UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
| |
☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 20172023
OR
| |
☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number 001-36548
ATARA BIOTHERAPEUTICS, INC.
(Exact name of Registrant as specified in its Charter)
| | |
Delaware |
| 46-0920988 |
(State or other jurisdiction of incorporation or organization) |
| (I.R.S. Employer
|
611 Gateway Blvd.2380 Conejo Spectrum Street, Suite 900
South San Francisco, 200
Thousand Oaks, CA
|
| 9408091320
|
(Address of principal executive offices) |
| (Zip Code) |
Registrant’s telephone number, including area code: (650) 278-8930(805) 623-4211
Securities registered pursuant to Section 12(b) of the Act: Common Stock, par value $0.0001 per share, traded on The Nasdaq Stock Market
| | | | |
Title of each class |
| Trading Symbol(s) |
| Name of each exchange on which registered |
Common Stock, par value $0.0001 per share, |
| ATRA |
| The Nasdaq Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. YES Yes☐NO☒ NO ☐
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. YES☐ NO No☒
Indicate by check mark whether the Registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. YES Yes☒NO☐
Indicate by check mark whether the Registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the Registrant was required to submit and post such files). YES Yes☒NO☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405) is not contained herein, and will not be contained, to the best of Registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☐
Indicate by check mark whether the Registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company, or an emerging growth company. See the definitiondefinitions of “large accelerated filer”, “accelerated filer”, “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one):
| | | | | | |
Large accelerated filer |
| ☐ |
| Accelerated filer |
| ☒☐
|
|
|
|
|
Non-accelerated filer |
| ☐ (Do not check if a small reporting company)☒
|
| Small reporting company |
| ☐☒
|
|
|
|
|
|
|
|
Emerging growth company |
| ☒☐
|
|
|
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). YES☐NO☒
The aggregate market value of common stock held by non-affiliates of the Registrant, based on the closing sales price for such stock on June 30, 20172023 as reported by The Nasdaq Stock Market, was $271,251,806.$161,602,451. This calculation excludes 10,528,420727,959 shares held by executive officers, directors and stockholders that the Registrant has concluded are affiliates of the Registrant. Exclusion of such shares should not be construed to indicate that any such person possesses the power, direct or indirect, to direct or cause the direction of the management or policies of the registrant or that such person is controlled by or under common control with the Registrant.
The number of outstanding shares of the Registrant’s Common Stock as of February 15, 2018March 20, 2024 was 38,825,835.119,359,230.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Registrant’s definitive proxy statement relating to its 20182024 Annual Meeting of Stockholders are incorporated by reference into Part III of this Report where indicated. Such proxy statement will be filed with the U.S. Securities and Exchange Commission within 120 days after the end of the fiscal year to which this report relates.
ATARA BIOTHERAPEUTICS, INC.
TABLE OF CONTENTS
FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains “forward-looking statements”forward-looking statements within the meaning of Section 27Athe safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act.1995. Such forward-looking statements, which represent our intent, belief or current expectations, involve risks and uncertainties and other factors that could cause actual results and the timing of certain events to differ materially from future results expressed or implied by such forward-looking statements. In some cases you can identify these statements by forward-looking words such as “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “could,” “would,” “project,” “predict,” “plan,” “expect” or the negative or plural of these words or similar expressions. Forward-lookingThe forward-looking statements in this Annual Report on Form 10-K include, but are not limited to, statements about:
•our expectations regarding the timing of initiating clinical trials,studies, opening client sites, enrolling clinical trialsstudies and reporting results of clinical trialsstudies for our T-cell programs;
programs, including our ATA3219 program; •the likelihood and timing of regulatory submissions or related approvals for our product candidates;
candidates, including the initiation, completion and expectations about the timing of approvals for a biologics license application (BLA) for tab-cel® for patients with Epstein-Barr virus with post-transplant lymphoproliferative disease (EBV+ PTLD); •the potential market opportunitiesindications for commercializing our product and product candidates;
•commercialization of tab-cel (Ebvallo™ in the United Kingdom (UK) and the European Union (EU)) worldwide and our amended and restated Commercialization Agreement with Pierre Fabre Medicament, including potential milestone and royalty payments under the agreement (Ebvallo in the UK and the EU subject to the Purchase and Sale Agreement with HCR Molag Fund, L.P.);
•our Purchase and Sale Agreement and related transactions with HCR Molag Fund, L.P.;
•our Commercial Manufacturing Services Agreement with Charles River Laboratories, Inc. (CRL) and other agreements we may enter into with CRL, including our ability to enter into a new drug supply agreement with CRL on terms favorable or acceptable to us, or at all;
•our Master Services and Supply Agreement and related transactions with FUJIFILM Diosynth Biotechnologies California, Inc.;
•our expectations regarding the potential commercial market opportunities, market size and the size of the patient populations for our product candidates, if approved for commercial use;and product candidates;
•estimates of our expenses, capital requirements and need for additional financing;
•our expectation regarding the length of time that our existing capital resources will be sufficient to enable us to fund our planned operations, into the first half of 2020;
including our going concern assessment; •our ability to enter into favorable commercialization arrangements with third parties to commercialize our product candidates;
•our ability to develop, acquire and advance product candidates into, and successfully complete, clinical trials;studies;
•the initiation, timing, costs, progress and results of future preclinical studies and clinical trialsstudies and our research and development programs;
•our ability to enter into and maintain contracts with clinical research organizations, contract manufacturing organizations (CMOs) and other vendors for clinical and preclinical studies, supplies and other services;
•the scope of protection we are able to obtain and maintain for ourthe intellectual property rights covering our product and product candidates;
•our financial performance;
•our election to rely on reduced reporting and disclosure requirements available to smaller reporting companies;
•developments and projections relating to our competitors and our industry;
•our ability to manufacturehave our product and product candidates manufactured for our clinical trials,studies or for commercial sale, including our Phase 3 trials;
our ability to sell or manufacture approved products at commercially reasonable values; and
•the impact of public health emergencies, such as COVID-19, to our business and operations, as well as the businesses and operations of third parties on which we rely;
•the impact of our workforce reductions on our ability to attract, retain and motivate qualified personnel and on our business, operations and financial condition; and
•timing and costs related to buildingthe qualification of the manufacturing facilities of our manufacturing plant.CMOs for commercial production.
These statements are only current predictions and are subject to known and unknown risks, uncertainties, including, without limitation, risks and uncertainties associated with the costly and time-consuming pharmaceutical product development process and the uncertainty of clinical success; the sufficiency of our cash resources and need for additional capital; and other factors that may cause our or our industry’s actual results, levels of activity, performance or achievements to be materially different from those anticipated by the forward-looking statements. We discuss many of these risks in this report in greater detail under the heading “1A. Risk Factors” and elsewhere in this report. You should not rely upon forward-looking statements as predictions of future events. New risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risks and uncertainties.
In this Annual Report on Form 10-K, unless the context requires otherwise, “Atara,” “Atara Biotherapeutics,” “Company,” “we,” “our,” and “us” means Atara Biotherapeutics, Inc. and, where appropriate, its subsidiaries.
Summary Risk Factors
Our business is subject to numerous risks and uncertainties that may have a material adverse effect on our business, financial condition or results of operations. These risks are more fully described under the heading “1A. Risk Factors” and elsewhere in this report and include, among others:
•we have incurred substantial losses since our inception and anticipate that we will continue to incur substantial losses for the foreseeable future;
•we have earned limited commercialization revenues to date, and we may never achieve profitability;
•we have one approved product, Ebvallo, in the EU and the UK, are generally early in our development efforts and have only a small number of product candidates in clinical development, and all of our other product candidates are still in preclinical development. If we or our collaborators are unable to successfully develop, manufacture and commercialize our product or product candidates or experience significant delays in doing so, our business may be materially harmed;
•we will require substantial additional financing on terms acceptable to us to achieve our goals, and a failure to obtain this necessary capital when needed could force us to delay, limit, reduce or terminate our product development or manufacturing efforts;
•our future success depends on our ability to retain our executive officers and to attract, retain and motivate qualified personnel;
•the results of preclinical studies or earlier clinical studies are not necessarily predictive of future results, and our existing product candidates in clinical studies, and any other product candidates we advance into clinical studies may not have favorable results in later clinical studies or receive regulatory approval;
•clinical drug development involves a lengthy and expensive process with an uncertain outcome;
•our T-cell immunotherapy product and product candidates and our next-generation CAR T programs represent new therapeutic approaches that could result in heightened regulatory scrutiny, delays in clinical development or our inability to achieve regulatory approval, commercialization or payor coverage of our product candidates;
•there can be no assurance that we will achieve all of the anticipated benefits of the Fujifilm Transaction and we could face unanticipated challenges;
•the market opportunities for our product and product candidates may be limited to those patients who are ineligible for or have failed prior treatments and may be small;
•we may not be able to obtain or maintain orphan drug exclusivity for our product candidates;
•the proposed revision of the European legislation on pharmaceuticals could lead to uncertainties over the regulatory framework that will be applicable to medicinal products in the EU, including orphan medicinal products;
•we have been affected by and could be adversely affected in the future by the effects of health epidemics and pandemics, such as the COVID-19 pandemic, which could materially and adversely affect our business and operations in the future, as well as the businesses and operations of third parties on which we rely;
•if we are unable to obtain and maintain sufficient intellectual property protection for our product candidates, or if the scope of the intellectual property protection is not sufficiently broad, our ability to commercialize our product candidates successfully and to compete effectively may be adversely affected;
•our principal stockholders own a significant percentage of our stock and will be able to exert control or significant influence over matters subject to stockholder approval;
•if we fail to continue to meet the listing standards of the Nasdaq Stock Market LLC (Nasdaq), our common stock may be delisted, which could have a material adverse effect on the liquidity of our common stock;
•we recently qualified as a “smaller reporting company” and a “non-accelerated filer,” and any decision on our part to comply only with certain reduced reporting and disclosure requirements applicable to such companies could make our stock less attractive to investors;
•our workforce reductions may not result in anticipated savings, could result in total costs and expenses that are greater than expected and could disrupt our business; and
•maintaining clinical and commercial timelines is dependent on our end-to-end supply chain network to support manufacturing; if we experience problems with our third party suppliers or CMOs, development and/or commercialization of our product and product candidates may be adversely affected.
PART I
Item 1. Business
Overview
Atara Biotherapeutics is a leadingleader in T-cell immunotherapy, company developingleveraging its novel treatmentsallogeneic Epstein-Barr virus (EBV) T-cell platform to develop transformative therapies for patients with cancer and autoimmune disease. Tab-cel (tabelecleucel), our lead program in Phase 3 clinical development in the U.S., has received marketing authorization approval (MAA) under the proprietary name Ebvallo™ for commercial sale in the European Union (EU) by the European Commission (EC) and viral diseases. The Company'sfor commercial sale and use in the United Kingdom (UK) by the Medicines and Healthcare products Regulatory Agency (MHRA). We are the most advanced allogeneic T-cell immunotherapy company and intend to rapidly deliver off-the-shelf or allogeneic, T-cells are bioengineered from donors with healthy immune function and allow for rapid delivery from inventorytreatments to patients withoutwith high unmet medical need. Our platform leverages the unique biology of EBV T cells and has the capability to treat a requirement for pretreatment. Atara'swide range of EBV-driven diseases or other serious diseases through incorporation of engineered chimeric antigen receptors (CARs) or T-cell immunotherapiesreceptors (TCRs). We are designedapplying this one platform, that does not require TCR or human leukocyte antigen (HLA) gene editing, to precisely recognize and eliminate cancerous or diseased cells without affecting normal, healthy cells. Atara'screate a robust pipeline. Our strategic priorities are:
•Tab-cel®: Our most advanced T-cell immunotherapy program, tab-cel, has received MAA for commercial sale in the EU and the UK under the proprietary name Ebvallo and is partnered with Pierre Fabre Medicament (Pierre Fabre) for commercialization in Europe and potential commercialization, if approved, worldwide, including in the U.S. Tab-cel is currently in Phase 3 development tabelecleucel (formerly known as ATA129), is being developedin the U.S. for the treatment of patients with Epstein-Barr virus, or EBV,EBV- associated post-transplant lymphoproliferative disorder, or EBV+ PTLD, disease (EBV+ PTLD) who have failed rituximab or rituximab plus chemotherapy, as well as other EBV-driven diseases; and •ATA3219: Allogeneic CAR T targeting CD19, currently in Phase 1 development is being developed as a potential best-in-class product intended to target B-cell malignancies and autoimmune diseases, based on a next generation 1XX co-stimulatory domain and the innate advantages of EBV associated hematologicT cells as the foundation for an allogeneic CAR T platform.
In addition to the aforementioned strategic priorities, we also have a number of preclinical programs, including ATA3431, an allogeneic dual CAR T immunotherapy targeting both CD19 and solid tumors, including nasopharyngeal carcinoma, or NPC. Off-the-shelfCD20 for B-cell malignancies; and a potential next generation EBV vaccine which is differentiated from earlier EBV vaccine efforts that solely focused on B cell responses to EBV. We have paused development on ATA188, and autologous, or patient derived, ATA190, the Company'san allogeneic T-cell immunotherapies using a complementary targeted antigen recognition technology, target specificimmunotherapy targeting EBV antigens believed to be important for the potential treatment of multiple sclerosis or MS. Atara's clinical pipeline also includes ATA520 targeting Wilms Tumor 1, or WT1,(MS), while we explore strategic options for this asset.
Our T-cell immunotherapy platform is potentially applicable to a broad array of targets and ATA230 directed against cytomegalovirus, or CMV.
diseases. Our technologyoff-the-shelf, allogeneic T-cell platform allows for rapid delivery of a T-cell immunotherapy product that has been manufactured in advance of patient need and stored in inventory, with each manufactured lot of cells providing therapy for numerous potential patients. This differs from autologous or patient-derived, treatments, in which each patient’s own cells must be extracted, genetically modified outside the body and then delivered back to the patient.patient, requiring a complex logistics network. We utilize a proprietary cell selection algorithm to select the appropriate set of cells for use based on a patient’s unique immune profile,profile. We estimate that only four and unlike manysix unique ATA3219 lots will be needed to cover approximately 90% of U.S. NHL and lupus patients, respectively. One of our contract manufacturing organizations (CMOs) has completed commercial production qualification activities for tab-cel and another of our CMOs is currently in the process of completing commercial production qualification activities for tab-cel while we manufacture inventory according to Pierre Fabre’s commercial product supply strategy.
In October 2021, we entered into the Commercialization Agreement with Pierre Fabre (Pierre Fabre Commercialization Agreement), pursuant to which we granted to Pierre Fabre an exclusive, field-limited license to commercialize and distribute Ebvallo in Europe and select emerging markets in the Middle East, Africa, Eastern Europe and Central Asia (the Initial Territory) following regulatory approval. As contemplated by the Pierre Fabre Commercialization Agreement, we entered into (i) a Manufacturing and Supply Agreement (ii) a Pharmacovigilance Agreement (iii) and a Quality Agreement, in each case, with Pierre Fabre to further advance our partnership with Pierre Fabre. In September 2022, we amended the Pierre Fabre Commercialization Agreement and received an additional $30 million milestone payment from Pierre Fabre following EC approval of Ebvallo for EBV+ PTLD and subsequent filing of the MAA transfer to Pierre Fabre, in exchange for, among other T-cell programs, there is neitherthings, a requirementreduction in: (i) royalties we are eligible to receive as a percentage of net sales of Ebvallo in the Initial Territory, and (ii) the supply price mark up on Ebvallo purchased by Pierre Fabre. Additionally, we agreed to extend the time period for pre-treatment beforeprovision of certain services to Pierre Fabre under the Pierre Fabre Commercialization Agreement. In December 2022, we entered into a Purchase and Sale Agreement (HCRx Agreement) with HCR Molag Fund L.P. (HCRx,) a Delaware limited partnership. Pursuant to the terms of the HCRx Agreement, we received a total investment amount of $31.0 million in exchange for granting HCRx the right to receive a portion of the tiered, sales-based royalties for Ebvallo, in amounts ranging from the mid-single digits to significant double digits, and certain milestone payments, both related to the Initial Territory and otherwise payable to us by Pierre Fabre. The total royalties and milestones payable to HCRx related to the Initial Territory under the HCRx Agreement are capped between 185% and 250% of the total investment amount by HCRx, dependent upon the timing of such royalty and milestone payments to HCRx.
On October 31, 2023, we entered into an amended and restated Pierre Fabre Commercialization Agreement (A&R Commercialization Agreement), pursuant to which we expanded Pierre Fabre’s exclusive rights to research, develop, manufacture, commercialize and distribute tab-cel (Ebvallo) to include all other countries in the world (Additional Territory) in addition to the Initial Territory (together, the Territory), subject to our cellsperformance of certain obligations as described below. In December 2023, upon the effective date of the A&R Commercialization Agreement, we met the contractual right to receive an additional upfront cash payment of $20.0 million for the expanded exclusive license grant, for which such cash was received in January 2024. We will also be entitled to receive an aggregate of up to $620.0 million in additional milestone payments upon achieving certain regulatory and commercial milestones relating to tab-cel in the Additional Territory including up to $100.0 million in potential regulatory milestones through approval by the United States Food and Drug Administration (FDA) of a biologics license application (BLA) for tab-cel. Of the $100.0 million in potential regulatory milestones, we expect to receive $20.0 million in April 2024 based on the positive pre-BLA meeting, an additional $20.0 million in connection with BLA acceptance, and up to $60.0 million in potential regulatory milestones in connection with BLA approval. We are administered nor is there extended monitoring following administration. For example,eligible to receive significant double-digit tiered royalties as a percentage of net sales of tab-cel (Ebvallo) in ourthe Territory until the later of 12 years after the first commercial sale in each such country, the expiration of specified patent rights in each such country, or the expiration of all regulatory exclusivity for tab-cel in each such country. Royalty payments may be reduced in certain specified customary circumstances. Royalties and milestones from the commercialization of Ebvallo in the Initial Territory remain subject to the HCRx Agreement.
During the applicable period specified in the A&R Commercialization Agreement, we will be responsible, at Pierre Fabre’s cost, to continue conducting the ongoing trials with our most advanced product candidate, tabelecleucel, patients are monitoredPhase 3 ALLELE clinical study and the Phase 2 multi-cohort clinical study. We will also be responsible, at Pierre Fabre’s cost, for onecertain other activities directed to two hours following receiptobtaining regulatory approval in the United States for tab-cel for EBV-associated post-transplant lymphoproliferative disease pursuant to the terms of tabelecleucel. Our T-cell immunotherapy platform is applicablethe A&R Commercialization Agreement. Pierre Fabre will be responsible, at its cost, for obtaining and maintaining all other required regulatory approvals and for commercialization and distribution of tab-cel in the Territory, including conducting any other clinical study required.
Prior to the transfer of manufacturing responsibility to Pierre Fabre, we will be responsible for manufacturing and supplying tab-cel to Pierre Fabre for commercialization in the Territory at cost plus a margin for orders placed after December 31, 2023, subject to a broad arraymaximum annual increase. Pierre Fabre will assume the responsibility and cost for the manufacture and supply of targetstab-cel in the Territory upon the Manufacturing Transition Date, which is defined as the earlier of i) the date on which all activities relating to the transfer of tab-cel manufacturing, pursuant to the A&R Commercialization Agreement, from Atara to Pierre Fabre have been completed to the reasonable satisfaction of both parties, or ii) December 31, 2025, throughout the remainder of the term of the A&R Commercialization Agreement. Pierre and diseases. With more than 200 patients treated acrosswe are to use commercially reasonable efforts to achieve such transition prior to the platform, we have observed clinical proofearlier transfer date from Atara to Pierre Fabre of concept across both viral and non-viral targetsthe first marketing authorization in conditions ranging from liquid and solid tumors to infectious and autoimmune diseases. the Additional Territory or the first BLA.
We have also observed a safety profile characterized by few treatment-related serious adverse events, or SAEs, and no evidence of cytokine release syndrome to date.
Our T-cell immunotherapy product candidates are engineered from cells donated by healthy individualsentered into research collaborations with normal immune function. Once cells are collected from a donor, they are bioengineered to expand those T-cells that recognize the antigens of interest. The resulting expanded T-cells are then characterized and heldleading academic institutions such as inventory. From inventory, these cells can be selected, distributed and prepared for infusion in a partially human leukocyte antigen, or HLA, matched patient in approximately 3-5 days. Following administration, our T-cells home to their target, undergo target-controlled proliferation, eliminate diseased cells and eventually recede. Target-controlled proliferation means that our T-cells expand in number when they encounter diseased cells in a patient’s body that express the antigen the cells are designed to recognize.
We have two technology platforms. One of our technology platforms was developed from more than a decade of experience at Memorial Sloan Kettering Cancer Center or MSK. The other was developed at QIMR Berghofer(MSK) and the Council of the Queensland Institute of Medical Research Institute, or QIMR Berghofer, in Australia. We licensed(QIMR Berghofer) pursuant to which we acquired rights to novel and proprietary technologies and programs.
Our research facilities in Thousand Oaks, California (ARC) and Aurora, Colorado contain our translational and preclinical sciences, analytical development and process science functions. These facilities support our product pipeline, process development and leverage our allogeneic cell therapy platform to drive innovation.
In January 2022, we entered into an asset purchase agreement with FUJIFILM Diosynth Biotechnologies California, Inc. (FDB) and, for certain know-howlimited purposes, FUJIFILM Holdings America Corporation, to sell all of the Company’s right, title and T-cellinterest in and to certain assets related to the Atara T-Cell Operations and Manufacturing facility (ATOM Facility) located in Thousand Oaks, California for $100 million in cash, subject to potential post-closing adjustments pursuant to the asset purchase agreement (the Fujifilm Transaction). The closing of the Fujifilm Transaction occurred on April 4, 2022. We also entered into a Master Services and Supply Agreement with FDB (Fujifilm MSA) that became effective upon the closing and could extend for up to ten years. Pursuant to the Fujifilm MSA, FDB will supply us with specified quantities of our cell therapy products (if approved) and product candidates, manufactured in accordance with cGMP standards. The Fujifilm MSA does not obligate us to purchase products and product candidates exclusively from MSKFDB.
We also work with Charles River Laboratories (CRL) pursuant to a Commercial Manufacturing Services Agreement (CRL MSA) that we entered into in June 2015. Our most advancedDecember 2019. Pursuant to the CRL MSA, CRL provides manufacturing services for our product candidate, tabelecleucel, targets EBV. Tabelecleucel received Breakthrough Therapy Designation,and certain intermediates. We further amended the CRL MSA to extend the term until the earlier of March 31, 2024 or BTD, fromupon receipt of certain batches of our product and product candidates. We are currently in negotiations with CRL for a new commercial drug product supply agreement to be effective upon the U.S. Foodexpiration of the CRL MSA. However, there can be no assurance that we will be able to enter into a new commercial drug product supply agreement with CRL on terms favorable or acceptable to us, or at all. If we are
unable to enter into a new commercial drug product supply agreement or extend the CRL MSA, we may need to identify alternative sources of drug product supply.
We have non-cancellable minimum commitments for products and Drug Administration, or FDA,services, subject to agreements with a term of greater than one year, with clinical research organizations and Priority Medicines, or PRIME, designation from the European Medicines Agency, or EMA,CMOs.
In November 2023, we announced a reduction in force of approximately 30% of our workforce at that time. This workforce reduction resulted in total restructuring charges of $6.7 million, comprised primarily of severance payments and is currently being evaluated as monotherapy in two Phase 3 trialswages for the treatment60-day notice period in accordance with the California Worker Adjustment and Retraining Notification (WARN) Act. In most cases, the severance payments were paid as a lump sum in January 2024. All of patientsthe severance costs represent cash expenditures.
In January 2024, we announced another reduction in force of approximately 25% of total workforce. We expect to recognize approximately $4.5 million in total severance and related benefits as a result of this reduction in force, consisting primarily of severance payments and wages for the 60-day notice period in accordance with EBV+ PTLD. We believe that tabelecleucel has the potential toCalifornia WARN Act. In most cases, the severance will be the first commercially available off-the-shelf T-cell immunotherapy and the first FDA and EMA approved therapy for EBV+ PTLD. With a European conditional marketing authorization application planned forpaid in the first half of 20192024. Certain of the notified employees had employment agreements which provided for separation benefits in the form of salary continuation; these benefits will be paid from February 2024 through January 2025. The majority of the associated costs represent cash expenditures.
Pipeline
Our pipeline is summarized below:
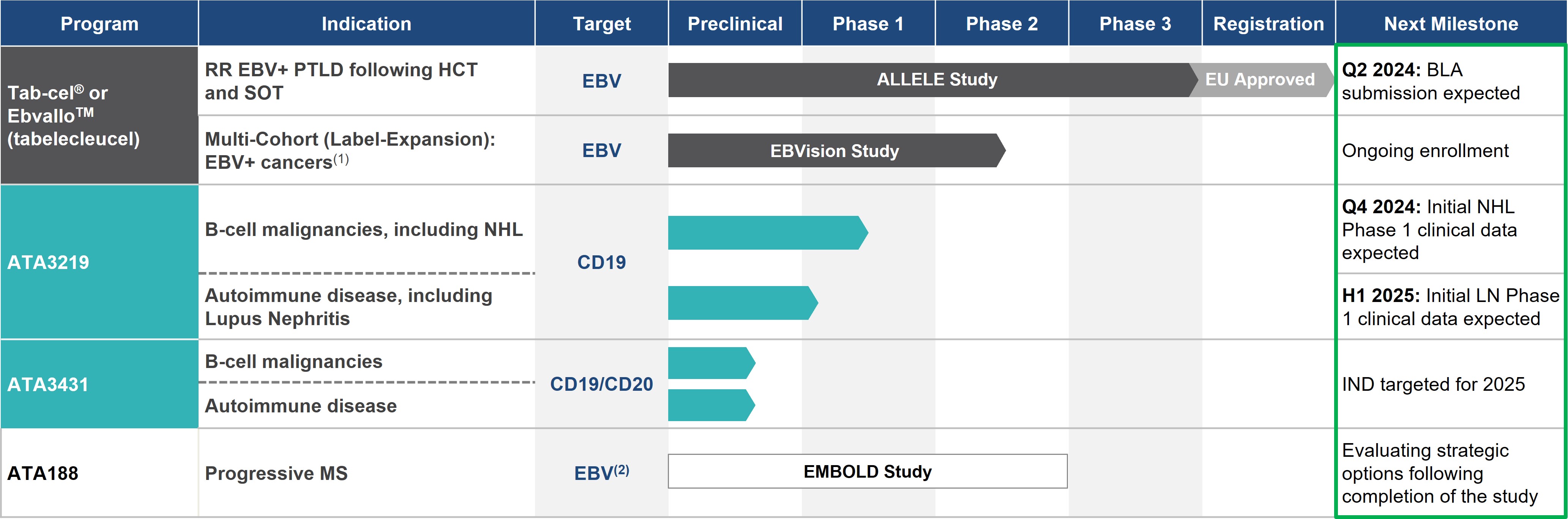
Excluding Ebvallo™ in EU, these investigational agents are not approved by any regulatory agencies and U.S. biologics licensing applications planned following the completion of one of our ongoing Phase 3 trials, we are currently developing the infrastructureefficacy and safety have not been established
EBV+ PTLD: EBV-Associated Post-Transplant Lymphoproliferative Disease; RR: rituximab relapsed/refractory; HCT: allogeneic hematopoietic cell transplant; SOT: solid organ transplant; NHL: non-Hodgkin’s lymphoma
Atara has entered into an agreement with Pierre Fabre to commercialize tabelecleucel globallytab-cel® for EBV+ cancers worldwide
Other programs: EBV vaccine and other hematological malignancies and solid tumor AlloCAR T programs
(1)Phase 2 multi-cohort initiated in Q3 2020, with possible indications including EBV+ PTLD with CNS involvement, front-line treatment in EBV+
PTLD. We are also evaluating thePTLD including front line with CNS involvement, EBV+ PID/AID LPD, and other potential
utility of tabelecleucel in patients with other EBV associated cancers, such as NPC, to continue its development in solid tumors. Additional product candidates derived from the collaboration with MSK are being developed to treat various cancers and severe viral infections.In October 2015 and September 2016, we licensed rights to certain know-how and technology from QIMR Berghofer that are complementary to those we which was licensed from MSK. This know-how and technology uses targetedEBV-associated diseases
(2)Targeted antigen recognition
to create off-the-shelf T-cell immunotherapy product candidates applicable to a variety of diseases, including autoimmune conditions such as multiple sclerosis, or MS. We are also working with QIMR Berghofer on the development of EBV and other virally targeted T-cells. Through this technology, we are expanding the role of immunotherapy beyond oncology and viral infections to autoimmune disease. Our most advanced off-the-shelf T-cell product candidate utilizing this technology, ATA188, targets select antigens of EBV and is currently being evaluated in atechnology; Phase
1 trial in an initial cohort for the treatment of patients with progressive MS. In connection with the initial license from QIMR Berghofer, we received an option to exclusively license an autologous version of ATA188, also known as ATA190, which recently demonstrated clinical activity in a Phase 1 trial in progressive MS. We expect to broadly explore the utility of our targeted antigen recognition technology in EBV and other virally driven diseases, and additional product candidates derived from our collaboration with QIMR Berghofer are being developed.
2 Randomized Controlled Trial
Overall, we believe that Atara is a leading allogeneic T-cell immunotherapy company with a robust and late stage oncology pipeline and potentially transformative T-cell immunotherapies for MS and other viral diseases. With tabelecleucel poised to potentially become the first approved off-the-shelf T-cell therapy and a robust pipeline of high potential candidates, our ambition is to be recognized as the leader in off-the-shelf T-cell immunotherapy.Ebvallo™ (Tab-cel®)
Tabelecleucel for EBV+ PTLD following HCT or SOT
Since its discovery as the first human oncovirus, EBV has been implicated in the development of a wide range of diseases, including lymphomas and other cancers. EBV is widespread in human populations and persists as a lifelong, asymptomatic infection. In healthy individuals, a small percentage of T-cellsT cells are devoted to keeping EBV in check. In contrast, immunocompromised patients, such as those undergoing hematopoietic cell transplants or HCT,(HCT) or solid organ transplants or SOT,(SOT) have a reduced ability to control EBV. Left without appropriate immune surveillance, EBV transformedEBV-transformed cells can, in some patients, proliferate and cause an aggressive, life-threatening cancer called EBV+ PTLD.PTLD. Nearly all cases of PTLD that occur following HCT are EBV positive while approximately 70%60% of PTLD cases that occur following SOT are EBV positive. Approximately 10-15% of
Historical studies suggest a high unmet medical need for improved therapies in patients with EBV+ PTLD patients are children. Patients with EBV+ PTLD are currently treated withwho have failed rituximab or rituximab plus chemotherapy, when systemic treatment is indicated, with approximately 50-60%40% to 60% of patients either not responding to or progressing following this first line of therapy. Historical studies suggest a high unmet medical need for improved therapies in patients with EBV+ PTLD who have failed rituximab. MedianExpected median overall survival in patients with EBV+ PTLD following HCT who have failed rituximab-based first line therapy is 16-56 days, with a one-year survival rate of approximately 23% based on our evaluation of available historical outcomes data. One-1.7 months, and two-year survival following incomplete response to rituximab infor patients with high-risk EBV+ PTLD after following SOT who have failed rituximab-based first line therapy, the median overall survival is 36% and 0%, respectively.approximately 3.3 months. The use of chemotherapy in patients with EBV+ PTLD who have failed rituximab is frequently associated with significant rates of treatment-related mortality due to the frailty of the patients and severe toxicities associated with chemotherapy.
We believe that the global commercial opportunity for PTLD is attractive. We expect the number of EBV+ PTLD patients to grow over time as a result of increases in the number of transplant procedures and an increasing rate of PTLD following these procedures. Based on our market research, we estimate that in 2019, approximately 164,000 HCT and SOT transplant procedures are expected to be performedthere were several hundred EBV+ PTLD patients who failed rituximab or rituximab plus chemotherapy in the United States,U.S. in 2019.
Tab-cel® (Ebvallo™) for EBV+ PTLD
In June 2015, we licensed certain patent rights, know-how and a library of T cells and cell lines specific to EBV from MSK under an exclusive license agreement. In accordance with the European Union, or EU, Australia, Canada, China, Japan, South Korealicense agreement, we agreed to use commercially reasonable efforts to commercialize the licensed products and Turkey,to make milestone payments with this number expected to increase to approximately 207,000 by 2024, predominantly due to increases in bone marrow, peripheral blood and umbilical cord blood donation and more haploidentical transplants. Similarly, the number of cases of EBV+ PTLD is expected to increase from approximately 4,700 in 2019 to 6,000 in 2024 duerespect to the uselicensed programs and to make royalty payments to MSK to the extent product candidates arising from the collaboration are commercialized. Our first commercial product, Ebvallo, is part of more potent immuno-suppression in haploidentical transplants.this MSK collaboration and targets EBV.
Our most advanced T-cell immunotherapy product candidate, tabelecleucel,Tab-cel® (Ebvallo™) is an allogeneic EBV-specific T-cell immunotherapy that is approved in the EU and UK and currently being investigatedin Phase 3 development in the U.S. for the treatment of patients with EBV+ PTLD who have failed rituximab. In February 2015, the FDA granted tabelecleucel BTD or rituximab plus chemotherapy. Tab-cel is also under development for other EBV+ diseases with significant unmet medical need through a Phase 2 multi-cohort study that was initiated in the treatmentthird quarter of patients with EBV+ PTLD after HCT who have failed rituximab. BTD is an FDA process designed to accelerate the development and review of drugs intended to treat a serious condition when early trials show that the drug may be substantially better than current treatment. In October 2016, tabelecleucel was accepted into the EMA PRIME regulatory pathway for the same indication, providing enhanced regulatory support. In addition, tabelecleucel2020.
Tab-cel has received orphan status inBreakthrough Therapy Designation (BTD) from the United StatesU.S. Food and European UnionDrug Administration (FDA) for the treatment of patients with EBV+ PTLD after HCT who have failed rituximab and orphan designation in the U.S. and European Union (EU) for the treatment of patients with EBV+ PTLD following HCT or SOT. In December 2016, we announced that we had reached agreement with the FDA on the designs of two Phase 3 trials for tabelecleucel intended to support approval in two separate indications, the treatment of EBV+ PTLD following HCT and SOT in patients who have failed rituximab. In December 2017, following discussion with the FDA of manufacturing and comparability data generated on material manufactured by our contract manufacturing organization, we initiated these trials in the United States. We expect to expand these trials geographically to include Europe, Canada, and Australia.
The Phase 3 MATCH trial (EBV+ PTLD following HCT) is a multicenter, open label, single arm trial designed to enroll approximately 35 patients with EBV+ PTLD following HCT who have failed rituximab. The Phase 3 ALLELE trial (EBV+ PTLD following SOT) is a multicenter, open label trial with two non-comparative cohorts. Each cohort is designed to enroll approximately 35 patients. The first cohort will include patients who previously received rituximab monotherapy, and the second cohort will include patients who previously received rituximab plus chemotherapy. Both cohorts are planned to enroll concurrently. The primary endpoint of both the MATCH and ALLELE trials is confirmed best objective response rate, or ORR, defined as the percent of patients achieving either a complete or partial response to treatment with tabelecleucel confirmed after the initial tumor assessment showing a response. The protocols are designed to rule out a 20% ORR as the null hypothesis. This means that if the lower bound of the 95% confidence interval on ORR among patients receiving at least one dose of tabelecleucel exceeds 20% at the end of the study, then the trial would be expected to meet the primary endpoint for the treatment of PTLD. For example, assuming anticipated enrollment of 35 patients in MATCH, an observed ORR above approximately 37% would be expected to meet the primary endpoint. In ALLELE, each of the two cohorts with an anticipated enrollment of 35 patients will be analyzed separately with respect to the primary endpoint and, similarly, as an example, with 35 patients enrolled in either cohort, an observed ORR above approximately 37% would be expected to meet the primary endpoint. Secondary endpoints for both trials include duration of response, overall survival, safety, quality of life metrics, and other measures to evaluate its health economic impact. A safety committee will meet periodically to monitor for safety. Results from the first tabelecleucel Phase 3 study, or cohort in the case of ALLELE, to reach the primary endpoint are expected to be available in the first half of 2019.
In clinical trialsstudies conducted at MSK that have enrolled patients with EBV+ PTLD following HCT and SOT, efficacy following treatment with tabelecleuceltab-cel monotherapy compared favorably with historical data in these patient populations. Patients with EBV+ PTLD after HCT who have failed rituximab and were treated with tabelecleuceltab-cel had one-yeartwo-year overall survival of approximately 70%83% in two separate clinical trials.studies. In the setting of EBV+ PTLD after SOT in patients who have failed rituximab,, similar results were observed, with one-yeartwo-year overall survival of approximately 60%86% in tabelecleucel-treatedtab-cel-treated patients. A response rate of greater than or equal to 50% was observed in HCT and SOT patients in these studies.
In June 2016,December 2017, we openedinitiated two Phase 3 studies for tab-cel intended to support approval in two separate indications, the treatment of EBV+ PTLD following HCT (which was referred to as the MATCH study) and SOT in patients who have failed rituximab (which was referred to as the ALLELE study). In 2019, after discussion and alignment with regulators, we combined MATCH and ALLELE into a single study (which we now refer to as the ALLELE study) that now consists of an HCT cohort for EBV+ PTLD patients who have failed rituximab, and a single SOT cohort for EBV+ PTLD patients who have failed prior treatment with rituximab with or without chemotherapy. Additionally, we expanded the ALLELE study geographically to include clinical sites in Europe and Canada.
In the third quarter of 2020, we completed an interim analysis for the ALLELE study. Data from the interim analysis showed a 50 percent objective response rate (ORR) to tab-cel with independent oncologic and radiographic assessment (IORA) in patients with relapsed-refractory EBV+ PTLD following HCT or SOT, that had reached at least six months follow-up after the ORR assessment. This ORR is consistent with previously published investigator assessed data. The tab-cel safety profile is also consistent with previously published data, with no new safety signals. In December 2022, we presented updated interim analysis and safety results from the ALLELE study and updated efficacy and safety data from two single-center, open-label studies, and multicenter expanded access protocol, or EAP, trial. The trial is currently open at more than ten clinical sitesprogram in the United States. The primary objective of this trial is to provide tabelecleucel monotherapy to patients with EBV-associated diseases or certain EBV positive malignancies for whom there are no other therapeutic options. Key secondary objectives include evaluation of efficacy and safety through a robust collection of data. We recently announced the presentation of positive interim results from this multicenter EAP trialEBV+ Leiomysosarcomas at the 59th2022 American Society of Hematology or ASH, Annual Meeting. Efficacy resultsIn December 2023, we presented new data for patients with relapsed or refractory (r/r) or treatment-naïve EBV+ PTLD involving the central nervous system following SOT or HCT. An ORR of 77.8% was observed in 1118 central nervous system (CNS) EBV+ PTLD patients including first line PTLD, and the estimated one-year overall survival rate (OS) was 70.6%. The one-year OS for responders was 85.7% versus 0% for non-responders. In January 2024, data from the planned Phase 3 populationsALLELE study that was published in The Lancet Oncology showed a 51.2% objective response rate and 23-month median duration of response in r/r EBV+ PTLD patients and that tab-cel was well tolerated with EBV+ PTLD following HCTno events of graft-versus-host disease as related to tab-cel.
In October 2021, we entered into the Pierre Fabre Commercialization Agreement, pursuant to which we granted to Pierre Fabre an exclusive, field-limited license to commercialize and SOT who had failed rituximab were consistent with the single-institution safety profile and response rates previously reported by our collaborating investigators at MSK. The response rates in both the five evaluable HCT patients treateddistribute Ebvallo in the EAPInitial Territory. In September 2022, we amended the Pierre Fabre Commercialization Agreement to receive an additional $30 million milestone payment from Pierre Fabre in exchange for a reduction in royalties and the six evaluable SOT patients was greater than 70%. Ansupply price mark up on Ebvallo purchased by Pierre Fabre. See section ‘Terms of Certain License and Collaboration Agreements’ below for additional patient with EBV+ PTLD following HCT remains alive but was not evaluable duedetails. In December 2022, we sold a portion of our right to lackreceive royalties and certain milestones in Ebvallo under the Pierre Fabre Commercialization Agreement to HCRx for a total investment amount of post-baseline assessment. We believe these results are consistent with$31.0 million, subject to a repayment cap between 185% and 250% of the tabelecleucel profile observedtotal investment amount by HCRx.
On October 31, 2023, we entered into the A&R Commercialization Agreement, which became effective in December 2023. Pursuant to the A&R Commercialization Agreement, Pierre Fabre’s exclusive rights to research, develop, manufacture, commercialize and distribute tab-cel (Ebvallo) will be expanded to include all other countries in the Phase 2 trials conducted at MSK. The Phase 3 trialsworld (Additional Territory) in addition to the Initial Territory (together, the Territory), subject to our performance of certain obligations as described below. In December 2023, upon the effective date of the A&R Commercialization Agreement, we met the contractual right to receive an additional upfront cash payment of $20.0 million for tabelecleucelthe expanded exclusive license grant, for which such cash was received in January 2024. We will also be entitled to receive an aggregate of up to $620.0 million in additional milestone payments upon achieving certain regulatory and commercial milestones relating to tab-cel in the Additional Territory, including up to $100.0 million in potential regulatory milestones through BLA approval. Of the $100.0 million in potential regulatory milestones, we expect to receive $20.0 million in April 2024 based on the positive pre-BLA meeting, an additional $20.0 million in connection with BLA acceptance, and up to $60.0 million in potential regulatory milestones in connection with BLA approval.
We are expectedeligible to enrollreceive significant double-digit tiered royalties as a percentage of net sales of tab-cel (Ebvallo) in the same EBV+ PTLD patient populations. Tabelecleucel was generally well toleratedTerritory until the later of 12 years after the first commercial sale in this study population. each such country, the expiration of specified patent rights in each such country, or the expiration of all regulatory exclusivity for tab-cel in each such country. Royalty payments may be reduced in certain specified customary circumstances. Royalties and milestones from the commercialization of Ebvallo in the Initial Territory remain subject to the HCRx Agreement.
In this study, five patients experienced treatment-related SAEs. One patient died due to PTLD disease progression. Two possibly related cases of graft versus host disease, or GvHD,November 2021, we submitted an EU marketing authorization application (MAA) for tab-cel in patients with EBV+ PTLD. In December 2022, the EC granted marketing authorization for Ebvallo under the “exceptional circumstances” regulatory pathway as a monotherapy for the treatment of adult and pediatric patients two years of age and older with r/r EBV+ PTLD following HCT were reported. A tumor flare who have received at least one prior therapy. For SOT patients, prior therapy includes chemotherapy unless chemotherapy is inappropriate. Our request to transfer the marketing authorization for Ebvallo to Pierre Fabre was observedadopted by the EC in one patient with EBV+HIV-associated plasmablastic lymphoma that resolved without clinical sequelae.
With respect to the total safety population following treatment with tabelecleucel, few treatment-related SAEs have been observed. Among 173 patients treated with tabelecleucel in clinical trials, there have been 12 patients with possibly related SAEs, with no infusion related toxicities, no cytokine release syndrome and three possibly related cases of GvHD.
We are also pursuing marketing approval of tabelecleucelFebruary 2023. Pierre Fabre commenced Ebvallo launch activities in the first European Union. countries in the first quarter of 2023 and is progressively launching Ebvallo on a country-by-country basis. Under the “exceptional circumstances” marketing authorization, Pierre Fabre is subject to annual reassessments of certain ongoing post-marketing obligations to continue confirmation of the benefits of Ebvallo. The annual reassessments will determine whether the Ebvallo marketing authorization should be maintained, changed, or suspended, based on Pierre Fabre’s fulfillment of post-marketing obligations and the risk/benefit profile of Ebvallo.
In March 2016,October 2022, we filed the EMA issued a positive opinionMAA for orphan drug designation for tabelecleucelEbvallo with the Medicines and Healthcare Products Regulatory Authority (MHRA) in the United Kingdom (UK). In May 2023, the MHRA granted Ebvallo marketing authorization in the UK for the treatment of patients with EBV+ PTLD. In October 2016, and the marketing authorization was subsequently transferred to Pierre Fabre.
We have performed extensive studies demonstrating analytical comparability between the tab-cel manufacturing process versions used for the pivotal ALLELE study and that intended for commercialization. Comprehensive comparability analyses covered 21 key attributes for potency, purity and alloreactivity. We believe analytic comparability between tab-cel process versions has been demonstrated based on well-established statistical methodology and application of International Council for Harmonization (ICH) guidelines and is further supported by significant and consistent clinical experience. These comparability data analyses were submitted to the EMA Committeethrough our MAA filing. EMA stated in its assessment report issued following approval of the MAA for Medicinal Productstab-cel by the EC that it considered comparability of the intended commercial product with the clinically used product to be shown.
We have been engaged in discussions with the FDA regarding a potential biologics license application (BLA) submission for Human Usetab-cel in the United States, including on (i) the content of chemistry, manufacturing and controls (CMC) module 3 and the Committeeassessment of comparability between the product used in the pivotal ALLELE study and that intended for Advanced Therapies granted tabelecleucel accesscommercialization and (ii) the clinical data package requirements.
In February 2022, we held a Type B CMC meeting with the FDA to discuss comparability between the intended commercial and pivotal clinical trial process versions. This meeting did not result in alignment on comparability and the FDA initially recommended we conduct a clinical study with commercial product as the FDA did not agree that comparability has been demonstrated between product used in the pivotal ALLELE study and the intended commercial product. Following further discussions, the FDA recommended a potential path to a BLA submission without the need for a new clinical study.
We subsequently held another meeting with the FDA to discuss topics relating to CMC, which culminated in clear guidance and agreement on specific CMC module 3 requirements for a potential BLA submission. Following this meeting, we filed an amendment to the EMA’sInvestigational New Drug (IND) application for tab-cel to provide additional CMC information requested by the FDA.
In 2023, we held a number of meetings with the FDA on clinical aspects and CMC for a potential BLA submission for tab-cel. Following such discussions, we aligned with the FDA on analytical comparability between manufacturing process versions of tab-cel. This alignment supports our ability to pool the pivotal clinical trial data from different process versions in a tab-cel BLA submission.
We recently established PRIMEheld a pre-BLA meeting with the FDA to discuss various aspects of our proposed BLA submission, which supports our plan to submit the tab-cel BLA in the second quarter of 2024. Of the $100.0 million in potential regulatory initiativemilestones that we may be entitled to receive through approval from Pierre Fabre pursuant to the terms of the A&R Commercialization Agreement, we expect to receive $20.0 million in April 2024 based on the positive pre-BLA meeting, an additional $20.0 million in connection with BLA acceptance, and up to $60.0 million in potential regulatory milestones in connection with BLA approval.
Tab-cel Multi-Cohort Study
We continue to pursue development of tab-cel in additional patient populations, with a primary focus on immunodeficiency-associated lymphoproliferative diseases (IA-LPDs), given the commonality of their EBV-driven mechanism of disease in immunocompromised patients, high unmet medical needs and positive clinical data to date with tab-cel. In patients where previous treatments have failed, the objective response rates, including complete response, were 33.3% (three out of nine patients) in AID-LPD and 37.5% (three out of eight patients) in PID-LPD groups. Tab-cel was generally well-tolerated with a favorable safety profile consistent with previously published clinical studies. These clinical data demonstrated that tab-cel was well-tolerated and showed encouraging clinical activity in this patient population, with objective response rates ranging from 50% (two out of four patients) to 80% (four out of five patients). The overall survival (OS) rate at one year in patients with EBV viremia treated in the EAP-201 study was 100 percent for a median follow-up of 14.6 months (min 12.2, max 17.8).
In the third quarter of 2020, we initiated a Phase 2 multi-cohort study which comprises a total of five patient populations, including IA-LPDs and other EBV-driven diseases, in both the U.S. and EU. We continue to enroll patients in this study. We are investigating additional label expansion opportunities with our Phase 2 multi-cohort study. In December 2023, we presented combined analysis that included the first reported data from our Phase 2 multi-cohort study at the European Society for Medical Oncology Immuno-Oncology annual congress that demonstrated a 77.8% ORR in 18 CNS EBV+ PTLD patients including first line PTLD.
ATA3219
We are developing ATA3219, an allogeneic CD19 CAR T immunotherapy targeting B-cell malignancies and autoimmune diseases, leveraging our next-generation 1XX CAR co-stimulatory domain and EBV T-cell platform and does not require TCR or HLA gene editing. ATA3219 combines the natural biology of unedited T cells with the benefits of an allogeneic therapy. It consists of allogeneic EBV-sensitized T cells that express a CD19 CAR construct for the treatment of CD19+ r/rB-cell malignancies, including B-cell non-Hodgkin’s lymphoma (NHL) and B-cell mediated autoimmune diseases including systemic lupus erythematosus (SLE) with kidney involvement (lupus nephritis (LN)). ATA3219 has been optimized to offer a potential best-in-class profile, featuring off-the-shelf availability. It incorporates multiple clinically validated technologies including a modified CD3ζ signaling domain (1XX) that optimizes expansion and mitigates exhaustion, provides enrichment during manufacturing for a less differentiated phenotype for robust expansion and persistence and retains the endogenous T-cell receptor without gene editing as a key survival signal for T cells which contribute to persistence.
Data from preclinical studies for ATA3219 suggest enhanced functional persistence, polyfunctional phenotype and efficient targeting of CD19-expressing tumor cells both in vitro and in vivo with a manufacturing process that focuses on T-cell stemness. Additional in vitro data demonstrate the CD19 antigen-specific functional activity of ATA3219 and CAR-mediated activity against B cells from SLE patients, leading to robust CD19-specific B-cell depletion compared to controls. This preclinical data was submitted as part of a late-breaking abstract which was accepted for poster presentation at the upcoming International Society for Cell & Gene Therapy meeting to be held May 29-June 1, 2024.
Based on academic data from a clinical study, an EBV T-cell platform has the potential to generate off-the-shelf, allogeneic CAR T immunotherapies with EBV+ PTLD following HCT who have failed rituximab. PRIME provides early enhanced regulatory supporthigh response rates, durable responses and low risk of toxicity that can be rapidly delivered to facilitate regulatory applicationspatients.
Our EBV CD19 CAR T program incorporates multiple clinically validated technologies designed for a memory phenotype, robust expansion, and accelerateretains the reviewendogenous T-cell receptor without gene editing as a key survival signal for T cells contributing to functional persistence. We continue to make progress on the ATA3219 manufacturing process for scale-up.
Data from an academic study (Curran et al ASH 2023) that used allogeneic EBV CAR T cells (CD28/CD3ζ co-stimulatory domain), demonstrated proof of medicinesprinciple for Atara’s allogeneic CAR T approach with overall survival up to 3 years in post-transplant B-cell malignancy patients. Atara’s ATA3219 builds upon this study with an improved process and construct that addressleverages multiple clinically validated technologies featuring a high unmet need. less differentiated phenotype and a novel 1XX costimulatory domain. We continue to make progress on the ATA3219 manufacturing process for scale-up.
In January 2017,July 2023, we received parallel scientific advicea Safe to Proceed letter from the EMA’s Scientific Advice Working Group and several national Health Technology Assessment agenciesFDA in response to our IND submission for ATA3219 in r/r B-cell NHL. We initiated enrollment of a multi-center, Phase 1 open-label, dose escalation clinical trial for ATA3219 in NHL, including large B-cell lymphomas, follicular lymphoma, or mantle cell lymphoma, with initial clinical data anticipated in the EU, including thosefourth quarter of 2024.
In February 2024, we received a Safe to Proceed letter from the FDA in the United Kingdom, Germany and France. Based on these discussions, we planresponse to submit an applicationour IND submission for Conditional Marketing Authorization, or CMA,use of tabelecleucel inATA3219 as a monotherapy for the treatment of patientsSLE with EBV+ PTLD following HCT who have failed rituximabLN and expect to initiate a Phase 1 study to evaluate the safety and preliminary efficacy of ATA3219 in the second half of 2024. Initial data is anticipated in the first half of 2019. The CMA will be based on clinical2025.
Additional Programs and Platform Expansion Activities
In addition to the prioritized programs described above, we have a number of preclinical programs.
Our CAR T immunotherapy pipeline includes ATA3431, an allogeneic, bispecific CAR directed against CD19 and CD20 for B-cell malignancies and autoimmune disease, leveraging our 1XX CAR co-stimulatory domain and EBV T-cell platform and does not require gene TCR or HLA gene editing. ATA3431 is enriched for central memory CAR T cells and in December 2023, we presented preclinical data from Phase 1that demonstrated early evidence of antitumor activity, long-term persistence, and 2 trials conducted at MSK and supported by available data from our Phase 3 MATCH and ALLELE trials in patients with EBV+ PTLD after HCT and SOT who have failed rituximab, which will be ongoingsuperior tumor growth inhibition compared to an autologous CD19/CD20 CAR T benchmark at the time65th American Society of filing.
In 2017, we began pre-commercial preparation to support the planned tabelecleucel EU CMA submission. For example, we are developing a proprietary, web-based, off-the-shelf delivery solution for commercial use that we call Atara MatchMe™. The Atara MatchMe system will be a portal for health care professionalsHematology Annual Meeting and institutions that allows for order input including the provision of required patient HLA and other information, the execution of our cell selection algorithm, product shipment and tracking, as well as the capture of data on outcomes following treatment. In the first quarter of 2017, we also signed a lease for an approximately 90,580 square foot facility in Thousand Oaks, California.Exposition 2023. We are building out a multi-product cellular therapy manufacturing facility with operations expected to commence in 2018. Overall, we believe that tabelecleucel monotherapy has a compelling value proposition in the treatment of patients with EBV+ PTLD who have failed rituximab. Weprogressing toward an IND submission and expect to pursue approvals globallyfile an IND submission for tabelecleucelATA3431 in patients2025.
We are also collaborating with EBV+ PTLD following HCT and SOT who have failed rituximab and may seek partnersQIMR Berghofer to aid in our commercializationdevelop a potential next generation EBV vaccine which is differentiated from earlier EBV vaccine efforts in select markets. In addition, we expectthat solely focused on B cell responses to pursue development of tabelecleucel in earlier lines of therapy, including first line EBV+ PTLD in combination with rituximab.
Tabelecleucel for nasopharyngeal carcinoma, or NPC
NPC, is a type of head and neck cancer that is primarily EBV associated. Standard treatment for NPC includes radiation therapy with or without platinum-based chemotherapy. InEBV. We also retain the setting of metastatic disease after the failure of chemotherapy, median survival is approximately fiverights to 11 months based on historical data, and there are no approved therapeutic agents available to treat this disease today. Based on our market research, we estimate that in 2015 there were approximately 9,400 patients with metastatic or recurrent Type III NPC in the United States, the United Kingdom, France, Germany, Italy and Spain and approximately 93,000 in Asia. Treatment with tabelecleucel as a monotherapy has been evaluated in 14 patients with metastatic NPC after failure of one to three lines of chemotherapy in the studies conducted by MSKCC. An ORR of 21% was observed in these patients with one complete response and two partial responses. In addition, 11 of the 14 patients were alive at a median follow up of 18 months with a Kaplan-
Meier survival estimate of 84% at two years. Tabelecleucel was administered to this immune competent patient population without prior lymphodepleting chemotherapy. Additionally, evidence of T-cell expansion following administration was observed. In April 2017, we entered intoATA188, an agreement with Merck (known as MSD outside of the United States and Canada) to provide drug supply for a trial sponsored and conducted by us to evaluate tabelecleucel in combination with Merck’s anti-PD-1 (programmed death receptor-1) therapy, KEYTRUDA ® (pembrolizumab), in patients with platinum-resistant or recurrent EBV-associated NPC. The Phase 1/2 trial will evaluate the safety, pharmacokinetics, pharmacodynamics, and preliminary efficacy of the combination and is planned for initiation in the second half of 2018.
Other T-Cell Programs
ATA188 and ATA190 for multiple sclerosis
MS is a chronic disorder of the central nervous system, or CNS, that disrupts the myelination and normal functioning of the brain, optic nerves and spinal cord through inflammation and tissue loss. The evolution of MS results in an increasing loss of both physical and cognitive (e.g., memory) function. This has a substantial negative impact on the approximately 2.3 million people worldwide affected by MS.
There are two categories of MS: progressive MS, or PMS; and relapsing-remitting MS, or RRMS. PMS is a severe form of MS with few therapeutic options. Within PMS there are two types of MS: secondary progressive MS, or SPMS; and primary progressive MS, or PPMS. According to the National Multiple Sclerosis Society, there are approximately one million people affected by PMS. Both types of PMS are characterized by persistent progression and worsening of MS symptoms and physical disability over time. PPMS occurs when the patient has a disease course characterized by steady and progressive worsening after disease onset. SPMS initially begins as RRMS, but once patients have continuous progression of their disease, they have developed SPMS. This is distinct from RRMS, where patients have flares of the disease that are followed by periods of recovery and quiescence during which the disease does not progress. There is substantial unmet medical need for new and effective therapies for patients with PMS. Most of the treatment options that work well in reducing the flares in RRMS have not been shown to be effective in slowing or reversing the progression of disability in PMS. The two approved therapeutic options for PMS patients have a modest impact on symptoms and disease progression and, therefore, we believe that unmet need remains. In the United States, mitoxantrone is approved for SPMS and ocrelizumab was approved in March 2017 for PPMS. Siponimod is currently being studied in Phase 3 trials for SPMS.
There is a strong biologic connection between EBV and MS. EBV is present in nearly all patients with MS. For example, in an international study of patients with clinically isolated syndrome, a CNS demyelinating event isolated in time that is compatible with the possible future development of MS, only one patient out of 1,407 was seronegative for, or not infected with, EBV. In addition, in separate studies, clusters of EBV infected B-cells and plasma cells were evident in the brains of MS patients but not found in brains of patients without MS. In these studies, the EBV infected B-cells and plasma cells were in close proximity to areas of active demyelination. Studies suggest that EBV positive B-cells and plasma cells in the CNS have the potential to catalyze an autoimmune response and the MS pathophysiology. In patients with MS, their T-cells may be unable to control EBV positive B-cells and plasma cells so that B-cells and plasma cells could then accumulate in the brain and generate antibodies that attack and destroy myelin, the protective layer that insulates nerves in the brain and spinal cord. This loss of myelin ultimately leads to MS symptoms. MS disease course has also been shown to correlate with measures of EBV activity. The role of B-cells in MS is supported by the recent approval by the FDA of ocrelizumab for PPMS which broadly targets B-cells through their expression of a cell surface marker known as CD20. Low vitamin D also suppresses T-cells and is associated with MS.
Our secondallogeneic T-cell immunotherapy product candidate, ATA188, is an off-the-shelf EBV-specific T-cell that utilizes a targeted antigen recognition technology that enables the T-cells we administer to selectively identify cells expressing thetargeting EBV antigens that we believe arebelieved to be important for the potential treatment of MS.multiple sclerosis (MS). We arehave paused development on ATA188 while we explore strategic options for this asset. We have also developing an autologous version of this product candidatediscontinued some programs and will return the programs to our collaborators. For example, in February 2024, we notified MSK that we call ATA190. ATA190 utilizeswill return the same approachATA2271 and ATA3271 programs targeting mesothelin to targeted antigen recognition as ATA188. These product candidates are designed to selectively target only those cells which are EBV positive while sparing those that are not. We believe that eliminating only EBV positive B-cells, including plasma cells, has the potential to benefit some patients with MS through enhanced efficacy and a better side-effect profile. In October 2015, we obtained an exclusive, worldwide license to develop and commercialize allogeneic T-cell immunotherapy product candidates targeting EBV, including ATA188, utilizing technology and know-how developed by QIMR Berghofer. In connection with this license, we also received an option to exclusively license the autologous version of EBV product candidates, including ATA190.MSK.
In the fourth quarter of 2017, we initiated an open label, single arm, multi-center, multi-national Phase 1 trial with allogeneic ATA188 for patients with MS and in January 2018 received clearance of our investigational new drug, or IND, application from the FDA to proceed with patient enrollment at U.S. sites. The primary objective of this Phase 1 trial is to assess the safety of ATA188 in patients followed for at least one year after the first dose. Key secondary endpoints in the trial include measures of clinical improvement such as Expanded Disability Status Scale, or EDSS, and annualized relapse rate, or ARR, as well as MRI imaging. The trial is expected to enroll a total of 60 patients across the United States, Australia and Europe: 30 patients with PMS, either PPMS or SPMS, and 30 patients with RRMS. We expect to announce results from our ATA188 Phase 1 trial in patients with PMS in the first half of 2019.
In addition, based on the Phase 1 clinical results observed to date with ATA190, we believe the continued development of ATA190 will enhance our understanding of the potential therapeutic utility of targeting EBV in the treatment of MS and further inform and complement our development of ATA188, and we are planning a multicenter Phase 1/2 trial with ATA190 in PMS.
Our collaborating investigators at QIMR Berghofer are currently conducting a Phase 1 trial utilizing autologous ATA190 for the treatment of patients with PMS. We believe this is the first clinical trial to prospectively explore both the feasibility and potential utility of targeting EBV in MS. The trial is designed to:
enroll 10 patients: five with PPMS and five with SPMS;
assess the safety and tolerability of ATA190 in patients with PMS;
document preliminary evidence of efficacy through the evaluation of both clinically measured and patient reported changes in MS symptoms during and following treatment; and
determine if autologous ATA190 can be generated to clinical scale from the blood of patients with PMS.
Each patient receives four escalating doses of ATA190 over six weeks, with each individual dose given once every two weeks. Patients are followed for 20 weeks after the last dose. An abstract from our collaborating investigators describing interim results from this Phase 1 trial was selected for inclusion in the Emerging Science Program during the 69th American Academy of Neurology Annual Meeting in April 2017 and updated interim results for all ten patients were recently presented at the MSParis 2017 Congress, the 7th Joint Meeting of the European Committee for Treatment and Research in Multiple Sclerosis and the Americas Committee for Treatment and Research in Multiple Sclerosis.
Results presented include data on five SPMS patients and five PPMS patients. Clinical improvements were reported in six of the ten patients treated and these improvements were observed within two to fourteen weeks after the first dose. Three patients improved their EDSS score. EDSS is a method for quantifying disability and monitoring changes over time. Reduction in fatigue was a consistent observation in responding patients. Five of the six patients who showed clinical improvements received ATA190 with greater than or equal to 7% EBV reactivity, or T-cell reactivity against target EBV antigens following manufacturing. This suggests that EBV reactivity may be an important product characterization metric for future development. ATA190 was well-tolerated, and no significant treatment-related adverse events were observed. A summary of study results is highlighted in the table below.
| | | | | | | | | | |
Subject Age/Gender
(MS Type)
| | EDSS1
BL2/
Post
Tx3
| | | CD8+
T cell
Reactivity
to EBV
| | | Observed Improvement
|
60 yo F (SPMS)
| | | 6.5/6.0
| | | | 47%
| | | Yes
|
60 yo M (PPMS)
| | | 5.0/3.5
| | | | 31%
| | | Yes
|
49 yo F (PPMS)
| | | 8.0/8.0
| | | | 15%
| | | Yes
|
61 yo M (SPMS)
| | | 6.5/6.5
| | | | 10%
| | | Equivocal
|
55 yo F (PPMS)
| | | 5.0/4.5
| | | | 8%
| | | Yes—still in follow up
|
46 yo M (SPMS)4
| | | 8.0/8.0
| | | | 7%
| | | Yes
|
42 yo F (PPMS)
| | | 6.5/7.0
| | | | 3%
| | | None
|
53 yo M (PPMS)
| | | 6.0/6.0
| | | | <1%
| | | None
|
54 yo F (SPMS)
| | | 6.5/6.5
| | | | <1%
| | | None
|
49 yo F (SPMS)
| | | 6.5/6.5
| | | | <1%
| | | Mild
|
|
| | |
1
| | EDSS = Expanded Disability Scale Score.
|
2
| | BL = Baseline EDSS score prior to treatment with ATA190.
|
3
| | Post Tx = EDSS score following treatment with ATA190.
|
4
| | This patient received ATA190 under a compassionate use protocol approximately 4 years prior to entry into the Phase 1 trial.
|
Overall, we believe these results are encouraging and support the continued development of ATA188 and ATA190 in MS.
ATA520 for hematologic malignancies
Our third T-cell immunotherapy product candidate, ATA520, is an off-the-shelf WT1 specific T-cell immunotherapy, that targets cancers expressing the antigen WT1 and is currently in Phase 1 clinical trials. WT1 is an intracellular protein that is overexpressed in a number of cancers, including hematological malignances as well as solid tumors. MSK has two Phase 1 clinical trials evaluating ATA520. The first trial is a dose escalation trial of ATA520 for residual or relapsed leukemia after HCT. The second trial is a dose escalation trial of ATA520 following T-cell depleted HCT for patients with relapsed or refractory multiple myeloma, including plasma cell leukemia, or PCL. Based on data from these trials, we intend to develop ATA520 in a select set of hematologic malignancies and solid tumors. Given the advances of our EBV-related pipeline programs in NPC and MS, as well as the opportunity to pursue a conditional marketing authorization in the EU for tabelecleucel, we expect to initiate an additional clinical trial with
ATA520 following the further process development of ATA520 as well as the clinical and regulatory advancement of tabelecleucel and ATA188.
ATA230 for CMV viremia and disease
Our fourth T-cell immunotherapy product candidate, ATA230, is an off-the-shelf CMV specific T-cell immunotherapy, that is in Phase 2 clinical trials for refractory CMV infection that occurs in some patients who have received an HCT or SOT or are otherwise immunocompromised. We met with the FDA for an end of Phase 2 meeting to discuss late stage development of ATA230 for the treatment of anti-viral refractory or resistant CMV infection following either HCT or SOT. Our collaborating investigators presented updated ATA230 results from 50 post-transplant patients with refractory CMV viremia and disease, including those with disease in the central nervous system, at the 59th ASH Annual Meeting in Atlanta, Georgia, in December 2017. Results reported include a response rate of approximately 60% in all patients, which was similar in those with CMV viremia and disease. Patients who responded to ATA230 showed improved 6- and 12-month survival rates of approximately 80% and 60%, respectively, versus those patients who did not respond to treatment. One of the 32 patients who responded died of CMV disease. ATA230 was generally well tolerated. Five patients experienced grade 4 or higher adverse events deemed possibility related to ATA230. Recently, the FDA granted orphan drug designation for ATA230 for the treatment of CMV viremia and disease in immunocompromised patients as well as Rare Pediatric Disease Designation for the treatment of congenital CMV infection. The EMA has also granted us orphan status for ATA230 for CMV infection in patients with impaired cell-mediated immunity. Given the opportunity to pursue a CMA in the EU for tabelecleucel, we have decided to prioritize our EBV related programs ahead of ATA230 at this time, and plan to further evaluate our development strategy for ATA230 in 2018.
ATA621 for BK and JC virus associated diseases
Through our ongoing collaboration with QIMR Berghofer, we recently developed a new T-cell immunotherapy product candidate, ATA621, for BK and JC virus associated diseases. These two viruses are closely related and there are no available antiviral agents approved for use in BK or JC associated diseases. JC virus is associated with progressive multifocal leukoencephalopathy, or PML, which occurs in transplant, HIV and cancer patients as well as in patients treated with other immunosuppressive therapies, including certain therapies utilized for the treatment of MS. Based on our market research, we estimate that there are approximately 7,800 cases of PML annually, worldwide. BK virus is associated with hemorrhagic cystitis, or BKVHC, which mainly occurs following HCT or cyclophosphamide treatment as well as BK virus associated nephropathy, or BKVAN, which is a disease most commonly associated with kidney transplant. Based on Atara market research, we estimate that there are approximately 2,100 cases of BKVAN and 2,300 cases of BKVHC annually, worldwide. We are currently conducting investigational new drug application enabling manufacturing process development and plan to initiate a Phase 1 trial with ATA621 in 2019.
Our pipeline of product candidates is highlighted in the figure below.

Additional Platform Expansion Activities
We believe our T-cell technology platform will have utility beyond the current set of targets to which it has been directed. We expectcontinue to further research and developevaluate additional cellular therapies, which may include T-cell programs targeted against other antigens as well as engineered T-cell immunotherapies such as chimeric antigen receptor, or CAR-T, cell programs. We believe that viral antigens are well suited to adoptive immunotherapy given that peopleproduct candidates, including those derived from collaborations with normal immune systems are able to mount robust responses to these viral targets, but immunocompromised patients and some cancer patients are not.our partners. We also intendcontinue to evaluate opportunities to license or acquire additional product candidates or technologies to enhance our existing T-cell technology platform.
Competition
The biotechnology and pharmaceutical industries are characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary products. While we believe that our innovative technology, knowledge, experience and scientific resources provide us with competitive advantages, weWe face potential competition from many different sources, including major pharmaceutical, specialtynumerous pharmaceutical and biotechnology companies,enterprises, as well as from academic institutions, government agencies and private and public and private research institutions.institutions for our current product candidates. Some of these competitors or potential competitors may have a moresignificantly greater established presencepresences in the market, financial resources, varied technologies, scientific tools and significantly greater financial, technical and human resourcesexpertise than we have.do. Our commercial opportunityopportunities will be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer side effects or are less expensive than any products that we may develop.
T-Cell Product Candidates
Should any of our T-cell product candidates be approved for use, we will face substantial competition. In addition to the current standard of care for patients, commercial and academic clinical trialsstudies are being pursued by a number of parties in the field of immunotherapy. Early results from these trialsstudies have fueled continued interest in T-cell immunotherapy. In addition, if approved, our T-cell programs would compete with currently marketed drugs and therapies used for treatment of the following indications we are addressing, and potentially with drugproduct candidates currently in development for the same indications.
EBV+ PTLD
There are currently no FDA or EMA approvedFDA-approved products for the treatment of EBV+ PTLD.PTLD, and there are no EC-approved products for this indication except for Ebvallo. However, we are aware that some approvedmarketed products and therapies are currently used off-label in this setting. Companiesby some healthcare professionals and academic institutions that may license therapies to companies in the future are or may be developing new treatments. These therapies, as well as promotional efforts by competitors and clinical trial results of competitive products, could diminish the ability to market and sell tabelecleucel. The current treatment for EBV+ PTLD involves administration of rituximab as a single agent or in the SOT setting, in combination with chemotherapy regimens. Additionally, companies and academic institutions are developing drug candidates for EBV+ PTLD and other EBV associated diseases, including Cell Medica Ltd., or Cell Medica, which is conducting Phase 1 clinical trials for baltaleucel-T, an autologous EBV specific T-cell therapy in post-transplant lymphoproliferative disorder and Viracta Therapeutics, Inc., or Viracta, which has initiated Phase 1b/2 clinical trials for VRx-3996 in relapsed/refractory EBV+lymphomas.
Multiple Sclerosis
Competition in the MS market is high with fourteen therapies approved for the treatment of RRMS in the United States and European Union. There are many U.S. and international competitors in the RRMS market, including major multi-national fully-integrated pharmaceutical companies and established biotechnology companies. Most recently, Ocrevus®, marketed by F. Hoffmann-La Roche, was approved for the treatment of relapsing MS in the United States and European Union. There are numerous other development candidates in Phase 3 trials for RRMS including Novartis’ anti-CD20 monoclonal antibody ofatumumab; Alkermes’ monomethyl fumarate prodrug ALKS 8700; Teva’s laquinimod, and Actelion’s next-generation sphingosine 1-phosphate receptor, or S1PR, agonist ponesimod. Celgene recently reported positive results from Phase 3 clinical trials evaluating ozamimod, a S1PR agonist, in relapsing MS.
Only three therapies have been approved for the treatment of progressive MS. Recently, Ocrevus® was approved in the United States and European Union for the treatment of PPMS. Extavia® (marketed by Novartis) is approved in the United States and European Union for the treatment of SPMS. Betaseron® (marketed by Bayer AG) is also approved in the European Union. Few therapies have been approved for progressive MS because many candidates have failed during clinical trial testing. In the United States, there is one drug (mitoxantrone) approved to treat SPMS.
The SPMS and PPMS markets have active development pipelines and additional novel agents could be approved in the future. Several development candidates are being evaluated in clinical trials including a number of Phase 3 programs: MedDay SA is evaluating MD-1003, a concentrated form of biotin, for progressive MS; AB Science is evaluating masitinib, a tyrosine kinase inhibitor, for progressive MS; and Novartis is evaluating siponimod, an S1P1 modulator, for SPMS; and Actelion Pharmaceuticals is evaluating ponesimod, for SPMS.
Multiple Myeloma including Plasma Cell Leukemia
Several products are approved for the treatment of relapsed or refractory multiple myeloma, or MM, including immunomodulatory drugs, or IMIDs,EBV+ PTLD, such as Thalomid® (Celgene Corporation), Revlimid® (Celgene Corporation)rituximab and Pomalyst® (Celgene Corporation); monoclonal antibodies such as Darzalex® (Janssen Research & Development, LLC) and Empliciti® (Bristol Myers Squibb); and proteasome inhibitors such as Velcade® (Millennium Pharmaceuticals, Inc.) and Kyprolis® (Amgen Inc.).
A number of companies and institutions are pursuing development programs for relapsed or refractory MM. These development programs include drug candidates being evaluated in clinical trials as a monotherapy or in combination with other approved agents. chemotherapy regimens.
In addition, many groups are developing novel T-cell therapies such as autologous CAR T-cell or autologous TCR T-cell candidates. These include bluebird bio, Inc., which is conducting Phase 2 clinical trials testing bb2121, an anti-BCMA CART; Gilead Sciences, Inc., which is testing KTE-585, an anti-BCMA CART in Phase 1 clinical trials; Juno Therapeutics, which is testing an anti-BCMA CART in Phase 1 clinical trials; Autolus Limited, which is testing AUTO-2, a bi-specific anti-BCMA/TACI CART in Phase 1 clinical trials and Adaptimmune Therapeutics PLC, which is testing an anti-NY-ESO TCR in Phase 1/2 clinical trials.
CMV Infection
There are numerous approved products and therapies for the treatment of CMV infection, and a number of companies and academic institutions that may license therapies to companies in the future are or may be developing new treatments for CMV infection. These therapies, as well as promotional efforts by competitors and clinical trial results of competitive products, could significantly diminish any ability to market and sell the CMV T-cell immunotherapy. Drug therapies approved or commonly used for CMV infection include antiviral compounds such as Prevymis®, marketed by Merck & Co. Inc.; ganciclovir, valganciclovir, cidofovir or foscarnet.
Additionally, a number of companies and academic institutions are developing drugproduct candidates for CMV infectionEBV+ PTLD and other CMV-associatedEBV-driven diseases including Shire Plcincluding: Viracta Therapeutics, Inc., which is conducting a pivotal, Phase 2 clinical study for nanatinostat (formerly named tractinostat, or VRx-3996) in combination with antiviral drug valganciclovir in relapsed/refractory EBV+ lymphomas; AlloVir (formerly known as ViraCyte), which has completed a Phase 2 clinical study for posoleucel (ALVR105), an allogeneic, multi-virus T-cell product that targets six viruses in allogeneic HSCT recipients with ≥1 treatment-refractory infection, including EBV, and is conducting two Phase 3 clinical trials of maribavir,for Virus-Associated Hemorrhagic-Cystitis, as well as a UL97 protein kinase inhibitor; Vical Inc., recently announced ASP0113, a therapeutic bivalent plasma DNA CMV vaccine being evaluated in patients undergoing an allogeneic stem cell transplant, failed to meet primary or secondary endpoints in Phase 3 trial for the prevention of BKV, CMV, AdV, EBV, HHV06 and JCV in post-allogeneic HSCT patients.
CAR T Program
There are currently six autologous CAR T therapies approved in the U.S. and/or EU: Novartis’ Kymriah® (tisagenlecleucel), Gilead/Kite’s Yescarta® (axicabtagene ciloleucel) and TecartusTM (brexucabtagene autoleucel) and Bristol-Myers Squibb’s Breyanzi® (lisocabtagene maraleucel) and Abecma (idecabtagene vicleucel) with 2seventy bio and Johnson & Johnson and Legend Biotech’s Carykti™ (ciltacabtagene autoleucel). There are many CAR-mediated cell therapies in development, and, although the majority are autologous, they also include allogeneic and off-the-shelf cell therapies. There are multiple allogeneic CAR platforms being developed with differences in approaches to minimize instances of donor cells recognizing the patient’s body as foreign or rejection of the donor cells by the patient’s body. These approaches include the use of gene-editing to remove or inhibit the TCR and the use of cell types without a TCR. The majority of clinical trials.stage allogeneic CAR programs utilize alpha beta T cells as the cell type and gene editing of the T-cell receptor and HLA as the preferred technology approach, however, other strategies are also in development. It is possible that some of these other approaches will have more favorable characteristics than the approach we utilize, which would result in them being favored by potential partners or customers over our products. Depending on the diseases (such as autoimmune diseases) that we target in the future, we may face competition from both autologous and allogeneic CAR therapies and other modalities (e.g., small molecules, antibodies, bispecifics) in the indication of interest.
Terms of Certain License and Collaboration Agreements
Out-licensing
Pierre Fabre Commercialization Agreement
In October 2021, we entered into the Pierre Fabre Commercialization Agreement, pursuant to which we granted to Pierre Fabre an exclusive, field-limited license to commercialize and distribute Ebvallo in Europe and select emerging markets in the Initial Territory following regulatory approval. In September 2022, we amended the Pierre Fabre Commercialization Agreement and received an additional $30 million milestone payment from Pierre Fabre following EC approval of Ebvallo for EBV+ PTLD and subsequent filing of the MAA transfer to Pierre Fabre, in exchange for, among other things, a reduction in: (i) royalties we are eligible to receive as a percentage of net sales of Ebvallo in the Initial Territory, and (ii) the supply price mark up on Ebvallo purchased by Pierre Fabre. Additionally, we agreed to extend the time period for provision of certain services to Pierre Fabre under the Pierre Fabre Commercialization Agreement. In December 2022, we sold a portion of our right to receive royalties and certain milestones in Ebvallo under the Pierre Fabre Commercialization Agreement to HCRx for a total investment amount of $31.0 million, subject to a repayment cap between 185% and 250% of the total investment amount by HCRx. See Note 6 – “Liability Related to the Sale of Future Revenues” in the Notes to Consolidated Financial Statements, included in Item 8. Financial Statement and Supplementary Data of this report for further information related to the agreement with HCRx.
In October 2023, we entered into an A&R Commercialization Agreement, pursuant to which we expanded Pierre Fabre’s exclusive rights to research, develop, manufacture, commercialize and distribute Ebvallo to include the Additional Territory in addition to the Initial Territory, subject to our performance of certain obligations as described below.
During the applicable period specified in the A&R Commercialization Agreement, we will be responsible, at Pierre Fabre’s cost, to continue conducting the ongoing Phase 3 ALLELE clinical study and the Phase 2 multi-cohort clinical study. We will also be responsible, at Pierre Fabre’s cost, for certain other activities directed to obtaining regulatory approval in the United States for tab-cel for EBV-associated post-transplant lymphoproliferative disease pursuant to the terms of the A&R Commercialization Agreement. Pierre Fabre will be responsible, at its cost, for obtaining and maintaining all other required regulatory approvals and for commercialization and distribution of tab-cel in the Additional Territory, including conducting any other clinical study required. We will own any intellectual property rights developed solely by us under the Agreement.
As part of the Pierre Fabre Commercialization Agreement, we formed a joint steering committee (JSC) with Pierre Fabre that provides oversight, decision making and implementation guidance regarding the commercialization activities, the responsibilities of which has been expanded to cover the incremental scope of the A&R Commercialization Agreement.
Pierre Fabre paid us an upfront cash payment of $45.0 million for the exclusive license grant for the Initial Territory in the fourth quarter of 2021. In December 2022, we met the contractual right to receive $40.0 million in milestone payments upon certain regulatory milestones, for which the cash was received in January 2023. Subject to the terms of the royalty purchase agreement with HCRx, as described in Note 6 – “Liability Related to the Sale of Future Revenues” in the Notes to Consolidated Financial Statements, included in Item 8. Financial Statements and Supplementary Data of this report, we are entitled to receive an aggregate of up to $308.0 million in remaining milestone payments upon achieving certain regulatory and commercial milestones in addition to double-digit tiered royalties as a percentage of net sales of Ebvallo in the Initial Territory, until the later of 12 years after the first commercial sale in such country, the expiration of specified patent rights, or the expiration of all regulatory exclusivity for such product on a country-by-country basis. In December 2023, upon the effective date of the A&R Commercialization Agreement, we met the contractual right to receive an additional upfront cash payment of $20.0 million for the expanded exclusive license grant, for which the cash was received in January 2024. We will also be entitled to receive an aggregate of up to $620.0 million in additional milestone payments upon achieving certain regulatory and commercial milestones relating to tab-cel in the Additional Territory, including up to $100.0 million in potential regulatory milestones through BLA approval. Of the $100.0 million in potential regulatory milestones, we expect to receive $20.0 million in April 2024 based on the positive pre-BLA meeting, an additional $20.0 million in connection with BLA acceptance, and up to $60.0 million in potential regulatory milestones in connection with BLA approval. In addition, Helocyte, Inc., is conducting two Phase 2 clinical trialswe will be eligible to receive significant double-digit tiered royalties as a percentage of net sales of tab-cel (Ebvallo) in the Additional Territory until the later of 12 years after the first commercial sale in such country, the expiration of specified patent rights in such country, or the expiration of all regulatory exclusivity for such Product in such country.
We have entered into a separate manufacturing and supply agreement with Pierre Fabre for us to manufacture Ebvallo for Pierre Fabre to use in the Initial Territory based on a fixed price through December 31, 2023 and at a price equal to cost plus a margin for orders placed after December 31, 2023, subject to a maximum annual increase. Prior to the transfer of manufacturing responsibility to Pierre Fabre, we will be responsible for manufacturing and supplying tab-cel to Pierre Fabre for commercialization in the Territory. Upon the Manufacturing Transition Date and through the remainder of the term of the A&R Commercialization Agreement, Pierre Fabre will be responsible, at its cost, for the manufacture and supply of tab-cel in the Territory. Without transfer of the manufacturing technology, no other party can manufacture tab-cel.
We are also responsible for cell selection services in the Initial Territory at our cost for a CMV MVA-vaccinecertain period of time, unless the parties agree to transfer the related cell selection technology to Pierre Fabre prior to this date, and we are responsible for cell selection services at the sole expense of Pierre Fabre for a CMV peptide vaccinecertain period of time in patients undergoing an allogeneic hematopoietic stemthe Additional Territory. After this period of time, if we agree to continue to provide cell transplant;Merckselection services in the Territory, it shall be at the sole expense of Pierre Fabre. Cell selection is conducting Phase 1 clinical trialsthe process of identifying the appropriate cell line from available inventory to be used for V160, a CMV DNA vaccine; VBI Vaccines Inc., has completed Phase 1 clinical trials for VBI-1501A, an eVLP vaccine; Hookipa Biotech, is conducting Phase 1 clinical trials for HB101, a bivalent vaccine, ViraCyte, is conducting Phase 1 clinical trials for Viralym-C, a CMV-specific allogeneicpatient. Without transfer of the cell therapy product; Fate Therapeutics is conducting a Phase 1/2 clinical trial for ProTmune, a small molecule programmed mobilized peripheral blood graft; Chimerix is conducting Phase 1 clinical trials for intravenous Brincidofovir (BCV IV), a nucleotide analog and Moderna Therapeutics is conducting Phase 1 clinical trials for mRNA-1647, an mRNA based prophylactic vaccine.selection technology, no other party can provide such services.
LicenseIn-licensing
MSK Agreements
MSK Option and License Agreement
In September 2014,June 2015, we entered into an exclusive optionlicense agreement with MSK under which we acquired the right to exclusively license from MSK the worldwide rights tofor three clinical stage T-cell programs. The initial option period was for 12 months. In exchange for the exclusive option, we paid MSK $1.25 million in cash and issued 59,761 shares of our common stocktherapies. We are required to MSK. We and MSK also agreed to collaborate on further research to develop additional cellular therapies, which may include T-cell programs targeted against other antigens and/or CAR-T, and which we also would hold an option to license, if developed.
In June 2015, we exercised the option and entered into a license agreement with MSK. Under the terms of the license agreement, MSK granted us a worldwide, exclusive license under certain patent rights, know-how and a library of T-cells and cell lines, to research, develop, manufacture and commercialize T-cell products specific to CMV, EBV or WT1 that comprise or are based on or made using such licensed rights. MSK also agreed to transfer certain INDs related to the licensed products to us. We have agreed to use commercially reasonable efforts to commercialize the licensed products and, if commercialized, continue active marketing efforts for any commercialized licensed product through the term of the license agreement.
In connection with the option exercise and the execution of the license agreement, we made an upfront cash paymentmake payments to MSK of $4.5 million. We are obligated to make additional milestone payments of up to $33.0 million with respect to the three licensed clinical stage T-cell programs based on achievement of specified development, regulatory and sales-related milestones. We are also required to make escalating mid to high single-digitmilestones, as well as mid-single-digit percentage tiered royalty payments to MSK based on future sales of anyproducts resulting from the development of the licensed products.product candidates, if any. In addition, under certain circumstances, we mustare required to make certain minimum annual royalty payments to MSK, which are creditable against earned royalties owed for the same annual period. We are also obligatedrequired to pay a low double-digit percentage of any consideration we receive for sublicensing the licensed rights.
The license agreement expires for each licensed T-cell product on a licensed product-by-licensed product basisproduct-by-product and a country-by-country basis on the latest of: (i) expiration of the last licensed patent rights related to sucheach licensed product, in such country, (ii) expiration of any market exclusivity period granted by law with respect to sucheach licensed product, in such country, and (iii) a specified number of years after the first commercial sale of the licensed product in sucheach country. Upon expiration of the license agreement, we will retain non-exclusive rights to the licensed products.
In May and December 2018, we licensed additional technology from MSK. We are obligated to make additional milestone payments based on achievement of specified development, regulatory and sales-related milestones, as well as mid-single-digit percentage tiered royalty payments based on future sales of products resulting from the development of the licensed product candidates, if any.
In March 2021, we amended and restated our license agreement with MSK to terminate our license to certain rights and license additional know-how rights not otherwise covered by our existing agreements.
In February 2024, we notified MSK that we intend to terminate our licenses granted to us will become non-exclusive royalty-free, perpetualthe ATA2271 and irrevocable. MSK may terminateATA3271 programs targeting mesothelin.
QIMR Berghofer Agreements
In October 2015, we entered into an exclusive license agreement and a research and development collaboration agreement with QIMR Berghofer. Under the terms of the license agreement, if we materially breachobtained an exclusive, worldwide license to develop and commercialize allogeneic T-cell therapy programs utilizing technology and know-how developed by QIMR Berghofer. In September 2016, the exclusive license agreement and does not cure such breach within a specified period orresearch and development collaboration agreement were amended and restated. Under the amended and restated agreements, we obtained an exclusive and worldwide license to develop and commercialize additional T-cell programs, as well as the option to license additional technology in June 2018. We further amended and restated our license agreement and research and development collaboration agreements with QIMR Berghofer in August 2019 to terminate our license to certain rights related to cytomegalovirus (CMV). In addition, we further amended and restated our license agreement and research and development collaboration agreements with QIMR Berghofer in August 2020 to terminate our license to certain rights related to BK polyomavirus and JC polyomavirus. In December 2021, we further amended and restated our license agreement and research and development collaboration agreements with QIMR Berghofer to terminate our license to certain rights related to HPV associated cancers. We refer to our December 2021 fourth amended and restated license agreement with QIMR Berghofer as the QIMR License Agreement and our December 2021 fourth amended and restated research and development collaboration agreement with QIMR Berghofer as our QIMR Collaboration Agreement.
The QIMR License Agreement provides for various milestone and low to mid single-digit royalty payments to QIMR Berghofer based on future product sales, if any. Under the terms of the QIMR Collaboration Agreement, we experienceare required to reimburse the cost of agreed-upon development activities related to programs developed under the collaboration. The QIMR Collaboration Agreement also provides for various milestone payments to QIMR Berghofer based on the achievement of certain insolvency events.developmental and regulatory milestones.
Intellectual Property
Patents
Our commercial success depends in part on our ability to obtain and maintain proprietary protection for our product candidates, to operate without infringing on the proprietary rights of others and to prevent others from infringing our proprietary rights. Our policy is toWe seek to protect our proprietary position by, among other methods, filing U.S. and non-U.S. patent applications and other regulatory filings related to our proprietary technology, inventions and improvements that are important to the development and implementation of our business. We also rely on trademarks, trade secrets, know-how, continuing technological innovation and potential in-licensing opportunities to develop and maintain our proprietary position. Some of patents, trademarks, trade secrets, know-how and other intellectual property rights we rely on are owned by us and others are in-licensed from our partners. When we refer to “our” technologies, inventions, patents, patent applications or other intellectual property rights, we are referring to both the rights that we own or possess as well as those that we in-license. Additionally, we expect to benefit from a variety of statutory frameworks in the United States,U.S., Europe and other countries that relate to the regulation of biosimilar molecules and orphan drug status. These statutory frameworks provide certain periods of regulatory exclusivity for qualifying molecules. See “Government Regulation.”
Patents
We seek composition-of-matter and/or associated method patents, including method-of-treatment patents, for each of our product candidates in key therapeutic areas. The United StatesU.S. patent system permits the filing of provisional and non-provisional patent applications. A provisional patent application is not examined for patentability by the USPTOU.S. Patent and Trademark Office (USPTO), and automatically expires 12
months after its filing date. As a result, a provisional patent application cannot mature into an issued patent. Provisional patent applications are often used, among other things, to establish an early effective filing date for a later-filed non-provisional patent application. A non-provisional patent application is examined by the USPTO and can mature into a patent once the USPTO determines that the claimed invention meets the standards of patentability.
Individual patents extend for varying periods of time depending on the date of filing of the patent application, the priority date claimed, and the legal term of patents as determined by the applicable law in the countries in which those patents are obtained. Generally, patents issued from applications filed in the United StatesU.S. are effective for 20 years from the earliest non-provisional filing date. In addition, in certain instances, a patent term can be extended to recapture a portion of the term effectively lost as a result of the FDA regulatory review period; however, the restoration period cannot be longer than five years and the total patent term including the restoration period must not exceed 14 years following FDA approval. Additionally, patent term adjustments can extend term to account for certain delays by the U.S. Patent and Trademark Office, or USPTO during prosecution before that office. The duration of non-U.S. patents varies in accordance with provisions of applicable local law, but typically, the life of a non-U.S. patent is 20 years from the earliest international filing date, not inclusive of any patent term extension that may be available. The actual protection afforded by a patent varies on a product-by-product basis, from country to country and depends upon many factors, including the type of patent, the scope of its coverage, the
availability of extensions of patent term, the availability of legal remedies in a particular country and the validity and enforceability of the patent.
National and international patent laws concerning protein-based biologics such as our products remain highly unsettled. No consistent policy regarding the patent-eligibility or the breadth of claims allowed in such patents in this field has emerged to date among the United States,U.S., Europe andor other countries. Changes in either the patent laws or in interpretations of patent laws in the United States andU.S. or other countries can diminish our ability to protect our inventions and enforce our intellectual property rights. Accordingly, we cannot predict the breadth or enforceability of claims that may be granted in our patents or in third-partythird party patents. The biotechnology and pharmaceutical industries are characterized by extensive intellectual property litigation. Our ability to maintain and solidify our proprietary position for our product candidates and technology will depend on our success in obtaining effective claims for any patentour patents and enforcing those claims once a patent is granted. We do not know whether any of theour patent applications that we may file or license from third parties will result in the issuance of any patents. TheOur issued patents that we own or may receive in the future may be challenged, invalidated or circumvented, and the rights granted under any issued patents may not provide us with sufficient protection or competitive advantages against competitors with similar technology. Furthermore, our competitors may independently develop and commercialize similar drugs or duplicate our technology, business model or strategy without infringing our patents. Because of the extensive time required for clinical development and regulatory review of any drug we may develop from our product candidates, it is possible that, before any of our drugs can be commercialized, any related patent may expire or remain in force for only a short period following commercialization, thereby reducing any advantage of any such patent. The patent positions for our product candidates are summarized below:
Our global patent estate consists of both issued patentssolely-owned and pending patent applications and includes wholly-owned patent applications, in-licensed patents and patent applications, to which we generally hold exclusive commercial rights, and patents and patent applications to which we hold an exclusive option to license commercial rights. Regarding our tabelecleucel for EBV+PTLD following HCT or SOT, tabelecleucel for NPC, ATA188, ATA190, ATA520, ATA230 and ATA621 programs, and our platform technologies underlying these programs, our global patent estate is directed to compositions of matter and/or associated methods, including methods of treatment, and consists of 2519 patent families having a total of 95more than 230 issued patents or patent applications. The issuedOur patents and patent applications (if issued) of our global patent estate are expected to expire between 20232024 and 2038,2044, not inclusive of any patent term extension that may be available isin any associated jurisdiction.
Trade Secrets
In addition to patents, we rely upon unpatented trade secrets and know-how and continuing technological innovation to develop and maintain our competitive position. We seek to protect our proprietary information, in part, using confidentiality agreements with our commercial partners, collaborators, employees and consultants and invention assignment agreements with our employees. These agreements are designed to protect our proprietary information and, in the case of the invention assignment agreements, to grant us ownership of technologies that are developed by an employee. These agreements may be breached, and we may not have adequate remedies for any such breach or any unauthorized disclosure of our proprietary information. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. To the extent that our commercial partners, collaborators, employees and consultants use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions.
Government Regulation
OverviewTrademarks
We also rely upon trademarks to develop and maintain our competitive position, and we continue to pursue and obtain trademark rights relating to our business. We have a vigorous global program of U.S. Government Regulation
The preclinical studiestrademark registration and clinical testing, manufacture, labeling, storage, recordkeeping, advertising, promotion, export, marketingenforcement to maintain and sales, among other things,strengthen the value of our product candidatestrademarks and prevent the unauthorized use of those trademarks. Our global trademark portfolio consists of six different trademark families comprised of more than 70 registrations and pending applications.
Government Regulation and Product Approval
As a biopharmaceutical company that operates in the United States, we are subject to extensive regulation by governmentalregulation. Our T-cell immunotherapies, if approved, will be products regulated as biological products, or biologics. With this classification, commercial production of our products will need to occur in registered facilities in compliance with current good manufacturing practice (cGMP) for biologics. The FDA categorizes human cell- or tissue-based products as either minimally manipulated or more than minimally manipulated and has determined that more than minimally manipulated products require clinical trials to demonstrate product safety and efficacy and the submission of a BLA for marketing authorization. Our product candidates are considered more than minimally manipulated and will require evaluation in clinical trials and the submission and approval of a BLA before we can market them.
Government authorities in the United States (at the federal, state and local level) and in other countries. Incountries extensively regulate, among other things, the United States, pharmaceuticalresearch, development, testing, manufacturing, quality control, approval, labeling, packaging, storage, record-keeping, promotion, advertising, distribution, tracking and tracing, post-approval monitoring and reporting, marketing and export and import of biopharmaceutical products such as those we are regulateddeveloping. Our product candidates must be approved by the FDA before they may be legally marketed in the U.S. and by the appropriate foreign regulatory agency before they may be legally marketed in foreign countries. Generally, our activities in other countries will be subject to regulation that is similar in nature and scope as that
imposed in the U.S., although there can be important differences. Additionally, some significant aspects of regulation in Europe are addressed in a centralized way, but country-specific regulation remains essential in many respects. The process for obtaining regulatory marketing approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations require the expenditure of substantial time and financial resources.
U.S. Product Development Process
In the U.S., the FDA regulates pharmaceutical and biological products under the Federal Food, Drug and Cosmetic Act and other laws, including, in the case of biologics,(FDCA), the Public Health Service Act.
Our T-cell immunotherapy product candidates, including tabelecleucel (formerly known as ATA129)Act (PHSA), are regulated byand their implementing regulations. The process of obtaining regulatory approvals and the FDA as biologics, reviewed by the Center for Biological Evaluationsubsequent compliance with appropriate federal, state, local and Research,foreign statutes and willregulations require the submissionexpenditure of BLAssubstantial time and approval by the FDA prior to being marketed in the United States. For T-cell immunotherapy trials conducted at, or sponsored by, institutions receiving NIH funding for recombinant DNA research, prior to the submission of an IND to the FDA, a protocol and related documents must be submitted to, and the study registered with, the NIH Office of Biotechnology Activities, or the OBA, pursuant to the NIH Guidelines for Research Involving Recombinant DNA Molecules, or the NIH Guidelines. Compliance with the NIH Guidelines is mandatory for investigators at institutions receiving NIH funds for research involving recombinant DNA. However, many companies and other institutions, not otherwise subject to the NIH Guidelines, voluntarily follow them. The NIH is responsible for convening the recombinant DNA advisory committee, or RAC, that discusses protocols that raise novel or particularly important scientific, safety or ethical considerations at one of its quarterly public meetings. The OBA will notify the FDA of the RAC’s decision regarding the necessity for full public review of a protocol. RAC proceedings and reports are posted to the OBA website and may be accessed by the public.
financial resources. Failure to comply with FDAthe applicable U.S. requirements both before andat any time during the product development process, approval process or after product approval, may subject us or our partners, contract manufacturers, and suppliersan applicant to administrative or judicial sanctions. FDA sanctions including FDAcould include, among other actions, refusal to approve pending applications, withdrawal of an approval, a clinical hold, warning or other enforcement letters, product recalls or withdrawals from the market, product seizures, total or partial suspension of production or distribution injunctions, fines, and/refusals of government contracts, restitution, disgorgement or civil or criminal prosecution.
penalties. Any agency or judicial enforcement action could have a material adverse effect on us. The stepsprocess required by the FDA before a biologicbiological product may be approved for marketing of an indicationmarketed in the United StatesU.S. generally include:involves the following:
•completion of preclinicalnonclinical laboratory tests and animal studies and formulation studies conducted according to good laboratory practices (GLPs), and applicable requirements for the humane use of laboratory animals or GLP, and other applicable regulations;
•submission to the FDA of an investigational new drug, or IND, application, which must become effective before human clinical trials may commence;
begin; •completionapproval by an independent Institutional Review Board (IRB), or ethics committee at each clinical site before the trial is commenced at such clinical site;
•performance of adequate and well-controlled human clinical trials in accordance withaccording to the FDA’s regulations commonly referred to as good clinical practices or GCP,(GCPs), and any additional requirements for the protection of human research patients and their health information, to establish that the biological product is “safe, pure and potent”, which is analogous to the safety and efficacy approval standard for a chemical drugof the proposed biological product for its intended use;
•submission to the FDA of a BLA;
BLA for marketing approval that includes substantial evidence of safety, purity, and potency of the drug from analytical (CMC) studies and from results of nonclinical testing and clinical trials; •satisfactory completion of an FDA preapprovalAdvisory Committee review, if applicable;
•satisfactory completion of an FDA inspection of the manufacturing facility or facilities at whichwhere the biological product is produced and tested to assess compliance with cGMP; to assure that the facilities, methods and controls are adequate to preserve the biological product’s identity, strength, quality and purity and; if applicable, current good manufacturing practices, or cGMP and in the case of our T-cell immunotherapy product candidates,FDA’s current good tissue practices or GTP;(GTPs) for the use of human cellular and tissue products;
•potential FDA inspection of the nonclinical study and clinical trial sites that generated the data in support of the BLA; and
•FDA review and approval, i.e., licensure of the BLA and issuanceproduct candidate that is the subject of a biologics license.the BLA.
Before conducting studiestesting any biological product candidate, including our product candidates, in humans, the product candidate enters the preclinical testing stage. Preclinical tests, also referred to as nonclinical studies, include laboratory evaluationevaluations of product chemistry, toxicity and formulation, as well as animal studies to assess the potential safety and efficacyactivity of the biologic candidateproduct candidate. The conduct of the preclinical tests must be conducted. Preclinical toxicology studies in animalscomply with federal regulations and requirements including applicable GLPs. The clinical trial sponsor must be conducted in compliance with FDA regulations. Thesubmit the results of the preclinical tests, together with manufacturing information, and analytical data, are submittedany available clinical data or literature and a proposed clinical trial protocol, to the FDA as part of anthe IND. Some preclinical testing may continue even after the IND is submitted. In addition to including the results of the preclinical testing, the IND will also include a protocol detailing, among other things, the objectives of the clinical trial, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated if the first phase or phases of the clinical trial lend themselves to an efficacy determination. The IND will automatically becomebecomes effective 30 days after receipt by the FDA, unless the FDA raises concerns or questions regarding the proposed clinical trials and places the trial on a clinical hold within thethat 30-day time period placesperiod. In such a case, the IND on clinical hold because of safety concerns about the product candidate or the conduct of the trial described in the clinical protocol included in the IND. The IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. The FDA may also impose clinical holds on a biological product candidate at any time before or during clinical trials can proceed.
Alldue to unacceptable and significant risks to clinical trial subjects or non-compliance with FDA requirements. If the FDA imposes a clinical hold, trials may not begin, continue or recommence in the U.S. without FDA authorization and then only under terms authorized by the FDA. Accordingly, we cannot be sure that submission of an IND will result in the FDA allowing clinical trials for new drugs and biologics must be conductedto begin, or that, once begun, issues will not arise that suspend or terminate the conduct of such trials in the U.S.
Clinical trials involve the administration of the biological product candidate to patients under the supervision of onequalified investigators, generally physicians not employed by or more qualified principal investigators in accordance with GCP. They must beunder the trial sponsor’s control. Clinical trials are conducted under protocols detailing, among other things, the objectives of the applicable phase of theclinical trial, dosing procedures, research subject selectioninclusion and exclusion criteria, and the safety and effectiveness criteriaparameters to be evaluated.used to monitor subject safety, including stopping rules that assure a clinical trial will be stopped if certain adverse events should occur. Each protocol and any amendments to the protocol must be submitted to the FDA as part of the IND,IND. Clinical trials must be conducted and monitored in accordance with the FDA’s regulations comprising the GCP requirements, including the requirement that all research patients provide informed consent. Further, the protocol and amendments, which describe the study design, for each clinical trial must be reviewed and approved by an independent IRB at or servicing each institution at which the clinical trial will be conducted. An IRB is charged with protecting the welfare and rights of trial participants and considers such items as whether the risks to individuals participating in the clinical trials are minimized and are reasonable in relation to anticipated benefits. The IRB also approves the form and content of the informed consent document that must be signed by each clinical trial subject or his or her legal representative and must monitor the clinical trial until completed. Some studies also include oversight by an independent group of qualified experts organized by the clinical study sponsor, known as a data safety monitoring board, which provides authorization for whether or not a study may move forward at designated check points based on access to certain data from the study and may halt the clinical trial if it determines that there is an unacceptable safety risk for subjects or other grounds, such as no demonstration of efficacy. There are also requirements governing the reporting of ongoing clinical studies and clinical study results to public registries.
Human clinical trials are typically conducted in three sequential phases that may overlap or be combined:
•Phase 1. The biological product is initially introduced into healthy human subjects and tested for safety. In the case of some products for severe or life-threatening diseases, especially when the product may be too inherently toxic to ethically administer to healthy volunteers, the initial human testing is often conducted in patients rather than healthy human volunteers.
•Phase 2. The biological product is evaluated in a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance, optimal dosage and dosing schedule.
•Phase 3. Clinical trials are undertaken to further evaluate dosage, clinical efficacy, potency, and safety in an expanded patient population at geographically dispersed clinical trial sites. These clinical trials are intended to establish the overall risk to benefit ratio of the product and provide an adequate basis for marketing approval and product labeling.
Post-approval clinical trials, sometimes referred to as Phase 4 clinical trials, may be conducted, and in some cases are required by the FDA, after initial marketing approval. These clinical trials are used to gain additional experience from the treatment of patients in the intended therapeutic indication, particularly for long-term safety follow-up. During all phases of clinical development, regulatory agencies require extensive monitoring and auditing of all clinical activities, clinical data, and clinical trial investigators. Annual progress reports detailing the statusresults of the clinical trials must be submitted to the FDA annually. SponsorsFDA. Written IND safety reports must also reportbe promptly submitted to the FDA within certain timeframes,and to investigators for serious and unexpected adverse reactions,events, findings from other studies that suggest a significant risk in humans exposed to the drug, laboratory animal testing or in vitro testing that suggest a significant risk to human patients, or any clinically important increase in the rate of a serious suspectedexpected adverse reaction over thatthe rate listed in the protocol or
investigator’s brochure, or any findings from other studies or animal or in vitro testing investigator brochure. The sponsor must submit an IND safety report within 15 calendar days after the sponsor determines that suggest a significant risk in humans exposed to the product candidate. An institutional review board, or IRB, at each institution participating in the clinical trial must review and approve the protocol before a clinical trial commences at that institution, approve the information regardingqualifies for reporting. The sponsor also must notify the trial andFDA of any unexpected fatal or life-threatening suspected adverse reaction within seven calendar days after the consent form that must be provided to each research subject or the subject’s legal representative, and monitor the trial until completed.
Clinical trials are typically conducted in three sequential phases, but the phases may overlap and different trials may be initiated with the same product candidate within the same phase of development in similar or differing patient populations.
Phase 1 clinical studies may be conducted in a limited number of patients or healthy volunteers, as appropriate. The product candidate is initially tested for safety and, as appropriate, for absorption, metabolism, distribution, excretion, pharmacodynamics and pharmacokinetics.
Phase 2 usually involves trials in a larger, but still limited, patient population to evaluate preliminarily the efficacysponsor’s initial receipt of the product candidate for specific, targeted indications to determine dosage tolerance and optimal dosage and to identify possible short-term adverse effects and safety risks.
Phase 3 trials are undertaken to further evaluate clinical efficacy of a specific endpoint and to test further for safety within an expanded patient population at geographically dispersed clinical trial sites.information. Phase 1, Phase 2 orand Phase 3 testing mightclinical trials may not be completed successfully within any specific timespecified period, if at all, with respect to any of our product candidates. Results from one trial are not necessarily predictive of results from later trials. Furthermore, theall. The FDA or the sponsor or its data safety monitoring board may suspend or terminate a clinical trialstrial at any time on various grounds, including a finding that the subjects orproduct candidate to which the research patients are being exposed toposes an unacceptable health risk.risk, including risks inferred from other unrelated immunotherapy trials. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the biological product candidate has been associated with unexpected serious harm to patients.
Prior to and concurrently with clinical trials, companies usually complete additional studies on and must also develop additional information about the physical characteristics of the biological product as well as finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements. To help reduce the risk of the introduction of adventitious agents with use of biological products, the FDCA, PHSA and FDA regulations emphasize the importance of manufacturing control for products whose attributes cannot be precisely defined. The manufacturing process must be capable of consistently producing quality batches of the product candidate and, among other things, the sponsor must develop methods for testing the identity, strength, quality, potency and purity of the final biological product. Additionally, appropriate packaging must be selected and tested, and stability studies must be conducted to demonstrate that the biological product candidate does not undergo unacceptable deterioration over its shelf life.
U.S. BLA Review and Approval Processes
After the completion of clinical trials of a biological product, FDA approval of a BLA for an innovator biological product must be obtained before commercial marketing of the biological product. The BLA submission must include results of the preclinicalproduct development, laboratory and animal studies, and clinicalhuman trials, together with other detailed information, including information on the manufacture and composition of the product, are submitted toproposed labeling and other relevant information. The testing and approval processes require substantial time and effort and there can be no assurance that the FDA as part ofwill accept the BLA for filing and, even if filed, that any approval will be granted on a BLA requesting approval to market the product candidate for a proposed indication. timely basis, if at all.
Under the Prescription Drug User Fee Act, as amended (PDUFA), each BLA must be accompanied by a significant user fee. The FDA adjusts the fees payable to the FDA for reviewing a BLA, as well as annualPDUFA user fees for commercial manufacturing establishments andlicensed innovator biological products on an annual basis. PDUFA also imposes an annual program fee for approvedinnovator biological products. Fee waivers or reductions are available in certain circumstances, including a waiver of the application fee for the first application filed by a small business. Additionally, no user fees are assessed on BLAs for innovator biological products can be substantial but are subject to certain limited deferrals, waivers and reductions that may be available. The fees typically increase each year. Each BLA submitted todesignated as orphan drugs, unless the FDA for approval is reviewed for administrative completeness and reviewability withinproduct also includes a non-orphan indication.
Within 60 days following receipt bysubmission of the application, the FDA ofreviews a BLA submission to determine if it is substantially complete before the application. If the BLA is found complete, the FDA will file the BLA, triggering a full review of the application.agency accepts it for filing. The FDA may refuse to file any BLA that it deems incomplete or not properly reviewable at the time of submission.submission and may request additional information. In this event, the BLA must be resubmitted with the additional information. The FDA’s established goalresubmitted application also is subject to review 90% of priority BLA applications within six months afterbefore the applicationFDA accepts it for filing. Once the submission is accepted for filing, and 90% of standard BLA applications within 10 monthsthe FDA begins an in-depth substantive review of the acceptance date, whereupon a review decision is to be made.BLA. The FDA however,reviews the BLA to determine, among other things, whether the proposed product is safe, potent, and/or effective for its intended use, and has an acceptable purity profile, and whether the product is being manufactured in accordance with cGMP to assure and preserve the product’s identity, safety, strength, quality, potency and purity. The FDA may refer applications for novel biological products or biological products that present difficult questions of safety or efficacy to an advisory committee, typically a panel that includes clinicians and other experts, for review, evaluation and a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions. During the biological product approval process, the FDA also determines whether a Risk Evaluation and Mitigation Strategy (REMS) is necessary to assure the safe use of the biological product. A REMS is a safety strategy to manage a known or potential serious risk associated with a medicine and to enable patients to have continued access to such medicines by managing their safe use, and could include medication guides, physician communication plans, or elements to assure safe use, such as restricted distribution methods, patient registries and other risk minimization tools. If the FDA concludes a REMS is needed, the sponsor of the BLA must submit and obtain approval for a proposed REMS. The FDA will not approve a product candidate within these established goals and its review goals are subject to change from time to time. Further, the outcome of the review, evenBLA without a REMS, if generally favorable, may not be an actual approval but a “complete response letter” that describes additional work that must be done before the application can be approved. required.
Before approving a BLA, the FDA maywill inspect the facility or facilities at which the product is manufactured andmanufactured. The FDA will not approve the product unless it determines that the facility compliesmanufacturing processes and facilities comply with cGMP.cGMP requirements and are adequate to assure consistent production of the product within required specifications. For immunotherapy products, the FDA also will not approve the product if the manufacturer is not in compliance with the GTPs, to the extent applicable. GTPs are FDA regulations and guidance documents that govern the methods used in, and the facilities and controls used for, the manufacture of human cells, tissue, and cellular and tissue-based products (HCT/Ps), which are human cells or tissue intended for implantation, transplant, infusion, or transfer into a human recipient. The primary intent of the GTP requirements is to ensure that cell and tissue-based products are manufactured in a manner designed to prevent the introduction, transmission and spread of communicable disease. FDA regulations also require tissue establishments to register and list their HCT/Ps with the FDA and, when applicable, to evaluate donors through screening and testing. Additionally, before approving a BLA, the FDA will typically inspect one or more clinical sites to assure that the clinical trials were conducted in compliance with IND trial requirements and GCP requirements. To assure cGMP, GTP and GCP compliance, an applicant must incur significant expenditure of time, money and effort in the areas of training, record keeping, production, and quality control.
Notwithstanding the submission of relevant data and information, the FDA may deny approval of aultimately decide that the BLA if applicable statutory ordoes not satisfy its regulatory criteria for approval and deny approval. Data obtained from clinical trials are not satisfied,always conclusive and the FDA may interpret data differently than we interpret the same data. If the agency decides not to approve the BLA after its review, the FDA will issue a complete response letter that describes the specific deficiencies in the BLA identified by the FDA. The deficiencies identified may be minor, for example, requiring labeling changes, or may requiremajor, for example, requiring additional testing or information, which can extendclinical trials. Additionally, the review process. FDA approval of any applicationcomplete response letter may include many delaysrecommended actions that the applicant might take to place the application in a condition for approval. If a complete response letter is issued, the applicant may either resubmit the BLA, addressing all of the deficiencies identified in the letter, or never be granted. withdraw the application.
If a product is approved,receives regulatory approval, the approval may impose limitations onbe limited to specific diseases and dosages or the usesindications for use may otherwise be limited, which could restrict the product may be marketed,commercial value of the product. Further, the FDA may require that warning statementscertain contraindications, warnings or precautions be included in the product labeling, may require that additional studies be conducted following approval as a condition of the approval, andlabeling. The FDA may impose restrictions and conditions on product distribution, prescribing, or dispensing in the form of a Risk Evaluation and Mitigation Strategy,risk management plan or REMS, or otherwise limit the scope of any approval. TheIn addition, the FDA must approvemay require post marketing clinical trials, sometimes referred to as Phase 4 clinical trials, designed to further assess a BLA supplement or a new BLA before a productbiological product’s safety and effectiveness, and may be marketed for other uses or before certain manufacturing or other changes may be made. Further post-marketingrequire testing and surveillance programs to monitor the safety or efficacy of a product is required. Also, product approvals may be withdrawn if compliance with regulatory standards is not maintained or if safety or manufacturing problems occur following initial marketing. approved products that have been commercialized.
In addition, new government requirements maythe Pediatric Research Equity Act (PREA) requires applicants to study certain drugs and biological products in relevant pediatric subpopulations, with the potential of obtaining pediatric labeling for the product, if the drug is found to be established that could delay or prevent regulatory approvalsafe and effective for use in children. With enactment of our product candidates under development.
Under the Biologics Price CompetitionFood and Drug Administration Safety and Innovation Act of 2009,2012, or FDASIA, sponsors must submit an initial pediatric study plan in the BLA. Pediatric study plans must contain an outline of the proposed pediatric study or studies the applicant plans to conduct, including study objectives and design, any deferral or waiver requests, and other information required by regulation and must be agreed upon by the FDA. The FDA or the BPCIA,applicant may request an amendment to the plan at any time.
The FDA may grant deferrals for submission of data or full or partial waivers for pediatric studies, including the study of all pediatric patients or subpopulations based on age on its own initiative or at the request of the applicant. Additional requirements and procedures relating to deferral requests and requests for extension of deferrals are contained in FDASIA. Unless otherwise required by regulation, the pediatric data requirements do not apply to products with orphan designation. For example, Section 505B of the U.S. Food, Drug and Cosmetic Act, as amended by the FDA Reauthorization Act in 2020, requires that any original NDA or BLA submitted on or after August 18, 2020 for a statutory pathwaynew active ingredient contain reports on molecularly targeted pediatric cancer investigations unless the requirement is waived or deferred, if the drug that is the subject of the application is: (1) intended for the treatment of an adult cancer, and (2) directed at a molecular target that the FDA determines to be substantially relevant to the growth or progression of a pediatric cancer. This requirement applies even if the adult cancer indication does not occur in the pediatric population and even if the drug is for an adult indication for which orphan designation has been createdgranted. Therefore, the BLA of any product we develop that is determined to be substantially relevant to the growth or progression of a pediatric cancer, even if the drug has been designated as an orphan drug for licensure,an adult indication, must contain reports on molecularly targeted pediatric cancer investigations unless such investigations are waived or approval, of biological products that are biosimilar to, and possibly interchangeable with, earlier biological products licensed under the Public Health Service Act. Also under the BPCIA, innovator manufacturers of original reference biological products are granted 12 years of exclusivity before biosimilars can be approved for marketingdeferred.
Orphan Drug Designation in the United States. The approval of a biologic product biosimilar to one of our products could have a material adverse impact on our business as it may be significantly less costly to bring to market and may be priced significantly lower than our products.
Both before and after the FDA approves a product, the manufacturer and the holder or holders of the BLA for the product are subject to comprehensive regulatory oversight. For example, quality control and manufacturing procedures must conform, on an ongoing basis, to cGMP and GTP requirements, as applicable and the FDA periodically inspects manufacturing facilities to assess compliance with these standards. Accordingly, manufacturers must continue to spend time, money and effort to maintain compliance.U.S.
Orphan Drug Act
TheUnder the Orphan Drug Act, provides incentivesthe FDA may grant orphan designation to manufacturersa drug or biologic intended to develop and market drugs fortreat a rare diseases and conditions affectingdisease or condition, which is generally a disease or condition that affects fewer than 200,000 personsindividuals in the United States, ator more than 200,000 individuals in the timeU.S. and for which there is no reasonable expectation that the cost of applicationdeveloping and making available in the U.S. a drug or biologic for orphanthis type of disease or condition will be recovered from sales in the U.S. for that drug designation.or biologic. Orphan drug designation must be requested before submitting a BLA. OrphanAfter the FDA grants orphan drug designation, the generic identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. The orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review andor approval process.
If a product that has orphan drug designation subsequently receives the first FDA approval for the disease for which it has such designation, the holder of the approvalproduct is entitled to a seven-year exclusive marketing period in the United States for thatorphan product except in very limited circumstances. For example, a drugexclusivity, which means that the FDA considersmay not approve any other applications, including a full BLA, to be clinically superior to, or different from, another approved orphan drug, even thoughmarket the same biologic for the same indication for seven years, except in limited circumstances, such as a showing of clinical superiority to the product with orphan drug exclusivity. Orphan drug exclusivity does not prevent the FDA from approving a different drug or biologic for the same disease or condition, or the same drug or biologic for a different disease or condition. Among the other benefits of orphan drug designation are tax credits for certain research and a waiver of the BLA application user fee.
A designated orphan drug may also obtain approvalnot receive orphan drug exclusivity if it is approved for a use that is broader than the indication for which it received orphan designation. In addition, exclusive marketing rights in the United States duringU.S. may be lost if the seven-year exclusive marketing period. In addition, holders of exclusivityFDA later determines that the request for orphan drugs are expecteddesignation was materially defective or if the manufacturer is unable to assure the availability of sufficient quantities of their orphan drugsthe product to meet the needs of patients. Failure to do so could resultpatients with the rare disease or condition.
Expedited Development and Review Programs in the withdrawal of marketing exclusivity for the drug.U.S.
Legislation similar to the Orphan Drug Act has been enacted outside the United States, including in the EU. The orphan legislation in the EU is available for therapies addressing chronic debilitating or life-threatening conditions that affect five or fewer out of 10,000 persons or are financially not viable to develop. The market exclusivity period is for ten years, although that period can be reduced to six years if, at the end of the fifth year, available evidence establishes that the product is sufficiently profitable not to justify maintenance of market exclusivity. The market exclusivity may be extended to 12 years if the sponsor completes a pediatric investigation plan agreed upon with the relevant committee of the EMA.
Expedited Review and Approval
The FDA has various programs, including Fast Track and Regenerative Medicine Advanced Therapy, or RMAT, priority review and accelerated approval, which area fast track program that is intended to expedite or simplifyfacilitate the process for developing and reviewing promising drugs, or to provide for the approval of a drug on the basis of a surrogate endpoint. Even if a drug qualifies for one or more of these programs, the FDA may later decidenew products that the drug no longer meets the conditions for qualification or that the time period for FDA review or approval will be shortened. Generally, drugs thatmeet certain criteria. Specifically, new products are eligible for these programsfast track designation if they are those for serious or life-threatening conditions, those with the potential to address unmet medical needs and those that offer meaningful benefits over existing treatments. For example, Fast Track and RMAT are processes designed to facilitate the development and expedite the review of drugs to treat serious or life-threatening diseases or conditions and fill unmet medical needs. Priority review is designed to give drugs that offer major advances in treatment or provide a treatment where no adequate therapy exists an initial review within six months as compared to a standard review time of 10 months. Although Fast Track, RMAT, and priority review do not affect the standards for approval, the FDA will attempt to facilitate early and frequent meetings with a sponsor of a Fast Track designated drug and expedite review of the application for a drug designated for priority review. Accelerated approval provides for an earlier approval for a new drug that is intended to treat a serious or life-threatening disease or condition and that fillsdemonstrate the potential to address an unmet medical need basedfor the disease or condition. Fast track designation applies to the combination of the product and the specific indication for which it is being studied. For a product that
has received fast track designation, the FDA may consider for review sections of the BLA on a rolling basis before the complete application is submitted. A sponsor seeking a rolling submission must provide a schedule for the submission of each section of the BLA, and the FDA must agree to the rolling submission and that the schedule is acceptable. In addition, the sponsor must pay any required user fees upon submission of the first section of the BLA. Submission of sections of a BLA on a rolling basis does not guarantee that the FDA will begin their review of these sections upon receipt or even before the BLA submission is deemed to be complete. Therefore, a rolling BLA submission may not result in a faster timeline to marketing approval. Additionally, a rolling BLA submission has no bearing on whether or not a product candidate is ultimately approved.
Any product, including a product with a fast track designation, may also be eligible for other types of FDA programs intended to expedite development and review, such as priority review and accelerated approval. A product is eligible for priority review if it is intended for treatment of a serious condition and has the potential to provide safe and effective therapy where no satisfactory alternative therapy exists or a significant improvement in the treatment, diagnosis or prevention of a disease compared to marketed products. The FDA will attempt to direct additional resources to the evaluation of an application for a new product designated for priority review in an effort to facilitate the review. The FDA intends to take action on applications under priority review within 6 months of the application filing date compared with 10 months from the filing date for standard review.
Additionally, a product may be eligible for accelerated approval. Products studied for their safety and effectiveness in treating serious or life-threatening diseases or conditions may receive accelerated approval upon a determination that the product has an effect on a surrogate endpoint. A surrogate endpoint that is reasonably likely to predict clinical benefit, or on a laboratory measurementclinical endpoint that can be measured earlier than irreversible morbidity or physical sign used asmortality, that is reasonably likely to predict an indirecteffect on irreversible morbidity or substitute measurement representing a clinically meaningful outcome.mortality or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments. As a condition of approval, the FDA may require that a sponsor of a drug or biological product candidate receiving accelerated approval perform adequate and well-controlled post-marketing clinical trialsstudies to confirm the clinically meaningful outcome as predicted by the surrogate marker trial.
demonstrate clinical benefit. In addition, to the Fast Track,FDA currently requires as a condition for accelerated approval pre-approval of promotional materials, which could adversely impact the timing of the commercial launch of the product.
Regenerative Medicine Advanced Therapy (RMAT) designation was established by FDA in 2017 to facilitate an efficient development program for, and priorityexpedite review programs discussed above, breakthrough therapy designation may be pursued. A breakthrough therapyof, any drug that meets the following criteria: (1) it qualifies as a RMAT, which is defined as a drug thatcell therapy, therapeutic tissue engineering product, human cell and tissue product, or any combination product using such therapies or products, with limited exceptions; (2) it is intended aloneto treat, modify, reverse, or in combination with one or more other drugs, to treatcure a serious or life-threatening disease or condition,condition; and (3) preliminary clinical evidence indicates that the drug has the potential to address unmet medical needs for such a disease or condition. RMAT designation provides potential benefits that include more frequent meetings with FDA to discuss the development plan for the product candidate and eligibility for rolling review and priority review. Products granted RMAT designation may demonstratealso be eligible for accelerated approval on the basis of a surrogate or intermediate endpoint reasonably likely to predict long-term clinical benefit, or reliance upon data obtained from a meaningful number of sites, including through expansion to additional sites. Once approved, when appropriate, the FDA can permit fulfillment of post-approval requirements under accelerated approval through the submission of clinical evidence, clinical studies, patient registries, or other sources of real-world evidence such as electronic health records; through the collection of larger confirmatory datasets; or through post-approval monitoring of all patients treated with the therapy prior to approval.
Breakthrough therapy designation is also intended to expedite the development and review of products that treat serious or life-threatening conditions. The designation by FDA requires preliminary clinical evidence that a product candidate, alone or in combination with other drugs and biologics, demonstrates substantial improvement over existing therapiescurrently available therapy on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. Drugs designated asBreakthrough therapy designation comes with all of the benefits of Fast Track designation, which means that the sponsor may file sections of the BLA for review on a rolling basis if certain conditions are satisfied, including an agreement with FDA on the proposed schedule for submission of portions of the application and the payment of applicable user fees before the FDA may initiate a review.
Fast Track designation, priority review, RMAT and breakthrough therapies are also eligibletherapy designation do not change the standards for accelerated approval.approval but may expedite the development or approval process. The FDA will seekmay revoke any of these designations if the product no longer meets applicable criteria.
Post-Approval Requirements in the U.S.
Any products for which we receive FDA approvals are subject to continuing regulation by the FDA, including, among other things, record-keeping requirements, reporting of adverse experiences with the product, providing the FDA with updated safety and efficacy information, product sampling and distribution requirements, and complying with FDA promotion and advertising requirements, which include, among others, standards for direct-to-consumer advertising, restrictions on promoting products for uses or in patient populations that are not described in the product’s approved uses (known as “off-label use”), limitations on industry-sponsored scientific and educational activities, and requirements for promotional activities involving the internet. Although a physician may prescribe a legally available product for an off-label use, if the physician deems such product to be appropriate in his/her professional medical judgment, a manufacturer may not market or promote off-label uses. However, it is permissible to share in certain circumstances truthful and not misleading information that is consistent with the product’s approved labeling.
In addition, the distribution of prescription drug products, including most biological products that require a prescription, are subject to the Prescription Drug Marketing Act, or the PDMA, which regulates the distribution of drug samples at the federal level, and sets minimum standards for the registration and regulation of drug distributors by the states. Both the PDMA and state laws limit the distribution of prescription drug product samples and impose requirements to ensure accountability in distribution.
Furthermore, quality control and manufacturing procedures must continue to conform to applicable manufacturing requirements after approval to ensure the sponsorlong-term stability of the product. cGMP regulations require among other things, quality control and quality assurance as well as the corresponding maintenance of records and documentation and the obligation to investigate and correct any deviations from cGMP. Manufacturers and other entities involved in the manufacture and distribution of approved products are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP and other laws. Accordingly, manufacturers must continue to expend time, money, and effort in production and quality control to maintain cGMP compliance. Discovery of problems with a product after approval may result in restrictions on a product, manufacturer, or holder of an approved BLA, including, among other things, recall or withdrawal of the product from the market. In addition, changes to the manufacturing process are strictly regulated, and depending on the significance of the change, may require prior FDA approval before being implemented. Other types of changes to the approved product, such as adding new indications and claims, are also subject to further FDA review and approval.
The FDA also may require post-marketing testing, known as Phase 4 testing, and surveillance to monitor the effects of an approved product. Discovery of previously unknown problems with a product or the failure to comply with applicable FDA requirements can have negative consequences, including adverse publicity, judicial or administrative enforcement, warning letters from the FDA, mandated corrective advertising or communications with doctors, and civil or criminal penalties, among others. Newly discovered or developed safety or effectiveness data may require changes to a product’s approved labeling, including the addition of new warnings and contraindications, and also may require the implementation of other risk management measures, for example, a REMS. Also, new government requirements, including those resulting from new legislation, may be established, or the FDA’s policies may change, which could delay or prevent regulatory approval of our products under development.
U.S. Marketing Exclusivity
The Biologics Price Competition and Innovation Act (BPCIA) amended the PHSA to authorize the FDA to approve similar versions of innovative biologics, commonly known as biosimilars. A competitor seeking approval of a breakthrough therapybiosimilar must file an application to establish that its molecule is highly similar to an approved innovator biologic, notwithstanding minor differences in clinically inactive components, and shows no clinically meaningful differences between the biological product candidate receives: intensive guidanceand the reference product in terms of safety, purity, and potency, which can generally be shown through analytical studies, animal studies, and a clinical study or studies. Separately, a product that is biosimilar to the reference product is considered interchangeable if the product demonstrates that it can be expected to produce the same clinical results as the reference product in any given patient and, for products that are administered multiple times to an individual, the interchangeable biosimilar and the reference biological product may be alternated or switched after one has been previously administered without increasing safety risks or risks of diminished efficacy relative to exclusive use of the reference biological product. If a product is shown to be biosimilar or interchangeable with an FDA-approved reference biologic, this can potentially reduce the cost and time required to obtain approval to market the biosimilar or interchangeable product. Complexities associated with the larger, and often more complex, structures of biological products, as well as the processes by which such products are manufactured, pose significant hurdles and have slowed implementation of the BPCIA by the FDA.
The BPCIA, however, bars the submission of BLAs for biosimilars to an approved application until four years after the licensure date for the reference biologic. In addition, the FDA may not approve biosimilar applications for 12 years after an innovator biological product receives initial marketing approval. During this 12-year period of reference product exclusivity, another company may obtain FDA licensure and market a competing version of the reference product if the FDA approves a full BLA for the competing
product containing that applicant’s own preclinical data and data from adequate and well controlled clinical trials to demonstrate the safety, purity and potency of its product. The BPCIA also created certain exclusivity periods for biosimilars approved as interchangeable products. At this juncture, it is unclear whether products deemed “interchangeable” by the FDA will, in fact, be readily substituted by pharmacies, which are governed by state pharmacy law. This 12-year period of data exclusivity may be extended by six months, for a total of 12.5 years, if the FDA requests, and the innovator company completes pediatric clinical investigations of the product.
The development and, if approved, marketing of biosimilars is subject to user fees under the Biosimilar User Fee Amendments of 2022 (BsUFA), which currently apply through September 2027 and may be renewed or amended thereafter. Sponsors must submit an initial biosimilar biological product development (BPD) fee on the earlier of the submission of an IND or within 7 calendar days of FDA granting a first BPD meeting, and annually thereafter until the sponsor submits a BLA that is accepted for filing, or the sponsor discontinues participation in the BPD program. FDA may also remove a sponsor from the BPD program if the sponsor has failed to pay annual BPD fees for a period of 2 consecutive fiscal years. Sponsors who discontinue participation in the BPD program but want to reengage FDA on product development must also pay all prior assessed BPD fees still owed and a reactivation fee and will be subject to annual BPD fees. Once a sponsor submits a BLA for a biosimilar, they are subject to application fees. And, once a biosimilar BLA is approved, the sponsor is subject to annual program fees. The FDA amends the specific fee amounts under BsUFA on an efficient drugannual basis.
The BPCIA is complex and continues to be interpreted and implemented by the FDA. In addition, there has been discussion of whether Congress should reduce the 12-year reference product exclusivity period. Other aspects of the BPCIA, some of which may impact the BPCIA exclusivity provisions, have also been the subject of recent litigation. As a result, the ultimate implementation of the BPCIA is subject to significant uncertainty.
Depending upon the timing, duration and specifics of the FDA approval of the use of our product candidates, some of our U.S. patents, if granted, may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984, commonly referred to as the Hatch-Waxman Act. The Hatch-Waxman Act permits a patent restoration term of up to five years, as compensation for patent term lost during product development program; intensive involvementand the FDA regulatory review process. However, patent term restoration cannot extend the remaining term of senior managersa patent beyond a total of 14 years from the product’s approval date. The patent term restoration period is generally one-half the time between the effective date of an IND and experienced staffthe submission date of a BLA plus the time between the submission date of a BLA and the approval of that application. Only one patent applicable to an approved product is eligible for the extension and the application for the extension must be submitted prior to the expiration of the patent. The U.S. Patent and Trademark Office, in consultation with the FDA, reviews and approves the application for any patent term extension or restoration. In the future, we may intend to apply for restoration of patent term for one of our currently owned or licensed patents to add patent life beyond its current expiration date, depending on a proactive, collaborativethe expected length of the clinical trials and cross-disciplinary review; and rolling review.other factors involved in the filing of the relevant BLA.
Reimbursement of Approved Products in the U.S.
In both domestic and foreign markets, sales and reimbursement of any approved products will depend, in part, on the extent to which the costs of such products will be covered by third-partythird party payors, such as government health programs, commercial insurance and managed healthcare organizations. Third party payors determine which medications they will cover and establish reimbursement amounts. There may be significant delays in obtaining coverage and reimbursement for newly approved drugs, and coverage may be more limited than the purposes for which the drug is approved by the FDA or comparable foreign regulatory authorities. Interim reimbursement amounts for new drugs, if applicable, may also be insufficient to cover our costs and may only be temporary. Reimbursement rates may vary according to the use of the drug and the clinical setting in which it is used, may be based on reimbursement levels already set for lower cost drugs and may be incorporated into existing payments for other services. Net prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future relaxation of laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the U.S. Coverage and reimbursement policies for drug products can differ significantly from payor to payor as there is no uniform policy of coverage and reimbursement for drug products among third party payors in the U.S. Third party payors in the U.S. often rely upon Medicare coverage policy and payment limitations in setting their own reimbursement policies. There may be significant delays in obtaining coverage and reimbursement as the process of determining coverage and reimbursement is often time consuming and costly which will require us to provide scientific and clinical support for the use of our products to each payor separately, with no assurance that coverage or adequate reimbursement will be obtained. It is difficult to predict at this time what government authorities and third party payors will decide with respect to coverage and reimbursement for our drug products.
These third-partythird party payors are increasingly challenging the prices charged for medical products and services and imposing controls to manage costs. The containment of healthcare costs has become a priority of federal and state governments and the prices of drugs have been a focus in this effort. Governments have shown significant interest in implementing
cost-containment programs,
including price controls, restrictions on reimbursement and requirements for substitution of generic products. For example,In the U.S. there have been several recent U.S. Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to drug pricing, review the relationship between pricing and manufacturer patient programs, reduce the cost of drugs under Medicare, and reform government program reimbursement methodologies for drugs. Further,For example, included in the Consolidated Appropriations Act of 2021 were several drug price reporting and transparency measures, such as a new requirement for certain Medicare plans to develop tools to display Medicare Part D prescription drug benefit information in real time and for group and health insurance issuers to report information on pharmacy benefit and drug costs to the Secretaries of the Departments of Health and Human Services, Labor and the Treasury. Additionally, on July 9, 2021, President Biden issued an Executive Order to promote competition in the U.S. economy that included several initiatives addressing prescription drugs. Among other provisions, the Executive Order stated that the Biden administration will “support aggressive legislative reforms that would lower prescription drugs, including by allowing Medicare to negotiate drug prices, by imposing inflation caps, and through other related reforms.” In response to the Executive Order, on September 9, 2021, the Department of Health and Human Services issued a Comprehensive Plan for Addressing High Drug Prices that identified potential legislative policies and administrative tools that Congress and the Trump administration have each indicatedagency can pursue in order to make drug prices more affordable and equitable, improve and promote competition throughout the prescription drug industry, and foster scientific innovation. Most recently, in August 2022, President Biden signed into law the Inflation Reduction Act of 2022 (IRA), which implements substantial changes to the Medicare program, including drug pricing reforms and changes to the Medicare Part D benefit design. Among other reforms, the IRA imposes inflation rebates on drug manufacturers for products reimbursed under Medicare Parts B and D if the prices of those products increase faster than inflation; implements changes to the Medicare Part D benefit that, itbeginning in 2025, will continue to seekcap benefit annual out-of-pocket spending at $2,000 while imposing new legislative and/or administrative measures to control drug costs.discount obligations for pharmaceutical manufacturers; and, beginning in 2026, establishes a “maximum fair price” for a fixed number of pharmaceutical and biological products covered under Medicare Parts B and D following a price negotiation process with the Centers for Medicare and Medicaid Services. At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, to encourage importation from other countries and bulk purchasing.Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our net revenue and results. In addition, there is significant uncertainty regarding the reimbursement status of newly approved healthcare products. We may need to conduct expensive pharmacoeconomic studies in order to demonstrate the cost-effectiveness of our products. If third-party payors do not consider our products to be cost-effective compared to other therapies, the payors may not cover our products after approved as a benefit under their plans or, if they do, the level of payment may not be sufficient to allow us to sell our products on a profitable basis.
Within the United States,U.S., if we obtain appropriate approval in the future to market any of our current product candidates, we may seek approval and coverage for those products under Medicaid, Medicare and the Public Health Service or PHS,(PHS), pharmaceutical pricing program and also seek to sell the products to federal agencies.
Medicaid is a joint federal and state program that is administered by the states for low income and disabled beneficiaries. Under the Medicaid Drug Rebate Program, manufacturers are required to pay a rebate for each unit of producta covered outpatient drug reimbursed by the state Medicaid programs. The amount of the rebate for each product is set by law and may be subject to an additional discount if certain pricing increases more than inflation.
Medicare is a federal program administered by the federal government that covers individuals age 65 and over as well as those with certain disabilities. Medicare Part D provides coverage to enrolled Medicare patients for self-administered, outpatient drugs (i.e., drugs typically dispensed by a pharmacy and that do not need to be administered by a physician). Medicare Part D is administered by private prescription drug plans approved by the U.S. government and each drug plan establishes its own Medicare Part D formulary for prescription drug coverage and pricing, subject to CMS rules and requirements, which the drug plan may modify from time-to-time.
Medicare Part B covers most injectable drugs given in an in-patient setting, and some drugs administered by a licensed medical provider in hospital outpatient departments and doctors’ offices. Medicare Part B is administered by Medicare Administrative Contractors, which generally have the responsibility of making coverage decisions.decisions in accordance with CMS rules and requirements. Subject to certain payment adjustments and limits, Medicare generally pays for Part B covered drugs based on a percentage of manufacturer-reported average sales price.
Drug products are subject to discounted pricing when purchased by federal agencies via the Federal Supply Schedule or FSS.
FFS(FSS). FSS participation is required for a drug product to be covered and paid for by certain federal agencies and for coverage under Medicaid, Medicare Part B and the PHS pharmaceutical pricing program.program, commonly referred to as the 340B Drug Pricing Program. FSS pricing is negotiated periodically with the Department of Veterans Affairs. FSS pricing is intended to not exceed the price that a manufacturer charges its most-favored non-federal customer for its product. In addition, prices for drugs purchased by the Veterans Administration, Department of Defense (including drugs purchased by military personnel and dependents through the TRICARE retail pharmacy program), Coast Guard, and PHS are subject to a cap on pricing (known as the “federal ceiling price”) and may be subject to an additional discount if pricing increases more than inflation.
To maintain coverage of drugs under the Medicaid Drug Rebate Program, manufacturers are required to extend discounts to certain purchasers under the PHS pharmaceutical pricing program. Purchasers eligible for discounts include hospitals that serve a
disproportionate share of financially needy patients, community health clinics and other entities that receive health services grants from the PHS.
The United States and state governments continue to propose and pass legislation designed to reduce the cost of healthcare. In March 2010, the United StatesU.S. Congress enacted the Patient Protection and Affordable Care Act, andas amended by the Health Care and Education Reconciliation Act or(collectively, the Affordable Care Act,Act), which included changes to the coverage and payment for drug products under government health care programs. Some of the provisions of the Affordable Care Act have yet to be implemented, andSince its enactment, there have been judicial and Congressional challenges to certain aspectsnumerous elements of the Affordable Care Act, as well as recent efforts by both the Trump administrationexecutive and legislative branches of the federal government to repeal or replace certain aspects of the Affordable Care Act. Since January 2017, President TrumpFor example, while Congress has signed two Executive Orders designed to delay the implementation ofnot passed comprehensive repeal legislation, it has enacted laws that modify certain provisions of the Affordable Care Act, or otherwise circumvent some of the requirementssuch as removing penalties, starting January 1, 2019, for health insurance mandated bynot complying with the Affordable Care Act. Concurrently, Congress has considered legislation that would repeal or repeal and replace all or part of the Affordable Care Act. While Congress has not passed comprehensive repeal legislation, two bills affecting the implementation of certain taxes under the Affordable Care Act have been
enacted. The Tax Cuts and Jobs Act of 2017 includes a provision repealing, effective January 1, 2019, the tax-based shared responsibility payment imposed by the Affordable Care Act on certain individuals who failAct’s individual mandate to maintain qualifyingcarry health coverage for all or part of a year that is commonly referred to as the “individual mandate”. Additionally, on January 22, 2018, President Trump signed a continuing resolution on appropriations for fiscal year 2018 that delayedinsurance, delaying the implementation of certain mandated fees, underand increasing the point-of-sale coverage gap discount that is owed by pharmaceutical manufacturers who participate in Medicare Part D. In June 2021, the U.S. Supreme Court dismissed a lawsuit challenging the constitutionality of certain aspects of the ACA, without ruling on the merits of the constitutionality arguments. Additionally, the American Rescue Plan of 2021, Pub. L. No. 117-2, enacted on March 11, 2021, temporarily increased premium tax credit assistance for those eligible for subsidies for 2021 and 2022 and removed the 400% federal poverty level limit that otherwise applies for purposes of eligibility to receive premium tax credits. Most recently, the IRA extended this increased tax credit assistance and removal of the 400% federal poverty limit through 2025.It is unclear how the healthcare reform measures of the Biden administration and any future litigation will impact the Affordable Care Act and our business.
U.S. Health Care Laws
Healthcare providers and third party payors will play a primary role in the recommendation and prescription of any product candidates for which we obtain regulatory approval. Our current and future arrangements with third party payors and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we conduct research and would market, sell and distribute our products. As a biopharmaceutical company, even though we do not and will not control referrals of healthcare services or bill directly to Medicare, Medicaid or other third party payors, federal and state healthcare laws and regulations pertaining to fraud and abuse and patients’ rights are and will be applicable to our business. Restrictions under applicable federal and state healthcare laws and regulations that may affect our ability to operate include the following:
•the federal healthcare Anti-Kickback Statute, which, for example, governs our marketing practices, educational programs, pricing policies, and relationships with healthcare providers or other entities, by prohibiting, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, overtly or covertly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under a federal healthcare program such as Medicare and Medicaid;
•federal civil and criminal false claims laws, including the so-called “Cadillac” taxcivil False Claims Act, which can be enforced through civil whistleblower or qui tam actions, and civil monetary penalty laws that impose criminal and civil penalties against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, including the Medicare and Medicaid programs, claims for payment or approval that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government; the FDCA and PHSA, which prohibit the misbranding and adulteration of biological products that are regulated as drugs, and which regulate the marketing of biological products;
•the federal Health Insurance Portability and Accountability Act of 1996 (HIPAA), which imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program and also created federal criminal laws that prohibit knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statements in connection with the delivery of or payment for healthcare benefits, items or services;
•HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act (HITECH), which also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information held by covered entities and their business associates and their subcontractors that use, disclose or otherwise process individually identifiable health information as well as their covered subcontractors;
•the federal Physician Payments Sunshine Act, implemented as the Open Payments Program, which requires certain manufacturers of drugs, devices, biologics and medical supplies to report annually to CMS information related to payments and other transfers of value to U.S.-licensed physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors), physician assistants, nurse practitioners, clinical nurse specialists, anesthesiologist assistants, certified registered nurse anesthetists, certified nurse midwives and U.S. teaching hospitals, as well as
ownership and investment interests held by physicians and their immediate family members and applicable group purchasing organizations;
•state and foreign laws and regulations that are analogous to the federal laws and regulations described in the preceding subsections of this risk factor, such as state anti-kickback and false claims laws, including but not limited to the UK Bribery Act 2010, which may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third party payors, including private insurers;
•state laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government; including those that require drug manufacturers to report information regarding pricing and marketing information related to payments and other transfers of value to physicians and other healthcare providers as well as those that require the registration of pharmaceutical sales representatives. Some state laws require the protection of the privacy and security of health information in a manner that may differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. For example, California enacted the California Consumer Privacy Act (CCPA), effective January 1, 2020, which was recently amended by the California Privacy Rights Act of 2020 (CPRA); and
•similar healthcare and privacy laws and regulations in the European Economic Area (EEA), the UK and other jurisdictions, such as, the General Data Protection Regulation (EU) 2016/679, which impose obligations and restrictions on certain high cost employer-sponsored insurance plans, the annual fee imposedcollection and use of personal information relating to individuals located in the EEA and the UK (including health information).
Efforts to ensure that our business arrangements with third parties comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices do not comply with current or future statutes, regulations or case law involving applicable healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations, we may be subject to significant civil, criminal and administrative penalties, damages, fines, imprisonment, exclusion from government-funded healthcare programs, such as Medicare and Medicaid, disgorgement, additional reporting requirements or oversight if we become subject to a corporate integrity agreement or similar agreement, and the curtailment or restructuring of our operations.
Foreign Regulation
In addition to regulations in the U.S., we are subject to a variety of foreign regulations governing clinical studies and commercial sales and distribution of our product candidates and interactions with healthcare professionals. Whether or not we obtain FDA approval for a product candidate, we must obtain approval from the comparable regulatory authorities of foreign countries or economic areas, such as the European Union, before we may commence clinical studies or market products in those countries or areas. The approval process and requirements governing the conduct of clinical studies, product licensing, pricing and reimbursement vary greatly from place to place, and the time may be longer or shorter than that required for FDA approval.
Certain countries outside of the U.S. have a process that requires the submission of a clinical trial application (CTA), which is similar to an IND in the U.S., prior to the commencement of human clinical studies. In the EU, for example, in accordance with the requirements of the EU Clinical Trials Regulation 536/2014 (CTR), a CTA must be submitted to the centralized EU Portal, Clinical Trials Information System (CTIS) for review by each country in which the sponsor intends to conduct the clinical study. As part of the application process under the CTR, the sponsor proposes a reporting Member State, which coordinates the validation and evaluation of the application. The reporting Member State shall consult and coordinate with the other concerned Member States. Ethics Committee (similar to an Investigational Review Board) review of the CTA is part of the process under the CTR. If an approval is issued, the sponsor can start the clinical trial in all concerned Member States. However, a concerned Member State can in limited circumstances declare an “opt-out” from an approval. In such a case, the clinical trial cannot be conducted in that concerned Member State. The CTR also aims to streamline and simplify the rules on certain health insurance providerssafety reporting and introduces enhanced transparency requirements such as mandatory submission of a summary of the clinical trial results to the EU Database.
Under the CTR, clinical trial sponsors were able to, but not obligated to, use the CTIS starting January 31, 2022. Beginning January 31, 2023, clinical trial sponsors were required to use the CTIS to submit a CTA for a new clinical trial in the EU or EEA, but clinical trials already approved under the previous law, the Clinical Trials Directive (CTD) can continue running under the CTD until January 31, 2025, at which time the sponsor must comply with the CTR and record information on these studies in the CTIS. National regulators in the EU Member States and EEA countries began to carry out their legal responsibilities in evaluating and overseeing clinical trials using the CTIS beginning January 31, 2022.
Under EU regulatory systems, a company may submit Marketing Authorization Applications (MAA) under national, centralized or decentralized, or mutual-recognition procedures. We expect to utilize the centralized procedure, which is compulsory for medicinal
products produced by biotechnology or those medicinal products containing new active substances for specific indications such as the treatment of AIDS, cancer, neurodegenerative disorders, diabetes, viral diseases and designated orphan medicines, and optional for other medicines which are highly innovative. Under the centralized procedure, a marketing application is submitted to the EMA, where it is evaluated by the Committee for Medicinal Products for Human Use. If this committee delivers a favorable opinion, this typically results in the grant by the EC of a single marketing authorization that is valid for all EU member states within 67 days of receipt of the opinion. The initial marketing authorization is valid for five years, but once renewed is usually valid for an unlimited period.
Conditional marketing authorization in the EU is permitted based on incomplete clinical data for a limited number of medicinal products for human use, including products designated as orphan medicinal products under EU law, if (1) the risk-benefit balance of the product is positive, (2) it is likely that the applicant will be in a position to provide the required comprehensive clinical study data, (3) unmet medical needs will be fulfilled and (4) the benefit to public health of the immediate availability on the market share,of the medicinal product outweighs the risk inherent in the fact that additional data are still required. Specific obligations, including with respect to the completion of ongoing or new studies, and with respect to the collection of pharmacovigilance data, may be specified in the conditional marketing authorization. Conditional marketing authorizations are valid for one year and may be renewed annually, if the risk-benefit balance remains positive, and after an assessment of the need for additional or modified conditions. A different marketing authorization pathway is also available to sponsors, called “exceptional circumstances” under which the EC grants marketing authorization of a product for a specific condition or disease when comprehensive data cannot be obtained even after authorization (e.g., for rare conditions or diseases). Sponsors who obtain marketing authorization for a drug product under exceptional circumstances are subject to ongoing post-marketing obligations to continue confirmation of the benefits of the product. Continuation of a marketing authorization granted under the “exceptional circumstances” regulatory pathway is subject to annual re-assessments. The annual re-assessment will determine whether the marketing authorization should be maintained, changed, or suspended, based on a sponsor’s fulfillment of its post-marketing obligations and the risk/benefit profile of product.
As in the U.S., we may apply for designation of a product as an orphan drug for the treatment of a specific indication in the EU before the MAA is submitted. Orphan drugs in Europe enjoy economic and marketing benefits, including up to 11 years of orphan market exclusivity for the approved indication unless another applicant can show that its product is safer, more effective or otherwise clinically superior to the orphan-designated product. The PRIME initiative was established by the EMA to help promote and foster the development of new medicines in the European Union that demonstrate potential for a major therapeutic advantage in areas of unmet medical device excise taxneed. Benefits of PRIME designation include early confirmation of potential for accelerated assessment, early dialogue and increased interaction with relevant regulatory committees to discuss development options, scientific advice at key development milestones, and proactive regulatory support from the EMA in order for the product to obtain a faster MAA.
In the EU, companies developing a new medicinal product must agree to a pediatric investigation plan (PIP) with the EMA and must conduct pediatric clinical trials in accordance with that PIP. The MAA for the product must include the results of pediatric clinical trials conducted in accordance with the PIP, unless a waiver applies, in which case studies in children are not required (for example, if the disease or condition occurs only in adults). or a deferral has been granted, in which case the pediatric clinical trials must be completed at a later date. Products granted a marketing authorization on non-exempt medical devices. Congress also could consider additional legislation to repeal or replace other elementsthe basis of pediatric clinical trials conducted in accordance with the PIP are eligible for a six month extension of the Affordable Care Act.protection under a supplementary protection certificate (if any is in effect at the time of approval) or, in the case of orphan medicinal products, a two year extension of the orphan market exclusivity. This pediatric reward is subject to specific conditions and is not automatically available when data in compliance with the PIP are developed and submitted.
Outside the United States,U.S., there are additional challenges in ensuring adequate coverage and payment for our products will face challenges.products. Pricing of prescription pharmaceuticals is subject to governmental control in many countries. Pricing negotiations with governmental authorities can extend well beyond the receipt of regulatory approval for a product and may require us to conduct a clinical trialstudy that compares the cost effectiveness of our product candidates or products to other available therapies. The conduct of such athis type of clinical trialstudy could be expensive and result in delays into our or our commercialization partners’ commercialization efforts. Third-partyThird party payors are challenging the prices charged for medical products and services, and many third-partythird party payors limit reimbursement for newly-approved health care products. Recent budgetaryBudgetary pressures in many European UnionEU countries are also causing governments to consider or implement various cost-containment measures, such as price freezes, increased price cuts and rebates. If budget pressures continue, governments may implement additional cost-containment measures. Cost-control initiatives could decrease the price we might establish for products that we may develop or sell, which would result in lower product revenues or royalties payable to us. There can be no assurance that any country that haswith price controls or reimbursement limitations for pharmaceutical products will allow favorable reimbursement and pricing arrangements for any of our products.
Foreign RegulationThe EC is currently conducting a wholesale review of the pharmaceutical legal framework, which includes the regulatory protection afforded to medicinal products such as data exclusivity, marketing protection, market exclusivity for orphan indications and pediatric extension. It is expected that the protection currently afforded in the EU will be reduced in the years to come and the new EU
legislative proposal is expected to be published by the EC in the second quarter of 2023, although this timeline may be further prolonged.
In addition to regulationsBrexit and the Regulatory Framework in the United States, we expectKingdom
Following the result of a referendum in 2016, the United Kingdom (UK) left the EU on January 31, 2020, commonly referred to beas Brexit. Pursuant to the formal withdrawal arrangements agreed between the UK and the EU, the UK was subject to a varietytransition period until December 31, 2020 (the Transition Period) during which EU rules continued to apply. A UK-EU Trade and Cooperation Agreement (the Deal) that outlines the future trading relationship between the UK and the EU was agreed in December 2020 and approved by each EU member state and the UK.
Since a significant proportion of foreignthe regulatory framework in the UK applicable to our business and our product candidates is derived from EU directives and regulations, governing clinical trialsUK Legislation has retained existing EU law. However, new UK legislation is being drafted and commercial salesthe UK has not implemented new EU law, such as the CTR; therefore Brexit has had, and distributionwill continue to have, a material impact upon the regulatory regime with respect to the development, manufacture, importation, approval and commercialization of our product candidates. Whethercandidates in the UK and the EU. Great Britain (made up of England, Scotland and Wales) is no longer covered by the EEA’s procedures for the grant of marketing authorizations (Northern Ireland will be covered by the centralized authorization procedure and can be covered under the decentralized or not we obtain FDA approval for a product candidate, we must obtain approval from the comparable regulatory authorities of foreign countries or economic areas, suchmutual recognition procedures, as the European Union, before we may commence clinical trialsEU legal framework continues to apply in Northern Ireland, under the Northern Ireland Protocol). A separate marketing authorization will be required to market drugs in Great Britain. It is currently unclear whether the Medical Healthcare products Regulatory Agency (MHRA) in the United Kingdom is sufficiently prepared to handle the increased volume of MAAs that it is likely to receive. Any delay in obtaining, or marketan inability to obtain, any marketing approvals, would delay or prevent us from commercializing our product candidates in the UK or the EU and restrict our ability to generate revenue and achieve and sustain profitability.
While the Deal provides for the tariff-free trade of medicinal products in those countries or areas. The approval process and requirements governingbetween the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from place to place,UK and the timeEU there may be longer or shorter than that required for FDA approval.
Certain countries outside of the United States have a process that requires the submission of a clinical trial application, or CTA, much like an INDadditional non-tariff costs to such trade which did not exist prior to the commencementend of human clinical trials. In Europe, for example,the Transition Period. Further, should the UK diverge from the EU from a CTA mustregulatory perspective in relation to medicinal products, tariffs could be submittedput into place in the future. We could therefore, both now and in the future, face significant additional expenses (when compared to the competent national health authority and to independent ethics committees in each country in which a company intends to conduct clinical trials. Once the CTA is approved in accordance with a country’s requirements, clinical trial development may proceed in that country. In all cases, the clinical trials must be conducted in accordance with GCP and other applicable regulatory requirements.
Under European Union regulatory systems, a company may submit marketing authorization applications either under a centralized or decentralized procedure. The centralized procedure is compulsory for medicinal products produced by biotechnology or those medicinal products containing new active substances for specific indications such as the treatment of AIDS, cancer, neurodegenerative disorders, diabetes, viral diseases and designated orphan medicines, and optional for other medicines which are highly innovative. Under the centralized procedure, a marketing application is submittedposition prior to the European Medicines Agency,end of the Transition Period) to operate our business, which could significantly and materially harm or EMA, wheredelay our ability to generate revenues or achieve profitability of our business. Any further changes in international trade, tariff and import/export regulations as a result of Brexit or otherwise may impose unexpected duty costs or other non-tariff barriers on us. These developments, or the perception that any of them could occur, may significantly reduce global trade and, in particular, trade between the impacted nations and the UK. It is also possible that Brexit may negatively affect our ability to attract and retain employees, particularly those from the EU.
Orphan designation in Great Britain following Brexit is granted on an essentially identical basis as it is in the EU, but is based on the prevalence of the condition in Great Britain. It is therefore possible that conditions currently designated as orphan conditions in Great Britain will no longer be designated as such and that conditions that are not currently designated as orphan conditions in the EU will be evaluated by the Committee for Medicinal Products for Human Use and a favorable opinion typically resultsdesignated as such in the grant by the European Commission of a single marketing authorization that is valid for all European Union member states within 67 days of receipt of the opinion. The initial marketing authorization is valid for five years, but once renewed is usually valid for an unlimited period. As with accelerated approval in the U.S., conditional marketing authorization in the European Union is permitted based on incomplete clinical data for a limited number of medicinal products for human use, including products designated as orphan medicinal products under EUlaw, if (1) the risk-benefit balance of the product is positive, (2) it is likely that the applicant will be in a position to provide the required comprehensive clinical trial data, (3) unmet medical needs will be fulfilled and (4) the benefit to public health of the immediate availability on the market of the medicinal product outweighs the risk inherent in the fact that additional data are still required. Specific obligations, including with respect to the completion of ongoing or new studies, and with respect to the collection of pharmacovigilance data, may be specified in the conditional marketing authorization. Conditional marketing authorizations are valid for one year and may be renewed annually, if the risk-benefit balance remains positive, and after an assessment of the need for additional or modified conditions.Great Britain.
As in the United States, we may apply for designation of a product as an orphan drug for the treatment of a specific indication in the European Union before the application for marketing authorization is made. Orphan drugs in Europe enjoy economic and marketing benefits, including up to 11 years of exclusivity for the approved indication unless another applicant can show that its product is safer, more effective or otherwise clinically superior to the orphan designated product. The PRIority MEdicines, or PRIME, initiative was established by the EMA to help promote and foster the development of new medicines in the European Union that demonstrate potential for a major therapeutic advantage in areas of unmet medical need. Benefits from the PRIME designation include early confirmation of potential for accelerated assessment, early dialogue and increased interaction with relevant regulatory committees to discuss development options, scientific advice at key development milestones, and proactive regulatory support from the EMA.
Additional Regulation
As a pharmaceuticalbiopharmaceutical company, even though we do not and will not control referrals of healthcare services or bill directly to Medicare, Medicaid or other third-partythird party payors, federal and state healthcare laws and regulations pertaining to fraud and abuse and patients’ rights are and will be applicable to our business. We are also subject to regulation under the Occupational Safety and Health Act, the Environmental Protection Act, the Toxic Substances Control Act, the Resource Conservation and Recovery Act and other present and potential federal, state or local regulations. These and other laws govern our use, handling and disposal of various biological and chemical substances used in, and waste generated by, our operations. Our research and development involvesinvolve the controlled use of hazardous materials, chemicals and viruses. Although we believe that our safety procedures for handling and disposing of such materials comply with the standards prescribed by state and federal regulations, the risk of accidental contamination or injury from these materials cannot be completely eliminated. In the event of such an accident, we could be held liable for any damages that result, and any such liability could exceed our resources.
There have been a numberManufacturing
In April 2022, we sold all of federalour right, title and state proposals during the last few years regarding the pricing of pharmaceuticalinterest in and biological products, government control and other changesto certain assets related to the healthcare systemAtara T-Cell Operations and Manufacturing facility (ATOM Facility) located in Thousand Oaks, California to FDB. We also entered into a Master Services and Supply Agreement with FDB (Fujifilm MSA) which became effective in April 2022 and could extend for up to ten years. Pursuant to
the Fujifilm MSA, FDB will supply us with specified quantities of our product and product candidates, manufactured in accordance with cGMP standards. The Fujifilm MSA does not obligate us to purchase our product and product candidates exclusively from FDB.
We continue to scale our EBV T-cell manufacturing platform to improve product yields from a single donor leukapheresis collection and have generated data confirming the use of stirred-tank bioreactors to improve yield and cell growth productivity. We believe our scalable technology can potentially be a key enabler to deliver biologic-like cost of goods manufactured and could be leveraged across our portfolio, including our CAR T programs.
In addition to FDB, we also work with Charles River Laboratories Inc. (CRL) pursuant to a Commercial Manufacturing Services Agreement (CRL MSA) that we entered into in December 2019. Pursuant to the CRL MSA, CRL provides manufacturing services for our product and certain intermediates. We further amended the CRL MSA to extend the term until the earlier of March 31, 2024 or upon receipt of certain batches of our product and intermediates. We are currently in negotiations with CRL for a new commercial drug product supply agreement to be effective upon the expiration of the United States. It is uncertain what legislative proposalsCRL MSA. However, there can be no assurance that we will be adoptedable to enter into a new commercial drug product supply agreement with CRL on terms favorable or what actions federal, stateacceptable to us, or private payers for medical goods and servicesat all. If we are unable to enter into a new commercial drug product supply agreement or extend the CRL MSA, we may take in responseneed to any healthcare reform proposals or legislation. We cannot predict the effect medical or healthcare reforms may have on our business, and no assurance can be given that any such reforms will not have a material adverse effect.identify alternative sources of drug product supply.
Manufacturing
Our initialcurrent manufacturing strategy is to outsourceevaluate each product candidate and determine which site in our manufacturing network provides the phase-appropriate technical, quality and regulatory compliance requirements. In addition, the long-range supply requirements of our product candidates are evaluated periodically to ensure we are planning manufacturing capacity and capabilities accordingly across our network. Our manufacturing network is comprised of our own laboratory facilities and the manufacturing packaging, labeling, storage,capabilities of our partners, including MSK and distribution for our preclinical studiesQ-Gen Cell Therapeutics, an affiliate of QIMR Berghofer, and clinical trials. In selecting contract manufacturing organizations or CMOs, to manufacture our product candidates, we generally strive to select(CMOs), including SAFC Carlsbad, Inc., FDB and CRL. This strategic approach provides us with the CMO based on the particular technical needs of the product candidate and quality and regulatory compliance. In addition, we aim to work with CMOs that possess the requisite scale, expertise and experienceflexibility to support our clinical and commercial production needs, address time or capacity constraints as well as commercial product manufacturing. We have transferred the manufacturing processes for tabelecleucel (formerly known as ATA129) from MSK to our CMO. The transfer of manufacturing processes to our CMO includes modifications to the processes, improvements in the manufacturing process as well as product testing. Moreover, we are currently developing commercial-scale manufacturing processes for tabelecleucel for the Phase 3 trials, with the proposed dose and schedule to be used in clinical practice and at a cost sufficient to support profitable commercialization. In December 2017, following discussion with the FDA of manufacturing and comparability data generated on material manufactured by our contract manufacturing organization, we initiated these trials in the United States.provide supply redundancy, where appropriate.
We are building our own manufacturing facility, which is designed to support clinical production and ultimately commercialization of tabelecleucel, if approved. In addition, we would expect our facility to support the supply needs for our other cellular therapy product candidates. We would expect to maintain our relationships with our CMOs in order to have redundant sources of supply. Our internal capabilities and experience in manufacturing encompass a broad range of activities including cell line development, process, analytical and formulation development, clinical and commercial scale GMP manufacturing, quality control and quality assurance. We are building the internal technical expertise in the manufacture of cellular therapeutics through hiring well experienced staff. This breadth of experience will allow us to effectively oversee our own manufacturing facility as well as direct the activities of our contract manufacturers and testing facilities. Benefits of owning our own facility may include improved cost of goods, increased control and oversight of manufacturing and supply chain activities, greater control over maintenance and management of production capacity across multiple products, development of redundant supply capabilities to reduce risk, and reduced reliance on third parties.
Our T-cell product candidates require bloodblood-derived starting materials which are received from healthy, consenting third-partythird party donors through FDA- and EMA-compliant collection centers. Our manufacturing operations are conducted under Code of Federal Regulations Good Manufacturing Practices (GMPs), as starting materials. The manufacturing process involves co-culturing and incubating viral or cancer specific antigen transformed B-cells collected from the blood of third party-donors with T-cells collected from the same donor, all underwell as Good Tissue Practices or GTPs.(GTPs). GTPs are FDA regulations and guidance documents that govern the methods used in, and the facilities and controls used for, the manufacture of human cells, tissues and cellular and tissue based products, or HCT/Ps, which are human cells or tissue intended for implantation, transplant, infusion or transfer into a human recipient. The primary intent of the GTP requirements is to ensure that cell and tissue basedtissue-based products are manufactured in a manner designed to prevent the introduction, transmission and spread of communicable disease. FDA regulations also require tissue establishments to register and list their HCT/Ps with the FDA and, when applicable, to evaluate donors through screening and testing.
Pursuant toThrough agreements with our June 2015 license agreement with MSK,partners, we have acquired the right to use certain manufacturing process know-how related to producing clinical research-related drug supply. This includedThese include materials to support the manufacturing of clinical trialstudy material, including key starting materials and intermediates as well as existing inventory of clinical trialstudy materials. We have also entered into athe ability to obtain supply agreement with afrom third partyparties to ensure we have the necessary bloodstarting materials donated from healthy consenting third-partythird party donors.
Employees
Human Capital Management
As of February 15, 2018,December 31, 2023, we had 185 full-time225 employees, consistingexcluding those impacted by the reduction in force in November 2023, which was subsequently reduced by approximately 25% as a result of researchthe January 2024 reduction in force. We believe that the success of our business will depend, in part, on our ability to attract and development, regulatory affairs, technical operations, commercial operations, medical affairsretain qualified personnel. Our human capital strategy is designed to enable successful execution of our business objectives, while fostering a collaborative and general administrative functions.innovative culture, that embraces diversity and inclusion. We monitor our success with insights across human capital metrics such as employee engagement, vacancy rates, time to hire, promotion rates, performance ratings, succession depth, retention, EEO compliance, pay equity, and diversity representation. The principal purposes of our compensation policies and equity incentive plans are to attract, retain and motivate employees and directors by paying for performance through the granting of stock-based compensation awards and cash-based performance bonus awards. None of our employees are represented by a labor union or are a party to a collective bargaining agreement and we consider our relations with our employees to be good.
COVID-19 Business Update
To date, the COVID-19 pandemic has not materially impacted our or our partners’ clinical, research and development, regulatory, and manufacturing operations or timelines. We have transitioned a portion of our workforce to a remote, work-from-home
model, while maintaining essential in-person laboratory functions in order to advance key research, development and manufacturing priorities. We implemented safety protocols and procedures to support our onsite workforce.
The full extent to which the COVID-19 pandemic may impact our business and operations is subject to future developments which are uncertain and difficult to predict.
For additional information about risks and uncertainties related to the COVID-19 pandemic that may impact our business, financial condition and operations, see the section titled “1A. Risk Factors” under Part I, Item 1A in this Annual Report on Form 10‑K.
Corporate Information
We were incorporated in Delaware in 2012 and completed our initial public offering in October 2014.2012. Our principal corporate offices are located at 611 Gateway Blvd.2380 Conejo Spectrum St., Suite 900, South San Francisco, CA 94080200, Thousand Oaks, California 91320 and our telephone number at that address is (650) 278-8930.
Available Information
(805) 623-4211. Our website address is www.atarabio.com.
Available Information
We file Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, proxy statements and other materials with the SEC.Securities and Exchange Commission (SEC). We are subject to the informational requirements of the Exchange Act and file or furnishmake these reports proxy statements, and other information with the SEC. Such reports and other information filed by the Company with the SEC are available free of charge onthrough our website at investors.atarabio.com.
as soon as reasonably practicable after we electronically file such reports with, or furnish such reports to, the SEC. The public may also read and copyinformation contained on, or that can be accessed through, our website is not a part of or incorporated by reference in this Annual Report on Form 10-K or in any materials filed by Ataraother filings we make with the SEC at the SEC’s Public Reference Room at 100 F Street, NE, Room 1580, Washington, DC 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. SEC.
The SEC also maintains a websitean internet site that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC at www.sec.gov.
Item 1A. Risk Factors
Investing in our common stock involves a high degree of risk. YouInvestors should carefully consider all of the risk factors and uncertainties described below, in addition to the other information contained in this Annual Report on Form 10-K, including the section of this report titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated and combined financial statements and related notes, before investing in our common stock.
The risks described below may not be the only ones relating to our company and additional risks that we currently believe are immaterial may also affect us. If any of the followingthese risks, including those described below, materialize, our business, competitive position, reputation, financial condition, and results of operations, cash flows and future prospects could be seriously harmed. In these circumstances, the market price of our common stocksecurities could decline, and youinvestors may lose all or a part of yourtheir investment.
Risks Related to Our Financial Results and Capital Needs
We have incurred substantial losses since our inception and anticipate that we will continue to incur substantial and increasing losses for the foreseeable future.
We are a clinical-stage biopharmaceutical company. Investment in biopharmaceutical product development is highly speculative because it entails substantial upfront capital expenditures and significant risk that a product candidatecandidates will fail to prove effective, gain regulatory approval or become commercially viable. We do not have any productsone product, Ebvallo, which is approved by regulatory authoritiesin the EU and the UK and have not generated anylimited revenues from product sales to date,commercialization, and have incurred significant research, development and other expenses related to our ongoing operations and expect to continue to incur such expenses. As a result, we have not been profitable and have incurred significant operating losses in every annual reporting period since our inception. For the year ended December 31, 2017,2023, we reported a net loss of $119.5 million and we had an accumulated deficit of $296.7 million as of December 31, 2017.$276.1 million.
We do not expectknow when, or if, we will generate sufficient revenue from commercialization to generate revenues for many years, if at all.offset our operating expenses. We expect to continue to incur significant expenses and operating losses for the foreseeable future. We anticipate these losses to increasefuture as we continue to research, develop and seek regulatory approvals for our product candidates and any additional product candidates we may acquire, and potentially begin to commercialize product candidates that may achieve regulatory approval.in-license or develop. We may encounter unforeseen expenses, difficulties, complications, delays and other unknown factors that may adversely affect our business. The size of our future net losses will depend, in part, on the rate of future growthchange of our expenses and our ability to generate revenues. If any of our product candidates fails in clinical trialsstudies or does not gain regulatory approval, or if approved, fails to achieve market acceptance, we may never become profitable. Even if we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods. We anticipate that ourOur expenses willmay increase in the future as we continue to invest in research and development of our existing product candidates, investigate and potentially acquire new product candidates and expand our manufacturing and commercialization activities.candidates.
We have a limited operating history, which may make it difficult for you to evaluate the success of our business to date and to assess our future viability.
Our company was formed in August 2012. Our operations to date have been limited to organizing and staffing our company, acquiring product and technology rights and conducting product development activities for our product candidates. We have not yet demonstrated our ability to successfully complete any Phase 2 or Phase 3 clinical trials,studies, obtain regulatory approval in the U.S., consistently manufacture a commercial scale product or arrange for a third party to do so on our behalf, or conduct sales and marketing activities necessary for successful commercialization for any of our product candidates.candidates or arrange for a third party to do so on our behalf. In addition, the adoptive immunotherapy technology underlying our T-cell product candidates, including our next-generation CAR T programs, is new and largely unproven. Any predictions about our future success, performance or viability, particularly in view of the rapidly evolving cancer immunotherapy field, may notprove to be as accurate as they could be if we had a longer operating history or approved products on the market.inaccurate.
In addition, as a young business, we may encounter unforeseen expenses, difficulties, complications, delays and other known and unknown factors. We will need to transition at some point from a company with a research and development focus to a company capable of supporting commercial activities. We may not be successful in such a transition. We expect our financial condition and operating results to continue to fluctuate significantly from quarter to quarter and year to year due to a variety of factors, many of which are beyond our control. Accordingly, any of our quarterly or annual periods’ results are not indicative of future operating performance.
We currently have no source of revenues.earned limited commercialization revenues to date. We may never generate revenues or achieve profitability.
To date, we have not generated anyonly limited revenues from product sales or otherwise. Even if we are able to successfully achievecommercialization. We have regulatory approval for ourone product, candidates,Ebvallo, in the EU and the UK. We have out-licensed the commercialization rights to tab-cel (Ebvallo in the EU and the UK) to Pierre Fabre under the A&R Commercialization Agreement and we do not know when we will generate revenues or become profitable, if at all.have sold certain royalty and milestone interests for the Initial Territory, subject to a specified cap, to HCRx pursuant to the HCRx Agreement. Our ability to generate revenues from product salescommercialization and achieve profitability will be subject to the A&R Commercialization Agreement, the HCRx Agreement and depend on our commercialization partners’ ability to successfully commercialize products, including any of our current product and product candidates, and other product candidates that we may develop, in-license or acquire in the future. Our ability to generate revenues from the sale of products and achieve profitability will also dependsdepend on a number of additional factors, including our ability to:
•successfully complete development activities, including the necessary clinical trials;
studies with positive results; •complete and submit BLAsregulatory submissions to the FDA, EMA or other agencies and obtain U.S. regulatory approval for indications for which there is a commercial market;
•complete and submit applications to, and obtain regulatory approval from, foreign regulatory authorities in Europe, Asia and other jurisdictions;
obtain coverage and adequate reimbursement from third parties, including government and private payors;
set commercially viable prices for our products, if any;
establish and maintain adequate supply with sufficient breadth to treat patients
establish and maintain manufacturing relationships with reliable third parties or build our own manufacturing facility and ensure adequate, legally globally compliant manufacturing of bulk drug substances and drug products to maintain that supply;
develop manufacturing and distribution processes for our novel T-cell immunotherapy product candidates;
•develop commercial quantities of our products, including at acceptable cost levels;
•establish and maintain adequate supply of our products, including cell lines with sufficient breadth to treat patients;
•establish and maintain manufacturing and commercialization relationships with reliable third parties;
•qualify our CMOs’ manufacturing facilities such that we can maintain the supply of our products by ensuring adequate manufacturing of bulk drug substances and drug products in a manner that is compliant with global legal and regulatory requirements;
•achieve market acceptance of and pricing and reimbursement for our products, if any;
•attract, hire and retain qualified personnel;
•protect our rights in our intellectual property and regulatory protections portfolio;
and •develop a commercial organization capable of sales, marketing and distribution for any products we intend to sell ourselves in the markets in which we choose to commercialize on our own; and
find suitable distributioncommercialization partners to help uswho can obtain coverage and adequate reimbursement from third parties, including government payors, set commercially viable prices, market, sell and distribute our approved products in other markets.
products.Our revenues forfrom Ebvallo or any product candidate for which regulatory approval is obtained will be dependent, in part, upon the size of the markets in the territories for which we gain regulatory approval, the accepted price for the product, the ability to get reimbursement at any price, and whether we own the commercial rightsterms and conditions of our commercialization agreement with our partner for that territory. We do not retain any meaningful milestones or royalty payments from Pierre Fabre for Ebvallo in the Initial Territory until the applicable royalty cap under the HCRx Agreement is met, which could take many years, if at all. If the number of our addressable disease patients is not as significant as we estimate, the indication approved by regulatory authorities is narrower than we expect, or the reasonably accepted population for treatment is narrowed by competition, physician choice, or treatment guidelines weor a reduction in the incidence of the addressable disease, our partners may not generate significant revenues from sales of suchsuccessfully commercialize our products, even if approved. In addition,The timing and amount of any milestone and royalty payments we anticipate incurring significant costs associated with commercializing any approved product candidate.may receive from our partners, as well as the commercial success of our products will depend on, among other things, the efforts, allocation of resources, negotiation of pricing and reimbursement and successful commercialization of our products by our partners. As a result, even if we generate product revenues, we may not become profitable and may need to obtain additional funding to continue operations. If we fail to become profitable or are unable to sustain profitability on a continuing basis, then we may be unable to continue our operations at planned levels and may be forced to reduce our operations.
We will require substantial additional financing on terms acceptable to us to achieve our goals, and a failure to obtain this necessary capital when needed could force us to delay, limit, reduce or terminate our product development or commercializationmanufacturing efforts.
We expect to expend substantial resources for the foreseeable future to continue the clinical development and manufacturing of our T-cell immunotherapy product candidates, and the advancement and expansion of our preclinical research pipeline. We also expect to continue to expend resources for the development and manufacturing of our product and product candidates and the technology we have licensed or have an exclusive right to license from QIMR Berghofer, including ATA188 and ATA190, which are in development for the treatment of MS.our partners. These expenditures will include costs associated with research and development, potentially acquiring or licensing new product candidates or technologies, conducting preclinical studies and clinical trialsstudies and potentially obtaining regulatory approvals and manufacturing products, as well as marketing and selling products approved for sale, if any.products. Under the terms of our license agreements with each of MSK and QIMR Berghofer,our in-license partners, we are obligated to make payments upon the achievement of certain development, regulatory and commercial milestones. In addition, other unanticipated costs may arise. Because the design and outcome of our ongoing, planned and
anticipated clinical trialsstudies is highly uncertain, we cannot reasonably estimate the actual amounts necessary to successfully complete the development and commercialization of our product and product candidates.
Our future capital requirements depend on many factors, including:
•the scope, progress, results and costs of researching and developing our product candidates, and conducting preclinical studies and clinical trials;
studies; •the timing of, and the costs involved in, obtaining regulatory approvals for our product candidates, if clinical trialsstudies are successful;
successful, including any costs from post-market requirements; •the cost of commercialization activitiescontracting for the manufacture of our product candidates, if any of these product candidates is approved for sale, including marketing, sales and distribution costs;
the cost of manufacturing our product candidates for clinical trialsstudies in preparation for regulatory approval and in preparation for commercialization;
•our ability to establish and maintain strategic licensing or other arrangements and the financial terms of such agreements;
•the costs to develop, acquire or in-license future product candidates or technologies;
•the costs involved in preparing, filing, prosecuting, maintaining, expanding, defending and enforcing patent claims, including litigation costs and the outcome of such litigation;
•the timing, receipt and amount of sales of, or royalties on, our product and future products, if any; and
•the emergence of competing technologies or other adverse market developments.
We believeexpect that our existing cash, cash equivalents and short-term investments, combined with certain anticipated payments from the A&R Commercialization Agreement, as well as cost reductions from completed workforce reductions and operating efficiencies resulting from our planned transition of substantially all activities relating to tab-cel at the time of the BLA transfer to Pierre Fabre, will be sufficientenable us to fund our planned operations into 2027. Such anticipated payments are estimates based on assumptions and plans that are subject to change and such changes could materially impact our cash runway. These assumptions include the first halfreceipt of 2020. Asfuture payments that are dependent upon the successful filing and approval of December 31, 2017, we had total cash, cash equivalentsthe tab-cel BLA, as well as the completion of specific development and short-term investments of $166.1 million,regulatory activities by us and in January 2018, we completed an underwritten public offering, resulting in net proceeds to us of approximately $131.4 million. However, ouractions taken by third parties, and are, therefore, uncertain at this time.
Our operating plan may change as a result of many factors currently unknown to us, and we may need additional funds sooner than planned. In addition, we may seek additional capital due to favorable market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. We do not have any committed external source of funds. Additional funds other than milestone and royalty payments that we may receive under the A&R Commercialization Agreement, subject to the terms of the HCRx Agreement. We do not retain any meaningful milestone or royalty payments related to the Initial Territory from Pierre Fabre until the applicable royalty cap under the HCRx Agreement is met, if at all.
As of December 31, 2023, we had total cash, cash equivalents and short-term investments of $51.7 million. Our existing cash, cash equivalents and short-term investments as of December 31, 2023 will not be available whensufficient to fund our planned operations for at least the next twelve months from the date of issuance of these financial statements. These conditions raise substantial doubt about our ability to continue as a going concern for at least 12 months after the issuance of the accompanying consolidated financial statements.
To alleviate the conditions that raise substantial doubt about our ability to continue as a going concern, we plan to secure additional capital, potentially through a combination of public or private security offerings; use of our ATM facility; and/or strategic transactions. We may also need themto raise additional funding as required based on the status of our development programs and our projected cash flows. Although we have been successful in raising capital in the past, and expect to continue to raise capital as required, there is no assurance that we will be successful in obtaining sufficient funding on terms that are acceptable to us to fund continuing operations, if at all, or at all.identify and enter into any strategic transactions that will provide the capital that we will require. If adequate fundswe are not availableunable to usobtain sufficient funding on a timely basis,acceptable terms, we maycould be requiredforced to delay, limit, reduce or terminate preclinical studies, clinical trialsstudies or other development activities for one or more of our product candidates, or delay, limit, reduce or terminatewhich could have a material adverse effect on our establishmentbusiness, results of salesoperations, and marketing capabilities or other activities that may be necessary to commercialize our product candidates.financial condition.
Raising additional capital may cause dilution to our existing stockholders, restrict our operations or require us to relinquish rights to our product candidates on terms that are unfavorable terms to us.
We mayplan to seek required additional capital, and may do so through a variety of means, including through private and public equity offerings and debt financings. For example, in December 2022, we sold certain of our royalty and milestone interests related to the Initial Territory under the amended and restated Pierre Fabre Commercialization Agreement, subject to a specified cap, to HCRx pursuant to the HCRx Agreement. To the extent that we raise additional capital through the sale of equity or convertible debt securities, youror if existing holders of warrants exercise their rights to purchase common stock, the ownership interest of existing
stockholders will be diluted, and the terms may include liquidation or other preferences that adversely affect yourthe rights of stockholders. To the extent equity valuations, including the trading price of our common stock, are depressed as a stockholder.result of economic disruptions or other uncertainties, for example due to rising inflationary pressures, the war in Ukraine, the war in the Middle East or other factors, the potential magnitude of this dilution will increase. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take certain actions, such asincluding incurring additional debt, making capital expenditures, entering into licensing arrangements, or declaring dividends. If we raise additional funds from third parties, we may have to relinquish valuable rights to our technologies or product candidates or grant licenses or other rights on terms that are not favorable to us. If we are unable to raise additional funds through equity or debt financing when needed, we may be required to delay, limit, reduce or terminate our product development or commercialization efforts for our product candidates, or grant to others the rights to develop and market product candidates that we would otherwise prefer to develop ourselves or take other actions that are adverse to our business.
If we fail to continue to meet the listing standards of Nasdaq, our common stock may be delisted, which could have a material adverse effect on the liquidity of our common stock.
Our common stock is currently listed on the Nasdaq Global Market. Nasdaq has requirements that a company must meet in order to remain listed on Nasdaq. In particular, Nasdaq rules require us to maintain a minimum bid price of $1.00 per share of our common stock (the “Bid Price Requirement”). If the closing bid price of our common stock falls below $1.00 per share for 30 consecutive trading days or we do not meet other listing requirements, we would fail to be in compliance with Nasdaq listing standards. There can be no assurance that we will continue to meet the Bid Price Requirement, or any other requirement in the future.
On January 8, 2024, we received a deficiency letter from the Listing Qualifications Department of Nasdaq notifying us that, for the last 30 consecutive business days, the bid price for our common stock had closed below the Bid Price Requirement.
In accordance with Nasdaq Listing Rule 5810(c)(3)(A) (Compliance Period Rule), we have been provided a period of 180 calendar days, or until July 8, 2024 (Compliance Date), to regain compliance with the Bid Price Requirement. If, at any time before the Compliance Date, the bid price for our common stock closes at $1.00 or more for a minimum of 10 consecutive business days, as required under the Compliance Period Rule, Nasdaq will provide us written communication that we have regained compliance with the Bid Price Requirement.
If we do not regain compliance with the Bid Price Requirement by the Compliance date, we may be eligible for an additional 180 calendar day compliance period. To qualify, we will be required to (i) transfer to the Nasdaq Capital Market, (ii) provide written notice of our intention to cure the deficiency during the additional 180 calendar day compliance period, by effecting a reverse stock split, if necessary, and (iii) and meet the continued listing requirement for market ourselves.value of its publicly held shares and all other initial listing standards for the Nasdaq Capital Market, with the exception of the Bid Price Requirement, which requirements include, among other things, a minimum stockholders’ equity of at least $4 million. For the year ended December 31, 2023, we reported total stockholders’ deficit of $99.2 million, which is below the stockholders’ equity requirement under the applicable standards.
If we do not regain compliance with the Bid Price Requirement by the Compliance Date and we are not eligible for an additional compliance period, or Nasdaq concludes that we will not be able to cure the deficiency during the additional compliance period, Nasdaq will provide us written notification that our common stock will be subject to delisting. At that time, we may appeal the delisting determination to a Nasdaq Hearings Panel. However, there can be no assurance that such appeal would be successful. If the Nasdaq Hearing Panel does not result in Nasdaq granting us an extension of time to achieve compliance with the Minimum Bid Price Rule, our common stock will be delisted from Nasdaq.
If our common stock were to be delisted, the actual and potential liquidity of our common stock and our ability to raise future capital would be adversely affected and the market price of our common stock could decrease. If, for any reason, we are unable to obtain listing on another national securities exchange or take action to restore our compliance with Nasdaq’s continued listing requirements, a reduction in some or all of the following may occur, each of which could have a material adverse effect on our stockholders:
•the liquidity of our common stock;
•the market price of our common stock;
•our ability to obtain financing for the continuation of our operations;
•the number of institutional and general investors that will consider investing in our securities;
•the number of market makers in our common stock;
•the availability of information concerning the trading prices and volume of our common stock; and
● the number of broker-dealers willing to execute trades in shares of our common stock
Our workforce reductions may not result in anticipated savings, could result in total costs and expenses that are greater than expected and could disrupt our business.
In August 2022, we reduced our workforce by approximately 20% across all areas of our company, including members of management. In November 2023, we further reduced our workforce by approximately 30%. In January 2024, we announced another reduction of our workforce by approximately 25%. The reductions in force reflect a prioritization around key research and development programs and the reduction of our expense profile. We may not realize, in full or in part, the anticipated benefits, savings and improvements in our cost structure from our restructuring efforts due to unforeseen difficulties, delays or unexpected costs. If we are unable to realize the expected operational efficiencies and cost savings from restructuring, our operating results and financial condition would be adversely affected. We also cannot be certain that we will not have to undertake additional workforce reductions or restructuring activities in the future. Furthermore, our cost savings plan may be disruptive to our operations, which could affect our ability to generate product revenue. In addition, our workforce reductions could yield unanticipated consequences, such as attrition beyond planned staff reductions, or disruptions in our day-to-day operations. Our workforce reductions could also harm our ability to attract and retain qualified management, scientific, clinical, and manufacturing personnel who are critical to our business. Any failure to attract or retain qualified personnel could prevent us from successfully developing and commercializing our product candidates in the future, including tab-cel, if approved.
There can be no assurance that we will achieve all of the anticipated benefits of the Fujifilm Transaction and we could face unanticipated challenges.
We may not realize some or all of the anticipated benefits from the Fujifilm Transaction and we may encounter post-closing risks, including associated with the provision of services to us by FDB pursuant to the Fujifilm MSA. We may experience increased difficulty and loss of institutional knowledge as a result of the transfer of ATOM Facility employees to FDB in connection with the Fujifilm Transaction, which could harm our business. Additionally, significant time and resources may be required from us, which could disrupt our business and distract management from other responsibilities, which may result in losses or continued financial involvement in the ATOM Facility, including through indemnification or other financial arrangements, which could adversely affect our financial results.
Risks Related to the Development of Our Product and Product Candidates
We have one approved product, Ebvallo, in the EU and the UK and are verygenerally early in our development efforts and have only a small number of product candidates in clinical development. All of our other product candidates are still in preclinical development. If we or our collaborators are unable to successfully develop, manufacture and commercialize our product or product candidates or experience significant delays in doing so, our business may be materially harmed.
We have one approved product, Ebvallo, in the EU and the UK and are verygenerally early in our development efforts, and only a small number of our product candidates are in clinical development. AllThe majority of our other product candidates are currently in preclinical development. We have invested substantially all of our efforts and financialsubstantial resources in identifying and developing potential product candidates, and conducting preclinical and clinical studies, clinical trialsmanufacturing activities, and manufacturing activities.preparing for the commercial launch of our product and product candidates. Our ability to generate revenues which we do not expect will occur for many years,from the sale of our product and product candidates, if ever,approved, will depend heavily on the successful development, manufacture and our partners' eventual commercialization of our product and product candidates.
The success of our product and product candidates will depend on severalmany factors, including the following:
•completion of preclinical studies and clinical trialsstudies with positive results;
results, including demonstrating the stability, safety, purity, and potency of our product candidates to the satisfaction of the FDA or other regulatory agencies; •receipt of regulatory approvals from applicable authorities;
authorities, including required authorizations for clinical trials and marketing authorizations; •protecting our rights in our intellectual property portfolio, including by obtaining and maintaining patent and trade secret protection and regulatory exclusivity for our product candidates;
•establishing or making successful arrangements with third-partythird party manufacturers or buildingand commercialization partners;
•qualifying our ownand our CMOs’ manufacturing facilityfacilities for clinical and commercial manufacturing purposes;
•developing manufacturing and distribution processes for our novel T-cell product candidates;
candidates and next-generation CAR T programs;
•contracting with third parties for the manufacture of our product candidates at an acceptable cost;
•launching commercial salescontracting with third parties for commercialization of our product candidates, if approved, whether alone or in collaboration with others;
acceptance of the product candidates,products on terms favorable to us, if approved by applicable regulatory authorities;
•acceptance of our products, if approved by applicable regulatory authorities, by patients and the medical communitycommunity;
•our partners’ ability to obtain and third-party payors;
effectively competing with other therapies;
obtaining and maintainingmaintain coverage and adequate reimbursement by third-party payors, including government payors, for our product candidates;
products, if approved by applicable regulatory authorities;•protecting our rights in our intellectual property portfolio;
effectively competing with other therapies; •maintaining a continued acceptable safetybenefit/risk profile of the products following approval; and
•maintaining and growing an organization of scientists and business peoplefunctional experts who can develop and commercialize our products and technology.
If we do not achieve one or more of these factors in a timely manner or at all, we could experience significant delays or an inability to successfully develop and commercialize our product candidates, which could materially harm our business.
We have been affected by and could be adversely affected in the future by the effects of health epidemics and pandemics, including the COVID-19 pandemic, which could materially and adversely affect our business and operations in the future, as well as the businesses and operations of third parties on which we rely.
Our business could be adversely affected by health epidemics and pandemics, including the COVID-19 pandemic, which presented a substantial public health and economic challenge around the world and has affected, and continues to affect, our employees, patients, communities and business operations, as well as the U.S. economy and financial markets. We continue to maintain essential in-person manufacturing and laboratory functions in order to advance key research, development and manufacturing priorities. In connection with these measures, we may be subject to claims based upon, arising out of, or related to COVID-19 and our actions and responses thereto. The effects of potential future state executive orders, local shelter-in-place orders, government-imposed quarantines, our work-from-home policies and other similar actions may negatively impact productivity, disrupt our business and delay our clinical programs and timelines, the magnitude of which will depend, in part, on the length and severity of the restrictions and other limitations on our ability to conduct our business in the ordinary course.
Further quarantines, shelter-in-place or similar restrictions and other actions taken by foreign, federal, state and local governments, or the perception that such orders, shutdowns or other restrictions on the conduct of business operations could be reinstated, related to COVID-19, other health epidemics and pandemics, or other infectious diseases, could impact our manufacturing capabilities and third party manufacturing facilities in the United States and other countries, or the availability or cost of materials, which would disrupt our supply chain. In particular, standard transportation channels were impacted and may again be impacted, and we and other manufacturing, testing, product disposition, CMOs and external testing laboratories are subject to enhanced risk assessment and mitigation measures. In addition, there were and may continue to be interruptions in the supply of leukapheresis collections, which supply raw materials used in our products.
Our clinical trials may also be affected by health epidemics and pandemics and have been affected by the COVID-19 pandemic. Clinical site initiation and patient enrollment experienced delays as a result of the COVID-19 pandemic, including due to the prioritization of hospital resources toward COVID-19 and away from clinical trials and as a result of changing practice patterns that impact the diseases our trials address. Some patients may not be able to comply with clinical trial protocols if quarantines impede patient movement or interrupt healthcare services or if patients are forced to quarantine due to a health epidemic or pandemic. For example, while most clinical trial sites for our studies, including our Phase 3 clinical trial of tab-cel in patients with EBV+ PTLD, remained open to enrollment for patients, some sites limited the screening and enrollment of new patients due to governmental orders related to COVID-19, or fear of infection of COVID-19, limited, and may limit in the future, patients’ abilities to access clinical sites. Pandemic-related travel restrictions may also interrupt key clinical trial activities, such as clinical trial site data monitoring and the collection, processing and analyses of efficacy, safety and translational data. For example, at the outset of the COVID-19 pandemic, we observed a temporary slow-down in stem cell and solid organ transplant volumes, which may have decreased the eligible patient population for the tab-cel Phase 3 study. In April 2020, we initiated a temporary pause in the screening and enrollment of patients in our EMBOLD study of ATA188 in patients with PMS. Although we were able to resume the screening and enrollment of patients in our EMBOLD study and enrolled the first patient in the study in June 2020, the COVID-19 pandemic required us to pause screening and enrollment of patients in our clinical studies. Similarly, our ability to recruit and retain principal investigators and site staff who, as healthcare providers, may have heightened exposure to health epidemics and pandemics, may be adversely impacted.
In addition, to the extent the COVID-19 pandemic adversely affected our business and results of operations, it may also have the effect of heightening many of the other risks and uncertainties described elsewhere in this “Risk Factors” section.
Our future success is dependent on the regulatory approval of our product candidates.
We do notonly have any productsone product, Ebvallo, that havehas gained regulatory approval.approval, an approval in the EU and the UK. Currently, our onlyprioritized clinical-stage product candidates are tabelecleucel (formerly known as ATA129), for which we recently initiated Phase 3 clinical trialsinclude tab-cel (tabelecleucel) in the United States, ATA188, which is in a Phase 1 clinical trial, ATA190, which is in a Phase 1 clinical trial conducted by QIMR Berghofer,U.S. and ATA230, which is in Phase 2 clinical trials.ATA3219 Our business is substantially dependent on our ability to obtain regulatory approval for, and, if approved, to find a partner who can successfully commercialize our product candidates in a timely manner. We cannot
Neither we nor our partners can commercialize product candidates in the United StatesU.S. without first obtaining regulatory approval for the product candidates from the FDA; similarly, neither we cannotnor our partners can commercialize product candidates outside of the United StatesU.S. without obtaining regulatory approval from comparable foreign regulatory authorities. Before obtaining regulatory approvals for the commercial sale of any product candidate for a target indication, we must demonstrate with substantial evidence gathered in preclinical studies and clinical trials, generally including two well-controlled Phase 3 trials,studies that the product candidate is safe and effective for use for that target indication and that the manufacturing facilities, processes and controls are adequate with respect to such product candidate.candidate to assure stability, safety, purity and potency.
The time required to obtain approval by the FDA and comparable foreign regulatory authorities is unpredictable but typically takes many years following the commencement of preclinical studies and clinical trialsstudies and depends upon numerous factors, including the substantial discretion of the regulatory authorities. The novel nature of our product candidates may create further challenges in obtaining regulatory approval. For example, the FDA and comparable foreign regulatory authorities have limited experience with regulating the development and commercialization of T-cell immunotherapies, particularly allogeneic T-cell product candidates, and CAR T therapies, including assessing the comparability of different versions of such product candidates. In addition, approval policies, regulations, regulatory positions or the type and amount of clinical and other data necessary to gain approval may change during the course of a product candidate’s clinical development and throughout regulatory interactions, and may vary among jurisdictions.jurisdictions, particularly for novel therapies. The EC and the MHRA has approved the MAA for Ebvallo as a monotherapy treatment for patients with EBV+ PTLD who have received at least one prior therapy under “exceptional circumstances,” which is a pathway under which EC and MHRA grant marketing authorization when “comprehensive data cannot be obtained even after authorization.” Under the exceptional circumstances marketing authorization, our commercial partner, Pierre Fabre, is subject to ongoing post-marketing obligations to continue confirmation of the benefits of Ebvallo, and if any of our other product candidates are approved under this pathway, we or our future commercial partners will be subject to this obligation. Continuation of the Ebvallo marketing authorization is subject to annual re-assessment. The annual re-assessment will determine whether the Ebvallo marketing authorization should be maintained, changed, or suspended, based on Pierre Fabre’s fulfillment of post-marketing obligations and the risk/benefit profile of Ebvallo. If we, or our commercial partners, do not satisfy the ongoing post-marketing obligations or the EC determines that the risk/benefit profile of Ebvallo is not acceptable, the EC may change or suspend the marketing approval for Ebvallo. We have not obtained regulatory approval for any other product candidate, and it is possible that none of our existing product candidates or any future product candidates will ever obtain regulatory approval.
Our product candidates could fail to receive regulatory approval from the FDA or a comparable foreign regulatory authority for many reasons, including:
•disagreement with the design or implementationconduct of our clinical trials;
studies; •failure to demonstrate that apositive benefit/risk profile of the product candidate is safe and effective for its proposed indication;
•failure to demonstrate the stability, safety, purity and potency of the product candidate;
•failure of clinical trialssites to conduct the study in accordance with applicable regulatory requirements;
•failure of clinical studies to meet the level of statistical significance required for approval;
•failure to demonstrate that a product candidate’s clinical and other benefits outweigh its safety risks;
disagreement with our interpretation of data from preclinical studies or clinical trials;
studies;•the insufficiency of data collected from clinical trialsstudies of our product candidates to support the submission and filing of a BLA or other submission or to obtain regulatory approval;
•inability to reach agreement with the FDA or comparable foreign regulatory authorities on the methodologies for, and assessment of, comparability of different versions of product candidates used in non-pivotal studies, pivotal studies and for intended commercial use;
•failure to obtain approval of our manufacturing processes or facilities of third-partythird party manufacturers with whom we contract for clinical and commercial supplies or our own manufacturing facility; or
•changes or inconsistencies in the requested or required methodologies, statistical analyses, specification criteria or regulatory submission requirements for a product candidate, including changes to, or inconsistencies with, applicable industry practice or precedent; or
•changes in the approval policies or regulations that render our preclinical and clinical data insufficient for approval.approval or in positions, guidance or feedback communicated by the FDA or comparable foreign regulatory authorities that have a negative impact on the potential approval of a product candidate.
The FDA or a comparable foreign regulatory authority may require more information beyond what we plan to provide in or expect to be required for a marketing application, including additional CMC information, preclinical or clinical data to support approval, whichapproval. These requirements may delay or prevent approval and our commercialization plans, or we may decide to abandon the development program. For example, at a Type B meeting in February 2022, we were not able to align with the FDA on comparability between tab-cel product versions used in the pivotal ALLELE study and the intended commercial product. The FDA initially recommended we conduct a new clinical trial with the commercial product to address the lack of alignment on comparability and to gain additional clinical experience with the intended commercial product. Throughout 2023, we held a number of meetings with the FDA on clinical aspects and CMC for a potential BLA submission for tab-cel. We recently held a pre-BLA meeting with the FDA that supports our plan to submit the tab-cel BLA in the second quarter of 2024. The FDA may not ultimately accept or approve a BLA submission with the current clinical dataset. In this case, the conduct of an additional clinical trial or trials in the lead indication or completing the ongoing ALLELE study may be necessary to support a BLA submission for tab-cel, which would result in considerable delay to a BLA submission or could lead us not to pursue a BLA submission. Conducting an additional clinical trial, if required, may prove too difficult or too expensive, and the process of designing a clinical trial, enrolling enough patients, and completing treatment and data collection under the protocol could take a significant amount of time, effort, and resources. Even if we complete the clinical trial, the study may not meet its prespecified endpoints, and even if it does, the FDA may still disagree that the clinical trial is sufficient to support submission and approval of a BLA for tab-cel, or may consider that the data, while adequate for BLA submission, can support only a more limited indication than that for which we initially applied.
Our development activities and/or commercialization planning with our partners could be harmed or delayed by governmental or regulatory delays due to a variety of factors. These factors include limitations on the availability of governmental and regulatory agency personnel to review regulatory filings or engage with us (caused by global health concerns or otherwise, including the COVID-19 pandemic); changes to governmental regulatory requirements, policies, guidelines or priorities, reallocation, or availability of government resources; or for other reasons, that may significantly delay the FDA’s, or other regulatory agencies', ability to review and process any submissions we have filed or may file or cause other regulatory delays. If global health concerns continue to prevent the FDA or other regulatory authorities from conducting their regular inspections, or impact reviews or other regulatory activities, it could significantly impact the ability of the FDA or other regulatory authorities to review and process our regulatory submissions in a timely fashion, which could have a material adverse effect on our business. For example, in response to the COVID-19 pandemic, on March 10, 2020, the FDA announced its intention to postpone most inspections of foreign manufacturing facilities and products inspections of domestic manufacturing facilities through April 2020. On March 18, 2020, the FDA announced its intention to postpone temporarily routine surveillance inspections of domestic manufacturing facilities and provided guidance regarding the conduct of clinical trials. On July 10, 2020, the FDA announced that it is working toward the goal of restarting on-site inspections it deems to be “mission critical.” In May 2021, the FDA updated its guidance, first published in August 2020, clarifying how it intends to conduct inspections during the COVID-19 pandemic, including how it plans to determine which inspections are “mission critical.” Additionally, on April 14, 2021, the FDA issued a guidance document in which the FDA described its plans to conduct voluntary remote interactive evaluations of certain drug manufacturing facilities and clinical research sites. According to the guidance, the FDA intends to request such remote interactive evaluations in situations where an in-person inspection would not be prioritized, deemed mission-critical, or where direct inspection is otherwise limited by travel restrictions, but where the FDA determines that remote evaluation would still be appropriate. The FDA intends to use this risk-based assessment system to identify the categories of regulatory activity that can occur within a given geographic area, ranging from mission critical inspections to resumption of all regulatory activities. The FDA has since adjusted its inspection activities in response to the COVID-19 pandemic. On December 29, 2021, the FDA implemented temporary changes to its inspection activities to ensure the safety of its employees and regulated firms. On February 2, 2022, FDA announced that it would resume domestic surveillance inspections across all product areas on February 7, 2022. In July 2022, FDA published draft guidance outlining its policies regarding remote regulatory assessments. On May 11, 2023, the COVID-19 Public Health Emergency (PHE) declared under the Public Health Services (PHS) Act expired. It is unclear how FDA’s and other health agencies’ policies and guidance will impact any inspections of our facilities or clinical trial sites involved with our clinical studies.
If we were todo obtain approval for a product candidate marketing application, regulatory authorities may approve any of our product candidates for fewer or more limited indications than we request (including failing to approve the most commercially promising
indications), may grant approval contingent on the performance of costly post-marketing clinical trials,studies, or may approve a product candidate with a label that does not include the labeling claims necessary or desirable for the successful commercialization of that product candidate.
In addition, the clinical study requirements of the FDA, EMA and other regulatory agencies and the criteria these regulators use to determine the safety and efficacy of a product candidate are determined according to the type, complexity, novelty and intended use and market of the potential products. The regulatory approval process for novel product candidates, such as our novel T-cell product candidates and next-generation CAR T programs, can be more complex and consequently more expensive and take longer than for other, better known or extensively studied pharmaceutical or other product candidates. Approvals by the EC and FDA of autologous CAR T therapies, such as Novartis’ Kymriah® and Gilead’s Yescarta®, may not be indicative of what these regulators may require for approval of our therapies. We have multiple clinical trials of our product candidates currently ongoing. If an adverse safety issue, clinical hold or other adverse finding occurs in one or more of our clinical trials, such event could adversely affect our other clinical trials of the same or related product candidates. Moreover, our product candidates may not perform successfully in clinical studies or may be associated with adverse events that distinguish them from those that have previously been approved, such as approved autologous CAR T therapies. For instance, allogeneic product candidates may result in adverse events not experienced with autologous products. Even if a product candidate were to successfully obtain approval fromis approved by the FDA and comparable foreign regulatory authorities, anythe approval might contain significant limitations related to use restrictions for specified age groups, warnings, precautions or contraindications, or may be subject to burdensome post-approval study or risk management requirements. If we are unable to obtain regulatory approval for one of our product candidates in one or more jurisdictions, or any approval contains significant limitations, we may not be able to obtain sufficient funding to continue the development of that product or generate revenues attributable to that product candidate. Also, any regulatory approval of our current or future product candidates, once obtained, may be withdrawn.withdrawn in a region or country by the respective regulatory agency.
Our T-cell immunotherapy product and product candidates and our next-generation CAR T programs represent new therapeutic approaches that could result in heightened regulatory scrutiny, delays in clinical development or delays in or our inability to achieve regulatory approval, commercialization or commercializationpayor coverage of our product candidates.
Our future success is dependent on the successful development and commercialization of T-cell immunotherapies and our next-generation CAR T programs in general and our development product candidates in particular. Because these programs, particularly our pipeline of allogeneic T-cell product and product candidates that are bioengineered from donors, represent a new approach to immunotherapy for the treatment of cancer and other diseases, developing and commercializing our product candidates subject us to a number of challenges, including:
•obtaining regulatory approval from the FDA and other regulatory authorities, which have limited experience with regulating the development and commercialization of T-cell immunotherapies;
immunotherapies, particularly allogeneic T-cell products and product candidates; •developing and deploying consistent and reliable processes for procuring blood from consenting third-partythird party donors, isolating T-cellsT cells from the blood of such donors, activating the isolated T-cellsT cells against a specific antigen, characterizing and storing the resulting activated T-cellsT cells for future therapeutic use, selecting and delivering a sufficient supply and breadth of appropriate partially HLA matchedHLA-matched cell line from among the available T-cell lines, and finally infusing these activated T-cellsT cells into patients;
•utilizing these product candidates in combination with other therapies (e.g., immunomodulatory approaches such as checkpoint inhibitors), which may increase the risk of adverse side effects;
•educating medical personnel regarding the potential side effect profile of our product and each of our product candidates;
candidates, particularly those that may be unique to our allogeneic T-cell product and product candidates and to our next-generation CAR T programs; •understanding and addressing variability in the quality of a donor’s T cells, which could ultimately affect our ability to manufacture products and product candidates in a reliable and consistent manner;
•developing processes for the safe administration of these products,product and product candidates, including long-term follow-up and registries, for all patients who receive these product candidates;
•establishing or making arrangements with third party manufacturers to manufacture, or manufacturing on our own, product and product candidates to our specifications and in a timely manner to support our clinical studies and, if approved, commercialization;
•sourcing clinical and, if approved by applicable regulatory authorities, commercial supplies for the materials used to manufacture and process these product and product candidates that are free from viruses and other pathogens that may increase the risk of adverse side effects;
•developing a manufacturing process and distribution network that can provide a stable supply with a cost of goods that allows for an attractive return on investment;
•establishing favorable terms with commercialization partners that possess appropriate sales and marketing capabilities ahead of and after obtaining any regulatory approval to gain market acceptance, and obtaining adequate coverage, reimbursement and pricing by third party payors and government authorities; and
| •
| establishing sales and marketing capabilities after obtaining any regulatory approval to gain market acceptance, and obtaining adequate coverage, reimbursement and pricing by third-party payors and government authorities; and
|
developing therapies for types of diseases beyond those initially addressed by our current product and product candidates.
We cannot be sure that the manufacturing processes used in connection with our T-cell immunotherapy product and product candidates will yield a sufficient supply of satisfactory products that are stable, safe, pure, and effective,potent, or comparable to those T-cellsT cells historically produced by MSK or QIMR Berghofer historically,our partners, be scalable or profitable.
Moreover, actual or perceived of safety issues, including adoption of new therapeutics or novel approaches to treatment, may adversely influence the willingness of subjects to participate in clinical trials,studies, or, if one of our product candidates is approved by applicable regulatory authorities, of physicians to subscribe to the novel treatment mechanics.mechanics or of patients to provide consent to receive a novel treatment despite its regulatory approval. The FDA or other applicable regulatory authorities may require specific post-market studies or additional information that communicates the benefits or risks of our products. New data may reveal new risks of our product candidates at any time prior to or after regulatory approval.
The FDA may also modify or enhance trial requirements which may affect enrollment. In August 2023, the FDA published a guidance document, Informed Consent, Guidance for IRBs, Clinical Investigators, and Sponsors, which supersedes past guidance and finalizes draft guidance on informed consent. The FDA’s new guidance presents evolving requirements for informed consent which may affect recruitment and retention of patients in clinical trials. Effects on recruitment and retention of patients may hinder or delay a clinical trial and could cause a significant setback to an applicable program.
Physicians, hospitals and third-partythird party payors often are slow to adoptutilize new products, technologies and treatment practices that require additional upfront costs and training. Physicians may not be willing to undergo training to adopton this novel therapy, may decide the therapy is too complex to adopt without appropriate training or not cost-efficient, and may choose not to administer the therapy. Based on these and other factors, hospitals and payors may decide that the benefits of this new therapy do not or will not outweigh its costs.
The results of preclinical studies or earlier clinical trialsstudies are not necessarily predictive of future results. Our existing product candidates in clinical trials,studies, and any other product candidate we advance into clinical trials,studies, may not have favorable results in later clinical trialsstudies or receive regulatory approval.
Success in preclinical studies and early clinical trialsstudies does not ensure that later clinical trialsstudies will generate adequate data to demonstrate the efficacy and safety of an investigational drug. ALikewise, a number of companies in the pharmaceutical and biotechnology industries, including those with greater resources and experience than us, have suffered significant setbacks in clinical trials,studies, even after seeing promising results in earlier preclinical studies or clinical trials. For example, in December 2015, we announced that our Phase 2 proof-of-concept trial of PINTA 745 did not meet its primary endpoint even though earlier clinical trials and preclinical studies had indicated that it might be effective to treat protein energy wasting in patients with end stage renal disease.studies. Despite the results reported in earlier preclinical studies or clinical trialsstudies for our product candidates, wethere can be significant variability in safety and efficacy results between different clinical trials of the same product candidate due to numerous factors. We do not know whether the clinical trialsstudies we may conduct, or clinical studies in progress, will demonstrate adequate efficacy and safety to result in regulatory approval to market tabelecleucel, ATA520, ATA188, ATA190, ATA230 or any of our other product candidates in any particular jurisdiction. Tabelecleucel
Tab-cel has been predominantly evaluated in a single-center trialstudies under investigator-sponsored INDsinvestigational new drug (INDs) applications held by MSK and in our Expanded Access Programs, utilizing a different response criteria and endpoints from those we have used or may utilize in later clinical trials. For example, the primary endpoint of both the MATCH and ALLELE trials is confirmed best objective response rate defined as the percent of patients achieving either a complete or partial response to treatment with tabelecleucel confirmed after the initial tumor assessment showing a response. In contrast, the prior MSK trials did not include response confirmation. The findings may not be reproducible in multi-center trials we conduct. For regulatory approvals, we are using independent radiologist and/or oncologist assessment of responses which may not correlate with the MSK reported assessments. In addition, thestudies. These Phase 2 clinical trialsstudies with tabelecleuceltab-cel also enrolled a heterogeneous group of patients with a variety of EBV-associatedEBV-driven malignancies, including but not limited to EBV+ PTLD after HCT and EBV+ PTLD after SOT. These Phase 2 trialsstudies were not prospectively designed to evaluate the efficacy of tabelecleuceltab-cel in the treatment of a single disease state for which we may later seek approval. Findings from early studies may not be reproducible in late phase studies we conduct. For instance, the current protocol for our ALLELE study in EBV+ PTLD is designed to rule out a 20% ORR as the null hypothesis. This means that if the lower bound of the 95% confidence interval on ORR among patients receiving at least one dose of tab-cel exceeds 20% at the end of the study, then the study would be expected to meet the primary endpoint for the treatment of PTLD. For example, assuming enrollment of 33 patients in a cohort of ALLELE, an observed ORR above approximately 37% would be expected to meet the primary endpoint for that cohort. In addition, our amended ALLELE study protocol includes an interim analysis as well as a final study analysis. We have previously received feedback from the FDA that an interim analysis of the ALLELE study may not be sufficient to support approval of a BLA. Moreover, final trialstudy results may not be consistent with interim trialstudy results. Furthermore, modifications to the total sample size of the ALLELE study and the statistical approach may be necessary in connection with the review of such a BLA submission by the FDA.
Efficacy data from prospectively designed trialsstudies may differ significantly from those obtained from retrospective subgroup analyses. In addition, clinical data obtained from a clinical trialstudy with an autologousallogeneic product candidate may not yield the same or better results withas compared to an allogeneicautologous product candidate. If later-stage clinical trialsstudies do not produce favorable results, our ability to achieve regulatory approval for any of our product candidates may be adversely impacted. Even if we believe that we have adequate data to support an application for regulatory approval to market any of our product candidates, the FDA or other regulatory authorities may not agree and may require that we conduct additional clinical trials.studies.
Ebvallo was approved under the exceptional circumstances regulatory pathway by the EMA and the MHRA, therefore continuation of the Ebvallo marketing authorizations in the EU and the UK are subject to annual reassessments. The annual reassessments will determine whether the Ebvallo marketing authorization should be maintained, changed, or suspended, based on Pierre Fabre’s fulfillment of post-marketing obligations and the risk/benefit profile of Ebvallo.
Interim “top line” and preliminary data from clinical studies that we or our partners may announce or share with regulatory authorities from time to time may change as more patient data become available and are subject to audit and verification procedures that could result in material changes in the final data.
From time to time, we or our partners may announce or share with regulatory authorities interim “top line” or preliminary data from clinical studies. Interim data from clinical studies are subject to the risk that one or more of the clinical outcomes may materially change as patient enrollment continues and more patient data become available. Preliminary or “top line” data also remain subject to audit and verification procedures that may result in the final data being materially different from the preliminary data previously announced. As a result, interim and preliminary data should be viewed with caution until the final data are available. Adverse differences between preliminary or interim data and final data could impact the regulatory approval of, and significantly harm the prospects of any product candidate that is impacted by the applicable data.
Clinical drug development involves a lengthy and expensive process with an uncertain outcome.
Clinical testing is expensive and can take many years to complete, and its outcome is inherently uncertain. Failure can occur at any time during the clinical trialstudy process. Product candidates in later stages of clinical trialsstudies may fail to show the desired safety and efficacy traits despite having progressed through preclinical and clinical trials.studies.
We may experience delays in our ongoing or future clinical trialsstudies and we do not know whether planned clinical trialsstudies will begin or enroll subjects on time, will need to be redesigned or will be completed on schedule, if at all. There can be no assurance that the FDA or comparable foreign regulatory authorities will not put clinical trialsstudies of any of our product candidates on clinical hold in the future. Clinical trialsstudies may be delayed, suspended or prematurely terminated for a variety of reasons, such as:
•delays in enrollment due to travel, shelter-in-place or quarantine policies, or other factors, related to the COVID-19 pandemic or other epidemics or pandemics;
•delay or failure in reaching agreement with the FDA or a comparable foreign regulatory authority on a trialstudy design that we are able to execute;
•delay or failure in obtaining authorization to commence a trialstudy or inability to comply with conditions imposed by a regulatory authority regarding the scope or design of a trial;
study; •delay or failure in reaching agreement on acceptable terms with prospective contract research organizations or CROs,(CROs) and clinical trialstudy sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trialstudy sites;
•delay or failure in obtaining institutional review board or IRB,(IRB) approval or the approval of other reviewing entities, including comparable foreign regulatory authorities, to conduct a clinical trialstudy at each site;
•withdrawal of clinical trialstudy sites from our clinical trialsstudies or the ineligibility of a site to participate in our clinical trials;
studies; •delay or failure in recruiting and enrolling suitableeligible subjects to participate in a trial;
study; •delay or failure in subjects completing a trialstudy or returning for post-treatment follow-up;
•clinical sites and investigators deviating from trialstudy protocol, failing to conduct the trialstudy in accordance with regulatory requirements, or dropping out of a trial;
study; •an FDA or other regulatory authority clinical site inspection reveals serious violations of regulations applicable to clinical investigations, which may result in requests for additional data analyses and/or rejection of data deemed unreliable;
•inability to identify and maintain a sufficient number of trialstudy sites, many of whichincluding because potential study sites may already be engaged in othercompeting clinical trialstudy programs including some that may be forenrolling the same indication;population;
•failure of our third-party clinical trialstudy managers to satisfy their contractual duties, meet expected deadlines or return trustworthy data;
•delay or failure in adding new trialstudy sites;
•interim results or data that are ambiguous or negative or are inconsistent with earlier results or data;
•feedback from the FDA, the IRB, data safety monitoring boards or a comparable foreign regulatory authority,authorities, or results from earlier stage or concurrent preclinical studies and clinical trials,studies, that might require modification to the protocol for a trial;
study; •a decision by the FDA, the IRB, a comparable foreign regulatory authority,authorities, or us, or a recommendation by a data safety monitoring board or comparable foreign regulatory authority, to suspend or terminate clinical trials at any timestudies for non-compliance with regulatory requirements, safety issues, including a finding that our product candidates have undesirable side effects or other unexpected characteristics, or a finding that the participants are being exposed to unacceptable health risk, or for any other reason;
•unacceptable risk-benefitbenefit/risk profile, unforeseen safety issues or adverse side effects;
•failure to demonstrate a benefit from using a product candidate;
•difficulties in manufacturing or obtaining from third parties sufficient quantities and breadth of appropriate partially HLA matched cell linelines from among the available T-cell lines to start or to use in clinical trials;
studies; •lack of adequate funding to continue a trial,study, including the incurrence of unforeseen costs due to enrollment delays, requirements to conduct additional studies or increased expenses associated with the services of our CROs and other third parties; or
•changes in governmental regulations or administrative actions or lack of adequate funding to continue a clinical trial.
study. Patient enrollment, a significant factor in the timing of clinical trials,studies, is affected by many factors including including:
•the size and nature of the patient population, population;
•the possibility that the rare diseases that many of our product candidates address are under-diagnosed;
•changing medical practice patterns or guidelines related to the diseases or conditions we are investigating;
•the severity of the disease under investigation, investigation;
•our ability to open clinical study sites;
•the proximity of subjects to clinical sites, sites;
•the patient referral practices of physicians, physicians;
•the design and eligibility criteria for the trial, the design of the clinical trial, study;
•ability to obtain and maintain patient consents, consents;
•risk that enrolled subjects will drop out or die before completion, completion;
•competition for patients from other clinical trials, studies;
•our or our partner’s ability to manufacture the requisite materials for a study;
•risk that we do not have an appropriately matched HLA cell line, competing clinical trials and lines;
•clinicians’ and patients’ perceptions as to the potential advantages and risks of the drug being studied in relation to other available therapies, including any new drugs that may be approved for the indicationsdiseases or conditions we are investigating.investigating; and
•disruptions caused by man-made or natural disasters or public health pandemics or epidemics, including, for example, the COVID-19 pandemic.
As an example, we activated additional clinical sites for the ALLELE study of tab-cel over the course of 2018 and increased HLA coverage during this period. As a result, enrollment in our studies was limited in the early part of 2018 and increased through the course of the year as we increased clinical sites and HLA coverage. However, in May 2019, we announced that enrollment in our Phase 3 studies of tab-cel for patients with EBV+ PTLD was proceeding slower than anticipated. Many of our product candidates are designed to treat rare diseases, and as a result, the pool of potential patients with respect to a given disease is small. We may not be able to initiate or continue to support clinical trialsstudies of tabelecleucel, ATA188, ATA520, ATA230tab-cel or any futureother product candidates if we are unable to locate and enroll a sufficient number of eligible participants in these trialsstudies as required by the FDA or other regulatory authorities. We experienced some transient delays in clinical trial site initiation and patient enrollment in certain of our clinical trials, including our ALLELE study, as a result of the COVID-19 pandemic. Even if we are able to enroll a sufficient number of patients in our clinical trials,studies, if the pace of enrollment is slower than we expect, the development costs for our product candidates may increase and
the completion of our trialsstudies may be delayed or our trialsstudies could become too expensive to complete.
We rely on CROs, other vendors and clinical trialstudy sites to ensure the proper and timely conduct of our clinical trials,studies, and while we have agreements governing their committed activities, we have limited influence over their actual performance. Furthermore, these third parties may also have relationships with other entities, some of which may be our competitors. Reliance on CROs entails risks to which we would not be subject if we conducted our clinical studies ourselves, including reliance on the CRO for clinical site initiation and monitoring, the possibility that the CRO does not maintain the financial resources to meet its obligations under our agreements, the possibility of breach of these agreements by the CRO because of factors beyond our control, including a failure to properly perform their obligations under these agreements, and the possibility of termination or non-renewal of the agreements by the CROs, based on their own business priorities, at a time that is costly or damaging to us. Our reliance on these third parties for research and development activities will reduce our control over these activities but will not relieve us of our regulatory responsibilities. For example, we will remain responsible for ensuring that each of our clinical trials is conducted in accordance with the general investigational plan, study protocols for the trial, statistical analysis plan and other study-specific documents (for example, monitoring and blinding plans). Moreover, the FDA requires us to comply with standards, commonly referred to as Good Clinical Practice (GCP), International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use, or ICH, guidelines, and regulations regarding the informed consent process, safety reporting requirements, data collection guidelines, and other regulations for conducting, recording and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of trial participants are protected. The EMA also requires us to comply with similar standards. Regulatory authorities enforce these GCP requirements through periodic inspections of trial sponsors, principal investigators and trial sites. If we or any of our CROs fail to comply with applicable GCP requirements, the clinical data generated in our clinical trials may be deemed unreliable and the FDA, EMA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. We cannot assure that upon inspection by a given regulatory authority, such regulatory authority will determine that any of our clinical trials comply with GCP and other applicable regulations. In addition, our clinical trials must be conducted with product produced under applicable current Good Manufacturing Practices (cGMP) and current Good Tissue Practices (cGTP) regulations. Our failure to comply with these regulations may require us to conduct new clinical trials, which would delay the marketing approval process. We also are required to register certain ongoing clinical trials and post the results of certain completed clinical trials on a government-sponsored database, ClinicalTrials.gov, within certain timeframes. Failure to do so can result in fines, adverse publicity and civil and criminal sanctions.
If we experience delays or quality issues in the conduct, completion or termination of any clinical trialstudy of our product candidates, the approval and commercial prospects of such product candidate will be harmed, and our ability to generate product revenues from such product candidate will be delayed. In addition, any delays in completing our clinical trialsstudies will increase our costs, slow down our product candidate development and approval process and jeopardize our ability to commence product sales and generate revenues. Any delays in completing our clinical trialsstudies for our product candidates may also decrease the period of commercial exclusivity. In addition, many of the factors that could cause a delay in the commencement or completion of clinical trialsstudies may also ultimately lead to the denial of regulatory approval of our product candidates.
Our product and product candidates, the methods used to deliver them or their dosage levels may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial profile of an approved label or result in significant negative consequences following any regulatory approval.
Undesirable side effects caused by our product and product candidates, their delivery methods or dosage levels could cause us or regulatory authorities to interrupt, delay or halt clinical trialsstudies and could result in a more restrictive label or the delay or denial of regulatory approval by the FDA or other comparable foreign regulatory authority. For example, hypoxia has been observed in some patients receiving ATA230 for the treatment of their CMV pneumonitis. As a result of safety or toxicity issues that we or our partners may experience in our clinical trials,studies, we or our partners may not receive approval to market any product candidates, which could prevent us from ever generating product or royalty revenues for such product candidates or achieving profitability. Results of our trialsstudies could reveal an unacceptably high severity and incidence of side effects.effects, or risks that outweigh the benefits of our product and product candidates. In such an event, our trialsstudies could be suspended or terminated, and the FDA or comparable foreign regulatory authorities could order us to cease further development of or deny approval of our product candidates for any or all targeted
indications. The drug-related side effects could affect patient recruitment or the ability of enrolled subjects to complete the trialstudy or result in potential product liability claims. Any of these occurrences may have a material adverse effect on our business, results of operations, financial condition, cash flows and future prospects.
Additionally, if any of our product candidates receives regulatory approval, and we or others later identify undesirable side effects caused by such product, a number of potentially significant negative consequences could result, including that:
•we may be forced to suspend marketing of suchthat product;
•regulatory authorities, IRBs, or other clinical trial oversight bodies may place a hold on any ongoing clinical trials;
•regulatory authorities may withdraw or change their approvals of suchthat product;
•regulatory authorities may require additional warnings on the label or limit access of suchthat product to selective specialized centers with additional safety reporting and with requirements that patients be geographically close to these centers for all or part of their treatment that could diminish the usage or otherwise limit the commercial success of such products;
treatment; •we may be required to conduct post-marketing studies;
•we may be required to change the way the product is administered;
•we could be sued and held liable for harm caused to subjects or patients; and
•our products may be seized, or we may be required to recall our products;
•our products may become less competitive in the marketplace; and
•our reputation may suffer.
Any of these events could diminish the usage or otherwise limit the commercial success of our product and product candidates and prevent us from achieving or maintaining market acceptance of the particularaffected product candidate, if approved.approved by applicable regulatory authorities.
The market opportunities for our product and product candidates may be limited to those patients who are ineligible for or have failed prior treatments and may be small.
The FDA often approves new cancer therapies initially only for use in patients with relapsed or refractory metastatic disease. We expect to seek initial approval of tab-cel and our other oncology product candidates in this setting. Subsequently, for those products that prove to be sufficiently beneficial, if any, we may seek approval for earlier lines of treatment and potentially as a first line therapy, but there is no guarantee that our product and product candidates, even if approved, would be approved for earlier lines of therapy, and, prior to any such approvals, we will have to conduct additional clinical trials.
Our projections of both the number of people who have the diseases we are targeting, as well as the subset of people with these diseases in a position to receive second or later lines of therapy, and who have the potential to benefit from treatment with our product and product candidates, are based on our current beliefs and estimates. These estimates have been derived from a variety of sources, including scientific literature, surveys of clinicians, patient foundations, or our own market research, and may prove to be incorrect. Further, new studies, product approvals, changes to the standard of care and diagnosis rates or scientific understanding of disease burden may change the estimated incidence or prevalence of these diseases, and the number of patients who could benefit from our products may turn out to be lower than expected. Additionally, the potentially addressable patient population for our product candidates may be limited or may not be amenable to treatment with our product candidates. For instance, we expect our product, tab-cel, to initially target a patient population that suffers from aggressive EBV+PTLD and has failed rituximab or rituximab plus chemotherapy. Our commercial partners may have different estimates of the market opportunities for our product or product candidates. At the outset of the COVID-19 pandemic, we initially observed a temporary slow-down in stem cell and solid organ transplant volumes. These reductions were transient, but if a reduction in such volumes resumes or if there are other disruptive factors that reduce PTLD incidence, such as changes in immunosuppression regimens or treatment of re-activated viremia, it could result in lower PTLD incidence and thus reduce the demand for tab-cel. Even if our product and product candidates obtain significant market share, because the potential target populations are small, we may never achieve profitability without obtaining regulatory approval for additional indications.
We may not be able to obtain or maintain orphan drug exclusivity for our product candidates.
Regulatory authorities in some jurisdictions, including the United StatesU.S., EU and Europe,the UK, may designate drugs for relatively small patient populations as orphan drugs. Under the Orphan Drug Act, the FDA may designate a product as an orphan drug if it is a drug intended to treat a rare disease or condition, which is generally defined as a patient population of fewer than 200,000 individuals annually in the United States. BothU.S. The FDA, the FDAEMA, and the EMAMHRA have granted us orphan statusdrug designation for tabelecleuceltab-cel for EBV+ PTLD after HCT or SOT. EMA has granted us orphan status for ATA230 for CMV infection in patients with impaired cell-mediated immunity and FDA has granted us orphan status for the ATA230 for the treatment of CMV viremia and disease in immunocompromised patients.PTLD.
Generally, if a product with an orphan drug designation subsequently receives the first regulatory approval for the indication for which it has such designation, the product is entitled to a period of marketing exclusivity, which precludes the FDA, the EMA, orand the FDAMHRA from approving another marketing application for the same drugbiologic for the same indication for that time period. The applicable period is seven years in the United StatesU.S. and ten years in Europe.the EU and the UK. The EuropeanEU and UK exclusivity periodperiods can be reduced to six years if a drug no longer meets the criteria for orphan drug designation or if the drug is sufficiently profitable so that market exclusivity is no longer justified. These periods may be reduced in the EU based on a new applicable legal framework, currently under review by the European Parliament and Council. Orphan drug exclusivity may be lost if the FDA, EMA or EMAMHRA determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantity of the drug to meet the needs of patients with the rare disease or condition. In the U.S., the FDA may still approve a later marketing application blocked by an ongoing period of orphan drug exclusivity in limited circumstances such as a showing of clinical superiority to the product with orphan drug exclusivity or if the FDA finds that the holder of the orphan drug exclusivity has not shown that it can assure the availability of sufficient quantities of the orphan drug to meet the needs of patients with the disease or condition for which the product was approved. As a result, even if one of our product candidates receives orphan exclusivity, the FDA can still approve or license other drugs or biological products that have a different active ingredient for use in treating the same indication or disease.
In addition, Congress is considering updates to the orphan drug provisions of the FDCA in response to a 2021 11th Circuit decision, Catalyst Pharms., Inc. v. Becerra, 14 F.4th 1299 (11th Cir. 2021), in which the court disagreed with the FDA’s longstanding position that the orphan drug exclusivity only applies to the approved use or indication within an eligible disease, and not to all uses or indications within the entire disease or condition. In particular, the circuit court held that that the orphan-drug exclusivity for the drug developed by Catalyst Pharmaceuticals, Inc. (Catalyst) blocked the FDA’s approval of another drug for all uses or indications within the same orphan-designated disease, Lambert-Eaton myasthenic syndrome (LEMS), even though Catalyst’s drug was approved at that time only for use in the treatment of LEMS in adults. Accordingly, the court ordered the FDA to set aside the approval of a drug indicated for LEMS in children. This decision created uncertainty in the application of the orphan drug exclusivity. Any changes to the orphan drug provisions could change our opportunities for, or likelihood of success in obtaining, orphan drug exclusivity and could materially adversely affect our business, financial condition, results of operations, cash flows and prospect.
Even if we obtain orphan drug exclusivity for a product, that exclusivity may not be maintained or effectively protect the product from competition because different drugs can be approved for the same condition. Even after an orphan drug is approved,
BTD by the FDA and PRIME designation by the EMA may not lead to a faster development or regulatory review or approval process and it does not increase the likelihood that our product candidates will receive marketing approval.
Although we have obtained BTD for tab-cel in the U.S. for treatment of patients with EBV+ PTLD who have failed rituximab, these designations may not lead to faster development or regulatory review and does not increase our likelihood of success. A breakthrough therapy is defined as a drug or biologic that is intended, alone or in combination with one or more other drugs or biologics, to treat a serious or life-threatening disease or condition and preliminary clinical evidence indicates that the drug, or biologic in our case, may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. For product candidates that have been designated as breakthrough therapies, interaction and communication between the FDA and the sponsor of the study can subsequently approvehelp to identify the most efficient path for clinical development while minimizing the number of patients placed in ineffective control regimens. Biologics designated as breakthrough therapies by the FDA may also be eligible for other expedited review programs, such as priority review. Based on our BTD, we may pursue a new drugrolling submission strategy for our BLA for tab-cel for EBV+ PTLD in the U.S. While a rolling review process may provide the opportunity for ongoing communications with and feedback from the FDA, it may not result in a faster timeline to marketing approval and has no bearing on whether or not tab-cel is ultimately approved. The FDA may raise issues and pose questions to us that may delay the initiation and completion of our BLA submission, acceptance of the complete BLA for filing, and approval of the BLA. We may not be able to provide a satisfactory or a timely response to FDA questions or we may not be able to gather the required data to prepare our BLA submissions as we plan. If we are unable to address all questions or concerns that the FDA may raise or if we do not have timely access to the data required for the same conditionpreparation of the BLA, we may not be able to initiate and complete our BLA in a timely manner and ultimately receive FDA approval. In addition, even if we submit our BLA under the rolling review process, the FDA concludesmay decide not to review portions of our BLA under the rolling review process until the submission is deemed to be complete.
PRIME designation supports the development and accelerated review by the EMA of new therapies to treat patients with unmet medical need. Despite this designation and the associated opportunity for accelerated assessment, the EMA may decide that additional time is needed for the MAA review and convert the MAA to a standard review timeline. For example, the EMA converted the tab-cel MAA review timeline from accelerated to standard.
Designation as a breakthrough therapy is at the discretion of the FDA, and access to PRIME is at the discretion of the EMA. Receipt of a BTD or PRIME designation for a product candidate may not result in a faster development process, review or approval compared to drugs considered for approval under non-expedited FDA or EMA review procedures and does not assure ultimate approval by either the FDA or EMA. In addition, the FDA or EMA, respectively, may later decide that the laterproduct no longer meets the conditions for qualification and rescind the BTD or PRIME designation or decide that the time period for FDA or EMA, respectively, review or approval will not be shortened. For example, in June 2022, FDA published a draft guidance document outlining considerations for the FDA in rescinding BTD for products that no longer meet the requirements for that designation.
A Fast Track designation by the FDA, even if granted for other current or future product candidates, may not lead to a faster development or regulatory review, licensure process and does not increase the likelihood that our product candidates will receive marketing licensure.
We may seek fast track designation for one or more of our future product candidates. If a drug or biological product is clinically superiorintended for the treatment of a serious or life-threatening disease or condition and it demonstrates the potential to address unmet medical needs for such a disease or condition, the drug sponsor may apply for FDA fast track designation for a particular indication. We may seek fast track designation for our product candidates, but there is no assurance that the FDA will grant this designation to any of our proposed product candidates. Marketing applications submitted by sponsors of products in fast track development may qualify for priority review under the policies and procedures offered by the FDA, but the fast track designation does not assure any such qualification or ultimate marketing licensure by the FDA. The FDA has broad discretion whether or not to grant fast track designation, so even if we believe a particular product candidate is eligible for this designation, there can be no assurance that the FDA would decide to grant it. Even if we do receive fast track designation, we may not experience a faster development process, review or licensure compared to conventional FDA procedures or pathways and receiving a fast track designation does not provide assurance of ultimate FDA licensure. In addition, the FDA may withdraw fast track designation at any time, including if it believes that the designation is shown to be safer, more effective or makes a major contribution to patient care.no longer supported by data from our clinical development program.
Failure to obtain regulatory or payor approval in international jurisdictions would prevent our product candidates from being marketed abroad.
In addition to regulations in the United States,U.S., to market and sell our products in the European Union,EU, the UK, many Asian countries and other jurisdictions, we, or our current or future commercialization partners, must obtain separate regulatory approvals and comply with numerous and varying regulatory requirements.requirements, both from a clinical and manufacturing perspective. The approval procedure varies among countries and can involve additional testing. The time required to obtain approval may differ substantially from that required to obtain FDA approval. The regulatory approval process outside the United StatesU.S. generally includes all of the risks associated with obtaining FDA approval.approval and may include additional risks. Clinical trialsstudies accepted in one country may not be accepted by regulatory authorities in other countries. In addition, many countries outside the United StatesU.S. require that a product be approved for reimbursement before it can be approved for sale in that country. A product candidate that has been approved for sale in a particular country may not receive reimbursement approval in that country. We or our partners may not be able to obtain approvals from regulatory authorities or payor authorities outside the United StatesU.S. on a timely basis, if at all. Approval by the FDAa regulatory agency or payor does not ensure approval by any other regulatory or payor authorities in other countries or jurisdictions, and approval by one regulatory authority outside the United States does not ensure approval by regulatory authorities in other countries or jurisdictions or by the FDA.jurisdictions. We may not be able to file for regulatory approvals and may not receive necessary approvals to commercialize our products in any market. If we or our partners are unable to obtain approval of any of our product candidates by regulatory or payor authorities in the European Union,US, EU, the UK, Asia or elsewhere, the commercial prospects of that product candidate may be significantly diminished, our business prospectsdiminished.
The proposed revision of the European legislation on pharmaceuticals could declinelead to uncertainties over the regulatory framework that will be applicable to medicinal products in the EU, including orphan medicinal products.
In April 2023, the EC published proposals to revise the existing European legislation on medicinal products (EU Pharma Law Review). The revisions consist of two proposals, a new directive and thisa new regulation (EU Pharma Law Proposal) that would amend and/or repeal and replace the relevant legislation concerning medicinal products for human use, including legislation concerning orphan medicinal products and medicinal products for pediatric use. The EU pharma Law Review could materially adversely affect our business, resultshave a significant impact on the designation of operations and financial condition.incentives offered to orphan medicinal products in the EU. If adopted in current form, the EU Pharma Law Proposal would introduce the possibility for the Commission, by way of delegated acts, to derogate from the current prevalence criterion, and introduce specific criteria for certain conditions, due to the characteristics of such conditions or other scientific reasons. The EU Pharma Law Proposal also proposes changes to the current orphan market exclusivity (OME) approach. If adopted in the
current form, the EU Pharma Law Proposal would in most cases reduce the duration of the OME and replace the current system of separate OME periods for each new indication with a system with a single OME period for each active substance.
Even if our product candidates receive regulatory approval, they may still face future development and regulatory difficulties.
Even if we, or our partners obtain regulatory approval for a product candidate, it would be subject to ongoing requirements by the FDA and comparable foreign regulatory authorities governing the manufacture, quality control, further development, labeling, packaging, storage, distribution, adverse event reporting, safety surveillance, import, export, advertising, promotion, recordkeeping and reporting of safety and other post-marketing information. These requirements include submissions of safety and other post-marketing information and reports, registration, as well as continued compliance by us and/or our contract manufacturing organizations, or CMOs and CROs for any post-approval clinical trialsstudies that we conduct. They also include any post-approval requirements or commitments imposed by FDA or comparable foreign regulatory authorities as a condition of approval, or any risk evaluation or mitigation strategies (REMS), if applicable. The safety profile of any product will continue to be closely monitored by the FDA and comparable foreign regulatory authorities after approval. If the FDA or comparable foreign regulatory authorities become aware of new safety information after approval of any of our product candidates, they may require labeling changes or establishment of a risk evaluation and mitigation strategy, impose significant restrictions on a product’s indicated uses or marketing or impose ongoing requirements for potentially costly post-approval studies or post-market surveillance.
In addition, manufacturers of drug products and their facilities are subject to initial and continual review and periodic inspections by the FDA and other regulatory authorities for compliance with current good manufacturing practices, or cGMP, current Good Clinical Practices, or GCP, current good tissue practices, or cGTP and other regulations. If we or a regulatory agency discover previously unknown problems with a product, such as adverse events of unanticipated severity or frequency, or problems with the facility where the product is manufactured, a regulatory agency may impose restrictions on that product, the manufacturing facility or us, including requiring recall or withdrawal of the product from the market or suspension of manufacturing. If we, our products, product candidates, or the manufacturing facilities for our products or product candidates fail to comply with applicable regulatory requirements, a regulatory agency may:
•issue warning letters or untitled letters;
•mandate modifications to promotional materials or require us to provide corrective information to healthcare practitioners;
•require us or our partners to enter into a consent decree, which can include imposition of various fines, reimbursements for inspection costs, required due dates for specific actions and penalties for noncompliance;
•seek an injunction or impose civil or criminal penalties or monetary fines;
| •
| seek an injunction or impose civil or criminal penalties or monetary fines;
|
•suspend, withdraw or modify regulatory approval;
•suspend or withdraw regulatory approval;
suspendmodify any ongoing clinical trials;
studies;•refuse to approve pending applications or supplements to applications filed by us;
•suspend or impose restrictions on operations, including costly new manufacturing requirements; or
•seize or detain products, refuse to permit the import or export of products, require us to withdraw product from the market, or require us to initiate a product recall.
The occurrence of any event or penalty described above may also generate negative publicity or inhibit our ability to successfully commercialize our products and generate revenues.products.
Advertising and promotion of any product candidate that obtains approval in the United StatesU.S. will be heavily scrutinized by the FDA, the Department of Justice or the DOJ,(DOJ), the Office of Inspector General of the Department of Health and Human Services or HHS,(HHS), state attorneys general, members of the U.S. Congress and the public. Additionally, advertising and promotion of any product candidate that obtains approval outside of the United StatesU.S. will be heavily scrutinized by comparable foreign entities and stakeholders. For example, a company may not promote “off-label” uses for its drug products. An off-label use is the use of a product for an indication that is not described in the product’s FDA-approved label in the U.S. or for uses in other jurisdictions that differ from those approved by the applicable regulatory authorities.agencies. Physicians, on the other hand, may prescribe products for off-label uses. Although the FDA and other regulatory agencies do not regulate a physician’s choice of drug treatment made in the physician’s independent medical judgment, they do restrict promotional communications from companies or their sales force with respect to off-label uses of products for which marketing clearance has not been issued. However, companies may share truthful and not misleading information that is otherwise consistent with a product’s FDA approved labeling. Violations, including actual or alleged promotion of our products for unapproved or off-label uses, are subject to enforcement letters, inquiries and investigations, and civil and criminal sanctions by the FDA.FDA or
comparable foreign bodies. Any actual or alleged failure to comply with labeling and promotion requirements may have a negative impact on our business.result in fines, warning letters, mandates to corrective information to healthcare practitioners, injunctions, or civil or criminal penalties.
Regulations, guidelines and recommendations published by various government agencies and organizations may affect the use of our product candidates.
AlthoughChanges to regulations, recommendations or other guidelines advocating alternative therapies for the indications we treat could result in decreased use of our products. For example, although treatment with EBV specific T-cellsEBV-specific T cells is recognized as a recommended treatment for persistent or progressive EBV+ PTLD as set forth in the 2017 National Comprehensive Cancer Network Guidelines, future guidelines from governmental agencies, professional societies, practice management groups, private health/science foundations and other organizations involved in various diseases may relatecould lead to such matters asdecreased ability to develop our product usage, dosage, and route of administration and use of relatedcandidates, or competing therapies. Changes to these recommendations or other guidelines advocating alternative therapies could result in decreased use of our product candidates, which may adversely affect our results of operations.products once approved by applicable regulatory authorities.
We may not successfully identify, acquire, develop or commercialize new potential product candidates.
Part of our business strategy is to expand our product candidate pipeline by identifying and validating new product candidates, which we may develop ourselves, in-license or otherwise acquire from others. In addition, in the event that our existing product candidates do not receive regulatory approval or are not successfully commercialized, then the success of our business will depend on our ability to expand our product pipeline through internal development, in-licensing or other acquisitions. We may be unable to identify relevant product candidates. If we do identify such product candidates, we may be unable to reach acceptable terms with any third party from which we desire to in-license or acquire them.
We may not realize the benefits of strategic alliances that Any product candidates we may form in the future.
We may form strategic alliances, create joint venturesidentify, acquire, in-license, develop, or collaborations or enter into licensing arrangements with third parties that we believe will complement or augment our existing business. These relationships, or those like them, may require us to incur nonrecurring and other charges, increase our near- and long-term expenditures, issue securities that dilute our existing stockholders or disrupt our management and business. In addition, we face significant competition in seeking appropriate strategic alliances and the negotiation process is time-consuming and complex. Moreover, wemanufacture may not be successful in our efforts to establish a strategic alliancesafe or other alternative arrangementseffective for any future product candidatestheir targeted diseases, and programs because our research and development pipeline may be insufficient, our product candidates and programs may be deemed to be at too early a stage of development for collaborative effort and third parties may not view our product candidates and programs as having the requisite potential to demonstrate safety and efficacy. If we license productsreceive marketing authorization in a timely manner, or acquire businesses, we may not be able to realize the benefit of such transactions if we are unable to successfully integrate them with our existing operations and company culture. We cannot be certain that, following a strategic transaction or license, we will achieve the revenues or specific net income that justifies such transaction. Any delays in entering into new strategic alliances agreements related to our product candidates could also delay the development and commercialization of our product candidates and reduce their competitiveness even if they reach the market.at all.
Risks Related to Manufacturing
We are subject to a multitude of manufacturing risks, any of which could substantially increase our costs and limit supply of our product and product candidates.
ConcurrentConcurrently with the licensein-license of our existing product and product candidates, we acquired manufacturing process know-how and, certainin some cases, inventory of process intermediates as well as certain supplies intended forand clinical use,materials from MSK and QIMR Berghofer. To facilitate the manufacture of additional drug product for our Phase 3 clinical trials using the MSKpartners. Transferring manufacturing processes, testing and process know-how, we undertook the process of transferring this know-how to our CMO. Transferring manufacturing testing and processes andassociated know-how is complex and involves review and incorporation of both documented and undocumented processes that may have evolved over time. In addition, transferring production to different facilities may require utilization of new or different processes and/or equipment to meet the specific requirements of a given facility. WeEach stage is retroactively and concurrently verified to be compliant with appropriate regulations. As a result, there is a risk that all relevant know-how was not adequately transferred to us from our CMOs willpartners or that previous execution was not compliant with applicable regulations.
In addition, we need to conduct significant development and scale-up work to transfer these processes and manufacture each of our product and product candidates for various studies, trialsclinical studies and commercial launch readiness. We cannot be certain that all relevant know-how has been adequately incorporated intoTo the extent we elect to transfer manufacturing process until the completionwithin our network of studies (and the related evaluations) intendedthird party CMOs, we are required to demonstrate that the comparability of material previously produced by MSK with that generated by our CMO.product manufactured in the new or “receiving” facility is comparable to the product manufactured in the original or “sending” facility. The inability to manufacturedemonstrate to each of the applicable regulatory authorities that comparable drug product by us or our CMOwas manufactured could delay the continued development of our product candidates. Although we believe we have manufactured material that is comparable to that previously produced by MSK, the FDA, EMA, and other comparable regulatory authorities may not agree.candidates or availability of additional commercial product supplies.
The processes by which some of our product and product candidates are manufactured were initially developed by MSK or QIMR Berghoferour partners for clinical purposes. We and our CMO intend to evolve these existingthe processes for moredeveloped by our partners and the processes developed by us to support advanced clinical trials or commercialization.studies and commercialization requirements. We similarly intend to evolve the processes originating at Atara to support advanced clinical studies and commercialization requirements. Developing commercially viable manufacturing processes is a difficult and uncertain task, and there are risks associated with scaling to the level required for advanced clinical trialsstudies or commercialization, including cost overruns, potential problems with process scale-up, process reproducibility, comparability issues, stability, safety, purity and potency issues, regulatory agency review and endorsement processes, consistency and timely availability of reagents or raw materials. The manufacturing facilities in which our product and product candidates will be made could be adversely affected by pandemics, earthquakes and other natural or man-made disasters, equipment failures, labor shortages, power failures, and numerous other factors.
Additionally, In addition, there have been, and there may continue to be, transient interruptions in the processsupply of raw materials and consumables used in the development and manufacturing biologicsof our preclinical and cellularclinical cell therapies is complex, highly regulatedrelated to raw material shortages due to the COVID-19 pandemic or other global pressures, including leukapheresis collections, which supply starting materials used in our product and subjectproduct candidates, and raw materials and consumables specialized for cell therapy manufacturing. If we are unable to several risks, including but not limited to:obtain such raw materials or other necessary raw materials in a timely manner, our business operations and manufacturing capabilities could be adversely affected.
The process of manufacturing cellular therapies is extremely susceptible to product loss due to contamination, equipment failure or improper installation or operation of equipment, or vendor or operator error. Even minor deviations from normal manufacturing and distribution processes for any of our product and product candidates could result in reduced production yields, impact to key product defects,quality attributes, and other supply disruptions. Product defects can also occur unexpectedly. If microbial, viral or other contaminations arecontamination is discovered in reagents or in our product and product candidates or in the manufacturing facilities in which our product and product candidates are made, suchthese manufacturing facilities may need to be closed for an extended period of time to allow us to investigate and remedy the contamination;contamination. For example, we were informed by a CMO of mold contamination in certain manufacturing suites related to the manufacture of finished Ebvallo and
because tab-cel product and intermediates at the CMO’s facility and we worked with the CMO to investigate and remediate such contamination issue while production continued in other manufacturing suites. Because our T-cell immunotherapy product and product candidates are manufactured from cells collected from the blood of third-partythird party donors, the process of manufacturing is susceptible to the availability of the third-partythird party donor material. The process of developing products that can be commercialized may be particularly challenging, even if they otherwise prove to be safe and effective. The manufacture of these product and product candidates involves complex processes. Some of these processes require specialized equipment and highly skilled and trained personnel. The process of manufacturing these product and product candidates will be susceptible to additional risks, given the need to maintain aseptic conditions throughout the manufacturing process. Contamination with viruses or other pathogens in either the donor material or materials utilized in the manufacturing process or ingress of microbiological material at any point in the process may result in contaminated or unusable product. SuchViral contaminants may also arise in recombinant viral reagent production systems used to manufacture viral reagents used to manufacture product and product candidates. These types of contaminations could result in delays in the manufacture of products which could result in delays in the development of our product candidates. SuchThese contaminations could also increase the risk of adverse side effects. Furthermore, the productour allogeneic products ultimately consistsconsist of many individual cell lines,intermediate or cell product lots, each with a different HLA profile. As a result, the selection and distribution of the appropriate cell lineproduct lot for therapeutic use in a patient will requirerequires close coordination between clinical operations, supply chain and manufacturingquality assurance personnel.
Any adverse developments affecting our, or our CMOs’ manufacturing operations for our product and product candidates may result in lot failures, inventory shortages, shipment delays, inventory shortages, lot failures,product withdrawals or recalls or other interruptions in the supply of our drug product which could delay the development of our product candidates.candidates or our ability to supply product to our commercial partners, including Pierre Fabre. We may also have to write off inventory, incur other charges and expenses for supply of drug product that fails to meet specifications, undertake costly remediation efforts, or seek more costly manufacturing alternatives. Inability to meet the demand for our product and product candidates could damage our reputation and the reputation of our products among physicians, healthcare payors, patients or the medical community and cancer treatment centers, which could adversely affect our ability to operate our business and our results of operations.
We intend to manufacture at least a portion ofthat supports our product candidates ourselves. development efforts, including hospitals and outpatient clinics.
Delays in building, commissioning and receiving regulatory approvals for product candidates produced at our manufacturing facilityCMOs’ facilities could delay our development plans and thereby limit our ability to generate revenues.
In February 2017, we entered into a lease to build a manufacturingThe research and development and process and analytical development labs within ARC and our facility in Thousand Oaks, California, which we intend to use to manufactureAurora, Colorado, currently support our preclinical and clinical trial materials for our product candidates. This new manufacturing facility is expected to be completedmid/late development activities. Product-specific qualification to support clinical development and commercial production in 2019. This project may result in unanticipated delays and cost more than expected due to a number of factors, includingqualification activities are ongoing for product candidates at our CMOs’ facilities. If the appropriate regulatory requirements. If construction or regulatory approval ofapprovals for manufacturing product candidates at our new facility isCMOs’ facilities are delayed, we may not be able to manufacture sufficient quantities of our drugproduct candidates, which would limit our development activities and our opportunities for growth. Cost overruns associated with constructing our manufacturing facility could require us to raise additional funds from other sources.
In addition to the similar manufacturing risks described in "Risks“Risks Related to Our Dependence on Third Parties,"” our manufacturing facilityfacilities, and our CMOs’ facilities, will be subject to ongoing, periodic inspection by the FDA, EMA or other comparable regulatory agencies to ensure compliance with cGMP and GTP. Our, or our partner’s, failure to follow and document our adherence to such cGMP and GTPthese regulations or other regulatory requirements may lead to significant delays in the availability of products for clinical or, in the future, commercial use, may result in the termination of or a hold on a clinical trial,study, or may delay or prevent filing or approval of commercial marketing applications for our drugs.product candidates. We also may encounter problems with the following:
•achieving adequate orinventory of clinical-grade materials that meet FDA, EMA or other comparable regulatory agency standards or specifications with consistent and acceptable production yield and costs;
•shortages of qualified personnel, raw materials or key contractors; and
•achieving and maintaining ongoing compliance with cGMP regulations and other requirements of the FDA, EMA or other comparable regulatory agencies.
Failure to comply with applicable regulations could also result in sanctions being imposed on us, including fines, injunctions, civil penalties, a requirement to suspend or put on hold one or more of our clinical trials,studies, failure of regulatory authorities to grant marketing approval of our drugproduct candidates, delays, suspension or withdrawal of approvals, license revocation, seizures or recalls of drugproduct candidates, operating restrictions and criminal prosecutions, any of which could harm our business.
Developing advanced manufacturing techniques and process controls is costly, time consuming and is required to fully utilize our facility. Advancesor our CMOs’ facilities. Failure to advance manufacturing techniques and process controls could lead to a delay in obtaining approval for our product candidates. Without further investment, advances in manufacturing techniques may render our facilitythe facilities and equipment that manufacture our product candidates inadequate or obsolete, without further investment.obsolete.
In order to produceA number of the product candidates in our drugs in the quantities that we believe will be requiredportfolio, if approved by applicable regulatory authorities, may require significant commercial supply to meet anticipated market demand of any of our drug candidates if approved,demand. In these cases, we willmay need to increase, or "scale“scale up,"” the production process by a significant factor over the initial level of production. If we are unable to do so, are delayed, or if the cost of this scale up is not economically feasible for us or we cannot find a third-partythird party supplier, we may not be able to produce our drugsproduct candidates in a sufficient quantityquantities to meet future demand.
If one or more of our sole clinical manufacturing facilityCMO’s facilities is damaged or destroyed or production at this facilitythese facilities is otherwise interrupted, our business and prospects would be negatively affected.
If any of our CMOs’ manufacturing facilityfacilities, or the equipment in itany such facilities, is either damaged or destroyed, we may not be able to quickly or inexpensively replace oursuch manufacturing capacity or replace it at all. In the event of a temporary or protracted disruption in operations or loss of thisa facility or its equipment, we may not be able to transfer manufacturing to aanother third party.party in the time required to maintain supply. Even if we could transfer manufacturing to aanother third party, the shift would likely be expensive and time-consuming, particularly since the new facility would need to comply with the necessary regulatory requirements and we would need FDAor may require regulatory approval before selling any products manufactured at that facility. Such an event could delay our clinical trialsstudies or reduce our commercial product sales.revenues.
Currently, we maintain insurance coverage against damage to our property and to cover business interruption and research and development restoration expenses. However, our insurance coverage may not reimburse us, or may not be sufficient to reimburse us, for any expenses or losses we may suffer. We may be unable to meet our requirements for our product candidates if there were a catastrophic event or failure of our current manufacturing facility or processes.
Risks Related to Our Dependence on Third Parties
We rely on third parties to conduct our preclinical studiesMaintaining clinical and clinical trials. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, or if we lose any of our CROs, we may not be able to obtain regulatory approval for or commercialize our product candidates on a timely basis, if at all.
We have relied upon and plan to continue to rely upon third-party CROs and contractors to monitor and manage data for our ongoing preclinical and clinical programs. For example, our collaborating investigators at MSK manage the conduct of the ongoing clinical trials for ATA520 as well as perform the analysis, publication and presentation of data and results related to this program and the tabelecleucel and ATA230 programs. We also rely on studies previously conducted by MSK. Our collaborating investigators at QIMR Berghofer manage the conduct of the ongoing clinical trials for ATA190. We are utilizing a CRO for our EAP trial of tabelecleucel and for our open Phase 3 trials for EBV+ PTLD after HCT and SOT. We rely on these parties for the execution of our preclinical studies and clinical trials, and we control only some aspects of their activities. Nevertheless, we are responsible for ensuring that each of our trialscommercial timelines is conducted in accordance with the applicable protocol and legal, regulatory and scientific standards, and our reliance on the CROs does not relieve us of our regulatory responsibilities. We also rely on third parties to assist in conducting our preclinical studies in accordance with Good Laboratory Practices, or GLP, and the Animal Welfare Act requirements. We and our CROs are required to comply with federal regulations, GCP, which are international standards meant to protect the rights and health of patients that are enforced by the FDA, the Competent Authorities of the Member States of the European Economic Area and comparable foreign regulatory authorities for all of our products in clinical development, and cGTP, which are standards designed to ensure that cell and tissue based products are manufactured in a manner designed to prevent the introduction, transmission and spread of communicable diseases. Regulatory authorities enforce GCP and cGTP through periodic inspections of trial sponsors, principal investigators and trial sites. If we, or any of our partners or CROs, fail to comply with applicable GCP or cGTP, the clinical data generated in our clinical trials may be deemed unreliable and the FDA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our regulatory applications. We cannot assure you that upon inspection by a given regulatory authority, such regulatory authority will determine that any of our clinical trials comply with GCP or cGTP requirements. In addition, our clinical trials must be conducted with product produced under cGMP and cGTP requirements. We are also required to register ongoing clinical trials and post the results of completed clinical trials on a government-sponsored database, clinicaltrials.gov, within a specified timeframe. Failure to comply with these regulations may require us to repeat preclinical studies and clinical trials, which would delay the regulatory approval process and result in adverse publicity.
Our CROs are not our employees, and except for remedies available to us under our agreements with such CROs, we cannot control whether or not they devote sufficient time and resources, including experienced staff, to our ongoing clinical, nonclinical and preclinical programs. They may also have relationships with other entities, some of which may be our competitors. If CROs do not successfully carry out their contractual duties or obligations or meet expected deadlines or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols, regulatory requirements or for other reasons, our clinical trials may be extended, delayed or terminated and we may not be able to obtain regulatory approval for or successfully commercialize our product candidates. CRO or contractor errors could cause our results of operations and the commercial prospects for our product candidates to be harmed, our costs to increase and our ability to generate revenues to be delayed.
Our internal capacity for clinical trial execution and management is limited and therefore we have relied on third parties. Outsourcing these functions involves risk that third parties may not perform to our standards, may not produce results or data in a timely manner or may fail to perform at all. Other data from studies or trials previously conducted by MSK or QIMR Berghofer may emerge in the future. In addition, the use of third-party service providers requires us to disclose our proprietary information to these parties, which could increase the risk that this information will be misappropriated. We currently have a small number of employees, which limits the internal resources we have available to identify and monitor our third-party providers. To the extent we are unable to identify and successfully manage the performance of third-party service providers in the future, our business may be adversely affected. Though we carefully manage our relationships with our CROs, there can be no assurance that we will not encounter challenges or delays in the future or that these delays or challenges will not have a material adverse impactdependent on our business, financial condition and prospects.
Our CROs have the right to terminate their agreements with us in the event of an uncured material breach. In addition, some of our CROs have an ability to terminate their respective agreements with us if it can be reasonably demonstrated that the safety of the subjects participating in our clinical trials warrants such termination, if we make a general assignment for the benefit of our creditors or if we are liquidated. Identifying, qualifying and managing performance of third-party service providers can be difficult, time consuming and cause delays in our development programs. In addition, there is a natural transition period when a new CRO commences work and the new CRO may not provide the same type or level of services as the original provider. If any of our relationships with our third-party CROs terminate, we may not be able to enter into arrangements with alternative CROs or to do so timely or on commercially reasonable terms.
We have limited experience manufacturing our product candidates on a clinical scale and no experience manufacturing on a commercial scale. We are, and expect to continue to be, dependent on third parties for the manufacturing of our product candidates and ourend-to-end supply chain andnetwork to support manufacturing; if we experience problems with any of theseour third parties, the manufacturingparty suppliers or CMOs we may delay development and/or commercialization of our product candidates could be delayed.and product candidates.
We do not currently operaterely on our own facilitiesCMOs or our partners for the manufacturingcurrent production of our product candidates. In the case of tabelecleucel, we currently rely on our CMO for the production of thisand product candidatecandidates and the acquisition of materials incorporated in or used in the manufacturing or testing of theseour product candidates. In the case of ATA230, we currently rely on MSK for the production of thisand product candidate and acquisition of the materials incorporated in or used in the manufacturing or testing. In the case of ATA520, we currently rely on our CMO for the production of this product candidate. In the case of ATA188 and ATA190, we currently rely on an affiliate of QIMR Berghofer for the production of these product candidates and acquisition of the materials incorporated in or used in the manufacturing or testing.candidates. Our CMOs or partners are not our employees, and except for remedies available to us under our agreements with suchour CMOs or partners, we cannot directly control whether or not they devote sufficient time and resources, including experienced staff, to the manufacturing of supply for our ongoing clinical, nonclinical and preclinical programs. Our CMOs for our product and product candidates will need to be prepared to undergo pre-approval inspection in connection with our regulatory filings, and we cannot be certain that we will be able to adequately support them through such inspection nor that they will successfully pass any such inspection.
To meet our projected supply needs for clinical and commercial materials to support our activities through regulatory approval and commercial manufacturing of ATA188, ATA190, ATA520 and ATA230,tab-cel, product candidates resulting from our next-generation CAR T programs or any other product candidates, we will need to transition the manufacturing of suchthese materials to a CMO and/or our own facility, and such CMOs orCMO. Regardless of where production occurs, we will need to develop relationships with suppliers of critical starting materials or other materials,reagents, increase the scale of production and demonstrate comparability of the material produced at these facilities to the material that was previously produced. Transferring manufacturing processes, analytical methods and know-how is complex and involves review and incorporation of both documented and undocumented processes that may have evolved over time.
In addition, transferring production to different facilities may require utilization of new or different processes to meet the specific requirements of a given facility. We would expect additional comparability work will also need to be conducted to support the transfer of certain manufacturing processes and process improvements. We cannot be certain that all relevant know-how and data has been adequately incorporated into the manufacturing process until the completion of studies (and the related evaluations) intended to demonstrate the comparability of material previously produced with that generated by us or our CMO. For example, we generated and evaluated data from new material manufactured by our CMO and identified certain assays that need refinement prior to initiating the Phase 3 trials. We have generated comparability data using our refined assays and cell lines produced by our CMO which data we believe supports the demonstration of comparability, and we recently initiated the Phase 3 trials in the U.S. following discussions with FDA.CMOs.
If we or our CMOs are not able to successfully transfer this know-how and produce comparable product and product candidates, our ability to further develop and manufacture our product and product candidates may be negatively impacted.
We still may need to identify additional CMOs for continued production of supply for allsome of our product and product candidates. In addition, givenGiven the nature of our manufacturing processes, for our T-cell immunotherapy product candidates, the number of CMOs who possess the requisite skill and capability to manufacture our T-cell immunotherapy product candidates, isand the critical intermediates or reagents used to manufacture such products, are limited. We have not yet identified alternate suppliers in the event the current CMOs that we utilize are unable to scale production, or if we otherwise experience any problems with them. In February 2017, we entered into a lease agreement to build
We rely on our own cellular therapyCMOs and manufacturing facility in Thousand Oaks, CA. At this facility, we intend to manufacturenetwork for the production of our product and product candidates. Our supply, and ability to maintain inventory, of these products and product candidates depends on the uninterrupted and efficient operation of these facilities, which could be adversely affected by equipment failures, failure to meet regulatory or cGMP requirements, labor or raw material shortages, public health epidemics, natural disasters, power failures, cyber-attacks and many other factors. If we encounter any manufacturing or supply chain difficulties, we may be unable to meet the demand for clinical or commercial use, if approved. our products and product candidates.
Manufacturing cellular therapies is complicated and tightly regulated by the FDA and comparable regulatory authorities around the world, and although alternative third-partythird party suppliers with the necessary manufacturing and regulatory expertise and facilities exist, it could be expensive and take a significant amount of time to arrange for alternative suppliers, transfer manufacturing procedures and analytical methods to these alternative suppliers, and demonstrate comparability of material produced by such new suppliers. New manufacturers of any product, product candidate or intermediate would be required to qualify under applicable regulatory requirements. These manufacturers may not be able to manufacture our compoundsproduct and product candidates at costs, or in sufficient quantities, or in a timely manner necessary to complete development of our product candidates or make commercially successful products. If we are unable to arrange for alternative third-partythird party manufacturing sources, or to do so on commercially reasonable terms or in a timely manner, we may not be able to complete development of our product candidates, or market or distribute them. In addition, should the FDA or comparable regulatory authorities not agree with our product candidate specifications and comparability methodologies or assessments for these materials, regulatory authorities may require that we conduct additional studies, including bridging comparability testing, and further clinical development or commercial launch of our product candidatecandidates could be substantially delayed and we would incur substantial additional expenses.delayed.
Reliance on third-partythird party manufacturers entails risks to which we would not be subject if we manufactured product and product candidates ourselves, including reliance on the third party for regulatory compliance and quality assurance, the possibility that the third-partythird party manufacturer does not maintain the financial resources to meet its obligations under the manufacturing agreement, the possibility of breach of the manufacturing agreement by the third party because of factors beyond our control, including a failure to synthesize and manufacture our product candidates or any products we or our partners may eventually commercialize in accordance with our specifications, misappropriation of our proprietary information, including our trade secrets and know-how, the possibility that the third party does not devote sufficient time or resources to our product candidates or any products we or our partners may eventually commercialize based on its own business priorities, the possibility that the third party is acquired by another party and changes its business priorities, and the possibility of termination or nonrenewal of the agreement by the third party, based on its own business priorities, at a time that is costly or damaging to us. For example, the CRL MSA expires on March 31, 2024. While we are in active negotiations with CRL for a new commercial manufacturing agreement, there can be no assurance that we will be able to enter into a new commercial drug product supply agreement with CRL on terms favorable or acceptable to us or at all. Any delays in entering into a new commercial manufacturing agreement and/or further extension of the current CRL MSA could delay the development and commercialization of our product and product candidates, if approved. In addition, if we are unable enter into a new commercial manufacturing agreement with CRL on acceptable terms, or at all, we would have to explore other options for the manufacture of our product and product candidates, which could harm our business. If Fujifilm does not perform its obligations under the Fujifilm MSA adequately or does not devote sufficient time or resources to our product or product candidates, our operations, including the commercialization of our products, may be adversely impacted. Similarly, if CRL does not perform its obligations under the CRL MSA adequately or does not devote sufficient time or resources to our product or product candidates, our operations, including the commercialization of our products, may be adversely impacted. We also have non-cancellable minimum purchase commitments for products and services in certain of our agreements with our CMOs, if we do not fulfill such minimum purchase commitments, we will need to pay such CMOs the difference between the applicable minimum purchase commitment and our actual purchases of products and services for a given period. In addition, the FDA and other regulatory authorities require that our product candidates and any products that we or our partners may eventually commercialize be manufactured according to cGMP, cGTP and similar regulatory jurisdictional standards. These requirements include, among other things, quality control, quality assurance and the maintenance of records and documentation. The FDA or similar foreign regulatory agencies may also implement new standards at any time or change their interpretations and enforcement of existing standards for manufacture, packaging or testing of products. We have littlelimited control over our manufacturers’ compliance with these regulations and standards.standards and although we monitor our manufacturers, we depend on them to provide honest and accurate information. Any failure by our third-partythird party manufacturers to comply with cGMP or cGTP or failure to scale up
manufacturing processes, including any failure to deliver sufficient quantities of product candidates in a timely
manner, could lead to a delay in, or failure to obtain, regulatory approval of any of our product candidates. In addition, such failure could be the basis for the FDA to issue a warning letter, withdraw approvals for product candidates previously granted to us, or take other regulatory or legal action, including recall or seizure of outside supplies of the product candidate, total or partial suspension of production, suspension of ongoing clinical trials,studies, refusal to approve pending applications or supplemental applications, detention or product, refusal to permit the import or export of products, injunction or imposing civil and criminal penalties.
We depend on third party suppliers and testing laboratories for key materials used to produce or test our product and product candidates. Any significant disruption in our supplier relationships could harm our business. Any significant delay in the supply of a product candidate for an ongoing clinical trialstudy could considerably delay initiation or completion of our clinical trials,studies, product testing and potential regulatory approval of our product candidates. If our manufacturersraw materials or we are unablecomponents cannot be purchased or fail to purchase key materials after regulatory approval has been obtained for our product candidates,meet approved specifications, the commercial launch of our product and product candidates could be delayed, or there could be a shortage in supply, which could impair our ability to generate revenues from the sale of our product and product candidates.
We are dependent on Pierre Fabre for the commercialization of tab-cel (Ebvallo in the EU, UK) worldwide, including the United States. The failure of Pierre Fabre to meet its contractual, regulatory or other obligations could adversely affect our business and our obligations under the HCRx Agreement.
We have entered into the A&R Commercialization Agreement for tab-cel (Ebvallo in the EU and the UK) worldwide for EBV-positive cancers and are in the process of negotiating amendments to certain ancillary agreements as contemplated by the A&R Commercialization Agreement to further advance our partnership with Pierre Fabre. The closing of the A&R Commercialization Agreement occurred in December 2023. As a result, we are entirely dependent on Pierre Fabre for marketing and commercialization, including negotiation of pricing and reimbursement, of tab-cel. The timing and amount of any milestone and royalty payments we may receive under the A&R Commercialization Agreement, as well as the commercial success of tab-cel, will depend on, among other things, the efforts, allocation of resources, negotiation of pricing and reimbursement and successful commercialization of tab-cel by Pierre Fabre.
Under the terms of the A&R Commercialization Agreement, if we receive US BLA approval for tab-cel in patients with EBV+ PTLD, we are required to transfer the BLA to Pierre Fabre. Pierre Fabre will be responsible for obtaining all other regulatory approvals and maintaining all regulatory approvals. We will depend on Pierre Fabre to comply with numerous and varying regulatory requirements governing, if and when applicable, the manufacture, quality control, further development, labeling, packaging, storage, distribution, adverse event reporting, safety surveillance, import, export, advertising, promotion, recordkeeping and reporting of safety and other post-marketing information. We do not control the individual efforts of Pierre Fabre and have limited ability to terminate the A&R Commercialization Agreement if Pierre Fabre does not perform as expected. The failure of Pierre Fabre to devote sufficient time and effort to comply with regulatory requirements and maintain the US BLA (if approved), the EU and UK marketing authorizations and other regulatory approvals and/or to meet their obligations to us, could have an adverse impact on our financial results and operations, and our obligations under the HCRx Agreement with respect to the Initial Territory.
We also depend on Pierre Fabre to comply with all applicable laws relative to the commercialization of tab-cel in the Additional Territory. The failure of Pierre Fabre to devote sufficient time and effort to the commercialization of tab-cel; to meet their obligations to us, including for future royalty and milestone payments; to adequately deploy business continuity plans in the event of a crisis; and/or to satisfactorily resolve significant disagreements with us or address other factors could have an adverse impact on our financial results and operations. In addition, if Pierre Fabre violates, or are alleged to have violated, any laws or regulations during the performance of their obligations for us, it is possible that we could suffer financial and reputational harm or other negative outcomes, including possible legal consequences.
Any termination, breach or expiration of the A&R Commercialization Agreement or ancillary agreements, could have a material adverse effect on our financial position, and our obligations under the HCRx Agreement with respect to the Initial Territory, by reducing or eliminating our right to receive fees, milestones and royalties. In such an event, we may be required to devote additional efforts and to incur additional costs associated with the transfer of regulatory approvals and commercialization of tab-cel. Alternatively, we may attempt to identify and transact with a new commercialization partner, but there can be no assurance that we would be able to identify a suitable partner or transact on terms similar to the A&R Commercialization Agreement or that are favorable to us.
We may not realize the benefits of strategic alliances that we may form in the future or of potential future product acquisitions or licenses.
We may desire to form additional strategic alliances, commercialization partnerships, create joint ventures or collaborations, enter into licensing arrangements with third parties or acquire products or business, in each case that we believe will complement or augment our existing business. These relationships or transactions, or those like them, may require us to incur nonrecurring and other
charges, increase our near- and long-term expenditures, issue securities that dilute our existing stockholders, reduce the potential profitability of the products that are the subject of the relationship or disrupt our management and business. In addition, we face significant competition in seeking appropriate strategic alliances and transactions and the negotiation process is time-consuming and complex and there can be no assurance that we can enter into any of these transactions even if we desire to do so. Moreover, we may not be successful in our efforts to establish a strategic alliance or other alternative arrangements for any future product candidates and programs because our research and development pipeline may be insufficient, our product candidates and programs may be deemed to be at too early a stage of development for collaborative effort and third parties may not view our product candidates and programs as having the requisite potential to demonstrate a positive benefit/risk profile. Any delays in entering into new strategic alliances agreements related to our product candidates could also delay the development and commercialization of our product candidates and reduce their competitiveness even if they reach the market. In addition, any termination of established strategic alliance agreements will terminate any potential future funding we may receive under the relevant agreements, and we would have to seek a new partner for development or commercialization, curtail or abandon that development or commercialization, or undertake and fund the development and commercialization of the relevant product. If we seek a new partner but are unable to do so on acceptable terms, or at all, or do not have sufficient funds to conduct the development or commercialization of products ourselves, we would have to explore other strategic options, including curtailing or abandoning that development or commercialization, which could harm our business. For example, effective July 31, 2022, we terminated the agreements with Bayer pursuant to the Termination, Amendment and Program Transfer Agreement (Bayer Termination Agreement).
If we license products or acquire businesses, we may not be able to realize the benefit of these transactions if we are unable to successfully integrate them with our existing operations and company culture. We cannot be certain that, following an acquisition or license, we will achieve the financial or strategic results that would justify the transaction.
Risks Related to Our Intellectual Property
If we are unable to obtain and maintain sufficient intellectual property protection for our product candidates, or if the scope of the intellectual property protection is not sufficiently broad, our ability to commercialize our product candidates successfully and to compete effectively may be adversely affected.
We rely upon a combination of patents, trademarks, trade secrets and confidentiality agreements – both that we own or possess or that are owned or possessed by our partners that are in-licensed to us – to protect the intellectual property related to our technology, product and product candidates. The T-cell immunotherapyWhen we refer to “our” technologies, inventions, patents, patent applications or other intellectual property rights, we are referring to both the rights that we own or possess as well as those that we in-license, many of which are critical to our intellectual property protection and our business. For example, the product, product candidates and platform technology we have licensed from MSK and QIMR Berghoferour partners are protected primarily by patent or patent applications of our partners that we have licensed and as confidential know-how and trade secrets. If the intellectual property that we dorely on is not adequately protect our intellectual property,protected, competitors may be able to use our technologies and erode or negate any competitive advantage we may have, which could harm our business and ability to achieve profitability. have.
The patentability of inventions and the validity, enforceability and scope of patents in the biotechnology field is generally uncertain because it involves complex legal, scientific and factual considerations, and it has in recent years been the subject of significant litigation. Moreover, the standards applied by the U.S. Patent and Trademark Office or USPTO,(USPTO) and non-USnon-U.S. patent offices in granting patents are not always applied uniformly or predictably. For example, there is no uniform worldwide policy regarding patentable subject matter or the scope of claims allowable in biotechnology patents.
Consequently, the patent applications that we own or in-license may fail to result in issued patents with claims that cover our product candidates in the United States or in other countries. There is no assurance that all potentially relevant prior art relating to our patents and patent applications is known to us or has been found in the instances where searching was done. We may be unaware of prior art that could be used to invalidate an issued patent or prevent a pending patent application from issuing as a patent. There also may be prior art of which we are aware, but which we do not believe affects the validity or enforceability of a claim of one of our patents or patent applications, which may, nonetheless, ultimately be found to affect the validity or enforceability of such claim. As a consequence of these and other factors, our patent applications may fail to result in issued patents with claims that cover our product and product candidates in the U.S. or in other countries.
Even if patents have issued or do successfully issue from patent applications, and even if suchthese patents cover our product and product candidates, third parties may challenge the validity, enforceability or scope thereof, which may result in suchthese patents being narrowed, invalidated or held to be unenforceable. No assurance can be given that if challenged, our patents would be declared by a court to be valid or enforceable. Furthermore, even
Even if they are unchallenged, our patents and patent applications or other intellectual property rights may not adequately protect our intellectual property, provide exclusivity for our product and product candidates or prevent others from designing around our claims. There is no guarantee that we will be able to obtain a license from this other entity on commercially reasonable terms, or at all. If this entity licenses its rights elsewhere, our competitors might gain access to this intellectual property. Also, theThe possibility exists that others will develop products on an independent basis which have the same effect as our product and product
candidates and which do not infringe our patents or other intellectual property rights, or that others will design around the claims of patents that we have had issued that cover our product and product candidates. If the breadth or strength of protection provided by theour patents and patent applications we hold, license or pursue with respect to our product and product candidates isare threatened, it could jeopardize our ability to commercialize our product candidates. and product candidates and dissuade companies from collaborating with us.
We may also desire to seek a license from a third party who owns intellectual property that may be useful for providing exclusivity for our product and product candidates, or for providing the ability to develop and commercialize a product candidate in an unrestricted manner. There is no guarantee that we will be able to obtain a license from such a third party on commercially reasonable terms, or at all.
In addition, the USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. While an inadvertent lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Any of these outcomes could have an adverse impact on
We and our business.
If patent applications that we hold or in-license with respect to our technology or product candidates fail to issue, if their breadth or strength of protection is threatened, or if they fail to provide meaningful exclusivity for our product candidates, it could dissuade companies from collaborating with us. Wepartners have filed a number of patent applications covering our product and product candidates or methods of using or making those product candidates. We cannot offer any assurances about which, if any, patents will be issued with respect to these pending patent applications, the breadth of any such patents that are ultimately issued or whether any issued patents will be found invalid and unenforceable or will be threatened by third parties. Any successful challenge to these patents or any other patents owned by or exclusively licensed to us could deprive us of rights necessary for the
successful commercialization of any product candidate that we or our collaborators may develop. Because patent applications in the United StatesU.S. and most other countries are confidential for a period of time after filing, and some remain so until issued, we cannot be certain that we or our partners were the first to file any patent application related to a product or product candidate. Furthermore, if third parties have filed such patent applications that have never had a claim with an effective filing date onWe or after March 16, 2013, an interference proceeding in the United States can be initiated by the USPTO to determine who was the first to invent any of the subject matter covered by the patent claims of our applications or patents. Similarly, we could become involved in derivation proceedings before the USPTO to determine inventorship with respect to our patent applications. We may become involved in inter partes review or post-grant review proceedings in the USPTO regarding our intellectual property rights. Wepartners may also become involved in opposition proceedings inregarding our patents, including patent infringement lawsuits, interference or derivation proceedings, oppositions, and inter partes and post-grant review proceedings before the USPTO the European Patent Office or counterpart offices inand other jurisdictions regarding our intellectual property rights. In addition,non-U.S. patent offices.
Even if granted, patents have a limited lifespan. In the United States,U.S., the natural expiration of a patent generally occurs 20 years after it is filed. Although various extensions may be available if certain conditions are met, the life of a patent and the protection it affords is limited. If we encounter delays in our clinical trialsstudies or in obtaining regulatory approvals, the period of time during which we could exclusively market any of our product and product candidates under patent protection, if approved, could be reduced. Even if patents covering our product and product candidates are obtained, once the patent life has expired for a product, we may be vulnerable to competition from biosimilar products. Any loss of patent protection could have a material adverse impact on our business. Weproducts, as we may be unable to prevent competitors from entering the market with a product that is similar or identical to our product candidates, which could harm our business and ability to achieve profitability.candidates.
Furthermore, the research resulting in certain of our licensed patent rights and technology was funded by the U.S. government. As a result, the government has certain rights such as march-in rights, to suchthese patent rights and technology. When new technologies are developed with government funding, the government generally obtains certain rights in any resulting patents, including a non-exclusive license authorizing the government to practice the invention for or on behalf of the United States.U.S. These rights may permit the government to disclose our confidential information on which we rely to third parties and to exercise march-in rights to use or allow third parties to use our licensed technology. The government can exercise its march-in rights if it determines that action is necessary because we fail to achieve practical application of the government-funded technology, because action is necessary to alleviate health or safety needs, to meet requirements of federal regulations or to give preference to U.S. industry. In addition, our rights in suchany inventions that result from government-funded research may be subject to certain requirements to manufacture products embodying suchthese inventions in the United States. Any exercise by the government of such rights could harm our competitive position, business, results of operations, financial condition and future prospects.U.S.
If we are sued for infringing the intellectual property rights of third parties, suchthe resulting litigation could be costly and time-consuming and could prevent or delay our or our partners’ development and commercialization efforts.
Our commercial success depends, in part, on us and our collaboratorspartners not infringing the patents and proprietary rights of third parties. There is a substantial amount of litigation and other adversarial proceedings, both within and outside the United States,U.S., involving patent and other intellectual property rights in the biotechnology and pharmaceutical industries, including patent infringement lawsuits, interference or derivation proceedings, oppositions, and inter partes and post-grant review proceedings before the USPTO and non-U.S. patent offices. Numerous U.S. and non-U.S. issued patents and pending patent applications owned by third parties exist in the fields in which we are developing and may develop our product and product candidates. As the biotechnology and pharmaceutical industries expand and more patents are issued, the risk increases that our product and product candidates may be subject to claims of infringement of third parties’ patent rights, as it may not always be clear to industry participants, including us, which patents cover various types of products or methods of use. The coverage of patents is subject to interpretation by the courts, and the interpretation is not always uniform or predictable.
Third parties may assert infringement claims against us based on existing or future intellectual property rights, alleging that we are employing their proprietary technology without authorization. There may be third-partythird party patents or patent applications with claims to materials, formulations, methods of manufacture or methods for treatment related to the use or manufacturing of our product and product candidates that we failed to identify. For example, applications filed before November 29, 2000, and certain applications filed on or after that date that will not be filed outside the United States, remain confidential until issued as patents. Except for the preceding exceptions, patent applications in the United States and elsewhere are generally published only after a waiting period of approximately 18 months after the earliest filing date. Therefore, patent applications covering our product and product candidates could have been filed by others without our knowledge. In addition,knowledge, since these applications generally remain confidential for some period of time after their filing date. Even pending patent applications that have been published, including some of which we are aware, could be later amended in a manner that could cover our product and product candidates or their use or manufacture. WeIn addition, we may analyzehave analyzed patents or patent applications of our competitorsthird parties that we believe are relevant to our activities and believe that we are free to operate in relation to any of our product and product candidates, but our competitors may obtain issued claims, including in patents we consider to be unrelated, which may block our efforts or potentially result in any of our product, product candidates or our activities infringing suchtheir claims.
If we or our partners are sued for patent infringement, we would need to demonstrate that our product candidates, products and methods either do not infringe the patent claims of the relevant patent or that the patent claims are invalid, and we may not be able to do this. Proving that a patent is invalid is difficult. For example, in the United States, proving invalidity in a district court proceeding requires a showing of cleardifficult and convincing evidence to overcome the presumption of validity enjoyed by issued patents, and proving invalidity in an inter partes
review proceeding in the USPTO requires a showing of a preponderance of the evidence. Eveneven if we are successful in thesethe relevant proceedings, we may incur substantial costs and the time and attention of our management and scientific personnel could be diverted which could have a material adverse effect on us.from other activities. If any issued third-partythird party patents were held by a court of competent jurisdiction to cover aspects of our materials, formulations, methods of manufacture or methods for treatment, we could be forced, including by court order, to cease developing, manufacturing or commercializing the relevant product or product candidate until suchthe relevant patent expired. Alternatively, we may desire or be required to obtain a license from such third party in order to use the infringing technology and to continue developing, manufacturing or marketing the infringing product or product candidate. However, we may not be able to obtain any required license on commercially reasonably terms, or at all. Even if we were able to obtain a license, the rights may be nonexclusive, which could result in our competitors gaining access to the same intellectual property licensed to us. Ultimately,
We may face claims that we couldmisappropriated the confidential information or trade secrets of a third party. If we are found to have misappropriated a third party’s trade secrets, we may be prevented from commercializing a product candidate, or be forced to cease some aspect of our business operations, if, as a result of actual or threatened patent infringement claims, we are unable to enter into licenses on acceptable terms. This could harm our business significantly.
Parties making claims against us may obtain injunctive or other equitable relief,further using these trade secrets, which could effectively blocklimit our ability to further develop and commercialize one or more of our product candidates.
Defending against intellectual property claims of patent infringement or misappropriation of trade secrets could be costly and time consuming, regardless of the outcome. Thus, even if we were to ultimately prevail, or to settle at an early stage, suchbefore a final judgment, any litigation could burden us with substantial unanticipated costs. In addition, litigation or threatened litigation could result in significant demands on the time and attention of our management team, distracting them from the pursuit of other company business. During the course of any intellectual property litigation, there could be public announcements of the results of hearings, rulings on motions, and other interim proceedings in the litigation and these announcements may have negative impact on the perceived value of our product, product candidates, programs or intellectual property. In the event of a successful intellectual property claim of infringement against us, we may have to pay substantial damages, including treble damages and attorneys’ fees if we are found to have willfully infringed a patent, or to redesign our infringing product and product candidates, which may be impossible or require substantial time and monetary expenditure. In addition to paying monetary damages, we may lose valuable intellectual property rights or personnel and the parties making claims against us may obtain injunctive or other equitable relief, which could impose limitations on the conduct of our business. We may also elect to enter into license agreements in order to settle patent infringement claims prior to litigation, and any suchof these license agreementagreements may require us to pay royalties and other fees that could be significant.
We may face claims that we misappropriated As a result of all of the confidential informationforegoing, any actual or trade secrets of a third party. If we are found to have misappropriated a third party’s trade secrets, we may be prevented from further using such trade secrets, which could limit our ability to develop our product candidates. We are not aware of any material threatened or pending claims related to these matters, but in the future litigation may be necessary to defend against such claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rightsclaim could prevent us or personnel. Even if we are successful in defending against such claims, litigation could result in substantial costs and beour partners from developing or commercializing a distractionproduct or product candidate or force us to management. During the course of any patent or other intellectual property litigation, there could be public announcements of the results of hearings, rulings on motions, and other interim proceedings in the litigation. If securities analysts or investors regard these announcements as negative, the perceived valuecease some aspect of our product candidates, programs or intellectual property could be diminished. Accordingly, the market price of our common stock may decline.business operations.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting, enforcing and defending patents on all of our product and product candidates in all countries throughout the world would be prohibitively expensive. Our or our licensors’ intellectual property rights in certain countries outside the United StatesU.S. may be less extensive than those in the United States.U.S. In addition, the laws of certain foreign countries do not protect intellectual property rights to the same extent as laws in the United States.U.S. Consequently, we and our licensorspartners may not be able to prevent third parties from practicing our and our licensors’ inventions in countries outside the United States,U.S., or from selling or importing infringing products made using our and our licensors’ inventions in and into the United StatesU.S. or other jurisdictions. Competitors may use our and our licensors’ technologies in jurisdictions where we have not obtained patent protection or where we do not have exclusive rights under the relevant patent(s)patents to develop their own products and, further, may export otherwise infringingotherwise-infringing products to territories where we and our licensorspartners have patent protection but where enforcement is not as strong as that in the United States.U.S. These infringing products may compete with our product and product candidates in jurisdictions where we or our licensorspartners have no issued patents or where we do not have exclusive rights under the relevant patent(s),patents, or our patent claims and other intellectual property rights may not be effective or sufficient to prevent them from so competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, particularly those relating to biopharmaceuticals, which could make it difficult for us and our licensorspartners to stop the infringement of our and our licensors’ patents or marketing of competing products in violation of our and our licensors’ proprietaryintellectual property rights generally. Proceedings to enforce our and our licensors’ patent rights in foreign jurisdictions could result in substantial costs and divert our attention from other aspects of our business, could put our and our licensors’ patents at risk of being invalidated or interpreted narrowly, could put our and our licensors’ patent applications at risk of not issuing, and could provoke third parties to assert claims against us or our licensors.partners. We or our licensorspartners may not prevail in any lawsuits that we or our licensors initiate, and even if we or our licensors are successful the damages or other remedies awarded, if any, may not be commercially meaningful.
We have in-licensed a significant portion of our intellectual property from MSK and QIMR Berghofer.our partners. If we breach any of our license agreements with MSK or QIMR Berghofer,these partners, we could lose the ability to continue the development and potential commercialization of one or more of our product candidates.
We hold rights under license agreements with our partners, including MSK and QIMR Berghofer that are important to our business. Our discovery and development platform is built, in part, around patent rights exclusively in-licensed from MSK and QIMR Berghofer. The MSK agreement generally grants us an exclusive license to research, develop, make, use, offer for sale, sell and import, tabelecleucel, ATA520 and ATA230.our partners. Under our existing MSK license agreement,agreements, we are subject to various obligations, including diligence obligations with respect to development and commercialization activities, payment obligations upon achievement of certain milestones and royalties on product sales, as well as other material obligations.sales. If there is any conflict, dispute, disagreement or issue of nonperformance between us and MSKour counterparties regarding our rights or obligations under thethese license agreements, including any such conflict, dispute or disagreement arising from our failure to satisfy diligence or payment obligations, under any such agreement, we may be liable to pay damages and MSKour counterparties may have a right to terminate the affected license. The loss ourtermination of any license agreement with MSK couldone of our partners would materially adversely affect our ability to proceed to utilize the affected intellectual property that is subject to that license agreement in our drug discovery and development efforts, our ability to enter into future collaboration, licensing and/or marketing agreements for one or more affected product and product candidates and our, or our partners’ ability to commercialize the affected product and product candidates. The risks described elsewhere pertaining toFurthermore, a disagreement under any of these license agreements may harm our patents and other intellectual property rights also apply torelationship with the intellectual property rights that we license, and any failure by us or our licensors to obtain, maintain and enforce these rightspartner, which could have a material adverse effectnegative impacts on other aspects of our business.
We may become involved in lawsuits to protect or enforce our intellectual property, which could be expensive, time-consuming and unsuccessful and have a material adverse effect on the success of our business and on our stock price.business.
Third parties may infringe our patents the patents of our licensors, or misappropriate or otherwise violate our or our licensor’s intellectual property rights. Our and our licensor’s patent applications cannot be enforced against third parties practicing the technology claimed in suchthese applications unless and until a patent issues from suchthe applications, and then only to the extent the issued claims cover the technology. In the future, we or our licensorspartners may elect to initiate legal proceedings to enforce or defend our or our licensors’partners’ intellectual property rights, to protect our or our licensor’spartners’ trade secrets or to determine the validity or scope of our intellectual property rights we own or control.rights. Any claims that we or our partners assert against perceived infringers could also provoke these parties to assert counterclaims against us or our partners alleging that we or our partners infringe their intellectual property rights or that our intellectual property rights are invalid. In addition, third parties may initiate legal proceedings against us or our licensors to challenge the validity or scope of intellectual property rights we own or control. The proceedings can be expensive and time-consuming. Many of our or our licensor’s adversaries in these proceedings may have the ability to dedicate substantially greater resources to prosecuting these legal actions than we or our licensors can. Accordingly, despite our or our licensors’ efforts, we or our licensors may not be able to prevent third parties from infringing upon or misappropriating intellectual property rights we own or control, particularly in countries where the laws may not protect our rights as fully as in the United States. Litigation could result in substantial costs and diversion of management resources, which could harm our business and financial results. In addition, in an infringement proceeding, a court may decide that a patent owned by or licensed to us is invalid or unenforceable, in whole or in part, or may refuse to stop the other party from using the technology at issue on the grounds that our or our licensors’ patents do not cover the technology in question. An adverse result in any litigation proceeding could put one or more of our or our licensors’ patents at risk of being invalidated, held unenforceable or interpreted narrowly.
Interference or derivation proceedings provoked by third parties, brought by us or our licensors or collaborators,partners, or brought by the USPTO or any non-USnon-U.S. patent authority may be necessary to determine the priority of inventions or matters of inventorship with respect to our patents or patent applications. We or our partners may also become involved in other proceedings, such as reexamination or opposition proceedings, inter partesreview, post-grant review or other preissuancepre-issuance or post-grant proceedings in the USPTO or its foreign counterparts relating to our intellectual property or the intellectual property of others. An unfavorable outcome in any such proceedingof these proceedings could require us or our licensorspartners to cease using the related technology and commercializing our product and product candidates, or to attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party does not offer us or our licensorspartners a license on commercially reasonable terms if any license is offered at all. Even if we or our licensors obtain a license, it may be non-exclusive, thereby giving our competitors access to the same technologies licensed to us or our licensors. In addition, if the breadth or strength of protection provided by our or our licensor’s patents and patent applications is threatened, it could dissuade companies from collaborating with us to license, develop or commercialize current or future product and product candidates.
Any intellectual property proceedings can be expensive and time-consuming. Our or our partners’ adversaries in these proceedings may have the ability to dedicate substantially greater resources to prosecuting these legal actions than we or our partners can. Accordingly, despite our or our partners’ efforts, we or our partners may not be able to prevent third parties from infringing upon or misappropriating our intellectual property rights, particularly in countries where the laws may not protect our rights as fully as in the U.S. Even if we successfully defend such litigation or proceeding,are successful in the relevant proceedings, we may incur substantial costs and it may distractthe time and attention of our management and scientific personnel could be diverted from other employees.activities. We could be found liable for monetary damages, including treble damages and attorneys’ fees, if we are found to have willfully infringed a patent. In addition, in an infringement proceeding, a court may decide that one or more of our patents is invalid or unenforceable, in whole or in part, or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse
result in any litigation proceeding could put one or more of our patents at risk of being invalidated, held unenforceable or interpreted narrowly.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of shares of our common stock.
Changes in patent law could diminish the value of patents in general, thereby impairing our ability to protect our product candidates.
As is the case with other biotechnology and pharmaceutical companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the biopharmaceutical industry involves both technological and legal complexity, and obtaining and enforcing biopharmaceutical patents are costly, time-consuming, and inherently uncertain. The Supreme Court has ruled on several patent cases in recent years, either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our and our licensors’ ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents once obtained. Depending on future decisions by the U.S. Congress, the federal courts and/or the USPTO, the laws and regulations governing patents could change in unpredictable ways that may weaken our and our licensors’ ability to obtain new patents or to enforce existing patents and patents we and our licensors or collaborators may obtain in the future.
Patent reform legislation that has occurred could increase the uncertainties and costs surrounding the prosecution of our and our licensors’ patent applications and the enforcement or defense of our or our licensors’ issued patents.
If we are unable to protect the confidentiality of our trade secrets and other proprietary information, the value of our technology could be materially adversely affected and our business could be harmed.
In addition to seeking the protection afforded by patents, we rely on trade secret protection and confidentiality agreements to protect proprietary know-how that is not patentable or that we elect not to patent, processes for which patents are difficult to enforce, and other elements of our technology, discovery and development processes that involve proprietary know-how, information or technology that is not covered by patents. The T-cell immunotherapy product candidates and platform technology we have licensed from MSK and QIMR Berghoferour partners are protected primarily as confidential know-how and trade secrets. Any disclosure to or misappropriation by third parties of our confidential proprietary information could enable competitors to quickly duplicate or surpass our technological achievements, including by enabling them to develop and commercialize products substantially similar to or competitive with our product candidates, thus eroding our competitive position in the market.
Trade secrets can be difficult to protect. We seek to protect our proprietary technology and processes, in part, by entering into confidentiality agreements and invention assignment agreements with our employees, consultants, CMOs, and outside scientific advisors, contractors and collaborators. These agreements are designed to protect our proprietary information. Although we use reasonable efforts to protect our trade secrets, our employees, consultants, contractors, or outside scientific advisors might intentionally or inadvertently disclose our trade secrets or confidential, proprietary information to competitors. In addition, competitors may otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. If any of our confidential proprietary information were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent such competitor from using that technology or information to compete with us, which could harm our competitive position.
Enforcing a claim that a third party illegally obtained and is using any of our trade secrets is expensive and time consuming, and the outcome is unpredictable. In addition, the laws of certain foreign countries do not protect proprietary rights such as trade secrets to the same extent or in the same manner as the laws of the United States.U.S. Misappropriation or unauthorized disclosure of our trade secrets to third parties could impair our competitive advantage in the market and could materially adversely affect our business, results of operations and financial condition.
Risks Related to Commercialization of Our Product and Product Candidates
Our commercial success depends upon attaining significant market acceptance of our product and product candidates, if approved, among physicians, patients, healthcare payors and cancer treatment centers.the medical community, including hospitals and outpatient clinics.
Even if we or our partners obtain regulatory approval for any of our product candidates that we may develop or acquire in the future, the product may not gain market acceptance among physicians, healthcare payors, patients or the medical community that supports our product development efforts, including cancer treatment centers.hospitals and outpatient clinics. Market acceptance of any of our product and product candidates for which we receive approval depends on a number of factors, including:
•the efficacy and safety of suchthe product candidates as demonstrated in clinical trials;
studies; •the clinical indications and patient populations for which the product candidate is approved;
•the inclusion into clinical treatment guidelines;
•acceptance by physicians major cancer treatment centers and patients of the drug as a safe and effective treatment;
•the adoptionadministrative and logistical burden of treating patients;
•the ability to identify in a timely manner the appropriate patients who will benefit from specific therapy;
•the consideration of novel cellular therapies by physicians, hospitals and third-partythird party payors;
•the potential and perceived advantages of product candidates over alternative treatments;
•the safety of product candidates seen in a broader patient group, including its use outside the approved indications;
| •
| any restrictions on use together with other medications;
|
•any restrictions on use together with other medications;
•the prevalence and severity of any side effects;
•product labeling or product insert requirements of the FDA or other regulatory authorities;
•the timing of market introduction of our products as well as competitive products;
•the development of manufacturing and distribution processes for our novel T-cell immunotherapyproduct and product candidates;
•the cost of treatment in relation to alternative treatments;
•the availability of coverage and adequate reimbursement from, and our commercialization partners’ ability to negotiate pricing bywith, third-party payors and government authorities;
•relative convenience and ease of administration; and
•the ability to achieve a pricing and reimbursement recommendation or commercial agreement with national payors; and
•the effectiveness of our commercialization partners’ sales and marketing efforts and those of our collaborators.efforts.
If any of our product candidates are approved but fail to achieve market acceptance among physicians, patients, healthcare payors or cancer treatment centers, we will not be able to generate significant revenues, which would compromise our ability to become profitable.
Even if we or our partners are able to commercialize our product and product candidates, the products may not receive coverage and adequate reimbursement from third-partythird party payors in the United StatesU.S. and in other countries in which weour partners seek to commercialize our products, which could harm our business.
Our ability to commercialize any product successfully will depend, in part, on the extent to which coverage and adequate reimbursement for these products and related treatments will be available from government health administration authorities, private health insurers and other organizations.
Government authorities and third-partythird party payors, such as private health insurers and health maintenance organizations, determine which medications they will cover and establish reimbursement levels. A primary trend in the healthcare industry is cost containment. Government authorities and third-partythird party payors have attemptedcontinue to support initiatives to control costs by limiting coverage and the amount of reimbursement for particular medications. Increasingly, third-partythird party payors are requiring that drug companies provide them with predetermined discounts from list prices and are challenging the prices charged for medical products. Third-partyThird party payors may also seek additional clinical evidence, beyond the data required to obtain regulatory approval, demonstrating clinical benefits and value in specific patient populations before covering our products for those patients. We cannot be sure that coverage and adequate reimbursement will be available for any product that weour partners commercialize and, if reimbursement is available, what the level of reimbursement will be. In some countries such as the U.S., greater cost-shifting from the payor to the patient is also a trend, and higher patient copayments or other administrative burdens could lead to reduced demand from patients or healthcare professionals. This could particularly be the case in a challenging economic climate. Coverage and reimbursement may impact the demand for, or the price of, any product candidate for which we obtain regulatory approval. If reimbursement is not available or is available only at limited levels, we may not be able to successfully commercialize any product candidate for which we obtain regulatory approval, and ultimately our partners’ ability to successfully commercialize any product or product candidate for which we obtain regulatory approval.
There may be significant delays in obtaining coverage and reimbursement for newly approved drugs, and coverage may be more limited than the purposes for which the drug is approved by the FDA or comparable foreign regulatory authorities. Moreover, eligibility for coverage and reimbursement does not imply that any drug will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture, sale and distribution. Interim reimbursement levels for new drugs, if applicable, may also not be sufficient to cover our costs and may only be temporary. Reimbursement rates may vary according to the use of the drug and the clinical setting in which it is used, may be based on reimbursement levels already set for lower cost drugs and may be incorporated into existing payments for other services. Net prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future relaxation of laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the United States. Third-partyU.S. Coverage and reimbursement policies for drug products can differ significantly from payor to payor as there is no uniform policy of coverage and reimbursement for drug products among third party payors in the United StatesU.S. Third party payors in the U.S. often rely upon Medicare coverage policy and payment limitations in setting their own reimbursement policies. The process of determining coverage and reimbursement is often time consuming and costly which will require us to provide scientific and clinical support for the use of our products to each payor separately, with no assurance that coverage or adequate reimbursement will be obtained. It is difficult to predict at this time what government authorities and third party payors will decide with respect to coverage and reimbursement for our drug products. Our partners’ inability to promptly obtain coverage and profitable reimbursement rates from both government-funded and private payors for any approved products that we develop could have a material adverse effect on our operating results, our ability to raise capital needed to commercialize products and our overall financial condition.
Current and future legislation, including potentially unfavorable pricing regulations or other healthcare reform initiatives, may increase the difficulty and cost for us to obtain regulatory approval of and commercialize our product candidates and affect the prices we may obtain.for our product and product candidates.
The regulations that govern, among other things, regulatory approvals, coverage, pricing and reimbursement for new drug products vary widely from country to country. In the United StatesU.S. and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay regulatory
approval of our product candidates, restrict or regulate post-approval activities and affect our ability to successfully sell any product and product candidates for which we obtain regulatory approval. In particular, in March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, collectively, the Affordable Care Act (ACA), was enacted, which substantially changeschanged the way health carehealthcare is financed by both governmental and private insurers, and continues to significantly impactsimpact the U.S. pharmaceutical industry. The Affordable Care Act and its implementing regulations, among other things, addressed a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for certain drugs and biologics, including our product candidates, that are inhaled, infused, instilled, implanted or injected, increased the minimum Medicaid rebates owed by manufacturers under the Medicaid Drug Rebate Program, extended the Medicaid Drug Rebate Program to utilization of prescriptions of individuals enrolled in Medicaid managed care organizations, subjected manufacturers to new annual fees and taxes for certain branded prescription drugs, provided incentives to programs that increase the federal government’s comparative effectiveness research and established a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% point-of-sale discounts off negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer’s outpatient drugs to be covered under Medicare Part D.research.
Other legislative changes have been proposed and adopted in the United StatesU.S. since the Affordable Care ActACA was enacted. In August 2011, the Budget Control Act of 2011, among other things, created measures for spending reductions by Congress. A Joint Select Committee on Deficit Reduction, tasked with recommending a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, was unable to reach required goals, thereby triggering the legislation’s automatic reduction to several government programs.U.S. Congress. This includes aggregate reductions of Medicare payments to providers of 2% per fiscal year, which went into effect in April 2013,2013. As a result of the COVID-19 pandemic, this reduction was temporarily suspended from May 1, 2020 through March 31, 2022, with subsequent reductions to 1% from April 1, 2022 until June 30, 2022. The 2% reduction was then reinstated and has been in effect since June 30, 2022, and will remain in effect through 2025(with additional reductions of 2.25% in the first half of 2030 and 3% in the second half of 2030 to offset the COVID-19 suspension) until 2031 unless additional Congressional action is taken. In January 2013, President Obama signed into law the American Taxpayer Relief Act of 2012 or the ATRA,was enacted which, among other things, further reduced Medicare payments to several providers, including hospitals and cancer treatment centers,outpatient clinics, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years.
There have been judicial and Congressional challenges to numerous elements of the ACA, as well as efforts by both the executive and legislative branches of the federal government to repeal or replace certain aspects of the ACA. While the U.S. Congress has not passed comprehensive repeal legislation, it has enacted laws that modify certain provisions of the ACA, such as removing penalties, starting January 1, 2019, for not complying with the ACA’s individual mandate to carry health insurance and eliminating the implementation of certain mandated fees. On June 17, 2021, the U.S. Supreme Court dismissed a legal challenge to the law brought by several states arguing that, without the individual mandate, the entire ACA was unconstitutional. The Supreme Court dismissed the lawsuit without ruling on the merits of the states’ constitutionality arguments. Further, the IRA, signed into law in August 2022, extended the provision of enhanced subsidies for individuals purchasing health coverage through the ACA marketplace. The enhanced subsidies, which were originally passed as part of the American Rescue Plan Act are now extended through 2025. In the future, there may be additional challenges and/or amendments to the ACA. It is unclear how future litigation and the healthcare reform measures of the Biden administration will impact the ACA and our business.
There have been, and likely will continue to be, legislative and regulatory proposals at the foreign, federal and state levels directed at broadening the availability of healthcare and containing or lowering the cost of healthcare. We cannot predict the initiatives that may be adopted in the future. The continuing efforts of the government,governments, insurance companies, managed care organizations and other payors of healthcare services to contain or reduce costs of healthcare, and/or imposeincluding by imposing price controls, may adversely affect:
| •
| the demand for our product candidates, if we obtain regulatory approval;
|
| •
| our ability to set a price that we believe is fair for our products;
|
| •
| our ability to generate revenue and achieve or maintain profitability;
|
| •
| the level of taxes that we are required to pay; and
|
| •
| the availability of capital.
|
affect the demand for our product and product candidates for which we obtain regulatory approval and our ability to set a price that we believe is fair for our products. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors, which may adversely affect our future profitability.payors.
Since its enactment, there have been judicialFor example, in April 2023, the European Commission adopted a wide-ranging proposal for a new Directive and Congressional challenges to numerous provisions of the Affordable Care Act, as well as recent efforts by the Trump administration to repeal or replace certain aspects of the Affordable Care Act. Since January 2017, President Trump has signed two Executive Orders designed to delay the implementation of certain provisions of the Affordable Care Act or otherwise circumvent some of the requirements for health insurance mandated by the Affordable Care Act. Concurrently, Congress has considered legislation that would repeal or repeala new Regulation. If made into law, this proposal will revise and replace all or part of the Affordable Care Act. While Congress has not passed comprehensive repeal legislation, two bills affecting the implementation of certain taxes under the Affordable Care Act have been enacted. The Tax Cuts and Jobs Act of 2017 includes a provision repealing, effective January 1, 2019, the tax-based shared responsibility payment imposed by the Affordable Care Act on certain individuals who fail to maintain qualifying health coverage for all or part of a year that is commonly referred to as the “individual mandate”. Additionally, on January 22, 2018, President Trump signed a continuing resolution on appropriations for fiscal year 2018 that delayed the implementation of certain mandated fees under the Affordable Care Act, including the so-called “Cadillac” tax on certain high cost employer-sponsored insurance plans, the annual fee imposed on certain health insurance providers based on market share, and the medical device excise tax on non-exempt medical devices. Congress may consider other legislation to repeal and replace elements of the Affordable Care Act. Any repeal and replace legislation may have the effect of limiting the amounts that government agenciesexisting general pharmaceutical legislation. This change will pay for healthcare products and services, which could result in reduced demand for our products or additional pricing pressure, or may lead to significant deregulation, which could make the introduction of competing products and technologies much easier. Policy changes, including potential modification or repeal of all or parts of the Affordable Care Act or the implementation of new health care legislation couldlikely result in significant changes to the health care system, which could have a material adverse effect on our business, resultspharmaceutical industry. In particular, it is expected that the new Directive and Regulations will, if made into law, affect the duration of operations and financial condition.the period of regulatory protection afforded to medicinal products including regulatory data protection (also called “data exclusivity”), marketing exclusivity afforded to orphan medicinal products, as well as the conditions of eligibility to the orphan designation.
Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. We cannot be sure whether additional legislative changes will be enacted, or whether the FDAU.S. or foreign regulations, guidance or interpretations will be changed, or what the impact of suchthese changes on the regulatory approvals of
our product and product candidates, if any, may be.
In the United States,U.S., the European UnionEU and other potentially significant markets for our product and product candidates, government authorities and third-partythird party payors are increasingly attempting to limit or regulate the price of medical products and services, particularly for new and innovative products and therapies, which has resulted in lower average selling prices. For example,prices for certain products in certain markets. In the United States,U.S., there have been several recent Congressional inquiries and proposed billsand enacted federal and state legislation designed to, among other things, bring more transparency to drug pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drugs. Further, CongressFor example, the Consolidated Appropriations Act of 2021 included several drug price reporting and transparency measures, such as a new requirement for certain Medicare plans to develop tools to display Medicare Part D prescription drug benefit information in real time and for group and health insurance issuers to report information on pharmacy benefit and drug costs to the Secretaries of Health and Human Services, Labor, and the TrumpTreasury. Additionally, the IRA allows Medicare to: beginning in 2026, establish a “maximum fair price” for certain pharmaceutical and biological products covered under Medicare Parts B and D; beginning in 2023, penalize drug companies that raise prices for products covered under Medicare Parts B and D faster than inflation; and beginning in 2025 impose new discount obligations on pharmaceutical and biological manufacturers for products covered under Medicare Part D. CMS has also taken steps to implement the IRA, including: on June 30, 2023, issuing guidance detailing the requirements and parameters of the first round of price negotiations, to take place during 2023 and 2024, for products subject to the “maximum fair price” provision that would become effective in 2026; on August 29, 2023, releasing the initial list of ten drugs subject to price negotiations; on November 17, 2023, releasing guidance outlining the methodology for identifying certain manufacturers eligible to participate in a phase-in period where discounts on applicable products will be lower than those required by the Medicare Part D Manufacturer Discount Program; and on December 14, 2023, releasing a list of 48 Medicare Part B products that had an adjusted coinsurance rate based on the inflationary rebate provisions of the IRA for the time period of January 1, 2024 to March 31, 2024. While it remains to be seen how the drug pricing provisions imposed by the IRA will affect the broader pharmaceutical industry, several pharmaceutical manufacturers and other industry stakeholders have challenged the law, including through lawsuits brought against the U.S. Department of Health and Human Services, the Secretary of the U.S. Department of Health and Human Services, CMS, and the CMS Administrator challenging the constitutionality and administrative implementation of the IRA’s drug price negotiation provisions.
There have also been administrative developments in the U.S. related to drug pricing. For example, on February 2, 2022, the Biden Administration signaled its continued commitment to the Cancer Moonshot initiative, which was initially launched in 2016. In its announcement, the administration noted that its new goals under the initiative include addressing inequities in order to ensure broader access to cutting-edge cancer therapeutics and investing in a robust pipeline for new treatments. On September 12, 2022, President Biden issued an Executive Order to promote biotechnology and biomanufacturing innovation. The Order noted several methods through which the Biden Administration would support the advancement of biotechnology and biomanufacturing in healthcare, and instructed the Department of Health and Human Service to submit, within 180 days of the Order, a report assessing how to use biotechnology and biomanufacturing to achieve medical breakthroughs, reduce the overall burden of disease, and improve health outcomes. On October 14, 2022 President Biden issued an Executive Order on Lowering Prescription Drug Costs for Americans, which instructed the Secretary of the Department of Health and Human Services to consider whether to select for testing by the CMS Innovation Center new health care payment and delivery models that would lower drug costs and promote access to innovative drug therapies for beneficiaries enrolled in the Medicare and Medicaid programs. The Executive Order further directed the Secretary of the Department of Health and Human Services to submit, within 90 days of the date of the Executive Order, a report regarding any models that may lead to lower cost-sharing for commonly used drugs and support value-based payment that promotes high-quality care. Most recently, on February 14, 2023, the Department of Health and Human Services issued a report in response to the October 14, 2022 Executive Order, which, among other things, selects three potential drug affordability and accessibility models to be tested by the CMS Innovation Center. Specifically, the report addresses: (1) a model that would allow Part D Sponsors to establish a “high-value drug list” setting the maximum co-payment amount for certain common generic drugs at $2; (2) a Medicaid-focused model that would establish a partnership between CMS, manufacturers, and state Medicaid agencies that would result in multi-state outcomes-based agreements for certain cell and gene therapy drugs; and (3) a model that would adjust Medicare Part B payment amounts for Accelerated Approval Program drugs to advance the developments of novel treatments. On August 29, 2023, CMS released an initial list of ten drugs subject to price negotiations. This negotiation process will occur during 2023 and 2024 and result in maximum prices that will be effective beginning in 2026. While it remains to be seen how the drug pricing provisions imposed by the Inflation Reduction Act (IRA) will affect the broader pharmaceutical industry, several pharmaceutical manufacturers and other industry stakeholders have each indicated that itchallenged the law, including through lawsuits brought against the U.S. Department of Health and Human Services, the Secretary of the U.S. Department of Health and Human Services, CMS, and the CMS Administrator challenging the constitutionality and administrative implementation of the IRA’s drug price negotiation provisions.
Other proposed administrative actions may affect our government pricing responsibilities. For example, CMS has issued proposals to amend the existing Medicaid Drug Rebate Program regulations. In addition, there are pending legal and legislative developments relating to the 340B drug pricing program, including ongoing litigation challenging federal enforcement actions against manufacturers and recently introduced and enacted state legislation. It remains to be seen how these drug pricing initiatives will continue to seek new legislative and/or administrative measures to control drug costs. affect the broader pharmaceutical industry.
At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, to encourage importation from other countries and bulk purchasing. Another emerging trend at the state level is the establishment of prescription drug affordability boards, some of which will prospectively permit certain states to establish upper payment limits for drugs that the state has determined to be “high-cost”. Furthermore, the increased emphasis on managed healthcare in the United StatesU.S. and on country and regional pricing and reimbursement controls in the European UnionEU will put additional pressure on product pricing, reimbursement and usage, which may adversely affect our future product sales and results of operations.sales. These pressures can arise from rules and practices of managed care groups, other insurers, judicial decisions and governmental laws and regulations related to Medicare, Medicaid and healthcare reform, pharmaceutical reimbursement policies and pricing in general.
In addition, there is significant uncertainty regarding the reimbursement status of newly approved healthcare products. We may need to conduct expensive pharmacoeconomic studies in order to demonstrate the cost-effectiveness of our product and product candidates. If third party payors do not consider our product and product candidates to be cost-effective compared to other therapies, the payors may not cover our product and product candidates after approval as a benefit under their plans or, if they do, the level of payment may not be sufficient to allow us to sell our products on a profitable basis.
Price controls may be imposed in foreign markets, which may adversely affect our future profitability.
In some countries, particularly member statesMember States of the European Union,EU and the UK, the pricing of prescription drugs is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take considerable time after receipt of regulatory approval for a product. In addition, there can be considerable pressure by governments and other stakeholders on prices and reimbursement levels, including as part of cost containment measures. Political, economic and regulatory developments may further complicate pricing negotiations, and pricing negotiations may continue after reimbursement has been obtained. Reference pricing used by various European Union member statesEU Member States and parallel distribution, or arbitrage between low-priced and high-priced member states,Member States, can further reduce prices. In some countries, we, or our collaborators, may be required to conduct a clinical trialstudy or other studies that compare the cost-effectiveness of our product and product candidates to other available therapies in order to obtain or maintain reimbursement or pricing approval. Publication of discounts by third-partythird party payors or authorities may lead to further pressure on the prices or reimbursement levels within the country of publication and other countries. If reimbursement of our products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, our business could be adversely affected.
We face substantial competition, which may result in others discovering, developing or commercializing products before or more successfully than we do.
We face competition from numerous pharmaceutical and biotechnology enterprises, as well as from academic institutions, government agencies and private and public research institutions for our current product and product candidates. Our commercial opportunities will be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer side effects or are less expensive than any products that we may develop. Additionally, our commercial opportunities will be reduced or eliminated if novel upstream products or changes in treatment protocols reduce the overall incidence or prevalence of our current or future target diseases. Competition could result in reduced sales and pricing pressure on our product and product candidates, if approved which in turn would reduce our ability to generate meaningful revenues and have a negative impact on our results of operations.by applicable regulatory authorities. In addition, significant delays in the development of our product candidates could allow our competitors to bring products to market before us and impair any ability to commercialize our product and product candidates.
There are currently no FDA or EMA approvedFDA-approved products for the treatment of EBV+ PTLD,. and there are no EC-approved products for this indication except for Ebvallo. However, we are aware some approvedmarketed products and therapies are used off-labelper global physician treatment guidelines in the US and the EU in the treatment of EBV+ PTLD, by some healthcare professionals and institutions, such as rituximab and combination chemotherapy regimens. In addition, a number of companies and academic institutions are developing drugproduct candidates for EBV+ PTLD and other EBV associatedEBV-driven diseases, including: Cell Medica Ltd., which is conducting Phase 1 clinical trials for baltaleucel-T, an autologous EBV specific T-cell therapy in post-transplant lymphoproliferative disorder andexample, Viracta Therapeutics, Inc., or Viracta, which has initiated Phase 1b/2 clinical trials for VRx-3996 in relapsed/refractory EBV+lymphomas. In addition, Tessa Therapeutics Pte Ltd. is developing TT10, an autologous EBV specific T-cell therapy, which is currently being evaluated in Phase 3 clinical trials for the treatment of nasopharyngeal carcinoma.
Drug therapies approved or commonly used for CMV infection include antiviral compounds such as ganciclovir, valganciclovir, cidofovir and foscarnet. In addition, a number of companies and academic institutions are developing drug candidates for CMV infection and other CMV-associated diseases. These companies and academic institutions are in various stages of development with their product candidates with Merck & Co, Inc. completing Phase 3 clinical trials of letermovir, a CMV terminase inhibitor; Shire Plc which is conducting Phase 3 clinical trials of maribavir, a UL97 protein kinase inhibitor; Vical Inc., recently announced ASP0113, a
therapeutic bivalent plasma DNA CMV vaccine being evaluated in patients undergoing an allogeneic stem cell transplant, failed to meet primary or secondary endpoints in Phase 3 clinical trials. In addition, Helocyte, Inc., is conducting two Phase 2 clinical trials for a CMV MVA-vaccine and a CMV peptide vaccine in patients undergoing an allogeneic hematopoietic stem cell transplant;a monoclonal antibody combination therapy; Merck is conducting Phase 1 clinical trials for V160, a CMV DNA vaccine; VBI Vaccines Inc., has completed Phase 1 clinical trials for VBI-1501A, an eVLP vaccine; Hookipa Biotech, is conducting Phase 1 clinical trials for HB101, a bivalent vaccine, ViraCyte, is conducting Phase 1 clinical trials for Viralym-C, a CMV-specific allogeneic cell therapy product; Fate Therapeutics is conducting a Phase 1/2 clinical trial for ProTmune, a small molecule programmed mobilized peripheral blood graft; Chimerix is conducting Phase 1 clinical trials for intravenous Brincidofovir (BCV IV), a nucleotide analog and Moderna Therapeutics is conducting Phase 1 clinical trials for mRNA-1647, an mRNA based prophylactic vaccine.
Competition in the MS market is high with fourteen therapies approved for the treatment of relapsing-remitting multiple sclerosis (RRMS) in the U.S. and European Union. There are many U.S. and international competitors in the RRMS market, including major multi-national fully-integrated pharmaceutical companies and established biotechnology companies. Most recently, Ocrevus®, marketed by F. Hoffmann-La Roche, was approved for the treatment of relapsing MS in the U.S. and European Union. There are numerous other development candidates in Phase 3 trials for RRMS including Novartis’ anti-CD20 monoclonal antibody ofatumumab; Alkermes’ monomethyl fumarate prodrug ALKS 8700; Teva’s laquinimod, and Actelion’s next-generation sphingosine 1-phosphate receptor (S1PR) agonist ponesimod. Celgene recently reported positive results from Phase 3 clinical trials evaluating ozamimod, a S1PR agonist, in relapsing MS.
Only three therapies have been approved for the treatment of progressive MS. Recently, Ocrevus® was approved in the U.S. and European Union for the treatment of primary progressive MS (PPMS). Extavia® (marketed by Novartis) is approved in the U.S. and European Union for the treatment of secondary progressive MS (SPMS). Betaseron® (marketed by Bayer AG) is also approved in the European Union. Few therapies have been approved for progressive MS because many candidates have failed during clinical trial testing. In the U.S., there is one drug (mitoxantrone) approved to treat SPMS.
The SPMS and PPMS markets have active development pipelines and additional novel agents could be approved in the future. Several development candidates are being evaluated in clinical trials including a number of Phase 3 programs: MedDay SA is evaluating MD-1003, a concentrated form of biotin, for progressive MS; AB Science is evaluating masitinib, a tyrosine kinase inhibitor, for progressive MS; and Novartis is evaluating siponimod, an S1P1 modulator, for SPMS; and Actelion Pharmaceuticals is evaluating ponesimod, for SPMS.
Several products are approved for the treatment of relapsed or refractory multiple myeloma (MM) including immunomodulatory drugs (IMIDs) such as Thalomid® (Celgene Corporation), Revlimid® (Celgene Corporation) and Pomalyst® (Celgene Corporation); monoclonal antibodies such as Darzalex® (Janssen Research & Development, LLC) and Empliciti® (Bristol Myers Squibb); and proteasome inhibitors such as Velcade® (Millennium Pharmaceuticals, Inc.) and Kyprolis® (Amgen Inc.).
A number of companies and institutions are pursuing development programs for relapsed or refractory MM. These development programs include drug candidates being evaluated in clinical trials as a monotherapy or in combination with other approved agents. In addition, many groups are developing novel T-cell therapies such as autologous CAR T-cell or autologous TCR T-cell candidates. These include bluebird bio, Inc., which is conducting a pivotal, Phase 2 clinical trials testing bb2121, an anti-BCMA CART; Gilead Sciences, Inc.study for nanatinostat (formerly named tractinostat, or VRx-3996) in combination with antiviral drug valganciclovir in relapsed/refractory EBV+ lymphomas.
There are currently six autologous CAR T therapies approved in the U.S. and/or EU: Novartis’ Kymriah® (tisagenlecleucel), Gilead/Kite’s Yescarta® (axicabtagene ciloleucel) and TecartusTM (brexucabtagene autoleucel) and Bristol-Myers Squibb’s Breyanzi® (lisocabtagene maraleucel) and Abecma (idecabtagene vicleucel) with 2seventy bio, Johnson & Johnson and Legend Biotech’s CarvyktiTM (ciltacabtagene autoleucel). There are many CAR-mediated cell therapies in development, and, although the majority are autologous, they also include allogeneic and off-the-shelf cell therapies. There are multiple allogeneic CAR platforms being developed with differences in approaches to minimize instances of donor cells recognizing the patient’s body as foreign or rejection of the donor cells by the patient’s body. These approaches include the use of gene-editing to remove or inhibit the TCR and the use of cell types
without a TCR. The majority of clinical stage allogeneic CAR programs utilize alpha beta T cells as the cell type and gene editing of the T-cell receptor and HLA as the preferred technology approach, however, other strategies are also in development such as Gamma Delta T cells and NK cells. It is possible that some of these other approaches will have more favorable characteristics than the approach we utilize, which is testing KTE-585, an anti-BCMA CARTwould result in Phase 1 clinical trials; Juno Therapeutics, which is testing an anti-BCMA CARTthem being favored by potential partners or customers over our products. Depending on the diseases (such as autoimmune diseases) that we target in Phase 1 clinical trials; Autolus Limited, which is testing AUTO-2, a bi-specific anti-BCMA/TACI CARTthe future, we may face competition from both autologous and allogeneic CAR therapies and other modalities (e.g., small molecules, antibodies, bispecifics) in Phase 1 clinical trials and Adaptimmune Therapeutics PLC, which is testing an anti-NY-ESO TCR in Phase 1/2 clinical trials.the indication of interest.
Many of the approved or commonly used drugs and therapies for our current or future target diseases, including EBV+ PTLD, CMV and relapsed or refractory multiple myelomaMS, are well-establishedwell established and are widely accepted by physicians, patients and third-partythird party payors. Some of these drugs are branded and subject to patent protection, and other drugs and nutritional supplements are available on a generic basis. Insurers and other third-partythird party payors may encourage the use of generic products or specific branded products. We expect that if any of theseour product and our product candidates, isif approved, it will be priced at a significant premium over competitive generic products. ThisAbsent differentiated and compelling clinical evidence, pricing premiumpremiums may make it difficult for us to differentiate theseimpede the adoption of our products fromover currently approved or commonly used therapies, and impede adoption of our product, which may adversely impact our business. In addition, many companies are developing new therapeutics, and we cannot predict what the standard of care will become as our products continue in clinical development.
Many of our competitors or potential competitors have significantly greater established presence in the market, financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials,studies, obtaining regulatory approvals and marketing approved products than we do, and as a result may have a competitive advantage over us. Smaller or early-stage companies may also prove to be significant competitors, particularlyincluding through collaborative arrangements with large and established companies or if they are acquired by larger companies. These third parties compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical trialstudy sites and patient registration for clinical trials,studies, establishing agreements with CROs and CMOs, as well as in acquiring technologies and technology licenses complementary to our programs or advantageous to our business.
As a result of these factors, these competitors may obtain regulatory approval of their products before we are able to obtain patent protection or other intellectual property rights, which will limit our ability to develop or commercialize our product candidates. Our competitors may also develop drugs that are safer, more effective, more widely used and cheaper than ours, and may also be more successful than us in manufacturing and marketing their products. These appreciable advantages could render our product candidates obsolete or noncompetitive before we can recover the expenses of development and commercialization.other expenses.
MergersWe are subject to certain contractual obligations under our royalty financing agreement with HealthCare Royalty Partners and acquisitionsmay be subject to claims for damages if we fail to fulfill these obligations.
In December 2022, we entered into a purchase and sale agreement (the HCRx Agreement) with HCR Molag Fund, L.P. (HCRx). Under the terms of the HCRx Agreement, we received $31.0 million in cash in consideration for our right to receive a portion of future royalty payments and certain milestones for Ebvallo in the pharmaceuticalInitial Territory due to us from Pierre Fabre under the A&R Commercialization Agreement. The HCRx Agreement contains certain customary terms and biotechnology industriesconditions, including representations and warranties, covenants, and indemnification obligations in favor of each party. Among these terms, there are certain covenants regarding our compliance with the A&R Commercialization Agreement. In the event of actual or alleged breaches of the A&R Commercialization Agreement or the HCRx Agreement, we could be subject to claims for damages from HCRx and could be subject to costly litigation.
We expect the product candidates we develop will be regulated as biological products (biologics) and therefore they may be subject to competition sooner than anticipated.
The Biologics Price Competition and Innovation Act of 2009 (BPCIA) was enacted as part of the Affordable Care Act to establish an abbreviated pathway for the approval of biosimilar and interchangeable biological products. The regulatory pathway establishes legal authority for the FDA to review and approve biosimilar biologics, including the possible designation of a biosimilar as “interchangeable” based on its similarity to an approved biologic. Under the BPCIA, an application for a biosimilar product cannot be approved by the FDA until 12 years after the reference product was approved under a BLA. During this 12-year period of exclusivity, another company may still market a competing version of the reference product if the FDA approves a full BLA for the competing product containing the sponsor’s own preclinical data and data from adequate and well-controlled clinical trials to demonstrate the safety, purity and potency of their product. The law is complex and is still being interpreted and implemented by the FDA. As a result, its ultimate impact, implementation, and meaning are subject to uncertainty. While it is uncertain when processes intended to implement BPCIA may be fully adopted by the FDA, any of these processes could have a material adverse effect on the future commercial prospects for our biological products.
We believe that our product and any of the product candidates we develop that are approved in even more resources being concentrated amongthe U.S. as a smallerbiological product under a BLA should qualify for the 12-year period of exclusivity. However, there is a risk that this exclusivity could be shortened due to Congressional action or otherwise, or that the FDA will not consider the subject product candidates to be reference products for competing products, potentially creating the opportunity for generic competition sooner than anticipated. Moreover, the extent to which a biosimilar, once approved, will be substituted for any one of the reference products in a way that is similar to traditional generic substitution for non-biological products is not yet clear, and will depend on a number of marketplace and regulatory factors that are still developing.
In addition, the approval of a biologic product biosimilar to one of our competitors. Smallerproducts could have a material adverse impact on our business as it may be significantly less costly to bring to market and other early stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These third parties compete with us in recruiting and retaining qualified scientific, management and commercial personnel, establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for,priced significantly lower than our programs.products.
If we are unable to establish sales and marketing capabilities or enter into agreements with third parties to market and sell our product and product candidates, we may be unable to generate any revenue.
We do not currently have an organization forrevenue from the sale marketing and distribution of pharmaceutical products and the cost of establishing and maintaining such an organization may exceed the cost-effectiveness of doing so. our products.
In order to market any products that may be approved by the FDA and comparable foreign regulatory authorities, we must build our sales, marketing, managerial and other non-technical capabilities or make arrangementsenter into agreements with third parties to perform these services.market and sell our product. There are significant risks involvedis no guarantee that we will be able to enter into such agreements with third parties or to do so on commercially reasonable terms or in building and managing a sales organization, including our ability to hire, retain and incentivize qualified individuals, generate sufficient sales leads, provide adequate training to sales and marketing personnel and effectively manage a geographically dispersed sales and marketing team.timely manner. Any failure or delay in the development ofentering into agreements with third parties to market and sell our internal sales, marketing and distribution capabilitiesproducts, would adversely impact the commercialization of these products. There can be no assurance that we would be able to identify a suitable third party to market and sell our product or agree upon terms with third parties that are favorable or acceptable to us, or at all. If we are unable to establish adequate sales, marketingidentify and distribution capabilities, whether independently orreach agreement with a third parties,party to market and commercialize our product, we may not be ableneed to generate product revenuesexplore other strategic options, including commercializing products ourselves, and may not become profitable.there is no guarantee we can successfully commercialize products ourselves. We willmay be competing with many companies that currently have extensive and well-funded sales and marketing operations. Without an internal commercial organization or the support of a sufficiently scaled, appropriately timed and trained third party to perform sales and marketing functions, we may be unable to compete successfully against these more established companies. If
We may encounter difficulties in managing our growth, including with respect to our employee base, and managing our operations successfully.
As of December 31, 2023, we are not successfulhad 225 employees, excluding those impacted by the reduction in commercializingforce announced in November 2023. In January 2024, we announced another reduction of our current or future product candidates either on our own or through collaborations with one or more third parties, our future product revenue will suffer and we would incur significant additional losses.
workforce by approximately 25%. We will need to growmay encounter difficulties in managing the size of our organization, and we may experience difficulties in managing this growth.
As of February 15, 2018, we had 185 employees. We need to grow the size of our organization in orderoperations to support our continuedcontinuing development activities and the commercialization of our product and potential commercialization of our product candidates. In particular, we will need to add substantial numbers of additional personnel and other resources to supportcandidates by our development and potential commercialization of our product candidates.partners. As our development and commercialization plans and strategies continue to develop,evolve, or as a result of any future acquisitions, we must continue to improve our need for additional managerial, operational, manufacturing, sales, marketing, financial and other resources will increase.procedures and processes to manage the size our of operations. Our management, personnel and systems currently in place may not be adequate to support thisany future growth. Future growth would impose significant added responsibilities on members of management, including:
•managing our preclinical studies and clinical trialsstudies effectively;
•managing CMC operations and our external manufacturing partners effectively;
•identifying, recruiting, maintaining, motivating and integrating additional employees;employees, including the additional personnel needed to support continued development and of our product candidates;
•managing our internal development efforts effectively while complying with our contractual obligations to licensors, licensees, contractors and other third parties;
•improving our managerial, development, operational, information technology, and finance systems; and
•expanding our facilities.
As our operations expand, we will also need to manage additional relationships with various strategic partners, suppliers and other third parties. Our future financial performance and our ability to commercialize our product candidates and to compete effectively will depend, in part, on our ability to manage any future growth effectively. To that end, we must be able to manage our development efforts and preclinical studies and clinical trialsstudies effectively and hire, train and integrate additional management, research and development, regulatory, manufacturing administrative and sales and marketingadministrative personnel. Our failure to accomplish any of these tasks could prevent us from successfully growing our company.
Our future success depends on our ability to retain our executive officers and to attract, retain and motivate qualified personnel.
We are highly dependent upon our personnel, including Isaac E. Ciechanover, M.D., our President, Chief Executive Officer and founder, and Christopher Haqq, Ph.D., M.D., our EVP, Chief Scientific Officer. Our employment agreements with Drs. Ciechanover
and Haqq are at-will and do not prevent them from terminating their employment with us at any time. The loss of the services of either of them could impede the achievement of our research, development and commercialization objectives.
Our future growth and success depend on our ability to recruit, retain, manage and motivate our employees. The loss of any member of our senior management team or the inability to hire or retain experienced management personnel could compromise our ability to execute our business plan and harm our operating results. Because of the specialized scientific and managerial nature of our business, we rely heavily on our ability to attract and retain qualified scientific, technical and managerial personnel. The competition for qualified personnel in the pharmaceutical field is intense and as a result, we may be unable to continue to attract and retain qualified personnel necessary for the development of our business.
Our relationships with customers and third-party payors will be subject to applicable anti-kickback, fraud and abuse, privacy and other laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings.
Healthcare providers, physicians and third-party payors will play a primary role in the recommendation and prescription of any product candidates for which we obtain regulatory approval. Our future arrangements with third-party payors and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we would market, sell and distribute our products. As a pharmaceutical company, even though we do not and will not control referrals of healthcare services or bill directly to Medicare, Medicaid or other third-party payors, federal and state healthcare laws and regulations pertaining to fraud and abuse and patients’ rights are and will be applicable to our business. Restrictions under applicable federal and state healthcare laws and regulations that may affect our ability to operate include the following:
the federal healthcare Anti-Kickback Statute will constrain our marketing practices, educational programs, pricing policies, and relationships with healthcare providers or other entities, by prohibiting, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, overtly or covertly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under a federal healthcare program such as Medicare and Medicaid;
federal civil and criminal false claims laws and civil monetary penalty laws impose criminal and civil penalties, including through civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, including the Medicare and Medicaid programs, claims for payment or approval that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government;
the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program and also created federal criminal laws that prohibit knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statements in connection with the delivery of or payment for healthcare benefits, items or services;
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information held by covered entities and their business associates;
the federal physician sunshine requirements under the Affordable Care Act requires manufacturers of drugs, devices, biologics and medical supplies to report annually to HHS information related to payments and other transfers of value to physicians, other healthcare providers, and teaching hospitals, and ownership and investment interests held by physicians and other healthcare providers and their immediate family members and applicable group purchasing organizations;
analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers; some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government and may require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers; and
marketing expenditures; and state and foreign laws govern the privacy and security of health information in specified circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply
with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, imprisonment, exclusion from government funded healthcare programs, such as Medicare and Medicaid, disgorgement, additional reporting requirements and/or oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of non-compliance with these laws, and the curtailment or restructuring of our operations. If any physicians or other healthcare providers or entities with whom we expect to do business are found to not be in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs.
Our employees may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements, which could cause significant liability for us and harm our reputation.
We are exposed to the risk of employee fraud or other misconduct, including intentional failures to comply with FDA regulations or similar regulations of comparable foreign regulatory authorities, provide accurate information to the FDA or comparable foreign regulatory authorities, comply with manufacturing standards we have established, comply with federal and state healthcare fraud and abuse laws and regulations and similar laws and regulations established and enforced by comparable foreign regulatory authorities, report financial information or data accurately or disclose unauthorized activities to us. Employee misconduct could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. It is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business and results of operations, including the imposition of significant fines or other sanctions.
Product liability lawsuits against us could cause us to incur substantial liabilities and to limit commercialization of any products that we may develop.
We face an inherent risk of product liability exposure related to the testing of our product candidates in human clinical trials and will face an even greater risk if we commercially sell any products that we may develop. Product liability claims may be brought against us by subjects enrolled in our clinical trials, patients, healthcare providers or others using, administering or selling our products. If we cannot successfully defend ourselves against claims that our product candidates or products caused injuries, we could incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
decreased demand for any product candidates or products that we may develop;
termination of clinical trial sites or entire trial programs;
injury to our reputation and significant negative media attention;
withdrawal of clinical trial participants;
significant costs to defend the related litigation;
substantial monetary awards to trial subjects or patients;
diversion of management and scientific resources from our business operations; and
the inability to commercialize any products that we may develop.
We currently hold product liability insurance coverage at a level that we believe is customary for similarly situated companies and adequate to provide us with insurance coverage for foreseeable risks, but which may not be adequate to cover all liabilities that we may incur. Insurance coverage is increasingly expensive. We may not be able to maintain insurance coverage at a reasonable cost or in an amount adequate to satisfy any liability that may arise. We intend to expand our insurance coverage for products to include the sale of commercial products if we obtain regulatory approval for our product candidates in development, but we may be unable to obtain commercially reasonable product liability insurance for any products that receive regulatory approval. Large judgments have been awarded in class action lawsuits based on drugs that had unanticipated side effects. A successful product liability claim or series of claims brought against us, particularly if judgments exceed our insurance coverage, could decrease our cash and adversely affect our business.
If we and our third-party manufacturers fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on the success of our business.
We and our third-party manufacturers are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes. Our operations involve the use of hazardous and flammable materials, including chemicals and biological materials. Our operations also produce hazardous waste products. We generally contract with third parties for the disposal of these materials and wastes. We cannot eliminate the risk of contamination or injury from these materials. In the event of contamination or injury resulting from our or our third-party manufacturers’ use of hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties.
Although we maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials with a policy limit that we believe is customary for similarly situated companies and adequate to provide us with insurance coverage for foreseeable risks, this insurance may not provide adequate cover age against potential liabilities. We do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of biological or hazardous materials.
In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations may impair our research, development or production efforts. Failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions, which could adversely affect our business, financial condition, results of operations and prospects.
Our business and operations would suffer in the event of computer system failures or security breaches.
Our internal computer systems, and those of MSK, QIMR Berghofer, our CROs, our CMOs, and other business vendors on which we rely, are vulnerable to damage from computer viruses, unauthorized access, natural disasters, fire, terrorism, war and telecommunication and electrical failures. We exercise little or no control over these third parties, which increases our vulnerability to problems with their systems. If such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our drug development programs. For example, the loss of clinical trial data from completed, ongoing or planned clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach results in a loss of or damage to our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability, the further development of our product candidates could be delayed and our business could be otherwise adversely affected.
The recently passed comprehensive tax reform bill could adversely affect our business and financial condition.
On December 22, 2017, President Trump signed into law new tax legislation, or the Tax Act, which significantly reforms the Internal Revenue Code of 1986, as amended. The Tax Act, among other things, contains significant changes to corporate taxation, including reduction of the corporate tax rate from a top marginal rate of 35% to a flat rate of 21%; limitation of the tax deduction for interest expense to 30% of adjusted earnings (except for certain small businesses); limitation of the deduction of net operating losses generated in tax years beginning after December 31, 2017 to 80% of taxable income, indefinite carryforward of net operating losses generated in tax years after 2018 and elimination of net operating loss carrybacks; changes in the treatment of offshore earnings regardless of whether they are repatriated; current inclusion in U.S. federal taxable income of certain earnings of controlled foreign corporations, mandatory capitalization of research and development expenses beginning in 2022; immediate deductions for certain new investments instead of deductions for depreciation expense over time; further deduction limits on executive compensation; and modifying, repealing and creating many other business deductions and credits, including the reduction in the orphan drug credit from 50% to 25% of qualifying expenditures. We continue to examine the impact this tax reform legislation may have on our business. Notwithstanding the reduction in the corporate income tax rate, the overall impact of the Tax Act is uncertain and our business and financial condition could be adversely affected. The impact of this tax reform on holders of our common stock is also uncertain and could be adverse. This periodic report does not discuss any such tax legislation or the manner in which it might affect us or our stockholders in the future. We urge our stockholders to consult with their legal and tax advisors with respect to such legislation.
Our ability to use net operating loss carryforwards to offset future taxable income, and our ability to use tax credit carryforwards, may be subject to certain limitations.
Our ability to use our federal and state net operating losses, or NOLs, to offset potential future taxable income and related income taxes that would otherwise be due is dependent upon our generation of future taxable income, and we cannot predict with certainty when, or whether, we will generate sufficient taxable income to use all of our NOLs.
As of December 31, 2017, we reported U.S. federal and state NOLs of approximately $76.0 million, $231.4 million, respectively. These federal NOLs generated prior to 2018 will continue to be governed by the NOL tax rules as they existed prior to the adoption of the new Tax Act, which means that generally they will expire 20 years after they were generated if not used prior thereto. Many states have similar laws. Accordingly, these federal and state NOLs could expire unused and be unavailable to offset future income tax
liabilities. Under the newly enacted Tax Act, federal NOLs incurred in 2018 and in future years may be carried forward indefinitely, but the deductibility of such federal NOL’s is limited to 80% of current year taxable income. It is uncertain if and to what extent various states will conform to the newly enacted federal tax law.
In addition, under Section 382 of the Internal Revenue Code of 1986, as amended, or the Code, our ability to utilize these NOLs and other tax attributes, such as federal tax credits, in any taxable year may be limited if we have experienced an “ownership change.” Generally, a Section 382 ownership change occurs if one or more stockholders or groups of stockholders who owns at least 5% of a corporation’s stock increases its ownership by more than 50 percentage points over its lowest ownership percentage within a three-year testing period. Similar rules may apply under state tax laws. We completed a Section 382 study of transactions in our stock through December 31, 2017 and concluded that we have experienced an ownership change that we believe under Section 382 of the Code will result in limitations in our ability to use certain of our NOLs and credits. In addition, we may experience future ownership changes as a result of future offerings or other changes in the ownership of our stock, some of which are beyond our control. As a result, the amount of the NOL’s and tax credit carryforwards presented in our financial statements could be limited and, in the case of NOL’s generated in 2017 and before, may expire unused. Any such material limitation or expiration of our NOL’s may harm our future operating results by effectively increasing our future tax obligations.
Business disruptions could seriously harm our future revenues and financial condition and increase our costs and expenses.
Our operations could be subject to earthquakes, power shortages, telecommunications failures, water shortages, floods, hurricanes, typhoons, fires, extreme weather conditions, medical epidemics and other natural or manmade disasters or business interruptions, for which we are predominantly self-insured. Two of our corporate locations are located in California, an area prone to earthquakes. The occurrence of any of these business disruptions could seriously harm our operations and financial condition and increase our costs and expenses. We rely on third-party manufacturers to produce our product candidates. Our ability to obtain clinical supplies of product candidates could be disrupted, if the operations of these suppliers are affected by a man-made or natural disaster or other business interruption. The ultimate impact on us, our significant suppliers and our general infrastructure is unknown, but our operations and financial condition could suffer in the event of a major earthquake, fire or other natural disaster.
Risks Related to Ownership of Our Common Stock
Our stock price has been and will likely continue to be volatile and may decline regardless of our operating performance.
Our stock price has fluctuated in the past and can be expected to be volatile in the future. From October 16, 2014, the first date of trading of our common stock,January 1, 2022 through December 31, 2017,2023, the reported sale price of our common stock has fluctuated between $9.66$0.20 and $65.56$16.93 per share. The stock market in general and the market for biotechnology companies in particular have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. In particular, during the COVID-19 pandemic the volatility of the stock market for biopharmaceutical companies was heightened. As a result of thisthe general volatility of the biopharmaceutical market, investors may experience losses on their investment in our common stock. The market price of our common stock may be influenced by many factors, including the following:
•the success of competitive products or technologies;
•regulatory actions with respect to our product candidates or products or our competitors’ product candidates or products;
•actual or anticipated changes in our growth rate relative to our competitors;
•announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures, collaborations or capital commitments;
•announcements of the results, including safety and efficacy of our product candidates, or progress of our clinical studies;
•results of clinical trialsstudies, including safety and efficacy, of our product candidates or those of our competitors;
•regulatory or legal developments in the United StatesU.S. and other countries;
•developments or disputes concerning patent applications, issued patents or other proprietary rights;
•the recruitment or departure of key personnel;
•the level of expenses related to any of our product candidates or clinical development programs;
•the results of our efforts to in-license or acquire additional product candidates or products;
•actual or anticipated changes in estimates as to financial results, development timelines or recommendations by securities analysts;
•variations in our financial results or those of companies that are perceived to be similar to us;
•fluctuations in the valuation of companies perceived by investors to be comparable to us;
•inconsistent or unusual trading volume levels of our shares;
shares or derivatives thereof; •announcement or expectation of additional financing efforts;
•sales of our common stock by us, our insiders or our other stockholders;
| •
| sales of our common stock by us, our insiders or our other stockholders;
|
changes in the structure of healthcare payment systems;
•market conditions in the pharmaceutical and biotechnology sectors;
•general economic, industry and market conditions; and
•the other risks described in this “Risk Factors” section.
WeIn addition, the stock markets in general, and the markets for biotechnology and pharmaceutical stocks in particular, have experienced significant volatility that has often been unrelated to the operating performance of particular companies, which has resulted in decreased stock prices for many companies. For example, negative publicity regarding drug pricing and price increases by pharmaceutical companies has negatively impacted, and may be subjectcontinue to securities litigation, which is expensivenegatively impact, the markets for biotechnology and pharmaceutical stocks. Likewise, as a result of significant changes in U.S. social, political, regulatory and economic conditions or in laws and policies governing foreign trade and healthcare spending and delivery, including the possible repeal and/or replacement of all or portions of the Affordable Care Act or changes in tariffs and other restrictions on free trade stemming from U.S. and foreign government policies, or for other reasons, the financial markets could divert management attention.
Theexperience significant volatility that could also negatively impact the markets for biotechnology and pharmaceutical stocks. These market fluctuations may adversely affect the trading price of our common stock has been volatile, and instock.
In the past, class action litigation has often been instituted against companies thatwhose securities have experienced periods of volatility in the market price of their stock have been subject to securities class action litigation. We may be the target of this type ofprice. Any such litigation in the future. Securities litigationbrought against us could result in substantial costs and divert our management’s attention from other business concerns,and resources, which could seriously harmresult in delays of our business.clinical studies or our partners’ commercialization efforts.
Our principal stockholders and management own a significant percentage of our stock and will be able to exert significant control or significant influence over matters subject to stockholder approval.
Our executive officers, directors andprincipal stockholders own a significant portion of our outstanding votingcommon stock. These stockholders may be able to determine the outcome of all matters requiring stockholder approval. For example, these stockholders may be able to control elections of directors, amendments of our organizational documents, or approval of any merger, sale of assets, or other major corporate transaction. This may prevent or discourage unsolicited acquisition proposals or offers for our common stock that you may feel are in your best interest as one of our stockholders.stock. The interests of this group ofour significant stockholders may not always coincide with your interests or the interests of other stockholders and they may act in a manner that advances their best interests and not necessarily those of other stockholders, including seeking a premium value for their common stock, and might affect the prevailing market price for our common stock.
Sales of a substantial number of shares of our common stock in the public market could cause our stock price to fall.
Sales of a substantial number of shares of our common stock in the public market could occur at any time. These sales, or the perception in the market that the holders of a large number of shares intend to sell shares, could reduce the market price of our common stock. Moreover, certain holders of shares of our common stock will have rights, subject to certain conditions, to require us to file registration statements covering their shares or to include their shares in registration statements that we may file for ourselves or other stockholders. We have registered and intend to continue to register all shares of common stock that we may issue under our equity compensation plans. Once we register these shares, they can be freely sold in the public market upon issuance, subject to volume limitations applicable to affiliates.
We are an “emerging growth company” and are taking advantage of reduced disclosure and governance requirements applicable to emerging growth companies, which could result in our common stock being less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act, and we are taking advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We cannot predict if investors will find our common stock less attractive because we will rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile. We may take advantage of these reporting exemptions until we are no longer an emerging growth company, which in certain circumstances could be for up to five years from the date of our initial public offering. We will cease to be an “emerging growth company” upon the earliest of: (1) December 31, 2019; (2) the last day of the first fiscal year in which our annual gross revenues are $1 billion or more; (3) the date on which we have, during the previous rolling three-year period, issued more than $1 billion in non-convertible debt securities; and (4) the date on which we are deemed to be a “large accelerated filer” as defined in the Exchange Act.
Our status as an “emerging growth company” under the JOBS Act may make it more difficult to raise capital as and when we need it.
Because of the exemptions from various reporting requirements provided to us as an “emerging growth company” we may be less attractive to investors and it may be difficult for us to raise additional capital as and when we need it. Investors may be unable to compare our business with other companies in our industry if they believe that our financial accounting is not as transparent as other
companies in our industry. If we are unable to raise additional capital as and when we need it, our financial condition and results of operations may be materially and adversely affected.
We have incurred and will continue to incur increased costs as a result of being a public company and our management expects to devote substantial time to public company compliance programs.
As a public company, we have incurred and will continue to incur significant legal, accounting and other expenses. We are subject to the reporting requirements of the Exchange Act, which require, among other things, that we file with the SEC annual, quarterly and current reports with respect to our business and financial condition. In addition, the Sarbanes-Oxley Act, as well as rules subsequently adopted by the SEC and The Nasdaq Stock Market to implement provisions of the Sarbanes-Oxley Act, impose significant requirements on public companies, including requiring establishment and maintenance of effective disclosure and financial controls and changes in corporate governance practices. Further, pursuant to the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010, the SEC has adopted and will adopt additional rules and regulations, such as mandatory “say on pay” voting requirements, that willnow apply to us when we cease to be an emerging growth company.us. Stockholder activism, the current political environment and the potential for future regulatory reform may lead to substantial new regulations and disclosure obligations, which may lead to additional compliance costs and impact the manner in which we operate our business in ways we cannot currently anticipate.
The rules and regulations applicable to public companies have substantially increased our legal and financial compliance costs and make some activities more time-consuming and costly. To the extent these requirements divert the attention of our management and personnel from other business concerns, they could have a material adverse effect on our business, financial condition and results of operations. The increased costs will decrease our net income or increase our net loss and may require us to reduce costs in other areas of our business or increase the prices of our products or services.
Because we do not anticipate paying any cash dividends on our capital stock in the foreseeable future, capital appreciation, if any, will be yourthe sole source of potential gain.gain for our stockholders.
We have never declared or paid cash dividends on our capital stock. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business. In addition, the terms of any future debt agreements may preclude us from paying dividends. As a result, capital appreciation, if any, of our common stock will be yourthe sole source of gain for our stockholders for the foreseeable future.
Future sales and issuances of our common stock or rights to purchase common stock, including pursuant to our equity incentive plans, could result in additional dilution of the percentage ownership of our stockholders and could cause our stock price to fall.
We expect that significant additional capital will be needed in the future to continue our planned operations. To raise capital, we may sell substantial amounts of common stock or securities convertible into or exchangeable for common stock.stock in one or more
transactions at prices and in a manner we determine from time to time. These future issuances of common stock or common stock-related securities, together with the exercise of outstanding options or warrants, and any additional shares issued in connection with acquisitions or in-licenses, if any, may result in material dilution to our investors. Such sales may also result in material dilution to our existing stockholders, and new investors could gain rights, preferences and privileges senior to those of holders of our common stock.
To the extent equity valuations, including the trading price of our common stock, are depressed as a result of economic disruptions or other factors, the potential magnitude of this dilution will increase. Pursuant to our equity incentive plans, our compensation committee is authorized to grant equity-based incentive awards to our employees, non-employee directors and consultants. Future grants of RSUs, options and other equity awards and issuances of common stock under our equity incentive plans will result in dilution and may have an adverse effect on the market price of our common stock.
Some provisionsterms of our charter documents and Delaware law may have anti-takeover effects that could discourage an acquisition of us by others, even if an acquisition would be beneficial to our stockholders and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in ourOur amended and restated certificate of incorporation or certificate(Certificate of incorporation,Incorporation) and amended and restated bylaws or bylaws,(Bylaws), as well as provisions of Delaware law, could make it more difficult for a third party to acquire us or increase the cost of acquiring us, even if doing so would benefit our stockholders, or remove our current management. These include provisions that will:terms that:
•permit our board of directors to issue up to 20,000,000 shares of preferred stock, with any rights, preferences and privileges as they may designate;
•provide that all vacancies on our board of directors, including as a result of newly created directorships, may, except as otherwise required by law, be filled by the affirmative vote of a majority of directors then in office, even if less than a quorum;
•establish that our board of directors is divided into three classes, with each class serving three-year staggered terms, which makes it more difficult to replace a majority of our directors in a short period of time;
•require that any action to be taken by our stockholders must be effected at a duly called annual or special meeting of stockholders and not be taken by written consent;
•provide that stockholders seeking to present proposals before a meeting of stockholders or to nominate candidates for election as directors at a meeting of stockholders must provide advance notice in writing, and also specify requirements as to the form and content of a stockholder’s notice;
•not provide for cumulative voting rights, thereby allowing the holders of a majority of the shares of common stock entitled to vote in any election of directors to elect all of the directors standing for election; and
•provide that special meetings of our stockholders may be called only by our board of directors, the chairperson of our board of directors or by such person or persons requested by a majorityour chief executive officer.
Any of the board of directors to call such meetings.
These provisionsfactors listed above may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors, who are responsible for appointing the members of our management. For example, our board is divided into three classes. Each class has a three-year term. These classes make it more difficult to replace a majority of our directors in a short period of time. Because
In addition, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which may discourage, delay or prevent someone from acquiring us or merging with us whether or not it is desired by or beneficial to our stockholders. Under Delaware law, a corporation may not, in general, engage in a business combination with any holder of 15% or more of its capital stock unless the holder has held the stock for three years or, among other things, the board of directors has approved the transaction. Any provisionterm of our amended and restated certificateCertificate of incorporationIncorporation or amended and restated bylawsBylaws or Delaware law that has the effect of delaying or deterring a change in control could limit the opportunity for our stockholders to receive a premium for their shares of our common stock and could also affect the price that some investors are willing to pay for our common stock.
Our Bylaws designate a state or federal court located within the State of Delaware as the sole and exclusive forum for substantially all disputes between us and our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our current or former directors, officers, stockholders, or other employees.
Our bylaws provide that, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware shall be the sole and exclusive forum for (i) any derivative action or proceeding brought on behalf of us under Delaware law, (ii) any action asserting a claim of breach of a fiduciary duty owed by any current or former director, officer, or other employee of the Company to us or our stockholders, (iii) any action asserting a claim against us or any of our directors, officers, or other employees arising pursuant to any provision of the DGCL or our Certificate of Incorporation or Bylaws (as either may be amended
from time to time), (iv) any action asserting a claim against us governed by the internal affairs doctrine, or (v) any other action asserting an “internal corporate claim,” as defined under Section 115 of the DGCL. The forgoing provisions do not apply to any claims arising under the Securities Act and, unless we consent in writing to the selection of an alternative forum, the federal district courts of the United States will be the sole and exclusive forum for resolving any action asserting a claim arising under the Securities Act.
These choice of forum provisions may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or any of our current or former directors, officers, or other employees, which may discourage lawsuits with respect to such claims. There is uncertainty as to whether a court would enforce such provisions, and the enforceability of similar choice of forum provisions in other companies’ charter documents has been challenged in legal proceedings. It is possible that a court could find these types of provisions to be inapplicable or unenforceable, and if a court were to find the choice of forum provision to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in other jurisdictions, which could harm our business, results of operations, and financial condition.
We recently qualified as a “smaller reporting company” and a “non-accelerated filer,” and any decision on our part to comply only with certain reduced reporting and disclosure requirements applicable to such companies could make our common shares less attractive to investors.
As a result of our public float (the market value of our common shares held by non-affiliates) as of June 30, 2023, we qualify as a “smaller reporting company,” as defined under the Exchange Act. In addition, we are a “non-accelerated filer” as defined under the Exchange Act. For as long as we continue to be a smaller reporting company or a non-accelerated filer, we may choose to take advantage of exemptions from various reporting requirements applicable to other public companies that are not smaller reporting companies or non-accelerated filers, as applicable, including, but not limited to, an exemption from the requirement that our independent registered public accounting firm attest to the effectiveness of our internal control over financial reporting under Section 404 of the Sarbanes-Oxley Act.
If we choose to rely on any of these reporting and disclosure exemptions, the information we provide stockholders will be different than the information that is available with respect to many other public companies. Moreover, if some investors find our common stock less attractive as a result of any choices to reduce future disclosure or have an independent review and attestation of our internal control over financial reporting, there may be a less active trading market for our common stock and the market price of our common stock may be more volatile.
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our stock price and trading volume could decline.
The trading market for our common stock will depend in part on the research and reports that securities or industry analysts publish about us orand our business. In the event securities or industry analysts who cover us downgrade our stock or publish inaccurate or unfavorable research about us or our business, our stock price would likely decline. If one or more of these analysts cease coverage of our company or fail to publish reports on us regularly, demand for our stock could decrease, which might cause our stock price and trading volume to decline.
General Risk Factors
Our future success depends on our ability to retain our executive officers and to attract, retain and motivate qualified personnel.
We are highly dependent upon our executive officers and other key employees and the loss of the services of any of our executive officers or other key employees, including scientific, technical or management personnel, could impede the achievement of our corporate objectives. In August 2022, we announced a reduction of our workforce by approximately 20% across all areas of our company, including members of management. In November 2023, we implemented a further reduction of our workforce by approximately 30%, and in January 2024, we announced an additional reduction of our workforce by approximately 25%, including a member of management. Losing members of management and other key personnel subjects us to a number of risks, including the failure to coordinate responsibilities and tasks, the necessity to create new management systems and processes, the impact on corporate culture, and the retention of historical knowledge.
Our success depends on our ability to recruit, retain, manage and motivate our employees. Although we enter into employment agreements or offer letters with our employees, these documents provide for “at-will” employment, which means that any of our employees could leave our employment at any time, with or without notice. Competition for skilled personnel in our industry and geographic regions is intense and may limit our ability to hire and retain qualified personnel on acceptable terms or at all. To induce valuable employees to remain at our company, in addition to salary and cash incentives, we have provided equity awards that vest
over time. The value to employees of equity awards may be significantly affected by movements in our stock price that are beyond our control and may at any time be insufficient to counteract more lucrative offers from other companies.
Our relationships with customers and third party payors will be subject to applicable anti-kickback, fraud and abuse, privacy and other laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings.
Healthcare providers, including physicians, and third party payors will play a primary role in the recommendation and prescription of our product and any product candidates for which we obtain regulatory approval. Our current and future arrangements with third party payors and customers may expose us to broadly applicable federal and state fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we conduct research and would market, sell and distribute our products. As a biopharmaceutical company, even though we do not and will not control referrals of healthcare services or bill directly to Medicare, Medicaid or other third party payors, federal and state healthcare laws and regulations pertaining to fraud and abuse and patients’ rights are and will be applicable to our business. If we obtain FDA approval of any of our product candidates and our partners begin commercializing those products in the United States, our potential exposure under such laws will increase significantly, and our costs associated with compliance with such laws are also likely to increase. These laws may impact, among other things, our current activities with principal investigators and research patients, as well as proposed and future sales, marketing and education programs, distribution agreements, discounting, commission compensation, certain patient support offerings, and other business arrangements generally. In addition, the approval and commercialization of our product and any of our product candidates outside the United States will also likely subject us to foreign equivalents of the healthcare laws mentioned here, among other foreign laws. Restrictions under applicable federal and state healthcare laws and regulations that may affect certain business arrangements and our ability to operate include, but are not limited to, the following:
•the federal healthcare Anti-Kickback Statute, a criminal law that governs, for example, our marketing practices, educational programs, pricing policies, and relationships with healthcare providers or other entities, by prohibiting, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, overtly or covertly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under a federal healthcare program such as Medicare and Medicaid;
•the FDCA and PHSA, which prohibit the misbranding and adulteration of biological products that are regulated as drugs, and which regulate the marketing of biological products;
•federal civil and criminal false claims laws, including the civil False Claims Act, which can be enforced through civil whistleblower or qui tam actions, and civil monetary penalty laws impose criminal and civil penalties against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, including the Medicare and Medicaid programs, claims for payment or approval that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government;
•provisions enacted under the federal HIPAA impose criminal and civil liability for knowingly and willfully executing or attempting to execute, a scheme or artifice to defraud any healthcare benefit program and also impose criminal liability for, among other things, knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statements in connection with the delivery of or payment for healthcare benefits, items or services;
•HIPAA, as amended by HITECH also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information held by covered entities and their business associates and their subcontractors that use, disclose or otherwise process individually identifiable health information;
•the federal Physician Payments Sunshine Act, implemented as the Open Payments Program, which requires certain manufacturers of drugs, devices, biologics and medical supplies to report annually to CMS information related to payments and other transfers of value to U.S.-licensed physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors), physician assistants, nurse practitioners, clinical nurse specialists, anesthesiologist assistants, certified registered nurse anesthetists and certified nurse midwives and U.S. teaching hospitals, and ownership and investment interests held by physicians and their immediate family members and applicable group purchasing organizations;
•state and foreign laws and regulations that are analogous to, and may be broader in scope than, the federal laws and regulations described in this risk factor, such as state anti-kickback and false claims laws, may apply to sales or marketing or other arrangements and claims involving healthcare items or services reimbursed by non-governmental third party payors, including private insurers; and
•state laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government; some state laws require drug manufacturers to report information regarding pricing and marketing information related to payments and other transfers of value to physicians and other healthcare providers as well as those that require the registration of pharmaceutical sales representatives; and some other state laws require the protection of the privacy and security of health information, which may differ from each other in significant ways and often are not preempted by HIPAA.
Efforts to ensure that our business arrangements with third parties comply with applicable healthcare laws and regulations will involve substantial costs. Item is possible that governmental authorities will conclude that our business practices do not comply with current or future statutes, regulations or case law involving applicable healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations, we may be subject to significant civil, criminal and administrative penalties, damages, fines, imprisonment, exclusion from government-funded healthcare programs, such as Medicare and Medicaid, disgorgement, additional reporting requirements or oversight if we become subject to a corporate integrity agreement or similar agreement, and the curtailment or restructuring of our operations, reputational harm, contractual damages, and diminished profits and future earnings, any of which could adversely affect our ability to operate our business and our results of operations. If any physicians or other healthcare providers or entities with whom we expect to do business are found to not be in compliance with applicable laws, they may be subject to significant criminal, civil or administrative sanctions, including exclusions from government-funded healthcare programs.
Our employees may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements, which could cause significant liability for us and harm our reputation.
We are exposed to the risk of employee fraud or other misconduct, including intentional failures to comply with FDA regulations or similar regulations of comparable foreign regulatory authorities, provide accurate information to the FDA or comparable foreign regulatory authorities, comply with manufacturing standards we have established, comply with federal and state healthcare fraud and abuse laws and regulations and similar laws and regulations established and enforced by comparable foreign regulatory authorities, report financial information or data accurately or disclose unauthorized activities to us. Employee misconduct could also involve the improper use of information obtained in the course of clinical studies, which could result in regulatory sanctions and serious harm to our reputation. It is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business and results of operations, including the imposition of significant fines or other sanctions.
Product liability lawsuits against us could cause us to incur substantial liabilities and to limit commercialization of any products that we may develop.
We face an inherent risk of product liability exposure related to the testing of our product candidates in human clinical studies and will face an even greater risk if we commercially sell any products that we may develop. Product liability claims may be brought against us by subjects enrolled in our clinical studies, patients, healthcare providers or others using, administering or selling our products. If we cannot successfully defend ourselves against claims that our product candidates or products caused injuries, we could incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
•decreased demand for any product candidates or products that we may develop;
•clinical holds or termination of clinical study sites or entire study programs;
•injury to our reputation and significant negative media attention;
•withdrawal of clinical study participants;
•significant costs to defend the related litigation;
•substantial monetary awards to study subjects or patients;
•diversion of management and scientific resources from our business operations; and
•the inability to commercialize any products that we may develop.
We currently hold product liability insurance coverage at a level that we believe is customary for similarly situated companies and adequate to provide us with insurance coverage for foreseeable risks, but which may not be adequate to cover all liabilities that we may incur. We may not be able to maintain insurance coverage at a reasonable cost or in an amount adequate to satisfy any liability that may arise. As deemed necessary, we may expand our insurance coverage for products to include the sale of commercial products if we obtain regulatory approval for our product candidates in development, but we may be unable to obtain commercially reasonable product liability insurance for any products that receive regulatory approval. Large judgments have been awarded in class action lawsuits based on drugs that had unanticipated side effects. A successful product liability claim or series of claims brought against us, particularly if judgments exceed our insurance coverage, could decrease our cash and adversely affect our business.
If we and our third party manufacturers fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on the success of our business.
We and our third party manufacturers are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes. Our operations involve the use of hazardous and flammable materials, including chemicals and biological materials. Our operations also produce hazardous waste products. We generally contract with third parties for the disposal of these materials and wastes. We cannot eliminate the risk of contamination or injury from these materials. In the event of contamination or injury resulting from our or our third party manufacturers’ use of hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties.
Although we maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials with a policy limit that we believe is customary for similarly situated companies and adequate to provide us with insurance coverage for foreseeable risks, this insurance may not provide adequate coverage against potential liabilities. We do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of biological or hazardous materials.
In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations may impair our research, development or production efforts. Failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions, which could adversely affect our business, financial condition, results of operations and prospects.
The actual or perceived failure by us, our customers, or vendors to comply with increasingly stringent laws, regulations and contractual obligations relating to privacy, data protection, and data security could harm our reputation, and subject us to significant fines and liability.
We are or may become subject to numerous domestic and foreign laws and regulations regarding privacy, data protection, and data security, the scope of which is changing, subject to differing applications and interpretations and may be inconsistent among countries, or conflict with other rules. We are also subject to the terms of our contractual obligations to customers and third parties related to privacy, data protection, and data security. The actual or perceived failure by us, our customers, our vendors, or other relevant third parties to address or comply with these laws, regulations, and obligations could increase our compliance and operational costs, expose us to regulatory scrutiny, actions, fines and penalties, cause regulators to reject, limit or disrupt our clinical trial activities, result in reputational harm, lead to a loss of customers, reduce the use of our products, result in litigation and liability, and could otherwise cause a material adverse effect on our business, financial condition, and results of operations.
For example, the EU General Data Protection Regulation (EU) 2016/679 (EU GDPR) imposes strict requirements on in-scope organizations regarding the processing of personal information (i.e., data which identifies an individual or from which an individual is identifiable) of individuals (or data subjects). The EU GDPR governs the collection, use, disclosure, transfer and other processing of personal information and has direct effect in all EU Member States and extraterritorial effect where organizations outside of the European Economic Area (EEA) process personal information of individuals in the EEA in relation to the offering of goods or services to those individuals or the monitoring of their behavior. The UK has implemented the EU GDPR into its national law by virtue of section 3 of the European Union (Withdrawal) Act as the UK GDPR (together, the UK GDPR and the EU GDPR, the GDPR). Under the UK GDPR, companies established in the UK and companies not established in the UK but who process personal information of individuals in the UK in relation to the offering of goods or services to those individuals, or to the monitoring of their behavior will be subject to the UK GDPR. As such, the GDPR applies to us to the extent we are established in an EU Member State or the UK, we are processing personal information in the context of an establishment in an EU Member State or the UK or we are processing personal information in relation to the offering of goods or services to individuals in the EEA or the UK or monitoring their behavior.
The GDPR imposes onerous and comprehensive privacy, data protection, and data security obligations onto controllers, including, as applicable: (i) contractual privacy, data protection, and data security commitments, including the requirement to implement appropriate technical and organizational measures to safeguard personal information processed; (ii) establishing means for individuals to exercise their data protection rights (e.g., the right to erasure of personal information); (iii) limitations on retention and the amount of personal information processed; (iv) additional requirements pertaining to sensitive information (such as health data); (v) data breach notification requirements to: (x) supervisory authorities without undue delay (and no later than 72 hours where feasible) after becoming aware of the breach, unless the breach is unlikely to result in a risk to the data subjects’ rights and freedoms; and/or (y) concerned individuals where the breach is likely to result in a high risk to their rights and freedoms; (vi) requirements to process personal information lawfully including specific requirements for obtaining valid consent from data subjects where consent is the lawful basis for processing; (vii) obligations to consider data protection as any new products or services are developed and designed; and (viii) accountability and transparency requirements, which require controllers to demonstrate and record compliance with the GDPR and to provide more detailed information to data subjects(such as clinical trial subjects and investigators) regarding processing. The GDPR also provides that EU Member States and the UK (as applicable) may introduce further laws and regulations limiting the processing of genetic, biometric, or health data, which could limit our ability to collect, use and share personal information subject to the GDPR, cause our compliance costs to increase, require us to change our practices, adversely impact our business, and harm our financial condition.
In addition, the EU GDPR also prohibits the transfer of personal information from the EEA to countries that the European Commission does not recognize as having an “adequate” level of data protection unless the parties to the transfer have implemented specific safeguards to protect the transferred personal information. Data protection laws in the UK (as discussed below) and Switzerland impose similar restrictions. One of the primary safeguards allowing U.S. companies to import personal information from the EU and Switzerland had historically been certification to the EU-U.S. Privacy Shield framework, which is administered by the U.S. Department of Commerce, and Swiss-U.S. Privacy Shield framework respectively. However, the EU-U.S. Privacy Shield framework was invalidated as a mechanism to legitimize international transfers in July 2020 in the “Schrems II” decision handed down by the Court of Justice of the EU and imposed further restrictions on the use of standard contractual clauses (SCCs). The Schrems II decision also led to a requirement for companies to carry out a transfer impact assessment (TIA) which, among other things, assess laws governing access to personal information in the recipient country and considers whether supplementary measures that provide privacy protections additional to those under the EU SCCs will need to be implemented to ensure an “essentially equivalent” level of data protection to that afforded in the EU. Similarly, the Swiss-U.S. Privacy Shield framework was declared as inadequate by the Swiss Federal Data Protection and Information Commissioner in light of the Schrems II decision.
On October 7, 2022, the U.S. President introduced an Executive Order to facilitate a new Trans-Atlantic Data Privacy Framework (DPF), and on July 10, 2023, the EC adopted its Final Implementing Decision granting the U.S. adequacy (Adequacy Decision) for EU-U.S. transfers of personal information for companies that self-certify to the DPF. Entities relying on EU SCCs for transfer to the U.S. are also able to rely on the analysis in the Adequacy Decision as support for their TIA regarding the equivalence of U.S. national security safeguards and redress. However, any transfers by us or our vendors of personal information subject to the EU GDPR may not comply with European data protection law, may increase our exposure to the EU GDPR’s heightened sanctions for violations of its cross-border data transfer restrictions, and may reduce demand from companies subject to European data protection laws.
Complying with the GDPR involves rigorous and time-intensive processes that may cause us to incur certain operational costs and/or require us to change our business practices. There may also be a risk that the measures will not be implemented correctly or that individuals within the business will not be fully compliant with the required procedures. If there are breaches of these measures, we could face significant administrative and monetary sanctions as well as reputational damage which may have a material adverse effect on our operations, financial condition and prospects. Assisting our customers, partners, and vendors in complying with the GDPR, or complying with the GDPR ourselves, may cause us to incur substantial operational costs or require us to change our business practices. There may also be a risk that the measures will not be implemented correctly or that individuals within the business will not be fully compliant with the required procedures. There is a risk that we could be impacted by a cybersecurity incident that results in loss or unauthorized disclosure of personal information, potentially resulting in us facing harms similar to those described above.
Companies that must comply with the GDPR face increased compliance obligations and risk, including more robust regulatory enforcement of data protection requirements, potential significant fines for non-compliance of up to the greater of €20 million (under the EU GDPR) or £17.5 million (under the UK GDPR) or 4% of consolidated annual global turnover and restrictions or prohibitions on data processing. The GDPR identifies a list of points to consider when determining the level of fines to impose (including the nature, gravity and duration of the infringement). The GDPR also confers a private right of action on data subjects and consumer associations to lodge complaints with supervisory authorities, seek judicial remedies and obtain compensation for damages resulting from violations of the GDPR.
The UK GDPR also imposes similar restrictions on transfers of personal information from the UK to jurisdictions that the UK Government does not consider “adequate”, including the U.S. It should also be noted that the UK Government has published its own form of EU SCCs known as the UK International Data Transfer Agreement together with an International Data Transfer Addendum to the new EU SCCs. The UK Information Commissioner’s Office (ICO) has also published its version of the TIA and guidance on international transfers, although entities may choose to adopt either the EU or UK style TIA. Further, on September 21, 2023, the UK Secretary of State of Science, Innovation and Technology established a UK-U.S. data bridge (i.e., a UK equivalent of the Adequacy Decision) and adopted UK regulations to implement the UK-U.S. data bridge (“UK Adequacy Regulations”). Personal information may now be transferred from the UK under the UK-U.S. data bridge through the UK extension to the DPF to organizations self-certified under the UK extension to DPF.
Other countries outside of Europe and the UK continue to enact or are considering enacting similar cross-border data transfer restrictions and laws requiring local data residency, which could increase the cost and complexity of delivering our services and operating our business. For example, Brazil recently enacted the General Data Protection Law (Lei Geral de Proteção de Dados Pessoais or LGPD) (Law No. 13,709/2018), which broadly regulates the processing of personal information and imposes compliance obligations and penalties comparable to those of the EU GDPR and the UK GDPR.
Regulation of privacy, data protection and data security has also become more stringent in the United States. HIPAA imposes requirements to protect the privacy and security of protected health information (“PHI”) and to provide notification in the event of a breach of PHI. Violations of HIPAA are punishable by civil money penalties and, in some cases, criminal penalties and imprisonment. HHS’ Office for Civil Rights (“OCR”), which is responsible for enforcing HIPAA, also may enter into resolution agreements requiring the payment of a civil money penalty and/or the establishment of a corrective action plan to address violations of HIPAA. Pursuant to HIPAA, HHS has adopted privacy regulations, known as the privacy rule, to govern the use and disclosure of PHI (the “Privacy Rule”). HHS has also adopted data security regulations (the “Security Rule”) that require Covered Entities (including health care providers) and Business Associates to implement administrative, physical and technical safeguards to protect the integrity, confidentiality and availability of PHI that is electronically created, received, maintained or transmitted (such as between us and our affiliated practices).
Numerous state and certain other federal laws protect the confidentiality of health information and other personal information, including but not limited to state medical privacy laws, state laws protecting personal information, state data breach notification laws, state genetic privacy laws, human subjects research laws and federal and state consumer protection laws. For example, the CCPA, which took effect on January 1, 2020, give California residents expanded rights to access and delete their personal information, opt out of certain personal information sharing, and receive detailed information about how their personal information is used. The CCPA provides for civil penalties for violations, as well as a private right of action for data breaches that is expected to increase data breach litigation. The CCPA may increase our compliance costs and potential liability. The CCPA was substantially expanded on January 1, 2023, when the CPRA amendments to the CCPA became fully operative. The CPRA amendments, among other things, give California residents the ability to limit use of certain sensitive personal information, further restrict the use of cross-contextual advertising, establish restrictions on the retention of personal information, expand the types of data breaches subject to the CCPA’s private right of action, provide for increased penalties for CCPA violations concerning California residents under the age of 16, and establish a new California Privacy Protection Agency to implement and enforce the new law. Regulation of privacy, data protection and data security has also become more stringent in the United States, with several new laws being enacted at the state level. For instance, additional states have enacted laws related to consumer privacy, such as Colorado, Connecticut, Utah, and Virginia. While these new laws generally include exemptions for HIPAA-covered data, they add layers of complexity to compliance in the U.S. market, and could increase our compliance costs and adversely affect our business. While these laws generally include exemptions for HIPAA-covered and clinical trial data, they impact the overall privacy landscape. Multiple other states and the federal government are considering enacting similar legislation, demonstrating a strong trend towards more stringent state privacy, data protection and data security legislation in the U.S., which could increase our potential liability and adversely affect our business. Other states have passed or amended existing state privacy laws to impose enhanced privacy and cybersecurity obligations for consumer health data, such as, the Washington My Health My Data Act and Nevada’s Consumer Health Data Privacy Law. For instance, Washington State passed the “My Health My Data Act” with respect to “consumer health data” which is defined as “personal information that is linked or reasonably linkable to a consumer and that identifies a consumer’s past, present, or future physical or mental health” and which will go into effect in 2024 with additional privacy rights and compliance obligations.
Lawmakers and regulatory bodies at the federal level have been considering more detailed regulation regarding these subjects and the privacy and security of personal information. For example, the FTC recently published an advance notice of proposed rulemaking on “commercial surveillance” and data security, and is seeking comment on whether it should implement new trade regulation rules or other regulatory alternatives concerning the ways in which companies (1) collect, aggregate, protect, use, analyze, and retain consumer data, as well as (2) transfer, share, sell, or otherwise monetize that data in ways that are unfair or deceptive. The FTC’s rulemaking may create change throughout the economy and broader data ecosystem. The FTC has also been active with respect to enforcement of its Health Breach Notification Rule and in scrutinizing the use and disclosure of sensitive personal information.
Additionally, the OCR has issued a Notice of Proposed Rulemaking, which propose a number of changes to the HIPAA Privacy Rule, with updates to the HIPAA Privacy Rule expected in 2024.
The Federal Trade Commission (FTC) has authority under Section 5 of the FTC Act to regulate unfair or deceptive practices, and has used this authority to initiate enforcement actions against companies that implement inadequate controls around privacy and information security in violation of their externally facing policies. The FTC has recently brought several cases alleging violations of Section 5 of the FTC Act with respect to health information, and has proposed rulemaking on privacy and data security.
Compliance with applicable U.S. and foreign privacy, data protection, and data security laws and regulations may result in government investigations or cause us to incur substantial costs or require us to change our business practices and compliance procedures in a manner adverse to our business. Moreover, complying with these various laws could require us to take on more onerous obligations in our contracts, restrict our ability to collect, use and disclose data, or in some cases, impact our ability to operate in certain jurisdictions. Failure to comply with U.S. and foreign privacy, data protection, and data security laws and regulations could result in government investigations or enforcement actions (which could include civil or criminal penalties), private litigation, claims, or public statements against us and/or adverse publicity and could negatively affect our operating results and business. Claims that we have violated individuals’ privacy rights, failed to comply with privacy, data protection, and data security laws, or breached our contractual obligations, even if we are not found liable, could be expensive and time consuming to defend, could result in adverse publicity and could have a material adverse effect on our business, reputation, financial performance and business, and operations. Furthermore, the costs of compliance with, and other burdens imposed by, the laws, regulations and policies that are applicable to the business of our customers may limit the adoption and use of, and reduce the overall demand for, our products and services.
If our security measures are compromised, or our information technology systems or those of our vendors, and other relevant third parties fail or suffer security breaches, loss or leakage of data, and other disruptions, this could result in a material disruption of our services, compromise sensitive information related to our business, harm our reputation, trigger our breach notification obligations, prevent us from accessing critical information, and expose us to liability or other adverse effects to our business.
In the ordinary course of our business, we may collect, process, and store proprietary, confidential, and sensitive information, including personal information (including health information), intellectual property, trade secrets, and proprietary business information owned or controlled by ourselves or other parties. It is critical that we do so in a secure manner to maintain the confidentiality, integrity, and availability of such information. We face several risks relative to protecting this critical information, including loss of access risk, inappropriate use or disclosure, inappropriate modification, and the risk of our being unable to adequately monitor, audit and modify our controls over our critical information. This risk extends to the third party service providers who handle elements of our operations.
We, our partners, our CROs, our CMOs, and other business vendors on which we rely depend on information technology and telecommunication systems for significant elements of our operations, including, for example, systems handling human resources, financial reporting and controls, regulatory compliance and other infrastructure operations. Notwithstanding the implementation of security measures, given the size and complexity of our information technology systems and those of our third party vendors and other contractors and consultants, and the increasing amounts of proprietary, confidential and sensitive information that they maintain, such information technology systems have been subject to and remain vulnerable to breakdown, service interruptions, system malfunction, natural disasters, terrorism, war and telecommunication and electrical failures, as well as security breaches from inadvertent or intentional actions by our personnel, third party vendors, contractors, consultants, business partners, and/or other third parties, or from cyber-attacks by malicious third parties (including the deployment of harmful malware, ransomware, denial-of-service attacks, social engineering, and other means to affect service reliability and threaten the confidentiality, integrity, and availability of information), which may compromise our system infrastructure, or that of our third party vendors and other contractors and consultants, or lead to data leakage. The risk of a security breach or disruption, particularly through accidental actions or omissions by trusted insiders, cyber attacks or cyber intrusions, including by computer hackers, viruses, foreign governments, and cyber terrorists, has generally increased as the number, intensity, and sophistication of attempted attacks and intrusions from around the world have increased. Additionally, the increased usage of computers operated on home networks due to the shelter-in-place or similar restrictions related to the COVID-19 pandemic may make our systems more susceptible to security breaches. For example, in March 2021, MSK provided notice that MSK was one of many customers impacted by a data breach at Accellion, Inc., which provides a document-sharing system. MSK subsequently notified us that certain documents related to one of our discontinued programs were subject to the breach, which compromise we deemed immaterial. Although we take measures to protect sensitive data from unauthorized access, use or disclosure, we and our third party service providers frequently defend against and respond to cyber attacks, and our information technology and infrastructure may be vulnerable to attacks by hackers or viruses or breached due to personnel error, malfeasance, or other malicious or inadvertent disruptions. Any such breach or interruption could compromise our networks and the information stored there could be accessed by unauthorized parties, manipulated, publicly disclosed, lost, or stolen.
Failures or significant downtime of our information technology or telecommunication systems or those used by our third party service providers could cause significant interruptions to our operations, including preventing us from conducting tests or research and development activities and preventing us from managing the administrative aspects of our business. For example, the loss of clinical study data from completed, ongoing or planned clinical studies could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. In addition, sophisticated operating system software and applications that we procure from third parties may contain defects in design or manufacture, including vulnerabilities, “bugs” and other problems that could unexpectedly interfere with the operation of our networks, system, or our processing of personal information or other data. To the extent that any disruption or security breach results in a loss of or damage to our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability, the further development of our product candidates could be delayed and our business could be otherwise adversely affected.
We may not be able to anticipate all types of security threats, and we may not be able to implement preventative measures effective against all such security threats. We also may not be effective in responding to, containing or mitigating the risks of an attack. The techniques used by cyber criminals change frequently, may not be recognized until launched, and can originate from a wide variety of sources, including outside groups such as external service providers, organized crime affiliates, terrorist organizations, hostile foreign governments or agencies, or cybersecurity researchers. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or those of our third party vendors and other contractors and consultants, or inappropriate disclosure of confidential or proprietary information, we could incur liability and reputational damage and the further development and commercialization of our products and services could be delayed.
The costs related to significant security breaches or disruptions could be material and could exceed the limits of the cybersecurity insurance we maintain, if any, against such risks. If the information technology systems of our third party vendors and other contractors and consultants become subject to disruptions or security breaches, we may have insufficient recourse against such third parties and may have to expend significant resources to mitigate the impact of such an event, and to develop and implement protections to prevent future events of this nature from occurring.
We cannot assure you that our data protection efforts and our investment in information technology will prevent significant breakdowns, data leakages, breaches in our systems, or those of our third party vendors and other contractors and consultants, or other cyber incidents that could have a material adverse effect upon our reputation, business, operations, or financial condition. For example, if such an event were to occur and cause interruptions in our operations, or those of our third party vendors and other contractors and consultants, it could result in a material disruption of our programs and the development of our services and technologies could be delayed. Furthermore, significant disruptions of our internal information technology systems or those of our third party vendors and other contractors and consultants, or security breaches could result in the loss, misappropriation, and/or unauthorized access, use, or disclosure of, or the prevention of access to, confidential information (including trade secrets or other intellectual property, proprietary business information, and personal information), which could result in financial, legal, business, and reputational harm to us. Any such event that leads to unauthorized access, use, or disclosure of personal information, including personal information regarding our customers or employees, could harm our reputation directly, compel us to comply with federal and/or state breach notification laws and foreign law equivalents, subject us to mandatory corrective action, and otherwise subject us to liability under laws and regulations that protect the privacy and security of personal information, which could result in significant legal and financial exposure and reputational damages that could potentially have an adverse effect on our business. For example, in November 2023, we experienced a cybersecurity incident which resulted in unauthorized access of certain systems within our IT environment and a third party obtaining certain of our documents. Such unauthorized access was detected and contained within several hours and it was determined the third party did not access any of our material confidential information. Following such incident, we’ve taken additional measures to strengthen our IT environment.
Although we take measures to protect sensitive data from unauthorized access, use or disclosure, our information technology and infrastructure may be vulnerable to attacks by hackers or viruses or breached due to personnel error, malfeasance, or other malicious or inadvertent disruptions. Any such breach or interruption could compromise our networks and the information stored there could be accessed by unauthorized parties, manipulated, publicly disclosed, lost, or stolen.
Any such access, breach, or other loss of information could result in legal claims or proceedings, liability under domestic or foreign privacy, data protection and data security laws such as HIPAA and HITECH, and penalties. Notice of certain security breaches must be made to affected individuals, the Secretary of HHS, and for extensive breaches, notice may need to be made to the media or state attorneys general. Such notice could harm our reputation and our ability to compete. Although we have implemented security measures, such data is currently accessible through multiple channels, and there is no guarantee we can protect our data from breach. Unauthorized access, loss or dissemination could also damage our reputation or disrupt our operations, including our ability to conduct our analyses, conduct research and development activities, collect, process and prepare company financial information, and manage the administrative aspects of our business.
Penalties for violations of these laws vary. For instance, penalties for failure to comply with a requirement of HIPAA and HITECH vary significantly, and include significant civil monetary penalties and, in certain circumstances, criminal penalties with fines up to $250,000 per violation and/or imprisonment. A person who knowingly obtains or discloses individually identifiable health information in violation of HIPAA may face a criminal penalty of up to $50,000 and up to one-year imprisonment. The criminal penalties increase if the wrongful conduct involves false pretenses or the intent to sell, transfer or use identifiable health information for commercial advantage, personal gain or malicious harm.
Further, various states, such as California and Massachusetts, have implemented similar privacy laws and regulations, such as the California Confidentiality of Medical Information Act, that impose restrictive requirements regulating the use and disclosure of health information and other personally identifiable information. These laws and regulations are not necessarily preempted by HIPAA, particularly if such a state law affords greater protection to individuals than HIPAA. Where state laws are more protective, we have to comply with the stricter provisions. In addition to fines and penalties imposed upon violators, some of these state laws also afford private rights of action to individuals who believe their personal information has been misused. California’s patient privacy laws, for example, provide for penalties of up to $250,000 and permit injured parties to sue for damages. Similarly, the CCPA allows consumers a private right of action when certain personal information is subject to unauthorized access and exfiltration, theft or disclosure due to a business’ failure to implement and maintain reasonable security procedures. The interplay of federal and state laws may be subject to varying interpretations by courts and government agencies, creating complex compliance issues for us and data we receive, use and share, potentially exposing us to additional expense, adverse publicity and liability. Further, as regulatory focus on privacy issues continues to increase and laws and regulations concerning the protection of personal information expand and become more complex, these potential risks to our business could intensify. Changes in laws or regulations associated with the enhanced protection of certain types of sensitive data, for the treatment of genetic data, along with increased customer demands for enhanced data security infrastructure, could greatly increase our cost of providing our products, decrease demand for our products, reduce our revenues and/or subject us to additional liabilities.
Changes in tax laws or regulations that are applied adversely to us or our customers may have an adverse effect on our business, cash flows, financial condition or results of operations.
We are subject to income and non-income based taxes in the U.S. and various jurisdictions outside the U.S. Our business and financial condition could be adversely affected by changes in federal, state, local or international tax laws, changes in taxing jurisdictions’ administrative interpretations, decisions, policies and positions, changes in accounting principles, applicability of withholding taxes, and changes to our business operations. For example, U.S. legislation such as the Tax Act, the Coronavirus Aid, Relief, and Economic Security Act (CARES Act), and the American Rescue Act, made significant changes to the corporate tax rate, the potential realization of net deferred tax assets relating to our operations, taxation of foreign earnings, and deductibility of expenses, and could have a material impact on our financial position or results of operations.
Our ability to use net operating loss carryforwards and certain tax assets to offset future taxable income or taxes may be subject to certain limitations.
Our ability to use our federal and state net operating losses (NOLs) and certain other tax attributes to offset potential future taxable income and related income taxes that would otherwise be due is dependent upon our generation of future taxable income, and we cannot predict with certainty when, or whether, we will generate sufficient taxable income to use all of our NOLs or other tax attributes.
As of December 31, 2023, we had significant U.S. federal and state NOLs due to prior period losses. Under the Tax Cuts and Jobs Act (the Tax Act), federal NOLs generated in tax years beginning on or after January 1, 2018 may be carried forward indefinitely, but the utilization of such federal NOLs is limited to 80% of current year taxable income. States vary in conformity to all or portions of the Tax Act. The Tax Act did not have a material impact to our financial statements.
In addition, under Section 382 of the Internal Revenue Code of 1986, as amended (the Code), our ability to utilize these NOLs and other tax attributes, such as federal tax credits, in any taxable year may be limited if we have experienced an “ownership change”. Generally, a Section 382 ownership change occurs if one or more stockholders or groups of stockholders who owns at least 5% of a corporation’s stock increases its ownership by more than 50 percentage points over its lowest ownership percentage within a three-year testing period. Similar rules may apply under state tax laws. We performed a Section 382 analysis of transactions in our stock through December 31, 2023 and concluded that we have experienced ownership changes since inception that we believe under Section 382 of the Code will result in limitations on our ability to use certain pre-change NOLs and credits. In addition, we may experience subsequent ownership changes as a result of future equity offerings or other changes in the ownership of our stock, some of which are beyond our control. As a result, the amount of the NOLs and tax credit carryforwards presented in our financial statements could be limited and, in the case of NOLs generated before January 1, 2018 may expire unused. Any such material limitation or expiration of our NOLs may harm our future operating results by effectively increasing our future tax obligations. Similar provisions of state tax
law may also apply to limit the use of accumulated state tax attributes. Regulatory changes, such as suspensions on the use of NOLs, or other unforeseen reasons, may cause our existing tax attributes to expire, decrease in value or otherwise be unavailable to offset future income tax liabilities.
Business disruptions could seriously harm our future revenues and financial condition and increase our costs and expenses.
Our operations could be subject to earthquakes, power shortages, telecommunications failures, water shortages, floods, hurricanes, typhoons, fires, extreme weather conditions, medical epidemics and other natural or man-made disasters or business interruptions, for which we are predominantly self-insured. Two of our corporate locations are located in California, an area prone to earthquakes and fires. The occurrence of any of these business disruptions could seriously harm our operations and financial condition and increase our costs and expenses. We rely on third-party manufacturers to produce our product candidates. Our ability to obtain clinical supplies of product candidates could be disrupted, if the operations of these suppliers are affected by a man-made or natural disaster or other business interruption, including, for example, the COVID-19 pandemic.
Item 1B. Unresolved Staff Comments
None.
Item 1C. Cybersecurity
Risk Management and Strategy
We have established policies and processes designed to assess, identify, and manage material risk from cybersecurity threats, and have integrated these processes into our overall risk management systems and processes.
We routinely assess material risks from cybersecurity threats, including any potential unauthorized occurrence on or conducted through our information systems that may result in adverse effects on the confidentiality, integrity, or availability of our information systems or any information residing therein.
We monitor our environments to identify cybersecurity threats, as well as assess our environments in the event of a material change in our business practices that may affect information systems that are vulnerable to such cybersecurity threats. These risk assessments include identification of reasonably foreseeable internal and external risks, the likelihood and potential damage that could result from such risks, and the sufficiency of existing policies, procedures, systems, and safeguards in place to manage such risks.
Following our monitoring, we adjust, implement and maintain reasonable safeguards to minimize identified risks; reasonably address any identified gaps in existing safeguards; and regularly monitor the effectiveness of our safeguards. Primary responsibility for assessing, monitoring and managing our cybersecurity risks rests with our Chief Information Officer, who reports to our Chief Financial Officer, to manage any identified risks and mitigation process. As part of our overall cyber security framework, we monitor and test our safeguards and train our employees on these safeguards, in collaboration with our information technology (IT) department and management. Personnel at all levels and departments are made aware of our cybersecurity policies through ongoing training.
We engage third-party vendors in connection with our cybersecurity risk monitoring and processes. These service providers assist in our design and implementation of our cybersecurity policies and procedures, as well as to monitor and test our safeguards. We require each third-party service provider to certify that it has the ability to implement and maintain appropriate security measures, consistent with all applicable laws, to implement and maintain reasonable security measures in connection with their work with us, and to promptly report any suspected breach of its security measures that may affect our company.
We also maintain insurance coverage that is intended to address certain aspects of cybersecurity risks.
Notwithstanding any of these measures, our systems and networks remain potentially vulnerable to known or unknown cybersecurity attacks and other threats, any of which could have a material adverse effect on our consolidated results of operations, financial condition and cash flows. We have experienced, and will continue to experience, cyber incidents in the normal course of our business. As of the date of this report, we have not identified any risks from cybersecurity threats, including those from any previous cybersecurity incidents, that have materially affected us, our business strategy, results of operation or financial condition. However, there can be no assurances that a cybersecurity threat or incident that could have a material impact on us will not occur in the future. For additional information on the risks we face from cybersecurity threats, please see the risk factor titled, "If our security measures are compromised, or our information technology systems or those of our vendors, and other relevant third parties fail or suffer security breaches, loss or leakage of data, and other disruptions, this could result in a material disruption of our services, compromise sensitive information related to our business, harm our reputation, trigger our breach notification obligations, prevent us from accessing critical information, and expose us to liability or other adverse effects to our business." in Item 1A. "Risk Factors."
Governance
The Audit Committee of the Board of Directors is responsible for the primary oversight of our information security programs, including relating to cybersecurity. The Audit Committee receives status updates on at least a semi-annual basis from our Chief Information Officer on, among other things, our cyber risks and threats, the status of projects to strengthen our information security systems, assessments of our security program, and our views of the emerging threat landscape. The Chair of the Audit Committee regularly reports to the Board on cybersecurity risks and other matters reviewed by the Audit Committee. In addition, all Board members have access to the materials for each Audit Committee meeting.
Our Chief Information Officer is responsible for the oversight of our cybersecurity risks. We have implemented a security incident response plan and use this incident response framework as part of the process we employ to keep the Audit Committee and our executive management informed about cybersecurity risks and to monitor the prevention, detection, mitigation and remediation of
cybersecurity incidents. The plan is a set of procedures and tasks that our incident response team, under the direction of the Chief Information Officer, executes with the goal of ensuring timely identification and appropriate resolution of cybersecurity incidents. In addition, we validate compliance with our internal data security controls through the use of security monitoring tools.
Our Chief Information Officer has over 25 years of IT experience and has a thorough understanding of enterprise level cyber security framework. Our Chief Information Officer has also participated in cybersecurity reviews and implementations including various tools and platforms for over 10 years and drives strategic cyber security implementations based on industry best practices that helps us strengthen our security posture on a continuous basis.
Item 2. Properties
Our corporate headquarters are currently located in South San Francisco,Thousand Oaks, California and consists of approximately 13,67051,160 square feet of leased office space. The lease is expected to expire in April 2021. We also lease office space in Westlake Village, California under a lease agreement that expires in April 2019. February 2026.
In February 2017,March 2021, we entered into a new lease agreement for approximately 33,659 square feet of office, lab and warehouse space in Thousand Oaks, California. The initial 10.5-year term of this lease commenced in August 2021. We have the option to extend this lease for two additional five-year periods after the initial term.
In November 2023, we entered into an amended lease agreement for our office and lab space in Aurora, Colorado, to extend the term of our lease agreement through April 2025.
In April 2022, we assigned our lease of approximately 90,580 square feet of office, lab and cellular therapy manufacturing space in Thousand Oaks, California. The termCalifornia, which expires in April 2033, to FDB as part of the Fujifilm Transaction. We remain joint and severally liable for obligations related to the assigned lease.
We lease commences uponoffice space in South San Francisco, California, under a non-cancellable lease agreement through May 2025. In October 2022, we entered into a sub-lease agreement with a third party for this office space. The sub-lease term commenced in November 2022 and expires in May 2025, with no option to extend the substantial completionsub-lease term.
We use our leased spaces in Thousand Oaks, California and Aurora, Colorado for our translational and preclinical sciences, analytical development and process science functions. These facilities support our product pipeline and process development and leverage our allogeneic cell therapy platform to drive innovation. We believe our existing facilities are in good operating condition and suitable for the conduct of landlord’s work as defined under the agreement, which is currently estimated to be in the second quarter of 2018. Upon the commencement of the lease, the initial term of the lease is fifteen years. our business.
Item 3. Legal Proceedings
None.
Item 4. Mine Safety Disclosures
Not applicable.
PARTPART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market Information
Our common stock has been listed on The Nasdaq Global Select Market under the symbol "ATRA"“ATRA” since October 16, 2014. Prior to that time, there was no public market for our common stock. The following table sets forth for the indicated periods the high and low intra-day sales prices per share for our common stock on The Nasdaq Global Select Market.
Year ended December 31, 2016 | | High | | | Low | |
First Quarter | | $ | 26.00 | | | $ | 13.31 | |
Second Quarter | | $ | 23.25 | | | $ | 14.29 | |
Third Quarter | | $ | 25.73 | | | $ | 19.00 | |
Fourth Quarter | | $ | 21.85 | | | $ | 12.45 | |
| | | | | | | | |
Year ended December 31, 2017 | | High | | | Low | |
First Quarter | | $ | 23.00 | | | $ | 12.45 | |
Second Quarter | | $ | 21.20 | | | $ | 11.80 | |
Third Quarter | | $ | 17.55 | | | $ | 13.00 | |
Fourth Quarter | | $ | 21.80 | | | $ | 12.65 | |
On February 15, 2018,March 20, 2024, there were 114 stockholders of record of our common stock and the closing price of our common stock was $47.05 per share as reported on The Nasdaq Global Select Market.stock. We are unable to estimate the total number of stockholders represented by these record holders, as many of our shares are held by brokers and other institutions on behalf of our stockholders.
Dividend Policy
We have never declared or paid cash dividends on our capital stock. We currently intend to retain anyall of our future earnings, for use inif any, to finance the operationgrowth and development of our business and do not intend to declare or pay any cash dividends in the foreseeable future. Any further determination to pay dividends on our capital stock will be at the discretion of our board of directors, subject to applicable laws, and will depend on our financial condition, results of operations, capital requirements, general business conditions and other factors that our board of directors considers relevant.
Securities Authorized for Issuance under Equity Compensation Plans
Information about our equity compensation plans is incorporated herein by reference to Item 12 of Part III of this Annual Report on Form 10-K.
Stock Performance Graph
Set forth below is a graph comparing the cumulative total return on an indexed basis of a $100 investment in the Company’s common stock, the Nasdaq Composite Index and the Nasdaq Biotechnology Index commencing on October 16, 2014 (the date our common stock began trading on The Nasdaq Global Select Market) and continuing through December 31, 2017. The graph assumes our closing sale price on October 16, 2014 of $10.65 per share as the initial value of our common stock for indexing purposes. Points on the graph represent the performance as of the last business day of each of the fiscal quarters indicated.Item 6. [Reserved]
This performance graph shall not be deemed “soliciting material” or to be “filed” with the SEC for purposes of Section 18 of the Exchange Act or incorporated by reference into any filing of Atara Biotherapeutics, Inc. under the Securities Act or the Exchange Act, except to the extent we specifically incorporate it by reference into such filing. The past performance of our common stock is no indication of future performance.Not Applicable.
COMPARISON OF CUMULATIVE TOTAL RETURN*
Among Atara Biotherapeutics, Inc., the Nasdaq Composite Index and the Nasdaq Biotechnology Index
*
| Assumes $100 invested in our common stock or the related index on October 16, 2014.
|
Item 6. Selected Consolidated and Combined Financial Data
The following selected consolidated and combined financial data of the Company for each of the periods indicated are derived from the Company’s audited consolidated and combined financial statements. The consolidated financial statements of the Company as of December 31, 2017 and 2016 and for the years ended December 31, 2017, 2016 and 2015, and the related reports of the independent registered public accounting firm are included elsewhere in this Annual Report on Form 10-K. The data presented below should be read in conjunction with the Company’s financial statements, the notes thereto, and "Management's Discussion and Analysis of Financial Condition and Results of Operations" included elsewhere in this report.
| | Year ended | | | Year ended | | | Year ended | | | Year ended | | | Year ended | |
Consolidated and Combined Statements of Operations | | December 31, | | | December 31, | | | December 31, | | | December 31, | | | December 31, | |
and Comprehensive Loss Data: | | 2017 | | | 2016 | | | 2015 | | | 2014 | | | 2013 | |
| | (In thousands, except per share amounts) | |
Operating expenses: | | | | | | | | | | | | | | | | | | | | |
Research and development | | $ | 81,206 | | | $ | 56,514 | | | $ | 41,618 | | | $ | 15,446 | | | $ | 4,859 | |
General and administrative | | | 40,326 | | | | 24,728 | | | | 16,830 | | | | 12,710 | | | | 3,756 | |
Total operating expenses | | | 121,532 | | | | 81,242 | | | | 58,448 | | | | 28,156 | | | | 8,615 | |
Loss from operations | | | (121,532 | ) | | | (81,242 | ) | | | (58,448 | ) | | | (28,156 | ) | | | (8,615 | ) |
Interest and other income, net | | | 2,027 | | | | 2,203 | | | | 1,218 | | | | 125 | | | | 12 | |
Loss before provision for income taxes | | | (119,505 | ) | | | (79,039 | ) | | | (57,230 | ) | | | (28,031 | ) | | | (8,603 | ) |
Provision (benefit) for income taxes | | | (14 | ) | | | 10 | | | | (9 | ) | | | (25 | ) | | | 170 | |
Net loss | | $ | (119,491 | ) | | $ | (79,049 | ) | | $ | (57,221 | ) | | $ | (28,006 | ) | | $ | (8,773 | ) |
Other comprehensive loss: | | | | | | | | | | | | | | | | | | | | |
Unrealized gain (loss) on available-for-sale securities | | | 32 | | | | 335 | | | | (418 | ) | | | (100 | ) | | | — | |
Comprehensive loss | | $ | (119,459 | ) | | $ | (78,714 | ) | | $ | (57,639 | ) | | $ | (28,106 | ) | | $ | (8,773 | ) |
Basic and diluted net loss per common share | | $ | (4.00 | ) | | $ | (2.75 | ) | | $ | (2.24 | ) | | $ | (5.62 | ) | | $ | (9.08 | ) |
| | | | | | | | | | | | | | | | | | | | |
| | As of December 31, | |
Consolidated and Combined Balance Sheet Data: | | 2017 | | | 2016 | | | 2015 | | | 2014 | | | 2013 | |
| | | | | | (In thousands) | |
Cash, cash equivalents and short-term investments | | $ | 166,096 | | | $ | 255,682 | | | $ | 320,482 | | | $ | 104,116 | | | $ | 51,615 | |
Working capital | | $ | 144,544 | | | $ | 250,878 | | | $ | 314,888 | | | $ | 103,302 | | | $ | 50,284 | |
Total assets | | $ | 217,779 | | | $ | 263,914 | | | $ | 324,975 | | | $ | 106,122 | | | $ | 51,828 | |
Long-term liabilities | | $ | 12,269 | | | $ | 503 | | | $ | 166 | | | $ | 216 | | | $ | 230 | |
Convertible preferred stock | | $ | - | | | $ | - | | | $ | - | | | $ | - | | | $ | 61,091 | |
Total stockholders' equity (deficit) | | $ | 177,864 | | | $ | 253,736 | | | $ | 315,100 | | | $ | 103,182 | | | $ | (11,017 | ) |
ItemItem 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
You should read the following discussion and analysis of our financial condition and results of operations together with our audited consolidated financial statements and related notes included elsewhere in this Annual Report on Form 10-K. This discussion and other parts of this Annual Report contain forward-looking statements that involve risk and uncertainties, such as statements of our plans, objectives, expectations and intentions. As a result of many factors, including those factors set forth in the “Risk Factors” section of this Annual Report, our actual results could differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Overview
Atara Biotherapeutics is a leadingleader in T-cell immunotherapy, company developingleveraging its novel treatmentsallogeneic Epstein-Barr virus (EBV) T-cell platform to develop transformative therapies for patients with cancer and autoimmune disease. Tab-cel (tabelecleucel), our lead program in Phase 3 clinical development in the U.S., has received marketing authorization approval (MAA) under the proprietary name Ebvallo™ for commercial sale in the European Union (EU) by the European Commission (EC) and viral diseases. The Company'sfor commercial sale and use in the United Kingdom (UK) by the Medicines and Healthcare products Regulatory Agency (MHRA). We are the most advanced allogeneic T-cell immunotherapy company and intend to rapidly deliver off-the-shelf or allogeneic, T-cells are bioengineered from donors with healthy immune function and allow for rapid delivery from inventorytreatments to patients withoutwith high unmet medical need. Our platform leverages the unique biology of EBV T cells and has the capability to treat a requirement for pretreatment. Atara'swide range of EBV-driven diseases or other serious diseases through incorporation of engineered chimeric antigen receptors (CARs) or T-cell immunotherapiesreceptors (TCRs). We are designedapplying this one platform, that does not require TCR or human leukocyte antigen (HLA) gene editing, to precisely recognize and eliminate cancerous or diseased cells without affecting normal, healthy cells. Atara'screate a robust pipeline. Our strategic priorities are:
•Tab-cel®: Our most advanced T-cell immunotherapy program, tab-cel, has received MAA for commercial sale in the EU and the UK under the proprietary name Ebvallo and is partnered with Pierre Fabre Medicament (Pierre Fabre) for commercialization in Europe and potential commercialization, if approved, worldwide, including in the U.S. Tab-cel is currently in Phase 3 development tabelecleucel (formerly known as ATA129), is being developedin the U.S. for the treatment of patients with Epstein-Barr virus (EBV)EBV- associated post-transplant lymphoproliferative disorderdisease (EBV+ PTLD), who have failed rituximab, or rituximab plus chemotherapy, as well as other EBV-driven diseases; and
•ATA3219: Allogeneic CAR T targeting CD19, currently in Phase 1 development is being developed as a potential best-in-class product intended to target B-cell malignancies and autoimmune diseases, based on a next generation 1XX co-stimulatory domain and the innate advantages of EBV associated hematologicT cells as the foundation for an allogeneic CAR T platform.
In addition to the aforementioned strategic priorities, we also have a number of preclinical programs, including ATA3431, an allogeneic dual CAR T immunotherapy targeting both CD19 and solid tumors, including nasopharyngeal carcinoma (NPC). Off-the-shelfCD20 for B-cell malignancies; and a potential next generation EBV vaccine which is differentiated from earlier EBV vaccine efforts that solely focused on B cell responses to EBV. We have paused development on ATA188, and autologous ATA190, the Company'san allogeneic T-cell immunotherapies using a complementary targeted antigen recognition technology, target specificimmunotherapy targeting EBV antigens believed to be important for the potential treatment of multiple sclerosis (MS). Atara's clinical pipeline also includes ATA520 targeting Wilms Tumor 1 (WT1), while we explore strategic options for this asset.
Our T-cell immunotherapy platform is potentially applicable to a broad array of targets and ATA230 directed against cytomegalovirus (CMV).
diseases. Our technologyoff-the-shelf, allogeneic T-cell platform allows for rapid delivery of a T-cell immunotherapy product that has been manufactured in advance of patient need and stored in inventory, with each manufactured lot of cells providing therapy for numerous potential patients. This differs from autologous or patient-derived, treatments, in which each patient’s own cells must be extracted, genetically modified outside the body and then delivered back to the patient.patient, requiring a complex logistics network. We utilize a proprietary cell selection algorithm to select the appropriate set of cells for use based on a patient’s unique immune profile,profile. We estimate that only four and unlike manysix unique ATA3219 lots will be needed to cover approximately 90% of U.S. NHL and lupus patients, respectively. One of our contract manufacturing organizations (CMOs) has completed commercial production qualification activities for tab-cel and another of our CMOs is currently in the process of completing commercial production qualification activities for tab-cel while we manufacture inventory according to Pierre Fabre’s commercial product supply strategy.
In October 2021, we entered into the Commercialization Agreement with Pierre Fabre (Pierre Fabre Commercialization Agreement), pursuant to which we granted to Pierre Fabre an exclusive, field-limited license to commercialize and distribute Ebvallo in Europe and select emerging markets in the Middle East, Africa, Eastern Europe and Central Asia (the Initial Territory) following regulatory approval. As contemplated by the Pierre Fabre Commercialization Agreement, we entered into (i) a Manufacturing and Supply Agreement (ii) a Pharmacovigilance Agreement (iii) and a Quality Agreement, in each case, with Pierre Fabre to further advance our partnership with Pierre Fabre. In September 2022, we amended the Pierre Fabre Commercialization Agreement and received an additional $30 million milestone payment from Pierre Fabre following EC approval of Ebvallo for EBV+ PTLD and subsequent filing of the MAA transfer to Pierre Fabre, in exchange for, among other T-cell programs, there is no requirementthings, a reduction in: (i) royalties we are eligible to receive as a percentage of net sales of Ebvallo in the Initial Territory, and (ii) the supply price mark up on Ebvallo purchased by Pierre Fabre. Additionally, we agreed to extend the time period for pre-treatment beforeprovision of certain services to Pierre Fabre under the Pierre Fabre Commercialization Agreement. In December 2022, we entered into a Purchase and Sale Agreement (HCRx Agreement) with HCR
Molag Fund L.P. (HCRx,) a Delaware limited partnership. Pursuant to the terms of the HCRx Agreement, we received a total investment amount of $31.0 million in exchange for HCRx being entitled to receive a portion of the tiered, sales-based royalties for Ebvallo, in amounts ranging from the mid-single digits to significant double digits, as well as certain milestone payments, both related to the Initial Territory and otherwise payable to us by Pierre Fabre. The total royalties and milestones payable to HCRx related to the Initial Territory under the HCRx Agreement are capped between 185% and 250% of the total investment amount by HCRx, dependent upon the timing of such royalty and milestone payments to HCRx.
On October 31, 2023, we entered into an amended and restated Pierre Fabre Commercialization Agreement (A&R Commercialization Agreement), pursuant to which we expanded Pierre Fabre’s exclusive rights to research, develop, manufacture, commercialize and distribute tab-cel (Ebvallo) to include all other countries in the world (Additional Territory) in addition to the Initial Territory (together, the Territory), subject to our cellsperformance of certain obligations as described below. In December 2023, upon the effective date of the A&R Commercialization Agreement, we met the contractual right to receive an additional upfront cash payment of $20.0 million for the expanded exclusive license grant, for which the cash was received in January 2024. We will also be entitled to receive an aggregate of up to $620.0 million in additional milestone payments upon achieving certain regulatory and commercial milestones relating to tab-cel in the Additional Territory including up to $100.0 million in potential regulatory milestones through approval by the United States Food and Drug Administration (FDA) of a biologics license application (BLA) for tab-cel. Of the $100.0 million in potential regulatory milestones, we expect to receive $20.0 million in April 2024 based on the positive pre-BLA meeting, an additional $20.0 million in connection with BLA acceptance, and up to $60.0 million in potential regulatory milestones in connection with BLA approval. We are administered nor is there extended monitoring following administration. For example,eligible to receive significant double-digit tiered royalties as a percentage of net sales of tab-cel (Ebvallo) in ourthe Territory until the later of 12 years after the first commercial sale in each such country, the expiration of specified patent rights in each such country, or the expiration of all regulatory exclusivity for tab-cel in each such country. Royalty payments may be reduced in certain specified customary circumstances. Royalties and milestones from the commercialization of Ebvallo in the Initial Territory remain subject to the HCRx Agreement.
During the applicable period specified in the A&R Commercialization Agreement, we will be responsible, at Pierre Fabre’s cost, to continue conducting the ongoing trials with our most advanced product candidate, tabelecleucel, patients are monitoredPhase 3 ALLELE clinical study and the Phase 2 multi-cohort clinical study. We will also be responsible, at Pierre Fabre’s cost, for two hours following receiptcertain other activities directed to obtaining regulatory approval in the United States for tab-cel for EBV-associated post-transplant lymphoproliferative disease pursuant to the terms of tabelecleucel. Our T-cell immunotherapy platform is applicablethe A&R Commercialization Agreement. Pierre Fabre will be responsible, at its cost, for obtaining and maintaining all other required regulatory approvals and for commercialization and distribution of tab-cel in the Territory, including conducting any other clinical study required.
Prior to the transfer of manufacturing responsibility to Pierre Fabre, we will be responsible for manufacturing and supplying tab-cel to Pierre Fabre for commercialization in the Territory at cost plus a margin for orders placed after December 31, 2023, subject to a broad arraymaximum annual increase. Pierre Fabre will assume the responsibility and cost for the manufacture and supply of targetstab-cel in the Territory upon the Manufacturing Transition Date, which is defined as the earlier of i) the date on which all activities relating to the transfer of tab-cel manufacturing, pursuant to the A&R Commercialization Agreement, from Atara to Pierre Fabre have been completed to the reasonable satisfaction of both parties, or ii) December 31, 2025, throughout the remainder of the term of the A&R Commercialization Agreement. Pierre and diseases. With more than 200 patients treated acrosswe are to use commercially reasonable efforts to achieve this prior to the platform, we have observed clinical proofearlier transfer date from Atara to Pierre Fabre of concept across both viral and non-viral targetsthe first marketing authorization in conditions ranging from liquid and solid tumors to infectious and autoimmune diseases. the Additional Territory or the first BLA.
We have also observed a safety profile characterized by few treatment-related serious adverse events, or SAEs, and no evidence of cytokine release syndrome to date.
Our T-cell immunotherapy product candidates are engineered from cells donated by healthy individualsentered into research collaborations with normal immune function. Once cells are collected from a donor, they are bioengineered to expand those T-cells that recognize the antigen of interest. The resulting expanded T-cells are then characterized and heldleading academic institutions such as inventory. From inventory, these cells can be selected, distributed and prepared for infusion in a partially human leukocyte antigen, or HLA, matched patient in approximately 3-5 days. Following administration, our T-cells home to their target, undergo target-controlled proliferation, eliminate diseased cells and eventually recede. Target-controlled proliferation means that our T-cells expand in number when they encounter diseased cells in a patient’s body that express the antigen the cells are designed to recognize.
We have two technology platforms. One of our technology platforms was developed from more than a decade of experience at Memorial Sloan Kettering Cancer Center or MSK. The other was developed at QIMR Berghofer(MSK) and the Council of the Queensland Institute of Medical Research Institute, or QIMR Berghofer, in Australia. We licensed(QIMR Berghofer) pursuant to which we acquired rights to novel and proprietary technologies and programs.
Our research facilities in Thousand Oaks, California (ARC) and Aurora, Colorado contain our translational and preclinical sciences, analytical development and process science functions. These facilities support our product pipeline, process development and leverage our allogeneic cell therapy platform to drive innovation.
In January 2022, we entered into an asset purchase agreement with FUJIFILM Diosynth Biotechnologies California, Inc. (FDB) and, for certain know-howlimited purposes, FUJIFILM Holdings America Corporation, to sell all of the Company’s right, title and T-cellinterest in and to certain assets related to the Atara T-Cell Operations and Manufacturing facility (ATOM Facility) located in Thousand Oaks, California for $100 million in cash, subject to potential post-closing adjustments pursuant to the asset purchase agreement (the Fujifilm Transaction). The closing of the Fujifilm Transaction occurred on April 4, 2022. We also entered into a Master Services and Supply Agreement with FDB (Fujifilm MSA) that became effective upon the closing and could extend for up to ten years. Pursuant to the Fujifilm MSA, FDB will supply us with specified quantities of our cell therapy products (if approved) and product candidates, manufactured in accordance with cGMP standards. The Fujifilm MSA does not obligate us to purchase products and product candidates exclusively from MSKFDB.
We also work with Charles River Laboratories (CRL) pursuant to a Commercial Manufacturing Services Agreement (CRL MSA) that we entered into in June 2015. Our most advancedDecember 2019. Pursuant to the CRL MSA, CRL provides manufacturing services for our product candidate, tabelecleucel, targets Epstein-Barr virus,and certain intermediates. We further amended the CRL MSA to extend the term until the earlier of March 31, 2024 or EBV. Tabelecleucel received Breakthrough Therapy Designation,upon receipt of certain batches of our product and product candidates. We are currently in negotiations with CRL for a new commercial drug product supply agreement to be effective upon the expiration of the CRL MSA. However, there can be no assurance that we will be able to enter into a new commercial drug product supply agreement with CRL on terms favorable or BTD, fromacceptable to us, or at all. If we are unable to enter into a new commercial drug product supply agreement or extend the U.S. FoodCRL MSA, we may need to identify alternative sources of drug product supply.
We have non-cancellable minimum commitments for products and Drug Administration, or FDA,services, subject to agreements with a term of greater than one year, with clinical research organizations and Priority Medicines, or PRIME, designation from the European Medicines Agency, or EMA,CMOs.
In November 2023, we announced a reduction in force of approximately 30% of our workforce at that time. This workforce reduction resulted in total restructuring charges of $6.7 million, comprised primarily of severance payments and is currently being evaluated as monotherapy in two Phase 3 trialswages for the treatment60-day notice period in accordance with the California Worker Adjustment and Retraining Notification (WARN) Act. In most cases, the severance payments were paid as a lump sum in January 2024. All of patientsthe severance costs represent cash expenditures.
In January 2024, we announced another reduction in force of approximately 25% of total workforce. We expect to recognize approximately $4.5 million in total severance and related benefits as a result of this reduction in force, consisting primarily of severance payments and wages for the 60-day notice period in accordance with EBV associated post-transplant lymphoproliferative disease, or EBV+ PTLD who have failed rituximab. We believe that tabelecleucel has the potential toCalifornia WARN Act. In most cases, the severance will be the first commercially available off-the-shelf T-cell immunotherapy and the first FDA and EMA approved therapy for EBV+ PTLD. With a European conditional marketing authorization application planned forpaid in the first half of 2019 and U.S. biologics licensing applications planned following2024. Certain of the completionnotified employees had employment agreements which provided for separation benefits in the form of onesalary continuation; these benefits will be paid from February 2024 through January 2025. The majority of our ongoing Phase 3 trials, we are currently developing the infrastructure to commercialize tabelecleucel globally in EBV+ PTLD. We are also evaluating the potential utility of tabelecleucel in patients with other EBV associated cancers, such as nasopharyngeal carcinoma, or NPC, to continue its development in solid tumors. Additional product candidates derived from the collaboration with MSK are being developed to treat various cancers and severe viral infections.costs represent cash expenditures.
In October 2015 and September 2016, we licensed rights to certain know-how and technology from QIMR Berghofer that is complementary to that which was licensed from MSK. This know-how and technology uses targeted antigen recognition to create off-the-shelf T-cell immunotherapy product candidates applicable to a variety of diseases, including autoimmune conditions such as multiple sclerosis, or MS. We are also working with QIMR Berghofer on the development of EBV and other virally targeted T-cell immunotherapies. Through this technology, we are expanding the role of immunotherapy beyond oncology and viral infections to
autoimmune disease. Our most advanced off-the-shelf T-cell product candidate utilizing this technology, ATA188, targets select antigens of EBV and is currently being evaluated in a Phase 1 trial initially for the treatment of patients with progressive MS. In connection with the initial license from QIMR Berghofer, we received an option to exclusively license an autologous version of ATA188, also known as ATA190, which recently demonstrated clinical activity in a Phase 1 trial in progressive MS. We expect to broadly explore the utility of our targeted antigen recognition technology in EBV and other virally driven diseases, and additional product candidates derived from our collaboration with QIMR Berghofer are being developed.Financial Overview
Overall, we believe that Atara is a leading allogeneic T-cell immunotherapy company with a robust and late stage oncology pipeline and potentially transformative T-cell immunotherapies for MS and other viral associated diseases. With tabelecleucel poised to potentially become the first approved off-the-shelf T-cell therapy and a robust pipeline of high potential candidates, our ambition is to be recognized as the leader in off-the-shelf T-cell immunotherapy.
We have a limited operating history. Since our inception in 2012, we have devoted substantially all of our resources to identify, acquire and develop our product candidates, including conducting preclinical studies and clinical trialsstudies, acquiring or manufacturing materials for clinical studies, and providing general and administrative support for these operations.
We have never generated revenues and have incurred losses since inception. Our net losses were $119.5 million, $79.0$276.1 million and $57.2$228.3 million for the years ended December 31, 2017, 20162023 and 2015,2022, respectively. As of December 31, 2017,2023, we had an accumulated deficit of $296.7 million.$2.0 billion. Substantially all of our net losses have resulted from costs incurred in connection with our research and development programs and from general and administrative expenses associated with our operations. As of December 31, 2017,2023, our cash, cash equivalents and short-term investments totaled $166.1$51.7 million, which we intend to use to fund our operations. In January 2018, we completed an underwritten public offering
Revenues
We have only just begun to generate commercialization revenues under the A&R Commercialization Agreement, following the December 2022 EC approval of 7,675,072 sharesEbvallo. Our commercialization revenue recognized to date is derived from agreements with Pierre Fabre, primarily related to upfront license fees, milestone payments and amounts recognized from the sale of common stock at $18.25 per share, resulting in net proceedszero-cost inventories for which all performance obligations are complete, and is subject to usthe terms of approximately $131.4 million.
Financial Overview
Revenues
To date, we have not generated any revenues.the HCRx Agreement. We do not retain any meaningful milestone or royalty payments related to the Initial Territory under the A&R Commercialization Agreement until the applicable royalty cap under the HCRx Agreement is met, if at all, or until regulatory approval is achieved in the US or for another market within the Additional Territory. Our license and collaboration revenue recognized to date is primarily derived from agreements with Bayer AG, which terminated as of July 31, 2022.
We expect that any revenue we generate from the A&R Commercialization Agreement, subject to receivethe terms of the HCRx Agreement, will fluctuate from period to period as a result of the timing and number of inventory purchases by Pierre Fabre, potential milestone achievement, any revenues from any product candidates thatpotential regulatory approvals and the timing of manufacturing and cell selection technology transfer to Pierre Fabre.
Cost of commercialization revenue
Cost of commercialization revenue consists primarily of expenses associated with cell selection services performed for Pierre Fabre, in-license sales-related milestone costs, period manufacturing expenses and the lower of cost or net realizable value adjustments to inventories. All Ebvallo sold to Pierre Fabre to date had been produced prior to receiving regulatory approval of Ebvallo. Costs incurred to produce Ebvallo prior to regulatory approval, referred to as zero cost inventories, have been recorded as
research and development expense in our consolidated statement of operations and comprehensive income (loss). Once we develop until we obtainbegin selling Ebvallo produced after receiving regulatory approval and commercialize our products or enter into collaborative agreementsin a qualified manufacturing facility, and as revenue is recognized on such Ebvallo shipments, cost of commercialization revenue will also include direct and indirect costs related to the production of Ebvallo. Such costs include, but are not limited to, CMO costs, quality testing and validation, materials used in production, and an allocation of compensation, benefits and overhead costs associated with third parties.employees involved with production.
Research and Development Expenses
The largest component of our total operating expenses since inception has been our investment in research and development activities, including the preclinical and clinical development of our product candidates. Research and development expenses consist primarily of compensation and benefits for research and development and regulatory support employees, including stock-based compensation; expenses incurred under agreements with contract research organizations and investigative sites that conduct clinical trialspreclinical and preclinicalclinical studies; the costs of acquiring and manufacturing clinical trialstudy materials and other supplies;supplies, including expenses incurred under agreements with CMOs; payments under licensing and research and development agreements; other outside services and consulting costs; and an allocation of facilities, information technology and overhead expenses. Research and development costs are expensed as incurred.
We plan to increase our research and development expenses as we continue investment in the development of our product candidates. Our current planned research and development activities include the following:
•initiating sites and enrollingcontinuing to enroll patients in tabelecleucelour Phase 3 clinical trialsstudy of tab-cel for the treatment of patients with EBV+ PTLD after HCT and SOT who have failed rituximab;
•process development, testing and manufacturing of drug supply to support clinical trials and IND-enabling studies;
•continuing development of ATA190 and enrolling patients to the Phase 1 trial of ATA188 in MS;
develop product candidates based on our next-generation CAR T programs; •continuing development of ATA520 for the treatment of hematologic malignancies, including PCL, and solid tumors;
continuing to develop our product candidates in additional indications, including tabelecleuceltab-cel for NPC;
EBV+ cancers;•continuing to develop other preclinical product candidates, including ATA621 for JCVcandidates; and BK-associated diseases; and
•leveraging our relationships and experience to in-license or acquire additional product candidates or technologies.
In addition, we believe it is important to invest in the development of new product candidates to continue to build the value of our product candidate pipeline and our business. We plan to continue to advance our most promising early product candidates into preclinical development with the objective to advanceof advancing these early-stage programs to human clinical trialsstudies over the next several years.
Our expenditures on current and future preclinical and clinical development programs are subject to numerous uncertainties in timing and cost to completion. The duration, costs, and timing of clinical trialsstudies and development of our product candidates will depend on a variety of factors, including:
•the availability of qualified drug supply for use in our plannedongoing Phase 3 or other clinical trials;
studies; •the scope, rate of progress, and expenses of our ongoing as well as anyclinical studies, potential additional clinical trialsstudies and other research and development activities;
•the potential review or reanalysis of our clinical study results;
•future clinical trialstudy results;
•uncertainties in clinical trialstudy enrollment rates or discontinuation rates of patients;
•potential additional safety monitoring or other studies requested by regulatory agencies;
•changing medical practice patterns related to the indications we are investigating;
•significant and changing government regulation; and
•disruptions caused by man-made or natural disasters or public health pandemics or epidemics, including, for example, the COVID-19 pandemic; and
•the timing and receipt of any regulatory approvals.approvals, as well as potential post-market requirements.
The process of conducting the necessary clinical research to obtain approval from the FDA approvaland other regulators is costly and time consuming and the successful development of our product candidates is highly uncertain. The risks and uncertainties associated with our research and development projects are discussed more fully in the section of this report titled “1A. Risk Factors.” As a result of these risks and uncertainties, we are unable to determine with any degree of certainty the duration and completion costs of our research and development projects, or if, when, or to what extent we will generate revenues from the commercialization and sale of any of our product candidates that obtain regulatory approval. We may never succeed in achieving regulatory approval for any of our product candidates.
General and Administrative Expenses
General and administrative expenses consist primarily of compensation and benefits for legal, human resources, finance and other general and administrative employees, including stock-based compensation; outside professional serviceservices costs, including legal, patent, human resources, audit and accounting services; other outside services and consulting costs; and allocated information technology and facilities costs. We anticipate that our general and administrative expenses will continue to increase inoverhead expenses.
Gain on sale of ATOM Facility
The gain on sale of the future as we increase our headcount to support our continued research and developmentATOM Facility consists of the consideration received from FDB, less transaction costs and the potential commercializationcarrying value of one or more of our product candidates.assets sold.
Interest and Other Income net
Interest and other income net consists primarily of interest earned on our cash, cash equivalents and short-term investments.
Interest Expense
Interest expense consists primarily of interest expense recorded in connection with the HCRx Agreement.
Provision for Income Taxes
Provision for income taxes consists primarily of income taxes in U.S. states and foreign jurisdictions. Our effective tax rate was 0% for the years ended December 31, 2023 and 2022.
Critical Accounting Policies and Significant Judgments and Estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States.U.S. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities and expenses. On an on-going basis, we evaluate our critical accounting policies and estimates. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable in the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions and conditions. Our significant judgments and estimates are detailed below, and our significant accounting policies are more fully described in Note 2 of the accompanying consolidated financial statements.statements.
Revenue Recognition
Revenue from out-license agreements is recognized as we satisfy performance obligations and when a customer obtains control of the promised goods or services, in an amount that reflects the consideration which we expect to receive in exchange for those goods or services. Revenue generated from our out-license agreements is not subject to repayment and typically includes upfront fees, development, regulatory and commercial milestone payments and royalties on the licensee’s future product sales.
Our out-license agreements may include the transfer of intellectual property rights in the form of licenses, promises to provide research and development services and promises to participate on certain development committees with the collaboration party. We assess whether the promises in these agreements are considered distinct performance obligations that should be accounted for separately. Judgment is required to determine whether these promises are distinct.
The transaction price in each agreement is allocated to the identified performance obligations based on the standalone selling price (SSP) of each distinct performance obligation.
Description
| Judgments and Uncertainties
| Effect if Actual Results Differ from Assumptions
|
Accrued Research and Development Expenses
| | |
As part of the process of preparing our financial statements, we are required to estimate and accrue expenses, the largest of which is related to research and development expenses, including those related to clinical trials and drug manufacturing. This process involves reviewing contracts and purchase orders, identifying and evaluating the services that have been performed on our behalf, and estimating the associated cost incurred for the services when we have not yet been invoiced or otherwise notified of the actual costs.
| Costs for preclinical studies, clinical trials
84
Revenue associated with nonrefundable upfront license fees where the license fees and other promises cannot be accounted for as separate performance obligations is deferred and recognized as revenue over the expected period of performance using an appropriate recognition method based on the nature of the performance obligations. We utilize judgment to assess the pattern of delivery of the performance obligation. We evaluate the measure of progress each reporting period and, if necessary, adjust the measure of performance and related revenue recognition. A significant change in the assumptions and estimates, such as forecasted costs or the extent and timing of patient demand, and expected dates of technology transfer, could have a material impact on the timing and amount of revenue recognized in future periods or adjustments to cumulative revenue recognized in the period of change. At the inception of each agreement that includes development, regulatory or commercial milestone payments, we evaluate whether the milestones are considered probable of being reached and estimate the amount to be included in the transaction price by using the most likely amount method. If it is probable that a significant revenue reversal would not occur, the associated milestone value is included in the transaction price. The transaction price is allocated to each performance obligation in the agreement based on relative SSP. We typically determine SSPs using a cost plus margin approach model. Milestone payments that are not within our or the licensee’s control, such as regulatory approvals, are typically not considered probable of being achieved until those approvals are received. At the end of each reporting period, we re-evaluate the probability of achievement of each such milestone and any related constraint, and if necessary, adjust our estimates of the overall transaction price. Any such adjustments are recorded on a cumulative catch-up basis, which would affect revenues and earnings in the period of adjustment. Certain judgments affect the application of our revenue recognition policy. For example, we record short-term and long-term deferred revenue based on our best estimate of when such revenue will be recognized. Short-term deferred revenue consists of amounts that are expected to be recognized as revenue in the next 12 months, and long-term deferred revenue consists of amounts that we expect will be recognized after the next 12 months. This estimate is based on forecasted patient demand, our current operating plan, and expected dates of technology transfer, and if these items should change in the future, we may recognize a different amount of deferred revenue over the next 12-month period. Accrued Research and Development Expenses As part of the process of preparing our financial statements, we are required to estimate and accrue expenses, the largest of which is related to research and development expenses, including those related to clinical studies and clinical product candidate manufacturing. This process involves reviewing contracts and purchase orders, identifying and evaluating the services that have been performed on our behalf, and estimating the associated cost incurred for the services which we have not yet been invoiced or otherwise notified of the actual costs incurred. Costs for preclinical studies, clinical studies and product candidate manufacturing activities are recognized based on an evaluation of our vendors’ progress towards completion of specific tasks, using data such as patient enrollment, clinical site activations or information provided to us by our vendors regarding their actual costs incurred. Payments for these activities are based on the terms of individual contracts and payment timing may differ significantly from the period in which the services were performed. We determine accrual estimates through reports from, and discussions with, applicable personnel and outside service providers as to the progress or state of completion of studies, or the goods and services delivered. Our estimates of accrued expenses as of each balance sheet date are based on the facts and circumstances known at the time. Costs that are paid in advance of performance are deferred as a prepaid asset and recognized as expense as the services are provided. For the years ended December 31, 2023 and 2022, there were no material changes from our estimates of accrued research and development expenses. We do not believe there is a reasonable likelihood that there will be a material change in the future estimates of accrued research and development expenses. However, if actual results are not consistent with our estimates, we may be exposed to changes in accrued research and development expenses that could be material or the accrued research and development expenses reported in our financial statements may not be representative of the actual economic cost of accrued research and development. Stock-based Compensation We have stock-based compensation programs, which include an employee incentive plan, an inducement plan and an employee stock purchase plan. See Note 2 – “Summary of Significant Accounting Policies” and Note 11 – “Stockholders' Equity” in the Notes to Consolidated Financial Statements, included in Item 8. Financial Statements and Supplementary Data of this report for a complete discussion of our stock-based compensation programs. We account for stock-based compensation expense, including the expense for grants of restricted stock units (RSUs) and stock options that may be settled in shares of our common stock, based on the fair values of the equity instruments issued. The fair value is determined on the measurement date, which is generally the date of grant. The fair value of our RSUs is measured at the market price of our common stock on the measurement date. The fair value for our stock option awards is determined at the grant date using the Black-Scholes valuation model.
Assumptions for the Black-Scholes valuation model used for employee stock awards include: •Expected term – We derived the expected term for employee stock awards using the “simplified” method (the expected term is determined as the average of the time-to-vesting and the contractual life of the options), as we have limited historical information to develop expectations about future exercise patterns and post vesting employment termination behavior. Expected term for non-employee awards is based on the remaining contractual term of an option on each measurement date. •Expected volatility – In 2021 and 2022, volatility was estimated using an average of our historical volatility and comparable public companies’ volatility for similar terms. Beginning in 2023, volatility is based solely on our stock price historical volatility. •Expected dividend rate – We have not historically declared or paid dividends to our stockholders and have no plans to pay dividends; therefore, we have assumed an expected dividend yield of 0%. •Risk-free interest rate – The risk-free interest rate is based on the yields of U.S. Treasury securities with expected terms similar to that of the associated award. •The fair value of our common stock is measured at the market price on the measurement date. For awards with performance-based vesting criteria, we assess the probability of the achievement of the performance conditions at the end of each reporting period and begin to recognize the share-based compensation costs when it becomes probable that the performance conditions will be met. For awards that are subject to both service and performance conditions, no expense is recognized until it is probable that performance conditions will be met. We do not believe there is a reasonable likelihood that there will be a material change in the future estimates or assumptions we use to determine stock-based compensation expense. However, if actual results are not consistent with our estimates or assumptions, we may be exposed to changes in stock-based compensation expense that could be material or the stock-based compensation expense reported in our financial statements may not be representative of the actual economic cost of the stock-based compensation. Accounting for Income Taxes See Note 12 – “Income Taxes” in the Notes to Consolidated Financial Statements, included in Item 8. Financial Statements and Supplementary Data of this report for a complete discussion of the components of our vendors’ progress towards completion of specific tasks, using data such as patient enrollment, clinical site activations or information provided to us by our vendors regarding their actual costs incurred. Payments for these activities are based on the terms of individual contracts and payment timing may differ significantly from the period in which the services were performed. We determine accrual estimates through reports from and discussions with applicable personnel and outside service providers as to the progress or state of completion of trials, or the services completed. Our estimates of accrued expenses as of each balance sheet date are based on the facts and circumstances known at the time. Costs that are paid in advance of performance are deferred as a prepaid expense and amortized over the service period as the services are provided. | For the years ended December 31, 2017 and 2016, there were no material changes from our estimates of accrued research and development expenses.
We do not believe there is a reasonable likelihood that there will be a material change in the future estimates of accrued research and development expenses. However, if actual results are not consistent with our estimates, we may be exposed to changes in accrued research and development expenses that could be material or the accrued research and development expenses reported in our financial statements may not be representative of the actual economic cost of accrued research and development.
A 10% change in accrued research and development expenses could have impacted our net loss by $0.4 million for 2017.
|
| | |
Stock-based Compensation
| | |
We have stock-based compensation programs, which include restricted stock agreements, or RSAs, restricted stock units, or RSUs, stock options and an employee stock purchase plan. See Note 2– “Summary of Significant Accounting Policies” and Note 8 – “Stockholders' Equity” in the Notes to Consolidated Financial Statements, included in Item 8. Financial Statements and Supplementary Data of this report for a complete discussion of our stock-based compensation programs. We account for stock-based compensation expense, including the expense for RSAs, grants of RSUs and stock options that may be settled in shares of our common stock, based on the fair values of the equity instruments issued. The fair value is determined on the measurement date, which is generally the date of grant for employee awards and the date when the service performance is completed for non-employees. The fair value for our RSAs is their intrinsic value, which is the difference between the fair value of the underlying stock at the measurement date and the purchase price. The fair value of our RSUs is the fair value of the underlying stock at the measurement date. The fair value for our stock option awards is determined at the grant date using the Black-Scholes valuation model. For employees’ awards with performance-based vesting criteria, we assess the probability of the achievement of the performance conditions at the end of each reporting period and recognize the share-based compensation costs when it becomes probable that the performance conditions will be met. For non-employees’ awards with performance-based vesting criteria, we assess all possible outcomes at the end of each reporting period and recognize the lowest aggregate fair value in the range of possible outcomes. The lowest value in the range of possible outcomes may be zero. For awards that are subject to both service and performance conditions, no expense is recognized until it is probable that performance conditions will be met. Stock-based compensation expense for awards with time-based vesting criteria is recognized as expense on a straight-line basis over the requisite service period. Stock-based compensation for awards with performance and other vesting criteria is recognized as expense under the accelerated graded vesting model.
| Key assumptions for the Black-Scholes valuation model used for employee stock awards include:
Expected term – We derived the expected term using the “simplified” method (the expected term is determined as the average of the time-to-vesting and the contractual life of the options), as we have limited historical information to develop expectations about future exercise patterns and post vesting employment termination behavior. Expected term for non-employee awards is based on the remaining contractual term of an option on each measurement date.
Expected volatility – Expected volatility is estimated using comparable public companies’ volatility for similar terms.
Expected dividend – We have not historically declared or paid dividends to our stockholders and have no plans to pay dividends; therefore we have assumed an expected dividend yield of 0%.
Risk-free interest rate – The risk-free interest rate is based on the yields of U.S. Treasury securities with expected terms similar to that of the associated award.
The fair value of non-employee stock options is estimated using the Black-Scholes valuation model with assumptions generally consistent with those used for employee stock options, with the exception of the expected term, which is the remaining contractual life at each measurement date.
Prior to our initial public offering in October 2014, due to the absence of an active market for our common stock, we estimated the fair value of our common stock in accordance with the framework of the American Institute of Certified Public Accountants Technical Practice Aid, Valuation of Privately-Held Company Equity Securities Issued as Compensation. Each valuation included estimates and assumptions that required management’s judgment, including assumptions regarding the probability and estimated time to completion of our initial public offering. Subsequent to the completion of our initial public offering in October 2014, the fair value of our common stock is based on observable market prices.
| We do not believe there is a reasonable likelihood that there will be a material change in the future estimates or assumptions we use to determine stock-based compensation expense. However, if actual results are not consistent with our estimates or assumptions, we may be exposed to changes in stock-based compensation expense that could be material or the stock-based compensation expense reported in our financial statements may not be representative of the actual economic cost of the stock-based compensation.
|
| | |
Accounting for Income Taxes
| | |
See Note 9 – “Income Taxes” in the Notes to Consolidated Financial Statements, included in Item 8. Financial Statements and Supplementary Data of this report for a complete discussion of the components of Atara's income tax expense, as well as the temporary differences that exist as of December 31, 2017.
| Our consolidated effective income tax rate is influenced by tax planning opportunities available to us in the various jurisdictions in which we conduct business. Significant judgment is required in determining our effective tax rate and in evaluating our tax positions, including those that may be uncertain.
Atara is also required to exercise judgment with respect to the realization of our net deferred tax assets. Management evaluates all positive and negative evidence and exercises judgment regarding past and future events to determine if it is more likely than not that all or some portion of the deferred tax assets may not be realized. If appropriate, a valuation allowance is recorded against deferred tax assets to offset future tax benefits that may not be realized.
| We do not believe that there is a reasonable likelihood that there will be a material change in our liability for uncertain income tax positions or our effective income tax rate. However, if actual results are not consistent with our estimates or assumptions, we may be exposed to losses that could be material. Atara recorded a valuation allowance of approximately $47.3 million as of December 31, 2017 related primarily to net operating losses and stock-based compensation.
|
Income Taxes
On December 22, 2017, President Trump signed into law new tax legislation, or the Tax Act, which significantly reforms the Internal Revenue Code of 1986, as amended. As of December 31, 2017, we have made a reasonable estimate of the effects on our existing deferred taxes and related disclosures for the reduction in corporate2023.
Our consolidated effective income tax rate and adjustmentsis influenced by tax planning opportunities available to us in the various jurisdictions in which we conduct business. Significant judgment is required in evaluating our tax positions, including those that may be uncertain. We are also required to exercise judgment with respect to the expected deductibilityrealization of executive compensation. Dueour net deferred tax assets. We evaluate all positive and negative evidence and exercise judgment regarding past and future events to current year taxable losses and our federal valuation allowance position, we diddetermine if it is more likely than not recognize any income tax expensethat all or benefit as a resultsome portion of the Tax Act. Due to accumulated foreign deficits the Company does not expect a current inclusion in U.S. federal taxable income for the transition tax on earnings of controlled foreign corporations.
The SEC staff has issued Staff Accounting Bulletin No. 118, Income Tax Accounting Implications of the Tax Cuts and Jobs Act (SAB 118), which allows us to record provisional amounts during a measurement period not to extend beyond one year of the enactment date. We consider the key estimates on the deferred tax remeasurementassets may not be realized. If appropriate, a valuation allowance is recorded against deferred tax assets to offset future tax benefits that may not be realized.
We do not believe that there is a reasonable likelihood that there will be a material change in our liability for uncertain income tax positions or our effective income tax rate. However, if actual results are not consistent with our estimates or assumptions, we may be exposed to losses that could be material. We recorded a valuation allowance of approximately $475.0 million as of December 31, 2023 related primarily to net operating loss carryforwards, capitalized research expenses, tax credit carryforwards and stock-based compensation.
Liability related to the impactsale of future revenues
To the extent we account for the sale of future revenues as debt in accordance with ASC 470, we amortize the liability and recognize interest expense related to the sale of future revenues using the effective interest rate method over the estimated life of the changes tounderlying agreement. The liability and related interest expense are based on our current estimate of expected future payments over the deductibilitylife of executive compensation to be provisional due tothe arrangement. We re-assess the amount and timing of expected forthcoming guidancepayments each reporting period using a combination of internal projections and forecasts from federalexternal resources and state tax authorities,record interest expense on the carrying value of the liability using the imputed effective interest rate. To the extent our continuing analysisestimates of final year-end data and tax positions,future payments are greater or less than previous estimates or the estimated timing of such payments is materially different than previous estimates, this could impact the amount of interest expense we record each period as well as further guidance expected for the associated income tax accounting. We expect to complete our analysis within the measurement period in accordance with SAB 118.
Emerging Growth Company Status
We are an “emerging growth company” as defined in the JOBS Act,amount and therefore we may take advantage of certain exemptions from various public company reporting requirements. As an “emerging growth company”,
we will avail ourselvesclassification of the exemption fromliability. We will account for any such changes by adjusting the requirement to obtain an attestation and report from our auditorseffective interest rate on a prospective basis. The assumptions used in determining the assessment of our internal control over financial reporting pursuant to the Sarbanes-Oxley Act;
we will provide less extensive disclosure about our executive compensation arrangements; and
we will not require stockholder non-binding advisory votes on executive compensation or golden parachute arrangements.
However, we are choosing to irrevocably opt outexpected repayment term of the extended transition periods available underliability and amortization period requires that we make estimates that could impact the JOBS Act for complying with new or revised accounting standards. We will remain an “emerging growth company” for up to five years, although we will cease to be an “emerging growth company” upon the earliest of: (1) December 31, 2019; (2) the last dayeffective interest rate, short-term and long-term classification of the first fiscal year inliability and the period over which our annual gross revenues are $1.07 billion or more; (3) the date on which we have, during the previous rolling three-year period, issued more than $1 billion in non-convertible debt securities; and (4) the date on which we are deemed toliability will be a “large accelerated filer” as defined in the Exchange Act.amortized.
Results of Operations
Comparison of the Years Ended December 31, 2017, 20162023 and 20152022
Research and development expenses by program forRevenues
Revenue consisted of the following in the periods indicatedpresented:
| | | | | | | | | | | | |
| | Year ended December 31, | | | | |
| | 2023 | | | 2022 | | | Increase (Decrease) | |
| | (in thousands) | |
Commercialization revenue | | $ | 7,886 | | | $ | — | | | $ | 7,886 | |
License and collaboration revenues | | | 687 | | | | 63,573 | | | | (62,886 | ) |
Total revenue | | $ | 8,573 | | | $ | 63,573 | | | $ | (55,000 | ) |
Commercialization revenues were as follows:
| Year Ended December 31, | | | Increase (Decrease) | | |
| 2017 | | | 2016 | | | 2015 | | | 2017 compared to 2016 | | | 2016 compared to 2015 | | |
| (in thousands) | | |
Tabelecleucel (formerly known as ATA129) | $ | 15,078 | | | $ | 8,821 | | | $ | 970 | | | $ | 6,257 | | | | 7,851 | | |
ATA188 | | 2,697 | | | | - | | | | - | | | | 2,697 | | | | - | | |
ATA230 | | 2,493 | | | | 2,572 | | | | 78 | | | | (79 | ) | | | 2,494 | | |
T-cell manufacturing and other T-cell program expenses | | 22,239 | | | | 18,673 | | | | 9,123 | | | | 3,566 | | | | 9,550 | | |
Molecular program expenses | | 389 | | | | 1,110 | | | | 18,511 | | | | (721 | ) | | | (17,401 | ) | |
Employee and overhead costs | | 38,310 | | | | 25,338 | | | | 12,936 | | | | 12,972 | | | | 12,402 | | |
Total research and development | $ | 81,206 | | | $ | 56,514 | | | $ | 41,618 | | | $ | 24,692 | | | $ | 14,896 | | |
Tabelecleucel costs were $15.1$7.9 million in 20172023 as compared to $8.8 millionnone in 2016 and $1.0 million in 2015.2022. The increases in 2017 and 2016 were primarily due to manufacturing and outside service costs related to the preparation for the two Phase 3 clinical trials of tabelecleucel in patients with EBV+ PTLD who have failed rituximab and ongoing costs for our tabelecleucel EAP clinical trial, which was initiated in mid-2016. We anticipate that tabelecleucel costs will increase in 20182023 was due to the initiationEC marketing authorization for commercial sale and use of two Phase 3 clinical trials forEbvallo in the EU was transferred to Pierre Fabre in February 2023 and no commercialization revenue could be recognized prior to this product candidate in December 2017.occurring.
ATA188 costsLicense and collaboration revenues were $2.7$0.7 million in 20172023 as compared to zero in 2016 and 2015. The increase in 2017 was primarily related to clinical manufacturing and preparations for the Phase 1 clinical trial of allogeneic ATA188, which was initiated in October 2017. We anticipate that ATA188 costs will increase in 2018 as the Phase 1 trial expands into additional territories.
ATA230 costs were $2.5$63.6 million in 2017 as compared to $2.6 million in 2016 and $0.1 million in 2015.2022. The decrease in 20172023 was primarily due to decreased clinical trial activityBayer having notified us in May 2022 of its decision to terminate the Bayer Agreements, which resulted in the year. The increase in 2016 was primarily related to outside services costs associated withrecognition of the Phase 2 clinical trial for this product candidate. We anticipate that ATA230 costs will decrease in 2018 as we are prioritizing our EBV related programs ahead of ATA230 at this time.
T-cell manufacturing and other T-cell program expenses were $22.2 million in 2017 as compared to $18.7 million in 2016 and $9.1 million in 2015. The increase of $3.5 million in 2017 was primarily due to increased tabelecleucel production to prepare for the pivotal trials and increased spending on our BKV and HPV programs through our collaboration with QIMR Berghofer. The increase of $9.6 million in 2016 was primarily due to an increase of $14.1 million for manufacturing-related activities, including the technical transfer of tabelecleucel manufacturing to a third party CMO, partially offset by a $4.5 million license payment made to MSK in 2015. Expenses in 2016 included cash payments to QIMR Berghofer of $3.3 millionremaining deferred revenue related to the Bayer Agreements in the second quarter of 2022. The only license of additional T-cell immunotherapy programs and the option to license additional technologies from them. Expenses in 2015 included a $4.5 million cash payment to MSK to exercise our option to license certain T-cell immunotherapy programs, and $3.0 million paid to QIMR Berghofer for an exclusive, worldwide license to develop and commercialize allogeneic T-cell immunotherapy programs utilizing technology and know-how developed by them. We anticipate that T-cell manufacturing and other T-cell program expenses will continue to increase in 2018 due to an increase in manufacturing activity, the continued development of our manufacturing processes, and the development of products obtained from our collaboration with QIMR Berghofer.
Molecular program expenses were $0.4 million in 2017 as compared to $1.1 million in 2016 and $18.5 million in 2015. The decrease of $0.7 million in 2017 and $17.4 million in 2016 were primarily duerevenue recognized subsequent to the suspensiontermination of the PINTA 745 and ATA 842 programs in December 2015 and the STM 434 programagreements with Bayer in the third quarter of 2017.2022 has been related to certain early access program cost reimbursements per the A&R Commercialization Agreement.
Employee and overhead costsCost of commercialization revenue
Cost of commercialization revenue consisted of the following in the periods presented:
| | | | | | | | | | | | |
| | Year ended December 31, | | | | |
| | 2023 | | | 2022 | | | Increase (Decrease) | |
| | (in thousands) | |
Cost of commercialization revenue | | $ | 8,886 | | | $ | — | | | $ | 8,886 | |
Costs of commercialization revenues were $38.3$8.9 million in 20172023 compared to none in 2022. The increase primarily related to adjustments to reflect current period work-in-process inventory at net realizable value, idle capacity period manufacturing costs and in-license sales-related milestone expense. Prior to receiving EC regulatory approval for Ebvallo in the EU in December 2022, we recorded all costs incurred in the manufacture of Ebvallo to be sold upon commercialization as research and development expense. As a result, Ebvallo inventories manufactured before EC regulatory approval, referred to as zero cost inventories, were expensed as research and development and are therefore excluded from the cost of commercialization revenue. Ebvallo manufacturing costs incurred after EC regulatory approval are capitalized into inventory. All commercialization revenue recognized to date relates to zero cost inventories and all Ebvallo sold to Pierre Fabre in the year ended December 31, 2023 were zero cost inventories.
Research and development expenses
Research and development expenses consisted of the following costs, by program, in the periods presented:
| | | | | | | | | | | | |
| | Year ended December 31, | | | | |
| | 2023 | | | 2022 | | | Increase (Decrease) | |
| | (in thousands) | |
Program-specific expenses | | | | | | | | | |
Tab-cel® expenses | | $ | 22,088 | | | $ | 40,597 | | | $ | (18,509 | ) |
ATA188 expenses | | | 21,308 | | | | 26,776 | | | | (5,468 | ) |
CAR T and other program expenses | | | 9,523 | | | | 10,728 | | | | (1,205 | ) |
Non program-specific expenses | | | | | | | | | |
Employee and overhead expenses | | | 159,437 | | | | 186,339 | | | | (26,902 | ) |
Other non program-specific expenses | | | 12,429 | | | | 8,093 | | | | 4,336 | |
Total research and development expenses | | $ | 224,785 | | | $ | 272,533 | | | $ | (47,748 | ) |
Tab-cel expenses were $22.1 million in 2023 as compared to $25.3$40.6 million in 20162022. The decrease in 2023 was primarily due to the capitalization of tab-cel manufacturing costs to inventory following EC regulatory approval in December 2022 and $12.9a decrease in European tab-cel regulatory filing and approval activities.
ATA188 expenses were $21.3 million in 2015. The increases of $13.02023 as compared to $26.8 million in 20172022. The decrease in 2023 is primarily due to lower product development and $12.4 million in 2016 were primarilymanufacturing activities as a result of us pausing such activities while we awaited the results of the Phase 2 EMBOLD clinical trial primary analysis data. Following the announcement in November 2023 that the study did not meet its primary endpoint and the subsequent pause in the development of ATA188, we expect ATA188 expenses to be lower in future periods.
CAR T and other program expenses were $9.5 million in 2023 as compared to $10.7 million in 2022. The decrease in 2023 was primarily driven by higher compensation-relatedmanufacturing, development and outside services spend in 2022 leading up to the submission of the ATA3219 IND in mid-2023, partially offset by costs from increased headcountrelated to ATA3219 clinical trial start-up activities in supportthe second half of our continuing expansion of research and development activities. In particular,2023.
Non program specific expenses were $171.9 million in 2023 as compared to $194.4 million in 2022. The decrease in 2023 was primarily due to lower payroll and related costs, driven by the sale of the ATOM facility and August 2022 reduction in force, partially offset by increased by $8.0 million in 2017costs associated with cross-program materials and outside services. In 2023 as compared to 2016,2022, payroll and by $8.5 million in 2016 as compared to 2015, from increased headcount. Also, facility related costs and otherdecreased by $26.1 million; outside servicesservice costs decreased by $2.3 million; facility-related costs increased by $3.4 million$1.6 million; and $0.8 million, respectively, in 2017 as compared to 2016, andother non-program specific costs increased by $2.3 million and $1.2 million, respectively, in 2016 as compared to 2015. We anticipate that employee and overhead costs will continue to increase in future periods as we continue to expand our research and development activities.$4.3 million.
General and administrative expenses
General and administrative expenses for the periods indicated were as follows:
| | | | | | | | | | | | |
| | Year ended December 31, | | | | |
| | 2023 | | | 2022 | | | (Decrease) Increase | |
| | (in thousands) | |
General and administrative expenses | | $ | 50,908 | | | $ | 71,553 | | | $ | (20,645 | ) |
| | Year ended December 31, | | | Increase (Decrease) | |
| | 2017 | | | 2016 | | | 2015 | | | 2017 compared to 2016 | | | 2016 compared to 2015 | |
| | (in thousands) | |
General and administrative | | $ | 40,326 | | | $ | 24,728 | | | $ | 16,830 | | | $ | 15,598 | | | $ | 7,898 | |
General and administrative expenses were $40.3$50.9 million in 20172023 as compared to $24.7$71.6 million in 20162022. The decrease in 2023 was primarily driven by lower payroll and $16.8related costs as a result of the August 2022 reduction in force and lower outside service costs related to a reduction in US tab-cel commercial activities.
Other income (expense), net
| | | | | | | | | | | | |
| | Year ended December 31, | | | | |
| | 2023 | | | 2022 | | | Increase (Decrease) | |
| | (in thousands) | | |
Interest income | | $ | 5,426 | | | $ | 3,059 | | | $ | 2,367 | |
Interest expense | | | (5,285 | ) | | | (373 | ) | | | (4,912 | ) |
Other income (expense), net | | | (246 | ) | | | (700 | ) | | | 454 | |
Gain on sale of ATOM Facility | | | — | | | | 50,237 | | | | (50,237 | ) |
Total other income (expense), net | | $ | (105 | ) | | $ | 52,223 | | | $ | (52,328 | ) |
Interest income was $5.4 million in 2015.2023, as compared to $3.1 million in 2022. The increase of $15.6 million in 20172023 was primarily due to a $10.1 million increase in compensation-related costs driven by increased headcount, a $4.7 million increase in professional services costs and a $0.8 million increase in travel and other related costs. The increase of $7.9 million in 2016 was primarily due to a $7.0 million increase in compensation-related costs driven by increased headcount, a $1.1 million increase in professional services costs,higher investment yields, partially offset by a $0.2lower balances of cash, cash equivalents and available-for-sale securities.
Interest expense was $5.3 million decrease in travel and other2023 as compared to $0.4 million in 2022. The higher interest expense in 2023 was due to interest expense recognized on the liability related costs. We expect that general and administrative costs will continue to increasethe sale of future revenues following us entering into the HCRx Agreement in 2018 as we continue to expand our operations.
Quarterly Results of Operations Data (unaudited)December 2022.
The following table sets forth our unaudited consolidated statement of operations data for each of the eight quarters in the period ended December 31, 2017. The unaudited quarterly statement of operations data set forth below have been prepared on a basis consistent with our audited annual consolidated financial statements in this Annual Report on Form 10-K and include, in our opinion, all normal recurring adjustments necessary for a fair statement of the financial information contained in those statements. Our historical results are not necessarily indicative of the results that may be expected in the future. The following quarterly financial data should be read in conjunction with our audited consolidated financial statements and the related notes included elsewhere in this Annual Report on Form 10-K.
| | Three months ended | |
| | March 31 | | | June 30 | | | September 30 | | | December 31 | |
2017 | | (In thousands) | |
Operating expenses: | | | | | | | | | | | | | | | | |
Research and development | | $ | 17,541 | | | $ | 18,296 | | | $ | 20,598 | | | $ | 24,771 | |
General and administrative | | | 8,620 | | | | 9,613 | | | | 11,062 | | | | 11,031 | |
Total operating expenses | | | 26,161 | | | | 27,909 | | | | 31,660 | | | | 35,802 | |
Loss from operations | | | (26,161 | ) | | | (27,909 | ) | | | (31,660 | ) | | | (35,802 | ) |
Interest and other income, net | | | 509 | | | | 481 | | | | 564 | | | | 473 | |
Loss before provision for income taxes | | | (25,652 | ) | | | (27,428 | ) | | | (31,096 | ) | | | (35,329 | ) |
Provision (benefit) for income taxes | | | 2 | | | | — | | | | — | | | | (16 | ) |
Net loss | | | (25,654 | ) | | | (27,428 | ) | | | (31,096 | ) | | | (35,313 | ) |
Other comprehensive loss: | | | | | | | | | | | | | | | | |
Unrealized gain (loss) on available-for-sale securities | | | 31 | | | | 38 | | | | 26 | | | | (63 | ) |
Comprehensive loss | | $ | (25,623 | ) | | $ | (27,390 | ) | | $ | (31,070 | ) | | $ | (35,376 | ) |
| | | | | | | | | | | | | | | | |
Basic and diluted net loss per common share | | $ | (0.88 | ) | | $ | (0.94 | ) | | $ | (1.02 | ) | | $ | (1.15 | ) |
| | | | | | | | | | | | | | | | |
| | Three months ended | |
| | March 31 | | | June 30 | | | September 30 | | | December 31(1) | |
2016 | | (In thousands) | |
Operating expenses: | | | | | | | | | | | | | | | | |
Research and development | | $ | 11,247 | | | $ | 12,991 | | | $ | 18,802 | | | $ | 13,474 | |
General and administrative | | | 5,814 | | | | 6,494 | | | | 7,140 | | | | 5,280 | |
Total operating expenses | | | 17,061 | | | | 19,485 | | | | 25,942 | | | | 18,754 | |
Loss from operations | | | (17,061 | ) | | | (19,485 | ) | | | (25,942 | ) | | | (18,754 | ) |
Interest and other income, net | | | 503 | | | | 605 | | | | 576 | | | | 519 | |
Loss before provision for income taxes | | | (16,558 | ) | | | (18,880 | ) | | | (25,366 | ) | | | (18,235 | ) |
Provision (benefit) for income taxes | | | 3 | | | | — | | | | 7 | | | | — | |
Net loss | | | (16,561 | ) | | | (18,880 | ) | | | (25,373 | ) | | | (18,235 | ) |
Other comprehensive loss: | | | | | | | | | | | | | | | | |
Unrealized gain (loss) on available-for-sale securities | | | 569 | | | | 142 | | | | (158 | ) | | | (218 | ) |
Comprehensive loss | | $ | (15,992 | ) | | $ | (18,738 | ) | | $ | (25,531 | ) | | $ | (18,453 | ) |
| | | | | | | | | | | | | | | | |
Basic and diluted net loss per common share | | $ | (0.58 | ) | | $ | (0.66 | ) | | $ | (0.88 | ) | | $ | (0.63 | ) |
(1)
| Subsequent to issuance of our interim consolidated financial statements for the three and nine months ended September 30, 2016, we identified certain share-based awards provided in 2016 and 2015 with only time-based vesting conditions for which we recorded stock based compensation expense using the graded accelerated expensing method instead of a straight-line expensing method in accordance with our accounting policy, certain share-based awards where stock-based compensation expense was not appropriately adjusted for unvested awards of terminated employees during 2016, and certain stock-based compensation expense related to non-employee options recorded incorrectly during the first quarter of 2016. We corrected for these errors by recording a $3.3 million out-of-period adjustment to stock-based compensation expense during the fourth quarter of 2016. The recorded adjustment included $0.7 million related to the three months ended September 30, 2016, $1.1 million related to the three months ended June 30, 2016, $0.7 million related to the three months ended March 31, 2016 and $0.7 million related to the fiscal year ended December 31, 2015. The adjustment was not considered material to the fiscal year ended December 31, 2016 or any previously issued interim or annual consolidated financial statements.
|
Liquidity and Capital Resources
Sources of Liquidity
Since our inception in 2012, we have funded our operations primarily through the issuance of common and preferred stock.stock, issuance of pre-funded warrants to purchase common stock, upfront fees and milestone payments from the Bayer License Agreement and the A&R Commercialization Agreement and the sale of our ATOM Facility.
In March 2017,the past three years, we have entered into atwo separate sales agreement, or the ATM facility,agreements with Cowen and Company, LLC (Cowen): in November 2021 (2021 ATM Facility) and in November 2023 (2023 ATM Facility). Each ATM facility provides or Cowen, under which we may offer and sell,provided for the sale, in our sole discretion, of shares of our common stock having an aggregate offering price of up to $75.0$100.0 million, through Cowen, as our sales agent. We will pay Cowenfiled a commissionregistration statement on Form S-3 registering the offer and sale of up to 3.0%these shares under the Securities Act (the 2023 Registration Statement). Upon the effectiveness of the gross2023 Registration Statement, the 2021 ATM Facility was terminated, and no further sales proceeds of any common stock soldcan be made under the 2021 ATM facility.Facility. The issuance and sale of these shares by us pursuant to the ATM facilityfacilities are deemed “at the market” offerings and are availabledefined in Rule 415 under the Securities Act of 1933, as amended.amended (Securities Act), and were registered under the Securities Act. Commissions of up to 3.0% are due on the gross sales proceeds of the common stock sold under each ATM facility.
During the fiscal year ended December 31, 2017,2023, we sold an aggregate of 1,349,8653,038,432 shares of common stock under theour ATM facility,facilities, at an average price of approximately $14.82$0.83 per share, for gross proceeds of $20.0$2.5 million and net proceeds of $19.2$2.2 million, after deducting commissions and other offering expenses. expenses payable by us. Approximately $0.1 million of the $2.2 million net proceeds were received on January 2, 2024.
As of December 31, 2017, $55.02023, we had $98.2 million of common stock remainedremaining and available to be sold under this facility,the 2023 ATM Facility.
From January 1, 2024 through March 15, 2024, we sold an additional 12,321,365 shares of common stock under the 2023 ATM Facility, at an average price of $0.77 per share, for gross proceeds of $9.5 million and net proceeds of $9.3 million, after deducting commissions and other offering expenses payable by us. As of March 15, 2024, we had $88.7 million of common stock remaining and available to be sold under the 2023 ATM Facility, subject to certain conditions as specified in the agreement.
In January 2018, we completed an underwritten public offering of 7,675,072 shares of common stock, including 675,072 from the exercise by the underwriters of their option to purchase additional shares, at an offering price of $18.25 per share. We received net proceeds of approximately $131.4 million, after deducting underwriting discounts and commissions and offering expenses.
We have incurred losses and negative cash flows from operations in each year since inception. Asinception and have only just begun to generate commercialization revenues from the A&R Commercialization Agreement, following the December 2022 EU regulatory approval of December 31, 2017, we had an accumulated deficitEbvallo, which is subject to the terms of $296.7 million. It will be several years,the HCRx Agreement. We do not maintain any meaningful milestone or royalty payments from Pierre Fabre relative to the Initial Territory until the applicable royalty cap under the HCRx Agreement is met, if ever, before we have a product candidate ready for commercialization,at all. We continue to incur significant research and we anticipate that we will continuedevelopment and other expenses related to our ongoing operations and expect to incur losses for at least the next several years. We expect that our research and development and general and administrative expenses will continue to increase and, asforeseeable future. As a result, we will need additional capital to fund our operations, which we may raise through a combination of equity offerings, debt financings, other third-partythird party funding marketing and distribution arrangements and other collaborations, strategic alliances and licensingpartnering arrangements. We may borrow funds on terms that may include restrictive covenants, including covenants that restrict the operation of our business, liens on assets, high effective interest rates and repayment provisions that reduce cash resources and limit future access to capital markets. In addition,addition, we expect to continue to opportunistically seek access to additional funds through additional public or private equity offerings or debt financings including by utilizing the equity capital markets to support our development efforts and operations.2023 ATM Facility, through potential collaboration, partnering or other strategic arrangements, or a combination of the foregoing. To the extent that we raise additional capital by issuing equity securities, our stockholders may experience substantial dilution. To the extent that we raise additional funds through collaboration or partnering arrangements, we may be required to relinquish some of our rights to our technologies or rights to market and sell our products in certain geographies or grant licenses or other rights on terms that are not favorable to us, or issue equity that may be substantially dilutive to our stockholders.us.
Cash in excess of immediate requirements is invested in accordance with our written investment policy, primarily with a view to liquidity and capital preservation. Currently, our cash, cash equivalents and short-term investments are held in bank and custodial accounts and consist of money market funds, U.S. Treasury, government agency and corporate debt obligations, commercial paper and asset-backed securities. In 2018, we expect to spend approximately $21.3 million of cash to complete the build-out of our office, lab and cellular therapy manufacturing space in Thousand Oaks, California. Management expects that our cash, cash equivalents and short-term investments as of December 31, 2017, along with the proceeds from the public offering completed in January 2018, will be sufficient to fund our planned operations into the first half of 2020. obligations.
Our cash, cash equivalents and short-term investments balances as of the dates indicated were as follows:
| | | | | | | | |
| | December 31, | | | December 31, | |
| | 2023 | | | 2022 | |
| | (in thousands) | |
Cash and cash equivalents | | $ | 25,841 | | | $ | 92,942 | |
Short-term investments | | | 25,884 | | | | 149,877 | |
Total cash, cash equivalents and short-term investments | | $ | 51,725 | | | $ | 242,819 | |
Contractual Obligations and Commitments
We lease our corporate headquarters in Thousand Oaks, California, under a non-cancellable lease agreement for approximately 51,160 square feet of office space. The initial term of this lease expires in February 2026. The contractual obligations during the initial term are $8.5 million in aggregate. We have the option to extend the lease for an additional period of five years after the initial term.
| | December 31, | | | December 31, | |
| | 2017 | | | 2016 | |
| | (in thousands) | |
Cash and cash equivalents | | $ | 79,223 | | | $ | 47,968 | |
Short-term investments | | | 86,873 | | | | 207,714 | |
Total cash, cash equivalents and short-term investments | | $ | 166,096 | | | $ | 255,682 | |
89
We lease office space in South San Francisco, California under a non-cancellable lease agreement. In December 2021, we entered into a second amendment to extend the lease term through May 2025. The amended lease agreement does not include an option to extend the lease term. In October 2022, we entered into a sub-lease agreement with a third party for this office space. The sub-lease term commenced in November 2022 and expires in May 2025, with no option to extend the sub-lease term. Subsequently, we have moved our corporate headquarters to our office space in Thousand Oaks, California.
In May 2019, we entered into a lease agreement for approximately 8,800 square feet of office and lab space in Aurora, Colorado. In February 2021, we further amended this lease to add an additional 2,861 square feet of lab space. In November 2023, we entered into an amended lease agreement for our office and lab space in Aurora, Colorado, to extend the term of our lease agreement through April 2025. The contractual obligations during the lease term are not material.
In March 2021, we entered into a lease agreement for approximately 33,659 square feet of office, lab and warehouse space in Thousand Oaks, California. The initial 10.5-year term of this lease commenced in August 2021 and the contractual obligations during the initial term are $21.0 million in aggregate. We have the option to extend this lease for two additional five-year periods after the initial term.
In February 2017, we entered into a lease agreement (ATOM Lease) for approximately 90,580 square feet of office, lab and cellular therapy manufacturing space in Thousand Oaks, California. The initial 15-year term of this lease commenced in February 2018, and the contractual obligations during the initial term are $16.4 million in aggregate. We have the option to extend this lease for two additional periods of ten and nine years, respectively, after the initial term. In April 2022, we assigned the ATOM Lease to FDB in connection with the closing of the sale of the ATOM Facility to FDB. We remain joint and severally liable for obligations related to the ATOM Lease. See Note 8 – “Leases” in the Notes to Consolidated Financial Statements, included in Item 8. Financial Statements and Supplementary Data of this report for further information on our lease obligations.
We enter into contracts in the normal course of business with clinical research organizations for clinical studies, with CMOs for clinical and commercial materials, and with other vendors for preclinical studies and supplies and other services and products for operating purposes. These contracts generally provide for termination for convenience following a notice period. We have non-cancellable minimum commitments for products and services, subject to agreements with a term of greater than one year with clinical research organizations and CMOs. See Note 10 – “Commitments and Contingencies” in the Notes to Consolidated Financial Statements, included in Item 8. Financial Statements and Supplementary Data of this report for further information on our contractual obligations and commitments.
Cash Flows
Comparison of the Years Ended December 31, 2023 and 2022
The following table details the primary sources and uses of cash for each of the periods set forth below:
| | | | | | | | |
| | Year Ended December 31, | |
| | 2023 | | | 2022 | |
| | (in thousands) | |
Net cash (used in) provided by: | |
| | | | |
Operating activities | | $ | (192,977 | ) | | $ | (270,430 | ) |
Investing activities | | | 123,866 | | | | 202,956 | |
Financing activities | | | 2,010 | | | | 53,084 | |
Net decrease in cash, cash equivalents and restricted cash | | $ | (67,101 | ) | | $ | (14,390 | ) |
| | Year Ended December 31, | |
| | 2017 | | | 2016 | | | 2015 | |
| | (in thousands) | |
Net cash provided by (used in): | | | | | | | | | | | | |
Operating activities | | $ | (87,502 | ) | | $ | (60,025 | ) | | $ | (37,156 | ) |
Investing activities | | | 98,709 | | | | 83,741 | | | | (220,127 | ) |
Financing activities | | | 20,048 | | | | 506 | | | | 259,226 | |
Effect of exchange rates on cash | | | — | | | | — | | | | (94 | ) |
Net increase in cash and cash equivalents | | $ | 31,255 | | | $ | 24,222 | | | $ | 1,849 | |
Operating activities
Net cash used in operating activities was $87.5$193.0 million in 20172023 as compared to $60.0$270.4 million in 2016.2022. The increasedecrease of $27.5$77.4 million was primarily due to a $40.4the $40.0 million received from Pierre Fabre in 2023 for development milestones met in December 2022, with no similar cash flows received in 2022.The remaining decrease was due to lower cash operating expenses in 2023 as compared to 2022, primarily due to lower compensation-related costs resulting from lower headcount driven by the sale of the ATOM facility in April 2022 and the August 2022 reduction in force, partially offset by an increase in net loss, partially offset by a $6.3 million increase stock-based compensation, a $4.3 million increase in accrued research and development expenses, a $1.2 million increase in accounts payable and a $0.8 million increased in accrued compensation.
Net cash used in operating activities was $60.0 million in 2016 as compared to $37.2 million in 2015. The increaseworking capital from the capitalization of $22.9 million was primarily due to a $21.8 million increase in net loss and a $7.0 million decrease in accrued research and development expenses, partially offset by a $6.5 million increase in stock-based compensation. costs into inventory following the December 2022 EC regulatory approval of Ebvallo.
Investing activities
Net cash provided by investing activities in 20172023 consisted primarily of $189.0$208.7 million received from maturities and $107.6 million from sales of available-for-sale securities, partially offset by $176.5$83.6 million used to purchase available-for-sale securities and $20.2$1.2 million used to purchasein purchases of property and equipment.
Net cash provided by investing activities in 20162022 consisted primarily of $149.0$293.0 million received from maturities and $242.6 million from sales of available-for-sale securities, partially offset by $304.9$180.6 million used to purchase available-for-sale securities and $3.0$4.2 million used to purchasein purchases of property and equipment.
Net cash used in investing activities in 2015 consisted primarily of $379.8 million of purchases of short-term available-for-sale securities, partially offset by $96.1 million received from maturities and $64.0 million from sales of available-for-sale securities.
Financing activities
Net cash provided by financing activities in the 20172023 consisted primarily of $19.2$2.1 million of net proceeds from the ATM facilityfacilities and $1.3$0.9 million of net proceeds from employee stock transactions, partially offset by $0.4 million of taxes paid related to the net share settlement of restricted stock. award transactions.
Net cash provided by financing activities in 20162022 consisted primarily of $0.5$21.9 million consists primarilyof net proceeds from ATM facilities, $30.6 million of net proceeds from the sale of future royalties and $1.9 million of net proceeds from employee stock award transactions. Net
Operating Capital Requirements and Plan of Operations
We expect that existing cash, provided by financing activities in 2015 consisted primarily of $263.4 million in aggregate net proceedscash equivalents and short-term investments, combined with certain anticipated payments from the saleA&R Commercialization Agreement, as well as cost reductions from completed workforce reductions and operating efficiencies resulting from our planned transition of common stock in two separate follow-on offerings.
Operating Capital Requirements
To date, we have not generated any revenue from product sales.substantially all activities relating to tab-cel at the time of the BLA transfer to Pierre Fabre, will enable us to fund our planned operations into 2027. Such anticipated payments are estimates based on assumptions and plans that are subject to change and such changes could materially impact our expected cash runway. These assumptions include the receipt of future payments that are dependent upon the successful filing and approval of the tab-cel BLA, as well as the completion of specific development and regulatory activities by us and actions taken by third parties, and are, therefore, uncertain at this time. We do not know when, or if, we will generate anysufficient revenue from product sales. We do not expectcommercialization to generate significant revenue from product sales unless and until we obtain regulatory approval of and commercialize one ofoffset our current or future product candidates.operating expenses. We anticipate that we will continue to generate losses for the foreseeable future, and we expect the accumulated losses to increase as we continue the development of, and seek regulatory approvals for, our product candidates and begin to commercialize any approved products. We are subject to all of the risks inherent in the development of new products, and we may encounter unforeseen expenses, difficulties, complications, delays and other unknown factors that may adversely affect our business. We anticipate that we will need to raise substantial additional funding to finance our planned operations in connection with our continuing operations.the long-term.
Our operating plan may change as a result of many factors currently unknown to us, and we may need additional funds sooner than planned. We expectdo not have any committed external source of funds other than milestone and royalty payments that ourwe may receive under the A&R Commercialization Agreement, subject to the terms of the HCRx Agreement. We do not retain any meaningful milestone or royalty payments related to the Initial Territory from Pierre Fabre until the applicable royalty cap under the HCRx Agreement is met, if at all.
Our existing cash, cash equivalents and short-term investments as of December 31, 2023 will not be sufficient to fund our planned operations intofor at least the first halfnext 12 months after the date of 2020. issuance of these financial statements. These conditions raise substantial doubt about our ability to continue as a going concern for at least 12 months after the issuance of the accompanying consolidated financial statements.
In order to complete the process of obtaining regulatory approval for any of our lead product candidates and to build the sales, marketing and distribution infrastructure that we believe will be necessary to commercialize our lead product candidates, if approved,have not received approval, we will require substantial additional funding. We expect to continue to opportunistically seek access to additional funds through additional public or private equity offerings or debt financings, through potential collaboration, partnering or other strategic arrangements, or a combination of the foregoing. If we are unable to obtain sufficient funding on acceptable terms, we could be forced to delay, limit, reduce or terminate preclinical studies, clinical studies or other development activities for one or more of our product candidates.
We have based our projections of operating capital requirements on assumptions that may prove to be incorrect and we may use all of our available capital resources sooner than we expect. Because of the numerous risks and uncertainties associated with research, development and commercialization of pharmaceutical products, we are unable to estimate the exact amount of our operating capital requirements. Our future funding requirements will depend on many factors, including, but not limited to:
•the timing, costs and costsresults of our ongoing and planned clinical trials and preclinical studies for our product candidates;
•our success in establishing and scaling commercialmaintaining manufacturing capabilities;relationships with CMOs;
•the number and characteristics of product candidates that we pursue;
•the outcome, timing and costs of seeking regulatory approvals;
•subject to receipt of regulatory approval, costs associated with the commercialization of our product candidates by our partners and the amount of revenues received from commercial sales of our product candidates;
•the timing of proceeds from, and our ability to perform under, the A&R Commercialization Agreement, subject to the HCRx Agreement, as well as the terms and timing of any future collaborations,commercialization, collaboration, licensing, consultingpartnering or other arrangements that we may establish;
•the amount and timing of any payments we may be required to make in connection with the licensing, filing, prosecution, maintenance, defense and enforcement of any patents or patent applications or other intellectual property rights;
•the extent to which we in-license or acquire other products and technologies; and
•the timing of capital expenditures, including the buildingqualification of our ownCMOs’ manufacturing facility.
facilities. Contractual Obligations and Commitments
We lease our current corporate headquarters in South San Francisco, California under a non-cancellable lease agreement for approximately 13,670 square feet of office space. The lease is expected to expire in April 2021.
In January 2015, we entered into a non-cancellable lease agreement for office and laboratory space in Westlake Village, California. In September 2015, we amended the lease agreement to add additional office space and extend the term of the agreement to April 2019.
In February 2017, we entered into a lease agreement for approximately 90,580 square feet of office, lab and cellular therapy manufacturing space in Thousand Oaks, California, or the Thousand Oaks lease. The term of the Thousand Oaks lease commences when the landlord delivers possession of the facility to us. Upon commencement, the initial term of the lease is fifteen years. We have the option to extend the lease for two additional periods of ten and nine years, respectively, after the initial term. We are accounting for this lease under build to suit accounting guidance, which requires us to capitalize the fair value of the building as well as the construction costs incurred on our consolidated balance sheet along with a corresponding financing liability for landlord-paid construction costs. Upon occupancy for build-to-suit leases,Until we are alsoable to generate a sufficient amount of net cash inflows from operations, which we may never do, meeting our long-term capital requirements is in large part reliant on access to public and private equity and debt capital markets, augmented by cash generated from operations and interest income earned on the investment of our cash balances. We expect to continue to seek access to the equity and debt capital markets to support our development efforts and operations. To the extent that we raise additional capital by issuing equity securities, our stockholders may experience substantial dilution. To the extent that we raise additional funds through commercialization, collaboration or partnering arrangements, we may be required to assess whether the circumstances qualify for sale recognition under “sale-leaseback” accounting guidance.
In fourth quarterrelinquish some of 2017, we entered into multiple lease agreements to lease certain equipment that have been accounted for as capital leases. The term of the lease agreements range between 2-3 years.
Aggregate future minimum commitments for our leases as of December 31, 2017 are as follows:
| Payments Due by Period | |
| | | | | | | Less than | | | | | | | More than | |
| | Total | | | | 1 Year | | | 1-3 Years | | | 3-5 Years | | | 5 Years | |
| (in thousands) | |
Operating lease obligations | $ | | 3,674 | | | | | 1,979 | | | | | 1,436 | | | | | 259 | | | | | — | |
Capital lease obligations | | | 1,204 | | | | | 546 | | | | | 658 | | | | | — | | | | | — | |
Financing lease obligations | | | 16,387 | | | | | 372 | | | | | 1,849 | | | | | 1,962 | | | | | 12,204 | |
Total contractual obligations | $ | | 21,265 | | | $ | | 2,897 | | | $ | | 3,943 | | | $ | | 2,221 | | | $ | | 12,204 | |
The above amounts exclude potential milestone and royalty payments relatedrights to our licensetechnologies or rights to market and collaboration agreements, as the achievementsell our products in certain geographies, grant licenses or other rights on terms that are not favorable to us, or issue equity that may be substantially dilutive to our stockholders.
As a result of these milestones is currently not fixed and determinable.
We may also enter into contracts in the normal course of business with clinical research organizations for clinical trials, with contract manufacturing organizations for clinical supplies, and with other vendors for preclinical studies and supplieseconomic conditions, general global economic uncertainty, political change and other services and products for operating purposes. These contracts generally provide for termination on notice, with the exception of potential termination charges related to one of our contract manufacturing agreements in the event certain minimum purchase volumes are not met. Payments in the table above are based on current operating forecasts, which are subject to change, and do not include any termination fees.
Off-Balance Sheet Arrangements
We did not have during the periods presented, andfactors, we do not currently have, any off-balance sheet arrangements, as defined inknow whether additional capital will be available when needed, or that, if available, we will be able to obtain additional capital on reasonable terms. If we are unable to raise additional capital due to the rules and regulationsvolatile global financial markets, general economic uncertainty or other factors, we will be forced to delay, limit, reduce or terminate preclinical studies, clinical studies or other development activities for one or more of the SEC.our product candidates.
Item 7A. Quantitative and Qualitative Disclosures about Market Risk
Interest Rate and Market Risk
We are exposed to market risk related to changes in interest rates. As of December 31, 2017,2023, we had total cash, and cash equivalents and short-term investments of $166.1$51.7 million. Our primary exposure to market risk is interest rate sensitivity, which is affected by changes in the general level of U.S. interest rates, particularly because our investments are in short-term securities. Our available-for-sale securities are subject to interest rate risk and will fall in value if market interest rates increase, which could result in a realized loss if we are forced to sell an investment before its scheduled maturity. maturity. We currently do not hedge our interest rate risk exposure. Due to the short-term duration of our investment portfolio and the low risk profile of our investments, an immediate change in interest rates of 10100 basis points would not result in a significantmaterial change in the fair market value of our portfolio.
The primary objectiveobjectives of our investment activities is to preserve principalare capital preservation and liquidity, while at the same time maximizing the income we receive from our investments without significantly increasing risk. To achieve this objective, we maintain our portfolio of cash equivalents and short-term and long-term investments in a variety of securities, including money market funds, U.S. Treasury, government agency and corporate debt obligations, commercial paper and asset-backed securities. These securities are all classified as available-for-sale and consequently are recorded on the balance sheet at fair value, with unrealized gains or losses reported as a separate component of accumulated other comprehensive income (loss). Our holdings of the securities of any one issuer, except for obligations of the U.S. Treasury, U.S. Treasury-guaranteed securities or U.S. Treasury guaranteed securities,money market funds, do not exceed 5% of our portfolio.
Item 8. Financial StatementsStatements and Supplementary Data
Index to Consolidated Financial Statements
ReportReport of Independent RegisteredRegistered Public Accounting Firm
To the stockholders and the Board of Directors of
Atara Biotherapeutics, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Atara Biotherapeutics, Inc. and subsidiaries (the "Company" or “Atara”) as of December 31, 20172023 and 2016,2022, the related consolidated statements of operations and comprehensive loss, stockholders’ equity (deficit), and cash flows, for each of the threetwo years in the period ended December 31, 2017,2023, and the related notes (collectively referred to as the "financial statements"). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 20172023 and 2016,2022, and the results of its operations and its cash flows for each of the threetwo years in the period ended December 31, 2017,2023, in conformity with accounting principles generally accepted in the United States of America (GAAP).America.
Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company’s recurring losses from operations raises substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB)PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current-period audit of the financial statements that was communicated or required to be communicated to the audit committee and that (1) relates to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing separate opinions on the critical audit matter or on the accounts or disclosures to which they relate.
Commercialization Revenue and Deferred Revenue – Accounting for Out- License Agreement – Refer to Notes 2 and 5 to the Financial Statements
Critical Audit Matter Description
The Company has entered into certain out-license agreements with Pierre Fabre.
During 2021, the Company entered into a Commercialization Agreement with Pierre Fabre Medicament (“Pierre Fabre”). Under the terms of the agreement, the Company granted Pierre Fabre a license to commercialize and distribute Ebvallo in an initial territory and became responsible for manufacturing and supplying Ebvallo to Pierre Fabre, along with related cell selection services.
In 2022, the Company entered into an Amendment Agreement to the Pierre Fabre Commercialization Agreement (the “PF Amendment No. 1”). Under the terms of the PF Amendment No. 1, Atara became entitled to an additional milestone payment in exchange for, among other things, a reduction in: (i) royalties Atara is eligible to receive, and (ii) the price mark up on supply purchased by Pierre Fabre. Additionally, Atara also agreed to extend the time period for provision of certain services to Pierre Fabre under the Pierre Fabre Commercialization Agreement.
In 2023, the Company entered into an amended and restatement Pierre Fabre Commercialization Agreement (the “A&R Commercialization Agreement”). Under the terms of the A&R Commercialization Agreement, the Company granted Pierre Fabre an expanded license to commercialize and distribute Ebvallo in all other countries in the world and remains responsible for manufacturing and supplying Ebvallo to Pierre Fabre, along with related cell selection services until transfer of responsibility to Pierre Fabre.
The Company recognizes revenue on the commercialization agreements with Pierre Fabre as they satisfy their performance obligations and when a customer obtains control of the promised goods or services. The revenue related to the commercialization agreements with Pierre Fabre is recognized within commercialization revenue within the Consolidated Statements of Operations and Comprehensive Loss. As of December 31, 2023, the Company recognized $7.89 million of commercialization revenue and deferred revenue amounted to $115.4 million, of which $77.8 million is included in current liabilities and $37.6 million is included in long-term liabilities.
We identified accounting for the commercialization agreements, the revenue recognized, and the estimated deferred revenue to be recognized as revenue as a critical audit matter. Given the judgments necessary to determine the accounting literature to apply to a commercialization agreement, the method to estimate and measure the progress toward the completion of the performance obligations and the estimated contractual term over which the performance obligations would be completed, auditing such judgments and estimates required extensive audit effort due to the complexity of the commercialization agreements and the high degree of auditor judgment applied when performing audit procedures and evaluating the results of those procedures.
How the Critical Audit Matter Was Addressed in the Audit
Our audit procedures related to determining the accounting literature to apply to the agreements, assessing management's method for measuring progress and evaluating management’s estimation of the contract term over which the performance obligations will be completed included the following, among others:
•We tested the operating effectiveness of controls over commercialization revenue, including those related to the evaluation of contract modifications, identification of performance obligations and the determination of the timing and amount of revenue recognized.
•We reviewed and obtained an understanding of the Company’s revenue generating agreements and related transactions during and at the end of the year via review of internal and external presentations, news and publications, and discussions with management.
•We evaluated the Company’s conclusions related to the accounting for contract modifications.
•We evaluated management’s determination that the agreements are within the scope of ASC 606 - Revenue from Contracts with Customers.
•We evaluated management's determination of the contractual term and the appropriateness of management's method to measure its progress over that term.
•We evaluated the assumptions used in the estimates of total costs and the estimated measure of progress for recognizing revenues by:
oPerforming corroborating inquiries with the Company's project and business development managers, and comparing the assumptions used in the estimates to management's work plans, cost estimates and costs reported to date, and material rights allocated, accumulated and earned.
oComparing costs incurred for activities completed to date to the costs forecasted for those activities.
oComparing material rights accumulated and earned for activities completed to date to the fulfilment of performance obligations forecasted for those activities.
oTesting the mathematical accuracy of management’s revenue and current and long-term deferred revenue balances based on the estimated revenue to be recognized over time.
/s/ DELOITTE & TOUCHE LLP
San Jose,Francisco, California
February 27, 2018March 28, 2024
We have served as the Company'sCompany’s auditor since 2013.
Atara Biotherapeutics, Inc.
Consolidated Balance Sheets
(In thousands, except per share amounts)
| | | | | | | | |
| | December 31, | | | December 31, | |
| | 2023 | | | 2022 | |
Assets | | | | | | |
Current assets: | | | | | | |
Cash and cash equivalents | | $ | 25,841 | | | $ | 92,942 | |
Short-term investments | | | 25,884 | | | | 149,877 | |
Restricted cash | | | 146 | | | | 146 | |
Accounts receivable | | | 34,108 | | | | 40,221 | |
Inventories | | | 9,706 | | | | 1,586 | |
Other current assets | | | 6,184 | | | | 10,308 | |
Total current assets | | | 101,869 | | | | 295,080 | |
Property and equipment, net | | | 3,856 | | | | 6,300 | |
Operating lease assets | | | 54,935 | | | | 68,022 | |
Other assets | | | 4,844 | | | | 7,018 | |
Total assets | | $ | 165,504 | | | $ | 376,420 | |
| | | | | | |
Liabilities and stockholders’ equity (deficit) | | | | | | |
Current liabilities: | | | | | | |
Accounts payable | | $ | 3,684 | | | $ | 6,871 | |
Accrued compensation | | | 11,519 | | | | 17,659 | |
Accrued research and development expenses | | | 17,364 | | | | 24,992 | |
Deferred revenue | | | 77,833 | | | | 8,000 | |
Other current liabilities | | | 31,826 | | | | 21,394 | |
Total current liabilities | | | 142,226 | | | | 78,916 | |
Deferred revenue – long-term | | | 37,562 | | | | 77,000 | |
Operating lease liabilities – long-term | | | 45,693 | | | | 58,064 | |
Liability related to the sale of future revenues – long-term | | | 34,623 | | | | 30,236 | |
Other long-term liabilities | | | 4,631 | | | | 5,564 | |
Total liabilities | | | 264,735 | | | | 249,780 | |
| | | | | | |
Commitments and contingencies (Note 10) | | | | | | |
| | | | | | |
Stockholders’ equity (deficit): | | | | | | |
Common stock—$0.0001 par value, 500,000 shares authorized as of December 31,
2023 and 2022, respectively; 106,447 and 95,927 shares issued and outstanding
as of December 31, 2023 and 2022, respectively | | | 11 | | | | 10 | |
Additional paid-in capital | | | 1,870,112 | | | | 1,821,721 | |
Accumulated other comprehensive (loss) income | | | (204 | ) | | | (2,067 | ) |
Accumulated deficit | | | (1,969,150 | ) | | | (1,693,024 | ) |
Total stockholders’ equity (deficit) | | | (99,231 | ) | | | 126,640 | |
Total liabilities and stockholders’ equity (deficit) | | $ | 165,504 | | | $ | 376,420 | |
See accompanying notes to the consolidated financial statements.
| | December 31, | | | December 31, | |
| | 2017 | | | 2016 | |
Assets | | | | | | | | |
Current assets: | | | | | | | | |
Cash and cash equivalents | | $ | 79,223 | | | $ | 47,968 | |
Short-term investments | | | 86,873 | | | | 207,714 | |
Restricted cash | | | 194 | | | | 194 | |
Prepaid expenses and other current assets | | | 5,900 | | | | 4,677 | |
Total current assets | | | 172,190 | | | | 260,553 | |
Property and equipment, net | | | 44,129 | | | | 3,259 | |
Restricted cash, long term | | | 1,200 | | | | — | |
Other assets | | | 260 | | | | 102 | |
Total assets | | $ | 217,779 | | | $ | 263,914 | |
| | | | | | | | |
Liabilities and stockholders’ equity | | | | | | | | |
Current liabilities: | | | | | | | | |
Accounts payable | | $ | 14,711 | | | $ | 2,778 | |
Accrued compensation | | | 5,664 | | | | 3,745 | |
Accrued research and development expenses | | | 4,006 | | | | 2,408 | |
Other current liabilities | | | 3,265 | | | | 744 | |
Total current liabilities | | | 27,646 | | | | 9,675 | |
Long-term liabilities | | | 12,269 | | | | 503 | |
Total liabilities | | | 39,915 | | | | 10,178 | |
| | | | | | | | |
Commitments and contingencies (Note 7) | | | | | | | | |
| | | | | | | | |
Stockholders’ equity: | | | | | | | | |
Common stock—$0.0001 par value, 500,000 shares authorized as of December 31, 2017 and December 31, 2016; 30,730 and 28,933 shares issued and outstanding as of December 31, 2017 and December 31, 2016, respectively | | | 3 | | | | 3 | |
Additional paid-in capital | | | 474,662 | | | | 431,075 | |
Accumulated other comprehensive loss | | | (151 | ) | | | (183 | ) |
Accumulated deficit | | | (296,650 | ) | | | (177,159 | ) |
Total stockholders’ equity | | | 177,864 | | | | 253,736 | |
Total liabilities and stockholders’ equity | | $ | 217,779 | | | $ | 263,914 | |
97
Atara Biotherapeutics, Inc.
Consolidated Statements of Operations and Comprehensive Loss
(In thousands, except per share amounts)
| | | | | | | | |
| | Years Ended December 31, | |
| | 2023 | | | 2022 | |
Commercialization revenue | | $ | 7,886 | | | $ | — | |
License and collaboration revenue | | | 687 | | | | 63,573 | |
Total revenue | | | 8,573 | | | | 63,573 | |
Costs and operating expenses: | | | | | | |
Cost of commercialization revenue | | | 8,886 | | | | — | |
Research and development expenses | | | 224,785 | | | | 272,533 | |
General and administrative expenses | | | 50,908 | | | | 71,553 | |
Total costs and operating expenses | | | 284,579 | | | | 344,086 | |
Loss from operations | | | (276,006 | ) | | | (280,513 | ) |
Other income (expense), net: | | | | | | |
Interest income | | | 5,426 | | | | 3,059 | |
Interest expense | | | (5,285 | ) | | | (373 | ) |
Gain on sale of ATOM Facility (See Note 7) | | | — | | | | 50,237 | |
Other income (expense), net: | | | (246 | ) | | | (700 | ) |
Total other income (expense), net | | | (105 | ) | | | 52,223 | |
Loss before provision for income taxes | | | (276,111 | ) | | | (228,290 | ) |
Provision for income taxes | | | 15 | | | | 12 | |
Net loss | | $ | (276,126 | ) | | $ | (228,302 | ) |
Other comprehensive gain (loss): | | | | | | |
Unrealized gain (loss) on available-for-sale securities | | | 1,863 | | | | (1,699 | ) |
Comprehensive loss | | $ | (274,263 | ) | | $ | (230,001 | ) |
| | | | | | |
Basic and diluted net loss per common share | | $ | (2.61 | ) | | $ | (2.24 | ) |
| | | | | | |
Basic and diluted weighted-average shares outstanding | | | 105,912 | | | | 101,990 | |
See accompanying notes to the consolidated financial statements.
| | Years Ended December 31, | |
| | 2017 | | | 2016 | | | 2015 | |
Operating expenses: | | | | | | | | | | | | |
Research and development | | $ | 81,206 | | | $ | 56,514 | | | $ | 41,618 | |
General and administrative | | | 40,326 | | | | 24,728 | | | | 16,830 | |
Total operating expenses | | | 121,532 | | | | 81,242 | | | | 58,448 | |
Loss from operations | | | (121,532 | ) | | | (81,242 | ) | | | (58,448 | ) |
Interest and other income, net | | | 2,027 | | | | 2,203 | | | | 1,218 | |
Loss before provision (benefit) for income taxes | | | (119,505 | ) | | | (79,039 | ) | | | (57,230 | ) |
Provision (benefit) for income taxes | | | (14 | ) | | | 10 | | | | (9 | ) |
Net loss | | $ | (119,491 | ) | | $ | (79,049 | ) | | $ | (57,221 | ) |
Other comprehensive gain (loss): | | | | | | | | | | | | |
Unrealized gain (loss) on available-for-sale securities | | | 32 | | | | 335 | | | | (418 | ) |
Comprehensive loss | | $ | (119,459 | ) | | $ | (78,714 | ) | | $ | (57,639 | ) |
Net loss per common share: | | | | | | | | | | | | |
Basic and diluted net loss per common share | | $ | (4.00 | ) | | $ | (2.75 | ) | | $ | (2.24 | ) |
| | | | | | | | | | | | |
Weighted-average common shares outstanding used to calculate basic and diluted net loss per common share | | | 29,863 | | | | 28,732 | | | | 25,583 | |
98
Atara Biotherapeutics, Inc.
Consolidated Statements of Changes in Stockholders’ Equity (Deficit)
(In thousands)
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | Accumulated | | | | | | | |
| | Common | | | Additional | | | Other | | | | | | Total | |
| | Stock | | | Paid-in | | | Comprehensive | | | Accumulated | | | Stockholders’ | |
| | Shares | | | Amount | | | Capital | | | (Loss) Income | | | Deficit | | | Equity (Deficit) | |
Balance as of January, 1 2022 | | | 91,671 | | | | 9 | | | | 1,744,695 | | | | (368 | ) | | | (1,464,722 | ) | | | 279,614 | |
Issuance of common stock through ATM facilities, net of
commissions and offering costs of $517 | | | 1,619 | | | | — | | | | 21,891 | | | | — | | | | — | | | | 21,891 | |
RSU settlements, net of shares withheld | | | 2,204 | | | | 1 | | | | (624 | ) | | | — | | | | — | | | | (623 | ) |
Issuance of common stock pursuant to employee stock awards | | | 433 | | | | — | | | | 1,921 | | | | — | | | | — | | | | 1,921 | |
Stock-based compensation expense | | | — | | | | — | | | | 53,838 | | | | — | | | | — | | | | 53,838 | |
Net loss | | | — | | | | — | | | | — | | | | — | | | | (228,302 | ) | | | (228,302 | ) |
Unrealized gain (loss) on available-for-sale securities | | | — | | | | — | | | | — | | | | (1,699 | ) | | | — | | | | (1,699 | ) |
Balance as of December 31, 2022 | | | 95,927 | | | | 10 | | | | 1,821,721 | | | | (2,067 | ) | | | (1,693,024 | ) | | | 126,640 | |
Issuance of common stock through ATM facilities, net of
commissions and offering costs of $351 | | | 3,038 | | | | 1 | | | | 2,171 | | | | — | | | | — | | | | 2,172 | |
Exercise of pre-funded warrants | | | 2,916 | | | | — | | | | — | | | | — | | | | — | | | | — | |
RSU settlements, net of shares withheld | | | 3,670 | | | | — | | | | (94 | ) | | | — | | | | — | | | | (94 | ) |
Issuance of common stock pursuant to employee stock awards | | | 896 | | | | — | | | | 928 | | | | — | | | | — | | | | 928 | |
Stock-based compensation expense | | | — | | | | — | | | | 45,386 | | | | — | | | | — | | | | 45,386 | |
Net loss | | | — | | | | — | | | | — | | | | — | | | | (276,126 | ) | | | (276,126 | ) |
Unrealized gain (loss) on available-for-sale securities | | | — | | | | — | | | | — | | | | 1,863 | | | | — | | | | 1,863 | |
Balance as of December 31, 2023 | | | 106,447 | | | $ | 11 | | | $ | 1,870,112 | | | $ | (204 | ) | | $ | (1,969,150 | ) | | $ | (99,231 | ) |
See accompanying notes to the consolidated financial statements.
| | | | | | | | | | | | | | Accumulated | | | | | | | | | |
| | Common | | | Additional | | | Other | | | | | | | Total | |
| | Stock | | | Paid-in | | | Comprehensive | | | Accumulated | | | Stockholders’ | |
| | Shares | | | Amount | | | Capital | | | Loss | | | Deficit | | | Equity | |
Balance as of December 31, 2014 | | | 19,693 | | | $ | 2 | | | $ | 144,169 | | | $ | (100 | ) | | $ | (40,889 | ) | | $ | 103,182 | |
Issuance of common stock in February 2015, net of discounts and offering costs of $5,166 | | | 4,147 | | | | 1 | | | | 69,486 | | | | — | | | | — | | | | 69,487 | |
Issuance of common stock in July 2015, net of discounts and offering costs of $13,053 | | | 3,981 | | | | — | | | | 193,947 | | | | — | | | | — | | | | 193,947 | |
Issuance of common stock upon vesting of restricted stock awards | | | 287 | | | | — | | | | 80 | | | | — | | | | — | | | | 80 | |
RSU settlements, net of shares withheld | | | 327 | | | | — | | | | (4,647 | ) | | | — | | | | — | | | | (4,647 | ) |
Issuance of common stock pursuant to stock option exercises | | | 24 | | | | — | | | | 439 | | | | — | | | | — | | | | 439 | |
Stock-based compensation expense | | | — | | | | — | | | | 10,251 | | | | — | | | | — | | | | 10,251 | |
Net loss | | | — | | | | — | | | | — | | | | — | | | | (57,221 | ) | | | (57,221 | ) |
Unrealized loss on available-for-sale securities | | | — | | | | — | | | | — | | | | (418 | ) | | | — | | | | (418 | ) |
Balance as of December 31, 2015 | | | 28,459 | | | | 3 | | | | 413,725 | | | | (518 | ) | | | (98,110 | ) | | | 315,100 | |
Issuance of common stock upon vesting of restricted stock awards | | | 233 | | | | — | | | | 60 | | | | — | | | | — | | | | 60 | |
RSU settlements, net of shares withheld | | | 199 | | | | — | | | | (94 | ) | | | — | | | | — | | | | (94 | ) |
Issuance of common stock pursuant to employee stock awards | | | 42 | | | | — | | | | 600 | | | | — | | | | — | | | | 600 | |
Stock-based compensation expense | | | — | | | | — | | | | 16,784 | | | | — | | | | — | | | | 16,784 | |
Net loss | | | — | | | | — | | | | — | | | | — | | | | (79,049 | ) | | | (79,049 | ) |
Unrealized gain on available-for-sale securities | | | — | | | | — | | | | — | | | | 335 | | | | — | | | | 335 | |
Balance as of December 31, 2016 | | | 28,933 | | | | 3 | | | | 431,075 | | | | (183 | ) | | | (177,159 | ) | | | 253,736 | |
Issuance of common stock through ATM facility, net of commissions and offering costs of $844 | | | 1,350 | | | | — | | | | 19,156 | | | | — | | | | — | | | | 19,156 | |
RSU settlements, net of shares withheld | | | 305 | | | | — | | | | (357 | ) | | | — | | | | — | | | | (357 | ) |
Issuance of common stock pursuant to employee stock awards | | | 142 | | | | — | | | | 1,688 | | | | — | | | | — | | | | 1,688 | |
Stock-based compensation expense | | | — | | | | — | | | | 23,100 | | | | — | | | | — | | | | 23,100 | |
Net loss | | | — | | | | — | | | | — | | | | — | | | | (119,491 | ) | | | (119,491 | ) |
Unrealized gain on available-for-sale securities | | | — | | | | — | | | | — | | | | 32 | | | | — | | | | 32 | |
Balance as of December 31, 2017 | | | 30,730 | | | $ | 3 | | | $ | 474,662 | | | $ | (151 | ) | | $ | (296,650 | ) | | $ | 177,864 | |
99
Atara Biotherapeutics, Inc.
Consolidated Statements of Cash Flows
(In thousands)
| | | | | | | | |
| | Year Ended December 31, | |
| | 2023 | | | 2022 | |
Operating activities | | | | | | |
Net loss | | $ | (276,126 | ) | | $ | (228,302 | ) |
Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | |
Gain on sale of ATOM Facility | | | — | | | | (50,237 | ) |
Stock-based compensation expense | | | 45,386 | | | | 53,838 | |
Depreciation and amortization expense | | | 4,829 | | | | 5,653 | |
Accretion of liability related to sale of future revenues | | | 4,792 | | | | — | |
Non-cash operating lease expense | | | 11,795 | | | | 8,915 | |
Amortization of investment premiums | | | 792 | | | | 1,024 | |
Other non-cash items, net | | | 214 | | | | 147 | |
Changes in operating assets and liabilities: | | | | | | |
Accounts receivable | | | 6,113 | | | | (39,235 | ) |
Inventories | | | (8,120 | ) | | | (1,586 | ) |
Other current assets | | | 273 | | | | 1,836 | |
Other assets | | | 1,043 | | | | (266 | ) |
Accounts payable | | | (3,130 | ) | | | (9,211 | ) |
Accrued compensation | | | (6,140 | ) | | | (7,491 | ) |
Accrued research and development expenses | | | (3,663 | ) | | | 11,541 | |
Other current liabilities | | | 11,064 | | | | 2,067 | |
Deferred revenue | | | 30,395 | | | | (11,468 | ) |
Operating lease liabilities | | | (12,636 | ) | | | (8,009 | ) |
Other long-term liabilities | | | 142 | | | | 354 | |
Net cash used in operating activities | | | (192,977 | ) | | | (270,430 | ) |
Investing activities | | | | | | |
Purchases of short-term investments | | | (83,648 | ) | | | (180,589 | ) |
Proceeds from maturities and sales of short-term investments | | | 208,712 | | | | 292,973 | |
Purchases of property and equipment | | | (1,223 | ) | | | (4,193 | ) |
Proceeds from sale of property and equipment | | | 25 | | | | — | |
Net proceeds from sale of ATOM Facility | | | — | | | | 94,765 | |
Net cash provided by (used in) investing activities | | | 123,866 | | | | 202,956 | |
Financing activities | | | | | | |
Proceeds from issuance of common stock through ATM facilities, net | | | 2,136 | | | | 21,891 | |
Proceeds from employee stock awards | | | 928 | | | | 1,921 | |
Proceeds from sale of future revenues, net | | | — | | | | 30,605 | |
Taxes paid related to net share settlement of restricted stock units | | | (94 | ) | | | (623 | ) |
Principal payments on finance lease obligations | | | (947 | ) | | | (518 | ) |
Other financing activities, net | | | (13 | ) | | | (192 | ) |
Net cash provided by financing activities | | | 2,010 | | | | 53,084 | |
Increase (decrease) in cash, cash equivalents and restricted cash | | | (67,101 | ) | | | (14,390 | ) |
Cash, cash equivalents and restricted cash at beginning of period | | | 93,088 | | | | 107,478 | |
Cash, cash equivalents and restricted cash at end of period | | $ | 25,987 | | | $ | 93,088 | |
Non-cash investing and financing activities | | | | | | |
Property and equipment purchases included in accounts payable and other
accrued liabilities | | $ | 132 | | | $ | 61 | |
Accrued costs related to ATM facilities | | $ | 78 | | | $ | — | |
Proceeds from issuance of common stock through ATM facilities not yet received | | $ | 114 | | | $ | — | |
Accrued transaction costs related to sale of future revenues | | $ | — | | | $ | 332 | |
Supplemental cash flow disclosure | | | | | | |
Cash paid for interest | | $ | 447 | | | $ | 335 | |
Cash paid for income taxes | | $ | 2 | | | $ | 19 | |
See accompanying notes to the consolidated financial statements.
| | | | | | | | | | | | |
| | Year Ended December 31, | |
| | 2017 | | | 2016 | | | 2015 | |
Operating activities | | | | | | | | | | | | |
Net loss | | $ | (119,491 | ) | | $ | (79,049 | ) | | $ | (57,221 | ) |
Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | | | | | |
Stock-based compensation expense | | | 23,100 | | | | 16,784 | | | | 10,251 | |
Amortization of investment premiums and discounts | | | 732 | | | | 2,582 | | | | 3,465 | |
Depreciation and amortization expense | | | 956 | | | | 383 | | | | 48 | |
Loss on foreign exchange | | | — | | | | — | | | | 94 | |
Write-off of property and equipment | | | — | | | | — | | | | 21 | |
Changes in operating assets and liabilities: | | | | | | | | | | | | |
Prepaid expenses and other current assets | | | (784 | ) | | | (742 | ) | | | (767 | ) |
Other assets | | | 2 | | | | 6 | | | | (61 | ) |
Accounts payable | | | 2,163 | | | | 981 | | | | 1,005 | |
Accrued compensation | | | 1,919 | | | | 1,121 | | | | 1,399 | |
Accrued research and development expenses | | | 1,598 | | | | (2,704 | ) | | | 4,288 | |
Other current liabilities | | | 1,896 | | | | 215 | | | | 293 | |
Long-term liabilities | | | 407 | | | | 398 | | | | 29 | |
Net cash used in operating activities | | | (87,502 | ) | | | (60,025 | ) | | | (37,156 | ) |
Investing activities | | | | | | | | | | | | |
Purchases of short-term investments | | | (176,459 | ) | | | (304,928 | ) | | | (379,776 | ) |
Sales of short-term investments | | | 107,627 | | | | 242,643 | | | | 64,020 | |
Maturities of short-term investments | | | 188,973 | | | | 149,046 | | | | 96,113 | |
Purchases of property and equipment | | | (20,232 | ) | | | (3,020 | ) | | | (290 | ) |
Restricted cash | | | (1,200 | ) | | | — | | | | (194 | ) |
Net cash provided by (used in) investing activities | | | 98,709 | | | | 83,741 | | | | (220,127 | ) |
Financing activities | | | | | | | | | | | | |
Proceeds from sale of common stock in underwritten offerings, net | | | — | | | | — | | | | 263,434 | |
Proceeds from issuance of common stock through ATM facility, net | | | 19,156 | | | | — | | | | — | |
Taxes paid related to net share settlement of restricted stock units | | | (357 | ) | | | (94 | ) | | | (4,647 | ) |
Proceeds from employee stock awards | | | 1,249 | | | | 600 | | | | 439 | |
Net cash provided by financing activities | | | 20,048 | | | | 506 | | | | 259,226 | |
Effect of exchange rates on cash | | | — | | | | — | | | | (94 | ) |
Increase in cash and cash equivalents | | | 31,255 | | | | 24,222 | | | | 1,849 | |
Cash and cash equivalents at beginning of period | | | 47,968 | | | | 23,746 | | | | 21,897 | |
Cash and cash equivalents at end of period | | $ | 79,223 | | | $ | 47,968 | | | $ | 23,746 | |
Non-cash investing and financing activities | | | | | | | | | | | | |
Property and equipment purchases included in accounts payable and other accrued liabilities | | $ | 10,122 | | | $ | 352 | | | $ | — | |
Capitalized lease obligations | | $ | 9,904 | | | $ | — | | | $ | — | |
Property & equipment acquired under capital leases | | $ | 1,076 | | | $ | — | | | $ | — | |
Asset retirement cost | | $ | 580 | | | $ | — | | | $ | — | |
Interest capitalized during construction period for build-to-suit lease transaction | | $ | 264 | | | $ | — | | | $ | — | |
Proceeds from options exercised not yet received | | $ | 439 | | | $ | — | | | $ | — | |
Accrued costs related to underwritten public offering | | $ | 160 | | | $ | — | | | $ | — | |
Issuance of common stock upon vesting of stock awards | | $ | — | | | $ | 60 | | | $ | 80 | |
Change in long-term liabilities related to non-vested stock awards | | $ | — | | | $ | (60 | ) | | $ | (80 | ) |
Supplemental cash flow disclosure | | | | | | | | | | | | |
Cash paid for taxes | | $ | — | | | $ | 10 | | | $ | 3 | |
100
Atara Biotherapeutics, Inc.
Notes to Consolidated Financial Statements
1.
| Description of Business
|
1.Description of Business
Atara Biotherapeutics, Inc. (“Atara”, “we”, “our”(Atara, we, our or “the Company”)the Company) was incorporated in August 2012 in Delaware.Delaware. Atara is a cell therapy company developingleader in T-cell immunotherapy, leveraging its novel treatmentsallogeneic Epstein-Barr Virus (EBV) T-cell platform to develop transformative therapies for patients with cancer and multiple sclerosis (“MS”). The Company’s off-the-shelf, or allogeneic, T-cells are engineered from donors with healthy immune function and allow for rapid delivery from inventory to patients without a requirement for pretreatment. Atara’sautoimmune disease.
We have several T-cell immunotherapies in clinical development and are designedprogressing multiple next-generation allogeneic chimeric antigen receptor T-cell (CAR T) programs. Our most advanced T-cell immunotherapy program, tab-cel® (tabelecleucel), has received marketing authorization approval under the proprietary name Ebvallo™ by the European Commission (EC) for commercial sale and use in the European Union (EU) and by the Medicines and Healthcare products Regulatory Agency (MHRA) for commercial sale and use in the United Kingdom (UK). Tab-cel is currently in Phase 3 development in the US. In October 2021, we entered into a commercialization agreement (Pierre Fabre Commercialization Agreement) with Pierre Fabre Medicament (Pierre Fabre), as amended in September 2022, pursuant to precisely recognizewhich we granted to Pierre Fabre an exclusive, field-limited license to commercialize and eliminate cancerous or diseased cells without affecting normal, healthy cells. distribute Ebvallo in Europe and select emerging markets in the Middle East, Africa, Eastern Europe and Central Asia (the Initial Territory), following regulatory approval. In October 2023, we amended and restated the Pierre Fabre Commercialization Agreement (A&R Commercialization Agreement). Pursuant to the A&R Commercialization Agreement, Pierre Fabre’s exclusive rights to research, develop, manufacture, commercialize and distribute Ebvallo are expanded to include all other countries in the world (Additional Territory) in addition to the Initial Territory (together, the Territory), subject to our performance of certain obligations. See Note 5 for further information. In December 2022, we sold a portion of our right to receive royalties and certain milestones in Ebvallo under the Pierre Fabre Commercialization Agreement to HCR Molag Fund L.P. (HCRx) for a total investment amount of $31.0 million, subject to a repayment cap between 185% and 250% of the total investment amount by HCRx. See Note 6 for further information.
We have licensed rights to T-cell product candidates from Memorial Sloan Kettering Cancer Center (“MSK”) in June 2015(MSK), rights related to our next-generation CAR T programs from MSK, and rights to know-how and technology from QIMR Berghoferthe Council of the Queensland Institute of Medical Research Institute (“QIMR Berghofer”) in October 2015 and September 2016.(QIMR Berghofer). See Note 68 for further information.
In 2017,January 2022, we received net proceedsentered into an asset purchase agreement with FUJIFILM Diosynth Biotechnologies California, Inc. (FDB) and, for certain limited purposes, FUJIFILM Holdings America Corporation, to sell all of $19.2the Company’s right, title and interest in and to certain assets related to the Atara T-Cell Operations and Manufacturing facility (ATOM Facility) located in Thousand Oaks, California for $100 million fromin cash, subject to potential post-closing adjustments pursuant to the aggregate saleasset purchase agreement (the Fujifilm Transaction). The closing of 1,349,865 sharesthe Fujifilm Transaction occurred on April 4, 2022, at which time 136 of our common stock throughATOM Facility employees transitioned to FDB as part of the transaction. We also entered into a Master Services and Supply Agreement and related Statements of Work with FDB (collectively, the Fujifilm MSA) which became effective upon the closing and could extend for up to ten years. Pursuant to the Fujifilm MSA, FDB will supply us with specified quantities of our ATM facility with Cowen (see Note 8). Further, in January 2018, we completed an underwritten public offering of 7,675,072 shares of common stock at an offering price of $18.25 per sharecell therapy product candidates and received net proceeds of $131.4 million (see Note 10).
2.
| Summary of Significant Accounting Policies
|
Basis of Presentation
The accompanying consolidated financial statements have been preparedany products approved by regulatory authorities, manufactured in accordance with accounting principles generally acceptedcGMP standards. See Note 8 for further information.
In November 2023, we announced a reduction in force that reduced our workforce by approximately 30%. We recognized $6.7 million in total for severance and related benefits for employees laid off under the reduction in force. These charges are one-time termination benefits and are all cash charges. Refer to Note 9 for further information.
Certain prior year amounts, which are not material, have been reclassified to conform to current year presentation in the United Statesconsolidated statements of America (“GAAP”)operations and comprehensive income (loss) and the rules and regulationsnotes to consolidated financial statements.
2.Summary of the U.S. Securities and Exchange Commission (the “SEC”).Significant Accounting Policies
Principles of Consolidation
The consolidated financial statements include the accounts of Atara and itsour wholly owned subsidiaries Nina Biotherapeutics, Inc., Santa Maria Biotherapeutics, Inc., Pinta Biotherapeutics, Inc., Atara Biotherapeutics Cayman Limited, a Cayman Islands corporation, Atara Biotherapeutics Ireland Limited, an Ireland corporation and Atara Biotherapeutics Switzerland GmbH, a Swiss corporation.subsidiaries. All intercompany balances and transactions have beenare eliminated in consolidation.
Segment and Geographic Information
We operate and manage our business as one reporting operating and one operatingreportable segment, which is the business of developing and commercializing therapeutics. Our Chief Executive Officer, who is our chief operating decision maker, reviews financial information on an aggregate basis for purposes of allocating resources and evaluating financial performance. Substantially all of our assets are located in the U.S.
All commercialization and collaboration revenue recognized in 2023 related to our agreements with Pierre Fabre, a French company. Of the $63.6 million license and collaboration revenue recognized in 2022, $61.8 million related to our agreements with Bayer, a German company, and $1.8 million related to our agreements with Pierre Fabre, a French company.
Use of Estimates
We prepare our consolidated financial statements in accordance with accounting principles generally accepted in the United States.States of America (U.S. GAAP), which requires us to make estimates and assumptions that affect the amounts reported in our consolidated financial statements and accompanying notes. The level of uncertainty in estimates and assumptions increases with the length of time until the underlying transactions are completed. Significant estimates and assumptions relied upon in preparing these financial statements include those related to revenue recognition, accrued research and development expenses, stock-based compensation expense, liability related to the sale of future revenues and income taxes. Additionally, we use available market information to assess the fair value of our short-term investments. Actual results could differ materially from those estimates. If actual amounts differ from estimates, we include the updates in our consolidated results of operations in the period the actual amounts become known. Historically, the aggregate differences, if any, between our estimates and actual amounts in any year have not had a material effect on our consolidated financial statements.
Liquidity Risk
We have incurred significant operating losses since inception and have relied primarily on public and private equity financings and receipts from commercialization and license and collaboration agreements to fund our operations. As of December 31, 2017, we had an accumulated deficit of $296.7 million. As we continue to incur losses, our transition to profitability will depend on the successful development, approval and commercialization of product candidates and on the achievement of sufficient revenues to support our cost structure. We may never achieve profitability,sustained operating cash inflows or profitability.
Going Concern
We have incurred operating losses since inception and unless and until we do, we will need to continue to raise additional capital. Management expectsexpect that ourexisting cash, cash equivalents and short-term investments as of December 31, 2017, along with the proceeds from the public offering completed in January 2018,2023, will not be sufficient to fund our planned operations for at least 12 months after the issuance of the accompanying consolidated financial statements. Although we anticipate the receipt of certain payments from the amended and restated Pierre Fabre Commercialization Agreement in 2024 and 2025, such payments are contingent upon the successful filing and approval of the tab-cel BLA, as well as the completion of specific development and regulatory activities by us and actions taken by third parties, and are, therefore, uncertain at this time.
To alleviate the conditions that raise substantial doubt about our ability to continue as a going concern, we plan to secure additional capital, potentially through a combination of public or private security offerings; use of our ATM facility as described in Note 9; and/or strategic transactions. We may also need to raise additional funding as required based on the status of our development programs and our projected cash flows. Although we have been successful in raising capital in the past, and expect to continue to raise capital as required, there is no assurance that we will be successful in obtaining sufficient funding on terms acceptable to us to fund continuing operations, if at all, or identify and enter into any strategic transactions that will provide the first halfcapital that we will require. If we are unable to obtain sufficient funding on acceptable terms, we could be forced to delay, limit, reduce or terminate preclinical studies, clinical studies or other development activities for one or more of 2020.our product candidates, which could have a material adverse effect on our business, results of operations, and financial condition. Accordingly, we have concluded that substantial doubt exists with respect to our ability to continue as a going concern for at least 12 months after the issuance of the accompanying consolidated financial statements. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Concentration of Credit Risk and Other Uncertainties
We place cash and cash equivalents in the custody of financial institutions that management believes are of high credit quality, the amount of which at times, may be in excess of the amount insured by the Federal Deposit Insurance Corporation. We also havemake short-term investments in money market funds,funds; U.S. Treasury, government agency and corporate debt obligations,obligations; commercial paperpaper; certificates of deposit; and asset-backed securities, which can be subject to certain credit risk. However, weWe strive to mitigate the risksthis credit risk by investing in high-grade instruments, limiting our exposure to any one issuer and monitoring the ongoing creditworthiness of the financial institutions and issuers.
We are subject to certain risks and uncertainties and believe that changes in any of the following areas could have a material adverse effect on future financial position or results of operations: our ability to obtain future financing; regulatory approval and market acceptance of, and reimbursement for, our product candidates, if approved; performance of third-party clinical research organizations and manufacturers upon which we rely; development of sales channels; protection of our intellectual property; litigation or claims against us based on intellectual property, patent, product, regulatory or other factors; and our ability to attract and retain employees necessary to support our growth.
Currency Translation
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions and judgments that affect the amounts reported in the financial statements and accompanying notes. Significant estimates relied upon in preparing these financial statements include estimates related to clinical trial and other accruals, stock-based compensation expense, fair value of investments and income taxes. Actual results could differ materially from those estimates.
Leases
We lease office space in multiple locations. In addition, we are constructing a manufacturing facility in Thousand Oaks, California under a non-cancelable lease agreement. The leases are reviewed for classification as operating or capital leases. For operating leases, rent is recognized on a straight-line basis over the lease period. For capital leases, we record the leased asset with a corresponding liability for principal and interest. Payments are recorded as reductions to these liabilities with interest being charged to interest expense in our statements of operations and comprehensive loss.
We analyzed the nature of the renovations and our involvement during the construction period of our manufacturing facility and determined that we are the deemed “owner” of the construction project during the construction period. As a result, we are required to capitalize the fair value of the building as well as the construction costs incurred on our consolidated balance sheet along with a corresponding financing liability for landlord-paid construction costs (i.e. “build-to-suit” accounting). Upon occupancy for build-to-suit leases, we are also required to assess whether the circumstances qualify for sale recognition under “sale-leaseback” accounting guidance.
Asset Retirement Obligations (“ARO”)
ARO are legal obligations associated with the retirement of long-lived assets pertaining to leasehold improvements. These liabilities are initially recorded at fair value and the related asset retirement costs are capitalized by increasing the carrying amount of the related assets by the same amount as the liability. Asset retirement costs are subsequently depreciated over the useful lives of the related assets. Subsequent to initial recognition, the Company records period-to-period changes in the ARO liability resulting from the passage of time and revisions to either the timing or the amount of the original estimate of undiscounted cash flows. The Company derecognizes ARO liabilities when the related obligations are settled.
Foreign Currency
Transactions and foreign currency-denominated monetary assets and liabilities that are denominated in a foreign currency are translated into U.S. dollars at the current exchange rate on the transaction date and as of each balance sheet date, respectively, with gains or losses on foreign exchange changes recognized in interest and other income (expense), net in the consolidated statements of operations and comprehensive loss. We held no foreign currencyForeign currency-denominated monetary assets and liabilities as of December 31, 2017 and 2016.2023 were not material.
Cash, Cash Equivalents and Short-Term Investments
Cash and cash equivalents are defined as short-term, highly liquid investments with original maturities of 90 days or less at the date of purchase, and generally consist of money market funds, U.S. Treasury, government agency and corporate debt obligations, and commercial paper.purchase.
Investments with original maturities of greater than 90 days are classified as short-term investments on the balance sheet, and consist primarily of U.S. Treasury, government agency and corporate debt obligations, commercial paper and asset-backed securities.sheet.
As our entire investment portfolio is considered available for use in current operations, we classify all investments as available-for-sale and as current assets, even though the stated maturity may be more than one year from the current balance sheet date. Available-for-sale securities are carried at fair value, with unrealized gains and losses reported in accumulated other comprehensive loss, which is a separate component of stockholders’ equity in the consolidated balance sheet.
The amortized cost of securities is adjusted for amortization of premiums and accretion of discounts to maturity, which are both recorded to interest and other income (expense), net in the consolidated statements of operations and comprehensive loss.
Changes in the fair value of available-for-sale securities impact the consolidated statements of operations and comprehensive loss only when such securities are sold, if an allowance for credit losses is recognized or if an other-than-temporary impairment is recognized. Realized gains and losses on the sale of securities are determined by specific identification of each security’s cost basis. We regularly review our investment portfolio to determine if any security is other-than-temporarily impaired, which would require us to record an allowance for credit losses or impairment charge in the period any such determination is made. In making this judgment, we evaluate, among other things, the duration and extent to which the fair value of a security is less than its cost, the financial condition of the issuer and any changes thereto, our intent to sell or whether it is more likely than not that we will be required to sell the security before recovery of its amortized cost basis. Ourbasis, the financial condition of the issuer and any changes thereto, and, as necessary, the portion of a decline in fair value that is credit-related. This assessment on whether a security is other-than-temporarily impaired could change in the future due to new developments or changes in assumptions related to any particular security. Realized gains and losses, allowances for credit losses and declines in value judged to be other-than-temporaryimpairments on available-for-sale securities, if any, are recorded to interest and other income (expense), net in the statements of operations and comprehensive loss.
Fair Value Measurement
The carrying amounts of certain of our financial instruments including cash equivalents, prepaid expenses,accounts receivable, other current assets, accounts payable and accrued liabilities approximate fair value due to their short maturities. Short-term investments are comprised of available-for-sale securities, which are carried at fair value.
Fair Value of Financial Instruments
Our financial assets and liabilities are measured at fair value on a recurring basis using the following hierarchy to prioritize valuation inputs, in accordance with applicable GAAP:
Level 1: Quoted prices in active markets for identical assets or liabilities that we have the ability to access
Level 1:
| | Quoted prices in active markets for identical assets or liabilities that we have the ability to access
|
Level 2:
| | Observable market based inputs or unobservable inputs that are corroborated by market data such as quoted prices, interest rates and yield curves
|
Level 3:
| | Inputs that are unobservable data points that are not corroborated by market data
|
Level 2: Observable market-based inputs or unobservable inputs that are corroborated by market data such as quoted prices, interest rates and yield curves
Level 3: Inputs that are unobservable data points that are not corroborated by market data
We review the fair value hierarchy classification on a quarterly basis. Changes in the ability to observe valuation inputs may result in a reclassification of levels of certain securities within the fair value hierarchy. We recognize transfers into and out of levels within the fair value hierarchy in the period in which the actual event or change in circumstances that caused the transfer occurs. There have been no transfers between Level 1, Level 2, and Level 3 in any periods presented.
Financial assets and liabilities are considered Level 2 when their fair values are determined using inputs that are observable in the market or can be derived principally from or corroborated by observable market data such as pricing for similar securities, recently executed transactions, cash flow models with yield curves, and benchmark securities. In addition, Level 2 financial instruments are
valued using comparisons to like-kind financial instruments and models that use readily observable market data as their basis. U.S. Treasury, government agency and corporate debt obligations, and commercial paper and asset-backed securities are valued primarily using market prices of comparable securities, bid/ask quotes, interest rate yields and prepayment spreads and are included in Level 2.
Financial assets and liabilities are considered Level 3 when their fair values are determined using pricing models, discounted cash flow methodologies, or similar techniques, and at least one significant model assumption or input is unobservable. We have no Level 3 financial assets or liabilities.
Accounts Receivable, net
Accounts receivable are recorded net of estimates of variable consideration for which reserves are established and which result from discounts and chargebacks that are offered within contracts between us and a limited number of specialty pharmacies and a specialty distributor in the United States. These reserves are classified as reductions of accounts receivable.
We estimate the allowance for doubtful accounts using the current expected credit loss model, or CECL model. Under the CECL model, the allowance for doubtful accounts reflects the net amount expected to be collected from the accounts receivable. We evaluate the collectability of these cash flow based on the asset’s amortized cost, the risk of loss even when that risk is remote, losses over an asset’s contractual life, and other relevant information available to us. Accounts receivable balances are written off against the allowance when it is probable that the receivable will not be collected. Given the nature and history of our accounts receivable, we determined that an allowance for doubtful accounts was not required for the periods presented.
Inventories
Inventories are stated at the lower of cost or estimated net realizable value, on a specific identification basis. We use actual costs to determine our cost basis for inventories. Inventories consist of raw materials, work-in-process and finished goods.
We begin capitalizing costs as inventory when the product candidate receives regulatory approval and when the manufacturing facility producing such inventory is qualified by the relevant regulatory agency. Prior to regulatory approval and qualification, we record such production costs related to product candidates as research and development expenses. Any manufactured product that is available for commercial sale is recorded to inventory; to the extent it is later used for clinical studies, such inventory costs are then recorded within research and development expenses.
We periodically assess the recoverability of our inventory and reduce the carrying value of the inventory when items are determined to be obsolete, defective or in excess of forecasted sales requirements. Inventory write-downs for excess, defective and obsolete inventory are recorded as a cost of sales.
Property and Equipment, net
Property and equipment are stated at cost and depreciated using the straight-line method over the estimated useful lives of the assets, ranging from three to five years.years, except for leasehold improvements, which are depreciated on a straight-line basis over the lesser of the estimated useful life of the leasehold improvement or the lease term. Costs incurred to acquire, construct or install property and equipment during the construction stage of a capital project or costs incurred to purchase and develop internal use software during the application development stage are recorded as construction in progress. Leasehold improvements are amortized over the lesser of the life of the leasehold improvements or the lease term. Equipment leased under capital leases is amortized over the shorter of the lease term or the asset’s estimated useful life. Maintenance and repairs are charged to operations as incurred.
Long-lived Assets
We evaluate the carrying amount of our long-lived assets whenever events or changes in circumstances indicate that the assets may not be recoverable. An impairment loss would be recognized when estimated future cash flows expected to result from the use of the asset and its eventual disposition are less than the carrying amount of the asset. To date, there have been no such impairment losses.
Asset Retirement Obligation (ARO)
Stock-Based Compensation Expense
We account for stock-based compensation expense, includingAn ARO is a legal obligation associated with the expenseretirement of restricted common stock awards (“RSAs”)long-lived assets pertaining to leasehold improvements. These liabilities are initially recorded at fair value and grants of restricted stock units (“RSUs”) and stock options that may be settled in shares of our common stock, based on the fair valuesrelated asset retirement costs are capitalized by increasing the carrying amount of the equity instruments issued. The fair value is determined onrelated assets by the measurement date, which is generallysame amount as the dateliability. Asset retirement costs are subsequently depreciated over the useful lives of grant for employee awardsthe related assets. Subsequent to initial recognition, we record period-to-period changes in the ARO liability resulting from the passage of time and revisions to either the datetiming or the amount of the original estimate of undiscounted cash flows. We derecognize ARO liabilities when the service performancerelated obligations are settled.
Leases
We determine if a contract is completedor contains a lease at contract inception. Operating leases are included in operating lease assets, other current liabilities, and operating lease liabilities on our consolidated balance sheets. Our policy is to not recognize right-of-use (ROU) assets and lease liabilities for non-employees. The fair value for our RSAs is their intrinsic value, which is the difference between the fair valueshort-term operating leases with terms of the underlying stock at the measurement date and the purchase price. The fair value of our RSUs is the fair value of the underlying stock at the measurement date. The fair value for our stock option awards is determined at the grant date using the Black-Scholes valuation model. For employees’ awards with performance-based vesting criteria,12 months or less; we assess the probability of the achievement of the performance conditions at the end of each reporting period and recognize the share-based compensation costs when it becomes probable that the performance conditions will be met. For non-employees’ awards with performance-based vesting criteria, we assess all possible outcomes at the end of each reporting period and recognize the lowest aggregate fair value in the range of possible outcomes. The lowest value in the range of possible outcomes may be zero. For awards that are subject to both service and performance conditions, no expense is recognized until it is probable that performance conditions will be met. Stock-based compensationshort-term lease expense for awards with time-based vesting criteria is recognized as expensethese leases on a straight-line basis over the requisite service period. Stock-based compensation expense for awards with performancelease term. Long-term operating lease ROU assets and long-term operating lease liabilities are presented separately and operating lease liabilities payable in the next twelve months are recorded in other current liabilities. Finance lease ROU assets are recorded in other assets and the related finance lease liabilities are presented in other current liabilities and other vesting criteria islong-term liabilities.
Lease assets and lease liabilities are recognized as expense under an accelerated graded vesting model.
Key assumptions used in the Black-Scholes valuation model used for employee stock awards include:
Expected term – We derived the expected term using the “simplified” method (the expected term is determined as the average of the time-to-vesting and the contractual life of the options), as we have limited historical information to develop expectations about future exercise patterns and post vesting employment termination behavior.
Expected volatility – Expected volatility is estimated using comparable public companies’ volatility for similar terms.
Expected dividend – We have not historically declared or paid dividends to our stockholders and have no plans to pay dividends; therefore we assumed an expected dividend yield of 0%.
Risk-free interest rate – The risk-free interest rate is based on the yield on U.S. Treasury securities with the expected termpresent value of the associated award.
future minimum lease payments over the lease term at commencement date. The fairlease term includes renewal options that we are reasonably certain of exercising as of the commencement date. None of the lease terms used to calculate the future minimum lease payments at commencement date include renewal options. As most of our leases do not provide an implicit rate, we use our incremental borrowing rate based on the information available at commencement date in determining the present value of non-employee stock optionsfuture payments. The incremental borrowing rate for our leases is estimated usingdetermined based on lease term and currency in which lease payments are made, adjusted for impacts of collateral. Lease assets also includes any lease payments made and excludes lease incentives and initial direct costs incurred. Operating lease expense for minimum lease payments is recognized on a straight-line basis over the Black-Scholes valuation model with assumptions generally consistent with those used for employee stock options, withlease term. Finance lease assets are amortized over the exceptionshorter of the expectedlease term whichor the asset’s estimated useful life.
Our facilities and equipment operating leases have lease and non-lease components and we have made a policy election to account for the lease and non-lease components as a single lease component.
We are considered the sub-lessor for certain of our leases where we have entered into a sub-lease agreement with or have assigned our lease to another party. Rental income was not material for any period presented and we record rental income as a reduction to rent expense within operating expenses.
We analyze whether or not amendments to existing leases classify as a lease modification or a full or partial termination of the existing lease. To the extent a partial lease termination is identified, our accounting policy is to decrease the remaining contractual life at each measurement date.
Prior to our IPO in October 2014, dueexisting right-of-use asset on a basis proportionate to the absencereduction in lease liability resulting from the partial termination.
Accruals of an active market for our common stock, we estimated the fair value of our common stock in accordance with the framework of the American Institute of Certified Public Accountants Technical Practice Aid, Valuation of Privately-Held Company Equity Securities Issued as Compensation. Each valuation included estimates and assumptions that required management’s judgment, including assumptions regarding the probability and estimated time to completion of our IPO. Subsequent to the completion of our IPO, the fair value of our common stock is based on observable market prices.
Research and Development ExpenseCosts
Research and development expense consists of costs incurred in performingWe record accruals for estimated research and development activities, including compensation and benefits for research and development employees, including stock-based compensation; expenses incurred under agreements with contract research organizations and investigative sites that conduct clinical trials and preclinical studies, the costs of acquiring and manufacturing clinical trial materials and other supplies; payments under licensing and research and development agreements; other outside services and consulting costs, and an allocation of facility, information technology and overhead expenses. Research and development costs are expensed as incurred.
Clinical Trial Accruals
Costs for preclinical study and clinical trial activities are recognized based on an evaluation of our vendors’ progress towards completion of specific tasks, using data such as patient enrollment, clinical site activations or information provided to us by our vendors regarding their actual costs incurred. Payments for these activities are based on the terms of individual contracts and payment timing may differ significantly from the period in which the services are performed. We determine accrual estimates through reports from and discussions with applicableinternal personnel and outside service providers as to the progress or state of completion of trials,studies, or the services completed. Our estimates of accrued expenses as of each balance sheet date are based on the facts and circumstances known at the time. Costs that are paid in advance of performance are deferred as a prepaid expenseasset and amortized over the service periodrecognized as expense as the services are provided.
Sale of Future Revenues
Other Current LiabilitiesTo the extent that we account for the sale of future revenues as debt in accordance with ASC 470, we amortize the liability and recognize interest expense related to the sale of future revenues using the effective interest rate method over the estimated life of the underlying agreement. The liability and related interest expense are based on our current estimate of expected future payments over the life of the arrangement. We re-assess the amount and timing of expected payments each reporting period using a combination of internal projections and forecasts from external resources and record interest expense on the carrying value of the liability using the imputed effective interest rate on a prospective basis.
Revenue Recognition
For contracts that are determined to be within the scope of Accounting Standards Codification Topic 606 (Accounting Standards Update (ASU) No. 2014-09), Revenue from Contracts with Customers, and all subsequent amendments (collectively, ASC 606), revenue is recognized as we satisfy performance obligations and when a customer obtains control of the promised goods or services. The amount of revenue recognized reflects the consideration to which we expect to be entitled to receive in exchange for these goods and services. To achieve this core principle, we apply the following five steps (i) identify the contract with the customer; (ii) identify the performance obligations in the contract; (iii) determine the transaction price; (iv) allocate the transaction price to the performance obligations in the contract; and (v) recognize revenue when or as we satisfy a performance obligation. We only apply the five-step model to contracts when we determine that collection of substantially all consideration for goods and services that are transferred is probable based on the customer’s intent and ability to pay the promised consideration.
Performance obligations promised in a contract are identified based on the goods and services that will be transferred to the customer that are both capable of being distinct and are distinct in the context of the contract. To the extent a contract includes multiple promised goods and services, we apply judgment to determine whether promised goods and services are both capable of being distinct and distinct in the context of the contract. If these criteria are not met, the promised goods and services are accounted for as a combined performance obligation.
The transaction price is determined based on the consideration to which we will be entitled in exchange for transferring goods and services to the customer. To the extent the transaction price includes variable consideration, we estimate the amount of variable consideration that should be included in the transaction price utilizing either the expected value method or the most likely amount method, depending on the nature of the variable consideration. Variable consideration is included in the transaction price if, in our judgment, it is probable that a significant future reversal of cumulative revenue under the contract will not occur. Any estimates, including the effect of the constraint on variable consideration, are evaluated at each reporting period for any changes. Determining the transaction price requires significant judgment, which is discussed in further detail for our out-license agreements in Note 5. Our out-license agreements do not contain a significant financing component.
If the contract contains a single performance obligation, the entire transaction price is allocated to the single performance obligation. Contracts that contain multiple performance obligations require an allocation of the transaction price to each performance obligation on a relative standalone selling price basis unless the transaction price is variable and meets the criteria to be allocated entirely to a performance obligation or to a distinct service that forms part of a single performance obligation. The consideration to be received is allocated among the separate performance obligations based on relative standalone selling prices. We typically determine standalone selling prices using an expected cost plus margin approach model.
We satisfy performance obligations either over time or at a point in time. Revenue is recognized over time if either (i) the customer simultaneously receives and consumes the benefits provided by our performance, (ii) our performance creates or enhances an asset that the customer controls as the asset is created or enhanced or (iii) our performance does not create an asset with an alternative use to the entity and we have an enforceable right to payment for performance completed to date. We evaluate the measure of progress each reporting period and, if necessary, adjust the measure of performance and related revenue recognition. If we do not satisfy a performance obligation over time, the related performance obligation is satisfied at a point in time by transferring control of a promised good or service to a customer.
As discussed in further detail in Note 5, the terms of our customer contracts include potential payments to us for some or all of the following: nonrefundable, upfront fees; development, regulatory, and commercial milestone payments; research and development funding payments; royalties on the net sales of licensed products; and transition plan cost reimbursements for certain development, safety, regulatory, and process science services. These payments relate to promised goods or services for which revenue will be recognized upon our satisfaction of the underlying performance obligations.
Licenses of intellectual property: If the license of our intellectual property is determined to be distinct from the other performance obligations identified in an arrangement, we recognize revenues from consideration allocated to the license when the license is transferred to the customer and the customer is able to use and benefit from the license. For licenses that are combined with other promises, we utilize judgment to assess the nature of the combined performance obligation to determine whether the combined performance obligation is satisfied over time or at a point in time and, if over time, the appropriate method of measuring progress for purposes of recognizing revenue.
Upfront payments: Upfront payments and fees are recorded as deferred revenue upon receipt or when due and may require deferral of revenue recognition to a future period until we have satisfied our obligations under these arrangements.
Milestone payments: At the inception of each arrangement that includes development milestone payments, we evaluate the probability of reaching the milestones and estimates the amount to be included in the transaction price using the most likely amount method. If it is probable that a significant revenue reversal would not occur in the future, the associated milestone value is included in the transaction price. The transaction price is then allocated to each performance obligation on a relative standalone selling price basis, for which we recognize revenue as or when the performance obligations under the contract are satisfied. At the end of each subsequent reporting period, we re-evaluate the probability of achievement of such development milestones and any related constraint and, if necessary, adjusts its estimate of the overall transaction price. Any such adjustments are recorded on a cumulative catch-up basis, which would affect license and collaboration revenues and the consolidated statements of operations and comprehensive loss in the period of adjustment.
Royalties: For arrangements that include sales-based royalties, including milestone payments based on levels of sales, if the license is deemed to be the predominant item to which the royalties relate, we recognize revenue at the later of (i) when the related sales occur, or (ii) when the performance obligation to which some or all of the royalty has been allocated has been satisfied, or partially satisfied. To date, we have not recognized material royalty revenue resulting from our out-licensing agreements.
Transition plan cost reimbursements: Reimbursements for certain development, safety, regulatory and manufacturing services are recorded as revenue as we perform the services and related obligations identified within the transition plans for our customers.
Certain judgments affect the application of our revenue recognition policy. For example, we record short-term and long-term deferred revenue based on our best estimate of when such revenue will be recognized. Short-term deferred revenue consists of amounts that are expected to be recognized as revenue in the next 12 months, and long-term deferred revenue consists of amounts that we expect will be recognized after the next 12 months. This estimate is based on forecasted patient demand, our current operating plan and expected dates of technology transfer, and if these items should change in the future, we may recognize a different amount of deferred revenue over the next 12-month period.
Cost of commercialization revenue
Cost of commercialization revenue consists primarily of expenses associated with cell selection services performed for Pierre Fabre, in-license sales-related milestone costs, period manufacturing expenses and adjustments to reduce inventory to the lower of cost or net realizable value. Costs incurred to produce Ebvallo prior to regulatory approval, referred to as zero cost inventories, have been recorded as research and development expense in our consolidated statement of operations and comprehensive income (loss). All tab-cel (Ebvallo) sold to Pierre Fabre in the year ended December 31, 2017,2023 was zero cost inventories. Once we begin selling Ebvallo produced after receiving regulatory approval and in a qualified manufacturing facility, and as revenue is recognized on such Ebvallo sales, cost of commercialization revenue will also include direct and indirect costs related to the production of Ebvallo. Such costs include, but are not limited to, CMO costs, quality testing and validation, materials used in production, and an allocation of compensation, benefits and overhead costs associated with employees involved with production.
In 2023 and 2022, cost of commercialization revenue included adjustments of $6.6 million and $0.0 million, respectively, to write-off of inventories and to reflect them at the lower of cost or net realizable value.
Research and Development Expense
Research and development expense consists of costs incurred in performing research and development activities, including compensation and benefits for research and development employees; expenses incurred under agreements with contract research organizations and investigative sites that conduct clinical and preclinical studies; expense incurred under agreements with contract manufacturing organizations related to acquiring and manufacturing clinical study materials and other current liabilities included $2.6 millionsupplies to support the manufacture of accrued operating expenses, $0.5 millionour product candidates; payments under licensing and research and development agreements; other outside services and consulting costs, and facilities, information technology and overhead expenses. Research and development costs are expensed as incurred.
Stock-Based Compensation Expense
We account for stock-based compensation expense, including the expense of current portionrestricted common stock awards, grants of capital lease obligationsrestricted stock units (RSUs), and $0.2 millionstock options that may be settled in shares of our common stock, based on the fair values of the equity instruments issued. The fair value is determined on the measurement date, which is generally the date of grant. The fair value of our RSUs is measured at the closing market price of our common stock on the measurement date. The fair value for our stock option awards is determined at the grant date using the Black-Scholes valuation model.
In determining the fair value of stock option awards granted, we use the Black-Scholes valuation model and assumptions include:
Expected term – We derived the expected term using the “simplified” method (the expected term is determined as the average of the time-to-vesting and the contractual life of the options), as we have limited historical information to develop expectations about future exercise patterns and post vesting employment termination behavior.
Expected volatility – In 2021 and 2022, volatility was estimated using an average of Atara’s historical volatility and comparable public companies’ volatility for similar terms. Beginning in 2023, volatility is based solely on Atara’s stock price historical volatility.
Expected dividend – We have not historically declared or paid dividends to our stockholders and have no plans to pay dividends; therefore, we assumed an expected dividend yield of 0%.
Risk-free interest rate – The risk-free interest rate is based on the yield on U.S. Treasury securities with the expected term of the associated award.
For awards with performance-based vesting criteria, we assess the probability of the achievement of the performance conditions at the end of each reporting period and begin to recognize the share-based compensation costs when it becomes probable that the performance conditions will be met. For awards that are subject to both service and performance conditions, no expense is recognized until it is probable that performance conditions will be met. Stock-based compensation expense for awards with time-based vesting criteria is recognized as expense on a straight-line basis over the requisite service period. Stock-based compensation expense for awards with performance and other accrued liabilities. Asvesting criteria is recognized as expense under an accelerated graded vesting model. We account for forfeitures of stock-based awards as they occur.
Defined Contribution Plan
We have one qualified 401(k) plan covering all eligible employees. Under the plan, employees may contribute up to the statutory allowable amount for any calendar year. We make matching contributions, equal to 50% of each dollar contributed up to the first 6% of an individual’s eligible earnings, up to the annual IRS maximum. For the years ended December 31, 2016, other current liabilities included $0.62023 and 2022 we recorded matching contributions of approximately $1.9 million of accrued operating expenses and $0.1$2.3 million, of other accrued liabilities.respectively.
Income Taxes
We use the assetsasset and liabilitiesliability method to account for income taxes. We record deferred tax assets and liabilities for the expected future tax consequences of temporary differences between the financial statement carrying amounts and the tax basis of assets and liabilities using enacted tax rates expected to be in effect when the differences are expected to reverse. Valuation allowances are provided when necessary to reduce net deferred tax assets to the amount that is more likely than not to be realized. Based on the available evidence, we are unable, at this time, to support the determination that it is more likely than not that our deferred tax assets will be utilized in the future. Accordingly, we recorded a full valuation allowance as of December 31, 20172023 and 2016.2022. We intend to maintain valuation allowances until sufficient evidence exists to support their reversal.
Tax benefits related to uncertain tax positions are recognized when it is more likely than not that a tax position will be sustained during an audit. Interest and penalties related to unrecognized tax benefits are included within the provision for income tax.
Comprehensive Income (Loss)
Comprehensive Loss
Comprehensive lossincome (loss) is defined as a change in equity of a business enterprise during a period resulting from transactions from non-owner sources. Our other comprehensive lossincome (loss) is comprised solely of unrealized gains (losses) on available-for-sale securities and is presented net of taxes. WeThere have not recordedbeen any material reclassifications from other comprehensive lossincome (loss) to net loss recorded during any period presented.
Recent Accounting Pronouncements
In February 2016,We consider the applicability and impact of any Accounting Standards Updates (ASUs) issued by the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2016-02, Leases (Topic 842), which is intended to increase(FASB). Other than the transparencyASUs listed below, all other ASUs were assessed and comparability in the reporting of leasing arrangements by generally requiring leased assets and liabilitiesdetermined to be recordedeither not applicable to Atara or are expected to have minimal impact on our consolidated financial statements.
In November 2023, the balance sheet.FASB issued ASU 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures. The new standard is effectiveamendment requires disclosure of significant segment expenses that are regularly provided to the chief operating decision maker and included within each reported measure of segment profit or loss, an amount and description of its composition for fiscal yearsother segment items, and interim periods within thosedisclosures of a reportable segment’s profit or loss and assets. Additionally, all disclosure requirements under the guidance are also required for public entities with a single reportable segment. The amendments are effective for fiscal years beginning after December 15, 2018, with early adoption permitted.2023, and for interim periods within fiscal years beginning after December 15, 2024. We have not yet determined the potential effect the new standard will haveare currently evaluating this ASU to determine its impact on our consolidated financial statements.disclosures.
In March 2016,December 2023, the FASB issued ASU No. 2016-09, 2023-09 Income Taxes (Topic 740): Improvements to Employee Share-Based Payment Accounting (Topic 718),Income Tax Disclosures, which simplifies several aspects of the accounting for share-based payment transactions, includingrequires enhancements to certain income tax disclosures, most notably the income tax consequences, classification of awards as either equity or liabilities,rate reconciliation and classification in the statement of cash flows. We prospectively adopted the new standard on January 1, 2017 and that adoption did not have a material effect on our consolidated financial statements due to the full valuation allowance of our deferred tax assets.
In June 2016, the FASB issued ASU No. 2016-13, Financial Instruments - Credit Losses: Measurement of Credit Losses on Financial Instruments. ASU 2016-13 requires that expected credit losses relating to both (a) financial assets measured on an amortized cost basis, and (b) available-for-sale debt securities be recorded through an allowance for credit losses. ASU 2016-13 limits the amount of credit losses to be recognized for available-for-sale debt securities to the amount by which carrying value exceeds fair value and also requires the reversal of previously recognized credit losses if fair value increases.income taxes paid. The new standard will beamendments are effective for us on January 1, 2020.fiscal years beginning after December 15, 2024. Early adoption will be available on January 1, 2019. We are currently evaluating the effect that the updated standard will have on our consolidated financial statements and related disclosures.
In August 2016, the FASB issued ASU No. 2016-15, Statement of Cash Flows (Topic 230): Classification of Certain Cash Receipts and Cash Payments, which clarifies how certain cash receipts and cash payments should be presented and classified in the statement of cash flows. The new standard will be effective for us on January 1, 2018.is permitted. We do not expect the adoption of this new standard towill have a significantmaterial impact on our consolidateddisclosures.
Additionally, on March 6, 2024, the Securities and Exchange Commission (SEC) issued Final Rule No. 33-11275, The Enhancement and Standardization of Climate-Related Disclosures for Investors. The rule requires registrants to provide climate related disclosures in their annual reports, including, but not limited to, material Scope 1 and Scope 2 GHG emissions (for large accelerated filers and accelerated filers); governance and oversight of material climate-related risks; the material impact of climate risks on the registrant’s strategy, business model, and outlook; risk management processes for material climate-related risks; and material climate targets and goals. Based on our current small reporting company and non-accelerated filer status, certain elements of the rule are effective for fiscal years beginning after December 15, 2026, with the remaining requirements effective for fiscal years beginning after December 15, 2027. We will evaluate the SEC rule to determine its impact on our future financial statementsreporting requirements and related disclosures.
In November 2016, the FASB issued ASU No. 2016-18 Statement of Cash Flows (Topic 230): Restricted Cash, which clarifies the statement of cash flow treatment of restricted cash or restricted cash equivalents. The new standard will be effective for us on January 1, 2018. The standard should be applied using a retrospective transition method to each period presented. We do not expect the adoption of this new standard to have a significant impact on our consolidated financial statements and related disclosures.
3.
| Net Loss per Common Share
|
3.Net Loss per Common Share
Basic net loss per common share is calculated by dividing the net loss by the weighted-average number of shares of common stock outstanding during the period, without consideration of common stock equivalents. Diluted net loss per common share is computed by dividing the net loss by the weighted-average number of shares of common stock and pre-funded warrants outstanding during the period, without consideration of common share equivalents. Diluted net loss per common share is computed by dividing net loss by the weighted-average number of shares of common stock, pre-funded warrants and common share equivalents outstanding for the period. The pre-funded warrants are included in the computation of basic and diluted net loss per common share as the exercise price is negligible and the pre-funded warrants are fully vested and exercisable. Common share equivalents are only included in the calculation of diluted net loss per common share when their effect is dilutive.
Potential dilutive securities, which include unvested RSAs,RSUs, unvested performance-based RSUs and performance-based options to purchase common stock for which established performance criteria have been achieved as of the end of the respective periods, vested and unvested options to purchase common stock and ESPP shareshares to be issued under our employee stock purchase rights,plan (ESPP), have been excluded from the computation of diluted net loss per share as theirthe effect is antidilutive. Therefore, the denominator used to calculate both basic and diluted net loss per common share is the same in all periods presented.
The following table represents the potential common shares issuable pursuant to outstanding securities as of the related period end dates listed that were excluded from the computation of diluted net loss per common share, as their inclusion would have an antidilutive effect:
| | | | | | | |
| As of December 31, | |
| 2023 | | | 2022 | |
Unvested RSUs | | 6,261,213 | | | | 6,698,858 | |
Vested and unvested options | | 10,706,651 | | | | 10,336,634 | |
ESPP share purchase rights | | 185,843 | | | | 86,782 | |
Total | | 17,153,707 | | | | 17,122,274 | |
| As of December 31, | |
| 2017 | | | 2016 | | | 2015 | |
Unvested RSAs | | — | | | | — | | | | 233,413 | |
Unvested RSUs | | 1,685,000 | | | | 1,286,262 | | | | 427,605 | |
Vested and unvested options | | 5,229,648 | | | | 3,733,847 | | | | 3,137,529 | |
ESPP share purchase rights | | 14,905 | | | | 7,037 | | | | — | |
Total | | 6,929,553 | | | | 5,027,146 | | | | 3,798,547 | |
109
The following tables summarize the estimated fair value and related valuation input hierarchy of our available-for-sale securities as of each period end:
| | | | | | | | | | | | | | | | | | |
| | | | Total | | | Total | | | Total | | | Total | |
| | | | Amortized | | | Unrealized | | | Unrealized | | | Estimated | |
As of December 31, 2023: | | Input Level | | Cost | | | Gain | | | Loss | | | Fair Value | |
| | | | (in thousands) | |
Money market funds | | Level 1 | | $ | 14,376 | | | $ | — | | | $ | — | | | $ | 14,376 | |
U.S. Treasury obligations | | Level 2 | | | 9,928 | | | | 1 | | | | — | | | | 9,929 | |
Corporate debt obligations | | Level 2 | | | 26,089 | | | | — | | | | (205 | ) | | | 25,884 | |
Total available-for-sale securities | | | | | 50,393 | | | | 1 | | | | (205 | ) | | | 50,189 | |
Less: amounts classified as cash equivalents | | | | | (24,304 | ) | | | (1 | ) | | | — | | | | (24,305 | ) |
Amounts classified as short-term investments | | | | $ | 26,089 | | | $ | — | | | $ | (205 | ) | | $ | 25,884 | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | Total | | | Total | | | Total | | | Total | |
| | | | Amortized | | | Unrealized | | | Unrealized | | | Estimated | |
As of December 31, 2022: | | Input Level | | Cost | | | Gain | | | Loss | | | Fair Value | |
| | | | (in thousands) | |
Money market funds | | Level 1 | | $ | 78,033 | | | $ | — | | | $ | — | | | $ | 78,033 | |
U.S. Treasury obligations | | Level 2 | | | 63,013 | | | | 3 | | | | (394 | ) | | | 62,622 | |
Government agency obligations | | Level 2 | | | 8,086 | | | | — | | | | (48 | ) | | | 8,038 | |
Corporate debt obligations | | Level 2 | | | 82,598 | | | | 4 | | | | (1,513 | ) | | | 81,089 | |
Commercial paper | | Level 2 | | | 996 | | | | — | | | | — | | | | 996 | |
Asset-backed securities | | Level 2 | | | 6,343 | | | | — | | | | (119 | ) | | | 6,224 | |
Total available-for-sale securities | | | | | 239,069 | | | | 7 | | | | (2,074 | ) | | | 237,002 | |
Less: amounts classified as cash equivalents | | | | | (87,122 | ) | | | (3 | ) | | | — | | | | (87,125 | ) |
Amounts classified as short-term investments | | | | $ | 151,947 | | | $ | 4 | | | $ | (2,074 | ) | | $ | 149,877 | |
| | | | Total | | | Total | | | Total | | | Total | |
| | | | Amortized | | | Unrealized | | | Unrealized | | | Estimated | |
As of December 31, 2017: | | Input Level | | Cost | | | Gain | | | Loss | | | Fair Value | |
| | | | (in thousands) | |
Money market funds | | Level 1 | | $ | 68,730 | | | $ | — | | | $ | — | | | $ | 68,730 | |
U.S. Treasury obligations | | Level 2 | | | 39,068 | | | | — | | | | (28 | ) | | | 39,040 | |
Government agency obligations | | Level 2 | | | 4,749 | | | | — | | | | (21 | ) | | | 4,728 | |
Corporate debt obligations | | Level 2 | | | 46,532 | | | | 2 | | | | (98 | ) | | | 46,436 | |
Commercial paper | | Level 2 | | | 1,592 | | | | — | | | | — | | | | 1,592 | |
Asset-backed securities | | Level 2 | | | 4,122 | | | | — | | | | (6 | ) | | | 4,116 | |
Total available-for-sale securities | | | | | 164,793 | | | | 2 | | | | (153 | ) | | | 164,642 | |
Less amounts classified as cash equivalents | | | | | (77,769 | ) | | | — | | | | — | | | | (77,769 | ) |
Amounts classified as short-term investments | | | | $ | 87,024 | | | $ | 2 | | | $ | (153 | ) | | $ | 86,873 | |
| | | | | | | | | | | | | | | | | | |
| | | | Total | | | Total | | | Total | | | Total | |
| | | | Amortized | | | Unrealized | | | Unrealized | | | Estimated | |
As of December 31, 2016: | | Input Level | | Cost | | | Gain | | | Loss | | | Fair Value | |
| | | | (in thousands) | |
Money market funds | | Level 1 | | $ | 28,816 | | | $ | — | | | $ | — | | | $ | 28,816 | |
U.S. Treasury obligations | | Level 2 | | | 65,403 | | | | 3 | | | | (21 | ) | | | 65,385 | |
Government agency obligations | | Level 2 | | | 23,860 | | | | 5 | | | | (5 | ) | | | 23,860 | |
Corporate debt obligations | | Level 2 | | | 113,649 | | | | 8 | | | | (172 | ) | | | 113,485 | |
Commercial paper | | Level 2 | | | 699 | | | | — | | | | — | | | | 699 | |
Asset-backed securities | | Level 2 | | | 13,414 | | | | 4 | | | | (6 | ) | | | 13,412 | |
Total available-for-sale securities | | | | | 245,841 | | | | 20 | | | | (204 | ) | | | 245,657 | |
Less amounts classified as cash equivalents | | | | | (37,944 | ) | | | — | | | | 1 | | | | (37,943 | ) |
Amounts classified as short-term investments | | | | $ | 207,897 | | | $ | 20 | | | $ | (203 | ) | | $ | 207,714 | |
The amortized cost and fair value of our available-for-sale securities by contractual maturity were as follows:
| | As of December 31, 2017 | | | As of December 31, 2016 | | As of December 31, 2023 | | | As of December 31, 2022 | |
| Amortized | | | Estimated | | | Amortized | | | Estimated | | Amortized | | | Estimated | | | Amortized | | | Estimated | |
| Cost | | | Fair Value | | | Cost | | | Fair Value | | Cost | | | Fair Value | | | Cost | | | Fair Value | |
| (in thousands) | | | (in thousands) | | (in thousands) | | | (in thousands) | |
Maturing within one year | $ | 151,938 | | | $ | 151,852 | | | $ | 198,022 | | | $ | 197,956 | | $ | 50,393 | | | $ | 50,189 | | | $ | 202,323 | | | $ | 201,359 | |
Maturing in one to five years | | 12,855 | | | | 12,790 | | | | 47,819 | | | | 47,701 | | | — | | | | — | | | | 36,746 | | | | 35,643 | |
Total available-for-sale securities | $ | 164,793 | | | $ | 164,642 | | | $ | 245,841 | | | $ | 245,657 | | $ | 50,393 | | | $ | 50,189 | | | $ | 239,069 | | | $ | 237,002 | |
We considered the current and expected future global economic and market conditions, including, but not limited to, the wars in Ukraine and the Middle East and increased tensions between the U.S. and China, and determined that our investments have not been significantly impacted. As of December 31, 2017, certain available-for-sale securities had been in a continuous unrealized loss position, each for less than twelve months. As of this date,2023, no significant facts or circumstances were present to indicate a deterioration in the creditworthiness of the respective issuers of the available-for-sale securities we hold, and the Company hadwe have no requirement or intention to sell these securities before maturity or recovery of their amortized cost basis. For all securities with a fair value less than its amortized cost basis, we determined the decline in fair value below amortized cost basis to be non-credit related and no allowance for losses has been recorded. During the years ended December 31, 2017, 20162023 and 2015,2022, we did not recognize any other-than-temporary impairment loss.losses on our investments.
We have elected the practical expedient to exclude the applicable accrued interest from both the fair value and the amortized cost basis of our available-for-sale securities for purposes of identifying and measuring an impairment. We present accrued interest receivable related to our available-for-sale securities in other current assets, separate from short-term investments, on our consolidated balance sheet. As of December 31, 2023 and 2022, accrued interest receivable was $0.3 million and $0.8 million, respectively. Our accounting policy is to not measure an allowance for credit losses for accrued interest receivables and to write-off any uncollectible accrued interest receivable as a reversal of interest income in a timely manner, which we consider to be in the period in which we determine the accrued interest will not be collected by us. We have not written off any accrued interest receivables for the years ended December 31, 2023 and 2022.
In addition, restricted cash collateralized by money market funds is a financial asset measured at fair value and is a Level 1 financial instrument under the fair value hierarchy.
The following table provides a reconciliation of cash, cash equivalents and restricted cash within the consolidated balance sheets that sum to the total of the same such amounts in the consolidated statement of cash flows:
| | | | | | | | |
| | December 31, | | | December 31, | |
| | 2023 | | | 2022 | |
| | (in thousands) | |
Cash and cash equivalents | | $ | 25,841 | | | $ | 92,942 | |
Restricted cash – short-term | | | 146 | | | | 146 | |
Total cash, cash equivalents and restricted cash | | $ | 25,987 | | | $ | 93,088 | |
| | | | | | |
Pierre Fabre Commercialization Agreement
In October 2021, we entered into the Pierre Fabre Commercialization Agreement, pursuant to which, we granted to Pierre Fabre an exclusive, field-limited license to commercialize and distribute Ebvallo in Europe and select emerging markets in the Initial Territory following regulatory approval. In September 2022, we entered into Amendment No. 1 to the Pierre Fabre Commercialization Agreement (the PF Amendment). Under the terms of the PF Amendment, following European Commission approval of Ebvallo for EBV+ PTLD and subsequent filing of the Marketing Authorization Application (MAA) transfer to Pierre Fabre, we are entitled to receive an additional $30 million milestone payment in exchange for, among other things, a reduction in: (i) royalties we are eligible to receive as a percentage of net sales of Ebvallo in the Territory, and (ii) the supply price mark up on Ebvallo purchased by Pierre Fabre. Additionally, we agreed to extend the time period for provision of certain services to Pierre Fabre in the Initial Territory at our cost pursuant to the Pierre Fabre Commercialization Agreement. In December 2022, we sold a portion of our right to receive royalties and certain milestone payments related to Ebvallo in the Initial Territory under the Pierre Fabre Commercialization Agreement to HCRx for a total investment amount of $31.0 million, subject to a repayment cap between 185% and 250% of the total investment amount by HCRx. See Note 6 for further information related to the agreement with HCRx.
In October 2023, we entered into the A&R Commercialization Agreement with Pierre Fabre. Pursuant to the A&R Commercialization Agreement, Pierre Fabre’s exclusive rights to research, develop, manufacture, commercialize and distribute tab-cel are expanded to include all other countries in the world (Additional Territory) in addition to the Initial Territory (together, the Territory), subject to our performance of certain obligations as described below.
Pierre Fabre paid us an upfront cash payment of $45.0 million for the exclusive license grant for the Initial Territory in the fourth quarter of 2021. In December 2022, we met the contractual right to receive $40.0 million in milestone payments upon certain regulatory milestones, for which the cash was received in January 2023. Subject to the terms of the royalty purchase agreement with HCRx, as described in Note 6, we are entitled to receive an aggregate of up to $308.0 million in remaining milestone payments upon achieving certain regulatory and commercial milestones in addition to double-digit tiered royalties as a percentage of net sales of Ebvallo in the Initial Territory, until the later of 12 years after the first commercial sale in each such country, the expiration of specified patent rights, or the expiration of all regulatory exclusivity for Ebvallo on a country-by-country basis. In December 2023, upon the effective date of the A&R Commercialization Agreement, we met the contractual right to receive an additional upfront cash payment of $20.0 million for the expanded exclusive license grant in the Additional Territory, for which the cash was received in January 2024. We will also be entitled to receive an aggregate of up to $620.0 million in additional payments upon achieving certain regulatory and commercial milestones relating to tab-cel in the Additional Territory. We will be eligible to receive significant double-digit tiered royalties as a percentage of net sales of tab-cel in the Additional Territory until the later of 12 years after the first commercial sale in each such country, the expiration of specified patent rights in each such country, or the expiration of all regulatory exclusivity for tab-cel on a country-by-country basis.
We have entered into a separate manufacturing and supply agreement with Pierre Fabre for us to manufacture Ebvallo for Pierre Fabre to use in the Initial Territory based on a fixed price through December 31, 2023 and at a price equal to cost plus a margin for orders placed after December 31, 2023, subject to a maximum annual increase. Pierre Fabre will assume the responsibility and cost for the manufacture and supply of tab-cel (Ebvallo) in the Territory upon the Manufacturing Transition Date, which is defined as the earlier of: i) all activities relating to the transfer of tab-cel manufacturing, pursuant to the A&R Commercialization Agreement, from Atara to Pierre Fabre have been completed to the reasonable satisfaction of both parties, or ii) December 31, 2025, through the remainder of the term of the A&R Commercialization Agreement. Pierre and we are to use commercially reasonable efforts to achieve this prior to the earlier transfer date from Atara to Pierre Fabre of the first marketing authorization in the Additional Territory or the
Propertyfirst BLA (PF Transfer Date). Prior to the Manufacturing Transition Date, we are responsible for manufacturing and equipment consistedsupplying tab-cel (Ebvallo) to Pierre Fabre in the Territory. Without transfer of the manufacturing technology, no other party can manufacture Ebvallo.
Cell selection is the process of identifying the appropriate cell line from available tab-cel inventory to be used for a patient. We are responsible for the performance of cell selection services in the Initial Territory at our cost through the earlier of the PF Transfer Date, June 30, 2025 or a date otherwise agreed to by Pierre Fabre and us. To the extent that the transfer of cell selection technology occurs subsequent to June 30, 2025, we will be responsible for the performance of cell selection services in the Initial Territory at Pierre Fabre’s cost until cell selection technology is transferred to Pierre Fabre. Without transfer of the cell selection technology, no other party can provide such services. We are responsible for the performance of cell selection services in the Additional Territory through the earlier of the PF Transfer Date or a date otherwise agreed to by Pierre Fabre and us, at the sole expense of Pierre Fabre.
As part of the Pierre Fabre Commercialization Agreement, we formed a joint steering committee (JSC) with Pierre Fabre that provides oversight, decision making and implementation guidance regarding the commercialization activities, the responsibilities of which has been expanded to cover the incremental scope of the A&R Commercialization Agreement.
During the applicable period specified in the A&R Commercialization Agreement, we will be responsible for various development, safety, process science, and regulatory activities, including obtaining regulatory approval in the United States for tab-cel for EBV-associated post-transplant lymphoproliferative disease. Pierre Fabre will pay us for these services in accordance with the A&R Commercialization Agreement. Pierre Fabre will be responsible, at its cost, for obtaining and maintaining all other required regulatory approvals and for commercialization and distribution of tab-cel in the Additional Territory, including conducting any other clinical study required. We will own any intellectual property rights developed solely by us under the Agreement.
Accounting Analysis
Identification of the Contract
We assessed this arrangement in accordance with ASC 606 and concluded that the promises in the A&R Commercialization Agreement represent transactions with a customer.
Identification of the Promises and Performance Obligations
We identified four performance obligations under the A&R Commercialization Agreement, consisting of the following material promises:
(1)the transfer of intellectual property rights in the form of a license in the Initial Territory, the obligation to participate in the JSC, a material right for purchases associated with the manufacture and supply of Ebvallo and the performance of cell-selection services. We concluded that the individual promises are not distinct because Pierre Fabre cannot benefit from the license without the other services and vice versa, since Pierre Fabre is not capable of carrying out the manufacturing and supply and cell selection services on their own, until the transfer of the related technologies occur. Consequently, these promises represent a single performance obligation, collectively referred to as the Initial Territory Obligation.
(2)the transfer of intellectual property rights in the form of a license in the Additional Territory, the manufacture and supply of tab-cel and the performance of cell-selection services, as well as the promises to transfer the related technologies, and perform certain development, safety and regulatory services. We concluded that the promises are not distinct because Pierre Fabre cannot benefit from the license without the other services and vice versa. Consequently, these promises represent a single performance obligation, collectively referred to as the Additional Territory Obligation.
(3)performance of certain process science services, referred to as the Process Sciences Obligation
(4)the sale of certain intermediate inventory used in the production of tab-cel in existence on the Manufacturing Transition Date, referred to as the Intermediate Inventory Obligation.
Determination of the Transaction Price
Under the Pierre Fabre Commercialization Agreement, we determined that the $45.0 million upfront payment constituted the entire consideration to be included in the transaction price at the outset of the arrangement, and the $40.0 million in development milestones achieved in December 2022 were added to the transaction price upon meeting the related milestone criteria. The remaining potential development and commercial milestone payments that we are eligible to receive were excluded from the transaction price, as the milestone amounts were fully constrained based on the probability of achievement or have not been earned. None of the future royalty and sales-based milestone payments were included in the transaction price, as the potential payments represent sales-based
consideration. We will reevaluate the transaction price at the end of each reporting period and as uncertain events are resolved or other changes in circumstances occur, and, as necessary, we will adjust our estimate of the transaction price.
Upon the effective date of the A&R Commercialization Agreement, the $20.0 million additional upfront payment received and estimated revenue for the development, safety, regulatory and process science services were added to the transaction price. The remaining potential development and commercial milestone payments that we are eligible to receive were excluded from the transaction price, as the milestone amounts were fully constrained based on the probability of achievement or have not been earned. None of the future royalty and sales-based milestone payments were included in the transaction price, as the potential payments represent sales-based consideration. Any consideration associated with the Intermediate Inventory Obligation was excluded from the transaction price as the amount was fully constrained based on uncertainty surrounding available inventory on the Manufacturing Transition Date.
We will reevaluate the transaction price at the end of each reporting period and as uncertain events are resolved or other changes in circumstances occur, and, as necessary, we will adjust our estimate of the transaction price.
Allocation of the Transaction Price to Performance Obligations
The transaction price was allocated to each performance obligation based on their relative standalone selling price. We developed the estimated standalone selling price for each of the A&R Commercialization Agreement performance obligations with the objective of determining the price at which we would sell such an item if it were to be sold regularly on a standalone basis.
Recognition of Revenue
Commercialization revenue associated with the Initial Territory Obligation will be recognized over the period during which the material right exists, which would end upon the Manufacturing Transition Date. Based on these considerations and our forecast of the timing and associated costs of the purchases related to the manufacture and supply of Ebvallo and estimated timing of technology transfer, we estimate the material right in the Initial Territory will exist for approximately one to two years. We reassess this evaluation each reporting period. Commercialization revenue associated with sales of Ebvallo to Pierre Fabre under the Initial Territory Obligation is deferred until we have performed the associated cell selection services, or once cell selection technology transfer to Pierre Fabre has been complete. At that point, Pierre Fabre would be able to utilize the Ebvallo it had purchased from us on its own.
Commercialization revenue associated with the Additional Territory Obligation and the Process Sciences Obligation will be recognized using a cost-based input method based on the amount of actual costs incurred relative to the total budgeted costs expected to be incurred for the respective performance obligations. A cost-based input method of revenue recognition requires us to make estimates of costs to complete our performance obligation. In making such estimates, significant judgment is required to evaluate assumptions related to cost estimates. The cumulative effect of revisions to estimated costs to complete our performance obligation will be recorded in the period in which changes are identified and amounts can be reasonably estimated. We expect to recognize revenue associated with the Additional Territory Obligation and the Process Sciences Obligation over a period of approximately two years. We reassess this evaluation each reporting period. The transfer of control occurs over the respective time period and, in our judgment, is the best measure of progress towards satisfying the performance obligation. A significant change in these assumptions and estimates could have a material impact on the timing and amount of revenue recognized in future periods. We expect to recognize revenue associated with the Intermediate Inventory Obligation at a point in time on the Manufacturing Transition Date, which is when title and risk of loss, and thus, control, of the intermediate inventory transfers to Pierre Fabre.
Deferred revenue activity related to commercialization revenue for the year ended December 31, 2023 was as follows:
| | | | |
| | Total | |
| | (in thousands) | |
Deferred revenue, December 31, 2022 | | $ | 85,000 | |
Additions | | | 38,170 | |
Recognized into commercialization revenue | | | (7,775 | ) |
Deferred revenue December 31, 2023 | | | 115,395 | |
Less: deferred revenue – current portion | | | (77,833 | ) |
Deferred revenue – long-term, December 31, 2023 | | $ | 37,562 | |
During the year ended December 31, 2023, we recognized $5.0 million of revenue that was included in the deferred revenue balance as of each period end:December 31, 2022.
| | December 31, | | | December 31, | |
| | 2017 | | | 2016 | |
| | (in thousands) | |
Construction in progress | | $ | 40,797 | | | $ | 970 | |
Lab equipment | | | 2,156 | | | | 1,506 | |
Manufacturing equipment | | | 885 | | | | - | |
Leasehold improvements | | | 623 | | | | 580 | |
Furniture and fixtures | | | 536 | | | | 526 | |
Computer equipment and software | | | 477 | | | | 66 | |
| | | 45,474 | | | | 3,648 | |
Less accumulated depreciation and amortization | | | (1,345 | ) | | | (389 | ) |
Property and equipment, net | | $ | 44,129 | | | $ | 3,259 | |
Costs incurred relating to performing the services within the Additional Territory Obligation and Process Sciences Obligation consist of third party expenses and for time incurred by our employees to satisfy requirements set forth by the A&R Commercialization Agreement. These costs are included in research and development expenses in the consolidated statements of operations and comprehensive income (loss) during the year ended December 31, 2023. Such costs were not material for the year ended December 31, 2023.
Construction in progress represents capitalized costs for our manufacturing facility in Thousand Oaks, CaliforniaUnder the A&R Commercialization Agreement, we conduct an early access program observational study at the sole cost and capitalizableexpense of Pierre Fabre. We recognize the costs incurred associated with this study within research and development expenses, which is directly offset by revenue recorded within license and collaboration revenue. The license and collaboration revenue associated with the early access program for the year ended December 31, 2023 was $0.7 million, as compared to $1.8 million for the year December 31, 2022.
Bayer Agreements
In December 2020, we entered into a research, development and license agreement (Bayer License Agreement) with Bayer AG (Bayer) pursuant to which we granted to Bayer an exclusive, field-limited license under the applicable patents and know-how owned or controlled by us and our affiliates covering or related to ATA2271 and ATA3271 (the Licensed Products).
Under the terms of the Bayer License Agreement, we were responsible at our cost for all mutually agreed preclinical and clinical activities for ATA2271 through the first in human Phase 1 clinical study in collaboration with MSK, following which Bayer was responsible for the further development of internalATA2271 at its cost. Bayer was responsible for the development of ATA3271, except for certain mutually agreed preclinical, translational, manufacturing and supply chain activities to be performed by us relating to ATA3271, in each case at Bayer’s cost. Bayer was also solely responsible for commercializing the Licensed Products at its cost.
In March 2021, we entered into a Technology Transfer Agreement with Bayer (the Bayer Tech Transfer Agreement), which was contemplated as part of the Bayer License Agreement, to transfer to Bayer the ATA3271 manufacturing process being developed as part of the CMC services in the Bayer License Agreement.
In March 2021, we also entered into a Manufacturing and Supply Agreement with Bayer (the Bayer Manufacturing Agreement), which was contemplated as part of the Bayer License Agreement, to manufacture Phase 1 and 2 allogeneic mesothelin-directed CAR T-cell therapies for Bayer to use software. Depreciationin clinical trials at a price based on our costs plus a reasonable margin, which is consistent with our standalone selling price. Collectively, the Bayer License Agreement, the Manufacturing and Supply Agreement and the Technology Transfer Agreement are referred to as “the Bayer Agreements”.
In May 2022, Bayer notified us of its decision to terminate the Bayer Agreements, and on August 2, 2022, we entered into the Termination, Amendment and Program Transfer Agreement with which terminated the Bayer Agreements (the Bayer Termination Agreement) with an effective date of July 31, 2022. Upon the termination effective date, full product development and commercialization rights related to ATA2271 and ATA3271 reverted to Atara. In return for certain activities performed by Atara prior to the termination effective date, Bayer paid Atara $4.2 million in September 2022. Utilizing the cost-based input method, we recognized license and collaboration revenue of $61.8 million for 2022. As a result of the termination, no license and collaboration revenue related to the Bayer Agreements was recognized for the year ended December 31, 2023, and there was no deferred revenue related to the Bayer Agreements as of December 31, 2023 or December 31, 2022.
6.Liability Related to the Sale of Future Revenues
In December 2022, we entered into a Purchase and Sale Agreement (the HCRx Agreement) with HCR Molag Fund, L.P., a Delaware limited partnership, (HCRx). In exchange for a payment of $31.0 million (the Investment Amount) to Atara, net of certain transaction expenses, HCRx obtained the right to receive certain Ebvallo royalties and milestone payments payable by Pierre Fabre under the Pierre Fabre Commercialization Agreement up to an agreed upon multiple of the Investment Amount.
Under the HCRx Agreement, HCRx is entitled to receive tiered royalties on net sales of Ebvallo in the Initial Territory in amounts ranging from the mid-single digits to double digits based on annual net sales. HCRx is also entitled to certain milestone payments due to Atara from Pierre Fabre. The total royalties and milestones payable to HCRx are capped between 185% and 250% of the Investment Amount, depending upon the timing of such royalties and milestones. Upon meeting the cap amount, HCRx’s right to receive royalties and milestone payments will terminate and all rights will revert to Atara. To the extent a certain milestone within the
Pierre Fabre Commercialization Agreement is not achieved on or prior to June 30, 2026, we will be required to make a one-time cash payment in the amount of $9.0 million to HCRx, and HCRx shall transfer all of its right, title and interest in this certain $9.0 million milestone payment to Atara. This payment, if required, would be included in the calculation of aggregate payments made to HCRx.
The gross proceeds of the Investment Amount of $31.0 million were recorded as a liability related to the sale of future revenues, net of transaction costs of $0.4 million, and is be amortized using the effective interest method over the life of the arrangement.
To determine the amortization of the recorded liability, we are required to estimate the total amount of future payments to be received by HCRx. The sum of these amounts less the $31.0 million proceeds we received will be recorded as interest expense over the life of the HCRx Agreement. We will estimate the effective interest rate used to record non-cash interest expense under the HCRx Agreement based on the estimate of future royalty payments to be received by HCRx. As of December 31, 2023, the annual effective interest rate was approximately 12%. Over the life of the arrangement, the actual effective interest rate will be affected by the amount and timing of the actual and forecasted royalty and milestone payments to HCRx. At each reporting date, we reassess our estimate of the timing and amounts of future payments made to HCRx, and prospectively adjust the effective interest rate and amortization expense was $1.0of the liability as necessary.
The following table presents the changes in the liability related to the sale of future revenues under the HCRx Agreement for the year ended December 31, 2023:
| | | | |
| | (in thousands) | |
Liability related to sale of future revenues as of December 31, 2022 | | $ | 30,236 | |
Accretion of interest expense on liability related to sale of future revenues | | | 4,792 | |
Amortization of debt discount and debt issuance costs | | | 79 | |
Repayment of the liability | | | (31 | ) |
Liability related to sale of future revenues as of December 31, 2023 | | | 35,076 | |
Less: current portion classified within other accrued liabilities | | | (453 | ) |
Long-term liability related to sale of future revenues | | $ | 34,623 | |
On April 4, 2022, we completed the sale of the ATOM Facility to FDB for net proceeds of $94.8 million, $0.4after deducting transaction costs of $4.6 million and $48,000other adjustments to the purchase price. The sale resulted in a gain of $50.2 million included within other income (expense), net for the year end December 31, 2022. As disclosed in Note 8, although we have assigned the lease for the ATOM Facility to FDB, we have not received novation from the landlord. Therefore, the lease-related assets and liabilities for the ATOM Facility remain on our balance sheet. Refer to the summary of assets sold and gain on sale of the ATOM Facility:
| | | | |
(in thousands) | | | |
Net proceeds from sale of ATOM Facility | | $ | 94,765 | |
| | | |
Assets sold: | | | |
Other current assets | | $ | 190 | |
Property and equipment, net | | | 44,299 | |
Other assets | | | 39 | |
Less: Assets sold | | | 44,528 | |
Gain on sale of ATOM Facility | | $ | 50,237 | |
In connection with the sale, we entered into a Transition Services Agreement (TSA) with FDB, pursuant to which we assisted FDB in the transition of certain functions, including, but not limited to, information technology, finance and technical operations. FDB reimbursed us at cost for all third party expenses incurred in conjunction with the TSA and for time incurred by our employees to satisfy requirements set forth by the TSA. The reimbursements are recorded as reductions to the related Operating expenses and the amounts associated with reimbursements for employee time incurred were not material for the years ended December 31, 2017, 20162023 and 2015, respectively.2022. The TSA was terminated in March 2023.
We lease office space for our corporate headquarters in Thousand Oaks, California. In November 2018, we entered into a lease agreement for this office space that expires in February 2026 and for which we have the option to extend the lease for an additional period of five years after the initial term.
6.
115
| License and Collaboration Agreements
|
MSK Agreements – In September 2014,March 2021, we entered into a new lease agreement for approximately 33,659 square feet of office, lab and warehouse space in Thousand Oaks, California. During the Companythird quarter of 2021, the initial 10.5-year lease term commenced, upon substantial completion of the landlord’s work as defined under the agreement. Base rent is subject to annual increases of 3% with each annual anniversary of the rent commencement date. We have the option to extend this lease for two additional five-year periods after the initial term. Additionally, in 2021, we entered into an exclusive optionamended lease agreement for our office and lab space in Aurora, Colorado, to add additional lab space.
In November 2023, we entered into an amended lease agreement for our office and lab space in Aurora, Colorado, to extend the term of the lease agreement to April 2025.
In February 2017, we entered into a lease agreement (the ATOM Lease) for approximately 90,580 square feet of office, lab and cellular therapy manufacturing space in Thousand Oaks, California. The initial 15-year term of the headlease commenced on February 15, 2018, upon the substantial completion of landlord’s work as defined under the agreement. In April 2022, we assigned the ATOM Lease to FDB in connection with MSKthe closing of the sale of the ATOM Facility to FDB. Under ASC 842, we are considered to be the sub-lessor of the ATOM Lease. We have not received novation from the landlord and therefore have not been relieved of our primary obligations under which it hadthe headlease. Therefore, the ROU asset and lease liability for the ATOM Facility remain on our balance sheet.
We evaluated our vendor contracts to identify embedded leases and determined that the Fujifilm MSA contained items that constituted a lease under ASC 842, Leases, as Atara has the right to acquiresubstantially all of the exclusive worldwide license rightseconomic benefits from the use of the asset and can direct the use of the asset. We concluded that the Fujifilm MSA contains an embedded operating lease for certain dedicated processing rooms for the manufacturing of Atara product and an embedded finance lease for certain freezers dedicated for our use. The Fujifilm MSA includes contractual obligations in the form of payments for the processing rooms and the freezers, each over a term of five years. As a result, we added ROU assets and lease liabilities for the processing rooms and freezers for the initial term of the lease in the amounts of $50.8 million and $4.8 million, respectively. In November 2023, we agreed to three clinical stage T-cell therapiesforego the use of one processing room for approximately one year in return for a reduction in contractual obligations under the Fujifilm MSA. We have the option to subsequently reclaim the processing room or release it to FDB for the remainder of the initial term.
We lease office space in South San Francisco, California under a non-cancellable lease agreement. In December 2021, we entered into a second amendment with the landlord to extend the lease term through May 2025. The amended lease agreement does not include an option to extend the lease term. In connection with the amended lease, we are required to maintain a letter of credit in the amount of $0.1 million to the landlord. In October 2022, we entered into a sub-lease agreement with a third party for this office space. The sub-lease term commenced in November 2022 and expires in May 2025, with no option to extend the sub-lease term. We have not received novation from MSK. the landlord and therefore have not been relieved of our primary obligations under the headlease. Therefore, the ROU asset and lease liability for the South San Francisco office remain on our balance sheet.
The maturities of lease liabilities under our operating and finance leases as of December 31, 2023 were as follows:
| | | | | | | | |
| Operating Leases | | Finance Leases | |
Years Ending December 31, | | (in thousands) | |
2024 | | $ | 17,209 | | | $ | 1,219 | |
2025 | | | 18,486 | | | | 1,263 | |
2026 | | | 17,271 | | | | 1,285 | |
2027 | | | 5,760 | | | | 436 | |
2028 | | | 3,319 | | | | — | |
Thereafter | | | 12,459 | | | | — | |
Total lease payments | | $ | 74,504 | | | $ | 4,203 | |
Less: amount representing interest | | | (17,547 | ) | | | (673 | ) |
Present value of lease liabilities | | $ | 56,957 | | | $ | 3,530 | |
| | | | | | |
Balance as of December 31, 2023 | | | | | | |
Other current liabilities | | $ | 11,264 | | | $ | 915 | |
Operating lease liabilities – long-term | | | 45,693 | | | | — | |
Other long-term liabilities | | | — | | | | 2,615 | |
Total | | $ | 56,957 | | | $ | 3,530 | |
The components of lease cost were as follows:
| | | | | | | | |
| | Year Ended | | | Year Ended | |
| | December 31, 2023 | | | December 31, 2022 | |
| | (in thousands) | |
Operating lease cost: | | | | | | |
Operating lease cost | | $ | 17,192 | | | $ | 14,245 | |
Short-term lease cost | | | 201 | | | | 386 | |
Total operating lease cost | | $ | 17,393 | | | $ | 14,631 | |
Finance lease cost: | | | | | | |
Amortization expense | | $ | 976 | | | $ | 872 | |
Interest on lease liabilities | | | 416 | | | | 373 | |
Total finance lease cost | | $ | 1,392 | | | $ | 1,245 | |
Other information related to leases was as follows:
| | | | | | | | |
| | Year Ended | | | Year Ended | |
| | December 31, 2023 | | | December 31, 2022 | |
| | (in thousands, except lease term and discount rate) | |
Supplemental Cash Flows Information | | | | | | |
Cash paid for amounts included in the measurement of
lease liabilities: | | | | | | |
Operating cash flows for operating leases | | $ | 16,660 | | | $ | 13,417 | |
Operating cash flows for finance leases | | | 416 | | | | 335 | |
Financing cash flows for finance leases | | | 947 | | | | 518 | |
| | | | | | |
Operating lease assets obtained in exchange for lease obligations: | | $ | 312 | | | $ | 50,779 | |
Finance lease assets obtained in exchange for lease obligations: | | | — | | | | 4,795 | |
Non-cash (decrease) increase to operating lease assets due to remeasurement of lease liabilities: | | | (1,589 | ) | | | — | |
| | | | | | |
Weighted Average Remaining Lease Term | | | | | | |
Operating leases | | 5.2 years | | | 5.9 years | |
Finance leases | | 3.3 years | | | 4.2 years | |
Weighted Average Discount Rate | | | | | | |
Operating leases | | | 11.4 | % | | | 9.9 | % |
Finance leases | | | 10.4 | % | | | 10.4 | % |
Asset Retirement Obligation
Our asset retirement obligation (ARO) consists of a contractual requirement to remove the tenant improvements at the ATOM Facility in Thousand Oaks, California and restore the facility to a condition specified in the lease agreement. Although we assigned the ATOM Lease to FDB in connection with the closing of the sale of the ATOM Facility to FDB in April 2022, we have not received novation from the landlord. Therefore, the ARO associated with the ATOM Facility remains on our balance sheet. We recorded an estimate of the fair value of our ARO liability in other long-term liabilities and the ARO asset as a long-term asset in the period incurred. The fair value of the ARO asset is amortized over the lease term. The fair value of our ARO was estimated by discounting projected cash flows over the estimated life of the related assets using our credit adjusted risk-free rate. As of December 31, 2023 and December 31, 2022, the ARO asset and liability were not material.
On August 8, 2022 we announced a strategic reduction in workforce of approximately 20% to focus our activities as an organization centered on research and development. The workforce reduction included total restructuring charges of $6.0 million, comprised primarily of severance payments, wages for the 60-day notice period in accordance with the California Worker Adjustment and Retraining Notification (WARN) Act and continuing health care coverage for a period of time after separation. In most cases, the severance payments were paid as a lump sum in October 2022. Certain of the notified employees had employment agreements which provided for separation benefits in the form of salary continuation; these benefits were paid between October 2022 and November 2023, and there are no further payments required for this reduction in workforce as of December 31, 2023. All of the costs were cash expenditures and represented one-time termination benefits.
On November 1, 2023 we announced a strategic reduction in workforce of approximately 30%. The workforce reduction resulted in total restructuring charges of $6.7 million, comprised primarily of severance payments and wages for the 60-day notice period in accordance with the California WARN Act. In most cases, the severance payments were paid as a lump sum in January 2024. All of the costs are cash expenditures and represent one-time termination benefits.
The following is a summary of restructuring charges associated with the reductions in force for the periods presented:
| | | | | | | |
| Year Ended December 31, | | | Year Ended December 31, | |
| 2023 | | | 2022 | |
| (in thousands) | |
| | | | | |
Research and development expense | $ | 5,619 | | | $ | 2,544 | |
General and administrative expense | | 1,111 | | | | 3,420 | |
Total restructuring charges | $ | 6,730 | | | $ | 5,964 | |
The following restructuring liability activity was recorded in connection with the reduction in force for the year ended December 31, 2023, with all of the $4.9 million liability balance as of December 31, 2023 included within other current liabilities on the accompanying consolidated balance sheet:
| | | | | | | | | |
| Year ended December 31, | | | Year ended December 31, | |
| 2023 | | | 2022 | |
| (in thousands) | |
Liability balance, January 1 | $ | | 1,545 | | | $ | | — | |
Restructuring charges | | | 6,730 | | | | | 5,964 | |
Cash payments | | | (3,352 | ) | | | | (4,419 | ) |
Liability balance, December 31 | $ | | 4,923 | | | $ | | 1,545 | |
10.Commitments and Contingencies
MSK Agreements
In June 2015, the Company exercised the option andwe entered into an exclusive license agreement with MSK. In connection with the execution of the license agreement, the Company paid $4.5 million in cash to MSK which was recorded as research and development expense in our consolidated statements of operations and comprehensive loss.for three clinical stage T-cell therapies. We are required to make additional payments of up to $33.0 million to MSK based on achievement of specified regulatory and sales-related milestones, as well as mid-single-digit percentage tiered royalty payments based on future sales of products resulting from the development of the licensed product candidates, if any. In addition, under certain circumstances, we are required to make certain minimum annual royalty payments to MSK, which are creditable against earned royalties owed for the same annual period. We are also required to pay a low double-digit percentage of any consideration we receive for sublicensing the licensed rights. The license agreement expires on a product-by-product and country-by-country basis on the laterlatest of: (i) expiration of the last licensed patent rights related to each licensed product, (ii) expiration of any market exclusivity period granted by law with respect to each licensed product, and (iii) a specified number of years after the first commercial sale of the licensed product in each country. Upon expiration of the license agreement, Atarawe will retain non-exclusive rights to the licensed products.
In May and December 2018, we licensed additional technology from MSK. We are obligated to make additional milestone payments based on achievement of specified development, regulatory and sales-related milestones as well as mid-single-digit percentage tiered royalty payments based on future sales of products resulting from the development of the licensed product candidates, if any.
In March 2021, we amended and restated our license agreement with MSK to terminate our license to certain rights and license additional know-how rights not otherwise covered by our existing agreements.
QIMR Berghofer Agreements –
In October 2015, the Companywe entered into an exclusive license agreement and a research and development collaboration agreement with QIMR Berghofer.
Under the terms of the license agreement, the Companywe obtained an exclusive, worldwide license to develop and commercialize allogeneic cytotoxic T-lymphocyte (“CTL”)T-cell therapy programs utilizing technology and know-how developed by QIMR Berghofer. In consideration for the exclusive license, we paid $3.0 million in cash to QIMR Berghofer, which was recorded as research and development expense in our consolidated statements of operations and comprehensive loss in the fourth quarter of 2015. In September 2016, the exclusive license agreement and research and development collaboration agreement were amended and restated. Under the amended and restated agreements, we obtained an exclusive, worldwide license to develop and commercialize additional CTLT-cell programs, as well as the option to license additional technology that we exercised in exchange for $3.3 million in cash, which was recorded asJune 2018. We further amended and restated our license agreement and research and development expensecollaboration agreements with QIMR Berghofer in August 2019, August 2020 and December 2021, in each case, to terminate our consolidated statement of operations and comprehensive loss in the third quarter of 2016 and paid in October 2016. The amended and restatedlicense to certain rights. Our current license agreement also provides for various milestone and royalty payments to QIMR Berghofer based on future product sales, if any.
Under the terms of the amended and restatedour current research and development collaboration agreement, we are also required to reimburse the cost of agreed-upon development activities related to programs developed under the collaboration. These payments are expensed as incurredon a straight-line basis over the related development periods and resulted in research and development expense of $2.9 million, $1.6 million and $0.2 million for the years ended December 31, 2017, 2016 and 2015, respectively.periods. The agreement also provides for various milestone payments to QIMR Berghofer based on achievement of certain developmental and regulatory milestones.
Other In-license and Collaboration Agreements
From time to time, we have entered into other license and collaboration agreements with other parties. For example, we licensed rights related to our MSK-partnered next-generation CAR T programs from the National Institutes of Health in December 2018.
Milestones and royalties under each of the above agreements are contingent upon future events and will be recorded as expense when it is probable that the underlying milestones will beare achieved or royalties are due.earned. Sales related milestone and royalty costs related to Ebvallo are recorded in cost of commercialization revenue, whereas regulatory milestone costs are recorded in research and development expense. As of December 31, 20172023 and 2016,2022, there were no material outstanding obligations for milestones and royalties to MSKunder our in-license and QIMR Berghofer.collaboration agreements.
Amgen License Agreements – CRL Manufacturing Agreement
In September 2012,December 2019, we entered into license agreementsa Commercial Manufacturing Services Agreement (the CRL MSA) with Amgen,Cognate BioServices, Inc., which was acquired by Charles River Laboratories Inc. (CRL) in March 2021.
Pursuant to the CRL MSA, CRL provides manufacturing services for several molecular programs, including PINTA745, ATA842our product and STM434. certain of our product candidates. We further amended the CRL MSA to extend the term until the earlier of March 31, 2024 or upon receipt of certain batches of our product and product candidates. We are currently in negotiations with CRL for a new commercial drug product supply agreement to be effective upon the expiration of the CRL MSA. However, there can be no assurance that we will be able to enter into a new commercial drug product supply agreement with CRL on terms favorable or acceptable to us, or at all. If we are unable to enter into a new commercial drug product supply agreement or extend the CRL MSA, we may need to identify alternative sources of drug product supply.
Fujifilm Master Services and Supply Agreement
In December 2015,January 2022, we announcedentered into the suspensionFujifilm MSA, which became effective upon the closing of further developmentthe sale of PINTA745the ATOM Facility on April 4, 2022 and could extend for up to ten years. Pursuant to the Fujifilm MSA, FDB will supply us with specified quantities of our cell therapy products and product candidates, manufactured in June 2016, we returnedaccordance with cGMP standards. We have certain non-cancellable minimum commitments to purchase products and services over the rights relatedfirst five years of the Fujifilm MSA. The Fujifilm MSA does not obligate us to thispurchase products and the ATA842 program to Amgen. In October 2017, we returned all remaining rights under the license agreements to Amgen.product candidates exclusively from FDB.
7.
| Commitments and Contingencies
|
License and Collaboration Agreements
Potential payments related to our license and collaboration agreements, including milestone and royalty payments, are detailed in Note 6.
Other Research, Development and DevelopmentManufacturing Agreements
We may also enter into other contracts in the normal course of business with clinical research organizations for clinical trials, with contract manufacturing organizationsCMOs for product, product candidates and clinical supplies, and with other vendors for pre-clinicalpreclinical studies, supplies and other services and products for our operating purposes. These contracts generally provide for termination on notice, with the exception of potential termination charges related to one of our contract manufacturing agreements in the event certain minimum purchase volumes are not met.notice. As of December 31, 20172023 and 2016,December 31, 2022, there were no material amounts accrued related to contract termination chargescharges.
Minimum Commitments
The non-cancellable minimum commitments for minimum purchase volumes not being met.
Leases
We leaseproducts and services, subject to agreements with a term of greater than one year with clinical research organizations and CMOs, excluding those recognized on our corporate headquarters in South San Francisco, California under a non-cancellable lease agreement that expires in April 2021. In connection with the lease, we are required to maintain a letter of credit in the amount of $0.2 million to the landlord, which expires and is renewed every 12 months, and is classified as restricted cash in our consolidated balance sheet. We also lease office space in Westlake Village, California under a lease agreement that expires in April 2019. Future minimum payments under our operating and capital leasessheet, as of December 31, 2017 were as follows:2023 are set forth below:
| | Operating Leases | | | | Capital Leases | |
Years Ending December 31, | | (in thousands) | |
2018 | | $ | 1,979 | | | | $ | 546 | |
2019 | | | 823 | | | | | 498 | |
2020 | | | 613 | | | | | 160 | |
2021 | | | 259 | | | | | — | |
2022 | | | — | | | | | — | |
Thereafter | | | — | | | | | — | |
Total minimum payments | | $ | 3,674 | | | | $ | 1,204 | |
Less: amount representing interest | | | | | | | | 128 | |
Present value of capital lease obligations | | | | | | | | 1,076 | |
Less: current portion | | | | | | | | 463 | |
Capital lease obligation, net of current portion | | | | | | | $ | 613 | |
| | | | |
Calendar Year | | Remaining Minimum Commitment as of December 31, 2023 | |
| | (in thousands) | |
2024 | | | 14,085 | |
2025 | | | 13,308 | |
2026 | | | 9,605 | |
2027 | | | 3,388 | |
Total | | $ | 40,386 | |
Rent expenses under operating leasesWe have incurred $19.4 million and $14.2 million against such minimum commitments for the years ended December 31 2017, 2016, 2023 and 2015 were $1.4 million, $1.2 million and $0.4 million,2022, respectively.
Financing Obligation—Build-to-Suit Lease
In February 2017, we entered into a lease agreement for approximately 90,580 square feet of office, lab and cellular therapy manufacturing space in Thousand Oaks, California. The initial 15-year term of the lease commences upon the substantial completion of landlord’s work as defined under the agreement. The contractual obligations during the initial term are $16.4 million in aggregate. We have the option to extend the lease for two additional periods of ten and nine years, respectively, after the initial term. In connection with the lease, we were required to issue a letter of credit in the amount of $1.2 million to the landlord, which is recorded as long-term restricted cash in our consolidated balance sheet.
Based on the terms of the lease agreement and due to our involvement in certain aspects of the construction, we have been deemed the owner of the building (for accounting purposes only) during the construction period in accordance with GAAP. Under this build-to-suit lease arrangement, we recognize construction in progress based on all construction costs incurred by both us and the landlord. We also recognize a financing obligation equal to all costs funded by the landlord.
As of December 31, 2017,2023 and December 31, 2022, we have recorded $9.9accrued $11.2 million of constructionand $9.2 million in progress relatingresearch and development expenses related to landlord’s costs of the building incurred through that date, and have recorded a corresponding long-term financing obligation for the same amount included in the long-term liabilities in our consolidated balance sheets. In addition, we have recorded $25.0 million of construction in progress for construction costs incurred by us and $0.3 million of capitalized interest during the construction period through December 31, 2017. Further, we recorded ground lease expense of $0.3 million for the year ended December 31, 2017, in our consolidated statement of operations and comprehensive loss, representing the estimated cost of renting the land during the construction period. Ground lease expense for the years ended December 31, 2016 and 2015 was zero.minimum purchase commitments.
Future minimum lease payments, under the Company’s facility lease financing obligations as of December 31, 2017 were as follows:
Years Ending December 31, | | (in thousands) | |
2018 | | $ | 372 | |
2019 | | | 911 | |
2020 | | | 938 | |
2021 | | | 966 | |
2022 | | | 996 | |
Thereafter | | | 12,204 | |
Total minimum payments | | $ | 16,387 | |
Asset Retirement Obligation
The Company recognizes its estimate of the fair value of its ARO in long-term liabilities in the period incurred. The fair value of the ARO is also capitalized as construction in progress. The fair value of our ARO was estimated by discounting projected cash flows over the estimated life of the related assets using our credit adjusted risk-free rate. The Company’s ARO consists of a contractual requirement to remove the tenant improvements at our manufacturing facility in Thousand Oaks, California and restore the facility to a condition specified in the lease agreement.
The following table presents the activity for our ARO liabilities:
| | (in thousands) | |
Balance as of December 31, 2016 | | $ | — | |
Liabilities incurred during the year | | | 580 | |
Balance as of December 31, 2017 | | $ | 580 | |
Indemnification Agreements
In the normal course of business, we enter into contracts and agreements that contain a variety of representations and warranties and provide for indemnification for certain liabilities. The exposure under these agreements is unknown because it involves claims that may be made against us in the future but have not yet been made. To date, we have not paid any claims or been required to defend any action related to our indemnification obligations. However, we may record charges in the future as a result of these indemnification obligations. We also have indemnification obligations to our directors and executive officers for specified events or occurrences, subject to some limits, while they are serving at our request in such capacities. There have been no claims to date and we believeconsider the fair value of these indemnification agreements isto be minimal. Accordingly, we have not recorded anydid not record liabilities for these agreements as of December 31, 20172023 and 2016.2022.
Contingencies
From time to time, we may be involved in legal proceedings, as well as demands, claims and threatened litigation, which arise in the normal course of our business or otherwise. The ultimate outcome of any litigation is uncertain and unfavorable outcomes could have a negative impact on our results of operations and financial condition. Regardless of outcome, litigation can have an adverse impact on us because of the defense costs, diversion of management resources and other factors. We are not currently involved in any material legal proceedings.
Our authorized capital stock consists of 520,000,000 shares, all with a par value of $0.0001$0.0001 per share, of which 500,000,000 shares are designated as common stock and 20,000,000 shares are designated as preferred stock. There were no shares of preferred stock outstanding as of December 31, 20172023 and 2016.2022.
Equity Offerings
As part of our July 2019 underwritten public offering, we issued and sold pre-funded warrants to purchase 2,945,026 shares of common stock in an underwritten public offering pursuant to a shelf registration on Form S-3.
Each pre-funded warrant entitles the holder to purchase one share of common stock at an exercise price of $0.0001 per share and expires seven years from the date of issuance. These warrants were recorded as a component of stockholders’ equity within additional paid-in capital. Per the terms of the warrant agreement, a holder of the outstanding warrants is not entitled to exercise any portion of any pre-funded warrant if, upon exercise of the warrant, the holder’s ownership (together with its affiliates) of our common stock or combined voting power of our securities beneficially owned by such holder (together with its affiliates) would exceed 9.99% after giving effect to the exercise (the Maximum Ownership Percentage). Upon at least 61 days’ prior notice to us by the holder, any holder may increase or decrease the Maximum Ownership Percentage to any other percentage not to exceed 19.99%. During the year ended December 31, 2023, 361,260 of the July 2019 pre-funded warrants were exercised, and, as of December 31, 2023, pre-funded warrants to purchase 2,527,266 shares of our common stock from the July 2019 offering were outstanding.
In May 2020, we issued and sold pre-funded warrants to purchase 2,866,961 shares of common stock at a public offering price of $11.3199 per warrant in an underwritten public offering pursuant to a shelf registration on Form S-3.
In December 2020, we issued and sold pre-funded warrants to purchase 2,040,816 shares of common stock at a public offering price of $24.4999 per warrant in an underwritten public offering pursuant to a shelf registration on Form S-3.
The terms of the pre-funded warrants issued and sold as part of the 2020 public offerings were similar to those issued and sold in 2019. During the year ended December 31, 2023, 1,898,578 and 656,107 of the May 2020 and December 2020 pre-funded warrants, respectively, were exercised. As of December 31, 2023, 968,383 and 1,384,709 of the pre-funded warrants to purchase shares of our common stock issued and sold as part of the May 2020 and December 2020 underwritten public offerings, respectively, were outstanding.
In January 2024, we issued and sold pre-funded warrants to purchase 27,272,727 shares of common stock at a price of $0.5499 per warrant in a registered direct offering pursuant to a shelf registration on Form S-3. The gross proceeds from this sale were $15.0 million, resulting in net proceeds of $14.8 million, after deducting underwriting discounts and commissions and estimated offering expenses payable by us.
Each of the January 2024 pre-funded warrants issued entitles the holder to purchase one share of common stock at an exercise price of $0.0001 per share, with no expiration date. These warrants were recorded as a component of stockholders’ equity within additional paid-in capital. Per the terms of the warrant agreement, a holder of the outstanding warrants is not entitled to exercise any portion of any pre-funded warrant if, upon exercise of the warrant, the holder’s ownership (together with its affiliates) of our common stock or combined voting power of our securities beneficially owned by such holder (together with its affiliates) would exceed 9.99% after giving effect to the exercise (Maximum Ownership Percentage). Upon at least 61 days’ prior notice to us by the holder, any holder may increase or decrease the Maximum Ownership Percentage to any other percentage not to exceed 19.99%.
ATM FacilityFacilities
In March 2017,the past three years, we have entered into atwo separate sales agreement (the “ATM facility”)agreements with Cowen under which we may offer and sell,Company, LLC (Cowen): in November 2021 (the 2021 ATM Facility) and in November 2023 (the 2023 ATM Facility). Each ATM facility provides or provided for the sale, in our sole discretion, of shares of our common stock having an aggregate offering price of up to $75.0$100.0 million, through Cowen, as our sales agent. We will pay Cowenfiled a commissionregistration statement on Form S-3 registering the offer and sale of up to 3.0%these shares under the Securities Act (the 2023 Registration Statement). Upon the effectiveness of the gross2023 Registration Statement, the 2021 ATM Facility was terminated, and no further sales proceeds of any common stock soldcan be made under the 2021 ATM facility.Facility. The issuance and sale of these shares by us pursuant to the ATM facilityfacilities are deemed “at the market” offerings and are availabledefined in Rule 415 under the Securities Act of 1933, as amended.amended (the Securities Act), and were registered under the Securities Act. Commissions of up to 3.0% are due on the gross sales proceeds of the common stock sold under each ATM facility.
During the fiscal year ended December 31, 2017,2022, we sold an aggregate of 1,349,8651,618,672 shares of common stock under the ATM facility,facilities, at an average price of approximately $14.82$13.84 per share, for gross proceeds of $20.0$22.4 million and net proceeds of $19.2$22.0 million, after deducting commissions and other offering expenses. expenses payable by us.
During the year ended December 31, 2023, we sold an aggregate of 3,038,432 shares of common stock under the ATM facilities, at an average price of $0.83 per share, for gross proceeds of $2.5 million and net proceeds of $2.2 million, after deducting commissions and other offering expenses payable by us. Approximately $0.1 million of the $2.2 million net proceeds were received on January 2, 2024.
As of December 31, 2017, $55.02023, we had $98.2 million of common stock remainedremaining and available to be sold under this facility,the 2023 ATM Facility.
From January 1, 2024 through March 15, 2024, we sold an additional 12,321,365 shares of common stock under the 2023 ATM Facility, at an average price of $0.77 per share, for gross proceeds of $9.5 million and net proceeds of $9.3 million, after deducting commissions and other offering expenses payable by us. As of March 15, 2024, we had $88.7 million of common stock remaining and available to be sold under the 2023 ATM Facility, subject to certain conditions as specified in the agreement.
Restricted Stock Awards121
In August 2012, in connection with our formation, our CEO purchased 1,066,154 post-recap, post-split shares of restricted common stock at a nominal per share purchase price. The shares were issued subject to certain vesting conditions, restrictions on transfer and a Company right of repurchase of any unvested share at their original purchase price. The combined grant date intrinsic value for this award was $1.7 million.
In March 2013, an Atara employee purchased 269,230 post-recap, post-split shares of restricted common stock for $0.3 million. The shares were issued under our 2012 Equity Incentive Plan and were subject to certain vesting conditions, restrictions on transfer and a Company right of repurchase of any unvested shares at their original purchase price.Plans
The amounts paid for both RSAs were initially recorded as other long-term liabilities. As the shares vested, we reclassified liabilities to equity. As of December 31, 2016, all of these shares had vested and are reported as common stock shares outstanding in the consolidated financial statements.
There were no grants of RSAs in the years ended December 31, 2017, 2016 and 2015. Stock-based compensation expense related to the RSAs is recorded using the accelerated graded vesting model and was none, $0.2 million and $0.8 million for the years ended December 31, 2017, 2016 and 2015, respectively.
Equity Incentive Plan
In March 2014, we adopted the 2014 Equity Incentive Plan (the “2014 EIP”)(2014 EIP), which was amended and restated on October 15, 2014 upon the pricing of our IPO.initial public offering (IPO).
The 2014 EIP provides for annual increases in the number of shares available for issuance thereunder on the first business day of each fiscal year, beginning with 2015 and ending in 2024, equal to five percent of the number of shares of the Company’s common stock outstanding as of such date or a lesser number of shares as determined by our board of directors.
Under the terms of the 2014 EIP, we may grant stock options, RSAs and RSUs to employees, directors, consultants and other service providers. RSUs typically require settlement bygenerally vest over two to four years. The fair value of RSUs, including those with performance conditions, is determined as the earlier of seven years fromclosing stock price on the date of grant or the service termination (or, for RSUsgrant. The 2014 EIP expires March 31, 2024, after which no new awards can be granted. All awards granted prior to the 2014 EIP expiration continue to remain outstanding and governed in accordance with the rules set forth in the 2014 EIP and the terms of the associated grant notice.
In February 2014, two years following2018, we adopted the service termination date). 2018 Inducement Plan (Inducement Plan), under which we may grant options, stock appreciation rights, RSAs and RSUs to new employees. In November 2020, September 2021 and June 2022, we amended the Inducement Plan to reserve an additional 1,500,000 shares of the Company’s common stock for issuance under the Inducement Plan in each case.
Stock options are granted with exercise prices at prices no less than 100%100% of the estimated fair value of the shares on the date of grant as determined by the board of directors, provided, however, that the exercise price of an option granted to a 10% shareholder cannot be less than 110% of the estimated fair value of the shares on the date of grant. The estimated fair value of the shares is generally equal to the closing market price of the Company’s common stock on the measurement date. Options granted to employees and non-employees generally vest over two to four years and expire in seven years. to ten years.
In 2022, we granted performance-based stock options to certain of our employees that provide for the issuance of stock options to purchase common stock if specified Company performance criteria related to business development initiatives are achieved. The number of performance-based awards that ultimately vests depends upon if performance criteria are achieved within a specified timeline, as well as the employee’s continuous service, as defined in the 2014 EIP, through the date of vesting. None of the performance criteria were achieved and the awards were subsequently forfeited.
As of December 31, 2017,2023, a total of 10,214,17420,326,356 shares of common stock were reserved for issuance under the 2014 Plan,EIP, of which 3,557,0415,714,772 shares were available for future grant and 6,657,13314,611,584 shares were subject to outstanding options and RSUs, including performance-based awards. As of December 31, 2023, 4,773,147 shares of common stock were reserved for issuance under the Inducement Plan, of which 2,397,366 shares were available for future grant and 2,375,781 shares were subject to outstanding options and RSUs.
Restricted Stock Units
The following is a summary of RSU activity under our 2014 EIP and AwardsInducement Plan:
| | | | | | | | |
| | RSUs | |
| | Shares | | | Weighted
Average
Grant Date
Fair Value | |
Balance as of December 31, 2022 | | | 6,708,608 | | | $ | 10.61 | |
Granted | | | 5,635,916 | | | $ | 3.57 | |
Forfeited | | | (2,388,808 | ) | | $ | 7.91 | |
Vested | | | (3,688,003 | ) | | $ | 8.89 | |
Balance as of December 31, 2023 | | | 6,267,713 | | | $ | 6.32 | |
The weighted average grant date fair value of RSUs granted during the years ended December 31, 2017, 20162023 and 20152022 was $15.07, $17.83$3.57 and $25.15,$8.26, respectively. The estimated fair value of RSUs that vested in the years ended December 31, 2023 and 2022 was $32.8 million and $34.4 million, respectively. As of December 31, 2017,2023, there was $20.6$35.9 million of unrecognized stock-based compensation expense related to RSUs that is expected to be recognized over a weighted average period of 2.62.0 years. The aggregate intrinsic value of the RSUs outstanding as of December 31, 20172023 was $30.8$3.2 million.
The following is a summary of RSU activity under our 2014 EIP:
| | RSUs | |
| | Shares | | | Weighted Average Grant Date Fair Value | |
Unvested as of December 31, 2016 | | | 1,286,262 | | | $ | 16.61 | |
Granted | | | 782,413 | | | | 15.07 | |
Forfeited | | | (64,313 | ) | | | 15.33 | |
Vested | | | (319,362 | ) | | | 11.57 | |
Unvested as of December 31, 2017 | | | 1,685,000 | | | $ | 16.90 | |
Vested and unreleased | | | 17,485 | | | | | |
Outstanding as of December 31, 2017 | | | 1,702,485 | | | | | |
122
Under our RSU net settlement procedures, for some of the RSUs granted to our employees, we withhold shares at settlement to cover the minimumestimated payroll withholding tax obligations. During 2017,2023, we settled 327,2823,688,003 shares underlying RSUs, of which 52,62451,244 shares underlying RSUs were net settled by withholding 22,27418,203 shares. The value of the shares underlying RSUs withheld was $0.4$0.1 million, based on the closing price of our common stock on the settlement date. During 2016,2022, we settled 204,6112,247,296 shares underlying RSUs, of which 13,573114,444 shares underlying RSUs were net settled by withholding 5,22243,524 shares. The value of the shares underlying RSUs withheld was $0.1$0.6 million, based on the closing price of our common stock on the settlement date. The value of RSUs withheld in each period was remitted to the appropriate taxing authorities and has been reflected as a financing activity in our consolidated statements of cash flows.
Stock Options
The following is a summary of stock option activity under our 2014 EIP:EIP and Inducement Plan:
| | Shares | | | Weighted Average Exercise Price | | | Weighted Average Remaining Contractual Term (Years) | | | Aggregate Intrinsic Value (in thousands) | |
Outstanding as of December 31, 2016 | | | 3,733,847 | | | $ | 24.14 | | | | | | | | | |
Granted | | | 1,694,000 | | | | 15.79 | | | | | | | | | |
Exercised | | | (60,125 | ) | | | 12.37 | | | | | | | | | |
Forfeited or expired | | | (413,074 | ) | | | 23.77 | | | | | | | | | |
Outstanding as of December 31, 2017 | | | 4,954,648 | | | $ | 21.46 | | | | 5.21 | | | $ | 7,433 | |
Vested and expected to vest as of December 31, 2017 | | | 4,954,648 | | | $ | 21.46 | | | | 5.21 | | | $ | 7,433 | |
Exercisable as of December 31, 2017 | | | 2,030,454 | | | $ | 24.06 | | | | 4.39 | | | $ | 2,465 | |
| | | | | | | | | | | | | | | | |
| | Shares | | | Weighted Average Exercise Price | | | Weighted Average
Remaining
Contractual
Term (Years) | | | Aggregate
Intrinsic Value
(in thousands) | |
Balance as of December 31, 2022 | | | 10,645,555 | | | $ | 16.88 | | | | 6.4 | | | $ | 42 | |
Granted | | | 4,693,897 | | | | 3.64 | | | | | | | |
Exercised | | | — | | | | — | | | | | | | |
Forfeited or expired | | | (4,619,801 | ) | | | 15.60 | | | | | | | |
Balance as of December 31, 2023 | | | 10,719,651 | | | $ | 11.63 | | | | 6.5 | | | $ | — | |
Vested and expected to vest as of
December 31, 2023 | | | 10,719,651 | | | $ | 11.63 | | | | 6.5 | | | $ | — | |
Exercisable as of December 31, 2023 | | | 6,389,752 | | | $ | 15.40 | | | | 5.1 | | | $ | — | |
Aggregate intrinsic value represents the difference between the closing stock price of our common stock on December 31, 20172023 and the exercise price of outstanding, in-the-money options. As of December 31, 2017,2023, there was $30.3$16.6 million of unrecognized stock-based compensation expense related to stock options that is expected to be recognized over a weighted average period of 2.61.9 years. This excludes unrecognized stock-based compensation expense for performance-based stock options that were deemed not probable of vesting in accordance with U.S. GAAP.
OptionsNo options for 60,125, 18,947 and 23,822 shares of our common stock were exercised during the yearsyear ended December 31, 2017, 2016 and 2015,2023. Options for 15,989 shares of our common stock were exercised during the year ended December 31, 2022, with an intrinsic value of $0.2 million, $0.2 million and $0.6 million, respectively.$0.1 million. As we believe it is more likely than not that no stock option related tax benefits will be realized, we do notnot record any net tax benefits related to exercised options.
The fair value of each option issued was estimated at the date of grant using the Black-Scholes valuation model. The following table summarizes the weighted-average assumptions used as inputs to the Black-Scholes model and resulting weighted-average grant date fair values of stock options granted to employees during the periods indicated:
| | | Year ended December 31, | | | Year ended December 31, | |
| | 2017 | | | 2016 | | | 2015 | | | 2023 | | | 2022 | |
Assumptions: | | | | | | | | | | | | | | | | | |
Expected term (years) | | | 4.5 | | | | 4.5 | | | | 4.5 | | | | 5.80 | | | | 6.0 | |
Expected volatility | | | 71.3 | % | | | 69.0 | % | | | 72.4 | % | | | 83.9 | % | | | 73.2 | % |
Risk-free interest rate | | | 1.9 | % | | | 1.3 | % | | | 1.6 | % | | | 4.2 | % | | | 2.1 | % |
Expected dividend yield | | | 0.0 | % | | | 0.0 | % | | | 0.0 | % | | | 0.0 | % | | | 0.0 | % |
Fair Value: | | | | | | | | | | | | | | | | | |
Weighted-average estimated grant date fair value per share | | $ | 9.01 | | | $ | 11.02 | | | $ | 16.63 | | | $ | 2.62 | | | $ | 5.88 | |
Options granted | | | 1,694,000 | | | | 966,250 | | | | 2,601,174 | | | | 4,693,897 | | | | 3,615,971 | |
Total estimated grant date fair value | | $ | 15,263,000 | | | $ | 10,648,000 | | | $ | 43,258,000 | | | $ | 12,298,010 | | | $ | 21,261,909 | |
The estimated fair value of stock options that vested in the years ended December 31, 2017, 20162023 and 20152022 was $14.0 million, $14.0$17.0 million and $2.9$23.2 million, respectively.
Employee Stock Purchase Plan
In May 2014, we adopted the 2014 Employee Stock Purchase Plan (“2014 ESPP”), which became effective on October 15, 2014 upon the pricing of our IPO. The 2014 ESPP permits eligible employees to purchase common stock at a discount through payroll deductions during defined offering periods. Eligible employees can purchase shares of the Company’s common stock at 85%85% of the lower of the fair market value of the common stock at (i) the beginning of a 12-monththe offering period or (ii) at the end of one of the two related 6-month purchase periods. No participant in the 2014 ESPP may be issued or transferred shares of common stock valued at more than $25,000 per calendar year. On June 1, 2016, the first offering under the 2014 ESPP commenced,period. We recorded $0.7 million and the Company recorded $0.4 million of expense in the year ended December 31, 2016. A total of 22,844 shares were purchased at the end of the first purchase period on November 30, 2016. The 2017 offering period commenced on June 1, 2017 and will end on May 31, 2018. The Company recorded $0.6$1.1 million of expense related to the 2014 ESPP in the yearyears ended December 31, 2017.2023 and 2022, respectively. A total of 81,922896,246 and 417,081 shares were purchased under the ESPP during the yearyears ended December 31, 2017.2023 and 2022, respectively.
As of December 31, 2017,2023, there was $0.2$0.1 million of unrecognized stock-based compensation expense related to the ESPP that is expected to be recognized by the end of second quarter of 2018.2024.
The 2014 ESPP provides for annual increases in the number of shares available for issuance thereunder on the first business day of each fiscal year, beginning with 2015 and ending in 2024, equal to the lower of (i) one percent of the number of shares of our common stock outstanding as of such date, (ii) 230,769 shares of our common stock, or (iii) a lesser number of shares as determined by our board of directors. As of December 31, 2017,2023, there were 789,6692,279,049 shares available for purchaseauthorized under the 2014 ESPP.
Reserved Shares
Options issued outside the 2014 EIP
During the year ended December 31, 2017, we granted 275,000 options, at a weighted average exercise price of $13.96 per share, outside of our 2014 EIP. These options have terms similar to the options granted under the 2014 EIP. The weighted average grant date fair value of such grants was $2.2 million. No options were granted outside the 2014 EIP during the years ended December 31, 2016 and 2015. As of December 31, 2017, there was $2.0 million of unrecognized stock-based compensation expense related to options issued outside the 2014 EIP that is expected to be recognized over a weighted average period of 3.5 years. The aggregate intrinsic value of such options as of December 31, 2017 was $1.1 million.
The following shares of common stock were reserved for future issuance under our equity incentive plans as of December 31, 2017: 2023:
| | | | |
|
| Total Shares Reserved |
|
2014 Equity Incentive Plan |
| 10,214,174
| 20,326,356 |
|
2018 Inducement Plan |
|
| 4,773,147 |
|
2014 Employee Stock Purchase Plan |
| 789,669
| 10,593 |
|
Options issued outside the 2014 EIP
| | 275,000
| |
Total reserved shares of common stock |
| 11,278,843
| 25,110,096 |
|
Stock-based Compensation Expense
TotalThe following is a summary of stock-based compensation expense related to all employee and non-employee awards was as follows:for the periods presented:
| | | | | | | | |
| | Year Ended December 31, | |
| | 2023 | | | 2022 | |
| | (in thousands) | |
Research and development | | $ | 26,529 | | | $ | 31,363 | |
General and administrative | | | 18,857 | | | | 22,475 | |
Total stock-based compensation expense | | $ | 45,386 | | | $ | 53,838 | |
| | Year Ended December 31, | |
| | 2017 | | | 2016 | | | 2015 | |
| | (in thousands) | |
Research and development | | $ | 8,778 | | | $ | 7,612 | | | $ | 4,822 | |
General and administrative | | | 14,322 | | | | 9,172 | | | | 5,429 | |
Total stock-based compensation expense | | $ | 23,100 | | | $ | 16,784 | | | $ | 10,251 | |
Losses before provision for income taxes were as follows in each period presented:
| | | | | | | | |
| | Year Ended December 31, | |
| | 2023 | | | 2022 | |
| | (in thousands) | |
United States | | $ | (276,360 | ) | | $ | (228,395 | ) |
Foreign | | | 249 | | | | 105 | |
Total loss before provision for income taxes | | $ | (276,111 | ) | | $ | (228,290 | ) |
| | Year Ended December 31, | |
| | 2017 | | | 2016 | | | 2015 | |
| | (in thousands) | |
United States | | $ | (12,894 | ) | | $ | (48,795 | ) | | $ | (57,230 | ) |
Foreign | | | (106,611 | ) | | | (30,244 | ) | | | — | |
Total loss before provision for income taxes | | $ | (119,505 | ) | | $ | (79,039 | ) | | $ | (57,230 | ) |
The components of provision for income tax provision (benefit)taxes were as follows in each period presented:
| | | | | | | | |
| | Year Ended December 31, | |
| | 2023 | | | 2022 | |
| | (in thousands) | |
Current provision for income taxes: | | | | | | |
State | | $ | 1 | | | $ | — | |
Foreign | | | 14 | | | | 12 | |
Total current provision for income taxes | | $ | 15 | | | $ | 12 | |
| | Year Ended December 31, | |
| | 2017 | | | 2016 | | | 2015 | |
Current provision (benefit) for income taxes: | | (in thousands) | |
Federal | | $ | (14 | ) | | $ | — | | | $ | (1 | ) |
State | | | — | | | | 10 | | | | (8 | ) |
Total current provision (benefit) for income taxes | | $ | (14 | ) | | $ | 10 | | | $ | (9 | ) |
124
A reconciliation of statutory tax rates to effective tax rates were as follows in each of the periods presented:
| | | Year Ended December 31, | | | Year Ended December 31, | |
| | 2017 | | | 2016 | | | 2015 | | | 2023 | | | 2022 | |
Federal income taxes at statutory rate | | | 34.0 | % | | | 34.0 | % | | | 34.0 | % | | | 21.0 | % | | | 21.0 | % |
Impact of stock compensation | | | (1.5 | %) | | | (1.3 | %) | | | (0.6 | %) |
Foreign income tax at different rate | | | (30.3 | %) | | | (13.0 | %) | | | — | |
Impact of US tax reform | | | (11.3 | %) | | | — | | | | — | |
Research tax credits | | | | 4.3 | % | | | 7.8 | % |
Stock-based compensation | | | | (4.5 | %) | | | (3.8 | %) |
Other | | | (3.8 | %) | | | (0.9 | %) | | | — | | | | (0.4 | %) | | | (0.2 | %) |
Change in valuation allowance | | | 12.9 | % | | | (18.8 | %) | | | (33.4 | %) | | | (20.4 | %) | | | (24.8 | %) |
Effective tax rate | | | 0.0 | % | | | 0.0 | % | | | 0.0 | % | | | 0.0 | % | | | 0.0 | % |
In the first quarter of 2017, the Company adopted ASU 2016-09, which requires excess tax benefits and tax deficiencies generated by the settlement of share-based awards to be recognized as part of the income tax provision. The adoption of ASU 2016-09 did not have an impact on our balance sheet, results of operations, cash flows or statement of stockholders’ equity because we have a full valuation allowance on our deferred tax assets. Upon adoption, the Company recognized the previously unrecognized excess tax benefits. The previously unrecognized excess tax effects were recorded as a deferred tax asset, which was fully offset by a valuation allowance. Upon adoption, the Company recorded additional federal and state net operating losses of $10.5 million. These net operating losses are offset with a valuation allowance. Beginning in 2017, the impact of excess tax benefits and tax deficiencies are included in the Impact of Stock Compensation tax rate line.
Deferred tax assets and liabilities reflect the net tax effecteffects of (a) temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. purposes and (b) operating loss and tax credit carryforwards. Significant components of our deferred tax assets and liabilities were as follows asfor each of the dates indicated:presented:
| | | | | | | | |
| | As of December 31, | |
| | 2023 | | | 2022 | |
| | (in thousands) | |
Deferred tax assets: | | | | | | |
Net operating loss carryforwards | | $ | 323,581 | | | $ | 302,591 | |
Capitalized research expenses | | | 72,229 | | | | 50,522 | |
Tax credit carryforwards | | | 30,580 | | | | 19,427 | |
Stock-based compensation | | | 14,033 | | | | 19,201 | |
Deferred revenue | | | 17,018 | | | | 10,069 | |
Operating lease liabilities | | | 12,035 | | | | 15,857 | |
License fees | | | 5,926 | | | | 6,877 | |
Other | | | 11,187 | | | | 13,169 | |
Total deferred tax assets | | | 486,589 | | | | 437,713 | |
Valuation allowance | | | (474,982 | ) | | | (422,493 | ) |
Total deferred tax assets | | | 11,607 | | | | 15,220 | |
| | | | | | |
Deferred tax liabilities: | | | | | | |
Operating lease assets | | | (11,607 | ) | | | (15,220 | ) |
Total deferred tax liabilities | | | (11,607 | ) | | | (15,220 | ) |
| | | | | | |
Net deferred tax assets (liabilities) | | $ | — | | | $ | — | |
Beginning January 1, 2022, the Tax Cuts and Jobs Act (the Tax Act) eliminated the option to deduct research and development expenditures in the current year and requires taxpayers to capitalize such expenses pursuant to Internal Revenue Code (IRC) Section 174. The capitalized expenses are amortized over a 5-year period for domestic expenses and a 15-year period for foreign expenses. As a result of this provision of the Tax Act, deferred tax assets related to capitalized research expenses pursuant to IRC Section 174 increased by $21.7 million and $43.7 million for the years ended December 31, 2023 and 2022, respectively. These increases were partially offset by amortization on research expenses capitalized in prior years.
| | As of December 31, | |
| | 2017 | | | 2016 | |
Deferred tax assets: | | (in thousands) | |
Net operating losses | | $ | 31,110 | | | $ | 36,911 | |
License fees | | | 2,940 | | | | 5,800 | |
Stock-based compensation | | | 10,489 | | | | 9,600 | |
Legal fees | | | 1,490 | | | | 1,933 | |
Other | | | 1,268 | | | | 1,643 | |
Total deferred tax assets | | | 47,297 | | | | 55,887 | |
Valuation allowance | | | (47,297 | ) | | | (55,887 | ) |
Net deferred tax assets | | $ | — | | | $ | — | |
Our tax credit carryforwards increased by $11.2 million, as compared to 2022, due to research and development and orphan drug credits generated during the current year.
We recognize deferred income taxes for temporary differences between the basis of assets and liabilities for financial statement and income tax purposes, as well as for tax attribute carryforwards. We regularly evaluate the positive and negative evidence in determining the realizability of our deferred tax assets. Based upon the weight of available evidence, which includes our historical operating performance and reported cumulative net losses since inception, we maintained a full valuation allowance on the net deferred tax assets as of December 31, 20172023 and 2016.2022. We intend to maintain a full valuation allowance on our deferred tax assets until sufficient positive evidence exists to support reversal of the valuation allowance. The valuation allowance decreased by $8.6 million and increased by $18.9$52.5 million for the yearsyear ended December 31, 20172023 due to the increase in our net deferred tax assets.
In August 2022, the CHIPS and 2016, respectively.Science Act (CHIPS Act) and the IRA were enacted, neither of which are expected to have a material impact to our financial statements.
The American Rescue Plan Act (ARA) was signed into law on March 11, 2021. We do not expect the ARA to have a material impact on our financial statements, however, given the potential changes to IRC Section 162(m) effective in 2027 as a result of the ARA, we will continue to monitor and assess.
Under the Tax Act, federal NOLs generated in tax years beginning on or after January 1, 2018 may be carried forward indefinitely, but the utilization of such federal NOLs is limited to 80% of taxable income in future years. Since enactment, the IRS and Treasury have issued final and proposed regulations including clarifying guidance on several topics addressed by the Tax Act. Not all states conform to the Tax Act or and other states have varying conformity to the Tax Act.
As of December 31, 2017,2023, for federal income tax purposes, we had federal and state net operating lossNOL carryforwards for tax return purposes of $76.0approximately $1.1 billion of which $23.3 million and $231.4 million, respectively. The federal and state net operating loss carryforwards begin to expire in 20322035 and the remaining may be carried forward indefinitely, research & development tax credits of $34.6 million which begin to expire in various amounts if not utilized.2032, and orphan drug tax credits of $111.8 million which begin to expire in 2035. For state income tax purposes, we had NOL carryforwards of approximately $1.3 billion which begin to expire in 2030, research & development tax credits of $43.5 million which may be carried forward indefinitely, and California Completes tax credit of $2.0 million, which begins to expire in 2025.
Under IRC Section 382, of the Internal Revenue Code of 1986, as amended, (the “Code”), our ability to utilize net operating losssubstantial restrictions exist on the utilization of NOL and tax credit carryforwards or other tax attributes in any taxable year may be limited if we havethe event a corporation experienced an “ownership change.” Generally, a Section 382 “ownership change” occurs if one or more stockholders or groups of stockholders who owns at least 5% of a corporation’s stock increases its ownership by more than 50%50 percentage points over its lowest ownership percentage within a specified testing period. Similar rules may apply under state tax laws. Accordingly, our ability to utilize NOL and tax credit carryforwards may be limited as a result of such ownership changes, and such a limitation could result in the expiration of carryforwards before they are utilized.
We have completed a Section 382 study of transactions in our stock through December 31, 2015.2023. The study concluded that we have experienced at least one ownership changechanges since inception and that our utilization of net operating loss carryforwards will be subject to annual limitations.inception. However, itthis is not expected that these limitations willto result in the expiration of tax attribute carryforwards prior to utilization.
The Company has also completed an updated analysis since the previous ownership change through December 31, 2017. As a result, no new ownership changes have occurred that would limit the current generated NOLs.
On December 22, 2017, the Tax Act was enacted into law. The Tax Act, among other things, contains significant changes to corporate taxation, including reduction of the corporate tax rate from a top marginal rate of 35% to a flat rate of 21%; limitation of the tax deduction for interest expense to 30% of adjusted earnings (except for certain small businesses); limitation of the deduction of net operating losses generated in tax years beginning after December 31, 2017 to 80% of taxable income, indefinite carryforward of net operating losses generated in tax years after 2018 and elimination of net operating loss carrybacks; changes in the treatment of offshore earnings regardless of whether they are repatriated; current inclusion in U.S. federal taxable income of certain earnings of controlled foreign corporations, mandatory capitalization of research and development expenses beginning in 2022; immediate deductions for certain new investments instead of deductions for depreciation expense over time; further deduction limits on executive compensation; and modifying, repealing and creating many other business deductions and credits, including the reduction in the orphan drug credit from 50% to 25% of qualifying expenditures. As of December 31, 2017, we have made a reasonable estimate of the effects on our existing deferred taxes and related disclosures by reducing the expected deductibility of certain executive compensation by $0.6 million due to the application of new limitations under Internal Revenue Section 162(m) and reducing our net federal and state deferred tax assets by $13.5 million for the reduction in corporate tax rate. These adjustments to our deferred tax assets are offset against the valuation allowance. Due to accumulated foreign deficits the Company does not expect a current inclusion in U.S. federal taxable income for the transition tax on earnings of controlled foreign corporations.
Additionally, the SEC staff has issued SAB 118, which allows us to record provisional amounts during a measurement period not to extend beyond one year of the enactment date. On December 22, 2017, Staff Accounting Bulletin No. 118 ("SAB 118") was issued to address the application of U.S. GAAP in situations when a registrant does not have the necessary information available,
prepared, or analyzed (including computations) in reasonable detail to complete the accounting for certain income tax effects of the Act. Because the Company is still in the process of analyzing certain provisions of the Act including the application of new executive compensation limitation provisions under Internal Revenue Section 162(m) in accordance with SAB 118, the Company has determined that the adjustment to its deferred taxes was a provisional amount and a reasonable estimate at December 31, 2017.
A reconciliation of the beginning and ending amountbalance of gross unrecognized tax benefits, iswhich excludes interest and penalties, for the years ended December 31, 2022 and 2023 are as follows:
| (In thousands) | |
Balance as of December 31, 2014 | $ | 1,643 | |
Gross increases for tax positions related to current year | | 2,671 | |
Balance as of December 31, 2015 | | 4,314 | |
Gross increases for tax positions related to current year | | 4,971 | |
Balance as of December 31, 2016 | | 9,285 | |
| | (In thousands) | |
Balance as of January 1, 2022 | | | 144,067 | |
Gross increases for tax positions related to current year | | 16,371 | | | 7,683 | |
Gross increases for tax positions related to prior year | | 9,534 | | | — | |
Gross decreases for tax positions related to prior year | | (4,643 | ) | | (785 | ) |
Impact of change in tax rate | | (496 | ) |
Balance as of December 31, 2017 | $ | 30,051 | |
Balance as of December 31, 2022 | | | 150,965 | |
Gross increases for tax positions related to current year | | | 5,538 | |
Gross increases for tax positions related to prior year | | | 3,843 | |
Gross decreases for tax positions related to prior year | | | — | |
Balance as of December 31, 2023 | | $ | 160,346 | |
The CompanyWe currently hashave a full valuation allowance against itsour U.S. net deferred tax assets, which would impact the timing of the effective tax rate benefit should any uncertain tax position be favorably settled in the future. Of the $30.1 million totalThe reversal of unrecognized tax benefits as of December 31, 2017, $0.1 million, if recognized, would not affect the Company’sour effective tax rate.
During July 2016, the Company licensed certain intellectual property rights to a wholly-owned subsidiary outside the United States. Although the license of intellectual property rights between consolidated entities did not result in any gain in the consolidated statements of operations and comprehensive loss, the transaction generated a taxable gain in the United States. However, as this gain is offset by current and existing tax losses, there was no cash tax impact from the transaction in the periods presented. As a result of the transaction, there was an increase of $0.4 million and $0.8 million in unrecognized tax benefits during the year ended December 31, 2016 and December 31, 2017, respectively. The remaining increases and decreases in unrecognized tax benefits related to changes to federal and state research and development and orphan drug tax credit carryforwards. The Company expects to record an uncertain tax benefit of $0.8 million during the next 12 months relatedrate to the licensed intellectual property rights. The Company’sextent we continue to maintain a full valuation allowance against our deferred tax assets.
Our policy is to account for interest and penalties related to uncertain tax positions as a component of the income tax provision. The amount ofWe have no accrued interest and penalties as of December 31, 20172023 and for the years ended December 31, 2017, 2016 and 2015 was immaterial.2022 due to available tax losses.
Our significant jurisdictions are the Cayman Islands, the U.S. federal jurisdiction and the California state jurisdiction. All of our tax years remain open to examination by the U.S. federal and California tax authorities. We also file in other state, local and foreign jurisdictions in which we operate, and such tax years remain open to examination.
As of December 31, 2023, we are not permanently reinvested with respect to its foreign earnings and have not recorded deferred income taxes and withholding taxes as these taxes are immaterial to the financial statements.
13.Supplemental Balance Sheet Information
Inventories
Inventories consist of the following as of each period:
| | | | | | | | |
| | December 31, | | | December 31, | |
| | 2023 | | | 2022 | |
| | (in thousands) | |
Raw Materials | | $ | 2,335 | | | $ | 1,214 | |
Work-in-process | | | 7,371 | | | | 372 | |
Total inventories | | $ | 9,706 | | | $ | 1,586 | |
Property and equipment, net
Property and equipment consisted of the following as of each period end:
| | | | | | | | |
| | December 31, | | | December 31, | |
| | 2023 | | | 2022 | |
| | (in thousands) | |
Leasehold improvements | | $ | 904 | | | $ | 875 | |
Lab equipment | | | 15,540 | | | | 14,797 | |
Machinery and equipment | | | 572 | | | | 572 | |
Computer equipment and software | | | 1,279 | | | | 1,149 | |
Furniture and fixtures | | | 1,272 | | | | 1,297 | |
Construction in progress | | | 158 | | | | 32 | |
Property and equipment, gross | | | 19,725 | | | | 18,722 | |
Less: accumulated depreciation | | | (15,869 | ) | | | (12,422 | ) |
Property and equipment, net | | $ | 3,856 | | | $ | 6,300 | |
Depreciation expense was $3.7 million and $4.7 million for the years ended December 31, 2023 and 2022, respectively.
Other current liabilities
Other current liabilities consisted of the following as of each period end:
| | | | | | | | |
| | December 31, | | | December 31, | |
| | 2023 | | | 2022 | |
| | (in thousands) | |
Accrued operating expenses | | $ | 19,007 | | | $ | 7,435 | |
Current portion of operating lease liabilities | | | 11,264 | | | | 12,806 | |
Current portion of finance lease liabilities | | | 915 | | | | 834 | |
Other accrued liabilities | | | 640 | | | | 319 | |
Total other current liabilities | | $ | 31,826 | | | $ | 21,394 | |
In January 2018,2024, we completed an underwritten public offering of 7,675,072 shares of common stock, including 675,072 from the exercise by the underwriters of their option to purchase additional shares, at an offering price of $18.25 per share. We received net proceedsannounced a reduction in force of approximately $131.425% of total workforce. We expect to recognize approximately $4.5 million after deducting underwriting discountsin total severance and commissionsrelated benefits as a result of this reduction in force, consisting primarily of severance payments and offering expenses.wages for the 60-day notice period in accordance with the California WARN Act. In most cases, the severance will be paid in the first half of 2024. Certain of the notified employees had employment agreements which provided for separation benefits in the form of salary continuation; these benefits will be paid from February 2024 through January 2025. The majority of the associated costs represent cash expenditures.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Under the supervision of our Chief Executive Officer and Chief Financial Officer, we evaluated the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) of the Exchange ActAct) as of December 31, 2017.2023. Based on that evaluation,our Chief Executive Officer and Chief Financial Officer have concluded that our disclosure controls and procedures were effective as of December 31, 20172023 to ensure that information required to be disclosed by us in the reports we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate to allow timely discussion regarding required disclosures. In designing and evaluating our disclosure
controls and procedures, management recognizes that any disclosure controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives. In addition, the design of disclosure controls and procedures must reflect the fact that there are resource constraints and that management is required to apply its judgment in evaluating the benefits of possible controls and procedures relative to their costs.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rules 13a-15(f) and 15d-15(f). Our management conducted an evaluation of the effectiveness of our internal control over financial reporting as of December 31, 20172023 based on the criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission.
Based on the results of its evaluation, management concluded that our internal control over financial reporting was effective as of December 31, 2017.2023.
Inherent Limitations on Controls and Procedures
Our management, including the Chief Executive and Financial Officer and Principal AccountingChief Financial Officer, does not expect that our disclosure controls and procedures and our internal controls will prevent all error and all fraud. A control system, no matter how well designed and operated, can only provide reasonable assurances that the objectives of the control system are met. The design of a control system reflects resource constraints; the benefits of controls must be considered relative to their costs. Because there are inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within the Company have been or will be detected. As these inherent limitations are known features of the financial reporting process, it is possible to design into the process safeguards to reduce, though not eliminate, these risks. These inherent limitations include the realities that judgments in decision-making can be faulty and that breakdowns occur because of simple error or mistake. Controls can be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of the control. The design of any system of controls is based in part upon certain assumptions about the likelihood of future events. While our disclosure controls and procedures are designed to provide reasonable assurance of achieving their objectives, there can be no assurance that any design will succeed in achieving its stated goals under all future conditions. Over time, controls may become inadequate because of changes in conditions or deterioration in the degree of compliance with the policies or procedures. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected.
We intend to review and evaluate the design and effectiveness of our disclosure controls and procedures on an ongoing basis and to improve our controls and procedures over time and to correct any deficiencies that we may discover in the future. While our Chief Executive and Financial Officer and Principal AccountingChief Financial Officer have concluded that, as of December 31, 2017,2023, the design of our disclosure controls and procedures, as defined in Rule 13a-15(e) under the Exchange Act, was effective, future events affecting our business may cause us to significantly modify our disclosure controls and procedures.
Changes in Internal Control over Financial Reporting
There waswere no changechanges in our internal control over financial reporting during the three months ended December 31, 2023 which were identified in connection with theour evaluation required by Rules 13a-15(d) and 15d-15(d) of the Exchange Act, that occurred during the three months ended December 31, 2017 that hashave materially affected, or isare reasonably likely to materially affect, our internal control over financial reporting.
Report of We have not experienced any material impact to our internal controls over financial reporting despite the Independent Registered Public Accounting Firm
This Annual Report on Form 10-K does not include an attestation reportfact that many of our registered public accounting firm dueemployees are working remotely. We are continually monitoring and assessing our remote working situation to an exemption established byminimize the JOBS Act for “emerging growth companies.”impact to the design and operating effectiveness of our internal controls.
Item 9B. Other Information
None.During the three months ended December 31, 2023, none of the Company's directors or executive officers adopted or terminated any contract, instruction or written plan for the purchase or sale of Company securities that was intended to satisfy the affirmative defense conditions of Rule 10b5-1(c) or any “non-Rule 10b5-1 trading arrangement” as defined in Item 408 of Regulation S-K under the Securities Exchange Act of 1934, as amended.
Item 9C. Disclosure Regarding Foreign Jurisdictions That Prevent Inspections
Not Applicable.
PART III
PART III
Certain information required by Part III is omitted from this Annual Report on Form 10-K since we intend to file our definitive proxy statement for our 20172024 annual meeting of stockholders or the(the Definitive Proxy Statement,Statement), pursuant to Regulation 14A of the Securities Exchange Act of 1934, as amended, not later than 120 days after December 31, 2017,2023, and certain information to be included in the Definitive Proxy Statement is incorporated herein by reference.
Item 10. Directors, Executive Officers and Corporate Governance
Information required by this Item is hereby incorporated by reference to our Definitive Proxy Statement.
We have adopted a Code of Conduct that applies to our officers, directors and employees which is available on our internet website at www.atarabio.com. The Code of Conduct contains general guidelines for conducting the business of our company consistent with the highest standards of business ethics, and is intended to qualify as a “code of ethics” within the meaning of Section 406 of the Sarbanes-Oxley Act of 2002 and Item 406 of Regulation S-K. In addition, we intend to promptly disclose (1) the nature of any amendment to our Code of Conduct that applies to our principal executive officer, principal financial officer, principal accounting officer or controller or persons performing similar functions and (2) the nature of any waiver, including an implicit waiver, from a provision of our code of ethics that is granted to one of these specified officers, the name of such person who is granted the waiver and the date of the waiver on our website in the future.
Item 11. Executive Compensation
Information required by this Item is hereby incorporated by reference to our Definitive Proxy Statement.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
Information required by this Item is hereby incorporated by reference to our Definitive Proxy Statement.
Item 13. Certain Relationships and Related Transactions, and Director Independence
Information required by this Item is hereby incorporated by reference to our Definitive Proxy Statement.
Item 14. Principal AccountingAccountant Fees and Services
Information required by this Item is hereby incorporated by reference to our Definitive Proxy Statement.
PARTPART IV
Item 15. Exhibits and Financial Statement Schedules
(a)(1)
| Financial Statements.
|
(a)(1) Financial Statements.
The response to this portion of Item 15 is set forth under Item 8 above.
(a)(2)
| Financial Statement Schedules.
|
(a)(2) Financial Statement Schedules.
All schedules have been omitted because they are not required or because the required information is given in the financial statements or notes thereto set forth under Item 8 above.
(a)(3) Exhibits.
EXHIBIT INDEX
| | | | | | | | | | | |
Exhibit | | | | Incorporated by Reference | | Filed |
Number | | Exhibit Description | | Form | | | Exhibit | | Filing Date | | Herewith |
3.1 |
| Amended and Restated Certificate of Incorporation of Atara Biotherapeutics, Inc. |
| S-1 |
|
| 3.2 |
| 06/20/2014 |
| |
| | | | | | | | | | | |
3.2 |
| Second Amended and Restated Bylaws of Atara Biotherapeutics, Inc. |
| 8-K |
|
| 3.1 |
| 09/27/2022 |
| |
| | | | | | | | | | | |
4.1 |
| Form of Common Stock Certificate |
| S-1/A |
|
| 4.1 |
| 07/10/2014 |
| |
| |
| | | | | | | | | |
4.2 |
| Form of 2019 Pre-Funded Warrant |
| 8-K |
|
| 4.1 |
| 07/22/2019 |
| |
| |
| | | | | | | | | |
4.3 |
| Form of May 2020 Pre-Funded Warrant |
| 8-K |
|
| 4.1 |
| 05/28/2020 |
| |
| |
| | | | | | | | | |
4.4 |
| Form of December 2020 Pre-Funded Warrant |
| 8-K |
|
| 4.1 |
| 12/09/2020 |
| |
| |
| | | | | | | | | |
4.5 |
| Description of Securities |
| 10-K |
|
| 4.4 |
| 02/27/2020 |
| |
| | | | | | | | | | | |
10.1* |
| Amended and Restated 2014 Equity Incentive Plan |
| 10-Q |
|
| 10.2 |
| 08/08/2016 |
| |
| | | | | | | | | | | |
10.2* |
| Forms of Option Agreement and Option Grant Notice under the 2014 Equity Incentive Plan |
| S-1 |
|
| 10.2 |
| 06/20/2014 |
| |
| | | | | | | | | | | |
10.3* |
| Form of Restricted Stock Unit Agreement and Restricted Stock Unit Grant Notice |
| 10-Q |
|
| 10.2 |
| 11/01/2023 |
| |
| | | | | | | | | | | |
10.4* |
| 2014 Employee Stock Purchase Plan |
| S-1/A |
|
| 10.8 |
| 07/10/2014 |
| |
| | | | | | | | | | | |
10.5* | | Atara Biotherapeutics, Inc. Third Amended and Restated 2018 Inducement Plan | | S-8 | | | 4.3 | | 07/22/2022 | | |
| | | | | | | | | | | |
10.6* | | Form of Restricted Stock Unit Agreement and Restricted Stock Unit Grant Notice under the Inducement Plan | | 10-Q | | | 10.2 | | 11/07/2019 | | |
| | | | | | | | | | | |
10.7* | | Form of Stock Option Agreement and Stock Option Grant Notice under the Inducement Plan | | 10-Q | | | 10.3 | | 05/08/2018 | | |
| | | | | | | | | | | |
10.8* | | Forms of Inducement Grant Notice and Inducement Grant Agreement | | 10-Q | | | 10.3 | | 08/07/2017 | | |
| | | | | | | | | | | |
10.9* |
| Form of Indemnification Agreement made by and between Atara Biotherapeutics, Inc. and each of its directors and executive officers |
| S-1 |
|
| 10.9 |
| 06/20/2014 |
| |
| | | | | | | | | | | |
10.10* | | Form of Atara Biotherapeutics, Inc. Executive Employment Agreement | | 10-K | | | 10.39 | | 02/28/2022 | | |
| | | | | | | | | | | |
10.11* | | Executive Employment Agreement, dated May 23, 2019, by and between Pascal Touchon and Atara Biotherapeutics, Inc. | | 8-K | | | 10.1 | | 05/28/2019 | | |
| | | | | | | | | | | |
10.12† |
| Exclusive License Agreement, by and between Atara Biotherapeutics, Inc. and Memorial Sloan Kettering Cancer Center, dated as of June 12, 2015 |
| S-1 |
|
| 10.30 |
| 06/29/2015 |
| |
| | | | | | | | | | | |
10.13† | | Amendment No. 1 to the Exclusive License Agreement, by and between Atara Biotherapeutics, Inc. and Memorial Sloan Kettering Cancer Center, dated as of August 30, 2018 | | 10-K | | | 10.14 | | 02/26/2019 | | |
| | | | | | | | | | | |
10.14 | | Office Lease, by and between BXP 611 Gateway Center LP and Atara Biotherapeutics, Inc., dated as of December 9, 2015 | | 10-K | | | 10.29 | | 03/04/2016 | | |
| | | | | | | | | | | |
10.15 | | First Amendment to Lease, by and between BXP 611 Gateway Center LP and Atara Biotherapeutics, Inc., dated October 21, 2020 | | 10-Q | | | 10.4 | | 11/09/2020 | | |
| | | | | | | | | | | |
10.16 | | Second Amendment to Lease, by and between Atara Biotherapeutics, Inc. and 611 Gateway Center LP, LLC, dated December 9, 2021 | | 10-K | | | 10.36 | | 02/28/2022 | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Exhibit | | | | Incorporated by Reference | | Filed |
Number | | Exhibit Description | | Form | | | Exhibit | | Filing Date | | Herewith |
10.17 | | Standard Industrial Lease by and between Thousand Oaks Industrial Portfolio, LLC and Atara Biotherapeutics, Inc. dated February 6, 2017 | | 10-Q | | | 10.1 | | 05/04/2017 | | |
| | | | | | | | | | | |
10.18 | | Lease Agreement between LA Region No. 2, LLC and Atara Biotherapeutics, Inc. dated March 17, 2021 | | 10-Q | | | 10.2 | | 05/04/2021 | | |
| | | | | | | | | | | |
10.19 | | First Amended and Restated Exclusive License Agreement by and between Atara Biotherapeutics, Inc. and Memorial Sloan Kettering Cancer Center, dated March 22, 2021 | | 10-Q | | | 10.3 | | 05/04/2021 | | |
| | | | | | | | | | | |
10.20 | | Fourth Amended and Restated Research and Development Collaboration Agreement between Atara Biotherapeutics, Inc. and the Counsel of the Queensland Institute of Medical Research, dated December 17, 2021 | | 10-K | | | 10.37 | | 02/28/2022 | | |
| | | | | | | | | | | |
10.21 | | Fourth Amended and Restated Exclusive License Agreement between Atara Biotherapeutics, Inc. and the Counsel of the Queensland Institute of Medical Research, dated December 17, 2021 | | 10-K | | | 10.38 | | 02/28/2022 | | |
| | | | | | | | | | | |
10.22 | | Asset Purchase Agreement, dated as of January 26, 2022, by and between Atara Biotherapeutics, Inc., FUJIFILM Diosynth Biotechnologies California, Inc., and certain limited purposes, FUJIFILM Holdings America Corporation | | 8-K | | | 2.1 | | 04/04/2022 | | |
| | | | | | | | | | | |
10.23 | | Master Services and Supply Agreement dated as of January 26, 2022 by and between Atara Biotherapeutics, Inc., and FUJIFILM Diosynth Biotechnologies California, Inc. | | 10-Q | | | 10.1 | | 05/05/2022 | | |
| | | | | | | | | | | |
10.24+ | | Purchase and Sale Agreement between Atara Biotherapeutics, Inc., and HCR Molag Fund, L.P., dated December 20, 2022 | | 10-K | | | 10.46 | | 02/08/2023 | | |
| | | | | | | | | | | |
10.25 | | Amendment No. 2 to Commercial Manufacturing Services Agreement dated as of May 31, 2022 by and between Atara Biotherapeutics, Inc. and Charles River Laboratories, Inc. | | 10-Q | | | 10.1 | | 08/08/2022 | | |
| | | | | | | | | | | |
10.26+ | | Amendment No. 3 to Commercial Manufacturing Services Agreement dated as of August 1, 2022 by and between Atara Biotherapeutics, Inc. and Charles River Laboratories, Inc. | | 10-Q | | | 10.1 | | 11/08/2022 | | |
| | | | | | | | | | | |
10.27+ | | Amendment No. 4 to Commercial Manufacturing Services Agreement between Atara Biotherapeutics, Inc. and Charles River Laboratories, Inc. dated January 30, 2023 | | 10-Q | | | 10.1 | | 05/08/2023 | | |
| | | | | | | | | | | |
10.28+ | | Amendment No. 5 to Commercial Manufacturing Services Agreement between Atara Biotherapeutics, Inc. and Charles River Laboratories, Inc. dated September 27, 2023 | | 10-Q | | | 10.1 | | 11/01/2023 | | |
| | | | | | | | | | | |
10.29+ | | Amendment No. 6 to Commercial Manufacturing Services Agreement between Atara Biotherapeutics, Inc. and Charles River Laboratories, Inc. dated December 30, 2023 | | | | | | | | | X |
| | | | | | | | | | | |
10.30+ | | Amendment No. 7 to Commercial Manufacturing Services Agreement between Atara Biotherapeutics, Inc. and Charles River Laboratories, Inc. dated January 31, 2024 | | | | | | | | | X |
| | | | | | | | | | | |
10.31+ | | Amended and Restated Commercialization Agreement by and between Atara Biotherapeutics, Inc. and Pierre Fabre Medicament dated October 31, 2023 | | | | | | | | | X |
| | | | | | | | | | | |
21.1 | | List of Subsidiaries | | | | | | | | | X |
| | | | | | | | | | | |
23.1 |
| Consent of Independent Registered Public Accounting Firm |
| |
|
| |
| |
| X |
| | | | | | | | | | | |
24.1 |
| Power of Attorney (included on signature page) |
| |
|
| |
| |
| |
| | | | | | | | | | | |
A list of exhibits filed with this report or incorporated herein by reference can be found in the Exhibit Index immediately following the signature page of this Report.
EXHIBIT INDEX
Exhibit
Number | | | | Incorporated by Reference | | Filed |
| Exhibit Description | | Form | | File No. | | Exhibit | | Filing Date | | Herewith |
| | | | | | | | | | | | |
3.1 | | Amended and Restated Certificate of Incorporation of Atara Biotherapeutics, Inc. | | S-1 | | 333-196936 | | 3.2 | | 06/20/2014 | | |
| | | | | | | | | | | | |
3.2 | | Amended and Restated Bylaws of Atara Biotherapeutics, Inc. | | S-1 | | 333-196936 | | 3.4 | | 06/20/2014 | | |
| | | | | | | | | | | | |
4.1 | | Form of Common Stock Certificate | | S-1/A | | 333-196936 | | 4.1 | | 07/10/2014 | | |
| | | | | | | | | | | | |
4.2 | | Investors’ Rights Agreement, by and among Atara Biotherapeutics, Inc. and the stockholders named therein, dated March 31, 2014 | | S-1 | | 333-196936 | | 4.2 | | 06/20/2014 | | |
| | | | | | | | | | | | |
10.1* | | Amended and Restated 2014 Equity Incentive Plan | | 10-Q | | 001-36548 | | 10.2 | | 08/08/2016 | | |
| | | | | | | | | | | | |
10.2* | | Forms of Option Agreement and Option Grant Notice under the 2014 Equity Incentive Plan | | S-1 | | 333-196936 | | 10.2 | | 06/20/2014 | | |
| | | | | | | | | | | | |
10.3* | | Form of Restricted Stock Unit Agreement and Restricted Stock Unit Grant Notice under the 2014 Equity Incentive Plan | | S-1 | | 333-196936 | | 10.3 | | 06/20/2014 | | |
| | | | | | | | | | | | |
10.4* | | Nina Biotherapeutics, Inc. 2012 Equity Incentive Plan | | S-1 | | 333-196936 | | 10.4 | | 06/20/2014 | | |
| | | | | | | | | | | | |
10.5* | | Pinta Biotherapeutics, Inc. 2012 Equity Incentive Plan | | S-1 | | 333-196936 | | 10.5 | | 06/20/2014 | | |
| | | | | | | | | | | | |
10.6* | | Santa Maria Biotherapeutics, Inc. 2012 Equity Incentive Plan | | S-1 | | 333-196936 | | 10.6 | | 06/20/2014 | | |
| | | | | | | | | | | | |
10.7* | | Form of Stock Unit Agreement under the Nina Biotherapeutics, Inc. 2012 Equity Incentive Plan, Pinta Biotherapeutics, Inc. 2012 Equity Incentive Plan and Santa Maria Biotherapeutics, Inc. 2012 Equity Incentive Plan | | S-1 | | 333-196936 | | 10.7 | | 06/20/2014 | | |
| | | | | | | | | | | | |
10.8* | | 2014 Employee Stock Purchase Plan | | S-1/A | | 333-196936 | | 10.8 | | 07/10/2014 | | |
| | | | | | | | | | | | |
10.9* | | Form of Indemnification Agreement made by and between Atara Biotherapeutics, Inc. and each of its directors and executive officers | | S-1 | | 333-196936 | | 10.9 | | 06/20/2014 | | |
| | | | | | | | | | | | |
10.10* | | Amended and Restated Executive Employment Agreement by and between Atara Biotherapeutics, Inc. and Isaac E. Ciechanover, dated October 12, 2015 | | 8-K | | 001-36548 | | 10.1 | | 10/16/2015 | | |
| | | | | | | | | | | | |
10.11* | | Amended and Restated Executive Employment Agreement between Atara Biotherapeutics, Inc. and John F. McGrath, Jr., dated October 12, 2015 | | 8-K | | 001-36548 | | 10.2 | | 10/16/2015 | | |
| | | | | | | | | | | | |
10.12* | | Amended and Restated Executive Employment Agreement between Atara Biotherapeutics, Inc. and Christopher M. Haqq, dated October 12, 2015 | | 8-K | | 001-36548 | | 10.3 | | 10/16/2015 | | |
| | | | | | | | | | | | |
10.13* | | Amended and Restated Executive Employment Agreement between Atara Biotherapeutics, Inc. and Mitchall Clark, dated October 12, 2015 | | 8-K | | 001-36548 | | 10.4 | | 10/16/2015 | | |
| | | | | | | | | | | | |
10.14* | | Amended and Restated Executive Employment Agreement between Atara Biotherapeutics, Inc. and Heather D. Turner, dated October 12, 2015 | | 10-Q | | 001-36548 | | 10.1 | | 05/06/2016 | | |
| | | | | | | | | | | | |
10.15† | | Exclusive Option Agreement, by and between Atara Biotherapeutics, Inc. and Memorial Sloan Kettering Cancer Center, dated as of September 19, 2014 | | 10-Q | | 001-36548 | | 10.29 | | 05/11/2015 | | |
| | | | | | | | | | | | |
10.16† | | Amendment Number One to the Exclusive Option Agreement, by and between Atara Biotherapeutics, Inc. and Memorial Sloan Kettering Cancer Center, dated as of June 12, 2015 | | 10-Q | | 001-36548 | | 10.32 | | 08/07/2015 | | |
| | | | | | | | | | | | |
Exhibit
Number | | | | Incorporated by Reference | | Filed |
| Exhibit Description | | Form | | File No. | | Exhibit | | Filing Date | | Herewith |
| | | | | | | | | | | | |
10.17† | | Exclusive License Agreement, by and between Atara Biotherapeutics, Inc. and Memorial Sloan Kettering Cancer Center, dated as of June 12, 2015 | | S-1 | | 333-205347 | | 10.30 | | 06/29/2015 | | |
| | | | | | | | | | | | |
10.18 | | Office Lease, by and between Atara Biotherapeutics, Inc. and BPG Rock Westlake, LLC, dated January 7, 2015 | | 10-Q | | 001-36548 | | 10.33 | | 11/06/2015 | | |
| | | | | | | | | | | | |
10.19 | | First Amendment to Lease, by and between BPG Rock Westlake, LLC and Atara Biotherapeutics, Inc., dated as of September 9, 2015 | | 10-Q | | 001-36548 | | 10.34 | | 11/06/2015 | | |
| | | | | | | | | | | | |
10.20 | | Office Lease, by and between BXP 611 Gateway Center LP and Atara Biotherapeutics, Inc., dated as of December 9, 2015 | | 10-K | | 001-36548 | | 10.29 | | 03/04/2016 | | |
| | | | | | | | | | | | |
10.21* | | Amended and Restated Executive Employment Agreement between Atara Biotherapeutics, Inc. and Gad Soffer, dated October 12, 2015 | | 10-K | | 001-36548 | | 10.21 | | 03/09/2017 | | |
10.22* | | Standard Industrial Lease by and between Thousand Oaks Industrial Portfolio, LLC and Atara Biotherapeutics, Inc. dated February 6, 2017 | | 10-Q | | 001-36548 | | 10.1 | | 05/04/2017 | | |
| | | | | | | | | | | | |
10.23* | | Executive Employment Agreement between Atara Biotherapeutics, Inc. and Joe Newell, dated March 20, 2017 | | 10-Q | | 001-36548 | | 10.1 | | 08/07/2017 | | |
| | | | | | | | | | | | |
10.24* | | Executive Employment Agreement between Atara Biotherapeutics, Inc. and Derrell Porter, M.D. dated March 23, 2017 | | 10-Q | | 001-36548 | | 10.2 | | 08/07/2017 | | |
| | | | | | | | | | | | |
10.25* | | Forms of Inducement Grant Notice and Inducement Grant Agreement | | 10-Q | | 001-36548 | | 10.3 | | 08/07/2017 | | |
21.1 | | List of Subsidiaries | | | | | | | | | | X |
| | | | | | | | | | | | |
23.1 | | Consent of Independent Registered Public Accounting Firm | | | | | | | | | | X |
| | | | | | | | | | | | |
24.1 | | Power of Attorney (included on signature page) | | | | | | | | | | |
| | | | | | | | | | | | |
31.1 | | Certification of the Chief Executive Officer pursuant to Securities Exchange Act Rules 13A-14A and 15D-14A | | | | | | | | | | X |
| | | | | | | | | | | | |
31.2 | | Certification of the Chief Financial Officer pursuant to Securities Exchange Act Rules 13A-14A and 15D-14A | | | | | | | | | | X |
| | | | | | | | | | | | |
32.1(1) | | Certifications of Chief Executive Officer and Chief Financial Officer pursuant to 18 USC Section 1350 as adopted pursuant to Section 906 of The Sarbanes-Oxley Act of 2002. | | | | | | | | | | X |
| | | | | | | | | | | | |
101.INS | | XBRL Instance Document. | | | | | | | | | | X |
| | | | | | | | | | | | |
101.SCH | | XBRL Taxonomy Extension Schema Document. | | | | | | | | | | X |
| | | | | | | | | | | | |
101.CAL | | XBRL Taxonomy Extension Calculation Linkbase Document. | | | | | | | | | | X |
| | | | | | | | | | | | |
101.DEF | | XBRL Taxonomy Extension Definition Linkbase Document. | | | | | | | | | | X |
| | | | | | | | | | | | |
101.LAB | | XBRL Taxonomy Extension Labels Linkbase Document. | | | | | | | | | | X |
| | | | | | | | | | | | |
101.PRE | | XBRL Taxonomy Extension Presentation LinkbaseDocument. | | | | | | | | | | X |
†
| Confidential treatment has been requested or granted for a portion of this exhibit.
|
*
| Indicates management contract or compensatory plan or arrangement.
|
† Confidential treatment has been granted for a portion of this exhibit.
+ Portions of this exhibit have been omitted as being both (i) not material and (ii) would likely cause competitive harm if publicly disclosed.
* Indicates management contract or compensatory plan or arrangement.
(1)The certifications attached as Exhibit 32.1 accompany this Annual Report on Form 10-K pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, and shall not be deemed “filed” by the Registrant for purposes of Section 18 of the Securities Exchange Act of 1934, as amended.
SIGNATURESItem 16. Form 10-K Summary
None.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of South San Francisco,Thousand Oaks, State of California, on the 27th28th day of February, 2018.March, 2024.
| | | |
| Atara Biotherapeutics, Inc. |
|
|
|
|
By:
| By: |
| /s/ Isaac E. CiechanoverPascal Touchon |
|
| Isaac E. Ciechanover, M.D.
| Pascal Touchon |
|
|
| President and Chief Executive Officer |
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Isaac E. CiechanoverPascal Touchon and John F. McGrath, Jr.,Eric Hyllengren, and each of them, as his or her true and lawful attorneys-in-fact and agents, each with the full power of substitution, for him or her and in his or her name, place or stead, in any and all capacities, to sign any and all amendments (including post-effective amendments) to this Annual Report on Form 10-K, and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or their, his or her substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| | | | |
Signature |
| Title |
| Date |
|
|
|
|
|
/s/ Isaac E. CiechanoverPascal Touchon |
|
|
|
|
Isaac E. Ciechanover, M.D.Pascal Touchon
|
| President, and Chief Executive Officer and Director
(principal executive officerPrincipal Executive Officer) |
| February 27, 2018March 28, 2024
|
/s/ Eric Hyllengren |
|
|
|
|
/s/ John F. McGrath, Jr.Eric Hyllengren
|
| Chief Financial Officer
(Duly Authorized Officer and Principle Financial and Accounting Officer) |
| March 28, 2024 |
John F. McGrath, Jr.
|
| Executive Vice President and Chief Financial Officer (principal financial and accounting officer)
|
| February 27, 2018
|
/s/ Carol G. Gallagher |
|
|
|
|
/s/ Eric DobmeierCarol G. Gallagher, Pharm. D.
|
| Director, Chair |
| March 28, 2024 |
Eric Dobmeier
|
| Director
|
| February 27, 2018
|
/s/ Eric L. Dobmeier |
|
|
|
|
Eric L. Dobmeier |
| Director |
| March 28, 2024 |
|
|
|
|
|
/s/ Matthew K. Fust |
|
|
|
|
Matthew K. Fust |
| Director |
| February 27, 2018March 28, 2024
|
|
|
|
|
|
/s/ Carol G. GallagherWilliam K. Heiden |
|
|
|
|
Carol G. Gallagher, Pharm.D.William K. Heiden
|
| Director |
| February 27, 2018March 28, 2024
|
|
|
|
|
|
/s/ William HeidenAmeet Mallik |
|
|
|
|
William HeidenAmeet Mallik
|
| Director |
| February 27, 2018March 28, 2024
|
|
|
|
|
|
/s/ Joel S. Marcus
|
|
|
|
|
Joel S. MarcusMaria Grazia Roncarolo, M.D.
|
| Director |
| February 27, 2018
|
|
|
|
| |
/s/ Beth Seidenberg
| | | | |
Beth Seidenberg, M.D.
| | Director
| | February 27, 2018
|
96


