SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
☒
| ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
xANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934For the Fiscal Year Ended December 31,
20202023
☐
| TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
oTRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934For the Transition Period from to
Commission File Number: 001-38085
(Exact name of Registrant as specified in its charter)
| | | | | | | | | | | | | | |
| Delaware | | 2834 | | | 46-5270895 |
Delaware
| | 2834
| | 46-5270895
|
(State or Other Jurisdiction of Incorporation or Organization) | | (Primary Standard Industrial Classification Code Number) | | Identification Number) |
441 Ninth Avenue, 14th Floor1460 Broadway, Suite 15021
New York, New York
1003610001
(Address, Including Zip Code, and Telephone Number, Including Area Code, of Registrant’s Principal Executive Offices)
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | | | | | | | |
| Title of each class | | Symbol(s) | | Name of each exchange on which registered |
Common Stock, par value $0.001 per share | | OVID | | The Nasdaq Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐o No ☒x Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Act. Yes ☐o No ☒x Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to the filing requirements for the past 90 days. Yes ☒x No ☐o Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒x No ☐o Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.:
| | | | | | | | | | | |
Large Accelerated Filer | o | ☐
| | Accelerated Filer | | ☐
o |
Non-accelerated Filer | x | ☒
| | Smaller Reporting Company | | ☒
x |
Emerging growth company | o | | ☒
| | | |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act ☒o Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. Yes ☐ No ☒¨ If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ¨
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to § 240.10D-1(b). ¨
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act.) Yes ☐o No ☒x As of June 30,
2020,2023, the last day of the registrant’s most recently completed second fiscal quarter, the aggregate market value of the Common Stock held by non-affiliates of the registrant was approximately
$293.7$191.1 million based on the closing price of the registrant’s common stock on June 30,
2020.2023. The calculation excludes shares of the registrant’s common stock held by current executive officers, directors and stockholders that the registrant has concluded are affiliates of the registrant. This determination of affiliate status is not a determination for other purposes.
As of March
8, 2021,5, 2024, there were
65,753,57070,709,857 shares of common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s definitive proxy statement for its
20212024 Annual Meeting of Stockholders, which the registrant intends to file pursuant to Regulation 14A with the Securities and Exchange Commission not later than 120 days after the registrant’s fiscal year ended December 31,
2020,2023, are incorporated by reference into Part III of this Annual Report on Form 10-K.
| | | | | | | | |
| | Page |
| | ii
|
| | | | |
| | | |
| | |
| | Business
| | 1
|
Item 1A.
| | | | 23
|
| | | | 59
|
Item 2. 1C. | |
| | 59
|
| | |
| | | | 59
|
| | | | 59
|
| | | | |
| | | | |
| | | | 60
|
| | | | 61
|
| | | | |
| | | | 62
|
| | | | |
| | | | 70
|
| | | | 70
|
| | | | 70
|
| | | | 70
|
| | | | |
| | | | |
| | | | 71
|
| | | | 71
|
| | | | 71
|
| | | | 71
|
| | | | 71
|
| | | | |
| | | | |
| | | | 72
|
| | | | 74
|
i
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act. All statements other than statements of historical fact are “forward-looking statements” for purposes of this Annual Report on Form 10-K. In some cases, you can identify forward-looking statements by terminology such as “aim,” “anticipate,” “assume,” “believe,” “contemplate,” continue,” “could,” “design,” “due,” “estimate,” “expect,” “goal,” “intend,” “may,” “objective,” “plan,” “positioned,” “potential,” “predict,” “project,” “should,” “target,” “will,” “would” or the negative or plural of those terms, and similar expressions.
Forward-looking statements include, but are not limited to, statements about:
statements regarding the impact of the COVID-19 pandemic and its effects on our operations, access to capital, research and development and clinical trials and potential disruption in the operations and business of third-party manufacturers, contract research organizations, or CROs, other service providers, and collaborators with whom we conduct business;
our estimates regarding expenses, future revenue, capital requirements and needs for additional financing;
•our ability to identify additional novel compounds with significant commercial potential to acquire or in-license;
•our ability to successfully acquire or in-license additional drug candidates on reasonable terms;
•our estimates regarding expenses, future revenue, including any royalty or milestone payments, capital requirements and needs for additional financing;
•our ability to obtain regulatory approval of our current and future drug candidates;
•our expectations regarding the timing of clinical trials and potential regulatory filings;
•our expectations regarding the potential market size and the rate and degree of market acceptance of such drug candidates;
•our ability to fund our working capital requirements;
•the implementation of our business model and strategic plans for our business and drug candidates;
•developments or disputes concerning our intellectual property or other proprietary rights;
•our ability to maintain and establish collaborations or obtain additional funding;
•our expectations regarding government and third-party payor coverage and reimbursement;
•our ability to compete in the markets we serve;
•the impact of government laws and regulations;
•developments relating to our competitors and our industry;
•the impact of geopolitical tensions, including war or the perception that hostilities may be imminent, adverse global economic conditions, terrorism, natural disasters or public health crises on our operations, research and
development and clinical trials and potential disruption in the operations and business of third parties and collaborators with whom we conduct business; and•the factors that may impact our financial results.
Factors that may cause actual results to differ materially from current expectations include, among other things, those set forth in Part I, Item 1A, “Risk Factors,” herein and for the reasons described elsewhere in this Annual Report on Form 10-K. Any forward-looking statement in this Annual Report on Form 10-K reflects our current view with respect to future events and is subject to these and other risks, uncertainties and assumptions relating to our operations, results of operations, industry and future growth. Given these uncertainties, you should not rely on these forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. Except as required by law, we assume no obligation to update or revise these forward-looking statements for any reason, even if new information becomes available in the future.
This Annual Report on Form 10-K also contains estimates, projections and other information concerning our industry, our business and the markets for certain drugs and consumer products, including data regarding the estimated size of those markets, their projected growth rates and the incidence of certain medical conditions. Information that is based on estimates, forecasts, projections or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from events and circumstances reflected in this information. Unless otherwise expressly stated, we obtained these industry, business, market and other data from reports, research surveys, studies and similar data prepared by third parties, industry, medical and general publications, government data and similar sources and
we have not independently verified the data from third party sources. In some cases, we do not expressly refer to the sources from which these data are derived.
In this Annual Report on Form 10-K, unless otherwise stated or as the context otherwise requires, references to “Ovid,” “the Company,” “we,” “us,” “our” and similar references refer to Ovid Therapeutics Inc. and its wholly owned
subsidiaries.subsidiary. This Annual Report on Form 10-K also contains references to our trademarks and to trademarks belonging to other entities. Solely for convenience, trademarks and trade names referred to, including logos, artwork and other visual displays, may appear without the ® or
TM™ symbols, but such references are not intended to indicate, in any way, that their respective owners will not assert, to the fullest extent under
ii
applicable law, their rights thereto. We do not intend our use or display of other companies’ trade names or trademarks to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
Summary of Selected Risks Associated with Our Business
Our business is subject to numerous risks and uncertainties, including those discussed at length in the section titled “Risk Factors.” These risks include, among others, the following:
We have incurred significant operating losses since inception and expect to continue to incur substantial operating losses for the foreseeable future.
2
We have never generated any revenue from drug sales. Our operating history may make it difficult to evaluate the success of our business to date and to assess our future viability.
We will require additional capital to finance our operations, which may not be available on acceptable terms, if at all. Failure to obtain this necessary capital when needed may force us to delay, limit or terminate certain of our drug development efforts or other operations.
Our future success is dependent on the successful clinical development, regulatory approval and commercialization of our current and future drug candidates. If we, or our licensees, are not able to obtain required regulatory approvals, we will not be able to commercialize our drug candidates, and our ability to generate revenue will be adversely affected.
Because the results of preclinical studies or earlier clinical trials are not necessarily predictive of future results, our drug candidates may not have favorable results in planned or future preclinical studies or clinical trials, or may not receive regulatory approval.
Interim topline and preliminary results from our clinical trials that we announce or publish from time to time may change as more patient data become available and are subject to audit and verification procedures, which could result in material changes in the final data.
We may encounter substantial delays in our clinical trials or we may fail to demonstrate safety and efficacy to the satisfaction of applicable regulatory authorities.
Angelman syndrome has no treatments approved by the U.S. Food and Drug Administration, and the primary clinical endpoint, CGI-I-AS, has not previously been used as a sole primary endpoint in a pivotal clinical trial.
If we are not successful in discovering, developing and commercializing additional drug candidates, our ability to expand our business and achieve our strategic objectives would be impaired.
Our drug candidates may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial potential or result in significant negative consequences following any potential marketing approval.
Even if our current or future drug candidates receive marketing approval, they may fail to achieve market acceptance by physicians, patients, third-party payors or others in the medical community necessary for commercial success.
If we are unable to establish sales and marketing capabilities, or enter into agreements with third parties to market and sell our current or any future drug candidates, we may be unable to generate any revenue from drug sales.
We are heavily dependent on our relationship with Takeda Pharmaceutical Company Limited (“Takeda”) for the development and commercialization of OV935. Any disruption in our relationship with Takeda could lead to delays in, or the termination of, the development of OV935, which would materially harm our business.
We are dependent on our relationship with Angelini Pharma Rare Diseases AG (“Angelini”) for the development and commercialization of OV101 in the European Economic Area as well as Switzerland, the United Kingdom, Russia and Turkey. Any disruption in our relationship with Angelini could lead to delays in the development and achievement of regulatory approval in these countries, which would materially harm our business.
We may be required to make significant payments in connection with our licenses of OV101 from H. Lundbeck A/S and OV935 from Takeda.
Our relationships with customers, physicians, and third-party payors may be subject, directly or indirectly, to federal and state healthcare fraud and abuse laws, false claims laws, health information privacy and security laws, and other healthcare laws and regulations. If we are unable to comply, or have not fully complied, with such laws, we could face substantial penalties.
Coverage and adequate reimbursement may not be available for our current or any future drug candidates, which could make it difficult for us to sell profitably, if approved.
If we are unable to obtain and maintain patent protection for our current or any future drug candidates, or if the scope of the patent protection obtained is not sufficiently broad, we may not be able to compete effectively in our markets.
We may be involved in lawsuits to protect or enforce our patents, the patents of our licensors or our other intellectual property rights, which could be expensive, time consuming and unsuccessful.
We do not have our own manufacturing capabilities and will rely on third parties to produce clinical and commercial supplies of our current and any future drug candidates.
We intend to rely on third parties to conduct, supervise and monitor our preclinical studies and clinical trials, and if those third parties perform in an unsatisfactory manner, it may harm our business.
iii
COVID-19 could adversely impact our business, including our clinical trials and access to capital.
We may need to expand our organization, and we may experience difficulties in managing this growth, which could disrupt our operations.
We may be subject to numerous and varying privacy and security laws, and our failure to comply could result in penalties and reputational damage.
iv
PART I
Ovid is a biopharmaceutical company focused on developing impactful medicines for patientsthat is dedicated to meaningfully improving the lives of people affected by certain epilepsies and families livingbrain conditions with rare neurological disorders.seizure symptoms. We believe that addressing these disorders represent an attractive area for drug development asrepresents a substantial scientific, medical and commercial opportunity. Over the last decade, scientific understanding of the underlying biology of neuronal hyperexcitability and the related pathophysiology of epilepsy and many neurological disorders has grown meaningfullyimproved. This understanding of disease, coupled with advances in preclinical research tools, is improving the predictive potential of translational research, and thereby, may increase the probability of successful clinical development of anti-seizure medicines (“ASMs”). Emerging science also indicates that addressing the underlying causes of hyperexcitability may have therapeutic applications in broad neurological disease well beyond epilepsy.
The large global epilepsy market opportunity reflects significant unmet medical need and economic potential. Epilepsy therapeutics today represent an approximately $8 billion market globally. Evidence supporting the opportunity includes the number of recent acquisitions of epilepsy assets and companies, several of which have been acquired for values greater than $1.0 billion.The unmet need of people affected by seizures remains significant. Approximately three million Americans live with epilepsies today and approximately 50 million people suffer from epilepsy worldwide.
We have proven capabilities and expertise in the successful clinical development of ASMs. We have applied our knowledge to build a differentiated pipeline of medicines with potential first-in-class or best-in-class drug mechanisms of action (“MoA”) to treat epilepsies and brain disorders with seizure symptoms. Our pipeline has produced three programs with potential first-in-class MoAs, and one program with a potential best-in-class MoA. Currently, three of these programs are in clinical trials in humans. The fourth is in preclinical studies and is anticipated to advance into human safety studies in 2024. An overview of these programs includes:
•Soticlestat, a novel cholesterol 24 hydroxylase (“CH24”) inhibitor, which is currently being evaluated in two pivotal Phase 3 trials for Dravet syndrome and Lennox-Gastaut syndrome by Takeda Company Limited (“Takeda”). Takeda purchased our rights to soticlestat, and is conducting the pivotal trials. We maintain a significant financial interest in the potential approval and commercialization of soticlestat via potential regulatory and commercial milestones of up to $660.0 million and net sales-based royalty payments from low double digits up to 20%. We sold a 13% stake in the royalty, regulatory and commercial milestone payments that we are eligible to receive from Takeda to Ligand Pharmaceuticals Incorporated for $30.0 million.
•OV888 (GV101), a highly selective inhibitor of rho associated coiled-coil containing protein kinase 2 (“ROCK2”), which is being developed as a potential first-in-class medicine to treat cerebral cavernous malformations (“CCM”), of which seizures are among common symptoms. OV888 (GV101) is completing a Phase 1 double-blind, multiple-ascending dose trial and is expected to initiate its Phase 2 program in 2024. A higher dose cohort has been added to the Phase 1 trial and no serious adverse events have been observed.
•OV329, a highly potent next-generation GABA-aminotransferase inhibitor (“GABA-AT”), that is thought to potentially deliver preferable seizure reduction and dosing relative to prior medicines in the class. An oral formulation of OV329 is currently being evaluated in a Phase 1 study that uses biomarkers for efficacy and target engagement. An intravenous formulation of OV329 is also in development for acute seizures and is expected to enter human safety studies in 2025.
•OV350, a potential first-in-class direct activator of the central nervous system (“CNS”), specific K-Cl co-transporter (“KCC2”) and is the most advanced of a unique portfolio of drugs leads which are direct KCC2 activator compounds. Oral and intravenous (“IV”) formulations of several of these compounds have been prepared and have demonstrated activity in multiple preclinical disease models. We expect to file the first investigational new drug application (“IND”) from this portfolio in 2024. We believe this portfolio has the potential to deliver multiple INDs over the years to come.
Collectively, these development programs are anticipated to create a range of value-creating milestones in the near- and mid-term for investors.
The Opportunity: Epilepsies and Neurological Disorders
Although it is one of the earliest known maladies documented by humanity four millennia ago, epilepsy remains a common, and often intractable, medical diagnosis. Approximately 50 million people globally experience epilepsy, including an estimated three million adults living with epilepsy in the United States.
While modern drug discovery efforts have produced more than 30 ASMs over the last few100 years, and today represent a substantial opportunity medicallynumber of epilepsy patients continue to experience breakthrough seizures that can cause enduring damage to the brain. Individuals who suffer from rare forms of refractory epilepsies may experience persistent seizure rates ranging from 50 - 90%. The seizures they suffer can have a devastating impact both upon patients and commercially. Based ontheir families, by triggering permanent motor, cognitive and developmental delays, as well as epileptogenesis, which is a cascade of seizures begetting more seizures. Some patients with developmental epileptic encephalopathies experience even greater rates of refractory seizures that are resistant to drug therapy.
With an estimated 70% of epilepsy diagnoses occurring in people less than 20 years of age, the rapid increaseneed to treat seizures early and effectively is critical to mitigate worsening and permanent later-life disabilities. In the search for seizure control, approximately half of patients take a polypharmacy regimen of five or more ASMs, requiring careful management of drug side effects and interactions. The large population of patients requiring multiple drug therapies to control seizures, and persistent rates of breakthrough seizures, signal the urgent need for effective new medicines. For these patients, new mechanisms of action that demonstrate improved efficacy, safety and tolerability profiles are optimal, as they may be more easily incorporated into existing treatment regimens without the fear of drug-to-drug interactions (“DDIs”).
Scientific progress, including the availability of genetic testing, improved radiographic scanning tools, and more accurate means of measuring non-seizure symptoms are illuminating the underpinning seizure disorders. The great unmet medical need and scientific advancements have set the stage for a potential era of neurotherapeutics, which we believe will be led by ASMs.
The Ovid Strategy
The science underlying the discovery and development of new drugs for the brain has changed fundamentally over the last decade. We believe that major developments in scientificthe understanding of the rolebiology of geneticsthese diseases means that key areas of unmet need, including many epilepsies and key biological pathways relevantseizure disorders, are now addressable and offer significant medical potential. Our team has proven expertise in understanding MoAs that underlie seizures and shaping potential therapies to diseasestreat rare epilepsies and disorders with seizures. Specifically, we have built a pipeline focused on treating the extrinsic or intrinsic causes of neuronal hyperexcitability. This know-how affords us an ability to build Ovid in a manner focused on delivering successive, novel medicines for epilepsies and seizure-related neurological conditions.
Our strategy is to create sustainable, long-term value by advancing an exciting and differentiated pipeline of small molecules that culminates in a fully integrated neurotherapeutics company with multiple commercial medicines and clinical stage programs. Over time, we intend to seek to expand our current pipeline of predominantly ASMs to include additional franchises of neurology programs via focused clinical development and business development activities. This corporate strategy is underpinned by specific research and development, financial and business development strategies. In addition, our Company seeks to protect shareholder value by creating multiple sources of potential revenue via clinical and commercial milestones from our pipeline, strategic collaborations and partnerships.
Our approach to building an epilepsy franchise has already resulted in success, namely the brain,development and subsequent repurchase of our rights to soticlestat by Takeda. In 2017, we aimin-licensed a 50% stake in soticlestat for $26.0 million, and further invested $57.0 million in designing and executing soticlestat’s early and mid-stage clinical trials. In 2021, following encouraging Phase 2 findings, which we delivered six months ahead of schedule, we entered into a Royalty, License and Termination Agreement (“RLT Agreement”) through which we sold back our rights to identify, discoversoticlestat to Takeda. The RLT Agreement provided us with $196.0 million paid in Q1 2021 and, develop novel compoundsif soticlestat is approved and successfully commercialized, we are eligible to receive up to $660.0 million in sales and regulatory milestone payments and low double-digits up to 20% of potential net sales-based tiered royalty payments. The RLT Agreement provides us with a potential stream of non-dilutive capital. The funds received from this transaction have enabled us to invest in and secure what we believe is a world-leading pipeline during a period when we believe the cost of capital would have been unduly expensive. In 2023, we sold a 13% stake in the royalty, regulatory and commercial milestone payments that we are eligible to receive under the RLT Agreement to Ligand Pharmaceuticals Incorporated (“Ligand”) for $30.0 million.
Our near-term strategy is focused on building a franchise of potential small molecule medicines to treat certain epilepsies and conditions with seizure symptoms. In alignment with this focus, in 2023, we made the treatment of rare neurological disorders. decision to both out-
license and halt certain non-core activities, including several preclinical genetic medicines programs. For additional information see the section below under the heading “Genetic Research Programs.” We simultaneously streamlined our organization and reduced headcount related mostly to the genetic programs.
Expert Team and Fit-for-Purpose Infrastructure
We have built a highly specialized, efficient, and focused infrastructure that supports us in our chosen area of neurotherapeutics development. This infrastructure spans the critical domains of research and development, commercial and market access strategy. Importantly, it positions us to be a potential partner of choice for leading biopharmaceutical companies who wish to pursue valuable drug candidates and research platforms in neurology.
We have recruited a team of professionals with deep knowledgesubject-matter expertise in seizures and neurological conditions. This includes epileptologists, physicians, academic scientists, commercial and biopharmaceutical industry leaders. In total, we have five individuals with M.D. degrees and 13 professionals with Ph.D. degrees specializing in the sciences. Our operational and commercial leaders have extensive experience fostering market access and sales for leading neurological medicines. In total, our team’s collective professional experience has involved the successful development or commercial launch of more than 25 CNS medicines, including several epilepsy products.
Our cohesive focus in epilepsies and conditions with seizure symptoms, clear strategy reinforced by our professional experience and pipeline of differentiated assets, gives us confidence we can succeed in our mission.
Research & Development Strategy: Potential First-In-Class or Best-in-Class Mechanism of Action
Our research and development strategy is focused on designing medicines that can ameliorate neuronal hyperexcitability and return neurons to a state of homeostasis, or “electrophysiological balance.” Many factors can contribute to neuronal hyperexcitability including those extrinsic to the cell such diseases, howas anatomical abnormalities, trauma, and even infectious disease. Other factors are intrinsic to the neuron (for example, genetic conditions or the disruption of neuronal metabolism) and others (for example lack of stimulus of the GABA receptor) are caused by imbalances outside the surface of the neuron. Whatever its origin, imbalance of electrophysiological homeostasis and resultant neuronal hyperexcitability manifests in a range of debilitating neurological conditions, including seizures and epilepsies.
Treatment of epilepsy and seizures today often requires multiple medicines.Whereas some epilepsy developers expressly focus on one biological target, we believe that until a definitive cure for epilepsy is discovered, multiple MoAs will be needed to successfully treat themthe heterogeneous causes of seizures. Accordingly, our pipeline seeks to curate and howdevelop a unique set of compounds that can be effective by itself and/or can provide the necessary intervention to impact multiple mechanisms. We believe this approach has the potential to provide the basis of a modern differentiated and leading epilepsy franchise. Core tenets of our approach include a focus on:
•Small molecule compounds. Ovid’s pipeline is comprised of small molecule programs that can potentially be delivered as a pill, injection, or intravenously. Recent advances in synthetic methodology, formulation technology, generative artificial intelligence and biology target research have unlocked more opportunities for innovative and creative medicines. The compounds we seek to develop are brain-permeable and are designed to modulate specific biological targets. We seek to take advantage of the clinicallyversatility small molecules have to offer in terms of manufacturing, chemistry, and dosing to potentially deliver novel and next-generation medicines that will make meaningful endpoints required for developmentimprovements in the lives of a compoundpatients.
•Unique biological targets implicated in these disorders. As a resultneuronal regulation. There is compelling evidence that the novel targets we pursue directly or indirectly modulate hyperexcitability. These targets are associated with regulating inherent intracellular excitatory/inhibitory balance within neurons.
•Differentiated potential first-in-class or best-in-class mechanisms of this knowledge, we have developedaction. We cultivate a pipeline of potential first-in-class compoundsor potential best-in-class MoAs to intercept and mitigate the underlying cause of seizures or brain disease. Collectively, our differentiated pipeline has produced four active potential value-creating drug programs. Several of these therapeutic development programs (soticlestat, OV888 (GV101) and have demonstrated our model by progressing compounds throughOV329) seek to late-stage development. We continueaffect metabolic, signaling, and enzymatic pathways to executemodulate extrinsic causes of hyperexcitability, thereby restoring neural balance. In essence, they seek to change the environment surrounding the neuron.Our KCC2 mechanisms of action act on our strategy to build this pipeline by discovering, in-licensing and collaborating with leading biopharmaceutical companies and academic institutions.Our Focus: Rare Neurological Disorders
Rare neurological disorders are among the most devastating in their impact on patients and their families. Patients suffering from these disorders typically require full-time care, and yet are among the most underserved. We believe that there are at least 100 neurodevelopmental disorders, epileptic encephalopathies and other related rare neurological disorders that we may be able to target. These disorders are characterized by impairments in the growth, development and functioningintrinsic excitability of the brain. Dueneuron.
•Sentinel indication clinical development. Our drug development approach generally pursues rare, resistant conditions as initial “sentinel” indications. Pursuing rare acute conditions can enable us to a historical overwhelming preference indemonstrate the drug industry to develop drugsrapid proof-of-concept (“POC”) for broader neurological indications, many of these disorders have no approved therapies. As a result, recent scientific advancements have been overlooked, which we believe presents us with an opportunity to pursue these indications. These reasons include:our compounds, while additionally exploring efficient regulatory pathways and
High penetrance linking genetic defect to disorder pathology. Some rare neurological disorders have a genetic origin and typically have a strong correlation, or penetrance, between the presence of a gene and the manifestation
5
incentives. Case studies of the corresponding disease pathology. As a result, we believelife-cycle management for prior ASMs suggest that demonstration of refractory seizure reduction is often indicative of therapeutic effect in those cases we canmore common and tractable seizure types. Similarly, many ASMs were later proven to have clinical efficacy in other neurological conditions. Simply put, effective therapeutic outcomes in highly pharmaco-resistant indications often bodes well for treating broader neuropathologies.
•Prioritization of the total drug profile. We strive to develop drug candidates that willdeliver therapeutic efficacy while maintaining safe and well-tolerated side effect profiles. As noted above, for neurology patients and clinicians who must regularly manage side effects associated with polypharmacy regimens, it is preferable to have medicines that are well tolerated and exhibit few DDIs. In addition, we strive to impact the broader symptomatology of disease, including for example, the measurement of behavior and communication change.
As the field of ASM advances, we believe connections may be efficacious in patients with a given genetic profile.
Predictive geneticestablished between the root cause of hyperexcitability and other models. Recent advancesneurological conditions affecting larger populations. These include neuropsychiatric and neuro degenerative disorders and pain. However, our strategy focuses initially on evaluating our investigational medicines for pathways and targets impacting rare brain conditions. If effective in genetics enable usrare disease, our intent is to employ predictive explore expanding on that success to broader conditions of the brain for which the MoA may hold therapeutic relevance. Accordingly, in vitro and in vivo genetic models of certain of these disorders. These models allow usthe future, we expect to evaluate and observe a drug candidate’s potential activity prior to initiation of clinical trials. Through these models, we believe we will be able to select the most relevant clinical endpoints forextend our trialsknowledge and increase the potential for clinical success.
Overlapping pathophysiology and symptoms. Neurological disorders are often characterized by a number of overlapping symptoms, such as seizures, sleep disturbances, movement deficiencies and behavioral manifestations. We believe these commonalities will enable us to employ clinical endpoints that may be translatable from one disorder to another, and to develop drugs that may provide a clinical benefit across multiple indications.
Early observation of proof-of-concept. By employing clinical endpoints that are highly relevant and are designed to detect meaningful clinical benefits, we anticipate that many of our studies may provide early proof-of-concept in clinical development.
Potential ability to affect disease progression. We are focusing on disorders that are typically diagnosed in early childhood when the brain is still developing. We believe that we may be able to meaningfully address symptoms and potentially alter the progression of disease, especially if the drug can be administered early in life.
Motivated and accessible patient populations. We are targeting our programs for disorders with motivated and accessible patient populations. We believe that the patients and caregivers affected by these disorders are avid users of social media, in order to learn about and share relevant information and experiences. We use digital platforms to efficiently identify new patients for our clinical trials, raise disease awareness and help connect the patient and caregiver communities.
The Ovid Strategy
Our strategy is to pursue drug discovery and development for rare neurological disorders in a manner that is scientifically driven, patient focused and is coupled with an integrated and discipled approach to research, clinical development and business development. As we build on our understanding of these rare neurological conditions, we gain an appreciation of the way the different molecular mechanisms and pathways underlying these disorders help drive the symptoms patients suffer. This, in turn, benefits us with the knowledge gained about genetics, relevant molecular pathways, physiological impact and clinical endpoints from one disorder to another, which we believe will enable us to build a scalable scientific platform and efficient development capabilities. Ovid has set out to be a leader in this field, and by keeping our focus on neurology, it is our belief that this offers us the potential to produce multiple medicines in the future, and thereby succeed in our mission.
Scientifically Driven
We take a scientifically driven approach to identify promising drug candidates for our pipeline. We are building our portfolio based on the existence of
clearknown biological rationales
including a focus on disorders that
have, where possible, a direct genetic linkage. We use our deep understanding of the area to identify mechanisms of action, appropriateare associated with targets, and
initial drug candidates.which can be evaluated using validated biomarkers and/or clear endpoints, for study in clinical trials. As we advance our drug candidates
intothrough nonclinical and
through the clinical evaluation, we
are building on theapply a systematic approach for de-risking compounds using emerging
body oftools, animal models and trial designs. This paradigm is continuously informed and refined with emerging scientific and clinical insights
developed by usto strengthen and
others in the biopharmaceutical industry to target these important disease pathways of the brain. As we evaluate data from previousde-risk development for prospective programs and
ongoing preclinical studies and clinical trials, we intend to refine and improve our scientific approach and apply these insights to continue to build our pipeline and conduct our clinical trials.
In particular,
Specifically, our approach is driven by the following scientific principles:
identify the genetic origin•Pursuit of the disorder;
develop understandingvalidated and emerging targets. We are building a pipeline of gene expressiontherapeutic development programs representing distinct MoAs, including validated and linkemerging biological targets in epilepsy and neurovascular disorders such as CCMs. We seek to pathophysiology;
target biological pathways or genes for which proof-of-conceptPOC has already been established viain vitro or animal models;
•Blood brain barrier (“BBB”) penetration. The brain is one of the most difficult organs in the body to treat, in part due to the challenge of penetrating the BBB. Ovid’s drug development programs include potential small molecule therapies that demonstrate penetration of the BBB.
•Clinically translatable preclinical models. Recent advances in genetics enable us to employ predictive in vitro and in vivo genetic models of specific brain diseases. We believe these predictive models will allow us to evaluate and observe a drug candidate’s potential activity prior to initiation of human trials. Applying these models, we believe we will be able to select the most relevant indication and seizure endpoints for our studies and increase the potential for clinical success.
•Clear primary endpoints and scales. We primarily focus on the pathwaysdisorders that are characterized by epilepsy-related symptoms and mechanismsseizures. Many seizure types afford clear observable endpoints and biomarkers that cause the pathology of the disorderhelp capture and that generate the symptoms that we can target;
target optimal mechanism of action for drug candidates; and
utilize biomarkers if present that can providemeasure evidence of the activityclinical impact of our drug candidates.
Our team of development experts has extensive experience designing scales to measure other symptoms that are common among seizure-disorders, such as cognitive declines, movement deficiencies and behavioral manifestations. These skills support our desire and ability to develop medicines that may provide a clinical benefit across multiple aspects of patient health.•Trial design enables early observation of proof-of-concept. By employing surrogate biomarkers and endpoints that are highly relevant and designed to detect meaningful clinical benefits, we anticipate that many of our studies may provide early POC in clinical development, thereby directing the use of our capital to projects with higher probability of later-stage success.
•Motivated and accessible patient populations.We are focused ontargeting our programs for disorders with motivated and accessible patient populations. We believe that the patient communitiespatients and caregivers affected by epilepsies and brain disorders have increasing access to diagnostics and genetic testing. Additionally, many are avid users of social media, through which they learn new insights about their conditions and share relevant information and experiences. We conduct patient disease community outreach and activities on digital platforms to efficiently identify new patients for our clinical trials, raise disease awareness and help connect with patients and caregivers.
Pipeline Enhanced by Academic Collaborations and Disciplined Business Development
To support our strategy we continue to enhance and expand our pipeline via two complementary efforts: (1) internal research and development efforts in collaboration with external leaders in the rare neurological disordersfield and academic collaborators; and (2) business development activities to partner assets that have promising potential in and outside our chosen therapeutic areas.
Ovid conducts focused internal drug discovery, which helps us maintain lower costs for laboratory facilities. We identify compounds with what we address.believe is untapped value sitting in other organizations’ pipelines and look to in-license or enter into collaborative agreements to secure such assets and advance clinical development. This strategy directs our efforts where we excel at creating value, which is specifically shaping translational and clinical-stage development in our therapeutic area. An integral part of our process is establishing collaborations with academic research centers to support translational expertise for our programs, including the Stephen Moss Lab of Neuropharmacology at Tufts University.
The multiple programs in our diversified pipeline create optionality to pursue disciplined business development to expand our opportunities. As the pipeline progresses, we may endeavor to partner the development of our compounds in non-core indications or extend regional market rights outside the United States. We believe thisthat we are well positioned to execute on our business development strategy due to the extensive experience and networks of our management team. Collectively, our senior management has transacted hundreds of in-licensing deals and collaborations.
Patient-Focused
Ovid is developing product candidates that we hope will create new possibilities and more good days for people living with rare epilepsies and brain conditions. For example, we believe that our therapeutic candidates may be able to meaningfully reduce harmful seizures, mitigate burdensome symptoms, and potentially slow down the underlying progression of disease.
Patient communities are critical to informing every aspect of our
approachapproach. Each disorder for which we are developing potential neurotherapeutics is
critical. Eacha condition that carries serious risk of
these disorders affect small populations of patients, but carry serious morbiditiesmorbidity and
requirerequires extensive and specialized involvement from the patients’ families, caregivers, physicians and
from patient advocacy groups.
Our strategy is enhanced by the following patient-focused principles:
•pursue under-addressed rare conditions that can be evaluated via scaled clinical trials;
•develop close relationships with patients,patient communities, caregivers, families, disease foundations and key opinion leaders, to better understand the history of these disorders, raise awareness, identify patients and facilitate enrollment of clinical trials;
•identify clinically meaningful endpoints, including seizures, symptoms, and cognitive and behavioral scales that are based on input from patientsthe patient communities and their physicians and caregivers; and
•develop digital capabilities to engage, fosterbe deeply informed and maintain close relationshipsengaged with the patient communities.
communities we serve.Financial Strategy
Research and Development Coupled with Business Development
We have builtare focused on delivering long-term value for shareholders. Our financial strategy seeks to apply our capital in a broadfocused manner to advance a differentiated pipeline of potential drug candidatesneurotherapeutics, which we believe may generate multiple value-creating milestones from data and ultimately, commercial sales.
Management believes that we have sufficient cash on hand to
treat rare neurological disorders. Initiallyfund Ovid's operations into the first half of 2026. To achieve this cash runway, in October 2023, we
in-licensed or partnered drug candidates. More recentlysold 13% of our
pipeline has been built through our internal research and development efforts in collaboration with external leadersinterest in the
field. The intended result is to develop a diversified pipeline to mitigate development riskmilestones and
provide for scientific and medical opportunity. Central to the success of this process is coupling a highly focused and disciplined effort to link our research effort in key laboratories around the world aimed at discovering and securing relevant assets in selected rare neurological disorders.As a result, we have built a specialized, scalable and robust infrastructure that we believe will make us a leader in rare neurological disorders and the partner of choice for leading biopharmaceutical companies or academic institutions that wish to maximize the value of their neurology drug candidates or research platforms in these areas. This infrastructure spans across critical domains which include but are not exclusive of target discovery, drug delivery and clinical development. If and when our drug candidates are approved, we also plan to establish over time a focused commercial and distribution network dedicated to rare neurological disorders in the United States and Europe, where we believe the patient populations and medical specialists are sufficiently concentrated to effectively market our drug candidates. We believeroyalties that we are particularly well positioned eligible
to
executereceive from soticlestat's potential approval and commercialization to Ligand for $30.0 million. We retain 87% interest in soticlestat's regulatory and commercial milestones and royalties on
sales if the drug is successfully approved and commercialized by Takeda. These future payments may contribute to funding our
operations and business development
strategy becauseactivities. For additional information, see the description of
both the
extensive network of our ChairmanLigand Agreement below under the heading “License and
Chief Executive Officer Dr. Jeremy LevinCollaboration Agreements – 2023 Ligand Pharmaceuticals Milestones and
other members of our management team, as well as our demonstrated success in collaboration with partners including Takeda. Royalties Monetization.”
Ovid Pipeline
Our Pipeline
Our efforts have already brought two drug candidates from proofPOC into human clinical trials. Today, we are one of conceptthe few companies that has researched and either through pivotal trialsdeveloped three distinct MoAs to target seizures and one of the only clinical development programs to evaluate a highly selective ROCK2 inhibitor for neurological disease. We believe this pipeline of potential first-in-class or topotential best-in-class mechanisms differentiates us and provides the initiationbasis of pivotal trials. an attractive franchise of potential small molecule neurotherapeutics.
The following table
(Figure 1) sets forth
the status and mechanism of action of our drug
candidates: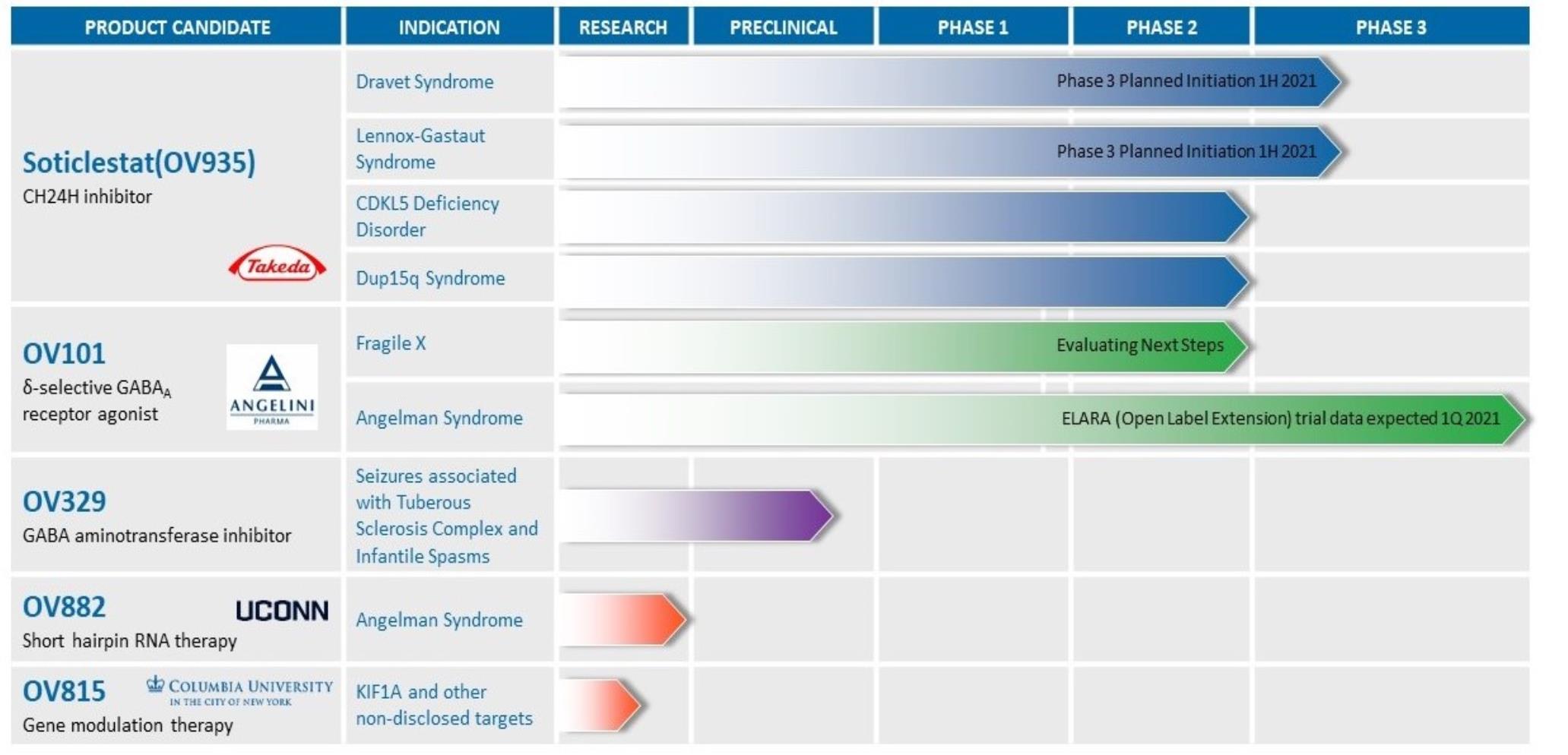
OV935 (soticlestat)
OV935 is being developedcandidate programs and their development status, MoA, and anticipated near-term milestones:
Figure 1. Ovid Therapeutics Pipeline
Soticlestat: Eligible for Non-Dilutive Capital from Takeda Pharmaceuticals
We retain significant financial interest in soticlestat, a joint collaboration with Takedanovel CH24 inhibitor for the potential treatment of rare epileptic encephalopathies.patients with resistant epilepsies, following our role in its successful early and mid-stage development program. We believe soticlestat has the potential to become a first-in-class medicine targeting the metabolism of cholesterol in the brain. It has been shown to gradually reduce inflammation in the brain as well as indirectly acting on the N-methyl D-aspartate pathway. We believe that this dual mechanism plays an important role in modulating excitatory signals involved in epilepsy, and thereby suppressing seizures.
Soticlestat is currently being studied by Takeda in two global, pivotal Phase 3 trials for people living with Lennox-Gastaut syndrome (“LGS”) and Dravet syndrome (“DS”). Takeda has stated two pivotal Phase 3 clinical trials studying soticlestat as a treatment for Lennox-Gastaut syndrome and Dravet syndrome completed enrollment, and it anticipates topline data readout by September 2024. Takeda has stated that it anticipates filing soticlestat for regulatory approval in its fiscal year 2024 (April 2024 – March 2025). If soticlestat receives regulatory approval and is commercialized, under the RLT Agreement, we are eligible to receive regulatory and commercial milestones payments of up to $660.0 million, in addition to potential net sales based tiered royalties of up to 20%. In 2023, we sold a 13% stake in the royalty, regulatory and commercial milestone payments that we are eligible to receive under the RLT Agreement to Ligand for $30.0 million. Royalty payments are eligible on net sales across all regions and all future indications. The future milestones payments do not include an initial upfront payment that we received from Takeda in March 2021 of $196.0 million.
Background on Takeda Royalty, License & Termination Agreement
The soticlestat development program began in January 2017
we entered intoas a license and collaboration agreement
withbetween us and Takeda
orfor rare epilepsies. Under this original agreement, Ovid held a 50% ownership stake in soticlestat and Takeda retained the
Takeda collaboration agreement, to develop and commercialize OV935 (soticlestat or TAK-935)remaining 50%.
Under the Takeda collaboration agreement, all costs and all profits are shared onFollowing a
worldwide basis and Ovid leadssuccessful Phase 2 development
andprogram led by us, in
addition is responsible for commercialization in North America, Europe and Israel.In March 2021, we entered into a royalty,the RLT Agreement. Under the terms of the RLT Agreement, we terminated our original collaboration agreement with Takeda, and Takeda subsequently secured an exclusive license and terminationintellectual property rights to repurchase our 50% share in soticlestat. In exchange, we received an upfront payment of $196.0 million. In addition, if soticlestat achieves regulatory approval, and is successfully commercialized, we are eligible to receive up to an additional $660.0 million in regulatory and commercial milestone payments and potential tiered royalties on net sales of soticlestat at percentages ranging from the low double-digits up to 20%, subject to standard reductions in certain circumstances. Royalties are payable on a country-by-country and product-by-product basis during the period beginning on the date of the first commercial sale of such product and ending on the expiration of patent rights in such country.
As a result of this agreement, orTakeda secured all the Takeda License and Termination Agreement, with Takeda under which Takeda will secure global rights at closing from us to develop and commercialize the investigational medicine OV935soticlestat for the treatment of developmental and epileptic encephalopathies, including DS and LGS. ClosingIn addition, Takeda has assumed responsibility for all development and commercialization costs associated with soticlestat. We have no ongoing costs or obligations.
OV888 (GV101) – A highly selective ROCK2 inhibitor
In May 2023, we invested in and entered a collaboration with Graviton Bioscience Corporation (“Graviton”) to develop a portfolio of highly selective inhibitors of ROCK2 for the treatment of rare neurological conditions. The collaboration includes the development of lead program OV888 (GV101), which is a small molecule ROCK2 inhibitor that is currently completing a Phase 1, double-blind multiple-ascending dose trial. The trial is progressing on track, a higher dose has been added and no serious adverse events have been reported. For additional information, see the description of the
Takeda License and Termination Agreement is subject toGraviton collaboration agreement below under the
satisfaction of customary closing conditions. See “Business—Licenseheading “License and Collaboration
Agreements—Agreements
with Takeda—2021 Royalty, License– 2023 In-licensing and
TerminationCollaboration Agreement with
Takeda” for a discussion of the terms of the Takeda License and Termination Agreement.OV935Graviton Bioscience Corporation.”
OV888 (GV101) is a potent,
BBB penetrant inhibitor that is highly selective
inhibitor of the enzyme cholesterol 24-hydroxylase, or CH24H. We believe, if approved, OV935 has the potential to become a first-in-class and only-in-class compound targeting the metabolism of cholesterolfor ROCK2. ROCK2 is expressed abundantly in the brain. We believe that OV935 inhibits a key enzyme in cholesterol metabolism pathwayskeletal muscles and in the brain and may modulate the excitatory signals involved in epilepsy, which may suppress seizures. CH24H is
predominantly expressed in the brain, where it plays a central role in cholesterol homeostasis and neuronal physiology. Recent literature suggests that in additionbelieved to
its impact on membrane cholesterol homeostasis in the central nervous system, modulation of CH24H may have an impact on over-activation of neurotransmitter pathways that have been implicated in a number of neurological disorders, such as epilepsy. Preclinical data suggest that inhibition of brain CH24H indirectly reduces glutamatergic signaling via NMDA receptors and modulates glialprimarily function and inflammation, which may impact disease pathology and epileptogenesis.to regulate intracellular cytoskeletal organization. We believe that because OV935 is an inhibitor of CH24H itthe ROCK2 signaling pathway may modulate the excitatory signals involvedbe hyperactivated in epilepsy, which may suppress seizures. In addition to these effects onmultiple neurological diseases, including disorders involving vascular structures and nerve myelination diseases that can result in seizures, and excitability of the brain, we believe that OV935 may reduce inflammation in and neurotoxic damage to the brain, which may lead to long-term, disease modifying effect. As a result, OV935 is being developed in rare and difficult to treat epilepsies with the goal to develop OV935 not just as a potential medicine to treat the seizures but also one that may have long-term disease-modifying potential. We believe that OV935 if successful in these areas may have utility in other areas as well. OV935 is initially being studied in those suffering from severe and often intractable forms of DEE, including Dravet syndrome, or DS, Lennox-Gastaut syndrome, or LGS, CDKL5 Deficiency Disorder, or CDD, and Duplication 15q, or Dup15q syndrome. There are limited or no therapeutic options in each of these disorders.
We have completed multiple trials on OV935 and expect to initiate pivotal phase 3 trials in second quarter of 2021. Previously we completed a Phase 1b/2a clinical trial of OV935 in a mixed group of adults with DEE and announced the results in December 2018. The trial achieved its primary endpoint of safety and tolerability, dose proportional reduction in a potential plasma biomarker called 24HC,spasms and a robust reduction in seizure frequency (61% at day 92), with two patients becoming seizure-free at the endvariety of the treatment period. Followingother symptoms. Despite this trial and as further discussed below, we reported the initial data from the Phase 2 open-label extension study (which we refer to as the ENDYMION trial) of OV935 in six patients who previously completed our 12-week Phase 1b/2a clinical trial of OV935 in adults with DEE.
On August 25, 2020, we, together with Takeda, announced positive topline results from the ELEKTRA trial which was a phase 2 trial in DS and LGS, along with updated findings from the ENDYMION trial. Based on the trial results of ELEKTRA and after presentation of the data and review of the phase 3 pivotal trial approach with regulatory authorities in the US (FDA), EU (CHMP), and Japan (PMDA), we expect to initiate two separate multinational Phase 3 pivotal registrational trials in Dravet syndrome and LGS with OV935 in the second quarter of 2021.
The FDA has granted orphan drug designation for OV935 for the treatment of Dravet syndrome and LGS.
Dravet Syndrome
Dravet syndrome is a severe form of childhood epilepsy largely genetically driven by the mutation of the SCN1A gene that typically presents during the first year of life. Eighty percent of patients have a mutation of the SCN1A-gene. Children experience frequent seizures, loss of muscle control, cognitive deficits and, in approximately 10% of cases, death before the age of 12 years. Children continue to suffer from seizures and severe cognitive and developmental impairment throughout their lifetime. While some patients may survive into adulthood, their long-term intellectual development and seizure outcomes are typically extremely poor. The incidence of Dravet syndrome in the United States ranges from 1 in 15,700 to 1 in 20,900 births.
Lennox-Gastaut Syndrome
Lennox-Gastaut syndrome is a rare disorder that is often diagnosed between three and five years of age. Patients diagnosed with Lennox-Gastaut syndrome experience a multitude of seizure types that are difficult to manage and have many of the same symptomologies as other rare pediatric epilepsies. Lennox-Gastaut syndrome affects over 30,000 people in the United States with approximately half being children under the age of 18. Some patients have de novo genetic mutations, including a mutation of the SCN2A gene. The annual incidence of Lennox-Gastaut syndrome in childhood is estimated to be two per 100,000 children. It is also estimated that between 1% and 4% of childhood epilepsies are a result of Lennox-Gastaut syndrome.
CDKL5 Deficiency Disorder
Patients with cyclin-dependent kinase-like 5 (CDKL5) mutations present with early epilepsy. In particular, early drug-resistant epilepsy, usually starting in the first months of life, tends to be the most common feature. Complex partial seizures, infantile spasms, myoclonic, generalized tonic-clonic, and tonic seizures have all been reported. Stereotypic hand movements, severe hypotonia, and impaired psychomotor development are usually associated with CDKL5 mutations and common to the general clinical manifestations.
Dup15q syndrome
Duplications of the proximal arm of chromosome 15q11.2-q13.1 result in the genetic condition Dup15q syndrome. Multiple genes including UBE3A from this region are implicated in the pathogenesis of autism spectrum disorders, epilepsy, and schizophrenia. Increased expression of the UBE3A gene contributes to epilepsy in Dup15q syndrome and it is thought to be the underlying cause of the autistic features of the syndrome as well. The most devastating feature of Dup15q syndrome is difficult to control seizures. The most common seizure types are infantile spasm and generalized tonic–clonic seizures followed by atonic, myoclonic, focal-onset, and tonic seizures. Poorly controlled seizures severely impact the quality of life of both affected individuals and their caregivers. Current treatment options for Dup15q syndrome-associated epilepsy are often ineffective. GABAergic promoting antiepileptics are typically ineffective while broad-spectrum antiepileptic medications such as valproic acid and rufinamide provide some relief.
OV935 Clinical Data
Phase 1 Trials
OV935link, there has been tested in 86 healthy volunteers across four Phase 1 trials. Single oral doses of up to 1,350mg of OV935 were well-tolerated. The most frequently reported adverse events were headache, ECG electrode application site dermatitis and nausea. All reported events were mild with no apparent dose-response. In a 14-day repeat dosing trial, doses of 100mg QD, 300mg QD and 400mg QD were well-tolerated. One volunteer at the 300mg BID experienced an event of confusional state and another volunteer at the 600mg QD dose experienced acute psychosis. Both volunteers discontinued the trial at day 11. One volunteer receiving placebo reported events of nightmares, spatial disorientation, insomnia and dizziness. All AEs resolved with continued dosing through day 15.
No SAEs were reported. Overall, no safety issues of concern were identified in the Phase 1 trials based on assessments of physical examinations, vital sign measurements, clinical laboratory values or 12-lead electrocardiogram findings.
The following table summarizes each Phase 1 trial:
Trial | | Purpose | | Design | | Number of Volunteers | | Dosage |
1 | | Safety and tolerability | | Phase 1, randomized, double-blind, placebo-controlled, single ascending dose trial | | 48 | | 15-1,350mg, oral |
| | | | | | | | |
2 | | Safety and tolerability | | Phase 1, randomized, double-blind, placebo-controlled, multiple ascending dose trial | | 40 | | 100-600mg QD, and 300mg BID, 14 days, oral |
| | | | | | | | |
3 | | Brain CH24H enzyme occupancy using positron emission tomography, or PET | | Open-label, non-randomized | | 11 | | 50-600mg, oral |
| | | | | | | | |
4 | | Relative bioavailability of tablet versus solution formulation; effect of food | | Phase 1, randomized, open-label, single dose trial | | 9 | | 300mg (tablet), oral; 300mg (solution), oral |
Phase 1b/2a Trial - 2001
The Phase 1b/2a trial of OV935 achieved its primary endpoint of safety and tolerability and showed OV935 was generally well tolerated. The trial was designed to have two parts. Part 1 was a randomized, double-blind, placebo-controlled (OV935 vs. placebo with a ratio of 4:1), 30-day phase that included a titration period (20 days: 100 mg, 200 mg twice daily), and treatment period (10 days).
OV935 achieved the primary endpoint of safety and tolerability as measured by incidence of AEs. AEs in patients treated with OV935 were similar to those who received placebo in Part 1. The majority of AEs in both treatment arms were mild. Overall, the data are consistent with a favorable safety and tolerability profile and support the continuedlimited clinical development of OV935.
AEs that occurred more frequently in the OV935-treatment group versus the placebo group were: dysarthria, insomnia, lethargy, seizure cluster, and upper respiratory infection. Four patients discontinuedROCK2 inhibitors due to an AE or an SAE inchallenges penetrating the OV935 treatment arm. Of these, in PartBBB and the inherent challenge of avoiding inhibition of rho-associated coiled-coil-containing protein kinase 1, one patient discontinued due to difficulty with walking and worsening lethargy and a second discontinued due to weakness. In Part 2, one patient discontinued due to a single episode of seizure cluster and a second experienced multiple seizure clusters.
An increase in seizure frequency was seen in three patients, all of whom were on perampanel. This suggests the potentialwhich can have unwanted side effects.
The initial indication for
a drug-drug interaction between medicines acting on different glutamatergic receptors. Accordingly, changes in seizure frequency data for the Phase 1b/2a trial are now reported with the inclusion and exclusion of the three patients on perampanel, respectively.ENDYMION
ENDYMIONOV888 (GV101) is a Phase 2 prospective, multi-center, open-label extension study of OV935 in patients with DEE who have participated in a previous OV935 clinical study. The primary objective is to assess the long-term safety and tolerability of OV935 over four years of treatment in patients with rare epilepsies. A secondary endpoint evaluates the effect of OV935 on seizure frequency over time. ENDYMION enrolled eligible patients from the ELEKTRA and ARCADE trials, each discussed below.
In September 2019, we announced initial data from the first six patients in ENDYMION who were previously enrolled in the Phase 1b/2a clinical trial of OV935 in adults with DEE and subsequently ceased taking OV935 for a period of between 6 weeks and 12 months. Therefore, in most cases the seizure frequency increased from the end of the adult DEE trial until they were enrolled in ENDYMION. As shown in Table 1, longer-term data from ENDYMION out to 48 weeks suggest increased seizure reduction with prolonged treatment of OV935 and is consistent with the believed mechanism of action of OV935. Median seizure frequency reductions were 84% following 25 to 36 weeks (n=6) and 90% following 37 to 48 weeks (n=4) of treatment.
Table 1: % Reduction from Baseline in Seizure Frequency
| | | | | | | | |
| | Weeks 1-12
| | Weeks 13-24
| | Weeks 25-36
| | Weeks 37-48
|
Overall median % reduction in seizure frequency from baseline
| | 48%
(n=6)
| | 65%
(n=6)
| | 84%
(n=6)
| | 90%
(n=4)*
|
% of Patients with ³50% reduction in seizure frequency from baseline
| | 50%
| | 50%
| | 67%
| | 75%
|
*
| At the time of data analysis, two patients had not yet completed 48 weeks of dosing.
|
Patient baseline seizure frequency ranged from 2 to 71 (median=11.5). In general, a greater reduction in seizure frequency was observed in those with higher baseline seizure frequency. In terms of overall seizure-free interval during treatment, one patient experienced 264 consecutive days and one patient experienced 150 consecutive days without a seizure.
In addition to the six patients from the Phase 1b/2a adult DEE trial included in this data analysis, all patients who have completed the ARCADE and ELEKTRA trials to date have enrolled in ENDYMION. Data from patients who have completed ARCADE, our Phase 2, multi-center, open-label, pilot study evaluating the treatment of OV935 in patients with epileptic seizures associated with CDD or Dup15q syndrome, are not included the above analysis due to their limited treatment duration in ENDYMION. Data from patients previously treated in ELEKTRA are not included in the above analysis due to the ELEKTRA trial being double-blinded and placebo-controlled at the time of the analysis.
Overall, at 48 weeks, safety observations were consistent with the completed Phase 1b/2a clinical trial in adults with DEE. OV935 continues to show a favorable safety and tolerability profile. The majority of adverse events were mild and comparable with those from the Phase 1b/2a trial. Specifically, adverse events that occurred included upper abdominal pain, pyrexia, bronchial wall thickening and rales. There was one treatment-related adverse event of nausea. No serious adverse events were observed.
As discussed below, in August 2020 and September 2020, we announced updated findings from ENDYMION from patients who completed the ELEKTRA and ARCADE studies, respectively, and elected to enroll in ENDYMION.
ELEKTRA
ELEKTRA was an international, multi-center, randomized, double-blind, placebo-controlled study designed to evaluate treatment with soticlestat in pediatric patients, aged 2 to 17 years, with highly refractory epileptic seizures associated with convulsive seizures (DS) or drop seizures (LGS). The study consisted of a four- to six-week screening period to establish baseline seizure frequency, followed by a 20-week double-blind treatment period, including an 8-week dose optimization period and a 12-week maintenance period. During the 8-week dose optimization period, patients were titrated from 100mg twice daily (BID), to 200mg BID to 300mg BID (mg/kg dosing for <60 kg) of orally administered soticlestat.
A total of 141 patients were enrolled in ELEKTRA and 126 completed the study. A modified intent-to-treat, or mITT, analysis of 139 patients was performed to evaluate the efficacy endpoints, which includes any patient who enrolled in the study and received at least one dose of study drug. Patients in the study were allowedanticipated to be on one to four concomitant anti-epileptic drugs, or AEDs, with the majority of patients concomitantly treated with at least three AEDs. The most common AEDs taken by the patients were valproate, clobazam, levetiracetam and topiramate. Further, all patients who completed ELEKTRA enrolled in the ENDYMION open-label extension study.
On August 25, 2020, we, together with Takeda, announced positive topline results from ELEKTRA and updated findings from ENDYMION. The ELEKTRA study achieved its primary endpoint with high statistical significance, demonstrating a 27.8% median reduction from baseline in convulsive seizure (DS) and drop seizure (LGS) frequency compared to a 3.1% median increase in patients taking placebo during the 12-week maintenance period (median placebo-adjusted reduction=30.5%; p=0.0007, based on the efficacy analysis set of 120 patients with seizure data in the maintenance period)CCM for which there is strong mechanistic evidence for inhibiting ROCK2 (see Figure 2 below). In addition, DS and LGS patients treated with soticlestat demonstrated a 29.8% median reduction in convulsive seizure (DS) and drop seizure (LGS) frequency compared to 0.0% change in median seizure frequency in patients taking placebo during the full 20-week treatment period (titration plus maintenance) of the ELEKTRA study (placebo-adjusted reduction=25.1%; p=0.0024). Soticlestat was generally well-tolerated in the ELEKTRA study and demonstrated a safety profile consistent with those of previous studies, with no new safety signals identified. All patients who completed the ELEKTRA study elected to enroll into the ENDYMION open-label extension study and findings from ENDYMION were also reported on August 25, 2020. The ENDYMION study data of rolled-over ELEKTRA patients support results in the ELEKTRA study. The ENDYMION data indicated maintenance of effect over 6 months in those patients originally randomized to soticlestat, and similarly reduced seizure frequency as compared to baseline in those patients previously assigned to the placebo arm. No new safety signals were identified in ENDYMION.
ARCADE
ARCADE is a Phase 2 open-label, signal-finding pilot study designed to inform the potential for future development of soticlestat in CDD and Dup15q syndrome. The study enrolled 20 patients, ages 2 to 55 years, with refractory epileptic seizures associated with CDD (n=12) or Dup15q (n=8) and consisted of a four- to six-week screening period to establish baseline seizure frequency, followed by a 20-week treatment period, including an eight-week titration/dose optimization period and a 12-week maintenance period. Patients in the study were allowed to be on one to six concomitant AEDs, with the majority of patients
concomitantly treated with at least four AEDs, representing a highly refractory patient population. The primary objective of the ARCADE study was to determine percent change from baseline in motor seizure frequency during the 12-week maintenance period. Further, all patients who completed ARCADE enrolled in the ENDYMION open-label extension study.
On September 30, 2020, we announced results from ARCADE and updated findings from ENDYMION. Together, data from the ARCADE and ENDYMION studies showed seizure frequency reduction over time. In CDD patients (n=12), median motor seizure frequency reduction was 24% during the 12-week maintenance period in the ARCADE study, increasing to a 50% reduction in the ENDYMION long-term extension study in the five CDD patients who reached nine months of continuous treatment. In Dup15q patients (n=8), there was an increase in median motor seizure frequency in the ARCADE study during the 12-week maintenance period; however, longer-term data from the four Dup15q patients who reached nine months of continuous treatment showed a 74% reduction in median motor seizure frequency. Soticlestat was generally well tolerated in both studies and continues to demonstrate a favorable safety profile. We believe the resultsCCMs are encouraging and next steps are being evaluated with Takeda.
OV101 (gaboxadol)
To date, we have been developing OV101 for the treatment of Angelman syndrome and Fragile X syndrome, two neurodevelopmental disorders that are characterized by similar symptoms. In December 2020, we reported the primary endpoint of the Pivotal Phase 3 trial in pediatric individuals with Angelman syndrome (the NEPTUNE trial) was not achieved. Based on the results of the NEPTUNE trial, further development of OV101, other than the ongoing long-term extension study with Angelman syndrome patients who had previously been in a trial for OV101 (the ELARA trial), is currently on hold. The data from all trials is under review and further development of OV101, if any, will be the subject of full analysis of this data.
Angelman syndrome and Fragile X syndrome have overlapping symptoms, including sleep disorder, aberrant behavior, anxiety and cognitive or intellectual disabilities thought to be caused by decreased tonic inhibition, an important mechanism whereby it is believed that the brain distinguishes signal from noise. Both of these disorders are typically diagnosable in early childhood and require full-time care for the patients affected. In September 2016, the FDA granted orphan drug designation for OV101 for the treatment of Angelman syndrome; in October 2017 the FDA granted orphan drug designation for OV101 for the treatment of Fragile X syndrome; in December 2017 the FDA granted fast track designation for the treatment of Angelman syndrome; and in March 2018, the FDA granted fast track designation for the treatment of Fragile X syndrome. In June 2019, the European Commission granted OV101 orphan drug designation for the treatment of Angelman syndrome based on the results of the STARS clinical trial.
OV101 and Tonic Inhibition
Tonic inhibition is a critical physiological regulatory mechanism that allows a healthy human brain to decipher excitatory and inhibitory neurological signals correctly without being overloaded. Defects of this system are thought to play a role in multiple disease states, including Angelman syndrome, Fragile X syndrome and insomnia. Because of its unique mode of action, OV101 represents a promising compound targeting this mechanism.
Decreased tonic inhibition results in an imbalance in the ratio of excitation to inhibition. If tonic inhibition is reduced, the brain becomes inundated with signals and loses the ability to separate background noise from critical information. This imbalance disrupts normal brain functioning, including sensory processing and integration. Disruption of tonic inhibition can lead to a multiplicity of symptoms including, but not limited to, sleep abnormalities, motor deficiencies, behavioral manifestations, delayed development, intellectual disability and severe speech impairment. By modulating tonic inhibition, OV101 may have the potential to alleviate important symptoms and provide a meaningful clinical benefit to patients across several disorders.
We believe that OV101 modulates tonic inhibition. Disruption of tonic inhibition can lead to a multiplicity of symptoms including, but not limited to, sleep abnormalities, motor deficiencies, behavioral manifestations, delayed development, intellectual disability and severe speech impairment. We believe modulating tonic inhibition may have a meaningful clinical impact in patients with Angelman syndrome and Fragile X syndrome.
Angelman Syndrome
Overview. Angelman syndrome is a rare genetic disorder that is typically diagnosed in the United States after one year of age when parents notice severe developmental delays or the child suffers seizures. Characteristic features of this disorder include delayed development, intellectual disability, severe speech impairment, problems with movement and balance, seizures, sleep disorders and anxiety. Individual patients with Angelman syndrome can have varied symptoms, including the inability to walk or control motor movement, which can limit their ability to handle daily functions such as feeding, dressing or bathing. These patients are also often hyperactive, leading to various behavioral problems. Angelman syndrome symptoms, such as poor sleeping patterns, can lead to serious consequences, including increased frequency of seizures and exacerbation of behavioral manifestations. Different patients with identical genetic defects can show different degrees of these symptoms. Most Angelman syndrome patients require full-time care and are unable to live independently, which can represent a substantial emotional and financial burden on their families. In addition, Angelman syndrome has been associated with poor parental sleep and high parental stress.
According to the National Organization for Rare Disorders, the approximate prevalence of Angelman syndrome is between 1 in 12,000 and 1 in 20,000 people. There are currently no approved therapies in the United States or ex-US specifically for the treatment of Angelman syndrome. No standard of care exists for Angelman syndrome, and current therapeutic options are symptomatic, suggesting a high unmet clinical need. Given the likely high burden of Angelman syndrome, new treatments targeting the aetiology of the syndrome that result in even small improvements in features of the syndrome may be clinically meaningful for patients and their families.
Fragile X Syndrome
Overview. Fragile X syndrome is a genetic condition that results in intellectual disability, anxiety disorders, behavioral and learning challenges and various physical disabilities. Patients with Fragile X syndrome exhibit autism-like symptoms, including cognitive impairment, anxiety, mood swings, hyperactivity, attention deficit and heightened sensitivity to various stimuli, such as sound. The severity of an individual patient’s impairment can range from mild learning disabilities to more severe cognitive or intellectual disabilities. Fragile X syndrome is one of the most commonly inherited intellectual disability disorders. Childrencommon intracranial vascular malformation in humans, presenting as mulberry-shaped abnormal blood vessels with Fragile X syndrome also often have unusual sleep patterns and may have difficulty with routine activities such as feeding and dressing. The challenges presented by Fragile X syndrome often extend beyond the patient and can lead to significant hardships on the emotional and financial health of their families.
Fragile X syndrome is caused by mutationsthin, leaky walls located in the fragile X mental retardation gene,brain and or FMR1 gene. FMR1 spinal cord. It is a gene that leads tothought CCMs affect approximately 1:250 individuals. At diagnosis, the synthesismajority of the fragile X mental retardation protein, FMRP, which is needed for normal brain development. The FMR1 gene normally contains in its sequence between 5 and 44 copies of a short, repeated motif, or recurring pattern in DNA. In Fragile X syndrome, there are more than 200 copies of this motif in the FMR1 gene, a genetic change that prevents the synthesis of FMRP. PatientsCCM patients present symptomatically with intermediate numbers of repeats are able to make some FMRP and have milder symptoms.
According to the National Fragile X Foundation, Fragile X syndrome affects approximately 1 in 3,600 to 4,000 males and 1 in 4,000 to 6,000 females. The average age of diagnosis of Fragile X syndrome is approximately three years. Currently, there are no approved therapies for the treatment of Fragile X syndrome. The current standard of care for the psychiatric challenges of Fragile X syndrome is tailored to each patient and may include antipsychotics, antidepressants and drugs to treat attentionhemorrhage, focal neurologic deficit and sleep disorders. Special education and symptomatic treatments for anxiety and irritability are often employed to lessen the burden of illness. Fragile X syndrome patients also may experienceand/or seizures, which are treated with traditional anticonvulsants.
Wereflect areas of core competency for our development acumen.
To date, preclinical and human safety studies evaluating OV888 (GV101) have
conducted a number of clinical trials with OV101. In 2017, we completed a successful Phase 1 clinical trial in adolescent patients with Angelman and Fragile X syndrome. In 2018, we completed a Phase 2 trial of OV101 in adults and adolescents with Angelman syndrome, which we refer to as the STARS clinical trial. The STARS clinical trial achieved its primary endpoint of safety and tolerability and showed statistically significant improvement in the once-daily OV101 dosing group on the pre-specified physician-rated Clinical Global Impressions-Improvement, or CGI-I, exploratory endpoint as well as improvements in relevant symptoms such as sleep, motor function and behavior.Based on the results of the NEPTUNE trial, further development of OV101, other than the ELARA trial, is currently on hold. The data is under review and further development, if any, will be the subject of full analysis of the data from this trial together with the data from ELARA.
Angelman Syndrome
STARS
In July of 2018, we completed the Phase 2 STARS trial, a 12-week, double blind, placebo-controlled study in adults and adolescents with Angelman syndrome that randomized 88 patients aged 13 to 49 years. The study achieved its primary endpoint of safety and tolerability with a similar incidence of adverse events, or AEs, across the OV101 and placebo treatment arms. The investigational medicine showedshown it has a favorable safetytoxicology profile and was well tolerated in adults and adolescents with Angelman syndrome through the 12 weeks of treatment. The most common AEs reported in the trial were vomiting, somnolence, irritability, aggression, and pyrexia. Serious adverse events, or SAEs, of seizure were reported in two patients with a previous history of seizures: one patient
in the once-daily, QD, dose group experienced a seizure that was deemed unrelated to study drug; one patient experienced a seizure in the twice-daily, BID, dose group that was assessed as possibly related to study drug by the investigator. At the prespecified efficacy analysis at 12 weeks of treatment, OV101 QD and BID dose groups showed a statistically significant improvement compared to placebo in the CGI-I. CGI-I was ranked first in the prespecified hierarchy of the statistical analysis plan. Data from the full analysis indicate that OV101 positively impacts several relevant clinical features of Angelman syndrome (global functioning, sleep, motor disruption). We met with the FDA in November 2018 to discuss OV101 as part of our End of Phase 2 Meeting.
NEPTUNE
Following the End of Phase 2 Meeting, we initiated the NEPTUNE trial and enrolled our first pediatric patient in September 2019. NEPTUNE was a randomized, double-blind, placebo-controlled, Phase 3 study that enrolled and treated 97 pediatric patients diagnosed with Angelman syndrome, four to 12 years of age, and seven patients diagnosed with Angelman syndrome ages two to three years for safety and pharmacokinetic evaluation only. The study was designed to assess the effects of treatment with OV101 (oral, once-daily dosing) versus placebo over 12 weeks. The sole primary endpoint was change in overall score on the Clinical Global Impression-Improvement-Angelman syndrome, or CGI-I-AS, scale. Secondary endpoints included sleep, communication, motor function, socialization, daily living skills and behaviour domains.
The primary endpoint of the NEPTUNE study was not achieved. Patients given OV101 showed a 0.7-point improvement in CGI-I-AS over baseline while placebo also showed a 0.8-point improvement in CGI-I-AS (p=NS). Secondary endpoints continue to be evaluated, although initial results show no difference between OV101 and placebo.
OV101 was well-tolerated, with no significant safety issues observed. We plan to complete a full analysis of the results of the NEPTUNE study and discuss these results with the FDA to determine next steps, if any, for the program. We will continue to offer study drug to patients enrolled in the open-label extension trial, ELARA, pending further analysis of the NEPTUNE study.
ELARA
Based on the STARS clinical trial data, we also initiated ELARA, an open-label extension trial which enrolled its first patient in February 2019, and enrollment is ongoing. We expect to report data from the ELARA study in the first half of 2021.
Based on the results of the NEPTUNE trial, further development of OV101, other than the ELARA trial, is currently on hold. The data is under review and further development, if any, will be the subject of full analysis of the data from this trial together with the data from ELARA.
Fragile X Syndrome
ROCKET
In May 2020, we completed a Phase 2 trial evaluating OV101 in adolescent and young male adults with Fragile X syndrome, which we refer to as the ROCKET clinical trial. The trial met its primary objective and OV101 appeared to be well tolerated over 12 weeks of treatment with no serious adverse events reported across all three dose cohorts. OV101 demonstrated a statistically significant effect on secondary behavioral endpointsreported. We anticipate initiating the Phase 2 program for OV888 (GV101) in the three combined study groups as follows: 26.2% mean improvement insecond half of 2024. Throughout OV888's (GV101) clinical development, we intend to harness the Aberrant Behavior Checklist for Fragile X (ABC-CFXS) total score from baseline to week 12 (p=0.002); and a 21.6% mean improvement in the Anxiety, Depression and Mood Scale (ADAMS) total score from baseline to week 12 (p=0.004). Statistically significant improvements were also observed across various ABC-FXS and ADAMS subscales. In addition, OV101 demonstrated a statistically significant mean reduction of 0.4 in the Clinical Global Impressions Scale-Severity (CGI-S) total score (p=0.002) from baseline to week 12.
SKYROCKET
We also initiated the SKYROCKET study in the fourth quarter of 2018. SKYROCKET was a 12-week, non-drug study to assess the suitability of several behavioral scales in individuals with Fragile X syndrome. The trial was an observational study designed to provide additional data on the key endpoints that are being explored in the ROCKET trial as well as provide contextual data on the benefit offeredexpertise held by the standard of care. The participating clinicians and caregivers were aware thatteam at Graviton, who previously pioneered the trial was non-interventional. The mean changes from baseline to week 12 were evaluated in the ABC total and subscale scores, the ADAMS subscale scores, and the CGI-S subscale scores as well as the mean change in CGI-I score at week 12. Other exploratory scales were also assessed. High variability was seen among caregiver-administered assessments (ABC-c, ADAMS) compared to clinician-assessed scales (CGI-I, CGI-S). The caregiver-administered assessments showed a placebo response as seen with previous Fragile X syndrome trials. In these other trials, placebo response rates were highly variable. Therefore, the SKYROCKET trial data will help inform future study design, including potential endpoints and measures to mitigate placebo response.
Based on the results of the NEPTUNE trial, development of OV101Rezurock, the first approved ROCK2 inhibitor for graft vs. host disease.
Figure 2. Believed mechanism of action for OV888 (GV101) in Fragile X syndromeCCM
OV329 – A next-generation GABA-AT inhibitor
OV329 is under review to enable us to fully understand the ramifications if any, for any potential future trial in Fragile X syndrome.
Previous Clinical Development of OV101 in Insomnia
We acquired worldwide rights to OV101 from H. Lundbeck A/S, or Lundbeck, in March 2015. Prior to the acquisition, Lundbeck filed an investigational, new drug application, or IND, with the FDAnext-generation GABA-AT inhibitor that we are developing for the treatment of insomnia. Pursuantadult and pediatric epilepsy disorders. OV329 represents a potential best-in-class GABA-AT inhibitor and was designed to this IND, Lundbeck and Merck & Co., Inc., or Merck, partnered to conduct several Phase 3 trials for primary insomnia between 2004 and 2007. Over the course of the development of OV101, over 4,000 adults were administered OV101, resulting insupplant vigabatrin, which is an OV101 exposure of approximately 950 patient years. These trials were primarily randomized, placebo-controlled short-term and long-term safety and efficacy clinical studies, using classic sleep parameters such as total sleep time, time to sleep onset, wakefulness after sleep onset, and number of nocturnal awakenings as clinical endpoints.
The Phase 3 program consisted of three trials: two 3-month placebo-controlled trials conductedapproved therapeutic in the United States and one 2-week trial conducted in Europe and Canada, each evaluating OV101 against placebo and the active comparator, zolpidem (Ambien). The primary endpoints of the trials included total sleep time and time-to-sleep onset and the secondary endpoints included number of nocturnal awakenings, wakefulness after sleep onset and daytime function. In Phase 3 trials, which were conducted for durations of up to 12 months, OV101 was observed to have efficacy that was largely comparable to zolpidem (Ambien) on several sleep metrics. In the first 3-month trial, a dose of 15mg of OV101 met both of the primary endpoints as well as the secondary endpoints at week one and month three compared to placebo. In the second 3-month trial, the same dose met only total sleep time and number of awakenings at week one, but significance was lost after adjusting for multiplicity at month three. The 2-week trial met all primary endpoints and wakefulness after sleep onset, for 15mg of OV101 at weeks one and two. Additionally, subjects who were administered OV101 showed no evidence of withdrawal symptoms or rebound insomnia after discontinuation of short-term treatment, whereas transient rebound insomnia was observed in subjects receiving zolpidem. In addition, clear differences were observed between OV101 and zolpidem from a sleep architecture perspective. OV101 has shown consistent increases in slow wave sleep compared to zolpidem with no significant effect on stage 2 or REM sleep in healthy adult, elderly subjects. It is believed that slow wave sleep is important for encoding long-term, fact-based memories. Slow wave sleep has been associated with physical changes in neuronal connections.
Safety and Tolerability
Overall, OV101 was observed to be well-tolerated in adult patients aged 18-64 years in the Phase 2 and Phase 3 insomnia trials at doses of 5mg to 15mg given as evening doses. Success in these previous trials does not ensure that our clinical trials in OV101 will generate the same results or otherwise provide adequate data to demonstrate the efficacy and safety of OV101. The most common reported adverse events were headache, nausea, vomiting, somnolence and dizziness. In general, the adverse events appeared to be dose-related. The majority of the serious adverse events observed were considered to not be related to treatment with OV101. One SAE, fatigue, was considered by Lundbeck and Merck as probably related to OV101 treatment. Among the nine SAEs considered by Lundbeck and Merck to be possibly related to OV101 treatment, there were three cases of fainting and one case each of: radius fracture, abnormal QRS axis, transient ischemic attack, non-cardiac chest pain, unresponsive to stimuli and atrial fibrillation. Across trials there were no apparent clinical trends regarding SAEs with respect to frequency, distribution across system organ classes or preferred terms. Also, there were no apparent clinical differences versus placebo. In the Phase 3 trials at the 15mg dose, at least one SAE was observed in 1.3% of subjects at two weeks and 3 months and 3.0% of subjects at 12 months versus 0.0% to 1.0% on placebo over the same time frame.
Consistent with the clinical development of other insomnia drugs, the FDA requested that Lundbeck and Merck conduct a series of preclinical and clinical abuse studies as part of their development program. In preclinical studies, OV101 demonstrated low abuse potential. In one clinical trial, the abuse potential of OV101 was investigated in doses up to 45mg in male and female subjects with a history of hypnotic/sedative abuse and other drug abuse. Safety results showed that OV101 administered at doses of 30mg and 45mg in women and 45mg in men was not tolerated in this population of drug abusers, contrary to previous experience with the same doses in healthy volunteers. This indicated that a history of drug abuse decreased tolerability to OV101 in these subjects. Adverse events that were associated with a dose-dependent lack of tolerability in this trial included psychiatric, nervous system, musculoskeletal and gastrointestinal disorders.
In 2007, following the completion of all clinical trials for OV101 in insomnia, Lundbeck and Merck discontinued the development program for insomnia, and announced that the overall clinical profile did not support further development of OV101 for insomnia.
Early-stage Research Programs
OV329 (GABA aminotransferase inhibitor) is a preclinical compound being developed by usEuropean Union for the treatment of infantile spasms. Vigabatrin has demonstrated substantial seizure reduction, which led to sales of more than $300 million by Lundbeck in the United States alone. However,
vigabatrin's clinical and commercial use has been limited by lack of a therapeutic window. Specifically, vigabatrin has generated deleterious ocular effects in some patients, including retinal degradation and irreversible vision loss that led to significant post-market restrictions and monitoring. We believe OV329 to be an improved GABA-AT inhibitor with a different chemical structure, potency and inhibition profile than vigabatrin. We have observed OV329 to deliver increased potency and efficiency in the target binding site and has been shown in our preclinical research to be more than 200-fold more potent. We are actively studying two formulations of OV329: (1) an oral formulation for chronic seizures, which is currently in a Phase 1 trial and 2) an intravenous formulation for acute seizures, which is being readied for an IND (or an equivalent regulatory application) expected in late 2024. We believe that OV329 has the potential to be therapeutic in refractory and drug-resistant epilepsies such as seizures associated with Tuberous Sclerosis Complextuberous sclerosis and Infantile Spasms.refractory status epilepticus (“SE”).
A benefit of our OV329 functionsprogram is that it acts upon a validated drug target for seizures. Specifically, it works by substantially reducing the activity of GABA aminotransferase (GABA-AT),GABA-AT, a key enzyme responsible for the degradation of the brain’s major inhibitory neurotransmitter, GABA. OV329 leads to increased concentrations of GABA by inhibiting its metabolism.metabolism. Given that epilepsy is characterized by excessive neuronal excitation, the increased levels of GABA may suppress this excitatory signaling and may reduce seizures. OV329 oral formulation
Based upon preclinical data supporting OV329, we believe that the oral formulation has the potential to provide (in comparison to vigabatrin) preferred (lower) dosing. We believe that these preclinical data suggests that OV329 could allow for seizure reduction efficacy and improved tolerability in comparison to existing therapeutics.
To support this profile, seven animal seizure models presented at medical conferences in 2022 and 2023 demonstrate the seizure reduction potential of OV329 (see Figure 3 below). These findings in both chronic and acute seizure models provide additional confidence about the therapeutic potential of OV329 in humans.
Figure 3. Seven preclinical animal models reaffirm OV329 seizure reduction activity, including resistant seizure models
To further differentiate OV329’s potential profile from vigabatrin, our preclinical efforts sought to extensively characterize its safety and tolerability, including any potential ocular effects. We have demonstrated that the tissue clearance of OV329 is rapid, leading us to believe that the accumulation in the back of the eye may be less likely than vigabatrin, which has a longer half- life. The pharmaco-dynamic profile of OV329 is also highly differentiated from vigabatrin. Preclinical research has shown that OV329 induces phasic (synaptic) and tonic (extrasynaptic) inhibition of GABA-AT. This produced more GABA in the synapse and environmental milieu, potentially contributing to prolonged inhibitory effects.
Additionally, we applied a clinically translatable rodent model of albino Sprague-Dawley rats to determine if any ocular changes could be observed associated with the predicted therapeutic doses of OV329 and vigabatrin, as compared to placebo. This rodent model is an accepted proxy by the U.S. Food and Drug Administration (“FDA”) for the ocular effects seen in humans treated with vigabatrin. Figure 3 (below) demonstrates the results of our research.
After 45 days of dosing with the therapeutic dose of vigabatrin and an expected therapeutic dose of OV329 (3 mg/kg), the model showed no ocular effect in animals taking OV329, whereas disruption in retinal cells was seen in animals taking the therapeutic dose of vigabatrin (300 mg/kg). In this short-term model, OV329’s ocular profile appears similar to placebo, and no disruption to the retina was seen at the anticipated therapeutic dose. These models must be confirmed in human studies, though they lead us to believe that OV329 may offer significant seizure reduction benefit with a therapeutic window not provided by vigabatrin.
Figure 4. No ocular changes seen in rodents treated with expected therapeutic dose of OV329 (3 mg/kg)

In late 2022, our IND for OV329 was cleared by the FDA and subsequently, we initiated a Phase 1 trial. That study is currently ongoing at the Nucleus Network at Alfred Hospital in Australia and is expected to complete in the second half of 2024. The trial is being conducted in two parts, including: a single-ascending dose and a multiple-ascending dose portion. Endpoints will evaluate the pharmacokinetic profile, safety, tolerability and target engagement associated with escalating doses of OV329 in healthy volunteers. Two surrogate biomarkers will be applied in the study, including: (1) transcranial magnetic stimulation (“TMS”) will be measured and is a corollary for clinical efficacy and (2) magnetic resonance spectrometry (“MRS”) will be used to measure target engagement. Previous studies have reported MRS measurement of GABA concentration levels increase following treatment with GABA-AT inhibitors, which has been shown to correlate with seizure reduction efficacy in existing GABA-AT inhibitor programs. If successful,OV329 proves to effectively engage the target and exhibits a tolerable safety profile in the Phase 1 study, these metrics may inform mid-to-late-stage development of the program.
Upon results from the Phase 1 program, we anticipate OV329 could be used to treatfurther studied for the treatment of seizures associated with Tuberous Sclerosis Complextuberous sclerosis complex, infantile spasms and Infantile Spasmsother epilepsies associated with focal onset seizures. If the safety and efficacy profile of OV329 is positive, we will also consider lifecycle management strategies in broader epilepsy indications.
OV329 Intravenous Formulation
We are currently assessingactively formulating and conducting IND-enabling studies for an intravenous use of OV329, which would be intended for the treatment of acute seizures, such as refractory status epilepticus (“RSE”). It is thought that approximately 35,000 Americans experience RSE. RSE is defined as a medical emergency where patients experiencing seizures of five minutes of duration or longer do not respond to first or second-line therapies. Status epilepticus (“SE”) and RSE can lead to enduring brain damage and increased rates of mortality.
While there is no intravenous formulation of vigabatrin for acute use, some investigator led studies have shown that the application of this approachGABA-AT inhibitor via a nasal-gastric tube has produced strong results that have led to the cessation of status epilepticus. Drawing on these findings, Ovid intends to submit an IND (or an equivalent regulatory application) for the IV formulation of OV39 in late 2024 and advance it in human studies for potential use in refractory SE.
Portfolio of KCC2 Transporter Direct Activators, Including OV350
We in-licensed a large portfolio of compounds from AstraZeneca AB (“AstraZeneca”) in December 2021 that are direct activators of a biological target expressed exclusively in the pre-clinicalCNS: the KCC2. We believe this portfolio represents the only small molecule program in the biopharmaceutical industry that directly activates the KCC2 transporter. KCC2 is a channel that regulates chloride homeostasis in neurons, and thereby is potentially important in the control of neuronal excitability and seizures. The portfolio includes a lead compound, OV350, and several other compounds that we believe are suitable for pharmaceutical development. We intend to analyze multiple candidates from the KCC2 portfolio for development in epilepsy as well as other possible neurological conditions associated with behavior, neuropathic pain or neurodegeneration. Since several compounds within the portfolio appear to be amenable for multiple formulations, the broad KCC2 program may provide Ovid with the ability to partner compounds for neurology indications outside our core competencies.
OV350
In vivo proof-of-concept studies in animals have established that restoring KCC2 activity leads to reduced seizure sensitivity and seizure-induced mortality. In one preclinical model, designed to mimic the acute seizure state of SE, OV350 terminated status and restored the efficacy of diazepam in SE seizures, whereas diazepam treatment alone failed to halt the seizures (see Figure 5 below). Preclinical mechanistic studies have also demonstrated that OV350 was well-tolerated and did not induce sedation. These findings must now be studied and demonstrated in humans.
Figure 5. OV350 Terminates Benzodiazepine Resistant Status Epilepticus
In 2023, we evaluated several compounds in the KCC2 portfolio and began optimizing the lead candidate, OV350, for possible intravenous and oral administration formulations. Dual formulations are optimal for patients who are treated acutely in the hospital and need chronic dosing to maintain outcomes in an out-patient setting.
To support human studies, we initiated a range of disease model experiments in seizure and psychosis models. As noted above, we believe that the anticonvulsant properties of OV350 are compelling in preclinical SE models. Additionally, two different animal models that are a proxy for human psychosis suggest that OV350 has a profile akin to atypical antipsychotics, though, it does not appear to carry the undesirable tolerability issues common in medicines for mental health conditions. Such a profile may represent an exciting profile for future psychosis medicines. Further research and IND-enabling studies are ongoing. We expect to submit the first IND from the KCC2 program by the end of 2024.
Figure 6. OV350 Demonstrates Antipsychotic Effects in Schizophrenia Model
The library of early-stage small molecules that target the KCC2 transporter, including OV350, are included in a pending composition-of-matter application that was filed globally and, if issued, will expire in 2041 excluding any potential regulatory extensions.
Genetic Research Programs
The majority of our development activities are dedicated to small molecule programs. However, we believe that genetic medicine will play an important role in the long-term future of treating genetic epilepsies and neurological disorders. Accordingly, we engage in appropriately scaled, early-stage research programs with certain collaborators, including Gensaic, Inc., formerly M13 Therapeutics, Inc. (“Gensaic”), a next-generation gene therapy developer.
Phage-Based Scaffolds: Gensaic Research Collaboration
In December 2016,August 2022, we entered into an investment in and research collaboration with Gensaic (the “Gensaic Collaboration Agreement”), a license agreementprivate biotech company that is developing gene therapies. Specifically, Gensaic is applying phage-display science that uses M13 phages as platforms to deliver genetic sequences. Though still in its nascence, we believe phage-based scaffolds may offer significant advantages for the delivery of genes, as compared to the current platform alternatives of adeno-associated viral (“AAV”) vectors. This collaboration is a part of a low cost and long-term strategy to potentially enable unique treatment modalities in genetic disorders with Northwestern University, or Northwestern, pursuanta potentially unique and proprietary, low-cost alternative to current therapies.
AAV gene therapies are not optimal for treating neurological conditions. Specifically, AAV gene therapies have limited cloning capacity, which Northwestern granted us an exclusive, worldwide license to patent rights in certain inventions, or the Northwestern Patent Rights, which relate to a specific compound and related methods ofrestricts their use for such compound, alongdelivering large genetic cargo. Additionally, they are immuno-reactive, have poor BBB permeability, and provide relatively poor tropism to target cells. In contrast, we believe phages offer the potential to: deliver larger genes (up to 20kb); be engineered to cross the BBB and deliver cargo to specific cell types; avert the immune system response to enable redosing; and be produced in a more cost-effective manner. As a result, we believe phage-based gene therapy has an improved potential for the treatment of genetic epilepsies and neurological diseases. We can pursue up to three targets in collaboration with certain Know-How relatedGensaic, which are not yet disclosed.
Genetic Programs: OV815, OV825 and OV882
Prior to
our strategic pipeline review in 2022, we had developed a series of early candidates for antisense oligonucleotide (“ASO”) and RNAi medicines as part of our collaborations with Columbia University and the
practiceUniversity of
Connecticut. These research programs included: OV815, which was in development for the
inventions claimedtreatment of mutations associated with KIF1A-associated neurological disorder (KAND); HNRNPH2 (also known as Bain Syndrome), which was a potential treatment for an X linked gene predominantly occurring in
the Northwestern Patents.females; and OV882, is a short hairpin RNA (shRNA-551) we are evaluating as a potential disease-modifying gene therapy for Angelman syndrome. The most common cause of Angelman syndrome is the loss of functional UBE3A protein duethat used short hairpin RNA to deliver a defect in the maternal copy of the UBE3A gene. Our aim is to develop a disease-modifying noncoding RNA vector that reducesreduced expression of UBE3A-antisense and restores UBE3A expression viaexpression.
At year-end 2023, we determined to pause these programs to prioritize our expanding clinical footprint in small molecule programs. We will continue to seek collaborations or out-licensing opportunities for these programs.
License and Collaboration Agreements
2023 Ligand Pharmaceuticals Milestones and Royalties Monetization
In October 2023, we sold Ligand a 13% stake in the paternal gene copy.royalties and milestones owed to Ovid related to the potential approval and commercialization of soticlestat (the “Ligand Agreement”). In return, we received a $30.0 million payment, less certain reimbursable expenses. We are in early stagesretained 87% of our research. This researchinterest in soticlestat's potential royalties and milestones. In the event that soticlestat is not approved and commercialized, we have no continuing debt or other obligations to Ligand.
2023 In-licensing and Collaboration Agreement with Graviton Bioscience Corporation
In April 2023, we entered into a Collaboration and License Agreement with Graviton, a privately held early-stage drug development company specializing in therapeutics that inhibit ROCK2. Under the terms of the agreement, we purchased shares of Graviton's preferred stock for $10.0 million and secured rights to develop their lead program
is being conductedOV888 (GV101) as well as a portfolio of ROCK2 inhibitors in
collaboration with the University of Connecticut School of MedicineIn June 2020, we began a strategic research collaboration with Columbia University Irving Medical Center, or Columbia, to advance genetic based therapies for a range ofmutually agreed upon rare neurological conditions, complementary of our pipeline. This collaborationCNS indications, excluding amyotrophic lateral sclerosis. The agreement provides us with worldwide rights, excluding China, Hong Kong, Macau and Taiwan.
Graviton is responsible for conducting the potential to expand our future drug development portfolioof the products through the end of Phase 2 trials under the oversight of a joint development committee from both companies. We are responsible for development and impact individuals living with rare genetic neurological conditions by working closely with the Precision Medicine Resourcecommercialization costs, post-Phase 2 development and commercialization of subsequent products. The collaboration has no milestone payments and Graviton will be eligible for percentage royalties in the Irving Institute at Columbia. Our first research program with Columbia is OV815 and focusesmid- to high-teens based on net sales in territories where the kinesin-familyproducts are marketed. Graviton retains rights to licensed products in all fields of proteins and KIF1A-associated neurological disorder (KAND), under this research and translational development alliance, Columbia will align its expertise in rare disease genetics and deep clinical understandingstudy outside of rare neurological diseases with our discovery, translational,brain disorders.
2022 Research Collaboration and
clinical development expertiseEquity Investment in
neurodevelopmental disorders and rare epilepsies.License and Collaboration Agreements
Collaboration Agreement with Angelini Pharma
On July 9, 2020,Gensaic
Under the terms of an equity agreement we entered into as an investor in Gensaic, we invested a total of $5.1 million in exchange for convertible preferred stock in Gensaic. We also entered into a collaboration agreement with Gensaic (the “Gensaic Collaboration Agreement”) to potentially develop up to three genetic medicines for neurological indications of interest to us, harnessing Gensaic’s proprietary phage-derived particle platform. Gensaic retains full rights to its platform technology. We will have commercial rights to license and develop any resulting phage-derived gene therapies that emerge from this collaboration subject to agreed-upon terms. We also retained rights to invest in future equity financing rounds.
2022 Out-License Agreement with Marinus Pharmaceuticals
In March 2022, we entered into an exclusive patent license agreement with Marinus, (“Marinus License Agreement”). Under the AngeliniMarinus License Agreement, with Angelini, pursuant to which we granted Marinus an exclusive, non-transferable (except as expressly provided therein), royalty-bearing right and license under certain Ovid patents relating to Angelini exclusive rightsganaxolone to develop, make, have made, commercialize, promote, distribute, sell, offer for sale and commercialize OV101, a selective agonistimport licensed products in the territory (which consists of the GABAA receptor,United States, the European Economic Area, United Kingdom and Switzerland) for the treatment of Angelman syndromeCDKL5 deficiency disorders. Following the date of regulatory approval by the FDA of the first licensed product in the European Economic Area as well as Switzerland,territory, which was received on March 18, 2022, Marinus issued, at the United Kingdom, Russia and Turkey, or the European Territory.Company's option, 123,255 shares of Marinus common stock, par value $0.001 per share. The licenses granted to Angelini include sublicenses under the Lundbeck Agreement, as well as licenses under our patents and know-how covering OV101. Angelini will be responsible for conducting any clinical trials necessary to obtain regulatory approval for OV101 for Angelman syndrome in the European Territory, and we will be responsible for bearing a portion of the costs for such trials. We will also be responsible, at our expense, for the completion of certain ongoing clinical trials for OV101, to the extent applicable to obtaining regulatory approval for OV101 in the European Territory. Angelini has the exclusive right, at its election, to develop and commercialize OV101 for the treatment of Fragile X Syndrome in the European Territory. The parties may also mutually agree to pursue additional indications for OV101 in the European Territory, and in such case, Angelini would have the exclusive rights to commercialize in such additional indications. Angelini is required to use commercially reasonable efforts to conduct development activities for OV101, and following regulatory approval, to commercialize OV101 in each approved indication.In conjunction with the entry into the Angelini License Agreement, the parties entered into a separate supply agreement, pursuant to which we will be responsible for supply of OV101 to Angelini for development and commercialization in the European Territory, through Angelini’s existing supply relationship with Lundbeck. The AngeliniMarinus License Agreement also provides for a transfer, at Angelini’s expense,payment of the relevant manufacturing technologyroyalties from us and Lundbeck to Angelini, in order to enable Angelini to assume responsibility for its own manufacture and supply of OV101 in the future.
Under the Angelini License Agreement, Angelini made an upfront payment and a milestone payment related to the transfer of a specified amount of compound and related information to the Company of $25.0 million during the year ended December 31, 2020. Angelini will be required to make additional milestone paymentsMarinus to us upon the completion of the specified components of the technology transfer and achievement of specified regulatory milestones for OV101 in Angelman syndrome of up to $55.0 million in the aggregate, as well as up to €162.5 million ($199.9 million) in sales milestone payments for achievement of specified levels of net sales in the European Territory. In addition, Angelini will be required to pay tiered royaltiessingle-digits on net sales by Angelini, its affiliates or sublicensees at double-digit percentages above the teens, subject to certain reductionsof each such licensed product sold.
2022 License and
offsets. Royalties will be payable on a product-by-product and country-by-country basis until the latest of the expiration of the licensed patents covering such product in such country, the expiration of market exclusivity for such product in such country, and fifteen years from first commercial sale of such product in such country.
Either party may terminate the Angelini License Agreement for an uncured material breach of the other party or in the case of insolvency. We may terminate the Angelini License Agreement if Angelini challenges any of the licensed patents. Angelini may terminate the Angelini License Agreement for convenience during specified notice periods, which are determined based upon whether the product has been commercially launched in the European Territory.
LicenseOption Agreement with H. Lundbeck A/S
Healx
In March 2015,February 2022, we entered into a license agreement with Lundbeck, which we subsequently amended in May 2019, or the Lundbeck agreement, pursuant to which we obtained from Lundbeck an exclusive (subject to certain reserved non-commercial rights), worldwide license to develop, manufacture, and commercialize OV101, also known as gaboxadol, for the treatment of human disease. Under the Lundbeck agreement, we are responsible for and will use commercially reasonable efforts to carry out all future development and commercialization of OV101. Initially, we will purchase OV101 compound from Lundbeck’s existing inventory at a specified price. Following the depletion of the existing inventory, we may purchase the compound from a third party or, if the parties agree, Lundbeck may continue to supply the compound to us. We are also obligated to make certain manufacturing-related payments to Lundbeck, including for its preparation of a drug master file for OV101.In connection with the Lundbeck agreement, we issued 489,756 shares of our common stock to Lundbeck. We also agreed to pay to Lundbeck milestone payments up to an aggregate of $189.0 million upon the achievement of certain global development, regulatory and sales milestone events. Pursuant to the Lundbeck agreement, the first milestone payment is $1.0 million, which is due upon the successful completion of the first Phase 3 trial for a product in which OV101 is an active ingredient. In addition, if we successfully develop and commercialize OV101, we will be obligated to pay to Lundbeck tiered royalties based on single-digit and low-double digit percentage of net sales of OV101, subject to certain reductions for generic product sales and for royalties paid for licenses to third party intellectual property. If Lundbeck manufactures OV101 compound for us after the expiration of the royalty term, we will pay to Lundbeck, in addition to the fully burdened cost of such manufacture, a low, single-digit manufacturing royalty on the net sales of OV101 manufactured by Lundbeck.
The Lundbeck agreement will continue until the expiration of all relevant royalty terms and may be earlier terminated by either party for the other party’s uncured material breach or insolvency. In addition, we can terminate the Lundbeck agreement upon advance notice for convenience at any time prior to first regulatory approval of OV101. If the Lundbeck agreement is terminated by us for convenience or by Lundbeck for our breach or insolvency, the OV101 compound will revert to Lundbeck and we will grant Lundbeck an exclusive license to develop and commercialize OV101; such license will be royalty-bearing if we filed an application for regulatory approval prior to termination. If we terminate the Lundbeckoption agreement for Lundbeck’s breach or insolvency, our license will continue and our obligations to make royalty payments to Lundbeck and to share Asian Partner payments(“Healx Agreement”) with Lundbeck will continue but we will not be obligated to make further milestone payments to Lundbeck or to purchase any additional quantities of OV101 compound from Lundbeck’s existing inventory.
Agreements with Takeda
2017 License and Collaboration Agreement with Takeda
In January 2017, we entered into the Takeda collaboration agreement with Takeda. All activities of the collaboration regarding OV935 will be guided by the Takeda/Ovid “One Team” concept, an integrated and interdisciplinary team from both companies devoted to the successful advancement of OV935 across rare epilepsy syndromes. Pursuant to the Takeda collaboration agreement, we will take the lead in nonclinical and clinical development activities and commercialization of the compound OV935 and products containing this compound (as well as certain other similar compounds, including any prodrug where TAK-935 is the primary pharmacologically active metabolite) for the treatment of certain rare neurological diseases in the United States, Canada, the European Union and Israel. Takeda will take the lead in commercialization of OV935 in Japan and has the option to lead in Asia and the rest of the world, or the Takeda Territory. While we and Takeda have agreed to initially focus on certain rare neurological disorders, the scope of the collaboration may in the future include other mutually agreed upon rare neurological disorders.
Under the Takeda collaboration agreement, Takeda granted to us an exclusive license in our territory under certain patents and other intellectual property controlled by Takeda to commercialize OV935 and products containing OV935 for the treatment of certain rare neurological disorders. Takeda also granted to us a worldwide, co-exclusive license to develop, manufacture and otherwise exploit (but not commercialize) OV935 and products containing OV935 for the treatment of certain rare neurological disorders.
Under the Takeda collaboration agreement, we granted to Takeda an exclusive license in the Takeda Territory under certain patents and other intellectual property controlled by us to commercialize OV935 and products containing OV935 for the treatment of certain rare neurological disorders. We also granted to Takeda a worldwide, co-exclusive license to develop, manufacture and otherwise exploit (but not commercialize) OV935 and products containing OV935 for the treatment of certain rare neurological disorders and a co-exclusive license in certain countries to commercialize OV935 and products containing OV935 for the treatment of certain rare neurological disorders that are subsequently included in the collaboration.
We and Takeda are collaborating in the development of OV935. Pursuant to the terms of the Takeda collaboration agreement, each party is required to use commercially reasonable efforts to develop OV935 for the treatment of certain rare neurological disorders in accordance with a mutually agreed upon development plan. We are primarily responsible for activities related to the development of OV935, and as such Takeda will transition certain development activities to us. Takeda is initially responsible for regulatory activities in all countries (excluding Israel)Healx, Ltd (“Healx”
). We are initially responsible for regulatory activities in Israel, and, upon regulatory approval in the United States, Canada, and the European Union, we will assume responsibility for further regulatory activities in such jurisdictions.We and Takeda are collaborating in the commercialization of OV935. Pursuant to the terms of the Takeda collaboration agreement, each party is required to use commercially reasonable efforts to commercialize OV935 for the treatment of certain rare neurological disorders in its territory. We are responsible for commercialization of OV935 in the United States, Canada, the European Union and Israel, and Takeda is responsible for commercialization of OV935 in Japan and has the first right to elect to commercialize the products in the Takeda Territory. Additionally, Takeda has the right to jointly commercialize the products with us in the United States and/or the European Union for any additional mutually agreed upon rare neurological indication.
Under the Takeda collaboration agreement, we and Takeda initially share equally all development and commercialization costs and expenses prior to launch of a product and all revenues and commercialization costs and expenses after launch. In the event that we and Takeda agree to expand the scope of the collaboration to include additional rare neurological disorders, either party may elect not to fund all or a portion of the development of such indication, in which case such party’s overall share of revenues and commercialization costs and expenses after launch of a product may be reduced under certain circumstances.
During the period commencing on the effective date of the Takeda collaboration agreement, we and Takeda have both agreed that we will not, directly or indirectly, and will cause all of our respective affiliates, not to, alone or with others, commercialize any competing product in the field of rare neurological disorders. For these purposes, a competing product is any product or compound directed against CH24H as its primary, intended mode of action. If, during such period, we or any of our affiliates is acquired by a third party that is commercializing a competing product, then we must divest our interest or terminate the commercialization of the competing product or cause our affiliate to do so.
The Takeda collaboration agreement will expire upon the cessation of commercialization of the products by both us and Takeda. Either party may terminate the Takeda collaboration agreement as a result of the other party’s uncured material breach or insolvency, for safety reasons, or, after completion of the first proof of mechanism clinical trial, for convenience. Takeda may terminate the Takeda collaboration agreement for our (or our sublicensee’s) challenge to the patents licensed under the Takeda collaboration agreement. If the agreement is terminated by Takeda for our material breach, bankruptcy or patent challenge or by us for convenience or safety reasons, our rights to the products will cease, we will transition all activities related to the products to Takeda, and we will grant Takeda an exclusive, royalty-bearing license under certain patents and other intellectual property controlled by us to commercialize OV935 and products containing OV935 for the treatment of certain rare neurological disorders. If the agreement is terminated by us for Takeda’s material breach or bankruptcy or by Takeda for convenience or safety reasons, Takeda’s rights to the products will cease, Takeda will transition all activities related to the products to us, and Takeda will grant us an exclusive, royalty-bearing license under certain patents and other intellectual property controlled by Takeda to commercialize OV935 and products containing OV935 for the treatment of certain rare neurological disorders.
Under the Takeda collaboration agreement, in the event of an acquisition of us by certain types of acquirers prior to the final dosing of a patient in the first Phase 3 trial, Takeda would have the right to elect to take over all development and commercialization activities with respect to the products, so long as Takeda at such time has (or will have) sufficient commercial infrastructure to commercialize the products. Even if Takeda exercises such right to take over all development and commercialization activities with respect to the products, we and Takeda will continue to share equally all development and commercialization costs and expenses prior to launch of a product and all revenues and commercialization costs and expenses after launch, unless otherwise set forth in the agreement.
In connection with the Takeda collaboration agreement and in consideration of certain license rights granted to us by Takeda, we issued 1,781,996 shares of our Series B-1 convertible preferred stock to Takeda. The shares of Series B-1 preferred stock held by Takeda automatically converted into 1,781,996 shares of our common stock in May 2017. Under the Takeda collaboration agreement, we are obligated to pay Takeda future payments if and when certain milestones are achieved. Upon the first patient enrollment in the first Phase 3 trial for the first of the initial disorders we and Takeda are focusing on, we are obligated to issue to Takeda the number of unregistered shares of our common stock equal to the lesser of (a) 8% of our outstanding capital stock on the issuance date or (b) $50.0 million divided by the applicable share price, unless certain events occur. In the event such payment would cause Takeda to own over 19.99% of our outstanding capital stock or other events occur, such payment must be paid in cash. The remaining potential global commercial and regulatory milestone payments equal approximately $35.0 million and can be satisfied in cash or unregistered shares of our common stock at our election.
2021 Royalty, License and Termination Agreement with Takeda
On March 2, 2021, we entered into a royalty, license and termination agreement, or the Takeda License and Termination Agreement, with Takeda, relating to the Takeda collaboration agreement described above. Under the terms of the Takeda LicenseHealx Agreement, Healx has secured a one-year option to investigate gaboxadol (OV101) as part of a potential combination therapy for Fragile X syndrome in a Phase 2A clinical trial, and Terminationas a treatment for other indications, for an upfront payment of $0.5 million, and fees to support prosecution and maintenance of our relevant intellectual property rights. In February 2023, we amended the Healx Agreement, upon closing to extend the option period by three months. At the end of the transaction,option period, Healx has the Takeda collaboration agreement will be terminated by mutual agreement, and Takeda willoption to secure rights to our 50% global share in OV935 (soticlestat), and an exclusive license under our relevant intellectual property rights, in exchange for an upfrontadditional payment of $2.0 million (“Option Fee”), development and commercial milestone payments, and royalties. Takeda will
assume all responsibility for, and costs of, both development and commercialization of soticlestat following closing. At closing, we will receive an upfront payment of $196.0 million and are eligiblelow to receive up to an additional $660.0 million in development, regulatory and sales milestones. In addition, if soticlestat achieves regulatory approval, we will receive tiered royalties on net sales of soticlestat at percentages ranging from the low double-digits up to 20%, subject to standard reductions in certain circumstances.mid-tier double digit royalties. Royalties are payable on a country-by-country and product-by-product basis during the period beginning on the date of the first commercial sale of such product in such country and ending on the later to occur of the expiration of patent rights covering the product in such country and a specified anniversary of such first commercial sale. The Takeda collaborationIn June 2023, the Healx Agreement was further amended to: (i) grant Healx a development license; (ii) grant a commercial license upon payment of the Option Fee to us; and (iii) restructure the timing of the Option Fee payable to us.
Healx assumed all responsibility for and costs of development of gaboxadol in June 2023. Healx will also be responsible for all commercialization costs of gaboxadol following the grant of a commercial license upon payment of the Option Fee. We retain the option to co-develop and co-commercialize the program with Healx (“Ovid Opt-In Right”) at the end of a positive readout of clinical phase 2B, and, in such case, would share net profits and losses in lieu of the milestones and royalty payments. We do not plan to conduct further trials of gaboxadol.
2021 Exclusive In-Licensing Agreement with AstraZeneca
In December 2021, we entered into an exclusive license agreement will remain(“AstraZeneca Exclusive License Agreement”) with AstraZeneca. Under the terms of the AstraZeneca Exclusive License Agreement, we have obtained worldwide rights to a portfolio of early-stage, small molecule compounds targeting the KCC2 transporter, including our lead compound, OV350. In exchange for an upfront payment of $5.0 million in effect until Takeda’s cessationcash and $7.3 million in shares of our common stock to AstraZeneca, we are responsible for using commercially reasonable efforts to carry out all future development and commercialization of soticlestat. KCC2 transporter activators in epilepsies and potentially other neuropathic conditions. We expectare obligated to closepay AstraZeneca potential clinical development milestones of up to $8.0 million, regulatory milestones of up to $45.0 million and total commercial milestones of up to $150.0 million, as well as tiered royalty payments ranging from the Takeda Licensesingle-digits up to ten percent on net sales. At the time of proof of clinical efficacy, AstraZeneca will have the right of first negotiation to opt in to co-develop and Termination Agreement inco-commercialize KCC2 transporter activators with Ovid. The license option will continue until the first halfexpiration of 2021, subject to the satisfaction of customary closing conditions, including regulatory review by the appropriate regulatory agencies under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended. The Takeda License and Termination Agreement may be terminated upon the mutual agreement of the parties, or by either Takeda or the Company, if the closing has not occurred on or before May 14, 2021. all relevant royalty terms.
2016 Northwestern License
for OV329
In December 2016, we entered into a license agreement
(“Northwestern Agreement”) with Northwestern
University (“Northwestern”), pursuant to which Northwestern granted us an exclusive, worldwide license to patent rights in certain inventions
or the (“Northwestern Patent
Rights,Rights”) which relate to a specific compound
(OV329) and related methods of use for such compound, along with certain
Know-Howknow-how related to the practice of the inventions claimed in the Northwestern
Patents.Patent Rights.
Under the Northwestern agreement, we were granted exclusive rights to research, develop, manufacture and commercialize products utilizing the Northwestern Patent Rights for all
uses. We have agreed that we will not use the Northwestern Patent Rights to develop any products for the treatment of cancer, but Northwestern may not grant rights in the technology to others for use inuses, other than cancer.
We also have an option, exercisable during the term of the agreement to an exclusive license under certain intellectual property rights covering novel compounds with the same or similar mechanism of action as the primary compound that is the subject of the license agreement. Northwestern has retained the right, on behalf of itself and other non-profit institutions, to use the Northwestern Patent Rights and practice the inventions claimed therein for educational and research purposes and to publish information about the inventions covered by the Northwestern Patent Rights. Upon entry into the Northwestern
agreement,Agreement, we paid an upfront non-creditable one-time license issuance fee of $75,000, and we are required to pay an annual license maintenance fee of $20,000, which will be creditable against any royalties payable to Northwestern following first commercial sale of licensed products under the agreement. We are responsible for all ongoing costs of filing, prosecuting and maintaining the Northwestern
Patents,Patent Rights, but we also have the right to control such activities using our own patent counsel. In consideration for the rights granted to us under the Northwestern agreement, we are required to pay to Northwestern up to an aggregate of $5.3 million upon the achievement of certain development and regulatory milestones for the first product covered by the Northwestern Patents, and, upon commercialization of any such products, will be required to pay to Northwestern a tiered royalty on net sales of such products by the Company, its affiliates or sublicensees, at percentages in the low to mid-single-digits, subject to standard reductions and offsets. Our royalty obligations continue on a product-by-product and country-by-country basis until the later of the expiration of the last-to-expire valid claim in a licensed patent covering the applicable product in such country and
10ten years following the first commercial sale of such product in such country. If Ovid sublicenses a Northwestern Patent Right, it will be obligated to pay to Northwestern a specified percentage of sublicense revenue received by us, ranging from the high
single digitssingle-digits to the low-teens.
The Northwestern
agreementAgreement requires that we use commercially reasonable efforts to develop and commercialize at least one product that is covered by the Northwestern Patent Rights.
Unless earlier terminated, the Northwestern
agreementAgreement will remain in force until the expiration of our payment obligations thereunder. We have the right to terminate the agreement for any reason upon prior written notice or for an uncured material breach by Northwestern. Northwestern may terminate the agreement for our uncured material breach or insolvency.
2015 License Agreement with H. Lundbeck A/S
In March 2015, we entered into a license agreement with Lundbeck, which we subsequently amended in May 2019, and July 2020 (collectively, the “Lundbeck Agreement”). As part of the Lundbeck Agreement, we obtained from Lundbeck an exclusive (subject to certain reserved non-commercial rights), worldwide license to develop, manufacture and commercialize OV101, also known as gaboxadol.
We subsequently closed our OV101 (gaboxadol) program in Angelman syndrome in early 2021. In February 2022, we entered into Amendment No. 3 to the Lundbeck Agreement (the “Amended Lundbeck Agreement”) to permit our performance under the Healx Agreement. Under the terms of the Amended Lundbeck Agreement, if Healx exercises its option, we will owe Lundbeck an equal share of all milestone and royalty payments received from Healx, if we choose not to exercise the Ovid Opt-In Right. If we choose to exercise the Ovid Opt-In Right, to co-develop and co-commercialize the program with Healx, we will owe an equal share of the net profit share to Lundbeck.
Given our stage of development, we have not yet established a commercial organization or distribution
capabilities. However, we do have internal market access and commercial strategy capabilities
however,that inform our pipeline strategy and execution. As our pipeline assets move into the clinic in
November 2019the future, we
hired a Chief Commercial Officer to begin planning to establish a commercial organization. We planintend to build focused capabilities
in the United States and European Union to commercialize our
development programs focused on
rare disorders of the brain.epilepsies and seizure-related disorders. In
other markets for which commercialization may be less capital efficient for us, we may selectively pursue strategic collaborations with third parties in order to maximize the commercial potential of our drug candidates.
We currently outsource all manufacturing, and we intend to use our collaborators and contract manufacturers for the foreseeable future. However, certain members of our management have broad experience in manufacturing, which we believe may provide a competitive advantage.
Competition
Competition
We believe
Zogenix, Inc. (acquired by UCB in 2022), Jazz Pharmaceuticals plc (acquired by UCB in 2022), Sage Therapeutics, Inc., Marinus Pharmaceuticals, Inc.
, Mallinckrodt plc, SK Biopharmaceuticals Inc., Epygenix Therapeutics, Inc., Stoke Therapeutics, Inc. and
ZynerbaXenon Pharmaceuticals, Inc. are our most direct competitors with respect to
OV101 in Angelman syndromesoticlestat, OV329 and
Fragile X syndrome. BiogenOV350. As it relates to OV888 (GV101), we believe Neurelis Inc. in collaboration with Ionis Pharmaceuticals, Inc., Roche Holding AG, PTC Therapeutics, Inc. and Ultragenyx Pharmaceutical Inc. in collaboration with GeneTx Biotherapeutics LLC are working on genetic approaches to Angelman syndrome.We believe Zogenix, Inc., GW Pharmaceuticals plc, Sage Therapeutics, Inc., Marinus Pharmaceuticals, Inc., Zynerba Pharmaceuticals, Inc., Stoke Therapeutics, Inc. and PTC Therapeutics, Inc. areis our most direct competitors with respect to OV935.
competitor.
Drug development is highly competitive and subject to rapid and significant technological advancements. Our ability to compete will significantly depend upon our ability to complete necessary clinical trials and regulatory approval processes, and effectively market any drug that we may successfully develop. Our current and potential future competitors include pharmaceutical and biotechnology companies, academic institutions and government agencies. The primary competitive factors that will affect the commercial success of any drug candidate for which we may receive marketing approval include efficacy, safety and tolerability profile, dosing convenience, price, coverage and reimbursement. Many of our existing or potential competitors have substantially greater financial, technical and human resources than we do and significantly greater experience in the discovery and development of drug candidates, as well as in obtaining regulatory approvals of those drug candidates in the United States and in foreign countries.
Our current and potential future competitors also have significantly more experience commercializing drugs that have been approved for marketing. Mergers and acquisitions in the pharmaceutical and biotechnology industries could result in even more resources being concentrated among a small number of our competitors.
Accordingly, our competitors may be more successful than us in obtaining regulatory approval for therapies and in achieving widespread market acceptance of their drugs. It is also possible that the development of a cure or more effective treatment method for the disorders we are targeting by a competitor could render our current or future drug candidates non-competitive or obsolete or reduce the demand for our drug candidates before we can recover our development and commercialization expenses.
Our commercial success depends in part on our ability to obtain and maintain proprietary protection for our current and future drug candidates, novel discoveries, product development technologies and know-how, to operate without infringing on the proprietary rights of others and to prevent others from infringing our proprietary rights. Our policy is to seek to protect our proprietary position by, among other methods, filing or in-licensing U.S. and foreign patents and patent applications related to technology, inventions and improvements that are important to the development and implementation of our business. We also rely on trademarks, trade secrets, copyright protection, know-how, continuing technological innovation and potential in-licensing opportunities to develop and maintain our proprietary position.
For example, the proprietary map of disease-relevant biological pathways underlying orphan disorders of the brain that we developed would not be appropriate for patent protection and, as a result, we rely on trade secrets to protect this aspect of our business.While we seek broad coverage under our existing patent applications, there is always a risk that an alteration to the product or process may provide sufficient basis for a competitor to avoid infringement claims. In addition, the coverage claimed in a patent application can be significantly reduced before a patent is issued and courts can reinterpret patent scope after issuance. Moreover, many jurisdictions including the United States permit third parties to challenge issued patents in administrative proceedings, which may result in further narrowing or even cancellation of patent claims. Moreover, we cannot provide any assurance that any patents will be issued from our pending or any future applications or that any potentially issued patents will adequately protect our intellectual property.
We currently own issued U.S. patents directed to treatment of Angelman syndrome and Fragile X syndrome with OV101 that expire in 2035, excluding any regulatory extensions. We also have exclusively licensed a portfolio of issued U.S. and international patents from Lundbeck directed to polymorphic forms of OV101 and their preparation and these patents expire on dates ranging from 2025 to 2028. In addition, we have exclusively licensed from Lundbeck an issued U.S. patent that will expire in 2036 and a pending application directed to an OV101methods of manufacturing processes that, if issued, would have a statutory expiration in 2036.OV101. We have also filed, and own, multiple patent families directed to methods of treatment and formulations with OV101. Additional issued patentsSubsequently, we have licensed much of this portfolio to Healx under the Amended Healx Agreement.
OV329 was in-licensed from Northwestern. OV329's composition of matter patent expires in 2036, excluding regulatory extensions. We have also filed, and
pending applications are directed to methods of treating neurodegenerative diseases and developmental disorders. We are seeking or will seekown, multiple patent
protection for these inventions in numerous countries and regions including, among others, Europe, Australia, Canada, Mexico, Israel, Japan, China, and Korea.
We licensed from Takeda a portfolio of U.S. and international patents and applicationsfamilies directed to the OV935synthesis of OV329 and methods of treatment with OV329.
OV888 (GV101) was licensed from Graviton. The composition of matter patent for OV88 expires in October 2038 excluding any potential regulatory extensions. Graviton has also filed patents on methods of use with OV888 (GV101).
A library of early-stage small molecules that target the KCC2 transporter, including OV350 was in-licensed from AstraZeneca. The molecules are included in a pending composition-of-matter application that was filed globally and,
these patents and applicationsif issued, will expire in
2032,2041, excluding any
potential regulatory extensions.
In addition, we have a library of proprietary genetic sequences that target UBE3A, KIF1A, HNRNPH2, and PDP2RD. We continue to expand our intellectual property portfolio to protect our library of potential development candidates.
Individual patents extend for varying periods depending on the date of filing of the patent application or the date of patent issuance and the legal term of patents in the countries in which they are obtained. Generally, utility patents issued for applications filed in the United States are granted a term of 20 years from the earliest effective filing date of a non-provisional patent application. In addition, in certain instances, a patent term can be extended to recapture a portion of the U.S. Patent and Trademark Office
or the USPTO,(the “USPTO”) delay in issuing the patent as well as a portion of the term effectively lost as a result of the FDA regulatory review period. However, as to the FDA component, the restoration period cannot be longer than five years and the total patent term including the restoration period must not exceed 14 years following FDA approval. The duration of foreign patents varies in accordance with provisions of applicable local law, but typically is also 20 years from the earliest effective filing date. The actual protection afforded by a patent may vary on a product-by-product basis, from country to country and can depend upon many factors, including the type of patent, the scope of its coverage, the availability of regulatory-related extensions, the availability of legal remedies in a particular country and the validity and enforceability of the patent.
Furthermore, we rely upon trade secrets and know-how and continuing technological innovation to develop and maintain our competitive position. We seek to protect our proprietary information, in part, using confidentiality agreements with our employees and consultants and any potential commercial partners and collaborators and invention assignment agreements with our employees. We also have or intend to implement confidentiality agreements or invention assignment agreements with our selected consultants and any potential commercial partners. These agreements are designed to protect our proprietary information and, in the case of the invention assignment agreements, to grant us ownership of technologies that are developed through a relationship with a third party. These agreements may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. To the extent that our commercial partners, collaborators, employees and consultants use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions.
Our commercial success will also depend in part on not infringing upon the proprietary rights of third parties. It is uncertain whether the issuance of any third-party patent would require us to alter our development or commercial strategies, or our drugs or processes, obtain licenses or cease certain activities. Our breach of any license agreements or failure to obtain a license to proprietary rights that we may require to develop or commercialize our future drugs may have an adverse impact on us. Since patent applications in the United States and certain other jurisdictions are maintained in secrecy for 18 months or potentially longer, and since publication of discoveries in the scientific or patent literature often lags behind actual discoveries, we cannot be certain of the priority of inventions covered by pending patent applications. Moreover, we may have to participate in Interference, Derivation, Reexam, Post-Grant Review, Inter Partes Review, or Opposition proceedings brought by third parties or declared by the USPTO.
The FDA and regulatory authorities in state and local jurisdictions and in other countries impose substantial and burdensome requirements upon companies involved in the clinical development, manufacture, marketing and distribution of drugs, such as those we are developing. These agencies and other federal, state and local entities regulate, among other things, the research and development, testing, manufacture, quality control, safety, effectiveness, labeling, storage, record keeping, approval, advertising and promotion, distribution, post-approval monitoring and reporting, sampling and export and import of drugs and drug candidates.
U.S. Government Regulation
In the United States, the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act or (“FDCA”) and its implementing regulations. The process of obtaining regulatory approvals and the subsequent compliance with applicable
federal, state, local and foreign statutes and regulations requires the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval, may subject an applicant to a variety of administrative or judicial sanctions, such as the FDA’s refusal to approve pending New Drug Applications (NDAs)(“NDAs”) or Biologics License Applications (BLAs)(“BLAs”), withdrawal of an approval, imposition of a clinical hold, issuance of warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement or civil or criminal penalties. The process required by the FDA before a drug product may be marketed in the United States generally involves the following:
•completion of preclinical laboratory tests, animal studies and formulation studies in compliance with the FDA’s good laboratory practice or (“GLP regulations.
•submission to the FDA of an IND which must become effective before human clinical trials may begin.
•approval by an independent institutional review board or (“IRB”) at each clinical site before each trial may be initiated.
•performance of adequate and well controlled human clinical trials in accordance with good clinical practice or (“GCP”) requirements to establish the safety and efficacy of the proposed drug product for each indication.
•submission to the FDA of an NDA or BLA.
•satisfactory completion of an FDA advisory committee review, if applicable.
•satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the product is produced to assess compliance with current good manufacturing practice or (“cGMP”), requirements and to assure that the facilities, methods and controls are adequate to preserve the drug’s identity, strength, quality and purity; and
•FDA review and approval of the NDA or BLA.
Preclinical studies include laboratory evaluation of product chemistry, toxicity and formulation, as well as animal studies to assess potential safety and efficacy. An IND sponsor must submit the results of the preclinical tests, together with manufacturing information, analytical data and any available clinical data or literature, among other things, to the FDA as part of an IND. Some preclinical testing may continue even after the IND is submitted. An IND automatically becomes effective 30 days after receipt by the FDA, unless before that time the FDA raises concerns or questions related to one or more proposed clinical trials and places the clinical trial on a clinical hold. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. As a result, submission of an IND may not result in the FDA allowing clinical trials to commence.
Clinical trials involve the administration of the investigational new drug to human patients under the supervision of qualified investigators in accordance with GCP requirements, which include the requirement that all research patients provide their informed consent in writing for their participation in any clinical trial. Clinical trials are conducted under protocols detailing, among other things, the objectives of the trial, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. A protocol for each clinical trial and any subsequent protocol amendments must be submitted to the FDA as part of the IND. In addition, an IRB at each institution participating in the clinical trial must review and approve the plan for any clinical trial before it commences at that institution. Information about certain clinical trials must be submitted within specific timeframes to the National Institutes of Health or (“NIH”) for public dissemination on their www.clinicaltrials.gov website. Human clinical trials are typically conducted in three sequential phases, which may overlap or be combined:
•Phase 1 clinical trial: The drug is initially introduced into healthy human volunteers or patients with the target disease or condition and tested for safety, dosage tolerance, absorption, metabolism, distribution, excretion and, if possible, to gain an early indication of its effectiveness.
•Phase 2 clinical trial: The drug is administered to a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage.
•Phase 3 clinical trial: The drug is administered to an expanded patient population, generally at geographically dispersed clinical trial sites, in well controlled clinical trials to generate enough data to statistically evaluate the efficacy and safety of the product for approval, to establish the overall risk-benefit profile of the product, and to provide adequate information for the labeling of the product.
Progress reports detailing the results of the clinical trials must be submitted at least annually to the FDA and more frequently if serious adverse events occur. Each of Phase 1, Phase 2 and Phase 3 clinical trials may not be completed successfully within any specified period, or at all. Furthermore, the FDA or the sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research patients are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the drug has been associated with unexpected serious harm to patients.
Assuming successful completion of the required clinical testing, the results of the preclinical studies and clinical trials, together with detailed information relating to the product’s chemistry, manufacture, controls and proposed labeling, among other things, are submitted to the FDA as part of an NDA or BLA requesting approval to market the product for one or more indications. In most cases, the submission of an NDA or BLA is subject to a substantial application user fee. Under the Prescription Drug User Fee Act or (“PDUFA”), guidelines that are currently in effect, the FDA has a goal of ten months from the date of “filing” of a standard NDA for a new molecular entity to review and act on the submission. This review typically takes twelve months from the date the NDA is submitted to FDA because the FDA has approximately two months to make a “filing” decision. The FDA conducts a preliminary review of all NDAs within the first 60 days after submission, before accepting them for filing, to determine whether they are sufficiently complete to permit substantive review. The FDA may request additional information rather than accept an NDA for filing. In this event, the application must be resubmitted with the additional information. The resubmitted application is also subject to review before the FDA accepts it for filing. Once the submission is accepted for filing, the FDA begins an in-depth substantive review. The FDA reviews an NDA to determine, among other things, whether the drug is safe and effective and whether the facility in which it is manufactured, processed, packaged or held meets standards designed to assure the product’s continued safety, quality and purity.
In addition, under the Pediatric Research Equity Act of 2003 or (“PREA”) as amended and reauthorized, certain research must contain data that are adequate to assess the safety and effectiveness of the drug for the claimed indications in all relevant pediatric subpopulations, and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. The FDA may, on its own initiative or at the request of the applicant, grant deferrals for submission of some or all pediatric data until after approval of the product for use in adults, or full or partial waivers from the pediatric data requirements. The FDA also may require submission of a risk evaluation and mitigation strategy or (“REMS”) plan to ensure that the benefits of the drug outweigh its risks. The REMS plan could include medication guides, physician communication plans, assessment plans, or elements to assure safe use, such as restricted distribution methods, patient registries, or other risk minimization tools. The FDA may refer an application for a novel drug to an advisory committee. An advisory committee is a panel of independent experts, including clinicians and other scientific experts, that reviews, evaluates and provides a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
Before approving an NDA or BLA, the FDA typically will inspect the facility or facilities where the product is manufactured. The FDA will not approve an application unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, before approving an NDA or BLA, the FDA may inspect one or more clinical trial sites to assure compliance with GCP requirements.
After evaluating the application and all related information, including the advisory committee recommendation, if any, and inspection reports regarding the manufacturing facilities and clinical trial sites, the FDA may issue an approval letter, or, in some cases, a complete response letter. A complete response letter generally contains a statement of specific conditions that must be met in order to secure final approval of the NDA or BLA and may require additional clinical or preclinical testing in order for
the FDA to reconsider the application. Even with submission of this additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval. If and when those conditions have been met to the FDA’s satisfaction, the FDA will typically issue an approval letter. An approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications.
Even if the FDA approves a product, it may limit the approved indications for use of the product, require that particular contraindications, warnings or precautions be included in the product labeling, require that post-approval studies, including Phase 4 clinical trials, be conducted to further assess a drug’s safety after approval, require testing and surveillance programs to monitor the product after commercialization, or impose other conditions, including distribution and use restrictions or other risk management mechanisms under a REMS, which can materially affect the potential market and profitability of the product. The FDA may prevent or limit further marketing of a product based on the results of post-marketing studies or surveillance programs. After approval, some types of changes to the approved product, such as adding new indications, manufacturing changes, and additional labeling claims, are subject to further testing requirements and FDA review and approval.
Orphan Drug Act
Under the Orphan Drug Act of 1983, the FDA may grant orphan designation to a drug or biologic intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States, or more than 200,000 individuals in the United States and for which there is no reasonable expectation that the cost of developing and making available in the United States a drug for this type of disease or condition will be recovered from sales in the United States for that drug. Orphan drug designation must be requested before submitting an NDA or BLA. After the FDA grants orphan drug designation, the name of the sponsor, identity of the drug or biologic and its potential orphan use are disclosed publicly by the FDA. The orphan drug designation does not shorten the duration of the regulatory review or approval process, but does provide certain advantages, such as a waiver of PDUFA fees, enhanced access to FDA staff and potential waiver of pediatric research requirements.
If a product that has orphan drug designation subsequently receives the first FDA approval for the disease for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications, including a full NDA or BLA, or an abbreviated NDA
(ANDA)(“ANDA”) or Biosimilar application, to market a drug or biologic with the same active moiety for the same indication for seven years, except in limited circumstances, such as a showing of clinical superiority to the product with orphan drug exclusivity. Orphan drug exclusivity does not prevent FDA from approving a different drug or biologic for the same disease or condition, or the same drug or biologic for a different disease or condition. Among the other benefits of orphan drug designation are tax credits for certain research and a waiver of the application user fee. A designated orphan drug may not receive orphan drug exclusivity if it is approved for a use that is broader than the indication for which it received orphan designation. In addition, exclusive marketing rights in the United States may be lost if the FDA later determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantities of the product to meet the needs of patients with the rare disease or condition.
Fast Track Designation
The FDA is required to facilitate the development and expedite the review of pharmaceutical products that are intended for the treatment of a serious or life-threatening condition for which there is no effective treatment, and which demonstrate the potential to address unmet medical needs for the condition. Under the fast-track program, the sponsor of a new drug candidate may request the FDA to designate the product for a specific indication as a fast-track product concurrent with or after the filing of the IND for the product candidate. The FDA must determine if the product candidate qualifies for fast-track designation within 60 days after receipt of the sponsor’s request.
In addition to other benefits, such as the ability to have more frequent interactions with the FDA, the agency may initiate review of sections of a fast-track product’s NDA or BLA before the application is complete. This rolling review is available if the applicant provides and the FDA approves a schedule for the submission of the remaining information and the applicant pays applicable user fees. However, the FDA’s PDUFA review period for a fast-track application does not begin until the last section of the application is submitted. In addition, the fast-track designation may be withdrawn by the FDA if the agency believes that the designation is no longer supported by data emerging in the clinical trial process.
Post-Approval Requirements
Drugs manufactured or distributed pursuant to FDA approvals are subject to pervasive and continuing regulation by the FDA, including, among other things, requirements relating to recordkeeping, periodic reporting, product sampling and distribution, advertising and promotion and reporting of adverse experiences with the product. After approval, most changes to the approved product, such as adding new indications or other labeling claims are subject to prior FDA review and approval. There also are continuing, annual user fee requirements for any marketed products and the establishments at which such products are manufactured, as well as new application fees for supplemental applications with clinical data.
The FDA may impose a number of post-approval requirements as a condition of approval of a marketing authorization. For example, the FDA may require post-marketing testing, including Phase 4 clinical trials, and surveillance to further assess and monitor the product’s safety and effectiveness after commercialization.
In addition, drug manufacturers and other entities involved in the manufacture and distribution of approved drugs are required to register their establishments with the FDA and state agencies and are subject to periodic unannounced inspections by the FDA and these state agencies for compliance with cGMP requirements. Changes to the manufacturing process are strictly regulated and often require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP requirements and impose reporting and documentation requirements upon the sponsor and any third-party manufacturers that the sponsor may decide to use. Accordingly, manufacturers must continue to expend time, money, and effort in the area of production and quality control to maintain cGMP compliance.
Once an approval is granted, the FDA may withdraw the approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in mandatory revisions to the approved labeling to add new safety information; imposition of
post-market studies or clinical trials to assess new safety risks; or imposition of distribution or other restrictions under a REMS program. Other potential consequences include, among other things:
•restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls.
•fines, warning letters or holds on post-approval clinical trials;
•refusal of the FDA to approve related pending applications or supplements to approved applications, or suspension or revocation of product approvals;
•product seizure or detention, or refusal to permit the import or export of products; or
•injunctions or the imposition of civil or criminal penalties.
The FDA strictly regulates marketing, labeling, advertising and promotion of products that are placed on the market. Drugs may be promoted only for the approved indications and in accordance with the provisions of the approved label. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant liability.
Coverage and Reimbursement
Sales of our drug candidates, if approved, will depend, in part, on the extent to which such products will be covered by third-party payors, such as government health care programs, commercial insurance and managed healthcare organizations. These third-party payors are increasingly limiting coverage or reducing reimbursements for medical products and services. In addition, the U.S. government, state legislatures, and foreign governments have continued implementing cost-containment programs, including price controls, restrictions on reimbursement and requirements for substitution of generic products. Third-party payors decide which therapies they will pay for and establish reimbursement levels. Third-party payors often rely upon Medicare coverage policy and payment limitations in setting their own coverage and reimbursement policies. Further, no uniform policy for coverage and reimbursement exists in the United States. Therefore, decisions regarding the extent of coverage and amount of reimbursement to be provided for any drug candidates that we develop will be made on a payor-by-payor basis. Each payor determines whether or not
it will provide coverage for a therapy, what amount it will pay the manufacturer for the therapy, and on what tier of its formulary it will be placed. The position on a payor’s list of covered drugs, or formulary, generally determines the co-payment that a patient will need to make to obtain the therapy and can strongly influence the adoption of such therapy by patients and physicians. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our net revenue and results. Decreases in third-party reimbursement for our drug candidates or a decision by a third-party payor to not cover our drug candidates could reduce physician usage of our drug candidates, once approved, and have a material adverse effect on our sales, results of operations and financial condition. Coverage policies and third-party payor reimbursement rates may change at any time. Therefore, even if favorable coverage and reimbursement status is attained, less favorable coverage policies and reimbursement rates may be implemented in the future.
Because of our current and future arrangements with healthcare professionals, principal investigators, consultants, customers and third-party payors, we will also be subject to healthcare regulation and enforcement by the federal government and the
statesstate and foreign governments in which we will conduct our business, including our clinical research, proposed sales, marketing and educational programs.
Failure to comply with these laws, where applicable, can result in the imposition of significant civil, criminal, and administrative penalties.
The U.S. laws that may affect our ability to operate, among others, include: the federal Health Insurance Portability and Accountability Act of 1996 or HIPAA,(“HIPPA”), as amended by the Health Information Technology for Economic and Clinical Health Act (“HITECH”), which governs the conduct of “covered entities,” including certain healthcare providers, health plans, and healthcare clearinghouses, as well as their respective “business associates,” including their covered subcontractors, that create, receive, maintain or transmit individually identifiable health information for or on behalf of a covered entity, with respect to certain electronic healthcare transactions and protectsprotecting the security and privacy of protected health information; certain state laws governing the privacy and security of health information in certain circumstances, some of which are more stringent than HIPAA and many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts; the federal Anti-Kickback Statute, which prohibits, among other things, individuals or entities from knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual for, or the purchase, order or recommendation of, any good or service for which payment may be made under federal healthcare programs such as the Medicare and Medicaid programs; federal false claims laws and civil monetary penalty laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payors that are false or fraudulent; federal criminal laws that prohibit executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters; the Physician Payments Sunshine Act, which requires certain manufacturers of drugs, devices, biologics, and medical supplies to report annually to the U.S. Department of Health and Human Services (“HHS”) information related to payments and other transfers of value to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors), certain other healthcare professionals (such as physician assistants and nurse practitioners) and teaching hospitals, and ownership and investment
interests held by physicians and their immediate family members. Beginning in 2022, applicable manufacturers also will be required to report such information regarding their payments and other transfers of value to physician assistants, nurse practitioners, clinical nurse specialists, anesthesiologist assistants, certified registered nurse anesthetists and certified nurse midwives during the previous year.
In addition, many states have similar laws and regulations, such as anti-kickback and false claims laws that may be broader in scope and may apply regardless of payor, in addition to items and services reimbursed under Medicaid and other state programs. Additionally, to the extent that our product is sold in a foreign country, we may be subject to similar foreign laws.
Failure to comply with these laws, where applicable, can result in the imposition of significant penalties, including civil, criminal, and administrative penalties, damages, disgorgement, monetary fines, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, imprisonment, and integrity oversight and reporting obligations.
Current and future legislative proposals to further reform healthcare or reduce healthcare costs may result in lower reimbursement for our products. The cost containment measures that payors and providers are instituting and the effect of any healthcare reform initiative implemented in the future could significantly reduce our revenues from the sale of our products.
For example, implementation of the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, collectively the Affordable Care Act or the (“PPACA”), has substantially changed healthcare financing and delivery by both governmental and private insurers, and significantly impacted the pharmaceutical industry. The PPACA, among other things, established an annual, nondeductible fee on any entity that manufactures or imports certain specified branded prescription drugs and biologic agents, revised the methodology by which rebates owed by manufacturers to the state and federal government for covered outpatient drugs under the Medicaid Drug Rebate Program are calculated, increased the minimum Medicaid rebates owed by most manufacturers under the Medicaid Drug Rebate Program, extended the Medicaid Drug Rebate program to utilization of prescriptions of individuals enrolled in Medicaid managed care organizations, provided incentives to programs that increase the federal government’s comparative effectiveness research and created a licensure frame work for follow-on biologic products. Since its enactment there have been executive, judicial and Congressional challenges to certain aspects of the PPACA. For example, President Trump has signed several Executive Orders and other directives designed to delayon June 17, 2021, the implementation of certain provisions of the PPACA or otherwise circumvent some of the requirements for health insurance mandated by the PPACA. Concurrently, Congress considered legislation to repeal or repeal and replace all or part of the PPACA. While Congress has not passed comprehensive repeal legislation, several bills affecting the implementation of certain taxes under the PPACA have been signed into law. The Tax Cuts and Jobs Act of 2017 includesU.S. Supreme Court dismissed a provision repealing, effective January 1, 2019, the tax-based shared responsibility payment imposed by the PPACAchallenge on certain individuals who fail to maintain qualifying health coverage for all or part of a yearprocedural grounds that is commonly referred to as the “individual mandate”. Additionally, the 2020 federal spending package permanently eliminated, effective January 1, 2020, the PPACA-mandated “Cadillac” tax on high-cost employer-sponsored health coverage and medical device tax and, effective January 1, 2021, also eliminated the health insurer tax. Further, the Bipartisan Budget Act of 2018, or the BBA, among other things, amends the PPACA, effective January 1, 2019, to increase from 50 percent to 70 percent the point-of-sale discount that is owed by pharmaceutical manufacturers who participate in Medicare Part D and to close the coverage gap in most Medicare drug plans, commonly referred to as the “donut hole.” Moreover, on December 14, 2018, a Texas U.S. District Court Judge ruled thatargued the PPACA is unconstitutional in its entirety because the “individual mandate” was repealed by Congress as part of the Tax Act. Additionally,Congress. Further, on December 18, 2019, the U.S. Court of Appeals for the 5th Circuit upheld the District Court ruling that the individual mandate was unconstitutional and remanded the case back to the District Court to determine whether the remaining provisions of the PPACA are invalid as well. The U.S. Supreme Court is currently reviewing this case, but it is unknown when a decision will be reached. Although the Supreme Court has not yet ruled on the constitutionality of the PPACA, on January 28, 2021,August 16, 2022, President Biden issued an executive order to initiate a special enrollment period from February 15, 2021 through May 15, 2021signed the
Inflation Reduction Act of 2022 (“IRA”) into law, which among other things, extends enhanced subsidies for purposes of obtainingindividuals purchasing health insurance coverage in PPACA marketplaces through plan year 2025. The IRA also eliminates the “donut hole” under the Medicare Part D program beginning in 2025 by significantly lowering the beneficiary maximum out-of-pocket cost and creating a new manufacturer discount program. It is possible that the PPACA marketplace. The executive order also instructs certain governmental agencieswill be subject to review and reconsider their existing policies and rules that limit access to healthcare, including among others, reexamining Medicaid demonstration projects and waiver programs that include work requirements, and policies that create barriers to obtaining access to health insurance coverage through Medicaidjudicial or Congressional challenges in the PPACA.future. It is unclear how the Supreme Court ruling, otherany such litigation,challenges and the healthcare reform measures of the Biden administration will impact the PPACA.
In addition, other legislative changes have been proposed and adopted since the PPACA was enacted. In August 2011, then President Obama signed into law the Budget Control Act of 2011, which, among other things, created the Joint Select Committee on Deficit Reduction to recommend to Congress proposals for spending reductions. The Joint Select Committee did not achieve a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, triggering the legislation’s automatic reduction to several government programs. This includes reductions to Medicare payments to providers of 2% per fiscal year, which went into effect in April 2013 and, due to subsequent legislative amendments, including the BBA, will remain in effect
through 2030until 2032 unless additional Congressional action is taken.
However, COVID-19 relief support legislation suspended the 2% Medicare sequester from May 1, 2020 through March 31, 2021. Additionally, in January 2013, then President Obama signed into law the American Taxpayer Relief Act of 2012, which, among other things, reduced Medicare payments to several providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years.
Congress is also considering additional health reform measures.Further, there has been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products. For example, there have been presidential executive orders,
and several recent Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to drug pricing, review the relationship between pricing and
manufacturer patient programs, and reform government program reimbursement methodologies for drug products. For example, in July 2021, the Biden administration released an executive order, “Promoting Competition in the American Economy,” with multiple provisions aimed at prescription drugs. In response to Biden’s executive order, on September 9, 2021, HHS released a Comprehensive Plan for Addressing High Drug Prices that outlines principles for drug pricing reform and sets out a variety of potential legislative policies that Congress could pursue as well as potential administrative actions HHS can take to advance these principles. Further, the IRA, among other things (i) directs HHS to negotiate the price of certain high-expenditure, single-source drugs and biologics covered under Medicare and (ii) imposes rebates under Medicare Part B and Medicare Part D to penalize price increases that outpace inflation. These provisions started to take effect in fiscal year 2023 and will continue to take effect progressively thereafter, although may be subject to legal challenges. On August 29, 2023, HHS announced the list of the first ten drugs that will be subject to price negotiations, although the Medicare drug price negotiation program is currently subject to legal challenges. It is currently unclear how the IRA will be implemented but is likely to have a significant impact on the pharmaceutical industry. In response to the Biden administration’s October 2022 executive order, on February 14, 2023, HHS released a report outlining three new models for testing by the CMS Innovation Center which will be evaluated on their ability to lower the cost of drugs, promote accessibility, and improve quality of care. It is unclear whether the models will be utilized in any health reform measures in the future. Further, on December 7, 2023, the Biden administration announced an initiative to control the price of prescription drugs through the use of march-in rights under the Bayh-Dole Act. On December 8, 2023, the National Institute of Standards and Technology published for comment a Draft Interagency Guidance Framework for Considering the Exercise of March-In Rights which for the first time includes the price of a product as one factor an agency can use when deciding to exercise march-in rights. While march-in rights have not previously been exercised, it is uncertain if that will work to reverse these measures or pursue similar policy initiatives.
We expect that additional federal and state, as well as foreign, healthcare reform measures will be adopted in the future, particularly in light ofcontinue under the new presidential administration, any of which could result in reduced demand for our products or additional pricing pressure. Further, it is possible that additional governmental action is taken in response to the COVID-19 pandemic.
Employees and framework.
Human Capital
ResourcesManagement
Our employees are dedicated to our mission of developing and delivering medicines that create new possibilities and more good days for people living with epilepsies and brain conditions.As of December 31, 2020,2023, we had 6740 full-time employees, 40the majority of whom were primarily engaged in research and development activities, including five individuals with M.D. degrees and 1613 professionals with Ph.D. degrees specializing in the sciences. Many of whom had an MDthese professionals have extensive epilepsy and neurology experience. In total, within our management team, we have colleagues who worked to shape the development or PhD degree.Ourcommercialization of a number of important marketed neurology and ASMs, including: Ztalmy, Fintepla, Brineura, Gilenya, Tysabri and Tecfidera.
We believe that our future success largely depends upon our continued ability to attract and retain highly skilled employees. We emphasize a number of measures and objectives in managing our human capital resources objectives include,assets, including, among others: employee engagement, development and training, talent acquisition and retention, employee wellness, diversity, inclusion, and compensation, benefits and equity.
We believe that developing a diverse and inclusive culture is central to continuing to attract and retain the top talent necessary to deliver on our growth strategy. As such, we are investing in a work environment in which our employees feel inspired, included and enjoy a strong sense of belonging. This includes a focus on extending our diversity,
equity, inclusion and belonging (“DEIB”) initiatives across our entire workforce, with specific employee engagement via the DEIB Committee. Approximately half of our employees are female, as applicable, identifying, recruiting, retaining, incentivizingare one-third of our board of directors. Approximately half of our organization is multicultural.
We value our employees’ insatiable curiosity to translate scientific discoveries into innovative medicines and integratingtheir courage and perseverance to overcome obstacles and operate with a sense of purpose and urgency on behalf of patients. Grounded in these guiding principles, we believe we have developed a collaborative environment where our existingcolleagues feel respected, valued, and additional employees. The principal purposes of ourcan contribute to their fullest potential.
We have equity incentive plans
that are
designed to attract, retain and motivate selected employees, consultants and directors through the granting of equity-based compensation awards and cash-based compensation awards, in order to increase stockholder value and the success of our company by motivating such individuals to perform to the best of their abilities and achieve our objectives.
In addition, our governance is overseen by an independent and diverse Board of Directors who provide and complement our expertise to help oversee the strategy and performance of our Company. Among our Board of Directors, five out of six members are independent. Collectively, our Board of Directors provides insight and expertise in areas of importance to the performance and growth of our enterprise, including experience as: senior operators of public companies; financial, transactional and oversight experience at public companies; proven biopharmaceutical and neuroscience experience; research and regulatory acumen in drug development; and corporate governance.
We lease the space for our principal executive offices, which are located at 1460 Broadway,441 Ninth Avenue, 14th Floor, New York, New York. In June 2022, we formally instituted our hybrid work policies. In 2022, we executed what we believe was a smooth transition to a hybrid work environment while ensuring that ample resources, support and flexibility were available to our employees.
Our headquarter office facilities in New York, New York
on a monthly basis. We believe that our facilities are adequate to meet our current needs.have received LEED Platinum certification.
Corporate and Other Information
We were incorporated in Delaware in April 2014. Our principal executive offices are located at
1460 Broadway, Suite 15021441 Ninth Avenue, 14th Floor, New York, New York
1003610001 and our telephone number is (646) 661-7661. Our corporate website address is www.ovidrx.com. Information contained on or accessible through our website is not a part of this Annual Report, and the inclusion of our website address in this Annual Report is an inactive textual reference only.
We file electronically with the Securities and Exchange Commission or the SEC,(“SEC”) our annual reports on Form 10-K, Annualquarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act. We make available on our website at www.ovidrx.com under “Investors,” free of charge, copies of these reports as soon as reasonably practicable after filing or furnishing these reports with the SEC.
An investment in our securities involves a high degree of risk. You should carefully consider the following information about these risks, together with the other information appearing elsewhere in this Annual Report on Form 10-K, including our audited condensedconsolidated financial statements and related notes hereto, before deciding to invest in our common stock. The occurrence of any of the following risks could have a material adverse effect on our business, financial condition, results of operations and future growth prospects or cause our actual results to differ materially from those contained in forward-looking statements we have made in this report and those we may make from time to time. In these circumstances, the market price of our common stock could decline and you may lose all or part of your investment. We cannot assure you that any of the events discussed below will not occur. In addition, such
Summary of Selected Risks Associated with Our Business
Our business faces significant risks
mayand uncertainties. If any of the following risks are realized, our business, financial condition and results of operations could be
amplified bymaterially and adversely affected. Some of the
COVID-19 pandemic and its potential impact on Ovid’s business andmore significant risks we face include the
global economy.Risks Related to Our Financial Position and Need for Additional Capital
Wefollowing:
•Historically, we have incurred significant operating losses since inception and expect to continue to incur substantial operating losses for the foreseeable future. These factors raise substantial doubt aboutfuture and may never achieve or maintain profitability.
•Our operating history may make it difficult to evaluate the success of our business to date and to assess our future viability.
• We will require additional capital to finance our operations, which may not be available on acceptable terms, if at all. Failure to obtain this necessary capital when needed may force us to delay, limit or terminate certain of our drug development efforts or other operations.
• We are early in our development efforts of our current drug candidates and all our drug candidates are in clinical trials or preclinical development. If we are unable to successfully develop, receive regulatory approval for and commercialize our drug candidates, or successfully develop any other drug candidates, or experience significant delays in doing so, our business will be harmed.
• Our future success is dependent on the successful clinical development, regulatory approval and commercialization of our current and future drug candidates. If we, or our licensees, are not able to obtain the required regulatory approvals, we, or our licensees, will not be able to commercialize our drug candidates, and our ability to continuegenerate revenue will be adversely affected.
• Because the results of preclinical studies or earlier clinical trials are not necessarily predictive of future results, our drug candidates may not have favorable results in planned or future preclinical studies or clinical trials, or may not receive regulatory approval.
• Interim topline and preliminary results from our clinical trials that we announce or publish from time to time may change as a going concern ifmore patient data become available and are subject to audit and verification procedures, which could result in material changes in the final data.
• Preclinical studies and clinical trials are very expensive, time consuming and difficult to design and implement and involve uncertain outcomes. Further, we may encounter substantial delays in our clinical trials or we may fail to demonstrate safety and efficacy in our preclinical studies and clinical trials to the satisfaction of applicable regulatory authorities.
• If we are
unsuccessful raisingnot successful in discovering, developing and commercializing additional
capital.Since inceptiondrug candidates, our ability to expand our business and achieve our strategic objectives would be impaired.
• Our drug candidates may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial potential or result in April 2014,significant negative consequences following any potential marketing approval.
• Even if our current or future drug candidates receive marketing approval, they may fail to achieve market acceptance by physicians, patients, third-party payors or others in the medical community necessary for commercial success.
• Under the RLT Agreement, we are entitled to receive royalty and milestone payments in connection with the development and commercialization of soticlestat. If Takeda fails to progress, delays or discontinues the development of soticlestat, we may not receive some or all of such payments, which would materially harm our business.
•Our relationships with customers, physicians, and third-party payors may be subject, directly or indirectly, to federal and state healthcare fraud and abuse laws, false claims laws, health information privacy and security laws, and other healthcare laws and regulations. If we are unable to comply, or have not fully complied, with such laws, we could face substantial penalties.
• Coverage and adequate reimbursement may not be available for our current or any future drug candidates, which could make it difficult for us to sell profitably, if approved.
• If we are unable to obtain and maintain patent protection for our current or any future drug candidates, or if the scope of the patent protection obtained is not sufficiently broad, we may not be able to compete effectively in our markets.
• We may be involved in lawsuits to protect or enforce our patents, the patents of our licensors or our other intellectual property rights, which could be expensive, time consuming and unsuccessful.
• We do not have our own manufacturing capabilities and will rely on third parties to produce clinical and commercial supplies of our current and any future drug candidates.
• We intend to rely on third parties to conduct, supervise and monitor our preclinical studies and clinical trials, and if those third parties perform in an unsatisfactory manner, it may harm our business.
• We may need to expand our organization, and we may experience difficulties in managing this growth, which could disrupt our operations.
• We may be subject to numerous and varying privacy and security laws, and our failure to comply could result in penalties and reputational damage.
Risks Related to Our Financial Position and Need for Additional Capital
We expect to continue to incur substantial operating losses for the foreseeable future and may never achieve or maintain profitability.
We have historically incurred significant operating losses. Our net loss was $81.0$52.3 million for the year ended December 31, 2020.2023. As of December 31, 2020,2023, we had an accumulated deficit of $294.2$277.9 million. We expect to continue to incur significant expenses and increasing operating losses for the foreseeable future. Since inception, we have devoted substantially all of our efforts to research and preclinical and clinical development of our drug candidates, as well as hiring employees and building our infrastructure.
We have no drugs approved for commercialization and have never generated any revenue from drug sales. Most of our drug candidates are still in the preclinical testing stage. It could be several years, if ever, before we have a commercialized drug. TheWe expect to continue to incur significant expenses and operating losses over the next several years, and the net losses we incur may fluctuate significantly from quarter to quarter and year to year. We anticipate that our expenses will increase substantially if, and as, we:
•continue the ongoing and planned preclinical and clinical development of our drug candidates;
•continue to build a portfolio of drug candidates through the acquisition or in-license of drugs, drug candidates or technologies;
•initiate preclinical studies and clinical trials for any additional drug candidates that we may pursue in the future;
experience further delays in our preclinical studies and clinical trials due to the ongoing COVID-19 pandemic;
•seek marketing approvals for our current and future drug candidates that successfully complete clinical trials;
•establish a sales, marketing and distribution infrastructure to commercialize any drug candidate for which we may obtain marketing approval;
•develop, maintain, expand and protect our intellectual property portfolio;
•implement operational, financial and management systems; and
•attract, hire and retain additional administrative, clinical, regulatory and scientific personnel.
Management has identified certain conditions or events, which, considered in the aggregate, raise substantial doubt about our ability to continue as a going concern, including the risk that we will be unable to raise adequate additional capital to fund our operations through at least the 12 months following the filing date of the this annual report on Form 10-K. Substantial doubt about our ability to continue as a going concern may create negative reactions to the price of our common stock. If we are unable to raise capital, we may be required to delay, reduce the scope of or eliminate research and development programs, or obtain funds through arrangements with collaborators or others that may require us to relinquish rights to certain drug candidates that we might otherwise seek to develop or commercialize independently. In addition,
Even if we
are unable to continue as a going concern,complete the development and regulatory processes described above, we
may have to liquidateanticipate incurring significant costs associated with launching and commercializing our
assetscurrent and
may receive less than the value at which those assets are carried on our financial statements, and it is likely that investors will lose all or a part of their investment. Further, the perception that we may be unable to continue as a going concern may impede our ability to pursue strategic opportunities or operate our business due to concerns regarding our ability to discharge our contractual obligations.Even iffuture drug candidates.
If we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our failure to become and remain profitable would decrease the value of our company and could impair our ability to raise capital, maintain our research and development efforts, expand our business or continue our operations.
We have never generated any revenue from drug sales.
Our operating history may make it difficult to evaluate the success of our business to date and to assess our future viability.
Our operations have consumed substantial amounts of cash since our inception,
in April 2014, primarily due to
research and development of our drug candidates, organizing and staffing our company, business planning, raising capital,
and acquiring
assets and undertaking the development of OV101 and OV935.assets. We have not yet demonstrated the ability to obtain marketing approvals, manufacture a commercial-scale drug or conduct sales and
marketing activities necessary for successful commercialization. Consequently, any predictions about our future success or viability may not be as accurate as they could be if we had more experience developing drug candidates.
Our ability to generate revenue from drug sales and achieve profitability depends on our ability, alone or with any current or future collaborative partners, to successfully complete the development of, and obtain the regulatory approvals necessary to commercialize, our current and future drug candidates. We do not anticipate generating revenue from drug sales for the next several years, if ever. Our ability to generate revenue from drug sales depends heavily on our, or any current or future collaborators’, success in the following areas, including but not limited to:
timely and successfully completing preclinical and clinical development of our current and future drug candidates;
obtaining regulatory approvals for our current and future drug candidates for which we successfully complete clinical trials;
launching and commercializing any drug candidates for which we obtain regulatory approval by establishing a sales force, marketing and distribution infrastructure or, alternatively, collaborating with a commercialization partner;
qualifying for coverage and adequate reimbursement by government and third-party payors for any drug candidates for which we obtain regulatory approval, both in the United States and internationally;
developing, validating and maintaining a commercially viable, sustainable, scalable, reproducible and transferable manufacturing process for our current and future drug candidates that is compliant with current good manufacturing practices, (“cGMP”);
establishing and maintaining supply and manufacturing relationships with third parties that can provide an adequate amount and quality of drugs and services to support clinical development, as well as the market demand for our current and future drug candidates, if approved;
obtaining market acceptance, if and when approved, of our current or any future drug candidates as a viable treatment option by physicians, patients, third-party payors and others in the medical community;
effectively addressing any competing technological and market developments;
implementing additional internal systems and infrastructure, as needed;
negotiating favorable terms in any collaboration, licensing or other arrangements into which we may enter and performing our obligations pursuant to such arrangements;
our ability to obtain and maintain orphan drug exclusivity for any of our current and future drug candidates for which we obtain regulatory approval;
maintaining, protecting and expanding our portfolio of intellectual property rights, including patents, trade secrets and know-how;
avoiding and defending against third-party interference or infringement claims; and
securing appropriate pricing in the United States, the European Union and other countries.
We expect our financial condition and operating results to continue to fluctuate from quarter to quarter and year to year due to a variety of factors, many of which are beyond our control. We will need to eventually transition from a company with a research and development focus to a company capable of undertaking commercial activities. We may encounter unforeseen expenses, difficulties, complications and delays and may not be successful in such a transition.
We will require additional capital to finance our operations, which may not be available on acceptable terms, if at all. Failure to obtain this necessary capital when needed may force us to delay, limit or terminate certain of our drug development efforts or other operations.
Our operations have consumed substantial amounts of cash since our inception. We expect our expenses to increase
in connection with our ongoing and planned activities, particularly as we
continue to developadvance our current and
future drug candidates through preclinical studies and clinical trials, commercialize our drug candidates,
in addition to costs associated withand pursue the acquisition or in-licensing of any additional drug
candidates we may pursue.candidates. Our expenses could increase beyond expectations if the FDA or other regulatory authorities require us to perform
preclinical studies or clinical
and other studiestrials in addition to those that we currently anticipate. In addition,
even if we obtain marketing approval for our drug candidates,
they may not achieve commercial success. Our revenue, if any, will be derived from sales of drugs that we do not expect to be commercially available for a number of years, if at all. If we obtain marketing approval for any drug candidates that we develop or otherwise acquire, we expect to incur significant expenses related to manufacturing, marketing, sales and distribution.
Furthermore, we expect to continue to incur additional costs associated with operating as a public company.As of December 31,
2020,2023, our cash,
and cash equivalents
and marketable securities were
$72.0$105.8 million and we had an accumulated deficit of
$294.2$277.9 million.
Management has identified certain conditions or events, which, considered in the aggregate, raise substantial doubt aboutWe believe that our
ability to continue as a going concern, including the risk that weexisting cash, cash equivalents and marketable securities will
be unable to raise adequate additional capital to fund our
operations current operating plans through at least the 12 months followingfrom the filing date of this annual reportAnnual Report on Form 10-K. Substantial doubt aboutHowever, our abilityoperating plans may change because of many factors currently unknown to continue as a going concern may create negative reactions to the price of our common stock. If we are unable to raise capital,us, and we may be requiredneed to delay, reduce the scopeseek additional funds sooner than planned, through public or private equity or debt financings, third-party funding, marketing and distribution arrangements, as well as other collaborations, strategic alliances and licensing arrangements, or any combination of or eliminate research and development programs, or obtain funds through arrangements with collaborators or others that may require us to relinquish rights to certain drug candidates that we might otherwise seek to develop or commercialize independently. In addition, if we are unable to continue as a going concern, we may have to liquidate our assets and may receive less than the value at which those assets are carried on our financial statements, and it is likely that investors will lose all or a part of their investment. Further, the perception that we may be unable to continue as a going concern may impede our ability to pursue strategic opportunities or operate our business due to concerns regarding our ability to discharge our contractual obligations.
these approaches.
We will require more capital in order to continue ouradvance the preclinical and clinical activities, todevelopment, obtain regulatory approval and, for the commercialization offollowing regulatory approval, commercialize our current or future drug candidates. Any additional capital raising efforts may divert our management from their day-to-day activities, which may adversely affect our ability to develop and commercialize our current and future drug candidates. The COVID-19 pandemic
While the long-term economic impacts associated with public health crises and geopolitical tensions, like the ongoing war between Russia and Ukraine and war in Israel, are difficult to assess or predict, each of these events has
already resulted in acaused significant
disruption ofdisruptions to the global financial
markets.markets and contributed to a general global economic slowdown. Furthermore, inflation rates have increased recently to levels not seen in decades. Increased inflation may result in increased operating costs (including labor costs) and may affect our operating budgets. In addition, the U.S. Federal Reserve has raised interest rates in response to concerns about inflation. High interest rates, especially if coupled with reduced government spending and volatility in financial markets, may further increase economic uncertainty and heighten these risks. If the
disruption persistsdisruptions and
deepens,slowdown deepen or persist, we
could experience an inabilitymay not be able to access additional
capital. If we do not raise additional capital
on favorable terms, or at all, which could in
sufficient amounts, or on terms acceptable to us, we may be prevented from pursuing developmentthe future negatively affect our financial condition and
commercialization efforts, which will harm our business, operating results and prospects.Raising additional capital or acquiring or licensing assets by issuing equity or debt securities may cause dilution to our stockholders, and raising funds through lending and licensing arrangements may restrict our operations or require us to relinquish proprietary rights.
Until such time as we can generate substantial revenue from drug sales, if ever, we expect to finance our cash needs through a combination of equity and debt financings, strategic alliances, and license and development agreements in connection with any collaborations. We do not have any committed external source of funds. To the extent that we issue additional equity securities, our stockholders may experience substantial dilution, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a stockholder. In addition, we may issue equity or debt securities as consideration for obtaining rights to additional compounds. For example, in our arrangement with Takeda, upon the achievement of a certain development milestone, we will be obligated to issue to Takeda additional securities equal to up to 8% of our outstanding capital stock in certain situations which will dilute our stockholders. In addition, further dilution may occur if we elect to issue shares of common stock to Takeda as payment for the remaining potential global commercial and regulatory milestone payments, which aggregate to approximately $35.0 million. In March 2021, we entered into a royalty, license and termination agreement (“Takeda License and Termination Agreement”) with Takeda under which Takeda will secure global rights at closing from us to develop and commercialize the investigational medicine OV935 for the treatment of developmental and epileptic encephalopathies, including DS and LGS. Closing of the Takeda License and Termination Agreement is subject to satisfaction of customary closing conditions. See “Business—License and Collaboration Agreements—Agreements with Takeda—2021 Royalty, License and Termination Agreement with Takeda” for a discussion of the terms of the Takeda License and Termination Agreement.
Debt and equity financings, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as redeemingpursue our shares, making investments, issuing additional equity, incurring additional debt, making capital expenditures, declaring dividends or placing limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could negatively impact our ability to conduct our business. If we raise additional capital through future collaborations, strategic alliances or third-party licensing arrangements, we may have to relinquish valuable rights to our intellectual property, future revenue streams, research programs or drug candidates, or grant licenses on terms that may not be favorable to us. For example, on July 9, 2020, we entered into a collaboration and license agreement (the “Angelini License Agreement”) with Angelini Pharma Rare Diseases AG, pursuant to which we granted to Angelini exclusive rights to develop and commercialize OV101 in the European Economic Area as well as Switzerland, the United Kingdom, Russia and Turkey. The licenses granted to Angelini include sublicenses under our existing license agreement with H. Lundbeck A/S (“Lundbeck”), as well as licenses under our patents and know-how covering OV101.
business strategy.
If we are unable to raise additional capital when needed, we may be required to delay, limit, reduce or terminate our drug development or future commercialization efforts, or grant rights to develop and market drug candidates that we would otherwise develop and market ourselves.
Our ability to use our net operating loss (“NOL”) carryforwards and certain other tax attributes to offset future taxable income may be subject to limitation.
Our NOL carryforwards could expire unused and be unavailable to offset future income tax liabilities because of their limited duration or because of restrictions under U.S. tax law. Our federal NOLs generated in tax years beginning on or before December 31, 2017 are permitted to be carried forward for only 20 years under applicable U.S. tax law. Under the Tax Cuts and Jobs Act, or the Tax Act, as modified by the Coronavirus Aid, Relief, and Economic Security Act, or the CARES Act, federal NOLs incurred in taxable years beginning after December 31, 2017 may be carried forward indefinitely, but the utilization of such federal NOLs incurred in the taxable year beginning after December 31, 2020 is limited. It is uncertain how various states will respond to the Tax Act and CARES Act.
In addition, under Section 382 and Section 383 of the Internal Revenue Code of 1986, as amended (the “Code”), and corresponding provisions of state law, if a corporation undergoes an “ownership change,” its ability to use its pre-change NOL carryforwards and other pre-change tax attributes (such as research tax credits) to offset its post-change income may be limited. A Section 382 “ownership change” generally occurs if one or more stockholders or groups of stockholders who own at least 5% of our stock increase their ownership by more than 50 percentage points (by value) over their lowest ownership percentage over a rolling three-year period. We may have experienced ownership changes in the past and may experience ownership changes in the future as a result of shifts in our stock ownership (some of which are outside our control). As a result, if we earn net taxable income, our ability to use our pre-change NOLs and certain other
tax attributes to offset such taxable income may be subject to limitations. Similar provisions of state tax law may also apply to limit our use of accumulated state tax attributes. In addition, at the state level, there may be periods during which the use of NOLs is suspended or otherwise limited, which could accelerate or permanently increase state taxes owed.
Consequently, even
For the years ended December 31, 2023 and 2022, we recorded no U.S. federal or state income tax provision, based on pre-tax losses of $52.3 million and $54.2 million, respectively. As of December 31, 2023, we had available approximately $150.2 million of unused NOL carryforwards for U.S. federal income tax purposes, $12.6 million of unused NOL carryforwards for Massachusetts income tax purposes, $164.1 million of unused NOL carryforwards for New York income tax purposes, and $163.9 million of unused NOL carryforwards for New York City income tax purposes, that may be applied against future taxable income. Our NOL carryforwards are significantly limited such that if we achieve profitability
in future periods, we may not be able to utilize
a material portionmost of
ourthe NOL carryforwards,
and certain other tax attributes, which could have a material adverse effect on cash flow and results of operations.
Changes in tax laws or regulations that are applied adversely to us or our customers may have a material adverse effect on our business, cash flow, financial condition, or results of operations.
New tax laws, statutes, rules, regulations, or ordinances could be enacted at any time. For instance, the recently enacted Inflation Reduction Act of 2022 (the “IRA”) imposes, among other rules, a 15% minimum tax on the book income of certain large corporations and a 1% excise tax on certain corporate stock repurchases. Further, existing tax laws, statutes, rules, regulations, or ordinances could be interpreted differently, changed, repealed, or modified at any time. Any such enactment, interpretation, change, repeal, or modification could adversely affect us, possibly with retroactive effect. In particular, changes in corporate tax rates, the realization of our net deferred tax assets, the taxation of foreign earnings, and the deductibility of expenses under the Tax Act, as amended by the CARES Act or any future tax reform legislation, could have a material impact on the value of our deferred tax assets, result in significant one-time charges, and increase our future tax expenses.
Risks Related to the Development and Commercialization of Our Drug Candidates
We are very early in our development efforts. If we are unable to successfully develop, receive regulatory approval for and commercialize our drug candidates, or successfully develop any other drug candidates, or experience significant delays in doing so, our business will be harmed.
We are early in our development efforts. We previously publicly announced we anticipate filing three INDs in three years, beginning in 2022; however, we cannot guarantee success of preclinical development to achieve all such INDs. Following IND acceptance, each of our drug candidates will need to be progressed through clinical development in order to achieve regulatory approval, and we will also need to address issues relating to manufacture and supply, which may involve building our own capacity and expertise. In order to commercialize any product that achieves regulatory approval, we will need to build a commercial organization or successfully outsource commercialization, all of which will require substantial investment and significant marketing efforts before we have the ability to generate any revenue from drug sales. We do not have any drugs that are approved for commercial sale, and we may never be able to develop or commercialize marketable drugs.
Our ability to generate revenue from drug sales and achieve profitability depends on our ability, alone or with any current or future collaborative partners, to successfully complete the development of, and obtain the regulatory approvals necessary to commercialize, our current and future drug candidates. We do not anticipate generating revenue from drug sales for the next several years, if ever. Our ability to generate revenue from drug sales depends heavily on our, or any current or future collaborators’, success in the following areas, including but not limited to:
•timely and successfully completing preclinical and clinical development of our current and future drug candidates;
•obtaining regulatory approvals for our current and future drug candidates for which we successfully complete clinical trials;
•launching and commercializing any drug candidates for which we obtain regulatory approval by establishing a sales force, marketing and distribution infrastructure or, alternatively, collaborating with a commercialization partner;
•qualifying for coverage and adequate reimbursement by government and third-party payors for any drug candidates for which we obtain regulatory approval, both in the United States and internationally;
•developing, validating and maintaining a commercially viable, sustainable, scalable, reproducible and transferable manufacturing process for our current and future drug candidates that is compliant with current good manufacturing practices (“cGMP”);
•establishing and maintaining supply and manufacturing relationships with third parties that can provide an adequate amount and quality of drugs and services to support clinical development, as well as the market demand for our current and future drug candidates, if approved;
•obtaining market acceptance, if and when approved, of our current or any future drug candidates as a viable treatment option by physicians, patients, third-party payors and others in the medical community;
•effectively addressing any competing technological and market developments;
•implementing additional internal systems and infrastructure, as needed;
•negotiating favorable terms in any collaboration, licensing or other arrangements into which we may enter and performing our obligations pursuant to such arrangements;
•obtaining and maintaining orphan drug exclusivity for any of our current and future drug candidates for which we obtain regulatory approval;
•maintaining, protecting and expanding our portfolio of intellectual property rights, including patents, trade secrets and know-how;
•avoiding and defending against third-party interference or infringement claims; and
•securing appropriate pricing in the United States, the European Union and other countries.
If we are not successful with respect to one or more of these factors in a timely manner or at all, we could experience significant delays or an inability to successfully commercialize the drug candidates we develop, which would materially harm our business. If we do not receive marketing approvals for any drug candidate we develop, we may not be able to continue our operations.
Our future success is dependent on the successful clinical development, regulatory approval and commercialization of our current and future drug candidates. If we, or our licensees, are not able to obtain
the required regulatory approvals, we,
or our licensees, will not be able to commercialize our drug candidates, and our ability to generate revenue will be adversely affected.
We do not have any drugs that have received regulatory approval. Our business is dependent on our ability to successfully complete preclinical and clinical development of, obtain regulatory approval for, and, if approved, successfully commercialize our current and future drug candidates in a timely manner. Activities associated with the development and commercialization of our current and future drug candidates are subject to comprehensive regulation by the FDA and other regulatory agencies in the United States and similar regulatory authorities outside the United States. Failure to obtain regulatory approval in the United States or other jurisdictions would prevent us from commercializing and marketing our current and future drug candidates. An inability to effectively develop and commercialize our current and future drug candidates
whether due to the impacts of the ongoing COVID-19 pandemic or otherwise, could have an adverse effect on our business, financial condition, results of operations and growth prospects.
Soticlestat, the most advanced compound we helped to develop, is continuing to be developed by Takeda and is currently in a pivotal trial program. If the pivotal trials are unsuccessful, or the compound is not approved, we will not receive the milestone payments and royalties from the RLT Agreement. Without those funds, we may need to raise significant additional capital to pursue the development and commercialization of our current and future pipeline.
Further, activities associated with the development and commercialization of our current and future drug candidates are subject to comprehensive regulation by the FDA and other regulatory agencies in the United States and similar regulatory authorities outside the United States. Failure to obtain regulatory approval in the United States or other jurisdictions would prevent us from commercializing and marketing our current and future drug candidates.
Even if we obtain approval from the FDA and comparable foreign regulatory authorities for our current and future drug candidates, any approval might contain significant limitations related to use restrictions for specified age groups, warnings, precautions or contraindications, or may be subject to burdensome post-approval study or risk management requirements. If we are unable to obtain regulatory approval, or any approval contains significant limitations, we may not be able to obtain sufficient funding or generate sufficient revenue to continue the development of that drug candidate or any other drug candidate that we may in-license, develop or acquire in the future. In certain circumstances, including under our license agreement with Angelini for OV101, our third-party
licensees are responsible for obtaining regulatory approvals in the countries covered by the license, and we are dependent on their efforts in order to achieve the necessary approvals in order to commercialize our products. If
Angelini, or any future licensees fail to perform their obligations to develop and obtain regulatory approvals for the licensed products, we may not be able to commercialize our products in the affected countries, or our ability to do so may be substantially delayed.
Furthermore, even if we obtain regulatory approval for our current and future drug candidates, we will still need to develop a commercial organization, establish a commercially viable pricing structure and obtain approval for adequate reimbursement from third-party and government payors. If we are unable to successfully commercialize our current and future drug candidates, we may not be able to generate sufficient revenue to continue our business.
Because the results of preclinical studies or earlier clinical trials are not necessarily predictive of future results, our drug candidates may not have favorable results in planned or future preclinical studies or clinical trials, or may not receive regulatory approval.
Success in preclinical testing and early clinical trials does not ensure that subsequent clinical trials will generate similar results or otherwise provide adequate data to demonstrate the efficacy and safety of a drug candidate. Frequently, drug candidates that have shown promising results in early clinical trials have subsequently suffered significant setbacks in later clinical trials. For instance, our STARS trial was the first clinical trial evaluating efficacy of OV101 in patients with Angelman syndrome and OV101 has not been evaluated in a clinical trial to treat Fragile X syndrome. We may be unable to demonstrate efficacy in any future trials, including any future clinical trials of OV101 to treat Angelman syndrome. For instance, in December 2020, we reported the primary endpoint of the NEPTUNE study was not achieved. Based on the results of the NEPTUNE trial, further development of OV101, other than the ELARA trial, is currently on hold. Similarly, our Phase 1b/2a adult study in OV935 showed exploratory signals of efficacy in seizure frequency reduction, but we may be unable to demonstrate efficacy in future trials in patients with DEE, or the related indications of Dravet syndrome, Lennox-Gastaut syndrome, CDKL5 Deficiency Disorder or Duplication 15q (“Dup15q”) syndrome, and the FDA has not yet made any determination regarding safety and efficacy of OV935 in any of these indications. The results from preclinical studies of OV101our current and OV935 in animal models and the results from our STARS clinical trial of OV101 in patients with Angelman syndrome and clinical trials of OV101 in patients with primary insomniafuture drug candidates may not be predictive of the effects of these compounds in later stage clinical trials. Our approach of targeting the extrasynaptic GABAA receptor with OV101, and cholesterol 24-hydroxylase (CH24H) with OV935, are both novel and unproven, and as such, the cost and time needed to develop OV101 and OV935 is difficult to predict and our efforts may not be successful. If we do not observe favorable results in clinical trials of one of our drug candidates, we may decide to delay or abandon clinical development of that drug candidate. Any such delay or abandonment could harm our business, financial condition, results of operations and prospects.
Interim topline and preliminary results from our clinical trials that we announce or publish from time to time may change as more patient data become available and are subject to audit and verification procedures, which could result in material changes in the final data.
From time to time, we have and may in the future publish or report preliminary or interim data from our clinical
trials, such as the initial data we announced from the ENDYMION open label extension trial for OV935 in September 2019, which involved data from the first six patients enrolled in that extension trial which showed promising signs of efficacy over the treatment period, or the topline data from the ELEKTRA trial for OV935 in August 2020.trials. Preliminary or interim data from our clinical trials and those of our partners may not be indicative of the final results of the trial and are subject to the risk that one or more of the clinical outcomes may materially change as patient enrollment continues and/or more patient data become available. Preliminary or topline results also remain subject to audit and verification procedures that may result in the final data being materially different from the preliminary data we previously published or reported. As a result, preliminary or interim data should be considered carefully and with caution until final data are available.
Differences between preliminary or interim data and final data could significantly harm our business prospects and may cause the trading price of our common stock to fluctuate significantly.We
Preclinical studies and clinical trials are very expensive, time-consuming and difficult to design and implement and involve uncertain outcomes. Further, we may encounter substantial delays in our clinical trials or we may fail to demonstrate safety and efficacy
in our preclinical studies and clinical trials to the satisfaction of applicable regulatory authorities.
Before obtaining marketing approval from regulatory authorities for the sale
All of our current drug candidates are in early clinical or preclinical development and their risk of failure is high. We must demonstrate through lengthy, complex and expensive preclinical testing and clinical trials that each of our drug candidates we must conduct extensive clinical trials to demonstrate the safetyare safe and efficacy of the drug candidateeffective for its intended indications. Clinicalindications before we are prepared to submit an NDA or BLA for regulatory approval. We cannot predict with any certainty if or when we might submit an NDA or BLA for any of our product candidates or whether any such application will be approved by the FDA. Human clinical trials are very expensive time-consuming and uncertain asdifficult to outcome. Further, delaysdesign and interruptionsimplement, in part because they are subject to ongoing trials relatedrigorous review and regulatory requirements by numerous government authorities in the United States and in other countries where we intend to test and market our product candidates. For instance, the COVID-19 pandemicFDA may also increasenot agree with our proposed endpoints for any future clinical trial of our product candidates, which may delay the duration and costscommencement of such trials.clinical trial.
We estimate that the successful completion of clinical trials of our product candidates will take at least several years to complete, if not longer. We cannot guarantee that any clinical trials will be conducted as planned or completed on schedule, if at all.
AFurthermore, failure
of one or more clinical trials can occur at any stage
of testing.and we could encounter problems that cause us to abandon or repeat clinical trials. Events that may prevent successful or timely completion of clinical development include:
•our inability to generate sufficient preclinical, toxicology or other data to support the initiation of clinical trials;
•our inability to develop and validate disease-relevant clinical endpoints;
•delays in reaching a consensus with regulatory authorities on trial design;
•delays in reaching agreement on acceptable terms with prospective clinical research organizations (“CROs”) and clinical trial sites;
•delays in opening investigational sites;
•delays or difficulty in recruiting and enrollment of suitable patients to participate in our clinical trials, whether as a result of the COVID-19 pandemic or otherwise;
•imposition of a clinical hold by regulatory authorities because of a serious adverse event, concerns with a class of drug candidates or after an inspection of our clinical trial operations or trial sites;
•delays in having patients complete participation in a trial or return for post-treatment follow-up;
•occurrence of serious adverse events associated with the drug candidate that are viewed to outweigh its potential benefits;
•changes in regulatory requirements and guidance that require amending or submitting new clinical protocols; or
•business interruptions resulting from geo-political actions,global geopolitical tensions, including the ongoing war between Russia and Ukraine and war in Israel, any other war or the perception that hostilities may be imminent, including terrorism, or natural disasters andor public health epidemics.
crises.In addition, our clinical trials may be affected by the COVID-19 pandemic.
Clinical site initiation and patient enrollment may be delayed due to prioritization of hospital resources toward the COVID-19 pandemic. Patients may not be able to comply with clinical trial protocols if quarantines impede patient movement or interrupt healthcare services. Similarly, our ability to recruit and retain patients and principal investigators and site staff who, as healthcare providers, may have heightened exposure to COVID-19, could be limited, which in turn could adversely impact our clinical trial operations. Additionally, we may experience interruption of key clinical trial activities, such as clinical trial site monitoring, due to limitations on travel, quarantines or social distancing protocols imposed or recommended by federal or state governments, employers and others in connection with the ongoing COVID-19 pandemic. As a result of the COVID-19 pandemic, we have faced and may continue to face delays in meeting our anticipated timelines for our ongoing and planned clinical trials.
Further, clinical endpoints for certain diseases we are targeting, such as
Angelman syndrome and Fragile X syndrome,CCM, have not been established, and accordingly we may have to develop new modalities or modify existing endpoints to measure efficacy, which may increase the time it takes for us to commence or complete clinical trials. In addition, we believe investigators in this area may be inexperienced in conducting trials in this area due to the current lack of drugs to treat these disorders, which may result in increased time and expense to train investigators and open clinical sites.
Any inability to successfully complete preclinical and clinical development could result in additional costs to us or impair our ability to generate revenue from future drug sales and regulatory and commercialization milestones. In addition, if we make manufacturing or formulation changes to our drug candidates, we may need to conduct additional testing to bridge our modified drug candidate to earlier versions. Clinical trial delays could also shorten any periods during which we may have the exclusive right to commercialize our drug candidates, if approved, or allow our competitors to bring comparable drugs to market before we do, which could impair our ability to successfully commercialize our drug candidates and may harm our business, financial condition, results of operations and prospects.
Additionally, if the results of our clinical trials are inconclusive or if there are safety concerns or serious adverse events associated with our drug candidates, we may:
•be delayed in obtaining marketing approval, if at all;
•obtain approval for indications or patient populations that are not as broad as intended or desired;
•obtain approval with labeling that includes significant use or distribution restrictions or safety warnings;
•be subject to additional post-marketing testing requirements;
•be required to perform additional clinical trials to support approval or be subject to additional post-marketing testing requirements;
•have regulatory authorities withdraw, or suspend, their approval of the drug or impose restrictions on its distribution in the form of a modified risk evaluation and mitigation strategy (“REMS”);
•be subject to the addition of labeling statements, such as warnings or contraindications;
•experience damage to our reputation.
Our drug development costs will also increase if we experience delays in testing or obtaining marketing approvals. We do not know whether any of our preclinical studies or clinical trials will begin as planned, need to be restructured or be completed on schedule, if at all.
Further, we, the FDA or an institutional review board (“IRB”)IRB may suspend our clinical trials at any time if it appears that we or our collaborators are failing to conduct a trial in accordance with regulatory requirements, including the FDA’s current Good Clinical Practice (“GCP)GCP regulations, that we are exposing participants to unacceptable health risks, or if the FDA finds deficiencies in our investigational new drug (“IND”)IND applications or the conduct of these trials. Therefore, we cannot predict with any certainty the schedule for commencement
and completion of future clinical trials. If we experience delays in the commencement or completion of our clinical trials, or if we terminate a clinical trial prior to completion, the commercial prospects of our drug candidates could be negatively impacted, and our ability to generate revenues from our drug candidates may be delayed.
Angelman syndrome has no treatments approved by the U.S. Food and Drug Administration, and the primary clinical endpoint, CGI-I-AS, has not previously been used as a sole primary endpoint in a pivotal clinical trial.
Angelman syndrome is characterized by a variety of signs and symptoms, such as delayed development, intellectual disability, severe speech impairment, problems with movement and balance, seizures, sleep disorders and anxiety. In order to obtain a broad indication for treatment of Angelman syndrome from the FDA, we may need to demonstrate efficacy on several of the key symptoms of Angelman syndrome. If we fail to do so, our clinical development may be delayed and/or our label may be limited. In December 2020, we reported the primary endpoint of the NEPTUNE study was not achieved. Based on the results of the NEPTUNE trial, further development of OV101, other than the ELARA trial, is currently on hold.
We may need to develop a new liquid formulation of OV101 for use in infant patients if our existing formulation in capsules that can be opened and sprinkled on semi-solid foods is not acceptable to the regulatory authorities, and we may be unable to successfully develop an appropriate liquid formulation
Our existing formulation of OV101 is an oral capsule. We have recently developed lower strength capsules that can be opened and sprinkled on applesauce or similar semi-solid foods. However, we may need to develop an oral liquid formulation of OV101 for use in very young pediatric patients. While we have begun developing this formulation, we do not know if our efforts will be successful or if the FDA will agree that the new formulation is comparable to our current formulation. We may experience manufacturing problems that may affect solubility or stability, or we may discover that the new formulation is less effective than an oral capsule. In addition, we will need to conduct bridging studies to demonstrate that the new formulation is equivalent to our oral capsule, which could result in delays in development and additional costs.
If we are not successful in discovering, developing and commercializing additional drug candidates, our ability to expand our business and achieve our strategic objectives would be impaired.
A key element of our
current strategy is to
discover, develop and potentially commercialize a portfolio of drug candidates to treat rare
epilepsies, seizure-related disorders, and rare neurological disorders.
However, our business development activities and research activities may present attractive opportunities outside of epilepsies and seizure-related disorders and we may choose to pursue drug candidates in other areas of interest including other disorders that we believe would be in the best interest of the Company and our stockholders. We plan to continuously review our strategies and modify as necessary based on attractive areas of interest and assets that we choose to pursue. We intend to
do sodevelop our portfolio of drug candidates by in-licensing and entering into collaborations with leading biopharmaceutical companies or academic institutions for new drug candidates. Identifying new drug candidates requires substantial technical, financial and human resources, whether or not any drug candidates are ultimately identified.
Our approach to business development, including our efforts to map the biological pathways related to orphan disorders of the brain and our relationships among the pharmaceutical industry, may not result in viable drug candidates for clinical development. The COVID-19 pandemic could also impact our ability to do in person due diligence, negotiations and other interactions to identify new opportunities. Even if we identify drug candidates that initially show promise, we may fail to in-license or acquire these assets and may also fail to successfully develop and commercialize such drug candidates for many reasons, including the following:
•the research methodology used may not be successful in identifying potential drug candidates;
•competitors may develop alternatives that render any drug candidate we develop obsolete;
•any drug candidate we develop may nevertheless be covered by third parties’ patents or other exclusive rights;
•a drug candidate may, on further study, be shown to have harmful side effects or other characteristics that indicate it is unlikely to be effective or otherwise does not meet applicable regulatory criteria;
•a drug candidate may not be capable of being produced in commercial quantities at an acceptable cost, or at all; and
•a drug candidate may not be accepted as safe and effective by physicians, patients, the medical community or third-party payors.
payors, even if approved.We have limited financial and management resources and, as a result, we may forego or delay
the pursuit of opportunities with other drug candidates or for other indications that later prove to have greater market potential. Our resource allocation decisions may cause us to fail to capitalize on viable commercial drugs or profitable market opportunities. If we do not accurately evaluate the commercial potential or target market for a particular drug candidate, we may relinquish valuable rights to that drug candidate through collaboration, licensing or other royalty arrangements in circumstances under which it would have been more advantageous for us to retain sole development and commercialization rights to such drug candidate.
If we are unsuccessful in identifying and developing additional drug candidates or are unable to do so, our key growth strategy and business will be harmed.
Clinical trials are very expensive, time-consuming and difficult to design and implement.
Our drug candidates will require clinical testing before we are prepared to submit a new drug application (“NDA”) for regulatory approval. We cannot predict with any certainty if or when we might submit an NDA for regulatory approval for any of our drug candidates or whether any such NDA will be approved by the FDA. Human clinical trials are very expensive and difficult to design and implement, in part because they are subject to rigorous regulatory requirements. For instance, the FDA may not agree with our proposed endpoints for any future clinical trial of our drug candidates, which may delay the commencement of our clinical trials. In addition, we may not succeed in developing and validating disease-relevant clinical endpoints based on insights regarding biological pathways for the disorders we are studying. The clinical trial process is also time-consuming. We estimate that the successful completion of clinical trials of our drug candidates will take at least several years to complete, if not longer. Furthermore, failure can occur at any stage and we could encounter problems that cause us to abandon or repeat clinical trials.
Enrollment and retention of patients in clinical trials is an expensive and time-consuming process and could be made more difficult or rendered impossible by multiple factors outside our control.
Identifying and qualifying patients to participate in our clinical trials is critical to our success. The number of patients suffering from Angelman syndrome, Fragile X syndromesome of the seizure-related disorders and DEE, such as Dravet syndrome, Lennox-Gastaut syndrome, Dup15q syndrome and CDKL5 deficiency disorderrare neurological disorders we are pursuing is small and has not been established with precision. If the actual number of patients with these disorders is smaller than we anticipate, we may encounter difficulties in enrolling patients in our clinical trials, thereby delaying or preventing development and approval of our drug candidates. Even once enrolled we may be unable to retain a sufficient number of patients to complete any of our trials. Patient enrollment and retention in clinical trials depends on many factors, including the size of the patient population, the nature of the trial protocol, the existing body of safety and efficacy data, the number and nature of competing treatments and ongoing clinical trials of competing therapies for the same indication, the proximity of patients to clinical sites and the eligibility criteria for the trial, any such enrollment issues could cause delays or prevent development and approval of our drug candidates. Because we are focused on addressing seizure-related disorders and rare neurological disorders, there are limited patient pools from which to draw in order to complete our clinical trials in a timely and cost-effective manner. Furthermore, our efforts to build relationships with patient communities may not succeed, which could result in delays in patient enrollment in our clinical trials. In addition, any negative results we may report in clinical trials
of our drug candidate may make it difficult or impossible to recruit and retain patients in other clinical trials of that same drug candidate. Delays or failures in planned patient enrollment or retention may result in increased costs, program delays or both, which could have a harmful effect on our ability to develop our drug candidates or could render further development impossible.For example, the impact of public health pandemics, such as COVID-19, may delay or prevent patients from enrolling or from receiving treatment in accordance with the protocol and the required timelines, which could delay our clinical trials, or prevent us or our partners from completing our clinical trials at all, and harm our ability to obtain approval for such product candidate. In addition, we may rely on CROs and clinical trial sites to ensure proper and timely conduct of our future clinical trials and, while we intend to enter into agreements governing their services, we will be limited in our ability to compel their actual performance.
Our drug candidates may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial potential or result in significant negative consequences following any potential marketing approval.
During the conduct of clinical trials, patients report changes in their health, including illnesses, injuries and discomforts, to their doctor. Often, it is not possible to determine whether or not the drug candidate being studied caused these conditions. Regulatory authorities may draw different conclusions or require additional testing to confirm these determinations, if they occur. In addition, it is possible that as we test our drug candidates in larger, longer and more extensive clinical programs, or as use of these drug candidates becomes more widespread if they receive regulatory approval, illnesses, injuries, discomforts and other adverse events that were observed in earlier trials, as well as conditions that did not occur or went undetected in previous trials, will be reported by subjects. Many times, side effects are only detectable after investigational drugs are tested in large-scale, Phase 3 trials or, in some cases, after they are made available to patients on a commercial scale after approval. For example,
in one of the trials conducted by Lundbeck, there were reports of hallucinations in drug abusers at 30mg and 45mg doses of OV101, which are higher than the 10mg and 15mg doses that were effective for insomnia. In addition, some patients treated with OV101 in the Lundbeck Phase 3 trials experienced headaches, nausea and dizziness. In the STARS study, the most frequent adverse events
were reported in certain clinical trials for OV101,
treated arms that were greater than placebo arm included pyrexia, rash, seizure, enuresis, myoclonic epilepsy, otitis mediaour former drug candidate, and
viral infection. Patients in our ongoing or planned clinicalsoticlestat. Clinical trials may
experience similar or other adverse events after treatment with OV101. In the Phase 1b/2a OV935 trial, adverse events that occurred more frequently in the OV935-treatment group versus the placebo group were dysarthria, insomnia, lethargy, seizure cluster, and upper respiratory infection.not demonstrate any ocular safety benefits for OV329 relative to vigabatrin. If
additional clinical experience indicates that any of our
current drug candidates
including OV101 and OV935, or any future drug candidates hascauses adverse events or
causes serious or life-threatening adverse events, the development of that drug candidate may fail or be delayed, or, if the drug candidate has received regulatory approval, such approval may be revoked, which would harm our business, prospects, operating results and financial condition.
Moreover, if we elect, or are required, to delay, suspend or terminate any clinical trial of our drug candidates, the commercial prospects of our drug candidates may be harmed and our ability to generate revenue through their sale may be delayed or eliminated. Any of these occurrences may harm our business, financial condition and prospects significantly.
Additionally, if any of our drug candidates receive marketing approval, the FDA could require us to include a black box warning in our label or adopt REMS to ensure that the benefits outweigh its risks, which may include, among other things, a medication guide outlining the risks of the drug for distribution to patients and a communication plan to health care practitioners. Furthermore, if we or others later identify undesirable side effects caused by our drug candidates, several potentially significant negative consequences could result, including:
•regulatory authorities may suspend or withdraw approvals of such drug candidate;
•regulatory authorities may require additional warnings on the label;
•we may be required to change the way a drug candidate is administered or conduct additional clinical trials;
•we could be sued and held liable for harm caused to patients;
•we may need to conduct a recall; and
•our reputation may suffer.
Any of these events could prevent us from achieving or maintaining market acceptance of our drug candidates and could significantly harm our business, prospects, financial condition and results of operations.
If the market opportunities for our drug candidates are smaller than we believe they are, even assuming approval of a drug candidate, our business may suffer. Because the patient populations in the market for our drug candidates may be small
and difficult to assess, we must be able to successfully identify patients and acquire a significant market share to achieve profitability and growth.
We focus our research and drug development on treatments for rare epilepsies, seizure-related disorders and rare neurological disorders. Given the small number of patients who have the disorders that we are targeting, our eligible patient population and pricing estimates may differ significantly from the actual market addressable by our drug candidates. Our projections of both the number of people who have these disorders, as well as the subset of people with these disorders who have the potential to benefit from treatment with our drug candidates, are based on our beliefs and estimates. These estimates have been derived from a variety of sources, including the scientific literature, patient foundations, or market research, and may prove to be incorrect. Further, new studies may change the estimated incidence or prevalence of these
disorders. The number of patients may turn out to be lower than expected. Likewise, the potentially addressable patient population for each of our drug candidates may be limited or may not be amenable to treatment with our drug candidates, and new patients may become increasingly difficult to identify or gain access to, which would adversely affect our results of operations and our business.
We face substantial competition, which may result in others developing or commercializing drugs before or more successfully than us.
The development and commercialization of new drugs is highly competitive. We face competition with respect to our current drug candidates and will face competition with respect to any other drug candidates that we may seek to develop or commercialize in the future, from major pharmaceutical companies, specialty pharmaceutical companies and biotechnology companies worldwide. There are a number of large pharmaceutical and biotechnology companies that currently market and sell drugs or are pursuing the development of drug candidates for the treatment of the indications that we are pursuing. Potential competitors also include academic institutions, government agencies and other public and private research organizations that conduct research, seek patent protection and establish collaborative arrangements for research, development, manufacturing and commercialization.
More established companies may have a competitive advantage over us due to their greater size, resources and institutional experience. In particular, these companies have greater experience and expertise in securing
collaboration or partnering relationships, reimbursement, government contracts, relationships with key opinion leaders, conducting testing and clinical trials, obtaining and maintaining regulatory approvals and distribution relationships to market products, and marketing approved drugs. These companies also have significantly greater research and marketing capabilities than we do. If we are not able to compete effectively against existing and potential competitors, our business and financial condition may be harmed.
As a result of these factors, our competitors may obtain regulatory approval of their drugs before we are able to, which may limit our ability to develop or commercialize our drug candidates. Our competitors may also develop therapies that are safer, more effective, more widely accepted and cheaper than ours, and may also be more successful than us in manufacturing and marketing their drugs. These appreciable advantages could render our drug candidates obsolete or non-competitive before we can recover the expenses of such drug candidates’ development and commercialization.
Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller and other early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These third parties compete with us in recruiting and retaining qualified scientific, management and commercial personnel, establishing clinical trial sites and subject registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs.
Even if our current or future drug candidates receive marketing approval, they may fail to achieve market acceptance by physicians, patients, third-party payors or others in the medical community necessary for commercial success.
Even if our current or future drug candidates receive marketing approval, they may fail to gain sufficient market acceptance by physicians, patients, third-party payors and others in the medical community. If they do not achieve an adequate level of acceptance, we may not generate significant drug revenue and may not become profitable. The degree of market acceptance of our current or future drug candidates, if approved for commercial sale, will depend on a number of factors, including but not limited to:
•the efficacy and potential advantages compared to alternative treatments and therapies;
•the safety profile of our drug candidate compared to alternative treatments and therapies;
•effectiveness of sales and marketing efforts;
•the strength of our relationships with patient communities;
•the cost of treatment in relation to alternative treatments and therapies, including any similar generic treatments;
•our ability to offer such drug for sale at competitive prices;
•the convenience and ease of administration compared to alternative treatments and therapies;
•the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies;
•the strength of marketing and distribution support;
•the availability of third-party coverage and adequate reimbursement;
•the prevalence and severity of any side effects; and
•any restrictions on the use of the drug together with other medications.
Our efforts to educate physicians, patients, third-party payors and others in the medical community on the benefits of our drug candidates may require significant resources and may never be successful. Such efforts may require more resources than are typically required due to the complexity and uniqueness of our drug candidates. Because we expect sales of our drug candidates, if approved, to
generate substantially all of our drug revenues for the foreseeable future, the failure of our drugs to find market acceptance would harm our business and could require us to seek additional financing.
If we are unable to establish sales and marketing capabilities, or enter into agreements with third parties to market and sell our current or any future drug candidates, we may be unable to generate any revenue from drug sales.
To successfully commercialize any drug candidate that may result from our development programs, we will need to build out our sales and marketing capabilities, either on our own or with others. The establishment and development of our own commercial team or the establishment of a contract sales force to market any drug candidate we may develop will be expensive and time-consuming and could delay any drug launch. Moreover, we cannot be certain that we will be able to successfully develop this capability. We may seek to enter into additional collaborations with other entities to utilize their established marketing and distribution capabilities, but we may be unable to enter into such agreements on favorable terms, if at all. If any current or future collaborators do not commit sufficient resources to commercialize our drug candidates, or we are unable to develop the necessary capabilities on our own, we will be unable to generate sufficient revenue to sustain our business. We compete with many companies that currently have extensive, experienced and well-funded marketing and sales operations to recruit, hire, train and retain marketing and sales personnel. We also face competition in our search for third parties to assist us with the sales and marketing efforts of our current and future drug candidates. Without an internal team or the support of a third party to perform marketing and sales functions, we may be unable to compete successfully against these more established companies.
Even if we obtain and maintain approval for our current or future drug candidates from the FDA, we may never obtain approval for our current or future drug candidates outside of the United States, which would limit our market opportunities and could harm our business.
Approval of a drug candidate in the United States by the FDA does not ensure approval of such drug candidate by regulatory authorities in other countries or jurisdictions, and approval by one foreign regulatory authority does not ensure approval by regulatory authorities in other foreign countries or by the FDA. Sales of our current and future drug candidates outside of the United States will be subject to foreign regulatory requirements governing clinical trials and marketing approval. Even if the FDA grants marketing approval for a drug candidate, comparable regulatory authorities of foreign countries also must approve the manufacturing and marketing of the drug candidate in those countries. Approval procedures vary among jurisdictions and can involve requirements and administrative review periods different from, and more onerous than, those in the United States,
includingwhich may require additional preclinical studies or clinical trials. In many countries outside the United States, a drug candidate must be approved for reimbursement before it can be approved for sale in that country. In some cases, the price that we intend to charge for any drug candidates, if approved, is also subject to approval. Obtaining approval for our current and future drug candidates in the European Union from the European Commission following the opinion of the
EMA,European Medicines Agency, if we choose to submit a marketing authorization application there, would be a lengthy and expensive process.
Even if a drug candidate is approved,The FDA and comparable foreign regulatory authorities have the
FDA or the European Commission, as the case may be, mayability to limit the indications for which the drug may be marketed, require extensive warnings on the drug labeling or require expensive and time-consuming additional clinical trials or reporting as conditions of approval. Obtaining foreign regulatory approvals and compliance with foreign regulatory requirements could result in significant delays, difficulties and costs for us and could delay or prevent the introduction of our current and future drug candidates in certain countries. In certain cases,
including under our license with Angelini, which covers the European Economic Area as well as Switzerland, the United Kingdom, Russia and Turkey, we are dependent on third parties to obtain such foreign regulatory approvals, and any delay or failure of performance of such third parties could delay or prevent our ability to commercialize our products in the affected countries.
Due to the ongoing COVID-19 pandemic, it is possible that we could experience delays in the timing of our interactions with regulatory authorities due to absenteeism by governmental employees, inability to conduct planned physical inspections related to regulatory approval, or the diversion of regulatory authority efforts and attention to approval of other therapeutics or other activities related to COVID-19, which could delay anticipated approval decisions and otherwise delay or limit our ability to make planned regulatory submissions or obtain new product approvals.Further, clinical trials conducted in one country may not be accepted by regulatory authorities in other countries. Also, regulatory approval for our drug candidates may be withdrawn. If we fail to comply with the regulatory requirements, our target market will be reduced and our ability to realize the full market potential of our current and future drug candidates will be harmed and our business, financial condition, results of operations and prospects could be harmed.
If we seek approval to commercialize our current or future drug candidates outside of the United States,
in particular in the European Union and Israel, a variety of risks associated with international operations could harm our business.
If we seek approval of our current or future drug candidates outside of the United States, we expect that we will be subject to additional risks in commercialization including:
•different regulatory requirements for approval of therapies in foreign countries;
•reduced protection for intellectual property rights;
•the potential requirement of additional clinical studies in international jurisdictions;
•unexpected changes in tariffs, trade barriers and regulatory requirements;
•economic weakness, including inflation, or political instability in particular foreign economies and markets;
•compliance with tax, employment, immigration and labor laws for employees living or traveling abroad;
•foreign currency fluctuations, which could result in increased operating expenses and reduced revenues, and other obligations incident to doing business in another country;
•foreign reimbursement, pricing and insurance regimes;
•workforce uncertainty in countries where labor unrest is more common than in the United States;
•production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and
•business interruptions resulting from geopolitical actions,tensions, including the ongoing war between Russia and Ukraine and the war in Israel, any other war or the perception that hostilities may be imminent, terrorism, or natural disasters andor public health pandemics, such as COVID-19;
crises.We have no prior experience in these areas. In addition, there are complex regulatory, tax, labor and other legal requirements imposed by
both the European Union, Israel and many of the individual countries in and outside of Europe with which we will need to comply. Many biopharmaceutical companies have found the process of marketing their own products in foreign countries to be very challenging.
Product liability lawsuits against us could cause us to incur substantial liabilities and could limit commercialization of any drug candidate that we may develop.
We face an inherent risk of product liability exposure related to the testing of our current and any future drug candidates in clinical trials and may face an even greater risk if we commercialize any drug candidate that we may develop. If we cannot successfully defend ourselves against claims that any such drug candidates caused injuries, we could incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
•decreased demand for any drug candidate that we may develop;
•substantial monetary awards to trial participants or patients;
•significant time and costs to defend the related litigation;
•withdrawal of clinical trial participants;
•the inability to commercialize any drug candidate that we may develop; and
•injury to our reputation and significant negative media attention.
Although we maintain product liability insurance coverage, such insurance may not be adequate to cover all liabilities that we may incur. We anticipate that we will need to increase our insurance coverage each time we commence a clinical trial and if we successfully commercialize any drug candidate. Insurance coverage is increasingly expensive. We may not be able to maintain insurance coverage at a reasonable cost or in an amount adequate to satisfy any liability that may arise.
Risks Related to Licensing and Collaboration Arrangements
We
Under the RLT Agreement, we are
heavily dependent on our relationshipentitled to receive royalty and milestone payments in connection with
Takeda for the development and commercialization of
OV935. Any disruption in our relationship withsoticlestat. If Takeda
could leadfails to
delays in,progress or
the termination of,discontinues the development of
OV935,soticlestat, we may not receive some or all of such payments, which would materially harm our business.
We are jointly developing OV935 with Takeda pursuant to the Takeda collaboration, which also granted us intellectual property rights to OV935. The development and commercialization of OV935 is highly dependent upon our relationship with Takeda, including Takeda’s submission of the IND to the FDA. If for any reason the Takeda collaboration is terminated, or we otherwise lose the intellectual property rights to OV935, our business would be adversely affected. The Takeda collaboration imposes on us rights and obligations, including but not limited to exclusivity, territorial rights, development, commercialization, funding, payment, diligence, sublicensing, insurance and intellectual property protection. After a negotiated time period, each party has the right to
terminate the license for convenience upon six to twelve months’ notice to the other party, which would result in us being unable to co-develop and sell OV935. Further, if we breach any material obligations, or use the intellectual property licensed to us in an unauthorized manner, we may be required to pay damages to Takeda, and Takeda may have the right to terminate the license. Takeda could also breach its obligations under the agreement or may not commit a sufficient amount of resources to satisfy its obligations, which would result in the development of OV935 being materially delayed or terminated. In March 2021, we entered into the Takeda License and TerminationRLT Agreement, underpursuant to which Takeda will securesecured rights to our 50% global rights at closingshare in soticlestat, which we had originally licensed from usTakeda, and we granted to Takeda an exclusive worldwide license under our relevant intellectual property rights to develop and commercialize the investigational medicine OV935soticlestat for the treatment of developmental and epileptic encephalopathies, including DSDravet syndrome and LGS. ClosingLennox-Gastaut syndrome. All rights in soticlestat are now owned by Takeda or exclusively licensed to Takeda by us. Following the closing date of the RLT Agreement, Takeda Licenseassumed all responsibility for, and Terminationcosts of, both development and commercialization of soticlestat, and we will no longer have any financial obligation to Takeda under the original collaboration agreement, including for milestone payments or any future development and commercialization costs. Upon closing of the RLT Agreement, we received a one-time, upfront payment of $196.0 million and, if soticlestat is subjectsuccessfully developed, we will be eligible to satisfactionreceive up to an additional $660.0 million upon Takeda achieving specified regulatory and sales milestones. In addition, if soticlestat achieves regulatory approval, we will be entitled to receive tiered royalties at percentages ranging from the low double-digits up to 20% on sales of customary closing conditions. See “Business—Licensesoticlestat. Royalties will be payable on a country-by-country and Collaboration Agreements—Agreements with Takeda—2021 Royalty, Licenseproduct-by-product basis during the period beginning on the date of the first commercial sale of such
product in such country and Terminationending on the later to occur of the expiration of patent rights covering the product in such country and a specified anniversary of such first commercial sale. Pursuant to the Ligand Agreement, with Takeda” forLigand will receive a discussion13% portion of the royalties and milestones owed to us pursuant to the RLT Agreement.
Under the terms of the
RLT Agreement, Takeda
License and Termination Agreement.We are dependent on our relationship with Angelini fornow has sole discretion over the conduct of the development and commercialization of OV101 in European Economic Area as well as Switzerland, the United Kingdom, Russia and Turkey. Any disruption in our relationship with Angelini could lead to delays in the development and achievement of regulatory approval in these countries, which would materially harm our business.
Under our license agreement with Angelini, Angelini obtained exclusive rights to develop and commercialize OV101 in the European Economic Area as well as Switzerland, the United Kingdom, Russia and Turkey. The development and commercialization of OV101 is highly dependent upon our relationship with Angelini, and on Angelini’s performance of its obligations under the agreement, including with respect to the preparation and submission of applications for marketing approval in Europe and the other licensed countries.soticlestat. If for any reason, AngeliniTakeda fails to perform its obligations,progress, or elects to terminate the development of soticlestat as contemplated by the RLT Agreement, or if the development or commercialization of soticlestat is delayed or deprioritized by Takeda, we may not be ablereceive some or all of the royalty and milestone payments under such agreement. We are dependent upon Takeda’s progression of such development and the resulting payments to achievefund the regulatory approval for OV101 indevelopment of our current and future drug candidates. If we are unable to find alternative sources of revenue, our inability to receive royalty or milestone payments under the licensed countries, or may be materially delayed in doing so, andRLT Agreement would negatively impact our business would be adversely affected.
We may be required to make significant payments in connection with our licensesand results of OV101 from Lundbeck and OV935 from Takeda.
We acquired rights to OV101, pursuant to a license agreement with Lundbeck in March 2015 (the “Lundbeck Agreement”), as amended on May 10, 2019. Under the Lundbeck Agreement, as amended, we are subject to significant obligations, including payment obligations upon achievement of specified milestones and royalties on drug sales, as well as other material obligations. We are obligated to pay Lundbeck milestone payments up to an aggregate of $189.0 million upon the achievement of certain development, regulatory and sales milestone events. In addition, we are obligated to pay Lundbeck tiered royalties based on net sales of OV101. If these payments become due under the terms of the Lundbeck agreement, we may not have sufficient funds available to meet our obligations and our development efforts may be harmed.
We also acquired rights to OV935 pursuant to a license and collaboration agreement with Takeda (the “Takeda collaboration”) in January 2017. Under the Takeda collaboration, we are obligated to pay Takeda future payments upon achievement of specified milestones. Upon the first patient enrollment in the first Phase 3 trial for the first of the initial indications we and Takeda are focusing on pursuant to the Takeda collaboration, we are obligated to issue to Takeda the number of unregistered shares of our common stock equal to the lesser of (i) 8% of our outstanding capital stock on the issuance date or (ii) $50.0 million divided by the applicable share price, unless certain events occur. The remaining potential global commercial and regulatory milestone payments equal approximately $35.0 million and can be satisfied in cash or unregistered shares of our common stock at our election, unless certain events occur in which Takeda can require us to pay such payments in cash. In the event a payment settled in shares of our common stock would cause Takeda to own over 19.99% of our outstanding capital stock or other events occur, such payment must be paid in cash. If these payments become due under the terms of the Takeda collaboration and we can only pay, or choose to pay, these payments in cash, we may not have sufficient funds available to meet our obligations and our development efforts may be harmed. In March 2021, we entered into the Takeda License and Termination Agreement under which Takeda will secure global rights at closing from us to develop and commercialize the investigational medicine OV935 for the treatment of developmental and epileptic encephalopathies, including DS and LGS. Closing of the Takeda License and Termination Agreement is subject to satisfaction of customary closing conditions. See “Business—License and Collaboration Agreements—Agreements with Takeda—2021 Royalty, License and Termination Agreement with Takeda” for a discussion of the terms of the Takeda License and Termination Agreement.
operations.
Risks associated with the in-licensing or acquisition of drug candidates could cause substantial delays in the preclinical and clinical development of our drug candidates.
Prior to March 2015, we had no involvement with or control over the preclinical and clinical research and development of OV101.
We have
relied on Lundbeck or its prior licensee to have conducted such researchpreviously acquired and
development in accordance with the applicable protocol, legal, regulatory and scientific standards, having accurately reported the results of all clinical trials conducted prior to our acquisition of OV101 and having correctly collected and interpreted the data from these trials. If the research and development processes or the results of the development programs prior to our acquisition of OV101 prove to be unreliable, this could result in increased costs and delays in the development of OV101, which could adversely affect any future revenue from this drug candidate.
Similarly, we acquired rights to OV935 from Takeda in January 2017. Because we were not involved in the development of OV935 prior to January 2017, we may experience difficulties in the transition of certain development activities from Takeda and its affiliates to us, which may result in delays in clinical trials, as well as problems in our development efforts. In March 2021, we entered into the Takeda License and Termination Agreement under which Takeda will secure global rights at closing from us to develop and commercialize the investigational medicine OV935 for the treatment of developmental and epileptic encephalopathies, including DS and LGS. Closing of the Takeda License and Termination Agreement is subject to satisfaction of customary closing conditions. See “Business—License and Collaboration Agreements—Agreements with Takeda—2021 Royalty, License and Termination Agreement with Takeda” for a discussion of the terms of the Takeda License and Termination Agreement.
We may also acquire or in-license additional drug candidates for preclinical or clinical development in the future as we continue to build our pipeline. The risks associatedSuch arrangements with acquiringthird parties may impose, diligence, development and commercialization obligations, milestone payments, royalty payments, indemnification and other obligations on us. Our obligations to pay milestone, royalty and other payments to our licensors may be substantial, and the amount and timing of such payments may impact our ability to progress the development and commercialization of our drug candidates. Our rights to use any licensed intellectual property may be subject to the continuation of and our compliance with the terms of any such agreements. Additionally, disputes may arise regarding our rights to intellectual property licensed to us or in-licensing currentacquired by us from a third party, including but not limited to:
•the scope of intellectual property rights included in, and rights granted under, any license or other agreement;
•the sublicensing of patent and other rights under such agreements;
•our compliance with our diligence obligations under any license agreement;
•the ownership of inventions and know-how resulting from the creation or use of intellectual property by us, alone or with our licensors and collaborators;
•the scope and duration of our payment obligations, and our ability to make such payments when they are owed;
•our need to acquire additional intellectual property rights from third parties that may impact payments due under such agreements;
•the rights of our licensors to terminate any such agreement;
•our rights and obligations upon termination of such agreement; and
•the scope and duration of exclusivity obligations of each party to the agreement.
Disputes over intellectual property and other rights that we have licensed or acquired, or may license or acquire in the future,
drug candidatesfrom third parties could
prevent or impair our ability to maintain any such arrangements on acceptable terms, result in delays in the commencement or completion of our preclinical studies and clinical trials
if ever, and
impact our ability to
generate revenues fromsuccessfully develop and commercialize the affected drug candidates. If we fail to comply with our
drug candidatesobligations under any future licensing agreements, these agreements may be
delayed.terminated or the scope of our rights under them may be reduced and we might be unable to develop, manufacture or market any product that is licensed under these agreements.
We may be required to relinquish important rights to and control over the development and commercialization of our drug candidates to any future collaborators.
Our current and future collaborations could subject us to a number of risks, including:
•we may be required to undertake the expenditure of substantial operational, financial and management resources;
•we may be required to issue equity securities that would dilute our stockholders’ percentage of ownership;
•we may be required to assume substantial actual or contingent liabilities;
•we may not be able to control the amount and timing of resources that our strategic collaborators devote to the development or commercialization of our drug candidates;
•strategic collaborators may delay clinical trials, provide insufficient funding, terminate a clinical trial or abandon a drug candidate, repeat or conduct new clinical trials or require a new version of a drug candidate for clinical testing;
•strategic collaborators may not pursue further development and commercialization of products resulting from the strategic collaboration arrangement or may elect to discontinue research and development programs;
•strategic collaborators may not commit adequate resources to the marketing and distribution of our drug candidates, limiting our potential revenues from these products;
•we rely on our current collaborators to manufacture drug substance and drug product and may do so with respect to future collaborators, which could result in disputes or delays;
•disputes may arise between us and our strategic collaborators that result in the delay or termination of the research, development or commercialization of our drug candidates or that result in costly litigation or arbitration that diverts management’s attention and consumes resources;
•disputes may arise between us and our current or future collaborators regarding any termination of any collaboration, license, or other business development arrangement in which we may enter;
•strategic collaborators may experience financial difficulties;
•strategic collaborators may not properly maintain or defend our intellectual property rights or may use our proprietary information in a manner that could jeopardize or invalidate our proprietary information or expose us to potential litigation;
•business combinations or significant changes in a strategic collaborator’s business strategy may also adversely affect a strategic collaborator’s willingness or ability to complete its obligations under any arrangement;
•strategic collaborators could decide to move forward with a competing drug candidate developed either independently or in collaboration with others, including our competitors; and
•strategic collaborators could terminate the arrangement or allow it to expire, which would delay the development and may increase the cost of developing our drug candidates.
If we engage in future acquisitions or strategic partnerships, this may increase our capital requirements, dilute our stockholders, cause us to incur debt or assume contingent liabilities and subject us to other risks.
Our business plan is to continue to evaluate various acquisitions and strategic partnerships, including licensing or acquiring complementary drugs, intellectual property rights, technologies, or businesses. Any potential acquisition or strategic partnership may entail numerous risks, including:
•increased operating expenses and cash requirements;
•the assumption of additional indebtedness or contingent liabilities;
•assimilation of operations, intellectual property and drugs of an acquired company, including difficulties associated with integrating new personnel;
•the diversion of our management’s attention from our existing drug programs and initiatives in pursuing such a strategic partnership, merger or acquisition;
•retention of key employees, the loss of key personnel, and uncertainties in our ability to maintain key business relationships;
•risks and uncertainties associated with the other party to such a transaction, including the prospects of that party and their existing drugs or drug candidates and regulatory approvals;
•our inability to generate revenue from acquired technology and/or drugs sufficient to meet our objectives in undertaking the acquisition or even to offset the associated acquisition and maintenance costs;
•challenges related to integrating acquired businesses or entering into or realizing the benefits of strategic transactions generally; and
•risks associated with potential international acquisition transactions, including in countries where we do not currently have a material presence.
In addition, if we engage in future acquisitions or strategic partnerships, we may issue dilutive securities, assume or incur debt obligations, incur large one-time expenses and acquire intangible assets that could result in significant future amortization expense. Moreover, we may not be able to locate suitable acquisition opportunities and this inability could impair our ability to grow or obtain access to technology or drugs that may be important to the development of our business.
We may explore additional strategic collaborations that may never materialize or may fail.
Our business strategy is based on acquiring or in-licensing compounds directed at rare epilepsies, seizure-related disorders, and rare neurological disorders. As a result, we intend to periodically explore a variety of possible additional strategic collaborations in an effort to gain access to additional drug candidates or resources. At the current time, we cannot predict what form such a strategic collaboration might take. We are likely to face significant competition in seeking appropriate strategic collaborators, and strategic collaborations can be complicated and time consuming to negotiate and document. We may not be able to negotiate strategic collaborations on acceptable terms, or at all. We are unable to predict when, if ever, we will enter into any additional strategic collaborations because of the numerous risks and uncertainties associated with establishing them. Further, our business development activities and research activities may present attractive opportunities outside of rare epilepsies and seizure-related disorders and we may choose to pursue drug candidates in other areas of interest including other disorders and diseases that we believe would be in the best interest of the Company and our stockholders. We plan to continuously review our strategies and modify as necessary based on attractive areas of interest and assets that we choose to pursue.
Risks Related to Regulatory Compliance
Our relationships with customers, physicians, and third-party payors may be subject, directly or indirectly, to federal and state healthcare fraud and abuse laws, false claims laws, health information privacy and security laws, and other healthcare laws and regulations. If we are unable to comply, or have not fully complied, with such laws, we could face substantial penalties.
Healthcare providers and third-party payors in the United States and elsewhere will play a primary role in the recommendation and prescription of any drug candidates for which we obtain marketing approval. Our current and future arrangements with healthcare professionals, principal investigators, consultants, customers and third-party payors may subject us to various federal and state fraud and abuse laws and other healthcare laws, including, without limitation, the federal Anti-Kickback Statute, the federal civil and criminal false claims laws and the law commonly referred to as the Physician Payments Sunshine Act and regulations. These laws will impact, among other things, our clinical research, proposed sales, marketing and educational programs. In addition, we may be subject to patient privacy laws by both the federal government and the states in which we conduct or may conduct our business. The laws that will affect our operations include, but are not limited to:
•the federal Anti-Kickback Statute, which prohibits, among other things, persons or entities from knowingly and willfully soliciting, receiving, offering or paying any remuneration (including any kickback, bribe or rebate), directly or indirectly, overtly or covertly, in cash or in kind, in return for the purchase, recommendation, leasing or furnishing of an item or service reimbursable under a federal healthcare program, such as the Medicare and Medicaid programs. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on the one hand, and prescribers, purchasers and formulary managers on the other. The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act (collectively, the “PPACA”), amended the intent requirement of the federal Anti-Kickback Statute. A person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it in order to have committed a violation;
•federal civil and criminal false claims laws, including, without limitation, the False Claims Act, and civil monetary penalty laws which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment or approval from Medicare, Medicaid or other government payors that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government. The PPACA provides, and recent government cases against pharmaceutical and medical device manufacturers support, the view that federal Anti-KickbackAnti-
Kickback Statute violations and certain marketing practices, including off-label promotion, may implicate the False Claims Act;
•the federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), which created additional federal criminal statutes that prohibit a person from knowingly and willfully executing a scheme or making false or fraudulent statements to defraud any healthcare benefit program, regardless of the payor (e.g., public or private);
•HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act (“HITECH”), and their implementing regulations, and as amended again by the final HIPAA omnibus rule, Modifications to the HIPAA Privacy, Security, Enforcement, and Breach Notification Rules Under HITECH and the Genetic Information Nondiscrimination Act; Other Modifications to HIPAA, published in January 2013, which imposes certain requirements relating to the privacy, security and transmission of individually identifiable health information without appropriate authorization by entities subject to the rule, such as health plans, healthcare clearinghouses and certain healthcare providers, known as covered entities, and their respective business associates, individuals or entities that perform certain services on behalf of a covered entity that involves the use or disclosure of individually identifiable health information and their subcontractors that use, disclose or otherwise process individually identifiable health information;
•Physician Payments Sunshine Act, which is part of the PPACA, that require certain manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program, with specific exceptions, to report annually to the Centers for Medicare & Medicaid Services (“CMS”), information related to: (i) payments or other “transfers of value” made to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors), certain other healthcare professionals (such as physician assistants and nurse practitioners), and teaching hospitals; and (ii) ownership and investment interests held by physicians and their immediate family members which will be expanded beginning in 2022, to require applicable manufacturers to report information regarding payments and other transfers of value provided to physician assistants, nurse practitioners, clinical nurse specialists, anesthesiologist assistants, certified registered nurse anesthetists and certified nurse midwives during the previous year;
•state and foreign law equivalents of each of the above federal laws, state laws that require manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures and/or information regarding drug pricing, state laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government or to adopt compliance programs as prescribed by state laws and regulations, or that otherwise restrict payments that may be made to healthcare providers, state laws and regulations that require drug manufacturers to file reports relating to drug pricing and marketing information, and state and local laws that require the registration of pharmaceutical sales representatives; and
•state and foreign laws that govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.
Because of the breadth of these laws and the narrowness of the statutory exceptions and regulatory safe harbors available, it is possible that some of our business activities could be subject to challenge under one or more of such laws.
It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, disgorgement, imprisonment, exclusion from participation in government funded healthcare programs, such as Medicare and Medicaid, additional reporting requirements and oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of non-compliance with these laws and the curtailment or restructuring of our operations.
The risk of our being found in violation of these laws is increased by the fact that many of them have not been fully interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations. Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. The shifting compliance environment and the need to build and maintain robust and expandable systems to
comply with multiple jurisdictions with different compliance and/or reporting requirements increases the possibility that a healthcare company may run afoul of one or more of the requirements.
Coverage and adequate reimbursement may not be available for our current or any future drug candidates, which could make it difficult for us to sell profitably, if approved.
Market acceptance and sales of any drug candidates that we commercialize, if approved, will depend in part on the extent to which coverage and adequate reimbursement for these drugs and related treatments will be available from third-party payors, including government health administration authorities, managed care organizations and other private health insurers. Third-party payors decide which therapies they will pay for and establish reimbursement levels. Third-party payors often rely upon Medicare coverage policy and payment limitations in setting their own coverage and reimbursement policies. However, decisions regarding the extent of coverage and amount of reimbursement to be provided for any drug candidates that we develop will be made on a payor-by-payor basis. One third-party payor’s determination to provide coverage for a drug does not assure that other payors will also provide coverage, and adequate reimbursement, for the drug. Additionally, a third-party payor’s decision to provide coverage for a therapy does not imply that an adequate reimbursement rate will be approved. Each third-party payor determines whether or not it will provide coverage for a therapy, what amount it will pay the manufacturer for the therapy, and on what tier of its formulary it will be placed. The position on a third-party payor’s list of covered drugs, or formulary, generally determines the co-payment that a patient will need to make to obtain the therapy and can strongly influence the adoption of such therapy by patients and physicians. Patients who are prescribed treatments for their conditions and providers prescribing such services generally rely on third-party payors to reimburse all or part of the associated healthcare costs. Patients are unlikely to use our drugs unless coverage is provided and reimbursement is adequate to cover a significant portion of the cost of our drugs.
A primary trend in the U.S. healthcare industry and elsewhere is cost containment. Third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications. We cannot be sure that coverage and reimbursement will be available for any drug that we commercialize and, if reimbursement is available, what the level of reimbursement will be. Inadequate coverage and reimbursement may impact the demand for, or the price of, any drug for which we obtain marketing approval. If coverage and adequate reimbursement are not available, or are available only to limited levels, we may not be able to successfully commercialize our current and any future drug candidates that we develop. Further, coverage policies and third-party payor reimbursement rates may change at any time. Even if favorable coverage and reimbursement status is attained, less favorable coverage policies and reimbursement rates may be implemented in the future.
Healthcare legislative reform measures may have a negative impact on our business and results of operations.
In the United States and some foreign jurisdictions, there have been, and continue to be, several legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of drug candidates, restrict or regulate post-approval activities, and affect our ability to profitably sell any drug candidates for which we obtain marketing approval.
Among policy makers and payors in the United States and elsewhere, there is significant interest in promoting changes in healthcare systems with the stated goals of containing healthcare costs, improving quality and/or expanding access. In the United States, the pharmaceutical industry has been a particular focus of these efforts and has been significantly affected by major legislative initiatives. In March 2010, the PPACA was passed, which substantially changed the way healthcare is financed by both the government and private insurers, and significantly impacts the U.S. pharmaceutical industry.
There have been executive, judicial, Congressional and executive branch challenges to certain aspects of the PPACA. For example,
President Trump signed Executive Orders and other directives designed to delayon June 17, 2021 the
implementation of certain provisions of the PPACA or otherwise circumvent some of the requirements for health insurance mandated by the PPACA. Concurrently, Congress considered legislation to repeal or repeal and replace all or part of the PPACA. While Congress has not passed comprehensive repeal legislation, it has enacted lawsU.S. Supreme Court dismissed a challenge on procedural grounds that
modify certain provisions of the PPACA such as removing penalties, starting January 1, 2019, for not complying with the PPACA’s individual mandate to carry health insurance, delaying the implementation of certain PPACA-mandated fees, and increasing the point-of-sale discount that is owed by pharmaceutical manufacturers who participate in Medicare Part D. Additionally, the 2020 federal spending package permanently eliminated, effective January 1, 2020, the PPACA-mandated “Cadillac” tax on high-cost employer-sponsored health coverage and medical device tax and, effective January 1, 2021, also eliminated the health insurer tax. On December 14, 2018, a Texas U.S. District Court Judge ruled thatargued the PPACA is unconstitutional in its entirety because the “individual mandate” was repealed by
Congress as partCongress. Further, there have been a number of
health reform measures by the
Tax Cuts and Jobs Act of 2017.Biden administration that have impacted the PPACA. On
December 18, 2019, the U.S. Court of Appeals for the 5th Circuit upheld the District Court ruling that the individual mandate was
unconstitutional and remanded the case back to the District Court to determine whether the remaining provisions of the PPACA are invalid as well. The United States Supreme Court is currently reviewing this case, but it is unknown when a decision will be reached. Although the Supreme Court has not yet ruled on the constitutionality of the PPACA, on January 28, 2021,August 16, 2022, President Biden issued an executive order to initiate a special enrollment period from February 15, 2021 through May 15, 2021signed the IRA into law, which among other things, extends enhanced subsidies for purposes of obtainingindividuals purchasing health insurance coverage in PPACA marketplaces through plan year 2025. The IRA also eliminates the “donut hole” under the Medicare Part D program beginning in 2025 by significantly lowering the beneficiary maximum out-of-pocket cost and by creating a new manufacturer discount program. It is possible that the PPACA marketplace. The executive order also instructs certain governmental agencieswill be subject to review and reconsider their existing policies and rules that limit access to healthcare, including among others, reexamining Medicaid demonstration projects and waiver programs that include work requirements, and policies that create barriers to obtaining access to health insurance coverage through Medicaidjudicial or Congressional challenges in the PPACA.future. It is unclear how the Supreme Court ruling, otherany such litigationchallenges and the healthcare reform measures of the Biden administration will impact the PPACA and our business.
Other legislative changes have been proposed and adopted since the PPACA was enacted. These changes include aggregate reductions to Medicare payments to providers of up to 2% per fiscal year pursuant to the Budget Control Act of
2011, which began in 2013, and due to subsequent legislative amendments to the statute,
including the BBA, will remain in effect
through 2030until 2032 unless additional Congressional action is taken.
However, COVID-19 relief legislation suspended the 2% Medicare sequester from May 1, 2020 through March 31, 2021. The American Taxpayer Relief Act of 2012, among other things, further reduced Medicare payments to several providers, including hospitals and cancer treatment centers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years.
Additional changes that may affect our business include the expansion of new programs such as Medicare payment for performance initiatives for physicians under the Medicare Access and CHIP Reauthorization Act of 2015 (“MACRA”), which ended the use of the statutory formula and established a quality payment program, also referred to as the Quality Payment Program.
In November 2019, CMS issued a final rule finalizingThis program provides clinicians with two ways to participate, including through the
changes toAdvanced Alternative Payment Models (“APMs”) and the
QualityMerit-based Incentive Payment
Program. At this time, the full impact to overall physician reimbursement as a result of the introduction of the Quality Payment Program remains unclear.System (“MIPS”). Under both APMs and MIPS, performance data collected each performance year will affect Medicare payments in later years, including potentially reducing payments.
Also, there has been heightened governmental scrutiny recently over the manner in which drug manufacturers set prices for their marketed products, which have resulted in several
Presidential executive orders, Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drug products. At the federal level,
, in July 2021, the
TrumpBiden administration
used several meansreleased an executive order, “Promoting Competition in the American Economy,” with multiple provisions aimed at prescription drugs. In response to propose or implementBiden’s executive order, on September 9, 2021, the U.S. Department of Health and Human Services (“HHS”) released a Comprehensive Plan for Addressing High Drug Prices that outlines principles for drug pricing reform including through federal budget proposals, executive orders and policy initiatives. For example, on July 24, 2020sets out a variety of potential legislative policies that Congress could pursue as well as potential administrative actions HHS can take to advance these principles. Further, the IRA, among other things (i) directs HHS to negotiate the price of certain high-expenditure, single-source drugs and September 13, 2020,biologics covered under Medicare and (ii) imposes rebates under Medicare Part B and Medicare Part D to penalize price increases that outpace inflation. These provisions take effect progressively starting in fiscal year 2023. On August 29, 2023, HHS announced the Trump administration announced several executive orders related to prescription drug pricing that attempt to implement severallist of the administration’s proposals. The FDA also released a final rule, effective November 30, 2020, implementing a portion offirst ten drugs that will be subject to price negotiations, although the importation executive order providing guidance for statesMedicare drug price negotiation program is currently subject to build and submit importation plans for drugs from Canada. Further, on November 20, 2020, HHS finalized a regulation removing safe harbor protection for price reductions from pharmaceutical manufacturers to plan sponsors under Part D, either directly or through pharmacy benefit managers, unless the price reduction is required by law. The implementation of the rule has been delayed bylegal challenges. Additionally, the Biden administration from January 1, 2022 to January 1, 2023 in response to ongoing litigation. The rule also creates a new safe harbor for price reductions reflected at the point-of-sale, as well as a new safe harbor for certain fixed fee arrangements between pharmacy benefit managers and manufacturers, the implementation of which have also been delayed pending review by the Biden administration until March 22, 2021. On November 20, 2020, CMS issuedreleased an interim final rule implementing President Trump’s Most Favored Nationadditional executive order which would tieon October 14, 2022, directing HHS to report on how the Center for Medicare Part B paymentsand Medicaid Innovation can be further leveraged to test new models for certain physician-administered drugs to the lowest price paid in other economically advanced countries, effective January 1, 2021. On December 28, 2020, the United States District Court in Northern California issued a nationwide preliminary injunction against implementation of the interim final rule. It is unclear whether the Biden administration will work to reverse these measures or pursue similar policy initiatives.lowering drug costs for Medicare and Medicaid beneficiaries. At the state level, legislatures have increasingly passed and implemented regulations designed to control pharmaceutical and biological product pricing, including pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. We expect that these and other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and in additional downward pressure on the price that we receive for any approved drug.
For example, in response to the Biden administration’s October 2022 executive order, on February 14, 2023, HHS released a report outlining three new models for testing by the Centers for Medicare & Medicaid Services Innovation Center which will be evaluated on their ability to lower the cost of drugs, promote accessibility, and improve quality of care. It is unclear whether the models will be utilized in any health reform measures in the future. Further, on December 7, 2023, the Biden administration announced an initiative to control the price of prescription drugs through the use of march-in rights under the Bayh-Dole Act. On December 8, 2023, the National Institute of Standards and Technology published for comment a Draft Interagency Guidance Framework for Considering the Exercise of March-In Rights which for the first time includes the price of a product as one factor an agency can use when deciding to exercise march-in rights. While march-in rights have not previously been exercised, it is uncertain if that will continue under the new framework. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability, or commercialize our drugs.
It is possible that additional governmental action is taken in response to the ongoing COVID-19 pandemic.
We may not be able to obtain or maintain orphan drug designations or exclusivity for our drug candidates, which could limit the potential profitability of our drug candidates.
Regulatory authorities in some jurisdictions, including the United States, may designate drugs for relatively small patient populations as orphan drugs. Under the Orphan Drug Act of 1983, the FDA may designate a drug as an orphan drug if it is a drug intended to treat a rare disease or condition, which is generally defined as a patient population of fewer than 200,000 individuals in the United States. Generally, if a drug with an orphan drug designation subsequently receives the first marketing approval for an indication for which it receives the designation, then the drug is entitled to a period of marketing exclusivity that precludes the
applicable regulatory authority from approving another marketing application for the same drug for the same indication for the exclusivity period except in limited situations. For purposes of small molecule drugs, the FDA defines “same drug” as a drug that contains the same active moiety and is intended for the same use as the drug in question. A designated orphan drug may not receive orphan drug exclusivity if it is approved for a use that is broader than the indication for which it received orphan designation.
For OV101, the FDA granted orphan drug designation for OV101 for the treatment of Angelman syndrome and for the treatment of Fragile X syndrome in September 2016 and October 2017, respectively. The EMA granted orphan designation for OV101 for the treatment of Angelman syndrome in June 2019. The FDA granted orphan drug designation for OV935 for the treatment of Dravet syndrome and Lennox-Gastaut syndrome both in December 2017. We intend to pursue orphan drug designation for OV101 in additional indications, pending our further analysis of the NEPTUNE trial data and analysis of data from ELARA, for OV935 in additional indications, pending closing of the Takeda License and Termination Agreement, and for potential other future drug candidates.
Obtaining orphan drug designations is important to our business strategy; however, obtaining an orphan drug designation can be difficult and we may not be successful in doing so. Even if we were to obtain orphan drug designation for a drug candidate, we may not obtain orphan exclusivity and that exclusivity may not effectively protect the drug from the competition of different drugs for the same condition, which could be approved during the exclusivity period. Additionally, after an orphan drug is approved, the FDA could subsequently approve another application for the same drug for the same indication if the FDA concludes that the later drug is shown to be safer, more effective or makes a major contribution to patient care. Orphan drug exclusive marketing rights in the United States also may be lost if the FDA later determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantity of the drug to meet the needs of patients with the rare disease or condition. The failure to obtain an orphan drug designation for any drug candidates we may develop, the inability to maintain that designation for the duration of the applicable period, or the inability to obtain or maintain orphan drug exclusivity could reduce our ability to make sufficient sales of the applicable drug candidate to balance our expenses incurred to develop it, which would have a negative impact on our operational results and financial condition.
We have received Fast Track designations for OV101 for the treatment of Angelman syndrome and Fragile X syndrome, but such designations may not actually lead to a faster development or regulatory review or approval process.
The FDA granted Fast Track designations to OV101 for the treatment of Angelman syndrome and Fragile X syndrome in December 2017 and March 2018, respectively. If a drug is intended for the treatment of a serious condition and nonclinical or clinical data demonstrate the potential to address unmet medical need for such condition, a sponsor may apply for FDA Fast Track designation. Even though we received Fast Track designations for OV101, such Fast Track designations do not ensure that we will receive marketing approval or that approval will be granted within any particular timeframe for any of these fast track-designated indications. We may not experience a faster development or regulatory review or approval process with Fast Track designation compared to conventional FDA procedures. In addition, the FDA may withdraw Fast Track designation if it believes that the designation is no longer supported by data from our clinical development program. Fast Track designation alone does not guarantee qualification for the FDA’s priority review procedures.
Although the FDA has granted Rare Pediatric Disease Designation for OV101 for the treatment of Angelman Syndrome, an NDA for OV101, if approved, may not meet the eligibility criteria for a priority review voucher.
Rare Pediatric Disease Designation has been granted for OV101 for the treatment of Angelman Syndrome. In 2012, Congress authorized the FDA to award priority review vouchers to sponsors of certain rare pediatric disease product applications. This provision is designed to encourage development of new drug and biological products for prevention and treatment of certain rare pediatric diseases. Specifically, under this program, a sponsor who receives an approval for a drug or biologic for a “rare pediatric disease” may qualify for a voucher that can be redeemed to receive a priority review of a subsequent marketing application for a different product. The sponsor of a rare pediatric disease drug product receiving a priority review voucher may transfer (including by sale) the voucher to another sponsor. The voucher may be further transferred any number of times before the voucher is used, as long as the sponsor making the transfer has not yet submitted the application. The FDA may also revoke any priority review voucher if the rare pediatric disease drug for which the voucher was awarded is not marketed in the U.S. within one year following the date of approval. For the purposes of this program, a “rare pediatric disease” is a (a) serious or life-threatening disease in which the serious or life-threatening manifestations primarily affect individuals aged from birth to 18 years, including age groups often called neonates, infants, children, and adolescents; and (b) rare disease or conditions within the meaning of the Orphan Drug Act. Congress has only authorized the Rare Pediatric Disease Priority Review Voucher program until September 30, 2024. However, if a drug candidate receives Rare Pediatric Disease Designation before September 30, 2024, it is eligible to receive a voucher if it is approved before September 30, 2026.
However, OV101 for the treatment of Angelman Syndrome may not be approved by that date, or at all, and, therefore, we may not be in a position to obtain a priority review voucher prior to expiration of the program, unless Congress further reauthorizes the program. Additionally, designation of a drug for a rare pediatric disease does not guarantee that an NDA will meet the eligibility criteria for a rare pediatric disease priority review voucher at the time the application is approved. Finally, a Rare Pediatric Disease Designation does not lead to faster development or regulatory review of the product, or increase the likelihood that it will receive marketing approval. We may or may not realize any benefit from receiving designation or a voucher.
Even if we obtain regulatory approval for our current or future drug candidates, they will remain subject to ongoing regulatory oversight.
Even if we obtain any regulatory approval for our current or future drug candidates, such approvals will be subject to ongoing regulatory requirements for manufacturing, labeling, packaging, storage, advertising, promotion, sampling, record-keeping and submission of safety and other post-market information. Any regulatory approvals that we receive for our current or future drug candidates may also be subject to a REMS, limitations on the approved indicated uses for which the drug may be marketed or to the conditions of approval, or contain requirements for potentially costly post-marketing testing, including Phase 4 trials, and surveillance to monitor the quality, safety and efficacy of the drug.
In addition, drug manufacturers and their facilities are subject to payment of user fees and continual review and periodic inspections by the FDA and other regulatory authorities for compliance with cGMP requirements and adherence to commitments made in the NDA,
BLA or foreign marketing application. If we, or a regulatory authority, discover previously unknown problems with a drug, such as adverse events of unanticipated severity or frequency, or problems with the facility where the drug is manufactured or if a regulatory authority disagrees with the promotion, marketing or labeling of that drug, a regulatory authority may impose restrictions relative to that drug, the manufacturing facility or us, including requesting a recall or requiring withdrawal of the drug from the market or suspension of manufacturing.
If we fail to comply with applicable regulatory requirements following approval of our current or future drug candidates, a regulatory authority may:
•issue an untitled letter or warning letter asserting that we are in violation of the law;
•seek an injunction or impose administrative, civil or criminal penalties or monetary fines;
•suspend or withdraw regulatory approval;
•suspend any ongoing clinical trials;
•refuse to approve a pending NDA or comparable foreign marketing application (or any supplements thereto) submitted by us or our strategic partners;
•restrict the marketing or manufacturing of the drug;
•seize or detain the drug or otherwise require the withdrawal of the drug from the market;
•refuse to permit the import or export of drug candidates; or
•refuse to allow us to enter into supply contracts, including government contracts.
Moreover, the FDA strictly regulates the promotional claims that may be made about drug products. In particular, a product may not be promoted for uses that are not approved by the FDA as reflected in the product’s approved labeling. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant civil, criminal and administrative penalties.
Any government investigation of alleged violations of law could require us to expend significant time and resources in response and could generate negative publicity. The occurrence of any event or penalty described above may inhibit our ability to commercialize our current or future drug candidates and harm our business, financial condition, results of operations and prospects.
In addition, the FDA’s policies, and those of equivalent foreign regulatory agencies, may change and additional government regulations may be enacted that could cause changes to or delays in the drug review process, or suspend or restrict regulatory approval of our drug candidates. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained and we may not achieve or sustain profitability, which would harm our business, financial condition, results of operations and prospects.
Risks Related to Our Intellectual Property
If we are unable to obtain and maintain patent protection for our current or any future drug candidates, or if the scope of the patent protection obtained is not sufficiently broad, we may not be able to compete effectively in our markets.
We rely upon a combination of patents, trade secret protection and confidentiality agreements to protect the intellectual property related to our development programs and drug candidates. Our success depends in large part on our ability to obtain and maintain patent protection in the United States and other countries with respect to our current and any future drug candidates. We seek to protect our proprietary position by filing patent applications in the United States and abroad related to our current and future development programs and drug candidates. The patent prosecution process is expensive and time-consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner.
Pursuant to the Lundbeck Agreement, as amended, we obtained an exclusive, worldwide license to develop, manufacture and commercialize OV101 for the treatment of human disease. However, the Lundbeck Agreement, as amended, permits Lundbeck and certain other entities to manufacture and research OV101 and, in certain situations, to perform additional non-commercial activities involving OV101, all of which could result in new patentable inventions concerning the manufacture or use of OV101. While the Lundbeck Agreement, as amended, prohibits Lundbeck from filing certain patent applications regarding OV101 and obligates Lundbeck to include certain newly filed patents in the license granted to us, if new patents issue that cover valuable methods for making or using OV101, we would be prohibited from employing such methods to manufacture or use OV101 unless we obtain a license to such patents.
It is also possible that we will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection. The patent applications that we own or in-license may fail to result in issued patents with claims that cover our current or any future drug candidates in the United States or in other foreign countries. There is no assurance that all of the potentially relevant prior art relating to our patents and patent applications has been found, which can invalidate a patent or prevent a patent from issuing from a pending patent application. Even if patents do successfully issue and even if such patents cover our current or any future drug candidates, third parties may challenge their validity, enforceability or scope, which may result in such patents being narrowed, invalidated, or held unenforceable. Any successful opposition to these patents or any other patents owned by or licensed to us could deprive us of rights necessary for the successful commercialization of any drug candidates or companion diagnostic that we may develop. Further, if we encounter delays in regulatory approvals, the period of time during which we could market a drug candidate and companion diagnostic under patent protection could be reduced.
If the patent applications we hold or have in-licensed with respect to our development programs and drug candidates fail to issue, if their breadth or strength of protection is threatened, or if they fail to provide meaningful exclusivity for our current or any future drug candidates, it could dissuade companies from collaborating with us to develop drug candidates, and threaten our ability to commercialize, future drugs. Any such outcome could have a negative effect on our business.
The patent position of biotechnology and pharmaceutical companies generally is highly uncertain, involves complex legal and factual questions and has in recent years been the subject of much litigation. In addition, the laws of foreign countries may not protect our rights to the same extent as the laws of the United States. For example, European patent law restricts the patentability of methods of treatment of the human body more than United States law does. Publications of discoveries in scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. Therefore, we cannot know with certainty whether we were the first to make the inventions claimed in our owned or licensed patents or pending patent applications, or that we were the first to file for patent protection of such inventions. As a result, the issuance, scope, validity, enforceability and commercial value of our patent rights are highly uncertain. Our pending and future patent applications may not result in patents being issued which protect our technology or drugs, in whole or in part, or which effectively prevent others from commercializing competitive technologies and drugs. Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of our patents or narrow the scope of our patent protection.
Recent patent reform legislation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents. On December 16, 2011, the Leahy-Smith America Invents Act (the “Leahy-Smith(“Leahy-Smith Act”) was signed into law. The Leahy-Smith Act includes a number of significant changes to United States patent law. These include provisions that affect the way patent applications are prosecuted and may also affect patent litigation. The United States Patent Office recently developed new regulations and procedures to govern
administration of the Leahy-Smith Act, and many of the substantive changes to patent law associated with the Leahy-Smith Act, and in particular, the first to file provisions, only became effective on March 16, 2013. Accordingly, it is not clear what, if any, impact the Leahy-Smith Act will have on the operation of our business. However, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could harm our business and financial condition.
Moreover, we may be subject to a third-party pre-issuance submission of prior art to the
U.S. Patent and Trademark Office (the “USPTO”)USPTO or become involved in opposition, derivation, reexamination, inter partes review, post-grant review or interference
proceedings challenging our patent rights or the patent rights of others. An adverse determination in any such submission, proceeding or litigation could reduce the scope of, or invalidate, our patent rights, allow third parties to commercialize our technology or drugs and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize drugs without infringing third-party patent rights. In addition, if the breadth or strength of protection provided by our patents and patent applications is threatened, it could dissuade companies from collaborating with us to license, develop or commercialize current or future drug candidates.
The issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, and our owned and licensed patents may be challenged in the courts or patent offices in the United States and abroad. An adverse determination in any such challenges may result in loss of exclusivity or in patent claims being narrowed, invalidated or held unenforceable, in whole or in part, which could limit our ability to stop others from using or commercializing similar or identical technology and drugs, or limit the duration of the patent protection of our technology and drugs. Moreover, patents have a limited lifespan. In the United States, the natural expiration of a patent is generally 20 years from the earliest filing date of a non-provisional patent application. Various extensions may be available; however, the life of a patent, and the protection it affords, is limited. Without patent protection for our current or future drug candidates, we may be open to competition from generic versions of such drugs. Given the amount of time required for the development, testing and regulatory review of new drug candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing drugs similar or identical to ours.
We may be unable to prevent third parties from selling, making, promoting, manufacturing, or distributing alternative polymorphic forms of OV101.
We currently have issued patents directed to polymorphic forms of OV101. These patents would not prevent a third-party from creating, making and marketing alternative polymorphic forms that fall outside the scope of these patent claims. There can be no assurance that any such alternative polymorphic forms will not be therapeutically equivalent and/or commercially feasible. In the event an alternative polymorphic form of OV101 is developed and approved for use in indications that we may seek approval for, the marketability and commercial success of OV101, if approved, could be materially harmed.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by government patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees and various other government fees on patents and/or applications will be due to be paid to the USPTO and various government patent agencies outside of the United States over the lifetime of our owned and licensed patents and/or applications and any patent rights we may own or license in the future. We rely on our outside counsel or our licensing partners to pay these fees due to non-U.S. patent agencies. The USPTO and various non-U.S. government patent agencies require compliance with several procedural, documentary, fee payment and other similar provisions during the patent application process. We employ reputable law firms and other professionals to help us comply and we are also dependent on our licensors to take the necessary action to comply with these requirements with respect to our licensed intellectual property. In many cases, an inadvertent lapse can be cured by payment of a late fee or by other means in accordance with the applicable rules. There are situations, however, in which non-compliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, potential competitors might be able to enter the market and this circumstance could harm our business.
Patent terms may be inadequate to protect our competitive position on our drug candidates for an adequate amount of time.
Given the amount of time required for the development, testing and regulatory review of new drug candidates,
such as OV101, patents protecting such candidates might expire before or shortly after such candidates are commercialized. We expect to seek extensions of patent terms in the United States and, if available, in other countries where we are prosecuting patents. In the United States, the Drug Price Competition and Patent Term Restoration Act of 1984 permits a patent term extension of up to five years beyond the normal expiration of the patent, which is limited to the approved indication (or any additional indications approved during the period of extension). However, the applicable authorities, including the FDA and the USPTO in the United States, and any equivalent regulatory authority in other countries, may not agree with our assessment of whether such extensions are available, and may refuse to grant extensions to our patents, or may grant more limited extensions than we request. If this occurs, our competitors may be able to take advantage of our investment in development and clinical trials by referencing our clinical and preclinical data and launch their drug earlier than might otherwise be the case.
Intellectual property rights do not necessarily address all potential threats to our business.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations, and may not adequately protect our business. The following examples are illustrative:
•others may be able to make compounds or formulations that are similar to our drug candidates but that are not covered by the claims of any patents, should they issue, that we own or control;
•we or any strategic partners might not have been the first to make the inventions covered by the issued patents or pending patent applications that we own or control;
•we might not have been the first to file patent applications covering certain of our inventions;
•others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our intellectual property rights;
•it is possible that our pending patent applications will not lead to issued patents;
•issued patents that we own or control may not provide us with any competitive advantages, or may be held invalid or unenforceable because of legal challenges;
•our competitors might conduct research and development activities in the United States and other countries that provide a safe harbor from patent infringement claims for certain research and development activities, as well as in countries where we do not have patent rights and then use the information learned from such activities to develop competitive drugs for sale in our major commercial markets;
•we may not develop additional proprietary technologies that are patentable; and
•the patents of others may have an adverse effect on our business.
The proprietary map of disease-relevant biological pathways underlying orphan disorders of the brain that we developed would not be appropriate for patent protection and, as a result, we rely on trade secrets to protect this aspect of our business.
Third parties may initiate legal proceedings alleging that we are infringing their intellectual property rights, the outcome of which would be uncertain and could have a negative impact on the success of our business.
Our commercial success depends, in part, upon our ability and the ability of our current or future collaborators to develop, manufacture, market and sell our current and any future drug candidates and use our proprietary technologies without infringing the proprietary rights and intellectual property of third parties. The biotechnology and pharmaceutical industries are characterized by extensive and complex litigation regarding patents and other intellectual property rights. We may in the future become party to, or be threatened with, adversarial proceedings or litigation regarding intellectual property rights with respect to our current and any future drug candidates and technology, including interference proceedings, post grant review and inter partes review before the USPTO. Third parties may assert infringement claims against us based on existing patents or patents that may be granted in the future, regardless of their merit. There is a risk that third parties may choose to engage in litigation with us to enforce or to otherwise assert their patent rights against us. Even if we believe such claims are without merit, a court of competent jurisdiction could hold that these third-party patents are valid, enforceable and infringed, which could have a negative impact on our ability to commercialize our current and any future drug candidates. In order to successfully challenge the validity of any such U.S. patent in federal court, we would need to overcome a presumption of validity. As this burden is a high one requiring us to present clear and convincing evidence as to the invalidity of any such U.S. patent claim, there is no assurance that a court of competent jurisdiction would invalidate the claims of any such U.S. patent. If we are found to infringe a third party’s valid and enforceable intellectual property rights, we could be required to obtain a license from such third party to continue developing, manufacturing and marketing our drug candidate(s) and technology. However, we may not be able to obtain any required license on commercially reasonable terms or at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors and other third parties access to the same technologies licensed to us, and it could require us to make substantial licensing and royalty payments. We could be forced, including by court order, to cease developing, manufacturing and commercializing the infringing technology or drug candidate. In addition, we could be found liable for monetary damages, including treble damages and attorneys’ fees, if we are found to have willfully infringed a patent or other intellectual property right. A finding of infringement could prevent us from manufacturing and commercializing our current or any future drug candidates or force us to cease some or all of our business operations, which could materially harm our business. Claims that we have misappropriated the confidential information or trade
secrets of third parties could have a similar negative impact on our business, financial condition, results of operations and prospects. See the section herein titled “Legal Proceedings” for additional information.
We may be subject to claims asserting that our employees, consultants or advisors have wrongfully used or disclosed alleged trade secrets of their current or former employers or claims asserting ownership of what we regard as our own intellectual property.
Certain of our employees, consultants or advisors are currently, or were previously, employed at universities or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we try to ensure that our employees, consultants and advisors do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that these individuals or we have used or disclosed intellectual property, including trade secrets or other proprietary
information, of any such individual’s current or former employer. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management.
In addition, while it is our policy to require our employees and contractors who may be involved in the conception or development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who, in fact, conceives or develops intellectual property that we regard as our own. The assignment of intellectual property rights may not be self-executing or the assignment agreements may be breached, and we may be forced to bring claims against third parties, or defend claims that they may bring against us, to determine the ownership of what we regard as our intellectual property.
We may be involved in lawsuits to protect or enforce our patents, the patents of our licensors or our other intellectual property rights, which could be expensive, time consuming and unsuccessful.
Competitors may infringe or otherwise violate our patents, the patents of our licensors or our other intellectual property rights. To counter infringement or unauthorized use, we may be required to file legal claims, which can be expensive and time-consuming. In addition, in an infringement proceeding, a court may decide that a patent of ours or our licensors is not valid or is unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation or defense proceedings could put one or more of our patents at risk of being invalidated or interpreted narrowly and could put our patent applications at risk of not issuing. The initiation of a claim against a third party may also cause the third party to bring counter claims against us such as claims asserting that our patents are invalid or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness, non-enablement or lack of statutory subject matter. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant material information from the USPTO, or made a materially misleading statement, during prosecution. Third parties may also raise similar validity claims before the USPTO in post-grant proceedings such as ex parte reexaminations, inter partes review, or post-grant review, or oppositions or similar proceedings outside the United States, in parallel with litigation or even outside the context of litigation. The outcome following legal assertions of invalidity and unenforceability is unpredictable. We cannot be certain that there is no invalidating prior art, of which we and the patent examiner were unaware during prosecution. For the patents and patent applications that we have licensed, we may have limited or no right to participate in the defense of any licensed patents against challenge by a third party. If a defendant were to prevail on a legal assertion of invalidity or unenforceability, we would lose at least part, and perhaps all, of any future patent protection on our current or future drug candidates. Such a loss of patent protection could harm our business.
We may not be able to prevent, alone or with our licensors, misappropriation of our intellectual property rights, particularly in countries where the laws may not protect those rights as fully as in the United States. Our business could be harmed if in litigation the prevailing party does not offer us a license on commercially reasonable terms. Any litigation or other proceedings to enforce our intellectual property rights may fail, and even if successful, may result in substantial costs and distract our management and other employees.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. There could also be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have an adverse effect on the price of our common stock.
Changes in U.S. patent law or the patent law of other countries or jurisdictions could diminish the value of patents in general, thereby impairing our ability to protect our current and any future drug candidates.
The United States has recently enacted and implemented wide-ranging patent reform legislation. The U.S. Supreme Court has ruled on several patent cases in recent years, either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on actions by the U.S. Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce patents that we have licensed or that we might obtain in the future. Similarly, changes in patent law and regulations in other countries or jurisdictions or changes in the governmental bodies that enforce them or changes in how the relevant governmental authority enforces patent laws or regulations may weaken our ability to obtain new patents or to enforce patents that we have licensed or that we may obtain in the future.
We may not be able to protect our intellectual property rights throughout the world, which could negatively impact our business.
Filing, prosecuting and defending patents covering our current and any future drug candidates throughout the world would be prohibitively expensive. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own drugs and, further, may export otherwise infringing drugs to territories where we may obtain patent protection, but where patent enforcement is not as strong as that in the United States. These drugs may compete with our drugs in jurisdictions where we do not have any issued or licensed patents and any future patent claims or other intellectual property rights may not be effective or sufficient to prevent them from so competing.
Reliance on third parties requires us to share our trade secrets, which increases the possibility that a competitor will discover them or that our trade secrets will be misappropriated or disclosed.
If we rely on third parties to manufacture or commercialize our current or any future drug candidates, or if we collaborate with additional third parties for the development of our current or any future drug candidates, we must, at times, share trade secrets with them. We may also conduct joint research and development programs that may require us to share trade secrets under the terms of our research and development partnerships or similar agreements. We seek to protect our proprietary technology in part by entering into confidentiality agreements and, if applicable, material transfer agreements, consulting agreements or other similar agreements with our advisors, employees, third-party contractors and consultants prior to beginning research or disclosing proprietary information. These agreements typically limit the rights of the third parties to use or disclose our confidential information, including our trade secrets. Despite the contractual provisions employed when working with third parties, the need to share trade secrets and other confidential information increases the risk that such trade secrets become known by our competitors, are inadvertently incorporated into the technology of others, or are disclosed or used in violation of these agreements. Given that our proprietary position is based, in part, on our know-how and trade secrets, a competitor’s discovery of our trade secrets or other unauthorized use or disclosure could have an adverse effect on our business and results of operations.
In addition, these agreements typically restrict the ability of our advisors, employees, third-party contractors and consultants to publish data potentially relating to our trade secrets. Despite our efforts to protect our trade secrets, our competitors may discover our trade secrets, either through breach of our agreements with third parties, independent development or publication of information by any third-party collaborators. A competitor’s discovery of our trade secrets would harm our business.
Risks Related to Our Dependence on Third Parties
We do not have our own manufacturing capabilities and will rely on third parties to produce clinical and commercial supplies of our current and any future drug candidates.
We do not own or operate, and we do not expect to own or operate, facilities for drug manufacturing,
drug formulation, storage and distribution or testing. We
have been in the past, and will
continue to be, dependent on third parties to manufacture the clinical supplies of our drug candidates.
The drug substance for OV101 was manufactured by Lundbeck. We believe that the drug substance transferred from Lundbeck under the Lundbeck Agreement will be sufficient for us to complete our ongoing and future clinical trials. Until the closing of the Takeda License and Termination Agreement, we will also continue to rely on Takeda to provide the drug product supply for our planned clinical trials of OV935.Further, we also will rely on third-party manufacturers to supply us with sufficient quantities of our drug candidates including OV101 and OV935, to be used, if approved, for commercialization. Any significant delay in the supply of a drug candidate, or the raw material components thereof, for an ongoing clinical trial due to the need to replace a third-party manufacturer could
considerably delay completion of our clinical trials, product testing and potential regulatory approval of our drug candidates.
Further, our reliance on third-party manufacturers entails risks to which we would not be subject if we manufactured drug candidates ourselves including:
•inability to meet our drug specifications and quality requirements consistently;
•delay or inability to procure or expand sufficient manufacturing capacity;
•issues related to scale-up of manufacturing;
•costs and validation of new equipment and facilities required for scale-up;
•failure to comply with cGMP and similar foreign standards;
•inability to negotiate manufacturing agreements with third parties under commercially reasonable terms, if at all;
•termination or nonrenewal of manufacturing agreements with third parties in a manner or at a time that is costly or damaging to us;
•reliance on single sources for drug components;
•lack of qualified backup suppliers for those components that are currently purchased from a sole or single source supplier;
•operations of our third-party manufacturers or suppliers could be disrupted by conditions unrelated to our business or operations, including the bankruptcy of the manufacturer or supplier; and
•carrier disruptions or increased costs that are beyond our control.
Any of these events could lead to clinical trial delays, failure to obtain regulatory approval or impact our ability to successfully commercialize our current or any future drug candidates once approved. Some of these events could be the basis for FDA action, including injunction, request for recall, seizure, or total or partial suspension of production.
We intend to rely on third parties to conduct, supervise and monitor our preclinical studies and clinical trials, and if those third parties perform in an unsatisfactory manner, it may harm our business.
We do not currently have the ability to independently conduct any
preclinical studies or clinical trials. We intend to rely on CROs and clinical trial sites to ensure the proper and timely conduct of our preclinical studies and clinical trials, and we expect to have limited influence over their actual performance. We intend to rely upon CROs to monitor and manage data for our clinical programs, as well as the execution of future
nonclinicalpreclinical studies. We expect to control only certain aspects of our CROs’ activities. Nevertheless, we will be responsible for ensuring that each of our preclinical studies or clinical trials are conducted in accordance with the applicable protocol, legal, regulatory and scientific standards and our reliance on the CROs does not relieve us of our regulatory responsibilities.
We and our CROs will be required to comply with good laboratory practices (“GLPs”) and
good clinical practices (“GCPs”),GCPs, which are regulations and guidelines enforced by the FDA and are also required by the Competent Authorities of the Member States of the European Economic Area and comparable foreign regulatory authorities in the form of International
Conference onCouncil for Harmonization guidelines for any of our drug candidates that are in preclinical and clinical development. The regulatory authorities enforce GCPs through periodic inspections of trial sponsors, principal investigators and clinical trial sites. Although we will rely on CROs to conduct GCP-compliant clinical trials, we remain responsible for ensuring that each of our GLP preclinical studies and clinical trials is conducted in accordance with its investigational plan and protocol and applicable laws and regulations, and our reliance on the CROs does not relieve us of our regulatory responsibilities. If we or our CROs fail to comply with GCPs, the clinical data generated in our clinical trials may be deemed unreliable and the FDA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. Accordingly, if our CROs fail to comply with these regulations or fail to recruit a sufficient number of subjects, we may be required to repeat clinical trials, which would delay the regulatory approval process.
While we will have agreements governing their activities, our CROs will not be our employees, and we will not control whether or not they devote sufficient time and resources to our future clinical and nonclinicalpreclinical programs. These CROs may also have relationships with other commercial entities, including our competitors, for whom they may also be conducting clinical trials, or other drug development activities which could harm our business. We face the risk of potential unauthorized disclosure or misappropriation of our intellectual property by CROs, which may reduce our trade secret
protection and allow our potential competitors to access and exploit our proprietary technology. If our CROs do not successfully carry out their contractual duties or obligations, fail to meet expected deadlines, or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements or for any other reasons, our clinical trials may be extended, delayed or terminated, and we may not be able to obtain regulatory approval for, or successfully commercialize any drug candidate that we develop. As a result, our financial results and the commercial prospects for any drug candidate that we develop would be harmed, our costs could increase, and our ability to generate revenue could be delayed.
If our relationship with these CROs terminates, we may not be able to enter into arrangements with alternative CROs or do so on commercially reasonable terms. Switching or adding additional CROs involves substantial cost and requires management time and
focus. In addition, there is a natural transition period when a new CRO commences work. As a result, delays occur, which can negatively impact our ability to meet our desired clinical development timelines. Though we intend to carefully manage our relationships with our CROs, there can be no assurance that we will not encounter challenges or delays in the future or that these delays or challenges will not have a negative impact on our business, financial condition and prospects.
In addition, principal investigators for our clinical trials may serve as scientific advisors or consultants to us from time to time and receive compensation in connection with such services. Under certain circumstances, we may be required to report some of these relationships to the FDA. The FDA may conclude that a financial relationship between us and a principal investigator has created a conflict of interest or otherwise affected interpretation of the trial. The FDA may therefore question the integrity of the data generated at the applicable clinical trial site and the utility of the clinical trial itself may be jeopardized. This could result in a delay in approval, or rejection, of our marketing applications by the FDA and may ultimately lead to the denial of marketing approval of our current and future drug candidates.
If our
contemplated royalty, licenserelationship with these CROs terminates, we may not be able to enter into arrangements with alternative CROs or do so on commercially reasonable terms. Switching or adding additional CROs involves substantial cost and
repurchase agreement with Takeda is not successfully closed or if we fail to realize the benefits we anticipate from such strategic alliance, it may harm our business.In March 2021, we entered into the Takeda Licenserequires management time and Termination Agreement under which Takeda will secure global rights at closing from us to develop and commercialize the investigational medicine OV935 for the treatment of developmental and epileptic encephalopathies, including DS and LGS.
Under the Takeda collaboration, Takeda received equity in us and was eligible to receive up to $85.0 million in payments for regulatory milestones, including the initiation of Phase 3 clinical trials. Since signing the agreement, we led global development of OV935.
Should we close the Takeda License and Termination Agreement, all rights in OV935 will be owned by Takeda, or exclusively licensed to Takeda by us. Takeda will assume sole responsibility for further worldwide development and commercialization, and we will no longer have any financial obligation to Takeda under the original collaboration agreement, including for milestone payments or any future development and commercialization costs. Under the Takeda License and Termination Agreement we will be eligible to receive an upfront payment of $196.0 million at closing and eligible to receive up to an additional $660.0 million upon Takeda achieving development, regulatory and sales milestones.focus. In addition, there is a natural transition period when a new CRO commences work. As a result, delays occur, which can negatively impact our ability to meet our desired clinical development timelines. Though we wouldintend to carefully manage our relationships with our CROs, there can be entitled to receive tiered royalties beginning in the low double-digits, and up to 20% on sales of OV935, if approved and commercialized.
We expect to close the Takeda License and Termination Agreement in the first half of 2021, subject to the satisfaction of customary closing conditions, including regulatory review by the appropriate regulatory agencies under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended (“HSR Act”). We cannot assure youno assurance that such closing conditions will be satisfied or that the post-closing filing will be approved by the appropriate regulatory agencies under the HSR Act. If the regulatory agencies do not approve our filing, such agencies may request us to rescind the transactions related to the Takeda License and Termination Agreement. If we fail to close the Takeda License and Termination Agreement, then we will not be entitled to any ofencounter challenges or delays in the payments described above, and the current terms of collaboration and related risksfuture or that these delays or challenges will remain in effect andnot have a negative impact on our business, and financial condition may be harmed.
and prospects.
Risks Related to Our Business Operations, Employee Matters and Managing Growth
COVID-19 could adversely impact our business, including our clinical trials and access to capital.
The ongoing COVID-19 pandemic has resulted in travel and other restrictions in order to reduce the spread of the disease, including state and local orders across the country, which, among other things, direct individuals to shelter at their places of residence, direct businesses and governmental agencies to cease non-essential operations at physical locations, prohibit certain non-essential gatherings, and order cessation of non-essential travel. In response to these public health directives and orders, we have implemented work-from-home policies for all employees. Our current plans to return to the office remain fluid as federal, state and local guidelines, rules and regulations continue to evolve. The effects of the executive orders, the shelter-in-place orders and our work-from-home policies may negatively impact productivity, disrupt our business and delay our clinical programs and timelines, the magnitude of which will depend, in part, on the length and severity of the restrictions and other limitations on our ability to conduct our business in the ordinary course. These and similar, and perhaps more severe, disruptions in our operations could negatively impact our business, operating results and financial condition.
Quarantines, shelter-in-place and similar government orders related to COVID-19 may adversely impact our business operations and the business operations of our contract research organizations conducting our clinical trials and our third-party manufacturing facilities in the United States and other countries. In particular, some of our third-party manufacturers which we use for the supply of materials for product candidates or other materials necessary to manufacture product to conduct preclinical studies and clinical trials are located in countries affected by COVID-19, and should they experience disruptions, such as temporary closures or
suspension of services, we would likely experience delays in advancing these tests and trials. Currently, we expect no material impact on the clinical supply of any of our product candidates.
In addition, our clinical trials may be affected by the COVID-19 pandemic.
Clinical site initiation and patient enrollment may be delayed due to prioritization of hospital resources toward the COVID-19 pandemic. Some patients may not be willing or able to comply with clinical trial protocols if quarantines impede patient movement or interrupt healthcare services. Similarly, our ability to recruit and retain patients and principal investigators and site staff who, as healthcare providers, may have heightened exposure to COVID-19 and adversely impact our clinical trial operations. As a result of the COVID-19 pandemic, we have faced and may continue to face delays in meeting our anticipated timelines for our ongoing and planned clinical trials.
The spread of COVID-19, which has caused a broad impact globally, may materially affect us economically. While the potential economic impact brought by, and the duration of, COVID-19 may be difficult to assess or predict, a widespread pandemic could result in significant disruption of global financial markets, reducing our ability to access capital, which could in the future negatively affect our liquidity. In addition, a recession or market correction resulting from the spread of COVID-19 could materially affect our business and the value of our common stock.
The global pandemic of COVID-19 continues to rapidly evolve. The extent to which the COVID-19 pandemic impacts our business, our clinical development and regulatory efforts will depend on future developments that are highly uncertain and cannot be predicted with confidence, such as the duration of the outbreak, travel restrictions, quarantines, social distancing requirements and business closures in the United States and other countries, and business disruptions, and the effectiveness of actions taken in the United States and other countries to contain and treat the disease. Accordingly, we do not yet know the full extent of potential delays or impacts on our business, our clinical and regulatory activities, healthcare systems or the global economy as a whole. However, these impacts could adversely affect our business, financial condition, results of operations and growth prospects.
In addition, to the extent the ongoing COVID-19 pandemic adversely affects our business and results of operations, it may also have the effect of heightening many of the other risks and uncertainties described in this ‘‘Risk Factors’’ section.
We are highly dependent on the services of our senior management team, including our Chairman and Chief Executive Officer, Dr. Jeremy Levin, and if we are not able to retain these members of our management team or recruit and retain additional management, clinical and scientific personnel, our business will be harmed.
We are highly dependent on our senior management team, including our Chairman and Chief Executive Officer, Dr. Levin. The employment agreements we have with these officers do not prevent such persons from terminating their employment with us at any time. The loss of the services of any of these persons could impede the achievement of our research, development,
operational, financial and commercialization objectives.
In addition, we are dependent on our continued ability to attract, retain and motivate highly qualified additional management, clinical and scientific personnel. If we are not able to retain our management and to attract, on acceptable terms, additional qualified personnel necessary for the continued development of our business, we may not be able to sustain our operations or grow. This risk may be further amplified given the particularly competitive hiring market in New York City, the location of our corporate headquarters.
We may not be able to attract or retain qualified personnel in the future due to the intense competition for qualified personnel among biotechnology, pharmaceutical and other businesses. Many of the other pharmaceutical companies that we compete against for qualified personnel and consultants have greater financial and other resources, different risk profiles and a longer history in the industry than we do. They also may provide more diverse opportunities and better chances for career advancement. Some of these characteristics may be more appealing to high-quality candidates and consultants than what we have to offer. If we are unable to continue to attract, retain and motivate high-quality personnel and consultants to accomplish our business objectives, the rate and success at which we can discover and develop drug candidates and our business will be limited and we may experience constraints on our development objectives.
Our future performance will also depend, in part, on our ability to successfully integrate newly hired executive officers into our management team and our ability to develop an effective working relationship among senior management. Our failure to integrate these individuals and create effective working relationships among them and other members of management could result in inefficiencies in the development and commercialization of our drug candidates, harming future regulatory approvals, sales of our drug candidates and our results of operations. Additionally, we do not currently maintain “key person” life insurance on the lives of our executives or any of our employees.
We may need to expand our organization, and we may experience difficulties in managing this growth, which could disrupt our operations.
As of December 31,
2020,2023, we had
6740 full-time employees. As our development and commercialization plans and strategies
for our current pipeline of product candidates develop, we expect to need additional managerial, operational, sales, marketing, financial, legal and other resources. Our management may need to divert a disproportionate amount of its attention away from our day-to-day operations and devote a substantial amount of time to managing these growth activities. We may not be able to effectively manage the expansion of our operations, which may result in weaknesses in our infrastructure, operational inefficiencies, loss of business opportunities, loss of employees and reduced productivity among remaining employees. Our expected growth could require significant capital expenditures and may divert financial resources from other projects, such as the development of our current and potential future drug candidates. If our management is unable to effectively manage our growth, our expenses may increase more than expected, our ability to generate and grow revenue could be reduced and we may not be able to implement our business strategy. Our future financial performance, our ability to commercialize drug candidates, develop a scalable infrastructure and compete effectively will depend, in part, on our ability to effectively manage any future growth.
Our employees, principal investigators, consultants and commercial partners may engage in misconduct or other improper activities, including non-compliance with regulatory standards and requirements and insider trading.
We are exposed to the risk that our employees, consultants, distributors, and collaborators may engage in fraudulent or illegal activity. Misconduct by these parties could include intentional, reckless or negligent conduct or disclosure of unauthorized activities to us that violates the regulations of the FDA and non-U.S. regulators, including those laws requiring the reporting of true, complete and accurate information to such regulators, manufacturing standards, healthcare fraud and abuse laws and regulations in the United States and abroad or laws that require the true, complete and accurate reporting of financial information or data. In particular, sales, marketing and business arrangements in the healthcare industry, including the sale of pharmaceuticals, are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. It is not always possible to identify and deter misconduct by our employees and other third parties, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. Further, because of
the work from homeour hybrid-work policies,
we implemented due to COVID-19, information that is normally protected, including company confidential information, may be less secure. If actions are instituted against us and we are not successful in defending ourselves or asserting our rights, those actions could result in the imposition of significant fines or other sanctions, including the imposition of civil, criminal and administrative penalties, damages, monetary fines, imprisonment, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, additional reporting obligations and oversight if we become subject to a corporate integrity agreement or other agreement to resolve allegations of non-compliance with these laws, contractual damages, reputational harm, diminished profits and future earnings and curtailment of operations, any of which could adversely affect our ability to operate our business and our results of operations. Whether or not we are successful in defending against such actions or investigations, we could incur substantial costs, including legal fees, and divert the attention of management in defending ourselves against any of these claims or investigations.
Significant disruptions of our information technology systems or data security incidents could result in significant financial, legal, regulatory, business and reputational harm to us.
We are increasingly dependent on information technology systems and infrastructure, including mobile technologies, to operate our business. In the ordinary course of our business, we collect, store, process and transmit large amounts of sensitive information, including intellectual property, proprietary business information, personal informationdata, and, other confidential information. It is criticalas a result, we and the third parties upon which we rely face a variety of evolving threats that we do so in a secure manner to maintain the confidentiality, integrity and availability of such sensitive information.could cause security incidents. We have also outsourced elements of our operations (including elements of our information technology infrastructure) to third parties, and as a result, we manage a number of third-party vendors who may or could have access to our computer networks or our confidential information.sensitive data. In addition, many of those third parties in turn subcontract or outsource some of their responsibilities to other third parties. While all information technology operations are inherently vulnerable to inadvertent or intentional security breaches, incidents, attacks and exposures, the accessibility and distributed nature of our information technology systems, and the sensitive informationdata stored on those systems, make such systems potentially vulnerable to unintentional or malicious, internal and external attacks on our technology environment. Furthermore, our ability to monitor the aforementioned third parties’ information security practices is limited, and these third parties may not have adequate information security measures in place. If our third-party service providers experience a security incident or other interruption, we could experience adverse consequences. While we may be entitled to damages
if our third-party service providers fail to satisfy their privacy or security-related obligations to us, any award may be insufficient to cover our damages, or we may be unable to recover such award. In addition, supply-chain attacks have increased in frequency and severity, and we cannot guarantee that third parties’ infrastructure in our supply chain or our third-party partners’ supply chains have not been compromised.
In addition, due to the COVID-19 pandemic,our hybrid-work environment, we have enabled allmay be more vulnerable to cyberattacks as more of our employees utilize network connections, computers, and devices outside our premises or network, including working at home, while in transit and in public locations. Additionally, future or past business transactions (such as acquisitions or integrations) could expose us to work remotely, whichadditional cybersecurity risks and vulnerabilities, as our systems could be negatively affected by vulnerabilities present in acquired or integrated entities’ systems and technologies. Furthermore, we may make us more vulnerablediscover security issues that were not found during due diligence of such acquired or integrated entities, and it may be difficult to cyberattacks. integrate companies into our information technology environment and security program.
Potential vulnerabilities can be exploited from inadvertent or intentional actions of our employees, third-party vendors, business partners, or by malicious third parties. AttacksWe take steps designed to detect, mitigate, and remediate vulnerabilities in our information systems (such as our hardware and/or software, including that of this naturethird parties upon which we rely); however we may not detect and remediate all such vulnerabilities on a timely basis. Further, we may experience delays in deploying remedial measures and patches designed to address identified vulnerabilities. Vulnerabilities could be exploited and result in a security incident.
Cyberattacks, malicious internet-based activity, online and offline fraud, and other similar activities are increasing in their frequency, levels of persistence, sophistication and intensity, and are
also being conducted by sophisticated and organized groups and individuals with a wide range of motives (including, but not limited to, industrial espionage) and expertise, including organized criminal groups, “hacktivists,” nation states and others.
In addition to the extraction of sensitive information, suchSuch attacks could include the deployment of harmful malware
(including as a result of advanced persistent threat intrusions), ransomware
attacks, denial-of-service attacks,
credential stuffing and/or harvesting, social engineering
(including through deep fakes, which may be increasingly more difficult to identify as fake, and phishing attacks), supply-chain attacks, software bugs, server malfunctions, software or hardware failures, loss of sensitive data or other information technology assets, adware, attacks enhanced or facilitated by artificial intelligence, telecommunications failures, earthquakes, fires, floods and other means to affect service reliability and threaten the
confidentiality, integrity and availability of information.our information systems and sensitive data. In addition,particular, severe ransomware attacks are becoming increasingly prevalent and can lead to significant interruptions in our operations, ability to provide our products or services, loss of sensitive data and income, reputational harm, and diversion of funds. Extortion payments may alleviate the prevalent usenegative impact of mobile devices increases the risk of data security incidents.
a ransomware attack, but we may be unwilling or unable to make such payments due to, for example, applicable laws or regulations prohibiting such payments.
Significant disruptions of our, our third-party vendors’ and/or business partners’ information technology systems or other similar data security incidents could adversely affect our business operations and/or result in the loss, misappropriation, and/or unauthorized access, use or disclosure of, or the prevention of access to, sensitive
information,data, which could result in financial, legal, regulatory, business and reputational harm to us. In addition, information technology system disruptions, whether from attacks on our technology environment or from computer viruses, natural disasters, terrorism, war and telecommunication and electrical failures, could result in a material disruption of our development programs and our business operations. For example, the loss of clinical trial data from completed or future clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data.
There is no way
We may expend significant resources or modify our business activities to try to protect against security incidents. Additionally, certain data privacy and security obligations may require us to implement and maintain specific security measures or industry-standard or reasonable security measures to protect our information technology systems and sensitive data.
Applicable data privacy and security obligations may require us to notify relevant stakeholders, including affected individuals, customers, regulators, and investors, of knowingsecurity incidents. Such disclosures are costly, and the disclosure or the failure to comply with certainty whethersuch requirements could lead to adverse consequences.
If we (or a third party upon whom we rely) experience a security incident or are perceived to have experienced any dataa security incidents that have not been discovered. While we have no reason to believe this to be the case, attackers have become very sophisticated in the way they conceal access to systems, and many companies that have been attacked are not aware that they have been attacked. Any event that leads to unauthorized access, use or disclosure of personal information,incident, including but not limited to a security incident involving personal information regarding our patients or employees, could disruptwe may experience adverse consequences, such as disruptions to our business, harm to our reputation, compel us to comply with applicable federalgovernment enforcement actions (for example, investigations, fines, penalties, audits, and inspections), additional reporting requirements, and/or state breach notification laws and foreign law equivalents,oversight, or we may otherwise be subject us to time consuming, distracting and expensive litigation, regulatory investigation and oversight, mandatory corrective action, require us to verify the correctness of database contents, or otherwise subject us to liability under laws, regulations and contractual obligations, including those that protect the privacy and security of personal information. This could result in increased
costs to us, and result in significant legal and financial exposure and/or reputational harm. In addition, any failure or perceived failure by us or our vendors or business partners to comply with our privacy, confidentiality or data security-related legal or other obligations to third parties, or any further security incidents or other inappropriate access events that result in the unauthorized access, release or transfer of sensitive information, which could include personally identifiable information,data, may result in governmental investigations, enforcement actions, regulatory fines, litigation, or public statements against us by advocacy groups or others, and could cause third parties, including clinical sites, regulators or current and potential partners, to lose trust in us or we could be subject to claims by third parties that we have breached our privacy- or confidentiality-related obligations, which could materially and adversely affect our business and prospects. Moreover, data security incidents and other inappropriate access can be difficult to detect, and any delay in identifying them may lead to increased harm of the type described above.
While we have implemented security measures intended to protect our information technology systems and infrastructure, there can be no assurance that such measures will
successfully prevent service interruptionsbe effective. Our contracts may not contain limitations of liability, and even where they do, there can be no assurance that limitations of liability in our contracts are sufficient to protect us from liabilities, damages, or
claims related to our data privacy and security
incidents.obligations.
We
may beare subject to
numerousstringent and
varyingevolving privacy and security laws,
regulations, contractual obligations, industry standards, policies, and other obligations, and our failure
or perceived failure to comply
with such obligations could result in
regulatory investigations or actions, litigation (including class actions), fines and penalties,
disruptions of our business operations, loss of revenue or profits, reputational damage and
reputational damage.We areother adverse business consequences.
In the ordinary course of business, we collect, receive, store, process, generate, use, transfer, disclose, make accessible, protect, secure, dispose of, transmit, and share (collectively, process) personal data and other sensitive information, including proprietary and confidential business data, trade secrets, intellectual property, sensitive third-party data, business plans, transactions, clinical trial data and financial information (collectively, sensitive data).
Our data processing activities subject
us to laws and regulations covering data privacy and the protection of personal information
including health information.and other sensitive data. The legislative and regulatory landscape for privacy and data protection continues to evolve, and there has been an increasing focus on privacy and data protection issues which may affect our business. In the
U.S.,United States, we may be subject to state security breach notification laws, state health information privacy laws and federal and state consumer protections laws which impose requirements for the collection, use, disclosure and transmission of personal information. Each of these laws is subject to varying interpretations by courts and government agencies, creating complex compliance issues for us. If we fail to comply with applicable laws and regulations, we could be subject to penalties or sanctions, including criminal penalties if we knowingly obtain individually identifiable health information from a covered entity in a manner that is not authorized or permitted by HIPAA or for aiding and abetting the violation of HIPAA.
Numerous other countries have, or are developing, laws governing the collection, use and transmission of personal information as well. EU member states and other jurisdictions have adopted data protection laws and regulations, which impose significant compliance obligations. For example, in May 2016, the EU formally adopted the General Data Protection Regulation
or GDPR,(“GDPR”), which applies to all EU member states as of May 25, 2018 and replaces the former EU Data Protection Directive. The regulation introduces new data protection requirements in the EU and imposes substantial fines for breaches of the data protection rules. The GDPR must be implemented into national laws by the EU member states imposes strict obligations and restrictions on the ability to collect, analyze, and transfer personal data, including health data from clinical trials and adverse event reporting. Data protection authorities from different EU member states have interpreted the privacy laws differently, which adds to the complexity of processing personal data in the EU, and guidance on implementation and compliance practices are often updated or otherwise revised. Any failure to comply with the rules arising from the GDPR and related national laws of EU member states could lead to government enforcement actions and
significant penalties against us,fines of up to 20 million Euros or 4% of annual global revenue, whichever is greater, and adversely impact our operating results. The GDPR will increase our responsibility and liability in relation to personal data that we process and we may be required to put in place additional mechanisms ensuring compliance with EU data protection rules.
Additionally, California enacted the California Consumer Privacy Act
of 2018, as amended by the California Privacy Rights Act of 2020 (the “CCPA”)
legislation thatwhich has been dubbed the first “GDPR-like” law in the United States.
In the past few years, numerous other U.S. states—including Virginia, Colorado, Connecticut, and Utah—have also enacted comprehensive privacy laws that impose certain obligations on covered businesses, including providing specific disclosures in privacy notices and affording residents with certain rights concerning their personal data. The CCPA gives California residents expanded rights to access, correct and delete their personal
information, opt out of certain personal information sharing and receive detailed information about how their personal information is used by requiring covered companies to provide new disclosures to California consumers (as that term is broadly defined) and provide such consumers new ways to opt-out of certain sales of personal information. The CCPA provides for civil penalties for
violations, as well as a private right of action for data breaches that is expected to increase data breach litigation. TheAlthough there are limited exemptions for clinical trial data under the CCPA (and the other similar state privacy laws), the CCPA and other similar laws may increaseimpact (possibly significantly) our compliance costsbusiness activities depending on how it is interpreted, should we become subject to the CCPA in the future.
In addition to data privacy and
potential liability.security laws, we may be bound by other contractual obligations related to data privacy and security, and our efforts to comply with such obligations may not be successful. We also publish privacy policies, marketing materials, and other statements regarding data privacy and security and if these policies, materials, or statements are found to be deficient, lacking in transparency, deceptive, unfair, or misrepresentative of our practices, we may be subject to investigation, enforcement actions by regulators, or other adverse consequences.
We may at times fail (or be perceived to have failed) in our efforts to comply with our data privacy and security obligations. Moreover, despite our efforts, our personnel or third parties on whom we rely may fail to comply with such obligations, which could negatively impact our business operations. If we or the third parties on which we rely fail, or are perceived to have failed, to address or comply with applicable data privacy and security obligations, we could face significant consequences, including but not limited to: government enforcement actions (e.g., investigations, fines, penalties, audits, inspections, and similar); litigation (including class-action claims) and mass arbitration demands; additional reporting requirements and/or oversight; bans on processing personal data (including clinical trial data); and orders to destroy or not use personal data.
Risks Related to Being a Public Company
We are
an “emerging growth company” and a “smaller reporting company” and the reduced disclosure requirements applicable to such companies may make our common stock less attractive to investors.
We are an emerging growth company (“EGC”),currently a “smaller reporting company” as defined in the Jumpstart Our Business StartupsSecurities Exchange Act of 20121934, as amended (the “JOBS“Exchange Act”). We will remain an EGC untilbe a smaller reporting company and may take advantage of the earlier of:scaled-back disclosures available to smaller reporting companies for so long as (i) the market value of our voting and non-voting ordinary shares held by non-affiliates is less than $250.0 million measured on the last business day of our second fiscal quarter or (ii) (a) our annual revenue is less than $100.0 million during the most recently completed fiscal year in which we have total annual gross revenuesand (b) the market value of $1.07 billion or more; (ii) December 31, 2022,our voting and non-voting ordinary shares held by non-affiliates is less than $700.0 million measured on the last business day of theour second fiscal year following the fifth anniversary of the date of the completion of our IPO; (iii) the date on which we have issued more than $1.0 billion in nonconvertible debt during the previous three years; or (iv) the date on whichquarter.
As a smaller reporting company, we are deemedpermitted to comply with scaled-back disclosure obligations in our SEC filings compared to other issuers, including with respect to disclosure obligations regarding executive compensation in our periodic reports and proxy statements. We have elected to adopt the accommodations available to smaller reporting companies. Until we cease to be a large accelerated filer undersmaller reporting company, the rulesscaled-back disclosure in our SEC filings will result in less information about our company being available than for other public companies. If investors consider our common shares less attractive as a result of our election to use the scaled-back disclosure permitted for smaller reporting companies, there may be a less active trading market for our common shares and our share price may be more volatile.
We may take advantage of certain of the Securitiesscaled-back disclosures available to smaller reporting companies, including but not limited to:
•reduced disclosure obligations regarding executive compensation arrangements; and Exchange Commission (“SEC”). For so long as we remain an EGC, we are permitted and intend to rely on exemptions from certain disclosure requirements that are applicable to other public companies that are not emerging growth companies. These exemptions include:not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002 (“Section 404”);
not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements;
• being permitted to provide only two years of audited financial statements, in addition to any required unaudited interim financial statements, with correspondingly reduced “Management’s Discussion and Analysis of Financial Condition and Results of Operations” disclosure;
reduced disclosure obligations regarding executive compensation arrangements; and
exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved.
We currently intend to take advantage of some, but not all, of the reduced regulatory and reporting requirements that will be available to us so long as we qualify as an EGC. For example, our independent registered public accounting firm will not be required to provide an attestation report on the effectiveness of our internal control over financial reporting so long as we qualify as an EGC, which may increase the risk that material weaknesses or significant deficiencies in our internal control over financial reporting go undetected. Likewise, so long as we qualify as an EGC, we may elect not to provide you with certain information, including certain financial information and certain information regarding compensation of our executive officers, that we would otherwise have been required to provide in filings we make with the SEC, which may make it more difficult for investors and securities analysts to evaluate our company. We cannot predict if investors will find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock, and our stock price may be more volatile and may decline.
In addition, the JOBS Act provides that an EGC may take advantage of an extended transition period for complying with new or revised accounting standards. This allows an EGC to delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, we will be subject to the same new or revised accounting standards as other public companies that are not an EGC.
We are also a smaller reporting company as defined in the Exchange Act. We may continue to be a smaller reporting company even after we are no longer an emerging growth company. We may take advantage of certain of the scaled disclosures available to smaller reporting companies and will be able to take advantage of these scaled disclosures for so long as (i) our voting and non-voting common stock held by nonaffiliates is less than $250.0 million measured on the last business day of our second fiscal quarter or (ii) our annual revenue is less than $100.0 million during the most recently completed fiscal year and our voting and non-voting common stock held by non-affiliates is less than $700.0 million measured on the last business day of our second fiscal quarter.
We will continue to incur increased costs as a result of operating as a public company, and our management will devote substantial time to new compliance initiatives.
As a public company, and particularly after we are no longer an EGC, we will incur significant legal, accounting and other expenses that we did not incur as a private company. In addition, the Sarbanes-Oxley Act of 2002 and rules subsequently implemented by the SEC and The Nasdaq Stock Market LLC have imposed various requirements on public companies, including establishment and maintenance of effective disclosure and financial controls and corporate governance practices. Our management and other personnel devote a substantial amount of time to these and other compliance initiatives. Moreover, these rules and regulations will continue to increase our legal and financial compliance costs and will make some activities more time-consuming and costly.
If we fail to maintain an effective system of internal control over financial reporting in the future, we may not be able to accurately report our financial condition, results of operations or cash flows, which may adversely affect investor confidence in us and, as a result, the value of our common stock.
The Sarbanes-Oxley Act requires, among other things, that we maintain effective internal controls for financial reporting and disclosure controls and procedures. We are required, under Section 404, to furnish a report by management on, among other things, the effectiveness of our internal control over financial reporting. This assessment will need to include disclosure of any material weaknesses identified by our management in our internal control over financial reporting. A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting that results in more than a reasonable possibility that a material misstatement of annual or interim financial statements will not be prevented or detected on a timely basis. Section 404 also generally requires an attestation from our independent registered public accounting firm on the effectiveness of our internal control over financial reporting. However,
As a public company and large accelerated filer for
as long asthe year ended December 31, 2022, we
remain an emerging growth company as defined in the JOBS Act, we intendwere required to
take advantageprovide management’s attestation on internal controls pursuant to Section 404 of the
exemption permitting us not to comply with theSarbanes-Oxley Act, and our independent registered public accounting firm
was required to attest to the effectiveness of our internal control over financial reporting pursuant to Section 404 of the Sarbanes-Oxley Act. However, as of the last business day of our second fiscal quarter of 2023, we determined that we requalify as a smaller reporting company and as a non-accelerated filer for the year ended December 31, 2023. We are therefore no longer required to include an attestation
requirement.report on internal control over financial reporting issued by our independent registered public accounting firm in this Annual Report on Form 10-K for the fiscal year ended December 31, 2023.
Our compliance with Section 404
willin future periods may require that we incur substantial expense and expend significant management efforts. We currently do not have an internal audit group, and
we willrely on experienced consultants to support this function. We may need to hire additional
consultants or accounting and financial staff with appropriate public company experience and technical accounting knowledge
and compile the system and process documentation necessaryin order to
perform the evaluation needed tocontinually comply with Section 404. We may not be able to complete our evaluation, testing and any required remediation in a timely fashion. During the evaluation and testing process, if we identify one or more material weaknesses in our internal control over financial reporting, we will be unable to assert that our internal control over financial reporting is effective. We cannot assure you that there will not be material weaknesses or significant deficiencies in our internal control over financial reporting in the future. Any failure to maintain internal control over financial reporting could severely inhibit our ability to accurately report our financial condition, results of operations or cash flows. If we are unable to conclude that our internal control over financial reporting is effective, or if our independent registered public accounting firm determines we have a material weakness
or significant deficiency in our internal control over financial reporting,
once that firm begins its Section 404 reviews, we could lose investor confidence in the accuracy and completeness of our financial reports, the market price of our common stock could decline, and we could be subject to sanctions or investigations by The Nasdaq Stock Market LLC, the SEC or other regulatory authorities. Failure to remedy any material weakness in our internal control over financial reporting, or to implement or maintain other effective control systems required of public companies, could also restrict our future access to the capital markets.
Risks Related to the Ownership of Our Common Stock and Other General Matters
The market price of our common stock may be volatile and fluctuate substantially, which could result in substantial losses for our common stock. The market price of our common stock
ishas been and likely
to bewill remain volatile. The stock market in general and the market for biopharmaceutical or pharmaceutical companies in particular, has experienced extreme volatility that has often been unrelated to the operating performance of particular companies,
including very recently in connection with the ongoing COVID-19 pandemic, which has resulted in decreased stock prices for many companies notwithstanding the lack of a fundamental change in their underlying business models or prospects. Broad market and industry factors, including potentially worsening economic conditions and other adverse effects or developments relating to
thenew or ongoing
COVID-19 pandemic,public health crises or other inflationary factors, may negatively affect the market price of our common stock, regardless of our actual operating performance. As a result of this volatility, you may lose all or part of your investment in our common stock since you might be unable to sell your shares at or above the price you paid for the shares. The market price for our common stock may be influenced by many factors, including:
•results of clinical trials of our current and any future drug candidates or those of our competitors;
•the success of competitive drugs or therapies;
•regulatory or legal developments in the United States and other countries;
•developments or disputes concerning patent applications, issued patents or other proprietary rights;
•the recruitment or departure of key personnel;
•the level of expenses related to our current and any future drug candidates or clinical development programs;
•the results of our efforts to discover, develop, acquire or in-license additional drug candidates;
•actual or anticipated changes in estimates as to financial results, development timelines or recommendations by securities analysts;
•our inability to obtain or delays in obtaining adequate drug supply for any approved drug or inability to do so at acceptable prices;
•disputes or other developments relating to proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our technologies;
•significant lawsuits, including patent or stockholder litigation;
•variations in our financial results or those of companies that are perceived to be similar to us;
•changes in the structure of healthcare payment systems;
•market conditions in the pharmaceutical and biotechnology sectors;
•general economic, industry and market conditions; and
•the other factors described in this “Risk Factors” section.
In addition, in the past, stockholders have initiated class action lawsuits against companies following periods of volatility in the market prices of these companies’ stock. Such litigation, if instituted against us, could cause us to incur substantial costs and divert management’s attention and resources.
Unstable market and economic conditions may have serious adverse consequences on our business, financial condition and share price.
The global economy, including credit and financial markets, has experienced extreme volatility and disruptions, including severely diminished liquidity and credit availability, declines in consumer confidence, declines in economic growth, increases in unemployment rates, increases in inflation rates and uncertainty about economic stability. Global geopolitical tensions have created extreme volatility in the global capital markets and are expected to have further global economic consequences, including disruptions of the global supply chain and energy markets. Any such volatility and disruptions may have adverse consequences on us or the third parties on whom we rely. If the equity and credit markets deteriorate, including as a result of political unrest or war, it may make any necessary debt or equity financing more difficult to obtain in a timely manner or on favorable terms, more costly or more dilutive.
There is no public market for our Series A convertible preferred stock. There is no established public trading market for our Series A convertible preferred stock, and we do not expect a market to develop. In addition, we do not intend to apply for listing of the Series A convertible preferred stock on any national securities exchange or other nationally recognized trading system. Without an active market, the liquidity of the Series A convertible preferred stock will be limited.
We may sell additional equity or debt securities or enter into other arrangements to fund our operations, which may result in dilution to our stockholders and impose restrictions or limitations on our business.
Until such time as we can generate a sufficient amount ofsubstantial revenue from our products,drug sales, if ever, we expect to finance futureour cash needs through publica combination of equity and debt financings, strategic alliances, and license and development agreements in connection with any collaborations. We do not have any committed external source of funds. To the extent that we issue additional equity securities, our stockholders may experience substantial dilution, and the terms of these securities may include liquidation or privateother preferences that adversely affect your rights as a stockholder. In addition, we may issue equity or debt offerings. securities as consideration for obtaining rights to additional compounds.
In November 2020, we filed a shelf registration statement on Form S-3 (Registration No. 333-250054) (the “Prior Registration Statement”). In November 2023, upon expiration of the of the Prior Registration Statement, we filed a new shelf registration statement on Form S-3 (Registration No. 333-275307) that allows us to sell up to an aggregate of $250.0 million of our common stock, preferred stock, debt securities and/or warrants (the “S-3“Current S-3 Registration Statement”), which includes a prospectus covering the issuance and sale of up to $75.0 million of common stock pursuant to an at-the-market (“ATM”) offering program. As of December 31, 2020,2023, we had $250.0 million available under our Current S-3 Registration Statement, including $75.0 million available pursuant to our ATM program. Financing activitiesDebt and equity financings, if available, may have an adverse impact oninvolve agreements that include covenants limiting or restricting our stockholders’ rights as well as on our operations, and such additional funding may not be available on reasonable terms, if at all. If we raise additional funds through the issuance of additional debt or equity securities, it may result in dilutionability to our existing stockholders and/or increased fixed payment obligations. Furthermore, these securities may have rights senior to those of our common stock and could contain covenants that would restrict our operations and potentially impair our competitiveness,take specific actions, such as redeeming our shares, making investments, issuing additional equity, limitations on our ability to incurincurring additional debt, making capital expenditures, declaring dividends or placing limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could adverselynegatively impact our ability to conduct our business. Additionally, ifIf we seek fundsraise additional capital through future collaborations, strategic alliances or third-party licensing arrangements, with collaborative partners, these arrangementswe may require ushave to relinquish valuable rights to some of our technologiesintellectual property, future revenue streams, research programs or productdrug candidates, or otherwise agree togrant licenses on terms unfavorablethat may not be favorable to us. Any of these events could significantly harm our business, financial condition and prospects.
You will be diluted by any conversions of outstanding Series A convertible preferred stock and exercises of outstanding options.
As of December 31, 2020,2023, we had outstanding options to purchase an aggregate of 10,403,42015,124,546 shares of our common stock at a weighted average exercise price of $5.26$3.87 per share and 3,250,0001,250,000 shares of common stock issuable upon conversion of outstanding Series A convertible preferred stock for no additional consideration. Such Series A convertible preferred stock is convertible any time at the option of the holder thereof subject to the beneficial ownership limitations described in Note 67 to the consolidated financial statements contained in this Annual Report on Form 10-K. The exercise of such options and conversion of the Series A convertible preferred stock for shares of our common stock will result in further dilution of your investment and could negatively affect the market price of our common stock. In addition, you may experience further dilution if we issue common stock, or securities convertible into common stock, in the future. As a result of this dilution, you may receive significantly less than the full purchase price you paid for the shares in the event of liquidation.
Concentration of ownership of our common stock among our executive officers, directors and principal stockholders may prevent new investors from influencing significant corporate decisions.
Based upon
our shares of our common stock outstanding as of
March 8, 2021,December 31, 2023, our executive officers, directors and stockholders who owned more than 5% of our outstanding common stock, in the aggregate, beneficially own shares representing approximately
35.54%52.4% of our outstanding common stock.
Takeda, a greater than 5% holder,
may receive additional securities upon the achievement of certain development, commercial and regulatory milestones pursuant to the Takeda collaboration. Specifically, we will be obligated to issue additional securities to Takeda equal to the lesser of 8% of our outstanding capital stock or $50.0 million unless certain events occur, and may issue, at our discretion, additional securities to Takeda upon the achievement of other milestones. Further, pursuant to the Series B-1 preferred stock purchase agreement entered into with Takeda in January 2017, or the Takeda stock purchase agreement, Takeda has agreed to, among other things, (i) a standstill provision, (ii) restrictions on its ability to sell or otherwise transfer
itits shares of our stock, (iii) vote its shares on certain matters in accordance with the holders of a majority of shares of our common stock and (iv) restrictions on the percentage of our outstanding common stock it may
own.own, in accordance with the terms of the RLT Agreement.
If our executive officers, directors and stockholders who owned more than 5% of our outstanding common stock acted together, they may be able to significantly influence all matters requiring stockholder approval, including the election and removal of directors and approval of any merger, consolidation or sale of all or substantially all of our assets. The concentration of voting power, Takeda standstill provisions, voting obligations and transfer restrictions could delay or prevent an acquisition of our company on terms that other stockholders may desire or result in the management of our company in ways with which other stockholders
disagree with.disagree.
If securities analysts do not publish research or reports about our business or if they publish negative evaluations of our stock, the price of our stock could decline.
The trading market for our common stock relies, in part, on the research and reports that industry or financial analysts publish about us or our business. We do currently have research coverage offered by several industry or financial
analysts, although two analysts have withdrawn research coverage recently. We do not have any control over these analysts. If one or more of the analysts covering our business downgrade their evaluations of our stock, the price of our stock could decline. If
one or more of theseadditional analysts cease to cover our stock
or fail to regularly publish reports, we could lose visibility in the market for our stock, which in turn could cause our stock price to decline.
Because we do not anticipate paying any cash dividends on our capital stock in the foreseeable future, capital appreciation, if any, will be your sole source of gain.
We have never declared or paid cash dividends on our capital stock. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business. In addition, the terms of any future debt agreements may preclude us from paying dividends. As a result, capital appreciation, if any, of our common stock will be your sole source of gain for the foreseeable future.
Provisions in our corporate charter documents and under Delaware law could make an acquisition of us, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our corporate charter and our bylaws may discourage, delay or prevent a merger, acquisition or other change in control of us that stockholders may consider favorable, including transactions in which you might otherwise receive a premium for your shares. These provisions also could limit the price that investors might be willing to pay in the future for shares of our common stock, thereby depressing the market price of our common stock. In addition, because our board of directors is responsible for appointing the members of our management team, these provisions may frustrate or
prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors. Among other things, these provisions:
•establish a classified board of directors such that not all members of the board are elected at one time;
•allow the authorized number of our directors to be changed only by resolution of our board of directors;
•limit the manner in which stockholders can remove directors from the board;
•establish advance notice requirements for stockholder proposals that can be acted on at stockholder meetings and nominations to our board of directors;
•require that stockholder actions must be effected at a duly called stockholder meeting and prohibit actions by our stockholders by written consent;
•limit who may call stockholder meetings;
•authorize our board of directors to issue preferred stock without stockholder approval, which could be used to institute a stockholder rights plan, or so-called “poison pill,” that would work to dilute the stock ownership of a potential hostile acquirer, effectively preventing acquisitions that have not been approved by our board of directors; and
•require the approval of the holders of at least 66 2/3% of the votes that all our stockholders would be entitled to cast to amend or repeal certain provisions of our charter or bylaws.
Moreover, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which prohibits a person who owns in excess of 15% of our outstanding voting stock from merging or combining with us for a period of three years after the date of the transaction in which the person acquired in excess of 15% of our outstanding voting stock, unless the merger or combination is approved in a prescribed manner.
Additionally, the Takeda standstill provisions and transfer restrictions in the
Takeda Stock PurchaseRLT Agreement may delay or prevent a merger, acquisition or other change in control of us that stockholders may consider favorable, including transactions in which you might otherwise receive a premium for your shares.
We may be subject to securities litigation, which is expensive and could divert management attention.
The market price of our common stock may be volatile. For example, on August 25, 2020, we announced the topline results of our ELEKTRA clinical trial, and our stock experienced a material decline. In the past, companies that have experienced volatility in the market price of their stock have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Securities litigation against us could result in substantial costs and divert our management’s attention from other business concerns, which could seriously harm our business.
Some provisions of our charter documents and Delaware law may have anti-takeover effects that could discourage an acquisition of us by others, even if an acquisition would benefit our stockholders and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our amended and restated certificate of incorporation and amended and restated bylaws, as well as provisions of Delaware law, could make it more difficult for a third party to acquire us or increase the cost of acquiring us, even if doing so would benefit our stockholders, or remove our current management. These provisions include:
•authorizing the issuance of “blank check” preferred stock, the terms of which we may establish and shares of which we may issue without stockholder approval;
•prohibiting cumulative voting in the election of directors, which would otherwise allow for less than a majority of stockholders to elect director candidates;
•prohibiting stockholder action by written consent, thereby requiring all stockholder actions to be taken at a meeting of our stockholders;
•eliminating the ability of stockholders to call a special meeting of stockholders; and
•establishing advance notice requirements for nominations for election to the board of directors or for proposing matters that can be acted upon at stockholder meetings.
These provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors, who are responsible for appointing the members of our management. Because we are incorporated in Delaware, we are governed by the
provisions of Section 203 of the Delaware General Corporation Law (the “DGCL”), which may discourage, delay or prevent someone from acquiring us or merging with us whether or not it is desired by or beneficial to our stockholders. Under the DGCL, a corporation may not, in general, engage in a business combination with any holder of 15% or more of its capital stock unless the holder has held the stock for three years or, among other things, the board of directors has approved the transaction. Any provision of our amended and restated certificate of incorporation or amended and restated bylaws or Delaware law that has the effect of delaying or deterring a change of control could limit the opportunity for our stockholders to receive a premium for their shares of our common stock and could also affect the price that some investors are willing to pay for our common stock.
If we engage in future acquisitions or strategic partnerships, this may increase our capital requirements, dilute our stockholders, cause us to incur debt or assume contingent liabilities and subject us to other risks.
Our business plan is to continue to evaluate various acquisitions and strategic partnerships, including licensing or acquiring complementary drugs, intellectual property rights, technologies, or businesses. Any potential acquisition or strategic partnership may entail numerous risks, including:
increased operating expenses and cash requirements;
the assumption of additional indebtedness or contingent liabilities;
assimilation of operations, intellectual property and drugs of an acquired company, including difficulties associated with integrating new personnel;
the diversion of our management’s attention from our existing drug programs and initiatives in pursuing such a strategic partnership, merger or acquisition;
retention of key employees, the loss of key personnel, and uncertainties in our ability to maintain key business relationships;
risks and uncertainties associated with the other party to such a transaction, including the prospects of that party and their existing drugs or drug candidates and regulatory approvals; and
our inability to generate revenue from acquired technology and/or drugs sufficient to meet our objectives in undertaking the acquisition or even to offset the associated acquisition and maintenance costs.
In addition, if we engage in future acquisitions or strategic partnerships, we may issue dilutive securities, assume or incur debt obligations, incur large one-time expenses and acquire intangible assets that could result in significant future amortization expense. Moreover, we may not be able to locate suitable acquisition opportunities and this inability could impair our ability to grow or obtain access to technology or drugs that may be important to the development of our business.
Sales of a substantial number of shares of our common stock in the public market could cause the market price of our common stock to drop significantly.
Sales of a substantial number of shares of our common stock in the public market could occur at any time. These sales, or the perception in the market that the holders of a large number of shares intend to sell shares, could reduce the market price of our common stock. Some of the holders of our securities have rights, subject to certain conditions, to require us to file registration statements covering their shares or to include their shares in registration statements that we may file for ourselves or other stockholders. Registration of these shares would result in the shares becoming freely tradable without restriction under the Securities Act except for shares held by our affiliates. Any sales of securities by these stockholders could have a material adverse effect on the trading price of our common stock.
Item 1B. Unresolved Staff Comments
None.
Item 1C. Cybersecurity
Risk Management and Strategy
We have implemented and maintain various information security processes designed to identify, assess and manage material risks from cybersecurity threats to our critical computer networks, third-party hosted services, communications systems, hardware and software, and our critical data, including intellectual property, and confidential information that is proprietary, strategic or competitive in nature (“Information Systems and Data”). Our information security function is led by our Director of Information Technology, with the support of our third-party specialists. Our information security function helps identify, assess and manage risks from cybersecurity threats by monitoring and evaluating our threat environment using various methods, including automated tools, domain name system filtering, antivirus protection, vulnerability scanning and penetration testing.
Depending on the environment, systems and data, we implement and maintain various technical, physical and organizational measures, processes, standards and policies designed to manage and mitigate material risks from cybersecurity threats to our Information Systems and Data. These measures include an incident response policy, encryption of certain data, network security controls, segregation of certain data and system monitoring. Our incident response policy provides the framework for the execution of cybersecurity incident response procedures and internal communications while adhering to applicable legal reporting and disclosure requirements.
Our assessment and management of material risks from cybersecurity threats are integrated into our overall risk management processes. Our information security function works with management to prioritize risk management processes and mitigate cybersecurity threats that are more likely to lead to a material impact to our business.
We use third-party service providers to assist us from time to time to identify, assess and manage material risks from cybersecurity threats. In addition, we use third-party service providers to perform a variety of functions throughout our business, including, for example, hosting and processing data, manufacturing our product candidates and assisting with R&D and clinical trial activities. Depending on the nature of the services provided, the sensitivity of the systems and data at issue, and the identity of the provider, our vendor contracting processes may include imposing certain contractual provisions related to privacy and cybersecurity.
For a description about our cybersecurity risks, refer to Item 1A. “Risk Factors,” to this Annual Report on Form 10-K “Significant disruptions of our information technology systems or data security incidents could result in significant financial, legal, regulatory, business and reputational harm to us.”
Governance
Our board of directors maintains oversight of our most significant risks and our processes to identify, manage and mitigate those risks. The audit committee of our board of directors (the “Audit Committee”) has responsibility for oversight of our management of cybersecurity risks.
Our cybersecurity risk assessment and management processes are implemented and maintained by certain management, including our Director of Information Technology, who reports to our Chief Operating Officer. Our Director of Information Technology is responsible for monitoring third-party specialists, helping to integrate cybersecurity risk considerations into our overall risk management strategy and communicating key priorities to relevant personnel. Our Chief Operating Officer is responsible for approving budgets, helping prepare for cybersecurity incidents, approving cybersecurity processes and reviewing security assessments and other security-related reports.
Our Incident Response Policy is designed to escalate certain cybersecurity incidents to members of management depending on the circumstances, including our Chief Operating Officer. Our Chief Operating Officer and other senior management work with our incident response team to help mitigate and remediate cybersecurity incidents in which case they are notified. In addition, our Incident Response Policy includes reporting to the Audit Committee for certain cybersecurity incidents. The Incident Response Policy is managed and maintained by our Director of Information Technology, with oversight from our Chief Operating Officer. The Audit Committee receives periodic updates from our Chief Operating Officer regarding the status of our cybersecurity program and our posture to identify and mitigate risks to our information security.
We currently lease the space for our principal executive offices, which are located at 1460 Broadway,441 Ninth Avenue, New York, New York, onunder a monthly basis.ten-year lease agreement which commenced in March 2022. We believe that our facilities are adequate to meet our current needs.
Item 3. Legal Proceedings
We are not currently subject to any material legal proceedings.proceedings.
Item 4. Mine Safety Disclosures
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Our common stock began trading on The Nasdaq Global Select Market on May 5, 2017, under the symbol “OVID.”
Comparative Stock Performance Graph
The following performance graph and related information shall not be deemed “soliciting material” or to be “filed” with the SEC, nor shall such information be incorporated by reference into any future filing under the Securities Act or Exchange Act. The following graph shows a comparison from
May 5, 2017 (the date our common stock commenced trading on the Nasdaq Global Select Market)December 31, 2018 through December 31,
2020,2023, of the cumulative total return for our common stock, the Nasdaq Composite Index and the Nasdaq
Biotechnology.
Biotechnology Index.
The graph assumes an initial investment of $100 on
May 5, 2017.December 31, 2018. The comparisons in the graph are not intended to forecast or be indicative of possible future performance of our common stock.
As of March
8, 2021,5, 2024, we had approximately
1114 holders of record of our common stock. Certain shares are held in
“street” name“street name” and accordingly, the number of beneficial owners of such shares is not known or included in the foregoing number. This number of holders of record also does not include stockholders whose shares may be held in trust by other entities.
We have never declared or paid any cash dividends on our common stock. We currently intend to retain future earnings to fund the development and growth of our business. We do not expect to pay any cash dividends in the foreseeable future. Any future determination to pay dividends will be made at the discretion of our board of directors and will depend on then-existing conditions, including our financial conditions, operating results, contractual restrictions, capital requirements, business prospects and other factors our board of directors may deem relevant.
Recent Sales of Unregistered Securities
Item 6.
Selected Financial DataThis item is no longer required as we have elected to early adopt the changes to Item 301 of Regulation S-K contained in SEC Release No. 33-10890.
[Reserved]
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion and analysis should be read in conjunction with our financial statements and related notes included elsewhere in this Annual Report on Form 10-K. This discussion and analysis and other parts of this Annual Report on Form 10-K contain forward-looking statements based upon current beliefs, plans and expectations that involve risks, uncertainties and assumptions, such as statements regarding our plans, objectives, expectations, intentions and projections. Our actual results and the timing of selected events could differ materially from those anticipated in these forward-looking statements as a result of several factors, including those set forth under “Risk Factors” and elsewhere in this Annual Report on Form 10-K. You should carefully read the “Risk Factors” section of this Annual Report on Form 10-K to gain an understanding of the important factors that could cause actual results to differ materially from our forward-looking statements. Please also see the section entitled “Special Note Regarding Forward-Looking Statements.”
We are a biopharmaceutical company
dedicated to meaningfully improving the lives of people affected by certain epilepsies and brain conditions with seizure symptoms. Our approach to achieve this goal is scientifically driven, patient focused,
on developing impactful medicines for patients and
families livingcoupled with
an integrated and disciplined approach to research, clinical development and business development. Our team has significant experience with and understanding of rare
epilepsies and neurological
disorders. We believeconditions, and we continue to gain insight into the ways the different molecular mechanisms and pathways underlying these disorders
represent an attractive area for drug development asimpact the
understandingsymptoms patients suffer. We have set out to be a leader in the field, and have developed a differentiated pipeline containing four novel mechanisms of
the underlying biology has grown meaningfully over the last few yearsaction to target different causes of certain epilepsies and
today represent a substantial opportunity medically and commercially. Based on the rapid increase in scientific understanding of the role of genetics and key biological pathways relevant to diseases of the brain
we aim to identify, discover and develop novel compounds for the treatment of rare neurological disorders.conditions with seizure symptoms. We have built a
deep knowledgescalable scientific platform with efficient development capabilities in epilepsies and conditions with seizure symptoms that focuses on clear, clinical endpoints. Three of
such diseases, howour programs are in clinical trials in humans, and the fourth is in preclinical development and anticipated to
treat themadvance into human safety studies in 2024. We are initially pursuing therapeutic assets for rare disorders as they can leverage accelerated development programs. If successfully developed and
howmarketed in rare conditions, we intend to
explore these assets for broader neurologic indications. Our cohesive focus in certain epilepsies and brain conditions with seizure symptoms reinforces our belief that we can develop
the clinically meaningful endpoints required for development of a compoundand produce multiple novel medicines, scale our infrastructure, and thereby succeed in
these disorders. As a result of this knowledge, we have developed a pipeline of first-in-class compounds and programs and have demonstrated our
model by progressing compounds through to late-stage development. We continue to execute on our strategy to build this pipeline by discovering in-licensing and collaborating with leading biopharmaceutical companies and academic institutions.Our latest pipeline includes two late-stage programs and several earlier stage programs.
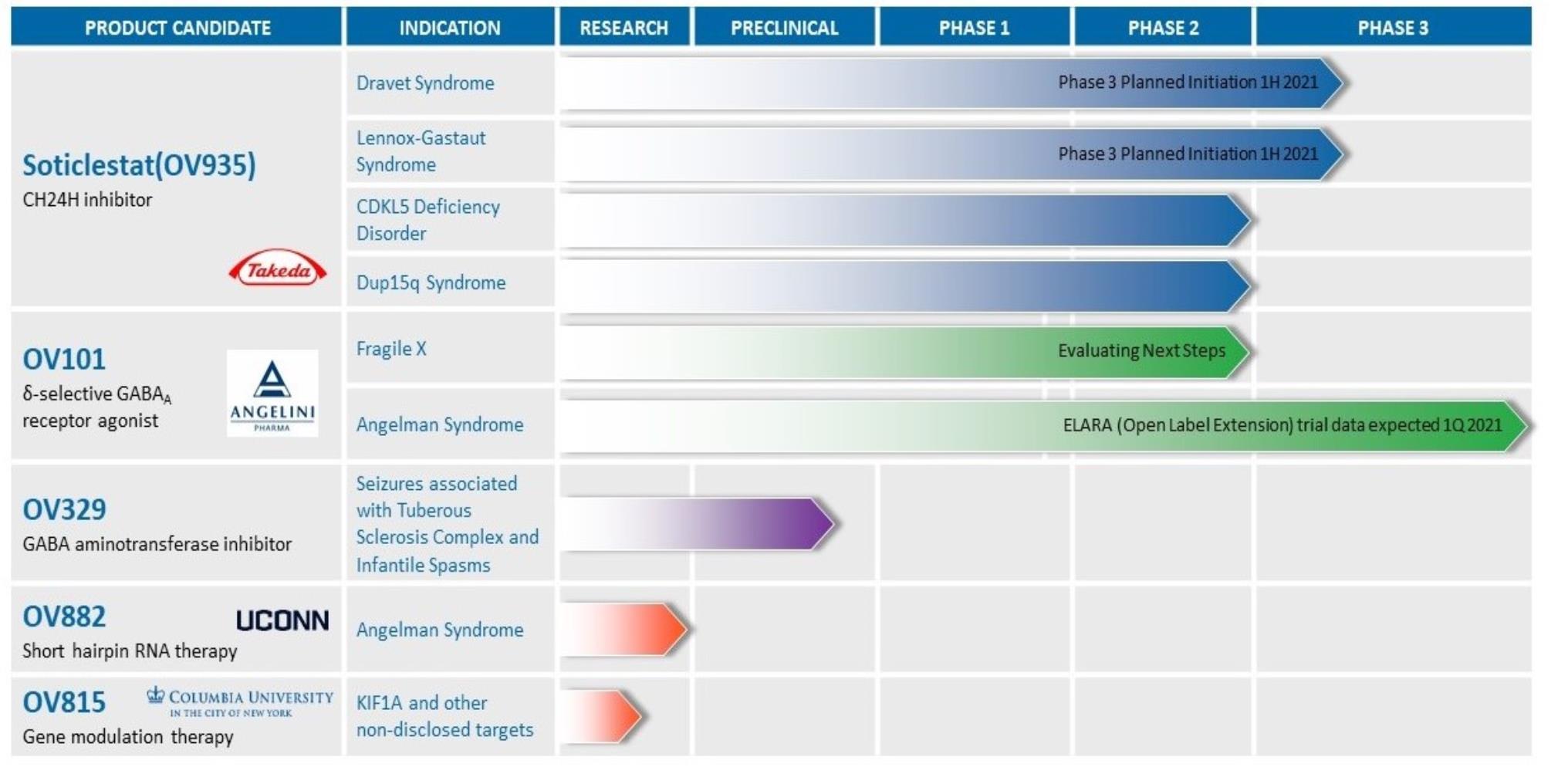
mission.
Since our inception in April 2014, we have devoted substantially all of our efforts to organizing and planning our business, building our management and technical team, acquiring operating assets and raising capital.
During the yearyears ended December 31, 2020,2023 and 2022, we generated $12.6$0.4 million and $1.5 million of licenseroyalty and otherlicensing revenue, through our Collaboration and License Agreement, or the Angelini License Agreement, with Angelini Pharma Rare Diseases AG, or Angelini, andrespectively. We have otherwise primarily funded our business primarily through the sale of our capital stock.stock and through the closing of the RLT Agreement with Takeda, which resulted in a one-time up-front payment of $196.0 million in 2021. Through December 31, 2020,2023, we have raised net proceeds of $275.4 million from the sale of our convertible preferred stock and our common stock. As of December 31, 2020,2023, we had $72.0$105.8 million in cash, cash equivalents and cash equivalents.marketable securities. We recorded net lossesloss of $81.0$52.3 million and $60.5$54.2 million for the years ended December 31, 20202023 and 2019,2022, respectively. As of December 31, 2020,2023, we had an accumulated deficit of $294.2$277.9 million.
We expect to continue to incur significant expenses and increasing operating losses for at least the next several years. Our net losses may fluctuate significantly from period to period, depending on the timing of our planned clinical trials and expenditures on our other research and development and commercial development activities. We expect our expenses will increase substantially over time as we:
•continue the ongoing and planned preclinical and clinical development of our drug candidates;
•build a portfolio of drug candidates through the development, acquisition or in-license of drugs, drug candidates or technologies;
•initiate preclinical studies and clinical trials for any additional drug candidates that we may pursue in the future;
•seek marketing approvals for our current and future drug candidates that successfully complete clinical trials;
•establish a sales, marketing and distribution infrastructure to commercialize any drug candidate for which we may obtain marketing approval;
•develop, maintain, expand and protect our intellectual property portfolio;
•implement operational, financial and management systems; and
•attract, hire and retain additional administrative, clinical, regulatory, manufacturing, commercial and scientific personnel.
Recent Developments
Royalty, License
Significant Risks and Termination AgreementUncertainties
The global economic slowdown, the overall disruption of global healthcare systems and other risks and uncertainties associated with
TakedaOn March 2, 2021, we entered intopublic health crises and global geopolitical tensions, like the ongoing war between Russia and Ukraine and the war in Israel, may have a royalty, licensematerial adverse effect on our business, financial condition, results of operations and termination agreement,growth prospects. The resulting high inflation rates may materially affect our business and corresponding financial position and cash flows. Inflationary factors, such as increases in the cost of our clinical trial materials and supplies, interest rates and overhead costs may adversely affect our operating results. High interest rates also present a recent challenge impacting the U.S. economy and could make it more difficult for us to obtain traditional financing on acceptable terms, if at all, in the future. Furthermore, economic conditions have produced downward pressure on share prices. Although we do not believe that inflation has had a material impact on our financial position or results of operations to date, we may experience increases in the Takeda Licensenear future (especially if inflation rates remain high or begin to rise again) on our operating costs, including our labor costs and Termination Agreement, with Takeda, relatingresearch and development costs, due to the Takeda collaboration agreement described above in Item 1. Business section. Under the termssupply chain constraints, global geopolitical tensions as a result of the Takeda Licenseongoing war between Russia and Termination Agreement, upon closing ofUkraine and the transaction, the Takeda collaboration agreement will be terminated by mutual agreement,war in Israel, worsening global macroeconomic conditions and Takeda will secure rightsemployee availability and wage increases, which may result in additional stress on our working capital resources.
In addition, we are subject to other challenges and risks specific to our
50% global sharebusiness and our ability to execute on our strategy, as well as risks and uncertainties common to companies in
soticlestat, and an exclusive license under our relevant intellectual property rights, in exchange for an upfront payment,the pharmaceutical industry with development and commercial
milestone payments,operations, including, without limitation, risks and
royalties. Takeda will assume all responsibility for, and costsuncertainties associated with: identifying, acquiring or in-licensing products or product candidates; obtaining regulatory approval of
bothproduct candidates; pharmaceutical product development and
commercializationthe inherent uncertainty of
soticlestat following closing. At closing, we will receive an upfront paymentclinical success; and the challenges of
$196.0 millionprotecting and
are eligible to receive up to an additional $660.0 million in development,enhancing our intellectual property rights; complying with applicable regulatory
and sales milestones. In addition, if soticlestat achieves regulatory approval, we will receive tiered royalties on net sales of soticlestat at percentages ranging from the low double-digits up to 20%, subject to standard reductions in certain circumstances. Royalties are payable on a country-by-country and product-by-product basis during the period beginning on the date of the first commercial sale of such product in such country and ending on the later to occur of the expiration of patent rights covering the product in such country and a specified anniversary of such first commercial sale. The Takeda License and Termination Agreement will remain in effect until Takeda’s cessation of commercialization of soticlestat. We expect to close the Takeda License and Termination Agreement in the first half of 2021, subject to the satisfaction of customary closing conditions, including regulatory review by the appropriate regulatory agencies under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended. The Takeda License and Termination Agreement may be terminated upon the mutual agreement of the parties, or by either Takeda or the Company, if the closing has not occurred on or before May 14, 2021. COVID-19 Update
We have implemented business continuity plans designed to address and mitigate the impact of the ongoing COVID-19 pandemic on our employees and our business. We continue to operate normally with the exception of enabling all of our employees to work productively at home and abiding by travel restrictions issued by federal, state and local governments. Our current plans to return to the office remain fluid as federal, state and local guidelines, rules and regulations continue to evolve.
requirements.
Financial Operations Overview
We have generated limited revenue primarily under the Angelini LicenseRLT Agreement, as well as nominal amounts from other licensing agreements and expect to recognize additional revenue as we satisfy our performance obligations.royalties. We have not generated any revenue from commercial drug sales and we do not expect to generate any further revenue from commercial drug sales unless or until we obtain regulatory approval of and commercialize one or more of our current or future drug candidates.candidates, or if we become entitled to revenue from our licensing agreements. In the future, we may also seek to generate revenue from a combination of research and development payments, license fees and other upfront or milestone payments.
Research and Development Expenses
Research and development expenses consist primarily of costs incurred for our research activities, including our product discovery efforts and the development of our product candidates, which include, among other things:
•employee-related expenses, including salaries, benefits and stock-based compensation expense;
•fees paid to consultants for services directly related to our drug development and regulatory effort;
•expenses incurred under agreements with contract research organizations, as well as contract manufacturing organizations and consultants that conduct preclinical studies and clinical trials;
•costs associated with preclinical activities and development activities;
•costs associated with technology and intellectual property licenses;
•milestone payments and other costs and payments under licensing agreements, research agreements and collaboration agreements; and
•depreciation expense for assets used in research and development activities.
Costs incurred in connection with research and development activities are expensed as incurred. Costs for certain development activities, such as clinical trials, are recognized based on an evaluation of the progress to completion of
specific tasks using data such as patient enrollment, clinical site activations or other information provided to us by our vendors.
Research and development activities are and will continue to be central to our business model. We expect our research and development expenses to increase for the foreseeable future as we advance our current and future drug candidates through preclinical studies and clinical trials. The process of conducting preclinical studies and clinical trials necessary to obtain regulatory approval is costly and time-consuming. It is difficult to determine with certainty the duration and costs of any preclinical study or clinical trial that we may conduct. The duration, costs and timing of clinical trial programs and development of our current and future drug candidates will depend on a variety of factors that include, but are not limited to, the following:
•number of clinical trials required for approval and any requirement for extension trials;
•per patient trial costs;
•number of patients who participate in the clinical trials;
•number of sites included in the clinical trials;
•countries in which the clinical trial is conducted;
•length of time required to enroll eligible patients;
•number of doses that patients receive;
•drop-out or discontinuation rates of patients;
•potential additional safety monitoring or other studies requested by regulatory agencies;
•duration of patient follow-up; and
•efficacy and safety profile of the drug candidate.
In addition, the probability of success for any of our current or future drug candidates will depend on numerous factors, including competition, manufacturing capability and commercial viability. We will determine which programs to pursue and how much to fund each program in response to the scientific and clinical success of each drug candidate, as well as an assessment of each drug candidate’s commercial potential.
General and Administrative Expenses
General and administrative expenses consist primarily of employee-related expenses, including salaries, benefits and stock-based compensation expense, related to our executive, finance, business development and support functions. Other general and administrative expenses include costs associated with operating as a public company,
described below, travel expenses, conferences, professional fees for auditing, tax and legal services and facility-related costs.
Other Income
Net(Expense), net
Other income (expense), net, consists primarily of interest income earnedand accretion of discount on our cash and cash equivalents maintained in money market funds and prior short-term investments that were maintained in U.S. treasury notes.and unrealized gains/losses on long-term equity investments.
Comparison of the Years Ended December 31,
20202023 and
20192022
The following table summarizes the results of our operations for the periods indicated:
| | Year Ended | | | Year Ended | | | | | |
| | December 31, | | | December 31, | | | | | |
| | 2020 | | | 2019 | | | Change $ | |
| | (in thousands) | |
| Year Ended December 31, 2023 | | | Year Ended December 31, 2023 | | Year Ended December 31, 2022 | | Change $ |
| (in thousands) | | | (in thousands) |
Revenue: | | | | | | | | | | | | |
License and other revenue | | $ | 12,617 | | | $ | - | | | $ | 12,617 | |
| License and other revenue | |
| License and other revenue | |
| Total revenue | |
Operating expenses: | | | | | | | | | | | | |
| Research and development | |
| Research and development | |
Research and development | | | 63,417 | | | | 42,157 | | | | 21,260 | |
General and administrative | | | 30,631 | | | | 19,252 | | | | 11,379 | |
Total operating expenses | | | 94,048 | | | | 61,409 | | | | 32,639 | |
Loss from operations | | | (81,431 | ) | | | (61,409 | ) | | | (20,022 | ) |
Other income, net | | | 395 | | | | 948 | | | | (553 | ) |
| Other income (expense), net | |
| Loss before provision for income taxes | |
| Provision for income taxes | |
Net loss | | $ | (81,036 | ) | | $ | (60,461 | ) | | $ | (20,575 | ) |
Revenue of $0.4 million was
$12.6recognized for the year ended December 31, 2023 related to royalties. Revenue was $1.5 million for the year ended December 31,
2020 as a result of revenue recorded in connection with the Angelini License Agreement. We did not generate any revenue during the year ended December 31, 2019.2022 related to licensing and other agreements.
Research and Development Expenses
| | Year Ended | | | Year Ended | | | | | |
| | December 31, | | | December 31, | | | | | |
| | 2020 | | | 2019 | | | Change $ | |
| | (in thousands) | |
| Year Ended December 31, 2023 | | | Year Ended December 31, 2023 | | Year Ended December 31, 2022 | | Change $ |
| (in thousands) | | | (in thousands) |
Preclinical and development expenses | | $ | 43,612 | | | $ | 27,034 | | | $ | 16,578 | |
Payroll and payroll-related expenses | | | 15,439 | | | | 11,496 | | | | 3,943 | |
Other expenses | | | 4,366 | | | | 3,627 | | | | 739 | | Other expenses | 3,442 | | 3,405 | | 37 |
Total research and development | | $ | 63,417 | | | $ | 42,157 | | | $ | 21,261 | |
Research and development expenses were
$63.4$28.6 million for the year ended December 31,
20202023 compared to
$42.2$24.6 million for the year ended December 31,
2019. 2022. The increase of $21.3$4.9 million in preclinical and development expenses was primarily due to an increase in developmentadditional activities related to our ongoing development programs,. During the year ended December 31, 2020, total research primarily relating to OV888 (GV101) and
development expenses consistedOV329. The decrease of
$43.6 million in preclinical and development expenses, including a credit of $0.7 million representing costs reimbursable to the Company from Takeda in respect of the Takeda collaboration, $15.4$1.0 million in payroll and payroll-related expenses
was primarily due to the impact of
a reorganization in early 2022 which
$2.8 million related to stock-based compensation, and $4.4resulted in approximately $1.0 million in
other expenses. Duringseverance costs during the year ended December 31, 2019, total research and development expenses consisted of $27.0 million in preclinical and development expenses, including a credit of $4.7 million representing costs reimbursed to us from Takeda in respect of the Takeda collaboration, $11.5 million in payroll and payroll-related expenses, of which $2.4 million related to stock-based compensation, and $3.6 million in other expenses.period.
General and Administrative Expenses
| | Year Ended | | | Year Ended | | | | | |
| | December 31, | | | December 31, | | | | | |
| | 2020 | | | 2019 | | | Change $ | |
| | (in thousands) | |
| Year Ended December 31, 2023 | | | Year Ended December 31, 2023 | | Year Ended December 31, 2022 | | Change $ |
| (in thousands) | | | (in thousands) |
Payroll and payroll-related expenses | | $ | 13,390 | | | $ | 9,888 | | | $ | 3,502 | |
Legal and professional fees | | | 13,824 | | | | 5,623 | | | | 8,201 | |
General office expenses | | | 3,417 | | | | 3,741 | | | | (324 | ) |
Total general and administrative | | $ | 30,631 | | | $ | 19,252 | | | $ | 11,379 | |
General and administrative expenses were
$30.6$31.1 million for the year ended December 31,
20202023 compared to
$19.3$32.4 million for the year ended December 31,
2019. 2022. The increasedecrease of $11.4$1.3 million was primarily consisted of increases in due to reduced legal
fees, compliance and pre-commercialization expenses and professional fees
of $8.2 million and payroll-relatedgeneral office expenses, of $3.5 million.partially offset by an increase in non-cash compensation expenses.
Other Income Net(Expense), net
Other income includes interest income of $0.4(expense), net was $6.9 million for the year ended December 31, 20202023, comprised of $4.9 million in interest and $0.9accretion income on investments in U.S. treasuries and $2.0 million forof unrealized gain on long-term equity investments. For the year ended December 31, 2019.
Income Taxes
There2022, other income (expense), net of $1.4 million was no provision forcomprised of $1.8 million in interest and accretion income taxes for the years ended December 31, 2020on investments in U.S. treasuries and 2019 because we have historically incurred operating losses and we maintain a full valuation allowance against our net deferred tax assets. The valuation allowance was approximately $89.8$0.4 million and $76.5 million at December 31, 2020 and 2019, respectively.
of unrealized loss on long-term equity investments.
Liquidity and Capital Resources
As of December 31,
2020,2023 and 2022, we had total cash, cash equivalents and
short-term investmentsmarketable securities of
$72.0$105.8 million
as compared to $76.7and $129.0 million,
respectively. We believe that our cash, cash equivalents and marketable securities as of December 31,
2019.On July 9, 2020, we entered into2023 will fund our projected operating expenses and capital expenditure requirements for at least 12 months from the Angelini License Agreement with Angelini, pursuant to which we granted to Angelini exclusive rights to develop and commercialize OV101 in the European Territory. Under the Angelini License Agreement, Angelini made an upfront payment and a milestone payment related to the transferissuance of a specified amount of compound and related information to the Company of $25.0 million during the year ended December 31, 2020. In addition, Angelini will be required to make additional milestone payments to us upon the completion of the specified components of the technology transfer, and achievement of specified regulatory milestones for OV101 in Angelman syndrome of up to $55.0 million in the aggregate, as well as up to €162.5 million ($199.9 million) in sales milestone payments for achievement of specified levels of net sales in the European Territory. Angelini also will be required to pay tiered royalties on net sales by Angelini, its affiliates or sublicensees at double-digit percentages above the teens, subject to certain standard reductions and offsets.
In November 2020, we filed a new shelf registration statementthis Annual Report on Form S-3 (Registration No. 333-250054) that allows us to sell up to an aggregate of $250.0 million of our common stock, preferred stock, debt securities and/or warrants (the “S-3 Registration Statement”), which includes a prospectus covering the issuance and sale of up to $75.0 million of common stock pursuant to an at-the-market (“ATM”) offering program. As of December 31, 2020, we had $250.0 million available under our S-3 Registration Statement, including $75.0 million available pursuant to our ATM program.
In August 2020, we sold 6,250,000 shares of our common stock at a public offering price of $8.00 per share, for net proceeds of $46.7 million, after deducting underwriting discounts and commissions and other offering expenses payable by us, or the August 2020 Offering.
In February 2019, we sold 13,993,778 shares of our common stock and 2,500 shares of Series A Convertible Preferred Stock at a public offering price of $2.00 and $2,000 per share, respectively, for net proceeds of $30.5 million, after deducting underwriting discounts and commissions and other offering expenses payable by us, or the February 2019 Offering. In October and November 2019, we sold 10,350,000 shares of our common stock, which included the full exercise of the underwriters’ option to purchase additional shares, and 4,000 shares of Series A Convertible Preferred Stock at a public offering price of $2.50 and $2,500 per share, respectively, for net proceeds of $33.5 million after deducting underwriting discounts and commissions and other offering expenses payable by us, or the October 2019 Offering.
During the year ended December 31, 2019, we sold 6,893,888 shares of our common stock under our previous ATM program for net proceeds of $22.3 million after deducting sales agent commissions and other offering expenses payable by us, or the ATM Offering.
10-K.
Similar to other development-stage biotechnology companies, we have generated limited
revenue, which has been through the Angelini License Agreement.revenue. We have incurred losses and experienced negative operating cash flows
in most years since our inception and anticipate that we will continue to incur losses for at least the next several years. We incurred net losses of
approximately $81.0$52.3 million and
$60.5$54.2 million for the
yearyears ended December 31,
20202023 and
2019,2022, respectively. As of December 31,
2020,2023, we had an accumulated deficit of
$294.2$277.9 million and working capital of
$52.8$98.1 million.
Management has identified these conditions or events, which, considered in the aggregate, raise substantial doubt about
Future Funding Requirements
We believe that our ability to continue as a going concern, including the risk that we will be unable to raise adequate additional capitalavailable cash, cash equivalents and marketable securities are sufficient to fund operations through at least the next 12 months from the date of filing of this Annual Report on Form 10-K. Management’s response to these conditionsexisting and events is that the successful closing of the Takeda License and Termination Agreement with Takeda (see note 12 of our Consolidated Financial Statements) will provide the liquidity needed to alleviate the substantial doubt referred to above. As described in note 12 of our Consolidated Financial Statements, there are certain conditions to close the Takeda License and Termination Agreement, however, management has determined that the likelihood of not closing inplanned cash requirements into the first half of 20212026. Our primary uses of capital are, and we expect will continue to be, compensation and related expenses, third-party clinical research and development services, clinical costs, legal and other regulatory expenses and general overhead costs. We have based our estimates on assumptions that may prove to be incorrect, and we could use our capital resources sooner than we currently expect. Additionally, the process of testing drug candidates in clinical trials is remote. Incostly, and the event thattiming of progress in these trials is uncertain. We cannot estimate the actual amounts necessary to successfully complete the development and commercialization of our product candidates or whether, or when, we are unable to close the Takeda License and Termination Agreement,may achieve profitability.
As of December 31, 2023, we had no material non-cancelable purchase commitments with service providers, as we have generally contracted on a cancelable, purchase order basis. We cannot estimate whether we will
need to raise additional capital in order to
alleviate substantial doubt about our ability to continuereceive or the timing of any potential contingent payments upon the achievement by us of clinical, regulatory and commercial events, as a going concern. The failure to raise capital as and when needed could have a negative impact on our financial condition and ability to pursue our business strategy. If we are unable to raise capital,applicable, or royalty payments that we may be required to delay, reducemake under license agreements we have entered into with various entities pursuant to which we have in-licensed certain intellectual property as contractual obligations or commitments, including agreements with AstraZeneca, Gensaic and Northwestern. Pursuant to these license agreements, we have agreed to make milestone payments up to an aggregate of $660.3 million upon the scopeachievement of or eliminate researchcertain development, regulatory and development programs, or obtain funds through arrangementssales milestones. We excluded these contingent payments from the consolidated financial statements given that the timing, probability, and amount, if any, of such payments cannot be reasonably estimated at this time.
In September 2021, we entered into a 10-year lease agreement for our corporate headquarters with
collaborators or others that may require us to relinquish rights to certain drug candidates that we might otherwise seek to develop or commercialize independently.In addition toa term commencing March 10, 2022, for approximately 19,000 square feet of office space at Hudson Commons in New York, New York. The lease provides for monthly rental payments over the proceeds we expect to receive upon closinglease term. The base rent under the lease is currently $2.3 million per year. Rent payments commenced January 10, 2023, and will continue for ten years following the rent commencement date. We issued a letter of credit in the amount of $1.9 million in association with the execution of the Takeda Licenselease agreement, which is reflected as restricted cash on the consolidated balance sheets. Payment obligations under the lease agreement include approximately $2.3 million in the 12 months subsequent to December 31, 2023 and Termination Agreement,approximately $23.5 million over the term of the agreement. For additional information see Note 5 to our consolidated financial statements under the heading 'Leases.'
We have no products approved for commercial sale and have not generated any revenues from product sales to date. Until such time, if ever, as we
plancan generate substantial product revenues, we expect to finance our cash needs through
eithera combination of equity offerings, debt financings
collaborations, strategic alliances,and additional funding from license and collaboration arrangements. Except for any obligations of our collaborators to reimburse us for research and development expenses or
licensingto make milestone or royalty payments under our agreements
or a combinationwith them, we will not have any committed external source of
any such transactions.liquidity. To the extent that we raise additional capital through future equity offerings or debt financings, ownership
interests may be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a common stockholder. Debt and equity financings, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. There can be no assurance that such financings will be obtained on terms acceptable to us, if at all. The ongoing COVID-19 pandemic continuesAdditionally, while the long-term economic impact of geopolitical tensions, including the war between Russia and Ukraine and war in Israel, is difficult to
rapidly evolve andassess or predict, each of these events has
already resulted in acaused significant
disruption ofdisruptions to the global financial
markets.markets and contributed to a general global economic slowdown. Furthermore, inflation rates have increased recently to levels not seen in decades. In addition, the U.S. Federal Reserve has raised interest rates in response to concerns about inflation. High interest rates, especially if coupled with reduced government spending and volatility in financial markets, may further increase economic uncertainty and heighten these risks. If the
disruption persistsdisruptions and
deepens,slowdown deepen or persist, we
could experience an inabilitymay not be able to access additional capital
on favorable terms, or at all, which could in the future negatively affect our
operations. ability to pursue our business strategy. If we raise additional funds through collaborations, strategic alliances or licensing agreements with third parties for one or more of our current or future drug candidates, we may be required to relinquish valuable rights to our technologies, future revenue streams, research programs or drug candidates or to grant licenses on terms that may not be favorable to us. Our failure to raise capital as and when needed would have a material adverse effect on our financial condition and our ability to pursue our business strategy. See “Risk Factors” for additional risks associated with our capital requirements.
At-the-Market Offering Program
In November 2020, we filed a shelf registration statement on Form S-3 (Registration No. 333-250054) (the “Prior S-3 Registration Statement”). In November 2023, upon expiration of the of the Prior Registration Statement, we filed a new shelf registration statement on Form S-3 (Registration No. 333-275307) that allows us to sell up to an aggregate of $250.0 million of our common stock, preferred stock, debt securities and/or warrants (the “Current S-3 Registration Statement”), which includes a prospectus covering the issuance and sale of up to $75.0 million of common stock pursuant to an at-the-market (“ATM”) offering program, including the unsold securities under the Prior Registration Statement. During the years ended December 31, 2023 and 2022, we did not sell any shares under our ATM program. As of December 31, 2023, we had $250.0 million available under our Current S-3 Registration Statement, including $75.0 million available pursuant to our ATM program.
The following table summarizes our cash flows for the periods indicated:
| | Year Ended | | | Year Ended | |
| | December 31, | | | December 31, | |
| | 2020 | | | 2019 | |
| | (in thousands) | |
Net cash provided by (used in): | | | | | | | | |
| Year Ended December 31, 2023 | | | Year Ended December 31, 2023 | | Year Ended December 31, 2022 |
| (in thousands) | | | (in thousands) |
| Net cash (used in) provided by: | |
| Operating activities | |
| Operating activities | |
Operating activities | | $ | (51,584 | ) | | $ | (51,091 | ) |
Investing activities | | | 34,648 | | | | (30,041 | ) |
Financing activities | | | 47,072 | | | | 86,539 | |
Net increase in cash and cash equivalents | | $ | 30,136 | | | $ | 5,407 | |
| Net decrease in cash, cash equivalents, and restricted cash | |
Net Cash Used in Operating Activities
Net cash
used in operating activities was
$51.6$45.8 million for the year ended December 31,
2020,2023, which consisted of
a net loss of
$81.0$52.3 million offset by a net of
$29.5$6.6 million of
various non-cash charges and
indirectoperating cash changes,
primarily related to $7.5most significantly $7.3 million
ofin stock-based
compensation expense and $12.4 million of deferred revenue.compensation. Net cash used in operating activities was
$51.1$55.2 million for the year ended December 31,
2019,2022, which consisted of
a net loss of
$60.5$54.2 million
and $8.3 million decrease in accounts payable and accrued expenses, partially offset by
a net of $9.4 million ofvarious non-cash charges and
indirect cash
changes, primarily related to $5.2 million of stock-based compensation expense.changes.
Net Cash
Provided by (Used in) in Investing Activities
Net cash
provided byused in investing activities was
$34.6$2.6 million for the year ended December 31,
2020, compared2023, which was primarily related to
$30.0 million usedour purchases and sales/maturities of investments in
investing activities forU.S. treasury funds and the purchase of a long-term equity investment. For the year ended December 31,
2019. The change2022, $87.9 million was used in
net cash provided by investing activities,
was primarily
due to the higher comprised of purchases and sales/maturities of
short-term investments
during the year ended December 31, 2020 compared to net purchases during the year ended December 31, 2019.in U.S. treasury funds.
Net Cash Provided by Financing Activities
Net cash provided by financing activities
of $47.0was $30.5 million for the year ended December 31,
20202023, which was primarily due to the
net proceeds from$30.0 million received in connection with the
August 2020 Offering. NetLigand Agreement. For the same period in 2022, cash provided by financing activities
of $86.5was $0.2 million,
for the year ended December 31, 2019 was primarily duerelated to
the net proceeds from the
February 2019 Offering, October 2019 Offeringexercise of options and
ATM Offering.Contractual Obligationspurchases made under the employee stock purchase plan.
Critical Accounting Estimates and
CommitmentsAsPolicies
Our management’s discussion and analysis of
December 31, 2020, we agreedfinancial condition and results of operations is based on our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these consolidated financial statements requires us to
continue certain studiesmake estimates and assumptions that
were ongoingaffect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the
time of signing the Angelini License Agreement. In March 2021, we entered into the Takeda License and Termination Agreement under which Takeda will secure global rights at closing from us to develop and commercialize the investigational medicine OV935 for the treatment of developmental and epileptic encephalopathies, including DS and LGS. Closingdate of the Takeda Licenseconsolidated financial statements, as well as the revenue and Termination Agreement is subject to satisfaction of customary closing conditions, including regulatory review byexpenses incurred during the
appropriate regulatory agenciesreported periods. On an ongoing basis, we evaluate our estimates and judgments. We base our estimates on historical experience and on various other factors that we believe are reasonable under the
HSR Act. We had no other material contractual obligations or commitments. We had no long-term debt or leasescircumstances, the results of which form the basis for making judgments about the carrying value of assets and
no material non-cancelable
purchase commitments with service providers, as we have generally contracted on a cancelable, purchase order basis. We excluded any potential contingent payments upon the achievement by us of clinical, regulatory and commercial events, as applicable, or royalty payments that we may be required to make under license agreements we have entered into with various entities pursuant to which we have in-licensed certain intellectual property as contractual obligations or commitments, including agreements with H. Lundbeck A/S, Northwestern, and our Takeda license agreement. Pursuant to these license agreements, we have agreed to make milestone payments up to an aggregate of $279.3 million upon the achievement of certain development, regulatory and sales milestones. We excluded these contingent payments given that the timing, probability, and amount, if any, of such payments cannot be reasonably estimated at this time.
Off-Balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements, as defined in the rules and regulations of the SEC.
Emerging Growth Company Status and Smaller Reporting Company Status
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, and may remain an emerging growth company until December 31, 2022. For so long as we remain an emerging growth company, we are permitted and intend to rely on exemptions from certain disclosure requirements that are applicable to other public companiesliabilities that are not emerging growth companies. These exemptions include:
apparent from other sources. Changes in estimates are reflected in reported results for the period in which they become known. reduced disclosure aboutActual results may differ from these estimates under different assumptions or conditions. In making estimates and judgments, management employs critical accounting policies. Our critical accounting policies are described in greater detail in Note 2 to our executive compensation arrangements;
no non-binding stockholder advisory votes on executive compensation or golden parachute arrangements; and
exemption from the auditor attestation requirement in the assessment of our internal control overaudited consolidated financial reporting.
We have taken advantage of reduced reporting requirementsstatements included in this Annual Report on Form 10-K and may continue to do so until such time10-K.We have listed below our critical accounting estimates that we are no longer an emerging growth company. believe to have the greatest potential impact on our consolidated financial statements. Historically, our assumptions, judgments and estimates relative to our critical accounting estimates have not differed materially from actual results.
Revenue Recognition
We will remain an “emerging growth company” untilrecognize revenue under sublicense agreements in accordance with ASC 606, Revenue Recognition, which is applicable to the earliest of (a) the last dayRLT Agreement. The terms of the fiscalagreements within this scope may contain multiple performance obligations, including but not limited to licenses and research and development activities. ASC 606 requires that we evaluate these agreements to determine the distinct performance obligations. Non-refundable, upfront fees that are not contingent on any future performance and require no consequential continuing involvement by us, are recognized as revenue when the license term commences and the licensed data, technology or product is delivered. We defer recognition of non-refundable upfront fees if the performance obligations are not satisfied.
During the year in whichended December 31, 2023, we recognized revenue of approximately $0.4 million related to royalty agreements.
Research and Development Accrual
When preparing our consolidated financial statements, we are required to estimate our accrued research and development expenses. This process involves reviewing open contracts and communicating with our personnel to identify services that have been performed on our behalf and estimating the level of service performed and the associated cost incurred for the service when we have total annual gross revenues of $1.07 billionnot yet been invoiced or more, (b) December 31, 2022, the last dayotherwise notified of the fiscal year followingactual cost. Payments under certain contracts we have with third parties depend on factors, such as the fifth anniversarysuccessful enrollment of certain numbers of patients, site initiation and the completion of clinical trial milestones.
When accruing research and development expenses, we estimate the our IPO, (c)time period over which services will be performed and the date on which we have issued more than $1.0 billion in nonconvertible debt during the previous three years or (d) the date on which we are deemedlevel of effort to be expended in each period. If possible, we obtain information regarding unbilled services directly from our service providers. However, we may be required to estimate the cost of these services based only on information available to us. If we underestimate or overestimate the cost associated with a large accelerated filer under the rules of the SEC. Section 107 of the JOBS Act provides that an emerging growth company can take advantage of the extended transition period for complying with newtrial or revised accounting standards. service at a given point in time, adjustments to research and development expenses may be necessary in future periods. Historically, our estimated accrued research and development expenses have approximated actual expense incurred.
Smaller Reporting Company Status
We
have irrevocably elected not to avail ourselves of this extended transition period and, as a result, we will adopt new or revised accounting standards on the relevant dates on which adoption of such standards is required for other public companies.In addition, we are also a smaller reporting company as defined in the Exchange Act. We may continue to be a smaller reporting company even after we are no longer an emerging growth company. We may take advantage of certain of the scaled disclosures available to smaller reporting companies and will be able to take advantage of these scaled disclosures for so long as (i) our voting and non-voting common stock held by non-affiliates is less than $250.0 million measured on the last business day of our second fiscal quarter or (ii) our annual revenue is less than $100.0 million during the most recently completed fiscal year and our voting and non-voting common stock held by non-affiliates is less than $700.0 million measured on the last business day of our second fiscal quarter.
Critical Accounting Policies and Estimates
Our management’s discussion and analysis of financial condition and results of operations is based on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the revenue and expenses incurred during the reported periods. On an ongoing basis, we evaluate our estimates and judgments, including those related to accrued expenses and stock-based compensation. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not apparent from other sources. Changes in estimates are reflected in reported results for the period in which they become known. Actual results may differ from these estimates under different assumptions or conditions.
During the year ended December 31, 2020, we recognized revenue. In addition, see Note 2 of our Consolidated Financial Statements under the heading “Recent Accounting Pronouncements” for new accounting pronouncements or changes to the accounting pronouncements during the year ended December 31, 2020.
Accrued Clinical Expenses
When preparing our consolidated financial statements,
As a smaller reporting company, we are
requiredpermitted to
estimatecomply with scaled-back disclosure obligations in our
accrued clinical expenses. This process involves reviewing open contractsSEC filings compared to other issuers, including with respect to disclosure obligations regarding executive compensation in our periodic reports and
communicating with our personnelproxy statements. We have elected to
identify services that have been performed on our behalf and estimatingadopt the
level of service performed and the associated cost incurred for the service when we have not yet been invoiced or
otherwise notified of actual cost. Payments under some of the contracts we have with third parties depend on factors, such as successful enrollment of certain numbers of patients, site initiation and the completion of clinical trial milestones.
When accruing clinical expenses, we estimate the time period over which services will be performed and the level of effort to be expended in each period. If possible, we obtain information regarding unbilled services directly from our service providers. However, we may be required to estimate the cost of these services based only on informationaccommodations available to us. If we underestimate or overestimate the cost associated with a trial or service at a given point in time, adjustments to research and development expenses may be necessary in future periods. Historically, our estimated accrued clinical expenses have approximated actual expense incurred.
Stock-Based Compensation
We account for stock-based compensation awards in accordance with the Financial Accounting Standards Board Accounting Standards Codification, or ASC, Topic 718, Compensation—Stock Compensation, or ASC 718 (see Note 2 of our Consolidated Financial Statements). ASC 718 requires all stock-based compensation awards to be recognized as expense based on their grant date fair values. We recognized expenses over the requisite service period, which is generally the vesting period of the award under the straight-line method. We account for forfeitures as they occur. We record the expense for stock-based compensation awards subject to performance-based milestone vesting over the remaining service period when management determines that achievement of the milestone is probable. Management evaluates when the achievement of a performance-based milestone is probable based on the expected satisfaction of the performance conditions at eachsmaller reporting date.
We measure the grant-date fair value based on the Black-Scholes option-pricing model, which uses subjective assumptions and other inputs. These assumptions and inputs include:
Expected Volatility. Due to the lack of sufficient volatility data, the expected volatility is estimated using weighted average measures of the historical volatility of a representative group of small, publicly traded drug development companies, at a similar stage of development as ourselves.
Expected Term. The expected term of the options outstanding is determined using the “simplified” method for “plain vanilla” options based on the mid-point between the vesting date and the end of the contractual term as prescribed by Staff Accounting Bulletin No. 107, Share-Based Payment.
Risk-Free Interest Rate. The risk-free interest rate is based on U.S. Treasury notes with remaining terms similar to the expected term of the option.
Expected Dividends. The dividend yield assumption is zero since we have never paid cash dividends and do not plan to pay cash dividends in the foreseeable future.
Revenue Recognition
We recognize revenue under sublicense agreements in accordance with the ASC 606. We currently have one such agreement, the Angelini License Agreement. The terms of the agreement within this scope may contain multiple performance obligations, including but not limited to:
•reduced disclosure obligations regarding executive compensation arrangements; and
• being permitted to
licensesprovide only two years of audited financial statements, in addition to any required unaudited interim financial statements, with correspondingly reduced “Management’s Discussion and
researchAnalysis of Financial Condition and
development activities. ASC 606 requires that we evaluate these agreements to determine the distinct performance obligations. Non-refundable, up-front fees that are not contingent on any future performance and require no consequential continuing involvement by us, are recognized as revenue when the license term commences and the licensed data, technology or product is delivered. We defer recognitionResults of
non-refundable upfront fees if the performance obligations are not satisfied.Prior to recognizing revenue, we make estimates of the transaction price, including variable consideration that is subject to a constraint. Amounts of variable consideration are included in the transaction price to the extent that it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved. Variable consideration may include all or portions of upfront license fees, payments for research and development activities, reimbursement of certain third-party costs, payments based upon the achievement of specified milestones, royalty payments based on product sales derived from collaboration and other payments.
If there are multiple distinct performance obligations, we allocate the transaction price to each distinct performance obligation based on its relative standalone selling price. For the Angelini License Agreement, the transaction price was allocated based on the standalone selling prices of the license and ongoing trials. The portion of the upfront payment allocated to the license was recognized in full as it was non-refundable and not contingent on any future performance and requires no consequential continuing involvement by the Company. Revenue related to ongoing trials is recognized by measuring the progress toward complete satisfaction of the performance obligations over time based on the portion of estimated total trial costs to be incurred. Milestone payments are considered contingent variable consideration which are not accounted for until the contingencies are met.
Operations” disclosure.
Item 7A. Quantitative and QualitativeQualitative Disclosures About Market Risk.Risk
The primary objectives of our investment activities are to ensure liquidity and to preserve capital. As of December 31, 2020,2023, we had cash, and cash equivalents and marketable securities of $72.0 million that were held in an interest-bearing money market account.$105.8 million. Our primary exposure to market risk is interest rate sensitivity, which is affected by changes in the general level of U.S. interest rates. Due to the short-term maturities of our cash equivalents and marketable securities and the low risk profile of our investments, an immediate 100 basis point change in interest rates would not have a material effect on the fair market value of our cash equivalents.equivalents and marketable securities. To minimize the risk in the future, we intend to maintain our portfolio of cash equivalents and marketable securities in institutional market funds that are comprised of U.S. Treasury and U.S. Treasury-backed repurchase agreements.agreements as well as treasury notes and high quality short-term corporate bonds.
Item 8.Financial Statements and Supplementary Data Our financial statements, together with the report of our independent registered public accounting firm, appear in this Annual Report on Form 10-K beginning on page F-1.
Item 9.Changes in and Disagreements with Accountants on Accounting and Financial Disclosure None.
Item 9A. Controls and
Procedures.Procedures
Management’s Evaluation of our Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in the reports that we file or submit under the Securities Exchange Act of 1934, as amended (the “Exchange Act”) is (1) recorded, processed, summarized, and reported within the time periods specified in the SEC’s rules and forms and (2) accumulated and communicated to our management, including our principal executive officer and principal financial officer, to allow timely decisions regarding required disclosure.
As of December 31,
2020,2023, our management, with the participation of our principal executive officer and principal financial officer, evaluated the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act). Our management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives, and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Our principal executive officer and principal financial officer have concluded based upon the evaluation described above that, as of December 31,
20202023 our disclosure controls and procedures were effective at the reasonable assurance level.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Rules 13a-15(f) and 15d-15(f) of the Exchange Act. Our management, under the supervision and with the participation of our Chief Executive Officer and Principal Financial and Accounting Officer, conducted an evaluation of the effectiveness of our internal control over financial reporting as of December 31,
20202023 based on the framework in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 Framework). Based on the results of its evaluation, management concluded that our internal control over financial reporting was effective as of December 31,
2020.2023.
Attestation Report of the Registered Public Accounting Firm
This Annual Report on Form 10-K does not include an attestation report
ofon our internal control over financial reporting from our registered public accounting firm due to an exemption
established byas a smaller reporting company and as a non-accelerated filer for the
JOBS Act for “emerging growth companies.”year ended December 31, 2023.
Changes in Internal Control over Financial Reporting
There have been no changes in
our internal
controlcontrols over financial reporting during our most recent quarter ended December 31,
20202023, that materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information
.None.
During the three months ended December 31, 2023, no director or officer of the Company adopted or terminated a “Rule 10b5-1 trading arrangement” or “non-Rule 10b5-1 trading arrangement,” as each term is defined in Item 408(a) of Regulation S-K.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections
Not Applicable.
PART III
Certain information required by Part III is omitted from this Annual Report because we will file with the SEC a definitive proxy statement pursuant to Regulation 14A, or the 2024 Proxy Statement, no later than 120 days after the end of our fiscal year, and certain information included therein is incorporated herein by reference.
Item 10. Directors, Executive Officers, and Corporate Governance
The information required by this item is incorporated by reference to the information set forth in the sections titled “Proposal 1 – Election of Directors,” “Executive Officers,” and “Information Regarding the Board and Corporate Governance” and “Delinquent Section 16(a) Reports,” if any, in our 20212024 Proxy Statement.
Information regarding our Code of Business Conduct and Ethics, or the Code of Conduct, required by this item will be contained in our 2024 Proxy Statement under the caption “Information Regarding the Board and Corporate Governance – Code of Business Conduct and Ethics,” and is hereby incorporated by reference. If we make any substantive amendments to the Code of Conduct or grant any waiver from a provision of the Code of Conduct to any executive officer or director, we will promptly disclose the nature of the amendment or waiver on its website. The full text of our Code of Conduct is available at the Investors section of our website at www.ovidrx.com. The reference to our website address does not constitute incorporation by reference of the information contained at or available through our website, and you should not consider it to be a part of this Annual Report.
Item 11. Executive Compensation
The information required by this item is incorporated by reference to the information set forth in the section titled “Executive Officer and Director Compensation” in our 20212024 Proxy Statement.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The information required by this item is incorporated by reference to the information set forth in the section titled “Security Ownership of Certain Beneficial Owners and ManagementManagement” and “Equity Compensation Plan Information” in our 20212024 Proxy Statement.
Item 13. Certain Relationships and Related Transactions and Director Independence
The information required by this item is incorporated by reference to the information set forth in the section titled “Transactions with Related Persons” and “Information Regarding the Board and Corporate Governance – Board Independence” in our 20212024 Proxy Statement.
Item 14. Principal Accountant Fees and Services
The information required by this item is incorporated by reference to the information set forth in
Proposal 3 under the section titled “Independent Registered Public Accounting Firm Fees”
and “Pre-Approval Policies and Procedures” contained in our
20212024 Proxy Statement.
PART IV
Item 15.
Exhibits,Exhibit and Financial Statements and Schedules
(a)(1) Financial Statements.
The response to this portion of Item 15 is set forth under Item 8 hereof.
(a)(2) Financial Statement Schedules.
All schedules have been omitted because they are not required or because the required information is given in the Financial Statements or Notes thereto.
The exhibits listed below are filed as part of this Annual Report on Form 10-K.
| | | | | | | | |
| 10.7+ | | |
| | |
10.8+ | | |
| | |
10.9+ | | |
| | |
10.10+ | | |
| | |
10.11+ | | |
| | |
10.12+ | | |
| | |
10.13+ | | |
| | |
10.14+ | | |
| | |
10.15+ | | |
| | |
10.16+ | | |
| | |
10.17+ | | Executive Employment Agreement between the Company and Jeff Rona, effective September 30, 2020
|
| | |
10.18+
| | Executive Employment Agreement between the Company and Jason Tardio, effective October 21, 2019
|
| | |
10.19+
| | |
| | |
10.20†
| | License Agreement by and between H. Lundbeck A/S and the Company, dated March 25, 2015 (incorporated herein by reference to Exhibit 10.16 to the Company’s Registration Statement on Form S-1 (File No. 333-217245), filed with the Commission on May 2, 2017).
|
| | |
10.21†
| | Collaboration and License Agreement, by and between the Company and Takeda Pharmaceutical Company Limited, effective January 6, 2017 (incorporated herein by reference to Exhibit 10.18 to the Company’s Registration Statement on Form S-1 (File No. 333-217245), filed with the Commission on April 10, 2017).
|
| | |
10.22†
| | Series B-1 Preferred Stock Purchase Agreement, by and between the Company and Takeda Pharmaceutical Company Limited, dated January 6, 2017 (incorporated herein by reference to Exhibit 10.19 to the Company’s Registration StatementAnnual Report on Form S-110-K (File No. 333-217245)001-38085), filed with the Commission on April 10, 2017)March 15, 2021).
|
| | |
10.23†
10.18+ | | |
| | |
10.24^
10.19+ | | |
| | |
| 10.20† | | |
| | |
| 10.21^ | | |
| | | | | | | | |
| | |
31.1 | | |
| | |
31.2 | | |
| | |
32.1*
32.1 | | |
| | |
| 97 | | |
| | |
| 101.INS | | Inline XBRL Instance Document |
| | |
101.SCH | | Inline XBRL Taxonomy Extension Schema Document |
| | |
101.CAL | | Inline XBRL Taxonomy Extension Calculation Linkbase Document |
| | |
101.DEF | | Inline XBRL Taxonomy Extension Definition Linkbase Document |
| | |
101.LAB | | Inline XBRL Taxonomy Extension Label Linkbase Document |
| | |
101.PRE | | Inline XBRL Taxonomy Extension Presentation Linkbase Document |
| *
| |
| 104 | | Furnished herewith
Cover Page Interactive Data File (formatted as Inline XBRL and not deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and shall not be deemed to be incorporated by reference into any filing under the Securities Act of 1933, as amended, or the Exchange Act (whether made before or after the date of the Form 10-K), irrespective of any general incorporation language contained in such filing.within Exhibit 101) |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
+
| Indicates a management contract or compensatory plan.
|
* Furnished herewith and not deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and shall not be deemed to be incorporated by reference into any filing under the Securities Act of 1933, as amended, or the Exchange Act (whether made before or after the date of the Form 10-K), irrespective of any general incorporation language contained in such filing.†
| Confidential treatment has been granted for certain portions of this exhibit. These portions have been omitted and filed separately with the SEC.
|
+ Indicates a management contract or compensatory plan.^
| Pursuant to Item 601(b)(10)(iv) of Regulation S-K promulgated by the Securities and Exchange Commission, certain portions of this exhibit have been redacted. The Registrant hereby agrees to furnish supplementally to the Securities and Exchange Commission, upon its request, an unredacted copy of this exhibit.
|
† Confidential treatment has been granted for certain portions of this exhibit. These portions have been omitted and filed separately with the SEC.^ Pursuant to Item 601(b)(10)(iv) of Regulation S-K promulgated by the Securities and Exchange Commission, certain portions of this exhibit have been redacted. The Registrant hereby agrees to furnish supplementally to the Securities and Exchange Commission, upon its request, an unredacted copy of this exhibit.
Item 16. Form 10-K Summary
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report on Form 10-K to be signed on its behalf by the undersigned thereunto duly authorized.
| | | | | | | | |
| OVID THERAPEUTICS INC. |
| | | | |
Date: March 15, 2021 8, 2024 | By: | By:
| | /s/ Jeremy M. Levin |
| | | | Jeremy M. Levin |
| | | | (Principal Executive Officer) |
| | |
| Date: March 8, 2024 | By: | | | /s/ Jeffrey Rona |
| Date: March 15, 2021
| By:
| | /s/ Timothy Daly
|
| | | | Timothy Daly
Executive Vice President, Finance, Corporate Controller
Jeffrey Rona Chief Business & TreasurerFinancial Officer (Principal Financial and Accounting Officer) |
POWER OF ATTORNEY
Each person whose individual signature appears below hereby authorizes and appoints Jeremy M. Levin, DPhil, MB BChir and Jeffrey Rona, and each of them, with full power of substitution and resubstitution and full power to act without the other, as his or her true and lawful attorney-in-fact and agent to act in his or her name, place and stead and to execute in the name and on behalf of each person, individually and in each capacity stated below, and to file any and all amendments to this report on Form 10-K, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing, ratifying and confirming all that said attorneys-in-fact and agents or any of them or their or his or her substitute or substitutes may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| | | | | | | | | | | | | | |
| Signature | | Title | | Date |
| POWER OF ATTORNEY
Each person whose individual signature appears below hereby authorizes and appoints Jeremy M. Levin, DPhil, MB BChir and Timothy Daly, and each of them, with full power of substitution and resubstitution and full power to act without the other, as his or her true and lawful attorney-in-fact and agent to act in his or her name, place and stead and to execute in the name and on behalf of each person, individually and in each capacity stated below, and to file any and all amendments to this report on Form 10-K, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing, ratifying and confirming all that said attorneys-in-fact and agents or any of them or their or his or her substitute or substitutes may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature
| | Title
| Date
|
| | |
/s/ Jeremy M. Levin, DPhil, MB BChir | | Chief Executive Officer and Director (Principal Executive Officer) | | March 8, 2024 |
| Jeremy M. Levin, DPhil, MB BChir | | | Chief Executive Officer and Director
(Principal Executive Officer)
| March 15, 2021
|
| | | | |
/s/ Timothy Daly Timothy Daly
Jeffrey Rona | | Executive Vice President, Finance
Chief Business and Corporate ControllerFinancial Officer (Principal Financial and Accounting Officer) | | March 15, 2021 8, 2024 |
| Jeffrey Rona | | | |
| | | | |
| /s/ Karen Bernstein, PhD | | Director | | March 8, 2024 |
| Karen Bernstein, PhD | | | | Director
| March 15, 2021
|
| | | | |
/s/ Barbara Duncan | | Director | | Director
| March 15, 2021 8, 2024 |
Barbara Duncan | | | | |
| | | | |
/s/ Bart Friedman Bart Friedman
| | Director | | Director
| March 15, 2021 8, 2024 |
| Bart Friedman | | | | |
| | | | |
| /s/ Kevin Fitzgerald, PhD | | Director | | March 8, 2024 |
Kevin Fitzgerald, PhD | | | | |
| | | | |
/s/ Douglas Williams, PhDDouglas Williams, PhD
Robert Michael Poole, MD, FACP | | Director | | Director
March 8, 2024 |
| Robert Michael Poole, MD, FACP | | | March 15, 2021
| |
Index to Consolidated Financial Statements
| | | | | |
| | F-2
| |
| | F-3
| |
| | F-4
| |
| | F-5
| |
| | F-6
| |
| | F-7
| |
| | F-8–F-23
| |
Report of Independent Registered Public Accounting Firm
To the Stockholders and Board of Directors
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Ovid Therapeutics Inc. and subsidiary (the Company) as of December 31,
20202023 and
2019,2022, the related consolidated statements of operations, comprehensive
loss,income (loss), changes in stockholders’ equity, and cash flows for the years then ended, and the related notes (collectively, the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31,
20202023 and
2019,2022, and the results of its operations and its cash flows for the years then ended, in conformity with U.S. generally accepted accounting principles.
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
. Critical Audit Matters
Critical audit matters are matters arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. We determined that there are no critical audit matters.
We have served as the Company’s auditor since 2015.
PART I—FINANCIALFINANCIAL INFORMATION
Item 1. Financial Statements.
Consolidated Balance Sheets
| | December 31, | | | December 31, | |
| | 2020 | | | 2019 | |
| December 31,
2023 | |
| December 31,
2023 | |
| December 31,
2023 | | | December 31,
2022 |
Assets | | | | | | | | |
Current assets: | | | | | | | | |
| Current assets: | |
| Current assets: | |
Cash and cash equivalents | | $ | 72,033,930 | | | $ | 41,897,144 | |
Short-term investments | | | - | | | | 34,841,969 | |
Related party receivable | | | 141,763 | | | | 1,131,146 | |
| Cash and cash equivalents | |
| Cash and cash equivalents | |
| Marketable securities | |
Prepaid expenses and other current assets | | | 2,667,508 | | | | 1,942,933 | |
Total current assets | | | 74,843,201 | | | | 79,813,192 | |
| | | | | | | | |
Long-term prepaid expenses | | | 477,171 | | | | 359,539 | |
Security deposit | | | 150,626 | | | | 135,390 | |
| Long-term equity investments | |
| Long-term equity investments | |
| Long-term equity investments | |
| Restricted cash | |
| Right-of-use asset, net | |
Property and equipment, net | | | 135,620 | | | | 68,363 | |
Other assets | | | 318,900 | | | | 467,247 | |
Total assets | | $ | 75,925,518 | | | $ | 80,843,731 | |
| | | | | | | | |
Liabilities and Stockholders' Equity | | | | | | | | |
| Liabilities and Stockholders' Equity | |
| Liabilities and Stockholders' Equity | |
| Current liabilities: | |
| Current liabilities: | |
Current liabilities: | | | | | | | | |
Accounts payable | | $ | 5,446,206 | | | $ | 3,256,098 | |
| Accounts payable | |
| Accounts payable | |
Accrued expenses | | | 12,032,685 | | | | 7,266,706 | |
Related party payable | | | 2,370,992 | | | | 10,804 | |
Deferred revenue - current | | | 2,212,892 | | | | - | |
| Current portion, lease liability | |
Total current liabilities | | | 22,062,775 | | | | 10,533,608 | |
Related party payable - noncurrent | | | 61,200 | | | | 286,562 | |
Deferred revenue, net of current portion | | | 10,169,887 | | | | - | |
| | Long-term liabilities: | |
| Long-term liabilities: | |
| Long-term liabilities: | |
| Lease liability | |
| Lease liability | |
| Lease liability | |
| Royalty monetization liability | |
Total liabilities | | | 32,293,862 | | | | 10,820,170 | |
| | | | | | | | |
| | | | | | | | |
Stockholders' equity: | | | | | | | | |
Preferred stock, $0.001 par value; 10,000,000 shares authorized; Series A convertible preferred stock, 10,000 shares designated, 3,250 and 7,762 shares issued and outstanding at December 31, 2020 and 2019, respectively | | | 3 | | | | 8 | |
Common stock, $0.001 par value; 125,000,000 shares authorized; 65,743,170 and 54,710,322 shares issued and outstanding at December 31, 2020 and 2019, respectively | | | 65,743 | | | | 54,711 | |
| Stockholders' equity: | |
| Stockholders' equity: | |
| Preferred stock, $0.001 par value; 10,000,000 shares authorized; Series A convertible preferred stock, 10,000 shares designated, 1,250 shares issued and outstanding at December 31, 2023 and 2022 | |
| Preferred stock, $0.001 par value; 10,000,000 shares authorized; Series A convertible preferred stock, 10,000 shares designated, 1,250 shares issued and outstanding at December 31, 2023 and 2022 | |
| Preferred stock, $0.001 par value; 10,000,000 shares authorized; Series A convertible preferred stock, 10,000 shares designated, 1,250 shares issued and outstanding at December 31, 2023 and 2022 | |
| Common stock, $0.001 par value; 125,000,000 shares authorized; 70,691,992 and 70,466,885 shares issued and outstanding at December 31, 2023 and 2022, respectively | |
Additional paid-in-capital | | | 337,758,007 | | | | 283,122,894 | |
Accumulated other comprehensive income | | | - | | | | 2,469 | |
| Accumulated other comprehensive income (loss) | |
Accumulated deficit | | | (294,192,097 | ) | | | (213,156,521 | ) |
Total stockholders' equity | | | 43,631,656 | | | | 70,023,561 | |
Total liabilities and stockholders' equity | | $ | 75,925,518 | | | $ | 80,843,731 | |
See accompanying notes to these consolidated financial statements
Consolidated Statements of Operations
| | For the Year Ended December 31, | | | For the Year Ended December 31, | |
| | 2020 | | | 2019 | |
| For the Year Ended December 31, 2023 | | | For the Year Ended December 31, 2023 | | For the Year Ended December 31,
2022 |
Revenue: | | | | | | | | |
License and other revenue | | $ | 12,617,221 | | | $ | - | |
| License and other revenue | |
| License and other revenue | |
| | Total revenue | |
| Total revenue | |
| Total revenue | |
Operating expenses: | | | | | | | | |
| Research and development | |
| Research and development | |
Research and development | | | 63,417,394 | | | | 42,157,641 | |
General and administrative | | | 30,630,804 | | | | 19,251,826 | |
Total operating expenses | | | 94,048,198 | | | | 61,409,467 | |
Loss from operations | | | (81,430,977 | ) | | | (61,409,467 | ) |
Other income, net | | | 395,401 | | | | 948,224 | |
| Other income (expense), net | |
| Loss before provision for income taxes | |
| Provision for income taxes | |
Net loss | | $ | (81,035,576 | ) | | $ | (60,461,243 | ) |
Net loss attributable to common stockholders | | $ | (81,035,576 | ) | | $ | (60,461,243 | ) |
Net loss per share attributable to common stockholders, basic and diluted | | $ | (1.39 | ) | | $ | (1.54 | ) |
Weighted-average common shares outstanding basic and diluted | | | 58,451,293 | | | | 39,217,223 | |
| Net loss per share, basic | |
| Net loss per share, diluted | |
| Weighted-average common shares outstanding, basic | |
| Weighted-average common shares outstanding, diluted | |
See accompanying notes to these consolidated financial statements
Consolidated Statements of Comprehensive
LossIncome (Loss) | | For the Year Ended December 31, | | | For the Year Ended December 31, | |
| | 2020 | | | 2019 | |
| For the Year Ended December 31,
2023 | | | For the Year Ended December 31,
2023 | | For the Year Ended December 31,
2022 |
Net loss | | $ | (81,035,576 | ) | | $ | (60,461,243 | ) |
Other comprehensive (loss) income: | | | | | | | | |
Unrealized (loss) gain on available-for-sale securities | | | (2,469 | ) | | | 4,298 | |
| Other comprehensive gain (loss): | |
| Unrealized gain (loss) on available-for-sale securities | |
| Unrealized gain (loss) on available-for-sale securities | |
| Unrealized gain (loss) on available-for-sale securities | |
Comprehensive loss | | $ | (81,038,045 | ) | | $ | (60,456,945 | ) |
See accompanying notes to these consolidated financial statements
Consolidated Statement of Changes in Stockholders’ Equity
| | Convertible Preferred Stock | | | Common Stock | | | Additional Paid-In | | | Accumulated Other Comprehensive | | | Accumulated | | | | | |
| | Shares | | | Amount | | | Shares | | | Amount | | | Capital | | | Income/(Loss) | | | Deficit | | | Total | |
Balance, December 31, 2019 | | | 7,762 | | | $ | 8 | | | | 54,710,322 | | | $ | 54,711 | | | $ | 283,122,894 | | | $ | 2,469 | | | $ | (213,156,521 | ) | | $ | 70,023,561 | |
ATM costs | | | - | | | | - | | | | - | | | | - | | | | (114,936 | ) | | | - | | | | - | | | | (114,936 | ) |
Issuance of common stock from employee stock purchase plan | | | - | | | | - | | | | 105,464 | | | | 105 | | | | 203,361 | | | | - | | | | - | | | | 203,466 | |
Issuance of common stock from exercise of stock options | | | - | | | | - | | | | 165,384 | | | | 165 | | | | 300,656 | | | | | | | | | | | | 300,821 | |
Conversion of series A convertible preferred stock to common stock | | | (4,512 | ) | | | (5 | ) | | | 4,512,000 | | | | 4,512 | | | | (4,507 | ) | | | - | | | | - | | | | - | |
Proceeds from August 2020 Offering, net of underwriting costs and commissions | | | - | | | | - | | | | 6,250,000 | | | | 6,250 | | | | 46,725,185 | | | | - | | | | - | | | | 46,731,435 | |
Stock-based compensation expense | | | - | | | | - | | | | - | | | | - | | | | 7,525,354 | | | | - | | | | - | | | | 7,525,354 | |
Other comprehensive loss | | | - | | | | - | | | | - | | | | - | | | | - | | | | (2,469 | ) | | | - | | | | (2,469 | ) |
Net loss | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | (81,035,576 | ) | | | (81,035,576 | ) |
Balance, December 31, 2020 | | | 3,250 | | | $ | 3 | | | | 65,743,170 | | | $ | 65,743 | | | $ | 337,758,007 | | | $ | - | | | $ | (294,192,097 | ) | | $ | 43,631,656 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Convertible Preferred Stock | | | Common Stock | | | Additional Paid-In | | | Accumulated Other Comprehensive | | | Accumulated | | | | | |
| | Shares | | | Amount | | | Shares | | | Amount | | | Capital | | | Income/(Loss) | | | Deficit | | | Total | |
Balance, December 31, 2018 | | | - | | | $ | - | | | | 24,654,114 | �� | | $ | 24,654 | | | $ | 191,477,598 | | | $ | (1,829 | ) | | $ | (152,695,278 | ) | | $ | 38,805,145 | |
Proceeds from February Offering, net of underwriting costs and commissions | | | 2,500 | | | | 3 | | | | 13,993,778 | | | | 13,994 | | | | 30,506,838 | | | | - | | | | - | | | | 30,520,835 | |
Issuance of common stock from employee stock purchase plan | | | - | | | | - | | | | 80,542 | | | | 81 | | | | 131,814 | | | | - | | | | - | | | | 131,895 | |
Conversion of common stock to series A convertible preferred stock | | | 1,262 | | | | 1 | | | | (1,262,000 | ) | | | (1,262 | ) | | | 1,261 | | | | - | | | | - | | | | - | |
Proceeds from October Offering, net of underwriting costs and commissions | | | 4,000 | | | | 4 | | | | 10,350,000 | | | | 10,350 | | | | 33,519,919 | | | | - | | | | - | | | | 33,530,273 | |
Proceeds from ATM Offerings, net of underwriting costs and commissions | | | - | | | | - | | | | 6,893,888 | | | | 6,894 | | | | 22,279,672 | | | | - | | | | - | | | | 22,286,566 | |
Stock-based compensation expense | | | - | | | | - | | | | - | | | | - | | | | 5,205,792 | | | | - | | | | - | | | | 5,205,792 | |
Other comprehensive income | | | - | | | | - | | | | - | | | | - | | | | - | | | | 4,298 | | | | - | | | | 4,298 | |
Net loss | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | (60,461,243 | ) | | | (60,461,243 | ) |
Balance, December 31, 2019 | | | 7,762 | | | $ | 8 | | | | 54,710,322 | | | $ | 54,711 | | | $ | 283,122,894 | | | $ | 2,469 | | | $ | (213,156,521 | ) | | $ | 70,023,561 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Convertible
Preferred Stock | | Common Stock | | Additional
Paid-In
Capital | | Accumulated Other Comprehensive Income (Loss) | | Accumulated
Deficit | | Total |
| Shares | | Amount | | Shares | | Amount | | | | |
| Balance, December 31, 2022 | 1,250 | | | $ | 1 | | | 70,466,885 | | | $ | 70,467 | | | $ | 357,770,825 | | | $ | (42,187) | | | $ | (225,526,542) | | | $ | 132,272,564 | |
| Issuance of common stock from exercise of stock options and employee stock purchase plan | — | | | — | | | 225,107 | | | 225 | | | 534,976 | | | — | | | — | | | 535,201 | |
| Stock-based compensation expense | — | | | — | | | — | | | — | | | 7,285,192 | | | — | | | — | | | 7,285,192 | |
| Other comprehensive income | — | | | — | | | — | | | — | | | — | | | 42,889 | | | — | | | 42,889 | |
| Net loss | — | | | — | | | — | | | — | | | — | | | — | | | (52,338,959) | | | (52,338,959) | |
| Balance, December 31, 2023 | 1,250 | | | $ | 1 | | | 70,691,992 | | | $ | 70,692 | | | $ | 365,590,993 | | | $ | 702 | | | $ | (277,865,501) | | | $ | 87,796,887 | |
| | | | | | | | | | | | | | | |
| Convertible
Preferred Stock | | Common Stock | | Additional
Paid-In
Capital | | Accumulated Other Comprehensive Income (Loss) | | Accumulated
Deficit | | Total |
| Shares | | Amount | | Shares | | Amount | | | | |
| Balance, December 31, 2021 | 1,250 | | | $ | 1 | | | 70,364,912 | | | $ | 70,359 | | | $ | 351,033,589 | | | $ | — | | | $ | (171,357,513) | | | $ | 179,746,436 | |
| Issuance of common stock from exercise of stock options and employee stock purchase plan | — | | | — | | | 101,973 | | | 108 | | | 180,550 | | | — | | | — | | | 180,658 | |
| Stock-based compensation expense | — | | | — | | | — | | | — | | | 6,556,686 | | | — | | | — | | | 6,556,686 | |
| Other comprehensive loss | — | | | — | | | — | | | — | | | — | | | (42,187) | | | — | | | (42,187) | |
| Net loss | — | | | — | | | — | | | — | | | — | | | — | | | (54,169,029) | | | (54,169,029) | |
| Balance, December 31, 2022 | 1,250 | | | $ | 1 | | | 70,466,885 | | | $ | 70,467 | | | $ | 357,770,825 | | | $ | (42,187) | | | $ | (225,526,542) | | | $ | 132,272,564 | |
See accompanying notes to these consolidated financial statements
Consolidated Statements of Cash Flows
| | Year Ended December 31, | | | Year Ended December 31, | |
| | 2020 | | | 2019 | |
Cash flows from operating activities: | | | | | | | | |
Net loss | | $ | (81,035,576 | ) | | $ | (60,461,243 | ) |
Adjustments to reconcile net loss to cash used in operating activities: | | | | | | | | |
Stock-based compensation expense | | | 7,525,354 | | | | 5,205,792 | |
Depreciation and amortization expense | | | 306,848 | | | | 255,002 | |
Change in accrued interest and accretion of discount on short-term investments | | | (199,408 | ) | | | (29,633 | ) |
Change in operating assets and liabilities: | | | | | | | | |
Prepaid expenses and other current assets | | | (724,575 | ) | | | 224,458 | |
Security deposit | | | (15,236 | ) | | | (13,235 | ) |
Related party receivable | | | 989,383 | | | | (531,042 | ) |
Long-term prepaid expenses | | | (117,632 | ) | | | 2,438,022 | |
Accounts payable | | | 2,333,716 | | | | (653,953 | ) |
Accrued expenses | | | 4,835,607 | | | | 2,177,844 | |
Deferred revenue | | | 12,382,779 | | | | - | |
Related party payable | | | 2,134,826 | | | | 297,366 | |
Net cash used in operating activities | | | (51,583,914 | ) | | | (51,090,622 | ) |
| | | | | | | | |
Cash flows from investing activities: | | | | | | | | |
Purchases of short-term investments | | | (9,961,092 | ) | | | (34,797,004 | ) |
Proceeds from maturities of short-term investments | | | 45,000,000 | | | | 5,000,000 | |
Purchase of property and equipment | | | (127,240 | ) | | | (57,156 | ) |
Software development and other assets | | | (263,440 | ) | | | (186,889 | ) |
Net cash provided by (used in) investing activities | | | 34,648,228 | | | | (30,041,049 | ) |
| | | | | | | | |
Cash flows from financing activities: | | | | | | | | |
Proceeds from August 2020 Offering, net of offering expenses | | | 46,731,435 | | | | - | |
Proceeds from February and October 2019 Offerings, net of offering expenses | | | - | | | | 64,120,736 | |
Proceeds from ATM Offerings, net of offering expenses | | | - | | | | 22,286,566 | |
ATM costs | | | (163,250 | ) | | | - | |
Proceeds from employee stock purchase plan | | | 203,466 | | | | 131,895 | |
Proceeds from exercise of options | | | 300,821 | | | | - | |
Net cash provided by financing activities | | | 47,072,472 | | | | 86,539,197 | |
| | | | | | | | |
Net increase in cash and cash equivalents | | | 30,136,786 | | | | 5,407,526 | |
Cash and cash equivalents, at beginning of period | | | 41,897,144 | | | | 36,489,618 | |
Cash and cash equivalents, at end of period | | $ | 72,033,930 | | | $ | 41,897,144 | |
| | | | | | | | |
Non-cash investing and financing activities: | | | | | | | | |
Software development and other costs in accrued expenses and accounts payable | | $ | — | | | $ | 164,922 | |
Offering costs in accrued expenses and accounts payable | | $ | 21,314 | | | $ | 69,628 | |
| | | | | | | | | | | |
| | | |
| Year Ended December 31,
2023 | | Year Ended December 31,
2022 |
| Cash flows from operating activities: | | | |
| Net loss | $ | (52,338,959) | | | $ | (54,169,029) | |
| Adjustments to reconcile net loss to cash used in operating activities: | | | |
| Non-cash consideration received in licensing agreement transaction | — | | | (945,366) | |
| Unrealized (gain) loss on equity investments | (2,003,073) | | | 454,811 | |
| Change in accrued interest income and accretion of discount on marketable securities | (2,172,254) | | | (1,211,311) | |
| Stock-based compensation expense | 7,285,192 | | | 6,556,686 | |
| Depreciation and amortization expense | 568,282 | | | 512,505 | |
| Amortization of right-of-use asset | 1,028,293 | | | 869,100 | |
| Change in lease liability | (533,946) | | | 936,927 | |
| Change in operating assets and liabilities: | | | |
| Prepaid expenses and other current assets | (1,385,052) | | | 109,292 | |
| Accounts payable | 1,750,026 | | | (5,174,136) | |
| Accrued expenses | 2,020,566 | | | (3,166,606) | |
| Net cash used in operating activities | (45,780,925) | | | (55,227,127) | |
| | | |
| Cash flows from investing activities: | | | |
| Purchase of marketable securities | (112,443,150) | | | (172,964,441) | |
| Sales/maturities of marketable securities | 120,000,000 | | | 90,000,000 | |
| Purchase of long-term equity investments | (10,000,000) | | | (2,500,000) | |
| Issuance of convertible short-term note receivable | — | | | (1,000,000) | |
| Purchases of property and equipment | (40,308) | | | (1,224,379) | |
| Software development and other costs | (97,147) | | | (194,397) | |
| Net cash used in investing activities | (2,580,605) | | | (87,883,217) | |
| | | |
| Cash flows from financing activities: | | | |
| Proceeds from exercise of options and employee stock purchase plan | 535,201 | | | 180,658 | |
| Proceeds from royalty monetization agreement | 30,000,000 | | | — | |
| Net cash provided by financing activities | 30,535,201 | | | 180,658 | |
| | | |
| | | |
| Net decrease in cash, cash equivalents and restricted cash | (17,826,329) | | | (142,929,686) | |
| Cash, cash equivalents and restricted cash, at beginning of period | 46,798,599 | | | 189,728,285 | |
| Cash, cash equivalents and restricted cash, at end of period | $ | 28,972,270 | | | $ | 46,798,599 | |
| | | |
| Non-cash investing and financing activities: | | | |
| Right-of-use asset in exchange for lease liability | $ | — | | | $ | 15,791,769 | |
| Conversion of short-term note receivable to long-term equity investment | $ | — | | | $ | 1,000,000 | |
See accompanying notes to these consolidated financial statements
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1
– NATURE OF OPERATIONSOvid Therapeutics Inc. (the “Company”) was incorporated under the laws of the state of Delaware,
commenced operations on April 1, 2014, and maintains its principal executive office in New York, New York. The Company
commenced operations on April 1, 2014 (date of inception). The Company is a biopharmaceutical company
focused exclusively on developing impactful medicines for patientsthat is dedicated to meaningfully improving the lives of people affected by certain epilepsies and
families livingbrain conditions with
rare neurological disorders.seizure symptoms.
Since its inception, the Company has devoted substantially all of its efforts to business development, research and development, recruiting management and technical staff, and raising capital, and has financed its operations through
the issuance of convertible preferred stock,
(“Preferred Stock”), common stock and other equity
instruments.instruments, the sale and/or licensing of certain assets and the licensing of certain intellectual property. The Company is subject to risks and uncertainties common to early-stage companies in the biotechnology industry, including, but not limited to, development and regulatory success, development by competitors of new technological innovations, dependence on key personnel, protection of proprietary technology, compliance with government regulations, and
the ability to secure additional capital to fund operations.
Historically, the
The Company’s major sources of cash have been
comprised oflicensing revenue, proceeds from various public and private offerings of its capital stock,
option exercises and interest income. As of December 31,
2020,2023, the Company had approximately
$72.0$105.8 million in cash,
cash equivalents and
cash equivalents.marketable securities. Since inception, the Company has generated
$12.6$222.8 million in revenue,
as part ofprimarily from the Company’s
royalty, license and
collaborationtermination agreement
(the “Angelini License(“RLT Agreement”) with
Angelini Pharma Rare Diseases AGTakeda Pharmaceutical Company Limited (“
Angelini”Takeda”).
TheHistorically, the Company has incurred recurring losses, has experienced recurring negative operating cash flows and
requireshas required significant cash resources to execute its business
plans. plans, which the Company expects will continue for the foreseeable future. The Company has an accumulated deficit of $294.2$277.9 million as of December 31, 2020,2023, working capital of
$52.8$98.1 million
and had cash outflows fromused in operating activities of $51.6$45.8 million for the year ended December 31, 2020.
2023.
The Company
has incurred operating losses since inceptionrecorded a net loss of $52.3 million during the year ended December 31, 2023 and expects to
continue to incur
net losses
in subsequent periods for at least the next several
years and ongoing operations areyears. The Company is highly dependent on
the Company’sits ability to
obtainfind additional sources of
funding.funding through either equity offerings, debt financings, collaborations, strategic alliances, licensing agreements or a combination of any such transactions. Management
has identified these conditions or events, which, considered in the aggregate, raise substantial doubt about our ability to continue as a going concern, including the riskbelieves that the
CompanyCompany’s existing cash, cash equivalents and marketable securities as of December 31, 2023 will be
unable to raise adequate additional capitalsufficient to fund
operationsits current operating plans through at least
the next 12 months from the date of filing of
thisthe Company’s Annual Report on Form 10-K.
Management’s responseAdequate additional funding may not be available to
these conditionsthe Company on acceptable terms or at all. The failure to raise capital as and
events is that the successful closing of the Takeda License and Termination Agreement with Takeda (see note 12) will provide the liquiditywhen needed
to alleviate the substantial doubt referred to above. As described in note 12, there are certain conditions to close the Takeda License and Termination Agreement, however management has determined that the likelihood of not closing in the first half of 2021 is remote. We have implemented business continuity plans designed to address and mitigate the impact of the COVID-19 pandemic on our business. The extent to which the ongoing COVID-19 pandemic impacts our business, our clinical development and regulatory efforts, our corporate development objectives and the value of and market for our common stock, will depend on future developments that are highly uncertain and cannot be predicted with confidence at this time, such as the ultimate duration of the pandemic, travel restrictions, quarantines, social distancing and business closure requirements in the U.S., Europe and other countries, and the effectiveness of actions taken globally to contain and treat the disease. The global economic slowdown, the overall disruption of global healthcare systems and the other risks and uncertainties associated with the pandemic could have a material adverse effectnegative impact on our business,the Company’s financial condition resultsand ability to pursue its business strategy. The Company may be required to delay, reduce the scope of operationsor eliminate research and growth prospects.
In addition, we aredevelopment programs, or obtain funds through arrangements with collaborators or others that may require the Company to relinquish rights to certain drug candidates that the Company might otherwise seek to develop or commercialize independently.
The Company is subject to other challenges and risks specific to ourits business and ourits ability to execute on ourits strategy, as well as risks and uncertainties common to companies in the pharmaceutical industry with development and commercial operations, including, without limitation, risks and uncertainties associated with: obtaining regulatory approval of our late-stage product candidates; delays or problems in the supply of our products,the Company's product candidates, loss of single source suppliers or failure to comply with manufacturing regulations; identifying, acquiring or in-licensing additional products or product candidates; pharmaceutical product development and the inherent uncertainty of clinical success; and the challenges of protecting and enhancing our intellectual property rights; complying with applicable regulatory requirements. In addition, to the extent the ongoing COVID-19 pandemic adversely affects our businessrequirements; and resultsobtaining regulatory approval of operations, it may also have the effect of heightening manyany of the other risks and uncertainties discussed above.Company's product candidates.
NOTE 2
– SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES(A) Basis of Presentation and Consolidation
The accompanying consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America (“GAAP”) and include the accounts of Ovid Therapeutics Inc. and its wholly-ownedwholly owned subsidiary, Ovid Therapeutics Hong Kong Limited. All intercompany transactions and balances have been eliminated in consolidation.
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reporting period. Actual results could differ materially from those estimates.
(C)
Risks and UncertaintiesMarketable Securities
Marketable securities consist of investments in U.S. treasury instruments which are considered available-for-sale securities. The Company
is subject to risks common to companies in the development stage including, but not limited to, dependency on the clinical and commercial success ofclassifies its
drug candidates, ability to obtain regulatory approval of its drug candidates, the need for substantial additional financing to achieve its goals, uncertainty of broad adoption of its approved products, if any, by physicians and consumers, and significant competition and untested manufacturing capabilities.(D) Deferred Transaction Costs
Deferred transaction costs, primarily consisted of direct incremental legal, accounting, and other fees related to the Company’s offering of its capital stock are capitalized as incurred. The deferred transaction costs are offset against proceeds upon the consummation of an offering.
(E) Comprehensive Loss
Comprehensive loss includes net loss as well as other changes in stockholders’ equity that result from transactions and economic events other than those with stockholders.
(F) Collaboration Arrangement
License and Collaboration Agreement with Takeda Pharmaceutical Company Limited
Under the terms of the current license and collaboration agreement with Takeda Ovid and Takeda are sharing 50/50 in the drug development, the Company records 50% of the development costs in research and development. When Ovid incurs the majority of the costs and Takeda transfers a payment to Ovid to equalize the costs, Ovid records the participation by Takeda as a reduction of its research and development expenses, as the parties under the collaboration are sharing in the costs and the payment represents reimbursement of costs by Takeda. When Takeda incurs the majority of the costs and Ovid transfers a payment to Takeda (to equalize the costs), Ovid records the participation in Takeda’s expenses as research and development costs in its statement of operations, as Ovid and Takeda are sharing in the research and development activities and this participation represents Ovid’s share of the research and development costs in the specific period.
(G) Cash and Cash Equivalents
The Company’s cash and cash equivalents consist of cash held in checking accounts and money market funds. The Company considers all highly liquid investments with an original maturity date of three months or less to be cash and cash equivalents. Accounts held at U.S. financial institutions are insured by the FDIC up to $250,000. Cash balances could exceed insured amounts at any given time.
(H) Short-term Investments
Short-term investments consist of debtmarketable securities with maturities greaterof less than three monthsone year from the balance sheet date of purchase.as current assets on its consolidated balance sheets. The Company classifies all its marketable securities with original maturities of less than three months as cash equivalents on its investments as available-for-sale securities. Debt securities are recorded at fair value with unrealizedconsolidated balance sheets. Unrealized gains and losses included inon these securities that are determined to be temporary are reported as a separate component of accumulated other comprehensive income (loss) in stockholders' equity.
(D) Restricted Cash
The Company classifies as restricted cash all cash pledged as collateral to secure long-term obligations and all cash for which use is otherwise limited by contractual provisions. Amounts are reported as non-current unless restrictions are expected to be released in the next 12 months.
(E) Long-term Equity Investments
Long-term equity investments consist of equity investments in the preferred shares of Gensaic, Inc., formerly M13 Therapeutics, Inc. (“Gensaic”), and Graviton Bioscience Corporation (“Graviton”), both privately held corporations. The preferred shares are not considered in-substance common stock, and the investments are accounted for at cost, with adjustments for observable changes in prices or impairments, and are classified within long-term equity investments on the consolidated balance sheets with adjustments recognized in other income (expense), net on the consolidated statements of operations. The Company has determined that these equity investments do not have a readily determinable fair value and elected the measurement alternative. Therefore, the carrying amount of the equity investments will be adjusted to fair value at the time of the next observable price change for the identical or similar investment of the same issuer, or when an impairment is recognized. Each reporting period, the Company performs a qualitative assessment to evaluate whether the investments are impaired. The assessment includes a review of recent operating results and trends, recent sales/acquisitions of the investees' securities, and other publicly available data. If an investment is determined to be impaired, the Company will then write it down to its estimated fair value. As of December 31, 2023 and 2022, the equity investment in Gensaic had a carrying value of $5.1 million. As of December 31, 2023, the equity investment in Graviton had a carrying value of $11.2 million, which reflects a $1.2 million unrealized gain recognized during the year and recorded in other income (expense), net, in the consolidated statements of operations due to an observable change in price.
Long-term equity investments also consist of an equity investment in the common shares of Marinus Pharmaceuticals, Inc. (“Marinus”) that was received as non-cash consideration via the terms of a licensing agreement executed between the two companies effective March 2022. The equity shares are marked-to-market at each reporting date with changes in the fair value being reflected in the carrying value of the investment on the Company's consolidated balance sheets and other income (expense), net on the Company's consolidated statements of operations. As of December 31, 2023 and 2022, the equity investment in Marinus had a carrying value of approximately $1.3 million and $0.5 million, respectively.
No impairments were recognized in the years ending December 31, 2023 and 2022.
(F) Note Receivable
On March 17, 2022, the Company issued a convertible promissory note with a principal amount of $1.0 million to Gensaic. The note included features that permitted the Company to acquire additional equity or to settle the note in cash. In August 2022, the Company executed an agreement with Gensaic which resulted in the conversion of the note into additional equity and was recorded as a componentlong-term equity investment in the consolidated balance sheets. The Company received interest on the convertible promissory note at the rate of stockholders’1.5% per annum through the date of conversion.
(G) Fair Value of Financial Instruments
Financial Accounting Standards Board guidance specifies a hierarchy of valuation techniques based on whether the inputs to those valuation techniques are observable or unobservable. Observable inputs reflect market data obtained
from independent sources, while unobservable inputs reflect market assumptions. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurement) and the lowest priority to unobservable inputs (Level 3 measurement).
The three levels of the fair value hierarchy are as follows:
•Level 1—Unadjusted quoted prices in active markets for identical assets or liabilities that the reporting entity has the ability to access at the measurement date. Level 1 primarily consists of financial instruments whose value is based on quoted market prices such as exchange-traded instruments and listed equities. The Company's Level 1 assets consisted of investments in a U.S. treasury money market fund and equity until realized. Realized gainssecurities totaling approximately $25.7 million and losses, amortization$42.5 million, respectively, as of December 31, 2023 and accretion2022.
•Level 2—Inputs other than quoted prices included within Level 1 that are observable for the asset or liability, either directly or indirectly (e.g., quoted prices of premiumssimilar assets or liabilities in active markets, or quoted prices for identical or similar assets or liabilities in markets that are not active). Level 2 includes financial instruments that are valued using models or other valuation methodologies. The Company's Level 2 assets consisted of U.S. treasury bills totaling approximately $78.8 million and discounts$84.1 million, respectively, as of December 31, 2023 and 2022.
•Level 3—Unobservable inputs for the asset or liability. Financial instruments are considered Level 3 when the fair values are determined using pricing models, discounted cash flows or similar techniques and at least one significant model assumption or input is unobservable. The Company's Level 3 liabilities consist of a royalty monetization liability totaling $30.0 million at December 31, 2023. There were no Level 3 assets or liabilities as of December 31, 2022.
The carrying amounts reported in the consolidated balance sheets for cash, cash equivalents and marketable securities, other current assets, accounts payable, and accrued expenses approximate their fair values based on the short-term maturity of these instruments.
(H) Leases
The Company determines if an arrangement is a lease at inception and recognizes the lease in accordance with ASC 842, Lease Accounting. Operating leases are included
within net loss.in right-of-use (“ROU”) assets, current portion, lease liability and long-term lease liability in the Company's consolidated balance sheets. ROU assets represent the right to use an underlying asset for the lease term and lease liabilities represent the Company's obligation to make lease payments arising from the lease. Operating lease ROU assets and liabilities are recognized at the lease commencement date based on the present value of the lease payments over the lease term. The Company determines the portion of the lease liability that is current as the difference between the calculated lease liability at the end of the current period and the lease liability that is projected 12 months from the current period.
(I) Property and Equipment
Property and equipment are stated at cost and depreciated over their estimated useful lives of three years using the straight-line method.
RepairsRepair and maintenance costs are expensed. The Company reviews the recoverability of all long-lived assets, including the related useful life, whenever events or changes in circumstances indicate that the carrying amount of a long-lived asset might not be recoverable.
(J) Research and Development Expenses
The Company expenses the cost of research and development as incurred. Research and development expenses compriseare comprised of costs incurred in performing research and development activities, including clinical trial costs, manufacturing costs for both clinical and pre-clinicalpreclinical materials as well as other contracted services, license fees, and other external costs. Research and development expenses also include the cost of licensing agreements acquired from third-parties. Nonrefundable advance payments for goods and services that will be used in future research and development activities are expensed when the activity is performed or when the goods have been received rather than when the cost is incurred, in accordance with ASC 730, Research and Development.
(K) Stock-BasedStock-based Compensation
The Company accounts for its stock-based compensation in accordance with ASC 718, Compensation—Stock Compensation, which establishes accounting for stock-based awards granted to employees for services and requires
companies to expense the estimated fair value of these awards over the requisite service period. The Company estimates the fair value of all awards granted using the Black-Scholes valuation model. Key inputs and assumptions include the expected term of the option, stock price volatility, risk-free interest rate, dividend yield, stock price and exercise price. Many of the assumptions require significant judgment and any changes could have
a materialan impact in the determination of stock-based compensation expense. The Company elected an accounting policy to record forfeitures as they occur. The Company recognizes employee stock-based compensation expense based on the fair value of the award on the date of the grant. The compensation expense is recognized over the vesting period under the straight-line method.
The Company accounts for
optionsoption awards granted to nonemployee consultants and directors in accordance with
ASU 2018-07, Compensation – Stock Compensation (Topic 718): Improvements to Nonemployee Share-Based Payment Accounting.ASC 718. The fair value of the option issued or committed to be issued is used to measure the transaction, as this is more reliable than the fair value of the services received. The fair value is measured at the value of the Company’s common stock award at the earlier of the date that the commitment for performance by the counterparty has been reached or the counterparty’s performance is complete.
(L) Fair ValueRoyalty Monetization Liability
The Company accounted for its sale to Ligand Pharmaceuticals Incorporated (“Ligand”) of
a 13% share of royalties and milestones owed to the Company related to the potential approval and commercialization of soticlestat in accordance with ASC 470, Debt, which addresses situations in which an entity receives cash from an investor in return for an agreement to pay the investor a specified percentage of the revenue from a contractual right. The Company classified the proceeds received from the sale to Ligand as debt as the Company determined that it had significant continuing involvement in the generation of the cash flows to Ligand. The Company further elected to account for the debt at fair value in accordance with ASC 825, Financial Instruments,
Financial Accounting Standards Board (“FASB”) guidance specifies which permits a hierarchy of valuation techniques based on whether the inputscompany to those valuation techniques are observable or unobservable. Observable inputs reflect market data obtained from independent sources, while unobservable inputs reflect market assumptions. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurement) and the lowest priority to unobservable inputs (Level 3 measurement).
The three levels ofelect the fair value hierarchy areoption on an instrument specific basis for a recognized financial liability that is not specifically excluded.
If commercialized, the Company will recognize 100% of the royalties and milestones received for sales of soticlestat as
follows:Level 1—Unadjusted quoted pricesrevenue and the 13% share of royalties payable to Ligand Pharmaceuticals as a cash outflow from financing activities in active marketsthe consolidated statements of cash flows. Changes in the fair value of the debt will be classified as a component of other income / expense in the consolidated statements of operations. The change in fair value of the debt was immaterial for identical assets or liabilities that the reporting entity has the ability to access at the measurement date. Level 1 primarily consists of financial instruments whose value is based on quoted market prices such as exchange-traded instruments and listed equities. The Company’s Level 1 assets consisted of money market funds and short-term investments totaling $70.1 million and $76.2 million as ofyear-ended December 31, 2020 and 2019, respectively.
2023.Level 2—Inputs other than quoted prices included within Level 1 that are observable for the asset or liability, either directly or indirectly (e.g., quoted prices of similar assets or liabilities in active markets, or quoted prices for identical or similar assets or liabilities in markets that are not active). Level 2 includes financial instruments that are valued using models or other valuation methodologies. The Company had no Level 2 assets or liabilities as of December 31, 2020 and 2019.
Level 3—Unobservable inputs for the asset or liability. Financial instruments are considered Level 3 when their fair values are determined using pricing models, discounted cash flows or similar techniques and at least one significant model assumption or input is unobservable. The Company had no Level 3 assets or liabilities as of December 31, 2020 and 2019.
The carrying amounts reported in the balance sheets for cash and cash equivalents, related-party receivables, other current assets, accounts payable, accrued expenses, and current related-party payables approximate their fair value based on the short-term maturity of these instruments.
(M) Income Taxes
The Company accounts for income taxes under the asset and liability method, which requires deferred tax assets and liabilities to be recognized for the estimated future tax consequences attributable to differences between financial statement carrying amounts and respective tax bases of existing assets and liabilities, as well as for net operating loss carryforwards and research and development
credit.credits. Valuation allowances are provided if it is more likely than not that some portion or all of the deferred tax assets will not be realized. The impact of a change in
the tax laws is recorded in the period in which the law is enacted.
(N) Net Loss
Per Commonper Share
Basic and diluted net
Net loss per common share is determined by dividing net loss attributable to common stockholders by the basic and diluted weighted-average common shares outstanding during the period. For all periods presented,The Company applies the two-class method to allocate earnings between common stock and participating securities.
Net loss per diluted share attributable to common stockholders adjusts the basic earnings per share attributable to common stockholders and the weighted-average number of shares of common stock outstanding for the potential dilutive impact of stock options
have been excluded fromusing the
calculation because their effect would be anti-dilutive. Therefore,treasury-stock method and the
weighted-average shares outstanding used to calculate both basic and diluted loss per common share arepotential impact of preferred stock using the
same. Under the terms of the Series A Preferred Stock issued in 2019, Preferred stockholders do not share in losses of the Company and have no obligation to fund losses or transfer assets. Since there is a loss, diluted EPS should be computed in the same manner as basic EPS and because no potential common shares shall be included in the computation of any diluted per-share amounts when a loss exists, the Series A Preferred Stock should be excluded from the computation of basic and diluted EPS.if-converted method.
The following potentially dilutive securities have been excluded from the computations of diluted weighted-average shares outstanding as they would be anti-dilutive:
| | For the Year Ended December 31, | |
| | 2020 | | | 2019 | |
Stock options to purchase common stock | | | 10,403,420 | | | | 7,405,295 | |
Series A convertible preferred stock | | | 3,250 | | | | 7,762 | |
(O) Segment Data
The Company manages its operations as a single segment for the purposes of assessing performance and making operating decisions.
(P) Retirement Plan
The Company maintains a 401(k)-retirement plan for its employees that is intended to qualify under Sections 401(a) and 501(a) of the U.S. Internal Revenue Code of 1986, as amended (“Code”). The Company provides all active employees with a 100% matching contribution equal to 3% of an employee’s eligible
deferred compensation
deferred and
a 50% matching
contributionscontribution on employee contributions that are between 3% and 5% of an employee’s eligible
compensation deferred.deferred compensation. These safe harbor contributions vest immediately. For the years ended December 31,
20202023 and
20192022 the Company contributed
$321,000$311,640 and
$285,000,$339,405, respectively.
(Q)
Under ASC 606,
Revenue from Contracts with Customers, an entity recognizes revenue when its customer obtains control of promised goods or services, in an amount that reflects the consideration that the entity expects to receive in exchange for those goods or services. In applying ASC 606, the Company performs the following five steps: (i) identify the contract(s) with a customer; (ii) identify the promises and performance obligations in the contract; (iii) determine the transaction price; (iv) allocate the transaction price to the performance obligations in the contract; and (v) recognize revenue when (or as)
it satisfies the performance
obligations.obligations are satisfied. The Company only applies the five-step model to contracts when it is probable that it will collect the consideration to which it is entitled in exchange for the goods or services
we transfertransferred to the customer. At contract inception, once the contract is determined to be within the scope of ASC 606, the Company assesses the goods or services promised within each contract, determines those that are performance obligations and assesses whether each promised good or service is distinct. The Company then recognizes as revenue the amount of the transaction price that is allocated to the respective performance obligation when (or as) the performance obligation is satisfied.
Prior to recognizing revenue, the Company makes estimates of the transaction price, including variable consideration that is subject to a constraint. Amounts of variable consideration are included in the transaction price to the extent that it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur and when the uncertainty associated with the variable consideration is subsequently resolved.
If there are multiple distinct performance obligations, the Company allocates the transaction price to each distinct performance obligation based on its relative standalone selling price. The standalone selling price is generally determined using expected cost and comparable transactions. Revenue for performance obligations recognized over time is recognized by measuring the progress toward complete satisfaction of the performance obligations using an input measure.
Non-refundable upfront fees allocated to licenses that are not contingent on any future performance and require no consequential continuing involvement by the Company, are recognized as revenue when the license term commences and the licensed data, technology or product is delivered. The Company defers recognition of upfront license fees if the performance obligations are not satisfied.
(R)
(Q) Recent Accounting Pronouncements
Recent
The Company has reviewed recently issued accounting standards
which have been adoptedIn June 2016,and plans to adopt those that are applicable. The Company does not expect the FASB issued ASU No. 2016-13, Financial Instruments – Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments. This new standard requires the measurement and recognition of expected credit losses for financial assets held at amortized cost, including loans and trade and other receivables. ASU 2016-13 replaces the existing incurred loss impairment model with an expected loss methodology, which will result in more timely recognition of credit losses. The standard also amends the impairment model for available-for-sale debt securities and requires entities to determine whether all or a portion of the unrealized loss on an available-for-sale debt security is a credit loss. Under the new guidance, an entity recognizes an allowance for credit losses on available-for-sale debt securities as a contra-account to the amortized cost basis rather than as a direct reduction of the amortized cost basis of the investment, as was previously required. ASU 2016-13 is effective for annual reporting periods, and interim periods within those years, beginning after December 15, 2019. As of December 31, 2020, the Company did not hold any debt securities with
credit losses, nor does it have any trade receivables. The adoption of this standard effective January 1, 2020 did notthose standards to have a material impact on the Company’s consolidatedits financial statements.
On August 29, 2018, the FASB issued ASU No. 2018-15, Intangibles – Goodwill and Other - Internal-Use Software (Subtopic 350-40) - which amends ASC 350-40position, results of operations or cash flows.
The Company adopts new pronouncements relating to
address a customer’s accounting for implementation costs incurred in a cloud computing arrangement (“CCA”) that is a service contract. ASU No. 2018-15 aligns the accounting for costs incurred to implement a CCA that is a service arrangement with the guidance on capitalizing costs associated with developing or obtaining internal-use software. Specifically, the ASU amends ASC 350 to include in its scope implementation costs of a CCA that is a service contract and clarifies that a customer should apply ASC 350-40 to determine which implementation costs should be capitalized in a CCA that is considered a service contract. AccordingGAAP applicable to the
standardCompany as they are issued, and based upon the
balance sheet line item foreffective dates included in the
presentation of capitalized implementation costs should be the same aspronouncements. Management does not believe that
for the prepayment of fees related to the hosting arrangement and the manner in which an entity classifies the cash flows related to capitalized implementation costs should be the same as that in which it classifies the cash flows for the fees related to the hosting arrangement. ASU 2018-15 isany recently issued, but not yet effective
for the Company for fiscal years beginning after December 15, 2019, including interim periods therein. Entities are permitted to apply either a retrospective or prospective transition approach to adopt the guidance. The adoption of this standard effective January 1, 2020 did not have a material impact on the Company’s consolidated financial statements and wasaccounting standards, if currently adopted,
prospectively.On November 5, 2018, the FASB issued ASU 2018-18, Collaborative Arrangements (Topic 808), - which amends ASC 808 to clarify when transactions between participants in a collaborative arrangement under ASC 808 are within the scope of the FASB’s new revenue standard, ASU 2014-09 (codified in ASC 606). The amendments require the application of ASC 606 existing guidance to determine the units of account that are distinct in a collaborative arrangement for purposes of identifying transactions with customers. If a unit of account within the collaborative arrangement is distinct and is with a customer, an entity shall apply the guidance in Topic 606 to that unit of account. In a transaction between collaborative participants, an entity is precluded by ASU 2018-18 from presenting a transaction together with “revenue from contracts with customers” unless the unit of account is within the scope of ASC 606 and the entity applies the guidance in ASC 606 to such unit of account. The amended guidance is effective for public business entities for fiscal years beginning after December 15, 2019, and interim periods within those fiscal years. The retrospective adoption of this standard effective January 1, 2020 did not have a material impact on the Company’s consolidated financial statements.
In October 2020, the FASB issued ASU 2020-10, Codification Improvements, which updates various codification topics by clarifying or improving disclosure requirements. ASU 2020-10 is effective for annual and interim periods beginning after December 15, 2020. The Company early adopted ASU 2020-10 for the reporting period ending December 31, 2020. The adoption of this update did notwould have a material effect on the Company saccompanying consolidated financial statements.
NOTE 3 – CASH, CASH EQUIVALENTS AND SHORT-TERM INVESTMENTSAll short-term investments are classified as available-for-sale. MARKETABLE SECURITIES
The following tables summarize the fair value of cash, cash equivalents and
short-term investments,marketable securities as well as gross unrealized holding gains and losses as of December 31,
20202023 and
December 31, 2019: | | December 31, 2020 | |
| | Amortized | | | Gross unrealized | | | Gross unrealized | | | Fair | |
| | cost | | | holding gains | | | holding losses | | | value | |
Cash | | $ | 1,977,320 | | | $ | - | | | $ | - | | | $ | 1,977,320 | |
Money market funds | | | 70,056,610 | | | | - | | | | - | | | | 70,056,610 | |
Total cash and cash equivalents | | $ | 72,033,930 | | | $ | - | | | $ | - | | | $ | 72,033,930 | |
| | | | | | | | | | | | | | | | |
U.S. treasury notes | | $ | - | | | $ | - | | | $ | - | | | | - | |
Total short-term investments | | $ | - | | | $ | - | | | $ | - | | | $ | - | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2023 |
| Amortized cost | | Gross unrealized holding gains | | Gross unrealized holding losses | | Fair value |
| Cash | $ | 2,701,396 | | | $ | — | | | $ | — | | | $ | 2,701,396 | |
| Money market funds | 24,340,121 | | | — | | | — | | | 24,340,121 | |
| Marketable securities | 78,791,156 | | | 702 | | | — | | | 78,791,858 | |
| Total cash, cash equivalents and marketable securities | $ | 105,832,673 | | | $ | 702 | | | $ | — | | | $ | 105,833,375 | |
| | December 31, 2019 | |
| | Amortized | | | Gross unrealized | | | Gross unrealized | | | Fair | |
| | cost | | | holding gains | | | holding losses | | | value | |
Cash | | $ | 501,537 | | | $ | - | | | $ | - | | | $ | 501,537 | |
Money market funds | | | 41,395,607 | | | | - | | | | - | | | | 41,395,607 | |
Total cash and cash equivalents | | $ | 41,897,144 | | | $ | - | | | $ | - | | | $ | 41,897,144 | |
| | | | | | | | | | | | | | | | |
U.S. treasury notes | | $ | 34,839,500 | | | $ | 2,469 | | | $ | - | | | | 34,841,969 | |
Total short-term investments | | $ | 34,839,500 | | | $ | 2,469 | | | $ | - | | | $ | 34,841,969 | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2022 |
| Amortized cost | | Gross unrealized holding gains | | Gross unrealized holding losses | | Fair value |
| Cash | $ | 2,853,042 | | | $ | — | | | $ | — | | | $ | 2,853,042 | |
| Money market funds | 42,014,804 | | | — | | | — | | | 42,014,804 | |
| Marketable securities | 84,175,752 | | | — | | | (42,187) | | | 84,133,565 | |
| Total cash, cash equivalents and marketable securities | $ | 129,043,598 | | | $ | — | | | $ | (42,187) | | | $ | 129,001,411 | |
The Company did not hold any securities that were in an unrealized loss position for more than 12 months as of December 31,
20202023 and
2019.2022.
There were no material realized gains or losses on available-for-sale securities during the years ended December 31,
20202023 and
2019. 2022.
NOTE
4 – PROPERTY AND EQUIPMENT AND INTANGIBLE ASSETSProperty and equipment is summarized as follows:
| | December 31, | | | December 31, | |
| | 2020 | | | 2019 | |
| December 31,
2023 | | | December 31,
2023 | | December 31,
2022 |
Furniture and equipment | | $ | 320,957 | | | $ | 193,717 | |
| Leasehold improvements | |
Less accumulated depreciation | | | (185,337 | ) | | | (125,354 | ) |
Total property, plant and equipment, net | | $ | 135,620 | | | $ | 68,363 | |
| Total property and equipment, net | |
Depreciation expense was
$60,000$419,518 and
$39,000$319,173 for the years ended December 31,
20202023 and
20192022 respectively.
Intangible assets, net of accumulated amortization, were $319,000$182,974 and $467,000$222,100 as of December 31, 20202023 and December 31, 2019,2022, respectively, and are included in other assets. Amortization expense was $247,000$148,764 and $216,000$193,333 for the years ended December 31, 20202023 and 2019,2022, respectively.
NOTE 5 – LEASES
During September 2021, the Company entered into a 10-year lease agreement for its corporate headquarters with a term commencing March 10, 2022, for approximately 19,000 square feet of office space at Hudson Commons in New York, NY. The lease provides for monthly rental payments over the lease term. The base rent under the lease is currently $2.3 million per year. Rent payments commenced 10 months following the commencement date of the lease, or January 10, 2023, and continue for 10 years following the rent commencement date. Rent also includes two months of free rent in the sixth and seventh months following the rent commencement date. The Company issued a letter of credit in the amount of $1.9 million in association with the execution of the lease agreement; the letter of credit is characterized as restricted cash on the Company's consolidated balance sheets.
The Hudson Commons lease has a remaining lease term of approximately nine years and includes a single renewal option for an additional five years. The Company did not include the renewal option in the lease term when calculating the lease liability as the Company is not reasonably certain that it will exercise the renewal option. The present value of the lease payments is calculated using an incremental borrowing rate of 7.02%. Lease expense is included in general and administrative and research and development expenses in the consolidated statements of operations.
ROU asset and lease liabilities related to the Company's operating lease are as follows:
| | | | | | | | |
| December 31,
2023 | December 31,
2022 |
| ROU asset | $ | 13,894,376 | | $ | 14,922,669 | |
| Current lease liability | $ | 1,246,119 | | $ | 533,946 | |
| Long-term lease liability | $ | 14,755,606 | | $ | 16,001,725 | |
The components of operating lease cost for the year ended December 31, 2023 and 2022 were as follows:
| | | | | | | | |
| December 31,
2023 | December 31,
2022 |
| Operating lease cost | $ | 2,167,233 | | $ | 1,806,028 | |
| Variable lease cost | — | | — | |
| Short-term lease cost | — | | — | |
Future minimum commitments under the non-cancelable operating lease are as follows:
| | | | | |
| 2024 | $ | 2,316,303 | |
| 2025 | 2,316,303 | |
| 2026 | 2,316,303 | |
| 2027 | 2,316,303 | |
| 2028 | 2,469,447 | |
| Thereafter | 9,877,788 | |
| $ | 21,612,447 | |
NOTE
56 – ACCRUED EXPENSES
Accrued expenses consist of the following:
| | December 31, | | | December 31, | |
| | 2020 | | | 2019 | |
Clinical trials accrual | | $ | 4,175,497 | | | $ | 3,235,527 | |
| December 31,
2023 | | | December 31,
2023 | | December 31,
2022 |
Payroll and bonus accrual | | | 3,845,441 | | | | 2,728,495 | |
| Research and development accrual | |
Professional fees accrual | | | 3,846,211 | | | | 1,070,589 | |
Other | | | 165,536 | | | | 232,095 | |
Total | | $ | 12,032,685 | | | $ | 7,266,706 | |
NOTE
67 – STOCKHOLDERS’ EQUITY
AND PREFERRED STOCKThe Company’s capital structure consists of common stock and
Preferred Stock.preferred stock. Pursuant to the Company’s amended and restated certificate of incorporation, as amended, the Company is authorized to issue up to 125,000,000 shares of common stock and 10,000,000 shares of
Preferred Stock.preferred stock. The Company has designated 10,000 of the 10,000,000 authorized shares of
Preferred Stockpreferred stock as non-voting Series A Convertible Preferred Stock (“Series A Preferred Stock”).
The holders of common stock are entitled to one vote for each share held. The holders of common stock have no preemptive or other subscription rights, and there are no redemption or sinking fund provisions with respect to such shares. Subject to preferences that may apply to any outstanding series of Preferred Stock,preferred stock, holders of the common stock are entitled to receive ratably any dividends declared on a non-cumulative basis. Shares of Series A Preferred Stock will be entitled to receive dividends at a rate equal to (on an as-if-converted-to-common stock basis), and in the same form and manner as, dividends actually paid on shares of common stock. The common stock is subordinate to all series of Preferred Stock with respect to rights upon liquidation, winding up and dissolution of the Company. The holders of common stock are entitled to liquidation proceeds after all liquidation preferences for the Preferred Stockpreferred stock are satisfied.
In June 2018, the Company entered into a sales agreement (the “2018 ATM agreement”) with Cowen and Company, LLC (“Cowen”) under which the Company offered and sold in “at the market offerings,” from time to time at its sole discretion, shares of its common stock having an aggregate offering price of up to $50.0 million through Cowen acting as sales agent. During the year ended December 31, 2019, the Company sold 6,893,888 shares of its common stock under the 2018 ATM agreement for net proceeds of $22.3 million after deducting sales agent commissions and other offering expenses payable by the Company, (the “ATM Offerings”).
In November 2020, the Company entered into a new sales agreement (the “2020 ATM agreement”) with Cowen and under which the Company may offer and sell in “at the market offerings,” from time to time at its sole discretion, shares of its common stock having an aggregate offering price of up to $75.0 million through Cowen acting as sales agent.
There were 3,250 and 7,7621,250 shares of Series A Preferred Stock outstanding as of December 31, 20202023 and December 31, 2019, respectively.2022. Each share of Series A Preferred Stock is convertible into 1,000 shares of common stock at any time at the holder’s option. However, the holder will be prohibited, subject to certain exceptions, from converting shares of Series A Preferred Stock into shares of common stock if, as a result of such conversion, the holder, together with its affiliates, would own more than, at the written election of the holder, either 9.99% or 14.99% of the total number of shares of common stock then issued and outstanding, which percentage may be changed at the holder’s election to any other number less than or equal to 19.99% upon 61 days’ notice to the Company; provided, however, that effective 61 days after delivery of such notice, such beneficial ownership limitations shall not be applicable to any holder that beneficially owns either 10.0% or 15.0%, as applicable based on the holder’s initial written election noted above, of the total number of shares of common stock issued and outstanding immediately prior to delivery of such notice. In the event of a liquidation, dissolution, or winding up of the
Company, holders of Series A Preferred Stock will receive a payment equal to $0.001 per share of Series A Preferred Stock before any proceeds are distributed to the holders of common stock.
In
Dividends
Through December
2020, entities affiliated with Biotechnology Value Fund, L.P. elected to convert an aggregate of 2,256 shares of Series A Preferred Stock owned by such holders into an aggregate of 2,256,000 shares of the Company’s common stock.In August 2020,31, 2023, the Company sold 6,250,000 shares of its common stock at a public offering price of $8.00 per share, for net proceeds of $46.7 million after deducting underwriting discounts and commissions and other offering expenses payable by the Company, (the “August 2020 Offering”).
In May 2020, entities affiliated with Biotechnology Value Fund, L.P. elected to convert an aggregate of 2,256 shares of Series A Preferred Stock owned by such holders into an aggregate of 2,256,000 shares of the Company’s common stock.
In October and November 2019, the Company sold 10,350,000 shares of its common stock, which included the full exercise of the underwriters’ option to purchase additional shares, and 4,000 shares of Series A Preferred Stock at a public offering price of $2.50 and $2,500 per share, respectively, for net proceeds of $33.5 million after deducting underwriting discounts and commissions and other offering expenses payable by the Company, (the “October 2019 Offering”).
In September 2019, the Company entered into an exchange agreement with entities affiliated with Biotechnology Value Fund, L.P. (the “Exchanging Stockholders”), pursuant to which the Company exchanged an aggregate of 1,262,000 shares of the Company’s common stock owned by the Exchanging Stockholders for an aggregate of 1,262 shares of the Company’s Series A Preferred Stock (the “Exchange Shares”). The Exchange Shares were issued without registration under the Securities Act of 1933, as amended, in reliance on the exemption from registration contained in Section 3(a)(9) of the Securities Act.
In February 2019, the Company sold 13,993,778 shares of its common stock and 2,500 shares of Series A Preferred Stock at a public offering price of $2.00 and $2,000 per share, respectively, for net proceeds of $30.5 million after deducting underwriting discounts and commission and other offering expenses payable by the Company (the “February 2019 Offering”).
Dividends
has not declared any dividends. No dividends on the common stock shall be declared and paid unless dividends on the Preferred Stockpreferred stock have been declared and paid. Through December 31, 2020, the Company has not declared any dividends.
NOTE
78 – STOCK-BASED COMPENSATION
On August 29, 2014, the
The Company’s Board of Directors (the “Board”) adopted and approved the 2014 Equity Incentive Plan (the “2014(“2014 Plan”), which authorized the Company to grant shares of common stock in the form of incentive stock options, nonstatutory stock options, stock appreciation rights, restricted stock and restricted stock units. The types of stock-based awards, including share purchase rights amount, terms, and exercisability provisions offor exercising grants arewere determined by the Company’sBoard.
The Board
of Directors.The Company's Board of Directors adopted, and the Company's stockholders approved, the 2017 equity incentive plan (“2017 Plan”), which became effective immediately prior to the execution of the underwriting agreement related to the IPO on May 4, 2017. The initial reserve of shares of common stock that may be issued under the 2017 Plan was 3,052,059 shares. The 2017 Plan provides
for the grant of incentive stock options, non-statutory stock options, restricted stock awards, restricted stock unit awards, stock appreciation rights, performance-based stock awards, and other forms of stock-based awards. Additionally, the 2017 Plan provides for the grant of performance cash awards. The Company's employees, officers, directors, and consultants and advisors are eligible to receive awards under the 2017 Plan. Upon the adoption of the 2017 Plan, no further awards will bewere granted under the 2014 Plan. Pursuant to the terms of the 2017 Plan, on each January 1st, the plan limit shall be increased by the lesser of (x) 5% of the number of shares of common stock outstanding as of the immediately preceding December 31 and (y) such lesser number as the Board of Directors may determine in its discretion. On January 1, 2019,2022, an additional 1,232,7051,000,000 shares were reserved for issuance under the 2017 Plan. On January 1, 2023, an additional 3,523,344 shares were reserved for issuance under the 2017 Plan. As of December 31, 2019,2023, there were 2,199,7654,400,759 shares of the Company’s common stock reserved for issuance under the 2017 Plan. On January 1, 2020,2024, an additional 2,735,5163,534,600 shares were reserved for issuance under the 2017 Plan. As of December 31, 2020, there were 1,771,772 shares of the Company’s common stock reserved for issuance under the 2017 Plan.
The
Company's Board
of Directors adopted, and the Company's stockholders approved the 2017 employee stock purchase plan
(the “2017 (“ESPP”), which became effective
immediately prior to the execution of the underwriting agreement related to the IPO on May 4, 2017. The initial reserve of shares of common stock that may be issued under the
2017 ESPP was 279,069 shares.
On September 20, 2017, the Company’s Compensation Committee approved an offering period under the 2017 ESPP, which began on October 20, 2017. The ESPP allows employees to purchase common stock of the Company at a 15% discount to the market price on designated purchase dates. During the years ended December 31,
20202023 and
2019, 105,4642022, 63,761 and
80,54276,455 shares were purchased under the ESPP and the Company recorded expense of
$152,973$57,148 and
$109,319,$85,319, respectively. The number of shares of common stock reserved for issuance under the
2017 ESPP will automatically increase on January 1 of each year, beginning on January 1, 2018 and continuing through and including January 1, 2027, by the lesser of (i) 1% of the total number of shares of the Company’s capital stock outstanding on December 31 of the preceding calendar year, (ii) 550,000 shares or (iii) such lesser number of shares determined by
ourthe Board.
OnThe Board acted prior to January 1,
2019, an additional 246,5412024 to provide that there be no increase in the number of shares
were reserved for issuance under the
2017 ESPP.
No shares were reserved for issuance under the 2017 ESPP during the year ended December 31, 2020. As of December 31,
2020,2023 and 2022, there were
553,552352,846 and 416,607 shares of the Company’s common stock reserved for issuance under the
2017 ESPP.
Unless specified otherwise in an individual option agreement, stock options granted under the 2014 Plan and 2017 Plan
generally have a ten-year term and a four-year graded vesting period. The vesting requirement is generally conditioned upon the grantee’s continued service with the Company during the vesting period. Once vested, all
awardsoptions granted are exercisable from the date of grant until they expire. The option grants are non-transferable. Vested options
generally remain exercisable for 90 days
under the 2017 Plan and 30 days under the 2014 Plan subsequent to the termination of the option holder’s service with the Company. In the event of
the option holder’s death or disability while employed by or providing service to the Company, the exercisable period extends to
18 months or 12
months.months, respectively under the 2017 Plan and 6 months under the 2014 Plan..
Performance-based option awards generally have similar vesting terms, with vesting occurring on the date the performance condition is achieved and expire in accordance towith the specific terms of the agreement. At December 31, 2020,2023 and 2022, there were 125,000zero and 100,000 performance-based options outstanding and unvested, respectively, that include options to vest upon the achievement of certain research and development milestones. milestones.
The fair value of options granted during the years ended December 31, 20202023 and 20192022 was estimated using the Black-Scholes option valuation model. The inputs for the Black-Scholes option valuation model require management’s significant assumptions made by management and are detailed in the table below. The risk-free interest rates were based on the rate for U.S. Treasury securities at the date of grant with maturity dates approximately equal to the expected life at the grant
date. The expected life was based on the simplified method in accordance with the SEC Staff Accounting Bulletin No. Topic 14D. The expected volatility was estimated based on
the Company's published historical
volatility information of peer companies that is publicly available.stock prices.
All assumptions used to calculate the grant date fair value of nonemployee options are generally consistent with the assumptions used for options granted to employees. In the event the Company terminates any of its consulting agreements, the unvested options underlying the agreements would also be cancelled.
The Company granted
10,00045,000 and
175,000zero stock options to nonemployee consultants for services rendered during the years ended December 31,
20202023 and
2019,2022, respectively. There were
32,50098,542 and
145,204127,459 unvested nonemployee options outstanding as of December 31,
20202023 and
2019,2022, respectively. Total expense recognized related to the nonemployee stock options for the years ended December 31,
20202023 and
20192022 was
$257,431$202,114 and
$51,666,$575,995, respectively.
During the year ended December 31, 2019, the Company recognized a credit of $17,000 related to the nonemployee stock options including the modification of certain options in connection with the separation and consulting agreement with Dr. During (see Note 11). Total unrecognized compensation expenses related to the nonemployee stock options was
$186,823$133,769 and $626,977 as of December 31,
2020.2023 and 2022, respectively. During the years ended December 31,
2020,2023 and
2019, the Company recognized $115,240 and zero, respectively, in2022, there were no expenses for nonemployee performance-based option
awards.awards recognized.
The Company granted
3,573,1602,993,000 and
3,372,5134,575,641 stock options to employees during the years ended December 31,
20202023 and
2019,2022, respectively. There were
4,975,2625,376,910 and
3,963,5446,090,889 unvested employee options outstanding as of December 31,
20202023 and
2019,2022, respectively. Total expense recognized related to the employee stock options for the years ended December 31,
20202023 and
20192022 was
$7.1$7.5 million and
$5.0$5.9 million, respectively. Total unrecognized compensation expense related to employee stock options was
$12.2$9.3 million and $11.5 million as of December 31,
2020.2023 and 2022, respectively. During the years ended December 31,
20202023 and
2019,2022, the Company recognized
$2,340,808zero and
$9,391$0.1 million, respectively, in expenses for employee performance-based option awards.
The Company’s stock-based compensation expense was recognized in operating expenses as follows:
| | For the Year Ended December 31, | |
| | 2020 | | | 2019 | |
| For the Year Ended December 31, | | | For the Year Ended December 31, |
| 2023 | | | 2023 | | 2022 |
Research and development | | $ | 2,779,758 | | | $ | 2,391,909 | |
General and administrative | | | 4,745,596 | | | | 2,813,883 | |
Total | | $ | 7,525,354 | | | $ | 5,205,792 | |
| | For the Year Ended December 31, | |
| | 2020 | | | 2019 | |
| For the Year Ended December 31, | | | For the Year Ended December 31, |
| 2023 | | | 2023 | | 2022 |
Stock options | | $ | 7,372,381 | | | $ | 5,096,473 | |
Employee Stock Purchase Plan | | | 152,973 | | | | 109,319 | |
| Employee stock purchase plan | |
Total | | $ | 7,525,354 | | | $ | 5,205,792 | |
The fair value of employee options granted during the years ended December 31,
20202023 and
2019,2022, respectively, was estimated by utilizing the following assumptions:
| | For the Year Ended December 31, | |
| | 2020 | | | 2019 | |
| | Weighted Average | | | Weighted Average | |
| For the Year Ended December 31, | | | For the Year Ended December 31, |
| 2023 | | | 2023 | | 2022 |
| Weighted
Average | | | Weighted
Average |
Volatility | | | 81.48 | % | | | 81.87 | % | Volatility | 84.52 | % | | 87.16 | % |
Expected term in years | | | 5.88 | | | | 6.07 | | Expected term in years | 6.07 | | 6.07 |
Dividend rate | | | 0.00 | % | | | 0.00 | % | Dividend rate | 0.00 | % | | 0.00 | % |
Risk-free interest rate | | | 0.58 | % | | | 2.04 | % | Risk-free interest rate | 3.97 | % | | 2.21 | % |
Fair value of option on grant date | | $ | 2.62 | | | $ | 2.26 | |
The fair value of nonemployee options granted and remeasured during the years ended December 31, 20202023 and 2019,2022, respectively, was estimated by utilizing the following assumptions:
| | For the Year Ended December 31, | |
| | 2020 | | | 2019 | |
| | Weighted Average | | | Weighted Average | |
| For the Year Ended December 31, | | | For the Year Ended December 31, |
| 2023 | | | 2023 | | 2022 |
| Weighted
Average | | | Weighted
Average |
Volatility | | | 74.37 | % | | | 74.56 | % | Volatility | 83.73 | % | | — | % |
Expected term in years | | | 5.64 | | | | 5.30 | | Expected term in years | 5.32 | | 0.00 |
Dividend rate | | | 0.00 | % | | | 0.00 | % | Dividend rate | 0.00 | % | | 0.00 | % |
Risk-free interest rate | | | 2.32 | % | | | 2.37 | % | Risk-free interest rate | 3.86 | % | | — | % |
Fair value of option on grant date | | $ | 1.18 | | | $ | 1.07 | |
The following table summarizes the number of options outstanding and the weighted average exercise price:
| | | | | | | | | | Weighted | | | | | |
| | | | | | Weighted | | | Average | | | | | |
| | | | | | Average | | | Remaining | | | Aggregate | |
| | Number of | | | Exercise | | | Contractual | | | Intrinsic | |
| | Shares | | | Price | | | Life in Years | | | Value | |
Options Outstanding at December 31, 2018 | | | 4,714,383 | | | $ | 8.10 | | | | 7.89 | | | $ | 153,485 | |
Vested and exercisable at December 31, 2018 | | | 2,337,931 | | | $ | 7.88 | | | | 7.20 | | | $ | 153,485 | |
Granted | | | 3,547,513 | | | | 2.93 | | | | 9.56 | | | | | |
Exercised | | | - | | | | - | | | | | | | | | |
Forfeited | | | (856,601 | ) | | | 6.43 | | | | | | | | | |
Options Outstanding December 31, 2019 | | | 7,405,295 | | | $ | 5.82 | | | | 8.01 | | | $ | 4,488,930 | |
Vested and exercisable at December 31, 2019 | | | 3,296,547 | | | $ | 7.93 | | | | 6.53 | | | $ | 356,443 | |
Granted | | | 3,583,160 | | | | 3.93 | | | | 9.68 | | | | | |
Exercised | | | (165,384 | ) | | | 1.82 | | | | - | | | | | |
Forfeited | | | (415,526 | ) | | | 5.00 | | | | | | | | | |
Options Outstanding December 31, 2020 | | | 10,403,420 | | | $ | 5.26 | | | | 7.59 | | | $ | 652,438 | |
Vested and exercisable at December 31, 2020 | | | 5,395,658 | | | $ | 6.45 | | | | 6.13 | | | $ | 445,599 | |
| | | | | | | | | | | | | | | | | | | | | | | |
| Number of
Shares | | Weighted
Average
Exercise
Price | | Weighted
Average
Remaining
Contractual
Life in Years | | Aggregate
Intrinsic
Value |
| Options outstanding at December 31, 2021 | 10,776,758 | | | $ | 4.97 | | | 6.07 | | $ | 2,389,890 | |
| Vested and exercisable at December 31, 2021 | 6,188,200 | | | $ | 5.98 | | | 4.63 | | $ | 1,531,907 | |
| Granted | 4,575,641 | | | 2.89 | | | 9.26 | | |
| Exercised | (25,518) | | | 1.91 | | | | | |
| Forfeited or expired | (2,365,643) | | | 5.56 | | | | | |
| Options outstanding December 31, 2022 | 12,961,238 | | | $ | 4.13 | | | 7.42 | | $ | 62,158 | |
| Vested and exercisable at December 31, 2022 | 6,742,890 | | | $ | 5.05 | | | 6.20 | | $ | 61,214 | |
| Granted | 3,038,000 | | | 2.63 | | | 9.20 | | |
| Exercised | (146,346) | | | 2.76 | | | | | |
| Forfeited or expired | (728,346) | | | 3.44 | | | | | |
| Options outstanding December 31, 2023 | 15,124,546 | | | $ | 3.87 | | | 6.90 | | $ | 5,212,586 | |
| Vested and exercisable at December 31, 2023 | 9,649,094 | | | $ | 4.47 | | | 5.97 | | $ | 2,464,620 | |
At December 31,
2020,2023, there was
$12.3$9.4 million of unamortized
share–basedstock-based compensation expense, which is expected to be recognized over a remaining average vesting period of
3.042.23 years.
At December 31, 2022, there was $12.1 million of unamortized stock-based compensation expense, which is expected to be recognized over a remaining average vesting period of 2.26 years.
At December 31, 2020,2023, the Company has available approximately $238.6$150.2 million and $186.7$190.2 million of unused NOLnet operating loss (“NOL”) carryforwards for federal and state tax purposes, respectively, that may be applied against future taxable income. The Company also has approximately $165.4$163.9 million of unused NOL carryforwards for New York City purposes. The NOL carryforwards will begin to expire in the year 20352037 if not utilized prior to that date. There is no provision for
Under Section 382 and Section 383 of the Internal Revenue Code of 1986, if a corporation undergoes an ownership change, the corporation’s ability to use its pre-change NOL carryforwards and other pre-change tax attributes to offset its post-change income taxes becausemay be limited. On each of August 10, 2015 and February 22, 2019 the Company experienced an ownership change. The Company anticipates a significant portion of its pre-change NOLs to be limited, however has historically incurred operating losses andnot yet completed a formal Section 382 analysis subsequent to the last ownership change.
The Company maintains a full valuation allowance against its net deferred tax assets.
The valuation allowance increased by approximately $13.3$18.9 million and $22.5$10.4 million during the years 20202023 and 2019,2022, respectively.The Company may be subjectincrease in valuation allowance in 2023 is primarily due to theincreases in NOL utilization provisionscarryforwards and capitalized research and experimental costs.
The tax effects of Section 382temporary differences that gave rise to significant portions of the Code. The effect of an ownership change would be the imposition of an annual limitation on the use of NOL carryforwards attributable to periods before the change. The amountdeferred tax assets and liabilities were as follows:
| | | | | | | | | | | |
| December 31, |
| 2023 | | 2022 |
| Deferred tax assets/liabilities: | | | |
| Net operating loss carryovers | $ | 55,653,286 | | | $ | 55,472,573 | |
| Intangible assets | 7,522,067 | | | 6,332,176 | |
| Capitalized research and experimental costs | 10,909,248 | | | 4,246,071 | |
| Stock-based compensation | 7,248,199 | | | 4,384,751 | |
| Royalty monetization liability | 8,427,970 | | | — | |
| Lease liability | 4,495,402 | | | 3,544,624 | |
| Research and development tax credits | 2,877,649 | | | 3,046,253 | |
| Accrued compensation | — | | | — | |
| Charitable contributions | — | | | — | |
| Depreciation | (166,075) | | | (241,096) | |
| Right-of-use asset | (3,903,379) | | | (3,198,857) | |
| Other | (434,957) | | | 97,494 | |
| Total gross deferred tax assets/liabilities | 92,629,410 | | | 73,683,989 | |
| Valuation allowance | (92,629,410) | | | (73,683,989) | |
| Net deferred tax assets (liabilities) | $ | — | | | $ | — | |
A reconciliation of the
annual limitation depends upon the value of the Company immediately before the change, changesstatutory U.S. Federal rate to the Company’s
capital during a specified period prior to the change, and the federal published interest rate. The Company has not completed a Section 382 analysis to determine if a change in ownership has occurred. Until an analysiseffective tax rate is
completed, there can be no assurance that the existing net operating loss carry-forwards or credits are not subject to significant limitation.as follows:
| | | | | | | | | | | |
| December 31, |
| 2023 | | 2022 |
| Federal income tax benefit at statutory rate | 21.00 | | | 21.00 | |
| State income tax, net of federal benefit | 9.12 | | | (0.27) | |
| Permanent items | (1.32) | | | (1.26) | |
| Change in valuation allowance | (29.23) | | | (18.50) | |
| Research and development tax credits | 1.05 | | | 1.28 | |
| Other | (0.62) | | | (2.25) | |
| Effective income tax expense rate | 0.00 | % | | 0.00 | % |
The Company’s reserves related to taxes are based on a determination of whether and how much of a tax benefit taken by the Company in its tax filings or positions is more likely than not to be realized following resolution of any potential contingencies related to the tax benefit. For the years ended December 31,
20202023 and
2019,2022, the Company had no unrecognized tax benefits or related interest and penalties accrued. The Company
has not yet conducted a study of research and development credit carryforwards. This study may result in an adjustment to the Company’s research and development credit carryforwards; however, until a study is completed, and any adjustment is known, no amounts are being presented as an uncertain tax position. A full valuation allowance has been provided against the Company’s research and development credits and, if an adjustment is required, this adjustment would be offset by an adjustment to the valuation allowance. Thus, there would be no impact to the balance sheet or statement of operations if an adjustment were required. The Company would recognize both accrued interest and penalties related to unrecognized benefits in
provision for income
tax
expense.taxes. The Company’s uncertain tax positions yet to be determined would be related to years that remain subject to examination by relevant tax authorities. Since the Company is in a loss carryforward position, the Company is generally subject to examination by the U.S. federal, state and local income tax authorities for all tax years in which a loss carryforward is available.
The tax effects of temporary differences that gave rise to significant portions of the deferred tax assets and liabilities were as follows:
| | December 31, | |
| | 2020 | | | 2019 | |
Deferred tax assets/liabilities: | | | | | | | | |
Net operating loss carryovers | | $ | 74,064,673 | | | $ | 57,288,334 | |
Research and development tax credits | | | 5,458,004 | | | | 3,351,315 | |
Share-based compensation | | | 4,978,370 | | | | 6,606,889 | |
Accrued compensation | | | 514,316 | | | | 898,067 | |
Depreciation | | | (29,760 | ) | | | (22,026 | ) |
Charitable contributions | | | 87,120 | | | | 132,072 | |
Intangible assets | | | 4,742,031 | | | | 8,208,519 | |
Total gross deferred tax assets/liabilities | | | 89,814,754 | | | | 76,463,170 | |
Valuation allowance | | | (89,814,754 | ) | | | (76,463,170 | ) |
Net deferred tax assets (liabilities) | | $ | - | | | $ | - | |
A reconciliation of the statutory U.S. Federal rate to the Company’s effective tax rate is as follows:
| | December 31, | |
| | 2020 | | | 2019 | |
Federal income tax benefit at statutory rate | | | (21.00 | ) | | | (21.00 | ) |
State income tax, net of federal benefit (1) | | | 6.15 | | | | (13.80 | ) |
Permanent items | | | 0.75 | | | | 0.50 | |
Change in valuation allowance | | | 16.72 | | | | 37.14 | |
Research and development tax credits | | | (2.64 | ) | | | (2.67 | ) |
Other | | | 0.02 | | | | (0.17 | ) |
Effective income tax (benefit) expense rate | | | 0 | % | | | 0 | % |
| | | | | | | | |
(1) Inclusive of $5.8 million deferred tax benefit due to change in apportionment | |
| | | | | | | | |
NOTE
910 – COMMITMENTS AND CONTINGENCIES
License Agreements
On March 26, 2015, the Company entered into an exclusive agreement with H. Lundbeck A/S (“Lundbeck”) for a worldwide perpetual licensing right related the research, development and commercialization of OV101. On May 10, 2019, the parties amended the license agreement.
Pursuant to the Lundbeck license agreement, as amended, the Company agreed to make milestone payments totaling up to $189.0 million upon the achievement of certain development, regulatory and sales milestones. The first payment of $1.0 million is due upon the successful completion of the first Phase 3 trial for a product in which OV101 is an active ingredient. In addition, the agreement calls for the Company to pay royalties for an initial term based on a low double-digit percentage of sales and provides for the reduction of royalties in certain limited circumstances. As of December 31, 2020, none of these contingent payments were considered probable.
Northwestern University License Agreement
In December 2016, the Company entered into a license agreement with(“Northwestern Agreement”) ///8with Northwestern University or Northwestern,(“Northwestern”), pursuant to which Northwestern granted usthe Company an exclusive, worldwide license to patent rights inof certain inventions or the (“Northwestern Patent Rights,Rights”) which relate to a specific compound and
related methods of use for such compound, along with certain
Know-Howknow-how related to the practice of the inventions claimed in the Northwestern
Patents. Patent Rights. The Company is developing OV329 under this agreement.
Under the Northwestern
agreement,Agreement, the Company was granted exclusive rights to research, develop, manufacture and commercialize products utilizing the Northwestern Patent Rights for all uses. The Company has agreed that it will not use the Northwestern Patent Rights to develop any products for the treatment of cancer, but Northwestern may not grant rights in the technology to others for use in cancer. The Company also has an option, exercisable during the term of the agreement to an exclusive license under certain intellectual property rights covering novel compounds with the same or similar mechanism of action as the primary compound that is
the subject of the license agreement. Northwestern has retained the right, on behalf of itself and other non-profit institutions, to use the Northwestern Patent Rights and practice the inventions claimed therein for educational and research purposes and to publish information about the inventions covered by the Northwestern Patent Rights.
Upon entry into the Northwestern
agreement,Agreement, the Company paid an upfront non-creditable one-time license issuance fee of $75,000 and is required to pay an annual license maintenance fee of $20,000, which will be creditable against any royalties payable to Northwestern following first commercial sale of licensed products under the agreement. The Company is responsible for all ongoing costs of filing, prosecuting and maintaining the Northwestern
Patents,Patent Rights, but also has the right to control such activities using its own patent counsel. In consideration for the rights granted to the Company under the Northwestern agreement, the Company is required to pay to Northwestern up to an aggregate of $5.3 million upon the achievement of certain development and regulatory milestones for the first product covered by the Northwestern
Patents,Patent Rights, and upon commercialization of any such products, will be required to pay to Northwestern a tiered royalty on net sales of such products by the Company, its affiliates or sublicensees, at percentages in the low to
mid single-digits,mid-single-digits, subject to standard reductions and offsets. The Company’s royalty obligations continue on a product-by-product and country-by-country basis until the later of the expiration of the last-to-expire valid claim in a licensed patent covering the applicable product in such country and 10 years following the first commercial sale of such product in such country. If the Company sublicenses a Northwestern Patent Right, it will be obligated to pay to Northwestern a specified percentage of sublicense revenue received by the Company, ranging from the high
single digitssingle-digits to the low-teens.
The Northwestern agreement requires that the Company use commercially reasonable efforts to develop and commercialize at least one product that is covered by the Northwestern Patent Rights.
Unless earlier terminated, the Northwestern agreement will remain in force until the expiration of the Company’s payment obligations thereunder. The Company has the right to terminate the agreement for any reason upon prior written notice or for an uncured material breach by Northwestern. Northwestern may terminate the agreement for the Company’s uncured material breach or insolvency.
AstraZeneca AB License Agreement
In December 2021, the Company entered into an exclusive license agreement with AstraZeneca AB (“AstraZeneca”), for a library of early-stage small molecules targeting the KCC2 transporter, including lead candidate OV350. Upon execution of the agreement, the Company was obligated to pay an upfront cash payment of $5.0 million and issued shares of the Company's common stock in an amount that equaled $7.3 million based on the volume-weighted average price of shares of the Company's common stock for the 30 business days immediately preceding the execution date of the transaction. Since the intangibles acquired in the AstraZeneca license agreement do not have an alternative future use, all costs incurred were treated as research and development expense. The Company recorded a total of $12.3 million as research and development expense related to this agreement during December 2021.
Pursuant to the AstraZeneca license agreement, the Company agreed to potential milestone payments of up to $203.0 million upon the achievement of certain developmental, regulatory and sales milestones. The first payment of $3.0 million is due upon the successful completion of the first Phase 2 clinical study of a licensed product following a positive biomarker readout in a Phase 1 clinical study.
Gensaic Collaboration and Option Agreement
In August 2022, the Company entered into a collaboration and option agreement with Gensaic (“Gensaic Collaboration Agreement”). The Gensaic Collaboration Agreement involves the research and development of phage-derived particle (“PDP”) products on Gensaic's proprietary platform for certain rare central nervous system (“CNS”) disorder targets.
Under the Collaboration Agreement, Gensaic grants the Company an option to obtain an exclusive license with respect to certain identified lead PDP products, which are exercisable at any time prior to the expiration of the option period. Once a product is identified by the Company that demonstrates sufficient efficacy, the Company may exercise its option with respect to the specific research program for that PDP product.
The Company shall reimburse Gensaic for Gensaic's research costs related to the specific research plan for PDP products identified. The research plan and budget shall be mutually agreed upon by the parties and shall not exceed $3.0 million in any research year. The Company will record these reimbursement payments as research and development costs in the period the research costs are incurred. In May 2023, the Company identified a lead PDP candidate for further research and provided $3.5 million to Gensaic to support the approved research plan and budget. The amount is expensed as the research and development occurs with the remaining amount included in prepaid expenses and other current assets in the condensed consolidated balance sheets.
If a product is ultimately commercialized under this agreement, the Company shall make tiered royalty payments to Gensaic in the mid-single to low double-digit range based on the net sales of all licensed PDP products during the royalty term. The Company is also responsible for potential tiered milestone payments of up to $452.0 million based upon the achievement of certain sales milestone events and developmental milestone approvals for three or more products. Gensaic also has the option to become a collaborative partner in the development and commercialization of PDP products in exchange for a fee based on a percentage of the costs incurred by the Company through the date Gensaic exercises its option. The Company would no longer be required to pay Gensaic royalty or milestone payments if Gensaic elects to exercise its option.
The Company may terminate this agreement by providing written notice to Gensaic 90 days in advance of the termination date.
As of December 31,
2020,2023, none of these contingent payments were considered probable.
Liabilities for loss contingencies arising from claims, assessments, litigation, fines, and penalties and other sources are recorded when it is probable that a liability has been incurred and the amount can be reasonably estimated. Legal costs incurred in connection with loss contingencies are expensed as incurred. The Company is not currently involved in any legal matters arising in the normal course of business.
Under the terms of their respective employment agreements, each of
ourthe Company's named executive officers is eligible to receive severance payments and benefits upon a termination without “cause” or due to “permanent disability,” or upon “resignation for good reason,” contingent upon the named executive officer’s delivery to the Company of a satisfactory release of claims, and subject to the named executive officer’s compliance with non-competition and non-solicitation restrictive covenants for two years following the termination date.
Pursuant to the Northwestern agreement, Northwestern granted the Company an exclusive license to certain patent rights and know-how, including a patent application covering a specified composition of matter (the “Patent Application”). Northwestern previously entered into a license agreement with Catalyst Pharmaceuticals, Inc. (“Catalyst”), dated August 27, 2009, pursuant to which Northwestern granted Catalyst rights under certain intellectual property rights covering a different composition of matter (the “Catalyst License”). In addition, the Company is a party to a confidential disclosure agreement with Catalyst, dated September 16, 2016 (the “CDA”). On June 25, 2018, Catalyst sent a letter to Northwestern and the Company alleging, among other things, that Northwestern breached the Catalyst License by licensing the Patent Application to the Company. Catalyst’s letter also asserted that the Company had breached its obligations under the CDA by allegedly failing to disclose that the Company had a license to the Patent Application, and that a further breach would occur if the Company makes any use of information obtained under the CDA in connection with its development program arising from the rights granted under the license agreement. Catalyst has asserted that the combined conduct of Northwestern and the Company gives rise to various claims, including breach of contract, fraud, and tortious interference. The Company believes that Catalyst’s claims are without merit and responded by letter dated June 28, 2018, which denies any and all liability to Catalyst, and further denies that Catalyst has been damaged in any way. On May 20, 2019, the Company entered into a Settlement Agreement with Catalyst (the “Settlement Agreement”), pursuant to which Catalyst released the Company from any and all claims, known or unknown, arising from or related to the dispute between Catalyst and Northwestern, the license agreement, and/or the claims that Catalyst asserted against the Company in the June 25, 2018 letter. Under the Settlement Agreement the Company retains all rights and privileges previously granted to the Company under the Northwestern license agreement.
NOTE
1011 – COLLABORATION
AGREEMENTAngelini Collaboration
On July 9, 2020, the Company entered into the Angelini License Agreement with Angelini, pursuant to which the Company granted to Angelini exclusive rights to develop and commercialize OV101, a selective agonist of the GABAA receptor, for the treatment of Angelman syndrome in the European Economic Area as well as Switzerland, the United Kingdom, Russia and Turkey (the “European Territory”). The licenses granted to Angelini include sublicenses under the Lundbeck Agreement, as well as licenses under the Company’s patents and know-how covering OV101. Angelini will be responsible for conducting any clinical trials necessary to obtain regulatory approval for OV101 for Angelman syndrome in the European Territory, and the Company will be responsible for bearing a portion of the costs for such trials. The Company will also be responsible, at its expense, for the completion of certain ongoing clinical trials for OV101, to the extent applicable to obtaining regulatory approval for OV101 in the European Territory. Angelini has the exclusive right, at its election, to develop and commercialize OV101 for the treatment of Fragile X Syndrome in the European Territory. The parties may also mutually agree to pursue additional indications for OV101 in the European Territory, and in such case, Angelini would have the exclusive rights to commercialize in such additional indications. Angelini is required to use commercially reasonable efforts to conduct development activities for OV101, and following regulatory approval, to commercialize OV101 in each approved indication.
In conjunction with the entry into the Angelini License Agreement, the parties entered into a separate supply agreement, pursuant to which the Company will be responsible for supply of OV101 to Angelini for development and commercialization in the European Territory, through its existing supply relationship with Lundbeck. The Angelini License Agreement also provides for a transfer, at Angelini’s expense, of the relevant manufacturing technology from the Company and Lundbeck to Angelini, in order to enable Angelini to assume responsibility for its own manufacture and supply of OV101 in the future.
Under the Angelini License Agreement, Angelini made an upfront payment and a milestone payment related to the transfer of a specified amount of compound and related information to the Company of $25.0 million during the year ended December 31, 2020. Angelini will be required to make additional milestone payments to the Company upon the completion of the specified components of the technology transfer and achievement of specified regulatory milestones for OV101 in Angelman syndrome of up to $55.0 million in the aggregate, as well as up to €162.5 million ($199.9 million) in sales milestone payments for achievement of specified levels of net sales in the European Territory. In addition, Angelini will be required to pay tiered royalties on net sales by Angelini, its affiliates or sublicensees at double-digit percentages above the teens, subject to certain standard reductions and offsets. Royalties will be payable on a product-by-product and country-by-country basis until the latest of the expiration of the licensed patents covering such product in such country, the expiration of market exclusivity for such product in such country, and fifteen years from first commercial sale of such product in such country.
Either party may terminate the Angelini License Agreement for an uncured material breach of the other party or in the case of insolvency. The Company may terminate the Angelini License Agreement if Angelini challenges any of the licensed patents. Angelini may terminate the Angelini License Agreement for convenience on specified notice periods, which are determined based upon whether the product has been commercially launched in the European Territory.
The Company identified the following material promises under the Angelini License Agreement: (1) licensing of intellectual property with respect to OV101 (2) completion of certain ongoing trials (3) transfer of a specified amount of compound and related information (4) potential for funding 35% of the cost for Angelini future trials limited to $7.0 million and (5) completion of the manufacturing process technology transfer.
The Company determined that the $7.0 million represents a potential payment to a customer and should be deferred. The transfer of compound and related information is considered a contingent milestone payment that will be recognized upon acceptance by Angelini of the milestone which was achieved and recognized in December 2020. The Company further determined that the license and the completion of ongoing trials are distinct from each other, as each has value without the other.
The Company determined the transaction price is equal to the up-front fee of $20.0 million. The transaction price was allocated based on the standalone selling price of the license, the ongoing trials and $7.0 million for potential future trials.
Upon the transfer of the specified amount of compound and related information and acceptance by Angelini, Angelini was required to and made a $5.0 million payment. This performance obligation was determined to be variable consideration which was constrained and not considered part of the upfront transaction price allocation.
Angelini will be required to make a separate $5.0 million tech transfer payment towards the $55.0 million aggregate tech transfer and regulatory milestone payments upon the successful completion of the manufacturing process technology transfer. The Company earning this is fully dependent on performance and cooperation of Angelini and Lundbeck in implementing the Technology Transfer. At this time, the Company cannot estimate if or when this milestone-related performance obligation might be achieved.
AGREEMENTS
During the year ended December 31, 2020, the Company recognized $6.1 million of license revenue and $1.5 million relating to the progress of the ongoing trials as well as $5.0 million for the transfer of the specified amount of compound and related information. The portion of the upfront payment allocated to License Revenue was recognized in full as it was non-refundable and not contingent on any future performance and requires no consequential continuing involvement by the Company. The Company did not have any such revenue during the year ended December 31, 2019. In addition, the Company recorded deferred revenue in the amount of approximately $12.4 million as of December 31, 2020 which will be recognized over the term of the ongoing trials based on the portion of total estimated expenses incurred. The Company has classified deferred revenue as current or noncurrent based on the expected timing of such expenses.
Takeda Collaboration
On
In January
6, 2017, the Company entered into a license and collaboration agreement with Takeda
pursuant to which Takeda granted the Company an exclusive license to commercialize the compound TAK-935,under which the Company
referslicensed from Takeda certain exclusive rights to
as OV935,develop and commercialize soticlestat in certain
territories, and a co-exclusive worldwide license, together with Takeda, to develop OV935. In consideration of certain license rights granted to the Company pursuant to the Takeda collaboration, the Company issued 1,781,996 shares of its Series B-1 Preferred Stock, pursuant to a Series B-1 preferred stock purchase agreement entered into on January 6, 2017, at an ascribed price per share of $14.513 on January 6, 2017 for an aggregate fair value of $25.9 million, which was recorded as research and development expense at the date of the transaction. The 1,781,996 shares of Series B-1 Preferred Stock held by Takeda automatically converted into 1,781,996 shares of the Company’s common stock upon the completion of its IPO in 2017. Under the Takeda collaboration, the Company is obligated to pay Takeda future payments if and when certain milestones are achieved. Upon the first patient enrollment in the first Phase 3 trial for the first of the initial indications the Company and Takeda are focusing on in the Takeda collaboration, the Company is obligated to issue to Takeda the number of unregistered shares of the Company’s common stock equal to the lesser of (a) 8% of the Company outstanding capital stock on the issuance date or (b) $50.0 million divided by the applicable share price, unless certain events occur. In the event such payment would cause Takeda to own over 19.99% of our outstanding capital stock or other events occur, such payment must be paid in cash. The remaining potential global commercial and regulatory milestone payments equal approximately $35.0 million and can be satisfied in cash or unregistered shares of the Company’s common stock at its election, unless certain events occur. As of December 31, 2020, none of these contingent payments have been triggered. During the year ended December 31, 2020, the Company recognized $0.7 million in research and development expenses representing research and development expenses reimbursable to the Company from Takeda, of which $0.1 million was included in related party receivable as of December 31, 2020 and $2.1 million was included in related party payable as of December 31, 2020. During the year ended December 31, 2019, the Company recognized a credit in research and development expenses of $4.7 million representing costs reimbursable to the Company from Takeda, of which $1.1 million was included in related party receivable as of December 31, 2019. The Takeda collaboration will expire upon the cessation of commercialization of the products by both the Company and Takeda. Either party may terminate the Takeda collaboration because of the other party’s uncured material breach or insolvency, for safety reasons, or, after completion of the first proof of mechanism clinical trial, for convenience. Takeda may terminate the Takeda collaboration for the Company’s (or the Company’s sublicensee’s) challenge to the patents licensed under the Takeda collaboration. If the collaboration is terminated by Takeda for material breach by the Company, bankruptcy or patent challenge or by the Company for convenience or safety reasons, the Company’s rights to the products will cease, the Company will transition all activities related to the products to Takeda, and the Company will grant Takeda an exclusive, royalty-bearing license under certain patents and other intellectual property controlled by the Company to commercialize OV935 and products containing OV935 for the treatment of certain rare neurological disorders. If the collaboration is terminated by the Company for Takeda’s material breach or bankruptcy or by Takeda for convenience or safety reasons, Takeda’s rights to the products will cease, Takeda will transition all activities related to the products to us, and Takeda will grant us an exclusive, royalty-bearing license under certain patents and other intellectual property controlled by Takeda to commercialize OV935 and products containing OV935 for the treatment of certain rare neurological disorders.
territories.
In March 2021, the Company entered into the RLT Agreement with Takeda, pursuant to which Takeda secured rights to the Company’s 50% global share in soticlestat, and the Company granted to Takeda an exclusive worldwide license under the Company’s relevant intellectual property rights to develop and commercialize the investigational medicine soticlestat for the treatment of developmental and epileptic encephalopathies, including Dravet syndrome and Lennox-Gastaut syndrome.
Under the RLT Agreement, all rights in soticlestat are owned by Takeda or exclusively licensed to Takeda by the Company. Takeda assumed all responsibility for, and costs of, both development and commercialization of soticlestat, and the Company will no longer have any financial obligation to Takeda under the original collaboration agreement, including milestone payments or any future development and commercialization costs. In March 2021, upon the closing of the RLT Agreement, the Company received an upfront payment of $196.0 million and, if soticlestat is successfully developed, will be eligible to receive up to an additional $660.0 million upon Takeda achieving developmental, regulatory and sales milestones. In addition, the Company will be entitled to receive tiered royalties beginning in the low double-digits, and up to 20% on sales of soticlestat if regulatory approval is achieved. Royalties will be payable on a country-by-country and product-by-product basis for any indications that soticlestat is approved for and sold during the period beginning on the date of the first commercial sale of such product in such country and ending on the later to occur of the expiration of patent rights covering the product in such country and a specified anniversary of such first commercial sale. In 2023, the Company sold a 13% stake in the royalty, regulatory and commercial milestone payments that the Company is eligible to receive under the RLT Agreement to Ligand for $30.0 million.
During the years ended December 31, 2023 and 2022, no income or expense was recognized pursuant to the RLT Agreement.
Healx License and Option Agreement
In February 2022, the Company entered an exclusive license option agreement (“Healx License and terminationOption Agreement”) with Healx, Ltd. (“Healx”). Under the terms of the Healx License and Option Agreement, Healx secured a one-year option to investigate gaboxadol (“OV101”) as part of a potential combination therapy for Fragile X syndrome in a Phase 1B/2A clinical trial, as well as a treatment for other indications, for an upfront payment of $0.5 million, and fees to support prosecution and maintenance of the Company's relevant intellectual property rights. At the end of the one-year option period, Healx had the option to secure rights to an exclusive license under the Company's relevant intellectual property rights, in exchange for an additional payment of $2.0 million, development and commercial milestone payments, and low to mid-tier double-digit royalties. In February 2023, the Company granted an extension of the option period for up to four months for Healx to continue to investigate gaboxadol. Royalties are payable on a country-by-country and product-by-product basis during the period beginning on the date of the first commercial sale of such product in such country and ending on the later to occur of the expiration of patent rights covering the product in such country and a specified anniversary of such first commercial sale.
Healx will assume all responsibility for, and costs of, both development and commercialization of gaboxadol following the exercise of the option. The Company will retain the option to co-develop and co-commercialize the program with Healx (“Ovid Opt-In Right”), at the end of a positive readout of clinical Phase 2B and would share net profits and losses in lieu of the milestones and royalty payments. If the Ovid-Opt-In Right were exercised, the Company would be required to pay Healx 50% of development costs. The Company does not plan to conduct further trials of gaboxadol. The term of the Healx License and Option Agreement will continue until the later of (a) the expiration of all relevant royalty terms, or in the event that Healx does not exercise its option during the option period defined in the Healx License and Option Agreement (“Option Period”), the expiration of such period, or (b) in the event that Healx does exercise its option during the Option Period, and the Company does not exercise the Ovid Opt-In Right during the period of time it has to opt-in (“Opt-In Period”) or the opt-in terms are otherwise terminated, upon the expiration of all payment obligations, or (c) in the event that Healx does exercise the Option during the Option Period, and the Company does exercise the Ovid Opt-In Right during the Opt-In Period, such time as neither Healx nor the Company is continuing to exploit gaboxadol. Further, if the Company exercises the Ovid Opt-In Right to co-develop and co-commercialize the program, it will owe an equal share of any net profits to a third party with which it previously established a licensing agreement. If the Company does not exercise the Ovid Opt-In Right, it will owe the third party an equal share of all milestone and royalty payments received.
In June 2023, the Company entered into an amendment to the Healx License and Option Agreement whereby revisions were made to terms regarding the timing of the option exercise fee payable by Healx to the Company, the clinical and regulatory milestone payment structure, and the royalty payment structure. Additionally, the parties agreed that following the exercise of the option, Healx would assume direct responsibility for patent maintenance and prosecution and that the Company would transfer to Healx all supply obligations with respect to the active pharmaceutical ingredient and finished gaboxadol products and any related licensed technology and know-how in the Company's possession that is relevant to the manufacture of such licensed products.
No revenue was recognized relating to this agreement during the year ended December 31, 2023. During the year ended December 31, 2022, the Company recorded revenue of $0.5 million associated with the Healx License and Option Agreement.
Marinus Pharmaceuticals Out-License Agreement
In March 2022 the Company entered into an exclusive patent license agreement with Takeda (see note 12)Marinus (“Marinus License Agreement”). Under the Marinus License Agreement, the Company granted Marinus an exclusive, non-transferable (except as expressly provided therein), royalty-bearing right and license under certain Ovid patents relating to ganaxolone to develop, make, have made, commercialize, promote, distribute, sell, offer for sale and import licensed products in the territory (which consists of the United States, the European Economic Area, United Kingdom and Switzerland) for the treatment of CDKL5 deficiency disorders. Following the date of regulatory approval by the FDA of the first licensed product in the territory which was received on March 18, 2022, Marinus issued, at the Company's option, 123,255 shares of Marinus common stock, par value $0.001 per share, as payment. The Marinus License Agreement also provides for payment of royalties from Marinus to the Company in single-digits on net sales of each such licensed product sold.
The Company recorded revenue and an associated investment in equity securities of approximately $0.9 million related to the Marinus License Agreement in March 2022, based on the price of Marinus common stock at that time. The Company had unrealized gains on the Marinus common stock of $0.8 million for the year ended December 31, 2023, and unrealized loss of $0.5 million for the year ended December 31, 2022, which were recorded as unrealized gains (losses) on equity securities and reflected in other income (expenses), net in the consolidated statements of operations.
Graviton License Agreement and Equity Purchase
In April 2023, the Company entered into a collaboration and license agreement with Graviton (“Graviton Agreement”), whereby it secured from Graviton an exclusive license to develop and commercialize Graviton's library of ROCK2 inhibitors including their lead program GV101 (OV888) in rare CNS disorders (excluding amyotrophic lateral sclerosis) worldwide (excluding China, Hong Kong, Macau and Taiwan). Under the Graviton Agreement, the Company and Graviton plan to investigate GV101 in cerebral cavernous malformations as well as Graviton's library of ROCK2 inhibitors in other rare CNS disorders. The Company will be responsible for all development and commercialization costs of the products. Should the Company receive regulatory approval and commercialize any of Graviton’s ROCK2 inhibitors, it will pay Graviton tiered royalties on net sales ranging from the mid to high teens. As part of the Graviton Agreement, the Company also purchased shares of Graviton's preferred stock for $10.0 million. The Company recorded the purchase of the preferred stock as a long-term equity investment on its consolidated balance sheets. In December 2023, the Company recognized an unrealized gain on the investment due to an observable change in price, and recorded the gain in other income (expense), net, in the consolidated statements of operations.
NOTE
1112 – RELATED PARTY TRANSACTIONS
As part of the Company’s collaboration agreement with Takeda the Company recognized a long-term liability representing long-term prepaid expenses to be reimbursed to Takeda.
On March 24, 2019, the Company entered into a separation and consulting agreement with Dr. Matthew During in connection with Dr. During’s resignation as President and Chief Scientific Officer with the Company effective as of April 1, 2019. Pursuant to the separation and consulting agreement, Dr. During agreed to non-solicit and non-compete covenants through such time as he remains a consultant to the Company, as well as a general release of claims in connection therewith. Dr. During agreed to a three-year consulting arrangement, pursuant to which he will be paid, amongst other specific milestone and meeting related fees, $150,000 per year for his role as the Chairman of the Company’s Scientific Advisory Board and $150,000 per year for other advisory and consulting services. Further, Dr. During was granted options to acquire 100,000 shares of common stock at an exercise price of $1.76 per share, the fair market value on April 1, 2019, which options shall vest in full upon completion of a specific clinical milestone, subject to Dr.
During’s continued service through such vesting date. In the event such option does not vest by December 31, 2020, the stock option will expire. Provided further, in recognition of Dr. During’s service on the Scientific Advisory Board, Dr. During was granted options to acquire 75,000 shares of common stock at an exercise price equal to $1.76 per share, the fair market value on April 1, 2019. Either Dr. During or the Company may terminate the consulting arrangements pursuant to the Consulting Agreement in accordance with its terms, at any time and for any reason, upon thirty (30) days written notice to the other party. Upon such termination, the Company will have no further obligations to Dr. During, including any obligation to pay further consulting fees.
In February 2019, the Company issued and sold an aggregate of 6,325,000 shares of common stock and 2,500 shares of Series A Preferred Stock to entities affiliated with Takeda, a collaboration partner and an existing stockholder, entities affiliated with Biotechnology Value Fund, L.P., an existing stockholder, and Dr. Jeremy M. Levin, its Chief Executive Officer and Chairman, for aggregate gross proceeds of $17.7 million.
In October and November 2019, the Company issued and sold an aggregate of 4,058,000 shares of common stock and 2,000 shares of Series A Preferred Stock to entities affiliated with Takeda, a collaboration partner and an existing stockholder, entities affiliated with Biotechnology Value Fund, L.P., an existing stockholder, and Dr. Jeremy M. Levin, its Chief Executive Officer and Chairman, for aggregate gross proceeds of $10.2 million.
In September 2019, the Company entered into an exchange agreement with the Exchanging Stockholders pursuant to which the Company exchanged an aggregate of 1,262,000 shares of the Company’s common stock owned by the Exchanging Stockholders for an aggregate of 1,262 shares of the Company’s Series A Preferred Stock.
In May 2020, entities affiliated with Biotechnology Value Fund, L.P. elected to convert an aggregate of 2,256 shares of Series A Preferred Stock owned by such holders into an aggregate of 2,256,000 shares of the Company’s common stock.
In August 2020, the Company issued and sold an aggregate of 1,250,000 shares of common stock to entities affiliated with Biotechnology Value Fund, L.P., an existing stockholder for aggregate gross proceeds of $10.0 million.
In December 2020, entities affiliated with Biotechnology Value Fund, L.P. elected to convert an aggregate of 2,256 shares of Series A Preferred Stock owned by such holders into an aggregate of 2,256,000 shares of the Company’s common stock.
NOTE 12 – SUBSEQUENT EVENTS
Equity Awards
From January 1, 2021 through the date of the filing of this Form 10-K, the Company has granted option awards for an aggregate of 643,600 shares of common stock to employees with a weighted average exercise price of $3.18.
Royalty, License and Termination Agreement with Takeda
In March 2021, the Company entered into the RLT Agreement with Takeda. For a royalty, licensedescription of the RLT Agreement, see Note 11.
NOTE 13 – NET LOSS PER SHARE
Basic net loss per share is calculated based upon the weighted-average number of common shares outstanding during the period, excluding outstanding stock options that have not yet vested. For any period in which the Company records net income, diluted net income per share is calculated based upon the weighted-average number of common shares outstanding during the period plus the dilutive impact of weighted-average common equivalent shares outstanding during the period resulting from the assumed exercise of outstanding stock options determined under the treasury stock method and termination agreement (the “Takeda License and Termination Agreement”) with Takeda under which Takeda will secure rightsthe assumed conversion of preferred stock into common shares determined using the if-converted method. Diluted net loss per share is equivalent to the basic net loss per share due to the exclusion of outstanding stock options and convertible preferred stock because the inclusion of these securities would result in an anti-dilutive effect on per share amounts.
The basic and diluted net loss per common share is presented in conformity with the two-class method required for participating securities and multiple classes of shares. The Company considers its preferred stock to be participating securities.
For any period in which the Company records net income, undistributed earnings allocated to the participating securities are subtracted from net income in determining net income attributable to common stockholders. The undistributed earnings have been allocated based on the participation rights of preferred stock and common shares as if the earnings for the year have been distributed. For periods in which the Company recognizes a net loss, undistributed losses are allocated only to common shares as the participating securities do not contractually participate in the Company’s 50% globallosses. Basic net loss per share in soticlestat,is computed by dividing the net loss attributable to common stockholders by the weighted-average number of common shares outstanding during the period. Participating securities are excluded from basic weighted-average common shares outstanding.
The following tables summarizes the calculation of basic and an exclusive license under our relevant intellectual property rights and global rights at closingdiluted net loss per share:
| | | | | | | | | | | |
| For the Year Ended December 31, |
| 2023 | | 2022 |
| Net loss | $ | (52,338,959) | | | $ | (54,169,029) | |
| Net income attributable to participating securities | — | | | — | |
| Net loss attributable to common stockholders | $ | (52,338,959) | | | $ | (54,169,029) | |
| | | | | | | | | | | |
| For the Year Ended December 31, |
| 2023 | | 2022 |
| Net loss attributable to common stockholders | $ | (52,338,959) | | | $ | (54,169,029) | |
| Weighted average common shares outstanding used in computing net loss per share - basic | 70,580,604 | | | 70,424,819 | |
| Weighted average common shares outstanding used in computing net loss per share - diluted | 70,580,604 | | | 70,424,819 | |
| Net loss per share, basic | $ | (0.74) | | | $ | (0.77) | |
| Net loss per share, diluted | $ | (0.74) | | | $ | (0.77) | |
The following potentially dilutive securities have been excluded from the
Company to develop and commercialize the investigational medicine OV935 for the treatmentcomputations of
developmental and epileptic encephalopathies, including Dravet syndrome and Lennox-Gastaut syndrome.Under the Takeda License and Termination Agreement, all rights in OV935 will be owned by Takeda, or exclusively licensed to Takeda by the Company. Takeda will assume sole responsibility for further worldwide development and commercialization, and the Company will no longer have any financial obligation to Takeda under the original collaboration agreement, including for milestone payments or any future development and commercialization costs. Should the Company close the Takeda License and Termination Agreement, the Company will receive an upfront payment of $196.0 million at closing and will be eligible to receive up to an additional $660.0 million upon achieving development, regulatory and sales milestones. In addition, the Companydiluted weighted-average shares outstanding as they would be entitled receive tiered royalties beginning in the low double-digits, and up to 20% on sales of OV935, if approved and commercialized.
The Company expects to close the Takeda License and Termination Agreement in the first half of 2021, subject to the satisfaction of closing conditions, including regulatory review by the appropriate regulatory agencies under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended. Either party may terminate the agreement if it does not close by May 14, 2021.
F-22
anti-dilutive: | | | | | | | | | | | |
| For the Year Ended December 31, |
| 2023 | | 2022 |
| Stock options to purchase common stock | 15,124,546 | | | 12,961,238 | |
| Common stock issuable upon conversion of Series A convertible preferred stock | 1,250,000 | | | 1,250,000 | |









