approval, the FDA or comparable foreign regulatory authorities may disagree and may not grant marketing approval of our product candidates.
In some instances, there can be significant variability in safety or efficacy results between different clinical trials of the same product candidate due to numerous factors, including changes in trial procedures set forth in protocols, differences in the size and type of the patient populations, changes in and adherence to the dosing regimen and other clinical trial protocols and the rate of dropout among clinical trial participants. If we fail to receive positive results in clinical trials of our producttamibarotene or any future candidates, the development timeline and regulatory approval and commercialization prospects for our most advancedsuch product candidates, and, correspondingly, our business and financial prospects would be negatively impacted.
We have never obtained marketing approval for a product candidate and we may be unable to obtain, or may be delayed in obtaining, marketing approval for our current product candidatestamibarotene or any future product candidates that we, or any future collaborators, may develop.
We have never obtained marketing approval for a product candidate. It is possible that the FDA may refuse to accept for substantive review any new drug applications, or NDAs, that we submit for ourtamibarotene or any future product candidates, or may conclude after review of our data that our application is insufficient to obtain marketing approval of oursuch product candidates. If the FDA does not accept or approve our NDAs for tamibarotene or any of ourfuture product candidates, it may require that we conduct additional clinical trials, preclinical studies or manufacturing validation studies and submit that data before it will reconsider our applications. Depending on the extent of these or any other FDA-required trials or studies, approval of any NDA or application that we submit may be delayed by several years, or may require us to expend more resources than we have available. It is also possible that additional trials or studies, if performed and completed, may not be considered sufficient by the FDA to approve our NDAs. In addition, our development programs contemplateprogram for tamibarotene contemplates the development of a companion diagnosticsdiagnostic by Qiagen, our third-party collaborators.collaborator. Companion diagnostics are subject to regulation as medical devices and must themselves be approved for marketing by the FDA or certain other foreign regulatory agencies before we may commercialize our product candidates.
Any delay in obtaining, or an inability to obtain, marketing approvals would prevent us from commercializing ourtamibarotene, any future product candidates, or any companion diagnostics, and from generating revenues and achieving and sustaining profitability. If any of these outcomes occur, we may be forced to abandon our development efforts for oursuch product candidates, which could significantly harm our business.
Even if any product candidates that we, or any future collaborators, may develop receive marketing approval, we or others may later discover that the product is less effective than previously believed or causes undesirable side effects that were not previously identified, which could compromise our ability, or that of any future collaborators, to market the product.
Clinical trials of SY-1425, SY-1365tamibarotene or any future product candidates that we, or any future collaborators, may develop will be conducted in carefully defined subsets of patients who have agreed to enter into clinical trials. Consequently, it is possible that our clinical trials, or those of any future collaborator,collaborators, may indicate an apparent positive effect of a product candidate that is greater than the actual positive effect, if any, or alternatively fail to identify
undesirable side effects. If, following approval of a product candidate, we, or others, discover that the product is less effective than previously believed or causes undesirable side effects that were not previously identified, we could be subject to the withdrawal of prior regulatory approvals and/or the imposition of additional regulatory requirements, restrictions on manufacturing, labelling and marketing, and product recalls. In addition, we our any collaborators could be sued and held liable for harm caused to patients and could become subject to fines, injunctions or the imposition of the following adverse events could occur:
| ·
| | regulatory authorities may withdraw their approval of the product or seize the product;
|
| ·
| | we, or any future collaborators, may be required to recall the product, change the way the product is administered or conduct additional clinical trials;
|
| ·
| | additional restrictions may be imposed on the marketing of, or the manufacturing processes for, the particular product;
|
| ·
| | we may be subject to fines, injunctions or the imposition of civil or criminal penalties;
|
| ·
| | regulatory authorities may require the addition of labeling statements, such as a "black box" warning or a contraindication;
|
| ·
| | we, or any future collaborators, may be required to create a Medication Guide outlining the risks of the previously unidentified side effects for distribution to patients;
|
| ·
| | we, or any future collaborators, could be sued and held liable for harm caused to patients;
|
| ·
| | the product may become less competitive; and
|
| ·
| | our reputation may suffer.
|
civil or criminal penalties. Any of these events could harm our reputation, business and operations and could negatively impact our stock price.
Even if our current product candidates,tamibarotene or any future product candidate that we, or any future collaborators, may develop receives marketing approval, it may fail to achieve the degree of market acceptance by physicians, patients, third-party payors and others in the medical community necessary for commercial success, in which case we may not generate significant revenues or become profitable.
We have never commercialized a product, and even if one of ourtamibarotene or any future product candidatescandidate is approved by the appropriate regulatory authorities for marketing and sale, it may nonetheless fail to gain sufficient market acceptance by physicians, patients, third-party payors and others in the medical community. Physicians are often reluctant to switch their patients from existing therapies even when new and potentially more effective or convenient treatments enter the market. Further, patients often acclimate to the therapy that they are currently taking and do not want to switch unless their physicians recommend switching products or they are required to switch therapies due to lack of reimbursement for existing therapies.
Efforts to educate the medical community and third-party payors on the benefits of ourtamibarotene and any future product candidates may require significant resources and may not be successful. If any of oursuch product candidatescandidate is approved but does not achieve an adequate level of market acceptance, we may not generate significant revenues and we may not become profitable. The degree of market acceptance of ourany product candidates, if approved for commercial sale, will depend on a number of factors, including:
| ·
| | including the efficacy and safety of the product; |
| ·
| | the potential advantages of the product compared to competitive therapies;
|
| ·
| | the prevalence and severity of any side effects;
|
| ·
| | whether the product is designated under physician treatment guidelines as a first-, second- or third-line therapy;
|
Table of Contentsthe product, the potential advantages of the product compared to competitive therapies, the prevalence and severity of any side effects, whether the product is designated under physician treatment guidelines as a first-, second- or third-line therapy, our ability, or the ability of any collaborators, to offer the product for sale at competitive prices, the product's convenience and ease of administration compared to alternative treatments, the willingness of the target patient population to try, and of physicians to prescribe, the product, limitations or warnings, including distribution or use restrictions, contained in the product's approved labeling, the strength of sales, marketing and distribution support, changes in the standard of care for the targeted indications for the product; and the availability and amount of coverage and reimbursement from government payors, managed care plans and other third-party payors.
| ·
| | our ability, or the ability of any future collaborators, to offer the product for sale at competitive prices;
|
| ·
| | the product's convenience and ease of administration compared to alternative treatments;
|
| ·
| | the willingness of the target patient population to try, and of physicians to prescribe, the product;
|
| ·
| | limitations or warnings, including distribution or use restrictions, contained in the product's approved labeling;
|
| ·
| | the strength of sales, marketing and distribution support;
|
| ·
| | changes in the standard of care for the targeted indications for the product; and
|
| ·
| | availability and amount of coverage and reimbursement from government payors, managed care plans and other third-party payors.
|
We may expend our limited resources to pursue a particular product candidate or indication and fail to capitalize on product candidates or indications that may be more profitable or for which there is a greater likelihood of success.
Because we have limited financial and managerial resources, we intend to focus on developing product candidates for specific indications that we identify as most likely to succeed, in terms of both their potential for marketing approval and commercialization. As a result, we may forego or delay pursuit of opportunities with other product candidates or for other indications that may prove to have greater commercial potential. In this regard, we announced in November 2022 that we have elected to seek a partnership for the further development of SY-5609. Further, in October 2023, we announced a strategic realignment to prioritize key development and pre-launch activities to advance tamibarotene for the treatment of newly diagnosed HR-MDS and newly diagnosed AML, and to stop further investment in the clinical development of SY-2101.
Our resource allocation decisionsdecision to cease development of SY-2101 and SY-5609 and to allocate our resources towards the development of tamibarotene may cause us to fail to capitalize on viable commercial products or profitable market opportunities. Our spending on current andtamibarotene or any future research and development programs and product candidates for specific indications may not yield any commercially viable product candidates. If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through collaboration, licensing or
other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to the product candidate.
If we are unable to establish sales marketing and distribution capabilities or enter into sales marketing and distribution arrangements with third parties, we may not be successful in commercializing any product candidates if approved.
We are planning our distribution, dispensing, commercial operations, and sales infrastructure strategy for a commercial launch of tamibarotene in the United States, subject to receiving marketing approval. We intend to build a focused and specialized sales and marketing organization in the United States to sell tamibarotene. We are building a marketing and commercial organization to create and implement marketing strategies for the anticipated commercial launch of tamibarotene and to oversee and support our sales force. We do not have a sales marketing or distribution infrastructure and have no experience in the sale marketing or distribution of pharmaceutical products. To achieve commercial success for any approved product, we must either develop a sales and marketing organization or outsource these functions to third parties. We plan to build focused and specialized capabilities to commercialize development programstamibarotene for certainthe HR-MDS and AML indications, where we believe that the medical specialists for the indications are sufficiently concentrated to allow us to effectively promote the product with a targeted sales team. The development of sales, marketing and distribution capabilities will requirerequires substantial resources, will beis time consuming and could delay any product launch. If the commercial launch of atamibarotene or any future product candidate for which we recruit a sales force and establish marketing and distribution capabilities is delayed or does not occur for any reason, we could have prematurely or unnecessarily incurred these commercialization costs. This may be costly, and our investment could be lost if we cannot retain or reposition our sales and marketing personnel. In addition, we may not be able to hire or retain a sales force in the United States that is sufficient in size or has adequate expertise in the medical markets that we plan to target. If we are unable to establish or retain a sales force and marketing and distribution capabilities, our operating results may be adversely affected. If a potential partner has development or commercialization expertise that we believe is particularly relevant to one of our products, then we may seek to collaborate with that potential partner even if we believe we could otherwise develop and commercialize the product independently.
In certain indications, we plan to seek to enter into collaborations that we believe may contribute to our ability to advance development and ultimately commercialize our product candidates. We also intend to seek to enter into collaborations where we believe that realizing the full commercial value of our development programs will require access to broader geographic markets or the pursuit of broader patient populations or indications. To that end, we intend to seek a partner to commercialize tamibarotene in the territories outside the United States. As a result of entering into arrangementsany such arrangement with a third partiesparty to perform sales, marketing and distribution services, our product revenues or the
profitability of these product revenues may be lower, perhaps substantially lower, than if we were to directly market and sell products in those markets. Furthermore, we may be unsuccessful in entering into the necessary arrangements with third parties or may be unable to do so on terms that are favorable to us. In addition, we may have little or no control over such third parties, and any of them may fail to devote the necessary resources and attention to sell and market our products effectively.
If we do not establish sales, marketing and distribution capabilities, either on our own or in collaboration with third parties, we will not be successful in commercializing tamibarotene or any of ourfuture product candidates that may receive marketing approval.
We face substantial competition, which may result in others discovering, developing or commercializing products before or more successfully than we do.
The development and commercialization of new products is highly competitive. We expect that we, and any future collaborators, will face significant competition from major pharmaceutical companies, specialty pharmaceutical companies and biotechnology companies worldwide with respect to any of our product candidates that we, or any future collaborators, may seek to develop or commercialize in the future. Specifically, there are a number of large pharmaceutical and biotechnology companies that currently market and sell products or are pursuing the development of product candidates for the treatment of the key indications oftargeted in our most advanced programs. clinical trials involving tamibarotene.
For example, we are aware of fourseveral new drugs approved by the FDA during 2017since 2018 for the treatment of newly diagnosed unfit AML or patient subsets within AML: midostaurin, enasidanib, daunorubicin + cytarabine liposome,newly diagnosed unfit AML (including ivosidenib, venetoclax, and gemtuzumab. SY-1425glasdegib), and one new drug approved by the FDA in 2020 for the treatment of MDS or patient subsets within MDS (decitabine/cedazuridine). Tamibarotene may also face competition from other investigational productsagents currently in clinical development for AML and MDS, including venetoclax, which is currently being evaluated bythose in late-stage development from AbbVie Inc. in two randomized pivotal studies in patients with AML, as well as investigational products in development from Takeda Pharmaceutical Co. Ltd., Daiichi Sankyo Company, Limited, Agios Pharmaceuticals,Roche Holding AG, Taiho Oncology, Inc., Novartis AG, Bristol-Myers Squibb Co., Eli Lilly & Co., Eisai Inc., Celgene Corporation,and Pfizer Inc., Incyte Corporation and FORMA Therapeutics, LLC. We are aware of only one other selective RARα program, a compound in development from Io Therapeutics, Inc. which, according to a government-sponsored website, is in an investigator initiated Phase 1/2 study in a non-selective patient group in relapsed and refractory AML, high-risk MDS and chronic myelomonocytic leukemia. In addition, we are aware of an oral CDK7 inhibitor being developed by Carrick Therapeutics Ltd. that has recently entered Phase 1 clinical development, and are aware of several other selective CDK7 inhibitor programs that we believe are in preclinical development, including programs from Aurigene Discovery Technologies Ltd., Ube Industries Ltd., Qurient Co. Ltd., and Beta Pharma, Inc. SY-1365 may face competition from these selective CDK7 inhibitors.
Our competitors may succeed in developing, acquiring or licensing technologies and products that are more effective, have fewer side effects or more tolerable side effects, have greater ease of access, or are less costly than any
product candidates that we are currently developing or that we may develop, which could render our product candidates obsolete and noncompetitive.
Our commercial opportunity could be reduced or eliminated if our competitors develop For example, the evolving standard of care for the treatment of patients with AML and commercialize products that are safer orthe response rates and duration of response seen with approved and investigational agents in this disease may result in a longer and more effective, have fewer or less severe side effects, are more convenient or are less expensive than any products that we, or any future collaborators, may develop.complex clinical development path for tamibarotene, which in turn will impact the potential return on investments in clinical trials of tamibarotene. Our competitors also may obtain FDA or other marketing approval for their products before we, or any future collaborators, are able to obtain approval for ours, which could result in our competitors establishing a strong market position before we, or any future collaborators, are able to enter the market.
Many of our existing and potential future competitors have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining marketing approvals and marketing approved products than we do. Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller or early stageearly-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, the development of our product candidates.
Even if we, or any future collaborators, are able to commercialize any product candidate that we, or they, develop, the product may become subject to unfavorable pricing regulations, third-party payor reimbursement practices or healthcare reform initiatives, any of which could harm our business.
The commercial success of ourtamibarotene or any future product candidates will depend substantially, both domestically and abroad, on the extent to which the costs of oursuch product candidates will be paid by third-party payors, including government health care programs and private health insurers. If coverage is not available, or reimbursement is limited, we, or any future collaborators, may not be able to successfully commercialize oursuch product candidates. Even if coverage is provided, the approved reimbursement amount may not be high enough to allow us, or any future collaborators, to establish or maintain pricing sufficient to realize a sufficient return on our or their investments. In the United States, no uniform policy of coverage and reimbursement for products exists among third-party payors and coverage and reimbursement levels for products can differ significantly from payor to payor. As a result, the coverage determination process is often a time consuming and costly process that may require us to provide scientific and clinical support for the use of our products to each payor separately, with no assurance that coverage and adequate reimbursement will be applied consistently or obtained in the first instance.
There is significant uncertainty related to third-party payor coverage and reimbursement of newly approved drugs. Marketing approvals, pricing and reimbursement for new drug products vary widely from country to country. Some countries require approval of the sale price of a drug before it can be marketed. In many countries, the pricing review period begins after marketing or product licensing approval is granted. In some foreign markets, prescription pharmaceutical pricing remains subject to continuing governmental control even after initial approval is granted. As a result, we, or any future collaborators, might obtain marketing approval for a product in a particular country, but then be subject to price regulations that delay commercial launch of the product, possibly for lengthy time periods, which may negatively impact the revenues we are able to generate from the sale of the product in that country. Adverse pricing limitations may hinder our ability or the ability of any future collaborators to recoup our or their investment in onetamibarotene or moreany future product candidates, even if oursuch product candidates obtain marketing approval.
Patients who are provided medical treatment for their conditions generally rely on third-party payors to reimburse all or part of the costs associated with their treatment. Therefore, our ability, and the ability of any future collaborators, to successfully commercialize successfullytamibarotene or any of ourfuture product candidates will depend in part on the extent to which coverage and adequate reimbursement for these products and related treatments will be available from third-party payors. Third-party payors decide which medications they will cover and establish reimbursement levels. The healthcare industry is acutely focused on cost containment, both in the United States and elsewhere. Government authorities and other third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications, which could affect our ability or that of any future collaborators to sell our product candidates profitably. These payors may not view our products, if any, as cost-effective, and coverage and reimbursement may not be available to our customers, or those of any future collaborators, or may not be sufficient to allow our products, if any, to be marketed on a competitive basis. Cost-control initiatives could cause us, or any future collaborators, to decrease the price we, or they, might establish for products, which could result in lower than anticipated product revenues. If the prices for our products, if any, decrease or if governmental and other third-party payors do not provide coverage or adequate reimbursement, our prospects for revenue and profitability will suffer.
There may also be delays in obtaining coverage and reimbursement for newly approved drugs, and coverage may be more limited than the indications for which the drug is approved by the FDA or comparable foreign regulatory authorities. Moreover, eligibility for reimbursement does not imply that any drug will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture, sale and distribution. Reimbursement rates may vary, by way of example, according to the use of the product and the clinical setting in which it is used. Reimbursement rates may also be based on reimbursement levels already set for lower cost drugs or may be incorporated into existing payments for other services.
In addition, increasingly, third-party payors are requiring higher levels of evidence of the benefits and clinical outcomes of new technologies and are challenging the prices charged. Further, the net reimbursement for drug products may be subject to additional reductions if there are changes to laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the United States. An inability to promptly obtain coverage and adequate payment rates from both government-funded and private payors for tamibarotene or any of ourfuture product candidates for which we, or any future collaborator, obtain marketing approval could significantly harm our operating results, our ability to raise capital needed to commercialize products and our overall financial condition.
Product liability lawsuits against us could divert our resources, cause us to incur substantial liabilities and limit commercialization of any products that we may develop.
We will face an inherent risk of product liability claims as a result of the clinical testing of ourtamibarotene and any future product candidates, despite obtaining appropriate informed consents from our clinical trial participants. We will face an even greater risk if we or any future collaborators commercially sell any product that we may or they may develop. For example, we may be sued if any product we develop allegedly causes injury or is found to be otherwise unsuitable during clinical testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, negligence, strict liability or a breach of warranties. Claims could also be asserted under state consumer protection acts. If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit commercialization of ourtamibarotene or any future product candidates. Regardless of the merits or eventual outcome, liability claims may result in:in, among other consequences, decreased demand for such product candidates or products that we may develop, injury to our reputation and significant negative media attention, withdrawal of clinical trial participants, significant costs to defend resulting litigation, substantial monetary awards to trial participants or patients, loss of revenue, reduced resources of our management to pursue our business strategy, and the inability to commercialize any products that we may develop.
| ·
| | decreased demand for our product candidates or products that we may develop;
|
| ·
| | injury to our reputation and significant negative media attention;
|
| ·
| | withdrawal of clinical trial participants;
|
| ·
| | significant costs to defend resulting litigation;
|
| ·
| | substantial monetary awards to trial participants or patients;
|
| ·
| | reduced resources of our management to pursue our business strategy; and
|
| ·
| | the inability to commercialize any products that we may develop.
|
Although we maintain clinical trial liability insurance coverage in the amount of up to $5.0$10.0 million in the aggregate, this insurance may not fully cover potential liabilities that we may incur. The cost of any product liability litigation or other proceeding, even if resolved in our favor, could be substantial. We will need to increase our insurance coverage if and when we commercialize any product that receives marketing approval. In addition, insurance coverage is becoming increasingly expensive. If we are unable to obtain or maintain sufficient insurance coverage at an acceptable cost or to otherwise protect against potential product liability claims, it could prevent or inhibit the development and commercial production and sale of ourtamibarotene or any future product candidates, which could harm our business, financial condition, results of operations and prospects.
If the FDA or comparable foreign regulatory authorities approve generic versions of any of our products that receive marketing approval, or such authorities do not grant our products appropriate periods of data exclusivity before approving generic versions of our products, the sales of our products could be adversely affected.
Once an NDA is approved, the product covered thereby becomes a "reference-listed drug" in the FDA's publication, "Approved Drug Products with Therapeutic Equivalence Evaluations," or the Orange Book. Manufacturers may seek approval of generic versions of reference-listed drugs through submission of abbreviated new drug applications, or ANDAs, in the United States. In support of an ANDA, a generic manufacturer need not conduct clinical trials. Rather, the applicant generally must show that its product has the same active ingredient(s), dosage form, strength, route of administration and conditions of use or labeling as the reference-listed drug and that the generic version is bioequivalent to the reference-listed drug, meaning it is absorbed in the body at the same rate and to the same extent. Generic products may be significantly less costly to bring to market than the reference-listed drug and companies that produce generic products are generally able to offer them at lower prices. Thus, following the introduction of a generic drug, a significant percentage of the sales of any branded product or reference-listed drug may be typically lost to the generic product.
The FDA may not approve an ANDA for a generic product until any applicable period of non-patent exclusivity for the reference-listed drug has expired. The Federal Food, Drug, and Cosmetic Act, or FDCA provides a period of five years of non-patent exclusivity for a new drug containing a new chemical entity, or NCE. Specifically, in cases where such exclusivity has been granted, an ANDA may not be filed with the FDA until the expiration of five years unless the submission is accompanied by a Paragraph IV certification that a patent covering the reference-listed drug is either invalid or will not be infringed by the generic product, in which case the applicant may submit its application four years following approval of the reference-listed drug. Because the composition of matter patent for SY-1425tamibarotene has expired and our license rights to SY-1425tamibarotene from TMRC are limited to human cancer indications, it is possible that another applicant could obtain approval of tamibarotenefor a similar product from the FDA before us, in which case our NDA for tamibarotene would not be eligible for NCE exclusivity. See "—Risks Related to Our Intellectual Property—We do not have composition of matter patent protection with respect to SY-1425.tamibarotene or the active pharmaceutical ingredient of SY-2101." If any product we develop does not receive five years of NCE exclusivity, the FDA may approve generic versions of such product three years after its date of approval, subject to the requirement that the ANDA applicant certifies to any patents listed for our products in the Orange Book. Manufacturers may seek to launch these generic products following the expiration of the applicable marketing exclusivity period, even if we still have patent protection for our product.
Competition that our products may face from generic versions of our products could negatively impact our future revenue, profitability and cash flows and substantially limit our ability to obtain a return on our investments in those product candidates.
Our internal computer systems, or those used by our third-party research institution collaborators, vendors or other contractors or consultants, may fail or suffer security breaches.
Despite the implementation of security measures, our internal computer systems and those of our vendors and other contractors and consultants may be vulnerable to damage from computer viruses and unauthorized access. Although to our knowledge we have not experienced any such material system failure or security breach to date, if such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our development programs and our business operations. For example, the loss of clinical trial data from completed, ongoing or future clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. Likewise, we rely on our third-party research institution collaborators for research and development of our product candidates and other third parties for the manufacture of our product candidates and to conduct clinical trials, and similar events relating to their computer systems could also have a material adverse effect on our business. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or systems, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the further development and commercialization of our product candidates could be delayed.
Risks Related to Our Dependence on Third Parties
We expect to rely on third parties to conduct our clinical trials and certain aspects of our research, and preclinical testing, and those third parties may not perform satisfactorily, including by failing to meet deadlines for the completion of such trials research or testing.research.
We expect to rely on third parties, such as contract research organizations, clinical data management organizations, medical institutions and clinical investigators, to conduct our clinical trials. We currently rely and expect to continue to rely on third parties such as consultants, clinical investigators, CROs, clinical data management organizations, medical institutions and other similar entities, to conductcomplete certain aspects of our researchclinical trials and preclinical testing.provide services in connection with such clinical trials. Any of these third parties on which we currently rely or may in the future rely may terminate their engagements with us at any time. If we need to enter into alternative arrangements, it wouldcould delay our product development activities. We additionally rely on other third parties to store and distribute drug supplies for our clinical trials. Any performance failure on the part of our distributors could delay clinical development or marketing approval of tamibarotene or any future product candidates or commercialization of our medicines, producing additional losses and depriving us of potential product revenue.
Our reliance on these third parties for research and development activities will reduce our control over these activities, but will not relieve us of our responsibilities. For example, we will remain responsible for ensuring that each of our clinical trials is conducted in accordance with the general investigational plan and protocols for the trial. Moreover, the FDA requires us to comply with standards, commonly referred to as good clinical practices, for conducting, recording and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of trial participants are protected. We also are required to register ongoing clinical trials and post the results of completed clinical trials on a government-sponsored database, ClinicalTrials.gov, within certain timeframes. Failure to do so can result in fines, adverse publicity and civil and criminal sanctions.
Furthermore, these third parties may also have relationships with other entities, some of which may be our competitors. If these third parties do not successfully carry out their contractual duties, meet expected deadlines or conduct our clinical trials in accordance with regulatory requirements or our stated protocols, we will not be able to obtain, or may be delayed in obtaining, marketing approvals for ourtamibarotene or any future product candidates and will not be able to, or may be delayed in our efforts to, successfully commercialize our medicines.
We also expect to rely on other third parties to store and distribute drug supplies for our clinical trials. Any performance failure on the part of our distributors could delay clinical development or marketing approval of our product candidates or commercialization of our medicines, producing additional losses and depriving us of potential product revenue.
We currently depend on third-party manufacturers to produce our preclinical and clinical drug supplies and we intend to rely upon third-party manufacturers to produce commercial supplies of any approved product candidates.
We do not have any manufacturing facilities. We currently rely, and expect to continue to rely, on third-party manufacturers for the manufacture of ourtamibarotene and any future product candidates for preclinical and clinical testing and for commercial supply of any of thesesuch product candidates for which we or our collaborators obtain marketing approval. We have engaged, and expect to continue engaging, third-party suppliers and manufacturers in China and India. Natural disasters such as earthquakes, tsunamis, power shortages or outages, floods or monsoons, public health crises such as COVID-19 or other pandemics or epidemics, political crises such as terrorism, war, political insecurity or other conflict, or other events outside of our control could adversely affect the ability of these third parties to perform their obligations as expected.
We also do not currently have a long-term supply agreement with any third-party manufacturers. We may be unable to establish any agreements with third-party manufacturers or to do so on acceptable terms. Even if we are able to establish agreements with third-party manufacturers, we face risks such as the possible breach of the agreement by the third party or termination or nonrenewal of the agreement by the third party at a time that is costly or inconvenient to us. We also face risks associated with reliance on third-party manufacturers entails additional risks, including:third parties for regulatory compliance, quality assurance, and safety and pharmacovigilance reporting.
| ·
| | reliance on the third party for regulatory compliance and quality assurance;
|
| ·
| | the possible breach of the manufacturing agreement by the third party;
|
| ·
| | the possible termination or nonrenewal of the agreement by the third party at a time that is costly or inconvenient for us; and
|
| ·
| | reliance on the third party for regulatory compliance, quality assurance, and safety and pharmacovigilance reporting.
|
Third-party manufacturers may not be able to comply with current good manufacturing practices, or cGMP, regulations or similar regulatory requirements outside the United States. Our failure, or the failure of our third-party manufacturers, to comply with applicable regulations could result in sanctions being imposed on us, including fines, injunctions, civil penalties, delays, suspension or withdrawal of approvals, license revocation, seizures or recalls of product candidates or medicines, operating restrictions and criminal prosecutions, any of which could significantly and adversely affect supplies of our medicines and harm our business and results of operations.
Any medicines that we may develop may compete with other product candidates and products for access to manufacturing facilities. There are a limited number of manufacturers that operate under cGMP regulations and that might be capable of manufacturing for us.
Any performance failure on the part of our existing or future manufacturers could delay clinical development or marketing approval. We do not currently have arrangements in place for redundant supply forof bulk drug substances.substance or drug product. If any one of our current contract manufacturers cannot perform as agreed, we may be required to replace that manufacturer. Although we believe that there are several potential alternative manufacturers who could manufacture our product candidates, we may incur added costs and delays in identifying and qualifying any such replacement.
Our currentWe currently depend on a third-party manufacturer to develop and anticipated future dependence upon othersvalidate the clinical trial assay being used to select patients with our proprietary RARA biomarker, and if this assay does not perform as designed, our clinical trials of tamibarotene may be adversely affected.
We are currently conducting SELECT-MDS-1, a Phase 3 clinical trial evaluating tamibarotene in combination with azacitidine in HR-MDS patients who have been prospectively selected using our proprietary RARA biomarker, and SELECT-AML-1, a randomized Phase 2 clinical trial evaluating tamibarotene in combination with venetoclax and azacitidine in newly diagnosed patients with AML who are positive for RARA overexpression and are not suitable candidates for standard intensive chemotherapy. We collaborate with a third party with respect to the clinical trial assay being used to select patients with the RARA biomarker for inclusion in these trials. The FDA has approved an investigational device exemption for the manufactureassay being used to select patients with the RARA biomarker, and we used this assay in our earlier Phase 2 trial evaluating the safety and efficacy of tamibarotene in certain AML and MDS patient populations. Based on data from over 175 patients screened in our product candidates or medicines mayclinical trials, we believe approximately 50% of MDS patients and approximately 30% of AML patients are positive for RARA overexpression. Our ability to continue to prospectively select patients who overexpress RARA for SELECT-MDS-1 and SELECT-AML-1 depends on the ability of this clinical trial assay to identify suitable patients for these clinical trials. If this assay does not perform as designed, it could adversely affect our future profit marginsestimated timelines to enroll patients, or adversely impact the results of these trials, which could significantly harm our business and commercial prospects.
Failure of Qiagen to successfully develop or commercialize a companion diagnostic test for use with tamibarotene to identify patients with RARA overexpression could harm our ability to commercialize tamibarotene.
We do not plan to internally develop a commercial companion diagnostic test to identify patients with RARA overexpression and, as a result, we will be dependent on the efforts of Qiagen to successfully develop and commercialize this test.
Qiagen may not perform its obligations as expected or as required under our agreement with Qiagen, may encounter production difficulties that could constrain the supply of the companion diagnostic test, may have difficulties gaining acceptance of the use of the companion diagnostic test in the clinical community, may not pursue commercialization of the companion diagnostic test even if they receive any medicinesrequired regulatory approvals, may elect not to continue the development of the companion diagnostic test based on changes in its strategic focus or available funding, or external factors such as an acquisition, that receivedivert resources or create competing priorities, may not commit sufficient resources to the marketing approvaland distribution of the companion diagnostic test, and may terminate their relationship with us.
If the companion diagnostic test that is developed for use with tamibarotene fails to gain market acceptance, our ability to derive revenues from sales from tamibarotene would be harmed. If Qiagen or any other third parties we engage fail to commercialize the companion diagnostic test, we may not be able to enter into arrangements with another diagnostic company to obtain supplies of an alternative test for use with tamibarotene or do so on a timely and competitive basis.
commercially reasonable terms, which
tamibarotene.
IfTo the extent that we enter into collaborations with third parties for the development and commercialization of any product candidates, our prospects with respect to those product candidates will depend in significant part on the success of those collaborations.
We expect to enter into collaborations for the development and commercialization of one or more product candidates we may develop, or to use our gene control platform to identify and validate targets in diseases beyond our current areas of focus, as we have with Incyte indevelop. To the field of myeloproliferative neoplasms. If and whenextent we enter into such collaborations, we will have limited control over the amount and timing of resources that our collaborators will dedicate to the development or commercialization of our product candidates. Our ability to generate revenues from these arrangements will depend on any collaborators' abilities to successfully perform the functions assigned to them in these arrangements. In addition, collaborators may have the right to abandon research or development projects and terminate applicable agreements, including funding obligations, prior to or upon the expiration of the agreed upon terms.
Collaborations involving our product candidates pose a number of risks, including the following:
| ·
| | collaborators have significant discretion in determining the efforts and resources that they will apply to these collaborations;
|
•collaborators have significant discretion in determining the efforts and resources that they will apply to these collaborations;
| ·
| | collaborators may not perform their obligations as expected;
|
•collaborators may not perform their obligations as expected;
| ·
| | collaborators may not pursue development and commercialization of our product candidates or may elect not to continue or renew development or commercialization programs, based on clinical trial results, changes in the collaborators' strategic focus or available funding or external factors, such as an acquisition, that divert resources or create competing priorities;
|
•collaborators may not pursue development and commercialization of our product candidates or may elect not to continue or renew development or commercialization programs, based on clinical trial results, changes in the collaborators' strategic focus or available funding or external factors, such as an acquisition, that divert resources or create competing priorities;
| ·
| | collaborators may delay clinical trials, provide insufficient funding for a clinical trial program, stop a clinical trial or abandon a product candidate, repeat or conduct new clinical trials or require a new formulation of a product candidate for clinical testing;
|
•collaborators may delay clinical trials, provide insufficient funding for a clinical trial program, stop a clinical trial or abandon a product candidate, repeat or conduct new clinical trials or require a new formulation of a product candidate for clinical testing;
| ·
| | collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with our product candidates;
|
•collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with our product candidates;
| ·
| | a collaborator with marketing and distribution rights to one or more products may not commit sufficient resources to the marketing and distribution of such product or products;
|
•a collaborator with marketing and distribution rights to one or more products may not commit sufficient resources to the marketing and distribution of such product or products;
| ·
| | disagreements with collaborators, including disagreements over proprietary rights, contract interpretation or the preferred course of development, might cause delays or termination of the research, development or commercialization of product candidates, might lead to additional responsibilities for us with respect to product candidates, or might result in litigation or arbitration, any of which would be time consuming and expensive;
|
•disagreements with collaborators, including disagreements over proprietary rights, contract interpretation or the preferred course of development, might cause delays or termination of the research, development or commercialization of product candidates, might lead to additional responsibilities for us with respect to product candidates, or might result in litigation or arbitration, any of which would be time consuming and expensive;
| ·
| | collaborators may not properly maintain or defend our intellectual property rights or may use our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to potential litigation;
|
•collaborators may not properly maintain or defend our intellectual property rights or may use our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to potential litigation;
| ·
| | collaborators may infringe the intellectual property rights of third parties, which may expose us to litigation and potential liability; and
|
•collaborators may infringe the intellectual property rights of third parties, which may expose us to litigation and potential liability;
| • collaboration agreements may contain restrictions on our ability to enter into potential collaborations, to conduct research or development in certain fields, or to otherwise develop specified product candidates; •there have been a significant number of recent business combinations among large pharmaceutical companies that have resulted in a reduced number of potential collaborators; and •collaborations may be terminated and, if terminated, may result in a need for additional capital to pursue further development or commercialization of the applicable product candidates.
| ·
| | collaborations may be terminated and, if terminated, may result in a need for additional capital to pursue further development or commercialization of the applicable product candidates.
|
Collaboration agreements may not lead to development or commercialization of product candidates in the most efficient manner or at all. If any future collaborator of ours is involved in a business combination, it could decide to delay, diminish or terminate the development or commercialization of any product candidate licensed to it by us.
We are currently seeking, and we expect to continue to seek to establish additional collaborations and, if we are not able to establish them on commercially reasonable terms, we may have to alter our development and commercialization plans.
We expect to seek one or more additional collaborators for the development and commercialization of one or more of our product candidates or to validate targets. Likely collaborators may include large and mid-size pharmaceutical companies, regional and national pharmaceutical companies and biotechnology companies. In addition, ifIf we are able to obtain marketing approval for tamibarotene or any future product candidates from foreign regulatory authorities, we intend to enter into strategic relationships with international biotechnology or pharmaceutical companies for the commercialization of such product candidates outside of the United States. In addition, we may seek to establish one or more additional collaborators for the development and commercialization of any future product candidates. Likely collaborators may include large and mid-size pharmaceutical companies, regional and national pharmaceutical companies and biotechnology companies.
We face significant competition in seeking appropriate collaborators. Whether we reach a definitive agreement for a collaboration will depend, among other things, upon our assessment of the collaborator's resources and expertise, the terms and conditions of the proposed collaboration, and the proposed collaborator's evaluation of a number of factors. Those factors may include the potential differentiation of our product candidatecandidates from competing product candidates, design or results of clinical trials, the likelihood of approval by the FDA or comparable foreign regulatory authorities and the regulatory pathway for any such approval, the potential market for the product candidate, the costs and complexities of manufacturing and delivering the product to patients and the potential of competing products. The collaborator may also consider alternative product candidates or technologies for similar indications that may be available for collaboration and whether such a collaboration could be more attractive than the one with us for our product candidate. If we elect to increase our expenditures to fund development or commercialization activities on our own, we may need to obtain additional capital, which may not be available to us on acceptable terms or at all. If we do not have sufficient funds, we may not be able to further develop our product candidates or bring them to market and generate product revenue.
Collaborations are complex and time consuming to negotiate and document.
Our target discovery collaboration with Incyte contains, and any collaboration agreement that we enter into in the future may contain, restrictions on our ability to enter into potential collaborations, to conduct research or development in certain fields, or to otherwise develop specified product candidates. Further, there have been a significant number of recent business combinations among large pharmaceutical companies that have resulted in a reduced number of potential future collaborators. We may not be able to negotiate new collaborations on a timely basis, on acceptable terms, or at all. If we are unable to do so, we may have to curtail the development of the product candidate for which we are seeking to collaborate, reduce or delay itsour development program or one or more of our other development programs, delay itsour potential commercialization or reduce the scope of any sales or marketing activities, or increase our expenditures and undertake development or commercialization activities at our own expense.
Risks Related to Our Intellectual Property
If we fail to comply with our obligations under our existing and any future intellectual property licenses with third parties, we could lose license rights that are important to our business.
We are party to several license agreements under which we license patent rights and other intellectual property related to orour business, including: a license agreement with Dana-Farber Cancer Institute, or Dana-Farber, under which we were granted an exclusive worldwide license under specified patents relating to CDK7 inhibitors and JNK inhibitors; a license agreement with the Whitehead Institute for Biomedical Research, or Whitehead, and Dana-Farber, pursuant to which we were granted a predominantly exclusive, with certain non-exclusive exceptions, license under specified U.S. patents relating to MYC modulators; a license agreement with Whitehead pursuant to which we were granted an exclusive worldwide license under specified patents relating to super-enhancers until April 2016, which license can remain exclusive thereafter, on a field-by-field basis, as long as we can demonstrate that we are using commercially reasonable efforts in the applicable field; andincluding the TMRC license agreement, pursuant to which we were granted a license under specified patent rights, data, regulatory filings and other intellectual property primarily for the North American and European development and commercialization of SY-1425tamibarotene for the treatment of human cancer indications. We may enter into additional license agreements in the future. Our license agreements impose, and we expect that future license agreements will impose, various diligence, milestone payment, royalty, insurance and other obligations on us. If we fail to comply with our obligations under these licenses, our licensors may have the right to terminate these license agreements, in which event we might not be able to market any product that is covered by these agreements, or our licensors may convert the license to a non-exclusive license, which could adversely affect the value of the product
candidate being developed under the license agreement. Termination of these license agreements or reduction or elimination of our licensed rights may also result in our having to negotiate new or reinstated licenses with less favorable terms.
We do not have composition of matter patent protection with respect to SY-1425.tamibarotene or the active pharmaceutical ingredient of SY-2101.
We own certain patents and patent applications with claims directed to specific methods of using SY-1425tamibarotene and we expect to have marketing exclusivity from the FDA and EMA for a period of no less than five and ten years, respectively, because SY-1425tamibarotene has not been approved in these markets. Composition of matter patent protection in the United States and elsewhere covering SY-1425tamibarotene has expired, however. In addition, we may in the future pursue further development of SY-2101, and we do not have composition of matter patent protection for arsenic trioxide, the active pharmaceutical ingredient of SY-2101. We may be limited in our ability to list our method patents in the FDA'sFDA’s Orange Book if the use of our product,products, consistent with its FDA-approved label, would not fall within the scope of our patent claims. Also, our competitors may be able to offer and sell products so long as these competitors do not infringe any other patents that we (or
third parties) hold, including patents with claims directed to the manufacture of SY-1425tamibarotene and/or method of use patents.patents, or to the formulation of SY-2101 drug product and/or methods of manufacture of SY-2101. In general, method of use patents are more difficult to enforce than composition of matter patents because, for example, of the risks that the FDA may approve alternative uses of the subject compounds not covered by the method of use, formulation or manufacturing method patents, and others may engage in off-label sale or use of the subject compounds. Physicians are permitted to prescribe an approved product for uses that are not described in the product's labeling. Although off-label prescriptions may infringe our method of use patents, the practice is common across medical specialties and such infringement is difficult to prevent or prosecute. FDA approval of uses of a generic version of SY-1425tamibarotene or SY-2101 that are not covered by our patents would limit our ability to generate revenue from the sale of SY-1425,such products, if approved for commercial sale. In addition, any off-label use of a generic version of SY-1425tamibarotene would limit our ability to generate revenue from the sale of SY-1425,tamibarotene, if approved for commercial sale.
Our intellectual property licenses with third parties may be subject to disagreements over contract interpretations, which could narrow the scope of our rights to the relevant intellectual property or technology or increase our financial or other obligations to our licensors.
The agreements under which we currently license intellectual property or technology from third parties are complex, and certain provisions in such agreements may be susceptible to multiple interpretations. The resolution of any contract interpretation disagreement that may arise could narrow what we believe to be the scope of our rights to the relevant intellectual property or technology, or increase what we believe to be our financial or other obligations under the relevant agreement, either of which could harm our business, financial condition, results of operations and prospects.
We may not be successful in obtaining or maintaining necessary rights to current or future product candidates through acquisitions and in-licenses.
We currently have rights to certain intellectual property, through ownership or licenses from third parties, to develop and commercialize SY-1425tamibarotene for human cancers in North and South America and Europe, Israel, Russia and SY-1365Australia, and for SY-2101 and SY-5609 for all potential uses worldwide.in North America and major markets in Europe and elsewhere. Because our programs may require the use of proprietary rights by third parties, the growth of our business likely will depend, in part, on our ability to acquire, in-license or use these proprietary rights. We may be unable to acquire or in-license from third parties any intellectual property rights directed to compositions, methods of use, processes or other intellectual property rights from third partiesprocesses that we identify as necessary for any product candidates. The licensing or acquisition of third-party intellectual property rights is a competitive area, and several more established companies may pursue strategies to license or acquire third-party intellectual property rights that we may consider attractive. These established companies may have a competitive advantage over us due to their size, capital resources and greater clinical development and commercialization capabilities. In addition, companies that perceive us to be a competitor may be unwilling to assign or license rights to us. We also may be unable to license or acquire third-party intellectual property rights on terms that would allow us to make an appropriate return on our investment.
We sometimes collaborate with non-profit and academic institutions to accelerate our preclinical research or development under written agreements with these institutions. Typically, these institutions provide us with an option to negotiate a license to any of the institution's rights in technology resulting from the collaboration. Regardless of such option, we may be unable to negotiate a license within the specified timeframe or under terms that are acceptable to us or we may decide not to execute such option if we believe such license is not necessary to pursue our program. If we are unable or opt not to do so, the institution may offer the intellectual property rights to other parties, potentially blocking our ability to pursue our program.
If we are unable to successfully obtain rights to required third-party intellectual property rights or maintain the existing intellectual property rights we have, we may be required to expend significant time and resources to redesign ourtamibarotene or any future product candidates or the methods for manufacturing them or to develop or license replacement technology, all of which may not be feasible on a technical or commercial basis. If we are unable to do so, we may be unable to develop or commercialize the affected product candidate, which could harm our business significantly.
We depend upon our license with TMRC, and we may not be able to maintain that license.
We have entered into a standby license with TMRC and Toko Pharmaceuticals Ind. Co. Ltd., or Toko, providing that if at any time the license agreement between Toko and TMRC relating to the SY-1425tamibarotene rights that TMRC has licensed to us terminates or otherwise ceases to be in effect for any reason, Toko will grant directly to us such rights and licenses with respect to SY-1425tamibarotene as are necessary for us to continue to develop SY-1425.tamibarotene. If the TMRC license agreement terminates and this standby license terminates, then we may lose rights to SY-1425tamibarotene that may be necessary to the development and commercialization of SY-1425,tamibarotene, which could have a material adverse impact on our business.
If we are unable to obtain and maintain sufficient patent protection for tamibarotene or any future product candidates, or if the scope of the patent protection is not sufficiently broad, our competitors could develop and commercialize products similar or identical to ours, and our ability to successfully commercialize oursuch product candidates may be adversely affected.
Our success depends in large part on our ability to obtain and maintain patent protection in the United States and
other countries with respect to ourtamibarotene and any future proprietary product candidates. If we do not adequately protect our intellectual property, our competitors may be able to erode or negate any competitive advantage we may have, which could harm our business and ability to achieve profitability. To protect our proprietary position, we file patent applications in the United States and abroad related to our noveltamibarotene and other potential product candidates that are important to our business. We also license or purchase patent applications filed by others. The patent application and approval process isprocesses are expensive and time consuming. We may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner.
Agreements through which we license patent rights may not give us control over patent prosecution or maintenance, so that we may not be able to control which claims or arguments are presented and may not be able to secure, maintain, or successfully enforce necessary or desirable patent protection from those patent rights. We have not had and do not have primary control over patent prosecution and maintenance for certain of the patents and patent applications we license, and therefore cannot guarantee that these patents and applications will be prosecuted in a manner consistent with the best interests of our business. We cannot be certain that patent prosecution and maintenance activities by our licensors have been or will be conducted in compliance with applicable laws and regulations or will result in valid and enforceable patents.
We, or any future partners, collaborators or licensees, may fail to identify patentable aspects of inventions made in the course of development and commercialization activities before it is too late to obtain patent protection for them. Therefore, we may miss potential opportunities to strengthen our patent position.
It is possible that defects of form in the preparation or filing of our patents or patent applications may exist, or may arise in the future, for example with respect to proper priority claims, inventorship, claim scope or patent term adjustments. If we or our partners, collaborators, licensees, or licensors, whether current or future, fail to establish, maintain or protect such patents and other intellectual property rights, such rights may be reduced or eliminated. If our partners, collaborators, licensees or licensors, are not fully cooperative or disagree with us as to the prosecution, maintenance or enforcement of any patent rights, such patent rights could be compromised. If there are material defects in the form, preparation, prosecution, or enforcement of our patents or patent applications, such patents may be invalid and/or unenforceable, and such applications may never result in valid, enforceable patents. Any of these outcomes could impair our ability to prevent competition from third parties, which may have an adverse impact on our business.
The patent position of biotechnology and pharmaceutical companies generally is highly uncertain. No consistent policy regarding the breadth of claims allowed in biotechnology and pharmaceutical patents has emerged to date in the United States or in many foreign jurisdictions. In addition, the determination of patent rights with respect to pharmaceutical compounds commonly involves complex legal and factual questions, which has in recent years been the subject of much litigation. As a result, the issuance, scope, validity, enforceability and commercial value of our patent rights are highly uncertain.
Pending patent applications cannot be enforced against third parties practicing the technology claimed in such applications unless and until a patent issues from such applications. Assuming the other requirements for patentability are met, currently, the first to file a patent application is generally entitled to the patent except that, prior to March 16, 2013 in the United States, the first to invent was entitled to the patent. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing, or in some cases, not at all. Therefore, we cannot be certain that we were the first to make the inventions claimed in our patents or pending patent applications, or that we were the first to file for patent protection of such inventions. Similarly, we cannot be certain that parties from whom we do or may license or purchase patent rights were the first to make relevant claimed inventions, or were the first to file for patent protection for them. If third parties have filed patent applications on inventions claimed in our patents or applications on or before March 15, 2013, an interference proceeding in the United States can be initiated by such third parties to determine who was the first to invent any of the subject matter covered by the patent claims of our applications. If third parties have filed such applications after March 15, 2013, a derivation proceeding in the United States can be initiated by such third parties to determine whether our invention was derived from theirs.
Moreover, because the issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, our patents or pending patent applications may be challenged in the courts or patent offices in the United States and abroad. There is no assurance that all of the potentially relevant prior art relating to our patents and patent applications has been found. If such prior art exists, it may be used to invalidate a patent, or may prevent a patent from issuing from a pending patent application. For example, such patent filings may be subject to a third-party preissuancepre-issuance submission of prior art to the U.S. Patent and Trademark Office, or USPTO or to other patent offices around the world. Alternately or additionally, we may become involved in post-grant review procedures, oppositions, derivations, proceedings, reexaminations, inter partes review or interference proceedings, in
the United States or elsewhere, challenging patents or patent applications in which we have rights, including patents on which we rely to protect our business. An adverse determination in any such challenges may result in loss of exclusivity or in patent claims being narrowed, invalidated or held unenforceable, in whole or in part, which could limit our ability to stop others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection of our technology and products. In addition, given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized.
Pending and future patent applications may not result in patents being issued which protect our business, in whole or in part, or which effectively prevent others from commercializing competitive products. Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of our patents or narrow the scope of our patent protection. In addition, the laws of foreign countries may not protect our rights to the same extent or in the same manner as the laws of the United States.
Issued patents that we have or may obtain or license may not provide us with any meaningful protection, prevent competitors from competing with us or otherwise provide us with any competitive advantage. Our competitors may be able to circumvent our patents by developing similar or alternative technologies or products in a non-infringing manner. Our competitors may also seek approval to market their own products similar to or otherwise competitive with our products. Alternatively, our competitors may seek to market generic versions of any approved products by submitting ANDAs to the FDA in which they claim that patents owned or licensed by us are invalid, unenforceable or not infringed. In these circumstances, we may need to defend or assert our patents, or both, including by filing lawsuits alleging patent infringement. In any of these types of proceedings, a court or other agency with jurisdiction may find our patents invalid or unenforceable, or that our competitors are competing in a non-infringing manner. Thus, even if we have valid and enforceable patents, these patents still may not provide protection against competing products or processes sufficient to achieve our business objectives. We are aware of a third party that is offering super-enhancer identification and analysis services, which we believe infringe our issued in-licensed United States patent relating to this subject matter. We are in communication with that third party regarding the timing under which we would grant them a license under our in-licensed patent. If we are unsuccessful in negotiating a license on acceptable terms, we may be required to file infringement claims against that party with all of the associated risks of patent infringement litigation set forth herein. If that party continues to offer these services, it may affect our ability to attract corporate partners who are interested in super-enhancer identification and analysis and may negatively affect the value of our technology platform and therefore harm our business.
Pursuant to the terms of some of our license agreements with third-parties,third parties, some of our third partythird-party licensors have the right, but not the obligation, in certain circumstances to control enforcement of our licensed patents or defense of any claims asserting the invalidity of these patents. Even if we are permitted to pursue such enforcement or defense, we will require the cooperation of our licensors, and cannot guarantee that we would receive it and on what terms. We cannot be certain that our licensors will allocate sufficient resources or prioritize their or our enforcement of such patents or defense of such claims to protect our interests in the licensed patents. If we cannot obtain patent protection, or enforce existing or future patents against third parties, our competitive position and our financial condition could suffer.
If we are unable to protect the confidentiality of our trade secrets, the value of our technology could be materially adversely affected and our business would be harmed.
In addition to the protection afforded by patents, we may also rely on trade secret protection for certain aspects of our technology platform, including certain aspects of our gene control platform.protection. We seek to protect these trade secrets, in part, by entering into non-disclosure and confidentiality agreements with parties who have access to them, such as our employees, consultants, independent contractors, advisors, contract manufacturers, suppliers and other third parties. We also enter into confidentiality and invention or patent assignment agreements with employees and certain consultants. Any party with whom we have executed such an agreement may breach that agreement and disclose our proprietary information, including ourany information we hold in confidence or as a trade secrets,secret, and we may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated confidential information or a trade secret is difficult, expensive and time consuming, and the outcome is unpredictable. Additionally, if the steps taken to maintain our trade secrets are deemed inadequate, we may have insufficient recourse against third parties for misappropriating the trade secret. Further, if any of our confidential information or trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent such third party, or those to whom they communicate such technology or information, from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed by a competitor, our business and competitive position could be harmed.
We may become involved in lawsuits to protect or enforce our patents or other intellectual property, which could be expensive, time consuming and unsuccessful.
Competitors may infringe our patents, trademarks, copyrights or other intellectual property. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time consuming and divert the time and attention of our management and scientific personnel. Any claims we assert against perceived infringers could provoke these parties to assert counterclaims against us alleging that we infringe their patents, in addition to counterclaims asserting that our patents are invalid or unenforceable, or both. In any patent infringement proceeding, there is a risk that a
court will decide that a patent of ours is invalid or unenforceable, in whole or in part, and that we do not have the right to stop the other party from using the invention at issue. There is also a risk that, even if the validity of such patents is upheld, the court will construe the patent's claims narrowly or decide that we do not have the right to stop the other party from using the invention at issue on the grounds that our patent claims do not cover the invention. An adverse outcome in a litigation or proceeding involving one or more of our patents could limit our ability to assert those patents against those parties or other competitors and may curtail or preclude our ability to exclude third parties from making and selling similar or competitive products. Similarly, if we assert trademark infringement claims, a court may determine that the marks we have asserted are invalid or unenforceable, or that the party against whom we have asserted trademark infringement has superior rights to the marks in question. In this case, we could ultimately be forced to cease use of such trademarks.
Even if we establish infringement, the court may decide not to grant an injunction against further infringing activity and instead award only monetary damages, which may or may not be an adequate remedy. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during litigation. There could also be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could adversely affect the price of shares of our common stock. Moreover, there can be no assurance that we will have sufficient financial or other resources to file and pursue such infringement claims, which typically last for years before they are concluded. Even if we ultimately prevail in such claims, the monetary cost of such litigation and the diversion of the attention of our management and scientific personnel could outweigh any benefit we receive as a result of the proceedings.
If we are sued for infringing intellectual property rights of third parties, such litigation could be costly and time consuming and could prevent or delay us from developing or commercializing ourtamibarotene or any future product candidates.
Our commercial success depends, in part, on our ability to develop, manufacture, market and sell ourtamibarotene and any future product candidates and use our gene control technology without infringing the intellectual property and other proprietary rights of third parties. Third parties may have U.S. and non-U.S. issued patents and pending patent applications relating to compounds and methods of use for the treatment of the disease indications for which we are developing ourtamibarotene and any future product candidates. If any third-party patents or patent applications are found to cover oursuch product candidates or their methods of use, we may not be free to manufacture or market oursuch product candidates as planned without obtaining a license, which may not be available on commercially reasonable terms, or at all.
There is a substantial amount of intellectual property litigation in the biotechnology and pharmaceutical industries, and we may become party to, or threatened with, litigation or other adversarial proceedings regarding intellectual property rights with respect to ourtamibarotene or any future product candidates, including interference proceedings before the USPTO. There may be third-party patents or patent applications with claims to materials, formulations, methods of manufacture or methods for treatment related to the use or manufacture of oursuch product candidates. Because patent applications can take many years to issue, there may be currently pending patent applications which may later result in issued patents that oursuch product candidates may be accused of infringing. In addition, third parties may obtain patents in the future and claim that use of our technologies infringes upon these patents. Accordingly, third parties may assert infringement claims against us based on existing or future intellectual property rights. The outcome of intellectual property litigation is subject to uncertainties that cannot be adequately quantified in advance. The pharmaceutical and biotechnology industries have produced a significant number of patents, and it may not always be clear to industry participants, including us, which patents cover various types of products or methods of use. The coverage of patents is subject to interpretation by the courts, and the interpretation is not always uniform. If we were sued for patent infringement, we would need to demonstrate that oursuch product candidates, products or methods either do not infringe the patent claims of the relevant patent or that the patent claims are invalid or unenforceable, and we may not be able to do this. Proving invalidity is difficult. For example, in the United States, proving invalidity requires a showing of clear and convincing evidence to overcome the presumption of validity enjoyed by issued patents. Even if we are successful in these proceedings, we may incur substantial costs and the time and attention of our management and scientific personnel could be diverted in pursuing these proceedings, which could significantly harm our business and operating results. In addition, we may not have sufficient resources to bring these actions to a successful conclusion.
If we are found to infringe a third party's intellectual property rights, we could be forced, including by court order, to cease developing, manufacturing or commercializing the infringing product candidate or product. Alternatively, we may be required to obtain a license from such third party in order to use the infringing technology and continue developing, manufacturing or marketing the infringing product candidate or product. It is possible, however, that we would be unable to
obtain any required license on commercially reasonable terms or at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. Alternatively, or additionally, it could include terms that impede or destroy our ability to compete successfully in the commercial marketplace. In addition, we could be found liable for monetary damages, including treble damages and attorneys' fees if we are found to have willfully infringed a patent. A finding of infringement could prevent us from commercializing ourtamibarotene or any future product candidates or force us to cease some of our business operations, which could harm our business. Claims that we have misappropriated the confidential information or trade secrets of third parties could have a similar negative impact on our business.
Changes to the patent law in the United States and other jurisdictions could diminish the value of patents in general, thereby impairing our ability to protect our products.
As is the case with other biopharmaceutical companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the biopharmaceutical industry involves both technological and legal complexity and is therefore costly, time consuming and inherently uncertain. Recent patentPatent reform legislation in the United States, including the Leahy-Smith America Invents Act, or the America Invents Act, could increase those uncertainties and costs. The America Invents Act was signed into law on September 16, 2011, and many of the substantive changes became effective on March 16, 2013. The America Invents Act reforms United States patent law in part by changing the U.S. patent system from a "first to invent" system to a "first inventor to file" system, expanding the definition of prior art, and developing a post-grant review system. This legislation changes United States
patent law in a way that may weaken our ability to obtain patent protection in the United States for those applications filed after March 16, 2013.
Further, the America Invents Act created new procedures to challenge the validity of issued patents in the United States, including post-grant review and inter partes review proceedings, which some third parties have been using to cause the cancellation of selected or all claims of issued patents of competitors. For a patent with an effective filing date of March 16, 2013 or later, a petition for post-grant review can be filed by a third party in a nine monthnine-month window from issuance of the patent. A petition for inter partes review can be filed immediately following the issuance of a patent if the patent has an effective filing date prior to March 16, 2013. A petition for inter partes review can be filed after the nine monthnine-month period for filing a post-grant review petition has expired for a patent with an effective filing date of March 16, 2013 or later. Post-grant review proceedings can be brought on any ground of invalidity, whereas inter partes review proceedings can only raise an invalidity challenge based on published prior art and patents. These adversarial actions at the USPTO review patent claims without the presumption of validity afforded to U.S. patents in lawsuits in U.S. federal courts and use a lower burden of proof than used in litigation in U.S. federal courts. Therefore, it is generally considered easier for a competitor or third party to have a U.S. patent invalidated in a USPTO post-grant review or inter partes review proceeding than invalidated in a litigation in a U.S. federal court. If any of our patents are challenged by a third party in such a USPTO proceeding, there is no guarantee that we or our licensors or collaborators will be successful in defending the patent, which would result in a loss of the challenged patent right to us.
In addition, recent court rulingsthe USPTO continues to modify its guidelines regarding subject matter eligibility, a process that began with decisions rendered in cases such as Association for Molecular Pathology v. Myriad Genetics, Inc.; BRCA1- & BRCA2-Based Hereditary Cancer Test Patent Litigation; and Promega Corp. v. Life Technologies Corp. Those court decisions have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents once obtained. Depending on future actions by the U.S. Congress, the U.S. courts, the USPTO and the relevant law-making bodies in other countries, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future.
We may not be able to enforce our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on ourtamibarotene or any future product candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States can be less extensive than those in the United States. The requirements for patentability may differ in certain countries, particularly in developing countries; thus, even in countries where we do pursue patent protection, there can be no assurance that any patents will issue with claims that cover our products.
Moreover, our ability to protect and enforce our intellectual property rights may be adversely affected by unforeseen changes in foreign intellectual property laws. Additionally, laws of some countries outside of the United States and Europe do not afford intellectual property protection to the same extent as the laws of the United States and Europe. Many companies
have encountered significant problems in protecting and defending intellectual property rights in certain foreign jurisdictions. The legal systems of some countries, including India, China and other developing countries, do not favor the enforcement of patents and other intellectual property rights. This could make it difficult for us to stop the infringement of our patents or the misappropriation of our other intellectual property rights. For example, many foreign countries have compulsory licensing laws under which a patent owner must grant licenses to third parties. Consequently, we may not be able to prevent third parties from practicing our inventions in certain countries outside the United States and Europe. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop and market their own products and, further, may export otherwise infringing products to territories where we have patent protection, if our ability to enforce our patents to stop infringing activities is inadequate. These products may compete with our products, and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Further, a decree was adopted by the Russian government in March 2022 allowing Russian companies and individuals to exploit inventions owned by patent holders from the United States without consent or compensation. Consequently, we would not be able to prevent third parties from practicing our inventions in Russia or from selling or importing products made using our inventions in and into Russia.
Agreements through which we license patent rights may not give us sufficient rights to permit us to pursue enforcement of our licensed patents or defense of any claims asserting the invalidity of these patents (or control of enforcement or defense) of such patent rights in all relevant jurisdictions as requirements may vary.
Proceedings to enforce our patent rights in foreign jurisdictions, whether or not successful, could result in substantial costs and divert our efforts and resources from other aspects of our business. Moreover, such proceedings
could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate, and the damages or other remedies awarded, if any, may not be commercially meaningful. Furthermore, while we intend to protect our intellectual property rights in major markets for our products, we cannot ensure that we will be able to initiate or maintain similar efforts in all jurisdictions in which we may wish to market our products. Accordingly, our efforts to protect our intellectual property rights in such countries may be inadequate.
Patent terms may be inadequate to protect our competitive position on our products for an adequate amount of time.
Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. We expect to seek extensions of patent terms in the United States and, if available, in other countries where we are prosecuting patents. In the United States, the Drug Price Competition and Patent Term Restoration Act of 1984 permits a patent term extension of up to five years beyond the normal expiration of the patent, which is limited to the approved indication (or any additional indications approved during the period of extension). The applicable authorities, including the FDA and the USPTO in the United States, and any equivalent regulatory authority in other countries, may not agree, however, with our assessment of whether such extensions are available, and may refuse to grant extensions to our patents, or may grant more limited extensions than we request. If this occurs, our competitors may be able to take advantage of our investment in development and clinical trials by referencing our clinical and preclinical data and launch their product earlier than might otherwise be the case.
We may be subject to claims by third parties asserting that our employees or we have misappropriated their intellectual property or claiming ownership of what we regard as our own intellectual property.
Many of our employees and our licensors' employees, including our senior management, were previously employed at universities or at other biotechnology or pharmaceutical companies, some of which may be competitors or potential competitors. Some of these employees, including each member of our senior management, executed proprietary rights, non-disclosure and non-competition agreements, or similar agreements, in connection with such previous employment. Although we try to ensure that our employees do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or these employees have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such third party. Litigation may be necessary to defend against such claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel or sustain damages. Such intellectual property rights could be awarded to a third party, and we could be required to obtain a license from such third party to commercialize our technology or products. Such a license may not be available on commercially reasonable terms or at all. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management.
In addition, while we typically require our employees, consultants, contractors and vendors who may be involved in the development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who in fact develops intellectual property that we regard as our own, which may result in claims by or against us related to the ownership of such intellectual property. If we fail in prosecuting or defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights. Even if we are successful in prosecuting or defending against such claims, litigation could result in substantial costs and be a distraction to our senior management and scientific personnel.
Obtaining and maintaining patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees and various other governmental fees on patents and applications are required to be paid to the USPTO and various governmental patent agencies outside of the United States in several stages over the lifetime of the patents and applications. The USPTO and various non-U.S. governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process and after a patent has issued. There are situations in which non-compliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction.
Risks Related to Regulatory Approval and Marketing of OurTamibarotene or Any Future Product Candidates and Other Legal Compliance Matters
Even if we complete the necessary preclinical studies and clinical trials, the regulatory approval process is expensive, time consuming and uncertain and may prevent us or any future collaborators from obtaining approvals for the commercialization of sometamibarotene or all of ourany future product candidates. As a result, we cannot predict when or if, and in which territories, we, or any future collaborators, will obtain marketing approval to commercialize a product candidate.
The research, testing, manufacturing, labeling, approval, selling, marketing, promotion and distribution of products are subject to extensive regulation by the FDA and comparable foreign regulatory authorities. We, and any future collaborators, are not permitted to market ourany product candidates in the United States or in other countries until we, or they, receive approval of an NDA from the FDA or marketing approval from applicable regulatory authorities outside the United States. OurTamibarotene and any future product candidates are in various stages of development and are subject to the risks of failure inherent in drug development. We have not submitted an application for or received marketing approval for any of our product candidates in the United States or in any other jurisdiction. We have limited experience in conducting and managing the clinical trials necessary to obtain marketing approvals, including FDA approval of an NDA.
The process of obtaining marketing approvals, both in the United States and abroad, is lengthy, expensive and uncertain. It may take many years, if approval is obtained at all, and can vary substantially based upon a variety of factors, including the type, complexity and novelty of the product candidates involved. Securing marketing approval requires the submission of extensive preclinical and clinical data and supporting information, including manufacturing information, to regulatory authorities for each therapeutic indication to establish the product candidate's safety and efficacy. Securing marketing approval also requires the submission of information about the product manufacturing process to, and inspection of manufacturing facilities by, the regulatory authorities. The FDA or other regulatory authorities may determine that ourtamibarotene or any future product candidates are not safe and effective, only moderately effective or have undesirable or unintended side effects, toxicities or other characteristics that preclude our obtaining marketing approval or prevent or limit commercial use. Moreover, the FDA or other regulatory authorities may fail to approve the commercial companion diagnostic that Qiagen is developing to identify patients with RARA overexpression or any other companion diagnostics that we contemplate developingmay develop with partners.partners in the future. Any marketing approval we ultimately obtain may be limited or subject to restrictions or post-approval commitments that render the approved product not commercially viable.
In addition, changes in marketing approval policies during the development period, changes in or the enactment or promulgation of additional statutes, regulations or guidance or changes in regulatory review for each submitted product application, may cause delays in the approval or rejection of an application. Regulatory authorities have substantial discretion in the approval process and may refuse to accept any application or may decide that our data are insufficient for approval and require additional preclinical, clinical or other studies. In addition, varying interpretations of the data obtained from preclinical and clinical testing could delay, limit or prevent marketing approval of a product candidate. Any marketing approval we, or any future collaborators, ultimately obtain may be limited or subject to restrictions or post-approval commitments that render the approved product not commercially viable.
Any delay in obtaining or failure to obtain required approvals could negatively affect our ability or that of any future collaborators to generate revenue from the particulartamibarotene or any future product candidate, which likely would result in significant harm to our financial position and adversely impact our stock price.
If we are required by the FDA to obtain approval of a companion diagnostic in connection with approval of a candidate therapeutic product, and we do not obtain or there are delays in obtaining FDA approval of a diagnostic device, we will not be able to commercialize the product candidate and our ability to generate revenue will be materially impaired.
In August 2014, the FDA issued final guidance clarifying the requirements that will apply to approval of therapeutic products and in vitro companion diagnostics. According to the guidance, if the FDA determines that a companion diagnostic device is essential to the safe and effective use of a novel therapeutic product or indication, the FDA generally will not approve the therapeutic product or new therapeutic product indication if the companion diagnostic is not also approved or cleared for that indication. Under the Federal Food, Drug, and Cosmetic Act, or FDCA, companion diagnostics are regulated as medical devices and the FDA has generally required companion diagnostics intended to select the patients who will respond to cancer treatment to obtain premarket approval, or a PMA. Consequently, we anticipate that certain of our companion diagnostics may require us or our collaborators to obtain a PMA. The PMA process, including the gathering of clinical and preclinical data and the submission to and review by the
FDA, involves a rigorous premarket review during which the applicant must prepare and provide the FDA with reasonable assurance of the device's safety and effectiveness and information about the device and its components regarding, among other things, device design, manufacturing and labeling. PMA approval is not guaranteed and may take considerable time, and the FDA may ultimately respond to a PMA submission with a not approvable determination based on deficiencies in the application and require additional clinical trial or other data that may be expensive and time-consuming to generate and that can substantially delay approval. As a result, if we or our collaborators are required by the FDA to obtain approval of a companion diagnostic for a candidate therapeutic product, and we or our collaborators do not obtain or there are delays in obtaining FDA approval of a diagnostic device, we will not be able to commercialize the product candidate and our ability to generate revenue will be materially impaired.
In its August 2014 guidance, the FDA also indicated that companion diagnostics used to make treatment decisions in clinical trials of a therapeutic product generally will be considered investigational devices. When a companion diagnostic is used to make critical treatment decisions, such as patient selection, the FDA stated that the diagnostic will be considered a significant risk device requiring an investigational device exemption. The FDA may find that a companion diagnostic that we, alone or with a third party, plan to develop does not comply with those requirements and, if this were to occur, we would not be able to proceed with our planned trial of the applicable product candidate in these patient populations.
Failure to obtain marketing approval in foreign jurisdictions would prevent ourtamibarotene or any future product candidates from being marketed abroad. Any approval we are granted for oura product candidatescandidate in the United States would not assure approval of ourthat product candidatescandidate in foreign jurisdictions.
In order to market and sell our products in the European Union and other foreign jurisdictions, we, and any future collaborators, must obtain separate marketing approvals and comply with numerous and varying regulatory requirements. The approval procedure varies among countries and can involve additional testing. The time required to obtain approval may differ substantially from that required to obtain FDA approval. The marketing approval process outside the United States generally includes all of the risks associated with obtaining FDA approval. In addition, in many countries outside the United States, a product must be approved for reimbursement before the product can be approved for sale in that country. We, and any future collaborators, may not obtain approvals from regulatory authorities outside the United States on a timely basis, if at all. Approval by the FDA does not ensure approval by regulatory authorities in other countries or jurisdictions, and approval by one regulatory authority outside the United States does not ensure approval by regulatory authorities in other countries or jurisdictions or by the FDA. We may file for marketing approvals but not receive necessary approvals to commercialize our products in any market.
Additionally, on June 23, 2016,In many countries outside the electorateUnited States, a product candidate must also be approved for reimbursement before it can be sold in that country. In some cases, the price that we intend to charge for our products, if approved, is also subject to approval. Obtaining non-U.S. regulatory approvals and compliance with non-U.S. regulatory requirements could result in significant delays, difficulties and costs for us and any collaborators and could delay or prevent the introduction of tamibarotene or any future product candidates in certain countries. In addition, if we or any collaborators fail to obtain the non-U.S. approvals required to market such product candidates outside the United States or if we or any collaborators fail to comply with applicable non-U.S. regulatory requirements, our target market will be reduced and our ability to realize the full market potential of such product candidates will be harmed and our business, financial condition, results of operations and prospects may be adversely affected.
Further, we could face heightened risks with respect to obtaining marketing authorization in the United Kingdom voted in favorU.K. as a result of leaving the European Union,withdrawal of the U.K. from the EU, commonly referred to as Brexit. On March 29, 2017, the country formally notifiedThe U.K. is no longer part of the European UnionSingle Market and EU Customs Union. As of its intention to withdraw pursuant to Article 50January 1, 2021, the Medicines and Healthcare products Regulatory Agency, or MHRA, became responsible for supervising medicines and medical devices in Great Britain, comprising England, Scotland and Wales under domestic law, whereas under the terms of the Lisbon Treaty. Since a significant proportion ofNorthern Ireland Protocol, Northern Ireland is currently subject to EU rules. The U.K. and EU have however agreed to the regulatory framework inWindsor Framework which fundamentally changes the United Kingdom is derived from European Union directives and regulations,existing system under the referendum could materially impact the regulatory regimeNorthern Ireland Protocol, including with respect to the regulation of medicinal products in the U.K. Once implemented, the changes introduced by the Windsor Framework will see the MHRA be responsible for approving all medicinal products destined for the U.K. market (i.e., Great Britain and Northern Ireland), and the EMA will no longer have any role in approving medicinal products destined for Northern Ireland.
In addition, foreign regulatory authorities may change their approval policies and new regulations may be enacted. For instance, the EU pharmaceutical legislation is currently undergoing a complete review process, in the context of the Pharmaceutical Strategy for Europe initiative, launched by the European Commission in November 2020. The European Commission’s proposal for revision of several legislative instruments related to medicinal products (potentially reducing the duration of regulatory data protection, revising the eligibility for expedited pathways, etc.) was published on April 26, 2023. The proposed revisions remain to be agreed and adopted by the European Parliament and European Council and the proposals may therefore be substantially revised before adoption, which is not anticipated before early 2026. The revisions may however have a significant impact on the pharmaceutical industry and our business in the long term.
We expect that we will be subject to additional risks in commercializing any of our product candidates that receive marketing approval outside the United States, including tariffs, trade barriers and regulatory requirements; economic weakness, including inflation, or political instability in particular foreign economies and markets (such as the ongoing conflict between Ukraine and Russia); compliance with tax, employment, immigration and labor laws for employees living or traveling abroad; foreign currency fluctuations, which could result in increased operating expenses and reduced revenue, and other obligations incident to doing business in another country; and workforce uncertainty in countries where labor unrest is more common than in the United KingdomStates.
We are conducting and intend to continue conducting certain of our clinical trials globally. However, the FDA and other foreign equivalents may not accept data from such trials, in which case our development plans will be delayed, which could materially harm our business.
We are conducting and intend to continue conducting certain of our clinical trials globally. The acceptance by the FDA or the European Union. Any delay in obtaining, or an inability to obtain, any marketing approvals, as a resultother regulatory authorities of Brexit or otherwise, would prevent usstudy data from commercializing our product candidates in the United Kingdom and/or the European Union and restrict our ability to generate revenue and achieve and sustain profitability. If any of these outcomes occur, weclinical trials conducted outside their jurisdiction may be forcedsubject to restrictcertain conditions or delay effortsmay not be accepted at all. In cases where data from foreign clinical trials are intended to seek regulatoryserve as the sole basis for marketing approval in the United Kingdom and/States, the FDA will generally not approve the application on the basis of foreign data alone unless (i) the data are applicable to the U.S. population and U.S. medical practice; (ii) the trials were performed by clinical investigators of recognized competence and pursuant to good clinical practices, or European UnionGCP, regulations; and (iii) the data may be considered valid without the need for ouran on-site inspection by the FDA, or if the FDA considers such inspection to be necessary, the FDA is able to validate the data through an on-site inspection or other appropriate means.
In addition, even where the foreign study data are not intended to serve as the sole basis for approval, the FDA will not accept the data as support for an application for marketing approval unless the study is well-designed and well-conducted in accordance with GCP requirements and the FDA is able to validate the data from the study through an onsite inspection if deemed necessary. Many foreign regulatory authorities have similar approval requirements. In addition, such foreign trials would be subject to the applicable local laws of the foreign jurisdictions where the trials are conducted. There can be no assurance that the FDA or any comparable foreign regulatory authority will accept data from trials conducted outside of the United States or the applicable jurisdiction. If the FDA or any comparable foreign regulatory authority does not accept such data, it would result in the need for additional trials, which could be costly and time-consuming, and which may result in current or future product candidates which could significantlythat we may develop not receiving approval for commercialization in the applicable jurisdiction.
Conducting clinical trials outside the United States also exposes us to additional risks, including risks associated with:
•additional foreign regulatory requirements;
•foreign exchange fluctuations;
•compliance with foreign manufacturing, customs, shipment and materially harmstorage requirements;
•cultural differences in medical practice and clinical research;
•diminished protection of intellectual property in some countries; and
•interruptions or delays in our business.trials resulting from geopolitical events, such as war or terrorism.
We, or any future collaborators, may not be able to obtain orphan drug designation or orphan drug exclusivity for ourany future product candidates and, even if we do, that exclusivity may not prevent the FDA or the EMA from approving competing products.
Regulatory authorities in some jurisdictions, including the United States and Europe, may designate drugs for relatively small patient populations as orphan drugs. Under the Orphan Drug Act, the FDA may designate a product as an orphan drug if it is a drug intended to treat a rare disease or condition, which is generally defined as a patient population of fewer than 200,000 individuals annually in the United States. We have obtained orphan drug designation for SY-1425 for the treatment of AML in the United States. In the future, however, we or any future collaborators may seek orphan drug designations for SY-1425 in other indications or territories or for other product candidates and may be unable to obtain such designations.
Even if we, or any future collaborators, obtain orphan drug designation for a product candidate, we, or they, may not be able to obtain orphan drug exclusivity for that product candidate. Generally, a product with orphan drug designation only becomes entitled to orphan drug exclusivity if it receives the first marketing approval for the indication for which it has such designation, in which case the FDA or the EMA will be precluded from approving another marketing application for the same drugproduct for that indication for the applicable exclusivity period. The applicable exclusivity period is seven years in the United States and ten years in Europe. The European exclusivity period can be reduced to six years if a drugproduct no longer meets the criteria for orphan drug designation or if the drugproduct is sufficiently profitable so that market exclusivity is no longer justified. Orphan
We have obtained orphan drug designation for tamibarotene for the treatment of MDS in the United States, and for the treatment of AML in the United States and in Europe. In addition, the EMA has issued a positive opinion on our application for orphan drug designation for tamibarotene for the treatment of MDS in Europe. In the future, we or any collaborators may seek orphan drug designations for tamibarotene in other indications or territories or for other product
candidates and may be unable to obtain such designations. Even if we do secure such designations and orphan drug exclusivity for a product, that exclusivity may not effectively protect the product from competition because different products can be approved for the same condition. Further, the FDA can subsequently approve the same drug for the same condition if the FDA concludes that the later product is clinically superior in that it is shown to be safer, to be more effective or to make a major contribution to patient care. Finally, orphan drug exclusivity may be lost if the FDA or the EMA determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantity of the drugproduct to meet the needs of patients with the rare disease or condition.
Even if we, or any future collaborators, obtain orphan drug exclusivity for a product, that exclusivity may not effectively protect the product from competition because different drugs can be approved for the same condition and the same drug can be approved for different conditions. Even after an orphan drug is approved, the FDA can subsequently approve the same drug for the same condition if the FDA concludes that the later drug is clinically superior in that it is shown to be safer, more effective or makes a major contribution to patient care.
On August 3, 2017, Congress passed the FDA Reauthorization Act of 2017 which, among other things, codified the FDA’s pre-existing regulatory interpretation, to require that a drug sponsor demonstrate the clinical superiority of an orphan drug that is otherwise the same as a previously approved drug for the same rare disease in order to receive orphan drug exclusivity. The new legislation reverses prior precedent holding that the Orphan Drug Act unambiguously requires that the FDA recognize the orphan exclusivity period regardless of a showing of clinical superiority. The FDA and Congress may further reevaluate the Orphan Drug Act and its regulations and policies. This may be particularly true in light of a decision from the Court of Appeals for the 11th Circuit in September 2021 finding that, for the purpose of determining the scope of exclusivity, the term “same disease or condition” means the designated “rare disease or condition” and could not be interpreted by the Agency to mean the “indication or use.” Although there have been legislative proposals to overrule this decision, they have not been enacted into law. On January 23, 2023, the FDA announced that, in matters beyond the scope of the court’s order, the FDA will continue to apply its existing regulations tying orphan-drug exclusivity to the uses or indications for which the orphan drug was approved. We do not know if, when, or how the FDA may change the orphan drug regulations and policies in the future, and it is uncertain how any changes might affect our business. Depending on what changes the FDA may make to its orphan drug regulations and policies, our business could be adversely impacted.
Even if we, or any future collaborators, obtain marketing approvals for ourAny product candidates, the terms of approvals and ongoing regulation of our products may limit how we manufacture and market our products, which could impair our ability to generate revenue.
Once marketing approval has been granted, an approved product and its manufacturer and marketer are subject to ongoing review and extensive regulation. We, and any future collaborators, must therefore comply with requirements concerning advertising and promotion for any of our product candidatescandidate for which we or theyour collaborators obtain marketing approval. Promotional communications with respect to prescription drugs areapproval is subject to a variety of legalongoing regulation and regulatory restrictions and must be consistent with the information in the product's approved labeling. Thus, we and any future collaborators will not be able to promote any products we develop for indications or uses for which they are not approved.
In addition, manufacturers of approved products and those manufacturers' facilities are required to comply with extensive FDA requirements, including ensuring that quality control and manufacturing procedures conform to cGMPs, which include requirements relating to quality control and quality assurance as well as the corresponding maintenance of records and documentation and reporting requirements. We, our contract manufacturers, any future collaborators and their contract manufacturers could be subject to periodic unannounced inspections by the FDA to monitor and ensure compliance with cGMPs.
Accordingly, assuming we, or any future collaborators, receive marketing approval for one or more of our product candidates, we, and any future collaborators, and our and their contract manufacturers will continue to expend time, money and effort in all areas of regulatory compliance, including manufacturing, production, product surveillance and quality control.
If we, and any future collaborators, are not able to comply with post-approval regulatory requirements, we, and any future collaborators, could have the marketing approvals for our products withdrawn by regulatory authorities and our, or any future collaborators', ability to market any future products could be limited, which could adversely affect our ability to achieve or sustain profitability. Further, the cost of compliance with post-approval regulations may have a negative effect on our operating results and financial condition.
Any of our product candidates for which we, or any future collaborators, obtain marketing approval in the future could be subject to post-marketing restrictions or withdrawal from the market, and we or any future collaborators, may be subject to substantial penalties if we or they, fail to comply with regulatory requirements, when and if tamibarotene or if we, or they, experience unanticipated problems with our products following approval.
Any of ourany future product candidates are approved.
Any product candidate for which we or any futureour collaborators obtain marketing approval as well as the manufacturing processes, post-approval studies and measures, labeling, advertising and promotional activities for such product, among other things, will be subject to ongoingcontinual requirements of and review by the FDA and other regulatory authorities. These requirements include submissions of safety and other post-marketing information and reports, registration and listing requirements, cGMP requirements relating to manufacturing, quality control and manufacturing, quality assurance and corresponding maintenance of records and documents, and requirements regarding the distribution of samples to physicians and recordkeeping. Even if marketing approval of a product candidate is granted,In addition, the approval may be subject to limitations on the indicated uses for which the product may be marketed or to the conditions of approval, including the requirement to implement a Risk Evaluation and Mitigation Strategy.
The FDA may also imposeor contain requirements for costly post-marketing studies or clinical trialstesting and surveillance to monitor the safety or efficacy of a product. The FDA and other agencies,the medicine, including the Department of Justice, closely regulaterequirement to implement a risk evaluation and monitor the post-approval marketing and promotion of products to ensure that they are manufactured, marketed and distributed only for the approved indications and in accordance with the provisions of the approved labeling. The FDA imposes stringent restrictions on manufacturers' communications regarding off-label use andmitigation strategy. Accordingly, if we or any future collaborators, do not market any of our product candidates for which we, or they, receive marketing approval for only their approved indications,tamibarotene or any future product candidates, we or they, may be subjectwould continue to warnings or enforcement actionexpend time, money and effort in all areas of regulatory compliance, including manufacturing, production, product surveillance and quality control. If we fail to comply with these requirements, we could have the marketing approvals for off-label marketing. Violation of the FDCA and other statutes, including the False Claims Act, relating to the promotion and advertising of prescription drugs may lead to investigations or allegations of violations of federal and state health care fraud and abuse laws and state consumer protection laws.
In addition, later discovery of previously unknown adverse events or other problems with our products withdrawn by regulatory authorities and our ability to market any products could be limited, which could adversely affect our ability to achieve or their manufacturers or manufacturing processes, or failuresustain profitability.
Failure to comply with regulatory requirements, may yield various results, including:
| ·
| | • restrictions on such products, manufacturers or manufacturing processes; |
| ·
| | restrictions on the labeling or marketing of a product;
|
| ·
| | restrictions on product distribution or use;
|
| ·
| | requirements to conduct post-marketing studies or clinical trials;
|
| ·
| | warning letters or untitled letters;
|
| ·
| | withdrawal of the products from the market;
|
| ·
| | refusal to approve pending applications or supplements to approved applications that we submit;
|
| ·
| | restrictions on coverage by third-party payors;
|
| ·
| | fines, restitution or disgorgement of profits or revenues;
|
| ·
| | suspension or withdrawal of marketing approvals;
|
| ·
| | refusal to permit the import or export of products;
|
•restrictions on the labeling or marketing of a product;
•requirements to conduct post-marketing studies or clinical trials;
•warning letters or untitled letters;
•withdrawal of the products from the market;
•refusal to approve pending applications or supplements to approved applications that we submit;
•damage to relationships with collaborators;
•unfavorable press coverage and damage to our reputation;
•fines, restitution or disgorgement of profits or revenues;
•suspension or withdrawal of marketing approvals;
•refusal to permit the import or export of our products;
•injunctions or the imposition of civil or criminal penalties; and
•litigation involving patients using our products.
Non-compliance with EU requirements regarding safety monitoring or pharmacovigilance, and with requirements related to the development of products for the pediatric population, can also result in significant financial penalties. Similarly, failure to comply with the EU’s requirements regarding the protection of personal information can also lead to significant penalties and sanctions.
The FDA, EMA and other regulatory authorities actively enforce the laws and regulations prohibiting the promotion of off-label uses.
If tamibarotene or any future product candidates are approved and we are found to have improperly promoted off-label uses of those products, we may become subject to significant liability. The FDA, EMA and other regulatory authorities strictly regulate the promotional claims that may be made about prescription products, such as tamibarotene or any future product candidate, if approved. In particular, a product may not be promoted in the United States for uses that are not approved by the FDA as reflected in the product’s approved labelling, or in other jurisdictions for uses that differ from the labelling or uses approved by the applicable regulatory authorities. While physicians may prescribe products for off-label uses, the FDA, EMA and other regulatory authorities actively enforce laws and regulations that prohibit the promotion of off-label uses by companies, including promotional communications made by companies’ sales force with respect to off-label uses that are not consistent with the approved labelling, and a company that is found to have improperly promoted off-label uses may be subject to significant civil, criminal and administrative penalties.
Notwithstanding the regulatory restrictions on off-label promotion, the FDA and other regulatory authorities allow companies to engage in truthful, non-misleading, and non-promotional scientific communications concerning their products in certain circumstances. For example, in October 2023, the FDA published draft guidance outlining the agency’s non-binding policies governing the distribution of scientific information on unapproved uses to healthcare providers. This draft guidance calls for such communications to be truthful, non-misleading, factual, and unbiased and include all information necessary for healthcare providers to interpret the strengths and weaknesses and validity and utility of the information about the unapproved use. In addition, under some relatively recent guidance from the FDA and the Pre-Approval Information Exchange Act, signed into law as part of the Consolidated Appropriations Act of 2023, companies may also promote information that is consistent with the prescribing information and proactively speak to formulary committee members of payors regarding data for an unapproved drug or unapproved uses of an approved drug. We may engage in these discussions and communicate with healthcare providers, payors and other constituencies in compliance with all applicable laws, regulatory guidance and industry best practices. We will need to carefully navigate the FDA’s various regulations, guidance and policies, along with recently enacted legislation, to ensure compliance with restrictions governing promotion of our products.
If we are found to have promoted such off-label uses, we may become subject to significant liability. The U.S. federal government has levied large civil and criminal fines against companies for alleged improper promotion of off-label use and has enjoined several companies from engaging in off-label promotion. The FDA has also requested that companies enter into consent decrees or permanent injunctions under which specified promotional conduct is changed or curtailed. If we cannot successfully manage the promotion of tamibarotene or any future product candidates, if approved, we could become subject to significant liability, which would materially adversely affect our business and financial condition.
62
| ·
| | injunctions or the imposition of civil or criminal penalties.
|
We may seek acertain designations for product candidates, including Breakthrough Therapy designation for one or more of our product candidates,and Fast Track designations, but we might not receive such designation,designations, and even if we do, such designationdesignations may not lead to a faster development or regulatory review or approval process.
We may seek acertain designations for product candidates that could expedite review and approval by the FDA. A Breakthrough Therapy designation for one or more of our product candidates. A Breakthrough Therapy is defined as a product that is intended, alone or in combination with one or more other products, to treat a serious condition, and preliminary clinical evidence indicates that the product may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. For products that have been designated as Breakthrough Therapies, interaction and communication between the FDA and the sponsor of the trial can help to identify the most efficient path for clinical development while minimizing the number of patients placed in ineffective control regimens. Products designated as Breakthrough Therapies by the FDA may also be eligible for priority review if supported by clinical data at the time the NDA is submitted to the FDA.
The FDA may also designate a product for Fast Track review if it is intended, whether alone or in combination with one or more other products, for the treatment of a serious or life‑threatening disease or condition, and it demonstrates the potential to address unmet medical needs for such a disease or condition. For example, the FDA has granted Fast Track designation to tamibarotene for the treatment of HR-MDS. For Fast Track products, sponsors may have greater interactions with the FDA and the FDA may initiate review of sections of a Fast Track product’s application before the application is complete. This rolling review may be available if the FDA determines, after preliminary evaluation of clinical data submitted by the sponsor, that a Fast Track product may be effective. The sponsor must also provide, and the FDA must approve, a schedule for the submission of the remaining information and the sponsor must pay applicable user fees.
Designation as a Breakthrough Therapy or Fast Track is within the discretion of the FDA. Accordingly, even if we believe that one of oura product candidatescandidate meets the criteria for designation as a Breakthrough Therapy,these designations, the FDA may disagree and instead determine not to make such designation. EvenFurther, even though we have received Fast Track designation for tamibarotene for the treatment of HR-MDS, and even if we receive Breakthrough Therapy or Fast Track designation for another product candidate, the receipt of such designation for a product candidatedesignations may not result in a faster development or regulatory review or approval process compared to products considered for approval under conventional FDA procedures and does not assure ultimate approval by the FDA. In addition, even if one or more of our product candidates qualify as Breakthrough Therapies, the FDA may later decide that thetamibarotene or one or more of our future product candidates no longer meetmeets the conditions for qualification or decide that the time period for FDA review or approval will not be shortened.
We may seek Fast Track designationInadequate funding for onethe FDA, the SEC and other government agencies, including from government shut downs, or moreother disruptions to these agencies’ operations, could hinder their ability to hire and retain key leadership and other personnel, prevent new products and services from being developed or commercialized in a timely manner or otherwise prevent those agencies from performing normal business functions on which the operation of our business may rely, which could negatively impact our business.
The ability of the FDA to review and approve new products can be affected by a variety of factors, including government budget and funding levels, ability to hire and retain key personnel and accept the payment of user fees, and statutory, regulatory and policy changes. Average review times at the agency have fluctuated in recent years as a result. Disruptions at the FDA and other agencies may also slow the time necessary for new product candidates but we might not receive such designation, and even if we do, such designation may not actually lead to a faster development be reviewed and/or regulatory review or approval process.
If a product is intended for the treatment of a serious condition and nonclinical or clinical data demonstrate the potential to address unmet medical need for this condition, a product sponsor may apply for FDA Fast Track designation. If we seek Fast Track designation for a product candidate, we may not receive it from the FDA. Even if we receive Fast Track designation, however, Fast Track designation does not ensure that we will receive marketing approval or that approval will be granted within any particular timeframe. We may not experience a faster development or regulatory review or approval process with Fast Track designation compared to conventional FDA procedures. In addition, the FDA may withdraw Fast Track designation if it believes that the designation is no longer supportedapproved by data from our clinical development program. Fast Track designation alone does not guarantee qualification for the FDA's priority review procedures.
Under the Cures Act and the Trump Administration’s regulatory reform initiatives, the FDA’s policies, regulations and guidance may be revised or revoked and that could prevent, limit or delay regulatory approval of our product candidates, which would impact our ability to generate revenue.
In December 2016, the 21st Century Cures Act, or Cures Act, was signed into law. The Cures Act, among other things, is intended to modernize the regulation of drugs and spur innovation, but its ultimate implementation is unclear. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained and we may not achieve or sustain profitability,necessary government agencies, which would adversely affect our business, prospects, financial conditionbusiness. In addition, government funding of the SEC and resultsother government agencies on which our operations may rely, including those that fund research and development activities, is subject to the political process, which is inherently fluid and unpredictable. For example, over the last several years the U.S. government has shut down several times and certain regulatory agencies, such as the FDA and the SEC, have had to furlough critical FDA, SEC and other government employees and stop critical activities.
During the COVID-19 pandemic, a number of operations.
We also cannot predictcomplete response letters due to the likelihood, nature or extentFDA’s inability to complete required inspections for their applications. In the event of government regulation thata similar public health emergency in the future, the FDA may arise from future legislation or administrative or executive action, either innot be able to continue its current pace and review timelines could be extended. Regulatory authorities outside the United States facing similar circumstances may adopt similar restrictions or abroad. For example, certain policiesother policy measures in response to a similar public health emergency and may experience delays in their regulatory activities.
If a prolonged government shutdown occurs, it could significantly impact the ability of the Trump administration mayFDA to timely review and process our regulatory submissions, which could have a material adverse effect on our business. Further, future government shutdowns could impact our business and industry. Namely, the Trump administration has taken several executive actions, including the issuance of a number of Executive Orders, that could impose significant burdens on, or otherwise materially delay, the FDA’s ability to engageaccess the public markets and obtain necessary capital in routine regulatoryorder to properly capitalize and oversight activities such as implementing statutes through rulemaking, issuance of guidance, and review and approval of marketing applications. An under-staffed FDA could result in delays in the FDA’s responsiveness or in its ability to review submissions or applications, issue regulations or guidance, or implement or enforce regulatory requirements in a timely fashion or at all. Moreover, on January 30, 2017, President Trump issued an Executive Order, applicable to all executive agencies, including the FDA, which requires that for each notice of proposed rulemaking or final regulation to be issued in fiscal year 2017, the agency shall identify at least two existing regulations to be repealed, unless prohibited by law. These requirements are referred to as the “two-for-one” provisions. This Executive Order includes a budget neutrality provision that requires the total incremental cost of all new regulations in the 2017 fiscal year, including repealed regulations, to be no greater than zero, except in limited circumstances. For fiscal years 2018 and beyond, the Executive Order requires agencies to identify regulations to offset any incremental cost of a new regulation and approximate the total costs or savings associated with each new regulation or repealed regulation. In interim guidance issued by the Office of Information and Regulatory Affairs within Office of Management and Budget on February 2, 2017, the administration indicates that the “two-for-one” provisions may apply not only to agency regulations, but also to significant agency guidance documents. In addition, on February 24, 2017, President Trump issued an executive order directing each affected agency to designate an agency official as a “Regulatory Reform Officer” and establish a “Regulatory Reform Task Force” to implement the two-for-one provisions and other previously issued executive orders relating to the review of federal regulations, however it is difficult to predict how these requirements will be implemented, and the extent to which they will impact the FDA’s ability to exercise its regulatory authority. If these executive actions impose constraints on the FDA’s ability to engage in oversight and implementation activities in the normal course,continue our business may be negatively impactedoperations.
Current and future legislation may result in more rigorous coverage and reimbursement criteria for product candidates, which could increase the difficulty and cost for us and any future collaborators to obtain marketing approval of ourtamibarotene or any future product candidates and affect the prices we, or they, may obtain.candidates.
In the United States and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could among other things, prevent or delay marketing approval of ourtamibarotene or any future product candidates, restrict or regulate post-approval activities and affect our ability or the ability of any collaborators, to profitably sell any productsproduct candidates for which we or they, obtain marketing approval. We expect that current laws, as well as other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and in additional downward pressure on the price that we, or any future collaborators, may receive for any approved products.
In March 2010, President Obama signed into law the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, or collectively the ACA. Among the provisions of the ACA of potential importance to our business and our product candidates are the following:
| ·
| | an annual, non-deductible fee on any entity that manufactures or imports specified branded prescription drugs and biologic agents;
|
| ·
| | an increase in the statutory minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program;
|
| ·
| | expansion of healthcare fraud and abuse laws, including the civil False Claims Act and the federal Anti-Kickback Statute, new government investigative powers and enhanced penalties for noncompliance;
|
| ·
| | a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% point-of-sale discounts off negotiated prices;
|
| ·
| | extension of manufacturers' Medicaid rebate liability;
|
| ·
| | expansion of eligibility criteria for Medicaid programs;
|
| ·
| | expansion of the entities eligible for discounts under the Public Health Service pharmaceutical pricing program;
|
| ·
| | new requirements to report certain financial arrangements with physicians and teaching hospitals;
|
| ·
| | a new requirement to annually report drug samples that manufacturers and distributors provide to physicians; and
|
| ·
| | a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research.
|
In addition, other legislative changes have been proposed and adopted since the ACA was enacted. These changes includeIn August 2011, the Budget Control Act of 2011, which, among other things, ledcreated measures for spending reductions by Congress. A Joint Select Committee on Deficit Reduction, tasked with recommending a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, was unable to reach required goals, thereby triggering the legislation’s automatic reduction to several government programs. These changes included aggregate reductions to Medicare payments to providers of up to 2% per fiscal year that startedwhich went into effect in April 2013 and due to subsequent legislative amendments to the statute, will stayremain in effect through 2025 unless additional congressional action is taken,2031 under the Coronavirus Aid, Relief, and Economic Security Act, or the CARES Act. The American Taxpayer Relief Act of 2012, which, among other things, reduced Medicare payments to several types of providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. These new laws may result in additional reductions in Medicare and other healthcare funding and otherwise affect the prices we may obtain for tamibarotene or any of ourfuture product candidates for which we may obtain regulatory approval or the frequency with which any such product candidate is prescribed or used. Further, there have been several recent U.S. congressional inquiries and proposed state and federalIndeed, under current legislation, designed to, among other things, bring more transparency to drug pricing, review the relationship between pricing and manufacturer patient programs, reduce the costs of drugs under Medicare and reform government program reimbursement methodologies for drug products.
We expect that these healthcare reforms, as well as other healthcare reform measures that may be adopted in the future, may result in additionalactual reductions in Medicare payments may vary up to 4%. The Consolidated Appropriations Act, which was signed into law by President Biden in December 2022, made several changes to sequestration of the Medicare program. Section 1001 of the Consolidated Appropriations Act delays the 4% Statutory Pay-As-You-Go Act of 2010 (PAYGO) sequester for two years, through the end of calendar year 2024. Triggered by enactment of the American Rescue Plan Act of 2021, the 4% cut to the Medicare program would have taken effect in January 2023. The Consolidated Appropriations Act’s health care offset title includes Section 4163, which extends the 2% Budget Control Act of 2011 Medicare sequester for six months into fiscal year 2032 and other healthcare funding, more rigorous coverage criteria, newlowers the payment methodologiesreduction percentages in fiscal years 2030 and additional downward pressure on the price that we receive for any approved product and/or the level of reimbursement physicians receive for administering any approved product we might bring to market. Reductions in reimbursement levels may negatively impact the prices we receive or the frequency with which our products are prescribed or administered. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors.2031.
Since enactment of the ACA, there have been and continue to be, numerous legal challenges and Congressional actions to repeal and replace provisions of the law. In MayFor example, with enactment of the Tax Cuts and Jobs Act, or TCJA, in 2017, Congress repealed the “individual mandate.” The repeal of this provision, which requires most Americans to carry a minimal level of health insurance, became effective in 2019. On June 17, 2021, the U.S. House of Representatives passed legislation known asSupreme Court dismissed the American Health Care Act of 2017. Thereafter, the Senate Republicans introduced and then updated a billmost recent judicial challenge to replace the ACA known asbrought by several states without specifically ruling on the Better Care Reconciliation Actconstitutionality of 2017. The Senate Republicans also introducedthe ACA. Litigation and legislation to repealover the ACA without companion legislationare likely to replace it,continue, with unpredictable and a “skinny” version of the Better Care Reconciliation Act of 2017. In addition, the Senate considered proposed healthcare reform legislation known as the Graham-Cassidy bill. None of these measures was passed by the U.S. Senate.uncertain results.
The Trump Administration hasadministration also takentook executive actions to undermine or delay implementation of the ACA. In January 2017, President Trump signed an Executive OrderACA, including directing federal agencies with authorities and responsibilities under the ACA to waive, defer, grant exemptions from, or delay the implementation of any provision of the ACA that would impose a fiscal or regulatory burden on states, individuals, healthcare providers, health insurers, or manufacturers of pharmaceuticals or medical devices. In October 2017, theOn January 28, 2021, however, President signedBiden issued a secondnew Executive Order allowingwhich directs federal agencies to reconsider rules and other policies that limit Americans’ access to health care, and consider actions that will protect and strengthen that access. Under this Order, federal agencies are directed to re-examine: policies that undermine protections for the use of association health planspeople with pre-existing conditions, including complications related to COVID-19; demonstrations and short-term health insurance, which may provide fewer health benefits than the plans sold throughwaivers under Medicaid and the ACA exchanges. Atthat may reduce coverage or undermine the same time,programs, including work requirements; policies that undermine the Administration announcedHealth Insurance Marketplace or other markets for health insurance; policies that make it will discontinuemore difficult to enroll in Medicaid and the paymentACA; and policies that reduce affordability of cost-sharing reduction,coverage or CSR, payments to insurance companies until Congress approves the appropriation of fundsfinancial assistance, including for such CSR payments. The loss of the CSR payments is expected to increase premiums on certain policies issued by qualified health plans under the ACA.dependents.
A bipartisan bill to appropriate funds for CSR payments was introduced
Current laws, as well as other healthcare reform measures that may be adopted in the Senate, but the future, of that bill is uncertain. More recently, with enactment of the Tax Cuts and Jobs Act of 2017, which was signed by the President on December 22, 2017, Congress repealed the “individual mandate.” The repeal of this provision, which requires most Americans to carry a minimal level of health insurance, will become effective in 2019. According to the Congressional Budget Office, the repeal of the individual mandate will cause 13 million fewer Americans to be insured in 2027 and premiums in insurance markets may rise. Further, each chamber of Congress has put forth multiple bills designed to
repeal or repeal and replace portions of the ACA. Although none of these measures has been enacted by Congress to date, Congress may consider other legislation to repeal and replace elements of the ACA. Congress will likely consider other legislation to replace elements of the ACA during the next Congressional session.
We will continue to evaluate the effect that the ACA and its possible repeal and replacement could have on our business. It is possible that repeal and replacement initiatives, if enacted into law, could ultimately result in fewer individuals having health insurancemore rigorous coverage orcriteria and in individuals having insurance coverage with less generous benefits. Whileadditional downward pressure on the timing and scope of any potential future legislation to repeal and replace ACA provisions is highly uncertain in many respects, it is also possible that some of the ACA provisions that generally are not favorable for the research-based pharmaceutical industry could also be repealed along with ACA coverage expansion provisions Accordingly, such reforms, if enacted, could have an adverse effect on anticipated revenue from product candidatesprice that we, or any collaborators, may successfully develop andreceive for which we may obtain marketing approval and may affect our overall financial condition and ability to develop commercialize product candidates.
The costs of prescription pharmaceuticals in the United States has also been the subject of considerable discussion in the United States, and members of Congress and the Administration have stated that they will address such costs through new legislative and administrative measures. The pricing of prescription pharmaceuticals is also subject to governmental control outside the United States. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a product. To obtain reimbursement or pricing approval in some countries, we may be required to conduct a clinical trial that compares the cost effectiveness of our product candidates to other available therapies.any approved products. If reimbursement of our products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, our ability to generate revenues and become profitablebusiness could be impaired. In the European Union, similar political, economic and regulatory developmentsmaterially harmed.
Changes in tax laws or in their implementation or interpretation may adversely affect our abilitybusiness and financial condition.
The TCJA, as amended by the CARES Act, additionally contains changes in tax law that could adversely affect our business or financial condition. The TCJA contains significant changes to profitably commercialize our products.corporate taxation, including a reduction of the corporate tax rate from a top marginal rate of 35% to a flat rate of 21%, and the limitation of the deduction for net operating losses to 80% of current year taxable income for losses arising in taxable years beginning after December 31, 2017 (though any such net operating losses may be carried forward indefinitely). In addition, beginning in 2022, the TCJA eliminates the option to deduct research and development expenditures currently and requires corporations to capitalize and amortize them over five years or 15 years (for expenditures attributable to foreign research). In addition to continuing pressurethe CARES Act, as part of Congress’s response to the COVID-19 pandemic, economic relief legislation was enacted in 2020 and 2021 containing tax provisions. The IRA also introduced new tax provisions, including a one percent excise tax imposed on certain stock repurchases by publicly traded companies. The one percent excise tax generally applies to any acquisition of stock by the publicly traded company (or certain of its affiliates) from a stockholder of the company in exchange for money or other property (other than stock of the company itself), subject to a de minimis exception. Thus, the excise tax could apply to certain transactions that are not traditional stock repurchases. Regulatory guidance under the TCJA, the IRA, and such additional legislation is and continues to be forthcoming, and such guidance could ultimately increase or lessen the impact of these laws on our business and financial condition. In addition, it is uncertain if and to what extent various states will conform to the TCJA, the IRA and such additional legislation.
Current and future legislation designed to reduce prescription drug costs may affect the prices we and cost containment measures, legislative developments at the European Unionany collaborators may obtain for tamibarotene or member state level may result in significant additional requirements or obstacles that may increase our operating costs. any future product candidates.
Moreover, legislative and regulatory proposalsThe prices of prescription pharmaceuticals have also been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. We cannot be sure whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or whatsubject of considerable discussion in the impact of such changes on the marketing approvals of our product candidates, if any, may be. In addition, increased scrutiny by theUnited States. There have been several recent U.S. Congress of the FDA’s approval process may significantly delay or prevent marketing approval,congressional inquiries, as well as proposed and enacted state and federal legislation designed to, among other things, bring more transparency to pharmaceutical pricing, review the relationship between pricing and manufacturer patient programs, and reduce the costs of pharmaceuticals under Medicare and Medicaid. In 2020, President Trump issued several executive orders intended to lower the costs of prescription products and certain provisions in these orders have been incorporated into regulations. These regulations include an interim final rule implementing a most favored nation model for prices that would tie Medicare Part B payments for certain physician-administered pharmaceuticals to the lowest price paid in other economically advanced countries, effective January 1, 2021. That rule, however, has been subject usto a nationwide preliminary injunction and, on December 29, 2021, CMS issued a final rule to rescind it. With issuance of this rule, CMS stated that it will explore all options to incorporate value into payments for Medicare Part B pharmaceuticals and improve beneficiaries' access to evidence-based care.
In addition, in October 2020, the Department of Health and Human Services, or HHS, and the FDA published a final rule allowing states and other entities to develop a Section 804 Importation Program, to import certain prescription drugs from Canada into the United States. That regulation was challenged in a lawsuit by the Pharmaceutical Research and Manufacturers of America, or PhRMA, but the case was dismissed by a federal district court in February 2023 after the court found that PhRMA did not have standing to sue HHS. Nine states (Colorado, Florida, Maine, New Hampshire, New Mexico, North Dakota, Texas, Vermont and Wisconsin) have passed laws allowing for the importation of drugs from Canada. Certain of these states have submitted Section 804 Importation Program proposals and are awaiting FDA approval. On January 5, 2024, the FDA approved Florida’s plan for Canadian drug importation.
Further, on November 20, 2020, HHS finalized a regulation removing safe harbor protection for price reductions from pharmaceutical manufacturers to plan sponsors under Part D, either directly or through pharmacy benefit managers, unless the price reduction is required by law. The rule also creates a new safe harbor for price reductions reflected at the point-of-sale, as well as a safe harbor for certain fixed fee arrangements between pharmacy benefit managers and manufacturers.Pursuant to court order, the removal and addition of the aforementioned safe harbors were delayed and recent legislation imposed a moratorium on implementation of the rule until January 1, 2026. The IRA further delayed implementation of this rule to January 1, 2032.
More recently, on August 16, 2022, the IRA was signed into law by President Biden. The new legislation has implications for Medicare Part D, which is a program available to individuals who are entitled to Medicare Part A or enrolled in Medicare Part B to give them the option of paying a monthly premium for outpatient prescription drug coverage. Among other things, the IRA requires manufacturers of certain drugs to engage in price negotiations with Medicare (beginning in 2026), with prices that can be negotiated subject to a cap; imposes rebates under Medicare Part B and Medicare Part D to penalize price increases that outpace inflation (first due in 2023); and replaces the Part D coverage gap discount program with a new discounting program (beginning in 2025). The IRA permits the Secretary of HHS to implement many of these provisions through guidance, as opposed to regulation, for the initial years.
Specifically, with respect to price negotiations, Congress authorized Medicare to negotiate lower prices for certain costly single-source drug and biologic products that do not have competing generics or biosimilars and are reimbursed under Medicare Part B and Part D. CMS may negotiate prices for ten high-cost drugs paid for by Medicare Part D starting in 2026, followed by 15 Part D drugs in 2027, 15 Part B or Part D drugs in 2028, and 20 Part B or Part D drugs in 2029 and beyond. This provision applies to drug products that have been approved for at least 9 years and biologics that have been licensed for 13 years, but it does not apply to drugs and biologics that have been approved for a single rare disease or condition. Nonetheless, since CMS may establish a maximum price for these products in price negotiations, we would be fully at risk of government action if our products are the subject of Medicare price negotiations. Moreover, given the risk that could be the case, these provisions of the IRA may also further heighten the risk that we would not be able to achieve the expected return on our drug products or full value of our patents protecting our products if prices are set after such products have been on the market for nine years.
Further, the legislation subjects drug manufacturers to civil monetary penalties and a potential excise tax for failing to comply with the legislation by offering a price that is not equal to or less than the negotiated “maximum fair price” under the law or for taking price increases that exceed inflation. The legislation also requires manufacturers to pay rebates for drugs in Medicare Part D whose price increases exceed inflation. The new law also caps Medicare out-of-pocket drug costs at an estimated $4,000 a year in 2024 and, thereafter beginning in 2025, at $2,000 a year.
On June 6, 2023, Merck & Co. filed a lawsuit against HHS and CMS asserting that, among other things, the IRA’s Drug Price Negotiation Program for Medicare constitutes an uncompensated taking in violation of the Fifth Amendment of the Constitution. Subsequently, a number of other parties, including the U.S. Chamber of Commerce, Bristol Myers Squibb Company, PhRMA, Astellas, Novo Nordisk, Janssen Pharmaceuticals, Novartis, AstraZeneca and Boehringer Ingelheim, also filed lawsuits in various courts with similar constitutional claims against HHS and CMS. We expect that litigation involving these and other provisions of the IRA will continue, with unpredictable and uncertain results.
At the state level, legislatures are increasingly passing legislation and implementing regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. In addition, regional health care authorities and individual hospitals are increasingly using bidding procedures to determine what pharmaceutical products and which suppliers will be included in their prescription drug and other health care programs. These measures could reduce the ultimate demand for our products, once approved, or put pressure on our product pricing. We expect that additional state and federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, which could result in reduced demand for tamibarotene or any future collaborators to more stringent product labeling and post-marketing testing and other requirements.candidates or additional pricing pressures.
GovernmentsFinally, outside the United States, tend to impose strict price controls, which may adversely affect our revenues, if any.
Inin some countries, such as the countriesnations, including those of the European Union,EU, the pricing of prescription pharmaceuticals is subject to governmental control.control and access. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a product. To obtain reimbursement or pricing approval in some countries, we, or any futureour collaborators may be required to conduct a clinical trial that compares the cost-effectiveness of our product to other available therapies. If reimbursement of our products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, our business could be materially harmed.
We may be subject to certain healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm, fines, disgorgement, exclusion from participation in government healthcare programs, curtailment or restricting of our operations, and diminished profits and future earnings.
Healthcare providers, third-party payors and others will play a primary role in the recommendation and prescription of any products for which we obtain marketing approval. Our future arrangements with healthcare providers and third-party payors will expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute
any products for which we obtain marketing approval. Potentially applicable U.S. federal and state healthcare laws and regulations include the following:
Anti-Kickback Statute. The federal Anti-Kickback Statute prohibits, among other things, persons and entities from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under federal healthcare programs such as Medicare and Medicaid;
False Claims Laws. The federal false claims laws, including the civil False Claims Act, impose criminal and civil penalties, including those from civil whistleblower or qui tam actions against individuals or entities for knowingly presenting, or causing to be presented to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government;
HIPAA. The federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, imposes criminal and civil liability for executing or attempting to execute a scheme to defraud any healthcare benefit program;
HIPAA and HITECH. HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or the HITECH, Act, also imposes obligations on certain types of individuals and entities, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information;information. While these provisions likely will not apply to us directly, they will apply to many of our partners and other entities assisting with our clinical trials and future activities, and therefore may impact our relationships with these entities and related costs;
False Statements Statute. The federal false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services;
Transparency Requirements. The federal Physician Payments Sunshine Act requires certain manufacturers of drugs, devices, biologics, and medical supplies for which payment is available under Medicare, Medicaid, or the Children's Health Insurance Program, with specific exceptions, to report annually to the U.S. Department of Health and Human ServicesHHS information related to physician payments and other transfers of value, and physicianincluding ownership and investment interests;interests, to physicians and their family members; and
Analogous State and Foreign Laws. Analogous state laws and regulations, such as state anti-kickback and false claims laws, and transparency laws, may apply to sales or marketing arrangements, and claims involving healthcare items or services reimbursed by non-governmental third party payors, including private insurers, and some state laws require pharmaceutical companies to comply with the pharmaceutical industry's voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, in addition to requiring drug manufacturers to report information related to payments to physicians and other healthcare providers or marketing expenditures. Many state laws also govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. Foreign laws also govern the privacy and security of health information in many circumstances.
Efforts to ensure that our business arrangements with third parties, and our business generally, will comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, imprisonment, exclusion of products from government funded healthcare programs, such as Medicare and Medicaid, disgorgement, contractual damages, and reputational harm, any of which could substantially disrupt our operations. If any of the physicians or other providers or entities with whom we expect to do business is found not to be in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs.
Compliance with global privacy and data security requirements could result in additional costs and liabilities to us or inhibit our ability to collect and process data globally, and the failure to comply with such requirements could subject us to significant fines and penalties, which may have a material adverse effect on our business, financial condition or results of operations.
We are subject to data privacy and protection laws and regulations that apply to the collection, transmission, storage and use of personally identifying information, which among other things, impose certain requirements relating to the privacy, security and transmission of personal information, including comprehensive regulatory systems in the U.S., EU and United Kingdom. The legislative and regulatory landscape for privacy and data protection continues to evolve in jurisdictions worldwide, and there has been an increasing focus on privacy and data protection issues with the potential to affect our business. Failure to comply with any of these laws and regulations could result in enforcement action against us, including fines, claims for damages by affected individuals, damage to our reputation and loss of goodwill, any of which could have a material adverse effect on our business, financial condition, results of operations or prospects.
There are numerous U.S. federal and state laws and regulations related to the privacy and security of personal information. In particular, regulations promulgated pursuant to HIPAA establish privacy and security standards that limit the
use and disclosure of individually identifiable health information, or protected health information, and require the implementation of administrative, physical and technological safeguards to protect the privacy of protected health information and ensure the confidentiality, integrity and availability of electronic protected health information. Determining whether protected health information has been handled in compliance with applicable privacy standards and our contractual obligations can be complex and may be subject to changing interpretation. These obligations may be applicable to some or all of our business activities now or in the future.
If we are unable to properly protect the privacy and security of protected health information, we could be found to have breached our contracts. Further, if we fail to comply with applicable privacy laws, including applicable HIPAA privacy and security standards, we could face civil and criminal penalties. HHS enforcement activity can result in financial liability and reputational harm, and responses to such enforcement activity can consume significant internal resources. In addition, state attorneys general are authorized to bring civil actions seeking either injunctions or damages in response to violations that threaten the privacy of state residents. We cannot be sure how these regulations will be interpreted, enforced or applied to our operations. In addition to the risks associated with enforcement activities and potential contractual liabilities, our ongoing efforts to comply with evolving laws and regulations at the federal and state level may be costly and require ongoing modifications to our policies, procedures and systems.
States also are passing privacy laws that may impact our business operations. In 2018, California passed into law the California Consumer Privacy Act, or the CCPA, which took effect on January 1, 2020 and imposed many requirements on businesses that process the personal information of California residents. Many of the CCPA’s requirements are similar to those found in the General Data Protection Regulation, or the GDPR, including requiring businesses to provide notice to data subjects regarding the information collected about them and how such information is used and shared, and providing data subjects the right to request access to such personal information and, in certain cases, request the erasure of such personal information. The CCPA also affords California residents the right to opt-out of “sales” of their personal information. The CCPA contains significant penalties for companies that violate its requirements. In November 2020, California voters passed a ballot initiative for the California Privacy Rights Act, or the CPRA, which went into effect on January 1, 2023 and significantly expanded the CCPA to incorporate additional GDPR-like provisions including requiring that the use, retention, and sharing of personal information of California residents be reasonably necessary and proportionate to the purposes of collection or processing, granting additional protections for sensitive personal information, and requiring greater disclosures related to notice to residents regarding retention of information. The CPRA also created a new enforcement agency – the California Privacy Protection Agency – whose sole responsibility is to enforce the CPRA, which will further increase compliance risk. The provisions in the CPRA may apply to some of our business activities. These laws may impact our business activities, including our identification of research subjects, relationships with business partners and ultimately the marketing and distribution of our products.
In addition to California, eleven other states, have passed comprehensive privacy laws similar to the CCPA and CPRA. These laws are either in effect or will go into effect sometime before the end of 2026. Like the CCPA and CPRA, these laws create obligations related to the processing of personal information, as well as special obligations for the processing of “sensitive” data, which includes health data in some cases. Some of the provisions of these laws may apply to our business activities. There are also states that are strongly considering or have already passed comprehensive privacy laws during the 2024 legislative sessions that will go into effect in 2025 and beyond. Other states will be considering similar laws in the future, and Congress has also been debating passing a federal privacy law. There are also states that are specifically regulating health information that may affect our business. For example, the State of Washington passed the My Health My Data Act in 2023 which specifically regulated health information that is not otherwise regulated by the HIPAA rules, and the law also has a private right of action, which further increases the relevant compliance risk. Connecticut and Nevada have also passed similar laws regulating consumer health data, and more states are considering such legislation in 2024. These laws may impact our business activities, including our identification of research subjects, relationships with business partners and ultimately the marketing and distribution of our products.
Similar to the laws in the U.S., there are significant privacy and data security laws that apply in Europe and other countries. The collection, use, disclosure, transfer, or other processing of personal data, including personal health data, regarding individuals who are located in the European Economic Area, or the EEA, and the processing of personal data that takes place in the EEA, is regulated by the GDPR, which went into effect in May 2018 and which imposes obligations on companies that operate in our industry with respect to the processing of personal data and the cross-border transfer of such data. The GDPR imposes onerous accountability obligations requiring data controllers and processors to maintain a record of their data processing and policies. If our or our partners’ or service providers’ privacy or data security measures fail to comply with the GDPR requirements, we may be subject to litigation, regulatory investigations, enforcement notices
requiring us to change the way we use personal data and/or fines of Contentsup to 20 million Euros or up to 4% of the total worldwide annual turnover of the preceding financial year, whichever is higher, as well as compensation claims by affected individuals, negative publicity, reputational harm and a potential loss of business and goodwill.
The GDPR places restrictions on the cross-border transfer of personal data from the EU to countries that have not been found by the EC to offer adequate data protection legislation, such as the U.S. There are ongoing concerns about the ability of companies to transfer personal data from the EU to other countries. In July 2020, the Court of Justice of the European Union, or the CJEU, invalidated the EU-U.S. Privacy Shield, one of the mechanisms used to legitimize the transfer of personal data from the EEA to the U.S. The CJEU decision also drew into question the long-term viability of an alternative means of data transfer, the standard contractual clauses, for transfers of personal data from the EEA to the U.S. While we were not self-certified under the Privacy Shield, this CJEU decision may lead to increased scrutiny on data transfers from the EEA to the U.S. generally and increase our costs of compliance with data privacy legislation as well as our costs of negotiating appropriate privacy and security agreements with our vendors and business partners.
Following the CJEU decision, in October 2022, President Biden signed an executive order to implement the EU-U.S. Data Privacy Framework, which would serve as a replacement to the EU-US Privacy Shield. The European Union initiated the process to adopt an adequacy decision for the EU-U.S. Data Privacy Framework in December 2022, and the European Commission adopted the adequacy decision in July 2023. The adequacy decision permits U.S. companies who self-certify to the EU-U.S. Data Privacy Framework to rely on it as a valid data transfer mechanism for data transfers from the European Union to the United States. However, some privacy advocacy groups have already suggested that they will be challenging the EU-U.S. Data Privacy Framework. If these challenges are successful, they may not only impact the EU-U.S. Data Privacy Framework, but also further limit the viability of the standard contractual clauses and other data transfer mechanisms. The uncertainty around this issue has the potential to impact our business.
Following the withdrawal of the U.K. from the EU, the U.K. Data Protection Act 2018 applies to the processing of personal data that takes place in the U.K. and includes parallel obligations to those set forth by GDPR. In relation to data transfers, both the U.K. and the EU have determined, through separate “adequacy” decisions, that data transfers between the two jurisdictions are in compliance with the U.K. Data Protection Act and the GDPR, respectively. The U.K. and the U.S. have also agreed to a U.S.-U.K. “Data Bridge,” which functions similarly to the EU-U.S. Data Privacy Framework and provides an additional legal mechanism for companies to transfer data from the U.K. to the United States. In addition to the U.K., Switzerland is also in the process of approving an adequacy decision in relation to the Swiss-U.S. Data Privacy Framework (which would function similarly to the EU-U.S. Data Privacy Framework and the U.S.-U.K. Data Bridge in relation to data transfers from Switzerland to the United States). Any changes or updates to these developments have the potential to impact our business.
Beyond GDPR, there are privacy and data security laws in a growing number of countries around the world. While many loosely follow GDPR as a model, other laws contain different or conflicting provisions. These laws will impact our ability to conduct our business activities, including both our clinical trials and the sale and distribution of commercial products, through increased compliance costs, costs associated with contracting and potential enforcement actions.
While we continue to address the implications of the recent changes to data privacy regulations, data privacy remains an evolving landscape at both the domestic and international level, with new regulations coming into effect and continued legal challenges, and our efforts to comply with the evolving data protection rules may be unsuccessful. It is possible that these laws may be interpreted and applied in a manner that is inconsistent with our practices. We must devote significant resources to understanding and complying with this changing landscape. Failure to comply with laws regarding data protection would expose us to risk of enforcement actions taken by data protection authorities in the EEA and elsewhere and carries with it the potential for significant penalties if we are found to be non-compliant. Similarly, failure to comply with federal and state laws in the U.S. regarding privacy and security of personal information could expose us to penalties under such laws. Any such failure to comply with data protection and privacy laws could result in government-imposed fines or orders requiring that we change our practices, claims for damages or other liabilities, regulatory investigations and enforcement action, litigation and significant costs for remediation, any of which could adversely affect our business. Even if we are not determined to have violated these laws, government investigations into these issues typically require the expenditure of significant resources and generate negative publicity, which could harm our business, financial condition, results of operations or prospects.
We are subject to U.S. and foreign anti-corruption and anti-money laundering laws with respect to our operations and non-compliance with such laws can subject us to criminal and/or civil liability and harm our business.
We are subject to the U.S. Foreign Corrupt Practices Act of 1977, as amended, or the FCPA, the U.S. domestic bribery statute contained in 18 U.S.C. § 201, the U.S. Travel Act, the USA PATRIOT Act, and possibly other state and national anti-bribery and anti-money laundering laws in countries in which we conduct activities. Anti-corruption laws are interpreted broadly and prohibit companies and their employees, agents, third-party intermediaries, joint venture partners and collaborators from authorizing, promising, offering, or providing, directly or indirectly, improper payments or benefits to recipients in the public or private sector. We may have direct or indirect interactions with officials and employees of government agencies or government-affiliated hospitals, universities, and other organizations. In addition, we may engage third party intermediaries to promote our clinical research activities abroad and/or to obtain necessary permits, licenses, and other regulatory approvals. We can be held liable for the corrupt or other illegal activities of these third-party intermediaries, our employees, representatives, contractors, partners, and agents, even if we do not explicitly authorize or have actual knowledge of such activities.
We have adopted a Code of Business Conduct and Ethics that mandates compliance with the FCPA and other anti-corruption laws applicable to our business throughout the world. We cannot assure you, however, that our employees and third partythird-party intermediaries will comply with this code or such anti-corruption laws. Noncompliance with anti-corruption and anti-money laundering laws could subject us to whistleblower complaints, investigations, sanctions, settlements, prosecution, other enforcement actions, disgorgement of profits, significant fines, damages, other civil and criminal penalties or injunctions, suspension and/or debarment from contracting with certain persons, the loss of export privileges, reputational harm, adverse media coverage, and other collateral consequences. If any subpoenas, investigations, or other enforcement actions are launched, or governmental or other sanctions are imposed, or if we do not prevail in any possible civil or criminal litigation, our business, results of operations and financial condition could be materially harmed. In addition, responding to any action will likely result in a materially significant diversion of management's attention and resources and significant defense and compliance costs and other professional fees. In certain cases, enforcement authorities may even cause us to appoint an independent compliance monitor which can result in added costs and administrative burdens.
We are subject to governmental export and import controls that could impair our ability to compete in international markets due to licensing requirements and subject us to liability if we are not in compliance with applicable laws.
Our products and solutions are subject to export control and import laws and regulations, including the U.S. Export Administration Regulations, U.S. Customs regulations, and various economic and trade sanctions regulations administered by the U.S. Treasury Department's Office of Foreign Assets Controls. Exports of our products and solutions outside of the United States must be made in compliance with these laws and regulations. If we fail to comply with these laws and regulations, we and certain of our employees could be subject to substantial civil or criminal penalties, including the possible loss of export or import privileges; fines, which may be imposed on us and responsible employees or managers; and, in extreme cases, the incarceration of responsible employees or managers.
In addition, changes in our products or solutions or changes in applicable export or import laws and regulations may create delays in the introduction, provision, or sale of our products and solutions in international markets, prevent customers from using our products and solutions or, in some cases, prevent the export or import of our products and solutions to certain countries, governments or persons altogether. Any limitation on our ability to export, provide, or sell our products and solutions could adversely affect our business, financial condition and results of operations.
If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could harm our business.
We are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes. From time to time and in the future, our operations may involve the use of hazardous and flammable materials, including chemicals and biological materials, and may also produce hazardous waste products. Even if we contract with third parties for the disposal of these materials and waste products, we cannot completely eliminate the risk of contamination or injury resulting from these materials. In the event of contamination or injury resulting from the use or disposal of our hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. We
also could incur significant costs associated with civil or criminal fines and penalties for failure to comply with such laws and regulations.
We maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials, but this insurance may not provide adequate coverage against potential liabilities. We do not, however, maintain insurance for environmental liability or toxic tort claims that may be asserted against us.
In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. Current or future environmental laws and regulations may impair our research, development or production efforts. In addition, failure to comply with these laws and regulations may result in substantial fines, penalties or other sanctions.
Unfavorable global economic conditions could adversely affect our business, financial condition or results of operations.
Our results of operations could be adversely affected by general conditions in the global economy and in the global financial markets. The most recent global financial crisis caused extreme volatility and disruptions in the capital and credit markets, and it is unclear what impact the decision by the United Kingdom to leave the European Union will have on the global economy. A severe or prolonged economic downturn, such as the most recent global financial crisis, could result in a variety of risks to our business, including weakened demand for ourtamibarotene or any future product candidates and our ability to raise additional capital when needed on acceptable terms, if at all. A weak or declining economy could strain our suppliers, possibly resulting in supply disruption, or cause delays in payments for our services by third-party payors or our collaborators. Any of the foregoing could harm our business and we cannot anticipate all of the ways in which the current economic climate and financial market conditions could adversely impact our business.
Our internal computer systems, or those of ourany collaborators or other contractors or consultants, may fail or suffer security breaches, which could result in a material disruption of our product development programs.
OurDespite the implementation of security measures and certain data recovery measures, our internal computer systems and those of our current and any future collaborators and other contractors or consultants are vulnerable to damage from cyber-attacks, computer viruses, unauthorized access, sabotage, natural disasters, terrorism, war and telecommunication and electrical failures. While weWe have not experienced, any such materialand may experience in the future, security breaches of our information technology systems. Any system failure, accident or security breach to date, if such an event were to occur and causethat causes interruptions in our operations, itfor us or those third parties with which we contract, could result in a material disruption of our product development programs and our business operations, whether due to a loss of our trade secrets or other proprietary information or other similar disruptions.disruptions, in addition to possibly requiring substantial expenditures of resources to remedy. For example, the loss of clinical trial data from an ongoing, completed or future clinical trialstrial could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach were to resultresults in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we couldmay incur liability,liabilities, our competitive position could be harmed and the further development and commercialization of ourtamibarotene or any future product candidates may be delayed. In addition, we may not have adequate insurance coverage to provide compensation for any losses associated with such events.
We could be delayed.subject to risks caused by misappropriation, misuse, leakage, falsification or intentional or accidental release or loss of information maintained in the information systems and networks of our company, including personal information of our employees. In addition, outside parties have attempted, and may in the future attempt, to penetrate our systems or those of our vendors or fraudulently induce our employees or employees of our vendors to disclose sensitive information to gain access to our data. Like other companies, we may experience threats to our data and systems, including malicious codes and viruses, and other cyber-attacks. The number and complexity of these threats continue to increase over time. If a material breach of our security or that of our vendors occurs, the market perception of the effectiveness of our security measures could be harmed, we could lose business and our reputation and credibility could be damaged. We could be required to expend significant amounts of money and other resources to repair or replace information systems or networks. Although we develop and maintain systems and controls designed to prevent these events from occurring, and we have a process to identify and mitigate threats, the development and maintenance of these systems, controls and processes is costly and requires ongoing monitoring and updating as technologies change and efforts to overcome security measures become more sophisticated. Moreover, our ability to oversee and identify risks from cybersecurity threats associated with the use of third-party service providers is limited. Despite our efforts, the possibility of these events occurring to our internal computer systems or to those of the third parties on which we rely cannot be eliminated entirely. Additionally, the risk of cyber-attacks or other privacy or data security incidents may be heightened as a result of our moving increasingly towards a remote working environment as a result of public health outbreaks, which may be less secure and more susceptible to hacking attacks.
Our employees, independent contractors, CROs, consultants, commercial partners, vendors, and principal investigators may engage in misconduct or other improper activities, including non-compliance with regulatory standards and requirements, which could cause significant liability for us and harm our reputation.
We are exposed to the risk of employee fraud or other misconduct by our employees, independent contractors, CROs, consultants, commercial partners, vendors and principal investigators, including intentional failures to comply with FDA regulations or similar regulations of comparable foreign regulatory authorities, provide accurate information to the FDA or comparable foreign regulatory authorities, comply with manufacturing standards we have established, comply with federal and state healthcare fraud and abuse laws and regulations and similar laws and regulations established and enforced by comparable foreign regulatory authorities, report financial information or data accurately or disclose unauthorized activities to us. Employee misconductMisconduct could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. It is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws, standards or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business and results of operations, including the imposition of significant fines or other sanctions.
Risks Related to Our Business Operations, Employee Matters and Managing Growth
Public health epidemics or outbreaks, including COVID-19, have had, and may continue to have, an adverse impact on our business.
Public health crises such as pandemics, epidemics and outbreaks could adversely impact our business. For example, COVID-19 has impacted, and it or another public health epidemic or outbreak may impact in the future, our operations and those of our third-party partners. The ultimate impact of any such public health epidemic or outbreak will depend on future developments which are highly uncertain and cannot be predicted with confidence, including the scope, severity, duration and any recurrence of such pandemic, actions taken to contain the pandemic or mitigate its impact, the direct and indirect economic effects of the pandemic and containment measures, the effectiveness of vaccination and booster vaccination campaigns, work from home and return-to-work arrangements, compliance with governmental measures in connection with such pandemic, among others. Such pandemic or a similar public health epidemic or outbreak could adversely impact our ability to conduct clinical trials and our business generally and could have a material adverse impact on our operations and financial condition and results. In addition, a recession, depression or other sustained adverse market event resulting from COVID-19 or a similar public health epidemic or outbreak could materially and adversely affect our business and the value of our common stock.
Our future success depends on our ability to attract and retain key management and scientists, development, medical and commercial staff, consultants and advisors.
Our ability to compete in the biotechnology and pharmaceuticals industries depends upon our executive officers andability to attract and retain highly qualified managerial, scientific and motivate qualifiedmedical personnel.
We are highly dependent on the pharmaceutical research and development and business development expertise of Nancy Simonian, M.D.,Conley Chee, our president and chief executive officer; [insert name],Jason Haas, our chief financial officer; Eric R. Olson, Ph.D., our chief scientific officer; Gerald E. Quirk, Esq., our chief legal officer and administrative officer;head of business development; David A. Roth, M.D., our chief medical officer; and Jeremy P. Springhorn, Ph.D.,Kristin Stephens, our chief businessdevelopment officer. Each of our executive officers isare employed "at will," meaning any of them may terminate his or her employment with us at any time with or without notice and for any reason or no reason.
Our ability to compete in the biotechnology We also rely on consultants and pharmaceuticals industries depends upon our ability to attract and retain highly qualified managerial,advisors, including scientific and medical personnel. clinical advisors, to assist us in formulating our research and development and commercialization strategy.
Our industry has experienced a high rate of turnover of management, scientific, clinical, medical and commercial personnel in recent years. If we lose one or more of our executive officers or other key employees, our ability to implement our business strategy successfully could be seriously harmed. Replacing executive officers or other key employees may be difficult and may take an extended period of time because of the limited number of individuals in our industry with the breadth of skills and experience required to develop, gain marketing approval of and commercialize products successfully. Competition to hireWe face intense competition for qualified individuals from this limited pool is intense, and we may be unable to hire, train, retain or motivate these additional key employees on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies, for similar personnel. We also experience competition for the hiring of scientific and clinical personnel from universities, and research institutions.
We rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our research and development and commercialization strategy. Our consultants and advisors may be employed by othergovernmental entities and other research institutions, many of which have substantially greater resources with which to attract and reward qualified individuals than we do. In addition, due to the risks associated with developing a new class of medicine, we may have commitments under consulting or advisory contracts with those entities that may limit their availability to us.face additional challenges in attracting and retaining employees. If we are unable to continue to attract and retain highly qualified personnel, our ability to develop and commercialize ourtamibarotene or any future product candidates will be limited.
We expect to expand our organization, and as a result, we may encounter difficulties in managing our growth, which could disrupt our operations.
WeIf we receive marketing approval for tamibarotene, we expect to experience significant growth in the number of our employees and the scope of our operations, particularly in the areas of clinical development, drug manufacturing, regulatory affairs and sales, marketing and distribution. To manage these growth activities, we must continue to implement and improve our managerial, operational and financial systems expand our facilities and continue to recruit and train additional qualified personnel. Our management may need to devote a significant amount of its attention to managing these growth activities. Due to our limited financial resources and the limited experience of our management team in managing a company with such anticipated growth, we may not be able to effectively manage the expansion or relocation of our operations, retain key employees, or identify, recruit and train additional qualified personnel. Our inability to manage the expansion or relocation of our operations effectively may result in weaknesses in our infrastructure, give rise to operational mistakes, loss of business opportunities, loss of employees and reduced productivity among remaining employees. Our expected growth could also require significant capital expenditures and may divert financial resources from other projects, such as the development of additional product candidates. If we are unable to effectively manage our expected growth, our expenses may increase more than expected, our ability to generate revenues could be reduced and we may not be able to implement our business strategy, including the successful commercialization of ourtamibarotene or any future product candidates.
We may engage in acquisitions that could disrupt our business, cause dilution to our stockholders or reduce our financial resources.
In the future, we may enter into transactions to acquire other businesses, products or technologies. Because we have not made any acquisitions to date, our ability to do so successfully is unproven. If we do identify suitable candidates, we may not be able to make such acquisitions on favorable terms, or at all. Any acquisitions we make may not strengthen our competitive position, and these transactions may be viewed negatively by customers or investors. We may decide to incur debt in connection with an acquisition or issue our common stock or other equity securities to the stockholders of the acquired company, as we did in connection with our acquisition of Tyme Technologies, Inc. in 2022, which would reduce the percentage ownership of our existing stockholders. We could incur losses resulting from undiscovered liabilities of the acquired business that are not covered by the
indemnification we may obtain from the seller. In addition, we may not be able to successfully integrate the acquired personnel, technologies and operations into our existing business in an effective, timely and non-disruptive manner. Acquisitions may also divert management attention from day-to-day responsibilities, increase our expenses and reduce our cash available for operations and other uses. We cannot predict the number, timing or size of future acquisitions or the effect that any such transactions might have on our operating results.
Our operations or those of the third parties upon whom we depend might be affected by the occurrence of a catastrophic event, such as a terrorist attack, war or other armed conflict, geopolitical tensions or trade wars, pandemic or natural disaster.
We depend on our employees, consultants, CROs, as well as regulatory agencies and other parties, for the continued operation of our business. Despite any precautions that we or any third parties on whom we depend take for catastrophic events, including terrorist attacks, wars or other armed conflicts, geopolitical tensions or trade wars, pandemics or natural disasters, these events could result in significant disruptions to our research and development, manufacturing, preclinical studies, clinical trials, and, ultimately, if approved, the commercialization of tamibarotene or any other future products. Long-term disruptions in the infrastructure caused by these types of events, such as natural disasters, which are increasing in frequency due to the impacts of climate change, the outbreak of wars or other armed conflicts, the escalation of hostilities, geopolitical tensions or trade wars, acts of terrorism or “acts of God,” particularly involving geographies in which we or third parties on whom we depend have offices, manufacturing or clinical trial sites, could adversely affect our businesses. We cannot be certain what the overall impact of such events will be on our business or on the business of any third parties on whom we depend. Although we carry business interruption insurance policies and typically have provisions in our contracts that protect us in certain events, our coverage might not include or be adequate to compensate us for all losses that may occur. Any catastrophic event affecting us, our CROs, regulatory agencies or other parties with which we are engaged could have a material adverse effect on our operations and financial performance.
Risks Related to Our Common Stock
An active trading market for our common stock may not be sustained.
Our shares of common stock began trading on the Nasdaq Global Select Market on June 30, 2016. Given the limited trading history of our common stock, there is a risk that an active trading market for our shares will not be sustained, which could put downward pressure on the market price of our common stock and thereby affect the ability of our stockholders to sell their shares. An inactive trading market may also impair our ability to raise capital to continue to fund operations by selling shares and may impair our ability to acquire other companies or technologies by using our shares as consideration.
The price of our common stock is likely to be highly volatile, which could result in substantial losses for our stockholders.
Our stock price is likely to be highly volatile. The stock market in general and the market for smaller pharmaceutical and biotechnology companies in particular have experienced extreme volatility that has often been unrelated to the operating
performance of particular companies. As a result of this volatility, you may lose some or all of your investment. The market price for our common stock may be influenced by many factors, including:
| ·
| | the timing and results of clinical trials of SY-1425 and SY-1365;
|
| ·
| | the success of existing or new competitive products or technologies;
|
| ·
| | regulatory actions with respect to our product candidates or our competitors' productsthe timing and results of clinical trials of tamibarotene or any future product candidates;
|
| ·
| | announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures, collaborations or capital commitments;
|
| ·
| | commencement or termination of collaborations for our development programs;
|
| ·
| | failure or discontinuation of any of our development programs;
|
| ·
| | results of clinical trials of product candidates of our competitors;
|
| ·
| | regulatory or legal developments in the United States and other countries;
|
| ·
| | developments or disputes concerning patent applications, issued patents or other proprietary rights;
|
| ·
| | the recruitment or departure of key personnel;
|
| ·
| | the level of expenses related to any of our product candidates or clinical development programs;
|
| ·
| | the results of our efforts to develop additional product candidates or products;
|
| ·
| | actual or anticipated changes in estimates as to financial results or development timelines or recommendations by securities analysts;
|
| ·
| | announcement or expectation of additional financing efforts;
|
• | ·
| | sales of our common stock by us, our insiders or other stockholders;
|
regulatory actions with respect to tamibarotene or any future product candidates or our competitors' products and product candidates; | ·
| | • announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures, collaborations or capital commitments; •commencement or termination of collaborations for our research or development programs; •failure or discontinuation of any of our development programs; •results of clinical trials of product candidates of our competitors; •regulatory or legal developments in the United States and other countries; •developments or disputes concerning patent applications, issued patents or other proprietary rights; •the recruitment or departure of key personnel; •the level of expenses related to any of our product candidates or clinical development programs; •the results of our efforts to develop additional product candidates or products; •actual or anticipated changes in estimates as to financial results or development timelines or recommendations by securities analysts; •announcement or expectation of additional financing efforts; •sales of our common stock by us, our insiders or other stockholders; •variations in our financial results or those of companies that are perceived to be similar to us; |
| ·
| | changes in estimates or recommendations by securities analysts, if any, that cover our stock;
|
| ·
| | changes in the structure of healthcare payment systems;
|
| ·
| | market conditions in the pharmaceutical and biotechnology sectors;
|
| ·
| | general economic, industry and market conditions; and
|
| ·
| | the other factors described in this “Risk Factors” section.
|
Additionally, our stock price is likely to be volatile,similar to us;
•changes in estimates or recommendations by securities analysts, if any, that cover our stock;
•changes in the structure of healthcare payment systems;
•market conditions in the pharmaceutical and biotechnology sectors;
•general economic, industry and market conditions;
•actual or threatened public health emergencies and outbreaks of disease (including, for example, COVID-19); and
•the other factors described in this “Risk Factors” section and elsewhere in this Annual Report.
In the past, companies that have experienced volatility in the market price of their stock have been subject to an increased incidence of securities class action litigation. This risk is especially relevant for us because pharmaceutical companies have experienced significant stock price volatility in recent years. If we face such litigation, it could result in substantial costs and a diversion of management’s attention and resources, which could harm our business.
We are an “emerging growth company,” andmay also face other material adverse consequences due to volatility or a sustained decrease in the reduced disclosure requirements applicable to emerging growth companies may makeprice of our common stock, less attractivesuch as negative publicity, a decreased ability to investors.
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012, obtain additional financing, diminished investor and/or the JOBS Act, and may remain an emerging growth company for up to five years from the date of our initial public offering. For so long as we remain an emerging growth company, we are permitted and plan to rely on exemptions from certain disclosure requirements that are applicable to other public companies that are not emerging growth companies. These exemptions include not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, or SOX Section 404, not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor's report providing additional information about the auditemployee confidence, and the financial statements, reduced disclosure obligations regarding executive compensation and exemptions from the requirementsloss of holdingbusiness development opportunities, some or all of which may contribute to a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We cannot predict whether investors will find our common stock less attractive if we rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock andfurther decline in our stock price may be more volatile.price.
74
In addition, the JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. This allows an emerging growth company to delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, we will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
We must maintain effective internal control over financial reporting, and if we are unable to do so, the accuracy and timeliness of our financial reporting may be adversely affected, which could have a material adverse effect on our business and stock price.
We must maintain effective internal control over financial reporting in order to accurately and timely report our results of operations and financial condition. In addition, as a public company, the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, requires, among other things, that we assess the effectiveness of our disclosure controls and procedures quarterly and the effectiveness of our internal control over financial reporting at the end of each fiscal year.
The rules governing the standards that must be met for our management to assess our internal control over financial reporting pursuant to Section 404 of the Sarbanes-Oxley Act are complex and require significant documentation, testing and possible remediation. These standards require that our audit committee be advised and
regularly updated on management’s review of internal control over financial reporting. Our management may not be able to effectively and timely implement controls and procedures that comply with the increased regulatory compliance and reporting requirements that are applicable to us as a public company. If we fail to staff our accounting and finance function adequately or maintain internal control over financial reporting adequate to meet the demands that are placed upon us as a public company, including the requirements of the Sarbanes-Oxley Act, our business and reputation may be harmed and our stock price may decline.
We might not be able to utilize a significant portion of our net operating loss carryforwards and research and development tax credit carryforwards.
As of December 31, 2017,2023, we had federal and state net operating loss carryforwards of $136.4$348.7 million and $138.8$349.0 million, respectively, and federal and state research and development tax credit carryforwards of $6.1$10.6 million and $1.8$1.5 million, respectively, each of which if not utilized will expire at various dates through 2037.respectively. These net operating loss and tax credit carryforwards could expire unused and be unavailable to offset future income tax liabilities. Our net operating loss carryforwards generated before 2018 will generally expire at various dates through 2037 and our research and development tax credit carryforwards will generally expire at various dates through 2043.
As described above in “Changes in tax laws or in their implementation or interpretation may adversely affect our business and financial condition,” the TCJA, as amended by the CARES Act, includes changes to U.S. federal tax rates and the rules governing net operating loss carryforwards that have significantly impacted our ability to utilize our net operating losses to offset taxable income in the future.
In addition, the net operating loss and tax credit carryforwards are subject to review and possible adjustment by the Internal Revenue Service and state tax authorities. Furthermore, under SectionSections 382 and 383 of the Internal Revenue Code of 1986, as amended, and corresponding provisions of state law, if a corporation undergoes an "ownership change," which is generally defined as a cumulative change in ownership of significant shareholders of greater than 50% change,, by value, in its equity ownership over a three-year period, the corporation's ability to use its pre-change net operating loss carryforwards and other pre-changeresearch and development tax attributescredit carryforwards to offset its post-change income may be limited. We have not determined if we have experienced Section 382 ownership changesOur acquisition of Tyme Technologies, Inc. and concurrent private financing in the past and if a portion of our net operating loss and tax credit carryforwards are subject to an annual limitation under Section 382. In addition, we may experience ownership changesSeptember 2022 resulted in the future as a result of subsequent shifts in our stock ownership, some of which may be outside of our control. We have not conducted a detailed study to document whether our historical activities qualify to support the research and development credit carryforwards. A detailed study could result in adjustment to our research and development credit carryforwards. If we determine that an ownership change has occurredfor purposes of Section 382, and as a result our ability to use our historical net operating loss and tax credit carryforwards iswill be materially limited,limited. Such limitation, or ifany adjustments to our research and development carryforwards are adjusted, it wouldmade by the Internal Revenue Service or state tax authorities, could harm our future operating results by effectively increasing our future tax obligations.
The recently passed comprehensive tax reform bill could adversely affect our business and financial condition.
On December 22, 2017, President Trump signed into law new legislation that significantly revised the Internal Revenue Code of 1986, as amended. The newly enacted federal income tax law, among other things, contains significant changes to corporate taxation, including reduction of the corporate tax rate from a top marginal rate of 35% to a flat rate of 21%, limitation of the tax deduction for interest expense to 30% of adjusted earnings (except for certain small businesses), limitation of the deduction for net operating losses to 80% of current year taxable income and elimination of net operating loss carrybacks, one time taxation of offshore earnings at reduced rates regardless of whether they are repatriated, elimination of U.S. tax on foreign earnings (subject to certain important exceptions), immediate deductions for certain new investments instead of deductions for depreciation expense over time, and modifying or repealing many business deductions and credits. Notwithstanding the reduction in the corporate income tax rate, the overall impact of the new federal tax law is uncertain and our business and financial condition could be adversely affected. In addition, it is uncertain how various states will respond to the newly enacted federal tax law. The impact of this tax reform on holders of our common stock is also uncertain and could be adverse. We urge you to consult with your legal and tax advisors with respect to this legislation and the potential tax consequences of investing in or holding our common stock.
We do not anticipate paying any cash dividends on our capital stock in the foreseeable future. Accordingly, stockholders must rely on capital appreciation, if any, for any return on their investment.
We have never declared nor paid cash dividends on our capital stock. We currently plan to retain all of our future earnings, if any, to finance the operation, development and growth of our business. In addition, the terms of any future debt or credit agreements may precludeour term loan facility with Oxford precludes us from paying dividends.cash dividends to our stockholders without Oxford’s consent. As a result, capital appreciation, if any, of our common stock will be your sole source of gain for the foreseeable future.
Concentration of ownership of our common stock among our existing executive officers, directors and principal stockholders may prevent new investors from influencing significant corporate decisions.
Our executive officers and directors, combined with our stockholders who own more than 5% of our outstanding common stock and their affiliates, in the aggregate, beneficially own a majoritysignificant portion of our common stock. As a
result, if these stockholders were to choose to act together, they would be able to controlsubstantially influence all matters submitted to our stockholders for approval, as well as our management and affairs. For example, these persons, if they choose to act together, would controlsubstantially influence the election
of directors and approval of any merger, consolidation or sale of all or substantially all of our assets. This concentration of ownership control may:
| ·
| | delay, defer or prevent a change in control;
|
•delay, defer or prevent a change in control;
| ·
| | entrench our management or the board of directors; or
|
•entrench our management or the board of directors; or
| ·
| | impede a merger, consolidation, takeover or other business combination involving us that other stockholders may desire.
|
•impede a merger, consolidation, takeover or other business combination involving us that other stockholders may desire.
Provisions in our certificate of incorporation and bylaws and under Delaware law may prevent or frustrate attempts by our stockholders to change our management or hinder efforts to acquire a controlling interest in us.
Provisions in our certificate of incorporation and our bylaws may discourage, delay or prevent a merger, acquisition or other change in control of us that stockholders may consider favorable, including transactions in which you might otherwise receive a premium for your shares. These provisions could also limit the price that investors might be willing to pay in the future for shares of our common stock, thereby depressing the market price of our common stock. In addition, because our board of directors is responsible for appointing the members of our management team, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors. Among other things, these provisions:
| ·
| | establish a classified board of directors such that all members of the board are not elected at one time;
|
•establish a classified board of directors such that all members of the board are not elected at one time;
| ·
| | allow the authorized number of our directors to be changed only by resolution of our board of directors;
|
•allow the authorized number of our directors to be changed only by resolution of our board of directors;
| ·
| | limit the manner in which stockholders can remove directors from the board;
|
•limit the manner in which stockholders can remove directors from the board;
| ·
| | establish advance notice requirements for nominations for election to the board of directors or for proposing matters that can be acted on at stockholder meetings;
|
•establish advance notice requirements for nominations for election to the board of directors or for proposing matters that can be acted on at stockholder meetings;
| ·
| | require that stockholder actions must be effected at a duly called stockholder meeting and prohibit actions by our stockholders by written consent;
|
•require that stockholder actions must be effected at a duly called stockholder meeting and prohibit actions by our stockholders by written consent;
| ·
| | limit who may call a special meeting of stockholders;
|
•limit who may call a special meeting of stockholders;
| ·
| | authorize our board of directors to issue preferred stock without stockholder approval, which could be used to institute a "poison pill" that would work to dilute the stock ownership of a potential hostile acquirer, effectively preventing acquisitions that have not been approved by our board of directors; and
|
•authorize our board of directors to issue preferred stock without stockholder approval, which could be used to institute a "poison pill" that would work to dilute the stock ownership of a potential hostile acquirer, effectively preventing acquisitions that have not been approved by our board of directors; and
| ·
| | require the approval of the holders of at least 75% of the votes that all our stockholders would be entitled to cast to amend or repeal certain provisions of our certificate of incorporation or bylaws.
|
•require the approval of the holders of at least 75% of the votes that all our stockholders would be entitled to cast to amend or repeal certain provisions of our certificate of incorporation or bylaws.
Moreover, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the General Corporation Law of the State of Delaware, which prohibits a person who owns in excess of 15% of our outstanding voting stock from merging or combining with us for a period of three years after the date of the transaction in which the person acquired in excess of 15% of our outstanding voting stock, unless the merger or combination is approved in a prescribed manner. This could discourage, delay or prevent someone from acquiring us or merging with us, whether or not it is desired by, or beneficial to, our stockholders. This could also have the effect of discouraging others from making tender offers for our common stock, including transactions that may be in your best interests. These provisions may also prevent changes in our management or limit the price that investors are willing to pay for our stock.
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our share price and trading volume could decline.
The trading market for our common stock will likely depend in part on the research and reports that securities or industry analysts publish about us or our business. We do not have any control over these analysts. There can be no assurance that analysts will cover us, or provide favorable coverage. Securities or industry analysts may elect not to provide research coverage of our common stock, and such lack of research coverage may negatively impact the market price of our common
stock. In the event we do have analyst coverage, if one or more analysts downgrade our stock or change their opinion of our stock, our share price would likely decline. In addition, if one or more analysts cease coverage of our company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which could cause our share price or trading volume to decline.
None.Not applicable.
ITEM 1C. CYBERSECURITY
We have certain processes for assessing, identifying, and managing cybersecurity risks, which are built into our information technology function and are designed to help protect our information assets and operations from internal and external cyber threats, protect employee, customer and patient information from unauthorized access or attack, as well as secure our networks and systems. Such processes include physical, procedural, and technical safeguards, response plans, regular vulnerability testing on our systems, incident simulations and routine review of our policies and procedures to identify risks and adapt our practices. We engage certain external parties, including a managed security service provider, independent privacy assessors, computer security firms and risk management, peer companies, industry groups and governance experts, to ensure our cybersecurity oversight.
Our board of directors does not believe that there are currently any known risks from cybersecurity threats that are reasonably likely to materially affect the Company or its business strategy, results of operations or financial condition.
The Audit Committee of the board of directors provides direct oversight over cybersecurity risk and provides updates to the board of directors regarding such oversight. The Audit Committee receives quarterly updates from management regarding cybersecurity matters.
Our Senior Director of Information Technology leads the operational oversight of company-wide cybersecurity strategy, policy, standards, and processes and works across relevant departments to assess and help prepare us and our employees to maintain awareness of cybersecurity risks, including email, web, and data security. The Senior Director of Information Technology has over 25 years of experience designing, implementing, and running information technology and cybersecurity programs and processes using the National Institute of Standards and Technology, or NIST, Framework.
To deter and detect cyber threats, we annually provide all employees, including part-time and temporary employees, with a data protection, cybersecurity and incident response and prevention training and compliance program, which covers timely and relevant topics, including social engineering, phishing, password protection, confidential data protection, asset use and mobile security, and educates employees on the importance of reporting all incidents immediately. To mitigate cybersecurity risks and bolster our employee-based cybersecurity programs, we monitor all user traffic and restrict access by country. In addition, access to web sites is filtered and monitored, and credentials for systems and services require multi-factor authentication whenever possible.
ITEM 2. PROPERTIES
We currently occupy approximately 21,48852,859 rentable square feet of office and laboratory space in Cambridge, Massachusetts under a lease that expires on October 31, 2020.in February 2030. We have an option to extend the lease term for five10 additional years. We believe that thisour office and laboratory space is sufficient to meet our current needs and that suitable additional space will be available as and when needed.
ITEM 3. LEGAL PROCEEDINGS
None.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS, AND ISSUER PURCHASES OF EQUITY SECURITIES
Certain Information Regarding the Trading of Our Common Stock
Our common stock trades under the symbol “SYRS” on the Nasdaq Global Select Market and has been publicly traded since June 30, 2016. Prior to this time, there was no public market for our common stock The following table sets forth the high and low sales price of our common stock as reported on the Nasdaq Global Select Market for the periods indicated:
| | | | | | | |
| | High | | Low | |
Year ended December 31, 2016: | | | | | | | |
Second Quarter (beginning June 30, 2016) | | $ | 19.80 | | $ | 14.58 | |
Third Quarter | | $ | 21.50 | | $ | 8.16 | |
Fourth Quarter | | $ | 16.85 | | $ | 11.31 | |
Year ended December 31, 2017: | | | | | | | |
First Quarter | | $ | 16.74 | | $ | 10.22 | |
Second Quarter | | $ | 19.22 | | $ | 13.27 | |
Third Quarter | | $ | 24.38 | | $ | 11.21 | |
Fourth Quarter | | $ | 17.62 | | $ | 6.30 | |
stock.
Holders of Our Common Stock
As of February 28, 2018,March 6, 2024, there were approximately 6173 holders of record of shares of our common stock. This number does not include stockholders for whom shares are held in “nominee” or “street” name.
Dividend Policy
We have never declared nor paid cash dividends on our common stock. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business. We do not intend to pay cash dividends in respect of our common stock in the foreseeable future. Any future determination to pay cash dividends will be made at the discretion of our board of directors and will depend on restrictions and other factors our board of directors may deem relevant. Investors should not purchase our common stock with the expectation of receiving cash dividends.
Stock Performance Graph
The following performance graph and related information shall not be deemed to be “soliciting material” or to be “filed” with the Securities and Exchange Commission, or SEC, for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or the Exchange Act, nor shall such information be incorporated by reference into any future filing under the Exchange Act or Securities Act of 1933, as amended, or the Securities Act, except to the extent that we specifically incorporate it by reference into such filing.
The following graph compares the performance of our common stock to the Nasdaq Composite Index and to the Nasdaq Biotechnology Index from June 30, 2016 (the first date that shares of our common stock were publicly traded) through December 29, 2017, which was the last trading day of the year. The comparison assumes $100 was invested in our common stock and in each of the foregoing indices after the market closed on June 30, 2016, and it assumes reinvestment of dividends, if any. The stock price performance included in this graph is not necessarily indicative of future stock price performance.
Use of Proceeds from Registered Securities
On July 6, 2016, we closed our initial public offering, or our IPO, in which we issued and sold 4,600,000 shares of our common stock at a public offering price of $12.50 per share, including 600,000 shares of common stock sold pursuant to the underwriters’ exercise of their option to purchase additional shares of common stock, for aggregate gross proceeds of $57.5 million. All of the shares issued and sold in the IPO were registered under the Securities Act pursuant to a Registration Statement on Form S-1 (File No. 333-211818), which was declared effective by the SEC on June 29, 2016. Cowen and Company, LLC and Piper Jaffray & Co. acted as joint book-running managers of the offering and as representatives of the underwriters. JMP Securities LLC and Wedbush Securities Inc. acted as co-managers for the offering. The offering commenced on June 29, 2016 and did not terminate until the sale of all of the shares offered.
The net offering proceeds to us, after deducting underwriting discounts and offering expenses payable by us totaling $7.6 million, were approximately $49.9 million. No offering expenses were paid directly or indirectly to any of our directors or officers (or their associates) or persons owning 10.0% or more of any class of our equity securities or to any other affiliates.
As of December 31, 2017, we estimate that we have used all of the net proceeds from the IPO to fund manufacturing and clinical development activities for SY-1425, IND-enabling studies and manufacturing activities for SY-1365, and other research activities in support of our preclinical programs and gene control platform, and for working capital and other general corporate purposes.
ITEM 6. SELECTED CONSOLIDATED FINANCIAL DATARESERVED
The following selected consolidated financial data should be read together with our financial statements and the related notes appearing at the end of this Annual Report on Form 10-K and Item 7 “Management’s Discussion and Analysis of Financial Condition and Results of Operations” of this Annual Report on Form 10-K. We have derived the consolidated statement of operations data for the years ended December 31, 2017, 2016 and 2015 and the consolidated balance sheet data as of December 31, 2017 and 2016 from our audited consolidated financial statements included elsewhere in this Annual Report on Form 10-K. We have derived consolidated statement of operations data for the years ended December 31, 2014 and 2013, and consolidated balance sheet data as of December 31, 2015 and 2014 from our audited consolidated financial statements and related notes not included in this Annual Report on Form 10-K. The selected historical financial information in this section is not intended to replace our financial statements and the related notes thereto. Our historical results for any prior period are not necessarily indicative of results to be expected in any future period.
| | | | | | | | | | | | | | | |
| | Year ended December 31 |
| | 2017 | | 2016 | | 2015 | | 2014 | | 2013 |
| | (in thousands, except for per share data) |
Statements of operations data: | | | | | | | | | | | | | | | |
Revenue | | $ | 1,101 | | $ | 317 | | $ | 317 | | $ | — | | $ | — |
Operating expenses: | | | | | | | | | | | | | | | |
Research and development | | | 41,896 | | $ | 37,817 | | $ | 24,408 | | | 10,923 | | | 6,266 |
General and administrative | | | 13,891 | | $ | 10,463 | | $ | 5,729 | | | 2,512 | | | 2,367 |
Total operating expenses | | | 55,787 | | | 48,280 | | | 30,137 | | | 13,435 | | | 8,633 |
Loss from operations | | | (54,686) | | | (47,963) | | | (29,820) | | | (13,435) | | | (8,633) |
Other income (expense), net | | | 676 | | $ | 220 | | $ | 2 | | | 4 | | | (32) |
Net loss | | $ | (54,010) | | $ | (47,743) | | $ | (29,818) | | $ | (13,431) | | $ | (8,665) |
Net loss per share applicable to common stockholders - basic and diluted (1) | | $ | (2.13) | | $ | (4.05) | | $ | (17.55) | | $ | (10.26) | | $ | (8.45) |
| | | | | | | | | | | | | | | |
Weighted-average number of common shares used in net loss per share applicable to common stockholders - basic and diluted (1) | | | 25,406,845 | | | 12,696,414 | | | 1,980,286 | | | 1,525,018 | | | 1,095,973 |
Not applicable.
| | | | | | | | | | | | |
| | | As of December 31, |
| | 2017 | | 2016 | | 2015 | | 2014 |
| | | (in thousands) |
Balance sheet data: | | | | | | | | | | | | |
Cash, cash equivalents and marketable securities | | $ | 72,049 | | $ | 83,593 | | $ | 35,909 | | $ | 60,393 |
Working capital (2) | | | 60,746 | | | 75,941 | | | 28,493 | | | 59,291 |
Total assets | | | 78,488 | | | 91,323 | | | 43,631 | | | 61,494 |
Convertible preferred stock (3) | | | — | | | — | | | 82,013 | | | 82,013 |
Total stockholders’ equity (deficit) | | | 65,324 | | | 80,602 | | | (47,964) | | | (21,772) |
| (1)
| | See Note 2 to our consolidated financial statements for a description of the method used to calculate basic and diluted net loss per share as well as the weighted-average number of common shares used in the calculation of the per share amounts.
|
| (2)
| | We define working capital as current assets less current liabilities. See our consolidated financial statements for further details regarding our current assets and current liabilities.
|
| (3)
| | On July 6, 2016, upon the closing of our IPO, all of the then-outstanding shares of our convertible preferred stock converted into 15,988,800 shares of common stock.
|
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our audited financial statements and related notes appearing elsewhere in this Annual Report on Form 10-K.10-K, or Annual Report. Some of the information contained in this discussion and analysis and set forth elsewhere in this Annual Report, including information with respect to our plans and strategy for our business, includes forward-looking statements that involve risks and uncertainties. You should review the section titled “Risk Factors” in Part I, Item 1A of this Annual Report for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Overview
We are a biopharmaceutical company pioneering an understandingcommitted to developing new standards of care for the non-coding regulatory regionfrontline treatment of patients with hematologic malignancies. Driven by the genome controlling the activation and repression of genes. Our goal ismotivation to advancehelp patients with blood disorders that have largely eluded other targeted approaches, we are advancing tamibarotene, a new wave of medicines to control the expression of genes. We have builtselective retinoic acid receptor alpha, or RARa, agonist for which we are conducting SELECT-MDS-1, a proprietary gene control platform designed to systematically and efficiently analyze this unexploited region of DNAPhase 3 clinical trial evaluating tamibarotene in human disease tissue to identify novel targets linked tocombination with azacitidine in a genomically defined patient populationssubset of patients with higher-risk myelodysplastic syndrome, or HR-MDS, and to develop drugs against those targets based on our expertisefor which we are conducting SELECT-AML-1, a randomized Phase 2 clinical trial evaluating tamibarotene in transcriptional chemistry. Because gene expression is fundamental to the functioncombination with venetoclax and azacitidine in a genomically defined subset of all cells, we believe that our gene control platform has broad potential to create medicines that achieve profound and durable benefit across therapeutic areas and a range of diseases. Wenewly diagnosed patients with acute myeloid leukemia, or AML, who are currently focused on developing treatmentsnot suitable candidates for cancer and diseases resulting from modifications of a single gene, also known as monogenic diseases, and are building a pipeline of gene control medicines.standard intensive chemotherapy.
Our lead product candidates are:
| ·
| | SY-1425, a selective retinoic acid receptor alpha, or RARα, agonist that is being evaluated in combination with azacitidine, a hypomethylating agent frequently used to treat acute myeloid leukemia, or AML, and myelodysplastic syndrome, or MDS, patients, and with daratumumab, an anti-CD38 therapeutic antibody approved to treat multiple myeloma, in a Phase 2 clinical trial in genomically defined subsets of patients with AML and MDS; and
|
| ·
| | SY-1365, a selective inhibitor of cyclin-dependent kinase 7, or CDK7, in a Phase 1 clinical trial in patients with advanced solid tumors for which expansions in ovarian and breast cancer are planned.
|
We also have multiple programs in earlier stages of research and development in oncology, including immuno-oncology, and monogenic diseases. Our goal is to build a fully integrated biopharmaceutical company based on our leadership position in gene control.
At the 59th62nd American Society of Hematology Annual Meeting and Exposition held in December 2017, or ASH 2017,2020, we presented clinical data from our fully enrolled Phase 2 clinical trial evaluating SY-1425 as a single agent in defined subsets of AML and MDS patients with our proprietary RARA and IRF8 biomarkers. In the trial, we observed that chronic daily dosing of SY-1425 administered at 6 mg/m2 orally divided in two doses was generally well-tolerated and that clinical and biological activity was observed in patients enrolled in the trial. Specifically, clinical activity was observed in ten of 23 (43%) evaluable patients with relapsed or refractory AML and higher-risk MDS, including improvement in blood counts, reduction in leukemic blasts and one bone marrow complete response. Thirteen of the 23 (57%) evaluable relapsed or refractory AML and higher-risk MDS patients had stable disease. Myeloid differentiation was also observed in the bone marrow, consistent with the underlying mechanism of action of SY-1425 as a differentiating agent. Induction of CD38, a marker of cell differentiation, was observed after one 28-day cycle of treatment in 11 of 13 (85%) patients with pre- and post-treatment immunophenotyping samples.
We believe that these clinical data, when combined with preclinical data showing the tumor-killing activity of SY-1425 in combination with azacitidine and with daratumumab support the ongoing development of SY-1425 as a combination agent. SY-1425 has shown synergistic tumor-killing activity in combination with azacitidine as well as with daratumumab in preclinical models of RARA biomarker-positive AML. In combination with azacitidine, SY-1425 demonstrated greater clearance of tumor cells in bone marrow and other tissues and greater depth and duration of tumor response in preclinical models, compared to either azacitidine or SY-1425 alone. In combination with daratumumab, SY-1425 induced robust immune cell-mediated tumor death in vitro. Notably, AML cells do not normally express high
levels of CD38. We have shown that by inducing CD38 expression, SY-1425 sensitizes biomarker-positive AML models to the tumor-killing effects of daratumumab.
Our ongoing Phase 2 clinical trial is assessing the safety and efficacy of SY-1425tamibarotene in combination with azacitidine in approximately 25 newly diagnosed AML patients who are not suitable candidates for standard intensive chemotherapy, andas well as in combination with daratumumab in approximately 12 relapsed or refractory AML and higher-risk MDS patients. All patients enrolled or to be enrolled in the trialwho have been or will be prospectively selected using our proprietary RARA, the gene that codes for RARa, biomarker. As of an October 1, 2020 data cut-off, 51 newly diagnosed unfit AML patients, including patients with and without RARA gene overexpression, were eligible for a safety analysis. Among these patients, tamibarotene in combination with azacitidine was generally well-tolerated, with no evidence of increased toxicity relative to either as a single agent, including rates of myelosuppression that were comparable to single agent azacitidine. As of the data cut-off, of the 18 patients with RARA overexpression that were evaluable for clinical response, 50% of patients achieved complete response, or IRF8 biomarkers.CR, and 11% achieved a complete response with incomplete blood count recovery, or CRi, for a total CR/CRi rate of 61%. The median time to initial CR/CRi response was 1.2 months, the median duration of CR/CRi response was 10.8 months, and the median overall survival, or OS, among patients who achieved a CR or CRi was 18.0 months. As of the data cut-off, of the 28 patients without RARA overexpression that were evaluable for clinical response, the overall response rate was 43%, with a CR/CRi rate of 32%, with 25% of patients achieving CR and 7% achieving CRi. The median time to initial CR/CRi response was 3.0 months, and the median duration of CR/CRi response was 10.3 months. Approximately 25,000 patients are diagnosed with unfit AML in the United States and Europe annually and we expect the overall total global market for all AML patients to grow to approximately $7.5 billion by 2028.
Based on these data and our assessment of ongoing areas of high unmet need, we advanced tamibarotene in combination with azacitidine into a registration-enabling Phase 3 clinical trial in newly diagnosed HR-MDS patients with RARA overexpression, which we refer to as SELECT-MDS-1. HR-MDS is a hematologic malignancy that is closely related to AML, and we believe that approximately 50% of HR-MDS patients overexpress RARA. We believe that approximately 18,500 patients are diagnosed with HR-MDS in the United States and Europe annually and we expect the total global market for myelodysplastic syndrome, or MDS, patients of all risk groups to grow to approximately $4.7 billion by 2028. The SELECT-MDS-1 trial is evaluating newly diagnosed HR-MDS patients with RARA overexpression in a double-blind placebo-controlled study design, randomized 2:1 to receive tamibarotene in combination with azacitidine, or placebo in combination with azacitidine, respectively. The primary efficacy endpoint is based on 190 patients to provide over 90% power to detect a difference in CR rates between the experimental and control arms with a one-sided alpha of 0.025. The United States Food and Drug Administration, or FDA, has expressed that the CR rate is an acceptable efficacy endpoint for either full or accelerated approval for treatment of newly diagnosed HR-MDS with supporting data on durability of remission. Informed by feedback from the FDA, we amended the SELECT-MDS-1 clinical trial protocol in March 2023 to include a total of approximately 550 patients to enable us to assess OS as a key secondary endpoint, which could allow the trial to serve as a confirmatory study if needed to convert an accelerated approval to a full approval in the future. The amended clinical trial protocol is designed with 80% power to detect a difference in OS rates for the key secondary endpoint between the experimental and control arms, also with a one-sided alpha of 0.025. In January 2023, the FDA granted Fast Track Designation to tamibarotene in combination with azacitidine for the treatment of adults with newly diagnosed HR-MDS who are positive for RARA overexpression. In the first quarter of 2024, we completed enrollment of the 190 patients necessary to support the CR primary endpoint analysis, and we expect to report pivotal CR data from the SELECT-MDS-1 trial by the middle of the fourth quarter of 2024.
In addition, we are advancing tamibarotene in combination with venetoclax and azacitidine in newly diagnosed unfit AML patients who are positive for RARA overexpression. Our ongoing Phase 2 clinical trial, known as SELECT-AML-1, included a single-arm safety lead-in to confirm the dosing regimen of the triplet to be used in the randomized portion of the trial, which is evaluating the safety and efficacy of tamibarotene in combination with venetoclax and azacitidine compared to venetoclax and azacitidine in approximately 80 patients randomized 1:1. The trial is also evaluating the triplet as a salvage strategy for patients in the control arm who do not respond to venetoclax and azacitidine. The primary endpoint of the trial is the CR/CRi rate and the study is powered at 80% to detect a difference between the experimental and control arms. In December 2017,2022, we reported data from the safety lead-in portion of SELECT-AML-1. As of the data cut-off, eight newly diagnosed, unfit patients who were positive for RARA overexpression had been enrolled in the trial, including six who were evaluable for response. In this population, tamibarotene in combination with venetoclax and azacitidine administered at approved doses showed no evidence of increased toxicity relative to the doublet combination of venetoclax and azacitidine. This includes rates of myelosuppression which were comparable to reports with venetoclax and azacitidine in this population. Among these patients, the CR/CRi rate was 83%, consisting of two patients (33%) who achieved a CR and three patients (50%) who achieved a CRi. These data supported our decision to initiate the randomized portion of the SELECT-AML-1 trial.
On December 6, 2023, we announced initial data from the randomized portion of SELECT-AML-1. As of November 13, 2023, 23 newly diagnosed unfit AML patients positive for RARA overexpression had enrolled in the randomized portion of the trial, including 19 who were evaluable for response. The CR/CRi rate was 100% among response evaluable patients (nine of nine) treated with the combination of tamibarotene, venetoclax and azacitidine, as compared to 70% of patients (seven of ten) treated with the control arm of venetoclax and azacitidine. Seven of the nine response evaluable patients (78%) treated with the combination of tamibarotene, venetoclax and azacitidine achieved a CR and two patients (22%) achieved a CRi. Three of the ten response evaluable patients (30%) treated with the control achieved a CR and four patients (40%) achieved a CRi. The median time to CR/CRi response was 21 days (ranging from 14-28) among patients treated with the combination of tamibarotene, venetoclax and azacitidine, as compared to 25 days (ranging from 17-56) among patients treated with the control, with the CR/CRi being reached by 100% of patients in the triplet arm by the end of cycle one, compared with 60% of patients in the doublet control arm. Consistent with prior clinical experience from the safety lead-in portion of this study, tamibarotene administered in combination with approved doses of venetoclax and azacitidine was generally well tolerated, and the overall safety profile demonstrated no additive toxicities or new safety signals, or evidence of increased myelosuppression compared to treatment with the doublet combination of venetoclax and azacitidine. The majority of non-hematologic adverse events were low-grade and reversible, and rates of serious adverse events were comparable between the study arms. As of the data cut-off, there was comparable exposure across the treatment arms, consisting of 66 days (ranging from 8-188) among patients treated with the combination of tamibarotene, venetoclax and azacitidine, and 75 days (ranging from 7-227) for patients treated with the control. Patients will be followed for duration of response, minimal residual disease-negative response, and survival. We continue to enroll patients in SELECT-AML-1 and anticipate reporting additional data from the trial in 2024. There can be no guarantee that this additional data will replicate the results of the initial data that was reported in December 2023.
Financings
On July 3, 2022, we entered into an Agreement and Plan of Merger, or the Merger Agreement, with Tack Acquisition Corp., a Delaware corporation and a wholly-owned subsidiary of us, or the Merger Sub, and Tyme Technologies, Inc., a Delaware corporation, or Tyme, providing for the merger of the Merger Sub with and into Tyme, with Tyme surviving the merger as our wholly-owned subsidiary, or the Merger. In connection with the closing of the Merger on September 16, 2022, we acquired net cash, cash equivalents and marketable securities of $67.1 million, before deducting severance costs and other commitments entered into by Tyme management prior to the consummation of the Merger of approximately $4.5 million.
Also on July 3, 2022, immediately prior to the execution and delivery of the Merger Agreement, we entered into a clinical supply agreementSecurities Purchase Agreement with Janssen Research and Development, LLC, or Janssen,certain accredited investors, pursuant to which Janssenthe investors agreed to supply us daratumumab for usepurchase shares of our common stock and/or pre-funded warrants to purchase shares of our common stock, and accompanying warrants to purchase additional shares of our common stock (or pre-funded warrants in lieu thereof), or the trial. We are no longer dosing patients inPIPE Financing.
On September 16, 2022, the cohortsPIPE Financing closed concurrently with the Merger. At the closing of the trial in which SY-1425 was being evaluated as a single agent. We expect to report initial clinical data from the combination cohorts of the trial in the fourth quarter of 2018.
We are continuing to dose patients in the dose-escalation phase of our ongoing Phase 1 clinical trial of SY-1365 and expect to report data from this phase of the trial in the fourth quarter of 2018. Once a maximum tolerated dose is reached in the dose escalation phase of the Phase 1 clinical trial and the recommended dosing schedule is identified,Merger, we intend to open expansion cohorts evaluating SY-1365 in multiple ovarian cancer populations as a single agent as well as in combination with carboplatin, a chemotherapeutic agent. The ovarian cancer populations include a 24-patient cohort evaluating SY-1365 as a single agent in patients who have relapsed after three or more prior therapies, a 24-patient cohort evaluating SY-1365 in combination with carboplatin in patients who relapsed after one or more prior therapies but who may still benefit from additional platinum-based treatment, and a 12-patient pilot cohort evaluating SY-1365 as a single agent in primary platinum-refractory disease. We also plan to evaluate SY-1365 in combination with fulvestrant, a hormonal medicine, in 12 patients with hormone-receptor positive, HER-2 negative metastatic breast cancer who have progressed after treatment with a CDK4/6 inhibitor plus an aromatase inhibitor. We also plan to evaluate the mechanism of action of SY-1365 in ten patients with any solid tumor accessible for biopsies. We expect that the expansion cohorts in our Phase 1 clinical trial will be open to enrollment in mid-2018.
We currently have five programs in our preclinical and discovery pipeline, including preclinical programs directed to the development of a CDK7 inhibitor that can be administered orally, inhibitors of cyclin-dependent kinase 12/13, and inhibitors of an immuno-oncology target, as well as discovery programs related to a gene control target to treat sickle cell disease and in the field of cancer. We plan to nominate a development candidate from one of these programs during 2018 that we can advance into preclinical studies to support a potential investigational new drug application, or IND, filing in 2019. We have and are continuing to use our gene control platform in collaboration with third parties to identify and validate targets in diseases beyond our current areas of focus. To this end, we entered a target discovery, research collaboration and option agreement with Incyte Corporation, or Incyte, in January 2018 under which we will use our platform to identify novel therapeutic targets with a focus on myeloproliferative neoplasms.
Recent Developments
Incyte Corporation
In January 2018, we entered into a target discovery, research collaboration and option agreement with Incyte. Under this agreement, we will use our gene control platform to identify novel therapeutic targets with a focus on myeloproliferative neoplasms, and Incyte will receive options to obtain exclusive worldwide rights to intellectual property resulting from the collaboration for the development and commercialization of therapeutic products directed to up to seven validated targets. Incyte will have exclusive worldwide rights to develop and commercialize any therapies under the collaboration that modulate those validated targets.
Under the terms of the collaboration agreement, Incyte paid us $10.0 million in up-front consideration, consisting of $2.5 million in cash and $7.5 million in pre-paid research funding. Our activities under this agreement are subject to a joint research plan and, subject to certain exceptions, Incyte will be responsible for funding our activities under the research plan, including amounts in excess of the pre-paid research funding amount. We are eligible to receive target selection milestone payments and option exercise fees of up toissued an aggregate of $54.0 million if Incyte selects the maximum number of targets for validation and exercises its option to obtain exclusive rights to collaboration intellectual property for therapeutic products directed to all seven validated targets. Should any therapeutic product be developed by Incyte against a target as to which Incyte has exercised its option to obtain exclusive rights to collaboration intellectual property, we will be eligible to receive milestone payments and, if approved and commercialized, royalty payments from Incyte. For each of the seven validated targets, we would become eligible to receive from Incyte a total of up to $50.0
million in development and regulatory milestone payments. If products arising from the collaboration are approved, we would become eligible to receive from Incyte, for each validated target, a total of up to $65.0 million in commercial milestone payments. Upon approval and commercialization of any therapeutic product resulting from the collaboration, we would become eligible to receive low single-digit royalties on net sales of such product.
In connection with the collaboration agreement, we sold 793,0217,546,014 shares of our common stock to Incyte forTyme stockholders. In the PIPE Financing, we issued an aggregate purchase price of $10.0 million in cash, or $12.61 per share, in a private placement. In addition, from the closing of this sale until the earlier of the second anniversary of such closing or the expiration or termination of the collaboration agreement, we have granted to Incyte the right to purchase up to its pro rata share of the securities offered in certain subsequent offerings of our common stock or common stock equivalents, subject to the terms and conditions set forth in the stock purchase agreement. In February 2018, we sold 144,505 additional6,387,173 shares of our common stock and, in lieu of shares to Incyte at a pricecertain investors, pre-funded warrants to purchase an aggregate of $9.55 per share, resulting7,426,739 shares of common stock, and, in each case, accompanying warrants to purchase an aggregate of up to 13,813,912 additional shares of common stock (or pre-funded warrants to purchase common stock in lieu thereof). We received aggregate gross proceeds tofrom the PIPE Financing of $129.9 million, before deducting estimated offering expenses payable by us not inclusive of $1.4 million.any exercise of the warrants.
On January 30, 2018,December 21, 2023, we issued and sold an aggregate of 4,188,4814,939,591 shares of our common stock in a public offering at a price of $4.42 per share, $9.55and, in lieu of our common stock to certain investors who so chose, pre-funded warrants to purchase an aggregate of 5,242,588 shares of our common stock at a price of $4.419 per share,pre-funded warrant, in an underwritten offering resulting in gross proceeds of $40.0approximately $45.0 million, before deducting underwriting commissionsfees and fees estimatedother transaction costs of approximately $3.2 million. The offering was made pursuant to be approximately $2.7 million. Additionally,an underwriting agreement between us and Cowen and Company, LLC and Piper Sandler & Co. on February 2, 2018,December 18, 2023. Pursuant to the underwriting agreement, the underwriters exercised their option to purchase an additional 628,272purchased the shares of common stock from us at a price of $4.1548 per share and the pre-funded warrants from us at a price of $9.55, resulting in additional gross proceeds$4.15386 per share underlying each pre-funded warrant. The shares of $6.0 million.common stock and the pre-funded warrants were issued, and any shares of common stock issuable upon exercise of the pre-funded warrants will be issued, pursuant to a shelf registration statement on Form S-3 that was filed with the U.S. Securities Exchange Commission, or SEC, on April 6, 2023 and declared effective by the SEC on April 28, 2023.
Financial Operations Overview
Revenue
To date, we have not generated any revenue from product sales and do not expect to generate any revenue from product sales for the foreseeable future. Our only source of revenue to date has been a research agreement with a multinational pharmaceutical company. For the year ended December 31, 2017 and 2016,2023, we recognized $1.1$9.9 million of revenue, all of which was attributable to our collaboration with Global Blood Therapeutics, now a subsidiary of Pfizer, Inc., or GBT. For the year ended December 31, 2022, we recognized $14.9 million of revenue,$13.6 million of which was attributable to our collaboration with GBT and $0.3$1.3 million respectively,of which was attributable to our target discovery collaboration with Incyte Collaboration, or Incyte. Our collaborations with GBT and Incyte were terminated effective October 2023, and we do not expect to recognize collaboration revenue from GBT or Incyte in revenue related to this agreement. This research agreement expired on March 31, 2017 in accordance with its terms.subsequent reporting periods.
Expenses
Expenses
Research and Development Expenses
Research and development expenses consist primarily of costs incurred for our research activities, including the preclinical and clinical development of our gene control platform and the development of product candidates, which include:
•employee-related expenses, including salaries and benefits;
•stock-based compensation expense;
•external costs of funding activities performed by third parties that conduct research and development on our behalf and of purchasing supplies used in designing, developing and manufacturing preclinical study and clinical trial materials;
•consulting, licensing and professional fees related to research and development activities; and
•facilities costs, depreciation and amortization and other expenses, which include direct and allocated expenses for rent and maintenance of facilities, insurance and other operating costs.
| ·
| | employee-related expenses including salaries and benefits;
|
| ·
| | stock-based compensation expense;
|
| ·
| | external costs of funding activities performed by third parties that conduct research and development on our behalf and of purchasing supplies used in designing, developing and manufacturing preclinical study and clinical trial materials;
|
| ·
| | consulting, licensing and professional fees related to research and development activities; and
|
| ·
| | facilities costs, depreciation and other expenses, which include direct and allocated expenses for rent and maintenance of facilities, insurance and other operating costs.
|
Research and development costs are expensed as incurred. Nonrefundable advance payments made to vendors for goods or services that will be received in the future for use in research and development activities are deferred and capitalized, even when there is no alternative future use for the research and development, until related goods or services are provided.
We typically use our employee, consultant and infrastructure resources across our research and development programs. We track outsourced development costs by product candidate or development program, but we do not allocate personnel costs, other internal costs or certain external consultant costs to specific product candidates or development programs.
The following table summarizes our external research and development expenses by program, as well as expenses not allocated to programs, for the years ended December 31, 20172023 and 20162022 (in thousands):
| | | | | | | |
| | Year Ended | |
| | December 31, | |
| | 2017 | | 2016 | |
| | | |
SY-1425 external costs | | $ | 9,227 | | $ | 7,940 | |
SY-1365 and other CDK7 program external costs | | | 8,289 | | | 8,129 | |
Other research and platform programs external costs | | | 8,161 | | | 7,184 | |
Employee-related expenses, including stock-based compensation | | | 11,719 | | | 11,214 | |
Facilities and other expenses | | | 4,500 | | | 3,350 | |
Total research and development expenses | | $ | 41,896 | | $ | 37,817 | |
| | | | | | | | |
| | Year Ended | |
| | December 31, | |
| | 2023 | | | 2022 | |
Tamibarotene external costs | | $ | 55,309 | | | $ | 41,549 | |
SY-5609 program external costs | | | 2,708 | | | | 6,754 | |
SY-2101 program external costs | | | 4,998 | | | | 4,211 | |
Other research program external costs | | | 7,059 | | | | 15,658 | |
Employee-related expenses, excluding stock-based compensation | | | 26,679 | | | | 30,321 | |
Stock-based compensation | | | 4,746 | | | | 5,946 | |
Facilities and other expenses | | | 6,654 | | | | 7,505 | |
Total research and development expenses | | $ | 108,153 | | | $ | 111,944 | |
We expect ourto incur significant research and development expenses will increase for the foreseeable future as we seek to advance our programs.clinical trials involving tamibarotene. At this time, we cannot reasonably estimate or know the nature, timing and estimated costs of the efforts that will be necessary to complete the development of ourtamibarotene or any future product candidates. We are also unable to predict when, if ever, material net cash inflows will commence from sales of ourany product candidates. This is due to the numerous risks and uncertainties associated with developing such product candidates, including the uncertainty of:
| ·
| | successful completion of preclinical studies, including activities related to an IND and minimally efficacious dose studies in animals, where applicable and requested under the good laboratory practice, or GLP, requirements of the FDA;
|
•approval of INDs for our product candidates to commence planned or future clinical trials;
| ·
| | approval of INDs for our product candidates to commence planned or future clinical trials;
|
•successful enrollment in, and completion of, clinical trials;
| ·
| | successful enrollment in, and completion of, clinical trials;
|
•successful data from our clinical programs that support an acceptable benefit-risk profile of our product candidates in the intended populations;
| ·
| | successful data from our clinical programs that support an acceptable benefit-risk profile of our product candidates in the intended populations;
|
•successful development, and subsequent clearance or approval, of companion diagnostic tests for use in identifying potential patients;
| ·
| | successful development, and subsequent clearance or approval, of companion diagnostic tests for use in identifying potential patients;
|
•receipt of regulatory approvals from applicable regulatory authorities;
| ·
| | receipt of regulatory approvals from applicable regulatory authorities;
|
•establishment of arrangements with third-party manufacturers for clinical supply and commercial manufacturing and, where applicable, commercial manufacturing capabilities;
| ·
| | establishment of arrangements with third-party manufacturers for clinical supply and commercial manufacturing and, where applicable, commercial manufacturing capabilities;
|
•establishment and maintenance of patent and trade secret protection or regulatory exclusivity for our product candidates;
| ·
| | establishment and maintenance of patent and trade secret protection or regulatory exclusivity for our product candidates;
|
•commercial launch of our product candidates, if and when approved, whether alone or in collaboration with others;
| ·
| | commercial launch of our product candidates, if and when approved, whether alone or in collaboration with others;
|
•enforcement and defense of intellectual property rights and claims;
| ·
| | enforcement and defense of intellectual property rights and claims;
|
•maintenance of a continued acceptable safety profile of the product candidates following approval;
| ·
| | maintenance of a continued acceptable safety profile of the product candidates following approval; and
|
•retention of key personnel;
| ·
| | retention of key research and development personnel.
|
•the impact of public health crises, including epidemics and pandemics such as the COVID-19 pandemic; and
•general economic conditions, including inflation, recession risk and increasing interest rates.
Any changes in the outcome of any of these variables with respect to the development of our product candidates in preclinical and clinical development could mean a significant change in the costs and timing associated with the development of these product candidates. For example, if the FDA or another regulatory authority were to delay our planned start of clinical trials or require us to conduct clinical trials or other testing beyond those that we currently expect or if we experience significant delays in enrollment in any of our planned clinical trials, we could be required to expend significant additional financial resources and time on the completion of clinical development of our product candidates.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and other related costs, including stock-based compensation, for personnel in executive, finance, information technology and administrative functions. Other significant costs include corporate facility costs not otherwise included in research and development expenses, legal fees related to patent and corporate matters, and fees for accounting and consulting services.
Restructuring Costs
We anticipateRestructuring costs consist primarily of severance, post-employment benefit, outplacement services, impairment charges and any other expenses that our general and administrative expenses will increase inwe incur related to the future as we increase our headcount to support our continued research activities and developmentrealignment of our product candidates. We also anticipatestrategy and cost reduction measures.
Transaction Related Expenses
Transaction related expenses primarily consist of incurred costs allocated to the warrants issued in connection with the PIPE Financing that we will incur increased accounting, audit, legal, compliancewere accounted for as liabilities, and director and officer insurance costs, as well as investor and public relations expenses, associated with operating as a public company.severance paid to former Tyme employees.
Interest Income
Other Income, Net
OtherInterest income net consists of interest income on our cash, and cash equivalents, interest, dividends,and investments in marketable securities, including the related amortization of premiumspremium and discounts, realized gainsdiscounts.
Interest Expense
Interest expense consists of interest, amortization of debt discount, and losses on salesamortization of marketable securitiesdeferred financing costs associated with our loans payable, and interest expense related toon finance lease arrangements.
Change in Fair Value of Warrant Liabilities
Change in fair value of warrant liabilities is the result of the remeasurement of the fair value of our equipment financing arrangement.warrant liabilities at each reporting period end.
Critical Accounting Policies and Estimates
Our management’s discussion and analysis of our financial condition and results of operations are based on our financial statements, which have been prepared in accordance with U.S. generally accepted accounting principles, or U.S. GAAP. The preparation of these financial statements requires us to make judgments and estimates that affect the reported amounts of assets, liabilities, revenues and expenses and the disclosure of contingent assets and liabilities in our financial statements. We base our estimates on historical experience, known trends and events and various other factors that are believed to be reasonable under the circumstances. Actual results may differ from these estimates under different assumptions or conditions. On an ongoing basis, we evaluate our judgments and estimates in light of changes in circumstances, facts and experience. The effects of material revisions in estimates, if any, will be reflected in the financial statements prospectively from the date of the change in estimates.
While our significant accounting policies are described in more detail in the notes to our financial statements appearing elsewhere in this report,Annual Report, we believe the following accounting policies used in the preparation of our financial statements require the most significant judgments and estimates.
Revenue
Revenue
To date our only revenue has consisted of collaboration and license revenue. We have not generated any revenue from product sales and do not expect to generate any revenue from product sales for the foreseeable future. For the year ended December 31, 2023, we recognized $9.9 million of revenue, all of which was attributed to our collaboration with GBT. For the year ended December 31, 2022, we recognized $14.9 million of revenue, $13.6 million of which was attributable to our collaboration with GBT and $1.3 million of which was attributable to our target discovery collaboration with Incyte.
We recognize revenue in accordanceconformity with Financial Accounting Standards Board, or FASB, Accounting Standards Codification, or ASC, Topic 605 Revenue Recognition, from Contracts with Customers, or ASC 605. Accordingly,606. ASC 606 applies to all contracts with customers, except for contracts that are within the scope of other standards, such as leases, insurance, collaboration arrangements and financial instruments. Under ASC 606, an entity recognizes revenue when its customer obtains control of promised goods or services, in an amount that reflects the consideration the entity expects to receive in exchange for those goods or services. To determine revenue recognition for arrangements that an entity determines are within the scope of ASC 606, the entity performs the following five steps:
(i)identify the contract(s) with a customer;
(ii)identify the performance obligations in the contract;
(iii)determine the transaction price;
(iv)allocate the transaction price to the performance obligations in the contract; and
(v)recognize revenue when (or as) the entity satisfies a performance obligation.
We only apply the five-step model to contracts when it is recognizedprobable that we will collect the consideration to which we are entitled in exchange for the goods or services we transfer to the customer. At contract inception, once the contract is determined to be within the scope of ASC 606, we assess the goods or services promised within each unit of accounting when allcontract and determine those that are performance obligations, and assess whether each promised good or service is distinct. We then recognize as revenue the amount of the following criteriatransaction price that is allocated to the respective performance obligation when (or as) the performance obligation is satisfied.
From time to time, we may enter into agreements that are met:within the scope of ASC 606. The terms of these arrangements typically include payment to us of one or more of the following: non-refundable, up-front license fees, prepaid research and development services, development, regulatory and commercial milestone payments; and royalties on net sales of licensed products. Each of these payments would result in license and collaboration revenues, except for revenues from royalties on net sales of licensed products, which will be classified as royalty revenues.
| ·
| | persuasive evidence of an arrangement exists;
|
| ·
| | delivery has occurred or services have been rendered;
|
| ·
| | the seller’s price to the buyer is fixed or determinable; and
|
| ·
| | collectability is reasonably assured.
|
We analyze multiple elementour collaboration arrangements to assess whether they are within the scope of ASC 808, Collaborative Arrangements, or ASC 808, to determine whether such arrangements involve joint operating activities performed by parties that are both active participants in the activities and exposed to significant risks and rewards dependent on the commercial success of such activities. This assessment is performed throughout the life of the arrangement based on the guidance in ASC Topic 605-25, Revenue Recognition—Multiple Element Arrangements, or ASC 605-25. Pursuant to the guidance in ASC 605-25, we evaluate multiple element arrangements to determine (1) the deliverables includedchanges in the arrangementresponsibilities of all parties in the arrangement. For collaboration arrangements within the scope of ASC 808 that contain multiple elements, we first determine which elements of the collaboration are deemed to be within the scope of ASC 808 and (2) whetherthose that are more reflective of a vendor-customer relationship and therefore within the individual deliverables represent separate unitsscope of accounting or whether they must beASC 606. For elements of collaboration arrangements that are accounted for as a combined unitpursuant to ASC 808, an appropriate recognition method is determined and applied consistently, generally by analogy to ASC 606. For those elements of accounting. This evaluation involves subjective determinationsthe arrangement that are accounted for pursuant to ASC 606, we apply the five-step model described above.
Research and Development Expenses
Expenditures relating to research and development are expensed in the period incurred. Research and development expenses consist of both internal and external costs associated with the development of our product candidates. Research and development costs include salaries and benefits, materials and supplies, external research, preclinical and clinical development expenses, stock-based compensation expense and facilities costs. Facilities costs primarily include the allocation of rent, utilities, depreciation and amortization.
In certain circumstances, we are required to make judgements about the individual deliverables and whether such deliverables are separate from other aspects of the contractual relationship. Deliverables are considered separate units of accounting provided that: (i) the delivered item(s) has value to the customer on a standalone basis and (ii) the arrangement includes a general right of return relative to the delivered item(s), delivery or performance of the undelivered item(s) is considered probable and substantially within our control.
We recognize arrangement consideration allocated to each unit of accounting when all of the revenue recognition criteria in ASC 605 are satisfied for that particular unit of accounting. In the event that a deliverable does not represent a separate unit of accounting, we recognize revenue from the combined unit of accounting over the contractual or estimated performance period for the undelivered items, which is typically the term of our research and development obligations. If there is no discernible pattern of performance or objectively measurable performance measures do not exist, then we recognize revenue under the arrangement on a straight-line basis over the period we are expected to
complete our performance obligations or upon completion when the final act is of such significance to the overall arrangement that performance has not substantively occurred until the completion of that act. Conversely, if the pattern of performance over which the service is provided to the customer can be determined and objectively measurable performance measures exist, then we recognize revenue under the arrangement using the proportional performance method.
The research agreement we entered with a multinational pharmaceutical company contained a single unit of accounting and we recognized service revenue based upon the completed performance method of revenue recognition as we are unable to reasonably estimate the period of performance of the services and the delivery of the final study report was significant to the arrangement.
Research and Development Expenses
As part of the process of preparing our financial statements, we are required to estimate our accrued expenses as of each balance sheet date. This process involves reviewing open contracts and purchase orders, communicating with our personnel to identify services that have been performed on our behalf and estimating the level of service performed and the associated cost incurred for the service when we have not yet been invoiced or otherwise notified of the actual cost. The majority of our service providers invoice us monthly in arrears for services performed or when contractual
milestones are met. We make estimates of our accrued expenses as of each balance sheet date based on facts and circumstances known to us at that time. We periodically confirm the accuracy of our estimates with the service providers and make adjustments if necessary. The significant estimates in our accrued research and development expenses include the costs incurred for services performed by our service providers in connection with research and development activities for which we have not yet been invoiced.
We record our expenses related to research and development activities based on our estimates of the services received and efforts expended pursuant to contracts with vendors that conduct research and development on our behalf. The financial terms of these agreements are subject to negotiation, vary from contract to contract and may result in uneven payment flows. There may be instances in which payments made to our vendors will exceed the level of services provided and result in a prepayment of the research and development expense. In accruing service fees, we estimate the time period over which services will be performed and the level of effort to be expended in each period. If the actual timing of the performance of services or the level of effort varies from our estimate, we adjust the accrual or prepaid accordingly. Non-refundablenonrefundable advance payments to vendors for goods andor services that will be usedreceived in the future for use in research and development activitiesactivities. In such circumstances, the nonrefundable advance payments are deferred and capitalized, even when there is no alternative future use for the research and development, until related goods or services are provided.
Although we do not expect our estimates to be materially different from amounts actually incurred, if our estimatesWe record accruals for estimated ongoing research costs. When evaluating the adequacy of the statusaccrued liabilities, we analyze progress of the work being performed, including the phase or completion of the event, invoices received and timingcosts. Significant judgments and estimates may be made in determining the accrued balances at the end of services performedany reporting period. Actual results could differ from the actual status and timing of services performed, it could result in us reporting amounts that are too high or too low in any particular period. To date, there have been no material differences between our estimates of such expenses and the amounts actually incurred.estimates.
We have and may in the future in-license the rights to develop and commercialize product candidates. For each in-license transaction, we evaluate whether we have acquired processes or activities along with inputs that would be sufficient to constitute a ‘‘business’’“business” as defined under U.S. GAAP. A “business” as defined under U.S. GAAP consists of inputs and processes applied to those inputs that have the ability to create outputs. Although businesses usually have outputs, outputs are not required for an integrated set of activities to qualify as a business. When we determine that we have not acquired sufficient processes or activities to constitute a business, any up-front payments, as well as milestone payments, are immediately expensed as acquired research and development in the period in which they are incurred.
Warrants
Stock-Based Compensation
We applyboth Liabilities and Equity, or ASC 480-10, or ASC 815-40, Accounting for Derivative Financial Instruments Indexed to, and Potentially Settled in, a Company’s Own Stock, or ASC 815-40. Under ASC 480-10, warrants are considered liabilities if they are mandatorily redeemable and they require settlement in cash or other assets, or a variable number of shares. If warrants do not meet liability classification under ASC 480-10, we consider the requirements of ASC 815-40 to determine whether the warrants should be classified as liability or equity. Under ASC 815-40, contracts that may require settlement for cash are liabilities, regardless of the probability of the occurrence of the triggering event. Liability classified warrants are measured at fair value on the issuance date and at the end of each reporting period. Any change in the fair value recognition provisions of the warrants after the issuance date is recorded in the consolidated statements of operations as a gain or loss. If warrants do not require liability classification under ASC Topic815-40, in order to conclude warrants should be classified as equity, we assess whether the warrants are indexed to our common stock and whether the warrants are classified as equity under ASC 815-40 or other applicable GAAP. Equity classified warrants are accounted for at fair value on the issuance date with no changes in fair value recognized after the issuance date.
On December 21, 2023, we issued and sold an aggregate of 4,939,591 shares of common stock at a price of $4.42 per share, and, in lieu of shares of common stock, pre-funded warrants to purchase an aggregate of 5,242,588 shares of common stock, or the 2023 Pre-Funded Warrants, at a price of $4.419 per 2023 Pre-Funded Warrant, resulting in gross proceeds of approximately $45.0 million, before deducting underwriting fees and other transaction costs of approximately $3.2 million.
On September 16, 2022, through the PIPE Financing, we issued 6,387,173 shares of common stock, and, in lieu of shares of common stock, pre-funded warrants to purchase an aggregate of 7,426,739 shares of common stock, or the 2022 Pre-Funded Warrants, and, in each case, accompanying warrants, or the 2022 Warrants, to purchase an aggregate of up to 13,813,912 additional shares of common stock (or 2022 Pre-Funded Warrants to purchase common stock in lieu thereof) at a price of $10.34 per share and accompanying 2022 Warrant (or $10.33 per 2022 Pre-Funded Warrant and accompanying 2022 Warrant). The PIPE Financing resulted in aggregate gross proceeds of $129.9 million, before $10.1 million of transaction costs.
On December 8, 2020, through a private placement, we issued 1,031,250 shares of our common stock and, in lieu of common stock, pre-funded warrants to purchase an aggregate of 100,000 shares of common stock, or the 2020 Pre-Funded Warrants, and, in each case, accompanying warrants, or the 2020 Warrants, to purchase an aggregate of up to 282,809 additional shares of common stock (or 2020 Pre-Funded Warrants to purchase common stock in lieu thereof) at a price of $80.00 per share and accompanying warrant (or $79.90 per pre-funded warrant and accompanying warrant). The private placement resulted in aggregate gross proceeds of $90.5 million, before $0.4 million of transaction costs.
In the event of certain fundamental transactions involving the Company, the holders of 2022 Warrants and 2020 Warrants may require us to make a payment based on a Black-Scholes valuation, using specified inputs. The holders of 2023 Pre-Funded Warrants, 2022 Pre-Funded Warrants and 2020 Pre-Funded Warrants do not have similar rights. Therefore, we
accounted for the 2022 Warrants and 2020 Warrants as liabilities, while the 2023 Pre-Funded Warrants, 2022 Pre-Funded Warrants and 2020 Pre-Funded Warrants met the permanent equity criteria classification. The 2023 Pre-Funded Warrants, 2022 Pre-Funded Warrants and 2020 Pre-Funded Warrants are classified as a component of permanent equity because they are freestanding financial instruments that are legally detachable and separately exercisable from the shares of common stock with which they were issued, are immediately exercisable, do not embody an obligation for us to repurchase our shares, and permit the holders to receive a fixed number of shares of common stock upon exercise. In addition, the 2023 Pre-Funded Warrants, 2022 Pre-Funded Warrants and 2020 Pre-Funded Warrants do not provide any guarantee of value or return. The initial fair value of the 2022 Warrants and 2020 Warrants was $64.7 million and $19.3 million, respectively, determined using the Black-Scholes valuation model. We remeasured the aggregate fair value of the 2022 Warrants and 2020 Warrants at December 31, 2023 and 2022 as $61.7 million and $24.5 million, respectively. The change in fair value of $37.3 million (loss) and $43.2 million (gain) was recorded in our statement of operations for the years ended December 31, 2023 and 2022, respectively.
Stock-Based Compensation
We account for our stock-based compensation awards in accordance with ASC 718, Compensation—Stock Compensation, or ASC 718. Determining the amountASC 718 requires all stock-based payments to employees and directors, including grants of stock-based compensationrestricted stock units and stock option awards, to be recorded requires us to develop estimatesrecognized as expense in the consolidated statements of operations based on their grant date fair values.
We estimate the fair value of stock options as of their grant date. Calculating the fair value of stock-based awards requires that we make highly subjective assumptions.
We usegranted using the Black-Scholes option pricing model to valueoption-pricing model. We estimate our expected stock option awards. Use of this valuation methodology requires that we make assumptions as to the volatility ofbased on our common stock, thehistorical volatility. The expected term of our stock options has been determined utilizing the risk free interest rate“simplified” method for a periodawards that approximatesqualify as “plain-vanilla” options. The expected term of stock options to non-employees can be determined using either the contractual term of the option award or the “simplified” method. We elected to continue to use the contractual term in determining the expected term of our stock options and our expected dividend yield. Prioroption to June 30, 2016, we were a privately-held company and lacked company-specific historical and implied volatility information. As such, we utilize data from a representative groupnon-employees. The risk-free interest rate is determined by reference to the U.S. Treasury yield curve in effect at the time of public companies to estimate expected stock price volatility. For purposes of identifying representative companies, we considered characteristics such as number of product candidates in early stages of product development, area of therapeutic focus, length of trading history and similar vesting provisions. The expected volatility was determined using an averagegrant of the historical volatilities of the representative group of companiesaward for a periodtime periods approximately equal to the expected term of the option grant. We intend to consistently apply this process using the same representative companies until a sufficient amount of historical information regarding the volatility of our own share price becomes available or until circumstances change, such that the identified entities are no longer representative companies. In the latter case, more suitable, similar entities whose share prices are publicly available would be utilized in the calculation.
We use the ‘‘simplified method’’ to estimate the expected term of stock option grants to employees. Under this approach, the weighted-average expected option term is presumed to be the average of the contractual term (ten years) and the vesting term (generally four years) of our stock options. We utilize this method due to lack of historical exercise data and the ‘‘plain-vanilla’’ nature of our stock-based awards. The risk-free rateaward. Expected dividend yield is based on the yield curve of U.S. Treasury securities in effect at the time of grant with periods commensurate with the expected term of the options being
valued. Wefact that we have never paid cash dividends and do not anticipate paying,expect to pay any cash dividends in the foreseeable future, and thereforefuture. We use an expected dividend yieldthe value of zero inour common stock to determine the option-pricing model.fair value of restricted stock awards.
Prior to becoming a public company, we determinedWe expense the fair value of our common stock using the option pricing method, or OPM, or a hybrid of the probability-weighted expected return method and OPM. The fair value of our common stock underlying our stock-based awards was determinedto employees and non-employees on each grant date by our board of directors. Upon becoming a public company,straight-line basis over the fair value ofassociated service period, which is generally the underlying shares of common stock equals the closing price of our stock on The Nasdaq Global Select Market on the date of grant.
The amount of stock-based compensation expense recognized is based on the fair value of the award on the date of grant. As a result of the adoption of ASU 2016-09, effective January 1, 2017, wevesting period. We account for forfeitures as they occur instead of estimating forfeitures at the time of grant and revising those estimates in subsequent periods if actual forfeitures differ from its estimates.grant. Ultimately, the actual expense recognized over the vesting period will be for only those options that vest.
We have computedCompensation expense for discounted purchases under the employee stock purchase plan is measured using the Black-Scholes model to compute the fair value of stock options at the date of grant usinglookback provision plus the following weighted-average assumptions:
| | | | | | | |
| | Year Ended December 31, | |
| | 2017 | | 2016 | | 2015 | |
Weighted-average risk-free interest rate | | 2.07 | % | 1.36 | % | 1.78 | % |
Expected dividend yield | | — | % | — | % | — | % |
Expected option term | | 6.05 | | 5.98 | | 6.09 | |
Volatility | | 87.83 | % | 85.39 | % | 82.71 | % |
We expense the fair value of our stock-based awards to employees on a straight-line basis over the associated service period, whichpurchase discount and is generally the vesting period. For stock-based awards granted to consultants and non-employees, we recognizerecognized as compensation expense over the period during which the services are rendered by such consultants and non-employees. At the end of each financial reporting period prior to completion of the service, the fair value of these awards is remeasured using the then-current fair value of our common stock for restricted stock and updated assumptions in the Black-Scholes option-pricing model for stock options.offering period.
We record the expense forFor stock-based awards that contain performance-based milestones, we record stock-based compensation expense in accordance with the accelerated attribution model. Management evaluates when the achievement of a performance-based milestone is probable based on the expected satisfaction of the performance conditions as of the reporting date. For certain
We have computed the fair value of our performance-based awards, notwithstanding any vesting in accordance withstock options at the achievementdate of performance-based milestones, such awards vest in full ongrant using the sixth anniversary of the vesting commencement date. Compensation expense for such awards is recognized over the six-year vesting period unless management determines that the achievement of any performance-based milestones are probable, in which case expense is accelerated.following weighted-average assumptions:
| | | | | | | | | |
| | Year Ended December 31, |
| | 2023 | | | 2022 | | |
Weighted-average risk-free interest rate | | | 3.93 | | % | | 3.56 | | % |
Expected dividend yield | | | — | | % | | — | | % |
Expected option term (in years) | | | 5.32 | | | | 5.80 | | |
Volatility | | | 84.44 | | % | | 83.32 | | % |
Results of Operations
Comparison of Years Ended December 31, 20172023 and 20162022
The following table summarizes our results of operations for the years ended December 31, 20172023 and 2016,2022, together with the changes in those items in dollars (in thousands):
| | | | | | | | | | | | | |
| | Year Ended December 31, | | | | | | | |
| | 2017 | | 2016 | | Dollar Change | | % Change | |
| | | | | | | | | |
Statements of Operations Data: | | | | | | | | | | | | | |
Revenue | | $ | 1,101 | | $ | 317 | | $ | 784 | | | 247 | % |
Operating expenses: | | | | | | | | | | | | | |
Research and development | | | 41,896 | | | 37,817 | | | 4,079 | | | 11 | % |
General and administrative | | | 13,891 | | | 10,463 | | | 3,428 | | | 33 | % |
Total operating expenses | | | 55,787 | | | 48,280 | | | 7,507 | | | 16 | % |
Other income, net | | | 676 | | | 220 | | | 456 | | | 207 | % |
Net loss | | $ | (54,010) | | $ | (47,743) | | $ | 6,267 | | | 13 | % |
Revenue
| | | | | | | | | | | | | | | | | |
| | Year Ended December 31, | | | | | | | | |
| | 2023 | | | 2022 | | | Dollar Change | | | % Change | | |
Statements of Operations Data: | | | | | | | | | | | | | |
Revenue | | $ | 9,936 | | | $ | 14,880 | | | $ | (4,944 | ) | | $ | (33 | ) | % |
Operating expenses: | | | | | | | | | | | | | |
Research and development | | | 108,153 | | | | 111,944 | | | | (3,791 | ) | | | (3 | ) | % |
General and administrative | | | 28,282 | | | | 29,299 | | | | (1,017 | ) | | | (3 | ) | % |
Transaction related expenses | | | — | | | | 9,510 | | | | (9,510 | ) | | | (100 | ) | % |
Restructuring costs | | | 2,489 | | | | — | | | | 2,489 | | | | — | | % |
Total operating expenses | | | 138,924 | | | | 150,753 | | | | (11,829 | ) | | | (8 | ) | % |
Loss from operations | | | (128,988 | ) | | | (135,873 | ) | | | 6,885 | | | | (5 | ) | % |
Interest income | | | 6,816 | | | | 2,132 | | | | 4,684 | | | | 220 | | % |
Interest expense | | | (5,127 | ) | | | (4,134 | ) | | | (993 | ) | | | 24 | | % |
Change in fair value of warrant liabilities | | | (37,275 | ) | | | 43,221 | | | | (80,496 | ) | | | (186 | ) | % |
Net loss | | $ | (164,574 | ) | | $ | (94,654 | ) | | $ | (69,920 | ) | | $ | 74 | | % |
In November 2014, we entered into a research agreement with a multinational pharmaceutical company for purposes of mapping immune cell super-enhancers and transcriptional targets in autoimmune disease. UnderRevenue
For the research agreement, we were responsible for the conduct of all activities under separate projects, as defined in the research agreement. We recognized revenue on a completed performance basis for each project performed under the agreement. We recognized revenue of $1.1 million and $0.3 million during the yearsyear ended December 31, 2017 and2023, we recognized approximately $9.9 million of revenue, all of which was attributable to our collaboration with GBT. For the year ended December 31, 2016, respectively.2022, we recognized approximately $14.9 million of revenue, $13.6 million of which was attributable to our collaboration with GBT and $1.3 million of which was attributable to our target discovery collaboration with Incyte. The agreementdecrease in revenue for the year ended December 31, 2023 was primarily attributable to the decrease in the percentage of completion achieved related to our collaboration with GBT during the multinational pharmaceutical company expired on Marchyear ended December 31, 2017 in accordance with its terms.2023 compared to the year ended December 31, 2022.
Research and Development Expense
Research and development expense increaseddecreased by approximately $4.1$3.7 million, or 11%3%, from $37.8$111.9 million for the year ended December 31, 20162022 to $41.9$108.2 million for the year ended December 31, 2017.2023. The following table summarizes our research and development expenses for the years ended December 31, 20172023 and 2016,2022, together with the changes to those items in dollars (in thousands):
| | | | | | | | | | | | |
| | Year Ended December 31, | | | | | | |
| | 2017 | | 2016 | | Dollar Change | | % Change | |
| | | | | | | | | | | | |
External research and development | | $ | 23,785 | | $ | 20,802 | | $ | 2,983 | | 14 | % |
Employee-related expenses, excluding stock-based compensation | | | 10,053 | | | 8,234 | | | 1,819 | | 22 | % |
Stock-based compensation | | | 1,666 | | | 2,980 | | | (1,314) | | (44) | % |
Consulting, licensing and professional fees | | | 1,892 | | | 2,451 | | | (559) | | (23) | % |
Facilities and other expenses | | | 4,500 | | | 3,350 | | | 1,150 | | 34 | % |
Total research and development expenses | | $ | 41,896 | | $ | 37,817 | | $ | 4,079 | | 11 | % |
| | | | | | | | | | | | | | | | | |
| | Year Ended December 31, | | | | | | | | |
| | 2023 | | | 2022 | | | Dollar Change | | | % Change | | |
External research and development | | $ | 61,280 | | | $ | 61,370 | | | $ | (90 | ) | | | (0 | ) | % |
Employee-related expenses, excluding stock-based compensation | | | 26,679 | | | | 30,321 | | | | (3,642 | ) | | | (12 | ) | % |
Stock-based compensation | | | 4,746 | | | | 5,946 | | | | (1,200 | ) | | | (20 | ) | % |
Consulting, licensing and professional fees | | | 8,794 | | | | 6,802 | | | | 1,992 | | | | 29 | | % |
Facilities and other expenses | | | 6,654 | | | | 7,505 | | | | (851 | ) | | | (11 | ) | % |
Total research and development expenses | | $ | 108,153 | | | $ | 111,944 | | | $ | (3,791 | ) | | | (3 | ) | % |
The change in research and development expense was primarily attributable to research and development activities associated with advancing our lead clinical and preclinical programs and enhancing our internal capabilities, and included the following:
| ·
| | an increase of approximately $3.0 million, or 14%, for expenses from third parties that conduct research and development and preclinical activities on our behalf, including an increase of approximately $5.5 million in clinical development for SY-1425 and SY-1365, offset by a decrease of $2.2 million in preclinical development work for SY-1365 as toxicology studies were completed and the Phase 1 clinical trial was initiated;
|
•a decrease of approximately $3.6 million, or 12%, for employee-related expenses, excluding stock-based compensation, primarily due to the decrease in headcount associated with the restructuring of our operations;
88
| ·
| | an increase of approximately $1.8 million, or 22%, for increased personnel related expenses, including increased salary and benefits primarily due to the hire of key research and development personnel;
|
| ·
| | a decrease of approximately $1.3 million, or 44%, for decreased stock-based compensation expense primarily related to the recognition of expense related to performance triggers achieved during 2016 for which no corresponding expense was recognized during 2017;
|
•a decrease of approximately $1.2 million, or 20%, for stock-based compensation, primarily due to the decrease in headcount associated to the restructuring of our operations;
| ·
| | a decrease of approximately $0.6 million, or 23% in consulting, licensing, and professional fees, due to the $0.5 million license payment paid to TMRC Co., Ltd., or TMRC, in 2016 under our license agreement with TMRC, which we refer to as the TMRC license agreement; and
|
•an increase of approximately $2.0 million, or 29%, for consulting, licensing and professional fees, primarily related to the advancement of our existing clinical trials of tamibarotene, and;
| ·
| | an increase of approximately $1.2 million, or 34%, for increases in the proportion of costs allocated to research and development, as well as increases in facilities costs including depreciation and maintenance expenses associated with our operating lease at our corporate headquarters beginning in August 2015.
|
•a decrease of approximately $0.9 million, or 11%, in facilities and other expenses primarily due to the closure of our laboratory facilities as part of the restructuring of our operations.
General and Administrative Expense
General and administrative expense increaseddecreased by approximately $3.4$1.0 million, or 33%3%, from $10.5$29.3 million for the year ended December 31, 20162022 to $13.9$28.3 million for the year ended December 31, 2017. 2023. The change in general and administrative expense was primarily attributable to an increasea decrease in employee-related costs, including salary, benefitsconsulting and stock-based compensation, as well as increased consulting, licensing, andother professional fees, to support our overall growth asand a company. decrease in facilities costs.
Restructuring Costs
Other Income, Net
Other income, net consists of interest income on our cash and cash equivalents, interest, amortization of premiums and discounts on marketable securities, and interest expense related to our equipment financing arrangement. The increase in other income from the year ended December 31, 2016 to the year ended December 31, 2017 is due to a full year of investing activities in 2017, as we started investing in marketable securities beginning in October 2016.
Comparison of Years Ended December 31, 2016 and 2015
The following table summarizes our results of operations for the years ended December 31, 2016 and 2015, together with the changes in those items in dollars (in thousands):
| | | | | | | | | | | | | |
| | Year Ended December 31, | | | | | | | |
| | 2016 | | 2015 | | Dollar Change | | Dollar Change | |
| | | | | | | | | | | |
Statements of Operations Data: | | | | | | | | | | | | | |
Revenue | | $ | 317 | | $ | 317 | | $ | — | | | — | % |
Operating expenses: | | | | | | | | | | | | | |
Research and development | | | 37,817 | | | 24,408 | | | 13,409 | | | 55 | % |
General and administrative | | | 10,463 | | | 5,729 | | | 4,734 | | | 83 | % |
Total operating expenses | | | 48,280 | | | 30,137 | | | 18,143 | | | 60 | % |
Other income, net | | | 220 | | | 2 | | | 218 | | | 10,900 | % |
Net loss | | $ | (47,743) | | $ | (29,818) | | $ | 17,925 | | | 60 | % |
Revenue
In November 2014, we entered into a research agreement with a multinational pharmaceutical company for purposes of mapping immune cell super-enhancers and transcriptional targets in autoimmune disease. Under the research agreement, we were responsible for the conduct of all activities under separate projects, as defined in the research agreement. We recognized revenue on a completed performance basis for each project performed under the agreement. We recognized revenue of $0.3 million during each of the years ended December 31, 2016 and December 31, 2015.
Research and Development Expense
Research and development expense increased by approximately $13.4 million, or 55%, from $24.4 millionRestructuring costs for the year ended December 31, 20152023 consist primarily of severance, post-employment benefit, outplacement services, impairment charges and any other expenses that we incur related to $37.8 million for the year ended December 31, 2016. The following table summarizesrealignment of our researchstrategy and developmentcost reduction measures.
Transaction Related Expenses
Transaction related expenses for the year ended December 31, 2016 and 2015, together2022 primarily consist of incurred costs allocated to the warrants issued in connection with the changesPIPE Financing that were accounted for as liabilities, and severance paid to those itemsformer Tyme employees.
Interest Income
Interest income was derived generally from our investments in dollars (in thousands):
| | | | | | | | | | | | |
| | Year Ended December 31, | | | | | | |
| | 2016 | | 2015 | | Dollar Change | | % Change | |
| | | | | | | | | | | | |
External research and development | | $ | 20,802 | | $ | 12,749 | | $ | 8,053 | | 63 | % |
Employee-related expenses, excluding stock-based compensation | | | 8,234 | | | 5,344 | | | 2,890 | | 54 | % |
Stock-based compensation | | | 2,980 | | | 2,733 | | | 247 | | 9 | % |
Consulting, licensing and professional fees | | | 2,451 | | | 1,972 | | | 479 | | 24 | % |
Facilities and other expenses | | | 3,350 | | | 1,610 | | | 1,740 | | 108 | % |
Total research and development expenses | | $ | 37,817 | | $ | 24,408 | | $ | 13,409 | | 55 | % |
cash, cash equivalents and marketable securities. The changeincrease in research and development expense was primarily attributable to research and development activities associated with advancing our lead preclinical programs and enhancing our internal capabilities, and included the following:
| ·
| | an increase of approximately $8.1 million, or 63% for expenses from third parties that conduct research and development and preclinical activities on our behalf, including approximately $6.1 million in contract manufacturing and clinical development for SY-1425, a $1.0 million milestone payment made under our license agreement with TMRC in September 2016, and approximately $0.9 million for preclinical development for SY-1365 and advancement of the CDK7 program;
|
| ·
| | an increase of approximately $2.9 million, or 54% for increased personnel related expenses, including increased salary and benefits primarily due to the hire of research and development personnel;
|
| ·
| | an increase of approximately $0.2 million, or 9% for increased stock-based compensation expense;
|
| ·
| | an increase of approximately $0.5 million, or 24% in consulting, licensing, and professional fees, due to increased preclinical, clinical and regulatory consulting fees for SY-1425 and SY-1365; and
|
| ·
| | an increase of approximately $1.7 million, or 108% for increases in facilities costs including depreciation and maintenance expenses associated with our operating lease at our corporate headquarters beginning in August 2015.
|
General and Administrative Expense
General and administrative expense increased by approximately $4.7 million, or 83% from $5.7 million forinterest income during the year ended December 31, 2015 to $10.5 million for the year ended December 31, 2016. The change in general and administrative expense was primarily attributable to an increase in employee-related costs, including salary, benefits and stock-based compensation,2023 as well as increased consulting, licensing, and professional fees, including increased
corporate legal fees in support of the negotiations of the TMRC license agreement and the negotiations of our operating lease agreement for office space and increased public relations expenses.
Other Income, Net
Other income, net consists of interest income on our cash, cash equivalents and marketable securities, offset by interest expense related to our equipment financing arrangement. The increase in other income from the year ended December 31, 2015compared to the year ended December 31, 2016 is2022 was due to the higher interest rate during the year ended December 31, 2023 compared to the year ended December 31, 2022.
Interest Expense
Interest expense was related to our credit facility with Oxford Finance LLC, or Oxford. Interest expense increased during the year ended December 31, 2023 as compared to the year ended December 31, 2022 due to a higher levelaverage carrying value of invested cashthe credit facility and cash equivalentsthe increasing interest rates during the year ended December 31, 2023 compared to the year ended December 31, 2022.
Change in Fair Value of Warrant Liabilities
The change in fair value of warrant liabilities (loss) during the year ended December 31, 2023 was primarily driven by the increase in the price of our common stock from our proceeds.December 31, 2022 to December 31, 2023.
Liquidity and Capital Resources
Sources of Liquidity
We funded our operations from inception through December 31, 20172023 primarily through the issuance of equity securities, through license and collaboration agreements, and through the credit facility with Oxford.
On February 12, 2020, we entered into a Loan and Security Agreement, or the Loan Agreement, with Oxford. Pursuant to the Loan Agreement, a term loan of up to an aggregate principal amount of $60.0 million is available to us. A $20.0 million term loan was funded on February 12, 2020, and another $20.0 million term loan was funded on December 23, 2020. On July 3, 2022, we entered into an amendment to the Loan Agreement with Oxford, or the Loan Amendment. Pursuant to the Loan Amendment, Oxford has agreed to modify the Loan Agreement in order to, among other things, extend the interest only period from March 1, 2023 to March 1, 2024 and extend the maturity date from February 1, 2025 to February 1, 2026, and upon the achievement of certain milestones and subject to the payment of certain fees, further extend
the interest only period to September 1, 2024 and maturity date to August 1, 2026. As of December 31, 2023, $20.0 million remains available under the Loan Agreement, at the sole discretion of Oxford.
In January 2021, we issued shares of our common stock in an underwritten public offering resulting in gross proceeds of $122.2$75.6 million, from salesbefore deducting underwriting discounts and commissions and other transaction expenses of our preferred stockapproximately $5.1 million, pursuant to a universal shelf registration statement on Form S-3 that was filed with the SEC on June 12, 2020.
On July 3, 2022, we entered into the Merger Agreement with Tyme. Also on July 3, 2022, immediately prior to the execution and delivery of the issuanceMerger Agreement, we entered into the Securities Purchase Agreement with certain accredited investors. In connection with the closing of convertible notes that subsequently convertedthe Merger on September 16, 2022, we acquired net cash, cash equivalents and marketable securities of $67.1 million, before deducting severance costs and other commitments entered into preferred stock, $57.5 million inby Tyme management prior to the consummation of the Merger of approximately $4.5 million. The PIPE Financing closed concurrently with the Merger on September 16, 2022, pursuant to which we received aggregate gross proceeds fromof $129.9 million, before deducting offering expenses payable by us, and not inclusive of any exercise of the sale of common stockwarrants issued in our initial public offering, or IPO, and $35.0 million in gross proceeds from the sale of common stock in a private placement inPIPE Financing.
On April 2017.
On July 20, 2017,6, 2023, we filed a universal shelf registration statement on Form S-3, or the 2023 Registration Statement, with the SEC to register for sale from time to time up to $225.0$250.0 million of common stock, preferred stock, debt securities, warrants and/or units in one or more registered offerings. The shelf registration statement2023 Registration Statement was declared effective on July 31, 2017.April 28, 2023. Further, in July 2017,April 2023, we entered into aan at-the-market sales agreement, or the 2023 sales agreement, with Cowen and Company, LLC, or Cowen, pursuant to which from time to time, we may offer and sell shares of our common stock having an aggregate offering price of up to $50.0 million through Cowen pursuant to the 2023 Registration Statement. During the year ended December 31, 2023, we issued and sold 350,000 shares of common stock pursuant to the 2023 sales agreement for gross proceeds of $1.4 million, before deducting underwriting fees.
Upon entry into the 2023 sales agreement, we terminated our prior at-the-market program pursuant to the original sales agreement dated July 12, 2020. At the time of such universal shelf registration statement. termination, the entire $75.0 million available under such agreement remained unsold.
In December 2023, we issued shares of our common stock and, in lieu of common stock to certain investors, pre-funded warrants to purchase our common stock, pursuant to the 2023 Registration Statement, in an underwritten offering resulting in gross proceeds of $45.0 million, before deducting underwriting fees and other transaction costs of approximately $3.2 million.
As of December 31, 2017,2023, $48.6 million of our common stock remained available for future issuance under the 2023 sales agreement.
As of December 31, 2023, $205.0 million of securities remained available for issuance under the 2023 Registration Statement.
As of December 31, 2023, we had cash and cash equivalents and marketable securities of approximately $72.0$139.5 million.
Cash Flows
The following table provides information regarding our cash flows for the years ended December 31, 2017, 20162023 and 20152022 (in thousands):
| | | | | | | | |
| | Year Ended December 31, | |
| | 2023 | | | 2022 | |
| | | | | | |
Net cash (used in) provided by: | | | | | | |
Operating activities | | $ | (109,707 | ) | | $ | (123,065 | ) |
Investing activities | | | 37,337 | | | | 67,185 | |
Financing activities | | | 43,462 | | | | 131,045 | |
Net (decrease) increase in cash, cash equivalents and restricted cash | | $ | (28,908 | ) | | $ | 75,165 | |
| | | | | | | | | | |
| | Year Ended December 31, | |
| | 2017 | | 2016 | | 2015 | |
| | | | | | | | | | |
Net cash provided by (used in): | | | | | | | | | | |
Operating activities | | $ | (44,729) | | $ | (40,536) | | $ | (23,030) | |
Investing activities | | | (15,591) | | | (27,342) | | | (1,176) | |
Financing activities | | | 33,937 | | | 90,557 | | | (278) | |
Net increase (decrease) in cash and cash equivalents | | $ | (26,383) | | $ | 22,679 | | $ | (24,484) | |
90
Net Cash Used in Operating Activities
The use of cash in operating activities in all periods presented resulted primarily from our net losses adjusted for non-cash charges and changes in components of working capital.
Net cash used in operating activities was $44.7$109.7 million during the year ended December 31, 20172023, compared to $40.5$123.1 million during the year ended December 31, 2016.2022. The increasedecrease in cash used in operating activities was primarily due to an increasea $6.9 million decrease in our netthe loss of $6.3 million forfrom operations during the year ended December 31, 2017 as compared2023, a $3.1 million increase in interest income, and a $10.6 million increase in the change in net operating assets and liabilities, partially offset by a $0.9 million decrease in stock-based compensation, a $1.1 million increase in interest expense, and $5.0 million of transaction costs allocated to warrants issued in connection with the year ended December 31, 2016.PIPE Financing.
Net Cash Provided by (Used in) Investing Activities
Net cash used in operatingprovided by investing activities was $40.5$37.3 million during the year ended December 31, 20162023, compared to $23.0net cash provided by investing activities of $67.2 million during the year ended December 31, 2015.2022. The increase innet cash used in operatingprovided by investing activities was primarily due to an increase in net loss of $17.9 million forduring the year ended December 31, 2016 as compared2023 was primarily due to maturities of our investment in marketable securities of $87.0 million and proceeds from disposal of assets-held-for-sale of $1.6 million, partially offset by the purchase of $51.0 million of marketable securities and $0.3 million of property and equipment.
Net cash provided by investing activities during the year ended December 31, 2015.2022 was primarily due to maturities of our investment in marketable securities of $68.4 million, partially offset by the purchase of $1.2 million of property and equipment.
Net Cash Used in InvestingProvided by Financing Activities and Merger
Net cash used in investingprovided by financing activities was $15.6$43.5 million during the year ended December 31, 2017 compared to $27.3 million during the year ended December 31, 2016. The decrease in cash used in investing activities was primarily due to sales and maturities of marketable securities of $27.0 million during 2017 compared to no sales or maturities of marketable securities during 2016, offset by an increase in purchases of marketable securities during 2017.
Net cash used in investing activities was $27.3 million during the year ended December 31, 2016 compared to $1.2 million during the year ended December 31, 2015. The increase in cash used in investing activities was due to purchases of marketable securities as well as increased purchases of property and equipment associated with our corporate headquarters.
Net Cash Provided by (Used in) Financing Activities
Net cash provided by financing activities was $33.9 million during the year ended December 31, 2017,2023, compared to net cash provided by financing activities of $90.6$131.1 million during the year ended December 31, 2016. The decrease in cash2022. Cash provided by financing activities isfor the year ended December 31, 2023 was primarily due to $41.9 million of proceeds from the issuance of common stock and accompanying pre-funded warrants in connection with the IPO in July 2016, as well asan underwritten registered offering, net of issuance costs, $1.4 million of proceeds from the issuance of $39.8common stock pursuant to the 2023 sales agreement, net of issuance costs, and $0.3 million in a preferred stock financing in January 2016, offset by grossof proceeds of $35.0 million in connection withfrom the saleissuance of common stock through private placement in April 2017.our employee stock purchase plan, partially offset by $0.1 million of payments made under our financing lease.
Net cashCash provided by financing activities was $90.6 million duringfor the year ended December 31, 2016 compared to net cash used in financing activities of $0.3 million during the year ended December 31, 2015. The increase in cash provided by financing activities2022 was primarily due to $119.8 million of proceeds from the issuance of common stock and accompanying 2022 Warrants and 2022 Pre-Funded Warrants in connection with the IPO in July 2016, as well asPIPE Financing, net of issuance costs and $11.7 million of proceeds from the Merger (recapitalization), net of issuance costs, and $0.2 million from the issuance of $39.8common stock through our employee stock purchase plan, partially offset by the payment of $0.3 million to Oxford related to the Loan Amendment, $0.3 million of payments made under our financing lease, and payment of $0.1 million in lieu of fractional shares due to the reverse stock split of our common stock.
Material Cash Requirements from Known Contractual Obligations
Our material cash requirements from known contractual obligations as of December 31, 2023 consisted of:
•Principal and interest payments under our loan and security agreement with Oxford. For additional information regarding the terms of the debt and interest payable, see Note 8 to the consolidated financial statements in Item 8 of this Annual Report.
•Operating lease liabilities with respect to our lease of approximately 52,859 square feet of space in Cambridge, Massachusetts for a preferred stock financinglease term ending in January 2016. February 2030. For additional information regarding the terms of this operating lease, see Note 11 to the consolidated financial statements in Item 8 of this Annual Report.
•Contingent milestone obligations that may become payable pursuant to the asset purchase agreement with Orsenix. For additional information regarding these contingent milestone obligations, see Note 11 to the consolidated financial statements in Item 8 of this Annual Report.
•Obligations pursuant to our license agreement and supply management agreement with TMRC Co. Ltd, or TMRC. For additional information regarding these obligations, see Note 11 to the consolidated financial statements in Item 8 of this Annual Report.
Funding Requirements
We expect ourto incur significant expenses to increase in connection with our ongoing activities, particularly as we continue to advance our clinical trials of SY-1425 and SY-1365,tamibarotene, seek to develop companion diagnostic tests for use with our product candidates, initiate new research and preclinical development effortstamibarotene, and seek marketing approval for tamibarotene or any future product candidates that we successfully develop. In addition, if we obtain marketing approval for tamibarotene or any of ourother product candidates,candidate, we expect to incur significant commercialization expenses related to establishing sales, marketing, distribution and other commercial infrastructure to commercialize such products. Furthermore, we expect to incur additional costs associated with operating as a public company. Accordingly, weWe will need to obtain substantial additional funding in connection with our continuing operations. If we are unable to raise capital when needed or on favorable terms, we would be forced to delay, reduce, eliminate, or out-license our research and development programs or future commercialization rights to our product candidates.
We believe that our cash and cash equivalents and marketable securities as of December 31, 2017, together with amounts received from Incyte in connection with our collaboration and option agreement executed in January 2018 and the net proceeds from the underwritten public offering of our common stock and concurrent private placement of our common stock to Incyte that closed in February 2018,2023, will enable us to fund our planned operating expense and capital expenditure requirements into 2020.the second quarter of 2025. Our future capitalfunding requirements, both short-term and long-term, will depend on many factors, including:
| ·
| | • the scope, progress, timing, costs and results of clinical trials of tamibarotene and results of clinical trials of SY-1425 and SY-1365 and any associated companion diagnostic tests; |
| ·
| | research and preclinical development efforts for any future product candidates that we may develop;
|
| ·
| | our ability to enter into and the terms and timing of any collaborations, licensing agreements or other arrangements;
|
| ·
| | whether our collaboration with Incyte will yield any validated targets, whether Incyte will exercise any of its options to exclusively license intellectual property directed to such targets, and whether and when any of the target validation fees, option exercise fees, milestone payments or royalties under the collaboration agreement with Incyte will ever be paid;
|
Table of Contents
development efforts for any future product candidates that we may develop;• | ·
| | the number of future product candidates that we pursue and their development requirements;
|
the number of future product candidates that we pursue and their development requirements; | ·
| | the outcome, timing and costs of seeking regulatory approvals;
|
•our ability to enter into, and the terms and timing of, any collaborations, licensing agreements or other arrangements;
| ·
| | the costs of commercialization activities for any of our product candidates that receive marketing approval to the extent such costs are not the responsibility of any future collaborators, including the costs and timing of establishing product sales, marketing, distribution and manufacturing capabilities;
|
•the outcome, timing and costs of seeking regulatory approvals;
| ·
| | the costs of acquiring potential new product candidates or technology;
|
•the costs of commercialization activities for any of our product candidates that receive marketing approval to the extent such costs are not the responsibility of any collaborators, including the costs and timing of establishing product sales, marketing, distribution and manufacturing capabilities;
| ·
| | the costs of any physician education programs relating to selecting and treating genomically defined patient populations;
|
•the costs of acquiring potential new product candidates or technology;
| ·
| | the timing and amount of milestone and other payments due to licensors for patent and technology rights used in our development platform;
|
•the costs of any physician education programs relating to selecting and treating genomically defined patient populations;
| ·
| | revenue received from commercial sales, if any, of our current and future product candidates;
|
•the timing and amount of milestone and other payments due to TMRC associated with the development, manufacture and commercialization of tamibarotene;
| ·
| | our headcount growth and associated costs as we expand our research and development, operate as a public company, and establish a commercial infrastructure;
|
•revenue received from commercial sales, if any, of our current and future product candidates;
| ·
| | the costs of preparing, filing and prosecuting patent applications, maintaining and protecting our intellectual property rights and defending against intellectual property related claims; and
|
•our employment-related costs as we advance our clinical pipeline and establish a commercial infrastructure;
| ·
| | the costs of operating as a public company.
|
•the costs of preparing, filing and prosecuting patent applications, maintaining and protecting our intellectual property rights and defending against intellectual property related claims; and
•the impact of public health crises, including epidemics and pandemics such as the COVID-19 pandemic.
Identifying potential product candidates and conducting preclinical studies and clinical trials is a time-consuming, expensive and uncertain process that takes many years to complete, and we may never generate the necessary data or results required to obtain marketing approval and achieve product sales. In addition, ourtamibarotene or any future product candidates,candidate, if approved, may not achieve commercial success. Accordingly, we will need to continue to rely on additional financing to achieve our business objectives. Adequate additional financing may not be available to us on acceptable terms, or at all.
Until such time, if ever, as we can generate substantial product revenues, we expect to finance our cash needs through a combination of equity offerings, debt financings, collaborations, strategic alliances and licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interests of our common stockholders will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect the rights of our common stockholders. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends.
If we raise funds through additional collaborations, strategic alliances or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or to grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
Contractual Obligations and Commitments
As December 31, 2017, we have a capital lease for laboratory equipment that expires in March 2018 and a capital lease for office equipment that ends in April 2020. Additionally, we lease office space at 620 Memorial Drive in Cambridge, Massachusetts under a non-cancellable operating lease that expires in October 2020. The following table summarizes our significant contractual obligations as of payment due date by period at December 31, 2017:
| | | | | | | | | | | | | | | | |
| | Payments due by period | |
| | | | | | | | | | | | | | More than 5 | |
(in thousands) | | Total | | Less than 1 year | | 1 to 3 years | | 3 to 5 years | | years | |
Capital lease payments | | $ | 55 | | $ | 48 | | $ | 7 | | $ | — | | $ | — | |
Operating lease payments | | | 3,743 | | | 1,288 | | | 2,455 | | | — | | | — | |
Total | | $ | 3,798 | | $ | 1,336 | | $ | 2,462 | | $ | — | | $ | — | |
We enter into agreements in the normal course of business with our contract research organizations and institutions to license intellectual property. We have not included these future payments in the table of contractual obligations above since the contracts are cancelable at any time by us, generally upon 30 to 90 days prior written notice.
Our license agreements include potential milestone payments that are contingent upon the successful development and commercialization of products using the intellectual property licensed under such agreements. Under our agreements with Dana-Farber and Whitehead, the maximum aggregate potential milestone payments payable by us total approximately $6.9 million. Under the applicable agreement, we are also required to pay annual maintenance fees, as well as tiered, single digit percentage royalties, on a country-by-country, product-by-product basis, on net product sales.
Under the amended and restated TMRC license agreement, we may make additional payments upon the successful achievement of pre-specified clinical and regulatory milestones in an aggregate amount of approximately $13.0 million per indication. In May 2016, we paid TMRC $0.5 million representing the balance of the remaining upfront license fee and in September 2016, we made a $1.0 million milestone payment to TMRC upon the successful dosing of the first patient in our Phase 2 clinical trial of SY-1425.
We also entered into a supply arrangement with TMRC, under which the Company agreed to pay TMRC a fee for each kilogram of SY-1425 active pharmaceutical ingredient that is produced. During the year ended December 31, 2017, we paid TMRC $0.4 million in payments related to this agreement. No payments were made under this supply management arrangement during the year ended December 31, 2016.
Off-Balance Sheet Arrangements
We did not have, during the periods presented, and we do not currently have, any off-balance sheet arrangements, as defined under applicable Securities and Exchange Commission, or SEC, rules.
JOBS Act
In April 2012, the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, was enacted. Section 107 of the JOBS Act provides that an emerging growth company, or EGC, can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act of 1933, as amended, or the Securities Act, for complying with new or revised accounting standards. Thus, an EGC can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have irrevocably elected not to avail ourselves of this extended transition period and, as a result, we will adopt new or revised accounting standards on the relevant dates on which adoption of such standards is required for other public companies.
As an EGC, we intend to rely on the exemption from the requirement to provide an auditor’s attestation report on our system of internal controls over financial reporting pursuant to Section 404(b) of the Sarbanes‑Oxley Act and with the exemption from any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements, known as the auditor discussion and analysis. We will remain an EGC until the earlier of (i) the last day of the fiscal year in which we have total annual gross revenues of $1.0 billion or more; (ii) the
last day of the fiscal year following the fifth anniversary of the date of the closing of our IPO; (iii) the date on which we have issued more than $1.0 billion in nonconvertible debt during the previous three years; or (iv) the date on which we are deemed to be a large accelerated filer under SEC rules.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We are exposed to market risk related to changes in interest rates. Our primary exposure to market risk is interest rate sensitivity, which is affected by changes in the general level of U.S. interest rates, particularly because our investments, including cash equivalents, are in the form of a money market fundfunds and marketable securities and are invested in U.S. Treasurytreasury or government obligations. However, because of the short-term nature of the duration of our portfolio and the low-risk profile of our investments, we believe an immediate 10% change in market interest rates would not be expected to have a material impact on the fair market value of our investments portfolio or on our financial condition or results of operations.
We are also exposed to market risk related to changes in foreign currency exchange rates. We contract with vendors that are located in Asia and Europe and certain invoices are denominated in foreign currencies. We are subject to fluctuations in foreign currency rates in connection with these arrangements. We do not currently hedge our foreign currency exchange rate risk. As of December 31, 2017,2023, we had minimal or no significant liabilities denominated in foreign currencies.
Inflation generally affects us by increasing our cost of labor and clinical trial costs. We do not believe that inflation had a material effect on our business, financial condition or results of operations during the year ended December 31, 2017.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
SYROS PHARMACEUTICALS, INC.
INDEX TO FINANCIAL STATEMENTS
Report of Independent Registered Public Accounting Firm
To the Stockholders and the Board of Directors of Syros Pharmaceuticals, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Syros Pharmaceuticals, Inc. (the Company) as of December 31, 20172023 and 2016,2022, the related consolidated statements of operations, comprehensive loss, convertible preferred stock and stockholders' equity (deficit) and cash flows for each of the threetwo years in the period ended December 31, 2017,2023, and the related notes (collectively referred to as the “consolidated financial statements“statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 20172023 and 2016,2022, and the results of its operations and its cash flows for each of the threetwo years in the period ended December 31, 2017,2023, in conformity with US generally accepted accounting principles.
Basis for Opinion
These financial statements are the responsibility of the Company‘sCompany’s management. Our responsibility is to express an opinion on the Company‘sCompany’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the financial statements that was communicated or required to be communicated to the audit committee and that: (1) relates to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective or complex judgments. The communication of the critical audit matter does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
| | |
|
| Phase 3 and Phase 2 Tamibarotene Prepaid and Accrued and Research & Development Costs |
Description of the Matter |
| The Company’s accrued expenses totaled $16.1 million as of December 31, 2023 inclusive of accrued research and development expenses related to the Company’s Phase 3 and Phase 2 Tamibarotene clinical trials. In addition, the Company’s prepaid expenses and other current assets were $5.5 million, which included amounts that were paid in advance of services incurred pursuant to the Company’s Phase 3 and Phase 2 Tamibarotene clinical trials. As discussed in Note 2 to the consolidated financial statements, the Company analyzes the progress of the clinical trials, including the phase or completion of events, invoices received and contracted costs. Significant judgments and estimates are made in determining the prepaid and accrued balances at the end of any reporting period for the Company’s Phase 3 and Phase 2 Tamibarotene clinical trials. The Company is required to estimate such prepaids and accruals using judgment based on certain information, including actual costs incurred or level of effort expended, as provided by its vendors. Payments for such activities are based on the terms of the individual arrangements, which may differ from the pattern of costs incurred, and are reflected on the consolidated balance sheet within prepaid or accrued expenses and other current assets.
Auditing the Company’s prepaid and accrued research and development costs for the Phase 3 and Phase 2 Tamibarotene clinical trials was complex, as accounting for the costs associated with this clinical trial required subjective estimates of the level of services performed and the associated costs incurred by service providers. Furthermore, due to the duration of the Company’s Phase 3 and Phase 2 Tamibarotene clinical trials, and the timing of information received from third parties, the actual amounts incurred are not typically known at the time the consolidated financial statements are issued.
|
How We Addressed the Matter in Our Audit |
| To evaluate the prepaid and accrued research and development costs for the Phase 3 and Phase 2 Tamibarotene clinical trials, our audit procedures included, among others, testing the accuracy and completeness of the underlying data used in the estimates and evaluating the significant judgments and estimates made by management to determine the recorded accruals and prepayments. To test the significant judgments and estimates, we corroborated the progress of research and development activities through discussion with the Company’s research and development personnel that oversee the research and development projects and inspected the Company’s contracts with third parties and any pending change orders to assess the impact on amounts recorded. In addition, we reviewed estimates of costs incurred to date provided to the Company from third parties. We also analyzed fluctuations in accruals by vendor and by trial throughout the period subject to audit, evaluated the costs incurred per trial, site and/or patient for reasonableness and tested subsequent invoices received from third parties. |
/s/ Ernst & Young LLP
We have served as the Company’s auditor since 2014.
Boston, Massachusetts
March 12, 2018
SYROS PHARMACEUTICALS, INC.
CONSOLIDATED BALANCE SHEETS
(in thousands, except share and per share data)
| | | | | | | |
| | December 31, | | December 31, | |
| | 2017 | | 2016 | |
Assets | | | | | | | |
Current assets: | | | | | | | |
Cash and cash equivalents | | $ | 32,205 | | $ | 58,588 | |
Marketable securities | | | 39,844 | | | 25,005 | |
Accounts receivable | | | — | | | 867 | |
Prepaid expenses and other current assets | | | 917 | | | 1,048 | |
Restricted cash | | | 193 | | | — | |
Total current assets | | | 73,159 | | | 85,508 | |
Property and equipment, net | | | 3,938 | | | 4,850 | |
Other long-term assets | | | 1,101 | | | 482 | |
Restricted cash | | | 290 | | | 483 | |
Total assets | | $ | 78,488 | | $ | 91,323 | |
Liabilities and stockholders' equity | | | | | | | |
Current liabilities: | | | | | | | |
Accounts payable | | $ | 2,283 | | $ | 2,415 | |
Accrued expenses | | | 9,728 | | | 6,115 | |
Deferred revenue | | | — | | | 550 | |
Deferred rent, current portion | | | 355 | | | 319 | |
Capital lease obligations, current portion | | | 47 | | | 168 | |
Total current liabilities | | | 12,413 | | | 9,567 | |
Deferred rent, net of current portion | | | 745 | | | 1,101 | |
Capital lease obligations, net of current portion | | | 6 | | | 53 | |
| | | | | | | |
Commitments and contingencies (Note 9) | | | | | | | |
| | | | | | | |
Stockholders' equity: | | | | | | | |
| | | | | | | |
Preferred stock, $0.001 par value; 10,000,000 shares authorized at December 31, 2017 and December 31, 2016, 0 shares issued and outstanding at December 31, 2017 and December 31, 2016 | | | — | | | — | |
Common stock, $0.001 par value; 200,000,000 shares authorized at December 31, 2017 and December 31, 2016; 26,423,375 and 23,380,888 shares issued and outstanding at December 31, 2017 and December 31, 2016, respectively | | | 26 | | | 23 | |
Additional paid-in capital | | | 220,606 | | | 181,844 | |
Accumulated other comprehensive loss | | | (42) | | | (9) | |
Accumulated deficit | | | (155,266) | | | (101,256) | |
Total stockholders' equity | | | 65,324 | | | 80,602 | |
Total liabilities and stockholders' equity | | $ | 78,488 | | $ | 91,323 | |
| | | | | | | | |
| | December 31, | | | December 31, | |
| | 2023 | | | 2022 | |
Assets | | | | | | |
Current assets: | | | | | | |
Cash and cash equivalents | | $ | 139,526 | | | $ | 167,467 | |
Marketable securities | | | — | | | | 34,837 | |
Unbilled receivable | | | — | | | | 1,694 | |
Prepaid expenses and other current assets | | | 5,454 | | | | 7,394 | |
Total current assets | | | 144,980 | | | | 211,392 | |
Property and equipment, net | | | 7,298 | | | | 11,353 | |
Other long-term assets | | | 1,592 | | | | 5,348 | |
Restricted cash | | | 2,119 | | | | 3,086 | |
Right-of-use asset – operating lease | | | 12,185 | | | | 13,231 | |
Right-of-use assets – financing leases | | | — | | | | 76 | |
Total assets | | $ | 168,174 | | | $ | 244,486 | |
Liabilities and stockholders' equity | | | | | | |
Current liabilities: | | | | | | |
Accounts payable | | $ | 11,544 | | | $ | 6,411 | |
Accrued expenses | | | 16,146 | | | | 17,966 | |
Deferred revenue | | | — | | | | 4,330 | |
Financing lease obligations | | | — | | | | 65 | |
Operating lease obligation, current portion | | | 2,324 | | | | 2,006 | |
Debt, current portion | | | 6,667 | | | | — | |
Total current liabilities | | | 36,681 | | | | 30,778 | |
Operating lease obligation, net of current portion | | | 18,528 | | | | 20,851 | |
Warrant liabilities | | | 61,747 | | | | 24,472 | |
Debt, net of debt discount, net of current portion | | | 34,556 | | | | 40,649 | |
Commitments and contingencies (See Note 11) | | | | | | |
Stockholders' equity: | | | | | | |
Preferred stock, $0.001 par value; 10,000,000 shares authorized at December 31, 2023 and December 31, 2022; 0 shares issued and outstanding at December 31, 2023 and December 31, 2022 | | | — | | | | — | |
Common stock, $0.001 par value; 70,000,000 shares authorized at December 31, 2023 and December 31, 2022; 26,448,678 and 20,263,116 shares issued and outstanding at December 31, 2023 and December 31, 2022, respectively | | | 26 | | | | 20 | |
Additional paid-in capital | | | 739,443 | | | | 685,847 | |
Accumulated other comprehensive income | | | — | | | | 102 | |
Accumulated deficit | | | (722,807 | ) | | | (558,233 | ) |
Total stockholders' equity | | | 16,662 | | | | 127,736 | |
Total liabilities and stockholders' equity | | $ | 168,174 | | | $ | 244,486 | |
See accompanying notes to consolidated financial statements.
SYROS PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands, except share and per share data)
| | | | | | | | | | |
| | Year Ended | |
| | December 31, | |
| | 2017 | | 2016 | | 2015 | |
| | | | | | | | | | |
| | | | | | | | | | |
Revenue | | $ | 1,101 | | $ | 317 | | $ | 317 | |
Operating expenses: | | | | | | | | | | |
Research and development | | | 41,896 | | | 37,817 | | | 24,408 | |
General and administrative | | | 13,891 | | | 10,463 | | | 5,729 | |
Total operating expenses | | | 55,787 | | | 48,280 | | | 30,137 | |
Loss from operations | | | (54,686) | | | (47,963) | | | (29,820) | |
| | | | | | | | | | |
Other income, net | | | 676 | | | 220 | | | 2 | |
Net loss | | $ | (54,010) | | $ | (47,743) | | $ | (29,818) | |
Accrued dividends on preferred stock | | | — | | | (3,681) | | | (4,934) | |
Net loss applicable to common stockholders | | $ | (54,010) | | $ | (51,424) | | $ | (34,752) | |
| | | | | | | | | | |
Net loss per share applicable to common stockholders - basic and diluted | | $ | (2.13) | | $ | (4.05) | | $ | (17.55) | |
| | | | | | | | | | |
Weighted-average number of common shares used in net loss per share applicable to common stockholders - basic and diluted | | | 25,406,845 | | | 12,696,414 | | | 1,980,286 | |
| | | | | | | | |
| | Year Ended | |
| | December 31, | |
| | 2023 | | | 2022 | |
Revenue | | $ | 9,936 | | | $ | 14,880 | |
Operating expenses: | | | | | | |
Research and development | | | 108,153 | | | | 111,944 | |
General and administrative | | | 28,282 | | | | 29,299 | |
Transaction related expenses | | | — | | | | 9,510 | |
Restructuring (Note 14) | | | 2,489 | | | | — | |
Total operating expenses | | | 138,924 | | | | 150,753 | |
Loss from operations | | | (128,988 | ) | | | (135,873 | ) |
Interest income | | | 6,816 | | | | 2,132 | |
Interest expense | | | (5,127 | ) | | | (4,134 | ) |
Change in fair value of warrant liabilities | | | (37,275 | ) | | | 43,221 | |
Net loss applicable to common stockholders | | $ | (164,574 | ) | | $ | (94,654 | ) |
Net loss per share applicable to common stockholders - basic and diluted | | $ | (5.81 | ) | | $ | (7.49 | ) |
Weighted-average number of common shares used in net loss per share applicable to common stockholders - basic and diluted | | | 28,325,779 | | | | 12,631,968 | |
See accompanying notes to consolidated financial statements.
SYROS PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
(in thousands, except share and per share data)
| | | | | | | | | | |
| | Year Ended | |
| | December 31, | |
| | 2017 | | 2016 | | 2015 | |
Net loss | | $ | (54,010) | | $ | (47,743) | | $ | (29,818) | |
Other comprehensive loss: | | | | | | | | | | |
Unrealized holding losses on marketable securities | | | (33) | | | (9) | | | — | |
Comprehensive loss | | $ | (54,043) | | $ | (47,752) | | $ | (29,818) | |
thousands)
| | | | | | | | |
| | Year Ended | |
| | December 31, | |
| | 2023 | | | 2022 | |
Net loss | | $ | (164,574 | ) | | $ | (94,654 | ) |
Other comprehensive (loss) gain: | | | | | | |
Unrealized holding (loss) gain on marketable securities, net of tax | | | (102 | ) | | | 181 | |
Comprehensive loss | | $ | (164,676 | ) | | $ | (94,473 | ) |
See accompanying notes to consolidated financial statements.
Table of Contents
SYROS PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS’ EQUITY (DEFICIT)
(in thousands except share data)
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | |
| | Common Stock | | | | | | Accumulated | | | | | | | | |
| | | | | | | | Additional | | | Other | | | | | | | | |
| | Number of | | | Par | | | Paid-In | | | Comprehensive | | | Accumulated | | | Stockholders’ | | |
| | Shares | | | Value | | | Capital | | | (Loss) Gain | | | Deficit | | | Equity | |
|
Balance at December 31, 2021 | | | 6,202,403 | | | $ | 6 | | | $ | 548,870 | | | $ | (79 | ) | | $ | (463,579 | ) | | $ | 85,218 | | |
Exercise of stock options | | | 3,770 | | | | — | | | | — | | | | — | | | | — | | | | — | | |
Vesting of restricted stock units | | | 86,066 | | | | 1 | | | | — | | | | — | | | | — | | | | 1 | | |
Issuance of shares under Employee Stock Purchase Plan | | | 48,560 | | | | — | | | | 217 | | | | — | | | | — | | | | 217 | | |
Issuance of shares in private placement, net of issuance cost of $5,068 | | | 6,387,173 | | | | 6 | | | | 60,106 | | | | — | | | | — | | | | 60,112 | | |
Issuance of shares in merger, net of issuance cost of $3,136 | | | 7,546,014 | | | | 7 | | | | 65,325 | | | | — | | | | — | | | | 65,332 | | |
Cancellation of fractional shares due to reverse stock split | | | (10,870 | ) | | | — | | | | (81 | ) | | | — | | | | — | | | | (81 | ) | |
Stock-based compensation expense | | | — | | | | — | | | | 11,410 | | | | — | | | | — | | | | 11,410 | | |
Other comprehensive gain | | | — | | | | — | | | | — | | | | 181 | | | | — | | | | 181 | | |
Net loss | | | — | | | | — | | | | — | | | | — | | | | (94,654 | ) | | | (94,654 | ) | |
Balance at December 31, 2022 | | | 20,263,116 | | | $ | 20 | | | $ | 685,847 | | | $ | 102 | | | $ | (558,233 | ) | | $ | 127,736 | | |
Vesting of restricted stock units | | | 481,142 | | | | 1 | | | | (1 | ) | | | — | | | | — | | | | — | | |
Issuance of shares under Employee Stock Purchase Plan | | | 95,998 | | | | — | | | | 257 | | | | — | | | | — | | | | 257 | | |
Exercise of Pre-funded warrants | | | 246,831 | | | | — | | | | — | | | | — | | | | — | | | | — | | |
Issuance of restricted stock awards | | | 72,000 | | | | — | | | | — | | | | — | | | | — | | | | — | | |
Issuance of shares in an underwritten registered direct offering, net of issuance cost of $3,446 | | | 4,939,591 | | | | 5 | | | | 41,549 | | | | — | | | | — | | | | 41,554 | | |
Issuance of common stock at-the-market, net of issuance costs | | | 350,000 | | | | — | | | | 1,357 | | | | — | | | | — | | | | 1,357 | | |
Stock-based compensation expense | | | — | | | | — | | | | 10,434 | | | | — | | | | — | | | | 10,434 | | |
Other comprehensive loss | | | — | | | | — | | | | — | | | | (102 | ) | | | — | | | | (102 | ) | |
Net loss | | | — | | | | — | | | | — | | | | — | | | | (164,574 | ) | | | (164,574 | ) | |
Balance at December 31, 2023 | | | 26,448,678 | | | $ | 26 | | | $ | 739,443 | | | $ | — | | | $ | (722,807 | ) | | $ | 16,662 | | |
| | | | | | | | | | | | | | | | | | | |
See accompanying notes to consolidated financial statements
SYROS PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Series A Convertible | | Series B Convertible | | | | | | | | | | Accumulated | | | | | | | |
| | Preferred Stock | | Preferred Stock | | Common Stock | | Additional | | Other | | | | | Stockholders’ | |
| | # of | | | | | # of | | | | | # of | | Par | | Paid-In | | Comprehensive | | Accumulated | | (Deficit) | |
| | Shares | | Amount | | Shares | | Amount | | Shares | | Value | | Capital | | Loss | | Deficit | | Equity | |
Balance at December 31, 2014 | | 30,350,000 | | $ | 29,015 | | 16,893,931 | | $ | 52,998 | | 1,640,009 | | $ | 1 | | $ | 1,922 | | $ | — | | $ | (23,695) | | $ | (21,772) | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Exercise of stock options and vesting of restricted stock awards | | — | | | — | | — | | | — | | 723,009 | | | 1 | | | 392 | | | — | | | — | | | 393 | |
Stock-based compensation expense | | — | | | — | | — | | | — | | — | | | — | | | 3,233 | | | — | | | — | | | 3,233 | |
Net loss | | — | | | — | | — | | | — | | — | | | — | | | — | | | — | | | (29,818) | | | (29,818) | |
Balance at December 31, 2015 | | 30,350,000 | | | 29,015 | | 16,893,931 | | | 52,998 | | 2,363,018 | | | 2 | | | 5,547 | | | — | | | (53,513) | | | (47,964) | |
Issuance of Series B convertible preferred stock, net of issuance costs of $206 | | — | | | — | | 12,714,150 | | | 39,794 | | — | | | — | | | — | | | — | | | — | | | — | |
Conversion of Series A convertible preferred stock into common stock | | (30,350,000) | | | (29,015) | | — | | | — | | 8,093,326 | | | 8 | | | 29,007 | | | — | | | — | | | 29,015 | |
Conversion of Series B convertible preferred stock into common stock | | — | | | — | | (29,608,081) | | | (92,792) | | 7,895,474 | | | 8 | | | 92,784 | | | — | | | — | | | 92,792 | |
Exercise of stock options and vesting of restricted stock awards | | — | | | — | | — | | | — | | 429,070 | | | — | | | 395 | | | — | | | — | | | 395 | |
Issuance of common stock under initial public offering, net of issuance costs of $7.6 million | | — | | | — | | — | | | — | | 4,600,000 | | | 5 | | | 49,877 | | | — | | | — | | | 49,882 | |
Stock-based compensation expense | | — | | | — | | — | | | — | | — | | | — | | | 4,234 | | | — | | | — | | | 4,234 | |
Other comprehensive loss | | — | | | — | | — | | | — | | — | | | — | | | — | | | (9) | | | — | | | (9) | |
Net loss | | — | | | — | | — | | | — | | — | | | — | | | — | | | — | | | (47,743) | | | (47,743) | |
Balance at December 31, 2016 | | — | | $ | — | | — | | $ | — | | 23,380,888 | | $ | 23 | | $ | 181,844 | | $ | (9) | | $ | (101,256) | | $ | 80,602 | |
Exercise of stock options and vesting of restricted stock awards | | — | | | — | | — | | | — | | 449,896 | | | — | | | 1,799 | | | — | | | — | | | 1,799 | |
Issuance of common stock through private placement, net of issuance costs of $2.4 million | | — | | | — | | — | | | — | | 2,592,591 | | | 3 | | | 32,544 | | | — | | | — | | | 32,547 | |
Stock-based compensation expense | | — | | | — | | — | | | — | | — | | | — | | | 4,419 | | | — | | | — | | | 4,419 | |
Other comprehensive loss | | — | | | — | | — | | | — | | — | | | — | | | — | | | (33) | | | — | | | (33) | |
Net loss | | — | | | — | | — | | | — | | — | | | — | | | — | | | — | | | (54,010) | | | (54,010) | |
Balance at December 31, 2017 | | — | | $ | — | | — | | $ | — | | 26,423,375 | | $ | 26 | | $ | 220,606 | | $ | (42) | | $ | (155,266) | | $ | 65,324 | |
(in thousands)
| | | | | | | | |
| | Year Ended | |
| | December 31, | |
| | 2023 | | | 2022 | |
Operating activities | | | | | | |
Net loss | | $ | (164,574 | ) | | $ | (94,654 | ) |
Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | |
Depreciation and amortization | | | 2,252 | | | | 2,706 | |
Non-cash lease expense | | | 76 | | | | 261 | |
Impairment of long-lived assets | | | 515 | | | | — | |
Transaction costs allocated to warrants issued in connection with private placement | | | — | | | | 5,015 | |
Stock-based compensation expense | | | 10,434 | | | | 11,410 | |
Change in fair value of warrant liabilities | | | 37,275 | | | | (43,221 | ) |
Net amortization of premiums and discounts on marketable securities | | | (1,314 | ) | | | 243 | |
Amortization of debt-discount and accretion of deferred debt costs | | | 574 | | | | 692 | |
Changes in operating assets and liabilities: | | | | | | |
Prepaid expenses and other current assets | | | 1,940 | | | | (2,807 | ) |
Unbilled receivable | | | 1,694 | | | | 1,285 | |
Other long-term assets | | | 3,756 | | | | (2,407 | ) |
Accounts payable | | | 5,133 | | | | 2,766 | |
Accrued expenses | | | (2,179 | ) | | | 2,345 | |
Deferred revenue | | | (4,330 | ) | | | (5,851 | ) |
Operating lease liabilities | | | (959 | ) | | | (848 | ) |
Net cash used in operating activities | | | (109,707 | ) | | | (123,065 | ) |
Investing activities | | | | | | |
Purchases of property and equipment | | | (272 | ) | | | (1,241 | ) |
Proceeds from the disposition of property and equipment | | | 1,560 | | | | — | |
Purchases of marketable securities | | | (50,968 | ) | | | — | |
Maturities of marketable securities | | | 87,017 | | | | 68,426 | |
Net cash provided by (used in) investing activities | | | 37,337 | | | | 67,185 | |
Financing activities | | | | | | |
Payments on financing lease obligations | | | (65 | ) | | | (291 | ) |
Proceeds from the issuance of common stock through employee stock purchase plan | | | 257 | | | | 217 | |
Proceeds from the issuance of common stock through exercise of stock options | | | — | | | | 1 | |
Payment to creditor related to debt modification | | | — | | | | (300 | ) |
Proceeds from issuance of common stock through at-the-market sales agreement, net of issuance costs | | | 1,357 | | | | — | |
Proceeds from issuance of common stock and pre-funded warrants in an underwritten registered direct offering, net of issuance costs | | | 41,913 | | | | — | |
Cash and cash equivalents acquired in connection with merger, net of issuance costs paid | | | — | | | | 11,762 | |
Proceeds from issuance of common stock and accompanying warrants and pre-funded warrants in private placement, net of issuance costs | | | — | | | | 119,761 | |
Redemption of fractional shares due to the reverse stock split | | | — | | | | (81 | ) |
Payment of issuance costs related to out of period offering | | | — | | | | (24 | ) |
Net cash provided by financing activities | | | 43,462 | | | | 131,045 | |
Net (decrease) increase in cash, cash equivalents and restricted cash | | | (28,908 | ) | | | 75,165 | |
Cash, cash equivalents and restricted cash (See reconciliation in Note 7) | | | | | | |
Beginning of year | | | 170,553 | | | | 95,388 | |
End of year | | | 141,645 | | | | 170,553 | |
Supplemental disclosure of cash flow information: | | | | | | |
Cash paid for interest | | $ | 4,552 | | | $ | 3,408 | |
Cash paid for income tax | | $ | — | | | $ | — | |
Non-cash investing and financing activities: | | | | | | |
Offering costs incurred but unpaid as of year end | | $ | 138 | | | $ | 10 | |
See accompanying notes to consolidated financial statements.
SYROS PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF CASH FLOWS
(in thousands)
| | | | | | | | | | |
| | Year Ended | |
| | December 31, | |
| | 2017 | | 2016 | | 2015 | |
Operating activities | | | | | | | | | | |
Net loss | | $ | (54,010) | | $ | (47,743) | | $ | (29,818) | |
Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | | | |
Depreciation and amortization | | | 1,527 | | | 1,273 | | | 602 | |
Loss on disposal of assets | | | — | | | 4 | | | 17 | |
Stock-based compensation expense | | | 4,419 | | | 4,234 | | | 3,233 | |
Net amortization of premiums and discounts on marketable securities | | | (102) | | | 6 | | | — | |
Changes in operating assets and liabilities: | | | | | | | | | | |
Prepaid expenses and other current assets | | | 131 | | | (508) | | | (390) | |
Accounts receivable | | | 867 | | | (317) | | | — | |
Other long-term assets | | | (365) | | | (482) | | | — | |
Restricted cash | | | — | | | — | | | (413) | |
Accounts payable | | | 141 | | | (404) | | | 2,022 | |
Accrued expenses | | | 3,533 | | | 3,685 | | | 1,663 | |
Deferred revenue | | | (550) | | | — | | | — | |
Deferred rent and lease incentive | | | (320) | | | (284) | | | 54 | |
Net cash used in operating activities | | | (44,729) | | | (40,536) | | | (23,030) | |
Investing activities | | | | | | | | | | |
Purchases of property and equipment | | | (821) | | | (2,322) | | | (1,176) | |
Purchases of marketable securities | | | (41,770) | | | (25,020) | | | — | |
Sales or maturities of marketable securities | | | 27,000 | | | — | | | — | |
Net cash used in investing activities | | | (15,591) | | | (27,342) | | | (1,176) | |
Financing activities | | | | | | | | | | |
Payments on capital lease obligations | | | (168) | | | (135) | | | (50) | |
Proceeds from issuance of common stock through employee benefit plans | | | 1,799 | | | 395 | | | 392 | |
Proceeds from issuance of convertible preferred stock, net of issuance costs | | | — | | | 39,813 | | | — | |
Proceeds from issuance of common stock, net of issuance costs | | | 32,306 | | | 50,484 | | | (620) | |
Net cash provided by (used in) financing activities | | | 33,937 | | | 90,557 | | | (278) | |
(Decrease) increase in cash and cash equivalents | | | (26,383) | | | 22,679 | | | (24,484) | |
Cash and cash equivalents | | | | | | | | | | |
Beginning of period | | | 58,588 | | | 35,909 | | | 60,393 | |
End of period | | $ | 32,205 | | $ | 58,588 | | $ | 35,909 | |
Supplemental disclosure of cash flow information: | | | | | | | | | | |
Cash paid for interest | | $ | 9 | | $ | 19 | | $ | 18 | |
Non-cash investing and financing activities | | | | | | | | | | |
Conversion of convertible preferred stock into common stock | | $ | — | | $ | 82,013 | | $ | — | |
Property and equipment received but unpaid as of period end | | $ | 143 | | $ | 349 | | $ | 1,359 | |
Assets acquired under capital lease | | $ | — | | $ | 17 | | $ | 389 | |
Assets acquired through lease incentive | | $ | — | | $ | — | | $ | 1,612 | |
Offering costs incurred but unpaid as of period end | | $ | 13 | | $ | — | | $ | 1,280 | |
See accompanying notes to consolidated financial statements.
Table of Contents
Syros Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements
1. Nature of Business
Syros Pharmaceuticals, Inc. (the "Company"), a Delaware corporation formed in November 2011, is a biopharmaceutical company seeking an understandingcommitted to developing new standards of care for the non-coding regulatory regionfrontline treatment of the genome to advance new medicines to control the expression of disease-driving genes. The Company has built a proprietary platform designed to analyze this unexploited region of DNA in human disease tissue to identify and drug novel targets linked to genomically defined patient populations.patients with hematologic malignancies.
The Company is subject to a number of risks similar to those of other early stage companies, including dependence on key individuals; risks inherent in the development and commercialization of medicines to treat human disease; competition from other companies, many of which are larger and better capitalized; risks relating to obtaining and maintaining necessary intellectual property protection; and the need to obtain adequate additional financing to fund the development of its product candidates and discovery activities.candidates. If the Company is unable to raise capital when needed or on favorable terms, it would be forced to delay, reduce, eliminate or out-license certain of its research and development programs or future commercialization rights to its product candidates.
On JulyApril 6, 2016,2023, the Company completedfiled a universal shelf registration statement on Form S-3, (the “2023 Registration Statement”), with the Securities and Exchange Commission (the “SEC”) to register for sale from time to time up to $250.0 million of common stock, preferred stock, debt securities, warrants and/or units in one or more registered offerings. The 2023 Registration Statement was declared effective on April 28, 2023. Further, in April 2023, the Company entered into an initial publicat-the-market sales agreement (the “2023 Sales Agreement”) with Cowen and Company, LLC (“Cowen”) pursuant to which the Company may offer and sell shares of its common stock having an aggregate offering in whichprice of up to $50.0 million through Cowen pursuant to the 2023 Registration Statement. During the year ended December 31, 2023, the Company issued and sold 4,600,000350,000 shares of common stock pursuant to the 2023 Sales Agreement for gross proceeds of $1.4 million, before deducting underwriting fees.
In December 2023, the Company issued 4.9 million shares of common stock and, in lieu of its common stock to certain investors who so chose, pre-funded warrants to purchase 5.2 million shares of its common stock at(the “2023 Pre-Funded Warrants”), pursuant to the 2023 Registration Statement, in an underwritten registered direct offering for gross proceeds of $45.0 million, before deducting underwriting fees and other transaction costs.
On October 2, 2023, the Company announced a public offering pricestrategic realignment to prioritize key development and pre-launch activities to advance tamibarotene for the treatment of $12.50newly diagnosed higher-risk myelodysplastic syndrome and newly diagnosed acute myeloid leukemia, and to stop further investment in the clinical development of SY-2101 (oral arsenic trioxide) for the treatment of newly diagnosed acute promyelocytic leukemia, as well as in the Company’s preclinical and discovery-stage programs. In connection with these decisions, the Company instituted certain expense reduction measures (the “Restructuring”), including a reduction of approximately 35% of the Company’s employee base excluding members of the Company’s drug discovery organization whose employment ended concurrently with the termination, effective October 16, 2023, of its collaboration with Pfizer, Inc. (“Pfizer”) related to the discovery, development and commercialization of novel therapies for sickle cell disease and beta thalassemia. The Restructuring was completed by February 2024 (refer to Note 14).
On September 16, 2022, the Company filed an amendment to its Restated Certificate of Incorporation (the “Restated Certificate of Incorporation”) with the Secretary of State of the State of Delaware to effect the reverse stock split of its common stock, such that every 10 shares of the Company’s common stock held by a stockholder immediately prior to the reverse stock split were combined and reclassified into one share of the Company’s common stock (the “Reverse Stock Split”). Except where otherwise indicated, all share and per share including 600,000amounts in the accompanying financial statements, related footnotes, and management’s discussion and analysis have been adjusted retroactively to reflect the Reverse Stock Split as if it had occurred at the beginning of the earliest period presented.
On September 16, 2022, the Company completed its acquisition of Tyme Technologies, Inc., a Delaware corporation (“Tyme”), in accordance with an Agreement and Plan of Merger, dated as of July 3, 2022 (the “Merger Agreement”). The Company issued approximately 7.5 million shares of its common stock to the former Tyme stockholders in exchange for all of the shares of Tyme common stock issued and outstanding immediately prior to the merger, with Tyme surviving as a wholly-owned subsidiary of the Company (the “Merger”). In connection with the closing of the Merger, and in accordance with the terms of the Merger Agreement, the Company acquired net cash, cash equivalents and marketable securities of approximately $67.1 million.
Syros Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
On September 16, 2022, the Company issued in a private placement (the “2022 Private Placement”) 6,387,173 shares of common stock, sold pursuantand, in lieu of shares of common stock, pre-funded warrants (the “2022 Pre-Funded Warrants”) to the underwriters’ exercisepurchase an aggregate of their optionup to 7,426,739 shares of common stock, and, in each case, accompanying warrants (the “2022 Warrants”) to purchase an aggregate of up to 13,813,912 additional shares of common stock for(or 2022 Pre-Funded Warrants to purchase common stock in lieu thereof) at a price of $10.34 per share and accompanying 2022 Warrant (or $10.33 per 2022 Pre-Funded Warrant and accompanying 2022 Warrant). The 2022 Private Placement resulted in aggregate gross proceeds of $57.5$129.9 million, (the “IPO”). The Company received approximately $49.9 million in net proceeds from the IPO after deducting $7.6before $10.1 million of underwriting discounts and commissions and offeringtransaction costs. Upon the closing of the IPO, all of the outstanding shares of the Company’s convertible preferred stock automatically converted into 15,988,800 shares of common stock at the applicable conversion ratio then in effect. Subsequent to the closing of the IPO, there were no shares of preferred stock outstanding. In connection with the IPO, the Company amended and restated its certificate of incorporation to change the authorized capital stock to 200,000,000 shares designated as common stock, and 10,000,000 shares designated as preferred stock, all with a par value of $0.001 per share. The significant increase in common stock outstanding in July 2016 relating to the IPO and conversion of convertible preferred stock had an impact on the year-over-year comparability of the Company’s net loss per share calculations throughout 2017.
On April 26, 2017, the Company issued and sold an aggregate of 2,592,591 shares of its common stock in a private placement at an offering price of $13.50 per share, for aggregate gross proceeds of $35.0 million, before deducting placement agent fees of $2.1 million and other offering expenses of $0.3 million.
The Company has incurred significant annual net operating losses in every year since its inception. It expects to continue to incur significant and increasing net operating losses for at least the next several years. The Company’s net losses were $54.0 million, $47.7$164.6 million and $29.8$94.7 million for the years ended December 31, 2017, 20162023 and 2015,2022, respectively. As of December 31, 2017,2023, the Company had an accumulated deficit of $155.3$722.8 million. The Company has not generated any revenues from product sales, has not completed the development of any product candidate and may never have a product candidate approved for commercialization. The Company has financed its operations to date primarily through private placementsa credit facility, the issuance of its preferred stock, the sale of common stock in the IPO,equity securities and a private placement of common stock in April 2017.through license and collaboration agreements. The Company has devoted substantially all of its financial resources and efforts to research and development and general and administrative expense to support such research and development. The Company’s net losses may fluctuate significantly from quarter to quarter and year to year. Net losses and negative cash flows have had, and will continue to have, an adverse effect on the Company’s stockholders' equity and working capital. The Company believes that its cash and cash equivalents and marketable securities of $72.0$139.5 million as of December 31, 2017, together with amounts received from Incyte Corporation (“Incyte”) in connection with the Company’s collaboration and option agreement with Incyte executed in January 2018 and the net proceeds from the underwritten public offering of the Company’s common stock and concurrent private placement of the Company’s common stock to Incyte that closed in February 2018,2023 will be sufficient to allow the Company to fund its current operating plan for a period of at least 12 months past the issuance date of these consolidated financial statements.
Table of Contents
Syros Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
2. Summary of Significant Accounting Policies
Basis of Presentation
The Company’s consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States (“U.S. GAAP”). Any reference in these notes to applicable guidance is meant to refer to the authoritative United States generally accepted accounting principlesU.S. GAAP as found in the Accounting Standards Codification (“ASC”) and Accounting Standards Updates (“ASU”) of the Financial Accounting Standards Board (“FASB”).
In connection with preparing for its IPO, the Company effected a one-for-3.75 reverse stock split of the Company’s common stock. The reverse stock split became effective on June 17, 2016. The par value and authorized shares of common stock and convertible preferred stock were not adjusted as a result of the reverse stock split. All share and per share amounts in the financial statements and notes thereto have been retroactively adjusted for all periods presented to give effect to this reverse stock split, including reclassifying an amount equal to the reduction in par value of common stock to additional paid-in capital. The financial statements have also been retroactively adjusted to reflect adjustments to the conversion price for each series of convertible preferred stock effected in connection with the reverse stock split.
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of Syros Pharmaceuticals, Inc.the Company and its wholly owned subsidiary,wholly-owned subsidiaries, (i) Syros Securities Corporation, which is a Massachusetts subsidiarycorporation formed by the Company in December 2014 to exclusively engage in buying, selling and holding securities on its own behalf.behalf, (ii) Syros Pharmaceuticals (Ireland) Limited, an Irish limited liability company formed by the Company in January 2019, and (iii) Tyme Technologies, Inc., a Delaware corporation, which is the surviving corporation in connection with the filing of a certificate of merger with the Secretary of State of the State of Delaware on September 16, 2022, pursuant to which Tack Acquisition Corp., a Delaware corporation formed by the Company in June 2022 to effect the Merger, merged with and into Tyme Technologies, Inc. (refer to Note 1). All intercompany transactions and balances have been eliminated.eliminated in consolidation.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts in the financial statements and accompanying notes. Management considers many factors in selecting appropriate financial accounting policies and in developing the estimates and assumptions that are used in the preparation of the financial statements. Management must apply significant judgment in this process. In addition, other factors may affect estimates, which include, but are not limited to, expected business and operational changes, sensitivity and volatility associated with the assumptions used in developing estimates and whether historical trends are expected to be representative of future trends. Management’s estimation process often may yield a range of potentially reasonable estimates and management must select an amount that falls within that range of reasonable estimates. On an ongoing basis, the Company’s management evaluates its estimates, which include, but are not limited to, estimates related to revenue recognition, valuation of warrant liabilities, stock-based compensation expense, including estimating the fair value of the Company’s common stock prior to the completion of the IPO, accrued expenses, and income taxes. Actual results may differ from those estimates or assumptions.
Syros Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
Segment Information
Operating segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation by the chief operating decision maker, or decision-making group, in making decisions on how to allocate resources and assess performance. The Company's chief operating decision maker is the Chief Executive Officer. The Company and the chief operating decision maker view the Company's operations and manage its business in one operating segment. The Company operates only in the United States.
Cash and Cash Equivalents
The Company considers all highly liquid instruments that have original maturities of three months or less when acquired to be cash equivalents. Cash equivalents, which consist of money market funds that invest in U.S. Treasury
Table of Contents
Syros Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
obligations, as well as overnight repurchase agreements, are stated at fair value. The Company maintains its bank accounts at onein two major financial institution.institutions.
Marketable Securities
The Company determines the appropriate classification of its marketable securities, which consist primarily of debt securities, at the time of purchase and reevaluates such designation at each balance sheet date. All of the Company’s marketable securities are considered available-for-sale and carried at estimated fair values and reported in short-term marketable securities. Unrealized gains and losses on available-for-sale securities are excluded from net income and reported in accumulated other comprehensive income (loss) as a separate component of stockholders’ equity (deficit). Other income, net, includes interest, dividends, amortization of premiums and discounts, realized gains and losses on sales of securities and other-than-temporary declines in the fair value of securities, if any. The cost of securities sold is based on the specific identification method. The Company regularly reviews all of its investments for other-than-temporary declines in fair value. The Company’s review includes the consideration of the cause of the impairment, including the creditworthiness of the security issuers, the number of securities in an unrealized loss position, the severity and duration of the unrealized losses, whether the Company has the intent to sell the securities and whether it is more likely than not that it will be required to sell the securities before the recovery of their amortized cost basis. If the Company were to determine that the decline in fair value of an investment is below its accounting basis and the decline is other-than-temporary, the Company would reduce the carrying value of the security and record a loss for the amount of such decline.
Off-Balance Sheet Risk and Concentrations of Credit Risk
The Company has no financial instruments with off-balance sheet risk, such as foreign exchange contracts, option contracts, or other foreign hedging arrangements. Financial instruments that potentially subject the Company to concentrations of credit risk primarily consist of cash equivalents and marketable securities. Under its investment policy, the Company limits amounts invested in such securities by credit rating, maturity, industry group, investment type and issuer, except for securities issued by the U.S. government. The Company is not exposed to any significant concentrations of credit risk from these financial instruments. The goals of the Company’s investment policy, in order of priority, are safety and preservation of principal and liquidity of investments sufficient to meet cash flow requirements.
Fair Value of Financial Instruments
ASC Topic 820, Fair Value Measurement (“ASC 820”), establishesestablished a fair value hierarchy for instruments measured at fair value that distinguisheddistinguishes between assumptions based on market data (observable inputs) and the Company’s own assumptions (unobservable inputs). Observable inputs are inputs that market participants would use in pricing the asset or liability based on market data obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company’s assumption about the inputs that market participants would use in pricing the asset or liability, andliability. These are developed based on the best information available inunder the circumstances.
ASC 820 identified fair value as the exchange price, or exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As a basis for considering market participant assumptions in fair value measurements, ASC 820 established a three-tier fair value hierarchy that distinguishes between the following:
Level 1—Quoted market prices (unadjusted) in active markets for identical assets or liabilities.
Level 2—Inputs other than quoted prices included within Level 1 that are either directly or indirectly observable, such as quoted market prices, interest rates and yield curves.
Level 3—Unobservable inputs developed using estimates or assumptions developed by the Company, which reflect those that a market participant would use.
Table of Contents
Syros Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
To the extent that the valuation is based on models or inputs that are less observable or unobservable in the market, the determination of fair value requires more judgment. Accordingly, the degree of judgment exercised by the Company in determining fair value is greatest for instruments categorized as Level 3. A financial instrument’s level within the fair value hierarchy is based on the lowest level of any input that is significant to the fair value measurement.
The carrying amounts reflected in the consolidated balance sheets for cash and cash equivalents, prepaid expenses, other current assets, restricted cash, accounts payable, and accrued expenses, approximate their respective fair values due to their short-term nature.
Syros Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
Property and Equipment
Property and equipment consists of laboratory equipment, computer equipment, furniture and fixtures and leasehold improvements, all of which are stated at cost, less accumulated depreciation. Expenditures for maintenance and repairs that do not improve or extend the lives of the respective assets are recorded to expense as incurred. Major betterments are capitalized as additions to property and equipment. Depreciation isand amortization are recognized over the estimated useful lives of the assets using the straight-line method.
Construction-in-progress is stated at cost, which relates to the cost of research equipmentleasehold improvements not yet placed into service. No depreciation expense is recorded on construction-in-progress until such time as the relevant assets are completed and put into use.
Impairment of Long-Lived Assets
The Company continually evaluates long-lived assets for potential impairment when events or changes in circumstances indicate the carrying value of the assets may not be recoverable. Recoverability is measured by comparing the bookcarrying values of the assets to the expected future net undiscounted cash flows that the assets are expected to generate. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the bookcarrying values of the assets exceed their fair value.
A long-lived asset classified as held for sale is measured at the lower of its carrying amount or fair value less cost to sell. A long-lived asset is not depreciated or amortized while it is classified as held for sale, and an impairment loss would be recognized to the extent the carrying amount exceeds the asset's fair value less cost to sell.
In connection with the Restructuring, the Company entered into an exclusive auction agreement in October 2023 to sell all of its laboratory equipment by public auction. The disposal of the assets was completed in November 2024 and the Company has not recognized anyrecorded $0.5 million of related impairment lossesand loss from inception
throughdisposal, included in the restructuring charges in the Company’s consolidated statement of operations for year ended December 31, 2017.2023.
Other Long-Term Assets
At December 31, 2017, other long-term assets consisted of deferred issuance costs, which included direct and incremental legal and accounting fees related to the shelf registration filed in July 2017, as well as advanced payments made to the contract research organization responsible for conducting the Company’s clinical trial of SY-1425 and SY-1365. At December 31, 2016, other long-term assets primarily consisted of advanced payments made to the contract research organization responsible for conducting the Company’s clinical trial of SY-1425.
Revenue Recognition
To date, the Company’s only source of revenue has been a research agreement with a multinational pharmaceutical company, which expired on March 31, 2017 in accordance with its terms..consisted of collaboration and license revenue. The Company has not generated any revenue from product sales and does not expect to generate any revenue from product sales for the foreseeable future.
The Company recognizes revenue in accordance with ASC Topic 605, 606, Revenue Recognition (“from Contracts with Customers (“ASC 605”606”). Accordingly,ASC 606 applies to all contracts with customers, except for contracts that are within the scope of other standards, such as leases, insurance, collaboration arrangements and financial instruments. Under ASC 606, an entity recognizes revenue when its customer obtains control of promised goods or services, in an amount that reflects the consideration the entity expects to receive in exchange for those goods or services. To determine revenue recognition for arrangements that an entity determines are within the scope of ASC 606, the entity performs the following five steps:
(i)identify the contract(s) with a customer;
(ii)identify the performance obligations in the contract;
(iii)determine the transaction price;
(iv)allocate the transaction price to the performance obligations in the contract; and
(v)recognize revenue when (or as) the entity satisfies a performance obligation.
The Company only applies the five-step model to contracts when it is recognized when allprobable that the Company will collect the consideration it is entitled to in exchange for the goods or services it transfers to the customer. If a contract is determined to be within the scope of ASC 606 at inception, the Company assesses the goods or services promised within such contract, determines which of those goods and services are performance obligations, and assesses whether each promised good or service is distinct. The Company then recognizes as revenue the amount of the following criteria are met:
| ·
| | persuasive evidence of an arrangement exists;
|
| ·
| | delivery has occurred or services have been rendered;
|
| ·
| | the seller’s price to the buyer is fixed or determinable; and
|
| ·
| | collectability is reasonably assured.
|
transaction price that is allocated to the respective performance obligation when (or as) the performance obligation is satisfied.
Table of Contents
Syros Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
Amounts received priorIf the Company performs by transferring goods or services to satisfyinga customer before the revenue recognition criteria are recognizedcustomer pays consideration or before payment is due, the Company records a contract asset, excluding any amounts presented as deferred revenue in the Company’saccounts receivable. The Company includes contract assets as unbilled accounts receivable on its consolidated balance sheets. Amounts expectedThe Company records accounts receivable for amounts billed to be recognized as revenuethe customer for which the Company has an unconditional right to consideration. The Company assesses contract assets and accounts receivable for impairment and, to date, no impairment losses have been recorded.
From time to time, the Company may enter into agreements that are within the 12 months followingscope of ASC 606. The terms of these arrangements typically include payment to the balance sheet date are classifiedCompany of one or more of the following: non-refundable, up-front license fees or prepaid research and development services; development, regulatory and commercial milestone payments; and royalties on net sales of licensed products. Each of these payments results in current liabilities. Amounts not expected tolicense and collaboration revenues, except for revenues from royalties on net sales of licensed products, which will be recognized as revenue within the 12 months following the balance sheet date are classified as deferred revenue, net of current portion.royalty revenues.
The Company analyzes its collaboration arrangements with multiple deliverablesto assess whether they are within the scope of ASC 808, Collaborative Arrangements (“ASC 808”), to determine whether such arrangements involve joint operating activities performed by parties that are both active participants in the activities and exposed to significant risks and rewards dependent on the commercial success of such activities. This assessment is performed throughout the life of the arrangement based on changes in the guidanceresponsibilities of all parties in the arrangement. For collaboration arrangements within the scope of ASC Topic 605-25, Revenue Recognition—Multiple Element Arrangements (“ASC 605-25”). Pursuant to the guidance in ASC 605-25,808 that contain multiple elements, the Company evaluates multiple elementfirst determines which elements of the collaboration are deemed to be within the scope of ASC 808 and those that are more reflective of a vendor-customer relationship and therefore within the scope of ASC 606. For elements of collaboration arrangements that are accounted for pursuant to determine (1) the deliverables included inASC 808, an appropriate recognition method is determined and applied consistently, generally by analogy to ASC 606. For those elements of the arrangement and (2) whether the individual deliverables represent separate units of accounting or whether they must bethat are accounted for as a combined unit of accounting. This evaluation involves subjective determinations and requires managementpursuant to make judgements about the individual deliverables and whether such deliverables are separate from other aspects of the contractual relationship. Deliverables are considered separate units of accounting provided that: (i) the delivered item(s) has value to the customer on a standalone basis and (ii) if the arrangement includes a general right of return relative to the delivered item(s), delivery or performance of the undelivered item(s) is considered probable and substantially within control of the Company. The Company’s research agreement contained a single unit of accounting.
The Company recognizes arrangement consideration allocated to each unit of accounting when all of the revenue recognition criteria in ASC 605 are satisfied for that particular unit of accounting. In the event that a deliverable does not represent a separate unit of accounting,606, the Company would recognize revenue fromapplies the combined unit of accounting over the contractual or estimated performance period for the undelivered items, which is typically the term of its research and development obligations. If there is no discernible pattern of performance or objectively measurable performance measures do not exist, then the Company would recognize revenue under the arrangement on a straight-line basis over the period it expects to complete its performance obligations or upon completion when the final act is of such significance to the overall arrangement that performance has not substantively occurred until the completion of that act. Conversely, if the pattern of performance over which the service is provided to the customer can be determined and objectively measurable performance measures exist, then the Company would recognize revenue under the arrangement using the proportional performance method.five-step model described above.
The Company recognized revenue under its research agreement based upon the completed performance method of revenue recognition as it was unable to reasonably estimate the period of performance of the services and the delivery of the final study report was significant to the arrangement.
Research and Development
Expenditures relating to research and development are expensed in the period incurred. Research and development expenses consist of both internal and external costs associated with the development of the Company’s gene control platform and product candidates. Research and development costs include salaries and benefits, materials and supplies, external research, preclinical and clinical development expenses, stock-based compensation expense and facilities costs. Facilities costs primarily include the allocation of rent, utilities, depreciation and depreciation.amortization.
In certain circumstances, the Company is required to make nonrefundable advance payments to vendors for goods or services that will be received in the future for use in research and development activities. In such circumstances, the nonrefundable advance payments are deferred and capitalized, even when there is no alternative future use for the research and development, until related goods or services are provided.
The Company records accruals for estimated ongoing research costs. When evaluating the adequacy of the accrued liabilities, the Company analyzes progress of the work being performed, including the phase or completion of the event, invoices received and costs. Significant judgementsjudgments and estimates may be made in determining the accrued balances at the end of any reporting period. Actual results could differ from the Company’s estimates.
The Company may in the future in-license the rights to develop and commercialize product candidates. For each in-license transaction the Company evaluates whether it has acquired processes or activities along with inputs that would be sufficient to constitute a “business” as defined under U.S. GAAP. A “business” as defined under U.S. GAAP consists of inputs and processes applied to those inputs that have the ability to create outputs. Although businesses
Table of Contents
Syros Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
usually have outputs, outputs are not required for an integrated set of activities to qualify as a business. When the Company determines that it has not acquired sufficient processes or activities to constitute a business, any up-front payments, as well as milestone payments, are immediately expensed as acquired research and development in the period in which they are incurred.
Warrants
The Company accounts for issued warrants either as a liability or equity in accordance with ASC 480-10, Accounting for Certain Financial Instruments with Characteristics of both Liabilities and Equity (“ASC 480-10”) or
Syros Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
ASC 815-40, Accounting for Derivative Financial Instruments Indexed to, and Potentially Settled in, a Company’s Own Stock (“ASC 815-40”). Under ASC 480-10, warrants are considered liabilities if they are mandatorily redeemable and they require settlement in cash, other assets, or a variable number of shares. If warrants do not meet liability classification under ASC 480-10, the Company considers the requirements of ASC 815-40 to determine whether the warrants should be classified as liability or equity. Under ASC 815-40, contracts that may require settlement for cash are liabilities, regardless of the probability of the occurrence of the triggering event. Liability classified warrants are measured at fair value on the issuance date and at the end of each reporting period. Any change in the fair value of the warrants after the issuance date is recorded in the consolidated statements of operations as a gain or loss. If warrants do not require liability classification under ASC 815-40, in order to conclude warrants should be classified as equity, the Company assesses whether the warrants are indexed to its common stock and whether the warrants are classified as equity under ASC 815-40 or other applicable GAAP. Equity classified warrants are accounted for at fair value on the issuance date with no changes in fair value recognized after the issuance date.
Stock-Based Compensation Expense
The Company accounts for its stock-based compensation awards in accordance with ASC Topic 718, Compensation—Stock Compensation (“ASC 718”). ASC 718 requires all stock-based payments to employees and directors, including grants of restricted stock units and stock options,option awards, to be recognized as expense in the consolidated statements of operations based on their grant date fair values. Grants of restricted stock and stock options to other service providers, referred to as non-employees, are required to be recognized as expense in the consolidated statements of operations based on their vesting date fair values. The Company estimates the fair value of stock options granted using the Black-Scholes option-pricing model. Prior to June 30, 2016, theThe Company was a private company and as such lacks Company-specific historical and implied volatility information. Therefore, it estimates its expected stock volatility based on theits historical volatility of a publicly traded set of peer companies and expects to continue to do so until such time as it has adequate historical data regarding the volatility of its own traded stock price.volatility. The expected term of the Company’s stock options granted to employees has been determined utilizing the “simplified” method for awards that qualify as “plain-vanilla” options. The Company uses the contractual term in determining the expected term of the stock options granted to non-employees is equal to the contractual term of the option award.non-employees. The risk-free interest rate is determined by reference to the U.S. Treasury yield curve in effect at the time of grant of the award for time periods approximately equal to the expected term of the award. Expected dividend yield is based on the fact that the Company has never paid cash dividends and does not expect to pay any cash dividends in the foreseeable future. The Company uses the value of its common stock at the grant date to determine the fair value of restricted stock awards.
Additionally, in March 2016, the FASB issued ASU No. 2016-09, Compensation—Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting (“ASU 2016-09”). ASU 2016-09 simplifies several aspects of the accounting for share-based payment transactions, including the income tax consequences, classification of awards as either equity or liabilities, and an option to recognize gross stock-based compensation expense with actual forfeitures as they occur, as well as certain classification on the statement of cash flows. For public entities, ASU 2016-09 is effective for fiscal years beginning after December 15, 2016, and interim periods within those fiscal years. The Company has adopted ASU 2016-09 as of January 1, 2017. The Company has applied ASU 2016-09 using a modified retrospective approach and has adopted the option to recognize stock compensation expense with actual forfeitures recognized as they occur. The adoption of this standard had an immaterial impact to the Company’s financial statements. The adoption of ASU 2016-09 also requires all excess tax benefit on stock options to be recorded in the consolidated statements of operations. The adoption did not have a material impact since the expected increase in net deferred tax assets is fully offset by a corresponding increase in the deferred tax asset valuation allowance. The amount of deferred tax assets that had not been previously recognized due to the recognition of excess tax benefits upon adoption was $0.4 million.
The Company expenses the fair value of its stock-based awards to employees and non-employees on a straight-line basis over the associated service period, which is generally the vesting period. As a result of the adoption of ASU 2016-09, effective January 1, 2017, theThe Company accounts for forfeitures as they occur instead of estimating forfeitures at the time of grant and revising those estimates in subsequent periods if actual forfeitures differ from its estimates.grant. Ultimately, the actual expense recognized over the vesting period will be for only those options that vest.For stock-based awards granted
Compensation expense for discounted purchases under the employee stock purchase plan is measured using the Black-Scholes model to consultants and non-employees, compensation expense is recognized over the period during which services are rendered by such consultants and non-employees until completed. At the end of each financial reporting period prior to completion of the service,compute the fair value of these awardsthe lookback provision plus the purchase discount and is remeasured usingrecognized as compensation expense over the then-current fair value of such awards.offering period.
For stock-based awards that contain performance-based milestones, the Company records stock-based compensation expense in accordance with the accelerated attribution model. Management evaluates when the achievement of a performance-based milestone is probable based on the expected satisfaction of the performance conditions as of the reporting date. For certain of the Company’s performance-based awards, notwithstanding any vesting in accordance with the achievement of performance-based milestones, such awards vest in full on the sixth anniversary of the vesting commencement date.
Table of Contents
Syros Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
Income Taxes
The Company accounts for income taxes in accordance with ASC Topic 740, Income Taxes (‘‘ASC 740’’). The Company recognizes deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in the Company’s financial statements or tax returns. Under this method, deferred tax assets and liabilities are determined based on differences between the financial statement carrying amounts and the tax bases of the assets and liabilities using the enacted tax rates in effect in the years in which the differences are expected to reverse. A valuation allowance against deferred tax assets is recorded if, based on the weight of the available evidence, it is more likely than not that some or all of the deferred tax assets will not be realized.
The Company accounts for uncertain tax positions using a more-likely-than-not threshold for recognizing and resolving uncertain tax positions. The evaluation of uncertain tax positions is based on factors including, but not limited to, changes in the law, the measurement of tax positions taken or expected to be taken in tax returns, the effective settlement of matters subject to audit, new audit activity, and changes in facts or circumstances related to a tax position.
Net Loss per Share
Basic net lossearnings per share applicable to common stockholders is calculated by dividing net lossearnings applicable to common stockholders by the weighted average shares outstanding during the period, without consideration for common stock equivalents. Diluted net lossearnings per share applicable to common stockholders is calculated by adjusting the weighted average shares outstanding for the dilutive effect of common stock equivalents outstanding for the period, determined using the treasury-stock method and the if-converted method. For purposes of the calculation of dilutive net loss per share applicable to common stockholders, calculation, convertible preferred stock, stock options, and unvested restricted stock units, and
Syros Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
warrants are considered to be common stock equivalents but are excluded from the calculation of diluted net loss per share applicable to common stockholders, as their effect would be anti-dilutive; therefore, basic and diluted net loss per share applicable to common stockholders were the same for all periods presented.
The following outstanding pre-funded warrants as of December 31, 2023 and 2022,were included in the basic and diluted net loss per share calculation:
| | | | | | | | |
| | As of December 31, | |
| | 2023 | | | 2022 | |
2020 Pre-Funded Warrants, issued in the 2020 Private Placement (refer to Note 12) | | | 100,000 | | | | 100,000 | |
2022 Pre-Funded Warrants, issued in the 2022 Private Placement (refer to Note 12) | | | 7,179,819 | | | | 7,426,739 | |
2023 Pre-Funded Warrants, issued in December 2023 registered direct offering (refer to Note 12) | | | 5,242,588 | | | | — | |
Total | | | 12,522,407 | | | | 7,526,739 | |
| | | | | | |
The following common stock equivalents were excluded from the calculation of diluted net loss per share applicable to common stockholders for the periods indicated because including them would have had an anti-dilutive effect.effect:
| | | | | | | |
| | As of December 31, | |
| | 2017 | | 2016 | | 2015 | |
Convertible preferred stock | | — | | — | | 12,598,370 | |
Stock options | | 2,846,668 | | 2,543,435 | | 2,226,698 | |
Unvested restricted stock | | — | | 4,885 | | 256,881 | |
| | 2,846,668 | | 2,548,320 | | 15,081,949 | |
| | | | | | | | |
| | As of December 31, | |
| | 2023 | | | 2022 | |
Stock options | | | 1,548,642 | | | | 1,727,327 | |
Unvested restricted stock units | | | 1,706,614 | | | | 1,204,421 | |
Warrants* | | | 14,142,298 | | | | 14,142,298 | |
Total | | | 17,397,554 | | | | 17,074,046 | |
* As of December 31, 2023 and December 31, 2022, this is comprised of 2,754 warrants to purchase common stock issued in connection with the execution and first draw of the Loan Agreement in February 2020 (refer to Note 8), 1,738 warrants to purchase common stock issued in connection with the second draw on the Loan Agreement in December 2020 (refer to Note 8), 282,809 warrants to purchase common stock issued in connection with the private placement in December 2020 (refer to Note 12), 13,813,912 warrants to purchase common stock issued in connection with the private placement in September 2022 (refer to Note 12), and 41,085 warrants to purchase common stock that were issued upon the assumption and conversion of Tyme warrants in connection with the Merger (refer to Note 3).
The weighted average number of common shares used in net loss per share applicable to common stockholders on a basic and diluted basis were 25,406,845, 12,696,41428,325,779 and 1,980,28612,631,968 shares for the years ended December 31, 2017, 20162023 and 2015,2022, respectively.
Recent Accounting Pronouncements
In May 2014, the FASB issued ASU No. 2014-09, Revenue From Contracts With Customers (“ASU 2014-09”). ASU 2014-09 amends ASC 605, Revenue Recognition, by outlining a single comprehensive model for entities to use in accounting for revenue arising from contracts with customers. ASU 2014-09 will be effective for the Company for interim and annual periods beginning after December 15, 2017. Entities have the option of using either a full retrospective or a modified retrospective approach to adopt this new guidance. The FASB issued supplemental adoption guidance and clarification to ASU 2014-09 in March 2016, April 2016, May 2016, and December 2016 within ASU 2016-08 “Revenue from Contracts with Customers: Principal vs. Agent Considerations,” ASU 2016-10 “Revenue from Contracts with Customers: Identifying Performance Obligations and Licensing,” ASU 2016-12 “Revenue from Contracts with Customers: Narrow-Scope Improvements and Practical Expedients,” and ASU 2016-20 “Technical Corrections and
Table of ContentsRecently Adopted Accounting Pronouncements
Syros Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
Improvements to Topic 606, Revenue from Contracts with Customers,” respectively. As of December 31, 2017, the Company had one revenue arrangement, which was completed on March 31, 2017, prior to adoption. The Company plans to use the modified retrospective approach in adopting this standard.
As described further in Note 14 – Subsequent Events, the Company entered into a target discovery, research collaboration and option agreement with Incyte in January 2018 under which the Company will use its gene control platform to identify novel therapeutic targets with a focus on myeloproliferative neoplasms, and Incyte will receive options to obtain exclusive worldwide rights to intellectual property resulting from the collaboration for the development and commercialization of therapeutic products directed to up to seven validated targets. Upon execution of the agreement, Incyte paid the Company $10.0 million in up-front consideration, consisting of $2.5 million in cash and $7.5 million in pre-paid research funding. The Company is eligible to receive target selection milestone payments and option exercise fees of up to an aggregate of $54.0 million, and if products arising from the collaboration are approved, the Company would become eligible to receive from Incyte, for each validated target, a total of up to $50.0 million in development and regulatory milestone payments and up to $65.0 million in commercial milestone payments. No revenue was recognized under this agreement during the year ended December 31, 2017. The Company will recognize revenue related to this agreement using the new standard during 2018.
In FebruaryJune 2016, the FASB issued ASU No. 2016-02, Leases (“2016-13, Financial Instruments-Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments (“ASU 2016-02”2016-13”), which appliesrequires the measurement and recognition of expected credit losses for financial assets held at amortized cost. ASU 2016-13 replaces the existing incurred loss impairment model with an expected loss model that requires the use of forward-looking information to all leasescalculate credit loss estimates. It also eliminates the concept of other-than-temporary impairment and will require lesseesrequires credit losses on available-for-sale debt securities to put most leases onbe recorded through an allowance for credit losses instead of as a reduction in the balance sheet, but recognize expense in a manner similar toamortized cost basis of the current standard.securities. ASU 2016-02 is2016-13 became effective for the Company for fiscal years beginning after December 15, 20182022. The Company adopted this new standard on January 1, 2023, and interim periods within those years, and, as such, will be effective for the year ended December 31, 2019 for the Company. Entities are required to use a modified retrospective approach of adoption for leases that exist or are entered into after the beginning of the earliest comparative period in the financial statements. Full retrospective application is prohibited. The Company is evaluating the new guidance and the expected effect on the Company’s consolidated financial statements. However, the Company anticipates recognition of additional assets and corresponding liabilities related to its operating leases. To date, the Company has one operating lease for its office and laboratory space in Cambridge, Massachusetts (Note 9).
In August 2016, the FASB issued ASU No. 2016-15,Statement of Cash Flows (Topic 230)(“ASU No. 2016-15”),which simplifies certain elements of cash flow classification. The new guidance is intended to reduce diversity of practice in how certain transactions are classified in the statement of cash flows. ASU No. 2016-15 is effective for annual periods beginning after December 15, 2017. The Company is currently evaluating the impact the adoption of ASU No. 2016-15 will haveon its consolidated financial statements.
In November 2016, the FASB issued ASU No. 2016-18,Restricted Cash(“ASU No. 2016-18”). The amendments in ASU No. 2016-18 require an entity to reconcile and explain the period-over-period change in total cash, cash equivalents and restricted cash within its statements of cash flows. ASU No. 2016-18 is effective for fiscal years (including interim reporting periods within those years) beginning after December 15, 2017. Early adoption is permitted. A reporting entity must apply the amendments in ASU No. 2016-18 using a full retrospective approach. The Company believes that the adoption of this guidance will notit did not have a significantmaterial impact on its consolidated financial statements and related disclosures.
3. Recapitalization
In January 2017,On September 16, 2022, the FASBCompany issued ASU No. 2017-01, Business Combinations (Topic 805): Clarifyingapproximately 7.5 million shares of its common stock to the Definition of a Business (“ASU No. 2017-01”). The amended guidance clarifies the definition of a businessformer Tyme stockholders in connection with the objectiveMerger. The Company also issued options and warrants to purchase
Syros Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
733,545 shares of adding guidancethe Company’s common stock to assist entities with evaluating whether transactions should becertain holders of Tyme options and warrants that were outstanding immediately before the consummation of the Merger. The Merger is accounted for as acquisitions (or disposals)a recapitalization because the Company was determined to be a legal and accounting acquirer under Financial Accounting Standards Board’s Accounting Standards Codification Topic 805, Business Combinations (“ASC 805”). This determination was primarily based on the following facts and circumstances:
•The pre-combination equity holders of the Company hold the relative majority of voting rights in the combined entity;
•The pre-combination equity holders of the Company have the right to appoint the majority of the directors on the combined entity’s board of directors;
•Senior management of the Company comprises the senior management of the combined entity;
•Operations of the Company comprise the ongoing operations of the combined entity; and
•Upon effectiveness of the Merger, the primary assets or businesses. of Tyme at the effective date were primarily cash, cash equivalents and marketable securities.
Under the recapitalization accounting model, the net assets acquired are recognized at fair value and any excess consideration transferred over the fair value of the net assets are reflected as a reduction to equity. Transaction costs incurred attributable to the Merger are also reflected as a reduction to the equity.
The new accounting guidancecarrying value of Tyme’s net assets as of September 16, 2022, which approximates fair value because of its short-term nature, is effective for annual periods beginning after December 15, 2017, including interim periods within those periods. Early adoption is permitted. set forth below:
| | | | | | |
|
|
|
| Fair Value |
|
Cash and cash equivalents |
|
|
| $ | 14,898 |
|
Marketable securities |
|
|
|
| 52,220 |
|
Prepaid expenses |
|
|
|
| 1,350 |
|
Total |
|
|
| $ | 68,468 |
|
No value has been ascribed to the development programs acquired from Tyme in the Merger.
The Company willincurred $3.1 million of transaction costs attributable to the Merger which are reflected as a reduction of additional paid-in capital. In addition, the Company paid $4.5 million of severance to former Tyme employees which is included in the Company's statement of operations as transaction related expenses.
4. Collaboration and Research Arrangements
Collaboration with Global Blood Therapeutics
On December 17, 2019, the Company entered into a license and collaboration agreement (the “GBT Collaboration Agreement”) with Global Blood Therapeutics, Inc. (“GBT”), now a subsidiary of Pfizer Inc. (“Pfizer”), pursuant to which the parties agreed to a research collaboration to discover novel targets that induce fetal hemoglobin in order to develop new small molecule treatments for sickle cell disease and beta thalassemia. The research term (the “Research Term”) was for an initial period of three years and could be extended for up to two additional one-year terms upon mutual agreement. In November 2022, the Company and GBT agreed to extend the Research Term for an additional one-year period. In July 2023, Pfizer, as successor to GBT, elected to exercise its right to terminate the GBT Collaboration Agreement, effective October 16, 2023.
Pursuant to the terms of the GBT Collaboration Agreement, GBT paid the Company an upfront payment of $20.0 million. GBT also agreed to reimburse the Company for full-time employee and out-of-pocket costs and expenses incurred by the Company in accordance with the agreed-upon research budget, which was anticipated to total approximately $40.0 million over the initial Research Term.
The Company granted to GBT an option (the “Option”) to obtain an exclusive, worldwide license, with the right to sublicense, under relevant intellectual property rights and know-how of the Company arising from the
Syros Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
collaboration to develop, manufacture and commercialize any compounds or products resulting from the collaboration. This Option terminated simultaneously with the effective date of termination of the GBT Collaboration Agreement, and the Company is no longer eligible to receive any milestone or royalty-based payments from GBT.
GBT Collaboration Revenue
The Company analyzed the GBT Collaboration Agreement and concluded that it represented a contract with a customer within the scope of ASC 606.
The Company identified a single performance obligation, which included a (i) non-exclusive research license that GBT had access to during the initial Research Term and (ii) research and development services provided during the initial Research Term. The non-exclusive research license only allowed GBT to evaluate the impactcandidate compounds developed under the research plan or to conduct work allocated to it during the Research Term. GBT could not extract any benefit from the non-exclusive research license without the research and development services performed by the Company, including the provision of data package information. As such, these two promises are inputs to a combined output (the delivery of data package allowing GBT to make an Option exercise decision) and are bundled into a single performance obligation (the non-exclusive research license and research and development service performance obligation).
ASC 606 requires an entity to recognize revenue only when it satisfies a performance obligation by transferring a promised good or service to a customer. A good or service is considered to be transferred when the customer obtains control. As the non-exclusive research license and research and development services represent one performance obligation, the Company has determined that it would satisfy its performance obligation over a period of time as services are performed and GBT receives the adoptionbenefit of ASU No. 2017-01 will havethe services, as the overall purpose of the arrangement is for the Company to perform the services. The Company recognizes revenue associated with the performance obligation as the research and development services are provided using an input method, according to the costs incurred as related to the research and development activities and the costs expected to be incurred in the future to satisfy the performance obligation. The transfer of control occurs during this time and is the best measure of progress towards satisfying the performance obligation.
The Company recognized revenue under the GBT Collaboration Agreement of $9.9 million and $13.6 million during the years ended December 31, 2023 and 2022, respectively. As of December 31, 2023, the GBT Collaboration Agreement has been terminated, and the Company has completed all performance obligations under the GBT Collaboration Agreement and has no receivable or deferred revenue outstanding under the GBT Collaboration Agreement.
Agreement with Incyte Corporation
In January 2018, the Company and Incyte entered into a Target Discovery, Research Collaboration and Option Agreement (the “Incyte Collaboration Agreement”). The Incyte Collaboration Agreement was amended in November 2019. Under the terms of the Incyte Collaboration Agreement, Incyte paid the Company $10.0 million in up-front consideration, consisting of $2.5 million in cash and $7.5 million in pre-paid research funding (the “Prepaid Research Amount”). On August 9, 2023, Incyte elected to terminate the Incyte Collaboration Agreement.
Incyte Collaboration Revenue
The Company analyzed the Incyte Collaboration Agreement and concluded that it represents a contract with a customer within the scope of ASC 606.
The Company identified a single performance obligation which included (i) a research license that Incyte retained as long as there remained an unexercised option (the “Research License”), and (ii) research and development services provided during the research term. The Incyte Collaboration Agreement included options to (x) obtain additional time to exercise the license options for certain targets designated as definitive validation targets, and (y) obtain license rights to each validated target, both of which were not considered by the Company’s management to be material rights, and therefore not performance obligations, at inception.
Syros Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
The total transaction price following the November 2019 amendment was $12.8 million, consisting of a $2.5 million upfront non-refundable and non-creditable payment, the $7.5 million Prepaid Research Amount, $2.3 million in premium paid on the equity investment made pursuant the Stock Purchase Agreement, and $0.5 million of additional consideration. The Company accounted for the contract amendment as a modification as if it were part of the existing contract as the remaining goods and services are not distinct, and therefore form part of a single performance obligation that was partially satisfied at the date of the amendment.
The Company recognizes revenue associated with the performance obligation as the research and development services are provided using an input method, according to the costs incurred as related to the research and development activities and the costs expected to be incurred in the future transactions.to satisfy the performance obligation. The transfer of control occurs during this time and is the best measure of progress towards satisfying the performance obligation.
The Company did not recognize any revenue under the Incyte Collaboration Agreement during the year ended December 31, 2023. During the year ended December 31, 2022, the Company recognized $1.3 million of revenue under the Incyte Collaboration Agreement. As of December 31, 2023, the Company has no deferred revenue outstanding under the Incyte Collaboration Agreement.
The following table presents the changes in contract liabilities for the year ended December 31, 2023 (in thousands):
| | | | | | | | | | | | | | | | |
| | Balance at
December 31, 2022 | | | Additions | | | Deductions | | | Balance at December 31, 2023 | |
Contract liabilities: | | | | | | | | | | | | |
Deferred revenue - GBT | | $ | 4,330 | | | $ | — | | | $ | 4,330 | | | $ | — | |
Total contract liabilities | | $ | 4,330 | | | $ | — | | | $ | 4,330 | | | $ | — | |
3.The Company recognized the balance of the deferred revenue as of December 31, 2022 into revenue during the year ended December 31, 2023 as the GBT Collaboration Agreement was terminated in October 2023.
5. Cash, Cash Equivalents and Marketable Securities
Cash equivalents are highly liquid investments that are readily convertible into cash with original maturities of three months or less when purchased. Marketable securities consist of securities with original maturities greater than 90 days when purchased. The Company classifies these marketable securities as available-for-sale and records them at fair value in the accompanying consolidated balance sheets. Unrealized gains or losses are included in accumulated other
Table of Contents
Syros Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
comprehensive income (loss).loss. Premiums or discounts from par value are amortized to investmentother income over the life of the underlying security.
Cash, cash equivalents and marketable securities, available-for-sale, consisted of the following at December 31, 20172023 and December 31, 20162022 (in thousands):
| | | | | | | | | | | | | | | | |
| | | | | Unrealized | | | Unrealized | | | Fair | |
December 31, 2023 | | Amortized Cost | | | Gains | | | Losses | | | Value | |
Cash and cash equivalents: | | | | | | | | | | | | |
Cash and money market funds | | $ | 139,526 | | | $ | — | | | $ | — | | | $ | 139,526 | |
Total: | | $ | 139,526 | | | $ | — | | | $ | — | | | $ | 139,526 | |
| | | | | | | | | | | | |
| | | | | | | | | | | | | |
| | | | | Unrealized | | Unrealized | | Fair | |
December 31, 2017 | | Amortized Cost | | Gains | | Losses | | Value | |
Cash Equivalents: | | | | | | | | | | | | | |
Money market funds | | $ | 17,205 | | $ | — | | $ | — | | $ | 17,205 | |
Overnight repurchase agreements | | | 15,000 | | | — | | | — | | | 15,000 | |
Marketable Securities: | | | | | | | | | | | | | |
U.S. treasury obligations | | | 39,886 | | | — | | | (42) | | | 39,844 | |
Total: | | $ | 72,091 | | $ | — | | $ | (42) | | $ | 72,049 | |
| | | | | | | | | | | | | |
111
Syros Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
| | | | | | | | | | | | | |
| | | | | Unrealized | | Unrealized | | Fair | |
December 31, 2016 | | Amortized Cost | | Gains | | Losses | | Value | |
Cash Equivalents: | | | | | | | | | | | | | |
Money market funds | | $ | 58,588 | | $ | — | | $ | — | | $ | 58,588 | |
Marketable Securities: | | | | | | | | | | | | | |
U.S. treasury obligations | | | 25,014 | | | — | | | (9) | | | 25,005 | |
Total: | | $ | 83,602 | | $ | — | | $ | (9) | | $ | 83,593 | |
| | | | | | | | | | | | | | | | |
| | | | | Unrealized | | | Unrealized | | | Fair | |
December 31, 2022 | | Amortized Cost | | | Gains | | | Losses | | | Value | |
Cash and cash equivalents: | | | | | | | | | | | | |
Cash and money market funds | | $ | 167,467 | | | $ | — | | | $ | — | | | $ | 167,467 | |
Marketable securities: | | | | | | | | | | | | |
Corporate debt securities - due in one year or less | | | 22,257 | | | | 116 | | | | (53 | ) | | | 22,320 | |
Commercial paper - due in one year or less | | | 2,491 | | | | — | | | | — | | | | 2,491 | |
Municipal bonds - due in one year or less | | | 5,987 | | | | 51 | | | | — | | | | 6,038 | |
US Treasury obligation - due in one year or less | | | 4,000 | | | | — | | | | (12 | ) | | | 3,988 | |
Total | | $ | 202,202 | | | $ | 167 | | | $ | (65 | ) | | $ | 202,304 | |
Although available to be sold to meet operating needs or otherwise, securities are generally held through maturity. The cost of securities sold is determined based on the specific identification method for purposes of recording realized gains and losses. During the yearyears ended December 31, 2017,2023 and 2022, there were no realized gains or losses on sales of investments, and no investments were adjusted for other than temporary declines in fair value.
At December 31, 2017, the Company held 17 securities that were in an unrealized loss position. The aggregate fair value of securities held by the Company in an unrealized loss position for less than twelve months asAs of December 31, 2017 was $39.8 million and there were no securities held by2023, the Company had no investments in an unrealized loss position for more than twelve months. The Company has the intent and ability to hold such securities until recovery. The Company determined that there was no material change in the credit risk of the above marketable securities. As a result, the Company determined it did not hold anyof December 31, 2022, marketable securities with an other-than temporary impairment asmaturities of December 31, 2017.one year or less when purchased are presented in current assets in the accompanying consolidated balance sheet.
4.6. Fair Value Measurements
Assets and liabilities measured at fair value on a recurring basis areas of December 31, 2023 and 2022 were as follows (in thousands):
| | | | | | | | | | | | | |
| | | | | Active | | Observable | | Unobservable | |
| | | | | Markets | | Inputs | | Inputs | |
Description | | December 31, 2017 | | (Level 1) | | (Level 2) | | (Level 3) | |
Cash equivalents: | | | | | | | | | | | | | |
Money market funds | | $ | 17,205 | | $ | 17,205 | | $ | — | | $ | — | |
Overnight repurchase agreements | | | 15,000 | | | — | | | 15,000 | | | — | |
Marketable securities: | | | | | | | | | | | | | |
U.S. treasury obligations | | | 39,844 | | | 39,844 | | | — | | | — | |
| | $ | 72,049 | | $ | 57,049 | | $ | 15,000 | | $ | — | |
| | | | | | | | | | | | | | | | |
| | | | | Active | | | Observable | | | Unobservable | |
| | | | | Markets | | | Inputs | | | Inputs | |
Description | | December 31, 2023 | | | (Level 1) | | | (Level 2) | | | (Level 3) | |
Assets: | | | | | | | | | | | | |
Cash and cash equivalents: | | | | | | | | | | | | |
Cash and money market funds | | $ | 139,526 | | | $ | 139,526 | | | $ | — | | | $ | — | |
Total | | $ | 139,526 | | | $ | 139,526 | | | $ | — | | | $ | — | |
Liabilities: | | | | | | | | | | | | |
Warrant liabilities | | $ | 61,747 | | | $ | — | | | $ | — | | | $ | 61,747 | |
Total | | $ | 61,747 | | | $ | — | | | $ | — | | | $ | 61,747 | |
| | | | | | | | | | | | | | | | |
| | | | | Active | | | Observable | | | Unobservable | |
| | | | | Markets | | | Inputs | | | Inputs | |
Description | | December 31, 2022 | | | (Level 1) | | | (Level 2) | | | (Level 3) | |
Assets: | | | | | | | | | | | | |
Cash and cash equivalents: | | | | | | | | | | | | |
Cash and money market funds | | $ | 167,467 | | | $ | 167,467 | | | $ | — | | | $ | — | |
Corporate debt securities - due in one year or less | | | 22,320 | | | | — | | | | 22,320 | | | — | |
Commercial paper | | | 2,491 | | | | — | | | | 2,491 | | | | — | |
Municipal bonds | | | 6,038 | | | | — | | | | 6,038 | | | | — | |
US Treasury obligation - due in one year or less | | | 3,988 | | | | 3,988 | | | | — | | | | — | |
Total | | $ | 202,304 | | | $ | 171,455 | | | $ | 30,849 | | | $ | — | |
Liabilities: | | | | | | | | | | | | |
Warrant liabilities | | $ | 24,472 | | | $ | — | | | $ | — | | | $ | 24,472 | |
Total | | $ | 24,472 | | | $ | — | | | $ | — | | | $ | 24,472 | |
Table of Contents
Syros Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
| | | | | | | | | | | | | |
| | | | | Active | | Observable | | Unobservable | |
| | | | | Markets | | Inputs | | Inputs | |
Description | | December 31, 2016 | | (Level 1) | | (Level 2) | | (Level 3) | |
Cash equivalents: | | | | | | | | | | | | | |
Money market funds | | $ | 58,588 | | $ | 58,588 | | $ | — | | $ | — | |
Marketable securities: | | | | | | | | | | | | | |
U.S. treasury obligations | | | 25,005 | | | 25,005 | | | — | | | — | |
| | $ | 83,593 | | $ | 83,593 | | $ | — | | $ | — | |
Assumptions Used in Determining Fair Value of Warrants
5. Restricted Cash
AtThe Company issued the 2022 Warrants to purchase an aggregate of up to 13,813,912 shares of common stock in connection with the 2022 Private Placement (see Note 12) and the 2020 Warrants to purchase an aggregate of up to 282,809 shares of common stock in connection with a private placement in December 31, 20172020 (see Note 12). The Company accounted for the 2022 Warrants and December 31, 2016,2020 Warrants as liabilities. The Company recorded the Company had $0.5 millionfair value of these warrants upon issuance using the Black-Scholes valuation model and is required to revalue these warrants at each reporting date with any changes in restricted cash that serves as the security depositfair value recorded on the leaseour statement of operations. The valuation of the Company’s current facility in Cambridge, Massachusetts. At December 31, 2017, approximately $0.2 million2022 Warrants and 2020 Warrants is considered under Level 3 of the restricted cash was classified as current as it is expected to be refunded tofair value hierarchy and influenced by the Company under the termsfair value of the lease agreement.
6. Property and Equipment
Property and Equipment consistsunderlying common stock of the Company.
A summary of the Black Scholes pricing model assumptions used to record the fair value of the warrants is as follows:
| | | | | | | | | | |
| | December 31, 2023 | | | | December 31, 2022 |
Stock price | | $ | 7.79 | | | | $ | 3.59 | | |
Average risk-free interest rate | | | 3.96 | | % | | | 4.02 | | % |
Average expected life (in years) | | | 3.67 | | | | | 4.67 | | |
Average expected volatility | | | 87.63 | | % | | | 86.79 | | % |
Changes in Level 3 Liabilities Measured at Fair Value on a Recurring Basis
The following (in thousands):
| | | | | | | | | |
| | Estimated useful life | | | | | | | |
| | (in years) | | December 31, 2017 | | December 31, 2016 | |
Laboratory equipment | | 5 | | $ | 3,978 | | $ | 3,612 | |
Computer equipment | | 3 | | | 651 | | | 401 | |
Furniture and fixtures | | 4 | | | 396 | | | 395 | |
Leasehold improvements | | Shorter of 7 years or life of lease | | | 2,613 | | | 2,599 | |
Construction in process | | | | | — | | | 18 | |
| | | | $ | 7,638 | | $ | 7,025 | |
Less: Accumulated depreciation | | | | | (3,700) | | | (2,175) | |
Total property and equipment, net | | | | $ | 3,938 | | $ | 4,850 | |
Depreciation expense, including depreciation expense for assets recorded under capital leases,table reflects the change in the Company’s Level 3 Warrant liabilities for the years ended December 31, 2017, 20162023 and 2015 was $1.5 million, $1.32022 (in thousands):
| | | | | | | | | |
| | December 31, 2023 | | | | December 31, 2022 | |
Fair value of warrant liabilities as of beginning of year | | $ | 24,472 | | | | $ | 3,029 | |
Warrants issued in connection with 2022 Private Placement | | | — | | | | | 64,664 | |
Change in fair value | | | 37,275 | | | | | (43,221 | ) |
Fair value of warrant liabilities as of end of year | | $ | 61,747 | | | | $ | 24,472 | |
7. Restricted Cash
As of December 31, 2023 and 2022, the Company had $2.1 million and $0.6$3.1 million, respectively. Laboratory equipment included assets recorded under capital leasesrespectively, in restricted cash, which was classified as long-term on the Company’s consolidated balance sheets, and all of $0.4which was attributable to the HQ Lease (as defined in Note 11).
In connection with the execution of the HQ Lease, the Company was required to provide the landlord with a letter of credit in the amount of $3.1 million atthat will expire 95 days after expiration or early termination of the HQ Lease. Pursuant to the HQ Lease, the Company exercised its right to reduce the amount of the letter of credit to $2.1 million during the year ended December 31, 2017 (Note 8). Accumulated depreciation from assets recorded under capital leases was $0.2 million at2023.
The following table provides a reconciliation of cash, cash equivalents, and restricted cash reported within the Company’s consolidated balance sheets that sum to the total of the amounts shown in the consolidated statement of cash flows as of December 31, 2017. 2023 and 2022 (in thousands):
| | | | | | | | |
| | December 31, | |
| | 2023 | | | 2022 | |
Cash and cash equivalents | | $ | 139,526 | | | $ | 167,467 | |
Restricted cash | | $ | 2,119 | | | | 3,086 | |
Total cash, cash equivalents and restricted cash | | $ | 141,645 | | | $ | 170,553 | |
Syros Pharmaceuticals, Inc.
7.Notes to Consolidated Financial Statements (Continued)
8. Oxford Finance Loan Agreement
On February 12, 2020, the Company entered into a Loan and Security Agreement (the “Loan Agreement”) with Oxford Finance LLC (the “Lender”). Pursuant to the Loan Agreement, a term loan of up to an aggregate principal amount of $60.0 million is available to the Company. A $20.0 million term loan (first tranche) was funded on February 12, 2020, and another $20.0 million term loan (second tranche) was funded on December 23, 2020. As of December 31, 2023, the final $20.0 million tranche remained available under the Loan Agreement, at the sole discretion of the Lender.
The term loan initially bore interest at an annual rate equal to the greater of (i) 7.75% and (ii) the sum of 5.98% and the greater of (A) one-month LIBOR or (B) 1.77%. The Loan Agreement initially provided for interest-only payments until March 1, 2023, and repayment of the aggregate outstanding principal balance of the term loan in monthly installments starting on March 1, 2023 and continuing through February 1, 2025 (the “Maturity Date”). Pursuant to the terms of an amendment to the Loan Agreement dated July 3, 2022 (the “Loan Agreement Amendment”), effective September 16, 2022, Oxford agreed to extend the interest-only period from March 1, 2023 to March 1, 2024 and to extend the Maturity Date from February 1, 2025 to February 1, 2026, and upon the achievement of certain milestones and subject to the payment of certain fees, further extend the interest only period to September 1, 2024 and the Maturity Date to August 1, 2026. Pursuant to the terms of a subsequent amendment to the Loan Agreement dated November 15, 2022, the floating annual rate for each term loan was amended to equal the greater of (i) 7.75% and (ii) the sum of (a) the 1-month CME Term SOFR reference rate, (b) 0.10%, and (c) 5.98%.
The Company paid a facility fee of $0.1 million upon the issuance of the first tranche, paid a facility fee of $75,000 upon the issuance of the second tranche, and must pay a $50,000 facility fee if and when the third tranche is issued. The Company also paid fees of $300,000 related to the Loan Agreement Amendment. The Company is required to make a final payment equal to 5.00% of the amount of the term loan drawn payable on the earlier of (i) the prepayment of the term loan or (ii) the Maturity Date. At the Company’s option, the Company may elect to prepay the loans subject to a prepayment fee equal to the following percentage of the principal amount being prepaid: 2% if an advance is prepaid during the first 12 months following the applicable advance date, 1% if an advance is prepaid after 12 months but prior to 24 months following the applicable advance date, and 0.5% if an advance is prepaid any time after 24 months following the applicable advance date but prior to the Maturity Date.
In connection with the Loan Agreement, the Company granted the Lender a security interest in all of the Company’s personal property now owned or hereafter acquired, excluding intellectual property (but including the right to payments and proceeds of intellectual property), and a negative pledge on intellectual property. The Loan Agreement also contains certain events of default, representations, warranties and non-financial covenants of the Company.
In connection with the issuance of the first tranche, the Company issued the Lender warrants to purchase 2,754 shares of the Company’s common stock at an exercise price per share of $72.60 in February 2020. In connection with the issuance of the second tranche, the Company issued the Lender warrants to purchase 1,738 shares of the Company’s common stock at an exercise price of $115.00 per share in December 2020 (collectively, the “Oxford Warrants”). The Oxford Warrants are exercisable within five years from the respective dates of issuance.
The Oxford Warrants are classified as a component of permanent equity because they are freestanding financial instruments that are legally detachable and separately exercisable from the shares of common stock with which they were issued, are immediately exercisable, do not embody an obligation for the Company to repurchase its shares, and permit the holders to receive a fixed number of shares of common stock upon exercise. In addition, the Oxford Warrants do not provide any guarantee of value or return.
Syros Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
The Company has the following minimum aggregate future loan payments as of December 31, 2023 (in thousands):
| | | | |
Year ending December 31, 2024 | | $ | 6,667 | |
Year ending December 31, 2025 | | | 20,000 | |
Year ending December 31, 2026 | | | 13,333 | |
Total minimum payments | | | 40,000 | |
Less unamortized debt discount | | | (335 | ) |
Plus accumulated accretion of final fees | | | 1,558 | |
Less current portion | | | (6,667 | ) |
Long-term debt, net of current portion | | $ | 34,556 | |
For the years ended December 31, 2023 and 2022 interest expense related to the Loan Agreement was approximately $5.1 million and $4.1 million, respectively.
9. Property and Equipment
Property and Equipment consist of the following as of December 31, 2023 and 2022 (in thousands):
| | | | | | | | | | |
| | | | | | | | |
| | Estimated useful life
(in years) | | December 31, 2023 | | | December 31, 2022 | |
Laboratory equipment | | 5 | | $ | — | | | $ | 9,140 | |
Computer equipment | | 3 | | | 2,200 | | | | 2,036 | |
Furniture and fixtures | | 4 | | | 1,075 | | | | 1,075 | |
Leasehold improvements | | * | | | 11,657 | | | | 11,657 | |
| | | | | 14,932 | | | | 23,908 | |
Less: Accumulated depreciation | | | | | (7,634 | ) | | | (12,555 | ) |
Total property and equipment, net | | | | $ | 7,298 | | | $ | 11,353 | |
* Leasehold improvements are depreciated over the shorter of the life of the asset and the term of the lease at 6.2 years and 7.2 years as of December 31, 2023 and 2022, respectively.
Depreciation expense for the years ended December 31, 2023 and 2022 was $2.3 million and $2.7 million, respectively.
10. Accrued Expenses
Accrued expenses consist of the following as of December 31, 2023 and 2022 (in thousands):
| | | | | | | |
| | December 31, 2017 | | December 31, 2016 | |
External research and preclinical development | | $ | 5,875 | | $ | 3,290 | |
Employee compensation and benefits | | | 2,494 | | | 1,911 | |
Professional fees | | | 1,225 | | | 819 | |
Facilities | | | 134 | | | 90 | |
Restricted stock liability | | | — | | | 5 | |
| | $ | 9,728 | | $ | 6,115 | |
| | | | | | | | | |
| | | December 31, 2023 | | | December 31, 2022 | |
External research and preclinical development | | | $ | 8,001 | | | $ | 8,219 | |
Employee compensation and benefits | | | | 6,993 | | | | 8,529 | |
Professional fees | | | | 1,015 | | | | 1,164 | |
Facilities and other | | | | 137 | | | | 54 | |
Accrued expenses | | | $ | 16,146 | | | $ | 17,966 | |
Table of Contents11. Commitments and Contingencies
Syros Pharmaceuticals, Inc.Operating Lease
Notes to Consolidated Financial Statements (Continued)
8. Indebtedness
Equipment Financing
In March 2015,On January 8, 2019, the Company entered into a lease agreement with a vendor for certain laboratory equipment. The Company financed $0.4 million of the amount owed under the lease agreement and is required to make consecutive monthly payments of principal, plus accrued interest at 6.44%, over 36 months through March 2018. During the year ended December 31, 2017, the Company made payments of $0.2 million, of which $9,000 related to interest. At December 31, 2017, $0.1 million of principal was outstanding(the “HQ Lease”) with respect to the equipment financing arrangement.
The following table sets forth the Company’s future minimum payments due under capital leases as of December 31, 2017:
| | | | |
Year | | (in thousands) | |
2018 | | $ | 48 | |
2019 | | | 5 | |
2020 | | | 2 | |
| | $ | 55 | |
9. Commitments and Contingencies
Operating Leases
In March 2015, the Company entered into an operating lease for approximately 21,488 rentable52,859 square feet of office and laboratory space in Cambridge, Massachusetts (the “2015 Lease”), withfor a lease term commencing in August 2015January 2019 and ending in October 2020.February 2030. The Company has anthe option to extend the lease term for fiveone additional years.ten (10) year period. The 2015HQ Lease has escalating rent payments, lease incentives and rent-free periods and the Company records rent expense on a straight-line basis over the term of the lease,HQ Lease, including any rent-free periods. The Company recorded rent expense
Syros Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
In connection with the execution of $0.9 million, $0.9 million, and $0.3 million for the years ended December 31, 2017, 2016 and 2015, respectively, related to the 2015 Lease. The 2015HQ Lease, agreement required the Company was required to issue an originalprovide the landlord with a letter of credit in the amount of $0.5$3.1 million which(See Note 7). The Company determined that, for purposes of applying the lease accounting guidance codified in ASU No. 2016-02, Leases (Topic 842) (“ASC 842”), the commencement date of the HQ Lease occurred on May 1, 2019. The Company recorded a right-of-use asset and lease liability of $15.8 million using an incremental borrowing rate of 9.3%, net of tenant allowances expected to be received of $9.3 million, on the May 1, 2019 lease commencement date. The Company is included in restricted cash inamortizing the accompanyingtenant allowance to offset rent expenses over the term of the HQ Lease starting at the lease commencement date on a straight-line basis. On the Company’s consolidated balance sheet at December 31, 2017sheets, the Company classified $2.3 million and December 31, 2016. At December 31, 2017, approximately $0.2$2.0 million of the restrictedlease liability as short-term and $18.5 million and $20.9 million of the lease liability as long-term as of December 31, 2023 and 2022, respectively.
The Company elected the practical expedient provided under ASC 842 and therefore has combined all lease and non-lease components when determining the right-of-use asset and lease liability for the HQ Lease.
The following is a maturity analysis of the annual undiscounted cash was classified as current as it is expected to be refundedflows reconciled to the carrying value of the operating lease liabilities as of December 31, 2023 (in thousands):
| | | | |
| | Operating | |
| | | |
Year ending December 31, 2024 | | $ | 4,166 | |
Year ending December 31, 2025 | | | 4,287 | |
Year ending December 31, 2026 | | | 4,412 | |
Year ending December 31, 2027 | | | 4,541 | |
Year ending December 31, 2028 and beyond | | | 10,303 | |
Total minimum lease payments | | | 27,709 | |
Less imputed interest | | | (6,857 | ) |
Total lease liability | | $ | 20,852 | |
The following table outlines the total lease cost for the Company’s operating lease as well as weighted average information for this lease as of December 31, 2023 (in thousands):
| | | | |
| | Year Ended December 31, 2023 | |
Lease cost: | | | |
Operating lease cost | | $ | 3,088 | |
Cash paid for amounts included in the measurement of liabilities: | | | |
Operating cash flows from operating lease | | $ | 4,048 | |
| | | |
Other information: | | Year Ended December 31, 2023 | |
Weighted-average remaining lease term (in years) - operating lease | | | 6.17 | |
Weighted-average discount rate - operating lease | | | 9.30 | |
Following the adoption of ASC 842, the Company underhas a right-of-use asset and lease liability that results in recording a temporary tax difference. This temporary tax difference is the result of recognizing a right-of-use asset and related lease liability while such asset and liability have no corresponding tax basis.
Asset Purchase Agreement
Orsenix, LLC
On December 4, 2020, the Company entered into an asset purchase agreement (the “Asset Purchase Agreement”) with Orsenix, LLC (“Orsenix”), pursuant to which the Company acquired all of Orsenix’s assets related to a novel oral form of arsenic trioxide, which the Company refers to as SY-2101. Under the terms of the lease agreement.
The 2015 Lease includes certain lease incentives inAsset Purchase Agreement, the formCompany is required to pay to Orsenix:
•an upfront fee of tenant allowances. The Company has capitalized$12.0 million, which was paid with cash on hand upon the improvements made withclosing of the tenant allowance into fixed assets and established a liability for the deferred lease incentive upon occupancy. The Company recorded these incentives as a component of deferred rent and will amortize these incentives as a reduction of rent expense over the lease term. The related fixed assets will be amortized over the lease term.The following table sets forth the Company’s future minimum payments due under operating leases as of December 31, 2017:
| | | | |
Year | | (in thousands) | |
2018 | | $ | 1,288 | |
2019 | | | 1,325 | |
2020 | | | 1,130 | |
| | $ | 3,743 | |
Table of Contents
Syros Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
License Agreements
Dana-Farber Cancer Institute, Inc. and Whitehead Institute for Biomedical Research
In February 2013, the Company entered into a license agreement with Dana-Farber Cancer Institute, Inc. ("Dana-Farber") pursuant to which the Company was granted an exclusive worldwide, sublicensable license under specified patents relating to CDK7 inhibitors and JNK inhibitors owned or controlled by Dana-Farber. Payments totaling $3.4•single-digit million are due to Dana-Farber if and when the Company achieves certain clinical and regulatory milestones for any licensed product, none of which have been achieved as of December 31, 2017. No future potentialdollar milestone payments have been accrued asrelated to the development of December 31, 2017 or December 31, 2016, respectively, as noSY-2101 in indications other than APL;
•$6.0 million following the achievement of a regulatory milestone related to the development of SY-2101 in APL; and
•up to $10.0 million upon the achievement of certain commercial milestones have been achieved and the agreement can be cancelled at the Company's option. Therefore, the Company had nowith respect to SY-2101.
The Company’s obligation to pay anythe commercial milestone payments expires following the tenth anniversary of these amounts.the first commercial sale of SY-2101. The Asset Purchase Agreement requires the Company to use commercially reasonable efforts to develop and commercialize SY-2101 for APL in the United States during such period, and to use commercially reasonable efforts to dose the first patient in a Phase 3 clinical trial of SY-2101 on or before the third anniversary of the closing of the transaction; however, the Company retains sole discretion to operate the acquired assets as it determines. The assets acquired from Orsenix do not meet the definition of a business under ASC 805, Business Combinations (“ASC 805”) because substantially all of the fair value of the assets acquired is concentrated in a single identifiable asset, the rights to SY-2101. Furthermore, as the acquired asset does not include a substantive process, the asset does not meet the minimum requirements to be considered a business under ASC 805. As SY-2101 does not have an alternative future use, the Company recorded the $12.0 million upfront cash payment as research and development expense on the date of acquisition in December 2020. The Company is obligated to pay a tiered royalty on net sales for licensed products inwill expense any country subjectfuture milestone payments made prior to the license. Royalty payments, if any, would continuetime an alternative future use for the duration of the licensed patents.
In April 2013,SY-2101 has been established. Once an alternative future use for SY-2101 has been established, the Company entered into a license agreement with the Whitehead Institute for Biomedical Research ("Whitehead") and Dana-Farber, pursuant to which the Company was granted a worldwide, sublicensable license under specified patents relating to MYC modulators owned or controlled by Whitehead and Dana-Farber.
In April 2013, the Company entered into an additional license agreement with Whitehead, pursuant to which the Company was granted a worldwide license under specified patents relating to super-enhancers owned or controlled by Whitehead.
In connection with the Whitehead agreements, the Company issued 171,674 shares of its common stock to Whitehead in April 2013. Payments totaling $3.6 million are due under the Whitehead agreements when the Company achieves certain milestones. The future potentialwill capitalize milestone payments due underas an addition to the Whitehead agreements have not been accrued ascarrying value of December 31, 2017 and December 31, 2016, respectively, as no milestones have been achieved and the agreement can be cancelled at the Company's option. Therefore, the Company had no obligation to pay any of these amounts. The Company paid Whitehead and the Whitehead Institute for Genome Technology Core $0.9 million and $1.0 million during the years ended December 31, 2017 and 2016, respectively, for annual license maintenance fees and research services. Additionally, at December 31, 2017, the Company had $0.2 million in accounts payable and accrued expenses due to Whitehead and the Whitehead Institute for Genome Technology Core for research services performed during 2017.SY-2101.
License Agreement
TMRC Co. Ltd.
In September 2015, the Company entered into an exclusive license agreement with the Japanese oncology company TMRC Co. Ltd. ("TMRC"(“TMRC”) to develop and commercialize tamibarotene in North America and Europe for the treatment of cancer. This agreement was amended and restated in April 2016.2016, and further amended in January 2021 to expand the territory under which the Company is licensed to include Central and South America, Australia, Israel, and Russia.
In exchange for this license, the Company agreed to a non-refundable upfront payment of $1.0$1.0 million, for which $0.5$0.5 million was paid in September 2015 upon execution of the agreement, and the remaining $0.5$0.5 million was paid in May 2016. Under the agreement, the Company is also obligated to make payments upon the successful achievement of clinical and regulatory milestones totaling approximately $13.0$13.0 million per indication, defined as a distinct tumor type. In September 2016, theThe Company paid $1.0$1.0 million to TMRC for a development milestone achieved upon the successful dosing of the first patient in its Phase 2 clinical trial of SY-1425.tamibarotene in 2016. In May 2021, the Company paid $2.0 million to TMRC for a development milestone achieved upon the successful dosing of the first patient in its Phase 3 clinical trial of tamibarotene in MDS patients. In September 2021, the Company paid $1.0 million to TMRC for a development milestone achieved upon the successful dosing of the first patient in its Phase 2 clinical trial of tamibarotene in AML patients. In addition, the Company is obligated to pay TMRC a single-digit percentage royalty, on a country-by-country and product-by-product basis, on net product sales of SY-1425tamibarotene using know-how and patents licensed from TMRC in North America and Europe for a defined royalty term.
The Company also entered into a supply management agreement with TMRC under which the Company agreed to pay TMRC a fee for each kilogram of SY-1425 active pharmaceutical ingredienttamibarotene that is produced. The Company made paymentsincurred fees of $0.4$1.8 million under this supply management agreement during each of the yearyears ended December 31, 2017. No payments were made under this supply management agreement during2023 and 2022.
Legal Contingencies
From time to time, the year ended December 31, 2016.
Litigation
business. The Company does not have any ongoing legal proceedings that, based on its estimates, could have a material effect on its consolidated financial statements. The Company is not party to any litigation and does not have contingency reserves established for any litigation liabilities as of December 31, 20172023 or December 31, 2016.2022.
Syros Pharmaceuticals, Inc.
10. Stock-Based PaymentsNotes to Consolidated Financial Statements (Continued)
12. Stockholders’ equity
2016 Stock Incentive PlanIssuance of Securities through an Underwritten Registered Direct Offering
The 2016 Stock Incentive Plan (the “2016 Plan”) was adopted by the board of directors onIn December 15, 2015 and approved by the stockholders on June 17, 2016, and became effective on July 6, 2016 upon the closing of the IPO. The 2016 Plan replaced the 2012 Equity Incentive Plan (the "2012 Plan"). Any options or awards outstanding under the 2012 Plan remained outstanding and effective. Under the 2016 Plan,2023, the Company may grant incentiveissued 4.9 million shares of common stock options, nonstatutoryand, in lieu of its common stock options, stock appreciation rights, restricted stock, restricted stock unitsto certain investors who so chose, the 2023 Pre-Funded Warrants, pursuant to the 2023 Registration Statement, in an underwritten registered direct offering for gross proceeds of $45.0 million, before deducting underwriting fees and other stock-based awards. transaction costs of $3.4 million.
The Company determined that the 2023 Pre-Funded Warrants are freestanding financial instruments because they are both legally detachable and separately exercisable from the common stock sold in the offering. As such, the Company evaluated the 2023 Pre-Funded Warrants to determine whether they represent instruments that require liability classification pursuant to the guidance in ASC 480. However, the Company concluded that the 2023 Pre-Funded Warrants are not a liability within the scope of ASC 480 due to their characteristics. Further, the Company determined that the 2023 Pre-Funded Warrants do not meet the definition of a derivative under ASC 815 because they do not meet the criteria regarding no or little initial net investment. Accordingly, the Company assessed the 2023 Pre-Funded Warrants relative to the guidance in ASC No. 815-40, Contracts in Entity’s Own Equity, to determine the appropriate treatment. The Company concluded that the 2023 Pre-Funded Warrants are both indexed to its own stock and meet all other conditions for equity classification. Accordingly, the Company has classified the 2023 Pre-Funded Warrants as permanent equity.
Increase of Authorized Shares and Reverse Stock Split
Effective on September 15, 2022, the number of authorized shares of the Company’s common stock reserved for issuance underwas increased from 200,000,000 shares (on a pre-split basis) to 700,000,000 shares (on a pre-split basis).
On September 16, 2022, the 2016 Plan will automatically increase on January 1number of each calendar year, commencing on January 1, 2017authorized shares of the Company’s common stock was proportionately adjusted from 700,000,000 to 70,000,000 as a result of the Reverse Stock Split. Immediately following the Reverse Stock Split, and ending on December 31, 2025, in an amount equalwithout giving effect to the leastshares of (i) 1,600,000the Company’s common stock issued in connection with the Merger and the 2022 Private Placement, there were approximately 6.3 million shares of the Company’s common stock outstanding. The Company’s common stock began trading on The Nasdaq Global Select Market on a split-adjusted basis on September 19, 2022.
No fractional shares were issued in connection with the Reverse Stock Split. Any fractional shares resulting from the Reverse Stock Split were rounded down to the nearest whole number, and each stockholder who would have otherwise been entitled to a fraction of a share of common stock upon the Reverse Stock Split (after aggregating all fractions of a share to which such stockholder would have otherwise been entitled) was, in lieu thereof, entitled to receive a cash payment.
Issuance of Securities through a Private Placement
On September 16, 2022, the Company issued in a private placement 6,387,173 shares of common stock, (ii) 4.0%and, in lieu of the outstanding shares of common stock, asthe 2022 Pre-Funded Warrants to purchase an aggregate of such date, or (iii) such lesser amount as specified by7,426,739 shares of common stock, and, in each case, the compensation committeeaccompanying 2022 Warrants to purchase an aggregate of up to 13,813,912 additional shares of common stock (or 2022 Pre-Funded Warrants to purchase common stock in lieu thereof) at a price of $10.34 per share and accompanying 2022 Warrant (or $10.33 per 2022 Pre-Funded Warrant and accompanying 2022 Warrant). The 2022 Private Placement resulted in aggregate gross proceeds of $129.9 million, before $10.1 million of transaction costs.
On December 8, 2020, through a private placement, the boardCompany issued 1,031,250 shares of directors. This number is subjectcommon stock, and, in lieu of shares of common stock, pre-funded warrants to adjustmentpurchase an aggregate of 100,000 shares of common stock (the “2020 Pre-Funded Warrants”), and, in each case, accompanying warrants (the “2020 Warrants”) to purchase an aggregate of up to 282,809 additional shares of common stock (or 2020 Pre-Funded Warrants to purchase common stock in lieu thereof) at a price of $80.00 per share and accompanying 2020 Warrant (or $79.90 per 2020 Pre-Funded
Syros Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
Warrant and accompanying 2020 Warrant). The 2020 Private Placement resulted in aggregate gross proceeds of $90.5 million, before $0.4 million of transaction costs.
In the event of certain fundamental transactions involving the Company, holders of the 2022 Warrants and 2020 Warrants may require the Company to make a payment based on a Black-Scholes valuation, using specified inputs. The holders of 2022 Pre-Funded Warrants and 2020 Pre-Funded Warrants do not have similar rights. Therefore, the Company accounted for the 2022 Warrants and 2020 Warrants as liabilities, while the 2022 Pre-Funded Warrants and 2020 Pre-Funded Warrants met the permanent equity criteria classification. The 2022 Pre-Funded Warrants and 2020 Pre-Funded Warrants are classified as a component of permanent equity because they are freestanding financial instruments that are legally detachable and separately exercisable from the shares of common stock split,with which they were issued, are immediately exercisable, do not embody an obligation for the Company to repurchase its shares, and permit the holders to receive a fixed number of shares of common stock dividendupon exercise. In addition, the 2022 Pre-Funded Warrants and 2020 Pre-Funded Warrants do not provide any guarantee of value or otherreturn. The initial fair value of the 2022 Warrants and 2020 Warrants was $64.7 million and $19.3 million, respectively, determined using the Black-Scholes valuation model. The Company remeasured the aggregate fair value of the 2022 Warrants and 2020 Warrants at December 31, 2023 and 2022 as $61.7 million and $24.5 million, respectively. The change in fair value of $37.3 million (loss) and $43.2 million (gain) was recorded in the Company’s capitalization. Forconsolidated statement of operations for the calendar year beginning January 1, 2017, the number of shares reserved for issuance under the 2016 Plan was increased by 935,430 shares. Atyears ended December 31, 2017, 2,880,493 shares remained available for future issuance under the 2016 Plan. Under the 2016 Plan, stock options may not be granted at less than fair value on the date of grant.2023 and 2022, respectively.
13. Stock-Based Payments
Terms of stock option agreements, including vesting requirements, are determined by the board of directors, subject to the provisions of the 2016 Plan. Stock option awards granted by the Company generally vest over four years, with 25% vesting on the first anniversary of the vesting commencement date and 75% vesting ratably, on a monthly basis, over the remaining three years. Such awards are exercisable from the date of grant for a period of ten years. The Company may grant performance-based stock option awards for which vesting accelerates upon the achievement of performance-based milestones. For certain of such awards, notwithstanding any vesting in accordance with the achievement of performance-based milestones, such awards may vest in full on the sixth anniversary of the vesting commencement date.
2016 Employee Stock Purchase Plan
The 2016 Employee Stock Purchase Plan (the “2016 ESPP”) was adopted by the board of directors on December 15, 2015, and approved by the stockholders on June 17, 2016, and became effective on July 6, 2016 upon the closing of the IPO. The number of shares of the Company’s common stock reserved for issuance under the 2016 ESPP will automatically increaseincreases on the first day of each fiscalcalendar year commencing on January 1, 2017 and ending on December 31,through the 2025 calendar year, in an amount equal to the least of (i) 1,173,333117,333 shares of the Company’s common stock, (ii) 1.0%1.0% of the total number of shares of the Company’s common stock outstanding on the first day of the applicable year, and (iii) an amount determined by the Company’s board of directors. For the calendar year beginning January 1, 2017,2023, the number of shares reserved for issuance under the 2016 ESPP was increased by 233,857117,333 shares. At December 31, 2017, 820,5232023, 258,504 shares remained available for future issuance under the 2016 ESPP.
2022 Inducement Stock Incentive Plan
On January 25, 2022, the Company’s board of directors adopted the 2022 Inducement Stock Options
Performance-Based Stock Options
TheIncentive Plan (the “2022 Plan”), pursuant to which the Company has grantedmay grant non-statutory stock options, stock appreciation rights, restricted stock, restricted stock units and other stock-based awards. Awards under the 2022 Plan may only be granted to management for which vesting accelerates uponpersons who (i) were not previously an employee or director of the achievementCompany or (ii) are commencing employment with the Company following a bona fide period of performance-based criteria. Milestone events are specificnon-employment, in either case as an inducement material to the Company’s corporate goals, including but not limited to certain preclinicalindividual’s entering into employment with the Company and clinical development milestones andin accordance with the requirements of Nasdaq Stock Market Rule 5635(c)(4). In January 2023, the Company’s abilityboard of directors amended the 2022 Plan to executeincrease the aggregate number of shares that can be granted by 750,000 shares. At December 31, 2023, 710,392 shares remained available for future issuance under the 2022 Plan.
2022 Equity Incentive Plan
The 2022 Stock Incentive Plan (the “2022 EIP”) was adopted by the board of directors on its corporate developmentJuly 14, 2022, approved by the stockholders and financing strategies. Stock-based compensation expense associated with these performance-basedbecame effective on September 15, 2022. The 2022 EIP replaced the 2016 Plan. Any options or awards outstanding under the 2016 Plan remained outstanding and effective. Under the 2022 EIP, the Company may grant incentive stock options, is recognized basednon-statutory stock options, stock appreciation rights, restricted stock, restricted stock units and other stock-based awards. At December 31, 2023, 1,170,425 shares remained available for future issuance under the 2022 EIP. Under the 2022 EIP, stock options may not be granted at less than fair value on the accelerated attribution model. Management evaluates when the achievementdate of
Table of Contents
Syros Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
a performance-based milestone is probable basedStock Options
Terms of stock option agreements, including vesting requirements, are determined by the board of directors, subject to the provisions of the applicable plan. Stock option awards granted by the Company generally vest over four years, with 25% vesting on the expected satisfaction of the performance conditions as of the reporting date. Notwithstanding any vesting in accordance with the achievement of performance-based milestones, such awards vest in full on the sixthfirst anniversary of the vesting commencement date. Duringdate and 75% vesting ratably, on a monthly basis, over the year ended December 31, 2016,remaining three years. Such awards have a contractual term of ten years from the Company recorded additional stock-based compensation expense of $0.2 million related to the achievement of certain performance-based milestones. grant date.
The Company did not record any additional stock-based compensation expense related to the achievement of performance-based milestones for the year ended December 31, 2017. As of December 31, 2017, there was $1.0 million of unrecognized stock-based compensation expense related to the performance-based stock options granted to management, with an expected recognition period of 3.2 years.
During the year ended December 31, 2016, the Companyhas granted options to purchase 75,0007,500 shares of common stock to an advisor for which the vesting acceleratesthat vest solely upon the achievement of performance-based criteria. As of December 31, 2017, no such2022, none of these performance-based criteria werehad been achieved. As of December 31, 2017,2023, there was $0.6$0.3 million of unrecognized stock-based compensation expensecost related to the performance-based stock options granted to management,this option, with an expected recognitiona remaining contractual period of 8.72.7 years.
A summary of the status of stock options as of December 31, 20172023 and December 31, 20162022 and changes during the year ended December 31, 20172023 is presented below:
| | | | | | | | | | | |
| | | | | | | | | Aggregate | |
| | | | Weighted | | Remaining | | Intrinsic | |
| | | | Average | | Contractual | | Value | |
| | Shares | | Exercise Price | | Life (in years) | | (in thousands) | |
Outstanding at December 31, 2016 | | 2,543,435 | | $ | 6.44 | | 8.3 | | $ | 14,898 | |
Granted | | 1,368,000 | | | 11.87 | | | | | | |
Exercised | | (445,012) | | | 4.03 | | | | | | |
Cancelled | | (619,755) | | | 7.22 | | | | | | |
Outstanding at December 31, 2017 | | 2,846,668 | | $ | 9.25 | | 8.2 | | $ | 5,713 | |
Exercisable at December 31, 2017 | | 858,927 | | $ | 6.06 | | 7.0 | | $ | 3,827 | |
Vested and expected to vest at December 31, 2017 | | 2,846,668 | | $ | 9.25 | | 8.2 | | $ | 5,713 | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | Aggregate | |
| | | | | Weighted | | | Remaining | | | Intrinsic | |
| | | | | Average | | | Contractual | | | Value | |
| | Shares | | | Exercise Price | | | Life (in years) | | | (in thousands) | |
Outstanding at December 31, 2022 | | | 1,727,237 | | | $ | 39.94 | | | | 5.7 | | | $ | — | |
Granted | | | 54,000 | | | | 3.70 | | | | | | | |
Exercised | | | — | | | | — | | | | | | | |
Cancelled | | | (232,595 | ) | |
| | | | | | | |
Outstanding at December 31, 2023 | | | 1,548,642 | | | $ | 34.80 | | | | 4.5 | | | $ | 646 | |
Exercisable at December 31, 2023 | | | 1,243,692 | | | $ | 39.18 | | | | 3.5 | | | $ | 362 | |
Pursuant to the terms of the Merger Agreement, the Company assumed certain Tyme stock options that were outstanding and unexercised immediately prior to the completion of the Merger. The Company issued options to purchase 692,460 shares of the Company’s common stock at the completion of the Merger on September 16, 2022. The original terms and restrictions on such Tyme options shall continue in full force and effect except for certain options held by certain Tyme employees which were modified to extend the exercise period to up to two years. The Company recorded $0.4 million of one-time additional stock-based compensation expense related to the modification.
There were no stock options exercised during the year ended December 31, 2023. The intrinsic value of stock options exercised during the yearsyear ended December 31, 2017, 2016 and 20152022 was $4.6 million, $2.4 million, and $2.0 million, respectively.
$0.1 million. Cash received from option exercises during the yearsyear ended December 31, 2017, 2016,2022, was $0.1 million.
As of December 31, 2023, there was $3.7 million of total unrecognized compensation cost related to non-vested stock options granted to employees, which is expected to be recognized over a weighted-average period of 1.3 years.
Restricted Stock Units and 2015 was $1.8 million, $0.4 million, and $0.4 million, respectively.Restricted Stock Awards
Restricted Common Stock
From time to time, upon approval by the Company’s board of directors, certain employees and advisors have been granted restricted shares of common stock units with time- and performance-basedtime-based vesting criteria. These sharesThe majority of these restricted stock units vest annually over a three-year or four-year term. In addition, pursuant to the Company’s director compensation policy, members of the Company’s board of directors have been granted, at their election, either restricted stock units or restricted stock awards, which awards vest annually over a three-year term with 33.33% vesting on each anniversary of the grant date. The fair value of restricted stock are subject to repurchase rights. Accordingly, the Company has recorded the proceeds from the issuance ofunits and restricted stock as a liability in the consolidated balance sheets included as a component of accrued expenses or other long term liabilitiesawards are calculated based on the scheduled vesting dates. closing sale price of the Company’s common stock on the date of grant.
The Company has granted performance-based restricted stock liability is reclassified into stockholders’ equity (deficit) as the restricted stock vests over time orunits to management for which vesting occurs upon the achievement of performance.
certain clinical development milestones. Stock-based compensation expense associated with these performance-based restricted stock units is recognized when the achievement of the vesting conditions becomes probable. The Company did not recognize any stock-based compensation expense relating to the achievement of performance-based milestones during the year ended December 31, 2023.
Table of Contents
Syros Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
A summary of the status of unvested restricted common stock units as of December 31, 20172022 and December 31, 20162023 and changes during the year ended December 31, 20172023 is presented below:
| | | | | | |
| | | | Weighted | |
| | | | Average Grant | |
| | Shares | | Date Fair Value | |
Unvested at December 31, 2016 | | 4,885 | | $ | 0.98 | |
Vested | | (4,885) | | | 0.98 | |
Repurchased | | — | | | — | |
Unvested at December 31, 2017 | | — | | $ | — | |
| | | | | | | | |
| | Shares | | | | |
| | Subject to | | | | |
| | Restricted Stock | | | | |
| | Units and | | | Weighted | |
| | Restricted Stock | | | Average Grant | |
| | Awards | | | Date Fair Value | |
Outstanding at December 31, 2022 | | | 1,204,421 | | | $ | 14.68 | |
Granted | | | 1,634,636 | | | | 3.85 | |
Vested | | | (481,142 | ) | | | 14.71 | |
Forfeited | | | (579,301 | ) | | | 9.11 | |
Outstanding at December 31, 2023 | | | 1,778,614 | | | $ | 6.29 | |
The total fair valueAs of December 31, 2023, there was $8.0 million of unrecognized stock-based compensation expense related to outstanding restricted stock vested during the years ended December 31, 2017, 2016, and 2015 was $1,000, $0.1 million, and $0.1 million, respectively, based upon the numberunits, with an expected recognition period of restricted stock awards vested multiplied by the fair value of the Company’s common stock on the grant date.1.85 years.
Stock-based Compensation Expense
The fair value of each stock option granted was estimated on the date of grant using the Black-Scholes option-pricing model based on the following weighted-average assumptions:
| | | | | | | |
| | Year Ended December 31, | |
| | 2017 | | 2016 | | 2015 | |
Weighted-average risk-free interest rate | | 2.07 | % | 1.36 | % | 1.78 | % |
Expected dividend yield | | — | % | — | % | — | % |
Expected option term | | 6.05 | | 5.98 | | 6.09 | |
Volatility | | 87.83 | % | 85.39 | % | 82.71 | % |
| | | | | | | | | |
| | Year Ended December 31, |
| | 2023 | | | 2022 | | |
Weighted-average risk-free interest rate | | | 3.93 | | % | | 3.56 | | % |
Expected dividend yield | | | — | | % | | — | | % |
Expected option term (in years) | | | 5.32 | | | | 5.80 | | |
Volatility | | | 84.44 | | % | | 83.32 | | % |
The weighted‑average grant date fair value per share of options granted in the years ended December 31, 2017, 20162023 and 20152022 was $8.75, $8.58$2.60 and $4.88,$5.99, respectively.
The following table summarizes the stock-based compensation expense for stock options, restricted stock units and restricted common stock granted to employees and non-employees and from the 2016 ESPP recorded in the Company’s statements of operations:
| | | | | | | | | |
| | Year Ended December 31, | | |
| | 2023 | | | 2022 | | |
Research and development | | $ | 4,746 | | | $ | 5,946 | | |
General and administrative | | | 5,522 | | | | 5,464 | | |
Restructuring | | | 166 | | | | — | | |
Total stock-based compensation expense | | $ | 10,434 | | | $ | 11,410 | | |
| | | | | | | | | | |
| | Year Ended December 31, | |
| | 2017 | | 2016 | | 2015 | |
Research and development | | $ | 1,666 | | $ | 2,980 | | $ | 2,733 | |
General and administrative | | | 2,753 | | | 1,254 | | | 500 | |
Total stock-based compensation expense | | $ | 4,419 | | $ | 4,234 | | $ | 3,233 | |
14. Restructuring
As a result of the Restructuring, the Company incurred $2.5 million in costs for the year ended December 31, 2017, there was $11.92023. Restructuring costs were comprised of $2.0 million of total unrecognizedseverance, post-employment benefits, stock-based compensation costand outplacement services, and $0.5 million of asset impairment charge and loss on disposal related to non-vested stock options grantedthe laboratory equipment that was classified as assets held for sale.
Syros Pharmaceuticals, Inc.
Notes to employees, which is expected to be recognized over a weighted-average period of 2.9 years. Additionally, as of December 31, 2017, there was $0.1 million of total unrecognized compensation cost related to non-vested stock options granted to non-employees, excluding those subject to performance-based criteria described above. Due to an operating loss, the Company does not record tax benefits associated with stock‑based compensation or option exercises. Tax benefits will be recorded when realized.Consolidated Financial Statements (Continued)
11.15. Income Taxes
The Company accounts for income taxes under FASB Accounting Standards Codification 740 ("ASC 740"). Deferred income tax assets and liabilities are determined based upon differences between financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the
Table of Contents
Syros Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
differences are expected to reverse. The components of the income tax provision for the years ended December 31, 20172023 and 20162022 are as follows:
| | | | | | | |
| | Year Ended December 31, | |
| | 2017 | | 2016 | |
Current | | $ | 7 | | $ | 2 | |
Deferred | | | — | | | — | |
Total | | $ | 7 | | $ | 2 | |
| | | | | | | | |
| | Year Ended December 31, | |
| | 2023 | | | 2022 | |
Current | | $ | 1 | | | $ | 3 | |
Deferred | | | — | | | | — | |
Total | | $ | 1 | | | $ | 3 | |
A reconciliation of the U.S. statutory income tax rate to the Company’s effective tax rate is as follows for the years ended December 31, 2017, 20162023 and 2015:2022:
| | | | | | | | | | |
| | Year ended |
| | December 31, |
| | 2023 | | | | 2022 | | |
Federal income tax computed at federal statutory tax rate | | | 21.00 | | % | | | 21.00 | | % |
State income tax, net of federal benefit | | | 4.49 | | | | | 8.09 | | |
Permanent items | | | (4.86 | ) | | | | 7.92 | | |
Federal and state research and development credits | | | 5.83 | | | | | 7.29 | | |
Expiring tax attributed - IRC 382 | | | — | | | | | (65.82 | ) | |
Stock option cancellations | | | (1.87 | ) | | | | (2.98 | ) | |
Other | | | 0.14 | | | | | (0.27 | ) | |
Change in valuation allowance | | | (24.73 | ) | | | | 24.77 | | |
Effective income tax rate | | | — | | % | | | — | | % |
| | | | | | | | |
| | Year ended | | |
| | December 31, | | |
| | 2017 | | 2016 | | 2015 | | |
Federal income tax computed at federal statutory tax rate | | 34.00 | % | 34.00 | % | 34.00 | % | |
State income tax, net of federal benefit | | 5.35 | | 4.79 | | 4.70 | | |
Permanent items | | (1.57) | | (2.83) | | (3.33) | | |
Federal and state research and development credits | | 7.36 | | 2.93 | | 2.66 | | |
Rate change | | (31.79) | | — | | — | | |
Other | | 0.66 | | (0.08) | | 0.12 | | |
Change in valuation allowance | | (14.02) | | (38.81) | | (38.15) | | |
Effective income tax rate | | (0.01) | % | 0.00 | % | 0.00 | % | |
On December 22, 2017, H.R.1., formerly known as the Tax Cuts and Jobs Act, was signed into law. The new law did not have a significant impact on the Company’s consolidated financial statements for the year ended December 31, 2017 because it maintains a valuation allowance on the majority of its net operating losses and other deferred tax assets. However, the reduction of the U.S. federal corporate tax rate from 35% to 21% resulted in increases to the amounts reflected in the effective tax rate attributable to "change in valuation allowance” and “rate change” in the Company’s effective tax rate reconciliation table above for the year ended December 31, 2017 compared to the years ended December 31, 2016 and 2015. The change in the U.S. federal corporate tax rate, which is effective January 1, 2018, is also reflected in the Company’s deferred tax table below. Lastly, the Company has discussed the possible impact of Staff Accounting Bulletin No. 118 (“SAB 118”) on the Company’s consolidated financial statements below.
The principal components of the Company’s deferred tax assets and liabilities consist of the following at December 31, 20172023 and 20162022 (in thousands):
| | | | | | | | |
| | Year ended | |
| | December 31, | |
| | 2017 | | 2016 | | |
Deferred tax assets: | | | | | | | | |
Federal and state net operating loss carryforwards | | $ | 37,411 | | $ | 33,846 | | |
Tax credit carryforwards | | | 7,573 | | | 3,350 | | |
Intangible assets | | | 56 | | | 197 | | |
Stock-based compensation | | | 1,059 | | | 247 | | |
Leasehold incentive | | | 240 | | | 467 | | |
Other | | | 1,237 | | | 1,605 | | |
Total deferred tax assets | | | 47,576 | | | 39,712 | | |
Less valuation allowance | | | (47,576) | | | (39,624) | | |
Net deferred tax assets | | | — | | | 88 | | |
Deferred tax liabilities: | | | | | | | | |
Fixed assets | | | — | | | (88) | | |
Total deferred tax liabilities | | | — | | | (88) | | |
Net deferred taxes | | $ | — | | $ | — | | |
| | | | | | | | |
| | Year ended | |
| | December 31, | |
| | 2023 | | | 2022 | |
Deferred tax assets: | | | | | | |
Federal and state net operating loss carryforwards | | $ | 95,290 | | | $ | 81,989 | |
Tax credit carryforwards | | | 11,853 | | | | 2,263 | |
Capitalized R&D | | | 48,483 | | | | 27,227 | |
Intangible assets | | | 2,563 | | | | 2,782 | |
Stock-based compensation | | | 7,486 | | | | 9,035 | |
Deferred revenue | | | — | | | | 1,183 | |
Capital lease | | | 5,697 | | | | 6,262 | |
Other | | | 2,216 | | | | 2,948 | |
Total deferred tax assets | | | 173,588 | | | | 133,689 | |
Less valuation allowance | | | (168,864 | ) | | | (128,186 | ) |
Net deferred tax assets | | | 4,724 | | | | 5,503 | |
Deferred tax liabilities: | | | | | | |
Right-of-use asset | | | (3,329 | ) | | | (3,635 | ) |
Fixed assets | | | (1,395 | ) | | | (1,868 | ) |
Total deferred tax liabilities | | | (4,724 | ) | | | (5,503 | ) |
Net deferred taxes | | $ | — | | | $ | — | |
Table of Contents
Syros Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
As of December 31, 2017 the Company had federal net operating loss (“NOL”) carryforwards of approximately $136.4 million and state net operating loss carryforwards of $138.8 million which are available to reduce future taxable income. The Company also had federal tax credits of approximately $6.1 million and state tax credits of $1.8 million which may be used to offset future tax liabilities. The NOL and tax credit carryforwards will expire at various dates through 2037. The NOL and tax credit carryforwards are subject to review and possible adjustment by the Internal Revenue Service and state tax authorities. Net operating loss and tax credit carryforwards may become subject to an annual limitation in the event of certain cumulative changes in the ownership interest of significant shareholders over a three-year period in excess of 50%, as defined under Sections 382 and 383 of the Internal Revenue Code, respectively, as well as similar state provisions. This could limit the amount of tax attributes that can be utilized annually to offset future taxable income or tax liabilities. The amount of the annual limitation is determined based on the value of the Company immediately prior to the ownership change. Subsequent ownership changes may further affect the limitation in future years. The Company has not determined whether an ownership change has occurred and as such, the Company's NOLs may be limited.
ASC 740 requires a valuation allowance to reduce the deferred tax assets reported if, based on the weight of the evidence, it is more likely than not that some portion or all of the deferred tax assets will not be realized. After consideration of all the evidence, both positive and negative, the Company has recorded a valuation allowance against its deferred tax assets at December 31, 20172023 and 2018,2022, respectively because the Company's management has determined that it is more likely than not that these assets will not be fully realized. The increase in the valuation allowance of $8.0$40.7 million in 20172023 and $18.5$23.5 million in 20162022 primarily relates to the net loss incurred by the Company.Company and capitalized R&D expenses.
As of December 31, 2023, the Company had federal net operating loss (“NOL”) carryforwards of approximately $348.7 million and state net operating loss carryforwards of $349.0 million which are available to reduce future taxable income. The Company also had federal tax credits of approximately $10.6 million and state tax credits of $1.5 million which may be used to offset future tax liabilities. Federal net operating losses generated before 2018 of approximately $8.2 million will expire at various dates through 2037, and net operating loss carryforwards of approximately $340.5 million, which were generated after 2017 have an indefinite carryforward period. Federal tax credits will expire at various dates through 2043. State net operating losses will expire at various dates through 2043. State tax credits will expire at various dates through 2038. The NOL and tax credit carryforwards are subject to review and possible adjustment by the Internal Revenue Service and state tax authorities. Net operating loss and tax credit carryforwards are subject to an annual limitation due to certain cumulative changes in the ownership interest of significant shareholders over a three-year period in excess of 50%, as defined under Sections 382 and 383 of the Internal Revenue Code, respectively, as well as similar state provisions. This limits the amount of tax attributes that can be utilized annually to offset future taxable income or tax liabilities. The amount of the annual limitation is determined based on the value of the Company immediately prior to the ownership change. Subsequent ownership changes may further affect the limitation in future years. The Company has determined that ownership changes have occurred and as such, the Company's NOLs are limited.
The Company’s reserves related to taxes are based on a determination of whether and how much of a tax benefit taken by the Company in its tax filings or positions is more likely than not to be realized following resolution of any potential contingencies present related to the tax benefit. As of December 31, 20172023 and 20162022, the Company had no unrecognized tax benefits or accrued interest and penalties related to unrecognized tax benefits. The Company’s policy is to recognize both interest and penalties related to unrecognized tax benefits in income tax expense.
The Company completed a study to document its qualifying research credits for all years ending before December 31, 2018. For the years ending after December 31, 2017, the Company generated research credits but has not conducted a study to document the qualified activities. This study may result in an adjustment to the Company’s research and development credit carryforwards; however, until a study is completed and any adjustment is known, no amounts are being presented as an unrecognized tax benefit for the year ended December 31, 2023. A full valuation allowance has been provided against the Company’s research and development credits and, if an adjustment is required, this adjustment would be offset by an adjustment to the deferred tax asset established for the research and development credit carryforwards and the valuation allowance.
The federal and state income tax returns are generally subject to examinations for the tax years ended December 31, 20142020 through December 31, 2017.2023. To the extent the Company has tax attribute carryforwards, the tax years in which the attribute was generated may still be adjusted upon examination by the Internal Revenue Service or state tax authorities to the extent utilized in a future period. The Company files income tax returns in the U.S. federal and Massachusettsvarious state jurisdictions. There are currently no federal or state audits in process.
On December 22, 2017, the SEC staff issued SAB 118 to address the application of GAAP in situations when a registrant does not have the necessary information available, prepared, or analyzed (including computations) in reasonable detail to complete the accounting for certain income tax effects of H.R.1. The Company has recognized the provisional tax impacts related the revaluation of deferred tax assets and liabilities and included these amounts in its financial statements for the year ended December 31, 2017. The ultimate impact may differ from these provisional amounts, possibly materially, due to, among other things, additional analysis, changes in interpretations and assumptions the Company has made, additional regulatory guidance that may be issued, and actions the Company may take as a result of H.R.1. The Company’s accounting treatment is expected to be complete when the 2017 U.S. corporate income tax return is filed in 2018.
12. Research Agreement
In November 2014, the Company entered into a research agreement with a multinational pharmaceutical company (the “Counterparty”) for purposes of mapping immune cell super-enhancers (“SE”) and transcriptional targets in autoimmune disease. Under the research agreement, the Company is responsible for the conduct of all activities under separate projects, as defined in the research agreement, associated with generating SE and transcriptional maps of the cell/tissue supplied by the Counterparty. Upon the completion of each project, the Counterparty determined whether to commence the next project under the research agreement upon written notification.
The research agreement was amended in November 2016 to extend the term to March 31, 2017. The research agreement terminated automatically on March 31, 2017 in accordance with its terms.
Table of Contents
Syros Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
The Company recognized revenue on a completed performance basis for each project performed under the agreement, as the Company does not have the ability to reasonably estimate the period of performance and the final study report for each project is significant to the overall arrangement. The Company recognized revenue of $1.1 million and $0.3 million during the years ended December 31, 2017 and December 31, 2016, respectively, under the agreement.
13.16. Defined Contribution Plan
The Company established a defined contribution savings plan under Section 401(k) of the Internal Revenue Code. This plan covers substantially all employees who meet minimum age and service requirements and allows participants to defer a portion of their annual compensation on a pretax or post-tax basis. Company contributions to the plan may be made at the discretion of the board of directors. Effective September 1, 2017, the Company institutedThe 401(k) plan includes an employer match of 100%100% of the amount youthe employees contribute to the Plan401(k) plan for each payroll period up to the first 1%2% of Plan Compensationplan compensation plus 50%50% of the amount youthe employees contribute between 1%2% and 6%6% of Plan Compensation.plan compensation. For the yearyears ended December 31, 2017,2023 and 2022, the Company contributed $0.3$0.9 million and $1.1 million, respectively, to the 401(k) Plan.
14. Subsequent Events
Collaboration Agreement
On January 8, 2018, the Company and Incyte Corporation entered into a Target Discovery, Research Collaboration and Option Agreement (the “Collaboration Agreement”). Under the Collaboration Agreement, the Company will use its proprietary gene control platform to identify novel therapeutic targets with a focus on myeloproliferative neoplasms, and Incyte will receive options to obtain exclusive worldwide rights to intellectual property resulting from the collaboration for the development and commercialization of therapeutic products directed to up to seven validated targets. Incyte will have exclusive worldwide rights to develop and commercialize any therapies under the collaboration that modulate those validated targets.
Under the terms of the Collaboration Agreement, Incyte paid the Company $10.0 million in up-front consideration, consisting of $2.5 million in cash and $7.5 million in pre-paid research funding. The Company’s activities under the Collaboration Agreement are subject to a joint research plan and, subject to certain exceptions, Incyte will be responsible for funding the Company’s activities under the research plan, including amounts in excess of the pre-paid research funding amount. The Company is obligated to make a payment to Whitehead representing a percentage of the up-front cash consideration received under the Collaboration Agreement.
The Company will be eligible to receive target selection milestone payments and option exercise fees of up to an aggregate of $54.0 million if Incyte selects the maximum number of targets for validation and exercises its option to obtain exclusive rights to collaboration intellectual property for therapeutic products directed to all seven validated targets. Should any therapeutic product be developed by Incyte against a target as to which Incyte has exercised its option to obtain exclusive rights to collaboration intellectual property, the Company will be eligible to receive milestone payments and, if approved and commercialized, royalty payments from Incyte. For each of the seven validated targets, the Company would become eligible to receive from Incyte a total of up to $50.0 million in development and regulatory milestone payments. If products arising from the collaboration are approved, the Company would become eligible to receive from Incyte, for each validated target, a total of up to $65.0 million in commercial milestone payments. Upon approval and commercialization of any therapeutic product resulting from the collaboration, the Company would become eligible to receive low single-digit royalties on net sales of such product.
The term of Collaboration Agreement began on January 8, 2018 and, unless terminated by a party early, will continue until all royalty obligations for products arising from the collaboration expire. The Collaboration Agreement may be terminated by Incyte for convenience on sixty (60) days’ prior written notice to the Company, or by the Company on thirty (30) days’ written notice in the event Incyte or one of its affiliates or sublicensees challenges the validity or enforceability of certain patent rights controlled by the Company. The Collaboration Agreement may also be terminated by either of the parties on thirty (30) days’ prior written notice in the event of an uncured material breach of the Collaboration Agreement by the other party or immediately in the case of certain bankruptcy events. Incyte’s right toplan.
Table of Contents
Syros Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
terminate for convenience and each party’s right to terminate for uncured material breach may be exercised either with respect to the Collaboration Agreement in its entirety or, as applicable, in relation to the relevant validated target and associated therapeutic products.
Following entry into the Collaboration Agreement, the Company’s Board of Directors accelerated the vesting of 63,793 shares underlying performance-based stock options granted to members of the Company’s management team.
Stock Purchase Agreement
On January 8, 2018, the Company entered into a Stock Purchase Agreement with Incyte (the “SPA”), pursuant to which Incyte agreed to purchase 793,021 shares of the Company’s common stock, par value $0.001 per share, for an aggregate purchase price of $10.0 million in cash, or $12.61 per share, in a private placement. The purchase price represents a thirty percent (30%) premium to the volume-weighted sale price of the shares of the Company’s common stock over the fifteen (15) trading day period immediately preceding the date of the SPA. The Company is obligated to make a payment to Whitehead representing a percentage of the equity premium received under the SPA. The Shares are subject to a lock-up restriction and a market stand-off agreement for a period of 12 months following the closing of the sale of the shares (the “Closing”).Pursuant to the terms of the SPA, the Company filed a registration statement covering the resale by Incyte of the Shares on January 19, 2018.
In addition, from the Closing until the earlier of the second anniversary of the Closing or the expiration or termination of the Collaboration Agreement, the Company has granted to Incyte the right to purchase up to its pro rata share of the securities offered in certain subsequent offerings of the Company’s common stock or common stock equivalents, subject to the terms and conditions set forth in the SPA.
Sale of Securities through Public Offering
On January 30, 2018, the Company issued and sold an aggregate of 4,188,481 shares of its common stock in a public offering at a price per share $9.55 per share, resulting in gross proceeds of $40.0 million before deducting underwriting commissions and fees estimated to be approximately $2.7 million. Additionally, on February 2, 2018, the underwriters exercised their option to purchase an additional 628,272 shares at a price per share of $9.55, resulting in additional gross proceeds of $6.0 million.
In addition, on February 2, 2018, we also closed a concurrent private placement of 125,656 shares of our common stock to Incyte Corporation at a price of $9.55 per share, resulting in proceeds to us of $1.2 million. The Company closed an additional private placement of 18,849 shares of its common stock to Incyte on February 7, 2018 at a price of $9.55 per share, resulting in gross proceeds to the Company of $0.2 million.
15. Selected Quarterly Financial Data (unaudited)
The following table contains selected quarterly financial information for 2017 and 2016. The Company believes that the following information reflects all normal recurring adjustments necessary for a fair statement of the information
Table of Contents
Syros Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements (Continued)
for the periods presented. The operating results for any quarter are not necessarily indicative of results for any future period.
| | | | | | | | | | | | | |
| | Three Months Ended | |
| | March 31, 2017 | | June 30, 2017 | | September 30, 2017 | | December 31, 2017 | |
| | | | | | | | | | | | | |
Revenue | | $ | 1,101 | | $ | — | | $ | — | | $ | — | |
Operating expenses: | | | | | | | | | | | | | |
Research and development | | | 9,628 | | | 10,041 | | | 10,447 | | | 11,780 | |
General and administrative | | | 3,086 | | | 3,472 | | | 3,593 | | | 3,740 | |
Total operating expenses | | | 12,714 | | | 13,513 | | | 14,040 | | | 15,520 | |
Loss from operations | | | (11,613) | | | (13,513) | | | (14,040) | | | (15,520) | |
| | | | | | | | | | | | | |
Other income, net | | | 98 | | | 145 | | | 215 | | | 218 | |
Net loss applicable to common stockholders | | $ | (11,515) | | $ | (13,368) | | $ | (13,825) | | $ | (15,302) | |
| | | | | | | | | | | | | |
Net loss per share applicable to common stockholders - basic and diluted | | $ | (0.49) | | $ | (0.52) | | $ | (0.53) | | $ | (0.58) | |
| | | | | | | | | | | | | |
Weighted-average number of common shares used in net loss per share applicable to common stockholders - basic and diluted | | | 23,393,448 | | | 25,584,147 | | | 26,259,216 | | | 26,316,550 | |
| | | | | | | | | | | | | |
| | Three Months Ended | |
| | March 31, 2016 | | June 30, 2016 | | September 30, 2016 | | December 31, 2016 | |
| | | | | | | | | | | | | |
Revenue | | $ | — | | $ | — | | $ | — | | $ | 317 | |
Operating expenses: | | | | | | | | | | | | | |
Research and development | | | 8,265 | | | 9,525 | | | 11,584 | | | 8,443 | |
General and administrative | | | 2,371 | | | 2,540 | | | 2,633 | | | 2,919 | |
Total operating expenses | | | 10,636 | | | 12,065 | | | 14,217 | | | 11,362 | |
Loss from operations | | | (10,636) | | | (12,065) | | | (14,217) | | | (11,045) | |
| | | | | | | | | | | | | |
Other income, net | | | 48 | | | 44 | | | 48 | | | 80 | |
Net loss | | $ | (10,588) | | $ | (12,021) | | $ | (14,169) | | $ | (10,965) | |
Accrued dividends on preferred stock | | | (1,737) | | | (1,823) | | | (121) | | | — | |
Net loss applicable to common stockholders | | $ | (12,325) | | $ | (13,844) | | $ | (14,290) | | $ | (10,965) | |
| | | | | | | | | | | | | |
Net loss per share applicable to common stockholders - basic and diluted | | $ | (5.15) | | $ | (5.42) | | $ | (0.65) | | $ | (0.47) | |
| | | | | | | | | | | | | |
Weighted-average number of common shares used in net loss per share applicable to common stockholders - basic and diluted | | | 2,394,470 | | | 2,553,146 | | | 22,012,743 | | | 23,374,734 | |
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Management’s Evaluation of Disclosure Controls and Procedures
We maintain “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended, or the Exchange Act, that are designed to ensure that information required to be disclosed in the reports that we file or submit under the Exchange Act is (1) recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms and (2) accumulated and communicated to our management, including our principal executive and principal financial officer,officers, as appropriate to allow timely decisions regarding required disclosure. Our management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and our management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Our disclosure controls and procedures are designed to provide reasonable assurance of achieving their control objectives.
Our management, with the participation of our Chief Executive Officer, who serves as our principal executive officer, and our Chief Financial Officer, who serves as our principal financial officer, has evaluated the effectiveness of our disclosure controls and procedures as of December 31, 2017.2023, the end of the period covered by this Annual Report. Based upon such evaluation, our Chief Executive Officerchief executive officer and Principal Financial Officer haschief financial officer have concluded that our disclosure controls and procedures were effective at the reasonable assurance level as of such date.
Internal Control Over Financial Reporting
Management’s Report on Internal Control Over Financial Reporting
This Annual Report on Form 10-K does not includeOur management is responsible for establishing and maintaining adequate internal control over our financial reporting. Internal control over financial reporting is defined in Rules 13a-15(f) and 15d-15(f) promulgated under the Exchange Act as a reportprocess designed by, or under the supervision of, management’s assessmentour principal executive and principal financial officers and effected by our board of directors, management and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with U.S. GAAP. Our internal control over financial reporting or an attestation reportincludes those policies and procedures that:
•pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect our transactions and dispositions of our independent registered public accounting firm dueassets;
•provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. GAAP, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and
•provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a transition period establishedmaterial effect on our financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2023. In making this assessment, management used the criteria set forth by rulesthe Committee of Sponsoring Organizations of the SEC for newly public companies.Treadway Commission in its 2013 Internal Control — Integrated Framework. Based on our assessment, our management has concluded that, as of December 31, 2023, our internal control over financial reporting is effective based on those criteria.
Changes in Internal Control Over Financial Reporting
During the year ended December 31, 2017, we implemented an accounting system for purposes of tracking and accounting for stock-based awards, as well as an enterprise resource system for the purposes of maintaining our general ledger and reporting. There2023, there were no other changes in our internal control over financial reporting (as(as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) during the year ended December 31, 2017 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
ITEM 9B. OTHER INFORMATION
None.(b) None of our directors or officers adopted or terminated a Rule 10b5-1 trading arrangement or a non-Rule 10b5-1 trading arrangement (as defined in Item 408(c) of Regulation S-K) during the fourth quarter of 2023.
ITEM 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The information required by this Item 10 will be included under the captions “Executive Officers,” “Election of Directors” and “Section“Delinquent Section 16(a) Beneficial Ownership Reporting Compliance”Reports,” if applicable, in our definitive proxy statement to be filed with the SEC with respect to our 20182024 Annual Meeting of Stockholders and is incorporated herein by reference.
We have adopted a Code of Business Conduct and Ethics that applies to our officers, including our principal executive, financial and accounting officers, and our directors and employees. We have posted the text of our Code of Business Conduct and Ethics under the “News & Investors— Corporate Governance” section of our website, www.syros.com. We intend to disclose on our website any amendments to, or waivers from, the Code of Business Conduct and Ethics that are required to be disclosed pursuant to the disclosure requirements of Item 5.05 of Form 8-K.
ITEM 11. EXECUTIVE COMPENSATION
The information required by this Item 11 will be included under the captions “Executive and Director Compensation” and “Compensation Committee Interlocks and Insider Participation” in our definitive proxy statement to be filed with the SEC with respect to our 20182024 Annual Meeting of Stockholders and, other than the information required by Item 402(v) of Regulation S-K, is incorporated herein by reference.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERSHIP AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information required by this Item 12 will be included under the captions “Security Ownership of Certain Beneficial Owners and Management” and “Securities Authorized for Issuance Under Equity Compensation Plans” in our definitive proxy statement to be filed with the SEC with respect to our 20182024 Annual Meeting of Stockholders and is incorporated herein by reference.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required by this Item 13 will be included, as applicable, under the captions “Employment Agreements,” “Potential Payments Upon Termination or Change in Control,” “Board Determination of Independence” and “Related Person Transactions” in our definitive proxy statement to be filed with the SEC with respect to our 20182024 Annual Meeting of Stockholders and is incorporated herein by reference.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The information required by this Item 14 will be included under the captions “Audit Fees and Services” and “Pre-Approval Policies and Procedures” in our definitive proxy statement to be filed with the SEC with respect to our 20182024 Annual Meeting of Stockholders and is incorporated herein by reference.
PART IV
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
For a list of the consolidated financial statements and report of Independent Registered Public Accounting Firm (PCAOB ID:42) included herein, see Index to the Consolidated Financial Statements on page 11394 of this Annual Report, on Form 10-K, which is incorporated into this Item by reference.
| | | | | |
| | Incorporation by Reference | |
Exhibit No. | Description | Form | SEC
Filing
Date | Exhibit
Number | Filed
with this
10-K |
| | | | | |
Organizational Documents and Documents Related to Common Stock | | | | |
2.1 | Asset Purchase Agreement, dated December 4, 2020, by and between the Registrant and Orsenix, LLC | 8-K | 12/7/20 | 2.1 | |
2.2++ | Agreement and Plan of Merger, dated July 3, 2022, by and among the Registrant, Tack Acquisition Corp. and Tyme Technologies, Inc. | 8-K | 7/5/22 | 2.1 | |
3.1 | Restated Certificate of Incorporation of the Registrant, as amended | 10-Q | 11/14/22 | 3.1 | |
3.2 | Second Amended and Restated Bylaws of the Registrant | 10-Q | 8/5/21 | 3.2 | |
4.1 | Description of Securities Registered under Section 12 of the Exchange Act | | | | X |
4.2 | Form of Common Stock Certificate | S-1^ | 6/3/16 | 4.1 | |
4.3 | Form of 2020 Warrant to Purchase Common Stock or 2020 Pre-Funded Warrants | 8-K | 12/7/20 | 4.1 | |
4.4 | Form of 2020 Pre-Funded Warrant to Purchase Common Stock | 8-K | 12/7/20 | 4.2 | |
4.5 | Securities Purchase Agreement, dated December 4, 2020, by and among the Registrant and the persons party thereto | 8-K | 12/7/20 | 10.1 | |
4.6 | Registration Rights Agreement, dated December 4, 2020, by and among the Registrant and the persons party thereto | 8-K | 12/7/20 | 10.2 | |
4.7 | Form of 2022 Warrant to Purchase Common Stock or 2022 Pre-Funded Warrants | 8-K | 7/5/22 | 4.1 | |
4.8 | Form of 2022 Pre-Funded Warrant to Purchase Common Stock | 8-K | 7/5/22 | 4.2 | |
4.9 | Securities Purchase Agreement, dated July 3, 2022, by and among the Registrant and the persons party thereto | 8-K | 7/5/22 | 10.4 | |
4.10 | Registration Rights Agreement, dated July 3, 2022, by and among the Registrant and the persons party thereto | 8-K | 7/5/22 | 10.5 | |
4.11 | Registration Rights Agreement, dated July 3, 2022, by and among the Registrant, 667, L.P. and Baker Brothers Life Sciences, L.P. | 8-K | 7/5/22 | 10.5 | |
4.12 | Form of 2023 Pre-Funded Warrant to Purchase Common Stock | 8-K | 12/19/23 | 4.1 | |
| | | | | |
Equity Plan Documents | | | | |
10.1* | 2012 Equity Incentive Plan, as amended | S-1^ | 6/3/16 | 10.1 | |
10.2* | Form of Incentive Stock Option Agreement under 2012 Equity Incentive Plan | S-1^ | 6/3/16 | 10.2 | |
10.3* | Form of Nonstatutory Stock Option Agreement under 2012 Equity Incentive Plan | S-1^ | 6/3/16 | 10.3 | |
10.4* | Form of Restricted Stock Agreement under 2012 Equity Incentive Plan | S-1^ | 6/3/16 | 10.4 | |
10.5* | 2016 Stock Incentive Plan | S-1^ | 6/3/16 | 10.5 | |
10.6* | Form of Incentive Stock Option Agreement under 2016 Stock Incentive Plan | S-1^ | 6/3/16 | 10.6 | |
10.7* | Form of Nonstatutory Stock Option Agreement under 2016 Stock Incentive Plan | S-1^ | 6/3/16 | 10.7 | |
10.8* | Form of Restricted Stock Unit Agreement under 2016 Stock Incentive Plan | 10-K | 3/7/19 | 10.8 | |
10.9* | 2016 Employee Stock Purchase Plan | S-1^ | 6/3/16 | 10.8 | |
10.10* | 2022 Inducement Stock Incentive Plan, as amended on January 30, 2023 | 10-K | 3/2/23 | 10.10 | |
10.11* | Form of Nonstatutory Stock Option Agreement under 2022 Inducement Stock Incentive Plan | 10-K | 3/15/22 | 10.11 | |
| | | | | |
| | Incorporation by Reference | |
Exhibit No. | Description | Form | SEC
Filing
Date | Exhibit
Number | Filed
with this
10-K |
| | | | | |
Organizational Documents and Documents Related to Common Stock | | | | |
3.1 | Restated Certificate of Incorporation of the Registrant | 8-K | 7/6/16 | 3.1 | |
3.2 | Amended and Restated Bylaws of the Registrant | 8-K | 7/6/16 | 3.2 | |
4.1 | Form of common stock certificate | S-1^ | 6/3/16 | 4.1 | |
4.2 | Second Amended and Restated Investors’ Rights Agreement dated October 9, 2014, as amended, among the Registrant and the other parties thereto | S-1^ | 6/3/16 | 4.2 | |
4.3 | Sales Agreement dated July 20, 2017 by and between the Registrant and Cowen and Company LLC | S-3^^ | 7/20/17 | 1.2 | |
4.4 | Stock Purchase Agreement dated January 8, 2018 by and between the Registrant and Incyte Corporation, as amended | | | | X |
4.5 | Underwriting Agreement dated January 30, 2018 by and among the Registrant, J.P. Morgan Securities LLC, Cowen and Company, LLC and Piper Jaffray and Co., as representatives of the several underwriters named thereing | 8-K | 1/31/18 | 1.1 | |
4.6 | Securities Purchase Agreement dated April 20, 2017 by and among the Registrant and the persons party thereto | 8-K | 4/21/17 | 10.1 | |
4.7 | Registration Rights Agreement, dated April 20, 2017, by and among the Registrant and the persons party thereto | 8-K | 4/21/17 | 10.2 | |
| | | | | |
Equity Plan Documents | | | | |
10.1* | 2012 Equity Incentive Plan, as amended | S-1^ | 6/3/16 | 10.1 | |
10.2* | Form of Incentive Stock Option Agreement under 2012 Equity Incentive Plan | S-1^ | 6/3/16 | 10.2 | |
10.3* | Form of Nonstatutory Stock Option Agreement under 2012 Equity Incentive Plan | S-1^ | 6/3/16 | 10.3 | |
10.4* | Form of Restricted Stock Agreement under 2012 Equity Incentive Plan | S-1^ | 6/3/16 | 10.4 | |
10.5* | 2016 Stock Incentive Plan | S-1^ | 6/3/16 | 10.5 | |
10.6* | Form of Incentive Stock Option Agreement under 2016 Stock Incentive Plan | S-1^ | 6/3/16 | 10.6 | |
10.7* | Form of Nonstatutory Stock Option Agreement under 2016 Stock Incentive Plan | S-1^ | 6/3/16 | 10.7 | |
10.8* | 2016 Employee Stock Purchase Plan | S-1^ | 6/3/16 | 10.8 | |
| | | | | |
Agreements with Directors and Executive Officers | | | | |
10.9* | Offer Letter, dated November 13, 2012 and effective as of July 2, 2012 by and between the Registrant and Nancy Simonian, M.D., as amended | S-1^ | 6/3/16 | 10.9 | |
10.10* | Offer Letter dated August 25, 2015 by and between the Registrant and Kyle D. Kuvalanka, as amended | S-1^ | 6/3/16 | 10.10 | |
10.11* | Offer Letter dated December 2, 2015 by and between the Registrant and David A. Roth, M.D., as amended | S-1^ | 6/3/16 | 10.11 | |
10.12* | Offer Letter dated September 9, 2016 by and between the Registrant and Gerald E. Quirk, Esq. | 10-K | 3/20/17 | 10.12 | |
10.13* | Offer Letter dated November 6, 2017 by and between the Registrant and Jeremy Springhorn, Ph.D. | | | | X |
10.14* | Consulting Agreement dated August 8, 2012 by and between the Registrant and Richard A. Young, Ph.D., as amended | 10-K | 3/20/17 | 10.13 | |
| | | | | |
10.12* | Form of Restricted Stock Unit Agreement under 2022 Inducement Stock Incentive Plan | 10-K | 3/15/22 | 10.12 | |
10.13* | 2022 Equity Incentive Plan | 8-K | 9/15/22 | 99.1 | |
10.14* | Form of Stock Option Agreement Under 2022 Equity Incentive Plan | 10-Q | 11/14/22 | 10.10 | |
10.15* | Form of Restricted Stock Unit Agreement Under 2022 Equity Incentive Plan | 10-Q | 11/14/22 | 10.11 | |
10.16* | Form of Restricted Stock Agreement Under 2022 Equity Incentive Plan | 10-Q | 11/14/22 | 10.12 | |
10.17* | Amended and Restated Director Compensation Policy | 10-Q | 11/14/22 | 10.13 | |
Agreements with Directors and Executive Officers | | | | |
10.18* | Offer Letter, dated November 13, 2012 and effective as of July 2, 2012 by and between the Registrant and Nancy Simonian, M.D., as amended | S-1^ | 6/3/16 | 10.9 | |
10.19* | Consulting Agreement dated August 8, 2012 by and between the Registrant and Richard A. Young, Ph.D., as amended | 10-Q | 11/12/19 | 10.1 | |
10.20* | Form of Director and Officer Indemnification Agreement by and between the Registrant and each of the directors and executive officers of the Registrant | S-1^ | 6/3/16 | 10.12 | |
10.21* | Offer Letter dated April 24, 2013 by and between the Registrant and Eric R. Olson, Ph.D. | 10-K | 3/5/20 | 10.14 | |
10.22* | Offer Letter dated December 2, 2015 by and between the Registrant and David A. Roth, M.D., as amended | S-1^ | 6/3/16 | 10.11 | |
10.23* | Retirement and Transition Agreement, dated September 28, 2023, by and between the Registrant and Nancy Simonian, M.D. | 10-Q | 11/14/23 | 10.1 | |
10.24* | Amended and Restated Offer Letter, dated September 28, 2023, by and between the Registrant and Conley Chee | 10-Q | 11/14/23 | 10.2 | |
10.25* | Offer Letter dated September 8, 2021 by and between the Registrant and Jason Haas | 8-K | 10/13/21 | 10.1 | |
10.26* | Separation Agreement, dated October 16, 2023, by and between the Registrant and Eric. R. Olson, Ph.D. | | | | X |
10.27* | Form of Nonstatutory Stock Option Agreement for Inducement Awards to Executive Officers | 8-K | 10/13/21 | 10.2 | |
| | | | | |
License and Collaboration Agreements | | | | |
10.28+ | Amended and Restated Cancer License Agreement dated April 28, 2016 by and between the Registrant and TMRC Co., Ltd. | 10-K | 3/7/19 | 10.16 | |
10.29++ | Amendment No. 1 to Amended and Restated Cancer License Agreement, dated January 8, 2021, by and between the Registrant and TMRC Co., Ltd. | 10-Q | 5/6/21 | 10.1 | |
10.30+ | Supply Management Agreement dated April 28, 2016 by and between the Registrant and TMRC Co., Ltd. | S-1^ | 6/3/16 | 10.18 | |
10.31 | Consent and Stand-by License Agreement dated April 28, 2016 by and among the Registrant, Toko Pharmaceutical Ind. Co., Ltd. and TMRC Co., Ltd. | S-1^ | 6/3/16 | 10.19 | |
10.32++ | Master Collaboration Agreement dated March 7, 2022 between the Registrant and Qiagen Manchester Limited | 10-Q | 5/16/22 | 10.1 | |
| | | | | |
Leases and Loan Documents | | | | |
10.33 | Lease dated January 8, 2019 by and between the Registrant and DIV 35 CPD, LLC | 8-K | 1/11/19 | 10.1 | |
10.34 | Loan and Security Agreement dated February 12, 2020 by and between the Registrant and Oxford Finance LLC, as collateral agent and lender, as amended | 10-K | 3/2/23 | 10.33 | |
| | | | | |
Financing Agreements | | | | |
10.35 | Sales Agreement, dated April 6, 2023, by and between the Registrant and Cowen and Company, LLC | S-3^^ | 4/6/23 | 1.2 | |
| | | | | |
Subsidiaries, Consents and Certifications | | | | |
21.1 | Subsidiaries of the Registrant | | | | X |
23.1 | Consent of Ernst & Young LLP, independent public accounting firm | | | | X |
31.1 | Certification of principal executive officer pursuant to Rule 13a-14(a)/15d-14(a) of the Securities Exchange Act of 1934, as amended | | | | X |
| | | | | |
| | Incorporation by Reference | |
Exhibit No. | Description | Form | SEC
Filing
Date | Exhibit
Number | Filed
with this
10-K |
10.15* | Form of Director and Officer Indemnification Agreement by and between the Registrant and each of the directors and executive officers of the Registrant | S-1^ | 6/3/16 | 10.12 | |
| | | | | |
License and Collaboration Agreements | | | | |
10.16+ | Exclusive License Agreement dated February 22, 2013 by and between the Registrant and the Dana-Farber Cancer Institute, Inc. | S-1^ | 6/3/16 | 10.13 | |
10.17+ | Exclusive License Agreement dated April 1, 2013 by and among the Registrant, the Whitehead Institute for Biomedical Research and the Dana-Farber Cancer Institute, Inc. | S-1^ | 6/3/16 | 10.14 | |
10.18+ | Exclusive License Agreement dated April 4, 2013 by and between the Registrant and the Whitehead Institute for Biomedical Research | S-1^ | 6/3/16 | 10.15 | |
10.19+ | Amended and Restated Cancer License Agreement dated April 28, 2016 by and between the Registrant and TMRC Co., Ltd. | S-1^ | 6/3/16 | 10.16 | |
10.20+ | Supply Management Agreement dated April 28, 2016 by and between the Registrant and TMRC Co., Ltd. | S-1^ | 6/3/16 | 10.18 | |
10.21 | Consent and Stand-by License Agreement dated April 28, 2016 by and among the Registrant, Toko Pharmaceutical Ind. Co., Ltd. and TMRC Co., Ltd. | S-1^ | 6/3/16 | 10.19 | |
10.22+ | Target Discovery, Research Collaboration and Option Agreement dated January 8, 2018 by and between the Registrant and Incyte Corporation | | | | X |
| | | | | |
Leases | | | | | |
10.23 | Lease dated March 13, 2015 by and between the Registrant and 620 Memorial Leasehold LLC | S-1^ | 6/3/16 | 10.17 | |
| | | | | |
Subsidiaries, Consents and Certifications | | | | |
21.1 | Subsidiaries of the Registrant | | | | X |
23.1 | Consent of Ernst & Young LLP, independent public accounting firm | | | | X |
31.1 | Certification of principal executive officer pursuant to Rule 13a-14(a)/15d-14(a) of the Securities Exchange Act of 1934, as amended | | | | X |
31.2 | Certification of principal financial officer pursuant to Rule 13a-14(a)/15d-14(a) of the Securities Exchange Act of 1934, as amended | | | | X |
32.1# | Statement of principal executive officer pursuant to 18 U.S.C. §1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | | | | X |
32.2# | Statement of principal financial officer pursuant to 18 U.S.C. §1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | | | | X |
| | | | | |
XBRL Documents | | | | |
101.INS | XBRL Instance Document | | | | |
101.SCH | XBRL Taxonomy Extension Schema Document | | | | |
101.CAL | XBRL Calculation Linkbase Document | | | | |
101.DEF | XBRL Taxonomy Extension Definition Linkbase Document | | | | |
101.LAB | XBRL Label Linkbase Document | | | | |
101.PRE | XBRL Taxonomy Presentation Linkbase Document | | | | |
* Indicates management contract or compensatory plan.
+Confidential treatment has been requested and/or granted as to certain portions, which portions have been omitted and filed separately with the U.S. Securities and Exchange CommissionCommission.
++ Portions of this exhibit have been omitted pursuant to Item 601 of Regulation S-K.
^ SEC File No. 333-211818
^^SEC File No. 333-219369333-271160
#This# This certification will not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liability of that section. Such certification will not be deemed to be incorporated by reference into any filing under the Securities Act of 1933, as amended, or the Exchange Act, except to the extent specifically incorporated by reference into such filing.
(c)Financial Statement Schedules
No financial statement schedules have been submitted because they are not required or are not applicable or because the information required is included in the consolidated financial statements or the notes thereto.
ITEM 16. FORM 10-K SUMMARY
None.
SIGNATURES
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | |
| SYROS PHARMACEUTICALS, INC. |
|
|
|
|
|
|
Date: March 12, 201827, 2024 | By: | /s/ Nancy Simonian, M.D.Conley Chee |
|
| Nancy Simonian, M.D.Conley Chee
|
|
| President and Chief Executive Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| | | | |
Signature |
| Title |
| Date |
|
|
|
|
|
/s/ Nancy Simonian, M.D.Conley Chee |
| President, Chief Executive Officer and Director |
| March 12, 201827, 2024 |
Nancy Simonian, M.D.Conley Chee
|
| (Principal Executive and Financial Officer) |
|
|
|
|
|
|
|
/s/ Michael InbarJason Haas |
| ControllerChief Financial Officer
|
| March 12, 201827, 2024 |
Michael InbarJason Haas
|
| (Principal Financial Officer and Principal Accounting Officer) |
|
|
|
|
|
|
|
/s/ Peter Wirth |
| Chair of the Board of Directors |
| March 12, 201827, 2024 |
Peter Wirth |
|
|
|
|
|
|
|
|
|
/s/ Srinivas Akkaraju, M.D., Ph.D. |
| Director |
| March 12, 201827, 2024 |
Srinivas Akkaraju, M.D., Ph.D. |
|
|
|
|
|
|
|
|
|
/s/ Deborah Dunsire, M.D. |
| Director |
| March 27, 2024 |
Deborah Dunsire, M.D. |
|
|
|
|
|
|
|
|
|
/s/ S. Gail Eckhardt, M.D. |
| Director |
| March 27, 2024 |
S. Gail Eckhardt, M.D. |
|
|
|
|
|
|
|
|
|
/s/ Marsha H. Fanucci |
| Director |
| March 12, 201827, 2024 |
Marsha H. Fanucci |
|
|
|
|
|
|
|
|
|
/s/ Amir Nashat, Ph.D.Andrew Oh |
| Director |
| March 12, 201827, 2024 |
Amir Nashat, Ph.D.Andrew Oh
|
|
|
|
|
|
|
|
|
|
/s/ Robert T. NelsenNancy Simonian, M.D. |
| Director |
| March 12, 201827, 2024 |
Robert T. NelsenNancy Simonian, M.D
|
|
|
|
|
|
|
|
|
|
/s/ Sanj K. PatelTimothy Tyson |
| Director |
| March 12, 201827, 2024 |
Sanj K. PatelTimothy Tyson
|
|
|
|
|
|
|
|
|
|
/s/ Vicki L Sato, Ph.D.
| | Director
| | March 12, 2018
|
Vicki L. Sato, Ph.D.
| | | | |
| | | | |
/s/ Phillip A. Sharp, Ph.D.
| | Director
| | March 12, 2018
|
Phillip A. Sharp, Ph.D.
| | | | |
| | | | |
/s/ Richard A. Young, Ph.D. |
| Director |
| March 12, 201827, 2024 |
Richard A. Young, Ph.D. |
|
|
|
|












