96666666
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
Form 10-K
(Mark One)
| |
☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31 2021, 2023
or
| |
☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 FOR THE TRANSITION PERIOD FROM TO |
Commission File Number 000-52024
ALPHATEC HOLDINGS, INC.
(Exact Name of Registrant as Specified in its Charter)
| | |
Delaware |
| 20-2463898 |
(State or Other Jurisdiction of Incorporation or Organization) |
| (I.R.S. Employer Identification No.) |
|
|
|
1950 Camino Vida Roble, Carlsbad, California |
| 92008 |
(Address of Principal Executive Offices) |
| (Zip Code) |
(760) (760) 431-9286
(Registrant’s Telephone Number, Including Area Code)
Securities registered pursuant to Section 12(b) of the Exchange Act:
| | |
Title of Each Class | Trading Symbol(s) | Name of Each Exchange on Which Registered |
Common Stock, par value $0.0001 per share | ATEC | The NASDAQ Global Select Market |
Securities registered pursuant to Section 12(g) of the Exchange Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes☒ No ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Exchange Act. Yes ☐No☒
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§(§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| | | |
Large accelerated filer | ☒ | Accelerated filer | ☐ |
Non-accelerated filer | ☐ | Smaller reporting company | ☒☐
|
| | Emerging growth company | ☐ |
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes Oxley Act (15 U.S.C. 7262 (b)) by the registered public accounting firm that prepared or issued its audit report. ☒
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☒
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by a check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
The aggregate market value of the registrant’s common stock held by non-affiliates of the registrant (without admitting that any person whose shares are not included in such calculation is an affiliate) computed by reference to the price at which the common stock was last sold as of the last business day of the registrant's most recently completed second fiscal quarter (June 30, 2021)2023), was approximately $1.0$1.6 billion.
The number of outstanding shares of the registrant’s common stock, par value $0.0001 per share, as of February 24, 202219, 2024 was 99,786,612.137,979,126.
DOCUMENTS INCORPORATED BY REFERENCE
The following documents (or parts thereof) are incorporated by reference into the following parts of this Form 10-K: Certain information required in Part III of this Annual Report on Form 10-K is incorporated from the Registrant’s Proxy Statement for the 20222024 Annual Meeting of Stockholders.
| | | | | |
Auditor Firm Id: | 34 | Auditor Name: | Deloitte & Touche LLPLLP | Auditor Location: | New York, New York, United States |
ALPHATEC HOLDINGS, INC.
FORM 10-K—ANNUAL REPORT
For the Fiscal Year Ended December 31, 20212023
Table of Contents
In this Annual Report on Form 10-K, the terms “we,” “us,” “our,” "ATEC," “Alphatec Holdings” and “Alphatec” mean Alphatec Holdings, Inc., our subsidiaries and their subsidiaries. “Alphatec Spine” refers to our wholly-owned operating subsidiary Alphatec Spine, Inc. “SafeOp” refers to our wholly owned operating subsidiary SafeOp Surgical, Inc. “EOS” refers to our wholly owned operating subsidiary EOS imaging S.A.S.A.S.
PART I
FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K and, in particular, the description of our "Business" set forth in Item 1, the "Risk Factors" set forth in this Item 1A and our "Management’s Discussion and Analysis of Financial Condition and Results of Operations" set forth in Item 7 contain or incorporate a number of forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended or the Securities Act,(the "Securities Act"), and Section 21E of the Securities Exchange Act of 1934, as amended or the Exchange Act,(the "Exchange Act"), including statements regarding:
| • our estimates regarding anticipated operating losses, future revenue, expenses, capital requirements, uses and sources of cash and liquidity, including our anticipated revenue growth and cost savings; •our ability to achieve profitability, and the potential need to raise additional funding; •our ability to ensure that we have effective disclosure controls and procedures; •our ability to meet, and potential liability from not meeting, any outstanding commitments and contractual obligations; •our ability to maintain compliance with the quality requirements of the United States ("U.S.") Food and Drug Administration ("FDA") and similar foreign regulatory requirements; •our ability to market, improve, grow, commercialize and achieve market acceptance of any of our products or any product candidates that we are developing or may develop in the future; •our ability to continue to enhance our product offerings, and to commercialize and achieve market acceptance of any of our products or product candidates; •the effect of any existing or future federal, state or international regulations on our ability to effectively conduct our business; •our business strategy and our underlying assumptions about market data, demographic trends, reimbursement trends and pricing trends; •our ability to maintain an adequate global sales network for our products, including to attract and retain independent sales agents and direct sales representatives; •our ability to increase the use and promotion of our products by training and educating spine surgeons and our global sales network; •our ability to attract and retain a qualified management team, as well as other qualified personnel and advisors; •our ability to enter into licensing and business combination agreements with third parties and to successfully integrate the acquired technology and/or businesses; •our ability to successfully incorporate Valence into our business; •the impact of global economic and political conditions and public health crises on our business and industry; and •other factors discussed elsewhere in this Annual Report on Form 10-K or any document incorporated by reference herein or therein. •
| our estimates regarding anticipated operating losses, future revenue, expenses, capital requirements, uses and sources of cash and liquidity, including our anticipated revenue growth and cost savings;
|
| •
| our ability to ensure that we have effective disclosure controls and procedures;
|
| •
| our ability to meet, and potential liability from not meeting, the payment obligations under the Orthotec settlement agreement;
|
| •
| our ability to maintain compliance with the quality requirements of the FDA;
|
| •
| our ability to market, improve, grow, commercialize and achieve market acceptance of any of our products or any product candidates that we are developing or may develop in the future;
|
| •
| our beliefs about the features, strengths and benefits of our products;
|
| •
| our ability to continue to enhance our product offerings, outsource our manufacturing operations and expand the commercialization of our products, and the effect of our strategy;
|
| •
| our ability to successfully integrate, and realize benefits from licenses and acquisitions;
|
| •
| the effect of any existing or future federal, state or international regulations on our ability to effectively conduct our business;
|
| •
| our estimates of market sizes and anticipated uses of our products;
|
| •
| our business strategy and our underlying assumptions about market data, demographic trends, reimbursement trends and pricing trends;
|
| •
| our ability to achieve profitability, and the potential need to raise additional funding;
|
| •
| our ability to maintain an adequate sales network for our products, including to attract and retain independent distributors;
|
| •
| our ability to enhance our U.S. distribution network;
|
| •
| our ability to increase the use and promotion of our products by training and educating spine surgeons and our sales network;
|
| •
| our ability to attract and retain a qualified management team, as well as other qualified personnel and advisors;
|
| •
| our ability to enter into licensing and business combination agreements with third parties and to successfully integrate the acquired technology and/or businesses;
|
| •
| other factors discussed elsewhere in this Annual Report on Form 10-K or any document incorporated by reference herein or therein.
|
Any or all of our forward-looking statements in this Annual Report may turn out to be wrong. They can be affected by inaccurate assumptions by known or unknown risks and uncertainties. Many factors mentioned in our discussion in this Annual Report on Form 10-K will be important in determining future results. Consequently, no forward-looking statement can be guaranteed. Actual future results may vary materially from expected results.
We also provide a cautionary discussion of risks and uncertainties under “Risk Factors” in Item 1A of this Annual Report. These are factors that we think could cause our actual results to differ materially from expected results. Other factors besides those listed there could also adversely affect us.
Without limiting the foregoing, the words “believe,” “anticipate,” “plan,” “expect,” “may,” “could,” “would,” “seek,” “intend,” and similar expressions are intended to identify forward-looking statements. There are a number of factors and uncertainties that could cause actual events or results to differ materially from those indicated by such forward-looking statements, many of which are beyond our control, including the factors set forth under “Item 1A Risk Factors.” In addition, the forward-looking statements contained herein represent our estimate only as of the date of this filing and should not be relied upon as representing our estimate as of any subsequent date. While we may elect to update these forward-looking statements at some point in the future, we specifically disclaim any obligation to do so to reflect actual results, changes in assumptions or changes in other factors affecting such forward-looking statements, except as required by applicable law.
Item 1.B Businessusiness
We are a medical technology company, headquartered in Carlsbad, California, focused on the design, development, and advancement of technology for better surgical treatment of spinalspine disorders. ThroughBy applying our wholly owned subsidiaries, Alphatec Spine, Inc., SafeOp Surgical, Inc.unique, 100% spine focus and EOS imaging S.A., our mission isdeep, collective industry know-how, we aim to revolutionize the approach to spine surgery through clinical distinction. We are focused on developing new The sophisticated approaches that we create from the ground up integrate seamlessly with our expanding Alpha InformatiX™ ("AIX") product platform to betterobjectively inform surgery and to achieve the goalgoals of spine surgery more safelypredictably and more reproducibly. We have a broadcomprehensive product portfolio designed to address the spine’s various pathologies. Ourpathologies, and are perpetually innovating to accomplish our ultimate vision, which is to be the standard bearer in spine.
In 2018, we embarked on a business transformation, replacing 100% of the executive team, 90% of our Board of Directors, and over 65% of the remaining team with spine-experienced professionals. Efforts that year founded our Organic Innovation Machine™, our in-house product design, development and testing capabilities aimed at the creation of clinical distinction, and furthered the strategic transition of our sales force to a more dedicated and clinically astute team. Since 2018, we believe that we have achieved the highest organic U.S. growth of any public spine company in every quarter by capitalizing on the collective spine experience that we have amassed and by investing in research and development to continually bring to market differentiated products and procedures designed to better meet the requirements of spine surgery.
Revenue from products and servicesTotal revenue was $242.3$482.3 million for the year ended December 31, 2021,2023, representing an increase of $101.2$131.4 million, or 72%37% compared to $141.1$350.9 million for the year ended December 31, 2020.2022. We believe our future success will continue to be fueled by the introduction and tractionincreasing adoption of the distinct productsnew and proceduresexisting integrated technologies that we have developed anddistinguish our approach-specific procedures. We feel that we areATEC is well-positioned to continue to capitalize on current spine market dynamics.
Recent DevelopmentsBackground
0.75% Senior Convertible Notes due 2026The year 2018 marked the beginning of a business transformation that replaced 100% of our executive team, 92% of our Board of Directors, and 96% of the remaining team with experienced professionals, infusing industry-leading spine know-how throughout our organization. Efforts that year founded the ATEC Organic Innovation Machine™, in-house product design, development and testing capabilities that harnessed the team’s collective spine expertise to create clinical distinction.
In AugustFrom 2019 through 2021, we issued $316.3 million principal amountfocused on building a foundation capable of unsecured senior convertible notes withsupporting the organization as we scale. We invested in new headquarters to substantially increase surgeon and sales training capacity and opened a stated interest rate of 0.75% (the “2026 Notes”). Interest on the 2026 Notes is payable semi-annuallydistribution facility in arrears on February 1Memphis, Tennessee, to ensure predictable and August 1 of each year, beginning on February 1, 2022. The 2026 Notes are convertible into sharesexpedient surgical support as our footprint expands. We developed and released several key elements of our common stock based upon an initial conversion rateapproach-based portfolio, including a comprehensive posterior fixation system and approach-specific IdentiTi™ porous titanium implants. We also acquired and integrated SafeOp™, technology that integrates uniquely with our approaches to provide information about both the location and the health of 54.5316 shares of our common stock per $1,000 principal amount of 2026 Notes (equivalent to an initial conversion price of approximately $18.34 per share). The initial conversion pricenerves intra-operatively. SafeOp became the informational foundation of the 2026 Notes represents a premiumProne TransPsoas (“PTP”) approach, which we developed and launched in 2020 to advance first-generation lateral spine surgery. We also acquired EOS®, technology that enables full-body, calibrated, 3D images that integrate throughout the span of approximately 100% overpatient spine care to influence procedure planning and improve and quantify the closing priceunderstanding of global alignment. As we execute our common stock on August 5, 2021, the date the 2026 Notes offering was priced. The net proceeds from the sale of the 2026 Notes were approximately $306.2 million after deducting the offering expenses. The 2026 Notesvision for EOS, we believe it will mature on August 1, 2026, unless earlier converted, redeemed, or repurchased.set new standards for spine care.
From 2022 to 2023, the momentum of PTP™ was robust, as both lateral-experienced and surgeons new to lateral surgery adopted the approach. We applied learnings from PTP to develop and introduce the Lateral TransPsoas ("LTP") and Midline ALIF approaches. Like PTP, the approaches were built from the ground up and integrated with SafeOp, and are designed to enable single-position surgery for the most commonly treated levels in spine. We believe that our lateral franchise boasts unparalleled optionality, the capacity to address the clinical requirements for every pathology and surgeon preference regardless of patient position. The lateral sophistication that we have created is earning surgeons’ confidence and loyalty, and that is fueling portfolio-wide utilization of even our most conventional procedures.
The application of our team’s deep spine know-how, coupled with a willingness to invest holistically in each of the technologies integrated into all of ATEC’s procedural approaches continues to increasingly compel surgeons and sales talent to partner with us. That adoption-driven validation has been the source of industry-leading market share expansion, which has delivered an approximately 40% revenue compound annual growth rate since our transformation commenced in 2018.
Recent Developments
Public Offering
On October 27, 2023, we completed an underwritten public offering (the “Public Offering”) of 14,300,000 shares of our common stock at a price of $10.50 per share. In connection with the Public Offering, we granted the underwriters a 30-day option (the "Green-Shoe") to purchase additional shares of common stock in the offering at the public offering price, less underwriting discounts and commissions. The net proceeds from the Public Offering and Green-Shoe, were approximately $145.8 million, including the underwriting discounts and commissions and offering expenses paid by us. We used $39.9 million ofexpect to use the net proceeds from the 2026 Notes offeringPublic Offering to enterfund general corporate purposes, including working capital, capital expenditures, acquisitions, or research and development.
Asset Purchase Agreement
On April 19, 2023, we entered into separate capped call instrumentsan Asset Purchase Agreement with Integrity Implants Inc. and Fusion Robotics, LLC (collectively, the “Sellers”), whereby we acquired certain financial institutions (the “Capped Call Transactions”). The Capped Call Transactions are generally expected to reduce potential dilution to our common stock beyond the conversion price upassets, liabilities, employees, and contracts related to the capped price on any conversionSellers’ navigation-enabled robotics platform ("Valence"). We paid the Sellers cash consideration of $55.0 million for the 2026 Notes and/or offset any paymentspurchase of Valence.
Underwritten Offering
On April 19, 2023, we make in excesscompleted a registered securities offering (the “Offering”) of the principal amount upon conversion.
In addition, we repurchased 1,806,3584,285,715 shares of our common stock forat a price of $14.00 per share. The net proceeds from the Offering were approximately $25.0$57.5 million, concurrently withincluding the issuance of the 2026 Notes.underwriting discounts and commissions and offering expenses paid by us. We also used approximately $53.4 milliona portion of the net proceeds of the offering to repay all obligations under our secured term loan with Squadron Medical Finance Solutions, LLC (the “Term Loan”) and our inventory finance agreement with an inventory supplier (the “Inventory Financing Agreement”).fund the purchase of Valence. We intendexpect to use the remainder ofremaining proceeds to fund the net proceeds fromcosts related to the 2026 Notes forpost-closing integration and research and development activities related to Valence, as well as to fund general corporate purposes.purposes, including working capital, capital expenditures, acquisitions, or research and development.
Term Loan
AcquisitionOn January 6, 2023, we entered into a $150.0 million term loan credit facility with Braidwell Transaction Holdings, LLC (the “Braidwell Term Loan”). The Braidwell Term Loan provided for an initial term loan of EOS
$100.0 million which was funded on the closing date. On May 13, 2021,September 28, 2023, we acquireddrew the additional $50.0 million (the “delayed draw term loan(s)” or the “DDTL”). The Braidwell Term Loan matures on January 6, 2028. Borrowings under the Braidwell Term Loan bear interest at a controlling interest in EOS imaging S.A. (“EOS”), pursuantrate per annum equal to the Tender Offer Agreement (the “Tender Offer Agreement”Term Secured Overnight Financing Rate for such SOFR business day ("SOFR") we entered on December 16, 2020, and in June 2021, we purchased the remaining issued and outstanding ordinary shares forsubject to a 100% interest in EOS. 3% floor, plus 5.75%.EOS, which now operates as our wholly owned subsidiary,
is a global medical device company that designs, develops and markets innovative, low-dose 2D/3D full-body scans for biplanar weight-bearing imaging, rapid 3D modeling
Strategy
Since the beginning of the COVID-19 pandemic, we have seen volatility in sales trends as the elective surgeries that employ our products and services have been impacted to varying degrees.
We continue to monitor the impact of the COVID-19 pandemic on our business and recognize it may continue to negatively impact our business and results of operations during 2022 and beyond. Given the present uncertainty surrounding the pandemic, we expect continued volatility through at least the remaining duration of the pandemic as the impact on individual markets and responses to conditions by international, state and local governments varies.
Strategy
Our vision is to be the standard bearer in spine. By leveraging our team’s extensive spine experience to createcreating clinically distinct solutions that improve surgical outcomes, we believe that we are positioned to take a greatercontinue to earn share of the U.S. spine market, becoming the partner of choice for spine surgeons, hospitals, healthcare systems, and payors.
To achieve our vision and buildunlock long-term value, we have, and will continue to prioritize the following vitalthree key strategic initiatives:
1. Create Clinical Distinction
Clinical distinction is paramount to our value creation strategy. We are committed to continuing to invest in the development, launch, and promotion of approaches and technologies intended to improverevolutionize spine surgery. We have developed, and continue to seek to develop, next-generation surgical approaches that advance spine care with seamlessly integrated access systems, implants, positioners, biologics and enabling technologies, that integrate seamlessly andeach specifically designed to beget objective decision-making and to more successfully address the core spine pathologies, regardless of surgical approach.
pathologies.1 | Yeh, Kuang-Ting MD, PhD; Lee, Ru-Ping RN, PhD; Chen, Ing-Ho MD; Yu, Tzai-Chiu MD; Liu, Kuan-Lin MD, PhD; Peng, Cheng-Huan MD, Wang, Jen-Hung MD; Wu, Wen-Tien MD, PhD. October 2018. Correlation of Functional Outcomes and Sagittal Alignment After Long Instrumented Fusion for Degenerative Thoracolumbar Spinal Disease. Spine: Volume 43, Issue 19.
|
3
Table of Contents
We continue to make investments to advance the clinical distinction of our product portfolio and accelerate revenue growth. During the year ended December 31, 2021,2023, we continued to champion adoption of our lateral franchise, including both PTP and LTP, and launched Calibrate LTX™, a totallateral expandable interbody implant system. We also continued to foster development of 10 new productsAlpha InformatiX, a workflow-integrated informatic ecosystem being designed to automatically and proceduresobjectively inform spine patient care before, during and beganafter surgery. A foundation of that ecosystem, the EOS imaging system, drove revenue growth of over 20%, while significant progress was made on initiatives intended to materially advance the system’s capabilities. We also acquired a robotic-enabled navigation system, branded "Valence", which is currently being developed to integrate EOS imaging technology into our Alpha InformatiX product platform. Our comprehensive portfolio now offers over 80 products across various product categories, of which roughly 40 were launched between July 2018 and December 2021.lateral procedural workflow.
With the expansion and increasing capabilities of our product portfolio, we continue to drive year-over-year increasesgrowth in revenue contributions from new products, increases inthe number of product categories used per surgery, surgical volume, and average revenue per surgery, and revenue per surgeon. For the year ended December 31, 2021, the percentage of revenue contribution from new products was 82%, compared to 67% in the prior year.surgery. Aggregate product categories used per surgery expanded to 2.02.4 for the full-year 2021,2023, compared to 1.82.2 in the prior year. For the full year 2023, surgical volume grew 31% and average revenue per surgery expanded 7% compared to 2022.
Looking to 20222024 and beyond, we intend to continue to be a pioneer of industry innovation.spine innovation that improves surgical outcomes. As such, we expect continued growth will continue to be driven by new technologies that improve the reproducibility and predictability of surgery, expanding surgeon adoption of our procedures and increasingin the number of our products sold into each surgery, surgical volume and revenue per surgery.
2. Compel Surgeon Adoption
An integral part of our strategy is to compel surgeon adoption ofwith the innovative productsclinical distinction that we have, and will continue to introduce. A key component ofCentral to inspiring surgeon interest in our drive to inspire surgeon interestapproaches is the “ATEC Experience”. The ATEC Experience, is” an outcome-basedoutcomes-based educational program for visiting surgeons facilitated at our headquarters in Carlsbad, CA.California. The program provides an interactive learning environment tailored to surgeon needs through both a peer-to-peer and subject matter expert approach. We leverage our state-of-the-art, 7-station cadaveric lab to enable visiting surgeons to gain deep practical experience with our procedural solutions and educate participants on our role in shaping innovation.
The surgeon relationships we are creating through ourthat educational program continue to drivefuel strong growth, evidenced by the increase in surgeon participation in the program, as well as the continued growth of surgeon adoption.growth. Over 400500 new and existing surgeons participated in the ATEC Experience in 2021,2023, driving 27% growth in our surgeon user basebase. Over time, we cultivate the relationships created, partnering with each surgeon in 2021,an increasing number of surgeries and increasing average revenue per surgeonfostering training to inspire partnership in increasingly complex surgeries. We seek to gradually expand utilization and average revenue per surgery in 2021. Revenue attributable to new surgeon customers has continued to contribute meaningfully to revenue growth overall.position us well for sustained long-term growth.
Revitalize the Sales Channel3. Elevate Distribution
We market and sell our products through a strategic network of independent distributorssales agents and direct sales representatives.
Distributors. To deliver increasingly consistent, predictable growth, we have added, and intend to continue to add, clinically astute and exclusive independent sales agents and direct distributors to form a strategic U.S. sales network. Consolidation in the industry continues to facilitate the process as large, seasoned agents are seeking opportunities to partner with a spine-focused company at the forefront of innovation. The expansion and professionalization of our sales team is allowing usrepresentatives to reach untapped surgeons, hospitals, and national accounts across the U.S., as well asand better penetrate existing accounts and territories.
During 2021, We believe the percentage of U.S. revenue driven by strategic independent distributors increasedopportunity to 97% of our U.S. revenue, up from 92% in 2020. Revenue fromexpand our strategic sales channel grew by 59% in 2021, compared to 2020.
National Accounts. We employ a national accounts team thatnetwork is responsible for securing access at hospitals and group purchasing organizations (“GPOs”) acrossvast, with approximately one third of U.S. territories, including many of the U.S. We have been very successful securing access to hospitals and GPOs and a majority of our business is achieved through these accounts. We will continue to focus on developing and maintaining relationships with key GPOs and hospital networks to secure favorable contracts and develop strategies to convert' most significant spine markets, still under- or grow business within these existing accounts.completely unrepresented.
Sales Training and Education. We continually enhance our sales training and education programs for independent distributors and direct sales representatives to optimize sales productivity.
EOS Sales Team Integration.With our acquisition of EOS, in May 2021, we aligned EOS’ U.S.-based capital sales team with our regional sales teams and leadership. The EOS sales team will continue to focusfocuses on hospital administrators, now with the benefitbenefits of leads generated by our broadening sales team. team and enhanced service support.
In 2023, we continued to enhance the expertise within our Memphis distribution facility. With expedient, flexible access to nearby distribution centers, we believe the facility will foster centralized, predictable surgical support as we grow.
We are also in the nascent stages of building an international footprint. Beginning in 2022, we partnered with surgeons to treat our first patients in Australia and New Zealand. Looking forward, we intend to focus our international investments in economically attractive markets with strong surgeon influence and reasonable regulatory pathways where we believe we can build a direct sales team that mirrors the sophistication of our U.S. network.
Spine Anatomy
The spine is the core of the human skeleton, and providesproviding important structural support and alignment while remaining flexible to allow movement. The spine is aA column of 33 bones thatvertebrae, it protects the spinal cord and provides the main support for the body. Each bony segment of the spine is referred to as a vertebra (two or more are called vertebrae). The spine has five regions containing groups of similar bones, listed from top to bottom: seven cervical vertebrae in the neck, twelve thoracic vertebrae in the mid-back (each attached to a rib), five lumbar vertebrae in the lower back, five sacral vertebrae fused together to form one bone called the sacrum, which sits in the pelvis, and four coccygeal bones fused together that form the tailbone. At the front of each vertebra is a block of bone called the vertebral body. Vertebrae are stacked on top of each other and separated from each other through a cushioning intervertebral disc in the front, and bony joints in the back, which create the stability and mobility needed for sitting, standing, and walking. Strong muscles and bones, flexible tendons and ligaments, and sensitive nerves contribute to a healthy spine.
Pain can be caused when any of thesethe spine’s structures is affected by strain, injury, or disease.disease, and spine surgery seeks to alleviate that pain. While the spine has been surgically intervened upon for decades, research demonstrates that surgical outcomes in spine are generally inferior to surgical outcomes delivered by most other orthopedic specialties, particularly in terms of durability, predictability and reproducibility. ATEC’s procedural offerings are designed to treat the various spine pathologies by better achieving the three goals of surgery including: (1) decompression, (2) stabilization, and (3) alignment. We believe there is vast opportunity to create value by innovating to improve surgical outcomes in spine.
The Alphatec Solution
Our mission to improve outcomes by revolutionizing spine surgery affords a differentiated procedural investment thesis. Unlike most of our peers, we take a holistic approach to the approaches that we bring to market, investing not just in the highest dollar components of the approach, but in each of the technologies that integrate to enhance the clinical predictability and reproducibility of an approach. Our procedures seamlessly incorporate technology engendered by that thesis, including an expanding informatic ecosystem designed to automatically and objectively inform spine patient care before, during and after surgery, as well as approach-specific, ergonomic patient positioning and surgical access technology.
Our principal procedural offerings include a wide varietycomprehensive approaches designed and developed to specifically meet the requirements of Approach Technologies designed to achieve clinical success inthe various spine approaches that treat conditions ranging from degenerative disc disease to complex deformity and trauma. Our Approach TechnologiesDepending on the approach, our solutions may comprise intraoperativepre-, intra-, and post-operative information, and neuromonitoring technologies,positioners, access systems, interbody implants, fixation systems, and various biologics offerings; all designed to more reproducibly and predictably improve patient outcomes by achieving the three tenets of spine surgery: (1) decompression, (2) stabilization,outcomes.
Our flagship approach, PTP, was designed and (3) alignment.
We continue to execute on our communicated product strategy, leveraging investments made to provide the sales channel with a differentiated portfolio and to build a strong pipeline capable of launching 8-10 solutions each year going forward (10 released in 2021 and 11 in 2020). Integrated new surgical approaches such as PTP™, enabling technologies such as EOS imaging equipment and our SafeOp Neural InformatiX System™, and the continued advancement of innovations like IdentiTi™, a differentiated portfolio of titanium interbody cages, and InVictus®, a next-generation pedicle screw system capable of addressing the spine from occiput to ilium, continue to gain traction and are delivering on our goal of driving above-market revenue growth. While new products launched since new management took over in late 2017 accounted for less than 10% of total revenue in 2018, that percentage increased to 82% in 2021.
5
Table of Contents
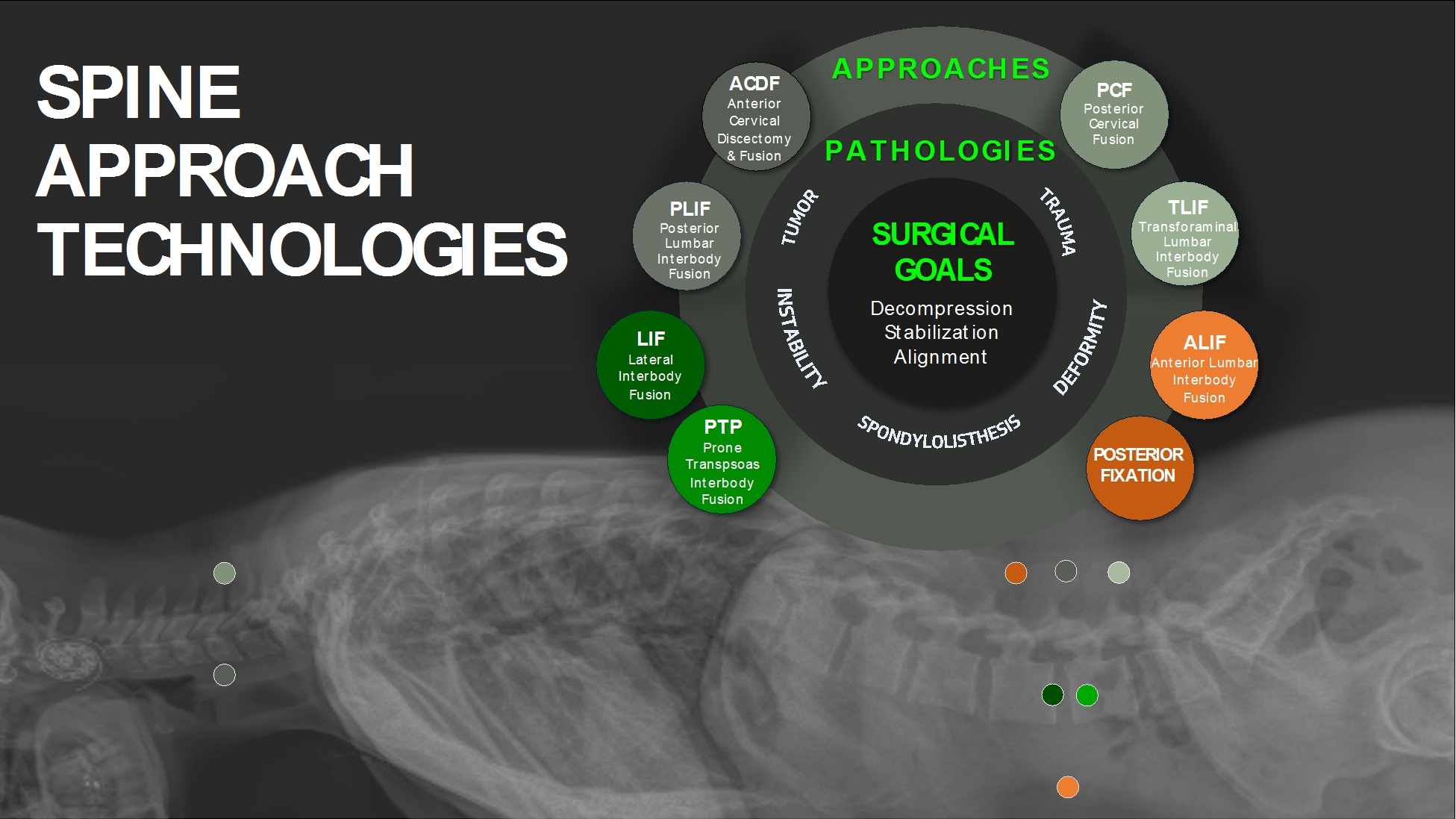
Figure 1: Our portfolio of access systems, implants, technologies and biologics are designed to seamlessly integrate and enhance clinical outcomes across multiple pathologies, regardless of a surgeon’s preferred surgical approach.
The prone transpsoas (“PTP”) approach, was designed2020 by the team that created the first-generation lateral approach for spinal fusion to treat a wide rangedirectly address the known challenges that limited earlier adoption of patient pathologies. The technique has been designedthe technique. Engineered to leverage the benefits achieved by lateral spinal fusion procedures, such as reduced blood loss, shorter hospital stays, and quicker recovery times, while addressing the known challenges that have limited adoptionPTP safely treats a wide range of the technique2. patient pathologies.
Compared to a standard lateral procedure, the PTP approach positions the patient in a prone (face down) position, allowing simultaneous access to the spine laterally (from the side) and posteriorly (from the back), all while in a position that is more familiar surgical positionto surgeons and offering a more streamlined, more orthogonal approach. The PTP techniqueSingle-position lateral surgery in the prone position minimizes unnecessary patient repositioning, enhances time efficiencies, provides surgeons with increased optionality, and achieves spinal alignment objectives more reproducibly. To date, surgeons have performed over 2,500
The PTP procedures.approach is enabled through a combination of purposefully developed technologies that address the unique challenges of approaching the spine laterally while prone. One such challenge, and probably the greatest limit to earlier adoption of lateral approaches overall, is the need to safely and predictably navigate across the lumbar plexus, an essential collection of nerves, to access the lumbar spine during surgery. Key to our PTP approach is the integration of SafeOp Advanced Neuromonitoring, a proprietary technology that couples automated electromyographic (“EMG”) and somatosensory evoked potential (“SSEP”) monitoring. SafeOp technology, as a result, is designed to uniquely beget real-time, surgeon-directed intra-operative information about both the location and the health of the patient’s nerves, enhancing the predictability and reproducibility of lateral approach outcomes.
TheOur Technology
Alpha InformatiX
Designed to provide actionable information that controls clinical variables in spine care, our AIX™ product platform comprises our EOS imaging system and VEA™ alignment mobile application, our SafeOp Neural InformatiX System and Valence. While some AIX applications are commercially available, significant development is underway to integrate and interconnect these technologies and bring unprecedented functionalities to market in 2024 and beyond.
Our EOS imaging system is designed to provide unbiased, high-quality, and calibrated full-body imaging that enables a 3D model of patients’ skeletal systems and to provide valuable diagnostic and surgical planning capabilities. The integration of our approach-specific solutions into EOS’ 3D surgical planning platform is expected to better inform surgery and enhance the predictability of outcomes. Development is expected to allow surgeons to more effectively and efficiently assess patients’ full-body alignment, assess level-specific bone quality, establish surgical objectives, bend patient-specific rods pre-operatively, reconcile to surgical objectives intra-operatively, and determine whether surgical objectives were met post-operatively. Ultimately, we believe the standardized images uniquely made possible by EOS pre-, intra-, and post-operatively can be the foundation for predictive care in spine.
Our SafeOp Neural InformatiX System was the first advanced technology to launch from our AlphaInformatiXthe AIX product platform, uniquely deliversplatform. SafeOp is a patented technology that automates both EMG and SSEP monitoring. As a result, the system can provide surgeons with objective, real-time, objective, and actionable information about both nerve location and nerve health to surgeons.intra-operatively on a small, easy-to-use tablet platform. Integration of the information with our advanced access, implant, and implantfixation technologies equips surgeons with procedural solutions designed to enhance safety, efficiency, and reproducibility. The VEA mobile alignment application is designed to leverage EOS technology to more quickly quantify alignment parameters on a mobile device. Further development is aimed at enabling broad EOS image access for the integration of intraoperative solutions.
Valence was acquired in 2023. An intra-operative system developed by spine experts with deep navigation and robotics know-how, Valence integrates navigation and robotics into spine procedures utilizing either a 3D imaging scan or 2D fluoroscopic images of the patient. Utilizing a small, table-mounted navigation system, a robotic arm guides instrumentation and implants to a pre-determined destination during surgery. While we achieved regulatory clearance to place Invictus® screws through the system late in 2023, we are seeking to further develop and commercialize our vision for Valence, which we expect to integrate the navigation and robotics into our lateral procedures to improve surgical predictability, reduce radiation exposure, and enhance intra-operative precision.
Positioners
We have developed approach-specific patient positioning systems that integrate with our other access systems, providing for a more rigid construct and enhanced reproducibility. The PTP Patient Positioning System™, for example,was developed specifically for the PTP procedure as an adjunct to the Sigma™-PTP Access System. Designed to maximize the positional effects of having the patient in a prone position while streamlining operating room setup, PTP enables a single-position surgery. Key features include bi-lateral structural support to minimize patient movement, adjustable side paddle position to accommodate varying patient habitus, an integrated bed-rail system and compatibility with the Jackson frame. In addition, the system’s ultra-radiolucent carbon fiber frame is designed to help enhance fluoroscopic visibility and its coronal bending mechanism is designed to create reproducible access to L4-5 and upper lumbar regions.
Access Systems
We have differentiated surgical access instruments that are designed to maximize patient outcomes through enhanced visibility and rigidity, intuitive orthogonality, and approach-specific exposure. We offer several split-blade retractors which allow for direct, illuminated visualization and freedom of maneuverability within the operative corridor. Our retractors also provide for stable positioning by attaching directly to the surgical table. We also offer procedure-specific access systems, including our Sigma-ALIF Access System which allows for custom anterior abdominal exposure through freehand placement of dissecting blades and connection to a ringed frame. The Sigma-ALIF Access System provides an unobstructed working corridor with custom features to enable an ALIF approach in either supine or lateral decubitus.
Implants and Fixation Systems
Our portfolio of specialized spinal implants and fixation systems are designed to specifically meet the requirements of each approach. Available in varying shapes, sizes, and lordosis options, our spinal implants include implants made from allograft, PEEK, and porous titanium. We offer NanoTec™ surface modifications to our interbody systems to increase the surface area for cell adhesion and proliferation. Customization can be enhanced with our lordotic expandable intervertebral body fusion system, Calibrate™ PSX, which was released in 2022. We also offer several standalone implants designed to provide for height restoration and stabilization in one integrated solution.
Invictus is our next-generation comprehensive spinal fixation solution, designed to treat the range of pathologies, with intraoperative adaptability and surgical predictability through an open, minimally invasive, or hybrid approach. The sophistication of the InVictusInvictus Posterior Fixation System continues to be expanded, providing adaptable, predictable surgical treatment of a range of pathologies through open, MIS, or hybrid approaches.expand. The commercial release of InVictusInvictus OCT in early 2021 extended the system’s proficiencies to include the thoracicocciput and cervical spine, creating a single system capable of addressing the entire spine from occiput to ilium with familiar and consistent instrument design, simplified screw insertion, and intraoperative adaptability. The launch of InVictusInvictus OsseoScrew® later in 2021 advanced the system with the first and only expandable screw commercially available in the United States.U.S. OsseoScrew has been designed to optimize fixation and address fixation failure in compromised bone.
2
| Data on file: LIT-85034.
|
6
Table of Contents
Biologics
Current Product Portfolio

Figure 2: We are creating clinical distinction with our portfolio of procedurally integrated approach-based products and technologies.
Alpha InformatiX
The SafeOp Neural InformatiX Systemlaunched in November 2019 and is the first installment from our Alpha InformatiX product platform. Our Alpha InformatiX product platform is an advanced neuromonitoring solution, which is designed to reduce the risk of intraoperative nerve injury. The SafeOp Neural InformatiX System is our patented next-generation technology which automates somatosensory evoked potential (“SSEP”) monitoring and is designed to provide surgeons with objective, real-time feedback on an easy-to-use mobile platform, while providing increased intraoperative information that monitors nerve health during a surgical procedure.
Key features of the SafeOp Neural InformatiX System include:
| •
| Proprietary peripheral devices designed to integrate critical neural information into our approaches
|
| •
| Real-time triggered electromyography (“tEMG”) nerve detection designed to provide reliable information regarding the location, direction, and proximity of relevant neural anatomy, in posterior fixation and lateral approach spine procedures
|
| •
| Validated Response Thresholding (“VRT”) algorithm designed to deliver industry-leading tEMG nerve detection while reducing the incidence of false positive responses due to electrical noise
|
| •
| Novel SSEP technology leveraging advanced signal processing and unique waveform averaging to provide an unparalleled ability to monitor femoral nerve health throughout lateral approach procedures
|
| •
| Seamless integration of critical neural information into our InVictus posterior fixation instruments, like SingleStep™
|
7
Table of Contents
The EOS imaging portfolio provides unbiased, calibrated full-body imaging that enable a 3D model of patients’ skeletal systems and provide unprecedented diagnostic and surgical planning capabilities. The integration of ATEC’s approach-specific solutions into EOS’ 3D surgical planning platform is expected to better inform surgery and enhance the predictability of outcomes by allowing surgeons to more effectively assess patients’ full-body alignment, establish surgical objectives, and simulate surgery with optimized implants.
Key features of the EOS portfolio include:
| •
| Standing full-body assessment. Head-to-toe biplanar exams in the weight-bearing position for accurate assessment of factors causing pain and disability to better guide treatment and surgical decisions. Surgical planning from a standing position enables alignment parameters that more closely match functional posture
|
| •
| High image quality. EOSedge® is the first X-ray system with a high-resolution photon-counting detector that delivers outstanding-quality images, reinforcing diagnostic capabilities while addressing a broad range of imaging and orthopedic surgery challenges
|
| •
| Reduced radiation exposure. Driven by the ALADA (as low as diagnostically acceptable) principle, the EOS or EOSedge exam delivers a minimal dose of radiation to reduce the long-term impact of repeated imaging. With the introduction of the proprietary EOSedge Flex Dose™ feature, the dose automatically adjusts along the patient’s body habitus to further optimize the radiation as well as delivering homogeneous image quality by avoiding over/under exposure in the thinner/larger parts of the body
|
| •
| Precise 3D measurements. Patient-specific measurements, dimensions, and angles to make informed clinical decisions at all stages of care
|
| •
| EOSapps and EOSlink for surgical planning and operating room integration. Pre-operative planning software to anticipate surgical results and select components for spine surgery; pairs with surgical technologies for precise execution with EOSlink
|
Access Systems
The Sigma-TLIF Pedicle-based AccessSystem™ provides direct visualization of key anatomical landmarks to help create a reproducible transforaminal lumbar interbody fusion (“TLIF”) approach. Key features include:
| •
| Vertebral body distraction which facilitates access to a collapsed disc space
|
| •
| Integrated fiber-optic light source designed to improve illumination
|
| •
| Modular shank and blade help provide direct visualization of facet, pars, and lamina
|
| •
| Independent cranial and caudal retraction which enable customized exposure
|
| •
| Surgeon-guided medial blade to help support differing patient pathologies
|
| •
| Quick-connect engagement which provides for a more streamlined assembly
|
The Sigma PTP Access System™was developed specifically for the PTP procedure and is designed to maximize efficiency and help achieve alignment through increased rigidity, customizable exposure, and intuitive orthogonality. Key features include:
| •
| Singular titanium construct designed to maximize rigidity and reduce weight
|
| •
| Independent anterior and posterior retraction mechanisms designed to enable customized exposure
|
| •
| Intuitive fluoroscopic indicators that provide reinforced orthogonality
|
| •
| Low-profile rounded blade design to enhance fluoroscopic visibility to the disc space
|
| •
| Contoured blade tips help establish optimal psoas retraction
|
| •
| Integrated fiber-optic light source designed to provide improved illumination of the exposure site
|
8
Table of Contents
| •
| A quick-connect articulating arm post for more streamlined engagement
|
The PTP Patient Positioning System™was developed specifically for the PTP procedure as an adjunct to the Sigma-PTP Access System. Designed to maximize the positional effects of having the patient in a prone position while streamlining operating room setup and provide a fully integrated rigid construct, the system’s key features include:
| •
| Ultra-radiolucent carbon fiber frame to help enhance fluoroscopic visibility
|
| •
| Bi-lateral structural support to minimize patient movement
|
| •
| Adjustable side paddle position to accommodate varying patient habitus
|
| •
| Coronal bending mechanism to create reproducible access to L4-5 and upper lumbar regions
|
| •
| Integrated nylon straps to eliminate need for taping patient to the table
|
| •
| Integrated bed-rail system which enables fixation of the Sigma-PTP Access System to facilitate a singular rigid construct
|
| •
| Compatibility with Jackson frame to help simplify pre-operational setup
|
The Squadron® Lateral Retractoris designed to maximize patient outcomes during lateral-approach surgery with the patient in the lateral decubitus position. The retractor offers multiple features to accommodatehave a variety of surgical techniques, as well as more quickly establish access, leading to minimized retraction times. Key features include:
| •
| Robust construction that provides a stable corridor with the ability to replace blades in-situ
|
| •
| Independent cranial and caudal blade movement which enables more precise surgical aperture
|
| •
| Telescoping blades and a fourth blade articulation that allows surgeons to traverse challenging anatomy
|
| •
| LevelToe™ mechanics that provide a parallel toe up to 15° to reduce tissue creep
|
Fixation Systems
The InVictus Spinal Fixation Systems (Open and MIS), which were introduced in 2019, are comprehensive thoracolumbar fixation systems that arebiologics designed to treat a rangefacilitate the process of pathologies. Fully integrated with our SafeOp electromyography (“EMG technology”), InVictus assists surgeons with intraoperative adaptability and surgical efficiency through a variety of surgical approaches including open, minimally invasive (“MIS”) or hybrid approaches. Key system features include:
| •
| Helical Flange®: construct confidence provided by the InVictus thread form designed to reduce the potential to cross-thread and eliminate tulip splay
|
| •
| Adaptability to surgical needs with a variety of implants designed to accept multiple rod diameters and materials
|
| •
| Instrumentation designed to provide more predictable surgical outcomes in the most challenging or complex procedural scenarios
|
The InVictus MIS SingleStepSystem is an extension of the InVictus platform, which offers a simplified approach to traditional minimally invasive pedicle screw placement through utilization of an all-in-one driver which is designed to improve surgical efficiency without compromising accuracy. SingleStep eliminates guidewire management and targeting needles, while reducing instrument passes, procedural steps, screw insertion time, and reliance on fluoroscopy.3Key features include:
| •
| Integrated, steerable stylet which enables robust pedicle targeting
|
3
| Data on file – LIT-17021
|
9
Table of Contents
| •
| Surgeon-controlled stylet advancement with visual indication of stylet depth
|
| •
| Robust, low-profile, extended tab design to accommodate complex manipulations
|
| •
| Quick-connect ratcheting handle which inserts the screw over the stylet
|
| •
| Leverages screws with InVictus thread-form designed to reduce cross-threading and tulip splay
|
| •
| Serrated, self-starting screw tip which is intended to eliminate the need for tapping
|
| •
| When combined with SafeOp automated EMG technology, the SingleStep approach offers a real-time trajectory and placement confirmation during stylet and screw insertion, helping to reinforce confidence of safe screw placement
|
The InVictus Modular Fixation Systems (Open and MIS) are extensions of the InVictus platform and are designed to enhance adaptability with the power of screw modularity. Key features include:
| •
| Screws with InVictus thread-form designed to help form reduced cross-threading and tulip splay
|
| •
| Modular tulip interconnection strength which is 4.5x greater than the average pull-out strength of pedicle screws4,5
|
| •
| Robust instruments and customizable modular implants designed to accept multiple rod diameters and materials to adapt intraoperatively to surgical techniques
|
| •
| Guidewire-less SingleStep technique designed to advance the standard of modular fixation to deliver modular shank and Sigma blade with one instrument pass
|
| •
| Integrates with SafeOp Neural InformatiX System and is intended to provide surgeons with more predictable real-time and actionable information which helps detect and monitor the health of at-risk nerves during posterior fixation procedures
|
| •
| Audible, tactile, and visual confirmations of tulip-to-shank attachment designed to instill confidence
|
The InVictus OsseoScrew System is an expandable screw system used in conjunction with the InVictus platform, and as an alternative to the use of cemented fenestrated screws. OsseoScrew is designed to restore the integrity of the spinal column in the absence of fusion (for a limited period) in patients with advanced stage tumors involving the thoracic and lumbar spine, and whose life expectancy is of insufficient duration to permit achievement of fusion. Key features include:
| •
| 29% greater pull-out strength over conventional pedicle screws6
|
| •
| Expansion zone location which is designed to optimize pedicle fixation
|
| •
| Stabilization in patients with compromised bone structures
|
The Arsenal® Spinal Fixation System is a comprehensive thoracolumbar fixation platform with components to support procedures aimed to fix a range of degenerative to deformity pathologies and both primary and revision surgical procedures. The Arsenal Spinal Fixation System also contains thread forms to accommodate both traditional and medialized (cortical) trajectories. Key features include:
| •
| Ergonomically designed instrumentation
|
| •
| Multiple instrument options designed to accommodate anatomical and pathological diversity
|
| •
| Multiple screw options which include polyaxial, uniplanar, monoaxial, reduction, and sacral screws
|
4
| Liljenqvist U, Hackenberg L, Link T, Halm H. Pullout strength of pedicle screws versus pedicle and laminar hooks in the thoracic spine. Acta Orthop Belg. 2001;67(2):157-63.
|
6
| Vishnubhotla S, McGarry WB, Mahar AT, et al. A titanium expandable pedicle screw improves initial pullout strength as compared with standard pedicle screws. Spine J 2011;11:777-81.
|
10
Table of Contents
| •
| Multiple pelvic fixation options
|
| •
| Low-profile, dual-lead screws
|
The Aspida Anterior Lumbar Plating System™ is a fixation system for anterior lumbar interbody fusion (“ALIF”) and consists of specifically designed lumbar and lumbo-sacral anterior plates and dual-lead self-drilling and self-tapping screws. Its intuitive instrument design, which is complemented by the AnchorMax™ locking mechanism is designed to provide efficient and effective anterior plating. Key features include:
| •
| Consistent 3.5 mm thickness which provide a low-profile design for reduced risk of vascular interference
|
| •
| An integrated passive locking mechanism
|
| •
| Dual-lead self-drilling and self-tapping screws for improved surgical efficiency
|
| •
| Intuitive instrumentation
|
The AMP Anti-Migration Plate™ is a plating system designed to be used with our lateral interbody spacer system and is designed to provide integrated fixation for lateral interbody fusion (“LIF”) constructs. Key features include:
| •
| One and two-screw plate options
|
| •
| Zero-step screw locking with audible, tactile, and visible indicators
|
| •
| Divergent screw angulation of 25°
|
| •
| Convergent screw angulation of 5°
|
| •
| Compatibility with IdentiTi and Transcend® lateral implants
|
| •
| Ability to implant as assembled with or after placement of the interbody implant
|
The InVictus OCT Spinal Fixation Systemis an extension of the InVictus platform with implant solutions to span the occipital-cervical-thoracic regions and is compatible with our Arsenal® and InVictus Spinal Fixation Systems using various rod-to-rod connectors and/or transitional rods.
The Trestle Luxe® Anterior Cervical Plate Systemis a fixation system used in anterior cervical discectomy and fusion procedures (“ACDF”). Key features include:
| •
| Low-profile design intended to reduce the irritation of the tissue adjacent to the plate following surgery
|
| •
| Large window design intended to enable enhanced graft site and end plate visualization, which ease plate placement
|
| •
| Self-retaining screw-locking mechanism which provides quick and easy plate locking
|
| •
| Flush profile upon screw insertion
|
The Insignia Anterior Cervical Plate System™ is our next-generation ACDF fixation system. Key features include:
| •
| Industry-leading screw angle and trajectory capabilities with a full range of screw and plate options to meet varied clinical requirements
|
| •
| Low-profile, attached active locking mechanism which allows for visual and tactile confirmation of secure blocker locking as well as compatible bone screw drivers which minimize the number of passes into a surgical site
|
11
Table of Contents
| •
| Locking screwdriver that allows for improved axial retention of the screw when compared to traditional tapered drivers and simplified usability when compared to traditional threaded driver and screw interfaces
|
| •
| A single-level plate technique, which allows for single-pass placement of plate and cage into surgical site as well as selection of optimized plate length, reproducible screw placement and optimized plate alignment
|
Interbody Systems
IdentiTi Porous Ti Interbody Implants™ are designed to provide the biological, biomechanical, and imaging characteristics that surgeons seek in a fusion construct. The subtractive process used to manufacture each IdentiTi Implant results in more predictable mechanical performance and enhanced imaging characteristics. IdentiTi implants take advantage of bone’s affinity for titanium and because of their porosity, have a surface roughness that enhances stability.7 Key features include:
| •
| Commercially pure titanium
|
| •
| Multiple lordosis and footprint options to accommodate varying surgical requirements across all interbody fusion procedures including ACDF, ALIF, LIF, PLIF, and TLIF
|
| •
| Fully interconnected porosity to promote bony on-growth and in-growth (as seen in animal model)89
|
| •
| 60% porosity which provides for reduced density and designed to enhance intraoperative and postoperative imaging
|
| •
| Porous titanium has a bone-like stiffness10
|
Transcend® Lateral Interbody Implants are polyetheretherketone (“PEEK”) interbody spacers for use in LIF procedures. Transcend and IdentiTi Lateral Implants are designed to function with the same instrumentation, providing surgeons with a more seamless experience regardless of implant material. The Transcend implant offering provides continuity in lordotic options with a refined design to meet a surgeon’s lateral needs. Key features include:
| •
| Quick-connect inserter feature which is designed to eliminate point loading
|
| •
| Bulleted distal tip which helps provide smooth disc-space insertion
|
| •
| Directional anti-migration teeth to help resist expulsion
|
| •
| Tantalum markers designed to enhance imaging via fluoroscopy
|
Battalion® Posterior Interbody Implants combine a PEEK body with our patented TiTec™ (titanium) coating technology to take advantage of the characteristics of both materials. The PEEK material allows surgeons to assess fusion through the implant while the titanium-coating provides initial stability due to the roughened surface. Key features include:
| •
| Straight (“PS”) and curved (“PC”) options to accommodate PLIF and TLIF surgical approaches
|
| •
| Multiple length options to accommodate varying surgical requirements
|
| •
| Patented TiTec coating helps improve expulsion strength when compared to PEEK11
|
| •
| TiTec coating combines visualization and stiffness benefits of PEEK with the initial stability characteristics of titanium
|
7
| Data on file – LIT-84895
|
8
| Data on file – LIT-84894
|
9
| Data on file – LIT-84890
|
10
| Data on file – LIT-84898
|
11
| Data on file – LIT-84701
|
12
Table of Contents
| •
| Uncoated nose structured to help combat delamination and wear debris issues
|
Novel®is a PEEK intervertebral body fusion system consisting of varying lengths, widths, and heights to accommodate individual patient anatomies and procedural approaches. Key features include:
| •
| Various size and shape options to accommodate different surgical approaches including PLIF, TLIF, ALIF, ACDF
|
| •
| Bulleted nose designed to facilitate easy insertion and matches anatomy
|
| •
| Multiple footprint options to accommodate different anatomy and surgical procedures
|
| •
| Tooth pattern helps to prevent migration and adds stability
|
| •
| Large contact area intended to increase subsidence resistance
|
| •
| PEEK radiographic markers which ease visual assessment of implant placement and fusion process
|
| •
| Color-coded titanium color-coding by size to help simplify identification
|
Biologics
Cervical Structural Allograft Spacers™Our biologics offerings consist of our portfolioseveral allograft (donated human tissue) options, including 3D ProFuse™ Osteoconductive Bioscaffold, and a family of allograft spacers which are available in a range of shapes and sizes, each with corresponding instrumentation, and are intended for use in the cervical spine.AlphaGRAFT
® products. 3D ProFuse Demineralized Bone Scaffold™consistsOsteoconductive Bioscaffold is highly compressible when hydrated, allowing for ease of a sponge-like demineralized bone matrix that has been pre-cut into sizes to fit within a spinal spacer. The 3D ProFuse Demineralized Bone Scaffold provides a natural scaffold derived entirely from bone that can be placed into a void within a spinal spacer or around a spinal spacer. The sponge-like qualities of the scaffold allow a surgeon to compress the scaffoldhandling and place it into a small space. Following placement, the scaffold expands for maximum contact between the spinal spacer and the endplate of the vertebral body and is designed to promote fusion.
Neocore® Osteoconductive Matrix is designed to provide an effective core environment for bone growth through a synthetic scaffold. When hydrated with patient bone marrow aspirate (“BMA”), Neocore becomes a complete bone graft, which possesses all the necessary components of bone growth. Engineered to perform like natural bone, Neocore’s composition and porosity provide the benefits of rapid revascularization throughout graft and supports replacement of three-dimensional matrix with healthy new bone growth. Offering excellent handling characteristics, these pre-formed strips are flexible to conform to adjacent structures, compressible, and moldable.
better endplate-to-endplate contact. Our AlphaGRAFT® Demineralized Bone Matrix (DBM)(“DBM”) consists of demineralized human tissue that is mixed with a bioabsorbable carrier and intended for use in surgery for bone grafting and is available in gel, putty, and fiber forms. AlphaGRAFT DBM Fibers combine the regenerative capacity of interconnected fibers with the maximum availability of growth factors endogenous to bone. Composed of 100% demineralized fibers, AlphaGRAFT DBM Fibers offer moldable, cohesive handling characteristics and provide an osteoconductive scaffold for the delivery of autologous stem cells.
characteristics. AlphaGRAFT Cellular Bone Matrix is our most recent addition to this family of products and(“CBM”) is a growth factor-enriched cellular bone matrix (“CBM”) with two differentiating technologies. Cellular activity via retention of endogenous mesenchymal stem cells and osteoprogenitor cells; and intracellular growth factors from the bone and bone marrow stroma contribute to amplify growth factors bound to the extracellular matrix of the bone, resulting in a product that exhibits the angiogenic, osteoinductive, and mitogenic growth factors necessary for bone growth. AlphaGRAFT CBM may be delivered in granular, fiber, or structural form. We also offer BioCORE
™ Moldable Bioactive Graft which is a synthetic mineral-collagen composite matrix that can be molded to fit the bone defect. OurAmnioshield® Amniotic Tissue Barrier is an allograft for spinal surgical barrier applications. The composite amniotic membrane reduces inflammationis intended to act as a biological barrier and enhances healing at the surgical site, reduces scar tissue formation, and providesprovide an excellent dissection plane.
13
Table of Contents
Products and Technologies Under Development
Internally Developed Products and Technologies
We are expanding our portfolio of products and technologies to enhance clinical outcomes across multiple pathologies, regardless of a surgeon’s preferred surgical approach. We expect to launch 8-10 new products during 2022.2024.
Research and Development
Our research and development team seeks to better meet the requirements of each surgical approach and design and release new products that increase our penetration of the U.S. spine market. We are focused on developing technology platforms and products that span the largest market segments addressing degenerative and deformity spine pathologies. We have transformed our development process by focusing our programs and leveraging integrated teams to reduce the time frame from product concept to market commercialization. We also collaborate with surgeon partners to design products that are intended to enhance the clinical experience, simplify surgical techniques, and reduce overall costs, while improving patient outcomes. Most of our product development efforts are fully integrated in a singular location, our Carlsbad headquarters, which allows us to bring products from concept to market rapidly responding to surgeon and patient needs. Our resources include a technology advancement cell for rapid prototyping, a cadaveric lab, and mechanical testing laboratory.
Sales and Marketing
We market and sell our products through a sales force consisting of dedicated and non-dedicated independent distributorssales agents and dedicated employee direct sales representatives. We employ a team of area vice presidents, (“AVPs”),sales directors, and regional business managers, (“RBMs”), who are responsible for overseeing the sales channel process in their territories. Although surgeons in the U.S. typically make the ultimate decision to use our products, we generally invoice the hospital for the products that are used and pay commissions to the sales representative, or the sales agent based on payment received from the hospital. We compensate our direct sales employees AVPs, and RBMs through salaries and incentive bonuses based on performance measures.
We evaluate and select our distributionindependent sales agent partners and sales employees based upon their expertise in selling spinal devices, reputation within the surgeon community, geographical coverage, and established sales network.
We market our products at various industry conferences, organized surgical training courses, and in industry trade journals and periodicals.
Surgeon Training and Education
We focus our surgeon training efforts on delivering critical technical skills needed to perform the entire spinal fusion procedure through a peer-to-peer approach for qualified surgeon customers. Well-timed surgeon education programs drive customer conversion and loyalty by focusing on delivering value through improved clinical outcomes. We devote significant resources to training and education and are committed to a culture of scientific excellence and ethics.
We believe that one of the most effective ways to introduce and build market demand for our products is by training and educating spine surgeons, independent distributors,sales agents, and direct sales representatives on the benefits and use of our products. Sales training programs are a platform for learning and organizational development, ensuring the sales force is clinically competitive and considered an essential resource to all stakeholders. We focus on cross-functional collaboration and alignment to deliver timely and relevant programs to meet surgeon and representative needs and positively impact the business.
Our training and education programs are designed to support new product introductions to the market as well as ongoing portfolio advancement. Our resources are nimble and responsive and include field-based engagements to supplement our core curriculum. We believe this is an effective way to increase overall surgeon adoption of our new products.
14
Table of Contents
We believe that surgeons, independent distributors,sales agents, and direct sales representatives will become exposed to the merits and distinguishing features of our products through our training and education programs, and that such exposure will increase the use and promotion of our products. With a focus on the entire procedure, we expect to build awareness of the breadth of our product offering. We are conscientious in the pursuit of delivering value to all stakeholders. Our goal is to provide surgeon education programs, coupled with a growing and comprehensive sales training platform that create a sustainable competitive advantage for our organization.
Manufacture and Supply
We rely on third-party suppliers for the manufacture of all our implants and instruments. Outsourcing implant manufacturing reduces our need for capital investment and reduces operational expense. Additionally, outsourcing provides expertise and capacity necessary to scale up or down based on demand for our products. We select our suppliers to ensure that all of our products are safe, effective, adhere to all applicable regulations, are of the highest quality, and meet our supply needs. We employ a rigorous supplier assessment, qualification, and selection process targeted to suppliers that meet the requirements of the U.S. Food and Drug Administration (“FDA”),FDA, and International Organization for Standardization (“ISO”), and quality standards supported by internal policies and procedures. Our quality assurance process monitors and maintains supplier performance through qualification and periodic supplier reviews and audits.
The raw materials used in the manufacture of our non-biologic products are principally titanium, titanium alloys, stainless steel, cobalt chrome, ceramic, allograft, and PEEK. With the exception of PEEK, none of our raw material requirements is limited to any significant extent by critical supply. We are subject to the risk that Invibio, one of a limited number of PEEK suppliers, will be unable to supply PEEK in adequate amounts and in a timely manner. We believe our supplier relationships, alternative product offerings, vendor-managed inventory, and quality processes will support our potential capacity needs for the foreseeable future.
With respect to biologics products, we are FDA-registered and licensed in the states of California, New York, and Florida, the only states that currently require licenses. Our facility and the facilities of the third-party suppliers we use are subject to periodic unannounced inspections by regulatory authorities and may undergo compliance inspections conducted by the FDA and corresponding state and foreign agencies. Because our biologics products are processed from human tissue, maintaining a steady supply can sometimes be challenging. We have not experienced significant difficulty in locating and obtaining the materials necessary to fulfill our production requirements and we have not experienced a meaningful disruption to sales orders.
Table of Contents
In connection with the sale of the previous international distribution business, the Company entered into a product manufacture and supply agreement (the “Supply Agreement”) with Globus Medical Ireland, Ltd., a subsidiary of Globus Medical, Inc., and its affiliated entities (collectively “Globus Medical”), pursuant to which the Company supplied to Globus
MedicalCompetition certain of its implants and instruments, previously offered for sale by the Company in international markets at agreed-upon prices. The Supply Agreement expired and terminated on August 31, 2021.
Competition
Although we believe that our current broad product portfolio and development pipeline is differentiated and has numerous competitive advantages, the spinal implant industry is highly competitive, subject to rapid technological change, and significantly affected by new product introductions. We believe that the principal competitive factors in our market include:
• improved outcomes for spine pathology procedures; •ease of use, quality, and reliability of product portfolio; •effective and efficient sales, marketing, and distribution; •quality service and an educated and knowledgeable sales network; •technical leadership and superiority; •surgeon services, such as training and education; •responsiveness to the needs of surgeons; •acceptance by spine surgeons; •product price and qualification for reimbursement; and
| •
| improved outcomes for spine pathology procedures
|
| •
| ease of use, quality, and reliability of product portfolio
|
| •
| effective and efficient sales, marketing, and distribution
|
| •
| quality service and an educated and knowledgeable sales network
|
| •
| technical leadership and superiority
|
| •
| surgeon services, such as training and education
|
15
Table of Contents
| •
| responsiveness to the needs of surgeons
|
| •
| acceptance by spine surgeons
|
| •
| product price and qualification for reimbursement
|
Both our currently marketed products and any future products we commercialize are subject to intense competition. We believe that our most significant competitors are Medtronic (Sofamor Danek), Johnson & Johnson (DePuy Spine), Stryker, NuVasive, Zimmer Biomet, Globus Medical, and others, many of which have substantially greater financial resources than we do. In addition, these companies may have more established distribution networks, entrenched relationships with physicians and greater experience in developing, launching, marketing, distributing, and selling spinal implant products.
Some of our competitors also provide non-operative therapies for spine disorder conditions. While these non-operative treatments are considered to be an alternative to surgery, surgery is typically performed in the event that non-operative treatments are unsuccessful. We believe that, to date, these non-operative treatments have not caused a material reduction in the demand for surgical treatment of spinal disorders.
Intellectual Property
We rely on a combination of patent, trademark, copyright, trade secret and other intellectual property laws, nondisclosure agreements, proprietary information ownership agreements, and other measures to protect our intellectual property rights. We believe that in order to have a competitive advantage, we must develop, maintain, and enforce the proprietary aspects of our technologies. We require our employees, consultants, co-developers, distributorssales agents and advisors to execute agreements governing the ownership of proprietary information and use and disclosure of confidential information in connection with their relationship with us. In general, these agreements require these individuals and entities to agree to disclose and assign to us all inventions that were conceived on our behalf, or which relate to our property or business and to keep our confidential information confidential and only use such confidential information in connection with our business.
Patents. As of December 31, 2021,2023, we and our affiliates owned, or we exclusively owned 184156 issued U.S. patents, 3642 pending U.S. patent applications and 214257 issued or pending foreign patents. We own multiple patents relating to unique aspects and improvements for several of our products. Patents for individual products extend for varying periods according to the date of filing or grant and legal term of patents in various countries where a patent is issued. We do not believe that the expiration of any single patent is likely to significantly affect our intellectual property position.
Trademarks. As of December 31, 2021,2023, we and our affiliates owned 2730 registered U.S. trademarks and 1526 registered trademarks outside of the U.S.
Government Regulation
Our products are subject to extensive regulation by the FDA and other U.S. federal and state regulatory bodies and comparable authorities in other countries. Our products are subject to regulation under the Federal Food, Drug and Cosmetic Act (“FDCA”), and in the case of our tissue products, also under the Public Health Service Act (“PHSA”). To ensure that our products are safe and effective for their intended use, the FDA regulates, among other things, the following activities that we or our partners perform and will continue to performperform::
| • product design and development; •non-clinical and clinical research; •premarket clearance or approval; •advertising and promotion; •product marketing, sales and distribution; •post-market surveillance, including reporting deaths or serious injuries related to products and certain product malfunctions. •
| product design and development;
|
| •
| non-clinical and clinical research;
|
16
Table of Contents
| •
| premarket clearance or approval;
|
| •
| advertising and promotion;
|
| •
| product marketing, sales and distribution;
|
| •
| post-market surveillance, including reporting deaths or serious injuries related to products and certain product malfunctions.
|
Government Regulation—Medical Devices
FDA’s Premarket Clearance and Approval Requirements. Unless an exemption applies, each medical device we wish to commercially distribute in the United StatesU.S. will require either FDA clearance of a premarket notification requesting permission for commercial distribution under Section 510(k) of the FDCA, also referred to as a 510(k) clearance, or approval of a premarket approval application (“PMA”). The information that must be submitted to the FDA in order to obtain clearance or approval to market a new medical device varies depending on how the medical device is classified by the FDA. Under the FDCA, medical devices are classified into one of three classes - Class I, Class II or Class III-dependingIII - depending on the degree of risk associated with the use of the device and the extent of manufacturer and regulatory controls deemed to be necessary by the FDA to reasonably ensure their safety and effectiveness.
Class I devices are those with the lowest risk to the patient for which safety and effectiveness can be reasonably assured by adherence to a set of regulations, referred to as General Controls, which require compliance with the applicable portions of the FDA’s Quality System Regulation (“QSR”), facility registration and product listing, reporting of adverse events and malfunctions, and appropriate, truthful and non-misleading labeling and promotional materials. Some Class I devices also require 510(k) clearance by the FDA, though most Class I devices are exempt from the premarket notification requirements. Class II devices are those that are subject to the General Controls, as well as Special Controls, which can include performance standards, product-specific guidance documents and post-market surveillance. Manufacturers of most Class II devices are required to submit to the FDA a premarket notification under Section 510(k) of the FDCA. Class III devices include devices deemed by the FDA to pose the greatest risk such as life-supporting or life-sustaininglife-sustaining devices, or implantable devices, in addition to those deemed not substantially equivalent following the 510(k) process. The safety and effectiveness of Class III devices cannot be reasonably assured solely by compliance with the General Controls and Special Controls described above. Therefore, these devices must be the subject of an approved PMA. Both 510(k)s and PMAs are subject to the payment of user fees at the time of submission for FDA review.
If the FDA determines that the device is not “substantially equivalent” to a predicate device following submission and review of a 510(k) premarket notification, or if the manufacturer is unable to identify an appropriate predicate device and the new device or new use of the device presents a moderate or low risk, the device sponsor may either pursue a PMA approval or seek reclassification of the device through the de novo process. Our current products on the market in the U.S. areinclude Class II devicesspinal implants, instruments, neuromonitoring systems, robotic navigation systems, x-ray imaging systems and software as a medical device (SaMD) marketed under FDA 510(k) premarket clearance.clearance, as well as Class I 510(k) exempt spinal instruments and devices.
510(k) Clearance Pathway. To obtain 510(k) clearance, we must submit a premarket notification demonstrating that the proposed device is substantially equivalent to a device legally marketed in the United States.U.S. A predicate device is a legally marketed device that is not subject to premarket approval, i.e., a device that was legally marketed prior to May 28, 1976 (pre-amendments device) and for which a PMA is not required, a device that has been reclassified from Class III to Class II or I, or a device that was found substantially equivalent through the 510(k) process. To be “substantially equivalent,” the proposed device must have the same intended use as the predicate device, and either have the same technological characteristics as the predicate device or have different technological characteristics and not raise different questions of safety or effectiveness than the predicate device. Clinical data is sometimes required to support substantial equivalence.
17
Table of Contents
The FDA’s goal is to review and act on each 510(k) within 90 days of submission, but on average the process usually takes approximately six months. It may take less time depending on the type of device and it may take longer if the FDA requests additional information. Most 510(k)s do not require supporting data from clinical trials, but the FDA may request such data. If the FDA agrees that the device is substantially equivalent, it will grant clearance to commercially market the device.
After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a new or major change in its intended use, will require a new 510(k) clearance or, depending on the modification, require premarket approval. The FDA requires each manufacturer to determine whether the proposed change requires the submission of a 510(k) or PMA, but the FDA can review any such decision and can disagree with a manufacturer’s determination. If the FDA disagrees with a manufacturer’s determination, the FDA can require the manufacturer to cease marketing and/or recall the modified device until 510(k) clearance or PMA is obtained. If the FDA requires us to seek a new 510(k) clearance or PMA for any modifications to a previously cleared product, we may be required to cease marketing or recall the modified device until we obtain this clearance or approval. Also, in these circumstances, we may be subject to significant fines or penalties. We have made and plan to continue to make enhancements to our products for which we have not submitted 510(k)s or PMAs, and we will consider on a case-by-case basis whether a new 510(k) or PMA is necessary.
The FDA began to consider proposals to reform its 510(k) marketing clearance process in 2011, and such proposals could include increased requirements for clinical data and a longer review period. Specifically, in response to industry and healthcare provider concerns regarding the predictability, consistency and rigor of the 510(k) regulatory pathway, the FDA initiated an evaluation of the 510(k) program, and as part of the Food and Drug Administration Safety and Innovation Act, (“FDASIA”), Congress reauthorized the Medical Device User Fee Amendments with various FDA performance goal commitments and enacted several “Medical Device Regulatory Improvements” and miscellaneous reforms, which are further intended to clarify and improve medical device regulation both pre- and post-clearance and approval. Further, in December 2016, the 21st Century Cures Act (“Cures Act”) was signed into law. The Cures Act, among other things, is intended to modernize the regulation of devices and spur innovation but its ultimate implementation is unclear.
Premarket Approval Pathway. Class III devices require PMA approval before they can be marketed, although some pre-amendment Class III devices for which the FDA has not yet required a PMA are cleared through the 510(k) process. The PMA process is generally more complex, costly and time consuming than the 510(k) process. A PMA must be supported by extensive data including, but not limited to, extensive technical information regarding device design and development, preclinical and clinical trials, manufacturing, and labeling information to demonstrate to the FDA’s satisfaction the safety and effectiveness of the device for its intended use. The PMA application must provide valid scientific evidence that demonstrates to the FDA’s satisfaction reasonable assurance of the safety and effectiveness of the device for its intended use. Following receipt of a PMA, the FDA determines whether the application is sufficiently complete to permit a substantive review. If the FDA accepts the application for review, it has 180 days under the FDCA to complete its review of the PMA, although in practice, the FDA’s review often takes significantly longer, and can take up to several years. During this review period, the FDA may request additional information or clarification of information already provided, and the FDA may issue a major deficiency letter to the applicant, requesting the applicant’s response to deficiencies communicated by the FDA. Also, during the review period, an advisory panel of experts from outside the FDA may be convened to review and evaluate the application and provide recommendations to the FDA as to the approvability of the device. The FDA may or may not accept the panel’s recommendation. In addition, the FDA will conduct a preapproval inspection of the manufacturing facility to ensure compliance with quality system regulation (QSR).QSR. The PMA process can be expensive, uncertain, and lengthy, and a number of devices for which FDA approval has been sought by other companies have never been approved by the FDA for marketing.
18
Table of Contents
Clinical Trials. Clinical trials are almost always required to support a PMA and are sometimes required for a 510(k). All clinical investigations of investigational devices to determine safety and effectiveness must be conducted in accordance with the FDA’s investigational device exemption (“IDE”), regulations which govern investigational device labeling, prohibit promotion of the investigational device, and specify an array of recordkeeping, reporting, and monitoring responsibilities of study sponsors and study investigators. If the device is determined to present a “significant risk” to human health, the manufacturer may not begin a clinical trial until it submits an IDE application to the FDA and obtains approval of the IDE from the FDA. The IDE must be supported by appropriate data, such as animal and laboratory testing results, showing that it is safe to test the device in humans and that the testing protocol is scientifically sound. In addition, the study must be approved by, and conducted under the oversight of, an Institutional Review Board (“IRB”), for each clinical site. The IRB is responsible for the initial and continuing review of the IDE and may pose additional requirements for the conduct of the study. If an IDE application is approved by the FDA and one or more IRBs, human clinical trials may begin at a specific number of investigational sites with a specific number of patients, as approved by the FDA. If the device presents a non-significant risk to the patient, a sponsor may begin the clinical trial after obtaining approval for the trial by one or more IRBs without separate approval from the FDA, but must still follow abbreviated IDE requirements, such as monitoring the investigation, ensuring that the investigators obtain informed consent, and labeling and record-keeping requirements. A clinical trial may be suspended by FDA, the sponsor, or an IRB at any time for various reasons, including a belief that the risks to the study participants outweigh the benefits of participation in the trial. Even if a clinical trial is completed, the results may not demonstrate the safety and efficacy of a device to the satisfaction of the FDA or may be equivocal or otherwise not be sufficient to obtain approval of a device. ATEC isWe are not currently undertaking any FDA IDE trials, as all of our existing products are FDA-cleared through the 510k510(k) pathway. It is possible, however, that future device development may require IDE clinical trial for approval.
Pervasive and Continuing FDA Regulation. After a device is placed on the market, numerous FDA and other regulatory requirements continue to apply. These include:
| •
| registration and listing requirements, which require manufacturers to register all manufacturing facilities and list all medical devices placed into commercial distribution;
|
•registration and listing requirements, which require manufacturers to register all manufacturing facilities and list all medical devices placed into commercial distribution;
| •
| the QSR, which requires manufacturers, including third-party contract manufacturers, to follow stringent design, testing, control, supplier/contractor selection, documentation, record maintenance and other quality assurance controls, during all aspects of the manufacturing process and to maintain and investigate complaints;
|
•the QSR, which requires manufacturers, including third-party contract manufacturers, to follow stringent design, testing, control, supplier/contractor selection, documentation, record maintenance and other quality assurance controls, during all aspects of the manufacturing process and to maintain and investigate complaints;
| •
| labeling regulations and unique device identification requirements;
|
•labeling regulations and unique device identification requirements;
| •
| advertising and promotion requirements;
|
•advertising and promotion requirements;
| •
| restrictions on sale, distribution, or use of a device;
|
•restrictions on sale, distribution, or use of a device;
| •13
| FDA prohibitions against the promotion of products for uncleared or unapproved (“off-label”) uses;
|
| •
| medical device reporting obligations, which require that manufacturers submit reports to the FDA of device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to reoccur;
|
| •
| medical device correction and removal reporting regulations, which require that manufacturers report to the FDA field corrections and product recalls or removals if undertaken to reduce a risk to health posed by the device or to remedy a violation of the FDCA that may present a risk to health;
|
| •
| device tracking requirements; and
|
| •
| other post-market surveillance requirements, which apply when necessary to protect the public health or to provide additional safety and effectiveness data for the device.
|
19
Table of Contents
•FDA prohibitions against the promotion of products for uncleared or unapproved (“off-label”) uses;
•medical device reporting obligations, which require that manufacturers submit reports to the FDA of device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to reoccur;
•medical device correction and removal reporting regulations, which require that manufacturers report to the FDA field corrections and product recalls or removals if undertaken to reduce a risk to health posed by the device or to remedy a violation of the FDCA that may present a risk to health;
•device tracking requirements; and
•other post-market surveillance requirements, which apply when necessary to protect the public health or to provide additional safety and effectiveness data for the device.
Failure to comply with applicable regulatory requirements can result in enforcement action by the FDA, which may include any of the following:
| • warning letters and untitled letters; •fines, injunctions, consent decrees, and civil penalties; •recalls, withdrawals, administrative detention, or seizure of products; •operating restrictions, partial suspension, or total shutdown of production; •withdrawals of 510(k) clearances or PMAs that have already been granted; •refusal to grant 510(k) clearance or PMAs of new products; and/or •
| warning letters and untitled letters;
|
| •
| fines, injunctions, consent decrees, and civil penalties;
|
| •
| recalls, withdrawals, administrative detention, or seizure of products;
|
| •
| operating restrictions, partial suspension, or total shutdown of production;
|
| •
| withdrawals of 510(k) clearances or PMA approvals that have already been granted;
|
| •
| refusal to grant 510(k) clearance or PMA approvals of new products; and/or
|
Our facilities, records and manufacturing processes are subject to periodic announced and unannounced inspections by the FDA to evaluate compliance with applicable regulatory requirements.
Regulation of Human Cells, Tissues, and Cellular and Tissue-based Products. Certain of our products are regulated as human cells, tissues, and cellular and tissue-based products (“HCT/Ps”). Section 361 of the PHSA authorizes the FDA to issue regulations to prevent the introduction, transmission or spread of communicable disease. HCT/Ps regulated as “361” HCT/Ps are subject to requirements relating to registering facilities and listing products with the FDA, screening and testing for tissue donor eligibility, or Good Tissue Practice, when processing, storing, labeling, and distributing HCT/Ps, including required labeling information, stringent record keeping, and adverse event reporting, among other applicable requirements and laws. If the HCT/P is minimally manipulated, is intended for homologous use only and meets other requirements, the HCT/P will not require 510(k) clearance, PMA, approval, a Biologics License Applications,Application, or other premarket authorization from the FDA before marketing.
Environmental Matters
Our facilities and operations are subject to extensive federal, state, and local environmental and occupational health and safety laws and regulations. These laws and regulations govern, among other things, air emissions; wastewater discharges; the generation, storage, handling, use and transportation of hazardous materials; the handling and disposal of hazardous wastes; the cleanup of contamination; and the health and safety of our employees. Under such laws and regulations, we are required to obtain permits from governmental authorities for some of our operations. If we violate or fail to comply with these laws, regulations or permits, we could be fined or otherwise sanctioned by regulators. We could also be held responsible for costs and damages arising from any contamination at our past or present facilities or at third-party waste disposal sites. We cannot completely eliminate the risk of contamination or injury resulting from hazardous materials, and we may incur material liability as a result of any contamination or injury.
Compliance with Certain Applicable Statutes
We are subject to various federal and state laws pertaining to healthcare fraud and abuse, including anti-kickback laws, false claims laws, criminal health care fraud laws, physician payment transparency laws, data privacy and security laws, and foreign corrupt practice laws. Violations of these laws are punishable by criminal and/or civil sanctions, including, in some instances, fines, imprisonment and, within the United States,U.S., exclusion from participation in government healthcare programs, including Medicare, Medicaid and Veterans Administration health programs. These laws are administered by, among others, the U.S. Department of Justice, the Office of Inspector General of the Department of Health and Human Services and state attorneys general. Many of these agencies have increased their enforcement activities with respect to medical device manufacturers in recent years.
20
Table of Contents
The federal Anti-Kickback Statute prohibits persons from knowingly and willfully soliciting, offering, receiving, or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing, arranging for or recommending a good or service, for which payment may be made in whole or part under federal healthcare programs, such as the Medicare and Medicaid programs. The Anti-Kickback Statute is broad and prohibits many arrangements and practices that are lawful in businesses outside of the healthcare industry. For example, the definition of “remuneration” has been broadly interpreted to include anything of value, including, gifts, discounts, the furnishing of supplies or equipment, credit arrangements, payments of cash, waivers of payments, ownership interests and providing anything at less than its fair market value. In addition, among other things, the Patient Protection and Affordable Health Care Act, as amended by the Health Care and Education Reconciliation Act (collectively referred to as “ACA”), amendsamended the intent requirement of the federal Anti-Kickback Statute. Pursuant to the ACA, a person or entity no longer needs to have actual knowledge of the Anti-Kickback Statute or specific intent to violate it. Furthermore, the ACA provides that the government may assert that a claim including items or services resulting from a violation of the Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal False Claims Act.
In implementing the Anti-Kickback Statute, the Department of Health and Human Services Office of Inspector General (“OIG”), has issued a series of regulations, known as the safe harbors, which began in July 1991. These safe harbors set forth provisions that, in circumstances where all the applicable requirements are met, will assure healthcare providers and other parties that they will not be prosecuted under the Anti-Kickback Statute. The failure of a transaction or arrangement to fit precisely within one or more safe harbors does not necessarily mean that it is illegal or that prosecution will be pursued. However, conduct and business arrangements that do not fully satisfy all requirements of an applicable safe harbor may result in increased scrutiny by government enforcement authorities such as the OIG. Penalties for violations of the Anti-Kickback Statute include criminal penalties and civil sanctions such as fines, imprisonment and possible exclusion from Medicare, Medicaid and other federal healthcare programs. Many states have anti-kickback laws that are similar to the federal law, some of which apply to the referral of patients for healthcare items or services reimbursed by any source, and may also result in penalties, fines, sanctions for violations, and exclusions from state or commercial programs.
We have entered into various agreements with certain surgeons that perform services for us, including some who make clinical decisions to use our products. Some of our referring surgeons own our stock, which they may have received from us as consideration for services performed. We frequently review these arrangements to determine whether they are in compliance with applicable laws and regulations. In addition, physician-owned distribution companies (“PODs”) have become decreasingly involved in the sale and distribution of medical devices, including products for the surgical treatment of spine disorders. In many cases, these distribution companies enter into arrangements with hospitals that bill Medicare or Medicaid for the furnishing of medical services, and the physician-owners are among the physicians who refer patients to the hospitals for surgery. On March 26, 2013, the OIG issued a Special Fraud Alert entitled “Physician-Owned Entities,” (the “Fraud Alert”), in which the OIG concluded, among other things, that PODs are “inherently suspect under the anti-kickback statute” and that PODs present “substantial fraud and abuse risk and pose dangers of patient safety.” Since 2013, the OIG has further increased its scrutiny of PODs and the Department of Justice has brought several high-profile cases against physician owners.
The federal False Claims Act prohibits persons from knowingly filing or causing to be filed a false or fraudulent claim to, or the knowing use of false statements to obtain payment from, the federal government. Private suits filed under the False Claims Act, known as qui tam actions, can be brought by individuals on behalf of the government. These individuals, sometimes known as “relators” or, more commonly, as “whistleblowers,” may share in any amounts paid by the entity to the government in fines or settlement. The number of filings of qui tam actions has increased significantly in recent years, causing more healthcare companies to have to defend a False Claim Act action. If an entity is determined to have violated the federal False Claims Act, it may be required to pay up to three times the actual damages sustained by the government, plus civil penalties of between $10,000 to $22,000$13,964 and $27,894 for each separate false claim and may be subject to exclusion from Medicare, Medicaid, and other federal healthcare programs. Various states have also enacted similar laws modeled after the federal False Claims Act which apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor.
21
Table of Contents
The Health Insurance Portability and Accountability Act (“HIPAA”) created two new federal crimes: healthcare fraud and false statements relating to healthcare matters. The healthcare fraud statute prohibits knowingly and willfully executing a scheme to defraud any healthcare benefit program, including private payors. The ACA changed the intent requirement of the healthcare fraud statute to such that a person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it. A violation of this statute is a felony and may result in fines, imprisonment or possible exclusion from Medicare, Medicaid, and other federal healthcare programs. The false statements statute prohibits knowingly and willfully falsifying, concealing, or covering up a material fact or making any materially false, fictitious, or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items, or services. A violation of this statute is a felony and may result in similar sanctions.
ACA also includes various provisions designed to significantly strengthen fraud and abuse enforcement in addition to those changes discussed above. Among these additional provisions include increased funding for enforcement efforts and new “sunshine” provisions to require us to report and disclose to the Centers for Medicare and Medicaid Services (“CMS”), any payment or “transfer of value” made or distributed to physicians or teaching hospitals. These sunshine provisions also require certain group purchasing organizations, including physician-owned distributors, to disclose physician ownership information to CMS. We and other device manufacturers are required to collect and annually report specific data on payments and other transfers of value to physicians and teaching hospitals. There are various state laws and initiatives that require device manufacturers to disclose to the appropriate regulatory agency certain payments or other transfers of value made to physicians, and in certain cases prohibit some forms of these payments, with the risk of fines for any violation of such requirements.
HIPAA also includes privacy and security provisions designed to regulate the use and disclosure of “protected health information” (“PHI”), which is health information that identifies a patient and that is held by a health care provider, a health plan or health care clearinghouse. We are not directly regulated by HIPAA, but our ability to access PHI for purposes such as marketing, product development, clinical research or other uses is controlled by HIPAA and restrictions placed on health care providers and other covered entities. HIPAA was amended in 2009 by the Health Information Technology for Economic and Clinical Health Act (“HITECH”) which strengthened the rule, increased penalties for violations, and added a requirement for the disclosure of breaches to affected individuals, the government, and in some cases the media. We must carefully structure any transaction involving PHI to avoid violation of HIPAA and HITECH requirements.
Almost all states have adopted data security laws protecting personal information including social security numbers, state issued identification numbers, credit card or financial account information coupled with individuals’ names or initials. We must comply with all applicable state data security laws, even though they vary extensively, and must ensure that any breaches or accidental disclosures of personal information are promptly reported to affected individuals and responsible government entities. We must also ensure that we maintain compliant, written information security programs or run the risk of civil or even criminal sanctions for non-compliance as well as reputational harm for publicly reported breaches or violations.
If any of our operations are found to have violated or be in violation of any of the laws described above and other applicable state and federal fraud and abuse laws, we may be subject to penalties, among them being civil and criminal penalties, damages, fines, exclusion from government healthcare programs, and the curtailment or restructuring of our operations.
Third-Party Reimbursement
In the U.S., healthcare providers generally rely on third-party payors, principally private insurers, and governmental payors such as Medicare and Medicaid, to cover and pay for all or part of the cost of a spine surgery in which our medical devices are used. We expect that sales volumes and prices of our products will depend in large part on the continued availability of reimbursement from such third-party payors. These third-party payors may deny reimbursement if they determine that a device used in a procedure was not medically necessary in accordance with cost-effective treatment methods, as determined by the third-party payor, or was used for an unapproved indication. Particularly in the U.S., third-party payors continue to carefully review, and increasingly challenge, the prices charged for procedures and medical products. Medicare coverage and reimbursement policies are developed by CMS, the federal agency responsible for administering the Medicare program, and its contractors. CMS establishes these Medicare policies for medical products and procedures and such policies are periodically reviewed and updated. While private payors vary in their coverage and payment policies, the Medicare program is viewed as a benchmark. Medicare payment rates for the same or similar procedures vary due to geographic location, nature of the facility in which the procedure is performed (i.e., teaching or community hospital) and other factors. We cannot provide assurance that government or private third-party payors will cover and provide adequate payment for the procedures in which our products are used. ACA and other reform proposals contain significant changes regarding Medicare, Medicaid, and other third-party payors.
In addition, other legislative changes have been proposed and adopted since the ACA was enacted. These changes include the Budget Control Act of 2011, which resulted in reductions to Medicare payments to providers of 2% per fiscal year, which went into effect on April 1, 2013 and will stay in effect through 2025 unless additional Congressional action is taken, as well as, the American Taxpayer Relief Act of 2012, which, among other things, further reduced Medicare payments to several types of providers, including hospitals and imaging centers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. An expansion in government’s role in the U.S. healthcare industry may lower reimbursements for procedures using our products, reduce medical procedure volumes, and adversely affect our business and results of operations, possibly materially.
We believe that the overall escalating cost of medical products and services has led to, and will continue to lead to, increased pressures on the healthcare industry to reduce the costs of products and services. We cannot assure that government or private third-party payors will cover and provide adequate payment for the procedures using our products. In addition, it is possible that future legislation, regulation, or reimbursement policies of third-party payors will adversely affect the demand for procedures using our products or our ability to sell our products on a profitable basis. The unavailability or inadequacy of third-party payor coverage or reimbursement could have a significant adverse effect on our business, operating results, and financial condition.
Human Capital
As of December 31, 2021,2023, we had 561839 employees worldwide. Approximately 451675 employees were located in the U.S. and 110164 employees were located outside of the U.S. Of our U.S. employees, 295428 were based in our Carlsbad, California headquarters, covering all of the following functional areas: sales, customer service, marketing, clinical education, advanced manufacturing, quality assurance, regulatory affairs, research and development, human resources, finance, legal, information technology, and administration.
Our workforce is highly educated and diverse, which we believe is important for our continued success as a leading innovator in the medical device market. We employ a number of strategies to best enable us to attract, retain, and engage our team members. To build a steady and diverse pipeline of talent, we have a robust recruiting program, which is focused on attracting and retaining the talent we believe is necessary to help achieve our strategy and mission. Further, we employ recruiting processes that mitigate unconscious biases and promote diverse candidate pools. Our employee base is comprised of men, women, underrepresented individuals, individuals with disabilities, and protected veterans.
To attract and retain employees, we offer competitive, performance-based compensation and benefits, opportunities for discounted equity ownership, employee recognition programs, career development opportunities, and access to continual growth through in-house live trainings, as well as support and reimbursement for external trainings and educational programs. In addition, to further expand employee enrichment and engagement, we periodically survey our employees regarding their satisfaction levels. We use these survey results to determine how we can continue to create work environments that energize our employees and enable them to develop and maintain a positive working culture. We completed a survey in December 2021,2023, in which over 95%88% of respondents indicated a willingness to recommend the Company to friends and family as a desirable place to work. High employee satisfaction is also reflected in our high employee engagement and low undesired turnover, which was below 6%approximately 3.7% for 2021.2023.
We also provide opportunities forhave never experienced a work stoppage due to labor difficulties and believe that our relations with our employees to participate in community volunteerare good. We currently have no employees working under collective bargaining agreements.
Health and clean-up programs, as well asWellness
We offer various health and wellness programs to promote a healthy and active lifestyle for our employees and foster camaraderie within our employee base.employees. In addition to our health and wellness program offerings, our corporate headquarters includes indoor and outdoor workout spaces, which our employees are able to access throughout the day, as well as various fitness and workout classes.
We have never experienced a work stoppage due to labor difficulties and believe that our relations with our employees are good. We currently have no employees working under collective bargaining agreements.
Health and Safety
We have taken steps to best ensure the health and safety of our employees globally during the COVID-19 pandemic. We have provided learning resources to enable our employee workforce to transition to/from a virtual or remote workplace, as necessary and appropriate. We have provided health and wellness initiatives throughout the year to promote the continued wellbeing of our employees. Further, despite the global pandemic, we have been ableemployees, as well as opportunities for our employees to maintainparticipate in community volunteer and clean-up programs to foster camaraderie within our employee workforce without material contraction.base.
Corporate and Available Information
We are a Delaware corporation incorporated in March 2005. Our principal executive office is located at 1950 Camino Vida Roble, Carlsbad, California 92008 and our telephone number is (760) 431-9286. Our Internet address is www.atecspine.com. We are not including the information contained on our website as a part of, or incorporating it by reference into, this Annual Report on Form 10-K. Our
We file our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and all amendments to those reports, areelectronically with the Securities and Exchange Commission ("SEC"). We make these reports available to you free of charge through the Investor Relations section of our website as soon as reasonably practicable after such materials have been electronically filed with, or furnished to, the Securities and Exchange Commission (“SEC”).SEC. The public can also obtain any documents that we file with the SEC at http://www.sec.gov.
Table of Contents
Item 1A. Risk Factors Item 1A.
| Risk Factors
|
Investing in our common stock involves a high degree of risk. You should carefully consider the following risk factors and all other information contained or incorporated by reference in this Annual Report on Form 10-K. The risks and uncertainties described below are not the only risks faced by the Company. Additional risks and uncertainties that we are unaware of, or that we currently deem immaterial may become important factors that affect us. If any of such risks or the risks described below occur, either alone or taken together occur, our business, financial condition or results of operations could be materially and adversely affected. In that case, the trading price of our common stock could decline, and you may lose some or all of your investment.
Risks Related to Our Business and Industry
Our business plan relies on certain assumptions pertaining to the market for our products that, if incorrect, may adversely affect our growth and profitability.
We allocate resources based on assumptions about trends in the development of and treatment for spine disorders and the resulting demand for our products. Our assumptions may not be accurate. Increasing awareness and use of non-invasive treatments and other shifts in technologies and treatments, emergence of new materials and acceptance of emerging technologies and procedures could adversely affect demand for our products. If our assumptions prove to be incorrect or if alternative treatments to those we offer gain further acceptance, then demand for our products could be significantly less than we anticipate and we may not be able to achieve or sustain growth or profitability.
We operate in a highly competitive market segment, face competition from large, well-established medical device companies with significant resources, and may not be able to compete effectively.
The market in which we operate is highly competitive, subject to rapid technological change and affected by new products and market activities of industry participants. Our competitors include numerous large and well-capitalized companies such as Medtronic Sofamor Danek, a subsidiary of Medtronic; Depuy Spine, a subsidiary of Johnson & Johnson; Stryker; NuVasive; Zimmer Biomet; and Globus Medical. Several of our competitors enjoy competitive advantages over us, including:
| • more established relationships with healthcare providers, distribution networks and healthcare payers; •broader product offerings and intellectual property portfolios, better name recognition, and more recognizable product trademarks; •greater resources for product research and development, clinical data, patent litigation, and launching, marketing, distributing and selling our products; and •greater experience in obtaining and maintaining FDA and other regulatory clearances or approvals for products and product enhancements. •
| more established relationships with healthcare providers, distribution networks and healthcare payers;
|
| •
| broader product offerings and intellectual property portfolios, better name recognition, and more recognizable product trademarks;
|
| •
| greater resources for product research and development, clinical data, patent litigation, and launching, marketing, distributing and selling our products; and
|
| •
| greater experience in obtaining and maintaining FDA and other regulatory clearances or approvals for products and product enhancements.
|
In addition, at any time our current competitors or new industry participants may develop alternative treatments, products or procedures for the treatment of spine disorders that compete directly or indirectly with our products, including ones that may be superior to our spine surgery products. For these reasons, we may not be able to compete successfully against our existing or potential competitors. Any such failure could lead us to further modify our strategy, lower our prices, increase our sales commissions and could have a significant adverse effect on our business, financial condition and results of operations.
A significant percentage of our revenues are derived from sales of our systems that include polyaxial pedicle screws.
Net sales of our systems that include polyaxial pedicle screws represented approximately 47%41% and 42% our net sales for both 2021the years ended December 31, 2023 and 20202022, respectively, and are expected to continue to be significant in the future. A decline in sales of these systems for any reason would have a significant adverse impact on our business, financial condition and results of operations. We rely on third-party licenses related to our polyaxial pedicle screw systems in order to use various proprietary technologies that are material to these systems, including the enforceability of the intellectual property rights in such technologies. Certain of our licenses may be terminated upon specific conditions. Our rights under each of the licenses are subject to our continued compliance with the terms of the license, including certain diligence, disclosure and confidentiality obligations and the payment of royalties and other fees. Because of the complexity of our product and the patents we have licensed, determining the scope of the license and related obligations can be difficult and can lead to disputes between us and the licensor. An unfavorable resolution of such a dispute could lead to an increase in the royalties payable pursuant to the license or termination of the license. Any action that would prevent us from manufacturing, marketing and selling these systems or increase the costs associated with these systems would have a significant adverse effect on our business, financial condition and results of operations.
Our reliance on sales and marketing efforts are largely dependent upon third parties, many of which are non-exclusive and freeagents could affect our ability to market products that compete with our products.
Most of our independent distributor arrangements are non-exclusive and our distributors are not obligated to buy our products efficiently and can represent competing products. Many of our independent distributors also market and sell competitive products. Our competitors may be able, by offering more favorable terms, to convince our independent distributors to terminate or reduce their relationships with us. Our independent distributors have varying expertise in marketing and selling specialty medical devices. To the extent that our independent distributors are distracted from selling our products or do not employ sufficient efforts in managing and selling our products, our sales and results of operations could be adversely affected.profitably.
The development of a large distribution network may be expensive and time consuming. Because of the intense competition for their services, we may be unable to recruit or retain qualified independent distributors. Somesales agents. Like us, some of our competitors enter into exclusive distribution agreements. Further, we may not be able to enter into agreements with independent distributorssales agents on commercially reasonable terms. Even if we do enter into agreements with new independent distributors,sales agents, it may take 90 to 120 days or even longer for new distributorssales agents to reach full operational effectiveness. Some distributorssales agents may not generate revenue as quickly as we expect, may not commit the necessary resources to effectively market and sell our products and may not ultimately be successful in selling our products. Our business, financial condition and results of operations will be materially adversely affected if we do not attract and retain new distributorssales agents or if the marketing and sales efforts of our distributorssales agents are unsuccessful.
To be commercially successful, we must convince the spine surgeon community that our products are an attractive alternative to competitive products.
In order for us to sell our products, spine surgeons must be convinced that our products are superior to competing products. Acceptance of our products depends on educating the spine surgeon community as to the distinctive characteristics, perceived benefits, safety and cost-effectiveness of our products compared to competitive products and on training spine surgeons in the proper application of our products. If we are not successful in convincing the spine surgeon community of the merit of our products, our sales will decline, and we will be unable to increase or achieve and sustain growth or profitability. Additionally, if surgeons are not properly trained, they may misuse or ineffectively use our products, which may result in unsatisfactory patient outcomes, patient injury, negative publicity or lawsuits against us, any of which could have a significant adverse effect on our business, financial condition and results of operations.
We rely on a limited number of third parties to manufacture and supply our products. Any problems experienced by these manufacturers could result in a delay or interruption in the supply of our products until such manufacturer cures the problem or until we locate and qualify an alternative source of supply.
We rely on third party manufacturers of our implants, instruments, imaging equipment and instruments.spare parts. We currently rely on a limited number of third parties and any prolonged disruption in the operations of our third-party suppliers could have a negative impact on our ability to supply products to customers. We may suffer losses as a result of business interruptions that exceed coverage under our manufacturer’s insurance policies. Other events beyond our control could also disrupt our product development and commercialization efforts until such events can be resolved or we can put in place third-party contract manufacturers to assume this manufacturing role. In addition, if we are required to change manufacturers for any reason, we will be required to verify that the new manufacturer maintains facilities and procedures that comply with quality standards and with all applicable regulations and guidelines. Delays associated with the verification of a new manufacturer or the re-verification of an existing manufacturer could negatively affect our ability to develop products or supply products to customers in a timely manner. Any disruption in the manufacture of our products by our third-party suppliers could have a material adverse impact on our business, financial condition and results of operations.
We depend on third-party suppliers, and in one case a single supplier, for key raw materials and the loss of any of these third-party suppliers, or their inability to supply us with adequate raw materials, could harm our business.
We rely on a number of suppliers and in one case on a single source vendor, Invibio, to provide the raw materials used in the production of our products. We have a supply agreement with Invibio, pursuant to which it supplies us with PEEK, a biocompatible plastic that we use in some of our spacers. Invibio is one of a limited number of companies approved to distribute PEEK in the United StatesU.S. for use in implantable devices. We depend on a limited number of sources of human tissue for use in our biologics products. Our supply of human tissue from our current suppliers and our current inventory of biologics products may not be available at current levels or may not be sufficient to meet our needs. Our dependence on a single third-party PEEK supplier and the challenges we may face in obtaining adequate supplies of biologics products involve several risks, including limited control over pricing, availability, quality and delivery schedules. Any supply interruption in a limited or sole sourced component or raw material could materially harm our ability to source manufactured products until a new source of supply could be found. We may be unable to find a sufficient alternative supply channel in a reasonable time period or on commercially reasonable terms, if at all, which would have a significant adverse effect on our business, financial condition and results of operations.
If we or our suppliers fail to comply with applicable regulations, the manufacture of our products could be delayed.
We and our suppliers are subject to extensive regulation by the FDA and other regulatory agencies both inside and outside of the United States.U.S. The FDA, and other regulatory agencies, audit compliance with some of these regulations. If significant non-compliance issues arise or if a corrective action plan is not sufficient, the manufacture or sale of our products may be limited until such problems are corrected to the regulatory body’s satisfaction, which could have a material adverse effect on our business, financial condition and results of operations. Further, our products could be subject to recall if the regulatory body determines, for any reason, that our products are not safe or effective. Any recall or additional regulatory approval or clearance requirements could result in delays, costs associated with modification of a product, loss of revenue and potential operating restrictions imposed by the regulatory body, all of which could have a material adverse effect on our business, financial condition and results of operations.
Demand for our products, and prices at which customers and patients are willing to pay for our products depend upon the ability of our customers to obtain adequate third-party coverage and reimbursement product purchases.
Sales of our products depend in part on the availability of adequate coverage and reimbursement from third-party payers, principally Medicare, Medicaid and private health insurance plans, to pay for all or a portion of the costs and fees associated with the use of our products. While procedures using our currently marketed products are eligible for reimbursement in the United States,U.S., if surgical procedures utilizing our products are performed on an outpatient basis, it is possible that private payers may no longer provide reimbursement for the procedures using our products without further supporting data on the procedure. Any delays in obtaining, or an inability to obtain, adequate coverage or reimbursement for procedures using our products could significantly affect the acceptance of our products and have a significant adverse effect on our business. Additionally, third-party payers continue to review their coverage policies carefully for existing and new therapies and can, without notice, deny coverage for treatments that include the use of our products. Our business would be negatively impacted if there are any changes that reduce reimbursement for our products.
Operation of our business internationally is subject to our continued compliance with the laws and regulations of each country in which we operate, as well as the business and legal customs in those jurisdictions and geographies.
Our operations, both inside and outside the United States,U.S., are subject to risks inherent in conducting business globally and under the laws, regulations and customs of various jurisdictions and geographies. Our operations outside the United StatesU.S. are subject to special risks and restrictions, including, without limitation: fluctuations in currency values and foreign-currency exchange rates; exchange control regulations; changes in local political or economic conditions; governmental pricing directives; import and trade restrictions; import or export licensing requirements and trade policy; restrictions on the ability to repatriate funds; and other potentially detrimental domestic and foreign governmental practices or policies affecting U.S. companies doing business abroad, including the United StatesU.S. Foreign Corrupt Practices Act and the trade sanctions laws and regulations administered by the United StatesU.S. Department of the Treasury’s Office of Foreign Assets Control. Acts of terror or war may impair our ability to operate in particular countries or regions and may impede the flow of goods and services between countries. Customers in weakened economies may be unable to purchase our products, or it could become more expensive for them to purchase imported products in their local currency, or sell at competitive prices, and we may be unable to collect receivables from such customers. Further, changes in exchange rates may affect our net earnings, the book value of our assets outside the United StatesU.S. and our stockholders’ equity. Failure to comply with the laws and regulations that affect our global operations could have an adverse effect on our business, financial condition, or results of operations.
We may fail to realize the anticipated benefits of the Valence Transaction, as defined below.
The success of our acquisition of Valence (the "Valence Transaction") will depend on, among other things, our ability to incorporate Valence into our business in a manner that enhances our value proposition to clients and facilitates other growth opportunities. If we are unable to successfully achieve these objectives, the anticipated benefits of the Valence Transaction may not be realized fully, if at all, or may take longer to realize than expected. Additionally, management may face challenges in incorporating certain elements and functions of Valence with our business, and this process may result in additional and unforeseen expenses. The integration of Valence Transaction may also disrupt our ongoing business or cause inconsistencies in standards, controls, procedures and policies that adversely affect our relationships with third party partners, employees, suppliers, customers and others with whom we or the business related to Valence have business or other dealings or limit our ability to achieve the anticipated benefits of the Valance Transaction. If we are unable to successfully integrate Valence into our existing business in an efficient, effective and timely manner, anticipated benefits, including the opportunities for expected growth from the Valence Transaction, may not be realized fully, if at all, or may take longer to realize than expected, and our cash flow and financial condition may be negatively affected.
Consolidation in the healthcare industry could lead to price concessions or exclusion of some suppliers from some markets, which could have an adverse effect on our business, financial condition or results of operations.
Continued consolidation in the healthcare industry is expected to increase competition among providers of products and services to industry participants. This in turn has resulted and will likely continue to result in greater pricing pressures and the exclusion of certain suppliers from important market segments as GPOs, independent delivery networks and large single accounts continue to use their market power to consolidate purchasing decisions for some of our customers. We expect that market demand, government regulation, third-party reimbursement policies and societal pressures will continue to impact the worldwide healthcare industry, resulting in further business consolidations and alliances among our customers, which may reduce competition, exert further downward pressure on the prices of our products and may adversely impact our business, financial condition or results of operations.
28
Table of Contents
We may be subject to or otherwise affected by federal and state healthcare laws, including fraud and abuse, health information privacy and security, and disclosure laws, and could face substantial penalties if we are unable to fully comply with such laws.
Although we do not provide healthcare services, submit claims for third-party reimbursement, or receive payments directly from any third-party payers for our products or the procedures in which our products are used, healthcare regulation significantly impacts our business. Healthcare fraud and abuse, health information privacy and security, and disclosure laws potentially applicable to our operations include:
| • the federal Anti-Kickback Statute, as well as state analogs, which prohibits, among other things, knowingly and willfully soliciting, receiving, offering or providing remuneration, intended to induce the purchase or recommendation of an item or service reimbursable under a federal (or state or commercial) healthcare program (such as the Medicare or Medicaid programs); •federal and state bans on physician self-referrals, which prohibits, subject to exceptions, physician referrals of Medicare and Medicaid patients to an entity providing certain “designated health services” if the physician or its immediate family member has any financial relationship with the entity; •false claims laws that prohibit, among other things, knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payers that are false or fraudulent; •HIPAA, and its implementing regulations, which created federal criminal laws that prohibit executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters; •the state and federal laws “sunshine” provisions that require detailed reporting and disclosures to the CMS and applicable states of any payments or “transfer of value” made or distributed to prescribers and other health care providers, and for certain states prohibit some forms of these payments, require the adoption of marketing codes of conduct, require the reporting of marketing expenditures and pricing information and constrain relationships with physicians and other referral sources; •the HITECH, which impose restrictions on uses and disclosures of protected health information and civil and criminal penalties for non-compliance and require the reporting of breaches to affected individuals, the government and in some cases the media in the event of a violation; and •a variety of state-imposed privacy and data security laws which require the protection of personal information beyond health information and which require reporting to state officials in the event of breach or violation and which impose both civil and criminal penalties. •
| the federal Anti-Kickback Statute, as well as state analogs, which prohibits, among other things, knowingly and willfully soliciting, receiving, offering or providing remuneration, intended to induce the purchase or recommendation of an item or service reimbursable under a federal (or state or commercial) healthcare program (such as the Medicare or Medicaid programs);
|
| •
| federal and state bans on physician self-referrals, which prohibits, subject to exceptions, physician referrals of Medicare and Medicaid patients to an entity providing certain “designated health services” if the physician or its immediate family member has any financial relationship with the entity;
|
| •
| false claims laws that prohibit, among other things, knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payers that are false or fraudulent;
|
| •
| The Health Insurance Portability and Accountability Act, or HIPAA, and its implementing regulations, which created federal criminal laws that prohibit executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters;
|
| •
| the state and federal laws “sunshine” provisions that require detailed reporting and disclosures to the CMS and applicable states of any payments or “transfer of value” made or distributed to prescribers and other health care providers, and for certain states prohibit some forms of these payments, require the adoption of marketing codes of conduct, require the reporting of marketing expenditures and pricing information and constrain relationships with physicians and other referral sources;
|
| •
| the Health Information Technology for Economic and Clinical Health Act (“HITECH”), which impose restrictions on uses and disclosures of protected health information and civil and criminal penalties for non-compliance and require the reporting of breaches to affected individuals, the government and in some cases the media in the event of a violation; and
|
| •
| a variety of state-imposed privacy and data security laws which require the protection of personal information beyond health information and which require reporting to state officials in the event of breach or violation and which impose both civil and criminal penalties.
|
If our operations, or those of our independent sales agents and distributors violate any of such laws or any regulations that may apply to us, we may be subject to civil and criminal penalties, damages, fines, exclusion from federal healthcare programs and/or the curtailment or restructuring of our operations. If the healthcare providers, sales agents distributors or other entities with which we do business are found to violate applicable laws, they may be subject to sanctions, which could also have a negative impact on us. Any penalties, damages, fines, curtailment or restructuring of our operations could adversely affect our ability to operate our business and our financial results.
Sales and marketing practices in the healthcare industry have been the subject of increased scrutiny from governmental agencies, and we believe that this trend will continue. Prosecutorial scrutiny and governmental oversight over the retention of healthcare professionals as consultants has affected and may continue to affect how medical device companies retain healthcare professionals as consultants. Our efforts to detect and prevent noncompliance with applicable laws may not be effective in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. Any action against us for violation of these laws, even if we successfully defend against them, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business.
29
Table of Contents
If we fail to timely obtain governmental clearances or approvals for our future products or modifications to our products, our ability to commercially distribute and market our products could suffer.
Our products are subject to extensive governmental regulations. The clearance and approval process, particularly with the FDA, can be costly and time consuming, and such clearances or approvals may not be granted on a timely basis, if at all. In particular, the FDA permits commercial distribution of most new medical devices only after clearance under Sectionreceiving 510(k) of the Federal Food, Drug and Cosmetic Act (“510(k)”),clearance, or approval of a premarket approval application (“PMA”).PMA. The FDA may make its 510(k) clearance process more restrictive and increase the time or expense required to obtain clearances or could make it unavailable for some of our products. A PMA must be submitted if the device cannot be cleared through the 510(k) process or is not exempt from premarket review by the FDA and must be supported by extensive data, including results of preclinical studies and clinical trials, manufacturing and control data and proposed labeling, to demonstrate to the FDA’s satisfaction the safety and effectiveness of the device for its intended use. The PMA process is more costly and uncertain than the 510(k) clearance process. In addition, any modification to a 510(k)-cleared device that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, design or manufacture, requires a new 510(k) clearance or possibly a PMA.
Commercial distribution and marketing of any of our products or product modifications will be delayed until regulatory clearance or approval is obtained which may take significantly longer than anticipated. Governmental authorities can delay, limit or deny clearance or approval of a device for many reasons, including:
| • our inability to demonstrate to the satisfaction of the applicable regulatory authority that our products are safe or effective for their intended uses, or that the clinical and other benefits of the device outweigh the risks; •disagreement of the applicable regulatory authority with the design or implementation of our clinical trials or the interpretation of data from pre-clinical studies or clinical trials; •serious and unexpected adverse effects experienced by participants in our clinical trials; •data from our pre-clinical studies and clinical trials may be insufficient to support clearance or approval, where required; •our manufacturing process or facilities we use may not meet applicable requirements; or •approval policies or regulations of the applicable regulatory authorities change significantly in a manner rendering our clinical data or regulatory filings insufficient for clearance or approval. •
| our inability to demonstrate to the satisfaction of the applicable regulatory authority that our products are safe or effective for their intended uses, or that the clinical and other benefits of the device outweigh the risks;
|
| •
| disagreement of the applicable regulatory authority with the design or implementation of our clinical trials or the interpretation of data from pre-clinical studies or clinical trials;
|
| •
| serious and unexpected adverse effects experienced by participants in our clinical trials;
|
| •
| data from our pre-clinical studies and clinical trials may be insufficient to support clearance or approval, where required;
|
| •
| our manufacturing process or facilities we use may not meet applicable requirements; or
|
| •
| approval policies or regulations of the applicable regulatory authorities change significantly in a manner rendering our clinical data or regulatory filings insufficient for clearance or approval.
|
Delays in obtaining regulatory clearances and approvals may delay or prevent commercialization of products we develop, require us to perform costly tests or studies, diminish any competitive advantages that we might otherwise have obtained and reduce our ability to generate revenues.
If we choose to acquire new and complementary businesses, products or technologies, we may be unable to complete these acquisitions or successfully integrate them in a cost-effective and non-disruptive manner.
Our success depends in part on our ability to continually enhance and broaden our product offering. Accordingly, we have pursued and intend to pursue the acquisition of complementary businesses, products or technologies. We do not know if we will be able to successfully complete any acquisitions or successfully integrate any acquired business. Our ability to successfully grow through acquisitions depends upon our ability to identify, negotiate, complete and integrate suitable acquisition targets. These efforts could be expensive and time consuming, disrupt our ongoing business and distract management. If we are unable to integrate any future or recently acquired businesses, products or technologies effectively, our business, financial condition and results of operations will be materially adversely affected.
30
Table of Contents
We are dependent on our senior management team, sales and marketing team, engineering team and key surgeon advisors, and the loss of any of them could harm our business.
Our continued success depends in part upon the continued availability and contributions of our senior management, sales and marketing team and engineering team and the continued participation of our key surgeon advisors. We compete for personnel and advisors with other companies and organizations, many of which have greater name recognition and resources than we do. Changes to our senior management team, sales and marketing team, engineering team and key surgeon advisors, or our inability to attract or retain other qualified personnel or advisors, could have a significant adverse effect on our business, financial condition and results of operations.
Our business is dependent upon the effective operation of our information systems, software, or information security practices and those of our business partners or third-party service providers. Security breaches, loss of data and other disruptions could compromise sensitive information related to our business, prevent us from accessing critical information or expose us to liability, which could adversely affect our business and our reputation.
We utilize many information systems and other software that are critical to our business, some of which are managed by third parties. We regularly use these information systems or software to collect and store sensitive data, including legally protected patient health and personally identifiable information, intellectual property information, and proprietary business information. We managemay be unable to maintain or improve our information systems and maintainsoftware or experience unanticipated delays, complications, or expenses in implementing, integrating, and operating our applicationssystems or incur substantial expenditures or interruptions in operations in connection with system improvements or implementations. The failure of our information systems or software or those of our business partners or third-party service providers to perform properly could disrupt our business and data utilizing on-site systems. harm our reputation, which may result in decreased sales, increased overhead costs, excess or obsolete inventory, and product shortages, causing our business, reputation, financial condition, and operating results to suffer.
The secure processing, storage, maintenance and transmission of this critical information is vital to our operations and business strategy, and we devote significant resources to protecting such information. Although we take measures to protect sensitive information from unauthorized access or disclosure, our information technology and infrastructure may be vulnerable to theft, loss, damage, and interruption from a number of potential sources and events, including unauthorized access or security breaches, data privacy breaches, natural or man-made disasters, cyber attacks, by hackers,computer viruses, breachesmalware, phishing, denial of service attacks, power loss, or interruptions.other disruptive events. Any such security incidents could compromise our networks and the information stored there could be accessed by unauthorized parties, disclosed, lost or stolen. Any such security incidents could also result in legal claims or proceedings, liability under laws that protect the privacy of personal information, government enforcement actions and regulatory penalties. Unauthorized access, loss or disclosure could also interrupt our operations and result in damage to our reputation, each of which could adversely affect our business. As a result of new SEC rules and regulations, we are required to disclose, on a current basis pursuant to new Item 1.05 of SEC Form 8-K, any cybersecurity incident that we determine to be material and describe the material aspects of the nature, scope, and timing of the incident, as well as the material impact or reasonably likely material impact of the incident on us, including our financial condition and results of operations. We will also be required to describe, on a periodic basis, our processes, if any, for the assessment, identification, and management of material risks from cybersecurity threats, and describe whether any risks from cybersecurity threats have materially affected or are reasonably likely to materially affect our business strategy, results of operations, or financial condition, our board’s oversight of risks from cybersecurity threats and management’s role in assessing and managing material risks from cybersecurity threats. We have incurred significant costs in an effort to detect and prevent security breaches and incidents, and we may face increased costs and requirements to expend substantial resources in the event of an actual or perceived security breach or incident and to comply with this new SEC cybersecurity rule. Additionally, our insurance policies may not be adequate to compensate us for the potential damages arising from any such disruption, failure or security breach or incident. In addition, such insurance may not be available to us in the future on economically reasonable terms, or at all. Further, our insurance may not cover all claims made against us and could have high deductibles in any event, and defending a suit, regardless of its merit, could be costly and divert management attention.
Nearly all of our operations are currently conducted in locations that may be at risk of damage from fire, earthquakes or other natural disasters.
We conduct nearly all of our business activities in or near known wildfire areas and earthquake fault zones. We have taken precautions to safeguard our facilities, including obtaining property and casualty insurance, and implementing health and safety protocols. We have developed an information technology disaster recovery plan. However, any future natural disaster could cause substantial delays in our operations, damage or destroy our equipment or inventory and cause us to incur additional expenses. A disaster could seriously harm our business, financial condition and results of operations. Our insurance against earthquakes, fires, and other natural disasters would not be adequate to cover a total loss of our facilities, may not be adequate to cover our losses in any particular case and may not continue to be available to us on acceptable terms, or at all.
Public health crises, political crises, and other catastrophic events or other events outside of our control may impact our business.
A natural disaster (such as tsunami, power shortage, or flood), public health crisis (such as a pandemic or epidemic), political crisis (such as terrorism, war, political instability or other conflict), or other events outside of our control that may occur and may adversely impact our business and operating results. Moreover, these types of events could negatively impact surgeon or patient spending in the impacted region(s), which could adversely impact our operating results. We monitor such events and take actions that we deem reasonable given the circumstances. In the future other types of crises, may create an environment of business uncertainty around the world, which may hinder sales and/or supplies of our products nationally and internationally.
The spread of COVID-19 has disrupted the United States’ and international healthcare and healthcare regulatory systems and diverted healthcare resources away from, and delayed, governmental approval with respect some products. It is unknown how long these disruptions could continue. Additionally, COVID-19’s spread, which has had a broad global impact, including restrictions on travel and quarantine polices put into place by businesses and governments, may materially affect us economically by causing disruptions in our supply chain or distribution channels, or, by causing delays or cancellations of elective surgical procedures due to lack of hospital resources or staffing. As the global response to the COVID-19 pandemic continues to evolve, the extent to which it may impact our business will depend on future developments, which are highly uncertain and cannot be predicted.
Alphatec Holdings is a holding company with no operations, and unless it receives dividends or other payments from its subsidiaries, it will be unable to fulfill its cash obligations.
As a holding company with no business operations, Alphatec Holdings’ material assets consist only of the common stock of its subsidiaries, dividends and other payments received from time to time from its subsidiaries, and the proceeds raised from the sale of debt and equity securities. Alphatec Holdings’ subsidiaries are legally distinct from Alphatec Holdings and have no obligation, contingent or otherwise, to make funds available to Alphatec Holdings. Alphatec Holdings will have to rely upon dividends and other payments from its subsidiaries to generate the funds necessary to fulfill its cash obligations. Alphatec Holdings may not be able to access cash generated by its subsidiaries in order to fulfill cash commitments. The ability of Alphatec Spine, SafeOp, or EOS to make dividend and other payments to Alphatec Holdings is subject to the availability of funds after taking into account its subsidiaries’ funding requirements, the terms of its subsidiaries’ indebtedness and applicable state laws.
If we fail to properly manage our anticipated growth, our business could suffer.
While we intend to continue to pursue growth in our business, such anticipated growth is expected to place significant demands on our managerial, operational and financial resources and systems. Our management may need to divert a disproportionate amount of its attention from day-to-day activities to managing these anticipated growth activities. If we do not manage our anticipated growth effectively, the quality of our products, our relationships with physicians, distributorssales agents and hospitals, and our reputation could suffer, which would have a significant adverse effect on our business, financial condition and results of operations.
If we decrease prices for our goods and services and we are unable to compensate for such reductions through changes in our product mix or reductions to our expenses, our results of operations will suffer.
We may be forced to decrease prices for our goods and services due to pricing pressure exerted by our customers in response to increased cost containment efforts from managed care organizations and other third-party payers and increased market power of our customers as the medical device industry consolidates. If we are unable to offset such price reductions through changes in our product mix or reductions in our expenses, our business, financial condition, results of operations and cash flows will be adversely affected.
The EOS business combination may have an adverse effect on our business.
The EOS business combination combined two companies that previously operated as independent public companies. The combined company will be required to devote significant management attention and resources to integrating our business practices and operations. In addition, we have incurred transaction-related and restructuring costs in connection with the business combination and will continue to incur such costs in connection with our integration. These expenses could, particularly in the near term, reduce the cost synergies that we achieve from the elimination of duplicative expenses and the realization of economies of scale and cost synergies related to the integration of the businesses following the completion of the business combination, and accordingly, any net synergies may not be achieved in the near term or at all. These integration expenses may result in us taking significant charges against earnings following the completion of the business combination, which could adversely affect our cash flows and operating results.
32
Table of Contents
Risks Related to Our Financial Results, Credit and Certain Financial Obligations and Need for Financing
We may need to raise additional funds in the future and such funds may not be available on acceptable terms, if at all.
At December 31, 2021,2023, our principal sources of liquidity consisted of cash and cash equivalents of $187.2$221.0 million, accounts receivable, net, and cash from operations.operations and available borrowings under our revolving credit facility with entities affiliated with MidCap Financial Trust ("Revolving Credit Facility"). We believe that our current sources of liquidity will be sufficient to fund our planned expenditures and meet our obligations for at least 12 months subsequent to the date the consolidated financial statements are issued. If needed, we will seek additional funds from public and private equity or debt financings, borrowings under the Revolving Credit Facility, new debt facilities or other sources to fund our projected operating requirements. Our capital requirements will depend on many factors, including:
| • the revenues generated by sales of our products; •the costs associated with expanding our sales and marketing efforts; •the expenses that we incur from the manufacture of our products by third parties and that we incur from selling our products; •the costs of developing new products or technologies; •the cost of obtaining and maintaining FDA or other regulatory approval or clearance for our products and products in development; •the cost of filing and prosecuting patent applications and defending and enforcing our patent and other intellectual property rights; •the number and timing of acquisitions and other strategic transactions; •the costs and any payments we may make related to our pending litigation matters; •the costs associated with increased capital expenditures; and •the costs associated with our employee retention programs and related benefits. •
| the payments due in connection with the settlement agreement enter into with Orthotec LLC;
|
| •
| the revenues generated by sales of our products;
|
| •
| the costs associated with expanding our sales and marketing efforts;
|
| •
| the expenses that we incur from the manufacture of our products by third parties and that we incur from selling our products;
|
| •
| the costs of developing new products or technologies;
|
| •
| the cost of obtaining and maintaining FDA or other regulatory approval or clearance for our products and products in development;
|
| •
| the cost of filing and prosecuting patent applications and defending and enforcing our patent and other intellectual property rights;
|
| •
| the number and timing of acquisitions and other strategic transactions;
|
| •
| the costs and any payments we may make related to our pending litigation matters;
|
| •
| the costs associated with increased capital expenditures; and
|
| •
| the costs associated with our employee retention programs and related benefits.
|
As a result of these factors, we may need to raise additional funds and such funds may not be available on favorable terms, if at all. In addition, rules and regulations of the SEC may restrict our ability to conduct certain types of financing activities or may affect the timing of and the amounts we can raise by undertaking such activities.
Furthermore, if we issue additional equity or debt securities to raise additional funds, our existing stockholders may experience dilution and the new equity or debt securities may have rights, preferences and privileges senior to those of our existing stockholders. In addition, if we raise additional funds through collaboration, licensing or other similar arrangements, it may be necessary to relinquish valuable rights to our potential products or proprietary technologies, or to grant licenses on terms that are not favorable to us. If we cannot raise funds on acceptable terms, we may not be able to repay debt or other liabilities, develop or enhance our products, execute our business plan, take advantage of future opportunities, or respond to competitive pressures or unanticipated customer requirements. Any of these events could adversely affect our ability to achieve our development and commercialization goals and have a significant adverse effect on our business, financial condition and results of operations.
If we default onCovenants in our obligations to make settlement payments to Orthotec LLC, the amounts due under the settlement agreements accelerateloan documents and become due and payable.
Any default of our payment obligation under the settlement agreements we entered into with Orthotec LLC (“Orthotec”), would give Orthotec the right to declare all of the future payments to be immediately payable. As of December 31, 2021, the outstanding amount to be paid to Orthotec through January 2024, which consists of accrued interest only, was $8.5 million. If acceleration of payments occurs,indenture may restrict our business and operations and if we do not effectively manage our covenants, our financial condition and results of operations could be materiallyadversely affected.
The loan agreements we entered into in connection with our Revolving Credit Facility and the Braidwell Term Loan as well as the indenture governing our outstanding 0.75% Convertible Senior Notes due 2026 (the "2026 Notes") contain certain affirmative, operating or financial covenants. These covenants could adversely affected.
33
Tableaffect our ability to operate our business, our liquidity or our results of Contentsoperations, and our inability to comply with any of these covenants could result in a default under the applicable loan agreement or indenture, which could result in an increase the applicable interest rate or all amounts borrowed under the applicable debt instrument, together with accrued interest and other fees, to become due and payable or, with respect to our Revolving Credit Facility, could result in MidCap refusing to make further extensions of credit to it. If our indebtedness under the Revolving Credit Facility, the Braidwell Term Loan or the 2026 Notes were to be accelerated, if the amount of interest owing under such debt or, in the case of the Revolving Credit Facility, if MidCap refuses to make further extensions of credit to us, we may not have sufficient cash available to repay the amounts due, and we may be forced to seek an amendment to the applicable loan terms or obtain alternative financing, which may not be available to us on acceptable terms, if at all. In addition, if we are unable to repay outstanding borrowings when due or upon an event of default, in the case of the Revolving Credit Facility and Braidwell Term Loan, the lender would also have the right to proceed against the collateral, including substantially all of our assets, granted to secure the indebtedness under the debt obligation. If the applicable lender proceeds against the collateral, such assets would no longer be available for use in our business, which would have a significant adverse effect our business, financial condition and results of operations.
We have a history of net losses, we expect to continue to incur net losses in the near future, and we may not achieve or maintain profitability.
We have typically incurred net losses from our continuing operations since our inception. As of December 31, 2021,2023, we had an accumulated deficit of $782.4 million.$1.1 billion. We have incurred significant net losses since inception and have relied on our ability to fund our operations through revenues from the sale of our products and equity and debt financings. Successful transition to profitability is dependent upon achieving a level of revenues adequate to support our cost structure. This may not occur and, unless and until it does, we will continue to need to raise additional capital. We may seek additional funds from public and private equity or debt financings, borrowings under new debt facilities or other sources to fund our projected operating requirements. However, we may not be able to obtain further financing on reasonable terms or at all. If we are unable to raise additional funds on a timely basis, or at all, we would be materially adversely affected.
A sudden and significant economic downturn or volatility in the economy in the United States and our other major marketsany market in which we operate could have a material adverse impact on our business, financial condition, results of operations, or cash flows.
We operate primarily in the United States but also globally and asAs a result of our domestic and global business andoperations, our revenues are impacted by changes in domestic and global macroeconomic conditions. A weakening of economic conditions, including from a worsening of the ongoing labor shortage or rising in inflation, could lead to increased costs to our business and reductions in demand for our products. Weakened economic conditions or a recession could reduce the amounts that customers are willing or able to spend on our products. Furthermore, a high percentage of our expenses, including those related to inventory, capital investments, and operating costs are generally fixed in nature in the short term. If we are not able to timely and appropriately adapt to changes resulting from a weak or uncertain economic environment, our business, financial condition, results of operations and cash flows could be adversely impacted.
Our quarterly financial results could fluctuate significantly.
Our quarterly financial results are difficult to predict and may fluctuate significantly from period to period, particularly because our sales prospects are uncertain. The level of our revenues and results of operations at any given time will be based primarily on the following factors:
| • acceptance of our products by spine surgeons, patients, hospitals and third-party payers; •demand and pricing of our products, and the mix of our products sold, because profit margins differ among our products; •timing of new product offerings, acquisitions, licenses or other significant events by us or our competitors; •our ability to grow and maintain a productive sales and marketing organization and independent sales agent network; •regulatory approvals and legislative changes affecting the products we may offer or those of our competitors; •successful integration of newly acquired businesses, technology and personnel into our business operations; •the effect of competing technological and market developments; •levels of third-party reimbursement for our products; •interruption in the manufacturing or distribution of our products or our ability to produce or obtain products of satisfactory quality or in sufficient quantities to meet demand; and •changes in our ability to obtain FDA, state and international approval or clearance for our products. •
| acceptance of our products by spine surgeons, patients, hospitals and third-party payers;
|
| •
| demand and pricing of our products, and the mix of our products sold, because profit margins differ among our products;
|
| •
| timing of new product offerings, acquisitions, licenses or other significant events by us or our competitors;
|
| •
| our ability to grow and maintain a productive sales and marketing organization and independent distributor network;
|
| •
| regulatory approvals and legislative changes affecting the products we may offer or those of our competitors;
|
| •
| the effect of competing technological and market developments;
|
| •
| levels of third-party reimbursement for our products;
|
| •
| interruption in the manufacturing or distribution of our products or our ability to produce or obtain products of satisfactory quality or in sufficient quantities to meet demand; and
|
| •
| changes in our ability to obtain FDA, state and international approval or clearance for our products.
|
In addition, until we have a larger base of spine surgeons using our products, occasional fluctuations in the use of our products by individual surgeons or small groups of surgeons will have a proportionately larger impact on our revenues than for companies with a larger customer base.
34
Table of Contents
We cannot begin to commercialize any products that we seek to introduce in the United StatesU.S. without FDA approval or clearance. As a result, it will be difficult for us to forecast demand for these products with any degree of certainty. Any shortfalls in revenue or earnings from levels expected by our stockholders or by industry analysts could have a significant adverse effect on the trading price of our common stock in any given period.
Risks Related to Our Intellectual Property, Regulatory Penalties and Litigation
If our patents and other intellectual property rights do not adequately protect our products, we may lose market share to our competitors and be unable to operate our business profitably.
Our success depends significantly on our ability to protect our proprietary rights in the technologies used in our products. We rely on patent protection, as well as a combination of copyright, trade secret and trademark laws, and confidentiality and other contractual restrictions to protect our proprietary technology. These legal means afford only limited protection and may not adequately protect our rights or permit us to gain or keep any competitive advantage. For example, our pending patent applications may not result in issued patents. The U.S. Patent and Trademark Office (“PTO”) may deny or require significant narrowing of claims in our pending patent applications, and patents issued as a result of the pending patent applications, if any, may not provide us with significant commercial protection or be issued in a form that is advantageous to us. We could also incur substantial costs in proceedings before the PTO. These proceedings could result in adverse decisions as to the priority of our inventions and the narrowing or invalidation of claims in issued patents. Issued patents could subsequently be successfully challenged by others and invalidated or rendered unenforceable, which could limit our ability to prevent competitors from marketing and selling related products. In addition, our pending patent applications include claims to aspects of our products and procedures that are not currently protected by issued patents.
Both the patent application process and the process of managing patent disputes can be time consuming and expensive. Competitors may design around our patents or develop products that provide outcomes that are comparable to our products but fall outside of the scope of our patent protection. Although we have entered into confidentiality agreements and intellectual property assignment agreements with certain of our employees, consultants and advisors as one of the ways we seek to protect our intellectual property and other proprietary technology, such agreements may not be enforceable or may not provide meaningful protection for our trade secrets or other proprietary information in the event of unauthorized use or disclosure or other breaches of the agreements. In the event a competitor infringes upon one of our patents or other intellectual property rights, enforcing those patents and rights may be difficult and time consuming. Even if successful, litigation to defend our patents against challenges or to enforce our intellectual property rights could be expensive and time consuming and could divert management’s attention from managing our business. Moreover, we may not have sufficient resources to defend our patents against challenges or to enforce our intellectual property rights.
The medical device industry is characterized by patent and other intellectual property litigation and we could become subject to litigation that could be costly, result in the diversion of management’s time and efforts, require us to pay damages, and/or prevent us from marketing our existing or future products.
The medical device industry is characterized by extensive litigation and administrative proceedings over patent and other intellectual property rights. Determining whether a product infringes a patent involves complex legal and factual issues, the determination of which is often uncertain. Our competitors may assert that our products, components of those products, methods of using those products, or methods we employ to manufacture or process those products are covered by patents held by them. In addition, they may claim that their patents have priority over ours because their patents were filed first. Because patent applications can take many years to issue, there may be applications now pending of which we are unaware, which may later result in issued patents that our products may infringe. There could also be existing patents that one or more components of our products may be inadvertently infringing, of which we are unaware. As the number of participants in the market for spine disorder devices and treatments increases, the possibility of patent infringement claims against us also increases.
35
Table of Contents
Any such claim against us, even those without merit, may cause us to incur substantial costs, and could place a significant strain on our financial resources, divert the attention of management from our core business and harm our reputation. If the relevant patents are upheld as valid and enforceable and we are found to infringe, we could be required to pay substantial damages and/or royalties and we could be prevented from selling our products unless we obtain a license or redesign our products to avoid infringement. Any such license may not be available on reasonable terms, if at all, and we may be unable to redesign our products to not infringe those patents, and any such redesign, if possible, may be costly. If we fail to obtain any required licenses or make any necessary changes to our products, we may have to withdraw existing products from the market or may be unable to commercialize one or more of our products, either of which could have a significant adverse effect on our business, financial condition and results of operations. We may lose market share to our competitors if we fail to protect our intellectual property rights.
In addition, we enter into agreements with spine surgeons to develop new products. As consideration for product development activities rendered pursuant to these agreements, in some instances we have agreed to pay royalties on products developed by cooperative involvement between us and such surgeons. The surgeons with whom we have entered into such an arrangement might claim to be entitled to a royalty even if we do not believe that such products were developed by cooperative involvement between us and such surgeons. Any such claim, even those without merit, may cause us to incur substantial costs, and could place a significant strain on our financial resources, divert the attention of management from our core business and harm our reputation.
Table of Contents
We are currently involved in a patent litigation action involving NuVasive, Inc. and, if we do not prevail in this action, we could be liable for past damages and might be prevented from marketing or selling some products.
NuVasive has filed suit against us in the U.S. District Court for the Southern District of California, alleging that certain of our products infringe, or contribute to the infringement of, United States patents owned by NuVasive. NuVasive is a large, publicly-traded corporation with significantly greater financial resources than us.
An unfavorable outcome for us in this patent litigation could significantly harm our business if such outcome makes us unable to commercialize some of our current or potential products or cease some of our business operations. In addition, costs of defense and any damages resulting from the litigation may materially adversely affect our business and financial results. The litigation may also harm our relationships with existing customers and subject us to negative publicity, each of which could harm our business and financial results.
If we become subject to product liability claims, we may be required to pay damages that exceed our insurance coverage.
Our business exposes us to potential product liability claims that are inherent in the manufacture and sale of medical devices for spine surgery procedures. Spine surgery involves significant risk of serious complications, including paralysis and even death. We carry product liability insurance. However, our product liability insurance coverage may be inadequate to satisfy liabilities we might incur. Any product liability claim brought against us could result in the increase of our product liability insurance rates or our inability to secure coverage in the future on commercially reasonable terms. If our product liability insurance proves to be inadequate to pay a damage award, we may have to pay the excess out of our cash reserves, which could harm our financial condition. If longer-term patient results and experience indicate that our products or any component of our products cause tissue damage, motor impairment or other adverse effects, we could be subject to significant liability. Even a meritless or unsuccessful product liability claim could harm our reputation in the industry, lead to significant legal fees and result in the diversion of management’s attention from managing our business. If a product liability claim or series of claims is brought against us in excess of our insurance coverage limits, our business could suffer and our financial condition, results of operations and cash flow could be materially adversely impacted.
36
Table of Contents
Because biologics products entail a potential risk of communicable disease to human recipients, we may be the subject of product liability claims regarding our biologics products.
Our biologics products may expose us to additional potential product liability claims. The development of biologics products entails the risk of transmitting disease to human recipients, and substantial product liability claims may be asserted against us. In addition, successful product liability claims made against one of our competitors could cause claims to be made against us or expose us to a perception that we are vulnerable to similar claims. Even a meritless or unsuccessful product liability claim could harm our reputation in the industry, lead to significant legal fees and result in the diversion of management’s attention from managing our business.
Any claims relating to our improper handling, storage or disposal of biological, hazardous and radioactive materials could be time consuming and costly.
The manufacture of certain of our products, including our biologics products, involves the controlled use of biological, hazardous and/or radioactive materials and waste. Our business and facilities and those of our suppliers are subject to laws and regulations governing the use, manufacture, storage, handling and disposal of these materials and waste. Although we believe that our safety procedures comply with legally prescribed standards, we cannot completely eliminate the risk of accidental contamination or injury from these materials. In the event of an accident, we could be held liable for damages or penalized with fines, which could exceed our resources and insurance. We may incur significant expenses in the future relating to any failure to comply with applicable laws and regulations, which could have a significant negative impact on our business, financial condition and results of operations.
Risks Related to Our Common Stock
Our stock price may fluctuate significantly, particularly if holders of substantial amounts of our stock attempt to sell, and holders may have difficulty selling their shares based on trading volumes of our stock.
The market price of our common stock is likely to be highly volatile and may fluctuate substantially due to many factors, including those described elsewhere in this “Risk Factors” section and the following:
| •
| volume and timing of orders for our products;
|
•volume and timing of orders for our products;
| •
| quarterly variations in our or our competitors’ results of operations;
|
•quarterly variations in our or our competitors’ results of operations;
| •
| our announcement or our competitors’ announcements regarding new or enhanced products, product enhancements, significant contracts, number of distributors, number of hospitals and spine surgeons using products, acquisitions, and collaborative or strategic investments;
|
•our announcement or our competitors’ announcements regarding new or enhanced products, product enhancements, significant contracts, number of sales agents, number of hospitals and spine surgeons using products, acquisitions, and collaborative or strategic investments;
| •
| announcements of technological or medical innovations for the treatment of spine pathology;
|
•announcements of technological or medical innovations for the treatment of spine pathology;
| •
| changes in earnings estimates or recommendations by securities analysts;
|
•changes in earnings estimates or recommendations by securities analysts;
| •31
| our ability to develop, obtain regulatory clearance or approval for, and market new and enhanced products on a timely basis;
|
| •
| changes in healthcare policy in the United States, including changes in governmental regulations or in the status of our regulatory approvals, clearances or applications, and changes in the availability of third-party reimbursement in the United States;
|
| •
| product liability claims or other litigation involving us, including disputes or other developments with respect to intellectual property rights;
|
| •
| sales of large blocks of our common stock, including sales by our executive officers, directors and significant stockholders;
|
| •
| changes in accounting principles; and
|
| •
| general market conditions and other factors, including factors unrelated to our operating performance or the operating performance of our competitors.
|
37
Table of Contents
•our ability to develop, obtain regulatory clearance or approval for, and market new and enhanced products on a timely basis;
•changes in healthcare policy in the U.S., including changes in governmental regulations or in the status of our regulatory approvals, clearances or applications, and changes in the availability of third-party reimbursement in the U.S.;
•product liability claims or other litigation involving us, including disputes or other developments with respect to intellectual property rights;
•sales of large blocks of our common stock, including sales by our executive officers, directors and significant stockholders;
•changes in accounting principles; and
•general market conditions and other factors, including factors unrelated to our operating performance or the operating performance of our competitors.
We may become involved in securities class action litigation that could divert management’s attention and harm our business.
The stock market in general, the NASDAQ Global Select Market and the market for medical device companies in particular, has experienced price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of those companies. In the past, following periods of volatility in the market price of a particular company’s securities, the company becomes subject to securities class action litigation. We may become involved in this type of litigation. Litigation is often expensive and diverts management’s attention and resources, which could materially harm our financial condition, results of operations and business.
Securities analysts may not provide coverage of our common stock or may issue negative reports, which may have a negative impact on the market price of our common stock.
Securities analysts may not provide research coverage of our common stock. The trading market for our common stock may be affected in part by the research and reports that analysts publish about our business. If one or more of the analysts who elects to cover us downgrades our stock, our stock price could likely decline rapidly. If one or more of these analysts ceases coverage of us, we could lose visibility in the market, which in turn could cause our stock price to decline.
Because of their significant stock ownership, our executive officers, directors and principal stockholders will be able to exert control over us and our significant corporate decisions.
Based on shares outstanding at February 24, 2022,19, 2024, our executive officers, directors and stockholders holding more than 5% of our outstanding common stock and their affiliates, in the aggregate, beneficially own approximately 30% of our outstanding common stock. As a result, these persons will have the ability to impact significantly the outcome of all matters requiring stockholder approval, including the election and removal of directors and any merger, consolidation, or sale of all or substantially all of our assets. This concentration of ownership may harm the market price of our common stock by delaying, deferring or preventing our change in control, causing us to enter into transactions or agreements that are not in the best interests of all of our stockholders, or reducing our public float held by non-affiliates.
Anti-takeover provisions in our organizational documents and change of control provisions in some of our employment agreements and agreements with distributors,sales agents, and in some of our outstanding debt agreements, as well as the terms of our redeemable preferred stock, may discourage or prevent a change of control, even if an acquisition would be beneficial to our stockholders, which could affect our stock price adversely.
Certain provisions of our amended and restated certificate of incorporation and restated by-laws could discourage, delay or prevent a merger, acquisition or other change in control that stockholders may consider favorable, including transactions in which our stockholders might otherwise receive a premium for their shares. These provisions also could limit the price that investors might be willing to pay in the future for shares of our common stock, thereby depressing the market price of our common stock. Stockholders who wish to participate in these transactions may not have the opportunity to do so. Furthermore, these provisions could prevent or frustrate attempts by our stockholders to replace or remove our management. These provisions:
| • allow the authorized number of directors to be changed only by resolution of our Board of Directors; •allow vacancies on our Board of Directors to be filled only by resolution of our Board of Directors; •authorize our Board of Directors to issue, without stockholder approval, blank check preferred stock that, if issued, could operate as a “poison pill” to dilute the stock ownership of a potential hostile acquirer to prevent an acquisition that is not approved by our Board of Directors; •require that stockholder actions must be effected at a duly called stockholder meeting and prohibit stockholder action by written consent; •establish advance notice requirements for stockholder nominations to our Board of Directors and for stockholder proposals that can be acted on at stockholder meetings; and •limit who may call stockholder meetings. •
| allow the authorized number of directors to be changed only by resolution of our Board of Directors;
|
| •
| allow vacancies on our Board of Directors to be filled only by resolution of our Board of Directors;
|
| •
| authorize our Board of Directors to issue, without stockholder approval, blank check preferred stock that, if issued, could operate as a “poison pill” to dilute the stock ownership of a potential hostile acquirer to prevent an acquisition that is not approved by our Board of Directors;
|
| •
| require that stockholder actions must be effected at a duly called stockholder meeting and prohibit stockholder action by written consent;
|
| •
| establish advance notice requirements for stockholder nominations to our Board of Directors and for stockholder proposals that can be acted on at stockholder meetings; and
|
| •
| limit who may call stockholder meetings.
|
38
Table of Contents
These provisions may frustrate or prevent attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our Board of Directors, which is responsible for appointing our management. In addition, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which limits the ability of stockholders owning in excess of 15% of our outstanding voting stock to merge or combine with us.
Some of our agreements provide for accelerated vesting of benefits, including full vesting of restricted stock and options, upon a change of control, or extends the term of the agreement upon a change in control and make it more difficult for us or our successor to terminate the agreement. These provisions may discourage or prevent a change of control.
In addition, in the event of a change of control, we would be required to redeem all outstanding shares of our redeemable preferred stock for an aggregate of $29.9 million, at the price of $9.00 per share. Further, our amended and restated certificate of incorporation permits us to issue additional shares of preferred stock. The terms of our redeemable preferred stock or any new preferred stock we may issue could have the effect of delaying, deterring or preventing a change in control.
Our ability to use our net operating loss carryforwards and certain other tax attributes may be limited.
Under Sections 382 and 383 of the Internal Revenue Code of 1986, as amended, if a corporation undergoes an “ownership change,” generally defined as a cumulative change in its equity ownership by “5-percent shareholders” of greater than 50 percentage points (by value) over a three-year period, the corporation’s ability to use its pre-change net operating loss carryforwards (“NOLs”), and certain other pre-change tax attributes (such as research tax credits) to offset its post-change taxable income and taxes may be limited. We have completed multiple rounds of financing and entered into transactions which may subject us to the Section 382 limitations. We may also experience ownership changes in the future. As a result, our ability to use our NOLs and research and development credits to offset our U.S. federal taxable income and taxes may be subject to limitations, which could potentially result in increased future tax liability to us. In addition, similar rules may also apply at the state level, and there may be periods during which the use of NOLs is suspended or limited, which could accelerate or permanently increase state taxes owed.
We could be subject to changes in our tax rates, new tax legislation or additional tax liabilities.
We are subject to taxes in the United StatesU.S. and foreign jurisdictions. Significant judgment is required to determine and estimate our worldwide tax liabilities. Due to economic and political conditions, tax rates in various jurisdictions may be subject to significant change. Our effective income tax rates have been, and could in the future be, adversely affected by changes in tax laws or interpretations of those tax laws; by stock-based compensation and other non-deductible expenses; by changes in the mix of earnings in countries with differing statutory tax rates; or by changes in the valuation of our deferred tax assets and liabilities.
Our tax returns and other tax matters also are subject to examination by the U.S. Internal Revenue Service and other tax authorities and governmental bodies. We regularly assess the likelihood of an adverse outcome resulting from these examinations to determine the adequacy of our provision for taxes. We cannot guarantee the outcome of these examinations. If our effective tax rates were to increase, particularly in the United States,U.S., or if the ultimate determination of our taxes owed is for an amount in excess of amounts previously accrued, our financial condition, operating results and cash flows could be adversely affected.
None.
Item 1C. Cybersecurity
Cybersecurity Risk Management and Strategy
We recognize the need to maintain the security and confidentiality of personal information, protected health information, and other confidential data that we collect and use in connection with our business, and the importance of assessing, identifying, and managing various cybersecurity risks that may impact our business. We have developed and implemented a cybersecurity risk management program intended to protect the confidentiality, integrity, and availability of our critical systems and information. Our cybersecurity risk management program includes a cybersecurity incident response plan.
We design and assess our program based on various cybersecurity frameworks, most prominently the Health Information Trust Alliance (“HITRUST”) Common Security Framework, and Service Organization Controls (“SOC”) 2, developed by the American Institute of CPAs. In 2023, our cybersecurity systems and services issued a SOC 2 Type 1 report for the design of our security processes. We use this cybersecurity framework and information security controls as a guide to help us identify, assess, and manage cybersecurity risks relevant to our business. Our cybersecurity program includes annual review and assessment by external, independent third parties, who certify and report on these programs.
As part of our enterprise risk management process, we assess the various cybersecurity risks that may impact our business and implement plans and initiatives that are intended to mitigate those risks.
Our information security program includes: (i) risk assessments designed to help identify material cybersecurity risks to our critical systems, information, products, software, and services; (ii) an information security team principally responsible for managing our (1) information security risk assessment processes, (2) security controls, and (3) response to cybersecurity incidents; (iii) risk assessments and security tests, conducted internally and by external security and risk audit providers, as appropriate; (iv) new-hire and annual cybersecurity awareness training of our employees; (v) a cybersecurity incident response plan that includes procedures for responding to cybersecurity incidents; and (vi) third-party risk assessment procedures to review material third-party vendors and applications for information security.
We have not identified any risks from known cybersecurity threats, including as a result of any prior cybersecurity incidents, that have materially affected or are reasonably likely to materially affect us, including our operations, business strategy, results of operations, or financial condition.
None.
39
Table of Contents
Cybersecurity Governance Our Board considers cybersecurity risk as part of its risk oversight function and has delegated to the Audit Committee oversight over our information security and technology risks, including our information security, cybersecurity and related risk management programs. The Audit Committee oversees management’s implementation of our information security program and receives periodic reports from management on our material cybersecurity risks. Additionally, management updates the Audit Committee, as necessary, regarding material cybersecurity incidents. The full Board receives quarterly updates from management on our information security program. Our management team, including our IT management team, is responsible for assessing and managing our material risks from cybersecurity threats. The team has primary responsibility for our overall cybersecurity risk management program and supervises both our internal cybersecurity personnel and our retained external cybersecurity consultants. To support data security, we have established an integrated risk management framework with practices that are derived from industry standards, including ISO 27001, HITRUST Common Security Framework (CSF) 11.2 certification, the NIST Cybersecurity Framework, and data privacy regulations, including HIPAA and the General Data Protection Regulation. The data security controls from these standards and regulations are evaluated for our risk management framework based on the needs of our business and our clients, the nature of our industry, and applicable regulations. Our management team oversees efforts to prevent, detect, mitigate, and remediate cybersecurity risks and incidents through various means, which may include briefings from internal security personnel, threat intelligence and other information obtained from governmental, public, or private sources, including external consultants engaged by us, and alerts and reports produced by security tools deployed in the information technology environment. Item 2. Properties Item 2.
| Properties
|
Our corporate office is located in Carlsbad, California. The table below provides selected information regarding the leased principal properties used in our operations.
| | | | | | |
Location |
| Use |
| Approximate
Square
Footage
|
|
Carlsbad, California |
| Corporate headquarters |
|
| 121,541 |
|
Memphis, Tennessee |
| Distribution facility |
|
| 75,643 |
|
Paris, France | | Office facilities
| | | 15,156
| |
Item 3.
| Legal Proceedings
| Office facilities |
|
| 15,156 |
|
We are and may become involved in various
Item 3. Legal Proceedings
For a description of our material legal proceedings, arising from our business activities. While the Company has no material accruals for pending litigation or claims for which accrual amounts are not disclosed in the Company’s consolidated financial statements, litigation is inherently unpredictable, and depending on the nature and timing of a proceeding, an unfavorable resolution could materially affect our future consolidated results of operations, cash flows or financial position in a particular period. We assess contingencies to determine the degree of probability and range of possible loss for potential accrual or disclosure in our consolidated financial statements. An estimated loss contingency is accrued in our consolidated financial statements if it is probable that a liability has been incurred and the amount of the loss can be reasonably estimated. Because litigation is inherently unpredictable and unfavorable resolutions could occur, assessing contingencies is highly subjective and requires judgments about future events. When evaluating contingencies, we may be unable to provide a meaningful estimate due to a number of factors, including the procedural status of the matter in question, the presence of complex or novel legal theories, and/or the ongoing discovery and development of information important to the matters. In addition, damage amounts claimed in litigation against us may be unsupported, exaggerated, or unrelated to reasonably possible outcomes, and as such are not meaningful indicators of our potential liability.
Referrefer to Note 7 of our Notes to Consolidated Financial Statements included elsewhere in this Annual Report on Form 10-K, which is incorporated herein by reference.for further information regarding the
NuVasive, Inc. Item 4. Mine Safety Disclosureslitigation.
Item 4.
| Mine Safety Disclosures
|
Not applicable.
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities Item 5.
| Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
|
Market Information
Our common stock is traded on The NASDAQ Global Select Market under the symbol “ATEC.”
Stockholders
As of February 24, 2022,19, 2024, there were approximately 304381 holders of record of an aggregate 99,786,612137,979,126 outstanding shares of our common stock.
Dividend Policy
We have never declared or paid cash dividends on our capital stock. We currently intend to retain all available funds and any future earnings for use in the operation and expansion of our business and do not anticipate paying any cash dividends in the foreseeable future.
Unregistered Sales of Equity Securities and Use of Proceeds
During the three months ended December 31, 2023, the Company issued unregistered equity securities as described below:
On October 2, 2023 and November 14, 2023, the Company issued 625 and 1,250 restricted shares of the Company’s common stock with a grant date fair values of $12.58 and $9.94, respectively, based on the market price of common stock on the respective grant dates, to an independent sales agent for distribution and related services rendered to the Company.
On October 2, 2023, the Company issued 795 restricted shares of the Company’s common stock with a grant date fair values of $12.58 based on the market price of common stock on the grant date, to an independent sales agent for distribution and related services rendered to the Company.
The issuances of the foregoing securities were made in reliance on the exemption from registration provided by Section 4(a)(2) of the Securities Act of 1933, as amended, as there was no general solicitation and the transactions did not involve a public offering.
Purchases of Equity Securities
Under the terms of our 2016 Equity Incentive Plan and our Amended and Restated 2005 Employee, Director and Consultant Stock Plan, as amended, which we refer to collectively as the Stock Plans, and prior to the expiration of the Stock Plans in May 2026, we are permitted to award shares of restricted stock to our employees, directors, and consultants. These shares of restricted stock are subject to a lapsing right of repurchase by us. We may exercise this right of repurchase in the event that a restricted stock recipient’s employment, directorship or consulting relationship with us terminates prior to the end of the vesting period. If we exercise this right, we are required to repay the purchase price paid by or on behalf of the recipient for the repurchased restricted shares. Repurchased shares are returned to the Stock Plans and are available for future awards under the terms of the Stock Plans.
On August 3, 2021, our BoardThere were no repurchases of Directors authorized the repurchase of an aggregate of up to $25.0 million of shares of our common stock. On August 10, 2021, in connection with the issuance of our 2026 Notes, we repurchased 1,806,358 shares of our common stock for approximately $25.0 million.
during the year ended December 31, 2023.
41
Stock Performance Graph
The following graph compares the cumulative total stockholder return data on our common stock with the cumulative return of two indices: (i) The Nasdaq Stock Market Composite Index, and (ii) The Nasdaq Medical Equipment Index over the five-year period ending December 31, 2023. The graph assumes that $100 was invested on December 31, 2018 in our common stock and in each of the comparative indices, and the reinvestment of any dividends. The stock price performance on the following graph is not necessarily indicative of future stock price performance.
The following graph and related information shall not be deemed "soliciting material" or deemed to be "filed" with the SEC, nor shall such information be incorporated by reference into any future filing, except to the extent that we specifically incorporate it by reference into such filing.
COMPARISON OF 5 YEAR CUMULATIVE TOTAL RETURN*
AMONG ALPHATEC HOLDINGS, INC.,
THE NASDAQ COMPOSITE INDEX
AND THE NASDAQ MEDICAL EQUIPMENT INDEX

*$100 invested on December 31, 2018 in stock or index, including reinvestment of dividends.
Item 6. Reserved
37
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations Item 7.
| Management’s Discussion and Analysis of Financial Condition and Results of Operations
|
The following discussion of our financial condition and results of operations should be read in conjunction with the financial statemen
tsstatements and the notes to those statements appearing elsewhere in this Annual Report on Form 10-K. A discussion regarding our financial condition and results of operations for 2023 compared to 2022 is presented under “Results of Operations” further below in this Item 7. For discussion regarding our financial condition and the results of operations for 2022 compared to 2021, refer to Part II, Item 7 Management’s Discussion and Analysis of Financial Condition and Results of Operations in our Annual Report on Form 10-K for the year ended December 31, 2022.
Some of the information contained in this discussion and analysis or set forth elsewhere in this report include the identification of certain trends and other statements that may predict or anticipate future business or financial results that are subject to important factors that could cause our actual results to differ materially from those indicated. See “Item 1A Risk Factors” included elsewhere in this Annual Report on Form 10-K.
Overview
We are a medical technology company, headquartered in Carlsbad, California, focused on the design, development, and advancement of technology for better surgical treatment of spinalspine disorders. We are dedicatedBy applying our unique, 100% spine focus and deep, collective industry know-how, we aim to revolutionizingrevolutionize the approach to spine surgery through clinical distinction. We are focused on developing newThe sophisticated approaches that we create from the ground up integrate seamlessly with our expanding Alpha InformatiX™InformatiX™ product platform to betterobjectively inform surgery and to achieve the goalgoals of spine surgery more safelypredictably and more reproducibly. We have a broadcomprehensive product portfolio designed to address the spine’s various pathologies. Ourpathologies, and are perpetually innovating to accomplish our ultimate vision, which is to be the standard bearer in spine.
We intendThe application of our team’s deep spine know-how, coupled with a willingness to driveinvest holistically in each of the technologies integrated into all of ATEC’s procedural approaches continues to increasingly compel surgeons and sales talent to partner with us. That adoption-driven validation has been the source of industry-leading market share expansion, which has delivered an approximately 40% revenue compound annual growth by capitalizing onrate since our collective spine experience and investingtransformation commenced in the research and development to continually differentiate our solutions and improve spine surgery. We believe our future success will be fueled by introducing market-shifting innovation to the spine market, and that we are well-positioned to capitalize on current spine market dynamics.2018.
We market and sell our products through a network of independent distributorssales agents and direct sales representatives. An objective of our leadership team is toTo deliver increasingly consistent, predictable growth. To accomplish this,growth, we have partnered more closely with new and existing distributors to create a more dedicated and loyal sales channel for the future. We have added, and intend to continue to add, new high-qualityclinically astute and exclusive and dedicated distributors to expand future growth. We believe this will allow ussales team members to reach an untapped market of surgeons, hospitals, and national accounts across the U.S., as well asand better penetrate existing accounts and territories.
We have continued to make progress in the transition of our sales channel since late 2017, driving the percent of sales contributed by our strategic independent distributors from approximately 92% for the year ended December 31, 2020 to 97% for the year ended December 31, 2021. Going forward, we intend to continue to relentlessly drive toward a fully exclusive network of independent and direct sales agents. Consolidation in the industry has facilitated this process, as large, seasoned agents seek opportunities to partner with spine-focused companies that have broad, growing product portfolios.
Recent Developments
0.75% Senior Convertible Notes due 2026
In August 2021, we issued $316.3 million principal amount of unsecured senior convertible notes with a stated interest rate of 0.75%, (the “2026 Notes”). Interest on the 2026 Notes is payable semi-annually in arrears on February 1 and August 1 of each year, beginning on February 1, 2022. The 2026 Notes are convertible into shares of our common stock based upon an initial conversion rate of 54.5316 shares of our common stock per $1,000 principal amount of 2026 Notes (equivalent to an initial conversion price of approximately $18.34 per share). The initial conversion price of the 2026 Notes represents a premium of approximately 100% over the closing price of our common stock on August 5, 2021, the date the 2026 Notes offering was priced. The net proceeds from the sale of the 2026 Notes were approximately $306.2 million after deducting the offering expenses. The 2026 Notes will mature on August 1, 2026, unless earlier converted, redeemed, or repurchased.
42
Table of Contents
We used $39.9 millionPublic Offering
On October 27, 2023, we completed an underwritten public offering (the “Public Offering”) of the net proceeds from the 2026 Notes offering to enter into separate capped call instruments with certain financial institutions. The Capped Call Transactions are generally expected to reduce potential dilution to our common stock beyond the conversion prices up to the cap prices on any conversion of the 2026 Notes and/or offset any payments we make in excess of the principal amount upon conversion.
In addition, we repurchased 1,806,358 shares of our common stock in privately negotiated transactions for approximately $25.0 million concurrently with the issuance of the 2026 Notes. We also used approximately $53.4 million of the net proceeds to repay all obligations under our secured term loan with Squadron Medical Finance Solutions, LLC (the “Term Loan”) and our inventory finance agreement with an inventory supplier (the “Inventory Financing Agreement”). We intend to use the remainder of the net proceeds from the 2026 Notes for general corporate purposes.
Acquisition of EOS
On May 13, 2021, we acquired a controlling interest in EOS imaging S.A. (“EOS”), pursuant to the Tender Offer Agreement (the “Tender Offer Agreement”) we entered into on December 16, 2020, and in June 2021, we purchased the remaining issued and outstanding ordinary shares for a 100% interest in EOS. EOS, which now operates as our wholly owned subsidiary, is a global medical device company that designs, develops and markets innovative, low dose 2D/3D full body scans for biplanar weight-bearing imaging, rapid 3D modeling of EOS patient X-ray images, web-based patient-specific surgical planning, and integration of surgical plan into the operating room. We plan to integrate this technology into our Alpha InformatixTM product platform to better inform and better achieve spinal alignment objectives in surgery.
In connection with the Tender Offer Agreement, we entered into a Securities Purchase Agreement with certain purchasers, providing for the sale of 12,421,24214,300,000 shares of our common stock at a purchase price of $11.11$10.50 per share for aggregate gross proceedsshare. In connection with the Public Offering, we granted the underwriters a 30-day option (the "Green-Shoe") to purchase additional shares of $138.0 million.common stock in the offering at the public offering price, less underwriting discounts and commissions. The private placement closed on March 1, 2021, and generated net proceeds offrom the Public Offering and Green-Shoe, were approximately $131.8$145.8 million, net of fees related toincluding the private placement.
COVID-19 Pandemic
Since the beginning of the COVID-19 pandemic, we have seen volatility in sales trends since elective surgeries that use our productsunderwriting discounts and services have been impacted to varying degrees.
We continue to monitor the impact of the COVID-19 pandemic on our businesscommissions and recognize it may continue to negatively impact our business and results of operations during the remainder of 2022 and beyond. Given the present uncertainty surrounding the pandemic, weoffering expenses paid by us. We expect to continueuse the net proceeds from the Public Offering to see volatility through at least the remaining duration of the pandemic as the impact on individual markets and responses to conditions by international, state and local governments continues to vary.
We continue to believe that existing funds, cash generated from operations and existing sources of and access to financing are adequate to satisfy our needs forfund general corporate purposes, including working capital, capital expenditures, acquisitions, or research and debt service requirementsdevelopment.
Asset Purchase Agreement
On April 19, 2023, we entered into an Asset Purchase Agreement with Integrity Implants Inc. and Fusion Robotics, LLC (collectively, the “Sellers”), whereby we acquired certain assets, liabilities, employees, and contracts in connection with the Sellers’ navigation-enabled robotics platform (“Valence”). We paid the Sellers cash consideration of $55.0 million for the purchase of Valence.
Underwritten Offering
On April 19, 2023, we completed a registered securities offering (the “Offering”) of 4,285,715 shares of our common stock at a price of $14.00 per share. The net proceeds from the Offering were approximately $57.5 million, including the underwriting discounts and commissions and offering expenses paid by us. We used a portion of the net proceeds of the offering to fund the purchase of Valence. We expect to use the remaining proceeds to fund the costs related to the post-closing integration and research and development activities related to Valence, as well as to engage in otherfund general corporate purposes, including working capital, capital expenditures, acquisitions, or research and development.
Term Loan
On January 6, 2023, we entered into a $150.0 million term loan credit facility with Braidwell Transaction Holdings, LLC (the “Braidwell Term Loan”). The Braidwell Term Loan provided for an initial term loan of $100.0 million which was funded on the closing date. On September 28, 2023, we drew the additional $50.0 million (the “delayed draw term loan(s)” or the “DDTL”). The Braidwell Term Loan matures on January 6, 2028. Borrowings under the Braidwell Term Loan bear interest at a rate per annum equal to the Term Secured Overnight Financing Rate for such SOFR business initiatives that we planday ("SOFR") subject to strategically pursue.a 3% floor, plus 5.75%.
Revenue and Expense Components
The following is a description of the primary components of our revenue and expenses:
Revenue. We derive our revenue primarily from the sale of spinal surgery implants used in the treatment of spine disorders as well as the sale of medical imaging equipment which is used for surgical planning and post-operative assessment. Spinal implant products include pedicle screws and complementary implants, interbody devices, plates, and tissue-based materials. Medical imaging equipment includes our EOS full-body and weight-bearing x-ray imaging devices, and related services. Our revenue is generated by our direct sales force and independent distributors.sales agents. Our products are shipped and invoiced to hospitals and surgical centers. Currently, most of our business is conducted with customers within markets in which we have experience and with payment terms that are customary to our business. We may defer revenue until the time of collection if circumstances related to payment terms, regional market risk or customer history indicate that collectability is not certain.
43
Table of Contents
Cost of sales. Cost of sales consists primarily of direct product costs, royalties, milestonesservice labor hours, and the amortization of purchased intangibles.parts. Our product costs consist primarily of raw materials, component parts, direct labor, overhead, and raw materials and components.overhead. The product costs of certain of our biologics products include the cost of procuring and processing human tissue. We incur royalties related to the technologies that we license from others and the products that are developed in part by surgeons with whom we collaborate in the product development process. Amortization of purchased intangibles consists of amortization of developed product technology.
Research and development expenses. Research and development expenses consist of costs associated with the design, development, testing, and enhancement of our products. Research and development expenses also include salaries and related employee benefits, research-related overhead expenses, and fees paid to external service providers and development consultants in the form of both cash and equity.
Sales, general and administrative expenses. Sales, general and administrative expenses consist primarily of salaries and related employee benefits, sales commissions and other variable costs, depreciation of our surgical instruments, regulatory affairs, quality assurance costs, professional service fees, travel, medical education, trade show and marketing costs, insurance, and legalinsurance expenses.
Litigation-related expenses. Litigation-related expenses are costs incurred for our ongoing litigation, primarily with NuVasive, Inc.and settled litigation.
Amortization of acquired intangible assets. Amortization of acquired intangible assets consists of intangible assets acquired in business combinations and asset purchases.
Transaction-related expenses. Transaction-related expenses areconsist of certain costs incurred related primarily to the acquisition and integration of EOS.Valence.
Restructuring expenses. Restructuring expenses primarily consist of severance, social plan benefits and related taxestax costs incurred in connection with cost rationalization efforts, as well as costs associated with the opening or closing of our Memphis distribution centeroffice and closing costs related to our old headquarters office in Carlsbad, California.warehouse facilities.
Loss on debt extinguishment, net. Loss on debt extinguishment, net is associated with debt extinguishment, including the write-off of the unamortized debt issuance costs. These are primarily non-cash and are associated with debt paydown transactions which are non-recurring.
Total interest and other expense, net. Total interest and other expense, net includes interest income, interest expense, gains and losses from foreign currency exchanges and other non-operating gains and losses.
Income tax provision. Income tax provision from continuing operations primarily consists of an estimate of federal, state, and foreign income taxes based on enacted state and foreign tax rates, as adjusted for allowable credits, deductions, uncertain tax positions, changes in the valuation of our deferred tax assets and liabilities, and changes in tax laws.
Results of Operations
Total revenue
| | | Year Ended December 31, | | | Change | | | Year Ended December 31, | | | Change | |
(in thousands, except %) | | 2021 | | | 2020 | | | $ | | | % | | | 2023 | | | 2022 | | | $ | | | % | |
Revenue: | | | | | | | | | | | | | | | | | | | | | | | | | |
Revenue from products and services | | $ | 242,258 | | | $ | 141,079 | | | $ | 101,179 | | | | 72 | % | | $ | 482,262 | | | $ | 350,852 | | | $ | 131,410 | | | | 37 | % |
Revenue from international supply agreement | | | 954 | | | | 3,782 | | | | (2,828 | ) | | | (75 | )% | | | — | | | | 15 | | | | (15 | ) | | | (100 | )% |
Total revenue | | $ | 243,212 | | | $ | 144,861 | | | $ | 98,351 | | | | 68 | % | | $ | 482,262 | | | $ | 350,867 | | | $ | 131,395 | | | | 37 | % |
44
Table of Contents
Total revenue was $243.2Revenue from products and services increased by $131.4 million, foror 37%, during the year ended December 31, 2021, compared to $144.9 million for the year ended December 31, 2020, representing an increase of $98.4 million, or 68%. Revenue associated with our acquisition of EOS accounted for approximately 21% of the increase in total revenue for the year ended December 31, 2021, compared to the same period in 2020. Product volume for our business, excluding the EOS acquisition, increased our revenue by approximately 47% for the year ended December 31, 2021,2023, compared to the year ended December 31, 2020,2022. The increase was primarily due to an increase in product volume that was due to the continued expansion of our new product portfolio, increasesincrease in our surgeon user base, and progress related to the transformationcontinued expansion of our product portfolio, and increasing adoption of our technology.
Cost of sales
| | | | | | | | | | | | | | | | |
| | Year Ended December 31, | | | Change | |
(in thousands, except %) | | 2023 | | | 2022 | | | $ | | | % | |
Cost of sales | | $ | 172,059 | | | $ | 117,808 | | | $ | 54,251 | | | | 46 | % |
Cost of sales network.
Revenue from international supply agreement, which is attributed to sales to Globus Medical Ireland, Ltd., a subsidiary of Globus Medical, Inc., and its affiliated entities (collectively “Globus Medical”), under which we supplied to Globus Medical certain of its implants and instruments at agreed-upon prices for a minimum term of three years, decreasedincreased by $2.8$54.3 million, or 75%46%, during the year ended December 31, 2021,2023, compared to the year ended December 31, 2020.2022. The decrease in revenue from the international supplyincrease was primarily due to an increase in product volume and an increase in stock-based compensation. We have entered into Development Service Agreements for the expirationdevelopment of a wide variety of potential products and terminationintellectual property. Under these agreements, future payments for product and/or intellectual property rights may be paid in either cash or restricted shares of our common stock at the election of the international supply agreement with Globus Medicaldeveloper, depending on August 31, 2021.
Costthe terms of sales
| | Year Ended December 31, | | | Change | |
(in thousands, except %) | | 2021 | | | 2020 | | | $ | | | % | |
Cost of sales | | $ | 85,450 | | | $ | 42,360 | | | $ | 43,090 | | | | 102 | % |
Total costthe agreement. Certain of sales, excluding EOS, forthese agreements have been amended to remove the cash option and require settlement in restricted shares of our common stock. During the year ended December 31, 2021 increased by $18.0 million, or 43%, primarily due to product volume. Expense related to2023, the purchase accounting fair value mark-upvesting conditions of inventory as partcertain of the EOS acquisition accounted for approximately 15% of the totalthese amended awards were deemed probable, resulting in an increase in stock-based compensation for the year ended December 31, 2021. Costyear.
Operating expenses
| | | | | | | | | | | | | | | | |
| | Year Ended December 31, | | | Change | |
(in thousands, except %) | | 2023 | | | 2022 | | | $ | | | % | |
Operating expenses: | | | | | | | | | | | | |
Research and development | | $ | 70,115 | | | $ | 44,033 | | | $ | 26,082 | | | | 59 | % |
Sales, general and administrative | | | 374,080 | | | | 300,013 | | | | 74,067 | | | | 25 | % |
Litigation-related expenses | | | 22,287 | | | | 23,943 | | | | (1,656 | ) | | | (7 | )% |
Amortization of acquired intangible assets | | | 14,284 | | | | 10,115 | | | | 4,169 | | | | 41 | % |
Transaction-related expenses | | | 2,113 | | | | 120 | | | | 1,993 | | | | 1,661 | % |
Restructuring expenses | | | 719 | | | | 1,810 | | | | (1,091 | ) | | | (60 | )% |
Total operating expenses | | $ | 483,598 | | | $ | 380,034 | | | $ | 103,564 | | | | 27 | % |
Operating expenses
| | Year Ended December 31, | | | Change | |
(in thousands, except %) | | 2021 | | | 2020 | | | $ | | | % | |
Operating expenses: | | | | | | | | | | | | | | | | |
Research and development | | $ | 32,015 | | | $ | 18,745 | | | $ | 13,270 | | | | 71 | % |
Sales, general and administrative | | | 229,271 | | | | 129,156 | | | | 100,115 | | | | 78 | % |
Litigation-related expenses | | | 11,123 | | | | 8,552 | | | | 2,571 | | | | 30 | % |
Amortization of acquired intangible assets | | | 5,348 | | | | 688 | | | | 4,660 | | | | 677 | % |
Transaction-related expenses | | | 6,365 | | | | 4,223 | | | | 2,142 | | | | 51 | % |
Restructuring expenses | | | 1,697 | | | | — | | | | 1,697 | | | | 100 | % |
Total operating expenses | | $ | 285,819 | | | $ | 161,364 | | | $ | 124,455 | | | | 77 | % |
Research and development expenses. Research and development expenses increased by $13.3$26.1 million, or 71%59%, primarily related to the hiring of new personnel and new project costs. Research and development costs associated with EOS accounted for approximately 17% of the total increase forduring the year ended December 31, 2021, as2023, compared to the year ended December 31, 2020.
45
Table2022. The increase was primarily due to an increase in personnel to support the expansion of Contentsour new product portfolio and an increase in stock-based compensation associated with Development Service Agreements described above.
Sales, general and administrative expenses. Sales, general and administrative expenses excluding EOS, increased by $89.3$74.1 million, or 69%25%, during the year ended December 31, 2021, as2023, compared to the year ended December 30, 2020.31, 2022. The increase was primarily due to higher compensation-related costs and variable selling expenses associated with the increase in revenue, and our continued investment in building our strategic distributionsales channel. Additionally, we have increased our investment in our sales and marketing functions by increasing headcount to support the growth of our business. Sales, general and administrative
Litigation-related expenses. Litigation-related expenses associated with EOS accounted for approximately 9% of the total increase fordecreased by $1.7 million, or 7%, during the year ended December 31, 2021, as2023, compared to the year ended December 31, 2020.
Litigation-related expenses. Litigation-related expenses increased by $2.6 million, or 30%, and2022. The decrease was primarily related to a decrease in legal fees associated with our previously settled litigation matters. Refer to Note 7 of our Notes to Consolidated Financial Statementsongoingincluded elsewhere in this Annual Report on Form 10-K for further information regarding litigation with NuVasive, Inc. and fluctuations related to the timing of related legal activities. matters.
Amortization of acquired intangible assets. AmortizationThe increase of amortization of acquired intangible assets is primarily includesdue to the amortization of intangible assets acquired in the EOSValence acquisition.
Transaction-related expenses. The increase in transaction-related expenses wasfor the year ended December 31, 2023 is primarily due to third-party advisory and legal fees related to our acquisitionthe closing of EOS, which closed on May 13, 2021, as well as costs to support our ongoing integration activities.the Valence acquisition.
Restructuring expenses. The increasedecrease in restructuring costs for the year ended December 31, 2021 was primarily2023 is due to severance, social plan benefits and related taxes in connection with cost rationalization efforts as well as costs associated with the opening of our Memphis distribution center and closing costs related to our old headquarters office.
Total interest and other expense, net
| | Year Ended December 31, | | | Change | |
(in thousands, except %) | | 2021 | | | 2020 | | | $ | | | % | |
Interest and other expense, net: | | | | | | | | | | | | | | | | |
Interest expense, net | | $ | (7,108 | ) | | $ | (12,374 | ) | | $ | 5,266 | | | | (43 | )% |
Loss on debt extinguishment, net | | | (7,434 | ) | | | (7,612 | ) | | | 178 | | | | (2 | )% |
Other expense, net | | | (1,563 | ) | | | — | | | | (1,563 | ) | | | 100 | % |
Total interest and other expense, net | | $ | (16,105 | ) | | $ | (19,986 | ) | | $ | 3,881 | | | | (19 | )% |
The decreasethat were completed during the year ended December 31, 2021 as2022.
Total interest and other expense, net
| | | | | | | | | | | | | | | | |
| | Year Ended December 31, | | | Change | |
(in thousands, except %) | | 2023 | | | 2022 | | | $ | | | % | |
Interest and other income, net: | | | | | | | | | | | | |
Interest expense, net | | $ | (16,641 | ) | | $ | (5,505 | ) | | $ | (11,136 | ) | | | 202 | % |
Other income, net | | | 3,121 | | | | 471 | | | | 2,650 | | | | 563 | % |
Total interest and other income, net | | $ | (13,520 | ) | | $ | (5,034 | ) | | $ | (8,486 | ) | | | 169 | % |
The increase in interest expense, net, during the year ended December 31, 2023, compared to the year ended December 30, 20202022, was primarily due to lower interest expense relatedon our revolving credit facility and Braidwell Term Loan which were executed in September 2022 and January 2023, respectively. The change in other income, net, compared to the early payoff ofsame in 2022, was primarily due to an employee retention credit received during the Term Loan, offset by foreign currency losses related to forward contract settlements.year ended December 31, 2023.
Income tax provision
| | | Year Ended December 31, | | | Change | | | Year Ended December 31, | | | Change | |
(in thousands, except %) | | 2021 | | | 2020 | | | $ | | | % | | | 2023 | | | 2022 | | | $ | | | % | |
Income tax provision | | $ | 164 | | | $ | 145 | | | $ | 19 | | | | 13 | % | | $ | (277 | ) | | $ | (716 | ) | | $ | 439 | | | | (61 | )% |
Income tax provision for the year ended December 31, 20212023 was negligible and remained consistent compared to the year ended December 31, 2020.2022.
Liquidity and Capital Resources
Our principal sources of liquidity are our existing cash and cash equivalents, our Revolving Credit Facility, and cash from operations. Our liquidity and capital structure are evaluated regularly within the context of our annual operating and strategic planning process. We consider the liquidity necessary to fund our operations, which includeincludes working capital needs, investments in research and development, investments in inventory and instrument sets to support our customers, as well as other operating costs. Our future capital requirements will depend on many factors including our rate of revenue growth, the timing and extent of spending to support development efforts, the expansion of sales, marketing and administrative activities, and the timing of introductions of new products and enhancements to existing products. products, and the international expansions of our business.
As current borrowing sources become due, we may be required to access the capital markets for additional funding. If we are required to access the debt market,markets, we expect to be able to secure reasonable borrowing rates. As part of our liquidity strategy, we will continue to monitor our current level of spending and cash use as well as our ability to secure additional credit facilities, term loans, or other similar arrangements in light of our spending levels and general financial market conditions.
A substantial portion of our operations are in the U.S., and most of our net sales have been made in the U.S. Accordingly, we do not have material exposures to foreign currency rate fluctuations from operations. However, as our business in markets outside of the U.S. continues to increase, we will be exposed to foreign currency exchange risk related to our foreign operations.
We do not have any material financial exposure to one customer or one country that would significantly hinder our liquidity. We are and may become involved in various legal proceedings arising from our business activities. While we have no material accruals for pending litigation or claims for which accrual amounts are not disclosed in our consolidated financial statements, litigation is inherently unpredictable, and depending on the nature and timing of a proceeding, an unfavorable resolution could materially affect our future consolidated results of operations, cash flows or financial position in a particular period. We assess contingencies to determine the degree of probability and range of possible loss for potential accrual or disclosure in our consolidated financial statements. An estimated loss contingency is accrued in our consolidated financial statements if it is probable that a liability has been incurred and the amount of the loss can be reasonably estimated. Assessing contingencies is highly subjective and requires judgments about future events because litigation is inherently unpredictable, and unfavorable resolutions could occur. When evaluating contingencies, we may be unable to provide a meaningful estimate due to a number of factors, including the procedural status of the matter in question, the presence of complex or novel legal theories, and/or the ongoing discovery and development of information important to the matters. In addition, damage amounts claimed in litigation against us may be unsupported, exaggerated, or unrelated to reasonably possible outcomes, and as such are not meaningful indicators of our potential liability. We have disclosed all material accruals for pending litigation or investigations in Note 7, Commitments and Contingencies, in the Notes to Consolidated Financial Statements included in this Annual Report.
Cash and cash equivalents were $187.2$221.0 million and $107.8$84.7 million at December 31, 20212023 and December 31, 2020,2022, respectively. We have available borrowings under the Revolving Credit Facility discussed above. We believe that our existing funds, cash generated from our operations and our existing sources of and access to financing are adequate to satisfy our needs for working capital, capital expenditure, and debt service requirements and other business initiatives we plan to strategically pursue.
Summary of Cash Flows
The following is a summary of cash (used in) provided by (used in) operating, investing, and financing activities, the effect of exchange rate changes on cash and cash equivalents, and the net change in cash and cash equivalents:
| | Year Ended December 31, | |
| | 2021 | | | 2020 | | Year Ended December 31, | |
Cash provided by (used in): | | | | | | | | |
| | | 2023 | | | 2022 | | | 2021 | |
Cash (used in) provided by: | | | | | | | | | | |
Operating activities | | $ | (73,432 | ) | | $ | (46,412 | ) | | $ | (78,485 | ) | | $ | (75,134 | ) | | $ | (73,317 | ) |
Investing activities | | | (157,762 | ) | | | (23,859 | ) | | | (141,975 | ) | | | (58,280 | ) | | | (157,762 | ) |
Financing activities | | | 311,966 | | | | 130,829 | | | | 356,919 | | | | 31,228 | | | | 311,966 | |
Effect of exchange rate changes on cash | | | (1,289 | ) | | | 94 | | | | (185 | ) | | | (366 | ) | | | (1,404 | ) |
Net increase in cash and cash equivalents | | $ | 79,483 | | | $ | 60,652 | |
Net change in cash and cash equivalents | | | $ | 136,274 | | | $ | (102,552 | ) | | $ | 79,483 | |
Operating Activities
We used net cash of $73.4$78.5 million from operating activities for the year ended December 31, 2021.2023. The cash used in operating activities primarily related to costs associated with the continued expansion of our business and inventory purchases, and the general timing of cash receipts, offset by the timing of cash distributions.payments and receipts.
Investing Activities
We used cash of $157.7$142.0 million in investing activities for the year ended December 31, 2021,2023, which is primarily related to ourthe acquisition of EOS, including the purchase of its convertible bonds (“OCEANEs”), theValence and purchase of surgical instruments to support our business growth and the commercial launch of new products capital expenditures associated with product development machinery and tools and the build-outgrowth of our distribution facility in Memphis, and other investments.business.
Financing Activities
Financing activities provided net cash of $312.0$356.9 million for the year ended December 31, 2021,2023, which is primarily related to thenet proceeds from the issuance of our 2026 NotesBraidwell Term Loan, the Revolving Credit Facility, and the closing of the Private Placement on March 1, 2021, partiallyequity offerings described above, offset by cash paid for the full repaymentpayment of our obligations under the Term Loan and Inventory Financing Agreement, purchase of the Capped Calls, and repurchase of our common stock.
47
Table of Contentsoutstanding OCEANEs, defined below.
Debt and Commitments
As of December 31, 2021,2023, we had $150.0 million outstanding under the Braidwell Term Loan. The outstanding loans under the Braidwell Term Loan bear interest at the sum of SOFR plus 5.75% per annum. The Braidwell Term Loan matures on January 6, 2028.
As of December 31, 2023, we had $49.7 million outstanding under the Revolving Credit Facility. The outstanding loans bear interest at the sum of SOFR plus 3.5% per annum. The Revolving Credit Facility matures on the earlier of September 29, 2027, or 90 days prior to the final maturity date of any of our outstanding 0.75% Convertible Senior Notes due 2026 (the "2026 Notes").
As of December 31, 2023, we had $316.3 million outstanding under the 2026 Notes. The 2026 Notes accrue interest at a rate of 0.75%, payable semi-annually in arrears on February 1 and August 1 of each year. Prior to maturity in August 2026, the holders of the 2026 Notes may, under certain circumstances, choose to convert their notes into shares of our common stock. Based on the terms, we have the option to pay or deliver cash, shares of our common stock, or a combination thereof, when a conversion notice is received.
We assumed the OCEANE convertible bonds issued by EOS in connection with our acquisitionAs of EOS. The OCEANEs bear interest at 6% per year, payable semi-annually in arrears on MayDecember 31, and November 30 of each year. Unless either earlier converted or repurchased, the outstanding OCEANEs of $14.12023, we had $4.5 million (€12.5 million) will mature on May 31, 2023.
We also assumed $5.3 million (€4.8 million) in other debts with the acquisition of EOS that are due in monthly and quarterly installments beginning in 2023 through maturity in 2027.
As of December 31, 2021, weWe have made $49.0 million in Orthotec settlement payments and there remains an outstanding balance of $8.5 million in Orthotec settlement payments to be paid by us in quarterly installments through October 2023.
With the acquisition of EOS, we assumed its inventory purchase commitment agreement with a third-party supplier. The Company issupplier, where we are obligated to certain minimum purchase commitment requirements through December 2025. As of December 31, 2021,2023, the remaining minimum purchase commitment under the agreement was $33.5$13.9 million.
Contractual obligations and commercial commitments
Total contractual obligations and commercial commitments as of December 31, 20212023 are summarized in the following table (in thousands):
| | | | | | | | | | | | |
| | Payments Due by Period | |
| | Total | | | 1 Year or Less | | | More than 1 Year | |
2026 Notes | | $ | 316,250 | | | $ | — | | | $ | 316,250 | |
Braidwell Term Loan, including final payment fee of $4,875 | | | 154,875 | | | | — | | | | 154,875 | |
Interest expense(1) | | | 76,577 | | | | 19,760 | | | | 56,817 | |
Revolving Credit Facility | | | 49,720 | | | | — | | | | 49,720 | |
Facility lease obligations (2) | | | 34,209 | | | | 5,505 | | | | 28,704 | |
Purchase commitments (3) | | | 13,887 | | | | 4,529 | | | | 9,358 | |
Other (4) | | | 4,811 | | | | 1,584 | | | | 3,227 | |
Development services plans | | | 1,248 | | | | — | | | | 1,248 | |
Total | | $ | 651,577 | | | $ | 31,378 | | | $ | 620,199 | |
| | Payments Due by Period | |
| | Total | | | 1 Year or Less | | | More than 1 Year | |
Senior Convertible Notes | | $ | 316,250 | | | $ | — | | | $ | 316,250 | |
Facility lease obligations (1) | | | 40,764 | | | | 4,405 | | | | 36,359 | |
Purchase commitments (2) | | | 33,542 | | | | 4,485 | | | | 29,057 | |
OCEANEs | | | 14,113 | | | | — | | | | 14,113 | |
Interest expense | | | 14,991 | | | | 4,265 | | | | 10,726 | |
Litigation settlement obligations, gross (3) | | | 8,465 | | | | 4,400 | | | | 4,065 | |
Other (4) | | | 5,341 | | | | — | | | | 5,341 | |
Development services plans | | | 3,854 | | | | — | | | | 3,854 | |
License agreement milestones (5) | | | 2,740 | | | | 690 | | | | 2,050 | |
Operating lease obligations | | | 2,503 | | | | 670 | | | | 1,833 | |
Guaranteed minimum royalty obligations | | | 2,450 | | | | 320 | | | | 2,130 | |
Total | | $ | 445,013 | | | $ | 19,235 | | | $ | 425,778 | |
Represents interest expense from our debt that we expect to pay in the future.
(1)
| (2) Includes our new headquarters building lease that commenced in February 2021. |
(2)
| Includes inventory purchase commitments of $33.5 million assumed with our acquisition of EOS.
|
(3)
| Represents gross payments due to Orthotec, LLC pursuant to a Settlement and Release Agreement, dated as of August 13, 2014, by and among the Company and its direct subsidiaries, including Alphatec Spine, Inc., Alphatec Holdings International C.V., Scient'x S.A.S. and Surgiview S.A.S.; HealthpointCapital, LLC, HealthpointCapital Partners, L.P., HealthpointCapital Partners II, L.P., John H. Foster and Mortimer Berkowitz III; and Orthotec, LLC and Patrick Bertranou.
|
(4)
| Commitments representing cash repayments of government sponsored COVID relief initiatives at EOS.
|
(5)
| Commitments representing payments in cash that are subject to attaining certain sales milestones which we believe are reasonably likely to be achieved.
|
48
Table of Contents
Real Property Leases
On April 9, 2021, we entered into 7-year operating lease agreement for a new distribution center which consists of approximately 75,643 square feet of office and warehouse space in Memphis, Tennessee. The term of the lease commenced on May 1, 2021 and will terminate on May 1, 2028, subject to two thirty-six-month options to renew. Base rent under the building lease will be commensurate with our proportionate share of occupancy of the building as we expand and will increase annually by 3.0% throughout the remainder of the lease.
With the acquisition of EOS, we assumed its right-of-use assets and lease liabilities in the amount of $4.3 million. EOS occupies its main office in Paris, France. The EOS office in Paris, France is an operating lease that commenced in 2019 and will terminate in September 2028.
On December 4, 2019, we entered into a new lease agreement, or New Building Lease, for our headquarters location which consistsFebruary 2021.
(3)Includes inventory purchase commitments of 121,541 square feet$13.9 million.
(4)Represents cash repayments of office, engineering, and research and development space in Carlsbad, California. The term of the New Building Lease commenced on February 1, 2021 and terminates January 31, 2031, subject to two sixty-month options to renew. Base rent under the New Building Lease for the first twelve months of the term will be government sponsored COVID relief initiatives at EOS.
$0.2 million per month subject to full abatement during months two through ten, and thereafter will increase annually by 3.0% throughout the remainder of the lease.
Off-Balance Sheet Arrangements
As of December 31, 2021,2023, we did not have any off-balance sheet arrangements.
Critical Accounting Policies and Estimates
Our discussion and analysis of our financial condition and results of operations is based upon our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues, expenses and related disclosures. On an on-going basis, we evaluate our estimates and assumptions, including those related to revenue recognition, allowances for accounts receivable, inventories and intangible assets, stock-based compensation and income taxes. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumption conditions.
We believe the following accounting policies to be critical to the judgments and estimates used in the preparation of our consolidated financial statements.
Revenue Recognition
We recognize revenue from product sales in accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) Revenue from Contracts with Customers (“Topic 606”). This standard applies to all contracts with customers, except for contracts that are within the scope of other standards, such as leases, insurance, collaboration arrangements, and financial instruments. Under Topic 606, an entity recognizes revenue when its customer obtains control of promised goods or services, in an amount that reflects the consideration that the entity expects to receive in exchange for those goods or services. To determine revenue recognition for arrangements that an entity determines are within the scope of Topic 606, the entity performs the following five steps: (i) identify the contract(s) with a customer; (ii) identify the performance obligations in the contract; (iii) determine the transaction price; (iv) allocate the transaction price to the performance obligations in the contract; and (v) recognize revenue when (or as) the entity satisfies a performance obligation. We only apply the five-step model to contracts when it is probable that we will collect the consideration we are entitled to in exchange for the goods or services that we transfer to the customer.
49
Table of Contents
Sales are derived primarily from the sale of spinal implant products, imaging equipment, and related services to hospitals and medical centers through direct sales representatives and independent distributor agents, and with the acquisition of EOS, includes imaging equipment and related services.sales agents. Revenue is recognized when obligations under the terms of a contract with customers are satisfied, which occurs with the transfer of control of products to customers, either upon shipment of the product or delivery of the product to the customer depending on the shipping terms, or when the products are used in a surgical procedure (implanted in a patient). Revenue from the sale of imaging equipment is recognized as each distinct performance obligation is fulfilled and control transfers to the customer, beginning with shipment or delivery, depending on the terms. Revenue from other distinct performance obligations, such as maintenance on imaging equipment, and other imaging related services, is recognized in the period the service is performed, and makes up less than 10% of our total revenue. Revenue is measured based on the amount of consideration expected to be received in exchange for the transfer of the goods or services specified in the contract with each customer. In certain cases, we offer the ability for customers to lease our imaging equipment primarily on a non-sales type basis, but such arrangements are immaterial to total revenue in the years presented. We generally do not allow returns of products that have been delivered. Costs incurred by us associated with sales contracts with customers are deferred over the performance obligation period and recognized in the same period as the related revenue, except for contracts that complete within one year or less, in which case the associated costs are expensed as incurred. Payment terms for sales to customers may vary but are commensurate with the general business practices in the country of sale.
To the extent that the transaction price includes variable consideration, such as discounts, rebates, and customer payment penalties, we estimate the amount of variable consideration that should be included in the transaction price utilizing either the expected value method or the most likely amount method depending on the nature of the variable consideration. Variable consideration is included in the transaction price if, in our judgment, it is probable that a significant future reversal of cumulative revenue under the contract will not occur. Estimates of variable consideration and determination of whether to include estimated amounts in the transaction price are based largely on an assessment of our anticipated performance and all information that is reasonably available, including historical, current, and forecasted information.
We record a contract asset when one or more performance obligations have been completed and revenue has been recognized, but the customer's payment is contingent on the satisfaction of additional performance obligations. We record a contract liability, or deferred revenue, when we have an obligation to provide a product or service to the customer and payment is received in advance of our performance. When we sell a product or service with a future performance obligation, revenue is deferred on the unfulfilled performance obligation and recognized over the related performance period. Generally, we do not have observable evidence of the standalone selling price related to our future service obligations; therefore, we estimate the selling price using an expected cost plus a margin approach. The transaction price is allocated using the relative standalone selling price method. The use of alternative estimates could result in a different amount of revenue deferral.
Excess and Obsolete Inventory
Most of our inventory is comprised of finished goods, and we primarily utilize third-party suppliers to produce our products. Specialized implants, fixation products, biologics, and disposablesbiologics are determined by utilizing a standard cost method, which includes capitalized variances, which approximates the weighted average cost. Imaging equipment and related parts are valued at weighted average cost. Inventories are stated at the lower of cost or net realizable value. We review the components of inventory on a periodic basis for excess and obsolescence and adjust inventory to its net realizable value as necessary.
We record a lower of cost or net realizable value inventory reserve (“LCNRV”) for estimated excess and obsolete inventory based upon our expected use of inventory on hand. Our inventory, which consists primarily of specialized implants, fixation products, biologics, and disposablesbiologics is at risk of obsolescence due to the need to maintain substantial levels of inventory. In order to market our products effectively and meet the demands of interoperative product placement, we maintain and provide surgeons and hospitals with a variety of inventory products and sizes. For each surgery, fewer than all components will be consumed. The need to maintain and provide such a variety of inventory causes inventory to be held that is not likely to be used.
50
Table of Contents
Our estimates and assumptions for excess and obsolete inventory are reviewed and updated on a quarterly basis. The estimates and assumptions are determined primarily based on current usage of inventory and the age of inventory quantities on hand. Additionally, we consider recent sales experience to develop assumptions about future demand for our products, while considering market conditions, product life cycles and new product launches. Increases in the LCNRV reserve for excess and obsolete inventory result in a corresponding charge to cost of sales.
For the year ended December 31, 2021 and December 31, 2020, we recorded a write-down for excess and obsolete inventory of $11.1 million and $7.0 million, respectively.
Valuation of Goodwill
Our goodwill represents the excess of the cost over the fair value of net assets acquired from our business combinations. The determination of the value of goodwill and intangible assets arising from business combinations and asset acquisitions requires extensive use of accounting estimates and judgments to allocate the purchase price to the fair value of the net tangible and intangible assets acquired. Goodwill is assessed for impairment using fair value measurement techniques on an annual basis or more frequently if facts and circumstance warrant such a review. Goodwill is considered to be impaired if we determine that the carrying value of the reporting unit exceeds its respective fair value.
Valuation of Intangible Assets
Our intangible assets are comprised primarily of purchased technology, customer relationships, trade name, trademarks, and in-process research and development. We make significant judgments in relation to the valuation of intangible assets resulting from business combinations and asset acquisitions. Intangible assets are generally amortized on a straight-line basis over their estimated useful lives of 2 to 12 years. We base the useful lives and related amortization expense on the period of time we estimate the assets will generate net sales or otherwise be used. We also periodically review the lives assigned to our intangible assets to ensure that our initial estimates do not exceed any revised estimated periods from which we expect to realize cash flows. If a change were to occur in any of the above-mentioned factors or estimates, the likelihood of a material change in our reported results would increase. We evaluate our intangible assets with finite lives for indications of impairment whenever events or changes in circumstances indicate that the carrying value may not be recoverable. Factors that could trigger an impairment review include significant under-performance relative to expected historical or projected future operating results, significant changes in the manner of our use of the acquired assets or the strategy for our overall business or significant negative industry or economic trends. If this evaluation indicates that the value of the intangible asset may be impaired, we make an assessment of the recoverability of the net carrying value of the asset over its remaining useful life. If this assessment indicates that the intangible asset is not recoverable, based on the estimated undiscounted future cash flows of the asset over the remaining amortization period, we reduce the net carrying value of the related intangible asset to fair value and may adjust the remaining amortization period. Significant judgment is required in the forecasts of future operating results that are used in the discounted cash flow valuation models. It is possible that plans may change and estimates used may prove to be inaccurate. If our actual results, or the plans and estimates used in future impairment analyses, are lower than the original estimates used to assess the recoverability of these assets, we could incur additional impairment charges.
In-process research and development ("IPR&D") and software in development have indefinite lives and are not amortized until the related products reach full commercial launch or when the projects are complete and their assets are ready for their intended use. Indefinite-lived intangible assets are considered to be impaired if the products do not reach commercial launch, if the project is not completed or not completed in a timely manner, or if the related products or projects are no longer technologically feasible. Impairment related to IPR&D and software in development is calculated as the excess of the asset's carrying value over its fair value.
Valuation of Stock-Based Compensation
Stock-based compensation expense for equity-classified awards, principally related to restricted stock units or RSUs,("RSUs") and performance restricted stock units or PRSUs,("PRSUs") is measured at the grant date based on the estimated fair value of the award. The fair value of equity instruments that are expected to vest is recognized and amortized over the requisite service period. We have granted awards with up to four year graded or cliff vesting terms. No exercise price or other monetary payment is required for receipt of the shares issued in settlement of the respective award; instead, consideration is furnished in the form of the participant’s service.
51
Table of Contents
The fair value of RSUs including PRSUs with pre-defined performance criteria is based on the stock price on the date of grant whereas the expense for PRSUs with pre-defined performance criteria is adjusted with the probability of achievement of such performance criteria at each periodyear end. The fair value of the PRSUs that are earned based on the achievement of pre-defined market conditions, is estimated on the date of grant using a Monte Carlo valuation model. The key assumptions in applying this model are an expected volatility and a risk-free interest rate.
Stock-based compensation recorded in our consolidated statements of operations is based on awards expected to ultimately vest and has been reduced for estimated forfeitures. Our estimated forfeiture rates may differ from our actual forfeitures. We consider our historical experience of pre-vesting forfeitures on awards by each homogenous group of employees as the basis to arrive at our estimated annual pre-vesting forfeiture rates.
We estimate the fair value of stock options issued under our equity incentive plans and shares issued to employees under our employee stock purchase plan or ESPP,("ESPP"), using a Black-Scholes option-pricing model on the date of grant. The Black-Scholes option-pricing model incorporates various assumptions including expected volatility, expected term and risk-free interest rates. The expected volatility is based on the historical volatility of our common stock over the most recent period commensurate with the estimated expected term of our stock options and ESPP offering period which is derived from historical experience. The risk-free interest rate for periods within the contractual life of the option is based on the U.S. Treasury yield in effect at the time of grant. We have never declared or paid dividends and have no plans to do so in the foreseeable future.
We account for stock option grantsAwards to non-employees are accounted for under the same stock-based compensation provisions as employees, which require that the fair value of these instruments be recognized as an expense overwhen earned. For Development Service Agreements, where the periodfuture payments for product and/or intellectual property rights may be paid in whicheither cash or restricted shares of our common stock at the relatedelection of the developer we estimate the fair value of those awards similar to a stock option, using a Black-Scholes option-pricing model on the date of grant. For Development Service Agreements where the future payments for product and/or intellectual property rights will be paid in restricted shares of our common stock, the fair value is based on the stock price on the date of grant. The stock-based compensation expense is recognized as earned once the award is deemed probable of achieving the pre-defined performance criteria. The stock-based compensation expense is included in cost of sales or research and development expense on the consolidated statements of operations commensurate with the nature of services are rendered.performed.
Recent Accounting PronouncementsPronouncements.
See “Notes to Financial Statements - Note 21 - RecentRecently Adopted and Issued Accounting Pronouncements” included elsewhere in this Annual Report on Form 10-K.
Table of Contents
Item 7A. Quantitative and Qualitative Disclosures About Market Risk Interest Rate Risk We are exposed to interest rate risks related to our cash, cash equivalents and borrowings. We had cash and cash equivalents of 221.0 million as of December 31, 2023, which consist of cash and money market funds. Interest-earning deposit accounts and money market funds carry a degree of interest rate risk; however, historical fluctuations in interest income have not been significant. Loans under the Revolving Credit Facility and the Braidwell Term Loan bear interest at floating rates tied to SOFR. As a result, changes in SOFR can affect our results of operation and cash flows. As of December 31, 2023, the outstanding balance under the Braidwell Term Loan and Revolving Credit Facility was $150.0 million and $49.7 million, respectively. The interest rates for the Braidwell Term Loan and Revolving Credit Facility as of December 31, 2023 were 11.2% and 9.0%, respectively. Item 7A.
| Quantitative and Qualitative Disclosures About Market Risk
|
Foreign Currency Exchange Risk
As our business in markets outside of the United StatesU.S. continues to increase, we may be exposed to foreign currency exchange risks related to our foreign operations. Fluctuations in the rate of exchange between the United StatesU.S. and foreign currencies, primarily the euro, could adversely affect our financial results. We do not have any material financial exposure to one customer or one country that would significantly hinder our liquidity.
Commodity Price Risk
We purchase raw materials that are processed from commodities, such as titanium and stainless steel. These purchases expose us to fluctuations in commodity prices. Given the historical volatility of certain commodity prices, this exposure can impact our product costs. However, because our raw material prices comprise a small portion of our cost of sales, we have not experienced any material impact on our results of operations from changes in commodity prices. A 10% change in commodity prices would not have had a material impact on our results of operations for the year ended December 31, 2021.2023.
Item 8. Financial Statements and Supplementary Data Item 8.
| Financial Statements and Supplementary Data
|
The consolidated financial statements and supplementary data required by this item are set forth at the pages indicated in Item 15.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure None. Item 9A. Controls and Procedures Item 9.
| Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
|
None.
Item 9A.
| Controls and Procedures
|
Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our reports under the Exchange Act is recorded, processed, summarized and reported within the timelines specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure. In designing and evaluating the disclosure controls and procedures, management recognized that any controls and procedures, no matter how well designed and operated, can only provide reasonable assurance of achieving the desired control objectives, and in reaching a reasonable level of assurance, management necessarily was required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
Under the supervision and with the participation of our management, including our Chief Executive Officer and our Chief Financial Officer, we carried out an evaluation of the effectiveness of the Company’s disclosure controls and procedures (as defined in SEC Rules 13a - 15(e) and 15d - 15(e) of the Exchange Act) as of December 31, 2021.2023. Based on such evaluation, our management has concluded as of December 31, 2021,2023, the Company’s disclosure controls and procedures are effective at the reasonable assurance level.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rule 13a-15(f) under the Exchange Act. Internal control over financial reporting refers to the process designed by, or under the supervision of, our Chief Executive Officer and Chief Financial Officer, and effected by our Board of Directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with U.S. generally accepted accounting principles.
53
Table of Contents
Management has used the framework set forth in the report entitled Internal Control - Integrated Framework published by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) to evaluate the effectiveness of the Company’s internal control over financial reporting. Management has concluded that the Company’s internal control over financial reporting was effective as of December 31, 2021,2023, based on those criteria.
Management excluded from its assessment of the Company's internal control over financial reporting as of December 31, 2021, the internal control over financial reporting of EOS which was acquired by the Company on May 13, 2021. This exclusion is consistent with guidance issued by the SEC that an assessment of a recently acquired business may be omitted from the scope of management's report on internal control over financial reporting in the year of acquisition. Total assets and net liabilities of EOS as of December 31, 2021 (excluding goodwill and other intangible assets, which were included in management's assessment of internal control over financial reporting as of December 31, 2021) were approximately $52.1 million and $13.8 million, respectively. EOS represented $30.0 million of our consolidated net revenue for the year ended December 31, 2021. See a discussion of this acquisition in Note 3, Business Combination, of the Notes to the Consolidated Financial Statements contained in Item 8 of this Annual Report on Form 10-K.
Deloitte & Touche LLP, the Company’s independent registered public accounting firm, who audited the consolidated financial statements included in this Annual Report on Form 10-K, has issued an attestation report on the Company’s internal control over financial reporting as of December 31, 2021.2023. This report states that internal control over financial reporting was effective and appears in “Report of Independent Registered Public Accounting Firm” in Part IV, Item 15 of this Annual Report on Form 10-K.
Changes in Internal Control over Financial Reporting
We are involved in ongoing evaluations of internal controls. In anticipation of the filing of this Annual Report on Form 10-K, our Chief Executive Officer and Chief Financial Officer, with the assistance of other members of our management, performed an evaluation of any change in internal control over financial reporting that occurred during our last fiscal quarter that has materially affected, or is reasonably likely to materially affect, our internal controls over financial reporting. We are in the process of implementing a new enterprise resource planning (“ERP”) system that affects many of our financial processes and is expected to improve the efficiency and effectiveness of certain financial and business transaction processes, as well as the underlying systems environment. There has been no change to our internal control over financial reporting during our most recent fiscal quarter that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information
Item 9B.Adoption, Modification or Termination of Trading Arrangements A portion of the compensation of our directors and officers is in the form of equity awards, and, from time to time, directors and officers engage in open-market transactions with respect to the securities they acquire pursuant to such equity awards we have issued. Transactions in our securities by directors and officers are required to be made in accordance with our insider trading policy, which requires that the transactions comply with applicable U.S. federal securities laws that prohibit trading while in possession of material nonpublic information. Rule 10b5-1 under the Exchange Act provides an affirmative defense that enables directors and officers to prearrange transactions in our securities in a manner that avoids concerns about initiating transactions while in possession of material nonpublic information. The following table describes the contracts, instructions or written plans for the purchase or sale of securities adopted by our directors or officers (as defined in Rule 16a-1(f) under the Exchange Act) during the three months ended December 31, 2023, that are intended to satisfy the affirmative defense conditions of Rule 10b5-1(c). No other Rule 10b5-1 trading arrangements or “non-Rule 10b5-1 trading arrangements” (as defined by S-K Item 408(c)) were entered into or terminated by our directors or officers during such period:
| Other Information
|
None.
Item 9C.49
| Disclosure Regarding Foreign Jurisdictions that Prevent Inspections
|
Not applicable.
54
PART III
| | | | | | | |
Item 10.Name
| Directors, Title of Director or Officer
| Action | Date | Total Shares to be Sold |
| Expiration Date |
J. Todd Koning | Executive OfficersVice President and Chief Financial Officer | Modify | November 13, 2023 |
| 138,738 |
| April 30, 2024 |
Tyson Marshall | General Counsel and Corporate GovernanceSecretary | Adopt | November 14, 2023 |
| 28,700 |
| August 14, 2024 |
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections
Not applicable.
PART III
Item 10. Directors, Executive Officers and Corporate Governance
The information required by this item is incorporated herein by reference to our Proxy Statement with respect to our 20222024 Annual Meeting of Stockholders to be filed with the SEC within 120 days of the end of the fiscal year covered by this Annual Report on Form 10-K.
Item 11. Executive Compensation Item 11.
| Executive Compensation
|
The information required by this item is incorporated herein by reference to our Proxy Statement with respect to our 20222024 Annual Meeting of Stockholders to be filed with the SEC within 120 days of the end of the fiscal year covered by this Annual Report on Form 10-K.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters Item 12.
| Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
|
The information required by this item is incorporated herein by reference to our Proxy Statement with respect to our 20222024 Annual Meeting of Stockholders to be filed with the SEC within 120 days of the end of the fiscal year covered by this Annual Report on Form 10-K.
Item 13. Certain Relationships and Related Transactions, and Director Independence Item 13.
| Certain Relationships and Related Transactions, and Director Independence
|
The information required by this item is incorporated herein by reference to our Proxy Statement with respect to our 20222024 Annual Meeting of Stockholders to be filed with the SEC within 120 days of the end of the fiscal year covered by this Annual Report on Form 10-K.
Item 14. Principal Accounting Fees and Services Item 14.
| Principal Accounting Fees and Services
|
The information required by this item is incorporated herein by reference to our Proxy Statement with respect to our 20222024 Annual Meeting of Stockholders to be filed with the SEC within 120 days of the end of the fiscal year covered by this Annual Report on Form 10-K.
PART IV
Item 15. Exhibits, Financial Statement Schedules Item 15.
| Exhibits, Financial Statement Schedules
|
Item 15 (a) The following documents are filed as part of this Annual Report on Form 10-K:
(1) Financial Statements:
(2) Financial Statement Schedules
Item 15(a)Schedule II. Valuation and Qualifying Accounts:
All other financial statement schedules have been omitted because they are not applicable, not required or the information required by such schedules is shown in the financial statements or the notes thereto.
(3) Exhibits List
The following is a list of exhibits filed as part of this Annual Report on Form 10-K.
| | | | | | | | | | |
Exhibit Number |
| Exhibit Description |
| Filed with this Report |
| Incorporated by Reference herein from Form or Schedule |
| Filing Date |
| SEC File/ Reg. Number |
|
|
|
|
|
|
|
|
|
|
|
2.13.1
| | Purchase and Sale Agreement, dated as of July 25, 2016, by and between Alphatec Holdings, Inc. and Globus Medical Ireland, Ltd.
| | | | Form 8-K
(Exhibit 2.1)
| | 07/26/16
| | 000-52024
|
| | | | | | | | | | |
2.1
| | First Amendment to Purchase and Sale Agreement, dated as of September 1, 2016, by and between Alphatec Holdings, Inc. and Globus Medical Ireland, Ltd.
| | | | Form 8-K
(Exhibit 99.1)
| | 09/08/16
| | 000-52024
|
| | | | | | | | | | |
| | Second Amendment to Purchase and Sale Agreement and First Amendment to Product Manufacture and Supply Agreement, dated as of February 9, 2017, by and between Alphatec Holdings, Inc. and Globus Medical Ireland, Ltd.
| | | | Form 10-K
(Exhibit 2.3)
| | 03/31/17
| | 000-52024
|
| | | | | | | | | | |
3.1
| | Amended and Restated Certificate of Incorporation of Alphatec Holdings, Inc. |
|
|
| Amendment No. 2 to Form S-1 (Exhibit 3.2) |
| 04/20/06 |
| 333-131609 |
|
|
|
|
|
|
|
|
|
|
|
3.2 |
| Amendment to the Certificate of Incorporation of Alphatec Holdings, Inc. |
|
|
| Form 8-K (Exhibit 3.1(B)) |
| 08/24/16 |
| 000-52024 |
|
|
|
|
|
|
|
|
|
|
|
3.3 |
| Restated Bylaws of Alphatec Holdings, Inc. |
|
|
| Amendment No. 5 to Form S-1 (Exhibit 3.4) |
| 05/26/06 |
| 333-131609
|
|
|
|
|
|
| |
3.4 |
| Form of Certificate of Designation of Preferences, Rights and Limitations of Series A convertible Preferred Stock of Alphatec Holdings, Inc. |
|
|
| Form 8-K (Exhibit 3.1) |
| 03/23/17 |
| 000-52024 |
56
Table of Contents
| | | | | | | | | | |
Exhibit Number |
| Exhibit Description |
| Filed with this Report |
| Incorporated by Reference herein from Form or Schedule |
| Filing Date |
| SEC File/ Reg. Number |
4.3 |
| Registration Rights Agreement, dated November 6, 2018, by and among Alphatec Holdings, Inc. and the other signatories thereto |
|
|
| Form S-3/A (Exhibit 4.5) |
| 11/13/18 |
| 333-221085 |
|
|
|
|
|
|
|
|
|
|
|
4.44.5
| | Form of Warrant issued to certain investors on March 28, 2017
| | | | Form 8-K
(Exhibit 4.1)
| | 03/23/17
| | 000-52024
|
| | | | | | | | | | |
4.5
| | Form of Warrant issued to certain investors on March 8, 2018
| | | | Form 8-K
(Exhibit 4.1)
| | 03/12/18
| | 000-52024
|
| | | | | | | | | | |
4.6
| | Form of Registration Rights Agreement |
|
|
| Form 8-K (Exhibit 4.2) |
| 03/23/17 |
| 000-52024 |
|
|
|
|
|
|
|
|
|
|
|
4.74.6
|
| Second Amended and Restated Warrant to Purchase Common Stock of Alphatec Holdings, Inc. issued to Patrick S. Miles |
|
|
| Form 10-Q10-K (Exhibit 4.1)4.6) |
| 11/05/202/28/23
|
| 000-52024 |
|
|
|
|
|
|
|
|
|
|
|
4.84.7
|
| Form of Warrant to Purchase Common Stock of Alphatec Holdings, Inc. issued in connection with financing dated November 6, 2018 |
|
|
| Form S-3/A (Exhibit 4.11) |
| 11/13/18 |
| 333-221085 |
|
|
|
|
|
|
|
|
|
|
|
4.94.8
|
| Form of Warrant to Purchase Common Stock of Alphatec Holdings, Inc. issued in connection with financing dated June 21, 2019 |
|
|
| Form 8-K (Exhibit 10.1) |
| 06/27/19 |
| 000-52024 |
|
|
|
|
|
|
|
|
|
|
|
4.104.9
| | Form of Merger Warrant
| | | | Form 8-K
(Exhibit 4.3)
| | 03/12/18
| | 000-52024
|
| | | | | | | | | | |
4.11
| | Registration Rights Agreement between Alphatec Holdings, Inc., and Squadron Medical Finance Solutions LLC and Tawani Holdings LLC, dated November 6, 2018 |
|
|
| Form S-3/A (Exhibit 4.5) |
| 11/13/18 |
| 333-221085 |
|
|
|
|
|
|
|
|
|
|
|
4.124.10
|
| Registration Rights Agreement between Alphatec Holdings, Inc., and Squadron Medical Finance Solutions LLC and Tawani Holdings LLC, dated June 21, 2019 |
|
|
| Form 8-K (Exhibit 10.2) |
| 06/27/19 |
| 000-52024 |
57
Table of Contents
Exhibit
Number
| | Exhibit Description
| | Filed
with this
Report
| | Incorporated by
Reference herein
from Form or
Schedule
| | Filing Date
| | SEC File/
Reg.
Number
|
|
|
|
|
|
|
|
|
|
|
|
4.134.11
|
| Description of the Registrant’s Securities Registered Pursuant to Section 12 of the Securities and Exchange Act of 1934 |
|
|
| Form 10-K (Exhibit 4.15) |
| 03/17/20 |
| 000-52024 |
|
|
|
|
|
|
|
|
|
|
|
4.144.12
|
| Form of Common Stock Purchase Warrant |
|
|
| Form 8-K (Exhibit 4.1) |
| 06/04/20 |
| 000-52024 |
|
|
|
|
|
|
|
|
|
|
|
4.154.13
|
| Form of Amendment to Warrant |
|
|
| Form 8-K (Exhibit 4.2) |
| 06/04/20 |
| 000-52024 |
|
|
|
|
|
|
|
|
|
|
|
4.164.14
|
| Form of Second Amendment to Warrant |
|
|
| Form 8-K (Exhibit 4.3) |
| 06/04/20 |
| 000-52024 |
|
|
|
|
|
|
|
|
|
|
|
4.174.15
|
| Registration Rights Agreement between Alphatec Holdings, Inc., and Squadron Medical Finance Solutions LLC and Tawani Holdings LLC, dated May 29, 2020 |
|
|
| Form 8-K (Exhibit 4.4) |
| 06/04/20 |
| 000-52024 |
|
|
|
|
|
|
|
|
|
|
|
4.184.16
|
| Registration Rights Agreement, dated December 16, 2020 |
|
|
| Form 8-K (Exhibit 4.1) |
| 12/17/20 |
| 000-52024 |
|
|
|
|
|
|
|
|
|
|
|
4.204.17
|
| Indenture, dated as of August 10, 2021, between Alphatec Holdings, Inc. and U.S. Bank National Association, as trustee. |
|
|
| Form 8-K (Exhibit 4.1) |
| 8/10/21 |
| 000-52024 |
| | | | | | | | | | |
Exhibit Number |
| Exhibit Description |
| Filed with this Report |
| Incorporated by Reference herein from Form or Schedule |
| Filing Date |
| SEC File/ Reg. Number |
|
|
|
|
|
|
|
|
|
|
|
4.214.18
|
| Form of certificate representing the 0.75% Convertible Senior Notes due 2026. |
|
|
| Form 8-K (Exhibit 4.2) |
| 8/10/21 |
| 000-52024 |
|
|
|
|
|
|
|
|
|
|
|
|
| Securities Purchase Agreements |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.1 |
| Securities Purchase Agreement dated as of March 8, 2018, between Alphatec Holdings, Inc. and each purchaser named in the signature pages thereto |
|
|
| Form 8-K (Exhibit 10.1) |
| 03/12/18 |
| 000-52024 |
|
|
|
|
|
|
|
|
|
|
|
|
| Real Property Lease Agreements | |
|
|
| |
10.410.2
|
| Lease Agreement by and between Alphatec Spine, Inc. and RAF Pacifica Group - Real Estate Fund IV, LLC; ARKA Monterey Park, LLC, and 170 Arrowhead Partners, LLC, dated as of December 4, 2019 |
|
|
| Form 10-K (Exhibit 10.310.3) |
| 03/17/20 |
| 000-52024 |
|
|
|
|
|
|
|
|
|
|
|
|
| Capped Call Agreements |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.410.3
|
| Form of Confirmation of Call Option Transaction |
|
|
| Form 8-K (Exhibit 10.1) |
| 8/10/21 |
| 000-52024 |
|
|
|
|
|
|
|
|
|
|
|
|
| Agreements with Respect to Product Supply, Collaborations, Licenses, Research and Development | | |
| | | | | | | | | | |
10.12†
|
|
|
|
|
|
|
|
|
|
|
10.4† |
| Supply Agreement by and between Alphatec Spine, Inc. and Invibio, Inc., dated as of October 18, 2004 and amended by Letter of Amendment in respect of the Supply Agreement, dated as of December 13, 2004 |
|
|
| Amendment No. 4 to Form S-1 (Exhibit 10.29) |
| 05/15/06 |
| 333-131609 |
58
Table of Contents
Exhibit
Number
| | Exhibit Description
| | Filed
with this
Report
| | Incorporated by
Reference herein
from Form or
Schedule
| | Filing Date
| | SEC File/
Reg.
Number
|
|
|
|
|
|
|
|
|
|
|
|
10.13†10.5†
|
| Letter Amendment between Alphatec Spine, Inc. and Invibio, Inc., dated November 24, 2010 |
|
|
| Form 10-Q (Exhibit 10.3) |
| 05/06/11 |
| 000-52024 |
|
|
|
|
|
|
|
|
|
|
|
|
| Agreements with Officers and Directors |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.15*10.6*
|
| Employment Agreement with J. Todd Koning dated April 6, 2021 |
|
|
| Form 8-K (Exhibit 10.1) |
| 04/8/21 |
| 000-52024 |
|
|
|
|
|
|
|
|
|
|
|
10.16*10.7*
| | Employment Agreement with Jon Allen dated October December 10, 2016
| | | | Form 10-Q
(Exhibit 10.4)
| | 05/12/17
| | 000-52024
|
| | | | | | | | | | |
10.17*
| | Employment Agreement with Craig E. Hunsaker dated September 14, 2016 |
|
|
| Form 10-Q (Exhibit 10.5) |
| 05/12/17 |
| 000-52024 |
|
|
|
|
|
|
|
|
|
|
|
10.18*10.8*
| | Employment Agreement with Brian Snider dated February 27, 2017
| | | | Form 10-Q
(Exhibit 10.6)
| | 05/12/17
| | 000-52024
|
| | | | | | | | | | |
10.19*
| | Employment Agreement by and among Patrick S. Miles, Alphatec Spine, Inc., and Alphatec Holdings, Inc., dated, October 2, 2017 |
|
|
| Form 10-K (Exhibit 10.26) |
| 03/09/18 |
| 000-52024 |
|
|
|
|
|
|
|
|
|
|
|
10.20*10.9*
| | Employment Agreement by and among Mark Ojeda, Alphatec Spine, Inc., and Alphatec Holdings, Inc., dated, September 17, 2018
| | | | Form 10-K
(Exhibit 10.28)
| | 03/17/20
| | 000-52024
|
| | | | | | | | | | |
10.21*
| | Employment Agreement by and among Eric Dasso, Alphatec Spine, Inc., and Alphatec Holdings, Inc., dated, August 2, 2019 |
|
|
| Form 10-K (Exhibit 10.29) |
| 03/17/20 |
| 000-52024 |
|
|
|
|
|
|
|
|
|
|
|
| | | | | | | | | | |
Exhibit Number |
| Exhibit Description |
| Filed with this Report |
| Incorporated by Reference herein from Form or Schedule |
| Filing Date |
| SEC File/ Reg. Number |
10.22*10.10*
| | Employment Agreement by and among Kelli Howell, Alphatec Spine, Inc., and Alphatec Holdings, Inc., dated March 10, 2018
| | | | Form 10-K
(Exhibit 10.30)
| | 03/17/20
| | 000-52024
|
| | | | | | | | | | |
10.23*
| | Employment Agreement by and among Dave Sponsel, Alphatec Spine, Inc., and Alphatec Holdings, Inc., dated March 4, 2018 |
|
|
| Form 10-K (Exhibit 10.31) |
| 03/17/20 |
| 000-52024 |
|
|
|
|
|
|
|
|
|
|
|
10.24*10.11*
|
| Form of Severance Agreement between J. Todd Koning, Dave Sponsel and Alphatec Spine, Inc dated March 11, 2019 | | | | Form 10-Q
(Exhibit 10.2)
| | 05/11/20
| | 000-52024
|
| | | | | | | | | | |
10.25*
| | Severance Agreement between Eric Dasso and Alphatec Spine, Inc dated March 11, 2019July 19, 2023
|
|
|
| Form 10-Q (Exhibit 10.3)10.2) |
| 05/11/2007/19/23
|
| 000-52024 |
|
|
|
|
|
|
|
|
|
|
|
10.26*10.12*
| | Severance Agreement between Kelli Howell and Alphatec Spine, Inc dated March 11, 2019
| | | | Form 10-Q
(Exhibit 10.4)
| | 05/11/20
| | 000-52024
|
| | | | | | | | | | |
10.27*
| | Severance Agreement between Mark Ojeda and Alphatec Spine, Inc dated March 11, 2019
| | | | Form 10-Q
(Exhibit 10.5)
| | 05/11/20
| | 000-52024
|
| | | | | | | | | | |
10.28*
| | Severance Agreement between Patrick S. Miles and Alphatec Spine, Inc dated February 18, 2021 |
|
|
| Form 8-K (Exhibit 10.1) |
| 02/22/21 |
| 000-52024 |
|
|
|
|
|
|
|
|
|
|
|
10.29*10.13*
|
| Severance Agreement between Craig E. Hunsaker and Alphatec Spine, Inc dated February 18, 2021 |
|
|
| Form 8-K (Exhibit 10.2) |
| 02/22/21 |
| 000-52024 |
59
Table of Contents
60
Table of Contents
| | | | | | | | | | |
Exhibit Number |
| Exhibit Description |
| Filed with this Report |
| Incorporated by Reference herein from Form or Schedule |
| Filing Date |
| SEC File/ Reg. Number |
10.52*10.41*
|
| Form of Stock Option Grant Notice and Stock Option Agreement under the 2016 Employment Inducement Award Plan |
|
|
| Form S-8 (Exhibit 10.4) |
| 10/05/16 |
| 333-213981 |
|
|
|
|
|
|
|
|
|
|
|
10.53*10.42*
|
| Form of Performance Stock-Based Award Grant Notice and Performance Stock-Based Award Agreement under the 2016 Employment Inducement Award Plan |
|
|
| Form S-8 (Exhibit 10.5) |
| 10/05/16 |
| 333-213981 |
|
|
|
|
|
|
|
|
|
|
|
|
| SettlementLoan Agreements
|
|
|
|
|
|
|
|
|
10.43 | | | | | | |
10.54
|
| SettlementCredit, Security and ReleaseGuaranty Agreement, dated as of August 13, 2014,January 6, 2023, by and among Alphatec Holdings, Inc., as borrower, the guarantors from time to time party thereto, the lenders from time to time party thereto, and its directWilmington Trust, National Association, as agent
|
|
|
| Form 8-K (Exhibit 10.1) |
| 01/09/23 |
| 000-52024 |
|
|
|
|
|
|
|
|
|
|
|
10.44 |
| Credit Agreement, dated as of September 29, 2022, by and indirect subsidiaries and affiliates, Orthotec, LLC, Patrick Bertranouamong Alphatec Holdings, Inc., Alphatec Spine, Inc. and the other parties named thereinborrowers from time to time party thereto, the guarantors from time to time party thereto, MidCap Financial Trust and the other lenders from time to time party thereto, and MidCap Funding IV Trust, as administrative agent |
|
|
| Form 10-Q8-K (Exhibit 10.3)10.1) |
| 10/30/1403/22 |
| 000-52024 |
61
Table of Contents
Exhibit
Number
| | Exhibit Description
| | Filed
with this
Report
| | Incorporated by
Reference herein
from Form or
Schedule
| | Filing Date
| | SEC File/
Reg.
Number
|
|
|
|
|
|
|
|
|
|
|
|
21.110.45
|
| Omnibus Joinder and Amendment No. 1 to Credit, Security and Guaranty Agreement, dated as of January 6, 2023, by and among Alphatec Holdings, Inc., Alphatec Spine, Inc., SafeOp Surgical, Inc., MidCap Funding IV Trust, as agent and the lenders party thereto |
|
|
| Form 8-K (Exhibit 10.2) |
| 01/09/23 |
| 000-52024 |
|
|
|
|
|
|
|
|
|
|
|
19 |
| Insider Trading Policy |
| X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
21 |
| Subsidiaries of the Registrant and Wholly Owned Subsidiaries of the Registrant's Subsidiaries |
| X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
23.123
| | Consent of Mayer Hoffman McCann P.C., Independent Registered Public Accounting Firm
| | X
| | | | | | |
| | | | | | | | | | |
23.2
| | Consent of Deloitte & Touche LLP, Independent Registered Public Accounting Firm |
| X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31.1 |
| Certification of Principal Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| X |
|
|
|
|
|
|
|
|
|
|
|
| |
31.2 |
| Certification of Principal Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| X |
|
|
|
|
|
|
| | | | | | | | | | |
Exhibit Number |
| Exhibit Description |
| Filed with this Report |
| Incorporated by Reference herein from Form or Schedule |
| Filing Date |
| SEC File/ Reg. Number |
|
|
|
|
|
| |
32 |
| Certification pursuant to 18 U.S.C. 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
101.INS97
|
| Clawback Policy |
| X |
|
|
|
|
|
|
|
|
|
|
|
|
101.INS |
| XBRL Instance Document-the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document. |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
101.SCH |
| Inline XBRL Taxonomy Extension Schema Documentwith Embedded Linkbase Documents |
|
|
|
|
|
|
|
|
|
|
|
|
| | |
101.CAL
|
| Inline XBRL Taxonomy Extension Calculation Linkbase Document
|
|
| | | | | |
|
104 |
| | | | | |
101.DEF
| | Inline XBRL Taxonomy Extension Definition Linkbase Document
| | | | | | | | |
| | | | | | |
101.LAB
| | Inline XBRL Taxonomy Extension Label Linkbase Document
| | | | | | | | |
| | | | | | |
101.PRE
| | Inline XBRL Taxonomy Extension Presentation Linkbase Document
| | | | | | | | |
| | | | | | | | | | |
104
| | Cover page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101). | | | | | | | | |
(*)
| Management contract or compensatory plan or arrangement.
|
(†)
| Confidential treatment has been granted by the Securities and Exchange Commission as to certain portions.
|
|
|
|
|
|
|
(*) Management contract or compensatory plan or arrangement.
(†) Confidential treatment has been granted by the Securities and Exchange Commission as to certain portions.
62
SIGNATURES
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| | | | | | |
|
|
|
| ALPHATEC HOLDINGS, INC. |
|
|
|
|
|
Dated: |
| March 1, 2022February 27, 2024
|
| By: |
| /s/ Patrick S. Miles |
|
|
|
|
|
| Patrick S. Miles |
|
|
|
|
|
| Chairman and Chief Executive Officer |
|
|
|
|
|
| (principal executive officer) |
|
|
|
|
|
|
|
Dated: |
| March 1, 2022February 27, 2024
|
| By: |
| /s/ J. Todd Koning |
|
|
|
|
|
| J. Todd Koning |
|
|
|
|
|
| J. Todd Koning
|
| | | | | | Executive Vice President and Chief Financial Officer |
|
|
|
|
|
| (principal financial officer and principal accounting officer) |
SIGNATURES AND POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Patrick S. Miles and J. Todd Koning, and each of them, as his or her true and lawful attorneys-in-fact and agents, each with full power of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K and to file the same, with all exhibits thereto and all documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that such attorneys-in-fact and agents or any of them, or his or her or their substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| | | | |
Signature |
| Title |
| Date |
|
|
|
|
|
/s/ Patrick S. Miles |
| Chairman and Chief Executive Officer (Principal Executive Officer) |
| March 1, 2022February 27, 2024
|
Patrick S. Miles |
|
|
|
|
|
|
|
|
|
/s/Mortimer Berkowitz III |
| Lead Director |
| March 1, 2022February 27, 2024
|
Mortimer Berkowitz III |
|
|
|
|
|
|
|
|
|
/s/Beth Altman |
| Director |
| March 1, 2022February 27, 2024
|
Beth Altman |
|
|
|
|
|
|
|
|
|
/s/Evan Bakst |
| Director |
| March 1, 2022February 27, 2024
|
Evan Bakst |
|
|
|
|
|
|
|
|
|
/s/Quentin Blackford |
| Director |
| March 1, 2022February 27, 2024
|
Quentin Blackford |
|
|
|
|
|
|
|
|
|
/s/Jason HochbergDavid Demski |
| Director |
| March 1, 2022February 27, 2024
|
Jason HochbergDavid Demski
|
|
|
|
|
|
|
|
|
|
/s/Karen K. McGinnis |
| Director |
| March 1, 2022February 27, 2024
|
Karen K. McGinnis |
|
|
|
|
|
|
|
|
|
/s/Marie Meynadier |
| Director |
| March 1, 2022February 27, 2024
|
Marie Meynadier |
|
|
|
|
|
|
|
|
|
/s/David H. Mowry |
| Director |
| March 1, 2022February 27, 2024
|
David H. Mowry |
|
|
|
|
|
|
|
|
|
63
Table of Contents
| | | | |
Signature |
| Title |
| Date |
|
|
|
|
|
/s/David R. Pelizzon |
| Director |
| March 1, 2022February 27, 2024
|
David R. Pelizzon |
|
|
|
|
|
|
|
|
|
/s/Jeffrey P. Rydin |
| Director |
| March 1, 2022February 27, 2024
|
Jeffrey P. Rydin |
|
|
|
|
|
|
|
|
|
/s/James L. L. Tullis |
| Director |
| March 1, 2022February 27, 2024
|
James L.L. Tullis |
|
|
|
|
|
|
|
|
|
/s/Ward W. Woods |
| Director |
| March 1, 2022February 27, 2024
|
Ward W. Woods |
|
|
|
|
64
ALPHATEC HOLDINGS, INC.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the stockholders and the Board of Directors of Alphatec Holdings, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheetsheets of Alphatec Holdings, Inc. and subsidiaries (the "Company") as of December 31, 2021,2023 and 2022, the related consolidated statements of operations, comprehensive loss, stockholders' equity (deficit), and cash flows, for each of the yearthree years in the period ended December 31, 2021,2023, and the related notes (collectively referred to as the "financial statements"). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2021,2023 and 2022, and the results of its operations and its cash flows for each of the yearthree years in the period ended December 31, 2021,2023, in conformity with accounting principles generally accepted in the United States of America.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company's internal control over financial reporting as of December 31, 2021,2023, based on criteria established in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated March 1, 2022,February 27, 2024, expressed an unqualified opinion on the Company's internal control over financial reporting.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current-period audit of the financial statements that was communicated or required to be communicated to the audit committee and that (1) relates to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Valuation of Inventories - Refer to Note 21 to the Financial Statements
Critical Audit Matter Description
The Company records its inventories at the lower of cost or net realizable value (“LCNRV”). A portion of the Company’s LCNRV reserve represents an amount for specialized implants and fixation products (collectively “implant inventory”). Quarterly, the Company records an adjustment to its LCNRV inventory reserve for estimated excess and obsolete implant inventory based upon its expected use of implant inventory on hand. To determine the expected use of implant inventory, management develops estimates and assumptions primarily considering ifbased on the current usage of implant inventory, items are currently being commercially marketed, and the age of implant inventory quantities on hand. Additionally, the Company considers recent sales experience to develop assumptions about future demand for its products, while considering product life cycles and new product launches.
F-2
Table of Contents
Inventories, net as of December 31, 2021 are $91.7 million. During the year ended December 31, 2021, the Company recognized $11.1 million of LCNRV charges related to excess and obsolete inventory.
We identified management’s estimation of excess and obsolete inventories, and the relatedimplant inventory LCNRV inventory reserve as a critical audit matter due to management’s significant manual process used to determine the estimate and the significant judgments required by management to estimate future use of their products. This required a high degree of auditor judgment and an increased extent of effort when performing audit procedures to evaluate the reasonableness of management’s assumptions related to the expected use of implant inventory in future operations.
How the Critical Audit Matter Was Addressed in the Audit
Our audit procedures related to management’s judgments used to estimate excess and obsoletethe implant inventory and the related LCNRV inventory reserve included the following, among others:
• We tested the effectiveness of controls over management’s estimate of the implant inventory LCNRV reserve, including: omanagement’s assessment of assumptions used to identify excess and obsolete implant inventory and to estimate the related LCNRV reserve. othe completeness and accuracy of data used in the calculation. •We evaluated the reasonableness of the methodology used by the Company to estimate the implant inventory LCNRV reserve by comparing actual results to the historical estimates. •We evaluated the key assumptions used in identifying the population of implant inventory with excess or obsolescence exposure that require a reserve and determining the amount of reserve to record. •We evaluated the appropriateness of the underlying data utilized in management’s analysis, including current implant inventory usage, product aging, recent sales, and product life cycle.
| •
| We tested the effectiveness of controls over management’s estimate of excess and obsolete inventories, including:
|
| o
| management’s assessment of assumptions used to identify excess and obsolete inventory and to estimate the related LCNRV inventory reserve.
|
| o
| the completeness and accuracy of data used in the calculation.
|
| •
| We evaluated the reasonableness of the methodology used by the Company to estimate the excess and obsolete inventories and related LCNRV reserve, by comparing actual results to the historical estimates.
|
| •
| We evaluated the key assumptions used in identifying the population of inventory with excess or obsolescence exposure that require a reserve and determining the amount of reserve to record.
|
| •
| We evaluated the appropriateness of the underlying data utilized in management’s analysis, including current inventory usage, product aging, recent sales and product life cycle.
|
/s/ Deloitte & Touche LLP
United States of America San Diego, California
March 1, 2022February 27, 2024
We have served as the Company's auditor since 2021.
Table of Contents
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of
Alphatec Holdings, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheet of Alphatec Holdings, Inc. (“Company”) as of December 31, 2020, and the related consolidated statement of operations, comprehensive loss, stockholders’ equity, and cash flows for the year ended December 31, 2020, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2020, and the results of its operations and its cash flows for the year ended December 31, 2020, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) ("PCAOB") and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
We served as the Company’s auditor since 2017, which ended in 2021.
/s/ Mayer Hoffman McCann P.C.
San Diego, California
March 5, 2021
F-4
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the stockholders and the Board of Directors of Alphatec Holdings, Inc.
Opinion on Internal Control over Financial Reporting
We have audited the internal control over financial reporting of Alphatec Holdings, Inc. and subsidiaries (the “Company”) as of December 31, 2021,2023, based on criteria established in Internal Control — Integrated Framework (2013)issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2021,2023, based on criteria established in Internal Control — Integrated Framework (2013) issued by COSO.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated financial statements as of and for the year ended December 31, 2021,2023, of the Company and our report dated March 1, 2022,February 27, 2024, expressed an unqualified opinion on those financial statements.
As described in Management’s Report on Internal Control over Financial Reporting, management excluded from its assessment the internal control over financial reporting at EOS imaging S.A., which was acquired on May 13, 2021, and whose financial statements constitute total assets and net liabilities of $52.1 million and $13.8 million, respectively, and revenue of $30.0 million of the consolidated financial statement amounts as of and for the year ended December 31, 2021. Accordingly, our audit did not include the internal control over financial reporting at EOS imaging S.A.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management's Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become
F-5
Table of Contents
inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ Deloitte & Touche LLP
San Diego, California
March 1, 2022 February 27, 2024
ALPHATEC HOLDINGS, INC.
CONSOLIDATED BALANCE SHEETS
(In thousands, except par value data)
| | | December 31, | | | December 31, | |
| | 2021 | | | 2020 | | | 2023 | | | 2022 | |
Assets | | | | | | | | | | | | | | |
Current assets: | | | | | | | | | | | | | | |
Cash and cash equivalents | | $ | 187,248 | | | $ | 107,765 | | | $ | 220,970 | | | $ | 84,696 | |
Accounts receivable, net | | | 41,893 | | | | 23,527 | |
Accounts receivable, net of allowances of $910 and $679, respectively | | | | 72,613 | | | | 60,060 | |
Inventories | | | 91,703 | | | | 46,001 | | | | 136,842 | | | | 101,521 | |
Prepaid expenses and other current assets | | | 10,313 | | | | 5,439 | | | | 20,666 | | | | 9,357 | |
Withholding tax receivable from Officer | | | — | | | | 1,076 | |
Current assets of discontinued operations | | | — | | | | 352 | |
Total current assets | | | 331,157 | | | | 184,160 | | | | 451,091 | | | | 255,634 | |
Property and equipment, net | | | 87,401 | | | | 36,670 | | | | 149,835 | | | | 101,952 | |
Right-of-use assets | | | 25,283 | | | | 1,177 | | | | 26,410 | | | | 28,360 | |
Goodwill | | | 39,689 | | | | 13,897 | | | | 73,003 | | | | 47,367 | |
Intangible assets, net | | | 85,274 | | | | 24,720 | | | | 102,451 | | | | 82,781 | |
Other assets | | | 3,249 | | | | 541 | | | | 2,418 | | | | 4,874 | |
Noncurrent assets of discontinued operations | | | — | | | | 58 | |
Total assets | | $ | 572,053 | | | $ | 261,223 | | | $ | 805,208 | | | $ | 520,968 | |
Liabilities and Stockholders’ Equity | | | | | | | | |
Liabilities and Stockholders’ Equity (Deficit) | | | | | | | |
Current liabilities: | | | | | | | | | | | | | | |
Accounts payable | | $ | 25,737 | | | $ | 17,599 | | | $ | 48,985 | | | $ | 34,742 | |
Accrued expenses and other current liabilities | | | 55,549 | | | | 35,264 | | | | 87,712 | | | | 72,382 | |
Contract liability | | | 15,255 | | | | — | |
Contract liabilities | | | | 13,910 | | | | 11,956 | |
Short-term debt | | | 342 | | | | 4,167 | | | | 1,808 | | | | 14,948 | |
Current portion of operating lease liability | | | 4,212 | | | | 885 | |
Current liabilities of discontinued operations | | | — | | | | 397 | |
Current portion of operating lease liabilities | | | | 5,159 | | | | 4,842 | |
Total current liabilities | | | 101,095 | | | | 58,312 | | | | 157,574 | | | | 138,870 | |
Long-term debt | | | 326,489 | | | | 37,999 | | | | 511,035 | | | | 349,511 | |
Operating lease liability, less current portion | | | 24,383 | | | | 41 | |
Operating lease liabilities, less current portion | | | | 23,677 | | | | 26,562 | |
Other long-term liabilities | | | 17,061 | | | | 11,388 | | | | 11,203 | | | | 17,089 | |
Redeemable preferred stock, $0.0001 par value; 20,000 shares authorized, and 3,319 shares issued and outstanding at December 31, 2021 and 2020 | | | 23,603 | | | | 23,603 | |
Redeemable preferred stock, $0.0001 par value; 20,000 shares authorized, and 3,319 shares issued and outstanding at December 31, 2023 and 2022 | | | | 23,603 | | | | 23,603 | |
Commitments and contingencies (Note 7) | | | | | | | | | | | | | | |
Stockholders’ equity: | | | | | | | | |
Series A convertible preferred stock, $0.0001 par value; 15 shares authorized, and 0 shares issued and outstanding at December 31, 2021 and 2020 | | | — | | | | — | |
Common stock, $0.0001 par value; 200,000 authorized; 99,627 shares issued and 99,537 outstanding at December 31, 2021, and 82,294 shares issued and 82,104 shares outstanding at December 31, 2020 | | | 10 | | | | 8 | |
Treasury stock, 1,808 shares at December 31, 2021 and 2 shares at December 31, 2020 | | | (25,097 | ) | | | (97 | ) |
Stockholders’ equity (deficit): | | | | | | | |
Series A convertible preferred stock, $0.0001 par value; 15 shares authorized, and 0 shares issued and outstanding at December 31, 2023 and 2022 | | | | — | | | | — | |
Common stock, $0.0001 par value; 200,000 authorized; 139,257 shares issued and 139,245 outstanding at December 31, 2023, and 106,673 shares issued and 106,640 shares outstanding at December 31, 2022 | | | | 14 | | | | 11 | |
Treasury stock, 1,808 shares at December 31, 2023 and December 31, 2022 | | | | (25,097 | ) | | | (25,097 | ) |
Additional paid-in capital | | | 892,828 | | | | 770,764 | | | | 1,230,484 | | | | 933,537 | |
Shareholder note receivable | | | — | | | | (4,000 | ) |
Accumulated other comprehensive (deficit) income | | | (5,994 | ) | | | 1,204 | |
Accumulated other comprehensive loss | | | | (8,323 | ) | | | (10,794 | ) |
Accumulated deficit | | | (782,325 | ) | | | (637,999 | ) | | | (1,118,962 | ) | | | (932,324 | ) |
Total stockholders’ equity | | | 79,422 | | | | 129,880 | |
Total liabilities and stockholders’ equity | | $ | 572,053 | | | $ | 261,223 | |
Total stockholders’ equity (deficit) | | | | 78,116 | | | | (34,667 | ) |
Total liabilities and stockholders’ equity (deficit) | | | $ | 805,208 | | | $ | 520,968 | |
See accompanying notes to consolidated financial statements.
ALPHATEC HOLDINGS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except per share amounts)
| | | Year Ended December 31, | | Year Ended December 31, | |
| | 2021 | | | 2020 | | | 2023 | | | 2022 | | | 2021 | |
Revenue: | | | | | | | | | | | | | | | | |
Revenue from products and services | | $ | 242,258 | | | $ | 141,079 | | | $ | 482,262 | | | $ | 350,852 | | | $ | 242,258 | |
Revenue from international supply agreement | | | 954 | | | | 3,782 | | | | — | | | | 15 | | | | 954 | |
Total revenue | | | 243,212 | | | | 144,861 | | | | 482,262 | | | | 350,867 | | | | 243,212 | |
Cost of sales | | | 85,450 | | | | 42,360 | | | | 172,059 | | | | 117,808 | | | | 85,450 | |
Gross profit | | | 157,762 | | | | 102,501 | | | | 310,203 | | | | 233,059 | | | | 157,762 | |
Operating expenses: | | | | | | | | | | | | | | | | | |
Research and development | | | 32,015 | | | | 18,745 | | | | 70,115 | | | | 44,033 | | | | 32,015 | |
Sales, general and administrative | | | 229,271 | | | | 129,156 | | | | 374,080 | | | | 300,013 | | | | 229,271 | |
Litigation-related expenses | | | 11,123 | | | | 8,552 | | | | 22,287 | | | | 23,943 | | | | 11,123 | |
Amortization of acquired intangible assets | | | 5,348 | | | | 688 | | | | 14,284 | | | | 10,115 | | | | 5,348 | |
Transaction-related expenses | | | 6,365 | | | | 4,223 | | | | 2,113 | | | | 120 | | | | 6,365 | |
Restructuring expenses | | | 1,697 | | | | — | | | | 719 | | | | 1,810 | | | | 1,697 | |
Total operating expenses | | | 285,819 | | | | 161,364 | | | | 483,598 | | | | 380,034 | | | | 285,819 | |
Operating loss | | | (128,057 | ) | | | (58,863 | ) | | | (173,395 | ) | | | (146,975 | ) | | | (128,057 | ) |
Interest and other expense, net: | | | | | | | | | | | | | | | | | |
Interest expense, net | | | (7,108 | ) | | | (12,374 | ) | | | (16,641 | ) | | | (5,505 | ) | | | (7,108 | ) |
Loss on debt extinguishment, net | | | (7,434 | ) | | | (7,612 | ) | | | — | | | | — | | | | (7,434 | ) |
Other expense, net | | | (1,563 | ) | | | — | |
Other income (expense), net | | | | 3,121 | | | | 471 | | | | (1,563 | ) |
Total interest and other expense, net | | | (16,105 | ) | | | (19,986 | ) | | | (13,520 | ) | | | (5,034 | ) | | | (16,105 | ) |
Net loss before taxes | | | (144,162 | ) | | | (78,849 | ) | | | (186,915 | ) | | | (152,009 | ) | | | (144,162 | ) |
Income tax provision | | | 164 | | | | 145 | |
Income tax benefit | | | | (277 | ) | | | (716 | ) | | | (1,130 | ) |
Net loss | | $ | (144,326 | ) | | $ | (78,994 | ) | | $ | (186,638 | ) | | $ | (151,293 | ) | | $ | (143,032 | ) |
Net loss per share, basic and diluted | | $ | (1.50 | ) | | $ | (1.18 | ) | | $ | (1.54 | ) | | $ | (1.46 | ) | | $ | (1.49 | ) |
Weighted average shares outstanding, basic and diluted | | | 96,197 | | | | 67,020 | | | | 121,242 | | | | 103,373 | | | | 96,197 | |
See accompanying notes to consolidated financial statements.
ALPHATEC HOLDINGS, INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
(in thousands)
| | | Year Ended December 31, | | Year Ended December 31, | |
| | 2021 | | | 2020 | | | 2023 | | | 2022 | | | 2021 | |
Net loss | | $ | (144,326 | ) | | $ | (78,994 | ) | | $ | (186,638 | ) | | $ | (151,293 | ) | | $ | (143,032 | ) |
Foreign currency translation adjustments | | | (7,198 | ) | | | 116 | | | | 2,471 | | | | (4,758 | ) | | | (7,240 | ) |
Comprehensive loss | | $ | (151,524 | ) | | $ | (78,878 | ) | | $ | (184,167 | ) | | $ | (156,051 | ) | | $ | (150,272 | ) |
See accompanying notes to consolidated financial statements.
ALPHATEC HOLDINGS, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)
(In thousands)
| | Common stock | | | | Additional | | | Shareholder | | | | | | | Accumulated other | | | | | | | Total stockholders’ | |
| | Shares | | | Par Value | | | | paid-in capital | | | note receivable | | | Treasury stock | | | comprehensive income | | | Accumulated deficit | | | equity (deficit) | |
Balance at December 31, 2020 | | | 82,104 | | | $ | 8 | | | | $ | 770,764 | | | $ | (4,000 | ) | | $ | (97 | ) | | $ | 1,204 | | | $ | (637,999 | ) | | $ | 129,880 | |
Stock-based compensation | | | — | | | | — | | | | | 34,728 | | | | — | | | | — | | | | — | | | | — | | | | 34,728 | |
Distributor equity incentives | | | 164 | | | | — | | | | | 1,339 | | | | — | | | | — | | | | — | | | | — | | | | 1,339 | |
Common stock issued for warrant exercises | | | 3,943 | | | | — | | | | | 3,254 | | | | — | | | | — | | | | — | | | | — | | | | 3,254 | |
Common stock issued for employee stock purchase plan and stock option exercises | | | 796 | | | | — | | | | | 3,584 | | | | — | | | | — | | | | — | | | | — | | | | 3,584 | |
Common stock issued for vesting of restricted stock units, net of shares withheld for tax liability | | | 1,915 | | | | — | | | | | (12,801 | ) | | | — | | | | — | | | | — | | | | — | | | | (12,801 | ) |
Issuance of common stock for private placement, net of offering costs of $6,200 | | | 12,421 | | | | 2 | | | | | 131,826 | | | | — | | | | — | | | | — | | | | — | | | | 131,828 | |
Shareholder note receivable | | | — | | | | — | | | | | — | | | | 4,000 | | | | — | | | | — | | | | — | | | | 4,000 | |
Repurchase of common stock | | | (1,806 | ) | | | — | | | | | — | | | | — | | | | (25,000 | ) | | | — | | | | — | | | | (25,000 | ) |
Purchase of capped calls | | | — | | | | — | | | | | (39,866 | ) | | | — | | | | — | | | | — | | | | — | | | | (39,866 | ) |
Foreign currency translation adjustments | | | — | | | | — | | | | | — | | | | — | | | | — | | | | (7,198 | ) | | | — | | | | (7,198 | ) |
Net loss | | | — | | | | — | | | | | — | | | | — | | | | — | | | | — | | | | (144,326 | ) | | | (144,326 | ) |
Balance at December 31, 2021 | | | 99,537 | | | $ | 10 | | | | $ | 892,828 | | | $ | — | | | $ | (25,097 | ) | | $ | (5,994 | ) | | $ | (782,325 | ) | | $ | 79,422 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Common stock | | | Additional
paid-in | | | Treasury | | | Accumulated other | | | Accumulated | | | Total
stockholders’ | |
| | Shares | | | Par Value | | | capital | | | stock | | | comprehensive loss | | | deficit | | | equity (deficit) | |
Balance at December 31, 2022 | | | 106,640 | | | $ | 11 | | | $ | 933,537 | | | $ | (25,097 | ) | | $ | (10,794 | ) | | $ | (932,324 | ) | | $ | (34,667 | ) |
Stock-based compensation | | | — | | | | — | | | | 81,244 | | | | — | | | | — | | | | — | | | | 81,244 | |
Common stock issued for warrant exercises | | | 5,571 | | | | 1 | | | | 669 | | | | — | | | | — | | | | — | | | | 670 | |
Common stock issued for employee
stock purchase plan and stock option
exercises | | | 774 | | | | — | | | | 4,353 | | | | — | | | | — | | | | — | | | | 4,353 | |
Common stock issued for vesting of
restricted stock units, net of
shares withheld for tax liability | | | 6,535 | | | | — | | | | (6,061 | ) | | | — | | | | — | | | | — | | | | (6,061 | ) |
Reclassification of equity-based liability | | | — | | | | — | | | | 3,561 | | | | — | | | | — | | | | — | | | | 3,561 | |
Common stock offerings, net of offering costs of $12,136 | | | 19,725 | | | | 2 | | | | 213,181 | | | | — | | | | — | | | | — | | | | 213,183 | |
Foreign currency translation adjustments | | | — | | | | — | | | | — | | | | — | | | | 2,471 | | | | — | | | | 2,471 | |
Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | (186,638 | ) | | | (186,638 | ) |
Balance at December 31, 2023 | | | 139,245 | | | $ | 14 | | | $ | 1,230,484 | | | $ | (25,097 | ) | | $ | (8,323 | ) | | $ | (1,118,962 | ) | | $ | 78,116 | |
See accompanying notes to consolidated financial statements.
ALPHATEC HOLDINGS, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)
(In thousands)
| | Common stock | | | | Additional | | | Shareholder | | | | | | | Accumulated other | | | | | | | Total stockholders’ | |
| | Shares | | | Par Value | | | | paid-in capital | | | note receivable | | | Treasury stock | | | comprehensive income | | | Accumulated deficit | | | equity (deficit) | |
Balance at December 31, 2019 | | | 61,400 | | | $ | 6 | | | | $ | 606,558 | | | $ | (5,000 | ) | | $ | (97 | ) | | $ | 1,088 | | | $ | (558,924 | ) | | $ | 43,631 | |
Cumulative effect of change in accounting principle | | | — | | | | — | | | | | — | | | | — | | | | — | | | | — | | | | (81 | ) | | | (81 | ) |
Stock-based compensation | | | — | | | | — | | | | | 15,730 | | | | — | | | | — | | | | — | | | | — | | | | 15,730 | |
Common stock issued for conversion of Series A preferred stock | | | 39 | | | | — | | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
Distributor equity incentives | | | — | | | | — | | | | | 521 | | | | — | | | | — | | | | — | | | | — | | | | 521 | |
Common stock issued for warrant exercises | | | 1,907 | | | | — | | | | | 2,368 | | | | — | | | | — | | | | — | | | | — | | | | 2,368 | |
Common stock issued for employee stock purchase plan and stock option exercises | | | 665 | | | | — | | | | | 1,970 | | | | — | | | | — | | | | — | | | | — | | | | 1,970 | |
Common stock issued for vesting of restricted stock units, net of shares withheld for tax liability | | | 2,238 | | | | — | | | | | (983 | ) | | | — | | | | — | | | | — | | | | — | | | | (983 | ) |
Issuance of common stock warrants, net | | | — | | | | — | | | | | 2,974 | | | | — | | | | — | | | | — | | | | — | | | | 2,974 | |
Issuance of common stock for public offering, net of offering costs of $7,300 | | | 13,143 | | | | 2 | | | | | 107,696 | | | | — | | | | — | | | | — | | | | — | | | | 107,698 | |
Shareholder note receivable | | | — | | | | — | | | | | — | | | | 1,000 | | | | — | | | | — | | | | — | | | | 1,000 | |
Issuance of common stock for other services | | | 12 | | | | — | | | | | 123 | | | | — | | | | — | | | | — | | | | — | | | | 123 | |
Issuance of common stock for prepayment of debt | | | 2,700 | | | | — | | | | | 33,807 | | | | — | | | | — | | | | — | | | | — | | | | 33,807 | |
Foreign currency translation adjustments | | | — | | | | — | | | | | — | | | | — | | | | — | | | | 116 | | | | — | | | | 116 | |
Net loss | | | — | | | | — | | | | | — | | | | — | | | | — | | | | — | | | | (78,994 | ) | | | (78,994 | ) |
Balance at December 31, 2020 | | | 82,104 | | | $ | 8 | | | | $ | 770,764 | | | $ | (4,000 | ) | | $ | (97 | ) | | $ | 1,204 | | | $ | (637,999 | ) | | $ | 129,880 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Common stock | | | Additional
paid-in | | | Treasury | | | Accumulated other | | | Accumulated | | | Total
stockholders’ | |
| | Shares | | | Par Value | | | capital | | | stock | | | comprehensive loss | | | deficit | | | (deficit) equity | |
Balance at December 31, 2021 | | | 99,537 | | | $ | 10 | | | $ | 892,828 | | | $ | (25,097 | ) | | $ | (6,036 | ) | | $ | (781,031 | ) | | $ | 80,674 | |
Stock-based compensation | | | — | | | | — | | | | 37,591 | | | | — | | | | — | | | | — | | | | 37,591 | |
Sales agent equity incentives | | | 221 | | | | — | | | | 3,068 | | | | — | | | | — | | | | — | | | | 3,068 | |
Common stock issued for conversion
of Series A preferred stock | | | 29 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
Common stock issued for warrant exercises | | | 3,820 | | | | 1 | | | | 4,159 | | | | — | | | | — | | | | — | | | | 4,160 | |
Common stock issued for employee
stock purchase plan and stock option
exercises | | | 806 | | | | — | | | | 4,020 | | | | — | | | | — | | | | — | | | | 4,020 | |
Common stock issued for vesting of
restricted stock units, net of
shares withheld for tax liability | | | 2,204 | | | | — | | | | (11,220 | ) | | | — | | | | — | | | | — | | | | (11,220 | ) |
Reclassification of liability-classified
awards | | | | | | | | | 2,841 | | | | | | | | | | | | | 2,841 | |
Common stock issued for asset acquisition | | | 23 | | | | — | | | | 250 | | | | — | | | | — | | | | — | | | | 250 | |
Foreign currency translation adjustments | | | — | | | | — | | | | — | | | | — | | | | (4,758 | ) | | | — | | | | (4,758 | ) |
Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | (151,293 | ) | | | (151,293 | ) |
Balance at December 31, 2022 | | | 106,640 | | | $ | 11 | | | $ | 933,537 | | | $ | (25,097 | ) | | $ | (10,794 | ) | | $ | (932,324 | ) | | $ | (34,667 | ) |
See accompanying notes to consolidated financial statements.
ALPHATEC HOLDINGS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWSSTOCKHOLDERS’ EQUITY
(In thousands)
| | Year Ended December 31, | |
| | 2021 | | | 2020 | |
Operating activities: | | | | | | | | |
Net loss | | $ | (144,326 | ) | | $ | (78,994 | ) |
Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | |
Depreciation and amortization | | | 26,756 | | | | 10,949 | |
Stock-based compensation | | | 36,450 | | | | 17,659 | |
Amortization of debt discount and debt issuance costs | | | 1,868 | | | | 3,974 | |
Amortization of right-of-use assets | | | 3,418 | | | | 683 | |
Write-down for excess and obsolete inventories | | | 11,147 | | | | 7,044 | |
Loss on disposal of assets | | | 1,976 | | | | 498 | |
Loss on debt extinguishment, net | | | 7,434 | | | | 7,612 | |
Impairment of investment | | | 3,000 | | | | — | |
Other | | | 2,721 | | | | 116 | |
Changes in operating assets and liabilities: | | | | | | | | |
Accounts receivable | | | (10,141 | ) | | | (7,484 | ) |
Inventories | | | (27,746 | ) | | | (18,192 | ) |
Prepaid expenses and other current assets | | | 1,258 | | | | (2,930 | ) |
Other assets | | | 11 | | | | (51 | ) |
Accounts payable | | | 757 | | | | 7,130 | |
Accrued expenses and other current liabilities | | | 6,983 | | | | 8,812 | |
Lease liability | | | 150 | | | | (1,312 | ) |
Other long-term liabilities | | | 4,852 | | | | (1,926 | ) |
Net cash used in operating activities | | | (73,432 | ) | | | (46,412 | ) |
Investing activities: | | | | | | | | |
Purchases of property and equipment | | | (68,544 | ) | | | (23,131 | ) |
Acquisition of business, net of cash acquired | | | (62,133 | ) | | | — | |
Purchase of OCEANE | | | (21,097 | ) | | | — | |
Cash paid for investments | | | (3,000 | ) | | | — | |
Cash paid for acquisition of intangible assets | | | — | | | | (755 | ) |
Cash received from sale of assets | | | — | | | | 27 | |
Settlement of forward contract | | | (2,988 | ) | | | — | |
Net cash used in investing activities | | | (157,762 | ) | | | (23,859 | ) |
Financing activities: | | | | | | | | |
Proceeds from common stock offering, net | | | 131,828 | | | | 107,698 | |
Proceeds from issuance of convertible notes | | | 316,250 | | | | — | |
Payment of debt issuance costs | | | (10,028 | ) | | | — | |
Net cash (paid) received from common stock exercises | | | (5,963 | ) | | | 3,341 | |
Borrowings under lines of credit | | | — | | | | 42,455 | |
Repayments under lines of credit | | | — | | | | (56,615 | ) |
Purchase of capped calls | | | (39,866 | ) | | | — | |
Repurchase of common stock | | | (25,000 | ) | | | — | |
Proceeds from issuance of term debt, net | | | — | | | | 34,008 | |
Repayment of Squadron Medical Term Loan | | | (45,000 | ) | | | — | |
Repayment of Inventory Financing Agreement | | | (8,088 | ) | | | — | |
Other | | | (2,167 | ) | | | (58 | ) |
Net cash provided by financing activities | | | 311,966 | | | | 130,829 | |
Effect of exchange rate changes on cash | | | (1,289 | ) | | | 94 | |
Net increase in cash and cash equivalents | | | 79,483 | | | | 60,652 | |
Cash and cash equivalents at beginning of year | | | 107,765 | | | | 47,113 | |
Cash and cash equivalents at end of year | | $ | 187,248 | | | $ | 107,765 | |
Supplemental disclosure of cash flow information: | | | | | | | | |
Cash paid for interest | | $ | 5,027 | | | $ | 6,330 | |
Cash paid for income taxes | | $ | 223 | | | $ | 190 | |
Supplemental disclosure of noncash investing and financing activities: | | | | | | | | |
Common stock warrants issued with term loan draw | | $ | — | | | $ | 2,974 | |
Common stock issued for partial extinguishment of debt | | $ | — | | | $ | 33,807 | |
PPP Loan Forgiveness | | $ | 4,271 | | | $ | — | |
Common stock issued for development of intangible assets | | $ | — | | | $ | 123 | |
Purchases of property and equipment in accounts payable | | $ | 2,577 | | | $ | 3,527 | |
Financed inventory | | $ | 4,015 | | | $ | — | |
Recognition of lease liability | | $ | 23,403 | | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Common stock | | | Additional
paid-in | | | Shareholder note | | | Treasury | | | Accumulated other comprehensive | | | Accumulated | | | Total
stockholders’ | |
| | Shares | | | Par Value | | | capital | | | receivable | | | stock | | | income (loss) | | | deficit | | | equity | |
Balance at December 31, 2020 | | | 82,104 | | | $ | 8 | | | $ | 770,764 | | | $ | (4,000 | ) | | $ | (97 | ) | | $ | 1,204 | | | $ | (637,999 | ) | | $ | 129,880 | |
Stock-based compensation | | | — | | | | — | | | | 34,728 | | | | — | | | | — | | | | — | | | | — | | | | 34,728 | |
Sales agent equity incentives | | | 164 | | | | — | | | | 1,339 | | | | — | | | | — | | | | — | | | | — | | | | 1,339 | |
Common stock issued for warrant
exercises | | | 3,943 | | | | — | | | | 3,254 | | | | — | | | | — | | | | — | | | | — | | | | 3,254 | |
Common stock issued for employee
stock purchase plan and stock option
exercises | | | 796 | | | | — | | | | 3,584 | | | | — | | | | — | | | | — | | | | — | | | | 3,584 | |
Common stock issued for vesting of
restricted stock units, net of
shares withheld for tax liability | | | 1,915 | | | | — | | | | (12,801 | ) | | | — | | | | — | | | | — | | | | — | | | | (12,801 | ) |
Issuance of common stock for private
placement, net of offering costs
of $6,200 | | | 12,421 | | | | 2 | | | | 131,826 | | | | — | | | | — | | | | — | | | | — | | | | 131,828 | |
Shareholder note receivable | | | — | | | | — | | | | — | | | | 4,000 | | | | — | | | | — | | | | — | | | | 4,000 | |
Repurchase of common stock | | | (1,806 | ) | | | — | | | | — | | | | — | | | | (25,000 | ) | | | — | | | | — | | | | (25,000 | ) |
Purchase of capped calls | | | — | | | | — | | | | (39,866 | ) | | | — | | | | — | | | | — | | | | — | | | | (39,866 | ) |
Foreign currency translation adjustments | | | — | | | | — | | | | — | | | | — | | | | — | | | | (7,240 | ) | | | — | | | | (7,240 | ) |
Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (143,032 | ) | | | (143,032 | ) |
Balance at December 31, 2021 | | | 99,537 | | | $ | 10 | | | $ | 892,828 | | | $ | — | | | $ | (25,097 | ) | | $ | (6,036 | ) | | $ | (781,031 | ) | | $ | 80,674 | |
See accompanying notes to consolidated financial statements.
ALPHATEC HOLDINGS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
| | | | | | | | | | | | |
| Year Ended December 31, | |
| | 2023 | | | 2022 | | | 2021 | |
Operating activities: | | | | | | | | | |
Net loss | | $ | (186,638 | ) | | $ | (151,293 | ) | | $ | (143,032 | ) |
Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | | |
Depreciation and amortization | | | 56,139 | | | | 41,168 | | | | 26,756 | |
Stock-based compensation | | | 81,244 | | | | 40,556 | | | | 36,450 | |
Amortization of debt discount and debt issuance costs | | | 3,634 | | | | 2,038 | | | | 1,868 | |
Amortization of right-of-use assets | | | 3,543 | | | | 2,760 | | | | 3,418 | |
Write-down for excess and obsolete inventories | | | 13,608 | | | | 9,792 | | | | 11,147 | |
Loss on disposal of assets | | | 3,708 | | | | 2,594 | | | | 1,976 | |
Loss on debt extinguishment, net | | | — | | | | — | | | | 7,434 | |
Impairment of investment | | | — | | | | — | | | | 3,000 | |
Other | | | 455 | | | | 1,187 | | | | 2,721 | |
Changes in operating assets and liabilities: | | | | | | | | | |
Accounts receivable | | | (12,795 | ) | | | (18,832 | ) | | | (10,141 | ) |
Inventories | | | (45,562 | ) | | | (20,704 | ) | | | (27,746 | ) |
Prepaid expenses and other current assets | | | (11,098 | ) | | | 552 | | | | 1,258 | |
Other assets | | | (541 | ) | | | (109 | ) | | | 11 | |
Accounts payable | | | 6,989 | | | | 9,796 | | | | 757 | |
Accrued expenses and other current liabilities | | | 17,000 | | | | 13,508 | | | | 8,023 | |
Lease liabilities | | | (3,602 | ) | | | (2,678 | ) | | | 150 | |
Contract liabilities | | | 1,793 | | | | (2,280 | ) | | | (1,040 | ) |
Other long-term liabilities | | | (6,362 | ) | | | (3,189 | ) | | | 3,673 | |
Net cash used in operating activities | | | (78,485 | ) | | | (75,134 | ) | | | (73,317 | ) |
Investing activities: | | | | | | | | | |
Purchases of property and equipment | | | (80,508 | ) | | | (49,453 | ) | | | (68,544 | ) |
Purchase of intangible assets | | | (6,467 | ) | | | (8,827 | ) | | | — | |
Acquisition of business, net of cash acquired | | | (55,000 | ) | | | — | | | | (62,133 | ) |
Purchase of OCEANE | | | — | | | | — | | | | (21,097 | ) |
Cash paid for investments | | | — | | | | — | | | | (3,000 | ) |
Settlement of forward contract | | | — | | | | — | | | | (2,988 | ) |
Net cash used in investing activities | | | (141,975 | ) | | | (58,280 | ) | | | (157,762 | ) |
Financing activities: | | | | | | | | | |
Proceeds from Revolving Credit Facility and line of credit | | | 134,000 | | | | 62,500 | | | | — | |
Payment of debt issuance costs | | | (3,321 | ) | | | (1,315 | ) | | | (10,028 | ) |
Proceeds from common stock offerings, net of offering costs | | | 213,181 | | | | — | | | | 131,828 | |
Proceeds from issuance of convertible notes | | | — | | | | — | | | | 316,250 | |
Net cash paid for common stock exercises | | | (1,064 | ) | | | (3,041 | ) | | | (5,963 | ) |
Purchase of capped calls | | | — | | | | — | | | | (39,866 | ) |
Repurchase of common stock | | | — | | | | — | | | | (25,000 | ) |
Proceeds from term debt, net of debt discount | | | 148,473 | | | | — | | | | — | |
Repayment of Revolving Credit Facility and line of credit | | | (119,500 | ) | | | (27,500 | ) | | | — | |
Repayment of OCEANEs | | | (13,315 | ) | | | — | | | | — | |
Repayment of Squadron Medical Term Loan | | | — | | | | — | | | | (45,000 | ) |
Repayment of Inventory Financing Agreement | | | — | | | | — | | | | (8,088 | ) |
Other | | | (1,535 | ) | | | 584 | | | | (2,167 | ) |
Net cash provided by financing activities | | | 356,919 | | | | 31,228 | | | | 311,966 | |
Effect of exchange rate changes on cash | | | (185 | ) | | | (366 | ) | | | (1,404 | ) |
Net change in cash and cash equivalents | | | 136,274 | | | | (102,552 | ) | | | 79,483 | |
Cash and cash equivalents at beginning of year | | | 84,696 | | | | 187,248 | | | | 107,765 | |
Cash and cash equivalents at end of year | | $ | 220,970 | | | $ | 84,696 | | | $ | 187,248 | |
Supplemental disclosure of cash flow information: | | | | | | | | | |
Cash paid for interest | | $ | 17,269 | | | $ | 3,860 | | | $ | 5,027 | |
Cash paid for income taxes | | $ | 333 | | | $ | 272 | | | $ | 223 | |
Supplemental disclosure of noncash investing and financing activities: | | | | | | | | | |
PPP Loan Forgiveness | | $ | - | | | $ | - | | | $ | 4,271 | |
Debt issuance costs | | $ | - | | | $ | 2,760 | | | $ | - | |
Financed insurance | | $ | 1,328 | | | $ | 1,959 | | | $ | - | |
Financed property and equipment | | $ | - | | | $ | 600 | | | $ | - | |
Financed inventory | | $ | - | | | $ | - | | | $ | 4,015 | |
Purchases of property and equipment in accounts payable and accrued expenses | | $ | 10,406 | | | $ | 2,128 | | | $ | 2,577 | |
Purchases of intangible assets | | $ | 99 | | | $ | 750 | | | $ | - | |
Recognition of lease liability | | $ | 424 | | | $ | 1,694 | | | $ | 23,403 | |
Modification of lease liability for lease amendment | | $ | - | | | $ | 4,288 | | | $ | - | |
See accompanying notes to consolidated financial statements.
ALPHATEC HOLDINGS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Organization and Significant Accounting Policies
The Company and Basis of Presentation
The Company
Alphatec Holdings, Inc. (the “Company”), through its wholly owned subsidiaries, Alphatec Spine, Inc. (“Alphatec Spine”), SafeOp Surgical, Inc. (“SafeOp”), and EOS imaging S.A.S.A.S. (“EOS”), is a medical technology company that designs, develops,focused on the design, development, and marketsadvancement of technology for the better surgical treatment of spinal disorders associated with disease and degeneration, congenital deformities, and trauma.disorders. The Company, headquartered in Carlsbad, California, markets its products in the United States ("U.S.") and internationally via a network of independent distributorssales agents and direct sales representatives.
Basis of Presentation and Principles of Consolidation
The consolidated financial statements have been prepared in accordance with generally accepted accounting principles in the United States of America ("U.S. GAAP") and include the accounts of the Company and its wholly owned subsidiaries. The Company translates the financial statements of its foreign subsidiaries using end-of-period exchange rates for assets and liabilities and average exchange rates during each reporting period for results of operations. All intercompany balances and transactions have been eliminated in consolidation. The Company operates in 1one reportable business segment.
Prior-year Adjustment
ReclassificationSubsequent to the issuance of the Company’s consolidated financial statements as of and for the year ended December 31, 2022, the Company identified that a deferred tax liability and additional goodwill related to the acquisition of EOS should have been recorded at the time of the acquisition. The Company corrected the errors in the accompanying consolidated financial statements as of the earliest year presented and has concluded that the correction of these errors is not material to the previously issued financial statements.
The correction to the accompanying consolidated balance sheets, consolidated statements of operations, consolidated statements of comprehensive loss, consolidated statements of stockholders' equity, and consolidated statements of cash flows are as follows (in thousands):
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | At December 31, 2022 | |
Consolidated Balance Sheets | | | | | | | | | As Reported | | Adjustment | | As Corrected | |
Goodwill | | | | | | | | | $ | 39,775 | | | 7,592 | | $ | 47,367 | |
Total assets | | | | | | | | | $ | 513,376 | | | 7,592 | | $ | 520,968 | |
Other long-term liabilities | | | | | | | | | $ | 11,543 | | | 5,546 | | $ | 17,089 | |
Accumulated other comprehensive loss | | | | | | | | | $ | (10,690 | ) | | (104 | ) | $ | (10,794 | ) |
Accumulated deficit | | | | | | | | | $ | (934,474 | ) | | 2,150 | | $ | (932,324 | ) |
Total stockholders' deficit | | | | | | | | | $ | (36,713 | ) | | 2,046 | | $ | (34,667 | ) |
Total liabilities and stockholders' deficit | | | | | | | | | $ | 513,376 | | | 7,592 | | $ | 520,968 | |
| | | | | | | | | | | | | | |
| | Year Ended December 31, 2022 | | | Year Ended December 31, 2021 | |
Consolidated Statements of Operations | | As Reported | | Adjustment | | As Corrected | | | As Reported | | Adjustment | | As Corrected | |
Income tax provision (benefit) | | $ | 140 | | | (856 | ) | $ | (716 | ) | | $ | 164 | | | (1,294 | ) | $ | (1,130 | ) |
Net loss | | $ | (152,149 | ) | | 856 | | $ | (151,293 | ) | | $ | (144,326 | ) | | 1,294 | | $ | (143,032 | ) |
Net loss per share, basic and diluted | | $ | (1.47 | ) | $ | 0.01 | | $ | (1.46 | ) | | $ | (1.50 | ) | $ | 0.01 | | $ | (1.49 | ) |
| | Year Ended December 31, 2022 | | | Year Ended December 31, 2021 | |
Consolidated Statements of Comprehensive Loss | | As Reported | | Adjustment | | As Corrected | | | As Reported | | Adjustment | | As Corrected | |
Net loss | | $ | (152,149 | ) | | 856 | | $ | (151,293 | ) | | $ | (144,326 | ) | | 1,294 | | $ | (143,032 | ) |
Foreign currency translation adjustments | | $ | (4,696 | ) | | (62 | ) | $ | (4,758 | ) | | $ | (7,198 | ) | | (42 | ) | $ | (7,240 | ) |
Comprehensive loss | | $ | (156,845 | ) | | 794 | | $ | (156,051 | ) | | $ | (151,524 | ) | | 1,252 | | $ | (150,272 | ) |
| | | | | | | | | | | | | | |
| | At December 31, 2022 | | | At December 31, 2021 | |
Consolidated Statements of Stockholders' (Deficit) Equity | | As Reported | | Adjustment | | As Corrected | | | As Reported | | Adjustment | | As Corrected | |
Foreign currency translation adjustments | | $ | (4,696 | ) | | (62 | ) | $ | (4,758 | ) | | $ | (7,198 | ) | | (42 | ) | $ | (7,240 | ) |
Net loss | | $ | (152,149 | ) | | 856 | | $ | (151,293 | ) | | $ | (144,326 | ) | | 1,294 | | $ | (143,032 | ) |
Accumulated other comprehensive loss | | $ | (10,690 | ) | | (104 | ) | $ | (10,794 | ) | | $ | (5,994 | ) | | (42 | ) | $ | (6,036 | ) |
Accumulated deficit | | $ | (934,474 | ) | | 2,150 | | $ | (932,324 | ) | | $ | (782,325 | ) | | 1,294 | | $ | (781,031 | ) |
Total stockholders' (deficit) equity | | $ | (36,713 | ) | | 2,046 | | $ | (34,667 | ) | | $ | 79,422 | | | 1,252 | | $ | 80,674 | |
| | | | | | | | | | | | | | |
| | Year Ended December 31, 2022 | | | Year Ended December 31, 2021 | |
Consolidated Statements of Cash Flows | | As Reported | | Adjustment | | As Corrected | | | As Reported | | Adjustment | | As Corrected | |
Net loss | | $ | (152,149 | ) | | 856 | | $ | (151,293 | ) | | $ | (144,326 | ) | | 1,294 | | $ | (143,032 | ) |
Other long-term liabilities | | $ | (2,342 | ) | | (847 | ) | $ | (3,189 | ) | | $ | 4,852 | | | (1,179 | ) | $ | 3,673 | |
Net cash used for operating activities | | $ | (75,143 | ) | | 9 | | $ | (75,134 | ) | | $ | (73,432 | ) | | 115 | | $ | (73,317 | ) |
Effect of exchange rate changes on cash | | $ | (357 | ) | | (9 | ) | $ | (366 | ) | | $ | (1,289 | ) | | (115 | ) | $ | (1,404 | ) |
Reclassification
Certain amountsfinancial statement line items in the consolidated financial statements for the year ended December 31, 20202022 have been reclassifieddisaggregated to conform to the current year’s presentation. These reclassifications were immaterial and have no impact on previously reported results of operations or accumulated deficit.
Recent Developments
0.75% Senior Convertible Notes due 2026
In August 2021, the Company issued $316.3 million principal amount of unsecured senior convertible notes (the “2026 Notes”) with a stated interest rate of 0.75% and a maturity date of August 1, 2026.The net proceeds from the sale of the 2026 Notes were approximately $306.2 million after deducting the offering expenses. See Note 6 for additional information on the 2026 Notes.
Acquisition of EOSF-13
On May 13, 2021, the Company acquired a controlling interest in EOS, pursuant to the Tender Offer Agreement (the “Tender Offer Agreement”) it entered into on December 16, 2020, and in June 2021 purchased the remaining issued and outstanding ordinary shares for a 100% interest in EOS. EOS, which now operates as a wholly owned subsidiary of the Company, is a global medical device company that designs, develops and markets innovative, low dose 2D/3D full body and weight-bearing imaging, rapid 3D modeling of EOS patient X-ray images, web-based patient-specific surgical planning, and integration of surgical plan into the operating room that collectively bridge the entire spectrum of care from imaging to post-operative assessment capabilities for orthopedic surgery. See Note 3 for additional information on the business combination.
F-13
COVID-19 Pandemic
In 2020, a novel strain of Coronavirus, which causes COVID-19, was identified and declared by the World Health Organization to be a pandemic. The virus causing COVID-19 has since rapidly spread across the globe to all countries, including to the United States. To slow the spread of COVID-19, governments have implemented measures, which include the mandatory closure of businesses, and restrictions on travel. In addition, many government agencies in conjunction with hospitals and healthcare systems have, to varying degrees, deferred or suspended elective surgical procedures. While certain spine surgeries are deemed essential and certain surgeries cannot be delayed, the Company has seen and may continue to see a reduction in procedural volumes as hospital systems and/or patients elect to defer spine surgery procedures and hospital systems experience staffing shortages.
The cumulative effect of these disruptions had an impact on the Company’s business during the years ended December 31, 2021 and 2020. The COVID-19 pandemic continues to evolve and its full impact on the Company’s business will depend on several factors that are uncertain and unpredictable, including, the efficacy and adoption of vaccines, future resurgences of the virus and its variants, the speed at which government restrictions are lifted or enacted, and patient capacity at hospitals and healthcare systems.
2. Summary of Significant Accounting Policies
Use of Estimates
The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities, and the reported amounts of revenues and expenses. Actual results could differ from those estimates. Significant items subject to such estimates and assumptions include the useful lives of property and equipment, goodwill, intangible assets, allowances for doubtful accounts, the valuation of share-based liabilities, deferred tax assets, inventory, stock-based compensation, revenues, restructuring liabilities, income tax uncertainties, and other contingencies.
Concentrations of Credit Risk and Significant Customers
Financial instruments, which potentially subject the Company to concentrations of credit risk, consist primarily of cash, cash equivalents, and accounts receivable. The Company limits its exposure to credit loss by depositing its cash and investments with established financial institutions. As of December 31, 2021, a substantial portion of the Company’s available cash funds is held in business accounts. Although the Company deposits its cash with multiple financial institutions, its deposits, at times, may exceed federally insured limits.
The Company’s customers are primarily hospitals and surgical centers, and distributors. NaNcenters. No one single customer represented greater than 10 percent of consolidated revenues and accounts receivable for the years presented. Credit to customers is granted based on an analysis of the customers’ credit worthiness. Credit losses have not been significant.
Cash and Cash Equivalents
The company considers all highly liquid investments that are readily convertible into cash and have an original maturity of three months or less at the time of purchase to be cash equivalents.
Accounts Receivable, net
Accounts receivable are presented net of allowance for doubtful accounts. The Company makes judgments as to its ability to collect outstanding receivables and provides allowances for a portion of receivables when collection becomes doubtful. Provisions are made based upon a specific review of all significant outstanding invoices. In determining the provision for invoices not specifically reviewed, the Company analyzes historical collection experience. If the historical data used to calculate the allowance provided for doubtful accounts does not reflect the Company’s future ability to collect outstanding receivables or if the financial condition of customers were to deteriorate, resulting in impairment of their ability to make payments, an increase in the provision for doubtful accounts may be required.
F-14
Table of Contents
The Company’s accounts receivable generally have net 30-day30-day payment terms. The Company generally does not allow returns of products that have been delivered. The Company offers standard quality assurance warranty on its products. The Company had no material bad debt expense during the years presented.
Excess and there were no material contract assets as of December 31, 2021.
InventoriesObsolete Inventory
Most of the Company’s inventory is comprised of finished goods, which is primarily produced by third-party suppliers. Specialized implants, fixation products, biologics, and disposablesbiologics are determined by utilizing a standard cost method that includes capitalized variances which approximates the weighted average cost. Imaging equipment and related parts are valued at weighted average cost. Inventories are stated at the lower of cost or net realizable value. The Company reviews the components of its inventory on a periodic basis for excess and obsolescence and adjusts inventory to its net realizable value as necessary.
The Company records a lower of cost or net realizable value (“LCNRV”) inventory reserve (“LCNRV”) for estimated excess and obsolete inventory based upon its expected use of inventory on hand. The Company’s inventory, which consists primarily of specialized implants, fixation products, biologics, and disposablesbiologics is at risk of obsolescence due to the need to maintain substantial levels of inventory. In order to market its products effectively and meet the demands of interoperative product placement, the Company maintains and provides surgeons and hospitals with a variety of inventory products and sizes. For each surgery, fewer than all components will be consumed. The need to maintain and provide such a wide variety of inventory causes inventory to be held that is not likely to be used.
The Company’s estimates and assumptions for excess and obsolete inventory are reviewed and updated on a quarterly basis. The estimates and assumptions are determined primarily based on current usage of inventory and the age of inventory quantities on hand. Additionally, the Company considers recent sales experience to develop assumptions about future demand for its products, while considering market conditions, product life cycles and new product launches. Increases in the LCNRV reserve for excess and obsolete inventory result in a corresponding charge to cost of sales. For the years ended December 31, 20212023, 2022 and 2020,2021, the Company recorded a write-downLCNRV charges for excess and obsolete inventory of $11.1$13.6 million, $9.8 million and $7.0$11.1 million, respectively, net of inventory sold of $0.2 million, $1.5 million and $2.5 million, respectively. For the years ended December 31, 2023, 2022 and 2021, the Company recorded a reduction of reserve for inventory that was disposed of $12.9 million, $8.2 million and $2.1 million, respectively.
Property and Equipment, net
Property and equipment are stated at cost, net of accumulated depreciation. Depreciation is computed using the straight-line method over the estimated useful lives of the related assets, generally ranging from three to seven years.years. Leasehold improvements and assets acquired under capitalfinancing leases are amortized over the shorter of their useful lives or the remaining terms of the related leases.
Operating Lease
The Company determines whether a contract is a lease or contains a lease at inception by assessing whether there is an identified asset and whether the contract conveys the right to control the use of the identified asset in exchange for consideration over a period of time. If both criteria are met, the Company determines the initial classification and measurement of its right-of-use (“ROU”) asset and lease liabilitiesliability at the lease commencement date and thereafter, if modified. The Company recognizes a ROU asset and lease liability for its operating leases with lease terms greater than 12 months. The lease term includes any renewal options and termination options that the Company is reasonably assured to exercise. ROU assets and lease liabilities are based on the present value of lease payments over the lease term. The present value of operating lease payments is determined by using the incremental borrowing rate of interest that the Company would borrow on a collateralized basis for an amount equal to the lease payments in a similar economic environment.
Rent expense for operating leases is recognized on a straight-line basis over the reasonably assured lease term based on the total lease payments and is included in cost of sales, research and development, and sales, general and administrative expenses in the consolidated statements of operations.
F-15
Table of Contents
The Company aggregates all lease and non-lease components for each class of underlying assets into a single lease component and variable charges for common area maintenance and other variable costs are recognized as expense as incurred. Total variable costs associated with leases were immaterial for theall years ended December 31, 2021 and 2020 were immaterial.presented. The Company had an immaterial amount of financing leases as of December 31, 20212023 and 2020,2022, which is included in property and equipment, net, accrued expenses and other current liabilities, and other long-term liabilities on the consolidated balance sheets.
Valuation of Goodwill
Goodwill represents the excess of the cost over the fair value of net assets acquired from the Company’s business combinations. The determination of the value of goodwill and intangible assets arising from business combinations and asset acquisitions requires extensive use of accounting estimates and judgments to allocate the purchase price to the fair value of the net tangible and intangible assets acquired. Goodwill is assessed for impairment using fair value measurement techniques on an annual basis or more frequently if facts and circumstance warrant such a review. Goodwill is considered to be impaired if the Company determines that the carrying value of the reporting unit exceeds its respective fair value.
The Company’s annual evaluation for impairment of goodwill consists of one reporting unit. The Company completed its most recent annual evaluation for impairment of goodwill as of October 1, 20212023 and determined that no impairment existed. In addition, no indicators of impairment were noted through December 31, 2021,2023, and consequently 0no impairment charge was recorded during the years ended December 31, 20212023, 2022 and 2020.2021.
Valuation of Intangible Assets
Intangible assets are comprised primarily of purchased technology, customer relationships, trade name, trademarks, and in-process research and development. The Company makes significant judgments in relation to the valuation of intangible assets resulting from business combinations and asset acquisitions. Intangible assets are generally amortized on a straight-line basis over their estimated useful lives of 2 to 12 years.years. The Company bases the useful lives and related amortization expense on the period of time it estimates the assets will generate net sales or otherwise be used. The Company also periodically reviews the lives assigned to its intangible assets to ensure that its initial estimates do not exceed any revised estimated periods from which the Company expects to realize cash flows. If a change were to occur in any of the above-mentioned factors or estimates, the likelihood of a material change in the Company’s reported results would increase. The Company evaluates its intangible assets with finite lives for indications of impairment whenever events or changes in circumstances indicate that the carrying value may not be recoverable. Factors that could trigger an impairment review include significant under-performance relative to expected historical or projected future operating results, significant changes in the manner of the Company’s use of the acquired assets or the strategy for its overall business or significant negative industry or economic trends. If this evaluation indicates that the value of the intangible asset may be impaired, the Company makes an assessment of the recoverability of the net carrying value of the asset over its remaining useful life. If this assessment indicates that the intangible asset is not recoverable, based on the estimated undiscounted future cash flows of the asset over the remaining amortization period, the Company reduces the net carrying value of the related intangible asset to fair value and may adjust the remaining amortization period. Significant judgment is required in the forecasts of future operating results that are used in the discounted cash flow valuation models. It is possible that plans may change and estimates used may prove to be inaccurate. If actual results, or the plans and estimates used in future impairment analyses, are lower than the original estimates used to assess the recoverability of these assets, the Company could incur additional impairment charges. There were 0no impairment charges during the years ended December 31, 20212023, 2022 or 2020.2021.
In-process research and development ("IPR&D") and software in development have indefinite lives and are not amortized until the related products reach full commercial launch or when the projects are complete and their assets are ready for their intended use. Indefinite-lived intangible assets are considered to be impaired if the products do not reach commercial launch, if the project is not completed or not completed in a timely manner, or if the related products or projects are no longer technologically feasible. Impairment related to IPR&D and software in development is calculated as the excess of the asset's carrying value over its fair value. There were no impairment charges during the years ended December 31, 2023, 2022 or 2021.
Impairment of Long-Lived Assets
The Company assesses potential impairment to its long-lived assets when there is evidence that events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. An impairment loss is recognized when estimated future undiscounted cash flows related to the asset are less than its carrying amount. Any required impairment loss is measured as the amount by which the carrying amount of a long-lived asset exceeds its fair value and is recorded as a reduction in the carrying value of the related asset and a charge to operating results. There were 0no material impairment charges during the years ended December 31, 20212023, 2022 or 2020.2021.
Warrants to Purchase Common Stock
Warrants are accounted for in accordance with the applicable accounting guidance as either derivative liabilities or as equity instruments depending on the specific terms of the agreements.
All warrants issued during the year ended December 31, 2020 qualifiedqualify for classification within stockholders’stockholders' equity. There were 0 warrants issued during the year ended December 31, 2021.
Fair Value Measurements
The carrying amount of financial instruments consisting of cash and cash equivalents, accounts receivable, prepaid expenses and other current assets, accounts payable, accrued expenses, and short-term debt included in the Company’s consolidated financial statements are reasonable estimates of fair value due to their short maturities.
Authoritative guidance establishes a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value as follows:
| Level 1:
| Quoted prices in active markets for identical assets or liabilities.
|
Level 1: Quoted prices in active markets for identical assets or liabilities.
| Level 2:
|
Level 2: Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities, quoted prices in markets that are not active; or other inputs that can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3: Unobservable inputs that are supported by observable market data for substantially the full term of the assets or liabilities. |
| Level 3:
| Unobservable inputs in which there is little or no market activity and that are significant to the fair value of the assets or liabilities.
|
The following table presents information related to the Company’s assets measured at fair value on a recurring basis as of December 31, 2021 (in thousands):
| | December 31, 2021 | |
| | Level 1 | | | Level 2 | | | Level 3 | | | Total | |
Cash equivalents: | | | | | | | | | | | | | | | | |
Money market funds | | $ | 140,010 | | | | — | | | | — | | | $ | 140,010 | |
Total cash equivalents | | $ | 140,010 | | | | — | | | | — | | | $ | 140,010 | |
There were no assets measured at fair value on a recurring basis as of December 31, 2020.
The following table presents information related to the Company’s liabilities measured at fair value on a recurring basis as of December 31, 2021 and December 31, 2020 (in thousands):
| | December 31, 2021 | |
| | Level 1 | | | Level 2 | | | Level 3 | | | Total | |
Liability classified equity award | | $ | — | | | | — | | | | 2,052 | | | $ | 2,052 | |
| | | | | | | | | | | | | | | | |
| | December 31, 2020 | |
| | Level 1 | | | Level 2 | | | Level 3 | | | Total | |
Liability classified equity award (1) | | $ | — | | | | — | | | | 4,108 | | | $ | 4,108 | |
Foreign currency forward contract | | | — | | | | 878 | | | | — | | | | 878 | |
Total | | $ | — | | | | 878 | | | | 4,108 | | | $ | 4,986 | |
(1) A portion of this award is being accreted over the requisite service period. The amount in the above table includes the fair value of the vested and unvested portion of the award.
The Company did not have any transfers of assets and liabilities between the levels of the fair value measurement hierarchy during the periods presented.or liabilities.
F-17
Table of Contents
On March 16, 2021, the Company entered into 2 foreign currency forward contracts, with a notional amount of $8.0 million total, $4.0 million each (€6.7 million total and €3.3 million each), to mitigate the foreign currency exchange risk related to its EOS subsidiary. The contracts are not designated as hedging instruments. The Company classified the derivative liabilities within Level 2 of the fair value hierarchy as observable inputs are available for the full term of the derivative instruments. The fair value of the forward contracts was developed using a market approach based on publicly available market yield curves and the term of the contracts. During the year ended December 31, 2021, the foreign currency forward contracts were settled for $7.6 million (€6.7 million). The Company recognized a nominal loss from the change in fair value of the contracts during the year ended December 31, 2021. The loss on the contract settlement was recorded within other expense, net on the consolidated statements of operations and the cash settlement is included in investing activities in the consolidated statements of cash flows for the year ended December 31, 2021.
On December 18, 2020, the Company entered into a foreign currency forward contract, with a notional amount of $117.9 million (€95.6 million) to mitigate the foreign currency exchange risk related to the Tender Offer Agreement, denominated in Euros. The contract was not designated as a hedging instrument. The Company classified the derivative liability within Level 2 of the fair value hierarchy as observable inputs were available for the full term of the derivative instrument. The fair value of the forward contract was developed using a market approach based on publicly available market yield curves and the term of the contract. On March 2, 2021, the foreign currency forward contract was settled for $115.3 million (€95.6 million). The Company recognized a $1.7 million loss from the change in fair value of the contract during the year ended December 31, 2021. The loss on the contract settlement was recorded as other expense on the consolidated statement of operations and the cash settlement is included in investing activities in the consolidated statements of cash flows for the year ended December 31, 2021.
The Company issued a liability classified equity award to one of its executive officers. The award vests in 2023 subject to continued service and a specific market condition. As the award will be settled in cash, it is classified as a liability within Level 3 of the fair value hierarchy as the Company is using a probability-weighted income approach, utilizing significant unobservable inputs including the probability of achieving the specific market condition with the valuation updated at each reporting period. The full fair value of the award was $3.1 million as of December 31, 2021 and is being recognized ratably as the underlying service period is provided.Revenue Recognition
The following table provides a reconciliation of liabilities measured at fair value using significant unobservable inputs (Level 3) as of December 31, 2021 and 2020 (in thousands):
| | Level 3 Liabilities | |
Balance at December 31, 2019 | | $ | 266 | |
Straight-line recognition of liability classified equity award | | | 371 | |
Change in fair value measurement | | | 1,031 | |
Balance at December 31, 2020 | | $ | 1,668 | |
Straight-line recognition of liability classified equity award | | | 1,028 | |
Change in fair value measurement | | | (644 | ) |
Balance at December 31, 2021 | | $ | 2,052 | |
Fair Value of Long-term Debt
The fair value, based on a quoted market price (Level 1), of the Company’s 2026 Notes at December 31, 2021 was approximately $308.1 million. The fair value based on a quoted market price (Level 1), of the Company’s outstanding OCEANEs at December 31, 2021 was approximately $14.1 million. See Note 6 for further information.
F-18
Table of Contents
Revenue Recognition
The Company recognizes revenue from product sales in accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) Revenue from Contracts with Customers (“Topic 606”). This standard applies to all contracts with customers, except for contracts that are within the scope of other standards, such as leases, insurance, collaboration arrangements, and financial instruments. Under Topic 606, an entity recognizes revenue when its customer obtains control of promised goods or services, in an amount that reflects the consideration that the entity expects to receive in exchange for those goods or services. To determine revenue recognition for arrangements that an entity determines are within the scope of Topic 606, the entity performs the following five steps: (i) identify the contract(s) with a customer; (ii) identify the performance obligations in the contract; (iii) determine the transaction price; (iv) allocate the transaction price to the performance obligations in the contract; and (v) recognize revenue when (or as) the entity satisfies a performance obligation. The Company only applies the five-step model to contracts when it is probable that the entity will collect the consideration it is entitled to in exchange for the goods or services it transfers to the customer.
Sales are derived primarily from the sale of spinal implant products, imaging equipment, and related services to hospitals and medical centers through direct sales representatives and independent distributor agents, and with the acquisition of EOS, includes imaging equipment and related services.sales agents. Revenue is recognized when obligations under the terms of a contract with customers are satisfied, which occurs with the transfer of control of products to customers, either upon shipment of the product or delivery of the product to the customer depending on the shipping terms, or when the products are used in a surgical procedure (implanted in a patient). Revenue from the sale of imaging equipment is recognized as each distinct performance obligation is fulfilled and control transfers to the customer, beginning with shipment or delivery, depending on the terms. Revenue from other distinct performance obligations, such as maintenance on imaging equipment and other imaging related services, is recognized in the period the service is performed, and makes up less than 10%10% of the Company’s total revenue. Revenue is measured based on the amount of consideration expected to be received in exchange for the transfer of the goods or services specified in the contract with each customer. In certain cases, the Company does offer the ability for customers to lease its imaging equipment primarily on a non-sales type basis, but such arrangements are immaterial to total revenue in the periodsyears presented. The Company generally does not allow returns of products that have been delivered. Costs incurred by the Company associated with sales contracts with customers are deferred over the performance obligation period and recognized in the same period as the related revenue, except for contracts that complete within one year or less, in which case the associated costs are expensed as incurred. Payment terms for sales to customers may vary but are commensurate with the general business practices in the country of sale.
To the extent that the transaction price includes variable consideration, such as discounts, rebates, and customer payment penalties, the Company estimates the amount of variable consideration that should be included in the transaction price utilizing either the expected value method or the most likely amount method depending on the nature of the variable consideration. Variable consideration is included in the transaction price if, in the Company’s judgment, it is probable that a significant future reversal of cumulative revenue under the contract will not occur. Estimates of variable consideration and determination of whether to include estimated amounts in the transaction price are based largely on an assessment of the Company’s anticipated performance and all information that is reasonably available, including historical, current, and forecasted information.
The Company records a contract asset when one or more performance obligations have been completed and revenue has been recognized, but the customer's payment is contingent on the satisfaction of additional performance obligations. The Company records a contract liability, or deferred revenue, when it has an obligation to provide a product or service to the customer and payment is received in advance of its performance. When the Company sells a product or service with a future performance obligation, revenue is deferred on the unfulfilled performance obligation and recognized over the related performance period. Generally, the Company does not have observable evidence of the standalone selling price related to its future service obligations; therefore, the Company estimates the selling price using an expected cost plus a margin approach. The transaction price is allocated using the relative standalone selling price method. The use of alternative estimates could result in a different amount of revenue deferral. The Company had current and non-current contract liability balances totaling $15.3 million and $2.9 million, respectively, as of December 31, 2021. The Company had no contract liability balances as of December 31, 2020. The non-current contract liability balance is included in other long-term liabilities on the consolidated balance sheets. The Company recognized $14.6 million of revenue from its contract liabilities during the year ended December 31, 2021. The Company did not recognize revenue related to contract liabilities during the year ended December 31, 2020.
F-19
Table of Contents
The opening and closing balances of the Company’s contract liability are as follows:
Balance at January 1, 2021 | | $ | — | |
Contract liability assumed in acquisition of EOS | | | 21,196 | |
Payments received | | | 11,590 | |
Revenue recognized | | | (14,635 | ) |
Balance at December 31, 2021 | | $ | 18,151 | |
Research and Development Expenses
Research and development costs are expensed as incurred. Research and development expenses consist of costs associated with the design, development, testing, and enhancement of the Company’s products and technologies.Company's products. Research and development costsexpenses also include salaries and related employee benefits, research-related overhead expenses, and fees paid to external service providers. Researchproviders and development consultants in the form of both cash and equity.
Transaction-related Expenses
Transaction-related costs are expensed as incurred.
Litigation-related Expenses
Litigation-related Transaction-related expenses are costs incurred for the ongoing litigation, primarily with NuVasive, Inc. See Note 7 for additional information on ongoing litigation.
Transaction-related Expenses
The Company expensedconsist of certain costs incurred throughout the year related primarily to the acquisition and integration of EOS. These expenses primarily include third-party advisoryValence, as defined below, and legal feesEOS..
Product Shipment Cost
Product shipment costs for surgical sets are included in sales, general and administrative expenses in the accompanying consolidated statements of operations. Product shipment costs totaled $8.3$17.3 million, $14.8 million and $5.3$8.3 million for the years ended December 31, 2023, 2022 and 2021 and 2020, respectively.
Stock-Based Compensation
Stock-based compensation expense for equity-classified awards, principally related to restricted stock units or RSUs,("RSUs") and performance restricted stock units or PRSUs,("PRSUs") is measured at the grant date based on the estimated fair value of the award. The fair value of equity instruments that are expected to vest is recognized and amortized over the requisite service period. The Company has granted awards with up to four year graded or cliff vesting terms. No exercise price or other monetary payment is required for receipt of the shares issued in settlement of the respective award; instead, consideration is furnished in the form of the participant’s service.
The fair value of RSUs including PRSUs with pre-defined performance criteria is based on the stock price on the date of grant whereas the expense for PRSUs with pre-defined performance criteria is adjusted with the probability of achievement of such performance criteria at each period end. The fair value of the PRSUs that are earned based on the achievement of pre-defined market conditions, is estimated on the date of grant using a Monte Carlo valuation model. The key assumptions in applying this model are an expected volatility and a risk-free interest rate.grant.
Stock-based compensation recorded in the Company’s consolidated statements of operations is based on awards expected to ultimately vest and has been reduced for estimated forfeitures. The Company’s estimated forfeiture rates may differ from its actual forfeitures. The Company considers its historical experience of pre-vesting forfeitures on awards by each homogenous group of employees as the basis to arrive at its estimated annual pre-vesting forfeiture rates.
F-20
Table of Contents
The Company estimates the fair value of stock options issued under the Company’s equity incentive plans and shares issued to employees under the Company’s employee stock purchase plan (“ESPP”) using a Black-Scholes option-pricing model on the date of grant. The Black-Scholes option-pricing model incorporates various assumptions including expected volatility, expected term and risk-free interest rates. The expected volatility is based on the historical volatility of the Company’s common stock over the most recent period commensurate with the estimated expected term of the Company’s stock options and ESPP offering period which is derived from historical experience. The risk-free interest rate for periods within the contractual life of the option is based on the U.S. Treasury yield in effect at the time of grant. The Company has never declared or paid dividends and havehas no plans to do so in the foreseeable future.
The Company accounts for stock option grantsAwards to non-employees in accordance withare accounted for under the same stock-based compensation provisions as employees, which require that the fair value of these instruments be recognized as an expense overwhen earned. For Development Service Agreements, where the periodfuture payments for product and/or intellectual property rights may be paid in whicheither cash or restricted shares of our common stock at the relatedelection of the developer, we estimate the fair value of those awards similar to a stock option, using a Black-Scholes option-pricing model on the date of grant. For Development Service Agreements where the future payments for product and/or intellectual property rights will be paid in restricted shares of our common stock, the fair value is based on the stock price on the date of grant. The stock-based compensation expense is recognized as earned once the award is deemed probable of achieving the pre-defined performance criteria. The stock-based compensation expense is included in cost of sales or research and development expense on the consolidated statements of operations commensurate with the nature of the services are rendered.performed.
Income Taxes
The Company accounts for income taxes in accordance with provisions which set forth an asset and liability approach that requires the recognition of deferred tax assets and deferred tax liabilities for the expected future tax consequences of temporary differences between the carrying amounts and the tax bases of assets and liabilities. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized. In making such determination, a review of all available positive and negative evidence must be considered, including scheduled reversal of deferred tax liabilities, projected future taxable income, tax planning strategies, and recent financial performance.
The Company recognizes interest and penalties related to uncertain tax positions as a component of the income tax provision.
Net Loss per Share
Basic earningsnet loss per share (“EPS”) is calculated by dividing the net loss available to common stockholders by the weighted averageweighted-average number of shares of common stock issued andshares outstanding for the period. Diluted EPSyear. If applicable, diluted net loss per share attributable to common stockholders is computedcalculated by dividing the net loss available to common stockholders by the weighted averagediluted weighted-average number of shares of common stockshares outstanding for the period and the weighted average number of dilutive common stock equivalents outstanding for the periodyear determined using the treasury-stock method and the if-converted method for convertible debt. For purposes of this calculation, common stock subject to repurchase by the Company, common stock issuable upon conversion or exercise of convertible notes, preferred shares, options, and warrants are considered to be common stock equivalents and are only included in the calculation of diluted earnings per share when their effect is dilutive. Due to the Company’s net loss position, the effect of including common stock equivalents in the EPSearnings per share calculation is anti-dilutive, and therefore not included.
The following table sets forth the computation of basic and diluted loss per share (in thousands, except per share data):
| | | | | | | | | | | | |
| | Year Ended December 31, | |
| | 2023 | | | 2022 | | | 2021 | |
Numerator: | | | | | | | | | |
Net loss | | $ | (186,638 | ) | | $ | (151,293 | ) | | $ | (143,032 | ) |
Denominator: | | | | | | | | | |
Weighted average common shares outstanding | | | 121,242 | | | | 103,373 | | | | 96,197 | |
Net loss per share, basic and diluted | | $ | (1.54 | ) | | $ | (1.46 | ) | | $ | (1.49 | ) |
| | Year Ended December 31, | |
| | 2021 | | | 2020 | |
Numerator: | | | | | | | | |
Net loss | | $ | (144,326 | ) | | $ | (78,994 | ) |
Denominator: | | | | | | | | |
Weighted average common shares outstanding | | | 96,197 | | | | 67,020 | |
Net loss per share, basic and diluted | | $ | (1.50 | ) | | $ | (1.18 | ) |
F-21
Table of Contents
The following potentially dilutive shares of common stock were excluded from the calculation of diluted net loss per share because their effect would have been anti-dilutive for the years presented (in thousands):
| | | | | | | | | | | | |
| | Year Ended December 31, | |
| | 2023 | | | 2022 | | | 2021 | |
Series A convertible preferred stock | | | — | | | | — | | | | 29 | |
Options to purchase common stock and employee stock purchase plan | | | 2,555 | | | | 2,991 | | | | 3,416 | |
Unvested restricted stock units | | | 7,713 | | | | 8,533 | | | | 8,703 | |
Warrants to purchase common stock | | | 8,219 | | | | 15,491 | | | | 20,184 | |
Senior convertible notes | | | 17,246 | | | | 17,246 | | | | 17,246 | |
| | | 35,733 | | | | 44,261 | | | | 49,578 | |
| | Year Ended December 31, | |
| | 2021 | | | 2020 | |
Series A convertible preferred stock | | | 29 | | | | 29 | |
Options to purchase common stock and employee stock purchase plan | | | 3,416 | | | | 3,951 | |
Unvested restricted stock units | | | 8,703 | | | | 8,216 | |
Warrants to purchase common stock | | | 20,184 | | | | 24,881 | |
Senior convertible notes | | | 17,246 | | | | — | |
| | | 49,578 | | | | 37,077 | |
Recent Accounting Pronouncements
Recently Adopted Accounting Pronouncements
In May 2021, the FASB issued Accounting Standard Update (“ASU”) No. 2021-04, Earnings Per Share (Topic 260), Debt—Modifications and Extinguishments (Subtopic 470-50), Compensation—Stock Compensation (Topic 718), and Derivatives and Hedging—Contracts in Entity’s Own Equity (Subtopic 815-40): Issuer’s Accounting for Certain Modifications or Exchanges of Freestanding Equity-Classified Written Call Options. The guidance clarifies and reduces diversity in an issuer’s accounting for modifications or exchanges of freestanding equity-classified written call options (i.e., warrants) that remain equity classified after a modification or exchange and provides guidance that clarifies whether an issuer should account for a modification or an exchange of a freestanding equity-classified written call option that remains equity classified after modification or exchange as (1) an adjustment to equity and, if so, the related earnings per share (EPS) effects, if any, or (2) an expense and, if so, the manner and pattern of recognition. The new guidance is effective for annual and interim periods beginning after December 15, 2021, and early adoption is permitted, including adoption in an interim period. The Company early adopted ASU 2021-04 on January 1, 2021 on a prospective basis. There were no changes to the consolidated financial statements as of January 1, 2021 as a result of the adoption.
In August 2020, the FASB issued ASU No. 2020-06, Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging-Contracts in Entity’s Own Equity (Subtopic 815-40) (“ASU 2020-06”), which simplifies the accounting for convertible instruments. The guidance removes certain accounting models that separate the embedded conversion features from the host contract for convertible instruments. ASU 2020-06 allows for a modified or full retrospective method of transition. This update is effective for fiscal years beginning after December 15, 2021, and interim periods within those fiscal years, and early adoption is permitted. The Company early adopted ASU 2020-06 on January 1, 2021, electing the modified transition method that allows for a cumulative-effect adjustment in the period of adoption. There were no changes to the consolidated financial statements as of January 1, 2021 as a result of the adoption.
Recently Issued Accounting Pronouncements
In August 2021, the FASB issued ASUAccounting Standards Update ("ASU") No. 2021-08, Business Combinations (Topic 805): Accounting for Contract Assets and Contract Liabilities from Contracts with Customers. The guidance requires application of ASCTopic 606, “RevenueRevenue from Contracts with Customers”Customers to recognize and measure contract assets and contract liabilities acquired in a business combination. ASU No. 2021-08 adds an exception to the general recognition and measurement principle in ASCTopic 805 where assets acquired and liabilities assumed in a business combination, including contract assets and contract liabilities arising from contracts with customers, are measured at fair value on the acquisition date. Under the new guidance, the acquirer will recognize acquired contract assets and contract liabilities as if the acquirer had originated the contract. The standard is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2022, with early adoption permitted. The Company adoptedASU No. 2021-08 as of January 1, 2023, on a prospective basis. The adoption of ASU No. 2021-08 did not have a material impact on the Company's consolidated financial statements and related disclosures.
In December 2023, the FASB issued ASU No. 2023-09, Income Taxes (Topic 740): Improvement to Income Tax Disclosures to enhance the transparency of income tax disclosures. The guidance in ASU No. 2023-09 allows for a prospective method of transition, with the option to apply the standard retrospectively. The standard is effective for fiscal years beginning after December 15, 2024, with early adoption permitted. The Company does not intend to early adopt the standard and is in the process of assessing the impact if any, on its consolidated financial statements and related disclosures.
In November 2023, the FASB issued ASU No. 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures that expands disclosure requirements for reportable segments, primarily through enhanced disclosure of significant segment expenses. The guidance in ASU No. 2023-07 allows for a retrospective method of transition. The standard is effective for fiscal years beginning after December 15, 2023 and interim periods in fiscal years beginning after December 15, 2024, with early adoption permitted. The Company does not intend to early adopt the standard and is in the process of assessing the impact on its consolidated financial statements and related disclosures.
3.In October 2023, the FASB issued ASU No. 2023-06, Disclosure Improvements - Codification Amendments in Response to the SEC’s Disclosure Update and Simplification Initiative. The guidance amends the ASC to incorporate certain disclosure requirements from SEC Release No. 33-10532 Disclosure Update and Simplification issued in 2018. The effective date for each amendment will be the date on which the Securities and Exchange Commission's removal of that related disclosure from Regulation S-X or Regulation S-K becomes effective, with early adoption prohibited. ASU No. 2023-06 is not expected to have a material impact on the Company's financial statements.
2. Business Combination
The Company recognizes assets acquired, liabilities assumed, and any noncontrolling interest at fair value at the date of acquisition.
On December 16, 2020,April 19, 2023, the Company entered into the Tender Offeran Asset Purchase Agreement with EOS, pursuant to whichIntegrity Implants Inc. and Fusion Robotics, LLC (collectively, the “Sellers”), whereby the Company agreed to commenceacquired certain assets, liabilities, employees, and contracts in connection with the Sellers’ navigation-enabled robotics platform (“Valence”). The Company paid the Sellers cash consideration of $55.0 million at closing, which represented the total purchase consideration. The acquisition was accounted for as a public tender offer (the “Offer”) to purchase all of the issuedbusiness combination and outstanding ordinary shares, nominal value €0.01 per share (collectively, the “EOS Shares”) for a cash offer of €2.45 per EOS Share, and outstanding convertible bonds of EOS (“OCEANEs”) for a cash offer of €7.01 per OCEANE, which included accrued but unpaid interest. On May 13, 2021 (the “Change in Control Date”), the Company substantially completed the Offer, pursuant to which the Company purchased 59% of the issued and outstanding EOS Shares and 53% of the OCEANEs for $66.5 milliondid not acquire any material assets or assume any material liabilities in cash pursuant to the Offer. In addition, prior to the Change in Control Date, the Company had also acquired 30% of the issued and outstanding EOS Shares and 4% of the OCEANEs on the open market for $25.0 million in cash. After the Change in Control Date, the Company held a controlling financial interest in EOS representing 89% of issued and outstanding EOS Shares and 57% of OCEANEs, equal to approximately 80% of the capital and voting rights of EOS on a fully diluted basis. The Offer was reopened on May 17, 2021 to purchase the remaining EOS Shares for $8.5 million, ultimately resulting inconnection with the acquisition, of 100% of EOS Sharesexcluding intangible assets and 57% ofgoodwill. The acquisition is treated as an asset purchase for income tax purposes; therefore, the OCEANEs as of June 2, 2021. As of June 2, 2021, the total cash paidgoodwill recorded is considered deductible for income tax purposes. Refer to acquire 100% of the EOS SharesNote 4 for further details on intangible assets and 57% of the OCEANEs was $100.0 million.goodwill acquired.
EOS, which now operates as a wholly owned subsidiary of the Company, is a global medical device company that designs, develops and markets innovative, low dose 2D/3D full body and biplanar weight-bearing imaging, rapid 3D modeling of EOS patient X-ray images, web-based patient-specific surgical planning, and integration of surgical plan into the operating room that collectively bridge the entire spectrum of care from imaging to post-operative assessment capabilities for orthopedic surgery. The Company plans to integrate this technology into its procedural approach to spine surgery to better inform and better achieve spinal alignment objectives in surgery.
F-23
Table of Contents
The Company is still in the process of finalizing the purchase price allocation given the timing of the acquisition and the size and scope of the assets and liabilities subject to valuation.allocation. While the Company does not expect material changes in the valuation outcome, of the valuation, certain assumptions and findings that were in place at the date of acquisition maycould result in changes in the purchase price allocation.
In May 2021, the Company completed the acquisition of EOS. The allocationCompany paid cash of $100.0 million to acquire 100% of all issued and outstanding ordinary shares and 57% of the purchase price to the assets acquiredoutstanding convertible bonds of EOS ("OCEANEs").
3. Fair Value Measurements
Assets and liabilities assumed basedmeasured at fair value on their fair values werea recurring basis include the following as follows:of December 31, 2023 and 2022 (in thousands):
(in thousands) | | As of May 13, 2021 | |
Cash paid for purchase of EOS shares at Change in Control Date | | $ | 46,908 | |
Cash paid for purchase of OCEANEs at Change in Control Date | | | 19,620 | |
Total cash paid at Change in Control Date | | | 66,528 | |
Fair value of investment in EOS Shares held prior to Change in Control Date | | | 23,549 | |
Fair value of investment in OCEANEs held prior to Change in Control Date | | | 1,477 | |
Total fair value of investment in EOS held prior to Change in Control Date | | | 25,026 | |
Fair value of noncontrolling interest acquired after Change in Control Date | | | 8,454 | |
| | $ | 100,008 | |
Cash and cash equivalents | | $ | 16,778 | |
Accounts receivable | | | 9,083 | |
Inventory | | | 26,531 | |
Other current assets | | | 4,422 | |
Property, plant and equipment, net | | | 1,650 | |
Deferred tax assets | | | 2,314 | |
Right-of-use asset | | | 4,341 | |
Goodwill | | | 27,841 | |
Definite-lived intangible assets: | | | | |
Developed technology | | | 56,000 | |
Customer relationships | | | 9,500 | |
Trade names | | | 6,000 | |
Other noncurrent assets | | | 395 | |
Contract liabilities | | | 21,196 | |
Long-term debt | | | 15,297 | |
Other liabilities assumed | | | 28,354 | |
Total identifiable net assets | | $ | 100,008 | |
| | | | | | | | | | | | | | | | |
| | December 31, 2023 | |
| | Level 1 | | | Level 2 | | | Level 3 | | | Total | |
Cash equivalents: | | | | | | | | | | | | |
Money market funds | | $ | 76,662 | | | | — | | | | — | | | $ | 76,662 | |
Total cash equivalents | | $ | 76,662 | | | | — | | | | — | | | $ | 76,662 | |
| | | | | | | | | | | | |
| | December 31, 2022 | |
| | Level 1 | | | Level 2 | | | Level 3 | | | Total | |
Cash equivalents: | | | | | | | | | | | | |
Money market funds | | $ | 62,956 | | | | — | | | | — | | | $ | 62,956 | |
Total cash equivalents | | $ | 62,956 | | | | — | | | | — | | | $ | 62,956 | |
The purchase price, including cash paid atCompany did not have any transfers of assets and liabilities between the Change in Control Date,levels of the fair value measurement hierarchy during the years presented.
Fair Value of Convertible Debt
The fair value, based on a quoted market price (Level 1), of the investment held prior to the Change in Control Date,Company’s outstanding 0.75% Convertible Senior Notes due 2026 (the "2026 Notes") was approximately $335.4 million at December 31, 2023 and theapproximately $288.8 million at December 31, 2022. The fair value, based on a quoted market price (Level 1), of the noncontrolling interest acquired, exceeded the fair value of the net tangible and identifiable intangible assets acquired as part of the acquisition. As a result, the Company recorded goodwill in connection with the acquisition. Goodwill primarily consists of expected revenue synergies resulting from the combination of product portfolios and cost synergies related to elimination of redundant facilities and functions associated with the combined entity. Goodwill recognized in this transaction is not deductible for tax purposes. The intangible assets acquired will be amortized on a straight-line basis over useful lives of ten years, seven years and ten years for technology-based, customer-related, and trade name related intangible assets, respectively. The estimated fair values of the intangible assets acquired were primarily determined using the income approach based on significant inputs that were not observable in the market.
Acquisition related costs of $5.8Company’s outstanding OCEANEs was approximately $13.3 million were recognized during the year endedat December 31, 2021, as transaction-related expenses on the consolidated statements2022. As of operations. The Company’s results of operations for the year ended December 31, 2021 included the operating results of EOS of $30.0 million of revenue and a net loss of $17.6 million in the consolidated statement of operations.2023, no OCEANEs remained outstanding. See Note 6 for further information.
The following table presents the unaudited pro forma results for the years ended December 31, 2021 and 2020, which combines the historical results of operations of the Company and its wholly owned subsidiaries as though the companies had been combined as of January 1, 2020. The pro forma information is presented for informational purposes only and is not indicative of the results of operations that may have been achieved if the acquisition had taken place at such time. The unaudited pro forma results presented include non-recurring adjustments directly attributable to the business combination, including $3.1 million in amortization charges for acquired intangible assets, a $2.0 million adjustment related to the increased fair value of acquired inventory and $14.1 million in acquisition related expenses. The unaudited pro forma results include IFRS to U.S. GAAP adjustments for EOS historical results and adjustments for accounting policy alignment, which were materially similar to the Company. Any differences in accounting policies were adjusted to reflect the accounting policies of the Company in the unaudited pro forma results presented.
| | December 31, | |
(in thousands, except per share amounts) | | 2021 | | | 2020 | |
Total revenue | | $ | 251,906 | | | $ | 172,052 | |
Net loss | | | (140,441 | ) | | | (111,967 | ) |
Net loss per share, basic and diluted | | $ | (1.46 | ) | | $ | (1.17 | ) |
4. Balance Sheet Details
Accounts Receivable, net
Accounts receivable, net consist of the following (in thousands):
Inventories
| | December 31, | |
| | 2021 | | | 2020 | |
Accounts receivable | | $ | 44,200 | | | $ | 23,887 | |
Allowance for doubtful accounts | | | (2,307 | ) | | | (360 | ) |
Accounts receivable, net | | $ | 41,893 | | | $ | 23,527 | |
Inventories
Inventories consist of the following (in thousands):
| | | December 31, | | | December 31, | |
| | 2021 | | | 2020 | | | 2023 | | | 2022 | |
Raw materials | | $ | 14,671 | | | $ | 6,064 | | | $ | 23,394 | | | $ | 13,928 | |
Work-in-process | | | 5,712 | | | | 1,982 | | | | 950 | | | | 3,032 | |
Finished goods | | | 71,320 | | | | 37,955 | | | | 112,498 | | | | 84,561 | |
Inventories | | $ | 91,703 | | | $ | 46,001 | | | $ | 136,842 | | | $ | 101,521 | |
F-25
Table of Contents
Property and Equipment, net
Property and equipment, net consist of the following (in thousands, except as indicated):
| | | Useful lives | | | December 31, | | | Useful Life | | December 31, | |
| | (in years) | | | 2021 | | | 2020 | | | (in years) | | 2023 | | | 2022 | |
Surgical instruments | | | 4 | | | $ | 130,432 | | | $ | 76,669 | | | 4 | | $ | 224,357 | | | $ | 158,906 | |
Machinery and equipment | | | 7 | | | | 11,092 | | | | 6,562 | | | 7 | | | 11,633 | | | | 9,502 | |
Computer equipment | | | 3 | | | | 5,694 | | | | 4,206 | | | 3 | | | 5,778 | | | | 4,753 | |
Office furniture and equipment | | | 5 | | | | 3,861 | | | | 1,380 | | | 5 | | | 6,225 | | | | 4,760 | |
Leasehold improvements | | various | | | | 1,754 | | | | 1,761 | | | various | | | 3,986 | | | | 2,965 | |
Construction in progress | | n/a | | | | 7,292 | | | | 2,738 | | | n/a | | | 24,732 | | | | 15,360 | |
| | | | | | | 160,125 | | | | 93,316 | | | | 276,711 | | | | 196,246 | |
Less: accumulated depreciation | | | | | | | (72,724 | ) | | | (56,646 | ) | | | (126,876 | ) | | | (94,294 | ) |
Property and equipment, net | | | | | | $ | 87,401 | | | $ | 36,670 | | | $ | 149,835 | | | $ | 101,952 | |
Total depreciation expense was $20.3$40.9 million, $31.0 million and $9.2$20.3 million for the years ended December 31, 20212023, 2022 and 2020,2021 respectively. At December 31, 20212023 and 2020,2022, assets recorded under capitalfinancing leases of $0.4$1.1 million and $0.1$0.4 million, respectively, were included in the machineryproperty and equipment, net, balance. Amortization of assets under capitalfinancing leases is included in depreciation expense.
Intangible Assets, net
Intangible assets, net consist of the following (in thousands, except as indicated):
Goodwill
| | Remaining Avg. Useful lives | | | Gross | | | Accumulated | | | Intangible | |
December 31, 2021: | | (in years) | | | Amount | | | Amortization | | | Assets, net | |
Developed product technology | | | 12 | | | $ | 74,543 | | | $ | (5,768 | ) | | $ | 68,775 | |
Trademarks and trade names | | | 10 | | | | 5,732 | | | | (477 | ) | | | 5,255 | |
Customer relationships | | | 5 | | | | 14,732 | | | | (5,264 | ) | | | 9,468 | |
Distribution network | | | 3 | | | | 2,413 | | | | (1,840 | ) | | | 573 | |
In-process research and development | | n/a | | | | 1,203 | | | | — | | | | 1,203 | |
Total | | | | | | $ | 98,623 | | | $ | (13,349 | ) | | $ | 85,274 | |
| | | | | | | | | | | | | | | | |
| | Remaining Avg. Useful lives | | | Gross | | | Accumulated | | | Intangible | |
December 31, 2020: | | (in years) | | | Amount | | | Amortization | | | Assets, net | |
Developed product technology | | | 12 | | | $ | 35,376 | | | $ | (23,056 | ) | | $ | 12,320 | |
License agreements | | | 1 | | | | 5,536 | | | | (965 | ) | | | 4,571 | |
Trademarks and trade names | | | — | | | | 792 | | | | (119 | ) | | | 673 | |
Customer relationships | | | 3 | | | | 7,458 | | | | (3,968 | ) | | | 3,490 | |
Distribution network | | | 2 | | | | 4,027 | | | | (1,639 | ) | | | 2,388 | |
In-process research and development | | n/a | | | | 1,278 | | | | — | | | | 1,278 | |
Total | | | | | | $ | 54,467 | | | $ | (29,747 | ) | | $ | 24,720 | |
During the year ended December 31, 2021, in connection with the expiration and termination of the Supply Agreement with Globus Medical, defined below, the Company wrote off $32.6 million in fully amortized intangible assets. During the year ended December 31, 2021, in connection with the Company’s acquisition of EOS, as further described in Note 3, the Company recorded additions to definite-lived intangible assets in the amount of $71.5 million.
Total expense related to amortization of intangible assets was $6.4 million and $1.8 million for the years ended December 31, 2021 and 2020, respectively. In-process research and development intangibles begin amortizing when the relevant products reach full commercial launch.
F-26
Table of Contents
Future amortization expense related to intangible assets as of December 31, 2021 is as follows (in thousands):
Year Ending December 31, | | | | |
2022 | | $ | 9,436 | |
2023 | | | 9,436 | |
2024 | | | 9,333 | |
2025 | | | 8,748 | |
2026 | | | 8,748 | |
Thereafter | | | 39,573 | |
Total | | $ | 85,274 | |
Goodwill
The change in the carrying amount of goodwill during the year ended December 31, 20212023 included the following (in thousands):
| | | | |
December 31, 2022 | | $ | 47,367 | |
Goodwill acquired during the year | | | 24,582 | |
Foreign currency fluctuation | | | 1,054 | |
December 31, 2023 | | $ | 73,003 | |
December 31, 2020 | | $ | 13,897 | |
Additions | | | 27,841 | |
Foreign currency fluctuation | | | (2,049 | ) |
December 31, 2021 | | $ | 39,689 | |
F-23
Intangible Assets, net
Intangible assets, net consist of the following (in thousands, except as indicated):
There were 0 changes in the carrying amount of goodwill during
| | | | | | | | | | | | | | |
| | Remaining Weighted Avg. Useful Life | | Gross | | | Accumulated | | | Intangible | |
December 31, 2023: | | (in years) | | Amount | | | Amortization | | | Assets, net | |
Developed product technology | | 6 | | $ | 106,782 | | | $ | (26,560 | ) | | $ | 80,222 | |
Trademarks and trade names | | 7 | | | 5,588 | | | | (1,561 | ) | | | 4,027 | |
Customer relationships | | 3 | | | 14,504 | | | | (8,692 | ) | | | 5,812 | |
Distribution network | | 1 | | | 2,413 | | | | (2,242 | ) | | | 171 | |
Total amortized intangible assets | | | | | 129,287 | | | | (39,055 | ) | | | 90,232 | |
| | | | | | | | | | | |
Software in development | | n/a | | | 7,934 | | | | — | | | | 7,934 | |
In-process research and development | | n/a | | | 4,285 | | | | — | | | | 4,285 | |
Total intangible assets | | | | $ | 141,506 | | | $ | (39,055 | ) | | $ | 102,451 | |
| | | | . | | | | | | | |
| | Remaining Weighted Avg. Useful Life | | Gross | | | Accumulated | | | Intangible | |
December 31, 2022: | | (in years) | | Amount | | | Amortization | | | Assets, net | |
Developed product technology | | 7 | | $ | 75,896 | | | $ | (13,420 | ) | | $ | 62,476 | |
Trademarks and trade names | | 8 | | | 5,421 | | | | (987 | ) | | | 4,434 | |
Customer relationships | | 4 | | | 14,240 | | | | (6,906 | ) | | | 7,334 | |
Distribution network | | 2 | | | 2,413 | | | | (2,041 | ) | | | 372 | |
Total amortized intangible assets | | | | | 97,970 | | | | (23,354 | ) | | | 74,616 | |
| | | | | | | | | | | |
Software in development | | n/a | | | 2,503 | | | | | | | 2,503 | |
In-process research and development | | n/a | | | 5,662 | | | | | | | 5,662 | |
Total intangible assets | | | | $ | 106,135 | | | $ | (23,354 | ) | | $ | 82,781 | |
During the year ended December 31, 2020.2023, in connection with the Company's acquisition of Valence, as further described in Note 2, the Company recorded additions to developed product technology and goodwill in the amount of $26.9 million and $24.6 million, respectively. The intangible asset acquired will be amortized on a straight-line basis over a useful life of seven years.
Total expense related to amortization of intangible assets was $15.2 million, $10.2 million and $6.4 million for the years ended December 31, 2023, 2022 and 2021, respectively. Software in development assets begin amortizing when the projects are complete and the assets are ready for their intended use. In-process research and development assets and software in development assets both begin amortizing when the related products reach full commercial launch or are approved and ready for their intended use.
Future amortization expense related to intangible assets as of December 31, 2023 is as follows (in thousands):
| | | | |
Year Ending December 31, | | | |
2024 | | $ | 16,699 | |
2025 | | | 15,211 | |
2026 | | | 15,211 | |
2027 | | | 12,989 | |
2028 | | | 11,057 | |
Thereafter | | | 19,065 | |
Total | | $ | 90,232 | |
Accrued Expenses and Other Current Liabilities
Accrued expenses and other current liabilities consist of the following (in thousands):
| | | December 31, | | | December 31, | |
| | 2021 | | | 2020 | | | 2023 | | | 2022 | |
Payroll and payroll related | | $ | 18,449 | | | $ | 13,552 | | | $ | 29,207 | | | $ | 19,764 | |
Commissions and sales milestones | | | 12,420 | | | | 7,038 | | | | 21,414 | | | | 17,444 | |
Accrued legal expenses | | | | 10,994 | | | | 8,345 | |
Royalties | | | | 7,968 | | | | 4,758 | |
Administration fees | | | | 2,732 | | | | 1,912 | |
Inventory in-transit | | | | 2,251 | | | | 3,548 | |
Other taxes payable | | | | 1,985 | | | | 1,955 | |
Interest | | | | 1,753 | | | | 1,122 | |
Professional fees | | | 4,684 | | | | 3,551 | | | | 1,384 | | | | 1,238 | |
Litigation settlement obligation | | | 4,400 | | | | 4,000 | |
Royalties | | | 3,466 | | | | 2,293 | |
Other taxes payable | | | 3,004 | | | | 248 | |
Inventory in-transit | | | 2,089 | | | | 1,771 | |
Administration fees | | | 1,698 | | | | 442 | |
Interest | | | 1,045 | | | | 619 | |
Orthotec litigation settlement obligation | | | | — | | | | 3,968 | |
Debt issuance costs | | | | — | | | | 2,690 | |
Other | | | 4,294 | | | | 1,750 | | | | 8,024 | | | | 5,638 | |
Total accrued expenses and other current liabilities | | $ | 55,549 | | | $ | 35,264 | | | $ | 87,712 | | | $ | 72,382 | |
F-27
Table of Contents
Other Long-Term Liabilities
Other long-term liabilities consist of the following (in thousands):
| | | | | | | | |
| | December 31, | |
| | 2023 | | | 2022 | |
Income tax-related liabilities | | $ | 5,838 | | | $ | 6,526 | |
Contract liabilities | | | 2,561 | | | | 3,047 | |
Royalties | | | 1,065 | | | | 5,739 | |
Other | | | 1,739 | | | | 1,777 | |
Other long-term liabilities | | $ | 11,203 | | | $ | 17,089 | |
| | December 31, | |
| | 2021 | | | 2020 | |
Royalties | | $ | 5,833 | | | $ | 1,678 | |
Litigation settlement obligation - long-term portion | | | 3,587 | | | | 7,634 | |
Income tax-related liabilities | | | 1,522 | | | | 373 | |
Contract liability | | | 2,897 | | | | — | |
Other | | | 3,222 | | | | 1,703 | |
Other long-term liabilities | | $ | 17,061 | | | $ | 11,388 | |
F-25
5. Discontinued OperationsContract Assets and Contract Liabilities
On September 1, 2016, the Company completed the sale of its international distribution operationsContract assets are included within prepaid expenses and agreements (collectively, the “International Business”) to Globus Medical Ireland, Ltd., a subsidiary of Globus Medical, Inc., and its affiliated entities (collectively “Globus Medical”). As a result of this transaction, the International Business has been excluded from continuing operations for all periods presentedother current assets in the consolidated financial statementsbalance sheets. Contract assets relate to contracts with customers for which one or more performance obligations have been completed and revenue has been recognized, but the customer's payment is reported as discontinued operations.
In connection withcontingent on the salesatisfaction of additional performance obligations. The opening and closing balances of the International Business,Company's contract assets are as follows (in thousands):
| | | | | | | | |
| | December 31,
2023 | | | December 31,
2022 | |
Contract assets | | $ | 3,865 | | | $ | — | |
The Company had current and non-current contract liabilities totaling $13.9 million and $2.6 million, respectively, as of December 31, 2023. The Company had current and non-current contract liabilities totaling $12.0 million and $3.0 million, respectively, as of December 31, 2022. The non-current contract liabilities balance is included in other long-term liabilities on the consolidated balance sheets. Contract liabilities relates to contracts with customers for which partial or complete payment of the transaction price has been received from the customer and the related obligations must be completed before revenue can be recognized. These amounts primarily relate to undelivered equipment, services, or maintenance agreements. The Company recognized $28.2 million of revenue from its contract liabilities during the year ended December 31, 2023, of which $10.9 million was recognized from the beginning contract liabilities balance. The Company recognized $21.6 million of revenue from its contract liabilities during the year ended December 31, 2022, of which $12.9 million was recognized from the beginning contract liabilities balance. The Company recognized $14.6 million of revenue from its contract liabilities during the years ended December 31, 2021. The opening and closing balances of the Company’s contract liabilities are as follows (in thousands):
| | | | |
Balance at December 31, 2022 | | $ | 15,003 | |
Payments received | | | 29,482 | |
Revenue recognized | | | (28,180 | ) |
Foreign currency fluctuation | | | 169 | |
Balance at December 31, 2023 | | $ | 16,474 | |
6. Debt
Term Loan
On January 6, 2023, the Company entered into a product manufacture and supply agreement$150.0 million term loan credit facility with Braidwell Transaction Holdings, LLC (the “Supply Agreement”“Braidwell Term Loan”) with Globus Medical, pursuant to. The Braidwell Term Loan provides for an initial term loan of $100.0 million which was funded on the closing date. On September 28, 2023, the Company supplied to Globus Medical certaindrew an additional $50.0 million (the “delayed draw term loan(s)” or the “DDTL”). The Braidwell Term Loan matures on January 6, 2028. As of its implants and instruments, previously offered for sale byDecember 31, 2023, the outstanding balance under the Braidwell Term Loan was $150.0 million.
In conjunction with the issuance of the Braidwell Term Loan, the Company incurred $3.4 million in international markets at agreed-upon prices.debt issuance costs and $1.5 million in commitment fees. Commitment fees paid to the lender were accounted for as a debt discount. The Supply Agreement expireddebt issuance costs and terminateddebt discount were recorded as a direct reduction of the carrying amount of the loan on August 31, 2021 and all associated discontinued operations balances were removed from the consolidated balance sheets and are being amortized over the life of the loan. As of December 31, 2023, debt issuance costs and debt discount, net of accumulated amortization, associated with the Braidwell Term Loan were $3.0 million and $1.3 million, respectively.
Borrowings under the Braidwell Term Loan bear interest at a rate per annum equal to the Term Secured Overnight Financing Rate for such SOFR business day ("SOFR") subject to a 3% floor, plus 5.75%. The applicable interest rate as of December 31, 2023 was 11.2%. The loan agreement includes an undrawn commitment fee, which is calculated as 1% per annum of the average daily undrawn portion of the DDTL. Interest and undrawn commitment fees incurred are due quarterly. The Company is also required to pay fees on any prepayment of the Braidwell Term Loan, ranging from 1.0% to 3.0% depending on the date of prepayment, and a final payment fee equal to 3.25% of the principal amount of the loans drawn. The effective interest rate as of December 31, 2023 was 12.2%. During the year ended December 31, 2021.
In accordance with authoritative guidance, sales to Globus Medical were reported under continuing operations as2023, the Company had continuing involvementrecognized interest expense on the Braidwell Term Loan of $13.2 million, which includes $0.4 million for the amortization of debt issuance costs and $0.2 million for the debt discount. Upon the Braidwell Term Loan’s maturity, any outstanding principal balance, unpaid accrued interest, and all other obligations under the Supply Agreement.Braidwell Term Loan will be due and payable.
The Braidwell Term Loan is secured by substantially all of the Company’s assets with the priority interest of the lenders in the Braidwell Term Loan and the Revolving Credit Facility, as defined below, subject to terms of a customary intercreditor agreement, which provides that the lenders under the Revolving Credit Facility have a priority with respect to the Company's accounts receivable, inventory, medical instruments, and items related to the foregoing, and the lenders under the Braidwell Term Loan have priority with respect to the remainder of the Company's assets. The loan agreement contains customary representations and warranties and affirmative and negative covenants. Under the loan agreement, the Company is required to maintain a minimum level of liquidity. The loan agreement also includes certain events of default, and upon the occurrence of such events of default, all outstanding loans under the Braidwell Term Loan may be accelerated and/or the lenders’ commitments terminated. The Company recorded $1.0is in compliance with all required financial covenants as of December 31, 2023.
Revolving Credit Facility
In September 2022, the Company entered into a revolving credit facility (the “Revolving Credit Facility”) with entities affiliated with MidCap Financial Trust (“MidCap”). The Revolving Credit Facility provides up to $50.0 million in revenueborrowing capacity to the Company based on a borrowing base. The borrowing base is calculated based on certain accounts receivable and $1.1inventory assets. The Company may request a $25.0 million increase in the Revolving Credit Facility for a total commitment of up to $75.0 million. The Revolving Credit Facility matures on the earlier of September 29, 2027, or 90 days prior to the final maturity date of the Company’s 2026 Notes, as defined below. As of December 31, 2023, the outstanding balance under the Revolving Credit Facility was $49.7 million. In January 2024, the Company repaid $42.0 million of the outstanding balance.
In conjunction with obtaining the Revolving Credit Facility, the Company incurred $1.4 million in costdebt issuance costs. These costs were capitalized to other assets on the consolidated balance sheets and are being amortized over the life of sales from the Supply Agreement in continuing operations for year endedRevolving Credit Facility. As of December 31 2021.2023, and 2022, debt issuance costs, net of accumulated amortization, associated with the Revolving Credit Facility were $1.0 million and $1.3 million, respectively.
The outstanding loans bear interest at the sum of SOFR plus 3.5% per annum. The interest rate as of December 31, 2023 was 9.0%. The loan agreements include an unused line fee, which is calculated as 0.5% per annum of either the unused Revolving Credit Facility or a minimum balance. Interest and unused line fees incurred are due and capitalized to the outstanding principal balance monthly. The Company recorded $3.8recognized interest expense on the Revolving Credit Facility of $2.0 million in revenue and $3.5 million in cost of sales from the Supply Agreement in continuing operations forduring the year ended December 31, 2020.2023, which includes $0.3 million for the amortization of debt issuance costs. The Company recognized interest expense on the Revolving Credit Facility of $0.2 million during the year ended December 31, 2022, which includes $0.1 million for the amortization of debt issuance costs. Upon the Revolving Credit Facility’s maturity, any outstanding principal balance, unpaid accrued interest, and all other obligations under the Revolving Credit Facility will be due and payable.
6. DebtThe Revolving Credit Facility contains a lockbox arrangement clause requiring the Company to maintain a lockbox bank account. If the revolving loan availability is less than 30% of the revolving loan limit for five consecutive business days, or the Company is in default, MidCap will apply funds collected from the Company's lockbox account to reduce the outstanding balance of the Revolving Credit Facility. As of December 31, 2023, the Company's loan availability level has not activated lockbox deductions, nor is it expected to for the next 12 months; therefore, the Company has determined that the outstanding balance under the Revolving Credit Facility is long-term debt on the consolidated balance sheets.
The outstanding loans are secured by substantially all of the Company’s assets with the priority interest of the lenders subject to terms of a customary intercreditor agreement in connection with the Braidwell Term Loan. The loan agreements and other ancillary documents contain customary representations and warranties and affirmative and negative covenants. Under the loan agreements, the Company is required to maintain a minimum level of liquidity. The loan agreements also include certain events of default, and upon the occurrence of such events of default, all outstanding loans under the Revolving Credit Facility may be accelerated and/or the lenders’ commitments terminated. The Company is in compliance with all required financial covenants as of December 31, 2023.
0.75% Convertible Senior Convertible Notes due 2026
In August 2021, the Company issued $ $316.3316.3 million aggregate principal amount of unsecured Senior Convertible Senior2026 Notes with a stated interest rate of 0.75%0.75% and a maturity date of August 1, 2026. Interest on the 2026 Notes is payable semi-annually in arrears on February 1 and August 1 of each year, beginning on February 1, 2022.2022. The net proceeds from the sale of the 2026 Notes were approximately $306.2$306.2 million after deducting the initial purchasers’ offering expenses and before cash used for the Cappedprivately negotiated capped call transactions (the "Capped Call Transactions,Transactions"), as described below, the repurchase of stock, as described in Note 9, and the repayment of the outstanding unsecured term loan (the “Term Loan”) with Squadron Medical Finance Solutions, LLC (“Squadron Medical”(the “Squadron Medical Term Loan") and outstanding obligation under the Inventory Financing Agreement, as described below. The 2026 Notes do not contain any financial covenants.covenants.
The 2026 Notes are convertible into shares of the Company’s common stock based upon an initial conversion rate of 54.5316 shares of the Company’s common stock per $1,000$1,000 principal amount of 2026 Notes (equivalent to an initial conversion price of approximately $18.34$18.34 per share). The conversion rate will be subject to adjustment upon the occurrence of certain specified events, including certain distributions and dividends to all or substantially all of the holders of the Company’s common stock. Based on the terms of the 2026 Notes, when a conversion notice is received, the Company has the option to pay or deliver cash, shares of the Company’s common stock, or a combination thereof.
F-28
Table of Contents
Holders of the 2026 Notes have the right to convert their notes in certain circumstances and during specified periods. Prior to the close of business on the business day immediately preceding February 2, 2026, holders may convert all or a portion of their 2026 Notes only under the following circumstances: (1) during any calendar quarter (and only during such calendar quarter) commencing after the calendar quarter ending on September 30, 2021, if the last reported sale price of the Company’s common stock for at least 20 trading days (whether or not consecutive) during a period of 30 consecutive trading days ending on, and including, the last trading day of the immediately preceding calendar quarter is greater than or equal to 130%130% of the conversion price on each applicable trading day; (2) during the 5 consecutive business days immediately after any 10 consecutive trading day period (the “measurement period”) in which the trading price per $1,000$1,000 principal amount of 2026 Notes for each trading day of the measurement period was less than 98%98% of the product of the last reported sale price of the Company’s common stock and the conversion rate on each such trading day; or (3) upon the occurrence of specified corporate events. From and after February 2, 2026, holders of the 2026 Notes may convert their notes at any time at their election until the close of business on the second scheduled trading day immediately before the maturity date. As of December 31, 2021,2023, none of the conditions permitting the holders of the 2026 Notes to convert have been met. The 2026 Notes are classified as long-term debt on the consolidated balance sheets as of December 31, 2021.2023 and 2022.
The 2026 Notes are redeemable, in whole or in part, at the Company’s option at any time, and from time to time, on or after August 6, 2024 and on or before the 40th scheduled trading day immediately before the maturity date, at a cash redemption price equal to the principal amount of the 2026 Notes to be redeemed, plus accrued and unpaid interest, if any, but only if the last reported sale price per share of the Company’s common stock exceeds 130%130% of the conversion price for a specified period of time. In addition, calling any of the 2026 Notesnotes for redemption will constitute a “make-whole fundamental change” with respect to that note, in which case the conversion rate applicable to the conversion of that note will be increased in certain circumstances if such note is converted after it is called for redemption.
If a fundamental change occurs prior to the maturity date, holders may require the Company to repurchase all or a portion of their 2026 Notes for cash at a price equal to 100%100% of the principal amount of the 2026 Notes plus accrued and unpaid interest. NaNNo principal payments are otherwise due on the 2026 Notes prior to maturity.
The Company recorded the full principal amount of the 2026 Notes as a long-term liability net of deferred issuance costs. The annual effective interest rate for the 2026 Notes is 1.4%1.4%. TotalThe Company recognized interest expense foron the 2026 Notes was $1.7of $4.4 million, $4.4 million and $1.7 million during the yearyears ended December 31, 2021.2023, 2022 and 2021, respectively, which includes $2.0 million, $2.0 million and $0.8 million for the amortization of debt issuance costs during the years ended December 31, 2023, 2022 and 2021, respectively. The Company uses the if-converted method for assumed conversion of the 2026 Notes to compute the weighted average shares of common stock outstanding for diluted earnings per share, if applicable.
The outstanding principal amount and carrying value of the 2026 Notes consist of the following (in thousands):
| | | December 31, 2021 | | | December 31,
2023 | |
Principal | | $ | 316,250 | | | $ | 316,250 | |
Unamortized debt issuance costs | | | (9,259 | ) | | | (5,293 | ) |
Net carrying value | | $ | 306,991 | | | $ | 310,957 | |
F-29
Table of Contents
Capped Call Transactions
In connection with the offering of the 2026 Notes, the Company entered into privately negotiated capped call transactions (the “Cappedthe Capped Call Transactions”)Transactions with certain financial institutions. The Capped Call Transactions are expected generally to reduce the potential dilution and/or offset the cash payments the Company is required to make in excess of the principal amount of the 2026 Notes upon conversion of the 2026 Notes in the event that the market price per share of the Company’s common stock is greater than the strike price of the Capped Call Transactions with such reduction and/or offset subject to a cap. The Capped Call Transactions have an initial cap price of $27.68$27.68 per share of the Company’s common stock, which represents a premium of 100%100% over the last reported sale price of the Company’s common stock on August 5, 2021, and is subject to certain adjustments under the terms of the Capped Call Transactions. Collectively, the Capped Call Transactions cover, initially, the number of shares of the Company’s common stock underlying the 2026 Notes, subject to anti-dilution adjustments substantially similar to those applicable to the 2026 Notes. The cost of the Capped Call Transactions was approximately $39.9$39.9 million.
The Capped Call Transactions are separate transactions and are not part of the terms of the 2026 Notes and will not affect any holder’s rights under the 2026 Notes.notes. Holders of the 2026 Notes will not have any rights with respect to the Capped Call Transactions.
The Capped Call Transactions meet all of the applicable criteria for equity classification and, as a result, the related $39.9$39.9 million cost was recorded as a reduction to additional paid-in capital on the Company’s consolidated statements of shareholders’ equity.(deficit) equity for the year ended December 31, 2021.
OCEANE Convertible Bonds
On May 31, 2018, EOS issued 4,344,651 OCEANE convertible bonds, OCEANEs denominated in Euros, due May 2023 for aggregate gross proceeds of $34.3 million (€29.5 million). $34.3 million. The OCEANEs arewere unsecured obligations of EOS, rankranked equally with all other unsecured and unsubordinated obligations of EOS, and paypaid interest at a rate equal to 6%6% per year, payable semiannually in arrears on May 31 and November 30 of each year, beginning November 30, 2018. Unless either earlier converted or repurchased,2018. The OCEANEs matured on May 31, 2023 and the outstanding OCEANEs will matureand accrued interest were paid in full on May 31, 2023. As of December 31, 2023, no OCEANEs remained outstanding. Interest expense was $0.7$0.3 million and $0.8 million for the periodyears ended December 31, 2023 and 2022, respectively, and $0.7 million from May 13, 2021the date of the EOS acquisition to December 31, 2021.
As discussed in Note 3, in connection with the Offer to acquire EOS, the Company purchased 2,486,135 OCEANEs, and as such, 1,858,516 OCEANEs with a principal amount of $15.3 million (€12.6 million) remained outstanding at the time of acquisition.F-29
The OCEANEs are convertible by their holders into new EOS Shares or exchangeable for existing EOS Shares, at the Company’s option, at an initial conversion rate of 1 share per OCEANE, and the initial conversion rate is subject to customary anti-dilution adjustments. The OCEANEs are convertible at any time until the seventh business day prior to maturity or seventh business day prior to an earlier redemption of the OCEANE. If the number of shares calculated is not a whole number, the holder may request allocation of either the whole number of shares immediately below the number and receive an amount in cash equal to the remaining fractional share value, or the whole number of shares immediately above the number and pay an amount in cash equal to the remaining fractional share value. Holders of the OCEANEs have the option to convert all or any portion of such OCEANEs, regardless of any conditions, at any time until the close of seventh business day immediately preceding the maturity date.
EOS has a right to redeem all of the OCEANEs at its option any time after June 20, 2021 at a cash redemption price equal to the par value of the OCEANEs plus accrued and unpaid interest if the product of the volume-weighted-average price of the shares and the conversion ratio as specified in the agreement in effect on each trading day exceeds 150% of the par value of each OCEANE on each of at least 20 consecutive trading days during any 40 consecutive trading days, if EOS redeems the OCEANEs when the number of OCEANEs outstanding is 15% or less of the number of OCEANEs originally issued, or the occurrence of a tender or exchange offer. As a result of the Company’s acquisition of EOS, the OCEANEs are now convertible into new shares of EOS, as a wholly-owned subsidiary of the Company. OCEANE holders can redeem the notes upon the occurrence of an event of default or upon the occurrence of a change of control. In July 2021, in connection with the change of control, holders of 25,971 OCEANEs chose to redeem their bonds for approximately $0.2 million (€0.2 million).
F-30
The carrying value of the outstanding OCEANEs was $14.1 million (€12.5 million) as of December 31, 2021.
Other Debt Agreements
In January and April 2021, prior to the acquisition, EOS obtained 2The Company has two loan agreements denominated in Euros, under French government sponsored COVID-19 relief initiatives (pret garanti par l’etat or “PGE”(“PGE” loans). Each PGE loan containsinitially contained a 12-month12-month term, with an option to extend, and 90%90% of the principal balance of each loan is state guaranteed. The cost of the state guaranty is 0.25%0.25% of the loan amounts. The loans carry an interest-free rate from the commercial banks (€3.3 million)loan of $3.6 million is interest free and a 1.75%the lenders loan of $1.6 million bears interest from the lender (€1.5 million)of 1.75%. The loan capital and loan guaranty costs are payable in full at the end of the 12-month term or the loan may be extended up to 5 additional years. If the Company chooses to extend the debt, the election must be made by the Company between months 8 and 11 of the 12-month term. The extension will carry an interest rate at the banks’ refinancing cost, to be applied from year 2 to year 6 and an increased state guaranty cost (50 to 200 bps, as per a scale with company size and extension year).
In February 2022, the Company extended the maturity for each loan agreement to 2027.2027. Each loan shall havehas a 12-month12-month period from the applicable extension date where interest only payments will occur (the “Interest Only Period”). Following the Interest Only Period, monthly and quarterly installments of principal and interest under each loan agreement will beis due until the original principal amounts and applicable interest is fully repaid in 2027. The Company has recorded the debt as long-term debt on the consolidated balance sheets as of December 31, 2021. The outstanding obligation under each loan as of December 31, 20212023 was $3.7$3.1 million and $1.6$1.4 million (€3.3 million and €1.5 million) at weighted average interest rates of 0.69%0.98% and 1.25%1.25%, respectively, and weighted average costs of the state guaranty of 0.69%0.69% and 1.00%1.00%, respectively.
Debt consists of the following (in thousands):
| | | | | | | | |
| | December 31, | |
| | 2023 | | | 2022 | |
2026 Notes | | $ | 316,250 | | | $ | 316,250 | |
OCEANEs | | | — | | | | 13,333 | |
Other notes payable | | | 1,175 | | | | 1,869 | |
EOS PGE Loans | | | 4,537 | | | | 5,046 | |
Braidwell Term Loan, including final payment fee of $4,875 | | | 154,875 | | | | — | |
Revolving Credit Facility | | | 49,720 | | | | 35,251 | |
Total | | | 526,557 | | | | 371,749 | |
Less: unamortized debt discount and debt issuance costs | | | (13,714 | ) | | | (7,290 | ) |
Total | | | 512,843 | | | | 364,459 | |
Less: short-term debt | | | (1,808 | ) | | | (14,948 | ) |
Total long-term debt | | $ | 511,035 | | | $ | 349,511 | |
| | December 31, | |
| | 2021 | | | 2020 | |
2026 Notes | | $ | 316,250 | | | $ | — | |
OCEANEs | | | 14,113 | | | | — | |
Other notes payable | | | 386 | | | | 1,887 | |
EOS PGE Loans | | | 5,341 | | | | — | |
Squadron Medical Term Loan | | | — | | | | 45,000 | |
Inventory Financing | | | — | | | | 3,821 | |
PPP Loan | | | — | | | | 4,271 | |
Total | | | 336,090 | | | | 54,979 | |
Less: debt discount | | | (9,259 | ) | | | (12,813 | ) |
Total | | | 326,831 | | | | 42,166 | |
Less: current portion of long-term debt | | | (342 | ) | | | (4,167 | ) |
Total long-term debt, net of current portion | | $ | 326,489 | | | $ | 37,999 | |
Principal payments on debt are as follows as of December 31, 20212023 (in thousands):
2022 | | $ | 342 | |
2023 | | | 14,834 | |
2024 | | | 1,353 | |
2025 | | | 1,335 | |
2026 | | | 317,586 | |
Thereafter | | | 640 | |
Total | | | 336,090 | |
Less: debt discount | | | (9,259 | ) |
Total | | | 326,831 | |
Less: current portion of long-term debt | | | (342 | ) |
Long-term debt, net of current portion | | $ | 326,489 | |
| | | | |
2024 | | $ | 1,804 | |
2025 | | | 1,733 | |
2026 | | | 317,802 | |
2027 | | | 50,343 | |
2028 | | | 154,875 | |
Total | | | 526,557 | |
Less: unamortized debt discount and debt issuance costs | | | (13,714 | ) |
Total | | | 512,843 | |
Less: short-term debt | | | (1,808 | ) |
Long-term debt | | $ | 511,035 | |
F-31
Table of Contents
Paycheck Protection Loan
On April 23, 2020, the Company received the proceeds from a loan in the amount of $4.3$4.3 million (the “PPP Loan”) from Silicon Valley Bank, as lender, pursuant to the Paycheck Protection Program (“PPP”) of the Coronavirus Aid, Relief, and Economic Security Act (the “CARES Act”). The PPP Loan matures on April 21, 2022 and bears interest at a rate of 1.0% per annum. Commencing August 21, 2021, the Company was required to pay the lender equal monthly payments of principal and interest as required to fully amortize the principal amount outstanding on the PPP Loan by April 21, 2022, the date prescribed by guidance issued by U.S. Small Business Administration (“SBA”). The PPP Loan is evidenced by a promissory note dated April 21, 2020 (the “Note”), which contains customary events of default relating to, among other things, payment defaults and breaches of representations and warranties.
Under the CARES Act, loan forgiveness is available for the sum of documented payroll costs, covered rent payments, covered mortgage interest and covered utilities during the twenty-four-week period, beginning on the date of loan approval. For purposes of the CARES Act, payroll costs exclude compensation of an individual employee in excess of $100,000, prorated annually. Not more than 25% of the forgiven amount may be for non-payroll costs. Forgiveness is reduced if full-time headcount declines, or if salaries and wages for employees with salaries of $100,000 or less annually are reduced by more than 25%. In the event the PPP Loan, or any portion thereof, is forgiven pursuant to the PPP, the amount forgiven is applied to outstanding principal.Act. The Company used allthe loan proceeds for purposes consistent with the terms of the proceeds from the PPP Loan to retain employees and maintain payroll. In July 2021, the Company received confirmation from the SBA thatapplied for forgiveness of the entire PPP Loan$4.3 million loan balance, which was forgiven andgranted in July 2021. The Company recorded a gain on debt extinguishment of $4.3$4.3 million, which is included in loss on debt extinguishment, net, on the consolidated statements of operations for the year ended December 31, 2021.
Squadron Medical Credit Agreement
On November 6, 2018, the Company entered into athe Squadron Medical Term Loan (the “Term Loan”) with Squadron Medical Finance Solutions, LLC, a provider of debt financing to growing companies in the orthopedic industry. The Term Loanthat was subsequently amended in March 2019, May 29, 2020 and December 16, 2020 to expand the availability of additional term loans, extend the maturity, remove all financial covenant requirements and, in the December 2020 amendment, to incorporate a debt exchange. OnIn December 16, 2020, the Company amended the Squadron Medical Term Loan to expand the credit facility by an additional $15.0$15.0 million and to extend the maturity of the Squadron Medical Term Loan to June 30, 2026.2026. In conjunction with the Squadron Medical Term Loan amendment onin December 16, 2020, the Company entered into a debt exchange agreement whereby the Company exchanged $30.0$30.0 million of the Company’s outstanding debt obligations pursuant tounder the Squadron Medical Term Loan dated as of November 6, 2018, as amended, for the issuance of 2,700,270 shares of the Company’s Common Stock to Squadron Capital LLC and a participant lender, based on a price of $11.11$11.11 per share. The debt exchange resulted in additional debt issuance costs of $3.8$3.8 million calculated as the difference between the Company’s stock price on the date of issuance and the issuance price.
The Company accounted for the March 2019, May 2020, and December 2020 amendments of the Squadron Medical Term Loan as debt modifications with continued amortization of the existing and inclusion of the new debt issuance costs amortized into interest expense utilizing the effective interest rate method. The Company determined that the $30.0$30.0 million pre-payment associated with the December 16, 2020 amendment should be accounted for as a partial extinguishment of the November 6, 2018 Squadron Medical Term Loan, as amended. As a result of the partial extinguishment the Company elected, as an accounting policy in accordance with ASC 470-50-40-2, to write off a proportionate amount of the unamortized fees at the time that the financing was partially settled in accordance with the terms of the Squadron Medical Term Loan dated November 6, 2018, as amended.Loan. The unamortized debt issuance costs were allocated between the remaining original loan balance and the portion of the loan paid down on a pro-rata basis. At the time of prepayment, the Company recorded a loss on extinguishment of $6.1$6.1 million, which was included in loss on debt extinguishment, net, on the consolidated statements of operations for the year ended December 31, 2020 and capitalized $3.8$3.8 million in non-cash debt issuance closing costs.
On August 10, 2021, the Company terminated and repaid all obligations under the Squadron Medical Term Loan, which consisted of the $45.0$45.0 million outstanding principal and $0.2$0.2 million accrued interest. As a result of the early termination of the Squadron Medical Term Loan, the Company recorded a loss on debt extinguishment associated with the unamortized debt issuance costs of $11.7$11.7 million, which is included in loss on debt extinguishment, net, on the consolidated statements of operations for the year ended December 31, 2021.
F-32
Table of Contents
In connection with the initial 2018 Squadron Medical Term Loan and subsequent amendments, the Company issued an aggregate of 6,759,530 warrants to Squadron Medical and a participant lender. See Note 9 for further information on the warrants issued.
Inventory Financing
In November 2018, the Company entered into an Inventory Financing Agreement with an inventory supplier whereby the Company was originally permitted to draw up to $3.0 million for the purchase of inventory. In November 2020 and May 2021, the Company amended the Inventory Financing Agreement with the supplier to increase the available draw to $6.0 million and then to $9.0 million for the purchase of inventory. On August 10, 2021, the Company terminated and repaid all obligations under the Inventory Financing Agreement, which consisted of $8.1 million outstanding principal and $0.1 million accrued interest.
MidCap Facility Agreement
On May 29, 2020, the Company repaid in full all amounts outstanding under the Amended Credit Facility with MidCap Funding IV, LLC (“MidCap”), including the outstanding balance of $9.6 million, which consisted of outstanding principal and accrued interest. As a result of the early termination of the Credit Facility with MidCap, the Company recorded a loss on debt extinguishment in its consolidated statements of operations for the year ended December 31, 2020.
7. Commitments and Contingencies
Leases
The Company determines if an arrangement is a lease at inception by assessing whether there is an identified asset and whether the contract conveys the right to control the use of the identified asset in exchange for consideration over a period of time. TheIf both criteria are met, the Company recognizesrecords the associated lease liability and corresponding ROU assets andasset upon commencement of the lease liabilitiesusing a discount rate based the incremental borrowing rate of interest that the Company would borrow on a collateralized basis for office buildings and certain equipment withan amount equal to the lease terms of 1 year to 10 years, some of which include options to extend and/or terminate the leases. payments in a similar economic environment. Any short-term leases defined as twelve months or less or month-to-month leases wereare excluded and continue to be expensed each month. Total costs associated with these short-term leases is immaterial to all periodsyears presented.
The Company leases office and storage facilities and equipment under various operating and financing lease agreements. The initial terms of these leases range from 1 to 10 years and generally provide for periodic rent increases. The Company’s lease agreements do not contain any material variable lease payments, residual value guarantees or material restrictive covenants. The Company aggregates all lease and non-lease components for each class of underlying assets into a single lease component and variable charges for common area maintenance and other variable costs are recognized as expense as incurred. Total variable costs associated with leases for the year ended December 31, 20212023 were immaterial. The Company had an immaterial amount of financing leases as of December 31, 2021,2023, which is included in property and equipment, net, accrued expenses and other current liabilities, and other long-term liabilities, on the consolidated balance sheets.
Operating Lease
The Company occupies approximately 121,541 square feet of office engineering, and research and development space as its headquarters in Carlsbad, California. On December 4, 2019, the Company entered into a 10-year10-year operating lease that commenced on February 1, 2021 and will terminate on January 31, 2031, subject to two sixty-month options to renew which are not reasonably certain to be exercised. The Company recognized a $21.1 million ROU asset and $21.5 million lease liability on the consolidated balance sheet upon taking control of the premises on the lease commencement date. Base rent under the building lease for the first twelve months of the term will be $0.2 million per month subject to full abatement during months two through ten, and thereafter will increaseincreases annually by 3.0%3% throughout the remainder of the lease.
F-33
Table of Contents
On April 9, 2021,May 11, 2022, the Company entered into a 7-year operating lease agreementamendment for the build out of additional space within the building which resulted in a new distribution center which consists of approximately 75,643 square feet of office and warehouse space in Memphis, Tennessee. The term oflease modification increasing the lease commenced on May 1, 2021 and will terminate on May 1, 2028, subject to two thirty-six-month options to renew which are not reasonably certain to be exercised. The Company recognized a $1.7 million ROU asset and $1.6 million lease liability upon taking control of the premises on the lease commencement date. Base rent under the new building lease will be commensurate with the Company’s proportionate share of occupancy of the new building and will increase annually by 3.0% throughout the remainder of the lease.liability.
With the acquisition of EOS, the Company assumed its ROU assets and lease liabilities in the amount of $4.3 million. EOS occupies its main office in Paris, France. The EOS office in Paris, France is a 10-year operating lease that commenced in 2019 and will terminate in September 2028. Base rent under the lease is approximately $0.6 million per year.
Future minimum annual lease payments for all operating leases of the Company are as follows (inas of December 31, 2023 (in thousands):
| | | | |
2024 | | $ | 5,505 | |
2025 | | | 5,246 | |
2026 | | | 5,187 | |
2027 | | | 5,175 | |
2028 | | | 4,514 | |
Thereafter | | | 8,582 | |
Total undiscounted lease payments | | | 34,209 | |
Less: imputed interest | | | (5,373 | ) |
Operating lease liability | | | 28,836 | |
Less: current portion of operating lease liability | | | (5,159 | ) |
Operating lease liability, less current portion | | $ | 23,677 | |
2022 | | $ | 4,405 | |
2023 | | | 4,609 | |
2024 | | | 4,620 | |
2025 | | | 4,590 | |
2026 | | | 4,694 | |
Thereafter | | | 17,846 | |
Total undiscounted lease payments | | | 40,764 | |
Less: imputed interest | | | (12,169 | ) |
Operating lease liability | | | 28,595 | |
Less: current portion of operating lease liability | | | (4,212 | ) |
Operating lease liability, less current portion | | $ | 24,383 | |
The Company’s weighted average remaining lease term and weighted average discount rate as of December 31, 20212023 and December 31, 20202022 are as follows:
| | | | | | | |
| December 31, | |
| 2023 | | | 2022 | |
Weighted average remaining lease term (years) | | 6.5 | | | | 7.7 | |
Weighted average discount rate | | 5.5 | % | | | 5.5 | % |
| | December 31, 2021 | | | December 31, 2020 | |
Weighted average remaining lease term (years) | | | 8.6 | | | | 0.7 | |
Weighted average discount rate | | | 8.5 | % | | | 10.5 | % |
Information related to the Company’s operating leaseleases is as follows (in thousands):
| | | December 31, | | Year Ended December 31, | |
| | 2021 | | | 2020 | | 2023 | | | 2022 | | | 2021 | |
Rent expense | | $ | 4,482 | | | $ | 1,300 | | $ | 5,045 | | | $ | 4,643 | | | $ | 4,482 | |
Cash paid for amounts included in measurement of lease liabilities | | $ | 1,795 | | | $ | 1,400 | | $ | 5,120 | | | $ | 4,409 | | | $ | 1,795 | |
Purchase Commitments
With the acquisition of EOS, the Company assumed its inventory purchase commitment agreement with a third-party supplier. The Company is obligated to certain minimum inventory purchase commitment requirements with a third-party supplier through December 2025.2026. As of December 31, 2021,2023, the remaining minimum purchase commitment required by the Company under the agreement was $33.5$13.9 million.
Litigation
Litigation
The Company is and may become involved in various legal proceedings arising from its business activities. While management is not aware of any litigation matter that in and of itself would have a material adverse impact on the Company’s consolidated results of operations, cash flows or financial position, litigation is inherently unpredictable, and depending on the nature and timing of a proceeding, an unfavorable resolution could materially affect the Company’s future consolidated results of operations, cash flows or financial position in a particular period.year. The Company assesses contingencies to determine the degree of probability and range of possible loss for potential accrual or disclosure in the Company’s consolidated financial statements. An estimated loss contingency is accrued in the Company’s consolidated financial statements if it is probable that a liability has been incurred and the amount of the loss can be reasonably estimated. Because litigation is inherently unpredictable and unfavorable resolutions could occur, assessing contingencies is highly subjective and requires judgments about future events. When evaluating contingencies, the Company may be unable to provide a meaningful estimate due to a number of factors, including the procedural status of the matter in question, the presence of complex or novel legal theories, and/or the ongoing discovery and development of information important to the matters. In addition, damage amounts claimed in litigation against the Company may be unsupported, exaggerated or unrelated to reasonably possible outcomes, and as such are not meaningful indicators of the Company’s potential liability.
In February 2018, NuVasive, Inc. filed suit against the Company in the United States District Court for the Southern District of California (NuVasive,(NuVasive, Inc. v. Alphatec Holdings, Inc. et al., Case No. 3:18-cv-00347-CAB-MDD (S.D. Cal.)), alleging that certain of the Company’s products, (including components of its Battalion™ Lateral System), infringe,infringed, or contributecontributed to the infringement of, U.S. Patent Nos. 7,819,801, 8,355,780, 8,361,156, 8,439,832, 8,753,270, 9,833,227 (entitled “Surgical access system and related methods”), U.S. Patent No. 8,361,156 (entitled “Systems and methods for spinal fusion”), and U.S. Design Patent Nos. D652,519 (“Dilator”) and D750,252 (“Intervertebral Implant”). NuVasive seeks unspecified monetary damagesD750,252. In June 2022, the parties reached a final resolution of all matters and an injunction against future purported infringement.
In March 2018,executed a confidential settlement agreement. The Court dismissed the Company moved to dismiss NuVasive’s claims of infringement of its design patents for failure to state a cognizable legal claim. In May 2018, the Court ruled that NuVasive failed to state a plausible claim for infringement of the asserted design patents and dismissed those claimsaction with prejudice. The Company filed its answer, affirmative defenses and counterclaims to NuVasive’s remaining claims in May 2018.
Also in March 2018, NuVasive moved for a preliminary injunction. In March 2018, the Court denied that motion without prejudice for failure to comply with the Court’s chambers rules. In April 2018, NuVasive again moved for a preliminary injunction. In July 2018, after a hearing on the matter in June 2018, the Court denied that motion on the grounds that NuVasive failed to establish either likelihood of success on the merits or that it would suffer irreparable harm absent injunction.
In September 2018, NuVasive filed an Amended Complaint, asserting additional infringement claims of U.S. Patent Nos. 9,924,859, 9,974,531 and 8,187,334. The Company filed its answer, affirmative defenses and counterclaims to these claims in October 2018. Also in October 2018, NuVasive moved to dismiss the Company’s counterclaims that NuVasive intentionally had misled the U.S. Patent and Trademark Office as a means of obtaining certain patents asserted against the Company. In January 2019, the Court denied NuVasive’s motion as to all but one counterclaim, but granted the Company leave to amend that counterclaim to cure dismissal. The Company amended that counterclaim in February 2019 and, that same month, NuVasive again moved to dismiss it. In March 2019, the Court denied NuVasive’s motion. NuVasive filed its Answer to the amended counterclaim in April 2019.
In December 2018, the Company filed a petition with the Patent Trial and Appeal Board (“PTAB”) challenging the validity of certain claims of the ’156 and ’334 Patents. In July 2019, PTAB instituted inter partes review (“IPR”) of the validity of asserted claims of the two patents at issue. In July 2019, PTAB instituted IPR of the validity of asserted claims of the two patents at issue and held a hearing on the matter in April 2020. In July 2020, the PTAB ruled that all challenged claims of the ‘156 Patent were valid (not unpatentable) and ruled that several challenged claims of the ‘334 Patent were invalid, while finding that other challenged claims of the ‘334 Patent valid. NuVasive and the Company both appealed the PTAB’s written decision on the matter. In February 2022, the U.S. Court of Appeals for the Federal Circuit affirmed the PTAB’s ruling without opinion.
F-35
Table of Contents
In January 2020, NuVasive filed a Motion for Partial Summary Judgment of infringement and validity of the ’832, ’780 and ’270 Patents and the Company filed a Motion for Summary Judgment of non-infringement of all asserted claims and of invalidity of the ’832 Patent and for dismissal of NuVasive’s claim for lost profits and its allegations of assignor estoppel. In April 2020, the Court granted NuVasive’s Motion as to the alleged infringement of the ’832 Patent only and denied NuVasive’s Motion in all other respects. Also, in April 2020, the Court granted the Company’s Motion as to dismissal of the allegations of assignor estoppel and denied the Company’s Motion in all other respects.
In November 2020, NuVasive filed a Motion to Strike the Company’s Invalidity Contentions concerning the ’156 and ’334 Implant Patents. In April 2021, the Court denied NuVasive’s motion.
In January 2021, NuVasive filed a Motion for Partial Summary Judgment of infringement and validity of the ’156 and ’334 Implant Patents and the Company filed a Motion for Summary Judgment of invalidity of those same patents. These motions were argued to the Court on June 29, 2021. In August 2021, the Court denied NuVasive’s motion and granted the Company’s motion for summary judgment of invalidity of the ’156 Patent. In September 2021, NuVasive elected not to proceed with its remaining claims for the ’334 Patent, ’780 Patent, ’270 Patent, ’227 Patent, and ’859 Patent. Trial on the remaining patents (’801 Patent, ’832 Patent, and ’531 Patent) has been rescheduled several times due to the COVID-19 pandemic and is now set to begin March 1, 2022.
The Company believes that the allegations lack merit and intends to vigorously defend all claims asserted. A liability is recorded in the consolidated financial statements if it is believed to be probable that a loss has been incurred and the amount of the loss can be reasonably estimated. It is impossible at this time to assess whether the outcome of this proceeding willthese proceedings did not have aany material adverse effect on the Company’s consolidated results of operations, cash flows or financial position. Therefore, in accordance with authoritative accounting guidance, the Company has not recorded any accrual for a contingent liability associated with this legal proceeding based on its belief that a liability, while possible, is not probable and any range of potential future charge cannot be reasonably estimated at this time.
Indemnifications
In the normal course of business, the Company enters into agreements under which it occasionally indemnifies third-parties for intellectual property infringement claims or claims arising from breaches of representations or warranties. In addition, from time to time, the Company provides indemnity protection to third-parties for claims relating to past performance arising from undisclosed liabilities, product liabilities, environmental obligations, representations and warranties, and other claims. In these agreements, the scope and amount of remedy, or the period in which claims can be made, may be limited. It is not possible to determine the maximum potential amount of future payments, if any, due under these indemnities due to the conditional nature of the obligations and the unique facts and circumstances involved in each agreement.
In October 2017, NuVasive filed a lawsuit in Delaware Chancery Court against Mr. Miles, the Company’s Chairman and CEO, who was a former officer and board member of NuVasive. The Company itself was not initially a named defendant in this lawsuit; however, onin June 28, 2018, NuVasive amended its complaint to add the Company as a defendant. As of December 31, 2021, the Company has 0t recorded any liability on the consolidated balance sheet related to this matter. OnIn October 12, 2018, the Delaware Court ordered that NuVasive begin advancingadvance legal fees for Mr. Miles’ defense in the lawsuit, as well as Mr. Miles’ legal fees incurred in pursuing advancement of his fees, pursuant to an indemnification agreement between NuVasive and Mr. Miles. As of December 31, 2021, the Company has 0t recorded any liability on the consolidated balance sheet related to this matter.
Royalties
The Company has entered into various intellectual property agreements requiring the payment of royalties based on the sale of products that utilize such intellectual property. These royalties primarily relate to products sold by Alphatec Spine and are based on fixed fees or calculated either as a percentage of net sales or on a per-unit sold basis. Royalties are included on the accompanying consolidated statements of operations as a component of cost of sales.
8. Orthotec Settlement
On September 26, 2014, the Company entered into a Settlement and Release Agreement, dated as of August 13, 2014, by and among the Company and its direct subsidiaries, including Alphatec Spine, Inc., Alphatec Holdings International C.V., Scient'x S.A.S. and Surgiview S.A.S.; HealthpointCapital, LLC, HealthpointCapital Partners, L.P., HealthpointCapital Partners II, L.P., John H. Foster and Mortimer Berkowitz III; and Orthotec, LLC and Patrick Bertranou, (the “Settlement Agreement”). Pursuant to the Settlement Agreement, the Company agreed to pay Orthotec, LLC $49.0$49.0 million in cash, including initial cash payments totaling $1.75$1.75 million, which the Company previously paid in March 2014, and an additional lump sum payment of $15.75$15.75 million, which the Company previously paid in April 2014. The Company agreed to pay the remaining $31.5$31.5 million in 28 quarterly installments of $1.1$1.1 million and 1one additional quarterly installment of $0.7$0.7 million, commencing October 1, 2014. The unpaid amountsbalance of the principal amount due accrueaccrued interest at the rate of 7.0%7% per year until paid in full. The accrued but unpaid interest will bewas paid in quarterly installments of $1.1$1.1 million (or the full amount of the accrued but unpaid interest if less than $1.1$1.1 million) following the full payment of the $31.5$31.5 million in quarterly installments.
The payments set forth above are guaranteed by Stipulated Judgments held against the Company, HealthpointCapital Partners, L.P., HealthpointCapital Partners II, L.P., HealthpointCapital, LLC, John H. Foster and Mortimer Berkowitz III and, in the event of a default, will be entered and enforced against these entities and/or individuals in that order. In September 2014, the Company and HealthpointCapital entered into an agreement for joint payment of settlement whereby HealthpointCapital has agreed to contribute $5.0 million to the $49.0 million settlement amount. In October 2020, HealthpointCapital made its first $1.0 million payment. During the year ended December 31, 2021 HealthpointCapital made an additional $4.0 million in payments. As of December 31, 2021, there were 0 amounts due from HealthpointCapital. See Note 12 for further information regarding HealthpointCapital.
As of December 31, 2021,2023, the Company has madepaid all installment payments indue, including imputed interest, under the aggregate of $49.0 million, with a remaining outstanding balance of $8.5 million (including imputed interest). The Settlement Agreement provides for mutual releases of all claims in the Orthotec, LLC v. Surgiview, S.A.S, et al. matter in the Superior Court of California, Los Angeles County and all other related litigation matters involving the Company and its directors and affiliates.no balance remains outstanding.
A reconciliation of the total net settlement obligation is as follows (in thousands):
| | | December 31, 2021 | | | December 31, 2020 | |
Litigation settlement obligation - short-term portion | | | $ | 4,400 | | | $ | 4,000 | |
Litigation settlement obligation - long-term portion | | | | 3,587 | | | | 7,634 | |
Total | | | | 7,987 | | | | 11,634 | |
Future imputed interest | | | | 478 | | | | 1,199 | |
Total settlement obligation, gross | | | | 8,465 | | | | 12,833 | |
Related party receivable - included in stockholders' equity | | | | — | | | | (4,000 | ) |
Total settlement obligation, net | | | $ | 8,465 | | | $ | 8,833 | |
9. Equity
Common Stock
There were 200,000,000 shares of common stock authorized at December 31, 20212023 and 2020. 2022. On October 27, 2023, the Company completed an underwritten public offering (the "Public Offering") of 14,300,000 shares of the Company’s common stock at a public offering price of $10.50 per share. On November 17, 2023, the underwriters exercised their option to purchase 470,769 additional shares. The net proceeds from the offering were approximately $145.8 million, including the underwriting discounts and commissions and offering expenses paid by the Company. On August 11, 2023, the Company completed an at the market offering of 668,484 shares of the Company’s common stock. The net proceeds from the offering were approximately $9.9 million, including the underwriting discounts and commissions and offering expenses paid by the Company. On April 19, 2023, the Company completed a registered securities offering (the “Offering”) of 4,285,715 shares of the Company’s common stock at a price of $14.00 per share. The net proceeds from the Offering were approximately $57.5 million, including the underwriting discounts and commissions and offering expenses paid by the Company.
On March 1, 2021, the Company completed the sale of 12,421,242 shares of common stock for gross proceeds of $138.0$138.0 million, and net proceeds of approximately $131.8$131.8 million, net of $6.2$6.2 million in fees. On October 16, 2020 the Company completed the sale of 13,142,855 shares of common stock for gross proceeds of $115.0 million, resulting in net proceeds of approximately $107.7 million, net of $7.3 million in fees.
On August 3, 2021, the Company’s Board of Directors authorized the Company to repurchase an aggregate of up to $25.0$25.0 million of shares of the Company’s common stock. On August 10, 2021, The Company repurchased 1,806,358 shares of its common stock for approximately $25.0$25.0 million in privately negotiated transactions. There were 0no repurchases of common stock repurchases in 2020.during the years ended December 31, 2023 or 2022.
Redeemable Preferred Stock
The Company issued shares of redeemable preferred stock in connection with its initial public offering in June 2006. As of December 31, 2021,2023, and 2020,2022, the redeemable preferred stock carrying value was $23.6$23.6 million and there were 20 million shares of redeemable preferred stock authorized. The redeemable preferred stock is not convertible into common stock but is redeemable at $9.00$9.00 per share, (i) upon the Company’s liquidation, dissolution or winding up, or the occurrence of certain mergers, consolidations or sales of all or substantially all of the Company’s assets, before any payment to the holders of the Company’s common stock, or (ii) at the Company’s option at any time. Holders of redeemable preferred stock are generally not entitled to vote on matters submitted to the stockholders, except with respect to certain matters that will affect them adversely as a class and are not entitled to receive dividends. The carrying value of the redeemable preferred stock was $7.11$7.11 per share at December 31, 20212023 and 2020.2022. The redeemable preferred stock is presented separately from stockholders’ equity in the consolidated balance sheets and any adjustments to its carrying value up to its redemption value of $9.00$9.00 per share are reported as a dividend.
2017 PIPE Warrants
The 2017 Common Stock Warrantscommon stock warrants (the “2017 PIPE Warrants”) havehad a five-year life and arewere exercisable for cash.by cash exercise only. The 2017 PIPE Warrants expired in 2022, and no 2017 PIPE warrants remained outstanding as of December 31, 2022. During the year ended December 31, 2021,2022, there were 795,000approximately 2,312,000 2017 PIPE Warrant exercises for total cash proceeds of $1.6$3.5 million.
2018 PIPE Warrants
The 2018 common stock warrants (the “2018 PIPE Warrants”) had a five-year life and were exercisable by cash or cashless exercise. The 2018 PIPE Warrants expired in May 2023, and no 2018 PIPE warrants remained outstanding as of December 31, 2023. During the year ended December 31, 2020, 2023, there were 273,554 2017 PIPE Warrant exercises, for total cash proceeds of $0.6 million.approximately As of December 31, 2021, there were 2,312,000 2017 PIPE Warrants outstanding.
2018 PIPE Warrants
The 2018 Common Stock Warrants (the “2018 PIPE Warrants”) have a five-year6,311,000 life and are exercisable for cash or by cashless exercise. During the year ended December 31, 2021, there were 2,900,660 2018 PIPE Warrant exercises for total cash proceeds of $1.5$0.4 million. During the year ended December 31, 2020,2022, there were 2,342,986approximately 2,168,000 2018 PIPE Warrant exercises for total cash proceeds of $1.3$0.4 million.A total of 8,479,025 2018 PIPE
SafeOp Surgical Merger Warrants
The SafeOp common stock warrants (the “SafeOp Warrants”), had a five-year life and were exercisable by cash or cashless exercise. The SafeOp Warrants expired in May 2023, and no SafeOp Warrants remained outstanding as of December 31, 2021.
SafeOp Surgical Merger Warrants
In conjunction with the Company’s 2018 acquisition of SafeOp, the Company issued warrants to purchase 2,200,000 shares of common stock at an exercise price of $3.50 per share and contain a five-year life and are exercisable for cash or by cashless exercise.2023. During the year ended December 31, 2021,2023, there were 969,932937,000 cashless SafeOp Surgical Merger Warrant exercises for cash proceeds of $0.1 million.exercises. During the year ended December 31, 2020,2022, there were 34,807approximately 257,000 cashless SafeOp Surgical Merger Warrant exercises for 0 cash proceeds. As of December 31, 2021, there were 1,194,943 SafeOp Surgical Merger Warrants outstanding.exercises.
F-38
Table of Contents
Squadron Medical Warrants
During the year ended December 31, 2018, inIn connection with debt financing entered into with Squadron Medical in 2018, and amended in 2019 and 2020, the Term Loan withCompany issued common stock warrants to Squadron Medical and a participant lender the Company issued warrants to purchase 845,000 shares of common stock at an exercise price of $3.15 per share. An additional 4,838,710 warrants were issued at an exercise price of $2.17 per share during the second quarter of 2019, in conjunction with the Company’s draw on the expanded credit facility. In May 2020, an additional 1,075,820 warrants were issued at an exercise price of $4.88 per share in conjunction with the Company’s second amendment to the Term Loan for total warrants outstanding to(the “Squadron Medical Warrants”). The Squadron Medical Warrants expire in May 2027 and the participant lender of 6,759,530. In conjunction with the second amendment, the expiration dates for all existing warrants were extended to May 29, 2027 to align all outstanding warrant expiration dates. In accordance with authoritative accounting guidance, the warrants qualified for equity treatment upon issuance and were recorded as a debt discount to the face of the debt liability based on fair value to be amortized into interest expense over the life of the debt agreement. The fair value assigned to the warrant amendment was also allocated as a debt issuance cost and amortized into interest expense. As the warrants provide for partial price protection that allow for a reduction in the price in the event of a lower per share priced issuance, the warrants were valued utilizing a Monte Carlo simulation that considers the probabilities of future financings. The Monte Carlo model simulates the present value of the potential outcomes of future stock prices of the Company over the seven-year life of the warrants. The projection of stock prices is based on the risk-free rate of return and the volatility of the stock price of the Company and correlates future equity raises based on the probabilities provided. NaNare exercisable by cash exercise. No Squadron Medical Warrants have been exercised as of December 31, 2021.2023.
Executive Warrants
In December 2017 theThe Company issued warrants to Mr. Patrick S. Miles, the Company’s Chairman and Chief Executive Officer to purchase 1,327,434 shares of the Company’s common stock for $5.00 per share (the “Executive Warrants”). The warrants haveExecutive Warrants had a five-year term and are exercisable by cash or cashless exercise. The warrants issuedIn October 2022, the term was extended to Mr. Miles were accounted for as share based compensation, and the fair value of the warrants of approximately $1.4 million were recognized in full in the statement of operations for the year ended December 31, 2017 as the warrants were immediately vested upon issuance. NaNseven years. No Executive Warrants have been exercised as of December 31, 2021.2023.
A summary of all outstanding warrants as of December 31, 2023 is as follows (in thousands):
| | | | | | | | | |
| | Number of
Warrants | | | Strike Price | | Expiration |
2018 Squadron Medical Warrants | | | 845 | | | $ | 3.15 | | May 2027 |
2019 Squadron Medical Warrants | | | 4,839 | | | $ | 2.17 | | May 2027 |
2020 Squadron Medical Warrants | | | 1,076 | | | $ | 4.88 | | May 2027 |
Executive Warrants | | | 1,327 | | | $ | 5.00 | | December 2024 |
Other* | | | 132 | | | $ | 7.45 | | Various through June 2026 |
Total | | | 8,219 | | | | | |
| | | | | | | |
*Represents weighted average strike price | | | | | | | |
| | Number of Warrants | | | Strike Price | | Expiration |
2017 PIPE Warrants | | | 2,312 | | | $ | 2.00 | | June 2022 |
2018 PIPE Warrants | | | 8,479 | | | $ | 3.50 | | May 2023 |
SafeOp Surgical Merger Warrants | | | 1,195 | | | $ | 3.50 | | May 2023 |
2018 Squadron Medical Warrants | | | 845 | | | $ | 3.15 | | May 2027 |
2019 Squadron Medical Warrants | | | 4,839 | | | $ | 2.17 | | May 2027 |
2020 Squadron Medical Warrants | | | 1,076 | | | $ | 4.88 | | May 2027 |
Executive Warrants | | | 1,327 | | | $ | 5.00 | | December 2022 |
Other* | | | 111 | | | $ | 5.40 | | Various through December 2024 |
Total | | | 20,184 | | | | | | |
| | | | | | | | | |
*Represents weighted average strike price | | | | | | | | | |
F-39
Table of Contents
10. Stock Benefit Plans and Stock-Based Compensation
2016 Equity Incentive Plan
In 2016 the Company adopted its 2016 Equity Incentive Plan (the “2016 Plan”), which replaced the Company’s 2005 Employee, Director and Consultant Stock Plan. On October 25, 2018, the Company’s Board of Directors adopted an amendment to the Company’s 2016 Equity Incentive Award Plan. The 2016 Plan allows for the grant of options, restricted stock, restricted stock unit awards and performance unit awards to employees, directors, and consultants of the Company. Upon its adoption, the 2016 Plan had 1,083,333 shares of common stock reserved for issuance. The Board of Directors determines the terms of the grants made under the 2016 Plan. Options granted under the 2016 Plan expire no later than ten years from the date of grant (five(five years for incentive stock options granted to holders of more than 10% of the Company’s voting stock). Options generally vest over a four-year period and may be immediately exercisable upon a change of control of the Company. The exercise price of incentive stock options may not be less than 100%100% of the fair value of the Company’s common stock on the date of grant. The exercise price of any option granted to a 10% stockholder may be no less than 110%110% of the fair value of the Company’s common stock on the date of grant. Restricted stock unit awards and performance unit awards generally vest over a three or four year period and vest immediately upon a change in control of the Company. On June 17, 2020,14, 2023, the Company’s shareholders approved an amendment to the Company’s 2016 Equity Incentive Award Plan which increased the shares of common stock available for issuance under the Equity2016 Plan by 7,000,0009,300,000 shares, resulting in total common stock reserved for issuance under the 2016 Plan of 26,383,333 shares. AtAs of December 31, 2021, 3,329,2472023, approximately 7,097,000 shares of common stock remained available for issuance under the 2016 Plan. The 2016 Plan will expireexpires in May 2026.
Salary-to-Equity Conversion Program
On April 5, 2020, the Company implemented a voluntary salary-to-equity conversion program for certain employees whose annual payroll costs exceed $100,000, including the Company’s executive officers. The program permitted each participant to make a voluntary election to reduce the participant’s compensation rate through July 11, 2020 from 10% to 75%. In exchange for the compensation reduction, each participant was granted a restricted stock unit from the Company’s 2016 Equity Incentive Plan, equal to the dollar amount of compensation reduction divided by the 30-day volume weighted average price of the Company’s common stock as of close of market on April 3, 2020. The restricted stock units granted under the program fully vested on July 10, 2020. The temporary reduction in compensation to the participants shall not be treated as a reduction in base annual salary rate for purposes of any other benefits plans in which the participants are enrolled or eligible to participate, including in any bonus plans of the Company. As the plan allows for a cash payment of the deferred amount in the event the employee separated from the Company prior to the completion date of the program, the amounts were recorded as a liability instrument through its settlement date with a corresponding fair value adjustment at each reporting period. In 2020, the full fair value of $0.9 million was reclassified into equity upon settlement of the program and issuance of the common stock. A stock compensation charge of $0.9 million related to the program was recorded during the year ended December 31, 2020.
2016 Employment Inducement Award Plan
On October 4, 2016, the Company’s Board of Directors adopted the 2016 Employment Inducement Award Plan (the “Inducement Plan”). The Inducement Plan allows for the grant of options, restricted stock, restricted stock unit awards and performance unit awards to new employees of the Company by granting an award to such new employee as an inducement for the employee to begin employment with the Company. On October 25, 2023, the Company’s Board of Directors approved an amendment to the Company’s Inducement Plan which increased the shares of common stock available for issuance under the Inducement Plan by 600,000 shares, resulting in total common stock available for issuance under the plan of 4,150,000 shares. As of December 31, 20212023 the Inducement Plan had 535,125approximately 567,000 shares of common stock reserved for issuance, which may only be granted to an employee who has not previously been an employee or member of the board of directors of the Company. The terms of the Inducement Plan are substantially similar to the terms of the Company’s 2016 Plan with two principal exceptions: (i) incentive stock options may not be granted under the Inducement Plan; and (ii) the annual compensation paid by the Company to specified executives will be deductible only to the extent that it does not exceed $1.0$1.0 million.
2019 Management Objective Strategic Incentive Plan
Under the 2019 Management Objective Strategic Incentive Plan, the Company is authorized to grant up to 500,000 shares of common stock to third-party individuals or entities that do not qualify under the Company’s other existing equity plans, with a maximum grant of 50,000 shares per participant.plans. As of December 31, 2021, 122,5002023, approximately 476,000 restricted shares and a warrant to purchase up to approximately 12,500 restricted common stock shareswarrants have been granted under the 2019 Management Objective Strategic Incentive Plan.
2017 Distributor Inducement Plan
Under the 2017 Distributor Inducement Plan, the Company is authorized to grant up to 1,000,000 shares of common stock to independent third-party distributorssales agents whereby, upon the achievement of certain Company sales and/or distribution milestones the Company may grant to a distributoran independent sales agent shares of common stock or warrants to purchase shares of common stock. The warrants and restricted stock units issued under the plan are subject to time based or net sales-based vesting conditions. On October 26, 2023, the Company’s Board of Directors approved an amendment to the Company’s Distributor Inducement Plan which increased the shares of common stock available for issuance under the Distributor Inducement Plan by 500,000 shares, resulting in total common stock available for issuance under the plan of 1,500,000 shares. As of December 31, 2021, 325,0002023, approximately 345,000 warrants and 229,900approximately 595,000 shares of restricted common stock were granted under the 2017 Distributor Inducement Plan. As of December 31, 2021, 205,0002023, approximately 338,000 warrants and 146,100approximately 579,000 shares of common stock were earned or issued.
2017 Development Services Plan
Under the 2017 Development Services Plan, the Company is authorized to issue up to 7,000,0008,000,000 shares of common stock to third-parties upon the achievement of certain revenue milestones associated with certain developed royalty-bearing products. Future royalty payments for product and/or intellectual property development work may be paid in either cash or restricted shares of the Company’s common stock at the election of the developer, depending on the terms of the agreement. Each common stock issuance is contingent on net sales-based criteria and other provisions, including the satisfaction of applicable laws and market regulations regarding the issuance of restricted shares to such developers. The Company has entered into Development Services Agreements for development of a wide variety of potential products and intellectual property, with the possibility of issuing shares of common stock. On October 26, 2023, the Company’s Board of Directors approved an amendment to the Company’s Development Services Plan which increased the shares of common stock available for issuance under the Development Services Plan by 2,000,000 shares, resulting in total common stock available for issuance under the plan of 10,000,000 shares. As of December 31, 2021, 02023, 2,362,000 shares have been earned or issued andunder the majority of the agreements are not deemed probable of common stock issuance at this time.Development Services Plan. The Company recognizes stock-based compensation once the achievement of the performance criteria and vesting conditions are deemed probable.
Stock-Based Compensation Costs
The compensation cost that has been included in the Company’s consolidated statements of operations for all stock-based compensation arrangements is detailed as follows (in thousands):
| | | | | | | | | | | | |
| | Year Ended December 31, | |
| | 2023 | | | 2022 | | | 2021 | |
Cost of sales | | $ | 25,082 | | | $ | 2,597 | | | $ | 737 | |
Research and development | | | 18,741 | | | | 5,016 | | | | 4,056 | |
Sales, general and administrative | | | 37,421 | | | | 32,943 | | | | 31,657 | |
Total | | $ | 81,244 | | | $ | 40,556 | | | $ | 36,450 | |
| | Year Ended December 31, | |
| | 2021 | | | 2020 | |
Cost of sales | | $ | 737 | | | $ | 512 | |
Research and development | | | 4,056 | | | | 2,114 | |
Sales, general and administrative | | | 31,657 | | | | 15,033 | |
Total | | $ | 36,450 | | | $ | 17,659 | |
F-37
F-41
Stock Options
A summary of the Company’s stock option activity under the Plansequity plans and related information is as follows (in thousands, except as indicated and per share data):
| | Shares | | | Weighted average exercise price | | | Weighted average remaining contractual term (in years) | | | Aggregate intrinsic value | |
Outstanding at December 31, 2020 | | | 3,951 | | | $ | 3.21 | | | | | | | | | |
Granted | | | 85 | | | | 13.62 | | | | | | | | | |
Exercised | | | (569 | ) | | | 2.63 | | | | | | | | | |
Forfeited | | | (116 | ) | | | 5.28 | | | | | | | | | |
Outstanding at December 31, 2021 | | | 3,351 | | | $ | 3.50 | | | | 6.03 | | | $ | 27,619 | |
Options vested and exercisable at December 31, 2021 | | | 2,973 | | | $ | 3.24 | | | | 5.86 | | | $ | 25,162 | |
Options vested and expected to vest at December 31, 2021 | | | 3,351 | | | $ | 3.50 | | | | 6.03 | | | $ | 27,619 | |
| | | | | | | | | | | | | | | | |
| | Shares | | | Weighted
average
exercise
price | | | Weighted
average
remaining
contractual
term
(in years) | | | Aggregate
intrinsic
value | |
Outstanding at December 31, 2022 | | | 2,916 | | | $ | 3.38 | | | | | | | |
Exercised | | | (399 | ) | | | 2.40 | | | | | | | |
Forfeited | | | (48 | ) | | | 20.46 | | | | | | | |
Outstanding at December 31, 2023 | | | 2,469 | | | $ | 3.20 | | | | 4.22 | | | $ | 29,446 | |
Options vested and expected to vest at
December 31, 2023 | | | 2,469 | | | $ | 3.20 | | | | 4.22 | | | $ | 29,466 | |
Options exercisable at
December 31, 2023 | | | 2,444 | | | $ | 3.11 | | | | 4.19 | | | $ | 29,371 | |
The weighted-average grant-date fair value per share of stock options granted during the year ended December 31, 2021 was $9.88. There were no stock options granted during the years ended December 31, 20212023 and 2020 was $9.88 and $4.71, respectively.2022. The total intrinsic value of stock options exercised was $6.8$5.2 million, $3.4 million and $2.3$6.8 million for the years ended December 31, 20212023, 2022 and 2020,2021, respectively. The aggregate intrinsic value of options at December 31, 20212023 is based on the Company’s closing stock price on the last business day of 20212023 of $11.43$15.11 per share.
The weighted average assumptions used to compute the stock-based compensation costs for the stock options granted during the yearsyear ended December 31, 2021 and 2020 are as follows:
| | Year Ended December 31, | |
| | 2021 | | | 2020 | |
Risk-free interest rate | | | 0.84 | % | | | 1.03 | % |
Expected dividend yield | | | — | | | | — | |
Weighted average expected life (years) | | | 6.07 | | | | 6.08 | |
Volatility | | | 87.38 | % | | | 84.00 | % |
| | | | |
|
| Year Ended December 31, |
|
|
| 2021 |
|
Risk-free interest rate |
|
| 0.84 | % |
Expected dividend yield |
|
| — |
|
Weighted average expected life (years) |
|
| 6.07 |
|
Volatility |
|
| 87.38 | % |
As of December 31, 2021,2023, there was $1.3$0.2 million of unrecognized compensation expense for stock options which is expected to be recognized on a straight-line basis over a weighted average period of approximately 1.401.18 years.
F-42
Table of Contents
Restricted Stock AwardsUnits and Performance Based Restricted Stock Units
The following table summarizes information about the restricted stock awards, restricted stock units and performance-based restricted units activity (in thousands, except as indicated and per share data):
| | | | | | | | | | | | |
| | Shares | | | Weighted
average
grant
date fair
value | | | Weighted
average
remaining
recognition
period
(in years) | |
Unvested at December 31, 2022 | | | 8,533 | | | $ | 6.63 | | | | |
Awarded | | | 6,162 | | | | 15.39 | | | | |
Vested | | | (6,724 | ) | | | 10.72 | | | | |
Forfeited | | | (258 | ) | | | 11.30 | | | | |
Unvested at December 31, 2023 | | | 7,713 | | | $ | 13.56 | | | | 1.01 | |
| | Shares | | | Weighted average grant date fair value | | | Weighted average remaining recognition period (in years) | |
Unvested at December 31, 2020 | | | 8,216 | | | $ | 3.13 | | | | | |
Awarded | | | 3,980 | | | | 12.79 | | | | | |
Vested | | | (2,824 | ) | | | 3.88 | | | | | |
Forfeited | | | (669 | ) | | | 4.01 | | | | | |
Unvested at December 31, 2021 | | | 8,703 | | | $ | 6.34 | | | | 1.66 | |
F-38
The weighted averageweighted-average grant-date fair value per share of awards granted during the years ended December 31, 2023, 2022 and 2021 was $15.39, $8.13and 2020 was $12.79 and $4.87,$12.79, respectively. The total fair value of RSUs that vested during the years ended December 31, 2023, 2022 and 2021 and 2020 was $43.9$104.3 million, $35.2 million and $13.1$43.9 million, respectively.
As of December 31, 2021,2023, there was $42.0$58.9 million of unrecognized compensation expense for restricted stock awards, restricted stock units, and performance-based restricted units which is expected to be recognized on a straight-line basis over a weighted average period of approximately 1.661.52 years.
Employee Stock Purchase Plan
In 2007, the Company adopted the Alphatec Holdings, Inc. 2007 Amended and Restated Employee Stock Purchase Plan (the “ESPP”), which was first amended in May 2017.ESPP. On June 16, 2021,14, 2023, the Company’s shareholders approved a secondthird amendment to the ESPP which increased the amountnumber of shares of common stock available for purchase under the ESPP by 500,0001,500,000 shares, resulting in total common stock reserved for issuance under the ESPP of 3,637,449 shares. As of December 31, 2023, approximately 1,243,000 shares were available under the ESPP for future issuance.
The ESPP provides eligible employees with a means of acquiring equity in the Company at a discounted purchase price using their own accumulated payroll deductions. Under the terms of the ESPP, employees can elect to have up to 20%20% of their annual compensation, up to a maximum of $21,250$21,250 per year, withheld to purchase shares of Company common stock for a purchase price equal to 85%85% of the lower of the fair market value per share (at closing) of Company common stock on (i) the commencement date of the six-month offering period or (ii) the respective purchase datedate.
During the years ended December 31, 20212023, 2022 and 2020,2021 there were 227,245approximately 375,000, 429,000 and 379,166227,000 shares of common stock, respectively, purchasedissued under the ESPP.ESPP, respectively. The Company recognized $1.1$1.7 million, $1.6 million and $1.0$1.1 million in expense related to the ESPP for the years ended December 31, 2023, 2022 and 2021, and 2020, respectively. As of December 31, 2021, 547,170 shares were available under the ESPP for future issuance.
The Company estimates the fair value of shares issued to employees under the ESPP using the Black-Scholes option-pricing model.model. The assumptions used to estimate the fair value of stock options granted and stock purchase rights under the ESPP are as follows:
| | | Year Ended December 31, | | | Year Ended December 31, | |
| | 2021 | | | 2020 | | | 2023 | | | 2022 | | | 2021 | |
Risk-free interest rate | | 0.04% - 0.12% | | | 0.12% - 1.58% | | | 4.54% - 5.41% | | | 0.07% - 4.54% | | 0.04% - 0.12% | |
Expected dividend yield | | | — | | | | — | | | | — | | | | — | | | | — | |
Expected term (years) | | | 0.50 | | | | 0.50 | | | 0.41 - 0.60 | | | 0.50 - 0.60 | | | 0.50 | |
Volatility | | 49.98% - 78.37% | | | 54.96% - 102.5% | | | 40.87% - 62.77% | | | 50.29% - 64.53% | | | 49.98% - 78.37% | |
F-43
Table of Contents
Common Stock Reserved for Future Issuance
Common stock reserved for future issuance consists of the following (in thousands):
| | | | |
|
| December 31, 20212023 |
|
Stock options outstanding |
|
| 3,3512,469
|
|
Unvested restricted stock units |
|
| 8,7037,713
|
|
Employee stock purchase plan |
|
| 5471,243
|
|
Series ASenior convertible preferred stocknotes
|
|
| 2917,246
|
|
Senior convertible notesWarrants outstanding
|
|
| 17,2468,219
|
|
Warrants outstanding
| | | 20,184
| |
Authorized for future grant under the Distributor and
Development Services plans
|
|
| 445560
|
|
Authorized for future grant under the Management
Objective Strategic Incentive Plan
|
|
| 36511
|
|
Authorized for future grant under the Company
Equity plans
|
|
| 4,0257,665
|
|
|
|
| 54,89545,126
|
|
11. Income Taxes
The components of the pretax income (loss)loss are presented in the following table (in thousands):
| | | | | | | | | | | | |
| | Year Ended December 31, | |
| | 2023 | | | 2022 | | | 2021 | |
U.S. Domestic | | $ | (178,313 | ) | | $ | (146,627 | ) | | $ | (127,943 | ) |
Foreign | | | (8,602 | ) | | | (5,382 | ) | | | (16,219 | ) |
Net loss before taxes | | $ | (186,915 | ) | | $ | (152,009 | ) | | $ | (144,162 | ) |
| | Year Ended December 31, | |
| | 2021 | | | 2020 | |
U.S. Domestic | | $ | (127,943 | ) | | $ | (78,849 | ) |
Foreign | | | (16,219 | ) | | | — | |
Net loss before taxes | | $ | (144,162 | ) | | $ | (78,849 | ) |
The components of the provision for income taxes from continuing operations are presented in the following table (in thousands):
| | | | | | | | | | | | |
| | Year Ended December 31, | |
| | 2023 | | | 2022 | | | 2021 | |
Current income tax provision: | | | | | | | | | |
Federal | | $ | 76 | | | $ | (764 | ) | | $ | 123 | |
State | | | 332 | | | | (140 | ) | | | 166 | |
Foreign | | | 145 | | | | 301 | | | | 66 | |
Total current | | | 553 | | | | (603 | ) | | | 355 | |
Deferred income tax provision: | | | | | | | | | |
Federal | | | 37 | | | | 583 | | | | (159 | ) |
State | | | — | | | | 160 | | | | (32 | ) |
Foreign | | | (867 | ) | | | (856 | ) | | | (1,294 | ) |
Total deferred | | | (830 | ) | | | (113 | ) | | | (1,485 | ) |
Total income tax provision | | $ | (277 | ) | | $ | (716 | ) | | $ | (1,130 | ) |
| | Year Ended December 31, | |
| | 2021 | | | 2020 | |
Current income tax provision: | | | | | | | | |
Federal | | $ | 123 | | | $ | — | |
State | | | 166 | | | | 100 | |
Foreign | | | 66 | | | | 35 | |
Total current | | | 355 | | | | 135 | |
Deferred income tax provision: | | | | | | | | |
Federal | | | (159 | ) | | | (2 | ) |
State | | | (32 | ) | | | 12 | |
Total deferred | | | (191 | ) | | | 10 | |
Total income tax provision | | $ | 164 | | | $ | 145 | |
F-44
Table of Contents
The provision for income taxes differs from the amount of income tax determined by applying the applicable U.S. statutory federal income tax rate to pretax loss from continuing operations as a result of the following differences:
| | | | | | | | | | | | |
| | December 31, | |
| | 2023 | | | 2022 | | | 2021 | |
Federal statutory rate | | | 21.00 | % | | | 21.00 | % | | | 21.00 | % |
Adjustments for tax effects of: | | | | | | | | | |
State taxes, net | | | (0.13 | ) | | | 0.03 | | | | (0.07 | ) |
Stock-based compensation | | | (1.12 | ) | | | (1.79 | ) | | | (0.48 | ) |
Rate differential | | | 0.18 | | | | 0.43 | | | | 0.43 | |
Foreign taxes | | | (0.07 | ) | | | (0.05 | ) | | | (0.05 | ) |
Other permanent adjustments | | | (0.21 | ) | | | 0.21 | | | | 0.08 | |
Credits | | | 1.53 | | | | 0.00 | | | | 0.00 | |
Federal uncertain tax positions | | | (1.50 | ) | | | 0.03 | | | | (0.08 | ) |
Expiration of tax attribute | | | (0.09 | ) | | | (1.60 | ) | | | 0.00 | |
Liquidation entries | | | 0.00 | | | | 0.86 | | | | 0.00 | |
Other | | | 0.70 | | | | (0.27 | ) | | | (0.13 | ) |
Valuation allowance | | | (20.12 | ) | | | (18.38 | ) | | | (19.91 | ) |
Effective income tax rate | | | 0.17 | % | | | 0.47 | % | | | 0.79 | % |
| | December 31, | |
| | 2021 | | | 2020 | |
Federal statutory rate | | | 21.00 | % | | | 21.00 | % |
Adjustments for tax effects of: | | | | | | | | |
State taxes, net | | | (0.07 | ) | | | (0.11 | ) |
Stock-based compensation | | | (0.48 | ) | | | (0.93 | ) |
Foreign rate differential | | | 0.43 | | | | 0.00 | |
Foreign taxes | | | (0.05 | ) | | | (0.04 | ) |
Other permanent adjustments | | | 0.08 | | | | (1.70 | ) |
Federal uncertain tax positions | | | (0.08 | ) | | | 0.00 | |
Other | | | (0.13 | ) | | | (0.15 | ) |
Valuation allowance | | | (20.81 | ) | | | (18.25 | ) |
Effective income tax rate | | | (0.11 | )% | | | (0.18 | )% |
F-40
Significant components of the Company’s deferred tax assets and liabilities as of December 31, 20212023 and 20202022 are as follows (in thousands):
| | | December 31, | | | December 31, | |
| | 2021 | | | 2020 | | | 2023 | | | 2022 | |
Deferred tax assets: | | | | | | | | | | | | | | |
Net operating losses | | $ | 131,734 | | | $ | 70,220 | | | $ | 167,389 | | | $ | 146,464 | |
Interest | | | 11,095 | | | | 8,193 | | | | 16,230 | | | | 12,979 | |
Capitalized research and development expenses | | | | 25,633 | | | | 12,062 | |
Inventory | | | 8,784 | | | | 8,117 | | | | 12,095 | | | | 11,298 | |
Accruals and reserves | | | 7,145 | | | | 3,437 | |
Lease liability | | | 6,216 | | | | 228 | | | | 7,268 | | | | 7,975 | |
Stock-based compensation | | | 4,318 | | | | 2,464 | | | | 14,024 | | | | 5,786 | |
Accruals and reserves | | | | 6,162 | | | | 4,806 | |
Legal settlement | | | 1,998 | | | | 2,875 | | | | 440 | | | | 2,302 | |
Income tax credit carryforwards | | | 1,574 | | | | 1,582 | | | | 4,231 | | | | 1,566 | |
Total deferred tax assets | | | 172,864 | | | | 97,116 | | | | 253,472 | | | | 205,238 | |
Valuation allowance | | | (127,209 | ) | | | (87,489 | ) | | | (211,454 | ) | | | (167,860 | ) |
Total deferred tax assets, net of valuation allowance | | | 45,655 | | | | 9,627 | | | | 42,018 | | | | 37,378 | |
Deferred tax liabilities: | | | | | | | | | | | | | | |
Property and equipment | | | | (26,367 | ) | | | (19,610 | ) |
Goodwill and intangibles | | | (18,439 | ) | | | (2,483 | ) | | | (13,399 | ) | | | (15,518 | ) |
Property and equipment | | | (18,404 | ) | | | (6,803 | ) |
Right-of-use assets | | | (6,256 | ) | | | (291 | ) | | | (6,837 | ) | | | (7,250 | ) |
Unrealized foreign exchange gain | | | | (254 | ) | | | (514 | ) |
Total deferred tax liabilities | | | (43,099 | ) | | | (9,577 | ) | | | (46,857 | ) | | | (42,892 | ) |
Net deferred tax assets | | $ | 2,556 | | | $ | 50 | | | $ | (4,839 | ) | | $ | (5,514 | ) |
The realization of deferred tax assets is dependent on the Company’s ability to generate sufficient taxable income in future years in the associated jurisdiction to which the deferred tax assets relate. As of December 31, 2021,2023, a valuation allowance of $127.2$211.5 million has been established against the deferred tax assets, as the Company has determined that it is currently not likely that these assets will be realized. During the yearyears ended December 31, 2023, 2022 and 2021, the valuation allowance increased by $39.7 million.$43.6 million, $39.4 million and $41.0 million, respectively.
F-45
Table of Contents
In determining the need for a valuation allowance, the Company considers all available positive and negative evidence, including scheduled reversals of deferred tax liabilities, projected future taxable income, tax planning strategies, and recent financial performance. Based on the review of all positive and negative evidence, including a three-year cumulative pretax loss, the Company determined that a full valuation allowance should be recorded against its deferred tax assets, with the exception of the net indefinite lived deferred tax liabilities and the Company’s Texas Temporary Credit for Business Loss Carryforwards and deferred tax assets in certain jurisdictions associated with EOS. There are 0 indefinite lived assets.Carryforwards.
The following table summarizes the changes to unrecognized tax benefits (in thousands):
| | | | | | | | | | | | |
| | Year ended December 31, | |
| | 2023 | | | 2022 | | | 2021 | |
Unrecognized tax benefit at the beginning of the year | | $ | 6,079 | | | $ | 15,165 | | | $ | 2,452 | |
Increases in tax positions for current year relating to
acquisitions | | | — | | | | — | | | | 12,713 | |
Increases in tax positions for prior years | | | 1,632 | | | | — | | | | — | |
Decreases in tax positions for prior years | | | — | | | | (8,929 | ) | | | — | |
Increases in tax positions for current year relating to ongoing operations | | | 1,435 | | | | 173 | | | | — | |
Decreases in tax positions as a result of a lapse of statute of limitations | | | (81 | ) | | | (330 | ) | | | — | |
Unrecognized tax benefits at the end of the year | | $ | 9,065 | | | $ | 6,079 | | | $ | 15,165 | |
| | Year ended December 31, | |
| | 2021 | | | 2020 | |
Unrecognized tax benefit at the beginning of the year | | $ | 2,452 | | | $ | 2,452 | |
Increases in tax positions for current year relating to acquisitions | | | 12,713 | | | | 0 | |
Unrecognized tax benefits at the end of the year | | $ | 15,165 | | | $ | 2,452 | |
F-41
At December 31, 2023, 2022 and 2021, and 2020, $14.5$8.6 million, $5.6 million and $2.0$14.5 million, respectively, of the Company’s total unrecognized tax benefits, if recognized, would impact the effective income tax rate.
In accordance with the disclosure requirements as described in ASC Topic 740, Income Taxes, the Company classifies uncertain tax positions as non-current income tax liabilities unless they are expected to be paid within one year. The Company recognizes interest and penalties related to income tax matters as a component of the income tax provision. As of December 31, 2023, 2022 and 2021, there were $0.2$0.1 million, $0.04 million and $0.2 million in accrued interest and penalties. There were 0 accrued interest and penalties, as of December 31, 2020.respectively.
The Company and its subsidiaries are subject to federal income tax as well as income tax of multiple state and foreign jurisdictions. With few exceptions, the Company is no longer subject to income tax examination by tax authorities in major jurisdictions for years prior to 2016.2019. However, to the extent allowed by law, the taxing authorities may have the right to examine prior periods where net operating losses and tax credits were generated and carried forward and make adjustments up to the amount of the carryforwards. The Company is not currently under examination by the Internal Revenue Service, foreign or state and local tax authorities.
At December 31, 2021,2023, the Company had federal, state, and foreign net operating loss carryforwards of $448.8$567.3 million, $316.9$448.3 million and $122.4$117.7 million, respectively. Federal and state net operating losses generated after December 31, 2017 of $437.4 million and $95.4 million, respectively, can be carried forward indefinitely. The remaining federal and state net operating losses begin expiring at various dates beginning in 20222024 through 2042,2043, while foreign net operating losses in France carryforward indefinitely. Federal and some state net operating losses generated in years ending after December 31, 2017 can be carried forward indefinitely. At December 31, 2021,2023, the Company had federal and state research and development tax credit carryforwards of $3.2 million.$5.4 million and $3.5 million, respectively. The federal research and development tax credits begin expiring in 2042 and the state research and development tax credits do not have an expiration date and may be carried forward indefinitely. At December 31, 2023, the Company also had interest expense carryovers of $67.2 million which can be carried forward indefinitely. Utilization of the net operating loss and tax credit carryforwards may become subject to annual limitations due to ownership change limitations that could occur in the future as provided by Section 382 and 383 of the Internal Revenue Code of 1986, as amended (the “Internal Revenue Code”), as well as similar state provisions. These ownership changes may limit the amount of the net operating loss and tax credit carryforwards that can be utilized annually to offset future taxable income if the Company experiences a cumulative change in ownership of more than 50%50% within a three-year testing period. The Company completed formal study through the year ended December 31, 2018 and determined ownership changes within the meaning of IRC Section 382 had occurred. The Company adjusted federal tax attribute carry forwards and deferred tax assets accordingly. The Company will make adjustments to the fully reserved attributes as further studies are completed.
12. Related Party Transactions
In July 2016, the Company entered into a forbearance agreement with HealthpointCapital, LLC, HealthpointCapital Partners, L.P., and HealthpointCapital Partners II, L.P. (collectively, "HealthpointCapital"), pursuant to which HealthpointCapital, on behalf of the Company, paid $1.0 million of the $1.1 million payment due and payable by the Company to Orthotec on July 1, 2016 and agreed to not exercise its contractual rights to seek an immediate repayment of such amount. Pursuant to this forbearance agreement, the Company repaid this amount in September 2016. The Company and HealthpointCapital also entered intopurchases inventory from an agreement for joint payment of settlement whereby HealthpointCapital has agreed to contribute $5.0 million to the $49.0 million Orthotec settlement amount. In October 2020, HealthpointCapital made its first quarterly $1.0 million payment. During the year ended December 31, 2021, HealthpointCapital made the remaining $4.0 million payments. As of December 31, 2021, there were 0 contributions due from HealthpointCapital.
In November 2018, the Company entered into the Term Loan and Inventory Financing Agreement with certain affiliatesaffiliate of Squadron Capital, LLC (“Squadron”), including an inventory supplier (the” Squadron(the “Squadron Supplier Affiliate”). The Term Loan was amended in March 2019, May 2020,David Pelizzon, President and December 2020. On August 10, 2021,Director of Squadron Capital, LLC, currently serves on the Company terminated and repaid all obligations under the Term Loan and the Inventory Financing Agreement. See Note 6 for further details regarding the Term Loan and Inventory Financing Agreement.Company’s Board of Directors. For the years ended December 31, 20212023, 2022 and 2020,2021, the Company purchased inventory in the amounts of $7.7$19.6 million, $10.3 million and $4.0$7.7 million, respectively, from the Squadron Supplier Affiliate. As of December 31, 20212023 and 2020,2022, the companyCompany had $0.8$5.4 million and $4.0$2.4 million, respectively, due to the Squadron Supplier Affiliate. Squadron was a lead investor in the Private Placement that was closed on March 1, 2021. David Pelizzon, President and Director of Squadron, currently serves on the Company’s Board of Directors.
13. Business Segment and Geographic Information
The Company operates in 1one segment based upon the Company’s organizational structure, the way in which the operations and investments are managed and evaluated by the chief operating decision maker (“CODM”) as well as the lack of available discrete financial information at a level lower than the consolidated level. The Company shares common, centralized support functions which report directly to the CODM and decision-making regarding the Company’s overall operating performance and allocation of Company resources is assessed on a consolidated basis.
Net revenue and property and equipment, net, by geographic region are as follows (in thousands):
| | | | | | | | | | | | | | | | | | | | |
| | Revenue | | | Property and equipment, net | |
| | Year Ended December 31, | | | December 31, | |
(in thousands) | | 2023 | | | 2022 | | | 2021 | | | 2023 | | | 2022 | |
United States | | $ | 445,351 | | | $ | 326,697 | | | $ | 223,911 | | | $ | 147,705 | | | $ | 99,050 | |
International | | | 36,911 | | | | 24,170 | | | | 19,301 | | | | 2,130 | | | | 2,902 | |
Total | | $ | 482,262 | | | $ | 350,867 | | | $ | 243,212 | | | $ | 149,835 | | | $ | 101,952 | |
| | Revenue | | | Property and equipment, net | |
| | December 31, | | | December 31, | |
(in thousands) | | 2021 | | | 2020 | | | 2021 | | | 2020 | |
United States | | $ | 223,911 | | | $ | 141,079 | | | $ | 85,320 | | | $ | 36,670 | |
International | | | 19,301 | | | | 3,782 | | | | 2,081 | | | | — | |
Total | | $ | 243,212 | | | $ | 144,861 | | | $ | 87,401 | | | $ | 36,670 | |
F-43
14. Subsequent Events
In February 2022, the Company extended the maturity for its outstanding PGE loans from 2022 to 2027. See Note 6 for further details regarding the PGE loans.
F-47


