UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
__________________
Form 10-K
__________________
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES
EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 20202023
or
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to .
Commission File No. 001-38403
__________________________
CRONOS GROUP INC.
(Exact name of Registrant as specified in its Charter)
__________________________
| | | | | | | | |
| British Columbia, Canada | | N/A |
| (State or other jurisdiction of | | (I.R.S. Employer |
| incorporation or organization) | | Identification No.) |
| | |
| 111 Peter St., Suite 300 | | |
| Toronto, Ontario | | M5V 2H1 |
| (Address of principal executive offices) | | (Zip Code) |
Registrant’s telephone number, including area code: 416-504-0004
____________________________
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | | | | | | | |
| Title of Each Class | | Trading Symbol | | Name of Each Exchange on Which Registered |
| Common Shares, no par value | | CRON | | The Nasdaq Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No x
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes o No x
Indicate by check mark whether the Registrant: (1) has filed all reports required to be filed by Section 13 or Section 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the Registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the Registrant was required to submit such files). Yes x No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| | | | | | | | | | | | | | |
| Large accelerated filer | xo | | Accelerated filer | ox |
| Non-accelerated filer | o | | Smaller reporting company | ☐o |
| Emerging growth company | ☐o | | | |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant's executive officers during the relevant recovery period pursuant to § 240.10D-1(b). ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. Yes ☒ No o
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No x
As of June 30, 2020,2023, the last business day of the Registrant’s most recently completed second fiscal quarter, the aggregate market value of common shares held by non-affiliates of the Registrant computed by reference to the closing price of $6.01$1.97 per common share on June 30, 20202023 was approximately $1,099,685,039.$391,676,759.
As of February 25, 2021,23, 2024, there were 360,258,680381,298,853 common shares of the Registrant issued and outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Certain information required by Part III of this Annual Report on Form 10-K will either be incorporated into this Annual Report on Form 10-K by reference to the registrant’s definitive proxy statement for its 20212024 Annual Meeting of Shareholders, or will be included in an amendment to this Annual Report on Form 10-K to be filed no later than 120 days after the registrant's fiscal year ended December 31, 2020.
| | | | | | | | | | | | | | |
| | Table of Contents | | |
| | | | |
| | PART I | | |
| Item 1. | | |
| Item 1A. | | |
| Item 1B. | | |
| Item 1C. | | |
| Item 2. | | |
| Item 3. | | |
| Item 4. | | |
| | | | |
| | PART II | | |
| Item 5. | | |
| Item 6. | Selected Financial DataReserved | |
| Item 7. | | |
| Item 7A. | | |
| Item 8. | | |
| Item 9. | | |
| Item 9A. | | |
| Item 9B. | | |
| Item 9C. | | | | |
| | PART III | | |
| Item 10. | | |
| Item 11. | | |
| Item 12. | | |
| Item 13. | | |
| Item 14. | | |
| | | | |
| | PART IV | | |
| Item 15. | | |
Unless otherwise noted or the context indicates otherwise, references in this Annual Report on Form 10-K (this “Annual Report”) to the “Company”, “Cronos Group”“Cronos”, “we”, “us” and “our” refer to Cronos Group Inc., its direct and indirect wholly owned subsidiaries and, if applicable, its joint ventures and investments accounted for by the equity method; the term “cannabis” means the plant of any species or subspecies of genus Cannabis and any part of that plant, including all derivatives, extracts, cannabinoids, isomers, acids, salts, and salts of isomers; the term “U.S. hemp” has the meaning given to the term “hemp” in the U.S. Agricultural Improvement Act of 2018 (the “2018 Farm Bill”), including hemp-derived cannabidiol (“CBD”); and the term “U.S. Schedule I cannabis” means cannabis excluding U.S. hemp.
This report contains references to our trademarks and trade names and to trademarks and trade names belonging to other entities. Solely for convenience, trademarks and trade names referred to in this report may appear without the ® or ™ symbols, but such references are not intended to indicate, in any way, that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto. We do not intend our use or display of other companies’ trademarks or trade names to imply a relationship with, or endorsement or sponsorship of us or our business by, any other companies.
All currency amounts in this Annual Report are stated in U.S. dollars, which is our reporting currency, unless otherwise noted. All references to “dollars” or “$” are to U.S. dollars; all references to “C$” are to Canadian dollars; all references to “A$” are to Australian dollars; and all references to “ILS” are to New Israeli Shekels.
| | (Exchange rates are shown as C$ per $) | (Exchange rates are shown as C$ per $) | As of December 31, | (Exchange rates are shown as C$ per $) | As of December 31, |
| 2020 | | 2019 | | 2018 |
| 2023 | | | 2023 | | 2022 | | 2021 |
| Average rate | Average rate | 1.3411 | | 1.3268 | | 1.2955 | Average rate | 1.3494 | | 1.3017 | | 1.2541 |
| Spot rate | Spot rate | 1.2751 | | 1.2990 | | 1.3639 | Spot rate | 1.3243 | | 1.3554 | | 1.2746 |
| | | | | | | | | | | | | | | | | |
| (Exchange rates are shown as ILS per $) | As of December 31, |
| 2023 | | 2022 | | 2021 |
| Average rate | 3.6819 | | 3.3566 | | 3.2297 |
| Spot rate | 3.6163 | | 3.5178 | | 3.1149 |
All summaries of agreements described herein are qualified by the full text of such agreements (certain of which are filed as exhibits hereto).
PART I
Special Note Regarding Forward-Looking Statements
This Annual Report, the documents incorporated into this Annual Report by reference, other reports we file with, or furnish to, the U.S. Securities and Exchange Commission (“SEC”) and other regulatory agencies, and statements by our directors, officers, other employees and other persons authorized to speak on our behalf contain information that may constitute forward-looking information and forward-looking statements within the meaning of applicable U.S. and Canadian securities laws and court decisions (collectively, “Forward-Looking Statements”), which are based upon our current internal expectations, estimates, projections, assumptions and beliefs. All information that is not clearly historical in nature may constitute Forward-Looking Statements. In some cases, Forward-Looking Statements can be identified by the use of forward-looking terminology, such as “expect”, “likely”, “may”, “will”, “should”, “intend”, “anticipate”, “potential”, “proposed”, “estimate” and other similar words, expressions and phrases, including negative and grammatical variations thereof, or statements that certain events or conditions “may” or “will” happen, or by discussion of strategy. Forward-Looking Statements include estimates, plans, expectations, opinions, forecasts, projections, targets, guidance or other statements that are not statements of historical fact.
Forward-Looking Statements include, but are not limited to, statements with respect to:
•expectations related to the uncertaintieswar involving Israel and Hamas (the “Israel-Hamas War”) and its impact on our operations in Israel, the supply of product in the market and the demand for product by medical patients in Israel, as well as any regional or global escalations to the Israel-Hamas War and its impact to global commerce and stability;
•expectations related to the German and Australian markets, including our strategic partnerships with Cansativa GmbH (“Cansativa”) and Vitura Health Limited (“Vitura”), respectively, and our plans to distribute the PEACE NATURALS® brand in Germany;
•expectations related to our announcement of cost-cutting measures, including our decision to wind down operations at our Winnipeg, Manitoba facility and list the facility for sale, the expected costs and benefits from the wind-down of production activities at the facility, challenges and effects related thereto as well as changes in strategy, metrics, investments, costs, operating expenses, employee turnover and other changes with respect thereto;
•expectations related to the impact of our decision to exit our U.S. hemp-derived cannabinoid product operations, including the costs, expenses and write-offs associated therewith, the impact on our operations and our financial statements and any future plans to re-enter the U.S. market;
•expectations related to our announced realignment (the “Realignment”) and any progress, challenges and effects related thereto as well as changes in strategy, metrics, investments, reporting structure, costs, operating expenses, employee turnover and other changes with respect thereto;
•the timing of the change in the nature of operations at, and the announced sale-leaseback of, our facility in Stayner, Ontario (the “Peace Naturals Campus”) and the expected costs and benefits from the wind-down of certain production activities at the Peace Naturals Campus;
•our ability to complete the sale and leaseback of the Peace Naturals Campus pursuant to the agreement with Future Farmco Canada Inc. (“Future Farmco”)
•our ability to acquire raw materials from suppliers, including Cronos Growing Company Inc. (“Cronos GrowCo”), and the costs and timing associated therewith;
•expectations regarding the potential success of, and the costs and benefits associated with, the COVID-19 pandemic, including our ability, and the abilities of our joint ventures, strategic alliances and equity investments, including the strategic partnership (the “Ginkgo Strategic Partnership”) with Ginkgo Bioworks Holdings, Inc. (“Ginkgo”);
•our ability or plans to identify, develop, commercialize or expand our technology and research and development (“R&D”) initiatives in cannabinoids, or the success thereof;
•expectations regarding revenues, expenses, gross margins and capital expenditures;
•expectations regarding our future production and manufacturing strategy and operations, the costs and timing associated therewith and the receipt of applicable production and sale licenses;
•the ongoing impact of the legalization of additional cannabis product types and forms for adult-use in Canada, including federal, provincial, territorial and municipal regulations pertaining thereto, the related timing and impact thereof and our suppliersintentions to participate in such markets;
•the legalization of the use of cannabis for medical or adult-use in jurisdictions outside of Canada, including the United States and distributors,Germany, the related timing and impact thereof and our intentions to effectively dealparticipate in such markets, if and when such use is legalized;
•the grant, renewal, withdrawal, suspension, delay and impact of any license or supplemental license to conduct activities with the restrictions, limitations and health issues presented by the COVID-19 pandemic, thecannabis or any amendments thereof;
•our ability to continue our production, distributionsuccessfully create and salelaunch brands and cannabis products;
•expectations related to the differentiation of our products, including through the utilization of rare cannabinoids;
•the benefits, viability, safety, efficacy, dosing and demand forsocial acceptance of cannabis, including CBD and the use of our products by consumers;other cannabinoids;
•laws and regulations and any amendments thereto applicable to our business and the impact thereof, including uncertainty regarding the application of United States (“U.S.”) state and federal law to cannabis and U.S. hemp (including CBD)CBD and other U.S. hemp-derived cannabinoids) products and the scope of any regulations by the U.S. Food and Drug Administration (the “FDA”), the U.S. Drug Enforcement Administration (the “DEA”), the U.S. Federal Trade Commission (the “FTC”), the U.S. Patent and Trademark Office (the “PTO”) and any state equivalent regulatory agencies over cannabis and U.S. hemp (including CBD) products;CBD and other U.S. hemp-derived cannabinoids) products, including the possibility marijuana is moved from Schedule I to Schedule III under the U.S. Controlled Substances Act;
•the lawsanticipated benefits and regulations and any amendments thereto relating to the U.S. hemp industryimpact of Altria Group Inc.’s investment in the U.S.Company (the “Altria Investment”), including the promulgation of regulations for the U.S. hemp industry by the U.S. Department of Agriculture (the “USDA”) and relevant state regulatory authorities;pursuant to a subscription agreement dated December 7, 2018;
•the grant, renewal and impact of any license or supplemental licenseuncertainties as to conduct activities with cannabis or any amendments thereof;
•our international activities and joint venture interests, including required regulatory approvals and licensing, anticipated costs and timing, and expected impact;
•our ability to successfully createexercise our option (the “PharmaCann Option”) in PharmaCann Inc. (“PharmaCann”), in the near term or the future, in full or in part, including the uncertainties as to the status and launch brands and further create, launch and scale U.S. hemp-derived consumer products and cannabis products;
•the benefits, viability, safety, efficacy, dosing and social acceptancefuture development of federal legalization of cannabis including CBDin the U.S. and other cannabinoids;our ability to realize the anticipated benefits of the transaction with PharmaCann;
•expectations regarding the implementation and effectiveness of key personnel changes;
•the anticipated benefits and impact of the Altria Investment (as defined herein);
•the potential exercise of the Altria Warrant (as defined herein), pre-emptive rights and/or top-up rights in connection with the Altria Investment, including proceeds to us that may result therefrom;
•expectations regarding the use of proceeds of equity financings, including the proceeds from the Altria Investment;
•the legalization of the use of cannabis for medical or adult-use in jurisdictions outside of Canada, the related timingacquisitions and impact thereof and our intentions to participate in such markets, if and when such use is legalized;
•expectations regarding the potential success of, and the costs and benefits associated with, our joint ventures, strategic alliances and equity investments, including the strategic partnership (the “Ginkgo Strategic Partnership”) with Ginkgo Bioworks, Inc. (“Ginkgo”);
•our ability to execute on our strategydispositions and the anticipated benefits of such strategy;therefrom;
•expectations of the amount or frequency of impairment losses, including as a result of the write-down of intangible assets, including goodwill;
•the ongoing impact of the legalizationongoing military conflict between Russia and Ukraine (and resulting sanctions) on our business, financial condition and results of additional cannabis product typesoperations or cash flows;
•our compliance with the terms of the settlement with the SEC (the “Settlement Order”) and forms for adult-use in Canada, including federal, provincial, territorialthe settlement agreement with the Ontario Securities Commission (the “Settlement Agreement”); and municipal regulations pertaining thereto, the related timing and impact thereof and our intentions to participate in such markets;
•the future performance of our business and operations;
•our competitive advantages and business strategies;
•the competitive conditionsimpact of the industry;
•the expected growth in the numberloss of customers using our products;
•our ability or plans to identify, develop, commercialize or expand our technology and R&D initiatives in cannabinoids, or the success thereof;
•expectations regarding acquisitions and dispositions and the anticipated benefits therefrom, including the proposed sale of our Original B.C. Ltd. (“OGBC”) production facility;
•expectations regarding revenues, expenses and anticipated cash needs;
•expectations regarding cash flow, liquidity and sources of funding;
•expectations regarding capital expenditures;
•the expansion of our production and manufacturing, the costs and timing associated therewith and the receipt of applicable production and sale licenses;
•the expected growth in our growing, production and supply chain capacities;
•expectations regarding the resolution of litigation and other legal and regulatory proceedings, reviews and investigations;
•expectations with respect to future production costs;
•expectations with respect to future sales and distribution channels and networks;
•the expected methods to be used to distribute and sell our products;
•the anticipated future gross margins of our operations;
•accounting standards and estimates;
•our ability to timelyrely on private offering exemptions under Regulation D of the Securities Act of 1933, as amended (the “Securities Act”), and effectively remediate any material weaknesses inthe loss of our internal control over financial reporting; and
•expectations regardingstatus as a well-known seasoned issuer, each as a result of the costs and benefits associated with our contracts and agreements with third parties, including under our third-party supply and manufacturing agreements.Settlement Order.
Certain of the Forward-Looking Statements contained herein concerning the industries in which we conduct our business are based on estimates prepared by us using data from publicly available governmental sources, market research, industry analysis and on assumptions based on data and knowledge of these industries, which we believe to be reasonable. However, although generally indicative of relative market positions, market shares and performance characteristics, such data is inherently imprecise. The industries in which we conduct our business involve risks and uncertainties that are subject to change based on various factors, which are described further below.
The Forward-Looking Statements contained herein are based upon certain material assumptions that were applied in drawing a conclusion or making a forecast or projection, including: (i) our ability to effectively navigate developments in the Israel-Hamas War and its impact on our employees and operations in Israel, the abilitiessupply of product in the market and demand for product by medical patients in Israel; (ii) our joint venturesability to efficiently and effectively distribute our PEACE NATURALS® brand in Germany with our strategic partner Cansativa and our suppliers and distributors, to effectively deal with the restrictions, limitations and health issues presented by the COVID-19 pandemic and the ability to continueefficiently and effectively distribute products in Australia with our production, distribution and sale of our products and customer demand for and use of our products; (ii) management’s perceptions of historical trends, current conditions and expected future developments;strategic partner Vitura; (iii) our ability to realize the expected cost-savings and other benefits related to the wind-down of our operations at our
Winnipeg, Manitoba facility, (iv) our ability to realize the expected cost-savings, efficiencies and other benefits of our Realignment and other announced cost-cutting measures and employee turnover related thereto; (v) our ability to efficiently and effectively wind down certain production activities at the Peace Naturals Campus, receive the benefits of the change in the nature of our operations at, and the announced sale-leaseback of, our Peace Naturals Campus and acquire raw materials on a timely and cost-effective basis from third parties, including Cronos GrowCo; (vi) our ability to satisfy all conditions for the sale and leaseback of the Peace Naturals Campus; (vii) our ability to realize anticipated benefits, synergies or generate cash flowrevenue, profits or value from operations; (iv) general economic, financial market, regulatoryour acquisitions and political conditions in which we operate; (v)strategic investments; (viii) the production and manufacturing capabilities and output from our facilities and our joint ventures, strategic alliances and equity investments; (vi) consumer interest in our products; (vii) competition; (viii) anticipated and unanticipated costs; (ix) government regulation of our activities and products including, but not limited to, the areas of cannabis taxation and environmental protection; (x) the timely receipt of any required regulatory authorizations, approvals, consents, permits and/or licenses; (xi) consumer interest in our products; (xii) our ability to obtain qualified staff, equipmentdifferentiate our products, including through the utilization of rare cannabinoids; (xiii) competition; (xiv) anticipated and services in a timely and cost-efficient manner; (xii)unanticipated costs; (xv) our ability to generate cash flow from operations; (xvi) our ability to conduct operations in a safe, efficient and effective manner; (xiii)(xvii) our ability to hire and retain qualified staff, and acquire equipment and services in a timely and cost-efficient manner; (xviii) our ability to exercise the PharmaCann Option and realize the anticipated benefits synergies or generate revenue, profits or value from our recent acquisitions into our existing operations; (xiv)of the transaction with PharmaCann; (xix) our ability to complete planned dispositions, including the sale of OGBC, and, if completed, obtain our anticipated sales price; (xx) general economic, financial market, regulatory and (xv)political conditions in which we operate; (xxi) management’s perceptions of historical trends, current conditions and expected future developments; and (xxii) other considerations that management believes to be appropriate in the circumstances. While our management considers these assumptions to be reasonable based on information currently available to management, there is no assurance that such expectations will prove to be correct.
By their nature, Forward-Looking Statements are subject to inherent risks and uncertainties that may be general or specific and which give rise to the possibility that expectations, forecasts, predictions, projections or conclusions will not prove to be accurate, that assumptions may not be correct and that objectives, strategic goals and priorities will not be achieved. A variety of factors, including known and unknown risks, many of which are beyond our control, could cause actual results to differ materially from the Forward-Looking Statements in this Annual Report and other reports we file with, or furnish to, the SEC and other regulatory agencies and made by our directors, officers, other employees and other persons authorized to speak on our behalf. Such factors include, without limitation, negative impacts on our employees, business and operations in Israel due to the riskIsrael-Hamas War, including that we may not be able to produce, import or sell our products or protect our people or facilities in Israel during the COVID-19 pandemicIsrael-Hamas War; the supply of product in the market and the demand for product by medical patients in Israel; that we may not be able to successfully continue to distribute our products in Germany and Australia or generate material revenue from sales in those markets; that we may not be able to achieve the anticipated benefits of the wind-down of our operations at our Winnipeg, Manitoba facility or be able to access raw materials on a timely and cost-effective basis from third-parties; that we may be unable to further streamline our operations and reduce expenses; that we may not be able to effectively and efficiently re-enter the U.S. market in the future; that we may not be able to wind down certain production activities at, and complete the sale-leaseback of, the Peace Naturals Campus in a disciplined manner or achieve the anticipated benefits of the change in the nature of our operations or be able to access raw materials on a timely and cost-effective basis from third-parties, including Cronos GrowCo;the military conflict between Russia and Ukraine may disrupt our operations and those of our suppliers and distribution channels and negatively impact the demand for and use of our products; the risk that cost savings and any other synergies from the Altria Investment may not be fully realized or may take longer to realize than expected; the risk that we will not complete planned dispositions, including the sale of OGBC, or, if completed, obtain our anticipated sales price; the implementation and effectiveness offailure to execute key personnel changes; futurethe risks that our Realignment, the change in the nature of our operations at the Peace Naturals Campus and our further leveraging of our strategic partnerships will not result in the expected cost-savings, efficiencies and other benefits or will result in greater than anticipated turnover in personnel; lower levels of revenues; the lack of consumer demand for cannabis and U.S. hempour products; our abilityinability to reduce expenses at the level needed to meet our projected net change in cash and cash equivalents; our inability to manage disruptions in credit markets or changes to our credit ratings;markets; unanticipated future levels of capital, environmental or maintenance expenditures, general and administrative and other expenses; the success or timing of completion of ongoing or anticipated capital or maintenance projects; business strategies, growth opportunities and expected investment;not turning out as expected; the adequacylack of our capital resources and liquidity, including but not limited to, availability of sufficient cash flow necessary to execute our business plan (either within the expected timeframe or at all); difficulty raising capital; the potential adverse effects of judicial, regulatory or other proceedings, or threatened litigation or proceedings, on our business, financial condition, results of operations and cash flows; volatility in and/or degradation of general economic, market, industry or business conditions; compliance with applicable environmental, economic, health and safety, energy and other policies and regulations and in particular health concerns with respect to vaping and the use of cannabis and U.S. hemp products in vaping devices; the anticipatedunexpected effects of actions of third parties such as competitors, activist investors or federal (including U.S. federal), state, provincial, territorial or local regulatory authorities or self-regulatory organizations; adverse changes in regulatory requirements in relation to our business and products; legal or regulatory obstacles that could prevent us from being able to exercise the PharmaCann Option and thereby realize the anticipated benefits of the transaction with PharmaCann; dilution of our fully diluted ownership of PharmaCann and the loss of our rights as a result of that dilution; our failure to improve our internal control environment and our systems, processes and procedures; and the factors discussed under the heading “Risk Factors”Part I, Item 1A “Risk Factors” in this Annual Report. Readers are cautioned to consider these and other factors, uncertainties and potential events carefully and not to put undue reliance on Forward-Looking Statements.
Forward-Looking Statements are provided for the purposes of assisting the reader in understanding our financial performance, financial position and cash flows as of and for periods ended on certain dates and to present information about management’s current expectations and plans relating to the future, and the reader is cautioned that thenot to place undue reliance on these Forward-Looking Statements may not be appropriatebecause of their inherent uncertainty and to appreciate the limited purposes for any other purpose. which they are being used by management.
While we believe that the assumptions and expectations reflected in the Forward-Looking Statements are reasonable based on information currently available to management, there is no assurance that such assumptions and expectations will prove to have been correct. Forward-Looking Statements are made as of the date they are made and are based on the beliefs, estimates, expectations and opinions of management on that date. We undertake no obligation to update or revise any Forward-Looking Statements, whether as a result of new information, estimates or opinions, future events or results or otherwise or to explain any material difference between subsequent actual events and such Forward-Looking Statements. The Forward-Looking Statements contained in this Annual Report and other reports we file with, or furnish to, the SEC and other regulatory agencies and made by our directors, officers, other employees and other persons authorized to speak on our behalf are expressly qualified in their entirety by these cautionary statements.
ITEM 1. BUSINESS
General
Cronos Group is incorporated under the laws of the Province of British Columbia with principal executive offices located at 111 Peter Street, Suite 300, Toronto, Ontario M5V 2H1. Our telephone number is +1-416-504-0004, our website is https://thecronosgroup.com/ and the investor relations section of our website is https://ir.thecronosgroup.com/. All references to our website are inactive references, are for informational purposes only and are not intended to incorporate any information from or referenced on our website into this Annual Report.
Our common shares are currently listed on the Toronto Stock Exchange (“TSX”) and on the NASDAQ Global Market (“Nasdaq”) under the trading symbol “CRON.”
Description of the Business
Overview
Cronos Group is an innovative global cannabinoid company with international production and distribution across five continents. Cronos Group is committed to building disruptive intellectual property by advancing cannabis research, technology and product development and aredevelopment. With a passion to responsibly elevate the consumer experience, Cronos is building an iconic brand portfolio. Cronos Group’sCronos’ diverse international brand portfolio includes Spinach®, PEACE NATURALS™, a global wellness platform; two adult-use brands, COVE™NATURALS® and Spinach™; and three U.S. hemp-derived consumer products brands, Lord Jones™, Happy Dance™ and PEACE+™Jones®.
Strategy
Cronos Group seeks to create value for shareholders by focusing on four core strategic priorities:
•growing a portfolio of iconic brands that responsibly elevate the consumer experience;
•developing a diversified global sales and distribution network;
•establishing an efficient global supply chain; and
•creating and monetizing disruptive intellectual property.
Discontinued Operations
In the second quarter of 2023, Cronos exited its U.S. hemp-derived cannabinoid product operations. The exit of the U.S. operations represented a strategic shift that has a major effect on Cronos’ operations and financial results, and as such, qualifies for reporting as discontinued operations in our consolidated statements of net loss and comprehensive loss. Prior period amounts have been reclassified to reflect the discontinued operations classification of the U.S. operations. For further detail on the discontinuation of the U.S. operations, see Note 2 “Discontinued Operations” to the consolidated financial statements under Item 8 of this Annual Report.
Business Segments
Cronos Group reports through two segments: “United States” and “Rest of World.” These two segments represent the geographic regions in which the Company operates and the different product offerings within each geographic region.
United States
On September 5, 2019, as a result of the acquisition (the “Redwood Acquisition”) of four Redwood Holding Group, LLC subsidiaries (collectively, “Redwood”), the Company established the United States segment. Redwood manufactures, markets and distributes U.S. hemp-derived supplements and cosmetic products through e-commerce, retail and hospitality partner channelsBeginning in the United States undersecond quarter of 2023, following the brands Lord Jones™exit of our U.S. operations, Cronos is reporting through one consolidated segment, which includes operations in both Canada and Happy Dance™.
No U.S. Schedule I Cannabis-Related Activities
On December 20, 2018, the 2018 Farm Bill was enacted in the U.S., removing U.S. hemp from the list of Schedule I controlled substances under the U.S. Controlled Substances Act (the “CSA”), and on January 19, 2021, the USDA issued a final rule establishing a domestic U.S. hemp production regulatory program, effective March 2021. Though a number of states in the U.S. have authorized the cultivation, distribution or possession of U.S. Schedule I cannabis and U.S. Schedule I cannabis containing products to various degrees and subject to various requirements or conditions, U.S. Schedule I cannabis continues to be a Schedule I controlled substance under the CSA. Therefore, the cultivation, manufacture, distribution and possession of U.S. Schedule I cannabis violates federal law in the U.S. unless a U.S. federal agency, such as the DEA, grants a registration for a specific activity, such as research, with U.S. Schedule I cannabis.
We do not engage in any activities related to U.S. Schedule I cannabis in the U.S. The Ginkgo Strategic Partnership contemplates the performance of licensed R&D activities in the U.S. in order to produce cultured cannabinoids, but such activities are conducted in compliance with all applicable laws regarding controlled substances.
Rest of World
The Rest of World operating segment is involved in the cultivation, manufacture, and marketing of cannabis and cannabis-derived products for the medical and adult-use markets.
Israel. In Canada, Cronos Group operates through atwo wholly owned license holderholders under the Cannabis Act (Canada) (the “Cannabis Act”), Peace Naturals Project Inc. (“Peace Naturals”), which has production facilities near Stayner, Ontario, (the “Peaceand Thanos Holdings Ltd., known as Cronos Fermentation (“Cronos Fermentation”), which has a production facility in Winnipeg, Manitoba. In November 2023, Cronos announced Peace Naturals Campus”). Cronos Group has established four strategic joint ventures in Canada, Israelhad entered into an agreement for the sale and Colombia and holds approximately 31%leaseback of the issued capitalPeace Naturals Campus. See “Operations and Investments—Sale and Leaseback of the Peace Naturals Campus” for more information. In August 2023, Cronos announced the planned wind-down of Cronos Australia Limited (“Cronos Australia”), which isFermentation, and has listed on the Australian Securities Exchangefacility for sale. In Israel, the Company operates under the trading symbol “CAU.” Cronos Group currently exports cannabis products to countries that permitIMC-GAP, IMC-GMP and IMC-GDP certifications required for the importcultivation, production and marketing of such products, such as Germany, Israeldried flower, pre-rolls and Australia.oils in the Israeli medical market.
Operations and Investments
Peace Naturals Campus / Cronos GrowCo
The production facilities at the Peace Naturals Campus and the production facilities of Cronos GrowCo are licensed by Health Canada under the Cannabis Act to engage in among other things, the cultivation, processing, distribution and sale of dried cannabis flower, cannabis resin, cannabis seeds, cannabis plants, cannabis extracts, cannabis topicals and cannabis edibles, among other prescribed activities.
Joint Ventures/Strategic InvestmentCronos Fermentation
We have established four strategic joint venturesThe production facility at Cronos Fermentation is licensed by Health Canada under the Cannabis Act to engage in Canada, Israelthe processing and Colombiadistribution and sale of dried flower, cannabis seeds, and cannabis plants, among other prescribed activities, which includes the production of cultured cannabinoids. The facility also hold approximately 31% ofholds a license for Analytical Testing under the issued capitalCannabis Regulations.As noted above, in August 2023, Cronos announced the planned wind-down of Cronos Australia, which we accountFermentation and has listed the facility for sale.
Israel
In Israel, the Company operates under the equity methodIMC-GAP, IMC-GMP and IMC-GDP certifications required for the cultivation, production and marketing of accounting.
Our ownership interest in each of our joint ventures is summarizeddried flower, pre-rolls and oils in the table below.Israeli medical market.
| | | | | | | | | | | | | | |
Joint Venture | | Jurisdiction | |
|
Cronos Israel(ii)
| | Israel | | 70%/90% |
Cronos Growing Company Inc. (“Cronos GrowCo”)(iii)
| | Canada | | 50% |
NatuEra S.à.r.l. (“Natuera”) (iv)
| | Colombia | | 50% |
MedMen Canada Inc. (“MedMen Canada”)(v)
| | Canada | | 50% |
(i)We define ownership interest as the proportionate share of net income to which we are entitled; equity interest may differ from ownership interest shown above. We consolidate the financial results of Cronos Israel and account for our other joint ventures under the equity method of accounting. See Note 2 and Note 6 of our audited consolidated financial statements included in Item 8 of this Annual Report.
(ii)A strategic joint venture with Kibbutz Gan Shmuel (“Gan Shmuel”), an Israeli agricultural collective settlement, for the production, manufacture and global distribution of medical cannabis, consisting of a cultivation company (Cronos Israel G.S. Cultivation Ltd.), a manufacturing company (Cronos Israel G.S. Manufacturing Ltd.), a distribution company (Cronos Israel G.S. Store Ltd.) and a pharmacy company (Cronos Israel G.S. Pharmacy Ltd., collectively, “Cronos Israel”). We hold a 70% equity interest in the cultivation company and a 90% equity interest in each of the manufacturing, distribution and pharmacy companies.
(iii)A strategic joint venture with a group of investors led by Bert Mucci (the “Greenhouse Partners”), a Canadian large-scale greenhouse operator. Each of Cronos Group and the Greenhouse Partners owns a 50% equity interest in Cronos GrowCo and has equal representation on its board of directors.
(iv)A strategic joint venture with an affiliate of Agroidea SAS (“AGI”), a Colombian agricultural services provider. Each of the Company and AGI owns a 50% equity interest in Natuera. Cronos Group has three manager nominees on the board of managers of Natuera, while AGI has four manager nominees on the board of managers. Natuera intends to develop, cultivate, manufacture, and export cannabis-based medical and consumer products for the Latin American and global markets.
(v)A strategic joint venture with MedMen Enterprises USA, LLC (“MedMen”) for retail in provinces in Canada that permit private retail. Each of the Company and MedMen owns a 50% equity interest in MedMen Canada, and has equal representation on the board of directors of MedMen Canada.
Operations Outside of Canada and the U.S.Israel
Cronos Group anticipates that it will continue expanding in the geographic markets outside of Canada and the U.S.Israel in which we currently participate and entering new geographic markets. By leveraging operational, manufacturing and regulatory expertise, quality standards and procedures and intellectual property, we believe that we are well-positioned to effectively access these markets. Subject to applicable regulatory approvals, strategic international business opportunities pursued by us could include:
•production, distribution, sales and marketing in jurisdictions whichthat have passed legislation to legalize the production, distribution and possession of cannabis and cannabis products at all relevant levels of government; and
•the export of cannabis and cannabis products to markets that permit the import of such products.
We distribute PEACE NATURALS® branded products along with other white-labeled cannabis products in the German medical market through our strategic partnership with Cansativa, a leading German cannabis company. In Australia, we distribute cannabis products through a distribution relationship with Vitura (formerly known as Cronos Australia Limited).
We seek to conduct business only in jurisdictions where we believe it is legal to do so and where such operations remain compliant with our listing obligations with the TSX and Nasdaq. Determining whether a business activity is legal in a jurisdiction may require judgment since laws, rules, regulations and licenses may not be clear and legal interpretation and advice of counsel may vary. If a business activity in which we engage in any jurisdiction is determined to be illegal, we could be subject to fines, penalties, reputational harm, delisting from securities exchanges and material civil, criminal and regulatory litigation and proceedings or be enjoined from doing business in the applicable jurisdiction. See “Risk Factors -Factors—Risks Relating to Regulation and Compliance - Compliance—We operate in highly regulated sectors where the regulatory environment is rapidly developing, and we may not always succeed in complying fully with applicable regulatory requirements in all jurisdictions where we carry on business.”
Sale and Leaseback of the Peace Naturals Campus
On November 27, 2023, the Company announced that Peace Naturals had entered into an agreement (the “Sale Agreement”) with Future Farmco Canada Inc. (“Future Farmco”) for the sale and leaseback of the Peace Naturals Campus. Pursuant to the terms of the Sale Agreement, Future Farmco has agreed to acquire the Peace Naturals Campus for an aggregate purchase price of C$23 million cash, subject to the terms and conditions set forth therein, including (a) Future Farmco having given written notice that it has satisfied itself in its sole, absolute and subjective discretion with respect to all aspects of the property, including title to the property, the physical condition of the property, zoning, environmental matters, financial matters including financing of the purchase price, and its review of the deliverables on or before the first business day that is 180 calendar days following the date of the Sale Agreement; (b) the Company having obtained all requisite approvals for the amendment of its licensed site perimeter from Health Canada on terms and conditions satisfactory to the Company, acting reasonably, prior to the end of the first business day following the later of: (i) 180 calendar days after the date of the Sale Agreement; or (ii) 75 calendar days after the satisfaction or waiver of Future Farmco’s condition described above; and (c) the parties having agreed, no later than 75 calendar days following the date of the Sale Agreement, to a form of lease to be entered into at closing for the Company to lease a portion of the Peace Naturals Campus (the “Lease Condition”). On February 29, 2024, the Company entered into a waiver and amending agreement with Future Farmco, pursuant to which the parties waived the Lease Condition. See “Risk Factors—Risks Relating to Our Growth Strategy—There can be no assurance that the regulatory approvals will be obtained or that the other closing conditions for the sale and leaseback of the Peace Naturals Campus will be satisfied or waived in a timely manner or at all.” As of the date of this Annual Report, the parties have agreed to a form of lease to be entered into at closing.
At closing, the parties expect to enter into a lease agreement for portions of the Peace Naturals Campus, which will include an initial five-year term and one five-year renewal option that may be exercised by the Company. The Company has the right to terminate the lease without penalty anytime after the second anniversary of the lease by giving written notice at least 12 months prior to termination. The leased premises will be identified and agreed between both parties prior to closing.
Joint Ventures/Strategic Investments
We have established two strategic joint ventures in Canada and Israel. Additionally, we hold approximately 9.6% of the issued capital of Vitura Health Limited (“Vitura”), which we account for as equity securities with a readily determinable fair value, and approximately 13.7% of the issued capital of NatuEra S.à.r.l. (“Natuera”), which we account for as equity securities without a readily determinable fair value, as of December 31, 2023.
Our ownership interest in each of our joint ventures is summarized in the table below.
| | | | | | | | | | | | | | |
| Joint Venture | | Jurisdiction | | Ownership Interest(i) |
Cronos Israel(ii) | | Israel | | 70%/90% |
Cronos GrowCo(iii) | | Canada | | 50% |
| | | | |
(i)We define ownership interest as the proportionate share of net income to which we are entitled; equity interest may differ from ownership interest shown above. We consolidate the financial results of Cronos Israel and account for our other joint ventures under the equity method of accounting. See Note 1 “Background, Basis of Presentation, and Summary of Significant Accounting Policies” and Note 4 “Investments” to our consolidated financial statements in Item 8 of this Annual Report.
(ii)A strategic joint venture with Kibbutz Gan Shmuel (“Gan Shmuel”), an Israeli agricultural collective settlement, for the production, manufacturing and global distribution of medical cannabis, consisting of a cultivation company (Cronos Israel G.S. Cultivation Ltd.), a manufacturing company (Cronos Israel G.S. Manufacturing Ltd.), a distribution company (Cronos Israel G.S. Store Ltd.) and a pharmacy company (Cronos Israel G.S. Pharmacy Ltd., collectively, “Cronos Israel”). We hold a 70% equity interest in the cultivation company and a 90% equity interest in each of the manufacturing, distribution and pharmacy companies.
(iii)A strategic joint venture with a group of investors led by Bert Mucci (the “Greenhouse Partners”), a Canadian large-scale greenhouse operator. Each of Cronos and the Greenhouse Partners owns a 50% equity interest in Cronos GrowCo and has equal representation on its board of directors.
Strategic Investment in PharmaCann, Inc.
On June 14, 2021, Cronos USA Holdings Inc., a wholly owned subsidiary of the Company, purchased an option (the “PharmaCann Option”), with an exercise price of $0.0001 per share, to acquire an approximately 10.5% ownership stake in PharmaCann, Inc. (“PharmaCann”) on a fully diluted basis for total consideration of approximately $110.4 million. PharmaCann is a leading vertically integrated U.S. cannabis company that has a broad geographic footprint in the U.S. and has built an efficient, effective and scalable operating model. The PharmaCann Option exercise will be based upon various factors, including the status of U.S. federal cannabis legalization, as well as regulatory approvals, including in the states where PharmaCann operates that may be required upon exercise. Following the exercise of the PharmaCann Option, the Company and PharmaCann will enter into commercial agreements that would permit each party to offer its products through the other party’s distribution channels.
As of December 31, 2023, the Company’s ownership percentage in PharmaCann on a fully diluted basis was approximately 5.9%. Under the terms of the Company’s investment in PharmaCann, the Company’s rights to nominate an observer or a director to the PharmaCann board of directors could be lost if the Company’s ownership drops below 6% on a fully diluted basis and it sells or transfers all or any portion of the option (subject to certain exceptions).
No U.S. Schedule I Cannabis-Related Activities
Though a number of states in the U.S. have authorized the cultivation, distribution or possession of U.S. Schedule I cannabis and U.S. Schedule I cannabis containing products to various degrees and subject to various requirements or conditions, U.S. Schedule I cannabis continues to be a Schedule I controlled substance under the U.S. Controlled Substances Act (the “CSA”). Therefore, the cultivation, manufacture, distribution and possession of U.S. Schedule I cannabis violates federal law in the U.S. unless a U.S. federal agency, such as the DEA, grants a registration for a specific activity, such as research, with U.S. Schedule I cannabis.
We do not engage in any activities related to U.S. Schedule I cannabis in the U.S. The Ginkgo Strategic Partnership contemplates the performance of licensed R&D activities in the U.S. in order to produce cultured cannabinoids, but such activities are conducted in compliance with all applicable laws regarding controlled substances.
Brand Portfolio
We are committed to building a portfolio of iconic brands that responsibly elevate the consumer experience.
In the U.S., we market and distribute solely U.S. hemp-derived supplements and cosmetic products through e-commerce, retail and hospitality channels under the brands Lord Jones™ and Happy Dance™.
In Canada, we sell a variety of cannabis and cannabis products including dried cannabis, pre-rolls and cannabis extracts (in the form of tinctures and vaporizers) through wholesale and direct-to-client channels under both our wellness platform, PEACE NATURALS™core adult-use brands, Spinach® and Lord Jones®, and under our two adult-use brands, COVE™ and Spinach™core wellness platform, PEACE NATURALS®. In addition, PEACE NATURALS™ driedNATURALS® cannabis and cannabis oilsproducts are currently exported for sale to Australia, Germanyavailable in the Israeli and Israel.German medical markets.
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | |
| Brand Positioning | Wellness | Premium adult-use | Mainstream adult-use | Prestige adult consumer goodsWellness | | Masstige adult consumer goods | Mass marketPremium adult-use |
| Product Offering | Dried cannabis,flower, pre-rolls, vaporizers, edibles | Dried flower, pre-rolls, cannabis tinctures | | | Pre-rolls, vaporizers, | Dried cannabis, cannabis tinctures,
pre-rolls, vaporizers
| Dried cannabis, pre-rolls, vaporizers | U.S. hemp-derived supplements, cosmetics | U.S hemp-derived cosmetics | In development (not yet offered for sale) edibles |
| Geographic Availability | Australia, Canada, Germany and Israel | Canada | Canada, Israel & Germany | | | Canada | U.S. | U.S. | Anticipated U.S. |
Core Adult-UseBrands
Spinach® is a mainstream adult-use cannabis brand focused on friends, fun and legendary cannabis. The Spinach® brand portfolio includes cannabinoid products in a wide range of formats including dried flower, pre-rolls, vaporizers and edibles.
The Spinach® brand also has two sub-brands, SOURZ by Spinach® and Spinach FEELZ™. SOURZ by Spinach® is a line of multi-colored, multi-flavored edibles in a variety of cannabinoid ratios in a distinctive “S” shaped gummy, featuring proprietary flavor masking technology. Spinach FEELZ™ prominently features rare cannabinoids in a range of product formats, designed to deliver unique and enhanced experiences made possible through proprietary blends of rare cannabinoids alongside common cannabinoids, like delta-9-tetrayhydrocannabinol (“THC”). Each product is formulated to help adult consumers, “Feelz. The Way You Want.”
Lord Jones® is a premium adult-use cannabis brand that goes above and beyond to unlock differentiated ways to experience cannabis. The Lord Jones® brand portfolio includes cannabis products in the pre-roll, vaporizer and edible categories.
Core Wellness BrandsBrand
We currently distribute products under PEACE NATURALS™ for the Canadian and non-U.S. international medical cannabis markets. PEACE NATURALS™NATURALS® is a global wellness platform committed to producing high-quality cannabis and cannabis products. PEACE NATURALS™It is focused on building and shaping the global cannabis wellness market and promoting a holistic approach to wellness. The brand’s goalCompany currently distributes products under PEACE NATURALS® for Canadian, Israeli and German medical markets.
Cronos Marketing Code
In 2021, Cronos released its Marketing Code, which is designed to improveresponsibly move the livesemerging cannabis industry forward. Cronos believes that those below the legal age of others, one client at a time.
Adult-UseBrands
COVE™consumption should not be targeted in an adult-use cannabis market. Cronos recognizes there is a premium positioned brand focused on creating crafted experiences. COVE™’s indoor, strain-specific grow rooms seek to maintain the highest quality standards throughout the entire process. The goal of this premium brand is to Make Each Experience a Discovery™.
Spinach™ is positioned as a mainstream adult-use brand with High Expectations™, which is geared towards a wide range of consumers who are lookingclear need for entertaining and fun ways to enhance activities. A lighthearted and playful brand, Spinach™ is focused on offering Farm-To-Bowl™ products that bring friends together and make experiences more enjoyable.
Adult Consumer Product Brandsstandards.
The Company operates Lord Jones™, a preeminent U.S. hemp-derived CBD brandprinciples in the U.S., for the adult consumer goods market. Lord Jones™ is a prestige beauty and lifestyle brand focusing on high-quality U.S. hemp-derived personal care products. Lord Jones™ U.S. hemp-derived supplements and cosmetics products are distributed online andCronos Marketing Code apply to approximately 900 premium stores and retail channels, including Sephora, Neiman Marcus and SoulCycle.
During the fourth quarterall marketing activities of 2020, the Company launched Happy Dance™, a U.S. hemp-derived CBD skincare and personal care brand, in partnership with Kristen Bell. Happy Dance™ products are made with CBD from premium full-spectrum hemp extract and provide consumers with high quality skincare at an accessible price point. Happy Dance™ launched with three product offerings: All-Over Whipped Body Butter +CBD, Head-To-Toe Coconut Melt +CBD and Stress Away Bath Bomb +CBD, all of which are currently available online. Happy Dance™ continues its expansion by securing its first major U.S. retailer, ULTA Beauty™. The full collection of Happy Dance™ products is expected to launch online at ULTA.com and in-store at over 550 ULTA Beauty™ locations across the U.S. in the coming weeks. Through the partnership with Ms. Bell, Cronos Group looks forward to future introductions of innovative products to the hemp-derived CBD market.
The Company launched PEACE+™, a U.S. hemp-derived CBD brand in the U.S. PEACE+™ U.S. hemp-derived CBD products are currently under developmentbrands globally and are not yet offeredcommunicated to all business partners in any work they do on the Company’s behalf. The Marketing Code represents Cronos’ commitment to responsible marketing standards. The code standards are:
•Our advertising will be targeted to adults.
•We will highlight responsible cannabis consumption and any people depicted in any imagery will be adults.
•Our brand websites and social media will be designed for sale. PEACE+™ is about more than making a better, high-quality U.S. hemp-derived CBD product; it stems from the belief that well-being can leadadults.
•Our marketing events will be targeted to a better world, full of positivityadults and possibility. It is a belief that extends beyond the productswill promote responsible cannabis consumption.
•We will provide our customers with facts and into everything the brand seeks to do and stand for. The brand intends to distribute its products through the mass market focused retail channel in the U.S. in the future.substantiate our claims.
Global Sales and Distribution - Principal Markets
Cronos Group has developed a diversified global sales and distribution network by leveraging established partners for their scale, sales force and market expertise.network. We have also built a distribution footprint in Canada through the direct-to-clientadult-use and medical market and the adult-use market,markets, as well as a distribution footprintchannel for U.S. hemp-derived consumer products in the U.S. through e-commerce, retail and hospitality channels.
United States Market and Distribution
Through Redwood, the Company manufactures, markets and distributes U.S. hemp-derived supplements and cosmetic products through e-commerce, retail and hospitality partner channels in the U.S. under the Lord Jones™ and Happy Dance™ brands. Redwood’s products use high-quality U.S. hemp extract that retains naturally occurring phytocannabinoids and terpenes found in the plant. We plan to use our resources to capitalize on market demand and to further create and scale U.S. hemp-derived consumer products and brands. We do not engage in any commercial activities related to the cultivation, distribution or possession of U.S. Schedule I cannabis in the U.S.
The Company has also launched its PEACE+™ brand for U.S. hemp-derived CBD products in the U.S. PEACE+™ U.S. hemp-derived CBD products are currently under development and are not yet offered for sale. The Company intends to access the U.S. mass market focused retail channel in the future.
Rest of WorldIsraeli medical market.
Canadian Market and Distribution
•Direct-to-ClientMedical Market.. Following the closure of the Medical Cannabis by Shoppers Drug Mart platform, our PEACE NATURALSWe currently sell dried® medical cannabis and cannabis extracts directly to clientsproducts are sold in Canada through our wellness platform, PEACE NATURALS™. These clients are typically sourced through physician and clinic referrals or word-of-mouth recommendations from existing clients.various third-party distributors.
•Adult-UseAdult-Use.. We currently sell dried flower, pre-rolls, vaporizers and cannabis extractsedibles through our core adult-use brands, COVE™ Spinach® and Spinach™Lord Jones®, to cannabis control authorities in variousall provinces including Ontario, Québec, British Columbia, Alberta, Manitoba, Nova Scotia, New Brunswick,of Canada and Prince Edward Island, as well asthe Yukon territory, except Saskatchewan, where we sell to private-sector retailers, in Saskatchewan, subject to the relevant province’s or territory’s product or other restrictions and requirements. As the Company’s supply chain grows, the Company continues to expand its portfolio of cannabis products for the existing markets in Canada. The rate
MarketsCronos Fermentation
The production facility at Cronos Fermentation is licensed by Health Canada under the Cannabis Act to engage in the processing and Distribution Outsidedistribution and sale of Canadadried flower, cannabis seeds, and cannabis plants, among other prescribed activities, which includes the production of cultured cannabinoids. The facility also holds a license for Analytical Testing under the Cannabis Regulations.As noted above, in August 2023, Cronos announced the planned wind-down of Cronos Fermentation and has listed the facility for sale.
•Israel. Cronos
In Israel, has receivedthe Company operates under the IMC-GAP, IMC-GMP and IMC-GDP certifications required for the cultivation, production and marketing of dried flower, pre-rolls and oils in Israel. In the second quarter of 2020, Cronos Israel began distributing PEACE NATURALS™ branded cannabis products to the Israeli medical cannabis market. See “- Licenses and Regulatory Framework in Israel.”
•Europe. We have distributed and anticipate continuing to distribute PEACE NATURALS™ branded cannabis products in Germany through an exclusive distribution relationship with G. Pohl-Boskamp GmbH & Co. KG (“Pohl-Boskamp”), an international pharmaceutical manufacturer and distributor with a distribution network of pharmacies. We have also entered into a strategic distribution partnership with Delfarma Sp. Zo.o (“Delfarma”), a pharmaceutical wholesaler in Poland. We and Delfarma are currently in the process of obtaining the necessary regulatory approvals to sell cannabis products in Poland.
•Latin America. We intend to distribute cannabis and cannabis products to the Latin American and other cannabis markets through the operations of Natuera. In 2020, Natuera began commercial cultivation of non-psychoactive (hemp) cultivars registered with the Colombian Agricultural Institute. Also in 2020, Natuera completed a number of test exports of hemp-derived CBD extract to the U.S. and United Kingdom for business development and R&D purposes, as well as its first export of hemp-derived CBD extract to the U.S. for commercial purposes.
•Australia and Asia-Pacific. Cronos Australia has received an import license from the Australian Office of Drug Control (the “ODC”), together with all necessary permits, to import PEACE NATURALS™ branded cannabis products for sale in the Australian medical market under the terms of the relevant permits. Cronos Australia facilitates distribution of the Company’s products in Australia, New Zealand and South East Asia, bolstering the Company’s distribution network in the Australia and Asia-Pacific region.
WeOperations Outside of Canada and Israel
Cronos anticipates that it will continue expanding in the geographic markets outside of Canada and Israel in which we currently participate and entering new geographic markets. By leveraging operational, manufacturing and regulatory expertise, quality standards and procedures and intellectual property, we believe that we are well-positioned to seek neweffectively access these markets. Subject to applicable regulatory approvals, strategic international business opportunities pursued by us could include:
•production, distribution, channelssales and marketing in jurisdictions that have legalizedpassed legislation to legalize the production, distribution and possession of cannabis and cannabis products at all relevant levels of government.government; and
Global Supply Chain•the export of cannabis products to markets that permit the import of such products.
We distribute PEACE NATURALS® branded products along with other white-labeled cannabis products in the German medical market through our strategic partnership with Cansativa, a leading German cannabis company. In Australia, we distribute cannabis products through a distribution relationship with Vitura (formerly known as Cronos GroupAustralia Limited).
We seek to conduct business only in jurisdictions where we believe it is focusedlegal to do so and where such operations remain compliant with our listing obligations with the TSX and Nasdaq. Determining whether a business activity is legal in a jurisdiction may require judgment since laws, rules, regulations and licenses may not be clear and legal interpretation and advice of counsel may vary. If a business activity in which we engage in any jurisdiction is determined to be illegal, we could be subject to fines, penalties, reputational harm, delisting from securities exchanges and material civil, criminal and regulatory litigation and proceedings or be enjoined from doing business in the applicable jurisdiction. See “Risk Factors—Risks Relating to Regulation and Compliance—We operate in highly regulated sectors where the regulatory environment is rapidly developing, and we may not always succeed in complying fully with applicable regulatory requirements in all jurisdictions where we carry on establishing an efficient global supply chain by seeking to develop industry-leading methodologiesbusiness.”
Sale and best practices atLeaseback of the Peace Naturals Campus and leveraging this expertise to create beneficial production partnerships. We plan to continue to develop a global supply chain, which will employ a combination of wholly owned production facilities, third-party suppliers and global production partnerships, all of which will support
On November 27, 2023, the manufacturing of cannabinoid-based consumer goods.
United States
In the ordinary course of our business, weenter into contract manufacturing agreements with suppliers of our cosmetic products. We supply these third-party manufacturers with U.S. hemp extract or infused bulk product, fragrances and/or packagingCompany announced that we source from other third-party suppliers. The contract manufacturers supply any other necessary ingredients to manufacture products using our formulas and fill and package our finished products. Our contract manufacturing and supply agreements generally do not require us to purchase minimum quantities of materials or products.
In producing our supplement products, we source our ingredients from our suppliers on an ongoing as-needed basis. We have notPeace Naturals had entered into any contracts that obligate us to purchase a minimum quantity or exclusively from any supplier. Our supplements are manufactured at our facilities in Los Angeles, California according to Good Manufacturing Practicesan agreement (the “Sale Agreement”) with Future Farmco Canada Inc. (“GMP”Future Farmco”).
We are obligated to purchase our supply for the sale and leaseback of certain U.S. hemp extract from one supplier unless that supplier cannot provide the agreed-upon quantities in relation to certain brands in the U.S.
Rest of World
Canadian Supply Chain
•Production Facilities at Peace Naturals. The Peace Naturals Campus is licensed for cannabis production andCampus. Pursuant to the manufacturingterms of certain cannabis products. The production processes atthe Sale Agreement, Future Farmco has agreed to acquire the Peace Naturals Campus are GMP-certified under relevant European Economic Area GMP directives by the national competent authorityfor an aggregate purchase price of Germany. The Peace Naturals Campus is engaged in cultivation, processing, finishing, packaging and shipping activities, as well as tissue culture and micro propagation, providing a year-round supply of cannabis. The Peace Naturals Campus also engages in R&D to pilot various production technologies, with any tests yielding favorable operational improvements evaluated for disseminationC$23 million cash, subject to the Company’s other partnership facilities. In addition,terms and conditions set forth therein, including (a) Future Farmco having given written notice that it has satisfied itself in its sole, absolute and subjective discretion with respect to all aspects of the property, including title to the property, the physical condition of the property, zoning, environmental matters, financial matters including financing of the purchase price, and its review of the deliverables on or before the first business day that is 180 calendar days following the date of the Sale Agreement; (b) the Company having obtained all requisite approvals for the amendment of its licensed site perimeter from Health Canada on terms and conditions satisfactory to the Company, acting reasonably, prior to the end of the first business day following the later of: (i) 180 calendar days after the date of the Sale Agreement; or (ii) 75 calendar days after the satisfaction or waiver of Future Farmco’s condition described above; and (c) the parties having agreed, no later than 75 calendar days following the date of the Sale Agreement, to a form of lease to be entered into at closing for the Company to lease a portion of the Peace Naturals Campus engages in R&D on cannabinoid formulations, delivery systems(the “Lease Condition”). On February 29, 2024, the Company entered into a waiver and product development.
•Cronos GrowCo. Cronos GrowCo completed construction of the structure of its greenhouse in Kingsville, Ontario in 2019. Full completion of construction of the facility, including all fixtures within the greenhouse and all post-harvest activity areas was completed in 2020. In November 2020, Cronos GrowCo obtained a cultivation license for the operations contemplated by the first phase of the project. The Company expects the facility to become operational in phases beginning in the first half of 2021. Full commencement of operations at Cronos GrowCo will be subject to obtaining the appropriate licenses and other customary approvals under applicable law.
•Third-Party Supply and Manufacturing Agreements. In the ordinary course of our business, we enter into spot market purchase agreements and supply agreementsamending agreement with suppliers of dried cannabis and other cannabis products. Our supply agreements, for the most part, do not obligate us to purchase minimum quantities of products and generally contain provisions permitting cancellation of orders or termination on notice. We also enter into contract manufacturing agreements with other license holders,Future Farmco, pursuant to which such license holders provide cannabis extract and services relatedthe parties waived the Lease Condition. See “Risk Factors—Risks Relating to Our Growth Strategy—There can be no assurance that the filling and packaging of vaporizer devicesregulatory approvals will be obtained or that the other closing conditions for the Canadian cannabis adult-usesale and wellness markets.leaseback of the Peace Naturals Campus will be satisfied or waived in a timely manner or at all.” As of the date of this Annual Report, the parties have agreed to a form of lease to be entered into at closing.
Supply Chain OutsideAt closing, the parties expect to enter into a lease agreement for portions of Canada
•Cronos Israel. Cronos Israelthe Peace Naturals Campus, which will include an initial five-year term and one five-year renewal option that may be exercised by the Company. The Company has received the IMC-GAP, IMC-GMP and IMC-GDP certifications required forright to terminate the cultivation, production and marketing of dried flower, pre-rolls and oils in Israel. Inlease without penalty anytime after the second quarteranniversary of 2020, Cronos Israel began distributing PEACE NATURALS™ branded cannabis productsthe lease by giving written notice at least 12 months prior to termination. The leased premises will be identified and agreed between both parties prior to closing.
Joint Ventures/Strategic Investments
We have established two strategic joint ventures in Canada and Israel. Additionally, we hold approximately 9.6% of the Israeli medical cannabis market. See “- Licensesissued capital of Vitura Health Limited (“Vitura”), which we account for as equity securities with a readily determinable fair value, and Regulatory Framework in Israel.”
•Natuera. Natuera is currently focused on accessing new markets and product development, including developing additional bulk offeringsapproximately 13.7% of hemp-derived CBD distillate and water-soluble hemp-derived CBD solutions. In 2020, Natuera began commercial cultivationthe issued capital of non-psychoactive (hemp) cultivars registered with the Colombian Agricultural Institute. Also in 2020, Natuera completedNatuEra S.à.r.l. (“Natuera”), which we account for as equity securities without a numberreadily determinable fair value, as of test exports of hemp-derived CBD extract to the U.S. and United Kingdom for business development and R&D purposes, as well as its first export of hemp-derived CBD extract to the U.S. for commercial purposes.December 31, 2023.
Major Customers
Four major customers (sales toOur ownership interest in each of our joint ventures is summarized in the table below.
| | | | | | | | | | | | | | |
| Joint Venture | | Jurisdiction | | Ownership Interest(i) |
Cronos Israel(ii) | | Israel | | 70%/90% |
Cronos GrowCo(iii) | | Canada | | 50% |
| | | | |
(i)We define ownership interest as the proportionate share of net income to which equaled or exceeded 10%we are entitled; equity interest may differ from ownership interest shown above. We consolidate the financial results of Cronos Israel and account for our other joint ventures under the equity method of accounting. See Note 1 “Background, Basis of Presentation, and Summary of Significant Accounting Policies” and Note 4 “Investments” to our consolidated financial statements in Item 8 of this Annual Report.
(ii)A strategic joint venture with Kibbutz Gan Shmuel (“Gan Shmuel”), an Israeli agricultural collective settlement, for the production, manufacturing and global distribution of medical cannabis, consisting of a cultivation company (Cronos Israel G.S. Cultivation Ltd.), a manufacturing company (Cronos Israel G.S. Manufacturing Ltd.), a distribution company (Cronos Israel G.S. Store Ltd.) and a pharmacy company (Cronos Israel G.S. Pharmacy Ltd., collectively, “Cronos Israel”). We hold a 70% equity interest in the cultivation company and a 90% equity interest in each of the manufacturing, distribution and pharmacy companies.
(iii)A strategic joint venture with a group of investors led by Bert Mucci (the “Greenhouse Partners”), a Canadian large-scale greenhouse operator. Each of Cronos and the Greenhouse Partners owns a 50% equity interest in Cronos GrowCo and has equal representation on its board of directors.
Strategic Investment in PharmaCann, Inc.
On June 14, 2021, Cronos USA Holdings Inc., a wholly owned subsidiary of the Company, purchased an option (the “PharmaCann Option”), with an exercise price of $0.0001 per share, to acquire an approximately 10.5% ownership stake in PharmaCann, Inc. (“PharmaCann”) on a fully diluted basis for total consideration of approximately $110.4 million. PharmaCann is a leading vertically integrated U.S. cannabis company that has a broad geographic footprint in the U.S. and has built an efficient, effective and scalable operating model. The PharmaCann Option exercise will be based upon various factors, including the status of U.S. federal cannabis legalization, as well as regulatory approvals, including in the states where PharmaCann operates that may be required upon exercise. Following the exercise of the PharmaCann Option, the Company and PharmaCann will enter into commercial agreements that would permit each party to offer its products through the other party’s distribution channels.
As of December 31, 2023, the Company’s ownership percentage in PharmaCann on a fully diluted basis was approximately 5.9%. Under the terms of the Company’s consolidated net revenues forinvestment in PharmaCann, the year ended December 31, 2020), Alberta GamblingCompany’s rights to nominate an observer or a director to the PharmaCann board of directors could be lost if the Company’s ownership drops below 6% on a fully diluted basis and Liquor Commission, B.C. Liquor Distribution Branch, Ontario Cannabis Retail Corporation, and Société Québécoise du Cannabis (the “SQDC”) (the cannabis control authorities in Alberta, British Columbia, Ontario and Québec, respectively), accounted for approximately 19%, 13%, 16% and 15%, respectively, of our consolidated net revenues for the year ended December 31, 2020. We mitigate credit risk through verificationit sells or transfers all or any portion of the customers’ liquidity prioroption (subject to certain exceptions).
No U.S. Schedule I Cannabis-Related Activities
Though a number of states in the authorizationU.S. have authorized the cultivation, distribution or possession of material transactions.
Government Contracts
In Canada, we sellU.S. Schedule I cannabis and U.S. Schedule I cannabis containing products to cannabis control authorities in various provinces, including, Ontario, Québec, British Columbia, Alberta, Manitoba, Nova Scotia, New Brunswickdegrees and Prince Edward Island, where each such cannabis control authority is the sole wholesale distributor and in certain provinces, the sole retailer, of cannabis and cannabis products. We sell these products to the various cannabis control authorities under supply agreements that are subject to terms that allow for renegotiationvarious requirements or conditions, U.S. Schedule I cannabis continues to be a Schedule I controlled substance under the U.S. Controlled Substances Act (the “CSA”). Therefore, the cultivation, manufacture, distribution and possession of sale prices and termination at the election of the applicableU.S. Schedule I cannabis control authority. In particular, the cannabis control authorities haveviolates federal law in the past and mayU.S. unless a U.S. federal agency, such as the DEA, grants a registration for a specific activity, such as research, with U.S. Schedule I cannabis.
We do not engage in any activities related to U.S. Schedule I cannabis in the future choose to stop purchasing our products, may change the prices at which they purchase our products, may return our products to us and, in certain circumstances, may cancel purchase orders at any time including after products have been shipped. For the year ended December 31, 2020 we had approximately $36.4 million in sales to cannabis control authorities.
Research and Development Activities and Intellectual Property
Cronos Research Labs
Cronos Research Labs Ltd. (“Cronos Research Labs”) is our Israel-based global research and development center for innovation. The state-of-the-art facility is equipped with advanced technology and analytical testing infrastructure and is home to an experienced team of scientific talent. The Cronos Research Labs team is comprised of scientific researchers, mechanical, electrical and software engineers, and analytical and formulation scientists. Cronos Group engages in both understanding the fundamental science behind the interactions of cannabinoids with each other and how those interactions can be leveraged to best deliver on the consumer’s needs. Cronos Group’s work spans many aspects of cannabis research from strain development, to growing conditions to extraction technology to biosynthesis to product development, all supported by advances in analytical sciences. This global R&D center is expected to significantly enhance Cronos Group’s innovation capabilities and accelerate development of the next-generation of cannabinoid products.
Ginkgo
The collaboration and license agreement between Ginkgo and the Company (the “Ginkgo Collaboration Agreement”) could enable us to produce certain cultured cannabinoids at commercial scale at a fraction of the cost compared to traditional cultivation practices. These cultured cannabinoid molecules are identical to those produced by plants grown using traditional cultivation but are created by leveraging the power of biological manufacturing via fermentation. In addition to tetrahydrocannabinol (“THC”) and CBD, these cultured cannabinoids include rare cannabinoids that are economically impractical or nearly impossible to produce at high purity and scale through traditional cultivation.
If the Ginkgo Strategic Partnership is ultimately successful, Cronos Group expects to be able to produce large volumes of these cultured cannabinoids from custom yeast strains by leveraging existing fermentation infrastructure at Cronos Fermentation in Winnipeg, Manitoba without incurring significant capital expenditures to build new cultivation and extraction facilities.
U.S. The Ginkgo Strategic Partnership contemplates the performance of licensed R&D activities in the U.S. in order to produce cultured cannabinoids, andbut such activities are to be conducted in compliance with all applicable laws regarding controlled substances.
Brand Portfolio
We intendare committed to produce building a portfolio of iconic brands that responsibly elevate the consumer experience.
In Canada, we sell a variety of cannabis products through wholesale channels under both our core adult-use brands, Spinach® and distributeLord Jones®, and under our core wellness platform, PEACE NATURALS®. In addition, PEACE NATURALS® cannabis products are currently available in the targetIsraeli and German medical markets.
| | | | | | | | | | | | | |
| | | | | |
| Brand Positioning | Mainstream adult-use | Wellness | | | Premium adult-use |
| Product Offering | Dried flower, pre-rolls, vaporizers, edibles | Dried flower, pre-rolls, cannabis tinctures | | | Pre-rolls, vaporizers, edibles |
| Geographic Availability | Canada | Canada, Israel & Germany | | | Canada |
Core Adult-UseBrands
Spinach® is a mainstream adult-use cannabis brand focused on friends, fun and legendary cannabis. The Spinach® brand portfolio includes cannabinoid products in a wide range of formats including dried flower, pre-rolls, vaporizers and edibles.
The Spinach® brand also has two sub-brands, SOURZ by Spinach® and Spinach FEELZ™. SOURZ by Spinach® is a line of multi-colored, multi-flavored edibles in a variety of cannabinoid ratios in a distinctive “S” shaped gummy, featuring proprietary flavor masking technology. Spinach FEELZ™ prominently features rare cannabinoids in a range of product formats, designed to deliver unique and enhanced experiences made possible through proprietary blends of rare cannabinoids alongside common cannabinoids, like delta-9-tetrayhydrocannabinol (“THC”). Each product is formulated to help adult consumers, “Feelz. The Way You Want.”
Lord Jones® is a premium adult-use cannabis brand that goes above and beyond to unlock differentiated ways to experience cannabis. The Lord Jones® brand portfolio includes cannabis products in the pre-roll, vaporizer and edible categories.
Core Wellness Brand
PEACE NATURALS® is a global wellness platform committed to producing high-quality cannabis products. It is focused on building and shaping the global cannabis wellness market and promoting a holistic approach to wellness. The Company currently distributes products under PEACE NATURALS® for Canadian, Israeli and German medical markets.
Cronos Marketing Code
In 2021, Cronos released its Marketing Code, which is designed to responsibly move the emerging cannabis industry forward. Cronos believes that those below the legal age of consumption should not be targeted in an adult-use cannabis market. Cronos recognizes there is a clear need for standards.
The principles in the Cronos Marketing Code apply to all marketing activities of all Cronos brands globally where permitted by applicable law, and are communicated to all business partners in any work they do on the Company’s behalf. The Marketing Code represents Cronos’ commitment to responsible marketing standards. The code standards are:
•Our advertising will be targeted to adults.
•We will highlight responsible cannabis consumption and any people depicted in any imagery will be adults.
•Our brand websites and social media will be designed for adults.
•Our marketing events will be targeted to adults and will promote responsible cannabis consumption.
•We will provide our customers with facts and substantiate our claims.
Global Sales and Distribution - Principal Markets
Cronos has developed a diversified global sales and distribution network. We have received confirmation from Healthbuilt a distribution footprint in Canada that this method of production is permitted underthrough the Cannabis Act.adult-use and medical markets, as well as a distribution channel for the Israeli medical market.
Ginkgo has filed certain patent applications pertaining to biosynthesis of cannabinoids to protectCanadian Market and Distribution
•Medical Market. Following the intellectual property developed as partclosure of the research progressing underMedical Cannabis by Shoppers Drug Mart platform, our PEACE NATURALS® medical cannabis products are sold in Canada through various third-party distributors.
•Adult-Use. We currently sell dried flower, pre-rolls, vaporizers and edibles through our core adult-use brands, Spinach® and Lord Jones®, to cannabis control authorities in all provinces of Canada and the Ginkgo Strategic Partnership. UnderYukon territory, except Saskatchewan, where we sell to private-sector retailers, subject to the partnership, Cronos Group isrelevant province’s or territory’s product or other restrictions and requirements. As the exclusive licenseeCompany’s supply chain grows, the Company continues to expand its portfolio of the intellectual property covered by the patent applicationscannabis products for the target cannabinoids.existing markets in Canada.
Cronos Fermentation
The production facility at Cronos Fermentation is licensed by Health Canada under the Cannabis Act to engage in the processing and distribution and sale of dried flower, cannabis seeds, and cannabis plants, among other prescribed activities, which includes the production of cultured cannabinoids. The facility also holds a license for Analytical Testing under the Cannabis Regulations.As noted above, in August 2023, Cronos announced the planned wind-down of Cronos Fermentation and has listed the facility for sale.
Israel
In Israel, the Company operates under the IMC-GAP, IMC-GMP and IMC-GDP certifications required for the cultivation, production and marketing of dried flower, pre-rolls and oils in the Israeli medical market.
Operations Outside of Canada and Israel
Cronos anticipates that it will continue expanding in the geographic markets outside of Canada and Israel in which we currently participate and entering new geographic markets. By leveraging operational, manufacturing and regulatory expertise, quality standards and procedures and intellectual property, we believe that we are well-positioned to effectively access these markets. Subject to applicable regulatory approvals, strategic international business opportunities pursued by us could include:
•production, distribution, sales and marketing in jurisdictions that have passed legislation to legalize the production, distribution and possession of cannabis products at all relevant levels of government; and
•the export of cannabis products to markets that permit the import of such products.
We distribute PEACE NATURALS® branded products along with other white-labeled cannabis products in the German medical market through our strategic partnership with Cansativa, a leading German cannabis company. In Australia, we distribute cannabis products through a distribution relationship with Vitura (formerly known as Cronos Australia Limited).
We seek to conduct business only in jurisdictions where we believe it is legal to do so and where such operations remain compliant with our listing obligations with the TSX and Nasdaq. Determining whether a business activity is legal in a jurisdiction may require judgment since laws, rules, regulations and licenses may not be clear and legal interpretation and advice of counsel may vary. If a business activity in which we engage in any jurisdiction is determined to be illegal, we could be subject to fines, penalties, reputational harm, delisting from securities exchanges and material civil, criminal and regulatory litigation and proceedings or be enjoined from doing business in the applicable jurisdiction. See “Risk Factors—Risks Relating to Regulation and Compliance—We operate in highly regulated sectors where the regulatory environment is rapidly developing, and we may not always succeed in complying fully with applicable regulatory requirements in all jurisdictions where we carry on business.”
Sale and Leaseback of the Peace Naturals Campus
On November 27, 2023, the Company announced that Peace Naturals had entered into an agreement (the “Sale Agreement”) with Future Farmco Canada Inc. (“Future Farmco”) for the sale and leaseback of the Peace Naturals Campus. Pursuant to the terms of the Sale Agreement, Future Farmco has agreed to acquire the Peace Naturals Campus for an aggregate purchase price of C$23 million cash, subject to the terms and conditions set forth therein, including (a) Future Farmco having given written notice that it has satisfied itself in its sole, absolute and subjective discretion with respect to all aspects of the property, including title to the property, the physical condition of the property, zoning, environmental matters, financial matters including financing of the purchase price, and its review of the deliverables on or before the first business day that is 180 calendar days following the date of the Sale Agreement; (b) the Company having obtained all requisite approvals for the amendment of its licensed site perimeter from Health Canada on terms and conditions satisfactory to the Company, acting reasonably, prior to the end of the first business day following the later of: (i) 180 calendar days after the date of the Sale Agreement; or (ii) 75 calendar days after the satisfaction or waiver of Future Farmco’s condition described above; and (c) the parties having agreed, no later than 75 calendar days following the date of the Sale Agreement, to a form of lease to be entered into at closing for the Company to lease a portion of the Peace Naturals Campus (the “Lease Condition”). On February 29, 2024, the Company entered into a waiver and amending agreement with Future Farmco, pursuant to which the parties waived the Lease Condition. See “Risk Factors—Risks Relating to Our Growth Strategy—There can be no assurance that the regulatory approvals will be obtained or that the other closing conditions for the sale and leaseback of the Peace Naturals Campus will be satisfied or waived in a timely manner or at all.” As of the date of this Annual Report, the parties have agreed to a form of lease to be entered into at closing.
At closing, the parties expect to enter into a lease agreement for portions of the Peace Naturals Campus, which will include an initial five-year term and one five-year renewal option that may be exercised by the Company. The Company has the right to terminate the lease without penalty anytime after the second anniversary of the lease by giving written notice at least 12 months prior to termination. The leased premises will be identified and agreed between both parties prior to closing.
Joint Ventures/Strategic Investments
We have established two strategic joint ventures in Canada and Israel. Additionally, we hold approximately 9.6% of the issued capital of Vitura Health Limited (“Vitura”), which we account for as equity securities with a readily determinable fair value, and approximately 13.7% of the issued capital of NatuEra S.à.r.l. (“Natuera”), which we account for as equity securities without a readily determinable fair value, as of December 31, 2023.
Our ownership interest in each of our joint ventures is summarized in the table below.
| | | | | | | | | | | | | | |
| Joint Venture | | Jurisdiction | | Ownership Interest(i) |
Cronos Israel(ii) | | Israel | | 70%/90% |
Cronos GrowCo(iii) | | Canada | | 50% |
| | | | |
(i)We define ownership interest as the proportionate share of net income to which we are entitled; equity interest may differ from ownership interest shown above. We consolidate the financial results of Cronos Israel and account for our other joint ventures under the equity method of accounting. See Note 1 “Background, Basis of Presentation, and Summary of Significant Accounting Policies” and Note 4 “Investments” to our consolidated financial statements in Item 8 of this Annual Report.
(ii)A strategic joint venture with Kibbutz Gan Shmuel (“Gan Shmuel”), an Israeli agricultural collective settlement, for the production, manufacturing and global distribution of medical cannabis, consisting of a cultivation company (Cronos Israel G.S. Cultivation Ltd.), a manufacturing company (Cronos Israel G.S. Manufacturing Ltd.), a distribution company (Cronos Israel G.S. Store Ltd.) and a pharmacy company (Cronos Israel G.S. Pharmacy Ltd., collectively, “Cronos Israel”). We hold a 70% equity interest in the cultivation company and a 90% equity interest in each of the manufacturing, distribution and pharmacy companies.
(iii)A strategic joint venture with a group of investors led by Bert Mucci (the “Greenhouse Partners”), a Canadian large-scale greenhouse operator. Each of Cronos and the Greenhouse Partners owns a 50% equity interest in Cronos GrowCo and has equal representation on its board of directors.
Strategic Investment in PharmaCann, Inc.
On June 14, 2021, Cronos USA Holdings Inc., a wholly owned subsidiary of the Company, purchased an option (the “PharmaCann Option”), with an exercise price of $0.0001 per share, to acquire an approximately 10.5% ownership stake in PharmaCann, Inc. (“PharmaCann”) on a fully diluted basis for total consideration of approximately $110.4 million. PharmaCann is a leading vertically integrated U.S. cannabis company that has a broad geographic footprint in the U.S. and has built an efficient, effective and scalable operating model. The PharmaCann Option exercise will be based upon various factors, including the status of U.S. federal cannabis legalization, as well as regulatory approvals, including in the states where PharmaCann operates that may be required upon exercise. Following the exercise of the PharmaCann Option, the Company and PharmaCann will enter into commercial agreements that would permit each party to offer its products through the other party’s distribution channels.
As of December 31, 2023, the Company’s ownership percentage in PharmaCann on a fully diluted basis was approximately 5.9%. Under the terms of the Company’s investment in PharmaCann, the Company’s rights to nominate an observer or a director to the PharmaCann board of directors could be lost if the Company’s ownership drops below 6% on a fully diluted basis and it sells or transfers all or any portion of the option (subject to certain exceptions).
No U.S. Schedule I Cannabis-Related Activities
Though a number of states in the U.S. have authorized the cultivation, distribution or possession of U.S. Schedule I cannabis and U.S. Schedule I cannabis containing products to various degrees and subject to various requirements or conditions, U.S. Schedule I cannabis continues to be a Schedule I controlled substance under the U.S. Controlled Substances Act (the “CSA”). Therefore, the cultivation, manufacture, distribution and possession of U.S. Schedule I cannabis violates federal law in the U.S. unless a U.S. federal agency, such as the DEA, grants a registration for a specific activity, such as research, with U.S. Schedule I cannabis.
We do not engage in any activities related to U.S. Schedule I cannabis in the U.S. The Ginkgo Strategic Partnership contemplates the performance of licensed R&D activities in the U.S. in order to produce cultured cannabinoids, but such activities are conducted in compliance with all applicable laws regarding controlled substances.
Brand Portfolio
We are committed to building a portfolio of iconic brands that responsibly elevate the consumer experience.
In Canada, we sell a variety of cannabis products through wholesale channels under both our core adult-use brands, Spinach® and Lord Jones®, and under our core wellness platform, PEACE NATURALS®. In addition, PEACE NATURALS® cannabis products are currently available in the Israeli and German medical markets.
| | | | | | | | | | | | | |
| | | | | |
| Brand Positioning | Mainstream adult-use | Wellness | | | Premium adult-use |
| Product Offering | Dried flower, pre-rolls, vaporizers, edibles | Dried flower, pre-rolls, cannabis tinctures | | | Pre-rolls, vaporizers, edibles |
| Geographic Availability | Canada | Canada, Israel & Germany | | | Canada |
Core Adult-UseBrands
Spinach® is a mainstream adult-use cannabis brand focused on friends, fun and legendary cannabis. The Spinach® brand portfolio includes cannabinoid products in a wide range of formats including dried flower, pre-rolls, vaporizers and edibles.
The Spinach® brand also has two sub-brands, SOURZ by Spinach® and Spinach FEELZ™. SOURZ by Spinach® is a line of multi-colored, multi-flavored edibles in a variety of cannabinoid ratios in a distinctive “S” shaped gummy, featuring proprietary flavor masking technology. Spinach FEELZ™ prominently features rare cannabinoids in a range of product formats, designed to deliver unique and enhanced experiences made possible through proprietary blends of rare cannabinoids alongside common cannabinoids, like delta-9-tetrayhydrocannabinol (“THC”). Each product is formulated to help adult consumers, “Feelz. The Way You Want.”
Lord Jones® is a premium adult-use cannabis brand that goes above and beyond to unlock differentiated ways to experience cannabis. The Lord Jones® brand portfolio includes cannabis products in the pre-roll, vaporizer and edible categories.
Core Wellness Brand
PEACE NATURALS® is a global wellness platform committed to producing high-quality cannabis products. It is focused on building and shaping the global cannabis wellness market and promoting a holistic approach to wellness. The Company currently distributes products under PEACE NATURALS® for Canadian, Israeli and German medical markets.
Cronos Marketing Code
In 2021, Cronos released its Marketing Code, which is designed to responsibly move the emerging cannabis industry forward. Cronos believes that those below the legal age of consumption should not be targeted in an adult-use cannabis market. Cronos recognizes there is a clear need for standards.
The principles in the Cronos Marketing Code apply to all marketing activities of all Cronos brands globally and are communicated to all business partners in any work they do on the Company’s behalf. The Marketing Code represents Cronos’ commitment to responsible marketing standards. The code standards are:
•Our advertising will be targeted to adults.
•We will highlight responsible cannabis consumption and any people depicted in any imagery will be adults.
•Our brand websites and social media will be designed for adults.
•Our marketing events will be targeted to adults and will promote responsible cannabis consumption.
•We will provide our customers with facts and substantiate our claims.
Global Sales and Distribution - Principal Markets
Cronos has developed a diversified global sales and distribution network. We have built a distribution footprint in Canada through the adult-use and medical markets, as well as a distribution channel for the Israeli medical market.
Canadian Market and Distribution
•Medical Market. Following the closure of the Medical Cannabis by Shoppers Drug Mart platform, our PEACE NATURALS® medical cannabis products are sold in Canada through various third-party distributors.
•Adult-Use. We currently sell dried flower, pre-rolls, vaporizers and edibles through our core adult-use brands, Spinach® and Lord Jones®, to cannabis control authorities in all provinces of Canada and the Yukon territory, except Saskatchewan, where we sell to private-sector retailers, subject to the relevant province’s or territory’s product or other restrictions and requirements. As the Company’s supply chain grows, the Company continues to expand its portfolio of cannabis products for the existing markets in Canada.
Markets and Distribution Outside of Canada
•Israel. Cronos Israel holds the IMC-GAP, IMC-GMP and IMC-GDP certifications required for the cultivation, production and marketing of dried flower, pre-rolls and oils in Israel. Cronos Israel distributes PEACE NATURALS® branded cannabis products to the Israeli medical market through pharmacies. See “—Licenses and Regulatory Framework in Israel.”
•Europe. In September 2023, Cronos established a strategic partnership with Cansativa, a leading German cannabis company, to distribute PEACE NATURALS® branded products along with other white-labeled cannabis products in the German medical market
•Australia. We distribute cannabis products in Australia through a distribution relationship with Vitura (formerly known as Cronos Australia Limited).
We continue to seek new international distribution channels in jurisdictions that have legalized the production, distribution and possession of cannabis products at all relevant levels of government.
Global Supply Chain
Cronos is focused on establishing an efficient global supply chain by seeking to develop industry-leading methodologies and best practices at Cronos Fermentation and the Peace Naturals Campus and leveraging this expertise to create beneficial production partnerships. We plan to continue to develop a global supply chain, which will employ a combination of wholly-owned production facilities, third party suppliers and global production partnerships, all of which will support the manufacturing of cannabinoid-based consumer goods.
Canadian Supply Chain
•Peace Naturals Campus. The Peace Naturals Campus is licensed for cannabis production and the manufacturing of certain cannabis products. The Peace Naturals Campus is engaged in processing, finishing, packaging and shipping activities, as well as R&D activities, including cannabinoid product formulation, product development, tissue culture and micro propagation. In the fourth quarter of 2023, Cronos announced that its wholly owned subsidiary entered into an agreement with Future Farmco, a vertical farming company, for the sale and leaseback of the Peace Naturals Campus, subject to the terms and conditions set forth therein. As described under “Operations and Investments–Sale and Leaseback of the Peace Naturals Campus” above, the parties plan to enter into a lease agreement upon closing for portions of the Peace Naturals Campus.
•Cronos GrowCo. The Cronos GrowCo production facility is licensed for cannabis production and the manufacturing of certain cannabis products. Under its current licenses, Cronos GrowCo is permitted to sell certain cannabis products to other license holders in the wholesale channel, as well as to provincial cannabis control authorities. Cronos GrowCo holds Global GAP and ICANN GAP certifications (equivalent to IMC-GAP) for the export of dried flower to Israel.
•Cronos Fermentation.Cronos Fermentation is licensed for cannabis production and the manufacturing of certain cannabis products. In August 2023, Cronos announced the planned wind-down of Cronos Fermentation and has listed the facility for sale. Cronos Fermentation engaged in R&D to produce high-quality cultured cannabinoids at commercial scale.
•Third-party Supply and Manufacturing Agreements. In the ordinary course of our business, we enter into spot market purchase agreements and supply agreements with suppliers of dried flower and other cannabis products. Our supply agreements, for the most part, do not obligate us to purchase minimum quantities of products and generally contain provisions permitting cancellation of orders or termination on notice. We also enter into contract manufacturing agreements with other license holders for certain manufacturing and processing services related to our products.
Supply Chain Outside of Canada
•Cronos Israel. Cronos Israel holds the IMC-GAP, IMC-GMP and IMC-GDP certifications required for the cultivation, production, distribution, and marketing of dried flower, pre-rolls and oils in Israel. Cronos Israel distributes PEACE NATURALS® branded cannabis products to the Israeli medical market. See “—Licenses and Regulatory Framework in Israel” for more information on our licenses in Israel.
Major Customers
Major customers are customers for which sales equaled or exceeded 10% of our consolidated net revenues for the year. We had three major customers, Ontario Cannabis Retail Corporation, Alberta Gaming, Liquor and Cannabis Commission, and BC Liquor Distribution Branch, which accounted for approximately 34%, 21%, and 11%, respectively, of our consolidated net revenues, before excises taxes, for the year ended December 31, 2023. We mitigate credit risk through verification of the customers’ liquidity prior to the authorization of material transactions.
Government Contracts
In Canada, we sell cannabis products to cannabis control authorities in all provinces of Canada and the Yukon territory, except for Saskatchewan (where we sell to private-sector retailers), where each such cannabis control authority is the sole wholesale distributor and in certain provinces, the sole retailer, of cannabis products. We sell these products to the various cannabis control authorities under supply agreements that are subject to terms that allow for renegotiation of sale prices and termination at the election of the applicable cannabis control authority. In particular, the cannabis control authorities have in the past and may in the future choose to stop purchasing our products, may change the prices at which they purchase our products, may return our products to us and, in certain circumstances, may cancel purchase orders at any time including after products have been shipped. For the year ended December 31, 2023, we had approximately $96.5 million in sales to cannabis control authorities in Canada.
Research and Development Activities and Intellectual Property
Ginkgo
The collaboration and license agreement between Ginkgo and the Company (the “Ginkgo Collaboration Agreement”) enabled us to produce certain cultured cannabinoids at commercial scale at a fraction of the cost compared to traditional cultivation practices. The Ginkgo Collaboration Agreement was amended in June 2021 to enable accelerated commercialization of such cultured cannabinoids, ultimately resulting in the Company’s launching of its first cultured cannabinoid product containing cannabigerol (“CBG”) in the second half of 2021. These cultured cannabinoid molecules are identical to those produced by plants grown using traditional cultivation but are created by leveraging the power of biological manufacturing via fermentation. These cultured cannabinoids include rare cannabinoids that are difficult to produce at high purity and scale through traditional cultivation.
The Ginkgo Strategic Partnership enabled Cronos to produce large volumes of these cultured cannabinoids from custom yeast strains by leveraging the fermentation infrastructure at Cronos Fermentation.However, in August 2023, Cronos announced the planned wind-down of the Cronos Fermentation facility and has listed the facility for sale.As a result, we are no longer operating facilities that leverage the patented intellectual property under the Ginkgo Strategic Partnership, although Cronos may leverage this intellectual property, either through production at the Peace Naturals Campus or through a contract manufacturer, and Cronos continues to utilize these cultured cannabinoids in our products sold in Canada.
Ginkgo has filed certain patent applications pertaining to biosynthesis of cannabinoids to protect the intellectual property developed as part of the research progressing under the Ginkgo Strategic Partnership. Under the partnership, Cronos is the exclusive licensee of the intellectual property covered by the patent applications for the target cannabinoids.
The Ginkgo Strategic Partnership contemplates the performance of licensed R&D activities in the U.S. in order to produce cultured cannabinoids, and such activities are to be conducted in compliance with all applicable laws regarding controlled substances. We intend to distribute the target cannabinoids globally, where permitted by applicable law, and have received confirmation from Health Canada that this method of production is permitted under the Cannabis Act.
Cronos Fermentation
Cronos Fermentation is a GMP-standard fermentation and manufacturing facility in Winnipeg, Manitoba. The state-of-the-art facility includes fully equipped laboratories covering microbiology, organic and analytical chemistry, quality control and method development as well as two large-scale microbial fermentation production areas, three downstream processing plants, and bulk product and packaging capabilities. The facility is expected to provideprovided the fermentation and manufacturing capabilities we need in orderused to capitalize on the progress underway with Ginkgo Strategic Partnership, by enabling us to produce the target cannabinoids contemplated under the Ginkgo Collaboration Agreement at commercial scale with high quality and high purity.
We have begun to work on developing scale-up and downstream processes atthe Cronos Fermentation while in parallel Ginkgo develops microorganisms for producing cultured compounds. As we develop the processesfacility and parameters, these learnings will be used for the strains that will be used for commercial production of cultured cannabinoids. Commercial production athas listed the facility is subject to completion of the equipment alignment for cannabinoid-based production, the receipt of the appropriate licenses from Health Canada for the production of cultured cannabinoids under the Cannabis Act and the achievement of the relevant milestones under the Ginkgo Strategic Partnership.
The Company is prioritizing rare cannabinoids, such as CBG, over common cannabinoids, such as THC and CBD, and will be sequencing commercial production and subsequent product launches based upon this approach. The Company currently expects to achieve its first equity milestone by the third quarter of 2021. The achievement of equity milestones for the remaining seven target cannabinoids will occur sequentially based on the Company’s future commercialization plans, which will depend on consumer insights, customer preferences and competitive opportunities.
Technion Skin Health Research Partnership
We have a sponsored research agreement (the “Technion Research Agreement”) with the Technion Research and Development Foundation of the Technion - Israel Institute of Technology (“Technion”) to explore the use of cannabinoids and their role in regulating skin health and skin disorders. Preclinical studies are being conducted by Technion over a three-year period and will focus on three skin conditions: acne, psoriasis and skin repair.
Research is led by Technion faculty members Dr. David “Dedi” Meiri and Dr. Yaron Fuchs, two of the world’s leading researchers in cannabis and skin stem cell research, respectively. Dr. Meiri heads the Laboratory of Cannabis and Cancer Research with vast experience in cannabis and endocannabinoid research. Dr. Fuchs heads the Laboratory of Stem Cell Biology and Regenerative Medicine with years of experience in the biology of the skin and its pathologies. Development and implementation of the research is being conducted at Technion’s Laboratory of Cancer Biology and Cannabis Research and the Lorry I. Lokey Interdisciplinary Center of Life Sciences and Engineering in Haifa, Israel.
Enterprise Initiatives
In the third quarter of 2020, the Company implemented a new enterprise resource planning (“ERP”) system across the Canadian business. Cronos Group has also commenced work to broaden the scope of our ERP system to the U.S. business, which is currently expected to be launched in the first half of 2021. The new ERP system will be a meaningful component of the Company’s internal control over financial reporting and is expected to enable us to realize efficiencies throughout our supply chain and operations.sale.
Competitive Conditions
Competitive Conditions in the United States
We face competition in all aspects of our business in the U.S. hemp market. In addition to numerous small companies and brands, we compete with larger, national companies that may have larger distribution capabilities with more developed and efficient supply chain operations. The principal factors on which we compete with other U.S. hemp brands are product quality, innovation, intellectual property, brand recognition and price. We believe the Company’s strong capitalization resulting from the Altria Investment, along with the Lord Jones™, Happy Dance™ and PEACE+™ brand equity, recognition and differentiation in the U.S. hemp retail channel, will enable us to provide better quality consumer products, grow our U.S. hemp business and strengthen our market position in the U.S. However, rapidly evolving and developing federal and state regulatory frameworks affect all areas of our business and could result in our inability to compete successfully against our current and future competitors. See “ -U.S. Hemp Regulatory Framework” for further information on regulatory framework on U.S. hemp.
Rest of World
Competitive Conditions in Canada
We face competition in all aspects of our business in the Canadian medicaladult-use and adult-usemedical markets. As the demand for cannabis increases as a result of the legalization of adult-use cannabis in Canada under the Cannabis Act, we believe that new competitors will continue to enter the market.
The principal factors on which we compete with other Canadian license holders are product quality, innovation, intellectual property, brand recognition and price. We believe the Company’s strong capitalization resulting from the Altria Investment, along with the Spinach® and Lord Jones® brand recognition and differentiation in the Canadian adult-use market, will enable us to provide better quality consumer products, grow our Canadian business and strengthen our market position in Canada. However, a rapidly evolving and stringent federal regulatory framework affects all areas of our business. See “—Regulatory Framework in Canada” for further information on the regulatory framework applicable to our Canadian business.
We also face competition from illegal market participants that are unlicensed and unregulated. As these illegal market participants do not comply with the regulations governing the cannabis industry, their operations may also have significantly lower costs. Any inability of the Canadian federal or provincial law enforcement authorities to enforce existing laws prohibiting the unlicensed cultivation and sale of cannabis and cannabis-based products could result in the perpetuation of the illegal market for cannabis.
In addition to competition from illegal market participants, we also face competition from licensed cannabis competitors that fail to comply with the regulations governing the cannabis industry when developing and selling cannabis products. If regulatory authorities are delayed in, or fail to, effectively restrict the sale and distribution of these non-compliant cannabis products, such regulatory non-compliance by our competitors may have adverse effects on our business and the perception of cannabis use.
Competitive Conditions in Europe and IsraelOutside of Canada
We face competition when entering new markets in Europe and in Israel.markets. The principal factors on which we compete are product quality, innovation, intellectual property, brand recognition, price and physician familiarity. We believe we are positioned to enter certain markets in Europe and Israel in a meaningful way while continuing to operate and penetrate the markets we currently serve, such as in Israel and Germany, due to our strong capitalization resulting from the Altria Investment, extensive experience and expertise in the highly regulated cannabis industry in Canada, which can be leveraged when entering new markets or growing existing operations, and strong partnerships with local pharmaceutical distributors. We believe these factors will enable us to develop greater market penetration, provide a greater variety of quality consumer products and enter into new markets and strengthen our existing market position in Europe, Australia and Israel. However, a patchwork of regulatory frameworks and federal regulations in these various regions also affectaffects our ability to compete in emerging markets as evolving regulations and federal frameworks have the potential to affect all areas of our business.
Altria Strategic Investment
Altria Investment and Investor Rights Agreement
As of December 31, 2020,2023, Altria beneficially owned 156,573,53741.1% of our common shares and had the right to acquire up to an additional 80,056,296 common shares on or prior to March 8, 2023 under the Altria Warrant, which has not been exercised, and the right to acquire additional common shares under its pre-emptive and top-up rights as discussed under “Pre-Emptive Rights and Top-Up Rights” below.
Investor Rights Agreement
In connection with the Altria Investment, we entered into the investor rights agreement (the “InvestorInvestor Rights Agreement”)Agreement with Altria pursuant to which Altria received certain governance rights whichthat are summarized below.
Board Representation
The Investor Rights Agreement provides that, for so long as Altria and certain of its affiliates (the “Altria Group”) continue to beneficially own at least 40% of our issued and outstanding common shares and the size of our board of directors (the “Board”) is seven directors, we agree to nominate for election as directors to the Board four individuals designated by Altria (the “Altria Nominees”). In addition, for so long as the Altria Group continues to beneficially own greater than 10% but less than 40% of our issued and outstanding common shares, Altria shall beis entitled to nominate a number of Altria Nominees that represents its proportionate share of the number of directors comprising the Board (rounded up to the next whole number) based on the percentage of our issued and outstanding common shares beneficially owned by the Altria Group at the relevant time. At least one Altria Nominee must be independent as long as Atria has the right to designate at least three Altria Nominees and the Altria Group’s beneficial ownership of our issued and outstanding common shares does not exceed 50%.
The Investor Rights Agreement also provides that, subject to certain exceptions, for so long as Altria is entitled to designate one or more Altria Nominees, we agree to appoint to each committee established by the Board such number of Altria Nominees that represents Altria’s proportionate share of the number of directors comprising the applicable Board committee (rounded up to the next whole number) based on the percentage of our issued and outstanding common shares beneficially owned by the Altria Group at the relevant time.
Approval Rights
The Investor Rights Agreement also grants Altria, until the Altria Group beneficially owns less than 10% of our issued and outstanding common shares, approval rights over certain transactions that may be undertaken by us. We have agreed that, among other things, we will not (and will use our commercially reasonable efforts to cause our affiliates not to), without the prior written consent of Altria:
•consolidate or merge into or with another person or enter into any similar business combination;
•acquire any shares or similar equity interests, instruments convertible into or exchangeable for shares or similar equity interests, assets, business or operations with an aggregate value of more than C$100,000,000, in a single transaction or a series of related transactions;
•sell, transfer, cause to be transferred, exclusively license, lease, pledge or otherwise dispose of any of our or any of our significant subsidiaries’ assets, business or operations in the aggregate with a value of more than C$60,000,000;
•except as required by applicable law, make any changes to our policy with respect to the declaration and payment of any dividends on our common shares;
•subject to certain exceptions, enter into any contract or other agreement, arrangement, or understanding with respect to, or consummate, any transaction or series of related transactions between us or any of our subsidiaries, on the one hand, and any related parties, on the other hand, involving consideration or any other transfer of value required to be disclosed pursuant to Item 404 of Regulation S-K promulgated pursuant to the United States Securities Act of 1933, as amended (the “Securities Act”);Act; or
•engage in the production, cultivation, advertisement, marketing, promotion, sale or distribution of cannabis or any Related Products and Services (as defined herein)in the Investor Rights Agreement) in any jurisdiction, including the U.S., where such activity is prohibited by applicable law as of the date of the Investor Rights Agreement (subject to certain limitations).
Exclusivity Covenant
Pursuant to the terms of the Investor Rights Agreement, until the earlier of:
(i) •the six-month anniversary of the date on which the Altria Group beneficially owns less than 10% of our issued and outstanding common shares; and
(ii) •the six-month anniversary of the termination of the Investor Rights Agreement,
Altria has agreed to make us its exclusive partner for pursuing cannabis opportunities throughout the world (subject to certain limited exceptions).
Pre-Emptive Rights and Top-Up Rights
Pursuant to the terms of the Investor Rights Agreement and provided the Altria Group continues to beneficially own at least 20% of our issued and outstanding common shares, Altria has a right to purchase, directly or indirectly by another member of the Altria Group, upon the occurrence of certain issuances of common shares by us (including issuances of common shares to Ginkgo under the Ginkgo Collaboration Agreement (each, a “Ginkgo Issuance”)) (each, a “Triggering Event”) and subject to obtaining the necessary approvals, up to such number of our common shares issuable in connection with the Triggering Event which will, when added to our common shares beneficially owned by the Altria Group immediately prior to the Triggering Event, result in the Altria Group beneficially owning the same percentage of our issued and outstanding common shares that the Altria Group beneficially owned immediately prior to the Triggering Event (in each case, calculated on a non-diluted basis). The price per common share to be paid by Altria pursuant to the exercise of these pre-emptive rights will be, subject to certain limited exceptions, the same price per common share at which the common shares are sold in the relevant Triggering Event; provided that if the consideration paid in connection with any such issuance is non-cash, the price per common share that would have been received had such common shares been issued for cash consideration will be determined by an independent committee (acting reasonably and in good faith); provided further that the price per common share to be paid by Altria pursuant to the exercise of its pre-emptive rights in connection with a Ginkgo Issuance will be C$16.25 per common share.
In addition to (and without duplication of) the aforementioned pre-emptive rights, the Investor Rights Agreement provides Altria with top-up rights, exercisable on a quarterly basis, whereby, subject to obtaining the necessary approvals and for so long as the Altria Group beneficially owns at least 20% of our issued and outstanding common shares, Altria has the right to subscribe for such number of common shares in connection with any Top-Up Securities (as defined below) that we may, from time to time, issue after the date of the Investor Rights Agreement, as will, when added to the common shares beneficially owned by the Altria Group prior to such issuance, result in the Altria Group beneficially owning the same percentage of our issued and outstanding common shares that the Altria Group beneficially owned immediately prior to such issuance. “Top-Up Securities” means any of our common shares issued:
•on the exercise, conversion or exchange of our convertible securities issued prior to the date of the Investor Rights Agreement or on the exercise, conversion or exchange of our convertible securities issued after the date of the Investor Rights Agreement in compliance with the terms of the Investor Rights Agreement, in each case, excluding any of our convertible securities owned by any member of the Altria Group;
•pursuant to any share incentive plan of the Company;
•on the exercise of any right granted by us pro rata to all shareholders to purchase additional common shares and/or other securities of the Company (other than a right issued in a rights offering in which Altria had the right to participate);
•in connection with bona fide bank debt, equipment financing or non-equity interim financing transactions with our lenders, in each case, with an equity component; or
•in connection with bona fide acquisitions (including acquisitions of assets or rights under a license or otherwise), mergers or similar business combination transactions or joint ventures undertaken and completed by us,
in each case, other than (A) common shares issued pursuant to Altria’s pre-emptive right and (B) common shares issued pursuant to the Ginkgo Collaboration Agreement.
The price per common share to be paid by Altria pursuant to the exercise of its top-up rights will be, subject to certain limited exceptions, the volume-weighted average price of our common shares on the TSX for the 10 full trading days preceding such exercise by Altria; provided that the price per common share to be paid by Altria pursuant to the exercise of its top-up rights in connection with the issuance of common shares pursuant to the exercise of options or warrants that were outstanding on the date of closing of the Altria Investment will be C$16.25 per common share without any set off, counterclaim, deduction or withholding.
For a period commencing on the date of the Investor Rights Agreement and ending on the earlier of (i) the date on which the Altria Warrant has been exercised in full by Altria, and (ii) the expiry or termination of the Altria Warrant, the Investor Rights Agreement provides that, without the prior approval of an independent committee of the Board, no member of theOpen Market Purchases
The Altria Group shall, directly or indirectly,is permitted to acquire our common shares (other than upon settlement ofin the open market at any of our common shares issued, soldtime and delivered pursuant to the proper exercise of rights contemplated by the Altria Warrant Certificate or the exercise of pre-emptive rights or top-up rights). The Altria Group, however, may make a take-over bid or commence a tender offer, in each case, to acquire not less than all of our issued and outstanding common shares (other than any such common shares beneficially owned by any member of the Altria Group and its affiliates) in accordance with applicable law.amount.
Registration Rights
The Investor Rights Agreement provides Altria with the right, subject to certain limitations and to the extent permitted by applicable law, to require us to use reasonable commercial efforts to file a prospectus under applicable securities laws and/or a registration statement, qualifying our common shares held by Altria for distribution in Canada and/or the U.S. In addition, the Investor Rights Agreement provides Altria with the right to require us to include our common shares held by Altria in any proposed distribution of common shares in Canada and/or the U.S. by us for our own account.
Commercial Arrangements
In connection with the Altria Investment, we and Altria have entered into certain commercial arrangements (the “Commercial Arrangements”), pursuant to which Altria providesmay provide us with consulting services on matters which may include R&D, marketing, advertising and brand management, government relations and regulatory affairs, finance, tax planning, logistics and other corporate administrative matters. The services under the Commercial Arrangements are provided on customary terms and for a services fee payable by us that is equal to Altria’s reasonably allocated costs plus 5%.
Protection of Intangible Assets
The ownership and protection of our intellectual property rights is a significant aspect of our future success. Currently, we rely on trademarks, patents, copyrights, trade secrets, technical know-how and proprietary information. We seek to protect our intellectual property by strategically seeking and obtaining registered protection where possible,appropriate, developing and implementing standard operating procedures to protect inventions, germplasm, trade secrets, technical know-how and proprietary information and entering into agreements with parties that have access to our inventions, germplasm, trade secrets, technical know-how and proprietary information, such as our partners, collaborators, employees and consultants, to protect confidentiality and ownership. We also seek to preserve the integrity and confidentiality of our inventions, germplasm, trade secrets, trademarks, technical know-how and proprietary information by maintaining physical security of our premises and physical and electronic security of our information technology systems.
In addition, we have sought trademark protection in many jurisdictions, including Canada, Australia, the U.S., China, Israel and Europe. Our ability to obtain registered trademark protection for cannabis-related goods and services, in particular for cannabis itself, may be limited in certain countries outside of Canada. For example, in the U.S., registered federal trademark protection is only available for goods and services that can be lawfully used in interstate commerce; the PTO is not currently approving any trademark applications for U.S. Schedule I cannabis, or certain goods containing U.S. hemp-derived CBD (such as dietary supplements and food) until the FDA provides clearer guidance on the regulation of such products. In Europe, trademarks cannot be obtained for products that are “contrary to public policy or accepted principles of morality.” Accordingly, our ability to obtain intellectual property rights and enforce intellectual property rights against third-party uses of similar trademarks may be limited in certain jurisdictions.
Human Capital Resources
Cronos Group is an innovative global cannabinoid company committed to building disruptive intellectual property by advancing cannabis research, technology and product development anddevelopment. With a passion to responsibly elevate the consumer experience, Cronos is seeking to buildbuilding an iconic brand portfolio. Our employees are critical to achieving this mission. In order to compete and succeed in our highly competitive and rapidly evolving industry, it is crucial that we continue to attract, develop, motivate and retain skilled, talented and passionate employees. The Company’s people strategy seeks to build a winning team and to foster a community where everyone feels included and empowered to do to their best work.
As of December 31, 2020,2023 we had 665356 full-time employees and 2 full-time contractors.employees. Of our full-time employees, 472231 were in Canada, 8649 were in the U.S., and 10776 were in Israel. None of our employees are represented by a labor union or covered by a collective bargaining agreement.
Compensation and Benefits. Our compensation program is designed to attract, motivate and reward talented individuals who possess the skills necessary to support our business objectives, assist in the achievement of our strategic goals and create long-term value for our shareholders. We believe we offer competitive compensation and benefits in each of our locations, including long-term equity awards to eligible employees under our 2020 Omnibus Equity Incentive Plan to reward and retain talented individuals and align employee and shareholder interests.
Safety, Health and Well-being. The safety, health and well-being of our employees are paramount to the Company. We provide our employees and their families with access to a variety of health and welfare programs, including benefits that support their physical and mental health by providing tools and resources to help them improve or maintain their health status. In responseSee “Risk Factors—Risks Relating to COVID-19, we implemented extensiveOperations in Israel” for risks to the safety, measures throughout the Company to protecthealth and well-being of our employees from COVID-19, including complying with social distancing and other health and safety standards as required by federal, provincial, state, local and municipal government agencies, taking into consideration guidelines of applicable public health authorities. These measures include reducingin Israel due to the number of employees working on-site at our production facilities to only the roles that are necessary to be performed on-site, implementing work-from-home policies for employees whose work can be performed off-site, and implementing other additional health and safety measures such as enhanced hygiene and sanitation procedures, modified work schedules, social distancing protocols at our production facilities and travel restrictions.Israel-Hamas War.
Employee Engagement, Development and Training. We are committed to developing our talent and building an agile and resilient organization with a workforce with the skillset to effectively adapt to changing business needs in order to best position the Company for success. We seek to foster a culture of employee learning, innovation and resiliencya drive to succeed through a talent development strategy
that adapts to changing business needs. Management is an active enabler of our people strategy as we seek to recruit, retain and engage top talent that will maximize our business performance. Employees are enabled to succeed through our communicated behaviors, development training opportunities, our performance management program and pay for performance philosophy, and using their voice in our employee engagement survey.
Diversity, Equity and Inclusion and Ethical Business Practices. We believe that a diverse, equitable and inclusive work environment mitigates the risk of group-think,groupthink, ensures that the Company has the opportunity to benefit from all available talent and enhances, among other things, our organizational strength, problem-solving ability and opportunity for innovation. We continue to focus on understanding our diversity and inclusion strengths and opportunities and executing on a strategy to support further progress. We are committed to hiring, developing, and promoting employees with diverse backgrounds. We are actively reviewing diversity across our Company to drive greater progress. We welcome, embrace, and celebrate all our employees. We seek to ensure this inclusivity is achieved through regular training and support for our employees. We maintain a whistleblower policy and anonymous hotline for the confidential reporting of any suspected policy violations and provide training and education to our global workforce with respect to our Code of Business Conduct and Ethics and related policies.
Regulatory Framework in the U.S.
U.S. Hemp Regulatory Framework
We derive a portion of our revenues from the manufacture, marketing and distribution of U.S. hemp-derived supplement and cosmetic consumer products through e-commerce, retail and hospitality channels in certain states in the U.S. All U.S. hemp-derived products produced and sold by us constitute “hemp” (i) under the 2018 Farm Bill and (ii) the applicable state-law equivalent in all states in which we produce and sell such U.S. hemp-derived products. The 2018 Farm Bill was enacted in the U.S. on December 20, 2018. Prior to this enactment, cannabis was scheduled as a controlled substance (marijuana) under the CSA with limited exemptions based on the portion of the cannabis plant. The 2018 Farm Bill, among other things, removed U.S. hemp (which is defined in the 2018 Farm Bill as “the plant Cannabis sativa L. and any part of that plant, including the seeds thereof and all derivatives, extracts, cannabinoids, isomers, acids, salts, and salts of isomers, whether growing or not, with a delta-9 tetrahydrocannabinol concentration of not more than 0.3 percent on a dry weight basis”) and its derivatives, extracts and cannabinoids, including CBD, derived from hemp, from the definition of “marijuana” in the CSA, thereby removing U.S. hemp and its derivatives as controlled substances. The 2018 Farm Bill also amended the Agricultural Marketing Act of 1946 to allow for production and sale of U.S. hemp and its derivatives in the U.S.
The 2018 Farm Bill tasks the USDA with promulgating regulations in relation to the cultivation and production of U.S. hemp. The 2018 Farm Bill also directs the USDA to promulgate federal regulations that would apply to the production of U.S. hemp in every state that does not put forth a state U.S. hemp plan for approval by the USDA. In January 2021, the USDA issued a final rule governing U.S. hemp production in the U.S. with an effective date of March 22, 2021. There remains some possibility that the effective date of the final rule could be extended, but the USDA has not so indicated that it intends to extend the effective date of the final rule.
The USDA’s final rule establishes a federal licensing plan for regulating U.S. hemp producers in states that do not have their own USDA-approved plans. In the absence of a state plan, U.S. hemp producers will be subject to regulation directly by the USDA unless the state prohibits U.S. hemp production. Additionally, the final rule includes requirements for maintaining information on the land where U.S. hemp is produced, testing U.S. hemp for THC levels, disposing of plants with more than 0.3 percent THC on a dry-weight basis and licensing for U.S. hemp producers. The USDA’s final rule requires hemp producers to use a laboratory that is registered with the DEA, although the USDA is delaying enforcement of this requirement until December 31, 2022. The final rule also includes provisions for producers to dispose or remediate violative hemp plants without the use of a DEA-registered reverse distributor or law enforcement.
States may adopt regulatory schemes that impose different levels of regulation and costs on the production of U.S. hemp. Moreover, the 2018 Farm Bill provides that its provisions do not pre-empt or limit state laws that regulate the production of U.S. hemp. Accordingly, some states may choose to restrict or prohibit some or all U.S. hemp production or sales within the state and variances in states’ laws and regulations on U.S. hemp are likely to persist.
Further, each state has discretion to develop and implement its own laws and regulations governing the manufacturing, marketing, labeling and sale of U.S. hemp products, which has created a patchwork of different regulatory schemes applicable to such products.
Under the 2018 Farm Bill, the FDA has retained authority over the Federal Food, Drug, and Cosmetic Act-regulated products (e.g., drugs, food, dietary supplements and cosmetics) containing U.S. hemp and U.S. hemp-derived ingredients, including CBD. Moreover, states have retained regulatory authority through their own analogues to the Federal Food, Drug, and Cosmetic Act, and the states may diverge from the federal treatment of the use of U.S. hemp as, or in, food, dietary supplements or cosmetic products.
The FDA has consistently taken the position that CBD, whether derived from U.S. hemp or U.S. Schedule I cannabis, is prohibited from use as an ingredient in food and dietary supplements. This stems from its interpretation of the exclusionary clauses in the Federal Food, Drug, and Cosmetic Act because CBD has been approved as a prescription drug and is the subject of substantial clinical investigations as a drug, which have been made public. The exclusionary clauses under the Federal Food, Drug, and Cosmetic Act provide that a substance that has been approved or has been subject to substantial clinical investigations as a drug may not be used in a food or dietary supplement, unless the substance was first marketed in a food or dietary supplement prior to the initiation of substantial clinical investigations of the substance as a drug. The exclusionary clause does not apply to cosmetics. Cosmetics containing CBD could be viewed as drug products by the FDA if disease claims are made, or if the FDA determines the use of CBD in the product has a structure or function effect on the body (i.e., a drug effect).
The FDA has not issued regulations that elaborate on the exclusionary clauses and the FDA has not taken any enforcement action in the courts asserting a violation of the exclusionary clauses. To date, the FDA has issued a number of warning letters to companies unlawfully marketing CBD products. In many of these cases, the manufacturers made unsubstantiated claims about the product being able to treat medical conditions (e.g., cancer, Alzheimer’s disease, opioid withdrawal, anxiety and COVID-19) and had not obtained drug approvals. Others were issued to companies marketing CBD products as dietary supplements despite those products which contain CBD not meeting the definition of a dietary supplement, adding CBD to human and animal foods and marketing CBD products for infants and children and other vulnerable populations. Some of these letters were co-signed with the FTC and cited the companies for making claims about the efficacy of CBD and other ingredients which were not substantiated by competent and reliable scientific evidence. In December 2020, the FTC announced it had entered into settlement agreements with six companies marketing CBD products including oils, gummies, creams, and others with deceptive health claims about serious health conditions. The settlements included monetary penalties ranging from $20,000 to $85,000. The FDA has also issued one warning letter to a dietary supplement manufacturer objecting to a CBD supplement on the basis that CBD was not a permissible dietary supplement ingredient.
In November 2019, the FDA published a revised “Consumer Update” on CBD. The update noted that, as at the time of the Consumer Update, the FDA has approved only one CBD product, a prescription drug product to treat two rare, severe forms of epilepsy. The update also stated that it is illegal to market CBD by adding it to a food or labeling it as a dietary supplement, that the FDA has seen only limited data about CBD safety and these data point to real risks that need to be considered before taking CBD for any reason and that some CBD products are being marketed with unproven medical claims and are of unknown quality. Lastly, the FDA stated that it continues to evaluate the regulatory frameworks that apply to certain cannabis-derived products that are intended for non-drug uses, including whether and/or how they might consider updating their regulations, as well as whether potential legislation might be appropriate.
The FDA has stated that it recognizes the potential opportunities and significant interest in drug and other consumer products containing CBD, is committed to evaluating the agency’s regulatory policies related to CBD and has established a dedicated internal working group to explore potential pathways for various types of CBD products to be lawfully marketed. The FDA held a public hearing in May 2019 to obtain scientific data and information about the safety, manufacturing, product quality, marketing, labeling and sale of products containing cannabis or cannabis-derived compounds. The rules and regulations and enforcement in this area continue to evolve and develop. In July 2020, the FDA sent to the White House Office of Management and Budget (the “OMB”) for review a draft guidance, “Cannabidiol Enforcement Policy,” the details of which were not made public. This guidance remained under review at the OMB until January 2021, when it was withdrawn by the FDA as a part of the regulatory moratorium Executive Order issued by President Biden. The timeline for further CBD policy development remains uncertain while the new administration is put into place and as the FDA faces competing regulatory priorities. In December 2020, the FDA announced a framework for leveraging real world evidence to better understand CBD safety and a number of research projects the agency plans to develop to address gaps in current CBD research. At the same time, the FDA reiterated that CBD remains subject to the same safety standard as any other ingredient based on its intended use, and that there remain a number of safety issues that need to be addressed in order to support the safety of CBD as a food or dietary supplement ingredient. The FDA also announced an expansion of its market sampling and analytical testing of CBD products, as well as issued a new series of Warning Letters against companies marketing CBD products with serious disease claims, including products that the agency viewed as posing serious health risks based on their route of administration, including nasal, ophthalmic and inhalable products.
For more information regarding certain risks facing our business in connection with the U.S. hemp regulatory framework in the U.S., see the section below entitled “Risk Factors - Risks Relating to Regulation and Compliance - Risks Related to U.S. Regulations and Compliance.”
Regulatory Framework in Canada
Licenses and Regulatory Framework
On October 17, 2018, theThe Cannabis Act and the Cannabis Regulations (the “Cannabis Regulations”) came into force. The Cannabis Regulations establish six classes of licenses:
•cultivation;
•processing;
•sale for medical purposes;
•analytical testing;
•research; and
•cannabis drug.
The Cannabis Regulations also create subclasses for cultivation licenses (standard cultivation, micro-cultivation and nursery) and processing licenses (standard processing and micro-processing). Different licenses and each sub-class therein carry differing rules and requirements that are intended to be proportional to the public health and safety risks posed by each category and sub-class.
Federal Regime
The Cannabis Act provides a licensing and permitting scheme for, among other things, the cultivation, processing, testing, packaging, labeling, distribution, sale, possession and disposal of adult-use cannabis, implemented by regulations promulgated under the Cannabis Act. The Cannabis Act and Cannabis Regulations include, among other things, strict specifications for the plain packaging and labeling and analytical testing of all cannabis products as well as stringent physical and personnel security requirements for all federally licensed cultivation, processing and sales sites.
On October 17, 2019, the Regulations Amending the Cannabis Regulations (the “Further Regulations”) came into effect. The Further Regulations amend the Cannabis Act and Cannabis Regulations to, among other things, permit the production and sale of cannabis extracts (including concentrates), cannabis topicals and cannabis edibles, in addition to dried cannabis, cannabis oil, fresh cannabis, cannabis plants and cannabis seeds for parties holding the appropriate licenses. The Cannabis Regulations set out certain requirements for the sale of cannabis products, including limiting the THC content and serving size of certain product forms.
Health Canada allows license holders to export cannabis and cannabis products with appropriate export permits. Export permits issued by Health Canada are specific to each shipment and may only be obtained for medical or scientific purposes. To apply for a permit to export cannabis, a license holder must submit significant information to the ministerHealth Canada including information about the substance to be exported (including description, intended use, quantity) and the importer. As part of the application, applicants are also generally required to provide a copy of the import permit issued by a competent authority in the jurisdiction of final destination and to make a declaration to the ministerHealth Canada that the shipment does not contravene the laws of the jurisdiction of the final destination or any country of transit or transshipment.
The Cannabis Act requires the federal government to conduct a review of the Cannabis Act beginningafter three years, which commenced in October 2021September 2022. ThisThe scope of this statutory review is required to include,includes, among other things, consideration of (i) the administration and operation of the Cannabis Act, (ii) the impact of the Cannabis Act on public health, (iii) the health and consumption habits of young persons, (iv) the impact of cannabis on indigenous persons and communities and (v) the impact of the cultivation of cannabis plants in a dwelling-house. ThisThe federal Minister of Health is expected to table a report in both Houses of Parliament by March 2024. The report resulting from the statutory review may recommend and/or lead to the amendment, removal or addition of provisions in or to the Cannabis Act which could adversely affect our business.
In addition to the current medical and adult-use regimes under the Cannabis Act, Health Canada has also been considering the implementation of a cannabis health product regime for products with potential therapeutic uses that would not require practitioner oversight. Between June and September 2019, Health Canada held a public consultation titled “Potential Market for Cannabis Health Products (CHPs) that would not Require Practitioner Oversight”.Oversight.” The consultation sought feedback from Canadians on the kinds of cannabis health products they would be interested in if such products were made available in Canada. A summary report of the consultation results was published by Health Canada in September 2020. Given the results of the consultation, Health Canada has indicated that it intends to obtain external scientific advice on the appropriate evidence standards required to demonstrate safety, efficacy and quality in cannabis health products, with the information it gathers informing the next steps on a potential implementation of a cannabis health product regime. Health Canada intends to engage with key stakeholders further in 2024.
In December 2023, Health Canada released guidance on cannabis products deliberately made with intoxicating cannabinoids other than delta-9-tetrayhydrocannabinol. Health Canada defines “intoxicating cannabinoids” as cannabinoids that bind to and activate the type 1 cannabinoid receptor (“CB1 receptor”). This guidance recommends that license holders apply the regulatory controls currently applicable to THC to all other cannabinoids that Health Canada defines as “intoxicating cannabinoids” in order to minimize the risks of accidental consumption, overconsumption and adverse effects. Health Canada’s guidance comes at a time when certain provincial cannabis regulators (such as those in Ontario, British Columbia and Alberta) are actively evaluating whether to permit the sale of, or whether to impose limits on the levels of, of certain cannabinoids.
Provincial and Territorial Developments
While the Cannabis Act provides for the regulation by the Canadian federal government of, among other things, the commercial cultivation and processingproduction of cannabis and the sale of medical cannabis, the various provinces and territories of Canada regulate certain aspects of adult-use cannabis, such asincluding the distribution, sale, minimum age requirements and places where cannabis can be consumed, and a range of other matters.consumed.
The governments of each Canadian province and territory have implemented their regulatory regimes for the distribution and sale of cannabis for adult-use purposes which continue to evolve over time. Most provinces and territories have announced a minimum age for possession and consumption of 19 years old, except for Québec and Alberta, where the minimum age is 21 and 18, respectively. In addition, provinces and territories may impose additional licensing requirements and restrictions on sales, distribution and promotion which are more stringent than those at the federal level. For example, the SQDC,Société Quebécoise du Cannabis (the “SQDC”), the exclusive distributor of cannabis in the province and the sole retail and online vendor in Québec, does not permit cannabis vaporizers or other high THC non-edible cannabis products to be sold through its channels. The SQDC has also placed significant restrictions on the types of edibles that may be sold through its channels, prohibiting edibles that are sweet, confectionary, dessert, chocolate or any other product SQDC considers attractive to persons under 21 years of age. Similarly, the Prince Edward Island Cannabis Management Corporation and the province of Newfoundland and Labrador also dodoes not allow cannabis vaporizers to be sold through theirits channels. Provincial distributors may also take different positions on the sale and distribution of products with various cannabinoids (including tetrahydrocannabivarin and cannabinol).
Licenses and Regulatory Framework in Israel
In Israel, cannabis is subject to the Israeli Dangerous Drugs Ordinance [New Version], 5733 -– 1973 (the “Ordinance”), and its sale and use are prohibited unless applicable licenses have been obtained. Licenses to cultivate, produce, possess and use cannabis for medical or research purposes in Israel are granted by the Israel Medical Cannabis Agency within the Israeli Ministry of Health (the “Yakar” and the “Israeli MOH”,MOH,” respectively). PatientsUntil the Reform Regulations (as described below) take effect, patients must also must obtain licenses either directly from physicians who have been authorized to grant patient licenses or from the Yakar following a request from the patient’s physician in order to purchase and consume medical cannabis.
In January 2019, the Israeli government approved, in principle, the export from Israel of medical cannabis products that meet applicable quality standards under the strict supervision of the Israeli authorities. Only products that can be directly marketed to patients (including smoking products, oils, and vaporizer products) may be exported, and only to those countries that have signed the United Nations Single Convention on Narcotic Drugs and that have explicitly approved the import of cannabis. The export of plant substances, including seeds and tissue cultures, is not permitted. In October 2020, the Israeli MOH initiated a pilot program in which certain medical cannabis companies were permitted to export their products, and the Yakar issued guidelines relating to the export of medical cannabis products. These guidelines set forth the process and conditions for obtaining an export license, which can only be issued to an applicant already holding a valid Yakar license.
In December 2021, the Director General of the Israeli MOH announced the appointment of a committee (the “CBD Committee”) intended to examine the possibility of excluding CBD from being considered a “dangerous drug” under the Ordinance. In February 2022, the CBD Committee published its recommendations. The CBD Committee concluded that it would be advisable to exclude CBD from the Ordinance and to allow CBD use, other than in food and cosmetics products, provided that the aggregate concentration of THC does not exceed 0.2%. The CBD Committee further recommended that during the next two years, CBD components not be approved as a component in food, food supplements, and cosmetics and that this issue be re-examined by a committee designated by the Israeli MOH. In March 2022, the Israeli MOH adopted the conclusions of the CBD Committee and published a draft order for public comments which excludes CBD from the Ordinance while setting the threshold of aggregate concentration of THC (or any structural derivative of THC) in any product, at 0.3%. Cosmetics and food products would be handled by specific regulations in such fields. As of January 2024, no final order was issued, and CBD has yet to be excluded from the Ordinance.
On January 31, 2022, the Economic Affairs Committee of the Israeli Parliament held a discussion regarding the adverse effect on the local cannabis industry of significant import of medical cannabis. The discussion was concluded with a request to the Israeli MOH to study the matter and consider banning medical cannabis import until a balance is reached in the market between the import and export of medical cannabis. The Israeli MOH has not yet publicly issued a study on the matter.
In July 2022, a committee appointed by the Director General of the Israeli MOH concluded that it would be advisable to reform the medical cannabis regulatory framework by transitioning from the grant of personal patient licenses to the issuance of prescriptions available through public healthcare services. In August 2022, the new regulations adopting this reform (the “Reform Regulations”) were presented for public comments. The Reform Regulations propose to amend the Ordinance to allow medical cannabis to be prescribed by trained and certified physicians and to be held and distributed by pharmacies. The Reform Regulations aim to ease the Yakar’s regulations relating to medical cannabis prescriptions and accelerate research and innovation in the field. The Reform Regulations were approved by the Israeli Parliament’s health committee in June 2023, and published in July 2023. The Reform Regulations were expected to enter into effect in December 2023, however, due to the situation in Israel and the Israel-Hamas War, its entrance into effect was postponed until March 29, 2024. On December 31, 2023, the Israeli MOH published proposed changes to the current directives and guidelines of the Yakar and Israeli MOH, which remained open for public comments until January 21, 2024.
In June 2022, 11 cannabis manufacturers (including Cronos Israel G.S Manufacturing Ltd.) (the “Petitioners”) filed an administrative petition to the District Court in Jerusalem regarding the Yakar’s decision to issue a constructive license (i.e., a license for dealing with medical cannabis, without a direct contact with the drug, such as brokering medical cannabis transactions) (the “Constructive License”). The Petitioners claim that the procedure and requirements for other licenses (e.g., for cultivation or production), granted by the Yakar, are unreasonably more stringent than those of the Constructive License. On January 5, 2023, the District Court in Jerusalem ordered the Israeli MOH to stop the issuance of Constructive Licenses for 60 days, and to formulate a clear policy regarding the issuance of such Constructive Licenses. On February 12, 2023, the director general of the Yakar published a decision regarding the Constructive Licenses, in response to the District Court’s order. The decision clarifies the Yakar’s authority to grant Constructive Licenses for the import/export of medical cannabis.
On August 30, 2023, following recommendation by the Israeli MOH’s committee, the Director General of the Israeli MOH appointed an additional committee (the “2023 Committee”). The 2023 Committee’s mandate is to comprehensively examine which cannabinoids and parts of the cannabis plant have a psychoactive-addictive effect and whether the classification of cannabis as a “dangerous drug” under the Ordinance is warranted. In September 2023, the Israeli MOH sought public comments and input about the 2023 Committee’s subject matter. The 2023 Committee was scheduled to publish its recommendations by January 1, 2024, but to date, has not done so.
Cronos Israel Licenses
DuringCronos Israel maintains the first quarter of 2020, the Yakar granted Cronos Israel:following certificates and corresponding permits: (1) full Good Agricultural Practices (“GAP”) certification, including a permit to propagate and cultivate at the full capacity of the greenhouse; and (2) GMPGood Manufacturing Practices (“GMP”) and Good Distribution Practices (“GDP”) certificates and permits to produce and distribute dried flower. During the third quarter of 2020, Cronos Israel also received GMP and GDP certificates and permits to produce and distributeflower, cannabis oils and pre-rolls.
Licenses and Regulatory Framework in Other Jurisdictions
We and our joint venture partners, strategic investments and strategic investmentspartners are subject to comprehensive and evolving regulations in each jurisdiction in which we and they operate. All aspects of the production, manufacture and distribution of cannabis products are
regulated and subject to licensing regimes. These regulations and licensing regimes vary by jurisdiction and we, our joint venture partners, strategic investments and strategic investmentspartners spend significant time, effort and money to comply with the applicable requirements. We seek to comply with export laws in connection with distributing our products in other jurisdictions by obtaining export permits from Health Canada. Our strategic partners are responsible for compliance with import laws and local regulatory requirements (including laws related to distribution of medical products) in jurisdictions in which they operate.
Available Information
We are subject to the informational requirements of the United States Securities Exchange Act of 1934, as amended (the “Exchange Act”), and, in accordance with the Exchange Act, we also file reports with and furnish other information to the SEC. The public may obtain any document that we file with or furnish to the SEC from the SEC’s Electronic Document Gathering, Analysis, and Retrieval system, which can be accessed at www.sec.gov, or via the System for Electronic Document Analysis and Retrieval Plus, which can be accessed at www.sedar.comwww.sedarplus.com, as well as from commercial document retrieval services.
Copies of this Annual Report may be obtained on request without charge from our Corporate Secretary, corporate.secretary@thecronosgroup.com, telephone: +1-416-504-0004. We also provide access without charge to all of our SEC filings, including copies of this Annual Report, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act, as well as Section 16 reports on Forms 3, 4 or 5, as soon as reasonably practicable after filing or furnishing, on our website located at https://thecronosgroup.com.
Business Conduct and Ethics. The Code of Business Conduct and Ethics is also available in print to any shareholder upon request without charge from our Corporate Secretary, corporate.secretary@thecronosgroup.com, telephone: +1-416-504-0004. Within the time period required by the SEC, we will post on our website any amendment to the Code of Business Conduct and Ethics and any waiver applicable to any executive officer, director or senior financial officer. From time to time, we use our website, as well as the following social media sites, as an additional means of disclosing public information to investors, the media and others interested in the Company.
•Facebook (https:(https://www.facebook.com/The-Cronos-Group-419168411987225)The-Cronos-Group-419168411987225);
•Twitter (https:X (f.k.a. Twitter) (https://twitter.com/cronosgroup)cronosgroup); and
•LinkedIn (https:(https://www.linkedin.com/company/cronosgroupcron/?viewAsMember=true)).
It is possible that certain information we post on our website or these social media sites could be deemed to be material information, and we encourage investors, the media and others interested in the Company to review the business and financial information we or our officers post on our website or these social media sites. None of the information on our website or disclosed through these social media sites is incorporated by reference into this Annual Report.
ITEM 1A. RISK FACTORS
An investment in us involves a number of risks. In addition to the other information contained in this Annual Report and in other filings we make, investors should give careful consideration to the following risk factors. Any of the matters highlighted in these risk factors could adversely affect our business, results of operations and financial condition, causing an investor to lose all, or part of, its, his or her investment. The risks and uncertainties described below are those we currently believe to be material, but they are not the only ones we face. If any of the following risks, or any other risks and uncertainties that we have not yet identified or that we currently consider not to be material, actually occur or become material risks, our business, prospects, financial condition, results of operations and cash flows and consequently the price of our securities could be materially and adversely affected.
Risk Factor Summary
•WeCertain of our subsidiaries and joint ventures have a limited operating historyhistories and our growth strategy may not be successful.
•We may not be able to achieve or maintain profitability and may continue to incur losses in the future.
•Our products are new; there is limited long-term data with respect to the effects and the safety of our products, which is subject to conflicting medical data; and our products have been and may be in the future subject to recalls.
•The production and distribution of our products is subject to disruption, the risks of an agricultural business and the risk third partythird-party suppliers and distributors may not perform their obligations to us.
•Intellectual property is key to our growth strategy, and we may be unable to obtain or enforce our intellectual property rights.
•Our entry into new markets is subject to risks normally associated with the conduct of business in foreign countries.
•We are subject to extensive regulation and licensing and may not successfully comply with all applicable laws and regulations.
•Our business has been and may be adversely affected by the COVID-19 pandemic.
•Our businesses face highly competitive conditions.
•Altria has significant influence over us and may acquire over 50% of our common shares.us.
•The price of our common shares has been and may continue to be highly volatile.
•We have had two restatements and seven material weaknesses in our internal control over financial reporting over the last five years.
•We are subject to other risks generally applicable to our industry and the conduct of our businesses.
Risks Relating to Our Growth Strategy.
We and certain of our subsidiaries have a limited operating history and therefore we are subject to many of the risks common to early-stage enterprises.
We began carrying on business in 2013; Peace Naturals began operations in 20122013 and generated itsour first revenues in 2013; OGBC began operations in 2014 and generated revenue in 2017 (inter-company bulk transfer); Redwood began operations in 2017.2013. In addition, many of our joint ventures are not yet operationalin the early stages of their operations and may not become operational for some time, if at all.have generated little or no revenue. We are therefore subject to many of the risks common to early-stage enterprises, including limitations with respect to personnel, financial, and other resources and lack of revenues.
We may not be able to achieve or maintain profitability and may continue to incur losses in the future.
We have incurred significant losses in recent periods. We hadperiods and have negative operating cash flow for the last five fiscal years ending December 31, 2020, December 31, 2019, December 31, 2018, December 31, 2017, December 31, 2016, December 31, 2015 and December 31, 2014.years. We may not be able to achieve or maintain profitability and may continue to incur significant losses in the future.future even in light of our Realignment, the pending sale-leaseback transaction and change in the nature of operations at the Peace Naturals Campus, the exit of our U.S. operations and the wind-down and exit of our operations at Cronos Fermentation. In addition, we expect to continue to increaseincur significant operating expenses as we implement initiatives to continue to grow our business. If our revenues do not increase to offset these expected increases in costs and operating expenses, we will not be profitable. If our revenue declines or fails to grow at a rate faster than our operating expenses, and we are unable to secure funding under terms that are favorable or acceptable to us, or at all, we will not be able to achieve and maintain profitability in future periods. As a result, we may continue to generate losses. We may not achieve profitability in the future and, even if we do become profitable, we might not be able to sustain that profitability.
We may not be able to successfully manage our growth.
We are currently in an early development stage and may be subject to growth-related risks, including capacity constraints and pressure on our internal systems and controls, which may place significant strain on our operational and managerial resources. While our revenue has generally grown in recent years, our ability to manage and sustain revenue growth will depend on a number of factors, many of which are beyond our control, including, but not limited to, changes in laws and regulations respecting the production of U.S. hemp and cannabis products, competition from other license holders, the size of the illegal market and the adult-use market in Canada, and our ability to produce sufficient volumes of our products to meet clientcustomer demand. In addition, we are subject to a variety of business risks generally associated with developing companies. Our ability to manage growth effectively will require us to continue to implement and improve our operational and financial systems and to expand, train and manage our employee base. There can be no assurances that we will be able to manage growth successfully. Any inability to manage growth successfully could have a material adverse effect on our business, financial condition and results of operations.
Our use of joint ventures may expose us to risks associated with jointly owned investments.
We currently operate parts of our business through joint ventures with other companies, and we may enter into additional joint ventures and strategic alliances in the future. Joint venture investments may involve risks not otherwise present for investments made solely by us, including: (i) we may not control the joint ventures, either by virtue of our economic or legal ownership share, or our ability to influence day-to-day operational decision-making; (ii) our joint venture partners may not agree to distributions that we believe are appropriate; (iii) where we do not have substantial decision-making authority, we may experience impasses or disputes with our joint venture partners on certain decisions, which could require us to expend additional resources to resolve such impasses or disputes, including litigation or arbitration; (iv) our joint venture partners may become insolvent or bankrupt, fail to fund their share of required capital contributions or fail to fulfill their obligations as a joint venture partner; (v) the arrangements governing our joint ventures may contain certain conditions or milestone events that may never be satisfied or achieved; (vi) our joint venture partners may have business or economic interests that are inconsistent with ours and may take actions contrary to our interests; (vii) we may suffer losses as a result of actions taken by our joint venture partners with respect to our joint venture investments; (viii) it may be difficult for us to exit a joint venture if an impasse arises or if we desire to sell our interest for any reason; (ix) our joint venture partners may exercise termination rights under the relevant agreements and (x) conflicts of interest may arise between our joint ventures and Company personnel who are directors of our joint ventures because of the fact that such directors are employed by us. In addition, we may, in certain circumstances, be liable for the actions of our joint ventures or joint venture partners. Any of the foregoing risks could have a material adverse effect on our business, financial condition and results of operations, and the magnitude of these material adverse effects could be greater to the extent we decide to rely on such joint ventures for certain goods or services, such as the receipt of raw materials from Cronos GrowCo, or decide to outsource certain operating activities to such joint ventures.
There can be no assurance of continued growth in Israel and our performance in Israel depends on, among other things, our ability to continue to import cannabis into Israel and our joint venture partners.
While our revenue in Israel has experienced periods of significant growth, our prior performance is not indicative of any potential future results in Israel. The Israel-Hamas War has created significant uncertainty with respect to our operations in Israel and may materially and adversely affect our sales and other activities in Israel. There can be no assurance that our growth in the Israeli market can be sustained or will continue. Our ability to manage and sustain revenue growth in Israel will depend on a number of factors, many of which are beyond our control, including, but not limited to, the impact of, and developments in, the Israel-Hamas War on our operations, our ability to continue to import cannabis into Israel (including the outcome of the anti-dumping investigation initiated by the Israel Ministry of Economy and Industry, see Part II, Note 10 “Commitments and Contingencies” to the consolidated financial statements in Item 8 of this Annual Report for further details), changes in laws and regulations respecting the cultivation, production, marketing and sale of dried flower, pre-rolls and oils in Israel, growth of the medical cannabis patient count in Israel, increased competition, our ability to produce sufficient volumes of our products to meet customer demand and our ability to maintain or grow our market share in Israel. Any of these factors could materially and negatively impact our growth in Israel.
We have identifiedbegun to further leverage our strategic joint venture with Cronos GrowCo. Our winddown of the cultivation and certain production activities at year-end 2020the Peace Naturals Campus as well as the previously-announced sale-leaseback of the Peace Naturals Campus and the Company’s intention to pursue a sale of Cronos Fermentation have increased the importance of Cronos GrowCo to our business and operations. Cronos GrowCo’s production facilities are our principal source of raw materials. Therefore, our performance in Israel is reliant on our ability to acquire such raw materials on a timely and cost-effective basis from Cronos GrowCo and to continue to import such raw materials and cannabis products to Israel from Cronos GrowCo’s production facilities. There is no guarantee that we will be able to successfully execute our strategy to expand production at Cronos GrowCo or that we will be able to obtain the regulatory approvals, licenses and permits required for both the export of cannabis from Canada and the import of cannabis into Israel. Further, there can be no assurance that the anti-dumping investigation initiated by the Israel Ministry of Economy and Industry will not result in the imposition of an anti-dumping duty on us or limit our imports into Israel, the impact of which could have a material weaknessadverse effect on our business in Israel.
Our acquisition strategy may not be successful, and we have in the past, and may in the future, need to write down the goodwill and indefinite-lived intangible assets recognized upon the acquisitions.
In the second quarter of 2021, we wrote off all of the goodwill and substantially all of the indefinite-lived intangible assets recognized upon the acquisition of Redwood and in the second quarter of 2023, we announced plans to cease our U.S. hemp operations. Acquisitions of companies or equity interests of companies operating in new markets are risky and speculative and may not produce the anticipated revenues and profits.
Our acquisition of the PharmaCann Option (the “PharmaCann Investment”) presents significant risks. See “Risk Factors—Risks Relating to Our Growth Strategy—Our U.S. strategy in part depends on the success of the PharmaCann Investment and there is no guarantee that we will exercise the PharmaCann Option in the near term, or at all, and, even if exercised, that the PharmaCann Investment will achieve the expected benefits of the transaction.”
We have had two restatements and seven material weaknesses in our internal control over financial reporting.reporting over the last five years. We had a material weakness in our control environment, and in 2021 and 2022, we experienced significant turnover, both voluntary and involuntary, in our accounting and financial reporting functions, as well as in our internal audit function. If we are unable to remediate this material weakness, or if we experience additional material weaknesses in the future,create and maintain an appropriate control environment, our business, mayresults of operations, financial condition, cash flows and reputation will be harmed.adversely affected.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rule 13a-15(f) under the Exchange Act) and for evaluating and reporting on the effectiveness of our system of internal control. Effective internal controls arecontrol is necessary for us to provide timely, reliable and accurate financial reports, identify and proactively correct any deficiencies, material weaknesses or fraud and meet our reporting obligations. As disclosedWe had two restatements and seven material weaknesses in Part II, Item 9A,the last five years and have had significant turnover, both voluntary and involuntary, in our accounting and financial reporting functions as well as in our internal audit function. Moreover, we identifiedhad a material weakness in the fourth quarterour control environment existing as of 2020.December 31, 2022. Remediation efforts have placed, and will continue to place, a significant burden on management and add increased pressure on our financial reporting resources and processes. If we are unable to successfully remediate this material weakness in a timely manner, or if any additional material weaknesses in our internal control over financial reporting are identified, theThe accuracy of our financial reporting and our ability to timely file with the SEC and the applicable securities regulatory authorities in Canada have in the past been, and may in the future be, adversely impacted.impacted if any additional material weaknesses in our internal control over financial reporting are identified. In addition, if our remedial efforts are insufficient, or if additional material weaknesses or significant deficiencies in our internal controlscontrol occur in the future, we could be required to restate our financial statements again, which could materially and adversely affect our business, results of operations and financial condition, restrict our ability to access the capital markets, require us to expend significant resources to correct the material weaknesses, or deficiencies, subject us to regulatory investigations and penalties, harm our reputation, cause a decline in investor confidence or otherwise cause a decline in our stock price.
We are subject to civil litigation relating to the restatements and we cannot predict the outcome of this litigation, but we have incurred and expect to continue to incur significant costs and expenses in defending against this civil litigation. For more information on this
civil litigation and proceedings, see Part II, Note 10(b) “Contingencies” to the consolidated financial statements under Item 8 of this Annual Report.
We are subject to disabilities as a result of our settlement with the SEC that may expose us to increased future litigation and adversely affect our ability to raise capital.
As of the date of our settlement with the SEC (the “SEC Order”), October 24, 2022, and for a period of three years thereafter, we are unable to rely on the safe harbor provisions regarding forward-looking statements provided by the Securities Act and the Exchange Act. Our inability to rely on these safe harbor provisions may expose us to increased future litigation in connection with forward-looking statements in our public disclosures.
Further, as of the date of the SEC Order, we have lost our status as a “well-known seasoned issuer” for a period of three years, which places limitations on the manner in which we can market our securities to the public, and we are unable to rely on the private offering exemptions provided by Regulations A and D under the Securities Act for a period of five years, which could impair our ability to raise additional capital in the private market quickly in response to changing requirements and market conditions.
There can be no assurance that our current and future strategic alliances or expansions of scope of existing relationships will have a beneficial impact on our business, financial condition and results of operations.
We currently have, and may in the future enter into additional, strategic alliances with third parties that we believe will complement or augment our existing business. Our ability to complete strategic alliances is dependent upon, and may be limited by, the availability of suitable candidates and capital. In addition, strategic alliances could present unforeseen integration or operational obstacles or costs, may not enhance our business and may involve risks that could adversely affect us, including significant amounts of management time that may be diverted from operations in order to pursue and complete such transactions or maintain such strategic alliances. Future strategic alliances could result in the incurrence of debt, costs and contingent liabilities, and there can be no assurance that future strategic alliances will achieve, or that our existing strategic alliances will achieve, the expected benefits to our business or that we will be able to consummate future strategic alliances on satisfactory terms, or at all. Any of the foregoing could have a material adverse effect on our business, financial condition and results of operations.
In the case of the Ginkgo Strategic Partnership, we have and will continue to obtain, pursuant to the Ginkgo Collaboration Agreement, the exclusive right to use and commercialize the key patented intellectual property related to the production of the target cannabinoids globally (referred to herein as the “Ginkgo exclusive licenses”). There can be no assurance that Ginkgo will be able to develop microorganisms that we will be able to commercialize or to obtain patents relating to production of the target cannabinoids, or that third parties will not develop similar microorganisms or obtain patents that may restrict our ability to commercialize the microorganisms developed by Ginkgo, and, as a result, there can be no assurance that we will be able to realize the expected benefits of the Ginkgo Strategic Partnership. Additionally, we have determined, and may determine in the future, that the production of certain cannabinoids is not economically feasible and in our best interests and we have abandoned, and may abandon in the future, the production efforts of Ginkgo with respect to certain target cannabinoids. Even if we are able to commercialize cultured cannabinoids, we may not be able to generate satisfactory returns on them or on the products that incorporate them, and there may not be demand for such cultured cannabinoid products.
In addition, pursuant to the Ginkgo Collaboration Agreement, if we undergo a change of control that is approved by the Board, Ginkgo may elect to receive cash payments, which, given the number of Equity Milestone Events (as defined in the Ginkgo Collaboration Agreement) that have occurred to date, could total up to $15.8 million, in lieu of the common shares that would otherwise become issuable in connection with any Equity Milestone Events achieved following such election (the “Milestone Cash Election”). If we undergo a change in control that has not been approved by the Board, then Ginkgo will have the ability to terminate the Ginkgo Collaboration Agreement immediately, in which case, among other things: (i) all rights or licenses granted to us by Ginkgo under the Ginkgo Collaboration Agreement will terminate; (ii) certain expenses and costs incurred by Ginkgo will be accelerated and become due and payable by us; (iii) the then-outstanding and unpaid portion of all cash payments from us to Ginkgo for the achievement of R&D milestones by Ginkgo shall be due immediately as if all R&D milestones had been achieved; and (iv) a lump sum cash payment equal to the aggregate of all Milestone Cash Election amounts in respect of which the relevant Equity Milestone Events have not yet been achieved will be immediately due and payable by us. In addition, should Ginkgo terminate the Ginkgo Collaboration Agreement upon a change of control, we will no longer be able to use or commercialize the key patented intellectual property related to the production of the target cannabinoids, which could have a material adverse effect on our business, financial condition and results of operations. See“Description of Business—Research and Development Activities and Intellectual Property.”
As additional equity milestones occur under the Ginkgo Collaboration Agreement, we are required by accounting rules to conduct an impairment analysis related to the new Ginkgo exclusive licenses. These analyses have resulted in impairment charges in the past and may do so in the future as additional equity milestones are achieved. For a discussion of our most recent impairments of the Ginkgo exclusive licenses, see Note 7 “Goodwill and Intangible Assets, net” to the consolidated financial statements in Item 8 of this Annual Report.
We may not successfully execute our production capacity expansion strategy.
We may not be successful in executing our strategy to expand production capacity at certain of our facilities and joint ventures.ventures and wind down of cultivation and certain production activities at the Peace Naturals Campus. Continuing and expanding operations at the productionour facilities of Cronos Israel and Natuerajoint ventures will be subject to obtaining and maintaining the appropriate licenses from the relevant regulatory agencies in those jurisdictions. In addition,particular, continuing and expanding operations at Cronos GrowCo’s production facilities will be subject to obtaining and maintaining the appropriate licenses from Health Canada. Construction delays or cost over-runs in respect of such operations, howsoever caused, could have a material adverse effect on our business, financial condition and results of operations. Moreover, with the pending sale-leaseback transaction and change in the nature of operations at the Peace Naturals Campus, the continued operations of the Cronos GrowCo production facilities will be more important to us. Additionally, we must obtain approval from Health Canada for changes to our site perimeter on the Peace Naturals Campus prior to closing the sale-leaseback transaction and there can be no assurance as to whether or when we might obtain such approval. Cronos GrowCo’s production facilities are our principal source of raw materials.
In addition, we may not be successful in obtaining the necessary approvals required to export or import our products to or from the jurisdictions in which we or our joint ventures operate. If we are unable to secure necessary production licenses in respect of our facilities and those of our joint ventures, the expectations of management with respect to the increased future cultivation and growing capacity may not be borne out, which could have a material adverse effect on our business, financial condition and results of operations.
We may not be able to successfully procure rare cannabinoids at commercially viable prices or in the quantities that we require.
As a result of our decision to wind down Cronos Fermentation, we no longer have the internal capacity to produce rare cannabinoids through the fermentation process developed with Ginkgo.To the extent we continue to utilize rare cannabinoids in our products, we may be required to engage third-party suppliers to obtain rights to new extraction methods or may be required to purchase rare cannabinoids in the open market.We may not be able to find third-party suppliers capable of producing rare cannabinoids at commercially viable prices or in the quantities we require.If we are unable to secure the necessary rare cannabinoids, we may experience product shortages and delays and we may be unable to launch new products, which could have a material adverse effect on our business, financial condition and results of operations.
There can be no assurance that the Realignment, the pending sale-leaseback and the change in the nature of operations at the Peace Naturals Campus, the exit of our U.S. operations and the wind-down and exit of our Cronos Fermentation facility will have a beneficial impact on our business, financial condition and results of operations. The timing, costs and benefits thereof cannot be guaranteed.
In the first quarter of 2022, we announced our Realignment to centralize functions under common leadership to increase efficient distribution of resources, improve strategic alignment and eliminate duplication of roles and costs; evaluate our global supply chain and perform product reviews and pricing and distribution optimization in order to reduce fixed expenses and reduce complexity; and implement an operating expense target to optimize cash deployment for activities such as margin accretive innovation and U.S. adult-use cannabis market entry in the future. Additionally, we announced a plan to leverage our strategic partnerships to improve supply chain efficiencies and reduce manufacturing overhead by partially exiting the Peace Naturals Campus. We subsequently decided to retain certain distribution, warehousing, R&D and manufacturing operations at the Peace Naturals Campus.
In the second quarter of 2023, we announced the exit of our U.S. operations. In the third quarter of 2023, we announced the wind-down and exit of our Cronos Fermentation facility.
There can be no assurance that these initiatives will achieve the expected benefits to our business or reduce costs or grow our revenue as intended and, if achieved at all, the timing thereof. The execution and implementation of these initiatives involve risk, including that significant amounts of management’s time and Company resources could be diverted from our core operations in order to complete such initiatives. Some risks, such as obtaining approval from Health Canada for changes to our site perimeter on the Peace Naturals Campus, are outside of our control. In addition, these initiatives could present unforeseen obstacles, lead to operating inefficiencies and negatively disrupt our corporate culture, which could lead to further employee attrition, any of which would have a material adverse effect on our business, financial condition and results of operations. We have and will continue to incur costs to implement these initiatives, and we could be subject to litigation risks and expenses. Our projected costs and expenses associated with the changes in operations described above may turn out to be too low by a material amount.
There can be no assurance that the regulatory approvals will be obtained or that the other closing conditions for the sale and leaseback of the Peace Naturals Campus will be satisfied or waived in a timely manner or at all.
Pursuant to the Sale Agreement, Future Farmco has agreed to acquire the Peace Naturals Campus subject to certain conditions. These conditions include, among other things, Future Farmco and the Company agreeing on the form of a lease, confirmation from Future Farmco that it has secured financing for the transaction and the Company receiving approval from Health Canada for site perimeter changes, each as set forth in the Sale Agreement. There can be no assurance that such closing conditions will be satisfied or waived or that the Company will obtain approval from Health Canada on commercially reasonable terms, in a timely manner, or at all, or that the
sale and leaseback of the Peace Naturals Campus will be completed. Additionally, there can be no assurance that the sale and leaseback of the Peace Naturals Campus will occur on the terms and conditions described herein or previously announced.
We may not be able to realize the expected cost-savings and other benefits related to the wind-down of operations at the Cronos Fermentation facility.
In the third quarter of 2023, we announced the decision to wind down operations at the Cronos Fermentation facility, list the Cronos Fermentation facility for sale, and implement additional organization-wide cost reductions as we continue our Realignment initiatives. There can be no assurance that we will be able to sell the Cronos Fermentation facility within an acceptable time frame and for an acceptable price or that these initiatives will achieve the expected benefits to our business within our expected time frame. The execution and implementation of these initiatives involve risk, including that significant amounts of management’s time and Company resources could be diverted from our core operations in order to complete such initiatives. In addition, these initiatives could present unforeseen obstacles, lead to operating inefficiencies and negatively disrupt our corporate culture, which could lead to further employee attrition, any of which would have a material adverse effect on our business, financial condition and results of operations. We have and will continue to incur costs to implement these initiatives, and we could be subject to litigation.
The industries and markets in which we operate are relatively new, and these industries and markets may not continue to exist or grow as anticipated or we may ultimately be unable to succeed in these industries and markets.
The cannabismedical and U.S. hempadult-use cannabis industries and markets in which we operate are relatively new, can beare highly speculative, are rapidly expanding and may ultimately not be successful. In addition to being subject to general business risks, we need to continue to build brand awareness in these industries and markets through significant investments in our strategy, our production capacity, quality assurance and compliance with regulations. These activities may not promote our brand and products as effectively as intended, or at all. Competitive conditions and consumer tastes, or client requirements, as applicable, and spending patterns in these new industries and markets are relatively unknown and may have unique circumstances that differ from existing industries and markets. We are subject to all of the business risks associated with a new business in a niche market, including risks of unforeseen capital requirements, failure of widespread market acceptance of our products, failure to establish business relationships and competitive disadvantages against larger and more established competitors.
Accordingly, there are no assurances that these industries and markets will continue to exist or grow as currently estimated or anticipated, or function and evolve in a manner consistent with management’s expectations and assumptions, and a failure to do so could have a material adverse effect on our business, financial condition and results of operations.
We may not be able to supply the provincial purchasers in various provinces and territories of Canada with our products in the quantities or prices anticipated, or at all.
We have entered into various supply arrangements for cannabis products with various provincial and territorial purchasers and have secured listings with various private retailers in thosecertain provinces. We have entered into such supply arrangements with eightall provinces in Canada (where the relevant provincial body is the sole wholesale distributor and retailer of cannabis and cannabis products in the province) and the Yukon Territory and with private retailers in Saskatchewan. Our supply arrangements with provincial and territorial purchasers each of which we understand to be substantially similar in all material respects with the supply arrangements entered into with the other license holders in the Canadian cannabis industry, do not contain any binding minimum purchase obligations on the part of the relevant provincial or territorial purchaser.
We expect purchase orders to be primarily driven by end-consumer demand for our products and the relevant provincial, territorial or private purchaser supply at the relevant time. Accordingly, we cannot predict the quantities of our products that will be purchased by the provincial, territorial and private purchasers, or if our products will be purchased at all. Provincial and territorial purchasers may change the terms of the supply agreements at any time during the supply relationship including pricing, have broad rights of return of products and are under no obligation to purchase our products or maintain any listings of our products for sale. As a result, provincial and territorial purchasers have a significant amount of control over the terms of the supply arrangements.
The effect of the legalization of adult-use cannabis in Canada on the medical cannabis market in Canada is still uncertain, and it may have a significant negative effect upon our medical cannabis business if our existing or future medical-use clients decide to purchase products available in the adult-use market instead of purchasing medical-use products from us.
The Cannabis Act allows individuals over the age of 18 to legally purchase, process and cultivate limited amounts of cannabis for adult-use in Canada, subject to Furthermore, provincial and territorial age restrictions whichpurchasers may increase the age of purchase in the provincealso decide to ban, limit or territory. As a result, individuals who rely upon the medical cannabis market to supply their medical cannabis and cannabis-based products may cease this reliance, and instead turn to the adult-use cannabis market to supply their cannabis and cannabis-based products. Factors that will influence this decision include the price of medical cannabis products in relation to similar adult-use cannabis products, the amount of active ingredients in medical cannabis products in relation to similar adult-use cannabis products,implement new guidance on the types of cannabis products availablepermitted for sale in each of their jurisdictions (including in response to adult users and limitationsHealth Canada’s guidance on access to adult-use cannabisintoxicating cannabinoids) which may result in some or all of our products imposed by the regulations under the Cannabis Act and the legislation governing the distribution and sale of cannabis that has been enacted by the individual provinces and territories of Canada.
The impact of the legalization of adult-use cannabis in Canada on the medical cannabis market is uncertain, and while we cannot predict its impact on our sales and revenue prospects, it may be adverse.being viewed as non-compliant with law or non-binding policy guidance.
The adult-use cannabis market in Canada has in the past been and may in the future become oversupplied following the recent implementation of the Cannabis Act and the related legalization of cannabis for adult-use.oversupplied.
As a result of the recent implementation of the Cannabis Act and the legalization of adult cannabis use, numerousNumerous additional cannabis producers have and may continue to enter the Canadian adult-use market. We and such other cannabis producers have in the past produced and may in the future produce more cannabis than is needed to satisfy the collective demand of the Canadian medical and adult-use markets, and we may be unable to export that over-supply into other markets. As a result, the available supply of cannabis could exceed demand, which couldhas in the past, and may in the future, result in a significant declineinventory write downs and decreases in the market price for cannabis, which could have a material adverse effect on our business, financial condition and results of operations.prices.
We may be unsuccessful in competing in the legal adult-use cannabis market in Canada.
We face competition from existing license holders licensed under the Cannabis Act. Certain of these competitors may have significantly greater financial, production, marketing, R&D and technical and human resources than we do. As a result, our competitors may be more successful than us in gaining market penetration and market share in the adult-use cannabis industry in Canada. Our commercial opportunity in the adult-use market could be reduced or eliminated if our competitors produce and commercialize products for the adult-use market that, among other things, are safer, more effective, more convenient or less expensive than the products that we may produce, have greater sales, marketing and distribution support than our products, enjoy enhanced timing of market introduction and perceived effectiveness advantages over our products and receive more favorable publicity than our products. If our adult-use products do not achieve an adequate level of acceptance by the adult-use market, we may not generate sufficient revenue from these products, and our adult-use business may not become profitable.
The Cannabis Act allows individuals over the age of 18 to cultivate, propagate, harvest and distribute up to four cannabis plants per household provided that each plant meets certain requirements, although various restrictions on these activities exist in certain provinces and territories. If we are unable to effectively compete with other license holders in the adult-use cannabis market, or a significant number of individuals take advantage of the ability to cultivate and use their own cannabis, our adult-use business may be negatively impacted.
We are subject to liability arising from any fraudulent or illegal activity by our employees, contractors, manufacturers and consultants.
We are exposed to the risk that our employees, independent contractors, manufacturers and consultants may engage in fraudulent or other illegal activity. Misconduct by these parties could include intentional, reckless and/or negligent conduct or disclosure ofengaging in unauthorized activities to us that violates:violate: (i) applicable laws and regulations; (ii) manufacturing standards; (iii) federal and provincial healthcare fraud and abuse of federal, state and provincial laws and regulations; or (iv) laws and regulations that require the true, complete and accurate reporting of financial information or data. It is not always possible for us to identify and deter misconduct by our employees and other third parties, and the precautions taken by us to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliancecomply with such laws or regulations. If any such actions are brought against us, and we are not successful in defending the Company or asserting our rights,them, those actions could have a significant impact on our business, including the imposition of civil, criminal and administrative penalties, damages, monetary fines, contractual damages, reputational harm, diminished profits and future earnings, loss or suspension of licenses and the curtailment of our operations, any of which could have a material adverse effect on our business, financial condition and results of operations.
Some jurisdictions may never develop markets for cannabis and U.S. hemp.
Many jurisdictions place restrictions on or prohibit commercial activities involving cannabis and U.S. hemp. Such restrictions or prohibitions may make it impossible or impractical for us to enter or expand our operations in such jurisdictions unless there is a change in law or regulation. For example, U.S. Schedule I cannabis remains illegal under U.S. federal law and may never become legal under U.S. federal law.
Our U.S. strategy in part depends on the success of the PharmaCann Investment and there is no guarantee that we will exercise the PharmaCann Option in the near term, or at all, and, even if exercised, that the PharmaCann Investment will achieve the expected benefits of the transaction.
Our ability to exercise the PharmaCann Option will depend on the satisfaction of several conditions, including U.S. federal cannabis legalization. In addition, our ability to exercise the PharmaCann Option is subject to the receipt of any required regulatory approvals, including in the states where PharmaCann operates that may be required upon exercise, as well as Altria’s approval under the Investor Rights Agreement. These conditions are outside of our control and therefore there can be no certainty that the PharmaCann Option will be exercised in the near term, or at all. If the PharmaCann Option is not exercised, we will not receive the benefits of the contemplated commercial arrangements between us and PharmaCann.
In addition, the regulatory approval processes in connection with the exercise of the PharmaCann Option may take a prolonged period of time to complete, which could significantly delay our ability to exercise the PharmaCann Option and realize the benefits of the PharmaCann Investment, or result in our not being able to exercise all or part of the PharmaCann Option. Furthermore, in connection with obtaining approvals from or otherwise satisfying the requests of the state regulators or applicable laws, we may be required to divest all or a portion of the PharmaCann Option, or if after the exercise of the PharmaCann Option, our shares of PharmaCann.
Even if we are able to and do exercise the PharmaCann Option, the intended benefits of the PharmaCann Investment may not be realized. We cannot assure you that the PharmaCann Investment will be accretive to us in the near term or at all. For example, if entered into, the commercial arrangements between us and PharmaCann may not be successful or beneficial to us. Furthermore, if we fail to realize the intended benefits of the PharmaCann Investment, our stock price could decline to the extent that the market price anticipates those benefits.
We are entitled to certain limited governance rights with respect to PharmaCann, including limited information rights and board observer rights. Therefore, we will have little to no ability to influence the strategy and material decisions of PharmaCann’s business. Furthermore, until such time as we exercise the PharmaCann Option, we will not have the ability to vote on matters requiring the vote of PharmaCann’s shareholders. In addition, we are subject to certain standstill restrictions, both prior to and after the exercise of the PharmaCann Option, which restrictions further limit our ability to influence decisions of PharmaCann.
Although we are entitled to certain anti-dilution protections with respect to our investment in PharmaCann, such protections are subject to various conditions, and our potential ownership in PharmaCann may be significantly diluted by, among other things, future issuances of PharmaCann securities or acquisition activity in which PharmaCann uses its equity as consideration. As of December 31, 2023, the Company’s ownership percentage in PharmaCann on a fully diluted basis was approximately 5.9%. Under the terms of our investment in PharmaCann, Cronos’ rights to nominate an observer to the PharmaCann board of directors could be lost if our ownership drops below 6% on a fully diluted basis and we sell or transfer all or any portion of the PharmaCann Option (subject to certain exceptions).
We must rely largely on our own market research to forecast sales and market demand and market prices which may differ from our forecasts.
We must rely largely on our own market research and internal data to forecast sales as detailed market researchdata is not generally obtainable from other sources at this early stage of the cannabis or U.S. hemp industries. Our market research andindustry. If our sales forecasts together with factors such asand our expectations regarding market conditions, including prices, influence capital expenditure levels, inventory levels, production and supply chain capacity and operating expenses, and if such forecasts and expectations prove to be inaccurate, this could have a material adverse effect on our business, financial condition and results of operations. For example, our forecastforecasts for product demand and market conditions waswere impacted by a decline in market prices for cannabis products in the Canadian market, which contributed to our inventory write-down in the second and fourth quarters of 2020.
We could have difficulty transitioningintegrating the operations of businesses that we have acquired and will acquire.
The success of our acquisitions including the Redwood Acquisition and the acquisition of Cronos Fermentation, depends upon our ability to transitionintegrate any businesses that we acquire. The transitioningintegration of acquired business operations could disrupt our business by causing unforeseen operating difficulties, diverting management’s attention from day-to-day operations and requiring significant financial resources that would otherwise be used for the ongoing development of our business. The difficulties of transitionsintegrations could be increased by the necessity of coordinating geographically dispersed organizations, coordinating personnel with disparate business backgrounds, and managing different corporate cultures, or discovering previously unknown liabilities. In addition, we could be unable to retain key employees or customers of the acquired businesses. We could face transitionintegration issues including those related to operations, internal controls,control and information systems and operational functions of the acquired companies and we also could fail to realize cost efficiencies or synergies that we anticipated when selecting our acquisition candidates or these acquisitions could fail to completecompete successfully. Any of these items could adversely affect our business, financial condition and results of operations. For more information on the risks associated with acquisitions, see “Risk Factors—Risks Relating to Our Growth Strategy—Our acquisition strategy may not be successful and we have in the past, and may in the future, need to write down the goodwill and indefinite-lived intangible assets recognized upon the acquisitions.”
We have been and may in the future be required to write down intangible assets, including goodwill, due to impairment, which could have a material adverse effect on our results of operations or financial position.
The Company has been and may in the future be required to write down intangible assets, including goodwill, due to impairment, which would reduce earnings. Indefinite-lived intangible assets are reviewed annually or more frequently when events or changes in circumstances indicate that the fair value of the indefinite-lived intangible assets have been reduced to less than their carrying amount. We periodically calculate the fair value of our reporting units and intangible assets to test for impairment. This calculation may be affected by several factors, including general economic conditions, regulatory developments, changes in category growth rates as a result of changing adult consumer preferences, success of planned new product introductions, and competitive activity. Certain events can also trigger an immediate review of goodwill and intangible assets. If the carrying amount of our reporting unit and other intangible assets exceed their fair value, the goodwill and other intangible assets are considered impaired, which would result in impairment losses and could have a material adverse effect on our consolidated financial position or results of operations.
For a discussion of previous write downs of indefinite-lived intangible assets and goodwill, see Note 7 “Goodwill and Intangible Assets, net” to the consolidated financial statements in Item 8 of this Annual Report.
Risks Relating to Operations in Israel
Conditions in Israel could materially and adversely affect our business, financial condition, and results of operations.
We have operations in Israel through a strategic joint venture, Cronos Israel.
On October 7, 2023, Hamas terrorists from the Gaza Strip launched a rocket barrage against Israel and engaged in incursions into Israeli territory, breaching the Gaza-Israel border, attacking military bases and slaughtering and kidnapping civilians in neighboring Israeli communities. Israel formally declared war on October 8. The Israel-Hamas War may further escalate, including due to an eruption of fighting between Hezbollah and Israel across Israel’s northern border, which would open a second front in the war, and may result in a broader conflict across the Middle East. The Israel-Hamas War (and any escalation) and any resulting regional political instability or interruption or curtailment of trade between Israel and its trading partners would likely materially and adversely affect our business, financial condition, and results of operations. On October 8, 2023, the Yakar issued guidelines for maintaining the business continuity in the field of medical cannabis, in light of the national effort relating to the Israel-Hamas War. Such guidelines include extensions of patient licenses and business licenses.
Our employees, including certain members of our management, operate from our offices located in Gan Shmuel, Israel and our manufacturing facilities located in Hadera, Israel. While our facilities have not been damaged by the war, rocket attacks continue, and our facilities could be damaged or destroyed. Imports into Israel have been severely affected by the war, and we may be unable to import materials into Israel. Further, our sales have been, and likely will continue to be, adversely affected by the war.
Our commercial insurance does not cover losses that may occur as a result of events associated with war and terrorism. Any losses or damage incurred by us could have an adverse effect on our business, financial condition, and results of operations.
Further, in the past, the State of Israel and Israeli companies have been subjected to economic boycotts. Several countries still restrict doing business with the State of Israel and with Israeli companies. A campaign of boycotts, divestment, and sanctions has been undertaken against Israel, which could also adversely impact our business, financial condition, and results of operations and the expansion of our business.
Our operations may be disrupted by the obligations of personnel to perform military service.
Some of our employees in Israel are obligated to perform annual reserve duty in the Israeli military for several days, and in some cases more, of annual military reserve duty each year until they reach the age of 40 (or older, for reservists who are military officers or who have certain occupations) and are subject to being called for additional active duty under emergency circumstances. In response to the Israel-Hamas War, a number of our employees have been called up to serve in the Israeli military. We cannot predict the full impact of these conditions on us in the future, particularly if emergency circumstances or an escalation in the political or military situation occurs. If many of our employees are called for active duty, our operations in Israel and our business may not be able to function at profitable levels, or at all, and our business in, results of operations from, Israel would be adversely affected.
Risks Relating to Our Products
There is limited long-term data with respect to the efficacy and side effects of our productscannabis, U.S. hemp and cannabinoids, and future clinical research studies on the effects of cannabis, U.S. hemp and cannabinoids may lead to conclusions that dispute or conflict with our understanding and belief regarding their benefits, viability, safety, efficacy, dosing and social acceptance.
Research in Canada, the U.S. and internationally regarding the benefits, viability, safety, efficacy, dosing and social acceptance of cannabis, U.S. hemp or isolated cannabinoids (such as CBD and THC) inhaled, in dietary supplements, food, or cosmetic products remains in early stages. There have been relatively few clinical trials on the potential benefits of cannabis, U.S. hemp or isolated cannabinoids in dietary supplements, food, or cosmetic products and there is limited long-term data with respect to efficacy, sidepotential benefits, effects and/or interaction of these substances with human or animal biochemistry. As a result, our products could have unexpected side effects or safety concerns, the discovery of which could lead to civil litigation, regulatory actions and even possibly criminal enforcement actions. In addition, if the products we sell do not or are not perceived to have the effects intended by the end user, this could have a material adverse effect on our business, financial condition and results of operations.
The statements made by the Company, including in this Annual Report, concerning the potential benefits of cannabis, U.S. hemp and isolated cannabinoids are based on published articles and reports and therefore are subject to the experimental parameters, qualifications and limitations in such studies that have been completed. Although we believe that the existing public scientific literature generally supports our beliefs regarding the benefits, viability, safety, efficacy, dosing and social acceptance of cannabis, U.S. hemp and cannabinoids in dietary supplements, food, or cosmetic products, future research and clinical trials may cast doubt or disprove such beliefs, or could raise or heighten concerns regarding, and perceptions relating to, cannabis, U.S. hemp and cannabinoids, which could have a material adverse effect on the demand for our products with the potential to lead to a material adverse effect on our business, financial condition and results of operations. Given these risks, uncertainties and assumptions, undue reliance should not be placed on such literature. In particular, the FDA has raised several questions regarding the safety of CBD and other cannabinoids, particularly in food and dietary supplements and gaps in the public scientific literature supporting the use of CBD and other cannabinoids by the general population.
Clinical trials of cannabis-based medical products and treatments are novel terrain with veryhave a limited or non-existent history, and any trials may not result in commercially viable products and treatments.
Clinical trials are expensive, time consuming and difficult to design and implement. Regulatory authorities may suspend, delay or terminate any clinical trials we commence at any time, may require us, for various reasons, to conduct additional clinical trials, or may require a particular clinical trial to continue for a longer duration than originally planned. Clinical trials face many risks, including, among others:
•lack of effectiveness of any formulation or delivery system during clinical trials;
•discovery of serious or unexpected toxicities or side effects experienced by trial participants or other safety issues;
•slower than expected subject recruitment and enrollment rates in clinical trials;
•delays or inability in manufacturing or in obtaining sufficient quantities of materials for use in clinical trials due to regulatory and manufacturing constraints;
•delays in obtaining regulatory authorization to commence a trial, including licenses required for obtaining and using cannabis, U.S. hemp or isolated cannabinoids for research, either before or after a trial is commenced;
•unfavorable results from ongoing pre-clinical studies and clinical trials;
•trial participants or investigators failing to comply with study protocols;
•trial participants failing to return for post-treatment follow-up at the expected rate;
•sites participating in an ongoing clinical study withdraw, requiring us to engage new sites; and
•third-party clinical investigators declining to participate in our clinical studies, not performing the clinical studies on the anticipated schedule, or acting in ways inconsistent with the established investigator agreement, clinical study protocol or good clinical practices.
Any of the foregoing could cause our products or treatments not to be commercially viable.viable, which could have a material adverse effect on our business, financial condition and results of operations.
The controversy surrounding vaporizers and vaporizer products may materially and adversely affect the market for vaporizer products and expose us to litigation and additional regulation.
There have been a number of highly publicized cases involving lung and other illnesses and deaths that appear to be related to vaporizer devices and/or products used in such devices (such as vaporizer liquids). The focus is currentlyhas been on the vaporizer devices, the manner in which the devices were used and the related vaporizer device products –THC,– THC, nicotine, other substances in vaporizer liquids, possibly adulterated products and other illegal unlicensed cannabis vaporizer products. Some states, provinces, territories and citiesmunicipalities in the U.S. and Canada have already taken steps to prohibit the sale or distribution of vaporizers, restrict the sale and distribution of such products or impose restrictions on flavors, substances and concentration of substances used, or use of such vaporizers. This trend may continue, accelerate and expand.
Cannabis vaporizers in Canada are regulated under the Cannabis Act, Cannabis Regulations and Cannabis Regulations.other laws and regulations of general application. Although this legislation sets rules and standards for the manufacture, composition, packaging, and marketing of cannabis vaporizer products, these rules and standards predate the spate of vaporizer-related health issues that have recently arisen in the U.S. These issues and accompanying negative public sentiment may prompt Health Canada or individual provinces/territories or municipalities to decide to further limit or defer the industry’s ability to sell cannabis vaporizer products and may also diminish consumer demand for such products. Currently, Québec Newfoundland and Labrador and Prince Edward Island do not allow the sale of cannabis vaporizers in their respective jurisdictions. jurisdictions and Health Canada is seeking to limit the flavors of inhaled cannabis extracts. In June 2021, Health Canada opened a consultation into the use of flavors in inhaled cannabis extracts as it claims that the availability of flavors is one of the factors that contributes to the increase in cannabis vaping in youth and young adults. As part of this consultation, Health Canada released proposed regulations that contemplate restricting the production, sale, promotion, packaging and labelling of inhaled cannabis extracts from having a flavor, other than the flavor of cannabis. The proposed amendments would apply equally to inhaled cannabis extracts sold for medical and non-medical purposes. The proposed amendments were pre-published in June 2021 and the consultation period closed in September 2021. No expected in-force date has been publicly announced.
There can be no assurance that thesethe jurisdictions in which we operate will allow the sale of cannabis vaporizers in the future, that other jurisdictions will not prohibit the sale of cannabis vaporizers, that we will be able to meet any additional compliance requirements or regulatory restrictions, or that we will remain competitive in face of unexpected changes in market conditionsconditions.
An extension of this controversy to non-nicotine vaporizer devices and other product formats could materially and adversely affect our business, financial condition, operating results, liquidity, cash flow and operational performance. In February 2020, the U.S. Centers for Disease Control reported that federal and state agencies were investigating an outbreak of over 2,807 lung injury cases associated with the use of vaporizer products, including non-nicotine containing products. Litigation pertaining to vaporizer products is acceleratingongoing and that litigation could potentially expand to include our products, which would materially and adversely affect our business, financial condition, operating results, liquidity, cash flow and operational performance.
Future research may lead to findings that vaporizers, electronic cigarettes and related products are not safe for their intended use.
Vaporizers, electronic cigarettes and related products were recently developed and therefore the scientific or medical communities have had a limited period of time to study the long-term health effects of their use. Currently, there is limited scientific or medical data on the safety of such products for their intended use and the medical community is still studying the health effects of the use of such products, including the long-term health effects. If a consensus were to develop among the scientific or medical community were to determine conclusively that the use of any or all of these products pose long-term health risks, market demand for these products and their use could materially decline. Such a determinationdevelopment could also lead to litigation, reputational harm and significant regulation. Loss of demand for our product,products, product liability claims and increased regulation stemming from unfavorable scientific studies on cannabis vaporizer products could have a material adverse effect on our business, financial condition, operating results, liquidity, cash flow and operational performance.
We, or the cannabis and U.S. hemp industries more generally, may receive unfavorable publicity or become subject to negative consumer perception.
We believe the cannabis and U.S. hemp industries are highly dependent upon broad social acceptance and consumer perception regarding the safety, efficacy and quality of the cannabis and U.S. hemp products, as well as consumer views concerning regulatory compliance. Consumer perception of our products can be significantly influenced by scientific research or findings, regulatory investigations, litigation, media attention, market rumors or speculation and other publicity regarding the consumption or effects thereof of cannabis and U.S. hemp products. There can be no assurance that future scientific research, findings, regulatory proceedings, litigation, media attention or other research findings or publicity will be favorable to the cannabis or U.S. hemp markets or any particular product, or consistent with earlier publicity. Future research reports, findings, regulatory proceedings, litigation, media attention or other publicity that are perceived as less favorable than, or that question, earlier research reports, findings or publicity could have a material adverse effect on the demand for our products and our business, financial condition and results of operations. Our dependence upon consumer perceptions means that adverse scientific research reports, findings, regulatory proceedings, litigation, media attention or other publicity, whether or not accurate or with merit, could have a material adverse effect on the demand for products, and our business, results of operations, financial condition and cash flows. Further, adverse publicity reports or other media attention regarding the safety, efficacy and quality of U.S. hemp or cannabis in general, or our products specifically, or associating the consumption or use of U.S. hemp or cannabis with illness or other negative effects or events, could have such a material adverse effect. Such adverse publicity reports or other media attention could arise even if the adverse effects associated with such products resulted from consumers’ failure to consume such products legally, appropriately or as directed.
The increased usage of social media, artificial intelligence and other web-based tools used to generate, publish and discuss user-generated content and to connect with other users has made it increasingly easier for individuals and groups to communicate and share opinions and views, whether or not true, on our operations and activities whether true or not, and the U.S. hemp and cannabis industries in general, whether true or not. Social media permits user-generated content to be distributed to a broad audience which can respond or react, in near real time, with comments that may be generated by automation and are often not filtered or checked for accuracy. In many cases, we do not have the ability to filter such comments or verify their accuracy. Accordingly, the speed with which negative publicity (whether true or not) can be disseminated has increased dramatically with the expansion of social media.media and artificial intelligence. The dissemination of negative or inaccurate posts, comments or other user-generated content about us on social media (including those published by third-parties) could damage our brand, image and reputation or how the U.S. hemp or cannabis industries are perceived generally, which could have a detrimental impact on the market for our products and thus on our business, financial condition and results of operations.
Certain well-funded and significant businesses may have strong economic opposition to the U.S. hemp or cannabis industries. Lobbying by such groups, and any resulting inroads they might make in halting or rolling back the U.S. hemp and cannabis movements, could affect how the U.S. hemp or cannabis industries are perceived by others and could have a detrimental impact on the market for our products and thus on our business, financial condition and results of operations.
The parties with which we do business, may perceive that they are exposed to reputational risk as a result of our cannabis or U.S. hemp business activities. Failure to establish or maintain business relationships could have a material adverse effect on our business, financial condition and results of operations. Any third-party service provider or supplier could suspend or withdraw its services to us or require increased fees or compensation if it perceives that the potential risks exceed the potential benefits to such services. For example, we face challenges making U.S. dollar wire transfers or engaging any third-party service provider or supplier with a substantial presence where cannabis is not federally legal (including the U.S.). WhileIn these circumstances, while we have other banking relationships and believe that thesuch services can be procured from other institutions, we may in the future have difficulty maintaining existing, or securing new, bank accounts or clearing services, service providers or other vendors.vendors or we may be forced to pay increased fees or compensation for such services.
Although we take care in protecting our image and reputation, we do not ultimately have control over how we or the U.S. hemp or cannabis industries are perceived by others. Reputation loss may result in decreased investor confidence, increased challenges in developing and maintaining community relations and an impediment to our overall ability to advance our business strategy and realize on our growth prospects, thereby having a material adverse impact on our business, financial condition and results of operations.
We may be subject to litigation in the ordinary course of our marketing, distribution and sale of our products.
We are subject to litigation, claims and other legal and regulatory proceedings from time to time in the ordinary course of our manufacturing, marketing, distribution and sale of our products, some of which may adversely affect our business, financial condition and results of operations. Several companies in the U.S. hemp-derived CBD industry, including the Company, have recently become party to an increasing number of purported class actions lawsuits relating to their food and dietary supplement products containing U.S. hemp-derived CBD. While theone such case against the Company was dismissed, similar class actions may be filed against us again, and the plaintiffs in such class action lawsuits, as well as in other lawsuits against us, may seek very large or indeterminate amounts, including punitive damages, which may remain unknown for substantial periods of time. Should any litigation in which we become involved be determined against us, such a decision could adversely affect our ability to continue operating, adversely affect the market price for theour common shares and require the use of significant resources. Even ifto the extent we are involvedultimately prevail in litigation, and win, litigation can consume and redirect significant resources. Litigation may also create a negative perception of our brands, which could have an adverse effect on our business, financial condition and results of operations. See Part II, Note 10(b) “Contingencies” to the consolidated financial statements under Item 3 in Part I8 of this Annual Report for more details ona discussion of our legal proceedings.
We may be subject to product liability claims.
As a manufacturer and distributor of products designed to be ingested by humans, we face an inherent risk of exposure to product liability claims, regulatory action and litigation if our products are alleged to have caused significant loss or injury. In addition, the manufacture and sale of cannabis and U.S. hemp products involve the risk of injury to consumers due to tampering by unauthorized third parties or product contamination. Previously unknown adverse reactions resulting from human consumption of cannabis or U.S. hemp products alone or in combination with other medications or substances could occur as described above under “—“— There is limited long-term data with respect to the efficacy and side effects of our productscannabis, U.S. hemp and cannabinoids and future clinical research studies on the effects of cannabis, U.S. hemp and cannabinoids may lead to conclusions that dispute or conflict with our understanding and belief regarding their benefits, viability, safety, efficacy, dosing and social acceptance.” We have been, and may in the future be, subject to various product liability claims that include, among others, our products caused injury or illness, incorrect labeling, inadequate instructions for use or inadequate warnings concerning possible side effects or interactions with other substances. A product liability claim or regulatory action against us could result in increased costs, could adversely affect our reputation with our clients and consumers generally, and could have a material adverse effect on our business, financial condition and results of operations. See Part I,II, Note 10(b) “Contingencies” to the consolidated financial statements under Item 3, Legal Proceedings,8 of this Annual Report for a discussion on our legal proceedings.
There can be no assurances that we will be able to obtain or maintain product liability insurance on acceptable terms or with adequate coverage against potential liabilities. Such insurance is expensive and may not be available in the future on acceptable terms, or at all. The inability to obtain sufficient insurance coverage on reasonable terms or to otherwise protect against potential product liability claims could prevent or inhibit the commercialization of products.
Our products have in the past and may in the future be subject to recalls.
Manufacturers and distributors of products are sometimes subject to the recall or return of their products for a variety of reasons, including, among other things, product defects, such as contamination, unintended harmful side effects or interactions with other substances, packaging safety and inadequate or inaccurate labelling disclosure. For example, on May 5, 2017, Peace Naturals announced a voluntary recall withSome of our products have been subject to recalls in the support of Health Canada for products sold between November 26, 2015 and March 13, 2017. Peace Naturals was notified by Health Canada that upon testing a random cannabis leaf sample, trace levels of Piperonyl Butoxide (“PBO”) were discovered at 0.78 parts per million (ppm). PBO is an organic compound known as a synergist. Root cause analysis conducted by Peace Naturals concluded that this was the result of cross-contamination. The source of the PBO was a Pest Management Regulatory Agency approved product that was used to sanitize empty rooms between harvests and which is no longer used.
If one or more of our products are recalled due to an alleged product defect or for any other reason, we could be required to incur the unexpected expense of the recall and any legal proceedings that might arise in connection with the recall. We may lose a significant amountnumber of sales and may not be able to replace those sales at an acceptable margin, or at all. In addition, a product recallrecalls have in the past and may in the future require significant management attention. Although we have detailed procedures in place for testing finished products, there can be no assurance that any quality, potency or contamination problems will be detected in time to avoid unforeseen product recalls, regulatory action or lawsuits. If one or more of our products were subject to recall, the public perception of that product and us could be harmed. A recall for any of the foregoing reasonsone of our products could lead to decreased demand for that product and our other products produced by us and could have a material adverse effect on our business, financial condition and results of operations. Additionally, product recalls may lead to increased scrutiny of our operations by Health Canada, the FDA, the California Department of Public Health (the “CDPH”), the DEA or other regulatory agencies, requiring further management attention and potential legal fees and other expenses. Furthermore, any product recall affecting the cannabis or U.S. hemp industries more broadly could lead consumers to lose confidence in the safety and security of the products sold by participants in these industries generally, which could have a material adverse effect on our business, financial condition and results of operations.
We rely on third-party testing and analytical methods which are validated but still being standardized.
For certain of our cannabis and U.S. hemp products, testing for cannabinoid levels, heavy metals and pesticides (among other things) is performed by independent third-party testing laboratories. Testing methods and analytical assays for cannabinoids and levels of detection vary among different testing laboratories in different jurisdictions. There is currently no industry consensus on standards for testing methods or an industry accepted compendium of analytical assays or standard levels of detection. The detected and reported cannabinoid content in our cannabis and U.S. hemp products therefore can differ depending on the laboratory and testing methods (analytical assays) used. Variations in reported cannabinoid content will likely continue until the relevant regulatory agencies and independent certification bodies (e.g., ISO, USP) collaborate to develop, publish and implement standardized analytical assays and levels of detection for cannabis, (including U.S. hemp), cannabinoids and their derivative products. SuchUntil such standardized analytical assays and levels of detection are developed, the existing differences could cause confusion with our consumers which could lead to a negative perception of us and our products, increase the risk of litigation regarding cannabinoid content and regulatory enforcement action and could make it more difficult for us to comply with regulatory requirements regarding contents of ingredients and packaging and labeling. For example, on June 16, 2020, an alleged consumer filed a Statement of Claim, which has since been dismissed as against the Company, on behalf of a class in the Court of Queen’sKing’s Bench of Alberta in Alberta, Canada, against the Company and other Canadian cannabis manufacturers and/orand distributors alleging claims related to the defendants’ advertised content of cannabinoids in cannabis products for medicinal use on or after June 16, 2010 and cannabis products for adult use on or after October 17, 2018. See Part I,II, Note 10(b) “Contingencies” to the consolidated financial statements under Item 3, Legal Proceedings,8 of this Annual Report.
The presence of trace amounts of THC in our U.S. hemp products may cause adverse consequences to users of such products that will expose us to the risk of litigation, liability and other consequences.
Some of our products that are intended to primarily contain U.S. hemp-derived CBD, or other products,U.S. hemp-derived cannabinoids, may contain trace amounts of THC. THC is a controlled substance in many jurisdictions, including under the federal laws of the U.S. if it exceeds the cut-off established in the U.S. definition of hemp. Whether or not ingestion of THC (at low levels or otherwise) is permitted in a particular jurisdiction, there may be adverse consequences to consumers of our U.S. hemp products who test positive for any amounts of THC because of the presence of trace amounts of THC in our U.S. hemp products. In addition, certain metabolic processes in the body may negatively affect the results of drug tests. Positive tests for THC may expose us to litigation from our consumers, adversely affect our reputation, our ability to obtain or retain customers and impact individuals’ participation in certain athletic, employment or other activities. A claim or regulatory action against us based on such positive test results could materially and adversely affect our business, financial condition, operating results, liquidity, cash flow and operational performance.
We may not be able to successfully develop new products or find a market for their sale.
The legal cannabis and U.S. hemp industries are in their early stages of development and it is likely that we, and our competitors, will seek to introduce new products, including products that contain cannabinoids other than THC and CBD, in the future. In attempting to keep pace with any new market developments, we may need to spend significant amounts of capital in order to successfully develop and generate revenues from new products we introduce. In addition, we may be required to obtain additional regulatory approvals from Health Canada, the FDA andand/or any other applicable regulatory authority, which may take significant amounts of time. We may not be successful in developing effective and safe new products, bringing such products to market in time to be effectively commercialized, or obtaining any required regulatory approvals, and, in the event we are successful, it is possible that there may be little or no demand for the products we develop (including products containing cannabinoids other than THC and CBD with which consumers may not be familiar or have significant reservations), which, together with any capital expenditures made in the course of such product development and regulatory approval processes, may have a material adverse effect on our business, financial condition and results of operations.
The Canadian excise duty framework may affect our profitability.
Canada’s excise duty framework imposes an excise duty and various regulatory-like restrictions on certain cannabis products sold in Canada. We currently hold licenses issued by the Canada Revenue Agency (“CRA”) required to comply with this excise framework. Any change in the rates or application of excise duty to cannabis products sold by us in Canada, and any restrictive interpretations by the CRA or the courts of the regulatory-like restrictions contained inprovisions of the Excise Act, 2001 (which may be different than those contained in the Cannabis Act) may affect our profitability and ability to compete in the market.
Our business may be impacted as a result of conditions in the global economy and financial markets, including changes in inflation, interest rates, and overall economic conditions.
Our results of operations could be adversely affected by general conditions in the global economy and in the global financial markets, including changes in inflation, interest rates and overall economic conditions.The worldwide economy continues to experience significant inflation and inflationary pressures, including, in particular, on wages. Inflation could reduce our purchasing power and negatively impact our ability to obtain goods and services at commercially viable prices. We may be unable to pass on rising costs, including increased employee costs, to our customers. To the extent that we are unable to offset such inflation through higher prices of our products or other cost savings, there would be a negative impact on our operating margins, net income, cash flows and the trading price of our common shares.
A period of sustained inflation across the markets in which we operate could result in higher operating costs and reduce our profitability. Despite efforts we may take to reduce the impact of inflation on our business across the markets in which we operate, it could become increasingly difficult to effectively mitigate the increases to our costs. In addition, the effects of inflation on consumers’ budgets could result in the reduction of our customers’ spending habits.
Additionally, interest rates directly affect the level of interest income we generate from investing our cash and cash equivalents and from our short-term investments. Interest rates are subject to fluctuation, and a decrease in interest rates could negatively impact our interest income. High interest rates have in the past had, and may in the future have, adverse effects on the disposable income of our customers and their spending habits.
Risks Relating to Production and Distribution of Products
Our production facilities, and those of our strategic joint ventures, are integral to our operations, and any adverse changes or developments affecting oursuch facilities may impact our business, financial condition and results of operations.
Our activities and resources are focused on various production and manufacturing facilities including in the U.S. (for U.S. hemp products), Canada and Israel.Israel and, prior to our exit of our U.S. operations, the U.S. Some licenses are specific to those facilities. Adverse changes or developments affecting our facilities and the facilities of our joint venture partners, including but not limited to a breach of security, an inability to successfully grow cannabis plants or produce finished goods, unanticipated cost overruns in growing or producing products, an outbreak of a communicable illness (such as COVID-19) or a force majeure event, could have a material and adverse effect on our business, financial condition and results of operations. As we proceed to complete the pending sale-leaseback of, and effect the change in the nature of operations at, the Peace Naturals Campus, the production and manufacturing facilities that we continue to use have become increasingly important to our business. Any breach of the security measures and other facility requirements, including any failure to comply with recommendations or requirements arising from inspections by regulatory agencies, could also have an impact on our ability to continue operating under our licenses or the prospect of renewing our licenses or could result in a revocation of our licenses.
If the sale and leaseback of the Peace Naturals Campus is completed, we anticipate that the tenant rights we are granted under the lease agreement will enable us to complete any necessary maintenance for the Peace Naturals Campus. To the extent that Future Farmco’s cooperation or facilitation is required for such maintenance, and Future Farmco fails to cooperate in a timely manner or at all, we may be unable to complete such maintenance, which may adversely impact our financial performance. Additionally, as we proceed to wind down certain production activities at the Peace Naturals Campus, the production and manufacturing facilities that we continue to use will become increasingly important to our business.
We bear the responsibility for all of the costs of maintenance and upkeep at our facilities and our operations and financial performance may be adversely affected if our facilities are unable to keep up with maintenance requirements.
We may experience breaches of security at our facilities, or fraudulent or unpermitted data access or other cyber-security breaches, which may cause our customers to lose confidence in our security and data protection measures and may expose us to the loss of inventory and risks related to breachesviolations of applicable privacy laws.laws and regulations.
Given the nature of our products and our lack of legal availability outside of certain legalized or regulated retail or distribution channels, as well as the concentration of inventory in our facilities, despite meetingwe are subject to the applicable security requirements under applicable law, there remains a risk of theft. A security breach at one of our facilities could expose us to additional liabilityscrutiny from regulators, increased expenses and to potentially costly litigation, increase expensesbusiness disruptions relating to the resolution and future prevention of these breachesbreaches.
We have in the past and may deter potential customers from choosingin the future experience unauthorized access to our products.
In addition, we collectinformation technology systems or other cybersecurity incidents, which may make us unable to access or operate business critical systems and store personal information aboutwhich may cause our customers to lose confidence in our cybersecurity, expose us to risks related to violations of applicable laws and are responsible for protecting that information from privacy breaches. regulations, and have a material adverse effect on our business, financial position and results of operations.
A privacycybersecurity incident or breach may occur throughin a variety of sources,ways, including, without limitation, a procedural or process failure, information technology malfunction, inadvertent disclosure of sensitive or private information, deliberate unauthorized intrusions,intrusion, computer viruses, cyber-attacksvirus, and direct or indirect cyberattack or other electronic security breaches.breach. Theft of data for competitive or fraudulent purposes, such as customer lists and preferences and other consumer and employee personal information, and trade secrets and other confidential intellectual property is an ongoing risk whether perpetrated via employee collusion or negligence or through deliberate cyber-attack.and growing risk. Any such theft or privacycybersecurity incident or breach wouldmay have a material adverse effect on our business, financial condition and results of operations.
We are dependent upon information technology systems in the conduct of our operations, and we collect, store and use certain sensitive data, intellectual property, our proprietary business information and certain personally identifiablepersonal information of our employees and customers on those systems, including cloud-based systems. We have been, and expect to continue to be, subject to various cyberattacks and phishing schemes. Additionally, we are undertaking an effort to modernize our networks.information technology systems, which could expose us to additional risks relating to our collection, storage and use of certain data on our systems.
There have been many highly publicized cyber-attacks over the last several years and we expect those to continue. Any fraudulent, malicious or accidental breach of our data securitysystems could result in unintentionalunintended disclosure of, or unauthorized access to, third-party, customer, vendor, employee or other confidential or sensitive data or information, whichand could potentially result in additional costs and business disruption to us, including without limitation, to repair or replace damaged systems, enhance security or to respond to occurrences, lost sales, violations of data privacy, security or other laws and regulations and subsequent penalties, fines, regulatory action or litigation. We also rely on third-party service providers, including cloud-based systems, for certainmost of our information technology systems, such as payment processing, and any data security breach at a third-party service provider could have similar effects. In addition, media or other reports of perceived security vulnerabilities to our systems or those of our third-party suppliers,providers, even if no breach has been attempted or occurred, could adversely impact our brand and reputation, and customers could lose confidence in our security measures and reliability, which would harm our ability to retain customers and gain new ones. If any of these were to occur, it could have a material adverse effect on our business, financial position and results of operations.
There can be no assurance that our systems and processes for overseeing and identifying cybersecurity risks will prevent or timely detect a cybersecurity incident. We rely on third-party service providers to assist with these measures. We and our third-party service providers may not have the resources or technical sophistication to anticipate, prevent, respond to, or mitigate cyberattacks or cybersecurity breaches or incidents, and we or they may face difficulties or delays in identifying and responding to cyberattacks, cybersecurity breaches and incidents.
We incur significant costs in an effort to detect and prevent cybersecurity breaches and incidents and we expect our costs will increase as we continue to implement systems and processes designed to prevent and otherwise address cybersecurity breaches and incidents. In the event of a significant or material cybersecurity breach or incident, we could be required to expend additional significant capital and other resources in an effort to respond to or prevent further breaches or incidents, which may require us to divert substantial resources from our business. Moreover, we could be required or otherwise find it appropriate to expend significant capital and other resources to respond to, notify third parties of, and otherwise address the breach or incident and its root cause.
In recent years, our Information Systems department, which oversees our cybersecurity systems and processes, has experienced high turnover, creating opportunities for knowledge and skill gaps, which can result in operational errors and security oversights. In addition, cybersecurity is not the sole focus of our Information Systems department, and no individual employee is specifically dedicated solely to cybersecurity; competing responsibilities may divert their attention from cybersecurity matters.
Any actual or perceived failure by us to comply with laws, regulations or any other obligations relating to privacy, data protection or the protection or transfer of personal data, could adversely affect our business.
We collect and store personal information about our customers and employees, including health information, and are responsible for protecting that information. In Canada, for example, we are required to retain certain customer personal information for prescribed periods of time pursuant to the Cannabis Act.In the U.S., for example, we must comply with Americans with Disability Act requirements for confidential employee medical records, including that they must be stored separately from other personnel records and access must be restricted to those who need access. With respect to customer health information, there are a number of federal, state and provincial laws and regulations protecting the confidentiality of certain clientcustomer health information, including clientcustomer records, and restricting the use and disclosure of that protected information. The privacy rules under the Personal Information Protection and Electronics Documents Act (Canada) (“PIPEDA”) and related provincial laws protect medical records and other personal health information by limiting theirthe use and disclosure of health information to the minimum level reasonably necessary to accomplish the intended purpose and apply to our operations globally. If we were to be found to be in violation of the privacy or securitydata protection rules under PIPEDA or other applicable laws and regulations protecting the confidentiality of client health information in jurisdictions we operate in, we could be subject to sanctions and civil or criminal penalties, which could increase our liabilities, harm our reputation and have a material adverse effect on our business, results of operations and financial condition. Additional
The jurisdictions in which we operate or which we may enter also have data privacy and securitydata protection laws and regulations that govern the collection, use, disclosure, transfer, storage, disposal, and protection of sensitive personal information (such as the California Consumer Privacy Rights Act and other similar state laws and regulations, and PIPEDA and related provincial laws in California)Canada (such as Bill 64 in Quebec)). We may incur significant expenses in an effort to comply with privacy, data protection and information security standards imposed by such laws and regulations, as well as contractual obligations.
New and modified laws, and other changes in laws or regulations relating to privacy, data protection and information security, may require us to modify our data collection or processing practices and policies, incur substantial costs and expenses to comply with these laws and regulations, and increase our potential exposure to regulatory enforcement and litigation. The interpretation and enforcement of such laws and regulations are uncertain and subject to change and may require substantial costs to monitor and implement compliance with any additional requirements.compliance. Failure to comply with data privacy and protection laws and regulations could result in government enforcement actions (which could include substantial civil and/orand criminal penalties), private litigation, and/orbusiness disruption, the diversion of management’s attention and adverse publicity and could negatively affect our operatingbusiness, results of operations and business.financial condition.
Our cannabis cultivation and U.S. hemp operations are subject to risks inherent in an agricultural business.
Our business and that of our joint venture partners and third-party suppliers involves the growing of cannabis, an agricultural product, in certain jurisdictions where that activity is permitted. As such, the business is subject to the risks inherent in the agricultural business, such as insects, plant diseases and similar agricultural risks that may create crop failures and supply interruptions for our customers. Although our current operational production facilities, and those of our joint venture partners and third-party suppliers, grow products indoors (including in greenhouses) under climate-controlled conditions and we and our joint venture partners and third-party suppliers carefully monitor the growing conditions with trained personnel, there can be no assurance that natural elements will not have a material adverse effect on the production of our products.
Our business also involves products containing U.S. hemp. U.S. hemp is typically harvested in To the extent we rely on third parties or around the month of October. U.S. hemp plantsour joint venture partners to grow cannabis that we intend to commercialize, we are exposed to similar risks and there can be vulnerable to various pathogens including bacteria, fungi, viruses and other miscellaneous pathogens. Such instances often lead to reduced crop quality, stunted growth and/or death of the plant. Moreover, U.S. hemp is “phytoremediative” (meaningno assurance that it may extract toxins or other undesirable chemicals or compounds from the ground in which it is planted). Various regulatory agencies have established maximum limits for pathogens, toxins, chemicals and other compounds that may be present in agricultural materials. If U.S. hemp used in our products is found to have levels of pathogens, toxins, chemicals or other undesirable compounds that exceed permitted limits, it may have to be destroyed. Should the U.S. hemp used in our products be lost due to pathogens, toxins, chemicals or other undesirable compounds, or if we or our suppliers are otherwise unable to obtain U.S. hemp for use in our products on an ongoing basis, it maysuch risks will not have a similarly material and adverse effect on our business, financial condition, operating results, liquidity, cash flow and operational performance.
The inabilitythe production of our customers to meet their financial or contractual obligations to us may result in disruption to our results of operations and could result in financial losses.
We have significant exposure to several customers and at least some of these customers are experiencing financial difficulties. We have in the past, and may in the future, need to take allowances against and need to write off receivables due to the creditworthiness of these customers. Further, the inability of these customers to purchase our products could materially adversely affect our results of operations.products.
The inability of our suppliers to meet their financial or contractual obligations to us may result in disruption to our supply chain and could result in financial losses.
We face exposure to our third-party cannabis suppliers that may face financial difficulties which would impact our supply of cannabis material.products. For example, supply chains throughout the world have been negatively impacted by COVID-19 and this has increased the costs of products and shipping. We have in the past, and may in the future, have disruptions in our supply chain.
We rely on third-party distributors and manufacturers to distribute and manufacture certain of our products, and those distributors and manufacturers may not perform their obligations.
We rely on third-party distributors, including pharmaceutical distributors and other courier services, and may in the future rely on other third parties, to distribute our products. We also rely on third-party manufacturers to manufacture certain of our products. If these distributors or manufacturers do not successfully carry out their contractual obligations or terminate or suspend their contractual arrangements with us, if there is a delay or interruption in the distribution or manufacturing of our products or if these third parties damage our products, it could negatively impact our revenue.revenue and may require significant management attention. In addition, any damage to our products due to acts or omissions of our third-party distributors or manufacturers, such as product spoilage or improper storage or handling, could expose us to potential product liability, damage our reputation and the reputation of our products or brands or otherwise harm our business.
Risks Relating to Intellectual Property
We are subject to risks related to the protection and enforcement of our intellectual property rights, and we may be unable to protect or enforce our intellectual property rights.
The ownership and protection of our intellectual property rights is a significant aspect of our future success. Currently we rely on trade secrets, technical know-how, proprietary information, trademarks, copyrights, designs and certain patent filings to maintain our competitive position. We try to protect our intellectual property by strategically seeking and obtaining registered protection where possible,appropriate, developing and implementing standard operating procedures to protect trade secrets, technical know-how and proprietary information, and entering into agreements with parties that have access to our inventions, trade secrets, technical know-how and proprietary information, such as our partners, collaborators, employees and consultants, to protect confidentiality and ownership. We also seek to preserve the integrity and confidentiality of our inventions, trade secrets, technical know-how and proprietary information by maintaining physical security of our premises and physical and electronic security of our information technology systems, and we seek to protect our trademarks and the goodwill associated therewith by monitoring and enforcing against unauthorized use of our trademarks.
It is possible that we will inadvertently disclose or otherwise fail or be unable to protect our inventions, trade secrets, technical know-how or proprietary information, or will fail to identify our inventions or trademarks as patentable or registrable intellectual property, or fail to obtain patent or registered trademark protection therefor. Any such disclosure or failure could have a material adverse effect on our business.
We may be unable to protect our inventions, trade secrets, and other intellectual property from discovery or unauthorized use.
In relation to our agreements with parties that have access to our intellectual property, any of these parties may breach their obligations to us, and we may not have adequate remedies for such breach. In relation to our security measures, such security measures may be breached and we may not have adequate remedies for such breach. In addition, our intellectual property that has not yet been applied for or registered may otherwise become known to, or be independently developed by, competitors, or may already be the subject of applications for intellectual property registrations filed by our competitors, which may have a material adverse effect on our business, financial condition and results of operations.
We cannot provide any assurances that our inventions, trade secrets, technical know-how and other proprietary information will not be disclosed in violation of agreements, or that competitors will not otherwise gain access to our intellectual property or independently develop and file applications for intellectual property rights in a manner that adversely impacts our intellectual property rights. For example, we have had employees misappropriate the Company’s confidential information, including intellectual property, including at least one employee who was subsequently employed by a competitor. Unauthorized parties may attempt to replicate or otherwise obtain and use our inventions, trade secrets, technical know-how and proprietary information. Policing the unauthorized use of our current or future intellectual property rights could beis difficult, expensive, time-consuming and unpredictable, as may beis enforcing these rights against unauthorized use by others. Identifying unauthorized use of intellectual property rights is difficult asdifficult. For example, we may be unable to effectively monitor and evaluate the products being distributed by our competitors, including parties such as unlicensed dispensaries, and the processes used to produce such products. If the steps taken to identify and protect our trade secrets are inadequate, we may be unable to enforce our rights in them against third parties.
Our intellectual property rights may be invalid or unenforceable under applicable laws, and we may be unable to have issued or registered, and unable to enforce, our intellectual property rights.
The laws and positions of intellectual property offices administering such laws regarding intellectual property rights relating to cannabis and cannabis-related products, and the positions of intellectual property offices administering such laws, are constantly evolving, and there is uncertainty regarding which countries will permit the filing, prosecution, issuance, registration and enforcement of intellectual property rights relating to cannabis and cannabis-related products.
Specifically, we have sought trademark protection in many countries, including Canada, the U.S. and others. Our ability to obtain registered trademark protection for cannabis and cannabis-related goods and services (including U.S. hemp and U.S. hemp-related goods and services) may be limited in certain countries outside of Canada, including the U.S., where registered federal trademark protection is currently unavailable for trademarks covering the sale of U.S. Schedule I cannabis products or certain goods containing U.S. hemp-derived CBD (such as dietary supplements and foods) until the FDA provides clearer guidance on the regulation of such products;products, and including Europe, where laws on the legality of cannabis use are not uniform, and trademarks cannot be obtained for products that are “contrary to public policy or accepted principles of morality.” Accordingly, our ability to obtain intellectual property rights or enforce intellectual property rights against third-party uses of similar trademarks may be limited in certain countries.
Moreover, in any infringement proceeding, some or all of our current or future trademarks, patents or other intellectual property rights or other proprietary know-how, or arrangements or agreements seeking to protect the same for our benefit, may be found invalid, unenforceable, anti-competitive or not infringed. An adverse result in any litigation or defense proceedings could put one or more of our current or future trademarks, patents or other intellectual property rights at risk of being invalidated or interpreted narrowly and could put existing intellectual property applications at risk of not being issued. Any or all of these events could materially and adversely affect our business, financial condition and results of operations.
There is no guarantee that any patent or other intellectual property applications that we file will result in registration or any enforceable intellectual property rights or the breadth of any such protection. Further, with respect to any patent applications that we file, there is no assurance that we will find all potentially relevant prior art relating to any patentsuch applications, that we file, which may prevent a patent from issuing from a patentsuch application or invalidate any patent that issues from such application. Even if patents do successfully issue, and cover our products and processes, third parties may challenge their validity, enforceability or scope, which may result in such patents being narrowed, found unenforceable or invalidated. Even if they are unchallenged, any patent applications and future patents may not adequately protect our intellectual property rights, provide exclusivity for our products or processes or prevent others from designing around any issued patent claims. Any of these outcomes could impair our ability to prevent competition from third parties, which could materially and adversely affect our business, financial condition and results of operations.
We may be subject to allegations that we are in violation of third-party intellectual property rights, and we may be found to infringe third-party intellectual property rights, possibly without the ability to obtain licenses necessary to use such third-party intellectual property rights.
Other parties may claim that our products infringe on their intellectual property rights, including with respect to patents, and our operation of our business, including our development, manufacture and sale of our goods and services, may be found to infringe third-party intellectual property rights. There may be third-party patents or patent applications with claims to products or processes related to the manufacture, use or sale of our products and processes. There may be currently pending patent applications, some of which may still be confidential, that may later result in issued patents that our products or processes may infringe. In addition, third parties may obtain patents in the future and claim that use of our inventions, trade secrets, technical know-how and proprietary information, or the manufacture, use or sale of our products, infringes upon those patents. Third parties may also claim that our use of our trademarks infringes upon their trademark rights. Such claims, whether or not meritorious, may result in the expenditure of significant financial and managerial resources, legal fees, injunctions, temporary restraining orders, other equitable relief, and/orand require the payment of damages, any or all of which may have an adverse impact on our business.business, financial condition and results of operations. In addition, we may need to obtain licenses from third parties who allege that we have infringed on their lawful rights.purported rights, whether or not such allegations have merit. Such licenses may not be available on terms acceptable to us, and we may be unable to obtain any licenses or other necessary or useful rights underto such third-party intellectual property.
Our germplasm relies heavily on intellectual property, and we may be unable to protect, register or enforce our intellectual property rights in germplasm, and may infringe third-party intellectual property rights with respect to germplasm, possibly without the ability to obtain licenses necessary to use such third-party intellectual property rights.
Germplasm, including seeds, clones and cuttings, is the genetic material used in new cannabis varieties and hybrids. We use advanced breeding technologies to produce cannabis germplasm (hybrids and varieties) with superior performance.. We rely on parental varieties for the success of our breeding program. Although we believe that the parental germplasm is proprietary to us, we may need to obtain licenses from third parties who may allege that we have appropriated their germplasm or their rights to such germplasm.germplasm, whether or not such allegations have merit. Such licenses may not be available on terms acceptable to us, and we may be unable to obtain any licenses or other necessary or useful rights under third-party intellectual property. We may seek to protect our parental germplasm, as appropriate, relying on intellectual property rights, including rights related to inventions (patents and plant breeders’ rights), trade secrets, technical know-how, and proprietary information. There is a risk that we will fail to protect such germplasm or that we will fail to register rights in relation to such germplasm. We have also licensed certain of our germplasm strains to Cronos GrowCo and may be unable to maintain control of these strains if we do not purchase all product derived from such strains.
We also seek to protect our parental germplasm, hybrids and varieties from pests and diseases and enhance plant productivity and fertility, and we research products to protect against crop pests and fungus. There are several reasons why new product concepts in these areas may be abandoned, including greater than anticipated development costs, technical difficulties, regulatory obstacles, competition, inability to prove the original concept, lack of demand and the need to divert focus, from time to time, to other initiatives with perceived opportunities for better returns.initiatives. The processes of breeding, development and trait integration are lengthy, and the germplasm we test may not be selected for commercialization. The length of time and the risk associated with breeding may affect our business. Our sales depend, in part, on our germplasm. Commercial success frequently depends on being the first company to the market, and many of our competitors are also making considerable investments in similar new and improved cannabis germplasm products. Consequently, there is no assurance that we will successfully develop and deliver new cannabis germplasm products to the point of commercial viability in the markets we serve on a timely basis.
Finally, we seek to protect our germplasm, hybrids and varieties from accidental release, theft, misappropriation and sabotage by maintaining physical security of our premises and through contractual rights with our employees and certain of our suppliers, independent contractors, consultants and licensees. However, such security measures may be insufficient or breached, and our employees, independent contractors, consultants and licensees may engage in the inadvertent disclosure, theft, misappropriation or sabotage. We may not have adequate remedies in the case of any such security breach, inadvertent disclosure, theft, misappropriation or sabotage.
We receive licenses to use some third-party intellectual property rights;rights and germplasm; the failure of the owner of such intellectual property or germplasm to properly maintain or enforce the intellectual property underlying such licenses or germplasm, as the case may be, or our inability to obtain or maintain such licenses, could have a material adverse effect on our business, financial condition and performance.
We are party to licenses granted by third parties, including with actress Kristen Bell and through the Ginkgo Strategic Partnership, thatwhich give us rights to use third-party intellectual property and germplasm that is necessary or useful to our business. Our success will depend, in part, on the ability of the applicable licensor to maintain and enforce its licensed intellectual property, including intellectual property underlying licensed germplasm, against other third parties, particularly intellectual property rights to which we have secured exclusive rights. Without protection for the intellectual property we have licensed, or underlying germplasm that we have licensed, as the case may be, other companies might be able to offer substantially similar products for sale or utilize substantially similar processes, or publicity and marketing rights or other intellectual property, any of which could have a material adverse effect on our business, financial condition and results of operations. Our success will also depend, in part, on our ability to obtain licenses to certain intellectual property and germplasm that we believe are necessary or useful for our business. Such licenses may not be available on terms acceptable to us, or at all, which could adversely affect our ability to commercialize our products or services, as well as have a material adverse effect on our business, financial condition and results of operations.
Any of our licensors may allege that we have breached our license agreements with those licensors, whether with or without merit, and accordingly seek to terminate our applicable licenses. If successful, this could result in our loss of the right to use applicable licensed intellectual property or germplasm, which could adversely affect our ability to commercialize our products or services, as well as have a material adverse effect on our business, financial condition and results of operations.
The technologies, process and formulations we use may face competition or become obsolete.
Rapidly changing markets, technology, emerging industry standards and frequent introduction of new products characterize our business. The introduction of new products embodying new technologies, including new manufacturing processes or formulations, and the emergence of new industry standards may render our products obsolete, less competitive or less marketable. The process of developing our products is complex and requires significant continuing costs, development efforts and third-party commitments, including licensees, researchers, collaborators and lenders.collaborators. Our failure to develop new technologies and products and the obsolescence of existing technologies or processes could adversely affect our business, financial condition and results of operations. We may be unable to anticipate changes in our potential customer preferences or requirements that could make our existing technology, processes or formulations obsolete. Our success will depend, in part, on our ability to continue to enhance our existing technologies, develop new technology that addresses the increasing sophistication and varied newsviews of the market, and respond to technological advances and emerging industry standards and practices on a timely and cost-effective basis. The development of our proprietary technology, processes and formulations entails significant technical and business risks. We may not be successful in using our new technologies or exploiting our niche markets effectively or adapting our business to evolving customer or medical requirements or preferencepreferences or emerging industry standards.
Risks Relating to Entry into New Markets
Entering into new jurisdictions is inherently risky, may not be successful and could be costly.
From time to time, we enter into additional jurisdictions throughout the world, whether directly or through strategic partnerships with local operators who distribute our products. These expansion efforts involve significant risks and uncertainties, including risks related to the ability to obtain and maintain governmental permits and licenses, consumer reception of our products in such jurisdictions, increases in operational complexity, increases in the complexity involved in ensuring our products consistently meet our quality standards, unanticipated delays or challenges, increased strain on our operational and internal resources, our dependence on strategic commercial partnerships, and negative public reception.
Our expansion efforts have required, and may in the future require, the dedication of substantial resources. In particular, we may need to make additional investments in management and personnel, infrastructure, operations and compliance systems. Expanding into additional jurisdictions may involve significant up-front capital investments and such investments may not generate our expected return on investment or any return at all. Further, from time to time we may reevaluate and discontinue our participation in such jurisdictions, which could result in write-offs, asset, intangible asset and goodwill impairments, and could otherwise adversely affect our business, financial condition and results of operations.
We will also face new operational risks and challenges as we enter into new markets. Expansion into foreign jurisdictions subjects us to legal, regulatory, reputational and political risks that may be different from and additional to those that we face in jurisdictions in which we currently operate, and we may be at a disadvantage relative to competitors who are more familiar with local markets and local laws and regulations. Similarly, consumer preferences in jurisdictions we enter may differ from those in our existing markets, and our products may not be received by consumers as well as competing products in such jurisdictions. These factors may cause our expansion efforts to be unsuccessful, which may result in write-offs, asset and intangible asset and goodwill impairments, and may otherwise have a material negative impact on our business, results of operations and financial condition.
Controlled substance and other legislation and treaties may restrict or limit our ability to research, manufacture and develop a commercial market for our products outside of the jurisdictions in which we currently operate, and our expansion into such jurisdictions is subject to risks.
Approximately 250 substances, including cannabis, are listed in the Schedules annexed to the UN Single Convention on Narcotic Drugs (New York, 1961), the Convention on Psychotropic Substances (Vienna, 1971) and the Convention against Illicit Traffic in Narcotic Drugs and Psychotropic Substances (introducing control on precursors) (Vienna, 1988). The purpose of these listings is to control and limit the use of these drugs according to a classification of their therapeutic value, risk of abuse and health dangers, and to minimize the diversion of precursor chemicals to illegal drug manufacturers. The 1961 UN Single Convention on Narcotic Drugs, as amended in 1972 classifies cannabis as a Schedule I (“substances with addictive properties, presenting a serious risk of abuse”) narcotic drug. In December 2020, the Commission on Narcotic Drugs voted to remove cannabis from Schedule IV (“the most dangerous substances, already listed in Schedule I, which are particularly harmful and of extremely limited medical or therapeutic value”). The 1971 UN Convention on Psychotropic Substances classifies tetrahydrocannabinols, which includes delta-9 THC, as a Schedule I psychotropic substance (substances presenting a high risk of abuse, posing a particularly serious threat to public health which are of very little or no therapeutic value). Many countries are parties to these conventions, which govern international trade and domestic control of these substances, including cannabis. They may interpret and implement their obligations in a way that creates legal obstacles to our obtaining manufacturing and/or marketing approval for our products in those countries. These countries may not be willing or able to amend or otherwise modify their laws and regulations to permit our products to be manufactured and/or marketed, and achieving such amendments to the laws and regulations may take a prolonged period of time. There can be no assurance that any market for our products will develop in any jurisdiction in which we do not currently have operations. We may face new or unexpected risks or significantly increase our exposure to one or more existing risk factors, including economic instability, political instability, changes in laws and regulations and the effects of competition. These factors may limit our capability to successfully expand our operations into such jurisdictions and may have a material adverse effect on our business, financial condition and results of operations.
Investments and joint ventures outside of Canada and the U.S. are subject to the risks normally associated with any conduct of business in foreign countries, including varying degrees of political, legal, regulatory and economic risk.
Much of our exposure to markets in jurisdictions outside of Canada and the U.S. is through investments and joint ventures. These investments and joint ventures are subject to the risks normally associated with any conduct of business in foreign and/or emerging countries including political risks; civil disturbance risks; changes in laws, regulations or policies of particular countries, including those relating to royalties, duties, imports, exports and currency; the cancellation or renegotiation of contracts; the imposition of royalties, net profits payments, tax increases or other claims by government entities, including retroactive claims; a disregard for due process and the rule of law by local courts; the risk of expropriation and nationalization; delays in obtaining or the inability to obtain necessary governmental permits or the reimbursement of refundable tax from fiscal authorities.
Threats or instability in a country or region caused by political events including elections, change in government, changes in personnel or legislative bodies, foreign relations or military control present serious political and social risk and instability causing interruptions to the flow of business negotiations and influencing relationships with government officials. Changes in policy or law may have a material adverse effect on our business, financial condition and results of operations. The risks include increased “unpaid” state participation, higher energy costs, higher taxation levels and potential expropriation.
Other risks include the potential for fraud and corruption by suppliers or personnel or government officials which may implicate us, compliance with applicable anti-corruption laws and regulations, including the U.S. Foreign Corrupt Practices Act and the Corruption of Foreign Public Officials Act (Canada), by virtue of our or our joint ventures and strategic alliances operating in jurisdictions that may be vulnerable to the possibility of bribery, collusion, kickbacks, theft, improper commissions, facilitation payments, conflicts of interest and related party transactions or our joint ventures’ and ourstrategic alliances’ possible failure to identify, manage and mitigate instances of fraud, corruption or violations of our codeCode of conductBusiness Conduct and Ethics and applicable regulatory requirements.
There is also the risk of increased disclosure requirements; currency fluctuations; restrictions on the ability of local operating companies to hold Canadian dollars, U.S. dollars or other foreign currencies in offshore bank accounts; import and export regulations;restrictions; increased regulatory requirements and restrictions; increased health-related regulations; limitations on the repatriation of earnings or on our ability to assist in minimizing our expatriate workforce’s exposure to double taxation in both the home and host jurisdictions; and increased financing costs.
These risks may limit or disrupt our joint ventures, strategic alliances or investments, restrict the movement of funds, cause us to have to expend more funds than previously expected or required or result in the deprivation of contract rights or the taking of property by nationalization or expropriation without fair compensation, and may materially adversely affect our businesses,business, financial position and/or results of operations. In addition, the enforcement by us of our legal rights in foreign countries, including rights to exploit our properties or utilize our permits and licenses and contractual rights may not be recognized by the court systems in such foreign countries or enforced in accordance with the rule of law.
We currently do, and may in the future, invest in companies, or engage in joint ventures, in countries with developing economies. It is difficult to predict the future political, social and economic direction of the countries in which we or our joint ventures operate, and the impact government decisions may have on our business. Any political or economic instability in the countries in which we operate could have a material and adverse effect on our business, financial condition and results of operations.
Our use of joint ventures may expose us to risks associated with jointly owned investments.
We currently operate parts of our business through joint ventures with other companies, and we may enter into additional joint ventures and strategic alliances in the future. Joint venture investments may involve risks not otherwise present for investments made solely by us, including: (i) we may not control the joint ventures; (ii) our joint venture partners may not agree to distributions that we believe are appropriate; (iii) where we do not have substantial decision-making authority, we may experience impasses or disputes with our joint venture partners on certain decisions, which could require us to expend additional resources to resolve such impasses or disputes, including litigation or arbitration; (iv) our joint venture partners may become insolvent or bankrupt, fail to fund their share of required capital contributions or fail to fulfil their obligations as a joint venture partner; (v) the arrangements governing our joint ventures may contain certain conditions or milestone events that may never be satisfied or achieved; (vi) our joint venture partners may have business or economic interests that are inconsistent with ours and may take actions contrary to our interests; (vii) we may suffer losses as a result of actions taken by our joint venture partners with respect to our joint venture investments; (viii) it may be difficult for us to exit a joint venture if an impasse arises or if we desire to sell our interest for any reason; and (ix) our joint venture partners may exercise termination rights under the relevant agreements. In addition, we may, in certain circumstances, be liable for the actions of our joint ventures or joint venture partners. Any of the foregoing risks could have a material adverse effect on our business, financial condition and results of operations.
There can be no assurance that our current and future strategic alliances or expansions of scope of existing relationships will have a beneficial impact on our business, financial condition and results of operations.
We currently have, and may in the future enter into additional, strategic alliances with third parties that we believe will complement or augment our existing business. Our ability to complete strategic alliances is dependent upon, and may be limited by, the availability of suitable candidates and capital. In addition, strategic alliances could present unforeseen integration obstacles or costs, may not enhance our business and may involve risks that could adversely affect us, including significant amounts of management time that may be diverted from operations in order to pursue and complete such transactions or maintain such strategic alliances. Future strategic alliances could result in the incurrence of debt, costs and contingent liabilities, and there can be no assurance that future strategic alliances will achieve, or that our existing strategic alliances will continue to achieve, the expected benefits to our business or that we will be able to consummate future strategic alliances on satisfactory terms, or at all. Any of the foregoing could have a material adverse effect on our business, financial condition and results of operations.
In the case of the Ginkgo Strategic Partnership, we will have, pursuant to the Ginkgo Collaboration Agreement, the exclusive right to use and commercialize the key patented intellectual property related to the production of the target cannabinoids globally. There can be no assurance that Ginkgo will be able to develop microorganisms that we will be able to commercialize or to obtain patents relating to production of the target cannabinoids, or that third parties will not develop similar microorganisms or obtain patents that may restrict our ability to commercialize the microorganisms developed by Ginkgo, and, as a result, there can be no assurance that we will be able to realize the expected benefits of the Ginkgo Strategic Partnership. Even if we are able to commercialize, there may not be demand for such products or the cultured cannabinoids developed therefrom.
In addition, pursuant to the Ginkgo Collaboration Agreement, if we undergo a change of control that is approved by the Board, including a change of control resulting from the exercise of the Altria Warrant, Ginkgo may elect to receive cash payments, totaling up to $100 million, in lieu of the common shares that would otherwise become issuable in connection with any Equity Milestone Events (as defined in the Ginkgo Collaboration Agreement) achieved following such election (the “Milestone Cash Election”). If we undergo a change in control that has not been approved by the Board, then Ginkgo will have the ability to terminate the Ginkgo Collaboration Agreement immediately, in which case, among other things: (i) all rights or licenses granted to us by Ginkgo under the Ginkgo Collaboration Agreement will terminate; (ii) certain expenses and costs incurred by Ginkgo will be accelerated and become due and payable by us; (iii) the then-outstanding and unpaid portion of all cash payments from us to Ginkgo for the achievement of R&D milestones by Ginkgo shall be due immediately as if all R&D milestones had been achieved; and (iv) a lump sum cash payment equal to the aggregate of all Milestone Cash Election amounts in respect of which the relevant Equity Milestone Events have not yet been achieved will be immediately due and payable by us. In addition, should Ginkgo terminate the Ginkgo Collaboration Agreement upon a change of control, we will no longer be able to use or commercialize the key patented intellectual property related to the production of the target cannabinoids, which could have a material adverse effect on our business, financial condition and results of operations. See“Description of Business - Research and Development Activities and Intellectual Property.”
With respect to the Technion Research Agreement, we will have access to the results of preclinical studies conducted by Technion over a three-year period, focusing on acne, psoriasis and skin repair. However, there can be no assurance that the preclinical studies will provide any actionable findings. As a result, there can be no assurance that we will be able to realize the expected benefits of the Technion Research Agreement. Even if the results are actionable, and we are able to develop commercial products based on such research, there may not be demand for such products. See “Description of Business - Research and Development Activities and Intellectual Property - Technion Skin Health and Research Partnership.”
Risks Relating to Regulation and Compliance
We operate in highly regulated sectors where the regulatory environment is rapidly developing, and we may not always succeed in complying fully with applicable regulatory requirements in all jurisdictions where we carry on business.
Our business and activities are heavily regulated in all jurisdictions where we carry on business. Our operations are subject to various laws, regulations and guidelines by governmental authorities (including, in Canada, Health Canada and analogousother federal, provincial and local regulatory agencies and, in the U.S., the FDA, the USDA, CDPH, DEA, PTO and FTC and analogousother federal and state agencies) relating to the cultivation, manufacture, processing, marketing, labeling, packaging, management, transportation, distribution, import, export, storage, sale, pricing and disposal of cannabis and U.S. hemp, and also including laws, regulations and guidelines relating to health and safety, insurance coverage, the conduct of operations and the protection of the environment (including relating to emissions and discharges to water, air and land, and the handling and disposal of hazardous and non-hazardous materials and wastes). Our operations may also be affected in varying degrees by government regulations with respect to, but not limited to,among other things, price controls, import or export controls, controls on currency remittance, increased income taxes, restrictions on foreign investment and government policies rewarding contracts to local competitors or requiring domestic producers or vendors to purchase supplies from a particular jurisdiction. Laws, regulations and guidelines, applied generally, grant government agencies and self-regulatory bodies broad administrative discretion over our activities, including the power to limit or restrict business activities as well as impose additional disclosure requirements on our products and services.services, as well as on our personnel (including management and our board of directors).
Achievement of our business objectives is contingent, in part, upon compliance with regulatory requirements enacted by these governmental authorities and obtaining all necessary regulatory approvals for the cultivation, production, processing storage, transportation, distribution, sale, import and export, as applicable, of our products. The cannabis and U.S. hemp industries are still new, industries and in Canada in particular, the Cannabis Act is a new regime that has no close precedent in Canadian law. Similarly, the regulatory regimes in the jurisdictions in which we and our joint ventures operate outside of the U.S. and Canada the regulatory environments in jurisdictions legalizing the import, cultivation, production and sale of cannabis and cannabis products are new and are still being developed without close precedent in such jurisdictions. The effect of relevant governmental authorities’ administration, application and enforcement of their respective regulatory regimes and delays in obtaining, or failure to obtain, applicablenecessary regulatory approvals which may be required may significantly delay or impact the development of markets, products and sales initiatives and could have a material adverse effect on our business, financial condition and results of operations.
The regulatory environment for our products is rapidly developing, and the need to build and maintain robust systems to comply with different and changing regulations in multiple jurisdictions increases the possibility that we may violate one or more applicable requirements. While we endeavor to comply with all relevant laws, regulations and guidelines, any failure to comply with the regulatory requirements applicable to our operations could subject us to negative consequences, including, but not limited to, civil and criminal penalties, damages, fines, the curtailment or restructuring of our operations, asset seizures, revocation or imposition of additional conditions on licenses to operate our business, the denial of regulatory applications (including, in the U.S., by other regulatory regimes that rely on the positions of the DEA and FDA in the application of their respective regimes), the suspension or expulsion from a particular market or jurisdiction of our key personnel, or the imposition of additional or more stringent inspection, testing and reporting requirements, any of which could materially adversely affect our business, financial condition and financial results.results of operations. Additionally, scheduled or unscheduled inspections of our facilities or facilities of our joint ventures or third-party suppliers by applicable regulatory agencies could result in adverse findings that could require significant remediation efforts and/or temporary or permanent shutdown of our facilities or those of our joint ventures or third-party suppliers. In the U.S., failure to comply with FDA requirements (and analogous state agencies) may result in, among other things, injunctions, product withdrawals, recalls, product seizures, fines and criminal prosecutions. The outcome of any regulatory or agency proceedings, investigations, inspections, audits, and other contingencies could harm our reputation, require us to take, or refrain from taking, actions that could harm our operations or require us to pay substantial amounts of money, harming our results of operations, financial condition and cash flows. There can be no assurance that any pending or future regulatory or agency proceedings, investigations, inspections and audits will not result in substantial costs or a diversion of management’s attention and resources, negatively impact our future growth plans and opportunities or have a material adverse impact on our business, financial condition and results of operations.
IfThough the Company exited its U.S. operations in 2023, if the Company’s previous U.S. hemp business activities are found to be in violation of any of U.S. federal, state or local laws or any other governmental regulations, in addition to the items described above:
•above, the Company may be subject to “Warning Letters,” fines, penalties, administrative sanctions, settlements, injunctions, product recalls and/or other enforcement actions arising from civil, administrative or other proceedings initiated that could adversely affect the Company’s business, financial condition, operatingand results liquidity, cash flow and operational performance;
•the profits or revenues derived therefrom could be subject to money laundering statutes, including the Money Laundering Control Act, which could result in significant disruption to our U.S. hemp businessof operations and involve significant costs, expenses or other penalties; and
•the Company’s suppliers and service providers and distributors may elect, at any time, tocould breach, terminate or otherwise cease to participate in supply, service or distribution agreements, or other relationships, on which the Company’s operations rely.do business with us.
As it relates to U.S. Schedule I cannabis, in the U.S., despite cannabis possession and use having been legalized at the state level for medical use in many states and for adult-use in a number of states, marijuana as defined by the CSA continues to be categorized as a Schedule I controlled substance under the CSA and subject to the Controlled Substances Import and Export Act (“CSIEA”). Although we do not engage in any activities related to marijuana as defined by the CSA in the U.S., violations of any U.S. federal laws and regulations, including the CSA and the CSIEA, whether intentional or inadvertent, could result in civil, criminal and/or administrative enforcement actions, which could result in fines, penalties, and other sanctions, including but not limited to, cessation of business activities. Additionally, U.S. border officials could deny entry into the U.S. to those employed at or investing in legal and licensed non-U.S. cannabis companies and such persons could face detention, denial of entry or lifetime bans from the U.S. for their business associations with cannabis businesses.
We and our joint ventures and strategic investments are reliant on required licenses, authorizations, approvals and permits for our ability to grow, process, store and sell cannabis, and cannabinoids which are subject to ongoing compliance, reporting and renewal requirements, and we may also be required to obtain additional licenses, authorizations, approvals and permits in connection with our business.
Our ability to grow, process, store and sell cannabis in Canada is dependent on our licenses from Health Canada, and in particular the licenses currently held by Peace Naturals.Naturals, Cronos Fermentation and Cronos GrowCo. Failure to comply with the requirements of the licenses or failure to maintain the licenses would have a material adverse impact on our business, financial condition and results of operations. Although we believe Peace Naturals, Cronos Fermentation and Cronos GrowCo will meet the requirements of the Cannabis Act for extension of its license,their licenses, there can be no guarantee that Health Canada will extend or renew the licenses or, if they are extended or renewed, that they will be extended or renewed on the same or similar terms or that Health Canada will not revoke the licenses. Should we fail to comply with requirements of the licenses, should Health Canada not extend or renew the licenses, should we renew the licensesthey be renewed on different terms (including not allowing for anticipated capacity increases) or should the licenses be revoked or suspended, our business, financial condition and results of the operations will be materially adversely affected.
Our ability to grow, process, store and sell cannabis in Israel is dependent on maintaining our cannabis cultivation, production and productiondistribution licenses and our ability to export products to, or import products from, Cronos Israel is also dependent on obtaining the relevant export permits. Cronos GrowCo’s ability to grow, process, store and sell cannabis at its cannabisproduction facility in Kingsville, Ontario depends on obtaining and maintaining the appropriate licenses from Health Canada. Natuera’s ability to grow, process, store and sell cannabis in Colombia is dependent on obtaining and maintaining and being granted the appropriate licenses from the Ministry of Health and Social Security and Natuera’s ability to export products from Colombia is dependent on its ability to obtain the relevant export permits. Should we or our joint ventures fail to comply with the requirements of the licenses, or should they not be extended or renewed by the applicable regulatory authorities, or should they be renewed on different terms (including not allowing for anticipated capacity increases) or should the licenses be revoked, the business, financial condition and results of our and our joint ventures’ operations will be materially adversely affected. There is no assurance that we or our joint ventures will be able to obtain additionalnecessary permits or licenses on commercially reasonable terms or within expected time periods, if at all. Moreover, the pending sale-leaseback transaction of, and the change in the nature of operations at, the Peace Naturals Campus will require approval from Health Canada for changes to our site perimeter on the Peace Naturals Campus and will increase the importance of the licenses of Cronos GrowCo for our business and operations. We cannot provide any assurance that the required approval will be obtained from Health Canada on commercially reasonable terms or within expected time periods, if at all. Additionally, given Peace Naturals will no longer own the Peace Naturals Campus following the completion of the pending sale-leaseback transaction, if Future Farmco’s assistance is necessary to comply with the requirements of our Health Canada licenses, there can be no assurance that Future Farmco will assist us on commercially reasonable terms or at all, which could result in the revocation or suspension of such licenses.
In addition, Ginkgo’s ability to conduct certain R&D activities in the U.S. under the Ginkgo Collaboration Agreement is conditional on Ginkgo continuing to maintain all necessary licenses, permits and approvals required for Ginkgo to perform such R&D activities. There are no assurances that Ginkgo will be able to maintain required licenses, permits and approvals and, to the extent such licenses, permits and approvals are not maintained, we may not realize the expected benefits of the Ginkgo Strategic Partnership.
We may also be required to obtain and maintain certain permits, licenses and approvals in the jurisdictions where we source, process, or sell products derived from U.S. hemp. We may be unable to obtain or maintain any necessary licenses, permits or approvals. Additional government licenses are currently, and in the future may be required in connection with our operations, in addition to other unknown permits and approvals which may be required, including with respect to our other Rest of World operations.required. To the extent such permits, and approvals are required and not obtained, we may be prevented from operating and/or expanding our business, which could have a material adverse effect on our business, financial condition and results of operations.
Changes in the laws, regulations and guidelines governing cannabis and U.S. hemp may adversely impact our business.
Our current operations are and have been subject to various laws, regulations and guidelines promulgated by governmental authorities (including, in Canada, Health Canada and other federal, provincial and local regulatory agencies and, in the U.S., the FDA, the USDA, CDPH, DEA, FTC and PTO)PTO and other federal and state agencies) relating to the cultivation, processing, marketing, acquisition, manufacture, packaging/labeling, management, transportation, distribution, import, export, storage, sale and disposal of cannabis or U.S. hemp but also including laws and regulations relating to health and safety, the conduct of operations and the protection of the environment. Additionally, our growth strategy continues to evolve as regulations governing the cannabis industry in the jurisdictions other than Canada and the U.S. in which we and our joint ventures operate become more fully developed. Interpretation of these laws, rules and regulations and their application to our operations and those of our joint ventures is ongoing. No assurance can be given that new laws, regulations and guidelines will not be enacted or that existing laws, regulations and guidelines will not be amended, repealed or interpreted or applied in a manner which could require extensive changes to our operations, increase compliance costs, give rise to material liabilities or a revocation of our licenses and other permits, restrict the growth opportunities that we currently anticipate or otherwise limit or curtail our operations. For example, the Cannabis Act requires the Canadian federal government to conduct a review of the Cannabis Act beginningafter three years, which commenced in October 2021. ThisSeptember 2022. The scope of this statutory review is required to include,includes, among other things, consideration of (i) the administration and operation of the Cannabis Act, (ii) the impact of the Cannabis Act on public health, (iii) the health and consumption habits of young persons, (iv) the impact of cannabis on Indigenous persons and communities and (v) the impact of cultivation of cannabis plants in a dwelling-house. This report resulting from the statutory review may recommend and/or lead to the amendment, removal or addition of provisions in or to the Cannabis Act which could adversely affect our business. Amendments to current laws, regulations and guidelines governing the production, sale and use of cannabis and cannabis-based products, more stringent implementation or enforcement thereof or other unanticipated events, including changes in political regimes or political instability, currency controls, fluctuations in currency exchange rates and rates of inflation, labor unrest, changes in taxation laws, regulations and policies, restrictions on foreign exchange and repatriation, governmental regulations relating to foreign investment and the cannabis business more generally, and changes in attitudes toward cannabis, are beyond our control and could require extensive changes to our operations, which in turn may result in a material adverse effect on our business, financial condition and results of operations.
While the production of cannabis in Canada, among other things, is under the regulatory oversight of the federal government of Canada, the distribution and retail sale of adult-use cannabis in Canada falls within the jurisdiction of the provincial and territorial governments. The impact of the legislation regulating adult-use cannabis passed in the provinces and territories on the cannabis industry and our business plans and operations is uncertain. Provinces and territories have announced certain restrictions that are more stringent than the federal rules or regulations such as retail sale and marketing restrictions, bans on certain types of cannabis edibles,products, raising minimum age of purchase and flavor restrictions. For example, Québec Newfoundland and Labrador and Prince Edward Island do not currently permit sales of cannabis vaporizers, and Québec limits the sale of other high THC non-edible cannabis products. In April 2023, the Supreme Court of Canada affirmed the provinces’ power to enact regulations that are more restrictive than the federal regime. In addition, the distribution and retail channels and applicable rules and regulations in the provinces continue to evolve, and our ability to distribute and retail cannabis and cannabis products in Canada is dependent on the ability of the provinces and territories of Canada to establish licensed retail networks and outlets. In response to the COVID-19 pandemic, various provinces and territories have introduced a variety of regulatory changes to their respective cannabis regimes, which include in certain jurisdictions, forced store closures, restrictions or bans on in-store shopping experiences, and the authorization of private delivery services. There is no guarantee that the applicable legislation regulating the distribution and sale of cannabis for adult-use purposes including as amended to respond to the COVID-19 pandemic, will allow for the growth opportunities we currently anticipate.anticipate and may result in a material adverse effect on our business, financial condition and results of operations.
9 cannabinoids which can be revised as new evidence becomes available. This guidance recommends that license holders apply the regulatory controls (including limits on the amount of cannabinoids in certain products) currently applicable to delta-9-THC to all other cannabinoids that Health Canada considers to be “intoxicating cannabinoids” in order to minimize the risks of accidental consumption, overconsumption and adverse effects. This guidance comes at a time when various provincial regulators (such as those in Ontario, British Columbia and Alberta) are actively evaluating whether to permit the sale of or how to evaluate limits on the levels of certain cannabinoids (such as tetrahydrocannabivarin and cannabinol). Provincial and territorial distributors may take different positions on the sale and distribution of products with various cannabinoids and may decide to ban, limit or implement new guidance on the types of cannabis products permitted for sale in each of their jurisdictions (including in response to Health Canada’s guidance on intoxicating cannabinoids) which may result in some or all of our products being viewed as non-compliant with law or non-binding policy guidance. Furthermore, additional countries continue to pass laws that allow forwith respect to the production and distribution of cannabis in some form or another. We have subsidiaries, investments, joint ventures and strategic alliances in place outside of the U.S. and Canada, which may be affected if more countries legalize cannabis. Increased international competition and limitations placed on us by Canadian regulations might lower the demand for our products on a global scale. We also face competition in each jurisdiction outside of the U.S. and Canada where we have subsidiaries, investments, joint ventures and strategic alliances with local companies that have more experience, more in-depth knowledge of local markets or applicable laws, regulations and guidelines or longer operating histories in such jurisdictions.
We are subject to certain restrictions of the TSX and Nasdaq, which may constrain our ability to expand our business internationally.
Our common shares are listed on the TSX and Nasdaq. We must comply with the TSX and Nasdaq requirements or guidelines when conducting business.
On October 16, 2017, theThe TSX has provided clarity regarding the application of Section 306 (Minimum Listing Requirements), Section 325 (Management) and Part VII (Halting of Trading, Suspension and Delisting of Securities) of the TSX Company Manual (collectively, the “Requirements”) to TSX-listed issuers with business activities in the cannabis sector. In TSX Staff Notice 2017- 0009, the TSX notes that issuers with ongoing business activities that violate U.S. federal law regarding U.S. Schedule I cannabis are not in compliance with the Requirements. The TSX reminded issuers that, among other things, should the TSX find that a listed issuer is engaging in activities contrary to the Requirements, the TSX has the discretion to initiate a delisting review. Although we do not conduct any operations in the U.S. with respect to U.S. Schedule I cannabis, failure to comply with the Requirements could result in a delisting of our common shares from the TSX or the denial of an application for certain approvals, such as to have additional securities listed on the TSX, which could have a material adverse effect on the trading price of our common shares and have a material adverse effect on our business, financial condition and results of operations.shares.
While Nasdaq has not issued official rules specific to the cannabis or U.S. hemp industry, stock exchanges in the U.S., including Nasdaq, have historically refused to list certain U.S. Schedule I cannabis related businesses, including U.S. Schedule I cannabis retailers, that operate primarily in the U.S. Failure to comply with any requirements imposed by Nasdaq could result in the delisting of our common shares from Nasdaq or denial of any application to have additional securities listed on Nasdaq which could have a material adverse effect on the trading price of our common shares.
We are constrained by law in our ability to market and advertise our products.
Our marketing and advertising are subject to regulation by various regulatory bodies in the jurisdictions we operate. In Canada, the development of our business and related results of operations may be hindered by applicable regulatory restrictions on sales and marketing activities. For example, the regulatory environment in Canada limits our ability to compete for market share in a manner similar to other industries. Furthermore, the applicable regulatory restrictions on sales and marketing activities are not always clear, may be subject to interpretation and have in the past, and may in the future, be interpreted or applied inconsistently by the applicable Canadian regulatory agencies, which have broad interpretative and enforcement discretion with respect to such activities. This may result in such restrictions on sales and marketing activities being interpreted unfavorably by a regulatory agency against some market participants, including us, but not others. Furthermore, if our competitors fail to comply with applicable laws relating to sales and marketing activities with which we comply, and regulatory agencies delay or do not take enforcement action against such competitors, or take sporadic enforcement action, our ability to compete for market share and our sales and results of operations could be adversely affected. If we are unable to effectively market our products and compete for market share in Canada, or if the costs of compliance with government legislation and regulation cannot be absorbed through increased selling prices for our products, our sales and results of operations could be adversely affected. See “Business –Regulatory Framework in Canada.”
In the U.S., our advertising is subject to regulation by the FTC under the Federal Trade Commission Actas well as the FDA under the Federal Food, Drug, and Cosmetic Act, including as amended by the Dietary Supplement Health and Education Act of 1994, and by state agencies under analogous and similar state and local laws. In recent years, the FTC, the FDA and state agencies have initiated numerous investigations of food and dietary supplement products both because of their CBD or cannabinoid content and based on allegedly deceptive or misleading marketing claims and have, on occasion, issued “Warning Letters” or instituted enforcement actions due to such claims. Some U.S. states also permit content, advertising and labeling laws and regulations to be enforced by state attorneys general, who may seek civil and criminal penalties, relief for consumers, class action certifications, class wide damages and recalls of products sold by us. There has also been a recentan increase in private litigation that seeks, among other things, relief for consumers, class action certifications, class wide damages and recalls of products. We have been subject to such litigation and may be subject to additional private class action litigation. Any actions against us by governmental authorities or private litigants could have a material and adverse effect on our business, financial condition, operating results, liquidity, cash flow and operational performance.
Risks RelatedRelating to U.S. Regulation and Compliance
We are subject to uncertainty regarding the legal and regulatory status of U.S. hemp, including with respect to U.S. federal and state implementation of the 2018 Farm Bill and related laws and regulations, including the Federal Food, Drug, and Cosmetic Act,FFDCA, and the interpretation or application of or changes to, such laws and regulations may have material and adverse effects on our business, financial condition, operatingand results liquidity, cash flow and operational performance.
In 2014, U.S. Congress passed the 2014 Farm Bill, which permitted the domestic cultivation of “industrial hemp” (defined as the plant Cannabis sativa L. and any part of such plant, whether growing or not, with no more than 0.3% THC on a dry weight basis) as part of agricultural pilot programs adopted by individual states for the purposes of research by state departments of agriculture and institutions of higher education. There is significant uncertainty concerning the permissible scope of commercial activity under the 2014 Farm Bill. The 2014 Farm Bill only authorized institutions of higher education and state agricultural departments to cultivate industrial hemp, and only to do so for research purposes. However, it also gave significant discretion to states to regulate industrial hemp pilot programs. Many states that have adopted pilot programs have licensed private companies to cultivate and process industrial hemp. Additionally, many states have interpreted the 2014 Farm Bill to permit research concerning industrial hemp through, among other things, commercial marketing and sale of industrial hemp and industrial hemp products. In contrast, the DEA, FDA and the USDA have taken the position that, under the 2014 Farm Bill, industrial hemp products may not be sold for the purpose of general commercial activity or in states without agricultural pilot programs that permit their sale for research marketing purposes; these agencies have also taken the position that, under the 2014 Farm Bill, industrial hemp plants and seeds may not be transported across state lines.operations.
On December 20, 2018, the 2018 Farm Bill was signed into law. The 2018 Farm Bill, among other things, removes “hemp” (which we refer to as “U.S. hemp” in this Annual Report, defined as the plant Cannabis sativa L. and any part of that plant, including the seeds thereof and all derivatives, extracts, cannabinoids, isomers, acids, salts, and salts of isomers, whether growing or not, with a THC concentration of not more than 0.3% on a dry weight basis and its derivatives) from the U.S. federal Controlled Substances Act and amends the Agricultural Marketing Act of 1946 to permit the production and sale of U.S. hemp in the U.S. The 2018 Farm Bill tasks the USDA with promulgating regulations in relation to the cultivation and production of U.S. hemp. The 2018 Farm Bill also directs the USDA to promulgate federal regulations that would apply to the production of U.S. hemp in every state which does not put forth a state U.S. hemp plan for approval by the USDA.certain uses under certain conditions. The USDA issued a final rule in January 2021 that becomes effective March 2021. Various states are in the process of applying to the USDA for approval of their U.S. hemp production regulations which impose different levels of regulation and costs on the production of U.S. hemp, and certain state plans have been approved by the USDA. The USDA’s recently issued final rule, requires hemp producers to use a laboratory that is registered with the DEA for analytical testing of hemp plants; however, the USDA is delaying enforcement of this requirement until December 31, 2022. The final rule does provide some flexibility for producers to dispose or remediate non-compliant plants, subject to additional documentation requirements, without requiring the use of a DEA-registered reverse distributor or law enforcement to dispose of non-compliant plants. Moreover, the 2018 Farm Bill provides that its provisions do not preempt or limit state laws that regulate the production of U.S. hemp. Accordingly, some states may choose to restrict or prohibit some or all U.S. hemp production or sales within the state, and variances in states’ laws and regulations on U.S. hemp are likely to persist. Further, each state has discretion to develop and implement its own laws and regulations governing the manufacturing, marketing, labeling, and sale of U.S. hemp products, which has created a patchwork of different regulatory schemes applicable to such products. To the extent a farm bill enacted in the future changes the definition of “hemp” or the regulation thereof, including product format or type, our ability to re-enter the U.S. market and launch competitive U.S. hemp products could be negatively impacted.
The FDA or particular states may ultimately prohibit the sale of some or all dietary supplements or conventional foods containing U.S. hemp and U.S. hemp-derived ingredients, including CBD and other cannabinoids, and we may be required to submit a New Dietary Ingredient notification to the FDA, which may not be accepted without objection.
Under the 2018 Farm Bill, the FDA has retained authority over the Federal Food, Drug, and Cosmetic Act-regulatedFFDCA-regulated products (e.g., drugs (human and animal), food (human and animal), dietary supplements and cosmetics) containing U.S. hemp and U.S. hemp-derived ingredients, including CBD. The FDA has consistently taken the position that CBD, whether derived from U.S. hemp or U.S. Schedule I cannabis, is prohibited from use as an ingredient in food and dietary supplements. This stems from its interpretation of the exclusionary clauses in the Federal Food, Drug, and Cosmetic ActFFDCA because CBD is the active ingredient in a drug that has been approved as a prescription drug and is the subject of substantial clinical investigations as a drug, which have been made public. The exclusionary clauses under the Federal Food, Drug, and Cosmetic ActFFDCA provide that a substance that has been approved or has been subject to substantial clinical investigations as a drug may not be used in a food or dietary supplement, unless the substance was first marketed in a food or dietary supplement prior to the initiation of substantial clinical investigations of the substance as a drug.
TheTo date, the FDA has not issued regulations that elaborate on the exclusionary clauses, and the FDA has not taken any enforcement action in the courts asserting a violation of the exclusionary clauses due to the marketing of U.S. hemp, U.S. hemp extracts, CBD or CBD.other cannabinoids. Additionally, on January 26, 2023, the FDA stated its views publicly that a new regulatory pathway for CBD is needed and it is prepared to work with Congress to create such a pathway. To date, the FDA has issued several “Warning Letters” to companies unlawfully marketing CBD products. In many of these cases, the manufacturer made unsubstantiated claims about the product being able to treat medical conditions (e.g., cancer, Alzheimer’s disease, opioid withdrawal, anxiety and COVID-19) and had not obtained drug approvals. Some of these letters were co-signed with the FTC and cited the companies for making claims about the efficacy of CBD or other ingredients which were not substantiated by competent and reliable scientific evidence. In December 2020, the FTC announced it had entered into settlement agreements with six companies marketing CBD products including oils, gummies, creams, and others with deceptive health claims about serious health conditions. The settlements included monetary penalties ranging from $20,000 to $85,000. The FDA has also issued a “Warning Letter” to at least one dietary supplement manufacturer for a number of violations observed during an inspection, including manufacturing CBD supplements in a licensed facility. In November 2022, the FDA issued “Warning Letters” to five additional companies selling CBD-products in forms that the FDA asserted are appealing to children, including gummies, hard candies and cookies. And in December 2023, the FDA issued a “Warning Letter” that stated that the FDA considers neither delta-8 tetrahydrocannabinol nor CBD to be “generally recognized as safe” (GRAS) food additives. These letters also outlined additional violations of the FFDCA including that several of the companies made claims that CBD-containing products cure, mitigate, treat or prevent various diseases or were added to animal foods.
Until the FDA formally adopts regulations with respect to CBD or other U.S. hemp-derived cannabinoid products or announces an official position with respect to CBD or other U.S. hemp-derived cannabinoid products, there is a risk that the FDA could take enforcement action (e.g., a “Warning Letter,” seizure, or injunction) against the Company’sCompany in respect of its U.S. hemp-derived CBD products sold in the U.S.
Moreover, states have retained regulatory authority through their own analogues to the Federal Food, Drug, and Cosmetic Act,FFDCA, and the states may diverge from the federal treatment of the use of U.S. hemp as, or in, food, dietary supplements or cosmetic products. The FDA or applicable states (under their CSA or Federal Food, Drug, and Cosmetic Act analogues) may ultimately not permit the sale
Even if the exclusionary clause issue discussed above is resolved in a manner favorable to us and we decide to re-enter the U.S. hemp market, we could be required to submit a New Dietary Ingredient Notification (“NDIN”)NDIN to the FDA with respect to U.S. hemp-derived ingredients, including CBD and other cannabinoids we intend to include in our products, used in dietary supplement products. This could depend on whether we can establish that a particular ingredient was marketed as a dietary ingredient in a dietary supplement prior to October 15, 1994, or is otherwise currently in the food supply in the same chemical form as used in our dietary supplement products. If the FDA objects to oursuch an NDIN, notification, this could prevent us from producing, marketing and selling ingestible U.S. hemp products which would have a material adverse impact onproducts. Such an NDIN submitted by one of our business, financial condition and results of operations.
The FDA or particular U.S. states may seekcompetitors was objected to regulate our cosmetic products containing U.S. hemp-derived ingredients, including CBD, as drugs, medical devices, or drug-device combination products.
The FDA may seek to regulate our cosmetic products containing U.S. hemp-derived ingredients, including CBD, under its authorities for medical products (i.e., drugs, medical devices, or drug-device combination products). Specifically, the agency could assert that our lotions, oils, balms and creams are intended for use in diagnosing, treating, mitigating or preventing disease or for use in affecting the structure or any function of the body. In making classification decisions, the agency considers a wide variety of factors to determine a product’s intended use; indeed,by the FDA has sometimes asserted that a product qualifies as a drug based solely on the presence of an ingredient widely understood to have drug effects, even in the absence of express claims about them. Though we do not market our lotions, oils, balms and creams as drugs for use in the treatment of diseases or their symptoms, the FDA could still assert that the products are intended for use as drugs, including based on the understood or presumed physical effects of topically administered cannabinoids. Thus, we may not have the ability to successfully respond to such allegations simply by modifying labeling or advertising claims. Ultimately, if the FDA asserts one of its medical product authorities over our lotion, oil, balm and cream products, and we cannot or elect not to comply with the onerous regulatory requirements applicable to the asserted medical product category (e.g., drug), we could be prevented from producing, marketing and selling cosmetic products containing U.S. hemp-derived ingredients, including CBD. In addition, states may similarly seek to regulate our cosmetic products containing U.S. hemp-derived ingredients, including CBD, as medical products (i.e., drugs, medical devices, or drug-device combination products) under state analogues to the Federal Food, Drug, and Cosmetic Act or otherwise. States have also considered and established additional restrictions on, or requirements for, the marketing of cosmetic products containing U.S. hemp-derived ingredients. If states assert their medical product authorities over our cosmetic products containing U.S. hemp-derived ingredients, including CBD, in a manner that we cannot address simply by modifying labeling or advertising claims, and we cannot or elect not to comply with the onerous regulatory requirements applicable to the asserted medical product category (e.g., drug), we could be prevented from producing, marketing and selling cosmetic products containing U.S. hemp-derived ingredients, including CBD. Likewise, if states enforce or adopt regulatory interpretations or restrictions that limit our ability to market our cosmetic products containing U.S. hemp-derived ingredients, including CBD, in such states, it could materially and adversely affect our business, financial condition, operating results, liquidity, cash flow and operational performance.August 2021.
The DEA could take enforcement action against us or other participants in the U.S. Schedule I cannabis or U.S. hemp industry.Any rescheduling of U.S. Schedule I cannabis to Schedule III would have an uncertain impact on our business.
There is substantial uncertainty concerning the legal status of U.S. hemp and U.S. hemp products containing U.S. hemp-derived ingredients, including CBD.CBD and other cannabinoids. The status of products derived from the cannabis or hemp plant, under both federal and state law can depend on the THC content of the plant or derivative (including whether the plant meets the statutory definition of “industrial hemp” or “hemp”), the part of the plant from which an individual or entity produces the derivative (including whether the plant meets the statutory definition of “marihuana” under the CSA), the THC concentration during the manufacturing process, whether the cultivator, processor, manufacturer or product marketer engages in cannabis-related activities for research versus purely commercial purposes, as well as the form and intended use of the product. The mere presence of a cannabinoid (such as CBD) is not dispositive as to whether the product is legal or illegal. Under U.S. federal law, products containing CBD may be unlawful if derived from U.S. Schedule I cannabis (including hemp with a concentration greater than 0.3% THC on a dry weight basis), or if derived from U.S. hemp grown outside the parameters of an approved U.S. hemp pilot program or U.S. hemp cultivated in violation of the 2018 Farm Bill. Even after enactment of the 2018 Farm Bill, the DEA may not treat all products containing U.S. hemp-derived ingredients, including CBD and other cannabinoids, as exempt from the CSA. In September 2020, the DEA issued an interim final rule that purported to align the DEA’s regulations with the statutory changes to the CSA made effective by the 2018 Farm Bill. The DEA received a number of comments objecting to the interim final rule, and the interim final rule ishas been the subject of ongoing litigation. However, the litigation was dismissed by the D.C. Circuit Court in June 2022. If the DEA takes action against us or other participants in the U.S. hemp industry, this could have a material and adverse effect on our business, financial condition operating results, liquidity, cash flow and operational performance.
Risks Relating to COVID-19
Our business and results of operations have been adversely affected and will likely continue to be materially adversely impacted by the coronavirus pandemic (COVID-19).
The COVID-19 pandemic has severely restricted the level of economic activity around the world and in all countries in which we or our affiliates, investments and joint ventures operate (including the U.S., Canada, Australia, Colombia, and Israel). In response to the COVID-19 pandemic the governments of many countries, states, cities and other geographic regions have taken preventative or protective actions, such as imposing restrictions on travel and business operations, ordering temporary closures of businesses and advising or requiring individuals to limit or forego their time outside of their homes. Numerous businesses have temporarily closed voluntarily or closed permanently. Although some preventative or protective actions have been eased or lifted in varying degrees by different governments of various countries, states and cities, the continued spread of COVID-19 and increase in infection rates have caused, and may continue to cause, some jurisdictions to reinstitute such actions, including rolling back reopening plans. The duration and ultimate impact of the COVID-19 pandemic, including the extent to which the virus may re-emerge following the current outbreak, are highly uncertain.
The effects of the COVID-19 pandemic had a material impact on the growth of revenues and sales during the fiscal year ended December 31, 2020 related to the Company’s U.S. segment. With segments of the U.S. economy currently in an economic contraction, and a significant number of the Company’s customers’ stores continuing to be challenged by remaining temporarily closed or closing permanently, the U.S. segment is experiencing slower than expected revenue growth. The prolonged closures of retail stores as well as the changes in consumer purchasing during the COVID-19 pandemic have adversely affected our financial results. We anticipate further adverse effects on our financial results so long as the measures implemented to combat the COVID-19 pandemic stay in effect.
The effect of the COVID-19 pandemic could include closures of our facilities or the facilities of our suppliers and other vendors in our supply chain and other preventive and protective measures in our supply chain. For example, as a result of the COVID-19 pandemic, closures of manufacturers in China in early 2020 resulted in delays of deliveries of batteries and cartridges for our cannabis vaporizers and personal protective equipment, such as masks and gowns used in our GMP manufacturing processes, from such manufacturers in China. In addition, as a result of a rise in infection rates late in the fourth quarter of 2020 and in the first quarter of 2021 in the U.S., certain contract manufacturers that manufacture U.S. hemp finished products for our U.S. business have experienced temporary closures or reductions in their operations leading to shortages of finished product available via the e-commerce channel. We expect these closures or reductions to ease and product shortages to be ameliorated as the spread of COVID-19 and infection rates decline. If the pandemic persists, closures or other restrictions on the conduct of business operations of our third-party manufacturers, suppliers or vendors could disrupt our supply chain. We have experienced minor delays in shipping and the increased global demand on shipping and transport services, in addition to customs and border control policies put in place in response to COVID-19 that require shipments to undergo quarantine periods, may cause us to experience delays or increased costs in the future which could impact our ability to obtain materials or deliver our products in a timely manner, could otherwise disrupt our operations and could have an adverse effect on our business, financial condition and results of operations.
In various provinces in Canada, cannabis retailers have been reducing opening hours, staff onsiteAugust 2023, the U.S. Department of Health and Human Services (“HHS”) recommended that the DEA move marijuana from Schedule I to Schedule III under the CSA. There can be no assurance that the DEA will ultimately adopt HHS’s recommendation and the numberimpacts of customers allowed in-store for cannabis retailers that continueany such adoption on our business and competitive position are unclear. For example, rescheduling marijuana from Schedule I to Schedule III may be open. Further, retailers of our productsaccompanied by additional regulatory obligations as prerequisite to participate in the U.S. market, and Canada have in some cases determined to, andit may in other cases be required to close or choose to suspend or significantly curtail their operations due to health and safety concerns for their employees. Even if our production facilities remain open, mandatory or voluntary self-quarantines and travel restrictions may limit our employees’ ability to get to our facilities, and this, together with impacts on our supply chain and the uncertainty produced by the rapidly evolving nature of the COVID-19 pandemic, may result in reduced or suspended production. Those type of restrictions could also impact the abilities of customers in the U.S. or certain Canadian provinces to continue to have access to our products. Quarantines, shelter-in-place and similar government orders, or the perception that such orders, shutdowns or other restrictions on the conduct of business operations could occur, could impact personnel at third-party manufacturing facilities in the U.S. and Canada and other countries, or the availability or cost of materials, which would disrupt our supply chain, in particular in relation to our supply of masks, gowns and other protective equipment used at our GMP facilities dueprovide a greater benefit to the global shortage of such protective equipment and materials. As a result of COVID-19, we have implemented work-from-home policies for certain employees and the effectsbusinesses of our work-from-home policies may negatively impact productivity, disrupt access to books and records, increase cybersecurity risks and the risk of inadvertent disclosure of confidential information and disrupt our business. In addition, the effects of the COVID-19 pandemic may delay our R&D programs and our ability to execute on certain of our new product launches, line extensions and strategic plans involving construction or the receipt and installation of new equipment.
The global impact of the COVID-19 pandemic continues to evolve rapidly, and the extent of its effect on our operational and financial performance will depend on future developments, which are highly uncertain, including the duration, scope and severity of the pandemic, the actions taken to contain or mitigate its impact, and the direct and indirect economic effects of the pandemic and related containment measures, among others. Even after the COVID-19 pandemic subsides, our businesses could also be negatively impacted should the effects of the COVID-19 pandemic lead to changes in consumer behavior, including as a result of a decline in the level of vaping or demand for inhalable products in light of certain recent published articles and studies on the potential increased susceptibility of individuals who smoke or vaporize nicotine or cannabis to COVID-19, in light of changes in consumer behavior such as reduced spending on certain product formats historically used in shared experiences such as pre-rolls or in reductions in discretionary spending. In addition, a severe or prolonged recession resulting from the COVID-19 pandemic would likely materially affectcompetitors than our business, and the valueincluding by providing favorable tax treatment to their U.S. operations. The rescheduling of our common shares.
We have been and may in the future be requiredmarijuana from Schedule I to write down intangible assets, including goodwill, due to impairment, which could have a material adverse effect on our results of operations or financial position.
The Company has been and may in the future be required to write down intangible assets, including goodwill, due to impairment, which would reduce earnings. We periodically calculate the fair value of our reporting units and intangible assets to test for impairment. This calculation may be affected by several factors, including general economic conditions, regulatory developments, changes in category growth rates as a result of changing adult consumer preferences, success of planned new product introductions, and competitive activity. Certain events can also trigger an immediate review of goodwill and intangible assets. If the carrying value of our reporting unit and other intangible assets exceed their fair value, the goodwill and other intangible assets are considered impaired, which would result in impairment losses and could have a material adverse effect on our consolidated financial position or results of operations. We cannot provide any assurance that the U.S. segment will successfully execute its business plans and strategies.
The ongoing impact of the COVID-19 pandemic on the Company’s operating results for the U.S. segment has been difficult, if not impossible to predict. The longer than anticipated closures of retail stores have resulted in slower than anticipated revenue growth. In the second quarter of 2020, we performed an interim impairment test and the Company incurred $35 million of impairment charges on the U.S. reporting unit and $5 million on the Lord Jones™ brand.
It is possible that estimates in the Company’s financial statements will continue to change in the near-term as a result of the COVID-19 pandemic and the effect of any such changes could be material, whichSchedule III could result in among other things, further impairmentsignificant volatility in the market for our common stock. To the extent that market speculation results in an increase in the price of goodwill and intangible assets.our stock, our stock price could decline significantly thereafter if the DEA fails to act on the recommendation or investor optimism fades.
Risks Relating to Competition
The markets in which we operate are increasingly competitive, and we may compete for market share with other companies, both domestically and internationally, that may have longer operating histories and more financial resources, manufacturing and marketing experience than us.
The marketsmarket for cannabis and U.S. hemp areis competitive and evolving and we face strong competition from both existing and emerging companies that offer similar products. Some of our current and potential competitors may have longer operating histories, greater financial, marketing and other resources and larger customer bases than us.we have. In addition, there is potential that the cannabis and U.S. hemp industriesindustry will undergo consolidation, creating larger companies with financial resources, manufacturing and marketing capabilities and product offerings that are greater than ours. As a result of this competition, we may be unable to maintain our operations or develop them as currently proposed on terms we consider acceptable, or at all. Increased competition byfrom larger, better-financed competitors with geographic advantages could materially and adversely affect our business, financial condition and results of operations.
Given the rapid changes affecting global, national and regional economies generally, and the U.S. hemp industry in particular, we may not be able to create and maintain a competitive advantage in the marketplace. Our success will depend on our ability to respond to, among other things, changes in the economy, regulatory conditions, market conditions and competitive pressures. Any failure by us to anticipate or respond adequately to such changes could have a material and adverse effect on our business, financial condition, operating results, liquidity, cash flow and operational performance.
In Canada, the number of licenses granted by Health Canada could also have an impact on our operations. We expect to face additional competition from new market entrants that are granted licenses under the Cannabis Act or existing license holders which are not yet active in the industry. If a significant number of new licenses are granted by Health Canada in the near term, we may experience increased competition for market share and may experience downward price pressure on our products as new entrants increase production. If the number of users of cannabis in Canada increases, the demand for products will increase and we expect that competition will become more intense, as current and future competitors begin to offer an increasing number of diversified products. To remain competitive, we will require a continued high level of investment in R&D, sales and customer support. We may not have sufficient resources to maintain R&D, sales and customer support efforts on a competitive basis which could have a material adverse effect on our business, financial condition and results of operations. Furthermore, the Canadian federal authorization of home cultivation, outdoor grow, and the easing of other barriers to entry to the Canadian adult-use cannabis market, could materially and adversely affect our business, financial condition and results of operations.
In the U.S., the number of competitors in the U.S. hemp industry has increased significantly in recent years and is expected to continue to increase, which could negatively impact our market share and demand for our products. Additionally, if the U.S. takes steps to legalize U.S. Schedule I cannabis, the impact of such a development could result in new entrants into the market and increased levels of competition.
We face competition from the illegal cannabis market.
We face competition from illegal market operatorsparticipants that are unlicensed and unregulated. As these illegal market participants do not comply with the regulations governing the cannabis industry, their operations may also have significantly lower costs.costs and they may be able to sell products with significantly higher cannabinoid potencies or which include ingredients that are prohibited by law. The perpetuation of the illegal market for cannabis may have a material adverse effect on our business, results of operations, financial condition as well as the perception of cannabis use.
Regulatory non-compliance by licensed cannabis competitors may have an adverse effect on our business, results of operations and financial condition.
In addition to competition from illegal market participants, we may also face competition from licensed cannabis competitors that fail to comply with the regulations governing the cannabis industry when developing and selling cannabis products. These competitors may be able to produce and sell products with significantly higher cannabinoid potencies or which include ingredients that are prohibited by law. If regulatory authorities are delayed in, or fail to, effectively restrict the sale and distribution of such non-compliant cannabis products by our competitors, there may be a material adverse effect on our business, results of operations and financial condition, as well as the perception of cannabis use.
We have been and may in the future be required to write down inventory due to downward pressure on market prices, which could have a material adverse effect on our results of operations or financial position.
At the end of each reporting period, management performs an assessment of inventory obsolescence, prices and demand to measure inventory at the lower of cost and net realizable value. Net realizable value is defined as the estimated selling price in the ordinary course of business, less reasonably predictable costs of completion, disposal and transportation. We also consider factors such as slow-moving or non-marketable products in our determination of obsolescence. As a result of this assessment, inventory write-downs have occurred on a number of occasions in the past and may occur from period to period. Due to continuedin the future. Continued pricing pressures in the Canadian marketplace,markets in which we operate, may incurresult in further inventory write-downs in the future.write-downs. We have had a series of inventory write-downs due to price compression in the cannabis market. We expect these write-downs to continue as pricing pressures remain elevated. These inventory write-downs have in the past and may in the future materially adversely affect our results of operations and financial position.
We may be unable to attract or retain skilled labor and personnel with experience in the cannabis sector and may be unable to attract, develop and retain additional employees required for our operations and future developments.
We may be unable to attract or retain employees with sufficient experience in the cannabis industry, and may prove unable to attract, develop and retain additional employees required for our development and future success.
Our success is currently largely dependent on the performance of our skilled employees. Our future success depends on our continuing ability to attract, develop, motivate and retain highly qualified and skilled employees. Qualified individuals are in high demand, and we may incur significant costs to attract and retain them. In 2022, we announced a Realignment to, among other things, centralize functions under common leadership to increase efficient distribution of resources, optimize collaboration and strategic alignment and eliminate duplication of roles and costs, including a reduction in headcount impacting a number of employees. In 2023, we exited our U.S. operations, announced our intention to list the Cronos Fermentation facility for sale and entered into a transaction for the sale and leaseback of the Peace Naturals Campus. Any or all of the Realignment, the U.S. exit, the planned sale of the Cronos Fermentation facility and the pending sale-leaseback transaction could lead to increased attrition amongst those employees who were not directly affected by the associated reductions in headcount, and we may not be successful at retaining such employees or attracting new employees, which may have a material adverse effect on our business, results of operations and financial condition.
Further, certain shareholders, directors, officers, employees and employeescontractors in our Canadian operations may require security clearance from Health Canada.Canada or require analogous clearance by various provincial agencies. Under the Cannabis Act, a security clearance cannot be valid for more than five years and must be renewed before the expiry of a current security clearance. There is no assurance that any of our existing personnel who presently or may in the future require a security clearance will be able to obtain or renew such clearances or that new personnel who require a security clearance will be able to obtain one. A failure by an employeeany of our existing personnel to maintain or renew his or her security clearance may impair our business operations. In addition, if an employeeindividual with security
clearance leaves the service of the Company and we are unable to find a suitable replacement who has a security clearance required by the Cannabis Act in a timely manner, or at all, there could occur a material adverse effect on our business operations. Similar risks and potential effects apply to analogous security clearances required by various provincial agencies.
Risks Relating to the Altria Investment
Altria has significant influence over us following closing of the Altria Investment.
Altria is our single largest shareholder. As of December 31, 2020,2023, Altria beneficially owned approximately 43.5%41.1% of our issued and outstanding common shares (calculated on a non-diluted basis). In light of such ownership, Altria is in a position to exercise significant influence over matters affecting shareholders or requiring shareholder approval, including the election of the Board, amendments to our articles and the determination of significant corporate actions. In addition, pursuant to the Investor Rights Agreement, Altria has certain rights, including the right to nominate a specified number of directors to the Board, approval rights over certain Company actions and pre-emptive and top-up rights entitling Altria to maintain its pro rata beneficial ownership in us. Further, as of the date hereof, four of the seven directors on the Board are Altria Nominees. For more information, see “Business -AltriaBusiness—Altria Strategic Investment - Investment—Investor Rights Agreement.Agreement.”
Upon exercise of the Altria Warrant in full, assuming no other securities of ours are issued, Altria will beneficially hold in excess of a majority of the voting rights of the issued and outstanding common shares and would have the right to elect the entire Board and be able to exercise a controlling influence over our business and affairs, including the selection of our senior management, the acquisition or disposition of our assets, the payment of dividends and any change of control of us, such as a merger or take-over.
Accordingly, Altria currently has significant influence over us and has the ability to increase this influence at any time upon the exercise of the Altria Warrant.us. There can be no assurance that Altria’s interests will align with our interests or the interests of other shareholders. In addition, such influence could limit the price that an acquirer might be willing to pay in the future for our common shares and it may have the effect of delaying or preventing a change of control of us, such as a merger or take-over.
We have discretion in the use of net proceeds from the Altria Investment and may not use them effectively.
Under the Subscription Agreement, we have discretion in the use of net proceeds from the Altria Investment, subject to our obligation to consult with Altria, in certain circumstances, seek the approval of Altria (such approval not to be unreasonably conditioned, withheld or delayed) and certain other limitations regarding the use of net proceeds set forth in the Subscription Agreement. Accordingly, shareholders may not agree with the manner in which management chooses to allocate and spend the net proceeds. Our failure to apply the funds effectively could have a material adverse effect on our business, financial condition and results of operations.
We have cash on hand, including short-term investments, of approximately $1.3 billion$861 million as of December 31, 2020.2023. There can be no assurance that we will be able to deploy the available cash in an effective manner that is accretive to us, or at all. Until such time as we are able to deploy the cash available to us, we anticipate holding the net proceeds as cash balances in our bank account oraccounts, investing in certificates of deposit and other instruments issued by banks or obligations of or guaranteed by the Government of Canada or any province thereof, or investing in U.S. Treasury securities or other obligations issued or guaranteed by the U.S. Government, its agencies or instrumentalities. Based on the level of current interest rates, we will not earn any material revenue from such invested cash.
We may not realize the benefits of our strategic partnership with Altria, which could have an adverse effect on our business, financial condition and results of operations.
We believe that the strategic partnership between us and Altria provides us with additional financial resources, product development and commercialization capabilities, and deep regulatory expertise to better position us to compete, scale and lead the rapidly growing global cannabis industry. We believe that the growth opportunities for us are significant and could extend across the globe as new markets open. With Altria’s resources, we expect to be even better positioned to support cannabinoid innovation, create differentiated products and brands across medical and adult-use categories and expand our global footprint and growing production capacity. Nevertheless, a number of risks and uncertainties are associated with the expansion into such markets and the pursuit of these other growth opportunities. The successful implementation of the Altria Investment is critical to our growth and capital position. The failure to successfully implement or reap the anticipated benefits of Altria’s resources and expertise to realize growth and expansion opportunities could have a material adverse effect on our business, financial condition and results of operations.
Any common shares issued pursuant to the exercise of the Altria Warrant will dilute shareholders.
The Altria Warrant may be exercised in full or in part at any time on or prior to March 8, 2023, from time to time, and entitles the holder thereof, upon valid exercise in full thereof, to acquire, accept and receive from us an aggregate of 80,056,296 of our common shares (subject to adjustment in accordance with the terms of the Altria Warrant Certificate), which represents 10% of the issued and outstanding common shares as of December 31, 2020 (on a non-diluted basis). Any issuance of common shares pursuant to the exercise of the Altria Warrant would dilute all of our other shareholders and give Altria control of us.
Altria’s significant interest in us may impact the liquidity of theour common shares.
Our common shares may be less liquid and trade at a discount relative to the trading that could occur in circumstances where Altria did not have the ability to significantly influence or determine matters affecting us. Additionally, Altria’s significant voting interest in us may discourage transactions involving a change of control of us, including transactions in which an investor, as a shareholder, might otherwise receive a premium for its common shares over the then-current market price.
The change of control provisions in certain of our existing or future contractual arrangements may be triggered upon the exercise of the Altria Warrant in part or in full.
Certain of our existing or future contractual arrangements may include change of control provisions requiring us to make certain payments or triggering certain termination rights for our counterparties if the change of control trigger is fulfilled. The change of control provisions in certain of our existing arrangements, including, but not limited to, compensatory arrangements, or agreements we may enter into in the future, may be triggered upon the exercise of the Altria Warrant in part or in full.
Future sales of our common shares by Altria could cause the market price for our common shares to fall.
Sales of a substantial number of our common shares by Altria could occur at any time. Such sales, or the market perception of such sales, could significantly reduce the market price of our common shares. We cannot predict the effect, if any, that future public sales of our common shares beneficially owned by Altria or the availability of these common shares for sale will have on the market price of our common shares. If the market price of our common shares were to drop as a result, this might impede our ability to raise additional capital and might cause a significant decline in the value of the investments of our other shareholders.
The intentions of Altria regarding its long-term economic ownership of our common shares are subject to change as a result of changes in the circumstances of Altria or its affiliates, changes in our management and operation and changes in laws and regulations, market conditions and our financial performance.
Conflicts of interest may arise between us and our directors and officers, including as a result of the continuing involvement of certain of our directors with Altria and its affiliates.
We may be subject to various potential conflicts of interest because of the fact that some of our directors and officers may be engaged in a range of business activities, or have relationships with or are employed by Altria. One of our directors, Jason Adler, is the co-founder and Managing Member of Gotham Green Partners, a private equity firm focused primarily on early-stage investing in companies in the cannabis industry, and Michael Gorenstein, our Chairman, President, and Chief Executive ChairmanOfficer, is a co-founder and non-managing Member of Gotham Green Partners. Three of our directors, Jody Begley, Murray GarnickKamran Khan, Dominik Meier and Heather Newman,Elizabeth Seegar, are employed by Altria as Executive Vice President and Chief Operating Officer, ExecutiveAssociate General Counsel, Vice President of Consumer & Marketplace Insights & Innovation, and General Counsel, and Senior Vice President, Corporate Strategy,Financial Planning & Analysis , respectively. As a result of these relationships, conflicts of interests may arise between us and them, as described below.
We may also become involved in other transactions whichthat are inconsistent or conflict with the interests of our directors and officers, and/or our directors and officers may have interests in persons, firms, institutions, corporations or transactions that are inconsistent or in conflict with our interests and those of our shareholders. In addition, from time to time, Gotham Green Partners or Altria may be competing with us for available investment opportunities. Conflicts of interest, if any, will be subject to the procedures and remedies provided under applicable laws.laws and regulations. In particular, in the event that such a conflict of interest arises at a meeting of our directors, a director who has such a conflict will abstain from voting for or against the approval of the transaction and may recuse himself or herself from any related discussion or deliberation. In accordance with applicable laws and regulations, our directors are required to act honestly, in good faith and in our best interests.
Risks Relating to Our Common Shares
It is not anticipated that any dividend will be paid to holders of our common shares for the foreseeable future.
No dividends on theour common shares have been paid to date. We currently intend to retain future earnings, if any, for future operationoperations and expansion. Any decision to declare and pay dividends in the future will be made at the discretion of the Board and will depend on, among other things, financial results, cash requirements, contractual restrictions and other factors that the Board may deem relevant. Any changes to our policy with respect to the declaration and payment of any dividends requires Altria’s approval. As a result, investors may not receive any return on an investment in our common shares unless they sell their shares for a price greater than that which such investors paid for them.
The market price for theour common shares has in the past been volatile and may continue to be volatile and subject to fluctuation in response to numerous factors, many of which are beyond our control.significant fluctuation.
The market price for theour common shares has been volatile and subject to wide fluctuations and may continue to be volatile and subject to wide fluctuations in response to many factors, including:
•actual or anticipated fluctuations in our results of operations;
•changes in estimates of our future results of operations by us or securities research analysts;
•changes in the economic performance or market valuations of other companies that investors deem comparable to us;
•additions or departures of our executive officers and other key personnel;
•transfer restrictions on outstanding common shares;our restating financial results twice in the last five years;
•sales of additional common shares or the perception in the market that such sales might occur;
•significant acquisitions or business combinations, strategic partnerships, investments, joint ventures or capital commitments by or involving us or our competitors;
•increases in speculative trading activity by investors targeting publicly traded cannabis companies, which can further contribute to the volatility of the market price for our common shares if aggregate short exposure exceeds the number of our common shares available for purchase;
•news reports relating to trends, concerns or competitive developments, regulatory changes or enforcement actions and other related issues in our industry or target markets;
•the prospect of actual or perceived future changes to the legal and regulatory regimes that govern our products and our industries;
•investors’ general perception of us and the public’s reaction to our press releases, our other public announcements and our filings with the SEC and Canadian securities regulators;
•our failure to timely file our public filings with the SEC and Canadian securities regulators;
•our failure to comply with the Nasdaq and TSX rules and potential trading halts or delisting notices;
•reports by industry analysts, investor perceptions, and market rumors or speculation; and
•negative announcements by our customers, competitors or suppliers regarding their own performance.
For example, reports by industry analysts, investor perceptions, market rumors or speculation could trigger a sell-off in our common shares. Any sales of substantial numbers of theour common shares in the public market or the perception that such sales might occur may cause the market price of theour common shares to decline. In addition, to the extent that other large companies within our industries experience declines in their stock price, the share price of our common shares may decline as well. Moreover, if the market price of our common shares drops significantly, shareholders may institute securities class action lawsuits against us. Lawsuits against us could cause us to incur substantial costs and could divert the time and attention of our management and other resources.
Securities markets continue to experience significant price and volume fluctuations that have, in some cases, been unrelated to the operating performance, underlying asset values or prospects of public companies. Accordingly, the market price of our common shares may decline even if our results of operations, underlying asset values or prospects have not changed. In addition, certain institutional investors may base their investment decisions on consideration of our environmental, governance, diversity and social practices and performance against such institutions’ respective investment guidelines and criteria, and failure to meet such criteria may result in limited or no investment in our common shares by those institutions, which could adversely affect the trading price of our common shares. There can be no assurance that continuing fluctuations in price and volume will not occur. If such increased levels of volatility and market turmoil continue, the trading price of the common shares may be adversely affected.
Securities class action litigation often has been brought against companies following periods of volatility in the market price of their securities. We have been the target of such litigation and may in the future be the target of similar litigation. Regardless of merit, such litigation could result in substantial costs and damages and divert management’s attention and resources, which could adversely affect our business. Any adverse determination in litigation against us could also subject us to significant liabilities.
We may require additional capital in the future or be required to issue common shares pursuant to certain of our agreements, which may dilute holders of our securities.
We may be required to issue additional common shares pursuant to the Ginkgo Collaboration Agreement or to Kristen Bell pursuant to a publicity rights agreement entered into with the Company (the “Publicity Rights Agreement”).Agreement. Pursuant to the Ginkgo Collaboration Agreement, upon Ginkgo’s demonstration that the microorganisms they develop are capable of producing thecertain target cannabinoids above a minimum productivity level, we will issue to Ginkgo up to approximately 14.7 million common shares in the aggregate. TranchesTo date, we have issued approximately 7.1 million common shares to Ginkgo in respect of thesecertain Equity Milestone Events that have occurred. Additional tranches of common shares will be issued as each of theif and when additional Equity Milestone Events isare reached. The issuance of such common shares, if any, would dilute holders of our common shares. PursuantIn addition, Altria has pre-emptive rights to the Publicity Rights Agreement, if certain performance milestones are achieved, up to ansubscribe for additional $2 million common shares in us following any issuances we make to Ginkgo pursuant to the aggregate may be issued.Ginkgo Collaboration Agreement, and the issuance of such common shares, if any, would further dilute holders of our common shares.
Holders of common shares will have no pre-emptive rights in connection with such further issuances. Our Board has the discretion to determine if an issuance of common shares is warranted, the price at which such issuance is effected and the other terms of issue of common shares. Any additional capital raised through the sale of equity will dilute the percentage of ownership of holders of our common shares. Capital raised through debt financing would require us to make periodic interest payments and may impose restrictive covenants on the conduct of our business.
A substantial number of our securities are owned by a limited number of existing shareholders.
Our management, directors and employees own a substantial number of our outstanding common shares (on a fully diluted basis). In addition, as of December 31, 2020,2023, Altria beneficially owned approximately 43.5%41.1% of our outstanding common shares (calculated on a non-diluted basis). As such, our management, directors and employees, as a group, and Altria each are in a position to exercise significant influence over matters requiring shareholder approval, including the election of directors and the determination of significant corporate actions. In addition, these shareholders could delay or prevent a change in control that could otherwise be beneficial to holders of common shares.
Investors in the U.S. may have difficulty bringing actions and enforcing judgments against us and others based on securities law civil liability provisions.
We are incorporated under the laws of the Province of British Columbia and our head office is located in the Province of Ontario. Some of our directors and officers and some of the experts named in this Annual Report are residents of Canada or otherwise reside outside of the U.S., and a substantial portion of their assets and our assets are located outside the U.S. Consequently, it may be difficult for investors in the U.S. to bring an action against such directors, officers or experts or to enforce against those persons or us a judgment obtained in a U.S. court predicated upon the civil liability provisions of U.S. federal securities laws or other laws of the U.S. In addition, while statutory provisions exist in British Columbia for derivative actions to be brought in certain circumstances, the circumstances in which a derivative action may be brought, and the procedures and defenses that may be available in respect of any such action, may be different than those of shareholders of a company incorporated in the U.S.
If we are a passive foreign investment company for U.S. federal income tax purposes in any year, certain adverse tax rules could apply to U.S. holders of our common shares.
We will be classified as a passive foreign investment company (“PFIC”) for any taxable year for U.S. federal income tax purposes if for a taxable year, (i) 75% or more of our gross income is passive income, or (ii) 50% or more of the value of our assets either produce passive income or are held for the production of passive income, based on the quarterly average of the fair market value of such assets. The determination of PFIC status depends on interpretive rules and computational conventions that are often unclear. In particular, in making our determination, we are relying on the application of certain “look-through” rules, taking into account certain intercompany items.items (including our interests in subsidiaries). There is, however, no direct legal authority applying these look-through rules to our particular situation (including to what extent, they apply to intercompany items). Likewise, in light of the volatility of our common share price, we intend to take the position that the spot trading price of our stock at each quarter end, as adjusted by liabilities, does not dictate the determination of the fair market value of our assets. Based on current business plans and financial expectations, an independent valuation reportanalysis in respect of our assets, and the application of certain look-through rules (including the taking into account ofto certain intercompany items)items and to our interests in our subsidiaries), we do not expect to be a PFIC for the taxable year ending December 31, 2020 and do not expect to become a PFIC in the foreseeable future.2024. However, PFIC status is determined annually and depends upon the composition of our gross income and assets, both of which are subject to change. Moreover, there can be no assurance that the IRSInternal Revenue Service (“IRS”) or a court will agree with our interpretation of fair market value or its computation, or with our interpretation of the PFIC rules (including the “look-through” rules and the scope of their application, including in respect of intercompany items). Therefore, there can be no assurance as to our PFIC status for the current taxable year or for future taxable years, nor any assurance that the IRS or a court will agree with our determination of our PFIC status.
If securities or industry analysts do not publish research, or publish inaccurate or unfavorable research about our business, our share price and trading volume could decline.
The trading market for our common shares depends, in part, on the research and reports that securities or industry analysts publish about us or our business. If one or more of the analysts who cover us downgrade our common shares or publish inaccurate or unfavorable research about our business, the trading price of our common shares would likely decline. In addition, if our results of operations fail to meet the forecastforecasts of analysts, the trading price of our common shares would likely decline. If one or more of these analysts cease coverage of us or fail to publish reports on us regularly, demand for our common shares could decrease, which might cause our trading price and trading volume to decline.
General Risks
We are dependent on our senior management.
Our success is dependent upon the ability, expertise, judgment, discretion and good faith of our senior management. While employment agreements are customarily used as a primary method of retaining the services of key employees, these agreements cannot assure the continued services of our senior management team. Qualified individuals are in high demand, and we may incur significant costs to attract and retain them. The loss of the services of a member of senior management, or an inability to attract other suitably qualified persons when needed, could have a material adverse effect on our ability to execute on our business plan and strategy, and we may be unable to find adequate replacements on a timely basis, or at all. We do not maintain key-person insurance on the lives of any of our officers or employees.
We will seekmay be unable to maintain adequateobtain insurance coverage in respect of the risks we face; however, insurance premiums for such insurance may not continue to be commercially justifiableat acceptable rates and there may be coverage limitations and other exclusions which may not be sufficient to cover our potential liabilities.
We have insurance to protect our assets, operations and employees. While we believe ourOur insurance coverage, addresses all material risks to which we are exposed in our current state of operations, such insurancehowever, is subject to deductibles, coverage limits and exclusions and may not be available or adequate for the risks and hazards to which we are exposed. For example, certain wholesalers, distributors, retailers and other service providers may require suppliers of U.S. hemp products to provide an indemnification from liability in connection with such products, which may not be covered by insurance. In addition, noNo assurance can be given that such insurance will be adequate to cover our liabilities or will be generally available in the future or, if available, that premiums and deductibles will be commercially justifiable. If we were to incur substantial liability claims and such damages were not covered by insurance or were in excess of policy limits, or if we were to incur such liability at a time when we are not able to obtain liability insurance, there could be a material adverse effect on our business, financial condition and results of operations. Furthermore, our insurers have in the past and may in the future deny us coverage, whether or not such denial is with merit, and we have in the past and may in the future need to commence litigation against such insurers, which could be time consuming and expensive and divert significant management resources, with no assurance that we will be successful in any resulting proceedings.
Tax and accounting requirements may changebe interpreted or be interpretedchanged in ways that are unforeseen tocomplex and not necessarily anticipated by us, and we may face difficulty or be unable to implement and/or comply with any such interpretations or changes.
We are subject to numerous tax and accounting requirements, and changes in existing accounting or taxation rules or practices, or varying interpretations of current rules or practices, could have a significant adverse effect on our financial results, the manner in which we conduct our business or the marketability of any of our products. In many countries, including the U.S., we are subject to transfer pricing and other tax regulations designed to ensure that appropriate levels of income are reported as earned and are taxed accordingly. Although we believe that we are in substantial compliance with all applicable regulations and restrictions, we are subject to the risk that governmental authorities could audit our transfer pricing and related practices and assert that additional taxes are owed.owed or that various jurisdictions could assert that we should file tax returns in jurisdictions where we do not file and subject us to additional tax. In the future, the geographic scope of our business may expand, and such expansion will require us to comply with the tax laws and regulations of additional jurisdictions. Requirements as to taxation vary substantially among jurisdictions. Complying with the tax laws and regulations of these jurisdictions can be time consuming and expensive and could potentially subject us to penalties and fees in the future if we failed to comply. In the event that we failed to comply with applicable tax laws and regulations, this could have a material adverse effect on our business, financial condition and results of operations.
Natural disasters, unusual weather, pandemic outbreaks, boycotts and geo-politicalgeopolitical events or acts of terrorism could adversely affect our operations and financial results.
The occurrence of one or more natural disasters, such as hurricanes, floods and earthquakes, unusually adverse weather, pandemic outbreaks, such as the COVID-19 virus, influenza and other highly communicable diseases or viruses, boycotts and geo-politicalgeopolitical events, such as civil unrest in countries in which our or our joint ventures’ operations are located and acts of terrorism, or similar disruptions could adversely affect our business, financial condition and results of operations. These events could result in physical damage to one or more of our or our joint ventures’ properties, increases in fuel or other energy prices, the temporary or permanent closure of one or more of our or our joint ventures’ facilities, the temporary lack of an adequate workforce in a market, the temporary or long-term disruption in the supply of products from suppliers, the temporary disruption in the transport of goods, delay in the delivery of goods to our or our joint ventures’ facilities, and disruption to our information systems. Such events could also negatively impact consumer sentiment, reduce demand for consumer products like ours and cause general economic slowdown.
Our business is subject to evolving corporate governance and public disclosure regulations and expectations, including with respect to environmental, social and governance matters, which could expose us to numerous risks.
We are subject to changing rules and regulations promulgated by a number of governmental and self-regulatory organizations, including the SEC, Nasdaq and the Financial Accounting Standards Board. These rules and regulations continue to evolve in scope and complexity. In addition, increasingly regulators, customers, investors, employees and other stakeholders are focusing on environmental, social and governance (“ESG”) matters and related disclosures. These changing rules, regulations and stakeholder expectations have resulted in, and are likely to continue to result in, increased general and administrative expenses and increased management time and attention spent complying with or meeting such regulations and expectations. For example, developing and acting on initiatives within the scope of ESG, andcollecting, measuring and reporting ESG related information and metrics can be costly, difficult and time consuming and is subject to evolving reporting standards, including the SEC’s proposed climate-related reporting requirements, and similar proposals by other international regulatory bodies. We may also communicate certain initiatives and goals, regarding environmental matters, diversity, responsible sourcing and social investments and other ESG related matters, in our SEC filings or in other public disclosures. These initiatives and goals within the scope of ESG could be difficult and expensive to implement, the technologies needed to implement them may not be cost effective and may not advance at a sufficient pace, and we could be criticized for the accuracy, adequacy or completeness of the disclosure. Further, statements about our ESG related initiatives and goals, and progress against those goals, may be based on standards for measuring progress that are still developing, internal controls and processes that continue to evolve, and assumptions that are subject to change in the future. In addition, we could be criticized for the scope or nature of such initiatives or goals, or for any revisions to these goals. If our ESG-related data, processes and reporting are incomplete or inaccurate, or if we fail to achieve progress with respect to our initiatives or goals within the scope of ESG on a timely basis, or at all, our reputation, business, financial performance and growth could be adversely affected.
Climate change may disrupt our business and our efforts to address concerns relating to climate change could result in damage to our reputation.
Our business and that of our joint venture partners and third-party suppliers involves the growing of cannabis, an agricultural product, and adverse weather conditions have historically caused volatility in the agricultural industry and consequently in operating results by causing crop failures or significantly reduced harvests, which may negatively affect the supply and pricing of agricultural commodities, such as cannabis. Additionally, the potential physical impacts of climate change are uncertain and may vary by region. These potential effects could include changes in rainfall patterns, water shortages, changing sea levels, changing storm patterns and intensities, and changing temperature levels that could adversely impact our costs and business operations, the location, costs, and competitiveness of cannabis production and related storage and processing facilities and the supply of cannabis.
We are also exposed to risks resulting from changes in public policy, laws and regulations, or market and public perceptions and preferences in connection with the transition to a less carbon-dependent economy. These changes could adversely affect our business, results of operations and reputation.
Our financial performance is subject to risks of foreign exchange rate fluctuation, which could result in foreign exchange losses.
We may be exposed to fluctuations of the U.S. dollar against certain other currencies, particularly the Canadian dollar and Israeli Shekel, because we publish our financial statements in U.S. dollars, while a significant portion of our assets, liabilities, revenues and costs are or will be denominated in other currencies. Exchange rates for currencies of the countries in which we operate may fluctuate in relation to the U.S. dollar, and such fluctuations may have a material adverse effect on our earnings or assets when translating foreign currency into U.S. dollars. We do not hedge our exchange rate so any changes in exchange rates will directly affect our earnings.
Our business, financial condition, results of operations and cash flows could be adversely affected by disruptions in the global economy caused by the ongoing conflict between Russia and Ukraine.
The global economy has been negatively impacted by the military conflict between Russia and Ukraine. Furthermore, governments in the U.S., Canada, the United Kingdom and European Union have each imposed export controls on certain products and financial and economic sanctions on certain industry sectors and parties in Russia. Although we do not have any customers or direct supplier relationships in Russia or Ukraine, businesses globally have experienced shortages in materials and increased costs for transportation, energy, and raw material due in part to the negative impact of the Russia-Ukraine military conflict on the global economy. Further escalation of geopolitical tensions related to the military conflict, including increased trade barriers or restrictions on global trade, could result in, among other things, cyberattacks, supply disruptions, lower consumer demand, and changes to foreign exchange rates and financial markets, any of which may adversely affect our business, financial condition, results of operations and cash flows.
We are continuing to monitor the situation in Ukraine and globally and assessing its potential impact on our business. Although our business has not been, to the date of this Annual Report, materially impacted by the ongoing military conflict in Ukraine, it is impossible to predict the extent to which our operations, or those of our suppliers and vendors, will be impacted in the short and long term, or the ways in which the conflict may impact our business. The extent and duration of the military action, sanctions and resulting market disruptions are impossible to predict, but may be substantial. In addition, the effects of the ongoing conflict could heighten any of our known risks described above.
ITEM 1B. UNRESOLVED STAFF COMMENTS.
None.
None.
ITEM 1C. CYBERSECURITY.
Our cybersecurity processes include:
•Basic security awareness online training for personnel with company email, on an annual basis;
•Phishing tests for personnel with company email, on not less than an annual basis;
•Reviews of certain third-party vendors’ information security programs (as discussed below);
•Consultation with external advisors regarding opportunities and enhancements to strengthen our practices and policies, on an ad hoc basis;
•Electronic monitoring of the majority of our technology environments to identify cybersecurity events;
•Periodic assessments of existing technology hardware configurations, patches, security and lifecycle;
•Periodic assessments, in consultation with software providers, of existing software versions, configurations, patches and updates; and
•Periodic assessments of data management and handling, including data use and access reviews.
Certain information technology general controls are reviewed and tested as part of our internal control over financial reporting.
We use third-party services to assist with penetration testing, security incident monitoring, incident response preparation, end point protection, and security awareness online training.
Before engaging third-party service providers to whom we grant access to our information technology systems, we may review their information security programs, depending on the feasibility of such review and our assessment of the level of risk the third-party service provider poses to our business operations and our information technology and financial reporting systems. We determine risk level based on a set of internally developed criteria. We do not, however, review the information security programs of all third-party vendors. Where feasible, we also conduct periodic reviews (typically annual) of certain third-party service providers, particularly
service providers of financial, financial reporting and accounting systems, depending on our assessment of the level of risk to our business operations and our information technology and financial reporting systems.
To date, we are not aware of any cybersecurity incident that has had or is reasonably likely to have a materially adverse effect on our business, including our business strategy, results of operations and financial condition.However, there can be no assurance that our processes and procedures will prevent or timely detect a cybersecurity incident. For more information regarding risks from cybersecurity threats, see the section entitled “Risk Factors—Risks Relating to Our Products—Risks Relating to Production and Distribution of Products.”
In fiscal year 2024, as part of our overall enterprise risk management process, our Board received a report on our program for assessing, monitoring and mitigating cybersecurity risks and has delegated oversight of such program to our Audit Committee. Going forward, the Audit Committee will receive periodic reports on our program for assessing, monitoring and mitigating cybersecurity risks. In addition, as part of its overall responsibility for overseeing the adequacy of the Company’s internal control over financial reporting, our Audit Committee receives periodic reports about our financial reporting information system controls and security.
Our Information Systems department, in addition to managing our general information technology systems, is also responsible for managing our enterprise-wide cybersecurity processes. Personnel in our Information System department collectively have decades of experience in information security, information technology and cybersecurity operations. Our Information Systems department monitors, and receives notifications of, potential cybersecurity incidents detected through automated detection and monitoring tools. In the event we discover a material cybersecurity incident, Information Systems personnel reports such incident to our Chief Financial Officer, who then reports to our Chief Executive Officer and the Audit Committee, as appropriate. We do not currently have a Chief Information Security Officer or other senior security officer of a similar title.
ITEM 2. PROPERTIES.
Our executive offices are located in Toronto, Ontario in Canada, where we lease office space. As of December 31, 2020, our Rest of World segment2023, the Company owned various manufacturing facilities in the Canadian provinces of Manitoba, Ontario and British ColumbiaCanada and in Hadera, Israel, as well as a researchIsrael. The Company has announced the pending sale and developmentleaseback of the Peace Naturals Campus and is winding down its operations at the Cronos Fermentation facility in Beit Shemesh, Israel. As of December 31, 2020, our United States segment leased office spaceManitoba and a manufacturing facility in Los Angeles, California.has listed the property for sale. See “Operations and Investments.” Management believes that our existing facilities and the anticipated changes described herein are adequate to meet our current requirements and, to the extent that our facilities are leased, comparable space is readily available.
ITEM 3. LEGAL PROCEEDINGS.
The Company is subject to various legal proceedings in the ordinary course of its business and in connection with its marketing, distribution and sale of its products. Many of these legal proceedings are in the early stages of litigation and seek damages that are unspecified or not quantified. Although the outcome of these matters cannot be predicted with certainty, the Company does not believe these legal proceedings, individually or in the aggregate, will have a material adverse effect on its consolidated financial condition but could be material to its results of operations for any particular reporting period depending, in part, on its results for that period.
Class action complaints relating See Part II, Note 10(b) “Contingencies,” to restatement
On March 11 and 12, 2020, two alleged shareholders of the Company separately filed two putative class action complaints in the U.S. District Court for the Eastern District of New York against the Company and its former Chief Executive Officer (now Executive Chairman) and Chief Financial Officer. The court has consolidated the cases, and the consolidated amended complaint alleges violations of Section 10(b) of the Exchange Act and Rule 10b-5 promulgated thereunder against all defendants, and Section 20(a) of the Exchange Act against the individual defendants. The consolidated amended complaint generally alleges that certain of the Company’s prior public statements about revenues and internal controls were incorrect based on the Company’s March 2, 2020 disclosure that the Audit Committee of the Board was conducting a review of the appropriateness of revenue recognized in connection with certain bulk resin purchases and sales of products through the wholesale channel. The consolidated amended complaint does not quantify a damage request. Defendants moved to dismiss on February 8, 2021.
On June 3, 2020, an alleged shareholder filed a Statement of Claim, as amended on August 12, 2020, in the Ontario Superior Court of Justice in Toronto, Ontario, Canada, seeking, among other things, an order certifying the action as a class action on behalf of a putative class of shareholders and damages of an unspecified amount. The Amended Statement of Claim names the Company, its former Chief Executive Officer (now Executive Chairman), Chief Financial Officer, former Chief Financial Officer and Chief Commercial Officer, and current and former members of the Board as defendants and alleges breaches of the Ontario Securities Act, oppression under the Ontario Business Corporations Act and common law misrepresentation. The Amended Statement of Claim generally alleges that certain of the Company’s prior public statements about revenues and internal controls were misrepresentations based on the Company’s March 2, 2020 disclosure that the Audit Committee of the Board was conducting a review of the appropriateness of revenue recognized in connection with certain bulk resin purchases and sales of products through the wholesale channel, and the Company’s subsequent restatement. The Amended Statement of Claim does not quantify a damage request.
Regulatory reviews relating to restatement
The Company has been responding to requests for information from various regulatory authorities relating to its previously disclosed restatement of its financial statements for the first three quarters of 2019. The Company is responding to all such requests for information and cooperating with all regulatory authorities. The Company cannot predict the outcome of any such regulatory review or investigation and it is possible that additional investigations or one or more formal proceedings may be commenced against the Company and its current and former officers and directors in connection with these regulatory reviews and investigations.
Litigation relating to marketing, distribution and sale of products
On June 16, 2020, an alleged consumer filed a Statement of Claim on behalf of a class in the Court of Queen’s Bench of Alberta in Alberta, Canada, against the Company and other Canadian cannabis manufacturers and/or distributors. On December 4, 2020, a Third Amended Statement of Claim was filed, which added a second alleged consumer. The Third Amended Statement of Claim alleges claims related to the defendants’ advertised content of cannabinoids in cannabis products for medicinal use on or after June 16, 2010 and cannabis products for adult use on or after October 17, 2018. The Third Amended Statement of Claim seeks a total of C$500 million for breach of contract, compensatory damages, and unjust enrichment or such other amount as may be proven in trial and C$5 million in punitive damages against each defendant, including the Company. The Third Amended Statement of Claim also seeks interest and costs associated with the action. The Company has not responded to the Third Amended Statement of the Claim.
A number of claims, including purported class actions, have been brought in the U.S. against companies engaged in the U.S. hemp business alleging, among other things, violations of state consumer protection, health and advertising laws. On Aprilunder Item 8 2020, a putative class action complaint was filed in the U.S. District Court for the Central District of California against Redwood, alleging violations of California’s Unfair Competition Law, False Advertising Law, Consumers Legal Remedies Act, and breaches of the California Commercial Code for breach of express warranties and implied warranty of merchantability with respect to Redwood’s marketing and sale of U.S. hemp products. The complaint did not quantify a damage request. On April 10, 2020, the class action complaint was dismissed for certain pleading deficiencies and the plaintiff was granted leave until April 24, 2020 to amend the complaint to establish federal subject matter jurisdiction. On April 28, 2020, the action was dismissed without prejudice for failure to prosecute and for failure to comply with a court order. As of the date of this Annual Report the plaintiff has not refiled the complaint.
We expect litigation and regulatory proceedings relating to the marketing, distribution and salefor a description of our products to increase.legal proceedings.
ITEM 4. MINE SAFETY DISCLOSURE.
Not applicable.
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED SHAREHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES.
Our common shares are traded on Nasdaq and the TSX under the symbol “CRON.”
Holders
As of February 25, 2021,23, 2024, there were approximately 71131 holders of record of our common shares. This number of holders of record does not represent the actual number of beneficial owners of our common shares because shares are frequently held in “street name” by securities dealers and others for the benefit of individual owners who have the right to vote their shares.
Dividends
As of the date of this Annual Report, we have not declared any dividends or made any distributions on our common shares. Furthermore, we have no current intention to declare dividends on our common shares in the foreseeable future. Any decision to pay dividends on our common shares in the future will be at the discretion of the Board and will depend on, among other things, our results of operations, current and anticipated cash requirements and surplus, financial condition, any future contractual restrictions and financing agreement covenants, our ability to meet solvency tests imposed by corporate law and other factors that the Board may deem relevant.
Securities Authorized for Issuance under Equity Compensation Plans
Information concerning securities authorized for issuance under equity compensation plans will be set forth in the Company’s definitive proxy statement for its 20212024 Annual Meeting of Shareholders or an amendment to this Annual Report to be filed within 120 days of our fiscal year end.
Purchases of Equity Securities by the Issuer and Affiliated Persons
None.
Recent Sales of Unregistered Securities
None.
Performance Graph
The following performance graph compares the cumulative total shareholder return of our common shares as listed on Nasdaq with the cumulative total return of the S&P 500 Index and twoa market-weighted indicesindex of publicly traded peers over the 34-month60-month period beginning on February 27,December 31, 2018, and ending on December 31, 2020. Because the Company changed the composition of the2023. The new peer group for 2020 as described below, the peer group used for the corresponding disclosures in 2019 is shown for comparison.includes Aurora Cannabis Inc., Canopy Growth Corporation, Green Thumb Industries, Inc., Organigram Holdings Inc., Tilray Inc., and Trulieve Cannabis Corp., (the “New Peer Group”). The graph assumes that $100 is invested in each of our common shares, the S&P 500 Index, and the indices of publicly traded peers on February 27, 2018 and that all dividends, if applicable, were reinvested.
The publicly traded companies in the new peer group are Aphria Inc., Aurora Cannabis Inc., Canopy Growth Corporation, Green Thumb Industries Inc., GW Pharmaceuticals plc, HEXO Corporation, iAnthus Capital Holdings Inc., Organigram Holdings Inc. and Tilray Inc. (the “New Peer Group”). The old peer group also included CannTrust Holdings (the “Old Peer Group”) Inc., which has been excluded from the New Peer Group for the year ended December 31, 2020 because we no longer believe that its business model makes it comparable. Past performance may not be indicative of future performance.
| | | | | | | | | | | | | | |
| Date | Cronos Group Inc. | S&P 500 | 2019 Peer Group | 2020 Peer Group |
| February 27, 2018 | $ | 100.00 | | $ | 100.00 | | $ | 100.00 | | $ | 100.00 | |
| March 31, 2018 | $ | 88.32 | | $ | 97.46 | | $ | 104.89 | | $ | 105.16 | |
| June 30, 2018 | $ | 85.56 | | $ | 100.81 | | $ | 115.49 | | $ | 116.33 | |
| September 30, 2018 | $ | 145.93 | | $ | 108.58 | | $ | 170.60 | | $ | 171.08 | |
| December 31, 2018 | $ | 136.35 | | $ | 93.90 | | $ | 88.54 | | $ | 88.91 | |
| March 31, 2019 | $ | 241.86 | | $ | 106.71 | | $ | 135.59 | | $ | 136.03 | |
| June 30, 2019 | $ | 209.71 | | $ | 111.31 | | $ | 118.11 | | $ | 119.12 | |
| September 30, 2019 | $ | 118.77 | | $ | 113.20 | | $ | 68.69 | | $ | 70.14 | |
| December 31, 2019 | $ | 100.66 | | $ | 123.46 | | $ | 56.31 | | $ | 57.49 | |
| March 31, 2020 | $ | 74.41 | | $ | 99.27 | | $ | 35.98 | | $ | 36.71 | |
| June 30, 2020 | $ | 78.87 | | $ | 119.66 | | $ | 44.09 | | $ | 45.19 | |
| September 30, 2020 | $ | 65.75 | | $ | 130.35 | | $ | 37.58 | | $ | 38.52 | |
| December 31, 2020 | $ | 91.08 | | $ | 146.18 | | $ | 60.28 | | $ | 61.88 | |
*$100 invested on 2/27/18 in stock or 2/28/18 in index, including reinvestment of dividends. Fiscal year ending December 31.
Copyright© 2021 Standard & Poor’s, a division of S&P Global. All rights reserved.
Because Cronos Group’s common shares are also traded on the TSX, we are providing additional information in order to enhance the reader’s understanding of our trading history. The following performance graph compares the cumulative total shareholder return of our common shares as listed on the TSX with the cumulative total return of the S&P 500 Index, the New Peer Group and the Old Peer Group over the five-year period beginning on December 31, 2015 and ending on December 31, 2020. The graph assumes that $100 is invested in each of our common shares, the S&P 500 Index, and the indices of the New Peer Group and Old Peer Group2018, and that all dividends, if applicable, were reinvested. Past performance may not be indicative of future performance.
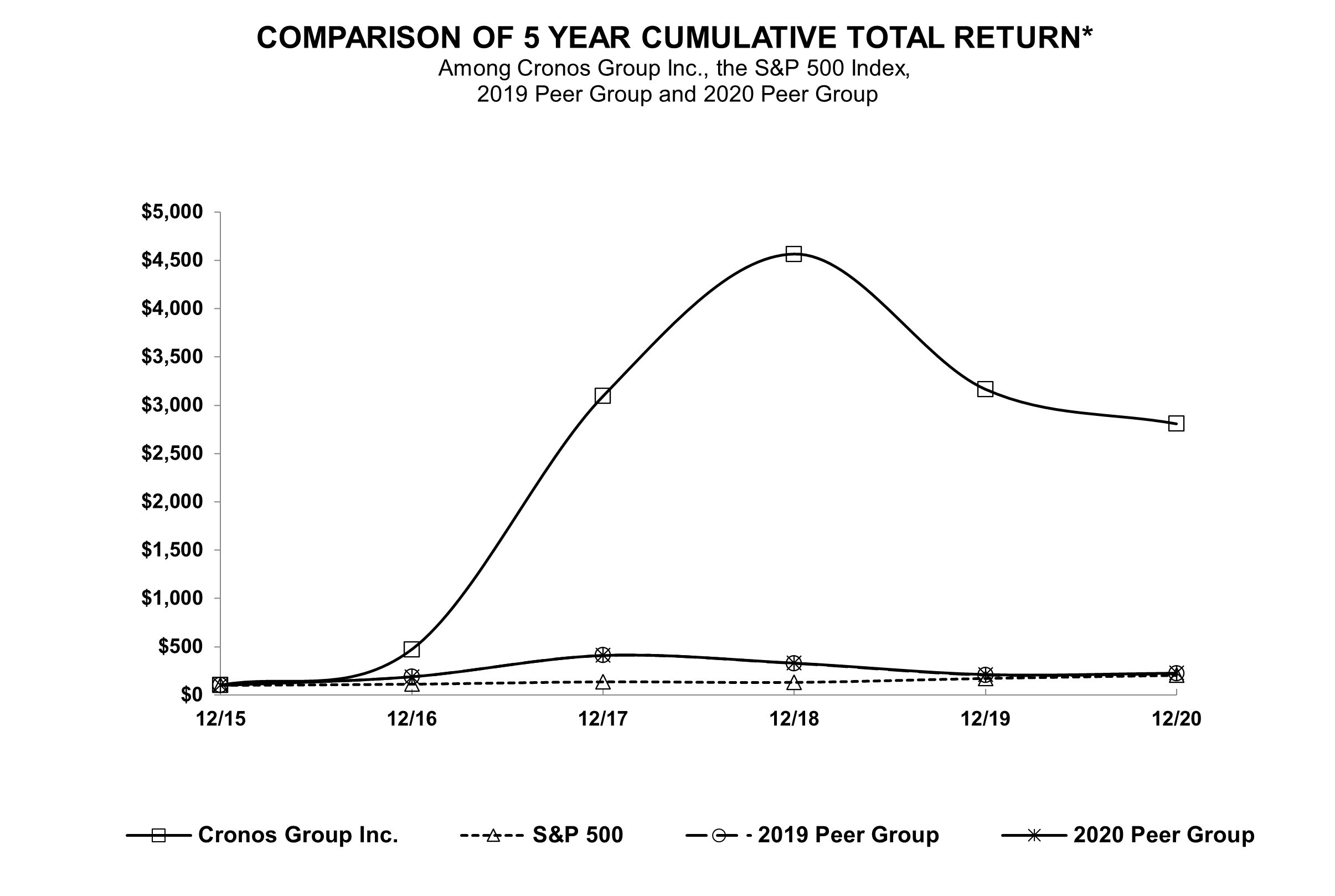
| | | | | | | | | | | | | | |
| Date | Cronos Group Inc. | S&P 500 | 2019 Peer Group | 2020 Peer Group |
| December 31, 2015 | $ | 100.00 | | $ | 100.00 | | $ | 100.00 | | $ | 100.00 | |
| December 31, 2016 | $ | 469.84 | | $ | 111.96 | | $ | 189.24 | | $ | 189.24 | |
| December 31, 2017 | $ | 3,092.06 | | $ | 136.40 | | $ | 410.21 | | $ | 410.21 | |
| December 31, 2018 | $ | 4,565.08 | | $ | 130.42 | | $ | 328.76 | | $ | 331.43 | |
| December 31, 2019 | $ | 3,165.08 | | $ | 171.49 | | $ | 207.65 | | $ | 212.29 | |
| December 31, 2020 | $ | 2,806.35 | | $ | 203.04 | | $ | 220.55 | | $ | 226.81 | |

| | | | | | | | | | | |
| Date | Cronos Group Inc. | S&P 500 | New Peer Group |
| December 31, 2018 | $ | 100.00 | | $ | 100.00 | | $ | 100.00 | |
| March 31, 2019 | $ | 177.38 | | $ | 113.65 | | $ | 146.46 | |
| June 30, 2019 | $ | 153.80 | | $ | 118.54 | | $ | 126.09 | |
| September 30, 2019 | $ | 87.10 | | $ | 120.55 | | $ | 72.34 | |
| December 31, 2019 | $ | 73.82 | | $ | 131.49 | | $ | 59.86 | |
| March 31, 2020 | $ | 54.54 | | $ | 105.72 | | $ | 36.55 | |
| June 30, 2020 | $ | 57.84 | | $ | 127.44 | | $ | 44.14 | |
| September 30, 2020 | $ | 48.22 | | $ | 138.81 | | $ | 41.80 | |
| December 31, 2020 | $ | 66.79 | | $ | 155.68 | | $ | 73.30 | |
| March 31, 2021 | $ | 91.05 | | $ | 165.29 | | $ | 101.70 | |
| June 30, 2021 | $ | 82.77 | | $ | 179.42 | | $ | 87.65 | |
| September 30, 2021 | $ | 54.48 | | $ | 180.47 | | $ | 60.03 | |
| December 31, 2021 | $ | 37.73 | | $ | 200.37 | | $ | 44.44 | |
| March 31, 2022 | $ | 37.44 | | $ | 191.15 | | $ | 39.24 | |
| June 30, 2022 | $ | 27.14 | | $ | 160.38 | | $ | 17.24 | |
| September 30, 2022 | $ | 27.14 | | $ | 152.55 | | $ | 15.91 | |
| December 31, 2022 | $ | 24.45 | | $ | 164.08 | | $ | 14.41 | |
| March 31, 2023 | $ | 18.67 | | $ | 176.38 | | $ | 12.07 | |
| June 30, 2023 | $ | 18.96 | | $ | 191.80 | | $ | 8.24 | |
| September 30, 2023 | $ | 19.24 | | $ | 185.52 | | $ | 12.29 | |
| December 31, 2023 | $ | 20.12 | | $ | 207.21 | | $ | 11.41 | |
*$100 invested on 12/31/152018 in stock or 12/31/2018 in index, including reinvestment of dividends. Fiscal year ending December 31.
20212024 Standard & Poor’s, a division of S&P Global. All rights reserved.
Share Information
| | | | | | | | |
| | As of February 25, 2021 |
Issued and outstanding shares | | |
Common shares | | 360,258,680 | |
Potentially issuable shares | | |
Stock options | | 13,749,800 | |
Warrants | | 7,987,354 | |
Restricted stock units | | 948,357 | |
Altria Warrant | | 80,056,296 | |
Exercisable Top-up Rights | | 3,557,226 | |
Total potentially issuable shares | | 106,299,033 | |
| | |
Total outstanding and potentially issuable shares | | 466,557,713 | |
ITEM 6. Selected Financial DataRESERVED
Omitted.Not applicable.
ITEM 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the followingManagement’s discussion and analysis togetherof financial condition and results of operations is provided as a supplement to, and should be read in conjunction with, ourthe consolidated financial statements and the related notes, to those statements, which are included in Item 8 of this Annual Report. Report on Form 10-K (this “Annual Report”), to enhance the understanding of our operations and our present business environment.
This discussion contains Forward-Looking Statementsforward-looking statements that involve risks and uncertainties. As a result of many factors, such as those set forth inuncertainties, see Part I, Item 1A “Risk Factors,1 “Business—Special Note Regarding Forward-Looking Statements” of this Annual Report and elsewhere in this Annual Report our actual results may differ materially from those anticipatedfor a discussion of the risks and uncertainties involved in thesethe Forward-Looking Statements.
For more information about our operations and the risks facing our business, see Part I, Item 1 “Business” and Part I, Item 1A “Risk Factors”, respectively, of this Annual Report.
Business Overview
Cronos Group is an innovative global cannabinoid company with international production and distribution across five continents. We are committed to building disruptive intellectual property by advancing cannabis research, technology and product development and are seekingdevelopment. With a passion to buildresponsibly elevate the consumer experience, Cronos is building an iconic brand portfolio. Cronos Group’sCronos’ diverse international brand portfolio includes Spinach®, PEACE NATURALS™NATURALS® and Lord Jones®.
Unless otherwise noted or the context indicates otherwise, references in this Annual Report to the “Company”, a global wellness platform; two adult-use brands, COVE™“Cronos”, “we”, “us” and Spinach™;“our” refer to Cronos Group Inc., its direct and threeindirect wholly owned subsidiaries and, if applicable, its joint ventures and investments accounted for by the equity method; the term “cannabis” means the plant of any species or subspecies of genus Cannabis and any part of that plant, including all derivatives, extracts, cannabinoids, isomers, acids, salts, and salts of isomers; the term “U.S. hemp” has the meaning given to the term “hemp” in the U.S. Agricultural Improvement Act of 2018, including hemp-derived consumer products brands, Lord Jones™, Happy Dance™ and PEACE+™cannabidiol (“CBD”).
Strategy
Cronos Group seeks to create value for shareholders by focusing on four core strategic priorities:
•growing a portfolio of iconic brands that responsibly elevate the consumer experience;
•developing a diversified global sales and distribution network;
•establishing an efficient global supply chain; and
•creating and monetizing disruptive intellectual property.
Business SegmentsDiscontinued Operations
Cronos Group reports through two segments: “United States” and “Rest of World.” These two segments represent the geographic regions in which the Company operates and the different product offerings within each geographic region. On September 5, 2019, as a result of the Redwood Acquisition, the Company established the United States segment, which includes only the results of Redwood since the date of acquisition. Redwood manufactures, markets and distributes U.S. hemp-derived supplements and cosmetic products through e-commerce, retail and hospitality partner channels in the United States (“U.S.”) under the brands Lord Jones™ and Happy Dance™.
Recent Developments
In December 2019, an outbreak of a novel strain of coronavirus, COVID-19, was identified in Wuhan, China. Since then, COVID-19 has spread across the globe, including the U.S., Canada and Israel, and other countries in which the Company or its affiliates operate (including Australia and Colombia) and was recognized as a pandemic by the World Health Organization. The COVID-19 pandemic has resulted in a sharp contraction in many areas of the global economy and increased volatility and uncertainty in the capital markets. In response to the pandemic, the governments of many countries, provinces, states, cities, and other geographic regions took preventative or protective actions, including closures of certain businesses, mandatory quarantines, limits on individuals’ time outside of their homes, travel restrictions and social distancing or other preventative measures. Such measures were eased or lifted in varying degrees by different governments of various countries, states and cities throughout 2020, but the continued spread of COVID-19 and increased infection rates has caused, and may continue to cause, some jurisdictions to roll back reopening plans that had been underway and re-impose quarantines, border closures, closure of certain businesses and stay-at-home orders. It is possible that jurisdictions in which the Company or its affiliates operate may reintroduce more stringent preventative or protective actions which could result in further closures of businesses. Governments in certain countries such as the U.S., Canada, and those in Europe have responded to the acute economic and market consequences with certain monetary and fiscal policy actions.
Impact on operating results
During the second quarter of 2020, the effects2023, Cronos exited its U.S. hemp-derived cannabinoid product operations. The exit of the COVID-19 pandemicU.S. operations represented a strategic shift that has a major effect on retail storesCronos’ operations and financial results, and as such, qualifies for reporting as discontinued operations in our consolidated statements of net loss and comprehensive loss. Prior period amounts have been reclassified to reflect the increasediscontinued operations classification of costs in production and sales in the U.S. had a material impactoperations. For further detail on the ratediscontinuation of growth of revenue in the U.S. segment, with revenue foroperations, see Note 2 “Discontinued Operations” to the segment remaining flat from the first quarter of 2020. As a result of these impacts and the expectation of future impacts, for the three months ended June 30, 2020, the Company recorded $35 million of impairment charges on its U.S. reporting unit and $5 million on the Lord Jones™ brand (refer to Note 11 of the Company’s consolidated financial statements for more information regarding intangibles assets and goodwill).
The continued negative impact on the Company’s results has been as expected during the three months ended December 31, 2020 and no further impairment charges were recorded to the U.S. reporting unit or brands as a result. The Company expects revenue growth and operating results in the U.S. segment to continue to be negatively impacted as retail closures are expected to continue as a result of the pandemic.
Revenue and the growth in revenue in the Rest of World segment was not materially impacted by the effects of COVID-19 during the year ended December 31, 2020. However, prolonged closures of retail stores due to government mandated lockdowns as well as the changes in consumer purchasing behavior in Canada during the COVID-19 pandemic are expected to have a negative impact on the Company’s short-term revenue growth in Canada.
In both segments, there were no material increases in the current expected credit loss in connection with COVID-19. The Company continues to closely monitor the effects of COVID-19 on its operating results.
Production and supply chain
The Company’s global production facilities currently remain operational. To comply with governmental orders or other health and safety requirements, the Company has: (i) reduced the number of personnel working on-site at its production facilities to only the roles that are necessary to be performed on-site, (ii) implemented work-from-home policies for other employees whose work can be performed off-site, and (iii) implemented other additional health and safety measures such as, among other things, enhanced hygiene and sanitation procedures, modified work schedules and social distancing protocols at its production facilities. Further, as recommended by certain jurisdictions in which it operates, the Company has implemented a documented COVID-19 prevention plan in these locations. The Company has and will continue to act in accordance with guidance from local, federal, and international health and governmental authorities, such as any government-mandated requirements for people to wear facemasks in all indoor communal spaces or at businesses, and is prepared to make additional operational adjustments, as necessary. Although the Company’s production facilities currently remain operational, governmental requirements, guidance and health and safety requirements continue to evolve as governmental and health authorities respond to the spread of the virus. Reopening plans in certain jurisdictions have been and may be further reversed, suspended or delayed and quarantines may be re-imposed. The Company’s production facilities may experience further temporary closures or quarantines if governmental or health authorities make changes to the businesses and workforces allowed to remain operational, which would result in reduced production or suspension of production if such closures or quarantines are mandated. In addition, to the extent the Company’s employees contract the virus, depending on the employee’s job duties and access to facilities, the Company’s ability to keep production and manufacturing facilities open may be impacted for health and safety reasons.
The impacts of certain closures or other restrictions on the conduct of business operations at the facilities of third-party manufacturers, suppliers and other vendors in the Company’s supply chain have not been significant to date but remain uncertain. Earlier closures at manufacturers in China in 2020 had resulted in delays of deliveries of batteries and cartridges for cannabis vaporizers and personal protective equipment, such as masks and gowns used in Cronos Group’s manufacturing facilities certified in accordance with Good Manufacturing Practices (“GMP”), from such manufacturers in China. Most delays have been ameliorated as manufacturers are now operational in China but other vendors or suppliers in the Company’s supply chain have been, and in the future could potentially be, impacted. Closures or other restrictions on the conduct of business operations on third-party manufacturers, suppliers or vendors may cause disruption in Cronos Group’s supply chain. As a result of a rise in infection rates late in the fourth quarter of 2020 and in the first quarter of 2021 in the U.S., certain contract manufacturers that manufacture U.S. hemp finished products for our U.S. business have experienced temporary closures or reductions in their operations leading to shortages of finished product available via the e-commerce channel. We expect these closures or reductions to ease and product shortages to be ameliorated as the spread of COVID-19 and infection rates decline. The Company has experienced delays in shipping and expects that the increased global demand on shipping and transport services, which has created challenges to container availability and port capacities, in addition to customs and border control policies put in place in response to the COVID-19 pandemic that require shipments to undergo a quarantine period, may cause the Company to experience further delays in the future which could impact the Company’s ability to obtain materials or deliver products in a timely and cost efficient manner. In addition, work-from-home policies for certain employees and the effects of the Company’s work-from-home policies may negatively impact productivity, disrupt access to books and records and disrupt the Company’s business. Even if the Company’s production facilities continue to remain open, mandatory or voluntary self-quarantines and travel restrictions may limit the Company’s employees’ ability to access facilities, and this together with impacts on the Company’s supply chain and the uncertainty produced by the rapidly evolving nature of the COVID-19 pandemic, may result in reduced or suspended production.Annual Report.
CustomersBusiness Segments
RetailersBeginning in the U.S. and Canada have been required to close at times or have curtailed their operations (such as reduced opening hours, reduced staff, limiting brand partner visits, and reduced traffic in stores) due to the implementation of health and safety measures. The slowdown or disruption faced by retailers, in addition to quarantine measures and travel restrictions, impacts the ability of customers to be able to access the Company’s products. These restrictions on retail stores are mandated by, and differ across, each state, province or territory and continue to change and evolve, which creates uncertainty in forecasting customer demand and sales velocity. In the U.S., while online sales have continued despite facing pressure, certain beauty and other retailers have temporarily closed physical boutiques. State specific limitations on retail capacity has also reduced the ability of larger retailers to offer in-store brand education for the Company’s products. In Canada, retailers have implemented a combination of measures from closing stores, offering curbside delivery (to the extent permitted by a province) and online sales only, reduced store opening hours and a reduction in the number of customers permitted in stores in light of social distancing measures. Suspensions of the ability of private retailers to offer click-and-collect or curbside delivery in certain provinces in Canada may further impact customer demand for products. Provincial purchasers have also similarly, among other things, reduced staff on-site leading to a decrease in delivery time slots for producers to deliver products or reduced frequency or size of their purchase orders. While various provinces in Canada have continued to increase the number of retail stores during the COVID-19 pandemic, we expect that the expansion of retail stores in various provinces in Canada will be delayed or slowed down in light of continued increases in COVID-19 cases. We anticipate that uncertainty created by these measures on forecasting customer demand and sales will continue as long as such measures are in place. Demand for the Company’s products could also be negatively impacted should the effects of COVID-19 lead to changes in consumer behavior, including as a result of a potential decline in the level of demand for vaporizer products or in discretionary spending as result of a general economic slowdown.
Macroeconomic impacts
The impacts of these current restrictions and measures on certain strategic projects continue to be uncertain. While facility design and expansion projects and product development initiatives are currently expected to proceed as planned, requirements and restrictions on the operation of businesses and workforces continue to evolve as governmental and health authorities respond to the spread of the virus and changes may result in delays or suspensions if authorities require such activities to be suspended.
In addition, a recession or market correction resulting from the spread of COVID-19 would likely materially affect the Company’s business and the value of its common shares. Collectively, the effects of the COVID-19 pandemic have adversely affected the Company’s results of operations and, if the effects continue unabated, could continue to do so as long as measures to combat the COVID-19 pandemic remain in effect. At this time, neither the duration nor scope of the disruption can be predicted; therefore, the ultimate impact to the Company’s business cannot be reasonably estimated but such impact could materially adversely affect the Company’s business and financial results.
Liquidity and capital resource impact
Despite the impacts of the COVID-19 pandemic, the Company believes that its significant cash on hand and short-term investments will be adequate to meet liquidity and capital requirements for at least the next twelve months. The impact of reduced interest rates has inhibited the Company’s ability to generate interest income in the short-term, but this has not, and is not expected to have, a material impact on the liquidity or capital resources of the Company.
2020 Business Highlights
Cronos Fermentation
In the second quarter of 2020,2023, following the exit of our U.S. operations, Cronos is reporting through one consolidated segment, which includes operations in both Canada and Israel. In Canada, Cronos operates two wholly owned license holder under the Cannabis Act (Canada) (the “Cannabis Act”), Peace Naturals Project Inc. (“Peace Naturals”), which has production facilities near Stayner, Ontario (the “Peace Naturals Campus”) and Thanos Holdings Ltd., known as Cronos Fermentation successfully fermented CBGA, one of(“Cronos Fermentation”), which has a production facility in Winnipeg, Manitoba. In Israel, the Company’s target cannabinoidsCompany operates under the Ginkgo Strategic Partnership, at research scale. Cronos Fermentation hasIMC-GAP, IMC-GMP and will continue to optimize downstream processing and scale up procedures in advance of receiving the final strains and commercial processing license, both of which areIMC-GDP certifications required for commercialization.
Management Appointments
On September 9, 2020, Cronos Group expanded its leadership structure to drive its next phasethe cultivation, production and marketing of growth by appointing Kurt Schmidt as Presidentdried flower, pre-rolls and Chief Executive Officer. This move coincided with Mike Gorenstein’s appointment to Executive Chairman. Mr. Schmidt brings deep experience in consumer products with decades of leadership experienceoils in the U.S. and overseas.Israeli medical market.
On August 31, 2020, the Company added Shannon Buggy as Senior Vice President, Global Head of People. With over 25 years of experience, Ms. Buggy has a proven track record of leading and managing global human resources teams and driving excellence in talent acquisition, development, retention, employee relations, compensation, benefits, talent management and labor relations.
On July 20, 2020, Summer Frein was named General Manager USA. Ms. Frein joined Cronos Group in January of 2020; however, she has worked with Cronos Group in various capacities since 2018. Under Ms. Frein’s leadership, the Company plans to further expand
its U.S. hemp-derived business, including introducing new product formats under both Lord Jones™ and Happy Dance™, that will target different retail channels and consumers.
Cronos Research Labs
In the third quarter of 2020, Cronos Device Labs expanded its scope for cannabinoid research and the R&D center was renamed to Cronos Research Labs. Cronos Group engages in both understanding the fundamental science behind the interactions of cannabinoids with each other and how those interactions can be leveraged to best deliver on the consumer’s needs. Additionally, the Company partners with leading scientific institutions engaged in fundamental cannabis science to augment and accelerate its internal efforts. Cronos Group’s work spans many aspects of cannabis research from strain development to growing conditions to extraction technology to biosynthesis to product development, all supported by advances in analytical sciences.
Recent Developments
Happy Dance™
Happy Dance™ continues its expansion by securing its first major U.S. retailer, ULTA Beauty™. The full collection of Happy Dance™ products is expected to launch online at ULTA.com and in-store at over 550 ULTA Beauty™ locations across the U.S. in the coming weeks. Happy Dance™ was co-founded by actress and New York Times best-selling author Kristen Bell and features an easy-to-use line of clean, vegan and cruelty-free U.S. hemp-derived CBD bath and body products including an All-Over Whipped Body Butter + CBD, Head-to-Toe Coconut Melt + CBD and Stress Away Bath Bomb + CBD.Israel-Hamas War
Cronos Israel
During 2020, Cronoscontinues to monitor the conflict in Israel received all certifications(the “Israel-Hamas War”) and licenses required forpotential impacts the cultivation, productionconflict could have on the Company’s personnel and marketing of dried flower, pre-rolls and oilsbusiness in Israel and currentlythe recorded amounts of assets and liabilities related to the Company’s operations in Israel. The extent to which the conflict may impact the Company’s personnel, business and activities will depend on future developments which remain highly uncertain and cannot be predicted. It is possible that the recorded amounts of assets and liabilities related to the Company’s operations in Israel could change materially in the near term.
New Israeli Shekel Fluctuation
In October 2023, with the onset of the aforementioned Israel-Hamas war, the New Israeli Shekel has fluctuated significantly against the U.S. dollar and Canadian dollar. As of January 31, 2024, the New Israeli Shekel has weakened to a position of 3.657 per U.S. dollar and a position of 2.729 per Canadian dollar, representing exchange rate increases of 4% and 3%, respectively, from the rates as of September 30, 2023. We cannot predict when or how the currency will fluctuate against the U.S. dollar and Canadian dollar and we have, as a result, experienced a volatile impact on our results of operations.
2023 Business Highlights
Brand and Product Portfolio
Throughout 2023, the Company expanded its brand and product portfolio globally with the following select new products:
Branded Product Portfolio Expansion in Canada:
•PEACE NATURALS™Q2 2023:
◦Spinach FEELZ™ Higher Dayz Mango Kiwi Haze THC:CBC pre-roll, infused with high potency cold filtered extract
◦Spinach FEELZ™ Deep Dreamz Blackberry Kush THC:CBN pre-roll, infused with high potency cold filtered extract
◦Spinach® Sonic Lemon fuel 3x0.5g pre-rolls & 3.5g dried flower
◦SOURZ by Spinach® Pink Lemonade gummy
◦Spinach® 28g Frosted Cream Puffs dried flower
•Q3 2023:
◦Six new Spinach® 1.2 gram vapes offerings, Peach Punch, Pink Lemonade, Strawberry Slurricane, Wavy Watermelon, Cotton Dandy Kush and oilsRocket Icicle
◦Spinach FEELZ® Full Tilt Blue Razz Durban THC:THCV vape and Spinach FEELZ™ Full Tilt Blue Raspberry Lemonade THC:THCV gummy products
◦Three new Spinach® Fully Charged infused pre-roll offerings, Peach Punch, Pink Lemonade and Strawberry Slurricane
◦Spinach® Atomic Sour Grapefruit 7g dried flower
◦Spinach® 3.5g Peach Gelato dried flower
•Q4 2023:
◦Lord Jones® Hash Fusions™, a line-up of premium ice water hash infused pre-rolls
◦SOURZ by Spinach® Strawberry Kiwi 5:1 CBD:THC 10-pack
◦Spinach FEELZ™ Deep Dreamz THC:CBN Drops infused oil
◦Spinach® 14g GMO Cookies dried flower
◦Spinach FEELZ™ Full Tilt Blue Razz Durban THC + THCV infused pre-roll
Branded Product Portfolio Expansion in market. On January 11, 2021, theIsrael:
•Q1 2023:
◦Two new PEACE NATURALS™ brand was recognized by the Israeli Marketing AssociationNATURALS® flower offerings - Atomic Sour Grapefruit and was given the 2020 Innovation Award forLemon Berry Cookies
•Q2 2023:
◦Two new PEACE NATURALS® pre-roll offerings - Wedding Rolls and Cocoa Bomba
•Q3 2023:
◦Five new PEACE NATURALS® flower offerings - Space Cake, Sticky Ape, Purple Punch, T-twist and Kobe OG
•Q4 2023
◦Three new PEACE NATURALS® flower offerings - Rockstar, Dancehall and Sonic Fuel
International Growth:
•Q3 2023: Cronos successfully completed its successful marketing strategy in 2020, which led to increased brand exposure. The marketing campaign gained this accolade for standing out amongst its peers by focusing on the high-quality naturefirst shipment of PEACE NATURALS™ products. In February 2021, Cronos Israel signed a distribution agreement with the largest pharmacy chain in Israel, Super-Pharm, which has over 250 branches in Israel. Cronos Israel continuesNATURALS® branded cannabis to build distribution and brand awarenessGermany through a growing network of pharmacies.strategic partnership with Cansativa GmbH, a leading German cannabis company.
Natuera•Q4 2023: Cronos successfully shipped cannabis flower to Vitura Health Limited for sale in the Australian medical market.
Throughout 2020, Natuera,Strategic and Organizational Update
In May 2023, Cronos simultaneously announced the Company’s joint venture in Latin America, a fully licensed operation in Colombia for hemp and cannabis derived bulk, consumer, and medicinal cannabinoid products, continued to achieve significant operational milestones. In addition to completing a numberdiscontinuation of test exports of hemp-derived CBD extract to both the U.S. segment and the United Kingdom for business development and R&D purposes overplanned repurposing of the course of 2020, duringLord Jones® brand by bringing the brand back to its adult-use roots in Canada. We launched the Lord Jones® brand in Canada in the fourth quarter of 2020, Natuera completed its first export2023.
In August 2023, following a careful evaluation of hemp-derived CBD extractthe Company’s global supply chain, the Company announced the planned wind-down of Thanos Holdings Ltd., known as Cronos Fermentation. The Company incurred $0.7 million in the year ended December 31, 2023, related to the U.S. for commercial purposes.wind-down of Cronos Fermentation and expects to incur charges of approximately $0.1 million of additional costs in connection with the ongoing exit. These charges include employee-related costs, such as severance, relocation and other termination benefits, as well as contract termination and other related costs. Cronos expects to continue to operate the Cronos Fermentation facility with a phased reduction and planned exit by the end of the first quarter 2024.
Cronos GrowCoAlso in August 2023, the Company announced organization-wide cost reductions. The Company incurred $0.8 million in the year ended December 31, 2023, related to the cost reductions and expects to incur additional restructuring costs of approximately $0.1 million in connection with the cost reductions, which include mostly one-time employee-related severance charges.
In 2020, Cronos GrowCo, the Company’s joint venture in Canada, fully completed constructionfourth quarter of its production facility, including all fixtures within2023, the greenhouse and all post-harvest activity areas. In November 2020, Cronos GrowCo obtained a cultivation licenseCompany entered into an agreement for the operations contemplatedsale and leaseback of the Peace Naturals Campus for an aggregate purchase price of C$23 million in cash, subject to certain terms and conditions. At closing the parties expect to enter into a lease agreement with respect to portions of Building 4 and Building 3 on the Peace Naturals Campus. The lease will have an initial term of five years with one five-year renewal option that may be exercised by the first phase of the project. The Company expects the facility to become operational in phases beginning in the first half of 2021.Company.
2019 Financial Results2022 Compared to 20182021
Cash Flows
For a discussion of our 2019 financial results2022 cash flows compared to 2018,2021, see Part II, Item 7 “Management’s Discussion and Analysis of Financial Condition and Results of Operations”,Operations,” in our Annual Report on Form 10K/A10-K for the year ended December 31, 2019.
Foreign currency exchange rates
All currency amounts in this Annual Report are stated in U.S. dollars, which is our reporting currency, unless otherwise noted. All references to “dollars” or “$” are to U.S. dollars. The assets and liabilities of the Company’sour foreign operations are translated into dollars at the exchange rate in effect as of December 31, 2020, December 31, 20192023 and December 31, 2018,2022, as reported on Bloomberg. Transactions affecting the shareholders’ equity (deficit) are translated at historical foreign exchange rates. The consolidated statements of net income (loss)loss and comprehensive income (loss)loss and consolidated statements of cash flows of the Company’sour foreign operations are translated into dollars by applying the average foreign exchange rate in effect for the reporting period,years ended December 31, 2023, December 31, 2022, and December 31, 2021, as reported on Bloomberg.
The exchange rates used to translate from Canadian dollars (“C$”) to dollars isare shown below:
| | (Exchange rates are shown as C$ per $) | (Exchange rates are shown as C$ per $) | Year ended December 31, | (Exchange rates are shown as C$ per $) | Year ended December 31, |
| 2020 | | 2019 | | 2018 |
| 2023 | | | 2023 | | 2022 | | 2021 |
| Average rate | Average rate | 1.3411 | | | 1.3268 | | 1.2955 | Average rate | 1.3494 | | 1.3017 | | 1.2541 |
| Spot rate | Spot rate | 1.2751 | | | 1.2990 | | 1.3639 | Spot rate | 1.3243 | | 1.3554 | | 1.2746 |
The exchange rates used to translate from New Israeli Shekels (“ILS”) to dollars are shown below:
| | | | | | | | | | | | | | | | | |
| (Exchange rates are shown as ILS per $) | Year ended December 31, |
| 2023 | | 2022 | | 2021 |
| Average rate | 3.6819 | | 3.3566 | | 3.2297 |
| Spot rate | 3.6163 | | 3.5178 | | 3.1149 |
Consolidated Results of Operations: FY 2020 compared with FY 2019Operations - 2023 Compared to 2022
The tables below set forth our consolidated results of operations, expressed in thousands of U.S. dollars for the periods presented. Our consolidated financial results for these periods are not necessarily indicative of the consolidated financial results that we will achieve in future periods.
| | | | | | | | | | | |
| Year ended December 31, |
| 2023 | | 2022 |
| Net revenue before excise taxes | $ | 120,270 | | $ | 109,301 |
| Excise taxes | (33,029) | | (22,552) |
| Net revenue | 87,241 | | 86,749 |
| Cost of sales | 74,527 | | 71,313 |
| Inventory write-down | 805 | | — |
| Gross profit | 11,909 | | 15,436 |
| Operating expenses: | | | |
| Sales and marketing | 22,701 | | 18,046 |
| Research and development | 5,843 | | 13,131 |
| General and administrative | 49,475 | | 67,674 |
| Restructuring costs | 1,524 | | 3,545 |
| Share-based compensation | 8,756 | | 15,008 |
| Depreciation and amortization | 5,044 | | 5,967 |
| | | |
| Impairment loss on long-lived assets | 3,366 | | 3,493 |
| Total operating expenses | 96,709 | | 126,864 |
| Operating loss | (84,800) | | (111,428) |
| Other income (expense) | 11,131 | | (9,575) |
| Income tax benefit (expense) | 3,230 | | (34,175) |
| Loss from discontinued operations | (4,114) | | (13,556) |
| Net loss | (74,553) | | (168,734) |
| Net loss attributable to non-controlling interest | (590) | | — |
| Net loss attributable to Cronos Group | $ | (73,963) | | $ | (168,734) |
Summary of select financial results - consolidated
| | | | | | | | | | | | | | | | | | | | | | | |
| (In thousands of U.S. dollars) | Year ended December 31, | | Change |
| 2020 | | 2019 | | $ | | % |
| Net revenue | $ | 46,719 | | | $ | 23,750 | | | $ | 22,969 | | | 97 | % |
| | | | | | | |
| Gross profit (loss) | (25,833) | | | (17,597) | | | (8,236) | | | 47 | % |
| Gross margin | (55) | % | | (74) | % | | N/A | | 19 | pp |
| | | | | | | |
Adjusted EBITDA (i) | $ | (147,253) | | | $ | (98,308) | | | $ | (48,945) | | | 50 | % |
| | | | | | | | | | | | | | | | | | | | | | | |
| Year ended December 31, | | Change |
| 2023 | | 2022 | | $ | | % |
| Net revenue | $ | 87,241 | | | $ | 86,749 | | | $ | 492 | | | 1 | % |
| Cost of sales | 74,527 | | | 71,313 | | | 3,214 | | | 5 | % |
| Inventory write-down | 805 | | | — | | | 805 | | | N/M |
| Gross profit | 11,909 | | | 15,436 | | | (3,527) | | | (23) | % |
Gross margin(i) | 14 | % | | 18 | % | | N/A | | (4) | pp |
(i) See “Non-GAAP Measures” for information related to Non-GAAP measure.
Net revenue - consolidated
| | | | | | | | | | | | | | | | | | | | | | | |
| (In thousands of U.S. dollars) | Year ended December 31, | | Change |
| 2020 | | 2019 | | $ | | % |
Net revenue, before excise taxes (i) | $ | 54,353 | | | $ | 25,639 | | | $ | 28,714 | | | 112 | % |
| Excise taxes | (7,634) | | | (1,889) | | | (5,745) | | | 304 | % |
| Net revenue | 46,719 | | | 23,750 | | | 22,969 | | | 97 | % |
(i) Net revenue, before excise taxes,Gross margin is calculateddefined as gross profit divided by net of sales returns and discounts as described under Note 4 to the consolidated financial statements.
For the fiscal year 2020 (“FY 2020”), we reported net revenue of $46.7 million, representing an increase of $23.0 million from the fiscal year 2019 (“FY 2019”). This change was primarily due to:
•An increase in sales in the Rest of World (“ROW”) segment in FY 2020 compared to FY 2019, due to the continued growth of the adult-use market in Canada and sales in the Israeli medical market, partially offset by non-recurring wholesale revenue in the Canadian market in FY 2019 and strategic price reductions on various adult-use cannabis products in Canada in FY 2020.
•An increase in net revenue in the U.S. segment of $6.1 million in FY 2020 compared to FY 2019 due to FY 2020 results including a full year of the Redwood business as opposed to 117 days in FY 2019.revenue.
Cost of sales and gross profit (loss) – consolidated
| | | | | | | | | | | | | | | | | | | | | | | |
| (In thousands of U.S. dollars) | Year ended December 31, | | Change |
| 2020 | | 2019 | | $ | | % |
| Cost of sales | $ | 46,497 | | | $ | 12,174 | | | $ | 34,323 | | | 282 | % |
| Inventory write-down | 26,055 | | | 29,173 | | | (3,118) | | | (11) | % |
| | | | | | | |
| Gross profit (loss) | (25,833) | | | (17,597) | | | (8,236) | | | 47 | % |
| Gross margin | (55) | % | | (74) | % | | N/A | | 19 | pp |
For FY 2020,2023, we reported gross lossconsolidated net revenue of $25.8$87.2 million, representing ana $0.5 million increase in losses of $8.2 million from FY 2019.2022. This change was primarily due to:to higher cannabis flower and extract sales in the Canadian adult-use market and the initiation of sales in Germany and Australia, partially offset by lower cannabis flower sales in Israel driven by pricing pressure as a result of competitive activity, the slowdown in patient permit authorizations and the Israel-Hamas War, an adverse price/mix in the Canadian cannabis flower category driving increased excise tax payments as a percentage of revenue, and the impact of the weakened Canadian dollar and New Israeli Shekel against the U.S. dollar during the period.
•An increase in ROWCost of sales
For 2023, we reported consolidated cost of sales primarily driven by third party purchased flower associated with adult-use products in Canada andof $74.5 million, representing a decline in wholesale sales in FY 2020 versus FY 2019.
•Partially offset by increased gross profit in the U.S. segment in FY 2020 compared to FY 2019,$3.2 million increase from 2022. This was primarily due to higher cannabis flower and extract sales in the Canadian adult-use market, partially offset by lower cannabis flower sales in the Israeli medical market, lower inventory reserves, lower cannabis biomass costs and the impact of the weakened Canadian dollar and New Israeli Shekel against the U.S. dollar during the period.
Inventory write-down
For 2023, we reported inventory write-downs of $0.8 million, as a full yearresult of the Company’s decision to wind down operations at a production facility in Winnipeg, Manitoba, held by license holder Cronos Fermentation. For further information, see Note 16 “Restructuring” to the consolidated financial statements in Item 8 of this Annual Report.
Gross profit
For 2023, we reported consolidated gross profit in FY 2020 versus 117 days in FY 2019 andof $11.9 million, representing a $3.5 million decrease from 2022. The decrease in inventory write-downsgross profit was primarily due to lower cannabis flower sales in the ROW segment. We may incur furtherIsraeli medical market, an adverse price/mix on cannabis flower sales in Canada resulting in higher excise taxes as a percentage of revenue and the inventory write-downswrite-down recognized as a result of the decision to wind down operations at Cronos Fermentation, partially offset by higher cannabis flower and extract sales in the Canadian adult-use market.
Operating expenses
| | | | | | | | | | | | | | | | | | | | | | | |
| Year ended December 31, | | Change |
| 2023 | | 2022 | | $ | | % |
| Sales and marketing | $ | 22,701 | | | $ | 18,046 | | | $ | 4,655 | | | 26 | % |
| Research and development | 5,843 | | | 13,131 | | | (7,288) | | | (56) | % |
| General and administrative | 49,475 | | | 67,674 | | | (18,199) | | | (27) | % |
| Restructuring costs | 1,524 | | | 3,545 | | | (2,021) | | | (57) | % |
| Share-based compensation | 8,756 | | | 15,008 | | | (6,252) | | | (42) | % |
| Depreciation and amortization | 5,044 | | | 5,967 | | | (923) | | | (15) | % |
| | | | | | | |
| Impairment loss on long-lived assets | 3,366 | | | 3,493 | | | (127) | | | (4) | % |
| Operating expenses | $ | 96,709 | | | $ | 126,864 | | | $ | (30,155) | | | (24) | % |
Sales and marketing
For 2023, we reported sales and marketing expenses of $22.7 million, representing an increase of $4.7 million from 2022. The increase was primarily due to pricing pressures in the marketplace.higher advertising and marketing spend.
Adjusted EBITDA – consolidated
| | | | | | | | | | | | | | | | | | | | | | | |
| (In thousands of U.S. dollars) | Year ended December 31, | | Change |
| 2020 | | 2019 | | $ | | % |
Adjusted EBITDA (i) | $ | (147,253) | | | $ | (98,308) | | | $ | (48,945) | | | 50 | % |
| | | | | | | |
(i)See “Non-GAAP Measures” for information related to non-GAAP measures.Research and development
For FY 2020,2023, we reported an adjusted earnings (loss) before interest, taxes, depreciationresearch and amortization (“Adjusted EBITDA”)development expenses of $(147.3)$5.8 million, representing a decrease in Adjusted EBITDA of $48.9$7.3 million from FY 2019.2022. This decrease was primarily due to lower costs associated with the collaboration and license agreement between Ginkgo Bioworks Holdings, Inc. (“Ginkgo”) and the Company (the “Ginkgo Collaboration Agreement”).
General and administrative
For 2023, we reported general and administrative expenses of $49.5 million, representing a decrease of $18.2 million from 2022. The decrease was primarily due to lower professional fees, largely related to lower financial statement review costs, and lower bonus, payroll and insurance costs.
Restructuring costs
For 2023, we reported restructuring costs of $1.5 million, representing a decrease of $2.0 million from 2022. The restructuring costs in 2023 and 2022 were related to Realignment activities. For further information, see Note 16 “Restructuring” to the consolidated financial statements in Item 8 of this Annual Report.
Share-based compensation
For 2023, we reported share-based compensation expenses of $8.8 million, representing a decrease of $6.3 million from 2022. The decrease was primarily due to the 2022 acceleration of expense on equity awards granted to certain executive employees in connection with their separation from the Company as well as the approval of the grant of previously held-back equity awards granted in 2022 to certain executives in connection with the settlements with the Securities and Exchange Commission (the “SEC”) and the Ontario Securities Commission (the “OSC”). For further information, see Note 11 “Share-based Compensation” to the consolidated financial statements in Item 8 of this Annual Report.
Depreciation and amortization
For 2023, depreciation and amortization expenses were $5.0 million, representing a decrease of $0.9 million from 2022. The decrease was primarily due to the recognition of certain tax credits related to the Company’s fixed assets, partially offset by higher amortization on Ginkgo-related intangible assets.
Impairment loss on long-lived assets
During 2023, we recorded a $3.4 million impairment charge related to our Ginkgo exclusive license for cannabichromevarinic acid (“CBCVA”). For 2022, we recorded $3.5 million of impairment charges related to the right-of-use lease asset and leasehold improvements associated with our corporate headquarters in Toronto, Ontario, Canada, which the Company has subleased in part. See Note 7 “Goodwill and Intangible Assets, net”, Note 6 “Property, plant and equipment, net” and Note 8 “Leases” to the consolidated financial statements in Item 8 of this Annual Report for additional information.
Total other income, income tax benefit (expense) and loss from discontinued operations
| | | | | | | | | | | | | | | | | | | | | | | |
| Year ended December 31, | | Change(i) |
| 2023 | | 2022 | | $ | | % |
| Interest income, net | $ | 51,235 | | | $ | 22,514 | | | $ | 28,721 | | | 128 | % |
| Gain (loss) on revaluation of derivative liabilities | (85) | | | 14,060 | | | (14,145) | | | N/M |
| Impairment loss on other investments | (23,350) | | | (61,392) | | | 38,042 | | | 62 | % |
| Share of income from equity method investments | 1,583 | | | 3,114 | | | (1,531) | | | (49) | % |
| Gain (loss) on revaluation of financial instruments | (12,042) | | | 14,739 | | | (26,781) | | | N/M |
| Foreign currency transaction loss | (7,324) | | | (2,286) | | | (5,038) | | | (220) | % |
| Other, net | 1,114 | | | (324) | | | 1,438 | | | N/M |
| Total other income | 11,131 | | | (9,575) | | | 20,706 | | | N/M |
| Income tax benefit (expense) | 3,230 | | | (34,175) | | | 37,405 | | | N/M |
| Loss from discontinued operations | (4,114) | | | (13,556) | | | 9,442 | | | 70 | % |
| Net loss | $ | (74,553) | | | $ | (168,734) | | | $ | 94,181 | | | 56 | % |
(i)“N/M” is defined as not meaningful.
Interest income, net
For 2023, we reported interest income, net of $51.2 million, representing an increase of $28.7 million from 2022 primarily due to higher interest rates and higher short-term investment balances during 2023.
Gain (loss) on revaluation of derivative liabilities
For 2023, we reported a loss on revaluation of derivative liabilities of $0.1 million, representing a change of $14.1 million compared to a gain in 2022 related to the irrevocable relinquishment by Altria of its warrant on December 16, 2022. We expect continued changes in derivative valuations as our share price fluctuates period to period and the remaining expected terms of our derivative instruments change over time. For further information, see Note 9 “Derivative Liabilities” to the consolidated financial statements in Item 8 of this Annual Report.
Impairment loss on other investments
For 2023, we recognized $23.4 million of impairment loss on other investments, driven by an impairment charge recorded on our PharmaCann Option for the difference between its estimated fair value and its carrying amount. For 2022, impairment loss on other investments was $61.4 million, driven by impairment charges recorded on our PharmaCann Option for the difference between its estimated fair value and its carrying amount. For more information, see Note 4 “Investments” to the consolidated financial statements in Item 8 of this Annual Report.
Share of income from equity method investments
For 2023, we reported share of income from equity method investments of $1.6 million, representing a decrease of $1.5 million from 2022. The decrease was primarily due to lower income pick-ups from our equity method investment in Cronos GrowCo. Periodic income and loss pickups from our equity method investment in Cronos GrowCo are impacted both by the profitability of Cronos GrowCo and any unsold inventory remaining at Cronos at the end of the period that originated from Cronos GrowCo.
Gain (loss) on revaluation of financial instruments
For 2023, we reported a loss on revaluation of financial instruments of $12.0 million, compared to a gain of $14.7 million in 2022. The change was due to the change in fair value of our investment in Vitura. See Note 4 “Investments” to the consolidated financial statements in Item 8 of this Annual Report for additional information.
Foreign currency transaction loss
For 2023, foreign currency transaction loss was $7.3 million, representing an increased loss of $5.0 million from 2022. The change was primarily due to certain foreign currency-denominated cash equivalents and short-term investments and certain foreign currency-denominated intercompany loans anticipated to be settled in the foreseeable future.
Other, net
For 2023, other, net was income of $1.1 million, compared to a loss of $0.3 million in 2022. The change was primarily driven by gains on the disposal of assets.
Income tax benefit (expense)
For 2023, we reported an income tax benefit of $3.2 million, compared to an income tax expense of $34.2 million in 2022. The change was due primarily to a capital gain for tax purposes of $479.8 million, which resulted in an income tax liability of $34.2 million, related to the irrevocable relinquishment by Altria of the Warrant on December 16, 2022.
Loss from discontinued operations
| | | | | | | | | | | | | | | | | | | | | | | |
| Year ended December 31, | | Change |
| 2023 | | 2022 | | $ | | % |
| Net revenue | $ | 1,029 | | $ | 5,155 | | $ | (4,126) | | (80)% |
| Cost of sales | 2,164 | | 8,622 | | (6,458) | | (75)% |
| Inventory write-down | 839 | | — | | 839 | | N/M |
| Gross profit | (1,974) | | (3,467) | | 1,493 | | 43% |
| Gross margin | (192)% | | (67)% | | N/A | | (125) | pp |
| Operating expenses | | | | | | | |
| Sales and marketing | 578 | | 4,236 | | (3,658) | | (86)% |
| Research and development | 32 | | 250 | | (218) | | (87)% |
| General and administrative | 668 | | 3,504 | | (2,836) | | (81)% |
| Restructuring costs | 523 | | 1,788 | | (1,265) | | (71)% |
| Share-based compensation | 13 | | 107 | | (94) | | (88)% |
| Depreciation and amortization | 13 | | 58 | | (45) | | (78)% |
| Impairment loss on long-lived assets | 205 | | — | | 205 | | N/M |
| Total operating expenses | 2,032 | | 9,943 | | (7,911) | | (80)% |
| Interest income | 10 | | 23 | | (13) | | (57)% |
| Other, net | (118) | | (169) | | 51 | | 30% |
| Total other loss | (108) | | (146) | | 38 | | 26% |
| Loss before income taxes | (4,114) | | (13,556) | | 9,442 | | 70% |
| Income tax expense (benefit) | — | | — | | — | | N/M |
| Net loss from discontinued operations | $ | (4,114) | | $ | (13,556) | | $ | 9,442 | | 70% |
For 2023, we reported a loss from discontinued operations of $4.1 million, compared to a loss from discontinued operations of $13.6 million in 2022. The decreased loss was driven by the decrease in operating expenses as a result of the exit of U.S. operations in the second quarter of 2023. See Note 2 “Discontinued Operations” to the consolidated financial statements in Item 8 of this Annual Report for additional information.
Consolidated Results of Operations - 2022 Compared to 2021
The tables below set forth our consolidated results of operations, expressed in thousands of U.S. dollars for the periods presented. Our consolidated financial results for these periods are not necessarily indicative of the consolidated financial results that we will achieve in future periods.
| | | | | | | | | | | |
| Year ended December 31, |
| 2022 | | 2021 |
| Net revenue before excise taxes | $ | 109,301 | | $ | 79,612 |
| Excise taxes | (22,552) | | (15,051) |
| Net revenue | 86,749 | | 64,561 |
| Cost of sales | 71,313 | | 70,193 |
| Inventory write-down | — | | 11,961 |
| Gross profit | 15,436 | | (17,593) |
| Operating expenses: | | | |
| Sales and marketing | 18,046 | | 20,917 |
| Research and development | 13,131 | | 21,841 |
| General and administrative | 67,674 | | 90,919 |
| Restructuring costs | 3,545 | | — |
| Share-based compensation | 15,008 | | 9,844 |
| Depreciation and amortization | 5,967 | | 4,413 |
| Impairment loss on goodwill and indefinite-lived intangible assets | — | | 37 |
| Impairment loss on long-lived assets | 3,493 | | 126,405 |
| Total operating expenses | 126,864 | | 274,376 |
| Operating loss | (111,428) | | (291,969) |
| Other income (expense) | (9,575) | | 163,459 |
| Income tax benefit (expense) | (34,175) | | 431 |
| Loss from discontinued operations | (13,556) | | (269,125) |
| Net loss | (168,734) | | (397,204) |
| Net loss attributable to non-controlling interest | — | | (1,097) |
| Net loss attributable to Cronos Group | $ | (168,734) | | $ | (396,107) |
Summary of select financial results
| | | | | | | | | | | | | | | | | | | | | | | |
| Year ended December 31, | | Change |
| 2022 | | 2021 | | $ | | % |
| Net revenue | $ | 86,749 | | | $ | 64,561 | | | $ | 22,188 | | | 34 | % |
| Cost of sales | 71,313 | | | 70,193 | | | 1,120 | | | 2 | % |
| Inventory write-down | — | | | 11,961 | | | (11,961) | | | (100) | % |
| Gross profit | 15,436 | | | (17,593) | | | 33,029 | | | 188 | % |
Gross margin(i) | 18 | % | | (27) | % | | N/A | | 45 | pp |
(i)Gross margin is defined as gross profit divided by net revenue.
Net revenue
For 2022, we reported consolidated net revenue of $86.7 million, representing a $22.2 million increase from 2021. This change was primarily due to:to higher cannabis flower sales in the Israeli medical market and higher cannabis extract sales in the Canadian adult-use market, partially offset by lower cannabis flower sales in the Canadian adult-use market driven by an unfavorable price/mix shift and the impact of the weakening Canadian dollar against the U.S. dollar during 2022.
Cost of sales
For 2022, we reported consolidated cost of sales of $71.3 million, representing a $1.1 million increase from 2021, despite a 34% increase in net revenue. This was primarily due to lower cannabis biomass costs and the impact of the weakening Canadian dollar against the U.S. dollar during the period, partially offset by higher sales volumes and lower fixed cost absorption due to the timing of the wind-down of cultivation and certain production activities associated with the change in the nature of operations at the Peace Naturals Campus.
Inventory write-down
For 2021, we reported inventory write-downs of $12.0 million, primarily related to cannabis strains and potency levels that were no longer in-line with consumer preferences in the Canadian market and adjustments for obsolete inventory in Canada. We reported no inventory write-downs for 2022.
Gross profit
For 2022, we reported consolidated gross profit of $15.4 million, representing a $33.0 million improvement from 2021. The improvement in gross profit is primarily due to increased revenue driven mainly by a favorable mix of cannabis extract products, which carry a higher gross profit and gross margin than other product categories, higher sales of cannabis flower in Israel, the absence of inventory write-downs in 2022, and lower cannabis biomass costs, partially offset by lower fixed cost absorption due to the timing of the wind-down of cultivation and certain production activities associated with the change in the nature of operations at the Peace Naturals Campus.
Operating expenses
| | | | | | | | | | | | | | | | | | | | | | | |
| Year ended December 31, | | Change |
| 2022 | | 2021 | | $ | | % |
| Sales and marketing | $ | 18,046 | | | $ | 20,917 | | | $ | (2,871) | | | (14) | % |
| Research and development | 13,131 | | | 21,841 | | | (8,710) | | | (40) | % |
| General and administrative | 67,674 | | | 90,919 | | | (23,245) | | | (26) | % |
| Restructuring costs | 3,545 | | | — | | | 3,545 | | | 100 | % |
| Share-based compensation | 15,008 | | | 9,844 | | | 5,164 | | | 52 | % |
| Depreciation and amortization | 5,967 | | | 4,413 | | | 1,554 | | | 35 | % |
| Impairment loss on goodwill and indefinite-lived intangible assets | — | | | 37 | | | (37) | | | (100) | % |
| Impairment loss on long-lived assets | 3,493 | | | 126,405 | | | (122,912) | | | (97) | % |
| Operating expenses | $ | 126,864 | | | $ | 274,376 | | | $ | (147,512) | | | (54) | % |
Sales and marketing
For 2022, we reported sales and marketing expenses of $18.0 million, representing a decrease of $2.9 million from 2021. The decrease was primarily due to lower advertising and marketing spend.
Research and development
For 2022, we reported research and development expenses of $13.1 million, representing a decrease of $8.7 million from 2021. This decrease was primarily due to lower costs associated with the Ginkgo Collaboration Agreement.
General and administrative
For 2022, we reported general and administrative expenses of $67.7 million, representing a decrease of $23.2 million from 2021. The decrease was primarily due to lower expected credit losses on our loans to joint venture partners when compared to 2021, lower legal and advisory fees associated with strategic initiatives and lower personnel-related costs associated with the Realignment.
Restructuring costs
For 2022, we reported restructuring costs of $3.5 million, compared to no restructuring costs in 2021. The restructuring costs in 2022 were related to Realignment activities. For further information, see Note 16 “Restructuring” to the consolidated financial statements in Item 8 of this Annual Report.
Share-based compensation
For 2022, we reported share-based compensation expenses of $15.0 million, representing an increase of $5.2 million from 2021. The increase was primarily due to the acceleration of expense on equity awards granted to certain executive employees in connection with their separation from the Company as well as the approval of the grant of previously held-back equity awards granted to certain executives in connection with the SEC and OSC settlements. For further information, see Note 11 “Share-based Compensation” to the consolidated financial statements in Item 8 of this Annual Report.
Depreciation and amortization
For 2022, depreciation and amortization expenses were $6.0 million, representing an increase of $1.6 million from 2021. The increase was primarily due to higher amortization on our Ginkgo exclusive license intangible assets.
Impairment loss on goodwill and indefinite-lived intangible assets
For 2021, we reported impairment loss on goodwill and intangible assets of $37 thousand due to impairment charges on our PEACE+™ trademark. For 2022, we reported no such impairment losses. For further information, see Note 7 “Goodwill and Intangible Assets, net” to the consolidated financial statements in Item 8 of this Annual Report.
Impairment loss on long-lived assets
During 2022, we recorded impairment charges of $3.5 million related to the right-of-use lease asset and leasehold improvements associated with our corporate headquarters in Toronto, Ontario, Canada, which the Company has subleased in part. For 2021, we recorded an impairment charge of $119.9 million on long-lived assets related to the previously announced planned exit from the Peace Naturals Campus. Additionally, in 2021, we recorded impairment charges of $4.8 million related to our Ginkgo exclusive licenses for cannabigerolic acid (“CBGA”) and cannabigerovarinic acid (“CBGVA”) for the difference between the fair value of the licenses and the consideration paid. See Note 6 “Property, plant and equipment, net”, Note 7 “Goodwill and Intangible Assets, net” and Note 8 “Leases” to the consolidated financial statements in Item 8 of this Annual Report for additional information.
Total other income, income tax benefit (expense) and loss from discontinued operations
| | | | | | | | | | | | | | | | | | | | | | | |
| Year ended December 31, | | Change(i) |
| 2022 | | 2021 | | $ | | % |
| Interest income, net | $ | 22,514 | | | $ | 9,068 | | | $ | 13,446 | | | 148 | % |
| Gain on revaluation of derivative liabilities | 14,060 | | | 151,360 | | | (137,300) | | | (91) | % |
| Impairment loss on other investments | (61,392) | | | — | | | (61,392) | | | N/M |
| Share of income (loss) from equity method investments | 3,114 | | | (6,313) | | | 9,427 | | | 149 | % |
| Gain on revaluation of financial instruments | 14,739 | | | 8,611 | | | 6,128 | | | 71 | % |
| Foreign currency transaction loss | (2,286) | | | — | | | (2,286) | | | N/M |
| Other, net | (324) | | | 733 | | | (1,057) | | | (144) | % |
| Total other income | (9,575) | | | 163,459 | | | (173,034) | | | (106) | % |
| Income tax benefit (expense) | (34,175) | | | 431 | | | (34,606) | | | N/M |
| Loss from discontinued operations | (13,556) | | | (269,125) | | | 255,569 | | | 95 | % |
| Net loss | $ | (168,734) | | | $ | (397,204) | | | $ | 228,470 | | | 58 | % |
(i)“N/M” is defined as not meaningful.
Interest income, net
For 2022, we reported interest income, net of $22.5 million, representing an increase of $13.4 million from 2021 primarily due to higher short-term investment balances and higher interest rates in 2022 when compared to 2021.
Gain on revaluation of derivative liabilities
For 2022, we reported a gain on revaluation of derivative liabilities of $14.1 million, representing a decrease of $137.3 million from 2021 primarily due to the greater impact on the derivative liabilities in 2021 from the decreased estimated term of the derivative instruments and the decreased price of Cronos common shares.
Impairment loss on other investments
For 2022, impairment loss on other investments was $61.4 million, driven by impairment charges recorded on our PharmaCann Option for the difference between its estimated fair value and its carrying amount. There were no such impairment losses on other investments during 2021. For more information, see Note 4 “Investments” to the consolidated financial statements in Item 8 of this Annual Report.
Share of income (loss) from equity method investments
For 2022, we reported share of income from equity method investments of $3.1 million, representing an increase of $9.4 million from 2021. The change was primarily due to improved results from our equity method investment in Cronos GrowCo.
Gain (loss) on revaluation of financial instruments
For 2022, we reported a gain on revaluation of financial instruments of $14.7 million, representing an increase of $6.1 million from 2021. The increase was due to the change in fair value of our investment in Vitura. See Note 4 “Investments” to the consolidated financial statements in Item 8 of this Annual Report for additional information.
Foreign currency transaction loss
For 2022, foreign currency transaction loss was $2.3 million, which related to certain foreign currency-denominated intercompany loans anticipated to be settled in the foreseeable future. There were no such foreign currency transaction gains or losses during 2021.
Other, net
For 2022, other, net was a loss of $0.3 million, compared to income of $0.7 million in 2021. The change was primarily due to loss on disposal of assets associated with the Realignment, partially offset by $0.4 million of dividend income from our Vitura investment during 2022.
Income tax benefit (expense)
For 2022, we reported income tax expense of $34.2 million, compared to an income tax benefit of $0.4 million in 2021. The change was due primarily to a capital gain for tax purposes of $479.8 million, which resulted in an income tax liability of $34.2 million, related to the irrevocable relinquishment by Altria of its warrant on December 16, 2022.
Loss from discontinued operations
| | | | | | | | | | | | | | | | | | | | | | | |
| Year ended December 31, | | Change(i) |
| 2022 | | 2021 | | $ | | % |
| Net revenue | $ | 5,155 | | $ | 9,874 | | $ | (4,719) | | | (48) | % |
| Cost of sales | 8,622 | | 9,850 | | (1,228) | | | (12) | % |
| | | | | | | |
| Gross profit | (3,467) | | 24 | | (3,491) | | | (14,546) | % |
| Gross margin | (67)% | | —% | | N/A | | (67) | pp |
| Operating expenses | | | | | | | |
| Sales and marketing | 4,236 | | 24,020 | | (19,784) | | | (82) | % |
| Research and development | 250 | | 1,490 | | (1,240) | | | (83) | % |
| General and administrative | 3,504 | | 6,133 | | (2,629) | | | (43) | % |
| Restructuring costs | 1,788 | | — | | 1,788 | | | N/M |
| Share-based compensation | 107 | | 307 | | (200) | | | (65) | % |
| Depreciation and amortization | 58 | | 71 | | (13) | | | (18) | % |
| Impairment loss on goodwill and indefinite-lived intangible assets | — | | 236,019 | | (236,019) | | | N/M |
| Impairment loss on long-lived assets | — | | 1,214 | | (1,214) | | | N/M |
| Total operating expenses | 9,943 | | 269,254 | | (259,311) | | | (96) | % |
| Interest income | 23 | | 4 | | 19 | | | 475 | % |
| Other, net | (169) | | 101 | | (270) | | | (267) | % |
| Total other loss | (146) | | 105 | | (251) | | | N/M |
| Loss before income taxes | (13,556) | | (269,125) | | 255,569 | | | 95 | % |
| Income tax expense (benefit) | — | | — | | — | | | N/M |
| Net loss from discontinued operations | $ | (13,556) | | $ | (269,125) | | $ | 255,569 | | | 95 | % |
For 2022, we reported loss from discontinued operations of $13.6 million, compared to a loss from discontinued operations of $269.1 million in 2021. The decreased loss was driven by the $236.0 impairment loss on goodwill and indefinite-lived intangible assets in 2021. See Note 2 “Discontinued Operations” to the consolidated financial statements in Item 8 of this Annual Report for additional information.
Non-GAAP Measures
Cronos reports its financial results in accordance with Generally Accepted Accounting Principles in the United States (“U.S. GAAP”). This Annual Report refers to measures not recognized under U.S. GAAP (“non-GAAP measures”). These non-GAAP measures do not have a standardized meaning prescribed by U.S. GAAP and are therefore unlikely to be comparable to similar measures presented by other companies. Rather, these non-GAAP measures are provided as a supplement to corresponding U.S. GAAP measures to provide additional information regarding our results of operations from management’s perspective. Accordingly, non-GAAP measures should not be considered a substitute for, or superior to, the financial information prepared and presented in accordance with U.S. GAAP. All non-GAAP measures presented in this Annual Report are reconciled to their closest reported U.S. GAAP measure. Reconciliations of historical adjusted financial measures to corresponding U.S. GAAP measures are provided below.
Adjusted EBITDA
Management reviews Adjusted EBITDA, a non-GAAP measure, which excludes non-cash items and items that do not reflect management’s assessment of ongoing business performance. Management defines Adjusted EBITDA as net income (loss) before interest, tax expense (benefit), depreciation and amortization adjusted for: share of (income) loss from equity method investments; impairment loss on goodwill and intangible assets; impairment loss on long-lived assets; (gain) loss on revaluation of derivative liabilities; (gain) loss on revaluation of financial instruments; transaction costs related to strategic projects; impairment loss on other investments; foreign currency transaction loss; other, net; loss from discontinued operations; restructuring costs; inventory write-downs resulting from restructuring actions; share-based compensation; and financial statement review costs and reserves related to the restatements of our 2019 and 2021 interim financial statements (the “Restatements”), including the costs related to the settlement of the SEC’s and the OSC’s investigations of the Restatements and legal costs defending shareholder class action complaints brought against us as a result of the 2019 restatement (see Note 10(b) “Contingencies,” to the consolidated financial statements under Item 8 of this Annual Report for a discussion of the shareholder class action complaints relating to the restatement of the 2019 interim financial statements and the settlement of the SEC’s and the OSC’s investigations of the Restatements). Results are reported as total consolidated results, reflecting our reporting structure of one reportable segment.
Management believes that Adjusted EBITDA provides the most useful insight into underlying business trends and results and provides a more meaningful comparison of period-over-period results. Management uses Adjusted EBITDA for planning, forecasting and evaluating business and financial performance, including allocating resources and evaluating results relative to employee compensation targets.
Adjusted EBITDA is reconciled to net loss as described above.follows:
| | | | | | | | | | | | | | | | | |
| (in thousands of U.S. dollars) | For the year ended December 31, 2023 |
| Continuing Operations | | Discontinued Operations | | Total |
| Net loss | $ | (70,439) | | | $ | (4,114) | | | $ | (74,553) | |
| Interest income, net | (51,235) | | | (10) | | | (51,245) | |
| Income tax expense (benefit) | (3,230) | | | — | | | (3,230) | |
| Depreciation and amortization | 7,866 | | | 244 | | | 8,110 | |
| EBITDA | (117,038) | | | (3,880) | | | (120,918) | |
| Share of (income) loss from equity method investments | (1,583) | | | — | | | (1,583) | |
Impairment loss on long-lived assets(ii) | 3,366 | | | 205 | | | 3,571 | |
Loss on revaluation of derivative liabilities(iii) | 85 | | | — | | | 85 | |
Loss on revaluation of financial instruments(iv) | 12,042 | | | — | | | 12,042 | |
Impairment loss on other investments(ix) | 23,350 | | | — | | | 23,350 | |
| Foreign currency transaction loss | 7,324 | | | — | | | 7,324 | |
Other, net(vi) | (1,114) | | | 118 | | | (996) | |
Restructuring costs(x) | 1,524 | | | 523 | | | 2,047 | |
Share-based compensation(vii) | 8,756 | | | 13 | | | 8,769 | |
Financial statement review costs(viii) | 919 | | | — | | | 919 | |
Inventory write-down(xi) | 805 | | | 839 | | | 1,644 | |
| Adjusted EBITDA | $ | (61,564) | | | $ | (2,182) | | | $ | (63,746) | |
| | | | | | | | | | | | | | | | | |
| (in thousands of U.S. dollars) | For the year ended December 31, 2022 |
| Continuing Operations | | Discontinued Operations | | Total |
| Net loss | $ | (155,178) | | | $ | (13,556) | | | $ | (168,734) | |
| Interest income, net | (22,514) | | | (23) | | | (22,537) | |
| Income tax expense (benefit) | 34,175 | | | — | | | 34,175 | |
| Depreciation and amortization | 11,924 | | | 1,198 | | | 13,122 | |
| EBITDA | (131,593) | | | (12,381) | | | (143,974) | |
| Share of income from equity method investments | (3,114) | | | — | | | (3,114) | |
Impairment loss on long-lived assets(ii) | 3,493 | | | — | | | 3,493 | |
Gain on revaluation of derivative liabilities(iii) | (14,060) | | | — | | | (14,060) | |
Gain on revaluation of financial instruments(iv) | (14,739) | | | — | | | (14,739) | |
Impairment loss on other investments(ix) | 61,392 | | | — | | | 61,392 | |
| Foreign currency transaction loss | 2,286 | | | — | | | 2,286 | |
Other, net(vi) | 324 | | | 169 | | | 493 | |
Restructuring costs(x) | 3,545 | | | 1,788 | | | 5,333 | |
Share-based compensation(vii) | 15,008 | | | 107 | | | 15,115 | |
Financial statement review costs(viii) | 7,167 | | | — | | | 7,167 | |
| | | | | |
| Adjusted EBITDA | $ | (70,291) | | | $ | (10,317) | | | $ | (80,608) | |
| | | | | | | | | | | | | | | | | |
| (in thousands of U.S. dollars) | For the year ended December 31, 2021 |
| Continuing Operations | | Discontinued Operations | | Total |
| Net loss | $ | (128,079) | | | $ | (269,125) | | | $ | (397,204) | |
| Interest income, net | (9,068) | | | (4) | | | (9,072) | |
| Income tax expense (benefit) | (431) | | | — | | | (431) | |
| Depreciation and amortization | 15,236 | | | 166 | | | 15,402 | |
| EBITDA | (122,342) | | | (268,963) | | | (391,305) | |
| Share of loss from equity method investments | 6,313 | | | — | | | 6,313 | |
Impairment loss on goodwill and indefinite-lived intangible assets(i) | 37 | | | 236,019 | | | 236,056 | |
Impairment loss on long-lived assets(ii) | 126,405 | | | 1,214 | | | 127,619 | |
Gain on revaluation of derivative liabilities(iii) | (151,360) | | | — | | | (151,360) | |
Gain on revaluation of financial instruments(iv) | (8,611) | | | — | | | (8,611) | |
Transaction costs(v) | 3,801 | | | — | | | 3,801 | |
Other, net(vi) | (733) | | | (101) | | | (834) | |
Share-based compensation(vii) | 9,844 | | | 307 | | | 10,151 | |
Financial statement review costs(viii) | 7,102 | | | — | | | 7,102 | |
| Adjusted EBITDA | $ | (129,544) | | | $ | (31,524) | | | $ | (161,068) | |
(i)For the year ended December 31, 2021, impairment loss on goodwill and indefinite-lived intangible assets relates primarily to impairment on goodwill and intangible assets related to our U.S. operations. See Note 7 “Goodwill and Intangible Assets, net” to the consolidated financial statements under Item 8 of this Annual Report.
•(ii)An increaseFor the year ended December 31, 2023, impairment loss on long-lived assets related to certain leased properties associated with the Company’s former U.S. operations and impairment of the Ginkgo Collaboration Agreement’s CBCVA exclusive license. For the year ended December 31, 2022, impairment loss on long-lived assets relates to the Company’s decision to seek a sublease for leased office space in Toronto, Ontario, Canada during the first quarter of 2022. For the year ended December 31, 2021, impairment loss on long-lived assets relates to impairment charges on property, plant and equipment and definite-lived intangible assets in the Canadian asset group, impairment charges for the differences between the consideration paid to Ginkgo for the achievement of two equity milestones in connection with the Ginkgo Collaboration Agreement and the fair values of the CBGA exclusive license and CBGVA exclusive license. See Note 6 “Property, plant and equipment, net” and Note 7 “Goodwill and Intangible Assets, net” to the consolidated financial statements in Item 8 of this Annual Report.
(iii)For the years ended December 31, 2023, 2022 and 2021, the (gain) loss on revaluation of derivative liabilities represents the fair value changes on the derivative liabilities. See Note 9 “Derivative Liabilities” to the consolidated financial statements in Item 8 of this Annual Report.
(iv)For the years ended December 31, 2023, 2022 and 2021, (gain) loss on revaluation of financial instruments relates primarily to our unrealized holding gain on our mark-to-market investment in Vitura as well as revaluations of financial liabilities resulting from deferred share units (“DSUs”) granted to directors. See Note 4 “Investments” and Note 11 “Share-based Compensation” to the consolidated financial statements in Item 8 of this Annual Report.
(v)For the year ended December 31, 2021, transaction costs represent legal, financial and other advisory fees and expenses incurred in connection with various strategic investments. These costs are included in general and administrative costs driven primarily by an increase in salaries and wages as a result of increased headcount in order to support the Company’s growth strategy, increased office and general expenses and increased costs due to a full year of U.S. segment results versus 117 days in 2019.
•An increase in sales and marketing costs, primarily related to brand development as well as increased costs due to a full year of U.S. segment results versus 117 days in the same period in 2019.
•An increase in R&D costs primarily related to the Ginkgo Strategic Partnership and increased spending on product development and developing cannabinoid intellectual property.
Other items affecting the comparability of net income (loss) during FY 2020 and FY 2019 – consolidated
Review costs related to restatement of 2019 interim financial statements
For FY 2020, we reported review costs related to the restatement of 2019 interim financial statements of $9.7 million within the general and administrative line in the consolidated statements of net income (loss). Theseloss and comprehensive loss.
(vi)For the years ended December 31, 2023 and 2022, other, net primarily related to related to (gain) loss on disposal of assets. For the year ended December 31, 2021, other, net is primarily related to (gain) loss on reclassification of held-for-sale assets and (gain) loss on disposal of assets.
(vii)For the years ended December 31, 2023, 2022 and 2021, share-based compensation relates to the vesting expenses of share-based compensation awarded to employees under our share-based award plans as described in Note 11 “Share-based Compensation” to the consolidated financial statements in Item 8 of this Annual Report.
(viii)For the years ended December 31, 2023, 2022 and 2021, financial statement review costs include costs related to the restatement of the Company’s 2019 interim financial statements,Restatement, costs related to the Company’s responses to requests for information from various regulatory authorities relating to such restatementthe Restatements, the costs related to the Settlement Order and Settlement Agreement and legal costs defending shareholder class action complaints brought against the Company as a result of the 2019 restatement. There were no costs
(ix)For the years ended December 31, 2023 and 2022, impairment loss on other investments related to the restatement ofPharmaCann Option for the Company’s 2019 interim financial statements incurred during FY 2019.
Repurposing charges
For FY 2019, we reported pre-tax charges of $7.3 million related to the Company’s decision to redesigndifference between its efforts at the Peace Naturals Campus, which includes impairment costs, inventory write-down,fair value and employee termination benefits. There were no repurposing costs incurred during FY 2020.
Interest income, net
For FY 2020, we reported interest income, net of $18.4 million representing a decrease of $9.6 million from FY 2019 primarily due to the impact of a decrease in interest rates on cash and cash equivalents and short-term investments during FY 2020 compared to FY 2019.
Gain on revaluation of derivative liabilities
For FY 2020, we reported a gain on revaluation of derivative liabilities of $129.3 million representing a decrease of $1,147.6 million from FY 2019 primarily driven by a decrease in our share price since December 31, 2019. The Company expects continued changes in derivative valuations as the Company’s share price fluctuates period-to-period. For further information, seecarrying amount. See Note 14 4 “Investments” to the consolidated financial statements in Item 8 of this Annual Report.
Impairment loss on goodwill and intangible assets
(x)For FY 2020, we reported an impairment loss on goodwill and intangible assets of $40.0 million representing an increase of $40.0 million from FY 2019 primarily driven by a $35.0 million impairment charge on the U.S. reporting unit and a $5.0 million impairment charge on the Lord Jones™ brand during the yearyears ended December 31, 2020. For further information, see2023 and 2022, restructuring costs related to the employee-related severance costs and other restructuring costs associated with the Realignment. See Note 11 16 “Restructuring” to the consolidated financial statements in Item 8 of this Annual Report.
Gain on disposal of other investments
(xi)For FY 2020, we reported a gain on disposal of other investments of $4.8 million representing a decrease of $11.5 millionthe year ended December 31, 2023, inventory write-downs from FY 2019 primarily driven by a decrease in the sale of sharesdiscontinued operations relate to product destruction and obsolescence associated with the Whistler Transaction (as defined below) during FY 2020 comparedexit of our U.S. operations as described in Note 2 “Discontinued Operations” and inventory write-downs from continuing operations relate to FY 2019. For further information, seeproduct destruction and obsolescence associated with the planned exit of Cronos Fermentation as described in Note 7 to16 “Restructuring.”
Constant Currency
To supplement the consolidated financial statements presented in Item 8accordance with U.S. GAAP, we have presented constant currency adjusted financial measures for net revenues, gross profit, gross profit margin, operating expenses, net income (loss) and Adjusted EBITDA for 2023, as well as cash and cash equivalents and short-term investment balances as of December 31, 2023 compared to December 31, 2022, which are considered non-GAAP financial measures. We present constant currency information to provide a framework for assessing how our underlying operations performed excluding the effect of foreign currency rate fluctuations. To present this information, current and prior period income statement results in currencies other than U.S. dollars are converted into U.S. dollars using the average exchange rates from the comparative period in 2022 rather than the actual average exchange rates in effect during 2023; constant currency current period balance sheet information is translated at the prior year-end spot rate rather than the current year-end spot rate. All growth comparisons relate to the corresponding period in 2022. We have provided this non-GAAP financial information to aid investors in better understanding the performance of our business. The non-GAAP financial measures presented in this Annual Report.Report should not be considered as a substitute for, or superior to, the measures of financial performance prepared in accordance with U.S. GAAP.
FinancingThe table below sets forth certain measures of consolidated results from continuing operations on an as-reported and transaction costsconstant currency basis for 2023 compared to 2022, as well as cash and cash equivalents and short-term investments as of December 31, 2023, compared to December 31, 2022, on an as-reported and constant currency basis (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As Reported | | As Adjusted for Constant Currency |
| Year ended December 31, | | As Reported Change | | Year ended December 31, | | Constant Currency Change |
| 2023 | | 2022 | | $ | | % | | 2023 | | $ | | % |
| Net revenue | $ | 87,241 | | | $ | 86,749 | | | $ | 492 | | | 1 | % | | $ | 91,711 | | | $ | 4,962 | | | 6 | % |
| Gross profit | 11,909 | | | 15,436 | | | (3,527) | | | (23) | % | | 12,662 | | | (2,774) | | | (18) | % |
| Gross margin | 14 | % | | 18 | % | | N/A | | (4) | pp | | 14 | % | | N/A | | (4) | pp |
| | | | | | | | | | | | | |
| Operating expenses | 96,709 | | | 126,864 | | | (30,155) | | | (24) | % | | 101,142 | | | (25,722) | | | (20) | % |
| Net loss from continuing operations | (70,439) | | | (155,178) | | | 84,739 | | | 55 | % | | (73,193) | | | 81,985 | | | 53 | % |
| Adjusted EBITDA | (61,564) | | | (70,291) | | | 8,727 | | | 12 | % | | (64,507) | | | 5,784 | | | 8 | % |
| | | | | | | | | | | | | |
| As of December 31, | | As Reported Change | | As of December 31, | | Constant Currency Change |
| 2023 | | 2022 | | $ | | % | | 2023 | | $ | | % |
| Cash and cash equivalents | $ | 669,291 | | | $ | 764,644 | | | $ | (95,353) | | | (12) | % | | $ | 656,647 | | | $ | (107,997) | | | (14) | % |
| Short-term investments | 192,237 | | | 113,077 | | | 79,160 | | | 70 | % | | 187,826 | | | 74,749 | | | 66 | % |
| Total cash and cash equivalents and short-term investments | $ | 861,528 | | | $ | 877,721 | | | $ | (16,193) | | | (2) | % | | $ | 844,473 | | | $ | (33,248) | | | (4) | % |
Net revenue
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As Reported | | As Adjusted for Constant Currency |
| Year ended December 31, | | As Reported Change | | Year ended December 31, | | Constant Currency Change |
| 2023 | | 2022 | | $ | | % | | 2023 | | $ | | % |
| Cannabis flower | $ | 62,071 | | | $ | 63,593 | | | $ | (1,522) | | | (2) | % | | $ | 65,573 | | | $ | 1,980 | | | 3 | % |
| Cannabis extracts | 24,569 | | | 22,522 | | | 2,047 | | | 9 | % | | 25,502 | | | 2,980 | | | 13 | % |
| Other | 601 | | | 634 | | | (33) | | | (5) | % | | 636 | | | 2 | | | — | % |
| Net revenue | $ | 87,241 | | | $ | 86,749 | | | $ | 492 | | | 1 | % | | $ | 91,711 | | | $ | 4,962 | | | 6 | % |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As Reported | | As Adjusted for Constant Currency |
| Year ended December 31, | | As Reported Change | | Year ended December 31, | | Constant Currency Change |
| 2023 | | 2022 | | $ | | % | | 2023 | | $ | | % |
| Canada | $ | 64,702 | | | $ | 56,233 | | | $ | 8,469 | | | 15 | % | | $ | 67,073 | | | $ | 10,840 | | | 19 | % |
| Israel | 21,134 | | | 30,516 | | | (9,382) | | | (31) | % | | 23,182 | | | (7,334) | | | (24) | % |
| | | | | | | | | | | | | |
| Other countries | 1,405 | | | — | | | 1,405 | | | N/M | | 1,456 | | | 1,456 | | | N/M |
| Net revenue | $ | 87,241 | | | $ | 86,749 | | | $ | 492 | | | 1 | % | | $ | 91,711 | | | $ | 4,962 | | | 6 | % |
Net Revenue
For FY 2020, we reported financing and transaction costs of $0.042023, net revenue on a constant currency basis was $91.7 million, representing a decrease of $32.2 million6% increase from FY 20192022. Net revenue increased on a constant currency basis primarily due to higher cannabis flower and extracts sales in the Canadian adult-use market, partially offset by lower cannabis flower sales in Israel driven by the increased financing and transaction costs incurred in FY 2019 compared to FY 2020pricing pressure as a result of competitive activity, the Altria Investmentslowdown in patient permit authorizations and the Redwood Acquisition. For further information see Note 27Israel-Hamas War, and Note 14 toan adverse price/mix in Canada in the consolidated financial statements in Item 8cannabis flower category driving increased excise tax payments as a percentage of this Annual Report.revenue.
Income tax expenseGross profit
For FY 2020, we reported an income tax expense of $1.32023, gross profit on a constant currency basis was $12.7 million, representing an increase of $1.3 million18% decrease from FY 2019 primarily driven by changes in valuation allowance, partially offset by2022. Gross profit decreased on a decrease in the fair value gain on financial liabilities.
Results of Operations by Business Segment: FY 2020 compared with FY 2019
Summary of financial results – ROW
| | | | | | | | | | | | | | | | | | | | | | | |
| (In thousands of U.S. dollars) | Year ended December 31, | | Change |
| 2020 | | 2019 | | $ | | % |
| Net revenue | $ | 37,224 | | | $ | 20,386 | | | $ | 16,838 | | | 83 | % |
| | | | | | | |
| Gross profit (loss) | (29,993) | | | (19,470) | | | (10,523) | | | 54 | % |
| Gross margin | (81) | % | | (96) | % | | N/A | | 15 | pp |
| | | | | | | |
Adjusted EBITDA (i) | $ | (98,349) | | | $ | (84,826) | | | $ | (13,523) | | | 16 | % |
(i)See “Non-GAAP Measures” for information related to non-GAAP measures.
Net revenue – ROW
| | | | | | | | | | | | | | | | | | | | | | | |
| (In thousands of U.S. dollars) | Year ended December 31, | | Change |
| 2020 | | 2019 | | $ | | % |
| Cannabis flower | $ | 27,932 | | | $ | 15,020 | | | $ | 12,912 | | | 86 | % |
| Cannabis extracts | 8,759 | | | 5,338 | | | 3,421 | | | 64 | % |
| Other | 533 | | | 28 | | | 505 | | | 1,804 | % |
| Net revenue | 37,224 | | | 20,386 | | | 16,838 | | | 83 | % |
For FY 2020, we reported net revenue of $37.2 million, representing an increase of $16.8 million from FY 2019. This change wasconstant currency basis primarily due to:
•An increase in sales due to the continued growth of the adult-use market in Canada andlower cannabis flower sales in the Israeli medical market, partially offset by non-recurring wholesale revenue in the Canadian market in FY 2019 and strategic price reductionsan adverse price/mix on various adult-use cannabis productsflower sales in Canada resulting in FY 2020.
Cost of salesrevenue and gross profit (loss) – ROW
| | | | | | | | | | | | | | | | | | | | | | | |
| (In thousands of U.S. dollars) | Year ended December 31, | | Change |
| 2020 | | 2019 | | $ | | % |
| Cost of sales | $ | 41,162 | | | $ | 10,683 | | | $ | 30,479 | | | 285 | % |
| Inventory write-down | 26,055 | | | 29,173 | | | (3,118) | | | (11) | % |
| | | | | | | |
| Gross profit (loss) | (29,993) | | | (19,470) | | | (10,523) | | | 54 | % |
| Gross margin | (81) | % | | (96) | % | | N/A | | 15 | pp |
For FY 2020, we reported gross loss of $30.0 million, representing an increase in losses of $10.5 million from FY 2019. This change was primarily due to:
•An increase in cost of sales primarily driven by third-party purchased flower associated with adult-use products in Canada and a decline in wholesale sales in FY 2020. The Company anticipates that gross margin will continue to fluctuate as price and mix change from quarter-to-quarter.
•Partially offset by an increase in net revenue from FY 2019, as described above and a decrease inthe inventory write-downs. We may incur further inventory write-downs due to pricing pressures in the marketplace.
Adjusted EBITDA – ROW
| | | | | | | | | | | | | | | | | | | | | | | |
| (In thousands of U.S. dollars) | Year ended December 31, | | Change |
| 2020 | | 2019 | | $ | | % |
| Adjusted EBITDA | $ | (98,349) | | | $ | (84,826) | | | $ | (13,523) | | | 16 | % |
| | | | | | | |
(i)See “Non-GAAP Measures” for information related to non-GAAP measures.
For FY 2020, we reported Adjusted EBITDA of $(98.3) million, representing a decrease in Adjusted EBITDA of $13.5 million from FY 2019. This change was primarily due to:
•An increase in ROW gross loss from FY 2019, as described above.
•An increase in R&D costs primarily related to the Ginkgo Strategic Partnership and costs related to increased spending on product development and cannabinoid intellectual property.
•An increase in general and administrative costs from FY 2019 driven by an increase in salaries and wageswrite-down recognized as a result of increased headcountthe decision to support the Company’s growth strategywind down operations at Cronos Fermentation, partially offset by higher cannabis flower and increased office and general expenses.
•An increase inextract sales and marketing costs primarily related to building and developing our brands and products in the Canadian adult-use market.
Summary of financial results – U.S.
| | | | | | | | | | | | | | | | | | | | | | | |
| (In thousands of U.S. dollars) | Year ended December 31, | | Change |
| 2020 | | 2019 | | $ | | % |
| Net revenue | $ | 9,495 | | | $ | 3,364 | | | $ | 6,131 | | | 182 | % |
| | | | | | | |
| Gross profit | 4,160 | | | 1,873 | | | 2,287 | | | 122 | % |
| Gross margin | 44 | % | | 56 | % | | N/A | | (12) | pp |
| | | | | | | |
Adjusted EBITDA (i) | $ | (28,019) | | | $ | (1,703) | | | $ | (26,316) | | | 1,545 | % |
(i)See “Non-GAAP Measures” for informationFor 2023, operating expenses on a constant currency basis was $101.1 million, representing a 20% decrease from 2022. Operating expenses decreased on a constant currency basis primarily due to lower professional fees, largely related to non-GAAP measures.financial statement review costs, lower costs associated with the achievement of Ginkgo milestones, the 2022 acceleration of expense on equity awards granted to certain executive employees in connection with their separation from the Company, as well as previously held-back equity awards granted in 2022 to certain executives, impairment loss on long-lived assets recognized in the prior year, lower bonus expense, lower payroll costs and lower insurance costs, partially offset by higher sales and marketing expenses.
Net revenue – U.S.
| | | | | | | | | | | | | | | | | | | | | | | |
| (In thousands of U.S. dollars) | Year ended December 31, | | Change |
| 2020 | | 2019 | | $ | | % |
| Net revenue | $ | 9,495 | | | $ | 3,364 | | | $ | 6,131 | | | 182 | % |
For FY 2020, the U.S. segment reported2023, net revenue of $9.5loss on a constant currency basis was $73.2 million, representing a 53% improvement from 2022.
Adjusted EBITDA
For 2023, Adjusted EBITDA on a constant currency basis was $64.5 million, representing an increase of $6.1 million8% improvement from FY 2019. The increase was2022. Adjusted EBITDA increased on a constant currency basis primarily due to:
•An increaseto higher cannabis flower and extracts sales in the Canadian adult-use market, decreases in general and administrative expenses and lower costs associated with the achievement of Ginkgo milestones, partially offset by lower cannabis flower sales due to a full year of U.S. segment results in FY 2020 as opposed to 117 days in FY 2019.
•The growth in existing product lines and introductions of new U.S. hemp-derived CBD products during FY 2020. A significant amount of the U.S. segment’s FY 2020 revenue was earned during the fourth quarterIsrael driven by pricing pressure as a result of increased salescompetitive activity, the slowdown in patient permit authorizations and the Israel-Hamas War, an adverse price/mix in Canada in the direct-to-consumer channel driven by holiday sales.
Costcannabis flower category driving increased excise tax payments as a percentage of revenue and higher sales and gross profit – U.S.marketing expenses.
| | | | | | | | | | | | | | | | | | | | | | | |
| (In thousands of U.S. dollars) | Year ended December 31, | | Change |
| 2020 | | 2019 | | $ | | % |
| Cost of sales | $ | 5,335 | | | $ | 1,491 | | | $ | 3,844 | | | 258 | % |
| | | | | | | |
| Gross profit (loss) | 4,160 | | | 1,873 | | | 2,287 | | | 122 | % |
| Gross profit margin | 44 | % | | 56 | % | | N/A | | (12) | pp |
For FY 2020, the U.S. segment reported gross profitCash and cash equivalents and short-term investments on a constant currency basis decreased 4% to $844.5 million as of $4.2December 31, 2023 from $877.7 million representing an increaseas of December 31, 2022. The decrease in gross profit of $2.3 million from FY 2019. This wascash and cash equivalents and short-term investments is primarily due to:
•The increaseto cash flows used in net revenue, as described above.
•Partially offset by an increaseoperating activities in cost of sales primarily driven by a full year of U.S. segment results in FY 2020 as opposed to 117 days in the same period in 2019.
Adjusted EBITDA – U.S.
| | | | | | | | | | | | | | | | | | | | | | | |
| (In thousands of U.S. dollars) | Year ended December 31, | | Change |
| 2020 | | 2019 | | $ | | % |
| Adjusted EBITDA | $ | (28,019) | | | $ | (1,703) | | | $ | (26,316) | | | 1,545 | % |
| | | | | | | |
(i)See “Non-GAAP Measures” for information related to non-GAAP measures.
For FY 2020, we reported an Adjusted EBITDA of $(28.0) million, representing a decrease in Adjusted EBITDA of $26.3 million from FY 2019. This change was primarily due to:
•Sales and marketing costs incurred in relation to the introduction of new U.S. hemp-derived CBD products.
•Increased general and administrative costs driven by increased headcount to support the Company’s growth strategy across a variety of functions.
•Partially offset by an increase in gross profit from FY 2019, as described above.2023.
LiquidityNon-GAAP Measures
Cronos reports its financial results in accordance with Generally Accepted Accounting Principles in the United States (“U.S. GAAP”). This Annual Report refers to measures not recognized under U.S. GAAP (“non-GAAP measures”). These non-GAAP measures do not have a standardized meaning prescribed by U.S. GAAP and Capital Resourcesare therefore unlikely to be comparable to similar measures presented by other companies. Rather, these non-GAAP measures are provided as a supplement to corresponding U.S. GAAP measures to provide additional information regarding our results of operations from management’s perspective. Accordingly, non-GAAP measures should not be considered a substitute for, or superior to, the financial information prepared and presented in accordance with U.S. GAAP. All non-GAAP measures presented in this Annual Report are reconciled to their closest reported U.S. GAAP measure. Reconciliations of historical adjusted financial measures to corresponding U.S. GAAP measures are provided below.
Adjusted EBITDA
Management reviews Adjusted EBITDA, a non-GAAP measure, which excludes non-cash items and items that do not reflect management’s assessment of ongoing business performance. Management defines Adjusted EBITDA as net income (loss) before interest, tax expense (benefit), depreciation and amortization adjusted for: share of (income) loss from equity method investments; impairment loss on goodwill and intangible assets; impairment loss on long-lived assets; (gain) loss on revaluation of derivative liabilities; (gain) loss on revaluation of financial instruments; transaction costs related to strategic projects; impairment loss on other investments; foreign currency transaction loss; other, net; loss from discontinued operations; restructuring costs; inventory write-downs resulting from restructuring actions; share-based compensation; and financial statement review costs and reserves related to the restatements of our 2019 and 2021 interim financial statements (the “Restatements”), including the costs related to the settlement of the SEC’s and the OSC’s investigations of the Restatements and legal costs defending shareholder class action complaints brought against us as a result of the 2019 restatement (see Note 10(b) “Contingencies,” to the consolidated financial statements under Item 8 of this Annual Report for a discussion of the shareholder class action complaints relating to the restatement of the 2019 interim financial statements and the settlement of the SEC’s and the OSC’s investigations of the Restatements). Results are reported as total consolidated results, reflecting our reporting structure of one reportable segment.
Management believes that Adjusted EBITDA provides the most useful insight into underlying business trends and results and provides a more meaningful comparison of period-over-period results. Management uses Adjusted EBITDA for planning, forecasting and evaluating business and financial performance, including allocating resources and evaluating results relative to employee compensation targets.
Adjusted EBITDA is reconciled to net loss as follows:
| | | | | | | | | | | | | | | | | |
| (in thousands of U.S. dollars) | For the year ended December 31, 2023 |
| Continuing Operations | | Discontinued Operations | | Total |
| Net loss | $ | (70,439) | | | $ | (4,114) | | | $ | (74,553) | |
| Interest income, net | (51,235) | | | (10) | | | (51,245) | |
| Income tax expense (benefit) | (3,230) | | | — | | | (3,230) | |
| Depreciation and amortization | 7,866 | | | 244 | | | 8,110 | |
| EBITDA | (117,038) | | | (3,880) | | | (120,918) | |
| Share of (income) loss from equity method investments | (1,583) | | | — | | | (1,583) | |
Impairment loss on long-lived assets(ii) | 3,366 | | | 205 | | | 3,571 | |
Loss on revaluation of derivative liabilities(iii) | 85 | | | — | | | 85 | |
Loss on revaluation of financial instruments(iv) | 12,042 | | | — | | | 12,042 | |
Impairment loss on other investments(ix) | 23,350 | | | — | | | 23,350 | |
| Foreign currency transaction loss | 7,324 | | | — | | | 7,324 | |
Other, net(vi) | (1,114) | | | 118 | | | (996) | |
Restructuring costs(x) | 1,524 | | | 523 | | | 2,047 | |
Share-based compensation(vii) | 8,756 | | | 13 | | | 8,769 | |
Financial statement review costs(viii) | 919 | | | — | | | 919 | |
Inventory write-down(xi) | 805 | | | 839 | | | 1,644 | |
| Adjusted EBITDA | $ | (61,564) | | | $ | (2,182) | | | $ | (63,746) | |
| | | | | | | | | | | | | | | | | |
| (in thousands of U.S. dollars) | For the year ended December 31, 2022 |
| Continuing Operations | | Discontinued Operations | | Total |
| Net loss | $ | (155,178) | | | $ | (13,556) | | | $ | (168,734) | |
| Interest income, net | (22,514) | | | (23) | | | (22,537) | |
| Income tax expense (benefit) | 34,175 | | | — | | | 34,175 | |
| Depreciation and amortization | 11,924 | | | 1,198 | | | 13,122 | |
| EBITDA | (131,593) | | | (12,381) | | | (143,974) | |
| Share of income from equity method investments | (3,114) | | | — | | | (3,114) | |
Impairment loss on long-lived assets(ii) | 3,493 | | | — | | | 3,493 | |
Gain on revaluation of derivative liabilities(iii) | (14,060) | | | — | | | (14,060) | |
Gain on revaluation of financial instruments(iv) | (14,739) | | | — | | | (14,739) | |
Impairment loss on other investments(ix) | 61,392 | | | — | | | 61,392 | |
| Foreign currency transaction loss | 2,286 | | | — | | | 2,286 | |
Other, net(vi) | 324 | | | 169 | | | 493 | |
Restructuring costs(x) | 3,545 | | | 1,788 | | | 5,333 | |
Share-based compensation(vii) | 15,008 | | | 107 | | | 15,115 | |
Financial statement review costs(viii) | 7,167 | | | — | | | 7,167 | |
| | | | | |
| Adjusted EBITDA | $ | (70,291) | | | $ | (10,317) | | | $ | (80,608) | |
| | | | | | | | | | | | | | | | | |
| (in thousands of U.S. dollars) | For the year ended December 31, 2021 |
| Continuing Operations | | Discontinued Operations | | Total |
| Net loss | $ | (128,079) | | | $ | (269,125) | | | $ | (397,204) | |
| Interest income, net | (9,068) | | | (4) | | | (9,072) | |
| Income tax expense (benefit) | (431) | | | — | | | (431) | |
| Depreciation and amortization | 15,236 | | | 166 | | | 15,402 | |
| EBITDA | (122,342) | | | (268,963) | | | (391,305) | |
| Share of loss from equity method investments | 6,313 | | | — | | | 6,313 | |
Impairment loss on goodwill and indefinite-lived intangible assets(i) | 37 | | | 236,019 | | | 236,056 | |
Impairment loss on long-lived assets(ii) | 126,405 | | | 1,214 | | | 127,619 | |
Gain on revaluation of derivative liabilities(iii) | (151,360) | | | — | | | (151,360) | |
Gain on revaluation of financial instruments(iv) | (8,611) | | | — | | | (8,611) | |
Transaction costs(v) | 3,801 | | | — | | | 3,801 | |
Other, net(vi) | (733) | | | (101) | | | (834) | |
Share-based compensation(vii) | 9,844 | | | 307 | | | 10,151 | |
Financial statement review costs(viii) | 7,102 | | | — | | | 7,102 | |
| Adjusted EBITDA | $ | (129,544) | | | $ | (31,524) | | | $ | (161,068) | |
(i)For the year ended December 31, 2021, impairment loss on goodwill and indefinite-lived intangible assets relates primarily to impairment on goodwill and intangible assets related to our U.S. operations. See Note 7 “Goodwill and Intangible Assets, net” to the consolidated financial statements under Item 8 of this Annual Report.
(ii)For the year ended December 31, 2023, impairment loss on long-lived assets related to certain leased properties associated with the Company’s former U.S. operations and impairment of the Ginkgo Collaboration Agreement’s CBCVA exclusive license. For the year ended December 31, 2022, impairment loss on long-lived assets relates to the Company’s decision to seek a sublease for leased office space in Toronto, Ontario, Canada during the first quarter of 2022. For the year ended December 31, 2021, impairment loss on long-lived assets relates to impairment charges on property, plant and equipment and definite-lived intangible assets in the Canadian asset group, impairment charges for the differences between the consideration paid to Ginkgo for the achievement of two equity milestones in connection with the Ginkgo Collaboration Agreement and the fair values of the CBGA exclusive license and CBGVA exclusive license. See Note 6 “Property, plant and equipment, net” and Note 7 “Goodwill and Intangible Assets, net” to the consolidated financial statements in Item 8 of this Annual Report.
(iii)For the years ended December 31, 2023, 2022 and 2021, the (gain) loss on revaluation of derivative liabilities represents the fair value changes on the derivative liabilities. See Note 9 “Derivative Liabilities” to the consolidated financial statements in Item 8 of this Annual Report.
(iv)For the years ended December 31, 2023, 2022 and 2021, (gain) loss on revaluation of financial instruments relates primarily to our unrealized holding gain on our mark-to-market investment in Vitura as well as revaluations of financial liabilities resulting from deferred share units (“DSUs”) granted to directors. See Note 4 “Investments” and Note 11 “Share-based Compensation” to the consolidated financial statements in Item 8 of this Annual Report.
(v)For the year ended December 31, 2021, transaction costs represent legal, financial and other advisory fees and expenses incurred in connection with various strategic investments. These costs are included in general and administrative expenses on the consolidated statements of net loss and comprehensive loss.
(vi)For the years ended December 31, 2023 and 2022, other, net primarily related to related to (gain) loss on disposal of assets. For the year ended December 31, 2021, other, net is primarily related to (gain) loss on reclassification of held-for-sale assets and (gain) loss on disposal of assets.
(vii)For the years ended December 31, 2023, 2022 and 2021, share-based compensation relates to the vesting expenses of share-based compensation awarded to employees under our share-based award plans as described in Note 11 “Share-based Compensation” to the consolidated financial statements in Item 8 of this Annual Report.
(viii)For the years ended December 31, 2023, 2022 and 2021, financial statement review costs include costs related to the Restatement, costs related to the Company’s responses to requests for information from various regulatory authorities relating to the Restatements, the costs related to the Settlement Order and Settlement Agreement and legal costs defending shareholder class action complaints brought against the Company as a result of the 2019 restatement.
(ix)For the years ended December 31, 2023 and 2022, impairment loss on other investments related to the PharmaCann Option for the difference between its fair value and carrying amount. See Note 4 “Investments” to the consolidated financial statements in Item 8 of this Annual Report.
(x)For the years ended December 31, 2023 and 2022, restructuring costs related to the employee-related severance costs and other restructuring costs associated with the Realignment. See Note 16 “Restructuring” to the consolidated financial statements in Item 8 of this Annual Report.
(xi)For the year ended December 31, 2023, inventory write-downs from discontinued operations relate to product destruction and obsolescence associated with the exit of our U.S. operations as described in Note 2 “Discontinued Operations” and inventory write-downs from continuing operations relate to product destruction and obsolescence associated with the planned exit of Cronos Fermentation as described in Note 16 “Restructuring.”
Constant Currency
To supplement the consolidated financial statements presented in accordance with U.S. GAAP, we have presented constant currency adjusted financial measures for net revenues, gross profit, gross profit margin, operating expenses, net income (loss) and Adjusted EBITDA for 2023, as well as cash and cash equivalents and short-term investment balances as of December 31, 2023 compared to December 31, 2022, which are considered non-GAAP financial measures. We believe thatpresent constant currency information to provide a framework for assessing how our existingunderlying operations performed excluding the effect of foreign currency rate fluctuations. To present this information, current and prior period income statement results in currencies other than U.S. dollars are converted into U.S. dollars using the average exchange rates from the comparative period in 2022 rather than the actual average exchange rates in effect during 2023; constant currency current period balance sheet information is translated at the prior year-end spot rate rather than the current year-end spot rate. All growth comparisons relate to the corresponding period in 2022. We have provided this non-GAAP financial information to aid investors in better understanding the performance of our business. The non-GAAP financial measures presented in this Annual Report should not be considered as a substitute for, or superior to, the measures of financial performance prepared in accordance with U.S. GAAP.
The table below sets forth certain measures of consolidated results from continuing operations on an as-reported and constant currency basis for 2023 compared to 2022, as well as cash and cash equivalents and short-term investments will be sufficientas of December 31, 2023, compared to fund our business operationsDecember 31, 2022, on an as-reported and capital expenditures over the next twelve months.constant currency basis (in thousands):
Liquidity
Our primary need for liquidity is to fund operations and capital expenditures. Our ability to fund operations and capital expenditures depends on, among other things, future operating performance and cash flows that are subject to general economic conditions and financial and other factors, including factors beyond our control. | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As Reported | | As Adjusted for Constant Currency |
| Year ended December 31, | | As Reported Change | | Year ended December 31, | | Constant Currency Change |
| 2023 | | 2022 | | $ | | % | | 2023 | | $ | | % |
| Net revenue | $ | 87,241 | | | $ | 86,749 | | | $ | 492 | | | 1 | % | | $ | 91,711 | | | $ | 4,962 | | | 6 | % |
| Gross profit | 11,909 | | | 15,436 | | | (3,527) | | | (23) | % | | 12,662 | | | (2,774) | | | (18) | % |
| Gross margin | 14 | % | | 18 | % | | N/A | | (4) | pp | | 14 | % | | N/A | | (4) | pp |
| | | | | | | | | | | | | |
| Operating expenses | 96,709 | | | 126,864 | | | (30,155) | | | (24) | % | | 101,142 | | | (25,722) | | | (20) | % |
| Net loss from continuing operations | (70,439) | | | (155,178) | | | 84,739 | | | 55 | % | | (73,193) | | | 81,985 | | | 53 | % |
| Adjusted EBITDA | (61,564) | | | (70,291) | | | 8,727 | | | 12 | % | | (64,507) | | | 5,784 | | | 8 | % |
| | | | | | | | | | | | | |
| As of December 31, | | As Reported Change | | As of December 31, | | Constant Currency Change |
| 2023 | | 2022 | | $ | | % | | 2023 | | $ | | % |
| Cash and cash equivalents | $ | 669,291 | | | $ | 764,644 | | | $ | (95,353) | | | (12) | % | | $ | 656,647 | | | $ | (107,997) | | | (14) | % |
| Short-term investments | 192,237 | | | 113,077 | | | 79,160 | | | 70 | % | | 187,826 | | | 74,749 | | | 66 | % |
| Total cash and cash equivalents and short-term investments | $ | 861,528 | | | $ | 877,721 | | | $ | (16,193) | | | (2) | % | | $ | 844,473 | | | $ | (33,248) | | | (4) | % |
Historically, we haveNet revenue
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As Reported | | As Adjusted for Constant Currency |
| Year ended December 31, | | As Reported Change | | Year ended December 31, | | Constant Currency Change |
| 2023 | | 2022 | | $ | | % | | 2023 | | $ | | % |
| Cannabis flower | $ | 62,071 | | | $ | 63,593 | | | $ | (1,522) | | | (2) | % | | $ | 65,573 | | | $ | 1,980 | | | 3 | % |
| Cannabis extracts | 24,569 | | | 22,522 | | | 2,047 | | | 9 | % | | 25,502 | | | 2,980 | | | 13 | % |
| Other | 601 | | | 634 | | | (33) | | | (5) | % | | 636 | | | 2 | | | — | % |
| Net revenue | $ | 87,241 | | | $ | 86,749 | | | $ | 492 | | | 1 | % | | $ | 91,711 | | | $ | 4,962 | | | 6 | % |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As Reported | | As Adjusted for Constant Currency |
| Year ended December 31, | | As Reported Change | | Year ended December 31, | | Constant Currency Change |
| 2023 | | 2022 | | $ | | % | | 2023 | | $ | | % |
| Canada | $ | 64,702 | | | $ | 56,233 | | | $ | 8,469 | | | 15 | % | | $ | 67,073 | | | $ | 10,840 | | | 19 | % |
| Israel | 21,134 | | | 30,516 | | | (9,382) | | | (31) | % | | 23,182 | | | (7,334) | | | (24) | % |
| | | | | | | | | | | | | |
| Other countries | 1,405 | | | — | | | 1,405 | | | N/M | | 1,456 | | | 1,456 | | | N/M |
| Net revenue | $ | 87,241 | | | $ | 86,749 | | | $ | 492 | | | 1 | % | | $ | 91,711 | | | $ | 4,962 | | | 6 | % |
Net Revenue
For 2023, net revenue on a constant currency basis was $91.7 million, representing a 6% increase from 2022. Net revenue increased on a constant currency basis primarily funded ourdue to higher cannabis flower and extracts sales in the Canadian adult-use market, partially offset by lower cannabis flower sales in Israel driven by pricing pressure as a result of competitive activity, the slowdown in patient permit authorizations and the Israel-Hamas War, and an adverse price/mix in Canada in the cannabis flower category driving increased excise tax payments as a percentage of revenue.
Gross profit
For 2023, gross profit on a constant currency basis was $12.7 million, representing an 18% decrease from 2022. Gross profit decreased on a constant currency basis primarily due to lower cannabis flower sales in the Israeli medical market, an adverse price/mix on cannabis flower sales in Canada resulting in higher excise taxes as a percentage of revenue and the inventory write-down recognized as a result of the decision to wind down operations throughat Cronos Fermentation, partially offset by higher cannabis flower and extract sales in the Canadian adult-use market.
Operating expenses
For 2023, operating expenses on a constant currency basis was $101.1 million, representing a 20% decrease from 2022. Operating expenses decreased on a constant currency basis primarily due to lower professional fees, largely related to financial statement review costs, lower costs associated with the achievement of Ginkgo milestones, the 2022 acceleration of expense on equity financing. In March 2019, Altria closed a C$2.4 billion (approximately $1.8 billion) investment in us, pursuant to which we issuedawards granted to certain wholly owned subsidiariesexecutive employees in connection with their separation from the Company, as well as previously held-back equity awards granted in 2022 to certain executives, impairment loss on long-lived assets recognized in the prior year, lower bonus expense, lower payroll costs and lower insurance costs, partially offset by higher sales and marketing expenses.
Net loss
For 2023, net loss on a constant currency basis was $73.2 million, representing a 53% improvement from 2022.
Adjusted EBITDA
For 2023, Adjusted EBITDA on a constant currency basis was $64.5 million, representing an 8% improvement from 2022. Adjusted EBITDA increased on a constant currency basis primarily due to higher cannabis flower and extracts sales in the Canadian adult-use market, decreases in general and administrative expenses and lower costs associated with the achievement of Altria 149,831,154Ginkgo milestones, partially offset by lower cannabis flower sales in Israel driven by pricing pressure as a result of our common sharescompetitive activity, the slowdown in patient permit authorizations and one warrant,the Israel-Hamas War, an adverse price/mix in Canada in the cannabis flower category driving increased excise tax payments as further discussed under “Altria Strategic Investment” herein. Asa percentage of February 23, 2021, we had a cashrevenue and higher sales and marketing expenses.
Cash and cash equivalents balance of $1.1 billion& short-term investments
Cash and $0.2 billion in short termcash equivalents and short-term investments on a constant currency basis decreased 4% to fund operations and capital expenditures.
Capital resources
As$844.5 million as of December 31, 2020, we had $1.1 billion2023 from $877.7 million as of December 31, 2022. The decrease in cash and cash equivalents and $0.2 billionshort-term investments is primarily due to cash flows used in short term investments. As of December 31, 2020, the Company had no external financing.
Contractual obligations
As of December 31, 2020, the Company had the following contractual obligations:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (In thousands of U.S. dollars) | Payments Due by Period |
| Total | | Less Than 1 Year | | 1-3 Years | | 4-5 Years | | After 5 Years |
| Long-term debt obligations | $ | — | | | $ | — | | | $ | — | | | $ | — | | | $ | — | |
| Capital (finance) lease obligations | 59 | | | 39 | | | 20 | | | — | | | — | |
| Operating lease obligations | 12,017 | | | 2,381 | | | 6,214 | | | 2,261 | | | 1,161 | |
| Purchase obligations | 18,942 | | | 14,489 | | | 3,341 | | | 1,112 | | | — | |
| Other long-term liabilities | 8,962 | | | 8,962 | | | — | | | — | | | — | |
| Total contractual obligations | $ | 39,980 | | | $ | 25,871 | | | $ | 9,575 | | | $ | 3,373 | | | $ | 1,161 | |
Finance lease obligations relate to equipment leases maturingoperating activities in June 2022. Operating lease obligations relate to land, buildings, vehicles, and office space in Canada, the U.S. and Israel. Purchase obligations include commitments for capital expenditure, information technology services, and other professional services. Other long-term liabilities relate to commitments for research and development such as Ginkgo foundry access fees, as well as undrawn but committed loans to our joint ventures.
Summary of cash flows
| | | | | | | | | | | | | | | | | |
| (In thousands of U.S. dollars) | Year ended December 31, |
| 2020 | | 2019 | | 2018 |
| Net cash provided by (used in) operating activities | $ | (142,457) | | | $ | (130,274) | | | $ | (7,517) | |
| Net cash provided by (used in) investing activities | 20,150 | | | (603,272) | | | (93,908) | |
| Net cash provided by (used in) financing activities | (5,465) | | | 1,856,941 | | | 122,112 | |
| Effect of foreign currency translation on cash and cash equivalents | 6,102 | | | 52,371 | | | (4,085) | |
| Net change in cash | $ | (121,670) | | | $ | 1,175,766 | | | $ | 16,602 | |
FY 2020 cash flows vs FY 2019 cash flows
Operating activities.
During FY 2020, we used $142.5 million of cash in operating activities as compared to $130.3 million in FY 2019, representing an increase of $12.2 million in cash used. This change is primarily driven by an increase in operating expenses in FY 2020 partially offset by increased revenues in FY 2020.
Investing activities.
During FY 2020, we received $20.2 million of cash in investing activities, as compared to $603.3 million of cash used during FY 2019, representing an increase of $623.5 million in net cash provided. This change is primarily driven by the Redwood Acquisition in FY 2019 for cash consideration of $224.3 million, as well as a $395.3 million increase from FY 2019 from the net effect of the purchase of and proceeds from maturity short-term investments.
Financing activities.
During FY 2020, cash used in financing activities was $5.5 million, as compared to $1,856.9 million of cash provided by financing activities in FY 2019, representing a net increase of $1,862.4 million. This change is primarily driven by proceeds from the strategic investment from Altria of $1,809.6 million as well as the proceeds from exercises Top-up Rights of $67.1 million by Altria partially offset by the $16.0 million repayment of the construction loan payable.
Non-GAAP Measures
Cronos Group reports its financial results in accordance with Generally Accepted Accounting Principles in the United States (“USU.S. GAAP”). This Annual Report refers to measures not recognized under USU.S. GAAP (“non-GAAP measures”). These non-GAAP measures do not have a standardized meaning prescribed by USU.S. GAAP and are therefore unlikely to be comparable to similar measures presented by other companies. Rather, these non-GAAP measures are provided as a supplement to corresponding USU.S. GAAP measures to provide additional information regarding our results of operations from management’s perspective. Accordingly, non- GAAPnon-GAAP measures should not be considered a substitute for, or superior to, the financial information prepared and presented in accordance with USU.S. GAAP. All non-GAAP measures presented in this Annual Report are reconciled to their closest reported U.S. GAAP measure. Reconciliations of historical adjusted financial measures to corresponding USU.S. GAAP measures are provided below.
Adjusted EBITDA
Management reviews Adjusted EBITDA, a non-GAAP measure, which excludes non-cash items orand items that do not reflect management’s assessment of on-goingongoing business performance. Management defines Adjusted EBITDA as net income (loss) before interest, tax expense (benefit), depreciation and amortization adjusted for: share of (income) loss from equity method investments; impairment loss on goodwill and intangible assets, repurposing charges, financing and transaction costs,assets; impairment loss on long-lived assets; (gain) loss on revaluation of derivative liabilities,liabilities; (gain) loss (gain) on disposalrevaluation of investments, share offinancial instruments; transaction costs related to strategic projects; impairment loss (income) from equity accounted investees,on other investments; foreign currency transaction loss; other, net; loss from discontinued operations, other loss (income)operations; restructuring costs; inventory write-downs resulting from restructuring actions; share-based compensation; and financial statement review costs and reserves related to the restatements of our 2019 and 2021 interim financial statements (the “Restatements”), reviewincluding the costs related to the restatementsettlement of the Company’s 2019 interim financial statements,SEC’s and the Company’s responses toOSC’s investigations of the reviews of such interim financial statements by various regulatory authoritiesRestatements and legal costs defending shareholder class action complaints brought against the Companyus as a result of the 2019 restatement (see Part I,Note 10(b) “Contingencies,” to the consolidated financial statements under Item 3, Legal Proceedings,8 of this Annual Report for a discussion of the regulatory reviews and shareholder class action complaints relating to the restatement of the 2019 interim financial statements),statements and share-based payments.the settlement of the SEC’s and the OSC’s investigations of the Restatements). Results are reported as total consolidated results, reflecting our reporting structure of one reportable segment.
Management believes that Adjusted EBITDA provides the most useful insight into underlying business trends and results and provides a more meaningful comparison of year-over-yearperiod-over-period results. Management uses Adjusted EBITDA for planning, forecasting and evaluating business and financial performance, including allocating resources and evaluating results relative to employee compensation targets. As a result of the appointment of Mr. Schmidt as President and Chief Executive Officer in September 2020 and a review of how management looks at the business,
Adjusted EBITDA is now the primary metric upon which management views the consolidated business performance and results year-over-year.reconciled to net loss as follows:
Adjusted EBITDA by segment
Management also reviews adjusted earnings (loss) before interest, tax, depreciation and amortization by segment (“Adjusted EBITDA by segment”), a non-GAAP measure which excludes non-cash items or items that do not reflect management’s assessment of on-going business performance. Corporate expenses are removed from Adjusted EBITDA by segment. Corporate expenses are expenses that relate to the consolidated business. The Company’s method of allocating corporate expenses is refined periodically. Management defines Adjusted EBITDA by segment as net income (loss) by segment before interest, tax expense, depreciation and amortization adjusted for the same items that are adjusted in consolidated Adjusted EBITDA.
Management believes that Adjusted EBITDA by segment provides useful insight into underlying segment trends and results and provides a more meaningful comparison of year-over-year segment results. Management uses Adjusted EBITDA by segment for planning, forecasting and evaluating business and financial performance, including allocating resources and evaluating results relative to employee compensation targets. As a result of the appointment of Mr. Schmidt as President and Chief Executive Officer in September 2020 and a review of how management looks at the business by segment, Adjusted EBITDA by segment is now the primary metric upon which management views the segment performance and results year-over-year. | | | | | | | | | | | | | | | | | |
| (in thousands of U.S. dollars) | For the year ended December 31, 2023 |
| Continuing Operations | | Discontinued Operations | | Total |
| Net loss | $ | (70,439) | | | $ | (4,114) | | | $ | (74,553) | |
| Interest income, net | (51,235) | | | (10) | | | (51,245) | |
| Income tax expense (benefit) | (3,230) | | | — | | | (3,230) | |
| Depreciation and amortization | 7,866 | | | 244 | | | 8,110 | |
| EBITDA | (117,038) | | | (3,880) | | | (120,918) | |
| Share of (income) loss from equity method investments | (1,583) | | | — | | | (1,583) | |
Impairment loss on long-lived assets(ii) | 3,366 | | | 205 | | | 3,571 | |
Loss on revaluation of derivative liabilities(iii) | 85 | | | — | | | 85 | |
Loss on revaluation of financial instruments(iv) | 12,042 | | | — | | | 12,042 | |
Impairment loss on other investments(ix) | 23,350 | | | — | | | 23,350 | |
| Foreign currency transaction loss | 7,324 | | | — | | | 7,324 | |
Other, net(vi) | (1,114) | | | 118 | | | (996) | |
Restructuring costs(x) | 1,524 | | | 523 | | | 2,047 | |
Share-based compensation(vii) | 8,756 | | | 13 | | | 8,769 | |
Financial statement review costs(viii) | 919 | | | — | | | 919 | |
Inventory write-down(xi) | 805 | | | 839 | | | 1,644 | |
| Adjusted EBITDA | $ | (61,564) | | | $ | (2,182) | | | $ | (63,746) | |
Adjusted EBITDA | | | | | | | | | | | | | | | | | |
| (in thousands of U.S. dollars) | For the year ended December 31, 2022 |
| Continuing Operations | | Discontinued Operations | | Total |
| Net loss | $ | (155,178) | | | $ | (13,556) | | | $ | (168,734) | |
| Interest income, net | (22,514) | | | (23) | | | (22,537) | |
| Income tax expense (benefit) | 34,175 | | | — | | | 34,175 | |
| Depreciation and amortization | 11,924 | | | 1,198 | | | 13,122 | |
| EBITDA | (131,593) | | | (12,381) | | | (143,974) | |
| Share of income from equity method investments | (3,114) | | | — | | | (3,114) | |
Impairment loss on long-lived assets(ii) | 3,493 | | | — | | | 3,493 | |
Gain on revaluation of derivative liabilities(iii) | (14,060) | | | — | | | (14,060) | |
Gain on revaluation of financial instruments(iv) | (14,739) | | | — | | | (14,739) | |
Impairment loss on other investments(ix) | 61,392 | | | — | | | 61,392 | |
| Foreign currency transaction loss | 2,286 | | | — | | | 2,286 | |
Other, net(vi) | 324 | | | 169 | | | 493 | |
Restructuring costs(x) | 3,545 | | | 1,788 | | | 5,333 | |
Share-based compensation(vii) | 15,008 | | | 107 | | | 15,115 | |
Financial statement review costs(viii) | 7,167 | | | — | | | 7,167 | |
| | | | | |
| Adjusted EBITDA | $ | (70,291) | | | $ | (10,317) | | | $ | (80,608) | |
| | | | | | | | | | | | | | | | | |
| (in thousands of U.S. dollars) | For the year ended December 31, 2021 |
| Continuing Operations | | Discontinued Operations | | Total |
| Net loss | $ | (128,079) | | | $ | (269,125) | | | $ | (397,204) | |
| Interest income, net | (9,068) | | | (4) | | | (9,072) | |
| Income tax expense (benefit) | (431) | | | — | | | (431) | |
| Depreciation and amortization | 15,236 | | | 166 | | | 15,402 | |
| EBITDA | (122,342) | | | (268,963) | | | (391,305) | |
| Share of loss from equity method investments | 6,313 | | | — | | | 6,313 | |
Impairment loss on goodwill and indefinite-lived intangible assets(i) | 37 | | | 236,019 | | | 236,056 | |
Impairment loss on long-lived assets(ii) | 126,405 | | | 1,214 | | | 127,619 | |
Gain on revaluation of derivative liabilities(iii) | (151,360) | | | — | | | (151,360) | |
Gain on revaluation of financial instruments(iv) | (8,611) | | | — | | | (8,611) | |
Transaction costs(v) | 3,801 | | | — | | | 3,801 | |
Other, net(vi) | (733) | | | (101) | | | (834) | |
Share-based compensation(vii) | 9,844 | | | 307 | | | 10,151 | |
Financial statement review costs(viii) | 7,102 | | | — | | | 7,102 | |
| Adjusted EBITDA | $ | (129,544) | | | $ | (31,524) | | | $ | (161,068) | |
(i)For the year ended December 31, 2021, impairment loss on goodwill and Adjusted EBITDA by segment is reconciledindefinite-lived intangible assets relates primarily to impairment on goodwill and intangible assets related to our U.S. operations. See Note 7 “Goodwill and Intangible Assets, net income (loss) as follows” to the consolidated financial statements under Item 8 of this Annual Report.
(ii)For the year ended December 31, 2023, impairment loss on long-lived assets related to certain leased properties associated with the Company’s former U.S. operations and impairment of the Ginkgo Collaboration Agreement’s CBCVA exclusive license. For the year ended December 31, 2022, impairment loss on long-lived assets relates to the Company’s decision to seek a sublease for leased office space in Toronto, Ontario, Canada during the first quarter of 2022. For the year ended December 31, 2021, impairment loss on long-lived assets relates to impairment charges on property, plant and equipment and definite-lived intangible assets in the Canadian asset group, impairment charges for the differences between the consideration paid to Ginkgo for the achievement of two equity milestones in connection with the Ginkgo Collaboration Agreement and the fair values of the CBGA exclusive license and CBGVA exclusive license. See Note 6 “Property, plant and equipment, net” and Note 7 “Goodwill and Intangible Assets, net” to the consolidated financial statements in Item 8 of this Annual Report.
(iii)For the years ended December 31, 20202023, 2022 and 2019:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| (in thousands of U.S. dollars) | | Year ended December 31, 2020 |
| | US | | ROW | | Corporate
Expenses | | Total |
| Net income (loss) | | $ | (77,368) | | | $ | 32,671 | | | $ | (30,573) | | | $ | (75,270) | |
| Adjustments | | | | | | | | |
| Interest expense (income), net | | 18 | | | (18,433) | | | — | | | (18,415) | |
| Income tax expense | | 323 | | | 1,024 | | | — | | | 1,347 | |
| Impairment loss on goodwill and intangible assets | | 40,000 | | | — | | | — | | | 40,000 | |
| | | | | | | | |
| Financing and transaction costs | | 40 | | | — | | | — | | | 40 | |
| Gain on revaluation of derivative liabilities | | — | | | (129,254) | | | — | | | (129,254) | |
| Gain on disposal of other investments | | — | | | (4,789) | | | — | | | (4,789) | |
| Share of loss from equity accounted investees | | — | | | 4,510 | | | — | | | 4,510 | |
| Loss from discontinued operations | | — | | | 650 | | | — | | | 650 | |
| Other loss (income) | | 20 | | | 1,814 | | | — | | | 1,834 | |
| Review costs related to restatement of 2019 interim financial statements | | — | | | — | | | 9,688 | | | 9,688 | |
| Share-based payments | | 8,714 | | | 6,647 | | | — | | | 15,361 | |
| Adjusted EBIT | | (28,253) | | | (105,160) | | | (20,885) | | | (154,298) | |
| Adjustments | | | | | | | | |
| Depreciation and amortization | | 234 | | | 6,811 | | | — | | | 7,045 | |
| Adjusted EBITDA | | $ | (28,019) | | | $ | (98,349) | | | $ | (20,885) | | | $ | (147,253) | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| (in thousands of U.S. dollars) | | Year ended December 31, 2019 |
| | US | | ROW | | Corporate
Expenses | | Total |
| Net income (loss) | | $ | (3,070) | | | $ | 1,180,241 | | | $ | (11,597) | | | $ | 1,165,574 | |
| Adjustments | | | | | | | | |
| Interest income, net | | (6) | | | (27,963) | | | — | | | (27,969) | |
| | | | | | | | |
| | | | | | | | |
| Repurposing charges | | — | | | 7,268 | | | — | | | 7,268 | |
| Financing and transaction costs | | 117 | | | 32,091 | | | — | | | 32,208 | |
| Gain on revaluation of derivative liabilities | | — | | | (1,276,819) | | | — | | | (1,276,819) | |
| Gain on disposal of other investments | | — | | | (16,277) | | | — | | | (16,277) | |
| Share of loss from equity accounted investees | | — | | | 2,009 | | | — | | | 2,009 | |
| | | | | | | | |
| | | | | | | | |
| Loss from discontinued operations | | — | | | 363 | | | — | | | 363 | |
| | | | | | | | |
| Other loss (income) | | 182 | | | (197) | | | (182) | | | (197) | |
| Share-based payments | | 900 | | | 10,719 | | | — | | | 11,619 | |
| Adjusted EBIT | | (1,877) | | | (88,565) | | | (11,779) | | | (102,221) | |
| Adjustments | | | | | | | | |
| Depreciation and amortization | | 174 | | | 3,739 | | | — | | | 3,913 | |
| Adjusted EBITDA | | $ | (1,703) | | | $ | (84,826) | | | $ | (11,779) | | | $ | (98,308) | |
Adjusted operating loss
Management previously reviewed operating2021, the (gain) loss on an adjusted basis, which excluded certain incomerevaluation of derivative liabilities represents the fair value changes on the derivative liabilities. See Note 9 “Derivative Liabilities” to the consolidated financial statements in Item 8 of this Annual Report.
(iv)For the years ended December 31, 2023, 2022 and expense items that management believed were not part2021, (gain) loss on revaluation of underlying operations.financial instruments relates primarily to our unrealized holding gain on our mark-to-market investment in Vitura as well as revaluations of financial liabilities resulting from deferred share units (“DSUs”) granted to directors. See Note 4 “Investments” and Note 11 “Share-based Compensation” to the consolidated financial statements in Item 8 of this Annual Report.
(v)For the year ended December 31, 2021, transaction costs represent legal, financial and other advisory fees and expenses incurred in connection with various strategic investments. These items typicallycosts are included repurposing chargesin general and non-recurring charges suchadministrative expenses on the consolidated statements of net loss and comprehensive loss.
(vi)For the years ended December 31, 2023 and 2022, other, net primarily related to related to (gain) loss on disposal of assets. For the year ended December 31, 2021, other, net is primarily related to (gain) loss on reclassification of held-for-sale assets and (gain) loss on disposal of assets.
(vii)For the years ended December 31, 2023, 2022 and 2021, share-based compensation relates to the vesting expenses of share-based compensation awarded to employees under our share-based award plans as described in Note 11 “Share-based Compensation” to the consolidated financial statements in Item 8 of this Annual Report.
(viii)For the years ended December 31, 2023, 2022 and 2021, financial statement review costs include costs related to the restatement of the Company’s 2019 interim financial statements,Restatement, costs related to the Company’s responses to the reviews of such interim financial statements byrequests for information from various regulatory authorities relating to the Restatements, the costs related to the Settlement Order and Settlement Agreement and legal costs defending shareholder class action complaints brought against the Company as a result of the restatement (see2019 restatement.
(ix)For the years ended December 31, 2023 and 2022, impairment loss on other investments related to the PharmaCann Option for the difference between its fair value and carrying amount. See Note 4 “Investments” to the consolidated financial statements in Item 8 of this Annual Report.
(x)For the years ended December 31, 2023 and 2022, restructuring costs related to the employee-related severance costs and other restructuring costs associated with the Realignment. See Note 16 “Restructuring” to the consolidated financial statements in Item 8 of this Annual Report.
(xi)For the year ended December 31, 2023, inventory write-downs from discontinued operations relate to product destruction and obsolescence associated with the exit of our U.S. operations as described in Note 2 “Discontinued Operations” and inventory write-downs from continuing operations relate to product destruction and obsolescence associated with the planned exit of Cronos Fermentation as described in Note 16 “Restructuring.”
Constant Currency
To supplement the consolidated financial statements presented in accordance with U.S. GAAP, we have presented constant currency adjusted financial measures for net revenues, gross profit, gross profit margin, operating expenses, net income (loss) and Adjusted EBITDA for 2023, as well as cash and cash equivalents and short-term investment balances as of December 31, 2023 compared to December 31, 2022, which are considered non-GAAP financial measures. We present constant currency information to provide a framework for assessing how our underlying operations performed excluding the effect of foreign currency rate fluctuations. To present this information, current and prior period income statement results in currencies other than U.S. dollars are converted into U.S. dollars using the average exchange rates from the comparative period in 2022 rather than the actual average exchange rates in effect during 2023; constant currency current period balance sheet information is translated at the prior year-end spot rate rather than the current year-end spot rate. All growth comparisons relate to the corresponding period in 2022. We have provided this non-GAAP financial information to aid investors in better understanding the performance of our business. The non-GAAP financial measures presented in this Annual Report should not be considered as a substitute for, or superior to, the measures of financial performance prepared in accordance with U.S. GAAP.
The table below sets forth certain measures of consolidated results from continuing operations on an as-reported and constant currency basis for 2023 compared to 2022, as well as cash and cash equivalents and short-term investments as of December 31, 2023, compared to December 31, 2022, on an as-reported and constant currency basis (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As Reported | | As Adjusted for Constant Currency |
| Year ended December 31, | | As Reported Change | | Year ended December 31, | | Constant Currency Change |
| 2023 | | 2022 | | $ | | % | | 2023 | | $ | | % |
| Net revenue | $ | 87,241 | | | $ | 86,749 | | | $ | 492 | | | 1 | % | | $ | 91,711 | | | $ | 4,962 | | | 6 | % |
| Gross profit | 11,909 | | | 15,436 | | | (3,527) | | | (23) | % | | 12,662 | | | (2,774) | | | (18) | % |
| Gross margin | 14 | % | | 18 | % | | N/A | | (4) | pp | | 14 | % | | N/A | | (4) | pp |
| | | | | | | | | | | | | |
| Operating expenses | 96,709 | | | 126,864 | | | (30,155) | | | (24) | % | | 101,142 | | | (25,722) | | | (20) | % |
| Net loss from continuing operations | (70,439) | | | (155,178) | | | 84,739 | | | 55 | % | | (73,193) | | | 81,985 | | | 53 | % |
| Adjusted EBITDA | (61,564) | | | (70,291) | | | 8,727 | | | 12 | % | | (64,507) | | | 5,784 | | | 8 | % |
| | | | | | | | | | | | | |
| As of December 31, | | As Reported Change | | As of December 31, | | Constant Currency Change |
| 2023 | | 2022 | | $ | | % | | 2023 | | $ | | % |
| Cash and cash equivalents | $ | 669,291 | | | $ | 764,644 | | | $ | (95,353) | | | (12) | % | | $ | 656,647 | | | $ | (107,997) | | | (14) | % |
| Short-term investments | 192,237 | | | 113,077 | | | 79,160 | | | 70 | % | | 187,826 | | | 74,749 | | | 66 | % |
| Total cash and cash equivalents and short-term investments | $ | 861,528 | | | $ | 877,721 | | | $ | (16,193) | | | (2) | % | | $ | 844,473 | | | $ | (33,248) | | | (4) | % |
Net revenue
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As Reported | | As Adjusted for Constant Currency |
| Year ended December 31, | | As Reported Change | | Year ended December 31, | | Constant Currency Change |
| 2023 | | 2022 | | $ | | % | | 2023 | | $ | | % |
| Cannabis flower | $ | 62,071 | | | $ | 63,593 | | | $ | (1,522) | | | (2) | % | | $ | 65,573 | | | $ | 1,980 | | | 3 | % |
| Cannabis extracts | 24,569 | | | 22,522 | | | 2,047 | | | 9 | % | | 25,502 | | | 2,980 | | | 13 | % |
| Other | 601 | | | 634 | | | (33) | | | (5) | % | | 636 | | | 2 | | | — | % |
| Net revenue | $ | 87,241 | | | $ | 86,749 | | | $ | 492 | | | 1 | % | | $ | 91,711 | | | $ | 4,962 | | | 6 | % |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As Reported | | As Adjusted for Constant Currency |
| Year ended December 31, | | As Reported Change | | Year ended December 31, | | Constant Currency Change |
| 2023 | | 2022 | | $ | | % | | 2023 | | $ | | % |
| Canada | $ | 64,702 | | | $ | 56,233 | | | $ | 8,469 | | | 15 | % | | $ | 67,073 | | | $ | 10,840 | | | 19 | % |
| Israel | 21,134 | | | 30,516 | | | (9,382) | | | (31) | % | | 23,182 | | | (7,334) | | | (24) | % |
| | | | | | | | | | | | | |
| Other countries | 1,405 | | | — | | | 1,405 | | | N/M | | 1,456 | | | 1,456 | | | N/M |
| Net revenue | $ | 87,241 | | | $ | 86,749 | | | $ | 492 | | | 1 | % | | $ | 91,711 | | | $ | 4,962 | | | 6 | % |
Net Revenue
For 2023, net revenue on a constant currency basis was $91.7 million, representing a 6% increase from 2022. Net revenue increased on a constant currency basis primarily due to higher cannabis flower and extracts sales in the Canadian adult-use market, partially offset by lower cannabis flower sales in Israel driven by pricing pressure as a result of competitive activity, the slowdown in patient permit authorizations and the Israel-Hamas War, and an adverse price/mix in Canada in the cannabis flower category driving increased excise tax payments as a percentage of revenue.
Gross profit
For 2023, gross profit on a constant currency basis was $12.7 million, representing an 18% decrease from 2022. Gross profit decreased on a constant currency basis primarily due to lower cannabis flower sales in the Israeli medical market, an adverse price/mix on cannabis flower sales in Canada resulting in higher excise taxes as a percentage of revenue and the inventory write-down recognized as a result of the decision to wind down operations at Cronos Fermentation, partially offset by higher cannabis flower and extract sales in the Canadian adult-use market.
Operating expenses
For 2023, operating expenses on a constant currency basis was $101.1 million, representing a 20% decrease from 2022. Operating expenses decreased on a constant currency basis primarily due to lower professional fees, largely related to financial statement review costs, lower costs associated with the achievement of Ginkgo milestones, the 2022 acceleration of expense on equity awards granted to certain executive employees in connection with their separation from the Company, as well as previously held-back equity awards granted in 2022 to certain executives, impairment loss on long-lived assets recognized in the prior year, lower bonus expense, lower payroll costs and lower insurance costs, partially offset by higher sales and marketing expenses.
Net loss
For 2023, net loss on a constant currency basis was $73.2 million, representing a 53% improvement from 2022.
Adjusted EBITDA
For 2023, Adjusted EBITDA on a constant currency basis was $64.5 million, representing an 8% improvement from 2022. Adjusted EBITDA increased on a constant currency basis primarily due to higher cannabis flower and extracts sales in the Canadian adult-use market, decreases in general and administrative expenses and lower costs associated with the achievement of Ginkgo milestones, partially offset by lower cannabis flower sales in Israel driven by pricing pressure as a result of competitive activity, the slowdown in patient permit authorizations and the Israel-Hamas War, an adverse price/mix in Canada in the cannabis flower category driving increased excise tax payments as a percentage of revenue and higher sales and marketing expenses.
Cash and cash equivalents & short-term investments
Cash and cash equivalents and short-term investments on a constant currency basis decreased 4% to $844.5 million as of December 31, 2023 from $877.7 million as of December 31, 2022. The decrease in cash and cash equivalents and short-term investments is primarily due to cash flows used in operating activities in 2023.
Liquidity and Capital Resources
We believe that our existing cash and cash equivalents and short-term investments will be sufficient to fund our business operations and capital expenditures over the next twelve months. Our primary need for liquidity is to fund operations and capital expenditures. Our ability to fund operations and capital expenditures depends on, among other things, future operating performance and cash flows that are subject to general economic conditions and financial and other factors, including factors beyond our control. Since 2019, we have been funded by the C$2.4 billion (approximately $1.8 billion) Altria investment in us as further discussed under “Business—Altria Strategic Investment” in Part I, Item 3, 1 of this Annual Report. As of December 31, 2023, we had $669.3 million in cash and cash equivalents and $192.2 million in short term investments, compared to $764.6 million in cash and cash equivalents and $113.1 million in short term investments as of December 31, 2022. As of both December 31, 2023 and December 31, 2022, we had no external financing.
Cash flows
| | | | | | | | | | | | | | | | | |
| (In thousands of U.S. dollars) | Year ended December 31, |
| 2023 | | 2022 | | 2021 |
| Net cash used in operating activities | $ | (42,835) | | | $ | (88,948) | | | $ | (153,616) | |
| Net cash used in investing activities | (59,499) | | | (1,842) | | | (28,898) | |
| Net cash used in financing activities | (1,030) | | | (2,897) | | | (13,442) | |
| Effect of foreign currency translation on cash and cash equivalents | 8,011 | | | (28,642) | | | 4,906 | |
| Net change in cash | $ | (95,353) | | | $ | (122,329) | | | $ | (191,050) | |
2023 cash flows vs 2022 cash flows
Operating activities
During 2023, we used $42.8 million of cash in operating activities, compared to $88.9 million in 2022, representing a decrease in cash used of $46.1 million. This change is primarily driven by an $88.3 million increase in net income after adjusting for non-cash items, such as impairment charges, share-based payments, depreciation and amortization, and share of loss from investments in equity method investments, partially offset by and a net decrease in changes in operating assets and liabilities of $42.2 million related to payments for income taxes, the timing of collections of receivables, payments for accruals and payables, and purchases of inventory.
Investing activitiesLegal Proceedings
During 2023, we used $59.5 million of cash in investing activities, compared to $1.8 million during 2022, representing an increase of $57.7 million in net cash used. This change is primarily driven by higher net purchases of short-term investments, partially offset by higher net repayments on loan receivables and lower purchases of property, plant and equipment.
Financing activities,
During 2023, cash used in financing activities was $1.0 million, as compared to $2.9 million in 2022, representing a decrease in net cash used of $1.9 million. This change is primarily driven by a decrease in withholding taxes paid on share-based awards.
Cash requirements
In the near term, we expect to use our available cash and investments to operate our core business and develop new ways to serve our customers as well as invest in our various strategic partnerships and in our investees. We believe we have adequate liquidity to meet working capital requirements.
Our material cash requirements include the following contractual and other obligations as of December 31, 2023:
Leases
We have operating leases for land, buildings and office space. As of December 31, 2023, the future minimum payments required under these leases totaled $3.0 million, with $1.1 million payable within 12 months. Refer to Note 8 “Leases” to the consolidated financial statements in Item 8 of this Annual Report for a discussionfurther information.
Loans receivable with related parties
We have entered into three loan agreements with affiliates. As of the regulatory reviews and shareholder class action complaints relating to the restatement of the 2019 interim financial statements).
Management did not view these itemsDecember 31, 2023, Cronos GrowCo had approximately $0.8 million undrawn on its credit facility, with no amounts expected to be part of underlying resultsdrawn within 12 months. The Mucci Promissory Note and Cannasoul Collaboration Loan (each as they maydefined below) have been highly variable, unusual or infrequent, were difficultfully drawn. Refer to predict or could distort underlying business trends and results. Management believed that adjusted operating loss provided useful insight into underlying business trends and results and provided a more meaningful comparison of year-over-year results. Management used to use adjusted operating loss for planning, forecasting and evaluating business and financial performance, including allocating resources and evaluating results relative to employee compensation targets. As a result of the appointment of Mr. Schmidt as President and Chief Executive Officer in September 2020 and a review of how management looks at the business, adjusted operating loss is no longer a primary metric upon which management views the consolidated business performance and results year-over-year.
| | | | | | | | | | | | | |
| (In thousands of U.S. dollars) | Year ended December 31, |
| 2020 | | 2019 | | |
| Reported operating loss | $ | (179,347) | | | $ | (121,108) | | | |
| Adjustments | | | | | |
| Review costs related to restatement of 2019 interim financial statements | 9,688 | | | — | | | |
| Repurposing charges | — | | | 7,268 | | | |
| Adjusted operating loss | $ | (169,659) | | | $ | (113,840) | | | |
Adjusted operating loss by segment
Note 5 “Management previously reviewed operating loss by segment, which excluded corporate expenses, and adjusted operating loss by segment, which further excluded certain income and expense items that management believed were not part of the underlying segment’s operations. Corporate expenses were expenses that relateLoans Receivable, net” to the consolidated business and not to an individual operating segment while the income and expense items typically included non-recurring charges such as repurposing charges and review costs related to the restatement of the Company’s 2019 interim financial statements, the Company’s responses to the reviews of such interim financial statements by various regulatory authorities and legal costs defending shareholder class action complaints brought against the Company as a result of the restatement (see Part I,statement in Item 3, Legal Proceedings,8 of this Annual Report for a discussion of the regulatory reviews and shareholder class action complaints relating to the restatement of the 2019 interim financial statements). Management did not view the income and expense items above to be part of underlying results of the segment as they may have been highly variable, unusual or infrequent, were difficult to predict and could distort underlying business trends and results.further information.
As a result of the appointment of Mr. Schmidt as President and Chief Executive Officer in September 2020 and a review of how management looks at the business, adjusted operating loss by segment is no longer the primary metric upon which management views the segment performance and results year-over-year.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (In thousands of U.S. dollars) | Year ended December 31, 2020 |
| US | | ROW | | Total Segments | | Corporate Expenses | | Total |
| Reported operating loss | $ | (36,967) | | | $ | (111,807) | | | $ | (148,774) | | | $ | (30,573) | | | $ | (179,347) | |
| Adjustments | | | | | | | | | |
| Review costs related to restatement of 2019 interim financial statements | — | | | — | | | — | | | 9,688 | | | 9,688 | |
| Adjusted operating loss | $ | (36,967) | | | $ | (111,807) | | | $ | (148,774) | | | $ | (20,885) | | | $ | (169,659) | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (In thousands of U.S. dollars) | Year ended December 31, 2019 |
| US | | ROW | | Total Segments | | Corporate Expenses | | Total |
| Reported operating loss | $ | (2,777) | | | $ | (106,552) | | | $ | (109,329) | | | $ | (11,779) | | | $ | (121,108) | |
| Adjustments | | | | | | | | | |
| Repurposing charges | — | | | 7,268 | | | 7,268 | | | — | | | 7,268 | |
| Adjusted operating loss | $ | (2,777) | | | $ | (99,284) | | | $ | (102,061) | | | $ | (11,779) | | | $ | (113,840) | |
Off-Balance Sheet Arrangements
We do not have any material off-balance sheet arrangements.
Purchase obligations
Our purchase obligations primarily consist of contractual obligations to maintain the ordinary course of business through information technology and capital expenditures related to computer software, agricultural supply services and data analytics. As of December 31, 2023, the Company had purchase obligations of $13.0 million, with $10.1 million payable within 12 months. Other purchase obligations consist of noncancellable obligations related to maintenance, internet, and telecommunication service. As of December 31, 2023, we had other purchase obligations of $4.4 million, with $2.2 million payable within 12 months.
Critical Accounting Estimates
Estimates and critical judgments by management
The preparation of the consolidated financial statements in conformity with USU.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the consolidated financial statements, and the reported amounts of revenues and expenses during the reporting period. These estimates are reviewed periodically, and adjustments are made as appropriate in the year they become known. Items for which actual results may differ materially from these estimates are described in the following section.
Refer to Note 21 “Background, Basis of Presentation, and Summary of Significant Accounting Policies” withinto the Consolidated Financial Statementsconsolidated financial statements in Item 8 of this Annual Report for further information on our critical accounting estimates and policies, which are as follows:
Revenue recognition
Revenue is recognized at the point in time when the control of the promised goods is transferred to the customer in an amount that reflects the consideration we expect to be entitled to in exchange for the performance obligation. Excise taxes remitted to tax authorities are government-imposed excise taxes on cannabis products. Excise taxes are recorded as a reduction of sales in net revenue in the consolidated statements of income (loss) and comprehensive income (loss) and are recognized as a current liability within accrued liabilities on the consolidated balance sheets, with the liability subsequently reduced when the taxes are remitted to the tax authority.
In addition, amounts disclosed as net revenue are net of allowances, discounts and rebates. In determining the transaction price for the sale of goods, the Company considers the effects of variable consideration and the existence of significant financing components, if any. Some contracts for the sale of goods may provide customers with a right of return, most-favored-customer rights, or early payment discounts. In addition, the Company may provide, in certain circumstances, a retroactive price reduction to a customer based primarily on inventory movement. These items give rise to variable consideration. The Company uses the expected value method to estimate the variable consideration because this method best predicts the amount of variable consideration to which the Company will be entitled. The Company uses historical evidence, current information and forecasts to estimate the variable consideration. The Company reduces revenue and recognizes a contract liability equal to the amount expected to be refunded to the customer in the form of a future rebate or credit for a retroactive price reduction, representing its obligation to return the customer’s consideration. The estimate is updated at each reporting period date.
Goodwill and indefinite-lived intangible assets
Goodwill and indefinite-lived intangible assets are not subject to amortization. We test goodwill and indefinite-lived intangible assets for impairment annually, or more frequently if an event occurs or circumstances change that could indicate a potential impairment. We compare the fair value of our reporting units with their carrying amount and recognize an impairment charge for the amount by which the carrying amount exceeds the reporting unit’s fair value.
We believe that the accounting estimate for goodwill and indefinite-lived intangible assets is a critical accounting estimate because of the judgment required in assessing the fair value of each of our reporting units. We estimate fair value through various valuation methods, including the use of discounted expected future cash flows of each reporting unit, as well as the use of the relief-from-royalty method on the Lord Jones™Jones® brand. Significant inputs include discount rates, growth rates, and cash flow projections, and, for the Lord Jones® brand, royalty rate. These valuation inputs are considered Level 3 inputs as defined by Accounting Standards Codification 820 Fair Value Measurement.The expected future cash flows for each reporting unit are significantly impacted by current market conditions. If these market conditions and resulting expected future cash flows for each reporting unit decline significantly, the actual results for each segmentreporting unit could differ from our estimate, which would cause goodwill to be impaired. Our accounting for goodwill and indefinite-lived intangible assets represents our best estimate of future events.
Based on our assessments and after considering potential triggering events, including COVID-19, we recognized an impairment loss related to goodwill and indefinite life intangible assets
Inventory valuation
We value our inventory at lower of cost or net realizable value determined using weighted average cost. Inventory is reflected at the lower of cost or net realizable value considering future demand, market conditions and market prices. Our estimates are based upon assumptions believed to be reasonable, but whichthat are inherently uncertain and unpredictable. These valuations require the use of management'smanagement’s assumptions whichthat do not reflect unanticipated events and circumstances that may occur. We record an inventory valuation adjustment for excess, slow moving, and obsolete inventory that is equal to the excess of the cost of the inventory over the estimated net realizable value. We also experience inventory write-downs due to reduced market prices. The inventory valuation adjustment to net realizable value establishes a new cost basis of the inventory that cannot be subsequently reversed. Inventory valuation adjustments are based on inventory levels, expected product life, and estimated product demand. In assessing the ultimate realization of inventories, we are required to make judgments as to future demand requirements compared with inventory levels.
Long-lived assets
Long-lived assets are primarily comprised of property, plant, and equipment and definite-lived intangible assets. We evaluate long-lived assets for impairment when events or changes in circumstances indicate, in management’s judgment, that the carrying amount of such assets may not be recoverable. Long-lived asset recoverability is assessed on an asset group basis. We group assets and liabilities for our asset groups at the reporting unit level, which is the lowest level for which cash flows are separately identifiable. Long-lived asset recoverability is measured by comparing the carrying amount of the asset group with its estimated future undiscounted pre-tax cash flows over the remaining life of the primary long-lived asset of the asset group. If the carrying amount exceeds the estimated future undiscounted cash flows as part of the recoverability assessment, an impairment charge is recognized equal to the difference between the carrying amount and fair value of the asset group. The impairment charge is allocated to the underlying long-lived assets in the asset group on a relative carrying amount basis; however, carrying amount after allocated impairment is subject to a floor of fair value on an individual asset basis.
We believe the accounting estimates used in the long-lived asset impairment assessment are critical accounting estimates because of the judgment required in identifying indicators of impairment, determining asset groups, assessing future undiscounted cash flows of the asset groups, and as applicable, evaluating the fair value of the determined asset groups as well as the underlying long-lived assets, once indicators of impairment have been identified.
We periodically evaluate whether impairment indicators related to our property, plant and equipment, operating leases and other long-lived assets are present. These impairment indicators may include a significant decrease in the market price of a long-lived asset or asset group, early termination of an operating lease, a significant adverse change to the extent or manner in which a long-lived asset or asset group is being used or in its physical condition, or a current-period operating or cash flow loss combined with a history of operating or cash flow losses or a forecast that demonstrates continuing losses associated with the use of a long-lived asset or asset group. If impairment indicators are present, we estimate the fair value for the asset or group of assets. We estimate fair value of long-lived assets through various valuation methods, including the use of the indirect cost approach, income approach, and direct comparison approach. The indirect cost approach is based on the estimated cost to reproduce the asset as if new, adjusted for physical deterioration and consideration of functional and economic obsolescence. The income approach is based on estimated rental and capitalization rates. The direct comparison approach is based on recent observable transactions of comparable assets. The estimation of future undiscounted cash flows of the asset groups as well as each of these fair value approaches are significantly impacted by market conditions. A significant adverse change in market conditions could result in fair values that differ from our estimates, which could adversely impact whether an impairment exists and the extent to which an asset group and underlying assets are impaired. The difference between the fair value and the carrying amount of the asset group is recorded as an impairment charge. Refer to Note 6 “Property, plant and equipment, net” to the consolidated financial statements in Item 8 of this Annual Report.
We periodically evaluate our long-lived assets that we plan to dispose of through sale for held-for-sale classification. To be classified as held-for-sale, management must have committed to a plan to sell, the asset (or asset group) must be available for immediate sale in its present condition, an active program to locate a buyer must have been initiated, the sale must be probable to close within one year, the asset (or asset group) must be marketed at a reasonable sales price, and it must be unlikely that significant changes to the plan will be made. Once an asset (or asset group) meets all of the above criteria, it is reclassified as assets held for sale on the consolidated balance sheet, and the asset(s) cease depreciation and are written down to their fair value, less costs to sell, if applicable.
The Company completed a review of its global supply chain and determined that it would wind down the Cronos Fermentation facility and list it for sale. This review involves significant complexities and judgments in making the accounting treatment determination. There are subjective and complex judgments in the determination of whether the Cronos Fermentation facility meets the criteria to be classified as held for sale, including: (1) whether the Cronos Fermentation facility is available for sale in its present condition subject only to terms that are usual and customary for sales of such businesses, (2) whether the sale of the Cronos Fermentation facility is probable and that the transfer of assets will be a completed sale within one year from period end, and (3) whether the Cronos Fermentation facility is being actively marketed at a reasonable price. See Note 6 “Property, plant and equipment, net” to the consolidated financial statements in Item 8 of this Annual Report for discussion regarding our evaluation of the Peace Naturals Campus and the Cronos Fermentation facility for held-for-sale classification as of December 31, 2023.
We account for the cannabinoid exclusive licenses originating from the Ginkgo Strategic Partnership as definite-lived intangible assets in accordance with the acquisition method of accounting. The cost of cash and equity in Cronos issued in exchange for the cannabinoid exclusive licenses is initially recognized and measured at the date of acquisition. On the date of acquisition, we then test each cannabinoid exclusive license for impairment by comparing the cost and fair value of each license. We believe that the accounting estimate for the cannabinoid exclusive licenses is a critical accounting estimate because of the judgment required in assessing their fair values and the expected future cash flows are significantly impacted by the future expectations for products containing each cannabinoid. We estimate the fair value using the relief-from-royalty method. Each cannabinoid exclusive license is subject to amortization. Refer to Note 7 “Goodwill and Intangible Assets, net” to the consolidated financial statements in Item 8 of this Annual Report.
Valuation of derivative liabilities
Prior to December 16, 2022, derivative liabilities consisted of the warrant issued to Altria, as well as Altria’s pre-emptive rights, and certain top-up rights. On December 16, 2022, Altria notified us that its wholly owned subsidiary, Altria Summit LLC, irrevocably relinquished its warrant and all rights that it may have held in the warrant or any common shares underlying the warrant for no consideration. As of December 31, 2023, derivative liabilities consisted of pre-emptive rights and certain top-up rights. We measure derivative liabilities at fair value at each reporting date until settlement with the re-measurement gain or loss being recognized immediately in net loss and comprehensive loss. We calculate fair value of the derivative liabilities using the Black-Scholes model. Significant assumptions are used in the valuation of derivative liabilities, including the expected term and our stock price. The assumptions used in computing the fair value of derivative liabilities reflect our best estimates, but involve uncertainties relating to market and other conditions, many of which are outside of our control. Sensitivity is performed on various inputs, refer to Note 9 “Derivative Liabilities” to the consolidated financial statements in Item 8 of this Annual Report.
Impairment of other investments without readily determinable fair values
We hold other investments without readily determinable fair values that are measured under the cost method less impairment, plus or minus changes resulting from observable price changes in orderly transactions for the identical or similar investment of the same investee. Each reporting period, we qualitatively assess if indicators of impairment are present, and, if present, we estimate the fair value of the investments and record impairment charges on the consolidated statements of income if the carrying value exceeds fair value. To estimate the fair value of the investments, we use a combination of the income and market approaches. Under the income approach, significant assumptions used in the discounted cash flow method that require the use of judgment are the discount rate, growth rates, cash flow projections, and the expectation of federal rescheduling and individual state legalization of cannabis in the U.S. Under the market valuation approach, the key assumptions that require judgment under the Guideline Public Companies method are cash flow projections, selected multiples and the discount for lack of marketability.
Share-based compensation
We measure the fair value of services received in exchange for all stock options granted based on the fair market value of the award as of the grant date. We compute the fair value of stock options with time-based vesting using the Black-Scholes option-pricing model and recognize the cost of the equity awards over the period that services are provided to earn the award. The Black-Scholes option-pricing model includes assumptions regarding dividend yields, expected volatility, expected option term and risk-free interest rates. The assumptions used in computing the fair value of share-based compensation expense reflect our best estimates, but involve uncertainties relating to market and other conditions, many of which are outside of our control. We estimate expected volatility based primarily on historical daily price changes of our stock and peers. The expected option term is the number of years that we estimate that the stock options will be outstanding prior to exercise.
Loans receivable, net
an allowance for credit losses. The probability of default rate is adjusted for current conditions and reasonable and supportable forecasts of future losses as necessary. We may also record a specific reserve for individual accounts when we become aware of specific customer circumstances, such as in the case of a bankruptcy filing or deterioration in the borrower’s operating results or financial condition. Valuation of derivative liabilitiesAssets held for sale
Derivative liabilities consistWe periodically evaluate our long-lived assets that we plan to dispose of through sale for held-for-sale classification. To be classified as held-for-sale, management must have committed to a plan to sell, the asset (or asset group) must be available for immediate sale in its present condition, an active program to locate a buyer must have been initiated, the sale must be probable to close within one year, the asset (or asset group) must be marketed at a reasonable sales price, and it must be unlikely that significant changes to the plan will be made. Once an asset (or asset group) meets all of the Altria Warrant, Pre-emptive Rights,above criteria, it is reclassified as assets held for sale on the consolidated balance sheet, and certain Top-up Rights. We measure derivative liabilities atthe asset(s) cease depreciation and are written down to their fair value, at each reporting date until settlement withless costs to sell, if applicable. See Note 6 “Property, plant and equipment, net” to the re-measurement gain or loss being recognized immediately in net income (loss) and comprehensive income (loss). We calculate fair value of the derivative liability using the Black-Scholes model. Significant assumptions are used in the valuation of derivative liabilities, including the volatility of the share price of our Company and our peers, expected dividend yield, expected term and expected risk-free interest rate. The assumptions used in computing the fair value of derivative liabilities reflect our best estimates, but involve uncertainties relating to market and other conditions, many of which are outside of our control. Sensitivity is performed on various inputs, refer to Note 14 “Derivative Liabilities”consolidated financial statements in Item 8 of this Annual Report.Report for discussion regarding our evaluation of the Peace Naturals Campus and the Cronos Fermentation facility for held-for-sale classification as of December 31, 2023.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
Interest rate risk
Interest rate risk is the risk that the value or yield of fixed-income investments may decline if interest rates change. Fluctuations in interest rates may impact the level of income and expense recorded on the cash equivalents and short-term investments, and the market value of all interest-earning assets, other than those which possess a short-termshort term to maturity. During the yearyears ended December 31, 2020, the Company2023 and December 31, 2022, we had net interest income, net of $18.4$51.2 million (December 31, 2019 – $28.0 million).and $22.5 million, respectively. A 10% change in the interest rate in effect on December 31, 20202023 would not have a material effect on the fair value of our cash equivalents and short-term investments as the majority of the portfolio had a maturity date of three months or less. A 10% change in the interest rate in effect for 2023 would have an effect of $5.4 million on interest income, net earned on our cash equivalents, short-term investments. A 10% change in the interest rate in effect on December 31, 2019,2022, would not have a material effect on (i) fair value of the cash equivalents and short-term investments as the majority of the portfolio hashad a maturity date of three months or less, or (ii) net interest income.income, net. Management continues to monitor external interest rates and revise the Company’sour investment strategy as a result.
During the year ended December 31, 2020, the Company’s2023, our average variable interest rate fell 1.49%, which resulted in a decrease of net interest income, of $15.7 million in the period.increased by approximately 1.45%. During the year ended December 31, 2019, the Company’s2022, our average variable interest rate did not materially change.
Currency rateForeign currency risk
Currency rate risk is the risk that the fair valueOur consolidated financial statements included in Part II, Item 8 “Financial Statements and Supplementary Data” of or future cash flows from, the Company’s financial instruments will significantly fluctuate due to changesthis Annual Report are expressed in U.S. dollars. In addition, we have net assets, liabilities, and revenues denominated in foreign exchange rates. The Company iscurrencies, including Canadian dollars and Israeli new shekels. As a result, we are exposed to this risk on advancesforeign currency translation gains and losses. Revenue and expenses of all foreign operations are translated into U.S. dollars at the foreign currency exchange rates that approximate the rates in effect during the period when such items are recognized. Appreciating foreign currencies relative to joint ventures denominated in A$. The Company is further exposed to this risk through subsidiaries operating in Israel and the U.S. asdollar will adversely impact operating income and net earnings, while depreciating foreign currencies relative to the Company’s functional currency is in Canadian dollars. The Company does not currently use foreign exchange contracts to hedge its exposure to currency rate risk. As such,U.S. dollar will have a positive impact.
For the Company’s financial position and financial results may be adversely affected by the unfavorable fluctuations in currency exchange rates.
As ofyears ended December 31, 2020, the Company2023 and December 31, 2022, we had foreign currency gain (loss) on translation of $15.0$21.5 million (December 31, 2019 – $37.7 million).and $(50.6) million, respectively. A 10% change in the exchange rates for the foreign currenciesCanadian dollar would affect the carrying valueamount of the net assets by approximately $170.8$97.7 million and $77.4 million as of December 31, 2020 (December2023 and December 31, 2019 – $174.9 million).2022, respectively. The corresponding impact would be recorded in accumulated other comprehensive income. We have not historically engaged in hedging transactions and do not currently contemplate engaging in hedging transactions to mitigate foreign exchange risks. As we continue to recognize gains and losses in foreign currency transactions, depending upon changes in future currency rates, such gains and losses could have a significant, and potentially adverse, effect on our results of operations.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
| | | | | |
| Table of Contents |
Reports of KPMG LLP, Independent Registered Public Accounting Firm (KPMG LLP, Vaughan, Ontario, Canada, Auditor Firm ID: 85) | |
| Consolidated Balance Sheets | |
Consolidated Statements of Net Income (Loss)Loss and Comprehensive Income (Loss)Loss | |
Consolidated Statements of Changes in Shareholders’ Equity (Deficit) | |
| Consolidated Statements of Cash Flows | |
| Notes to Consolidated Financial Statements | |
Report of Independent Registered Public Accounting Firm
To the Shareholders and Board of Directors
Cronos Group Inc.:
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Cronos Group Inc. and subsidiaries (the Company) as of December 31, 20202023 and December 31, 2019,2022, the related consolidated statements of net income (loss)loss and comprehensive income (loss),loss, changes in shareholders’ equity, (deficit), and cash flows for each of the years in the three‑year period ended December 31, 2020,2023 and the related notes (collectively, the consolidatedfinancial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 20202023 and December 31, 2019,2022, and the results of its operations and its cash flows for each of the years in the three‑year period ended December 31, 2020,2023, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company’s internal control over financial reporting as of December 31, 2020,2023, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission, and our report dated February 26, 2021,29, 2024 expressed an adverseunqualified opinion on the effectiveness of the Company’s internal control over financial reporting.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated to the Audit Committeeaudit committee and that: (1) relate to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.relate
EvaluationValuation of sufficiency of audit evidence over inventoryan option to purchase equity securities without a readily determinable fair value
As discusseddisclosed in Note 5 tonote 1(g) of the consolidated financial statements, inventory consists of raw materials; dry cannabisthe Company records its other investments without a readily determinable fair value using the cost method and cannabis extracts work-in-progress; dry cannabisassesses such investments for observable price changes and cannabis extract finished goods; and supplies and consumables. The Company’s total inventory as of December 31, 2020 was $44,002 thousand. The accuracy of inventory quantities is dependent upon the performance of an annual physical count and appropriately recording adjustments to inventory quantitiesother than temporary impairment on a periodic basis. Changes in the Company’s inventory process. The Company has also identified a material weakness related to inventory asreported value of December 31, 2020.
We identified the evaluation of the sufficiency of audit evidence over the accuracy of inventory quantities as a critical audit matter. Evaluating the sufficiency of the audit evidence obtained required especially subjective auditor judgment because of the material weakness described above.
The followingother investments are the primary procedures we performed to address this critical audit matter. We applied auditor judgment to determine the nature and extent of procedures to be performed over inventory. We increased the number of inventory samples selected to perform certain procedures compared to those we would have selected if the Company’s internal controls were operating effectively at year end. We performed independent test counts of inventory quantities. We compared the results of our independent test counts to the Company’s inventory records. We confirmed with third parties a selection of inventory quantities held by them. We evaluated the overall sufficiency of audit evidence obtained by assessing the results of procedures performed.
Evaluation of the impairment analysis for goodwillreported in the U.S. reporting unitconsolidated statements of net loss and the Lord JonesTM brand indefinite life intangible asset
comprehensive loss. As discusseddisclosed in Note 2(k) to the consolidated financial statements, goodwill and indefinite life intangible assets are not amortized but are reviewed for impairment annually or more frequently when events or changes in circumstances indicate that fair value of the reporting unit has been reduced to less than its carrying value. As discussed in Note 11note 4(b) to the consolidated financial statements, the Company had $178,414 thousandCompany’s investment in an option to purchase equity securities of goodwillPharmaCann, Inc. (the “PharmaCann Option”) is accounted for as an other investment without a readily determinable fair value. To estimate the fair value of the PharmaCann Option, management uses a combination of the income and market approaches. Under the income approach, significant assumptions used in the U.S. reporting unitdiscounted cash flow method that require the use of judgment are the discount rate, terminal growth rate, cash flow projections, and an indefinite life intangible assetthe expectation of $59,000 thousand from the Lord JonesTM brand. The Company recorded $35,000 thousandfederal rescheduling and individual state legalization of impairment charges on the goodwill recordedcannabis in the U.S. reporting unitUnited States. Under the market valuation approach, the key assumptions which require judgment under the Guideline Public Companies method are cash flow projections, selected multiples, and $5,000the discount for lack of marketability. As disclosed in note 14(a) of the consolidated financial statements, the Company’s other investment in PharmaCann Option amounted to $25,650 thousand as of impairment charges on the Lord JonesTM brand indefinite life intangible asset in the year ended December 31, 2020.2023.
We identified the evaluation of the impairment analysis for goodwill in the U.S. reporting unit and the Lord JonesTM brand indefinite life intangible assetassessment of PharmaCann Option as a critical audit matter. The evaluationAs the original cash flow projections of the Company’s key assumptions used in the discounted cash flow valuation model, including the growth rates, the discount rates, and forecasted royalty income from the Lord JonesTM brand indefinite life intangible asset, requiredinvestee were not prepared by management, a high degree of challenging auditor judgment.judgement was required to evaluate fair value of the investment. These assumptions were challenging to test as they represented subjective determinations of future market, economic, and legal conditions that are sensitive to variation. Minor changes to these assumptions could have had a significant
impact on the Company’s assessment of the fair value of the PharmaCann Option. Additionally, the audit effort associated with this estimate required specialized skills and knowledge.
The following are the primary procedures we performed to address this critical audit matter. We evaluated the design and tested the operating effectiveness of certainan internal controls over the Company’s impairment process, including a control related to the determination and assessmentcritical audit matter. We compared the historical cash flow projections of PharmaCann to actual results to assess the Company’s ability to accurately project PharmaCann cash flows. We evaluated the reasonableness of key assumptions used in the discounted cash flow valuation model.projections by comparing them to historical actual results, planned business initiatives, external industry reports, and peer data. We evaluated the key assumptions used inreasonableness of the discounted cash flow valuation model, including comparing forecasted growth rates against external analyst expectations for the industryexpectation of federal rescheduling and individual state legalization of cannabis in the United States. We also performed sensitivity analysis on the forecasted growth ratesStates by comparing management’s estimated timing with external industry reports and forecasted royalty income to assess the impact on the Company’s fair value estimate. We compared a selection of royalty rates of comparable entities used by the Company to determine the royalty rate used in the discounted cash flow valuation model to publicly availablemarket information. WeIn addition, we involved valuation professionals with specialized skills and knowledge, who assisted in:
•evaluatingassessing the discount rate by comparing itused in the income approach and the multiples used in the market approach against the internal rate of return and comparing the weighted average cost of capital to a rangeranges that waswere independently developed using publicly available marketpeer data, for comparable entities.
•evaluating the royaltyterminal growth rate which was appliedused in the income approach by comparing management’s assumption against external market information, and;
•independently developing a discount for lack of marketability and comparing to estimate forecasted revenues,management’s assumption used in the market approach.
Assessment of held for sale classification for the Cronos Fermentation facility
As discussed in Note 1(s) of the consolidated financial statements, the Company periodically evaluates its long-lived assets that it plans to calculate forecasted royalty income, using industry knowledgedispose of through sale for held-for-sale classification. To be classified as held-for-sale, management must have committed to a plan to sell, the asset (or asset group) must be available for immediate sale in its present condition, an active program to locate a buyer must have been initiated, the sale must be probable to close within one year, the asset (or asset group) must be marketed at a reasonable sales price, and consideration of comparable brand royalty rates and qualitative factors specificit must be unlikely that significant changes to the brand.plan will be made. Once an asset (or asset group) meets all the above criteria, it is reclassified as assets held for sale on the consolidated balance sheet, and the assets cease depreciation and are written down to their fair value, less costs to sell, if applicable.
As discussed in Note 6 of the consolidated financial statements, the Company has $59,468 thousand of property, plant and equipment, which includes the Fermentation facility located in Winnipeg, Manitoba, Canada. The Company completed a review of its global supply chain and determined that it would wind-down the Fermentation facility and list it for sale. This review involves significant complexities and judgments in making the accounting treatment determination. There are subjective and complex judgments in the determination of whether the Fermentation facility meets the criteria to be classified as held for sale, including: (1) whether the Fermentation facility is available for sale in its present condition subject only to terms that are usual and customary for sales of such businesses, (2) whether the sale of the Fermentation facility is probable and that the transfer of assets will be a completed sale within one year from period end, and (3) whether the Fermentation facility is being actively marketed at a reasonable price.
We identified the assessment of held for sale classification for the Fermentation facility as a critical audit matter because of the degree of subjectivity associated with significant judgments made in determining whether events have occurred indicating that the Fermentation facility should be presented as held for sale. This required a high degree of auditor judgment when performing audit procedures to evaluate whether management appropriately classified the assets of the Fermentation facility.
The following are the primary procedures we performed to address this critical audit matter. We evaluated the design and tested the operating effectiveness of an internal control related to the critical audit matter. We evaluated the Company’s assessment of held for sale criteria as it relates to the Fermentation facility, which included:
•interviewing key members of management, their real estate agents and the Board of Directors to obtain an understanding of the plans to sell the Fermentation facility, including the marketing efforts and pricing strategy, and;
•reading minutes from meetings of the Board of Directors and reading communications regarding the status of the sales process.
/s/ KPMG LLP
Chartered Professional Accountants, Licensed Public Accountants
We have served as the Company’s auditor since 2018.
Vaughan, Canada
February 26, 2021
Report of Independent Registered Public Accounting Firm
To the Shareholders and Board of Directors
Cronos Group Inc.:
Opinion on Internal Control Over Financial Reporting
We have audited Cronos Group Inc.’s and subsidiaries’ (the Company) internal control over financial reporting as of December 31, 2020,2023, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. In our opinion, because of the effect of the material weakness, described below, on the achievement of the objectives of the control criteria, the Company has not maintained, in all material respects, effective internal control over financial reporting as of December 31, 2020,2023, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated balance sheets of the Company as of December 31, 20202023 and December 31, 2019,2022, the related consolidated statements of net income (loss)loss and comprehensive income (loss),loss, changes in shareholders’ equity, (deficit), and cash flows for each of the years in the three-year period ended December 31, 2020,2023 and the related notes (collectively, the consolidated financial statements), and our report dated February 26, 202129, 2024 expressed an unqualified opinion on those consolidated financial statements.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the company’s annual or interim financial statements will not be prevented or detected on a timely basis. A material weakness related to the following has been identified and included in management’s assessment:
Inventory verification: Management failed to properly design and execute sufficient procedures to verify inventory quantities. Specifically, while inventory counts were performed in the fourth quarter, (i) the aggregate value of items excluded from the count exceeded the Company’s materiality threshold, and (ii) human error in count execution, data transposition and reconciliation analysis resulted in inaccurate adjustments.
The material weakness was considered in determining the nature, timing, and extent of audit tests applied in our audit of the 2020 consolidated financial statements, and this report does not affect our report on those consolidated financial statements.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control Over Financial Reporting (Item 9A(b)(i) of the Form 10-K).Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ KPMG LLP
Chartered Professional Accountants, Licensed Public Accountants
February 26, 2021
Vaughan, Canada
CRONOS GROUP INC.
CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 20202023 AND 20192022
Cronos Group Inc.
Consolidated Balance Sheets
As of December 31, 20202023 and 20192022
(In thousands of U.S. dollars)
| | | | | | | | | | | |
| As of December 31, |
| 2020 | | 2019 |
| Assets | | | |
| Current assets | | | |
| Cash and cash equivalents | $ | 1,078,023 | | | $ | 1,199,693 | |
| Short-term investments | 211,766 | | | 306,347 | |
Accounts receivable (i) | 8,928 | | | 4,638 | |
| Other receivables | 10,033 | | | 7,232 | |
| Current portion of loans receivable | 7,083 | | | 4,664 | |
| Prepaids and other assets | 11,161 | | | 9,395 | |
| Inventory, net | 44,002 | | | 38,043 | |
| Held-for-sale assets | 1,176 | | | 3,248 | |
| Total current assets | 1,372,172 | | | 1,573,260 | |
| Investments in equity accounted investees | 19,235 | | | 557 | |
| Advances to joint ventures | 467 | | | 19,437 | |
| | | |
| Loans receivable, net | 87,191 | | | 44,967 | |
| Property, plant and equipment | 187,599 | | | 159,948 | |
| Right-of-use assets | 9,776 | | | 6,546 | |
| Intangible assets | 69,720 | | | 71,235 | |
| Goodwill | 179,522 | | | 214,492 | |
| Total assets | $ | 1,925,682 | | | $ | 2,090,442 | |
| | | |
| Liabilities | | | |
| Current liabilities | | | |
| Accounts payable and other liabilities | $ | 42,102 | | | $ | 35,301 | |
| Current portion of lease obligation | 1,322 | | | 427 | |
Derivative liabilities(ii) | 163,410 | | | 297,160 | |
| Total current liabilities | 206,834 | | | 332,888 | |
| Due to non-controlling interests | 2,188 | | | 1,844 | |
| Lease obligation | 8,492 | | | 6,680 | |
| Total liabilities | 217,514 | | | 341,412 | |
Commitments and contingencies(iii) | 0 | | 0 |
| Shareholders’ equity | | | |
Share capital(iv, v) | 569,260 | | | 561,165 | |
| Additional paid-in capital | 34,596 | | | 23,234 | |
| Retained earnings | 1,064,509 | | | 1,137,646 | |
| Accumulated other comprehensive income | 42,999 | | | 27,838 | |
| Total equity attributable to shareholders of Cronos Group | 1,711,364 | | | 1,749,883 | |
| Non-controlling interests | (3,196) | | | (853) | |
| Total shareholders' equity | 1,708,168 | | | 1,749,030 | |
| Total liabilities and shareholders’ equity | $ | 1,925,682 | | | $ | 2,090,442 | |
(i)Net of current expected credit loss allowance of $74 as of December 31, 2020 (December 31, 2019 – $136)
(ii)Refer to Note 14 in the notes to the consolidated financial statements.
(iii)Refer to Note 20 and Note 21 in the notes to the consolidated financial statements.
(iv)Authorized for issuance as of December 31, 2020: unlimited (December 31, 2019 – unlimited).
(v)Shares issued as of December 31, 2020: 360,253,332 (as of December 31, 2019: 348,817,472) | | | | | | | | | | | | |
| As of December 31, | |
| 2023 | | 2022 | |
| Assets | | | | |
| Current assets | | | | |
| Cash and cash equivalents | $ | 669,291 | | | $ | 764,644 | | |
| Short-term investments | 192,237 | | | 113,077 | | |
| Accounts receivable, net | 13,984 | | | 23,113 | | |
| Interest receivable | 10,012 | | | 2,469 | | |
| Other receivables | 6,341 | | | 3,298 | | |
| Current portion of loans receivable, net | 5,541 | | | 8,890 | | |
| Inventory, net | 30,495 | | | 37,559 | | |
| Prepaids and other current assets | 5,405 | | | 7,106 | | |
| Total current assets | 933,306 | | | 960,156 | | |
| Equity method investments, net | 19,488 | | | 18,755 | | |
| Other investments | 35,251 | | | 70,993 | | |
| Non-current portion of loans receivable, net | 69,036 | | | 72,345 | | |
| Property, plant and equipment, net | 59,468 | | | 60,557 | | |
| Right-of-use assets | 1,356 | | | 2,273 | | |
| Goodwill | 1,057 | | | 1,033 | | |
| Intangible assets, net | 21,078 | | | 26,704 | | |
| Other | 45 | | | 193 | | |
| Total assets | $ | 1,140,085 | | | $ | 1,213,009 | | |
| Liabilities | | | | |
| Current liabilities | | | | |
| Accounts payable | $ | 12,130 | | | $ | 11,163 | | |
| Income taxes payable | 64 | | | 32,956 | | |
| Accrued liabilities | 27,736 | | | 22,268 | | |
| Current portion of lease obligation | 994 | | | 1,330 | | |
| Derivative liabilities | 102 | | | 15 | | |
| Current portion due to non-controlling interests | 373 | | | 384 | | |
| Total current liabilities | 41,399 | | | 68,116 | | |
| Non-current portion due to non-controlling interests | 1,003 | | | 1,383 | | |
| Non-current portion of lease obligation | 1,559 | | | 2,546 | | |
| | | | |
| Total liabilities | 43,961 | | | 72,045 | | |
| | | | |
| Shareholders’ equity | | | | |
| Share capital (authorized for issue as of December 31, 2023 and 2022: unlimited; shares outstanding as of December 31, 2023 and 2022: 381,298,853 and 380,575,403, respectively) | 613,725 | | | 611,318 | | |
| Additional paid-in capital | 48,449 | | | 42,682 | | |
| Retained earnings | 416,719 | | | 490,682 | | |
| Accumulated other comprehensive income (loss) | 20,678 | | | (797) | | |
| Total equity attributable to shareholders of Cronos Group | 1,099,571 | | | 1,143,885 | | |
| Non-controlling interests | (3,447) | | | (2,921) | | |
| Total shareholders’ equity | 1,096,124 | | | 1,140,964 | | |
| Total liabilities and shareholders’ equity | $ | 1,140,085 | | | $ | 1,213,009 | | |
See notes to consolidated financial statements.
Cronos Group Inc.
Consolidated Statements of Net Income (Loss)Loss and Comprehensive Income (Loss)Loss
For the years ended December 31, 2020, 2019,2023, 2022, and 20182021
(In thousands of U.S dollars, except share and per share amounts)
| | | | | | | | | | | | | | | | | | | |
| | | Year ended December 31, |
| | | 2020 | | 2019 | | 2018 |
| Net revenue, before excise taxes | | | $ | 54,353 | | | $ | 25,639 | | | $ | 13,234 | |
| Excise taxes | | | (7,634) | | | (1,889) | | | (1,113) | |
| Net revenue | | | 46,719 | | | 23,750 | | | 12,121 | |
| Cost of sales | | | 46,497 | | | 12,174 | | | 5,908 | |
| Inventory write-down | | | 26,055 | | | 29,173 | | | 0 | |
| Gross profit (loss) | | | (25,833) | | | (17,597) | | | 6,213 | |
| Operating expenses | | | | | | | |
| Sales and marketing | | | 34,386 | | | 23,048 | | | 2,537 | |
| Research and development | | | 20,366 | | | 12,155 | | | 1,814 | |
| General and administrative | | | 80,529 | | | 49,271 | | | 13,349 | |
| Share-based payments | | | 15,361 | | | 11,619 | | | 8,151 | |
| Depreciation and amortization | | | 2,872 | | | 2,090 | | | 807 | |
| Repurposing charges | | | 0 | | | 5,328 | | | 0 | |
| Total operating expenses | | | 153,514 | | | 103,511 | | | 26,658 | |
| Operating loss | | | (179,347) | | | (121,108) | | | (20,445) | |
| Other income (expense) | | | | | | | |
| Interest income, net | | | 18,415 | | | 27,969 | | | 81 | |
Gain on revaluation of derivative liabilities(i) | | | 129,254 | | | 1,276,819 | | | 0 | |
| Impairment loss on goodwill and intangible assets | | | (40,000) | | | 0 | | | 0 | |
| Gain on disposal of other investments | | | 4,789 | | | 16,277 | | | 164 | |
| Share of loss from investments in equity accounted investees | | | (4,510) | | | (2,009) | | | (723) | |
| Financing and transaction costs | | | (40) | | | (32,208) | | | 0 | |
| Other income (loss) | | | (1,834) | | | 197 | | | 0 | |
| Total other income (expense) | | | 106,074 | | | 1,287,045 | | | (478) | |
| Income (loss) before income taxes | | | (73,273) | | | 1,165,937 | | | (20,923) | |
| Income tax expense | | | 1,347 | | | 0 | | | 0 | |
| Income (loss) from continuing operations | | | (74,620) | | | 1,165,937 | | | (20,923) | |
| Loss from discontinued operations | | | (650) | | | (363) | | | (894) | |
| Net income (loss) | | | $ | (75,270) | | | $ | 1,165,574 | | | $ | (21,817) | |
| Net income (loss) attributable to: | | | | | | | |
| Cronos Group | | | $ | (73,137) | | | $ | 1,166,506 | | | $ | (21,636) | |
| Non-controlling interests | | | (2,133) | | | (932) | | | (181) | |
| | | $ | (75,270) | | | $ | 1,165,574 | | | $ | (21,817) | |
| | | | | | | |
| Net income (loss) per share | | | | | | | |
| Basic | | | $ | (0.21) | | | $ | 3.76 | | | $ | (0.12) | |
Diluted (ii) | | | (0.21) | | | 3.33 | | | (0.12) | |
| Weighted average number of outstanding shares | | | | | | | |
| Basic | | | 351,576,848 | | | 310,067,179 | | | 172,269,170 | |
Diluted (ii) | | | 351,576,848 | | | 342,811,992 | | | 172,269,170 | |
(i)Refer to Note 14 in the notes to the consolidated financial statements
(ii)In computing diluted earnings per share, incremental common shares are not considered in periods in which a net loss is reported, as the inclusion of the common share equivalents would be anti-dilutive. | | | | | | | | | | | | | | | | | | | |
| | | Year ended December 31, |
| | | 2023 | | 2022 | | 2021 |
| Net revenue, before excise taxes | | | $ | 120,270 | | | $ | 109,301 | | | $ | 79,612 | |
| Excise taxes | | | (33,029) | | | (22,552) | | | (15,051) | |
| Net revenue | | | 87,241 | | | 86,749 | | | 64,561 | |
| Cost of sales | | | 74,527 | | | 71,313 | | | 70,193 | |
| Inventory write-down | | | 805 | | | — | | | 11,961 | |
| Gross profit | | | 11,909 | | | 15,436 | | | (17,593) | |
| Operating expenses | | | | | | | |
| Sales and marketing | | | 22,701 | | | 18,046 | | | 20,917 | |
| Research and development | | | 5,843 | | | 13,131 | | | 21,841 | |
| General and administrative | | | 49,475 | | | 67,674 | | | 90,919 | |
| Restructuring costs | | | 1,524 | | | 3,545 | | | — | |
| Share-based compensation | | | 8,756 | | | 15,008 | | | 9,844 | |
| Depreciation and amortization | | | 5,044 | | | 5,967 | | | 4,413 | |
| Impairment loss on goodwill and indefinite-lived intangible assets | | | — | | | — | | | 37 | |
| Impairment loss on long-lived assets | | | 3,366 | | | 3,493 | | | 126,405 | |
| Total operating expenses | | | 96,709 | | | 126,864 | | | 274,376 | |
| Operating loss | | | (84,800) | | | (111,428) | | | (291,969) | |
| Other income (expense) | | | | | | | |
| Interest income, net | | | 51,235 | | | 22,514 | | | 9,068 | |
| Gain (loss) on revaluation of derivative liabilities | | | (85) | | | 14,060 | | | 151,360 | |
| Share of income (loss) from equity method investments | | | 1,583 | | | 3,114 | | | (6,313) | |
| Gain (loss) on revaluation of financial instruments | | | (12,042) | | | 14,739 | | | 8,611 | |
| Impairment loss on other investments | | | (23,350) | | | (61,392) | | | — | |
| Foreign currency transaction loss | | | (7,324) | | | (2,286) | | | — | |
| | | | | | | |
| Other, net | | | 1,114 | | | (324) | | | 733 | |
| Total other income (expense) | | | 11,131 | | | (9,575) | | | 163,459 | |
| Loss before income taxes | | | (73,669) | | | (121,003) | | | (128,510) | |
| Income tax expense (benefit) | | | (3,230) | | | 34,175 | | | (431) | |
| Loss from continuing operations | | | (70,439) | | | (155,178) | | | (128,079) | |
| Loss from discontinued operations | | | (4,114) | | | (13,556) | | | (269,125) | |
| Net loss | | | (74,553) | | | (168,734) | | | (397,204) | |
| Net loss attributable to non-controlling interest | | | (590) | | | — | | | (1,097) | |
| Net loss attributable to Cronos Group | | | $ | (73,963) | | | $ | (168,734) | | | $ | (396,107) | |
| | | | | | | |
| Comprehensive income (loss) | | | | | | | |
| Net loss | | | $ | (74,553) | | | $ | (168,734) | | | $ | (397,204) | |
| Foreign exchange gain (loss) on translation | | | 21,539 | | | (50,616) | | | 8,192 | |
| Comprehensive loss | | | (53,014) | | | (219,350) | | | (389,012) | |
| Comprehensive income (loss) attributable to non-controlling interest | | | (526) | | | 46 | | | 229 | |
| Comprehensive loss attributable to Cronos Group | | | $ | (52,488) | | | $ | (219,396) | | | $ | (389,241) | |
| Net loss per share | | | | | | | |
| Basic and diluted - continuing operations | | | $ | (0.18) | | | $ | (0.41) | | | $ | (0.34) | |
| Basic and diluted - discontinued operations | | | $ | (0.01) | | | $ | (0.04) | | | $ | (0.73) | |
| Basic and diluted - total | | | $ | (0.19) | | | $ | (0.45) | | | $ | (1.07) | |
See notes to consolidated financial statements.
Cronos Group Inc.
Consolidated Statements of Net Income (Loss) and Comprehensive Income (Loss)
For the years ended December 31, 2020, 2019, and 2018
(In thousands of U.S dollars, except share and per share amounts)
| | | | | | | | | | | | | | | | | | | |
| | | Year ended December 31, |
| | | 2020 | | 2019 | | 2018 |
| | | | | | | |
| Net income (loss) | | | $ | (75,270) | | | $ | 1,165,574 | | | $ | (21,817) | |
| | | | | | | |
| Other comprehensive income (loss) | | | | | | | |
| Foreign exchange gain (loss) on translation | | | 14,951 | | | 37,687 | | | (12,337) | |
| Gain on revaluation and disposal of other investments, net of tax | | | 0 | | | 0 | | | 3 | |
| | | | | | | |
| Total other comprehensive income (loss) | | | 14,951 | | | 37,687 | | | (12,334) | |
| Comprehensive income (loss) | | | $ | (60,319) | | | $ | 1,203,261 | | | $ | (34,151) | |
| Comprehensive income (loss) attributable to: | | | | | | | |
| Cronos Group | | | $ | (57,976) | | | $ | 1,204,214 | | | $ | (33,964) | |
| Non-controlling interests | | | (2,343) | | | (953) | | | (187) | |
| | | $ | (60,319) | | | $ | 1,203,261 | | | $ | (34,151) | |
See notes to consolidated financial statements.
Cronos Group Inc.
Consolidated Statements of Changes in Shareholders’ Equity (Deficit)
For the years ended December 31, 2020, 2019,2023, 2022, and 20182021
(In thousands of U.S dollars, except number of share amounts)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Number of shares | | Share capital | | Additional paid-in capital | | Retained earnings (accumulated deficit) | | Accumulated other comprehensive income (loss) | | Non-controlling interests | | Total shareholders’ equity |
| Balance as of January 1, 2018 | 149,360,603 | | | $ | 62,834 | | | $ | 4,734 | | | $ | (6,293) | | | $ | 2,458 | | | $ | 0 | | | $ | 63,733 | |
| Shares issued | 15,677,143 | | | 115,510 | | | — | | | — | | | — | | | — | | | 115,510 | |
| Share issuance costs | — | | | (7,577) | | | — | | | — | | | — | | | — | | | (7,577) | |
| Vesting of options | — | | | — | | | 8,151 | | | — | | | — | | | — | | | 8,151 | |
| Options exercised | 567,940 | | | 671 | | | (220) | | | (16) | | | — | | | — | | | 435 | |
| Warrants exercised | 13,114,336 | | | 3,563 | | | (1,402) | | | — | | | — | | | — | | | 2,161 | |
| Contribution by non-controlling interests | — | | | — | | | — | | | — | | | — | | | 287 | | | 287 | |
| Net income (loss) | — | | | — | | | — | | | (21,636) | | | — | | | (181) | | | (21,817) | |
| Other comprehensive income (loss) | — | | | — | | | — | | | — | | | (12,328) | | | (6) | | | (12,334) | |
| Balance as of December 31, 2018 | 178,720,022 | | | $ | 175,001 | | | $ | 11,263 | | | $ | (27,945) | | | $ | (9,870) | | | $ | 100 | | | $ | 148,549 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Number of shares | | Share capital | | Additional paid-in capital | | Retained earnings (accumulated deficit) | | Accumulated other comprehensive income (loss) | | Non-controlling interests | | Total shareholders’ equity |
| Balance as of January 1, 2019 | 178,720,022 | | | $ | 175,001 | | | $ | 11,263 | | | $ | (27,945) | | | $ | (9,870) | | | $ | 100 | | | $ | 148,549 | |
| Shares issued | 155,773,757 | | | 304,411 | | | 410 | | | — | | | — | | | — | | | 304,821 | |
| Share issuance costs | — | | | (3,722) | | | — | | | — | | | — | | | — | | | (3,722) | |
| Warrants exercised | 7,390,961 | | | 2,034 | | | (596) | | | — | | | — | | | — | | | 1,438 | |
| Vesting of options | — | | | — | | | 11,619 | | | — | | | — | | | — | | | 11,619 | |
| Options exercised | 190,349 | | | 368 | | | (351) | | | (915) | | | — | | | — | | | (898) | |
| Vesting of restricted share units | — | | | — | | | 889 | | | — | | | — | | | — | | | 889 | |
| Top-up Rights exercised | 6,742,383 | | | 83,073 | | | — | | | — | | | — | | | — | | | 83,073 | |
| Net income (loss) | — | | | — | | | — | | | 1,166,506 | | | — | | | (932) | | | 1,165,574 | |
| Other comprehensive income (loss) | — | | | — | | | — | | | — | | | 37,708 | | | (21) | | | 37,687 | |
| Balance as of December 31, 2019 | 348,817,472 | | | $ | 561,165 | | | $ | 23,234 | | | $ | 1,137,646 | | | $ | 27,838 | | | $ | (853) | | | $ | 1,749,030 | |
Cronos Group Inc.
Consolidated Statements of Changes in Shareholders’ Equity (Deficit)
For the years ended December 31, 2020, 2019, and 2018
(In thousands of U.S dollars, except number of share amounts)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Number of shares | | Share capital | | Additional paid-in capital | | Retained earnings (accumulated deficit) | | Accumulated other comprehensive income (loss) | | Non-controlling interests | | Total shareholders’ equity |
| Balance as of January 1, 2020 | 348,817,472 | | | $ | 561,165 | | | $ | 23,234 | | | $ | 1,137,646 | | | $ | 27,838 | | | $ | (853) | | | $ | 1,749,030 | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Warrants exercised | 9,755,642 | | | 1,244 | | | (1,137) | | | — | | | — | | | — | | | 107 | |
| Vesting of options | — | | | — | | | 7,185 | | | — | | | — | | | — | | | 7,185 | |
| Options exercised | 1,266,130 | | | 1,586 | | | (1,577) | | | — | | | — | | | — | | | 9 | |
| Restricted share units settled | 414,088 | | | — | | | — | | | — | | | — | | | | | — | |
| Vesting of restricted share units | — | | | — | | | 8,176 | | | — | | | — | | | — | | | 8,176 | |
| Taxes withheld on vesting of restricted share units | — | | | — | | | (2,148) | | | — | | | — | | | — | | | (2,148) | |
| Vesting of common shares issued in connection with the use of certain publicity rights in brand development | — | | | 2,000 | | | 863 | | | — | | | — | | | — | | | 2,863 | |
| Top-up Rights exercised | 0 | | | 3,265 | | | — | | | — | | | — | | | — | | | 3,265 | |
| Net income (loss) | — | | | — | | | — | | | (73,137) | | | — | | | (2,133) | | | (75,270) | |
| Other comprehensive income (loss) | — | | | — | | | — | | | — | | | 15,161 | | | (210) | | | 14,951 | |
| Balance as of December 31, 2020 | 360,253,332 | | | $ | 569,260 | | | $ | 34,596 | | | $ | 1,064,509 | | | $ | 42,999 | | | $ | (3,196) | | | $ | 1,708,168 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Number of shares | | Share capital | | Additional paid-in capital | | Retained earnings | | Accumulated other comprehensive income (loss) | | Non-controlling interests | | Total shareholders’ equity |
| Balance as of January 1, 2021 | 360,253,332 | | | $ | 569,260 | | | $ | 34,596 | | | $ | 1,064,509 | | | $ | 42,999 | | | $ | (3,196) | | | $ | 1,708,168 | |
| Shares issued | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Share issuance costs | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Activities relating to share-based compensation | 11,764,381 | | | 7,288 | | | 2,671 | | | (12,213) | | | — | | | — | | | (2,254) | |
| Share issuance pursuant to research and development milestones | 2,934,980 | | | 17,374 | | | — | | | — | | | — | | | — | | | 17,374 | |
| Accelerated restricted share units vesting out-of-period adjustment | — | | | 4,802 | | | (4,802) | | | — | | | — | | | — | | | — | |
| Top-up rights out-of-period adjustment | — | | | (3,227) | | | — | | | 3,227 | | | — | | | — | | | — | |
| Net loss | — | | | — | | | — | | | (396,107) | | | — | | | (1,097) | | | (397,204) | |
| Foreign exchange gain on translation | — | | | — | | | — | | | — | | | 6,866 | | | 1,326 | | | 8,192 | |
| Balance as of December 31, 2021 | 374,952,693 | | | 595,497 | | | 32,465 | | | 659,416 | | | 49,865 | | | (2,967) | | | 1,334,276 | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Activities relating to share-based compensation | 1,464,822 | | | 4,617 | | | 10,217 | | | — | | | — | | | — | | | 14,834 | |
| Share issuance pursuant to research and development milestones | 4,157,888 | | | 11,204 | | | — | | | — | | | — | | | — | | | 11,204 | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Net loss | — | | | — | | | — | | | (168,734) | | | — | | | — | | | (168,734) | |
| Foreign exchange gain on translation | — | | | — | | | — | | | — | | | (50,662) | | | 46 | | | (50,616) | |
| Balance as of December 31, 2022 | 380,575,403 | | | 611,318 | | | 42,682 | | | 490,682 | | | (797) | | | (2,921) | | | 1,140,964 | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Activities relating to share-based compensation | 723,450 | | | 2,407 | | | 5,767 | | | — | | | — | | | — | | | 8,174 | |
| | | | | | | | | | | | | |
| Net loss | — | | | — | | | — | | | (73,963) | | | — | | | (590) | | | (74,553) | |
| Foreign exchange gain on translation | — | | | — | | | — | | | — | | | 21,475 | | | 64 | | | 21,539 | |
| Balance as of December 31, 2023 | 381,298,853 | | | $ | 613,725 | | | $ | 48,449 | | | $ | 416,719 | | | $ | 20,678 | | | $ | (3,447) | | | $ | 1,096,124 | |
See notes to consolidated financial statements.
Cronos Group Inc.
Consolidated Statements of Cash Flows
For the years ended December 31, 2020, 2019,2023, 2022, and 20182021
(In thousands of U.S dollars)
| | | | | | | | | | | | | | | | | |
| Year ended December 31, |
| 2023 | | 2022 | | 2021 |
| Operating activities | | | | | |
| Net loss | $ | (74,553) | | | $ | (168,734) | | | $ | (397,204) | |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | |
| Share-based compensation | 8,769 | | | 15,115 | | | 10,151 | |
| Depreciation and amortization | 8,110 | | | 13,122 | | | 15,402 | |
| Impairment loss on goodwill and indefinite-lived intangible assets | — | | | — | | | 236,056 | |
| Impairment loss on long-lived assets | 3,571 | | | 3,493 | | 127,619 | |
| Impairment loss on other investments | 23,350 | | | 61,392 | | — | |
| Income from investments | 10,513 | | | (17,853) | | | (1,974) | |
| Loss (gain) on revaluation of derivative liabilities | 85 | | | (14,060) | | | (151,360) | |
| Changes in expected credit losses on long-term financial assets | (1,528) | | | (662) | | | 12,202 | |
| | | | | |
| Foreign currency transaction loss | 7,324 | | | 2,286 | | | — | |
| Other non-cash operating activities, net | (2,008) | | | 1,294 | | | 335 | |
| Changes in operating assets and liabilities: | | | | | |
| Accounts receivable, net | 9,206 | | | (2,711) | | | (13,163) | |
| Interest receivable | (14,344) | | | (6,985) | | | (2,497) | |
| Other receivables | (1,449) | | | 1,148 | | | 3,497 | |
| Prepaids and other current assets | 1,437 | | | 996 | | | 3,102 | |
| Inventory, net | 7,399 | | | (7,217) | | | 11,565 | |
| Accounts payable | (773) | | | (863) | | | (1,597) | |
| Income taxes payable | (33,104) | | | 34,212 | | | (776) | |
| Accrued liabilities | 5,160 | | | (2,921) | | | (4,974) | |
| Net cash used in operating activities | (42,835) | | | (88,948) | | | (153,616) | |
| | | | | |
| Investing activities | | | | | |
| Proceeds from short-term investments | 532,838 | | | 268,870 | | | 215,303 | |
| Purchase of short-term investments | (608,247) | | | (271,378) | | | (119,610) | |
| Dividends received from equity method investee | 1,297 | | | — | | | — | |
| Purchase of investments | — | | | — | | | (110,392) | |
| Dividend proceeds | 345 | | | 384 | | | — | |
| Repayments (advances) on loan receivables | 16,831 | | | 5,246 | | | (4,967) | |
| Purchase of property, plant and equipment, net of disposals | (2,505) | | | (3,451) | | | (11,144) | |
| Purchase of intangible assets, net of disposals | (918) | | | (1,581) | | | (1,118) | |
| Other investing activities | 860 | | | 68 | | | 3,030 | |
| Net cash used in investing activities | (59,499) | | | (1,842) | | | (28,898) | |
Cronos Group Inc.
Consolidated Statements of Cash Flows (continued)
For the years ended December 31, 2023, 2022, and 2021
(In thousands of U.S dollars)
| | | | | | | | | | | | | | | | | |
| Year ended December 31, |
| 2020 | | 2019 | | 2018 |
| Operating activities | | | | | |
| Net (loss) income | $ | (75,270) | | | $ | 1,165,574 | | | $ | (21,817) | |
| Items not affecting cash: | | | | | |
Gain on revaluation of derivative liabilities (i) | (129,254) | | | (1,276,819) | | | 0 | |
| Impairment on goodwill and intangible assets | 40,000 | | | 0 | | | 0 | |
| Inventory write-down | 26,055 | | | 29,173 | | | 0 | |
| Provisions for inventory and doubtful accounts | 3,741 | | | 136 | | | 0 | |
| Share-based payments | 15,361 | | | 11,619 | | | 8,151 | |
| Depreciation and amortization | 7,045 | | | 3,913 | | | 1,937 | |
| Share of loss from investments in equity accounted investees | 4,510 | | | 2,009 | | | 723 | |
| Gain on disposal of other investments | (4,789) | | | (16,277) | | | (164) | |
| Non-cash sales and marketing | 2,863 | | | 410 | | | 0 | |
| Income tax expense | 1,347 | | | 0 | | | 0 | |
| Non-cash repurposing costs | 0 | | | 4,439 | | | 0 | |
| Other non-cash operating activity expense (income) | (123) | | | (243) | | | (9) | |
| Net changes in non-cash working capital | (33,943) | | | (54,208) | | | 3,662 | |
| Net cash provided by (used in) operating activities | (142,457) | | | (130,274) | | | (7,517) | |
| Investing activities | | | | | |
| Proceeds from (purchase of) short-term investments, net | 95,404 | | | (299,923) | | | 0 | |
| Investments in equity accounted investees | 0 | | | (1,658) | | | (480) | |
| Proceeds from sale of other investments | 4,789 | | | 19,614 | | | 747 | |
| Advances to joint ventures | 0 | | | (15,135) | | | (5,358) | |
| Purchase of property, plant and equipment, net of disposals | (31,412) | | | (38,664) | | | (88,308) | |
| Purchase of intangible assets | (3,979) | | | (289) | | | (278) | |
| Acquisition of Redwood | 0 | | | (224,295) | | | 0 | |
| Advances on loans receivable | (44,652) | | | (43,337) | | | 0 | |
| Other non-cash investing activity expense (income) | 0 | | | 415 | | | (231) | |
| Net cash provided by (used in) investing activities | 20,150 | | | (603,272) | | | (93,908) | |
| Financing activities | | | | | |
| Advance to non-controlling interests | (1,019) | | | 0 | | | 0 | |
| Repayment of lease obligations | (2,414) | | | (919) | | | 0 | |
| Withholding taxes paid on equity awards | (2,148) | | | (915) | | | (16) | |
| Proceeds from Altria Investment | 0 | | | 1,809,556 | | | 0 | |
| Proceeds from exercise of Top-up Rights | 0 | | | 67,051 | | | 0 | |
| Proceeds from exercise of warrants and options | 116 | | | 1,455 | | | 2,612 | |
| Proceeds from share issuance | 0 | | | 0 | | | 115,510 | |
| Share issuance costs | 0 | | | (3,722) | | | (7,577) | |
| Proceeds from construction loan payable | 0 | | | 0 | | | 11,583 | |
| Repayment of construction loan payable | 0 | | | (15,971) | | | 0 | |
| Advance under Credit Facility | 0 | | | 48,715 | | | 0 | |
| Repayment of Credit Facility | 0 | | | (48,309) | | | 0 | |
| Net cash provided by (used in) financing activities | (5,465) | | | 1,856,941 | | | 122,112 | |
| Effect of foreign currency translation on cash and cash equivalents | 6,102 | | | 52,371 | | | (4,085) | |
| Increase in cash and cash equivalents | (121,670) | | | 1,175,766 | | | 16,602 | |
| Cash and cash equivalents, beginning of period | 1,199,693 | | | 23,927 | | | 7,325 | |
| Cash and cash equivalents, end of period | $ | 1,078,023 | | | $ | 1,199,693 | | | $ | 23,927 | |
| | | | | | | | | | | | | | | | | |
| Year ended December 31, |
| 2023 | | 2022 | | 2021 |
| Financing activities | | | | | |
| | | | | |
| Withholding taxes paid on equity awards | (1,030) | | | (2,829) | | | (13,458) | |
| Other financing activities, net | — | | | (68) | | | 16 | |
| Net cash used in financing activities | (1,030) | | | (2,897) | | | (13,442) | |
| Effect of foreign currency translation on cash and cash equivalents | 8,011 | | | (28,642) | | | 4,906 | |
| Net change in cash and cash equivalents | (95,353) | | | (122,329) | | | (191,050) | |
| Cash and cash equivalents, beginning of period | 764,644 | | | 886,973 | | | 1,078,023 | |
| Cash and cash equivalents, end of period | $ | 669,291 | | | $ | 764,644 | | | $ | 886,973 | |
| | | | | |
Supplementary cash flow information(i): | | | | | |
| Interest paid | $ | — | | | $ | — | | | $ | — | |
| Interest received | 36,501 | | | 15,548 | | | 8,988 | |
| Taxes paid | 33,013 | | | 177 | | | 892 | |
(i)Refer toSee Note 14 in the notes2 “Discontinued Operations” and Note 8 “Leases” for supplementary cash flow information related to the consolidated financial statementsCompany’s operating leases.
See notes to consolidated financial statements.
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020, 2019,2023, 2022, and 20182021
(In thousands of U.S. dollars, except for gram and share amounts)
1. Background, Basis of Presentation, and Summary of Significant Accounting Policies
(a)Background
Cronos Group Inc. (“Cronos Group”Cronos” or the “Company”) is incorporated in the Provinceprovince of British Columbia and under the British Columbia Business Corporations Act(British Columbia) with principal executive offices at 111 Peter St. Street,, Suite 300, Toronto, Ontario, M5V 2H1. The Company’s common shares are currently listed on the Toronto Stock Exchange (“TSX”) and Nasdaq Global Market (“Nasdaq”) under the ticker symbol “CRON.”
Cronos Group is an innovative global cannabinoid company with international production and distribution across 5 continents. The Company is committed to building disruptive intellectual property by advancing cannabis research, technology and product development anddevelopment. With a passion to responsibly elevate the consumer experience, Cronos is seeking to buildbuilding an iconic brand portfolio. Cronos Group’sCronos’ diverse international brand portfolio includes Spinach®, PEACE NATURALS™, a global wellness platform; 2 adult-use brands, COVE™NATURALS® and Spinach™; and 3 U.S hemp-derived consumer products brands, Lord Jones™, Happy Dance™ and PEACE+™Jones®.
Cronos Group has established 4 strategic joint ventures in Canada, Israel, and Colombia. Cronos Israel (as defined herein) is consolidated for financial reporting purposes. The Company also holds approximately 31% of the issued capital of Cronos Australia Limited (“Cronos Australia”) and accounts for its investment in Cronos Australia under the equity method of accounting. For additional discussion regarding the joint ventures and strategic investment, see Note 6.
2. Summary of Significant Accounting Policies
(a)(b)Basis of presentation
The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”). U.S. GAAP requires management to make estimates and assumptions that affect the reportedCertain prior year amounts of assets and liabilities, the disclosure of contingent liabilities at the dates of the consolidated financial statements and the reported amounts of net revenues and expenses during the reporting periods. The accounting policies adopted in the preparation of the consolidated financial statements were effective as of January 1, 2020.
(b)Segment structure
The Company undertook a realignment of its management structure along geographic regions in September 2019. As a result, effective September 2019, the Company’s results are reported through the following operating segments: United States and Rest of World. Prior period amounts contained in these consolidated financial statements have been adjustedreclassified to conform to the new segment presentation. Refer to Note 29 for additional information.current year presentation of our consolidated financial statements, which includes discontinued operations. These reclassifications had no effect on reported results of operations and ending shareholders’ equity.
(c)Basis of consolidation
The accompanying consolidated financial statements include the accounts of the Company, and all entities in which the Company has a controlling voting interest or is the primary beneficiary of a variable interest as of and for the fiscal years ended December 31, 2020, December 31, 2019 and December 31, 2018.reporting periods. The Company assesses control under the variable interest entity (“VIE”) model to determine whether the Company is the primary beneficiary of that entity’s operations. If an entity is not deemed to be a VIE, the Company consolidates the entity if the Company has a controlling voting interest. Subsidiaries are fully consolidated from the date on which control is transferred to the Company. They are deconsolidated from the date that control ceases. Investments in which the Company has the ability to exercise significant influence over the operating and financial policies of the investee, but does not have control, are accounted for under the equity method of accounting. The Company consolidates the financial results of the following entities, which the Company controls:controls but does not wholly own:
| | | | | | | | | | | | | | | | | | | | |
| Subsidiaries | | Jurisdiction of incorporation | | Incorporation date | | Ownership interest (ii) |
Cronos Israel G.S. Cultivation Ltd.(i) | | Israel | | February 4, 2018 | | 70% |
Cronos Israel G.S. Manufacturing Ltd.(i) | | Israel | | September 4, 2018 | | 90% |
Cronos Israel G.S. Store Ltd.(i) | | Israel | | June 28, 2018 | | 90% |
Cronos Israel G.S. Pharmacy Ltd.(i) | | Israel | | February 15, 2018 | | 90% |
(i)These Israeli entities are collectively referred to as “Cronos Israel.”
(ii)“Ownership interest” is defined as the proportionate share of net income to which the Company is entitled; equity interest may differ from ownership interest as described herein.
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020, 2019, and 2018
(In thousands of U.S. dollars, except for gram and share amounts)
In the consolidated statements of net income (loss)loss and comprehensive income (loss), theloss, net income (loss)loss and comprehensive income (loss)loss are attributed to the equity holders of the Company and to the non-controlling interests. Non-controlling interests in the equity of Cronos Israel are presented separately in the shareholders’ equity (deficit) section of the consolidated balance sheets and consolidated statements of shareholders’ equity (deficit).equity. All intercompany transactions and balances are eliminated upon consolidation.
(d)Use of estimates
The preparation of the consolidated financial statements in conformity with U.S. GAAP requires management to make estimates, judgments and assumptions that affect the reported amounts in the consolidated financial statements and accompanying notes. Significant estimates and assumptions include, among other things, valuation of derivative liabilities, fair valueexpected credit losses on long-term financial assets, impairment losses on goodwill and indefinite-lived intangible assets, impairment losses on long-lived assets,
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2023, 2022, and 2021
(In thousands of assets and liabilities assumed in business combinations, impairmentU.S. dollars, except for share amounts)
inventory write-downs, share-bared payments, valuation allowance on deferred income tax assets and uncertain tax liabilities. Actual results could differ from those estimates.
(e)Revenue recognitionCash and cash equivalents and short-term investments
The Company’s contractsCash and cash equivalents are comprised of cash and highly liquid short-term investments that are readily convertible into known amounts of cash, generally with customers for the saleoriginal maturities of dried cannabis, cannabis oil, cannabinoid-derived productsthree months or less. Cash and U.S. hemp-derived personal care productscash equivalents include amounts held in dollars, C$, and ILS and security deposits. Short-term investments consist of one performance obligation. Thedebt securities that (i) generally have original maturities of greater than three months and (ii) the Company has concluded that revenue from the sale of these products should be recognizedability to convert into cash within one year.
Short-term investments are classified as held-to-maturity and recorded at the pointcost. Interest earned on short-term investments is recorded in time when control is transferred to the customer, which is on shipment or delivery, dependingother receivables on the contract. Revenue is recognized at the transaction price, which is the amount of consideration to which the Company expects to be entitled in exchange for transferring promised goods to a customer.
Net revenue before excise taxes from sale of goods, as presented inconsolidated balance sheets and interest income, net on the consolidated statements of net income (loss)loss and comprehensive income (loss), represents revenue fromloss. Cash inflows and outflows related to the salepurchase and maturity of short-term investments are classified as investing activities in the Company’s consolidated statements of cash flows.
(f)Inventory
Inventory is comprised of raw materials, finished goods less expected price discounts, and allowances for customer returns. Net revenue before excise taxes excludes excise taxes, whichwork-in-progress, such as pre-harvested cannabis plants, dried flower, by-products to be extracted, cannabis extracts and by-products, dry cannabis and cannabis extract containers, and boxes. When the Company pays as principalcultivated cannabis, costs capitalized into inventory until the time of harvest included, but were not limited to, labor, utilities, nutrition and excludes dutiesirrigation.
Inventory is stated at the lower of cost and taxes collected on behalf of third parties. Excise taxes are a production tax classified as government remittances payable, which when applicable, become payable when a product is delivered to the customer and are notnet realizable value, determined using weighted average cost. Cost includes expenditures directly related to manufacturing and distribution of the products. Primary costs include consumables (insect control, fertilizers, soil), packaging, shipping, direct labor, contract manufacturer fees, overhead, supplies and small tools, and the depreciation of manufacturing equipment and production facilities determined at normal capacity. Manufacturing overhead and related expenses include salaries, wages, employee benefits, rent, utilities, security, and property taxes. Net realizable value is defined as the estimated selling price in the ordinary course of revenue.
The Company treats shippingbusiness, less reasonably predictable costs of completion, disposal and handling activities as a fulfillment cost, classified as costtransportation. At the end of sales. Accordingly,each reporting period, the Company accrues all fulfillment costs relatedperforms an assessment to the shipping and handling of consumer goodsmeasure inventory at the timelower of shipment. Within the Company's Rest of World segment, dried cannabis sales outside of Canada may include profit sharing arrangements with distributors which give rise to variable consideration. If the consideration in a contract includes a variable amount, the Company estimates the amount of consideration to which it will be entitled in exchange for transferring the goods to the customer. The variable consideration is estimated as the most likely amount, based on the Company’s historical information, at contract inception.
The Company’s payment terms vary by customercost and product type. For individual consumer sales, payment is due prior to the transfer of control.
The Company elected to treat the costs incurred to obtain a contract, primarily related to sales commissions, as an expensenet realizable value. Factors considered in the period incurred and not an asset to be capitalized, as the amortization perioddetermination of the related asset would be less than one year. Accordingly, the Company will expense the costs to obtain a contract in the period incurred.net realizable value include slow-moving or non-marketable products.
(f)(g)Investments
Variable interest entities (“VIE”)
A VIEvariable interest entity is an entity having either a total equity investment that is insufficient to finance its activities without additional subordinated financial support or equity investors at risk that lack the ability to control the entity’s activities. Variable interests are investments or other interests that will absorb portions of a VIE’s expected losses or receive portions of the VIE’s expected residual returns. The Company evaluates whether it is the primary beneficiary of each VIE it identifies on an ongoinga periodic basis and considers the impact of any reconsideration events. The primary beneficiary is the party that has both the power to direct the activities that most significantly impact the VIE and holds a variable interest that could potentially be significant to the VIE. To make this determination, the Company considers both quantitative and qualitative factors regarding the nature, size and form of its involvement with the VIE. The Company consolidates thea VIE when it is determined that it is the primary beneficiary of the VIE.
Equity method investments
The Company accounts for investments in companies over which it has the ability to exercise significant influence but does not hold a controlling financial interest using the equity method. Under the equity method, the Company records its proportionate share of income or lossesloss in share of income (loss) from equity method investments within the consolidated statements of net income (loss)loss and comprehensive loss. Cash payments to equity method investees such as additional investments or expenses incurred on behalf of investees, as well as income (loss).earned and payments from equity method investees such as dividends and distributions are recorded as adjustments to investment balances. If the current fair value of an investment falls below its carrying amount, this may indicate that an impairment loss should be recorded. Any impairment losses recognized in one period cannot be reversed in subsequent periods.
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020, 2019,2023, 2022, and 20182021
(In thousands of U.S. dollars, except for gram and share amounts)
Other investments include common stock and options in third-party entities in which the Company’s influence is deemed non-significant. The Company holds other investments with and without readily determinable fair values. Other investments with readily determinable fair values are recorded using the fair value method of accounting as of period-end on the consolidated balance sheets. Other investments without readily determinable fair values are recorded using the cost method of accounting on the consolidated balance sheets. Other investments without readily determinable fair values are assessed for observable price changes and other than temporary impairment on a periodic basis. Changes in the reported value of other investments are reported in the consolidated statements of net loss and comprehensive loss.
(g)(h)InventoryProperty, plant and equipment
Inventory is comprised of raw materials, finished goodsProperty, plant and work-in-progress such as pre-harvested cannabis plants, by-products to be extracted, oils, gel caps, tinctures, and boxes. The costs of growing cannabis, including but not limited to labor, utilities, nutrition and irrigation,equipment are capitalized into inventory until the time of harvest.
Inventory is stated at cost less accumulated depreciation and accumulated impairment losses. Depreciation is computed using the lowerstraight-line method over the estimated useful lives of the assets as follows:
| | | | | | | | |
| | | Rate |
| Building and leasehold improvements | | | | 15 to 20 years |
| Machinery and equipment | | | | 5 to 7 years |
| Furniture and fixtures | | | | 5 years |
When assets are disposed of, the cost and net realizable value, determined using weighted average cost. Cost includesaccumulated depreciation are removed from the respective accounts and any related gain or loss is recognized. Maintenance and repairs are charged to expense as incurred. Significant expenditures, directly related to manufacturing and distributionwhich increase productivity or extend the useful life of the products. Primary costs include consumables (insect control, fertilizers, soil), packaging, shipping, direct labor, overhead, supplies and small tools, and the depreciation of manufacturing equipment and production facilities determined at normal capacity. Manufacturing overhead and related expenses include salaries, wages, employee benefits, rent, utilities, security, and property taxes.asset, are capitalized.
Net realizable valueAvailable for use is defined as the estimated selling price inpoint at which the ordinary courserelated property, plant and equipment is operational, including the possession of business, less reasonably predictable costs of completion, disposal and transportation. At the end of each reporting period, the Company performs an assessment of inventory obsolescence to measure inventoryany requisite licenses. Depreciation commences at the lower of cost and net realizable value. Factors considered inpoint the determination of obsolescence include slow-moving or non-marketable products. During the year ended December 31, 2020, we incurred significant inventory write-downs driven by pricing pressures in the marketplace and will continue assessing pricing pressures on the value of inventory at the end of each reporting period. See Note 5.assets are available for use.
(h)(i)Definite lifeDefinite-lived intangible assets
Intangible assets are recorded at cost less any accumulated amortization and accumulated impairment losses. Intangible assets acquired through a business combination are measured at fair value at the acquisition date.
The Company capitalizes certain costs incurred in connection with its enterprise software, which include external direct costs of materials and services consumed in developing or obtaining internal-use software and payroll and payroll-related costs for employees who are directly associated with and who devote time to the development of the software for the function intended. All other costs are expensed as incurred.
Intangible assets with finitedefinite useful lives are amortized over their estimated useful lives using the following methods and rates:
| | | | | | | | | | | |
| Method | | Rate |
| Software | Straight-line | | 5 years |
Enterprise resource planning (“ERP”) software | Straight-line | | 5 years |
| Health Canada licenses | Straight-line | | Useful life of corresponding facilities |
| Ginkgo exclusive licenses | Straight-line | | 10 years |
Israeli codes(i) | Straight-line | | Useful life of corresponding facilities |
(i) The preliminary licenses granted to Kibbutz Gan Shmuel (the Cronos Israel joint venture partner) by the Medical Cannabis Unit of the Israeli Ministry of Health in early 2017 (the “Israeli codes”) were transferred by non-controlling interests to Cronos Israel in exchange for equity interests in the Cronos Israel entities specified above.
Amortization begins when assets become available for use. The estimated useful life, amortization method, and rate are reviewed at the end of each reporting period, with the effect of any changes in estimate being accounted for on a prospective basis.
(i)Property, plant & equipment
Property, plantIntangible assets originating from the strategic partnership (the “Ginkgo Strategic Partnership”) with Ginkgo Bioworks Holdings, Inc. (“Ginkgo”) are accounted for in accordance with the acquisition method of accounting. Equity interests issued in exchange for an asset are initially recognized and equipment are statedmeasured at cost less accumulated depreciation and accumulated impairment losses. Depreciation is computedthe date of acquisition at fair value. We estimate fair value using the straight-linerelief-from-royalty method overand key assumptions include the discount rate and estimated useful lives oflife. Definite-lived intangible assets, including intangible assets originating from the assets as follows:
| | | | | | | | |
| | | Rate |
Building | | | | 15 to 25 years |
Furniture and equipment | | | | 5 to 7 years |
Computer equipment | | | | 5 years |
Leasehold improvements | | | | Lesser of term of lease and useful life |
Equipment under finance lease | | | | Lesser of term of lease and useful life |
Ginkgo Strategic Partnership, are subject to amortization and reviewed for impairment annually or more frequently when events or changes in circumstances indicate that fair value has been reduced to less than its carrying amount.
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020, 2019,2023, 2022, and 20182021
(In thousands of U.S. dollars, except for gram and share amounts)
When assets are disposedAccrued liabilities consist of the costfollowing:
| | | | | | | | | | | |
| As of December 31, |
| 2023 | | 2022 |
| Accrued payroll and related expenses | $ | 8,970 | | | $ | 11,492 | |
| Accrued professional fees | 2,525 | | | 2,414 | |
| Accrued taxes | 11,695 | | | 4,132 | |
| Other accrued expenses | 4,546 | | | 4,230 | |
| Total accrued liabilities | $ | 27,736 | | | $ | 22,268 | |
Accrued payroll and accumulated depreciation are removed fromrelated expenses include salaries and wages, bonuses, and other related payroll expenses associated with the respective accountsCompany’s employees. Accrued professional fees include fees for legal expenses, litigation, consulting, marketing, and anyother related gain or loss is recognized. Maintenanceexpenses. Accrued taxes include sales, excise and repairs are chargedother taxes owed. Other accrued expenses include the fair value of deferred share units outstanding to expense as incurred. Significant expenditures, which increase productivity or extend the useful life of the asset, are capitalized.
Interest incurred relating to construction or expansion of buildings is capitalized to construction in progress. The Company ceases the capitalization of interest when construction activities are substantially completeddirectors and the facility is available for use. At this point, construction in progress is transferred to the appropriate asset class. Available for use is defined as the point at which the related building receives the requisite regulatory licenses to (i) possess cannabis, (ii) obtain dried cannabis, fresh cannabis, cannabis plants or cannabis plant seeds by cultivating, propagating and harvesting cannabis, and (iii) produce cannabis, other than obtaining it by cultivating, propagating, or harvesting. Depreciation commences at the point the assets are available for use.general expenses.
(j)(k)Leases
The Company enters into leases in the normal course of business, primarily for the land-use rights, office premises, and equipment used in the production of its products. At the inception of a contract, the Company assesses whether a contract is, or contains, a lease. A contract is, or contains, a lease if the contract conveys the right to control the use of an identified asset for a period of time in exchange for consideration. The Company performs an analysis over the classification of the lease agreement as either an operating lease or finance lease.
A right-of-use asset and the related lease obligation associated with the lease are recorded at the inception of the lease. The right-of-use asset’s recorded amount is based on the present value of future lease payments over the lease term at the commencement date plus any initial direct costs incurred. If the rate implicit in the lease is not readily determinable for the Company’s operating leases, an incremental borrowing rate is generally used based on information available at the lease commencement date to determine the present value of future lease payments. Subsequent changes to these lease payments due to rate updates are recorded as lease expense in the period incurred. Leases with a term of 12 months or less are not recorded on the balance sheet as a lease.
The right-of-use asset is subject to impairment testing whenever events or changes in circumstances indicate the carrying valueamount of the asset may not be recoverable. The leased asset is amortized over the shorter of the lease term or its estimated useful life if title does not transfer to the Company, while the leased asset is depreciated in accordance with the Company’s depreciation policy if the title is to eventually transfer to the Company.
The Company’s lease agreements generally exclude non-lease components. As a result, non-lease components are accounted for separately for all classes of assets and expensed as incurred. In addition, the Company’s lease agreements do not contain any material residual value guarantees or material restrictive covenants. For finance leases, from the commencement date to the earlier of the end of the useful life of the right-of-use asset or the end of the lease term, the right-of-use asset is amortized on a straight-line basis and the interest expense is recognized on the lease liability using the effective interest method. For operating leases, lease expense is recognized on a straight-line basis over the term of the lease and presented as a single charge in the consolidated statements of net income (loss)loss and comprehensive income (loss)loss.
(l)Derivative liabilities
For financial instruments classified as derivatives that are not designated as hedging instruments or do not qualify for hedge accounting, changes in fair value are recorded in the consolidated statements of net loss and comprehensive loss each period. The Company does not enter into or hold derivative financial instruments for trading or speculative purposes. Derivative liabilities are initially recognized at fair value at the date on which the derivative contract was entered into. Any attributable transaction costs are recognized in net loss as incurred. Subsequent to initial recognition, derivative liabilities are measured at fair value at each reporting date until settlement with the re-measurement gain or loss being recognized immediately in net loss and comprehensive loss. For more details on derivative liabilities consisting of the Altria Warrant, Pre-emptive Rights, and certain Top-up Rights, see Note 9 “Derivative Liabilities.”
(m)Capital stock
Capital stock is presented at the fair value at the time of issuance of the shares issued. Costs related to the issuance of shares are reported in equity, net of tax, as a deduction from the issuance proceeds.
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2023, 2022, and 2021
(In thousands of U.S. dollars, except for share amounts)
(n)Revenue recognition
Revenue is measured based on the consideration specified in a contract with a customer. The Company recognizes revenue when it satisfies a performance obligation by transferring control over a product or service to a customer. The Company’s contracts with customers for the sale of dried flower, cannabis oil, cannabinoid-derived products and “hemp” (as defined in the U.S. Agricultural Improvement Act of 2018 “U.S. hemp”) derived products consist of a single performance obligation.
The Company has concluded that revenue from the sale of these products should be recognized at the point in time when control is transferred to the customer, depending on the specific contractual terms. The Company has determined that the most definitive demonstration that control has transferred to a customer is physical shipment or delivery, depending on the contractual shipping terms, except for consignment transactions. Consignment transactions are arrangements where the Company transfers product to a customer or third-party location but retains ownership and control of such product until it is used by the customer. Revenue for consignment arrangements is recognized upon the customer’s usage.
Revenue is recognized at the transaction price, which is the amount of consideration to which the Company expects to be entitled in exchange for transferring promised goods to a customer. Net revenue before excise taxes from sale of goods, as presented in the consolidated statements of net loss and comprehensive loss, represents revenue from the sale of goods less expected price discounts, allowances for customer returns and other forms of variable consideration. If the consideration in a contract includes a variable amount, the Company estimates the amount of consideration to which it will be entitled in exchange for transferring the goods to the customer. The variable consideration is estimated using either the expected value or most likely amount method, based on the Company’s historical information, at contract inception. The Company’s payment terms vary by customer and product type.
The Company treats shipping and handling activities as a fulfillment cost, classified as cost of sales. Accordingly, the Company accrues all fulfillment costs related to the shipping and handling of consumer goods at the time of shipment.
The following table presents the Company's revenue by major product category for continuing operations:
| | | | | | | | | | | | | | | | | |
| Year ended December 31, |
| 2023 | | 2022 | | 2021 |
| Cannabis flower | $ | 62,070 | | | $ | 63,593 | | | $ | 55,194 | |
| Cannabis extracts | 24,569 | | | 22,522 | | | 8,807 | |
| Other | 602 | | | 634 | | | 560 | |
| Net revenue | $ | 87,241 | | | $ | 86,749 | | | $ | 64,561 | |
Net revenue attributed to a geographic region based on the location of the customer was as follows:
| | | | | | | | | | | | | | | | | |
| Year ended December 31, |
| 2023 | | 2022 | | 2021 |
| Canada | $ | 64,702 | | | $ | 56,233 | | | $ | 50,294 | |
| Israel | 21,134 | | | 30,516 | | | 13,376 | |
| | | | | |
| Other countries | 1,405 | | | — | | | 891 | |
| Net revenue | $ | 87,241 | | | $ | 86,749 | | | $ | 64,561 | |
(o)Research and development
The Company has a research and development center in Canada that performs scientific research on the interaction of cannabinoids as well as strain development, growing conditions and extraction technology. In 2023, fermentation and production related research was performed to further strategic initiatives around rare cannabinoids. In addition, the Company has a collaboration and license agreement with Ginkgo (the “Ginkgo Collaboration Agreement”) to research, produce, and commercialize cultured cannabinoids. Technological feasibility is considered to be established once productivity targets or commercialization are achieved, at which point the exclusive license is recognized at cost less impairment charges. As of the acquisition date of each exclusive license, cost less impairment charges is equal to the fair value. Refer to Note 7“Goodwill and Intangible Assets, net”for more information on the Ginkgo Collaboration Arrangement. Research and development costs associated with these collective efforts are expensed as incurred as part of operating expenses in the Company’s consolidated statements of net loss and comprehensive loss.
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2023, 2022, and 2021
(In thousands of U.S. dollars, except for share amounts)
(p)Advertising costs
Advertising costs include costs to sell the Company’s products and are expensed as incurred through sales and marketing expenses in the consolidated statements of net loss and comprehensive loss. Advertising costs were $1,382, $889 and $2,229 for the years ended December 31, 2023, 2022, and 2021, respectively.
(q)Share-based compensation
The Company has five share-based compensation plans under which awards have been made: the 2020 Omnibus Plan, the 2018 Stock Option Plan, the 2015 Stock Option Plan, the Employment Inducement Award Plan and the DSU Plan (each as defined below).
Share-based awards consists of equity-settled share-based awards such as stock options and restricted share units (“RSUs”) that are issued to eligible employees, non-executive directors, and non-employees. Cash-settled deferred share units (“DSUs”) that are issued to non-executive directors under the DSU Plan are recorded in accrued liabilities with the fair value adjustment recorded in other income.
Equity instruments granted are initially measured at fair value on the grant date. The fair value of stock options is determined using the Black-Scholes option pricing model. The fair value of RSUs and DSUs are determined using the market price of the Company’s common shares. Compensation expense related to options and RSUs is recognized on a straight-line basis in the consolidated statements of net loss and comprehensive loss over the vesting period for employees, and over the contractual term for non-employees. The fair value of the payout of cash-settled DSUs is determined at each reporting date based on the fair value of the Company’s common shares at the reporting date and is recorded within other liabilities. The related costs for all equity-settled share-based awards are reflected in additional paid-in capital until the awards are settled or exercised. Upon settlement or exercise, shares are issued and the amount previously reflected in additional paid-in capital is, along with any proceeds paid upon settlement or exercise, credited to a combination of share capital and additional paid-in capital. Forfeitures of share-based compensation awards are accounted for as reductions to share-based compensation and additional paid-in capital as they occur.
(k)(r)Impairment of long-lived assets
The Company reviews its long-lived assets, such as property, plant and equipment and definite-lived intangible assets, for impairment wheneverin accordance with Accounting Standards Codification (“ASC”) Topic 360, Property, Plant, and Equipment. In accordance with ASC Topic 360, long-lived assets to be held are reviewed for events or changes in circumstances that indicate that thetheir carrying value of an assetamount may not be recoverable. The Company periodically reviews for indicators and, if indicators are present, tests the carrying amount of long-lived assets, assessing their recoverable value based on estimated undiscounted cash flows over their remaining estimated useful lives. The Company groups assets at the lowest level for which cash flows are separately identifiable, referred to as an asset group. The Company prepares projected undiscounted cash flow analyses for each asset or asset group. If the sumcarrying amount of an asset (or asset group) exceeds its estimated undiscounted future cash flows, an impairment charge is measured as the amount by which the carrying amount of the undiscounted cash flow is less thanasset exceeds the carryingfair value of the asset group, based on discounted cash flows.
(s)Assets held for sale and discontinued operations
In accordance with ASC 205-20 Presentation of Financial Statements: Discontinued Operations, a disposal of a component of an entity or a group of components of an entity is required to be reported as discontinued operations if the disposal represents a strategic shift that has (or will have) a major effect on an entity’s operations and financial results when the components of an entity meet the criteria in paragraph ASC 205-20-45-10. In the period in which the component meets held-for-sale or discontinued operations criteria, the major current assets, other assets, current liabilities, and other liabilities are reported as components of total assets and liabilities separate from those balances of the continuing operations. At the same time, the results of all discontinued operations, less applicable income taxes (benefit), are reported as components of net loss separate from the net loss of continuing operations.
The Company periodically evaluates its long-lived assets that it plans to dispose of through sale for held-for-sale classification. To be classified as held-for-sale, management must have committed to a plan to sell, the asset group,(or asset group) must be available for immediate sale in its present condition, an impairment loss is recognized equalactive program to locate a buyer must have been initiated, the sale must be probable to close within one year, the asset (or asset group) must be marketed at a reasonable sales price, and it must be unlikely that significant changes to the excessplan will be made. Once an asset (or asset group) meets all of the carrying value over the fair value, if any. Impairment losses onabove criteria, it is reclassified as assets held for sale are based on the consolidated balance sheet, and the asset(s) cease depreciation and are written down to their fair value, less costs to sell.sell, if applicable.
The Company completed a review of its global supply chain and determined that it would wind down its Winnipeg, Manitoba facility (“Cronos Fermentation”) and list it for sale. This review involves significant complexities and judgments in making the accounting treatment determination. There are subjective and complex judgments in the determination of whether the Cronos Fermentation facility meets the criteria to be classified as held for sale, including: (1) whether the Cronos Fermentation facility is available for sale in its present condition subject only to terms that are usual and customary for sales of such businesses, (2) whether the sale of the
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2023, 2022, and 2021
(In thousands of U.S. dollars, except for share amounts)
Cronos Fermentation facility is probable and that the transfer of assets will be a completed sale within one year from period end, and (3) whether the Cronos Fermentation facility is being actively marketed at a reasonable price. See Note 6 “Property, plant and equipment, net” for discussion regarding our evaluation of the Peace Naturals Campus and the Cronos Fermentation facility for held-for-sale classification as of December 31, 2023.
(t)Impairment of goodwill and indefinite-lived intangible assets
Goodwill and indefinite lifeindefinite-lived intangible assets are not amortized. Goodwill and indefinite lifeindefinite-lived intangible assets are reviewed for impairment annually or more frequently when events or changes in circumstances indicate that fair value of the reporting unit has been reduced to less than its carrying value.amount in accordance with the provisions of ASC Topic 350, Intangibles—Goodwill and Other. The Company performs an impairment test annually in the fourth quarter by comparing the fair value of the reporting unit with its carrying amount, including goodwill. If the fair value of the reporting unit exceeds its carrying amount, goodwill is not considered to be impaired. An impairment charge would be recognized for the amount by which the carrying amount exceeds the reporting unit’s fair value. The Company determined that it has 2 reporting units: the U.S. reporting unit and the Rest of World reporting unit.
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020, 2019, and 2018
(In thousands of U.S. dollars, except for gram and share amounts)
(l)Repurposing charges
The Company’s repurposing charges primarily include employee termination benefits and refurbishment costs. The recognition of repurposing charges requires the Company make certain judgments and estimates regarding the nature, timing, and amount of costs associated with the planned activity. To the extent actual results differ from estimates and assumptions, a revision to the estimated liabilities, requiring the recognition of additional repurposing charges or the reduction of liabilities already recognized may be required. At the end of each reporting period, the Company evaluates the remaining accrued balances to ensure these balances are properly stated and the utilization of the reserves are for their intended purpose in accordance with planned activity.
(m)Capital stock
Capital stock is presented at the fair value at the time of issuance of the shares issued. Costs related to the issuance of shares are reported in equity, net of tax, as a deduction from the issuance proceeds.
(n)Foreign currency
The Company’s functional currency is the Canadian dollar (“C$”). Prior to the year ended December 31, 2019, the Company reported its financial results in C$, however following the change in the Company’s status from foreign private issuer to a United States (“U.S.”) domestic issuer, the financial results are now reported in U.S dollars (“dollars” or “$”). Functional currencies for the entities in these consolidated financial statements are their respective local currencies, including C$, Australian dollars (“A$”) and Israeli New Shekel (“ILS”). The assets and liabilities of the Company’s foreign operations are translated into dollars at the exchange rate in effect as of December 31, 2020 and 2019. Transactions affecting the shareholders’ equity (deficit) are translated at historical foreign exchange rates. The consolidated statements of net income (loss) and comprehensive income (loss) and the consolidated statements of cash flows of the Company’s foreign operations are translated into dollars by applying the average foreign exchange rate in effect for the reporting period. The cumulative translation adjustments (“CTA”) resulting from translating the consolidated financial statements into their dollar reporting currency are recorded in other comprehensive income (loss).
Monetary assets and liabilities denominated in foreign currencies are translated to the functional currency by applying the foreign exchange rate in effect at the balance sheet date. Revenues and expenses are translated using the average foreign exchange rate for the reporting period. Realized and unrealized foreign currency differences are recognized in the consolidated statements of net income (loss) and comprehensive income (loss).
Transactions in foreign currencies are translated into the functional currency using the exchange rate prevailing at the date of the transaction and are recorded in other comprehensive income. Exchange differences arising from operating transactions are recorded in net income for the period.
(o)(u)Income taxes
The Company uses the liability method of accounting for income taxes, under which deferred tax assets and liabilities are recognized for the future tax consequences attributable to the differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax basis. Deferred tax assets and liabilities are measured using enacted tax rates expected to be in effect when such assets and liabilities are recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the year that includes the enactment date. The Company determines deferred tax assets including net operating losses and liabilities, based on temporary differences between the book and tax bases of assets and liabilities.
A valuation allowance is established to reduce some or all net deferred tax assets to amounts that are more likely than not to be realized. The Company considers all available evidence, both positive and negative, including past operating results, estimates of future taxable income, and the feasibility of tax planning strategies, in assessing the need for a valuation allowance.
The Company has a fullA valuation allowance against its net deferred tax assets, and has concluded, based on the weightsome or all of all available evidence, that it is more likely than not that the net deferred tax assets will not be realized, primarily due to the historical net operating losses. The valuation allowance against the net deferred tax assets does not in any way impact the Company’s ability to use future tax deductions such as the Company’s net operating loss carryforwards; rather, the valuation allowance indicates, according to the provisions of Accounting Standards CodificationASC 740, Income Taxes, it is more likely than not that the deferred tax assets will not be realized. The valuation allowance that was established will be maintained until there is sufficient positive evidence to conclude that it is more likely than not that the net deferred tax assets will be realized. The Company’s income tax expense for future periods will be reduced to the extent of corresponding decreases in our valuation allowance. There is uncertainty regarding any future realization of the benefit by the Company of all or part of our net deferred tax assets.
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020, 2019, and 2018
(In thousands of U.S. dollars, except for gram and share amounts)
Judgment is required to determine the recognition and measurement attributes prescribed in the accounting guidance for uncertainty in income taxes. The Company uses a two-step approach for evaluating uncertain tax positions. Step one, recognition, requires us to determine ifwhether the weight of available evidence indicates that a tax position is more likely than not to be sustained upon audit, including resolution of related appeals or litigation processes, if any. If a tax position is not considered “more likely than not” to be sustained, no benefits of the position are recognized. If we determine that a position is “more likely than not” to be sustained, then we proceed to step two, measurement, which is based on the largest amount of benefit which is more likely than not to be realized on effective settlement. This process involves estimating our actual current tax exposure, including assessing the risks associated with income tax audits, together with assessing temporary differences resulting from the different treatment of items for tax and financial reporting purposes. If actual results differ from our estimates, our net operating loss and credit carryforwards, to the extent not covered by a valuation allowance, could be materially impacted in the period which such determination is made.
The Company recognizes uncertain income tax positions at the largest amount that is more-likely-than-not to be sustained upon examination by the relevant taxing authority. An uncertain income tax position will not be recognized if it has less than a 50% likelihood of being sustained. Recognition or measurement is reflected in the period in which the likelihood changes. Any interest and penalties related to unrecognized tax liabilities are presented within income tax expense in the consolidated statements of net income (loss)loss and comprehensive income (loss).loss. Accrued interest and penalties are included in accounts payable and other liabilities in the consolidated balance sheets.
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2023, 2022, and 2021
(In thousands of U.S. dollars, except for share amounts)
(v)Share-based compensationForeign currency
As described in more detail below,The Company’s functional currency is the Company has five share-based compensation plans under which awards have been made: the 2020 Omnibus Plan (as defined below), the 2018 Stock Option Plan (as defined below), the 2015 Stock Option Plan (as defined below), the Employment Inducement Award Plan #1 (the “Employment Inducement Award Plan”Canadian dollar (“C$”) and its reporting currency is the U.S. dollar. Functional currencies for the entities in these consolidated financial statements are their respective local currencies, including C$, U.S. dollar and Israeli New Shekel (“ILS”). All assets and liabilities of operations with a cash-settled deferred share unit (“DSU”) plan for non-executive directors.
Share-based compensation consists of equity-settled share-based awards such as stock optionsfunctional currency other than the Canadian dollar are translated into Canadian dollars at the period-end currency exchange rates and restricted share units (“RSUs”) that are issued to eligible employees, non-executive directors, and non-employees.
Cash-settled deferred share units (“DSUs”) that are issued to non-executive directorssubsequently translated into U.S. dollars at period-end currency exchange rates. The resulting translation adjustments are recorded in accounts payableaccumulated other comprehensive income (loss), net of tax. Revenues and expenses of operations, as well as all cash flows, with a functional currency other liabilities withthan the fair value adjustmentCanadian dollar are translated into Canadian dollars at the average exchange rates for the period and subsequently translated into U.S. dollars at the average exchange rates for the period. Transaction gains and losses resulting from changes in foreign currency exchange rates are recorded in other incomeeither cost of sales, general and administrative expenses, or foreign currency transaction gain (loss).
Equity instruments granted are initially measured at fair value on the grant date. The fair value of the stock options are determined using the Black-Scholes option pricing model. The fair value of RSUs and DSUs are determined using the market price. This is recognized on a straight-line basis in the consolidated statements of net income (loss)loss and comprehensive income (loss) over the vesting period for employees, and over the contractual term for non-employees. The fair value of the payout of cash-settled DSUsloss.
(w)Segments
Segment reporting is determined at each reporting date basedprepared on the fair valuesame basis that the Company’s chief operating decision maker (the “CODM”) manages the business, makes operating decisions and assesses the Company’s performance. Historically, the Company has reported results for two reportable segments, the U.S. and Rest of World. In the second quarter of 2023, as a result of the Company’s common shares at the reporting date and is recorded within other liabilities.
The related costs for all equity-settled share-based awards are reflected in additional paid-in capital until the awards are exercised. Upon exercise, shares are issued and the amount previously reflected in the additional paid-in capital is, along with any proceeds paid upon exercise, credited to share capital. Forfeitures are estimated at the timeexit of grant, andits then-existing U.S. operations, the Company revises these estimatesdetermined that it has one operating segment and therefore one reportable segment. All prior period segment disclosure information has been reclassified to conform to the current reporting structure in subsequent periods if there is a differencethis Annual Report. These reclassifications had no effect on our consolidated financial statements in actual forfeitures and the estimates.any period presented.
(q)(x)Net earningsEarnings (loss) per share
The Company presents basic and diluted net earnings (loss) per share data for its common shares. Basic net earnings (loss) per share is calculated by dividing the profit or loss attributable to common shareholders of the Company by the weighted average number of common shares outstanding during the period. Diluted net earnings (loss) per share is determined by adjusting the profit or loss attributable to common shareholders and the weighted average number of common shares outstanding for the effects of all potentially dilutive common shares that are issuable upon exercise of warrants, stock options and the Altria Warrant (as defined below) as well as the vesting of RSUs outstanding.shares.
(r)Cash and cash equivalents and short-term investments
Cash and cash equivalents are comprised of cash and highly liquid investments that are readily convertible into known amounts of cash with original maturities of three months or less. Cash and cash equivalents include amounts held in dollars, C$ and ILS and security deposits.
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020, 2019, and 2018
(In thousands of U.S. dollars, except for gram and share amounts)
Short-term investments consist of debt securities that (i) have original maturities of greater than three months and (ii) the Company has the ability to convert into cash within one year. Short-term investments are classified as held-to-maturity. Our investments classified as held-to-maturity are recorded at cost. Interest earned on short-term investments is recorded in other receivables on the consolidated balance sheet and interest income on the consolidated statement of comprehensive income (loss). Cash inflows and outflows related to the purchase and maturity of short-term investments are classified as investing activities in the Company’s consolidated statements of cash flows.
(s)Derivative liabilities
For financial instruments that are not designated as hedging instruments or do not qualify for hedge accounting, changes in fair value are recorded in the consolidated statements of net income (loss) and comprehensive income (loss) each period. The Company does not enter into or hold derivative financial instruments for trading or speculative purposes. Derivative liabilities are initially recognized at fair value at the date on which the derivative contract was entered into. Any attributable transaction costs are recognized in net income (loss) as incurred. Subsequent to initial recognition, derivative liabilities are measured at fair value at each reporting date until settlement with the re-measurement gain or loss being recognized immediately in comprehensive income.
For more details on derivative liabilities consisting of the Altria Warrant, Pre-emptive Rights, and certain Top-up Rights, see Note 14.
(t)(y)Fair value measurements
The carrying valueamount of the Company’s cash and cash equivalents, accounts receivable, other receivables, loanloans receivable, account payablesaccounts payable and other liabilities and holdbacks payable approximate fair value, given their short-term nature.
Cronos Group uses a fair value hierarchy, which gives the highest priority to unadjusted quoted prices in active markets for identical assets and liabilities, noted as Level 1 measurements, and the lowest priority to unobservable inputs, noted as Level 3 measurements.
The following are the three levels of inputs used to measure fair value:
•Level 1 – valuation based on quoted prices (unadjusted) in active markets for identical assets and liabilities.
•Level 2 – valuation techniques based on inputs other than quoted prices included in Level 1 that are observable for the asset or liability, either directly or indirectly.
•Level 3 – valuation techniques using the inputs for the asset or liability that are not based on observable market data.
The Company’s policy for determining when transfers between levels of the fair value hierarchy occur is based on the date of the event or changes in circumstances that caused the transfer.
(u)(z)AcquisitionsAdoption of new accounting pronouncements
On January 1, 2023, the Company adopted ASU 2022-02, Financial Instruments – Credit Losses (Topic 326): Troubled Debt Restructurings and Vintage Disclosures (“ASU 2022-02”). ASU 2022-02 eliminates the existing troubled debt restructuring recognition and measurement guidance, and instead aligns the accounting treatment to that of other loan modifications. The accounting basis for each acquisition is dependent on whether the integrated setamendments enhance existing disclosure requirements and introduce new requirements related to certain modifications of assets and activities acquired constitutes a business. A business consists of inputs and processes appliedreceivables made to those inputs that have the ability to contribute to the creation of outputs. The cost of acquisition is allocated to the assets acquired on a relative fair value basis. No goodwill is recognized in an asset acquisition. If it is determined that a business is not acquired, the transaction is accounted for as an asset acquisition and the relevant values are finalized prior to the next reporting period. On the other hand, when a business is acquired, the acquisition method of accounting in accordance with Accounting Standards Codification (“ASC”) 805 is applied, whichborrowers experiencing financial difficulty. ASU 2022-02 also requires that once controlentities disclose current-period gross write-offs by year of the business is obtained, the assetsorigination for financing receivables and liabilitiesnet investments in leases. The adoption of the acquired business, including amounts attributable to non-controlling interests, be recorded at their respective fair values as of the date of acquisition. Any excess of purchase consideration over the net fair value of tangible and identified intangible assets acquired less liabilities assumed is recorded as goodwill. The costs of business acquisitions, including fees for accounting, legal, professional consulting and valuation specialists, are expensed as incurred. Purchase price allocations may be preliminary and, during the measurement periodASU 2022-02 did not to exceed one year from the date of acquisition, changes in assumptions and estimates that result in adjustments to the fair value of assets acquired and liabilities assumed are recorded in the period the adjustments are determined.
For business combinations achieved in stages,have a material impact on the Company’s previously held interest in the acquiree is remeasured at its subsequent acquisition date fair value, with the resulting gain or loss recorded in the consolidated statements of net income (loss) and comprehensive income (loss). For a pre-existing relationship between the Company and acquiree that is not extinguished on the business combination, such a relationship is considered effectively settled as part of the business combination even if it is not legally cancelled. At the acquisition date, it becomes an intercompany relationship and is eliminated upon consolidation.financial statements.
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020, 2019,2023, 2022, and 20182021
(In thousands of U.S. dollars, except for gram and share amounts)
Contingent consideration in a business combination is initially measured at fair value. Subsequently all liability classified as contingent consideration is remeasured at fair value at each reporting period until the contingency is resolved and any change in the fair value following the date of acquisition is recorded in the consolidated statements of net income (loss) and comprehensive income (loss). All equity classified as contingent consideration is not remeasured and its subsequent settlement is accounted for within equity.
(v)Assets held for sale and discontinued operations
In accordance with ASC 205-20 Presentation of Financial Statements: Discontinued Operations, a disposal of a component of an entity or a group of components of an entity is required to be reported as discontinued operations if the disposal represents a strategic shift that has (or will have) a major effect on an entity’s operations and financial results when the components of an entity meet the criteria in paragraph 205-20-45-10. In the period in which the component meets held-for-sale or discontinued operations criteria the major current assets, other assets, current liabilities, and noncurrent liabilities shall be reported as components of total assets and liabilities separate from those balances of the continuing operations. At the same time, the results of all discontinued operations, less applicable income taxes (benefit), shall be reported as components of net income (loss) separate from the net income (loss) of continuing operations.
During the year ended December 31, 2020, OGBC, formerly included within the Rest of World segment, met the criteria for “held-for-sale”. As a result, the Company has reflected amounts relating to OGBC as a disposal group classified as held-for-sale on the consolidated balance sheet and included as part of discontinued operations on the consolidated statement of net income (loss) and comprehensive income (loss) for all periods presented in this Annual Report. OGBC is no longer included in the segment reporting following the reclassification to discontinued operations. The discontinued operations of OGBC are described further in Note 30.
3. New Accounting Pronouncements
(a)Adoption of new accounting pronouncements
On January 1, 2020, the Company adopted ASU No. 2018-13, Disclosure Framework – Changes to the Disclosure Requirements for Fair Value Measurement (Topic 820) (“ASU No. 2018-13”). ASU No. 2018-13 adds, modifies, and removes certain fair value measurement disclosure requirements. The adoption of ASU No. 2018-13 did not have a material impact on the Company’s consolidated financial statements.
On January 1, 2020, the Company adopted ASU No. 2018-15, Intangibles – Goodwill and Other Internal-use-software (Subtopic 350-40): Customer’s Accounting for Implementation Costs Incurred in a Cloud Computing Arrangement That Is a Service Contract (“ASU No. 2018-15”). ASU No. 2018-15 amends current guidance to align the accounting for costs incurred in a hosting arrangement that is a service contract with the requirements for capitalizing costs associated with developing or obtaining internal-use software. The guidance in ASU No. 2018-15 is effective for annual and interim periods beginning after December 15, 2019, with early adoption permitted. The adoption of ASU No. 2018-15 did not have a material impact on the Company’s consolidated financial statements.
On January 1, 2020, the Company adopted ASU No. 2017-04, Intangibles – Goodwill and Other (Topic 350) – Simplifying the Test for Goodwill Impairment (“ASU No. 2017-04”). ASU No. 2017-04 eliminates step 2 from the goodwill impairment test and instead requires an entity to measure the impairment of goodwill assigned to a reporting unit if the carrying value of assets and liabilities assigned to the reporting unit, including goodwill, exceeds the reporting unit’s fair value. The guidance in ASU No. 2017-04 is effective for annual and interim goodwill tests completed by the Company beginning on January 1, 2020. The adoption of ASU No. 2017-04 was applied prospectively and the Company follows a one-step model for goodwill impairment.
(b)(aa)New accounting pronouncements not yet adopted
In December 2019,June 2022, the FASBFinancial Accounting Standards Board (“FASB”) issued ASU 2019-12, Income Taxes2022-03, Fair Value Measurement (Topic 740)820): Simplifying the Accounting for Income TaxesFair Value Measurement of Equity Securities Subject to Contractual Sale Restrictions (“ASU No. 2019-12”2022-03”). ASU No. 2019-12 eliminates certain exceptions and simplifies2022-03 clarifies that a contractual restriction on the applicationsale of U.S. GAAP-related changesan equity security is not considered in enacted tax laws or rates and employee stock option plans.measuring fair value. The amendments also require additional disclosures for equity securities subject to contractual sale restrictions. ASU No. 2019-122022-03 is effective for annual and interim periodsfiscal years beginning after December 15, 2020. Early adoption is permitted.2023, and interim periods within those fiscal years, and we expect to adopt ASU 2022-03 prospectively. The Company has evaluateddoes not expect the impactadoption of ASU No. 2019-12 and the adoption will not2022-03 to have a material impact on the Company’sits consolidated financial statements.
In November 2023, the FASB issued ASU 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures (“ASU 2023-07”). ASU 2023-07 enhances reportable segment disclosures by requiring disclosures such as significant segment expenses, information on the CODM and disclosures for entities with a single reportable segment. Additionally, the amendments enhance interim disclosure requirements, clarify circumstances in which an entity can disclose multiple segment measures of profit or loss, and contain other disclosure requirements. ASU 2023-07 is effective for fiscal years beginning after December 15, 2023, and interim periods within fiscal years beginning after December 15, 2024, and we expect to adopt ASU 2023-07 retrospectively. The Company does not expect the adoption of ASU 2023-07 to have a material impact on its consolidated financial statements.
In December 2023, the FASB issued ASU 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures (“ASU 2023-09”). ASU 2023-09 enhances the existing income tax disclosures to provide additional information to better assess how an entity’s operations, related tax risks and tax planning, and operational opportunities affect its tax rate and prospects for future cash flows. ASU 2023-09 is effective for fiscal years beginning after December 15, 2023, and interim periods within fiscal years beginning after December 15, 2024, and we expect to adopt ASU 2023-09 prospectively. The Company does not expect the adoption of ASU 2023-09 to have a material impact on its consolidated financial statements.
2. Discontinued Operations
In the second quarter of 2023, the Company exited its then-existing U.S. hemp-derived cannabinoid product operations. The exit of the U.S. operations represented a strategic shift that has a major effect on the Company’s operations and financial results, and as such,
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020, 2019,2023, 2022, and 20182021
(In thousands of U.S. dollars, except for gram and share amounts)
In January 2020,qualifies for reporting as discontinued operations in our consolidated statements of net loss and comprehensive loss. Prior period amounts have been reclassified to reflect the Financial Accounting Standards Boardissued ASU No. 2020-01, Investments-Equity Securities (Topic 321), Investments-Equity Method and Joint Ventures (Topic 323), and Derivatives and Hedging (Topic 815) (“ASU No. 2020-01”). ASU No. 2020-01 clarifiesdiscontinued operations classification of the interactionU.S. operations.
The following table presents the major components comprising loss from discontinued operations in the consolidated statements of accountingoperations for the transition into and out of the equity method. The new standard also clarifies the accounting for measuring certain purchased options and forward contracts to acquire investments. The guidance in ASU No. 2020-01 is effective for annual and interim periods beginning after December 15, 2020. The Company has evaluated the impact of ASU No. 2020-01 and does not expect the adoption to have a material impact to the Company’s consolidated financial statements.
In August 2020, the FASB issued ASU 2020-06, Debt –Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging –Contracts in Entity’s Own Equity (Subtopic 815–40) (“ASU No. 2020-06”). ASU No. 2020-06 simplifies the accounting for certain financial instruments with characteristics of liabilities and equity, including convertible instruments and contracts on an entity’s own equity. ASU No 2020-06 is part of the FASB’s simplification initiative, which aims to reduce unnecessary complexity in U.S. GAAP. ASU No 2020-06 is effective for fiscal years beginning after December 15, 2021, and interim periods within those fiscal years. The Company is currently evaluating the impact of ASU No. 2020-06 on its consolidated financial statements and related disclosures.
4. Revenues from Contracts with Customers
Cronos Group disaggregates net revenues based on product type. For further discussion, see Note 29. Receivables were $8,928 atended December 31, 2020 (December 31, 2019 – $4,638). The Company recorded a current expected credit loss allowance of $74 as of December 31, 2020 (December 31, 2019 – $136).2023, 2022, and 2021:
Cronos Group offers discounts to customers for prompt payment and calculates cash discounts as a percentage of the list price based on historical experience and agreed-upon payment terms. Cronos Group records an allowance for cash discounts, which is included as a contra-asset against receivables on the Company’s consolidated balance sheets.
Revenue is measured net of returns. As a result, the Company is required to estimate the amount of returns based on the historical data by customer and product type, adjusted for forward-looking information. This is included as a provision against accounts receivable on the Company’s consolidated balance sheets. The Company estimates sales returns based principally on historical volume and return rates, as a reduction to revenues. The difference between actual sales and estimated sales returns is recorded in the period in which the actual amounts become known. These differences, if any, have not had a material impact on the Company’s consolidated financial statements. Upon return, products can be extracted from dried cannabis, resold, or destroyed depending on the nature of the product. The Company has assessed that the amount recoverable is immaterial. | | | | | | | | | | | | | | | | | |
| Year ended December 31, 2023 | | Year ended December 31, 2022 | | Year ended December 31, 2021(iv) |
| Net revenue | $ | 1,029 | | | $ | 5,155 | | | $ | 9,874 | |
| Cost of sales | 2,164 | | | 8,622 | | | 9,850 | |
Inventory write-down(i) | 839 | | | — | | | — | |
| Gross profit | (1,974) | | | (3,467) | | | 24 | |
| Operating expenses | | | | | |
| Sales and marketing | 578 | | | 4,236 | | | 24,020 | |
| Research and development | 32 | | | 250 | | | 1,490 | |
| General and administrative | 668 | | | 3,504 | | | 6,133 | |
| Restructuring costs | 523 | | | 1,788 | | | — | |
| Share-based compensation | 13 | | | 107 | | | 307 | |
| Depreciation and amortization | 13 | | | 58 | | | 71 | |
| Impairment loss on goodwill and indefinite-lived intangible assets | — | | | — | | | 236,019 | |
Impairment loss on long-lived assets(ii) | 205 | | | — | | | 1,214 | |
| Total operating expenses | 2,032 | | | 9,943 | | | 269,254 | |
| Interest income | 10 | | | 23 | | | 4 | |
Other, net(iii) | (118) | | | (169) | | | 101 | |
| Total other loss | (108) | | | (146) | | | 105 | |
| Loss before income taxes | (4,114) | | | (13,556) | | | (269,125) | |
| Income tax expense (benefit) | — | | | — | | | — | |
| Net loss from discontinued operations | (4,114) | | | (13,556) | | | (269,125) | |
5. Inventory
Inventory is comprised of the following items:
| | | | | | | | | | | | | | |
| | As of December 31, |
| | 2020 | | 2019 |
| Raw materials | | $ | 11,489 | | | $ | 2,469 | |
| Work-in-progress – dry cannabis | | 20,520 | | | 11,538 | |
| Work-in-progress – cannabis extracts | | 5,758 | | | 17,975 | |
| Finished goods – dry cannabis | | 4,894 | | | 1,798 | |
| Finished goods – cannabis extracts | | 1,011 | | | 2,624 | |
| Supplies and consumables | | 330 | | | 1,639 | |
| Total | | $ | 44,002 | | | $ | 38,043 | |
Inventory is written down for any obsolescence such as slow-moving or non-marketable products, or when the net realizable value of inventory is less than the carrying value. For the year ended December 31, 2020,2023, Inventory write-down relates to the disposal of obsolete inventory as a result of the exit of the U.S. operations.
(ii)During the year ended December 31, 2023, as a result of the exit of the U.S. operations, the Company recorded write-downsrecognized an impairment charge of $205 related to inventory of $26,055 (December 31, 2019 – $29,173,the right-of-use lease assets associated with the Company’s former U.S. manufacturing facility in Los Angeles, California. For the year ended December 31, 2018 - $NaN) driven by pricing pressures2021, the Company concluded that the carrying amount of the U.S. reporting unit exceeded its fair value, which resulted in the Canadian marketplace. Asrecognition of an impairment charge of $178,414 on goodwill and concluded the carrying amount of the Lord Jones® brand exceeded its fair value, which resulted in impairment charges of $57,500 on its Lord Jones® brand intangible asset.
(iii)For the years ended December 31, 2020, an inventory reserve2023 and December 31, 2022, Other, net related to loss on disposal of $1,229 was recorded (Decemberassets that were part of the U.S. operations. For the year ended December 31, 2019 – $NaN) in cost2021, Other, net includes a gain on disposal of sales.assets related to Original B.C. Ltd. (“OGBC”) as well as a loss from disposal of assets that were part of the U.S. operations.
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020, 2019,2023, 2022, and 20182021
(In thousands of U.S. dollars, except for gram and share amounts)
The following tables present the Company’s discontinued operations revenue by major product category:
| | | | | | | | | | | | | | | | | |
| |
| 2023 | | 2022 | | 2021 |
| Cannabis extracts | 1,029 | | | 5,155 | | | 9,874 | |
| Net revenue | $ | 1,029 | | | $ | 5,155 | | | $ | 9,874 | |
The following tables summarize the Company’s discontinued operations restructuring activity for the years ended December 31, 2023 and 2022: | | | | | | | | | | | | | | | | | | | | | | | | | |
| Accrual as of January 1, 2023 | | Expenses | | Payments/Write-offs | | | | Accrual as of December 31, 2023 |
| Employee Termination Benefits | $ | — | | | $ | 431 | | | $ | (431) | | | | | $ | — | |
| | | | | | | | | |
| Other Restructuring Costs | — | | | 92 | | | (92) | | | | | — | |
| Total | $ | — | | | $ | 523 | | | $ | (523) | | | | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Accrual as of January 1, 2022 | | Expenses | | Payments/Write-offs | | | | Accrual as of December 31, 2022 |
| Employee Termination Benefits | $ | — | | | $ | 1,788 | | | $ | (1,788) | | | | | $ | — | |
| | | | | | | | | |
| | | | | | | | | |
| Total | $ | — | | | $ | 1,788 | | | $ | (1,788) | | | | | $ | — | |
The Company’s discontinued operations incurred no restructuring costs for the year ended December 31, 2021.
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2023, 2022, and 2021
(In thousands of U.S. dollars, except for share amounts)
The following table presents a reconciliation of assets and liabilities of the discontinued operations presented in the consolidated balance sheets:
| | | | | | | | | | | |
| As of December 31, 2023 | | As of December 31, 2022 |
| Assets | | | |
| Current assets | | | |
| Cash and cash equivalents | $ | — | | | $ | 2,300 | |
| | | |
| Accounts receivable, net | — | | | 253 | |
| Other receivables | — | | | 775 | |
| | | |
| Prepaids and other current assets | — | | | 464 | |
| Inventory, net | — | | | 934 | |
| Current assets of discontinued operations | — | | | 4,726 | |
| | | |
| Non-current assets | | | |
| | | |
| | | |
| | | |
| Property, plant and equipment, net | — | | | 254 | |
| Right-of-use assets | — | | | 430 | |
| | | |
| Intangible assets, net | — | | | 1,594 | |
| | | |
| Non-current assets of discontinued operations | — | | | 2,278 | |
| | | |
| Liabilities | | | |
| Current liabilities | | | |
| Accounts payable | — | | | 166 | |
| | | |
| Accrued liabilities | — | | | 807 | |
| Current portion of lease obligation | — | | | 415 | |
| | | |
| | | |
| Current liabilities of discontinued operations | $ | — | | | $ | 1,388 | |
For the years ended December 31, 2023, 2022 and 2021, purchases of property plant and equipment related to discontinued operations were $67, $183 and $971, respectively.
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2023, 2022, and 2021
(In thousands of U.S. dollars, except for share amounts)
The following table presents information related to leases associated with the Company’s U.S. discontinued operations. As of December 31, 2023, the Company has no right-of-use assets or lease obligations associated with its U.S. discontinued operations. For the years ended December 31, 2023, 2022 and 2021, the aggregate depreciation expense on right-of-use assets associated with the Company’s U.S. discontinued operations was $198, $865 and $548, respectively, and was included in loss from discontinued operations on the consolidated statements of net loss and comprehensive loss.
| | | | | | | | | | | | | | | | | |
| As of December 31, |
| 2023 | | 2022 | | 2021 |
| Lease cost | | | | | |
| Operating lease cost | $ | 213 | | | $ | 1,102 | | | $ | 837 | |
| Short-term lease cost | — | | | — | | | — | |
| Total lease cost | $ | 213 | | | $ | 1,102 | | | $ | 837 | |
| | | | | |
| Supplemental cash flow and other information | | | | | |
| Operating cash flows - cash paid for operating lease obligations | $ | 525 | | | $ | 1,050 | | | $ | 752 | |
| Non-cash activity - right-of-use assets obtained in exchange for lease obligations | — | | | 443 | | | 3,277 | |
| | | | | |
| Weighted-average remaining lease term (years) – operating leases | 0.0 | | 1.0 | | 3.8 |
| Weighted-average discount rate – operating leases | — | % | | 5.62 | % | | 7.65 | % |
6.3. Inventory, net
Inventory, net is comprised of the following items:
| | | | | | | | | | | |
| As of December 31, |
| 2023 | | 2022 |
| Raw materials | $ | 4,795 | |
| $ | 7,421 | |
| Work-in-progress | 10,593 | | | 15,646 | |
| Finished goods | 14,819 | | | 13,503 | |
| Supplies and consumables | 288 | | | 989 | |
| Total | $ | 30,495 | | | $ | 37,559 | |
4. Investments
(a)Variable Interest Entitiesinterest entities and investments in equity method investments, net
The Company holds variable interests in Cronos Growing Company Inc. (“Cronos GrowCo”), Natuera S.à.r.l (“Natuera”), MedMen Canada Inc. (“MedMen Canada”) and Cannasoul Lab Services Ltd. (“CLS”).
Cronos GrowCo is a joint venture incorporated under the Canada Business Corporations Act (“CBCA”) on June 14, 2018 with the objective of building a cannabis production greenhouse, applying for cannabis licenses under the Cannabis Act (Canada), and growing, cultivating, extracting, producing and selling cannabis in accordance with such licenses. Cronos Group holds variable interests The Company’s investment in Cronos GrowCo through its ownership of 50% of Cronos GrowCo’s common shares and senior secured debt in Cronos GrowCo. Cronos GrowCo’s economic performance is driven by the quantity and strains of cannabis grown. The joint venture partners mutually determine the quantity and strains of cannabis grown.
MedMen Canada is a joint venture incorporated under the CBCA on March 13, 2018, with the objective of the retail sale and marketing of cannabis products in Canada. MedMen Canada holds the exclusive license to the MedMen brand in Canada for a minimum term of 20 years. Cronos holds variable interests in MedMen Canada through its ownership of 50% of MedMen Canada’s common shares and other subordinated debt in the entity. MedMen Canada’s economic performance is driven by the quantity and strains of cannabis sold. Subject to applicable law, the joint venture partners mutually determine the quantity and strains of cannabis to be sold in MedMen Canada’s retail stores, if and when stores are opened.
Natuera is a joint venture registered in Luxembourg with the objective of cultivating and commercializing medical cannabis to serve the export market. Cronos holds variable interests in Natuera through its ownership of 50% of Natuera’s common shares and other debt in the entity. Natuera’s economic performance is driven by the quantity and strains of cannabis to be grown. The joint venture partners mutually determine the quantity and strains of cannabis grown.
The Company’s investments in Cronos GrowCo, Natuera and MedMen Canada are exposed to economic variability from eachbased on the entity’s performance; however, the Company does not consolidate the entitiesentity as it does not have the power to direct the activities that most significantly impact each entities’the entity’s economic performance. Thus, Cronos Groupthe Company is not considered the primary beneficiary of eachthe entity. These investments areThe investment in Cronos GrowCo is accounted for as an equity method investmentsinvestment classified as “Investments in equity accounted investees”“Equity method investments, net” in the consolidated balance sheets.
Cronos GrowCo
Cronos GrowCo is a joint venture incorporated under the Canada Business Corporations Act on June 14, 2018, with the objective of cultivating and commercializing cannabis and cannabis products. Cronos GrowCo’s economic performance is driven by the day-to-day operations of Cronos GrowCo, which are controlled by 2645485 Ontario Inc. (“Mucci”), the Company’s joint venture partner.
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2023, 2022, and 2021
(In thousands of U.S. dollars, except for share amounts)
During the year ended December 31, 2021, the Company concluded that lower than expected sales forecasts combined with the increase to the aggregate principal amount of the GrowCo Credit Facility (as defined below) were indicators of impairment for the Company’s equity method investment in Cronos GrowCo. Accordingly, the Company performed a quantitative impairment assessment in the third quarter of 2021 to compare the fair value of the investment in Cronos GrowCo to its carrying amount. The fair value was estimated using the discounted cash flow method. Significant inputs included discount rate, growth rates, and cash flow projections. As a result of this analysis, the Company concluded that as of September 30, 2021, the estimated fair value was higher than the carrying amount and no impairment charges were recorded. There were no such indicators of impairment present for the years ended December 31, 2023 and 2022.
CLS
CLS is a wholly owned subsidiary of Cannasoul Analytics Ltd., incorporated with the purpose of establishing a commercial cannabis analytical testing laboratory located on the premises of Cronos Israel (the “Cannasoul Collaboration”). Cronos Israel willagreed to advance up to ILS 8,297 (approximately $2,574)($2,664) by a non-recourse loan (the “Cannasoul Collaboration Loan”) to CLS over a period of two years from April 1, 2020 for the capital and operating expenditures of the laboratory. The loan will bearbears interest at 3.5% annually. Cronos Israel will receive 70% of the profits of the laboratory until such time as it has recovered 150% of the amounts advanced to CLS, after which time it will receive 50% of the laboratory profits. As a result, the Company is exposed to economic variability from CLS’s performance. The Company does not consolidate CLS as it does not have the power to direct the activities that most significantly impact the entity’s economic performance; thus, the Company is not considered the primary beneficiary of the entity. The carrying amount of the non-recourse loan is recorded under loans receivable and the full loan amount, ILS 8,297, represents the Company’s maximum potential exposure to losses through the Cannasoul Collaboration. See Note 85 “Loans Receivable, net” for further information regarding loans receivablereceivable.
A reconciliation of the carrying amount of the investments in equity method investments, net is as follows:
| | | | | | | | | | | | | | | | | |
| Ownership interest | | As of December 31, |
| | 2023 | | 2022 |
| | | | | |
| Cronos GrowCo | 50% | | $ | 19,488 | | | $ | 18,755 | |
| | | | | |
| | | | | |
| | | | | |
The following is a summary of the Company’s share of net income (losses) from equity investments accounted for under the equity method of accounting:
| | | | | | | | | | | | | | | | | |
| Year ended December 31, |
| 2023 | | 2022 | | 2021 |
Vitura(i) | N/A | | N/A | | $ | (48) | |
| Cronos GrowCo | 1,583 | | | 3,114 | | | (2,518) | |
Natuera(ii) | N/A | | N/A | | (3,747) | |
| $ | 1,583 | | | $ | 3,114 | | | $ | (6,313) | |
(i).As of December 31, 2023 and December 31, 2022, the Company held a 9.6% and 9.9% ownership interest, respectively, in Vitura, and Vitura was no longer considered an equity-method investment. As of and up to December 16, 2021, the Company held a 31% ownership interest in Vitura and was considered an equity-method investment.
(ii)As of December 31, 2021, the combination of the Company’s share of accumulated net losses and impairment charges was in excess of its equity investment in Natuera, and the net book value of the investment was zero.
The following is a summary of financial information for the Company’s equity method investments:
| | | | | | | | | | | | | | | | | |
| As of December 31, |
| 2023 | | 2022 | | 2021 |
| Current assets | $ | 19,600 | | | $ | 21,158 | | | $ | 5,660 | |
| Non-current assets | 99,227 | | | 104,227 | | | 125,777 | |
| Current liabilities | 9,707 | | | 5,593 | | | 13,457 | |
| Non-current liabilities | 64,814 | | | 73,779 | | | 81,594 | |
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020, 2019,2023, 2022, and 20182021
(In thousands of U.S. dollars, except for gram and share amounts)
(a)Equity Method Investments
A reconciliation of the carrying amount of the investments in associates and joint ventures is as follows:
| | | | | | | | | | | | | | | | | |
| Ownership interest | | Carrying Amount |
| | December 31, 2020 | | December 31, 2019 |
Cronos Australia(i) | 31% | | $ | 0 | | | $ | (346) | |
Cronos GrowCo(ii) | 50% | | 19,235 | | | 1,501 | |
| | | | | |
| Natuera | 50% | | 0 | | | (598) | |
| | | $ | 19,235 | | | $ | 557 | |
(i)On October 25, 2019, Cronos Australia issued 40 million new shares in an initial public offering at an offering price of A$0.50 per share. The Company’s ownership in Cronos Australia decreased from 50% to 31% on November 7, 2019 when Cronos Australia began trading on the Australian Securities Exchange. This resulted in a reconsideration event, which required the reassessment of the Company’s variable interest entity conclusion. Upon reconsideration, the Company determined that the entity was no longer a variable interest entity as of December 31, 2019 and is now reported under the equity method.
(ii)On September 25, 2020, the Company and 2645485 Ontario Inc. (“Mucci”), the other joint venture partner of Cronos GrowCo, agreed to capitalize certain historical advances made by each shareholder to Cronos GrowCo. Total aggregate gross advances to Cronos GrowCo, excluding any amounts advanced by the Company to Cronos GrowCo under the GrowCo Credit Facility, were C$49,300 ($37,010), of which the Company advanced 50% and Mucci advanced the remaining 50% for an amount of C$24,650 ($18,505) each. As a result, the Company transferred the advances of C$24,650 ($18,505) to investments in equity accounted investees in respect of Cronos GrowCo.
The Company’s share of net earnings (losses) from equity investments accounted for under the equity method of accounting as of and for the years ended December 31:
| | | | | | | | | | | | | | | | | |
| 2020 | | 2019 | | 2018 |
Whistler Medicinal Marijuana Company (“Whistler”)(i) | $ | 0 | | | $ | 29 | | | $ | 178 | |
| Cronos Australia | (363) | | | (1,101) | | | (588) | |
| Cronos GrowCo | (1,537) | | | (167) | | | (100) | |
| MedMen Canada | 0 | | | 35 | | | (213) | |
Natuera(ii) | (2,610) | | | (805) | | | 0 | |
| $ | (4,510) | | | $ | (2,009) | | | $ | (723) | |
(i)Whistler was incorporated in British Columbia, Canada and is a license holder under the Cannabis Act (Canada) with production facilities in British Columbia, Canada. The Company fully divested its investment in Whistler during 2019. See Note 7.
(ii)The Company’s share of accumulated net losses in excess of its equity investment in Natuera has been applied as a loss allowance on the loan receivable. See Note 8.
| | | | | | | | | | | | | | | | | |
| Year ended December 31, |
| 2023 | | 2022 | | 2021 |
| Revenue | $ | 40,604 | | | $ | 39,110 | | | $ | 8,186 | |
| Gross profit | 21,565 | | | 24,379 | | | (5,059) | |
| Net income (loss) | 3,051 | | | 6,569 | | | (12,603) | |
The following is a summary of financial informationthe maximum exposure to loss from the Company’s investments in equity method investments:
| | | | | | | | | | | | | | | | | |
| Ownership interest | | Other Net Assets (Liabilities) | | Maximum Exposure to Loss |
| | | | | |
| Cronos GrowCo | 50% | | $ | 44,306 | | | $ | 19,488 | |
| | | | | |
| Balance as of December 31, 2023 | | | $ | 44,306 | | | $ | 19,488 | |
| | | | | | | | | | | | | | | | | |
| Ownership interest | | Other Net Assets (Liabilities) | | Maximum Exposure to Loss |
| Cronos GrowCo | 50% | | $ | 46,013 | | | $ | 18,755 | |
| | | | | |
| | | | | |
| Balance as of December 31, 2022 | | | $ | 46,013 | | | $ | 18,755 | |
The Company’s maximum exposure to loss is equal to the carrying amount of the investment.
(b)Other investments
Other investments consist of investments in common shares and options of two companies in the cannabis industry.
PharmaCann Option
On June 14, 2021, the Company purchased an option (the “PharmaCann Option”) to acquire 473,787 shares of Class A Common Stock of PharmaCann, Inc. (“PharmaCann”), a vertically integrated cannabis company in the United States, at an exercise price of $0.0001 per share, representing approximately 10.5% of PharmaCann’s issued and outstanding capital stock on a fully diluted basis as of the date of the PharmaCann Option, for an aggregate purchase price of approximately $110,392. The option exercise will be based upon various factors, including the status of U.S. federal cannabis legalization, as well as regulatory approvals, including in the states where PharmaCann operates that may be required upon exercise. The Company has deemed its influence in PharmaCann to be non-significant. The PharmaCann Option is classified as an investment in an equity security without a readily determinable fair value. The Company measures the PharmaCann Option at cost less accumulated impairment charges, if any, and subsequently adjusted for observable price changes in orderly transactions for the Company’s equity methodidentical or a similar investment of the same issuer. The PharmaCann Option is reported as Other investments ason the consolidated balance sheet for the periods ended December 31, 2023 and 2022.
As of December 31, 2021, the Company performed an assessment on the existence of impairment indicators on the PharmaCann Option and fornoted no indicators of impairment existed. As such, no impairment loss on the investment was recorded during the year ended December 31:31, 2021.
| | | | | | | | | | | | | | | | | |
| 2020 | | 2019 | | 2018 |
| Current assets | $ | 19,126 | | | $ | 23,200 | | | $ | 7,121 | |
| Non-current assets | 122,099 | | | 76,212 | | | 27,129 | |
| Current liabilities | 24,223 | | | 52,796 | | | 3,746 | |
| Non-current liabilities | 76,313 | | | 33,189 | | | 13,201 | |
| | | | | |
| Revenue | 367 | | | 52 | | | 5,344 | |
| Gross profit | (631) | | | 0 | | | 0 | |
| Net loss | (11,453) | | | (2,048) | | | (874) | |
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020, 2019,2023, 2022, and 20182021
(In thousands of U.S. dollars, except for gram and share amounts)
(b)Advances to Joint Ventures
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| MedMen Canada(i) | | Cronos GrowCo(ii) | | Cronos Australia(iii) | | Natuera | | Total |
| As of January 1, 2020 | $ | 471 | | | $ | 18,966 | | | $ | 0 | | | $ | 0 | | | $ | 19,437 | |
Transfer to investments in equity accounted investees(ii) | 0 | | | (18,505) | | | 0 | | | 0 | | | (18,505) | |
| Interest on advances | 0 | | | 0 | | | 37 | | | 0 | | | 37 | |
| Advances to joint ventures recovered from (applied to) carrying amount of investments | 0 | | | 0 | | | (38) | | | 0 | | | (38) | |
| Effect from foreign exchange | (4) | | | (461) | | | 1 | | | 0 | | | (464) | |
| As of December 31, 2020 | $ | 467 | | | $ | 0 | | | $ | 0 | | | $ | 0 | | | $ | 467 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| MedMen Canada(i) | | Cronos GrowCo(ii) | | Cronos Australia(iii) | | Natuera | | Total |
| As of January 1, 2019 | $ | 1,244 | | | $ | 2,970 | | | $ | 475 | | | $ | 0 | | | $ | 4,689 | |
| Advances (repayments) | (852) | | | 15,494 | | | 274 | | | 219 | | | 15,135 | |
| Advances to joint ventures recovered from (applied to) carrying amount of investments | 35 | | | 22 | | | (779) | | | (224) | | | (946) | |
| Effect from foreign exchange | 44 | | | 480 | | | 30 | | | 5 | | | 559 | |
| As of December 31, 2019 | $ | 471 | | | $ | 18,966 | | | $ | 0 | | | $ | 0 | | | $ | 19,437 | |
(i) Advance is unsecured, non-interest bearing, and there are no termsDuring the fourth quarter of repayment.
(ii)On September 25, 2020,2023, the Company and Mucci agreed to capitalize historical advances made by each shareholder to Cronos GrowCo. The Company capitalized C$24,650 ($18,505) through a transferidentified adverse forecast changes in the financial performance of aggregate gross advances from advances to joint ventures to investments in equity accounted investees in respectPharmaCann as an indicator of Cronos GrowCo. Refer to footnote (ii)impairment related to the first table set forth under Note 6(a).
(iii)A$1,500 is governed byPharmaCann Option and conducted an unsecured loan bearing interest atanalysis comparing the PharmaCann Option’s carrying amount to its estimated fair value. The fair value was estimated using a rate of 12% per annum, calculated and compounded daily, in arrears, on the amounts advanced from the date of each advance. The loan is due on January 1, 2022. If the loan is overdue, the outstanding amount bears interest at an additional 2% per annum.
The Company determined that the maximum exposure to loss on variable interest entities is limited to the Company’s initial investment, advances and/or loans for each variable interest entity. The following is a summarycombination of the maximum exposure toincome approach and the market approach. Under the income approach, significant inputs used in the discounted cash flow method include discount rate, growth rates, cash flow projections, and the expectation of federal rescheduling and individual state legalization of cannabis in the U.S. Under the market valuation approach, the key assumptions are the selected multiples and the discount for lack of marketability. As a result of this analysis, the Company recorded a non-cash impairment charge of $23,350 in the fourth quarter of 2023 as the difference between the carrying amount of the PharmaCann Option and its estimated fair value, in the consolidated statements of net loss and comprehensive loss for the year ended December 31, 20202023.
Vitura (formerly known as Cronos Australia)
On September 14, 2021, Cronos Australia (now, Vitura Health Limited) entered into a merger agreement to acquire 100% of the issued shares of CDA Health Pty Ltd, an Australian medicinal cannabis company, subject to customary closing conditions, including shareholder approval (the “Cronos Australia Merger”). The Cronos Australia Merger closed on December 16, 2021. In connection with the closing of the Cronos Australia Merger, all advances made under the Company’s A$1,500 unsecured loan to Cronos Australia, plus accrued interest and 2019:
| | | | | | | | | | | | | | | | | |
| Ownership interest | | Other Net Assets (Liabilities) | | Maximum Exposure to Loss |
| Cronos Australia | 31% | | $ | 8,976 | | | $ | 1,530 | |
| Cronos GrowCo | 50% | | 109,329 | | | 21,125 | |
| MedMen Canada | 50% | | 0 | | | 467 | |
| Natuera | 50% | | (6,849) | | | 8,154 | |
| Balance as of December 31, 2020 | | | $ | 111,456 | | | $ | 31,276 | |
certain royalties payable, were converted into ordinary shares of Cronos Australia. In addition, the Company’s ownership interest in Cronos Australia decreased to approximately 10% and the Company’s number of Cronos Australia board seats was reduced from two to zero. On November 29, 2022, the shareholders of Cronos Australia approved the proposal to change the name of the company to “Vitura Health Limited” (the “CAU Name Change”). On December 2, 2022, in connection with the CAU Name Change, Vitura and the Company mutually agreed to terminate the intellectual property license that granted Vitura the right to use certain intellectual property of the Company, including, but not limited to, the “Cronos” name and the PEACE NATURALS
| | | | | | | | | | | | | | | | | |
| Ownership interest | | Other Net Assets (Liabilities) | | Maximum Exposure to Loss |
| Cronos Australia | 31% | | $ | 10,900 | | | $ | 1,355 | |
| Cronos GrowCo | 50% | | 3,091 | | | 20,700 | |
| MedMen Canada | 50% | | (199) | | | 642 | |
| Natuera | 50% | | (358) | | | 4,888 | |
| Balance as of December 31, 2019 | | | $ | 13,434 | | | $ | 27,585 | |
The following table summarizes the Company’s other investments activity:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As of January 1, 2023 | | Unrealized loss | | Impairment charges | | Foreign exchange effect | | As of December 31, 2023 |
| PharmaCann | $ | 49,000 | | | $ | — | | | $ | (23,350) | | | $ | — | | | $ | 25,650 | |
| Vitura | 21,993 | | | (12,096) | | | — | | | (296) | | | 9,601 | |
| $ | 70,993 | | | $ | (12,096) | | | $ | (23,350) | | | $ | (296) | | | $ | 35,251 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As of January 1, 2022 | | Unrealized gain | | Impairment charges | | Foreign exchange effect | | As of December 31, 2022 |
| PharmaCann | $ | 110,392 | | | $ | — | | | $ | (61,392) | | | $ | — | | | $ | 49,000 | |
| Vitura | 8,000 | | | 14,739 | | | — | | | (746) | | | 21,993 | |
| $ | 118,392 | | | $ | 14,739 | | | $ | (61,392) | | | $ | (746) | | | $ | 70,993 | |
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020, 2019,2023, 2022, and 20182021
(In thousands of U.S. dollars, except for gram and share amounts)
7. Other Investments5. Loans Receivable, net
Other investments consistLoans receivable, net consists of investments in common shares and warrants of several companies in the cannabis industry. As of December 31, 2020 and December 31, 2019, the Company did not hold any other investments. The gains and losses on sale of other investments are classified as fair value through net income (loss).following:
The gains (losses) recognized in net income (loss) related to other investments were as follows:
| | | | | | | | | | | | | | | | | |
| Year ended December 31, |
| 2020 | | 2019 | | 2018 |
| Gain recognized in net income (loss) | | | | | |
Canopy Growth Corporation (“Canopy”)(i) | $ | 0 | | | $ | 51 | | | $ | 166 | |
Vivo Cannabis (“Vivo”) - shares(ii) | 0 | | | 0 | | | (173) | |
Vivo Cannabis - share warrants(ii) | 0 | | | 0 | | | 171 | |
Whistler(iii) | 0 | | | 15,530 | | | 0 | |
Aurora Cannabis Inc. (“Aurora”)(iv) | 4,789 | | | 696 | | | 0 | |
| $ | 4,789 | | | $ | 16,277 | | | $ | 164 | |
| | | | | | | | | | | |
| As of December 31, |
| 2023 | | 2022 |
| | | |
| | | |
| GrowCo Credit Facility | $ | 5,034 | | | $ | 4,427 | |
| Add: Accrued interest | 507 | | | 4,463 | |
| Total current portion of loans receivable | 5,541 | | | 8,890 | |
| GrowCo Credit Facility | 53,638 | | | 56,898 | |
| Mucci Promissory Note | 13,379 | | | 13,438 | |
| Cannasoul Collaboration Loan | 1,771 | | | 1,837 | |
| Add: Long-term portion of accrued interest | 248 | | | 172 | |
| Total long-term portion of loans receivable | 69,036 | | | 72,345 | |
| Total loans receivable | $ | 74,577 | | | $ | 81,235 | |
(i)During the year ended December 31, 2019, the Company sold all remaining 11,062 common shares of Canopy (2018 – 18,436 common shares) for net proceeds of $355 (2018 – $530) recorded as a gain on disposal of other investments in other income (expense). Upon adoption of ASU 2016-01 during the year ended December 31, 2018, the gains and losses on the Canopy investment were reclassified from fair value through other comprehensive income to fair value through net income.Cronos GrowCo Credit Facility
(ii)During the year ended December 31, 2018, the Company exercised 182,927 share warrants for aggregate consideration of $87 for additional shares of Vivo. During the year ended December 31, 2018, the Company then sold all 182,927 shares of Vivo for proceeds of $216. Upon adoption of ASU 2016-01 during the year ended December 31, 2018, the gains and losses on the Vivo Cannabis shares investment were reclassified from fair value through other comprehensive income to fair value through net income.
(iii)On March 4, 2019, the Company sold all 2,563 shares of Whistler, representing approximately 19.0% of Whistler’s issued and outstanding common shares, to Aurora, in connection with Aurora’s acquisition of Whistler (the “Whistler Transaction”). As a result of the closing of the Whistler Transaction, the Company received 2,524,341 Aurora common shares. During the year ended December 31, 2019, the Company sold all 2,524,341 common shares of Aurora, for gross proceeds of $19,259. A gain on disposal of other investments was recorded in other income (expense) as a result.
(iv)For the year ended December 31, 2020, in connection with the achievement of certain milestones related to the Whistler Transaction, the Company received 980,662 common shares of Aurora. During the year ended December 31, 2020, the Company sold all 980,662 of the Aurora common shares, for gross proceeds of approximately $4,789 (C$6,404), recorded as a gain on disposal of other investments in other income (expense).
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020, 2019, and 2018
(In thousands of U.S. dollars, except for gram and share amounts)
8. Loans Receivable
| | | | | | | | | | | | | | | | | |
| | As of |
| | December 31, 2020 | | December 31, 2019 |
| Current portion | | | | |
Natuera Series A Loan(i) | | $ | 3,518 | | | $ | 4,575 | |
GrowCo Credit Facility(ii) | | 3,137 | | | 0 | |
| Add: Accrued interest | | 428 | | | 89 | |
| Total current portion of loans receivable | | 7,083 | | | 4,664 | |
| Long term portion | | | | |
GrowCo Credit Facility(ii) | | 69,939 | | | 31,678 | |
Mucci Promissory Note(iii) | | 13,324 | | | 12,587 | |
Cannasoul Collaboration loan(iv) | | 1,261 | | | 0 | |
| Add: Accrued interest | | 2,667 | | | 702 | |
| Total long-term portion of loans receivable | | 87,191 | | | 44,967 | |
| Total loans receivable | | $ | 94,274 | | | $ | 49,631 | |
(i)On July 24, 2020, the Company and its joint venture partner entered into an amendment to the Series A Loan with Natuera to increase the principal amount of the Series A Loan by $6,350, to an aggregate principal amount of the Series A Loan of $15,500, of which the Company has committed to fund 50% and its joint venture partner has committed to fund the remaining 50%. Outstanding principal amounts continue to bear interest at a fixed annual rate of 5.67%. In December 2020, the Series A Loan was amended to extend its maturity date to September 1, 2021 from March 1, 2021.
As of December 31, 2020, a current expected credit loss allowance of $685 was recorded against the Natuera Series A loan. A loss allowance of $3,024 was recorded against the Natuera Series A Loan related to the Company’s share of net loss from Natuera in excess of the carrying value of the equity method investment. Refer to Note 6.
(ii)On August 23, 2019, the Company, as lender, and Cronos GrowCo, as borrower, entered into a senior secured credit agreement with Cronos GrowCo in respectfor an aggregate principal amount of a C$100,000 (approximately $78,430) secured non-revolving term loan credit facility (the “GrowCo Credit Facility”). The GrowCo Credit Facility will mature on March 31, 2031 and will bear interest at varying rates based on the Canadian prime rate as announced by the Bank of Montreal. Interest began to accrue as of the closing date of the GrowCo Credit Facility and is payable on a quarterly basis until maturity, except that any interest accrued prior to March 31, 2021 will be payable not later than December 31, 2021. Repayment of principal will be made on a quarterly basis commencing on March 31, 2021. The GrowCo Credit Facility is secured by substantially all present and after acquiredafter-acquired personal and real property of Cronos GrowCo. In August 2021, the GrowCo Credit Facility was amended to increase the aggregate principal amount available to C$105,000. As a result of the increase in the aggregate principal amount of the GrowCo Credit Facility and its subsidiaries. Mucci, the other 50% shareholder oflower than expected sales forecasts from Cronos GrowCo, has provided a limited recourse guaranteethe Company revalued its allowance for credit loss on the GrowCo Credit Facility resulting in favoran increase in the allowance of Cronos GrowCo, secured by Mucci’s shares$12,748, which was recorded in Cronos GrowCo.general and administrative expenses on the consolidated statements of net loss and comprehensive loss for the year ended December 31, 2021. As of both December 31, 2020,2023 and 2022, Cronos GrowCo had drawn C$95,150104,000 ($74,626)78,532 and $76,730), respectively, from the GrowCo Credit Facility. Subsequent toFor the years ended December 31, 2020,2023 and 2022, Cronos GrowCo repaid C$7,500 ($5,612) and C$4,000 ($3,073) in principal and C$13,462 ($10,287) and C$7,060 ($5,209) in interest, respectively. As of December 31, 2023, Cronos GrowCo had repaid, in the aggregate, C$11,500 ($8,685) and C$20,522 ($15,496) in principal and interest, respectively, under the terms of the GrowCo Credit Facility were amended, such that any interest accrued from April 1, 2021 to December 31, 2021 will be payable not later than December 31, 2022, and repayment of principal will be made on a quarterly basis commencing on March 31, 2022.
As of December 31, 2020, a current expected credit loss allowance of $1,470 was recorded against the GrowCo Credit Facility.
(iii)Mucci Promissory Note
On June 28, 2019, the Company entered into a promissory note receivable agreement (the “Mucci Promissory Note”) for C$16,350 (approximately $12,823)$12,063) with Mucci. The outstanding principal amount of the Mucci Promissory Note bears interest at 3.95% annually and is due within 90 days of demand. The Company does not intend to demand the loan within 12 months. Interest accrued under the Mucci Promissory Note until July 1, 2021 is payable by way of capitalization on the principal amount and interest thereafter must be paid in cash on a quarterly basis. The Mucci Promissory Note is secured by a general security agreement covering all the assets of Mucci. Subsequent to December 31, 2020, the terms ofOn September 30, 2022, the Mucci Promissory Note werewas amended such thatand restated to increase the interest rate from 3.95% to the Canadian Prime Rate plus 1.25%, change the interest payments from quarterly to annual, and defer Mucci’s initial cash interest payment from September 30, 2022 to July 1, 2023.
Prior to July 1, 2022, interest accrued underon the Mucci Promissory Note untilwas capitalized as part of the principal balance. As of July 1, 2022, is payable by way of capitalization on the principal amountinterest was accrued and interest thereafter mustto be paid in cash beginning on a quarterly basis.
AsJuly 1, 2023. Prior to 2023, there were no repayments of December 31, 2020, a current expected credit loss allowance of $259 has been recorded againstprincipal or interest on the Mucci Promissory Note.
(iv)On April 1, 2020, Cronos Israel entered into the Cannasoul Collaboration. Cronos Israel has agreed to advance approximately ILS 8,297 (approximately $2,574) by a non-recourse loan to CLS over a period of two years for the capital and operating expenditures of the laboratory. The outstanding principal on the loan bears interest at 3.5% annually and will be repaid through the profits generated from the Cannasoul Collaboration. For the year ended December 31, 2020,2023, Mucci repaid C$563 ($425) in principal and C$1,187 ($897) in interest related to the Mucci Promissory Note.
Cannasoul Collaboration Loan
As of both December 31, 2023 and 2022, CLS has received ILS $4,1488,297 (approximately $1,287).$2,294 and $2,359, respectively), from the Cannasoul Collaboration Loan. See Note 4 “Investments” for further information regarding the Cannasoul Collaboration Loan.
Expected credit loss allowances on the Company’s long-term financial assets were comprised of the following items:
| | | | | | | | | | | | | | | | | | | | | | | |
| As of January 1, 2023 | | Increase (decrease)(i) | | Foreign exchange effect | | As of December 31, 2023 |
| GrowCo Credit Facility | $ | 12,455 | | | $ | (1,542) | | | $ | 263 | | | $ | 11,176 | |
| | | | | | | |
| Mucci Promissory Note | 89 | | | (2) | | | 2 | | | 89 | |
| Cannasoul Collaboration Loan | 522 | | | 16 | | | (14) | | | 524 | |
| $ | 13,066 | | | $ | (1,528) | | | $ | 251 | | | $ | 11,789 | |
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2023, 2022, and 2021
(In thousands of U.S. dollars, except for share amounts)
| | | | | | | | | | | | | | | | | | | | | | | |
| As of January 1, 2022 | | Increase (decrease)(i) | | Foreign exchange effect | | As of December 31, 2022 |
| GrowCo Credit Facility | $ | 14,089 | | | $ | (827) | | | $ | (807) | | | $ | 12,455 | |
| | | | | | | |
| Mucci Promissory Note | 90 | | | 4 | | | (5) | | | 89 | |
| Cannasoul Collaboration Loan | 415 | | | 161 | | | (54) | | | 522 | |
| $ | 14,594 | | | $ | (662) | | | $ | (866) | | | $ | 13,066 | |
(i)During the years ended December 31, 2023 and 2022, $(1,528) and $(662), respectively, were recorded to general and administrative expenses on the consolidated statements of net loss and comprehensive loss as a result of adjustments to our expected credit losses.
6. Property, plant and equipment, net
Property, plant and equipment, net consisted of the following:
| | | | | | | | | | | | | | |
| As of December 31, |
| | 2023 | | 2022 |
| Cost | | | | |
| Land | | $ | 2,764 | | | $ | 2,556 | |
| Building and leasehold improvements | | 168,498 | | | 177,095 | |
| Machinery and equipment | | 21,322 | | | 19,130 | |
| Furniture and fixtures | | 3,385 | | | 5,345 | |
| Construction in progress | | 3,269 | | | 841 | |
| Less: accumulated depreciation | | (30,707) | | | (35,347) | |
| Less: accumulated impairment charges | | (109,063) | | | (109,063) | |
| Property, plant and equipment, net | | $ | 59,468 | | | $ | 60,557 | |
For the years ended December 31, 2023, 2022 and 2021, depreciation expense on property, plant and equipment was $3,913, $8,667 and $11,668, respectively, and was included in cost of sales as well as depreciation and amortization in operating expenses on the consolidated statements of net loss and comprehensive loss.
Impairment of Long-lived Assets
During the third quarter of 2023, as a result of the Company’s decision to wind down the operations at Cronos Fermentation, the Company performed an assessment of the recovery of the carrying value of the Canada asset group and determined the carrying value of the asset group was recoverable.
During the first quarter of 2022, the Company recognized an impairment charge of $1,507 related to leasehold improvements and other office equipment that it planned to include in any potential sublease agreement of the Company’s corporate headquarters in Toronto, Ontario, Canada. The determination to seek a sublease of the property and include leasehold improvements and other office equipment in any potential sublease agreement triggered the impairment charges. The impairment charge was recognized as impairment loss on long-lived assets on the consolidated statements of net loss and comprehensive loss.
During the fourth quarter of 2021, the Company concluded that indicators of impairment were present with respect to its Canadian asset group in connection with the previously anticipated wind-down and closure of the Company’s facility in Stayner, Ontario. As a result, the Company compared the sum of the undiscounted future cash flows attributable to the Canadian asset group to their respective carrying amounts and recorded a non-cash impairment charge on long-lived assets of $113,917 as the difference between the carrying amount of the asset group and its estimated fair value for the year ended December 31, 2021, in the consolidated statements of net loss and comprehensive loss.
Held-for-sale Assessments
The Company evaluated both the Peace Naturals Campus and the Cronos Fermentation facility for held-for-sale classification as of December 31, 2020,2023, and, as a current expected credit loss allowanceresult of $25 has been recorded against the Cannasoul Collaboration loan.assessments, determined that neither asset group met the criteria to be classified as held-for-sale as of December 31, 2023. The Peace Naturals Campus failed the criteria, as the facility is not immediately available for sale in its present condition due to ongoing construction related to the condition the facility must be in for the proposed leaseback to occur. The Cronos Fermentation facility failed the criteria as it was determined it was not probable to sell the property within one year, and the property was not being marketed at a reasonable price in relation to its fair value, as the property is currently being marketed unpriced.
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020, 2019,2023, 2022, and 20182021
(In thousands of U.S. dollars, except for gram and share amounts)
7. Goodwill and Intangible Assets, net
9. Loans Payable
As of December 31, 2020, the Company did not have any loans payable. On January 23, 2019, the Company entered into a credit agreement with the Canadian Imperial Bank of Commerce, as administrative agent and lender, and the Bank of Montreal, as lender, in respect of a C$65,000 ($48,715) secured non-revolving term loan credit facility (the “Credit Facility”). The loan was guaranteed by the Company’s wholly owned Canadian subsidiaries and secured by substantially all present and after-acquired propertyGoodwill is comprised of the Company and its wholly owned Canadian subsidiaries. The Company used the funds available under the Credit Facility to fully repay the construction loan payable, consisting of C$21,311 ($15,971) in loan principal and C$275 ($206) in accrued interest and fees, calculated for the period from January 1, 2019 to January 22, 2019.following items:
On March 8, 2019, the Credit Facility was fully repaid. In connection with the Credit Facility, the Company incurred financing costs of C$523 ($395) which were expensed upon repayment of the Credit Facility. | | | | | | | | | | | | | | | | | |
| As of December 31, 2023 |
| Cost | | Accumulated impairment charges | | Net |
| Peace Naturals | $ | 1,057 | | | $ | — | | | $ | 1,057 | |
| $ | 1,057 | | | $ | — | | | $ | 1,057 | |
| | | | | | | | | | | | | | | | | |
| As of December 31, 2022 |
| Cost | | Accumulated impairment charges | | Net |
| Peace Naturals | $ | 1,033 | | | $ | — | | | $ | 1,033 | |
| $ | 1,033 | | | $ | — | | | $ | 1,033 | |
10. Property, Plant and Equipment
Property, plant and equipment, net consisted of the following:
| | | | | | | | | | | | | | |
| As of December 31, |
| | 2020 | | 2019 |
| Cost | | | | |
| Land | | $ | 3,197 | | | $ | 3,071 | |
| Building | | 158,586 | | | 149,690 | |
| Furniture and equipment | | 18,824 | | | 10,079 | |
| Computer equipment | | 710 | | | 526 | |
| Leasehold improvements | | 3,740 | | | 1,758 | |
| Construction in progress | | 20,837 | | | 3,569 | |
| Less: accumulated depreciation and amortization | | (18,295) | | | (8,745) | |
| Balance as of December 31 | | $ | 187,599 | | | $ | 159,948 | |
Depreciation expense included in costs of sales relating to manufacturing equipment and production facilities for the year ended December 31, 2020 was $3,447 (December 31, 2019 – $1,812; 2018 – $374). Depreciation expense included in operating expenses related to general office space and equipment for the year ended December 31, 2020 was $2,058 (December 31, 2019 – $1,444; 2018 – $261). The remaining depreciation is included in inventory.
For the year ended December 31, 2020, there was 0 capitalized interest included in construction in progress (2019 – $NaN; 2018 –$89).
11. Intangible Assets and Goodwill
The ongoing restrictions and closures experienced by retail stores in the U.S. as a result of the COVID-19 pandemic have negatively impacted sales and demand which has resulted in slower than expected revenue growth in the U.S. reporting unit. The Company expects the revenue growth and operating results in the U.S. reporting unit to continue to be negatively impacted as the decrease in customer demand and retail closures are expected to continue as a result of the pandemic. The Company performed an interim impairment test as of June 30, 2020 on the U.S. reporting unit, which holds the Redwood goodwill, as well as the indefinite-lived intangible asset (Lord Jones™ brand) to determine whether the carrying amount of the reporting unit and intangible asset exceeded their respective fair values. The Company reassessed its estimates and forecasts during the second quarter of 2020 to determine the fair values of the reporting unit and intangible asset. The fair values were determined using a discounted cash flow method on the reporting unit and the relief-from-royalty method on the Lord Jones™ brand. Significant inputs include discount rate, growth rates, and cash flow projections. Based on these valuations, the carrying value exceeded the fair value resulting in an impairment on both the reporting unit as well as the Lord Jones™ brand.
The Company recorded $35,000 of impairment charges on the U.S. reporting unit and $5,000 of impairment charges on the Lord Jones™ brand during year ended December 31, 2020.
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020, 2019, and 2018
(In thousands of U.S. dollars, except for gram and share amounts)
(a)(b)Intangible assets, net
Intangible assets, net are comprised of the following items:
| | Weighted average amortization period (in years) | | As of December 31, 2020 |
| Cost | | Accumulated amortization | | Impairment charges | | Net |
| | | As of December 31, 2023 | |
| | | | As of December 31, 2023 | | | | | As of December 31, 2023 |
| | Cost | | Accumulated amortization | | Accumulated impairment charges | | Net |
| Software | Software | 5 | | $ | 610 | | | $ | (291) | | | $ | — | | | $ | 319 | |
| ERP system | 5 | | 3,955 | | | (274) | | | — | | | 3,681 | |
| Health Canada licenses | Health Canada licenses | 17 | | 7,526 | | | (1,254) | | | — | | | 6,272 | |
Lord JonesTM brand | N/A | | 64,000 | | | — | | | (5,000) | | | 59,000 | |
| Ginkgo exclusive licenses | |
Israeli codes(i) | |
| Total definite-lived intangible assets | |
Lord Jones® brand | |
| Trademarks | Trademarks | N/A | | 148 | | | — | | | — | | | 148 | |
Israeli codes(i) | 25 | | 322 | | | (22) | | | — | | | 300 | |
| $ | 76,561 | | | $ | (1,841) | | | $ | (5,000) | | | $ | 69,720 | |
| Total intangible assets | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | As of December 31, 2022 |
| Cost | | Accumulated amortization | | Accumulated impairment charges | | Net |
| Software | | | $ | 6,037 | | | $ | (2,388) | | | $ | (76) | | | $ | 3,573 | |
| Health Canada licenses | | | 8,269 | | | (1,771) | | | (6,498) | | | — | |
| Ginkgo exclusive licenses | | | 27,676 | | | (1,854) | | | (4,434) | | | 21,388 | |
Israeli codes(i) | | | 292 | | | (49) | | | — | | | 243 | |
| Total definite-lived intangible assets | | | 42,274 | | | (6,062) | | | (11,008) | | | 25,204 | |
Lord Jones® brand | | | 64,000 | | | | | (62,500) | | | 1,500 | |
| Trademarks | | | 142 | | | | | (142) | | | — | |
| Total intangible assets | | | $ | 106,416 | | | $ | (6,062) | | | $ | (73,650) | | | $ | 26,704 | |
(i)The Israeli codes were transferred by non-controlling interests to Cronos Israel in exchange for their equity interests in the Cronos Israel entities specified above.entities.
| | | | | | | | | | | | | | | | | | | | | | | |
| Weighted average amortization period (in years) | | As of December 31, 2019 |
| Cost | | Accumulated amortization | | Net |
| Software | 5 | | $ | 541 | | | $ | (202) | | | $ | 339 | |
| Health Canada licenses | 17 | | 7,387 | | | (821) | | | 6,566 | |
Lord JonesTM brand | N/A | | 64,000 | | | — | | | 64,000 | |
| Trademarks | N/A | | 36 | | | — | | | 36 | |
Israeli codes(i) | 25 | | 298 | | | (4) | | | 294 | |
| | | $ | 72,262 | | | $ | (1,027) | | | $ | 71,235 | |
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2023, 2022, and 2021
(In thousands of U.S. dollars, except for share amounts)
Ginkgo Exclusive Licenses
CBGA
In August 2021, the Company achieved the final productivity target in respect of cannabigerolic acid (“CBGA”), under the Ginkgo Collaboration Agreement. As a result, on August 21, 2021, the Company issued 1,467,490 common shares at a share price of C$7.90 for total consideration given of C$11,593 ($9,042) to Ginkgo for the achievement of commercialization and productivity milestones for CBGA. The Israeli codes were transferred by non-controlling interestsestimated fair value of the exclusive license for CBGA (the “CBGA Exclusive License”) was $7,300 determined using a variation of the income approach called the relief-from-royalty method, which requires an estimate or forecast of the expected future cash flows. The definite-lived intangible asset is being amortized using the straight-line method over its estimated useful life of 10 years. The difference between the consideration paid to Cronos Israel in exchange for their equity interests inGinkgo and the Cronos Israel entities specified above.
The aggregate amortizationfair value of the exclusive license intangible asset of $1,784 was $814recognized as an impairment charge on the consolidated statements of net loss and comprehensive loss for the year ended December 31, 2020 (December 31, 2019 – $646; 2018 – $546)2021.
CBGVA
In November, 2021, the Company achieved the final productivity target in respect of cannabigerovarinic acid (“CBGVA”). IntangibleAs a result, on November 12, 2021, the Company issued 1,467,490 common shares at a share price of C$7.12 for total consideration given of C$10,449 ($8,150) to Ginkgo. The estimated fair value of the exclusive license for CBGVA (the “CBGVA Exclusive License”) was $5,300 determined using a variation of the income approach called the relief-from-royalty method, which requires an estimate or forecast of the expected future cash flows. The definite-lived intangible asset additions in 2020 primarily relateis being amortized using the straight-line method over its estimated useful life of 10 years. The difference between the consideration paid to Ginkgo and the implementationfair value of a new ERP system. Intangiblethe exclusive license intangible asset additions inof $3,008 was recognized as an impairment charge on the consolidated statements of net loss and comprehensive loss for the year ended December 31, 2019 included2021.
THCVA
In June 2022, the Lord Jones™ brandCompany achieved the final productivity target in respect of tetrahydrocannabivaric acid (“THCVA”) under the Ginkgo Collaboration Agreement. As a result, the Company issued 2,201,236 common shares at a share price of C$3.47, and made a cash payment of $600, for $64 million. There weretotal consideration of C$8,412 ($6,522) to Ginkgo. The definite-lived intangible asset is being amortized using the straight-line method over its estimated useful life of 10 years.
CBCA
In November 2022, the Company achieved the early commercialization milestone in respect of cannabichromenic acid (“CBCA”) under the Ginkgo Collaboration Agreement. As a result, the Company issued 489,163 common shares at a share price of C$4.11 for total consideration of C$2,010 ($1,473) to Ginkgo. The definite-lived intangible asset is being amortized using the straight-line method over its estimated useful life of 10 years.
CBCVA
In December 2022, the Company achieved the final productivity target in respect of cannabichromevarinic acid (“CBCVA”) under the Ginkgo Collaboration Agreement. As a result, the Company issued 1,467,490 common shares at a share price of C$3.44 for total consideration of C$5,048 ($3,724) to Ginkgo. In December 2023, the Company determined that it had no material disposalsimmediate plans to monetize CBCVA and, therefore, recognized $3,366 in impairment charges on the consolidated statements of intangible assets in 2020 or 2019. The amortization expensenet loss and comprehensive loss for the next fiveyear ended December 31, 2023, which resulted in a $nil carrying value of the intangible asset as of December 31, 2023.
For the years ended December 31, 2023, 2022 and 2021, the aggregate amortization expense on intangible assets was $3,514, $2,751 and $1,800, respectively, and was included in usedepreciation and amortization in operating expenses on the consolidated statements of net loss and comprehensive loss.
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2023, 2022, and 2021
(In thousands of U.S. dollars, except for share amounts)
The estimated future amortization of definite-lived intangible assets is estimated to be as follows: 2021 – $1,047; 2022 – $998; 2023: $964; 2024 – $952; 2025 – $664.
(b)Goodwill | | | | | | | | |
| | As of December 31, 2023 |
| 2024 | | $ | 3,365 | |
| 2025 | | 3,020 | |
| 2026 | | 2,498 | |
| 2027 | | 2,297 | |
| 2028 | | 2,156 | |
| Thereafter | | 6,242 | |
| | $ | 19,578 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As of December 31, 2019 | | Additions | | Impairment charges | | Effect of foreign exchange | | As of December 31, 2020 |
| Peace Naturals | $ | 1,078 | | | $ | 0 | | | $ | 0 | | | $ | 30 | | | $ | 1,108 | |
| Redwood | 213,414 | | | 0 | | | (35,000) | | | 0 | | | 178,414 | |
| $ | 214,492 | | | $ | 0 | | | $ | (35,000) | | | $ | 30 | | | $ | 179,522 | |
Accumulated impairment charges on intangible assets, net consist of:
| | | | | | | | | | | | | | | | | | | | | | | |
| As of January 1, 2023 | | Impairment charges | | Foreign exchange effect | | As of December 31, 2023 |
| Software | $ | (76) | | | $ | — | | | $ | (2) | | | $ | (78) | |
| Health Canada licenses | (6,498) | | | — | | | (152) | | | (6,650) | |
| Ginkgo exclusive licenses | (4,434) | | | (3,366) | | | (168) | | | (7,968) | |
Lord Jones® brand | (62,500) | | | — | | | — | | | (62,500) | |
| Trademarks | (142) | | | — | | | — | | | (142) | |
| $ | (73,650) | | | $ | (3,366) | | | $ | (322) | | | $ | (77,338) | |
| | | | | | | | | | | | | | | | | | | | | | | |
| As of January 1, 2022 | | Impairment charges | | Foreign exchange effect | | As of December 31, 2022 |
| Software | $ | (4) | | | $ | (76) | | | $ | 4 | | | $ | (76) | |
| Health Canada licenses | (6,910) | | | — | | | 412 | | | (6,498) | |
| Ginkgo exclusive licenses | (4,752) | | | — | | | 318 | | | (4,434) | |
| Lord Jones® brand | (62,500) | | | — | | | — | | | (62,500) | |
| Trademarks | (142) | | | — | | | — | | | (142) | |
| $ | (74,308) | | | $ | (76) | | | $ | 734 | | | $ | (73,650) | |
| | | | | | | | | | | | | | | | | | | | | | | |
| As of January 1, 2021 | | Impairment charges | | Foreign exchange effect | | As of December 31, 2021 |
| Software | $ | — | | | $ | (4) | | | $ | — | | | $ | (4) | |
| Health Canada licenses | (1,053) | | | (5,951) | | | 94 | | | (6,910) | |
| Ginkgo exclusive licenses | — | | | (4,792) | | | 40 | | | (4,752) | |
| Lord Jones® brand | (5,000) | | | (57,500) | | | — | | | (62,500) | |
| Trademarks | — | | | (142) | | | — | | | (142) | |
| $ | (6,053) | | | $ | (68,389) | | | $ | 134 | | | $ | (74,308) | |
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020, 2019,2023, 2022, and 20182021
(In thousands of U.S. dollars, except for gram and share amounts)
12.8. Leases
The Company has entered into leases primarily for the land-use rights, office premises and equipment used in the production of cannabis U.S. hemp and other related products. The Company’s leases have terms whichthat range from three years to six years, excluding land use rights, which generally extend to 15 years. These leases often include options to extend the term of the lease for up to 10 years. When it is reasonably certain that the option will be exercised, the impact of the option is included in the lease term for purposes of determining total future lease payments.
Operating leases greater than one year are included in right-of-use assets and operating lease liabilities. Finance leases are included in property, plant and equipment on the Company’s consolidated balance sheet.
The Company’s finance leases were not material for any of the periods presented.
| | As of December 31, |
| 2020 | | 2019 |
| As of December 31, | |
| As of December 31, | |
| As of December 31, | |
| 2023 | |
| Lease cost | |
| Lease cost | |
| Lease cost | Lease cost | | | | |
| Operating lease cost | Operating lease cost | | $ | 2,479 | | | $ | 760 | |
| Operating lease cost | |
| Operating lease cost | |
| Short-term lease cost | |
| Short-term lease cost | |
| Short-term lease cost | Short-term lease cost | | 60 | | | 373 | |
| Total lease cost | Total lease cost | | $ | 2,539 | | | $ | 1,133 | |
| Total lease cost | |
| Total lease cost | |
| | Weighted-average remaining lease term – operating leases | | 6 | | 5 |
| Supplemental cash flow and other information | |
| | Supplemental cash flow and other information | |
| | Supplemental cash flow and other information | |
| Operating cash flows - cash paid for operating lease obligations | |
| Operating cash flows - cash paid for operating lease obligations | |
| Operating cash flows - cash paid for operating lease obligations | |
| Non-cash activity - right-of-use assets obtained in exchange for lease obligations | |
| Non-cash activity - right-of-use assets obtained in exchange for lease obligations | |
| Non-cash activity - right-of-use assets obtained in exchange for lease obligations | |
| | Weighted-average remaining lease term (years) – operating leases | |
| | Weighted-average remaining lease term (years) – operating leases | |
| | Weighted-average remaining lease term (years) – operating leases | |
| Weighted-average discount rate – operating leases | Weighted-average discount rate – operating leases | | 8.86 | % | | 12.00 | % |
| | Weighted-average discount rate – operating leases | |
| Weighted-average discount rate – operating leases | |
13. GeneralFor the years ended December 31, 2023, 2022 and Administrative Expenses
General2021, the aggregate depreciation expense on right-of-use assets was $455, $839 and $1,386, respectively, and was included in general and administrative expense are comprisedexpenses on the consolidated statements of net loss and comprehensive loss.
During the following items:
| | | | | | | | | | | | | | | | | |
| Year ended December 31, |
| 2020 | | 2019 | | 2018 |
| Salaries and wages | $ | 26,742 | | | $ | 17,829 | | | $ | 4,240 | |
| Professional and consulting | 15,135 | | | 15,369 | | | 5,242 | |
| Office and general | 17,845 | | | 13,407 | | | 3,713 | |
Review costs related to restatement of 2019 interim financial statements(i) | 9,688 | | | 0 | | | 0 | |
| Current expected credit loss | 2,512 | | | 136 | | | 0 | |
| Other | 8,607 | | | 2,530 | | | 154 | |
| Total | $ | 80,529 | | | $ | 49,271 | | | $ | 13,349 | |
(i)These financial statement review costs include costsyear ended December 31, 2022, the Company recognized an impairment charge of $1,986 related to the restatementright-of-use lease asset associated with the Company’s corporate headquarters in Toronto, Ontario, Canada, for which the Company determined it would seek a sublease.
The following is a summary of the Company’s 2019 interim financial statements, costs relatedfuture minimum lease payments under operating leases for its premises due in future fiscal years:
| | | | | |
| As of December 31, 2023 |
| 2024 | $ | 1,132 | |
| 2025 | 1,006 | |
| 2026 | 247 | |
| 2027 | 95 | |
| 2028 | 95 | |
| Thereafter | 412 | |
| Total lease payments | 2,987 | |
| Less: imputed interest | (434) | |
| Present value of lease liabilities | $ | 2,553 | |
In addition to the Company’s responses to requests for information from various regulatory authorities relating to such restatement and legal costs defending shareholder class action complaints brought againstminimum lease payments, the Company as a resultis required to pay realty taxes and other occupancy costs in accordance with the terms of the restatement.
lease agreements.
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020, 2019,2023, 2022, and 20182021
(In thousands of U.S. dollars, except for gram and share amounts)
14.9. Derivative Liabilities
On March 8, 2019, the Company closed the previously announced investment in the Company (the “Altria Investment”) by Altria Group Inc. (“Altria”), pursuant to a subscription agreement dated December 7, 2018. As of the closing date of the Altria Investment, the Altria Investment consisted of 149,831,154 common shares of the Company refer to Note 15, issued to a wholly owned subsidiary of Altria and 1one warrant of the Company (the “Altria Warrant”), refer to Note 16(a), issued to a wholly owned subsidiary of Altria. As of the closing date of the Altria Investment, Altria beneficially held an approximate 45% ownership interest in the Company (calculated on a non-diluted basis). As summarizedOn December 16, 2022, Altria notified the Company that it was irrevocably relinquishing all of its rights, title and interest in this note, if exercised in full on such date, the exercise of the Altria Warrant, would have resulted ineffective immediately. As a result, the Company’s liability associated with the Altria holding a total ownership interest inWarrant was reduced to nil, with the Companychange recorded to gain on revaluation of approximately 55% (calculated on a non-diluted basis). derivative liabilities for the year ended December 31, 2022.
Pursuant to the investor rights agreement between the Company and Altria, entered into in connection with the closing of the Altria Investment (the “Investor Rights Agreement”), the Company granted Altria certain rights, among others, summarized in this note.
The summaries below are qualified entirely by the terms and conditions fully set out in the Investor Rights Agreement and the Altria Warrant, as applicable.
a.The Altria Warrant entitles the holder, subject to certain qualifications and limitations, to subscribe for and purchase up to an additional 10% of the common shares of Cronos (approximately 80.1 million common shares as of December 31, 2020) at a per share exercise price of C$19.00, which expires at 5:00 p.m. (Toronto time) on March 8, 2023. The number of common shares of the Company to which the holder is entitled, and the corresponding exercise price, is subject to adjustment in the event of a share dividend, share issuance, distribution, or share subdivision, split or other division, share consolidation, reverse-split or other aggregation, share reclassification, a capital reorganization, consolidation, amalgamation, arrangement, binding share exchange, merger or other combination, certain securities issuances, repurchases, redemptions or certain other actions that would result in a reduction in the number of common shares of the Company outstanding, in each case, executed by the Company. If and whenever there is a reclassification of the common shares or a capital reorganization of the Company, or a consolidation, amalgamation, arrangement, binding share exchange or merger of the Company, in each case executed by the Company and pursuant to which (i) in the event the consideration received by the Company’s shareholders is exclusively cash, the Company or the successor entity (as applicable) is required to purchase the Altria Warrant in cash equal to the amount by which the purchase price per share paid for the common shares acquired exceeds the exercise price of the Altria Warrant multiplied by the number of common shares that would have been issuable upon exercise of the Altria Warrant immediately prior to any such transaction, and (ii) in the event the consideration received by the Company’s shareholders is not exclusively cash, the Altria Warrant will remain outstanding in accordance with its terms until any subsequent exercise of the Altria Warrant, at which time the holder thereof will receive in lieu of each share that would have been issuable upon the exercise of the Altria Warrant immediately prior to any such transaction, the kind and amount of cash, the number of shares or other securities or property resulting from any such transaction that such holder would have been entitled to receive had such holder been the registered holder of such shares that would have been issuable upon the exercise of the Altria Warrant on the record date or effective date of the transaction (as applicable).
b.The Company granted to Altria, subject to certain qualifications and limitations, upon the occurrence of certain issuances of common shares of the Company executed by the Company (including issuances pursuant to the research and development (“R&D&D”) partnership with Ginkgo, (the “Ginkgo Agreement”), refer(refer to Note 20(b)10 “Commitments and Contingencies”), the right to purchase up to such number of common shares of the Company in order to maintain their ownership percentage of issued and outstanding common shares of the Company immediately preceding any issuance of shares by the Company (“Pre-emptive Rights”), at the same price per common share of the Company at which the common shares are sold in the relevant issuance; provided that if the consideration paid in connection with any such issuance is non-cash, the price per common share of the Company that would have been received had such common shares been issued for cash consideration will be determined by an independent committee (acting reasonably and in good faith); provided further that the price per common share of the Company to be paid by Altria pursuant to its exercise of its Pre-emptive Rights related to the Ginkgo Collaboration Agreement will be C$16.25 per common share. These rights may not be exercised if Altria’s ownership percentage of the issued and outstanding shares of the Company falls below 20%.
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020, 2019, and 2018
(In thousands of U.S. dollars, except for gram and share amounts)
c.b.In addition to (and without duplication of) the Pre-emptive Rights, the Company granted to Altria, subject to certain qualifications and limitations, the right to subscribe for common shares of the Company issuable in connection with the exercise, conversion or exchange of convertible securities of the Company issued prior to March 8, 2019 or thereafter (excluding any convertible securities of the Company owned by Altria or any of its subsidiaries), a share incentive plan of the Company, the exercise of any right granted by the Company pro rata to all shareholders of the Company to purchase additional common shares and/or securities of the Company, bona fide bank debt, equipment financing or non-equity interim financing transactions that contemplate an equity component or bona fide acquisitions (including acquisitions of assets or rights under a license or otherwise), mergers or similar business combination transactions or joint ventures involving the Company in order to maintain their ownership percentage of issued and outstanding common shares of the Company immediately preceding any such transactions (“Top-up Rights”).
The price per common share to be paid by Altria pursuant to the exercise of its Top-up Rights will be, subject to certain limited exceptions, the 10-day volume-weighted average price of the common shares of the Company on the TSX for the 10 full days preceding such exercise by Altria; provided that the price per common share of the Company to be paid by Altria pursuant to the exercise of its Top-up Rights in connection with the issuance of common shares of the Company pursuant to the exercise of options or warrants that were outstanding as of March 8, 2019 will be C$16.25 per common share without any set off, counterclaim, deduction, or withholding. These rights may not be exercised if Altria’s ownership percentage of the issued and outstanding shares of the Company falls below 20%.
The Altria Warrant, Pre-emptive Rights, and fixed price Top-up Rights have been classified as derivative liabilities; related transaction costsliabilities on the Company’s consolidated balance sheet.
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2023, 2022, and 2021
(In thousands of $22,355 have been expensed as financing costsU.S. dollars, except for share amounts)
As of December 31, 2023, Altria beneficially held 156,573,537 of the Company’s common shares, an approximate 41.1% ownership interest in 2019.the Company (calculated on a non-diluted basis).
Reconciliations of the carrying amounts of the derivative liability are presented below:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As of January 1, 2020 | | Gain on revaluation | | Exercise of Rights | | Effect of FX | | As of December 31, 2020 |
| (a) Altria Warrant | $ | 234,428 | | | $ | (95,045) | | | $ | 0 | | | $ | (525) | | | $ | 138,858 | |
| (b) Pre-emptive Rights | 12,787 | | | (885) | | | 0 | | | 193 | | | 12,095 | |
| (c) Top-up Rights | 49,945 | | | (33,324) | | | (3,227) | | | (937) | | | 12,457 | |
| $ | 297,160 | | | $ | (129,254) | | | $ | (3,227) | | | $ | (1,269) | | | $ | 163,410 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| As of January 1, 2023 | | Loss (gain) on revaluation | | | | Foreign exchange effect | | As of December 31, 2023 |
| | | | | | | | | |
| Pre-emptive Rights | — | | | 100 | | | | | 2 | | | 102 | |
| Top-up Rights | 15 | | | (15) | | | | | — | | | — | |
| $ | 15 | | | $ | 85 | | | | | $ | 2 | | | $ | 102 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As of March 8, 2019 | | Gain on revaluation | | Exercise of Rights | | Effect of FX | | As of December 31, 2019 |
| (a) Altria Warrant | $ | 1,086,920 | | | $ | (869,630) | | | $ | 0 | | | $ | 17,138 | | | $ | 234,428 | |
| (b) Pre-emptive Rights | 92,548 | | | (81,070) | | | 0 | | | 1,309 | | | 12,787 | |
| (c) Top-up Rights | 386,152 | | | (326,119) | | | (15,478) | | | 5,390 | | | 49,945 | |
| $ | 1,565,620 | | | $ | (1,276,819) | | | $ | (15,478) | | | $ | 23,837 | | | $ | 297,160 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| As of January 1, 2022 | | Gain on revaluation | | | | Foreign exchange effect | | As of December 31, 2022 |
| Altria Warrant | $ | 13,720 | | | $ | (13,431) | | | | | $ | (289) | | | $ | — | |
| Pre-emptive Rights | 180 | | | (179) | | | | | (1) | | | — | |
| Top-up Rights | 475 | | | (450) | | | | | (10) | | | 15 | |
| $ | 14,375 | | | $ | (14,060) | | | | | $ | (300) | | | $ | 15 | |
Fluctuations in the expected life of the derivative instruments and the Company’s share price are a primary driverdrivers for the changes in the derivative valuations during each reporting period. As the period of time the derivative liability is expected to be outstanding decreases and the share price decreases, the fair value typically decreases for each of the related derivative instruments, the liability of the instrument generally decreases. Shareinstrument. Weighted-average expected life and share price is oneare two of the significant observable inputs used in the fair value measurement of each of the Company’s derivative instruments. During the year ended December 31, 2020, the Company’s share price decreased from December 31, 2019 resulting in a gain on revaluation of $129,254.
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020, 2019, and 2018
(In thousands of U.S. dollars, except for gram and share amounts)
The fair values of the derivative liabilities were determined using the Black-Scholes pricing model as of December 31, 2020 and December 31, 2019, applyingusing the following inputs:
| | As of December 31, 2020 |
| Altria Warrant | | Pre-emptive Rights | | Top-up Rights |
| Share price at grant date (per share in C$) | $8.84 | | $8.84 | | $8.84 |
| | | As of December 31, 2023 | |
| | | As of December 31, 2023 | |
| | | As of December 31, 2023 | |
| | | Pre-emptive Rights | | | | | Pre-emptive Rights | | Top-up Rights |
| Share price at valuation date (per share in C$) | | Share price at valuation date (per share in C$) | | | $2.77 | | $2.77 |
| Subscription price (per share in C$) | Subscription price (per share in C$) | $19.00 | | $16.25 | | $16.25 | Subscription price (per share in C$) | | | $16.25 | | $16.25 |
Weighted average risk-free interest rate(i) | Weighted average risk-free interest rate(i) | 0.21% | | 0.17% | | 0.13% | Weighted average risk-free interest rate(i) | | | 3.99% | | 4.76% |
Weight average expected life (in years)(ii) | Weight average expected life (in years)(ii) | 2.18 | | 1.50 | | 0.98 | Weight average expected life (in years)(ii) | | | 1.75 | | 0.60 |
Expected annualized volatility(iii) | Expected annualized volatility(iii) | 81% | | 81% | | 81% | Expected annualized volatility(iii) | | | 60% | | 58% |
| Expected dividend yield | Expected dividend yield | 0% | | 0% | | 0% | Expected dividend yield | | | —% | | —% |
| | As of December 31, 2019 |
| Altria Warrant | | Pre-emptive Rights | | Top-up Rights |
| Share price at grant date (per share in C$) | $9.97 | | $9.97 | | $9.97 |
| As of December 31, 2022 | | | As of December 31, 2022 |
| Pre-emptive Rights | | | Pre-emptive Rights | | Top-up Rights |
| Share price at valuation date (per share in C$) | | Share price at valuation date (per share in C$) | $3.44 | | $3.44 |
| Subscription price (per share in C$) | Subscription price (per share in C$) | $19.00 | | $16.25 | | $16.25 | Subscription price (per share in C$) | $16.25 | | $16.25 |
Weighted average risk-free interest rate(i) | Weighted average risk-free interest rate(i) | 1.69% | | 1.73% | | 1.71% | Weighted average risk-free interest rate(i) | 4.14% | | 4.28% |
Weight average expected life (in years)(ii) | Weight average expected life (in years)(ii) | 3.18 | | 1.25 | | 1.66 | Weight average expected life (in years)(ii) | 0.25 | | 0.59 |
Expected annualized volatility(iii) | Expected annualized volatility(iii) | 82% | | 82% | | 82% | Expected annualized volatility(iii) | 73% | | 73% |
| Expected dividend yield | Expected dividend yield | 0% | | 0% | | 0% | Expected dividend yield | —% | | —% |
(i) The risk-free interest rate was based on Bank of Canada government treasury bills and bonds with a remaining term equal to the expected life of the derivative liabilities. TheAs of December 31, 2023 and December 31, 2022, the risk-free interest rate uses a range of approximately 0.10%3.99% to 0.39% as of December 31, 2020 (December 31, 2019 – 1.66%4.82% and 3.81% to 1.73%)4.37%, respectively, for the Pre-emptive Rights and Top-up Rights.
(ii) The expected life in years represents the period of time, in years, that the derivative liabilities are expected to be outstanding. The expected life of the Pre-emptive Rights and Top-up Rights is determined based on the expected term of the underlying options, warrants, and shares, to which the Pre-emptive Rights and Top-up Rights are linked. TheAs of December 31, 2023 and December 31, 2022, the expected life uses a range of approximately 0.50 years to 51.75 years as of December 31, 2020 (December 31, 2019 –and 0.25 years to 6 years).2.75 years, respectively.
(iii) Volatility was based on thean equally weighted blended historical and implied volatility levelslevel of the Company and peer companies.
The following table quantifies eachunderlying equity securities of the significant inputs described above and provides a sensitivity analysis of the impact on the reported values of the derivative liabilities. The sensitivity analysis for each significant input is performed by assuming a 10% decrease in the input while other significant inputs remain constant at management’s best estimate as of the respective dates. As of December 31, 2020, there would be an equal but opposite impact on net income (loss), refer to Note 15, and as of December 31, 2019, there would be an equal but opposite impact on share capital.
| | | | | | | | | | | | | | | | | |
| Decrease as of December 31, 2020 |
| Altria Warrant | | Pre-emptive Rights | | Top-up Rights |
| Share price at issuance date | $ | 25,819 | | | $ | 2,527 | | | $ | 2,989 | |
| Weighted average expected life | 13,541 | | | 1,988 | | | 2,121 | |
| Expected annualized volatility | 26,183 | | | 2,269 | | | 2,602 | |
| | | | | | | | | | | | | | | | | |
| Decrease as of December 31, 2019 |
| Altria Warrant | | Pre-emptive Rights | | Top-up Rights |
| Share price at issuance date | $ | 36,436 | | | $ | 2,743 | | | $ | 9,577 | |
| Weighted average expected life | 17,471 | | | 2,366 | | | 2,178 | |
| Expected annualized volatility | 33,343 | | | 2,180 | | | 7,714 | |
These inputs are classified in Level 3 on the fair value hierarchy and are subject to volatility and several uncontrollable factors, which could significantly affect the fair value of these derivative liabilities in future periods.
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020, 2019,2023, 2022, and 20182021
(In thousands of U.S. dollars, except for gram and share amounts)
10. Commitments and Contingencies
R&D commitments
15. Capital Stock
TheOn September 4, 2018, the Company is authorizedannounced an R&D partnership with Ginkgo to develop scalable and consistent production of a variety of certain common and lesser-known cannabinoids. As part of this partnership, Cronos agreed to issue an unlimited number of no par valueup to 14,674,903 common shares. The holdersshares of the commonCompany (aggregate value of approximately $100,000 as of July 17, 2018 assuming all milestones are met, collectively the “Ginkgo Equity Milestones”) in tranches and $22,000 in cash subject to Ginkgo’s achievement of certain milestones and to fund certain R&D expenses, including foundry access fees. During the years ended December 31, 2022 and December 31, 2021, 4,157,888 shares are entitled to receive dividends, which may be declared from time to time, and are entitled to 1 vote per share at shareholder meetings2,934,980 shares, respectively, of the Company. AllCompany’s common stock were issued in conjunction with this partnership. No shares are ranked equally with regards to the Company’s residual net assets.
The following is a summary of common shareswere issued other than in connection with outstanding options and warrants (see Note 16):
| | | | | | | | | | | | | | | | | |
| 2020 | | 2019 | | 2018 |
Altria Investment(i) | 0 | | | 149,831,154 | | | 0 | |
Redwood Acquisition(ii) | 0 | | | 5,086,586 | | | 0 | |
Private placement(iii) | 0 | | | 856,017 | | 0 | |
Bought deal offering(iv) | 0 | | | 0 | | | 15,677,143 | |
| 0 | | | 155,773,757 | | | 15,677,143 | |
(i)Duringfor the year ended December 31, 2023.
Other commitments
On February 18, 2019, the Company issued 149,831,154 common sharesentered into an agreement with a wholly owned subsidiary of Altria (which agreement was subsequently amended and restated to substitute Altria for aggregate gross proceeds of $1,809,556, net of issuance costs of $3,722. The gross proceeds were first allocatedPinnacle as a party thereto), to receive strategic advisory and project management services from Altria Pinnacle (the “Services Agreement”). Pursuant to the derivative liabilities issuedServices Agreement, the Company will pay Altria Pinnacle a monthly fee equal to the product of 105% and the sum of: (i) all costs directly associated with the services incurred during the monthly period, and (ii) a reasonable and appropriate allocation of indirect costs incurred during the monthly period. The Company will also pay all third-party direct charges incurred during the monthly period in connection with the Altria Investment,services, including any reasonable and documented costs, fees and expenses associated with obtaining any consent, license or permit. The Services Agreement will remain in effect until terminated by either party. See Note 15 “Related Party Transactions.”
(b)Contingencies
The Company is subject to various legal proceedings in the ordinary course of its business and in connection with its marketing, distribution and sale of its products. Many of these legal proceedings are in the early stages of litigation and seek damages that are unspecified or not quantified. Although the outcome of these matters cannot be predicted with certainty, the Company does not believe these legal proceedings, individually or in the aggregate, will have a material adverse effect on its consolidated financial condition but could be material to its results of operations for any particular reporting period depending, in part, on its results for that period.
(i)Class action complaints relating to restatement of 2019 interim financial statements
On March 11 and 12, 2020, two alleged shareholders of the Company separately filed two putative class action complaints in the U.S. District Court for the Eastern District of New York against the Company and its Chief Executive Officer and former Chief Financial Officer. The court consolidated the cases, and the residualconsolidated amended complaint alleges violations of $248,302 was allocated to share capital. Pursuant toSection 10(b) of the Altria Investment,Securities Exchange Act of 1934 (the “Exchange Act”) and Rule 10b-5, promulgated thereunder, against all defendants, and Section 20(a) of the Company incurred transaction costsExchange Act against the individual defendants. The consolidated amended complaint generally alleges that certain of $25,223, of which $3,642 was allocated to share capitalthe Company’s prior public statements about revenues and $21,581 to the derivative liabilitiesinternal controls were incorrect based on the relative fair values assignedCompany’s disclosures relating to the respective components. Refer to Note 14 for additional information.
(ii)During the year ended December 31, 2019, the Company issued 5,086,586 common shares with a fair value of $56,109 related to the acquisition (the “Redwood Acquisition”) of certain subsidiaries of Redwood Holding Group, LLC (collectively, “Redwood”). Refer to Note 27 for additional information.
(iii)During the year ended December 31, 2019, the Company issued 856,017 common shares to Kristen Bell, an accredited investor in a private placement (“Private Placement”) in reliance on Section 4(a)(2)Audit Committee of the Securities ActBoard of Directors’ review of the appropriateness of revenue recognized in connection with certain bulk resin purchases and sales of products through the use of certain publicity rights in brand development.wholesale channel. The common shares vest in 3 equal installmentsconsolidated amended complaint does not quantify a damage request. The defendants moved to dismiss on each of (a) January 31, 2020, (b) June 23, 2021,February 8, 2021. On November 17, 2023, the court entered an order granting the motion and (c)dismissed the case with prejudice. On December 23, 2022. The issuance did not involve a public offering and was made without general solicitation or advertising. The total fair value1, 2023, the shareholder plaintiffs sought reconsideration of the consideration paid fordismissal, requesting that the issuance of such common shares was approximately $6,000.court instead dismiss the action without prejudice and permit the plaintiffs to seek leave to further amend the complaint. The fair value of the shares was calculated using the 10-day volume weighted average price per share of the Company’s common shares on Nasdaq.
(iv)During the year ended December 31, 2018, the Company issued 15,677,143 common shares for aggregate gross proceeds of $115,510 through bought deal offerings, net of issuance costs of $7,577.
There were 0 share repurchases during the year ended December 31, 2020, 2019 or 2018.
16. Share-based Payments
(a)Warrants
The followingreconsideration motion is a summary of the changes in warrants during the year ended December 31, 2020 and December 31, 2019:
| | | | | | | | | | | |
| Weighted average exercise price (C$) | | Number of warrants |
| Balance as of January 1, 2020 | $ | 0.26 | | | 18,066,662 | |
| Exercise of warrants | 0.27 | | | (10,079,313) | |
| Balance as of December 31, 2020 | $ | 0.25 | | | 7,987,349 | |
| | | | | | | | | | | |
| Weighted average exercise price (C$) | | Number of warrants |
| Balance as of January 1, 2019 | $ | 0.26 | | | 25,457,623 | |
| Exercise of warrants | 0.26 | | | (7,390,961) | |
| | | |
| Balance as of December 31, 2019 | $ | 0.26 | | | 18,066,662 | |
For a description of the Altria Warrant, see Note 14. As of December 31, 2020, the Company had outstanding warrants as follows:
| | | | | | | | | | | | | | | | | | | | |
| Grant date | | Expiry date | | Weighted average exercise price (C$) | | Number of warrants |
| May 13 – 27, 2016 | | May 13 – 27, 2021 | | $ | 0.25 | | | 7,987,349 | |
| As of December 31, 2020 | | $ | 0.25 | | | 7,987,349 | |
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020, 2019,2023, 2022, and 20182021
(In thousands of U.S. dollars, except for gram and share amounts)
On June 3, 2020, an alleged shareholder filed a Statement of Claim, as amended on August 12, 2020, in the Ontario Superior Court of Justice in Toronto, Ontario, Canada, seeking, among other things, an order certifying the action as a class action on behalf of a putative class of shareholders and damages of an unspecified amount. The Amended Statement of Claim named (i) the Company, (ii) its Chief Executive Officer, (iii) former Chief Financial Officer, (iv) former Chief Financial Officer and Chief Commercial Officer, and (v) current and former members of the Board as defendants and alleged breaches of the Ontario Securities Act, oppression under the Ontario Business Corporations Act and common law misrepresentation. The Amended Statement of Claim generally alleged that certain of the Company’s prior public statements about revenues and internal controls were misrepresentations based on the Company’s March 2, 2020 disclosure that the Audit Committee of the Board of Directors was conducting a review of the appropriateness of revenue recognized in connection with certain bulk resin purchases and sales of products through the wholesale channel, and the Company’s subsequent restatement. The Amended Statement of Claim did not quantify a damage request. On June 28, 2021, the Court dismissed motions brought by the plaintiff for leave to commence a claim for misrepresentation under the Ontario Securities Act and for certification of the action as a class action. The plaintiff appealed the Court’s dismissal of the motions only with respect to the Company, the Chief Executive Officer, and the now former Chief Financial Officer; the remaining defendants were dismissed from the matter with prejudice and the Company and all individual defendants agreed not to seek costs from plaintiff in connection with the dismissal of the motions. On September 26, 2022, the Court of Appeal for Ontario reversed the Superior Court’s dismissal of the leave and certification motions, granted the plaintiff leave to proceed to bring a claim for misrepresentation under the Ontario Securities Act, and remitted the certification motion back to the Superior Court. On April 11, 2023, the plaintiff filed a Fresh as Amended Statement of Claim, which reflected the dismissal of the defendants for which an appeal was not sought, the removal of the claims for oppression under the Ontario Business Corporations Act and common law misrepresentation, as well as shortening the proposed class period. On October 10, 2023, the Superior Court certified the action on behalf of a class of persons or entities who acquired shares in the secondary market, including on the TSX and Nasdaq, during the period from May 9, 2019 to March 30, 2020, other than certain excluded persons.
(ii)Regulatory Settlements
On October 24, 2022, the Company announced regulatory settlements as follows:
SEC Settlement
On October 24, 2022, the SEC issued an Order Instituting Cease-and-Desist Proceedings Pursuant to Section 8(a) of the Securities Act of 1933 (the “Securities Act”) and Section 21(c) of the Exchange Act, Making Findings, and Imposing a Cease-and-Desist Order (the “Settlement Order”) resolving the Restatements.
The Company agreed to settle with the SEC, without admitting or denying the allegations described in the Settlement Order. The Settlement Order fully and finally disposed of the investigation of the Company by the SEC into the Restatements without the payment of any civil penalty or other amount.
The Settlement Order required the Company to cease and desist from committing or causing any violations and any future violations of Section 17(a) of the Securities Act, Sections 10(b), 13(a), 13(b)(2)(B) of the Exchange Act and Rules 10b-5, 13a-13, 13a-15(a), 13a-16 and 12b-20 thereunder.
As a result of the Settlement Order, the Company (i) lost its status as a well-known seasoned issuer for a period of three years, (ii) is unable to rely on the private offering exemptions provided by Regulations A and D under the Securities Act for a period of five years and (iii) is unable to rely on the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995 for a period of three years.
OSC Settlement
On October 24, 2022, the Ontario Capital Markets Tribunal approved a settlement agreement (the “Settlement Agreement”) between the Company and the staff of the Ontario Securities Commission (the “OSC”), resolving the Restatements.
Pursuant to the terms of the Settlement Agreement, which fully and finally disposed the investigation of the Company by the OSC, Cronos agreed to pay a total of C$1.34 million to fully settle the matter, and acknowledged that it had failed to comply with the requirement under Section 77 of the Ontario Securities Act to file interim financial reports in the manner set out therein and had acted in a manner contrary to the public interest.
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2023, 2022, and 2021
(In thousands of U.S. dollars, except for share amounts)
(iii)Litigation and regulatory inquiries relating to marketing, distribution, import and sale of products
On April 17, 2023, a group of plaintiffs led by the Green Leaf (Ale Yarok) political party filed a Statement of Claim and Request for Approval of a Class Action on behalf of a purported class of Israeli cannabis consumers in the District Court of Tel Aviv, Israel, against 26 cannabis-related parties, including three Cronos Israel entities. The Statement of Claim alleges that the defendants violated certain laws relating to the marketing of medical cannabis products, including marketing to unlicensed cannabis consumers. The lawsuit seeks a total of ILS 420 million. The Cronos Israel defendants moved to dismiss the action on August 13, 2023.
On January 18, 2024, the Company was notified that the Trade Levies Commissioner of the Israel Ministry of Economy and Industry initiated a public investigation of alleged dumping of medical cannabis imports from Canada into Israel. The Company is responding to requests for information from the Ministry. The Company cannot predict the outcome of the investigation.
We expect litigation and regulatory proceedings relating to the marketing, distribution, import and sale of our products to increase.
11. Share-based Compensation
(b)(a)Stock options
(i)Stock optionShare-based award plans
The Company adopted an amendedhas granted stock options, RSUs and restated stock option planDSUs to employees and non-employee directors under the Stock Option Plan dated May 26, 2015 (the “2015 Stock Option Plan”), the 2018 Stock Option Plan dated June 28, 2018 (the “2018 Stock Option Plan” and, together with the 2015 Stock Option Plan, the “Prior Option Plans”), the Employment Inducement Award Plan #1 (the “Employment Inducement Award Plan”), the 2020 Omnibus Equity Incentive Plan dated March 29, 2020 (the “2020 Omnibus Plan”) and the DSU plan dated August 10, 2019 (the “DSU Plan”). The Company can no longer make grants under the Prior Option Plans or the Employment Inducement Award Plan.
The following table summarizes the total share-based compensation associated with the Company’s stock options and RSUs:
| | | | | | | | | | | | | | | | | |
| Year ended December 31, |
| 2023 | | 2022 | | 2021 |
| Stock options | $ | 1,175 | | | $ | 6,778 | | | $ | 7,604 | |
| RSUs | 7,581 | | | 7,633 | | | 2,240 | |
Liability-classified awards(i) | — | | | 597 | | | — | |
| Total share-based compensation | $ | 8,756 | | | $ | 15,008 | | | $ | 9,844 | |
(i)Represents share-based compensation awards conditionally approved for grant to one of the Company’s former executives for a fixed monetary value, but a variable number of shares. These awards were liability-classified until the number of shares was determined.
(b)Stock options
The Company adopted the 2015 Stock Option Plan, which was approved by shareholders of the Company at the annual general meeting of shareholders held on June 28, 2017. The 2015 Stock Option Plan allowed the Company’s Board of Directors (the “Board”) to award options to purchase shares to directors, officers, key employees and service providers of the Company. As of June 28, 2018, 0no further awards will be granted under the 2015 Stock Option Plan; however, shares may be purchased via option exercise by the holdersas of any outstandingDecember 31, 2023, no options previously issued under the 2015 Stock Option Plan.Plan remain outstanding.
On June 28, 2018, the shareholders of the Company approved a stock option plan (the “2018the 2018 Stock Option Plan”),Plan, which replaced the 2015 Stock Option Plan. The 2018 Stock Option Plan terminated the Company’s ability to grant equity under the 2015 Stock Option Plan. As of June 25, 2020, the date on which the 2020 Omnibus Plan (as defined below) was approved by the shareholders of the Company, 0no further awards will be granted under the 2018 Stock Option Plan; however, shares may be purchased via option exercise by the holders of any outstanding options previously issued under the 2018 Stock Option Plan.
On March 29, 2020, the Board adopted a new omnibus equity incentive plan (the “2020the 2020 Omnibus Plan”),Plan, which was approved by the shareholders of the Company at the annual and special meeting of shareholders held on June 25, 2020. The 2020 Omnibus Plan provides for grants of stock options, share appreciation rights, restricted shares, restricted share units (“RSUs”)RSUs and other share-based or cash-based awards, which are subject to terms as determined by the Compensation Committee of the Board (the “Compensation Committee”), and awards may be granted to eligible employees, non-employee directors and consultants. The 2020 Omnibus Plan terminated the Company’s ability to grant equity awards under the 2018 Stock Option Plan and restricted stock unitsRSUs under the Employment Inducement Award Plan.
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2023, 2022, and 2021
(In thousands of U.S. dollars, except for share amounts)
Options represent the right to purchase Company common shares on the date of exercise at a stated exercise price. The exercise price of an option generally must be at least equal to the fair market value of the Company common shares on the date of grant. The Compensation Committee of the Board (“Compensation Committee”) may provide for options to be exercised only as they vest or to be immediately exercisable with any shares issued on exercise being subject to the Company’s right of repurchase that lapses as the shares vest. Vesting conditions for grants of options are determined by the Compensation Committee. The typical vesting for stock option grants is quarterly vesting over three to five years. The maximum term of options granted under the 2020 Omnibus Plan is seven years. Participants under the 2020 Omnibus Plan are eligible to be granted options to purchase shares at an exercise price established upon approval of the grant by the Compensation Committee. When options are granted, the exercise price is, with respect to a particular date, the closing price as reported by the TSX or the Nasdaq and, if the shares are not traded on the TSX or the Nasdaq, any other stock exchange on which the Company’s common shares are traded (as selected by the Compensation Committee in good faith taking into account applicable legal and tax requirements) on the immediately preceding trading day (the “Fair Market Value”). The 2020 Omnibus Plan does not authorize grants of options with an exercise price below the Fair Market Value.
The equity plans described above have the following stock options outstanding:
| | | | | | | | | | | | | | | | | |
| Shares outstanding as of December 31, |
| 2020 | | 2019 | | 2018 |
| 2020 Omnibus Plan | 2,000,000 | | | 0 | | | 0 | |
| 2018 Stock Option Plan | 1,627,715 | | 1,817,287 | | 285,000 | |
| 2015 Stock Option Plan | 10,127,433 | | | 12,332,215 | | | 12,617,995 | |
| Total stock options outstanding | 13,755,148 | | 14,149,502 | | 12,902,995 | |
For the year ended December 31, 2020, the total stock-based compensation expense associated with the equity plans was $7,185 (December 31, 2019 – $10,278; December 31, 2018 - $8,151).
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020, 2019, and 2018
(In thousands of U.S. dollars, except for gram and share amounts)
(ii) Summary of changes
The following is a summary of the changes in options during the year ended December 31, 2020 and December 31, 2019:options:
| | | | | | | | | | | | | | | | | |
| Weighted average exercise price (C$) | | Number of options | | Weighted average remaining contractual term (years) |
| Balance as of January 1, 2020 | $ | 4.84 | | | 14,149,502 | | | 2.56 |
Issuance of options(i) | 6.96 | | | 2,000,000 | | | |
| Exercise of options | 2.03 | | | (2,131,939) | | | |
| Cancellation, forfeiture and expiry of options | 14.34 | | | (262,415) | | | |
| Balance as of December 31, 2020 | $ | 5.40 | | | 13,755,148 | | | 2.30 |
| Exercisable at December 31, 2020 | $ | 3.75 | | | 9,643,682 | | | 1.34 |
| | | | | | | | | | | | | | | | | |
| Weighted average exercise price (C$)(i) | | Number of options | | Weighted average remaining contractual term (years) |
| Balance as of January 1, 2023 | $ | 10.57 | | | 5,350,600 | | | 0.73 |
| Issuance of options | 2.96 | | | 188,317 | | | |
| | | | | |
| Cancellation, forfeiture and expiry of options | 7.75 | | | (3,435,716) | | | |
| Balance as of December 31, 2023 | $ | 14.50 | | | 2,103,201 | | | 1.84 |
| Exercisable as of December 31, 2023 | $ | 15.89 | | | 1,864,732 | | | 1.29 |
| | | | | | | | | | | | | | | | | |
| Weighted average exercise price (C$)(i) | | Number of options | | Weighted average remaining contractual term (years) |
| Balance as of January 1, 2022 | $ | 7.75 | | | 8,939,330 | | | 2.70 |
| Issuance of options | 4.20 | | | 113,947 | | | |
| Exercise of options | 2.79 | | | (2,735,985) | | | |
| Cancellation, forfeiture and expiry of options | 5.74 | | | (966,692) | | | |
| Balance as of December 31, 2022 | $ | 10.57 | | | 5,350,600 | | | 0.73 |
| Exercisable as of December 31, 2022 | $ | 11.48 | | | 3,867,887 | | | 0.66 |
(i)The weighted average exercise price reflects the conversion of foreign currency-denominated stock options attranslated into C$ using the average foreign exchange rates as of December 31, 2020. For foreign currency-denominated options, the weighted average exercise prices are translated using exchange ratesrate as of the settlement date.date of issuance.
| | | | | | | | | | | | | | | | | |
| Weighted average exercise price (C$) | | Number of options | | Weighted average remaining contractual term (years) |
| Balance as of January 1, 2019 | $ | 2.99 | | | 12,902,995 | | | 3.35 |
| Issuance of options | 20.08 | | | 1,534,162 | | | |
| Exercise of options | 3.48 | | | (282,572) | | | |
| Cancellation, forfeiture and expiry of options | 2.27 | | | (5,083) | | | |
| Balance as of December 31, 2019 | $ | 4.84 | | | 14,149,502 | | | 2.56 |
| Exercisable at December 31, 2019 | $ | 2.93 | | | 9,034,714 | | | 2.27 |
(iii)FairFor the years ended December 31, 2023 and 2022, the fair value of options issued
per option at grant date was C$2.07 and C$2.94, respectively. The fair value of the options issued during the year was determined using the Black-Scholes option pricing model, using the following inputs:
| | 2020 | | 2019 |
| 2023 | | | 2023 | | 2022 |
| Share price at grant date (per share) | Share price at grant date (per share) | C$6.96 | | C$15.34 – $24.75 | Share price at grant date (per share) | C$2.96 | | C$4.20 |
Exercise price (per option)(i) | C$6.96 | | C$15.34 – $24.75 |
| Risk-free interest rate | 0.43% | | 1.39% – 1.62% |
Expected life of options (in years)(ii) | 5 | | 5 |
| Expected annualized volatility | 91% | | 82% |
| Exercise price (per option) | | Exercise price (per option) | C$2.96 | | C$4.20 |
Risk-free interest rate(i) | | Risk-free interest rate(i) | 3.22% | | 3.14% |
Expected life of options (in years)(i) | | Expected life of options (in years)(i) | 7 | | 7 |
Expected annualized volatility(i) | | Expected annualized volatility(i) | 73% | | 73% |
| Expected dividend yield | Expected dividend yield | 0 | | 0 | Expected dividend yield | — | | — |
| Weighted average Black-Scholes value at grant date (per option) | Weighted average Black-Scholes value at grant date (per option) | C$4.84 | | C$13.03 | Weighted average Black-Scholes value at grant date (per option) | C$2.07 | | C$2.94 |
| Forfeiture rate | Forfeiture rate | 0 | | 0 | Forfeiture rate | — | | — |
(i)The weighted average exercise price reflects the conversion of foreign currency-denominated options at the exchange rates as of December 31, 2020. For foreign currency-denominated options, the weighted average exercise prices are translated using exchange rates as of the settlement date.
(ii)The expected life of the awards represents the period of time options are expected to be outstanding and is estimated considering vesting terms and employees’ and non-employees’ historical exercise and, where relevant, post-vesting employment termination behavior. Volatility was estimated by using the historical volatility of the Company’s share price, adjusted for the Company’s expectation of volatility going forward. The risk-free interest rate was based on the Bank of Canada government bonds with a remaining term equal to the expected life of the options at the grant date.
The weighted average fair value per share at grant date of
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the options during the yearyears ended December 31, 2020 was C$4.84 per2023, 2022, and 2021
(In thousands of U.S. dollars, except for share (December 31, 2019 – C$13.03 per share).amounts)
The following table summarizes stock options outstanding:
| | | | | | | | | | | | | | | | | |
| Options outstanding as of December 31, |
| 2023 | | 2022 | | 2021 |
| 2020 Omnibus Plan | 702,264 | | | 2,788,947 | | | 2,900,000 | |
| 2018 Stock Option Plan | 1,400,937 | | 1,422,069 | | 1,550,074 | |
| 2015 Stock Option Plan | — | | | 1,139,584 | | | 4,489,256 | |
| Total stock options outstanding | 2,103,201 | | 5,350,600 | | 8,939,330 | |
(c)Restricted share units
RSUs are granted under the 2020 Omnibus Plan. RSUs represent an equivalent amount of Company common shares on the date of issuance at fair value. Fair value is determined using the closing price of the trading day immediately preceding the date of grant. RSUs issued under the 2020 Omnibus Plan typically vest over a three-year period following the grant date and have no performance requirementsrequirements.
The following is a summary of the changes in RSUs:
| | | | | | | | | | | |
| Weighted average grant date fair value (C$)(iii) | | Number of RSUs |
| Balance as of January 1, 2023 | $ | 4.63 | | | 5,725,470 | |
Granted(i) | 2.64 | | | 3,292,586 | |
| Vested and issued | 5.00 | | | (1,041,097) | |
| Cancellation and forfeitures | 3.60 | | | (595,418) | |
| Balance as of December 31, 2023 | $ | 3.77 | | | 7,381,541 | |
| | | | | | | | | | | |
| Weighted average grant date fair value (C$)(iii) | | Number of RSUs |
| Balance as of January 1, 2022 | $ | 9.22 | | | 1,225,870 | |
Granted(i)(ii) | 4.27 | | | 6,140,492 | |
| Vested and issued | 7.40 | | | (1,151,292) | |
| Cancellation and forfeitures | 5.08 | | | (489,600) | |
| Balance as of December 31, 2022 | $ | 4.63 | | | 5,725,470 | |
(i)RSUs granted in the period vest annually in equal installments over a three-year period from either the grant date or after a three or five year “cliff-period.” All RSUs are subject to such holder’s continued employment through each vesting date. The vesting of such RSUs is not subject to the achievement of any performance criteria.
(ii)Equity grants for 2020, 2021 and no forfeiture rate.2022 were held back for certain executives of the Company in connection with ongoing investigations by the SEC and the OSC, which were subsequently settled on October 24, 2022. On August 5, 2022, the Compensation Committee approved the release of these held-back equity grants conditioned upon settlement of the SEC and OSC investigations. These RSUs vest in equal installments over a period of three years from what would have been their original grant dates had the grants not been withheld.
(iii)The weighted-average grant date fair value reflects the conversion of foreign currency-denominated RSUs translated into C$ using the foreign exchange rate as of the date of issuance.
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020, 2019,2023, 2022, and 20182021
(In thousands of U.S. dollars, except for gram and share amounts)
On July 20, 2020, the Company entered into separation agreements with Robert Rosenheck and another Redwood employee pursuant to which they resigned from their employment with Redwood. In connection with such separation agreements, 732,972 outstanding and unvested RSUs were accelerated and vested during the year ended December 31, 2020.
For the year ended December 31, 2020, the Company recorded $8,176 (December 31, 2019 – $889) in share-based compensation expense related to these RSUs. NaN RSUs were granted in 2018.
The following is a summary of the changes in RSUs:
| | | | | | | | | | | |
| Number of RSUs | | Weighted average grant date fair value (C$) |
| Balance at January 1, 2020 | 732,972 | | | $ | 15.34 | |
| Granted | 957,854 | | | 7.66 | |
| Exercised | (732,972) | | | 15.34 | |
| Cancellation and forfeitures | (9,497) | | | 7.52 | |
| Balance at December 31, 2020 | 948,357 | | | $ | 7.66 | |
| | | | | | | | | | | |
| Number of RSUs | | Weighted average grant date fair value (C$) |
| Balance at January 1, 2019 | 0 | | | $ | 0 | |
| Granted | 732,972 | | | 15.34 | |
| Balance at December 31, 2019 | 732,972 | | | $ | 15.34 | |
(d)Deferred share units
On August 10, 2019, the Company established a cash-settled deferred share unit plan (“the DSU Plan”)Plan pursuant to which its non-executive directors receive deferred share units (“DSUs”).DSUs for Board services. The DSU Plan is designed to promote a greater alignment of long-term interests between non-executive directors and shareholders. The number of DSUs granted under the DSU Plan (including fractional DSUs) is determined by dividing the amount of remuneration payable by the closing price as reported by the TSX for awards made prior to 2022 and as reported by Nasdaq for awards made in 2022 and 2023 on the trading day immediately preceding the date of grant. DSUs are payable at the time a non-executive director ceases to hold the office of director for any reason and are settled by a lump-sum cash payment, in accordance with the terms of the DSU Plan, based on the fair value of the DSUs at such time. The fair value of the cash payout is determined by multiplying the number of DSUs vested at the payout date by the closing price as reported by the TSX for awards made prior to 2022 and as reported by Nasdaq for awards made in 2022 and 2023 on the trading day immediately preceding the payout date. The fair value of the cash payout is determined at each reporting date based on the fair value of the Company’s common shares at the reporting date and is recorded within other liabilities. NaN DSUs were granted during 2018.
The following is a summary of the changes in DSUs:
| | | | | | | | | | | |
| Number of DSUs | | Financial liability |
| Balance at January 1, 2020 | 33,397 | | | $ | 255 | |
| Granting and vesting of DSUs | 58,380 | | | 338 | |
| Liabilities settled | (8,484) | | | (46) | |
| Loss (gain) on revaluation | — | | | 30 | |
| Balance at December 31, 2020 | 83,293 | | | $ | 577 | |
| | | | | | | | | | | |
| Financial liability | | Number of DSUs |
| Balance as of January 1, 2023 | $ | 674 | | | 265,732 | |
| Granting and vesting of DSUs | 450 | | | 255,947 | |
| | | |
| Gain on revaluation | (32) | | | — | |
| Balance as of December 31, 2023 | $ | 1,092 | | | 521,679 | |
| | | | | | | | | | | |
| Financial liability | | Number of DSUs |
| Balance as of January 1, 2022 | $ | 408 | | | 104,442 | |
| Granting and vesting of DSUs | 443 | | | 161,290 | |
| | | |
| Loss on revaluation | (177) | | | — | |
| Balance as of December 31, 2022 | $ | 674 | | | 265,732 | |
| | | | | | | | | | | |
| Number of DSUs | | Financial liability |
| Balance at January 1, 2019 | 0 | | | $ | 0 | |
| Granting and vesting of DSUs | 33,397 | | | 452 | |
| Loss (gain) on revaluation | — | | | (197) | |
| Balance at December 31, 2019 | 33,397 | | | $ | 255 | |
Cronos Group Inc.
Notes to Consolidated Financial Statements
ForDuring the yearsyear ended December 31, 2020, 2019, and 2018
(In thousands2022, the Compensation Committee conditionally approved the grant to one of U.S. dollars, exceptthe Company’s former executives of share-based compensation awards for gram and share amounts)
17. Earnings (loss) Per Share
Basic and diluted earnings (loss) per share from continuing operations are calculated using common shares. These awards were liability-classified until the following numerators and denominators:
| | | | | | | | | | | | | | | | | | | | |
| | 2020 | | 2019 | | 2018 |
| Basic earnings (loss) per share computation | | | | | | |
| Income (loss) from continuing operations attributable to common shareholders of Cronos Group | | $ | (72,487) | | | $ | 1,166,869 | | | $ | (20,742) | |
| Weighted average number of common shares outstanding | | 351,576,848 | | | 310,067,179 | | | 172,269,170 | |
| Basic earnings (loss) per share | | $ | (0.21) | | | $ | 3.76 | | | $ | (0.12) | |
| Diluted earnings (loss) per share computation | | | | | | |
| Income (loss) from continuing operations used in the computation of basic earnings (loss) per share | | $ | (72,487) | | | $ | 1,166,869 | | | $ | (20,742) | |
| Adjustment for gain on revaluation of derivative liabilities | | 0 | | | (24,416) | | | 0 | |
| Income (loss) from continuing operations used in the computation of diluted income (loss) per share | | (72,487) | | | 1,142,453 | | | (20,742) | |
| | | | | | |
| Weighted average number of common shares outstanding used in the computation of basic earnings (loss) per share | | 351,576,848 | | | 310,067,179 | | | 172,269,170 | |
| Dilutive effect of warrants | | 0 | | | 19,481,352 | | | 0 | |
| Dilutive effect of stock options | | 0 | | | 10,649,487 | | | 0 | |
| Dilutive effect of restricted share units | | 0 | | | 732,972 | | | 0 | |
| Dilutive effect of Altria Warrant | | 0 | | | 0 | | | 0 | |
| Dilutive effect of Top-up Rights – exercised and exercisable fixed price | | 0 | | | 1,881,002 | | | 0 | |
| Weighted average number of common shares for computation of diluted income (loss) per share | | 351,576,848 | | | 342,811,992 | | | 172,269,170 | |
Diluted earnings (loss) per share(i) | | $ | (0.21) | | | $ | 3.33 | | | $ | (0.12) | |
(i)In computing diluted earnings per share, incremental number of common shares are not considered in periods in which a net loss is reported,was determined.
| | | | | |
| Financial liability |
| Balance as of January 1, 2022 | $ | — | |
| Grants | 597 | |
| Transfers to equity awards | (597) | |
| |
| Balance as of December 31, 2022 | $ | — | |
During the inclusion of the common share equivalents would be anti-dilutive.
Basic and diluted loss per share from discontinued operations are calculated using the following numerators and denominators:
| | | | | | | | | | | | | | | | | | | | |
| | 2020 | | 2019 | | 2018 |
| Basic loss from discontinued operations per share computation | | | | | | |
| Loss from discontinued operations attributable to common shareholders of Cronos Group | | $ | (650) | | | $ | (363) | | | $ | (894) | |
| Weighted average number of common shares outstanding | | 351,576,848 | | 310,067,179 | | | 172,269,170 | |
Basic loss from discontinued operations per share(i) | | $ | 0.00 | | | $ | 0.00 | | | $ | (0.01) | |
(i)In computing diluted earnings per share, incremental common shares are not considered in periods in which a net loss is reported, as the inclusion of the common share equivalents would be anti-dilutive.
The following securities were not included in the computation of diluted shares outstanding because the effect would be anti-dilutive or because conditions for contingently issuable shares were not satisfied at the end of the reporting periods.
| | | | | | | | | | | | | | | | | | | | |
| | 2020 | | 2019 | | 2018 |
| Ginkgo Equity Milestones | | 14,674,904 | | | 14,674,904 | | | 0 | |
| Pre-emptive Rights | | 11,534,475 | | | 12,006,740 | | | 0 | |
| Top-up Rights – fixed price | | 17,214,621 | | | 25,103,456 | | | 0 | |
| Top-up Rights – market price | | 3,413,065 | | | 1,255,223 | | | 0 | |
| Altria Warrant | | 80,056,296 | | | 77,514,993 | | | 0 | |
| Warrants | | 15,147,116 | | | 0 | | | 25,457,623 | |
| Stock options | | 9,218,674 | | | 1,315,787 | | | 12,902,995 | |
| Restricted share units | | 79,611 | | | 0 | | | 0 | |
| Total anti-dilutive securities | | 151,338,762 | | | 131,871,103 | | | 38,360,618 | |
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the yearsyear ended December 31, 2020, 2019, and 2018
(In thousands of U.S. dollars, except for gram and share amounts)
18. Accumulated Other Comprehensive Income (Loss)
The following is a continuity schedule of accumulated other comprehensive income (loss):
| | | | | | | | | | | | | | | | | |
| 2020 | | 2019 | | 2018 |
| Net unrealized gain (loss) on revaluation and disposal of other investments | | | | | |
| Balance at January 1 | $ | 5 | | | $ | 5 | | | $ | 446 | |
| Cumulative effect from adoption of ASU 2016-01 | 0 | | | 0 | | | (444) | |
| Net unrealized (loss) gain | 0 | | | 0 | | | 3 | |
| | | | | |
| Balance at December 31 | 5 | | | 5 | | | 5 | |
| | | | | |
| Net foreign exchange gain (loss) on translation of foreign operations | | | | | |
| Balance at January 1 | 27,833 | | | (9,875) | | | 2,456 | |
| Net unrealized (loss) gain | 15,161 | | | 37,708 | | | (12,331) | |
| Balance at December 31 | 42,994 | | | 27,833 | | | (9,875) | |
| Total other comprehensive income (loss) | $ | 42,999 | | | $ | 27,838 | | | $ | (9,870) | |
19.12. Income Taxes
For financial reporting purposes, income (loss)loss from continuing operations before income taxes includes the following components:
| | | | | | | | | | | | | | | | | | | | |
| | Year ended December 31, |
| | 2020 | | 2019 | | 2018 |
| Rest of World | | $ | 12,679 | | | $ | 1,169,007 | | | $ | (20,923) | |
| United States | | (85,952) | | | (3,070) | | | 0 | |
| Total | | $ | (73,273) | | | $ | 1,165,937 | | | $ | (20,923) | |
| | | | | | | | | | | | | | | | | | | | |
| | Year ended December 31, |
| | 2023 | | 2022 | | 2021 |
| Loss before income taxes | | $ | (73,669) | | | $ | (121,003) | | | $ | (128,510) | |
The loss before income taxes above excludes losses from discontinued operations of $650$4,114, $13,556 and $269,125 for the yearyears ended December 31, 2020 (December 31, 2019 – $363; December 31, 2018 – $894).
Income tax expense consists of the following components:
| | | | | | | | | | | | | | | | | | | | |
| | Year ended December 31, |
| | 2020 | | 2019 | | 2018 |
| Current: | | | | | | |
| Rest of World | | $ | 1,024 | | | $ | 0 | | | $ | 0 | |
| United States | | 323 | | | 0 | | | 0 | |
| Total | | $ | 1,347 | | | $ | 0 | | | $ | 0 | |
| Deferred: | | | | | | |
| Rest of world | | $ | 0 | | | $ | 0 | | | $ | 0 | |
| United States | | 0 | | | 0 | | | 0 | |
| Total | | $ | 0 | | | $ | 0 | | | $ | 0 | |
Included in accounts payable2023, 2022 and other liabilities as of December 31, 2020 is $865 (December 31, 2019 – $NaN; December 31, 2018 – $NaN) related to current income tax expense above.2021, respectively.
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020, 2019,2023, 2022, and 20182021
(In thousands of U.S. dollars, except for gram and share amounts)
| | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| | 2023 | | 2022 | | 2021 |
| Current | | $ | (3,375) | | | $ | 34,416 | | | $ | (471) | |
| Deferred | | 145 | | | (241) | | | 40 | |
| Total | | $ | (3,230) | | | $ | 34,175 | | | $ | (431) | |
As of December 31, 2023, 2022 and 2021, the Company’s current income taxes payable were $64, $32,956 and $105, respectively, related to current income tax expense. Included in other receivables as of December 31, 2023, 2022 and 2021 is $3,374, $40 and $543, respectively, related to current income tax benefits.
Income tax differs from that computed using the combined Canadian federal and provincial statutory income tax rate of 26.5%. Reconciliation of the expected income tax to the effective tax rate in continuing operations is as follows:
| | | Year ended December 31, | | | | Year ended December 31, |
| | 2023 | | | | 2023 | | 2022 | | 2021 |
| Loss before income taxes | |
| Effective income tax rate | | Effective income tax rate | | 26.5 | % | | 26.5 | % | | 26.5 | % |
| Expected income tax benefit | |
| | Year ended, |
| 2020 | | 2019 | | 2018 |
| Income (loss) before income taxes | | $ | (73,273) | | | $ | 1,165,937 | | | $ | (20,923) | |
| Effective income tax rate | | 26.5 | % | | 26.5 | % | | 26.5 | % |
| Expected income tax expense (benefit) | | $ | (19,417) | | | $ | 308,973 | | | $ | (5,545) | |
| | Non-taxable income | | (711) | | | (2,156) | | | 14 | |
| Non-taxable income (loss) | |
| Non-taxable income (loss) | |
| Non-taxable income (loss) | |
| Non-deductible share-based compensation | Non-deductible share-based compensation | | 2,498 | | | 2,839 | | | 2,466 | |
| Non-deductible expenses | Non-deductible expenses | | 1,364 | | | 764 | | | 0 | |
| Non-deductible transaction costs | Non-deductible transaction costs | | 3,146 | | | 1,523 | | | 0 | |
| Effect of provincial tax rate difference | Effect of provincial tax rate difference | | (15) | | | (44) | | | (64) | |
| Effect of tax rates outside of Canada | Effect of tax rates outside of Canada | | (362) | | | 70 | | | 0 | |
| Fair value gain on financial liabilities | | (34,250) | | | (338,409) | | | 0 | |
| Effect of change in tax rates | |
| Fair value gain (loss) on financial liabilities | |
| Changes in valuation allowance | Changes in valuation allowance | | 48,227 | | | 25,808 | | | 3,437 | |
| Capital gain on financial liabilities | |
| Other | Other | | 867 | | | 632 | | | (308) | |
| Income tax expense (recovery), net | | $ | 1,347 | | | $ | 0 | | | $ | 0 | |
| Income tax expense (benefit), net | |
The valuation allowance recorded against the loss on discontinued operations is not reflected in the effective tax rate reconciliation presented above for continuing operations.
The following table summarizesFor the significant components of the Company’s deferred tax assets and liabilities as ofyear ended December 31, 2020 and2022, the Company realized a capital gain for tax purposes of $479,800 as a result of Altria’s irrevocable relinquishment of its warrant on December 16, 2022, resulting in an increase in the total income tax expense reported for the year ended December 31, 2019:
| | | | | | | | | | | | | | | | |
| | As of | | |
| | 2020 | | 2019 | | |
| Deferred assets: | | | | | | |
| Tax loss carryforwards | | $ | 67,476 | | | $ | 30,908 | | | |
| Interest expense carryforwards | | 1,407 | | | 0 | | | |
| Deferred financing costs | | 4,233 | | | 5,690 | | | |
| Share issuance cost | | 1,573 | | | 2,217 | | | |
| Finance lease obligation | | 1,953 | | | 1,491 | | | |
| Plant and equipment | | 5,945 | | | 871 | | | |
| Investment | | 307 | | | 395 | | | |
| Intangible asset | | 4,218 | | | 0 | | | |
| Reserve | | 1,858 | | | 0 | | | |
| Other | | 570 | | | 482 | | | |
| Total deferred tax assets | | 89,540 | | | 42,054 | | | |
| Less valuation allowance | | (85,935) | | | (36,948) | | | |
| Net deferred tax assets | | 3,605 | | | 5,106 | | | |
| | | | | | |
| Deferred tax liabilities: | | | | | | |
| Inventory | | 0 | | | (1,227) | | | |
| Plant and equipment | | 0 | | | 0 | | | |
| Intangible assets | | 0 | | | (2,126) | | | |
| Investment | | 0 | | | 0 | | | |
| License | | (1,662) | | | (293) | | | |
| Right-of-use assets | | (1,943) | | | (1,460) | | | |
| Total deferred tax liabilities | | (3,605) | | | (5,106) | | | |
| Net deferred tax liability | | $ | 0 | | | $ | 0 | | | |
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020, 2019,2023, 2022, and 20182021
(In thousands of U.S. dollars, except for gram and share amounts)
The following table summarizes the significant components of the Company’s deferred tax assets and liabilities:
| | | | | | | | | | | |
| As of December 31, |
| 2023 | | 2022 |
| Deferred assets: | | | |
| Tax loss carryforwards | $ | 111,516 | | | $ | 98,074 | |
| Interest expense carryforwards | 2,688 | | | 2,533 | |
| Deferred financing costs | — | | | 1,311 | |
| Share issuance cost | — | | | 196 | |
| Finance lease obligation | 519 | | | 690 | |
| Plant and equipment | 36,697 | | | 37,662 | |
| Investment | 19,745 | | | 13,359 | |
| Intangible asset | 37,203 | | | 53,365 | |
| Inventory | 556 | | | 1,287 | |
| Reserve | 2,430 | | | 2,972 | |
| Unrealized foreign exchange | 275 | | | — | |
| R&D investment tax credits | 567 | | | 293 | |
| Other | 4,271 | | | 3,099 | |
| Total deferred tax assets | 216,467 | | | 214,841 | |
| Less valuation allowance | (216,241) | | | (214,199) | |
| Net deferred tax assets | 226 | | | 642 | |
| Deferred tax liabilities: | | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| Right-of-use assets | (181) | | | (365) | |
| Unrealized foreign exchange | — | | | (84) | |
| Total deferred tax liabilities | (181) | | | (449) | |
Net deferred tax asset (liability)(i) | $ | 45 | | | $ | 193 | |
Net deferred tax asset (liability) is reported as Other assets within our consolidated balance sheet.The realization of deferred tax assets is dependent on the Company’s generating sufficient taxable income in the years that the temporary differences become deductible. A valuation allowance has been provided for the deferred tax assets that the Company determined did not meet the more-likely-than-not recognition threshold under U.S. GAAP.
As of December 31, 2020,2023 and 2022, the Company’s valuation allowance was $216,241 and $214,199, respectively. The valuation allowance increased by $2,042 during the year ended December 31, 2023, and decreased by $10,577 during the year ended December 31, 2022. The increase in the valuation allowance during the year ended December 31, 2023, was primarily due to an increase in net operating loss carryforwards. The decrease in the valuation allowance during the year ended December 31, 2022, was primarily due to the recognition of a portion of deferred tax assets for which a valuation allowance was recorded in the prior year.
As of December 31, 2023, the Company had net operating losses in Canada, the U.S., and Israel available to offset future years’ taxable income of approximately $177,651, $62,851, and $14,042,$253,521, $144,219, $20,794, respectively. As of December 31, 2019,2022, the Company had net operating losses in Canada, the U.S., and Israel available to offset future years’ taxable income of approximately $92,773, $14,374,$216,120, $134,593, and $8,763,$18,739, respectively. The net operating losses in Canada will begin to expire, for purposes of carryforward, in fiscal year 2032.2033. The net operating losses in the U.S. can be carried forward indefinitely for federal purposes. The net operating losses in Israel can be carried forward indefinitely.
Utilization of the net operating loss carryforwards may be subject to limitations under the tax laws applicable in each tax jurisdiction due to ownership changes that could occur in the future. These ownership changes could limit the amount of net operating loss carryforwards and other deferred tax assets that can be utilized to offset future taxable income and tax expense. Specifically, if Altria exercises its warrant the Company would recognize a change in control event and certain Canadian net operating loss carryforwards may be limited. Due to the existence of the valuation allowance, limitations created by ownership changes, if any, will not impact the Company’s effective tax rate.
As of December 31, 2023, the Company has various scientific research and experimental development investment tax credit carryforwards of $683 related to Canadian operations, which, if not utilized, will begin to expire in 2041. Investment tax credits are recognized when realization of the tax credits is more likely than not.
The Company files federal income tax returns in Canada, Israel and the U.S. The Company has open tax years with the taxation jurisdictions. These open years contain certain matters that could be subject to differing interpretations of applicable tax laws and
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2023, 2022, and 2021
(In thousands of U.S. dollars, except for share amounts)
regulations and tax treaties, as they relate to the amount, timing, or inclusion of revenue and expense. As of December 31, 2023, Peace Naturals and Hortican Inc. are under examination with the Canadian Revenue Agency for tax years 2019 and 2020.
| | | | | | | | |
| Jurisdiction | | Open Years |
| Canada | | 20162019 – 20202023 |
| United States | | 20182020 – 20202023 |
| Israel | | 20192020 – 20202023 |
The following table outlines the movements in the valuation allowance:
| | | | | | | | | | | | | | | | | | | | | | | |
| Balance at beginning of year | | Change due to foreign exchange | | Increase | | Balance at end of year |
| Year ended December 31, 2020 | $ | (36,948) | | | $ | (693) | | | $ | (48,294) | | | $ | (85,935) | |
| Year ended December 31, 2019 | (7,931) | | | (998) | | | (28,019) | | | (36,948) | |
As of December 31, 2020 and December 31, 2019, the Company recorded a valuation allowance of $85,935 and $36,948, respectively. The valuation allowance increased by $48,294 and $28,019 during the years ended December 31, 2020 and December 31, 2019, respectively. The increase in the valuation allowance during the years ended December 31, 2020 and December 31, 2019 was primarily due to an increase in net operating loss carryforwards, the utilization of which did not meet the more-likely-than-not recognition threshold.
Accounting guidance clarifies the accounting for uncertain tax positions and prescribes a recognition threshold and measurement process for recording in the financial statements uncertain tax positions taken or expected to be taken in a tax return. Additionally, the authoritative guidance addresses the de-recognition,derecognition, classification, accounting in interim periods and disclosure requirements for uncertain tax positions. Only tax positions that meet the more-likely-than-not recognition threshold may be recognized. There were no identified unrecognized tax benefits as of December 31, 20202023 or December 31, 2019.2022.
The Company considers all earnings and profits of its subsidiaries outside Canada to be indefinitely reinvested. As of December 31, 20202023 and December 31, 2019,2022, deferred income taxes have not been provided for any undistributed earnings from operations outside of Canada. The foreign subsidiaries have accumulated losses and as such the amount of undistributed earnings upon which income taxes have not been provided is immaterial to these consolidated financial statements.
13. Loss per Share
Basic and diluted earnings (loss) per share from continued and discontinued operations are calculated as follows:
| | | | | | | | | | | | | | | | | |
| Year ended December 31, |
| 2023 | | 2022 | | 2021 |
| Basic loss per share computation | | | | | |
| Net loss from continuing operations attributable to the shareholders of Cronos Group | $ | (69,849) | | | $ | (155,178) | | | $ | (126,982) | |
Weighted-average number of common shares outstanding for computation for basic and diluted earnings per share(i) | 380,964,739 | | | 376,961,797 | | | 370,390,965 | |
| Basic loss from continuing operations per share | $ | (0.18) | | | $ | (0.41) | | | $ | (0.34) | |
| Diluted loss per share from continuing operations | $ | (0.18) | | | $ | (0.41) | | | $ | (0.34) | |
| | | | | |
| Loss from discontinued operations attributable to the shareholders of Cronos Group | $ | (4,114) | | | $ | (13,556) | | | $ | (269,125) | |
Weighted-average number of common shares outstanding from computation for basic and diluted earnings per share(i) | 380,964,739 | | | 376,961,797 | | | 370,390,965 | |
| Basic loss from discontinued operations per share | $ | (0.01) | | | $ | (0.04) | | | $ | (0.73) | |
| Diluted loss from discontinued operations per share | $ | (0.01) | | | $ | (0.04) | | | $ | (0.73) | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
(i)In computing diluted earnings per share, incremental common shares are not considered in periods in which a net loss is reported, as the inclusion of the common share equivalents would be anti-dilutive.
Total securities of 25,426,119, 112,612,579 and 125,195,001 were not included in the computation of diluted shares outstanding for the years ended December 31, 2023, 2022 and 2021, respectively, because the effect would be anti-dilutive.
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020, 2019,2023, 2022, and 20182021
(In thousands of U.S. dollars, except for gram and share amounts)
20. Commitments14. Financial Instruments
(a)Lease CommitmentsFair value measurement
The following is a summary of the Company’s future minimum lease payments under operating leasesCompany complies with ASC 820 Fair Value Measurements for its premises due in future fiscal years:
| | | | | | | | |
| | As of December 31, |
| | 2020 |
| 2021 | | $ | 2,381 | |
| 2022 | | 2,302 | |
| 2023 | | 2,201 | |
| 2024 | | 1,711 | |
| 2025 | | 1,505 | |
| 2026 and thereafter | | 1,916 | |
financial assets and liabilities that are re-measured and reported at fair value at each reporting period and non-financial assets and liabilities that are re-measured and reported at fair value at least annually. In addition to the minimum lease payments, the Company is required to pay realty taxes and other occupancy costs in accordance with the terms of the lease agreements.
(b)R&D commitmentsgeneral, fair values are determined by:
(i) •Ginkgo. On September 4, 2018, the Company announced an R&D partnership with Ginkgo Bioworks Inc. (“Ginkgo”) to develop scalable and consistent production of 8 target cannabinoids, including THC, CBD and a variety of other lesser known and rarer cannabinoids. As part of this partnership, Cronos Group has agreed to issue up to 14,674,903 common shares of the Company (aggregate value of approximately $100,000 as of July 17, 2018 assuming all milestones are met, collectively the “Ginkgo Equity Milestones”)Level 1 inputs utilize quoted prices (unadjusted) in tranches and $22,000 in cash subject to Ginkgo’s achievement of certain milestones and to fund certain R&D expenses, including foundry access fees.active markets for identical assets or liabilities.
(ii) •Technion. On October 15, 2018, the Company entered into a sponsored research agreement with the Technion ResearchLevel 2 inputs utilize data points that are observable such as quoted prices, interest rates and Development Foundation of the Technion – Israel Institute of Technology (“Technion”). Research is focused on the use of cannabinoids and their role in regulating skin health and skin disorders. The Company has committed to $1,784 of research funding over a period of three years. An additional $4,900 of cash payments will be paid to Technion upon the achievement of certain milestones.yield curves.
(c)•Altria consulting services
On February 18, 2019, the Company entered into an agreement with a wholly owned subsidiary of Altria (which agreement was subsequently amended and restated to substitute Altria Pinnacle as a party thereto), to receive strategic advisory and project management services from Altria Pinnacle (the “Services Agreement”). Pursuant to the Services Agreement, the Company will pay Altria Pinnacle a monthly fee equal to the product of 105% and the sum of: (i) all costs directly associated with the services incurred during the monthly period, and (ii) a reasonable and appropriate allocation of indirect costs incurred during the monthly period. The Company will also pay all third-party direct charges incurred during the monthly period in connection with the services, including any reasonable and documented costs, fees and expenses associated with obtaining any consent, license or permit. The Services Agreement will remain in effect until terminated by either party. See Note 25.
(d)Use of publicity rights in brand development
On December 23, 2019, the Company issued 856,017 restricted common shares to Kristen Bell, an accredited investor, in a private placement (“Private Placement”) in reliance on Section 4(a)(2) of the Securities Act of 1933 in connection with the use of certain publicity rights in the brand development of Happy Dance™. One-third of such common shares vested on January 31, 2020 with the remaining shares vesting in two equal installments on June 23, 2021, and December 23, 2022. The issuance did not involve a public offering and was made without general solicitation or advertising. The total fair value of the consideration paidLevel 3 inputs are unobservable data points for the issuance of such common shares was approximately $6,000. The fair value ofasset or liability, and include situations where there is little, if any, market activity for the shares was calculated using the 10-day volume weighted average price per share of the Company’s common shares on Nasdaq.
Additional restricted common shares are issued when certain performance milestones are achieved:asset or liability.
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020, 2019,2023, 2022, and 20182021
(In thousands of U.S. dollars, except for gram and share amounts)
The following tables present information about the Company’s assets that are measured at fair value on a recurring basis and indicates the fair value hierarchy of the valuation techniques the Company utilized to determine such fair value:
| | | | | | | | | | | | | | | | | | | | | | | |
| As of December 31, 2023 |
| Level 1 | | Level 2 | | Level 3 | | Total |
| Cash and cash equivalents | $ | 669,291 | | | $ | — | | | $ | — | | | $ | 669,291 | |
| Short-term investments | 192,237 | | | — | | | — | | | 192,237 | |
Other investments(i) | 9,601 | | | — | | | — | | | 9,601 | |
| Derivative liabilities | — | | | — | | | 102 | | | 102 | |
| | | | | | | | | | | | | | | | | | | | | | | |
| As of December 31, 2022 |
| Level 1 | | Level 2 | | Level 3 | | Total |
| Cash and cash equivalents | $ | 764,644 | | | $ | — | | | $ | — | | | $ | 764,644 | |
| Short-term investments | 113,077 | | | — | | | — | | | 113,077 | |
Other investments(i) | 21,993 | | | — | | | — | | | 21,993 | |
| Derivative liabilities | — | | | — | | | 15 | | | 15 | |
(i) First Performance Issuance: if, prior toAs of December 23,31, 2023 and December 31, 2022, the product line generates at least $50,000 in net revenue,Company’s influence on Vitura is deemed non-significant and the investment is considered an equity security with a readily determinable fair value. See Note 4 “Investments” for additional common shares with an aggregateinformation.
There were no transfers between fair value of $1,000 will be issued.
(ii) Second Performance Issuance: if, prior to December 23, 2022,categories during the product line generates at least $100,000 in net revenue, additional common shares with an aggregate value of $1,000 will be issued (together with the First Performance Issuance noted above).periods presented.
The numberfollowing tables present information about the Company’s assets that are measured at fair value on a non-recurring basis and indicates the fair value hierarchy of commonthe valuation techniques the Company utilized to determine such fair value:
| | | | | | | | | | | | | | | | | | | | | | | |
| As of December 31, 2023 |
| Level 1 | | Level 2 | | Level 3 | | Total |
Other investments(i) | — | | | — | | | 25,650 | | | 25,650 | |
| | | | | | | | | | | | | | | | | | | | | | | |
| As of December 31, 2022 |
| Level 1 | | Level 2 | | Level 3 | | Total |
Other investments(i) | — | | | — | | | 49,000 | | | 49,000 | |
(i)On June 14, 2021, the Company purchased an option to acquire 473,787 shares that would be issued upon achievingof Class A Common Stock of PharmaCann, a vertically integrated cannabis company in the foregoing milestones will be determined based on the 10-day volume weighted averageUnited States, at an exercise price of $0.0001 per share, representing approximately 10.5% of the Company’s common sharesPharmaCann’s issued and outstanding capital stock on Nasdaqa fully diluted basis as of the trading day immediately prior to the date of filing with the Securities and Exchange Commission of the Company’s audited year-end financial statements for the first fiscal year during which such milestones are achieved.
21. Contingencies
The Company is subject to various legal proceedings in the ordinary course of its business and in connection with its marketing, distribution and sale of its products. Many of these legal proceedings are in the early stages of litigation and seek damages that are unspecified or not quantified. Although the outcome of these matters cannot be predicted with certainty, the Company does not believe these legal proceedings, individually or in the aggregate, will have a material adverse effect on its consolidated financial condition but could be material to its results of operations for any particular reporting period depending, in part, on its results for that period.
(a)Class action complaints relating to restatement
On March 11 and 12, 2020, 2 alleged shareholders of the Company separately filed 2 putative class action complaints in the U.S. District Court for the Eastern District of New York against the Company and its former Chief Executive Officer (now Executive Chairman) and Chief Financial Officer. The court has consolidated the cases, and the consolidated amended complaint alleges violations of Section 10(b) of the Exchange Act and Rule 10b-5 promulgated thereunder against all defendants, and Section 20(a) of the Exchange Act against the individual defendants. The consolidated amended complaint generally alleges that certain of the Company’s prior public statements about revenues and internal controls were incorrect based on the Company’s March 2, 2020 disclosure that the Audit Committee of the Board was conducting a review of the appropriateness of revenue recognized in connection with certain bulk resin purchases and sales of products through the wholesale channel. The consolidated amended complaint does not quantify a damage request. Defendants moved to dismiss on February 8, 2021.
On June 3, 2020, an alleged shareholder filed a Statement of Claim, as amended on August 12, 2020, in the Ontario Superior Court of Justice in Toronto, Ontario, Canada, seeking, among other things, an order certifying the action as a class action on behalf of a putative class of shareholders and damages of an unspecified amount. The Amended Statement of Claim names the Company, its former Chief Executive Officer (now Executive Chairman), Chief Financial Officer, former Chief Financial Officer and Chief Commercial Officer, and current and former members of the Board as defendants and alleges breaches of the Ontario Securities Act, oppression under the Ontario Business Corporations Act and common law misrepresentation. The Amended Statement of Claim generally alleges that certain of the Company’s prior public statements about revenues and internal controls were misrepresentations based on the Company’s March 2, 2020 disclosure that the Audit Committee of the Board was conducting a review of the appropriateness of revenue recognized in connection with certain bulk resin purchases and sales of products through the wholesale channel, and the Company’s subsequent restatement. The Amended Statement of Claim does not quantify a damage request.
(b)Regulatory reviews relating to restatement
The Company has been responding to requests for information from various regulatory authorities relating to its previously disclosed restatement of its financial statements for the first three quarters of 2019. The Company is responding to all such requests for information and cooperating with all regulatory authorities. The Company cannot predict the outcome of any such regulatory review or investigation and it is possible that additional investigations or one or more formal proceedings may be commenced against the Company and its current and former officers and directors in connection with these regulatory reviews and investigations.
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020, 2019, and 2018
(In thousands of U.S. dollars, except for gram and share amounts)
(c)Litigation relating to marketing, distribution and sale of products
On June 16, 2020, an alleged consumer filed a Statement of Claim on behalf of a class in the Court of Queen’s Bench of Alberta in Alberta, Canada, against the Company and other Canadian cannabis manufacturers and/or distributors. On December 4, 2020, a Third Amended Statement of Claim was filed, which added a second alleged consumer. The Third Amended Statement of Claim alleges claims related to the defendants’ advertised content of cannabinoids in cannabis products for medicinal use on or after June 16, 2010 and cannabis products for adult use on or after October 17, 2018. The Third Amended Statement of Claim seeks a total of C$500 million for breach of contract, compensatory damages, and unjust enrichment or such other amount as may be proven in trial and C$5 million in punitive damages against each defendant, including the Company. The Third Amended Statement of Claim also seeks interest and costs associated with the action. The Company has not responded to the Third Amended Statement of Claim.
A number of claims, including purported class actions, have been brought in the U.S. against companies engaged in the U.S. hemp business alleging, among other things, violations of state consumer protection, health and advertising laws. On April 8, 2020, a putative class action complaint was filed in the U.S. District Court for the Central District of California against Redwood, alleging violations of California’s Unfair Competition Law, False Advertising Law, Consumers Legal Remedies Act, and breaches of the California Commercial Code for breach of express warranties and implied warranty of merchantability with respect to Redwood’s marketing and sale of U.S. hemp products. The complaint did not quantify a damage request. On April 10, 2020, the class action complaint was dismissed for certain pleading deficiencies and the plaintiff was granted leave until April 24, 2020 to amend the complaint to establish federal subject matter jurisdiction. On April 28, 2020, the action was dismissed without prejudice for failure to prosecute and for failure to comply with a court order. As of the date of this Annual Report, the plaintiff has not refiledPharmaCann Option, for an aggregate purchase price of approximately $110,392. On February 28, 2022, PharmaCann closed its previously announced transaction with LivWell pursuant to which PharmaCann acquired LivWell. As a result of the complaint.LivWell Transaction, the Company’s ownership percentage in PharmaCann on a fully diluted basis decreased to approximately 6.4%. As of December 31, 2023 and December 31, 2022, the Company’s ownership percentage in PharmaCann on a fully diluted basis was approximately 5.9% and 6.3%, respectively. See Note 4 “Investments”.
There were no transfers between fair value categories during the periods presented.
22. Supplementary Cash Flow Information
The net changes in non-cash working capital items are as follow:
| | | | | | | | | | | | | | | | | | | |
| Year ended December 31, |
| 2020 | | 2019 | | 2018 | | |
| Accounts receivable | $ | (4,724) | | | $ | (702) | | | $ | (2,569) | | | |
| Prepaids and other receivables | (5,300) | | | (10,509) | | | (2,382) | | | |
| Current portion of loans receivable | 0 | | | (4,585) | | | 0 | | | |
| Inventory | (28,094) | | | (51,888) | | | (4,092) | | | |
| Accounts payable and other liabilities | 4,175 | | | 13,476 | | | 12,705 | | | |
| Total | $ | (33,943) | | | $ | (54,208) | | | $ | 3,662 | | | |
23. Financial Instrumentsrisks
The Company’s activities expose it to a variety of financial risks, including credit risk, liquidity risk, and market risk, (including interest rate risk)risk, and foreign currency rate risk.
(a)Credit risk
Credit risk is the risk of financial loss to the Company if a customer or counterparty to a financial instrument fails to meet its contractual obligations. The Company is exposed to credit risk from its operating activities, primarily accounts receivable and other receivables, and its investing activities, including cash held with banks and financial institutions, short term investments, loanloans receivable, and advances to joint ventures. The Company’s maximum exposure to this risk is equal to the carrying amount of these financial assets, which amounted to $1,403,491$966,442 and $987,836 as of December 31, 2020 (December2023 and December 31, 2019 – $1,586,978).2022, respectively.
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020, 2019,2023, 2022, and 20182021
(In thousands of U.S. dollars, except for gram and share amounts)
(i)Accounts receivable
The Company had accounts receivable of $8,928$13,984 and $23,113 as of December 31, 2020 (December2023 and December 31, 2019 – $4,638).2022, respectively. An impairment analysis is performed at each reporting date using a provision matrix to measure expected credit losses. The provision rates are based on the days past due for groupings of various customer segments with similar loss patterns. The calculation reflects the probability-weighted outcome, the time value of money and reasonable and supportable information that is available at the reporting date about past events, current conditions and forecasts of future economic conditions. Accounts receivable are written off when there is no reasonable expectation of recovery. Indicators that there is no reasonable expectation of recovery include, amongst others, the failure of a debtor to engage in a repayment plan, and a failure to make contractual payments for a period of greater than 120 days past due.
For the year ended As of December 31, 2020,2023 and 2022, the Company recognized an approximate CECLhad $3 and $219, respectively, in expected credit losses on receivables from contracts with customers.
As of $74 (DecemberDecember 31, 2019 – $136). The2023, the Company has assessed that there is a concentration of credit risk as 78%37% of the Company’s accounts receivable were due from four customers as of December 31, 2020 (December 31, 2019 – 56% due from two customers)one customer with an established credit history with the Company. As of December 31, 2022, 55% of the Company’s accounts receivable were due from three customers with an established credit history with the Company.
The Company sells products through a limited number of major customers. Major customers are defined as customers that each individually accounted for greater than 10% of the Company’s revenues. During the year ended December 31, 2023, the Company earned a total net revenue before excise taxes of $79,503 from three major customers, together accounting for 66% of the Company’s total net revenue before excise taxes. During the year ended December 31, 2022, the Company earned a total net revenue before excise taxes of $63,509 from three major customers, together accounting for 55% of the Company’s total net revenue before excise taxes. During the year ended December 31, 2021, the Company earned a total net revenue before excise taxes of $41,603 from three major customers, accounting for 56% of the Company’s total net revenues before excise taxes.
(ii) Cash and cash equivalents, short-term investments, and other receivables
The Company held cash and cash equivalents of $1,078,023 at$669,291 and $764,644 as of December 31, 2020 (December2023 and December 31, 2019 – $1,199,693).2022, respectively. The short-term investments and related interest receivable of $211,766 (December$192,237 and $113,077 as of December 31, 2019 – $306,347) represents2023 and December 31, 2022, respectively, represent short-term investments with a maturity of less than a year and accrued interest as of December 31, 2020.interest. The cash and cash equivalents and short-term investments, including guaranteed investment certificates and bankers’ acceptances, are held with central banks and financial institutions that are highly rated. In addition to interest receivable, other receivables include sales taxes receivable from the government. As such, the Company has assessed an insignificant loss allowance on these financial instruments.
(iii)Advances to joint ventures
The Company had advances to joint ventures of $467 as of December 31, 2020 (December 31, 2019 – $19,437). The Company has assessed the credit risk of advances to joint ventures based on the financial position of the borrowers, and the regulatory and economic environment of the borrowers. The expected loss rates are based on the historical credit losses experienced. Where appropriate, the historical loss rates are adjusted to reflect current and forward-looking information. The Company has assessed the loss allowance on these advances as of December 31, 2020 to be $NaN (December 31, 2019 – $NaN).
(b)Liquidity risk
Liquidity risk is the risk that the Company will not be able to meet its financial obligations as they become due and arises principally from the Company’s accounts payable and other liabilities.payable. The Company had trade accounts payable of $12,107$8,887 and $8,599 as of December 31, 2020 (December2023 and December 31, 2019 – $9,194).2022, respectively, included in accounts payable on the consolidated balance sheet. The Company’s policy is to review liquidity resources and ensure that sufficient funds are available to meet financial obligations as they become due. Further, the Company’s management is responsible for ensuring funds exist and are readily accessible to support business opportunities as they arise. The Company’s funding is primarily provided in the form of capital raised through the issuance of common shares and warrants. As of December 31, 2020, 64%2023, the Company has assessed a concentration of the Company’s payablesrisk of vendors as 26% of accounts payable were due to fourone vendor. As of December 31, 2022, the Company has assessed a concentration risk of vendors (December 31, 2019 – 42%as 27% of accounts payable due to three vendors).one vendor.
(c)Market risk
Market risk is the risk that the fair value of, or future cash flows from, the Company’s financial instruments will significantly fluctuate due to changes in market prices. The value of financial instruments can be affected by changes in interest rates, market and economic conditions, and equity and commodity prices. The Company is exposed to market risk in divesting its investments, such that unfavorable market conditions could result in dispositions of investments at less than their carrying values.amounts. Further, the revaluation of securities classified as fair value through net income could result in significant write-downs of the Company’s investments, which would have an adverse impact on the Company’s financial position.results of operations, unless these would flow through other comprehensive income.
The Company manages risk by having a portfolio of securities from multiple issuers, such that the Company was not materially exposed to any one issuer.
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020, 2019,2023, 2022, and 20182021
(In thousands of U.S. dollars, except for gram and share amounts)
(d)Interest rate risk
Interest rate risk is the risk that the value or yield of fixed-income investments may decline if interest rates change. Fluctuations in interest rates may impact the level of income and expense recorded on the cash equivalents and short-term investments, and the market value of all interest-earning assets, other than those whichthat possess a short term to maturity. During the year ended December 31, 2020, the Company had net interest income of $18,415 (December 31, 2019 – $27,969). A 10% change in the interest rate in effect on December 31, 20202023 would not have a material effect on the fair value of our cash equivalents and short-term investments as the majority of the portfolio had a maturity date of three months or less. A 10% change in the interest rate in effect for 2023 would have an effect of $5.4 million on interest income, net earned on our cash equivalents and short-term investments. A 10% change in the interest rate in effect on December 31, 2019,2022, would not have a material effect on (i) fair value of the cash equivalents and short-term investments as the majority of the portfolio hashad a maturity date of three months or less, or (ii) interest income.income, net. Management continues to monitor external interest rates and revise the Company’s investment strategy as a result.
During the years ended December 31, 2023 and December 31, 2022, the Company had net interest income of $51,235 and $22,514, respectively. During the year ended December 31, 2020,2023, the Company’s average variable interest rate fell 1.49%, which resulted in a decrease of net interest income of $15,671 in the period.increased approximately 1.45%. During the year ended December 31, 2019,2022, the Company’s average variable interest rate did not materially change.increased approximately 3.50%.
(e)Currency rateForeign currency risk
Currency rate risk is the risk that the fair value of, or future cash flows from, the Company’s financial instruments will significantly fluctuate due to changes in foreign exchange rates. The Company is exposed to this risk on advances to joint venturesinvestments in equity investments denominated in A$ and C$, and other assets and liabilities denominated in A$ and C$. The Company is further exposed to this risk through subsidiaries operating in Israel and the U.S. as the Company’s functional currency is in Canadian dollars. The Company does not currently use foreign exchange contracts to hedge its exposure to currency rate risk. As such, the Company’s financial position and financial results may be adversely affected by the unfavorable fluctuations in currency exchange rates.
As of December 31, 2020,2023 and December 31, 2022, the Company had foreign currency gain (loss) on translation of $14,951 (December 31, 2019 – $37,687).$21,539 and $(50,616), respectively. A 10% change in the exchange rates for the foreign currencies would affect the carrying valueamounts of net assets by approximately $170,817$97,678 and $77,414 as of December 31, 2020 (December2023 and December 31, 2019 – $174,902).2022, respectively.
24. Fair Value Measurement
The Company complies with ASC 820 Fair Value Measurements, for its financial assets and liabilities that are re-measured and reported at fair value at each reporting period, and non-financial assets and liabilities that are re-measured and reported at fair value at least annually. In general, fair values are determined by:
•Level 1 inputs utilize quoted prices (unadjusted) in active markets for identical assets or liabilities.
•Level 2 inputs utilize data points that are observable such as quoted prices, interest rates and yield curves.
•Level 3 inputs are unobservable data points for the asset or liability, and includes situations where there is little, if any, market activity for the asset or liability.
The following tables present information about the Company’s assets that are measured at fair value on a recurring basis as of December 31, 2020 and December 31, 2019 and indicates the fair value hierarchy of the valuation techniques the Company utilized to determine such fair value.
| | | | | | | | | | | | | | | | | | | | | | | |
| As of December 31, 2020 |
| Level 1 | | Level 2 | | Level 3 | | Total |
| Cash and cash equivalents | $ | 1,078,023 | | | $ | 0 | | | $ | 0 | | | $ | 1,078,023 | |
| Short-term investments | 211,766 | | | 0 | | | 0 | | | 211,766 | |
| Derivative liabilities | 0 | | | 0 | | | 163,410 | | | 163,410 | |
| | | | | | | | | | | | | | | | | | | | | | | |
| As of December 31, 2019 |
| Level 1 | | Level 2 | | Level 3 | | Total |
| Cash and cash equivalents | $ | 1,199,693 | | | $ | 0 | | | $ | 0 | | | $ | 1,199,693 | |
| Short-term investments | 306,347 | | | 0 | | | 0 | | | 306,347 | |
| Derivative liabilities | 0 | | | 0 | | | 297,160 | | | 297,160 | |
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020, 2019, and 2018
(In thousands of U.S. dollars, except for gram and share amounts)
25.15. Related Party Transactions and Balances
(a)Altria
On March 8, 2019, in connection with the Altria Investment, Altria, through certain of its wholly owned subsidiaries, purchased a 45% equity interest in the Company. As of December 31, 2023, Altria beneficially held an approximately 41.1% ownership interest in the Company (calculated on a non-diluted basis).
The Company incurred the following expenses for consulting services from Altria Pinnacle LLC, a subsidiary of Altria (“Altria Pinnacle”):
| | Year ended December 31, |
| 2020 | | 2019 |
| Year ended December 31, | | | Year ended December 31, |
| 2023 | | | 2023 | | 2022 | | 2021 |
| Altria Pinnacle – expense | Altria Pinnacle – expense | $ | 1,199 | | | $ | 3,479 | |
| Total | $ | 1,199 | | | $ | 3,479 | |
NaN expenses for consulting services from Altria Pinnacle during the year ended December 31, 2018.
The following is a summary ofThere were no amounts payable related to the consulting services with Altria Pinnacle:Pinnacle as of December 31, 2023 and 2022.
| | | | | | | | | | | |
| As of December 31, |
| 2020 | | 2019 |
| Altria Pinnacle – payable | $ | 0 | | | $ | 1,152 | |
| Total | $ | 0 | | | $ | 1,152 | |
During 2019, the Company purchased machinery and equipment amounting to $1,258 from a subsidiary of Altria, which was fully paid for during the year.
Refer to Note 149 “Derivative Liabilities” for further information on the derivative liabilities related to the Altria Investment.
There were no other material related party transactions during
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020,2023, 2022, and 2021
(In thousands of U.S. dollars, except for share amounts)
(b)Cronos GrowCo
The Company holds a variable interest in Cronos GrowCo through its ownership of 50% of Cronos GrowCo’s common shares and senior secured debt in Cronos GrowCo. See Note 4 “Investments” for further discussion.
The Company made the following purchases of cannabis products from Cronos GrowCo:
| | | | | | | | | | | | | | | | | |
| Year ended December 31, |
| 2023 | | 2022 | | 2021 |
| Cronos GrowCo – purchases | $ | 21,335 | | | $ | 18,144 | | | $ | 4,820 | |
The Company’s outstanding payable balance to Cronos GrowCo was $2,267 and $2,519 as of December 31, 20192023 and December 31, 2018.2022, respectively.
26. Non-monetary Transactions
The Company had 0 non-monetary transactions duringDuring the year ended December 31, 2020 or 2018.
On March 28, 2019,third quarter of 2023, the Company, as supplier, entered into 2 transactionsa cannabis germplasm supply agreement with Cronos GrowCo as buyer. During 2023, the Company received proceeds of $1,114 in relation to simultaneously purchase and sell inventory to a third party. Thethis agreement.
Also during 2023, the Company purchased cannabis resin from the third party and in turn sold cannabis dry flower to the third party. The transactions involved the exchange of work in progress inventory and were accountedcertain held for at thesale assets with carrying value of inventory transferred by the Company, which equaled the$332 and certain other previously expensed assets with a zero net book value to Cronos GrowCo for total proceeds of the cannabis resin received. NaN revenue was$761 and recognized as a result of this transaction and 0$436 gain or loss was recognizedin Other, net in the consolidated statements of net income (loss)loss and comprehensive income (loss)loss.
Additionally, on August 23, 2019, the Company, as lender, and Cronos GrowCo, as borrower, entered into the GrowCo Credit Facility. See additional information in Note 5 “Loans Receivable, net”.
(c)Vendor Agreement
In September 2019,November 2022, the Company entered into 3 transactionsan agreement with an external vendor whereby the vendor would provide certain manufacturing services to simultaneously purchase and sell inventorythe Company. The vendor then subcontracted out a portion of those services to a third party.another vendor whose chief executive officer is an immediate family member of an executive of the Company. The Company purchased cannabis resin$2,310 and cannabis tincture oil$645 of products and services under this subcontracted agreement for the years ended December 31, 2023 and December 31, 2022, respectively. The company had $28 in turn sold cannabis dry floweroutstanding accounts payables related to the third party. The transactions involvedsubcontracted agreement as of December 31, 2023 and no outstanding accounts payable related to the exchangesubcontracted agreement as of workDecember 31, 2022.
In November 2023, the Company negotiated a direct contract with the related-party vendor. During the year ended 2023, the Company purchased $42 of products and services directly from the related-party vendor and had $11 in progress inventory and were accounted for in accordance with ASC 845 Non-monetary transactions atoutstanding accounts payable to the carrying valuevendor as of inventory transferred by the Company. $2,300 was recognized in revenue as a result of this transaction and 0 gain or loss was recognized in the consolidated statements of net income (loss) and comprehensive income (loss).
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020, 2019,2023, 2022, and 20182021
(In thousands of U.S. dollars, except for gram and share amounts)
27. Business Combinations16. Restructuring
On September 5, 2019,In the first quarter of 2022, the Company closedinitiated a strategic plan to realign the Redwood Acquisition. Redwood manufactures, marketsbusiness around its brands, centralize functions and distributes U.S. hemp-derived supplements and cosmetics product through e-commerce, retail and hospitality channels inevaluate the U.S. underCompany’s supply chain (the “Realignment”). As part of the brand Lord Jones™. Redwood’s products use high quality U.S. hemp extract that retains naturally occurring phytocannabinoids and terpenes found inRealignment, on February 28, 2022, the plant. The CompanyBoard approved plans to leverage Redwood’s capabilitiesthe Company’s strategic partnerships to capitalize onimprove supply chain efficiencies and reduce manufacturing overhead by exiting its production facility in Stayner, Ontario, Canada (the “Peace Naturals Campus”). On February 27, 2023, the significant demandBoard approved revisions to further create and scale U.S. hemp-derived consumer products and brands.
In 2019,the Realignment, which are expected to result in the Company acquired allmaintaining select components of its operations at the issuedPeace Naturals Campus, namely distribution warehousing, certain research and outstanding sharesdevelopment activities and manufacturing of eachcertain of the 4 Redwood operating subsidiaries for an aggregate consideration of $283,300, which included $227,191 in cashCompany’s products, while seeking to sell and 5,086,586 common shareslease back all or some of the Peace Naturals Campus or to lease certain portions of the Peace Naturals Campus to third parties. In the third quarter of 2023, the Board approved revisions to the Realignment to wind down operations at its Winnipeg, Manitoba facility (“Cronos Fermentation”), list the Cronos Fermentation facility for sale, and implement additional organization-wide cost reductions as the Company with a fair valuecontinues its Realignment initiatives. During the third quarter of $56,109. The fair2023, the Company performed an assessment under ASC 360, Property Plant and Equipment, of the recovery of the carrying value of the shares issued as partCanada asset group, which includes Cronos Fermentation, and determined the carrying value of the consideration paidasset group was based onrecoverable. As of December 31, 2023, Cronos Fermentation did not meet the volume weighted average trading price ofcriteria to be classified as held-for-sale.
During the common shares on Nasdaq on each of the ten consecutive trading days prior to the date of the Membership Interest Purchase Agreement dated August 1, 2019 (the “MIPA”), by and amongyear ended December 31, 2023, the Company Redwood Holding Group, LLC, and certain Key Persons solely forincurred $1,524 of restructuring costs in its continuing operations in connection with the purposes as described inRealignment. During the MIPA, at C$14.74 per share.
The Redwood Acquisition was unanimously approved by the board of directors of Redwood Holdings Group, LLC and by the Board following the unanimous recommendation of a special committee of independent directors (“Special Committee”). A Special Committee composed entirely of independent directors ofyear ended December 31, 2022, the Company was formed to evaluaterecognized $3,545 of restructuring costs in continuing operations in connection with the Realignment. Charges related thereto include employee-related costs such as severance, relocation and make recommendations toother termination benefits, as well as contract termination and other related costs. During the Board since one of our directors, Jason Adler, and Michael Gorenstein, our Executive Chairman and former President and Chief Executive Officer, each held an indirect interest in Redwood Holding Group, LLC by way of their interest in certain funds affiliated with Gotham Green Partners, which funds were equity holders in Redwood Holding Group, LLC. Jason Adler is the co-founder and Managing Member of Gotham Green Partners, a private equity firm focused primarily on early-stage investing in companies in the cannabis industry, and Michael Gorenstein is a co-founder and non-managing Member of Gotham Green Partners. The Special Committee engaged Perella Weinberg Partners LPyear ended December 31, 2023, as financial advisor.
The Redwood Acquisition was accounted for as a business combination as defined in ASC 805 Business Combinations. As a result of the change in controldecision to wind down operations at Cronos Fermentation, the Company recognized an inventory write-down of Redwood, the assets$805 related to certain obsolete raw materials. Restructuring costs and liabilities of Redwood are recorded at fair valueinventory write-downs incurred in the consolidated financial statements ofCompany’s discontinued operations during the Company. years ended December 31, 2023 and 2022 is presented in Note 2 “Discontinued Operations”.
The following table summarizes the Company’s finalized allocation of the purchase price to assets acquired and liabilities assumed at the acquisition date.
| | | | | | | | | | | | | | |
| September 5, 2019 |
Fair value of net assets acquired | | |
Cash | $ | 2,896 | |
Accounts receivable (i)
| | 647 | |
Prepaid expenses and other assets | | 265 | |
Inventory | | 2,806 | |
Property and equipment | | 1,890 | |
Right-of-use assets | | 3,533 | |
Intangible assets (ii)
| | 64,037 | |
Goodwill | | 213,414 | |
Accounts payable and accrued liabilities | | (2,688) | |
Lease obligations | | (3,500) | |
| $ | 283,300 | |
(i) The fair value of acquired accounts receivable is $647. NaN loss allowance was recognized on acquisition.
(ii) Intangible assets include the fair value of brand name of $64,000, the remaining balance relates to software.
For the year ended December 31, 2019, acquisition-related costs of $8,531 were expensed. These costs are included in the consolidated statement of net income (loss) in financing and transaction costs.
The goodwill recognized represents the excess over the fair value of the net tangible and intangible assets acquired as a part of the Redwood acquisition. This goodwill is attributable to the expertise and reputation of the assembled workforce acquired, the expected synergies, and other intangible assets that do not qualify for separate recognition. The goodwill is 0t deductible for income tax purposes. The Relief-from-Royalty Method was used to value the intangible asset relating to the Lord Jones™ brand. Significant inputs include discount rate, growth rates, and cash flow projections.
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020, 2019, and 2018
(In thousands of U.S. dollars, except for gram and share amounts)
During the period from September 5, 2019 to December 31, 2019, the Company recognized $3,364 in revenues and a net loss of $2,613 from Redwood operations. If the acquisition had occurred on January 1, 2019, the Company estimates that for the year ended December 31, 2019, it would have recorded an increase of $12,266 in revenues and a decrease of $1,112 in net income and that for the year ended December 31, 2018, it would have recorded an increase of $7,630 in revenues and an increase of $1,533 in net income.
There were 0 business combinations during the years ended December 31, 2020 or December 31, 2018.
28. Repurposing Charges
During the year ended December 31, 2019, the Company commenced initiatives to better align its evolving business and its strategy. Certain facilities at the Peace Naturals campus were repurposed from cultivation activities to provide for the following activities: additional R&D activities focused on new technologies for value-added product manufacturing; production and manufacturing of derivative products; and increased vault and warehousing capabilities.
The activities associated with the repurposing were completed as of December 31, 2019. The following table presents information associated with this plan:
| | | | | |
| Year ended December 31, 2019 |
Employee termination benefits | $ | 889 | |
Impairment costs associated with plan | 4,439 | |
| $ | 5,328 | |
During the year ended December 31, 2019, an inventory write-down associated with the repurposing cost of $1,940 was included as part of inventory write-down on the consolidated statements of net income (loss) and comprehensive income (loss). The Company did not incur any further significant costs related to the repurposing activities.
NaN repurposing costs were incurred during the years ended December 31, 2020 or December 31, 2018.
As of December 31, 2020, there was 0 accrued liability associated with the Company’s repurposing initiative (December 31, 2019 - $907). All repurposing related charges were incurred within the Rest of World segment.
29. Segment Information
Segment reporting is prepared on the same basis that the Company’s chief operating decision makers (the “CODMs”) manage the business, make operating decisions and assess the Company’s performance. For the years ended December 31, 2020 and December 31, 2019, the Company determined that it has the following 2 reportable segments: United States and Rest of World. The United States operating segment consists of the manufacture and distribution of hemp-derived CBD infused products. The Rest of World operating segment is involved in the cultivation, manufacture, and marketing of cannabis and cannabis-derived products for the medical and adult-use markets. These 2 segments represent the geographic regions in which the Company operates and the different product offerings within each geographic region. The results of each segment are regularly reviewed by the CODMs to assess the performance of the segment and make decisions regarding the allocation of resources. The CODMs review adjusted earnings (loss) before interest, tax, depreciation and amortization (“Adjusted EBITDA”) as the measure of segment profit or loss to evaluate performance of and allocate resources for its reportable segments. Adjusted EBITDA is defined as earnings before interest, tax, depreciation, non-cash items and items that do not reflect management’s assessment of on-going business performance.
Reporting by operating segments follows the same accounting policies as those used to prepare the consolidated financial statements. The operating segments are presented in accordance with the same criteria used for internal reporting prepared for the CODMs. Inter-segment transactions are recorded at the stated values as agreed to by the segments.
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020, 2019, and 2018
(In thousands of U.S. dollars, except for gram and share amounts)
Segment data was as follows for the year ended December 31, 2020:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | United States | | Rest of World | | Corporate | | Total |
| Consolidated statements of net income (loss) and comprehensive income (loss) | | | | | | | | |
| Net revenue | | | | | | | | |
| Cannabis flower | | $ | 0 | | | $ | 27,932 | | | $ | 0 | | | $ | 27,932 | |
| Cannabis extracts | | 9,495 | | | 8,759 | | | 0 | | | 18,254 | |
| Other | | 0 | | | 533 | | | 0 | | | 533 | |
| Net revenue | | 9,495 | | | 37,224 | | | 0 | | | 46,719 | |
| | | | | | | | |
| Share of loss from equity accounted investees | | 0 | | | (4,510) | | | 0 | | | (4,510) | |
| | | | | | | | |
| Interest revenue | | 16 | | | 18,585 | | | 0 | | | 18,601 | |
| Interest expense | | (34) | | | (152) | | | 0 | | | (186) | |
| Interest income (expense), net | | (18) | | | 18,433 | | | 0 | | | 18,415 | |
| | | | | | | | |
| Depreciation and amortization | | 234 | | | 2,638 | | | 0 | | | 2,872 | |
| Income tax expense | | 323 | | | 1,024 | | | 0 | | | 1,347 | |
| Loss from discontinued operations | | 0 | | | (650) | | | 0 | | | (650) | |
| Adjusted EBITDA | | (28,019) | | | (98,349) | | | (20,885) | | | (147,253) | |
| | | | | | | | |
| Consolidated balance sheets | | | | | | | | |
| Total assets | | 253,745 | | | 388,351 | | | 1,283,586 | | | 1,925,682 | |
| Investments in equity accounted investees | | 0 | | | 19,235 | | | 0 | | | 19,235 | |
| Goodwill | | 178,414 | | | 1,108 | | | 0 | | | 179,522 | |
| Purchase of property, plant and equipment | | 385 | | | 31,027 | | | 0 | | | 31,412 | |
Segment data was as follows for the year ended December 31, 2019:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | United States | | Rest of World | | Corporate | | Total |
| Consolidated statements of net income (loss) and comprehensive income (loss) | | | | | | | | |
| Net revenue | | | | | | | | |
| Cannabis flower | | $ | 0 | | | $ | 15,020 | | | $ | 0 | | | $ | 15,020 | |
| Cannabis extracts | | 3,364 | | | 5,338 | | | 0 | | | 8,702 | |
| Other | | 0 | | | 28 | | | 0 | | | 28 | |
| Net revenue | | 3,364 | | | 20,386 | | | 0 | | | 23,750 | |
| | | | | | | | |
| Share of loss from equity accounted investees | | 0 | | | (2,009) | | | 0 | | | (2,009) | |
| | | | | | | | |
| Interest revenue | | 6 | | | 29,207 | | | 0 | | | 29,213 | |
| Interest expense | | 0 | | | (1,244) | | | 0 | | | (1,244) | |
| Interest income, net | | 6 | | | 27,963 | | | 0 | | | 27,969 | |
| | | | | | | | |
| Depreciation and amortization | | 46 | | | 2,044 | | | 0 | | | 2,090 | |
| Income tax expense | | 0 | | | 0 | | | 0 | | | 0 | |
| Loss from discontinued operations | | 0 | | | (363) | | | 0 | | | (363) | |
| Adjusted EBITDA | | (1,703) | | | (84,826) | | | (11,779) | | | (98,308) | |
| | | | | | | | |
| Consolidated balance sheets | | | | | | | | |
| Total assets | | 293,985 | | | 309,854 | | | 1,486,603 | | | 2,090,442 | |
| Investments in equity accounted investees | | 0 | | | 557 | | | 0 | | | 557 | |
| Goodwill | | 213,414 | | | 1,078 | | | 0 | | | 214,492 | |
| Purchase of property, plant and equipment | | 259 | | | 38,405 | | | 0 | | | 38,664 | |
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020, 2019, and 2018
(In thousands of U.S. dollars, except for gram and share amounts)
Segment data was as follows for the year ended December 31, 2018:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | United States | | Rest of World | | Corporate | | Total |
| Consolidated statements of net income (loss) and comprehensive income (loss) | | | | | | | | |
| Net revenue | | | | | | | | |
| Cannabis flower | | $ | 0 | | | $ | 9,210 | | | $ | 0 | | | $ | 9,210 | |
| Cannabis extracts | | 0 | | | 2,732 | | | 0 | | | 2,732 | |
| Other | | 0 | | | 179 | | | 0 | | | 179 | |
| Net revenue | | 0 | | | 12,121 | | | 0 | | | 12,121 | |
| | | | | | | | |
| Share of loss from equity accounted investees | | 0 | | | (723) | | | 0 | | | (723) | |
| Interest revenue | | 0 | | | 220 | | | 0 | | | 220 | |
| Interest expense | | 0 | | | (139) | | | 0 | | | (139) | |
| Interest income, net | | 0 | | | 81 | | | 0 | | | 81 | |
| | | | | | | | |
| Depreciation and amortization | | 0 | | | 807 | | | 0 | | | 807 | |
| Income tax expense | | 0 | | | 0 | | | 0 | | | 0 | |
| Loss from discontinued operations | | 0 | | | (894) | | | 0 | | | (894) | |
| Adjusted EBITDA | | 0 | | | (10,357) | | | 0 | | | (10,357) | |
| | | | | | | | |
| Consolidated balance sheets | | | | | | | | |
| Total assets | | 0 | | | 183,471 | | | 0 | | | 183,471 | |
| Investments in equity accounted investees | | 0 | | | 2,960 | | | 0 | | | 2,960 | |
| Goodwill | | 0 | | | 1,314 | | | 0 | | | 1,314 | |
| Purchase of property, plant and equipment | | 0 | | | 88,308 | | | 0 | | | 88,308 | |
Adjusted EBITDA is reconciled to net income (loss) as followsrestructuring activity for the years ended December 31, 2020, 20192023 and 2018:2022:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| (in thousands of U.S. dollars) | | Year ended December 31, 2020 |
| | US | | ROW | | Corporate
Expenses | | Total |
| Net income (loss) | | $ | (77,368) | | | $ | 32,671 | | | $ | (30,573) | | | $ | (75,270) | |
| Adjustments | | | | | | | | |
| Interest expense (income), net | | 18 | | | (18,433) | | | 0 | | | (18,415) | |
| Income tax expense | | 323 | | | 1,024 | | | 0 | | | 1,347 | |
| Impairment loss on goodwill and intangible assets | | 40,000 | | | 0 | | | 0 | | | 40,000 | |
| | | | | | | | |
| Financing and transaction costs | | 40 | | | 0 | | | 0 | | | 40 | |
| Gain on revaluation of derivative liabilities | | 0 | | | (129,254) | | | 0 | | | (129,254) | |
| Gain on disposal of other investments | | 0 | | | (4,789) | | | 0 | | | (4,789) | |
| Share of loss from equity accounted investees | | 0 | | | 4,510 | | | 0 | | | 4,510 | |
| Loss from discontinued operations | | 0 | | | 650 | | | 0 | | | 650 | |
| | | | | | | | |
| Other loss (income) | | 20 | | | 1,814 | | | 0 | | | 1,834 | |
| Review costs related to restatement of 2019 interim financial statements | | 0 | | | 0 | | | 9,688 | | | 9,688 | |
| Share-based payments | | 8,714 | | | 6,647 | | | 0 | | | 15,361 | |
| Adjusted EBIT | | (28,253) | | | (105,160) | | | (20,885) | | | (154,298) | |
| Adjustments | | | | | | | | |
| Depreciation and amortization | | 234 | | | 6,811 | | | 0 | | | 7,045 | |
| Adjusted EBITDA | | (28,019) | | | (98,349) | | | (20,885) | | | (147,253) | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| As of January 1, 2023 | | Expenses | | Payments/Write-offs | | | | As of December 31, 2023 |
| Employee Termination Benefits | $ | 403 | | | $ | 1,371 | | | $ | (1,624) | | | | | $ | 150 | |
| | | | | | | | | |
| Other Restructuring Costs | 21 | | | 153 | | | (174) | | | | | — | |
| Total | $ | 424 | | | $ | 1,524 | | | $ | (1,798) | | | | | $ | 150 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| As of January 1, 2022 | | Expenses | | Payments/Write-offs | | | | As of December 31, 2022 |
| Employee Termination Benefits | $ | — | | | $ | 1,883 | | | $ | (1,480) | | | | | $ | 403 | |
| | | | | | | | | |
| Other Restructuring Costs | — | | | 1,662 | | | (1,641) | | | | | 21 | |
| Total | $ | — | | | $ | 3,545 | | | $ | (3,121) | | | | | $ | 424 | |
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020, 2019, and 2018
(In thousands of U.S. dollars, except for gram and share amounts)
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| (in thousands of U.S. dollars) | | Year ended December 31, 2019 |
| | US | | ROW | | Corporate
Expenses | | Total |
| Net income (loss) | | $ | (3,070) | | | $ | 1,180,241 | | | $ | (11,597) | | | $ | 1,165,574 | |
| Adjustments | | | | | | | | |
| Interest income, net | | (6) | | | (27,963) | | | 0 | | | (27,969) | |
| | | | | | | | |
| Impairment loss on goodwill and intangible assets | | 0 | | | 0 | | | 0 | | | 0 | |
| Repurposing charges | | 0 | | | 7,268 | | | 0 | | | 7,268 | |
| Financing and transaction costs | | 117 | | | 32,091 | | | 0 | | | 32,208 | |
| Gain on revaluation of derivative liabilities | | 0 | | | (1,276,819) | | | 0 | | | (1,276,819) | |
| Gain on disposal of other investments | | 0 | | | (16,277) | | | 0 | | | (16,277) | |
| Share of loss from equity accounted investees | | 0 | | | 2,009 | | | 0 | | | 2,009 | |
| | | | | | | | |
| | | | | | | | |
| Loss from discontinued operations | | 0 | | | 363 | | | 0 | | | 363 | |
| | | | | | | | |
| Other loss (income) | | 182 | | | (197) | | | (182) | | | (197) | |
| Share-based payments | | 900 | | | 10,719 | | | 0 | | | 11,619 | |
| Adjusted EBIT | | (1,877) | | | (88,565) | | | (11,779) | | | (102,221) | |
| Adjustments | | | | | | | | |
| Depreciation and amortization | | 174 | | | 3,739 | | | 0 | | | 3,913 | |
| Adjusted EBITDA | | $ | (1,703) | | | $ | (84,826) | | | $ | (11,779) | | | $ | (98,308) | |
| | | | | | | | | | | |
(in thousands of U.S. dollars) | | | Year ended December 31, 2018 |
| | | | |
Net income (loss) | | | | | $ | (21,817) | |
Adjustments | | | | | |
Interest income, net | | | | | (81) | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
Loss (gain) on disposal of other investments | | | | | (164) | |
Share of loss (income) from equity accounted investees | | | | | 723 | |
| | | | | |
| | | | | |
Loss from discontinued operations | | | | | 894 | |
| | | | | |
| | | | | |
Share-based payments | | | | | 8,151 | |
| | | | | |
Adjusted EBIT | | | | | (12,294) | |
Adjustments | | | | | |
Depreciation and amortization | | | | | 1,937 | |
Adjusted EBITDA | | | | | $ | (10,357) | |
Sources of net revenue were as follows:
| | | | | | | | | | | |
| Year ended December 31, |
| 2020 | | 2019 |
| Cannabis flower | $ | 27,932 | | | $ | 15,020 | |
| Cannabis extracts | 18,254 | | | 8,702 | |
| Other | 533 | | | 28 | |
| Net revenue | $ | 46,719 | | | $ | 23,750 | |
Net revenue attributed to a geographic region based on the location of the customer were as follows:
| | | | | | | | | | | | | |
| Year ended December 31, |
| 2020 | | 2019 | | |
| Canada | $ | 34,538 | | | $ | 20,202 | | | |
| United States | 9,495 | | | 3,364 | | | |
| Other countries | 2,686 | | | 184 | | | |
| Total | $ | 46,719 | | | $ | 23,750 | | | |
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020, 2019, and 2018
(In thousands of U.S. dollars, except for gram and share amounts)
Property, plant and equipment assets were physically located in the following geographic regions:
| | | | | | | | | | | |
| As of December 31, |
| 2020 | | 2019 |
| Canada | $ | 162,163 | | | $ | 139,160 | |
| United States | 2,293 | | | 2,103 | |
| Other countries | 23,143 | | | 18,685 | |
| Total | $ | 187,599 | | | $ | 159,948 | |
The Company sells products through a limited number of major customers. Major customers are defined as customers that each individually accounted for greater than 10% of the Company’s annual revenues and greater than 10% of accounts receivable.
United States
During the years ended December 31, 2020 and December 31, 2019, the Company had no major customers in the U.S. segment.
As of December 31, 2020, $65 in expected credit losses has been recognized on receivables from contract with customers (December 31, 2019 – $12). Refer to Note 23(a).
Rest of World
During the year ended December 31, 2020, the Company earned a total net revenue before excise taxes of $34,295 from four major customers (December 31, 2019 – $7,597; December 31, 2018 – $2,186 from two and one major customers, respectively), accounting for 63% of the Company’s revenues (December 31, 2019 – 32%; December 31, 2018 – 17%).
As of December 31, 2020, $9 (December 31, 2019 – $124; December 31, 2018 – $NaN) in expected credit losses has been recognized on receivables from contract with customers. Refer to Note 23(a).
30. Discontinued Operations and Held-for-sale Assets
During the year ended December 31, 2020, the Company advanced its plans for the sale and disposal of substantially all of the assets of OGBC. As a result, OGBC’s results of operations have been reclassified as discontinued operations in the accompanying consolidated financial statements. Accordingly, the property, plant and equipment assets of OGBC are separately reported as assets and liabilities held-for-sale as of December 31, 2020 on the consolidated balance sheet. For comparative purposes, amounts in the prior periods have been reclassified to conform to current year presentation, as disclosed below. For the year ended December 31, 2020, the Company recorded $1,860 as a loss on held-for-sale assets, including $919 as a valuation allowance loss upon reclassification of property, plant and equipment assets as held-for-sale in Q3 2020, and a further $941 impairment charge on held-for-sale assets in Q4 2020 upon surrender of the OGBC Health Canada licenses. OGBC was formerly included within the Rest of World segment.
The following table summarizes the financial information for discontinued operations as of December 31, 2020:
| | | | | | | | | | | | | | | | | |
| Year ended December 31, |
| 2020 | | 2019 | | 2018 |
| Consolidated statements of net income (loss) and comprehensive income (loss) | | | | | |
| OGBC income (loss) from discontinued operations, net of income taxes | $ | (650) | | | $ | (363) | | | $ | (894) | |
| | | | | | | | | | | |
| As of December 31, |
| 2020 | | 2019 |
| Consolidated balance sheets | | | |
| Long-term assets classified as discontinued operations | $ | 1,176 | | | $ | 3,248 | |
Cronos Group Inc.
Notes to Consolidated Financial Statements
For the years ended December 31, 2020, 2019, and 2018
(In thousands of U.S. dollars, except for gram and share amounts)
The following table presents a reconciliation of the carrying amounts of major classes of assets and liabilities of the discontinued operations to total assets and liabilities of the disposal group classified as held-for-sale in the consolidated balance sheet of December 31, 2019:
| | | | | | | | | | | | | | | | | | | | |
| | As of December 31, 2019 |
| | As previously disclosed | | Held-for-sale adjustment | | Adjusted balance |
| Property, plant and equipment | | $ | 161,809 | | | $ | (1,861) | | | $ | 159,948 | |
| Intangible assets | | 72,320 | | | (1,085) | | | 71,235 | |
| Goodwill | | 214,794 | | | (302) | | | 214,492 | |
The following table presents a reconciliation of the major classes of line items constituting reported operating loss of discontinued operations to net loss of discontinued operations for the years ended December 31, 2019 and December 31, 2018:
| | | | | | | | | | | | | | | | | | | | |
| | Year ended December 31, 2019 |
| | As previously disclosed | | Held-for-sale adjustment | | Adjusted balance |
| Inventory write-down | | $ | (29,440) | | | $ | 267 | | | $ | (29,173) | |
| Operating expenses | | (103,620) | | | 109 | | | (103,511) | |
| Other income (loss) | | 1,287,058 | | | (13) | | | 1,287,045 | |
| Net income (loss) from continuing operations | | 1,165,574 | | | 363 | | | 1,165,937 | |
| | | | | | | | | | | | | | | | | | | | |
| | Year ended December 31, 2018 |
| | As previously disclosed | | Held-for-sale adjustment | | Adjusted balance |
| Operating expenses | | $ | (27,554) | | | $ | 896 | | | $ | (26,658) | |
| Other income (loss) | | (476) | | | (2) | | | (478) | |
| Net income (loss) from continuing operations | | (21,817) | | | 894 | | | (20,923) | |
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE.
None.
ITEM 9A. CONTROLS AND PROCEDURES.
(a)Evaluation of disclosure controlsDisclosure Controls and procedures.Procedures.
OurThe Company’s management, with the participation of the Chief Executive Officer and the Chief Financial Officer, performed an evaluation of ourthe disclosure controls and procedures, as defined in Rules 13a-15(e) and 15d-15(e) of the Securities Exchange Act. OurAct of 1934 (the “Exchange Act”), as of December 31, 2023. Based on that evaluation, management has concluded that, as of December 31, 2023, the disclosure controls and procedures are designedwere effective to ensureprovide reasonable assurance that the information required to be disclosed by us in reports we file or submit under the Exchange Act iswere recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC, and to ensure that the information required to be disclosed by us in reports that we file or submit under the Exchange Act, is accumulated and communicated to our management, including the principal executive officer and principal financial officer, as appropriate, to allow timely decisions regarding required disclosure. Based on this evaluation, the Chief Executive Officer and Chief Financial Officer have concluded that our disclosure controls and procedures were not effective as of December 31, 2020 due to the material weakness described below.
(b)(i) Management’s Report on Internal Control Over Financial Reporting.Reporting
The Company’s management is responsible for establishing and maintaining adequate internal control over financial reporting, (asas such term is defined in Rule 13a-15(f) underof the Exchange Act). ManagementAct. Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we conducted an assessmentevaluation of the effectiveness of our internal control over financial reporting based upon criteria established in Internal Control – Integrated Framework (2013) by the Committee of Sponsoring Organizations of the Treadway Commission. Based on that evaluation, our management concluded that our internal control over financial reporting was effective as of December 31, 2023.
Our independent registered public accounting firm, KPMG LLP, who audited the consolidated financial statements included in this Annual Report, issued an unqualified opinion on the effectiveness of the Company’s internal control over financial reporting based on the criteria set forthreporting.
(c)Changes in Internal Control - Integrated Framework issued byover Financial Reporting
Other than the Committee of Sponsoring Organizationsremediation of the Treadway Commission (2013 framework). Based on the Company’s assessment, management has concluded that its internal control over financial reporting was not effective as of December 31, 2020 to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with GAAP, due to the material weakness described below.
A material weakness is a deficiency, or combination of deficiencies in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis.
2020 Material Weakness – Inventory Verification
Management failed to properly design and execute sufficient procedures to verify inventory quantities. Specifically, while inventory counts were performed in the fourth quarter, (i) the aggregate value of items excluded from the count exceeded the Company’s materiality threshold, and (ii) human error in count execution, data transposition and reconciliation analysis resulted in inaccurate adjustments. This deficiency did not result in errors that were quantitatively material. Nevertheless, the deficiency creates a reasonable possibility that a material misstatement to the consolidated financial statements will not be prevented or detected on a timely basis.
The effectiveness of internal control over financial reporting has been audited by KPMG LLP, an independent registered public accounting firm, who has issued an adverse opinion on the effectiveness of our internal control over financial reporting as of December 31, 2020 as stated in their report which is included in the financial statements in Part II, Item 8 of this 10-K.
Internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
(b)(ii) Remediation of 2020 Material Weakness
Contributing factors included restrictions in the ability to perform physical inventory counts at third party logistics and subcontract manufacturing locations, resource turnover and the organization’s experience in processing count results within their new ERP system implemented at the beginning of the third quarter of 2020.
Management will (i) enhance their count procedures to ensure appropriate consideration and coverage of their total inventory balance, (ii) implement cycle counts as a redundant control to supplement the annual physical count, and (iii) provide training to inventory teams on count procedures and inventory management control expectations.
(c) Changes in internal control over financial reporting.
During the year, the Company implemented a new ERP system across the Canadian business. The new ERP system is a meaningful component of our internal control over financial reporting and is expected to enable us to realize efficiencies throughout our finance, supply chain and operations processes. In connection with the ERP implementation, we updated the processes and controls that constitute our internal control over financial reporting, as necessary, to accommodate related changes to our accounting procedures and business processes.
The Company has implemented its processes and internal controls over financial reporting in the U.S. segment for the year ended December 31, 2020.
Closure of 2019 Material Weakness
As disclosed in Part II Item 9A, Controls and Procedures, in our Annual Report on Form 10-K/A for the fiscal year ended December 31, 2019 (the “2019 Annual Report”), during the fourth quarter of 2019 we identified material weaknesses in internal controls related to risk assessment, segregation of duties, and non-routine transactions.
During 2020, management implemented our previously disclosed remediation plan that included: (i) enhancement of our quarterly review of the risk assessment model and its risk control matrices, (ii) establishing a risk committee, which reviews the risk assessment foridentified, there were no changes in the business on a quarterly basis, (iii) enhancement of our delegation of authority matrix, to further limit groups that may authorize sales or purchases of inventory, (iv) the establishment of a review process and internal database to identify entities that are both vendors and customers of the Company, in order to identify potential related transactions, (v) the implementation of revenue recognition training, (vi) the creation and implementation of a non-routine transaction policy, (vii) implementation of procedures for the preparation of business cases for all non-routine business-to-business unbranded sales and purchases, and (viii) expansion of the sub-certification processes to additional members of management to ensure that non routine transactions are identified.
During the fourth quarter of 2020, we completed our testing of the operating effectiveness of the implemented controls and found them to be effective. As a result, we have concluded the material weaknesses reported in the 2019 Annual Report have been remediated as of December 31, 2020.
The completed remediation to date is described below:
| | | | | | | | | | | | | | | |
Material Weakness | Control, Control Enhancement or Mitigant | Implementation Status | Management Testing Status | Remediation Status | |
Risk Assessment | •Enhance quarterly review of risk assessment model and risk control matrices
| Implemented | Tested | Remediated | |
•Establish a risk committee which reviews the risk assessment for changes in the business on a quarterly basis
| Implemented | Tested | Remediated | |
Segregation of Duties | •Enhance Delegation of Authority matrix to further limit groups that may authorize sales or purchases of inventory
| Implemented | Tested | Remediated | |
•Establish review process and internal database to identify entities that are both vendors and customers of the Company
| Implemented | Tested | Remediated | |
•Create and implement revenue recognition training
| Implemented | Tested | Remediated | |
Non-Routine Transactions | •Create and implement a Non-Routine Transactions Policy to include:
| Implemented | Tested | Remediated | |
◦Definition of Non-Routine Transactions
|
◦Accounting memorandum completion
|
◦CEO/CFO approval
|
•Formalization of business cases for all non-routine business to business unbranded sales and purchases to be reviewed on a quarterly basis to ensure alignment with the objectives of the business
| Implemented | Tested | Remediated | |
•Expand the sub-certification process to additional members of management to ensure that non routine transactions are identified
| Implemented | Tested | Remediated | |
In the fourth quarter of 2020, there was no change in ourCompany’s internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act), other than noted above.that occurred during the fourth quarter of the year ended December 31, 2023, that have materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting.
Remediation of Previously Reported Material Weakness
We had identified a material weakness related to Information Technology General Controls in user access management and the provisioning and monitoring of privileged access as of December 31, 2022.
As of December 31, 2023, the Company had remediated the identified material weakness.
ITEM 9B. OTHER INFORMATION.
None.
ITEM 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS.
Not applicable.
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The information required under this Item is incorporated herein by reference to our definitive proxy statement or to an amendment to this Annual Report on Form 10-K to be filed with the SEC no later than 120 days after the close of our fiscal year ended December 31, 2020.2023.
ITEM 11. EXECUTIVE COMPENSATION
The information required under this Item is incorporated herein by reference to our definitive proxy statement or to an amendment to this Annual Report on Form 10-K to be filed with the SEC no later than 120 days after the close of our fiscal year ended December 31, 2020.2023.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information required under this Item is incorporated herein by reference to our definitive proxy statement or to an amendment to this Annual Report on Form 10-K to be filed with the SEC no later than 120 days after the close of our fiscal year ended December 31, 20202023.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE.
The information required under this Item is incorporated herein by reference to our definitive proxy statement or to an amendment to this Annual Report on Form 10-K to be filed with the SEC no later than 120 days after the close of our fiscal year ended December 31, 2020.2023.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES.
The information required under this Item is incorporated herein by reference to our definitive proxy statement or to an amendment to this Annual Report on Form 10-K to be filed with the SEC no later than 120 days after the close of our fiscal year ended December 31, 2020.2023.
PART IV
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES.
The following documents are filed as part of this Annual Report on Form 10-K, or incorporated herein by reference:
(a)(1) Financial Statements. The following financial statements of Cronos Group Inc. are filed as part of this Annual Report on Form 10-K on the pages indicated.
| | | | | | | | |
| CRONOS GROUP INC. AND SUBSIDIARIES | | Page No. |
| Reports of Independent Registered Public Accounting Firm | | |
Consolidated Balance Sheets as of December 31, 20202023 and 20192022 | | |
Consolidated Statements of Net Income (Loss)Loss and Comprehensive Income (Loss)Loss for the years ended December 31, 2020, 2019,2023, 2022, and 20182021 | | |
Consolidated Statements of Changes in Shareholders’ (Deficit) Equity for the years ended December 31, 2020, 2019,2023, 2022, and 20182021 | | |
Consolidated Statements of Cash Flows for the years ended December 2020, 2019,31, 2023, 2022, and 20182021 | | |
| Notes to Consolidated Financial Statements | | |
(a)(2) Financial Statement Schedules. Schedules are omitted because the required information is inapplicable, not material, or the information is presented in the consolidated financial statements or related notes.
(a)(3) Exhibits. The exhibits listed in the Exhibit Index immediately below are filed as part of this Annual Report on Form 10-K or are incorporated by reference herein.
| | | | | | | | |
| Exhibit Number | | Exhibit Description |
| 2.1 | | Membership InterestOptions Purchase Agreement, amongdated June 14, 2021, by and between Cronos GroupUSA Holdings Inc., Redwood Holdings Group, LLC and certain key persons, dated as of August 1, 2019PharmaCann Inc. (incorporated by reference to Exhibit 99.12.1 to the Company’s Current Report on Form 8-K of Foreign Private Issuer,Cronos Group Inc. filed August 2, 2019)June 15, 2021). |
| 2.2 | | |
| 3.1 | | |
| 4.1 | | |
4.2*4.2 | | |
| 10.1 | | Subscription Agreement, dated as of December 7, 2018, by and among Cronos Group Inc., Altria Summit LLC, and, solely for the purposes specified therein, Altria Group, Inc. (incorporated by reference to Exhibit 99.1 to the Company’s Current Report of Foreign Private Issuer, filed December 10, 2018). |
| 10.2 | | |
| 10.3 | | |
| 10.4 | | |
10.5†10.5 | | |
| 10.6† | | |
10.6†10.7† | | |
10.7†10.8† | | |
| | | | | | | | |
10.10†10.11† | | |
10.11† | | |
| 10.12† | | ExecutiveDescription of Oral Amendment, effective as of June 2019, to Employment Agreement, by and among Hortican Inc., Jerry Barbato and, solely for the purposes specified therein,between Cronos Group Inc., (f/k/a PharmaCann Capital Corporation) and Michael Gorenstein, effective as of April 15, 2019.August 10, 2016 (incorporated by reference to the corresponding exhibitExhibit 10.11 to the Annual Report on Form 10-K of Cronos Group Inc., filed on March 2, 2020). |
| 10.13† | | |
| 10.14† | | |
10.15† | | |
10.16† | | |
10.17† | | |
10.18† | | |
10.19† | | |
10.20† | | |
10.21† | | |
10.22† | | |
10.23† | | |
10.24† | | |
10.25† | | |
10.26† | | |
10.27†10.15† | | |
10.28†10.16† | | |
10.29†10.17† | | |
| | | | | | | | |
10.30† | | |
| 10.18† | | |
| 10.19† | | |
| 10.20† | | |
| 10.21† | | |
| 10.22† | | |
| 10.23† | | |
| 10.24†* | | |
| 10.25† | | |
| 10.26 | | |
| 10.27† | | |
| 10.28† | | |
| 10.29 | | |
| 10.30* | | |
| | | | | | | | |
| 10.31* | | |
| 14.1 | | |
| 21.1* | | |
| 23.1* | | |
| 24.1* | | |
| 31.1* | | |
| 31.2* | | |
| 32.1** | | |
| 32.2** | | |
| 97.1* | | |
| 101.INS* | | XBRL Instance Document |
| 101.SCH* | | XBRL Taxonomy Extension Schema Document. |
| 101.CAL* | | XBRL Taxonomy Extension Calculation Linkbase Document. |
| 101.DEF* | | XBRL Taxonomy Extension Definition Linkbase Document. |
| 101.LAB* | | XBRL Taxonomy Extension Label Linkbase Document. |
| 101.PRE* | | XBRL Taxonomy Extension Presentation Linkbase Document. |
† Management contract or compensatory plan or arrangement.
* Filed herewith.
** Furnished herewith and not “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | | | | |
| CRONOS GROUP INC. |
| | |
| By: | /s/ Kurt SchmidtMichael Gorenstein |
| | Kurt SchmidtMichael Gorenstein
Chairman, President and Chief Executive Officer |
| Date: February 26, 2021 | |
Power of Attorney
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints each of Kurt SchmidtMichael Gorenstein and Jerry Barbato,James Holm, severally, his or her attorneys-in-fact, each with the power of substitution, for him or her in any and all capacities, to sign any amendments to this Annual Report on Form 10-K, and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, hereby ratifying and confirming all that each of said attorneys-in-fact, or his substitute or substitutes, may do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated:
| | | | | | | | | | | | | | |
| Name | | Title | | Date |
/s/ Kurt SchmidtMichael Gorenstein | | Chairman, President and Chief Executive Officer
(Principal Executive Officer) | | February 26, 202129, 2024 |
Kurt SchmidtMichael Gorenstein | | | | |
/s/ Jerry BarbatoJames Holm | | Chief Financial Officer
(Principal Financial Officer) | | February 26, 202129, 2024 |
Jerry BarbatoJames Holm | | | | |
/s/ Puneet MathurJimmy McGinness | | Vice President, Controller
(Principal Accounting Officer) | | February 26, 202129, 2024 |
Puneet MathurJimmy McGinness | | | | |
/s/ Bronwen EvansKendrick Ashton, Jr. | | Director | | February 26, 202129, 2024 |
Bronwen EvansKendrick Ashton, Jr. | | | | |
/s/ Heather NewmanKamran Khan | | Director | | February 26, 202129, 2024 |
Heather NewmanKamran Khan | | | | |
| /s/ James Rudyk | | Director | | February 26, 202129, 2024 |
| James Rudyk | | | | |
/s/ Jody BegleyDominik Meier | | Director | | February 26, 202129, 2024 |
Jody BegleyDominik Meier | | | | |
| /s/ Jason Adler | | Director | | February 26, 202129, 2024 |
| Jason Adler | | | | |
/s/ Michael GorensteinElizabeth Seegar | | Director | | Director, Executive Chairman | February 26, 202129, 2024 |
Michael GorensteinElizabeth Seegar | | | | |
/s/ Murray Garnick | | Director | | February 26, 2021 |
Murray Garnick | | | | |