UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
ý ☒ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 20172019
OR
o ☐TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from _______ to _______
Commission file number: 000-55039
BioTelemetry, Inc.
(Exact name of registrant as specified in its charter)
|
| | |
DELAWARE
Delaware | | 46-2568498 |
| (State or other jurisdiction of incorporation or organization) | | 46-2568498
(I.R.S.IRS Employer Identification No.) |
|
| | | |
| 1000 Cedar Hollow Road | | |
1000 Cedar Hollow Road #102
Malvern, | Pennsylvania | | 19355 |
| (Address of principal executive offices) | | 19355
(Zip Code) |
(610)729-7000
(Registrant’s telephone number, including area code)
(Former name, former address and former fiscal year, if changed since last report)
Securities registered pursuant to Section 12(b) of the Act:
|
| | |
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
Common Stock, $0.001 par value $0.001 per share | BEAT | NASDAQ Global Select Market |
Securities registered pursuant to Section 12(g) of the Act:None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yesý No o
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes oNoý
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yesý No o
Indicate by check mark whether the registrant has submitted electronically, and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yesý No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ý
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company, or an emerginggrowth company. See the definitions of “large accelerated filer,” “accelerated filer,” and “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one):
|
| | | | | | | | |
Large accelerated filerý | ☒ | Accelerated filer | ☐ | Emerging growth company | ☐ |
| Non-accelerated filer | ☐ | Smaller reporting company | ☐ | | Accelerated filer o
| | Non-accelerated filer o
(Do not check if a
smaller reporting company)
| | Smaller reporting company o
| | Emerging growth company o
|
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No ý
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. o
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ý
The aggregate market value of the registrant’s common stock held by non-affiliates of the registrant was $931.4 million$1.6 billion based on the closing sale price of the registrant’s common stock as reported by the NASDAQ Global Select Market on June 30, 2017, the last business day of the registrant’s most recently completed second fiscal quarter.
quarter ended June 30, 2019. As of February 15, 2018, 32,531,36517, 2020, 34,023,053 shares of the registrant’s common stock were outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s definitive proxy statement for its 20182020 annual meeting of stockholders, which proxy statement will be filed no later than 120 days after the close of the registrant’s fiscal year ended December 31, 2017,2019, are incorporated by reference into Part III of this Annual Report on Form 10-K to the extent stated herein.
BioTelemetry, Inc.
Annual Report on Form 10-K
For The Fiscal Year Ended December 31, 20172019
|
| | |
| | | Page |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | | |
| | |
| | |
| | |
| | |
| | |
| | |
| | | |
| | |
| | |
| Item 16. | Form 10-K Summary | |
| |
| |
Unless the context otherwise indicates or requires, the terms “we,“we,” “our,“our,” “us,“us,” “BioTelemetry”“BioTelemetry” and the “Company,“Company,” as used in this Annual Report on Form 10-K, refer to BioTelemetry, Inc. and its directly and indirectly owned subsidiaries as a combined entity, except where otherwise stated or where it is clear that the terms mean only BioTelemetry, Inc. exclusive of its subsidiaries. We do not use the ® or ™ symbol in each instance in which one of our registered or common law trademarks appears in this Annual Report on Form 10-K, but this should not be construed as any indication that we will not assert our rights thereto to the fullest extent permissible under applicable law.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This document includes certain forward-looking statements within the meaning of the “Safe Harbor”“Safe Harbor” provisions of the Private Securities Litigation Reform Act of 1995 regarding, among other things, our growth prospects, the prospects for our products and our confidence in our future. These statements may be identified by words such as “expect,“expect,” “anticipate,“anticipate,” “estimate,“estimate,” “intend,“intend,” “plan,“plan,” “believe,“believe,” “promises”“promises” and other words and terms of similar meaning. Examples of forward-looking statements include statements we make regarding our ability to increase demand for our products and services, to leverage our Mobile Cardiac Outpatient Telemetry platform, to expand into new markets, to grow our market share, our expectations regarding revenue trends in our segments and the achievement of cost efficiencies through process improvement and gross margin improvements.improvement. Such forward-looking statements are based on current expectations and involve inherent risks and uncertainties, including important factors that could delay, divert or change any of these expectations, and could cause actual outcomes and results to differ materially from current expectations. These factors include, among other things:
our ability to identify acquisition candidates, acquire them on attractive terms and integrate their operations into our business, including our recent acquisition of LifeWatch AG (“LifeWatch”);business;
our ability to educate physicians and continue to obtain prescriptions for our products and services;
changes to insurance coverage and reimbursement levels by Medicare and commercial payors for our products and services;
our ability to attract and retain talented executive management and sales personnel;
the commercialization of new competitive products;
| |
| • | acceptance of our new products and services, such as our mobile cardiac telemetry (“MCT”) patch; |
the impact of the October 2019 information technology incident;
our ability to obtain and maintain required regulatory approvals for our products, services and manufacturing facilities;
changes in governmental regulations and legislation;
adverse regulatory action;
our ability to obtain and maintain adequate protection of our intellectual property;
acceptance of our new products and services;
adverse regulatory action;
interruptions or delays in the telecommunications systems and/or information technology systems that we use;
our ability to successfully resolve outstanding legal proceedings; and
the other factors that are described in “Part I; Item 1A. Risk Factors” of this Annual Report on Form 10-K.
| |
| • | the other factors that are described in “Part I; Item 1A. Risk Factors” of this Annual Report on Form 10-K. |
We undertake no obligation to publicly update any forward-looking statement, whether as a result of new information, future events, or otherwise, except as may be required by law.
PART I
Item 1. Business
Overview
BioTelemetry, Inc. providesis the leading remote medical technology company focused on delivery of health information to improve quality of life and reduce cost of care. We provide remote cardiac monitoring, services and digital population health management for healthcare providers, medical device manufacturing and centralized core laboratory services for clinical research.trials, remote blood glucose monitoring, and original equipment manufacturing that serves both healthcare and clinical research customers.
With over 30,000 unique referring physicians per month, we provide cardiac monitoring and reporting for over one million patients per year, processing over four billion heart beats per day. More information can be found at www.gobio.com. Information on our website or linked to our website is not incorporated by reference into this Annual Report on Form 10-K.
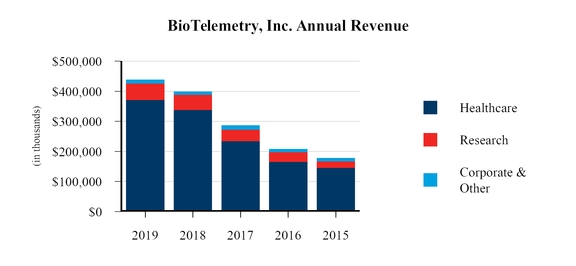
BioTelemetry operates under two reportable segments: Healthcare and Research. Our smaller brands are aggregated in the Corporate and Other category.
The Healthcare segment, which generated 85% of our revenue in 2019, is focused on remote cardiac monitoring to identify cardiac arrhythmias or heart rhythm disorders and to monitor the functionality of implantable cardiac devices. Since we became focusedfocusing on cardiac monitoring in 1999, we have developed a proprietary integrated patient management platform that incorporates a wireless data transmission, network, U.S. Food and Drug Administration (“FDA”FDA”) cleared algorithms, medical devices and 24-hour monitoring service centers.
In July 2017, we acquired LifeWatch, a supplier of mobile cardiac monitoring solutions, headquartered in Zug, Switzerland with U.S. operations based in Rosemont, IL. We believe the integration of LifeWatch will create one of the most comprehensive connected healthcare platforms in the world, by continuing to develop innovative remote cardiac monitoring solutions and delivering those solutions to meet today’s healthcare challenges. See “Part II; Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations; Recent Developments” and “Part II; Item 8. Financial Statements and Supplementary Data; Notes to Consolidated Financial Statements; Note 3. Acquisitions” below for further discussions related to this transaction.
BioTelemetry operates under three reportable segments: (1) Healthcare, (2) Research and (3) Technology. The Healthcare segment, which generated 81% of our revenue in 2017, is focused on the diagnosis and monitoring of cardiac arrhythmias or heart rhythm disorders. We offer cardiologists, and electrophysiologists, neurologists and primary care physicians a full spectrum of solutions, which provides them with a single source of remote cardiac monitoring services. These services range from the differentiated mobile cardiac telemetry service (“MCT”)
include MCT, to event, traditional Holter, extended-wearextended Holter, Pacemaker, and International Normalized Ratio (“INR”INR”), implantable loop recorder (“ILR”) and other implantable cardiac device monitoring. The majority of our Healthcare revenue is derived from the monitoring of devices that BioTelemetry has developed, manufactured and marketed. The Research segment, which generated 14%12% of our revenue in 2017,2019, is engaged in centralcentralized core laboratory services providing cardiac monitoring, imaging services, scientific consulting and data management services for drug and medical device trials. The Technology segment, which generated 5%
During the first quarter of 2018, as part of the LifeWatch AG (“LifeWatch”) integration, our forward-looking integration and rebranding plans, as well as re-evaluating the significance and materiality of our revenuesegments, we aggregated our Technology operating segment into the Corporate and Other category. Included in 2017, focuses on the development,Corporate and Other category is the manufacturing, testing and marketing of cardiovascularcardiac and blood glucose monitoring devices to medical companies, clinics and hospitals.hospitals and corporate overhead and other items not allocated to any of our reportable segments. See “Part II; Item 8. Financial Statements and Supplementary Data; Notes to Consolidated Financial Statements; Note 17.18. Segment Information” below of this Annual Report on Form 10-K for further discussions related to our segments.
As of July 31, 2013, we reorganized to create a holding company structure. CardioNet, Inc., which was previously the public company, became a wholly owned subsidiary of a newly formed entity, BioTelemetry, Inc., a Delaware corporation, and all the outstanding shares of CardioNet, Inc. were exchanged, on a one-for-one basis, for shares of BioTelemetry, Inc. Our new holding company began trading on August 1, 2013common stock is traded on the NASDAQ Global Select Market under our same symbolsymbol: “BEAT.”
Business Strategy
Our goals are to solidify our position as the leading provider of outpatient cardiac monitoring services, expand our presence in the research market and leverage our monitoring platform in new markets. The key elements of the business strategy by which we intend to achieve these goals include:
| |
| • | Increase Overall Demand for Our Cardiac Monitoring Services. We believe that we can increase demand for our comprehensive portfolio of cardiac monitoring solutions by educating cardiologists, electrophysiologists, neurologists and primary care physicians on the benefits of using our services, including MCT, to meet their arrhythmia monitoring needs, stressing the increased diagnostic yield and their ability to use the clinically significant data to make timely interventions and guide more effective treatments. We also believe we can become further incorporated into the medical practices’ workflow by remotely monitoring patients with implanted devices, such as pacemakers, defibrillators and loop recorders, and by offering solutions such as the bi-directional integration of our data into Electronic Medical Record systems. |
| |
| • | Expand Our Presence in the Clinical Research Market. We continue to focus our efforts on increasing our presence in the clinical research market, diversifying our service offerings and expanding our preferred global provider relationships with clinical trial sponsors. We have experienced an increase in dual-service studies that require both cardiac and imaging service, which we see as a key element of our strategic growth plan. We have had success incorporating our proprietary ePatch™ extended-wear monitor as an element of our new cardiac studies creating cross-segment, top-line synergies. |
| |
| • | Leverage Our Core Competencies to New Market Opportunities. We believe our core competencies can be leveraged for applications in multiple markets. While our initial focus has been on remote cardiac monitoring to identify cardiac arrhythmias or heart rhythm disorders and to monitor the functionality of implantable cardiac devices, we intend to expand into new market areas that require outpatient or ambulatory monitoring and management. During the second quarter of 2018, we announced the commercial introduction of our latest generation |
Increase Demand for Our Comprehensive Cardiac Monitoring Solutions. We believe that we can increase demand for our comprehensive portfolio of outpatient cardiac monitoring solutions by educating cardiologists, electrophysiologists and neurologists on the benefits of using mobile cardiac telemetry to meet their arrhythmia monitoring needs, stressing the increased diagnostic yield and their ability to use the clinically significant data to make timely interventions and guide more effective treatments.
Expand Our Presence in the Research Market. In December 2010, we entered the core lab services business through our acquisition of Agility Centralized Research. We later were able to expand our presence in clinical research with our acquisition of Cardiocore Lab, LLC (“Cardiocore”) in August 2012 and our purchase of the assets of RadCore Lab, LLC (“RadCore”) in June 2014. In 2016, we further expanded our core lab capabilities with the acquisition of VirtualScopics, Inc. (“VirtualScopics”), a leading provider of clinical trial imaging solutions. We continue to focus our efforts on increasing our presence in the research market and on becoming a preferred global provider as it provides us with the ability to diversify our service offerings.
Leverage Our Core Competencies to New Market Opportunities. We believe our core competencies can be leveraged for applications in multiple markets. While our initial focus has been on arrhythmia diagnosis and monitoring, we intend to expand into new market areas that require outpatient or ambulatory monitoring and management. In line with this goal, we acquired Telcare, the first company to receive FDA clearance for a cellular-enabledwireless Blood Glucose Monitoring (“BGM”BGM”) system, increasing our presence in the large and rapidly growing digital population health management market. This wireless BGM system transmits real-time results to a cloud-based analytical engine, which synthesizes the data, monitors trends and provides caregivers with critical information about a patient’s health status and the potential need to intervene. We have been leveraging our wireless platform and proprietary technology to develop new opportunities for growth in the digital population health management business through key partnerships and internal investments. We continue to evaluate numerous connected health technologies and solutions to better understand where we can best leverage our capabilities.
The Healthcare segment, or BioTel Heart, operating under the legal entities of CardioNet, LLC (“CardioNet”), LifeWatch and Heartcare Corporation of America, Inc. (“Heartcare”)Heart®, is focused on the diagnosis andremote cardiac monitoring ofto identify cardiac arrhythmias or heart rhythm disorders.disorders and to monitor the functionality of implantable cardiac devices. We provideoffer cardiologists, electrophysiologists, neurologists and primary care physicians who prefer to use a single source of cardiac monitoring services with a full spectrum of solutions, ranging from our differentiated MCTwhich provides them with a single source of remote cardiac monitoring services. These services toinclude MCT, event, to extended wear and Holter monitoring and traditional Holter, extended Holter, Pacemaker, INR, ILR and other implantable cardiac device monitoring. We also provide PacemakerThe majority of our Healthcare revenue is derived from the monitoring of devices that BioTelemetry has developed, manufactured and INR monitoring.marketed.
MCT
Our MCT services incorporate a lightweight patient-worn sensor attached to electrodes that capture two-channel electrocardiogram (“ECG”ECG”) data, measuring electrical activity of the heart, on a compact wireless handheld monitor. The monitor analyzes incoming heartbeat-by-heartbeat information from the sensor on a real-time basis by applying proprietary algorithms designed to detect arrhythmias. The monitor can detect an arrhythmic event even in the absence of symptoms noticed by the patient. When the monitor detects an arrhythmic event, it automatically transmits the ECG to our monitoring centers. At our 24/7 monitoring centers, which operate 24/7, experienced certifiedtrained cardiac monitoring specialiststechnicians analyze the sent data, respond to urgent events and report results in the manner prescribed by the physician. The MCT devices employ two-way wireless communications, enabling continuous transmission of patient data to the
monitoring centers and permitting physicians to remotely adjust monitoring parameters and request previous ECG data from the memory stored in the monitor. The MCT devices have the capability of storing 30 days of continuous ECG data, in contrast to a maximum of 10 minutes for a typical event monitor and a maximum of 24 hours for a typical Holter monitor.
|
| | |
In 2016, we obtained FDA approval of our next generation MCT in a patch form factor. The MCT patch is a four-lead, two-channel system which provides the same best in class technology as the current MCT device, in a patch form factor. The MCT patch is a four-lead, two-channel system that provides the same best-in-class technology as our traditional MCT devices, in a more convenient form factor. The MCT patch was commercially launched in limited accounts during 2017, with a full launch expected in the first quarter of 2018. | | |
Event
Our event monitoring services provide physicians with the flexibility to prescribe wireless event monitors, digital loop event monitors, memory loop event monitors and non-loop event monitors. Event data is transmitted, either through automatic transmission of event data with wireless event monitors or through telephonic transmission of stored event data with our traditional event monitors, to one of our 24/7 monitoring centers where our trained cardiac technicians analyze the data.
Traditional Holter and extended-wear Holter monitors store an image of the electrical impulses of every heartbeat or irregularity in digital format on a compact memory card. The memory card is mailed or the data is sent electronically through a secure web transfer to one of our Holter labs, where our trained cardiac technicians analyze the data. Our next generation Holter monitor, the CardioKey™ and ePatch™ are small, lightweight cardiac monitors, which can continuously store up to 7-14 days of cardiac images.
|
| | |
| Traditional Holter and extended Holter monitors locally store, on a compact memory card, ECG data of every heartbeat or irregularity. At the end of service, the device is returned. Our trained cardiac technicians then analyze the data. Our next generation Holter monitors, the CardioKey® and ePatch™ are small, lightweight cardiac monitors, which can continuously store up to 14 days of ECG data. | | |
Geneva
|
| | |
| Our Geneva platform is a cloud-based solution for point of care and remote monitoring data that consolidates and manages information from ILRs and other implantable cardiac devices of several manufacturers into a single workflow. When combined with Geneva’s cardiac monitoring services, our trained cardiac technicians analyze and report the data. | | |
We market our services generally throughout the United States and receive reimbursement for the monitoring provided to patients from Medicaregovernment and other third-party commercial payors.
The Research segment, or BioTel Research, operating under the legal entities of Cardiocore and VirtualScopics,Research™, is engaged in centralcentralized core laboratory services that provideproviding cardiac monitoring, imaging services, scientific consulting and data management services for drug and medical treatment and device trials. We entered the research field through the acquisition of Agility Centralized Research in December 2010, and later expanded our presence with the asset acquisition of Cardiocore in August 2012 and RadCore in June 2014. The centralized services include ECG, Holter monitoring, ambulatory blood pressure monitoring, echocardiography, multigated acquisition scan (“MUGA”MUGA”), a full range of imaging services, protocol development, expert reporting and statistical analysis. Our imaging servicesservice offerings were bolstered by our 2016 acquisition of VirtualScopics, Inc. (“VirtualScopics”), a leading provider of clinical trial imaging solutions and services in the cardiovascular,cardiac, oncology, metabolic, musculoskeletal and neurologic therapeutic areas. Through these acquisitions, we gained global experience in central core laboratory services, which includes experience in Phase I-IV and Thorough QT Trials. We also provide a full range of support services in Phase I-IV trials and Thorough QT Trials, that include project coordination, setup and management, equipment rental, data transfer, processing, analysis and 24/7 customer support and site training. Our data management systems enable
complete customization for sponsors’ preferred data specifications, and our web service, CardioPortal™, provides access to rich data from any web browser, without client-side plug-ins.browser. Our primary customers in this segment are pharmaceutical companies and contract research organizations. We operate locations domestically,organizations (“CROs”).
In late 2017, we collaborated with Apple, Stanford Medicine and American Well in the Apple Heart Study, to improve the technology used to identify irregular heart rhythms and advance heart science. The study enrolled over 400,000 participants and was expected to discover undiagnosed irregular heart rhythms, such as AFib, using the Apple Watch and dedicated “Apple Heart Study” App. As part of the study, participants who experienced an irregular pulse received BioTelemetry’s ePatch™ for additional monitoring. In November 2019, Stanford Medicine published its findings, “Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation” in the New England Journal of Medicine, which support both domestic and international operations.confirmed that wearable technology can safely identify heart rate irregularities that subsequent clinical evaluations confirmed to be AFib. This is an example of a customer outside the traditional CRO or pharmaceutical company utilizing our services.
The Technology segment,Corporate and Other category contains our other operating under the legal entities of Braemar Manufacturing, LLC (“Braemar”)business brands: BioTel Alliance™, Telcare (“Telcare”) and to a lesser extent, LifeWatch,which focuses on manufacturing, testing and marketing of cardiac devices to medical companies, clinics and hospitals, and BioTel Care®, which manufactures blood glucose monitoring devices and is actively working to expand our position in the manufacturing, engineering and development of non-invasive cardiac monitors for leading healthcare companies worldwide.digital population health management space. We have been able to build successful customer relationships by providing reliable, quality products and engineering services. We offer contract manufacturing services, developing and producing devices to the specific requirements set by customers.
Braemar manufacturesWe manufacture various devices, including but not limited to, cardiacMCT, event monitors, digitaland Holter monitors and MCT monitors utilized by our Healthcare segment. and Research segments. Our facilities located in San Diego, CA, and Eagan, MNConcord, MA, are responsible for research and product development under FDA guidelines. Manufacturing of devices is performed in part in our Eagan, MN, facility. We believe that our manufacturing facilitiescapacity will be sufficient to meet our manufacturing needs for the foreseeable future.
In addition, in December 2016, we acquired Telcare, the first company to receive FDA clearance for a cellular-enabled BGM system. This wireless BGM system transmits real-time results to a cloud-based analytical engine, which synthesizes the data, monitors trends and provides caregivers with critical information about the patients’ health status and the potential need to intervene.
We believe our manufacturing operations are in compliance with regulations mandated by the applicable regulatory governing bodies. We are subject to unannounced inspections by the FDA, and we successfully completed routine inspections by the FDA in October 2017 (Eagan, MN), May 2016 (Rosemont, IL) and February 2016 (Concord, MA),recent years with no significant findings noted or warnings issued. Our Eagan, MN, San Diego, CA, and Concord, MA, facilities are ISO 13485 certified13485-certified and registered with the FDA.FDA. ISO 13485 is aan international quality system standard used by medical companies providing design, development,device manufacturing installation and servicing,companies and is the basis for acquiring European Conformity Marking (“CE Marking”Marking”) for medical device product distribution in the European Union. ManyIn addition to FDA clearance, many of our devices also carry a CE Marking.Marking, which is a certification mark that indicates conformity with health, safety and environmental protection standards for products used and sold within the European Economic Area (“EEA”).
There are a number of critical components and sub-assemblies in the devices. The vendors for these materials are qualified through stringent evaluation and testing of their performance. We implement a strict no-change policy with our contract manufacturers to ensure that no components are changed without our approval.
Research and Development
For the years ended December 31, 2017, 2016 and 2015, we spent $11.1 million, $8.4 million and $7.1 million, respectively, onWe make significant investments in research and development activities focused on developing new products and enhancements to our existing products. We intend to continue to develop proof of superiority of our technology through clinical data. Our aim is to create products that are smaller, faster, more efficient and that provide more useful and relevant information to physicians on a more timely basis. We employ a dedicated internal core research and development team, primarily based in San Diego. We have been consolidating parts of the process across the organization to bring cross-company synergies and benefits. Our San Diego location also houses our rapid prototype lab, which has 3-D printing capability. In addition, we consult with external consultants and partners on certain projects or prototype work.
The three primary sources of clinical data that we have used to date to illustrate the clinical value of MCT include: (i) a randomized 300-patient clinical study; (ii) our cumulative actual monitoring experience from our databases; and (iii) numerous other published studies.
We sponsored and completed a 17-center, 300-patient randomized clinical trial in March 2007.2007 - Steven A. Rothman M.D. etal. “The Diagnosis of Cardiac Arrhythmias: A Prospective Multi-Center Randomized Study Comparing Mobile Cardiac Outpatient Telemetry Versus Standard Loop Event Monitoring,” Journal of Cardiovascular Electrophysiology. We believe this study at that time, represented the largest randomized study comparing two non-invasive arrhythmia monitoring methods. The study was designed to evaluate patients who were suspected to have an arrhythmic cause underlying their symptoms but who were a diagnostic challenge given that they had already had a non-diagnostic 24-hour Holter monitoring session or four hours of telemetry monitoring within 45 days prior to enrollment. Patients were randomized to either MCT or to a loop event monitor
for up to 30 days. Of the 300 patients who were randomized, 266 patients who completed a minimum of 25 days of monitoring were analyzed (134 patients using MCT and 132 patients using loop event monitors).
The study specifically compared the success of MCT against loop event monitors in detecting patients with clinically significant arrhythmias and demonstrated the superiority of MCT for confirming the diagnosis of these types of arrhythmias. The study also demonstrated the advantage of using MCT compared to the loop event monitor in the detection of asymptomatic atrial fibrillation (“AFib”) or flutter. Diagnosis and treatment of atrial fibrillationAFib is important because it can lead to many other medical problems, including stroke. The study concluded that MCT provided a significantly higher diagnostic yield, in detecting an arrhythmic event in patients with symptoms of cardiac arrhythmia, compared to traditional loop event monitoring, including such monitoring designed to automatically detect certain arrhythmias.
In addition to the aforementioned 300-patient randomized clinical trial, MCT has been cited and referenced in over 40 publications and abstracts, which lends support to its clinical efficacy.
In 2016, we obtained FDA approval of our next generation MCT device, in a patch form factor. The MCT patch is a four-lead, two-channel system that provides the same best-in-class technology as our traditional MCT devices, in a more convenient form factor. We continue to explore unique designs to improve patient experience while maintaining clinical efficacy.
We also continue to research study setup automation and workflow management for use in our Research segment. Our continued efforts to integrate machine learning and artificial intelligence to improve our automated patient management tracking in our Research segment services will drive efficiencies, and we believe it will help us acquire more dual-service studies from our partners.
Additionally, we continue to build out our coaching platform for our wireless BGM, through patient and third-party feedback. We are analyzing data to determine where machine learning can provide additional technological leverage and efficiencies to the physician and the patient.
Sales and Marketing
We market our cardiac monitoring solutions in our Healthcare segment through aour direct sales force primarily to cardiologists, electrophysiologists, neurologists and neurologists who are the physician specialistsprimary care physicians who most commonly diagnose and managetreat patients with arrhythmias. We sponsor peer-to-peer educational eventsdifferentiate ourselves through our clinical efficacy and participatethe seamless integration of our data in targeted public relations opportunities. the practice’s electronic medical records.
We are athe leading member of the Remote Cardiac Service Provider Group. Group (“RCSPG”), with our Senior Vice President of Medical Affairs being the current President of the RCSPG. The RCSPG collaborates with physician specialty societies as well as the American Telemedicine Association to advocate to the Centers for Medicare and Medicaid Services (“CMS”) and Congress for appropriate valuation of remote diagnostic services that the RCSPG members provide.
We market our researchResearch segment services to pharmaceutical companies, medical device companies, contract research organizationsCROs and academic research organizations. Cardiocore isWe are a founding member and the first cardiac core lablaboratory to join the Cardiac Safety Research Consortium (“CSRC”CSRC”). Through the CSRC, we are able to network with representativeskey thought leaders and decision makers of major pharmaceutical companies, as well as discuss key cardiac safety issues during the drug development process. Through the 2016 acquisitionour integration of VirtualScopics, we have broadenedexperienced an increase in acquiring studies that include both cardiac and imaging requirements. Expanding our research service offerings is a key element of our strategic growth plan, allowing us to more favorably compete for research studies requiring a wider range of research services. We marketOur team has also had success incorporating our proprietary ePatch™ monitor as a critical element of new cardiac studies creating cross-segment, top-line synergies.
Our BioTel Alliance™ brand, currently included in the Corporate and Other category, markets our manufactured products to physicians, hospitals and other cardiac monitoring providers. BioTel Care® is actively working to expand our position in the digital population health space, and continues to evaluate numerous connected health technologies and solutions to better understand where we can best leverage our capabilities. Specifically, we are engaged in increasing awareness and utilization of our wireless BGM and our diabetes management platform. Our commercial team is primarily focused on securing contracted relationships for our diabetes management services with:
commercial managed care plans;
accountable care organizations;
integrated delivery networks;
physicians groups;
durable medical equipment distributors; and
employer groups.
We attend trade shows and medical conferences to promote our various product and service offerings. The trade shows and conferences we attend are related to organizations such as: the Heart Rhythm Society,
American College of Cardiology, American Telemedicine Association, Society of Thoracic Surgeons, European Society of Cardiology, American Heart Association and the American Telemedicine Association.European Society of Cardiology. We also attend the Medica, DIADrug Information Association and Partnerships in Clinical Trials trade shows, as well as the annual Boston Atrial Fibrillation Conference.Symposium. We have had limited product and service-based advertising in certain national newspapers and medical journals.
Healthcare Reimbursement
Seasonality
Our Healthcare segment experiences some seasonality during the third quarter as well as during the year-end holiday season. We believe that this is the result of patients electing to delay our monitoring services during the summer months or holidays.
Healthcare Reimbursement
In the Healthcare segment, services are billed to government and commercial payors using specific codes describing the services. Those codes are part of the CommercialCurrent Procedural Terminology (“CPT”CPT”) coding system, which was established by the American Medical Association (“AMA”) to describe services provided by physicians and other suppliers. Physicians select the code that best describes the medical services being prescribed. Approximately 34%35% of our total revenue is subject to reimbursement directly from the Medicare program, a federal government health insurance program administered by the Centers for Medicare and Medicaid Services (“CMS”)CMS, at rates that are set nationally and adjusted for certain regional indices.
In addition to receiving reimbursement from Medicare,government payors, we enter into contracts with commercial payors to receive reimbursement at specified rates for our technical services. Such contracts typically provide for an initial term of between one and three years and provide for automatic renewal thereafter.
Either party can typically terminate these contracts by providing between 6030 and 120180 days’ prior notice to the other party at any time following the end of the initial term of the agreement. The contracts provide for an agreed upon reimbursement rate, which in some instances is tied to the rate of reimbursement we receive from Medicare.
In addition to receiving reimbursement from government and commercial payors, we have direct arrangements with physicians who may purchase our monitoring services and then submit claims for these services directly to commercialgovernment and governmentcommercial payors. In some cases, patients pay for their service out-of-pocket.
Competition
Although we believe that we have a leading market shareposition in the mobile cardiac monitoring industry,in the marketU.S., the industry in which our Healthcare segment operates is fragmented and characterized by a large number of smaller regional service providers. Additionally, several larger healthcare companies
Our Research segment competes directly with other core labs as well as CROs that offer certaincentralized core laboratory services. We believe that we compete favorably based on our comprehensive cardiac monitoring solutions, primarily Holter monitors. and imaging service offerings, the scale of our operation and our ability to support the entire life cycle of new drug development.
We also compete directly with other original diagnostic equipment manufacturers.
We believe that the principal competitive factors that impact the success of our cardiac monitoring solutionsbusinesses include some or all of the following:
quality of our algorithms used to detect symptoms;arrhythmias;
quality and accuracy of clinical data;
turnaround times;
ease of use and reliability of cardiac monitoring solutions for patients and physicians;
technology performance, innovation, flexibility and range of application;application generating the highest yields;
timeliness and clinical relevance of new product introductions;
quality and availability of superior customer support services;
size, experience, knowledge and training of sales and marketing staff;
reputation;
relationships with referring physicians, hospitals, managed care organizations and other third-party payors;
reporting capabilities;
| |
| • | providing a full spectrum of remote cardiac monitoring solutions, including MCT, event, traditional Holter, extended Holter, Pacemaker, INR, ILR and other implantable cardiac device monitoring; |
spectruma widening range of solutions, ranging from our differentiated MCTclinical cardiac and imaging services to event and Holter monitoring, making us a single source for cardiac monitoring services; andbest-in-class solutions;
perceived value.value; and
extensive industry expertise.
We believe that we compete favorably based on the factors described above. However, our industry is evolving rapidly and is becoming increasingly competitive, and the basis on which we compete may change over time. In addition, if companies with substantially greater resources than ours enter our market, we will face increased competition.
Our Research segment competes directly with other core labs
Charitable Giving
In recent years, we have supported the American Heart Association through sponsorship and employee fund-raising at local Heart Walk events in the Philadelphia area as well as contract research organizations that offer core lab services. We believe that we compete favorably based on our comprehensive cardiac and imaging service offerings,at various other locations across the scaleU.S. as a way to share a portion of our operation andsuccess. In 2019, we began our ability“Heart for Hope” initiative, whereby we committed to supportfund life-saving heart procedures for children in need. To date, we have funded 200 surgeries for children from parts of Southeast Asia whose families do not have the entire life cycle of new drug development.resources to do so. That funding also includes the follow-up care after the surgery is completed. More information about “Heart for Hope” can be found at www.gobio.com/heartforhope.
Our Technology segment competes directly with other original equipment manufacturers. We believe that we compete favorably based on our suite of quality products and innovative solutions, our superior customer service and our extensive industry experience.
Intellectual Property
We rely on a combination of intellectual property laws, non-disclosure agreements and other measures to protect our proprietary rights. We attempt to protect our intellectual property rights by filing patent applications for new features and products we develop. In addition, we also seek to maintain certain intellectual property and proprietary know-how as trade secrets, and generally require our partners to execute non-disclosure agreements prior to any substantive discussions or disclosures of our technology or business plans. Our business and competitive positions are dependent in part upon our ability to protect our proprietary technology and our ability to avoid infringing the patents or proprietary rights of others.
We hold patents in the United States as well as many international jurisdictions on our products, processes and related technologies. In furtherance of our overall global intellectual property strategy, we also have patent applications currently on file in the United States and internationally. While we have several patents expiring between 2018 andthrough 2032, including patents that relate, in part, to our key products, we do not believe such expirations will have a material impact on our ability to compete in the short term since our technology is typically covered by several patents, creating a system of protected technology.
Our trademarks, certain of which are material to our business, are registered or otherwise legally protected in the United States and in certain foreign countriesinternational jurisdictions and include, among others, the registered trademarks CardioNet®BioTelemetry®, BioTelemetry®BioTel Heart®, Geneva Healthcare®, BioTel Care®, and LifeWatch®BioTel Europe® and the unregistered trademarks Mobile Cardiac Outpatient Telemetry™, MCOT™, CardioPortal™ePatch™, BioTel Heart™, BioTel Care™CardioPortal™, BioTel Research™ and BioTel Technology™Alliance™. We also have a significant amount of copyright-protected materials.
Government Regulation
The health carehealthcare industry is highly regulated, with no guarantee that the regulatory environment in which we operate will not change significantly and adversely in the future. We believe that health carehealthcare legislation, rules, regulations and interpretations will change, and we expect we will have to modify our agreements and operations in response to these changes.
U.S. Food and Drug Administration. Administration
The medical devices that we use to provide patient monitoring services are regulated by the FDA under the Federal Food, Drug, and Cosmetic Act. The basic regulatory requirements that manufacturers ofAct (“FDCA”). Unless exempt, medical devices distributed in the United SatesStates must comply with arereceive marketing authorization by the FDA through either a full Premarket Approval (“PMA”) or the Premarket Notification 510(k), unless exempt, process. Based on the classification and characteristics of a medical device, it may receive marketing authorization through a PMA pathway, which requires the demonstration of safety and effectiveness through adequate and well-controlled clinical studies, or Premarket Approval,receive clearance under the 510(k) pathway after demonstrating substantial equivalence to a predicate device. In addition to marketing authorization requirements, device manufacturers must also comply generally with establishment registration, medical device listing, quality system regulation, labeling requirements and medical device reporting.reporting requirements, and any special controls specific to a particular device and used to support reclassification.
The algorithms we use in the MCT service maintain FDA 510(k) clearance as a Class II device (“510(k) Clearance”).device. On October 28, 2003, the FDA issued a guidance document entitled: “Class“Class II Special Controls Guidance Document: Arrhythmia Detector and Alarm.” In addition to conforming to the general requirements of the Federal Food, Drug, and Cosmetic Act,FDCA, including the Premarket Notification requirements described above, all of our cardiac related
510(k) submissions address the specific issues covered in this special controls guidance document. The algorithms we use in the BGM service also maintain FDA 510(k) Clearanceclearance as a Class II device.
Failure to comply with applicable regulatory requirements can result in enforcement action by the FDA which may include, including certain sanctions, such as fines, injunctions and civil penalties;penalties or injunctions; recall or seizure of our devices and intellectual property; operating restrictions; partial suspension or total shutdown of
production; withdrawal of 510(k) Clearanceclearance of new components or algorithms; withdrawal of 510(k) Clearanceclearance already granted to one or more of our existing components or algorithms; and criminal prosecution.
CE Marking. Marking
Medical devices distributed within the EEA require the CE Marking, which is a certification mark that indicates conformity with health, safety, and environmental protection standards for products sold within the EEA. The CE Marking is also found on products sold outside the EEA that are manufactured in, or designed to be sold in, the EEA. Although ISO 13485 certification is not a direct requirement for CE Marking medical devices under the European Economic Area requireMedical Device Directives, it is recognized as a CE Marking.harmonized standard by the European Commission. ISO 13485 is a quality system standard used by medical companies providing design, development, manufacturing, installation and servicing, and isaligned with the basis for acquiring CE Marking forthree European medical device product distributiondirectives that are applicable to different types of medical devices in the European Union. Europe.Failure to maintain appropriate CE Marking could have an adverse effect on our ability to use or sell our devices within the European Union.
Health CareHealthcare, Fraud, Waste and Abuse. Abuse
In the United States, there are state and federal laws and regulations restrict healthcare providers from billing and collecting for products or services connected with fraudulent, wasteful or abusive conduct. These state and federal laws include civil and criminal false claims provisions, health fraud and false statements provisions, anti-kickback provisions, physician self-referral provisions, and more, violation of which may subject us to criminal penalties as well as potential exclusion from federal healthcare programs and civil monetary penalties.
Federal and state anti-kickback laws that generally prohibit the payment or receipt of kickbacks, bribesanything of value to induce prescription or other remuneration in exchange for the referral of patientsreimbursed products or other health care-related business. In addition, federalservices. Stark law (e.g., the “Stark” law) and some state laws prohibit the existence oflimits certain financial relationships between referring physicians and health care providers and suppliers unless those relationships meet the requirements of specific exceptions to the law.certain designated healthcare services. Anti-kickback laws constrain our sales, marketing and promotional activities by limiting the kinds ofrestrict financial arrangements we may have with physicians, medical centers and otherscertain healthcare professionals in a position to purchase, recommend or refer patients for our cardiac monitoring services or other products or services we may develop and commercialize. Due to the breadth of some of these laws, it is possible that some of our current or future practices might be challenged under one or more of these laws.
Furthermore, federalFederal and state false claims laws prohibit anyone from presenting, or causing to be presented, claims for payment to third-party payors that are false or fraudulent. Violations may result in substantial civil penalties including treble damages, andas well as criminal penalties, including imprisonment, fines and exclusion from participation in federal health care programs. The Federal False Claims Act (“FCA”) also contains “whistleblower” or “qui tam” provisions that allow private individuals to bring actions on behalf of the government alleging that the defendant has defrauded the government.false claims and potentially other violations of fraud and abuse laws. Various states have enacted laws modeled after the Federal False Claims Act,FCA, including “qui tam” provisions, and some of these laws apply to claims filed with commercial insurers. Any violationsViolation of anti-kickbackfederal and false claimsstate fraud and abuse laws could have a material adverse effect on our business, financial condition and results of operations.
The Patient Protection and Affordable Care Act. Act
On March 23, 2010, the Patient Protection and Affordable Care Act was signed into law, and on March 30, 2010, the Health Care and Education Reconciliation Act of 2010 was signed into law. Together, the two measures, collectively known as the Affordable Care Act make the most sweeping and(“ACA”), made fundamental changes to the United States health care system since the creation of Medicare and Medicaid.healthcare system. The Affordable Care ActACA expanded Medicaid eligibility, required most individuals to have health insurance or pay a penalty, imposed new requirements for health plans and insurance policy standards, established health insurance exchanges, changed Medicare payment systems to encourage more cost-effective care and newnewly expanded tools to address fraud and abuse and required manufacturers of medical devices and other products reimbursed by Medicare to report annually to the government certain payments to physicians and teaching hospitals.
As a result In 2018, certain provisions of the passage ofACA were modified and repealed effective 2019 and beyond, and in December 2019, the Affordable Care Act, manufacturers of certain medical devices are subject to andevice excise tax applicable to sales of taxable medical devices beginning January 1, 2013. Several devices that are manufactured by our Technology segment are subject to these taxes. The tax equals 2.3% of the sale price of the applicable medical device. As a manufacturer, we are responsible for remitting these taxes to the federal government. The Consolidated Appropriations Act of 2016, enacted on December 18, 2015, included a moratorium on the medical devices tax commencing on January 1, 2016 and ending onwas permanently repealed.
December 31, 2017. Budget legislation signed in January 2018 extended that moratorium through December 31, 2019.
Health Insurance Portability and Accountability Act of 1996. 1996
The Health Insurance Portability and Accountability Act (“HIPAA”) was enacted in 1996, amended by the United States CongressHealth Information Technology for Economic and Clinical Health (“HITECH”) Act in 1996. Numerous state2009, and federalimplemented through regulation (collectively, “HIPAA”). HIPAA, together with other data privacy and security laws governsuch as the General Data Protection Regulations (“GDPR”) in Europe, dictate privacy and security standards governing the collection, storage, maintenance, dissemination, use and confidentiality of individually identifiable patient health information and other personal information. HIPAA applies directly to “covered entities,” which include health plans, healthcare clearinghouses and many healthcare providers, who electronically transmit health information includingand thereby engage in HIPAA “covered transactions.” HIPAA also applies to “business associates,” individuals who perform certain functions or activities for or on behalf of covered entities that require the administrative simplificationindividuals to create, receive, maintain, or transmit protected health information (“PHI”). HIPAA is concerned primarily with the privacy of PHI when it is used and/or disclosed; the confidentiality, integrity and availability of electronic PHI; notifying federal regulators and impacted patients in the event of a breach of unsecured PHI; and the content and format of certain identified electronic healthcare transactions. The laws governing healthcare information privacy provisionsand security impose civil and criminal penalties for their violation. Compliance with these laws requires substantial expenditures of HIPAA.financial and other resources for information technology system compliance, maintenance, monitoring, validation and evaluation. Historically, state law has governed confidentiality issues, and HIPAA preserves these laws to the extent they are more protective of a patient’s privacy or provide the patient with greater access to his or her health information. As a result of the implementation of the HIPAA regulations, manyMany states are consideringcontinue to consider revisions to their existing laws and regulations that may or may not be more stringent or burdensome than the federal HIPAA provisions. HIPAA applies directly to covered entities, which include health plans, health care clearinghouses and many health care providers. The HIPAA statute, as amended by the Health Information Technology for Economic and Clinical Health (“HITECH”) Act in 2009, and its implementation rules are concerned primarily with the privacy of protected health information when it is used and/or disclosed; the confidentiality, integrity and availability of electronic health information; notifying federal regulators and impacted patients in the event of a breach of unsecured protected health information; and the content and format of certain identified electronic health care transactions. The laws governing health care information privacy and security impose civil and criminal penalties for their violation and can require substantial expenditures of financial and other resources for information technology system modifications and for ongoing operational compliance.
Medicare. Medicare
Medicare is a federal program administered by CMS and its Medicare administrative contractors.Administrative Contractors (“MAC”). The Medicare program provides qualified persons with health carehealthcare benefits that cover the major costs of medical care within prescribed limits, subject to certain deductibles and co-payments. The Medicare program has established guidelines for local and national coverage determinations and reimbursement of certain equipment, supplies and services, which are subject to change. The methodology for determining coverage status and the basis and amount of Medicare reimbursement varies based upon, among other factors, the settinglocation in which a Medicare beneficiary receives health carehealthcare items and services.services, the type of items and services provided, and the benefits available to individual beneficiaries.
The Medicare program is subject to statutory and regulatory changes, retroactive and prospective rate adjustments, administrative rulings, interpretations of policy, Medicare administrativebilling guidelines, MAC contractor local coverage determinations and government funding restrictions. All of these policies may materially increase or decrease the rate of program payments to health carehealthcare facilities and other health carehealthcare suppliers and practitioners, including those paid for our cardiac monitoring or other services. Other regulations such as facility standards, billing requirements, rules of participation and other regulations affecting the provision of and reimbursement for products and services also affect the ability to provide, bill and receive reimbursement for services or products provided under the program. Any changes in federal legislation, regulations or other policies affecting Medicare coverage, reimbursement or reimbursementeligibility relative to our cardiac monitoring or other services could have an adverse effect on our performance.
Certain of our facilities are enrolled in Medicare as Independent Diagnostic Testing Facilities (“IDTFs”IDTFs”). An IDTF is defined by CMS as an entity independent of a hospital or physician’s office in which diagnostic tests are performed, often by licensed or certified non-physician personnel, and under appropriate physician supervision. Medicare has set veryprescribes detailed performancecertification standards that every IDTF must meet in order to obtain or maintain its billing privileges, including requirements to, among other things, operate in compliance with all applicable federal and state licensure and regulatory requirements for the health and safety of patients; maintain a physical facility on an appropriate site meeting specific criteria; have a comprehensive liability insurance policy of at least $0.3 million per location; disclose certain ownership information; have its testing equipment calibrated and maintained in accordance with specific standards; have technical staff on duty with the appropriate credentials to perform tests; and permit on-site inspections.
These requirements are subject to change. WeOur IDTF facilities are periodically inspected by CMS to confirm our compliance with the IDTF standards, and we believe that our facilities are in compliance with the IDTFthese standards.
Environmental Regulation. Regulation
We use materials and products regulated under environmental laws, primarily in the manufacturing and sterilization processes. While it is difficult to quantify, we believe the ongoing cost of compliance with environmental protection laws and regulations will not have a material impact on our business, financial position or results of operations.
Supply Chain Diligence and Transparency
Section 1502 of the Dodd Frank Wall Street Reform and Consumer Protection Act was adopted to further the humanitarian goal of ending the violent conflict and human rights abuses in the Democratic Republic of the Congo and adjoining countries (“DRC”). This conflict has been partially financed by the exploitation and trade of tantalum, tin, tungsten and gold (so called “conflict minerals”) that originate from mines or smelters in the region. United States Securities and Exchange Commission (“SEC”) rules adopted in August 2012 under Section 1502 require reporting companies to disclose annually on Form SD whether any such minerals that are necessary to the functionality or production of products they manufactured, or for which they contracted the manufacture, during the prior calendar year did, in fact, originate in the DRC and, if so, if the related revenue was used to support the conflict and/or abuses.Some of the products we manufacture may contain tantalum, tin, tungsten and/or gold. Consequently, in compliance with SEC rules, we have adopted a policy on conflict minerals, which can be found on our website, and have implemented a supply chain due diligence and risk mitigation process with reference to the Organization for Economic Cooperation and Development (“OECD”) guidance approved by the SEC to assess and report annually whether our products are “conflict free.”
We support efforts to end the violence and human rights abuses in the mining of certain minerals in the DRC. We expect our suppliers to comply with the OECD guidance and industry standards and to ensure that their supply chain conforms to our policy and the OECD guidance. We will mitigate identified risks by working directly with our suppliers; however, we may need to alter our sources of supply or modify our product design if circumstances require. We may incur certain costs in order to comply with these disclosure requirements, including for due diligence to determine the source of the subject minerals used in our products and other potential changes to products, processes or sources of supply as a consequence of such verification activities. In addition, these rules could adversely affect the sourcing, supply and pricing of materials used in our products throughout the supply chain beyond our control, whether or not the subject minerals are “conflict free.”
Product Liability and Insurance
The design, manufacture and marketing of medical devices and services of the types we produce entail an inherent risk of product liability claims. In addition, we provide information to health carehealthcare providers and payors upon which determinations affecting medical care are made, and claims may be made against us resulting from adverse medical consequences to patients allegedly resulting from the information we provide. To protect ourselves from product liability claims, we maintain professional liability and general liability insurance on a “claims made” basis. Insurance coverage under such policies is contingent upon a policy being in effect when a claim is made, regardless of when the events whichevent(s) that caused the claim occurred. While, as of the date of this Annual Report on Form 10-K, a material product liability claim has never been made against us and we believe our insurance policies are adequate in amount and coverage for our current operations, there can be no assurance that the coverage maintained by us is sufficient to cover all future
claims. In addition, there can be no assurance that we will be able to obtain such insurance on commercially reasonable terms in the future.
Employees
As of December 31, 2017,2019, we employedhad approximately 1,6001,700 employees. None of our employees are represented by a collective bargaining agreement. We consider our relationship with our employees to be good.
Available Information
We file electronically with the SEC our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934 (“Exchange Act”Act”). We make these reports available on our website at www.gobio.com, free of charge. Copies of these reports are made available as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. Further copies of these reports are located at the SEC’s Public Reference Room at 100 F Street, NE, Washington, D.C. 20549. Information on the operation of the Public Reference Room can be obtained by calling the SEC at 1-800-SEC-0330.. The SEC maintains a website that contains reports, proxy and information statements, and other information regarding our filings, at www.sec.gov. We do not utilize social media platforms as our primary means of distributing material company information.
Item 1A. Risk Factors
The risk factors discussed below are cautionary statements that identify important factors and risks that could cause actual results to differ materially from those anticipated by the forward-looking statements described under “Cautionary Note Regarding Forward-Looking Statements” contained in this Annual Report on Form 10-K. For more information regarding the forward-looking statements contained in this report, see the Table of Contents of this Annual Report on Form 10-K. You should carefully consider the risks and uncertainties described below, together with all of the other information included in this Annual Report on Form 10-K, in considering our business and prospects.prospects as the occurrence of any of the following risks could affect our business, liquidity, results of operations, financial condition or cash flows. The risks and uncertainties described below are not the only ones facing BioTelemetry. Additional risks and uncertainties not presently known to us may also impair our business operations. The occurrence of any of the following risks could affect our business, liquidity, results of operations, financial condition or cash flows.
Our businesses and those of many of our clients have been and continue to be subject to increased legislation and regulatory scrutiny, and we face the risk of changes to this regulatory environment and business in the future.
U.S. income tax reform efforts could have a material impact on our business. On December 22, 2017, the Tax Cuts and Jobs Act (“TCJA”) was signed into law. The TCJA enacts broad changes to the existing U.S. federal income tax code, including reducing the federal corporate income tax rate from 35% to 21% beginning in 2018, amongst many other complex provisions. The ultimate impact of such tax reforms may differ from our current estimates due to changes in interpretations and assumptions made by us as well as the issuance of any further regulations or guidance that may alter the operation of the U.S. federal income tax code. Various uncertainties also exist in terms of how U.S. states and any foreign countries within which we operate will react to these U.S. federal income tax reforms, which could have additional impacts on our business.
Reimbursement by Medicare is highly regulated, and subject to change and our failure to comply with applicable regulations could decrease our revenue, subject us to penalties or adversely affect our results of operations.
The Medicare program is administered by CMS, which imposes extensive and detailed requirements on medical product and services providers, including, but not limited to, rules that govern how we structure our relationships with physicians, how and when we submit reimbursement claims, how we operate our monitoring centers and how and where we provide our arrhythmia monitoring solutions. Our failure to comply with applicable Medicare rules could result in the discontinuation of our reimbursement under the Medicare payment program, a requirement to return funds already paid to us, civil monetary penalties, criminal penalties and/or exclusion from the Medicare program.
Changes in the reimbursement rate that commercial payors and Medicare will pay for our products and services could adversely affect our revenue.operating performance.
We receiveReductions in reimbursement for our products and servicesrates from consolidation of, or contract negotiations with, commercial payors and from Medicare administrative contractors with jurisdiction in the state where the services are performed. In addition,could adversely affect our prescribing physicians receive reimbursement for professional interpretation of the information provided by our products and services from commercial payors or Medicare. Average commercial reimbursement rates have declined over a three and five year period.operating performance. When commercial payors combine their operations, the combined company may electdecide to reimburse for our products and services at the lowest rate paid by any of the participants in the consolidation. If one of the payors participating in the consolidation
does not reimburse for one of our products or services, the combined company may electdecide not to reimburse for such product or service.
Additionally, our agreements with commercial payors can typically allow either party to the contract to terminate these contractsthe contract by providing between 6030 and 120180 days’ prior written notice to the other party at any time following the end of the initial term of the agreement. contract. Our commercial payors may elect to terminate or not to renew their contracts with us for any reason. A commercial payor who terminates or does not renew their contract with us may, or may not, alter their coverage for the type of services we provide. In the event any of our key commercial payors terminate their agreements with us, elect not to renew or enter into new agreements with us upon expiration of their current agreements, or do not renew or establish new agreements on terms as favorable as are currently contracted, our business, operating results and prospects would be adversely affected.
In addition, CMS may reduce the reimbursement rate for our services, as it has in the past. CMS updates the reimbursement rate via the Medicare physician fee schedule annually. Furthermore, CMS has adopted a complex new system for reimbursing Medicare physician services as required by the Medicare Access and CHIP Reauthorization Act of 2015. Under the new program, which began January 1, 2017, physicians will either report under the Merit-based Incentive Payment System or an Advanced Alternative Payment Model, and their 2017past performance will impact 2019future rates. CMS published a final rule on November 16, 2017, modifying program requirements for performance year 2018. The rule designates use of certain patient-generated health data with an active feedback loop as a “high” weighted activity for purposes of the Advancing Care Information bonus. We cannot predict the impact of this new framework or potential future revisions to physician payment policy on reimbursement for our services. A decrease in Medicare or commercial reimbursement rates or termination of commercial payor contracts would adversely affect our financial results.
The operationFinally, patients may continue to move to Medicare Advantage plans from traditional Medicare plans, which may change the nature of our monitoring centers is subject to rules and regulations governing IDTFs and state licensure requirements; failure to comply with these rules could preventthe reimbursements received by us from receivingtraditional Medicare programs and may negatively affect our revenue.
Our revenues could be affected by third-party reimbursement from Medicarepolicies and some commercial payors.potential cost constraints.
We have several monitoring centers throughoutIn the United States, that analyze the data obtained from cardiac monitors and report the results to physicians. In order for us towe receive reimbursement from Medicarefor our products and someservices from commercial payors our monitoring centers must be certified as IDTFs. Certification as an IDTF requires that we follow strict regulations governing how our monitoring centers operate, such as requirements regardingand from the experience and certifications of the technicians who review data transmitted from our monitors. These rules and regulations vary from location to location and are subject to change. If they change, we may have to change the operating procedures at our monitoring centers, which could increase our costs significantly. If we fail to obtain and maintain IDTF certification, our services may no
longer be reimbursed by Medicare and some commercial payors, which could have a material adverse impact on our business.
Our failure to maintain accreditation could impact our DMEPOS operations.
Accreditation is required by most of our managed care payors and became a mandatory requirement for all Medicare durable medical equipment, prosthetics, orthotics and supplies (“DMEPOS”) providers effective October 1, 2009. In 2016, we acquired Telcare, a diabetes care management company. In 2017, Telcare completed a nationwide accreditation renewal process conducted by the Healthcare Quality Association on Accreditation, which renewed our accreditation for another three years. The Company will undergo the next survey cycle in 2020. If we lose accreditation, our failure to maintain accreditation could have an adverse effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity.
Failure to appropriately track and report certain payments to physicians and teaching hospitals may violate certain federal reporting laws and subject us to fines and penalties.
Section 6002 of the Affordable Care Act requires certain medical device manufacturers that produce devices covered by the Medicare and Medicaid programs to report annually to the government certain payments to physicians and teaching hospitals. If we fail to appropriately track and report such payments to the government, we could be subject to civil fines and penalties, which could adversely affect the results of our operations.
Audits or denials of our claims by government agencies and private payors could reduce our revenue and have an adverse effect on our results of operations.
As part of our business operations, we submit claims on behalf of patients directly to, and receive payments from, Medicare, Medicaid and other third-party payors. We are subject to extensive government regulation, including requirements for submitting reimbursement claims under appropriate codes and maintaining certain documentation to support our claims. Medicare contractors and Medicaid agencies periodically conduct pre-and post-payment reviews and other audits of claims and are under increasing pressure to more closely scrutinize health care claims and supporting documentation. We have previously been subject to pre-and post-payment reviews as well as audits of claims under CMS’ Recovery Audit Program and may experience such reviews and audits of claimsMAC with jurisdiction in the future. Such reviews and similar audits of our claims could result in material delays in payment, as well as material recoupments or denials, which would reduce our net sales and profitability, or result in our exclusion from participation instate where the Medicare or Medicaid programs. Weservices are also subject to similar review and audits from private payors, which may result in material delays in payment and material recoupments and denials.performed. In addition, state agencies may conduct investigationsour prescribing physicians or submit requests for information relating to claims data submitted to private payors.
We have a concentrated number of payors and losing one of them would reduce our sales and adversely affect our business and operating results.
Medicare, our largest payor, represents a significant percentage of our revenue. For the year ended December 31, 2017, Medicare accounted for approximately 34% of our total revenue. No other payor accounted for more than 4% of total revenue. Our agreements with commercial payors typically allow either party to the contract to terminate the contract by providing between 60 and 120 days’ prior written notice to the other party at any time following the end of the initial term of the contract. Our commercial payors may elect to terminate or not to renew their contracts with us for any reason and, in some instances,
can unilaterally change the reimbursement rates they pay. A commercial payor who terminates or does not renew their contract with us may, or may not, alter their coverage of our services. In the event any of our key commercial payors terminate their agreements with us, elect not to renew or enter into new agreements with us upon expiration of their current agreements, or do not renew or establish new agreements on terms as favorable as are currently contracted, our business, operating results and prospects would be adversely affected.
Violation of federal and state laws regarding privacy and security of patient information may adversely affect our business, financial condition or operations.
The use and disclosure of certain health care information by health care providers receive reimbursement for professional interpretation of the information provided by our products and their business associates have come under increased public scrutiny. Federal standards under HIPAA establish rules concerning how individually-identifiable health information may be used, disclosedservices from commercial payors or Medicare. The overall escalating cost of medical products and protected. Historically, state law had governed confidentiality issues,services has led to, and HIPAA preserves these laws to the extent they are more protective of a patient’s privacy or provide the patient with more access to his or her health information. Additionally, HITECH and associated changes to HIPAA impose additional requirements relating to the privacy, security and transmission of individually identifiable health information. We must operate our business in a manner that complies with all applicable laws, both federal and state, and that does not jeopardize the ability of our customers to comply with all applicable laws. We believe that our operations are consistent with these legal standards. Nevertheless, these laws and regulations present risks for health care providers and their business associates that provide services to patients in multiple states. As wewill continue to see how government regulatorslead to, increased pressures on the healthcare industry, both foreign and courts interpretdomestic, to reduce the cost of products and enforce HIPAA’s requirements, weservices. Currently available levels of reimbursement may neednot continue to adjustbe available in the future for our interpretations of these lawsexisting products and regulations over time. If a challenge to our activities is successful, it could have an adverse effect on our operations,services or products or services under development. Third-party reimbursement and coverage may require us to forego relationships with customersnot be available or adequate in certain states and may restrict the territory available to us to expand our business. In addition, even if our interpretations of HIPAA and other federal and state laws and regulations are correct, we could be held liable for unauthorized uses or disclosures of patient information as a result of inadequate systems and controls to protect this information or as a result of the theft of information by unauthorized computer programmers who penetrate our network security.
Violation of these laws against us could have a material adverse effect on our business, financial condition and results of operations. For example, in 2011, we experienced the theft of two unencrypted laptop computers and, as a result, were required to provide notices under the HIPAA Breach Notification Rule and were subsequently investigated byeither the United States Department of Health and Human Services' Office for Civil Rights (“OCR”). Although we have been in compliance with our obligations stemming from these incidents, and believe that our operations are consistent with these legal standards imposed by HIPAA, to avoid the uncertainty of administrative enforcement proceedings or protracted litigation, we elected to settle the investigation by OCR in April 2017 by paying $2.5 million and entering into a 3-year corrective action plan. This settlement did not contain any admission of liability by the Company.
The FDAinternational markets, current reimbursement amounts may recommend a different approach to measuring the cardiac impact and safety of drugs as part of the approval process. Such changes could make the systems and processes of our research segment obsolete and adversely affect revenue and profitability.
As part of its approval process, the FDA has provided guidance reinforcing the need for cardiac safety testing of all compounds entering the blood stream. The requirements vary based on the type and history of each compound. This testing is accomplished by different methods, including cardiac imaging such as MUGA and ECG analysis, which involves measuring the QT/QTc interval for prolongation. We function as a core lab and have developed proprietary systems and processes to receive cardiac imaging
studies and ECGs for analysis. It is possible that,be decreased in the future the FDAand future legislation, and regulation or reimbursement policies of third-party payors may recommend a different approach for evaluating the cardiac impact and safety of compounds which may diminish the need for a core lab. This would considerably reduce the value ofdemand for our existing systems and processes and would substantially decrease our revenue and profitability in our Research segment.
In December 2015, the FDA published a report which called into question the need for certain QT studies. In a series of public meetings throughout 2016 discussing the report, FDA speakers indicated that certain studies were no longer mandatory and that future regulations will include some combination of traditional study types along with early phase Exposure Response modeling. Further guidance around the performance of QT studies from the FDA is expected. We cannot assess the impact of this expected guidance at this time, but it may substantially decrease our revenue and profitability in our Research segment.
We are subject to numerous FDA regulations and decisions and it may be costly to comply with these regulations and decisions and to develop compliant products and processes.
The devices that we manufacture are classified as medical devices and are subject to extensive regulation by the FDA. Further, we maintain establishment registration with the FDA as a distributor of medical devices. FDA regulations govern manufacturing, labeling, promotion, distribution, importing, exporting, shipping and sale of these devices. Our devices and our arrhythmia detection algorithms have 510(k) Clearance status from the FDA. Modifications to our devices or our algorithms that could significantly affect safety or effectiveness, or that could constituteability to sell our products on a significant change in intended use, would require a new clearance from the FDA. If in the future we make changes to our devices or our algorithms, the FDA could determine that such modifications require new FDA clearance, and we may not be able to obtain such FDA clearances timely, or at all.profitable basis.
We are subject to continuing regulation by the FDA, including quality regulations applicable to the manufacture of our devices and various reporting regulations, as well as regulations that govern the promotion and advertising of medical devices. The FDA could find that we have failed to comply with one of these requirements, which could result in a wide variety of enforcement actions, ranging from a warning letter to one or more severe sanctions. These sanctions could include fines, injunctions and civil penalties; recall or seizure of devices; operating restrictions, partial suspension or total shutdown of production; refusal to grant 510(k) Clearance of new components or algorithms; withdrawing 510(k) Clearance already granted to one or more of our existing components or algorithms; and criminal prosecution. Any of these enforcement actions could be costly and significantly harm our business, financial condition and results of operations.
Our operations and our interactions with our physicians and patients are subject to regulation aimed at preventing health carehealthcare fraud and abuse and, if we are unable to fully comply with such laws, we could face substantial penalties.
Our operations may be directly or indirectly affected by various broad state and federal health carehealthcare fraud and abuse laws, including the Federal Healthcare Programs’ Anti-Kickback Statute and the Federal False Claims Act.FCA. For
some of our services, we directly bill physicians or other health carehealthcare entities, that, in turn, bill payors. Although we believe such payments and practices are proper and in compliance with laws and regulations, we may be subject to claims asserting that we have violated these laws and regulations. If our past or present operations are found to be in violation of these laws, we or our officers may be subject to civil or criminal penalties, including large monetary penalties, damages, fines, imprisonment and exclusion from Medicare and Medicaid program participation. Furthermore, if we knowingly file, or “cause” the filing of, false claims for reimbursement with government programs such as Medicare and Medicaid, we may be subject to substantial civil penalties, including treble damages. The Federal False
Claims ActFCA also contains “whistleblower” or “qui tam” provisions that allow private individuals to bring actions on behalf of the government alleging that the defendant has defrauded the government. In recent years, the number of suits brought in the medical industry by private individuals has increased dramatically. Various states have enacted laws modeled after the Federal False Claims Act,FCA, including “qui tam” provisions, and some of these laws apply to claims filed with commercial insurers. Even if we are not found to have violated any of these federal or state anti-fraud or false claims acts, the costs of defending these claims could adversely affect our results of operations.
The operation of our monitoring centers is subject to rules and regulations governing IDTFs and state licensure requirements; failure to comply with these rules could prevent us from receiving reimbursement from Medicare and some commercial payors.
We have several monitoring centers throughout the United States that analyze the data obtained from cardiac monitors and report the results to physicians. In order for us to receive reimbursement from Medicare and some commercial payors, our monitoring centers must be certified as IDTFs. Certification as an IDTF requires that we follow strict regulations governing how our monitoring centers operate, such as requirements regarding qualifications of the technicians who review data transmitted from our monitors. These rules can vary from location to location and are subject to change. If they change, we may have to change the operating procedures at our monitoring centers, which could increase our costs significantly. If we fail to obtain and maintain IDTF certification, our services may no longer be reimbursed by Medicare and some commercial payors, which could have a material adverse impact on our business.
Our failure to maintain accreditation could impact our DMEPOS operations.
Accreditation is required by most of our managed care payors and became a mandatory requirement for all Medicare durable medical equipment, prosthetics, orthotics and supplies (“DMEPOS”) providers effective October 1, 2009. In 2017, we completed a nationwide accreditation renewal process conducted by the Healthcare Quality Association on Accreditation, which renewed our accreditation for another three years. We will undergo the next survey cycle in 2020. If we lose accreditation, our failure to maintain accreditation could have an adverse effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity.
Billing for our products and service is complex, and we must dedicate substantial time and resources to the billing process.
Billing for our products and services is complex, time consuming and expensive. Depending on the billing arrangement and applicable law, we bill several types of payors, including CMS, third-party commercial payors, institutions and patients, which may have different billing requirements procedures or expectations. We also must bill patient co-payments, co-insurance and deductibles. We face risk in our collection efforts, including potential write-offs of doubtful accounts and long collection cycles, which could adversely affect our business, financial condition and results of operations.
Several factors make the billing and collection process uncertain, including: differences between the submitted price for our products and services and the reimbursement rates of payors; compliance with complex federal and state regulations related to billing CMS; differences in coverage among payors and the effect of patient co-payments, co-insurance and deductibles; differences in information and billing requirements among payors; and incorrect or missing patient history, indications or billing information.
Additionally, our billing activities require us to implement compliance procedures and oversight, train and monitor our employees and undertake internal review procedures to evaluate compliance with applicable laws, regulations and internal policies. Payors also conduct audits to evaluate claims, which may add further cost and uncertainty to the billing process. These billing complexities, and the related uncertainty in obtaining payment for our products and services, could negatively affect our revenue and cash flow, our ability to achieve profitability, and the consistency and comparability of our results of operations.
Failure to appropriately track and report certain payments to physicians and teaching hospitals may violate certain federal reporting laws and subject us to fines and penalties.
Section 6002 of the ACA requires certain medical device manufacturers that produce devices covered by the Medicare and Medicaid programs to report annually to the government certain payments and transfers of value to physicians and teaching hospitals. If we fail to appropriately track and report such payments to the government, we could be subject to civil fines and penalties, which could adversely affect the results of our operations.
Audits or denials of our claims by government agencies and commercial payors could reduce our revenue and have an adverse effect on our results of operations.
As part of our business operations, we submit claims on behalf of patients directly to, and receive payments from, Medicare, Medicaid and other third-party payors. We are subject to extensive government regulation, including requirements for submitting reimbursement claims under appropriate codes and maintaining certain documentation to support our claims. Medicare contractors and Medicaid state agencies periodically conduct pre-and post-payment reviews and other audits of claims and are under increasing pressure to more closely scrutinize healthcare claims and supporting documentation. The Medicare and Medicaid programs also have broad authority to impose payment suspensions and supplier number revocations when they believe credible allegations of fraud or other supplier standard noncompliance issues exist. We have previously been subject to pre-and post-payment reviews as well as audits of claims under CMS’ Recovery Audit Program and may experience such reviews and audits of claims in the future. Such reviews and similar audits of our claims could result in restrictions on our ability to bill for our services, material delays in payment, as well as material recoupments or denials, which would reduce our net sales and profitability, or result in our exclusion from participation in the Medicare or Medicaid programs. We are also subject to similar review and audits from commercial payors, which may result in material delays in payment and material recoupments and denials. In addition, state agencies may conduct investigations or submit requests for information relating to claims data submitted to commercial payors.
We have a concentrated number of payors and losing one of them would reduce our sales and adversely affect our business and operating results.
Medicare, our largest payor, represents a significant percentage of our revenue. For the year ended December 31, 2019, Medicare (exclusive of Medicare Advantage) accounted for approximately 35% of our total revenue. No other payor accounted for more than 6% of total revenue. Our agreements with commercial payors typically allow either party to the contract to terminate the contract by providing between
30 and 180 days’ prior written notice to the other party at any time following the end of the initial term of the contract. Our commercial payors may elect to terminate or not to renew their contracts with us for any reason. A commercial payor who terminates or does not renew their contract with us may, or may not, alter their coverage for the type of services we provide. In the event any of our key commercial payors terminate their agreements with us, elect not to renew or enter into new agreements with us upon expiration of their current agreements, or do not renew or establish new agreements on terms as favorable as are currently contracted, our business, operating results and prospects would be adversely affected.
Our cardiac monitoring and INR testing businesses are dependent upon physicians prescribing our services and failure to obtain those prescriptions may adversely affect our revenue.
The success of our cardiac monitoring and INR testing businesses are dependent upon physicians prescribing our services. Our success in obtaining prescriptions will be directly influenced by a number of factors, including:
the ability of the physicians with whom we work to obtain sufficient reimbursement and be paid in a timely manner for the professional services they provide in connection with the use of our cardiac monitoring solutions;
| |
| • | our ability to continue to establish ourselves as a comprehensive cardiac monitoring and INR services provider; |
our ability to educate physicians regarding the benefits of our services over alternative diagnostic monitoring solutions; and
the clinical efficacy of our devices.
If we are unable to educate physicians regarding the benefits of our products and obtain sufficient prescriptions for our services, revenue from the provision of our cardiac monitoring and INR solutions could potentially decrease.
If we are unable to provide service in a timely manner, physicians may elect not to prescribe our services, and our revenue and growth prospects may be adversely affected.
While our goal is to provide each patient with the appropriate device in a timely manner, we have experienced, and may in the future experience, service delays or delays due to the availability of devices. This can occur when converting to a new generation of device or in connection with the increase in prescriptions following acquisitions of other companies.
We may also experience shortages of devices due to manufacturing difficulties. Multiple suppliers provide the components used in our devices, but our Minnesota and Massachusetts facilities are registered and approved by the FDA as the manufacturer of record of our devices. Our manufacturing operations could be disrupted by fire, earthquake or other natural disaster, a labor-related disruption, failure in supply or other logistical channels, network or electrical outages or other reasons. If there were a disruption to our facilities in Minnesota or Massachusetts, we would be unable to manufacture devices until we have restored and re-qualified our manufacturing capability or developed alternative manufacturing facilities.
Our success in obtaining future cardiac monitor prescriptions from physicians is dependent upon our ability to promptly deliver devices to our patients, and a failure in this regard would have an adverse effect on our revenue and growth prospects.
Cost-containment efforts of group purchasing organizations could adversely affect our selling prices, financial position and results of operations.
Some of our existing and potential customers may become members of group purchasing organizations (“GPOs”) and integrated delivery network (“IDN”) in an effort to reduce costs. GPOs and IDNs negotiate pricing arrangements with healthcare suppliers and distributors and offer the negotiated prices to affiliated hospitals and other members. GPOs and IDNs typically award contracts on a category-by-category basis through a competitive bidding process. Bids are generally solicited from multiple vendors with the intention of driving down pricing. Due to the highly competitive nature of the GPO and IDN contracting processes, we may not be able to obtain market prices for our products or obtain or maintain contract positions with major GPOs and IDNs, which could adversely impact our profitability. Also, sales through a GPO or IDN can be significant to our business and if we are unable to retain contracts with our customers, or acquire additional contracts, our financial results may be negatively impacted.
We are increasingly dependent on sophisticated information technology systems to operate our business, and if we fail to properly maintain the integrity of our data or if our products do not operate as intended or we experience a cyber-attack or other breach of these systems, our business could be materially affected.
We are increasingly dependent on sophisticated information technology for our products and infrastructure. We rely on information technology systems to process, transmit and store electronic information in our day-to-day operations. The size and complexity of our information technology systems makes them vulnerable to increasingly sophisticated cyber-attacks, malicious intrusion, breakdown, destruction, loss of data privacy or other significant disruption. For example, in October 2019, we detected suspicious activity on our information technology network, which required services and systems to be taken offline for a period of time. Our information systems require an ongoing commitment of significant resources to maintain, protect and enhance existing systems and develop new systems to keep pace with continuing changes in information processing technology, evolving systems and regulatory standards, the increasing need to protect patient and customer information and changing customer patterns. As a result of technology initiatives, recently enacted regulations, changes in our system platforms and integration of new business acquisitions, we have been consolidating and integrating the number of systems we operate and have upgraded and expanded our information systems capabilities.
In addition, third parties may attempt to hack into our products or systems and may obtain data relating to patients with our products or our proprietary information. If we fail to maintain or protect our information systems and data integrity effectively, we could lose existing customers, have difficulty attracting new customers, have problems in determining product cost estimates and establishing appropriate pricing, have difficulty preventing, detecting and controlling fraud, have disputes with customers, physicians and other healthcare professionals, have regulatory sanctions or penalties imposed, have increases in operating expenses, incur expenses or lose revenue as a result of a data privacy breach or suffer other adverse consequences. There can be no assurance that our process of consolidating the number of systems we operate, upgrading and expanding our information systems capabilities, protecting and enhancing our systems and developing new systems to keep pace with continuing changes in information processing technology will be successful or that additional systems issues will not arise in the future. Any significant breakdown, intrusion, interruption, corruption or destruction of these systems, as well as any data breaches, could have a material adverse effect on our business.
Violation of federal and state laws regarding privacy and security of patient information or other personal information may adversely affect our business, financial condition or operations.
The use and disclosure of certain healthcare information by HIPAA-covered entities and their business associates have come under increased public scrutiny. Federal standards under HIPAA establish rules concerning how individually-identifiable health information may be used, disclosed and protected. Historically, state law had governed confidentiality issues, and HIPAA preserves these laws to the extent they are more protective of a patient’s privacy or provide the patient with more access to his or her health information. Additionally, amendments to HIPAA (e.g. HITECH) impose additional requirements relating to the privacy, security and transmission of individually identifiable health information. Further, certain personal information that is not regulated by HIPAA may instead be subject to other state privacy laws, such as the California Consumer Protection Act. We must operate our business in a manner that complies with all applicable laws, both federal and state, and that does not jeopardize the ability of our customers to comply with all applicable laws. We believe that our operations are consistent with these legal standards. Nevertheless, these laws and regulations present risks for covered entities and their business associates that provide services to patients in multiple states. As we continue to see how government regulators and courts interpret and enforce HIPAA and other state law requirements, we may need to adjust our interpretations of these laws and regulations over time. If a challenge to our activities is successful, it could have an adverse effect on our operations, may require us to forgo relationships with customers in certain states and may restrict the territory available to us to expand our business. In addition, even if our interpretations of HIPAA and other federal and state laws and regulations are correct, we could be held liable for unauthorized uses or disclosures of patient information as a result of inadequate systems and controls to protect this information or as a result of the theft of information by unauthorized computer programmers who penetrate our network security.
Violation of these laws against us could have a material adverse effect on our business, financial condition and results of operations. For example, in 2011, we experienced the theft of two unencrypted laptop computers and, as a result, were required to provide notices under the HIPAA Breach Notification Rule and were subsequently investigated by the United States Department of Health and Human Services’ (“HHS”) Office for Civil Rights (“OCR”). Although we have been in compliance with our obligations stemming from these incidents, and believe that our operations are consistent with the legal standards imposed by HIPAA, to avoid the uncertainty of administrative enforcement proceedings or protracted litigation, we elected to settle the investigation by OCR in April 2017 by paying $2.5 million and entering into a three-year corrective action plan. This settlement did not contain any admission of liability by us.
The FDA may recommend a different approach to measuring the cardiac impact and safety of drugs as part of the approval process. Such changes could make the systems and processes of our research segment obsolete and adversely affect revenue and profitability.
As part of its approval process, the FDA has provided guidance reinforcing the need for cardiac safety testing of all compounds entering the blood stream. The requirements vary based on the type and history of each compound. This testing is accomplished by different methods, including cardiac imaging such as MUGA and ECG analysis, which involves measuring the QT/QTc interval for prolongation. We function as a core lab and have developed proprietary systems and processes to receive cardiac imaging studies and ECGs for analysis. It is possible that, in the future, the FDA may recommend a different approach for evaluating the cardiac impact and safety of compounds which may diminish the need for a core lab. This would considerably reduce the value of our existing systems and processes and would substantially decrease our revenue and profitability in our Research segment.
In December 2015, the FDA published a report which called into question the need for certain QT studies. In a series of public meetings throughout 2016 discussing the report, FDA speakers indicated that certain studies were no longer mandatory and that future regulations will include some combination of traditional study types along with early phase Exposure Response modeling. Further guidance around the performance of QT studies from the FDA is expected. We cannot assess the impact of this expected guidance at this time, but it may substantially decrease our revenue and profitability in our Research segment.
We are subject to numerous FDA regulations and decisions, and it may be costly to comply with these regulations and decisions and to develop compliant products and processes.
The devices that we manufacture are classified as medical devices and are subject to extensive regulation by the FDA. Further, we maintain establishment registration with the FDA as a distributor of medical devices. FDA regulations govern manufacturing, labeling, promotion, distribution, importing, exporting, shipping, advertising, promotion and sale of these devices. Our devices and our arrhythmia detection algorithms have 510(k) clearance status from the FDA. Modifications to our devices or our algorithms that could significantly affect safety or effectiveness, or that could constitute a significant change in intended use, would require a new clearance from the FDA. If in the future we make changes to our devices or our algorithms, the FDA could determine that such modifications require new FDA clearance, and we may not be able to obtain such FDA clearances timely, or at all.
We are subject to continuing regulation by the FDA, including general controls, special controls and quality system regulations applicable to the manufacture of our devices and various reporting regulations, as well as regulations that govern the promotion and advertising of medical devices. The FDA could find that we have failed to comply with one of these requirements, which could result in a wide variety of enforcement actions, ranging from a warning letter to one or more severe sanctions. These sanctions could include criminal fines, injunctions and civil money penalties; recall or seizure of devices; operating restrictions, partial suspension or total shutdown of production; refusal to grant 510(k) clearance of new components or algorithms; withdrawing 510(k) clearance already granted to one or more of our existing components or algorithms; and criminal prosecution. Any of these enforcement actions could be costly and significantly harm our business, financial condition and results of operations.
The healthcare industry is the subject of numerous governmental investigations into marketing and other business practices. These investigations could result in the commencement of civil and/or criminal proceedings, substantial fines, penalties, and/or administrative remedies, divert the attention of our management, and have an adverse effect on our financial condition and results of operations.
As mentioned above, we are subject to rigorous regulation by the FDA and numerous other federal, state and foreign governmental authorities. These authorities have been increasing their scrutiny of our industry. We occasionally receive subpoenas or other requests for information from state and federal governmental agencies, including, among others, the United States Department of Justice and the Office of Inspector General of the U.S. Department of Health and Human Services.HHS. These investigations typically relate primarily to financial arrangements with health carehealthcare providers, regulatory compliance and product promotional practices.
We cooperate with these investigations and respond to such requests. However, when an investigation begins, we cannot predict when it will be resolved, the outcome of the investigation or its impact on us. An adverse outcome in one or more of these investigations could include the commencement of civil and/or criminal proceedings, substantial fines, penalties and/or administrative remedies, including exclusion from government reimbursement programs and entry into Corporate Integrity Agreements with governmental agencies. In addition, resolution of any of these matters could involve the imposition of additional and costly compliance obligations. Finally, if these investigations continue over a long period
of time, they could divert the attention of management from the day-to-day operations of our business and impose significant administrative burdens, including cost, on us. These potential consequences, as well as any adverse outcome from these investigations or other investigations initiated by the government at any time, could have a material adverse effect on our financial condition and results of operations.
Resolution of income tax matters may impact our financial condition, results of operations and cash flows.
We are subject to income taxes in many U.S. and certain foreign jurisdictions, which requires significant judgment in determining our effective income tax rate and in evaluating tax positions, particularly those related to unrecognized tax benefits. We have provided for unrecognized tax benefits when such tax positions do not meet the recognition thresholds or measurement standards prescribed by the accounting standard for unrecognized tax benefits. Changes in unrecognized tax benefits or other adjustments resulting from tax audits and settlements with taxing authorities, including related interest and penalties, impact our effective tax rate. When particular tax matters arise, a number of years could elapse before such matters are audited and finally resolved. We believe our positions are appropriate, however, federal, state, or foreign tax authorities could disagree. If the settlement of any unrecognized tax reserves is different than accrued, it would impact our effective rate in the year of resolution. Any resolution of a tax matter may require the adjustment of tax assets or tax liabilities and/or the use of cash in the year of resolution.
Our business is subject to the risks of international operations.
Compliance with applicable United States and foreign laws and regulations, such as import and export requirements, anti-corruption laws, tax laws, foreign exchange controls and cash repatriation restrictions, data privacy requirements (e.g. GDPR), environmental laws, labor laws and anti-competition regulations, increases the costs of doing business in foreign jurisdictions. Although we have implemented policies and procedures to comply with these laws and regulations, a violation by our employees, contractors or agents could nevertheless occur. In some cases, compliance with the laws and regulations of one country could violate the laws and regulations of another country. Violations of these laws and regulations could materially adversely affect our brand, international growth efforts and business.
If our employees or agents violate the U.S. Foreign Corrupt Practices Act or anti-bribery laws in other jurisdictions, we may incur fines or penalties, or experience other adverse consequences.
We alsoare subject to the U.S. Foreign Corrupt Practices Act (“FCPA”) and similar anti-bribery laws in international jurisdictions, which generally prohibit companies and their intermediaries from making improper payments to non-U.S. officials for the purpose of obtaining or retaining business. Because of the predominance of government-sponsored healthcare systems around the world, many of our customer relationships outside of the United States are with governmental entities and are therefore subject to such anti-bribery laws. Our sales to customers and distributors outside of the United States have been increasing, and we expect them to continue to increase in the future. If our employees or agents violate the provisions of the FCPA or other anti-bribery laws, we may incur fines or penalties, we may be unable to market our products in other countries or we may experience other adverse consequences which could be affected byhave a material adverse effect on our operating results or financial condition.
In addition, the DOJ or other risks associated with international activitiesgovernmental agencies could impose a broad range of civil and criminal sanctions under the FCPA and other laws and regulations including, but not limited to, economicinjunctive relief, disgorgement, fines, penalties, modifications to business practices including the termination or modification of existing business relationships, the imposition of compliance programs and labor conditions, increased duties, taxes (including taxes related to our international operations) and other costs and political instability. Margins on salesthe retention of our products in foreign countries, and on sales of products that include components obtained from foreign suppliers, could be materially adversely affected by international trade regulations, including duties, tariffs and antidumping penalties. For the year ended December 31, 2017, our international operations comprised less than 1% of our totala monitor
revenue. We are also exposed to credit and collectability riskoversee compliance with the FCPA. The imposition of any of these sanctions or remedial measures could have a material adverse effect on our trade receivables with customers in certain international markets. There can be no assurance that we can effectively limit its credit riskbusiness and avoid losses.results of operations.
If we do not obtain and maintain adequate protection for our intellectual property, it may adversely affect the value of our technology and devices and future revenue and operating income.
Our business and competitive positions are in part dependent upon our ability to protect our proprietary technology. To protect our proprietary rights, we rely on a combination of trademark, copyright, patent, trade secret and other intellectual property laws, employment, confidentiality and invention assignment agreements with our employees and contractors, and confidentiality agreements and protective contractual provisions with other third parties. We attempt to protect our intellectual property position by filing trademark applications and United States and international patent applications related to our proprietary technology, inventions and improvements that are important to the development of our business.
We do not believe that any single patent, trademark or other intellectual property right of ours, or combination of our intellectual property rights, is likely to prevent others from competing with us using a similar business model. There are many issued patents and patent applications held by others in our industry and the electronics field. Our competitors may independently develop technologies that are substantially similar or superior to our technologies, or design around our patents or other intellectual property to avoid infringement. In addition, we may not apply for a patent relating to products or processes that are patentable, we may fail to receive any patent for which we apply or have applied, and any patent owned by us or issued to us could be circumvented, challenged, invalidated, or held to be unenforceable or rights granted thereunder may not adequately protect our technology or provide a competitive advantage to us. If a third-party challenges the validity of any patents or proprietary rights of ours, we may become involved in intellectual property disputes and litigation that would be costly and time-consuming. All of our patents will eventually expire. Some of our patents, including patents protecting significant elements of our technology, will expire between 2018 andhave expiration dates through 2032, at which point we can no longer enforce these against third parties to prevent them from making, using, selling, offering to sell or importing our current clinical device. While we have several patents expiring between 2018 andthat have expiration dates through 2032, including patents that relate, in part, to our key products, our technology is typically covered by several patents, creating a system of protected technology. The expiration of our patents could expose us to more competition and have an adverse impact on our business.
Although third parties may infringe on our patents and other intellectual property rights, we may not be aware of any such infringement, or we may be aware of potential infringement but elect not to seek to prevent such infringement or pursue any claim of infringement, and the third-party may continue its potentially infringing activities. Any decision whether or not to take further action in response to potential infringement of our patent or other intellectual property rights may be based on a variety of factors, such as the potential costs and benefits of taking such action, and business and legal issues and circumstances. Litigation of claims of infringement of a patent or other intellectual property rights may be costly and time-consuming, may divert the attention of key management personnel and may not be successful or result in any significant recovery of compensation for any infringement or enjoining of any infringing activity. Litigation or licensing discussions may also involve or lead to counterclaims that could be brought by a potential infringer to challenge the validity or enforceability of our patents and other intellectual property.
To protect our trade secrets and other proprietary information, we generally require our employees, consultants, contractors and outside collaborators to enter into written non-disclosure agreements. These agreements, however, may not provide adequate protection to prevent any unauthorized use, misappropriation or disclosure of our trade secrets, know-how or other proprietary information. These
agreements may be breached, and we may not become aware of, or have adequate remedies in the event of, any such breach. Also, others may independently develop the same or substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets.
Our ability to innovate or market our products may be impaired by the intellectual property rights of third parties.
Our success is dependent, in part, upon our ability to avoid infringing the patents or proprietary rights of others. The cardiac monitoring industry is characterized by a large number of patents and patent filings. Competitors may have filed applications for, or have been issued, patents and may obtain additional patents and proprietary rights related to devices, services or processes that we use to compete. We may not be aware of all of the patents or patent applications potentially adverse to our interests that may have been filed or issued to others.
United States patent applications may be kept confidential while pending in the Patent and Trademark Office. If other companies have or obtain patents relating to our products or services, we may be required to obtain licenses to those patents or to develop or obtain alternative technology. We may not be able to obtain any such licenses on acceptable terms, or at all. Any failure to obtain such licenses could impair or foreclose our ability to make, use, market or sell our products and services.
Based on the fact that we may pose a competitive threat to some companies who own or control various patents, it is possible that one or more third parties may assert a patent infringement claim seeking damages and to enjoin the manufacture, use, sale and marketing of our products and services. If a third-party asserts that we have infringed on its patent or proprietary rights, we may become involved in intellectual property disputes and litigation that would be costly and time-consuming and could impair or foreclose our ability to make, use, market or sell our products and services. Lawsuits may have already been filed against us without our knowledge. Additionally, we may receive notices from other third parties suggesting or asserting that we are infringing their patents and inviting us to license such patents. We do not believe that we are infringing on any other party’s patents or that a license to any such patents is necessary. Should litigation over such patents arise, we intend to vigorously defend against any allegation of infringement.
If we are found to infringe on the patents or intellectual property rights of others, we may be required to pay damages, stop the infringing activity or obtain licenses or rights to the patents or other intellectual property in order to use, manufacture, market or sell our products and services. Any required license may not be available to us on acceptable terms, or at all. If we succeed in obtaining such licenses, payments under such licenses would reduce any earnings from our products. In addition, licenses may be non-exclusive and, accordingly, our competitors may have access to the same technology as that which may be licensed to us. If we fail to obtain a required license or are unable to alter the design of our product candidates to make a license unnecessary, we may be unable to manufacture, use, market or sell our products and services, which could significantly affect our ability to achieve, sustain or grow our commercial business.
If we are unableWe may not be able to consummate future acquisitions or successfully integrate acquired companiesacquisitions into our business, which could result in unanticipated expenses and technology, we may not realize the benefits anticipated and our future growth may be adversely affected.losses.
We have grown, in part, through acquisitions of companies and technology, including our acquisitions of the assets of the ePatch Division of DELTA Danish Electronics, Light & Acoustics, VirtualScopics and Telcare Medical Supply, Inc. (“Telcare”) in April 2016, VirtualScopicsLifeWatch in May 2016, Telcare2017 and Geneva and ADEA Medical AB (“ADEA”) in December 20162019. Our strategy is largely based on our ability to grow through acquisitions of additional businesses to build an integrated group. Consummating acquisitions of related businesses, or our failure to integrate such businesses successfully into our existing businesses, could result
in unanticipated expenses and LifeWatchlosses. Furthermore, we may not be able to realize any of the anticipated benefits from acquisitions.
We anticipate that any future acquisitions we may pursue as part of our business strategy may be partially financed through additional debt or equity. If new debt is added to current debt levels, or if we incur other liabilities, including acquisition-related contingent consideration, in July 2017. Acquisitions involveconnection with an acquisition, the debt or liabilities could impose additional constraints and requirements on our business and operations, which could materially adversely affect our financial condition and results of operation. In addition, to the extent our common stock is used for all or a portion of the consideration to be paid for future acquisitions, dilution may be experienced by existing stockholders.
In connection with our completed and future acquisitions, the process of integrating acquired operations into our existing group operations may result in unforeseen operating difficulties and may require significant financial resources that would otherwise be available for the ongoing development or expansion of existing operations. Some of the risks associated with our assumptionacquisitions include:
unexpected losses of the liabilities of an acquired company, which may be liabilities that we werekey employees or are unaware of at the time of the acquisition, potential write-offs of acquired assets and potential losscustomers of the acquired company;
conforming the acquired company’s key employeesstandards, processes, procedures and controls with our operations;
negotiating with labor unions; and
increasing the scope, geographic diversity and complexity of our current operations.
We may encounter unforeseen obstacles or customers. Physician, patientcosts in the integration of businesses that we may acquire. In addition, general economic and customer satisfactionmarket conditions or performance problems with an acquired business,
technology, serviceother factors outside of our control could make our operating strategies difficult or deviceimpossible to implement. Any failure to implement these operational improvements successfully and/or the failure of these operational improvements to deliver the anticipated benefits could also have a material adverse effect on our reputation. Additionally,results of operations and financial condition.
Any due diligence by us in connection with an acquisition may not reveal all relevant considerations or liabilities of the target business, which could have a material adverse effect on our financial condition or results of operations.
We intend to conduct such due diligence as we deem reasonably practicable and appropriate based on the facts and circumstances applicable to any potential disputesacquisition. The objective of the due diligence process will be to identify material issues which may affect the decision to proceed with any one particular acquisition target or the sellerconsideration payable for an acquisition. We also intend to use information revealed during the due diligence process to formulate our business and operational planning for, and our valuation of, any target company or business. While conducting due diligence and assessing a potential acquisition, we may rely on publicly available information, if any, information provided by the relevant target company to the extent such company is willing or able to provide such information and, in some circumstances, third party investigations.
There can be no assurance that the due diligence undertaken with respect to an acquiredacquisition will reveal all relevant facts that may be necessary to evaluate such acquisition including the determination of the price we may pay for an acquisition target or to formulate a business strategy. Furthermore, the information provided during due diligence may be incomplete, inadequate or inaccurate. As part of the due diligence process, we will also make subjective judgments regarding the results of operations, financial condition and prospects of a potential target. If the due diligence investigation fails to correctly identify
material issues and liabilities that may be present in a target company or business, or its employees, suppliersif we consider such material risks to be commercially acceptable relative to the opportunity, and we proceed with an acquisition, we may subsequently incur substantial impairment charges or customers could adversely affect our business, operating results and financial condition. Ifother losses.
In addition, following an acquisition, we fail to properly evaluate and execute acquisitions, our business may be disrupted and our operating results and prospects may be harmed.
Furthermore, integrating acquired companies or new technologies into our business may prove more difficult than we anticipate. We may encounter difficulties in successfully integrating our operations, technologies, services and personnel with thatsubject to significant, previously undisclosed liabilities of the acquired business that were not identified during due diligence and which could contribute to poor operational performance, undermine any attempt to restructure the acquired company or business in line with our business plan and have a material adverse effect on our financial condition and management resources may be diverted from our existingresults of operations. Offices in multiple states create a strain on our ability to effectively manage our operations and key personnel. If we elect to consolidate our facilities, we may lose key personnel unwilling to relocate to the consolidated facility, may have difficulty hiring appropriate personnel at the consolidated facility and may have difficulty providing continuity of service through the consolidation.
The success of our business is partially dependent on our ability to raise capital, and failure to raise the necessary capital may adversely affect our results of operations, financial condition and stock price.
We believe that our existing cash and cash equivalents, together with our term loan andamended revolving credit facility pursuant to our Credit Agreement with SunTrust BankTruist and Lenders named therein (the “2020 Credit Agreement” and “Lenders”, respectively), will be sufficient to meet our anticipated cash requirements for the foreseeable future. However, our future funding requirements will depend on many factors, including:
the results of our operations;
the reimbursement rates associated with our products and services;
our ability to secure contracts with additional commercial payors providing for the reimbursement of our services;
the costs associated with manufacturing and building our inventory of our current and future generation monitors;
the costs of hiring additional personnel and investing in infrastructure to support future growth;
the costs of undertaking future strategic initiatives, such as acquisitions or joint ventures;
the emergence of competing technologies and products and other adverse market developments;
the costs of preparing, filing, prosecuting, maintaining and enforcing patent claims and other intellectual property rights or defending against claims of infringement by others; and
actions taken by the FDA, | |
| • | actions taken by the FDA, CMS and other regulatory authorities affecting cardiac monitoring devices and competitive products. |
If we decide to raise additional capital in the future, such capital may not be available on reasonable terms, or at all. If we raise additional funds by issuing equity securities, dilution to existing stockholders would result. If we raise additional funds by incurring debt financing, the terms of the debt may involve significant cash payment obligations as well as covenants and financial ratios that may restrict our ability to operate our business.
We have outstanding debt, and may incur other debt in the future, which could adversely affect our financial condition, liquidity and results of operations.
As of December 31, 2017,2019, we had an outstanding term loan pursuant to our SunTrust Credit Agreement with SunTrust Bank and the Lenders named therein of $199.4$194.7 million, net of $5.6$3.2 million of deferred financing costs. We may
borrow additional amounts in the future and use the proceeds from any future borrowing for general corporate purposes, future acquisitions or expansion of our business.
Our incurrence of this debt, and any increases in our levels of debt, may adversely affect our operating results and financial condition by, among other things:
requiring a portion of our cash flow from operations to make payments on this debt; or
limiting our flexibility in planning for, or reacting to, changes in our business and the industry.
Our current credit facility imposes restrictions on us, including restrictions on our ability to create liens on our assets, incur additional indebtedness, make acquisitions or dispose of assets, and also requires us to maintain compliance with specified financial ratios. Our ability to comply with these ratios may be affected by events beyond our control. If we breach any of the covenants and do not obtain a waiver from our lender, then, subject to applicable cure periods, our outstanding indebtedness could be declared immediately due and payable.
Our financing costs may be adversely affected by changes in LIBOR.
LIBOR, the London interbank offered rate, is the basic rate of interest used in lending between banks on the London interbank market and is widely used as a reference for setting the interest rate on loans globally. We use LIBOR as a reference rate in our amended revolving credit facility to calculate interest due to our lender. On July 27, 2017, the United Kingdom’s Financial Conduct Authority, which regulates LIBOR, announced its intention to phase out LIBOR by the end of 2021. It is unclear if LIBOR will cease to exist at that time or if new methods of calculating LIBOR will be established such that it continues to exist after 2021. If LIBOR ceases to exist, we may need to renegotiate our 2020 Credit Agreement. This could have an adverse effect on our financing costs.
Our business depends on our ability to attract and retain talented employees.
Our business is based on successfully attracting and retaining talented employees, including our executive team. The market for highly-skilled workers and leaders in our industry is extremely competitive. If we are less successful in our recruiting efforts, or if we are unable to retain key employees, our ability to develop and deliver successful products and services may be adversely affected.
Our cardiac monitoring and INR testing businesses are dependent upon physicians prescribing our services and failure to obtain those prescriptions may adversely affect our revenue.
The success of our cardiac monitoring and INR testing businesses are dependent upon physicians prescribing our services. Our success in obtaining prescriptions will be directly influenced by a number of factors, including:
the ability of the physicians with whom we work to obtain sufficient reimbursement and be paid in a timely manner for the professional services they provide in connection with the use of our cardiac monitoring solutions;
our ability to continue to establish ourselves as a comprehensive cardiac monitoring and INR services provider;
our ability to educate physicians regarding the benefits of our services over alternative diagnostic monitoring solutions; and
the clinical efficacy of our devices.
If we are unable to educate physicians regarding the benefits of our products and obtain sufficient prescriptions for our services, revenue from the provision of our cardiac monitoring and INR solutions could potentially decrease.
We may experience difficulty in obtaining reimbursement for our services from commercial payors that consider our technology to be experimental and investigational, which would adversely affect our revenue and operating results.
Many commercial payors refuse to enter into contracts to reimburse the fees associated with medical devices or services that such payors determine to be “experimental and investigational.” Commercial payors typically label medical devices or services as “experimental and investigational” until such devices or services have demonstrated product superiority evidenced by a randomized clinical trial. We completed a clinical trial in March 2007 that showed that MCT provided higher diagnostic yield than traditional loop event monitoring. Prior to our clinical trial, MCT was labeled “experimental and investigational” by numerous commercial payors. Since the trial was published in March 2007, we have obtained contracts with most of these commercial payors that previously labeled MCT as “experimental and investigational.” We have not obtained contracts with certain remaining commercial payors however, and these payors have informed us that they do not believe the data from this trial justifies the removal of the experimental designation. As a result, these commercial payors may refuse to reimburse the technical and professional fees associated with MCT.
If commercial payors decide not to reimburse our products or services or the related services provided by physicians, or the rates of such reimbursement change, or if we fail to properly administer claims, our revenue could be adversely affected.
We have a concentration of risk related to the accounts receivable from Medicare and failure to fully collect outstanding balances from this customer, or a combination of other customers, may adversely affect our results of operations.
As of December 31, 2017,2019, we have balances owed to us directly from one customer, Medicare representing approximately 21%22% of our total gross accounts receivable. We maintain an allowance for doubtful accounts based on the collections history and aging of outstanding receivables, as well as for any specific instances we become aware of that may preclude us from reasonably assuring collection on outstanding balances. Determining the allowance for doubtful accounts is judgmental in nature and often involves the use of significant estimates. A determination that requires a change in our estimatescollection trends could have a materiallyan adverse effect on our financial condition and operating results.
If we do not have enough equipment or experience delays in manufacturing, we may be unable to fill prescriptions for cardiac and diabetic monitoring in a timely manner, physicians may elect not to prescribe our services, and our revenue and growth prospects may be adversely affected.
When a physician prescribes cardiac monitoring to a patient, our customer service department begins the patient set-up process. While our goal is to provide each patient with the appropriate device in a timely manner, we have experienced, and may in the future experience, delays due to the availability of devices, primarily when converting to a new generation of device or in connection with the increase in prescriptions following potential acquisitions of other companies.
We may also experience shortages of devices due to manufacturing difficulties. Multiple suppliers provide the components used in our devices, but our Minnesota and Massachusetts facilities are registered and approved by the FDA as the manufacturer of record of our devices. Our manufacturing operations could be disrupted by fire, earthquake or other natural disaster, a labor-related disruption, failure in supply or other logistical channels, electrical outages or other reasons. If there were a disruption to our facilities in Minnesota or Massachusetts, we would be unable to manufacture devices until we have restored and re-qualified our manufacturing capability or developed alternative manufacturing facilities.
Our success in obtaining future cardiac monitor prescriptions from physicians is dependent upon our ability to promptly deliver devices to our patients, and a failure in this regard would have an adverse effect on our revenue and growth prospects.
Interruptions or delays in telecommunications systems could impair the delivery of our MCT, BGM and wireless event services.
The success of our MCT, BGM and wireless event services is dependent upon our ability to transmit and process data. Our MCT, BGM and wireless event devices rely on third-party wireless carriers to transmit data over their data networks. We are dependent upon these third-party wireless carriers to provide data transmission services to us through our various agreements. If we fail to maintain these relationships, or if we lose wireless carrier services, we would be forced to seek alternative providers of data transmission services, which might not be available on commercially reasonable terms, or at all.
As we expand our commercial activities, an increased burden will be placed upon our data processing systems and the equipment upon which they rely. Interruptions of our data networks, or the data networks of our wireless carriers for any extended length of time, loss of stored data or other computer problems could have a material adverse effect on our business and operating results. Frequent or persistent interruptions in our cardiac monitoring services could cause permanent harm to our reputation and could cause current or potential users of our remote monitoring services or prescribing physicians to believe that our systems are unreliable, leading them to switch to our competitors. Such interruptions could result in liability claims and litigation against us for damages or injuries resulting from the disruption in service.
Our business may be impacted by political events, war, terrorism, public health issues, natural disasters and other business interruptions.
War, terrorism, geopolitical uncertainties, public health issues and other business interruptions have caused and could cause damage or disruption to commerce and the economy, and thus could have a material adverse effect on us, our suppliers, logistics providers and customers. Our business operations are subject to interruption by, among others, natural disasters, whether as a result of climate change or otherwise, fire, power shortages, nuclear power plant accidents and other industrial accidents, terrorist attacks and other hostile acts, labor disputes, public health issues and other events beyond our control. Such events could decrease demand for our products, make it difficult or impossible for us to make and deliver products to our customers or to receive components from itsour suppliers, and create delays and inefficiencies in our supply chain. For example, we have suppliers based in China, which is currently facing a major health crisis related to the COVID-19 coronavirus. Our potential customers and monitoring centers could be impacted by natural disasters such as hurricanes, tornadostornadoes and earthquakes. In the event of a natural disaster, we could incur significant losses, require substantial recovery time and experience significant expenditures in order to resume operations.
New products and technological advances by our competitors may negatively affect our market share, commercial opportunities and results of operations.
The market for cardiac monitoring solutions is evolving rapidly and becoming increasingly competitive. OurThe mobile cardiac monitoring industry in the U.S. is highly fragmented and characterized by a small number of large providers and a large number of smaller regional service providers. These third parties compete with us in marketing to payors and prescribing physicians, recruiting and retaining qualified personnel, acquiring technology and developing solutions complementary to our programs. In addition, as companies with substantially greater resources than ours enter our market, we will face increased competition. If our competitors are better able to develop and patent cardiac monitoring solutions than us, or develop more effective or less expensive cardiac monitoring solutions that render our solutions obsolete or non-competitive, or deploy
larger or more effective marketing and sales resources than ours, our business would be harmed and our commercial opportunities would be reduced or eliminated.
Our efforts to develop new products may not be successful or the new products may not provide the revenue we expect.
We plan to add to our product portfolio through internal development efforts, acquisitions and other strategic partnerships. To be successful, we must continue to develop and commercialize new products and to enhance versions of our existing products. Our products are technologically complex and require significant research, planning, design, development and testing before they may be marketed. These new products and technologies may fail to reach the market or may only have limited commercial success because of:
efficacy or safety concerns;
the limited scope of approved uses;
excessive costs to manufacture, failure to establish or maintain intellectual property rights, or infringement of the intellectual property rights of others;
inability to:
| |
| ▪ | achieve positive clinical outcomes; |
| |
| ▪ | timely and accurately identify new market trends; |
| |
| ▪ | obtain necessary regulatory approvals or minimize related costs; |
| |
| ▪ | adopt competitive pricing; |
| |
| ▪ | timely manufacture and deliver products; |
| |
| ▪ | accurately predict and control costs associated with the development, manufacturing and support of our products; and |
| |
| ▪ | anticipate and compete effectively with our competitors’ efforts. |
Even if we successfully develop new products or enhancements or new generations of our existing products, they may be quickly rendered obsolete by changing customer preferences, changing industry standards, or competitors’ innovations. Innovations may not be accepted quickly in the marketplace because of, among other things, entrenched patterns of clinical practice or uncertainty over third-party reimbursement. We cannot provide certainty as to when or whether any of our products under development will be launched, whether we will be able to develop, license, or otherwise acquire products, or whether any products will be commercially successful. Failure to launch successful new products or technologies, or new indications or uses for existing products, may cause our products or technologies to become obsolete, causing our revenues and operating results to suffer.
We operate in an intensely competitive industry, and our failure to respond quickly to technological developments and incorporate new features into our products could harm our ability to compete.
We operate in an intensely competitive industry that experiences rapid technological developments, changes in industry standards, changes in patient requirements and frequent new product introductions and improvements. If we are unable to respond quickly and successfully to these developments, we may lose our competitive position, and our products or technologies may become uncompetitive or obsolete. To compete successfully, we must maintain a successful research and development effort, develop new products and production processes and improve our existing products and processes at the same pace or ahead of our competitors. Our research and development efforts are aimed at solving increasingly complex problems, as well as creating new technologies, and we do not expect that all of our projects will be successful. If our research and development efforts are unsuccessful, our future results of operations could be materially affected.
We are increasingly dependent on sophisticated information technology systems to operate our business, and if we fail to properly maintain the integrity of our data or if our products do not operate as intended or we experience a cyber-attack or other breach of these systems, our business could be materially affected.
We are increasingly dependent on sophisticated information technology for our products and infrastructure. We rely on information technology systems to process, transmit and store electronic information in our day-to-day operations. The size and complexity of our information technology systems makes them vulnerable to increasingly sophisticated cyber-attacks, malicious intrusion, breakdown, destruction, loss of data privacy or other significant disruption. Our information systems require an ongoing commitment of significant resources to maintain, protect and enhance existing systems and develop new systems to keep pace with continuing changes in information processing technology, evolving systems and regulatory standards, the increasing need to protect patient and customer information and changing customer patterns. As a result of technology initiatives, recently enacted regulations, changes in our system platforms and integration of new business acquisitions, we have been consolidating and integrating the number of systems we operate and have upgraded and expanded our information systems capabilities.
In addition, third parties may attempt to hack into our products or systems and may obtain data relating to patients with our products or our proprietary information. If we fail to maintain or protect our information systems and data integrity effectively, we could lose existing customers, have difficulty attracting new customers, have problems in determining product cost estimates and establishing appropriate pricing, have difficulty preventing, detecting and controlling fraud, have disputes with customers, physicians and other health care professionals, have regulatory sanctions or penalties imposed, have increases in operating expenses, incur expenses or lose revenue as a result of a data privacy breach or suffer other adverse consequences. There can be no assurance that our process of consolidating the number of systems we operate, upgrading and expanding our information systems capabilities, protecting and enhancing our systems and developing new systems to keep pace with continuing changes in information processing technology will be successful or that additional systems issues will not arise in the future. Any significant breakdown, intrusion, interruption, corruption or destruction of these systems, as well as any data breaches, could have a material adverse effect on our business.
Changes in the health carehealthcare industry or tort reform could reduce the number of cardiac monitoring solutions ordered by physicians, which could result in a decline in the demand for our solutions, pricing pressure and decreased revenue.
Changes in the health carehealthcare industry directed at controlling health carehealthcare costs or perceived over-utilization of cardiac monitoring solutions could reduce the volume of services ordered by physicians. If more health carehealthcare cost controls are broadly instituted throughout the health carehealthcare industry, the volume of cardiac monitoring solutions could decrease, resulting in pricing pressure and declining demand for our services, which could harm our operating results. In addition, it has been suggested that some physicians order cardiac monitoring solutions, even when the services may have limited clinical utility, primarily to establish a record for defense in the event of a claim of medical malpractice against the physician. Legal changes increasing the difficulty of initiating medical malpractice cases, known as tort reform, could reduce the number of our services prescribed as physicians respond to reduced risks of litigation, which could harm our operating results.
Legislation and policy changes reforming the United States health carehealthcare system may have a material adverse effect on our operating results and financial condition.
The Affordable Care ActACA makes the most sweeping and fundamental changes to the United States health carehealthcare system since the creation of Medicare and Medicaid. The Affordable Care ActACA includes a large number of health-related provisions expanding Medicaid eligibility, requiring most individuals to have health insurance, establishing new regulations on health plans, establishing health insurance exchanges, requiring manufacturers to report payments or other transfers of value made to physicians and teaching hospitals and modifying certain payment systems to encourage more cost-effective care.
Several provisions of the Affordable Care Act specifically affect the medical equipment industry. In addition to changes in Medicare DMEPOS reimbursement and an expansion of the DMEPOS competitive bidding program, the Affordable Care Act provides that for sales on or after January 1, 2013, manufacturers, producers and importers of taxable medical devices must pay an annual excise tax of 2.3% of the price for which the devices are sold. Subsequent legislation, the Consolidated Appropriations Act of 2016, includes a two-year moratorium on the medical device excise tax commencing on January 1, 2016 and ending on December 31, 2017. Budget legislation signed in January 2018 extended that moratorium through December 31, 2019.
The Affordable Care ActACA also establishes enhanced Medicare and Medicaid program integrity provisions, including expanded documentation requirements for Medicare DMEPOS orders, more stringent procedures for screening Medicare and Medicaid DMEPOS suppliers, and new disclosure requirements regarding manufacturer payments to physicians and teaching hospitals, along with broader expansion of federal fraud and abuse authorities. Subsequent legislation made additional changes to the DMEPOS reimbursement policy. For instance, the Consolidated Appropriations Act of 2016 caps Medicaid durable medical equipment reimbursement rates at Medicare fee-for-service rates applicable in the state, including applicable competitive bidding rates, beginning January 1, 2019, and the 21st Century Cures Act moved up implementation of this provision to January 1, 2018. There can be no assurances that future legislation will not adversely impact reimbursement for our products and services.
In addition, various health carehealthcare reform proposals have also emerged at the state level. We cannot predict the full effect that these laws or any future legislation or regulation will have on us. However, the implementation of new legislation and regulation may lower reimbursements for our products, reduce medical prescriptions for our services and adversely affect our business.
If we or our suppliers fail to achieve or maintain regulatory approval of manufacturing facilities, our growth could be limited and our business could be adversely affected.
We currently assemble and manufacture our cardiac monitoring and BGM devices. We purchase INR monitoring devices from third parties. In order to maintain compliance with FDA and other regulatory requirements, our manufacturing facilities must be periodically reevaluated and qualified under a quality system to ensure they meet production and quality standards. Suppliers of components and products used to manufacture MCT, BGM, event, and Holter devices and the manufacturers of the monitors used in INR services must also comply with FDA regulatory requirements, which often require significant resources
and subject us and our suppliers to potential regulatory inspections and stoppages. If we or our suppliers do not maintain regulatory approval for our manufacturing operations, our business could be adversely affected.
If we fail to meet Medicare accreditation and surety bond requirements or DMEPOS supplier standards, it could negatively affect our business operations.
Medicare DMEPOS suppliers (other than certain exempted professionals) must be accredited by an approved accreditation organization as meeting DMEPOS quality standards adopted by CMS.CMS. Medicare suppliers also are required to meet surety bond requirements. In addition, Medicare DMEPOS suppliers must comply with Medicare supplier standards in order to obtain and retain billing privileges, including meeting all applicable federal and state licensure and regulatory requirements. Furthermore, many of our managed care contracts for the provision of diabetes services require that we qualify as an accredited DMEPOS supplier. CMS periodically expands or otherwise clarifies the Medicare DMEPOS supplier standards. We believe we are in compliance with these requirements. If we fail to maintain our Medicare accreditation status and/or do not comply with Medicare surety bond or supplier standard requirements in the future, or if these requirements are changed or expanded, it could adversely affect our profits and results of operations.
Our dependence on a limited number of suppliers may prevent us from delivering our devices on a timely basis.
We currently rely on a limited number of suppliers of components for the devices that we manufacture. If these suppliers became unable to provide components in the volumes needed, which has occurred, or at an acceptable price, we would have to identify and qualify acceptable replacements from alternative sources of supply. The process of qualifying suppliers is lengthy. Delays or interruptions in the supply of our required components could limit or stop our ability to provide sufficient quantities of devices on a timely basis and meet demand for our services, which could have a material adverse effect on our business, financial condition and results of operations.
We could be subject to medical liability or product liability claims, which may not be covered by insurance and which would adversely affect our business and results of operations.
The design, manufacture and marketing of services of the types we provide entail an inherent risk of product liability claims. Any such claims against us may require us to incur significant defense costs, irrespective of whether such claims have merit. In addition, we provide information to health carehealthcare providers and payors upon which determinations affecting medical care are made, and claims may be made against us resulting from adverse medical consequences to patients resulting from the information we provide. In addition, we may become subject to liability in the event that the devices we use fail to correctly record or transfer patient information or if we provide incorrect information to patients or health carehealthcare providers using our services.
Our liability insurance is subject to deductibles and coverage limitations. In addition, our current insurance may not continue to be available to us on acceptable terms, if at all, and, if available, the coverage may not be adequate to protect us against any future claims. If we are unable to obtain insurance at an acceptable cost or on acceptable terms with adequate coverage or otherwise protect against any claims against us, we would be exposed to significant liabilities, which may adversely affect our business and results of operations.
Our products may in the future be subject to product recalls that could harm our reputation and product liability claims.
The FDA has the authority to require the recall of commercialized products in the event of material regulatory deficiencies or defects in design or manufacture. Any recalls of our products or products that we distribute would divert managerial and financial resources, harm our reputation with customers and have an adverse effect on our financial condition and results of operations.
Regulations related to conflict minerals may adversely impact our business.
The Dodd FrankDodd-Frank Wall Street Reform and Consumer Protection Act contains provisions to improve transparency and accountability concerning the supply of certain minerals, known as conflict minerals, originating from the DRC.Democratic Republic of the Congo and adjoining countries (“DRC”). Due to the materials used in certain of the products manufactured by our subsidiaries, we must comply with annual disclosure and reporting rules adopted by the SEC by assessing whether the subject minerals contained in our products originated in the DRC.DRC. Our supply chain is complex since we do not source our minerals directly from the original mine or smelter. Consequently, we incur costs in complying with these disclosure requirements, including for due diligence to determine the source of the subject minerals used in our products and other potential changes to products, processes or sources of supply as a consequence of such verification activities. The rules may adversely affect the sourcing, supply and pricing of materials used in our products throughout the supply chain beyond our control, whether or not the subject minerals are “conflict free.” Also, we may face reputational challenges if we determine that certain of our products contain minerals not determined to be conflict free or if we are unable to sufficiently verify the origins for all subject minerals used in our products through our diligence process.
We are reliant on the outsourcing of clinical research by pharmaceutical, clinical research and biotechnology companies.
We are reliant on the ability and willingness of pharmaceutical, clinical research and biotechnology companies to continue to outsource the types of research services that we provide. As such, we are impacted and subject to risks, uncertainties and trends that affect companies in these industries. Any downturn in these industries or reduction in spending or outsourcing could adversely affect our Research business.
Future sales of our common stock may depress our stock price.
Future issuanceissuances in connection with acquisitions and sales of a substantial number of shares of our common stock in the public market could occur at any time. These sales, or the perception in the market that the holders of a large number of shares intend to sell shares, could reduce the market price of our common stock. As of December 31, 2017,2019, we had 32,460,66834,023,053 outstanding shares of common stock. In addition, as of December 31, 2019, we had 3,574,4392,691,059 stock options, and 467,12930,000performance stock options, 270,052 restricted stock units (“RSUs”RSUs”) and 90,020 performance stock units (“PSUs”) outstanding to purchase shares of our common stock that willcould become exercisable over the next four years or vest over the next three years. Further, we had 150,00030,000 performance stock options that are exercisable.exercisable as of December 31, 2019. If exercised, vested or earned, additional shares would become available for sale.
In addition, in connection with the acquisition of Geneva Healthcare, Inc. (“Geneva”) discussed under “Item 7: Management’s Discussion and Analysis of Financial Condition and Results of Operations - Recent Developments,” each equity holder of Geneva shall be eligible to receive additional consideration on the third anniversary of the closing date based on Geneva’s performance following the closing (the “Earn-Out Amount”). The Earn-Out Amount will be payable in a combination of cash and
our common stock. Concurrent with the closing of the acquisition, the former Geneva security holders have made elections as to the percentage mix of their total additional consideration to be settled in cash or common stock. We expect approximately 45% of the Earn-Out Amount will be satisfied with the issuance of our common stock. The number of shares of BioTelemetry common stock issuable to each electing equity holder in partial payment of the Earn-Out Amount will be calculated based on a share price of $67.85.
Anti-takeover provisions in our charter documents and Delaware law might deter acquisition bids for us that our stockholders might consider favorable.
Our amended and restated certificate of incorporation and bylaws contain provisions that may make the acquisition of our CompanyBioTelemetry more difficult without the approval of our Board of Directors. These provisions:
establish a classified Board of Directors so that not all members of the board are elected at one time;
authorize the issuance of undesignated preferred stock, the terms of which may be established and shares of which may be issued without stockholder approval, and which may include rights superior to the rights of the holders of common stock;
prohibit stockholder action by written consent, which requires all stockholder actions to be taken at a meeting of our stockholders;
provide that the Board of Directors is expressly authorized to make, alter or repeal our bylaws; and
establish advance notice requirements for nominations for elections to our Board of Directors or for proposing matters that can be acted upon by stockholders at stockholder meetings.
In addition, because we are incorporated in Delaware, we are subject to Section 203 of the Delaware General Corporation Law which, subject to certain exceptions, prohibits stockholders owning in excess of 15% of our outstanding voting stock from merging or combining with us. These anti-takeover provisions and other provisions under Delaware law could discourage, delay or prevent a transaction involving a change of control of our Company,BioTelemetry, even if doing so would benefit our stockholders. These provisions could also discourage proxy contests and make it more difficult for our stockholders to elect directors of their choosing and cause us to take other corporate actions such stockholders desire.
We have a history of net losses andOur future profitability is uncertain.
We previously incurredIn recent years we have realized net losses for each annual period from our inception through December 31, 2014. For the years ended December 31, 2016 and 2015, we achieved net income attributable to the Company of $53.4 million and $7.4 million, respectively; for the year ended December 31, 2017 we realized a net loss attributable to the Company of $16.0 million.income. We may not, however, be able to sustain or increase our profitability in the future on a quarterly or annual basis. AsWhile our recent results have been positive, as of December 31, 2017,2019, we had a total accumulated deficit of approximately $158.7$86.0 million.
We may not be able to realize our net operating loss carryforwards.
We have deferred tax assets that include net operating loss carryforwards that can be used to offset taxable income in future periods and reduce income taxes payable in those future periods. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences are deductible. The timing and manner in which we can utilize our net operating loss carryforward and future income tax deductions in any year may be limited by provisions of the Internal Revenue Code (“IRC”IRC”) regarding the change in ownership of corporations. Such limitation may have an impact on the ultimate realization of our carryforwards and future tax deductions.
Section 382 of the IRC (“Section 382”382”) imposes limitations on a corporation’s ability to utilize net operating losses if it experiences an “ownership change.” In general terms, an ownership change may result from transactions increasing the ownership of certain stockholders in the stock of a corporation by more than 50 percentage points over a three-year period. Any unused annual limitation may be carried over to later years, and the amount of the limitation may under certain circumstances be increased by the built-in gains in assets held by us at the time of the change that are recognized in the five-year period after the change. Currently, a portion of our loss carryforwards is limited under Section 382.382.
Resolution of income tax matters may impact our financial condition, results of operations and cash flows.
We are subject to income taxes in many U.S. and certain foreign jurisdictions, which requires significant judgment in determining our effective income tax rate and in evaluating tax positions, particularly those related to uncertain tax positions. We have provided for uncertain tax positions when such tax positions do not meet the recognition thresholds or measurement standards prescribed by the accounting standard for uncertain tax positions. Changes in uncertain tax positions or other adjustments resulting from tax audits and settlements with taxing authorities, including related interest and penalties, impact our effective tax rate. When particular tax matters arise, a number of years could elapse before such matters are audited and finally resolved. We believe our positions are appropriate, however, federal, state, or foreign tax authorities could disagree. If the settlement of any unrecognized tax reserves is different than accrued, it would impact our effective rate in the year of resolution. Any resolution of a tax matter may require the adjustment of tax assets or tax liabilities and/or the use of cash in the year of resolution.
Item 1B. Unresolved Staff Comments
None.
Item 2. Properties
As of December 31, 2017, we operate2019, the following were our material leased facilities:
| | | Location | Use | Segment(s) | Square feet | Lease expiry | Use | Segment/ Category | Square feet | Lease expiry |
| Malvern, PA | Corporate shared services, operations and monitoring | C, H | 61,000 |
| 2021 | Corporate shared services, operations and monitoring | H, C&O | 61,000 |
| 2021 |
| Linwood, PA | | Distribution | H, R, C&O | 56,000 |
| 2030 |
| Rosemont, IL | Customer support center, distribution, and administrative | H, T | 56,000 |
| 2019 | Corporate shared services, operations, monitoring and distribution | H, C&O | 52,000 |
| 2024 |
| Ewing, NJ | Monitoring | H | 28,000 |
| 2018 | |
| Rochester, NY | | Research services | R | 27,000 |
| 2028 |
| Eagan, MN | Monitoring and manufacturing | H, T | 24,000 |
| 2022 | Manufacturing | C&O | 24,000 |
| 2022 |
| Rochester, NY | Research | R | 22,000 |
| 2028 | |
| Mercerville, NJ | | Monitoring | H | 22,000 |
| 2026 |
| Phoenix, AZ | | Distribution center | H | 22,000 |
| 2027 |
| San Francisco, CA | Monitoring | H | 17,000 |
| 2019 | Monitoring, research services | H, R | 20,000 |
| 2022 |
| Rehovot, Israel | Research, development and manufacturing | H | 17,000 |
| 2018 | |
| Chester, PA | Distribution center | H | 16,000 |
| 2020 | Distribution center | H | 16,000 |
| 2020 |
| Rockville, MD | Research | R | 16,000 |
| 2026 | Research services | R | 13,000 |
| 2026 |
| Phoenix, AZ | Distribution center | H | 11,000 |
| 2020 | |
| San Diego, CA | Research, development and engineering | T | 8,000 |
| 2020 | Research, development and engineering | C&O | 8,000 |
| 2020 |
| Concord, MA | Research and development and distribution | T | 7,000 |
| 2018 | |
| Norfolk, VA | Monitoring | H | 5,000 |
| 2018 | Monitoring | H | 8,000 |
| 2024 |
C = Corporate, H = Healthcare segment, R = Research Tsegment, C&O = TechnologyCorporate and Other category
We believe that all of our existing facilities are adequate to meet our current needs and that suitable additional alternative spaces will be available in the future on commercially reasonable terms. We have secured space, via renewal or alternate locations, for our leases that expire in the near term.
Item 3. Legal Proceedings
From time to time, in the ordinary course of business and like others in the industry, we receive requests for information from government agencies in connection with their regulatory or investigational authority or are involved in traditional employment or business litigation. We review such requests and notices and take appropriate action.
On April 5, 2019, a complaint filed under seal in the U.S. District Court for the Eastern District of Pennsylvania against the Company by private relators under the Federal False Claims Act, and analogous state acts, was unsealed. The U.S. Department of Justice notified the District Court of its decision not to intervene in the case at this time, and the case is currently stayed until May 2020 while the U.S. Department of Justice determines whether it will intervene in the case.
The relators’ complaint alleges, among other things, that the Company engaged in the offshoring of certain activities and improper performance of work at certain U.S. locations in violation of applicable law. The relators seek unspecified damages on behalf of the U.S. and various states and municipalities.
The Company is evaluating the complaint, but, at this point, it believes the allegations in the complaint are without merit and intends to vigorously defend the litigation. The Company also does not believe these claims will have a material impact on its business operations or strategic plans.
The final outcome of any current or future litigation or governmental or internal investigations cannot be accurately predicted, nor can we predict any resulting penalties, fines or other sanctions that may be imposed at the discretion of federal or state regulatory authorities. We record accruals for such contingencies to the extent that we conclude it is probable that a liability has been incurred and the amount of the loss can be estimated.
For further details on the material legal proceedings to which we are currently a party, which is incorporated herein by reference, please refer to “Part II; Item 8. Financial Statements and Supplementary Data; Notes to Consolidated Financial Statements; Note 18.19. Legal Proceedings” below.
Item 4. Mine Safety Disclosures
Not Applicable.
PART II
| |
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
Unregistered Sales of Equity Securities
In connection with the tender offer and subsequent acquisition of LifeWatch, from the acquisition date through December 31, 2017 we issued 3,635,646 shares of BioTelemetry Common Stock. The tender offer was subject to a Tier I exemption pursuant to Rule 14d-1(c) of the Securities Exchange Act of 1934, as amended, and the issuance of BioTelemetry Common Stock in connection therewith was exempt from registration under the Securities Act of 1933, as amended, pursuant to Rule 802 thereof, because Life Watch is a foreign private issuer and U.S. holders held less than 10% of the LifeWatch Shares that were the subject of the tender offer.
Subsequent to December 31, 2017, in accordance with the squeeze-out procedures under Swiss Law, we issued 58,786 shares to the remaining LifeWatch stockholders.None.
Market Information for Common Stock
Our common stock is traded on the NASDAQ Global Select Market under the symbol,our symbol: “BEAT.” The following table sets forth the range of high and low sale prices of our common stock for the periods indicated:
|
| | | | | | | | | | | | | | | |
| | 2017 | | 2016 |
| Quarter Ended | High | | Low | | High | | Low |
| March 31 | $ | 29.50 |
| | $ | 21.05 |
| | $ | 13.35 |
| | $ | 8.74 |
|
| June 30 | 34.00 |
| | 26.45 |
| | 17.68 |
| | 10.96 |
|
| September 30 | 39.20 |
| | 28.80 |
| | 21.42 |
| | 15.86 |
|
| December 31 | 34.70 |
| | 23.30 |
| | 24.10 |
| | 15.25 |
|
As of February 15, 2018,17, 2020, there were 32,531,36534,023,053 shares of our common stock outstanding. Also as of that date, we had 5552 holders of record (this does not include persons whose stock is in nominee or “street name” accounts through brokers).
Dividends
We have never declared or paid any cash dividends on our capitalcommon stock. We currently intend to retain all available funds and any future earnings to support our operations and finance the growth and development of our business. We do not intend to pay cash dividends on our common stock for the foreseeable future. Any future determination related to dividend policy will be made at the discretion of our Board of Directors.
Stock Performance Graph
The graph below compares the total stockholder return of an investment of $100 on December 31, 20122014 through December 31, 20172019 for (i) our common stock (ii) The NASDAQ Health Care Index and (iii) The Russell 2000 Index. Each of the three measures of cumulative total return assumes reinvestment of dividends, if any. The stock price performance shown on the graph below is based on historical data and is not indicative of future stock price performance.
Comparison of 5 Year Cumulative Total Return
Among BioTelemetry, Inc., The NASDAQ Health Care Index
and The Russell 2000 Index
| | | | | | | | | | | | | | | | Year Ended December 31, |
| Company/Index | Company/Index | Base Period Dec 31,
2012 | | Dec 31,
2013 | | Dec 31,
2014 | | Dec 31,
2015 | | Dec 31,
2016 | | Dec 31,
2017 | Company/Index | 2014* | | 2015 | | 2016 | | 2017 | | 2018 | | 2019 |
| BioTelemetry, Inc. | BioTelemetry, Inc. | 100.00 |
| | 348.25 |
| | 439.91 |
| | 512.28 |
| | 980.26 |
| | 1,311.40 |
| BioTelemetry, Inc. | 100.00 |
| | 116.45 |
| | 222.83 |
| | 298.10 |
| | 595.41 |
| | 461.62 |
|
| NASDAQ Health Care Index | NASDAQ Health Care Index | 100.00 |
| | 157.04 |
| | 201.75 |
| | 215.59 |
| | 180.19 |
| | 219.87 |
| NASDAQ Health Care Index | 100.00 |
| | 106.86 |
| | 89.31 |
| | 108.98 |
| | 105.02 |
| | 132.95 |
|
| Russell 2000 Index | Russell 2000 Index | 100.00 |
| | 138.82 |
| | 145.62 |
| | 139.19 |
| | 168.85 |
| | 193.58 |
| Russell 2000 Index | 100.00 |
| | 95.59 |
| | 115.95 |
| | 132.94 |
| | 118.30 |
| | 148.49 |
|
| * Base Period | | * Base Period | | | | | | | | | | | |
The foregoing graph and chart shall not be deemed incorporated by reference by any general statement incorporating by reference this Annual Report on Form 10-K into any filing under the Securities Act of 1933, as amended, or under the Securities Exchange Act of 1934, as amended, except to the extent we specifically incorporate this information by reference, and shall not otherwise be deemed filed under those acts.
Information regarding our equity compensation plans is incorporated by reference from our Proxy Statement, unless our Proxy Statement is not filed on or before May 1, 2018,April 30, 2020, in which case we will amend this Annual Report on Form 10-K to provide the omitted information in accordance with the requirements of Instruction G to Form 10-K.
Item 6. Selected Financial Data
The selected financial data set forth below are derived from our consolidated financial statements. The statement of operations data for the years ended December 31, 2017, 20162019, 2018 and 2015,2017, and the balance sheet data at December 31, 20172019 and 20162018 are derived from our audited consolidated financial statements included elsewhere in this Annual Report on Form 10-K. The statement of operations data for the years ended December 31, 20142016 and 20132015 and the balance sheet data at December 2015, 201431, 2017, 2016 and 20132015 are derived from our audited consolidated financial statements, which are not included herein. Certain reclassifications have been made below to prior period statements to conform to the current period presentation.
presentation. The selected financial data for the year ended December 31, 2019 reflects the adoption of ASC 842 - Leases using the optional modified transition method as of January 1, 2019, therefore prior period amounts are not restated.
The following selected financial data should be read in conjunction with “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Item 8. Financial Statements and Supplementary Data” included in this Annual Report on Form 10-K.
| | | Statement of Operations Data: | Year ended December 31, | Year Ended December 31, |
| (in thousands, except per share data) | 2017 | | 2016 | | 2015 | | 2014 | | 2013 | 2019 | | 2018 | | 2017 | | 2016 | | 2015 |
| Revenue: | | | | | | | | | | |
| Healthcare | $ | 234,385 |
| | $ | 165,664 |
| | $ | 145,963 |
| | $ | 133,178 |
| | $ | 100,386 |
| |
| Research | 38,790 |
| | 32,565 |
| | 21,853 |
| | 19,744 |
| | 20,329 |
| |
| Technology | 13,601 |
| | 10,103 |
| | 10,697 |
| | 13,656 |
| | 8,786 |
| |
| Total revenue | 286,776 |
| | 208,332 |
| | 178,513 |
| | 166,578 |
| | 129,501 |
| |
| Cost of revenue: | | | | | | | | | | |
| Healthcare | 81,356 |
| | 53,559 |
| | 51,693 |
| | 54,942 |
| | 35,177 |
| |
| Research | 22,881 |
| | 18,395 |
| | 12,728 |
| | 10,646 |
| | 11,317 |
| |
| Technology | 10,169 |
| | 6,928 |
| | 7,535 |
| | 7,526 |
| | 3,937 |
| |
| Total cost of revenue | 114,406 |
| | 78,882 |
| | 71,956 |
| | 73,114 |
| | 50,431 |
| |
| Revenue | | $ | 439,107 |
| | $ | 399,472 |
| | $ | 286,776 |
| | $ | 208,332 |
| | $ | 178,513 |
|
| Cost of revenue | | 164,833 |
| | 148,986 |
| | 114,406 |
| | 78,882 |
| | 71,956 |
|
| Gross profit | 172,370 |
| | 129,450 |
| | 106,557 |
| | 93,464 |
| | 79,070 |
| 274,274 |
| | 250,486 |
| | 172,370 |
| | 129,450 |
| | 106,557 |
|
| Operating expenses: | | | | | | | | | | | | | | | | | | |
| General and administrative | 82,983 |
| | 55,877 |
| | 47,882 |
| | 45,131 |
| | 36,569 |
| 120,093 |
| | 109,736 |
| | 82,983 |
| | 55,877 |
| | 47,882 |
|
| Sales and marketing | 35,322 |
| | 28,636 |
| | 27,936 |
| | 28,805 |
| | 26,275 |
| 50,664 |
| | 42,849 |
| | 35,322 |
| | 28,636 |
| | 27,936 |
|
| Bad debt expense | 13,291 |
| | 9,931 |
| | 8,047 |
| | 9,347 |
| | 7,787 |
| 21,768 |
| | 22,222 |
| | 13,291 |
| | 9,931 |
| | 8,047 |
|
| Research and development | 11,101 |
| | 8,355 |
| | 7,111 |
| | 7,396 |
| | 7,338 |
| 13,994 |
| | 11,206 |
| | 11,101 |
| | 8,355 |
| | 7,111 |
|
| Other charges | 31,436 |
| | 8,639 |
| | 6,063 |
| | 7,098 |
| | 7,982 |
| 15,004 |
| | 14,659 |
| | 31,436 |
| | 8,639 |
| | 6,063 |
|
| Total operating expenses | 174,133 |
| | 111,438 |
| | 97,039 |
| | 97,777 |
| | 85,951 |
| 221,523 |
| | 200,672 |
| | 174,133 |
| | 111,438 |
| | 97,039 |
|
| Income/(loss) from operations | (1,763 | ) | | 18,012 |
| | 9,518 |
| | (4,313 | ) | | (6,881 | ) | 52,751 |
| | 49,814 |
| | (1,763 | ) | | 18,012 |
| | 9,518 |
|
| Other expense: | | | | | | | | | | | | | | | | | | |
| Interest expense | (4,897 | ) | | (1,830 | ) | | (1,534 | ) | | (713 | ) | | (88 | ) | (9,482 | ) | | (9,429 | ) | | (4,897 | ) | | (1,830 | ) | | (1,534 | ) |
| Loss on extinguishment of debt | (543 | ) | | — |
| | — |
| | (372 | ) | | — |
| — |
| | — |
| | (543 | ) | | — |
| | — |
|
| Loss on equity method investment | (384 | ) | | (287 | ) | | — |
| | — |
| | — |
| (1,298 | ) | | (246 | ) | | (384 | ) | | (287 | ) | | — |
|
| Other non-operating expense, net | (2,809 | ) | | (125 | ) | | (88 | ) | | (6,708 | ) | | (135 | ) | |
| Other non-operating (expense)/income, net | | (2,243 | ) | | 1,365 |
| | (2,809 | ) | | (125 | ) | | (88 | ) |
| Total other expense | (8,633 | ) | | (2,242 | ) | | (1,622 | ) | | (7,793 | ) | | (223 | ) | (13,023 | ) | | (8,310 | ) | | (8,633 | ) | | (2,242 | ) | | (1,622 | ) |
| Income/(loss) before income taxes | (10,396 | ) | | 15,770 |
| | 7,896 |
| | (12,106 | ) | | (7,104 | ) | 39,728 |
| | 41,504 |
| | (10,396 | ) | | 15,770 |
| | 7,896 |
|
| Benefit from/(provision for) income taxes | (6,747 | ) | | 37,667 |
| | (468 | ) | | 2,313 |
| | (215 | ) | |
| (Provision for)/benefit from income taxes | | (9,884 | ) | | 370 |
| | (6,747 | ) | | 37,667 |
| | (468 | ) |
| Net income/(loss) | (17,143 | ) | | 53,437 |
| | 7,428 |
| | (9,793 | ) | | (7,319 | ) | 29,844 |
| | 41,874 |
| | (17,143 | ) | | 53,437 |
| | 7,428 |
|
| Net loss attributable to noncontrolling interests | (1,187 | ) | | — |
| | — |
| | — |
| | — |
| — |
| | (946 | ) | | (1,187 | ) | | — |
| | — |
|
| Net income/(loss) attributable to BioTelemetry, Inc. | $ | (15,956 | ) | | $ | 53,437 |
| | $ | 7,428 |
| | $ | (9,793 | ) | | $ | (7,319 | ) | $ | 29,844 |
| | $ | 42,820 |
| | $ | (15,956 | ) | | $ | 53,437 |
| | $ | 7,428 |
|
| Net income/(loss) per common share attributable to BioTelemetry, Inc.: | | | | | | | | | | | | | | | | | | |
| Basic | $ | (0.53 | ) | | $ | 1.91 |
| | $ | 0.27 |
| | $ | (0.37 | ) | | $ | (0.29 | ) | $ | 0.88 |
| | $ | 1.31 |
| | $ | (0.53 | ) | | $ | 1.91 |
| | $ | 0.27 |
|
| Diluted | $ | (0.53 | ) | | $ | 1.75 |
| | $ | 0.26 |
| | $ | (0.37 | ) | | $ | (0.29 | ) | $ | 0.82 |
| | $ | 1.20 |
| | $ | (0.53 | ) | | $ | 1.75 |
| | $ | 0.26 |
|
| Weighted average number of shares outstanding: | | | | | | | | | | | | | | | | | | |
| Basic | 30,386 |
| | 27,920 |
| | 27,116 |
| | 26,445 |
| | 25,544 |
| 33,948 |
| | 32,709 |
| | 30,386 |
| | 27,920 |
| | 27,116 |
|
| Diluted | 30,386 |
| | 30,489 |
| | 29,089 |
| | 26,445 |
| | 25,544 |
| 36,440 |
| | 35,783 |
| | 30,386 |
| | 30,489 |
| | 29,089 |
|
| | | Balance Sheet Data: | December 31, | December 31, |
| (in thousands) | 2017 | | 2016 | | 2015 | | 2014 | | 2013 | 2019 | | 2018 | | 2017 | | 2016 | | 2015 |
| Cash and cash equivalents | $ | 36,022 |
| | $ | 23,052 |
| | $ | 18,986 |
| | $ | 20,007 |
| | $ | 22,151 |
| $ | 68,614 |
| | $ | 80,889 |
| | $ | 36,022 |
| | $ | 23,052 |
| | $ | 18,986 |
|
| Working capital | 39,153 |
| | 28,053 |
| | 23,157 |
| | 13,879 |
| | 25,215 |
| 112,579 |
| | 97,037 |
| | 39,153 |
| | 28,053 |
| | 23,157 |
|
| Total assets | 524,562 |
| | 198,984 |
| | 124,143 |
| | 124,372 |
| | 87,546 |
| 685,720 |
| | 586,801 |
| | 524,562 |
| | 198,984 |
| | 124,143 |
|
| Total debt | 199,356 |
| | 25,161 |
| | 23,194 |
| | 23,873 |
| | — |
| |
| Total long-term obligations | | 263,049 |
| | 226,693 |
| | 223,904 |
| | 28,563 |
| | 24,329 |
|
| Total BioTelemetry, Inc.’s stockholders’ equity | 250,757 |
| | 138,914 |
| | 75,926 |
| | 63,676 |
| | 66,829 |
| 366,917 |
| | 310,485 |
| | 250,757 |
| | 138,914 |
| | 75,926 |
|
| Noncontrolling interests | (1,054 | ) | | — |
| | — |
| | — |
| | — |
| — |
| | — |
| | (1,054 | ) | | — |
| | — |
|
| Total equity | $ | 249,703 |
| | $ | 138,914 |
| | $ | 75,926 |
| | $ | 63,676 |
| | $ | 66,829 |
| $ | 366,917 |
| | $ | 310,485 |
| | $ | 249,703 |
| | $ | 138,914 |
| | $ | 75,926 |
|
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
You should read the following discussion and analysis of our financial condition and results of our operations in conjunction with our consolidated financial statements and the related notes to those statements as well as “Part I; Item 1. Business”included elsewhere in this Annual Report on Form 10-K. ThisThe following discussion contains forward-looking statements reflecting our current expectations that involve risks and uncertainties. Our actual results and the timing of events may differ materially from those contained in these forward-looking statements due to a number of factors—see “Cautionary Note Regarding Forward-Looking Statements”Statements” and “Part I; Item 1A; Risk Factors.” We report on a calendar year end, and except where otherwise indicated below, “2017”“2019” refers to the year ended December 31, 2017, “2016”2019, “2018” refers to the year ended December 31, 20162018 and “2015”“2017” refers to the year ended December 31, 2015.2017.
OverviewExecutive Summary
Company BackgroundThe following is a summary of certain financial highlights and trends related to 2019:
| |
| • | Recognized $439.1 million in revenue, or an increase of 9.9% over prior year period, with our 2019 fourth quarter representing our 30th consecutive quarter of year-over-year revenue growth. |
2019 revenue was negatively impacted by Medicare rate reductions; 2020 rates did not change significantly from 2019 rates.
| |
| • | Our sales force expansion during 2019 executed well, more than offsetting Medicare rate reductions, through growth in MCT and extended Holter. |
In 2019, the American Medical Association accepted industry recommendation for permanent coding for extended Holter, which should become effective January 1, 2021.
| |
| • | The acquisition of Geneva helps create additional sources of revenue, positions BioTelemetry as a more progressive data consolidation and solutions-oriented company and hedges against any potential shift in favor of implantable cardiac monitoring devices. |
| |
| • | Our ADEA acquisition, though currently immaterial, helps us expand our Healthcare service offerings into the Nordics and potentially other parts of Europe. |
We provide monitoring services andcontinued to invest internally to build our digital population health management business and obtain faster, more efficient processing systems to create greater efficiency and scalability.
In 2019, we began our “Heart for healthcare providers, medical device manufacturing and centralized core laboratory servicesHope” initiative, funding life-saving heart procedures for clinical research. We operate under three reportable segments: (1) Healthcare, (2) Research and (3) Technology. The Healthcare segment is focused on200 children from parts of Southeast Asia whose families do not have the diagnosis and monitoring of cardiac arrhythmias or heart rhythm disorders. We offer cardiologists and electrophysiologists, neurologists and primary care physicians a full spectrum of solutions which provides them with a single source of cardiac monitoring services. These services range fromresources to do so.
Subsequent to year end, we renegotiated our credit agreement to more favorable financial terms, giving us more flexibility in the differentiated MCT to event, Holter, extended wear Holter, Pacemaker and INR monitoring. The Research segment is engaged in central core laboratory services providing cardiac monitoring, imaging services, scientific consulting and data management services for drug and medical device trials. The Technology segment focuses on the development, manufacturing, testing and marketing of cardiovascular and blood glucose monitoring devices to medical companies, clinics and hospitals.future.
Recent Developments
Acquisitions
On July 12, 2017,March 1, 2019, we acquired throughGeneva as part of our wholly owned subsidiary Cardiac Monitoring Holding Company, LLC, approximately 97%business strategy to go deeper and wider into the cardiac monitoring market. Geneva has developed an innovative proprietary cloud-based platform that aggregates data from the leading cardiac device manufacturers, enabling us to remotely monitor a physician’s patients with implantable cardiac devices such as pacemakers, defibrillators and loop recorders. Geneva’s platform provides physicians a single portal to order patient monitoring, review monitoring results
and request routine device checks, helping drive significant in-office efficiencies and patient compliance. We have continued to merge this functionality with that of the outstanding shares of LifeWatch AG (“LifeWatch”) for aggregate consideration of 3,615,840 shares of BioTelemetry common stock with a fair value of $116.8 million and cash in the amount of $165.8 million. On that date, we acquired control of LifeWatch AG and began consolidating its financial statements. In September 2017, we purchased 343,525 additional shares of LifeWatch for cash consideration of $4.8 million and the issuance of 19,806 of our shares with a fair value of $0.6 million. We completed the acquisition of the remaining shares in December 2017, for aggregate consideration of $2.9 million in cash and 58,786 shares with a fair market value of $2.0 million which was settled in early January 2018. LifeWatch is included in the Healthcare segment.
On December 1, 2016, we entered into a Share and Asset Purchase Agreement (“Agreement”) with Telcare, Inc. (“Telcare”) pursuant to segment user interface, which we acquired the stock of Telcare Medical Supply, Inc.believe will drive greater workflow and certain assets of Telcare. The total consideration paid at closing amounted to $7.0 million in cash, with the potential for a performance-based earn out up to $5.0 million upon reaching certain milestones, as defined in the Agreement. The fair value of the total consideration transferred in the acquisition, including contingent consideration, was $9.7 million at the acquisition date. Telcare is included in the Technology segment.
On May 11, 2016, we completed the acquisition of VirtualScopics, Inc. (“VirtualScopics”), a leading provider of clinical trial imaging solutions. The all cash Tender Offer commenced on April 8, 2016 and
ended on May 9, 2016, pursuant to which we acquired the business and operations of VirtualScopics. The total consideration paid at closing amounted to $15.0 million, net of cash acquired of $0.8 million. VirtualScopics is included in the Research segment.
On April 1, 2016, we entered into an Asset Purchase Agreement (“APA”) with DELTA Danish Electronics, Light, and Acoustics (“DELTA”), pursuant to which we acquired substantially all of the assets of the ePatch division of DELTA, inclusive of all products and indications currently under development. The total consideration paid at closing amounted to $3.0 million in cash and 244,519 shares of our common stock valued at $2.9 million. In addition, there is the potential for a performance-based earn out up to $3.0 million upon reaching certain milestones, as defined in the APA. The fair value of the total consideration transferred in the acquisition, including contingent consideration, was $6.5 million at the acquisition date. ePatch is included in the Technology segment.
U.S. Tax Cuts and Jobs Act
On December 22, 2017, the Tax Cuts and Jobs Act was signed into law. The TCJA reduced the U.S. corporate income tax rate from 35% to 21% effective January 1, 2018. We are required to revalue our U.S. deferred tax assets and liabilities at the new federal corporate income tax rate as of the date of enactment of the TCJA and to include the rate change effect in the tax provision for the period ended December 31, 2017. As a result, we recognized a $8.0 million deferred tax expense based on a reasonable estimate of the re-measurement of our deferred tax assets and liabilities as of December 22, 2017. This significantly increased the effective tax rate for the period ended December 31, 2017 in comparisondata management efficiencies to the effective tax rates for the last two comparable periods.clients we serve.
In October 2019, we detected suspicious activity on our information technology network. As part of U.S. international tax reform,our comprehensive response plan, we immediately took certain systems offline to contain the TCJA imposes a transition tax on certain accumulated foreign earnings aggregated acrossactivity and engaged an outside forensics team to conduct an independent investigation. While the incident did temporarily disrupt services, substantially all non-U.S. subsidiaries, netsystems resumed operation in early November 2019 and our technical team continues to work closely with third-party consultants to further address this matter. Although we have insurance coverage for costs and business interruption resulting from cyber-attacks, disputes over the extent of foreign deficits. As we are in an aggregate net foreign deficit positioninsurance coverage for U.S. tax purposes, weclaims are not liable foruncommon. We have incurred approximately $3.0 million of direct expenses from the transition tax.incident. At this time, there is no evidence of any unauthorized transfer or misuse of customer or employee data.
On January 27, 2020, we amended our SunTrust Credit Agreement. The new agreement is summarized in “Part II; Item 8. Financial Statements and Supplementary Data; Notes to Consolidated Financial Statements; Note 21. Subsequent Event” in this Annual Report on Form 10-K.
Critical Accounting Policies and Estimates
The discussion and analysis of our financial condition and results of operations are based on our financial statements, which we have prepared in accordance with U.S. generally accepted accounting principles. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amount of assets and liabilities, revenues and expenses and related disclosures. We base our estimates and judgments on historical experience and on various other factors that we believe to be reasonable under the circumstances; however, actual results may differ from these estimates. We review our estimates and judgments on an ongoing basis.
We believe that the following accounting policies and estimates are most critical to a full understanding and evaluation of our reported financial results. Our significant accounting policies are more fully described in “Part II; Item 8. Financial Statements and Supplementary Data; Notes to Consolidated Financial Statements; Note 2. Summary of Significant Accounting Policies” below. in this Annual Report on Form 10-K.
Revenue Recognition
Healthcare
Healthcare segment revenue includes revenue from MCT, event, traditional Holter, extended Holter, Pacemaker, INR, ILRand INRother implantable cardiac device monitoring services. We receive aA significant portion of our revenue fromis paid for by third-party commercial insurance organizations and governmental entities. We also receive reimbursement directly from patients through co-pays, deductibles and self-pay arrangements. Billings for services reimbursed by
For contracted third-party payors, including Medicare, we determine revenue based on negotiated prices for the services provided. Based on our history, we have experience collecting substantially all of the negotiated contracted rates and are therefore not providing an implicit price concession. As a result, any adjustments to the revenue recognized are due to patient default and are recorded as revenue net of contractual allowances. Ifbad debt expense.
For non-contracted payors, we are providing an implicit price concession because we do not have
consistent historical information regarding collectability from a given payor to support revenue recognition atcontract with the time of service, revenue is recognized when cash is received. Adjustments to the estimated receipts for non-contractualunderlying payor. As a result, we estimate our expected revenue based on final settlement withhistorical cash collections. Subsequent adjustments to the third-party payors,revenue recognized are recorded upon settlement. Unearned amounts are appropriately deferred until the service has been completed. Medicare accounts for a significant portion of our as an adjustment to Healthcare revenue and total revenue.not as bad debt expense.
Research
Research segment revenue includes revenue for core laboratory services. Our Research segment revenue is provided on a fee-for-service basis, and revenue is recognized as the related services are performed. We also provide consulting services on a time and materials basis, and this revenue is recognized as the services are performed. Our site support revenue, consisting of equipment rentals and sales along with related supplies and logistics management, are recognized at the time of sale or over the rental period. Under a typical contract, customers pay us a portion of our fee for these services upon contract execution as an upfront deposit. Unearned revenue, including upfront deposits, is deferred, and then recognized as the services are performed.
For arrangements with multiple deliverables, the revenue is allocated to each element (both delivered and undelivered items) based on their relative selling prices or management’s best estimate of their selling prices, when vendor-specific or third-party evidence is unavailable.
We record reimbursements received for out-of-pocket expenses, including freight, as revenue in the accompanying consolidated statements of operationsoperations.
See “Part II; Item 8. Financial Statements and comprehensive income (loss).Supplementary Data; Notes to Consolidated Financial Statements; Note 3. Revenue Recognition” in this Annual Report on Form 10-K for more information.
Technology
Technology revenue includes revenue received from the sale of products, product repairs and supplies to medical companies, clinics and hospitals. Our Technology revenue is recognized when products are shipped, or as services are completed.
Accounts Receivable
Healthcare accounts receivable, including contract assets, are related to the Healthcare segment, and are recorded at the time revenue is recognized net of contractual allowances, and are presented on the consolidated balance sheet net of allowance for doubtful accounts. The ultimate collectionFor contracted payors, including Medicare, we determine revenue based on negotiated prices for the services provided. Based on our history, we have experience collecting substantially all of accounts receivable maythe negotiated contracted rates and are therefore not be known for several months after services have been provided and billed. Weproviding an implicit price concession. As a result, we record an allowance for doubtful accounts based on historical collection trends to account for the agingrisk of receivables using historical data. The percentages and amounts used to record bad debt expense and the allowance for doubtful accounts are supported by various methods and analyses, including current and historical cash collections and the aging of receivables by payor.patient default. Because of continuing changes in the health carehealthcare industry and third-party reimbursement, it is possible that our estimates could change, which could have a material impact on our operations and cash flows.
Other accounts receivable are related to the TechnologyResearch segment and Research segmentsCorporate and Other category and are recorded at the time revenue is recognized, or when products are shipped or services are performed. We estimate the allowance for doubtful accounts on a specific account basis, and consider several factors in our analysis including customer specific information and the aging of the account.information.
We will write off receivables when the likelihood for collection is remote and when we believe collection efforts have been fully exhausted and we do not intend to devote additional resources in attempting to collect. We perform write-offs on a monthly basis. In the Healthcare segment, we wrote off $8.8$15.3 million and $8.4$11.2 million of receivables for the years ended December 31, 20172019 and 2016,2018, respectively. The impact
was a reduction of gross receivables and a reduction in the allowance for doubtful accounts. There were no material write-offs in the Technology and Research segments.segment. We recorded bad debt expense of $13.3 million, $9.9$1.1 million and $8.0$0.8 million related to a specific customer bankruptcy in the Corporate and Other category during the years ended December 31, 2018 and 2017, respectively, and wrote off these amounts in 2018. We recorded consolidated bad debt expense of $21.8 million, $22.2 million and $13.3 million, for the years ended December 31, 2019, 2018 and 2017, 2016 and 2015, respectively.
Stock-Based Compensation
Accounting Standards Codification (“ASC”) 718 - Compensation—Stock Compensation (“ASC 718”718”), addresses the accounting for share-based payment transactions in which an enterprise receives employee services in exchange forfor: (i) equity instruments of the enterprise or (ii) liabilities that are based on the fair value of the enterprise’s equity instruments or that may be settled by the issuance of such equity instruments. ASC 718 requires that an entity measure the cost of equity-based service awards issued to employees, such as stock options and RSUs, based on the grant-date fair value of the award and recognize the cost of such awards over the requisite service period during which(generally, the employee is required to provide service in exchange for the award (the vesting period). ASC 718 requires that an entity measure the cost of liability-based service awards based on current fair value that is remeasured subsequently at each reporting date through the settlement date. We also use the provisions of ASC 505-50, Equity Based Payments to Non-Employees (“ASC 505-50”), to account for stock-based compensation awards issued to non-employees for services. Such awards for services are recorded at either the fair valueperiod of the services rendered or the instruments issued in exchange for such services, whichever is more readily determinable, using the measurement date guidelines enumerated in ASC 505-50.
Stock-basedaward). The compensation expense associated with PSUsis only recognized for outstanding performance stock units (“PSUs”) whereratably over the period between when the performance conditions are deemed probable for achievement. For PSUs deemed probable forof achievement stock-based compensation expense is recognized ratably overand when the expected vesting period.awards are vested. Performance stock options (“PSOs”PSOs”) are valued and stock-based compensation expense is only recognizedrecorded once the performance conditions of the outstanding PSOs have been met.achieved probability. Prior to our July 1, 2018 adoption of Accounting Standards Update (“ASU”) 2018-07, Compensation - Stock Compensation (Topic 718): Improvements to Nonemployee Share-Based Payment Accounting (“ASU 2018-07”), we accounted for equity awards issued to non-employees in accordance with ASC 505-50, Equity-Based Payments to Non-Employees.
We have historically recorded stock-based compensation expense based on the number of stock options or restricted stock unitsRSUs we expect to vest using our historical forfeiture experience and we periodically update those forfeiture rates to apply to new grants. While we early adopted ASU 2016-09 in the year ended December 31, 2016, we have elected to continue toWe estimate forfeitures under the true-up provision of ASC 718. ASC
718 requires forfeitures to be. We record additional expense if the actual forfeiture rate is lower than estimated, atand record a recovery of prior expense if the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from estimates. Forfeitures are estimated based on our historical experience.forfeiture rate is higher than estimated.
We estimate the fair value of our stock options to employees and directors using the Black-ScholesBlack‑Scholes option valuation model. The Black-ScholesBlack‑Scholes option valuation model requires the use of certain subjective assumptions. The most significant of these assumptions are the estimates of the expected volatility of the market price of our stock and the expected term of the award. We base our estimates of expected volatility on the historical volatilityaverage of our stock price. The expected term represents the period of time that stock-basedshare‑based awards granted are expected to be outstanding. Other assumptions used in the Black-ScholesBlack‑Scholes option valuation model include the risk-freerisk‑free interest rate and expected dividend yield. The risk-freerisk‑free interest rate for periods pertaining to the contractual lifeexpected term of each option is based on the United StatesU.S. Treasury yield of a similar duration in effect at the time of grant. We have never paid, and do not expect to pay, dividends in the foreseeable future.
We estimate the fair value of our PSUs using a Monte Carlo simulation. This model uses assumptions, including the risk free interest rate, expected volatility of our stock price and those of the performance group, dividends of the performance group members and expected life of the awards. As noted above, we continue to estimate forfeitures under the true-up provision of ASC 718. If it is deemed probable that the PSU performance targets will be met, compensation expense is recorded for these awards ratably over the requisite service period. The PSUs are forfeited to the extent the performance criteria are not met within the service period.
Goodwill and Acquired Intangible Assets
Goodwill is the excess of the purchase price of an acquired business over the amounts assigned to assets acquired and liabilities assumed in a business combination. In accordance with ASC 350, - Intangibles
— - Goodwill and Other (“ASC 350”350”), goodwill is reviewed for impairment annually, or when events arise that could indicate that an impairment exists. Initially, we qualitativelyIn order to test goodwill for impairment, ASC 350 allows reporting entities to take either a qualitative or quantitative approach to testing. Under the qualitative approach, an entity may assess qualitative factors to determine whether it is more-likely-than-not that an impairment exists for eachthe fair value of a reporting unit.unit is less than its carrying amount. Such qualitative factors can include, among others, industry and market conditions, present and anticipated sales and cost factors, overall financial performance and relevant entity-specific events. If we conclude based on our qualitative assessment that it is more-likely-than-not that the fair value of a reporting unit is less than its carrying value, we perform an impairment testa quantitative analysis in accordance with ASC 350. We compare350. Under the quantitative approach, an entity compares the fair value of ourits reporting units to their carrying value. If the reporting unit’s carrying value exceeds its fair value, an impairment loss equal to the difference is recognized. The loss recognized shall not exceed the total amount of goodwill allocated to the reporting unit, and the income tax effects from any deductible goodwill on the carrying value of the reporting unit when measuring the goodwill impairment loss, if any, are considered.
For the purpose of performing our goodwill impairment analysis, we consider our business to be composed of three reporting units: Healthcare Technology, Research and Research.Technology. When performing a quantitative analysis, we calculate the fair value of the reporting units utilizing a weighting of the income and market approaches. The income approach is based on a discounted cash flow methodology that includes assumptions for, among other things, forecasted income, cash flow, growth rates, income tax rates, expected tax benefits and long-term discount rates, all of which require significant judgment. The market approach utilizes our market data as well as market data from publicly-traded companies that are similar to us. There are inherent uncertainties related to these factors and the judgment applied in the analysis. We believe that the combination of an income and a market approach providesapproaches provide a reasonable basis to estimate the fair value of our reporting units.
Acquired intangible assets are recorded at fair value on the acquisition date. The estimated fair values and useful lives of intangible assets are determined by assessing many factors, including estimates of future operating performance and cash flows of the acquired business, the characteristics of the intangible assets and the experience of the acquired business. Independent appraisal firms may assist with the valuation of acquired assets. The impairment test for indefinite-lived intangible assets other than goodwill consists of a comparison of the fair value of the indefinite-lived intangible asset to the carrying value of the asset. We estimate the fair value of the indefinite-lived intangibles using the relief from royalty method. We amortize our finite-lived intangible assets over each asset’s estimated useful life using the straight-line method.
We performed an impairment analysis of goodwill and indefinite-lived intangible assets for the years ended December 31, 2017, 20162019, 2018 and 2015.2017. There was no goodwill impairment recorded as a result of these analyses.
During our impairment testing of our intangible assets for the year ended December 31, 2017, consideringgiving particular consideration to the LifeWatch integration and forward-looking integration plans, we determined that certain trade names and internally developed software costs ceased being used and were no longer going to be used and were therefore impaired, resultingimpaired. This resulted in $11.0 million of intangible asset impairment charges included within the Corporate and Other segmentcategory as a component of other costs within the other charges line in our consolidated statements of operations and comprehensive income/(loss).operations. There were no other intangible asset impairments for the year ended December 31, 2017.
At2017, and there were no intangible asset impairments for the years ended December 31, 20162019 and 2015, we performed2018.
Acquisition-Related Contingent Consideration
Acquisition-related contingent consideration is our required annual impairment testobligation, arising from a business combination, to transfer additional assets and/or equity interests to the seller if certain future events occur or conditions are met. The fair value of indefinite-lived intangible assets. Basedthe contingency is estimated as of the acquisition date using certain unobservable inputs (and therefore classified as Level 3 in the fair value hierarchy) and is recorded as a liability. We re-measure the estimated fair value of acquisition-related contingent consideration classified as a liability at each reporting date. Adjustments subsequent to the acquisition measurement period are recorded in other charges in the consolidated statements of operations. Changes to the inputs used in the measurement of acquisition-related contingent consideration include, but are not limited to: changes in the assumptions regarding probabilities of successful achievement of future events or conditions; estimated revenue projections; discounts for lack of marketability of our common stock; estimated stock price volatility; and the discount rate used to estimate the fair value of the liability. Acquisition-related contingent consideration may change significantly as our inputs and assumptions noted above evolve and additional data is obtained. The inputs and assumptions used in estimating fair value require significant judgment. The use of different assumptions and judgments could result in different fair value estimates that may have a material impact on these impairment tests, we determined that there was no impairment.our results from operations and financial position.
Income Taxes
We account for income taxes under the liability method, as described in ASC 740 - Income Taxes (“ASC 740”740”). Deferred income taxes are recognized for the tax consequences of temporary differences
between the tax and financial statement reporting bases of assets and liabilities. When we determine that we will not be able to realize our deferred tax assets, we adjust the carrying value of the deferred tax assetassets through the valuation allowance.
We record uncertainunrecognized tax positionsbenefits in accordance with ASC 740 on the basis of a two-step process in which (i) we determine whether it is more likely than not that the tax positions will be sustained on the basis of the technical merits of the position and (ii) for those tax positions that meet the more-likely-than-notmore-likely-than-
not recognition threshold, we recognize the largest amount of tax benefit that is more than 50 percent likely to be realized upon ultimate settlement with the related tax authority.
Statements of Operations Overview
Revenue
The vast majority of our revenue is derived from remote cardiac monitoring services in our Healthcare segment. The amount of Healthcare segment revenue generated is based on the number of patients enrolled through physician prescriptions and the rates reimbursed to us by Medicare, commercial payors, physicians patients and Medicare. patients. MCT Medicare pricing was downdeclined slightly in 20172019 after being stable in 2018 and will be flathas not changed significantly for 2018. Over time, we expect the price to remain relatively stable.2020. We expect volumes to grow in the long-term as the market grows and we continue to gain market share.
Revenue is generated in the Research segment through various study and consulting services, which include activities such as core lablaboratory services, project management, data management, equipment rental and customer support. Research segment revenue is driven by our ability to enter into service contracts at various phases of the pharmaceutical drug development and medical device life cycle.cycles. We expect volume to increase as a result of our growing capabilities as a multi-service provider. Negotiated pricing for service contracts is subject to market pressures, and as a result has decreased slightly over the last few years. With recent market consolidation, we are experiencing stabilization in our prices. We expect revenue from the Research segment to increase over the long-term as we continue to increase our study volume.
Revenue is generated in the Technology segmentCorporate and Other category from the sale of non-invasive cardiac andmonitors to healthcare companies, the sale of wireless blood glucose monitoring meters and test strips to wholesale distributors of diabetic supplies and diabetic patients as well as product repairs and is recognized when products to third-party distributors and service providers in our Technology segment. Technology revenue is driven by the number of the units purchased by our customers and the relative per unit pricing for various products. The sales volume for our Technology segment has increased in the current year with integration of Telcare.are shipped, or as services are completed. We expect our Technology segment revenue in the Corporate and Other category to increase over the long-term as we grow ourintroduce new products.
Gross Profit
Gross profit consists of revenue less the cost of revenue.
Cost of revenue for the Healthcare segment includes:
salaries and benefits for personnel providing various services and customer support to physicians and patients including customer service, monitoring services, distribution services (scheduling, packaging and delivery of the devices to the patients and practices), device repair and maintenance and quality assurance;
cost of patient-related services provided by third-party subcontractors including device transportation to and from the patients and practices and wireless communication charges related to transmission of data to the monitoring centers;
consumable supplies sent to patients along with the durable components of our devices; and
depreciation of our medical devices.
Cost of revenue for the Research segment includes:
cost of internal and third-party medical specialists and technicians;
salaries and benefits of personnel providing various services to customers including consulting, customer support, project management and certain information technology support;
depreciation of our medical devices; and
cost of materials and transportation related to the shipment of products and supplies.
Cost of revenue for the Technology segmentCorporate and Other category includes the cost of materials and labor related to the manufacture of our products and product repair services.
We expect multiple factors to influence our gross profit margins in the foreseeable future. Changes in reimbursement and payor mix are two such factors. While we expect reimbursement to be stable, payor mix is unpredictable and dependent on the insurance coverage of patients that are prescribed our services. We have a history of lowering the average cost of revenuesrevenue as we increase volume. We expect to continue to achieve efficiencies in cost of revenue through process improvements, as well as from a reduction in the cost of our devices. We also expect to realize synergies from our LifeWatch acquisition. Thesethese factors will have a favorable impact on our gross profit margins. While these factors could be offsetting,impact; however, it is difficult to predict how they will influence our gross profit margins.
We expect to achieve some efficiencies in our Research segment cost of revenue through process improvements and automation, and expect a favorable impact on gross margins due to the leveraging of the relatively fixed costfixed-cost infrastructure. If we experience increased service contract pricing or volume declines in our Research segment, it would have an adverse effect on our gross profit margin.
If we experience volume or selling price declines in our Technology segment, or service contract pricing, it would have an adverse effect on our gross profit margin. We expect the cost of products sold and repairs to remain relatively consistent.
General and Administrative
General and administrative expense consists primarily of salaries and benefits related to general and administrative personnel, management bonuses, professional fees primarily related to legal and audit fees,services, amortization related to intangible assets, facilities expenses and the related overhead.
Sales and Marketing
Sales and marketing expense consists primarily of salaries, benefits and commissions related to sales personnel, travel and entertainment costs and marketing and contracting personnel.costs. Also included are costs associated with marketing programs such as trade shows and advertising campaigns.
Research and Development
Research and development expense consists primarily of salaries and benefits of personnel, as well as subcontractors who work on new product development and sustaining engineering of our existing products.
Other Charges
We account for expenses associated with our acquisitions and certain litigation as other charges as incurred. These expenses wereare primarily a result of legal and professional fees related to activities surrounding our acquisitions, including integration activities subsequentincluding severance and asset impairments, as well as legal expense related to our acquisitions and patent litigation infor which we are the plaintiff. Other charges including intangible and fixed asset impairments, are costs that are not considered necessary toindicative of the ongoing business operations.
Interest Expense
Interest expense consists primarily of the interest accrued related to our term loan, capitalfinance leases and where applicable, our revolving credit agreement,facility, along with the amortization of deferred debt acquisitionissuance costs.
Loss on Extinguishment of Debt
Loss on extinguishment of debt consists primarily of the costs incurred related to the write offwrite-off of the unamortized debt issuance costs when we settledupon settlement of our prior credit agreements.
Loss on Equity Method Investment
Loss on equity method investment represents our portion of the results of operations of our equity method investee.investees.
Other Non-Operating Expense,Income/(Expense), net
Other non-operating expense,income/(expense), net represents other infrequently occurring non-operating items including settlement charges and foreign exchange gains/(losses).
Net Loss Attributable to Noncontrolling Interests
Net loss attributable to noncontrolling interests consists of the post-acquisition activity of the portion of LifeWatch that we had not yet acquired during the period from July 12, 2017 through December 31, 2017, as well as the 45% of LifeWatch Turkey Holding AG that we dodid not own over that same period.own.
Results of Operations
Years Ended December 31, 20172019 and 20162018
Revenue
| | | | Year Ended | | Change | Year Ended December 31, | | Change |
| (In thousands, except percentages) | December 31, 2017 | | December 31, 2016 | | $ | | % | |
| (in thousands, except percentages) | | 2019 | | 2018 | | $ | | % |
| Healthcare | $ | 234,385 |
| | $ | 165,664 |
| | $ | 68,721 |
| | 41.5 | % | $ | 372,014 |
| | $ | 338,812 |
| | $ | 33,202 |
| | 9.8 | % |
| Research | 38,790 |
| | 32,565 |
| | 6,225 |
| | 19.1 | % | 54,450 |
| | 50,561 |
| | 3,889 |
| | 7.7 | % |
| Technology | 13,601 |
| | 10,103 |
| | 3,498 |
| | 34.6 | % | |
| Other | | 12,643 |
| | 10,099 |
| | 2,544 |
| | 25.2 | % |
| Total revenue | $ | 286,776 |
| | $ | 208,332 |
| | $ | 78,444 |
| | 37.7 | % | $ | 439,107 |
| | $ | 399,472 |
| | $ | 39,635 |
| | 9.9 | % |
Total revenue for the year ended December 31, 20172019 increased 9.9%, due primarily to growth in revenue across all of our businesses. Healthcare revenue growth was driven by increased patient volume, related to our MCT and extended Holter services, as well as the Healthcare segment,addition of the implantable device monitoring revenue contributed by Geneva, which we acquired on March 1, 2019. The positive impact of the higher patient volume was affectedpartially offset by the acquisitionreduction of LifeWatch, along with increased patient volumesMCT Medicare reimbursement, which went into effect January 1, 2019, as well as a favorable service mix. While overall pricing was stable, we saw a declinechange in our Medicare rates.payor mix. Research revenue continues to benefit from new studies resulting from the utilization of ePatch™, our extended Holter device. Other revenue increased due to the full year impactcontinued volume growth of the acquisition of our imaging business, VirtualScopics, which was acquired in May 2016, partially offset by lower cardiac study volumes.diabetic product sales.
Technology revenue also increased due to the full year impact of Telcare, which was acquired in December 2016.
Gross Profit
| | | | Year Ended | | Change | Year Ended December 31, | | Change |
| (In thousands, except percentages) | December 31, 2017 | | December 31, 2016 | | $ | | % | |
| (in thousands, except percentages) | | 2019 | | 2018 | | $ | | % |
| Gross profit | $ | 172,370 |
| | $ | 129,450 |
| | $ | 42,920 |
| | 33.2 | % | $ | 274,274 |
| | $ | 250,486 |
| | $ | 23,788 |
| | 9.5 | % |
| Percentage of revenue | 60.1 | % | | 62.1 | % | | | | | 62.5 | % | | 62.7 | % | | | | |
Gross profit for the year ended December 31, 20172019 increased primarily due primarily to our recent acquisitions, organic growth, and realized synergies from our acquisitions, partially offset by the impact of the decrease in Medicare rates.higher revenue. The 20 basis point decrease in gross marginprofit percentage was due to the impact of our recent acquisitions, whose revenues carry lower profit margins than our existing business.the reduction of MCT Medicare reimbursement, which went into effect January 1, 2019, as well as increased costs to support Research studies. This was partially offset by the positive impact of Healthcare operational efficiencies.
General and Administrative Expense
| | | | Year Ended | | Change | Year Ended December 31, | | Change |
| (In thousands, except percentages) | December 31, 2017 | | December 31, 2016 | | $ | | % | |
| (in thousands, except percentages) | | 2019 | | 2018 | | $ | | % |
| General and administrative expense | $ | 82,983 |
| | $ | 55,877 |
| | $ | 27,106 |
| | 48.5 | % | $ | 120,093 |
| | $ | 109,736 |
| | $ | 10,357 |
| | 9.4 | % |
| Percentage of revenue | 28.9 | % | | 26.8 | % | | | | | 27.3 | % | | 27.5 | % | | | | |
General and administrative expense increased for the year ended December 31, 20172019 increased primarily due primarily to costs associated with the $19.9 million impact fromongoing investment in our recent acquisitions, primarily LifeWatch, a $6.0 million impact from intangible asset amortization attributed to LifeWatch,business systems and infrastructure as well as a $2.2 million increase in technology costs, offset partially by $0.7 million in headcount related savings. The increase in the general and administrative expense as a percentaddition of revenue was the result of the intangible asset amortization recorded stemming from the LifeWatch acquisition.Geneva.
Sales and Marketing Expense
| | | | Year Ended | | Change | Year Ended December 31, | | Change |
| (In thousands, except percentages) | December 31, 2017 | | December 31, 2016 | | $ | | % | |
| (in thousands, except percentages) | | 2019 | | 2018 | | $ | | % |
| Sales and marketing expense | $ | 35,322 |
| | $ | 28,636 |
| | $ | 6,686 |
| | 23.3 | % | $ | 50,664 |
| | $ | 42,849 |
| | $ | 7,815 |
| | 18.2 | % |
| Percentage of revenue | 12.3 | % | | 13.7 | % | | | | | 11.5 | % | | 10.7 | % | | | | |
The increase to salesSales and marketing expense for the year ended December 31, 2017 was due2019 increased primarily to the $10.7 million impact from our recent acquisitions, primarily LifeWatch, and $2.2 million in added employee related costs exclusive of our recent acquisitions, partially offset by approximately $5.0 million of synergies obtained from the recent acquisitions and $1.1 million due to lower sales meetings costs. Sales and marketing expense decreased as a percentage of revenueincreased headcount-related expenses due to the leverage and synergies obtained fromongoing investment in our acquired businesses.
Healthcare field sales force as well as the addition of Geneva.
Bad Debt Expense
| | | | Year Ended | | Change | Year Ended December 31, | | Change |
| (In thousands, except percentages) | December 31, 2017 | | December 31, 2016 | | $ | | % | |
| (in thousands, except percentages) | | 2019 | | 2018 | | $ | | % |
| Bad debt expense | $ | 13,291 |
| | $ | 9,931 |
| | $ | 3,360 |
| | 33.8 | % | $ | 21,768 |
| | $ | 22,222 |
| | $ | (454 | ) | | (2.0 | )% |
| Percentage of revenue | 4.6 | % | | 4.8 | % | | | | | 5.0 | % | | 5.6 | % | | | | |
The increase in badBad debt expense for the year ended December 31, 2017 was2019 decreased primarily due to a prior year $1.1 million specific reserve related to a customer bankruptcy in the Other category. This was partially offset by the increased Healthcarerevenue from our recently acquired business and the timing of revenue andHealthcare collections. Substantially all of our bad debt expense relates to the Healthcare segment. Bad debt expense for the year ended December 31, 2019 in Research and the Research segmentsOther category was minimal and is recorded on a specific account basis.
Research and Development Expense
| | | | Year Ended | | Change | Year Ended December 31, | | Change |
| (In thousands, except percentages) | December 31, 2017 | | December 31, 2016 | | $ | | % | |
| (in thousands, except percentages) | | 2019 | | 2018 | | $ | | % |
| Research and development expense | $ | 11,101 |
| | $ | 8,355 |
| | $ | 2,746 |
| | 32.9 | % | $ | 13,994 |
| | $ | 11,206 |
| | $ | 2,788 |
| | 24.9 | % |
| Percentage of revenue | 3.9 | % | | 4.0 | % | | | | | 3.2 | % | | 2.8 | % | | | | |
Research and development expense for the year ended December 31, 20172019 increased due primarily to increased headcount-related expenses related to our ongoing investment in new products and technologies, including the $5.2 million impact fromfurther incorporation of artificial intelligence and machine learning into our recent acquisitions, primarily LifeWatch, offset partially by $2.7 million of synergies obtained from the recent acquisitions and other cost savings.services.
Other Charges
| | | | Year Ended | | Change | Year Ended December 31, | | Change |
| (In thousands, except percentages) | December 31, 2017 | | December 31, 2016 | | $ | | % | |
| (in thousands, except percentages) | | 2019 | | 2018 | | $ | | % |
| Other charges | $ | 31,436 |
| | $ | 8,639 |
| | $ | 22,797 |
| | 263.9 | % | $ | 15,004 |
| | $ | 14,659 |
| | $ | 345 |
| | 2.4 | % |
| Percentage of revenue | 11.0 | % | | 4.1 | % | | | | | 3.4 | % | | 3.7 | % | | | | |
The increase in otherOther charges for the year ended December 31, 2017 was2019 increased primarily due to a $4.4 million increase from activity associated with our ongoing patent and other litigation, $3.0 million of charges related to $12.0our October 2019 information technology incident, and a $0.5 million impact from changes in acquisition-related contingent consideration. These increases were offset partially by a $5.2 million reduction of asset impairment charges, $4.9 million in additional professional fees, $4.1 million of severance and employee related costs, and $1.5 million of legal fees, primarilyintegration expense related to the acquisition of LifeWatch. These charges were partially offset byour acquisitions, a $2.6$1.8 million decreaseprior year reserve for a note receivable with a bankrupt customer and a small reduction in the fair value of acquisition-related consideration recognized for previous acquisitions.
other non-recurring charges. For further details, please see “Part II; Item 8, Financial Statements and Supplementary Data; Note 13. Other Charges.”
Other Expense
|
| | | | | | | | | | | | | | |
| | Year Ended | | Change |
| (In thousands, except percentages) | December 31, 2017 | | December 31, 2016 | | $ | | % |
| Interest expense | $ | (4,897 | ) | | $ | (1,830 | ) | | $ | (3,067 | ) | | 167.6 | % |
| Loss on extinguishment of debt | (543 | ) | | — |
| | (543 | ) | | n/a |
| Loss on equity method investment | (384 | ) | | (287 | ) | | (97 | ) | | 33.8 | % |
| Other non-operating expense, net | (2,809 | ) | | (125 | ) | | (2,684 | ) | | 2,147.2 | % |
| Total Other expense | $ | (8,633 | ) | | $ | (2,242 | ) | | $ | (6,391 | ) | | 285.1 | % |
| Percentage of revenue | 3.0 | % | | 1.1 | % | | | | |
|
| | | | | | | | | | | | | | |
| | Year Ended December 31, | | Change |
| (in thousands, except percentages) | 2019 | | 2018 | | $ | | % |
| Interest expense | $ | (9,482 | ) | | $ | (9,429 | ) | | $ | (53 | ) | | 0.6 | % |
| Loss on equity method investments | (1,298 | ) | | (246 | ) | | (1,052 | ) | | 427.6 | % |
| Other non-operating (expense)/income, net | (2,243 | ) | | 1,365 |
| | (3,608 | ) | | (264.3 | )% |
| Total Other expense | $ | (13,023 | ) | | $ | (8,310 | ) | | $ | (4,713 | ) | | 56.7 | % |
| Percentage of revenue | 3.0 | % | | 2.1 | % | | | | |
Total other expense for the year ended December 31, 20172019 increased due primarily to $3.1 million of interest expense resulting from our new credit agreement entered into concurrent with our LifeWatch acquisition and a $2.5 million non-operating charge for our settlement with the Office of Civil Rights relateddue to the thefteffect of two unencrypted laptop computers in 2011. Additionally, we incurred a $1.3 million non-operating charge related tonon-cash foreign currency transaction losses as well as the derivative instrument premium for the acquisition of LifeWatch, and a $0.5 million loss on the extinguishmentnon-cash impairment of our previous credit agreement with Healthcare Financial Solutions, LLC, offset partially by a non-operating gain of approximately $1.3 million related to the favorable settlement with the former owner of Mednet.equity method investment in Wellbridge Health, Inc.
Income Taxes
|
| | | | | | | | | | | | | | |
| | Year Ended | | Change |
| (In thousands, except percentages) | December 31, 2017 | | December 31, 2016 | | $ | | % |
| Benefit from/(provision for) income taxes | $ | (6,747 | ) | | $ | 37,667 |
| | $ | (44,414 | ) | | (117.9 | )% |
| Effective tax benefit/(provision) rate | (64.9 | )% | | 238.9 | % | | | | |
|
| | | | | | | | | | | | | |
| | Year Ended December 31, | | Change |
| (in thousands, except percentages) | 2019 | | 2018 | | $ | | % |
| (Provision for)/benefit from income taxes | $ | (9,884 | ) | | $ | 370 |
| | $ | (10,254 | ) | |
|
| Effective tax rate | 24.9 | % | | (0.9 | )% | | | | |
For the year ended December 31, 2017,2019, we recognized a netrecorded an income tax provision primarily due to the re-measurement of our deferred tax assets and liabilitiescomputed at the new federal corporate rate of 21 percent, which total $8.0 million.statutory and applicable state rates, partially offset by favorable tax deductions related to stock compensation.
For the year ended December 31, 2016,2018, we recognized a netrecorded an income tax benefit primarily due primarily to a $51.6 million benefitfavorable tax deductions related to the release of our valuation allowance. The release of our valuation allowance was partially offset by adjustments to deferred taxes and state taxes levied on fiscal 2016 taxable income.
stock compensation.
Years Ended December 31, 20162018 and 20152017
Revenue
|
| | | | | | | | | | | | | | |
| | Year Ended | | Change |
| (In thousands, except percentages) | December 31, 2016 | | December 31, 2015 | | $ | | % |
| Healthcare | $ | 165,664 |
| | $ | 145,963 |
| | $ | 19,701 |
| | 13.5 | % |
| Research | 32,565 |
| | 21,853 |
| | 10,712 |
| | 49.0 | % |
| Technology | 10,103 |
| | 10,697 |
| | (594 | ) | | (5.6 | )% |
| Total revenue | $ | 208,332 |
| | $ | 178,513 |
| | $ | 29,819 |
| | 16.7 | % |
Healthcare revenue increasedFor discussion related to the results of operations for the years ended December 31, 2018 and 2017, refer to “Part II – Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations” included in our Annual Report on Form 10-K for the fiscal year ended December 31, 2016 due to increased patient volumes as well as higher MCT Medicare pricing. In addition, Research revenue increased due to2018, which was filed with the acquisition of VirtualScopics. These increases were partially offset by a decrease in Technology revenue due to lower sales volume resulting from customers delaying purchases as they await the release of upgraded devices, partially offset by increases due to current year acquisitions.SEC on February 22, 2019.
Gross Profit
|
| | | | | | | | | | | | | | |
| | Year Ended | | Change |
| (In thousands, except percentages) | December 31, 2016 | | December 31, 2015 | | $ | | % |
| Gross profit | $ | 129,450 |
| | $ | 106,557 |
| | $ | 22,893 |
| | 21.5 | % |
| Percentage of revenue | 62.1 | % | | 59.7 | % | | | | |
The increase in gross margin percentage for the year ended December 31, 2016 was due to Healthcare volume efficiencies and higher Healthcare pricing, as well as reduced costs related to shipping and device communication. These increases were slightly offset by the impact of our acquisitions, which carry lower profit margins than our existing business.
General and Administrative Expense
|
| | | | | | | | | | | | | | |
| | Year Ended | | Change |
| (In thousands, except percentages) | December 31, 2016 | | December 31, 2015 | | $ | | % |
| General and administrative expense | $ | 55,877 |
| | $ | 47,882 |
| | $ | 7,995 |
| | 16.7 | % |
| Percentage of revenue | 26.8 | % | | 26.8 | % | | | | |
General and administrative expense for the year ended December 31, 2016 increased due to the addition of $3.8 million from our acquired businesses, as well as a $2.6 million increase in employee related costs and a $1.6 million increase in stock compensation expense. $1.3 million of the increase in stock compensation expense related to a performance bonus awarded to a third‑party.
Sales and Marketing Expense
|
| | | | | | | | | | | | | | |
| | Year Ended | | Change |
| (In thousands, except percentages) | December 31, 2016 | | December 31, 2015 | | $ | | % |
| Sales and marketing expense | $ | 28,636 |
| | $ | 27,936 |
| | $ | 700 |
| | 2.5 | % |
| Percentage of revenue | 13.7 | % | | 15.6 | % | | | | |
Sales and marketing expense for the year ended December 31, 2016 increased due to the addition of $0.9 million from our acquired businesses, partially offset by a $0.2 million decrease in employee related costs.
Bad Debt Expense
|
| | | | | | | | | | | | | | |
| | Year Ended | | Change |
| (In thousands, except percentages) | December 31, 2016 | | December 31, 2015 | | $ | | % |
| Bad debt expense | $ | 9,931 |
| | $ | 8,047 |
| | $ | 1,884 |
| | 23.4 | % |
| Percentage of revenue | 4.8 | % | | 4.5 | % | | | | |
Bad debt expense for the year ended December 31, 2016 increased due to the timing of revenue and collections. Substantially all of our bad debt expense relates to the Healthcare segment. Bad debt expense in the Technology and Research segments was minimal and is recorded on a specific account basis.
Research and Development Expense
|
| | | | | | | | | | | | | | |
| | Year Ended | | Change |
| (In thousands, except percentages) | December 31, 2016 | | December 31, 2015 | | $ | | % |
| Research and development expense | $ | 8,355 |
| | $ | 7,111 |
| | $ | 1,244 |
| | 17.5 | % |
| Percentage of revenue | 4.0 | % | | 4.0 | % | | | | |
Research and development expense for the year ended December 31, 2016 increased due to the addition of $1.0 million from our acquired businesses as well as a $0.2 million increase in consulting costs and a $0.1 million increase in employee related expenses.
Other Charges
|
| | | | | | | | | | | | | | |
| | Year Ended | | Change |
| (In thousands, except percentages) | December 31, 2016 | | December 31, 2015 | | $ | | % |
| Other charges | $ | 8,639 |
| | $ | 6,063 |
| | $ | 2,576 |
| | 42.5 | % |
| Percentage of revenue | 4.1 | % | | 3.4 | % | | | | |
Other charges for the year ended December 31, 2016 consisted of legal charges of $7.2 million related primarily to patent litigation cases in which we are the plaintiff, professional fees of $0.7 million, severance and employee related costs of $0.6 million and $0.1 million of other acquisition related costs from our 2016 acquisitions.
Other charges for the year ended December 31, 2015 consisted of legal charges of $5.8 million related primarily to patent litigation and severance and employee related costs of $0.3 million associated with activities surrounding our 2014 acquisitions.
Other Expense
|
| | | | | | | | | | | | | | |
| | Year Ended | | Change |
| (In thousands, except percentages) | December 31, 2016 | | December 31, 2015 | | $ | | % |
| Interest expense | $ | (1,830 | ) | | $ | (1,534 | ) | | $ | (296 | ) | | 19.3 | % |
| Loss on equity method investment | (287 | ) | | — |
| | (287 | ) | | n/a |
| Other non-operating expense, net | (125 | ) | | (88 | ) | | (37 | ) | | 42.0 | % |
| Total Other expense | $ | (2,242 | ) | | $ | (1,622 | ) | | $ | (620 | ) | | 38.2 | % |
| Percentage of revenue | 1.1 | % | | 0.9 | % | | | | |
Total other expense for the year ended December 31, 2016 increased due primarily to increased interest expense related to our borrowings under our Revolving Loans as well as due to recognizing our share of our equity method investee’s loss.
Income Taxes
|
| | | | | | | | | | | | | | |
| | Year Ended | | Change |
| (In thousands, except percentages) | December 31, 2016 | | December 31, 2015 | | $ | | % |
| Benefit from/(provision for) income taxes | $ | 37,667 |
| | $ | (468 | ) | | $ | 38,135 |
| | 8,148.5 | % |
| Effective tax benefit/(provision) rate | 238.9 | % | | (5.9 | )% | | | | |
For the year ended December 31, 2016, we recognized a net tax benefit due primarily to a $51.6 million benefit related to the release of our valuation allowance. The release of our valuation allowance was partially offset by adjustments to deferred taxes and state taxes levied on fiscal 2016 taxable income.
For the year ended December 31, 2015, we recognized a tax provision due primarily to Alternative Minimum Tax (“AMT”) levied on fiscal 2015 taxable income net of allowable AMT net operating loss carryovers, as well as an increase in the deferred tax liability created by the book to tax differences on indefinite‑lived assets.
Liquidity and Capital Resources
The following table highlights certain information related to our liquidity and capital resources:
| | | | Year Ended | | | | | |
| (In thousands, except ratios) | December 31, 2017 | | December 31, 2016 | December 31, 2019 | | December 31, 2018 |
| Cash and cash equivalents | $ | 36,022 |
| | $ | 23,052 |
| $ | 68,614 |
| | $ | 80,889 |
|
| Healthcare accounts receivable, net of allowance for doubtful accounts | 25,190 |
| | 14,594 |
| 71,851 |
| | 37,754 |
|
| Other accounts receivable, net of allowance for doubtful accounts | 13,296 |
| | 12,261 |
| 15,625 |
| | 14,874 |
|
| Availability under revolving credit facility | 50,000 |
| | 12,000 |
| 50,000 |
| | 50,000 |
|
| | | | | | | |
| Working capital | $ | 39,153 |
| | $ | 28,053 |
| $ | 112,579 |
| | $ | 97,037 |
|
| Current ratio | 1.8 |
| | 1.9 |
| 3.0 |
| | 3.0 |
|
| | | | | | | |
| Total capital lease obligations | $ | 5,509 |
| | $ | 288 |
| |
Total operating lease obligations(1) | | $ | 19,216 |
| | $ | — |
|
| Total finance lease obligations | | 683 |
| | 1,769 |
|
| Total debt | $ | 199,356 |
| | $ | 25,161 |
| $ | 194,667 |
| | $ | 198,549 |
|
________________
| |
(1) | We adopted ASC 842 - Leases, effective January 1, 2019, which resulted in the recognition of most of our operating leases on our balance sheet, both as a right-of-use asset and right-of-use liability. Since we adopted this standard using the optional modified retrospective method, we have not restated prior year amounts. |
The following table highlights certain cash flow activities:
| | | | Year Ended | Year Ended December 31, |
| (In thousands) | December 31, 2017 | | December 31, 2016 | 2019 | | 2018 |
| Net income/(loss) | $ | (17,143 | ) | | $ | 53,437 |
| |
| Net income | | $ | 29,844 |
| | $ | 41,874 |
|
| Non-cash adjustments to net income | 66,193 |
| | (6,765 | ) | 87,536 |
| | 70,154 |
|
| Cash used for working capital | (25,268 | ) | | (7,821 | ) | (49,830 | ) | | (39,282 | ) |
| Cash provided by operating activities | 23,782 |
| | 38,851 |
| 67,550 |
| | 72,746 |
|
| | | | | | | |
| Cash used for acquisitions of businesses, net of cash acquired | (161,479 | ) | | (24,970 | ) | (44,766 | ) | | (3,750 | ) |
| Purchases of property, equipment and investment in internally developed software | (13,697 | ) | | (10,899 | ) | (30,707 | ) | | (24,637 | ) |
| Cash used in investing activities | (177,188 | ) | | (36,181 | ) | (75,473 | ) | | (28,851 | ) |
| | | | | | | |
| Cash provided by financing activities | $ | 166,457 |
| | $ | 1,427 |
| |
| Cash (used in)/provided by financing activities | | $ | (4,379 | ) | | $ | 601 |
|
Cash Provided By Operating Activities
The decrease in cash provided by operating activities was primarily due to the decrease in net income. Non-cash adjustments to net income primarily relate to bad debt, depreciation, amortization and stock compensation expense offset by any non-cash tax benefit. Forincreased for the year ended December 31, 2016, we released a $51.6 million2019 primarily due to the change in deferred tax valuation allowance onexpense recognized during 2019 primarily due to the basisusage of management’s reassessmentnet operating losses, increases in stock-based compensation, depreciation and amortization of intangible assets. Cash used for working capital increased primarily due to the amounttiming of deferred income tax assets that are more‑likely‑than‑not to be realized, which was a component of the non-cash adjustments recognized.cash receipts and payments.
Cash Used In Investing Activities
During the year ended December 31, 20172019, we spent $44.8 million, net of cash acquired, LifeWatchprimarily related to our acquisition of Geneva. We increased our purchases for $161.5 million cash alongequipment and internally developed software in 2019, consistent with $117.4 million in common stock. During the year ended December 31, 2016, we acquired Telcare for $7.0 million cash, VirtualScopics for approximately $15.0 million,our higher Healthcare service volumes and the ePatch divisionlaunch of DELTA for $3.0 millionour next generation products used in our monitoring services.
Cash Used In Financing Activities
The increase in cash along with $2.9 millionused in common stock.financing activities was primarily due to the decrease in cash proceeds related to the exercise of stock options, the increase in principal payments on our long-term debt and the increase in payments of tax withholdings related to the vesting of share-based payments. These changes were offset partially by the 2018 impact of our acquistion of noncontrolling interests and the decrease in payments on finance lease obligations.
In conjunction with the LifeWatch acquisition,On January 27, 2020, we established a newamended our SunTrust Credit Agreement with SunTrust Bank and Lenders named therein in the amount of $205.0 million and extinguished the previous
Healthcare Financial Solutions Credit Agreement.. For further details regarding the Credit Agreements,credit agreements, please see “Part II; Item 8. Financial Statements and Supplementary Data; Notes to Consolidated Financial Statements; Note 10.11. Credit Agreement” below.Agreement and Note 21. Subsequent Event” in this Annual Report on Form 10-K.
Our primary sources of liquidity are cash on hand, cash flows from operating activities and a committed credit line. Based on our forecasted liquidity needs, we believe that our aforementioned current and projected sources of liquidity will be sufficient to meet our needs for the foreseeable future.
Contractual Obligations and Commitments
The following table describes our long-term contractual obligations and commitments as of December 31, 2017:2019:
| | | | | Payments due by period | | Payments due by period |
| (in thousands) | | Total | | Less than 1 year | | 1-3 Years | | 3-5 Years | | More than 5 Years | | Total | | Less than 1 year | | 1-3 Years | | 3-5 Years | | More than 5 Years |
| Operating lease obligations | | $ | 23,655 |
| | $ | 5,871 |
| | $ | 8,008 |
| | $ | 4,261 |
| | $ | 5,515 |
| | $ | 21,481 |
| | $ | 5,926 |
| | $ | 7,481 |
| | $ | 4,235 |
| | $ | 3,839 |
|
| Capital lease obligations | | 5,509 |
| | 4,023 |
| | 1,486 |
| | — |
| | — |
| |
Debt and interest obligations(1) | | 236,083 |
| | 8,197 |
| | 34,776 |
| | 193,110 |
| | — |
| |
| Purchase obligations | | 2,184 |
| | 2,184 |
| | — |
| | — |
| | — |
| |
| Finance lease obligations | | | 697 |
| | 412 |
| | 285 |
| | — |
| | — |
|
| Deferred consideration - cash | | | 11,068 |
| | — |
| | 11,068 |
| | — |
| | — |
|
Debt and interest obligations(1)(2) | | | 231,279 |
| | 13,111 |
| | 51,142 |
| | 167,026 |
| | — |
|
Total(3)(4) | | $ | 267,431 |
| | $ | 20,275 |
| | $ | 44,270 |
| | $ | 197,371 |
| | $ | 5,515 |
| | $ | 264,525 |
| | $ | 19,449 |
| | $ | 69,976 |
| | $ | 171,261 |
| | $ | 3,839 |
|
________________
| |
(1) | Our debt bears a variable interest rate, at theour election, of the Company, with an applicable margin determined by the credit agreement.SunTrust Credit Agreement. The amounts above assume the rate and margin as of December 31, 20172019 throughout the remaining term. The rate and margin may fluctuate, as may our election of LIBOR or Base Rate pricing, throughout the term of the loan. Excluded from the amounts in the table is the 0.3%0.2% commitment fee payable on the unused portion of our line of credit. |
| |
(2) | On January 27, 2020, we amended our SunTrust Credit Agreement, whereby the term loan portion of the agreement has been converted into a revolving credit facility, and the amendment does not require scheduled principal payments. See “Part II; Item 8. Financial Statements and Supplementary Data; Notes to Consolidated Financial Statements; Note 21. Subsequent Event” in this Annual Report on Form 10-K for further information. |
| |
(3) | In connection with certain acquisitions completed in 2016,2019 and 2018, we have acquisition-related contingent consideration obligations payable to the sellers in these transactions upon the achievement of certain milestones.milestones that are not reflected in the table above. The maximum aggregate undiscounted amounts potentially payable not included in the table above total $5.0 million.million, with the exception of Geneva, which is uncapped. As of December 31, 2019, the estimate of the cash portion of the Geneva contingent consideration is $8.2 million, which is scheduled to be paid in early 2022 and subject to certain indemnification obligations. See “Part II; Item 8. Financial Statements and Supplementary Data; Notes to Consolidated Financial Statements; Note 4. Acquisitions” in this Annual Report on Form 10-K for further discussion related to the Geneva contingent consideration. |
| |
(3)(4)
| OurAs of December 31, 2019, our other long-term liabilities in our consolidated balance sheets consist primarily ofsheet includes reserves for uncertainunrecognized tax positions, pension obligations and contingent consideration. As of December 31, 2017, webenefits. We are unable to make reasonably reliable estimates of both the timing of tax audit outcomes and if unfavorable, the timing of payments; therefore, such amounts are not included in the above contractual obligation table. See “Part II; Item 8. Financial Statements and Supplementary Data; Notes to Consolidated Financial Statements; Note 16.17. Income Taxes” in this Annual Report on Form 10-K for further discussion related to uncertain tax positions.
|
As of December 31, 2017,2019, we were bound under facility leases and several office equipment leases that are included in the table above.above, none of which utilize a variable interest rate. Our debt bears a variable interest rate of LIBOR plus the applicable margin, or the prime rate plus the applicable margin. If LIBOR rates, the prime rates, or the applicable margin increase, obligationsthe interest obligation amounts on our debt disclosed above will increase above the amounts disclosed above.be affected.
Recent Accounting Pronouncements
For further details on recently issued accounting pronouncements, please refer to “Part II; Item 8. Financial Statements and Supplementary Data; Notes to Consolidated Financial Statements; Note 2. Summary of Significant Accounting Policies; (t)w) Recent Accounting Pronouncements.”
Off-Balance Sheet Arrangements
As of December 31, 2017 and 2016,2019, we did not have any relationships with unconsolidated entities or financial partnerships, such as entities referred to as structured finance or special purpose entities, which would have been established for the purpose of facilitating off-balance sheet arrangements, as defined in Item 303(a)(4)(ii) of Regulation S-K, that have or other contractually narroware reasonably likely to have a current or limited purposes.future effect on our financial condition, changes in our financial condition, revenues, or expenses, results of operations, liquidity, capital expenditures, or capital resources that is material to investors.
Item 7A7A. Quantitative and Qualitative Disclosures About Market Risk
Our cash and cash equivalents as of December 31, 20172019 were $36.0$68.6 million. We do not invest in any short-term or long-term marketable securities, nor do we hold any derivative financial instruments for trading or speculative purposes.
At December 31, 2017,2019, we had $199.4$194.7 million of variable rate debt, inclusive of debt discounts and deferred charges, at a rate of LIBOR plus the applicable margin, or the prime rate plus the applicable margin. A 1.0% change in either the LIBOR rate, prime rate, or the applicable margin would result in a change in annual interest expense of approximately $2.1$1.9 million. For further details regarding the debt, rates or applicable margin, please refer to “Part II; Item 8. Financial Statements and Supplementary Data; Notes to Consolidated Financial Statements; Note 10.11. Credit Agreement” included in this Annual Report on Form 10-K.
Item 8. Financial Statements and Supplementary Data
Report of Independent Registered Public Accounting Firm
To the Stockholders and the Board of Directors of BioTelemetry, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of BioTelemetry, Inc. (the Company) as of December 31, 20172019 and 2016, and2018, the related consolidated statements of operations, and comprehensive income/income (loss), cash flows and equity for each of the three years in the period ended December 31, 2017,2019, and the related notes and the financial statement schedule listed in the Index at Item 15(a) (collectively referred to as the “financial“consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 20172019 and 2016,2018, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2017,2019, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company’s internal control over financial reporting as of December 31, 2017,2019, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) and our report dated February 26, 201827, 2020 expressed an unqualified opinion thereon.
Adoption of New Accounting Standard
As discussed in Note 2 to the consolidated financial statements, the Company changed its method of accounting for share-based payments to employees as a result of the adoption of the amendments to the FASB Accounting Standards Codification resulting from Accounting Standards Update No. 2016-09, “Improvements to Employee Share-Based Payment Accounting,” in 2016 and 2017.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures to respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
|
| | |
| | Healthcare Segment Revenue |
| Description of the Matter | | For the year ended December 31, 2019, the Company’s revenue derived from remote cardiac monitoring services in its Healthcare segment was $372.0 million. As explained in Note 3 to the consolidated financial statements, the Company measures and recognizes revenue for Contracted payors (including Medicare) at a transaction price negotiated with each payor for services provided, on a case rate basis. Auditing the Company’s Healthcare segment revenue is complex and required a high degree of judgment in the application of our audit procedures and evaluating the results of those audit procedures to address the completeness and accuracy of the underlying data used to recognize Healthcare segment revenue, which is compiled using end-user computing applications. |
| How We Addressed the Matter in Our Audit | | We tested the Company’s controls that address the risk of material misstatement relating to the occurrence and measurement of Healthcare segment revenue. For example, we tested management’s review of the contracted rates utilized to determine the transaction price for each service provided and controls over the completeness and accuracy of the underlying data used to recognize Healthcare segment revenue. To test the Company’s Healthcare segment revenue, our audit procedures included, among others, performing analytical review procedures over key financial ratios, and selecting a representative sample of healthcare segment revenue transactions and comparing the components of the revenue calculations to source data including remote cardiac monitoring results and contracted rates to test the completeness and accuracy of the data compiled from end-user computing applications. |
| | Accounting for acquisition of Geneva Healthcare, Inc. |
| Description of the Matter | | As explained in Note 4 to the consolidated financial statements, on March 1, 2019, the Company completed its acquisition of Geneva Healthcare, Inc. (“Geneva”) for a total purchase price of $77.9 million. The transaction was accounted for using the acquisition method of accounting whereby the total purchase price was allocated to tangible and intangible assets acquired and liabilities assumed based on the respective fair values. Auditing the Company's accounting for the acquisition of Geneva was complex due to the significant estimation uncertainty in determining the fair value of its identifiable intangible assets, which principally consisted of customer relationships, technology and trade names. The significant estimation uncertainty was primarily due to the sensitivity of the respective fair values to underlying assumptions about the future performance of the acquired business that rely upon limited historical data on which to base those assumptions. The significant assumptions used to estimate the fair value of the customer relationships included the future operating performance and cash flows generated by the customer relationships and a discount rate. The significant assumptions used to estimate the fair value of the technology included the projected revenues generated by the technology, a royalty rate, and a discount rate. The significant assumptions used to estimate the fair value of the trade name included projected revenues generated by the trade name, a royalty rate, and a discount rate. These significant assumptions are forward looking and could be affected by future economic and market conditions. |
|
| | |
| How We Addressed the Matter in Our Audit | | We tested the Company’s controls over its accounting for acquisitions, including the valuation of identifiable intangible assets. For example, we tested the Company's controls over management’s review of the identifiable intangible asset valuation models, as well as the significant assumptions used in the valuation models. To test the estimated fair value of the intangible assets acquired, we performed audit procedures that included, among others, evaluating the Company's selection of the valuation methodologies used, evaluating the significant assumptions described above, and evaluating the completeness and accuracy of the underlying data supporting the significant assumptions and estimates. For example, we compared the significant assumptions to current industry, market and economic trends, as well as to the historical results of the acquired business. We involved our valuation specialists to assist in our evaluation of the methodologies used by the Company and the significant assumptions included in the fair value estimates. Additionally, we performed sensitivity analyses to evaluate changes in the fair value of the intangible assets that would result from changes in the significant assumptions. |
| | Valuation of contingent consideration |
| Description of the Matter | | As explained in Note 2 to the consolidated financial statements, the Company measures and records the fair value of contingent consideration on a recurring basis. As explained in Note 4 to the consolidated financial statements, the total purchase price for the acquisition of Geneva included the acquisition date fair value of contingent consideration of $13.2 million. As explained in Note 6 to the consolidated financial statements, this liability was remeasured to $12.9 million as of December 31, 2019. Auditing the Company's valuations of the contingent consideration related to the Geneva acquisition was complex due to the significant estimation required by management. The significant estimation was primarily due to the complexity of the valuation model used by management to measure the fair value of the contingent consideration and the sensitivity of the respective fair values to the significant underlying assumptions. The Company used a Monte Carlo simulation to measure the fair value of the contingent consideration. The significant assumptions used in the simulation included estimated projected revenues, estimated stock price volatility in future periods, and discount rates. These significant assumptions are forward looking and could be affected by future economic and market conditions. |
| How We Addressed the Matter in Our Audit | | We tested the Company’s controls over the valuations of the contingent consideration. For example, we tested controls over management’s review of the contingent consideration valuation models, as well as the significant assumptions used in the valuation models. To test the estimated fair value of the contingent consideration related to the Geneva acquisition, our audit procedures included, among others, assessing the terms of the arrangement, including the conditions that must be met for the contingent consideration to become payable, and evaluating the completeness and accuracy of the underlying data supporting the significant assumptions and estimates. We also involved our valuation specialists to assist in evaluating the use of the Monte Carlo simulation for the contingent consideration and testing the significant assumptions used in the model. We compared the significant assumptions to current industry, market and economic trends and to the historical results for the acquired business. |
|
| |
| /s/ ERNST & YOUNG LLP | |
| | |
We have served as BioTelemetry Inc.’sthe Company’s auditors since 2004. |
| | |
| Philadelphia, Pennsylvania | |
February 26, 201827, 2020 | |
BIOTELEMETRY, INC.
CONSOLIDATED BALANCE SHEETS
| | | | December 31, | December 31, |
| (In thousands, except shares and par value) | 2017 | | 2016 | |
| Assets | | | | |
| (in thousands, except shares and par value data) | | 2019 | | 2018 |
| ASSETS | | | | |
| Current assets: | | | | | | |
| Cash and cash equivalents | $ | 36,022 |
| | $ | 23,052 |
| $ | 68,614 |
| | $ | 80,889 |
|
| Healthcare accounts receivable, net of allowance for doubtful accounts of $15,556 and $12,198 at December 31, 2017 and 2016, respectively | 25,190 |
| | 14,594 |
| |
| Other accounts receivable, net of allowance for doubtful accounts of $1,425 and $665 at December 31, 2017 and 2016, respectively | 13,296 |
| | 12,261 |
| |
| Healthcare accounts receivable, net of allowance for doubtful accounts of $31,780 and $25,345 at December 31, 2019 and 2018, respectively | | 71,851 |
| | 37,754 |
|
| Other accounts receivable, net of allowance for doubtful accounts of $201 and $268 at December 31, 2019 and 2018, respectively | | 15,625 |
| | 14,874 |
|
| Inventory | 5,332 |
| | 5,176 |
| 5,738 |
| | 7,323 |
|
| Prepaid expenses and other current assets | 10,268 |
| | 4,477 |
| 6,505 |
| | 5,820 |
|
| Total current assets | 90,108 |
| | 59,560 |
| 168,333 |
| | 146,660 |
|
| Property and equipment, net | 49,194 |
| | 25,823 |
| 56,380 |
| | 48,377 |
|
| Intangible assets, net | 141,707 |
| | 33,472 |
| 129,596 |
| | 129,653 |
|
| Goodwill | 223,105 |
| | 41,068 |
| 301,321 |
| | 238,814 |
|
| Deferred tax asset | 17,681 |
| | 36,636 |
| |
| Deferred tax assets | | 12,626 |
| | 19,975 |
|
| Other assets | 2,767 |
| | 2,425 |
| 17,464 |
| | 3,322 |
|
| Total assets | $ | 524,562 |
| | $ | 198,984 |
| $ | 685,720 |
| | $ | 586,801 |
|
| Liabilities and Equity | | | | |
| LIABILITIES AND EQUITY | | | | |
| Current liabilities: | | | | | | |
| Accounts payable | 13,227 |
| | 12,425 |
| 24,198 |
| | 18,157 |
|
| Accrued liabilities | 27,357 |
| | 13,698 |
| 27,318 |
| | 24,689 |
|
| Current portion of capital leases obligations | 4,023 |
| | 162 |
| |
| Current portion of finance lease obligations | | 394 |
| | 1,652 |
|
| Current portion of long-term debt | 2,050 |
| | 1,250 |
| 3,844 |
| | 5,125 |
|
| Deferred revenue | 4,298 |
| | 3,972 |
| |
| Total current liabilities | 50,955 |
| | 31,507 |
| 55,754 |
| | 49,623 |
|
| Long-term portion of capital lease obligations | 1,486 |
| | 126 |
| |
| Long-term portion of finance lease obligations | | 289 |
| | 117 |
|
| Long-term debt | 197,306 |
| | 23,911 |
| 190,823 |
| | 193,424 |
|
| Other long-term liabilities | 25,112 |
| | 4,526 |
| 71,937 |
| | 33,152 |
|
| Total liabilities | 274,859 |
| | 60,070 |
| 318,803 |
| | 276,316 |
|
| Stockholders’ equity: | | | | | | |
| Common stock—$.001 par value as of December 31, 2017 and 2016; 200,000,000 shares authorized as of December 31, 2017 and 2016; 32,460,668 and 28,261,503 shares issued and outstanding at December 31, 2017 and 2016, respectively | 32 |
| | 28 |
| |
| Common stock—$.001 par value as of December 31, 2019 and 2018; 200,000,000 shares authorized as of December 31, 2019 and 2018; 34,023,053 and 33,406,364 shares issued and outstanding at December 31, 2019 and 2018, respectively | | 34 |
| | 33 |
|
| Paid-in capital | 409,517 |
| | 281,642 |
| 453,366 |
| | 426,054 |
|
| Accumulated other comprehensive loss | (114 | ) | | (34 | ) | |
| Accumulated other comprehensive (loss)/income | | (469 | ) | | 256 |
|
| Accumulated deficit | (158,678 | ) | | (142,722 | ) | (86,014 | ) | | (115,858 | ) |
| Total BioTelemetry, Inc.’s stockholders’ equity | 250,757 |
| | 138,914 |
| |
| Noncontrolling interests | (1,054 | ) | | — |
| |
| Total equity | 249,703 |
| | 138,914 |
| 366,917 |
| | 310,485 |
|
| Total liabilities and equity | $ | 524,562 |
| | $ | 198,984 |
| $ | 685,720 |
| | $ | 586,801 |
|
See accompanying Notes to Consolidated Financial Statements.
BIOTELEMETRY, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE INCOME/(LOSS)
| | | | Year Ended December 31, | Year Ended December 31, |
| (In thousands, except per share amounts) | 2017 | | 2016 | | 2015 | |
| Revenue: | | | | | | |
| Healthcare | $ | 234,385 |
| | $ | 165,664 |
| | $ | 145,963 |
| |
| Research | 38,790 |
| | 32,565 |
| | 21,853 |
| |
| Technology | 13,601 |
| | 10,103 |
| | 10,697 |
| |
| Total revenue | 286,776 |
| | 208,332 |
| | 178,513 |
| |
| Cost of revenue: | | | | | | |
| Healthcare | 81,356 |
| | 53,559 |
| | 51,693 |
| |
| Research | 22,881 |
| | 18,395 |
| | 12,728 |
| |
| Technology | 10,169 |
| | 6,928 |
| | 7,535 |
| |
| Total cost of revenue | 114,406 |
| | 78,882 |
| | 71,956 |
| |
| (in thousands, except per share data) | | 2019 | | 2018 | | 2017 |
| Revenue | | $ | 439,107 |
| | $ | 399,472 |
| | $ | 286,776 |
|
| Cost of revenue | | 164,833 |
| | 148,986 |
| | 114,406 |
|
| Gross profit | 172,370 |
| | 129,450 |
| | 106,557 |
| 274,274 |
| | 250,486 |
| | 172,370 |
|
| Operating expenses: | | | | | | | | | | |
| General and administrative | 82,983 |
| | 55,877 |
| | 47,882 |
| 120,093 |
| | 109,736 |
| | 82,983 |
|
| Sales and marketing | 35,322 |
| | 28,636 |
| | 27,936 |
| 50,664 |
| | 42,849 |
| | 35,322 |
|
| Bad debt expense | 13,291 |
| | 9,931 |
| | 8,047 |
| 21,768 |
| | 22,222 |
| | 13,291 |
|
| Research and development | 11,101 |
| | 8,355 |
| | 7,111 |
| 13,994 |
| | 11,206 |
| | 11,101 |
|
| Other charges | 31,436 |
| | 8,639 |
| | 6,063 |
| 15,004 |
| | 14,659 |
| | 31,436 |
|
| Total operating expenses | 174,133 |
| | 111,438 |
| | 97,039 |
| 221,523 |
| | 200,672 |
| | 174,133 |
|
| Income/(loss) from operations | (1,763 | ) | | 18,012 |
| | 9,518 |
| 52,751 |
| | 49,814 |
| | (1,763 | ) |
| Other expense: | | | | | | | | | | |
| Interest expense | (4,897 | ) | | (1,830 | ) | | (1,534 | ) | (9,482 | ) | | (9,429 | ) | | (4,897 | ) |
| Loss on extinguishment of debt | (543 | ) | | — |
| | — |
| — |
| | — |
| | (543 | ) |
| Loss on equity method investment | (384 | ) | | (287 | ) | | — |
| |
| Other non-operating expense, net | (2,809 | ) | | (125 | ) | | (88 | ) | |
| Total other expense | (8,633 | ) | | (2,242 | ) | | (1,622 | ) | |
| Loss on equity method investments | | (1,298 | ) | | (246 | ) | | (384 | ) |
| Other non-operating (expense)/income, net | | (2,243 | ) | | 1,365 |
| | (2,809 | ) |
| Total other expense, net | | (13,023 | ) | | (8,310 | ) | | (8,633 | ) |
| Income/(loss) before income taxes | (10,396 | ) | | 15,770 |
| | 7,896 |
| 39,728 |
| | 41,504 |
| | (10,396 | ) |
| (Provision for)/benefit from income taxes | (6,747 | ) | | 37,667 |
| | (468 | ) | (9,884 | ) | | 370 |
| | (6,747 | ) |
| Net income/(loss) | (17,143 | ) | | 53,437 |
| | 7,428 |
| 29,844 |
| | 41,874 |
| | (17,143 | ) |
| Net loss attributable to noncontrolling interests | (1,187 | ) | | — |
| | — |
| — |
| | (946 | ) | | (1,187 | ) |
| Net income/(loss) attributable to BioTelemetry, Inc. | $ | (15,956 | ) | | $ | 53,437 |
| | $ | 7,428 |
| $ | 29,844 |
| | $ | 42,820 |
| | $ | (15,956 | ) |
| Other comprehensive income/(loss): | | | | | | |
| Foreign currency translation loss | (80 | ) | | (22 | ) | | (12 | ) | |
| Comprehensive income/(loss) attributable to BioTelemetry, Inc. | $ | (16,036 | ) | | $ | 53,415 |
| | $ | 7,416 |
| |
| | | | | | | |
| Net income/(loss) per common share attributable to BioTelemetry, Inc.: | | | | | | | | | | |
| Basic | $ | (0.53 | ) | | $ | 1.91 |
| | $ | 0.27 |
| $ | 0.88 |
| | $ | 1.31 |
| | $ | (0.53 | ) |
| Diluted | $ | (0.53 | ) | | $ | 1.75 |
| | $ | 0.26 |
| $ | 0.82 |
| | $ | 1.20 |
| | $ | (0.53 | ) |
| Weighted average number of common shares outstanding: | | | | | | | | | | |
| Basic | 30,386 |
| | 27,920 |
| | 27,116 |
| 33,948 |
| | 32,709 |
| | 30,386 |
|
| Dilutive stock options and restricted stock units | — |
| | 2,569 |
| | 1,973 |
| |
| Dilutive common stock equivalents | | 2,492 |
| | 3,074 |
| | — |
|
| Diluted | 30,386 |
| | 30,489 |
| | 29,089 |
| 36,440 |
| | 35,783 |
| | 30,386 |
|
| Anti-dilutive stock options and restricted stock units excluded from weighted average calculation | 463 |
| | 380 |
| | 1,103 |
| |
See accompanying Notes to Consolidated Financial Statements.
BIOTELEMETRY, INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME/(LOSS)
|
| | | | | | | | | | | |
| | Year Ended December 31, |
| (in thousands) | 2019 | | 2018 | | 2017 |
| Net income/(loss) attributable to BioTelemetry, Inc. | $ | 29,844 |
| | $ | 42,820 |
| | $ | (15,956 | ) |
| Other comprehensive income/(loss): | | | | | |
| Foreign currency translation (loss)/gain | (725 | ) | | 370 |
| | (80 | ) |
| Comprehensive income/(loss) attributable to BioTelemetry, Inc. | $ | 29,119 |
| | $ | 43,190 |
| | $ | (16,036 | ) |
See accompanying Notes to Consolidated Financial Statements.
BIOTELEMETRY, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
| | | | Year Ended December 31, | Year Ended December 31, |
| (in thousands) | 2017 | | 2016 | | 2015 | 2019 | | 2018 | | 2017 |
| Operating activities | | | | | | |
| OPERATING ACTIVITIES | | | | | | |
| Net income/(loss) | $ | (17,143 | ) | | $ | 53,437 |
| | $ | 7,428 |
| $ | 29,844 |
| | $ | 41,874 |
| | $ | (17,143 | ) |
| Adjustments to reconcile net income/(loss) to net cash provided by operating activities: | | | | | | | | | | |
| Bad debt expense | 13,291 |
| | 9,931 |
| | 8,047 |
| 21,768 |
| | 22,222 |
| | 13,291 |
|
| Depreciation | 18,337 |
| | 10,547 |
| | 8,987 |
| |
| Amortization of intangibles | 10,224 |
| | 3,722 |
| | 3,501 |
| |
| Depreciation and amortization | | 42,976 |
| | 40,168 |
| | 28,561 |
|
| Impairment charge | 12,045 |
| | — |
| | — |
| — |
| | — |
| | 12,045 |
|
| Stock-based compensation | 7,680 |
| | 6,502 |
| | 4,952 |
| 13,376 |
| | 9,261 |
| | 7,680 |
|
| Equity method investment loss | 384 |
| | 287 |
| | — |
| |
| Write off of derivative premium | | — |
| | — |
| | 1,322 |
|
| Accretion of debt discount | | 1,243 |
| | 1,243 |
| | 678 |
|
| Loss on extinguishment of debt | | — |
| | — |
| | 543 |
|
| Gain on legal settlement | | — |
| | — |
| | (1,333 | ) |
| Deferred income taxes | | 7,385 |
| | (2,294 | ) | | 6,050 |
|
| Change in fair value of acquisition-related contingent consideration | (2,605 | ) | | — |
| | — |
| (230 | ) | | (700 | ) | | (2,605 | ) |
| Write off of derivative premium | 1,322 |
| | — |
| | — |
| |
| Accretion of debt discount and amortization of deferred charges | 678 |
| | 217 |
| | 259 |
| |
| Loss on extinguishment of debt | 543 |
| | — |
| | — |
| |
| Non-cash gain on legal settlement | (1,333 | ) | | — |
| | — |
| |
| Non-cash lease (income)/expense | (423 | ) | | 170 |
| | (14 | ) | |
| Non-cash tax (benefit)/expense | 6,050 |
| | (38,141 | ) | | 245 |
| |
| Other non-cash items | | 1,018 |
| | 254 |
| | (39 | ) |
| Changes in operating assets and liabilities: | | | | | | | | | | |
| Healthcare and other accounts receivable | (15,455 | ) | | (8,707 | ) | | (7,677 | ) | (55,027 | ) | | (35,742 | ) | | (15,455 | ) |
| Inventory | 665 |
| | (753 | ) | | 188 |
| 1,585 |
| | (1,991 | ) | | 665 |
|
| Prepaid expenses and other assets | (694 | ) | | (1,050 | ) | | (3 | ) | 1,742 |
| | 3,517 |
| | (694 | ) |
| Accounts payable | (9,622 | ) | | 3,145 |
| | (4,699 | ) | 5,551 |
| | 3,760 |
| | (8,320 | ) |
| Accrued and other liabilities | (162 | ) | | (456 | ) | | (464 | ) | (3,681 | ) | | (8,826 | ) | | (1,464 | ) |
| Liability associated with the Civil Investigative Demand | — |
| | — |
| | (6,400 | ) | |
| Net cash provided by operating activities | 23,782 |
| | 38,851 |
| | 14,350 |
| 67,550 |
| | 72,746 |
| | 23,782 |
|
| Investing activities | | | | | | |
| INVESTING ACTIVITIES | | | | | | |
| Acquisition of businesses, net of cash acquired | (161,479 | ) | | (24,970 | ) | | — |
| (44,766 | ) | | (3,750 | ) | | (161,479 | ) |
| Purchases of property and equipment and investment in internally developed software | (13,697 | ) | | (10,899 | ) | | (13,600 | ) | (30,707 | ) | | (24,637 | ) | | (13,697 | ) |
| Purchase of derivative instrument | (1,322 | ) | | — |
| | — |
| — |
| | — |
| | (1,322 | ) |
| Investment in equity method investee | (690 | ) | | (312 | ) | | — |
| — |
| | (464 | ) | | (690 | ) |
| Net cash used in investing activities | (177,188 | ) | | (36,181 | ) | | (13,600 | ) | (75,473 | ) | | (28,851 | ) | | (177,188 | ) |
| Financing activities | | | | | | |
| FINANCING ACTIVITIES | | | | | | |
| Proceeds related to the exercising of stock options and employee stock purchase plan | 6,071 |
| | 2,519 |
| | 1,222 |
| 7,610 |
| | 12,186 |
| | 6,071 |
|
| Tax payments related to the vesting of shares | (1,933 | ) | | (2,333 | ) | | (1,575 | ) | |
| Payments of tax withholdings related to vesting of share-based awards | | (4,955 | ) | | (2,910 | ) | | (1,933 | ) |
| Issuance of long-term debt | 205,000 |
| | — |
| | — |
| — |
| | — |
| | 205,000 |
|
| Borrowings under revolving loans | — |
| | 14,500 |
| | — |
| |
| Principal payments on revolving loans | (3,000 | ) | | (11,500 | ) | | — |
| |
| Repayments on revolving loans | | — |
| | — |
| | (3,000 | ) |
| Payment of debt issuance costs | (6,213 | ) | | — |
| | — |
| — |
| | — |
| | (6,213 | ) |
| Principal payments on long-term debt | (25,840 | ) | | (1,438 | ) | | (938 | ) | (5,125 | ) | | (2,050 | ) | | (25,840 | ) |
| Principal payments on capital lease obligations | (2,863 | ) | | (321 | ) | | (480 | ) | |
| Principal payments on finance lease obligations | | (1,909 | ) | | (3,740 | ) | | (2,863 | ) |
| Acquisition of noncontrolling interests | (4,765 | ) | | — |
| | — |
| — |
| | (2,885 | ) | | (4,765 | ) |
| Net cash provided by/(used in) financing activities | 166,457 |
| | 1,427 |
| | (1,771 | ) | |
| Net cash (used in)/provided by financing activities | | (4,379 | ) | | 601 |
| | 166,457 |
|
| Effect of exchange rate changes on cash | (81 | ) | | (31 | ) | | — |
| 27 |
| | 371 |
| | (81 | ) |
| Net increase/(decrease) in cash and cash equivalents | 12,970 |
| | 4,066 |
| | (1,021 | ) | |
| Net (decrease)/increase in cash and cash equivalents | | (12,275 | ) | | 44,867 |
| | 12,970 |
|
| Cash and cash equivalents—beginning of period | 23,052 |
| | 18,986 |
| | 20,007 |
| 80,889 |
| | 36,022 |
| | 23,052 |
|
| Cash and cash equivalents—end of period | $ | 36,022 |
| | $ | 23,052 |
| | $ | 18,986 |
| $ | 68,614 |
| | $ | 80,889 |
| | $ | 36,022 |
|
| Supplemental disclosure of cash flow information | | | | | | |
| SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION | | | | | | |
| Non-cash purchases of property and equipment | $ | 498 |
| | $ | — |
| | $ | — |
| $ | 3,302 |
| | $ | 505 |
| | $ | 498 |
|
| Non-cash fair value of common stock returned in legal settlement | 2,753 |
| | — |
| | — |
| — |
| | — |
| | 2,753 |
|
| Non-cash fair value of equity issued for acquisition of business | 117,440 |
| | 2,885 |
| | — |
| 2,142 |
| | — |
| | 117,440 |
|
| Non-cash fair value of non-trade receivables exchanged for investment in equity method investee | | — |
| | 395 |
| | — |
|
| Cash paid for interest | 3,888 |
| | 1,273 |
| | 1,044 |
| 8,029 |
| | 7,836 |
| | 3,888 |
|
| Cash paid for taxes | $ | 1,648 |
| | $ | 359 |
| | $ | 384 |
| |
| Cash paid for taxes, net of refunds | | $ | (89 | ) | | $ | 763 |
| | $ | 1,648 |
|
See accompanying Notes to Consolidated Financial Statements.
BIOTELEMETRY, INC.
CONSOLIDATED STATEMENTS OF EQUITY
| | | | BioTelemetry, Inc. Equity | | | | | BioTelemetry, Inc. Equity | | | | |
| | | | | | | | Accumulated Other Comprehensive Income/(Loss) | | | | | | | | | | | | | Accumulated Other Comprehensive (Loss)/Income | | | | | | |
| | Common Stock | | Paid-in Capital | | Accumulated Deficit | | Noncontrolling Interests | | Total Equity | Common Stock | | Paid-in Capital | | Accumulated Deficit | | Noncontrolling Interests | | Total Equity |
| (in thousands, except shares) | Shares | | Amount | | Accumulated Other Comprehensive Income/(Loss) | Shares | | Amount | | Accumulated Other Comprehensive (Loss)/Income |
| Balance December 31, 2014 | 26,693,248 |
| | $ | 27 |
| | $ | 267,236 |
| | $ | — |
| $ | (203,587 | ) | $ | — |
| $ | 63,676 |
| |
| Share issuances related to stock compensation plans | 719,564 |
| | — |
| | 1,222 |
| | — |
| — |
| — |
| 1,222 |
| |
| Stock-based compensation | — |
| | — |
| | 4,952 |
| | — |
| | — |
| | — |
| | 4,952 |
| |
| Shares withheld to cover taxes on vesting of share based awards | (167,090 | ) | | — |
| | (1,575 | ) | | — |
| | — |
| | — |
| | (1,575 | ) | |
| Issuance of stock related to business combinations | 32,217 |
| | — |
| | 235 |
| | — |
| | — |
| | — |
| | 235 |
| |
| Currency translation adjustment | — |
| | — |
| | — |
| | (12 | ) | | — |
| | — |
| | (12 | ) | |
| Net income | — |
| | — |
| | — |
| | — |
| | 7,428 |
| | — |
| | 7,428 |
| |
| Balance December 31, 2015 | 27,277,939 |
| | 27 |
| | 272,070 |
| | (12 | ) | | (196,159 | ) | | — |
| | 75,926 |
| |
| Share issuances related to stock compensation plans | 917,912 |
| | 1 |
| | 2,518 |
| | — |
| | — |
| | — |
| | 2,519 |
| |
| Stock-based compensation | — |
| | — |
| | 6,502 |
| | — |
| | — |
| | — |
| | 6,502 |
| |
| Shares withheld to cover taxes on vesting of share based awards | (178,867 | ) | | — |
| | (2,333 | ) | | — |
| | — |
| | — |
| | (2,333 | ) | |
| Issuance of stock related to 2014 business combination | 244,519 |
| | — |
| | 2,885 |
| | — |
| | — |
| | — |
| | 2,885 |
| |
| Currency translation adjustment | — |
| | — |
| | — |
| | (22 | ) | | — |
| | — |
| | (22 | ) | |
| Net income | — |
| | — |
| | — |
| | — |
| | 53,437 |
| | — |
| | 53,437 |
| |
| Balance December 31, 2016 | 28,261,503 |
| | 28 |
| | 281,642 |
| | (34 | ) | | (142,722 | ) | | — |
| | 138,914 |
| 28,261,503 |
| | $ | 28 |
| | $ | 281,642 |
| | $ | (34 | ) | | $ | (142,722 | ) | | $ | — |
| | $ | 138,914 |
|
| Share issuances related to stock compensation plans | 722,441 |
| | — |
| | 6,071 |
| | — |
| | — |
| | — |
| | 6,071 |
| 722,441 |
| | — |
| | 6,071 |
| | — |
| | — |
| | — |
| | 6,071 |
|
| Stock-based compensation | — |
| | — |
| | 7,680 |
| | — |
| | — |
| | — |
| | 7,680 |
| — |
| | — |
| | 7,680 |
| | — |
| | — |
| | — |
| | 7,680 |
|
| Shares withheld to cover taxes on vesting of share based awards | (79,589 | ) | | — |
| | (1,933 | ) | | — |
| | — |
| | — |
| | (1,933 | ) | |
| Issuance of stock related to business combination | 3,615,840 |
| | 4 |
| | 116,788 |
| | — |
| | — |
| | 11,224 |
| | 128,016 |
| |
| Shares withheld to cover taxes on vesting of share-based awards | | (79,589 | ) | | — |
| | (1,933 | ) | | — |
| | — |
| | — |
| | (1,933 | ) |
| Issuance of stock related to business combinations | | 3,615,840 |
| | 4 |
| | 116,788 |
| | — |
| | — |
| | 11,224 |
| | 128,016 |
|
| Acquisition of noncontrolling interest | 19,806 |
| | — |
| | 2,022 |
| | — |
| | — |
| | (11,091 | ) | | (9,069 | ) | 19,806 |
| | — |
| | 2,022 |
| | — |
| | — |
| | (11,091 | ) | | (9,069 | ) |
| Common stock returned in legal settlement | (79,333 | ) | | — |
| | (2,753 | ) | | — |
| | — |
| | — |
| | (2,753 | ) | (79,333 | ) | | — |
| | (2,753 | ) | | — |
| | — |
| | — |
| | (2,753 | ) |
| Currency translation adjustment | — |
| | — |
| | — |
| | (80 | ) | | — |
| | — |
| | (80 | ) | — |
| | — |
| | — |
| | (80 | ) | | — |
| | — |
| | (80 | ) |
| Net loss | — |
| | — |
| | — |
| | — |
| | (15,956 | ) | | (1,187 | ) | | (17,143 | ) | — |
| | — |
| | — |
| | — |
| | (15,956 | ) | | (1,187 | ) | | (17,143 | ) |
| Balance December 31, 2017 | 32,460,668 |
| | $ | 32 |
| | $ | 409,517 |
| | $ | (114 | ) | | $ | (158,678 | ) | | $ | (1,054 | ) | | $ | 249,703 |
| 32,460,668 |
| | 32 |
| | 409,517 |
| | (114 | ) | | (158,678 | ) | | (1,054 | ) | | 249,703 |
|
| Share issuances related to stock compensation plans | | 972,415 |
| | 1 |
| | 12,185 |
| | — |
| | — |
| | — |
| | 12,186 |
|
| Stock-based compensation | | — |
| | — |
| | 9,261 |
| | — |
| | — |
| | — |
| | 9,261 |
|
| Shares withheld to cover taxes on vesting of share-based awards | | (85,505 | ) | | — |
| | (2,909 | ) | | — |
| | — |
| | — |
| | (2,909 | ) |
| Acquisition of noncontrolling interest | | 58,786 |
| | — |
| | (2,000 | ) | | — |
| | — |
| | 2,000 |
| | — |
|
| Currency translation adjustment | | — |
| | — |
| | — |
| | 370 |
| | — |
| | — |
| | 370 |
|
| Net income | | — |
| | — |
| | — |
| | — |
| | 42,820 |
| | (946 | ) | | 41,874 |
|
| Balance December 31, 2018 | | 33,406,364 |
| | 33 |
| | 426,054 |
| | 256 |
| | (115,858 | ) | | — |
| | 310,485 |
|
| Share issuances related to stock compensation plans | | 631,107 |
| | 1 |
| | 7,609 |
| | — |
| | — |
| | — |
| | 7,610 |
|
| Stock-based compensation | | — |
| | — |
| | 13,376 |
| | — |
| | — |
| | — |
| | 13,376 |
|
| Shares withheld to cover taxes on vesting of share-based awards | | (64,418 | ) | | — |
| | (4,955 | ) | | — |
| | — |
| | — |
| | (4,955 | ) |
| Issuance of stock related to business combinations | | 50,000 |
| | — |
| | 2,142 |
| | — |
| | — |
| | — |
| | 2,142 |
|
| Deferred purchase price consideration - equity portion | | — |
| | — |
| | 9,140 |
| | — |
| | — |
| | — |
| | 9,140 |
|
| Currency translation adjustment | | — |
| | — |
| | — |
| | (725 | ) | | — |
| | — |
| | (725 | ) |
| Net income | | — |
| | — |
| | — |
| | — |
| | 29,844 |
| | — |
| | 29,844 |
|
| Balance December 31, 2019 | | 34,023,053 |
| | $ | 34 |
| | $ | 453,366 |
| | $ | (469 | ) | | $ | (86,014 | ) | | $ | — |
| | $ | 366,917 |
|
See accompanying Notes to Consolidated Financial Statements.
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Organization and Description of Business
BioTelemetry, Inc. (“BioTelemetry,” “Company,“Company,” “we,“we,” “our”“our” or “us”“us”), a Delaware corporation, providesis the leading remote medical technology company focused on delivery of health information to improve quality of life and reduce cost of care. We provide remote cardiac monitoring, services and digital population health management for healthcare providers, medical device manufacturing and centralized core laboratory services for clinical research.trials, remote blood glucose monitoring and original equipment manufacturing that serves both healthcare and clinical research customers.
We operate under three2 reportable segments: (1) Healthcare (2) Research and (3) Technology.Research. During the first quarter of 2018, we aggregated our Technology operating segment into the Corporate and Other category. The Healthcare segment is focused on the diagnosis andremote cardiac monitoring ofto identify cardiac arrhythmias or heart rhythm disorders.disorders and to monitor the functionality of implantable cardiac devices. We offer cardiologists, and electrophysiologists, neurologists and primary care physicians a full spectrum of solutions, which provides them with a single source of remote cardiac monitoring services. These services range from the differentiatedinclude mobile cardiac telemetry service (“MCT”MCT”), to event, traditional Holter, extended-wearextended Holter, Pacemaker, and International Normalized Ratio (“INR”INR”), implantable loop recorder (“ILR”) monitoring. Since we became focused onand other implantable cardiac monitoring in 1999, we have developed a proprietary integrated patient management platform that incorporates a wireless data transmission network, U.S. Food and Drug Administration (“FDA”) cleared algorithms, medical devices and 24-hour monitoring service centers.device monitoring. The Research segment is engaged in centralcentralized core laboratory services providing cardiac monitoring, imaging services, scientific consulting and data management services for drug and medical device trials. The Technology segment focuses onIncluded in the development,Corporate and Other category is the manufacturing, testing and marketing of cardiovascularcardiac and blood glucose monitoring devices to medical companies, clinics and hospitals.hospitals and corporate overhead and other items not allocated to any of our reportable segments.
We have grown both organically and through recent acquisitions:
On July 12, 2017, we acquired approximately 97% of the outstanding shares of LifeWatch AG (“LifeWatch”). On that date, we acquired control of LifeWatch and began consolidating its financial statements. In September 2017, we purchased 343,525 additional shares of LifeWatchacquisitions; for cash consideration of $4.8 million and the issuance of 19,806 of our shares with a fair value of $0.6 million. We completed the acquisition of the remaining shares in December 2017, for aggregate consideration of $2.9 million in cash and 58,786 shares with a fair market value of $2.0 million which was settled in early January 2018. LifeWatch is included in the Healthcare segment.
On December 1, 2016, we acquired the stock of Telcare Medical Supply, Inc. and certain assets of Telcare Inc. (collectively, “Telcare”). Telcare is included in the Technology segment.
On May 11, 2016, we acquired VirtualScopics, Inc. (“VirtualScopics”), a leading provider of clinical trial imaging solutions. VirtualScopics is included in the Research segment.
On April 1, 2016, we acquired substantially all of the assets of the ePatch division (“ePatch”) of DELTA Danish Electronics, Light, and Acoustics (“DELTA”), inclusive of all products and indications under development. ePatch is included in the Technology segment.
For further details related to our recent acquisitions, please see “Note 3. Acquisitions” below.4. Acquisitions.”
Our common stock is traded on the NASDAQ Global Select Market under our symbol “BEAT.”
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
2. Summary of Significant Accounting Policies
a) Principles of Consolidation & Reclassifications
The accompanying consolidated financial statements have been prepared in accordance with U.S. generally accepted accounting principles (“U.S. GAAP”GAAP”) and include the accounts of BioTelemetry and its wholly owned subsidiaries. All intercompany transactions and balances have been eliminated in consolidation.
Certain reclassifications have been made to prior period statements to conform to the current period presentation. These consist of:
disaggregating the components of othercombining our non-cash depreciation and amortization expense in the consolidated statements of operations,
disaggregating the equity method investment loss from the change in prepaid expenses and other assets in theinto one line on our consolidated statements of cash flows,flows;
reclassifying researchseparating the non-cash operating item of change in fair value of acquisition-related contingent consideration from other non-cash items on our consolidated statements of cash flows; and development costs from the Corporate and Other segment to the Healthcare segment
combining our contract liabilities into accrued liabilities in our segment information disclosures.consolidated balance sheets.
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The reclassifications had no impact on previously reported working capital, consolidated net income/(loss),results of operations, cash flows from operating, financing and investing activities, or accumulated deficit.
b) Use of Estimates
The preparation of consolidated financial statements in conformity with U.S. GAAP requires that managementwe make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenue and expenses during the reporting period. Actual results may differ from those estimates.
c) Fair Value of Financial Instruments
Fair value is defined as the exit price, the price that would be received to sell an asset or transfer a liability in an orderly transaction between market participants at the measurement date. The fair value hierarchy prioritizes the inputs to valuation techniques used to measure fair value into three broad levels, as defined below. Observable inputs are inputs a market participant would use in valuing an asset or liability based on market data obtained from sources independent of us. Unobservable inputs are inputs that reflect our own assumptions about the factors a market participant would use in valuing an asset or liability developed using the best information available in the circumstances. The classification of an asset’s or liability’s level within the fair value hierarchy is determined based on the lowest level input that is significant to the fair value measurement.
| |
| Level 1 - | Quoted prices in active markets for an identical asset or liability. |
| |
| Level 2 - | Inputs that are observable for the asset or liability, either directly or indirectly through market corroboration, for substantially the full term of the asset or liability. |
| |
| Level 3 - | Inputs that are unobservable for the asset or liability, based on our own assumptions about the assumptions a market participant would use in pricing the asset or liability. |
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Our financial instruments consist primarily of cash and cash equivalents, Healthcare accounts receivable, other accounts receivable, accounts payable, acquisition-related contingent consideration, short-term debt and long-term debt. With the exception of theacquisition-related contingent consideration and long-term debt, the carrying value of these financial instruments approximates their fair value because of their short-term nature (classified as Level 1).
Our long-term debt (classified as Level 2) is measured using market prices for similar instruments, inputs such as the borrowing rates currently available, benchmark yields, actual trade data, broker/dealer quotes and other similar data obtained from quoted market prices or independent pricing vendors.
The fair value of acquisition-related contingent consideration (classified as Level 3) is measured on a recurring basis using unobservable inputs such asa Monte Carlo simulation. This model uses assumptions, including estimated projected payment dates, probabilitiesrevenues, estimated stock price volatility in future periods, estimated discount rates and discounts for the lack of meeting specified milestones and other such variables resulting in payment amounts which are discounted back to present value using a probability-weighted discounted cash flow model. Adjustments to contingent consideration are recorded in other charges in the consolidated statementsmarketability of operations and comprehensive income/(loss).
common stock. In addition to the recurring fair value measurements, the fair value of certain assets acquired and liabilities assumed in connection with a business combination are recorded at fair value, primarily using a discounted cash flow model (classified as Level 3). This valuation technique requires us to make certain assumptions, including but not limited to, future operating performance, and cash flows and revenue growth rates, royalty raterates and other such variables, which are discounted to present value using a discount rate that reflects the risk factors associated with future cash flow, the characteristics of the assets acquired and liabilities assumed and the experience of the acquired business. Non-financial assets such as
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
goodwill, intangible assets, and property plant, and equipment and right-of-use (“ROU”) assets are subsequently measured at fair value when there is an indicator of impairment and recorded at fair value only when an impairment is recognized. We assess the impairment of goodwill and intangible assets annually or whenever events or changes in circumstances indicate that the carrying amount of an intangible asset may not be recoverable.
d) Cash and Cash Equivalents
Cash and cash equivalents are held in financial institutions or in custodial accounts with financial institutions. Cash equivalents are defined as liquid investments and money market funds with maturity from date of purchase of 90 days or less that are readily convertible into cash and have minimal interest rate risk. We do not have restricted cash.
e) Accounts Receivable and Allowance for Doubtful Accounts
Healthcare accounts receivable, is related to the Healthcare segment andincluding contract assets, is recorded at the time Healthcare segment revenue is recognized net of contractual allowances, and is presented on the consolidated balance sheet net of an allowance for doubtful accounts. The ultimate collectionFor our contracted payors, we determine revenue based on negotiated prices for the services provided. Based on our history, we have experience collecting substantially all of accounts receivable maythe negotiated contracted rates and are therefore not be known for several months after services have been provided and billed. The percentages and amounts used to record bad debt expense and theproviding an implicit price concession. As a result, an allowance for doubtful accounts are supported by various methods and analyses, including current andis recorded based on historical cash collections andcollection trends to account for the agingrisk of receivables by payor.patient default. Because of continuing changes in the health carehealthcare industry and third-party reimbursement, it is possible that our estimates of collectability could change, which could have a material impact on our operations and cash flows.
Other accounts receivable is related to the TechnologyResearch segment and Research segmentsCorporate and Other category and is recorded at the time revenue is recognized, or when products are shipped or services are performed.
We estimate an
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
allowance for doubtful accounts on a specific account basis, and consider several factors in our analysis including customer specific information and the aging of the account.
We write-offwrite off receivables when the likelihood for collection is remote, and when we believe collection efforts have been fully exhausted and we do not intend to devote additional resources in attempting to collect. We perform write-offs on a monthly basis. In the Healthcare segment, we wrote-off $8.8$15.3 million and $8.4$11.2 million of receivables for the years ended December 31, 20172019 and 2016,2018, respectively. The impact was a reduction of gross accounts receivablereceivables and a reduction in the allowance for doubtful accounts. There were no material write-offs in the Technology and Research segments. Additionally, wesegment. We recorded bad debt expense of $13.3 million, $9.9$1.1 million and $8.0$0.8 million related to a customer bankruptcy in the Corporate and Other category during the years ended December 31, 2018 and 2017, respectively, and wrote off these amounts in 2018. We recorded consolidated bad debt expense of $21.8 million, $22.2 million and $13.3 million for the years ended December 31, 2019, 2018 and 2017, 2016 and 2015, respectively.
f) Acquisition-Related Contingent Consideration
Acquisition-related contingent consideration is our obligation, arising from a business combination, to transfer additional assets and/or equity interests to the seller if certain future events occur or conditions are met. The fair value of the contingency is estimated as of the acquisition date using certain unobservable inputs (and therefore classified as Level 3 in the fair value hierarchy) and is recorded as a liability. We re-measure the estimated fair value of acquisition-related contingent consideration at each reporting date. Adjustments subsequent to the acquisition measurement period are recorded in other charges in the consolidated statements of operations. Changes to the inputs used in the measurement of acquisition-related contingent consideration include, but are not limited to: changes in the assumptions regarding probabilities
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
of successful achievement of future events or conditions, estimated revenue projections; discounts for lack of marketability of our common stock, estimated stock price volatility, and the discount rate used to estimate the fair value of the liability. Acquisition-related contingent consideration may change significantly as our inputs and assumptions noted above evolve and additional data is obtained. The inputs and assumptions used in estimating fair value require significant judgment. The use of different assumptions and judgments could result in different fair value estimates that may have a material impact on our results from operations and financial position.
g) Concentrations of Credit Risk
Financial instruments that potentially subject us to concentrations of credit risk consist primarily of cash and cash equivalents, Healthcare accounts receivablesreceivable, including contract assets related to our cardiac monitoring services and other accounts receivables.receivable. We maintain our cash and cash equivalents with high quality financial institutions to mitigate this risk. We perform ongoing credit evaluations of our customers and generally do not require collateral. We record an allowance for doubtful accounts in accordance with the procedures described above. Past-due amounts are written-offwritten off against the allowance for doubtful accounts when collections are believed to be unlikely and all collection efforts have ceased.
At December 31, 2019, 2018 and 2017, 2016 and 2015, one1 payor, Medicare, accounted for 21%22%, 11%15% and 13%21%, respectively, of our gross healthcare accounts receivable.
g)h) Inventory
Inventory is valued at the lower of cost (using first-in, first-out cost method) or market (net realizable value or replacement cost). Management reviews inventory for specific future usage, and estimates of impairment of individual inventory items are recorded to reduce inventory to the lower of cost or market.
h)i) Property and Equipment
Property and equipment is recorded at cost, except for assets acquired in business combinations, which are recorded at fair value as of the acquisition date. Depreciation is recorded over the estimated useful life of each class of depreciable assets, and is computed using the straight-line method. Leasehold improvements and assets acquired under a finance lease are amortized over the shorter of the estimated asset life or term of the lease.lease and included in depreciation expense. Repairs and maintenance costs are charged to expense as incurred. Costs of additions and improvements are capitalized.
i)j) Impairment of Long-Lived Assets
The carrying value of long-lived assets, other than goodwill, and indefinite-lived intangible assets, is evaluated when events or changes in circumstances indicate the carrying value may not be recoverable or the useful life has changed. We consider historical performance and anticipated future results in our evaluation of potential impairment. Accordingly, when indicators of impairment are present, we evaluate the carrying value of these assets in relation to the operating performance of the business and the undiscounted cash flows expected to result from the use of these assets. If the carrying amount of a long-lived asset exceeds its expected undiscounted cash flows, an impairment charge is recognized to the extent the carrying amount exceeds its fair value.
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
j)k) Leases
We lease our administrative and service facilities, as well as certain office equipment, monitoring devices and information technology equipment under arrangements classified as leases under Accounting Standards Codification (“ASC”) 842 - Leases (“ASC 842”). We adopted ASC 842 using the optional modified retrospective transition method as of January 1, 2019, therefore prior period amounts are not restated.
We recognize ROU assets at the inception of the arrangement as the present value of the lease payments plus our initial direct costs (if any), less any lease incentives. The corresponding liability is computed as the present value of the lease payments at inception. Assets are classified as either operating or finance ROU assets according to the classification criteria in ASC 842. Upon the adoption of ASC 842, we elected the transition practical expedients to not reassess lease identification, lease classification and initial indirect costs related to those leases entered into prior to adoption of ASC 842 and to not separate lease and non-lease components where we are the lessor when the requisite criteria is met to be treated as such. The present value of the lease payments is computed using the rate implicit in the lease (if known) or our incremental borrowing rate, which is the rate incurred to borrow on a collateralized basis over a similar term at an amount equal to the lease payments.
Operating lease costs are charged to operations on a straight-line basis over the lease term. Interest charged on the finance lease liabilities is charged to interest expense, while the amortization of the finance lease ROU assets is also charged to operations on a straight-line basis.
Under our policy, we do not record an ROU asset or corresponding liability for arrangements where the initial lease term is one year or less. Those leases are expensed on a straight-line basis over the term of the lease.
Effective January 1, 2019, for our operating leases, we record the ROU assets as a component of other assets, the current lease liability as a component of accrued liabilities, and the long-term lease liability as a component of other long-term liabilities on our consolidated balance sheet. For our finance leases, we record the ROU asset and the accumulated amortization for the finance ROU asset as a component of property and equipment, net, with the current and long-term portions of the finance lease obligations as separate lines within our consolidated balance sheet. We amortize the finance ROU assets over the shorter of the remaining lease term or the estimated life of the asset.
l) Derivative InstrumentsInstrument
During the second quarter of 2017, we purchased a foreign currency option with a notional value of $194.2 million to mitigate the foreign exchange risk related to the Swiss Franc denominatedFranc-denominated purchase price of LifeWatch.LifeWatch AG (“LifeWatch”). This derivative instrument was not designated as a hedge for accounting purposes. We did not exercise this option and the contract expired during the third quarter of 2017, resulting in a charge of $1.3 million, which was recorded as a component of other non-operating income/(expense), net in the consolidated statements of operations and comprehensive income/(loss).operations. We have not entered into any derivative transactions since.
k)m) Equity Method Investments
We account for investments using the equity method of accounting if the investment provides us the ability to exercise significant influence, but not control, over the investee. Significant influence is
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
generally deemed to exist if our ownership interest in the voting stock of the investee ranges between 20% and 50%, although other factors, such as representation on the investee’s board of directors, are considered in determining whether the equity method of accounting is appropriate. Under the equity method of accounting, the investment is recorded at cost in the consolidated balance sheets under other assets and is periodically adjusted for capital contributions, dividends received and our share of the investee’s earnings or losses together with other-than-temporary impairments which are recorded through loss on equity method investment in the consolidated statements of operations and comprehensive income/(loss).operations.
l)n) Noncontrolling InterestInterests
The consolidated financial statements reflect the application of ASC 810 - Consolidations, which establishes accounting and reporting standards that require: (i) the ownership interest in subsidiaries held by parties other than the parent to be clearly identified and presented in the consolidated balance sheet within stockholder’s equity, but separate from the parent’s equity; (ii) the amount of consolidated net incomeincome/(loss) attributable to the parent and the noncontrolling interest to be clearly identified and presented on the face of the consolidated statements of income;operations; and (iii) changes in a parent’s ownership interest while the parent retains its controlling financial interest in its subsidiary to be accounted for consistently.
We acquired approximately 97% of LifeWatch on July 12, 2017. On that date, we acquired control of LifeWatch and began consolidating its financial statements. As of December 31, 2017, we owned 100% of LifeWatch.LifeWatch.
Also on July 12, 2017, LifeWatch owns owned 55% of LifeWatch Turkey Holding AG (“LifeWatch Turkey,” domiciled in Switzerland), with theira partner IKSIR TEKNOLOJI SAGLIK VE KIMYA SAN. ve TIC. A.S., a company located in Ankara, Turkey, to provide digital health solutions to the Turkish market. Concurrent with our acquisition of LifeWatch, we acquired control of LifeWatch Turkey and began consolidating their financial statements.
During 2018, after a formal restructuring of shareholdings approved by the board of directors of LifeWatch Turkey, we became the sole shareholder of LifeWatch Turkey. No cash or other consideration was exchanged to effect this transaction. As of December 31, 2017, LifeWatch Turkey’sa result, we no longer reflect a noncontrolling interest in our consolidated balance sheet; however, we continue to reflect the net assets were $3.6 million and their loss since July 12, 2017 was $2.3 million.
Amounts pertainingattributable to the noncontrolling ownership interest of LifeWatch Turkey held by third parties in our operating results are combined and reported as noncontrolling interests inconsolidated statement of operations for the accompanying consolidated financial statements.period of time where we did not own the entire entity.
m)o) Goodwill and Acquired Intangible Assets
Goodwill is the excess of the purchase price of an acquired business over the amounts assigned to assets acquired and liabilities assumed in a business combination. In accordance with ASC 350 - Intangibles
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
— Goodwill and Other (“ASC 350”350”), goodwill is reviewed for impairment annually, or when events arise that could indicate that an impairment exists. Initially, we qualitativelyIn order to test goodwill for impairment, ASC 350 allows reporting entities to take either a qualitative or quantitative approach to testing. Under the qualitative approach, an entity may assess qualitative factors to determine whether it is more-likely-than-not that an impairment exists for eachthe fair value of oura reporting units.unit is less than its carrying amount. Such qualitative factors can include, among others, industry and market conditions, present and anticipated sales and cost factors, overall financial performance and relevant entity-specific events. If we conclude based on our qualitative assessment that it is more-likely-than-not that the fair value of a reporting unit is less than its carrying value, we perform an impairment testa quantitative analysis in accordance with ASC 350. We compare350. Under the quantitative approach, an entity compares the fair value of ourits reporting units to their carrying value. If the reporting unit’s carrying value exceeds its fair value, an impairment loss equal to the difference is recognized. The loss recognized shall not exceed
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
the total amount of goodwill allocated to the reporting unit, and the income tax effects from any deductible goodwill on the carrying value of the reporting unit when measuring the goodwill impairment loss, if any, are considered.
For the purpose of performing our goodwill impairment analysis, we consider our business to be composed of three3 reporting units: Healthcare Technology, Research and Research.Technology. When performing a quantitative analysis, we calculate the fair value of the reporting units utilizing a weighting of the income and market approaches. The income approach is based on a discounted cash flow methodology that includes assumptions for, among other things, forecasted income, cash flow, growth rates, income tax rates, expected tax benefits and long-term discount rates, all of which require significant judgment. The market approach utilizes our market data as well as market data from publicly-traded companies that are similar to us. There are inherent uncertainties related to these factors and the judgment applied in the analysis. We believe that the combination of an income and a market approach providesapproaches provide a reasonable basis to estimate the fair value of our reporting units.
Acquired intangible assets are recorded at fair value on the acquisition date. The estimated fair values and useful lives of intangible assets are determined by assessing many factors including estimates of future operating performance and cash flows of the acquired business, the characteristics of the intangible assets and the experience of the acquired business. Independent appraisal firms may assist with the valuation of acquired assets. The impairment test for indefinite-lived intangible assets other than goodwill consists of a comparison of the fair value of the indefinite-lived intangible asset to the carrying value of the asset. We usedAs a qualitative approach to determineresult of our impairment analysis for the year ended December 31, 2017, we noted entity-specific events which resulted in the write-off of the remainder of our indefinite-lived as well asintangible assets other than goodwill, and a portion of our finite-lived intangible assets. See “Note 8. Goodwill and Intangible Assets” for further details regarding the impairment charges. We amortize our amortizablefinite-lived intangible assets.assets over each asset’s estimated useful life using the straight-line method and assess impairment indicators during each reporting period. Should an impairment indicator exist, we compare the carrying value of the asset to the undiscounted cash flows and record an impairment charge on the excess of the carrying value over the fair value.
n)p) Revenue Recognition
We recognize approximately 81%adopted ASC 606 - Revenue from Contracts with Customers (“ASC 606”) on January 1, 2018, which requires revenue recognized to represent the transfer of our totalpromised goods or services to customers at an amount that reflects the consideration that a company expects to receive in exchange for those goods or services.
We utilized the modified retrospective method for adoption, allowing us to not retrospectively adjust prior periods. We applied the modified retrospective method only to contracts that were not complete at January 1, 2018 and accounted for the aggregate effect of any contract modifications upon adoption. No cumulative adjustment to retained earnings was recorded.
Healthcare
Healthcare segment revenue includes revenue from patientMCT, event, traditional Holter, extended Holter, Pacemaker, INR and ILR monitoring services in our Healthcare segment. We receive aservices. A significant portion of thisour revenue fromis paid for by third-party commercial payorsinsurance organizations and governmental entities. We also receive reimbursement directly from patients through co-pays, deductibles and self-pay arrangements. Revenue from the Medicare program is based on reimbursement rates set by CMS. For the years ended December 31, 2017, 2016 and 2015, revenue from Medicare as a percentage of total revenue was 34%, 33% and 34%, respectively. Revenue from contracted commercial payors is recorded at the negotiated contractual rate. Revenue from non-contracted commercial payors is recorded at net realizable value based on historical payment patterns. Adjustments to the estimated net realizable value, based on final settlement with the third-party payors, are recorded upon settlement. If we do not have consistent historical information regarding collectability from a given payor, revenue is recognized when cash is received. Unearned amounts are appropriately deferred until service has been completed. For the years ended December 31, 2017 and 2016, deferred revenues related to the Healthcare segment were $2.4 million and $1.1 million, respectively; none of these deferred revenues were refundable.
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
For contracted payors, including Medicare, we determine revenue based on negotiated prices for the services provided. Based on our history, we have experience collecting substantially all of the negotiated contracted rates and are therefore not providing an implicit price concession. As a result, any adjustments to the revenue recognized are due to patient default and are recorded as bad debt expense.
For non-contracted payors, we are providing an implicit price concession because we do not have a contract with the underlying payor. As a result, we estimate our expected revenue based on historical cash collections. Subsequent adjustments to the revenue recognized are recorded as an adjustment to Healthcare revenue and not as bad debt expense.
Research
Research segment revenue includes revenue for centralized core laboratory services. Our Research segment revenue is provided on a fee-for-service basis, and revenue is recognized as the related services are performed. We also provide consulting services on a time and materials basis, and recognizethis revenue is recognized as we perform the services.services are performed. Our site support revenue, consisting of equipment rentals and sales along with related supplies and logistics management, are recognized at the time of saleservice or over the rental period. Under a typical contract, customers pay us a portionOur research customer contracts have legally enforceable terms that are predominately thirty days due to termination for convenience clauses, which are held by the customer with no significant penalty. Given the short-term nature of these contracts and the structure of our fee for these services upon contract executionbilling practices, our billing practices approximate our performance if measured by an output method, where each output is an individual occurrence of each performance obligation. Accordingly, we utilize the invoice practical expedient as an upfront deposit. Unearneddefined in ASC 606, resulting in recognition of revenue including upfront deposits, are deferred, and then recognized asin the services are performed. Foramount that we have the years ended December 31, 2017 and 2016, deferred revenues relatedright to the Research segment were $4.2 million and $2.7 million, respectively; these deferred revenues were refundable.
Revenue in our Technology segment is received from the sale of products, product repair and supplies which are recognized when shipped, or as service is completed. Deferred revenues related to our Technology segment were immaterial for the years ended December 31, 2017 and 2016.
For arrangements with multiple deliverables, the revenue is allocated to each element (both delivered and undelivered items) based on their relative selling prices or management’s best estimate of their selling prices, when vendor-specific or third-party evidence is unavailable.
invoice. We record reimbursements received for out-of-pocket expenses, including freight, incurred as revenue in the accompanying consolidated statements of operationsoperations.
Other
Other revenue is primarily derived from the sale of non-invasive cardiac monitors to healthcare companies, wireless blood glucose meters and comprehensive income (loss). test strips to wholesale distributors of diabetes supplies and employer health plans, as well as product repairs. Performance obligations are primarily the sale of devices, related goods and repairs provided by us. These contracts transfer control to a customer at a point in time based on the transfer of title for the underlying goods or service.
Revenue generally is recognized net of any taxes collected from customers and subsequently remitted to government authorities.
o)See “Note 3. Revenue Recognition” for more information.
q) Stock-Based Compensation
ASC 718 - Compensation—Stock Compensation (“ASC 718”718”), addresses the accounting for share-based payment transactions in which an enterprise receives employee services in exchange for: (i) equity instruments of the enterprise or (ii) liabilities that are based on the fair value of the enterprise’s equity instruments or that may be settled by the issuance of such equity instruments. ASC 718 requires that an entity measuresmeasure the cost of equity-based service awards issued to employees, such as stock options and restricted stock units (“RSUs”), based on the grant-date fair value of the award and recognizesrecognize the cost of such awards over the requisite service period (generally, the vesting period of the award). ASC 718 requires that an entity measure the cost of liability-based service awards based on current fair value that is remeasured subsequently at each reporting date through the settlement date. The compensation expense associated with performance stock units (“PSUs”) is recognized ratably over the period between when the performance conditions are deemed probable of achievement and when the awards are vested. We account
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Performance stock options (“PSOs”) are valued and stock-based compensation expense is recorded once the performance conditions of the outstanding PSOs have achieved probability. Prior to our July 1, 2018 adoption of Accounting Standards Update (“ASU”) 2018-07, Compensation - Stock Compensation (Topic 718): Improvements to Nonemployee Share-Based Payment Accounting, we accounted for equity awards issued to non-employees in accordance with ASC 505-50, Equity-Based Payments to Non-Employees.
Stock-based compensation expense is only recognized for outstanding performance stock units (“PSUs”) where the performance conditions are deemed probable for achievement. For PSUs deemed probable for achievement, stock-based compensation expense is recognized ratably over the expected vesting period. Performance stock options (“PSOs”) are valued and stock-based compensation expense is only recognized once the performance conditions of the outstanding PSOs have been met.
We have historically recorded stock-based compensation expense based on the number of stock options or restricted stock units (“RSUs”) we expect to vest using our historical forfeiture experience and we periodically update those forfeiture rates to apply to new grants. While we early adopted ASU 2016-09 in the year ended December 31, 2016, we have elected to continue toWe estimate forfeitures under the true-up
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
provision of ASC 718.718. We record additional expense if the actual forfeiture rate is lower than estimated and record a recovery of prior expense if the actual forfeiture rate is higher than estimated.
We estimate the fair value of our stock options using the Black‑Scholes option valuation model. The Black‑Scholes option valuation model requires the use of certain subjective assumptions. The most significant of these assumptions are the estimates of the expected volatility of the market price of our stock and the expected term of the award. We base our estimates of expected volatility on the historical average of our stock price. The expected term represents the period of time that share‑based awards granted are expected to be outstanding. Other assumptions used in the Black‑Scholes option valuation model include the risk‑free interest rate and expected dividend yield. The risk‑free interest rate for periods pertaining to the contractual lifeexpected term of each option is based on the U.S. Treasury yield of a similar duration in effect at the time of grant. We have never paid, and do not expect to pay, dividends in the foreseeable future.
p)We estimate the fair value of our PSUs using a Monte Carlo simulation. This model uses assumptions, including the risk free interest rate, expected volatility of our stock price and those of the performance group, dividends of the performance group members and expected life of the awards. As noted above, we continue to estimate forfeitures under the true-up provision of ASC 718. If it is deemed probable that the PSU performance targets will be met, compensation expense is recorded for these awards ratably over the requisite service period. The PSUs are forfeited to the extent the performance criteria are not met within the service period.
r) Research and Development Costs
Research and development costs are charged to expense as incurred.
q)s) Foreign Currency Translation
We primarily operate our businesses in the United States, however, we have certain international subsidiaries. Our primary functional currency is the U.S. dollar. Assets and liabilities of non-U.S. dollar functional currency entities are translated to U.S. dollars at period-end exchange rates, and the currency impacts arising from the translation of the assets and liabilities are recorded as a cumulative translation adjustment, a component of our accumulated other comprehensive (loss)/income within our consolidated balance sheets. Revenues and expenses for those entities are translated at the average monthly currency exchange rates in effect during the period. Currency transaction gains and losses are included in other non-operating (expense)/income, net in the consolidated statements of operations as incurred. We recognized a $2.8 million transaction loss, a $1.3 million transaction gain, and a $0.3 million transaction loss as a component of other non-operating (expense)/income, net for the years ended December 31, 2019, 2018 and 2017, respectively.
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
t) Income Taxes
We account for income taxes under the liability method, as described in ASC 740 - Income Taxes(Taxes (“ASC 740”740”). Deferred income taxes are recognized for the tax consequences of temporary differences between the tax and financial statement reporting bases of assets and liabilities. When we determine that we will not be able to realize our deferred tax assets, we adjust the carrying value of the deferred tax assetassets through the valuation allowance.
We record uncertainunrecognized tax positionsbenefits in accordance with ASC 740 on the basis of a two-step process in which (i) we determine whether it is more likely than not that the tax positions will be sustained on the basis of the technical merits of the position and (ii) for those tax positions that meet the more-likely-than-not recognition threshold, we recognize the largest amount of tax benefit that is more than 50 percent likely to be realized upon ultimate settlement with the related tax authority.
On December 22, 2017, the Tax Cuts and Jobs Act (the “TCJA”“TCJA”) was enacted in the United States.U.S. The TCJA represents sweeping changes in U.S. tax law. Under ASC 740, the effects of changes in tax rates and tax laws on deferred tax balances are recognized in the period in which the new legislation is enacted. The total effect of tax law changes on deferred tax balances is recorded as a component of income tax expense.
In response to the TCJA, the Staff of the U.S. Securities and Exchange Commission (“SEC”) issued Staff Accounting Bulletin No. 118 (“SAB 118”118”) to provide guidance to registrants in applying ASC 740 in connection with the TCJA. SAB 118 provides that in the period of enactment, the income tax effects of the TCJA may be reported as a provisional amount based on a reasonable estimate (to the extent a reasonable estimate can be determined), which would be subject to adjustment during a “measurement period.” The measurement period begins in the reporting period of the TCJA’s enactment and ends when a registrant has obtained, prepared, and analyzed the information that was needed in order to complete the accounting requirements under ASC 740.740. SAB 118 also describes supplemental disclosures that should accompany the provisional amounts. We have applied the guidance in SAB 118 to account for the financial accounting impacts of the TCJA as of December 31, 2017, and have provided the applicable supplemental disclosures in “Note 16.17. Income Taxes” below.Taxes.”
As of December 31, 2017, we recorded the provisional impact from the TCJA in accordance with SAB 118. We finalized our adjustments related to the impact of the TCJA during the year ended December 31, 2018.
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
r)u) Net Income/(Loss) Per Share
We compute net income/(loss) per share in accordance with ASC 260 - Earnings Per Share.Share. Basic net income/(loss) per share is computed by dividing net income/(loss) by the weighted average number of common shares outstanding during the period. Diluted net income/(loss)income per share is computed by giving effect to all potential dilutive common shares,stock equivalents, including stock options, RSUs, PSOsand restricted stock units (“RSUs”)PSUs, using the treasury stock method.method and shares expected to be issued in connection with acquisition-related contingent consideration arrangements when dilutive. Potentially dilutive common sharesstock equivalents are not included in the weighted-average shares outstanding for determining net loss per share for the year ended December 31, 2017, as the result would be anti-dilutive.
Certain stock options, which are priced higher than the market price of our shares as of December 31, 20172019, 20162018 and 20152017 would be anti-dilutive and therefore have been excluded from the weighted average
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
shares used in computing diluted net income per share. These options could become dilutive in future periods. Similarly, certain recently granted RSUs and PSUs are also excluded using the treasury stock method as their impact would be anti-dilutive. The dilutive effect of weighted average shares outstanding excludes approximately 0.5 million shares for each of the years ended December 31, 2019 and 2018, as their effect would have been anti-dilutive on our net income per share.
s)v) Segment Information
ASC 280 - Segment Reporting, establishes standards for reporting information regarding operating segments in annual consolidated financial statements. Operating segments are identified as components of an enterprise for which separate discrete financial information is available for evaluation by the chief operating decision maker, or decision-making group, in making decisions on how to allocate resources and assess performance.
We report our business under three2 segments: Healthcare Research and Technology.Research. The Healthcare segment is focused on theremote cardiac monitoring of cardiacto identify arrhythmias or heart rhythm disorders inand to monitor the functionality of implantable cardiac devices. We offer cardiologists, electrophysiologists, neurologists and primary care physicians a health care setting.full spectrum of solutions, which provides them with a single source of remote cardiac monitoring services. The Research segment provides centralis engaged in centralized core laboratory services providing cardiac monitoring, imaging services, scientific consulting and data management services for drug and medical device trials. During the first quarter of 2018, as part of the LifeWatch integration, our forward-looking integration and rebranding plans, as well as re-evaluating the significance and materiality of our segments, we aggregated the Technology operating segment into the Corporate and Other category. Included in a research environment, which includes certain equipment rentalthe Corporate and device sales. The Technology segment focuses onOther category is the development, manufacturing, testing and marketing of medicalcardiac and blood glucose monitoring devices to medical companies, clinics and hospitals.hospitals and corporate overhead and other items not allocated to any of our reportable segments.
t)w) Recent Accounting Pronouncements
Accounting Pronouncements Recently Adopted
In March 2016,August 2018, the FinancialFASB issued ASU 2018-15, Customer’s Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2016-09, Improvementsfor Implementation Costs Incurred in a Cloud Computing Arrangement That Is a Service Contract, to Employee Share-Based Payment Accounting.align the requirements for capitalizing implementation costs incurred in a hosting arrangement that is a service contract with the requirements for capitalizing implementation costs incurred to develop or obtain internal-use software. The updated guidance also requires an entity to expense the capitalized implementation costs of a hosting arrangement that is a service contract over the term of the hosting arrangement. Historically, our implementation costs incurred in hosting service contracts have not been material. We early adopted this standard revises the accounting for certain aspects of share-based compensation arrangements and requires any excess tax benefits or tax deficiencies to be recorded directly in the income statement when such awards vest or settle. In addition, the cash flows related to any excess tax benefits will no longer be separately classified as a financing activity, but will rather be classified as an operating activity, along with all other income tax cash flows. The standard also makes certain changes to the way the treasury stock method is applied when calculating diluted net income per share, as well as allows for a policy election to account for forfeitures as they occur, rather than using the estimation method currently prescribed by ASC 718, Compensation — Stock Compensation (“ASC 718”). The standard is effective for annual and interim periods beginning after December 15, 2016, with early adoption permitted.
We elected to early adopt the standard during the fourth quarter of 2016. The standard requires the recognition of any pre-adoption date net operating loss (“NOL”) carryforwards from share-based compensation arrangements to be recognizedApril 1, 2019 on a modified retrospective basis, through an opening retained earnings adjustment on January 1, 2016. Any income tax effects from share-based compensation
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
arrangements arising after January 1, 2016 will be recognized prospectively in the income statement during the period of adoption.
prospective basis. Upon adoption, we recognized all previously unrecognized tax benefits which resulted in a cumulative-effect adjustment of $1.8 million to our accumulated deficit. These previously unrecognized tax benefits wereeligible cloud computing implementation costs are capitalized and recorded as a deferred tax asset, which was fully offset by a valuation allowance on January 1, 2016, thus there was no net impact fromcomponent of technology within intangible assets in our consolidated balance sheet and amortized to selling, general and administrative costs over the adoption of ASU 2016-9 aslife of the same date. In addition, we recognized excess tax benefits as an adjustment to our previously reported benefit from/(provision for) income taxes of $0.1 million, $0.4 million and $0.1 million for the quarters ended March 31, 2016, June 30, 2016 and September 30, 2016, respectively. The weighted average number of common shares outstanding for calculating diluted net income per share increased by 340,000 to 550,000 for each quarter of 2016. Basic and diluted net income per share increased by $0.01 for the three months ended June 30, 2016. Net income per share for the three months ended March 30, 2016 and September 30, 2016 were not changed by the adoption of ASU 2016-9. Recast quarterly net income and basic and diluted net income per share for the first three quarters of 2016 is disclosed in “Note 19. Quarterly Financial Data” below.
Our adoption of the standard did not have any impact to our consolidated statements of cash flows as no NOL carryforwards from share-based compensation arrangements were recognized prior to January 1, 2016, due to our use of the “with and without” method of accounting for equity-generated NOL carryforwards. We have elected to continue to estimate forfeitures under the true-up provision of ASC 718. The adoption of this standard decreased our effective tax rate by 11.1% for the year ended December 31, 2016.
In July 2015, the FASB issued ASU 2015-11, Simplifying the Measurement of Inventory. The standard requires inventory to be measured at the lower of cost or net realizable value. The guidance will not apply to inventories for which cost is determined using the last-in, first-out method or the retail inventory method. Our adoption of this standard in the first quarter of 2017 did not have a material impactservice arrangement on our consolidated financial statements.
In January 2017, the FASB issued ASU 2017-04, Simplifying the Test for Goodwill Impairment. The standard eliminates step two in the current two-step impairment test under ASC 350. Under the new standard, a goodwill impairment is recorded for any excessstatement of a reporting unit’s carrying value over its fair value. A prospective transition approach is required. The standard is effective for annual and interim reporting periods beginning after December 15, 2019 with early adoption permitted for annual and interim goodwill impairment testing dates after January 1, 2017. Our adoption of this standard in the fourth quarter of 2017 did not have a material impact on our consolidated financial statements.
Accounting Pronouncements Not Yet Adopted
In May 2017, the FASB released ASU 2017-09, Scope of Modification Accounting, which clarifies the changes to terms or conditions of a share based payment award that requires application of modification accounting under Topic 718. A change to an award should be accounted for as a modification unless the fair value of the modified award is the same as the original award, the vesting conditions do not change and the classification as an equity or liability instrument does not change.operations. This update is effective for annual reporting periods, and interim periods within those annual periods, beginning after December 15, 2017. Early application is permitted and prospective application is required for awards modified on or
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
after the adoption date. We will adopt this standard effective January 1, 2018, and this standard willdid not have a material impact on our financial position, results of operations or disclosures.
In January 2017,August 2018, the FASB released ASU 2017-01, Business Combinations: ClarifyingU.S. Securities and Exchange Commission (“SEC”) adopted the Definitionfinal rule under SEC Release No. 33-10532, Disclosure Update and Simplification, amending certain disclosure requirements that were redundant, duplicative, overlapping, outdated or superseded. Additionally, the amendments expanded the disclosure requirements on the consolidated statements of a Business, which clarifies the definition of a business with the objective of adding guidance to assist entities with evaluating whether transactions should be accountedequity for as acquisitions or disposals of assets or businesses. The amendments in this ASU should be applied prospectively and are effective for fiscal years beginning after December 15, 2017, including interim periods within those fiscal years, with early adoption permitted. No disclosures are required at transition. We will adopt this standard effective January 1, 2018 and do not expect the standard to have a material impact on our consolidated financial statements. Under the amendments, a summary of changes in each caption of
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
stockholders’ equity presented in the consolidated balance sheets must be provided in a note or separate statement. The consolidated statements of equity should present a reconciliation of the beginning balance to the ending balance of each period for which the consolidated statement of comprehensive income is required to be filed. This final rule was effective in the fourth quarter of 2018. The SEC provided relief on the effective date until the first quarter of 2019, and we adopted this rule in the first quarter of 2019.
In February 2016, the FASB issued ASU 2016-02, Leases. TheThis standard, will requirealong with several subsequent updates, requires lessees to recognize most leases on their balance sheet, and makesmake selected changes to lessor accounting.accounting and disclose additional key information about leases. We adopted these updates on January 1, 2019, using the optional modified retrospective transition method and utilizing practical expedients available. The adoption of the new standard resulted in the recording, as of January 1, 2019, of additional ROU assets of $22.7 million as a component of other assets, current ROU liabilities of $6.2 million as a component of accrued liabilities and long-term ROU liabilities of $16.5 million, all of which relate to our operating leases. The adoption of the new standard did not materially impact our consolidated results of operations and had no impact on our cash flows.
Accounting Pronouncements Not Yet Adopted
In December 2019, the FASB issued ASU 2019-12, Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes (“ASU 2019-12”). This update eliminates certain exceptions related to the approach for intra-period tax allocation, the methodology for calculating income taxes in an interim period and the recognition of deferred tax liabilities for outside basis differences. The update also simplifies aspects of the accounting for franchise taxes and enacted changes in tax laws or rates and clarifies the accounting for transactions that result in a step-up in the tax basis of goodwill. Additionally, ASU 2019-12 clarifies that single-member limited liability companies and similar disregarded entities that are not subject to income tax are not required to recognize an allocation of consolidated income tax expense in their separate financial statements, but they can elect to do so. ASU 2019-12 is effective for annualfiscal years, and interim reporting periods within those fiscal years, beginning after December 15, 2018. A modified retrospective transition approach is required,2020, with certain practical expedients available.early adoption permitted. We are currentlyin the process of evaluating the impact the adoption of this standard will haveupdate on our consolidated financial statements.statements and related disclosures.
In May 2014,August 2018, the FASB issued ASU 2014-09, Revenue from Contracts with Customers2018-13, Fair Value Measurement (Topic 820): Disclosure Framework-Changes to the Disclosure Requirements for Fair Value Measurement (“ASU 2014-09), which has been updated through several revisions and clarifications since its original issuance (collectively, the “Standard”2018-13”). The StandardThis update eliminates certain disclosures related to transfers and valuation processes, clarifies the requirement for measurement uncertainty disclosures, and requires additional disclosures for Level 3 fair value measurements, including the range and weighted average of significant unobservable inputs used to develop Level 3 fair value measurements. ASU 2018-13 is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2019, with early adoption permitted. We will adopt this update on January 1, 2020, and this update will not impact our consolidated financial statements; however the update is expected to result in additional disclosures.
In June 2016, the FASB issued ASU 2016-13, Financial Instruments - Credit Losses. This update, along with subsequent amendments, introduces the current expected credit loss model, which will require an entity to measure credit losses for certain financial instruments and financial assets, including trade receivables. Under this update, upon initial recognition and at each reporting period, an entity will be required to recognize an allowance that reflects the entity’s current estimate of credit losses expected to be incurred over the life of the financial instrument. This update will be effective for fiscal years beginning after December 15, 2019 and interim periods within those fiscal years, and a modified retrospective approach
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
is required, with a cumulative-effect adjustment to retained earnings as of the beginning of the first reporting period in which the guidance is effective. We are currently in process of adopting this update as of January 1, 2020, and we currently do not anticipate that the adoption will have a material impact on our consolidated financial statements.
3. Revenue Recognition
We adopted ASC 606 on January 1, 2018, which requires revenue recognized to represent the transfer of promised goods or services to customers at an amount that reflects the consideration whichthat a company expects to receive in exchange for those goods or services. The Standard also requires new, expanded disclosures regarding revenue recognition. The Standard is effective
We utilized the modified retrospective method for adoption, allowing us to not retrospectively adjust prior periods. We applied the modified retrospective method only to contracts that were not complete at January 1, 2018.2018 and accounted for the aggregate effect of any contract modifications upon adoption. No cumulative adjustment to retained earnings was recorded.
Disaggregation of Revenue
We completed the detailed review of our contract portfoliodisaggregate revenue from contracts with customers by payor type and revenue streams to identify potential differences in accounting resulting from adopting the Standard.
We implemented the following controls with respect to assessing the potential impact of adopting the Standard:
Created an implementation working group, which includes internal and third-party resources;
Adopted implementation controls that will allow us to properly adopt the Standard;
Developed a detailed project plan with key milestone dates;
Outlined our revenue generating activities that fall within the scope of ASU 2014-09; and
Monitored and assessed the impact of changes to ASU 2014-09 and its interpretations.
We have determined the following pertaining to the impact of adopting ASU 2014-09:
Healthcare Revenue -major service line. We determined that contracts withindisaggregating revenue into these categories achieves the disclosure objective of illustrating the differences in the nature, amount, timing and uncertainty of our revenue streams. Disaggregated revenue by payor type and major service line was as follows:
|
| | | | | | | | | | | | | | | |
| | Year Ended December 31, 2019 |
| | Reporting Segment | | | | Total Consolidated |
| (in thousands) | Healthcare | | Research | | Other | |
| Payor/Service Line | | | | | | | |
| Remote cardiac monitoring services - Medicare | $ | 153,743 |
| | $ | — |
| | $ | — |
| | $ | 153,743 |
|
| Remote cardiac monitoring services - commercial payors | 218,271 |
| | — |
| | — |
| | 218,271 |
|
| Clinical trial support and related services | — |
| | 54,450 |
| | — |
| | 54,450 |
|
| Technology devices, consumable and related services | — |
| | — |
| | 12,643 |
| | 12,643 |
|
| Total | $ | 372,014 |
| | $ | 54,450 |
| | $ | 12,643 |
| | $ | 439,107 |
|
|
| | | | | | | | | | | | | | | |
| | Year Ended December 31, 2018 |
| | Reporting Segment | | | | Total Consolidated |
| (in thousands) | Healthcare | | Research | | Other | |
| Payor/Service Line | | | | | | | |
| Remote cardiac monitoring services - Medicare | $ | 137,600 |
| | $ | — |
| | $ | — |
| | $ | 137,600 |
|
| Remote cardiac monitoring services - commercial payors | 201,212 |
| | — |
| | — |
| | 201,212 |
|
| Clinical trial support and related services | — |
| | 50,561 |
| | — |
| | 50,561 |
|
| Technology devices, consumables and related services | — |
| | — |
| | 10,099 |
| | 10,099 |
|
| Total | $ | 338,812 |
| | $ | 50,561 |
| | $ | 10,099 |
| | $ | 399,472 |
|
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Remote Cardiac Monitoring Services Revenue (Healthcare segment)
Healthcare segment meetrevenue is generated by remote cardiac monitoring to identify cardiac arrhythmias or heart rhythm disorders and monitoring the definitionfunctionality of implantable cardiac devices. We offer cardiologists, electrophysiologists, neurologists and primary care physicians a contract underfull spectrum of solutions which provides them with a single source of remote cardiac monitoring services.
Performance obligations are determined based on the Standard.nature of the services provided. With our remote cardiac monitoring services, the patient receives the benefits of the service over time, resulting in revenue recognition over time based on the output method. We have elected to applybelieve that this method provides an accurate depiction of the transfer of value over the term of the performance obligation because the level of effort in providing these services is consistent during the service period.
A summary of the payment arrangements with payors is as follows:
| |
| • | Contracted payors (including Medicare): We determine the transaction price based on negotiated prices for services provided, on a case rate basis, as provided for under the relevant Current Procedural Terminology (“CPT”) codes. |
| |
| • | Non-contracted payors: Non-contracted commercial and government insurance carriers often reimburse out of network rates provided for under the relevant CPT codes on a case rate basis. Our transaction price includes implicit price concessions based on our historical collection experience for our non-contracted patients. |
We are utilizing the portfolio approach practical expedient toin ASC 606 for our patient contracts in the Healthcare segment and segment. We account for the contracts within each portfolio as a collective group, rather than individual contracts. Based on our history with these portfolios and the homogenoussimilar nature and characteristics of the patient accountspatients within each portfolio, we have concluded that the financial statement effects are not expected to be materially different than if accounting for revenue based on individual contracts. Ifa contract-by-contract basis.
For the Company hascontracted portfolio, we have historical experience of collecting substantially all of the negotiated contractual
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
rates and the Company has determined at contract inception that these customers have the intention and ability to pay the promised consideration, the Company has concluded that it hasconsideration. As such, we are not providedproviding an implicit price concession but, rather, that it hashave chosen to accept the risk of default, by the patient and adjustments to the transaction price would be presentedare recorded as bad debts. debt expense.
For our non-contracted portfolio, we have determined that we are providing an implicit price concession (a formbecause we do not have a contract with the underlying payor, the result of variable consideration), resulting in the needwhich requires us to continually estimate our transaction price based on historical cash collections utilizing the expected value method. Subsequent adjustments to the transaction price will beare recorded as an adjustment to Healthcare segment revenue and not as bad debt expense. Our current accounting policy is such that
We have not made any significant changes to judgments in applying ASC 606 during the years ended December 31, 2019 and 2018.
Clinical Trial Support and Related Services Revenue (Research segment)
Research segment revenue is generated by providing centralized core laboratory services, including cardiac monitoring, imaging services, scientific consulting and data management services for drug and medical device trials. These amounts are due from pharmaceutical companies and contract research organizations. We bill our customers on a fee for service basis. Under a typical contract, some customers
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
pay us a portion of our fee upon contract execution as an upfront refundable deposit. Upfront deposits are deferred and then recognized upon agreed upon reimbursement rates.as the services are performed. If we do not have agreed upon reimbursement rates, we recognize revenuea contract is canceled prior to service being provided, the upfront deposit is refunded.
Performance obligations are determined based on historical experience, or if no historical experience, when cash is received. Adjustments to the estimated net realizable value, based on final settlement with the third-party payors, are recorded upon settlement.
Research Revenue - We have concluded that our arrangements with customers meet the definition of a contract under the Standard. We are in the process of finalizing our assessment of whether our material promises within our contracts will represent a single or multiple performance obligations, as well as allocationnature of the transaction price toservices provided by us. Our core laboratory services are provided over time as the performance obligation(s). Wecustomer receives benefits resulting in revenue recognition over the term of the contract. Our research customer contracts have determined that the legally enforceable term of our research contractsterms that are predominately thirty days due to termination for convenience clauses, which are held by the customer. customer with no significant penalty. Given the short-term nature of these contracts and the structure of our billing practices, our billing practices approximate our performance if measured by an output method, where each output is an individual occurrence of each performance obligation. Accordingly, we utilize the invoice practical expedient as defined in ASC 606, resulting in recognition of revenue in the amount that we have the right to invoice.
We have not made any significant changes to judgments in applying ASC 606 during the years ended December 31, 2019 and 2018.
Other Revenue (Other category)
Our current accounting policy dictates that ResearchOther category revenue is recognized as the related services are performed. Our revenue is allocated to each element (both delivered and undelivered items) based on their relative selling prices as determined by our best estimate of our selling prices.
Technology Revenue - We determined that contracts within our Technology segment meet the definition of a contract under the Standard and that contracts are predominantly short-term in nature (i.e., approximately 30 daysprimarily derived from receipt of purchase order to shipment). We determined that the promised goods and services within our Technology segment revenue streams are broadly grouped into three categories: (1) the sale of non-invasive cardiac monitors to healthcare companies, wireless blood glucose meters and test strips to wholesale distributors of diabetes supplies and diabetic patients, as well as product repairs. Performance obligations are primarily the sale of devices, related goods producedand repairs provided by the Company (2) constructing, manufacturing, or developing an asset on behalf ofus. These contracts transfer control to a customer and (3) performing an agreed-upon service for a customer. We have determined the following: (1) That all of the transaction price with respect to our customer contracts consists of fixed consideration, (2) that our individual contracts consist of one performance obligation and thus, the allocation of contract consideration to separate performance obligations is not applicable and (3) that we will continue recognizing revenue at a point in time in the Technology segment when control transfers as dictated bybased on the transfer of title onfor the underlying good sold or service. We provide standard warranty provisions.
We determine the transaction price based on fixed consideration in our contractual agreements with our customers and allocate the transaction price to each performance obligation based on the relative stand-alone selling price. We determine the relative stand-alone selling price utilizing our observable prices for the sale of the underlying goods.
We have not made any significant changes to judgments in applying ASC 606 during the years ended December 31, 2019 and 2018.
Contract Assets and Contract Liabilities
ASC 606 requires an entity to present a revenue contract as a contract asset when the entity performs its obligations under the contract by transferring goods or services to a customer before the customer pays consideration or before payment is due. ASC 606 also requires an entity to present a revenue contract as a contract liability in instances when a customer pays consideration, or an entity has a right to an amount of consideration that is unconditional (e.g. receivable), before the entity transfers a good or service to the customer.
As of December 31, 2019 and 2018, we had contract assets of $15.1 million and $2.1 million, respectively, related to cardiac monitoring services, which are rendered.
Transition Method - We will be adopting ASU 2014-09 using the modified retrospective approach.
In addition, the remaining significant implementation matters to be addressed prior to fully adopting ASU 2014-09 include finalizing the transition adjustment analysisincluded as a component of Healthcare accounts receivable on our consolidated financial statements,balance sheets. We also had contract assets of $1.7 million and finalizing updates$0.2 million, respectively, related to our business processes, systems and controls to comply with ASU 2014-09.Other category revenue contracts, which are included as a component of Other accounts receivable on our consolidated balance sheets.
We expect to complete our assessment of the full financial impact of ASU 2014-09 before filing our 10-Q for the three months ended March 31, 2018 which will include the required financial reporting disclosures under ASU 2014-09.
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
3.As of December 31, 2019 and 2018, we had contract liabilities of $1.6 million and $3.1 million, respectively, primarily related to the Research segment where customers paid upfront deposits upon contract execution for future services to be performed by us. If the contract is canceled, these upfront deposits are refundable if service was not yet provided. For the year ended December 31, 2019, the amount recognized as revenue from the contract liability balance as of December 31, 2018 was $2.0 million. Similarly, for the year ended December 31, 2018, the amount recognized as revenue from the contract liability balance as of December 31, 2017 was $3.1 million. Our contract liabilities are included as a component of accrued liabilities on our consolidated balance sheets.
There were no significant changes or impairment losses related to our contract assets and contract liabilities for the years ended December 31, 2019 and 2018.
Practical Expedient Elections
We have elected the following practical expedients in applying ASC 606 across all reportable segments unless otherwise noted below.
Unsatisfied Performance Obligations: Because all of our performance obligations relate to contracts with a duration of less than one year, we have elected to apply the optional exemption provided in ASC 606 and, therefore, are not required to disclose the aggregate amount of the transaction price allocated to performance obligations that are unsatisfied or partially unsatisfied at the end of the reporting period.
Contract Costs: All incremental customer contract acquisition costs are expensed as they are incurred as the amortization period of the asset that we otherwise would have recognized is one year or less in duration.
Significant Financing Component: We do not adjust the promised amount of consideration for the effects of a significant financing component as we expect, at contract inception, that the period between when we transfer a promised good or service to a customer and when the customer pays for that good or service will be one year or less.
Sales Tax Exclusion from the Transaction Price: We exclude from the measurement of the transaction price all taxes assessed by a governmental authority that are both imposed on and concurrent with a specific revenue-producing transaction and collected by us from the customer.
Shipping and Handling Activities: For our other category revenue, we account for shipping and handling activities we perform after a customer obtains control of the good as activities to fulfill the promise to transfer the good.
4. Acquisitions
ADEA Medical AB
During the second quarter of 2019, we acquired all of the remaining outstanding equity of ADEA Medical AB, now known as BioTel Europe AB (“ADEA” or “BioTel Europe”), a limited company incorporated and registered under the laws of Sweden. BioTel Europe provides cardiac monitoring in northern Europe.
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Pursuant to the acquisition agreement, we agreed to issue the owners of ADEA 50,000 shares of our common stock, with a fair value of approximately $2.1 million, as well as to pay $0.2 million in cash. The shares are restricted, with the restrictions related to 10,000 shares that expired in the fourth quarter of 2019, and the restrictions on the remaining 40,000 shares are set to expire in the second quarter of 2022, and the shares are also available to satisfy indemnification obligations.
Prior to the second quarter of 2019, we accounted for our 23.8% stake in ADEA as an equity method investment. We accounted for the acquisition of the remaining equity of ADEA as a step acquisition, which required us to re-measure our previous ownership interest to fair value prior to application of purchase accounting, and we recognized the immaterial difference between the fair value and the carrying value of the equity method investment at that time. The total purchase price of ADEA was $3.3 million, primarily consisting of the equity and cash consideration paid in the second quarter of 2019, plus the amounts paid for our initial investment in ADEA in 2018. We then allocated this purchase price to the assets acquired and liabilities assumed. The acquired net assets consisted primarily of customer relationships and non-compete agreements. The excess of the fair value of the purchase price over the fair value of the net assets acquired has been recognized as goodwill, which represents the expected future benefits arising from the assembled workforce and other synergies attributable to cost savings opportunities. We have recognized $2.6 million of goodwill as a result of the acquisition, all of which has been assigned to the Healthcare segment. NaN of this goodwill will be deductible for tax purposes.
We finalized our fair value estimates related to the ADEA acquisition during the three months ended September 30, 2019. There were no changes to the total purchase price, and the measurement period adjustment related to deferred income taxes recorded during the three months ended September 30, 2019 was not material.
We do not consider this acquisition to be significant to our results of operations. The transaction costs related to this acquisition and revenues and results of operations of ADEA prior to our acquisition were all immaterial.
Geneva Healthcare, Inc.
On March 1, 2019, we acquired Geneva Healthcare, Inc., now known as Geneva Healthcare LLC (“Geneva”), for cash consideration of $45.9 million. In addition, pursuant to the terms of the Agreement and Plan of Merger, dated January 25, 2019, by and among Geneva, BioTelemetry, Inc., Tyersall Merger Sub, Inc., and the Securityholders’ Representative (the “Geneva Agreement”), on the third anniversary of the closing date, the Securityholders (as defined in the Geneva Agreement) are eligible to receive additional consideration in the form of cash payments, as well as shares of BioTelemetry common stock, with a total estimated present value of $32.0 million as of the March 1, 2019 acquisition date, for a total aggregate purchase price of $77.9 million. Concurrent with the closing of the acquisition, the Securityholders have made elections as to the percentage mix of their total additional consideration to be settled in cash or common stock.
The estimated additional consideration of $32.0 million, as of the March 1, 2019 acquisition date, consisted of the following:
| |
| • | The Securityholders will, subject to potential deductions pursuant to the Geneva Agreement, receive additional consideration of $20.0 million, a total of $11.1 million of which will be paid in cash, and the remaining value will be settled in shares. We will issue a total of 131,594 shares |
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
of our common stock to settle the share-related portion of the obligation, based on the elections made by the Securityholders and the formulas within the Geneva Agreement.
| |
| • | The estimated present value of the future cash payment of $11.1 million, which totaled $9.7 million as of the acquisition date, as well as the estimated fair value of our common stock of $9.1 million, has been included within the purchase price for Geneva. The estimated present value of the future cash payment is recorded as a component of other long-term liabilities and will be accreted to its redemption value through interest expense through the payment date. The estimated fair value of the 131,594 shares our common stock has been recorded within paid-in-capital. |
| |
| • | The Securityholders will also be eligible to receive additional consideration, in the form of both cash and shares, based on a predetermined formula that is driven by the future revenues of Geneva and does not have a predetermined limit. The total estimated acquisition-related contingent consideration as of the March 1, 2019 acquisition date was $13.2 million, which is also included in the purchase price of Geneva. The $13.2 million is recorded within other long-term liabilities and will be marked to market through earnings on a quarterly basis throughout the earn-out period. The equity portion of the acquisition-related contingent consideration requires liability classification and mark-to-market accounting pursuant to the provisions of ASC 815 - Derivatives and Hedging. |
We acquired Geneva as part of our business strategy to go deeper and wider into the cardiac monitoring market. Geneva has developed an innovative proprietary cloud-based platform that aggregates data from the leading cardiac device manufacturers, enabling the company to remotely monitor a physician’s patients with implantable cardiac devices such as pacemakers, defibrillators and loop recorders. Geneva’s platform provides physicians a single portal to order patient monitoring, review monitoring results and request routine device checks, helping drive significant in-office efficiencies and patient compliance. We have continued to merge this functionality with that of the Healthcare segment user interface, which we believe will drive greater workflow and data management efficiencies to the clients we serve.
We accounted for the transaction as a business combination, and as such, all assets acquired and liabilities assumed were recorded at their estimated fair values. The excess of the fair value of the purchase price over the fair value of the net assets acquired has been recognized as goodwill, which represents the expected future benefits arising from the assembled workforce and other synergies attributable to cost savings opportunities. We have recognized $59.9 million of goodwill as a result of the acquisition, all of which has been assigned to the Healthcare segment. None of this goodwill will be deductible for tax purposes.
The amounts in the table below represent our final fair value estimates related to the Geneva acquisition as of March 1, 2019. Measurement period adjustments recorded during 2019 consisted primarily of decreasing additional consideration by $2.2 million and increasing net deferred tax assets by $2.9 million. We finalized our fair value estimates related to the Geneva acquisition during the three months ended December 31, 2019.
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
|
| | | | | |
| (in thousands, except years) | March 1,
2019 | | Weighted Average Life (Years) |
| Fair value of assets acquired: | | | |
| Cash and cash equivalents | $ | 1,376 |
| | |
| Healthcare accounts receivable | 1,500 |
| | |
| Prepaid expenses and other current assets | 234 |
| | |
| Identifiable intangible assets: | | | |
| Customer relationships | 3,500 |
| | 12 |
| Technology | 8,900 |
| | 7 |
| Trade names | 2,500 |
| | 15 |
| Total identifiable intangible assets | 14,900 |
| | |
| Deferred tax assets | 1,013 |
| | |
| Total assets acquired | 19,023 |
| | |
| Fair value of liabilities assumed: | | | |
| Accounts payable | 215 |
| | |
| Accrued liabilities | 872 |
| | |
| Total liabilities assumed | 1,087 |
| | |
| | | | |
| Total identifiable net assets | 17,936 |
| | |
| Goodwill | 59,944 |
| | |
| Net assets acquired | $ | 77,880 |
| | |
We have incurred $1.4 million of acquisition related costs associated with Geneva for the year endedDecember 31, 2019. The revenues and income of Geneva for periods prior to our acquisition and for the period from the acquistion date through December 31, 2019 were immaterial to our consolidated operating results.
ActiveCare
On October 2, 2018, we acquired, through our subsidiary Telcare Medical Supply, LLC, certain assets of ActiveCare, Inc. (“ActiveCare”) for $3.8 million in cash. The purchase price also included a potential earn-out payment of $2.0 million, which is contingent on the achievement of certain revenue targets by November 1, 2020. We accounted for the transaction as a business combination, and as such, all assets acquired were recorded at their estimated fair values. The acquired net assets primarily consisted of customer relationships and software developed by ActiveCare. The earn-out was assigned 0 value as of the acquisition date as it was and is currently not probable of achievement. The excess of the fair value of the purchase price over the fair value of the net assets acquired has been recognized as goodwill, has been assigned to the Corporate and Other category and will be deductible for tax purposes. We finalized our fair value estimates related to the ActiveCare acquisition during the three months ended March 31, 2019, and there were no changes to the amounts initially recorded. The transaction costs related to this acquisition and revenues and income of ActiveCare prior to our acquisition were all immaterial.
LifeWatch AG
On July 12, 2017, the Company,we acquired, through itsour wholly owned subsidiary Cardiac Monitoring Holding Company, LLC, acquired approximately 97.0% of the outstanding shares of LifeWatch AG for aggregate consideration of 3,615,840 shares of BioTelemetry common stock with a fair value of $116.8 million and
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
cash in the amount of $165.8 million. On that date, we acquired control of LifeWatch and began consolidating its financial statements.
Through December 31, 2017, we purchased 343,525 additional shares of LifeWatch for cash consideration of $4.8 million and the issuance of 19,806 shares with a fair value of $0.6 million. We acquired the remaining untendered LifeWatch shares pursuant to a squeeze-out procedure in accordance with Swiss law and takeover regulationregulations related to the offering occurring in early January 2018, with the settlement of $2.9 million in cash, which was recorded as a component of accrued liabilities in our consolidated balance sheets, and 58,786 shares of our common stock with a fair market value of $2.0 million, which was recorded as a component of paid-in capital in our consolidated balance sheets, both as of December 31, 2017. As of December 31, 2017, we owned 100% of LifeWatch.LifeWatch.
Also on July 12, 2017, in connection with the closing of the acquisition of LifeWatch, and refinancing of itsour existing debt, we entered into a Credit Agreement pursuant to which the Company obtained loanscredit agreement with SunTrust Bank, as follows; (i) a term loan (funded on July 12, 2017) inlender and an aggregate principal amount equal to $205.0 million, the proceeds of which were used to (a) pay our existing General Electric Credit Agreement of $24.9 million and acquired LifeWatch debt of $3.0 million, (b) pay a portion of the cash considerationagent for the lenders (the “Lenders”) (together, the “SunTrust Credit Agreement”). For more information regarding the financing of this acquisition, of LifeWatch, and (c) pay related transaction fees and expenses of the acquisition of LifeWatch; and (ii) a $50.0 million revolving credit facility for ongoing working capital purposes, which remains undrawn. The term loan will be repaid in quarterly installments beginning January 1, 2018, with the remaining principal balance repaid on or before July 12, 2022.please refer to “Note 11. Credit Agreement.”
The acquisition of LifeWatch strengthens our position as the leader in wireless medicine,remote medical technology, creating the foremost connected health platform, significantly enhancing our ability to improve quality of life and reduce cost of care. We accounted for the transaction as a business combination, and as such, all assets acquired and liabilities assumed were recorded at their estimated fair values. The excess of the fair value of the purchase price over the fair value of the net assets acquired has been recognized as goodwill, which represents the expected future benefits arising from the assembled workforce and other synergies attributable to cost savings opportunities. We recognized $183.5$198.8 million of goodwill as a result of the acquisition, all of which has been assigned to the Healthcare segment. NoneNaN of this goodwill will be deductible for tax purposes.
We finalized our estimates related to the LifeWatch acquisition by July 12, 2018. The amounts below represent our preliminary fair value estimates as of December 31, 2017 and are subject to subsequent adjustment as additional information is obtained during the applicable measurement period. Measurement period adjustments recorded duringin 2018 were primarily due to a $5.7 million adjustment to increase accrued liabilities related to the fourth quarter of 2017 consisted primarily of increasing customer relationships by $17.5ZTech legal matter (see “Note 19. Legal Proceedings” for details) and an $8.9 million increasing acquired technology by $0.9 million, increasingincrease to other long-term liabilities by $21.7 million, decreasing deferred tax liabilities by $7.5 million and decreasing fixed assets by $2.0 million. The primary areas of these preliminary estimates that are not yet finalized related to certain tangible assets acquired and liabilities assumed, including deferred taxes, unrecorded tax provisions and identifiable intangible assets. We expect to finalize all accounting for the acquisition of LifeWatch within one year of the acquisition date.liabilities.
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
|
| | | | | |
| (in thousands, except years) | July 12,
2017 | | Weighted Average Life (Years) |
| Fair value of assets acquired: | | | |
| Cash and cash equivalents | $ | 4,303 |
| | |
| Healthcare accounts receivable | 10,089 |
| | |
| Inventory | 1,136 |
| | |
| Prepaid expenses and other current assets | 3,798 |
| | |
| Property and equipment | 27,507 |
| | |
| Other assets | 713 |
| | |
| Identifiable intangible assets: | | | |
| Customer relationships | 126,800 |
| | 10 |
| Technology | 3,217 |
| | 3 |
| Total identifiable intangible assets | 130,017 |
| | |
| Total assets acquired | 177,563 |
| | |
| Fair value of liabilities assumed: | | | |
| Accounts payable | 10,292 |
| | |
| Accrued liabilities | 15,579 |
| | |
| Current portion of capital lease obligations | 4,664 |
| | |
| Current portion of long-term debt | 3,027 |
| | |
| Long-term capital lease obligations | 3,420 |
| | |
| Deferred tax liabilities | 14,465 |
| | |
| Other long-term liabilities | 32,364 |
| | |
| Total liabilities assumed | 83,811 |
| | |
| | | | |
| Total identifiable net assets | 93,752 |
| | |
| Fair value of noncontrolling interest | (9,961 | ) | | |
| Goodwill | 198,783 |
| | |
| Net assets acquired | $ | 282,574 |
| | |
|
| | | | | |
| (in thousands, except lives) | Amount | | Weighted Average Life (Years) |
| Fair value of assets acquired: | | | |
| Cash and cash equivalents | $ | 4,303 |
| | |
| Healthcare accounts receivable | 9,467 |
| | |
| Inventory | 1,136 |
| | |
| Prepaid expenses and other current assets | 4,392 |
| | |
| Property and equipment | 28,241 |
| | |
| Other assets | 713 |
| | |
| Identifiable intangible assets: | | | |
| Customer relationships | 126,900 |
| | 10 |
| Technology | 3,005 |
| | 3 |
| Total identifiable intangible assets | 129,905 |
| | |
| Total assets acquired | 178,157 |
| | |
| Fair value of liabilities assumed: | | | |
| Accounts payable | 10,424 |
| | |
| Accrued liabilities | 9,747 |
| | |
| Current portion of capital lease obligations | 4,664 |
| | |
| Current portion of long-term debt | 3,027 |
| | |
| Long-term capital lease obligations | 3,420 |
| | |
| Deferred tax liabilities | 14,454 |
| | |
| Other long-term liabilities | 23,435 |
| | |
| Total liabilities assumed | 69,171 |
| | |
| | | | |
| Total identifiable net assets | 108,986 |
| | |
| Fair value of noncontrolling interest | (9,961 | ) | | |
| Goodwill | 183,549 |
| | |
| Net assets acquired | $ | 282,574 |
| | |
We have integrated the operations of LifeWatch which are included as components of into our Healthcare segment. As a result of this integration, it is impracticable to disclose the amount of revenue and earnings/income/(loss) attributable to LifeWatch for the period from July 12, 2017 to December 31, 2017..
We incurred $31.0 million of acquisition related costs related to LifeWatch for the year ended December 31, 2017. These costs were included in other charges in our consolidated statements of operations and comprehensive income/(loss).operations.
The following unaudited pro forma financial information has been prepared using historical financial results of BioTelemetry and LifeWatch as if the acquisition had occurred as of January 1, 2016. Certain adjustments related to the elimination of transaction costs, as well as the addition of depreciation and amortization related to fair value adjustments on the tangible and identifiable intangible assets acquired, have been reflected for the purposes of the unaudited pro forma financial information presented below. We
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
believe the assumptions used in preparing the unaudited pro forma financial information are reasonable, but not necessarily indicative of actual results should the acquisition have occurred on January 1, 2016.
Pro forma financial information for the periods presented is summarized as follows:
|
| | | | | | | | |
| | Year Ended December 31, |
| (pro forma, unaudited, in thousands, except share and per share amounts) | 2017 | | 2016 |
| Revenue | $ | 349,900 |
| | $ | 322,200 |
|
| Net income/(loss) | (1,800 | ) | | 23,400 |
|
| Net income/(loss) per common share: | | | |
| Basic | $ | (0.05 | ) | | $ | 0.74 |
|
| Diluted | $ | (0.05 | ) | | $ | 0.69 |
|
| Weighted average number of common shares outstanding: | | | |
| Basic | 34,022 |
| | 31,556 |
|
| Diluted | 34,022 |
| | 34,125 |
|
Telcare, Inc.
On December 1, 2016, the Company, through its wholly owned subsidiary BioTelemetry Care Management, LLC, entered into the Agreement with Telcare pursuant to which the Company acquired the stock of Telcare Medical Supply, Inc. and certain assets of Telcare Inc. The total consideration paid at closing amounted to $7.0 million in cash, with the potential for a performance-based earn out up to $5.0 million upon reaching certain revenue milestones. The fair value of the total consideration transferred in the acquisition, including the fair value of the contingent consideration, was $9.7 million at the acquisition date.
The acquisition of Telcare provides us the opportunity to apply our expertise in remote monitoring to the diabetes market and increases our presence in the digital population health management market. We accounted for the transaction as a business combination, and as such, all assets acquired and liabilities assumed were recorded at their estimated fair values. The excess of the fair value of the purchase price over the fair value of the net assets acquired has been recognized as goodwill, which represents the expected future benefits arising from the assembled workforce and other synergies attributable to cost savings opportunities. We recognized $2.2 million of goodwill as a result of the acquisition, all of which has been assigned to the Technology segment. We expect $0.3 million of this goodwill will be deductible for tax purposes.
The amounts below represent our final fair value estimates, which were completed in the fourth quarter ending December 31, 2017. Measurement period adjustments reducing the valuation of inventory of $0.3 million and $0.1 million were recorded in the second and third quarters of 2017, respectively, and a $1.5 million adjustment, increasing deferred tax assets and reducing deferred revenue, prior to completing our valuation during the fourth quarter of 2017.
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The total consideration and related allocation for Telcare is summarized as follows:
|
| | | | | |
| (in thousands, except lives) | Amount | | Weighted Average Life (Years) |
| Fair value of assets acquired: | | | |
| Other accounts receivable | $ | 235 |
| | |
| Inventory | 1,417 |
| | |
| Prepaid expenses and other current assets | 1,261 |
| | |
| Property and equipment | 55 |
| | |
| Other assets | 933 |
| | |
| Deferred tax assets | 1,463 |
| | |
| Identifiable intangible assets: | | | |
| Customer relationships | 400 |
| | 5 |
| Technology | 2,000 |
| | 5 |
| Tradename | 400 |
| | Indefinite |
| Total identifiable intangible assets | 2,800 |
| | |
| Total assets acquired | 8,164 |
| | |
| Fair value of liabilities assumed: | | | |
| Accounts payable | 459 |
| | |
| Accrued liabilities | 206 |
| | |
| Total liabilities assumed | 665 |
| | |
| Total identifiable net assets | 7,499 |
| | |
| Goodwill | 2,201 |
| | |
| Net assets acquired | $ | 9,700 |
| | |
The acquisition has been included within the consolidated results of operations and financial condition from the date of the acquisition.
The following unaudited pro forma financial information has been prepared using historical financial results of BioTelemetry and Telcare as if the acquisition had occurred as of January 1, 2015. Certain adjustments related to the elimination of transaction costs, as well as the addition of depreciation and amortization related to fair value adjustments on the tangible and identifiable intangible assets acquired, have been reflected for the purposes of the unaudited pro forma financial information presented below. We believe the assumptions used in preparing the unaudited pro forma financial information are reasonable, but not necessarily indicative of actual results should the acquisition have occurred on January 1, 2015.2016.
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Pro forma financial information for the periods presentedyear ended December 31, 2017 is summarized as follows:
|
| | | | |
| | Year Ended December 31, |
| (pro forma, unaudited, in thousands, except per share amounts) | 2017 |
| Revenue | $ | 349,900 |
|
| Net loss | (1,800 | ) |
| Net loss per common share: | |
| Basic | $ | (0.05 | ) |
| Diluted | $ | (0.05 | ) |
| Weighted average number of common shares outstanding: | |
| Basic | 34,022 |
|
| Diluted | 34,022 |
|
|
| | | | | | | | |
| (pro forma, unaudited, in thousands, except per share amounts) | Year Ended December 31,
2016 | | Year Ended December 31,
2015 |
| Revenue | $ | 212,538 |
| | $ | 182,755 |
|
| Net income | 50,693 |
| | 948 |
|
| Net income per common share: | | | |
| Basic | $ | 1.82 |
| | $ | 0.03 |
|
| Diluted | $ | 1.66 |
| | $ | 0.03 |
|
The Agreement includes the potential for a performance-based earn out up to $5.0 million upon reaching certain milestones. The fair value of the contingent consideration associated with the Telcare acquisition was $2.7 million as of the acquisition date and was included as a component of other liabilities in the accompanying consolidated balance sheets. For further details regarding contingent consideration, refer to “Note 5. Fair Value Measurements” below.
VirtualScopics, Inc.
On March 25, 2016, the Company, through its wholly owned subsidiary BioTelemetry Research Acquisition Corporation, entered into a definitive Agreement and Plan of Merger (“Merger Agreement”) with VirtualScopics, Inc. (“VirtualScopics”), a leading provider of clinical trial imaging solutions. Under the terms of the Merger Agreement, we purchased: (i) any and all outstanding shares of VirtualScopics’ $0.001 par value common stock for $4.05 per share; (ii) any and all outstanding shares of VirtualScopics’ $0.001 par value Series A and Series B Convertible Preferred Stock for $336.30 per share; and (iii) any and all outstanding shares of VirtualScopics’ $0.001 par value Series C-1 Convertible Preferred Stock for $920.00 per share. The all cash acquisition of VirtualScopics was completed on May 11, 2016. The total consideration paid at closing amounted to $15.0 million, net of cash acquired of $0.8 million.
The acquisition of VirtualScopics expands our existing clinical research offerings and gives us further access to established customer relationships. We accounted for the transaction as a business combination, and as such, all assets acquired and liabilities assumed were recorded at their estimated fair values. The excess of the consideration paid over the fair value of the net assets acquired has been recognized as goodwill, which represents the expected future benefits arising from the assembled workforce and other synergies attributable to cost savings opportunities. We recognized $4.3 million of goodwill as a result of the acquisition, all of which has been assigned to the Research segment. None of this goodwill will be deductible for tax purposes.
The amounts below represent our final fair value estimates, which were completed in the second quarter of 2017. Measurement period adjustments were recorded in the fourth quarter of 2016 related to the recognition of a $0.3 million deferred tax liability, and in the second quarter of 2017 primarily to recognize $0.3 million of deferred tax assets resulting from state net operating losses.
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The total consideration and related allocation for VirtualScopics is summarized as follows:
|
| | | | | |
| (in thousands, except lives) | Amount | | Weighted Average Life (Years) |
| Fair value of assets acquired: | | | |
| Cash and cash equivalents | $ | 849 |
| | |
| Other accounts receivable | 3,679 |
| | |
| Inventory | 111 |
| | |
| Prepaid expenses and other current assets | 396 |
| | |
| Property and equipment | 500 |
| | |
| Deferred taxes | 20 |
| | |
| Identifiable intangible assets: | | | |
| Customer relationships | 5,200 |
| | 12 |
| Technology | 2,000 |
| | 10 |
| Backlog | 3,100 |
| | 4 |
| Total identifiable intangible assets | 10,300 |
| | |
| Total assets acquired | 15,855 |
| | |
| Fair value of liabilities assumed: | | | |
| Accounts payable | 325 |
| | |
| Accrued liabilities | 2,945 |
| | |
| Current portion of capital lease obligations | 59 |
| | |
| Current portion of long-term debt | 91 |
| | |
| Deferred revenue | 700 |
| | |
| Long-term capital lease obligations | 162 |
| | |
| Long-term debt | 97 |
| | |
| Total liabilities assumed | 4,379 |
| | |
| Total identifiable net assets | 11,476 |
| | |
| Goodwill | 4,343 |
| | |
| Net assets acquired | $ | 15,819 |
| | |
The acquisition has been included within the consolidated results of operations and financial condition from the date of the acquisition. For the period from May 11, 2016 to December 31, 2016, VirtualScopics contributed revenue of approximately $12.3 million and net income of approximately $1.4 million to our consolidated results of operations.
The following unaudited pro forma financial information has been prepared using historical financial results of BioTelemetry and VirtualScopics as if the acquisition had occurred as of January 1, 2015. Certain adjustments related to the elimination of transaction costs and acquisition-related indebtedness, as well as the addition of depreciation and amortization related to fair value adjustments on the tangible and identifiable intangible assets acquired, have been reflected for the purposes of the unaudited pro forma financial information presented below. No adjustments for synergies or certain other expected benefits of the acquisition have been included. We believe the assumptions used in preparing the unaudited pro forma
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
financial information are reasonable, but not necessarily indicative of actual results should the acquisition have occurred on January 1, 2015.
Pro forma financial information for the periods presented is summarized as follows:
|
| | | | | | | |
| (pro forma, unaudited, in thousands, except per share amounts) | Year Ended December 31,
2016 | | Year Ended December 31,
2015 |
| Revenue | $ | 214,271 |
| | $ | 191,230 |
|
| Net income | 55,413 |
| | 7,232 |
|
| Net income per common share: | | | |
| Basic | $ | 1.98 |
| | $ | 0.27 |
|
| Diluted | $ | 1.82 |
| | $ | 0.25 |
|
ePatch Division of DELTA Danish Electronics, Light, and Acoustics
On April 1, 2016, we, through our wholly owned subsidiary BioTelemetry Technology ApS, entered into an Asset Purchase Agreement (“APA”) with DELTA, pursuant to which we acquired substantially all of the assets of the ePatch division of DELTA, inclusive of all products and indications currently under development. The total consideration paid at closing amounted to $3.0 million in cash and 244,519 shares of our common stock valued at $2.9 million. In addition, there is the potential for a performance-based earn out up to $3.0 million upon reaching certain regulatory and revenue milestones, as defined in the APA. The fair value of the total consideration transferred in the ePatch acquisition, including the fair value of the contingent consideration, was $6.5 million at the acquisition date.
The ePatch acquisition is expected to generate future cost savings for us and will provide control over proprietary components for our next generation MCT device. We accounted for the transaction as a business combination, and as such, all assets acquired and liabilities assumed were recorded at their estimated fair values. The excess of the fair value of the purchase price over the fair value of the net assets acquired has been recognized as goodwill, which represents the expected future benefits arising from the assembled workforce and other synergies attributable to cost savings opportunities. We recognized $3.2 million of goodwill as a result of the acquisition, all of which has been assigned to the Technology segment, and we expect all of this goodwill to be deductible for tax purposes.
The amounts below represent our final fair value estimates, which we completed in the first quarter of 2017. During the fourth quarter of 2016, we reduced the allocation to the technology intangible asset by $0.2 million as a result of additional information obtained during the measurement period.
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The total consideration and related allocation for the ePatch acquisition is summarized as follows:
|
| | | | | |
| (in thousands, except lives) | Amount | | Weighted Average Life (Years) |
| Fair value of assets acquired: | | | |
| Inventory | $ | 100 |
| | |
| Property and equipment | 175 |
| | |
| Identifiable intangible assets: | | | |
| Customer relationships | 400 |
| | 10 |
| Technology | 2,800 |
| | 10 |
| Trade names | 100 |
| | Indefinite |
| Total identifiable intangible assets | 3,300 |
| | |
| Total assets acquired | 3,575 |
| | |
| Fair value of liabilities assumed: | | | |
| Accrued liabilities | 266 |
| | |
| Total liabilities assumed | 266 |
| | |
| Total identifiable net assets | 3,309 |
| | |
| Goodwill | 3,181 |
| | |
| Net assets acquired | $ | 6,490 |
| | |
While the ePatch acquisition provides control over proprietary components of our next generation cardiac monitoring device, the acquisition did not have a material effect on our consolidated results of operations.
The APA includes the potential for a performance-based earn out up to $3.0 million upon reaching certain regulatory and revenue milestones. The fair value of the contingent consideration associated with the ePatch acquisition was $0.6 million as of the acquisition date and was included as a component of other liabilities in the accompanying consolidated balance sheets. For further details regarding contingent consideration, refer to “Note 5. Fair Value Measurements” below.
4.5. Inventory
Inventory consists of the following:
|
| | | | | | | |
| | December 31, |
| (in thousands) | 2019 | | 2018 |
| Raw materials and supplies | $ | 4,429 |
| | $ | 3,667 |
|
| Finished goods | 1,309 |
| | 3,656 |
|
| Total inventory | $ | 5,738 |
| | $ | 7,323 |
|
|
| | | | | | | |
| | December 31, |
| (In thousands) | 2017 | | 2016 |
| Raw materials and supplies | $ | 3,128 |
| | $ | 2,866 |
|
| Finished goods | 2,204 |
| | 2,310 |
|
| Total inventory | $ | 5,332 |
| | $ | 5,176 |
|
Inventory, which includes purchased parts, materials, direct labor and applied manufacturing overhead, is stated at the lower of cost or net realizable value, with cost determined by use of the first-in, first-out method.
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
5.6. Fair Value Measurements
The fair value of our liabilities measured at fair value on a recurring basis is summarized as follows:
|
| | | | | | | | | | | | | |
| (in thousands) | Balance at December 31,
2017 | | Level 1 | | Level 2 | | Level 3 |
| Liabilities | | | | | | | |
| Contingent consideration | $ | 700 |
| | — |
| | — |
| | $ | 700 |
|
|
| | | | | | | | | | | | | |
| (in thousands) | Balance at December 31,
2016 | | Level 1 | | Level 2 | | Level 3 |
| Liabilities | | | | | | | |
| Contingent consideration | $ | 3,305 |
| | — |
| | — |
| | $ | 3,305 |
|
We have determined that our long termlong-term debt, classified as Level 2, has a fair value consistent with its carrycarrying value, exclusive of debt discount and deferred charges, of $199.4$194.7 million and $25.2$198.5 million as of December 31, 20172019 and 2016,2018, respectively.
ContingentAcquisition-related contingent consideration represents our contingent milestone payment obligations related to our acquisitions and is measured at fair value, based on significant inputs not observable in the market, which represents a Level 3 measurement within the fair value hierarchy. The valuation of acquisition-related contingent consideration uses assumptions we believe would be made by a market participant. We assess these estimates on an ongoing basis as additional data impacting the assumptions is obtained. The balances of the fair value of acquisition-related contingent consideration are recognized within other long-term liabilities on our consolidated balance sheets. AdjustmentsChanges in the fair value of the acquisition-related contingent consideration, after the final determination as of the acquistion date, resulting from changes in the variables used to contingent considerationcompute the fair value, are recorded in other charges in the consolidated statements of operations and comprehensive income/(loss).operations.
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The following table provides a reconciliation of the beginning and ending balances of acquisition-related contingent payments associatedconsideration:
|
| | | | | | | |
| | Year Ended December 31, |
| (in thousands) | 2019 | | 2018 |
| Beginning balance | $ | — |
| | $ | 700 |
|
| Additional acquisition-related contingent consideration | 13,170 |
| | — |
|
| Changes in fair value of contingent consideration | (230 | ) | | (700 | ) |
| Ending balance | $ | 12,940 |
| | $ | — |
|
In conjunction with acquisitions during the years endedGeneva acquisition, we recognized $13.2 million of acquisition-related contingent consideration on March 1, 2019 as a component of other long-term liabilities as the contingency will be finalized after the third anniversary of the closing date. There was no value assigned to the acquisition-related contingent consideration related to the ActiveCare acquisition as the achievement of the contingency was not probable as of December 31, 20172019 and December 31, 2016:2018.
The estimated fair value of the acquisition-related contingent consideration related to the Geneva acquisition was estimated using a Monte Carlo simulation, that considered numerous variables, including estimated projected revenues, future stock price, discount rates and discounts for lack of marketability of common stock. These estimates are subject to a significant level of judgment. |
| | | | | | | |
| | Year ended |
| (in thousands) | December 31,
2017 | | December 31,
2016 |
| Beginning balance | $ | 3,305 |
| | $ | — |
|
| Purchase price contingent consideration | — |
| | 3,305 |
|
| Changes in fair value of contingent consideration | (2,605 | ) | | — |
|
| Ending balance | $ | 700 |
| | $ | 3,305 |
|
During the year ended December 31, 2017,2018, the fair value of the contingent consideration related to the ePatch acquisition decreased $0.6$0.7 million, as it iswas no longer probable that anycertain of the contingencies will be met. Additionally during the year, the fair value of the contingent consideration related to the Telcare acquisition was reduced by $2.0 million as a result of reducing the probability of attaining all the revenue contingencies.would be met.
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
6.7. Property and Equipment
Property and equipment consists of the following:
|
| | | | | | | | | |
| | Estimated Useful Life (Years) | | December 31, |
| (in thousands, except years) | | 2019 | | 2018 |
| Cardiac monitoring devices, device parts and components | 3 - 5 | | $ | 87,431 |
| | $ | 76,088 |
|
| Computers and purchased software | 3 - 5 | | 18,756 |
| | 16,800 |
|
| Equipment, tools and molds | 3 - 5 | | 6,492 |
| | 6,441 |
|
| Furniture, fixtures and other | 5 - 7 | | 4,582 |
| | 3,805 |
|
| Leasehold improvements | * | | 7,714 |
| | 5,877 |
|
| Equipment under finance leases | * | | 7,500 |
| | 6,568 |
|
| Total property and equipment, at cost | | | 132,475 |
| | 115,579 |
|
| Less accumulated depreciation | | | (76,095 | ) | | (67,202 | ) |
| Total property and equipment, net | | | $ | 56,380 |
| | $ | 48,377 |
|
| * shorter of useful life or term of lease | | | | | |
|
| | | | | | | | | |
| | Estimated Useful Life (Years) | | December 31, |
| (In thousands, except years) | | 2017 | | 2016 |
| Cardiac monitoring devices, device parts and components | 3 - 5 | | $ | 76,039 |
| | $ | 55,825 |
|
| Computers and purchased software | 3 - 5 | | 22,357 |
| | 18,027 |
|
| Equipment, tools and molds | 3 - 5 | | 7,857 |
| | 6,666 |
|
| Furniture, fixtures and other | 5 - 7 | | 2,104 |
| | 1,467 |
|
| Leasehold improvements | * | | 5,434 |
| | 3,171 |
|
| Capital leases | 3 - 7 | | 7,305 |
| | 737 |
|
| Total property and equipment, at cost | | | 121,096 |
| | 85,893 |
|
| Less accumulated depreciation | | | (71,902 | ) | | (60,070 | ) |
| Total property and equipment, net | | | $ | 49,194 |
| | $ | 25,823 |
|
| * shorter of useful life or term of lease | | | | | |
Depreciation expense associated with property and equipment, inclusive of amortization of assets recorded under capitalfinance leases, was $18.3$24.7 million, $10.5$23.0 million and $9.0$18.3 million, for the years ended December 31, 2017, 20162019, 2018 and 2015,2017, respectively.
During the year ended December 31, 2017, considering the LifeWatch integration and forward-looking integration plans, we determined that certain software ceased being used and was no longer going to be used and was
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
therefore impaired, resulting in $1.1 million of impairment charges included within the Corporate and Other segmentcategory as a component of other costs within the other charges line in our consolidated statements of operations and comprehensive income/(loss).operations. There were no fixed asset impairments for the yearyears ended December 31, 2016.2019 and 2018.
7.8. Goodwill and Intangible Assets
Goodwill was recognized at the time of our acquisitions. The following table presents the carrying amount of goodwill allocated to our reportable segments, as well as the changes to goodwill during the years ended December 31, 20172019 and 2016:2018:
|
| | | | | | | | | | | | | | | |
| | Reporting Segment | | Corporate and Other | | |
| (in thousands) | Healthcare | | Research | | | Total |
| Balance at December 31, 2017 | $ | 198,273 |
| | $ | 16,293 |
| | $ | 8,539 |
| | $ | 223,105 |
|
| Initial goodwill acquired | — |
| | — |
| | 475 |
| | 475 |
|
| Measurement period adjustments | 15,234 |
| | — |
| | — |
| | 15,234 |
|
| Balance at December 31, 2018 | 213,507 |
| | 16,293 |
| | 9,014 |
| | 238,814 |
|
| Initial goodwill acquired | 62,516 |
| | — |
| | — |
| | 62,516 |
|
| Currency translation | (9 | ) | | — |
| | — |
| | (9 | ) |
| Balance at December 31, 2019 | $ | 276,014 |
| | $ | 16,293 |
| | $ | 9,014 |
| | $ | 301,321 |
|
|
| | | | | | | | | | | | | | | |
| | Reporting Segment |
| (in thousands) | Healthcare | | Research | | Technology | | Total |
| Balance at December 31, 2015 | $ | 14,724 |
| | $ | 11,950 |
| | $ | 3,157 |
| | $ | 29,831 |
|
| Initial goodwill acquired | — |
| | 4,633 |
| | 6,171 |
| | 10,804 |
|
| Measurement period adjustments | — |
| | 60 |
| | 373 |
| | 433 |
|
| Balance at December 31, 2016 | 14,724 |
| | 16,643 |
| | 9,701 |
| | 41,068 |
|
| Initial goodwill acquired | 186,456 |
| | — |
| | — |
| | 186,456 |
|
| Measurement period adjustments | (2,907 | ) | | (350 | ) | | (1,162 | ) | | (4,419 | ) |
| Balance at December 31, 2017 | $ | 198,273 |
| | $ | 16,293 |
| | $ | 8,539 |
| | $ | 223,105 |
|
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The goodwill acquired and the measurement period adjustments in the Healthcare segment isare primarily due to the ADEA, Geneva and LifeWatch acquisition; acquisitions; Research segment goodwill relates to the 2016 VirtualScopics acquisition; Technologythe Corporate and Other category goodwill primarily represents our ePatch2018 ActiveCare acquisition and our 2016 Telcare and ePatch acquisitions. Refer to “Note 3.4. Acquisitions” above for details related to the GenevaandLifeWatchmeasurement period adjustments.
At December 31, 2017, 20162019, 2018 and 2015,2017, we performed our required annual impairment test of goodwill. Based on these impairment tests, we determined that there waswere no goodwill impairment.impairments. The carrying amount of our goodwill as of December 31, 20172019 and 20162018 was $223.1$301.3 million and $41.1$238.8 million, respectively.
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The gross carrying amounts and accumulated amortization of our intangible assets as of December 31, 20172019 and 20162018 are as follows:
|
| | | | | | | | | |
| | Weighted Average Life (Years) | | December 31, |
| (in thousands, except years) | | 2019 | | 2018 |
| Gross Carrying Value | | | | | |
| Customer relationships | 10.3 | | $ | 149,420 |
| | $ | 146,200 |
|
| Technology including internally developed software | 6.7 | | 21,892 |
| | 18,078 |
|
| Backlog | 4.0 | | 3,100 |
| | 6,860 |
|
| Trade names | 15.0 | | 2,500 |
| | — |
|
| Covenants not to compete | 4.7 | | 424 |
| | 1,040 |
|
| Total intangible assets, gross | | | 177,336 |
| | 172,178 |
|
| Accumulated Amortization | | | | | |
| Customer relationships | | | (38,270 | ) | | (24,870 | ) |
| Technology including internally developed software | | | (6,153 | ) | | (10,879 | ) |
| Backlog | | | (2,842 | ) | | (5,827 | ) |
| Trade names | | | (138 | ) | | — |
|
| Covenants not to compete | | | (337 | ) | | (949 | ) |
| Total accumulated amortization | | | (47,740 | ) | | (42,525 | ) |
| Total intangible assets, net | | | $ | 129,596 |
| | $ | 129,653 |
|
|
| | | | | | | | | |
| | Estimated Useful Life (Years) | | December 31, |
| (In thousands, except years) | | 2017 | | 2016 |
| Customer relationships | 5 - 15 | | $ | 143,174 |
| | $ | 16,700 |
|
| Technology including internally developed software | 3 - 10 | | 15,953 |
| | 21,135 |
|
| Backlog | 1 - 4 | | 6,860 |
| | 6,860 |
|
| Covenants not to compete | 5 - 7 | | 1,040 |
| | 1,040 |
|
| Total intangible assets, gross | | | 167,027 |
| | 45,735 |
|
| Customer relationships | | | (10,868 | ) | | (3,809 | ) |
| Technology including internally developed software | | | (8,573 | ) | | (6,588 | ) |
| Backlog | | | (5,052 | ) | | (4,176 | ) |
| Covenants not to compete | | | (827 | ) | | (690 | ) |
| Total accumulated amortization | | | (25,320 | ) | | (15,263 | ) |
| Indefinite-lived trade names | | | — |
| | 3,000 |
|
| Total intangible assets, net | | | $ | 141,707 |
| | $ | 33,472 |
|
During our intangible asset impairment testing for the year ended December 31, 2017, considering2019, we wrote off certain fully amortized intangible assets, primarily technology and backlog, and incurred an immaterial amount of foreign currency translation impact related to the LifeWatch integrationcustomer relationships and forward-looking integration plans, we determined that certain trade names and internally developed software costs ceased being used and were no longer goingcovenants not to be used and were therefore impaired, resulting in $11.0 million of intangible asset impairment charges included withincompete related to the Corporate and Other segment as a component of other costs within the other charges line in our consolidated statements of operations and comprehensive income/(loss). There were no other intangible asset impairments for the year ended December 31, 2017.
At December 31, 2016 and 2015, we performed our required annual impairment test of indefinite-lived intangible assets. Based on these impairment tests, we determined that there was no impairment.
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
BioTel Europe acquisition.
The estimated amortization expense for finite-lived intangible assets for the next five years and thereafter is summarized as follows at December 31, 2017:2019:
|
| | | | |
| (in thousands) | |
| 2020 | $ | 18,290 |
|
| 2021 | 17,925 |
|
| 2022 | 17,417 |
|
| 2023 | 16,959 |
|
| 2024 | 16,427 |
|
| Thereafter | 42,578 |
|
| Total estimated amortization | $ | 129,596 |
|
|
| | | | |
| (in thousands) | |
Fiscal Year | |
| 2018 | $ | 16,718 |
|
| 2019 | 16,231 |
|
| 2020 | 15,420 |
|
| 2021 | 14,687 |
|
| 2022 | 14,238 |
|
| Thereafter | 64,413 |
|
| Total estimated amortization | $ | 141,707 |
|
Amortization expense for the years ended December 31, 2019, 2018 and 2017 2016 and 2015 was $10.2$18.3 million, $3.7$17.2 million and $3.5$10.2 million, respectively. The 2017 amortization expense excludes impairment charges of $3.0 million related to indefinite-lived trade names and $8.0 million related to developed technology and customer relationships. See “Note. 12Note 13. Other Charges” below.
8. Equity Method Investment
In December 2015, we acquired an ownership interest in Well Bridge Health, Inc. (“WellBridge”) through the conversion of an outstanding note receivable and the related accrued interest. The investment is accounted for under the equity method. In December 2015, the equity method basis difference of $0.9 million was allocated to equity method goodwill. Our Chief Executive Officer sits on Wellbridge’s Board of Directors, and therefore WellBridge is considered a related party. Except for our continued investment in WellBridge through capital contributions, there There were no related party transactions.
As ofintangible asset impairments for the years ended December 31, 2017, our investment in WellBridge represented 32.1% of its outstanding stock. A summary of our investment in Wellbridge is as follows:2019 and 2018.
|
| | | | | | | |
| | Year ended December 31, |
| (in thousands) | 2017 | | 2016 |
| Beginning balance | $ | 1,125 |
| | $ | 1,100 |
|
| Capital contributions | 690 |
| | 312 |
|
| Loss in equity method investment | (384 | ) | | (287 | ) |
| Ending balance | $ | 1,431 |
| | $ | 1,125 |
|
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
9. Equity Method Investments
On October 31, 2018, we acquired an ownership interest in ADEA for approximately $0.9 million. This investment was accounted for under the equity method. During the second quarter of 2019, we acquired all of the remaining outstanding equity of ADEA. In conjunction with this step acquisition, we derecognized our equity method investment in ADEA and recognized the fair value of the assets acquired and liabilities assumed related to ADEA in our consolidated financial statements. For more information, see “Note 4. Acquisitions.”
In December 2015, we acquired an ownership interest in Wellbridge Health, Inc. (“Wellbridge”). The investment is accounted for under the equity method. Our Chief Executive Officer sits on Wellbridge’s board of directors, and therefore, Wellbridge is considered a related party. There were no material related-party transactions between the parties during the years ended December 31, 2019, 2018 and 2017. As of December 31, 2019, our investment in Wellbridge represented 32.2% of their outstanding stock.
During the fourth quarter of 2019, as part of our review of Wellbridge’s financial results, their forward-looking business plan and outlook, we determined that there was an other-than-temporary impairment of our investment. As a result, we recorded a non-cash impairment charge of $1.0 million to write-off the carrying value of our investment. This charge is included as a component of loss on equity method investments in our consolidated statement of operations for the year ended December 31, 2019.
A summary of our investments, recorded as a component of other assets in our consolidated balance sheets, is as follows:
|
| | | | | | | |
| | Year Ended December 31, |
| (in thousands) | 2019 | | 2018 |
| Beginning balance | $ | 2,044 |
| | $ | 1,431 |
|
| Capital contributions | — |
| | 859 |
|
| Derecognition of ADEA investment | (746 | ) | | — |
|
| Loss on equity method investments | (1,298 | ) | | (246 | ) |
| Ending balance | $ | — |
| | $ | 2,044 |
|
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
10. Accrued Liabilities
Accrued liabilities consists of the following:
|
| | | | | | | |
| | December 31, |
| (in thousands) | 2017 | | 2016 |
| Accrued compensation | $ | 13,086 |
| | $ | 7,831 |
|
| Accrued professional fees | 1,587 |
| | 2,841 |
|
| Accrued squeeze-out | 2,885 |
| | — |
|
| Accrued restructuring | 1,605 |
| | — |
|
| Accrued non-income taxes | 588 |
| | 250 |
|
| Accrued interest | 306 |
| | 330 |
|
| Other | 7,300 |
| | 2,446 |
|
| Total | $ | 27,357 |
| | $ | 13,698 |
|
|
| | | | | | | |
| | December 31, |
| (in thousands) | 2019 | | 2018 |
| Compensation | $ | 13,167 |
| | $ | 13,443 |
|
| Right-of-use liabilities - operating leases | 5,187 |
| | — |
|
| Professional fees | 4,128 |
| | 4,260 |
|
| Contract liabilities | 1,584 |
| | 3,080 |
|
| Non-income taxes | 427 |
| | 906 |
|
| Interest | 569 |
| | 702 |
|
| Operating costs | 637 |
| | 1,095 |
|
| Other | 1,619 |
| | 1,203 |
|
| Total | $ | 27,318 |
| | $ | 24,689 |
|
10.11. Credit Agreement
2017 SunTrust Credit AgreementsAgreement
Concurrent with the acquisition of LifeWatch as discussed in “Note 3.4. Acquisitions” above, we entered into a credit agreement with the SunTrust Bank, as a lender and an agent for the lenders (the “Lenders”) (together, the “SunTrust Credit Agreement”)Agreement. Pursuant to the credit agreement,SunTrust Credit Agreement, the Lenders agreed to make loans to the Companyus as follows;follows: (i) a term loan in an aggregate principal amount equal to $205.0 million; and (ii) a $50.0 million revolving credit facility for ongoing working capital purposes. The proceeds of the loans were used to pay our existing prior GE Credit Agreement of $24.9 million and acquired LifeWatch debt of $3.0 million, pay a portion of the consideration for the acquisition of LifeWatch and pay related transaction fees and expenses of the acquisition of LifeWatch.LifeWatch.
The loans bear interest at an annual rate, at theour election, of the Company, of (i) with respect to LIBOR rate loans, LIBOR plus the applicable margin and (ii) with respect to base rate loans, the Base Rate (the “prime rate” as published in the Wall Street Journal plus the applicable margin). The applicable margin for both LIBOR and Base Rate loans is determined by reference to the Company’sour Consolidated Total Net Leverage Ratio, as defined in the credit agreement.SunTrust Credit Agreement. As of December 31, 2017,2019, the applicable margin is 2.00%1.50% for LIBOR loans and 1.00%0.50% for base rate loans.
The outstanding principal of the loan willis scheduled, in accordance with the SunTrust Credit Agreement, to be paid as follows:
Beginning January 1, 2018, the principal amount of the term loan will be repaid, on a quarterly basis, in installments of approximately $0.5 million, plus accrued interest;
Beginning January 1, 2019, the principal amount of the term loan will be repaid, on a quarterly basis, in installments of approximately $1.3 million, plus accrued interest;
BeginningBeginning January 1, 2020, the principal amount of the term loan will be repaid, on a quarterly basis, in installments of approximately $3.8 million, plus accrued interest;
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Beginning January 1, 2021, the principal amount of the term loan will be repaid, on a quarterly basis, in installments of approximately $5.1 million, plus accrued interest;
| |
| • | The remaining principal balance is scheduled to be repaid on or before July 12, 2022 (or such earlier date upon an acceleration of the loans by Lenders upon an event of default or by our termination). |
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The remaining principal balance will be repaid on or before July 12, 2022 (or such earlier date upon an acceleration of the loans by Lenders upon an event of default or termination by the Company).
The loans are secured by substantially all of theour assets of the Company and by a pledge of theour capital stock of the Company’s U.S. based subsidiaries as well as a pledge of 65% of the capital stock of itsour first tier material foreign subsidiaries, including 65% of the capital stock the Company ownswe own of LifeWatch.LifeWatch.
The carrying amount of the term loan was $199.4$194.7 million as of December 31, 2017,2019, which is the principal amount outstanding, net of $5.6$3.2 million of unamortized deferred financing costs to be amortized over the remaining term of the credit facility. The revolving credit facility is subject to an unused commitment fee, which is determined by reference to the our Consolidated Total Net Leverage Ratio, as defined in the credit agreement.SunTrust Credit Agreement. Our unused commitment fee as of December 31, 20172019 was 0.3%0.2% and the revolving credit facility remains undrawn as of that date.
2014 GE Credit Agreement
On December 30, 2014, we entered into a Credit Agreement with Healthcare Financial Solutions, LLC, (“HFS”HFS”), previously The General Electric Capital Corporation (“GE Capital”Capital”), as agent for the lenders, (“Lenders”), and as a lender and swingline lender (the “General Electric“GE Credit Agreement”Agreement”). Pursuant to the General ElectricGE Credit Agreement, the Lenderslenders agreed to make loans to us as follows: (i) Term Loans in an amount of $25.0 million as of the closing date with an uncommitted ability to increase such Term Loans up to an amount not to exceed $10.0 million and (ii) Revolving Loans up to $15.0 million. As of December 31, 2016, $3.0 million was drawn on the Revolving Loans.
Covenants
The loan, inclusive of Term Loans and Revolving Loans, was recorded on our consolidated balance sheet as of December 31, 2016 in the amount of $25.2 million, which is net of a debt discount and deferred charges of $0.7 million.
The loans bore interest at an annual rate of LIBOR plus 4.0%, subject to a LIBOR floor of 1.0%. The outstanding principal of the Term Loans was to be paid as follows:
beginning April 1, 2015, the principal amount of the Term Loans were repaid, on a quarterly basis, in installments of $0.3 million, plus accrued interest;
The loan was secured by substantially all of our assets and by a pledge of the capital stock of our U.S. based subsidiaries as well as a pledge of 65% of the capital stock of our foreign subsidiaries. As noted above, this agreement was paid off with the proceeds of the SunTrust Credit Agreement in 2017.
Covenants
The SunTrust Credit Agreement contains affirmative and financial covenants regarding the operations of our business and certain negative covenants that, among other things, limit our ability to incur additional indebtedness, grant certain liens, make certain investments, merge or consolidate, make certain restricted payments and engage in certain asset dispositions, including a sale of all, or substantially all, of our property. As of December 31, 2017,2019, we were in compliance with our covenants.
Debt Extinguishment
In connection with the SunTrust Credit Agreement in 2017, we paid the $24.9 million outstanding indebtedness under the Credit Agreement between the CompanyBioTelemetry and Healthcare Financial Solutions, LLC,HFS, previously the General ElectricGE Capital Corporation,, as agent for the lenders, and as a lender, and we terminated the General ElectricGE Credit Agreement.Agreement. We wrote‑off the unamortized deferred financing fees
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
related to the existing debtGE Credit Agreement of $0.5 million, which is included in loss on extinguishment of debt in our consolidated statements of operations and comprehensive income/(loss).for the year ended December 31, 2017.
Amendment
On January 27, 2020, we amended our SunTrust Credit Agreement; see “Note 21. Subsequent Event” for further information. As a result of this amendment, as of December 31, 2019, in accordance with applicable U.S. GAAP, we have reclassified our debt as long-term, with the exception of the portion that has been paid prior to the issuance of this report.
11.BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
12. Leases
We lease our principal administrative and service facilities, as well as certain office equipment, monitoring devices and information technology equipment under arrangements classified as leases under ASC 842. We adopted ASC 842 using the optional modified retrospective transition method as of January 1, 2019; therefore prior period amounts are not restated.
We have non-cancelable operating leases expiring at various dates through 2028. The terms of theCertain leases are renewable at the end of the lease term. Payments madeterm at our option, none of which are certain at this time. We have also entered into and acquired finance leases with various expiration dates through 2022, which are used primarily to finance office equipment, monitoring devices and other information technology equipment.
The components of our lease expense under operating leasesASC 842 are charged to operations on a straight-line basis over the period of the lease. Differences between straight-line expense and cash payments are recorded as deferred rent. follows:
|
| | | |
| (in thousands) | Year Ended December 31,
2019 |
| Operating lease cost: | |
| Operating lease cost | $ | 5,828 |
|
| Short-term lease cost | 299 |
|
| Total operating lease cost | 6,127 |
|
| | |
| Finance lease cost: | |
| Amortization of right-of-use asset | 2,073 |
|
| Interest on lease liabilities | 58 |
|
| Total finance lease cost | 2,131 |
|
| | |
| Total lease cost | $ | 8,258 |
|
Rent expense under ASC 840, Leaseswas $5.8 million, $4.2$6.3 million and $3.8$5.8 million for the years ended December 31, 2018 and 2017, 2016 and 2015, respectively.
We have entered into and acquired capitalSupplemental balance sheet information related to leases with various expiration dates through 2020 which were used to finance equipment, furniture and monitoring devices.as of December 31, 2019 is as follows:
|
| | | | | | | |
| (in thousands, except percentage and years) | Operating Leases | | Finance Leases |
| Property and equipment, net | $ | — |
| | $ | 493 |
|
| Other assets | 16,400 |
| | — |
|
| Total right-of-use assets | 16,400 |
| | 493 |
|
| | | | |
| Accrued liabilities | 5,187 |
| | — |
|
| Current portion of finance lease obligations | — |
| | 394 |
|
| Long-term portion of finance lease obligations | — |
| | 289 |
|
| Other long-term liabilities | 14,029 |
| | — |
|
| Total lease obligations | $ | 19,216 |
| | $ | 683 |
|
| | | | |
| Weighted average remaining lease term (years) | 5.0 |
| | 1.9 |
|
| Weighted average discount rate | 4.4 | % | | 4.5 | % |
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Future minimummaturities of lease payments under non-cancelable operating and capital leases are summarizedliabilities as follows atof December 31, 2017:2019 are as follows:
|
| | | | | | | |
| (in thousands) | Operating Leases | | Finance Leases |
| 2020 | $ | 5,926 |
| | $ | 412 |
|
| 2021 | 4,423 |
| | 191 |
|
| 2022 | 3,058 |
| | 94 |
|
| 2023 | 2,268 |
| | — |
|
| 2024 | 1,967 |
| | — |
|
| Thereafter | 3,839 |
| | — |
|
| Total minimum lease payments | 21,481 |
| | 697 |
|
| Less imputed interest | (2,265 | ) | | (14 | ) |
| Present value of lease liabilities | $ | 19,216 |
| | $ | 683 |
|
|
| | | | | | | |
| (in thousands) | Operating Leases | | Capital Leases |
| 2018 | $ | 5,871 |
| | $ | 4,023 |
|
| 2019 | 4,240 |
| | 1,358 |
|
| 2020 | 3,768 |
| | 128 |
|
| 2021 | 2,535 |
| | — |
|
| 2022 | 1,726 |
| | — |
|
| Thereafter | 5,515 |
| | — |
|
| Total minimum lease payments | $ | 23,655 |
| | $ | 5,509 |
|
|
| | | |
| (in thousands) | Year Ended December 31,
2019 |
| Cash paid for amounts included in the measurement of lease liabilities: | |
| Operating cash flows from operating leases | $ | (6,026 | ) |
| Operating cash flows from finance leases | (58 | ) |
| Financing cash flows from finance leases | (1,909 | ) |
| | |
| Right-of-use assets obtained in exchange for lease obligations: | |
| Operating leases | 21,244 |
|
| Finance leases | 787 |
|
See “Note 2. Summary of Significant Accounting Policies; (w) Recent Accounting Pronouncements; Accounting Pronouncements Recently Adopted” for further discussion regarding the transition from ASC 840 to ASC 842 effective January 1, 2019.
12.13. Other Charges
We account for expenses associated with exit or disposal activities in accordance with ASC 420, Exit or Disposal Cost Obligations, and record the expenses in other charges in our consolidated statements of operations and comprehensive income/(loss), and record the related accrual in the accrued expenses line of our consolidated balance sheets.
We account for expenses associated with our acquisitions and activity associated with certain ongoing litigation as other charges as incurred. These expenses were primarily a result of activities surrounding our acquisitions and legal fees related to patent litigation in which we are the plaintiff and activities surrounding our acquisitions.plaintiff. Integration costs are primarily due to employee-related costs. Other charges are costs that are not considered necessary to the ongoing business operations. A summary of these expenses is as follows:
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
|
| | | | | | | | | | | |
| | Year Ended December 31, |
| (in thousands) | 2019 | | 2018 | | 2017 |
| Asset impairment charges | $ | — |
| | $ | — |
| | $ | 12,045 |
|
| Acquisition and integration costs | 4,863 |
| | 10,089 |
| | 18,656 |
|
| Information technology incident costs | 2,992 |
| | — |
| | — |
|
| Reserve for note receivable | — |
| | 1,793 |
| | — |
|
| Change in fair value of acquisition-related contingent consideration | (230 | ) | | (700 | ) | | (2,605 | ) |
| Patent and other litigation | 7,019 |
| | 2,583 |
| | 1,185 |
|
| Other costs | 360 |
| | 894 |
| | 2,155 |
|
| Total | $ | 15,004 |
| | $ | 14,659 |
| | $ | 31,436 |
|
|
| | | | | | | | | | | |
| | Year ended December 31, |
| (in thousands) | 2017 | | 2016 | | 2015 |
| Asset impairment charges | $ | 12,045 |
| | $ | — |
| | $ | — |
|
| Legal fees | 8,689 |
| | 7,177 |
| | 5,764 |
|
| Professional fees | 5,614 |
| | 719 |
| | 50 |
|
| Severance and employee related costs | 4,747 |
| | 645 |
| | 249 |
|
| Change in fair value of contingent consideration | (2,605 | ) | | — |
| | — |
|
| Other costs | 2,946 |
| | 98 |
| | — |
|
| Total | $ | 31,436 |
| | $ | 8,639 |
| | $ | 6,063 |
|
During our intangible asset impairment testing for the year ended December 31, 2017, in conjunction withconsidering the LifeWatch integration and forward-looking integration plans, we determined that certain trade names and certain internally developed software costs ceased being used and were no longer going to be used and were therefore impaired. We recognizedimpaired, resulting in impairment charges included within the Corporate and Other segmentcategory. There were no other asset impairments for the year ended December 31, 2017, and no intangible asset impairments for the years ended December 31, 2019 and 2018. See “Note 8. Goodwill and Intangible Assets” above.
In October 2019, we detected suspicious activity on our information technology network. As part of $1.1 million relatedour comprehensive response plan, we immediately took certain systems offline to purchased software,contain the activity and engaged an outside forensics team to conduct an independent investigation. As a result of the information technology incident, we incurred approximately $3.0 million related to indefinite-lived trade names and $8.0 million related to certain developed technology and customer relationships. Professional fees, severance and employee related costs increased primarily due to integration activities related toof direct expenses in the LifeWatch acquisition. Thefourth quarter of 2019.
In 2018, we recorded a reserve for a note receivable with a bankrupt customer.
For the year ended December 31, 2019, the change in fair value of acquisition-related contingent consideration is partially the result of the contingent consideration relatedrelates to the ePatch acquisition being written off as it is no longer probable that any of the contingencies will be met. Additionally duringour Geneva acquisition. For the year ended December 31, 2018 , the fair value ofchange relates to our Telcare acquisition, while the contingent consideration relatedchange for the year ended December 31, 2017 relates to the both our Telcare acquisition was reduced as a result of reducing the probability of attaining all the revenue contingencies. and ePatch acquisitions.
13.14. Equity
Common Stock
As of December 31, 20172019 and 2016,2018, we were authorized to issue 200,000,000 shares of common stock. As of December 31, 20172019 and 2016,2018, we had 32,460,66834,023,053 and 28,261,50333,406,364 shares issued and outstanding, respectively. Subsequent to December 31, 2017, in accordance with the squeeze-out procedures under Swiss Law, we issued 58,786 shares to the remaining stockholders of LifeWatch. See “Note 3. Acquisitions” above for further details related to the LifeWatch acquisition.
Preferred Stock
As of December 31, 2017,2019, we were authorized to issue 10,000,000 shares of preferred stock. As of December 31, 2016,2019, we maintained an unregistered blank check preferred stock class, and no shares were authorized. As of December 31, 20172019 and 2016,2018, there were no shares of preferred stock issued or outstanding.
Noncontrolling Interest
As of December 31, 2017, the noncontrolling interest of $1.1 million on our consolidated balance sheet represents our partner’s share of the accumulated deficit recorded within LifeWatch Turkey. See “Note 1. Summary of Significant Accounting Policies; l) Noncontrolling Interests” above for further details.
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
14.Noncontrolling Interest
During 2018, after a formal restructuring of shareholdings approved by the board of directors of LifeWatch Turkey, we became the sole shareholder of LifeWatch Turkey. No cash or other consideration was exchanged to effect this transaction. As a result, we no longer reflect a noncontrolling interest in our consolidated balance sheet; however, we continue to reflect the net loss attributable to the noncontrolling interest on our consolidated statement of operations for the period of time where we did not own the entire entity.
15. Stock-Based Compensation
We have three3 stock plans: our 2017 Omnibus Incentive Plan (“OIP”OIP”), our 2008 Equity Incentive Plan (the “2008 Plan”“2008 Plan”) and our 2003 Equity Incentive Plan (the “2003 Plan”“2003 Plan”) (collectively, the “Plans”). The OIP is the only remaining stock plan actively granting new stock options or units. The purpose of these stock plans was, and the OIP is, to grant incentive stock options to employees and non-qualified stock options, RSUs performance stock, PSOs, PSUs and other stock-based incentive awards to officers, directors, employees and consultants. The Plans are administered by our Board of Directors (the “Board”“Board”) or its delegates. The number, type, exercise price, and vesting terms of awards are determined by the Board or its delegates in accordance with the terms of the Plans. The stock options granted expire on a date specified by the Board but generally not more than ten years from the grant date. Stock option grants to employees generally vest over four years while RSUs generally vest overafter three years.
2017 Omnibus Incentive Plan
InOn May 11, 2017, theour stockholders and Board approved the OIP, which replacesreplaced the 2008 Plan.Plan, with 3,000,000 shares reserved for issuance. Stock options, RSUs, PSUs and PSOs are have been granted under the OIP. At December 31, 2017, 2,753,252OIP. Under the terms of the OIP, any cancellation, forfeiture or expiry of equity awards granted under the 2008 Plan roll into the availability under the OIP. There were 1,952,041 shares remain available for grant under the OIP.OIP as of December 31, 2019.
2008 Equity Incentive Plan
Our 2008 Plan became effective on March 18, 2008 and replaced our 2003 Plan.Plan. Under the terms of the 2008 Plan, all available shares in the 2003 Plan share reserve automatically rolled into the 2008 Plan.Plan. Any cancellations or forfeitures of granted stock options under the 2003 Plan also automatically rollrolled into the 2008 Plan. Beginning on January 1, 2009, and each year thereafter, the number of options available to be granted under the plan increased by the lesser of 4% of the total number of common shares outstanding or 1,500,000 shares. The 2008 Plan had 2,637,019 shares available for grant as of December 31, 2016; there. There are no0 shares available to grant under the 2008 Plan subsequent to the approval of the OIP.OIP.
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Stock option and PSO activity is summarized for the years ended December 31, 2017, 20162019, 2018 and 20152017 as follows:
|
| | | | | | | | | | | | | |
| | Stock Options | | Performance Stock Options |
| | Number of Shares | | Weighted Average Exercise Price | | Number of Shares | | Weighted Average Exercise Price |
| Outstanding as of December 31, 2014 | 3,250,852 |
| | $ | 6.40 |
| | — |
| | — |
|
| Granted | 427,786 |
| | 10.39 |
| | 200,000 |
| | $ | 19.89 |
|
| Forfeited | (181,777 | ) | | 11.32 |
| | — |
| | — |
|
| Exercised | (76,342 | ) | | 3.82 |
| | — |
| | — |
|
| Outstanding as of December 31, 2015 | 3,420,519 |
| | $ | 6.69 |
| | 200,000 |
| | $ | 19.89 |
|
| Granted | 519,770 |
| | 13.44 |
| | — |
| | — |
|
| Forfeited | (49,709 | ) | | 9.97 |
| | — |
| | — |
|
| Exercised | (322,146 | ) | | 4.56 |
| | — |
| | — |
|
| Outstanding as of December 31, 2016 | 3,568,434 |
| | $ | 7.82 |
| | 200,000 |
| | $ | 19.89 |
|
| Granted | 543,881 |
| | 31.12 |
| | — |
| | — |
|
| Forfeited | (154,510 | ) | | 16.22 |
| | — |
| | — |
|
| Exercised | (383,366 | ) | | 9.91 |
| | (50,000 | ) | | 18.33 |
|
| Outstanding as of December 31, 2017 | 3,574,439 |
| | $ | 10.78 |
| | 150,000 |
| | $ | 20.41 |
|
The PSOs met their performance criteria, vested, and were priced as follows: |
| | | | | | | | | | | | |
| Stock Options | Number of Stock Options | | Weighted Average Exercise Price | | Weighted Average Remaining Contractual Term
(Years) | | Aggregate Intrinsic Value
(in thousands) |
| Outstanding as of December 31, 2016 | 3,568,434 |
| | $ | 7.82 |
| | | | |
| Granted | 543,881 |
| | 31.12 |
| | | | |
| Forfeited | (154,510 | ) | | 16.22 |
| | | | |
| Exercised | (383,366 | ) | | 9.91 |
| | | | |
| Outstanding as of December 31, 2017 | 3,574,439 |
| | $ | 10.78 |
| | | | |
| Granted | 387,306 |
| | 43.82 |
| | | | |
| Forfeited | (114,769 | ) | | 30.64 |
| | | | |
| Exercised | (1,185,694 | ) | | 8.08 |
| | | | |
| Outstanding as of December 31, 2018 | 2,661,282 |
| | $ | 15.94 |
| | | | |
| Granted | 367,142 |
| | 63.27 |
| | | | |
| Forfeited | (71,534 | ) | | 31.61 |
| | | | |
| Exercised | (265,831 | ) | | 8.94 |
| | | | |
| Outstanding as of December 31, 2019 | 2,691,059 |
| | $ | 22.67 |
| | 5.7 | | $ | 72,621 |
|
| Exercisable as of December 31, 2019 | 1,852,025 |
| | $ | 10.92 |
| | 4.4 | | $ | 66,165 |
|
| Expected to vest as of December 31, 2019 | 761,428 |
| | $ | 48.62 |
| | 8.5 | | $ | 5,858 |
|
|
| | | | | | | |
| Performance Achievement Date | | Number of Shares | | Weighted Average Exercise Price |
| October 4, 2016 | | 100,000 |
| | $ | 18.33 |
|
| January 13, 2017 | | 100,000 |
| | $ | 21.45 |
|
|
| | | | | | | | | | | | |
| Performance Stock Options | Number of PSOs | | Weighted Average Exercise Price | | Weighted Average Remaining Contractual Term
(Years) | | Aggregate Intrinsic Value
(in thousands) |
| Outstanding as of December 31, 2016 | 200,000 |
| | $ | 19.89 |
| | | | |
| Granted | — |
| | — |
| | | | |
| Forfeited | — |
| | — |
| | | | |
| Exercised | (50,000 | ) | | 18.33 |
| | | | |
| Outstanding as of December 31, 2017 | 150,000 |
| | $ | 20.41 |
| | | | |
| Granted | — |
| | — |
| | | | |
| Forfeited | — |
| | — |
| | | | |
| Exercised | (15,000 | ) | | 18.33 |
| | | | |
| Outstanding as of December 31, 2018 | 135,000 |
| | $ | 20.64 |
| | | | |
| Granted | — |
| | — |
| | | | |
| Forfeited | — |
| | — |
| | | | |
| Exercised | (105,000 | ) | | 20.41 |
| | | | |
| Outstanding as of December 31, 2019 | 30,000 |
| | $ | 21.45 |
| | 7.0 | | $ | 746 |
|
| Exercisable as of December 31, 2019 | 30,000 |
| | $ | 21.45 |
| | 7.0 | | $ | 746 |
|
A summary of total outstanding stock options as of December 31, 2017 is as follows:
|
| | | | | | | | | | | | | | | | | | |
| | Options Outstanding | | Options Exercisable |
| Range of Exercise Prices | | Number Outstanding | | Weighted Average Remaining Contractual Life (in Years) | | Weighted Average Exercise Price | | Number Exercisable | | Weighted Average Remaining Contractual Life (in Years) | | Weighted Average Exercise Price |
| $1.93 - $6.50 | 1,296,231 |
| | 4.2 | | $ | 3.17 |
| | 1,296,231 |
| | 4.2 | | $ | 3.17 |
|
| $6.51 - $10.00 | 1,136,472 |
| | 4.8 | | 7.88 |
| | 952,756 |
| | 4.3 | | 7.62 |
|
| $10.01 - $20.00 | 606,190 |
| | 6.5 | | 14.02 |
| | 407,990 |
| | 5.8 | | 13.79 |
|
| $20.01 - $30.00 | 375,546 |
| | 8.2 | | 23.57 |
| | 174,068 |
| | 7.0 | | 21.93 |
|
| $30.01 - $37.15 | 310,000 |
| | 9.2 | | 36.10 |
| | 15,000 |
| | 0.6 | | 30.98 |
|
| $1.93 - $37.15 | 3,724,439 |
| | 5.6 | | $ | 11.17 |
| | 2,846,045 |
| | 4.6 | | $ | 7.48 |
|
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The PSOs met their performance criteria, vested, and were priced as follows:
|
| | | | | |
| Performance Achievement Date | | Number of Shares | | Weighted Average Exercise Price |
| October 4, 2016 | | 100,000 |
| | $18.33 |
| January 13, 2017 | | 100,000 |
| | $21.45 |
A summary of total outstanding stock options and PSOs as of December 31, 2019 is as follows:
|
| | | | | | | | | | | | | | | | | | |
| | Stock Options & PSOs Outstanding | | Stock Options & PSOs Exercisable |
| Range of Exercise Prices | | Number of Stock Options & PSOs Outstanding | | Weighted Average Remaining Contractual Life (in Years) | | Weighted Average Exercise Price | | Number of Stock Options & PSOs Exercisable | | Weighted Average Remaining Contractual Life (in Years) | | Weighted Average Exercise Price |
| $2.22 - $10.00 | 1,248,361 |
| | 3.4 | | $ | 5.23 |
| | 1,246,486 |
| | 3.4 | | $ | 5.22 |
|
| $10.01 - $25.00 | 529,091 |
| | 6.1 | | 16.26 |
| | 439,160 |
| | 5.9 | | 15.25 |
|
| $25.01 - $40.00 | 519,798 |
| | 8.1 | | 34.46 |
| | 167,129 |
| | 7.8 | | 33.93 |
|
| $40.01 - $70.00 | 136,000 |
| | 9.2 | | 53.79 |
| | 11,250 |
| | 8.7 | | 58.99 |
|
| $70.01 - $76.01 | 287,809 |
| | 9.1 | | 73.99 |
| | 18,000 |
| | 8.9 | | 73.62 |
|
| $2.22 - $76.01 | 2,721,059 |
| | 5.7 | | $ | 22.66 |
| | 1,882,025 |
| | 4.5 | | $ | 11.08 |
|
The table below summarizes certain additional information with respect to our stock options:
|
| | | | | | | | | | | | |
| | | Year Ended December 31, |
| (in thousands, except per option amounts) | | 2019 | | 2018 | | 2017 |
| Aggregate intrinsic value of stock options exercised during the year | $ | 19,062 |
| | $ | 49,188 |
| | $ | 7,562 |
|
| Cash received from the exercise of stock options | 4,519 |
| | 9,855 |
| | 4,714 |
|
| Weighted average grant date fair value per option | $ | 36.52 |
| | $ | 25.96 |
| | $ | 18.05 |
|
|
| | | | | | | | | | | | |
| | | Year Ended December 31, |
| (In thousands, except per share amounts) | | 2017 | | 2016 | | 2015 |
| Aggregate intrinsic value of options outstanding at year-end | $ | 71,680 |
| | $ | 52,671 |
| | $ | 19,436 |
|
| Aggregate intrinsic value of options exercisable at year-end | 63,834 |
| | 43,750 |
| | 16,124 |
|
| Aggregate intrinsic value of options exercised during the year | 7,562 |
| | 3,546 |
| | 662 |
|
| Cash received from the exercise of stock options | 4,714 |
| | 1,470 |
| | 291 |
|
| Weighted average grant date fair value per option | $ | 18.05 |
| | $ | 9.47 |
| | $ | 6.58 |
|
The total compensation cost of options granted but not yet vested at December 31, 20172019 was $11.0$19.8 million, which is expected to be recognized over a weighted average period of approximately three years.
The weighted average of the assumptions used to determine the fair value of stock options was estimated at the date of grant using the following weighted average assumptions:is as follows:
|
| | | | | | | | |
| | Year Ended December 31, |
| | 2019 | | 2018 | | 2017 |
| Expected volatility | 54.9 | % | | 55.2 | % | | 59.2 | % |
| Expected term (in years) | 7.2 |
| | 7.4 |
| | 7.3 |
|
| Risk-free interest rate | 2.30 | % | | 2.78 | % | | 2.08 | % |
| Expected dividends | 0.0 | % | | 0.0 | % | | 0.0 | % |
|
| | | | | | | | |
| | Year Ended December 31, |
| | 2017 | | 2016 | | 2015 |
| Expected volatility | 59.2 | % | | 64.4 | % | | 66.5 | % |
| Expected term (in years) | 7.3 |
| | 8.0 |
| | 6.7 |
|
| Weighted average risk-free interest rate | 2.08 | % | | 1.61 | % | | 1.68 | % |
| Expected dividends | 0.0 | % | | 0.0 | % | | 0.0 | % |
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
RSU and PSU activity is summarized for the years ended December 31, 2017, 20162019, 2018 and 20152017 as follows:
|
| | | | | | | | | | | | | |
| | Restricted Stock Units | | Performance Stock Units |
| | Number of RSUs | | Weighted Average Grant Date Fair Value | | Number of PSUs | | Weighted Average Grant Date Fair Value |
| Units outstanding as of December 31, 2016 | 592,349 |
| | $ | 9.86 |
| | 132,992 |
| | $ | 8.68 |
|
| Granted | 117,614 |
| | 25.98 |
| | — |
| | — |
|
| Forfeited | (48,974 | ) | | 13.57 |
| | (132,992 | ) | | 8.68 |
|
| Vested | (193,860 | ) | | 9.31 |
| | — |
| | — |
|
| Units outstanding as of December 31, 2017 | 467,129 |
| | $ | 13.76 |
| | — |
| | $ | — |
|
| Granted | 128,860 |
| | 35.14 |
| | 88,345 |
| | 37.79 |
|
| Forfeited | (12,466 | ) | | 22.88 |
| | (1,236 | ) | | 37.79 |
|
| Vested | (224,840 | ) | | 12.02 |
| | — |
| | — |
|
| Units outstanding as of December 31, 2018 | 358,683 |
| | $ | 22.22 |
| | 87,109 |
| | $ | 37.79 |
|
| Granted | 109,998 |
| | 59.16 |
| | 34,088 |
| | 86.29 |
|
| Forfeited | (24,965 | ) | | 33.24 |
| | (31,177 | ) | | 40.95 |
|
| Vested | (173,664 | ) | | 13.73 |
| | — |
| | — |
|
| Units outstanding as of December 31, 2019 | 270,052 |
| | $ | 41.70 |
| | 90,020 |
| | $ | 55.06 |
|
|
| | | | | | | | | | | | | |
| | Restricted Stock Units | | Performance Stock Units |
| | Number of Shares | | Weighted Average Grant Date Fair Value | | Number of Shares | | Weighted Average Grant Date Fair Value |
| Units outstanding as of December 31, 2014 | 864,634 |
| | $ | 4.23 |
| | 284,423 |
| | $ | 8.68 |
|
| Granted | 328,060 |
| | 9.70 |
| | — |
| | — |
|
| Forfeited | (50,642 | ) | | 6.90 |
| | (18,433 | ) | | 8.68 |
|
| Vested | (451,116 | ) | | 3.89 |
| | — |
| | — |
|
| Units outstanding as of December 31, 2015 | 690,936 |
| | 6.85 |
| | 265,990 |
| | 8.68 |
|
| Granted | 225,198 |
| | 11.06 |
| | — |
| | — |
|
| Forfeited | (11,905 | ) | | 9.50 |
| | — |
| | — |
|
| Vested | (311,880 | ) | | 4.08 |
| | (132,998 | ) | | 8.68 |
|
| Units outstanding as of December 31, 2016 | 592,349 |
| | 9.86 |
| | 132,992 |
| | 8.68 |
|
| Granted | 117,614 |
| | 25.98 |
| | — |
| | — |
|
| Forfeited | (48,974 | ) | | 13.57 |
| | (132,992 | ) | | 8.68 |
|
| Vested | (193,860 | ) | | 9.31 |
| | — |
| | — |
|
| Units outstanding as of December 31, 2017 | 467,129 |
| | $ | 13.76 |
| | — |
| | $ | — |
|
In addition, a summary of total outstanding RSUs and PSUs as of December 31, 20172019 is as follows:
|
| | | | | | |
| Range of Grant Date Fair Value | | RSUs Outstanding | | PSUs Outstanding* |
| $24.65 - $31.50 | | 83,827 |
| | — |
|
| $31.51 - $44.16 | | 108,508 |
| | 57,963 |
|
| $44.17 - $76.01 | | 77,717 |
| | — |
|
| $76.02 - $86.29 | | — |
| | 32,057 |
|
| $24.65 - $86.29 | | 270,052 |
| | 90,020 |
|
* See “Note 2. Summary of Significant Accounting Policies; q) Stock-Based Compensation” for discussion regarding the fair value of our PSUs.
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
|
| | | |
Range of Grant Date Fair Value | | RSUs
Outstanding
|
$8.93 - $9.75 | | 154,335 |
|
$9.76 - $10.36 | | 184,395 |
|
$10.37 - $33.05 | | 128,399 |
|
$8.93 - $33.05 | | 467,129 |
|
Additional information about our RSUs and PSUs is summarized as follows:
|
| | | | | | | | | | | | |
| | | Year Ended December 31, |
| (in thousands) | | 2019 | | 2018 | | 2017 |
| Aggregate market value of RSUs vested during the year | $ | 12,833 |
| | $ | 7,940 |
| | $ | 4,768 |
|
|
| | | | | | | | | | | | |
| | | Year Ended December 31, |
| (In thousands) | | 2017 | | 2016 | | 2015 |
| Aggregate market value of RSUs vested during the year | $ | 4,768 |
| | $ | 3,826 |
| | $ | 4,460 |
|
| Aggregate market value of PSUs vested during the year | — |
| | 2,093 |
| | — |
|
The total compensation cost of RSUs and PSUs granted but not yet vested, inclusive of the PSUs for which vesting has been deemed probable at December 31, 20172019, was $3.2$7.8 million, which is expected to be recognized over a weighted average period of approximately one year.
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
two years. Additionally, there were 588,359 RSUs vested but not released at December 31, 2019.
Employee Stock Purchase Plan
In July 2008, we made available anMay 2017, the stockholders approved the BioTelemetry, Inc. 2017 Employee Stock Purchase Plan (“2017 ESPP”), with 500,000 shares reserved for issuance, which replaced the 2008 ESPP”) in which substantiallyEmployee Stock Purchase Plan. Substantially all of our full-time employees becameare eligible to participate effective March 18, 2008.in the 2017 ESPP. Under the 20082017 ESPP employees, each participant may contributepurchase option value of our shares, through payroll deductions, upnot to 15%exceed $25,000 of their compensation toward thegrant date fair value in a calendar year. The purchase of our common stock, or $21,500, whichever is lower. The price per share is equal to the lower of 85% of the fairclosing market price on the first day of the offering period, or 85% of the fairclosing market price on the day of purchase. Proceeds received from the issuance of shares are credited to stockholders’ equity in the period that the shares are issued. In May 2017, the Board of Directors and stockholders approved the BioTelemetry, Inc. 2017 Employee Stock Purchase Plan (“2017 ESPP”), with 500,000 shares reserved for issuancePurchases under the 2017 ESPP which will replace the 2008 ESPP. The contribution limits, price discount are made in March and the offering periods remain the same under the 2017 ESPP.September. In 2017,2019, an aggregate of 95,21598,425 shares were purchased in accordance with the Plans.2017 ESPP. Net proceeds from the issuance of shares of common stock under the Plans2017 ESPP for the year ended December 31, 20172019 were $1.4$3.1 million. At December 31, 2017, 452,7512019, 232,671 shares remain available for purchase under the 2017 ESPP.ESPP.
Our aggregate stock-based compensation expense is summarized as follows:
|
| | | | | | | | | | | |
| | Year Ended December 31, |
| (in thousands) | 2019 | | 2018 | | 2017 |
| Stock options | $ | 7,307 |
| | $ | 4,550 |
| | $ | 3,183 |
|
| Performance stock options | — |
| | — |
| | 1,534 |
|
| Restricted stock units | 3,719 |
| | 3,052 |
| | 2,273 |
|
| Performance stock units | 867 |
| | 589 |
| | — |
|
| Employee stock purchase plan | 1,483 |
| | 1,070 |
| | 690 |
|
| Total stock-based compensation expense | $ | 13,376 |
| | $ | 9,261 |
| | $ | 7,680 |
|
|
| | | | | | | | | | | |
| | Year Ended December 31, |
| (In thousands) | 2017 | | 2016 | | 2015 |
| Stock options | $ | 3,183 |
| | $ | 2,030 |
| | $ | 1,782 |
|
| Performance stock options | 1,534 |
| | 1,297 |
| | — |
|
| Restricted stock units | 2,273 |
| | 2,211 |
| | 2,039 |
|
| Performance stock units | — |
| | 444 |
| | 711 |
|
| Employee stock purchase plan | 690 |
| | 520 |
| | 420 |
|
| Total stock-based compensation expense | $ | 7,680 |
| | $ | 6,502 |
| | $ | 4,952 |
|
For the years ended December 31, 2017,2019, 2018 and 2016,2017, we recognized $5.0 million, $11.6 million and $1.5 million, and $1.7 millionrespectively, of tax benefit from stock options exercised during the period as a component of our (provision for)/benefit from income tax provision/(benefit).taxes.
15.BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
16. Employee Benefit Plan
We sponsor a 401(k) Retirement Savings Plan (the “Plan”“401k Plan”) for all eligible employees who meet certain requirements. Participants may contribute, on a pre-tax basis, up to the maximum allowable amount pursuant to Section 401(k) of the Internal Revenue Code (“IRC”IRC”). The plan also includes a Roth feature, allowing after-tax contributions, up to the maximum allowable amount pursuant to Section 401(k) of the IRC. We are not required to contribute to the Plan. In January 2014, we adopted an amendment to theIRC. The 401k Plan that allowed allows for an employer matching contribution of 100% of the first 3% of the employees’ salary, and 50% of the next 2% of the employees’ salary. For the years ended December 31, 2017, 20162019, 2018 and 2015,2017, we contributed $2.6$4.1 million,, $2.1 $3.5 million and $1.8$2.6 million, respectively. Employer contributions vest immediately. Additionally, we sponsor an immaterial pension plan for five participants in Switzerland.
17. Income Taxes
The components of our (provision for)/benefit from income taxes are summarized as follows:
|
| | | | | | | | | | | |
| | Year Ended December 31, |
| (in thousands) | 2019 | | 2018 | | 2017 |
| Current: | | | | | |
| Federal | $ | (178 | ) | | $ | — |
| | $ | (273 | ) |
| State | (1,397 | ) | | (27 | ) | | (424 | ) |
| Foreign | (924 | ) | | (1,897 | ) | | — |
|
| Total (provision for) income taxes | (2,499 | ) | | (1,924 | ) | | (697 | ) |
| Deferred: | | | | | |
| Federal | (5,237 | ) | | 875 |
| | (4,353 | ) |
| State | (2,243 | ) | | 690 |
| | 151 |
|
| Foreign | 95 |
| | 729 |
| | (1,848 | ) |
| Total deferred (provision for)/benefit from income taxes | (7,385 | ) | | 2,294 |
| | (6,050 | ) |
| Total (provision for)/benefit from income taxes | $ | (9,884 | ) | | $ | 370 |
| | $ | (6,747 | ) |
Reconciliations between expected income taxes computed at the federal statutory rate for each of the years ended December 31, 2019, 2018 and 2017, and the (provision for)/benefit from income taxes is as follows:
|
| | | | | | | | | | | |
| | Year Ended December 31, |
| (in thousands) | 2019 | | 2018 | | 2017 |
| Income tax (provision)/benefit at statutory rate | $ | (8,342 | ) | | $ | (8,716 | ) | | $ | 3,638 |
|
| Permanent difference | 2,532 |
| | 9,127 |
| | 392 |
|
| (Increase)/decrease in valuation allowance | (1,305 | ) | | 714 |
| | (976 | ) |
| State income tax, net of federal benefit | (1,532 | ) | | 240 |
| | (213 | ) |
| Deferred tax asset adjustments | 826 |
| | 208 |
| | (485 | ) |
| Unrecognized tax benefit | (831 | ) | | (1,547 | ) | | — |
|
| Foreign rate differential | 12 |
| | (36 | ) | | (1,107 | ) |
| Tax Reform impact | — |
| | — |
| | (8,048 | ) |
| Rate change impact | (814 | ) | | 366 |
| | 36 |
|
| Other | (430 | ) | | 14 |
| | 16 |
|
| (Provision for)/benefit from income taxes | $ | (9,884 | ) | | $ | 370 |
| | $ | (6,747 | ) |
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
16. Income Taxes
The components of our provision for/(benefit from) income taxes are summarized as follows:
|
| | | | | | | | | | | |
| | Year Ended December 31, |
| (in thousands) | 2017 | | 2016 | | 2015 |
| Current: | | | | | |
| Federal | $ | 273 |
| | $ | 321 |
| | $ | 173 |
|
| State | 424 |
| | 153 |
| | 50 |
|
| Total provision for income taxes | 697 |
| | 474 |
| | 223 |
|
| Deferred: | | | | | |
| Federal | 6,201 |
| | (32,484 | ) | | 220 |
|
| State | (151 | ) | | (5,657 | ) | | 25 |
|
| Total deferred provision for/(benefit from) income taxes | 6,050 |
| | (38,141 | ) | | 245 |
|
| Total provision for/(benefit from) income taxes | $ | 6,747 |
| | $ | (37,667 | ) | | $ | 468 |
|
Reconciliations between expected income taxes computed at the federal rate of 35% for each ofFor the years ended December 31, 2019, 2018 and 2017, 2016we recognized $5.0 million, $11.6 million and 2015, and$1.5 million, respectively, of tax benefit from stock options exercised during the provision for/(period as a component of our (provision for)/benefit from)from income taxes is as follows:taxes.
|
| | | | | | | | | | | |
| | Years ended December 31, |
| (in thousands) | 2017 | | 2016 | | 2015 |
| Income tax (benefit)/provision at statutory rate | $ | (3,638 | ) | | $ | 5,520 |
| | $ | 2,763 |
|
| State income tax, net of federal benefit | 177 |
| | 259 |
| | (239 | ) |
| Research and development | — |
| | — |
| | 634 |
|
| Permanent difference | (392 | ) | | — |
| | — |
|
| Deferred tax asset adjustments | 485 |
| | 4,336 |
| | — |
|
| Tax Reform impact | 8,048 |
| | — |
| | — |
|
| Unrecognized tax benefit | — |
| | 3,559 |
| | — |
|
| Foreign rate differential | 1,107 |
| | — |
| | — |
|
| Other | (16 | ) | | 289 |
| | 549 |
|
| Increase/(decrease) in valuation allowance | 976 |
| | (51,630 | ) | | (3,239 | ) |
| Provision for/(benefit from) income taxes | $ | 6,747 |
| | $ | (37,667 | ) | | $ | 468 |
|
At December 31, 2017,2019, we had federal net operating loss carryforwards of approximately $140.4$146.9 million to offset future federal taxable income expiring in various years starting in 2023 through 2037. At December 31, 2017,2019, we had state net operating loss carryforwards of $83.5$73.7 million, which expire in various years starting in 20182020 through 20372039. We also had $121.2$121.1 million of foreign net operating loss carryforwards, for which weexpire in various years starting in 2020 through 2026. We have recorded a valuation allowance against mosta portion of theour foreign and state net operating loss balance and expire in various years starting in 2018 through 2024.losses.
The timing and manner in which we can utilize our net operating loss carryforwards and future income tax deductions in any year may be limited by provisions of the IRC.limited. Section 382 of the IRC (“Section 382”) imposes limitations on a corporation’s ability to utilize net operating losses if it experiences an “ownership change.”
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Section 383 of the IRC382 imposes similar limitations on other tax attributes such as research and development credits. Currently, a portion of our loss carryforwards is limited under Section 382 and therefore, is not included in the total net operating losses disclosed above.
The U.S. Internal Revenue Service concluded its examination of our U.S. federal tax returns for all years through 2011. Because of net operating losses, our U.S. federal tax returns statutes for those years will remain subject to examination until the losses are utilized.statute of limitations passes for the tax returns which utilized those losses. Additionally, state tax return statutes generally remain open due to operating losses.
We have deferred income tax assets totaling $57.7 million at December 31, 2017, consisting primarily of federal and state net operating loss and credit carryforwards, stock-based compensation, non-deductible accruals and allowance for doubtful accounts. Our provision from income taxes for 2017 of $6.7 million primarily relates to the re-measurement of our deferred tax assets and liabilities at the new federal corporate rate of 21 percent.
Deferred taxes result from temporary differences between the carrying amounts of assets and liabilities used for financial reporting purposes and the amounts used for income tax purposes. As of December 31, 2017, our deferred income tax assets were primarily the result of federal and state net operating losses, stock-based compensation, non-deductible accruals and allowance for doubtful accounts. A valuation allowance of $6.0 million and $0.1 million was recorded against our deferred income tax asset balance as of December 31, 2017 and 2016, respectively.
As of each reporting date, our management considers new evidence, both positive and negative, that could impact management’s view with regard to future realization of deferred income tax assets.
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The significant components of our deferred taxes are as follows:
|
| | | | | | | |
| | December 31, |
| (in thousands) | 2019 | | 2018 |
| Deferred tax assets: | | | |
| Net operating loss carryforwards | $ | 33,692 |
| | $ | 33,803 |
|
| Allowance for doubtful accounts | 8,107 |
| | 6,526 |
|
| Non-deductible accruals | 2,427 |
| | 4,980 |
|
| Operating lease obligations | 4,842 |
| | — |
|
| Stock based compensation expense | 4,191 |
| | 3,337 |
|
| Transaction costs | 2,030 |
| | 2,186 |
|
| Research and development and AMT credit carryforwards | 275 |
| | 1,092 |
|
| Deferred revenue and deferred rent | 111 |
| | 1,019 |
|
| Capital loss carryforwards | 2,718 |
| | 2,114 |
|
| Other, net | 785 |
| | 917 |
|
| Total deferred tax assets | 59,178 |
| | 55,974 |
|
| Less valuation allowance | (3,877 | ) | | (3,021 | ) |
| Net deferred tax assets | 55,301 |
| | 52,953 |
|
| Deferred tax liabilities: | | | |
| Intangible assets | (31,463 | ) | | (29,663 | ) |
| Property and equipment | (6,907 | ) | | (3,173 | ) |
| Right-of-use assets | (4,132 | ) | | — |
|
| Prepaid insurance | (173 | ) | | (142 | ) |
| Total deferred tax liabilities | (42,675 | ) | | (32,978 | ) |
| Net deferred tax assets | $ | 12,626 |
| | $ | 19,975 |
|
|
| | | | | | | |
| | December 31, |
| (in thousands) | 2017 | | 2016 |
| Deferred tax assets: | | | |
| Net operating loss carryforwards | $ | 38,245 |
| | $ | 33,404 |
|
| Research and development and AMT credit carryforwards | 1,198 |
| | 912 |
|
| Stock option grants | 4,300 |
| | 5,602 |
|
| Property and equipment | 690 |
| | — |
|
| Non-deductible accruals | 4,471 |
| | — |
|
| Transaction costs | 2,361 |
| | — |
|
| Allowance for doubtful accounts | 5,324 |
| | 4,965 |
|
| Deferred revenue | 937 |
| | 885 |
|
| Other, net | 158 |
| | 1,868 |
|
| Total deferred tax assets | 57,684 |
| | 47,636 |
|
| Less valuation allowance | (6,032 | ) | | (95 | ) |
| Net deferred tax assets | 51,652 |
| | 47,541 |
|
| Deferred tax liabilities: | | | |
| Property and equipment | — |
| | (3,604 | ) |
| Intangible assets | (33,854 | ) | | (7,124 | ) |
| Prepaid insurance | (117 | ) | | (177 | ) |
| Total deferred tax liabilities | (33,971 | ) | | (10,905 | ) |
| Net deferred tax asset/(liability) | $ | 17,681 |
| | $ | 36,636 |
|
On December 22, 2017, the U.S. government enacted comprehensive tax legislation commonly referred to as the Tax Cuts and Jobs Act (the “TCJA”).TCJA. The TCJA makes broad and complex changes to the U.S. tax code, including, but not limited to, (1) reducing the U.S. federal corporate tax rate from 35 percent to 21 percent; (2) requiring companies to pay a one-time transition tax on certain unrepatriated earnings of foreign subsidiaries; (3) generally eliminating U.S. federal income taxes on dividends from foreign subsidiaries; (4) requiring a current inclusion in U.S. federal taxable income of certain earnings of controlled foreign corporations; (5) eliminating the corporate alternative minimum tax (AMT)(“AMT”) and changing how existing AMT credits can be realized; (6) creating the base erosion anti-abuse tax, (BEAT), a new minimum tax; (7) creating a new limitation on deductible interest expense; and (8) changing rules related to uses and limitations of net operating loss carryforwards created in tax years beginning after December 31, 2017.
The SEC staff issued SAB 118, which provides guidance on accounting for the tax effects of the TCJA. SAB 118 provides a measurement period that should not extend beyond one year from the TCJA enactment date for companies to complete the accounting under ASC 740.740. In accordance with SAB 118, a company must reflect the income tax effects of those aspects of the TCJA for which the accounting under ASC 740 is complete. To the extent that a company’s accounting for certain income tax effects of the TCJA is incomplete but it is able to determine a reasonable estimate, it must record a provisional estimate in the financial statements. If a company cannot determine a provisional estimate to be included in the financial statements, it should continue to apply ASC 740 on the basis of the provisions of the tax laws that were in effect immediately before the enactment of the TCJA.
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Our accounting forIn 2018, we completed our analysis of the following elementsprovisional items of the TCJA is incomplete. However, we were able to make reasonable estimates of certain effects and, therefore, recorded provisionalunder SAB 118, resulting in immaterial adjustments, as follows:
Reduction of US federal corporate tax rate: The TCJA reduces the corporate tax rate to 21 percent, effective January 1, 2018. For certain of our DTAs and DTLs, we have recorded a provisional net decrease of $8.0 million, with a corresponding net adjustment to deferred income tax expense for the year ended December 31, 2017. While we are able to make a reasonable estimate of the impact of the reduced corporate rate, it may be affected by other analysesprimarily related to the TCJA, including, but not limited to, our calculation of deemed repatriation of deferred foreign income and the state tax effect of adjustments made to federalcumulative temporary differences.
Deemed Repatriation Transition Tax: As part of U.S. international tax reform, the TCJA imposes a transition tax on certain accumulated foreign earnings aggregated across all non-U.S. subsidiaries, net of foreign deficits. As we are in an aggregate net foreign deficit position for U.S. tax purposes, we are not liable for the transition tax. However, we are continuing to gather additional information to more precisely compute our aggregate net foreign deficit position.
Cost recovery: While we have not yet completed all of the computations necessary or completed an inventory of our 2017 expenditures that qualify for immediate expensing, we have recorded a provisional benefit of $1.1 million based on our current intent to fully expense all qualifying expenditures.
Global intangible low-taxed income: The TJCA subjects a U.S. shareholder to current tax on global intangible low-taxed income (“GILTI”) earned by certain foreign subsidiaries. The FASB Staff Q&A, Topic 740 No. 5, Accounting for Global Intangible Low-Taxed Income, states that an entity can make an accounting policy election to either recognize deferred taxes for temporary differences expected to reverse as GILTI in future years or provide for the tax expense related to GILTI in the year the tax is incurred. We have elected to recognize the tax on GILTI as a period expense in the period the tax is incurred.
During 2017, in connection with our acquisitions, we identified uncertain tax positions for periods prior to our ownership related to items recorded through purchase accounting. The following summarizes the changes in our unrecognized tax benefit:benefits:
|
| | | | | | | |
| | Year Ended December 31, |
| (in thousands) | 2019 | | 2018 |
| Unrecognized tax benefits at the beginning of the year | $ | 52,832 |
| | $ | 39,710 |
|
| Additions to unrecognized tax benefits related to current year | — |
| | — |
|
| Additions to unrecognized tax benefits related to prior years | 2,446 |
| | 13,122 |
|
| Unrecognized tax benefits at the end of the year | $ | 55,278 |
| | $ | 52,832 |
|
|
| | | | | | | |
| | Year ended |
| (in thousands) | December 31,
2017 | | December 31,
2016 |
| Unrecognized tax benefit at the beginning of the year | $ | 3,899 |
| | $ | — |
|
| Additions to uncertain tax positions related to current year | 35,811 |
| | — |
|
| Additions to uncertain tax positions related to prior years | — |
| | 3,899 |
|
| Unrecognized tax benefit at the end of the year | $ | 39,710 |
| | $ | 3,899 |
|
The balance of unrecognized tax benefits, if recognized, would affect the effective tax rate. As of December 31, 2017,2019 and 2018, we have recorded a net reserve of $22.0$34.8 million and $31.3 million, respectively, for uncertainunrecognized tax positionsbenefits as a component of other long-term liabilities within our consolidated balance sheets. The unrecognized tax benefit, orIn addition, a portion of anthe unrecognized tax benefit,benefits is presented in the financial statements as a reduction to a deferred tax asset for a net operating loss carryforward, a similar tax loss, or a tax credit carryforward.
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
December 31, 2019, a total of $34.8 million of unrecognized tax benefits, if recognized, would affect the effective tax rate.
We recognize interest and penalties related to unrecognized tax benefits on the (provision for)/benefit from income tax expensetaxes line in the accompanying consolidated statements of operations and comprehensive income/(loss).operations. As of December 31, 2017,2019, our accrued interest associated with our liability for unrecognized tax benefits was $2.7 million. In addition, we have not recorded any interest and penalties on our uncertain tax positions.positions for each of the years ended December 31, 2019 and 2018.
It is reasonably possible that a portion of these unrecognized tax benefits could be resolved within the next twelve months that may result in a decrease in our effective tax rate.
17.18. Segment Information
We operate under three2 reportable segments: Healthcare Research and Technology.Research. The Healthcare segment is focused on the diagnosis andremote cardiac monitoring ofto identify cardiac arrhythmias or heart rhythm disorders.disorders and to monitor the functionality of implantable cardiac devices. We offer cardiologists, and electrophysiologists, neurologists and primary care physicians a full spectrum of solutions, which provides them with a single source of remote cardiac monitoring services. These services include MCT, event, traditional Holter, extended Holter, Pacemaker, INR, ILR and other implantable cardiac device monitoring. The majority of our Healthcare revenue is derived from the monitoring of devices that BioTelemetry has developed, manufactured and marketed. The Research segment is engaged in centralcentralized core laboratory services providing cardiac monitoring, imaging services, scientific consulting and data management services for drug and medical device trials. TheDuring the first quarter of 2018, as part of the LifeWatch integration, our forward-looking integration and rebranding plans, as well as re-evaluating the significance and materiality of our segments, we aggregated our Technology operating segment focuses oninto our Corporate and Other category. Included in our Corporate and Other category is the development, manufacturing, testing and marketing of cardiovascularcardiac and blood glucose monitoring devices to medical companies, clinics and hospitals. Intercompany revenue relatinghospitals and corporate overhead and other items not allocated to the manufacturingany of devices by the Technology segment for the other segments is included on the intersegment revenue line.our reportable segments.
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Expenses that can be specifically identified with a segment have been included as deductions in determining pre-tax segment income.income/(loss). Any remaining expenses including integration, restructuring and other charges, as well as the elimination of costs associated with intercompany revenue, are included in our Corporate and Other.Other category. Also included in our Corporate and Other category is our net interest expense, and other financing expenses.expenses, and income taxes. We do not allocate assets to the individual segments.
During the year ended December 31, 2017 we reclassified research and development costs not utilized by our Research segment from the Corporate and Other segment to the Healthcare segment to synchronize our external reporting with the way our chief operating decision maker reviews the segment performance and makes decisions about the reportable segments. |
| | | | | | | | | | | | | | | |
| | Year Ended December 31, 2019 |
| | Reporting Segment | | Corporate and Other | | Consolidated |
| (in thousands) | Healthcare | | Research | | |
| Revenue | $ | 372,014 |
| | $ | 54,450 |
| | $ | 12,643 |
| | $ | 439,107 |
|
| Gross profit | 251,114 |
| | 20,164 |
| | 2,996 |
| | 274,274 |
|
| Income/(loss) before income taxes | 122,829 |
| | 4,653 |
| | (87,754 | ) | | 39,728 |
|
| Depreciation and amortization | 34,972 |
| | 4,047 |
| | 3,957 |
| | 42,976 |
|
| Capital expenditures | 25,543 |
| | 3,591 |
| | 1,573 |
| | 30,707 |
|
| | | | Year Ended December 31, 2018 |
| | | | | | | | | | | | Reporting Segment | | Corporate and Other | | Consolidated |
| (in thousands) | Healthcare | | Research | | Technology | | Corporate and Other | | Consolidated | Healthcare | | Research | |
| 2017 | | | | | | | | | | |
| Revenue | $ | 234,385 |
| | $ | 38,790 |
| | $ | 13,601 |
| | $ | — |
| | $ | 286,776 |
| $ | 338,812 |
| | $ | 50,561 |
| | $ | 10,099 |
| | $ | 399,472 |
|
| Intersegment revenue | — |
| | — |
| | 14,793 |
| | (14,793 | ) | | — |
| |
| Gross profit | | 220,883 |
| | 21,603 |
| | 8,000 |
| | 250,486 |
|
| Income/(loss) before income taxes | 52,054 |
| | 1,214 |
| | 3,807 |
| | (67,471 | ) | | (10,396 | ) | 98,135 |
| | 6,228 |
| | (62,859 | ) | | 41,504 |
|
| Depreciation and amortization | 29,255 |
| | 4,148 |
| | 1,045 |
| | (5,887 | ) | | 28,561 |
| 33,119 |
| | 3,723 |
| | 3,326 |
| | 40,168 |
|
| Capital expenditures | 12,542 |
| | 1,274 |
| | 749 |
| | (868 | ) | | 13,697 |
| 20,258 |
| | 3,272 |
| | 1,107 |
| | 24,637 |
|
97 |
| | | | | | | | | | | | | | | |
| | Year Ended December 31, 2017 |
| | Reporting Segment | | Corporate and Other | | Consolidated |
| (reclassified, in thousands) | Healthcare | | Research | | |
| Revenue | $ | 234,385 |
| | $ | 38,790 |
| | $ | 13,601 |
| | $ | 286,776 |
|
| Gross profit | 153,029 |
| | 15,909 |
| | 3,432 |
| | 172,370 |
|
| Income/(loss) before income taxes | 52,054 |
| | 1,214 |
| | (63,664 | ) | | (10,396 | ) |
| Depreciation and amortization | 29,255 |
| | 4,148 |
| | (4,842 | ) | | 28,561 |
|
| Capital expenditures | 12,542 |
| | 1,274 |
| | (119 | ) | | 13,697 |
|
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
|
| | | | | | | | | | | | | | | | | | | |
| (reclassified, in thousands) | Healthcare | | Research | | Technology | | Corporate and Other | | Consolidated |
| 2016 | | | | | | | | | |
| Revenue | $ | 165,664 |
| | $ | 32,565 |
| | $ | 10,103 |
| | $ | — |
| | $ | 208,332 |
|
| Intersegment revenue | — |
| | — |
| | 11,456 |
| | (11,456 | ) | | — |
|
| Income/(loss) before income taxes | 53,025 |
| | 2,229 |
| | 3,862 |
| | (43,346 | ) | | 15,770 |
|
| Depreciation and amortization | 10,216 |
| | 3,837 |
| | 517 |
| | (301 | ) | | 14,269 |
|
| Capital expenditures | 8,885 |
| | 1,941 |
| | 73 |
| | — |
| | 10,899 |
|
|
| | | | | | | | | | | | | | | | | | | |
| (reclassified, in thousands) | Healthcare | | Research | | Technology | | Corporate and Other | | Consolidated |
| 2015 | | | | | | | | | |
| Revenue | $ | 145,963 |
| | $ | 21,853 |
| | $ | 10,697 |
| | $ | — |
| | $ | 178,513 |
|
| Intersegment revenue | 7 |
| | — |
| | 10,224 |
| | (10,231 | ) | | — |
|
| Income/(loss) before income taxes | 38,322 |
| | 540 |
| | 4,390 |
| | (35,356 | ) | | 7,896 |
|
| Depreciation and amortization | 7,790 |
| | 3,676 |
| | 371 |
| | 651 |
| | 12,488 |
|
| Capital expenditures | 9,155 |
| | 4,373 |
| | 72 |
| | — |
| | 13,600 |
|
18.19. Legal Proceedings
The final outcome of any current or future litigation or governmental or internal investigations cannot be accurately predicted, nor can we predict any resulting penalties, fines or other sanctions that may be imposed at the discretion of federal or state regulatory authorities. We record accruals for such contingencies to the extent that we conclude it is probable that a liability has been incurred and the amount of the loss can be estimated.
Mednet Settlement
In the third quarter of 2017, a settlement was reached with the selling stockholder of Mednet Healthcare Technologies, Inc., Heartcare Corporation of America, Inc., Universal Medical, Inc., and Universal Medical Laboratory, Inc. (together, “Mednet”(collectively, “Mednet”), whereby 79,333 shares of BioTelemetry common stock with a fair value of $2.8 million were returned to the Company.us. These shares were part of the
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
consideration paid in the acquisition of Mednet and had been subject to certain terms and conditions set forth in the Stock Purchase Agreement (the “Agreement”“Mednet Agreement”). In accordance with the terms of the Mednet Agreement, we sought indemnification for alleged breaches of certain representations and warranties. Accordingly, in 2016 we recorded a $1.4 million indemnification asset. However, as a result of the settlement’s fair value exceeding the indemnification asset recorded, a gain of $1.3 million was recorded as a component of other non-operating expense,(expense)/income, net in theour consolidated statements of operations for the year ended December 31, 2017.
United States Department of Health and Human Services’ Office for Civil Rights Settlement
In 2011, we experienced the theft of two2 unencrypted laptop computers and, as a result, were required to provide notices under the HIPAAHealth Insurance Portability and Accountability Act Breach Notification Rule to the United States Department of Health and Human Services’ Office for Civil Rights (“OCR”OCR”). During the first quarter of 2017, the OCR concluded its investigation into the matter and reached a settlement agreement with us. Per the agreement, we paid
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
the OCR $2.5 million and agreed to submit a two-year corrective action plan. We did not admit any liability or wrongdoing. As a result of the settlement, we recorded a non-operating charge of $2.5 million to other non-operating expense,(expense)/income, net in theour consolidated statements of operations and comprehensive income/(loss) for the year ended December 31, 2017.
ZTech, Inc., Biorita LLC, and the Cleveland Clinic Foundation Arbitration
In January 2017, ZTech, Inc., Biorita LLC, and the Cleveland Clinic Foundation (the “Claimants”(collectively, the “Claimants”) filed an arbitration demand against LifeWatch with the American Arbitration Association. Claimants alleging alleged that LifeWatch violated the 2015 Stock Purchase Agreement for the purchase of FlexLife Health, Inc., a remote international normalized ratioINR monitoring business. The demand alleges alleged LifeWatch did not make commercially reasonable efforts to achieve certain conditions precedent and did not have a reasonable basis for terminating the business line. Claimants seek liquidated damages and attorneys’ fees. We are vigorously defendingOn May 9, 2018, the arbitration panel issued an award including accrued interest against these claims and are seeking recoveryLifeWatch in the amount of attorneys’ fees$6.0 million. The award liability, plus the accrued interest through July 12, 2017, was recorded as a measurement period adjustment related to our defense.LifeWatch acquisition due to new facts learned that existed as of the acquisition date (see “Note 4. Acquisitions”). The arbitration hearinginterest accrued since the acquisition date of LifeWatch was held in February 2018, and we are awaitingrecorded as a decision. While we believe that the riskcomponent of loss in this arbitration is improbable, we cannot determine, nor can we estimate, the rangeother non-operating (expense)/income, net within our consolidated statements of potential loss. Accordingly, as we do not believe that a loss is probable, in accordance with authoritative guidance on the evaluation of loss contingencies, we have not recorded an accrual related to this matter.
ScottCare Litigation
In May 2012, CardioNet, Inc. (“CardioNet”) filed suit against The ScottCare Corporation and Ambucor Health Solutions, Inc. (“ScottCare”) in the U.S. District Courtoperations for the Eastern District of Pennsylvania for patent infringement. We are seeking an injunction against each defendant, as well as monetary damages. ScottCare has asserted counterclaims alleging the patents in the suit are invalid and not infringed.year ended December 31, 2018. The trial court heard argument on motions for summary judgment and motions to limit expert testimony in June 2015, but has not yet issued rulings on these motions. ScottCare has dropped all invalidity challenges with respect to onetotal amount of the patents in the suit. The parties are awaiting a trial date. We are vigorously pursuing our claimsaward and defending against the counterclaims. The probable outcome of this matter cannot be determined, nor can we estimate a range of potential loss. Therefore, in accordance with authoritative guidance on the evaluation of loss contingencies, we have not recorded an accrual related to this matter.
InfoBionic Litigation
CardioNet, LLC and Braemar Manufacturing, LLC filed a patent infringement lawsuit against InfoBionic, Inc. (“InfoBionic”)accrued interest was paid in May 2015, in the U.S. District Court for the District of Massachusetts, and filed an amended complaint in March 2016. We are seeking an injunction and enhanced damages for willful infringement because InfoBionic had prior knowledge of some or all of the asserted patents. We are also asserting claims for unfair competition and misappropriation of trade secrets due to its discovery that InfoBionic is in unauthorized possession of confidential and proprietary materials of ours, including source code. A trial date has not been set.2018.
In March 2017, we filed a second infringement action in the same District Court asserting infringement of one additional patent seeking an injunction and enhanced damages for willful infringement. InfoBionic moved to dismiss the complaint in this action in June 2017, and the parties are awaiting a ruling from the Court.
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
We also initiated an arbitration proceeding against InfoBionic with the American Arbitration Association in July 2017 asserting claims of misappropriation of trade secrets, unfair competition, and unjust enrichment as a result of our discovery that InfoBionic is in unauthorized possession of our confidential and proprietary materials, including source code. We are seeking monetary and injunctive relief.
In response to our infringement assertion, InfoBionic filed several petitions at the United States Patent and Trademark Office (“USPTO”) for Inter Partes review (“IPR”) of certain of our patents. The USPTO denied institution of IPR regarding certain patents and found certain of our claims in our patents to be unpatentable. In July 2017 we filed an appeal with the Federal Circuit challenging the unpatentability findings.
BIOTELEMETRY, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
19.20. Quarterly Financial Data (Unaudited)
The following tables summarize the unaudited quarterly financial data for the last two fiscal years. Net Income, basic net income per shareyears:
|
| | | | | | | | | | | | | | | |
| (in thousands, except per share amounts) | First Quarter | | Second Quarter | | Third Quarter | | Fourth Quarter |
| 2019 | | | | | | | |
| Total revenue | $ | 103,979 |
| | $ | 111,803 |
| | $ | 111,291 |
| | $ | 112,034 |
|
| Gross profit | 64,778 |
| | 70,240 |
| | 69,339 |
| | 69,917 |
|
| Net income | 11,685 |
| | 8,300 |
| | 8,283 |
| | 1,576 |
|
| Net income attributable to BioTelemetry, Inc. | 11,685 |
| | 8,300 |
| | 8,283 |
| | 1,576 |
|
| Basic net income per share attributable to BioTelemetry, Inc. | $ | 0.35 |
| | $ | 0.25 |
| | $ | 0.24 |
| | $ | 0.05 |
|
| Diluted net income per share attributable to BioTelemetry, Inc. | $ | 0.32 |
| | $ | 0.23 |
| | $ | 0.23 |
| | $ | 0.04 |
|
| | | | | | | | |
| 2018 | | | | | | | |
| Total revenue | $ | 94,496 |
| | $ | 101,360 |
| | $ | 100,013 |
| | $ | 103,603 |
|
| Gross profit | 58,048 |
| | 65,755 |
| | 62,737 |
| | 63,946 |
|
| Net income | 5,036 |
| | 10,444 |
| | 16,001 |
| | 10,393 |
|
| Net income attributable to BioTelemetry, Inc. | 5,982 |
| | 10,444 |
| | 16,001 |
| | 10,393 |
|
| Basic net income per share attributable to BioTelemetry, Inc. | $ | 0.18 |
| | $ | 0.32 |
| | $ | 0.48 |
| | $ | 0.31 |
|
| Diluted net income per share attributable to BioTelemetry, Inc. | $ | 0.17 |
| | $ | 0.29 |
| | $ | 0.45 |
| | $ | 0.29 |
|
21. Subsequent Event
On January 27, 2020, we entered into an Amended and diluted net income per shareRestated Credit Agreement (the “2020 Credit Agreement”) with Truist Bank (successor to SunTrust Bank) as agent (the “Agent”) for the first three quarterslenders (the “Lenders”), and as issuing bank and swingline lender, which amends the SunTrust Credit Agreement entered into by the parties on July 12, 2017.
Pursuant to the 2020 Credit Agreement, the Lenders agreed to provide us a $400.0 million senior secured revolving credit facility, which includes a $25.0 million sublimit for the issuance of 2016 have been recast in accordance withstandby letters of credit and a $40.0 million sublimit for swingline loans. The proceeds of the adoptionrevolving facility was used to refinance indebtedness under the SunTrust Credit Agreement and to fund working capital and general corporate purposes. We may increase commitments to the revolving facility or establish new incremental term loans at any time on or before the final maturity date of ASU 2016-09.the revolving facility, which is January 27, 2025. Incremental term loans under the 2020 Credit Agreement will be used to fund future acquisitions, working capital and general corporate purposes.
|
| | | | | | | | | | | | | | | |
| (in thousands, except per share amounts) | First Quarter | | Second Quarter | | Third Quarter | | Fourth Quarter |
| 2017 | | | | | | | |
| Total revenue | $ | 55,881 |
| | $ | 58,129 |
| | $ | 81,023 |
| | $ | 91,743 |
|
| Gross profit | 32,909 |
| | 35,967 |
| | 49,069 |
| | 54,425 |
|
| Net income/(loss) | 196 |
| | 1,726 |
| | (2,564 | ) | | (16,501 | ) |
| Net income/(loss) attributable to BioTelementry, Inc. | 196 |
| | 1,726 |
| | (2,285 | ) | | (15,593 | ) |
| Basic net income/(loss) per share attributable to BioTelemetry, Inc. | $ | 0.01 |
| | $ | 0.06 |
| | $ | (0.07 | ) | | $ | (0.48 | ) |
| Diluted net income/(loss) per share attributable to BioTelemetry, Inc. | $ | 0.01 |
| | $ | 0.05 |
| | $ | (0.07 | ) | | $ | (0.48 | ) |
| | | | | | | | |
| 2016 | | | | | | | |
| Total revenue | $ | 48,640 |
| | $ | 52,680 |
| | $ | 53,055 |
| | $ | 53,957 |
|
| Gross profit | 30,627 |
| | 32,921 |
| | 32,866 |
| | 33,036 |
|
| Net income | 4,097 |
| | 4,697 |
| | 4,195 |
| | 40,448 |
|
| Net income attributable to BioTelementry, Inc. | 4,097 |
| | 4,697 |
| | 4,195 |
| | 40,448 |
|
| Basic net income per share attributable to BioTelemetry, Inc. | $ | 0.15 |
| | $ | 0.17 |
| | $ | 0.15 |
| | $ | 1.43 |
|
| Diluted net income per share attributable to BioTelemetry, Inc. | $ | 0.14 |
| | $ | 0.15 |
| | $ | 0.14 |
| | $ | 1.30 |
|
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Prior to the filing of this Annual Report on Form 10-K, an evaluation was performed under the supervision of and with the participation of our management, including the Chief Executive Officer (“CEO”CEO”) and Chief Financial Officer (“CFO”CFO”), of the effectiveness of our disclosure controls and procedures. Based on the evaluation, the CEO and CFO have concluded that, as of December 31, 2017,2019, our disclosure controls and procedures are effective to ensure that information required to be disclosed in reports that it fileswe file or submitssubmit under the Securities Exchange Act of 1934 (“Exchange Act”), is recorded, processed, summarized and reported within the time periods specified in SEC rules and forms, and is accumulated and communicated to our management, as appropriate, to allow timely decisions regarding required disclosure. It should be noted that the design of any system of controls is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions, regardless of how remote.
Changes in Internal Control over Financial Reporting
On July 12, 2017, we completed the acquisition of LifeWatch. We are in the process of integrating the acquired LifeWatch entities and our management is in the process of evaluating any related changes to our internal control over financial reporting as a result of this integration. Except for any changes relating to this integration, thereThere has been no change in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act)Act) for the yearquarter ended December 31, 2017,2019, that materially affected or areis reasonably likely to materially affect our internal control over financial reporting.
MANAGEMENT’S REPORT ON INTERNAL CONTROL OVER FINANCIAL REPORTING
Management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act.Act. Our internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with U.S. generally accepted accounting principles. Our internal control over financial reporting includes those policies and procedures that:
| |
| (i) | pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets; |
| |
| (ii) | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of management and directors; and |
| |
| (iii) | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements. |
Because of inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
The effectiveness of our internal control over financial reporting did not include the internal controls of LifeWatch,Geneva, which were included in our consolidated financial statements for the year ended December 31, 2017,2019, due to the timing of the acquisitions. LifeWatchacquisition. Based on the fair value of the net assets acquired on the date of acquisition, Geneva comprised 19%13% of total assets and 40%22% of net assets as of December 31, 2017.2019.
Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2017.2019. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”) in Internal Control—Integrated Framework (2013). Based on management’s assessment and those criteria, management has concluded that our internal control over financial reporting was effective as of December 31, 2017.2019.
The effectiveness of our internal control over financial reporting as of December 31, 20172019 has been audited by Ernst & Young LLP, an independent registered public accounting firm, as stated in their report included in this Annual Report on Form 10-K.
Report of Independent Registered Public Accounting Firm
The Stockholders and the Board of Directors of BioTelemetry, Inc.
Opinion on Internal Control over Financial Reporting
We have audited BioTelemetry, Inc.’s internal control over financial reporting as of December 31, 2017,2019, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the COSO criteria). In our opinion, BioTelemetry, Inc. (the Company) maintained, in all material respects, effective internal control over financial reporting as of December 31, 2017,2019, based on the COSO criteria.
As indicated in the accompanying Management’s Report on Internal Control over Financial Reporting, management’s assessment of and conclusion on the effectiveness of internal control over financial reporting did not include the internal controls of Lifewatch AG,Geneva Healthcare Inc., which is included in the 20172019 consolidated financial statements of the Company and constituted 19%13% and 40%22% of total and net assets, respectively as ofat December 31, 2017.2019. Our audit of internal control over financial reporting of the Company also did not include an evaluation of the internal control over financial reporting of Lifewatch AG.Geneva Healthcare Inc.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated balance sheets of the Company as of December 31, 20172019 and 2016,2018, the related consolidated statements of operations, and comprehensive income/income (loss), cash flows and equity for each of the three years in the period ended December 31, 2017,2019, and the related notes and schedule listed in the Index at Item 15(a) of the Company and our report dated February 26, 201827, 2020 expressed an unqualified opinion thereon.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects.
Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
|
| |
| /s/ ERNST & YOUNG LLP | |
| | |
| Philadelphia, Pennsylvania | |
February 26, 201827, 2020 | |
Item 9B. Other Information
None.
PART III
Item 10. Directors, Executive Officers and Corporate Governance
Information with respect to this Item is incorporated by reference from our definitive proxy statement in connection with the 20182020 Annual Meeting of Stockholders, or the Proxy Statement, unless the Proxy Statement is not filed by May 1, 2018,April 30, 2020, in which case we will amend this Form 10-K to provide the omitted information in accordance with the requirements of Instruction G to Form 10-K.
BioTelemetry emphasizes the importance of professional business conduct and ethics through its corporate governance initiatives. Our Board of Directors has adopted a code of business conduct and ethics that applies to all employees, directors and officers, including our principal executive officer and principal financial officer. Our corporate governance information and materials, including our Code of Business Conduct and Ethics, are posted under “Corporate Governance” in the Investors section of our website at www.gobio.com. Our Board of Directors regularly reviews corporate governance developments and modifies these materials and practices as warranted. To the extent we make amendments to or grant waivers from our Code of Business Conduct and Ethics in the future, we intend to disclose the amendments and waivers on our website.
Item 11. Executive Compensation
Information required by this Item is incorporated by reference from the Proxy Statement unless the Proxy Statement is not filed on or before May 1, 2018,April 30, 2020, in which case we will amend this Form 10-K to provide the omitted information in accordance with the requirements of Instruction G to Form 10-K.
| |
Item 1212. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
Information required by this Item is incorporated by reference from the Proxy Statement unless the Proxy Statement is not filed on or before May 1, 2018,April 30, 2020, in which case we will amend this Form 10-K to provide the omitted information in accordance with the requirements of Instruction G to Form 10-K.
Item 13. Certain Relationships and Related Transactions, and Director Independence
Information required by this Item is incorporated by reference from the Proxy Statement unless the Proxy Statement is not filed on or before May 1, 2018,April 30, 2020, in which case we will amend this Form 10-K to provide the omitted information in accordance with the requirements of Instruction G to Form 10-K.
Item 14. Principal Accountant Fees and Services
Information required by this Item is incorporated by reference from the Proxy Statement unless the Proxy Statement is not filed on or before May 1, 2018,April 30, 2020, in which case we will amend this Form 10-K to provide the omitted information in accordance with the requirements of Instruction G to Form 10-K.
PART IV
Item 15. Exhibits and Financial Statement Schedules
| |
| (a) | The following financial statements, schedules and exhibits are filed as part of this Annual Report on Form 10-K |
| |
| 1. | Financial Statements—The Financial Statements required by this item are listed on the Index to Financial Statements in Part II, Item 8 of this Annual Report on Form 10-K. |
| |
| 2. | Financial Statement Schedules |
Schedule II—Valuation and Qualifying Accounts and Reserves; and
Other financial statement schedules are not included because they are not required or the information is otherwise shown in the financial statements or notes thereto.
| |
| 3. | Exhibits—The exhibits listed on the accompanying Exhibit Index are filed as part of, or are incorporated by reference into, this Annual Report on Form 10-K. |
| |
| (b) | See Item 15(a)(3) above. |
| |
| (c) | See Item 15(a)(2) above. |
Item 16. Form 10-K Summary
None.
SCHEDULE II
| | | (in thousands) | Beginning Balance | | Additions
| | Deductions
| | Ending Balance | Beginning Balance | | Additions | | Deductions | | Ending Balance |
| Allowance for Doubtful Accounts | | | | | | | | | | | | | | |
| Year ended December 31, 2019 | | $ | 25,613 |
| | $ | 21,768 |
| | $ | (15,400 | ) | | $ | 31,981 |
|
| Year ended December 31, 2018 | | $ | 16,981 |
| | $ | 22,222 |
| | $ | (13,590 | ) | | $ | 25,613 |
|
| Year ended December 31, 2017 | $ | 12,863 |
| | $ | 13,291 |
| | $ | (9,173 | ) | | $ | 16,981 |
| $ | 12,863 |
| | $ | 13,291 |
| | $ | (9,173 | ) | | $ | 16,981 |
|
| Year ended December 31, 2016 | $ | 11,601 |
| | $ | 9,931 |
| | $ | (8,669 | ) | | $ | 12,863 |
| |
| Year ended December 31, 2015 | $ | 10,662 |
| | $ | 8,047 |
| | $ | (7,108 | ) | | $ | 11,601 |
| |
| | | (in thousands) | Beginning Balance | | Additions
| | Deductions
| | Ending Balance | Beginning Balance | | Additions | | Deductions | | Ending Balance |
| Tax Valuation Allowance | | | | | | | | | | | | | | |
| Year ended December 31, 2019 | | $ | 3,021 |
| | $ | 856 |
| | $ | — |
| | $ | 3,877 |
|
| Year ended December 31, 2018 | | $ | 6,032 |
| | $ | — |
| | $ | (3,011 | ) | | $ | 3,021 |
|
| Year ended December 31, 2017 | $ | 95 |
| | $ | 5,937 |
| | $ | — |
| | $ | 6,032 |
| $ | 95 |
| | $ | 5,937 |
| | $ | — |
| | $ | 6,032 |
|
| Year ended December 31, 2016 | $ | 49,759 |
| | $ | 1,966 |
| | $ | (51,630 | ) | | $ | 95 |
| |
| Year ended December 31, 2015 | $ | 52,998 |
| | $ | 1,734 |
| | $ | (4,973 | ) | | $ | 49,759 |
| |
EXHIBIT INDEX
|
| | | | | | | | | | | | |
| | | | | | Incorporated by Reference | |
Exhibit Number | | Description | | Form | | File No. | | Exhibit | | Filing Date | Filed Herewith |
| | 2.3 | | | | 10-K | | 000-55039 | | 2.3 | | February 22, 2017 | |
| | 2.4 | | | | 10-Q | | 000-55039 | | 2.1 | | November 7, 2017 | |
| | 3.1 | | | | 10-Q | | 000-55039 | | 3.1 | | August 8, 2017 | |
| | 3.2 | | | | 10-Q | | 000-55039 | | 3.2 | | August 8, 2017 | |
| | 10.1 | | | | S-1 | | 333-145547 | | 10.1 | | August 17, 2007 | |
| | 10.2* | | | | S-8 | | 333-218228 | | 10.1 | | May 25, 2017 | |
| | 10.3* | | | | S-1 | | 333-145547 | | 10.4 | | February 28, 2008 | |
| | 10.4* | | | | S-8 | | 333-218228 | | 10.2 | | May 25, 2017 | |
| | 10.5* | | | | 8-K | | 001-33993 | | 10.2 | | October 28, 2008 | |
| | 10.6* | | | | 8-K | | 001-33993 | | 99.5 | | January 28, 2009 | |
| | 10.7* | | | | 8-K | | 001-33993 | | 99.2 | | June 18, 2010 | |
| | 10.8* | | | | 10-K | | 001-33993 | | 10.36 | | February 23, 2010 | |
| | 10.9* | | | | 10-K | | 001-33993 | | 10.38 | | February 25, 2011 | |
| | 10.10* | | | | 10-Q | | 001-33993 | | 10.1 | | May 6, 2011 | |
| | 10.11* | | | | 10-K | | 001-33993 | | 10.26 | | February 22, 2013 | |
| | 10.12 | | | | 10-Q | | 000-55039 | | 10.1 | | November 7, 2017 | |
| | 21 | | | | | | | | | | | † |
| | 23 | | | | | | | | | | | † |
|
| | | | | | | | | | | | | |
| | | | | | Incorporated by Reference | | |
Exhibit Number | | Description | | Form | | File No. | | Exhibit | | Filing Date | | Filed/Furnished Herewith |
| | 2.1 | | | | 10-Q | | 000-55039 | | 2.1 | | April 25, 2019 | | |
| | 3.1 | | | | 10-Q | | 000-55039 | | 3.1 | | August 8, 2017 | | |
| | 3.2 | | | | 8-K | | 000-55039 | | 3.2 | | February 28, 2019 | | |
| | 4.1 | | | | | | | | | | | | † |
| | 10.1 | | | | S-1 | | 333-145547 | | 10.1 | | August 17, 2007 | | |
| | 10.2* | | | | S-8 | | 333-218228 | | 10.1 | | May 25, 2017 | | |
| | 10.2(a) | | | | 10-Q | | 000-55039 | | 10.2 | | April 27, 2018 | | |
| | 10.2(b) | | | | 10-Q | | 000-55039 | | 10.3 | | April 27, 2018 | | |
| | 10.2(c) | | | | 10-Q | | 000-55039 | | 10.4 | | April 27, 2018 | | |
| | 10.3* | | | | S-1 | | 333-145547 | | 10.4 | | February 28, 2008 | | |
| | 10.4* | | | | S-8 | | 333-218228 | | 10.2 | | May 25, 2017 | | |
| | 10.5* | | | | 10-Q | | 000-55039 | | 10.1 | | July 31, 2019 | | |
| | 10.6* | | | | 8-K | | 001-33993 | | 99.2 | | June 18, 2010 | | |
| | 10.7* | | | | 10-K | | 001-33993 | | 10.36 | | February 23, 2010 | | |
| | 10.8* | | | | 10-K | | 001-33993 | | 10.38 | | February 25, 2011 | | |
| | 10.9* | | | | 10-Q | | 001-33993 | | 10.1 | | May 6, 2011 | | |
| | 10.10* | | | | 10-K | | 001-33993 | | 10.26 | | February 22, 2013 | | |
| | 10.11* | | | | | | | | | | | | † |
| | | | | | | | | | | | | | |
|
| | | | | | | | | | | | | |
| | | | | | Incorporated by Reference | | |
Exhibit Number | | Description | | Form | | File No. | | Exhibit | | Filing Date | Filed | Filed/Furnished Herewith |
| 10.12* | | | | 8-K | | 000-55039 | | 10.1 | | August 22, 2019 | | |
| 10.13 | | | | | | | | | | | | † |
| 21 | | | | | | | | | | | | † |
| 23 | | | | | | | | | | | | † |
| | 31.1 | | | | | | | | | | | | † |
| | 31.2 | | | | | | | | | | | | † |
| | 32 | | | | | | | | | | | | + |
| 101.INS | | XBRL Instance Document | | | | | | | | | | † |
| | 101.INS101.DEF | | XBRL Instance Document.Taxonomy Extension Definition Linkbase Document | | | | | | | | | | † |
| | 101.DEF101.SCH | | XBRL Taxonomy Extension Definition Linkbase Document.Schema Document | | | | | | | | | | † |
| | 101.SCH101.CAL | | XBRL Taxonomy Extension Schema Document.Calculation Linkbase Document | | | | | | | | | | † |
| | 101.CAL101.LAB | | XBRL Taxonomy Extension CalculationLabel Linkbase Document.Document | | | | | | | | | | † |
| | 101.LAB101.PRE | | XBRL Taxonomy Extension LabelPresentation Linkbase Document.Document | | | | | | | | | |
| 101.PRE | | XBRL Taxonomy Extension Presentation Linkbase Document. | | | | | | | | | † |
| |
| * | Indicates a management plan or compensatory plan or arrangement. |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this reportAnnual Report on Form 10-K to be signed on its behalf by the undersigned, thereunto duly authorized.
|
| | | | |
| Date: | February 26, 201827, 2020 | BioTelemetry, Inc. |
| | | |
| | | By: | | /s/ JOSEPHJoseph H. CAPPERCapper |
| | | | | Joseph H. Capper President and Chief Executive Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this reportAnnual Report on Form 10-K has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
| | | | |
| Signature | | Title | | Date |
| | | |
/s/ JOSEPHJoseph H. CAPPERCapper | President and Chief Executive Officer (Principal Executive Officer); Director | February 26, 201827, 2020 |
| Joseph H. Capper |
| | | |
/s/ HEATHERHeather C. GETZGetz | Executive Vice President and Chief Financial and Administrative Officer (Principal Financial and Accounting Officer) | February 26, 201827, 2020 |
| Heather C. Getz, CPA |
| | | |
/s/ KIRKKirk E. GORMANGorman | Chairman and Director | February 26, 201827, 2020 |
| Kirk E. Gorman |
| | | |
/s/ ANTHONYAnthony J. CONTIConti | Director | February 26, 201827, 2020 |
| Anthony J. Conti |
| | | |
/s/ JOSEPH A. FRICKLaura Dietch | Director | February 26, 201827, 2020 |
| Laura Dietch |
| | |
| /s/ Joseph A. Frick | Director | February 27, 2020 |
| Joseph A. Frick |
| | | |
/s/ COLIN HILLColin Hill | Director | February 26, 201827, 2020 |
| Colin Hill |
| | | |
/s/ REBECCA RIMELTiffany Olson | Director | February 26, 201827, 2020 |
| Tiffany Olson |
| | |
| /s/ Stephan Rietiker | Director | February 27, 2020 |
| Stephan Rietiker, M.D. |
| | |
| /s/ Rebecca W. Rimel | Director | February 27, 2020 |
| Rebecca W. Rimel |
| | | |
/s/ ROBERTRobert J. RUBINRubin | Director | February 26, 201827, 2020 |
| Robert J. Rubin, M.D. |