·
| Academic Gene Editing Licenses. We in-licensed aβ-thalassemia/SCD Product Candidate Licenses. We have in-licensed patents and patent applications that are directed to certain specific compositions of matter and methods for treating β-thalassemia/SCD. As of January 31, 2018, we had an exclusive license to onepending U.S. patent application and 22 pending corresponding foreign applications. We expect any composition of matter or method patents, if issued from the pending patent applications, if applicable, and if the appropriate maintenance, renewal, annuity or other governmental fees are paid, to expire in 2035 (worldwide, excluding possible patent term extensions). We expect any other patents in this portfolio, if issued, and if the appropriate maintenance, renewal, annuity, or other governmental fees are paid, to expire from 2035 (worldwide, excluding possible patent term extensions). In addition, as of January 31, 2018, we had a non-exclusive license to two issued U.S. patents, one pending U.S. patent application, and 9 pending corresponding foreign patent applications and 19 issued foreign patents. We expect the issued composition of matter and method patents to expire in 2029 in the United States and in the rest of the world (excluding possible patent term extensions). We expect any composition of matter or method patents, if issued from the pending patent applications, if applicable, and if the appropriate maintenance, renewal, annuity or other governmental fees are paid, to expire in 2029 (worldwide, excluding possible patent term extensions). We expect any other patents in this portfolio, if issued, and if the appropriate maintenance, renewal, annuity, or other governmental fees are paid, to expire from 2029 (worldwide, excluding possible patent term extensions. Cerebral Adrenoleukodystrophy (CALD) The CALD program includes three patent portfolios, described below. Pasteur Institute. The in-licensed Pasteur patent portfolio contains the patents and patent applications described above directed towards aspects of our lentiviral vectors utilized to produce our Lenti-D product candidate for CALD. RDF. The in-licensed RDF patent portfolio contains the patents and patent applications described above directed towards aspects of our lentiviral vectors utilized to produce our Lenti-D product candidate for CALD. bluebird bio. The bluebird bio patent portfolio contains patent applications directed to compositions of matter for CALD gene therapy vectors and compositions and methods of using the vectors and compositions in cell-based gene therapy of adrenoleukodystrophy or adrenomyeloneuropathy. As of January 31, 2018, we owned three U.S. patents and six pending corresponding foreign applications and five issued foreign patents. We expect the issued composition of matter patents for CALD gene therapy vectors to expire in 2032 (excluding possible patent term extensions). Further, we expect composition of matter or method patents, if issued from the pending patent applications and if the appropriate maintenance, renewal, annuity or other governmental fees are paid, to expire in 2032 (worldwide, excluding possible patent term extensions). We expect any other patents in this portfolio, if issued, and if the appropriate maintenance, renewal, annuity, or other governmental fees are paid, to expire in 2032 (worldwide, excluding possible patent term extensions).
Multiple Myeloma The multiple myeloma program includes five patent portfolios, described below. Pasteur Institute. The in-licensed Pasteur patent portfolio contains patents and patent applications described above that are directed towards aspects of our lentiviral vectors utilized to produce our bb2121 product candidate for multiple myleoma. RDF. The in-licensed RDF patent portfolio contains the patents and patent applications described above directed towards aspects of our lentiviral vectors utilized to produce our bb2121 product candidate for multiple myleoma. In addition, the RDF portfolio contains additional patent applications directed to aspects of our oncology program. As of January 31, 2018, we had an exclusive license (from RDF) to four issued patents and two pending U.S. patent applications related to our oncology platform. We expect the issued patent to expire in 2021 (excluding possible patent term extensions). Further, we expect composition of matter or methods patents, if issued from the pending patent applications and if the appropriate maintenance, renewal, annuity or other governmental fees are paid, to expire in 2021-2022 (excluding possible patent term extensions). We expect any other patents and patent applications in this portfolio, if issued, and if the appropriate maintenance, renewal, annuity, or other governmental fees are paid, to expire in 2021-2022 (worldwide, excluding possible patent term extensions). Biogen. The in-licensed patent portfolio from Biogen Inc., formerly Biogen Idec MA Inc. and referred to herein as Biogen, contains patents and patent applications directed towards aspects of T cell-based products that target BCMA. As of January 31, 2018, we had a co-exclusive license to nine issued U.S. patents and two pending U.S. patent applications and six pending corresponding foreign applications and 104 issued corresponding foreign patents related to bb2121. We expect the issued patents to expire from 2020-2032 (excluding possible patent term extensions). Further, we expect composition of matter or methods patents, if issued from the pending patent applications and if the appropriate maintenance, renewal, annuity or other governmental fees are paid, to expire from 2020-2032 (excluding possible patent term extensions). We expect any other patents and patent applications in this portfolio, if issued, and if the appropriate maintenance, renewal, annuity, or other governmental fees are paid, to expire in 2020-2032 (worldwide, excluding possible patent term extensions). NIH. The in-licensed patent portfolio from NIH contains patent applications directed towards aspects of T cell-based products that target BCMA. As of January 31, 2018, we had an exclusive license to one issued U.S. Patent, one pending U.S. patent application and 18 corresponding foreign patent applications and two issued corresponding foreign patents related to bb2121. We expect the issued composition of matter patents to expire in 2034 (excluding possible patent term extensions). We expect any other composition of matter and methods patents, if issued from the pending patent applications and if the appropriate maintenance, renewal, annuity or other governmental fees are paid, to expire in 2033 (excluding possible patent term extensions). We expect any other patents and patent applications in this portfolio, if issued, and if the appropriate maintenance, renewal, annuity, or other governmental fees are paid, to expire in 2033 (worldwide, excluding possible patent term extensions). bluebird bio. The bluebird bio patent portfolio contains patent applications directed to certain specific compositions of matter for generating CAR T cells. As of January 31, 2018, we owned five pending U.S. patent applications, one pending U.S. provisional patent application, 86 corresponding pending foreign patent applications and two pending PCT applications. We expect any composition of matter or methods patents, if issued from a corresponding nonprovisional application or national stage application, or corresponding foreign applications, if applicable, and if the appropriate maintenance, renewal, annuity or other governmental fees are paid, to expire from 2035-2038 (worldwide, excluding possible patent term extensions). We expect any other patents and patent applications in this portfolio, if issued, and if the appropriate maintenance, renewal, annuity, or other governmental fees are paid, to expire from 2035-2038 (worldwide, excluding possible patent term extensions). Lentiviral platform (e.g., vectors, manufacturing, and cell therapy products) The lentiviral platform, which is potentially applicable to the β-thalassemia, SCD, CALD, oncology and other potential programs, includes three patent portfolios, described below. Pasteur Institute. The Pasteur patent portfolio contains the patents and patent applications described above. RDF. The in-licensed RDF patent portfolio contains the patents and patent applications described above. bluebird bio. Another component of the bluebird bio patent portfolio includes the vector manufacturing platform and is potentially applicable to the CALD, β-thalassemia, SCD, oncology, and other programs. This portion of the portfolio contains patents and patent applications directed to improved methods for transfection and transduction of therapeutic cells. As of January 31, 2018, we owned two PCT applications, one pending U.S. patent applications and 20 corresponding foreign patent applications and 18 issued corresponding foreign patents. We expect composition of matter and method patents, if issued from the pending patent applications and if the appropriate maintenance, renewal, annuity or other governmental fees are paid, to expire from 2032-2037 (excluding possible patent term extensions). We expect any other patents and patent applications in this portfolio, if issued, and if the appropriate maintenance, renewal, annuity, or other governmental fees are paid, to expire in 2032-2037 (worldwide, excluding possible patent term extensions).
Oncology platform (e.g., vectors, manufacturing, and T cell-based products) Our T cell-based oncology platform and oncology research program, which is applicable to our multiple myeloma program and other potential programs in cancer, includes four patent portfolios, described below. Pasteur Institute. The Pasteur patent portfolio contains the patents and patent applications described above. RDF. The in-licensed RDF patent portfolio described above contains patents and patent applications that are also applicable to our oncology platform. In addition, the RDF portfolio contains additional patent applications directed to aspects of our oncology program. As of January 31, 2018, we had an exclusive license (from RDF) to four issued patents and two pending U.S. patent applications related to our oncology platform. We expect the issued patent to expire in 2021 (excluding possible patent term extensions). Further, we expect composition of matter or methods patents, if issued from the pending patent applications and if the appropriate maintenance, renewal, annuity or other governmental fees are paid, to expire in 2021-2022 (excluding possible patent term extensions). We expect any other patents and patent applications in this portfolio, if issued, and if the appropriate maintenance, renewal, annuity, or other governmental fees are paid, to expire in 2021-2022 (worldwide, excluding possible patent term extensions). bluebird bio. One aspect of the bluebird bio patent portfolio contains patent applications directed to certain specific compositions of matter for generating CAR T cells directed against various cancers and improved CAR T cell compositions. As of January 31, 2018, we owned four pending U.S. patent applications and seven corresponding pending foreign patent applications; seven families of pending U.S. provisional applications; and five pending PCT applications. We expect any composition of matter or methods patents, if issued from a corresponding nonprovisional application or national stage application, or corresponding foreign applications, if applicable, and if the appropriate maintenance, renewal, annuity or other governmental fees are paid, to expire in 2033-2037 (worldwide, excluding possible patent term extensions). We expect any other patents and patent applications in this portfolio, if issued, and if the appropriate maintenance, renewal, annuity, or other governmental fees are paid, to expire from 2033-2037(worldwide, excluding possible patent term extensions). T Cell Manufacturing Methods License. We have in-licensed patents and patent applications that are directed to certain specific methods for generating CAR T cells. As of January 31, 2018, we had a nonexclusive license to one issued U.S. patent, onepending U.S. patent application, and 30 corresponding issued foreign patents. We expect the issued method patents to expire in 2026 (excluding possible patent term extensions). Further, we expect methods patents, if issued from the pending patent applications and if the appropriate maintenance, renewal, annuity or other governmental fees are paid, to expire in 2026 (excluding possible patent term extensions). We expect any other patents and patent applications in this portfolio, if issued, and if the appropriate maintenance, renewal, annuity, or other governmental fees are paid, to expire from 2026 (worldwide, excluding possible patent term extensions). T Cell Immunotherapy Product Candidate Licenses. We have in-licensed patents and patent applications that are directed to certain specific compositions of matter for generating CAR T cells directed against various cancers and related methods of treatment. As of January 31, 2018, we have an exclusive license to one issued U.S. patent and ten corresponding foreign patents and co-own a pending PCT application to a particular target antigen (due for national stage conversion in March 2018). We expect the issued composition of matter patent to expire in 2025 (excluding possible patent term extensions). We expect any composition of matter or methods patents, if issued from a corresponding nonprovisional application or national stage application, or corresponding foreign applications, if applicable, and if the appropriate maintenance, renewal, annuity or other governmental fees are paid, to expire in 2036 (worldwide, excluding possible patent term extensions). We expect any other patents and patent applications in this portfolio, if issued, and if the appropriate maintenance, renewal, annuity, or other governmental fees are paid, to expire from 2036 (worldwide, excluding possible patent term extensions). In addition, as of January 31, 2018, we have an exclusive license to two issued U.S. patents, one pending U.S. patent application and ten corresponding foreign patents to another particular target antigen. We expect the issued method of use patents to expire in 2029 (excluding possible patent term extensions). We expect any composition of matter or methods patents, if issued from a corresponding nonprovisional application or corresponding foreign applications, if applicable, and if the appropriate maintenance, renewal, annuity or other governmental fees are paid, to expire from 2029 (worldwide, excluding possible patent term extensions). We expect any other patents and patent applications in this portfolio, if issued, and if the appropriate maintenance, renewal, annuity, or other governmental fees are paid, to expire from 2029 (worldwide, excluding possible patent term extensions).
Gene editing platform (e.g., homing endonucleases, chimeric endonucleases, megaTALs, genetically modified cells) The gene editing platform includes five patent portfolios, described below. Pasteur Institute. The Pasteur patent portfolio described above may contain patents and patent applications that are potentially applicable to our gene editing platform. RDF. The in-licensed RDF patent portfolio described above may contain patents and patent applications that are potentially applicable to our gene editing platform. Gene Editing License. We in-licensed patent portfolios that contain patents and patent applications directed to aspects of our gene editing platform to produce genome modifying enzymes and genetically modified cells that are potentially applicable to our β-thalassemia, SCD, oncology and other programs. As of January 31, 2018, we had an exclusive/co-exclusive license to six issued U.S. patents and one pending U.S. patent application and 61 corresponding foreign patents and six corresponding patent applications related to our gene editing platform. We expect the issued composition of matter patents to expire in 2030 (excluding possible patent term extensions). Further, we expect composition of matter or methods patents, if issued from the pending patent applications and if the appropriate maintenance, renewal, annuity or other governmental fees are paid, to expire in 2030 (excluding possible patent term extensions). We expect any other patents and patent applications in this portfolio, if issued, and if the appropriate maintenance, renewal, annuity, or other governmental fees are paid, to expire in 2030 (worldwide, excluding possible patent term extensions). In addition, as of January 31, 2018, we had an exclusive license to two issued U.S. patents and six corresponding foreign patents related to our gene editing platform. We expect the issued composition of matter patent to expire in 2031 in the United States (excluding possible patent term extensions) and in 2027 in the rest of the world. Further, we expect composition of matter and methods patents, if issued from the pending patent applications and if the appropriate maintenance, renewal, annuity or other governmental fees are paid, to expire in 2027 (excluding possible patent term extensions). We expect any other patents and patent applications in this portfolio, if issued, and if the appropriate maintenance, renewal, annuity, or other governmental fees are paid, to expire in 2027 (worldwide, excluding possible patent term extensions). Academic Gene Editing Licenses. We in-licensed patent portfolios from multiple academic medical centers, each portfolio from an academic medical center containing patents and patent applications directed to aspects of our gene editing platform to produce genome modifying enzymes and genetically modified cells that are potentially applicable to our β-thalassemia, SCD, oncology and other programs.As of January 31, 2018, we had an exclusive license to one issued U.S. patent and seven pending U.S. patent applications and four corresponding foreign patents and four corresponding patent applications related to our gene editing platform. We expect the issued patent to expire in 2032 (excluding possible patent term extensions) in the U.S. and 2027-3032 in the rest of the world. We expect composition of matter or method patents, if issued from the pending patent applications and if the appropriate maintenance, renewal, annuity or other governmental fees are paid, to expire from 2027-2032 (excluding possible patent term extensions). We expect any other patents and patent applications in this portfolio, if issued, and if the appropriate maintenance, renewal, annuity, or other governmental fees are paid, to expire in 2027-2032 (worldwide, excluding possible patent term extensions). As of January 31, 2018, we also had a non-exclusive license to one pending U.S. patent application related to our gene editing platform. We expect any composition of matter or methods patents, if issued from a corresponding nonprovisional application or national stage application, or corresponding foreign applications, if applicable, and if the appropriate maintenance, renewal, annuity or other governmental fees are paid, to expire in 2036 (worldwide, excluding possible patent term extensions). We expect any other patents and patent applications in this portfolio, if issued, and if the appropriate maintenance, renewal, annuity, or other governmental fees are paid, to expire from 2036 (worldwide, excluding possible patent term extensions). In addition, as of January 31, 2018, we had an exclusive license to two pending U.S. applications and 14 corresponding issued foreign patents and 15 pending foreign patent applications related to our gene editing platform. We expect the issued composition of matter patens to expire in 2033 (excluding possible patent term extensions). We expect other composition of matter or method patents, if issued from the pending patent applications and if the appropriate maintenance, renewal, annuity or other governmental fees are paid, to expire from 2031-2033 (excluding possible patent term extensions). We expect any other patents and patent applications in this portfolio, if issued, and if the appropriate maintenance, renewal, annuity, or other governmental fees are paid, to expire in 2031-2033 (worldwide, excluding possible patent term extensions). As of January 31, 2018, we also had a non-exclusive license to one issued U.S. patent, one pending U.S. application and 19 corresponding foreign patent applications related to our gene editing platform. We expect the issued composition of matter patents to expire in 2033 (excluding possible patent term extensions). Further, we expect composition of matter or method patents, if issued from the pending patent applications and if the appropriate maintenance, renewal, annuity or other governmental fees are paid, to expire from 2033 (excluding possible patent term extensions). We expect any other patents and patent applications in this portfolio, if issued, and if the appropriate maintenance, renewal, annuity, or other governmental fees are paid, to expire in 2033 (worldwide, excluding possible patent term extensions).
| • | bluebird bio. One aspect of the bluebird bio patent portfolio contains patent applications that are potentially applicable to certain aspects of our gene editing platform to produce genome modifying enzymes and genetically modified cells that are potentially applicable to our ß-thalassemia, SCD, oncology and other programs. As of January 31, 2015,2018, we had an exclusive licenseowned seven families of PCT applications related to our gene editing platform. We expect any composition of matter or methods patents, if issued from a corresponding nonprovisional application or national stage application, or corresponding foreign applications, if applicable, and if the appropriate maintenance, renewal, annuity or other governmental fees are paid, to expire in 2037 (worldwide, excluding possible patent term extensions). We expect any other patents and patent applications in this portfolio, if issued, and if the appropriate maintenance, renewal, annuity, or other governmental fees are paid, to expire in 2037 (worldwide, excluding possible patent term extensions). As of January 31, 2018, we also owned five families of provisional applications related to our gene editing platform. We expect any composition of matter or methods patents, if issued from a corresponding nonprovisional application or national stage application, or corresponding foreign applications, if applicable, and if the appropriate maintenance, renewal, annuity or other governmental fees are paid, to expire in 2038 (worldwide, excluding possible patent term extensions). We expect any other patents and patent applications in this portfolio, if issued, and if the appropriate maintenance, renewal, annuity, or other governmental fees are paid, to expire in 2038 (worldwide, excluding possible patent term extensions). As of January 31, 2018, we co-owned (with Cellectis) two pending U.S. applications and 1912 corresponding pending foreign patent applications related to our gene editing platform. We expect composition of matter or method patents, if issued from the pending patent applications and if the appropriate maintenance, renewal, annuity or other governmental fees are paid, to expire from 2032-2033(excluding2034 (excluding possible patent term extensions). We expect the other patents and patent applications in this portfolio, if issued, and if the appropriate maintenance, renewal, annuity, or other governmental fees are paid, to expire in 2032-2033 (worldwide, excluding possible patent term extensions). |
·
| bluebird bio. One aspect of the bluebird bio patent portfolio contains patent applications that are potentially applicable to certain aspects of our gene editing platform to produce genome modifying enzymes and genetically modified cells that are potentially applicable to our ß-thalassemia, SCD, oncology, and other programs. As of January 31, 2015, we co-owned (with Cellectis) two pending PCT applications, both due for nationalization November 2015. We expect any composition of matter or methods patents, if issued from a corresponding national stage application, or corresponding foreign applications, if applicable, and if the appropriate maintenance, renewal, annuity or other governmental fees are paid, to expire from 2034 (worldwide, excluding possible patent term extensions).
|
The term of individual patents depends upon the legal term of the patents in the countries in which they are obtained. In most countries in which we file, the patent term is 20 years from the date of filing the non-provisional application. In the United States, a patent’s term may be lengthened by patent term adjustment, which compensates a patentee for administrative delays by the U.S. Patent and Trademark Office in granting a patent, or may be shortened if a patent is terminally disclaimed over an earlier-filed patent. The term of a patent that covers an FDA-approved drug may also be eligible for patent term extension, which permits patent term restoration of a U.S. patent as compensation for the patent term lost during the FDA regulatory review process. The Hatch-Waxman Act permits a patent term extension of up to five years beyond the expiration of the patent. The length of the patent term extension is related to the length of time the drug is under regulatory review. A patent term extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval and only one patent applicable to an approved drug may be extended. 25
Moreover, a patent can only be extended once, and thus, if a single patent is applicable to multiple products, it can only be extended based on one product. Similar provisions are available in Europe and other foreign jurisdictions to extend the term of a patent that covers an approved drug. When possible, depending upon the length of clinical trials and other factors involved in the filing of a new drug application, or NDA,BLA, we expect to apply for patent term extensions for patents covering our product candidates and their methods of use. We may rely, in some circumstances, on trade secrets to protect our technology. However, trade secrets can be difficult to protect. We seek to protect our proprietary technology and processes, in part, by entering into confidentiality agreements with our employees, consultants, scientific advisors and contractors.third parties. We also seek to preserve the integrity and confidentiality of our data and trade secrets by maintaining physical security of our premises and physical and electronic security of our information technology systems. While we have confidence in these individuals, organizations and systems, agreements or security measures may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. To the extent that our consultants contractors or collaborators use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions. License agreements Inserm-Transfert In May 2009, we entered into an exclusive license with Inserm-Transfert, which is a wholly-owned subsidiary of Institut national de la santé et de la recherche médicale, for use of certain patents and know-how related to the ABCD1 gene and corresponding protein, for use in the field of human ALD therapy. This agreement was amended once in 2012 and again in 2013. Inserm-Transfert is referred to herein as Inserm. The last patent in the Inserm licensed patent portfolio includes at least three U.S. and foreign patents and patent applications. This portfolio has no pending applications.expired in February of 2016. Inserm retains the right to practice the intellectual property licensed under the agreement for educational, clinical and preclinical studies purposes. Upon commercialization of our products covered by the in-licensed intellectual property, which we expect would include our Lenti-D product candidate, we will be obligated to pay Inserm a percentage of net sales as a royalty for the longer of the life of any patents covering the product or 10 years from first commercial sale. This royalty is in the low single digits. The royalties payable to Inserm are subject to reduction for any third party payments required to be made, with a minimum floor in the low single digits.
We are required to use all commercially reasonable efforts to develop licensed products and introduce them into the commercial market as soon as practical, consistent with our reasonable business practices and judgment in compliance with an agreed upon development plan. We have assumed certain development, regulatory and commercial milestone obligations and must report on our progress in achieving these milestones on an annual basis. We may unilaterally terminate the license agreement at any time. Either party may terminate the agreement in the event of the other party’s material breach which remains uncured after 60 days of receiving written notice of such breach or in the event the other party become subject of a voluntary or involuntary petition in bankruptcy and such petition is not dismissed with prejudice within 120 days after filing. In addition, Inserm may terminate the license agreement in the event that we cannot prove within 60 days of written notice from Inserm that we have been diligent in developing the licensed products and introducing them into the commercial market. Absent early termination, the agreement will automatically terminate upon the expiration of all issued patents and filed patent applications within the patent rights covered by the agreement or 10 years from the date of first commercial sale of a licensed product, whichever is later. The license grant ceases in connection with any such termination. The longest lived patent rights licensed to us under the agreement are currently expected to expire in 2016. Institut Pasteur In September 2011, weWe have entered into a license with Institut Pasteur for certain patents relating to the use of DNA sequences, lentiviral vectors and recombinant cells in the field of ex vivo gene therapy and CAR T cell-based therapy in a range of indications.indications, excluding vaccinations. This agreement was amended twice in 2012, again in 2013 and a third timemost recently in 2013.2015. The Institut Pasteur licensed patent portfolio includes at least 23107 U.S. and foreign patents and patent applications. Any patents within this portfolio that have issued or may yet issue would have a statutory expiration dates between 2019 and 2023. The license is exclusive for products containing human (HIV-1 and HIV-2) lentiviral vector and non-exclusive for products containing non-human lentiviral vector.vectors. Institut Pasteur retains the right, on behalf of itself, its licensees and research partners, to conduct research using the licensed intellectual property.
We have the right to grant sublicenses outright to third parties under the agreement. For the first sublicense including a product targeting β-hemoglobinopathies (including TDT and severe SCD) or ALD (including CALD and AMN), we must pay Institut Pasteur an additional payment of €3.0 million. If we receive any income (cash or non-cash) in connection with such sublicenses for products targeting indications other than β-hemoglobinopathies (including TDT and severe SCD) or ALD (including CCALDCALD and AMN), or β-hemoglobinopathies (including β-thalassemia, and SCD), we must pay Institut Pasteur a percentage of such income varying from low single digits if the sublicense also includes licenses to lower to midintellectual property controlled by us, and a percentage of sublicense income in the mid-range double digits depending onif the nature of the sublicense.sublicense does not include licenses to intellectual property controlled by us. 26
Upon commercialization of our products covered by the in-licensed intellectual property, which we expect would include our Lenti-DLentiGlobin and LentiGlobinLenti-D product candidates, we will be obligated to pay Institut Pasteur a percentage of net sales as a royalty. This royalty varies depending on the indication of the product but in any event is in the low single digits. In addition, starting in 2016 we must make under this agreement an annual maintenance payment which is creditable against royalty payments on a year-by-year basis. If the combined royalties we would be required to pay to Institut Pasteur and third parties is higher than a pre-specified percentage, we may ask Institut Pasteur to re-negotiate our royalty rates under this relationship. We are required to use all reasonable commercial efforts (as compared to a company of similar size and scope) to develop and commercialize one or more products in the license field and to obtain any necessary governmental approvals in respect of, and market the products in license field, if any. Additionally, we have assumed certain development and regulatory milestone obligations. We must report on our progress towards achieving these milestones on an annual basis. We may unilaterally terminate the license agreement at any time by sending Institut Pasteur 90 daydays prior written notice. Either party may terminate the license in the event of the other party’s substantial breach which remains uncured after 60 days of receiving written notice of such breach. Institut Pasteur may also terminate the agreement in the event bankruptcy proceedings are opened against us and not dismissed within 60 days. Absent early termination, the agreement will automatically terminate upon the expiration of the last licensed patents.patents or five years after first market authorization of the first product, whichever occurs later. In the event the agreement is terminated, while the license grant would cease, we would retain the right to manufacture, import, use and sell licensed products for a certain period of time post-termination. In addition, our ownership stake in certain jointly made improvements covered by the licensed patents would survive termination of the agreement. The longest lived patent rights licensed to us under the agreement are currently expected to expire in 2023.
Stanford University In July 2002, we entered into a non-exclusive license agreement with the Board of Trustees of the Leland Stanford Junior University, referred to herein as Stanford, which we amended and restated in April 2012. Under this agreement, we are granted a license to use the HEK293T cell line for any commercial or non-commercial use for research, non-clinicalnonclinical and clinical development purpose and human and animal gene therapy products. We have the right to grant sublicenses outright to third parties under the agreement. For each such sublicense we grant, we must pay Stanford a fee (unless the sublicense is to a collaborating partner, contract manufacturer or contract research organization). Upon commercialization of our products covered by the in-licensed intellectual property, which we expect would include our Lenti-D product candidate, we will be obligated to pay Stanford a percentage of net sales as a royalty. This royalty varies with net sales but in any event is in the low single digits and is reduced for each third-party license that requires payments by us with respect to a licensed product, provided that the royalty to Stanford is not less than a specified percentage which is less than one percent. Since April 2013, we have been paying Stanford an annual maintenance fee, which will be creditable against our royalty payments. We may unilaterally terminate the agreement by giving Stanford 30 days’ written notice. Stanford may also terminate the license agreement if after 30 days of providing notice we are delinquent on any report or payment, are not using commercially reasonable efforts to develop, manufacture and/or commercialize one or more licensed products, are in material breach of any provision or provide any false report. Termination of this agreement may require us to utilize different cell types for vector manufacturing, which could lead to delays. Absent early termination, the license will expire in April 2037. We may elect to extend the term for an additional 25 years so long as we have a commercial product on the market at that time and we are in material compliance with the license agreement. Massachusetts Institute of Technology In December 1996, we entered into an exclusive license with the Massachusetts Institute of Technology, referred to herein as MIT, for use of certain patents in any field. This license agreement was amended in December 2003, May 2004 and June 2011. The licensed patent portfolio includes at least 2618 U.S. and foreign patents and patent applications. Any patents within this portfolio that have issued or may yet issue would have a statutory expiration date in 2023.from 2017-2023. This license also has been amended to include a case jointly owned by MIT and us wherein we received the exclusive license to MIT’s rights in this case. MIT retains the right to practice the intellectual property licensed under the agreement for noncommercial research purposes. We have the right to grant sublicenses outright to third parties under the agreement. In the event we sublicense the patent rights, we must pay MIT a percentage of all payments we receive from by the sublicensee. This percentage varies from mid-single digits to low double digits. Upon commercialization of our products covered by the in-licensed intellectual property, which we expect would include our LentiGlobin product candidate, we will be obligated to pay MIT a percentage of net sales by us or our sublicensees as a royalty. This royalty is in the low single digits and is reduced for royalties payable to third parties, provided that the royalty to MIT is not less than 27
a specified percentage that is less than one-percent. In addition, we make under this agreement an annual maintenance payment which may be credited against the royalty payments. We are required to use diligent efforts to market licensed products and to continue active, diligent development and marketing efforts for licensed products during the term of the agreement. We have assumed certain milestones with respect to raising capital investment and regulatory progress. We must report on our progress on achieving these milestones on an annual basis. We may unilaterally terminate the license agreement upon six months’ notice to MIT. MIT may terminate the agreement if we cease to carry on our business, or in the event of our material breach which remains uncured after 90 days of receiving written notice of such breach (30 days in the case of nonpayment). In the event the agreement is terminated, while the license grant would cease, we would retain a right to complete manufacture of any licensed products in process and sell then-existing inventory. In addition, MIT would grant our sublicensees a direct license following such termination. With respect to jointly owned intellectual property, any termination would allow MIT to grant licenses to any third party to such intellectual property, without our approval, unless a sublicensee was already in place, in which case, MIT would grant our sublicensees a direct license.
Research Development Foundation In December 2011, we entered into an exclusive license with RDF to use certain patents that involve lentiviral vectors. The RDF licensed patent portfolio includes at least 1429 U.S. and foreign patents and patent applications. Any patents within this portfolio that have issued or may yet issue would have an expected statutory expiration date ofbetween 2021 or 2022.and 2027. RDF retains the right, on behalf of itself and other nonprofit academic research institutions, to practice and use the licensed patents for any academic, non-clinical research and educational purposes. We have the right to grant sublicenses outright to third parties under the agreement. Upon commercialization of our products covered by the in-licensed intellectual property, which we expect would include both our Lenti-D and LentiGlobin product candidates, we are obligated to pay RDF a percentage of net sales as a royalty. This royalty is in the low single digits and is reduced by half if during the following ten years from the first marketing approval the last valid claim within the licensed patent that covers the licensed product expires or ends. We are required to use commercially reasonable and diligent efforts for a company of our size and resources to develop or commercialize one or more licensed products, including our first licensed product by 2016 and a second licensed product by 2018. These diligence efforts include minimum annual royalty payments to RDF, which are creditable against earned royalties otherwise due to RDF, and payments upon regulatory milestones. RDF may terminate the agreement in the event of our material breach which remains uncured after 90 days of receiving written notice of such breach (30 days in the case of nonpayment) or in the event we become bankrupt, our business or assets or property are placed in the hands of a receiver, assignee or trustee, we institute or suffer to be instituted any procedure in bankruptcy court for reorganization or rearrangement of our financial affairs, make a general assignment for the benefit of creditors, or if we or an affiliate or a sublicensee institutes any procedure challenging the validity or patentability of any patent or patent application within the licensed patents, the agreement will immediately terminate. Absent early termination, the agreement will continue until its expiration upon the later of there being no more valid claims within the licensed patents or the expiration of our royalty obligations on licensed products that are subject to an earned royalty, if such earned royalty is based on the minimum 10-year royalty period described above. In the event the agreement is terminated, while the license grant would cease, RDF will grant our sublicensees a direct license. The longest lived patent rights licensed to us under the agreement are in one U.S. patent currently expected to expire in 2023.2027. Biogen In August 2014, we entered into a license agreement with Biogen, pursuant to which we co-exclusively licensed certain patents and patent applications directed towards aspects of T cell-based products that target BCMA. Any patents within this portfolio that have issued or may yet issue would have an expected statutory expiration date between 2020 and 2032. Biogen retains the right to practice and use the licensed patents in the licensed field and territory. We have the right to grant sublicenses to third parties, subject to certain conditions. Upon commercialization of our products covered by the in-licensed intellectual property, which we expect would include our bb2121 product candidate, we will be obligated to pay Biogen a percentage of net sales as a royalty in the low single digits. We are required to use commercially reasonable efforts to research and develop one or more licensed products in the license field during the term of the agreement. Additionally, we have assumed certain development and regulatory milestone obligations and must report on our progress in achieving those milestones on a periodic basis. We may be obligated to pay up to $24.0 million in the aggregate for each licensed product upon the achievement of these milestones. We may unilaterally terminate the license agreement at any time with prior written notice to Biogen. Either party may terminate the license in the event of the other party’s material breach upon notice and an opportunity for the breaching party to cure. Either party may also terminate the agreement in the event bankruptcy proceedings are opened against the other party and are not dismissed within a specified period of time. Absent early termination, the agreement will automatically terminate upon the expiration of all patent rights covered by the agreement or ten years from the date of first commercial sale of a licensed product, whichever is later. The license grant ceases in connection with any such termination. The longest lived patent rights licensed to us under the Agreement are in a U.S. patent, currently expected to expire in 2032. NIH In August 2015, we entered into a license agreement with the NIH, pursuant to which we exclusively licensed certain patents and patent applications directed towards aspects of T cell-based products that target BCMA. Any patents within this portfolio that have issued or may yet issue would have an expected statutory expiration date in 2033. NIH retains the right to practice the intellectual property licensed under the agreement on behalf of the government of the United States. We have the right to grant sublicenses to third parties, subject to certain conditions. For each such sublicense we grant we must pay the NIH a fee. Upon commercialization of our products covered by the in-licensed intellectual property, which we expect would include our bb2121 product candidate, we will be obligated to pay the NIH a percentage of net sales as a royalty in the low single digits. We are required to use commercially
reasonable efforts to research and develop one or more licensed products in the license field during the term of the agreement. Additionally, we have assumed certain development and regulatory milestone obligations and must report on our progress in achieving those milestones on a periodic basis. We may be obligated to pay up to $9.7 million in the aggregate for a licensed product upon the achievement of these milestones. We may unilaterally terminate the license agreement at any time with prior written notice to the NIH. The NIH may terminate the license in the event of our material breach upon notice and following an opportunity for us to cure the material breach. The NIH may also terminate the agreement in the event bankruptcy proceedings are opened against us and are not dismissed within a specified period of time. Absent early termination, the agreement will automatically terminate upon the expiration of the patent rights covered by the agreement. The license grant ceases in connection with any such termination. The longest lived patent rights licensed to us under the Agreement are currently expected to expire in 2033. GlaxoSmithKline In April 2017, we entered into a license agreement with GlaxoSmithKline Intellectual Property Development Limited, or GSK, pursuant to which GSK non-exclusively licensed certain of our patent rights related to lentiviral vector technology to develop and commercialize gene therapies for Wiscott-Aldrich syndrome and metachromatic leukodystrophy, two rare genetic diseases. Financial terms of the agreement include an upfront payment to us as well as potential development and regulatory milestone payments and low single digit royalties on net sales of covered products. Novartis Pharma AG In April 2017, we entered into a license agreement with Novartis Pharma AG, or Novartis, pursuant to which Novartis non-exclusively licensed certain of our patent rights related to lentiviral vector technology to develop and commercialize chimeric antigen receptor T cell (CAR T) therapies for oncology, including Novartis’ approved CAR-T therapy Kymriah. Financial terms of the agreement include an upfront payment to us as well as potential development and regulatory milestone payments and low single digit royalties on net sales of covered products. Competition The biotechnology and pharmaceutical industries are characterized by intense and rapidly changing competition to develop new technologies and proprietary products. We face potential competition from many different sources, including larger and better-funded pharmaceutical and biotechnology companies. Not only must we compete with other companies that are focused on gene therapy products but any products that we may commercialize will have to compete with existing therapies and new therapies that may become available in the future.
There are other organizations working to improve existing therapies or to develop new therapies for our initially selected indications. Depending on how successful these efforts are, it is possible they may increase the barriers to adoption and success for our LentiGlobin, Lenti-D, bb2121 and Lenti-Dbb21217 product candidates, if approved and our preclinical CAR T cell-based cancer immunotherapy product candidates. These efforts include the following: β-thalassemia: The current standard of care for the treatment of β-thalassemia in the developed world is chronic blood transfusions to address the patient’s anemia. In addition, such patients often receive iron chelation therapy to help manage the iron overload associated with their chronic blood transfusions. Novartis and ApoPharma Inc., who provide the leading iron chelation therapy, are seeking to develop improvements to their product profile and accessibility. A number of different approaches are under investigation that seek to improve the current standard of care treatment options, including, a protein that aims to improve red blood cell production and fetal hemoglobin regulators. Acceleron Pharma, Inc. (in collaboration with Celgene) is investigating Luspatercept (ACE-536), a subcutaneously-delivered protein therapeutic that targets molecules in the TGF-β superfamily, which is currently in two Phase III clinical trials in subjects with transfusion dependent β-thalassemia (TDT) and non-transfusion dependent β-thalassemia. Results of Luspatercept’s Phase III clinical trial in TDT are expected in 2018. In addition, some patients with β-thalassemia receive HSCT treatment, particularly if a sufficiently well-matched source of donor cells is identified. In addition, there are a number of academic and industry-sponsored research and development programs to improve the outcomes of allogeneic HSCT, or the tolerability and safety of haploidentical HSCT, while increasing the availability of suitable donors. These programs include a modified donor T cell therapy to be used in conjunction with haploidentical HSCT that is in an ongoing Phase I/II study supported by Bellicum Pharmaceuticals, Inc.; and an adjunctive T cell immunotherapy treatment in conjunction with allogeneic HSCT that is in an ongoing Phase I/II study supported by Kiadis Pharma. There are also several different groups developing other approaches for β-thalassemia, one that uses a similar ex vivo autologous gene therapy approach, but uses a different vector and different cell processing techniques and two that use gene editing approaches These include: the San Raffaele Telethon Institute for Gene Therapy (in collaboration with GSK) is currently investigating its gene therapy in a Phase 2 study of adults and pediatric patients with in transfusion dependent β-thalassemia (TDT) ; Sangamo BioSciences Inc. (in collaboration with Bioverativ) has announced that the FDA has accepted Sangamo’s IND for ST-400, using a zinc finger nuclease-mediated gene-editing approach, which enables anticipated initiation of Phase I/II trials in adults with TDT in the first half of 2018; and CRISPR Therapeutics, AG (in collaboration with Vertex Pharmaceuticals Incorporated) is investigating its CRISPR Cas9 gene editing platform in TDT and recently completed its GLP/toxicology studies and filed a CTA in December 2017, indicating plans to initiate a Phase I/II clinical trial in TDT during 2018. Sickle cell disease: The current standard of care for the treatment of SCD in the developed world is chronic blood transfusions or hydroxyurea (a generic drug). In addition, patients treated with chronic blood transfusions often receive iron chelation therapy to help manage the iron overload. Emmaus Life Sciences, Inc. recently received FDA approval for and have launched Endari (L-glutamine). We are aware of ongoing studies that continue to evaluate the efficacy and safety of hydroxyurea in various populations. In addition, a limited number of patients with SCD receive allogeneic HSCT treatment, particularly if a sufficiently well-matched source of donor cells is identified. In addition, there are a number of academic and industry-sponsored research and development programs to improve the tolerability and safety of allogeneic HSCT with less well-matched sources of donor cells, while increasing the availability of suitable donors. These programs include a modified donor T cell therapy to be used in conjunction with haploidentical HSCT that is in an ongoing Phase I/II study supported by Bellicum Pharmaceuticals, Inc. A number of different therapeutic approaches are under investigation targeting the various aspects of SCD pathophysiology, including: antibodies to p-selectin including crizanlizumab which recently completed a Phase II study supported by Novartis; hemoglobin modifiers to prevent the sickling of RBC, including GBT440 in a Phase III study supported by Global Blood Therapeutics, Inc.; pan-selectin inhibitors, including GMI-1070 in Phase III studies supported by GlycoMimetics Inc. (in 2011, Pfizer Inc. and GlycoMimetics Inc. entered a global collaboration to advance this compound); and also gene editing approaches being supported by Intellia Therapeutics, Inc. (in collaboration with Novartis), Editas Medicine, Inc. and CRISPR Therapeutics, AG (in collaboration with Vertex Pharmaceuticals Incorporated); and Sangamo BioSciences Inc. (in collaboration with Bioverativ, Inc.) which has announced plans to investigate the use of zinc finger nuclease-mediated gene-editing techniques in hemoglobinopothies including SCD, although to our knowledge no clinical studies have been initiated in SCD. There are also several different groups developing gene therapy approaches for SCD. Some of these groups use a similar ex vivo autologous approach, but make use of different vectors and different cell processing techniques. These include: UCLA, which has received funding from the California Institute of Regenerative Medicine to pursue a Phase I gene therapy study for SCD; and Cincinnati Children’s Hospital Medical Center, which is conducting a Phase I/II gene therapy study for SCD. CALD: The current standard of care for the treatment of CALD is allogeneic HSCT. We understand that various academic centers around the world are seeking to develop improvements to allogeneic HSCT. In addition, some physicians recommend glyceryl trierucate—better known as Lorenzo’s Oil—to patients diagnosed with ALD or AMN. However, Lorenzo’s Oil has not been clinically proven to address the cerebral symptoms of ALD, and has not been approved by any major regulatory agency as a prescription drug. There are efforts underway to obtain FDA approval for Lorenzo’s Oil as a prescription drug.
·
| • | CCALD: Relapsed/Refractory Multiple Myeloma:The current standard of care for the treatment of CCALD is allogeneicrelapsed/refractory multiple myeloma includes IMIDs (e.g., thalidomide, lenalidomide, pomalidomide), proteasome inhibitors (e.g., bortezomib, carfilzomib, ixazomib), monoclonal antibodies (e.g., daratumamab, elotumuzumab), cytotoxic agents, and in some cases, HSCT. We understand that various academic centers around the world are seeking to develop improvements to allogeneic HSCT. In addition, some physicians recommend
|
28
| glyceryl trierucate—better known as Lorenzo’s Oil—to patients diagnosed with ALD or AMN. However, Lorenzo’s Oil has not been clinically proven to address the cerebral symptoms of ALD, and has not been approved by any major regulatory agency as a prescription drug. There are efforts underway to obtain FDA approval for Lorenzo’s Oil as a prescription drug.
|
·
| ß-thalassemia: The current standard of care for the treatment of ß-thalassemia in the developed world is chronic blood transfusions to address the patient’s anemia. In addition, such patients often receive iron chelation therapy to help manage the iron overload associated with their chronic blood transfusions. We understand that established biopharmaceutical companies, such as Novartis AG and ApoPharma Inc., who provide the leading iron chelation therapy, are seeking to develop improvements to their product profile and accessibility. In addition, some patients with ß-thalassemia receive HCST treatment, particularly if a sufficiently well-matched source of donor cells is identified. We understand that various academic centers around the world are seeking to develop improvements to allogeneic HSCT. A number of different approaches are under investigation to improve treatment options, including iron modulating agents and fetal hemoglobin regulators. There are also several different groups developing gene therapy approachesautologous T cell therapies for ß-thalassemia. Some of these groupsrelapsed/refractory multiple myeloma that use a similar autologous ex vivo autologous approach, but make use ofa different vectors and differenttarget antigen, BCMA single-chain variable fragment or, we believe, cell processing techniques. These programs include: Memorial Sloan Kettering, which received clearance for its IND froman anti-BCMA CAR T cell therapy that is currently in a single-center Phase I study by the FDAUniversity of Pennsylvania (in collaboration with Novartis AG); an anti-BCMA CAR T cell therapy that is in 2012 forPhase I/II study in China and may soon be in clinical trials in the United States (Nanjing Legend in collaboration with Janssen Biotech); an anti-BCMA and TACI CAR T cell therapy that is currently in a Phase I/II genestudy (Autolus); an anti-BCMA CAR T cell therapy study; GlaxoSmithKline Plc, whichthat is in Phase I study (Poseida); and other anti-BCMA CAR T cell therapies in early clinical development (Phase I) are being sponsored by Gilead Sciences, Inc., and Juno Therapeutics, Inc. In addition to these autologous T cell-based approaches, Cellectis SA (in collaboration with Pfizer Inc.) has entered intodisclosed a preclinical program for an agreement with the San Raffaele Telethon Institute for Gene Therapy to advanceallogeneic BCMA CAR T cell therapy. There are also antibody-based therapies being developed by several genegroups, including a bispecific antibody therapy programs, including one for ß-thalassemia; Sangamo BioSciences Inc., through its partnership with Biogen Idec, which has announced plans to initiatecurrently in a Phase I clinical study using zinc finger nuclease-mediated gene-correction techniques in hemoglobinopathies including ß-thalassemia and Acceleron Pharma,supported by Amgen Inc., which is investigating Sotatercept, a protein therapeutic that targets TGF-β,an antibody drug conjugate therapy currently in a Phase II clinical trialI study supported by GSK, and those being developed in subjects with ß-thalassemia.preclinical programs.
|
·
| Sickle cell disease: The current standard of care for the treatment of SCD in the developed world is chronic blood transfusions or hydroxyurea (a generic drug). In addition, such patients often receive iron chelation therapy to help manage the iron overload associated with chronic blood transfusions. We are aware of ongoing studies that continue to evaluate the efficacy and safety of hydroxyurea in various populations. In addition, some patients with SCD receive allogeneic HSCT treatment, particularly if a sufficiently well-matched source of donor cells is identified. We understand that various academic centers around the world are seeking to develop improvements to allogeneic HSCT. A number of different therapeutic approaches are under investigation targeting the various aspects of SCD pathophysiology, including: fetal hemoglobin regulators, including HQK-1001 in Phase II studies supported by HemaQuest Pharmaceuticals Inc., and Vorinostat in Phase II studies supported by Merck & Co.; and pan-selectin inhibitors, including GMI-1070 in Phase II studies supported by GlycoMimetics Inc. (in 2011, Pfizer Inc. and GlycoMimetics Inc. entered a global collaboration to advance this compound). There are also several different groups developing gene therapy approaches for SCD. Some of these groups use a similar ex vivo autologous approach, but make use of different vectors and different cell processing techniques. These include: UCLA, which has received funding from the California Institute of Regenerative Medicine to pursue a Phase I/II gene therapy study for SCD, although to our knowledge no clinical studies have been initiated and Sangamo BioSciences Inc., through its partnership with Biogen Idec, which has announced plans to investigate the use of zinc finger nuclease-mediated gene-correction techniques in hemoglobinopathies including SCD, although to our knowledge no clinical studies have been initiated.T cell-based immunotherapies in oncology: A number of pharmaceutical companies and academic collaborators are researching and developing T cell-based immunotherapies in oncology, in addition to the multiple myeloma programs described above. These include: Novartis AG (in collaboration with the University of Pennsylvania), Adaptimmune Inc., Juno Therapeutics, Inc. (in collaboration with Celgene, Memorial Sloan Kettering and the Fred Hutchinson Cancer Research Center), Gilead Sciences, Inc. (in collaboration with Amgen, Inc. and the National Institutes of Health), Pfizer Inc. (through their collaboration with Cellectis SA and Servier), among others. Many of the T cell-based immunotherapy programs being developed by these companies are already in Phase I/II clinical trials for multiple indications.
|
·
| CAR T therapies for oncology:A number of pharmaceutical companies and academic collaborators are researching and developing CAR T therapies for oncology, including Novartis AG in collaboration with the University of Pennsylvania, Juno Therapeutics, Inc., in collaboration with Memorial Sloan Kettering and the Fred Hutchinson Cancer Research Center, Kite Pharma, Inc. in collaboration with Amgen, Inc. and the National Institutes of Health, among others. Many of the CAR T programs being developed by these companies are already in Phase I/II clinical trials for multiple indications.
|
Many of our competitors, either alone or with their strategic partners, have substantially greater financial, technical and human resources than we do and significantly greater experience in the discovery and development of product candidates, obtaining FDA and other regulatory approvals of treatments and the commercialization of those treatments. Accordingly, our competitors may be more successful than us in obtaining approval for treatments and achieving widespread market acceptance. Our competitors’ treatments may be more effective, or more effectively marketed and sold, than any treatment we may commercialize and may render our treatments obsolete or non-competitive before we can recover the expenses of developing and commercializing any of our treatments. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical study sites and patient registration for clinical studies, as well as in acquiring technologies complementary to, or necessary for, our programs. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. We anticipate that we will face intense and increasing competition as new drugs enter the market and advanced technologies become available. We expect any treatments that we develop and commercialize to compete on the basis of, among other things, efficacy, safety, convenience of administration and delivery, price, the level of generic competition and the availability of reimbursement from government and other third-party payors. 29
Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient or are less expensive than any products that we may develop. Our competitors also may obtain FDA or other regulatory approval for their products more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market. In addition, our ability to compete may be affected in many cases by insurers or other third-party payors seeking to encourage the use of generic products. If our therapeutic product candidates are approved, we expect that they will be priced at a significant premium over competitive generic products. Government regulation In the United States, biological products, including gene therapy products, are subject to regulation under the Federal Food, Drug, and Cosmetic Act, or FD&C Act, and the Public Health Service Act, or PHS Act, and other federal, state, local and foreign statutes and regulations. Both the FD&C Act and the PHS Act and their corresponding regulations govern, among other things, the testing, manufacturing, safety, efficacy, labeling, packaging, storage, record keeping, distribution, reporting, advertising and other promotional practices involving biological products. FDA approval must be obtained before clinical testing of biological products, and each clinical study protocol for a gene therapy product is reviewed by the FDA and, in some instances, the NIH, through its RAC. FDA approval also must be obtained before marketing of biological products. The process of obtaining regulatory approvals and the
subsequent compliance with appropriate federal, state, local and foreign statutes and regulations require the expenditure of substantial time and financial resources and we may not be able to obtain the required regulatory approvals. Within the FDA, the CBER regulates gene therapy products. The CBER works closely with the NIH and its RAC, which makes recommendations to the NIH on gene therapy issues and engages in a public discussion of scientific, safety, ethical and societal issues related to proposed and ongoing gene therapy protocols. The FDA and the NIH have published guidance documents with respect to the development and submission of gene therapy protocols. The FDA also has published guidance documents related to, among other things, gene therapy products in general, their preclinical assessment, observing subjects involved in gene therapy studies for delayed adverse events, potency testing, and chemistry, manufacturing and control information in gene therapy INDs. Ethical, social and legal concerns about gene therapy, genetic testing and genetic research could result in additional regulations restricting or prohibiting the processes we may use. Federal and state agencies, congressional committees and foreign governments have expressed interest in further regulating biotechnology. More restrictive regulations or claims that our products are unsafe or pose a hazard could prevent us from commercializing any products. New government requirements may be established that could delay or prevent regulatory approval of our product candidates under development. It is impossible to predict whether legislative changes will be enacted, regulations, policies or guidance changed, or interpretations by agencies or courts changed, or what the impact of such changes, if any, may be. U.S. biological products development process The process required by the FDA before a biological product may be marketed in the United States generally involves the following: ·
| completion of nonclinical laboratory tests and animal studies according to good laboratory practices, or GLPs, and applicable requirements for the humane use of laboratory animals or other applicable regulations;
|
completion of nonclinical laboratory tests and animal studies according to good laboratory practices, or GLPs, and applicable requirements for the humane use of laboratory animals or other applicable regulations; ·
| submission to the FDA of an application for an IND, which must become effective before human clinical studies may begin;
|
submission to the FDA of an application for an IND, which must become effective before human clinical studies may begin; ·
| performance of adequate and well-controlled human clinical studies according to the FDA’s regulations commonly referred to as good clinical practices, or GCPs, and any additional requirements for the protection of human research subjects and their health information, to establish the safety and efficacy of the proposed biological product for its intended use;
|
performance of adequate and well-controlled human clinical studies according to the FDA’s regulations commonly referred to as good clinical practices, or GCPs, and any additional requirements for the protection of human research subjects and their health information, to establish the safety and efficacy of the proposed biological product for its intended use; ·
| submission to the FDA of a Biologics License Application, or BLA, for marketing approval that includes substantive evidence of safety, purity, and potency from results of nonclinical testing and clinical studies;
|
submission to the FDA of a Biologics License Application, or BLA, for marketing approval that includes substantive evidence of safety, purity, and potency from results of nonclinical testing and clinical studies; ·
| satisfactory completion of an FDA inspection of the manufacturing facility or facilities where the biological product is produced to assess compliance with GMP, to assure that the facilities, methods and controls are adequate to preserve the biological product’s identity, strength, quality and purity and, if applicable, the FDA’s current good tissue practices, or GTPs, for the use of human cellular and tissue products;
|
satisfactory completion of an FDA inspection of the manufacturing facility or facilities where the biological product is produced to assess compliance with GMP, to assure that the facilities, methods and controls are adequate to preserve the biological product’s identity, strength, quality and purity and, if applicable, the FDA’s current good tissue practices, or GTPs, for the use of human cellular and tissue products; ·
| potential FDA audit of the nonclinical and clinical study sites that generated the data in support of the BLA; and
|
potential FDA audit of the nonclinical and clinical study sites that generated the data in support of the BLA; and ·
| FDA review and approval, or licensure, of the BLA.
|
FDA review and approval, or licensure, of the BLA. Before testing any biological product candidate, including a gene therapy product, in humans, the product candidate enters the preclinical testing stage. Preclinical tests, also referred to as nonclinical studies, include laboratory evaluations of product chemistry, 30
toxicity and formulation, as well as animal studies to assess the potential safety and activity of the product candidate. The conduct of the preclinical tests must comply with federal regulations and requirements including GLPs. Where a gene therapy study is conducted at, or sponsored by, institutions receiving NIH funding for recombinant DNA research, prior to the submission of an IND to the FDA, a protocol and related documentation is submitted to and the study is registered with the NIH Office of Biotechnology Activities, or OBA, pursuant to the NIH Guidelines for Research Involving Recombinant DNA Molecules, or NIH Guidelines. Compliance with the NIH Guidelines is mandatory for investigators at institutions receiving NIH funds for research involving recombinant DNA, however many companies and other institutions not otherwise subject to the NIH Guidelines voluntarily follow them. The NIH is responsible for convening the RAC, a federal advisory committee that discusses protocols that raise novel or particularly important scientific, safety or ethical considerations, at one of its quarterly public meetings. The OBA will notify the FDA of the RAC’s decision regarding the necessity for full public review of a gene therapy protocol. RAC proceedings and reports are posted to the OBA web site and may be accessed by the public. The clinical study sponsor must submit the results of the preclinical tests, together with manufacturing information, analytical data, any available clinical data or literature and a proposed clinical protocol, to the FDA as part of the IND. Some preclinical testing may continue even after the IND is submitted. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA
places the clinical study on a clinical hold within that 30-day time period. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical study can begin. With gene therapy protocols, if the FDA allows the IND to proceed, but the RAC decides that full public review of the protocol is warranted, the FDA will request at the completion of its IND review that sponsors delay initiation of the protocol until after completion of the RAC review process. The FDA may also impose clinical holds on a biological product candidate at any time before or during clinical studies due to safety concerns or non-compliance. If the FDA imposes a clinical hold, studies may not recommence without FDA authorization and then only under terms authorized by the FDA. Accordingly, we cannot be sure that submission of an IND will result in the FDA allowing clinical studies to begin, or that, once begun, issues will not arise that suspend or terminate such studies. Clinical studies involve the administration of the biological product candidate to healthy volunteers or patients under the supervision of qualified investigators, generally physicians not employed by or under the study sponsor’s control. Clinical studies are conducted under protocols detailing, among other things, the objectives of the clinical study, dosing procedures, subject selection and exclusion criteria, and the parameters to be used to monitor subject safety, including stopping rules that assure a clinical study will be stopped if certain adverse events should occur. Each protocol and any amendments to the protocol must be submitted to the FDA as part of the IND. Clinical studies must be conducted and monitored in accordance with the FDA’s regulations comprising the GCP requirements, including the requirement that all research subjects provide informed consent. Further, each clinical study must be reviewed and approved by an independent institutional review board, or IRB, at or servicing each institution at which the clinical study will be conducted. An IRB is charged with protecting the welfare and rights of study participants and considers such items as whether the risks to individuals participating in the clinical studies are minimized and are reasonable in relation to anticipated benefits. The IRB also approves the form and content of the informed consent that must be signed by each clinical study subject or his or her legal representative and must monitor the clinical study until completed. Clinical studies also must be reviewed by an institutional biosafety committee, or IBC, a local institutional committee that reviews and oversees basic and clinical research conducted at that institution. The IBC assesses the safety of the research and identifies any potential risk to public health or the environment. Human clinical studies are typically conducted in three sequential phases that may overlap or be combined: ·
| Phase I. The biological product is initially introduced into healthy human subjects and tested for safety. In the case of some products for severe or life-threatening diseases, especially when the product may be too inherently toxic to ethically administer to healthy volunteers, the initial human testing is often conducted in patients.
|
Phase I. The biological product is initially introduced into healthy human subjects and tested for safety. In the case of some products for severe or life-threatening diseases, especially when the product may be too inherently toxic to ethically administer to healthy volunteers, the initial human testing is often conducted in patients. ·
| Phase II. The biological product is evaluated in a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance, optimal dosage and dosing schedule.
|
Phase II. The biological product is evaluated in a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance, optimal dosage and dosing schedule. ·
| Phase III. Clinical studies are undertaken to further evaluate dosage, clinical efficacy, potency, and safety in an expanded patient population at geographically dispersed clinical study sites. These clinical studies are intended to establish the overall risk/benefit ratio of the product and provide an adequate basis for product labeling.
|
Phase III. Clinical studies are undertaken to further evaluate dosage, clinical efficacy, potency, and safety in an expanded patient population at geographically dispersed clinical study sites. These clinical studies are intended to establish the overall risk/benefit ratio of the product and provide an adequate basis for product labeling. Post-approval clinical studies, sometimes referred to as Phase IV clinical studies, may be conducted after initial marketing approval. These clinical studies are used to gain additional experience from the treatment of patients in the intended therapeutic indication, particularly for long-term safety follow-up. The FDA recommends that sponsors observe subjects for potential gene therapy-related delayed adverse events for a 15-year period, including a minimum of five years of annual examinations followed by ten years of annual queries, either in person or by questionnaire, of study subjects. During all phases of clinical development, regulatory agencies require extensive monitoring and auditing of all clinical activities, clinical data, and clinical study investigators. Annual progress reports detailing the results of the clinical studies must be submitted to 31
the FDA. Written IND safety reports must be promptly submitted to the FDA, the NIH and the investigators for serious and unexpected adverse events, any findings from other studies, tests in laboratory animals or in vitro testing that suggest a significant risk for human subjects, or any clinically important increase in the rate of a serious suspected adverse reaction over that listed in the protocol or investigator brochure. The sponsor must submit an IND safety report within 15 calendar days after the sponsor determines that the information qualifies for reporting. The sponsor also must notify the FDA of any unexpected fatal or life-threatening suspected adverse reaction within seven calendar days after the sponsor’s initial receipt of the information. Phase I, Phase II and Phase III clinical studies may not be completed successfully within any specified period, if at all. The FDA or the sponsor or its data safety monitoring board may suspend a clinical study at any time on various grounds, including a finding that the research subjects or patients are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical study at its institution if the clinical study is not being conducted in accordance with the IRB’s requirements or if the biological product has been associated with unexpected serious harm to patients. Human gene therapy products are a new category of therapeutics. Because this is a relatively new and expanding area of novel therapeutic interventions, there can be no assurance as to the length of the study period, the number of patients the FDA will require to
be enrolled in the studies in order to establish the safety, efficacy, purity and potency of human gene therapy products, or that the data generated in these studies will be acceptable to the FDA to support marketing approval. The NIH and the FDA havehas a publicly accessible database, the Genetic Modification Clinical Research Information System which includes information on gene transfer studies and serves as an electronic tool to facilitate the reporting and analysis of adverse events on these studies. Concurrent with clinical studies, companies usually complete additional animal studies and must also develop additional information about the physical characteristics of the biological product as well as finalize a process for manufacturing the product in commercial quantities in accordance with GMP requirements. To help reduce the risk of the introduction of adventitious agents with use of biological products, the PHS Act emphasizes the importance of manufacturing control for products whose attributes cannot be precisely defined. The manufacturing process must be capable of consistently producing quality batches of the product candidate and, among other things, the sponsor must develop methods for testing the identity, strength, quality, potency and purity of the final biological product. Additionally, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the biological product candidate does not undergo unacceptable deterioration over its shelf life. U.S. review and approval processes After the completion of clinical studies of a biological product, FDA approval of a BLA, must be obtained before commercial marketing of the biological product. The BLA must include results of product development, laboratory and animal studies, human studies, information on the manufacture and composition of the product, proposed labeling and other relevant information. In addition, under the Pediatric Research Equity Act, or PREA, a BLA or supplement to a BLA must contain data to assess the safety and effectiveness of the biological product for the claimed indications in all relevant pediatric subpopulations and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. The FDA may grant deferrals for submission of data or full or partial waivers. Unless otherwise required by regulation, PREA does not apply to any biological product for an indication for which orphan designation has been granted. The testing and approval processes require substantial time and effort and there can be no assurance that the FDA will accept the BLA for filing and, even if filed, that any approval will be granted on a timely basis, if at all. Within 60 days following submission of the application, the FDA reviews a BLA submitted to determine if it is substantially complete before the agency accepts it for filing. The FDA may refuse to file any BLA that it deems incomplete or not properly reviewable at the time of submission and may request additional information. In this event, the BLA must be resubmitted with the additional information. The resubmitted application also is subject to review before the FDA accepts it for filing. Once the submission is accepted for filing, the FDA begins an in-depth substantive review of the BLA. The FDA reviews the BLA to determine, among other things, whether the proposed product is safe and potent, or effective, for its intended use, and has an acceptable purity profile, and whether the product is being manufactured in accordance with GMP to assure and preserve the product’s identity, safety, strength, quality, potency and purity. The FDA may refer applications for novel biological products or biological products that present difficult questions of safety or efficacy to an advisory committee, typically a panel that includes clinicians and other experts, for review, evaluation and a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions. During the biological product approval process, the FDA also will determine whether a Risk Evaluation and Mitigation Strategy, or REMS, is necessary to assure the safe use of the biological product. If the FDA concludes a REMS is needed, the sponsor of the BLA must submit a proposed REMS; the FDA will not approve the BLA without a REMS, if required. Before approving a BLA, the FDA will inspect the facilities at which the product is manufactured. The FDA will not approve the product unless it determines that the manufacturing processes and facilities are in compliance with GMP requirements and adequate to assure consistent production of the product within required specifications. For a gene therapy product, the FDA also will not approve the product if the manufacturer is not in compliance with the GTPs. These are FDA regulations that govern the methods used in, and the facilities and controls used for, the manufacture of human cells, tissues, and cellular and tissue based products, or HCT/Ps, which are human cells or tissue intended for implantation, transplant, infusion, or transfer into a human recipient. The primary intent of the 32
GTP requirements is to ensure that cell and tissue based products are manufactured in a manner designed to prevent the introduction, transmission and spread of communicable disease. FDA regulations also require tissue establishments to register and list their HCT/Ps with the FDA and, when applicable, to evaluate donors through screening and testing. Additionally, before approving a BLA, the FDA will typically inspect one or more clinical sites to assure that the clinical studies were conducted in compliance with IND study requirements and GCP requirements. To assure GMP, GTP and GCP compliance, an applicant must incur significant expenditure of time, money and effort in the areas of training, record keeping, production, and quality control. Notwithstanding the submission of relevant data and information, the FDA may ultimately decide that the BLA does not satisfy its regulatory criteria for approval and deny approval. Data obtained from clinical studies are not always conclusive and the FDA may interpret data differently than we interpret the same data. If the agency decides not to approve the BLA in its present form, the FDA will issue a complete response letter that usually describes all of the specific deficiencies in the BLA identified by the FDA. The
deficiencies identified may be minor, for example, requiring labeling changes, or major, for example, requiring additional clinical studies. Additionally, the complete response letter may include recommended actions that the applicant might take to place the application in a condition for approval. If a complete response letter is issued, the applicant may either resubmit the BLA, addressing all of the deficiencies identified in the letter, or withdraw the application. If a product receives regulatory approval, the approval may be significantly limited to specific diseases and dosages or the indications for use may otherwise be limited, which could restrict the commercial value of the product. Further, the FDA may require that certain contraindications, warnings or precautions be included in the product labeling. The FDA may impose restrictions and conditions on product distribution, prescribing, or dispensing in the form of a risk management plan, or otherwise limit the scope of any approval. In addition, the FDA may require post marketing clinical studies, sometimes referred to as Phase IV clinical studies, designed to further assess a biological product’s safety and effectiveness, and testing and surveillance programs to monitor the safety of approved products that have been commercialized. One of the performance goals agreed to by the FDA under the PDUFA is to review 90% of standard BLAs in 10 months and 90% of priority BLAs in six months, whereupon a review decision is to be made. The FDA does not always meet its PDUFA goal dates for standard and priority BLAs and its review goals are subject to change from time to time. The review process and the PDUFA goal date may be extended by three months if the FDA requests or the BLA sponsor otherwise provides additional information or clarification regarding information already provided in the submission within the last three months before the PDUFA goal date. Orphan drug designation Under the Orphan Drug Act, the FDA may grant orphan designation to a drug or biological product intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States, or more than 200,000 individuals in the United States and for which there is no reasonable expectation that the cost of developing and making a drug or biological product available in the United States for this type of disease or condition will be recovered from sales of the product. Orphan product designation must be requested before submitting an NDA or BLA. After the FDA grants orphan product designation, the identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. Orphan product designation does not convey any advantage in or shorten the duration of the regulatory review and approval process. If a product that has orphan designation subsequently receives the first FDA approval for the disease or condition for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications to market the same drug or biological product for the same indication for seven years, except in limited circumstances, such as a showing of clinical superiority to the product with orphan exclusivity. Competitors, however, may receive approval of different products for the indication for which the orphan product has exclusivity or obtain approval for the same product but for a different indication for which the orphan product has exclusivity. Orphan product exclusivity also could block the approval of one of our products for seven years if a competitor obtains approval of the same biological product as defined by the FDA or if our product candidate is determined to be contained within the competitor’s product for the same indication or disease. If a drug or biological product designated as an orphan product receives marketing approval for an indication broader than what is designated, it may not be entitled to orphan product exclusivity. Orphan drug status in the European Union has similar, but not identical, benefits. Expedited development and review programs The FDA has a Fast Track program that is intended to expedite or facilitate the process for reviewing new drugs and biological products that meet certain criteria. Specifically, new drugs and biological products are eligible for Fast Track designation if they are intended to treat a serious or life-threatening condition and demonstrate the potential to address unmet medical needs for the condition. Fast Track designation applies to the combination of the product and the specific indication for which it is being studied. The sponsor of a new drug or biologic may request the FDA to designate the drug or biologic as a Fast Track product at any time during the clinical development of the product. Unique to a Fast Track product, the FDA may consider for review sections of the marketing application on a rolling basis before the complete application is submitted, if the sponsor provides a schedule for the submission of the sections of 33
the application, the FDA agrees to accept sections of the application and determines that the schedule is acceptable, and the sponsor pays any required user fees upon submission of the first section of the application. Any product submitted to the FDA for marketing, including under a Fast Track program, may be eligible for other types of FDA programs intended to expedite development and review, such as priority review and accelerated approval. Under the Breakthrough Therapy program, products intended to treat a serious or life-threatening disease or condition may be eligible for the benefits of the Fast Track program when preliminary clinical evidence demonstrates that such product may have substantial improvement on one or more clinically significant endpoints over existing therapies. Additionally, FDA will seek to ensure the sponsor of a breakthrough therapy product receives timely advice and interactive communications to help the sponsor design and conduct a development program as efficiently as possible. Any product is eligible for priority review if it has the potential to provide safe and effective
therapy where no satisfactory alternative therapy exists or a significant improvement in the treatment, diagnosis or prevention of a disease compared to marketed products. The FDA will attempt to direct additional resources to the evaluation of an application for a new drug or biological product designated for priority review in an effort to facilitate the review. Additionally, a product may be eligible for accelerated approval. Drug or biological products studied for their safety and effectiveness in treating serious or life-threatening illnesses and that provide meaningful therapeutic benefit over existing treatments may receive accelerated approval, which means that they may be approved on the basis of adequate and well-controlled clinical studies establishing that the product has an effect on a surrogate endpoint that is reasonably likely to predict a clinical benefit, or on the basis of an effect on a clinical endpoint other than survival or irreversible morbidity. As a condition of approval, the FDA may require that a sponsor of a drug or biological product receiving accelerated approval perform adequate and well-controlled post-marketing clinical studies. In addition, the FDA currently requires as a condition for accelerated approval pre-approval of promotional materials, which could adversely impact the timing of the commercial launch of the product. Fast Track designation, Breakthrough Therapy designation, priority review and accelerated approval do not change the standards for approval but may expedite the development or approval process. Regenerative medicine advanced therapies (RMAT) designation As part of the 21st Century Cures Act, Congress amended the FD&C Act to facilitate an efficient development program for, and expedite review of regenerative medicine advanced therapies, which include cell and gene therapies, therapeutic tissue engineering products, human cell and tissue products, and combination products using any such therapies or products. Regenerative medicine advanced therapies do not include those human cells, tissues, and cellular and tissue based products regulated solely under section 361 of the Public Health Service Act and 21 CFR Part 1271. This program is intended to facilitate efficient development and expedite review of regenerative medicine therapies, which are intended to treat, modify, reverse, or cure a serious or life-threatening disease or condition and qualify for RMAT designation. A drug sponsor may request that FDA designate a drug as a RMAT concurrently with or at any time after submission of an IND. FDA has 60 calendar days to determine whether the drug meets the criteria, including whether there is preliminary clinical evidence indicating that the drug has the potential to address unmet medical needs for a serious or life-threatening disease or condition. A BLA for a regenerative medicine therapy that has received RMAT designation may be eligible for priority review or accelerated approval through use of surrogate or intermediate endpoints reasonably likely to predict long-term clinical benefit, or reliance upon data obtained from a meaningful number of sites. Benefits of RMAT designation also include early interactions with FDA to discuss any potential surrogate or intermediate endpoint to be used to support accelerated approval. A regenerative medicine therapy with RMAT designation that is granted accelerated approval and is subject to post-approval requirements may fulfill such requirements through the submission of clinical evidence from clinical studies, patient registries, or other sources of real world evidence, such as electronic health records; the collection of larger confirmatory data sets; or post-approval monitoring of all patients treated with such therapy prior to its approval. Post-approval requirements Maintaining substantial compliance with applicable federal, state, and local statutes and regulations requires the expenditure of substantial time and financial resources. Rigorous and extensive FDA regulation of biological products continues after approval, particularly with respect to GMP. We will rely, and expect to continue to rely, on third parties for the production of clinical and commercial quantities of any products that we may commercialize. Manufacturers of our products are required to comply with applicable requirements in the GMP regulations, including quality control and quality assurance and maintenance of records and documentation. Other post-approval requirements applicable to biological products, include reporting of GMP deviations that may affect the identity, potency, purity and overall safety of a distributed product, record-keeping requirements, reporting of adverse effects, reporting updated safety and efficacy information, and complying with electronic record and signature requirements. After a BLA is approved, the product also may be subject to official lot release. As part of the manufacturing process, the manufacturer is required to perform certain tests on each lot of the product before it is released for distribution. If the product is subject to official release by the FDA, the manufacturer submits samples of each lot of product to the FDA together with a release protocol showing a summary of the history of manufacture of the lot and the results of all of the manufacturer’s tests performed on the lot. The FDA also may perform certain confirmatory tests on lots of some products, such as viral vaccines, before releasing the lots for distribution by the manufacturer. In addition, the FDA conducts laboratory research related to the regulatory standards on the safety, purity, potency, and effectiveness of biological products. We also must comply with the FDA’s advertising and promotion requirements, such as those related to direct-to-consumer advertising, the prohibition on promoting products for uses or in patient populations that are not described in the product’s approved labeling (known as “off-label use”), industry-sponsored scientific and educational activities, and promotional activities involving the internet. Discovery of previously unknown problems or the failure to comply with the applicable regulatory requirements may result in restrictions on the marketing of a product or withdrawal of the product from the market as well as possible civil or criminal sanctions. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval, may subject an applicant or manufacturer to administrative or judicial civil or criminal sanctions and adverse publicity. FDA sanctions could include refusal to approve pending applications, withdrawal of an approval, clinical hold, warning or
untitled letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, mandated corrective advertising or communications with doctors, debarment, restitution, disgorgement of profits, or civil or criminal penalties. Any agency or judicial enforcement action could have a material adverse effect on us. Biological product manufacturers and other entities involved in the manufacture and distribution of approved biological products are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with GMPs and other laws. Accordingly, manufacturers must continue to expend time, money, and effort in the area of production and quality control to maintain GMP compliance. Discovery of problems with a product after approval may result in restrictions on a product, manufacturer, or holder of an approved BLA, including withdrawal of the product from the market. In addition, changes to the manufacturing process or facility generally require prior FDA approval before being implemented and other types of changes to the approved product, such as adding new indications and additional labeling claims, are also subject to further FDA review and approval. 34
U.S. patent term restoration and marketing exclusivity Depending upon the timing, duration and specifics of the FDA approval of the use of our product candidates, some of our U.S. patents may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984, commonly referred to as the Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a patent restoration term of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. However, patent term restoration cannot extend the remaining term of a patent beyond a total of 14 years from the product’s approval date. The patent term restoration period is generally one-half the time between the effective date of an IND and the submission date of a BLA plus the time between the submission date of a BLA and the approval of that application. Only one patent applicable to an approved biological product is eligible for the extension and the application for the extension must be submitted prior to the expiration of the patent. The U.S. PTO, in consultation with the FDA, reviews and approves the application for any patent term extension or restoration. In the future, we may intend to apply for restoration of patent term for one of our currently owned or licensed patents to add patent life beyond its current expiration date, depending on the expected length of the clinical studies and other factors involved in the filing of the relevant BLA. A biological product can obtain pediatric market exclusivity in the United States. Pediatric exclusivity, if granted, adds six months to existing exclusivity periods and patent terms. This six-month exclusivity, which runs from the end of other exclusivity protection or patent term, may be granted based on the voluntary completion of a pediatric study in accordance with an FDA-issued “Written Request” for such a study. The Patient Protection and Affordable Care Act, or Affordable Care Act, signed into law on March 23, 2010, includes a subtitle called the Biologics Price Competition and Innovation Act of 2009 which created an abbreviated approval pathway for biological products shown to be similar to, or interchangeable with, an FDA-licensed reference biological product. This amendment to the PHS Act attempts to minimize duplicative testing. Biosimilarity, which requires that there be no clinically meaningful differences between the biological product and the reference product in terms of safety, purity, and potency, can be shown through analytical studies, animal studies, and a clinical study or studies. Interchangeability requires that a product is biosimilar to the reference product and the product must demonstrate that it can be expected to produce the same clinical results as the reference product and, for products administered multiple times, the biologic and the reference biologic may be switched after one has been previously administered without increasing safety risks or risks of diminished efficacy relative to exclusive use of the reference biologic. However, complexities associated with the larger, and often more complex, structure of biological products, as well as the process by which such products are manufactured, pose significant hurdles to implementation that are still being worked out by the FDA. A reference biologic is granted twelve years of exclusivity from the time of first licensure of the reference product. The first biologic product submitted under the abbreviated approval pathway that is determined to be interchangeable with the reference product has exclusivity against other biologics submitting under the abbreviated approval pathway for the lesser of (i) one year after the first commercial marketing, (ii) 18 months after approval if there is no legal challenge, (iii) 18 months after the resolution in the applicant’s favor of a lawsuit challenging the biologics’ patents if an application has been submitted, or (iv) 42 months after the application has been approved if a lawsuit is ongoing within the 42-month period. Additional regulation In addition to the foregoing, state and federal laws regarding environmental protection and hazardous substances, including the Occupational Safety and Health Act, the Resource Conservancy and Recovery Act and the Toxic Substances Control Act, affect our business. These and other laws govern our use, handling and disposal of various biological, chemical and radioactive substances used in, and wastes generated by, our operations. If our operations result in contamination of the environment or expose individuals to hazardous substances, we could be liable for damages and governmental fines. We believe that we are in material compliance with
applicable environmental laws and that continued compliance therewith will not have a material adverse effect on our business. We cannot predict, however, how changes in these laws may affect our future operations. U.S. Foreign Corrupt Practices Act The U.S. Foreign Corrupt Practices Act, to which we are subject, prohibits corporations and individuals from engaging in certain activities to obtain or retain business or to influence a person working in an official capacity. It is illegal to pay, offer to pay or authorize the payment of anything of value to any foreign government official, government staff member, political party or political candidate in an attempt to obtain or retain business or to otherwise influence a person working in an official capacity. Government regulation outside of the United States In addition to regulations in the United States, we will be subject to a variety of regulations in other jurisdictions governing, among other things, clinical studies and any commercial sales and distribution of our products. Because biologically sourced raw materials are subject to unique contamination risks, their use may be restricted in some countries. 35
Whether or not we obtain FDA approval for a product, we must obtain the requisite approvals from regulatory authorities in foreign countries prior to the commencement of clinical studies or marketing of the product in those countries. Certain countries outside of the United States have a similar process that requires the submission of a clinical study application much like the IND prior to the commencement of human clinical studies. In the European Union, for example, a CTA must be submitted for each clinical trial to each country’s national health authority and an independent ethics committee, much like the FDA and the IRB, respectively. Once the CTA is approved in accordance with a country’s requirements, the corresponding clinical study may proceed. The requirements and process governing the conduct of clinical studies, product licensing, pricing and reimbursement vary from country to country. In all cases, the clinical studies are conducted in accordance with GCP and the applicable regulatory requirements and the ethical principles that have their origin in the Declaration of Helsinki. To obtain regulatory approval of an investigational biological product under European Union regulatory systems, we must submit a marketing authorization application. The application used to file the BLA in the United States is similar to that required in the European Union, with the exception of, among other things, country-specificregion-specific document requirements. The European Union also provides opportunities for market exclusivity. For example, in the European Union, upon receiving marketing authorization, new chemical entitiesinnovative medicinal products generally receive eight years of data exclusivity and an additional two years of market exclusivity. If granted, data exclusivity prevents regulatory authorities in the European Union from referencing the innovator’s data to assess a generic or biosimilar application. During the additional two-year period of market exclusivity, a generic or biosimilar marketing authorization can be submitted, and the innovator’s data may be referenced, but no generic or biosimilar product can be marketed until the expiration of the market exclusivity. However, there is no guarantee that a product will be considered by the European Union’s regulatory authorities to be a new chemical entity,an innovative medicinal product, and products may not qualify for data exclusivity. Products receiving orphan designation in the European Union can receive ten years of market exclusivity, during which time no similar medicinal product for the same indication may be placed on the market. An orphan product can also obtain an additional two years of market exclusivity in the European Union for pediatric studies. No extension to any supplementary protection certificate can be granted on the basis of pediatric studies for orphan indications. The criteria for designating an “orphan medicinal product” in the European Union are similar in principle to those in the United States. Under Article 3 of Regulation (EC) 141/2000, a medicinal product may be designated as orphan if (1) it is intended for the diagnosis, prevention or treatment of a life-threatening or chronically debilitating condition; (2) either (a) such condition affects no more than five in 10,000 persons in the European Union when the application is made, or (b) the product, without the benefits derived from orphan status, would not generate sufficient return in the European Union to justify investment; and (3) there exists no satisfactory method of diagnosis, prevention or treatment of such condition authorized for marketing in the European Union, or if such a method exists, the product will be of significant benefit to those affected by the condition, as defined in Regulation (EC) 847/2000. Orphan medicinal products are eligible for financial incentives such as reduction of fees or fee waivers and are, upon grant of a marketing authorization, entitled to ten years of market exclusivity for the approved therapeutic indication. The application for orphan drug designation must be submitted before the application for marketing authorization. The applicant will receive a fee reduction for the marketing authorization application if the orphan drug designation has been granted, but not if the designation is still pending at the time the marketing authorization is submitted. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process.
The 10-year market exclusivity may be reduced to six years if, at the end of the fifth year, it is established that the product no longer meets the criteria for orphan designation, for example, if the product is sufficiently profitable not to justify maintenance of market exclusivity. Additionally, marketing authorization may be granted to a similar product for the same indication at any time if: ·
| The second applicant can establish that its product, although similar, is safer, more effective or otherwise clinically superior;
|
The second applicant can establish that its product, although similar, is safer, more effective or otherwise clinically superior; ·
| The applicant consents to a second orphan medicinal product application; or
|
The applicant consents to a second orphan medicinal product application; or ·
| The applicant cannot supply enough orphan medicinal product.
|
The applicant cannot supply enough orphan medicinal product. For other countries outside of the European Union, such as countries in Eastern Europe, Latin America or Asia, the requirements governing the conduct of clinical studies, product licensing, pricing and reimbursement vary from country to country. In all cases, again, the clinical studies are conducted in accordance with GCP and the applicable regulatory requirements and the ethical principles that have their origin in the Declaration of Helsinki. The EMA has established the Adaptive Pathways pilot program intended to expedite or facilitate either an initial approval of a medicinal product in a well-defined patient subgroup with a high medical need and subsequent iterative expansion of the indication to a larger patient population, or an early regulatory approval (e.g., conditional approval), which is prospectively planned, and where uncertainty is reduced through the collection of post-approval data on a medicinal product’s use in patients. The approach builds in regulatory processes already in place within the existing EU legal framework. If we fail to comply with applicable foreign regulatory requirements, we may be subject to, among other things, fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions and criminal prosecution. Employees As of January 31, 2015,2018, we had 143479 full-time employees, 41113 of whom have Ph.D., M.D. or Pharm.D. degrees. Of these full-time employees, 112360 employees are engaged in research and development activities and 31119 employees are engaged in commercial, finance, legal, business development, human resources, information technology, facilities and other general administrative functions. We have no collective bargaining agreements with our employees and we have not experienced any work stoppages. We consider our relations with our employees to be good. 36
Corporate Information We were incorporated in Delaware in April 1992 under the name Genetix Pharmaceuticals, Inc., and subsequently changed our name to bluebird bio, Inc. in September 2010. Our mailing address and executive offices are located at 150 Second60 Binney Street, Third Floor, Cambridge, Massachusetts and our telephone number at that address is (339) 499-9300. We maintain an Internet website at the following address: www.bluebirdbio.com. The information on our website is not incorporated by reference in this annual report on Form 10-K or in any other filings we make with the Securities and Exchange Commission, or SEC. We make available on or through our website certain reports and amendments to those reports that we file with or furnish to the SEC in accordance with the Securities Exchange Act of 1934, as amended. These include our annual reports on Form 10-K, our quarterly reports on Form 10-Q, and our current reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act. We make this information available on or through our website free of charge as soon as reasonably practicable after we electronically file the information with, or furnish it to, the SEC. Item 1A. Risk Factors An investment in shares of our common stock involves a high degree of risk. You should carefully consider the following information about these risks, together with the other information appearing elsewhere in this Annual Report on Form 10-K, including our financial statements and related notes hereto, before deciding to invest in our common stock. The occurrence of any of the following risks could have a material adverse effect on our business, financial condition, results of operations and future growth prospects. In these circumstances, the market price of our common stock could decline, and you may lose all or part of your investment.
Risks related to the discovery and development of our product candidates Our gene therapy product candidates are based on a novel technology, which makes it difficult to predict the time and cost of product candidate development and subsequently obtaining regulatory approval. At the moment, noOnly a few gene therapy products have been approved in the United States and only one product has been approved in the European Union, (EU).or EU. We have concentrated our therapeutic product research and development efforts on our gene therapy platform, and our future success depends on the successful development of this therapeutic approach. There can be no assurance that any development problems we experience in the future related to our gene therapy platform will not cause significant delays or unanticipated costs, or that such development problems can be solved. We may also experience delays in developing a sustainable, reproducible and commercial-scale manufacturing process or transferring that process to commercial partners, which may prevent us from completing our clinical studies or commercializing our products on a timely or profitable basis, if at all. In addition, the clinical study requirements of the U.S. Food and Drug Administration, or FDA, the European Medicines Agency, or EMA, and other regulatory agencies and the criteria these regulators use to determine the safety and efficacy of a product candidate vary substantially according to the type, complexity, novelty and intended use and market of the potential products. The regulatory approval process for novel product candidates such as ours can be more expensive and take longer than for other, better known or more extensively studied pharmaceutical or other product candidates. At the moment,Currently, only onea few gene therapy product, UniQure’s Glybera, which received marketing authorization in the EU in 2012, hasproducts have been approved in the Western world, including Spark’s gene therapy product, which makesreceived approval from the FDA in 2017, GlaxoSmithKline’s Strimvelis, and Novartis’s and Gilead’s CAR-T therapies, which received approval from the FDA in 2017. Given the few precedents of approved gene therapy products, it is difficult to determine how long it will take or how much it will cost to obtain regulatory approvals for our product candidates in the United States, the EU or other jurisdictions. Approvals by the EMA and the European Commission may not be indicative of what the FDA may require for approval. Regulatory requirements governing gene and cell therapy products have evolved and may continue to change in the future. For example, the FDA has established the Office of Cellular, Tissue and Gene Therapies within its Center for Biologics Evaluation and Research, or CBER, to consolidate the review of gene therapy and related products, and the Cellular, Tissue and Gene Therapies Advisory Committee to advise CBER on its review. Gene therapy clinical studies conducted at institutions that receive funding for recombinant DNA research from the U.S. National Institutes of Health, or the NIH, are also subject to review by the NIH Office of Biotechnology Activities’ Recombinant DNA Advisory Committee, or the RAC. Although the FDA decides whether individual gene therapy protocols may proceed, the RAC review process can impede the initiation of a clinical study, even if the FDA has reviewed the study and approved its initiation. Conversely,Clinical trial sites in the United States that receive NIH funding for research involving recombinant or synthetic nucleic acid molecules are required to follow RAC recommendations, or risk losing NIH funding for such research or needing NIH pre-approval before conducting such research. In addition, the FDA can put an investigational new drug application, or IND, on clinical hold even if the RAC has provided a favorable review. Also, beforeinformation in an IND is not sufficient to assess the risks in pediatric patients. Before a clinical study can begin at an NIH-fundedany institution, that institution’s institutional review board, or IRB, and its Institutional Biosafety Committee will have to review the proposed clinical study to assess the safety of the study. In addition,Moreover, serious adverse events or developments in clinical trials of gene therapy product candidates conducted by others may cause the FDA or other regulatory bodies to initiate a clinical hold on our clinical trials or otherwise change the requirements for approval of any of our product candidates. These regulatory review agencies, committees and advisory groups and the new requirements and guidelines they promulgate may lengthen the regulatory review process, require us to perform additional or larger studies, increase our development costs, lead to changes in regulatory positions and interpretations, delay or prevent approval and commercialization of these treatment candidates or lead to significant post-approval studies, limitations or restrictions. As we advance our product candidates, we will be required to consult with these 37
regulatory and advisory groups and comply with applicable requirements and guidelines. If we fail to do so, we may be required to delay or discontinue development of our product candidates. Delay or failure to obtain, or unexpected costs in obtaining, the regulatory approval necessary to bring a potential product to market could decrease our ability to generate sufficient product revenue to maintain our business. We may find it difficult to enroll patients in our clinical studies, which could delay or prevent clinical studies of our product candidates. Identifying and qualifying patients to participate in clinical studies of our product candidates is critical to our success. The timing of our clinical studies depends on the speed at which we can recruit eligible patients to participate in testing our product candidates. We have experienced delays in some of our clinical studies, and we may experience similar delays in the future. If patients are unwilling to participate in our gene therapy studies because of negative publicity from adverse events in the biotechnology or gene therapy industries or for other reasons, including competitive clinical studies for similar patient populations, the timeline for recruiting patients, conducting studies and obtaining regulatory approval of potential products may be delayed. These delays could result in increased costs, delays in advancing our product development, delays in testing the effectiveness of our technology or termination of the clinical studies altogether.
We may not be able to identify, recruit and enroll a sufficient number of patients, or those with required or desired characteristics to achieve diversity in a study, to complete our clinical studies in a timely manner. Patient enrollment is affected by factors including: ·
| severity of the disease under investigation;
|
severity of the disease under investigation; ·
| design of the study protocol;
|
design of the study protocol; ·
| size of the patient population;
|
size of the patient population; ·
| eligibility criteria for the study in question;
|
eligibility criteria for the study in question; ·
| perceived risks and benefits of the product candidate under study;
|
perceived risks and benefits of the product candidate under study, including as a result of adverse effects observed in similar or competing therapies; ·
| proximity and availability of clinical study sites for prospective patients;
|
proximity and availability of clinical study sites for prospective patients; ·
| availability of competing therapies and clinical studies;
|
availability of competing therapies and clinical studies; ·
| efforts to facilitate timely enrollment in clinical studies;
|
efforts to facilitate timely enrollment in clinical studies; ·
| patient referral practices of physicians; and
|
patient referral practices of physicians; and ·
| ability to monitor patients adequately during and after treatment.
|
ability to monitor patients adequately during and after treatment. In particular, each of the conditions for which we plan to evaluate our current hematopoietic stem cell, or HSC, product candidates are rare genetic disorders with limited patient pools from which to draw for clinical studies. It has been estimated that about 1.5% (80 to 90 million people) of the global population are carriers of ß-thalassemia, with about 60,000 symptomatic individuals born annually, the great majority in the developing world. According to Thalassemia International Federation, about 288,000 patients with ß-thalassemia major are alive and registered as receiving regular treatment around the world, of which we estimate that about 10,000-15,000 live in the United States and Europe. The global incidence of SCD is estimated to be 250,000-300,000 births annually with a global prevalence estimated to be about 20-25 million. The worldwide incidence rate for adrenoleukodystrophy, or ALD, the superset of CCALD, is approximately one in 20,000 newborn males. CCALD accounts for about 30-40% of patients diagnosed with ALD. Further, because newborn screening for CCALDCALD is not widely adopted, and it can be difficult to diagnose CCALDCALD in the absence of a genetic screen, we may have difficulty finding patients who are eligible to participate in our study. The eligibility criteria of our clinical studies will further limit the pool of available study participants. Additionally, the process of finding and diagnosing patients may prove costly. Finally, our treatment process requires that the patientprocurement of autologous cells from subjects be near oneconducted where the cells can be shipped to a transduction facility within the required timelines, as the HSCs and T cells, in the case of our transduction facilities, as the hematopoietic stem cells, or HSCs,oncology product candidate, have limited viability following harvest and cannot be transported long distances.harvest. Our current product candidates are being developed to treat rare conditionssevere genetic diseases and certain cancers. We plan to seek initial marketing approval in the United States and the European Union. We may not be able to initiate or continue clinical studies if we cannot enroll a sufficient number of eligible patients to participate in the clinical studies required by the FDA or the EMA or other regulatory agencies. Our ability to successfully initiate, enroll and complete a clinical study in any foreign country is subject to numerous risks unique to conducting business in foreign countries, including: ·
| difficulty in establishing or managing relationships with contract research organizations, or CROs, and physicians;
|
difficulty in establishing or managing relationships with contract research organizations, or CROs, and physicians; ·
| different standards for the conduct of clinical studies;
|
different standards for the conduct of clinical studies; ·
| our inability to locate qualified local consultants, physicians and partners; and |
38the potential burden of complying with a variety of foreign laws, medical standards and regulatory requirements, including the regulation of pharmaceutical and biotechnology products and treatment.
·
| the potential burden of complying with a variety of foreign laws, medical standards and regulatory requirements, including the regulation of pharmaceutical and biotechnology products and treatment.
|
If we have difficulty enrolling a sufficient number of patients to conduct our clinical studies as planned, we may need to delay, limit or terminate ongoing or planned clinical studies, any of which would have an adverse effect on our business. We may encounter substantial delays in our clinical studies or we may fail to demonstrate safety and efficacy to the satisfaction of applicable regulatory authorities. Before obtaining marketing approval from regulatory authorities for the sale of our product candidates, we must conduct extensive clinical studies to demonstrate the safety, purity and potency, or efficacy, of the product candidates in humans. Clinical testing is expensive, time-consuming and uncertain as to outcome. We cannot guarantee that any clinical studies will be conducted as planned or completed on schedule, if at all. A failure of one or more clinical studies can occur at any stage of testing. Events that may prevent successful or timely completion of clinical development include: ·
| delays in reaching a consensus with regulatory agencies on study design;
|
delays in reaching a consensus with regulatory agencies on study design; delays in obtaining required IRB or Institutional Ethics Committee approval at each clinical study site; delays in recruiting suitable patients to participate in our clinical studies;
·
| delays in obtaining required Institutional Review Board, or IRB, or Institutional Ethics Committee approval at each clinical study site;
|
·•
| delays in recruiting suitable patients to participate in our clinical studies;
|
·
| imposition of a clinical hold by regulatory agencies, after an inspection of our clinical study operations or study sites or due to unforeseen safety issues; |
·
| failure by our CROs, other third parties or us to adhere to clinical study requirements;
|
failure by our CROs, other third parties or us to adhere to clinical study requirements; ·
| failure to perform in accordance with the FDA’s good clinical practices, or GCP, or applicable regulatory requirements in other countries;
|
failure to perform in accordance with the FDA’s good clinical practices, or GCP, or applicable regulatory requirements in other countries; ·
| delays in the testing, validation, manufacturing and delivery of our product candidates to the clinical sites;
|
delays in the testing, validation, manufacturing and delivery of our product candidates to the clinical sites; ·
| failure to obtain sufficient cells from patients to manufacture enough drug product or achieve target cell doses;
|
failure to obtain sufficient cells from patients to manufacture enough drug product or achieve target cell doses; ·
| delays in having patients complete participation in a study or return for post-treatment follow-up;
|
delays in having patients complete participation in a study or return for post-treatment follow-up; ·
| clinical study sites or patients dropping out of a study;
|
clinical study sites or patients dropping out of a study; ·
| occurrence of serious adverse events associated with the product candidate that are viewed to outweigh its potential benefits; or
|
occurrence of serious adverse events associated with the product candidate that are viewed to outweigh its potential benefits; or ·
| changes in regulatory requirements and guidance that require amending or submitting new clinical protocols.
|
changes in regulatory requirements and guidance that require amending or submitting new clinical protocols. Any inability to successfully complete preclinical and clinical development could result in additional costs to us or impair our ability to generate revenues from product sales, regulatory and commercialization milestones and royalties. In addition, if we make manufacturing or formulation changes to our product candidates, we may need to conduct additional studies to demonstrate comparability of our modified product candidates to earlier versions. Clinical study delays could also shorten any periods during which we may have the exclusive right to commercialize our product candidates or allow our competitors to bring products to market before we do, which could impair our ability to successfully commercialize our product candidates and may harm our business and results of operations. If the results of our clinical studies are inconclusive or if there are safety concerns or adverse events associated with our product candidates, we may: ·
| be delayed in obtaining regulatory approval for our product candidates, if at all;
|
be delayed in obtaining regulatory approval for our product candidates, if at all; ·
| obtain approval for indications or patient populations that are not as broad as intended or desired;
|
obtain approval for indications or patient populations that are not as broad as intended or desired; ·
| obtain approval with labeling that includes significant use or distribution restrictions or safety warnings;
|
obtain approval with labeling that includes significant use or distribution restrictions or safety warnings; ·
| be required to perform additional clinical studies or clinical studies of longer duration to support approval or be subject to additional post-marketing testing requirements;
|
be required to perform additional clinical studies or clinical studies of longer duration to support approval or be subject to additional post-marketing testing requirements; ·
| have regulatory authorities withdraw their approval of the product or impose restrictions on its use;
|
have regulatory authorities withdraw their approval of the product or impose restrictions on its use; ·
| be subject to the addition of labeling statements, such as warnings or contraindications;
|
be subject to the addition of labeling statements, such as warnings or contraindications; 39experience damage to our reputation.
·
| experience damage to our reputation.
|
Treatment with our gene therapy product candidates involves chemotherapy and myeloablative treatments, which can cause side effects or adverse events that are unrelated to our product candidate,candidates, but may still impact the success of our clinical studies. Additionally, our product candidates could potentially cause other adverse events that have not yet been predicted. The inclusion of critically ill patients in our clinical studies may result in deaths or other adverse medical events due to other therapies or medications that such patients may be using.using, or the progression of their disease. As described above, any of these events could prevent us from achieving or maintaining market acceptance of our product candidates and impair our ability to commercialize our products. We have not completed any clinical studies of our current viral vectors or product candidates derived fromcandidates. Initial success in our ongoing clinical studies may not be indicative of results obtained when these viral vectors. Successstudies are completed. Furthermore, success in early clinical studies may not be indicative of results obtained in later studies. Our current viral vectors and our product candidates first initiated evaluation in human clinical studies in 2013, and we may experience unexpected results in the future. Earlier gene therapy clinical studies, which we believe serve as proof-of-concept for our product candidates, utilized lentiviral vectors similar to ours. However, these studies should not be relied upon as evidence that our future clinical studies will succeed. Study designs and resultsResults from previous or ongoing studies are not necessarily predictive of our future clinical study designs or results, and initial or interim results may not continue or be confirmed upon full analysiscompletion of the complete study data. Ourstudy. There is limited data concerning long-term safety and efficacy following treatment with our gene therapy and T cell-based product candidates. These data, or other positive data, may not continue or occur for these subjects or for any future subjects in our ongoing or future clinical studies, and may not be repeated or observed in ongoing or future studies involving our product candidates. For instance, while patients with TDT or severe SCD who have been
treated with our LentiGlobin product candidate may experience a reduction or temporary elimination of transfusion support, there can be no assurance that they will not require transfusion support in the future. Similarly, patients with relapsed/refractory multiple myeloma who have been treated with the bb2121 or the bb21217 product candidate may experience disease progression. Furthermore, our product candidates may also fail to show the desired safety and efficacy in later stages of clinical development despite having successfully advanced through initial clinical studies. There can be no assurance that any of these studies will ultimately be successful or support further clinical advancement or regulatory approval of our product candidates. There is a high failure rate for drugs and biologics proceeding through clinical studies. A number of companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in later stage clinical studies even after achieving promising results in earlier stage clinical studies. Data obtained from preclinical and clinical activities are subject to varying interpretations, which may delay, limit or prevent regulatory approval. In addition, regulatory delays or rejections may be encountered as a result of many factors, including changes in regulatory policy during the period of product development. Patients with different genotypes of TDT may experience different outcomes from treatment with our product candidates, which may result in the delay of our clinical development and commercialization plans. Initial success inresults from our ongoing clinical studies may not be indicative of results obtained when these studies are completed. In December 2014, at the Annual Meeting of the American Society of Hematology (ASH), we announced data from the first eight subjects treated with LentiGlobin BB305 drug product. In the first four subjects, each of whom had at least three months of follow up, treatment with LentiGlobin BB305 drug product resulted in sufficient hemoglobin production to reduce or eliminate the need for transfusion support amongsuggest that patients with ßTDT -thalassemia major who would otherwise require chronic blood transfusions. These data include the first five subjects treated in the Northstar Study and the first three subjects from the HGB-205 Study. Although the initial clinical data on these subjects are encouraging, the data are preliminary in nature, based on limited periods of time since patient infusion, and the Northstar and HGB-205 Studies are not complete. There is limited data concerning long-term safety and efficacy followinga non-β0/β0 genotype experienced better outcomes to treatment with LentiGlobin drug product. These data, or other positive data, may not continue or occur for these subjects or for any future subjects in this study, and may not be repeated or observed in ongoing or future studies involving our LentiGlobin product candidate includingthan patients with TDT and a β0/β0 genotype. Consequently, we expect to seek FDA approval of our LentiGlobin product candidate initially for the HGB-205 Study,treatment of patients with TDT and a non-β0/β0 genotype. In order to support an application for FDA approval of our LentiGlobin product candidate in patients with TDT and a β0/β0 genotype, we initiated the Northstar StudyHGB-212 study, but we do not know if or the HGB-206 Study in severe SCD. There canwhen our LentiGlobin product candidate may be no assurance that subjects for whom periodic transfusion support has been reduced or temporarily eliminated will not receive transfusion support in the future. Furthermore, there can be no assurance that any of these studies will ultimately be successful or support further clinical advancement of this product candidate. There is a high failure rate for drugs and biologics proceeding through clinical studies. A number of companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in clinical development even after achieving promising results in earlier studies, and any such setbacks in our clinical development could have a material adverse effect on our business and operating results. commercially available to patients with all genotypes.
The results from our Starbeam Study may not be sufficiently robust to support the submission of marketing approval for our Lenti-D product candidate. Before we submit our Lenti-D product candidate for marketing approval, the FDA and the EMA may require us to enroll additional subjects, conduct additional clinical studies, or evaluate subjects for an additional follow-up period. The FDA has advised us that our Starbeam Study, which is a single-arm, open-label study to evaluate the safety and efficacy of our Lenti-D product candidate to halt the progression of CCALD,CALD, may not be deemed to be a pivotal study or may not provide sufficient support for a Biologics License Application, or BLA, submission. The FDA normally requires two pivotal clinical studies to approve a drug or biologic product, and thus the FDA may require that we conduct larger or additional clinical studies of our Lenti-D product candidate prior to a BLA submission. The FDA typically does not consider a single clinical study to be adequate to serve as a pivotal study unless it is, among other things, well-controlled and demonstrates a clinically meaningful effect on mortality, irreversible morbidity, or prevention of a disease with potentially serious outcome, and a confirmatory study would be practically or ethically impossible. Due to the nature of CCALDCALD and the limited number of patients with this condition, we believe a placebo-controlled and blinded study is not practicable for ethical and other reasons. However, it is still possible that, even if we achieve favorable results in the Starbeam Study, the FDA may require us to enroll additional subjects or conduct additional clinical studies, possibly involving a larger sample size or a different clinical study design, particularly if the FDA does not find the results from the Starbeam Study to be sufficiently persuasive to support a BLA 40
submission. The FDA may also require that we conduct a longer follow-up period of subjects treated with our Lenti-D product candidate prior to accepting our BLA submission. In addition, the Starbeam Study was not designed to achieve a statistically significant efficacy determination. Rather, we anticipate that Lenti-Dthe safety and efficacy of our Lenti-D product candidate will be evaluated in light of the data collected in our retrospective ALD-101 Studystudy and potentially our observational ALD-103 study. However, due to the retrospective nature of the ALD-101 study, and the limited number of patients with this condition, the FDA has advised us that the ALD-101 Studystudy is not sufficiently robust to serve as a conventional historical control group and as a basis of comparison against the results of the Starbeam Study. Thus, we expect that the FDA will assess the totality of the safety and efficacy data from our CCALDCALD clinical studies in reviewing any future BLA submission for our Lenti-D product candidate. Based on this assessment, the FDA may require that we conduct additional preclinical or clinical studies prior to submitting or approving a BLA for this indication. It is possible that the FDA or the EMA may not consider the results of this study to be sufficient for approval of our Lenti-D product candidate for this indication. If the FDA or the EMA requires additional studies, we would incur increased costs and delays in the marketing approval process, which may require us to expend more resources than we have available. In addition, it is possible that the FDA and the EMA may have divergent opinions on the elements necessary for a successful BLA and Marketing Authorization Application, or MAA, respectively, which may cause us to alter our development, regulatory and/or commercialization strategies.
We cannot be certain that our Northstar-2 Study in patients with TDT and a non-β0/β0 genotype, or our Northstar-3 Study in patients with TDT and a β0/β0 genotype, together with data from our Northstar Study and HGB-205 study, will be sufficient to form the basis for a BLA submission for our LentiGlobin product candidate. In general, the FDA requires the successful completion of two pivotal trials to support approval of a BLA, but in certain circumstances, will approve a BLA based on only one pivotal trial. If successful, we believe the results from our ongoing Northstar-2 Study, together with data from our ongoing Northstar Study and HGB-205 study, could be sufficient to form the basis for a BLA submission for our LentiGlobin product candidate to treat adult and adoleschent patients with TDT and a non-β0/β0 genotype. In addition, if successful, we believe the results from our Northstar-3 Study, together with data from our ongoing Northstar Study and Northstar-2 Study, could be sufficient to form the basis for a BLA supplement submission for our LentiGlobin product candidate to treat patients with TDT and a β0/β0 genotype. However, it should be noted that our ability to submit and obtain approval of a BLA is ultimately an FDA review decision, which will be dependent upon the data available at such time, and the available data may not be sufficiently robust from a safety and/or efficacy perspective to support the submission or approval of a BLA. Depending on the outcome of these ongoing clinical studies, the FDA may require that we conduct additional or larger pivotal trials before we can submit or obtain approval for a BLA for our LentiGlobin product candidate for the treatment of TDT. There can be no assurance that we will ultimately receive conditional marketing approval of our LentiGlobin product candidate in the European Union, or the nature of the conditions that would be imposed on us if conditionally approved. The EMA Adaptive Pathways pilot program in which we are participating is intended to facilitate either an initial approval in a well-defined patient subgroup with a high medical need and subsequent widening of the indication to a larger patient population through iterative extension of the indication, or an early regulatory approval (e.g. conditional approval), which is prospectively planned, and where uncertainty is reduced through the collection of post-approval data on the use in of medicinal product in patients. Based on our discussions with the EMA, we believe that we may be able to seek conditional approval for our LentiGlobin product candidate, with our refined manufacturing process, for the treatment of adult and adolescent subjects with TDT and a non- β0/β0 genotype on the basis of the totality of the clinical data from our ongoing studies with LentiGlobin. For efficacy, we believe that the Northstar Study and supportive ongoing HGB-205 study, together with the data available from our ongoing Northstar-2 Study and our long-term follow-up study LTF-303, could support the filing of a marketing authorization application in the European Union. This plan is contingent upon all of the studies conducted in patients with TDT with the LentiGlobin product candidate demonstrating sufficient efficacy and safety, and in particular, transfusion independence and reduction in transfusion requirements, for efficacy analyses in the Northstar, HGB-205 and Northstar-2 studies. However, it should be noted that the EMA Adaptive Pathways program is a pilot program, and as such there is limited information and precedent regarding the potential outcomes for sponsors that participate in this program. Whether our LentiGlobin product candidate is eligible for conditional approval will ultimately be determined at the discretion of the EMA and will be dependent upon the data available at such time, and the available data may not be sufficiently robust from a safety and/or efficacy perspective to support conditional approval. Depending on the outcome of our planned and ongoing clinical trials, the EMA may require that we conduct additional or larger clinical trials before our LentiGlobin product candidate is eligible for conditional approval. Even if conditional approval is obtained, the conditions to be imposed on us under this program are unknown and will be imposed at the time of any such conditional approval. Changes in our manufacturing processes may cause delays in our clinical development and commercialization plans. The manufacturing processes for our lentiviral vectors and our product candidates are complex. We have developed and have implemented an improved manufacturing process of our LentiGlobin drug product that will be administered in our Northstar-2 Study in patients with TDT and a non-β0/β0 genotype, our amended ongoing HGB-206 study in patients with severe SCD, and our Northstar-3 Study in patients with TDT and a β0/β0 genotype. There can be no assurances that LentiGlobin drug product manufactured using the improved manufacturing process will lead to similar or improved efficacy or safety results in subjects, as compared to the LentiGlobin drug product used in the ongoing Northstar Study, the ongoing HGB-205 study, or the HGB-206 study under the original protocol. As we develop a commercial-scale manufacturing process for our LentiGlobin and Lenti-D product candidates, we are implementing improvements to the manufacturing process for both producing our lentiviral vectors and for our product candidates on a continual basis. In some circumstances, changes in the manufacturing process may require us to perform additional comparability studies or to collect additional clinical data from patients prior to undertaking additional clinical studies or filing for regulatory approval. These requirements may lead to delays in our clinical development and commercialization plans.
In previous clinical studies involving viral vectors for gene therapy, some subjects experienced serious adverse events, including the development of leukemia due to vector-related insertional oncogenesis. If our vectors demonstrate a similar effect, we may be required to halt or delay further clinical development of our product candidates. A significant risk in any gene therapy product based on viral vectors is that the vector will insert in or near cancer-causing oncogenes leading to uncontrolled clonal proliferation of mature cancer cells in the patient. For example, in 2003, 20 subjects treated for X-linked severe combined immunodeficiency in two gene therapy studies using a murine, or mouse-derived, gamma-retroviral vector showed correction of the disease, but the studies were terminated after five subjects developed leukemia (four of whom were subsequently cured). The cause of these adverse events was shown to be insertional oncogenesis, which is the process whereby the corrected gene inserts in or near a gene that is important in a critical cellular process like growth or division, and this insertion results in the development of a cancer (often leukemia). Using molecular diagnostic techniques, it was determined that clones from these subjects showed retrovirus insertion in proximity to the promoter of the LMO2 proto-oncogene. Earlier generation retroviruses like the one used in these two studies have been shown to preferentially integrate in regulatory regions of genes that control cell growth. These well-publicized adverse events led to the development of new viral vectors, such as lentiviral vectors, with improved safety profiles and also the requirement of enhanced safety monitoring in gene therapy clinical trials, including periodic analyses of the therapy’s genetic insertion sites. In published studies, lentiviral vectors have demonstrated an improved safety profile over gamma-retroviral vectors, with no disclosed events of gene therapy-related adverse events, which we believe is due to a number of factors including the tendency of these vectors to integrate within genes rather than in areas that control gene expression, as well as their lack of strong viral enhancers. However, it should be noted that in our Phase I/II study (the LG001 Study) of autologous HSCs transduced ex vivo using an earlier generation of our LentiGlobin vector, called HPV569, we initially observed in one patientsubject that a disproportionate number of the cells expressing our functional gene had the same insertion site. Tests showed that this partial clonal dominance contained an insertion of the functional gene in the HMGA2 gene that persisted for a period of two to three years. Although there was some initial concern that the observed clonal dominance might represent a pre-leukemic event, there have been no adverse clinical consequences of this event, or any signs of cancer, in over seven years since the observation was made. The presence of the HMGA2 clone has steadily declined in this patientsubject over time to the point that it is no longer the most common clone observed in this patient.subject. Notwithstanding the historical data regarding the potential safety improvements of lentiviral vectors, the risk of insertional oncogenesis remains a significant concern for gene therapy and we cannot assure that it will not occur in any of our ongoing or planned clinical studies. There is also the potential risk of delayed adverse events following exposure to gene therapy products due to persistent biological activity of the genetic material or other components of products used to carry the genetic material. The FDA has stated that lentiviral vectors possess characteristics that may pose high risks of delayed adverse events. If any such adverse events occur, further advancement of our clinical studies could be halted or delayed, which would have a material adverse effect on our business and operations. In previous clinical studies involving T cell-based immunotherapies, some subjects experienced serious adverse events. Our T cell-based immunotherapy product candidates may demonstrate a similar effect or have other properties that could halt their clinical development, prevent their regulatory approval, limit their commercial potential, or result in significant negative consequences. The bb2121 and bb21217 product candidates are chimeric antigen receptor, or CAR, T cell-based immunotherapy. In previous and ongoing clinical studies involving CAR T cell products, many subjects experienced side effects such as neurotoxicity and cytokine release syndrome, which have in some cases resulted in clinical holds in ongoing clinical trials of CAR T product candidates. There have been life threatening events related to severe neurotoxicity and cytokine release syndrome, requiring intense medical intervention such as intubation or pressor support, and in several cases, resulted in death. Severe neurotoxicity is a condition that is currently defined clinically by cerebral edema, confusion, drowsiness, speech impairment, tremors, seizures, or other central nervous system side effects, when such side effects are serious enough to lead to intensive care. In some cases, severe neurotoxicity was thought to be associated with the use of certain lymphodepletion regimens used prior to the administration of the CAR T cell products. Cytokine release syndrome is a condition that is currently defined clinically by certain symptoms related to the release of cytokines, which can include fever, chills, low blood pressure, when such side effects are serious enough to lead to intensive care with mechanical ventilation or significant vasopressor support. The exact cause or causes of cytokine release syndrome and severe neurotoxicity in connection with treatment of CAR T cell products is not fully understood at this time. In addition, subjects have experienced other adverse events in these studies, such as a reduction in the number of blood cells (in the form of neutropenia, thrombocytopenia, anemia or other cytopenias), febrile neutropenia, chemical laboratory abnormalities (including elevated liver enzymes), and renal failure. Undesirable side effects caused by the bb2121 or bb21217 product candidate, other CAR T product candidates targeting BCMA, or our other T cell-based immunotherapy product candidates, could cause us or regulatory authorities to interrupt, delay or halt clinical studies and could result in a more restrictive label or the delay or denial of regulatory approval by the FDA or other comparable
foreign regulatory authorities. Results of our studies could reveal a high and unacceptable severity and prevalence of side effects or unexpected characteristics. Treatment-related side effects could also affect patient recruitment or the ability of enrolled subjects to complete the studies or result in potential product liability claims. In addition, these side effects may not be appropriately recognized or managed by the treating medical staff, as toxicities resulting from T cell-based immunotherapies are not normally encountered in the general patient population and by medical personnel. We expect to have to train medical personnel regarding our T cell-based immunotherapy product candidates to understand their side effects for both our planned clinical trials and upon any commercialization of any T cell-based immunotherapy product candidates. Inadequate training in recognizing or managing the potential side effects of T cell-based immunotherapy product candidates could result in patient deaths. Any of these occurrences may harm our business, financial condition and prospects significantly. Even if we complete the necessary preclinical and clinical studies, we cannot predict when or if we will obtain regulatory approval to commercialize a product candidate or the approval may be for a more narrow indication than we expect. We cannot commercialize a product until the appropriate regulatory authorities have reviewed and approved the product candidate. Even if our product candidates demonstrate safety and efficacy in clinical studies, the regulatory agencies may not complete their review processes in a timely manner, or we may not be able to obtain regulatory approval. Additional delays may result if an FDA Advisory Committee or other regulatory advisory group or authority recommends non-approval or restrictions on approval. In 41
addition, we may experience delays or rejections based upon additional government regulation from future legislation or administrative action, or changes in regulatory agency policy during the period of product development, clinical studies and the review process. Regulatory agencies also may approve a treatment candidate for fewer or more limited indications than requested or may grant approval subject to the performance of post-marketing studies. In addition, regulatory agencies may not approve the labeling claims that are necessary or desirable for the successful commercialization of our treatment candidates. For example, the development of our product candidates for pediatric use is an important part of our current business strategy, and if we are unable to obtain regulatory approval for the desired age ranges, our business may suffer. Even if we obtain regulatory approval for a product candidate, our products will remain subject to regulatory scrutiny. Even if we obtain regulatory approval in a jurisdiction, the regulatory authority may still impose significant restrictions on the indicated uses or marketing of our product candidates, or impose ongoing requirements for potentially costly post-approval studies, post-market surveillance or post-market surveillance.patient or drug restrictions. For example, the FDA typically advises that patients treated with gene therapy undergo follow-up observations for potential adverse events for a 15-year period. Additionally, the holder of an approved BLA is obligated to monitor and report adverse events and any failure of a product to meet the specifications in the BLA. The holder of an approved BLA must also submit new or supplemental applications and obtain FDA approval for certain changes to the approved product, product labeling or manufacturing process. Advertising and promotional materials must comply with FDA rules and are subject to FDA review, in addition to other potentially applicable federal and state laws. In addition, product manufacturers and their facilities are subject to payment of user fees and continual review and periodic inspections by the FDA and other regulatory authorities for compliance with good manufacturing practices, or GMP, and adherence to commitments made in the BLA. If we or a regulatory agency discovers previously unknown problems with a product such as adverse events of unanticipated severity or frequency, or problems with the facility where the product is manufactured, a regulatory agency may impose restrictions relative to that product or the manufacturing facility, including requiring recall or withdrawal of the product from the market or suspension of manufacturing. If we fail to comply with applicable regulatory requirements following approval of any of our product candidates, a regulatory agency may: ·
| issue a warning letter asserting that we are in violation of the law;
|
issue a warning letter asserting that we are in violation of the law; ·
| seek an injunction or impose civil or criminal penalties or monetary fines;
|
seek an injunction or impose civil or criminal penalties or monetary fines; ·
| suspend or withdraw regulatory approval;
|
suspend or withdraw regulatory approval; ·
| suspend any ongoing clinical studies;
|
suspend any ongoing clinical studies; ·
| refuse to approve a pending marketing application, such as a BLA or supplements to a BLA submitted by us;
|
refuse to approve a pending marketing application, such as a BLA or supplements to a BLA submitted by us; ·
| refuse to allow us to enter into supply contracts, including government contracts.
|
refuse to allow us to enter into supply contracts, including government contracts.
Any government investigation of alleged violations of law could require us to expend significant time and resources in response and could generate negative publicity. The occurrence of any event or penalty described above may inhibit our ability to commercialize our product candidates and generate revenues. Risks related to our reliance on third parties We expect to rely on third parties to conduct some or all aspects of our viral vector production, drug product manufacturing, research and preclinical and clinical testing, and these third parties may not perform satisfactorily. We do not expect to independently conduct all aspects of our viral vector production, drug product manufacturing, research and preclinical and clinical testing. We currently rely, and expect to continue to rely, on third parties with respect to these items. In some cases these third parties are academic, research or similar institutions that may not apply the same quality control protocols utilized in certain commercial settings. Our reliance on these third parties for research and development activities will reduce our control over these activities but will not relieve us of our responsibility to ensure compliance with all required regulations and study protocols. For example, for product candidates that we develop and commercialize on our own, we will remain responsible for ensuring that each of our IND-enabling studies and clinical studies are conducted in accordance with the study plan and protocols.protocols, and that our viral vectors and drug products are manufactured in accordance with GMP as applied in the relevant jurisdictions. If these third parties do not successfully carry out their contractual duties, meet expected deadlines, or conduct our studies in accordance with regulatory requirements or our stated study plans and protocols, or manufacture our viral vectors and drug products in accordance with GMP, we will not be able to complete, or may be delayed in 42
completing, the preclinical and clinical studies and manufacturing process validation activities required to support future IND, MAA and BLA submissions and approval of our product candidates. Any of these third parties may terminate their engagements with us at any time. If we need to enter into alternative arrangements, it could delay our product development activities. Reliance on third-party manufacturers entails risks to which we would not be subject if we manufactured the product candidates ourselves, including: ·
| the inability to negotiate manufacturing agreements with third parties under commercially reasonable terms;
|
the inability to negotiate manufacturing agreements with third parties under commercially reasonable terms; ·
| reduced control as a result of using third-party manufacturers for all aspects of manufacturing activities;
|
reduced control as a result of using third-party manufacturers for all aspects of manufacturing activities; ·
| the risk that these activities are not conducted in accordance with our study plans and protocols;
|
the risk that these activities are not conducted in accordance with our study plans and protocols; ·
| termination or nonrenewal of manufacturing agreements with third parties in a manner or at a time that is costly or damaging to us; and
|
termination or nonrenewal of manufacturing agreements with third parties in a manner or at a time that is costly or damaging to us; and ·
| disruptions to the operations of our third-party manufacturers or suppliers caused by conditions unrelated to our business or operations, including the bankruptcy of the manufacturer or supplier.
|
disruptions to the operations of our third-party manufacturers or suppliers caused by conditions unrelated to our business or operations, including the bankruptcy of the manufacturer or supplier. Any of these events could lead to clinical study delays or failure to obtain regulatory approval, or impact our ability to successfully commercialize future products. Some of these events could be the basis for FDA action, including injunction, recall, seizure or total or partial suspension of production. We and our contract manufacturers are subject to significant regulation with respect to manufacturing our products. The manufacturing facilities on which we rely may not continue to meet regulatory requirements and have limited capacity. We currently have relationships with a limited number of suppliers for the manufacturing of our viral vectors and product candidates. Each supplier may require licenses to manufacture such components if such processes are not owned by the supplier or in the public domain and we may be unable to transfer or sublicense the intellectual property rights we may have with respect to such activities. All entities involved in the preparation of therapeutics for clinical studies or commercial sale, including our existing contract manufacturers for our product candidates, are subject to extensive regulation. Some components of a finished therapeutic product approved for commercial sale or used in late-stage clinical studies must be manufactured in accordance with GMP. These regulations govern manufacturing processes and procedures (including record keeping) and the implementation and operation of quality systems to control and assure the quality of investigational products and products approved for sale. Poor control of production processes can
lead to the introduction of adventitious agents or other contaminants, or to inadvertent changes in the properties or stability of our product candidates that may not be detectable in final product testing. We or our contract manufacturers must supply all necessary documentation in support of a BLA or MAA on a timely basis and where required, must adhere to the FDA’s or other regulator’s good laboratory practices, or GLP, and GMP regulations enforced by the FDA or other regulator through facilities inspection programs. Some of our contract manufacturers have not produced a commercially-approved product and therefore have not obtained the requisite FDA or other regulatory approvals to do so. Our facilities and quality systems and the facilities and quality systems of some or all of our third-party contractors must pass a pre-approval inspection for compliance with the applicable regulations as a condition of regulatory approval of our product candidates or any of our other potential products. In addition, the regulatory authorities may, at any time, audit or inspect a manufacturing facility involved with the preparation of our product candidates or our other potential products or the associated quality systems for compliance with the regulations applicable to the activities being conducted. If these facilities do not pass a pre-approval plant inspection, FDA or other regulatory approval of the products will not be granted. The regulatory authorities also may, at any time following approval of a product for sale, audit the manufacturing facilities of our third-party contractors. If any such inspection or audit identifies a failure to comply with applicable regulations or if a violation of our product specifications or applicable regulations occurs independent of such an inspection or audit, we or the relevant regulatory authority may require remedial measures that may be costly and/or time-consuming for us or a third party to implement and that may include the temporary or permanent suspension of a clinical study or commercial sales or the temporary or permanent closure of a facility. Any such remedial measures imposed upon us or third parties with whom we contract could materially harm our business. If we or any of our third-party manufacturers fail to maintain regulatory compliance, the FDA or other regulators can impose regulatory sanctions including, among other things, refusal to approve a pending application for a biologic product, or revocation of a pre-existing approval. As a result, our business, financial condition and results of operations may be materially harmed. Additionally, if supply from one approved manufacturer is interrupted, there could be a significant disruption in commercial supply. The number of manufacturers with the necessary manufacturing capabilities is limited. In addition, an alternative manufacturer would need to be qualified through a BLA supplement or similar regulatory submission which could result in further delay. The 43
regulatory agencies may also require additional studies if a new manufacturer is relied upon for commercial production. Switching manufacturers may involve substantial costs and is likely to result in a delay in our desired clinical and commercial timelines. These factors could cause the delay of clinical studies, regulatory submissions, required approvals or commercialization of our product candidates, cause us to incur higher costs and prevent us from commercializing our products successfully. Furthermore, if our suppliers fail to meet contractual requirements, and we are unable to secure one or more replacement suppliers capable of production at a substantially equivalent cost, our clinical studies may be delayed or we could lose potential revenue. We are dependent on Celgene for the successful development and commercialization of bb2121 and bb21217. If Celgene does not devote sufficient resources to the development of bb2121 and bb21217, is unsuccessful in its efforts, or chooses to terminate its agreements with us, our business will be materially harmed. We have exclusively licensed to Celgene the right to develop and commercialize the bb2121 and bb21217 product candidates, and we retain an option to co-develop and co-promote bb2121 and bb21217 in the United States under our license agreement and collaboration agreement with Celgene. With respect to bb2121, we are responsible for completing the ongoing CRB-401 study, but Celgene is responsible for further clinical development and commercialization costs, unless we choose to exercise our option to co-develop and co-promote bb2121 in the United States. If we exercise our option to co-develop and co-promote bb2121 in the United States, we and Celgene will share the obligation to develop and commercialize bb2121 in the United States, and we will be solely dependent on Celgene to develop and commercialize bb2121 outside of the United States. With respect to bb21217, we are responsible for completing the ongoing CRB-402 study, but Celgene is responsible for further clinical development and commercialization costs, unless we choose to exercise our option to co-develop and co-promote bb21217 in the United States. If we exercise our option to co-develop and co-promote bb21217 in the United States, we and Celgene will share the obligation to develop and commercialize bb21217 in the United States, and we will be solely dependent on Celgene to develop and commercialize bb21217 outside of the United States. Celgene is obligated to use commercially reasonable efforts to develop and commercialize bb2121 and bb21217. Celgene may determine however, that it is commercially reasonable to develop and commercialize a next-generation product candidate, rather than continue the development of bb2121 and bb21217. Alternatively, Celgene may determine that it is not commercially reasonable to continue development of any product candidates that arise from our collaboration. These outcomes may occur for many reasons, including internal business reasons or because of unfavorable regulatory feedback. Further, on review of the safety and efficacy data, the FDA may impose requirements on the clinical trial program that render such a program commercially nonviable. In addition, under our license agreement, Celgene may determine the development plan and activities for that product candidate. We may disagree with Celgene about the development strategy it employs, but we will have limited rights to impose our development strategy on
Celgene. Similarly, Celgene may decide to seek regulatory approval for, and limit commercialization of, bb2121 or bb21217 to narrower indications than we would pursue. More broadly, if Celgene elects to discontinue the development of bb2121 or bb21217, we may be unable to advance the product candidate ourselves. We would also be prevented from developing or commercializing another CAR T cell-based product candidate that targets BCMA outside of our collaboration with Celgene. This partnership may not be scientifically or commercially successful for us due to a number of important factors, including the following: Celgene has wide discretion in determining the efforts and resources that it will apply to its partnership with us. The timing and amount of any development milestones, and downstream commercial milestones and royalties that we may receive under such partnership will depend on, among other things, Celgene’s efforts, allocation of resources and successful development and commercialization of bb2121, bb21217 and other product candidates that are the subject of its collaboration with us. Celgene may develop and commercialize, either alone or with others, products that are similar to or competitive with bb2121, bb21217 and other product candidates that are the subject of its collaboration with us. For example, Celgene is currently commercializing certain of its existing products, including lenalidomide and pomalidomide, for certain patients with relapsed/refractory multiple myeloma. Celgene may terminate its partnership with us without cause and for circumstances outside of our control, which could make it difficult for us to attract new strategic partners or adversely affect how we are perceived in scientific and financial communities. Celgene may develop or commercialize our product candidates in such a way as to elicit litigation that could jeopardize or invalidate our intellectual property rights or expose us to potential liability. Celgene may not comply with all applicable regulatory requirements, or may fail to report safety data in accordance with all applicable regulatory requirements. If Celgene were to breach its arrangements with us, we may need to enforce our right to terminate the agreement in legal proceedings, which could be costly and cause delay in our ability to receive rights back to the relevant product candidates. If we were to terminate an agreement with Celgene due to Celgene's breach or Celgene terminated the agreement without cause, the development and commercialization of bb2121, bb21217 and other product candidates that are the subject of its collaboration with us could be delayed, curtailed or terminated because we may not have sufficient financial resources or capabilities to continue development and commercialization of these product candidates on our own if we choose not to, or are unable to, enter into a new collaboration for these product candidates. Celgene may enter into one or more transactions with third parties, including a merger, consolidation, reorganization, sale of substantial assets, sale of substantial stock or other change in control, which could divert the attention of its management and adversely affect Celgene's ability to retain and motivate key personnel who are important to the continued development of the programs under the strategic partnership with us. In addition, the third-party to any such transaction could determine to reprioritize Celgene's development programs such that Celgene ceases to diligently pursue the development of our programs and/or cause the respective collaboration with us to terminate. We expect to rely on third parties to conduct, supervise and monitor our clinical studies, and if these third parties perform in an unsatisfactory manner, it may harm our business. We expect to rely on CROs and clinical study sites to ensure our clinical studies are conducted properly and on time. While we will have agreements governing their activities, we will have limited influence over their actual performance. We will control only certain aspects of our CROs’ activities. Nevertheless, we will be responsible for ensuring that each of our clinical studies is conducted in accordance with the applicable protocol, legal, regulatory and scientific standards, and our reliance on the CROs does not relieve us of our regulatory responsibilities. We and our CROs are required to comply with the FDA’s GCPs for conducting, recording and reporting the results of clinical studies to assure that the data and reported results are credible and accurate and that the rights, integrity and confidentiality of clinical study participants are protected. The FDA enforces these GCPs through periodic inspections of study sponsors, principal investigators and clinical study sites. If we or our CROs fail to comply with applicable GCPs, the clinical data generated in our future clinical studies may be deemed unreliable and the FDA may require us to perform additional clinical studies before approving any marketing applications. Upon inspection, the FDA may determine that our clinical studies did not comply with GCPs. In addition, our future clinical studies will require a sufficient number of test subjects to evaluate the safety and efficacy of our product candidates. Accordingly, if our CROs fail to comply with these regulations or fail to recruit a sufficient number of patients, we may be required to repeat such clinical studies, which would delay the regulatory approval process.
Employees of our CROs are not our employees, and we are therefore unable to directly monitor whether or not they devote sufficient time and resources to our clinical and nonclinical programs, which must be conducted in accordance with GCPs and GLPs, respectively. These CROs may also have relationships with other commercial entities, including our competitors, for whom they may also be conducting clinical studies or other drug development activities that could harm our competitive position. If our CROs do not successfully carry out their contractual duties or obligations, fail to meet expected deadlines, or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements, or for any other reasons, our clinical studies may be extended, delayed or terminated, and we may not be able to obtain regulatory approval for, or successfully commercialize our product candidates. As a result, our financial results and the commercial prospects for our product candidates would be harmed, our costs could increase, and our ability to generate revenues could be delayed. We also expect to rely on other third parties to store and distribute our vectors and products for any clinical studies that we may conduct.conduct, as well as on third parties to administer our products to patients when and if our products are introduced into market. Any performance failure on the part of our distributorsthese third parties could delay clinical development or marketing approval of our product candidates or commercialization of our products, if approved, producing additional losses and depriving us of potential product revenue. Our reliance on third parties requires us to share our trade secrets, which increases the possibility that a competitor will discover them or that our trade secrets will be misappropriated or disclosed. Because we rely on third parties to manufacture our vectors and our product candidates, and because we collaborate with various organizations and academic institutions on the advancement of our gene therapy platform, we must, at times, share trade secrets with them. We seek to protect our proprietary technology in part by entering into confidentiality agreements and, if applicable, material transfer agreements, collaborative research agreements, consulting agreements or other similar agreements with our collaborators, advisors, employees and consultants prior to beginning research or disclosing proprietary information. These agreements typically limit the rights of the third parties to use or disclose our confidential information, such as trade secrets. Despite the contractual provisions employed when working with third parties, the need to share trade secrets and other confidential information increases the risk that such trade secrets become known by our competitors, are inadvertently incorporated into the technology of others, or are disclosed or used in violation of these agreements. Given that our proprietary position is based, in part, on our know-how and trade secrets, a competitor’s discovery of our trade secrets or other unauthorized use or disclosure would impair our competitive position and may have a material adverse effect on our business. In addition, these agreements typically restrict the ability of our collaborators, advisors, employees and consultants to publish data potentially relating to our trade secrets. Our academic collaborators typically have rights to publish data, provided that we are notified in advance and may delay publication for a specified time in order to secure our intellectual property rights arising from the collaboration. In other cases, publication rights are controlled exclusively by us, although in some cases we may share these rights 44
with other parties. We also conduct joint research and development programs that may require us to share trade secrets under the terms of our research and development partnerships or similar agreements. Despite our efforts to protect our trade secrets, our competitors may discover our trade secrets, either through breach of these agreements, independent development or publication of information including our trade secrets in cases where we do not have proprietary or otherwise protected rights at the time of publication. A competitor’s discovery of our trade secrets would impair our competitive position and have an adverse impact on our business. Risks related to our financial condition and capital requirements We have incurred significant losses since our inception and anticipate that we will continue to incur significant losses for the foreseeable future. We are a clinical-stage biotechnology company, and we have not yet generated significant revenues. We have incurred net losses in each year since our inception in 1992, including net losses of $48.7$335.6 million and $25.3$263.5 million for the years ended December 31, 20142017 and 2013,2016, respectively. As of December 31, 2014,2017, we had an accumulated deficit of $147.4$913.8 million. We have devoted most of our financial resources to research and development, including our clinical and preclinical development activities. To date, we have financed our operations primarily through the sale of equity securities and convertible debt and, to a lesser extent, through collaboration agreements and grants from governmental agencies and charitable foundations. The amount of our future net losses will depend, in part, on the rate of our future expenditures and our ability to obtain funding through equity or debt financings, strategic collaborations or additional grants. We have not completed pivotal clinical studies for any product candidate and it will be several years, if ever, before we have a product candidate ready for commercialization. Even if we obtain regulatory approval to market a product candidate, our future revenues will depend upon the size of any markets in which our product candidates have received approval, and our ability to achieve sufficient market acceptance, reimbursement from third-party payors and adequate market share for our product candidates in those markets.
We expect to continue to incur significant expenses and increasing operating losses for the foreseeable future. We anticipate that our expenses will increase substantially if and as we: ·
| continue our research and preclinical and clinical development of our product candidates;
|
continue our research and preclinical and clinical development of our product candidates; ·
| expand the scope of our current clinical studies for our product candidates;
|
expand the scope of our current clinical studies for our product candidates; ·
| initiate additional preclinical, clinical or other studies for our product candidates, including under our collaboration agreement with Celgene;
|
initiate additional preclinical, clinical or other studies for our oncology product candidates; ·
| further develop the manufacturing process for our vectors or our product candidates;
|
further develop the manufacturing process for our vectors or our product candidates; ·
| change or add additional manufacturers or suppliers;
|
change or add additional manufacturers or suppliers; ·
| seek regulatory and marketing approvals for our product candidates that successfully complete clinical studies;
|
seek regulatory and marketing approvals for our product candidates that successfully complete clinical studies; ·
| seek to identify and validate additional product candidates;
|
seek to identify and validate additional product candidates; ·
| acquire or in-license other product candidates and technologies;
|
acquire or in-license other product candidates and technologies; ·
| make milestone or other payments under any license agreements or our stock purchase agreement with the former equityholders of Pregenen;
|
make milestone or other payments under any license agreements or our stock purchase agreement with the former equityholders of Pregenen; ·
| maintain, protect and expand our intellectual property portfolio;
|
maintain, protect and expand our intellectual property portfolio; ·
| establish a sales, marketing and distribution infrastructure to commercialize any products for which we may obtain marketing approval;
|
establish a sales, marketing and distribution infrastructure in the United States and Europe to commercialize any products for which we may obtain marketing approval; ·
| attract and retain skilled personnel;
|
attract and retain skilled personnel; ·
| build additional infrastructure to support our operations as a public company and our product development and planned future commercialization efforts; and
|
build additional infrastructure to support our operations as a public company and our product development and planned future commercialization efforts, including manufacturing capacity at third-party manufacturers or potentially our own manufacturing facility; and ·
| experience any delays or encounter issues with any of the above.
|
experience any delays or encounter issues with any of the above. The net losses we incur may fluctuate significantly from quarter to quarter and year to year, such that a period-to-period comparison of our results of operations may not be a good indication of our future performance. In any particular quarter or quarters, our operating results could be below the expectations of securities analysts or investors, which could cause our stock price to decline. 45
We have never generated any revenue from product sales and may never be profitable. Our ability to generate revenue and achieve profitability depends on our ability, alone or with strategic collaboration partners, to successfully complete the development of, and obtain the regulatory, pricing and reimbursement approvals necessary to commercialize our product candidates. We do not anticipate generating revenues from product sales for the foreseeable future, if ever. Our ability to generate future revenues from product sales depends heavily on our success in: ·
| completing research and preclinical and clinical development of our product candidates;
|
completing research and preclinical and clinical development of our product candidates; seeking and obtaining regulatory and marketing approvals for product candidates for which we complete clinical studies; developing a sustainable, commercial-scale, reproducible, and transferable manufacturing process for our vectors and product candidates; establishing and maintaining supply and manufacturing relationships with third parties that can provide adequate (in amount and quality) products and services to support clinical development and the market demand for our product candidates, if approved; launching and commercializing product candidates for which we obtain regulatory and marketing approval, either by collaborating with a partner or, if launched independently, by establishing a sales force, marketing and distribution infrastructure; obtaining sufficient pricing and reimbursement for our product candidates from private and governmental payors; obtaining market acceptance and adoption of our product candidates and gene therapy as a viable treatment option; addressing any competing technological and market developments; identifying and validating new gene therapy product candidates;
·
| seeking and obtaining regulatory and marketing approvals for product candidates for which we complete clinical studies;
|
·•
| developing a sustainable, commercial-scale, reproducible, and transferable manufacturing process for our vectors and product candidates;
|
·
| establishing and maintaining supply and manufacturing relationships with third parties that can provide adequate (in amount and quality) products and services to support clinical development and the market demand for our product candidates, if approved;
|
·
| launching and commercializing product candidates for which we obtain regulatory and marketing approval, either by collaborating with a partner or, if launched independently, by establishing a sales force, marketing and distribution infrastructure;
|
·
| obtaining sufficient pricing and reimbursement for our product candidates from third-party and governmental payors;
|
·
| obtaining market acceptance of our product candidates and gene therapy as a viable treatment option;
|
·
| addressing any competing technological and market developments;
|
·
| identifying and validating new gene therapy product candidates;
|
·
| negotiating favorable terms in any collaboration, licensing or other arrangements into which we may enter; and |
·
| maintaining, protecting and expanding our portfolio of intellectual property rights, including patents, trade secrets and know-how.
|
maintaining, protecting and expanding our portfolio of intellectual property rights, including patents, trade secrets and know-how. We expect to continue to incur significant expenditures for the foreseeable future, and we expect these expenditures to increase as we prepare for any potential commercial launch. Our expenses could increase beyond expectations if we are required by the FDA, the EMA, or other regulatory agencies, domestic or foreign, to perform clinical and other studies in addition to those that we currently anticipate. Even if one or more of the product candidates that we develop is approved for commercial sale, we anticipate incurring significant costs associated with commercializing any approved product candidate. Our expenses couldcandidate, which costs may increase beyond expectations if we are required by the U.S. Food and Drug Administration, or the FDA, the European Medicines Agency, or the EMA, or other regulatory agencies, domestic or foreign, to perform clinical and other studies in addition to those that we currently anticipate.with any increased competition. Even if we are able to generate revenues from the sale of any approved products, we may not become profitable and may need to obtain additional funding to continue operations. From time to time, we will need to raise additional funding, which may not be available on acceptable terms, or at all. Failure to obtain this necessary capital when needed may force us to delay, limit or terminate our product development efforts or other operations. We are currently advancing our LentiGlobin, Lenti-D, bb2121 and LentiGlobinbb21217 product candidates through clinical development and other product candidates through preclinical development. Developing gene therapy products is expensive, and we expect our research and development expenses to increase substantially in connection with our ongoing activities, particularly as we advance our product candidates in clinical studies. As of December 31, 2014,2017, our cash, cash equivalents and marketable securities were $492.0 million.$1.6 billion. We expect that our existing cash, and cash equivalents, and marketable securities will be sufficient to fund our current operations through 2017.into 2021. However, our operating plan may change as a result of many factors currently unknown to us, and we may need to seek additional funds sooner than planned, through public or private equity or debt financings, government or other third-party funding, marketing and distribution arrangements and other collaborations, strategic alliances and licensing arrangements or a combination of these approaches. In any event, we will require additional capital to obtain regulatory approval for, and to commercialize, our product candidates. Even if we believe we have sufficient funds for our current or future operating plans, we may seek additional capital if market conditions are favorable or if we have specific strategic objectives. Any additional fundraising efforts may divert our management from their day-to-day activities, which may adversely affect our ability to develop and commercialize our product candidates. In addition, we cannot guarantee that future financing will be available in sufficient amounts or on terms acceptable to us, if at all. Moreover, the terms of any financing may adversely affect the holdings or the rights of our stockholders and the issuance of additional securities, whether equity or debt, by us, or the possibility of such issuance, may cause the market price of our shares to decline. The sale of additional equity or convertible securities would dilute all of our stockholders. The incurrence of indebtedness would result in increased fixed payment obligations and we may be required to agree 46
to certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. We could also be required to seek funds through arrangements with collaborative partners or otherwise at an earlier stage than otherwise would be desirable and we may be required to relinquish rights to some of our technologies or product candidates or otherwise agree to terms unfavorable to us, any of which may have a material adverse effect on our business, operating results and prospects. If we are unable to obtain funding on a timely basis, we may be required to significantly curtail, delay or discontinue one or more of our research or development programs or the commercialization of any product candidates or be unable to expand our operations or otherwise capitalize on our business opportunities, as desired, which could materially affect our business, financial condition and results of operations. Risks related to commercialization of our product candidates We intend to rely on a mix of internal and third-party manufacturers to produce our vector, product candidates and other key materials, but we are still in the process of building out our internal capacity and also have not entered into binding agreements with any suchall of the manufacturers needed to support commercialization. Additionally, neither we nor these manufacturers do not have experience producing our vectors and product candidates at commercial levels and may not achieve the necessary regulatory approvals or produce our vectors and products at the quality, quantities, locations and timing needed to support commercialization. We have not yet secured reliable manufacturing capabilities for commercial quantities of our viral vectors or established transduction facilities in all of the desired commercialization regions to support commercialization of our products. Although we intend to rely on a mix of internal and third-party manufacturers for commercialization, we are still in the process of building out our internal capacity and also have onlynot entered into binding agreements with suchall of the manufacturers needed to support our clinical studies. Weplanned commercialization activities,
and may not be unableable to timely or successfully build out our internal capacity, or negotiate binding agreements with the manufacturers to support our commercialization activities at commercially reasonable terms. No manufacturer currently has the experience or ability to produce our vectors andor drug product candidates at commercial levels. We are currently developing a commercial-scale manufacturing process for our LentiGlobin and Lenti-D product candidates, which we are beginning to transfertransferring to one or more contract manufacturers. We may run into technical or scientific issues related to manufacturing or development that we may be unable to resolve in a timely manner or with available funds. Although we have been able to produce our Lenti-D vector at commercial scale, we have not completed the characterization and validation activities necessary for commercial and regulatory approvals. If we or our manufacturing partners do not obtain such regulatory approvals, our commercialization efforts will be harmed. Additionally, since the HSCs and T cells have a limited window of stability following extractionprocurement from the patient,subject, we must set up transduction facilities in the regions where we wish to commercialize our product. Currently, we rely on third-party contract manufacturers in the United States and Europe to produce our product candidates for our clinical studies. Since a portion of our target patient populations will be outside the United States and Europe, we will need to set up additional transduction facilities that can replicate our transduction process. Establishment of such facilities may be financially impractical or impeded by technical, quality, or regulatory issues related to these new sites and we may also run into technical or scientific issues related to transfer of our transduction process or other developmental issues that we may be unable to resolve in a timely manner or with available funds. Even if we timely develop a manufacturing process and successfully transfer it to the third-party vector and product manufacturers or successfully and timely develop our internal capacity, if we or such third-party manufacturers are unable to produce the necessary quantities of viral vectors and our product candidates, or in compliance with GMP or other pertinent regulatory requirements, and within our planned time frame and cost parameters, the development and sales of our products, if approved, may be materially harmed. Furthermore, if we or our third-party manufacturers are unable to produce the necessary quantities of viral vectors or our product candidates in quantities, quality requirements, or within the time frames that we need to support our commercialization activities, it may result in delays in our development plans or increased capital expenditures. In addition, any significant disruption in our supplier relationships could harm our business. We source key materials from third parties, either directly through agreements with suppliers or indirectly through our manufacturers who have agreements with suppliers. There are a small number of suppliers for certain key materials that are used to manufacture our product candidates. Such suppliers may not sell these key materials to us or to our manufacturers at the times we need them or on commercially reasonable terms. We do not have any control over the process or timing of the acquisition of these key materials by our manufacturers. Moreover, we currently do not have any agreements for the commercial production of these key materials. Although we expect to begin building out our field team, we have no sales or distribution experience and only early capabilities for marketing and market access, and expect to invest significant financial and management resources to establish these capabilities. If we are unable to establish sales and marketingdistribution capabilities or enter into agreements with third parties to market and sell our product candidates, we may be unable to generate any revenues. WeAlthough we expect to begin building out our field team, we have no sales or distribution experience selling and only early capabilities for marketing our product candidates.and market access. To successfully commercialize any products that may result from our development programs, we will need to develop these capabilities in the United States, Europe and other regions, either on our own or with others. We may enter into collaborations with other entities to utilize their mature marketing and distribution capabilities, but we may be unable to enter into marketing agreements on favorable terms, if at all. If our future collaborative partners do not commit sufficient resources to commercialize our future products, if any, and we are unable to develop the necessary marketing capabilities on our own, we will be unable to generate sufficient product revenue to sustain our business. We will be competing with many companies that currently have
47
extensive and well-funded marketing and sales operations. Without ana significant internal team or the support of a third party to perform marketing and sales functions, we may be unable to compete successfully against these more established companies. We face intense competition and rapid technological change and the possibility that our competitors may develop therapies that are more advanced or effective than ours, which may adversely affect our financial condition and our ability to successfully commercialize our product candidates. We are engaged in gene therapy for severe genetic and rare diseases and in the field of CAR T cells in oncology,cell-based immunotherapy, both of which are competitive and rapidly changing fields. We have competitors both in the United States and internationally, including major multinational pharmaceutical companies, biotechnology companies and universities and other research institutions. Some of the pharmaceutical and biotechnology companies we expect to compete with include Bellicum Pharmaceuticals, Inc., Acceleron Pharma, Inc., GlaxoSmithKline plc through their collaboration with TIGET/MolMed, Sangamo BioSciences Inc. through their collaboration with Biogen Idec, Merck & Co.,Bioverativ Inc., Novartis AG, through their collaboration with the University of Pennsylvania,Global Blood Therapeutics, Inc., GlycoMimetics Inc., Acceleron Pharma,Gilead Sciences, Inc., Kite Pharma, Inc., Pfizer Inc. through
their collaboration with Cellectis SA, Adaptimmune Inc., Juno Therapeutics, Inc., including after their proposed acquisition by Celgene Corporation, and Juno Therapeutics.Acceleron Pharma Inc. through their collaboration with Celgene Corporation. In addition, many universities and private and public research institutes are active in our target disease areas. Many of our competitors have substantially greater financial, technical and other resources, such as larger research and development staff, andmanufacturing capabilities, experienced marketing and manufacturing organizations. Competition may increase further as a result of advances in the commercial applicability of technologies and greater availability of capital for investment in these industries. Our competitors may succeed in developing, acquiring or licensing on an exclusive basis, products that are more effective or less costly than any product candidate that we may develop, or achieve earlier patent protection, regulatory approval, product commercialization and market penetration than us. Additionally, technologies developed by our competitors may render our potential product candidates uneconomical or obsolete, and we may not be successful in marketing our product candidates against competitors. Even if we are successful in achieving regulatory approval to commercialize a product candidate faster than our competitors, we may face competition from biosimilars due to the changing regulatory environment. In the United States, the Biologics Price Competition and Innovation Act of 2009 created an abbreviated approval pathway for biological products that are demonstrated to be “highly similar,” or biosimilar, to or “interchangeable” with an FDA-approved biological product. This new pathway could allow competitors to reference data from biological products already approved after 12 years from the time of approval. In his proposed budget for fiscal year 2014, President Obama proposed to cut this 12-year period of exclusivity down to seven years. He also proposed to prohibit additional periods of exclusivity due to minor changes in product formulations, a practice often referred to as “evergreening.” In Europe, the European Commission has granted marketing authorizations for several biosimilars pursuant to a set of general and product class-specific guidelines for biosimilar approvals issued over the past few years. In Europe, a competitor may reference data from biological products already approved, but will not be able to get on the market until 10 years after the time of approval. This 10-year period will be extended to 11 years if, during the first eight of those 10 years, the marketing authorization holder obtains an approval for one or more new therapeutic indications that bring significant clinical benefits compared with existing therapies. In addition, companies may be developing biosimilars in other countries that could compete with our products. If competitors are able to obtain marketing approval for biosimilars referencing our products, our products may become subject to competition from such biosimilars, with the attendant competitive pressure and consequences. Expiration or successful challenge of our applicable patent rights could also trigger competition from other products, assuming any relevant exclusivity period has expired. In addition, although our product candidates have been granted orphan drug status by the FDA and EMA, there are limitations to the exclusivity. In the United States, the exclusivity period for orphan drugs is seven years, while pediatric exclusivity adds six months to any existing patents or exclusivity periods. In Europe, orphan drugs may be able to obtain 10 years of marketing exclusivity and up to an additional two years on the basis of qualifying pediatric studies. However, orphan exclusivity may be reduced to six years if the drug no longer satisfies the original designation criteria. Additionally, a marketing authorization holder may lose its orphan exclusivity if it consents to a second orphan drug application or cannot supply enough drug. Orphan drug exclusivity also can be lost when a second applicant demonstrates its drug is “clinically superior” to the original orphan drug. Finally, as a result of the expiration or successful challenge of our patent rights, we could face more litigation with respect to the validity and/or scope of patents relating to our competitors’ products. The availability of our competitors’ products could limit the demand, and the price we are able to charge, for any products that we may develop and commercialize. The commercial success of any current or future product candidate will depend upon the degree of market acceptance by physicians, patients, third-party payors and others in the medical community. Ethical, social and legal concerns about gene therapy and genetic research could result in additional regulations restricting or prohibiting the products and processes we may use. Even with the requisite approvals, the commercial success of our product candidates will depend in part on the medical community, patients, and third-party or governmental payors accepting gene therapy products in general, and our product candidates in particular, as medically useful, cost-effective, and safe. Any product that we bring to the market may not gain market acceptance by physicians, patients, third-party payors and others in the medical community. If 48
these products do not achieve an adequate level of acceptance, we may not generate significant product revenue and may not become profitable. The degree of market acceptance of theseour product candidates, if approved for commercial sale, will depend on a number of factors, including: ·
| the potential efficacy and potential advantages over alternative treatments;
|
the potential efficacy and potential advantages over alternative treatments; the prevalence and severity of any side effects, including any limitations or warnings contained in a product’s approved labeling; the prevalence and severity of any side effects resulting from the chemotherapy and myeloablative treatments associated with the procedure by which our product candidates are administered; relative convenience and ease of administration;
·
| the prevalence and severity of any side effects, including any limitations or warnings contained in a product’s approved labeling;
|
·•
| the prevalence and severity of any side effects resulting from the chemotherapy and myeloablative treatments associated with the procedure by which our product candidates are administered;
|
·
| relative convenience and ease of administration;
|
·
| the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies; |
·
| the strength of marketing and distribution support and timing of market introduction of competitive products;
|
the strength of marketing and distribution support and timing of market introduction of competitive products; ·
| the pricing of our products;
|
the pricing of our products; ·
| publicity concerning our products or competing products and treatments; and
|
publicity concerning our products or competing products and treatments; and ·
| sufficient third-party insurance coverage or reimbursement.
|
sufficient third-party insurance coverage or reimbursement. Even if a potential product displays a favorable efficacy and safety profile in preclinical and clinical studies, market acceptance of the product will not be known until after it is launched. Our efforts to educate the medical community and third-party payors on the benefits of the product candidates may require significant resources and may never be successful. SuchFor instance, UniQure encountered challenges in commercializing Glybera, the first approved gene therapy in Europe, and announced in 2017 that it will not seek renewal of Glybera’s marketing authorization in Europe when it expired in October 2017, due to limited use since it was approved in 2012, and forecasted patient demand. Our efforts to educate the marketplace may require more resources than are required by the conventional technologies marketed by our competitors. If we obtain approvalWe intend to commercializemarket our product candidatesproducts outside of the United States, a variety of risks associated with international operations could materially adversely affect our business.
If any of our product candidates are approved for commercialization, we may enter into agreements with third parties to market them on a worldwide basis or in more limited geographical regions. We expect thatand we will be subject to additionalthe risks relatedof doing business outside of the United States.
Because we intend to entering into internationalmarket our product candidates, if approved, outside of the United States, our business relationships,is subject to risks associated with doing business outside of the United States. Accordingly, our business and financial results in the future could be adversely affected due to a variety of factors, including: ·
| different regulatory requirements for approval of drugs and biologics in foreign countries;
|
efforts to develop an international sales, marketing and distribution organization may increase our expenses, divert our management’s attention from the acquisition or development of product candidates or cause us to forgo profitable licensing opportunities in these geographies; ·
| reduced protection for intellectual property rights;
|
changes in a specific country’s or region’s political and cultural climate or economic condition; ·
| economic weakness, including inflation, or political instability in particular foreign economies and markets; and
|
unexpected changes in foreign laws and regulatory requirements; ·
difficulty of effective enforcement of contractual provisions in local jurisdictions; inadequate intellectual property protection in foreign countries; trade-protection measures, import or export licensing requirements such as Export Administration Regulations promulgated by the U.S. Department of Commerce and fines, penalties or suspension or revocation of export privileges; the effects of applicable foreign tax structures and potentially adverse tax consequences; and significant adverse changes in foreign currency exchange rates. In addition to FDA and related regulatory requirements in the United States and abroad, we are subject to extensive additional federal, state and foreign anti-bribery regulation, which include the U.S. Foreign Corrupt Practices Act, the U.K. Bribery Act, and similar laws in other countries outside of the United States. We have developed and implemented a corporate compliance program based on what we believe are current best practices in the pharmaceutical industry for companies similar to ours, but we cannot guarantee that we, our employees, our consultants or our third-party contractors are or will be in compliance with all federal, state and foreign regulations regarding bribery and corruption. Moreover, our partners and third party contractors located outside the United States may have inadequate compliance programs or may fail to respect the laws and guidance of the territories in which they operate. Even if we are not determined to have violated these laws, government investigations into these issues typically require the expenditure of significant resources and generate negative publicity, which could also have an adverse effect on our business, financial condition and results of operations | foreign currency fluctuations, which could result in increased operating expenses and reduced revenues, and other obligations incident to doing business in another country.
|
The insurance coverage and reimbursement status of newly-approved products is uncertain. Failure to obtain or maintain adequate coverage and reimbursement for new or current products could limit our ability to market those products and decrease our ability to generate revenue. The availability and extent of reimbursement by governmental and private payors is essential for most patients to be able to afford expensive treatments, such as stem cell transplants or gene therapy. In addition, because our CAR and TCR T cell product candidates represent new approaches to the treatment of cancer, we cannot accurately estimate the potential revenue. Sales of our product candidates will depend substantially, both domestically and abroad, on the extent to which the costs of our product candidates will be paid by health maintenance, managed care, pharmacy benefit and similar healthcare management organizations, or reimbursed by
government health administration authorities, private health coverage insurers and other third-party payors. If reimbursement is not available, or is available only to limited levels, we may not be able to successfully commercialize our product candidates. Even if coverage is provided, the approved reimbursement amount may not be high enough to allow us to establish or maintain pricing sufficient to realize a sufficient return on our investment. There is significant uncertainty related to the insurance coverage and reimbursement of newly approved products, including gene therapies. In the United States, the principal decisions about reimbursement for new medicines are typically made by the Centers for Medicare & Medicaid Services, or CMS, an agency within the U.S. Department of Health and Human Services, as CMS decides whether and to what extent a new medicine will be covered and reimbursed under Medicare. Private payors tend to follow CMS to a substantial degree. It is difficult to predict what CMS will decide with respect to reimbursement for fundamentally novel products such as ours, as there is no body of established practices and precedents for these new products. Reimbursement agencies in Europe may be more conservative than CMS. For example, a number of cancer drugs have been approved for reimbursement in the United 49
States and have not been approved for reimbursement in certain European countries. In addition, costs or difficulties associated with the reimbursement of Glybera could create an adverse environment for reimbursement of other gene therapies. Outside the United States, international operationscertain countries, including a number of member states of the European Union, set prices and reimbursement for pharmaceutical products, or medicinal products, as they are generally subjectcommonly referred to extensive governmental price controlsin the European Union, with limited participation from the marketing authorization holders. We cannot be sure that such prices and other market regulations,reimbursement will be acceptable to us or our collaborators. If the regulatory authorities in these foreign jurisdictions set prices or reimbursement levels that are not commercially attractive for us or our collaborators, our revenues from sales by us or our collaborators, and we believe the increasing emphasis on cost-containment initiatives in Europe, Canada, and other countries has and will continue to put pressure on the pricing and usagepotential profitability of our drug products, in those countries would be negatively affected. An increasing number of countries are taking initiatives to attempt to reduce large budget deficits by focusing cost-cutting efforts on pharmaceuticals for their state-run health care systems. These international price control efforts have impacted all regions of the world, but have been most drastic in the European Union. Additionally, some countries require approval of the sale price of a product candidates.before it can be marketed. In many countries, the pricespricing review period begins after marketing or product licensing approval is granted. As a result, we might obtain marketing approval for a product in a particular country, but then may experience delays in the reimbursement approval of medical products areour product or be subject to varying price control mechanisms as partregulations that would delay our commercial launch of national health systems. In general, the prices of medicines under such systems are substantially lower than inproduct, possibly for lengthy time periods, which could negatively impact the United States. Other countries allow companies to fix their own prices for medicines, but monitor and control company profits. Additional foreign price controls or other changes in pricing regulation could restrict the amount thatrevenues we are able to charge for ourgenerate from the sale of the product candidates. Accordingly, in markets outside the United States, the reimbursement for our products may be reduced compared with the United States and may be insufficient to generate commercially reasonable revenues and profits.that particular country. Moreover, increasing efforts by governmental and third-party payors, in the United States and abroad, to cap or reduce healthcare costs may cause such organizations to limit both coverage and level of reimbursement for new products approved and, as a result, they may not cover or provide adequate payment for our product candidates. We expect to experience pricing pressures in connection with the sale of any of our product candidates, due to the trend toward managed healthcare, the increasing influence of health maintenance organizations and additional legislative changes. The downward pressure on healthcare costs in general, particularly prescription drugs and surgical procedures and other treatments, has become very intense. As a result, increasingly high barriers are being erected to the entry of new products. Healthcare legislative reform measures may have a material adverse effect on our business and results of operations. In the United States, there have been and continue to be a number of legislative initiatives to contain healthcare costs. For example, in March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010, or the Affordable Care Act, was passed, which substantially changes the way health care is financed by both governmental and private insurers, and significantly impacts the U.S. pharmaceutical industry. The Affordable Care Act, among other things, increased the minimum Medicaid rebates owed by manufacturers under the Medicaid Drug Rebate Program and extended the rebate program to individuals enrolled in Medicaid managed care organizations, established annual fees and taxes on manufacturers of certain branded prescription drugs, and promoted a new Medicare Part D coverage gap discount program. In addition, other legislative changes have been proposed and adopted in the United States since the Affordable Care Act was enacted. On August 2, 2011, the Budget Control Act of 2011, among other things, created measures for spending reductions by Congress. A Joint Select Committee on Deficit Reduction, tasked with recommending a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, was unable to reach required goals, thereby triggering the legislation’s automatic reduction to several government programs. This includes aggregate reductions of Medicare payments to providers up to 2% per fiscal year. On January 2, 2013, then President Obama signed into law the American Taxpayer Relief Act of 2012, which, among other things, delayed for another two months the budget cuts mandated by these sequestration provisions of the Budget Control Act of 2011. On March 1, 2013, the President signed an executive order implementing sequestration, and on April 1, 2013, the 2% Medicare payment reductions went into effect. We expect that additional state and federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, which could result in reduced demand for our product candidates or additional pricing pressures. For each state that does not choose to expand its Medicaid program, there may be fewer insured patients overall, which could impact the sales, business and financial condition of
manufacturers of branded prescription drugs or other therapies. Where patients receive insurance coverage under any of the new options made available through the Affordable Care Act, the possibility exists that manufacturers may be required to pay Medicaid rebates on that resulting drug utilization, a decision that could impact manufacturer revenues. Some of the provisions of the Affordable Care Act have yet to be fully implemented, while certain provisions have been subject to judicial and Congressional challenges. In January 2017, Congress voted to adopt a budget resolution for fiscal year 2017, that while not a law, is widely viewed as the first step toward the passage of legislation that would repeal certain aspects of the Affordable Care Act. Further, on January 20, 2017, President Trump signed an Executive Order directing federal agencies with authorities and responsibilities under the Affordable Care Act to waive, defer, grant exemptions from, or delay the implementation of any provision of the Affordable Care Act that would impose a fiscal burden on states or a cost, fee, tax, penalty or regulatory burden on individuals, healthcare providers, health insurers, or manufacturers of pharmaceuticals or medical devices. Congress also could consider subsequent legislation to replace elements of the Affordable Care Act that are repealed. With the new Administration and Congress, there will likely be additional administrative or legislative changes, including modification, repeal, or replacement of all, or certain provisions of, the Affordable Care Act. However, it remains to be seen whether new legislation modifying the Affordable Care Act is enacted and, if so, precisely what the new legislation will provide, when it will be enacted and what impact it will have on the availability of healthcare and containing or lowering the cost of healthcare.The implications of a potential repeal and/or replacement of the Affordable Care Act, for our and our partners’ business and financial condition, if any, are not yet clear. The delivery of healthcare in the European Union, including the establishment and operation of health services and the pricing and reimbursement of medicines, is almost exclusively a matter for national, rather than EU, law and policy. National governments and health service providers have different priorities and approaches to the delivery of health care and the pricing and reimbursement of products in that context. In general, however, the healthcare budgetary constraints in most EU member states have resulted in restrictions on the pricing and reimbursement of medicines by relevant health service providers. Coupled with ever-increasing EU and national regulatory burdens on those wishing to develop and market products, this could prevent or delay marketing approval of our product candidates, restrict or regulate post-approval activities and affect our ability to commercialize any products for which we obtain marketing approval. We cannot predict what healthcare reform initiatives may be adopted in the future. Further federal, state and foreign legislative and regulatory developments are likely, and we expect ongoing initiatives to increase pressure on drug pricing. Such reforms could have an adverse effect on anticipated revenues from product candidates that we may successfully develop and for which we may obtain regulatory approval and may affect our overall financial condition and ability to develop product candidates. Due to the novel nature of our technology and the potential for our product candidates to offer therapeutic benefit in a single administration, we face uncertainty related to pricing and reimbursement for these product candidates. Our target patient populations are relatively small, as a result, the pricing and reimbursement of our product candidates, if approved, must be adequate to support commercial infrastructure. If we are unable to obtain adequate levels of reimbursement, our ability to successfully market and sell our product candidates will be adversely affected. The manner and level at which reimbursement is provided for services related to our product candidates (e.g., for administration of our product to patients) is also important. Inadequate reimbursement for such services may lead to physician resistance and adversely affect our ability to market or sell our products. If the market opportunities for our product candidates are smaller than we believe they are, our revenues may be adversely affected and our business may suffer. Because the target patient populations of our product candidates are small, we must be able to successfully identify patients and achieve a significant market share to maintain profitability and growth. We focus our research and product development on treatments for severe genetic and rare diseases. Our projections of both the number of people who have these diseases, as well as the subset of people with these diseases who have the potential to benefit from treatment with our product candidates, are based on estimates. These estimates may prove to be incorrect and new studies may change the estimated incidence or prevalence of these diseases. The number of patients in the United States, Europe and elsewhere may turn out to be lower than expected, may not be otherwise amenable to treatment with our products, or new patients may become increasingly difficult to identify or gain access to, all of which would adversely affect our results of operations and our business. The market opportunities for our T cell-based immunotherapy product candidates may be limited to those patients who are ineligible for or have failed prior treatments and may be small. The FDA often approves new therapies initially only for use in patients with relapsed or refractory advanced disease. We expect to initially seek approval of our T cell-based product candidates in cancer in this context. Subsequently, for those products that prove to be sufficiently beneficial, if any, we would expect to seek approval in earlier lines of treatment and potentially as a first line therapy,
but there is no guarantee that our product candidates, even if approved, would be approved for earlier lines of therapy, and, prior to any such approvals, we may have to conduct additional clinical trials. Our projections of both the number of people who have the cancers we may be targeting, as well as the subset of people with these cancers in a position to receive second or third line therapy, and who have the potential to benefit from treatment with our product candidates, are based on our beliefs and estimates. These estimates have been derived from a variety of sources, including scientific literature, surveys of clinics, patient foundations, or market research, and may prove to be incorrect. Further, new studies may change the estimated incidence or prevalence of these cancers. The number of patients may turn out to be lower than expected. Additionally, the potentially addressable patient population for our product candidates may be limited or may not be amenable to treatment with our product candidates. Even if we obtain significant market share for our product candidates, because the potential target populations are small, we may never achieve profitability without obtaining regulatory approval for additional indications. Risks related to our business operations If we undertake business combinations, collaborations or similar strategic transactions, they may disrupt our business, divert management’s attention, dilute stockholder value or be difficult to integrate. On a regular basis, we consider various business combination transactions, collaborations, license agreements and strategic transactions with third parties, including transactions which may result in us acquiring, or being acquired by, a third party. The consummation or performance of any future business combination, collaboration or strategic transaction may involve risks, such as: ·
| diversion of managerial resources from day-to-day operations;
|
diversion of managerial resources from day-to-day operations; ·
| challenges associated with integrating acquired technologies and operations of acquired companies;
|
challenges associated with integrating acquired technologies and operations of acquired companies; ·
| exposure to unforeseen liabilities;
|
exposure to unforeseen liabilities; ·
| difficulties in the assimilation of different cultures and practices, as well as in the assimilation and retention of broad and geographically dispersed personnel and operations;
|
difficulties in the assimilation of different cultures and practices, as well as in the assimilation and retention of broad and geographically dispersed personnel and operations; ·
| misjudgment with respect to value, return on investment or strategic fit;
|
misjudgment with respect to value, return on investment or strategic fit; ·
| higher than expected transaction costs; and |
50additional dilution to our existing stockholders if we issue equity securities as consideration for any acquisitions.
·
| additional dilution to our existing stockholders if we issue equity securities as consideration for any acquisitions.
|
As a result of these risks, we may not be able to achieve the expected benefits of any such transaction. If we are unsuccessful in completing or integrating any acquisition, we may be required to reevaluate that component of our strategy only after we have incurred substantial expenses and devoted significant management time and resources in seeking to complete and integrate the acquisition. Future business combinations could involve the acquisition of significant intangible assets. We may need to record write-downs from future impairments of identified intangible assets and goodwill. These accounting charges would increase a reported loss or reduce any future reported earnings. In addition, we could use substantial portions of our available cash to pay the purchase price for company or product candidate acquisitions. Subject to the limitations under our existing indebtedness, it is possible that we could incur additional debt or issue additional equity securities as consideration for these acquisitions, which could cause our stockholders to suffer significant dilution. The failure to successfully integrate Precision Genome Engineering, Inc.’s business and operations or fully realize the benefits of this acquisition may adversely affect our future results.
On June 30, 2014, we acquired all of the outstanding capital stock of Precision Genome Engineering, Inc., or Pregenen. Based in Seattle, Washington, Pregenen is focused on the development of gene editing and cell signaling technologies. The success of our acquisition of Pregenen will depend, in part, on our ability to successfully integrate Pregenen’s business and operations and fully realize the anticipated benefits and synergies from combining our business with Pregenen’s business, in particular our ability to advance Pregenen’s gene editing and cell signaling technologies to the stage where they can be incorporated into our existing or new product candidates. However, to realize these anticipated benefits, we must successfully combine these businesses and continue the research and development activities previously undertaken by Pregenen as a stand-alone company. If we are unable to achieve these objectives, the anticipated benefits of our acquisition of Pregenen may not be realized fully or at all or may take longer to realize than expected. Any failure to timely realize these anticipated benefits could have a material adverse effect on our development programs, expenses and operating results.
Negative public opinion and increased regulatory scrutiny of gene therapy and genetic research may damage public perception of our product candidates or adversely affect our ability to conduct our business or obtain regulatory approvals for our product candidates. Public perception may be influenced by claims that gene therapy is unsafe, and gene therapy may not gain the acceptance of the public or the medical community. In particular, our success will depend upon physicians specializing in the treatment of those diseases that our product candidates target prescribing treatments that involve the use of our product candidates in lieu of, or in addition to, existing treatments they are already familiar with and for which greater clinical data may be available. More restrictive government regulations or negative public opinion would have a negative effect on our business or financial condition and may delay or impair the development and commercialization of our product candidates or demand for any products we may develop. For example, in 2003, 20 subjects treated for X-linked severe combined immunodeficiency in two gene therapy studies using a murine gamma-retroviral vector showed correction of the disease, but the studies were terminated after five subjects developed leukemia (four of whom were subsequently cured). Although none of our current product candidates utilize these gamma-retroviruses, our product candidates use a viral delivery system. Adverse events in our clinical studies, even if not ultimately attributable to our product candidates (such as the
many adverse events that typically arise from the transplant process) and the resulting publicity could result in increased governmental regulation, unfavorable public perception, potential regulatory delays in the testing or approval of our potential product candidates, stricter labeling requirements for those product candidates that are approved and a decrease in demand for any such product candidates. Our future success depends on our ability to retain key employees, consultants and advisors and to attract, retain and motivate qualified personnel. We are highly dependent on principal members of our executive team and key employees, the loss of whose services may adversely impact the achievement of our objectives. While we have entered into employment agreements with each of our executive officers, any of them could leave our employment at any time, as all of our employees are “at will” employees. Recruiting and retaining other qualified employees, consultants and advisors for our business, including scientific and technical personnel, will also be critical to our success. There is currently a shortage of skilled executives in our industry, which is likely to continue. As a result, competition for skilled personnel is intense and the turnover rate can be high. We may not be able to attract and retain personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for individuals with similar skill sets. In addition, failure to succeed in preclinical or clinical studies may make it more challenging to recruit and retain qualified personnel. The inability to recruit or loss of the services of any executive, key employee, consultant or advisor may impede the progress of our research, development and commercialization objectives. 51
We will need to expand our organization and we may experience difficulties in managing this growth, which could disrupt our operations. As of January 31, 2015,2018, we had 143479 full-time employees. As our business research and development activities expand, and as we continue the activities required under our collaboration with Celgene, we expect to expand our full-time employee base and to hire more consultants and contractors. Our management may need to divert a disproportionate amount of its attention away from our day-to-day activities and devote a substantial amount of time to managing these growth activities. We may not be able to effectively manage the expansion of our operations, which may result in weaknesses in our infrastructure, operational mistakes, loss of business opportunities, loss of employees and reduced productivity among remaining employees. Our expected growth could require significant capital expenditures and may divert financial resources from other projects, such as the development of additional product candidates. If our management is unable to effectively manage our growth, our expenses may increase more than expected, our ability to generate and/or grow revenues could be reduced, and we may not be able to implement our business strategy. Our employees, principal investigators, consultants and commercial partners may engage in misconduct or other improper activities, including non-compliance with regulatory standards and requirements and insider trading. We are exposed to the risk of fraud or other misconduct by our employees, principal investigators, consultants and commercial partners. Misconduct by these parties could include intentional failures to comply with the regulations of the FDA and non-U.S. regulators, provide accurate information to the FDA and non-U.S. regulators, comply with healthcare fraud and abuse laws and regulations in the United States and abroad, report financial information or data accurately or disclose unauthorized activities to us. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Such misconduct could also involve the improper use of information obtained in the course of clinical studies, which could result in regulatory sanctions and cause serious harm to our reputation or could cause regulatory agencies not to approve our product candidates. We have adopted a code of conduct applicable to all of our employees, but it is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of significant fines or other sanctions.
We face potential product liability, and, if successful claims are brought against us, we may incur substantial liability and costs. If the use of our product candidates harms patients, or is perceived to harm patients even when such harm is unrelated to our product candidates, our regulatory approvals could be revoked or otherwise negatively impacted and we could be subject to costly and damaging product liability claims. The use of our product candidates in clinical studies and the sale of any products for which we obtain marketing approval exposes us to the risk of product liability claims. Product liability claims might be brought against us by subjects participating in clinical trials, consumers, healthcare providers, pharmaceutical companies or others selling or otherwise coming into contact with our products. There is a risk that our product candidates may induce adverse events. If we cannot successfully defend against product liability claims, we could incur substantial liability and costs. In addition, regardless of merit or eventual outcome, product liability claims may result in: ·
| impairment of our business reputation;
|
impairment of our business reputation; ·
| withdrawal of clinical study participants;
|
withdrawal of clinical study participants; ·
| costs due to related litigation;
|
costs due to related litigation; ·
| distraction of management’s attention from our primary business;
|
distraction of management’s attention from our primary business; ·
| substantial monetary awards to patients or other claimants;
|
substantial monetary awards to patients or other claimants; ·
| the inability to commercialize our product candidates; and
|
the inability to commercialize our product candidates; and ·
| decreased demand for our product candidates, if approved for commercial sale.
|
decreased demand for our product candidates, if approved for commercial sale. We carry product liability insurance and we believe our product liability insurance coverage is sufficient in light of our current clinical programs; however, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses due to liability. If and when we obtain marketing approval for product candidates, we intend to expand our insurance coverage to include the sale of commercial products; however, we may be unable to obtain product liability insurance on commercially reasonable terms or in adequate amounts. On occasion, large judgments have been awarded in class action lawsuits based on drugs or medical treatments that had unanticipated adverse effects. A successful product liability claim or series of claims 52
brought against us could cause our stock price to decline and, if judgments exceed our insurance coverage, could adversely affect our results of operations and business. Patients with the diseases targeted by our product candidates are often already in severe and advanced stages of disease and have both known and unknown significant pre-existing and potentially life-threatening health risks. During the course of treatment, patients may suffer adverse events, including death, for reasons that may be related to our product candidates. Such events could subject us to costly litigation, require us to pay substantial amounts of money to injured patients, delay, negatively impact or end our opportunity to receive or maintain regulatory approval to market our products, or require us to suspend or abandon our commercialization efforts. Even in a circumstance in which we do not believe that an adverse event is related to our products, the investigation into the circumstance may be time-consuming or inconclusive. These investigations may interrupt our sales efforts, delay our regulatory approval process in other countries, or impact and limit the type of regulatory approvals our product candidates receive or maintain. As a result of these factors, a product liability claim, even if successfully defended, could have a material adverse effect on our business, financial condition or results of operations. If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on the success of our business. We are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes. Our operations involve the use of hazardous and flammable materials, including chemicals and biological materials. Our operations also produce hazardous waste products. We generally contract with third parties for the disposal of these materials and wastes. We cannot eliminate the risk of contamination or injury from these materials. In the event of contamination or injury resulting from our use of hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties. Although we maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials or other work-related injuries, this insurance may not provide adequate coverage against potential liabilities. In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations may impair our research,
development or production efforts. Failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions. We may not be successful in our efforts to identify or discover additional product candidates. The success of our business depends primarily upon our ability to identify, develop and commercialize products based on our gene therapy and gene editing platforms. Although our LentiGlobin, Lenti-D, bb2121 and LentiGlobinbb21217 product candidates are currently in clinical development, our research programs, including those subject to our collaboration with Celgene,oncology research programs, may fail to identify other potential product candidates for clinical development for a number of reasons. Our research methodology may be unsuccessful in identifying potential product candidates or our potential product candidates may be shown to have harmful side effects or may have other characteristics that may make the products unmarketable or unlikely to receive marketing approval. If any of these events occur, we may be forced to abandon our development efforts for a program or programs, which would have a material adverse effect on our business and could potentially cause us to cease operations. Research programs to identify new product candidates require substantial technical, financial and human resources. We may focus our efforts and resources on potential programs or product candidates that ultimately prove to be unsuccessful. We may use our financial and human resources to pursue a particular research program or product candidate and fail to capitalize on programs or product candidates that may be more profitable or for which there is a greater likelihood of success. Because we have limited resources, we may forego or delay pursuit of opportunities with certain programs or product candidates or for indications that later prove to have greater commercial potential. Our resource allocation decisions may cause us to fail to capitalize on viable commercial products or profitable market opportunities. Our spending on current and future research and development programs for product candidates may not yield any commercially viable products. If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through strategic collaboration, licensing or other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to such product candidate, or we may allocate internal resources to a product candidate in a therapeutic area in which it would have been more advantageous to enter into a partnering arrangement. 53
We incur significant increased costs as a result of operating as a public company, and our management devotes substantial time to new compliance initiatives. As a public company, we have incurred and will continue to incur significant legal, accounting and other expenses. In addition, the Sarbanes-Oxley Act, as well as rules subsequently implemented by the SEC, and The NasdaqNASDAQ Global Select Market have imposed various requirements on public companies. In July 2010, the Dodd-Frank Wall Street Reform and Consumer Protection Act, or the Dodd-Frank Act, was enacted, resulting in significant corporate governance and executive compensation-related regulations. Stockholder activism, the current political environment and the current high level of government intervention and regulatory reform may lead to substantial new regulations and disclosure obligations, which may lead to additional compliance costs and impact the manner in which we operate our business in ways we cannot currently anticipate. Our management and other personnel will need to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations will increase our legal and financial compliance costs and will make some activities more time-consuming and costly. For example, we expect these rules and regulations to make it more difficult and more expensive for us to obtain director and officer liability insurance and we may be required to incur substantial costs to maintain our current levels of such coverage. Comprehensive Tax Reform Legislation Could Adversely Affect Our Business And Financial Condition. On December 31, 2014, we ceased22, 2017, the “Tax Cuts and Jobs Act” (TCJA) was enacted. The TCJA significantly reforms the Internal Revenue Code of 1986, as amended (the “Code”). The TCJA, among other things, includes changes to be an “emerging growth company,”U.S. federal tax rates, imposes significant additional limitations on the deductibility of interest and net operating loss carryforwards, allows for the expensing of capital expenditures, and puts into effect the migration from a “worldwide” system of taxation to a territorial system. Our net deferred tax assets and liabilities have been revalued at the newly enacted U.S. corporate tax rate, and the reduced disclosure requirements applicableimpact of the reduction to emerging growth companies no longer apply to us. On December 31, 2014, we ceased to be an “emerging growth company,” as definedour deferred tax assets and associated valuation allowance was recognized in the Jumpstart Our Business Startups Actcurrent period. We continue to examine the impact this tax reform legislation may have on our business. The impact of 2012,this tax reform is uncertain and the reduced disclosure requirements applicable to emerging growth companies no longer apply to us. As a large accelerated filer, we are now subject to certain disclosure requirements that are applicable to other public companies that have not been applicable to us as an emerging growth company. These requirements include:could be adverse.
·
| compliance with the auditor attestation requirements in the assessment of our internal control over financial reporting;
|
·
| compliance with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements;
|
·
| full disclosure obligations regarding executive compensation; and
|
·
| compliance with the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved.
|
Risks related to our intellectual property If we are unable to obtain or protect intellectual property rights related to our product candidates, we may not be able to compete effectively in our markets. We rely upon a combination of patents, trade secret protection and confidentiality agreements to protect the intellectual property related to our product candidates. The strength of patents in the biotechnology and pharmaceutical field involves complex legal and scientific questions and can be uncertain. The patent applications that we own or in-license may fail to result in issued patents with claims that cover our product candidates in the United States or in other foreign countries. There is no assurance that all of the potentially relevant prior art relating to our patents and patent applications has been found, which can invalidate a patent or prevent a patent from issuing from a pending patent application. Even if patents do successfully issue and even if such patents cover our product candidates, third parties may challenge their validity, enforceability or scope, which may result in such patents being narrowed or invalidated. Furthermore, even if they are unchallenged, our patents and patent applications may not adequately protect our intellectual property, provide exclusivity for our product candidates or prevent others from designing around our claims. Any of these outcomes could impair our ability to prevent competition from third parties, which may have an adverse impact on our business. If the patent applications we hold or have in-licensed with respect to our programs or product candidates fail to issue, if their breadth or strength of protection is threatened, or if they fail to provide meaningful exclusivity for our product candidates, it could dissuade companies from collaborating with us to develop product candidates, and threaten our ability to commercialize, future products. Several patent applications covering our product candidates have been filed recently. We cannot offer any assurances about which, if any, patents will issue, the breadth of any such patent or whether any issued patents will be found invalid and unenforceable or will be threatened by third parties. Any successful opposition to these patents or any other patents owned by or licensed to us could deprive us of rights necessary for the successful commercialization of any product candidates that we may develop. Further, if we encounter delays in regulatory approvals, the period of time during which we could market a product candidate under patent protection could be reduced. Since patent applications in the United States and most other countries are confidential for a period of time after filing, and some remain so until issued, we cannot be certain that we were the first to file any patent application related to a product candidate. Furthermore, if third parties have filed such patent applications, an interference proceeding in the United States can be initiated by a third party to determine who was the first to invent any of the subject matter covered by the patent claims of our applications. In addition, patents have a limited lifespan. In the United States, the natural expiration of a patent is generally 20 years after it is filed. Various extensions may be available however the life of a patent, and the protection it affords, is limited. Even if 54
patents covering our product candidates are obtained, once the patent life has expired for a product, we may be open to competition from generic medications. In addition to the protection afforded by patents, we rely on trade secret protection and confidentiality agreements to protect proprietary know-how that is not patentable or that we elect not to patent, processes for which patents are difficult to enforce and any other elements of our product candidate discovery and development processes that involve proprietary know-how, information or technology that is not covered by patents. However, trade secrets can be difficult to protect. We seek to protect our proprietary technology and processes, in part, by entering into confidentiality agreements with our employees, consultants, scientific advisors and contractors. We also seek to preserve the integrity and confidentiality of our data and trade secrets by maintaining physical security of our premises and physical and electronic security of our information technology systems. While we have confidence in these individuals, organizations and systems, agreements or security measures may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. Although we expect all of our employees and consultants to assign their inventions to us, and all of our employees, consultants, advisors and any third parties who have access to our proprietary know-how, information or technology to enter into confidentiality agreements, we cannot provide any assurances that all such agreements have been duly executed or that our trade secrets and other confidential proprietary information will not be disclosed or that competitors will not otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. Misappropriation or unauthorized disclosure of our trade secrets could impair our competitive position and may have a material adverse effect on our business. Additionally, if the steps taken to maintain our trade secrets are deemed inadequate, we may have insufficient recourse against third parties for misappropriating the trade secret. In addition, others may independently discover our trade secrets and proprietary information. For example, the FDA, as part of its Transparency Initiative, is currently considering whether to make additional information publicly available on a routine basis, including information that we may consider to be trade secrets or other proprietary information, and it is not clear at the present time how the FDA’s disclosure policies may change in the future, if at all. Further, the laws of some foreign countries do not protect proprietary rights to the same extent or in the same manner as the laws of the United States. As a result, we may encounter significant problems in protecting and defending our intellectual property both in the United States and abroad. If we are unable to prevent material disclosure of the non-patented intellectual property related to our technologies to third parties, and there is no guarantee that we will have any such enforceable trade secret protection, we may not be
able to establish or maintain a competitive advantage in our market, which could materially adversely affect our business, results of operations and financial condition. Third-party claims of intellectual property infringement may prevent or delay our development and commercialization efforts. Our commercial success depends in part on our avoiding infringement of the patents and proprietary rights of third parties. There is a substantial amount of litigation, both within and outside the United States, involving patent and other intellectual property rights in the biotechnology and pharmaceutical industries, including patent infringement lawsuits, interferences, oppositions, ex parte reexaminations, post-grant review, and inter partes reexaminationreview proceedings before the U.S. Patent and Trademark Office, or U.S. PTO, and corresponding foreign patent offices. Numerous U.S. and foreign issued patents and pending patent applications, which are owned by third parties, exist in the fields in which we are pursuing development candidates. As the biotechnology and pharmaceutical industries expand and more patents are issued, the risk increases that our product candidates may be subject to claims of infringement of the patent rights of third parties. Third parties may assert that we are employing their proprietary technology without authorization. There may be third-party patents or patent applications with claims to materials, formulations, methods of manufacture or methods for treatment related to the use or manufacture of our product candidates. Because patent applications can take many years to issue, there may be currently pending patent applications which may later result in issued patents that our product candidates may infringe. In addition, third parties may obtain patents in the future and claim that use of our technologies infringes upon these patents. If any third-party patents were held by a court of competent jurisdiction to cover the manufacturing process of any of our product candidates, any molecules formed during the manufacturing process or any final product itself, the holders of any such patents may be able to block our ability to commercialize such product candidate unless we obtained a license under the applicable patents, or until such patents expire. Similarly, if any third-party patents were held by a court of competent jurisdiction to cover aspects of our formulations, processes for manufacture or methods of use, including combination therapy, the holders of any such patents may be able to block our ability to develop and commercialize the applicable product candidate unless we obtained a license or until such patent expires. In either case, such a license may not be available on commercially reasonable terms or at all. Parties making claims against us may obtain injunctive or other equitable relief, which could effectively block our ability to further develop and commercialize one or more of our product candidates. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources from our business. In the event of a successful claim of infringement against us, we may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement, pay royalties, redesign our infringing products or obtain one or more licenses from third parties, which may be impossible or require substantial time and monetary expenditure. 55
We may not be successful in obtaining or maintaining necessary rights to gene therapy product components and processes for our development pipeline through acquisitions and in-licenses. Presently we have rights to the intellectual property, through licenses from third parties and under patents that we own, to develop our gene therapy product candidates. Because our programs may involve additional product candidates that may require the use of proprietary rights held by third parties, the growth of our business will likely depend in part on our ability to acquire, in-license or use these proprietary rights. In addition, our product candidates may require specific formulations to work effectively and efficiently and these rights may be held by others. We may be unable to acquire or in-license any compositions, methods of use, processes or other third-party intellectual property rights from third parties that we identify. The licensing and acquisition of third-party intellectual property rights is a competitive area, and a number of more established companies are also pursuing strategies to license or acquire third-party intellectual property rights that we may consider attractive. These established companies may have a competitive advantage over us due to their size, cash resources and greater clinical development and commercialization capabilities. For example, we sometimes collaborate with U.S. and foreign academic institutions to accelerate our preclinical research or development under written agreements with these institutions. Typically, these institutions provide us with an option to negotiate a license to any of the institution’s rights in technology resulting from the collaboration. Regardless of such right of first negotiation for intellectual property, we may be unable to negotiate a license within the specified time frame or under terms that are acceptable to us. If we are unable to do so, the institution may offer the intellectual property rights to other parties, potentially blocking our ability to pursue our program. In addition, companies that perceive us to be a competitor may be unwilling to assign or license rights to us. We also may be unable to license or acquire third-party intellectual property rights on terms that would allow us to make an appropriate return on our investment. If we are unable to successfully obtain rights to required third-party intellectual property rights, our business, financial condition and prospects for growth could suffer.
If we fail to comply with our obligations in the agreements under which we license intellectual property rights from third parties or otherwise experience disruptions to our business relationships with our licensors, we could lose license rights that are important to our business. We are a party to a number of intellectual property license agreements that are important to our business and expect to enter into additional license agreements in the future. Our existing license agreements impose, and we expect that future license agreements will impose, various diligence, milestone payment, royalty and other obligations on us. If we fail to comply with our obligations under these agreements, or we are subject to a bankruptcy, the licensor may have the right to terminate the license, in which event we would not be able to market products covered by the license. We may need to obtain licenses from third parties to advance our research or allow commercialization of our product candidates, and we have done so from time to time. We may fail to obtain any of these licenses at a reasonable cost or on reasonable terms, if at all. In that event, we may be required to expend significant time and resources to develop or license replacement technology. If we are unable to do so, we may be unable to develop or commercialize the affected product candidates, which could harm our business significantly. We cannot provide any assurances that third-party patents do not exist which might be enforced against our current product candidates or future products, resulting in either an injunction prohibiting our sales, or, with respect to our sales, an obligation on our part to pay royalties and/or other forms of compensation to third parties. In many cases, patent prosecution of our licensed technology is controlled solely by the licensor. If our licensors fail to obtain and maintain patent or other protection for the proprietary intellectual property we license from them, we could lose our rights to the intellectual property or our exclusivity with respect to those rights, and our competitors could market competing products using the intellectual property. In certain cases, we control the prosecution of patents resulting from licensed technology. In the event we breach any of our obligations related to such prosecution, we may incur significant liability to our licensing partners. Licensing of intellectual property is of critical importance to our business and involves complex legal, business and scientific issues and is complicated by the rapid pace of scientific discovery in our industry. Disputes may arise regarding intellectual property subject to a licensing agreement, including: ·
| the scope of rights granted under the license agreement and other interpretation-related issues;
|
the scope of rights granted under the license agreement and other interpretation-related issues; ·
| the extent to which our technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement;
|
the extent to which our technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement; ·
| the sublicensing of patent and other rights under our collaborative development relationships;
|
the sublicensing of patent and other rights under our collaborative development relationships; ·
| our diligence obligations under the license agreement and what activities satisfy those diligence obligations;
|
our diligence obligations under the license agreement and what activities satisfy those diligence obligations; ·
| the ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and us and our partners; and |
56the priority of invention of patented technology.
·
| the priority of invention of patented technology.
|
If disputes over intellectual property that we have licensed prevent or impair our ability to maintain our current licensing arrangements on acceptable terms, we may be unable to successfully develop and commercialize the affected product candidates. We may be involved in lawsuits to protect or enforce our patents or the patents of our licensors, which could be expensive, time-consuming and unsuccessful. Competitors may infringe our patents or the patents of our licensors. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time-consuming. In addition, in an infringement proceeding, a court may decide that a patent of ours or our licensors is not valid, is unenforceable and/or is not infringed, or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation or defense proceedings could put one or more of our patents at risk of being invalidated or interpreted narrowly and could put our patent applications at risk of not issuing. Interference proceedings provoked by third parties or brought by us may be necessary to determine the priority of inventions with respect to our patents or patent applications or those of our licensors. An unfavorable outcome could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms. Our defense of litigation or interference proceedings may fail and, even if successful, may result in substantial costs and distract our management and other employees. We may not be able to prevent, alone or with our licensors, misappropriation of our intellectual property rights, particularly in countries where the laws may not protect those rights as fully as in the United States.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. There could also be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a material adverse effect on the price of our common stock. Recent patentPatent reform legislation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents.
On September 16, 2011, the Leahy-Smith America Invents Act, or the Leahy-Smith Act, was signed into law. The Leahy-Smith Act includes a number of significant changes to U.S. patent law, including provisions that affect the way patent applications will be prosecuted and may also affect patent litigation. The U.S. PTO is currently developing regulations and procedures to govern administration of the Leahy-Smith Act, and many of the substantive changes to patent law associated with the Leahy-Smith Act, and in particular, the first to file provisions, were enacted March 16, 2013. However, it is not clear what, if any, impact the Leahy-Smith Act will have on the operation of our business. However, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business and financial condition. We may be subject to claims that our employees, consultants or independent contractors have wrongfully used or disclosed confidential information of third parties or that our employees have wrongfully used or disclosed alleged trade secrets of their former employers. We employ individuals who were previously employed at universities or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we try to ensure that our employees, consultants and independent contractors do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise used or disclosed intellectual property, including trade secrets or other proprietary information, of any of our employee’s former employer or other third parties. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel, which could adversely impact our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees. We may be subject to claims challenging the inventorship or ownership of our patents and other intellectual property. We may also be subject to claims that former employees, collaborators or other third parties have an ownership interest in our patents or other intellectual property. We have had in the past, and we may also have to in the future, ownership disputes arising, for example, from conflicting obligations of consultants or others who are involved in developing our product candidates. Litigation may be necessary to defend against these and other claims challenging inventorship or ownership. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or right to 57
use, valuable intellectual property. Such an outcome could have a material adverse effect on our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees. Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements. Periodic maintenance fees, renewal fees, annuity fees and various other governmental fees on patents and/or applications will be due to be paid to the U.S. PTO and various governmental patent agencies outside of the United States in several stages over the lifetime of the patents and/or applications. We have systems in place to remind us to pay these fees, and we employ an outside firm and rely on our outside counsel to pay these fees due to non-U.S. patent agencies. The U.S. PTO and various non-U.S. governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. We employ reputable law firms and other professionals to help us comply, and in many cases, an inadvertent lapse can be cured by payment of a late fee or by other means in accordance with the applicable rules. However, there are situations in which non-compliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, our competitors might be able to enter the market and this circumstance would have a material adverse effect on our business. Issued patents covering our product candidates could be found invalid or unenforceable if challenged in court. If we or one of our licensing partners initiated legal proceedings against a third party to enforce a patent covering one of our product candidates, the defendant could counterclaim that the patent covering our product candidate is invalid and/or unenforceable. In patent
litigation in the United States, defendant counterclaims alleging invalidity and/or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including patent eligible subject matter, lack of novelty, obviousness or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the U.S. PTO, or made a misleading statement, during prosecution. Third parties may also raise similar claims before administrative bodies in the United States or abroad, even outside the context of litigation. Such mechanisms include re-examination, post grant review, and equivalent proceedings in foreign jurisdictions (e.g., opposition proceedings). Such proceedings could result in revocation or amendment to our patents in such a way that they no longer cover our product candidates. The outcome following legal assertions of invalidity and unenforceability is unpredictable. With respect to the validity question, for example, we cannot be certain that there is no invalidating prior art, of which we and the patent examiner were unaware during prosecution. If a defendant were to prevail on a legal assertion of invalidity and/or unenforceability, we would lose at least part, and perhaps all, of the patent protection on our product candidates. Such a loss of patent protection would have a material adverse impact on our business. Changes in U.S. patent law could diminish the value of patents in general, thereby impairing our ability to protect our products. As is the case with other biotechnology companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the biotechnology industry involve both technological and legal complexity, and is therefore obtaining and enforcing biotechnology patents is costly, time-consuming and inherently uncertain. In addition, the United States has recently enacted and is currently implementing wide-ranging patent reform legislation. Recent U.S. Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the federal courts, and the U.S. PTO, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future. We may not be able to protect our intellectual property rights throughout the world. Filing, prosecuting and defending patents on product candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States can be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and further, may export otherwise infringing products to territories where we have patent protection, but enforcement is not as strong as that in the United States. These products may compete with our products and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing. Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, 58
trade secrets and other intellectual property protection, particularly those relating to biotechnology products, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license. Risks related to ownership of our common stock The market price of our common stock may be highly volatile, and you may not be able to resell your shares at or above the price at which you purchase them. Companies trading in the stock market in general, and The NasdaqNASDAQ Global Select Market in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Broad market and biotechnology and pharmaceutical industry factors may negatively affect the market price of our common stock, regardless of our actual operating performance.
The market price of our common stock may be volatile. Our stock price could be subject to wide fluctuations in response to a variety of factors, including the following: ·
| adverse results or delays in preclinical or clinical studies;
|
adverse results or delays in preclinical or clinical studies; ·
| reports of adverse events in other gene therapy products or clinical studies of such products;
|
reports of adverse events in other gene therapy products or clinical studies of such products; ·
| inability to obtain additional funding;
|
inability to obtain additional funding; ·
| any delay in filing an IND or BLA for any of our product candidates and any adverse development or perceived adverse development with respect to the FDA’s review of that IND or BLA;
|
any delay in filing an IND, MAA or BLA for any of our product candidates and any adverse development or perceived adverse development with respect to the FDA’s review of that IND, MAA or BLA; ·
| failure to develop successfully and commercialize our product candidates;
|
failure to develop successfully and commercialize our product candidates; ·
| failure to maintain our existing strategic collaborations or enter into new collaborations;
|
failure to maintain our existing strategic collaborations or enter into new collaborations; ·
| failure by us or our licensors and strategic collaboration partners to prosecute, maintain or enforce our intellectual property rights;
|
failure by us or our licensors and strategic collaboration partners to prosecute, maintain or enforce our intellectual property rights; ·
| changes in laws or regulations applicable to future products;
|
changes in laws or regulations applicable to future products; ·
| inability to obtain adequate product supply for our product candidates or the inability to do so at acceptable prices;
|
inability to obtain adequate product supply for our product candidates or the inability to do so at acceptable prices; ·
| adverse regulatory decisions;
|
adverse regulatory decisions; ·
| introduction of new products, services or technologies by our competitors;
|
introduction of new products, services or technologies by our competitors; ·
| failure to meet or exceed financial projections we may provide to the public;
|
failure to meet or exceed financial projections we may provide to the public; ·
| failure to meet or exceed the financial projections of the investment community;
|
failure to meet or exceed the financial projections of the investment community; ·
| the perception of the pharmaceutical industry by the public, legislatures, regulators and the investment community;
|
the perception of the pharmaceutical industry by the public, legislatures, regulators and the investment community; ·
| announcements of significant acquisitions, strategic partnerships, joint ventures or capital commitments by us, our strategic collaboration partner or our competitors;
|
announcements of significant acquisitions, strategic partnerships, joint ventures or capital commitments by us, our strategic collaboration partner or our competitors; ·
| disputes or other developments relating to proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our technologies;
|
disputes or other developments relating to proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our technologies; ·
| additions or departures of key scientific or management personnel;
|
additions or departures of key scientific or management personnel; ·
| significant lawsuits, including patent or stockholder litigation;
|
significant lawsuits, including patent or stockholder litigation; ·
| changes in the market valuations of similar companies;
|
changes in the market valuations of similar companies; ·
| sales of our common stock by us or our stockholders in the future; and
|
sales of our common stock by us or our stockholders in the future; and ·
| trading volume of our common stock. |
59
Actual or potential sales of our common stock by our employees, including our executive officers, pursuant to pre-arranged stock trading plans could cause our stock price to fall or prevent it from increasing for numerous reasons, and actual or potential sales by such persons could be viewed negatively by other investors. In accordance with the guidelines specified under Rule 10b5-1 of the Securities Exchange Act of 1934, as amended, and our policies regarding stock transactions, a number of our employees, including executive officers and members of our board of directors, have adopted and may continue to adopt stock trading plans pursuant to which they have arranged to sell shares of our common stock from time to time in the future. Generally, sales under such plans by our executive officers and directors require public filings. Actual or potential sales of our common stock by such persons could cause the price of our common stock to fall or prevent it from increasing for numerous reasons. For example, a substantial number of shares of our common stock becoming available (or being perceived to become available) for sale in the public market could cause the market price of our common stock to fall or prevent it from increasing. Also, actual or potential sales by such persons could be viewed negatively by other investors. Future sales and issuances of our common stock or rights to purchase common stock, including pursuant to our equity incentive plans, could result in additional dilution of the percentage ownership of our stockholders and could cause our stock price to fall. Additional capital will be needed in the future to continue our planned operations. To the extent we raise additional capital by issuing equity securities, our stockholders may experience substantial dilution. We may sell common stock, convertible securities or other equity securities in one or more transactions at prices and in a manner we determine from time to time. If we sell common stock, convertible securities or other equity securities in more than one transaction, investors may be materially diluted by subsequent sales.
These sales may also result in material dilution to our existing stockholders, and new investors could gain rights superior to our existing stockholders. Pursuant to our 2013 Stock Option and Incentive Plan, or the 2013 Plan, our management is authorized to grant stock options and other equity-based awards to our employees, directors and consultants. The number of shares available for future grant under the 2013 Plan automatically increases each year by up to 4% of all shares of our capital stock outstanding as of December 31 of the prior calendar year, subject to the ability of our board of directors or compensation committee to take action to reduce the size of the increase in any given year. Currently, we plan to register the increased number of shares available for issuance under the 2013 Plan each year. If our board of directors or compensation committee elects to increase the number of shares available for future grant by the maximum amount each year, our stockholders may experience additional dilution, which could cause our stock price to fall. We also have an Employee Stock Purchase Plan and any shares of common stock purchased pursuant to that plan will also cause dilution. We could be subject to securities class action litigation. In the past, securities class action litigation has often been brought against a company following a decline in the market price of its securities. This risk is especially relevant for us because biotechnology and pharmaceutical companies have experienced significant stock price volatility in recent years. If we face such litigation, it could result in substantial costs and a diversion of management’s attention and resources, which could harm our business. Our ability to use our net operating loss carryforwards and certain other tax attributes may be limited. Under Section 382 of the Internal Revenue Code of 1986, as amended, if a corporation undergoes an “ownership change,” generally defined as a greater than 50% change (by value) in its equity ownership over a three-year period, the corporation’s ability to use its pre-change net operating loss carryforwards, or NOLs, and other pre-change tax attributes (such as research tax credits) to offset its post-change income may be limited. We have completed several financings since our inception which we believe have resulted in a change in control as defined by IRC Section 382. We may also experience ownership changes in the future as a result of subsequent shifts in our stock ownership. As a result, if we earn net taxable income, our ability to use our pre-change net operating loss carryforwards to offset U.S. federal taxable income may be subject to limitations, which could potentially result in increased future tax liability to us. The TCJA also reduced the corporate income tax rate to 21%, from a prior rate of 35%. This may cause a reduction in the economic benefit of our NOLs and other deferred tax assets available to us. In addition, at the state level, there may be periods during which the use of NOLs is suspended or otherwise limited, which could accelerate or permanently increase state taxes owed. We do not intend to pay dividends on our common stock so any returns will be limited to the value of our stock. We have never declared or paid any cash dividends on our common stock. We currently anticipate that we will retain future earnings for the development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends for the foreseeable future. Any return to stockholders will therefore be limited to the appreciation of their stock. 60
Provisions in our amended and restated certificate of incorporation and by-laws, as well as provisions of Delaware law, could make it more difficult for a third party to acquire us or increase the cost of acquiring us, even if doing so would benefit our stockholders or remove our current management. Our amended and restated certificate of incorporation, amended and restated by-laws and Delaware law contain provisions that may have the effect of delaying or preventing a change in control of us or changes in our management. Our amended and restated certificate of incorporation and by-laws, include provisions that: ·
| authorize “blank check” preferred stock, which could be issued by our board of directors without stockholder approval and may contain voting, liquidation, dividend and other rights superior to our common stock;
|
authorize “blank check” preferred stock, which could be issued by our board of directors without stockholder approval and may contain voting, liquidation, dividend and other rights superior to our common stock; create a classified board of directors whose members serve staggered three-year terms; specify that special meetings of our stockholders can be called only by our board of directors, the chairperson of our board of directors, our chief executive officer or our president; prohibit stockholder action by written consent; establish an advance notice procedure for stockholder approvals to be brought before an annual meeting of our stockholders, including proposed nominations of persons for election to our board of directors; provide that our directors may be removed only for cause;
·
| create a classified board of directors whose members serve staggered three-year terms;
|
·•
| specify that special meetings of our stockholders can be called only by our board of directors, the chairperson of our board of directors, our chief executive officer or our president;
|
·
| prohibit stockholder action by written consent;
|
·
| establish an advance notice procedure for stockholder approvals to be brought before an annual meeting of our stockholders, including proposed nominations of persons for election to our board of directors;
|
·
| provide that our directors may be removed only for cause;
|
·
| provide that vacancies on our board of directors may be filled only by a majority of directors then in office, even though less than a quorum; |
·
| specify that no stockholder is permitted to cumulate votes at any election of directors;
|
specify that no stockholder is permitted to cumulate votes at any election of directors; ·
| expressly authorize our board of directors to modify, alter or repeal our amended and restated by-laws; and
|
expressly authorize our board of directors to modify, alter or repeal our amended and restated by-laws; and ·
| require supermajority votes of the holders of our common stock to amend specified provisions of our amended and restated certificate of incorporation and amended and restated by-laws.
|
require supermajority votes of the holders of our common stock to amend specified provisions of our amended and restated certificate of incorporation and amended and restated by-laws. These provisions, alone or together, could delay or prevent hostile takeovers and changes in control or changes in our management. In addition, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which limits the ability of stockholders owning in excess of 15% of our outstanding voting stock to merge or combine with us. Any provision of our amended and restated certificate of incorporation or amended and restated by-laws or Delaware law that has the effect of delaying or deterring a change in control could limit the opportunity for our stockholders to receive a premium for their shares of our common stock, and could also affect the price that some investors are willing to pay for our common stock. Provisions in our collaboration agreement with Celgene Corporation may prevent or delay a change in control.
Our collaboration agreement with Celgene Corporation provides that during the initial three-year term of the collaboration and, if extended, during the first extension term of the collaboration which is two years, in the event that we engage in a change in control transaction, including for such purposes a merger or consolidation of bluebird bio or the sale of all or substantially all of our assets, or if another person or entity or group of persons or entities acquires at least 50% of our voting capital stock, then Celgene has the right, but not the obligation, to terminate the collaboration agreement and obtain perpetual, non-terminable, worldwide, exclusive, fully paid-up licenses to all, but not less than all, of the product candidates previously identified under the collaboration agreement. We refer to this right to acquire such licenses as the call option.
Under the call option, the product candidates to which Celgene would have the right to acquire fully paid-up licenses include any product candidate previously licensed out of the collaboration during the term of the collaboration, any product candidate for which we have exercised our right to co-develop and co-promote the product candidate within the United States, any product candidate for which Celgene previously declined its option to obtain a license and any product candidate for which at least in vivo efficacy studies have been initiated or authorized by the joint steering committee for the collaboration. The purchase price for such fully paid-up licenses would be determined pursuant to a binding arbitration process and would be paid on or about the consummation of the change of control transaction with our acquiror. The call option will lapse at the end of the three-year term of the collaboration, unless extended, in which case it will lapse at the end of the first extension term, which is two years, even if the collaboration is extended further.
61
In addition, during the initial three-year term of the collaboration, but not during any extension of the collaboration agreement, in the event that we engage in a change in control transaction described above and Celgene exercises the call option described above, then, in addition to the right to acquire the fully paid-up licenses described above, Celgene would also have the right to obtain a perpetual, non-terminable, worldwide, exclusive license to our intellectual property to develop one or more CAR T cell products targeting one or more oncology associated target antigens identified by Celgene following the third anniversary of the collaboration agreement. There is no limit to the number of oncology associated target antigens Celgene may select under this license. Upon commercialization of any such product candidate so licensed by Celgene, Celgene would be obligated to pay us a specified milestone payment upon regulatory approval and a percentage of net sales as a royalty. We refer to this license agreement to develop one or more CAR T cell products targeting one or more oncology associated target antigens as the target antigen license. The right to acquire a target antigen license will lapse after the initial three-year term of the collaboration, even if the collaboration is extended.
The call option and the right to acquire a target antigen license may have the effect of delaying or preventing a change in control transaction involving us, or may reduce the number of companies interested in acquiring us. If Celgene were to exercise the call option, it would gain exclusive development and marketing rights to the product candidates developed under the collaboration agreement, including any product for which we previously exercised our co-development and co-promotion rights. Were this to happen, our successor would not receive a royalty on net sales of any of the products out-licensed in connection with the call option, nor would it realize any value it may otherwise ascribe to our right to co-develop and co-promote within the United States any products developed during the collaboration. Moreover, if such event were to occur during the first three years of the collaboration, Celgene would also effectively have the exclusive right to develop and market an unlimited number of additional CAR T cell products using our gene therapy platform, whether or not these products were first identified or developed during the course of the collaboration, which product candidates would target a list of oncology associated target antigens that would not be known at the time we close our change in control transaction. This license could potentially give Celgene rights to our gene therapy platform for CAR T cell product candidates in the event we are acquired prior to the third anniversary of the collaboration.
These provisions could have the effect of delaying or preventing a change in control transaction involving bluebird bio, or could reduce the number of companies interested in acquiring us, in particular during the first three years of the collaboration. This risk may become particularly acute in the event either of our lead product candidates, Lenti-D or LentiGlobin, suffer material setbacks or delays in their clinical advancement, as a result of which the long-term strategic value potential acquirors may ascribe to us could increasingly be attributable to the potential long-term value of any CAR T cell products we develop under the collaboration.
Item 1B. Unresolved Staff Comments Not applicable. Item 2. Properties Our corporate headquarters are located in Cambridge, Massachusetts. Our current leased facility encompasses approximately 53,400253,108 square feet of office and laboratory space, located at 150 Second60 Binney Street, Cambridge, Massachusetts. The nine-year lease commenced on JanuaryOctober 1, 2014.2016 and will continue until March 31, 2027. We have the option to extend thisthe 60 Binney Street lease by an additional five years. We also lease our former corporate headquarters in Cambridge, Massachusetts, which lease expires on March 31, 2015, but we have fully subleased our former corporate headquarters through March 31, 2015.for two successive five-year terms. We also lease approximately 3,9007,800 square feet of office and laboratory space in Seattle, Washington, which lease expiresshall expire upon the effectiveness of a new lease agreement for approximately 18,000 square feet of office and laboratory space in December 2016.Seattle, Washington that will take effect upon the landlord’s delivery of the leased premises, which is anticipated to occur in the second quarter of 2018. The new lease will continue until the end of the 48th full calendar month following the date we occupy the building or other conditions specified in the lease occur. In November 2017, we purchased a 125,000 square foot manufacturing facility located in Durham, North Carolina to provide manufacturing capacity for lentiviral vector in support of our current and planned gene and cell therapies. We also lease office space in Zug, Switzerland, the location of our European headquarters. We believe that our existing facilities are adequate for our current needs. As additional space is needed in the future, we believe that suitable space will be available in the required locations on commercially reasonable terms. Item 3. Legal Proceedings In the ordinary course of business, we are from time to time involved in lawsuits, claims, investigations, proceedings, and threats of litigation relating to intellectual property, commercial arrangements and other matters. While the outcome of these proceedings and claims cannot be predicted with certainty, as of December 31, 2014,2017, we were not party to any legal or arbitration proceedings that may have, or have had in the recent past, significant effects on our financial position or profitability. No governmental proceedings are pending or, to our knowledge, contemplated against us. We are not a party to any material proceedings in which any director, member of senior management or affiliate of ours is either a party adverse to us or our subsidiaries or has a material interest adverse to us or our subsidiaries. Item 4. Mine Safety Disclosures Not applicable.
62
PART II Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities Our common stock has been traded on the Nasdaq Global Select Market under the symbol “BLUE” since our initial public offering on June 19, 2013. Prior to this time, there was no public market for our common stock.“BLUE.” The following table shows the high and low sale prices per share of our common stock as reported on the Nasdaq Global Select Market for the periods indicated: | | High | | | Low | | 2013 | | | | | | | | | Second Quarter 2013 (beginning June 19, 2013) | | $ | 26.91 | | | $ | 24.97 | | Third Quarter 2013 | | $ | 35.00 | | | $ | 24.43 | | Fourth Quarter 2013 | | $ | 27.11 | | | $ | 17.53 | | 2014 | | | | | | | | | First Quarter 2014 | | $ | 28.08 | | | $ | 19.34 | | Second Quarter 2014 | | $ | 41.75 | | | $ | 17.40 | | Third Quarter 2014 | | $ | 40.31 | | | $ | 30.33 | | Fourth Quarter 2014 | | $ | 94.77 | | | $ | 29.73 | |
| | High | | | Low | | 2016 | | | | | | | | | First Quarter 2016 | | $ | 65.00 | | | $ | 37.40 | | Second Quarter 2016 | | $ | 53.38 | | | $ | 35.37 | | Third Quarter 2016 | | $ | 74.95 | | | $ | 43.10 | | Fourth Quarter 2016 | | $ | 79.70 | | | $ | 37.05 | | 2017 | | | | | | | | | First Quarter 2017 | | $ | 100.40 | | | $ | 60.95 | | Second Quarter 2017 | | $ | 123.75 | | | $ | 74.45 | | Third Quarter 2017 | | $ | 143.50 | | | $ | 85.65 | | Fourth Quarter 2017 | | $ | 222.03 | | | $ | 119.90 | |
On February 18, 2015,16, 2018, the last reported sale price for our common stock on the Nasdaq Global Select Market was $91.89$213.35 per share. Stock Performance Graph The graph set forth below compares the cumulative total stockholder return on our common stock between June 19, 2013 (the date of our initial public offering) and December 31, 2014,2017, with the cumulative total return of (a) the Nasdaq Biotechnology Index and (b) the Nasdaq Composite Index, over the same period. This graph assumes the investment of $100 on June 19, 2013 in our common stock, the Nasdaq Biotechnology Index and the Nasdaq Composite Index and assumes the reinvestment of dividends, if any. The graph assumes our closing sales price on June 19, 2013 of $26.91 per share as the initial value of our common stock and not the initial offering price to the public of $17.00 per share.
63
The comparisons shown in the graph below are based upon historical data. We caution that the stock price performance shown in the graph below is not necessarily indicative of, nor is it intended to forecast, the potential future performance of our common stock. Information used in the graph was obtained from the Nasdaq Stock Market LLC, a financial data provider and a source believed to be reliable. The Nasdaq Stock Market LLC is not responsible for any errors or omissions in such information.  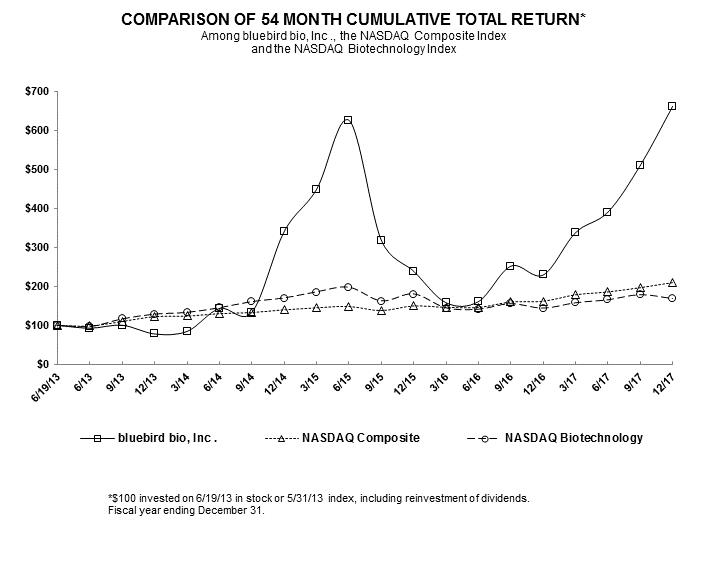
Holders As of February 18, 2015,16, 2018, there were approximately 228 holders of record of our common stock. The actual number of stockholders is greater than this number of record holders, and includes stockholders who are beneficial owners, but whose shares are held in street name by brokers and other nominees. This number of holders of record also does not include stockholders whose shares may be held in trust by other entities. Dividends We have not paid any cash dividends on our common stock since inception and do not anticipate paying cash dividends in the foreseeable future. Securities authorized for issuance under equity compensation plans Information about our equity compensation plans is incorporated herein by reference to Item 12 of Part III of this Annual Report on Form 10-K. Purchases of Equity Securities by the Issuer or Affiliated Purchasers
There were no repurchases of shares of common stock made during the year ended December 31, 2014.
64
Item 6. Selected Financial Data The following selected consolidated financial data should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations”, the consolidated financial statements and related notes, and other financial information included in this Annual Report on Form 10-K. We derived the consolidated financial data for the years ended December 31, 2014, 20132017, 2016 and 20122015 and as of December 31, 20142017 and 20132016 from our audited consolidated financial statements, which are included elsewhere in this Annual Report on Form 10-K. We derived the consolidated financial data for the yearyears ended December 31, 20112014 and 2013 and as of December 31, 20122015, 2014 and 20112013 from our audited consolidated financial statements that are not included elsewhere in this Annual Report on Form 10-K. Historical results are not necessarily indicative of the results to be expected in future periods. | | Years Ended December 31, | | | Year Ended December 31, | | | | 2014 | | | 2013 | | | 2012 | | | 2011 | | | 2017 | | | 2016 | | | 2015 | | | 2014 | | | 2013 | | | | (1) | | | | | | | | | | | | | | | | | | | | | | | | | | | (1) | | | | | | | | (in thousands, except per share amounts) | | | (in thousands, except per share amounts) | | Consolidated statements of operations data: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Revenue: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Collaboration revenue | | $ | 25,031 | | | $ | 19,792 | | | $ | — | | | $ | — | | | $ | 22,207 | | | $ | 6,155 | | | $ | 14,079 | | | $ | 25,031 | | | $ | 19,792 | | Research and license fees | | | 390 | | | | 389 | | | | 340 | | | | 640 | | | Grant revenue | | | — | | | | — | | | | — | | | | 242 | | | Total revenue | | | 25,421 | | | | 20,181 | | | | 340 | | | | 882 | | | Expenses: | | | | | | | | | | | | | | | | | | License and royalty revenue | | | | 13,220 | | | | — | | | | — | | | | 390 | | | | 389 | | Total revenues | | | | 35,427 | | | | 6,155 | | | | 14,079 | | | | 25,421 | | | | 20,181 | | Operating expenses: | | | | | | | | | | | | | | | | | | | | | | Research and development | | | 62,574 | | | | 31,002 | | | | 17,210 | | | | 11,409 | | | | 273,040 | | | | 204,775 | | | | 134,038 | | | | 62,574 | | | | 31,002 | | General and administrative | | | 23,227 | | | | 14,126 | | | | 6,846 | | | | 4,615 | | | | 93,550 | | | | 65,119 | | | | 46,209 | | | | 23,227 | | | | 14,126 | | Cost of license and royalty revenue | | | | 1,527 | | | | — | | | | — | | �� | | — | | | | — | | Change in fair value of contingent consideration | | | 246 | | | | — | | | | — | | | | — | | | | (525 | ) | | | 4,091 | | | | 2,869 | | | | 246 | | | | — | | Total operating expenses | | | 86,047 | | | | 45,128 | | | | 24,056 | | | | 16,024 | | | | 367,592 | | | | 273,985 | | | | 183,116 | | | | 86,047 | | | | 45,128 | | Loss from operations | | | (60,626 | ) | | | (24,947 | ) | | | (23,716 | ) | | | (15,142 | ) | | | (332,165 | ) | | | (267,830 | ) | | | (169,037 | ) | | | (60,626 | ) | | | (24,947 | ) | Other income (expense), net | | | 120 | | | | (374 | ) | | | 46 | | | | (456 | ) | | Benefit from income taxes | | | 11,797 | | | | — | | | | — | | | | — | | | Interest (expense) income, net | | | | (2,001 | ) | | | 3,782 | | | | 1,591 | | | | 290 | | | | 35 | | Other (expense) income, net | | | | (1,267 | ) | | | (71 | ) | | | 723 | | | | (170 | ) | | | (409 | ) | Income tax (expense) benefit | | | | (210 | ) | | | 612 | | | | (60 | ) | | | 11,797 | | | | — | | Net loss | | $ | (48,709 | ) | | $ | (25,321 | ) | | $ | (23,670 | ) | | $ | (15,598 | ) | | $ | (335,643 | ) | | $ | (263,507 | ) | | $ | (166,783 | ) | | $ | (48,709 | ) | | $ | (25,321 | ) | Net loss per share applicable to common stockholders - basic and diluted | | $ | (1.83 | ) | | $ | (2.02 | ) | | $ | (13.79 | ) | | $ | (171.59 | ) | | Weighted-average number of common shares used in net loss per share applicable to common stockholders - basic and diluted | | | 26,546 | | | | 12,555 | | | | 262 | | | | 120 | | | Net loss per share - basic and diluted | | | $ | (7.71 | ) | | $ | (7.07 | ) | | $ | (4.81 | ) | | $ | (1.83 | ) | | $ | (2.02 | ) | Weighted-average number of common shares used in net loss per share - basic and diluted | | | | 43,535 | | | | 37,284 | | | | 34,669 | | | | 26,546 | | | | 12,555 | |
| | As of December 31, | | | | 2014 | | | 2013 | | | 2012 | | | 2011 | | | | (1) | | | | | | | | | | | | | | | | (in thousands) | | Consolidated balance sheet data: | | | | | | | | | | | | | | | | | Cash and cash equivalents | | $ | 347,845 | | | $ | 206,279 | | | $ | 67,011 | | | $ | 25,604 | | Marketable securities | | | 144,158 | | | | - | | | | - | | | | 3,507 | | Working capital | | | 437,011 | | | | 177,113 | | | | 63,156 | | | | 27,087 | | Total assets | | | 556,739 | | | | 224,390 | | | | 69,322 | | | | 30,918 | | Contingent consideration, net of current portion | | | 6,321 | | | | — | | | | — | | | | — | | Preferred stock | | | — | | | | — | | | | 122,177 | | | | 82,403 | | Common stock and additional paid-in capital | | | 638,712 | | | | 250,342 | | | | 15,270 | | | | 7,734 | | Total stockholders' equity (deficit) | | | 491,257 | | | | 151,667 | | | | (55,747 | ) | | | (55,707 | ) |
(1)
| Starting in 2014, the selected financial data includes the impact of the acquisition of Pregenen in June 2014. See Note 12. “Business Combinations” in the accompanying notes to consolidated financial statements for additional information.
|
| | As of December 31, | | | | 2017 | | | 2016 | | | 2015 | | | 2014 | | | 2013 | | | | | | | | | | | | | | | | (1) | | | | | | | | (in thousands) | | Consolidated balance sheet data: | | | | | | | | | | | | | | | | | | | | | Cash, cash equivalents and marketable securities | | $ | 1,614,302 | | | $ | 884,830 | | | $ | 865,763 | | | $ | 492,003 | | | $ | 206,279 | | Total assets | | | 1,900,567 | | | | 1,118,122 | | | | 1,002,337 | | | | 556,739 | | | | 224,390 | | Total current liabilities | | | 95,612 | | | | 74,533 | | | | 40,368 | | | | 42,978 | | | | 34,874 | | Financing lease obligation, net of current portion | | | 154,749 | | | | 120,140 | | | | 61,901 | | | — | | | — | | Other long-term obligations | | | 26,774 | | | | 54,009 | | | | 49,572 | | | | 22,504 | | | | 37,849 | | Total stockholders' equity | | $ | 1,623,432 | | | $ | 869,440 | | | $ | 850,496 | | | $ | 491,257 | | | $ | 151,667 | |
65(1) Starting in 2014, the selected financial data includes the impact of the June 2014 acquisition of Pregenen.
Item 7. Management’sManagement’s Discussion and Analysis of Financial Condition and Results of Operations The following information should be read in conjunction with the consolidated financial statements and related notes thereto included in this Annual Report on Form 10-K10-K. Except for theIn addition to historical information, contained herein, the matters discussed in this Annual Report on Form 10-K may be deemed to bereport contains forward-looking statements that involve risks and uncertainties.uncertainties which may cause our actual results to differ materially from plans and results discussed in forward-looking statements. We make such forward-looking statements pursuantencourage you to review the safe harbor provisionsrisks and uncertainties discussed in the sections entitled Item 1A. “Risk Factors” and “Forward-Looking Statements” included at the beginning of the Private Securities Litigation Reform Act of 1995 and other federal securities laws. In this Annual Report on Form 10-K, words such as “may,” “will,” “expect,” “anticipate,” “estimate,” “intend,”10-K. The risks and similar expressions (as well as other words or expressions referencing future events, conditions or circumstances) are intended to identify forward-looking statements.
Ouruncertainties can cause actual results and the timing of certain events mayto differ materiallysignificantly from the results discussed, projected, anticipated, or indicatedthose forecast in any forward-looking statements. We caution you that forward-looking statements are not guarantees of future performanceor implied in historical results and that our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate may differ materially from the forward-looking statements contained in this Annual Report. In addition, even if our results of operations, financial condition and liquidity, and the development of the industry in which we operate are consistent with the forward-looking statements contained in this Annual Report, they may not be predictive of results or developments in future periods.
The following information and any forward-looking statements should be considered in light of factors discussed elsewhere in this Annual Report on Form 10-K, including those risks identified under Item 1A. Risk Factors.trends.
We caution readers not to place undue reliance on any forward-looking statements made by us, which speak only as of the date they are made. We disclaim any obligation, except as specifically required by law and the rules of the SEC, to publicly update or revise any such statements to reflect any change in our expectations or in events, conditions or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements. Overview We are a clinical-stage biotechnology company committed to developing potentially transformative gene therapies for severe genetic and rare diseases and in the field of T cell-based immunotherapy.cancer. With our lentiviral-based gene therapy and gene editing capabilities, we have built an integrated product platform with broad therapeutic potential application in these areas.a variety of indications. We believe that gene therapy for severe genetic diseases has the potential to change the way these patients are treated by correcting the underlying genetic defect that is the cause of their disease, rather than offering solutionstreatments that only address their symptoms. WeOur clinical programs in severe genetic diseases include our LentiGlobin® product candidate as a treatment for each of transfusion-dependent β-thalassemia, or TDT, and severe sickle cell disease, or severe SCD, and our scientific collaboratorsLenti-D™ product candidate as a treatment for cerebral adrenoleukodystrophy, or CALD, a rare hereditary neurological disorder. Our programs in oncology are built upon our leadership in lentiviral gene delivery and T cell engineering, with a focus on developing novel T cell-based immunotherapies, including chimeric antigen receptor (CAR) and T cell receptor (TCR) T cell therapies. bb2121 and bb21217, our lead product candidates in oncology, are CAR T cell product candidates for the treatment of multiple myeloma, which we have generated whatexclusively licensed to Celgene Corporation, or Celgene. We are developing our LentiGlobin product candidate with the goal of filing for regulatory approval in the US and EU for different genotypes of TDT and for severe SCD. Both TDT and severe SCD are rare, hereditary blood disorders that often lead to severe anemia and shortened lifespans. We are conducting five clinical studies of our LentiGlobin product candidate: a Phase I/II study in the United States, Australia, and Thailand for the treatment of subjects with TDT, called the Northstar Study (HGB-204); a multi-site, international, Phase III study for the treatment of subjects with TDT and a non-β0/β0 genotype, called the Northstar-2 Study (HGB-207); a multi-site, international, Phase III study for the treatment of subjects with TDT and a β0/β0 genotype, called the Northstar 3 Study (HGB-212); a single-center Phase I/II study in France for the treatment of subjects with TDT or severe SCD (HGB-205); and a multi-site Phase I study in the United States for the treatment of subjects with severe SCD (HGB-206). We have achieved our enrollment target of 18 patients in the Northstar Study, and we believehave achieved our enrollment target for the adult and adolescent cohort in the Northstar 2 Study. We anticipate filing a marketing authorization application in the EU for LentiGlobin for the treatment of adult and adolescent patients with TDT and a non-β0/β0 genotype during the second half of 2018, with a future BLA planned in the United States. We are also engaged with the U.S. Food and Drug Administration, or FDA, and the EMA in discussions regarding our proposed development plans for LentiGlobin in severe SCD. We are developing our Lenti-D product candidate with the goal of filing for regulatory approval in the US and EU for CALD, a rare, hereditary neurological disorder that is human proof-of-concept dataoften fatal. We are planning to submit our first filing for our gene therapy platformregulatory approval of Lenti-D in three underserved diseases, each of which has been granted orphan drug status by U.S. and European regulatory authorities. 2019. We are conducting a multi-site, international, Phase II/III clinical study of our Lenti-D™ product candidate, called the Starbeam Study (ALD-102), for the treatment of subjects with CALD. Seventeen subjects were treated with our most advancedLenti-D product candidate Lenti-D,in the initial cohort of the Starbeam Study, and we are enrolling up to evaluate its safety and efficacythirteen additional subjects in subjects with childhood cerebral adrenoleukodystrophy, or CCALD, a rare, hereditary neurological disorder affecting young boys that is often fatal. In October 2013, we announced that the first subject had been treated inan expansion cohort of this study. We are also planning to conductconducting an observational study of subjects with CCALDCALD treated by allogeneic hematopoietic stem-cell transplant referred to as the ALD-103 study. We are also conducting two Phase I/II clinical studies of If our next most advancedLenti-D product candidate LentiGlobin, to evaluate its safetyshows a sufficiently compelling treatment effect, and efficacy in subjectspending further discussion with ß-thalassemia major,regulatory authorities, the results from the Starbeam study could potentially form the basis of a hereditary blood disorder that often leads to severe anemiaBiologics License Application, or BLA, and shortened lifespans. In December 2013, we announced that the first subject with ß-thalassemia major had been treated in our European study of LentiGlobin, called the HGB-205 study, which also permits the enrollment of subjects with severe sickle cell disease,a Marketing Authorization Application, or SCD. In October 2014 we announced that the first subject with severe SCD had been treated in the European HGB-205 study. In March 2014, we announced that the first subject with ß-thalassemia major had been treated in our other study of LentiGlobin being conductedMAA, submission in the United States Australia and Thailand, calledEuropean Union, respectively.
We are developing, in collaboration with Celgene, our bb2121 and bb21217 product candidates with the Northstar Study.goal of filing for regulatory approval in multiple myeloma on a global basis, a hematologic malignancy that develops in the bone marrow that is fatal if untreated. We presented results from the HGB-205 study at the European Hematology Association Congress in June 2014 and presented results from both the Northstar Study and HGB-205 study at the American Society of Hematology Annual Meeting in December 2014. We have initiatedare conducting a multi-site Phase I clinical study in the United States calledof our bb2121 product candidate for the HGB-206 Study, to evaluate the safety and efficacytreatment of LentiGlobin in subjects with severe SCD.relapsed/refractory multiple myeloma (CRB-401). bb2121 is the lead product candidate arising from our multi-year
66
In March 2013, we announced a global strategic collaboration with Celgene Corporation, or Celgene, for the discovery, development and commercialization of CAR T cell therapies targeting B-cell maturation antigen, or BCMA. We have exclusively licensed to discover,Celgene the right to develop and commercialize chimeric antigen receptor-modified T cells, or CAR T cells, as potentially disease-altering therapiesour bb2121 product candidate, and we may exercise our option to co-develop and co-promote this product candidate in oncology. This collaborationthe United States. The FDA has an initial termgranted Breakthrough Therapy designation and the EMA has granted PRIME eligibility to the bb2121 product candidate for relapsed/refractory multiple myeloma. In February 2018, Celgene treated the first subject in a multi-site Phase II clinical study in the United States and Europe of three years, andour bb2121 product candidate for the treatment of subjects with relapsed/refractory multiple myeloma. Celgene has madeannounced plans to initiate an international Phase III study of bb2121 in third line multiple myeloma in 2018 and plans to file a $75.0 million up-front, non-refundable cash paymentBLA with the FDA in 2019 for bb2121 to us as consideration for entering intotreat relapsed/refractory multiple myeloma.
In September 2017, we initiated a Phase I clinical study of bb21217, the collaboration. During the year ended December 31, 2014, we recognized $25.0 million of revenue associated withsecond anti-BCMA product candidate arising from our collaboration with Celgene, relatedand Celgene exercised its option to obtain an exclusive worldwide license to develop and commercialize bb21217. The FDA has granted Orphan Drug status to both bb2121 and bb21217 product candidates for the researchtreatment of patients with relapsed/refractory multiple myeloma. Our collaboration now focuses exclusively on anti-BCMA CAR T product candidates. We advanced our development of our bb2121 product candidate, the first CAR product candidate from our collaboration with Celgene, into clinical trials in February 2016. In February 2016, we exclusively licensed to Celgene the right to develop and commercialize our bb2121 product candidate, pursuant to Celgene’s exercise of its exclusive option under the collaboration arrangement. We have the obligation to continue conducting our ongoing CRB-401 study, but Celgene has the responsibility for the costs of further development services performed. and of commercialization. We retain an option to co-develop and co-promote the bb2121 product candidate in the United States. In September 2017, we initiated a Phase I clinical study of bb21217, the second anti-BCMA cell product candidate arising from our collaboration with Celgene, and Celgene exercised its option to obtain an exclusive worldwide license to develop and commercialize bb21217. As of December 31, 2014, we have classified $30.72017 and December 31, 2016, there was $47.4 million and $46.4 million, respectively, of total deferred revenue related to ourthe Company’s collaboration with Celgene as current or long-term in the accompanying balance sheets based on the contractual term of the arrangement. We expect the first product candidate from this collaboration to enter clinical trials in early 2016.Celgene. In June 2014, we acquired Precision Genome Engineering, Inc., or Pregenen, a privately-held biotechnology company headquartered in Seattle, Washington. Through the acquisition, we obtained rights to Pregenen’s gene editing and cell signaling technology. Under the terms of the agreement, we paid the former Pregenen equityholders $5.1 million in cash and issued 405,400 shares of our common stock with a fair value of $15.6 million. The consideration for the transaction also includes an additional 94,117 shares of our common stock that will be held for a period of 18 months after the acquisition and may be used to settle certain claims for indemnification for breaches or inaccuracies in Pregenen’s representations and warranties, covenants, and agreements. Additionally, the total consideration was subject to post-closing adjustments relating to the working capital of Pregenen as of closing, and we issued an additional 2,119 shares with a fair value of $0.1 million in July 2014. The agreement also providesprovided for up to $135.0 million in future contingent cash payments by us upon the achievement of certain preclinical, clinical and commercial milestones related to the Pregenen technology,technology. As of which $15.0December 31, 2017, there are $120.0 million relates to preclinical milestones,in remaining future contingent cash payments, of which $20.1 million relates to clinical milestones and $99.9 million relates to commercial milestones. During each of 2017 and 2016, $5.0 million in preclinical milestones were achieved and paid to the former equityholders of Pregenen. We estimate these future contingent cash payments have a fair value of $6.8$2.2 million as of December 31, 2014.2017, which is classified as a non-current liability. As of December 31, 2014,2017, we had cash, cash equivalents and marketable securities of approximately $492.0 million.$1.6 billion. We expect that our existing cash, cash equivalents and marketable securities will be sufficient to fund our current operations through 2017.into 2021. Since our inception in 1992, we have devoted substantially all of our resources to research andour development efforts relating to our product candidates, including activities to manufacture product candidates in compliance with good manufacturing practices, or GMP, to conduct clinical studies of our product candidates, to perform preclinical research to identify new product candidates and to provide general and administrative support for these operations.operations and to protect our intellectual property. We do not have any products approved for sale and have not generated any revenue from product sales. We have funded our operations primarily through the sale of common stock in our public offerings, private placements of preferred stock and warrants and through collaborations. We have never been profitable and have incurred net losses in each year since inception. Our net loss was $48.7losses were $335.6 million for the year ended December 31, 20142017 and our accumulated deficit was $147.4$913.8 million as of December 31, 2014.2017. Substantially all our net losses resulted from costs incurred in connection with our research and development programs and from general and administrative costs associated with our operations. We expect to continue to incur significant expenses and increasing operating losses for at least the next several years. We expect our expenses will increase substantially in connection with our ongoing and planned activities, as we: ·
| • | conduct clinical studies for our Lenti-DLentiGlobin and LentiGlobinLenti-D product candidates, as well as for our anti-BCMA product candidates, bb2121 and bb21217, which are partnered with Celgene; |
·
| increase research and development-related activities for the discovery and development of oncology product candidates in connection with our strategic collaboration with Celgene;
|
increase research and development-related activities for the discovery and development of oncology product candidates; continue our research and development efforts; manufacture clinical study materials and develop large-scale manufacturing capabilities;
·
| continue our research and development efforts;
|
·•
| manufacture clinical study materials and develop large-scale manufacturing capabilities;
|
·
| seek regulatory approval for our product candidates; and |
·
add personnel to support our product development and commercialization efforts. | add personnel to support our product development and commercialization efforts; and
|
·
| operate as a public company.
|
We do not expect to generate revenue from product sales unless and until we successfully complete development and obtain regulatory approval for one or more of our product candidates, which we expect will take a number of years and is subject to significant uncertainty. We currently have no commercial-scale manufacturing facilities of our own, and all of our manufacturing activities are contracted out to third parties. Additionally, we currently utilize third-party contract research organizations, or CROs, to carry out our clinical development activities; andactivities. If we do not yet have a sales organization. If weseek to obtain regulatory approval for any of our product candidates, we expect to incur significant commercialization expenses related toas we prepare for product sales, marketing, manufacturing, and distribution. Accordingly, we will seek to fund our operations through public or private equity or debt financings, strategic collaborations, or other sources. However, we may be unable to raise additional funds or enter into such other arrangements when needed on favorable terms or at all. Our failure to raise capital or enter into such other arrangements as and when needed would have a negative impact on our financial condition and our ability to develop our products. 67
Because of the numerous risks and uncertainties associated with product development, we are unable to predict the timing or amount of increased expenses or when or if we will be able to achieve or maintain profitability. Even if we are able to generate revenues from the sale of our products, we may not become profitable. If we fail to become profitable or are unable to sustain profitability on a continuing basis, then we may be unable to continue our operations at planned levels and be forced to reduce our operations. Financial operations overview Revenue To date, we have not generated any revenues from the sale of products. Our revenues have been derived from collaboration arrangements, out-licensing arrangements including royalties on net sales of products to licensees or sublicensees, research fees, license fees, and grant revenues. Collaboration revenue is generated exclusively from our collaboration arrangement with Celgene.Celgene, which was amended in 2015. The terms of this amended arrangement contain multiple deliverables, which include at inception: (i) discovery, research and development services, (ii) participation on the joint steering committee, andor JSC, (iii) participation on the patent committee. We recognize arrangement consideration allocatedcommittee, (iv) a license to each unitbb2121, (v) manufacture of accounting when allvectors and associated payload for incorporation into bb2121 under the license, and (vi) participation on the joint governance committee, or JGC, under the co-development and co-promotion agreement for bb2121. As of September 2017, the revenue recognition criteria in Financial Accounting Standards Board, or FASB, Accounting Standards Codification, or ASC, Topic 605, Revenue Recognition, or ASC 605, are satisfied for that particular unitcollaboration also includes a license bb21217, the deliverables as part of accounting. Revenue from the Celgene arrangement associated with discovery,which include: (i) research and development services, joint steering committee services(ii) a license to bb21217, (iii) manufacture of vectors and patent committee servicesassociated payload for incorporation into bb21217, under the license, and (iv) participation on the JGC under the co-development and co-promotion agreement for bb21217. Nonrefundable license fees are recognized as revenue upon delivery provided there are no undelivered elements in the arrangement. License revenue is generated from our out-license agreements with Novartis Pharma AG, or Novartis, and GlaxoSmithKline Intellectual Property Development Limited, or GSK. Under our out-licensing agreements we may also recognize revenue from potential future milestone payments and royalties. Royalties are recognized ratably overin the associated period of sale of the related product(s), based on the underlying contract terms, provided that the reported sales are reliably measurable and we have no remaining performance which is initially three years.obligations, assuming all other revenue recognition criteria are met. Research and license fee revenue is primarily generated through license and research and development agreements with strategic partners and nonprofit organizations for the development and commercialization of our product candidates. There are no performance, cancellation, termination, or refund provisions in any of our arrangements that contain material financial consequences to us. Nonrefundable license fees are recognized as revenue upon delivery provided there are no undelivered elements in the arrangement. Research fees are recognized as revenue over the period we perform the associated services or on a straight-line basis if the pattern of performance cannot be estimated.more readily determined.
Research and development expenses Research and development expenses consist primarily of costs incurred for the development of our product candidates, which include: ·
| employee-related expenses, including salaries, benefits, travel and stock-based compensation expense; expenses incurred under agreements with CROs and clinical sites that conduct our clinical studies; costs of acquiring, developing, and manufacturing clinical study materials;
| • | reimbursable costs to our partners for collaborative activities; |
·
| expenses incurred under agreements with CROs and clinical sites that conduct our clinical studies;
|
facilities, depreciation, and other expenses, which include direct and allocated expenses for rent and maintenance of facilities, information technology, insurance, and other supplies in support of research and development activities; ·
| costs of acquiring, developing, and manufacturing clinical study materials;
|
costs associated with our research platform and preclinical activities; ·
| facilities, depreciation, and other expenses, which include direct and allocated expenses for rent and maintenance of facilities, insurance, and other supplies;
|
costs associated with our regulatory, quality assurance and quality control operations; and ·
amortization of intangible assets. | costs associated with our research platform and preclinical activities;
|
·
| costs associated with our regulatory, quality assurance and quality control operations; and
|
·
| amortization of intangible assets.
|
Research and development costs are expensed as incurred. Costs for certain development activities are recognized based on an evaluation of the progress to completion of specific tasks using information and data provided to us by our vendors and our clinical sites. We cannot determine with certainty the duration and completion costs of the current or future clinical studies of our product candidates or if, when, or to what extent we will generate revenues from the commercialization and sale of any of our product candidates that obtain regulatory approval. We may never succeed in achieving regulatory approval for any of our product candidates. The duration, costs, and timing of clinical studies and development of our product candidates will depend on a variety of factors, including: ·
| the scope, rate of progress, and expense of our ongoing as well as any additional clinical studies and other research and development activities we undertake;
|
·
| future clinical study results;
|
·
| uncertainties in clinical study enrollment rates;
|
·
| changing standards for regulatory approval; and
|
68
·
| the timing and receipt of any regulatory approvals.
|
A change in the outcome of any of these variables with respect to the development of a product candidatewhich could mean a significant change in the costs and timing associated with the development of thatour product candidate. For example, if candidates including:
the FDA, or another regulatory authority were to require us to conductscope, rate of progress, and expense of our ongoing as well as any additional clinical studies beyond those that we currently anticipate will be required for the completion of clinical development of a product candidate or if we experience significant delays in enrollment in any of our clinical studies, we could be required to expend significant additional financial resources and time on the completion of clinical development for our product candidates. From inception through December 31, 2014, we have incurred $158.1 million inother research and development expenses. activities we undertake;
future clinical study results; uncertainties in clinical study enrollment rates; changing standards for regulatory approval; and the timing and receipt of any regulatory approvals. We plan to increase our research and development expenses for the foreseeable future as we continue to advance the development of our Lenti-D, LentiGlobin, bb2121, and LentiGlobinbb21217 product candidates, conduct research and development activities in oncology, including under our strategic collaboration with Celgene, and continue the research and development of product candidates using the acquired Pregenenour gene editing technology platform. Our research and development activities include the following: ·
| We are conducting a Phase II/III clinical study to examine the safety and efficacy of our Lenti-D product candidate in the treatment of CCALD. In October 2013, we announced that the first subject had been treated in this study.
|
Starbeam Study (ALD-102) – We are conducting a Phase II/III clinical study in the United States, England and France to examine the safety and efficacy of our Lenti-D product candidate in the treatment of subjects with CALD. In January 2018, we announced that we are amending the protocol for this study to enroll up to a total of 30 subjects. ·
| Northstar Study (HGB-204) – We are conducting a Phase I/II clinical study in the United States, Australia and Thailand to study the safety and efficacy of our LentiGlobin product candidate in the treatment of subjects with TDT. In March 2014, we announced that the first subject had been treated in this study. In May 2016, we announced that this study has been fully enrolled. HGB-205 study – We are conducting a Phase I/II clinical study in France to study the safety and efficacy of our LentiGlobin product candidate in the treatment of subjects with TDT and of subjects with severe SCD. In December 2013, we announced that the first subject with TDT had been treated in this study and in October 2014, we announced that the first subject with severe SCD had been treated in this study. In February 2017, we announced that this study has been fully enrolled. HGB-206 study – We are conducting a Phase I clinical study in the United States to study the safety and efficacy of our LentiGlobin product candidate in the treatment of subjects with severe SCD. In June 2015, we announced that the first subject with severe SCD had been treated in this study. In October 2016, we announced that we have amended the protocol for this study to incorporate several process changes and to expand enrollment up to a total of 29 subjects. | • | Northstar-2 Study (HGB-207) – We are conducting a Phase III study at multiple sites internationally to examine the safety and efficacy of our LentiGlobin product candidate in the treatment of subjects with ß-thalassemia major.TDT and a non-β0/β0 genotype. In March 2014,December 2016, we announced that the first subject had been treated in this study. In August 2017, we announced that the adult and adolescent cohort for this study has been fully enrolled. |
·
| We are conducting a Phase I/II clinical study in France to
Northstar-3 Study (HGB-212) – We are conducting a Phase III study at multiple sites internationally to examine the safety and efficacy of our LentiGlobin product candidate in the treatment of subjects with TDT and a β0/β0 genotype. In November 2017, we announced that the first subject had been treated in this study. CRB-401 study – We are conducting a Phase I clinical study in the United States to study the safety and efficacy of our bb2121 product candidate in the treatment of subjects with relapsed/refractory multiple myeloma. In February 2016, we announced that the first subject with relapsed/refractory multiple myeloma had been treated in this study, and the final patient enrolled in the CRB-401 study was treated in February 2018.
| • | CRB-402 study – We are conducting a Phase I clinical study of our bb21217, our next-generation anti-BCMA product candidate in the treatment of subjects with ß-thalassemia major and severe SCD.relapsed/refractory multiple myeloma. In December 2013, we announced that the first subject ß-thalassemia major had been treated in this study and in October 2014,September 2017, we announced that the first subject with SCDrelapsed/refractory multiple myeloma had been treated in this study. |
·
We will continue to manufacture clinical study materials in support of our clinical studies. | We have initiated a Phase I clinical study in the United States to study the safety and efficacy of our LentiGlobin product candidate in the treatment of subjects with severe SCD.
|
·
| We will continue to manufacture clinical study materials in support of our clinical studies.
|
From inception through December 31, 2017, we have incurred $769.9 million in research and development expenses. Our direct research and development expenses consist principally of external costs, such as fees paid to investigators, consultants, central laboratories and CROs in connection with our clinical studies, and costs related to acquiring and manufacturing clinical study materials. Effective January 1, 2014, we began allocatingWe allocate salary and benefit costs directly related to specific programs. We do not allocate personnel-related discretionary bonus or stock-based compensation costs, costs associated with our general discovery platform improvements, depreciation or other indirect costs that are deployed across multiple projects under development and, as such, the costs are separately classified as personnelother research and otherdevelopment expenses in the table below: | Year ended December 31, | | | Year Ended December 31, | | | | 2014 | | | | 2013 | | | | 2012 | | | 2017 | | | 2016 | | | 2015 | | | (in thousands) | | | (in thousands) | | LentiGlobin | | | $ | 85,710 | | | $ | 67,154 | | | $ | 38,515 | | Lenti-D | $ | 12,137 | | | $ | 4,396 | | | $ | 3,966 | | | | 16,223 | | | | 18,612 | | | | 13,666 | | LentiGlobin | | 21,444 | | | | 8,490 | | | | 5,259 | | | bb2121 | | | | 32,144 | | | | 12,690 | | | | — | | bb21217 | | | | 7,402 | | | | — | | | | — | | Pre-clinical programs | | 6,651 | | | | 783 | | | | — | | | | 40,167 | | | | 32,771 | | | | 15,937 | | Total direct research and development expense | | 40,232 | | | | 13,669 | | | | 9,225 | | | | 181,646 | | | | 131,227 | | | | 68,118 | | Employee- and contractor-related expenses | | 6,771 | | | | 9,152 | | | | 5,742 | | | | 23,698 | | | | 17,047 | | | | 11,793 | | Stock-based compensation expense | | 5,151 | | | | 3,809 | | | | 408 | | | | 26,633 | | | | 19,690 | | | | 24,854 | | Platform-related expenses | | 5,112 | | | | 1,067 | | | | 727 | | | | 15,414 | | | | 15,359 | | | | 21,217 | | Facility expenses | | 5,292 | | | | 2,288 | | | | 709 | | | | 24,700 | | | | 20,301 | | | | 7,282 | | Other expenses | | 16 | | | | 1,017 | | | | 399 | | | | 949 | | | | 1,151 | | | | 774 | | Unallocated personnel and other expenses | | 22,342 | | | | 17,333 | | | | 7,985 | | | Total other research and development expenses | | | | 91,394 | | | | 73,548 | | | | 65,920 | | Total research and development expense | $ | 62,574 | | | $ | 31,002 | | | $ | 17,210 | | | $ | 273,040 | | | $ | 204,775 | | | $ | 134,038 | |
The costs associated with our bb2121 program were included within pre-clinical programs in the table shown above for the year ended December 31, 2015 and are separately shown for the years ended December 31, 2017 and 2016. The costs associated with our bb21217 program were included in pre-clinical programs in the table shown above through June 30, 2017. The costs associated with our bb21217 program are presented separately in the table above beginning in the third quarter of 2017 as we initiated the first clinical study for bb21217 in the third quarter of 2017. General and administrative expenses General and administrative expenses consist primarily of salaries and related costs for personnel, including stock-based compensation and travel expenses for our employees in executive, operational, finance, legal, business development, commercial, information technology, and human resource functions. Other general and administrative expenses include facility-related costs, professional fees for accounting, tax and legal and consulting services, directors’ fees and expenses associated with obtaining and maintaining patents. 69
We anticipate that our general and administrative expenses will increase in the future as we increase our headcount to support our continued research and development and the potential commercialization of our product candidates. We also anticipate increased expenses related to audit, legal, regulatory, and tax-related services associated with maintaining compliance with exchange listing and SEC requirements, director and officer insurance premiums, and investor relations costs associated with being a public company. Additionally, if and when we believe a regulatory approval of the first product candidate appears likely, we anticipate an increase in payroll and related expenses as a result of our preparation for commercial operations, especially as it relates to the sales and marketing of our product candidates. Other income (expense), netCost of license and royalty revenue
Other incomeCost of license and royalty revenue represents expense consists primarily of interest income earned on investments, the gain or loss associated with amounts owed to third party licensors as a result of revenue recognized under our out-license arrangements with Novartis and GSK.
We anticipate that our cost of license and royalty revenue will increase in the changefuture contingent upon the achievement of regulatory milestones by Novartis or GSK. Additionally, we anticipate that our cost of license and royalty revenue will increase in the future as we expect to continue to recognize royalty revenue related to Novartis’ commercial sale of Kymriah.
Change in fair value of contingent consideration On June 30, 2014, we acquired Pregenen. In connection with the acquisition, we recorded contingent consideration pertaining to the amounts potentially payable to Pregenen’s former equityholders pursuant to the Stock Purchase Agreement (the “Stock Purchase Agreement”) by and among us, Pregenen and Pregenen’s former equityholders. We assess these estimates on an on-going basis as additional data impacting the assumptions is obtained. Future changes in the fair value of preferred stock warrants, foreign currency gain or losscontingent consideration related to updated assumptions and tax incentives fromestimates are recognized within the Massachusetts Life Sciences Center.consolidated statements of operations and comprehensive loss. UntilContingent consideration may change significantly as development progresses and additional data are obtained, impacting our IPO in June 2013 when all our outstanding preferred stock warrants were converted into common stock warrants, we recognized the re-measurement gain or loss associated with the change in the fair valueassumptions regarding probabilities of the preferred stock warrant liability as a componentsuccessful achievement of other income (expense), net. Werelated milestones used the Black-Scholes option pricing model to estimate the fair value of preferred stock warrants. We based the estimatesliability and the timing in which they are expected to be achieved. The significant unobservable inputs used in the Black-Scholes option pricing model, in part, on subjective assumptions, including stock price volatility, risk-free interest rate, dividend yield, and themeasurement of fair value of our contingent consideration are probabilities of successful achievement of clinical and commercial milestones, the preferred stock underlyingperiod in which these milestones are expected to be achieved and discount rates. Significant increases or decreases in any of the warrants.probabilities of success would result in a significantly higher or lower fair value measurement, respectively. Significant increases or decreases in the other inputs would result in a significantly lower or higher fair value measurement, respectively.
Interest (expense) income, net Interest (expense) income, net consists primarily of interest expense on the 60 Binney Street financing lease obligation and interest income earned on investments. Other (expense) income, net Other (expense) income, net consists primarily of a loss on disposal of assets, realized gains and losses on investments, and gains and losses on foreign currency. Critical accounting policies and significant judgments and estimates Our management’s discussion and analysis of our financial condition and results of operations are based on our financial statements, which have been prepared in accordance with generally accepted accounting principles.principles in the U.S. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, and expenses and the disclosure of contingent assets and liabilities in our financial statements. On an ongoing basis, we evaluate our estimates and judgments, including those relatedexpected business and operational changes, sensitivity and volatility associated with the assumptions used in developing estimates, and whether historical trends are expected to accrued research and development expenses, stock-based compensation, and business combinations.be representative of future trends. We base our estimates on historical experience, known trends and events and various other factors that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions. In making estimates and judgments, management employs critical accounting policies. While our significant accounting policies are described in more detail in the notes to our financial statements appearing elsewhere in this annual report, we believe the following accounting policies to be most critical to the judgments and estimates used in the preparation of our financial statements. Revenue recognition We have primarily generated revenue through collaboration arrangements, out-licensing arrangements including royalties on net sales of products to licensees or sublicensees, research arrangementsfees, and license arrangements with strategic partners and nonprofit organizations for the development and commercialization of product candidates. Additionally, we have generated revenue from research and development grant programs.revenues. Collaboration revenue As of December 31, 2017, our collaboration revenue was generated exclusively from our collaboration arrangement with Celgene, which was originally entered into in March 2013 and was subsequently amended in June 2015. We recognize revenueanalyze our collaboration arrangements to assess whether they are within the scope of ASC 808 to determine whether such arrangements involve joint operating activities performed by parties that are both active participants in accordance withthe activities and exposed to significant risks and rewards dependent on the commercial success of such activities. This assessment is performed throughout the life of the arrangement based on changes in the responsibilities of all parties in the arrangement. For collaboration arrangements that are deemed to be within the scope of ASC 808, we first determine which elements of the collaboration are deemed to be within the scope of ASC 808 and those that are more reflective of a vendor-customer relationship and therefore within the scope of ASC 605. Accordingly,
For those elements of the arrangement that are accounted for pursuant to ASC 605, revenue is recognized for each unit of accounting when all of the following criteria are met: ·
| Persuasive evidence of an arrangement exists
|
Persuasive evidence of an arrangement exists ·
| Delivery has occurred or services have been rendered
|
Delivery has occurred or services have been rendered ·
| The seller’s price to the buyer is fixed or determinable
|
The seller’s price to the buyer is fixed or determinable ·
| Collectability is reasonably assured
|
Collectability is reasonably assured Amounts received prior to satisfying the revenue recognition criteria are recorded as deferred revenue in our consolidated balance sheets. Amounts expected to be recognized as revenue within the 12 months following the balance sheet date are classified as deferred revenue, current portion. Amounts not expected to be recognized as revenue within the 12 months following the balance sheet date are classified as deferred revenue, net of current portion.
70
Collaboration revenue
As of December 31, 2014, our collaboration revenue was generated exclusively from ourWhen a collaboration arrangement with Celgene. The terms of this arrangement contains multiple deliverables, which include at inception: (i) discovery, research and development services, (ii) participation on the joint steering committee and (iii) participation on the patent committee. The collaboration arrangement also provides Celgene with the option to obtain a license to any product candidates resulting from the collaboration. Moreover, Celgene has the option to extend the term of the collaboration arrangement, firstmultiple-elements accounted for a period of two years and then for an additional period of one year. Additionally,under ASC 605, we have the sole right to manufacture or have manufactured supplies of vectors and associated payloads manufactured for incorporation into the associated product candidate in the event a product candidate is licensed. Non-refundable payments to us under this arrangement may include: (i) up-front research fees, (ii) product candidate license fees, (iii) extension term research fees, (iv) payments for the manufacture and supply of vectors and payloads, (v) payments based on the achievement of certain milestones and (vi) royalties on product sales. Additionally, we may elect to share in the costs incurred from the development, commercialization and manufacture of product candidates licensed by our collaborators and earn our share of the net profits or bear our share of the net losses generated from the sale of product candidates licensed by our collaborators.
We analyze multiple-element arrangements based on the guidance in FASB ASC Topic 605-25, Revenue Recognition-Multiple-Element Arrangements, or ASC 605-25. Pursuant to the guidance in ASC 605-25, we evaluate multiple-element arrangements to determine (1) the deliverables included in the arrangement and (2) whether the individual deliverables represent separate units of accounting or whether they must be accounted for as a combined unit of accounting. This evaluation involves subjective determinations and requires usmanagement to make judgments about the individual deliverables and whether such deliverables are separable from the other aspects of the contractual relationship. Deliverables are considered separate units of accounting provided that: (i) the delivered item(s) has value to the customer on a standalone basis and (ii) if the arrangement includes a general right of return relative to the delivered item(s), delivery or performance of the undelivered item(s) is considered probable and substantially in our control.controlled by us. In assessing whether an item has standalone value, we considerconsidered factors such as the research, manufacturing and commercialization capabilities of the collaboration partner and the availability of the associated expertise in the general marketplace. In addition, we consider whether the collaboration partner can use the other deliverable(s) for their intended purpose without the receipt of the remaining element(s), whether the value of the deliverable is dependent on the undelivered item(s) and whether there are other vendors that can provide the undelivered element(s). The collaboration arrangement does not contain a general right of return relative to the delivered item(s).
Arrangement consideration that is fixed or determinable is allocated among the separate units of accounting using the relative selling price method. Then,method and the applicable revenue recognition criteria in ASC 605 are applied to each of the separate units of accounting in determiningdetermine the appropriate period and pattern of recognition. We determine the selling price of a unit of accounting following the hierarchy of evidence prescribed by ASC 605-25. Accordingly, we determine the estimated selling price for units of accounting within each arrangement using vendor-specific objective evidence or VSOE,(“VSOE”) of selling price, if available, third-party evidence or TPE,(“TPE”) of selling price if VSOE is not available, or best estimate of selling price or BESP,(“BESP”) if neither VSOE nor TPE is available. We typically use BESP to estimate the selling price, since we generally do not have VSOE or TPE of selling price for our units of accounting. Determining the BESP for a unit of accounting requires significant judgment. In developing the BESP for a unit of accounting, we consider applicable market conditions and relevant entity-specific factors, including factors that were contemplated in negotiating the agreement with the customer and estimated costs. We validate the BESP for units of accounting by evaluating whether changes in the key assumptions used to determine the BESP will have a significant effect on the allocation of arrangement consideration between multiple units of accounting. Options are considered substantive if, at the inception of the arrangement, we are at risk as to whether the collaboration partner will choose to exercise the option. Factors that we consider in evaluating whether an option is substantive include the overall objective of the arrangement, the benefit the collaborator might obtain from the arrangement without exercising the option, the cost to exercise the option and the likelihood that the option will be exercised. For arrangements under which an option is considered substantive, we do not consider the item underlying the option to be a deliverable at the inception of the arrangement and the associated option fees are not included in allocable arrangement consideration, assuming the option is not priced at a significant and incremental discount. Conversely, for arrangements under which an option is not considered substantive or if an option is priced at a significant and incremental discount, we would consider the item underlying the option to be a deliverable at the inception of the arrangement and a corresponding amount would be included in allocable arrangement consideration. All of the options included in our collaboration arrangement have been determined to be substantive, and none of the options are priced at a significant and incremental discount. We recognize arrangement consideration allocated to each unit of accounting when all of the revenue recognition criteria in ASC 605 are satisfied for that particular unit of accounting.satisfied. We will recognize as revenue arrangement consideration attributed to licenses that have standalone value from the other deliverables to be provided in an arrangementas revenue upon delivery. We will recognize as revenueWhen arrangement consideration attributed to licenses that do not have standalone value, fromwe will recognize revenue over the other deliverables to be provided in an arrangement over our estimated performance period asof the combined deliverable. For elements of the arrangement would be accounted for as a single unit of accounting. 71
We recognize revenue from the Celgene arrangement associated with discovery, researchthat are recognized over time, and development services, joint steering committee services and patent committee services ratably over the associated period of performance. If there is no discernible pattern of performance and/or objectively measurable performance measures do not exist, then we recognize revenue under the arrangement on a straight-line basis over the period we are expect to complete our performance obligations. Conversely, if the pattern of performance in which the service is provided to the customer can be determined and objectively measurable performance measures exist, then we recognize revenue under the arrangement using the proportional performance method. Revenue
For elements of collaboration arrangements that are accounted for pursuant to ASC 808, an appropriate recognition method is determined and applied consistently, generally by analogy to ASC 605. Amounts that are owed to collaboration partners are recognized is limitedas an offset to collaboration revenues as such amounts are incurred by the lesser of the cumulative amount of payments received or the cumulative amount of revenue earned,collaboration partner. Where amounts owed to a collaboration partner exceed our collaboration revenues in each quarterly period, such amounts are classified as determined using the straight-line method or proportional performance method, as applicable, as of the period ending date.research and development expense.
At the inception of an arrangement that includes milestone payments, we evaluate whether each milestone is substantive and at risk to both parties on the basis of the contingent nature of the milestone. This evaluation includes an assessment of whether: (i) the consideration is commensurate with either our performance to achieve the milestone or the enhancement of the value of the delivered item(s) as a result of a specific outcome resulting from our performance to achieve the milestone, (ii) the consideration relates solely to past performance and (iii) the consideration is reasonable relative to all of the deliverables and payment terms within the arrangement. We evaluate factors such as the scientific, clinical, regulatory, commercial and other risks that must be overcome to achieve the respective milestone and the level of effort and investment required to achieve the respective milestone in making this assessment. There is considerable judgment involved in determining whether a milestone satisfies all of the criteria required to conclude that a milestone is substantive. We have concluded that all of the clinical and regulatory milestones pursuant to itsour collaboration arrangement are substantive. Accordingly, in accordance with FASB ASC Topic 605-28, Revenue Recognition-Milestone Method,, revenue from clinical and regulatory milestone payments will be recognized in its entirety upon successful accomplishment of the milestone, assuming all other revenue recognition criteria are met. Milestones that are not considered substantive would be recognized as revenue over the remaining period of performance, assuming all other revenue recognition criteria are met. Revenue fromAll commercial milestone paymentsmilestones will be accounted for in the same manner as royalties and recorded as revenue upon achievement of the milestone, assuming all other revenue recognition criteria are met. We will recognize royalty revenue generated under collaboration arrangements in the period of sale of the related product(s), based on the underlying contract terms, provided that the reported sales are reliably measurable and we have no remaining performance obligations, assuming all other revenue recognition criteria are met. Business combinations
On June 30, 2014, we completed our acquisition of Pregenen for total consideration of $31.0 million, consisting of cash consideration of $5.1 million, common stock consideration of $19.3 million and contingent consideration with an estimated fair value of $6.6 million. The estimated fair value of the contingent consideration is based upon significant assumptions regarding probabilities of successful achievement of related milestones, the estimated timing in which the milestones are achieved and discount rates. The estimated fair value could materially differ from actual values or fair values determined using different assumptions.
This transaction was accounted for as a business combination under the acquisition method of accounting. Accordingly, the tangible assets and identifiable intangible assets acquired and liabilities assumed were recorded at fair value as of the date of acquisition, with the remaining purchase price recorded as goodwill. The estimated fair values of acquired assets and assumed liabilities were determined using the methods discussed in the following paragraphs and require significant judgment and estimates, which could materially differ from actual values and fair values determined using different methods or assumptions.
Goodwill
Goodwill represents the excess of the purchase price over the fair value of the net assets acquired when accounted for using the acquisition method of accounting for business combinations. Goodwill is not amortized but is evaluated for impairment within our single reporting unit on an annual basis, during the fourth quarter, or more frequently if an event occurs or circumstances change that would more-likely-than-not reduce the fair value of the reporting unit below its carrying amount. We have not recognized any impairment charges related to goodwill.obligations.
Intangible assets Intangible assets consist of acquired core technology with finite lives. We amortize intangible assets using the straight-line method over their estimated economic lives. We evaluate the potential impairment of intangible assets if events or changes in circumstances indicate that the carrying amount of the assets may not be fully recoverable or that the useful lives of these assets are no longer appropriate. The impairment test is based on a comparison of the undiscounted cash flows expected to be generated from the use of the asset group and its eventual disposition to the carrying value of the asset group. If impairment is indicated, the asset is written down by the amount by which the carrying value of the asset exceeds the related fair value of the asset. We have not recognized an impairment charge related to intangible assets. 72Financing lease obligation
Beginning in 2015 through construction completion in 2017, we recorded certain estimated construction costs incurred and reported to us by the landlord for our new corporate headquarters building, located at 60 Binney Street in Cambridge, Massachusetts, as an asset and corresponding construction financing lease obligation on our consolidated balance sheets because we were deemed to be the owner of the building during the construction period for accounting purposes. During construction, the Company periodically met with the landlord and its construction manager to review these estimates and observe construction progress before recording such amounts. Upon completion of the construction of the building in the first quarter of 2017, the Company evaluated the lease and determined that it did not meet the criteria for “sale-leaseback” treatment. Accordingly, the Company is depreciating the building over 40 years and incurring interest expense in its consolidated statement of operations and comprehensive loss related to the financing lease obligation recorded on its consolidated balance sheet. Any costs incurred by us that have been reimbursed by the landlord or that qualify for reimbursement by the landlord are recorded as an asset and financing lease obligation. Any incremental costs incurred directly by us that do not qualify for reimbursement by the landlord are also capitalized. We began occupying our new corporate headquarters building on March 27, 2017. Contingent consideration Each reporting period, we revalue the contingent consideration obligations associated with business combinations to their fair value and record increasesthe resulting change in their fair value as either contingent consideration expense and decreases in the fair value as contingent considerationor income. Changes in contingent consideration result from changes in the assumptions regarding probabilities of successful achievement of related milestones, the estimated timing in which the milestones are achieved and the discount rate used to estimate the fair value of the liability. Contingent consideration may change significantly as development of our programs in certain indications progress and additional data are obtained, impacting our assumptions. The assumptions used in estimating fair value require significant judgment and the use of different assumptions and judgments could result in a materially different estimate of fair value.
Accrued research and development expenses As part of the process of preparing our financial statements, we are required to estimate our accrued expenses. This process involves reviewing open contracts and purchase orders, communicating with our personnel to identify services that have been performed on our behalf and estimating the level of service performed and the associated cost incurred for the service when we have not yet been invoiced or otherwise notified of the actual cost. The majority of our service providers invoice us monthly in arrears for services performed or when contractual milestones are met. We make estimates of our accrued expenses as of each balance sheet date in our financial statements based on facts and circumstances known to us at that time. We periodically confirm the accuracy of our estimates with the service providers and make adjustments if necessary. Examples of estimated accrued research and development expenses include fees paid to: ·
| CROs in connection with clinical studies;
|
CROs in connection with clinical studies; ·
| investigative sites in connection with clinical studies;
|
investigative sites in connection with clinical studies; ·
| vendors in connection with preclinical development activities; and
|
vendors in connection with preclinical development activities; and ·
| vendors related to product manufacturing, development and distribution of clinical supplies.
|
vendors related to development, manufacturing, and distribution of clinical trial materials. We base our expenses related to clinical studies on our estimates of the services received and efforts expended pursuant to contracts with multiple CROs that conduct and manage clinical studies on our behalf. The financial terms of these agreements are subject to negotiation, vary from contract to contract and may result in uneven payment flows. There may be instances in which payments made to our vendors will exceed the level of services provided and result in a prepayment of the clinical expense. Payments under some of these contracts depend on factors such as the successful enrollment of subjects and the completion of clinical study milestones. In accruing service fees, we estimate the time period over which services will be performed and the level of effort to be expended in each period. If the actual timing of the performance of services or the level of effort varies from our estimate, weperiod and adjust the accrual or prepaid accordingly. Although we do not expect our estimates to be materially different from amounts actually incurred, if our estimates of the status and timing of services performed differs from the actual status and timing of services performed we may report amounts that are too high or too low in any particular period. To date, there has been no material differences from our estimates to the amount actually incurred. Stock-based compensation Stock-based awards
We issue stock-based awards to employees and non-employees, generally in the form of stock options and restricted stock units. We account for our stock-based awards in accordance with FASB ASC Topic 718, Compensation—Stock Compensation, or ASC 718. ASC 718 requires all stock-based payments to employees, including grants of employee stock options and modifications to existing stock options, to be recognized in the consolidated statements of operations and comprehensive loss based on their fair values. We account for stock-based awards to non-employees in accordance with FASB ASC Topic 505-50, Equity-Based Payments to Non-Employees, which requires the fair value of the award to be remeasuredre-measured at fair value as the award vests. Our stock-based awards are subject to either service or performance-based vesting conditions. Compensation expense related to awards to employees and directors with service-based vesting conditions is recognized on a straight-line basis based on the grant date fair value over the associated service period of the award, which is generally the vesting term. Compensation expense related to awards to non-employees with service-based vesting conditions is recognized on the then-current fair value at each financial reporting date prior to the measurement date over the associated service period of the award, which is generally the vesting term, using the accelerated attribution method. Compensation expense related to awards to employees with performance-based vesting conditions is recognized based on the grant date fair value over the requisite service period using the accelerated attribution method to the extent achievement of the performance condition is probable. Compensation expense related to awards to non-employees with performance-based vesting conditions is recognized based on the then-current fair value at each financial reporting date prior to the measurement date over the requisite service period using the accelerated attribution method to the extent achievement of the performance condition is probable. 73
Described below is the methodology we have utilized in measuring stock-based compensation expense. Following the consummation of our initial public offering, stock option and restricted stock unit values have been determined based on the quoted market price of our common stock.
We estimate the fair value of our stock-based awards to employees and non-employees using the Black-Scholes option pricing model, which requires the input of highly subjective assumptions, including (i) the expected volatility of our stock, (ii) the expected term of the award, (iii) the risk-free interest rate, and (iv) expected dividends. Due to the lack of a public market for the trading of our common stock and a lack of company specific historical and implied volatility data, we based our estimate of expected volatility on the historical volatilityestimate and expected volatilities of a representative group of similar companies that are publicly traded.traded companies. For these analyses, we select companies with comparable characteristics to ours including enterprise value, risk profiles, position within the industry, and with historical share price information sufficient to meet the expected life of the stock-based awards. We compute the historical volatility data using the daily closing prices for the selected companies’ shares during the equivalent period of the calculated expected term of our stock-based awards. We will continue to apply this process until a sufficient amount of historical information regarding the volatility of our own stock price becomes available. We estimate the expected life of our employee stock options using the “simplified” method, whereby, the expected life equals the average of the vesting term and the original contractual term of the option. The risk-free interest rates for periods within the expected life of the option were based on the U.S. Treasury yield curve in effect during the period the options were granted.
We are also requiredAs a result of the adoption of the FASB Accounting Standards Update (“ASU”) 2016-09, Improvements to estimateEmployee Share-Based Payment Accounting, effective January 1, 2017, we account for forfeitures as they occur instead of estimating forfeitures at the time of grant and reviserevising those estimates in subsequent periods if actual forfeitures differ from itsour estimates. We use historical data
Recent accounting pronouncements See Note 2 to estimate pre-vesting option forfeitures and record stock-based compensation expense only for those awards that are expected to vest. To the extent that actual forfeitures differ from our estimates, the difference is recorded as a cumulative adjustment in the period the estimates were revised. Stock-based compensation expense recognized in theconsolidated financial statements is basedincluded in Part II, Item 8, “Financial Statements and Supplementary Data,” of this Annual Report on awards that are ultimately expectedForm 10-K for a description of recent accounting pronouncements applicable to vest. We have computed the fair value of employee and director stock options at date of grant using the following weighted-average assumptions:
| | Year ended December 31, | | | | 2014 | | | 2013 | | | 2012 | | Expected volatility | | | 82.3 | % | | | 82.0 | % | | | 79.6 | % | Expected term (in years) | | | 6.0 | | | | 6.1 | | | | 6.1 | | Risk-free interest rate | | | 1.8 | % | | | 1.1 | % | | | 1.0 | % | Expected dividend yield | | 0.0% | | | | 0.0 | % | | | 0.0 | % | Weighted average exercise price per share | | $ | 26.92 | | | $ | 8.59 | | | $ | 2.20 | |
Stock-based compensation totaled approximately $10.8 million for the year ended December 31, 2014 and $6.5 million for the year ended December 31, 2013. As of December 31, 2014, we had $22.1 million of total unrecognized compensation expense related to unvested stock options, net of related forfeiture estimates, which is expected to be recognized over a weighted-average remaining vesting period of approximately 2.9 years and $3.9 million of total unrecognized compensation expenses related to unvested restricted stock units, net of related forfeiture estimates, which is expected to be recognized over a weighted-average remaining vesting period of 1.5 years. We expect the impact of our stock-based compensation expense for stock options and restricted stock units granted to employees and non-employees to grow in future periods due to the current year and potential future increases in the value of our common stock and headcount.business.
Recently adopted accounting pronouncements
In May 2014, the FASB issued ASU No. 2014-09, Revenue from Contracts with Customers (Topic 606), which supersedes all existing revenue recognition requirements, including most industry-specific guidance. The new standard requires a company to recognize revenue when it transfers goods or services to customers in an amount that reflects the consideration that the company expects to receive for those goods or services. The new standard will be effective for us on January 1, 2017. We are currently evaluating the method of adoption and the potential impact that Topic 606 may have on our financial position and results of operations.
In August 2014, the FASB issued ASU No. 2014-15, Presentation of Financial Statements – Going Concern (“ASU No. 2014-15”). The new guidance addresses management’s responsibility to evaluate whether there is substantial doubt about an entity’s ability to continue as a going concern and to provide related footnote disclosures. Management’s evaluation should be based on relevant conditions and events that are known and reasonably knowable at the date that the financial statements are issued. The standard will be effective for the first interim period within annual reporting periods beginning after December 15, 2016. Early adoption is permitted. We are currently evaluating the potential impact that ASU No. 2014-15 may have on our financial position and results of operations.
74
Results of Operations Comparison of the years ended December 31, 20142017 and 2013:2016: | Year ended December 31, | | | | | | | Year Ended December 31, | | | | | | | | 2014 | | | | 2013 | | | Change | | | 2017 | | | 2016 | | | Change | | | (in thousands) | | | (in thousands) | | Revenue: | | | | | | | | | | | | | | | | | | | | | | | | Collaboration revenue | $ | 25,031 | | | $ | 19,792 | | | $ | 5,239 | | | $ | 22,207 | | | $ | 6,155 | | | $ | 16,052 | | Research and license fees | | 390 | | | | 389 | | | | 1 | | | Total revenue | | 25,421 | | | | 20,181 | | | | 5,240 | | | License and royalty revenue | | | | 13,220 | | | | — | | | | 13,220 | | Total revenues | | | | 35,427 | | | | 6,155 | | | | 29,272 | | Operating expenses: | | | | | | | | | | | | | | | | | | | | | | | | Research and development | | 62,574 | | | | 31,002 | | | | 31,572 | | | | 273,040 | | | | 204,775 | | | | 68,265 | | General and administrative | | 23,227 | | | | 14,126 | | | | 9,101 | | | | 93,550 | | | | 65,119 | | | | 28,431 | | Cost of license and royalty revenue | | | | 1,527 | | | | — | | | | 1,527 | | Change in fair value of contingent consideration | | 246 | | | | — | | | | 246 | | | | (525 | ) | | | 4,091 | | | | (4,616 | ) | Total operating expenses | | 86,047 | | | | 45,128 | | | | 40,919 | | | | 367,592 | | | | 273,985 | | | | 93,607 | | Loss from operations | | (60,626 | ) | | | (24,947 | ) | | | 35,679 | | | | (332,165 | ) | | | (267,830 | ) | | | 64,335 | | Other income (expense), net | | 120 | | | | (374 | ) | | | (494 | ) | | Interest (expense) income, net | | | | (2,001 | ) | | | 3,782 | | | | 5,783 | | Other expense, net | | | | (1,267 | ) | | | (71 | ) | | | 1,196 | | Loss before income taxes | | (60,506 | ) | | | (25,321 | ) | | | 35,185 | | | | (335,433 | ) | | | (264,119 | ) | | | 71,314 | | Benefit from income taxes | | 11,797 | | | | — | | | | (11,797 | ) | | Income tax benefit (expense) | | | | (210 | ) | | | 612 | | | | 822 | | Net loss | $ | (48,709 | ) | | $ | (25,321 | ) | | $ | 23,388 | | | $ | (335,643 | ) | | $ | (263,507 | ) | | $ | 72,136 | |
Revenue. Total revenue was $25.4$35.4 million for the year ended December 31, 2014,2017, compared to $20.2$6.2 million for the year ended December 31, 2013.2016. The increase of $5.2$29.3 million was primarily dueattributable to a full yearcollaboration revenue recognized associated with the bb2121 license and manufacturing services which commenced in the first quarter of 2017 and revenue fromrecognized under our Celgene collaboration, which was signed on March 19, 2013out-licensing arrangements with Novartis and is expected to be recognized on a straight-line basis through March 2016.GSK.
Research and development expenses. Research and development expenses were $62.6$273.0 million for the year ended December 31, 2014,2017, compared to $31.0$204.8 million for the year ended December 31, 2013.2016. The increase of $31.6 $68.3 million was primarily attributable to the following: $22.1 million of employee compensation and benefits, inclusive of $6.9 million increased stock-based compensation expense, as well as $2.2 million in other headcount related costs primarily due to an increase in headcount to support overall growth; $17.7 million of manufacturing costs for our ongoing clinical and pre-clinical studies; $12.1 million of clinical trial-related costs to support the advancement of our clinical programs; $4.4 million of facility related costs; $3.5 million of license and milestone fees; and $3.0 million related to cost reimbursement to Celgene for costs incurred under our collaboration arrangement General and administrative expenses. General and administrative expenses were $93.6million for the year ended December 31, 2017, compared to $65.1 million for the year ended December 31, 2016. The increase of approximately $28.4 million was primarily due an increase of $14.9 million in employee compensation and benefits, inclusive of $6.6 million increased stock-based compensation expense, $2.3 million in other headcount related costs, increased commercial-related costs of $8.6 million primarily attributed to market research costs, and increased facility-related costs of $2.1 million. Cost of license and royalty revenue. Cost of license and royalty revenue was $1.5 million for the year ended December 31, 2017. The costs are attributable to expense associated with amounts owed to third party licensors in connection with revenue recognized under our out-license arrangements with Novartis and GSK. Change in fair value of contingent consideration. The change in fair value of contingent consideration of $4.6 million was primarily related to the successful achievement of one milestone in 2017 compared to the achievement of two milestones in 2016, and a decrease in the probability of successful achievement of future milestones within the next twelve months. Interest (expense) income, net. The change in interest (expense) income, net was primarily related to interest expense on the 60 Binney financing obligation partially offset by interest income earned on investments. Other expense, net. Other expense, net was $1.3 million for the year ended December 31, 2017, compared to $0.1 million for the year ended December 31, 2016. The increase was primarily related to a loss on the disposal of assets. Comparison of the years ended December 31, 2016 and 2015: | | Year Ended December 31, | | | | | | | | 2016 | | | 2015 | | | Change | | | | (in thousands) | | Revenue: | | | | | | | | | | | | | Collaboration revenue | | $ | 6,155 | | | $ | 14,079 | | | $ | (7,924 | ) | Total revenue | | | 6,155 | | | | 14,079 | | | | (7,924 | ) | Operating expenses: | | | | | | | | | | | | | Research and development | | | 204,775 | | | | 134,038 | | | | 70,737 | | General and administrative | | | 65,119 | | | | 46,209 | | | | 18,910 | | Change in fair value of contingent consideration | | | 4,091 | | | | 2,869 | | | | 1,222 | | Total operating expenses | | | 273,985 | | | | 183,116 | | | | 90,869 | | Loss from operations | | | (267,830 | ) | | | (169,037 | ) | | | 98,793 | | Interest income, net | | | 3,782 | | | | 1,591 | | | | (2,191 | ) | Other (expense) income, net | | | (71 | ) | | | 723 | | | | 794 | | Loss before income taxes | | | (264,119 | ) | | | (166,723 | ) | | | 97,396 | | Income tax (expense) benefit | | | 612 | | | | (60 | ) | | | (672 | ) | Net loss | | $ | (263,507 | ) | | $ | (166,783 | ) | | $ | 96,724 | |
Revenue. Total revenue was $6.2 million for the year ended December 31, 2016, compared to $14.1 million for the year ended December 31, 2015. The decrease of $7.9 million was primarily due to a change in revenue recognition resulting from the amendment to our Celgene collaboration in 2015.
Research and development expenses. Research and development expenses were $204.8 million for the year ended December 31, 2016, compared to $134.0 million for the year ended December 31, 2015. The increase of $70.7million was primarily due to the increase in headcount, in-licensing costs, clinical trial-related costs, and manufacturing-related expenses necessary to support the advancement of our product candidates into clinical trials, as well as expenses for the Celgene collaboration, and included in the following increasesincreases: $9.2 million of employee compensation and benefits, primarily due to a $14.3 million increase in expenses:payroll and payroll-related expense due to an increase in headcount, offset by a $5.2 million decrease in stock-based compensation expense due to non-recurring stock-based compensation charges incurred in 2015 and not in 2016 related to the modification of awards of our former Chief Scientific Officer and a non-employee founder. ·
| Direct research and development expenses:
|
·
| $8.4 million of employee compensation and benefits, of which $1.3 million was related to stock-based compensation expense.
|
$2.2 million of consulting and contractor costs to support our overall growth. ·
| $6.8 million of manufacturing costs for our ongoing clinical studies.
|
$26.1 million of manufacturing costs for our ongoing clinical and pre-clinical studies. ·
| $6.3 million of clinical trial-related costs.
|
$6.6 million of increased license and milestone fees, primarily due to a $15.0 million one-time upfront payment made in 2016 offset by other license and milestone fees incurred during 2015 and 2016. ·
| $2.9 million of direct project lab supplies related to increased headcount and process development activities.
|
$4.0 million of clinical trial-related costs to support the advancement of our clinical programs. ·
| $1.0 million of license fees.
|
$5.7 million of laboratory supplies related to increased headcount and process development activities. $13.0 million of facilities and information technology expenses. ·
$2.1 million of ongoing expenses related to sponsored research agreements. | $2.5 million in rent and other facility-related expenses related to our new corporate headquarters.
|
·
| $1.9 million in amortization of our gene editing platform intangible asset related to our acquisition of Pregenen.
|
·
| $0.8 million in expenses related to ongoing collaboration agreements.
|
·
| $0.8 million in costs associated with preclinical research activities.
|
General and administrative expenses. General and administrative expenses were $23.2 $65.1million for the year ended December 31, 2014,2016, compared to $14.1$46.2 million for the year ended December 31, 2013.2015. The increase of $9.1$18.9 million was primarily due to the following increasesan increase of $11.1 million in expenses: $5.9 million of employee-related costs to support our overall growth and $8.0 million of which $2.4consulting costs to support our overall growth as well as pre-commercial efforts. Change in fair value of contingent consideration. The change in fair value of contingent consideration of $1.2 million was primarily related to stock-based compensation expensethe successful achievement of two milestones in 2016 and $0.8 million was relatedan increase in the probability of successful achievement of a future milestone expected to a one-time severance charge; $1.0 million of professional fees to supportbe achieved within the requirements of being a public company; $0.2 million in rent and other facility-related expenses related to our new corporate headquarters to accommodate increased headcount; $0.7 million in general office expenses as a result of increased headcount and $0.9 million in depreciation and amortization relating to our fixed assets, including our new corporate headquartersnext twelve months. 75
OtherInterest income, (expense), net. OtherInterest income, (expense), net, was $0.1$3.8 million for the year ended December 31, 2014,2016, compared to $(0.4)$1.6 million for the year ended December 31, 2013.2015. The decrease of $0.5 millionincrease was primarily due to the re-measurement of fair value of our convertible preferred stock warrants in 2013, foreign currency gain and interest income.
Comparison of the years ended December 31, 2013 and 2012:
| Year ended December 31, | | | | | | | | 2013 | | | | 2012 | | | Change | | | (in thousands) | | Revenue: | | | | | | | | | | | | Collaboration revenue | $ | 19,792 | | | $ | — | | | $ | 19,792 | | Research and license fees | | 389 | | | | 340 | | | | 49 | | Total revenue | | 20,181 | | | | 340 | | | | 19,841 | | Operating expenses: | | | | | | | | | | | | Research and development | | 31,002 | | | | 17,210 | | | | 13,792 | | General and administrative | | 14,126 | | | | 6,846 | | | | 7,280 | | Total operating expenses | | 45,128 | | | | 24,056 | | | | 21,072 | | Loss from operations | | (24,947 | ) | | | (23,716 | ) | | | 1,231 | | Other income (expense), net | | (374 | ) | | | 46 | | | | 420 | | Net loss | $ | (25,321 | ) | | $ | (23,670 | ) | | $ | 1,651 | |
Revenue. Total revenue was $20.2 million for the year ended December 31, 2013, compared to $0.3 million for the year ended December 31, 2012. The increase of $19.8 million was primarily due to the Celgene collaboration. In the year ended December 31, 2013, we recorded $19.8 million of the up-front payment related to research and development services from the Celgene collaboration, which was entered into in March 2013 and is expected to be recognizedinterest income earned on a straight-line basis through March 2016, and $0.4 million of research and license fees.
Research and development expenses. Research and development expenses were $31.0 million for the year ended December 31, 2013, compared to $17.2 million for the year ended December 31, 2012. The increase of $13.8 million was primarily due to the increase in headcount and clinical trial related expenses to support the advancement of our programs, including the new Celgene collaboration, and included the following increases in expenses:
·
| Direct research and development expenses:
|
·
| $0.7 million of materials production costs in preparation for and upon initiation of the Starbeam, Northstar and HGB-205 clinical studies.
|
·
| $2.1 million of clinical trial related costs related to initiation of clinical studies in 2013.
|
·
| $0.5 million of costs for clinical and regulatory consultants to support regulatory filing and other clinical start-up activities.
|
·
| $1.6 million of platform and direct project lab supplies related to increased headcount and scale up process development activities.
|
·
| Personnel and other expenses:
|
·
| $6.8 million of employee compensation and benefits to support increased development activities related to the three clinical studies initiated and in support of preclinical programs in 2013. The increased headcount resulted in an incremental $0.2 million of recruiting and $0.4 million of travel expense.
|
·
| $1.6 million in facility-related expenses to accommodate increased lab headcount.
|
·
| $0.3 million in accelerated depreciation due to the shortened expected useful life of assets relating to our former corporate headquarters.
|
General and administrative expenses. General and administrative expenses were $14.1 million for the year ended December 31, 2013, compared to $6.8 million for the year ended December 31, 2012. The increase of $7.3 million was primarily due to the following increases in expenses: $4.3 million of employee-related costs to support our overall growth; $1.0 million of contractors and consultants expenses and $0.7 million of professional fees to support the requirements of being a public company; $0.5 million in general office expenses as a result of increased headcount; and $0.3 million in accelerated depreciation due to the shortened expected useful life of assets relating to our former corporate headquarters.
76
Other income (expense), net. Other income (expense), net, was $(0.4) million for the year ended December 31, 2013, compared to $0.05 million for the year ended December 31, 2012. The decrease of $0.4 million was primarily due to the re-measurement of fair value of our convertible preferred stock warrants and foreign currency gain.marketable securities.
Liquidity and Capital Resources As of December 31, 2014,2017, we had cash, cash equivalents and marketable securities of approximately $492.0 million.$1.6 billion. We expect cash, cash equivalents and marketable securities to fund operations through 2017.into 2021. Cash in excess of immediate requirements is invested in accordance with our investment policy, primarily with a view to liquidity and capital preservation. As of December 31, 2014,2017, our funds are primarily held inU.S. Treasury securities, U.S. government agency securities, federally insured deposits, certificates of deposit and money market mutual funds invested in U.S. Treasuries or U.S. government agency securities.accounts. We have incurred losses and cumulative negative cash flows from operations since our inception in April 1992, and as of December 31, 2014,2017, we had an accumulated deficit of $147.4$913.8 million. We anticipate that we will continue to incur losses for at least the next several years. We expect that our research and development and general and administrative expenses will continue to increase and, as a result, we will need additional capital to fund our operations, which we may raise through a combination ofpublic or private equity offerings,or debt financings, strategic collaborations, or other third-party funding, marketing and distribution arrangements and other collaborations, strategic alliances and licensing arrangements.sources. We have funded our operations principally from the sale of common stock, preferred stock and through the Celgene collaboration. On June 24, 2013, we completed our initial public offering, or IPO, whereby29, 2015, we sold 6,832,3522,941,176 shares of common stock through an underwritten public offering at a price of $17.00$170.00 per share for aggregate net proceeds received byto us of $104.9$477.2 million. On July 14, 2014,December 12, 2016, we sold 3,450,0003,289,473 shares of common stock through an underwritten public offering at a price of $76.00 per share for aggregate net proceeds to us of $234.7 million. On June 27, 2017, we sold 4,381,500 shares of common stock (inclusive of 450,000571,500 shares of common stock sold by us pursuant to the full exercise of an overallotment option granted to the underwriters in connection with the offering) through an underwritten public offering at a price of $34.00$105.00 per share for aggregate net proceeds to us of $109.8$436.8 million. On December 19, 2014,15, 2017, we sold 3,047,5003,243,244 shares of common stock (inclusive of 397,500(excluding any shares of common stock sold by us pursuant to the full exercise of an overallotment option granted to the underwriters in connection with the offering) through an underwritten public offering at a price of $85.00$185.00 per share for aggregate net proceeds to us of $243.3$569.8 million. In January 2018, we sold 277,109 shares of common stock pursuant to the partial exercise of an overallotment option granted to the underwriters
in connection with the December 2017 underwritten public offering at a price of $185.00 per share for aggregate net proceeds of $48.6 million. Sources of Liquidity Cash Flows The following table sets forth the primary sources and uses ofsummarizes our cash for each of the periods below:flow activity: | Year ended December 31, | | | | 2014 | | | | 2013 | | | | 2012 | | | (in thousands) | | Net cash provided by (used in): | | | | | | | | | | | | Operating activities | $ | (59,693 | ) | | $ | 43,450 | | | $ | (21,044 | ) | Investing activities | | (157,193 | ) | | | (9,823 | ) | | | 2,599 | | Financing activities | | 358,452 | | | | 105,641 | | | | 59,852 | | Net increase in cash and cash equivalents | $ | 141,566 | | | $ | 139,268 | | | $ | 41,407 | |
| Year Ended December 31, | | | | 2017 | | | | 2016 | | | | 2015 | | | (in thousands) | | Net cash used in operating activities | $ | (280,553 | ) | | $ | (189,647 | ) | | $ | (98,429 | ) | Net cash (used in) provided by investing activities | | (316,003 | ) | | | 62,731 | | | | (571,867 | ) | Net cash provided by financing activities | | 1,076,174 | | | | 241,534 | | | | 486,720 | | Net increase (decrease) in cash and cash equivalents | $ | 479,618 | | | $ | 114,618 | | | $ | (183,576 | ) |
Cash Flows from Operating Activities. The net cash used in operating activities was $59.7$280.6 million for the year ended December 31, 20142017 and primarily consisted of a net loss of $48.7 million adjusted for non-cash items including a noncash benefit on release of tax valuation allowance of $11.8 million, stock-based compensation of $10.8 million, depreciation and amortization of $4.2 and a net decrease in operating assets and liabilities of $14.7 million. The significant items in the decrease in operating assets and liabilities include a decrease in deferred revenue of $24.9 million due to amortization of the up-front payment related to the Celgene collaboration, a decrease in accounts payable of $2.2 million and a decrease in prepaid expenses and other assets of $0.3 million offset by an increase in accrued expenses and other liabilities of $10.0 million and an increase in deferred rent of $2.0 million.
The net cash provided by operating activities was $43.5 million for the year ended December 31, 2013 and primarily consisted of a net loss of $25.3$335.6 million adjusted for non-cash items including stock-based compensation of $6.5$53.3 million, depreciation and amortization of $0.9 million, re-measurement of warrants of $0.4$13.5 million and a net increase in operating assets and liabilities of $60.9$12.7 million. The increase in operating assets and liabilities is driven by an increase in prepaid expenses and other current assets of $20.1 million primarily driven by upfront payments to contract manufacturing organizations offset by an increase of $5.9 million in accounts payable, accrued expenses and other liabilities and an increase of $1.0 million in deferred revenue.
The net cash used in operating activities was $189.6 million for the year ended December 31, 2016 and primarily consisted of a net loss of $263.5 million adjusted for non-cash items including stock-based compensation of $39.8 million, depreciation and amortization of $9.6 million and a net increase in operating assets and liabilities of $19.0 million. The increase in operating assets and liabilities is driven by an increase in prepaid expenses and other current assets of $14.3 million for upfront payments to contract manufacturing organizations offset by an increase of $20.9 million in accrued expenses and other liabilities related to an increase in manufacturing and clinical-trial related costs for our ongoing clinical and pre-clinical studies. The net cash used in operating activities was $98.4 million for the year ended December 31, 2015 primarily consisted of a net loss of $166.8 million adjusted for non-cash items including stock-based compensation of $41.1 million, depreciation and amortization of $7.4 and a net increase in operating assets and liabilities of $16.0 million. The significant items in the increase in operating assets and liabilities include an increase in deferred revenue of $54.9$11.2 million due to the up-front payment related to the Celgeneamendment to our collaboration an increase in deferred rent of $7.4 million related to leasehold improvements at our new corporate headquarters,with Celgene and an increase in accounts payableaccrued expenses of $2.1$9.4 million slightlyrelated to an increase in accrued goods and services and an increase in the contingent consideration, offset by an increasea decrease in prepaid expenses and other assets of $4.2 million.$6.8 million due to purchases of marketable securities at a premium. The net cash used in operating activities was $21.0 million for the year ended December 31, 2012, and consisted primarily of a net loss of $23.7 million adjusted for non-cash items including stock-based compensation expense of $0.8 million and depreciation of
77
$0.3 million and a net increase in operating assets and liabilities of $1.5 million. The significant items in the change in operating assets and liabilities include an increase in accounts payable of $0.4 million and accrued expenses and other liabilities of $1.4 million and a decrease in prepaid expenses and current assets of $0.1 million, offset by a decrease in deferred revenue of $0.3 million.
Cash Flows from Investing Activities. Net cash used in investing activities for the year ended December 31, 20142017 was $157.2$316.0 million and was primarily due to the purchase of $175.0$686.2 million of available-for-sale marketable securities and the purchase of fixed assets$62.2 million of $8.7 millionproperty, plant and cash paid in connection with the acquisition of Pregenen of $4.7 million. The fixed asset purchases primarily consisted of leasehold improvements for the build-out of our new corporate headquarters. These decreases were partiallyequipment offset by $31.0 million in proceeds from the maturities of investments.available-for-sale marketable securities of $431.8 million.
Net cash provided by investing activities for the year ended December 31, 2016 was $62.7 million and was primarily due to proceeds from the maturities of available-for-sale marketable securities of $443.4 million offset by the purchase of $348.2 million of available-for-sale marketable securities. Net cash used in investing activities for the year ended December 31, 20132015 was $9.8$571.9 million and consistedwas primarily due to the purchase of purchases$755.2 million of property and equipment of $8.7available-for-sale marketable securities offset by $199.2 million and the new $1.3 million cash-collateralized irrevocable standby letter of credit on the corporate headquarters lease that we signed in June 2013. The fixed asset purchases primarily consisted of leasehold improvements at our new corporate headquarters and purchases of lab equipment for the additional lab space added during the first quarter of 2013 and lab equipment to support the start-up of the Celgene program. The new $1.3 million letter of credit, naming our landlord as beneficiary, is reduced to $1.0 million, $0.8 million, and $0.6 million upon the rent commencement date and the first and second anniversaries of the rent commencement date, respectively. Net cash provided by investing activities for the year ended December 31, 2012 was $2.6 million and consisted primarily of proceeds from the salematurities of available-for-sale marketable securities of $3.5 million slightly offset by purchases of property and equipment of $0.9 million.securities.
Cash Flows from Financing Activities: Net cash provided by financing activities for the year ended December 31, 20142017 was $358.5 million$1.1 billion and was primarily due to net cash proceeds from our JulyJune 2017 and December 20142017 common stock offerings.
Net cash provided by financing activities for the year ended December 31, 20132016 was $105.6$241.5 million and was primarily due to the issuance of 6,832,352net cash proceeds from our December 2016 common stock related to our IPO that closed on June 24, 2013, for total proceeds of $104.9 million, the repayment of a non-recourse note collateralized by restricted stock of $0.3 million, and proceeds from the exercise of common stock options of $0.4 million.offering.
Net cash provided by financing activities for the year ended December 31, 20122015 was $486.7 million and was primarily due to proceeds from our June 2015 common stock offering and $10.1 million proceeds from the resultissuance of common stock, primarily related to the saleexercise of 120.4 million shares of our Series D preferred stock for net proceeds of $59.8 million.options. Contractual Obligations and Commitments The following table summarizes our contractual obligations at December 31, 2014.2017. These do not include potential milestone payments and assume non-termination of agreements. | Total | | | 2015 | | | 2016 through 2017 | | | 2018 through 2019 | | | After 2019 | | | (in thousands) | | 150 Second Street Lease | $ | 28,152 | | | $ | 3,166 | | | $ | 6,619 | | | $ | 7,023 | | | $ | 11,344 | | Facility operating leases, excluding 150 Second Street Lease (1) | | 717 | | | | 486 | | | | 231 | | | | — | | | | — | | License costs (2) | | 3,086 | | | | 851 | | | | 1,400 | | | | 835 | | | | — | | Total | $ | 31,955 | | | $ | 4,503 | | | $ | 8,250 | | | $ | 7,858 | | | $ | 11,344 | |
| Total | | | 2018 | | | 2019 through 2020 | | | 2021 through 2022 | | | After 2023 | | | (in thousands) | | 60 Binney Street lease | $ | 185,444 | | | $ | 18,647 | | | $ | 38,279 | | | $ | 39,631 | | | $ | 88,887 | | Non-cancellable operating leases (1) | | 81,494 | | | | 13,941 | | | | 23,012 | | | | 16,916 | | | | 27,625 | | License costs (2) | | 6,080 | | | | 1,171 | | | | 2,387 | | | | 2,522 | | | | — | | Clinical and manufacturing development | | 24,000 | | | | 12,000 | | | | 12,000 | | | | — | | | | — | | Total contractual obligations | $ | 297,018 | | | $ | 45,759 | | | $ | 75,678 | | | $ | 59,069 | | | $ | 116,512 | |
(1) | Excludes $0.2 millionIncludes the lease of sublease receipts for 2015 for our 840 Memorial Drive leased space.lab and office space in Seattle, Washington and rental payments associated with two embedded operating leases at contract manufacturing organizations.
|
(2) | License costs include annual license maintenance fee payments. We have not included annual license maintenance fees or minimum royalty payments after December 31, 2019,2022, as we cannot estimate if they will occur. |
60 Binney Street Lease On September 21, 2015, we entered into a lease agreement for office and laboratory space located at 60 Binney Street, Cambridge, Massachusetts. Under the terms of the lease, starting on October 1, 2016, we leased approximately 253,108 square feet at $72.50 per square foot per year, or $18.4 million per year in base rent, which is subject to scheduled annual rent increases of 1.75% plus certain operating expenses and taxes. We also executed a $9.2 million letter of credit upon signing the lease, which was required to be collateralized with a bank account at a financial institution in accordance with the lease agreement. This letter of credit was increased to $13.8 million during the third quarter of 2016 as required under the terms of the lease. Subject to the terms of the lease and certain reduction requirements specified therein, including market capitalization requirements, this amount may decrease back to $9.2 million over time. The lease will continue until March 31, 2027. Pursuant to a work letter entered into in connection with the lease, the landlord will contribute an aggregate of $42.4 million toward the cost of construction and tenant improvements for the building. The purpose of the lease was to replace our previously leased premises at 150 Second Street and 215 First Street in Cambridge, Massachusetts, both of which were fully exited during the first half of 2017. We occupied the building at 60 Binney Street beginning on March 27, 2017. Operating Leases On June 3, 2013, we entered into a nine-year building lease for approximately 43,600 square feet of space located at 150 Second Street, Cambridge, Massachusetts, which commenced in December 2013. This lease was amended in June 2014 to add approximately 9,900 additional square feet. The lease originally had monthly lease payments of $0.2 million for the first 12 months, which increased to $0.3 million per month beginning in December 2014 due to the lease amendment, with annual rent escalations thereafter. Rent expense was recognized on a straight-line basis over the term of the lease through April 2017. The lease provided a contribution from the landlord towards the initial build-out of the space of up to $7.8 million. We capitalized the leasehold improvements as property, plant and equipment and recorded the landlord incentive payments received as deferred rent and amortized these amounts as reductions to rent expense over the lease term. In addition, in accordance with the lease, we entered into a cash-collateralized irrevocable standby letter of credit in the amount of $1.3 million, naming the landlord as beneficiary, which had a balance of $0.6 million as of December 31, 2016. On September 30, 2016, we entered into an Assignment and Assumption of Lease (“Assignment”) relating to this lease. Under the Assignment, we assigned all of our rights, interests, obligations and responsibilities under the lease, effective May 1, 2017. Accordingly, $8.3 million of tenant improvement assets were disposed and $8.0 million of non-current deferred rent was removed from the consolidated balance sheets as of December 31, 2017, with the resulting loss of $0.3 million recorded within the consolidated statement of operations and comprehensive loss during the year ended December 31, 2017. The $0.6 million letter of credit was also released during the year ended December 31, 2017.
On June 29, 2015, we entered into a lease agreement for additional office space located at 215 First Street, Cambridge, Massachusetts. Under the terms of the lease, we leased approximately 15,120 square feet starting on July 13, 2015 for $0.5 million per year in base rent, which was subject to a 3% annual rent increase plus certain operating expenses and taxes. The lease term was until August 31, 2020, and included early termination provisions that could allow us to terminate the lease without penalty at the end of the 20th full calendar month following the delivery of the premises if we met certain conditions specified within the lease. Under the terms of the lease, we also leased an additional 8,075 square feet of office space in the same premises starting on January 1, 2016 for an additional $0.3 million per year in base rent, which was subject to a 3% annual rent increase plus certain operating expenses and taxes. The Company terminated this lease effective April 12, 2017. On June 3, 2016, we entered into a strategic manufacturing agreement for the future commercial production of the our Lenti-D and LentiGlobin product candidates with a contract manufacturing organization. Under this 12 year agreement, the contract manufacturing organization will complete the design, construction, validation and process validation of the leased suites prior to anticipated commercial launch of the product candidates. During construction, we are required to pay $12.5 million upon the achievement of certain contractual milestones, and may pay up to $8.0 million in additional contractual milestones if we elect its option to lease additional suites. We paid $5.0 million for the achievement of the first and second contractual milestones during 2016 and paid the third milestone of $3.0 million during the first quarter of 2017. Additionally, the fourth milestone of $2.5 million was achieved in the fourth quarter of 2017 and is reflected as a component of accrued expenses and other current liabilities within the consolidated balance sheet at December 31, 2017. Following construction completion, we will pay $5.1 million per year in fixed suite fees as well as certain fixed labor, raw materials, testing and shipping costs for manufacturing services, and may pay additional suite fees if it elects its option to reserve or lease additional suites. We estimate completion will occur in 2018. We may terminate this agreement any time after upon payment of a one-time termination fee and up to 24 months of fixed suite and labor fees. We concluded that this agreement contains an embedded lease as the suites are designated for our exclusive use during the term of the agreement. We concluded that we are not the deemed owner during construction nor is it a capital lease under ASC 840-10, Leases – Overall. As a result, we account for the agreement as an operating lease and expenses the rental payments on a straight-line basis over the term of the embedded lease. On November 18, 2016, we entered into an agreement for future clinical and commercial production of our LentiGlobin gene therapy drug products with a contract manufacturing organization at an existing facility. The term of the agreement is five years with a three year renewal at the mutual option of each party. Under the agreement, we are required to pay an up-front fee of €3.0 million, €2.0 million of which was paid in the fourth quarter of 2016 and €1.0 million of which is expected to be paid in mid-2018, and annual maintenance and production fees of up to €9.8 million, depending on its production needs. We may terminate this agreement with six months’ notice and a one-time termination fee prior to July 1, 2018, or twelve months’ notice and a one-time termination fee thereafter. We concluded that this agreement contains an embedded lease as the clean rooms are designated for our exclusive use during the term of the agreement, and determined that it is not a capital lease under ASC 840-10, Leases – Overall. As a result, we will account for the agreement as an operating lease and expense the rental payments on a straight-line basis over the term of the embedded lease. We also have obligations to make future payments to third parties that become due and payable on the achievement of certain development, regulatory and commercial milestones (such as the start of a clinical trial, filing of a BLA, approval by the FDA or product launch). We have not included these commitments on our balance sheet or in the table above because the achievement and timing of these milestones is not fixed and determinable. These commitments include: Contingent Consideration Related to Business Combinations In connection with the Pregenen acquisition, we agreed to make contingent cash payments to the former equity holders of Pregenen. In accordance with accounting guidance for business combinations, these contingent cash payments are recorded as contingent consideration liabilities on our consolidated balance sheets at fair value. During the second quarter of 2017, a $5.0 million preclinical milestone was achieved, which resulted in a $5.0 million payment to the former equityholders of Pregenen during the third quarter of 2017. The aggregate remaining undiscounted amount of contingent consideration potentially payable is $120.0 million. We have not included these payments in the table above because the achievement and timing of these milestones is not fixed and determinable. As of December 31, 2017, $2.2 million is reflected as a non-current liability in the consolidated balance sheet, and as of December 31, 2016, $4.5 million and $3.3 million was reflected as a current and non-current liability, respectively, in the consolidated balance sheet, which represents the fair value of our contingent consideration obligations as of this date. ·
| In connection with the Pregenen acquisition, we agreed to make contingent cash payments to the former equityholders of Pregenen. In accordance with accounting for business combinations guidance, these contingent cash payments are recorded as contingent consideration liabilities on our consolidated balance sheets at fair value. The aggregate, undiscounted amount of contingent consideration potentially payable is $135.0 million.
|
Contingent Milestone and Royalty Payments Based on our development plans as of December 31, 2017, we may be obligated to make future development, regulatory and commercial milestone payments and royalty payments on future sales of specified products associated with our collaboration and license agreements. Payments under these agreements generally become due and payable upon achievement of such milestones or sales. Because the achievement of these milestones or sales had not occurred as of December 31, 2017, such contingencies have not been recorded in our financial statements. Amounts related to contingent milestone payments and sales-based royalties are not yet considered contractual obligations as they are contingent upon success. Under a license agreement with Inserm-Transfert pursuant to which we license certain patents and know-how for use in adrenoleukodystrophy therapy, we will be required to make payments based upon development, regulatory and commercial milestones for any products covered by the in-licensed intellectual property. The maximum aggregate payments we may be obligated to pay for each of these milestone categories per product is €0.3, €0.2 and €1.6 million, respectively. We will also be required to pay a royalty on net sales of products covered by the in-licensed intellectual property in the low single digits. The royalty is subject to reduction for any third-party payments required to be made, with a minimum floor in the low single digits. Under a license agreement with Institut Pasteur pursuant to which we license certain patents for use in ex vivo gene therapy, we will be required to make payments per product covered by the in-licensed intellectual property upon the achievement of development and regulatory milestones, depending on the indication and the method of treatment. The maximum aggregate payments we may be obligated to pay for each of these milestone categories per product is €1.5 and €2.0 million, respectively. We will also be required to pay a royalty on net sales of products covered by the in-licensed intellectual property in the low single digits, which varies slightly depending on the indication of the product. We have the right to sublicense our rights under this agreement, and we will be required to pay a percentage of such license income varying from the low single digits to mid-range double digits depending on the nature of the sublicense and stage of development. We are required to make an annual maintenance payment, which is creditable against royalty payments on a year-by-year basis. On April 1, 2015, we amended this license agreement with Institut Pasteur, which resulted in a payment of €3.0 million that was paid during the second quarter of 2015. During the year ended December 31, 2017 we paid Institut Pasteur €1.0 million in connection with amounts owed to us by sublicensees. Under a license agreement with the Board of Trustees of the Leland Stanford Junior University, or Stanford, pursuant to which we license the HEK293T cell line for use in gene therapy products, we are required to pay a royalty on net sales of products covered by the in-licensed intellectual property in the low single digits that varies with net sales. The royalty is reduced for each third-party license that requires payments by us with respect to a licensed product, provided that the royalty to Stanford is not less than a specified percentage that is less than one percent. We have been paying Stanford an annual maintenance fee, which will be creditable against our royalty payments. ·
| Under a license agreement with Inserm-Transfert pursuant to which we license certain patents for use in human adrenoleukodystrophy therapy, we will be required to make payments based upon development, regulatory and commercial milestones for any products covered by the in-licensed intellectual property. The maximum aggregate payments we may be
|
78
| obligated to pay for each of these milestone categories per product is €0.3, €0.2 and €1.6 million, respectively. We will also be required to pay a royalty on net sales of products covered by the in-licensed intellectual property in the low single digits. The royalty is subject to reduction for any third-party payments required to be made, with a minimum floor in the low single digits.
|
·•
| Under a license agreement with Institut Pasteur pursuant to which we license certain patents for use in ex vivo gene therapy, we will be required to make payments per product covered by the in-licensed intellectual property upon the achievement of development and regulatory milestones, depending on the indication and the method of treatment. The maximum aggregate payments we may be obligated to pay for each of these milestone categories per product is €1.5 and €2.0 million, respectively. We will also be required to pay a royalty on net sales of products covered by the in-licensed intellectual property in the low single digits, which varies slightly depending on the indication of the product. We have the right to sublicense our rights under this agreement, and we will be required to pay a percentage of such license income varying from the low single digits to mid-double digits depending on the nature of the sublicense. Starting in 2016, we will be required to make an annual maintenance payment, which is creditable against royalty payments on a year-by-year basis.
|
·
| Under a license agreement with the Board of Trustees of the Leland Stanford Junior University, or Stanford, pursuant to which we license the HEK293T cell line for use in gene therapy products, we are required to pay a royalty on net sales of products covered by the in-licensed intellectual property in the low single digits that varies with net sales. The royalty is reduced for each third-party license that requires payments by us with respect to a licensed product, provided that the royalty to Stanford is not less than a specified percentage that is less than one percent. We are required to pay Stanford an annual maintenance fee based on net sales of licensed products, which is creditable against our royalty payments.
|
·
| Under a license agreement with the Massachusetts Institute of Technology, or MIT, pursuant to which we license various patents, we will be required to make a payment of $0.1 million based upon a regulatory filing milestone. We will also be required to pay a royalty on net sales of products covered by the in-licensed intellectual property by us or our sublicensees. The royalty is in the low single digits and is reduced for royalties payable to third parties, provided that the royalty to MIT is not less than a specified percentage that is less than one percent. We have the right to sublicense our rights under this agreement, and we will be required to pay a percentage of such license income varying from the mid-single digits to low double digits. We are required to pay MIT an annual maintenance fee based on net sales of licensed products, which is creditable against our royalty payments. |
·
| Under a license agreement with Research Development Foundation pursuant to which we license patents that involve lentiviral vectors, we will be required to make payments of $1.0 million based upon a regulatory milestone for each product covered by the in-licensed intellectual property. We will also be required to pay a royalty on net sales of products covered by the in-licensed intellectual property in the low single digits, which is reduced by half if during the ten years following first marketing approval the last valid claim within the licensed patent that covers the licensed product expires or ends. During the year ended December 31, 2017 we paid Research Development Foundation $1.0 million based upon a regulatory milestone for a product covered by the in-licensed intellectual property. Under a license agreement with Biogen Inc., pursuant to which we license certain patents and patent applications related to our bb2121 and bb21217 product candidates, we will be required to make certain payments related to certain development milestone obligations and must report on our progress in achieving these milestones on a periodic basis. We may be obligated to pay up to $23.0 million in the aggregate for each licensed product upon the achievement of remaining milestones. Upon commercialization of our products covered by the in-licensed intellectual property, we will be obligated to pay a percentage of net sales as a royalty in the low single digits. During the year ended December 31, 2017 we paid Biogen $1.0 million based upon a clinical development milestone for a product covered by the in-licensed intellectual property.
| • | Under a license agreement with the National Institutes of Health, or NIH, pursuant to which we license certain patent applications related to our bb2121 and bb21217 product candidates, we have agreed to certain development and regulatory milestone obligations and must report on our progress in achieving these milestones on a periodic basis. We may be obligated to pay up to $9.7 million in the aggregate for a licensed product upon the achievement of these milestones. Upon commercialization of our products covered by the in-licensed intellectual property, we will be obligated to pay NIH a percentage of net sales as a royalty in the low single digits, which is reduced by half if duringdigits. The royalties payable under this license agreement are subject to reduction for any third party payments required to be made, with a minimum floor in the ten year following first marketing approval the last valid claim within the licensed patent that covers the licensed product expires or ends.low single digits. |
Under a license agreement related to certain aspects of our manufacturing process, we will be required to make certain payments related to certain development milestone obligations and must report on our progress in achieving these milestones on a periodic basis. We may be obligated to pay up to $13.4 million in the aggregate for each product covered by the in-licensed intellectual property. Upon commercialization of our products covered by the in-licensed intellectual property, we will be obligated to pay the third party a percentage of net sales as a royalty in the low single digits. The royalties payable under this license agreement are subject to reduction for any third party payments required to be made, with a minimum floor in the low single digits. During the year ended December 31, 2017 we paid the respective party $2.0 million based upon a development milestone for a product covered by the in-licensed intellectual property. Other Funding Commitments We enter into contracts in the normal course of business with CROs for preclinical research studies and clinical trials, research supplies and other services and products for operating purposes. Additionally, we enter into contracts in the normal course of business with contract manufacturers. These contracts generally provide for termination on notice, and therefore are cancelablenotice. Wherever contracts and notinclude stipulated commitment payments, we have included such payments in the table of contractual obligations and commitments. On June 3, 2013, we entered into a nine-year building lease for approximately 43,600 square feet of space in Cambridge, Massachusetts, commencing on the earlier of the substantial completion of our build-out work or January 1, 2014. This lease was amended in June 2014 to add an additional approximately 9,900 square feet. The lease has monthly lease payments of $0.2 million for the first 12 months, which increased to $0.3 million per month beginning in December 2014 due to the lease amendment, with annual rent escalations thereafter and provides a rent abatement of $0.2 million per month for the first six months. The total operating lease obligation of the noncancellable term of this agreement is $29.5 million. In addition, the lease provides a contribution from the landlord towards the initial build-out of the space of up to $7.8 million. We have the option to extend this lease by an additional five years. In accordance with the lease, we entered into a cash-collateralized irrevocable standby letter of credit in the amount of $1.3 million, naming the landlord as beneficiary. This letter of credit was reduced to $1.0 million during the third quarter of 2014 and may be further reduced to $0.8 million and $0.6 million upon the first and second anniversaries of the rent commencement date, respectively. The building lease for our former corporate headquarters in Cambridge, Massachusetts, expires on March 31, 2015. We relocated to the new space in December 2013 and ceased use of the facility during the first quarter of 2014.
We also lease approximately 3,900 square feet of office and laboratory space in Seattle, Washington, which lease expires in December 2016.
Off-Balance Sheet Arrangements As of December 31, 2014,2017, we did not have any off-balance sheet arrangements as defined in the rules and regulations of the SEC. 79
Item 7A. Quantitative and Qualitative Disclosures about Market Risks We are exposed to market risk related to changes in interest rates. As of December 31, 20142017 and 2013,2016, we had cash, cash equivalents and marketable securities of $492.0 million$1.6 billion and $206.3$884.8 million, respectively, primarily invested in U.S. Governmentgovernment agency securities, federally insured certificates of deposit and money market mutual funds invested in U.S. Treasuries or U.S. government agency securities. Our primary exposure to market risk is interest rate sensitivity, which is affected by changes in the general level of U.S. interest rates, particularly because our investments are in short-term securities. Our available for sale securities are subject to interest rate risk and will fall in value if market interest rates increase. If market interest rates were to increase immediately and uniformly by 100 basis points, or one percentage point, from levels at December 31, 2014,2017, the net fair value of our interest-sensitive marketable securities would have resulted in a hypothetical decline of $0.9 $7.3 million. Item 8. Financial Statements and Supplementary Data The financial statements required to be filed pursuant to this Item 8 are appended to this report. An index of those financial statements is found in Item 15. Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure None. Item 9A. Controls and Procedures Evaluation of Disclosure Controls and Procedures Our management, with the participation of our principal executive officer and principal financial officer, has evaluated the effectiveness of our disclosure controls and procedures (as defined in Rules 13a- 15(e) and 15d- 15(e) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”)), as of the end of the period covered by this Annual Report on Form 10-K. Based on such evaluation, our principal executive officer and principal financial officer have concluded that as of such date, our disclosure controls and procedures were effective.
Management’s Annual Report on Internal Control Over Financial Reporting Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rules 13a-15(f) and 15d-15(f) promulgated under the Exchange Act as a process designed by, or under the supervision of, our principal executive and principal financial officers and effected by our board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that: • Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets;
• Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and
• Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements.Understatements. Under the supervision and with the participation of management, including our principal executive and financial officers, we assessed our internal control over financial reporting as of December 31, 2014,2017, based on criteria for effective internal control over financial reporting established in Internal Control — Integrated Framework (2013), issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Our management’s assessment of the effectiveness of our internal control over financial reporting included testing and evaluating the design and operating effectiveness of our internal controls. In our management’s opinion, we have maintained effective internal control over financial reporting as of December 31, 2014,2017, based on criteria established in the COSO 2013 framework.
The effectiveness of the our internal control over financial reporting as of December 31, 20142017 has been audited by Ernst & Young LLP, an independent registered public accounting firm, as stated in their report which is included herein. Inherent Limitations of Internal Controls Our management, including our Chief Executive Officer and Chief Financial Officer, does not expect that our disclosure controls and procedures or our internal controls will prevent all errors and all fraud. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Because of the 80
inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within the Company have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because of a simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of the control. The design of any system of controls also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Over time, controls may become inadequate because of changes in conditions, or the degree of compliance with the policies or procedures may deteriorate. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected. Changes in Internal Control over Financial Reporting There werehave been no changes in our internal control over financial reporting, as such term is defined in Rules 13a-15(f) and 15(d)-15(f) promulgated under the Securities Exchange Act of 1934, during our most recent fiscalthe fourth quarter of 2017 that have materially affected, or areis reasonably likely to materially affect, our internal control over financial reporting. Report of Independent Registered Public Accounting Firm TheTo the Stockholders and the Board of Directors and Stockholders of
bluebird bio, Inc. Opinion on Internal Control over Financial Reporting We have audited bluebird bio, Inc.’s internal control over financial reporting as of December 31, 2014,2017, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the COSO criteria). In our opinion, bluebird bio, Inc.’s (the Company) maintained, in all material respects, effective internal control over financial reporting as of December 31, 2017, based on the COSO criteria. We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated balance sheets of the Company as of December 31, 2017 and 2016, the related consolidated statements of
operations and comprehensive loss, stockholders’ equity and cash flows for each of the three years in the period ended December 31, 2017, and the related notes and our report dated February 21, 2018 expressed an unqualified opinion thereon. Basis for Opinion The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Annual Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the company’sCompany’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB. We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States).PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion. Definition and Limitations of Internal Control Over Financial Reporting A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. In our opinion, bluebird bio, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2014, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of bluebird bio, Inc. as of December 31, 2014 and 2013, and the related consolidated statements of operations and comprehensive loss, convertible preferred stock and stockholders’ equity (deficit), and cash flows for each of the three years in the period ended December 31, 2014 of bluebird bio, Inc. and our report dated February 25, 2015 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP Boston, Massachusetts February 25, 201521, 2018 81
Item 9B. Other Information Our policy governing transactions in our securities by our directors, officers, and employees permits our officers, directors and certain other persons to enter into trading plans complying with Rule 10b5-1 under the Securities Exchange Act of 1934, as amended. We have been advised that certain of our officers (including Jeffrey Walsh, Chief Operating Officer, Mitchell Finer, Chief Scientific Officer, David Davidson Chief(Chief Medical Officer,Officer), Philip Gregory (Chief Scientific Officer), Jason Cole Senior Vice President(Chief Legal Officer) and General CounselSusanna High (Chief Operating Officer) and Eric Sullivan, Senior Director, Finance and Principal Accounting Officer)certain of our directors (including Mark Vachon) have entered into trading plans covering periods after the date of this annual report on Form 10-K in accordance with Rule 10b5-1 and our policy governing transactions in our securities. Generally, under these trading plans, the individual relinquishes control over the transactions once the trading plan is put into place. Accordingly, sales under these plans may occur at any time, including possibly before, simultaneously with, or immediately after significant events involving our company. We do not undertake to report Rule 10b5-1 trading plans that may be adopted by any officers or directors in the future, or to report any modifications or termination of any publicly announced trading plan, except to the extent required by law.
82
PART III Item 10. Directors, Executive Officers and Corporate Governance Incorporated by reference from the information in our Proxy Statement for our 20152018 Annual Meeting of Stockholders, which we will file with the SEC within 120 days of the end of the fiscal year to which this Annual Report on Form 10-K relates. Item 11. Executive Compensation Incorporated by reference from the information in our Proxy Statement for our 20152018 Annual Meeting of Stockholders, which we will file with the SEC within 120 days of the end of the fiscal year to which this Annual Report on Form 10-K relates. Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters Incorporated by reference from the information in our Proxy Statement for our 20152018 Annual Meeting of Stockholders, which we will file with the SEC within 120 days of the end of the fiscal year to which this Annual Report on Form 10-K relates. Item 13. Certain Relationships and Related Transactions and Director Independence Incorporated by reference from the information in our Proxy Statement for our 20152018 Annual Meeting of Stockholders, which we will file with the SEC within 120 days of the end of the fiscal year to which this Annual Report on Form 10-K relates. Item 14. Principal Accountant Fees and Services Incorporated by reference from the information in our Proxy Statement for our 20152018 Annual Meeting of Stockholders, which we will file with the SEC within 120 days of the end of the fiscal year to which this Annual Report on Form 10-K relates.
83
PART IV Item 15. Exhibits and Financial Statements andStatement Schedules (a)(1) Financial Statements. The response to this portion of Item 15 is set forth under Item 8 above. (a)(2) Financial Statement Schedules. All schedules have been omitted because they are not required or because the required information is given in the Consolidated Financial Statements or Notes thereto set forth under Item 8 above. (a)(3) Exhibits. See the Exhibit Index immediately followingbefore the signature page of this Annual Report on Form 10-K. The exhibits listed in the Exhibit Index below are filed or incorporated by reference as part of this Annual Report on Form 10-K. Item 16. Form 10-K Summary 84Not applicable.
bluebird bio, Inc. Index to consolidated financial statementsConsolidated Financial Statements
F-1
Report of independent registered public accounting firmIndependent Registered Public Accounting Firm The
To the Stockholders and the Board of Directors and Stockholders of bluebird bio, Inc. Opinion on the Financial Statements We have audited the accompanying consolidated balance sheets of bluebird bio, Inc. (the Company) as of December 31, 20142017 and 2013, and2016, the related consolidated statements of operations and comprehensive loss, convertible preferred stock and stockholders’ equity (deficit), and cash flows for each of the three years in the period ended December 31, 2014. These2017, and the related notes (collectively referred to as the “consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits. We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States)statements”). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of bluebird bio, Inc.the Company at December 31, 20142017 and 2013,2016, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2014,2017, in conformity with U.S. generally accepted accounting principles. We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), bluebird bio, Inc.’sthe Company's internal control over financial reporting as of December 31, 2014,2017, based on the criteria established in Internal Control—IntegratedControl-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) and our report dated February 25, 201521, 2018 expressed an unqualified opinion thereon. Basis for Opinion These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB. We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion. /s/ Ernst & Young LLP We have served as the Company’s auditor since 2012. Boston, Massachusetts February 25, 201521, 2018
F-2
bluebird bio, Inc. Consolidated Balance Sheets (in thousands, except per share data)amounts) | December 31, | | As of December 31, | | | 2014 | | | 2013 | | 2017 | | | 2016 | | Assets | | | | | | | | | | | | | | | Current assets: | | | | | | | | | Current Assets: | | | | | | | | | Cash and cash equivalents | $ | 347,845 | | | $ | 206,279 | | $ | 758,505 | | | $ | 278,887 | | Marketable securities | | 125,710 | | | | — | | | 531,604 | | | | 425,491 | | Deferred tax assets | | 1,913 | | | | 693 | | | Prepaid expenses and other current assets | | 4,521 | | | | 5,015 | | | Tenant improvement receivable | | | 3,112 | | | | 8,542 | | Prepaid expenses | | | 21,171 | | | | 8,209 | | Other current assets and receivables | | | 8,377 | | | | 3,085 | | Total current assets | | 479,989 | | | | 211,987 | | | 1,322,769 | | | | 724,214 | | Marketable securities | | 18,448 | | | | — | | | 324,193 | | | | 180,452 | | Property and equipment, net | | 15,740 | | | | 10,920 | | | Property, plant and equipment, net | | | 199,606 | | | | 156,955 | | Intangible assets, net | | 28,219 | | | | — | | | 16,931 | | | | 20,694 | | Goodwill | | 13,128 | | | | — | | | 13,128 | | | | 13,128 | | Restricted cash and other non-current assets | | 1,215 | | | | 1,483 | | | 23,940 | | | | 22,679 | | Total assets | $ | 556,739 | | | $ | 224,390 | | $ | 1,900,567 | | | $ | 1,118,122 | | Liabilities and stockholders' equity | | | | | | | | | Current liabilities: | | | | | | | | | Liabilities and Stockholders' Equity | | | | | | | | | Current Liabilities: | | | | | | | | | Accounts payable | $ | 2,954 | | | $ | 4,359 | | $ | 12,873 | | | $ | 13,664 | | Accrued expenses and other current liabilities | | 14,649 | | | | 5,175 | | | 57,065 | | | | 54,660 | | Deferred revenue, current portion | | 25,375 | | | | 25,340 | | | 25,674 | | | | 6,209 | | Total current liabilities | | 42,978 | | | | 34,874 | | | 95,612 | | | | 74,533 | | Deferred rent, net of current portion | | 8,674 | | | | 6,740 | | | 2,720 | | | | 10,408 | | Deferred revenue, net of current portion | | 5,302 | | | | 30,208 | | | 21,763 | | | | 40,204 | | Contingent consideration, net of current portion | | 6,321 | | | | — | | | 2,231 | | | | 3,277 | | Deferred tax liabilities | | 1,913 | | | | 693 | | | Financing lease obligation, net of current portion | | | 154,749 | | | | 120,140 | | Other non-current liabilities | | 294 | | | | 208 | | | 60 | | | | 120 | | Total liabilities | | 65,482 | | | | 72,723 | | | 277,135 | | | | 248,682 | | Commitments and contingencies (Note 9) | | | | | | | | | Stockholders' equity: | | | | | | | | | Preferred stock, $0.01 par value, 5,000 shares authorized; 0 shares issued and outstanding at December 31, 2014 and December 31, 2013 | | — | | | | — | | | Common stock, $0.01 par value, 125,000 shares authorized; 32,340 and 23,940 shares issued and outstanding at December 31, 2014 and December 31, 2013, respectively | | 323 | | | | 239 | | | Commitments and contingencies (Note 8) | | | | | | | | | Stockholders' Equity: | | | | | | | | | Preferred stock, $0.01 par value, 5,000 shares authorized; 0 shares issued and outstanding at December 31, 2017 and December 31, 2016 | | | — | | | | — | | Common stock, $0.01 par value, 125,000 shares authorized; 49,406 and 40,691 shares issued and outstanding at December 31, 2017 and December 31, 2016, respectively | | | 494 | | | | 407 | | Additional paid-in capital | | 638,389 | | | | 250,103 | | | 2,540,951 | | | | 1,447,856 | | Accumulated other comprehensive loss | | (71 | ) | | | — | | | (4,205 | ) | | | (1,149 | ) | Accumulated deficit | | (147,384 | ) | | | (98,675 | ) | | (913,808 | ) | | | (577,674 | ) | Total stockholders' equity | | 491,257 | | | | 151,667 | | | 1,623,432 | | | | 869,440 | | Total liabilities and stockholders' equity | $ | 556,739 | | | $ | 224,390 | | $ | 1,900,567 | | | $ | 1,118,122 | | | | | | | | | | |
See accompanying notes to consolidated financial statements.
F-3
bluebird bio, Inc. Consolidated Statements of Operations and Comprehensive Loss (in thousands, except per share data)amounts) | Year ended December 31, | | | | 2014 | | | | 2013 | | | | 2012 | | Revenue: | | | | | | | | | | | | Collaboration revenue | $ | 25,031 | | | $ | 19,792 | | | $ | — | | Research and license fees | | 390 | | | | 389 | | | | 340 | | Total revenue | | 25,421 | | | | 20,181 | | | | 340 | | Operating expenses: | | | | | | | | | | | | Research and development | | 62,574 | | | | 31,002 | | | | 17,210 | | General and administrative | | 23,227 | | | | 14,126 | | | | 6,846 | | Change in fair value of contingent consideration | | 246 | | | | — | | | | — | | Total operating expenses | | 86,047 | | | | 45,128 | | | | 24,056 | | Loss from operations | | (60,626 | ) | | | (24,947 | ) | | | (23,716 | ) | Other income (expense), net: | | | | | | | | | | | | Interest income | | 152 | | | | 29 | | | | 5 | | Foreign currency gains (losses) | | (163 | ) | | | 37 | | | | 13 | | Re-measurement of warrants | | — | | | | (440 | ) | | | 28 | | Other income | | 131 | | | | — | | | | — | | Total other income (expense), net | | 120 | | | | (374 | ) | | | 46 | | Loss before income taxes | | (60,506 | ) | | | (25,321 | ) | | | (23,670 | ) | Benefit from income taxes | | 11,797 | | | | — | | | | — | | Net loss | $ | (48,709 | ) | | $ | (25,321 | ) | | $ | (23,670 | ) | Other comprehensive loss: | | | | | | | | | | | | Unrealized loss on available-for-sale securities, net of tax | | (71 | ) | | | — | | | | (1 | ) | Total other comprehensive loss | | (71 | ) | | | — | | | | (1 | ) | Comprehensive loss | $ | (48,780 | ) | | $ | (25,321 | ) | | $ | (23,671 | ) | Reconciliation of net loss to net loss applicable to common stockholders: | | | | | | | | | | | | Net loss | $ | (48,709 | ) | | $ | (25,321 | ) | | $ | (23,670 | ) | Accretion and dividends on convertible preferred stock | | — | | | | — | | | | (3,057 | ) | Gain on extinguishment of convertible preferred stock | | — | | | | — | | | | 23,114 | | Net loss applicable to common stockholders | $ | (48,709 | ) | | $ | (25,321 | ) | | $ | (3,613 | ) | Net loss per share applicable to common stockholders - basic and diluted: | $ | (1.83 | ) | | $ | (2.02 | ) | | $ | (13.79 | ) | Weighted-average number of common shares used in computing net loss per share - basic and diluted: | | 26,546 | | | | 12,555 | | | | 262 | | | | | | | | | | | | | |
| Year Ended December 31, | | | | 2017 | | | | 2016 | | | | 2015 | | Revenue: | | | | | | | | | | | | Collaboration revenue | $ | 22,207 | | | $ | 6,155 | | | $ | 14,079 | | License and royalty revenue | | 13,220 | | | | — | | | | — | | Total revenues | | 35,427 | | | | 6,155 | | | | 14,079 | | Operating expenses: | | | | | | | | | | | | Research and development | | 273,040 | | | | 204,775 | | | | 134,038 | | General and administrative | | 93,550 | | | | 65,119 | | | | 46,209 | | Cost of license and royalty revenue | | 1,527 | | | | — | | | | — | | Change in fair value of contingent consideration | | (525 | ) | | | 4,091 | | | | 2,869 | | Total operating expenses | | 367,592 | | | | 273,985 | | | | 183,116 | | Loss from operations | | (332,165 | ) | | | (267,830 | ) | | | (169,037 | ) | Interest (expense) income, net | | (2,001 | ) | | | 3,782 | | | | 1,591 | | Other (expense) income, net | | (1,267 | ) | | | (71 | ) | | | 723 | | Loss before income taxes | | (335,433 | ) | | | (264,119 | ) | | | (166,723 | ) | Income tax (expense) benefit | | (210 | ) | | | 612 | | | | (60 | ) | Net loss | $ | (335,643 | ) | | $ | (263,507 | ) | | $ | (166,783 | ) | Net loss per share - basic and diluted | $ | (7.71 | ) | | $ | (7.07 | ) | | $ | (4.81 | ) | Weighted-average number of common shares used in computing net loss per share - basic and diluted | | 43,535 | | | | 37,284 | | | | 34,669 | | Other comprehensive income (loss): | | | | | | | | | | | | Other comprehensive income (loss), net of tax expense of $0.0, $0.6 and $0.0 million for the years ended December 31, 2017, 2016 and 2015, respectively | | (3,056 | ) | | | 1,142 | | | | (2,220 | ) | Total other comprehensive income (loss) | | (3,056 | ) | | | 1,142 | | | | (2,220 | ) | Comprehensive loss | $ | (338,699 | ) | | $ | (262,365 | ) | | $ | (169,003 | ) |
See accompanying notes to consolidated financial statements.
F-4
bluebirdbluebird bio, Inc.
Consolidated Statements of Convertible Preferred Stock and Stockholders’ Equity (Deficit) (in thousands) | | Series A-1 | | | Series A-2 | | | | | Series B | | | | | Series C | | | Series D | | | | convertible | | | convertible | | | | | convertible | | | | | convertible | | | convertible | | | | preferred stock | | | preferred stock | | | | | preferred stock | | | | | preferred stock | | | preferred stock | | | | Shares | | | Amount | | | Shares | | | Amount | | | | | Shares | | | Amount | | | | | Shares | | | Amount | | | Shares | | | Amount | | Balance at December 31, 2011 | | | 12,981 | | | $ | 9,217 | | | | 22,304 | | | $ | 15,837 | | | | | | 115,204 | | | $ | 41,495 | | | | | | 39,943 | | | $ | 15,854 | | | | - | | | $ | - | | Issuance of Series D Preferred Stock, net of issuance costs | | | - | | | | - | | | | - | | | | - | | | | | | - | | | | - | | | | | | - | | | | - | | | | 120,409 | | | | 59,831 | | Accretion and dividends on convertible preferred stock | | | - | | | | 194 | | | | - | | | | 332 | | | | | | - | | | | 1,689 | | | | | | - | | | | 673 | | | | - | | | | 169 | | Gain on extinguishment of convertible preferred stock | | | - | | | | (7,074 | ) | | | - | | | | (9,032 | ) | | | | | - | | | | (2,863 | ) | | | | | - | | | | (4,145 | ) | | | - | | | | - | | Reclassification of Series A-1 Preferred Stock | | | (12,981 | ) | | | (2,337 | ) | | | - | | | | - | | | | | | - | | | | - | | | | | | - | | | | - | | | | - | | | | - | | Reclassification of Series A-1 Preferred Stock warrants | | | - | | | | - | | | | - | | | | - | | | | | | - | | | | - | | | | | | - | | | | - | | | | - | | | | - | | Vesting of restricted stock issued in exchange for nonrecourse note | | | - | | | | - | | | | - | | | | - | | | | | | - | | | | - | | | | | | - | | | | - | | | | - | | | | - | | Vesting of restricted stock | | | - | | | | - | | | | - | | | | - | | | | | | - | | | | - | | | | | | - | | | | - | | | | - | | | | - | | Exercise of stock options | | | - | | | | - | | | | - | | | | - | | | | | | - | | | | - | | | | | | - | | | | - | | | | - | | | | - | | Realized gain on marketable securities | | | - | | | | - | | | | - | | | | - | | | | | | - | | | | - | | | | | | - | | | | - | | | | - | | | | - | | Stock-based compensation | | | - | | | | - | | | | - | | | | - | | | | | | - | | | | - | | | | | | - | | | | - | | | | - | | | | - | | Net loss | | | - | | | | - | | | | - | | | | - | | | | | | - | | | | - | | | | | | - | | | | - | | | | - | | | | - | | Balance at December 31, 2012 | | | - | | | $ | - | | | | 22,304 | | | $ | 7,137 | | | | | | 115,204 | | | $ | 40,321 | | | | | | 39,943 | | | $ | 12,382 | | | | 120,409 | | | $ | 60,000 | |
| | Series A-1 | | | | | | | | | | | | | | | Accumulated | | | | | | | | | Total | | | | convertible | | | | | | | | | | | Additional | | | other | | | | | | | | | stockholders' | | | | preferred stock | | | Common stock | | | paid-in | | | comprehensive | | | | | Accumulated | | | equity | | | | Shares | | | Amount | | | Shares | | | Amount | | | capital | | | income (loss) | | | | | deficit | | | (deficit) | | Balance at December 31, 2011 | | | - | | | $ | - | | | | 205 | | | $ | 2 | | | | 7,732 | | | $ | 1 | | | | | $ | (63,442 | ) | | $ | (55,707 | ) | Issuance of Series D Preferred Stock, net of issuance costs | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | | | - | | | | - | | Accretion and dividends on convertible preferred stock | | | - | | | | - | | | | - | | | | - | | | | (3,057 | ) | | | - | | | | | | - | | | | (3,057 | ) | Gain on extinguishment of convertible preferred stock | | | - | | | | - | | | | - | | | | - | | | | 9,356 | | | | - | | | | | | 13,758 | | | | 23,114 | | Reclassification of Series A-1 Preferred Stock | | | 12,981 | | | | 2,337 | | | | - | | | | - | | | | - | | | | - | | | | | | - | | | | 2,337 | | Reclassification of Series A-1 Preferred Stock warrants | | | - | | | | - | | | | - | | | | - | | | | 394 | | | | - | | | | | | - | | | | 394 | | Vesting of restricted stock issued in exchange for nonrecourse note | | | - | | | | - | | | | 82 | | | | 1 | | | | (1 | ) | | | - | | | | | | - | | | | - | | Vesting of restricted stock | | | - | | | | - | | | | 12 | | | | - | | | | - | | | | - | | | | | | - | | | | - | | Exercise of stock options | | | - | | | | - | | | | 10 | | | | - | | | | 21 | | | | - | | | | | | - | | | | 21 | | Realized gain on marketable securities | | | - | | | | - | | | | - | | | | - | | | | - | | | | (1 | ) | | | | | - | | | | (1 | ) | Stock-based compensation | | | - | | | | - | | | | - | | | | - | | | | 822 | | | | - | | | | | | - | | | | 822 | | Net loss | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | | | (23,670 | ) | | | (23,670 | ) | Balance at December 31, 2012 | | | 12,981 | | | $ | 2,337 | | | | 309 | | | $ | 3 | | | $ | 15,267 | | | $ | - | | | | | $ | (73,354 | ) | | $ | (55,747 | ) |
F-5
bluebird bio, Inc.
Consolidated Statements of Convertible Preferred Stock and Stockholders’ Equity (Deficit)
(in thousands)
| | Series A-1 | | | Series A-2 | | | Series B | | | Series C | | | Series D | | | | convertible | | | convertible | | | convertible | | | convertible | | | convertible | | | | preferred stock | | | preferred stock | | | preferred stock | | | preferred stock | | | preferred stock | | | | Shares | | | Amount | | | Shares | | | Amount | | | Shares | | | Amount | | | Shares | | | Amount | | | Shares | | | Amount | | Balance at December 31, 2012 | | | - | | | $ | - | | | | 22,304 | | | $ | 7,137 | | | | 115,204 | | | $ | 40,321 | | | | 39,943 | | | $ | 12,382 | | | | 120,409 | | | $ | 60,000 | | Vesting of restricted stock issued in exchange for nonrecourse note | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | Vesting of restricted stock | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | Proceeds from IPO, net of closing costs of $11,229 | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | Conversion of convertible preferred stock into common stock | | | - | | | | - | | | | (22,304 | ) | | | (7,137 | ) | | | (115,204 | ) | | | (40,321 | ) | | | (39,943 | ) | | | (12,382 | ) | | | (120,409 | ) | | | (60,000 | ) | Reclassification of warrants to purchase preferred stock to stockholders' equity | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | Repayment of nonrecourse note | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | Exercise of common stock warrants | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | Exercise of stock options | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | Stock-based compensation | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Net loss | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | Balance at December 31, 2013 | | | - | | | $ | - | | | | - | | | $ | - | | | | - | | | $ | - | | | | - | | | $ | - | | | | - | | | $ | - | |
| | Series A-1 | | | | | | | | | | | | | | | Accumulated | | | | | | | Total | | | | convertible | | | | | | | | | | | Additional | | | other | | | | | | | stockholders' | | | | preferred stock | | | Common stock | | | paid-in | | | comprehensive | | | Accumulated | | | equity | | | | Shares | | | Amount | | | Shares | | | Amount | | | capital | | | income (loss) | | | deficit | | | (deficit) | | Balance at December 31, 2012 | | | 12,981 | | | $ | 2,337 | | | | 309 | | | $ | 3 | | | $ | 15,267 | | | $ | - | | | $ | (73,354 | ) | | $ | (55,747 | ) | Vesting of restricted stock issued in exchange for nonrecourse note | | | - | | | | - | | | | 41 | | | | - | | | | - | | | | - | | | | - | | | | - | | Vesting of restricted stock | | | - | | | | - | | | | 45 | | | | - | | | | - | | | | - | | | | - | | | | - | | Proceeds from IPO, net of closing costs of $11,229 | | | - | | | | - | | | | 6,832 | | | | 69 | | | | 104,852 | | | | - | | | | - | | | | 104,921 | | Conversion of convertible preferred stock into common stock | | | (12,981 | ) | | | (2,337 | ) | | | 16,389 | | | | 164 | | | | 122,014 | | | | - | | | | - | | | | 119,841 | | Reclassification of warrants to purchase preferred stock to stockholders' equity | | | - | | | | - | | | | - | | | | - | | | | 655 | | | | - | | | | - | | | | 655 | | Repayment of nonrecourse note | | | - | | | | - | | | | - | | | | - | | | | 344 | | | | - | | | | - | | | | 344 | | Exercise of common stock warrants | | | - | | | | - | | | | 102 | | | | 1 | | | | (1 | ) | | | - | | | | - | | | | - | | Exercise of stock options | | | - | | | | - | | | | 222 | | | | 2 | | | | 481 | | | | - | | | | - | | | | 483 | | Stock-based compensation | | | | | | | | | | | | | | | | | | | 6,491 | | | | - | | | | - | | | | 6,491 | | Net loss | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | (25,321 | ) | | | (25,321 | ) | Balance at December 31, 2013 | | | - | | | $ | - | | | | 23,940 | | | $ | 239 | | | $ | 250,103 | | | $ | - | | | $ | (98,675 | ) | | $ | 151,667 | |
F-6
bluebird bio, Inc.
Consolidated Statements of Convertible Preferred Stock and Stockholders’ Equity (Deficit)
(in thousands)
| | Series A-1
| | | Series A-2
| | | Series B
| | | Series C
| | | Series D
| | | | convertible
| | | convertible
| | | convertible
| | | convertible
| | | convertible
| | | | preferred stock
| | | preferred stock
| | | preferred stock
| | | preferred stock
| | | preferred stock
| | | | Shares
| | | Amount
| | | Shares
| | | Amount
| | | Shares
| | | Amount
| | | Shares
| | | Amount
| | | Shares
| | | Amount
| | Balance at December 31, 2013
| | | -
| | | $
| -
| | | | -
| | | $
| -
| | | | -
| | | $
| -
| | | | -
| | | $
| -
| | | | -
| | | $
| -
| | Vesting of restricted stock issued
in exchange for nonrecourse note
| | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | Issuance of common stock upon public offering,
net of issuance costs of $23,295
| | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | Issuance of common stock in connection with
acquisition
| | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | Exercise of common stock warrants
| | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | Exercise of stock options
| | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | Issuance of common stock in exchange for
consulting services to non-employees
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Stock-based compensation
| | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | Unrealized loss on available-for-sale
securities, net of tax
| | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | Net loss
| | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | | | -
| | Balance at December 31, 2014
| | | -
| | | $
| -
| | | | -
| | | $
| -
| | | | -
| | | $
| -
| | | | -
| | | $
| -
| | | | -
| | | $
| -
| |
| | Series A-1 | | | | | | | | | | | | | | | | | Accumulated | | | | | | | Total | | | | convertible | | | | | | | | | | | | | Additional | | | other | | | | | | | stockholders' | | | | preferred stock | | | | | Common stock | | | paid-in | | | comprehensive | | | Accumulated | | | equity | | | | Shares | | | Amount | | | | | Shares | | | Amount | | | capital | | | income (loss) | | | deficit | | | (deficit) | | Balance at December 31, 2013 | | | - | | | $ | - | | | | | | 23,940 | | | $ | 239 | | | $ | 250,103 | | | $ | - | | | $ | (98,675 | ) | | $ | 151,667 | | Vesting of restricted stock issued in exchange for nonrecourse note | | | - | | | | - | | | | | | 69 | | | | 1 | | | | (1 | ) | | | - | | | | - | | | | - | | Issuance of common stock upon public offering, net of issuance costs of $23,295 | | | - | | | | - | | | | | | 6,498 | | | | 65 | | | | 352,977 | | | | - | | | | - | | | | 353,042 | | Issuance of common stock in connection with acquisition | | | - | | | | - | | | | | | 411 | | | | 4 | | | | 19,470 | | | | - | | | | - | | | | 19,474 | | Exercise of common stock warrants | | | - | | | | - | | | | | | 114 | | | | 1 | | | | (1 | ) | | | - | | | | - | | | | - | | Exercise of stock options | | | - | | | | - | | | | | | 1,306 | | | | 13 | | | | 5,036 | | | | - | | | | - | | | | 5,049 | | Issuance of common stock in exchange for consulting services to non-employees | | | - | | | | - | | | | | | 2 | | | | - | | | | 42 | | | | - | | | | - | | | | 42 | | Stock-based compensation | | | - | | | | - | | | | | | - | | | | - | | | | 10,763 | | | | - | | | | - | | | | 10,763 | | Unrealized loss on available-for-sale securities, net of tax | | | - | | | | - | | | | | | - | | | | - | | | | - | | | | (71 | ) | | | - | | | | (71 | ) | Net loss | | | - | | | | - | | | | | | - | | | | - | | | | - | | | | - | | | | (48,709 | ) | | | (48,709 | ) | Balance at December 31, 2014 | | | - | | | $ | - | | | | | | 32,340 | | | $ | 323 | | | $ | 638,389 | | | $ | (71 | ) | | $ | (147,384 | ) | | $ | 491,257 | |
| | | | | | | | | | | | | | Accumulated | | | | | | | | | | | | | | | | | | | | Additional | | | Other | | | | | | | Total | | | | Common Stock | | | Paid-In | | | Comprehensive | | | Accumulated | | | Stockholders' | | | | Shares | | | Amount | | | Capital | | | Loss | | | Deficit | | | Equity | | Balances at December 31, 2014 | | | 32,340 | | | $ | 323 | | | $ | 638,389 | | | $ | (71 | ) | | $ | (147,384 | ) | | $ | 491,257 | | Vesting of restricted stock units | | | 62 | | | | 1 | | | | (1 | ) | | | — | | | | — | | | | — | | Issuance of common stock upon public offering, net of issuance costs of $22,753 | | | 2,941 | | | | 29 | | | | 477,218 | | | | — | | | | — | | | | 477,247 | | Issuance of common stock to Pregenen equityholders | | | 94 | | | | 1 | | | | (1 | ) | | | — | | | | — | | | | — | | Exercise of common stock warrants | | | 164 | | | | 2 | | | | (2 | ) | | | — | | | | — | | | | — | | Exercise of stock options | | | 1,282 | | | | 13 | | | | 9,370 | | | | — | | | | — | | | | 9,383 | | Purchase of common stock under ESPP | | | 11 | | | | — | | | | 492 | | | | — | | | | — | | | | 492 | | Stock-based compensation | | | — | | | | — | | | | 41,120 | | | | — | | | | — | | | | 41,120 | | Unrealized loss on available-for-sale securities, net of tax | | | — | | | | — | | | | — | | | | (2,220 | ) | | | — | | | | (2,220 | ) | Net loss | | | — | | | | — | | | | — | | | | — | | | | (166,783 | ) | | | (166,783 | ) | Balances at December 31, 2015 | | | 36,894 | | | $ | 369 | | | $ | 1,166,585 | | | $ | (2,291 | ) | | $ | (314,167 | ) | | $ | 850,496 | | Vesting of restricted stock units | | | 113 | | | | 1 | | | | (1 | ) | | | — | | | | — | | | | — | | Issuance of common stock upon public offering, net of issuance costs of $15,269 | | | 3,289 | | | | 33 | | | | 234,698 | | | | — | | | | — | | | | 234,731 | | Exercise of stock options | | | 377 | | | | 4 | | | | 6,141 | | | | — | | | | — | | | | 6,145 | | Purchase of common stock under ESPP | | | 18 | | | | — | | | | 677 | | | | — | | | | — | | | | 677 | | Stock-based compensation | | | — | | | | — | | | | 39,756 | | | | — | | | | — | | | | 39,756 | | Unrealized gain on available-for-sale securities, net of tax | | | — | | | | — | | | | — | | | | 1,142 | | | | — | | | | 1,142 | | Net loss | | | — | | | | — | | | | — | | | | — | | | | (263,507 | ) | | | (263,507 | ) | Balances at December 31, 2016 | | | 40,691 | | | $ | 407 | | | $ | 1,447,856 | | | $ | (1,149 | ) | | $ | (577,674 | ) | | $ | 869,440 | | Retroactive adjustment to beginning accumulated deficit and additional paid-in capital resulting from adoption of ASU 2016-09 | | | — | | | | — | | | | 491 | | | | — | | | | (491 | ) | | | — | | Vesting of restricted stock units | | | 88 | | | | 1 | | | | (1 | ) | | | — | | | | — | | | | — | | Issuance of common stock upon public offering, net of issuance costs of $53,487 | | | 7,625 | | | | 76 | | | | 1,006,494 | | | | — | | | | — | | | | 1,006,570 | | Exercise of stock options | | | 981 | | | | 10 | | | | 31,676 | | | | — | | | | — | | | | 31,686 | | Purchase of common stock under ESPP | | | 21 | | | | — | | | | 1,153 | | | | — | | | | — | | | | 1,153 | | Stock-based compensation | | | | | | | | | | | 53,282 | | | | — | | | | — | | | | 53,282 | | Other comprehensive loss | | | — | | | | — | | | | — | | | | (3,056 | ) | | | — | | | | (3,056 | ) | Net loss | | | — | | | | — | | | | — | | | | — | | | | (335,643 | ) | | | (335,643 | ) | Balances at December 31, 2017 | | | 49,406 | | | $ | 494 | | | $ | 2,540,951 | | | $ | (4,205 | ) | | $ | (913,808 | ) | | $ | 1,623,432 | |
See accompanying notes to consolidated financial statements. F-7
bluebird bio, Inc. Consolidated Statements of Cash Flows (in thousands) | Year ended December 31, | | | | 2014 | | | | 2013 | | | | 2012 | | Operating activities | | | | | | | | | | | | Net loss | $ | (48,709 | ) | | $ | (25,321 | ) | | $ | (23,670 | ) | Adjustments to reconcile net loss to net cash (used in) provided by operating activities: | | | | | | | | | | | | Noncash benefit on release of income tax valuation allowance | | (11,797 | ) | | | — | | | | — | | Depreciation and amortization | | 4,228 | | | | 941 | | | | 301 | | Stock-based compensation expense | | 10,763 | | | | 6,491 | | | | 822 | | Remeasurement of contingent consideration | | 246 | | | | — | | | | — | | Issuance of restricted common stock in exchange for consulting services to non-employees | | 168 | | | | — | | | | — | | Re-measurement of warrants | | — | | | | 440 | | | | (28 | ) | Loss on disposal of equipment | | 3 | | | | 9 | | | | 10 | | Other non-cash items | | 139 | | | | — | | | | — | | Changes in operating assets and liabilities: | | | | | | | | | | | | Prepaid expenses and other assets | | 307 | | | | (4,214 | ) | | | 93 | | Accounts payable | | (2,249 | ) | | | 2,067 | | | | 380 | | Accrued expenses and other liabilities | | 9,969 | | | | 761 | | | | 1,336 | | Deferred revenue | | (24,871 | ) | | | 54,868 | | | | (340 | ) | Deferred rent | | 2,110 | | | | 7,408 | | | | 52 | | Net cash (used in) provided by operating activities | | (59,693 | ) | | | 43,450 | | | | (21,044 | ) | Investing activities | | | | | | | | | | | | Restricted cash | | 209 | | | | (1,153 | ) | | | (40 | ) | Purchase of property and equipment | | (8,708 | ) | | | (8,670 | ) | | | (867 | ) | Purchases of marketable securities | | (174,981 | ) | | | — | | | | — | | Proceeds from sales or maturities of marketable securities | | 30,960 | | | | — | | | | 3,506 | | Acquisition of business, net of cash acquired | | (4,673 | ) | | | — | | | | — | | Net cash (used in) provided by investing activities | | (157,193 | ) | | | (9,823 | ) | | | 2,599 | | Financing activities | | | | | | | | | | | | Proceeds from public offering of common stock, net of issuance costs | | 353,226 | | | | 104,921 | | | | — | | Proceeds from issuance of convertible preferred stock, net of issuance costs | | — | | | | — | | | | 59,831 | | Repayment of nonrecourse note collateralized by restricted stock | | — | | | | 344 | | | | — | | Proceeds from exercise of stock options and issuance of common stock | | 5,226 | | | | 376 | | | | 21 | | Net cash provided by financing activities | | 358,452 | | | | 105,641 | | | | 59,852 | | Increase in cash and cash equivalents | | 141,566 | | | | 139,268 | | | | 41,407 | | Cash and cash equivalents at beginning of period | | 206,279 | | | | 67,011 | | | | 25,604 | | Cash and cash equivalents at end of period | $ | 347,845 | | | $ | 206,279 | | | $ | 67,011 | | Non-cash investing and financing activities: | | | | | | | | | | | | Purchases of property and equipment included in accounts payable and accrued expenses | $ | 387 | | | $ | 1,924 | | | $ | — | | Offering expenses included in accounts payable and accrued expenses | $ | 183 | | | $ | — | | | $ | — | | Accretion and dividends on convertible preferred stock | $ | — | | | $ | — | | | $ | 3,057 | | Gain on extinguishment of convertible preferred stock | $ | — | | | $ | — | | | $ | 23,114 | | Reclassification of warrants to additional paid-in capital | $ | — | | | $ | 655 | | | $ | 394 | | Reclassification of Series A-1 Preferred Stock to common stock | $ | — | | | $ | - | | | $ | 2,337 | | Conversion of preferred stock to common stock upon closing of IPO | $ | — | | | $ | 122,178 | | | $ | — | | Stock option exercise proceeds receivable | $ | 98 | | | $ | 107 | | | $ | — | |
| Year Ended December 31, | | 2017 | | 2016 | | 2015 | Cash flows from operating activities: | | | | | | Net loss | $(335,643) | | $(263,507) | | $(166,783) | Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | Change in fair value of contingent consideration | (2,189) | | 2,675 | | 2,344 | Depreciation and amortization | 13,538 | | 9,648 | | 7,419 | Stock-based compensation expense | 53,282 | | 39,756 | | 41,120 | Other non-cash items | 3,153 | | 2,825 | | 1,513 | Changes in operating assets and liabilities: | | | | | | Prepaid expenses and other assets | (20,092) | | (14,318) | | (6,847) | Accounts payable | 526 | | 6,658 | | 2,541 | Accrued expenses and other liabilities | 5,406 | | 20,889 | | 9,423 | Deferred revenue | 1,024 | | 4,565 | | 11,171 | Deferred rent | 442 | | 1,162 | | (330) | Net cash used in operating activities | (280,553) | | (189,647) | | (98,429) | Cash flows from investing activities: | | | | | | Restricted cash | 627 | | (4,379) | | (8,816) | Purchase of property, plant and equipment, including assets under financing lease obligation | (62,242) | | (28,029) | | (7,055) | Purchases of marketable securities | (686,204) | | (348,225) | | (755,175) | Proceeds from maturities of marketable securities | 431,816 | | 443,364 | | 199,179 | Net cash (used in) provided by investing activities | (316,003) | | 62,731 | | (571,867) | Cash flows from financing activities: | | | | | | Cash paid for contingent purchase price consideration | (1,074) | | (2,025) | | (453) | Reimbursement of assets under financing lease obligation | 38,021 | | 1,663 | | — | Payments on financing lease obligation | (574) | | — | | — | Proceeds from public offering of common stock, net of issuance costs | 1,006,570 | | 234,962 | | 477,064 | Proceeds from issuance of common stock | 33,231 | | 6,934 | | 10,109 | Net cash provided by financing activities | 1,076,174 | | 241,534 | | 486,720 | Increase (decrease) in cash and cash equivalents | 479,618 | | 114,618 | | (183,576) | Cash and cash equivalents at beginning of year | 278,887 | | 164,269 | | 347,845 | Cash and cash equivalents at end of year | $758,505 | | $278,887 | | $164,269 | Supplemental cash flow disclosures: | | | | | | Cash paid for interest in connection with financing lease obligation | $11,411 | | $ — | | $ — | Supplemental cash flow disclosures from investing and financing activities: | | | | | | Assets acquired under financing lease obligation | $3,271 | | $48,034 | | $61,901 | Purchases of property, plant and equipment included in accounts payable and accrued expenses | $2,566 | | $6,363 | | $2,089 | Tenant improvements under financing lease included in tenant improvements receivable | $3,112 | | $8,542 | | $ — |
See accompanying notes to consolidated financial statements. F-8
bluebird bio, Inc. Notes to Consolidated Financial Statements For the Years Ended December 31, 2017, 2016 and 2015 1. Description of the business bluebird bio, Inc. (the “Company” or “bluebird”) was incorporated in Delaware on April 16, 1992, and is headquartered in Cambridge, Massachusetts. The Company was formedresearches, develops, manufactures and plans to develop, manufacturecommercialize gene therapies for severe genetic diseases and market therapies to safely and effectively deliver genes useful in the treatment of serious human diseases.cancer. Since its inception, the Company has devoted substantially all of its resources to its research and development efforts relating to its product candidates, including activities to manufacture product candidates, conduct clinical studies of its product candidates, perform preclinical research to identify new product candidates and provide general and administrative support for these operations. On June 30, 2014, the Company acquired all of the outstanding capital stock of Precision Genome Engineering, Inc. (“Pregenen”) and in connection therewith, obtained the rights to Pregenen’s gene editing and cell signaling technology. See Note 12, “Business combinations,” for additional information. In July 2014, the Company sold 3,450,000 shares of common stock (inclusive of 450,000 shares of common stock sold by the Company pursuant to the full exercise of an overallotment option granted to the underwriters in connection with the offering) through an underwritten public offering at a price of $34.00 per share. The aggregate net proceeds received by the Company from the offering were $109.8 million, net of underwriting discounts and commissions and offering expenses payable by the Company of approximately $7.5 million.
In December 2014, the Company sold 3,047,500 shares of common stock (inclusive of 397,500 shares of common stock sold by the Company pursuant to the full exercise of an overallotment option granted to the underwriters in connection with the offering) through an underwritten public offering at a price of $85.00 per share. The aggregate net proceeds received by the Company from the offering were $243.3 million, net of underwriting discounts and commissions and estimated offering expenses payable by the Company of approximately $15.8 million.
As of December 31, 2014,2017, the Company had cash, cash equivalents and marketable securities of $492.0 million.$1.6 billion. Although the Company has incurred recurring losses and expects to continue to incur losses for the foreseeable future, the Company expects its cash, cash equivalents and marketable securities will be sufficient to fund current operations for at least the next twelve months. 2. Summary of significant accounting policies and basis of presentation Basis of presentation and principles of consolidation The accompanying consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries: Precision Genome Engineering, Inc., bluebird bio France – SARL, bluebird bio Australia Pty Ltd. and bluebird bio Securities Corporation.subsidiaries. All intercompany balances and transactions have been eliminated in consolidation. The assets acquired and liabilities assumed in connection with the Company’s acquisition of Pregenen were recorded at their fair values as of June 30, 2014, the date of the acquisition, and the operating results of Pregenen have been consolidated with those of the Company from the date of acquisition. These consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America (“GAAP”). Any reference in these notes to applicable guidance is meant to refer to the authoritative United States generally accepted accounting principles as found in the Accounting Standards Codification (“ASC”) and Accounting Standards Update (“ASU”) of the Financial Accounting Standards Board (“FASB”). Foreign currency translation
The Company’s consolidated financial statements are prepared in U.S. dollars. Its foreign subsidiary usesCertain aggregations of prior year amounts have been made to conform to current year presentation. In the U.S. dollar as its functional currencyprior year balance sheet, tenant improvements receivable, prepaid expenses and maintains its records in the local currency. Nonmonetaryrestricted cash and other current assets and liabilities are re-measured at historical rates and monetary assets and liabilities are re-measured at exchange rates in effect at the end of the reporting period. Income statement accounts are re-measured at average exchange rates for the reporting period. The resulting gains or losses are included in foreign currency gains (losses) inwithin prepaid expenses and other current assets. In the consolidatedprior year statements of operations and comprehensive loss.loss, interest (expense) income, net and other (expense) income, net are aggregated. Additionally, the Company has combined in the statements of cash flows the purchase of property and equipment and tenant improvements.
Use of estimates The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts in the financial statements and accompanying notes. Actual results could materially differ from those estimates. Management considers many factors in selecting appropriate financial accounting policies and controls, and in developing the estimates and assumptions that are used in the preparation of these financial statements. Management must apply significant F-9
judgment in this process. In addition, other factors may affect estimates, including: expected business and operational changes, sensitivity and volatility associated with the assumptions used in developing estimates, and whether historical trends are expected to be representative of future trends. The estimation process often may yield a range of potentially reasonable estimates of the ultimate future outcomes and management must select an amount that falls within that range of reasonable estimates. This process may result in actual results differing materially from those estimated amounts used in the preparation of the financial statements. Estimates are used in the following areas, among others: acquisition-date fair value and subsequent fair value estimates used to assess potential impairment of long-lived assets, including goodwill and intangible assets, financing lease obligations, contingent consideration, stock-based compensation expense, accrued expenses, revenue and income taxes. Foreign currency translation The financial statements of the Company’s subsidiaries with functional currencies other than the U.S. dollar are translated into U.S. dollars using period-end exchange rates for assets and liabilities, historical exchange rates for stockholders’ equity and weighted average exchange rates for operating results. Translation gains and losses are included in accumulated other comprehensive income
(loss) in stockholders’ equity. Foreign currency transaction gains and losses are included in other (expense) income, net in the results of operations. Segment information Operating segments are identified as components
The Company operates in a single segment, focusing on the development of an enterprise about which separate discrete financial information is availablepotentially transformative gene therapies for evaluation by thesevere genetic diseases and cancer. Consistent with its operational structure, its chief operating decision maker or decision making group, in making decisionsmanages and allocates resources at a global, consolidated level. Therefore, results of our operations are reported on how to allocate resources and assess performance. The Company views its operations and manages its business in one operating segment.a consolidated basis for purposes of segment reporting, consistent with our management reporting. All material long-lived assets of the Company reside in the United States. Cash and cash equivalents The Company considers all highly liquid investments purchased with original final maturities of 90 days or less at acquisitionfrom the date of purchase to be cash equivalents. Cash and cash equivalents includecomprise funds in cash, held in banks and money market accounts.accounts, and federally insured deposits. Cash equivalents are reported at fair value. Marketable securities The Company classifies marketable securities with a remaining maturity when purchased of greater than three months as available-for-sale. Marketable securities with a remaining maturity date greater than one year are classified as non-current. Available-for-sale securities are maintained by an investment managermanagers and may consist of U.S. Treasury securities, U.S. government agency securities, federally insuredand certificates of deposit and money market funds invested in U.S. Treasuries or U.S. government agency securities.deposit. Available-for-sale securities are carried at fair value with the unrealized gains and losses included in other comprehensive income (loss) as a component of stockholders’ equity until realized. Any premium or discount arising at purchase is amortized and/or accreted to interest income and/or expense.expense over the life of the instrument. Realized gains and losses are determined using the specific identification method and are included in other (expense) income, (expense).net. If any adjustment to fair value reflects a decline in value of the investment, the Company considers all available evidence to evaluate the extent to which the decline is “other-than-temporary” and, if so, markmarks the investment to market through a charge to the Company’s statement of operations and comprehensive loss. Concentrations of credit risk and off-balance sheet risk Financial instruments that subject the Company to credit risk primarily consist of cash and cash equivalents and available-for-sale securities. The Company maintains its cash and cash equivalent balances with high-quality financial institutions and, consequently, the Company believes that such funds are subject to minimal credit risk. The Company’s available-for-sale investments primarily consist of U.S. Treasury securities, U.S. government agency securities and federally insured certificates of deposit, and potentially subject the Company to concentrations of credit risk. The Company has adopted an investment policy whichthat limits the amounts the Company may invest in any one type of investment and requires all investments held by the Company to be at least AA+/Aa1 rated, thereby reducing credit risk exposure. Fair value of financial instruments The Company is required to disclose information on allhas certain financial assets and liabilities reportedrecorded at fair value that enables an assessment of the inputs used in determining the reported fair values. FASB ASC Topic 820, Fair Value Measurements and Disclosures (“ASC 820”), establishes a hierarchy of inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the observable inputs be used when available. Observable inputs are inputs that market participants would use in pricing the assetwhich have been classified as Level 1, 2 or liability based on market data obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company’s assumptions about the inputs that market participants would use in pricing the asset or liability, and are developed based on the best information available in the circumstances. The fair value hierarchy applies only to the valuation inputs used in determining the reported fair value of the investments and is not a measure of the investment credit quality. The three levels of3 within the fair value hierarchy areas described below:in the accounting standards for fair value measurements F-10
Level 1—Valuations based on unadjustedFair values are determined utilizing quoted prices (unadjusted) in active markets for identical assets or liabilities that the Company has the ability to access at the measurement date. Level 2—Valuations based onFair values are determined utilizing quoted prices for identical or similar assets or liabilities in active markets that are not active or for which all significantother market observable inputs are observable, either directly or indirectly.such as interest rates, yield curves and foreign currency spot rates. Level 3—ValuationsPrices or valuations that require inputs that reflect the Company’s own assumptions that are both significant to the fair value measurement and unobservable. To the extent that valuation is based on models or inputs that are less observable or unobservable in the market, the determination of fair value requires more judgment. Accordingly, the degree of judgment exercised by the Company in determining fair value is greatest for instruments categorized in Level 3. A financial instrument’s level within the fair value hierarchy is based on the lowest level of any input that is significant to the fair value measurement.
Items measured at fair value on a recurring basis include a warrant liability (Note 8), marketable securities (Note 3)3 and Note 4) and contingent consideration (Note 12)4). The carrying amounts of accounts payable and accrued expenses approximate their fair values due to their short-term maturities.nature. Business combinations On June 30, 2014,Business combinations are accounted for using the acquisition method of accounting. Using this method, the tangible and intangible assets acquired and the liabilities assumed are recorded as of the acquisition date at their respective fair values. The Company completed itsevaluates a business as an integrated set of activities and assets that is capable of being managed for the purpose of providing a return in the form of dividends, lower costs or other economic benefits and consists of inputs and processes that provide or have the ability to provide outputs. In an acquisition of Pregenen for total considerationa business, the excess of $31.0 million, consisting of cash consideration of $5.1 million, common stock consideration of $19.3 million and contingent consideration with an estimated fair value of $6.6 million on the date of purchase. The estimated fair value of the contingent consideration is based upon significant assumptions regarding probabilities of successful achievement of related milestones,transferred over the estimated timing in which the milestones are achieved and discount rates. The estimated fair value could materially differ from actual values or fair values determined using different assumptions.of the net assets acquired is recorded as goodwill. In an acquisition of net assets that does not constitute a business, no goodwill is recognized.
The consolidated financial statements include the results of operations of an acquired business after the completion of the acquisition. See Note 4, “Fair value measurements,” for additional information. This transaction was accounted for as a business combination under the acquisition method of accounting. Accordingly, the tangible assets and identifiable intangible assets acquired and liabilities assumed were recorded at fair value as of the date of acquisition, with the excess purchase price recorded as goodwill. The estimated fair values of the assets acquired and liabilities assumed at the date of acquisition are summarized in Note 12, “Business combinations.” The estimated fair values of acquired assets and assumed liabilities were determined using the methods discussed in the following paragraphs and require significant judgment and estimates, which could materially differ from actual values and fair values determined using different methods or assumptions.
Goodwill Goodwill represents the excess of the purchase price over the fair value of the net assets acquired when accounted for using the acquisition method of accounting for business combinations. Goodwill is not amortized but is evaluated for impairment within the Company’s single reporting unit on an annual basis, during the fourth quarter, or more frequently if an event occurs or circumstances change that would more-likely-than-not reduce the fair value of the Company’s reporting unit below its carrying amount. The Company has not recognized any impairment charges related to goodwill.goodwill to date. Intangible assets Intangible assets consist of acquired core technology with finite lives. The Company amortizes its intangible assets using the straight-line method over their estimated economic lives. The Company evaluates the potential impairment of intangible assets if events or changes in circumstances indicate that the carrying amount of the assets may not be fully recoverable or that the useful lives of these assets are no longer appropriate. The impairment test is based on a comparison of the undiscounted cash flows expected to be generated from the use of the asset group and its eventual disposition to the carrying value of the asset group. If impairment is indicated, the asset is written down by the amount by which the carrying value of the asset exceeds the related fair value of the asset. The Company has not recognized an impairment charge related to intangible assets.periodically reviews for impairment. Contingent consideration Each reporting period, the Company revalues the contingent consideration obligations associated with business combinations to their fair value and records within operating expenses increases in their fair value as contingent consideration expense and decreases in the fair value as contingent consideration income. Changes in contingent consideration result from changes in the assumptions regarding probabilities of successful achievement of related milestones, the estimated timing in which the milestones are achieved and the discount rate used to estimate the fair value of the liability. Contingent consideration may change significantly as development of the Company’s programs in certain indications progress and additional data are obtained, impacting the Company’s assumptions. The assumptions used in estimating fair value require significant judgment. The use of different assumptions and judgments could result in a materially different estimate of fair value. See Note 4, “Fair value measurements,” for additional information. F-11
Property, plant and equipment Property, plant and equipment is stated at cost. Maintenance and repairs that do not improve or extend the lives of the respective assets are expensed to operations as incurred. Upon disposal, the related cost and accumulated depreciation is removed from the accounts and any resulting gain or loss is included in the results of operations. Depreciation and amortization is calculated using the straight-line method over the estimated useful lives of the assets, which are as follows: | | | Asset | | Estimated useful life | Building | | 40 years | Computer equipment and software | | 3 years | OfficeFurniture and laboratoryfixtures
| | 2-5 years | Laboratory equipment | | 2 -52-5 years
| Leasehold improvements | | Shorter of the useful life or remaining lease term |
The Company records certain estimated costs incurred and reported by a landlord as an asset and corresponding financing lease obligation on the consolidated balance sheets. See Note 8, “Commitments and contingencies,” for additional information.
Impairment of long-lived assets The Company reviews long-lived assets when events or changes in circumstances indicate the carrying value of the assets may not be recoverable. Recoverability is measured by comparison of the book values of the assets to future net undiscounted cash flows that the assets are expected to generate. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the book value of the assets exceed their fair value, which is measured based on the projected discounted future net cash flows arising from the assets. No impairment losses Financing lease obligation Beginning in 2015 and through construction completion in 2017, the Company recorded certain estimated construction costs incurred and reported to the Company by the landlord for its 60 Binney Street location as an asset and corresponding financing lease obligation on the consolidated balance sheets because it was deemed to be the owner of the building during the construction period for accounting purposes. Any costs incurred by the Company that have been reimbursed by the landlord or that qualify for reimbursement by the landlord are recorded during the years ended December 31, 2014, 2013as an asset and 2012. Warrants to purchase convertible preferred stock
In conjunction with various financing transactions,lease obligation. Any incremental costs incurred directly by the Company issued warrantsthat do not qualify for reimbursement by the landlord are also capitalized. In each reporting period, the landlord estimates and reports to purchase sharesthe Company any costs incurred to date related to its portion of the Company’s Series A-1 convertible preferred stock (“Series A-1 Preferred Stock”)building using allocation estimates. During construction, the Company periodically met with the landlord and Series B convertible preferred stock (“Series B Preferred Stock”). Priorits construction manager to July 23, 2012, the Company’s Series A-1 Preferred Stockreview these estimates and Series B Preferred Stock were subject to a redemption provision that was outsideobserve construction progress before recording such amounts. Upon completion of the Company’s control. Therefore, the associated shares were presented as temporary equity. Consequently, the warrants to purchase shares of Series A-1 Preferred Stock and Series B Preferred Stock were accounted for as liabilities through July 23, 2012 and adjusted to fair value at the end of each reporting period. The fair valueconstruction of the warrants classified as liabilities is estimated using the Black-Scholes option pricing model. The estimatesbuilding in the Black-Scholes option pricing model are based,first quarter of 2017, the Company evaluated the lease and determined that it did not meet the criteria for “sale-leaseback” treatment. Accordingly, the Company is depreciating the building over 40 years and incurring interest expense in part,its consolidated statement of operations and comprehensive loss related to the financing lease obligation recorded on subjective assumptions, including, stock price volatility, termits consolidated balance sheet. The Company bifurcates its lease payments pursuant to the lease into (i) a portion that is allocated to the financing obligation related to the building and (ii) a portion that is allocated to the land on which the building was constructed. The portion of the warrants, risk free interest rate, dividend yield, and fair value of the preferred stock underlying the warrants. Such assumptions could differ materially in the future. The re-measurement gain or loss associated with the change in the fair value of the preferred stock warrant liability from the prior period is recognized as a component of other income (expense), net.
On July 23, 2012, in connection with the sale of the Company’s Series D convertible preferred stock (“Series D Preferred Stock”) and the associated modificationslease obligation allocated to the rights, preferencesland is treated for accounting purposes as an operating lease that commenced in September 2015 and privileges ofis recorded on a straight-line basis over the then-existing series of preferred stock, the Series A-1 Preferred Stock was reclassified to permanent equity because the redemption rights were relinquishedinitial lease term. See Note 8, “Commitments and no liquidation preferences were obtained. Additionally, the fair value of the warrants to purchase shares of Series A-1 Preferred Stock as of July 23, 2012 were correspondingly reclassified tocontingencies,” for additional paid-in capital consistent with the treatment of the associated shares of preferred stock. All other classes of preferred stock remained classified within temporary equity as of December 31, 2012, due to their associated liquidation preferences. Due to these remaining liquidating preferences, the warrants to purchase shares of Series B Preferred Stock remained classified within liabilities as of December 31, 2012.
Upon closing of the IPO, all warrants exercisable for convertible preferred stock were automatically converted into warrants exercisable for common stock, resulting in the reclassification of the related convertible preferred stock warrant liability to additional paid-in capital.information.
Revenue recognition The Company has primarily generated revenue through collaboration arrangements researchand out-licensing arrangements including royalties on net sales of products to licensees or sublicensees. Collaboration revenue As of December 31, 2017, the Company’s collaboration revenue is generated exclusively from its collaboration arrangement with Celgene Corporation (“Celgene”), which was originally entered into in March 2013 and licensewas subsequently amended in June 2015 (the “Amended Collaboration Agreement”), as further described in Note 10. The Company analyzes its collaboration arrangements with strategic partnersto assess whether they are within the scope of ASC 808, Collaborative Arrangements (“ASC 808”) to determine whether such arrangements involve joint operating activities performed by parties that are both active participants in the activities and nonprofit organizations forexposed to significant risks and rewards dependent on the development and commercializationcommercial success of product candidates. Additionally,such activities. This assessment is performed throughout the life of the arrangement based on changes in the responsibilities of all parties in the arrangement. For collaboration arrangements that are deemed to be within the scope of ASC 808, the Company has generated revenue from researchfirst determines which elements of the collaboration are deemed to be within the scope of ASC 808 and development grant programs. The Company recognizes revenue in accordance with FASBthose that are more reflective of a vendor-customer relationship and therefore within the scope of ASC Topic 605, Revenue Recognition (“ASC 605”). Accordingly,
For those elements of the arrangement that are accounted for pursuant to ASC 605, revenue is recognized for each unit of accounting when all of the following criteria are met: ●
| Persuasive evidence of an arrangement exists
|
Persuasive evidence of an arrangement exists ●
| Delivery has occurred or services have been rendered
|
Delivery has occurred or services have been rendered ●
| The seller’s price to the buyer is fixed or determinable
|
The seller’s price to the buyer is fixed or determinable ●
| Collectability is reasonably assured
|
Collectability is reasonably assured F-12
Amounts received prior to satisfying the revenue recognition criteria are recorded as deferred revenue in the Company’s consolidated balance sheets. Amounts expected to be recognized as revenue within the 12 months following the balance sheet date are classified as deferred revenue, current portion. Amounts not expected to be recognized as revenue within the 12 months following the balance sheet date are classified as deferred revenue, net of current portion.
Collaboration revenue
As of December 31, 2014, the Company’s collaboration revenue is generated exclusively from itsWhen a collaboration arrangement with Celgene Corporation (“Celgene”). The terms of this arrangement contain multiple deliverables, which include at inception: (i) discovery, research and development services, (ii) participation on the joint steering committee and (iii) participation on the patent committee. The collaboration arrangement also provides Celgene with the option to obtain a license to any product candidates resulting from the collaboration. Moreover, Celgene has the option to extend the term of the collaboration arrangement, firstmultiple-elements accounted for a period of two years and then for an additional period of one year. Additionally,under ASC 605, the Company has the sole right to manufacture or have manufactured supplies of vectors and associated payloads manufactured for incorporation into the associated product candidate in the event a product candidate is licensed. Non-refundable payments to the Company under this arrangement may include: (i) up-front research fees, (ii) product candidate license fees, (iii) extension term research fees, (iv) payments for the manufacture and supply of vectors and payloads, (v) payments based on the achievement of certain milestones and (vi) royalties on product sales. Additionally, the Company may elect to share in the costs incurred from the development, commercialization and manufacture of product candidates licensed by its collaborators and earn its share of the net profits or bear its share of the net losses generated from the sale of product candidates licensed by its collaborators.
The Company analyzes multiple-element arrangements based on the guidance in FASB ASC Topic 605-25, Revenue Recognition-Multiple-Element Arrangements (“ASC 605-25”). Pursuant to the guidance in ASC 605-25, the Company evaluates multiple-element arrangements to determine (1) the deliverables included in the arrangement and (2)determines whether the individual deliverables represent separate units of accounting or whether they must be accounted for as a combined unit of accounting. This evaluation involves subjective determinations and requires management to make judgments about the individual deliverables and whether such deliverables are separable from the other aspects of the contractual relationship. Deliverables are considered separate
units of accounting provided that: (i) the delivered item(s) has value to the customer on a standalone basis and (ii) if the arrangement includes a general right of return relative to the delivered item(s), delivery or performance of the undelivered item(s) is considered probable and substantially in the control of the Company. In assessing whether an item has standalone value, the Company considers factors such as the research, manufacturing and commercialization capabilities of the collaboration partner and the availability of the associated expertise in the general marketplace. In addition, the Company considers whether the collaboration partner can use the other deliverable(s) for their intended purpose without the receipt of the remaining element(s), whether the value of the deliverable is dependent on the undelivered item(s) and whether there are other vendors that can provide the undelivered element(s). The Company’s collaboration arrangement does not contain a general right of return relative to the delivered item(s). Arrangement consideration that is fixed or determinable is allocated among the separate units of accounting using the relative selling price method. Then,method and the applicable revenue recognition criteria in ASC 605-25 are applied to each of the separate units of accounting in determiningdetermine the appropriate period and pattern of recognition. The Company determines the selling price of a unit of accounting following the hierarchy of evidence prescribed by ASC 605-25. Accordingly, the Company determines the estimated selling price for units of accounting within each arrangement using vendor-specific objective evidence (“VSOE”) of selling price, if available, third-party evidence (“TPE”) of selling price if VSOE is not available, or best estimate of selling price (“BESP”) if neither VSOE nor TPE is available. The Company typically uses BESP to estimate the selling price, since it generally does not have VSOE or TPE of selling price for its units of accounting. Determining the BESP for a unit of accounting requires significant judgment. In developing the BESP for a unit of accounting, the Company considers applicable market conditions and relevant entity-specific factors, including factors that were contemplated in negotiating the agreement with the customer and estimated costs. The Company validates the BESP for units of accounting by evaluating whether changes in the key assumptions used to determine the BESP will have a significant effect on the allocation of arrangement consideration between multiple units of accounting. Options are considered substantive if, at the inception of the arrangement, the Company is at risk as to whether the collaboration partner will choose to exercise the option. Factors that the Company considers in evaluating whether an option is substantive include the overall objective of the arrangement, the benefit the collaborator might obtain from the arrangement without exercising the option, the cost to exercise the option and the likelihood that the option will be exercised. For arrangements under which an option is considered substantive, the Company does not consider the item underlying the option to be a deliverable at the inception of the arrangement and the associated option fees are not included in allocable arrangement consideration, assuming the option is not priced at a significant and incremental discount. Conversely, for arrangements under which an option is not considered substantive or if an option is priced at a significant and incremental discount, the Company would consider the item underlying the option to be a deliverable at the inception of the arrangement and a corresponding amount would be included in allocable arrangement consideration. All of the options included in the Company’s collaboration arrangement have been determined to be substantive, and none of the options are priced at a significant and incremental discount. F-13
The Company recognizes arrangement consideration allocated to each unit of accounting when all of the revenue recognition criteria in ASC 605-25 are satisfied for that particular unit of accounting.satisfied. The Company will recognize as revenue arrangement consideration attributed to licenses that have standalone value from the other deliverables to be provided in an arrangementas revenue upon delivery. The Company will recognize as revenueWhen arrangement consideration attributed to licenses that do not have standalone value, from the other deliverables to be provided in an arrangementCompany will recognize revenue over the Company’s estimated performance period asof the combined deliverable. For elements of the arrangement would be accounted for as a single unit of accounting. The Company recognizes revenue from the Celgene arrangement associated with discovery, researchthat are recognized over time, and development services, joint steering committee services and patent committee services ratably over the associated period of performance. If there is no discernible pattern of performance and/or objectively measurable performance measures do not exist, then the Company recognizes revenue under the arrangement on a straight-line basis over the period the Company is expected to complete its performance obligations. Conversely, if the pattern of performance in which the service is provided to the customer can be determined and objectively measurable performance measures exist, then the Company recognizes revenue under the arrangement using the proportional performance method. Revenue
For elements of collaboration arrangements that are accounted for pursuant to ASC 808, an appropriate recognition method is determined and applied consistently, generally by analogy to ASC 605. Amounts that are owed to collaboration partners are recognized is limitedas an offset to collaboration revenues as such amounts are incurred by the lesser ofcollaboration partner. Where amounts owed to a collaboration partner exceed the cumulative amount of payments received or the cumulative amount of revenue earned,Company’s collaboration revenues in each quarterly period, such amounts are classified as determined using the straight-line method or proportional performance method, as applicable, as of the period ending date.research and development expense. At the inception of an arrangement that includes milestone payments, the Company evaluates whether each milestone is substantive and at risk to both parties on the basis of the contingent nature of the milestone. This evaluation includes an assessment of whether: (i) the consideration is commensurate with either the Company’s performance to achieve the milestone or the enhancement of the value of the delivered item(s) as a result of a specific outcome resulting from the Company’s performance to achieve the milestone, (ii) the consideration relates solely to past performance and (iii) the consideration is reasonable relative to all of the deliverables and payment terms within the arrangement. The Company evaluates factors such as the scientific, clinical, regulatory, commercial and other risks that must be overcome to achieve the respective milestone and the level of effort and investment required to achieve the respective milestone in making this assessment. There is considerable judgment involved in determining whether a milestone satisfies all of the criteria required to conclude that a milestone is substantive. The Company has concluded that all of the clinical and regulatory milestones pursuant to its collaboration arrangement are substantive. Accordingly, in accordance with FASB ASC Topic 605-28, Revenue Recognition-Milestone Method, revenue from clinical and regulatory milestone payments will be recognized in its entirety upon successful accomplishment of the milestone, assuming all other revenue recognition criteria are met. Milestones that are
not considered substantive would be recognized as revenue over the remaining period of performance, assuming all other revenue recognition criteria are met. Revenue fromAll commercial milestone paymentsmilestones will be accounted for in the same manner as royalties and recorded as revenue upon achievement of the milestone, assuming all other revenue recognition criteria are met. The Company recognizes royalty revenue generated under collaboration arrangements in the period of sale of the related product(s), based on the underlying contract terms, provided that the reported sales are reliably measurable and the Company has no remaining performance obligations. License and royalty revenue The terms of the Company’s license agreements include delivery of an intellectual property license or the performance of research and development activities. The Company does not have any material license arrangements that contain multiple deliverables. The Company is compensated under license arrangements through nonrefundable up-front payments, milestones, and future royalties on net product sales. Nonrefundable license fees are recognized as revenue upon delivery provided there are no undelivered elements in the arrangement. The Company will recognize royalty revenue in the period of sale of the related product(s), based on the underlying contract terms, provided that the reported sales are reliably measurable and the Company has no remaining performance obligations, assuming all other revenue recognition criteria are met. Research fees and license fees
The terms ofAmounts received prior to satisfying the revenue recognition criteria are recorded as deferred revenue in the Company’s research agreements and license agreements include delivery of an intellectual property license or the performance of research and development activities. The Company does not have any material research arrangements or license arrangements that contain multiple deliverables. The Company is compensated under research arrangements and license arrangements through nonrefundable up-front payments and future royalties on net product sales. Research fees areconsolidated balance sheets. Amounts expected to be recognized as revenue on a straight-line basis overwithin the period that12 months following the research servicesbalance sheet date are classified as deferred revenue, current portion. Amounts not expected to be performed unless the Company’s pattern of performance can be determined to be other than straight-line, in which case, the Company uses the proportional performance method. Nonrefundable license fees are recognized as revenue upon delivery provided therewithin the 12 months following the balance sheet date are no undelivered elements in the arrangement.classified as deferred revenue, net of current portion.
Research and development expenses Research and development costs are charged to expense as costs are incurred in performing research and development activities, including salaries and benefits, facilities costs, overhead costs, clinical study and related clinical manufacturing costs, contract services and other related costs. Research and development costs, including up-front fees and milestones paid to collaborators, are also expensed as incurred. In circumstances where amounts have been paid in excess of costs incurred, the Company records a prepaid expense. The Company accrues costs for clinical trial activities based upon estimates of the services received and related expenses incurred that have yet to be invoiced by the contract research organizations, clinical study sites, laboratories, consultants, or other clinical trial vendors that perform the activities. The Company recognizes the reimbursement associated with collaborative activities to its collaborative partners as research and development expense in the period the services are provided. Cost of license and royalty revenue Cost of license and royalty revenue represents expense associated with amounts owed to third parties as a result of revenue recognized under the Company’s out-license arrangements. Stock-based compensation The Company’s share-based compensation programs grant awards that have included stock options, restricted stock units, restricted stock awards, and shares issued under its employee stock purchase plan. Grants are awarded to employees, including directors, and non-employees. The Company accounts for its stock-based compensation awards in accordance with FASB ASC Topic 718, Compensation—Stock Compensation (“ASC 718”). ASC 718 requires all stock-based payments to employees, including grants of employee stock options and restricted stock units and modifications to existing stock options, to be recognized in the consolidated statements of operations and comprehensive loss based on their fair values. The Company uses the Black-Scholes option pricing model to determine the fair value of options granted. F-14
The Company’s stock-based awards are subject to either service or performance-based vesting conditions. Compensation expense related to awards to employees and directors with service-based vesting conditions is recognized on a straight-line basis based on the grant date fair value over the associated service period of the award, which is generally the vesting term. Compensation expense related to awards to non-employees with service-based vesting conditions is recognized based on the then-current fair value at each financial reporting date prior to the measurement date over the associated service period of the award, which is generally the vesting term, using the accelerated attribution method. Compensation expense related to awards to employees with performance-based vesting
conditions is recognized based on the grant date fair value over the requisite service period using the accelerated attribution method to the extent achievement of the performance condition is probable. Compensation expense related to awards to non-employees with performance-based vesting conditions is recognized based on the then-current fair value at each financial reporting date prior to the measurement date over the requisite service period using the accelerated attribution method to the extent achievement of the performance condition is probable. The Company expenses restricted stock unit awards to employees based on the fair value of the award on a straight-line basis over the associated service period of the award. Awards of restricted stock units to non-employees are adjusted through share-basedstock-based compensation expense at each reporting period end to reflect the current fair value of such awards and expensed using an accelerated attribution model. The Company estimates the fair value of its option awards to employees and directors using the Black-Scholes option pricing model, which requires the input of and subjective assumptions, including (i) the expected stock price volatility, (ii) the calculation of expected term of the award, (iii) the risk-free interest rate, and (iv) expected dividends. Due to the lack of company specificcomplete company-specific historical and implied volatility data for the full expected term of its common stock,the stock-based awards, the Company has basedbases its estimate of expected volatility on the historical volatility of a representative group of similarpublicly traded companies that are publicly traded. When selectingin addition to its own volatility data. For these public companies on which it has based its expected stock price volatility,analyses, the Company selected companies with comparable characteristics to it,its own, including enterprise value, risk profiles, position within the industry, and with historical share price information sufficient to meet the expected termlife of the stock-based awards. The Company computes historical volatility data using the daily closing prices for the selected companies’ shares during the equivalent period of the calculated expected term of the stock-based awards. The Company will continue to apply this process until a sufficient amount of historical information regarding the volatility of its own stock price becomes available. The Company has estimated the expected term of its employee stock options using the “simplified” method, whereby, the expected term equals the arithmetic average of the vesting term and the original contractual term of the option due to its lack of sufficient historical data. The risk-free interest rates for periods within the expected term of the option are based on the U.S. Treasury securities with a maturity date commensurate with the expected term of the associated award. The Company has never paid, and does not expect to pay dividends in the foreseeable future. TheAs a result of the adoption of ASU 2016-09, Improvements to Employee Share-Based Payment Accounting, effective January 1, 2017, the Company is also required to estimateaccounts for forfeitures as they occur instead of estimating forfeitures at the time of grant and reviserevising those estimates in subsequent periods if actual forfeitures differ from its estimates. The Company uses historical data to estimate pre-vesting option forfeitures and records stock-based compensation expense only for those awards that are expected to vest. To the extent that actual forfeitures differ from the Company’s estimates, the differences are recorded as a cumulative adjustment in the period the estimates were revised. Stock-based compensation expense recognized in the financial statements is based on awards thatfor which performance or service conditions are ultimately expected to vest.be satisfied.
Consistent with the guidance in FASB ASC Topic 505-50, Equity-Based Payments to Non-Employees, the fair value of each non-employee stock option and warrant award is estimated at the date of grant using the Black-Scholes option pricing model with assumptions generally consistent with those used for employee stock options, with the exception of expected term, which is over the contractual life. Other IncomeInterest (expense) income, net
Interest (expense) income, net consists primarily of interest expense on the Company’s 60 Binney Street financing lease obligation and Expense In March 2014,interest income earned on investments, net of amortization of premium and accretion of discount. Interest income was approximately $9.5 million, $3.8 million, and $1.6 million for the Company received an award of $0.3 million of tax incentives from the Massachusetts Life Sciences Center, which will allow the Company to monetize approximately $0.3 million of state research and development tax credits. In exchange for these incentives, the Company pledged to hire an incremental fifteen employees and to maintain the additional headcount through at leastyears ended December 31, 2018. Failure to do so could result in the Company being required to repay some or all of these incentives. As the Company met the additional headcount condition during the quarter ended June 30, 20142017, 2016, and continues to meet such condition during2015, respectively. Interest expense was $11.4 million for the year ended December 31, 2014,2017. Please refer to Note 8, “Commitments and contingencies,” for further discussion of interest expense incurred on the 60 Binney Street lease.
Other (expense) income, net Other (expense) income, net consists primarily of gains and losses on the disposal of fixed assets, realized gains and losses on investments, and gains and losses on foreign currency transactions and remeasurement. Net loss per share Basic net loss per share is calculated by dividing net loss attributable to common stockholders by the weighted average number of common shares outstanding during the period. Diluted net income per share is calculated by dividing the net income attributable to common stockholders by the weighted-average number of common equivalent shares outstanding for the period, including any dilutive effect from outstanding stock options, unvested restricted stock, restricted stock units, and employee stock purchase plan stock using the treasury stock method. Given that the Company continuesrecorded a net loss for each of the periods presented, there is no difference between basic and diluted net loss per share since the effect of common stock equivalents would be anti-dilutive and are, therefore, excluded from the diluted net loss per share calculation.
The Company follows the two-class method when computing net loss per share in periods when issued shares that meet the definition of participating securities are outstanding. The two-class method determines net loss per share for each class of common and participating securities according to deferdividends declared or accumulated and amortizeparticipation rights in undistributed earnings. The two-class method requires income available to common stockholders for the benefit of this monetization onperiod to be allocated between common and participating securities based upon their respective rights to received dividends as if all income for the period had been distributed. Accordingly, in periods in which the Company reports a straight-line basis overnet loss attributable to common stockholders when participating securities are outstanding, losses are not allocated to the remaining five-year performance period in other income (expense).participating securities. Income taxes Income taxes are recorded in accordance with FASB ASC Topic 740, Income Taxes (“ASC 740”), which provides for deferred taxes using an asset and liability approach. The Company recognizes deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. Deferred tax assets and liabilities are determined based on the difference between the financial statement and tax bases of assets and liabilities using enacted tax rates in F-15
effect for the year in which the differences are expected to reverse. Valuation allowances are provided, if based upon the weight of available evidence, it is more likely than not that some or all of the deferred tax assets will not be realized. The Company accounts for uncertain tax positions in accordance with the provisions of ASC 740. When uncertain tax positions exist, the Company recognizes the tax benefit of tax positions to the extent that the benefit will more likely than not be realized. The determination as to whether the tax benefit will more likely than not be realized is based upon the technical merits of the tax position as well as consideration of the available facts and circumstances. As of December 31, 20142017 and 2013,2016, the Company does not have any significant uncertain tax positions. Net loss per share
Basic net income (loss) per share is calculated by dividing net income (loss) attributable to common stockholders by the weighted average number of common shares outstanding during the period. Diluted net income per share is calculated by dividing the net income attributable to common stockholders by the weighted-average number of common equivalent shares outstanding for the period, including any dilutive effect from outstanding stock options, unvested restricted stock, restricted stock units, employee stock purchase plan, warrants and acquisition holdback shares using the treasury stock method.
The Company follows the two-class method when computing net income (loss) per share in periods when issued shares that meet the definition of participating securities are outstanding. The two-class method determines net income (loss) per share for each class of common and participating securities according to dividends declared or accumulated and participation rights in undistributed earnings. The two-class method requires income available to common stockholders for the period to be allocated between common and participating securities based upon their respective rights to received dividends as if all income for the period had been distributed. Accordingly, in periods in which the Company reports a net loss attributable to common stockholders when participating securities are outstanding, losses are not allocated to the participating securities.
Comprehensive loss Comprehensive loss is comprisedcomposed of net loss and other comprehensive income or loss.(loss). Other comprehensive income or loss(loss) consists of unrealized gains and losses on marketable securities and foreign currency translation adjustments. Subsequent events
The Company considers events or transactions that occur after the balance sheet date, but prior to the issuance of the financial statements to provide additional evidence relative to certain estimates or to identify matters that require additional disclosure.Recent accounting pronouncements
Recently adopted In March 2016, the FASB issued ASU 2016-09, Improvements to Employee Share-Based Payment Accounting, which simplifies share-based payment accounting pronouncementsthrough a variety of amendments. Upon adoption, all excess tax benefits and tax deficiencies (including tax benefits of dividends on share-based payment awards) are recognized as income tax expense or benefit in the statement of operations and comprehensive loss. The tax effects of exercised or vested awards are treated as discrete items in the reporting period in which they occur. The Company adopted this standard effective January 1, 2017 and applied the modified retrospective adoption approach beginning in 2017, and therefore prior periods have not been adjusted. The adoption impacted the income tax footnote disclosure and did not have a material impact on the Company’s consolidated financial statements. The Company recognizes excess tax benefits regardless of whether or not the benefit reduces taxes payable in the current period. As a result, the Company established a net operating loss deferred tax asset of $76.7 million to account for prior period excess tax benefits through retained earnings, however an offsetting valuation allowance of $76.7 million was also established through retained earnings because it is not more likely than not that the deferred tax asset will be realized due to historical and expected future losses, and as a result there was no impact on the Company’s consolidated financial statements. The Company also elected to account for forfeitures as they occur, and recorded a cumulative catch up of $0.5 million within additional paid-in capital and retained earnings upon adoption in the first quarter of 2017. During the quarter ended June 30,Not yet adopted
In May 2014, the FASB issued ASU No. 2014-09, Revenue from Contracts with Customers (“ASU No. 2014-09”ASC 606”), which supersedes all existing revenue recognition requirements, including most industry-specific guidance. The new standard requires a company to recognize revenue when it transfers goods or services to customers in an amount that reflects the consideration that the company expects to receive for those goods or services. The new standard will be effective on January 1, 2018 and earlier application is permitted only for annual reporting periods beginning after December 15, 2016, including interim reporting periods within that reporting period. ASC 606 allows for either a full retrospective adoption, in which the standard is applied to all of the periods presented, or a modified retrospective approach, in which the standard is applied to the most current period presented in the financial statements. The Company expects to adopt this standard using the modified retrospective approach. The revenue generated in the year ended December 31, 2017 relates to the Company’s collaboration arrangement with Celgene and the Company’s out-licensing arrangements. The Company is continuing to assess the potential impact that ASC 606 may have on its financial position and results of operations as it relates to the Celgene arrangement. The Company expects that certain of its accounting conclusions will require
further judgment, including, but not limited to, (1) the evaluation of variable consideration, and in particular, milestone payments due from Celgene as the inclusion of milestone payments in the transaction price could accelerate revenue recognized under ASC 606 compared to ASC 605, (2) allocation of variable consideration to one or more performance obligations, (3) evaluation of whether a significant financing component is present, and (4) determination of the revenue recognition method for services performed under the arrangement. As the Company is still in the process of completing its assessment of the Celgene arrangement, an estimate of the potential impact has not yet been made. The Company will complete its assessment in the first quarter of 2018. The Company has substantially completed its assessment of the ASC 606 impact on its two out-licensing arrangements and does not expect the adoption of ASC 606 to have a material impact on its financial position and results of operations when applied to its out-licensing arrangements. In February 2016, the FASB issued ASU 2016-02, Leases, (“ASU 2016-02”), which requires a lessee to recognize assets and liabilities on the balance sheet for operating leases and changes many key definitions, including the definition of a lease. The new standard includes a short-term lease exception for leases with a term of 12 months or less, as part of which a lessee can make an accounting policy election not to recognize lease assets and lease liabilities. Lessees will continue to differentiate between finance leases (previously referred to as capital leases) and operating leases using classification criteria that are substantially similar to the previous guidance. The new standard will be effective beginning January 1, 2019, and early adoption is not permitted for public entities. The Company is currently evaluating the potential impact that ASU No. 2014-092016-02 may have on its financial position and results of operations. During the quarter ended September 30, 2014,In August 2016, the FASB issued ASU No. 2014-15, Presentation2016-15, Statement of Financial Statements – Going Concern (“ASU No. 2014-15”).Cash Flows: Classification of Certain Cash Receipts and Cash Payments. The new guidance addresses management’s responsibilitystandard clarifies certain aspects of the statement of cash flows, including the classification of contingent consideration payments made after a business combination, the clarification of restricted cash, and several clarifications not currently applicable to evaluate whether therethe Company. The new standard also clarifies that an entity should determine each separately identifiable source or use within the cash receipts and cash payments on the basis of the nature of the underlying cash flows. In situations in which cash receipts and payments have aspects of more than one class of cash flows and cannot be separated by source or use, the appropriate classification should depend on the activity that is substantial doubt about an entity’s abilitylikely to continue as a going concern and to provide related footnote disclosures. Management’s evaluation should be based on relevant conditions and events that are known and reasonably knowable at the date thatpredominant source or use of cash flows for the financial statements are issued.item. The new standard will be effective for the first interim periodCompany on January 1, 2018. The Company is continuing to assess the impact that adoption of this standard is expected to have on the Company’s consolidated statements of cash flows upon adoption.
In November 2016, the FASB issued ASU 2016-18, Statement of Cash Flows: Restricted Cash (“ASU 2016-18”). The amendments in this update require that amounts generally described as restricted cash and restricted cash equivalents be included within annual reporting periods beginning after December 15, 2016. Earlycash and cash equivalents when reconciling the beginning-of-period and end-of-period total amounts shown on the statement of cash flows. ASU 2016-18 will be effective January 1, 2018 and early adoption is permitted. TheAs of December 31, 2017, the Company is currently evaluatinghas not elected to early adopt this guidance, but expects the potentialadoption to have an impact that ASU No. 2014-15 may have on its consolidated statement of cash flows as, upon adoption, it will include the Company’s restricted cash balance in the cash and cash equivalents reconciliation of operating, investing and financing activities. In January 2017, the FASB issued ASU 2017-04, Intangibles – Goodwill and Other (Topic 350): Simplifying the Test for Goodwill Impairment. To address concerns over the cost and complexity of the two-step goodwill impairment test, the amendments in this ASU remove the second step of the test. An entity will apply a one-step quantitative test and record the amount of goodwill impairment as the excess of a reporting unit's carrying amount over its fair value, not to exceed the total amount of goodwill allocated to the reporting unit. The new guidance does not amend the optional qualitative assessment of goodwill impairment. The new standard will be effective beginning January 1, 2020 and early adoption is permitted with measurement dates on or after January 1, 2017. The adoption of this standard is not expected to have a material impact on the Company’s financial position andor results of operations.operations upon adoption. In April 2017, the FASB issued ASU 2017-08, Receivables – Nonrefundable Fees and Other Costs (“Subtopic 310-20”). The new standard amends the amortization period for certain purchased callable debt securities held at a premium by shortening the amortization period for the premium to the earliest call date. Subtopic 310-20 calls for a modified retrospective application under which a cumulative-effect adjustment will be made to retained earnings as of the beginning of the first reporting period in which the guidance is adopted. The new standard will be effective beginning January 1, 2019 and early adoption is permitted for public entities. The adoption of this standard is not expected to have a material impact on the Company’s financial position or results of operations upon adoption. In May 2017, the FASB issued ASU 2017-09, Compensation – Stock Compensation (“Topic 718”): Scope Modification Accounting. The new standard is intended to reduce the diversity in practice and cost and complexity when applying the guidance in Topic 718 to a change to the terms or conditions of a share-based payment award. The new standard will be effective beginning January 1, 2019. The adoption of this standard is not expected to have a material impact on the Company’s financial position or results of operations upon adoption.
F-16
3. Marketable securities The following table summarizes the available-for-sale securities held at December 31, 20142017 and 2016 (in thousands): Description | | Amortized Cost | | | Unrealized Gains | | | Unrealized Losses | | | Fair Value | | | Amortized Cost | | | Unrealized Gains | | | Unrealized Losses | | | Fair Value | | December 31, 2014 | | | | | | | | | | | | | | | | | | U.S. government agency securities | | $ | 131,589 | | | $ | 6 | | | $ | (59 | ) | | $ | 131,536 | | | December 31, 2017 | | | | | | | | | | | | | | | | | | U.S. government agency securities and treasuries | | | $ | 841,895 | | | $ | — | | | $ | (3,579 | ) | | $ | 838,316 | | Certificates of deposit | | | 12,640 | | | | — | | | | (18 | ) | | | 12,622 | | | | 17,480 | | | | 1 | | | | — | | | | 17,481 | | Total | | $ | 144,229 | | | $ | 6 | | | $ | (77 | ) | | $ | 144,158 | | | $ | 859,375 | | | $ | 1 | | | $ | (3,579 | ) | | $ | 855,797 | | | | | | | | | | | | | | | | | | | | December 31, 2016 | | | | | | | | | | | | | | | | | | U.S. government agency securities and treasuries | | | $ | 600,001 | | | $ | 34 | | | $ | (575 | ) | | $ | 599,460 | | Certificates of deposit | | | | 6,480 | | | | 6 | | | | (3 | ) | | | 6,483 | | Total | | | $ | 606,481 | | | $ | 40 | | | $ | (578 | ) | | $ | 605,943 | |
The Company did not hold any available-for-sale securities as of December 31, 2013.
No available-for-sale securities held as of December 31, 20142017 or 2016 had remaining maturities greater than twothree years. 4. Fair value measurements The following table sets forth the Company’s assets and liabilities that are measured at fair value on a recurring basis as of December 31, 20142017 and 20132016 (in thousands): Description | | Total | | | Quoted prices in active markets (Level 1) | | | Significant other observable inputs (Level 2) | | | Significant unobservable inputs (Level 3) | | | Total | | | Quoted prices in active markets (Level 1) | | | Significant other observable inputs (Level 2) | | | Significant unobservable inputs (Level 3) | | December 31, 2014 | | | | | | | | | | | | | | | | | | December 31, 2017 | | | | | | | | | | | | | | | | | | Assets: | | | | | | | | | | | | | | | | | | Cash and cash equivalents | | | $ | 758,505 | | | $ | 758,505 | | | $ | — | | | $ | — | | Marketable securities: | | | | | | | | | | | | | | | | | | U.S. government agency securities and treasuries | | | | 838,316 | | | | — | | | | 838,316 | | | | — | | Certificates of deposit | | | | 17,481 | | | | — | | | | 17,481 | | | | — | | Total assets | | | $ | 1,614,302 | | | $ | 758,505 | | | $ | 855,797 | | | $ | — | | Liabilities: | | | | | | | | | | | | | | | | | | Contingent consideration | | | $ | 2,231 | | | $ | — | | | $ | — | | | $ | 2,231 | | Total liabilities | | | $ | 2,231 | | | $ | — | | | $ | — | | | $ | 2,231 | | December 31, 2016 | | | | | | | | | | | | | | | | | | Assets: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Cash and cash equivalents | | $ | 347,845 | | | $ | 347,845 | | | $ | — | | | $ | — | | | $ | 278,887 | | | $ | 278,887 | | | $ | — | | | $ | — | | Marketable securities: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | U.S. government agency securities | | | 131,536 | | | | — | | | | 131,536 | | | | — | | | | 599,460 | | | | — | | | | 599,460 | | | | — | | Certificates of deposit | | | 12,622 | | | | — | | | | 12,622 | | | | — | | | | 6,483 | | | | — | | | | 6,483 | | | | — | | Total assets | | $ | 492,003 | | | $ | 347,845 | | | $ | 144,158 | | | $ | — | | | $ | 884,830 | | | $ | 278,887 | | | $ | 605,943 | | | $ | — | | Liabilities: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Contingent consideration | | $ | 6,796 | | | $ | — | | | $ | — | | | $ | 6,796 | | | $ | 7,756 | | | $ | — | | | $ | — | | | $ | 7,756 | | Total liabilities | | $ | 6,796 | | | $ | — | | | $ | — | | | $ | 6,796 | | | $ | 7,756 | | | $ | — | | | $ | — | | | $ | 7,756 | | December 31, 2013 | | | | | | | | | | | | | | | | | | Total cash and cash equivalents | | $ | 206,279 | | | $ | 206,279 | | | $ | — | | | $ | — | | |
Cash and cash equivalents The Company considers all highly liquid securities with original final maturities of three months90 days or less from the date of purchase to be cash equivalents. As of December 31, 2014 and 2013,2017, cash and cash equivalents comprise funds in cash and money market accounts. As of December 31, 2016, cash and cash equivalents comprise funds in cash, money market accounts, and federally insured deposits.
Marketable securities The amortized cost of available-for-sale securities is adjusted for amortization of premiums and accretion of discounts to maturity. At December 31, 2014,2017 and 2016, the balance in the Company’s accumulated other comprehensive loss was composed solelyprimarily of activity related to the Company’s available-for-sale marketable securities. There were no material realized gains or losses recognized on the sale or maturity of available-for-sale securities during the years ended December 31, 20142017 or 2013,2016, and as a result, the Company did not reclassify any amount out of accumulated other comprehensive incomeloss for the same periods. The aggregate fair value of securities held by the Company in an unrealized loss position for less than twelve months as of December 31, 20142017 and 2016 was $704.1 million and $376.1 million, respectively. As of December 31, 2017 and 2016, there were $134.4 million which consisted of 52 certificates of deposit and 7 U.S. government agency securities.$95.5 million in securities held by the Company in an unrealized loss position for more than twelve months, respectively. The Company has the intent and ability to hold such securities until recovery. The Company determined that there was no material change in the credit risk of the above investments. As a result, the Company determined it did not hold any investments with an other-than-temporary impairment as of December 31, 2014.2017 and 2016. F-17
Contingent consideration On June 30, 2014, the Company acquired Pregenen. In connection with the acquisition of Pregenen, the Company recorded contingent consideration pertaining to the amounts potentially payable to Pregenen’s former equityholders pursuant to the Stock Purchase Agreement by and among the Company, Pregenen and Pregenen’s former equityholders. Contingent consideration is measured at fair value and is based on significant inputs not observable in the market, which represents a Level 3 measurement within the fair value hierarchy. The valuation of contingent consideration uses assumptions the Company believes would be made by a market participant. The Company assesses these estimates on an on-going basis as additional data impacting the assumptions is obtained. Future changes in the fair value of contingent consideration related to updated assumptions and estimates are recognized within the consolidated statements of operations and comprehensive loss. Contingent consideration may change significantly as development progresses and additional data are obtained, impacting the Company’s assumptions regarding probabilities of successful achievement of related milestones used to estimate the fair value of the liability and the timing in which they are expected to be achieved. In evaluating the fair value information, considerable judgment is required to interpret the market data used to develop the estimates. The estimates of fair value may not be indicative of the amounts that could be realized in a current market exchange. Accordingly, the use of different market assumptions and/or different valuation techniques could result in materially different fair value estimates. The significant unobservable inputs used in the measurement of fair value of the Company’s contingent consideration are probabilities of successful achievement of preclinical, clinical and commercial milestones, the period in which these milestones are expected to be achieved ranging from 20152021 to 20262028 and discount rates ranging from 10.8%19.0% to 14.8%20.0%. Significant increases or decreases in any of the probabilities of success would result in a significantly higher or lower fair value measurement, respectively. Significant increases or decreases in these other inputs would result in a significantly lower or higher fair value measurement, respectively. The table below provides a roll-forward of fair value of the Company’s contingent consideration obligations which include Level 3 inputs (in thousands): | | Year ended December 31, | | | Year ended December 31, 2014 | | 2017 | | | 2016 | | Beginning balance | $ | — | | $ | 7,756 | | | $ | 8,665 | | Additions | | 6,550 | | | — | | | | — | | Changes in fair value | | 246 | | | (525 | ) | | | 4,091 | | Payments | | — | | | (5,000 | ) | | | (5,000 | ) | Ending balance | $ | 6,796 | | $ | 2,231 | | | $ | 7,756 | |
A $5.0 million preclinical milestone was achieved and paid to the former equityholders of Pregenen in each of the years ended December 31, 2017 and 2016. As of December 31, 2014, $0.52017, the remaining $2.2 million contingent consideration obligation is reflected as a non-current liability in the consolidated balance sheet. As of December 31, 2016, $4.5 million and $3.3 million of the fair value of the Company’s total contingent consideration obligationsobligation was reflected as components of accrued expenses and other current liabilities and as a non-current liability, respectively, within the consolidated balance sheets, with the remaining balances of $6.3 million reflected as a non-current liability.sheet. Please refer to Note 8, “Commitments and contingencies,” for further information.
5. Property, plant and equipment, net Property, plant and equipment, net, consists of the following (in thousands): | | | | December 31, | | | | As of December 31, | | | 2014 | | | 2013 | | 2017 | | | 2016 | | Land | | $ | 1,210 | | | $ | — | | Building | | | 164,414 | | | | — | | Computer equipment and software | $ | 814 | | | $ | 576 | | | 5,134 | | | | 1,655 | | Office equipment | | 786 | | | | 780 | | | 4,478 | | | | 1,427 | | Laboratory equipment | | 7,223 | | | | 3,758 | | | 24,914 | | | | 16,305 | | Leasehold improvements | | 10,318 | | | | 7,260 | | | 116 | | | | 13,697 | | Total property and equipment | | 19,141 | | | | 12,374 | | | Construction-in-progress | | | 15,189 | | | | 136,315 | | Total property, plant and equipment | | | 215,455 | | | | 169,399 | | Less accumulated depreciation and amortization | | (3,401 | ) | | | (1,454 | ) | | (15,849 | ) | | | (12,444 | ) | Property and equipment, net | $ | 15,740 | | | $ | 10,920 | | | Property, plant and equipment, net | | $ | 199,606 | | | $ | 156,955 | |
In November 2017, the Company acquired a manufacturing facility, which is in the process of construction, in Durham, North Carolina for the future manufacture of lentiviral vector for the Company’s gene and cell therapies. Construction-in-progress as of December 31, 2017 includes $12.9 million related to the North Carolina manufacturing facility. As of December 31, 2017, total property, plant and equipment includes $164.4 million related to the Company’s headquarters at 60 Binney Street in Cambridge, Massachusetts, of which $156.0 was incurred by the landlord. Construction-in-progress as of December 31, 2016 includes $126.9 million related to the construction of the Company’s headquarters at 60 Binney Street in Cambridge, Massachusetts. Depreciation and amortization expense related to property, plant and equipment was $2.3$9.8 million, $0.9$5.9 million, and $0.3$3.7 million for the years endingended December 31, 2014, 2013,2017, 2016, and 2012,2015, respectively. Please refer to Note 8, “Commitments and contingencies,” for further information. F-18
6. Restricted cash As of December 31, 2014 and 2013,2017, the Company maintained letters of credit of $1.2 $13.8 million and $1.4 million, respectively, which are required to be collateralized with a bank account at a financial institution in accordance with the Company’s 60 Binney Street Lease agreement. As of December 31, 2016, the Company maintained letters of credit of $14.4 million which were required to be collateralized with a bank account at a financial institution in accordance with the Company’s current and formerprior headquarters’ lease agreements. Subject to the terms of the 60 Binney Street Lease agreement and certain reduction requirements specified therein, including market capitalization requirements, this amount may decrease by $1.5 million on the fourth, fifth and sixth anniversaries of the date the Company occupies the building. 7. Accrued expenses and other current liabilities Accrued expenses and other current liabilities consist of the following (in thousands): | | December 31, | | | As of December 31, | | | | 2014 | | | 2013 | | | 2017 | | | 2016 | | Employee compensation | | $ | 4,943 | | | $ | 1,740 | | | $ | 19,657 | | | $ | 11,296 | | Accrued goods and services | | | 7,358 | | | | 2,119 | | | | 29,533 | | | | 34,275 | | Accrued license and milestone fees | | | | 4,584 | | | | 2,464 | | Accrued professional fees | | | 428 | | | | 305 | | | | 1,402 | | | | 1,492 | | Deferred rent, current portion | | | 914 | | | | 738 | | | Financing lease obligation, current portion | | | | 1,051 | | | | — | | Contingent consideration, current portion | | | 475 | | | | — | | | | — | | | | 4,479 | | Other | | | 531 | | | | 273 | | | | 838 | | | | 654 | | Total accrued expenses and other current liabilities | | $ | 14,649 | | | $ | 5,175 | | | $ | 57,065 | | | $ | 54,660 | |
8. Warrants Warrants outstanding consist of the following (in thousands):
| | As of December 31, | | | | 2014 | | | 2013 | | Warrants to purchase Common Stock | | | 177 | | | | 338 | | | | | 177 | | | | 338 | |
As of December 31, 2012, the Company had outstanding warrants to purchase 6,512,650 shares of capital stock. Upon the closing of the Company’s IPO on June 24, 2013, all of the warrants exercisable for convertible preferred stock were automatically converted into warrants exercisable for 337,952 shares of common stock, of which 307,648Commitments and 30,304 were exercisable at $12.55 and $6.19 per share, respectively, and expire between November 16, 2015 and April 15, 2019. Each warrant is exercisable on either a physical settlement or net share settlement basis. During the years ended December 31, 2014 and 2013, there were 160,676 and 102,394 warrants exercised, respectively, and no cancellations or expirations.
In conjunction with the automatic conversion of all warrants exercisable for convertible preferred stock into warrants exercisable for common stock, the Company reclassified the related convertible preferred stock warrant liability to additional paid-in capital. The warrant liability was re-measured to fair value prior to reclassification to additional paid-in capital. As of December 31, 2014 and 2013, the Company had no outstanding warrant liability.
The following table sets forth a summary of changes in the fair value of the Company’s preferred stock warrant liability which represents a recurring measurement that is classified within Level 3 of the fair value hierarchy wherein fair value is estimated using significant unobservable inputs (in thousands):
| | Year ended December 31, 2013* | | Beginning balance | | $ | 215 | | Change in fair value | | | 440 | | Reclassification to equity | | | (655 | ) | Ending balance | | $ | — | |
*
| These warrants were re-measured to fair value and then reclassified to additional paid-in capital on June 24, 2013.
|
F-19
The fair value of each warrant to purchase shares of the Company’s Series B Preferred Stock was estimated using the Black-Scholes option pricing model with the following weighted-average assumptions:
| | Year ended December 31, 2013* | | Fair value of underlying instrument | | $ | 0.95 | | Expected volatility | | | 82.0 | % | Expected term (in years) | | | 5.93 | | Risk-free interest rate | | | 1.1 | % | Expected dividend yield | | | 0.0 | % |
*
| Series B warrants were re-measured to fair value and then reclassified to additional paid-in capital on June 24, 2013.
|
contingencies 9. Commitments and contingenciesOperating lease commitments
On June 3, 2013, the Company entered into a nine-year building lease for approximately 43,600 square feet of space for its new corporate headquarterslocated at 150 Second Street, Cambridge, Massachusetts, which commenced in December 2013. This lease was amended in June 2014 to add approximately 9,900 additional square feet. The lease originally had monthly lease payments of $0.2 million for the first 12 months, which increased to $0.3 million per month beginning in December 2014 due to the lease amendment, with annual rent escalations thereafter and provided a rent abatement of $0.2 million per month for the first six months. The Company has the option to extend this lease by an additional five years. As the Company obtained access to the newly leased space on July 22, 2013, this is considered the lease commencement date for accounting purposes, thus rentthereafter. Rent expense began on this date and iswas recognized on a straight-line basis over the term of the lease. In addition, thelease through April 2017. The lease provided a contribution from the landlord towards the initial build-out of the space of up to $6.5$7.8 million. The Company capitalizescapitalized the leasehold improvements as property, plant and equipment and recordsrecorded the landlord incentive payments received as deferred rent and amortizesamortized these amounts as reductions to rent expense over the lease term. In addition, in accordance with the lease, the Company entered into a cash-collateralized irrevocable standby letter of credit in the amount of $1.3 million, naming the landlord as beneficiary.beneficiary, which had a balance of $0.6 million as of December 31, 2016. On September 30, 2016, the Company entered into an Assignment and Assumption of Lease (“Assignment”) relating to this lease. Under the Assignment, the Company assigned all of its rights, interests, obligations and responsibilities under the lease, effective May 1, 2017. Accordingly, $8.3 million of tenant improvement assets were disposed and $8.0 million of non-current deferred rent was removed from the consolidated balance sheets as of December 31, 2017, with the resulting loss of $0.3 million recorded within the consolidated statement of operations and comprehensive loss during the year ended December 31, 2017. The $0.6 million letter of credit was also released during the year ended December 31, 2017. On June 29, 2015, the Company entered into a lease agreement for additional office space located at 215 First Street, Cambridge, Massachusetts. Under the terms of the lease, the Company leased approximately 15,120 square feet starting on July 13, 2015 for $0.5 million per year in base rent, which was subject to a 3% annual rent increase plus certain operating expenses and taxes. The lease term was until August 31, 2020, and included early termination provisions that could allow the Company to terminate the lease without penalty at the end of the 20th full calendar month following the delivery of the premises if the Company met certain conditions specified within the lease. Under the terms of the lease, the Company also leased an additional 8,075 square feet of office space in the same premises starting on January 1, 2016 for an additional $0.3 million per year in base rent, which was subject to a 3% annual rent increase plus certain operating expenses and taxes. The Company terminated this lease effective April 12, 2017. On June 3, 2016, the Company entered into a strategic manufacturing agreement for the future commercial production of the Company’s Lenti-D and LentiGlobin product candidates with a contract manufacturing organization. Under this 12 year agreement, the contract manufacturing organization will complete the design, construction, validation and process validation of the leased suites prior to anticipated commercial launch of the product candidates. During construction, the Company is required to pay $12.5 million upon the achievement of certain contractual milestones, and may pay up to $8.0 million in additional contractual milestones if the Company elects its option to lease additional suites. The Company paid $5.0 million for the achievement of the first and second contractual milestones during 2016 and paid the third milestone of $3.0 million during the first quarter of 2017. Additionally, the fourth milestone of $2.5 million was achieved in the fourth quarter of 2017 and is reflected as a component of accrued expenses and other current liabilities within the consolidated balance sheet at December 31, 2017. Following construction completion, the Company will pay $5.1 million per year in fixed suite fees as well as certain fixed labor, raw materials, testing and shipping costs for manufacturing services, and may pay additional suite fees if it elects its option to reserve or lease additional suites. The Company estimates completion will occur in 2018. The Company may terminate this agreement any time after upon payment of a one-time termination fee and up to 24 months of fixed suite and labor fees. The Company concluded that this agreement contains an embedded lease as the suites are designated for the Company’s exclusive use during the term of the agreement. The Company concluded that it is not the deemed owner during construction nor is it a capital lease under ASC 840-10, Leases – Overall. As a result, the Company accounts for the agreement as an operating lease and expenses the rental payments on a straight-line basis over the term of the embedded lease. On November 18, 2016, the Company entered into an agreement for future clinical and commercial production of the Company’s LentiGlobin gene therapy drug products with a contract manufacturing organization at an existing facility. The term of the agreement is five years with a three year renewal at the mutual option of each party. Under the agreement, the Company is required to pay an up-front fee of €3.0 million, €2.0 million of which was paid in the fourth quarter of 2016 and €1.0 million of which is expected to be paid in mid-2018, and annual maintenance and production fees of up to €9.8 million, depending on its production needs. The Company may terminate this agreement with six months’ notice and a one-time termination fee prior to July 1, 2018, or twelve months’ notice and a one-time termination fee thereafter. The Company concluded that this agreement contains an embedded lease as the clean rooms are designated for the Company’s exclusive use during the term of the agreement, and determined that it is not a capital lease under ASC 840-10, Leases – Overall. As a result, the Company will account for the agreement as an operating lease and expense the rental payments on a straight-line basis over the term of the embedded lease.
60 Binney Street Lease commitments On September 21, 2015, the Company entered into a lease agreement for office and laboratory space located in a building (the “Building”) at 60 Binney Street, Cambridge, Massachusetts (the “60 Binney Street Lease”) to become its new corporate headquarters. Under the terms of the 60 Binney Street Lease, starting on October 1, 2016, the Company leases approximately 253,108 square feet of office and laboratory space at $72.50 per square foot per year, or $18.4 million per year in base rent, which is subject to scheduled annual rent increases of 1.75% plus certain operating expenses and taxes. The Company also executed a $9.2 million letter of credit upon signing the 60 Binney Street Lease, which was required to be collateralized with a bank account at a financial institution in accordance with the 60 Binney Street Lease agreement. This letter of credit was reducedincreased to $1.0$13.8 million during the third quarter of 2014 and may be further reduced to $0.8 million and $0.6 million upon2016 as required under the first and second anniversariesterms of the rent commencement date, respectively.lease. Subject to the terms of the lease and certain reduction requirements specified therein, including market capitalization requirements, this amount may decrease back to $9.2 million over time. The Company relocated60 Binney Street Lease will continue until March 31, 2027. Pursuant to a work letter entered into its new corporate headquarters in December 2013connection with the 60 Binney Street Lease, the landlord will contribute an aggregate of $42.4 million toward the cost of construction and ceased use of its former facility during the first quarter of 2014. During the first quarter of 2014, the Company fully subleased its former facility, which decreased the rent abatement available under its new lease. The leasetenant improvements for the Building. The purpose of the 60 Binney Street Lease was to replace the Company’s former headquarters, located at 840 Memorial Drive, Cambridge, Massachusetts, expires on March 31, 2015. On June 9, 2014, the Company amended its lease agreement to add an additional 9,869 square feet of space for its corporate headquarterspreviously leased premises at 150 Second Street and 215 First Street in Cambridge, Massachusetts.Massachusetts, both of which were fully exited in the first half of 2017. The expansion increasedCompany has the yearlyoption to extend the 60 Binney Street Lease for two successive five-year terms. The Company occupied the Building beginning on March 27, 2017.
Because the Company is involved in the construction project, including having responsibility to pay for a portion of the costs of finish work and mechanical, electrical, and plumbing elements of the Building, among other items, the Company is deemed for accounting purposes to be the owner of the Building during the construction period. Accordingly, construction costs that have been incurred by the landlord directly or indirectly through reimbursement to the Company as part of its tenant improvement allowance have been recorded as an asset in “Property, plant and equipment, net” with a related financing obligation in “Accrued expenses and other current liabilities” and “Financing lease obligation, net of current portion” on the Company’s consolidated balance sheets. Tenant improvement costs that are reimbursable by the landlord and have not yet been paid to the Company are recorded in “Tenant improvements receivable” on the Company’s consolidated balance sheets. Tenant improvement costs that are not reimbursable by the landlord are recorded in “Property, plant and equipment, net” on the Company’s consolidated balance sheets. The Company evaluated the 60 Binney Street Lease upon occupancy on March 27, 2017 and determined that the 60 Binney Street Lease did not meet the criteria for “sale-leaseback” treatment. This determination was based on, among other things, the Company's continuing involvement with the property in the form of non-recourse financing to the lessor. Accordingly, upon occupancy, the Company commenced depreciating the portion of the building in service over a useful life of 40 years and incurred interest expense related to the financing obligation of $11.4 million for the year ended December 31, 2017. The Company made $0.6 million in principal payments, which are included in operating expense, for the year ended December 31, 2017. The Company bifurcates its lease payments by $0.6 million beginningpursuant to the 60 Binney Street Lease into (i) a portion that is allocated to the Building and (ii) a portion that is allocated to the land on which the Building is located, which is recorded as rental expense. The Company began making lease payments pursuant to the 60 Binney Street Lease in December 2014 with rent escalations thereafter. In addition,March 2017. The portion of the lease amendment provides a contribution fromobligation allocated to the landlord towards the build-outland is treated for accounting purposes as an operating lease that commenced upon execution of the additional space60 Binney Street Lease in September 2015. During the years ended December 31, 2017, 2016 and 2015, the Company recognized $1.9 million, $1.9 million, and $0.5 million of uprental expense attributable to $1.2 million.the land, respectively.
As of December 31, 2014,2017, future minimum commitments under the 60 Binney Street Lease and facility operating leases were as follows (in thousands): Years ended December 31, | Second Street Lease | | | Other Operating Leases | | | Total Lease Commitments | | 2015 | $ | 3,166 | | | $ | 486 | | | $ | 3,652 | | 2016 | | 3,261 | | | | 231 | | | | 3,492 | | 2017 | | 3,358 | | | | — | | | | 3,358 | | 2018 | | 3,460 | | | | — | | | | 3,460 | | 2019 | | 3,563 | | | | — | | | | 3,563 | | 2020 and thereafter | | 11,344 | | | | — | | | | 11,344 | | Total minimum lease payments | $ | 28,152 | | | $ | 717 | | | $ | 28,869 | |
Years Ended December 31, | | 60 Binney Street Lease | | Other Operating Leases (1) | | Total Lease Commitments | 2018 | | $18,647 | | $13,941 | | $32,588 | 2019 | | 18,974 | | 11,491 | | 30,465 | 2020 | | 19,306 | | 11,521 | | 30,827 | 2021 | | 19,642 | | 11,551 | | 31,193 | 2022 | | 19,987 | | 5,365 | | 25,352 | 2023 and thereafter | | 88,888 | | 27,625 | | 116,513 | Total minimum lease payments | | $185,444 | | $81,494 | | $266,938 |
(1) | Includes the lease of the Company’s lab and office space in Seattle, Washington and two embedded operating leases at contract manufacturing organizations. |
For the 60 Binney Street Lease, the table above sets forth the future minimum rental payments that the Company is obligated to pay, including amounts reflected on the consolidated balance sheet as part of the balance under the caption “Accrued expenses and other current liabilities” and “Financing lease obligation, net of current portion.” The Company commenced rental payments in April 2017. Rent expense is calculated on a straight-line basis over the term of the lease. Rent expense recognized under all operating leases, including additional rent charges for utilities, parking, maintenance, and real estate taxes, and including rental expense attributable to the 60 Binney Street Lease land was $4.3$9.0 million, $2.4$8.3 million, and $0.8$5.7 million for the years ended December 31, 2017, 2016 and 2015, respectively. Contingent consideration related to business combinations On June 30, 2014, 2013the Company acquired Pregenen. During 2017, one milestone under the Stock Purchase Agreement was achieved, which resulted in a $5.0 million payment to the former equityholders of Pregenen during 2017. During 2016, two milestones were achieved, which resulted in a $5.0 million payment to the former equityholders of Pregenen. The Company may be required to make up to an additional $120.0 million in remaining future contingent cash payments to the former equityholders of Pregenen upon the achievement of certain clinical and 2012, respectively.commercial milestones related to the Pregenen technology, of which $20.1 million relates to clinical milestones and $99.9 million relates to commercial milestones. In accordance with accounting guidance for business combinations, contingent consideration liabilities are required to be recognized on the consolidated balance sheets at fair value. Estimating the fair value of contingent consideration requires the use of significant assumptions primarily relating to probabilities of successful achievement of certain clinical and commercial milestones, the expected timing in which these milestones will be achieved and discount rates. The use of different assumptions could result in materially different estimates of fair value. See Note 4, “Fair value measurements” for additional information. Other funding commitments The Company is party to various agreements, principally relating to licensed technology, that require future payments relating to milestones not met at December 31, 20142017 and December 31, 20132016 or royalties on future sales of specified products. Additionally, the Company is party to various contracts with contract research organizations and contract manufacturers that generally provide for termination on notice, with the exact amounts in the event of termination to be based on the timing of the termination and the terms of the agreement. In each of 2018 and 2019, the Company expects to make payments of approximately $12.0 million under an agreement with a contract manufacturer. The Company enters into standard indemnification agreements in the ordinary course of business. Pursuant to the agreements, the Company indemnifies, holds harmless, and agrees to reimburse the indemnified party for losses suffered or incurred by the indemnified party, generally the Company’s business partners or customers.partners. The term of these indemnification agreements is generally perpetual any time after execution of the agreement. The maximum potential amount of future payments the Company could be F-20
required to make under these indemnification agreements is unlimited. The Company has never incurred costs to defend lawsuits or settle claims related to these indemnification agreements. The Company’s wholly-owned subsidiary bluebird bio France – SARL participates in the French Crédit d’Impôt Recherche (“CIR”) program which allows companies to monetize up to 30% of eligible research expenses. The Company received aggregate reimbursement of $1.4 million related to years 2011 through 2013. The Company recognized these amounts as reductions to research and development expense in the periods incurred. The years 2011 through 2013 are open and subject to examination. The Company has applied for but not yet received $0.9 million related to the year ended December 31, 2014 which are classified as current assets within the consolidated balance sheets as of December 31, 2014.
On June 30, 2014, the Company acquired Pregenen. The Company may be required to make up to an additional $135.0 million in future contingent cash payments to the former equityholders of Pregenen upon the achievement of certain preclinical, clinical and commercial milestones related to the Pregenen technology, of which $15.0 million relates to preclinical milestones, $20.1 million relates to clinical milestones and $99.9 million relates to commercial milestones. In accordance with accounting for business combinations guidance, contingent consideration liabilities are required to be recognized on the consolidated balance sheets at fair value. Estimating the fair value of contingent consideration requires the use of significant assumptions primarily relating to probabilities of successful achievement of certain preclinical, clinical and commercial milestones, the expected timing in which these milestones will be achieved and discount rates. The use of different assumptions could result in materially different estimates of fair value. See Note 4, “Fair value measurements,” and Note 12, “Business combinations,” for additional information.
10.9. Common stock and preferred stock
On June 18, 2013, theThe Company increased theis authorized capital stockto issue 125,000,000 shares of the Company to 125,000,000 shares.common stock. Holders of common stock are entitled to one vote per share. Holders of common stock are entitled to receive dividends, if and when declared by the Company’s Board of Directors, and to share ratably in the Company’s assets legally available for distribution to the Company’s shareholders in the event of liquidation. Holders of common stock have no preemptive, subscription, redemption or conversion rights. As of December 31, 2017 and 2016, the Company had 49,406,011 and 40,691,904 shares of common stock issued and outstanding, respectively.
In June 2015, the Company sold 2,941,176, shares of common stock through an underwritten public offering at a price of $170.00 per share for aggregate net proceeds of $477.2 million. In December 2016, the Company sold 3,289,473 shares of common stock through an underwritten public offering at a price of $76.00 per share for aggregate net proceeds of $234.7 million. On June 27, 2017, the Company sold 4,381,500 shares of common stock (inclusive of 571,500 shares of common stock sold by the Company pursuant to the full exercise of an overallotment option granted to the underwriters in connection with the offering) through an underwritten public offering at a price of $105.00 per share for aggregate net proceeds of $436.8 million. On December 15, 2017, the Company sold 3,243,244 shares of common stock (excluding any shares sold pursuant to an overallotment option granted to the underwriters in connection with the offering) through an underwritten public offering at a price of $185.00 per share for aggregate net proceeds of $569.8 million. In January 2018, the Company sold 277,109 shares of common stock pursuant to the partial exercise of an overallotment option granted to the underwriters in connection with the December 2017 underwritten public offering at a price of $185.00 per share for aggregate net proceeds of $48.6 million. The Company is authorized to issue 5,000,000 shares of preferred stock in one or more series and to fix the powers, designations, preferences and relative participating option or other rights thereof, including dividend rights, conversion rights, voting rights, redemption terms, liquidation preferences and the number of shares constituting any series, without any further vote or action by the Company’s shareholders. As of December 31, 20142017 and 2013,2016, the Company had no shares of preferred stock issued or outstanding. Reserved for future issuance The Company has reserved for future issuance the following number of shares of common stock (in thousands): | | December 31, 2014
| | Options to purchase common stock
| | | 3,652
| | Restricted stock units
| | | 179
| | 2013 Stock Option and Incentive Plan
| | | 465
| | Warrants to purchase common stock
| | | 177
| | Employee Stock Purchase Plan
| | | 238
| | Acquisition holdback (Note 12)
| | | 94
| | | | | 4,805
| |
| | As of December 31, | | | | 2017 | | | 2016 | | Options to purchase common stock | | | 3,755 | | | | 3,735 | | Restricted stock units | | | 477 | | | | 263 | | 2013 Stock Option and Incentive Plan | | | 1,550 | | | | 1,226 | | 2013 Employee Stock Purchase Plan | | | 188 | | | | 209 | | | | | 5,970 | | | | 5,433 | |
11.10. Significant agreements
Celgene Corporation Summary of theOriginal Collaboration Agreement
On March 19, 2013, the Company entered into a Master Collaboration Agreement (the “Collaboration Agreement”) with Celgene to discover, develop and commercialize potentially disease-altering gene therapies in oncology. The collaboration is focused on applying gene therapy technology to genetically modify a patient’s own T cells, known as chimeric antigen receptor, or CAR T cells, to target and destroy cancer cells. Additionally, on March 19, 2013, the Company entered into a Platform Technology Sublicense Agreement (the “Sublicense Agreement”) with Celgene pursuant to which the Company obtained a sublicense to certain intellectual property from Celgene, originating under Celgene’s license from Baylor College of Medicine, for use in the collaboration. Under the terms of the Collaboration Agreement, the Company received a $75.0 million up-front, non-refundable cash payment. The Company iswas responsible for conducting discovery, research and development activities through completion of Phase I clinical F-21
studies, if any, during the initial term of the agreement,Collaboration Agreement, or three years. The collaboration is governed by a joint steering committee (“JSC”) formed by an equal number of representatives from the Company and Celgene. The JSC, will, among other activities, reviewreviews the collaboration program, reviewreviews and evaluateevaluates product candidates and approveapproves regulatory plans. In addition to the JSC, the Collaboration Agreement provides that the Company and Celgene each appoint representatives to a patent committee, which is responsible for managing the intellectual property developed and used during the collaboration.
PriorAmended Collaboration Agreement
On June 3, 2015, the Company and Celgene amended and restated the Collaboration Agreement (the “Amended Collaboration Agreement”). Under the Amended Collaboration Agreement, the parties will now focus the collaboration exclusively on anti- B-cell maturation antigen (“BCMA”) product candidates for a new three-year term. In connection with the Amended Collaboration Agreement, the Company received an upfront, one-time, non-refundable, non-creditable payment of $25.0 million to expirationfund research and development under the collaboration. The collaboration will continue to be governed by the JSC. Under the terms of the Amended Collaboration Agreement, for up to two product candidates selected for development under the collaboration, the Company is responsible for conducting and funding all research and development activities performed up through completion of the initial termPhase I clinical study, if any, of the Collaboration Agreement, Celgene has two options to extend the term, through March 19, 2019, with the payment of significant extension fees. Separately, Celgene has an option to license an unlimited number ofsuch product candidates resulting from the collaboration duringcandidate. On a period commencing upon execution of the Collaboration Agreement and continuingproduct candidate-by-product candidate basis, up through a specified period following enrollment of the completion offirst patient in an initial Phase I clinical studiesstudy for each individualsuch product candidate. In the event such option is exercised,candidate (the “Option Period”), the Company would granthas granted Celgene an option to obtain an exclusive worldwide license to develop and commercialize such product candidate. Upon exercise ofproduct. In the option to license a product candidate,event that Celgene is required to pay an option fee, which is subject to reduction if the Company elects to co-develop and co-promote such product candidate in the United States. For any product candidates licensed by Celgene, the Company may be responsible, at Celgene’s election, to continue performing certain development activities contemplated as part of the collaboration plan. If Celgene does not exerciseexercises its option with respect to aany product candidate, prior to the expiration of the applicable option period (each a “declined product candidate”), then the Company has the right to develop the product candidate outside the scope of the collaboration, subject to a Celgene opt-in right to obtain a license to that declined product candidate for significant additional cash consideration. The opt-in right exists through a specified period following the completion of a pivotal study for the specific declined product candidate and functions in the same manner as the option to license any other product candidates resulting from the collaboration. In addition, Celgene would be required to make certain milestone payments upon the achievement of specified clinical, regulatory and commercial events. For each product candidate that is licensed by Celgene, the Company would be eligible to receive per product up to $20.0 million in option fees, up to $10.0 million in clinical milestone payments, up to $117.0 million in regulatory milestone payments and up to $78.0 million in commercial milestone payments. Clinical milestone payments are triggered upon initiation of a defined phase of clinical research for a product candidate. Regulatory milestone payments are triggered upon approval to market a product candidate by the FDA or other global regulatory authorities. Commercial milestone payments are triggered upon the first commercial sale of an approved pharmaceutical product and when an approved pharmaceutical product reaches certain defined levels of net sales by the licensee or receives approval to be marketed by certain global regulatory authorities in a specified number of countries outside of the United States. In addition, to the extent any of the product candidates licensed by Celgene are commercialized, the Company would be entitled to receive tiered royalty payments ranging from the mid-single digits to mid-teens based on a percentage of net sales. Royalty payments are subject to certain reductions, including for any royalty payments required to be made by Celgene to acquire patent rights, with an aggregate minimum floor. The Company is not eligible to receive either milestone payments or royalty payments unless and until Celgene exercises its option to license a product candidate resulting from the collaboration whereupon the parties will execute a license agreement, the terms of which are included as part of the collaboration arrangement.
Additionally, the Company may elect to co-develop and co-promote the product candidates licensed by Celgene. Ifcandidate in the United States, provided that, if the Company electsdoes not exercise its option to co-develop and co-promote the first product candidate in-licensed by Celgene under the Amended Collaboration Agreement, then the Company will not be permitted to exercise its option to co-develop and co-promote any future product candidates under the Amended Collaboration Agreement. If Celgene elects to exercise its option to exclusively in-license a product candidate, thenit must pay the Company an option fee in the amount of $10.0 million for the first product candidate and $15.0 million for any additional product candidates.
bb2121 License Agreement On February 10, 2016, Celgene exercised its option to obtain an exclusive worldwide license to develop and commercialize bb2121, the first product candidate under the Amended Collaboration Agreement, pursuant to an executed license agreement (“bb2121 License Agreement”) entered into by the parties wouldon February 16, 2016 and paid the associated $10.0 million option fee. Pursuant to the bb2121 License Agreement, Celgene is responsible for development and related funding of bb2121 after the substantial completion of the on-going Phase I clinical trial. The Company is responsible for the manufacture of vector and associated payload throughout development and commercialization, which is fully reimbursed by Celgene, and Celgene is responsible for the manufacture of drug product throughout development and commercialization. The Company may elect to co-develop and co-promote bb2121 within the United States, which it currently expects to elect. The Company’s election to co-develop and co-promote bb2121 must be made by the substantial completion of the ongoing Phase I trial of bb2121. If elected, the responsibilities of the parties remain largely unchanged, however, the Company will share equally in all costs incurred relating to the development, commercializationdeveloping, commercializing and manufacture of the product candidatemanufacturing bb2121 within the United States and share equallyhas the right to participate in the profits generated by such product candidatedevelopment and promotion of bb2121 in the United States. Additionally, if the Company elects to co-develop and co-promote a product candidate, then the option fees, milestones and royalties would decrease compared to those described above. Under this scenario, the Company wouldmay receive, per product, up to $10.0 million in option fees, up to $10.0 million in clinical milestone payments and, outside of the United States, up to $54.0 million in regulatory milestone payments, and up to $36.0 million in commercial milestone payments. Clinical milestone payments are triggered upon initiation of a defined phase of clinical research for a product candidate. Regulatory milestone payments are triggered upon approval to market a product candidate by global regulatory authorities. Commercial milestone payments are triggered when an approved pharmaceutical product reaches certain defined levels of net sales by the licensee or receives approval to be marketed by certain global regulatory authorities in a specified number of countries outside the United States. In addition, to the extent any of the product candidates licensed by Celgene and co-developed and co-promoted by the Company arebb2121 is commercialized, the Company would be entitled to receive tiered royalty payments ranging from the mid-single digits to mid-teenslow-teens based on a percentage of net sales generated outside of the United States, subject to certain reductions. In the event the Company does not exercise its option to co-develop and co-promote bb2121, the Company will receive an additional fee in the amount of $10.0 million. Under this scenario, the Company may be eligible to receive up to $10.0 million in clinical milestone payments, up to $117.0 million in regulatory milestone payments, and up to $78.0 million in commercial milestone payments. In addition, to the extent bb2121 is commercialized, the Company would be entitled to receive tiered royalty payments ranging from the mid-single digits to low-teens based on a percentage of net sales, subject to certain reductions. bb21217 License Agreement On September 22, 2017, Celgene exercised its option to obtain an exclusive worldwide license to develop and commercialize bb21217, the second product candidate under the Amended Collaboration Agreement, pursuant to an executed license agreement (“bb21217 License Agreement”) entered into by the parties on September 28, 2017 and paid the associated $15.0 million option fee. Pursuant to the license agreement, Celgene is responsible for development and related funding of bb21217 after the substantial completion of the on-going Phase I clinical trial. The Company is responsible for the manufacture of vector and associated payload throughout development and commercialization, which is fully reimbursed by Celgene, and Celgene is responsible for the manufacture of drug product throughout development and commercialization.
The Company may elect to exercise its option to co-develop and co-promote bb21217 within the United States. The Company’s election to co-develop and co-promote bb21217 must be made by the substantial completion of the ongoing Phase I trial of bb21217. If elected, responsibilities of the parties remain unchanged, however, the Company will share equally in all costs relating to developing, commercializing and manufacturing bb21217 within the United States and has the right to participate in the development and promotion of bb21217 in the United States. Under this scenario, the Company may receive, per product, up to $10.0 million in clinical milestone payments and, outside of the United States, up to $54.0 million in regulatory milestone payments, and up to $36.0 million in commercial milestone payments. In addition, to the extent bb21217 is commercialized, the Company would be entitled to receive tiered royalty payments ranging from the mid-single digits to low-teens based on a percentage of net sales generated outside of the United States. Royalty payments areStates, subject to certain reductions, including for any royalty payments required to be made by Celgene to acquire patent rights, with an aggregate minimum floor. The Company is not eligible to receive profit share payments, milestone payments or royalty payments unless and until Celgene exercises its option to license a product candidate resulting from the collaboration whereupon the parties will execute a co-development, co-promote and profit share agreement, the terms of which are included as part of the collaboration arrangement.reductions. In the event Celgene elects to license a product candidate discovered and developed as part of the Collaboration Agreement, Celgene would be solely responsible for all costs and expenses of manufacturing and supplying any product candidates. Subject to customary back-up supply rights granted to Celgene, the Company has the sole right to manufacture or have manufactured supplies of vectors and associated payloads manufactured for incorporation into the associated product candidate. Celgene would reimburse the Company for the costs incurred to manufacture and supply such vectors and associated payloads, plus a modest mark-up. The F-22
Company isdoes not obligated to manufacture or have manufactured supplies of vectors and associated payloads for incorporation into an optioned product candidate unless and until Celgene exercisesexercise its option to license a product candidate resulting from the collaboration whereupon the parties will execute a separate manufacturing and supply agreement.
The Collaboration Agreement may be terminated by either the Company or Celgene, upon written notice, in the event of the other party’s uncured material breach. Celgene may terminate the Collaboration Agreement for any reason upon written notice to the Company. If the Collaboration Agreement is terminated, rights to product candidates in development at the time of such termination will be allocated to the parties through a mechanism included in the Collaboration Agreement. In addition, if Celgene terminates the Collaboration Agreement as a result of a breach by the Company, then any then-existing co-development and co-promotion agreement will be automatically terminated and replaced with a license agreement for such product candidate and any amounts payable by Celgene under any then-existing product license agreements will be reduced.
Call Option
During the initial three-year term of the collaboration and, if extended, during the first two-year extension term of the collaboration, in the event that the Company engages in a change in control transaction, including for such purposes a merger or consolidation of the Company or the sale of all or substantially all of the Company’s assets, or if another person or entity or group of persons or entities acquires at least 50% of the Company’s voting capital stock, then Celgene has the right, but not the obligation, to terminate the Collaboration Agreement and obtain perpetual, non-terminable, worldwide, exclusive, fully paid-up licenses to all, but not less than all, of the product candidates previously identified under the Collaboration Agreement (the “Call Option”). Under the Call Option, the product candidates to which Celgene would have the right to acquire licenses include any product candidate previously licensed out of the collaboration during the term of the collaboration, any product candidate for which the Company has exercised the right to co-develop and co-promote withinbb21217, the United States, any product candidate for which Celgene previously declined its option to obtain a license and any product candidate for which at least in vivo efficacy studies have been initiated or authorized by the JSC. The purchase price for such licenses would be based on the fair value of these rights received and obligations assumed determined pursuant to a binding arbitration process.
In addition, during the initial three-year term of the collaboration, but not during any extension term,Company will receive an additional fee in the event that Celgene exercisesamount of $10.0 million. Under this scenario, the Call Option,Company may be eligible to receive up to $10.0 million in clinical milestone payments, up to $117.0 million in regulatory milestone payments, and up to $78.0 million in commercial milestone payments. In addition, to the right to acquireextent bb21217 is commercialized, the fully paid-up licenses described above, Celgene would obtain a perpetual, non-terminable, worldwide, exclusive license to the Company’s intellectual property to develop one or more CAR T cell products targeting one or more oncology associated target antigens for the remainder of the initial collaboration term. Following the initial collaboration term, the license to the Company’s intellectual property is limited to target antigens identified by Celgene promptly following the initial collaboration term for which Celgene reasonably intends to develop CAR T cell products. There is no limit to the number of oncology-related target antigens Celgene may select under this license. Upon commercialization of any such product candidate so licensed by Celgene, CelgeneCompany would be obligatedentitled to payreceive tiered royalty payments ranging from the Company a specified milestone payment upon regulatory approval andmid-single digits to low-teens based on a percentage of net sales, as a royalty.subject to certain reductions.
TheAccounting Analysis – bb2121
Upon execution of the Amended Collaboration Agreement, the Company concluded that the value of the Call Option is immaterial based primarily on the probability that the Call Option would become exercisable. Accounting Analysis
The Company’s arrangement with Celgene containscontained the following deliverables: (i) discovery, research and development services, (ii) participation on the JSC, and (iii) participation on the patent committee.committee, (iv) a license to the first product candidate, bb2121, (v) manufacture of vectors and associated payload for incorporation into bb2121, under the license, and (vi) participation on the JGC under the co-development and co-promotion agreement for bb2121.
The license to the first product candidate, bb2121, was considered a deliverable at the inception of the arrangement and therefore the associated option fee was included in allocable arrangement consideration as the Company believed there was minimal risk with regard to whether Celgene will exercise the option based on the successful completion of preclinical activities and proximity of enrollment of the first patient in an initial Phase I clinical study for this product candidate. The Company hasalso determined that the obligation to manufacture, or have manufactured, supplies of vector and associated payload (hereafter referred to as vector manufacturing services) was a deliverable. However, the Company determined that the options to extend the termlicense any additional product candidates were substantive options and therefore were not considered deliverables at execution of the agreement and the options to license product candidates, including those related to Celgene’s opt-in right for a declined product candidate, are substantive options.Amended Collaboration Agreement. Celgene iswas not contractually obligated to exercise the options. Additionally, as a result of the uncertain outcome of the discovery, research and development activities, the Company iswas at risk with regard to whether Celgene willwould exercise the options.options to license additional product candidates. Moreover, the Company has determined that the options arewere not priced at a significant and incremental discount. Accordingly, the options areto other product candidates were not considered deliverables at the inception of the arrangement and the associated option fees are not included in allocable arrangement consideration. The Company has determined that the potential obligation to manufacture or have manufactured supplies of vectors and associated payloads for incorporation into an optioned product candidate is contingent upon Celgene exercising its option to license a product candidate resulting from the collaboration. Therefore, consistent with the treatment of the options to license product candidates, the Company’s potential obligation under a manufacturing and supply agreement is not considered a deliverable at the inception of the arrangement and the associated fees arewere not included in allocable arrangement consideration. TheUpon execution of the Amended Collaboration Agreement in June 2015, the Company concluded that each of the three deliverables identifieddelivered elements at the inception of the arrangement (discovery, researchagreement (research and development services, participation on the JSC and participation on the patent committee) hashad standalone value from the other undelivered elements. Additionally, the Amended Collaboration Agreement does not include return rights related to the initial collaboration term. Accordingly, each deliverable qualifiesqualified as a separate unit of accounting.
F-23
The Company identified the allocable arrangement consideration as the $75.0 million up-front payment. The Company determined that each of the identified deliverables havedelivered elements had the same period of performance (the three yearthree-year term through projected initial term)Phase I clinical study substantial completion) and have the same pattern of revenue recognition, ratably over the period of performance. As a result,performance as there was no other discernible pattern of recognition. The Company identified the $75.0allocable arrangement consideration as the $25.0 million up-front research and development funding payment, $10.0 million option fee for the first product candidate, bb2121, $20.0 million related to remaining deferred revenue from the original Collaboration Agreement, and $54.1 million related to the estimated amounts that will be received from Celgene for manufacturing services. The $109.0 million total allocable arrangement consideration was allocated based on the relative estimated selling price of the separate units of accounting at the inception of the amended agreement, resulting in $17.3 million allocated to the three delivered elements at the inception of the agreement, which will be recognized over thea three year term.
The Company determined that each of the identified deliverables that qualify as a separate unit of accounting continue to have the same period of performance (the three year term through projected initial term.Phase I clinical study substantial completion) and the same pattern of revenue recognition, ratably over the period of performance as there is no other discernible pattern of recognition, and therefore there is no change in the recognition of $17.3 million allocated to these three elements. These services continue to be recognized over a three-year term that began in June 2015.
However, the Company concluded that the license to bb2121 did not have standalone value from the vector manufacturing services, because the manufacturing is essential to the use of the license. Accordingly, these two deliverables qualify as a single combined unit of accounting. The Company is required to reassess its conclusions on standalone value of deliverables each period end. As of December 31, 2017, the Company determined that there were no changes to its initial standalone value conclusion and that the bb2121 license agreement continues to not have standalone value from the manufacturing services. Accordingly, these two deliverables continue to qualify as a single combined unit of accounting. Based on the likelihood and the Company’s intent to execute the co-development and co-promotion agreement for bb2121, the Company determined that the operating activities involved in the co-development and co-promotion of bb2121 in the U.S., which include the Company’s vector manufacturing services for U.S. development, participation on the JGC, and Celgene’s U.S. development efforts, are deemed to be within the scope of ASC 808 given that both parties are active participants in the activities and both parties are exposed to significant risks and rewards dependent on the commercial success of the activities. The Company recognizes revenue for its U.S. manufacturing services by application of ASC 605 as it has deemed its vector manufacturing services to be essential to Celgene’s use of the license to bb2121 and therefore is a combined unit of accounting. The portion of Celgene’s U.S. development costs that bluebird is responsible for are recognized as a reduction to its collaboration revenues, or, if in excess of such revenues in a given quarter, as research and development expense. bb2121 research and development services The Company recognized revenue related to bb2121 research and development services for Celgene of $6.2 million for each of the years ended December 31, 2017 and 2016. bb2121 license and manufacturing services Revenue recognition for the combined unit of accounting commenced during the first quarter of 2017 after the Company reached agreement with Celgene regarding the budget and timing for vector manufacturing services. The Company recognizes revenue associated with the combined unit of accounting using the proportional performance method. In using this method, the Company estimated, through discussions with Celgene regarding their development plan for bb2121, the proportion of effort it incurred as a percentage of total effort it expects to incur and applied this ratio to the total estimated budget for bb2121 vector manufacturing services. In developing the total estimated budget, management assumed that the Company will exercise its option to co-develop and co-promote bb2121 and therefore is currently recognizing revenue related to 67.5% of worldwide development costs incurred, which represents the percentage the Company is contractually entitled to bill Celgene under the cost share provisions of the co-development and co-promotion agreement, upon its execution. The period of performance and recognition pattern will be revisited as the development plan changes or if other events impacting the deliverables occur. The Company recognized $10.4 million of revenue related to the combined unit of accounting for its rest of world license and vector manufacturing services for the year ended December 31, 2017 in accordance with ASC 605. With respect to the combined unit of accounting for its U.S. license and vector manufacturing services accounted for in accordance with ASC 808, $4.9 million was recognized as revenue (representative of gross revenue of $10.5 million offset by approximately $5.6 million of cost reimbursement to Celgene) and $3.0 million was recognized as research and development expense for the year ended December 31, 2017. As noted above, revenue recognition for the combined unit of accounting commenced during the first quarter of 2017 and as such there was no revenue recognized related to the combined unit of accounting in 2016. In the event the Company does not exercise its option to co-develop and co-promote bb2121, the Company expects to recognize the remaining 32.5% of worldwide development costs, as Celgene would be responsible for 100% of costs incurred, plus a markup. Actual costs could materially differ from these estimates, and management has applied significant judgment in the process of developing its budget estimates. Any changes to these estimates will be recognized in the period in which they change as a cumulative catch up. The Company evaluated all of the milestones that may be received in connection with Celgene’s optionbb2121 license to license a product candidate resulting from the collaboration. In evaluatingdetermine if a milestone isthey were substantive the Company assesses whether: (i) the consideration is commensurate with either the Company’s performance to achieve the milestone or the enhancement of the value of the delivered item(s) as a result of a specific outcome resulting from the Company’s performance to achieve the milestone, (ii) the consideration relates solely to past performance and (iii) the consideration is reasonable relative to all of the deliverables and payment terms within the arrangement.in nature. All clinical and regulatory milestones that may be received under the bb2121 license agreement are considered substantive on the basis of the contingent nature of the milestone, specifically reviewing factors such as the scientific, clinical, regulatory, commercial and other risks that must be overcome to achieve the milestone as well as the level of effort and investment required. Accordingly, such amounts will be recognized as revenue in full in the period in which the associated milestone is achieved, assuming all other revenue recognition criteria are met. All commercial milestones will be accounted for in the same manner as royalties and recorded as revenue upon achievement of the milestone, assuming all other revenue recognition criteria are met.
DuringAccounting Analysis – bb21217
On September 22, 2017, Celgene exercised its option to obtain an exclusive worldwide license to develop and commercialize bb21217, the years ended December 31, 2014second optioned product candidate, pursuant to the bb21217 License Agreement entered into by the parties on September 28, 2017, and 2013,paid the associated $15.0 million option fee. The Company’s bb21217 License Agreement with Celgene contains the following deliverables: (i) research and development services, (ii) a license to the second product candidate, bb21217, (iii) manufacture of vectors and associated payload for incorporation into bb21217, under the license, and (iv) participation on the JGC under the co-development and co-promotion agreement for bb21217. Upon execution of the bb21217 License Agreement in September 2017, the Company concluded that the research and development services, which commenced at the inception of the arrangement, have standalone value from the license to bb21217 and manufacture of vectors and associated payload under the license (hereafter referred to as bb21217 vector manufacturing services). However, the Company concluded that the license to bb21217 does not have standalone value from one of the undelivered elements, the bb21217 manufacturing services under the license, because the manufacturing is essential to the use of the license. Accordingly, these two deliverables qualify as a single combined unit of accounting. The Company determined that the period of performance of the research and development services was two years through projected initial Phase I clinical study substantial completion, and revenue will be recognized $25.0ratably over the period of performance as there was no other discernible pattern of recognition. The Company identified the allocable arrangement consideration as the $15.0 million option fee for the second product candidate and $19.8$26.7 million respectively, of revenue associated with its collaboration with Celgene related to the recognitionestimated amounts that will be received from Celgene for bb21217 vector manufacturing services. The $41.7 million total allocable arrangement consideration was allocated based on the relative estimated selling price of discovery,the separate units of accounting at the inception of the bb21217 License Agreement, resulting in $5.4 million allocated to the research and development services.services at the inception of the agreement, which will be recognized over an initial two-year term. As of December 31, 20142017, this will continue to be recognized over a two-year term that began in September 2017. bb21217 research and 2013,development services The Company recognized revenue related to research and development services of $0.7 million for the year ended December 31, 2017. As noted above, revenue recognition for the bb21217 research and development services for Celgene commenced in September 2017 and as such there was $30.7no revenue recognized in 2016. bb21217 license and manufacturing services As of December 31, 2017, the manufacture of vectors and associated payload for bb21217 had not yet commenced. Therefore, no revenue has been recognized for the combined unit of accounting for the year ended December 31, 2017. The Company currently expects that recognition may commence in 2018 but has classified deferred revenue associated with the combined unit of accounting as deferred revenue, net of current portion on its consolidated balance sheet given exact timing is currently unknown and the budget for such services has not yet been agreed to by the parties. The Company evaluated all milestones that may be received in connection with Celgene’s bb21217 license to determine if they were substantive in nature. All clinical and regulatory milestones that may be received under the bb21217 license agreement are considered substantive on the basis of the contingent nature of the milestone, specifically reviewing factors such as the scientific, clinical, regulatory, commercial and other risks that must be overcome to achieve the milestone as well as the level of effort and investment required. Accordingly, such amounts will be recognized as revenue in full in the period in which the associated milestone is achieved, assuming all other revenue recognition criteria are met. All commercial milestones will be accounted for in the same manner as royalties and recorded as revenue upon achievement of the milestone, assuming all other revenue recognition criteria are met. Balance sheet impact As of December 31, 2017 and December 31, 2016, there was $47.4 million and $55.2$46.4 million, respectively, of total deferred revenue respectively, related to the Company’s collaboration with Celgene, which is classified as current or non-current in the consolidated balance sheets based onsheets. As of December 31, 2017, total deferred revenue related to bb2121 is $32.6 million, of which $9.8 million is classified as non-
current. As of December 31, 2017, total deferred revenue related to bb21217 is $14.8 million, of which $12.0 million is classified as non-current. As of December 31, 2017, other current assets and receivables includes a $4.6 million receivable related to cost reimbursement from Celgene for bb2121 development costs incurred to date, net of costs incurred and billable by Celgene to the contractual termCompany. There was no receivable from Celgene as of the arrangement.December 31, 2016. Association Française contre les MyopathiesNovartis Pharma AG
In January 2011,On April 26, 2017, the Company entered into a research fundingworldwide license agreement with Novartis. Under the Association Française contre les Myopathies (“AFM”), a nonprofit organization dedicated to curing rare neuromuscular diseases and providing treatments to reduce the associated disabilities of such diseases. As partterms of the agreement, AFM funded the Company 1.0 million EurosNovartis non-exclusively licensed certain patent rights related to be usedlentiviral vector technology to advance the Company’s research, process development, manufacturing, preclinical development,develop and clinical development in gene therapycommercialize CAR T cell therapies for beta-hemoglobinopathies in ß-thalassemia and/or in Sickle Cell Disease.
The funding, or a portion thereof depending on timing, shall be repaid to AFM upon anyoncology, including Kymriah (formerly known as CTL019), Novartis’s anti-CD19 CAR T therapy. At contract inception, financial terms of the following events: (i)agreement included a $7.5 million payment upon out-licensing or saleexecution, $7.5 million of the program, (ii) upon obtaining the firstpotential future milestone payments associated with regulatory approvals, and $1.1 million of payments for each subsequently licensed product, authorization for the market, or (iii) upon saleas well as low single digit royalty payments on net sales of the Company, provided that the development is active at the time of such sale. The agreement is for a period of four years. The Company believes that repayment of the funds paid under the agreement is remote atcovered products. At the date of contract inception, only one deliverable was identified and accordingly the entire nonrefundable license fee of $7.5 million was recognized as revenue given there were no other undelivered elements in the arrangement.
Given that there were no further deliverables identified in the contract, all regulatory milestones will be recognized as revenue in full in the period in which the associated milestone is achieved, assuming all other revenue recognition criteria are met. In August 2017, Novartis received FDA approval for Kymriah and as a result the Company recognized revenue of $2.5 million as a result of the achievement of a related milestone. During the year ended December 31, 2017, the Company recognized license revenue of $10.0 million in connection with this arrangement, as there were no other undelivered elements in the arrangement. During the year ended December 31, 2017, the Company recognized royalty revenue of $0.1 million in connection with this arrangement. The cost of license revenue related to this agreement was $1.4 million for the year ended December 31, 2017. GlaxoSmithKline Intellectual Property Development Limited On April 28, 2017, the Company entered into a worldwide license agreement with GSK. Under the terms of the agreement, GSK non-exclusively licensed certain patent rights related to lentiviral vector technology to develop and commercialize gene therapies for Wiscott-Aldrich syndrome and metachromatic leukodystrophy, two rare genetic diseases. Financial terms of the agreement include a nonrefundable upfront payment of $3.0 million as well as $1.3 million of potential milestone payments for each marketing authorization for each indication in any country as well as low single digit royalties on net sales of covered products. At the date of contract inception, only one deliverable was identified and accordingly the entire nonrefundable license fee of $3.0 million was recognized as revenue given there were no other undelivered elements in the arrangement. Given that there were no further deliverables identified in the contract, all regulatory milestones will be recognized as revenue in full in the period in which the associated milestone is achieved, assuming all other revenue recognition criteria are met. During the year ended December 31, 2014 or2017, the Company recognized revenue of $3.0 million associated with the delivery of the license, as there were no other undelivered elements in the arrangement. The cost of license revenue related to this agreement was $0.1 million for the year ended December 31, 2013. The Company recognizes the revenue under this arrangement on a straight-line basis over the term of the agreement. The Company will reassess the probability of repayment at the end of each reporting period.2017. 12. Business combinations11. Intangible assets
On June 30, 2014, the Company completed its acquisition of Pregenen, a privately-held biotechnology company, upon which Pregenen became a wholly-owned subsidiary. As a result, the Company obtained gene editing and cell signaling technology with a broad range of potential therapeutic applications. Under the terms of the Stock Purchase Agreement, the Company purchased all of Pregenen’s outstanding capital stock in exchange for 405,401 unregistered shares of common stock and $5.1 million in cash. The consideration for the transaction also includes an additional 94,117 shares of common stock that will be held for a period of 18 months after the acquisition and may be used to settle certain claims for indemnification for breaches or inaccuracies in Pregenen’s representations and warranties, covenants, and agreements and an additional 2,119 shares relating to a working capital adjustment. The Stock Purchase Agreement also provides for up to $135.0 million in future contingent cash payments upon the achievement of certain preclinical, clinical and commercial milestones related to the Pregenen technology, of which $15.0 million relates to preclinical milestones, $20.1 million relates to clinical milestones and $99.9 million relates to commercial milestones.
The acquisition-date fair value of the purchase consideration is as follows (in thousands):
Cash | $ | 5,093 | | Common stock | | 19,348 | | Contingent consideration | | 6,550 | | Total purchase consideration | $ | 30,991 | |
F-24
Common stock in the table above is comprised of $15.6 million in common stock transferred, $3.6 million in common stock that will be held back for a period of 18 months and $0.1 million related to a working capital adjustment.
The transaction was accounted for as a business combination under the acquisition method of accounting. Accordingly, the tangible and identifiable intangible assets acquired and liabilities assumed were recorded at estimated fair value as of the date of acquisition, with the remaining consideration transferred recorded as goodwill.
The purchase price allocation has been finalized and the following table summarizes the estimated fair value of the assets acquired and liabilities assumed at the date of acquisition (in thousands):
| Acquisition date fair value
| | Cash
| $
| 420
| | Gene editing platform intangible asset
| | 30,100
| | Goodwill
| | 13,128
| | Other assets acquired
| | 111
| | Total assets acquired
| | 43,759
| | Deferred tax liabilities
| | 11,797
| | Other liabilities assumed
| | 971
| | Total liabilities assumed
| | 12,768
| | Net assets acquired
| $
| 30,991
| |
The fair value of the gene editing platform intangible asset was determined using a relief from royalty approach, including assumptions of projected revenues and royalty rate in addition to a discount rate of 15.5% applied to the projected cash flows. The Company considersconsidered the intangible asset acquired to be developed technology, as at the date of the acquisition it could be used the way it iswas intended to be used in certain ongoing research and development activities. The Company believes the assumptions are representative of those a market participant would use in estimating fair value. The gene editing platform intangible asset will beis being amortized to research and development expense over its expected useful life of approximately eight years.years from the date of the acquisition.
Amortization expense for the gene editing platform intangible asset was $1.9$3.8 million for each of the yearyears ended December 31, 20142017, 2016 and 2015, respectively, and accumulated amortization as of December 31, 20142017 and 2016 was $1.9 million. The estimated amortization of intangible assets for the year ended December 31, 2015$13.2 million and for each of the five succeeding years and thereafter is as follows (in thousands): 2015 | $ | 3,763 | | 2016 | | 3,763 | | 2017 | | 3,763 | | 2018 | | 3,763 | | 2019 | | 3,763 | | 2020 and thereafter | | 9,404 | | Total | $ | 28,219 | |
The deferred tax liabilities of $11.8$9.4 million, primarily relate to the tax impact of future amortization or impairments associated with the identifiedrespectively. The intangible asset which is not deductible for tax purposes. See Note 15 “Income taxes,” for additional information.
Goodwill is calculated as the difference between the acquisition-date fair value of the consideration transferred and the fair values of the assets acquired and liabilities assumed and is not expectedwill continue to be deductible for income tax purposes. Goodwill is recorded as an indefinite-lived asset and is not amortized but tested for impairment on an annuala straightline basis or when indicationsover its remaining useful life of impairment exist. Among the factors which resulted in goodwill for the Pregenen acquisition was the opportunity to recognize synergies with the Company’s existing gene insertion platform and deferred tax liabilities recognized in connection with the acquisition.
The Company incurred a total of $0.2 million in transaction costs in connection with the acquisition, which were included in general and administrative expenses within the consolidated statements of operations and comprehensive loss for the year ended December 31, 2014.
In connection with the acquisition, the Company issued 3,267 shares of common stock to a former consultant of Pregenen and recognized $0.2 million of expense within general and administrative expenses in the consolidated statements of operations and comprehensive loss for the year ended December 31, 2014.4.5 years.
F-25
13.12. Stock-based compensation
On June 3, 2013, the Company’s board of directors adopted its 2013 Stock Option and Incentive Plan (“2013 Plan”), which was subsequently approved by its stockholders and became effective upon the closing of the Company’s IPO on June 24, 2013. The 2013 Plan replaces the 2010 Stock Option and Grant Plan (“2010 Plan”). The 2013 Plan allows for the granting of incentive stock options, non-qualified stock options, restricted stock units and restricted stock awards to the Company’s employees, members of the board of directors, and consultants of the Company. The Company initially reserved 955,000 shares of its common stock for the issuance of awards under the 2013 Plan. The 2013 Plan provides that the number of shares reserved and available for issuance under the 2013 Plan will automatically increase each January 1, beginning on January 1, 2014, by four percent of the outstanding number of shares of common stock on the immediately preceding December 31 or such lesser number of shares as determined by the Company’s compensation committee. In January 2017 and January 2018, the number of common stock available for issuance under the 2013 Plan was increased by approximately 1.6 million and 2.0 million shares, respectively, as a result of this automatic increase provision. Any options or awards outstanding under the Company’s previous stock option plans, including both the 2010 Plan and the Second Amended and Restated 2002 Employee, Director and Consultant Stock Plan (“2002 Plan”), at the time of adoption of the 2013 Plan remain outstanding and effective. The shares of common stock underlying any awards that are forfeited, canceled, repurchased, expire or are otherwise terminated (other than by exercise) under the 2002 Plan and 2010 Plan are added to the shares of common stock available for issuance under the 2013 Plan. As of December 31, 2014,2017, the total number of common stock that may be issued under all equity award plans is 464,530.1.6 million. The Company does not currently hold any treasury shares. Upon stock option exercise, the Company issues new shares and delivers them to the participant. Stock-based compensation expense The Company recognized stock-based compensation expense totaling $10.8$53.3 million, $6.5$39.8 million, and $0.8$41.1 million during the years ended December 31, 2014, 20132017, 2016 and 2012,2015, respectively. Share-basedStock-based compensation expense recognized by award type is as follows (in thousands): | Year ended December 31, | | Year Ended December 31, | | | | 2014 | | | | 2013 | | | | 2012 | | 2017 | | | 2016 | | | 2015 | | Stock options | $ | 9,487 | | | $ | 6,399 | | | $ | 742 | | $ | 42,262 | | | $ | 33,966 | | | $ | 37,536 | | Restricted stock awards | | 52 | | | | 92 | | | | 80 | | | Restricted stock units | | 1,158 | | | | — | | | | — | | | 10,495 | | | | 5,374 | | | | 3,325 | | Employee stock purchase plan | | 66 | | | | — | | | | — | | | 525 | | | | 416 | | | | 259 | | | $ | 10,763 | | | $ | 6,491 | | | $ | 822 | | $ | 53,282 | | | $ | 39,756 | | | $ | 41,120 | |
Stock-based compensation expense by classification included within the consolidated statements of operations and comprehensive loss was as follows (in thousands): | Year Ended December 31, | | | 2017 | | | 2016 | | | 2015 | | Research and development | $ | 26,633 | | | $ | 19,690 | | | $ | 24,854 | | General and administrative | | 26,649 | | | | 20,066 | | | | 16,266 | | | $ | 53,282 | | | $ | 39,756 | | | $ | 41,120 | |
As of December 31, 2017, there was $94.5 million, $31.1 million and $0.1 million of unrecognized compensation expense related to unvested stock options, restricted stock units and the employee stock purchase plan, respectively, that is expected to be recognized over a weighted-average period of 2.6, 2.9, and 0.1 years. In 2015, the Company modified outstanding options held by its former Chief Scientific Officer as part of his separation agreement, modified the vesting conditions of a stock option award held by a non-employee founder, and modified the vesting conditions of stock option awards held by two employees immediately following their separation from the Company. As a result of these modifications, the Company recognized $10.3 million of incremental stock-based compensation expense during 2015.
Stock options The fair value of each option issued to employees was estimated at the date of grant using the Black-Scholes option pricing model with the following weighted-average assumptions: | | Year ended December 31, | | | Year Ended December 31, | | | | | 2014 | | | | 2013 | | | | 2012 | | | 2017 | | | 2016 | | | 2015 | | Expected volatility | | | 82.3 | % | | | 82.0 | % | | | 79.6 | % | | | 78.1 | % | | | 74.3 | % | | | 72.6 | % | Expected term (in years) | | | 6.0 | | | | 6.1 | | | | 6.1 | | | | 6.0 | | | | 6.0 | | | | 5.9 | | Risk-free interest rate | | | 1.8 | % | | | 1.1 | % | | | 1.0 | % | | | 2.1 | % | | | 1.5 | % | | | 1.7 | % | Expected dividend yield | | 0.0% | | | 0.0% | | | 0.0% | | | 0.0 | % | | 0.0 | % | | 0.0% | |
The intrinsic value of options exercised during the years ended December 31, 2014, 2013, and 2012, was $41.4 million, $3.9 million and $0, respectively.
The weighted-average fair values of options granted during 2014, 2013 and 2012 was $18.53, $7.36 and $1.51, respectively.
The aggregate fair value of restricted stock awards that vested during the years ended December 31, 2014, 2013 and 2012, based on the estimated fair value of the underlying stock on the day of vesting was $1.9 million, $1.5 million and $0.1 million, respectively.
As of December 31, 2014, there was $22.1 million and $3.9 million of unrecognized compensation expense related to unvested stock options and restricted stock units, respectively, that is expected to be recognized over a weighted-average period of 2.9 and 1.5 years.
F-26
During the year ended December 31, 2014, the Company issued 2,000 shares of restricted common stock in exchange for consulting services. The shares vested upon issuance and the Company recognized $0.1 million of expense related to the services provided.
During the year ended December 31, 2014, 123,263 options held by former employees were modified to accelerate vesting and extend the period in which the employees were allowed to exercise the vested options. The modification was valued using a Black-Scholes option valuation model and the Company accounted for the $0.6 million of incremental value within general and administrative expenses.
Restricted common stock
A summary of the Company’s restricted stock awards activity and related information is as follows:
| Shares | | | Weighted-average grant date fair value | | Unvested balance at December 31, 2013 | | 68,822 | | | $ | 0.95 | | Granted | | — | | | | — | | Vested | | (68,822 | ) | | | 0.95 | | Forfeited | | — | | | | — | | Unvested balance at December 31, 2014 | | — | | | $ | — | |
Restricted stock units
A summary of the Company’s restricted stock unit activity and related information is as follows:
| Shares | | | Weighted-average grant date fair value | | Unvested balance at December 31, 2013 | | — | | | $ | — | | Granted | | 185,850 | | | | 30.47 | | Vested | | — | | | | — | | Forfeited | | (6,430 | ) | | | 30.47 | | Unvested balance at December 31, 2014 | | 179,420 | | | $ | 30.47 | | | | | | | | | |
Stock options
The following table summarizes the stock option activity under the Plan:Company’s equity awards plans: | Shares | | | Weighted-average exercise price per share | | | Weighted-average contractual life (in years) | | | Aggregate intrinsic value (a) (in thousands) | | Outstanding at December 31, 2013 | | 3,957,673 | | | $ | 5.21 | | | | | | | | | | Granted | | 1,188,060 | | | $ | 26.80 | | | | | | | | | | Exercised | | (1,313,031 | ) | | $ | 3.95 | | | | | | | | | | Canceled or forfeited | | (180,465 | ) | | $ | 12.98 | | | | | | | | | | Outstanding at December 31, 2014 | | 3,652,237 | | | $ | 12.30 | | | | 8.1 | | | $ | 290,049 | | Exercisable at December 31, 2014 | | 1,251,045 | | | $ | 4.37 | | | | 7.2 | | | $ | 109,283 | | Vested and expected to vest at December 31, 2014 | | 3,597,828 | | | $ | 12.41 | | | | 8.1 | | | $ | 285,358 | |
| Shares (in thousands) | | | Weighted-average exercise price per share | | | Weighted-average contractual life (in years) | | | Aggregate intrinsic value (a) (in thousands) | | Outstanding at December 31, 2016 | | 3,735 | | | $ | 52.17 | | | | | | | | | | Granted | | 1,206 | | | $ | 90.95 | | | | | | | | | | Exercised | | (982 | ) | | $ | 32.39 | | | | | | | | | | Canceled or forfeited | | (204 | ) | | $ | 86.84 | | | | | | | | | | Outstanding at December 31, 2017 | | 3,755 | | | $ | 67.91 | | | | 7.5 | | | $ | 414,169 | | Exercisable at December 31, 2017 | | 1,834 | | | $ | 50.60 | | | | 6.4 | | | $ | 234,190 | | Vested and expected to vest at December 31, 2017 | | 3,755 | | | $ | 67.91 | | | | 7.5 | | | $ | 414,169 | |
(a) | The aggregate intrinsic value is calculated as the difference between the exercise price of the underlying options and the estimated fair value of the common stock for the options that were in the money at December 31, 2014.2017. |
Note receivable
In November 2010, the Company received a non-recourse note from its Chief Executive Officer (“CEO”) in exchange for the purchaseThe weighted-average fair values of 329,256 sharesoptions granted during 2017, 2016 and 2015 was $62.03, $34.22, and $74.65, respectively. The intrinsic value of restricted stock. Interest accrued on the note on an annual basis at a rate of four percent. In May 2013, prior to the initial filing of the registration statement in connection with the Company’s IPO, the CEO repaid the note in full plus all
F-27
accrued interest. The Company recorded stock-based compensation expense in connection with this restricted stock award of $0.1 million, $0.1 million and $0.1 million foroptions exercised during the years ended December 31, 2014, 20132017, 2016, and 2012,2015, was $91.0 million, $15.3 million and $147.9 million, respectively.
Restricted stock units The following table summarizes the restricted stock unit activity under the Company’s equity award plans (shares in thousands): | Shares | | | Weighted-average grant date fair value | | Unvested balance at December 31, 2016 | | 263 | | | $ | 63.07 | | Granted | | 332 | | | | 91.15 | | Vested | | (87 | ) | | | 72.91 | | Forfeited | | (31 | ) | | | 64.52 | | Unvested balance at December 31, 2017 | | 477 | | | $ | 80.72 | |
The intrinsic value of restricted stock units vested during the years ended December 31, 2017, 2016, and 2015 was $8.1 million, $5.3 million and $10.4 million, respectively. Employee Stock Purchase Plan On June 3, 2013, the Company’s board of directors adopted its 2013 Employee Stock Purchase Plan (“2013 ESPP”), which was subsequently approved by its stockholders and became effective upon the closing of the Company’s IPO on June 24, 2013. The 2013 ESPP authorizes the initial issuance of up to a total of 238,000 shares of the Company’s common stock to participating employees. The first offering periodDuring the years ended December 31, 2017 and 2016, 20,773 and 18,338 shares of common stock were issued under the 2013 ESPP, opened on August 1, 2014.respectively.
14.13. 401(k) Savings plan
In 1997, the Company established a defined-contribution savings plan under Section 401(k) of the Internal Revenue Code (“the 401(k) Plan”). The 401(k) Plan covers all employees who meet defined minimum age and service requirements, and allows participants to defer a portion of their annual compensation on a pretax basis. TheIn February 2018 and 2017, the Company has not made any contributions of approximately $1.5 million and $1.0 million, respectively, related to employee contributions made during 2017 and 2016, which is included in accrued expenses and other current liabilities as of December 31, 2017 and 2016. Expense related to the 401(k) Plan totaled $1.5 million, $1.0 million, $0.6 million for the three years ended December 31, 2014.2017, 2016, and 2015, respectively. 15.14. Income taxes
The components of loss before income taxes were as follows (in thousands): | | Year ended December 31, | | Year Ended December 31, | | | | 2014 | | | 2013 | | | 2012 | | 2017 | | | 2016 | | | 2015 | | U.S. | | $ | (61,118 | ) | | $ | (26,018 | ) | | $ | (23,700 | ) | $ | (266,236 | ) | | $ | (210,188 | ) | | $ | (162,287 | ) | Foreign | | | 612 | | | | 697 | | | | 30 | | | (69,197 | ) | | | (53,931 | ) | | | (4,436 | ) | Total | | $ | (60,506 | ) | | $ | (25,321 | ) | | $ | (23,670 | ) | $ | (335,433 | ) | | $ | (264,119 | ) | | $ | (166,723 | ) |
The benefitprovision for (benefit from) income taxes were as follows (in thousands): | | Year ended December 31, | | Year Ended December 31, | | | | 2014 | | | 2013 | | | 2012 | | 2017 | | | 2016 | | | 2015 | | Current | | | | | | | | | | | | | | Current: | | | | | | | | | | | | | Federal | | $ | — | | | $ | — | | | $ | — | | $ | — | | | $ | — | | | $ | — | | State | | | 1 | | | | — | | | | — | | | 115 | | | | — | | | | — | | Foreign | | | — | | | | — | | | | — | | | 95 | | | | — | | | | 60 | | Deferred | | | | | | | | | | | — | | | Deferred: | | | | | | | | | | | | | Federal | | | (9,390 | ) | | | — | | | | — | | | — | | | | (588 | ) | | | — | | State | | | (2,408 | ) | | | — | | | | — | | | — | | | | (24 | ) | | | — | | Foreign | | | — | | | | — | | | | — | | | — | | | | — | | | | — | | Total income tax benefit | | $ | (11,797 | ) | | $ | — | | | $ | — | | | | | | | | | | | | | | | | | Total income tax expense (benefit) | | $ | 210 | | | $ | (612 | ) | | $ | 60 | |
A reconciliation of income tax benefitprovision (benefit) computed at the statutory federal income tax rate to the Company’s effective income tax rate benefit(benefit) provision as reflected in the financial statements is as follows: | | Year ended December 31, | | | Year Ended December 31, | | | | 2014 | | | 2013 | | | 2012 | | | 2017 | | | 2016 | | | 2015 | | Federal income tax expense at statutory rate | | | 34.0 | % | | | 34.0 | % | | | 34.0 | % | | | 34.0 | % | | | 34.0 | % | | | 34.0 | % | State income tax, net of federal benefit | | | 4.0 | % | | | 4.5 | % | | | 4.4 | % | | | 4.3 | % | | | 3.3 | % | | | 4.2 | % | Permanent differences | | | (3.2 | %) | | | (0.6 | %) | | | (0.8 | %) | | | 2.7 | % | | | (5.3 | %) | | | (6.4 | %) | Research and development credit | | | 25.7 | % | | | 6.0 | % | | | 0.8 | % | | | 12.8 | % | | | 15.0 | % | | | 14.6 | % | Foreign differential | | | | (6.9 | %) | | | (7.0 | %) | | | (1.0 | %) | Federal tax rate change | | | | (31.6 | %) | | | 0.0 | % | | | 0.0 | % | Other | | | 0.0 | % | | | 0.0 | % | | | 0.6 | % | | | (0.8 | %) | | | 0.0 | % | | | (0.5 | %) | Change in valuation allowance | | | (41.0 | %) | | | (43.9 | %) | | | (39.0 | %) | | | (14.6 | %) | | | (39.9 | %) | | | (44.9 | %) | Effective income tax rate benefit | | | 19.5 | % | | | 0.0 | % | | | 0.0 | % | | Effective income tax rate (benefit) | | | | (0.1 | %) | | | 0.1 | % | | | 0.0 | % |
For the yearyears ended December 31, 2014,2017, 2016 and 2015, the Company recognized an income tax (expense) benefit of $11.8$(0.2) million or 19.5%. The Company recorded a non-recurring tax benefit of $11.8(0.1%), $0.6 million due to the release of a portion of the valuation allowance due to taxable F-28
temporary differences available as a source of income to realize certain pre-existing deferred tax assets as a result of the acquisition of Pregenen. Excluding the impact of this item, the Company’s overall tax provisionor 0.1%, and effective tax rate would be zero.
$(0.1) million or 0.0%, respectively. The Company did not recognize any significant tax benefit for the years ended December 31, 20132017, December 31, 2016, and December 31, 20122015 as the Company was subject to a full valuation allowance.
Deferred taxes are recognized for temporary differences between the basis of assets and liabilities for financial statement and income tax purposes. The significant components of the Company’s deferred tax assets and liabilities are comprisedcomposed of the following (in thousands): | | Year ended December 31, | | Year Ended December 31, | | | | 2014 | | | 2013 | | 2017 | | | 2016 | | Deferred tax assets: | | | | | | | | | | | | | | | | U.S. net operating loss carryforwards | | $ | 33,767 | | | $ | 11,010 | | | Foreign net operating loss carryforwards | | | 899 | | | | 659 | | | Tax credit carryforwards | | | 23,274 | | | | 3,638 | | | Capitalized research and development expenses, net | | | 1,372 | | | | 1,837 | | | U.S. net operating loss carryforwards (federal and state) | | $ | 194,160 | | | $ | 106,064 | | Tax credit carryforwards (federal and state) | | | 131,289 | | | | 87,117 | | Capitalized research and development expenses | | | 241 | | | | 631 | | 60 Binney Street lease | | | 42,025 | | | | 47,191 | | Deferred revenue | | | 12,050 | | | | 21,830 | | | 12,795 | | | | 18,231 | | Capitalized license fees | | | 13,388 | | | | 11,752 | | Accruals and other | | | 7,552 | | | | 4,177 | | | 25,781 | | | | 32,172 | | Total deferred tax assets | | | 78,914 | | | | 43,151 | | | 419,679 | | | | 303,158 | | Intangible assets | | | (11,084 | ) | | | - | | | (4,567 | ) | | | (8,129 | ) | Fixed assets | | | (2,526 | ) | | | (2,596 | ) | | (42,062 | ) | | | (48,902 | ) | Less valuation allowance | | | (65,304 | ) | | | (40,555 | ) | | (373,050 | ) | | | (246,127 | ) | Net deferred taxes | | $ | — | | | $ | — | | $ | — | | | $ | — | |
A valuation allowance is recorded against deferred tax assets if it is more likely than not that some or all of the deferred tax assets will not be realized. Due to the uncertainty surrounding the realization of the favorable tax attributes in future tax returns, the Company has recorded a full valuation allowance against the Company’s otherwise recognizable net deferred tax assets. The Company has allocated its valuation allowance in accordance with the provisions of ASC 740, which resulted in a current deferred tax asset of $1.9 million and a non-current deferred tax liability of $1.9 million as of December 31, 2014. The valuation allowance increased on a net basis by approximately $24.7$126.9 million during the year ended December 31, 20142017 due primarily to net operating losses and tax credit carryforwards. carryforwards, which are partially offset by the decrease in federal statutory rate due to tax reform. As of December 31, 2014, 20132017, 2016 and 2012,2015, the Company had U.S. federal net operating loss carryforwards of approximately $130.0$716.1 million, $30.3$466.8 million, and $62.6$347.5 million, respectively, which may be available to offset future income tax liabilities and expire at various dates through 2034.2037. As of December 31, 2014, 20132017, 2016 and 2012,2015, the Company also had U.S. state net operating loss carryforwards of approximately $115.5$692.9 million, $13.3$456.8 million, and $52.3$335.0 million, respectively, which may be available to offset future income tax liabilities and expire at various dates through 2034.2037. At December 31, 2014, $42.12016, $195.4 million and $42.1$195.4 million of federal and state net operating losses, respectively, relaterelated to excess equity based compensation tax deductions, the benefits for which will be recorded to additional paid-in capital when recognized through a reduction of cash taxes paid. As a result of adopting FASB ASU 2016-09 in the first quarter of 2017, the Company recorded a cumulative-effect adjustment to retained earnings of $76.7 million to record a net deferred tax asset relative to these tax attribute carryforwards. The deferred tax asset was offset by a corresponding adjustment to the valuation allowance. At December 31, 2014, 20132017, 2016 and 2012,2015, the Company also had approximately $2.7$0.0 million, $2.0$0.0 million, and $1.8$0.6 million, respectively, of foreign net operating loss carryforwards whichthat may be available to offset future income tax liabilities; these carryforwards do not expire. As of December 31, 2014, 20132017, 2016 and 2012,2015, the Company had federal research and development and orphan drug tax credit carryforwards of approximately $22.0$124.1 million, $2.7$83.2 million, and $1.3$44.9 million, respectively, available to reduce future tax liabilities which expire at various dates through 2034.2037. As of December 31, 2014, 20132017, 2016 and 2012,2015, the Company had state credit carryforwards of approximately $2.0$9.1 million, $1.4$6.0 million, and $0.9$3.8 million, respectively, available to reduce future tax liabilities which expire at various dates through 2029.2032. Under the provisions of the Internal Revenue Code, the net operating loss and tax credit carryforwards are subject to review and possible adjustment by the Internal Revenue Service and state tax authorities. Net operating loss and tax credit carryforwards may become subject to an annual limitation in the event of certain cumulative changes in the ownership interest of significant shareholders over a three-year period in excess of 50 percent, as defined under Sections 382 and 383 of the Internal Revenue Code, respectively, as well as similar state provisions. This could limit the amount of tax attributes that can be utilized annually to offset future taxable income or tax liabilities. The amount of the annual limitation is determined based on the value of the Company immediately prior to the ownership change. Subsequent ownership changes may further affect the limitation in future years. The Company has completed several financings since its inception which it believes has resulted in a change in control as defined by Sections 382 and 383 of the Internal Revenue Code. F-29
The Company will recognize interest and penalties related to uncertain tax positions in income tax expense. As of December 31, 2014, 20132017, 2016 and 2012,2015, the Company had no significant accrued interest or penalties related to uncertain tax positions and no significant amounts have been recognized in the Company’s consolidated statements of operations and comprehensive loss.
For all years through December 31, 2014,2017, the Company generated research credits but has not conducted a study to document the qualified activities. This study may result in an adjustment to the Company’s research and development credit carryforwards; however, until a study is completed and any adjustment is known, no amounts are being presented as an uncertain tax position. A full valuation allowance has been provided against the Company’s research and development credits and, if an adjustment is required, this adjustment would be offset by an adjustment to the deferred tax asset established for the research and development credit carryforwards and the valuation allowance. The Company or one of its subsidiaries files income tax returns in the United States, and various state and foreign jurisdictions. The federal, state and foreign income tax returns are generally subject to tax examinations for the tax years ended December 31, 20112014 through December 31, 2014.2016. To the extent the Company has tax attribute carryforwards, the tax years in which the attribute was generated may still be adjusted upon examination by the Internal Revenue Service, state or foreign tax authorities to the extent utilized in a future period. On December 22, 2017, the Tax Cuts and Jobs Act (“TCJA”) was enacted. This law substantially amended the Internal Revenue Code and among other things, permanently reduced the U.S. corporate income tax rate from 35% to 21%. On December 22, 2017, the SEC staff issued Staff Accounting Bulletin No. 118, Income Tax Accounting Implications of the Tax Cuts and Jobs Act (“SAB 118”), which allows the recording of provisional amounts during a measurement period not to extend beyond one year of the enactment date. In accordance with SAB 118, the Company has determined that its deferred tax asset value and associated valuation allowance reduction of $106.0 million is a provisional amount and a reasonable estimate at December 31, 2017. The final impact may differ from this provisional amount due to, among other things, changes in interpretations and assumptions the Company has made thus far and the issuance of additional regulatory or other guidance. The Company expects to complete the final impact within the measurement period. 16.
15. Net loss per share The following common stock equivalents were excluded from the calculation of diluted net loss per share for the periods indicated because including them would have had an anti-dilutive effect (in thousands): | Year ended December 31, | | | | 2014 | | | | 2013 | | | | 2012 | | Warrants | | 177 | | | | 338 | | | | 440 | | Outstanding stock options | | 3,652 | | | | 3,958 | | | | 2,201 | | Unvested restricted stock | | — | | | | 69 | | | | 155 | | Restricted stock units | | 179 | | | | — | | | | — | | ESPP shares | | 6 | | | | — | | | | — | | Acquisition holdback (Note 12) | | 94 | | | | — | | | | — | | Preferred stock | | — | | | | — | | | | 16,389 | | | | 4,108 | | | | 4,365 | | | | 19,185 | |
| Year Ended December 31, | | | | 2017 | | | | 2016 | | | | 2015 | | Outstanding stock options | | 3,755 | | | | 3,735 | | | | 3,532 | | Restricted stock units | | 477 | | | | 263 | | | | 148 | | ESPP shares | | 9 | | | | 11 | | | | 3 | | | | 4,241 | | | | 4,009 | | | | 3,683 | |
F-30
17.16. Selected Quarterly Financial Data (Unaudited)quarterly financial data (unaudited)
The following table contains quarterly financial information for 20142017 and 2013.2016. The Company believes that the following information reflects all normal recurring adjustments necessary for a fair statementpresentation of the information for the periods presented. The operating results for any quarter are not necessarily indicative of results for any future period. | | 2014 | | | 2017 | | | | First Quarter | | | Second Quarter | | | Third Quarter | | | Fourth Quarter | | | Total | | | First Quarter | | | Second Quarter | | | Third Quarter | | | Fourth Quarter | | | Total | | | | (in thousands, except per share data) | | | (in thousands, except per share data) | | Total revenue | | $ | 6,335 | | | $ | 6,335 | | | $ | 6,365 | | | $ | 6,386 | | | $ | 25,421 | | | $ | 6,832 | | | $ | 16,716 | | | $ | 7,711 | | | $ | 4,168 | | | $ | 35,427 | | Total operating expenses | | | 17,003 | | | | 19,669 | | | | 23,375 | | | | 26,000 | | | | 86,047 | | | | 76,745 | | | | 84,538 | | | | 85,369 | | | | 120,940 | | | | 367,592 | | Loss from operations | | | (10,668 | ) | | | (13,334 | ) | | | (17,010 | ) | | | (19,614 | ) | | | (60,626 | ) | | | (69,913 | ) | | | (67,822 | ) | | | (77,658 | ) | | | (116,772 | ) | | | (332,165 | ) | Net loss | | | (10,609 | ) | | | (1,526 | ) | (1) | | (17,030 | ) | | | (19,544 | ) | | | (48,709 | ) | | | (68,712 | ) | | | (70,898 | ) | | | (78,805 | ) | | | (117,228 | ) | | | (335,643 | ) | Net loss per share applicable to common stockholders - basic and diluted | | $ | (0.44 | ) | | $ | (0.06 | ) | | $ | (0.61 | ) | | $ | (0.67 | ) | | $ | (1.83 | ) | | $ | (1.68 | ) | | $ | (1.73 | ) | | $ | (1.73 | ) | | $ | (2.52 | ) | | $ | (7.71 | ) | | | | | | | | | | | | | | | | | | | | | | | | | 2013 | | | | | First Quarter | | | Second Quarter | | | Third Quarter | | | Fourth Quarter | | | Total | | | | | (in thousands, except per share data) | | | Total revenue | | $ | 1,127 | | | $ | 6,334 | | | $ | 6,385 | | | $ | 6,335 | | | $ | 20,181 | | | Total operating expenses | | | 7,608 | | | | 10,528 | | | | 12,542 | | | | 14,450 | | | | 45,128 | | | Loss from operations | | | (6,481 | ) | | | (4,194 | ) | | | (6,157 | ) | | | (8,115 | ) | | | (24,947 | ) | | Net loss | | | (6,544 | ) | | | (4,583 | ) | | | (6,113 | ) | | | (8,081 | ) | | | (25,321 | ) | | Net loss per share applicable to common stockholders - basic and diluted | | $ | (19.94 | ) | | $ | (2.13 | ) | | $ | (0.26 | ) | | $ | (0.34 | ) | | $ | (2.02 | ) | |
(1)
| During the second quarter of 2014, the Company recorded an income tax benefit of $11.8 million in connection with the acquisition of Pregenen completed in June 2014. See Note 12, “Business Combinations” for additional information.
|
| | 2016 | | | | First Quarter | | | Second Quarter | | | Third Quarter | | | Fourth Quarter | | | Total | | | | (in thousands, except per share data) | | Total revenue | | $ | 1,499 | | | $ | 1,552 | | | $ | 1,552 | | | $ | 1,552 | | | $ | 6,155 | | Total operating expenses | | | 58,879 | | | | 61,527 | | | | 79,692 | | | | 73,887 | | | | 273,985 | | Loss from operations | | | (57,380 | ) | | | (59,975 | ) | | | (78,140 | ) | | | (72,335 | ) | | | (267,830 | ) | Net loss | | | (56,274 | ) | | | (58,844 | ) | | | (77,025 | ) | | | (71,364 | ) | | | (263,507 | ) | Net loss per share applicable to common stockholders - basic and diluted | | $ | (1.52 | ) | | $ | (1.59 | ) | | $ | (2.07 | ) | | $ | (1.88 | ) | | $ | (7.07 | ) |
18.
17. Subsequent events The
As discussed in Note 9, “Common stock and preferred stock,” in January 2018, the Company has evaluated all events or transactions that occurred aftersold 277,109 shares of common stock pursuant to the partial exercise of an overallotment option granted to the underwriters in connection with the December 31, 2014. In the judgment2017 underwritten public offering at a price of management, there were no material events that impacted the consolidated financial statements or disclosures.$185.00 per share for aggregate net proceeds of $48.6 million.
Exhibit Index | | | | Incorporated by Reference | Exhibit Number | | Exhibit Title | | Form | | File no. | | | Exhibit | | | Filing Date | | | | | | | | | | | | | | | | 2.1 | | Stock Purchase Agreement by and between the Registrant and Precision Genome Engineering, Inc. | | 8-K | | | 001-35966 | | | | 2.1 | | | June 30, 2014 | | | | | | | | | | | | | | | | 3.1 | | Amended and Restated Certificate of Incorporation of the Registrant | | 8-K | | | 001-35966 | | | | 3.1 | | | June 24, 2013 | | | | | | | | | | | | | | | | 3.2 | | Amended and Restated By-laws of the Registrant | | 8-K | | | 001-35966 | | | | 3.2 | | | June 24, 2013 | | | | | | | | | | | | | | | | 3.3 | | Amendment No. 1 to Amended and Restated By-laws of the Registrant | | 8-K | | | 001-35966 | | | | 3.1 | | | February 11, 2016 | | | | | | | | | | | | | | | | 4.1 | | Specimen Common Stock Certificate | | S-1/A | | | 333-188605 | | | | 4.1 | | | June 4, 2013 | | | | | | | | | | | | | | | | 4.2 | | Amended and Restated Investors’ Rights Agreement, dated as of July 23, 2012, by and among the Registrant and the Investors listed therein. | | S-1/A | | | 333-188605 | | | | 4.5 | | | May 14, 2013 | | | | | | | | | | | | | | | | 4.3 | | Amendment to Amended and Restated Investors’ Rights Agreement, dated as of July 8, 2014, by and among the Registrant and the Investors listed therein. | | 10-Q | | | 001-35966 | | | | 4.6 | | | August 12, 2014 | | | | | | | | | | | | | | | | 10.1# | | Second Amended and Restated 2002 Employee, Director and Consultant Plan, as amended, and forms of award agreement thereunder | | S-1/A | | | 333-188605 | | | | 10.1 | | | May 14, 2013 | | | | | | | | | | | | | | | | 10.2# | | 2010 Stock Option and Grant Plan, as amended, and forms of award agreement thereunder | | S-1/A | | | 333-188605 | | | | 10.2 | | | May 14, 2013 | | | | | | | | | | | | | | | | 10.3# | | 2013 Stock Option and Incentive Plan and forms of award agreement thereunder | | S-1/A | | | 333-188605 | | | | 10.3 | | | June 4, 2013 | | | | | | | | | | | | | | | | 10.4 | | Form of Indemnification Agreement between the Registrant and each of its Executive Officers and Directors | | S-1/A | | | 333-188605 | | | | 10.4 | | | May 14, 2013 | | | | | | | | | | | | | | | | 10.5 | | Amended and Restated Lease Agreement, dated May 18, 2007, by and between the Registrant and Rivertech Associates II, LLC, as amended | | 10-Q | | | 001-35966 | | | | 10.1 | | | November 14, 2013 | | | | | | | | | | | | | | | | 10.6† | | Patent License Agreement, dated December 11, 1996, by and between the Registrant (formerly known as Genetix Pharmaceuticals Inc., successor-in-interest to Innogene Pharmaceuticals Inc.) and Massachusetts Institute of Technology, as amended | | S-1/A | | | 333-188605 | | | | 10.6 | | | May 14, 2013 | | | | | | | | | | | | | | | | 10.7† | | Fourth Amendment to Patent License Agreement, dated October 28, 2016, by and between the Registrant and Massachusetts Institute of Technology | | 10-K | | | 001-35966 | | | | 10.7 | | | February 22, 2017 | | | | | | | | | | | | | | | | 10.8† | | Patent and Know-How License Agreement No. 07554F30, dated May 14, 2009, by and between the Registrant (formerly known as Genetix Pharmaceuticals Inc.) and INSERM-TRANSFERT, as amended | | S-1/A | | | 333-188605 | | | | 10.7 | | | May 14, 2013 | | | | | | | | | | | | | | | | 10.9† | | License Agreement, dated September 13, 2011, by and between the Registrant and Institut Pasteur, as amended | | S-1/A | | | 333-188605 | | | | 10.8 | | | May 14, 2013 | | | | | | | | | | | | | | | | 10.10† | | Amendment No. 3 to License Agreement, dated September 10, 2013, by and between the Registrant and Institut Pasteur | | 10-Q | | | 001-35966 | | | | 10.2 | | | November 14, 2013 | | | | | | | | | | | | | | | | 10.11† | | Amendment No. 4 to License Agreement, dated April 1, 2015, by and between the Registrant and Institut Pasteur | | 10-Q | | | 001-35966 | | | | 10.10 | | | May 6, 2015 | | | | | | | | | | | | | | | | 10.12† | | License Agreement, dated December 7, 2011, by and between the Registrant and Research Development Foundation | | S-1/A | | | 333-188605 | | | | 10.9 | | | May 14, 2013 | | | | | | | | | | | | | | | | 10.13† | | Novation Agreement, dated April 2, 2012, by and between the Registrant and The Board of Trustees of the Leland Stanford Junior University | | S-1/A | | | 333-188605 | | | | 10.10 | | | May 14, 2013 | | | | | | | | | | | | | | | |
| | | | Incorporated by Reference | Exhibit Number | | Exhibit Title | | Form | | File no. | | | Exhibit | | | Filing Date | 10.14† | | Master Collaboration Agreement by and between the Registrant and Celgene Corporation, dated March 19, 2013 | | S-1/A | | | 333-188605 | | | | 10.11 | | | May 14, 2013 | | | | | | | | | | | | | | | | 10.15† | | Amended and Restated Master Collaboration Agreement by and between the Registrant and Celgene Corporation, dated June 3, 2015 | | 10-Q | | | 001-35966 | | | | 10.14 | | | August 7, 2015 | | | | | | | | | | | | | | | | 10.16 | | Amendment No. 1 to Amended and Restated Master Collaboration Agreement by and between the Registrant and Celgene Corporation, dated February 17, 2016 | | 10-Q | | | 001-35966 | | | | 10.15 | | | May 4, 2016 | | | | | | | | | | | | | | | | 10.17 | | Amendment No. 2 to Amended and Restated Master Collaboration Agreement by and between the Registrant and Celgene Corporation, dated September 28, 2017 | | 10-Q | | | 001-35966 | | | | 10.17 | | | November 1, 2017 | | | | | | | | | | | | | | | | 10.18† | | Amended and Restated License Agreement by and between the Registrant and Celgene Corporation, dated February 16, 2016 | | 10-Q | | | 001-35966 | | | | 10.16 | | | November 2, 2016 | | | | | | | | | | | | | | | | 10.19† | | Amended and Restated License Agreement by and between the Registrant and Celgene Corporation, dated September 28, 2017 | | 10-Q - | | | 001-35966 | | | | 10.19 | | | November 1, 2017 | | | | | | | | | | | | | | | | 10.20† | | License Agreement by and between the Registrant and Biogen Idec MA Inc., dated August 13, 2014 | | 10-Q | | | 001-35966 | | | | 10.17 | | | November 2, 2016 | | | | | | | | | | | | | | | | 10.21† | | Letter Agreement by and between the Registrant and Biogen MA Inc., dated September 29, 2017 | | 10-Q | | | 001-35966 | | | | 10.21 | | | November 1, 2017 | | | | | | | | | | | | | | | | 10.22† | | Exclusive Patent License Agreement by and between the Registrant and the National Institutes of Health, dated August 31, 2015 | | 10-Q | | | 001-35966 | | | | 10.18 | | | November 2, 2016 | | | | | | | | | | | | | | | | 10.23# | | Amended and Restated Employment Agreement by and between the Registrant and Nick Leschly | | S-1/A | | | 333-188605 | | | | 10.12 | | | June 4, 2013 | | | | | | | | | | | | | | | | 10.24# | | Amended and Restated Employment Agreement by and between the Registrant and Jeffrey T. Walsh | | S-1/A | | | 333-188605 | | | | 10.13 | | | June 4, 2013 | | | | | | | | | | | | | | | | 10.25# | | Amended and Restated Employment Agreement by and between the Registrant and Mitch Finer | | S-1/A | | | 333-188605 | | | | 10.14 | | | June 4, 2013 | | | | | | | | | | | | | | | | 10.26# | | Transitional Services and Separation Agreement by and between the Registrant and Mitch Finer | | 10-Q | | | 001-35966 | | | | 10.17 | | | May 6, 2015 | | | | | | | | | | | | | | | | 10.27# | | Amended and Restated Employment Agreement by and between the Registrant and David M. Davidson, M.D. | | S-1/A | | | 333-188605 | | | | 10.15 | | | June 4, 2013 | | | | | | | | | | | | | | | | 10.28# | | Employment Agreement, dated February 3, 2014, by and between the Registrant and Jason F. Cole | | 10-Q | | | 001-35966 | | | | 10.18 | | | May 13, 2014 | | | | | | | | | | | | | | | | 10.29# | | Amendment to Employment Agreement, dated March 7, 2016, by and between the Registrant and Jason F. Cole | | 10-Q | | | 001-35966 | | | | 10.25 | | | May 4, 2016 | | | | | | | | | | | | | | | | 10.30# | | Amendment No. 2 to Employment Agreement, dated November 3, 2016, by and between the Registrant and Jason F. Cole | | 10-K | | | 001-35966 | | | | 10.27 | | | February 22, 2017 | | | | | | | | | | | | | | | | 10.31# | | Employment Agreement, dated October 20, 2014, by and between the Registrant and James DeTore | | 8-K | | | 001-35966 | | | | 10.1 | | | November 10, 2014 | | | | | | | | | | | | | | | | 10.32# | | Separation Agreement, dated February 24, 2016, by and between the Registrant and James DeTore | | 10-Q | | | 001-35966 | | | | 10.27 | | | May 4, 2016 | | | | | | | | | | | | | | | | 10.33# | | Employment Agreement, dated May 30, 2015, by and between the Registrant and Philip D. Gregory | | 10-Q | | | 001-35966 | | | | 10.21 | | | August 7, 2015 | | | | | | | | | | | | | | | | 10.34# | | Amendment to Employment Agreement, dated November 3, 2016, by and between the Registrant and Philip D. Gregory | | 10-K | | | 001-35966 | | | | 10.31 | | | February 22, 2017 | | | | | | | | | | | | | | | | 10.35# | | Employment Agreement, dated November 23, 2016, by and between the Registrant and Susanna High | | 10-K | | | 001-35966 | | | | 10.32 | | | February 22, 2017 | | | | | | | | | | | | | | | | 10.36# | | Offer Letter, dated October 14, 2013, by and between the Registrant and Eric Sullivan | | 10-Q | | | 001-35966 | | | | 10.19 | | | May 13, 2014 | | | | | | | | | | | | | | | |
| | | | Incorporated by Reference | Exhibit Number | | Exhibit Title | | Form | | File no. | | | Exhibit | | | Filing Date | 10.37# | | 2013 Employee Stock Purchase Plan | | S-1/A | | | 333-188605 | | | | 10.17 | | | June 4, 2013 | | | | | | | | | | | | | | | | 10.38# | | First Amendment of the Bluebird Bio, Inc. 2013 Employee Stock Purchase Plan | | — | | | — | | | | — | | | Filed herewith | | | | | | | | | | | | | | | | 10.39# | | Offer Letter, dated November 16, 2017, by and between the Registrant and Kory Wentworth | | — | | | — | | | | — | | | Filed herewith | | | | | | | | | | | | | | | | 10.40# | | Executive Cash Incentive Bonus Plan | | S-1/A | | | 333-188605 | | | | 10.18 | | | May 14, 2013 | | | | | | | | | | | | | | | | 10.41 | | Lease, dated June 3, 2013, by and between the Registrant and 150 Second Street, LLC, as amended | | S-1/A | | | 333-188605 | | | | 10.19 | | | June 4, 2013 | | | | | | | | | | | | | | | | 10.42 | | Lease Amendment, dated November 15, 2013, by and between the Registrant and 150 Second Street, LLC, as amended | | 10-K | | | 001-35966 | | | | 10.19 | | | March 5, 2014 | | | | | | | | | | | | | | | | 10.43 | | Lease Amendment, dated June 9, 2014, by and between the Registrant and 150 Second Street, LLC, as amended | | 10-Q | | | 001-35966 | | | | 10.24 | | | August 12, 2014 | | | | | | | | | | | | | | | | 10.44 | | Consent to Assignment, dated September 30, 2016, by and among the Registrant, ARE-MA Region No. 50, LLC, and Foundation Medicine, Inc. | | 10-Q | | | 001-35966 | | | | 10.35 | | | November 2, 2016 | | | | | | | | | | | | | | | | 10.45 | | Assignment and Assumption of Lease, dated September 30, 2016, by and between the Registrant and Foundation Medicine, Inc. | | 10-Q | | | 001-35966 | | | | 10.36 | | | November 2, 2016 | | | | | | | | | | | | | | | | 10.46 | | Lease, dated June 29, 2015, by and between the Registrant and ARE-MA Region No. 38, LLC | | 10-Q | | | 001-35966 | | | | 10.29 | | | August 7, 2015 | | | | | | | | | | | | | | | | 10.47† | | Lease, dated September 21, 2015, by and between the Registrant and ARE-MA Region No. 40 LLC | | 10-Q | | | 001-35966 | | | | 10.30 | | | November 5, 2015 | | | | | | | | | | | | | | | | 10.48 | | First Amendment to Lease, dated June 21, 2016, by and between the Registrant and ARE-MA Region No. 40 LLC | | 10-Q | | | 001-35966 | | | | 10.37 | | | August 3, 2016 | | | | | | | | | | | | | | | | 10.49 | | Second Amendment to Lease, dated November 14, 2016, by and between the Registrant and ARE-MA Region No. 40 LLC | | 10-K | | | 001-35966 | | | | 10.44 | | | February 22, 2017 | | | | | | | | | | | | | | | | 10.50 | | Termination of Lease, dated February 10, 2017, by and between the Registrant and ARE-MA Region No. 38, LLC | | 10-K | | | 001-35966 | | | | 10.45 | | | February 22, 2017 | | | | | | | | | | | | | | | | 21.1 | | Subsidiaries of the Registrant | | — | | | — | | | | — | | | Filed herewith | | | | | | | | | | | | | | | | 23.1 | | Consent of Ernst & Young LLP | | — | | | — | | | | — | | | Filed herewith | | | | | | | | | | | | | | | | 31.1 | | Certification of Principal Executive Officer pursuant to Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | | — | | | — | | | | — | | | Filed herewith | | | | | | | | | | | | | | | | 31.2 | | Certification of Principal Financial Officer pursuant to Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | | — | | | — | | | | — | | | Filed herewith | | | | | | | | | | | | | | | | 32.1 | | Certification of Principal Executive Officer and Principal Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | | — | | | — | | | | — | | | Furnished herewith | | | | | | | | | | | | | | | | 101 | | The following materials from the Company’s Annual Report on Form 10-K for the year ended December 31, 2017, formatted in XBRL (eXtensible Business Reporting Language): (i) Consolidated Balance Sheets, (ii) Consolidated Statements of Operations and Comprehensive Loss, (iii) Consolidated Statements of Cash Flows and (iv) Notes to Consolidated Financial Statements. | | — | | | — | | | | — | | | Filed herewith |
† | Portions of this exhibit (indicated by asterisks) have been omitted pursuant to a request for confidential treatment and this exhibit has been submitted separately to the SEC. |
# | Indicates a management contract or any compensatory plan, contract or arrangement. |
F-31
SIGNATURESSIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized. bluebird bio, Inc. | | | By: | | /s/ Nick Leschly | | | Nick Leschly | | | President, Chief Executive Officer and Director |
SIGNATURES AND POWER OF ATTORNEY We, the undersigned directors and officers of bluebird bio, Inc. (the “Company”), hereby severally constitute and appoint Nick Leschly and James M. DeTore,Jeffrey Walsh, and each of them singly, our true and lawful attorneys, with full power to them, and to each of them singly, to sign for us and in our names in the capacities indicated below, any and all amendments to this Annual Report on Form 10-K, and to file or cause to be filed the same, with all exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as each of us might or could do in person, and hereby ratifying and confirming all that said attorneys, and each of them, or their substitute or substitutes, shall do or cause to be done by virtue of this Power of Attorney. Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated. Name | | Title | | Date | | | | /s/ Nick Leschly | | President, Chief Executive Officer and Director | | February 25, 201521, 2018 | Nick Leschly | | (Principal Executive Officer and Duly Authorized Officer) | | | | | | /s/ James M. DeToreJeffrey Walsh | | Chief Financial and Strategy Officer and Treasurer | | February 25, 201521, 2018 | James M. DeToreJeffrey Walsh
| | (Principal Financial Officer and Duly Authorized Officer) | | | | | | /s/ Eric SullivanKory Wentworth | | Senior Director,Vice President, Finance
| | February 25, 201521, 2018 | Eric SullivanKory Wentworth
| | (Principal Accounting Officer) | | | | | | /s/ Robert I. Tepper, M.D.
| | Director
| | February 25, 2015
| Robert I. Tepper, M.D.
| | | | | | | | /s/ Steven Gillis, Ph.D.
| | Director
| | February 25, 2015
| Steven Gillis, Ph.D.
| | | | | | | | /s/ Daniel S. Lynch | | Director | | February 25, 201521, 2018 | Daniel S. Lynch | | | | | | | | /s/ James Mandell,John O. Agwunobi, M.D. | | Director | | February 25, 201521, 2018 | John O. Agwunobi, M.D. | | | | | | | | | | /s/ Wendy L. Dixon, Ph.D. | | Director | | February 21, 2018 | Wendy L. Dixon, Ph.D. | | | | | | | | /s/ Mary Lynne Hedley, Ph.D. | | Director | | February 21, 2018 | Mary Lynne Hedley, Ph.D. | | | | | | | | | | /s/ James Mandell, M.D.. | | Director | | February 21, 2018 | James Mandell, M.D. | | | | | | | | /s/ John M. Maraganore,Douglas A. Melton, Ph.D. | | Director | | February 25, 201521, 2018 | John M. Maraganore, Ph.D.
| | | | | | | | /s/ Wendy L. Dixon, Ph.D.
| | Director
| | February 25, 2015
| Wendy L. Dixon,Douglas A. Melton, Ph.D.
| | | | | | | | /s/ David P. Schenkein, M.D. | | Director | | February 25, 201521, 2018 | David P. Schenkein, M.D. | | | | | | | | /s/ Mark Vachon | | Director | | February 25, 201521, 2018 | Mark Vachon | | | | |
Exhibit Index
| | | | Incorporated by Reference | Exhibit
Number | | Exhibit Title | | Form | | File no. | | | Exhibit | | | Filing Date | | | | | | | | | | | | | | | | 2.1 | | Stock Purchase Agreement by and between the Registrant and Precision Genome Engineering, Inc. | | 8-K | | | 001-35966 | | | | 2.1 | | | June 30, 2014 | | | | | | | | | | | | | | | | 3.1 | | Amended and Restated Certificate of Incorporation of the Registrant | | 8-K | | | 001-35966 | | | | 3.1 | | | June 24, 2013 | | | | | | | | | | | | | | | | 3.2 | | Amended and Restated By-laws of the Registrant | | 8-K | | | 001-35966 | | | | 3.2 | | | June 24, 2013 | | | | | | | | | | | | | | | | 4.1 | | Specimen Common Stock Certificate | | S-1/A | | | 333-188605 | | | | 4.1 | | | June 4, 2013 | | | | | | | | | | | | | | | | 4.2 | | Form of Common Stock Warrant | | S-1/A | | | 333-188605 | | | | 4.2 | | | May 14, 2013 | | | | | | | | | | | | | | | | 4.3 | | Form of Series A-1 Preferred Stock Warrant | | S-1/A | | | 333-188605 | | | | 4.3 | | | May 14, 2013 | | | | | | | | | | | | | | | | 4.4 | | Form of Series B Preferred Stock Warrant | | S-1/A | | | 333-188605 | | | | 4.4 | | | May 14, 2013 | | | | | | | | | | | | | | | | 4.5 | | Amended and Restated Investors’ Rights Agreement, dated as of July 23, 2012, by and among the Registrant and the Investors listed therein. | | S-1/A | | | 333-188605 | | | | 4.5 | | | May 14, 2013 | | | | | | | | | | | | | | | | 4.6 | | Amendment to Amended and Restated Investors’ Rights Agreement, dated as of July 8, 2014, by and among the Registrant and the Investors listed therein. | | 10-Q | | | 001-35966 | | | | 4.6 | | | August 12, 2014 | | | | | | | | | | | | | | | | 10.1 | | Second Amended and Restated 2002 Employee, Director and Consultant Plan, as amended, and forms of award agreement thereunder | | S-1/A | | | 333-188605 | | | | 10.1 | | | May 14, 2013 | | | | | | | | | | | | | | | | 10.2 | | 2010 Stock Option and Grant Plan, as amended, and forms of award agreement thereunder | | S-1/A | | | 333-188605 | | | | 10.2 | | | May 14, 2013 | | | | | | | | | | | | | | | | 10.3 | | 2013 Stock Option and Incentive Plan and forms of award agreement thereunder | | S-1/A | | | 333-188605 | | | | 10.3 | | | June 4, 2013 | | | | | | | | | | | | | | | | 10.4 | | Form of Indemnification Agreement between the Registrant and each of its Executive Officers and Directors | | S-1/A | | | 333-188605 | | | | 10.4 | | | May 14, 2013 | | | | | | | | | | | | | | | | 10.5 | | Amended and Restated Lease Agreement, dated May 18, 2007, by and between the Registrant and Rivertech Associates II, LLC, as amended | | 10-Q | | | 001-35966 | | | | 10.1 | | | November 14, 2013 | | | | | | | | | | | | | | | | 10.6† | | Patent License Agreement, dated December 11, 1996, by and between the Registrant (formerly known as Genetix Pharmaceuticals Inc., successor-in-interest to Innogene Pharmaceuticals Inc.) and Massachusetts Institute of Technology, as amended | | S-1/A | | | 333-188605 | | | | 10.6 | | | May 14, 2013 | | | | | | | | | | | | | | | | 10.7† | | Patent and Know-How License Agreement No. 07554F30, dated May 14, 2009, by and between the Registrant (formerly known as Genetix Pharmaceuticals Inc.) and INSERM-TRANSFERT, as amended | | S-1/A | | | 333-188605 | | | | 10.7 | | | May 14, 2013 | | | | | | | | | | | | | | | | 10.8† | | License Agreement, dated September 13, 2011, by and between the Registrant and Institut Pasteur, as amended | | S-1/A | | | 333-188605 | | | | 10.8 | | | May 14, 2013 | | | | | | | | | | | | | | | | 10.9† | | Amendment No. 3 to License Agreement, dated September 10, 2013, by and between the Registrant and Institut Pasteur | | 10-Q | | | 001-35966 | | | | 10.2 | | | November 14, 2013 | | | | | | | | | | | | | | | | 10.10† | | License Agreement, dated December 7, 2011, by and between the Registrant and Research Development Foundation | | S-1/A | | | 333-188605 | | | | 10.9 | | | May 14, 2013 | | | | | | | | | | | | | | | | 10.11† | | Novation Agreement, dated April 2, 2012, by and between the Registrant and The Board of Trustees of the Leland Stanford Junior University | | S-1/A | | | 333-188605 | | | | 10.10 | | | May 14, 2013 | | | | | | | | | | | | | | | | 10.12† | | Master Collaboration Agreement by and between the Registrant and Celgene Corporation, dated March 19, 2013 | | S-1/A | | | 333-188605 | | | | 10.11 | | | May 14, 2013 | | | | | | | | | | | | | | | |
| | | | Incorporated by Reference | Exhibit
Number | | Exhibit Title | | Form | | File no. | | | Exhibit | | | Filing Date | 10.13 | | Amended and Restated Employment Agreement by and between the Registrant and Nick Leschly | | S-1/A | | | 333-188605 | | | | 10.12 | | | June 4, 2013 | | | | | | | | | | | | | | | | 10.14 | | Amended and Restated Employment Agreement by and between the Registrant and Jeffrey T. Walsh | | S-1/A | | | 333-188605 | | | | 10.13 | | | June 4, 2013 | | | | | | | | | | | | | | | | 10.15 | | Amended and Restated Employment Agreement by and between the Registrant and Mitch Finer | | S-1/A | | | 333-188605 | | | | 10.14 | | | June 4, 2013 | | | | | | | | | | | | | | | | 10.16 | | Amended and Restated Employment Agreement by and between the Registrant and David M. Davidson, M.D. | | S-1/A | | | 333-188605 | | | | 10.15 | | | June 4, 2013 | | | | | | | | | | | | | | | | 10.17 | | Offer Letter, dated September 27, 2011 by and between the Registrant and Linda Bain | | S-1/A | | | 333-188605 | | | | 10.16 | | | June 4, 2013 | | | | | | | | | | | | | | | | 10.18 | | Employment Agreement, dated February 3, 2014, by and between the Registrant and Jason F. Cole | | 10-Q | | | 001-35966 | | | | 10.18 | | | May 13, 2014 | | | | | | | | | | | | | | | | 10.19 | | Employment Agreement, dated October 20, 2014, by and between the Registrant and James DeTore | | 8-K | | | 001-35966 | | | | 10.1 | | | November 10, 2014 | | | | | | | | | | | | | | | | 10.20 | | Offer Letter, dated October 14, 2013, by and between the Registrant and Eric Sullivan | | 10-Q | | | 001-35966 | | | | 10.19 | | | May 13, 2014 | | | | | | | | | | | | | | | | 10.21 | | 2013 Employee Stock Purchase Plan | | S-1/A | | | 333-188605 | | | | 10.17 | | | June 4, 2013 | | | | | | | | | | | | | | | | 10.22 | | Executive Cash Incentive Bonus Plan | | S-1/A | | | 333-188605 | | | | 10.18 | | | May 14, 2013 | | | | | | | | | | | | | | | | 10.23 | | Lease, dated June 3, 2013, by and between the Registrant and 150 Second Street, LLC, as amended | | S-1/A | | | 333-188605 | | | | 10.19 | | | June 4, 2013 | | | | | | | | | | | | | | | | 10.24 | | Lease Amendment, dated November 15, 2013, by and between the Registrant and 150 Second Street, LLC, as amended | | 10-K | | | 001-35966 | | | | 10.19 | | | March 5, 2014 | | | | | | | | | | | | | | | | 10.25 | | Lease Amendment, dated June 9, 2014, by and between the Registrant and 150 Second Street, LLC, as amended | | 10-Q | | | 011-35966 | | | | 10.24 | | | August 12, 2014 | | | | | | | | | | | | | | | | 21.1 | | Subsidiaries of the Registrant | | 10-Q | | | 011-35966 | | | | 21.1 | | | August 12, 2014 | | | | | | | | | | | | | | | | 23.1 | | Consent of Ernst & Young LLP | | — | | | — | | | | — | | | Filed herewith | | | | | | | | | | | | | | | | 31.1 | | Certification of Principal Executive Officer pursuant to Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | | — | | | — | | | | — | | | Filed herewith | | | | | | | | | | | | | | | | 31.2 | | Certification of Principal Financial Officer pursuant to Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | | — | | | — | | | | — | | | Filed herewith | | | | | | | | | | | | | | | | 32.1 | | Certification of Principal Executive Officer and Principal Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | | — | | | — | | | | — | | | Furnished herewith | | | | | | | | | | | | | | | | 101 | | The following materials from the Company’s Annual Report on Form10-K for the year ended December 31, 2014, formatted in XBRL (eXtensible Business Reporting Language): (i) Consolidated Balance Sheets, (ii) Consolidated Statements of Operations and Comprehensive Loss, (iii) Consolidated Statements of Convertible Preferred Stock and Stockholders’ Equity (Deficit), (iv) Consolidated Statements of Cash Flows and (v) Notes to Consolidated Financial Statements. | | — | | | — | | | | — | | | Filed herewith |
†
| Portions of this exhibit (indicated by asterisks) have been omitted pursuant to a request for confidential treatment and this exhibit has been submitted separately to the SEC.
|
|
|
|
|
|