UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
(Mark One)
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 20202023
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from _ to _
Commission File Number: 001-38753
Moderna, Inc.
(Exact Name of Registrant as Specified in Its Charter)
| | | | | | | | |
| Delaware | | 81-3467528 |
| (State or Other Jurisdiction of Incorporation or Organization) | | (IRS Employer Identification No.) |
| | |
200 Technology Square Cambridge, Massachusetts | | 02139 |
| (Address of Principal Executive Offices) | | (Zip Code) |
(617) 714-6500
(Registrant’s Telephone Number, Including Area Code)
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | |
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
| Common stock, par value $0.0001 per share | MRNA | The Nasdaq Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☑ No ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☑
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☑ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☑ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer”, “accelerated filer”, “smaller reporting company”, and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| | | | | | | | | | | | | | | | | | | | |
| Large accelerated filer ☑ | | Accelerated filer ☐ | | Non-accelerated filer ☐ | | Smaller reporting company ☐ |
| | | | | | Emerging growth company ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☑
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. . Yes ☑ No ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant's executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☑
As of June 30, 2020,2023, the aggregate market value of voting and non-voting common equity held by non-affiliates of the registrant was approximately $25.25$40.2 billion based on the closing sale price on that date of $64.21.$121.50. Shares of common stock held by each executive officer and director and by each other person who may be deemed to be an affiliate of the Registrant have been excluded from this computation. The determination of affiliate status for this purpose is not necessarily a conclusive determination for other purposes.
As of February 16, 2021,2024, there were 399,769,582382,073,208 shares of the registrant’s common stock, par value $0.0001 per share, outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s Definitive Proxy Statement relating to its 20212024 Annual Meeting of Stockholders to be filed hereafter are incorporated by reference into Part III of this Annual Report on Form 10-K where indicated.
Table of Contents
| | | | | | | | |
PART I. | | Page |
| Item 1. | Business | |
| Item 1A. | Risk Factors | |
| Item 1B. | Unresolved Staff Comments | |
| Item 1C. | Cybersecurity | |
| Item 2. | Properties | |
| Item 3. | Legal Proceedings | |
| Item 4. | Mine Safety Disclosures | |
PART II. | | |
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | |
| Item 6. | Reserved[Reserved] | |
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations | |
| Item 7A. | Quantitative and Qualitative Disclosures about Market Risk | |
| Item 8. | Financial Statements and Supplementary Data | |
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | |
| Item 9A. | Controls and Procedures | |
| Item 9B. | Other Information | |
| Item 9C. | Disclosure Regarding Foreign Jurisdictions that Prevent Inspections | |
PART III. | | |
| Item 10. | Directors, Executive Officers and Corporate Governance | |
| Item 11. | Executive Compensation | |
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | |
| Item 13. | Certain Relationships and Related Transactions, and Director Independence | |
| Item 14. | Principal Accountant Fees and Services | |
PART IV. | | |
| Item 15. | Exhibits, Financial Statement Schedules | |
| Item 16. | Form 10-K Summary | |
| Signatures | | |
SUMMARY OF THE MATERIAL RISKS ASSOCIATED WITH OUR BUSINESS
Our business is subject to numerous risks and uncertainties that you should be aware of before making an investment decision, including those highlighted in the section entitled “Risk Factors.” These risks include, but are not limited to, the following:
•WhileEvolving dynamics in the market for COVID-19 vaccines are likely to impact our financial results, which are likely to result in lower product revenues in 2024 than we have received Emergency Use Authorization, or EUA, from the U.S. Food and Drug Administration and other provisional, interim or conditional authorizations from regulatory authorities outside the United States for our COVID-19 vaccine, we may encounter difficulties manufacturing. producing shipping or successfully commercializing the vaccine consistent with our existing or potential contractual obligations, including due to delays or difficulties experienced by our commercial partners;in recent years;
•We are devoting significant resourcesmay encounter difficulties producing or shipping our products consistent with our projections or future contractual commitments;
•We have limited sales, distribution and marketing experience and may be unable to the scale-up, manufacturing, development and commercialization ofeffectively establish such capabilities or supplement our COVID-19 vaccine, including for usecapabilities by the U.S. government and other global governmental and commercial partners, and the distribution of our vaccine requires us to complyentering into agreements with additional regulatory requirements, including pharmacovigilance regimes that require us to monitor safety data and to identify, evaluate and potentially respond to adverse reactions to our COVID-19 vaccine;third parties;
•The positive interim data frompharmaceutical market is intensely competitive, and we may not compete effectively in the ongoing clinical studies ofmarket for existing products, new treatment methods and new technologies;
•We may be unsuccessful or delayed in updating our COVID-19 vaccine and the EUA granted by the FDA may not be predictive of the final results of the clinical trials, which is one of a number of factors that may delay or prevent us from receiving full regulatory approval of our vaccine;
•Our current COVID-19 vaccine (mRNA-1273) may prove ineffective at providing protectionto protect against infection by variant strainsfuture variants of the SARS-CoV-2 virus, and updated versions of our COVID-19 vaccine may not protect against such variants;
•The commercial success of our products will depend on the degree of market acceptance by physicians, patients, third-party payors and others in the medical community;
•Sales of pharmaceutical products depend on the availability and extent of reimbursement from third-party payors, and we may be unsuccessfuladversely impacted by changes to such reimbursement policies or rules;
•Preclinical development is lengthy and uncertain, especially for mRNA medicines, and our preclinical programs or product candidates may be delayed or terminated;
•Clinical development is lengthy and uncertain, and our clinical programs may be delayed or terminated, or may be more costly to conduct than we anticipate;
•If we cannot obtain, or are delayed in adapting our COVID-19 vaccineobtaining, required regulatory approvals, we will be unable to effectively protect against variant strains of the SARS-CoV-2 virus;commercialize, or will be delayed in commercializing, product candidates we may develop;
•Our mRNA products developmentand product candidates and investigational medicines are based on novel technologies and any development candidates and investigational medicines we develop may beare complex and difficult to manufacture. We or our third-party manufacturers may encounter difficulties in manufacturing, product release, shelf life, testing, storage, supply chain management or shipping for any of our medicines, includingproducts;
•As we grow as a commercial company and our COVID-19 vaccine. If we or any ofdrug development pipeline increases and matures, the increased demand for clinical and commercial supplies from our third-party manufacturers encounter such difficulties,facilities and third parties may impact our ability to supply commercial product or material for clinical trials could be delayed or stopped;operate. We rely on third-party service providers, all of whom have inherent risks in their operations;
•Other companies or organizations may challenge our patent rights or may assert patent rights that prevent us from developing and commercializing our products—including our COVID-19 vaccine—or these rights may beWe are subject to government action, including “march in rights” byoperational risks associated with the U.S. or foreign governments, which could harmphysical and digital infrastructure at our ability to commercializemanufacturing facilities and those of our products or fully realize the financial benefits of those products;external service providers;
•Our businessindividualized neoantigen therapy (INT) product candidates are uniquely manufactured for each patient using a novel, complex manufacturing process and we may continue to be adversely affected by the ongoing coronavirus pandemic;encounter difficulties in producing INT;
•mRNA drugs have only been authorizedWe are dependent on single-source suppliers for emergency use, or other provisional, interim or conditional use, for COVID-19,some of the components and there is no guarantee that any other mRNA drug will be granted an EUA or will be granted full approvalmaterials used in, and the future as a result of efforts by others or us. mRNA drug development has substantial clinical developmentprocesses required to develop, our products and regulatory risks due to the novel nature of this new class of medicines;product candidates;
•We have incurred significant losses since our inception and we may incur significant losses again in the future;
•Preclinical development is lengthy and uncertain, especially for a new class of medicines such as mRNA, and therefore our preclinical programs or development candidates may be delayed, terminated, or may never advance to the clinic, any of which may have a material adverse impact on our platform or our business;
•Clinical development is lengthy and uncertain, especially with a new class of medicines such as mRNA medicines. Clinical trials of our investigational medicines may be delayed, including as a result of the COVID-19 pandemic or other pandemics in the future, and certain programs may never advance in the clinic or may be more costly to conduct than we anticipate, any of which could have a material adverse impact on our platform or our business;
•mRNA medicines are a novel approach, and negative perception of the efficacy, safety, or tolerability of any investigational medicines that we develop could adversely affect our ability to conduct our business, advance our investigational medicines, or obtain regulatory approvals;
•If we are not able to obtain, or if there are delays in obtaining, required regulatory approvals, or if other regulatory requirements are introduced, we will not be able to commercialize, or will be delayed in commercializing, investigational medicines we may develop, and our ability to generate revenue will be materially impaired;
•We have in the past entered into, and in the future may enter into, strategic alliances with third parties for the development and commercialization of our products development candidates and investigational medicines.product candidates. If these strategic alliances are not successful, our business could be adversely affected;
•We have limited sales, distribution, and marketing experience, and have only recently invested significant financial and management resourcesmay seek to establish these capabilities as a result of our rapid developmentadditional strategic alliances and, commercialization of our COVID-19 vaccine. Ifif we are unable to effectively establish such capabilities or enter intothem on commercially reasonable terms, we may have to alter our development and commercialization plans. Certain of our strategic alliance agreements with third parties to market and sell our future products, if approved,may restrict our ability to generate revenues may be adversely affected;
•Certain of our customers for our COVID-19 vaccine prepay us for a portion of the product payment for the vaccine doses that they expect to receive from us, and under the terms ofdevelop certain of our supply agreements, we may be required to refund some or all of those prepayments if a customer reduces its purchase commitment or if we fail to deliver the purchased volume;products;
•We will needmay be unable to developobtain and expandenforce patent protection for our company,discoveries and the intellectual property rights therein, or protect the confidentiality of our trade secrets;
•Uncertainty over intellectual property in the pharmaceutical and biotechnology industry has been the source of litigation and other disputes, which is inherently costly and unpredictable and can have adverse financial and freedom-to-operate consequences;
•We incurred net losses in 2023 and we are likely to incur losses again in the future; we have a limited history of recognizing revenue from product sales and may be unable to achieve long-term sustainable profitability;
•We may encounter difficulties in managing thisthe development and expansion which could disruptof our operations;company;
•Our internal computer systems and physical premises, or those of our strategic collaborators, other contractors, consultants or regulatory agenciesthird parties with which we share sensitive data or information, may fail or suffer security breaches, including from cybersecurity incidents, which could result in a material disruption ofmaterially disrupt our product development programs and our manufacturing operations; and
•The price of our common stock has been volatile, and fluctuates substantially, which could result in substantial losses for stockholders; and
•Unfavorable U.S. or global economic conditions could adversely affect our business, financial condition, or results of operations.stockholders.
You should consider carefully the risks and uncertainties described below, in the section entitled “Risk Factors” and the other information contained in this Annual Report on Form 10-K, including our consolidated financial statements and the related notes, before you decide whether to purchase our common stock. The risks described above are not the only risks that we face. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also impair our business operations.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K, including the sections entitled “Business,” “Risk Factors,”Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” contains express or implied forward-looking statements within the
meaning of the federal securities laws, Section 27A of the Securities Act of 1933, as amended (the Securities Act), and Section 21E of the Securities Exchange Act of 1934, as amended. We intend these forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995 and are including this statement for purposes of complying with those safe harbor provisions.amended (the Exchange Act). All statements other than statements of historical facts contained in this Annual Report are forward-looking statements. TheseForward-looking statements in this Annual Report on Form 10-K include, but are not limited to, statements about:
•our expectations regarding the future profitability of our COVID-19 vaccine franchise, as well as our ability to grow and maintain market share;
•our expectations regarding the evolution of the endemic, commercial COVID-19 vaccine market and future demand for COVID-19 vaccines;
•our expectations regarding sales of our COVID-19 vaccine in 2024 and beyond;
•our ability to continue to develop variant-specific versions of our COVID-19 vaccine that are able to effectively combat COVID-19 as the SARS-CoV-2 virus continues to evolve;
•the potential launch, following regulatory approvals, of our respiratory syncytial virus (RSV) vaccine for adults in the first half of 2024, as well as additional respiratory product launches in 2024 and 2025;
•the durability of our individualized neoantigen therapy (INT) candidate, the ability of our INT candidate to address different types of cancer, our Phase 3 clinical trials in adjuvant melanoma and non-small cell lung cancer (NSCLC) and our plans to rapidly expand to additional tumor types;
•our goal to launch up to 15 new products over the next five years;
•our discussions with regulators regarding our first-generation seasonal influenza vaccine candidate (mRNA-1010), and our intent to file for regulatory approval in 2024;
•the potential of our platform to address rare genetic diseases, and our plans to advance propionic acidemia (PA) and methylmalonic acidemia (MMA) programs into pivotal studies in 2024;
•our ability to deliver on the next-generation pipeline and platform, including the commencement of additional clinical trials;
•our ability to successfully contract with third-party suppliers, distributors and manufacturers;
•our ability and the ability of third parties with whom we contract to successfully manufacture, supply and distribute our products, at scale, as well as drug substances, delivery vehicles and product candidates for preclinical and clinical use;
•the scope of protection we are able to establish and maintain for intellectual property rights covering our commercial products, product candidates and technology, including our ability to enter into license agreements, and our expectations regarding pending legal proceedings related to our intellectual property;
•the timing of initiation, progress, completion, results (including interim data) and cost of our clinical trials, preclinical studies and research and development programs, as well as those of our collaborators;
•participant enrollment in our clinical trials, including enrollment demographics and timing;
•potential advantages of mRNA as compared to traditional medicine;
•our ability to obtain and maintain regulatory approval for our products;
•our ability to successfully commercialize our products, if approved, including in light of the size and growth potential of the markets for our products and the degree of market acceptance of our products;
•the pricing and reimbursement of our medicines, if approved;
•the buildout of our manufacturing and commercial operations, including our partnerships with various governments to establish mRNA vaccine manufacturing facilities;
•our financial performance and estimates of our future expenses, revenues and capital requirements;
•the potential benefits of strategic collaboration agreements and our ability to enter into strategic collaborations or other agreements with collaborators with development, regulatory and commercialization expertise;
•legal and regulatory developments in the United States and foreign countries;
•our ability to produce our products or product candidates with advantages in turnaround times or manufacturing cost;
•our ability to attract and retain key scientific, manufacturing, regulatory, commercial and management personnel; and
•developments relating to our competitors and our industry.
In some cases, forward-looking statements can be identified by terminology such as “may,” “should,” “expects,” “intends,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “continue,” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these identifying words. Forward-looking statements are based on our management’s belief and assumptions and on information currently available to our management. Although we believe that the expectations reflected in these forward-looking statements are reasonable, these statements relate to future events or our future operational or financial performance, and involve known and unknown risks, uncertainties, and other factors that may cause our actual results, performance, or achievements to be materially different from any future results, performance, or achievements expressed or implied by these forward-looking statements. Forward-looking statementsWe may not actually achieve the plans, intentions or expectations disclosed in this Annual Report on Form 10-K include, but are not limited to, statements about:
•our activities with respect to our COVID-19 vaccine, and our plans and expectations regarding future generations of our COVID-19 vaccine that we may develop in response to variants of the SARS-CoV-2 virus, ongoing clinical development, manufacturing and supply, pricing, commercialization, if approved, regulatory matters and third-party and governmental arrangements and potential arrangements;
•the initiation, timing, progress, results, and cost of our research and development programs and our current and future preclinical studies and clinical trials, including statements regarding the timing of initiation and completion of studies or trials and related preparatory work, the period during which the results of the trials will become available, and our research and development programs;
•the ultimate impact of the current coronavirus pandemic, or the COVID-19 pandemic, or any other health epidemic, on our business, manufacturing, clinical trials, research programs, supply chain, regulatory review, healthcare systems or the global economy as a whole;
•risks related to the direct or indirect impact of the COVID-19 pandemic or any future large-scale adverse health event, such as the scope and duration of the outbreak, government actions and restrictive measures implemented in response, material delays in diagnoses, initiation or continuation of treatment for diseases that may be addressed by our development candidates and investigational medicines, or in patient enrollment in clinical trials, potential clinical trials, regulatory review or supply chain disruptions, and other potential impacts to our business, the effectiveness or timeliness of steps taken by us to mitigate the impact of the pandemic, and our ability to execute business continuity plans to address disruptions caused by the COVID-19 pandemic or future large-scale adverse health event;
•our anticipated next steps for our development candidates and investigational medicines that may be slowed down due to the impact of the COVID-19 pandemic, including our resources being significantly diverted towards our COVID-19 vaccine efforts, particularly if the federal government seeks to require us to divert such resources;
•our ability to identify research priorities and apply a risk-mitigated strategy to efficiently discover and develop development candidates and investigational medicines, including by applying learnings from one program to our other programs and from one modality to our other modalities;
•our ability and the potential to successfully manufacture our drug substances, delivery vehicles, development candidates, and investigational medicines for preclinical use, for clinical trials and on a larger scale for commercial use, if approved;
•the ability and willingness of our third-party strategic collaborators to continue research and development activities relating to our development candidates and investigational medicines;
•our ability to obtain funding for our operations necessary to complete further development and commercialization of our investigational medicines;
•our ability to obtain and maintain regulatory approval of our investigational medicines;
•our ability to commercialize our products, if approved;
•the pricing and reimbursement of our investigational medicines, if approved;
•the implementation of our business model, and strategic plans for our business, investigational medicines, and technology;
•the scope of protection we are able to establish and maintain for intellectual property rights covering our investigational medicines and technology;
•estimates of our future expenses, revenues, capital requirements, and our needs for additional financing;
•the potential benefits of strategic collaboration agreements, our ability to enter into strategic collaborations or arrangements, and our ability to attract collaborators with development, regulatory, and commercialization expertise;
•future agreements with third parties in connection with the commercialization of our investigational medicines, if approved;
•the size and growth potential of the markets for our investigational medicines, and our ability to serve those markets;
•our financial performance;
•the rate and degree of market acceptance of our investigational medicines;
•regulatory developments in the United States and foreign countries;
•our ability to contract with third-party suppliers and manufacturers and their ability to perform adequately;
•our ability to produce our products or investigational medicines with advantages in turnaround times or manufacturing cost;
•the success of competing therapies that are or may become available;
•our ability to attract and retain key scientific or management personnel;
•the impact of laws and regulations;
•developments relating to our competitors and our industry; and
•other risks and uncertainties, including those listed under the caption “Risk Factors.”
In some cases, forward-looking statements, can be identified by terminology such as “may,” “should,” “expects,” “intends,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “continue,” or the negative of these terms or other comparable terminology. The risks set forth above are not exhaustive. Other sections of this report may include additional factors that could adversely affect our business and financial performance. Moreover, we operate in a very competitive and rapidly changing environment. New risk factors emerge from time to time and it is not possible for management to predict all risk factors, nor can we assess the impact of all risk factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. These statements are only predictions. Youyou should not place undue reliance on forward-looking statements because they involve known and unknown risks, uncertainties, and other factors, which are, in some cases, beyond our control and which could materially affect results.statements. Factors that may cause actual results or events to differ materially from current expectations include, among other things, those listed under the section entitled “Risk Factors” and elsewhere in this Annual Report on Form 10-K. If one or more of these risks or uncertainties occur, or if our underlying assumptions prove to be incorrect, actual events or results may vary significantly from those expressed or implied by the forward-looking statements. No forward-looking statement is a guarantee of future performance.
The forward-looking statements in this Annual Report on Form 10-K represent our views as of the date of this Annual Report on Form 10-K. We anticipate that subsequent events and developments will cause our views to change. However, while we may elect to update these forward-looking statements at some point in the future, we have no current intention of doing so except to the extent required by applicable law. You should therefore not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this Annual Report on Form 10-K.
This Annual Report on Form 10-K includes statistical and other industry and market data that we obtained from industry publications and research, surveys, and studies conducted by third parties. Industry publications and third-party research, surveys, and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. We have not independently verified the information contained in such sources.
NOTE REGARDING COMPANY REFERENCES
Unless the context otherwise requires, the terms “Moderna,” “the Company,the “Company,” “we,” “us,”“us” and “our” in this Annual Report on Form 10-K refer to Moderna, Inc. and its consolidated subsidiaries.
TRADEMARKS
This Annual Report on Form 10-K contains references to our trademarks and to trademarks belonging to other entities. Solely for convenience, trademarks and trade names referred to, including logos, artwork and other visual displays, may appear without the ® or TM symbols, but such references are not intended to indicate, in any way, that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto. We do not intend our use or display of other companies’ trade names or trademarks to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
PART I
Item 1. Business
Moderna is a leader in the creation of the field of messenger RNA (mRNA) medicine. By working at the intersection of science, technology and health for more than a decade, we have developed medicines at unprecedented speed and efficiency, including one of the earliest and most effective COVID-19 vaccines.
Our mRNA platform has enabled the development of therapeutics and vaccines for infectious diseases, immuno-oncology, rare diseases and autoimmune diseases. With a unique culture and a global team driven by the Moderna values and mindsets to responsibly change the future of human health, we strive to deliver the greatest possible impact to people through mRNA medicines.
Our first commercial product, Spikevax (our COVID-19 vaccine), has helped hundreds of millions of people worldwide combat COVID-19. SARS-CoV-2, the virus that causes COVID-19, continues to evolve and in 2023, the COVID-19 vaccine market transitioned to an endemic, seasonal commercial market. To adapt to the evolving market, we significantly resized our manufacturing infrastructure to help position our COVID-19 franchise for future profitability. We achieved 2023 net product sales of $6.7 billion, with $6.1 billion of COVID-19 vaccine sales, and recognition of approximately $0.6 billion of deferred revenue related to our efforts with Gavi, The Vaccine Alliance. In the United States, we achieved 48% market share in the retail market for the 2023 fall season, compared to 37% in the 2022 fall season.
Beyond COVID-19, in 2023, we prepared for the potential 2024 launch of our investigational respiratory syncytial virus (RSV) vaccine for adults, which we expect to further demonstrate the commercial potential of our mRNA platform. In cancer, we reported additional data from our Phase 2b trial evaluating our individualized neoantigen therapy (INT) in combination with Merck’s KEYTRUDA in melanoma patients compared to KEYTRUDA alone. The treatment continued to show significant and clinically meaningful improvement in recurrence-free survival and reduced the risk of recurrence or death by 49%. We believe that these data, with a median follow-up of approximately three years, demonstrate the durability of the therapy, and we have initiated Phase 3 studies in the adjuvant setting in patients with high-risk melanoma and non-small cell lung cancer. We and Merck plan to rapidly expand our clinical trials to additional tumor types.
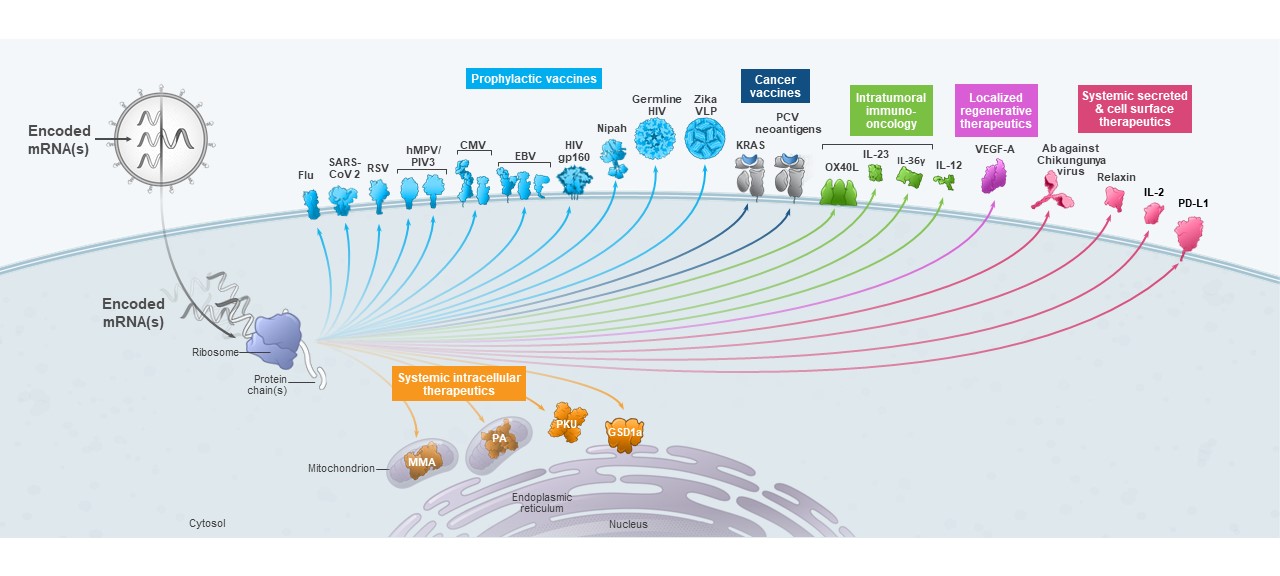
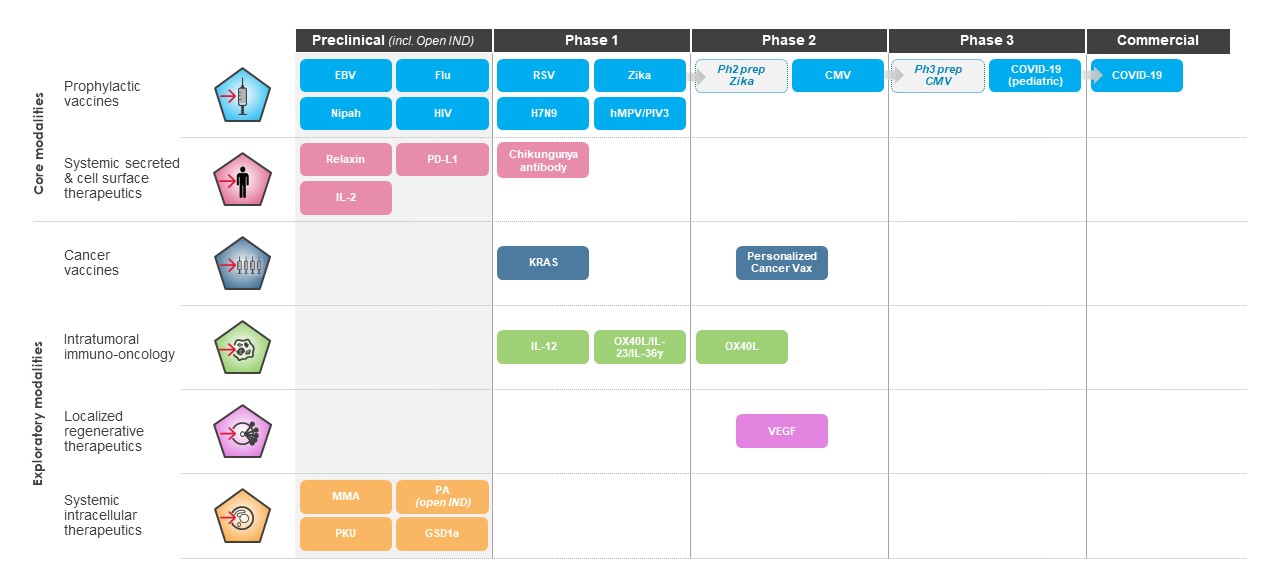
Having demonstrated clinical benefit in multiple infectious disease areas and skin cancer, as well as potential clinical benefit for several rare genetic diseases, we continue to advance a broad and diverse pipeline and are focused on execution to deliver for patients. Our pipeline includes 45 therapeutic and vaccine programs, nine of which are in late-stage development.
THE mRNA OPPORTUNITY
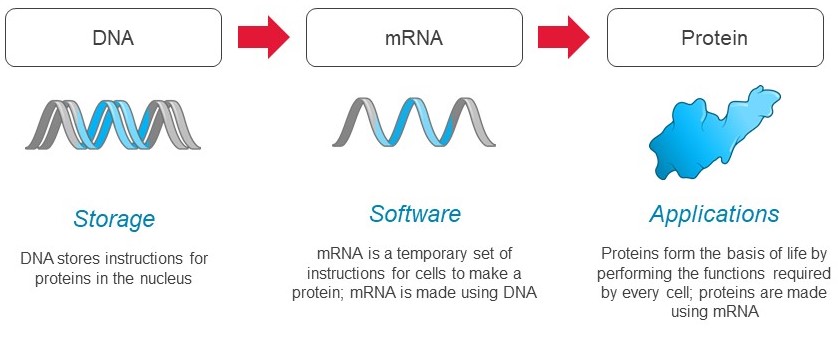
mRNA, the software of life
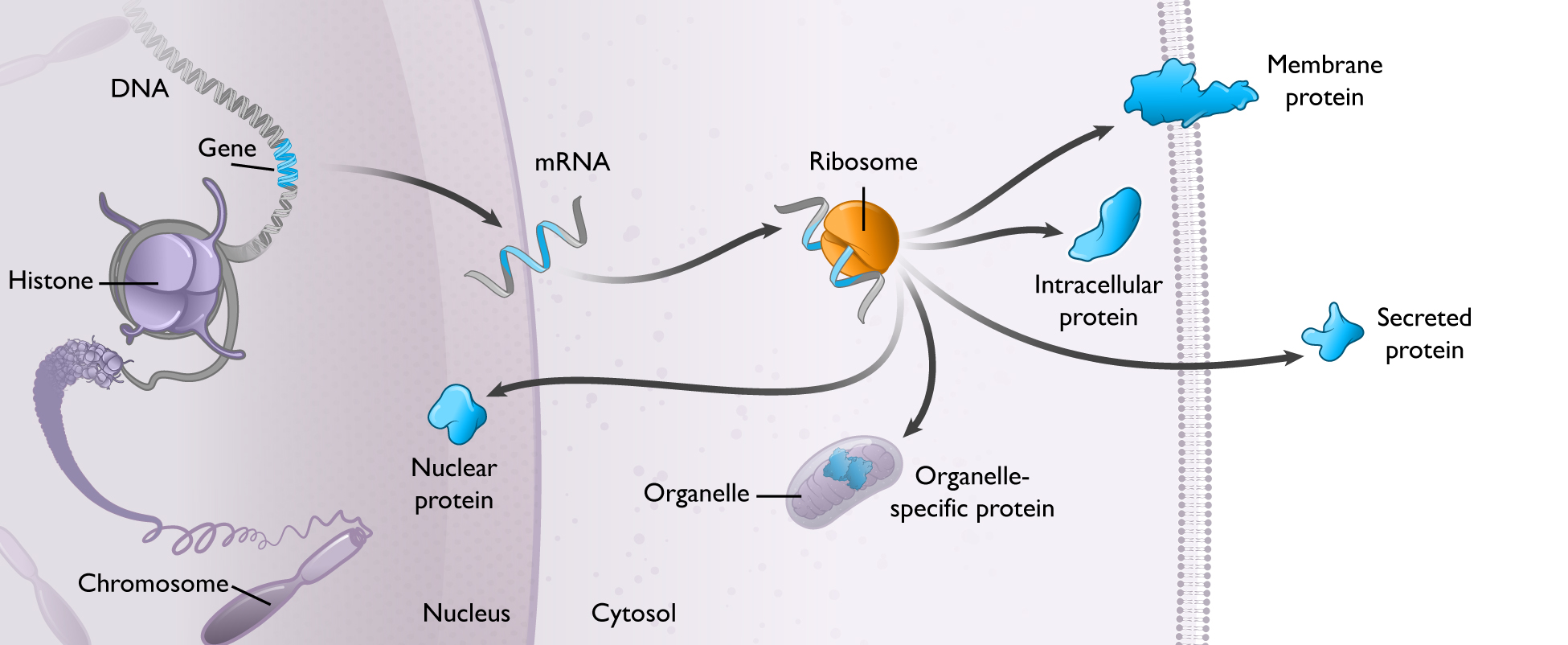
Messenger RNA, or mRNA transfers the information stored in our genes to the cellular machinery that makes all the proteins required for life. Our genes are stored as sequences of DNA which contain the instructions to make specific proteins. DNA serves as a hard drive, safely storing these instructions in the cell’s nucleus until they are needed by the cell.
When a cell needs to produce a protein, the instructions to make that protein are copied from the DNA to mRNA, which serves as the template for protein production. Each mRNA molecule contains the instructions to produce a specific protein with a distinct function in the body. mRNA transmits those instructions to cellular machinery, called ribosomes, that make copies of the required protein.
We see mRNA functioning as the “software of life.” Every cell uses mRNA to provide real time instructions to make the proteins necessary to drive all aspects of biology, including in human health and disease. This was codified as the central dogma of molecular biology over 5060 years ago, and is exemplified in the schematic below.
mRNA is used to make every type of protein, including secreted, membrane, and intracellular proteins, in varying quantities over time, in different locations, and in various combinations. This is shown in the figure below.
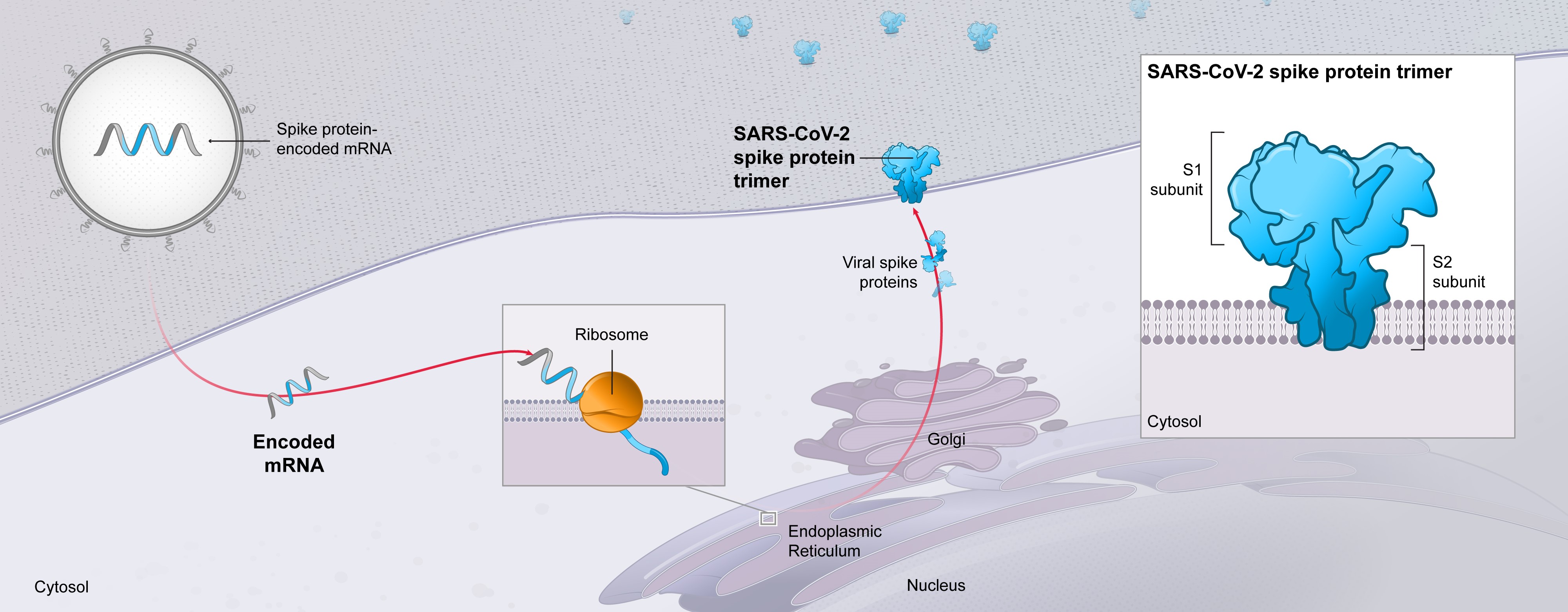
Since our founding in 2010, we have been inspired by the belief that mRNA could be used to create a new class of medicines, with significant potential to improve the lives of patients. In 2020, mRNA technology emerged as a new class of medicine, with the potential to help treat the ongoing global pandemic. In the span of just over 11 months, we designed a vaccine against COVID-19 (mRNA-1273) using mRNA-based technology, conducted Phase 1, Phase 2 and Phase 3 clinical trials, which demonstrated that the vaccine was highly effective at preventing COVID-19, and obtained an Emergency Use Authorization (EUA) for the vaccine from the U.S. Food and Drug Administration on December 18, 2020, followed a few days later by an Interim Order from Health Canada authorizing the distribution of the vaccine in Canada. By December 31, 2020, we had delivered nearly 17 million doses of the Moderna COVID-19 Vaccine to the U.S. government, and several hundred thousand doses to the Canadian government to help fight the pandemic. As of December 31, 2020, we had committed orders for approximately 520 million doses of the vaccine to be delivered in 2021 to governments around the world.
The success we experienced in 2020 builds on over 40 years of progress in the biotechnology industry. Our approach fundamentally differs from traditional approaches to medicine. Rather than introduce a protein or chemical to the body, we send tailored mRNA into cells to instruct them to produce specific proteins. We built Moderna on the guiding premise that if mRNA can be used as a medicine for one disease, it could work for many diseases. Instead of starting from scratch for each new vaccine or therapy, our mRNA approach leverages the technology and fundamental components that we have been researching and developing since our founding. By building off our prior research and learning, we believe we can improve how we discover, develop, and manufacture medicines.
Our success in developing the Moderna COVID-19 Vaccine further underpins our belief that mRNA-based medicines have the potential to help patients in ways that could equal or exceed the impact of traditional approaches to medicine.
Our strategic priorities
Our first priority for 2021 is to maximize the impact of our COVID-19 vaccine, both in terms of access and value creation of this product between now and the end of 2021. We are closely monitoring emerging variants of the SARS-CoV-2 virus as it continues to evolve and testing the performance of our vaccine against them. We are also studying potential booster shots, either of the existing vaccine or of a version that has been adjusted to address significant variants, as well as conducting further clinical trials in younger populations, with the hope of being able to provide the vaccine to adolescents aged 12 to 18 by fall 2021. Executing on this first priority will allow us to pursue our second priority, to accelerate vaccine development to advance our pipeline and bring new vaccines to market. In turn, this will make way for our third priority, to generate human proof-of-concept data in autoimmune diseases, cardiovascular diseases, oncology and rare diseases. And this will allow for our fourth priority, to continue to expand the use of mRNA technology to maximize the potential impact we can have on patients. We continue to believe that over time we will have a number of commercial products within our different modalities, which are described in more detail below.
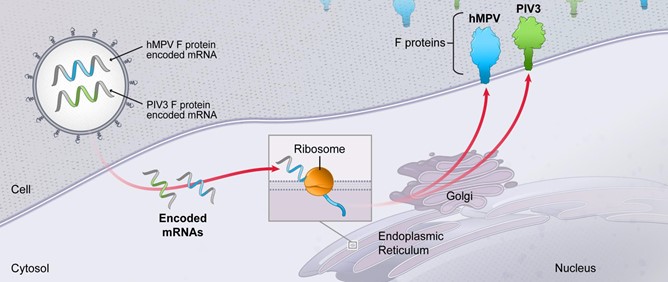
In January 2021, we announced the expansion of our pipeline of prophylactic vaccines with three new development programs: mRNA vaccine candidates against seasonal flu, human immunodeficiency virus (HIV) and the Nipah virus. We also announced our intent to expand our respiratory syncytial virus (RSV) vaccine program into older adults. We currently have 24 mRNA development programs in our portfolio, with 13 having entered the clinic.
As of February 15, 2021, we had received additional regulatory authorizations for use of our COVID-19 vaccine in Europe, the United Kingdom, Israel, Switzerland, Singapore and Qatar. We continue forging ahead with the rolling reviews of our COVID-19 vaccine that have already been initiated with several regulatory agencies across the globe and the WHO, which is important for obtaining regulatory authorization to distribute the vaccine in many middle- and low-income countries.
The early investment we made in our manufacturing and digital capabilities prepared us to rapidly scale our production. At present, we believe we will be able to produce between 700 million and 1 billion doses of our COVID-19 vaccine in 2021. We are continuing to invest and add staff to make this production possible. We are also working on increasing our potential supply to up to 1.4 billion doses for 2022. Much of the production for the supply of the U.S. market will be completed at our Moderna Technology Center facility, or MTC South, in Norwood, Massachusetts, with additional production by Lonza Ltd. for the U.S. market. We have also partnered with Lonza to complete all production in Switzerland of our COVID-19 vaccine (generally referred to as COVID-19 Vaccine Moderna outside the U.S.) for markets other than the U.S. Fill-finish services for our COVID-19 vaccine are provided by Catalent Inc. in the U.S., and by ROVI and Recipharm outside the U.S. We have also partnered with other contract manufacturing organizations for the production of and fill-finish services for our COVID-19 vaccine, and expect that we will enter into additional collaborations as we scale production.

The structure of mRNA
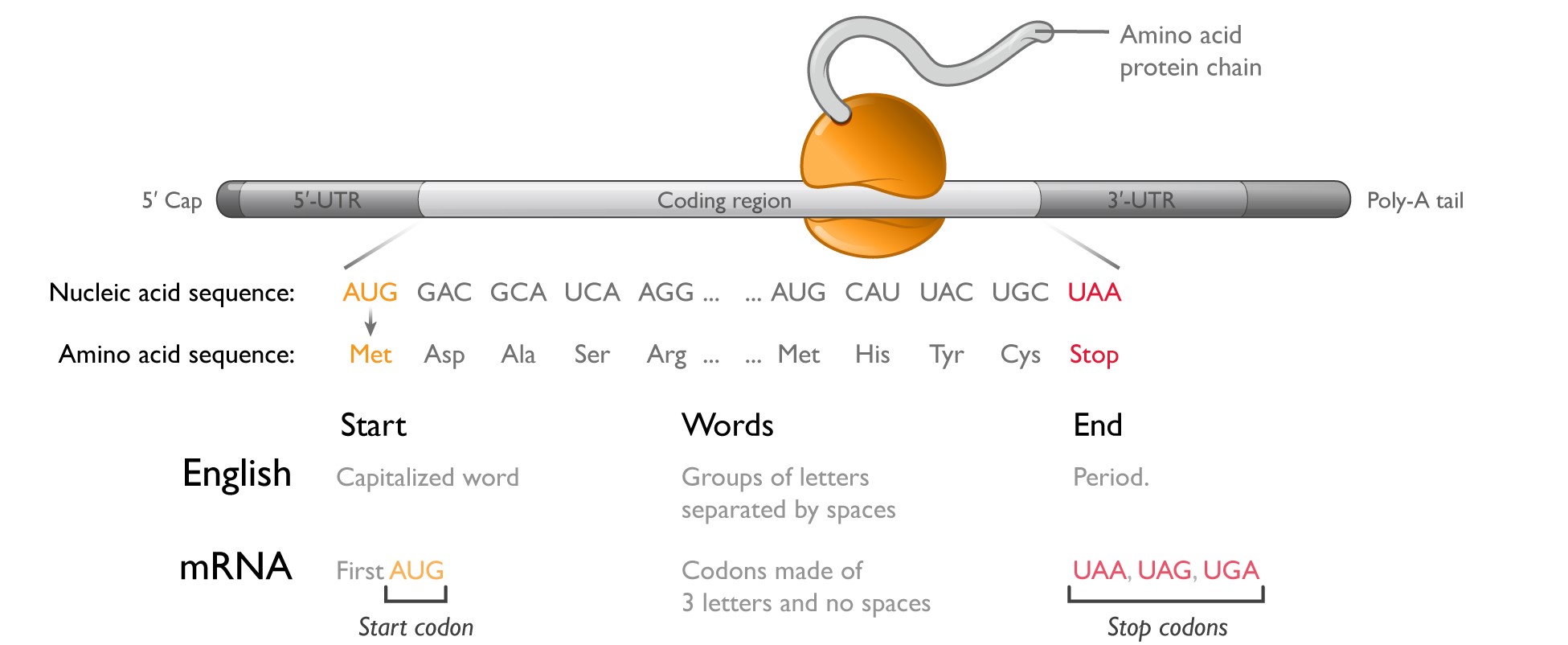
Messenger RNAmRNA is a linear polymer comprising four monomers called nucleotides: adenosine (A), guanosine (G), cytosine (C), and uridine (U). Within the region of the molecule that codes for a protein or the(the coding region,region), the sequence of these four nucleotides forms a language made up of three-letter words called codons. The first codon, or start codon (AUG), signals where the ribosome
should start protein synthesis. To know what protein to make, the ribosome then progresses along the mRNA one codon at a time, appending the appropriate amino acid to the growing protein. To end protein synthesis, three different codons (UAA, UAG, and UGA) serve as stop signals, telling the ribosome where to terminate protein synthesis. In total, there are 64 potential codons, but only 20 amino acids that are used to build proteins; therefore, multiple codons can encode for the same amino acid.
The process of protein production is called translation because the ribosome is reading in one language (a sequence of codons) and outputting in another language (a sequence of amino acids). As shown in the figure below, theThe coding region is analogous to a sentence in English. Much like a start codon, a capitalized word can indicate the start of a sentence. Codons within the coding region resemble groups of letters representing words. The end of the sentence is signaled by a period in English, or a stop codon for mRNA.
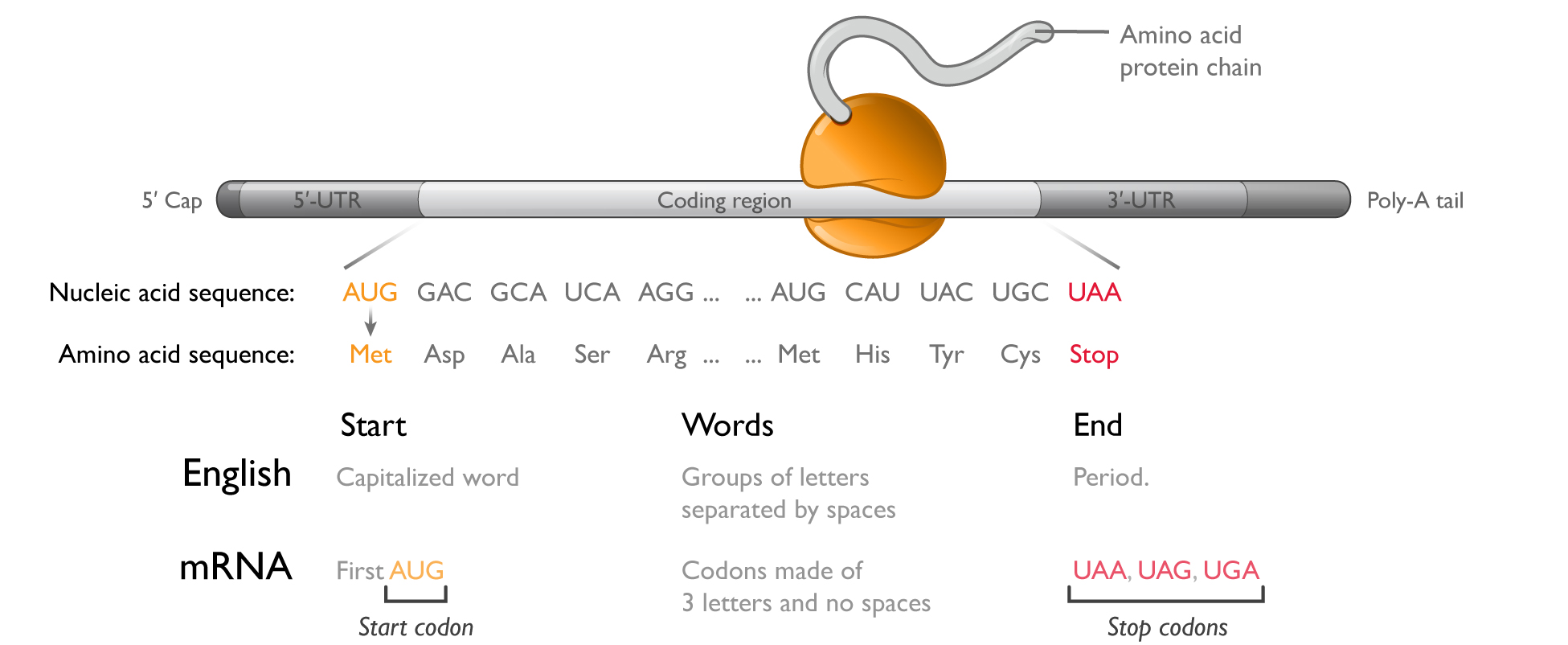

In every cell, hundreds of thousands of mRNAs make hundreds of millions of proteins every day. A typical protein contains 200-600 amino acids; therefore, a typical mRNA coding region ranges from 600-1,800 nucleotides. In addition to the coding region, mRNAs contain four other key features: (1) the 5’ untranslated region (5’-UTR); (2) the 3’ untranslated region (3’-UTR); (3) the 5’ cap; and (4) a 3’ polyadenosine (poly-A) tail. The sequence of nucleotides in the 5’-UTR influences how efficiently the ribosome initiates protein synthesis, whereas the sequence of nucleotides in the 3’-UTR contains information about which cell types should translate that mRNA and how long the mRNA should last. The 5’ cap and 3’ poly-A tail enhance ribosome engagement and protect the mRNA from attack by intracellular enzymes that digest mRNA from its ends.
The intrinsic advantages of using mRNA as a medicine
mRNA possesses inherent characteristics that we believe provideposition it withto have a strong foundation as a new class of medicines. These characteristics include:profound impact on human health:
1.•mRNA is used by every cell to produce all proteins:Cells in the human body use mRNA to make all types of proteins, including secreted, membrane,and intracellular proteins. mRNA is used by cells to vary the quantitiesmake every type of protein, producedincluding secreted, membrane and intracellular proteins, in varying quantities over time, in different locations and in various combinations. Given the universal role of mRNA in protein production, we believe that mRNA medicines could have broad applicability across human disease.
2.•Making proteins inside one’s own cells mimics human biology: Using a person’s ownTailored mRNA can be sent into cells to instruct them to produce specific protein therapeutics or vaccine antigens and provides certain advantages over existing technologies such as recombinant proteins, which are manufactured using processes that are foreigntraditional approaches to the human body. These advantages include the ability to:
•use multiple mRNAsmedicine, where a protein or chemical is introduced to produce multiple proteins;
•reduce or eliminate immunogenicity;
•create multi-protein complexes;
•produce therapeutic or vaccine proteins locally;
•harness native protein folding and glycosylation; and
•make proteins that are unstable outside the body.
3.•mRNA has a simple and flexible chemical structure:Each mRNA molecule comprises four chemically similar nucleotides to encode proteins made from up to 20 chemically different amino acids. To make the full diversity of possible proteins, only simple sequence changes are required in mRNA. A vast numbermRNA, instead of potential mRNA medicines can be developed, therefore, with only minor changes to the underlying chemical structure of the moleculestarting from scratch for each new vaccine or manufacturing processes, a significant advantage over small molecule or protein therapeutics.therapy.
4.•mRNA has classic pharmacologic features:The intrinsic properties of mRNA translate into attractive pharmacologic features,including:
•each mRNA encodes for a specific protein and no other protein;
•each mRNA molecule can produce many copies of a protein in the cell before being degraded;
•increasing mRNA levels in a cell generally leads to increasing protein levels; and
•the effects of mRNA in a cell can be transient and limits risk of irreversible changes to the cell’s DNA.
As a result, mRNA possesses many of the attractive pharmacologic features of most modern medicines, including reproducible activity, predictable potency and well-behaved dose dependency; andmRNA also provides the ability to adjust dosing based on an individual patient’s needs, including stopping or lowering the dose, to seek to ensurepromote safety and tolerability.
Our ability to rapidly develop, manufacture and commercialize vaccines against COVID-19 demonstrates the potential mRNA as a new classmedicines have to help people and patients in far-reaching ways that could exceed the impact of medicinestraditional approaches to medicine.
Based on these and other features, we have developed four core beliefs aboutWe believe that the value driversmain advantages of mRNA as a new class of medicines:compared to traditional medicine are:
1.mRNA has the potential tocould create an unprecedented abundance and diversity of medicines.Although only two infectious disease vaccines using mRNA technology have been authorized to date for emergency use, or other provisional, interim or conditional use in response to the COVID-19 pandemic, we believe this success further demonstrates the potential for mRNA medicines to provide patients orhealthy individuals with any therapeutic protein or vaccine, including those targeting intracellular and membrane proteins. ThismRNA’s breadth of applicability has the potential to create an extraordinary number of new mRNA-basedmRNA medicines that are currently beyond the reach of recombinant protein technology.
2.Advances in the development of our mRNA medicines can reduce risks across our portfolio. mRNA medicines share fundamental features that can be used to learn quicklyleveraged across aour portfolio. We believe that once safety and proof of protein production has been established in one program, the technology and biology risks of related programs that use similar mRNA technologies, delivery technologies and manufacturing processes will decrease significantly. We believe that the progress of our COVID-19 vaccine has helped mitigate risk associated with our prophylactic vaccine modality.
3.mRNA technology can accelerate discovery and development. The software-like features of mRNA enable rapid in silico design and the use of automated high-throughput synthesis processes that permit discovery to proceed in parallel rather than sequentially. We believe these mRNA features can also accelerate drug development by allowing the use of shared manufacturing processes and infrastructure.
4.The ability to leverage shared processes and infrastructure can drive significant capital efficiency over time. We believe the manufacturing requirements of different mRNA medicines are dramatically more similar than traditional recombinant protein-based drugs across a similarly diverse pipeline. When manufacturingand that at commercial scale, we believe a portfolio of mRNA medicines will benefit from shared capital expenditures, resulting in lower program-specific capital needs and an advantageous variable cost profile.expenditures.
OUR STRATEGY
We believe that the development of mRNA as a new class of medicines as evidenced by the development of mRNA-based vaccines during 2020, represents a significant breakthrough for patients, our industry and human health globally. Our success in developing one of the earliest and most effective COVID-19 vaccines, at unprecedented speed and efficiency, demonstrates the promise of mRNA medicine. Our COVID-19 vaccine has helped hundreds of millions of people worldwide combat COVID-19. Beyond COVID-19, our industry.platform continues to be highly productive, with 45 programs currently in development, spanning infectious diseases, immuno-oncology, rare diseases and autoimmune diseases.
Across our respiratory vaccines, latent and other vaccines, oncology and rare disease franchises, we are aiming to launch up to 15 new products over the next five years. We have formulated strategic objectives to help enable our near- and long-term goals:
OUR STRATEGIC PRINCIPLES AND APPROACH TO MANAGING RISK
Our strategyDeliver an unrivalled respiratory vaccine franchise. We are developing vaccines against COVID-19, seasonal flu and RSV individually, while pursuing parallel development of combination vaccines. Recognizing that COVID-19 is designedlikely to deliver onpose an ongoing health burden, we are making it an important piece of our business with our vaccines against COVID-19 and our investigational combination vaccine against flu and COVID-19. In parallel, we are preparing for the full scopepotential 2024 launch of our investigational RSV vaccine for older adults, which is expected to further demonstrate the commercial potential of our mRNA opportunity over the long-term. Reaching patients with mRNA medicines requiresplatform. We expect that our anticipated respiratory product launches in 2024 and 2025 will allow us to make complex choices, including: how much capital we devote to technology creation, drug discovery, drug development, commercial and global marketing and infrastructure; which programs we advance and how; whether we advance programs alone or with strategic collaborators; and which capabilities we build internally versus outsource.recognize efficiencies from our growing pipeline.
To navigate these choices, we established five strategic principles that guide our approach to creating long-term value for patients and investors. No single strategic principle dominates our choices. Embedded in every decision we make is also our assessment of the most important risks inherent in our business. We believe these risks fall into four categories: technology, biology, execution, and financing.
To increase our chances of success, we often find it necessary to balance our near-to-mid-term risks against the strategic principles that guide our approach to long-term value creation.
Our strategic principles
1.We seek to discover and develop a large pipeline in parallel.
Our goal is to address or prevent as many human diseases as our technology, talent,capital, and other resources permit. We do so as rapidly as we can, understanding both the urgency for patients and the need to be disciplined in our approach. We have a diverse pipeline of 24 development programs, with 13 of them having entered the clinic, many of which have the potential to be
first-in-class or best-in-class medicines. We have one commercial product, our COVID-19 vaccine, that is being distributed globally.
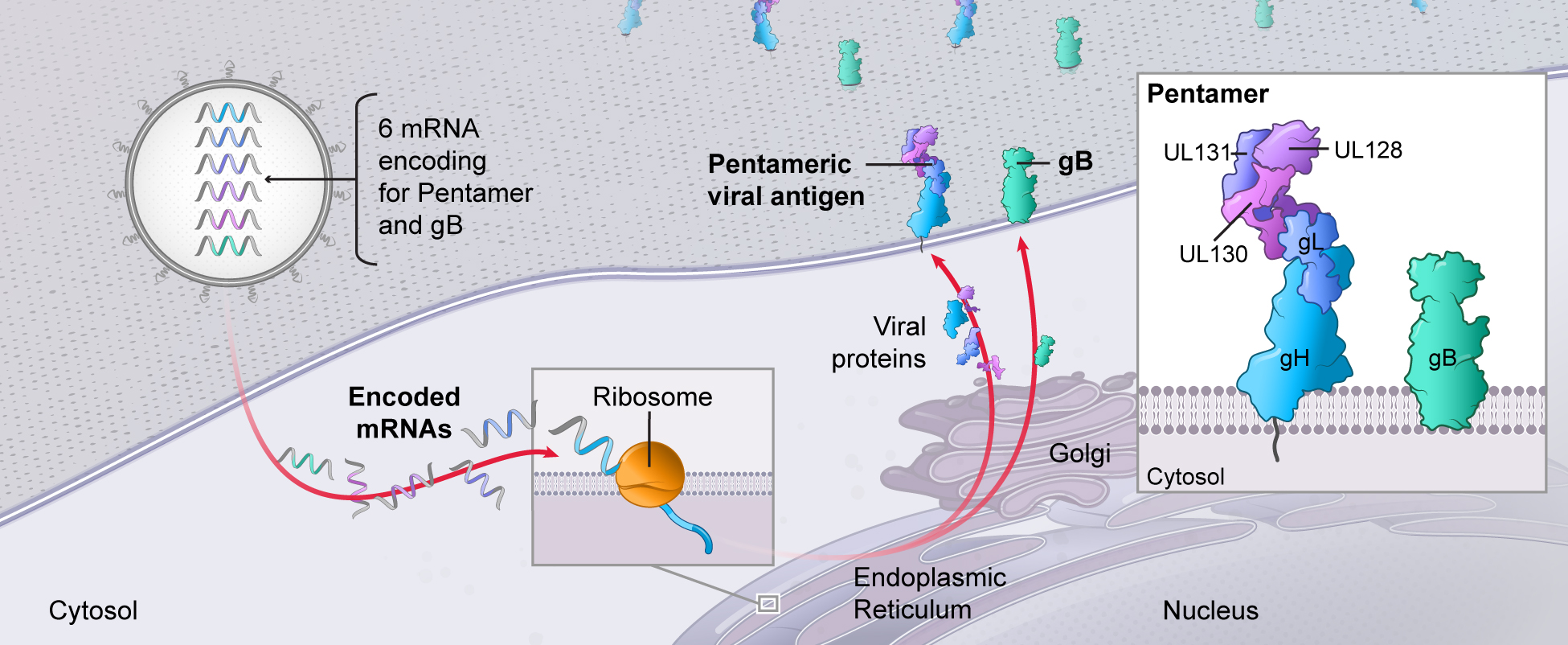
2.We undertake sustained, long-term investment in technology creation. Advance multiple latent virus and other vaccines.We aim to improve the performance of mRNA medicines in our currentmodalities, and to unlock new modalities, through investments within basic and applied science. We are committed to remaining atdeveloping vaccines against latent and other viruses with unmet or underserved needs, including cytomegalovirus (CMV), Epstein-Barr virus (EBV), herpes simplex virus (HSV), varicella zoster virus (VZV), norovirus, HIV and Lyme disease. We anticipate potential efficacy data from the forefrontpivotal Phase 3 study of mRNA science.our CMV vaccine candidate in 2024. CMV is the most common infectious cause of birth defects in the United States and has been designated as a top priority in new vaccine development by the U.S. National Academy of Medicine for more than two decades.
3.We focus on the pace and scaleAccelerate a large portfolio of our learning. We believe that time islate-stage clinical trials in INT to deliver a critical resource. We seek to accelerate our progress by solving numeroustransformative impact in cancer treatments. technical problems in parallel rather than in sequence. Our scientists pursue experiments based on how muchIn 2023, we can learn from the results, not just the probability of a positive outcome. We believe negative information is valuable and we can learnreported data from our setbacks.Phase 2b trial evaluating our INT in combination with Merck’s KEYTRUDA in melanoma patients compared to KEYTRUDA alone, which we believe demonstrates the durability of the therapy. We make significant investments in digital assetshave launched Phase 3 trials for both adjuvant melanoma and research infrastructurenon-small cell lung cancer (NSCLC), and plan to accelerateexpand the pace and scale of our learning.development program to additional tumor types.
4.We integrate across the most critical parts of our value chain. mRNA is a complex multicomponent system and we believe it demands integration.Accelerate investment in three rare disease programs to pursue potential launches. We believe that we must be directly engagedhave demonstrated the potential for clinical benefit in research, drug discovery, drug development, processthree different rare genetic diseases (propionic acidemia (PA), methylmalonic acidemia (MMA) and analytical development,glycogen storage disease type 1a (GSD1a)), and manufacturingexpect to accelerateadvance our learning, reduce our risk,PA and protect our critical know-how. Where appropriate, we seek out strategic collaborators that can augment our capabilities or expand our capacityMMA programs into pivotal studies in specific therapeutic areas, while being careful to resist the fragmentation of our core technology.2024.
5.We forward invest in core enabling capabilities and infrastructure. To execute across a broad pipeline, we need to invest at risk before we have allDeliver the answers. Our forward investments focus on areas where lead times are long and where early investments can reduce execution risk and accelerate future progress. We proactively invested in a dedicated manufacturing facility, Moderna Technology Center (MTC), in Norwood, MA, to support the anticipated growth of ournext-generation pipeline and this early investment greatly facilitated our ability to respond to the COVID-19 pandemic by allowing us to begin production of our vaccine even before we received regulatory authorization for its distribution.platform.
Our approach to managing risk
In conjunction with the strategic principles that guide our approach to long-term value creation, we actively manage the risks inherentWe have demonstrated clinical benefit in our business. At present, these categories of risk include: technology, biology, execution, and financing. We summarize our approach to managing these risks below:
1.Technology risk encompasses the challenges of developing the product features of mRNA medicines, including delivery, controlling interactions withthe immune system, optimizing therapeutic index, and manufacturing. We believe the best way to mitigate technology risk is to sustain long-term investments in our platform. In addition, we diversify our technology risk by compartmentalizing our pipeline into groups of programs with shared product features, which we call modalities. Lastly, we stage program development within a modality, leveraging the first program, whether successful or not, to generate insights that accelerate and reduce the risk of subsequent programs within the modality.
2.Biology risk entails the risk unique to each program based on its mechanism of action and of clinical development in the target patient population. Webelieve the best way to manage biology risk is to diversify it by pursuing multiple programs in parallel. In addition, within a modality we seek to initially pursue programs with well-understood biology. Lastly, we may seek strategic collaborators to share risk and upside in disease areas with high inherent biology risk, such as cancer and heart disease.
3.Execution risk refers to the challenge of executing against the scale of our mission. We solve for this risk by seeking to hire the right people, the besttalent in the industry. We seek to foster a culture of execution with a focus on quick review cycles and high velocity decision-making. We make forward investments in infrastructure, including manufacturing. Lastly, we have created a digital backbone to track all aspects of our programs and anticipate challenges before they arise.
4.Financing risk refers to our ability to access the capital required to fund the current breadth of our endeavor, as well as new opportunities. We managethis risk by attempting to maintain a strong balance sheet with several years of cash runway. As of December 31, 2020, we had cash, cash equivalents, and investments of $5.25 billion. This balance represents a significant increase in our liquidity over the prior year, driven primarily by customer deposits for the sale of our COVID-19 vaccine, as well as two equity offerings during the first half of the year (in February and May).
There is no single strategic principle nor single category of risk that dominates our decision-making, and universal rules do not exist across our portfolio. Our trade-offs generally involve balancing near-term risks and long-term value creation. Because development cycles are long, our choices are complex. We expect the weighting and types of risk we face will evolve as our business matures. We
believe that disciplined capital allocation across near- and long-term choices must be a core competency if we are to maximize the opportunity for patient impact and shareholder value creation.
Our progress
Our success in developing a highly effective vaccine against COVID-19, going from sequence selection, conducting clinical trials and to receipt of regulatory authorization for emergency use, all in less than a year, provides a visible example of the promise of mRNA-based medicine. Our COVID-19 vaccine is currently authorized or conditionally approved in over 30 countries, and has already been used to vaccinate millions of patients to help combat the pandemic. We believe our success in developing this vaccine has positive implications beyond infectious disease vaccines and acrossin skin cancer, as well as potential clinical benefit in three different rare genetic diseases. Based on these clinical successes, we continue to advance a broad and diverse pipeline and are focused on execution to deliver for patients. We plan to continue to invest in our six modalities. We currently have 24 programs in development,science and our pipeline spans five therapeutic areas: infectious diseases, immuno-oncology, rare diseases, cardiovascular diseasesplatform to expand mRNA applications and autoimmune diseases. We remain on track to produce between 700 million and 1 billion doses of our COVID-19 vaccine in 2021.advance new programs into clinical studies.
OUR PLATFORM
Overview of our platform
Our mRNA “platform” refers to our accumulated knowledge and capabilities in basic and applied sciencessciences. Our platform incorporates advances across three key components—mRNA, the delivery of mRNA to target tissues, and the manufacturing processes for making potential mRNAprocess— to advance our medicines. We invest in basic science to discover foundational mechanistic insights,integrate these components and we invest in applied sciences to invent technology that harnesses those insights. We use our platform to identify and develop new mRNA medicines. When we identify a combinationcombine different versions of platform technologies or programs across mRNA technologies, delivery technologies, and manufacturing processes that can enable shared product features across multiple potential mRNA medicines, we group those programs as a modality. The primary goal of our platform is to identify new modalities and to expand the utility of our existing modalities. We are committed to advancing the technological frontier of mRNA medicines over the long term.
We define success in our platform as achieving the following pharmacologic properties:
•predictable dose response;
•reproducible pharmacology, including upon repeat dosing;
•therapeutic potency, through achieving the intended pharmacologic activity in the target tissue;
•safety and tolerability; and
•scalability for development.
Achieving any of these pharmacologic properties requires many, often interdependent, technological solutions. We organize our efforts into three core scientific areas: mRNA delivery and manufacturing process as shown in the figure below:

We pursue mRNA science to minimize undesirable activation of the immune system by mRNA, to maximize the mRNA potency of mRNA once inside target cells, and to extend pharmacologyinto each of our therapeutics. We pursue delivery science to protect mRNA from extracellular enzymes that would degrade it, to avoid counterproductive interactions of our delivery vehicles with the immune system, deliver mRNA to desired tissues, and facilitate mRNA transport across cell membranes to the translational machinery within cells. Finally, we have learned that the methods for producing mRNA and lipid nanoparticle, or LNP, delivery systems can have profound positive and negative effects on pharmacology. We pursue process science to optimize these features for our future medicines and to develop technical capabilities to scale our potential mRNA medicines for clinical development.
We have incurred significant expense to advance our platform technology and our intellectual property. This investment has underpinned the creation of all six of our existing modalities and helped us to establish fundamental intellectual property. We intend to sustain our investment in our platform in the future because we believe we can establish new modalities and continue to make meaningful improvements in the performance of our current modalities.
Our COVID-19 vaccine demonstrates the success of our current platform. Our current pipeline, which consists of 24 programs, depends on hundreds of small advances and 10 years of research and investment in our three core scientific areas. Examples of many
critical advances that we have made are described below. These advances demonstrate our significant progress to date, and exemplify our approach to tackling hundreds of smaller scientific problems and organizing them into technological solutions.medicines.
Our platform: mRNA science
An overview of mRNA biology
Messenger RNA is a linear polymer comprised of four monomers called nucleotides: adenosine (A), guanosine (G), cytosine (C), and uridine (U). Within the region of the mRNA molecule that serves as instructions for protein synthesis, the coding region, the exact sequence of these four nucleotides forms a language made up of three-letter words called codons. One codon, the start codon (AUG), serves to signal where the ribosome should start protein synthesis. To know what protein to make, the ribosome then progresses along the mRNA one codon at a time, appending the appropriate amino acid to the growing protein chain. Because the ribosome is reading in one language (a sequence of codons) and outputting in another language (a sequence of amino acids), this process is called translation. Finally, three different codons (UAA, UAG, and UGA) can serve as stop signals, telling the ribosome where to terminate protein synthesis. The production of proteins from mRNA sequences is called translation and is used to make all human proteins. The production of mRNA from DNA is called transcription.
As shown in the figure below, the coding region in an mRNA molecule is analogous to a sentence in English. The start codon indicates the start of the protein, much like a capitalized word can indicate the start of a sentence. Codons within the coding region resemble groups of letters representing words. The end of the sentence is signaled by a period in English, or a stop codon for mRNA.
In every cell, hundreds of thousands of mRNAs make hundreds of millions of proteins every day. A typical protein contains 200-600 amino acids; therefore a typical mRNA coding region ranges from 600-1,800 nucleotides.
In addition to the coding region, mRNAs contain four other key features: (1) the 5’ untranslated region or 5’-UTR; (2) the 3’ untranslated region or 3’-UTR; (3) the 5’ cap; and (4) a 3’ polyadenosine, or poly-A, tail. The sequence of nucleotides in the 5’-UTR influences how efficiently the ribosome initiates protein synthesis, whereas the sequence of nucleotides in the 3’-UTR contains information about which cell types should translate that mRNA and how long the mRNA should last. The 5’ cap and 3’ poly-A tail enhance ribosome engagement and protect the mRNA from attack by intracellular enzymes that digest mRNA from its ends. As a result of this biology, mRNA has several key features. First, mRNA is exquisitely specific. There is a one-to-one correspondence between an mRNA molecule and the protein dictated by the coding sequence. Second, the biological effects of mRNA are amplified. Because each mRNA copy can be translated thousands of times, we believe that in some cases, a small number of mRNA copies per cell may be sufficient to induce a pharmacologic effect. Finally, mRNA is impermanent. mRNAs produce proteins for a defined and biologically-regulated period of time without risk of changing genes or cell DNA. If dosing of mRNA stops, protein production will stop and the biological effects generally can be reversed. advancements
We continue to invest in both appliedbasic and basicapplied research, seeking to advance both the state of our technology and the state of the scientific community’s understanding of mRNA. Examples of advances in mRNA science that combine nucleotide chemistry, sequence engineering and targeting elements are described below.
mRNA chemistry: Modified nucleotides to mitigate immune system activation
activation:
The innate immune system has evolved to protect cells from foreign RNA, such as viral RNA, by inducing inflammation and suppressing mRNA translation once detected. Many cells surveil their environment through sensors called toll-like-receptors or TLRs.(TLRs). These include types that are activated by the presence of double-stranded RNA (TLR3) or uridine containing RNA fragments (TLR7, TLR8). Additionally, all cells have cytosolic double-stranded RNA, sensors, including retinoic acid inducible gene-I or RIG-I(RIG-I) that are sensitive to foreign RNA inside the cell.
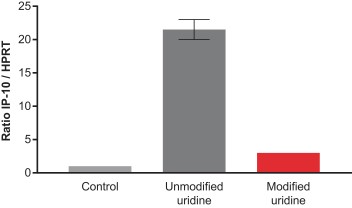
The immune and cellular response to mRNA is complex, context specific, and often linked to the sensing of uridine. To minimize undesired immune responses to our potential mRNA medicines, our platform employs chemically-modified uridine nucleotides to minimize recognition by both immune cell sensors such as TLR3/7/8, and broadly-distributed cytosolic receptors such as RIG-I. mRNA produced using our synthesis technologies and containing unmodified uridine results in significant upregulation of secreted cytokines such as IP-10, which is indicative of local immune cell activation. Administration of monocyte-derived macrophages, or MDMs, with unmodified mRNA formulated in LNPs results in an increased ratio of IP-10 transcripts relative to a housekeeping gene. By substituting unmodified uridine with a modified uridine, we can substantially reduce immune cell activation in this assay. The control contains only transfection agent and no mRNA. In multiple preclinical experiments we have demonstrated reduced immune cell activation, including of B cells, lower immunoglobulin secretion, and lower cytokine expression when administering mRNA made with modified uridine versus unmodified uridine. To date, when deploying these technologies, we have yet to observe dose-limiting toxicity attributable to the mRNA encoding proteins from our drug substance even at the exaggerated doses in IND-enabling GLP toxicology programs. Importantly, in preclinical testing, our chemically-modified uridine has not significantly affected the ribosome’s ability to read and translate the mRNA sequence.
Nucleotide chemistry of mRNA reduces immune activation in vitro (in monocyte-derived macrophages)
mRNA sequence engineering: Maximizing protein expression
expression:
mRNA exists transiently in the cytoplasm, during which time it can be translated into thousands of proteins before eventually being degraded. Our platform applies bioinformatic, biochemical, and biological screening capabilities, most of which have been invented internally that aim to optimize the amount of protein produced per mRNA. We have identified proprietary sequences for the 5’-UTR that have been observed to increase the likelihood that a ribosome bound to the 5’-end of the mRNA transcript will find the desired start codon and reliably initiate translation of the coding region.
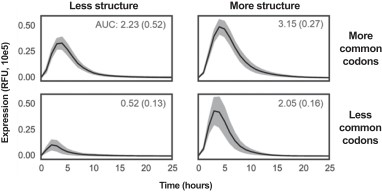
We additionally design the nucleotide sequence of the coding region to maximize its successful translation into protein. As previously described, there are often multiple codons that encode for a specific amino acid. The amount of protein produced by an mRNA sequence is known to be partly determined by the codons it uses, with certain codons being more or less common in endogenous mRNAs. We have found that the amount of protein produced is also determined by the secondary structure of mRNA, or the propensity of mRNA to fold on itself, with more structured mRNAs producing more protein. We designed a set of sequences which independently varied codon usage and structure of the mRNA. As shown in the figure below, protein expression in the Alpha mouse liver 12, cell line is highest for sequences containing more commonly occurring codons and also more structured mRNA. Both codon usage and structure have an independent and additive effect on protein expression, shown as mean expression (solid line), as measured by fluorescence of the expressed protein, with 95% confidence interval in gray. The total expression area under the curve, or AUC, and standard error of the mean for AUC are shown for each quadrant, in relative fluorescence units per hour. By optimizing translation initiation and efficiency, we have further increased the average number of full-length desired proteins expressed per molecule mRNA. This permits us to reduce the mRNA doses required to achieve the same therapeutic benefit.
Sequences with more structure and more common codons in mRNA maximize protein expression in vitro
Targeting elements: Enabling tissue-targeted translation
translation:
All nucleated cells in the body are capable of translating mRNA, resulting in pharmacologic activity in any cell in which mRNA is delivered and translated. To minimize or prevent potential off-target effects, our platform employs technologies that regulate mRNA translation in select cell types. Cells often contain short RNA sequences, called microRNAs or miRNAs, that bind to mRNA to regulate protein translation at the mRNA level. Different cell types have different concentrations of specific microRNAs, in effect giving cells a microRNA signature. microRNA binding directly to mRNA effectively silences or reduces mRNA translation and promotes mRNA degradation. We design microRNA binding sites into
the 3’-UTR of our potential mRNA medicines so that if our mRNA is delivered to cells with such microRNAs, it will be minimally translated and rapidly degraded.
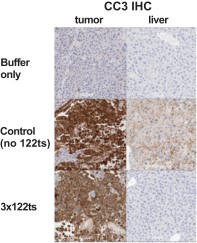
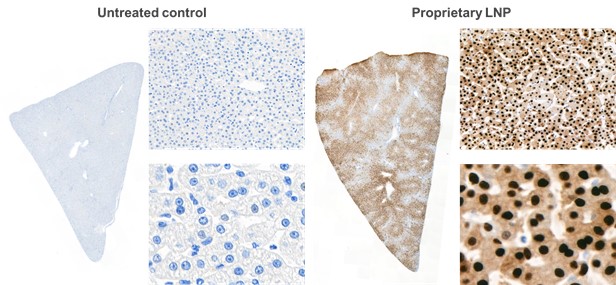
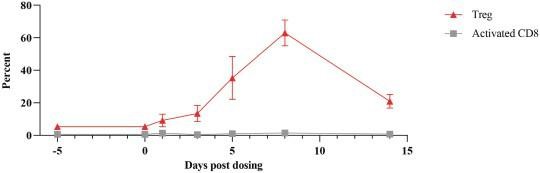
As an example, we have demonstrated by intratumoral administration in an animal model that an mRNA encoding a cytotoxic protein and containing a microRNA binding site can be used to selectively kill cancer cells, while protecting systemic tissues such as liver cells. In a mouse model of cancer (Hep3b subcutaneous xenograft mouse), liver enzyme levels and immunohistochemistry, or IHC, of cleaved caspase-3, indicate production of an apoptosis-inducing protein encoded by mRNA in tumor cells but not healthy liver cells when the mRNA has multiple miR-122 target sites. This is denoted as 3x122ts in the figure below; miR-122 is more prevalent in non-cancerous liver cells, but absent in the cancerous liver cells. We published this work in Nucleic AcidTherapeutics in 2018.
Tissue-targeted translation of mRNA encoding a pro-apoptotic protein
and microRNA binding sites in mouse study
Our platform: Delivery science
We focus on the delivery of our mRNA molecules to specific tissues. Our mRNA can, in specific instances, such as our VEGF therapeutic, be delivered by direct injection to a tissue in a simple saline formulation without lipid nanoparticles or LNPs,(LNPs) to locally produce small amounts of pharmacologically active protein. However, the blood and interstitial fluids in humans contain significant RNA degrading enzymes that rapidly degrade any extracellular mRNA and prevent broader distribution without LNPs. Additionally, cell membranes tend to act as a significant barrier to entry of large, negatively-charged molecules such as mRNA. We have therefore
invested heavily in delivery science and have developed LNP technologies as well as alternative nanoparticle approaches to enable delivery of larger quantities of mRNA to target tissues.
LNPs are generally composed of four components: an amino lipid, a phospholipid, cholesterol, and a pegylated-lipid or PEG-lipid.(PEG-lipid). Each component, as well as the overall composition, or mix of components, contributes to the properties of each LNP system. LNPs containing mRNA injected into the body rapidly bind proteins that can drive uptake of LNPs into cells. Once internalized in endosomes within cells, the LNPs are designed to escape the endosome and release their mRNA cargo into the cell cytoplasm, where the mRNA can be translated to make a protein and have the desired therapeutic effect. Any mRNA and LNP components that do not escape the endosome are typically delivered to lysosomes where they are degraded by the natural process of cellular digestion.
Examples of tools we developed by using our platform include proprietary LNP formulations that address the steps of mRNA delivery, including cell uptake, endosomal escape, and subsequent lipid metabolism, and for avoidance of counterproductive interactions with the immune system. Examples of delivery tools we have developed are described below.
Chemistry: Novel lipid chemistry to potentially improve safety and tolerability
tolerability:
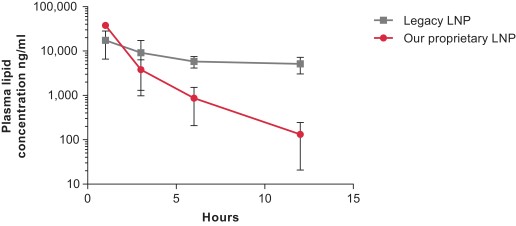
We initially used LNP formulations that were based on known lipid systems, which we refer to as “legacy LNPs.” A recognized limitation of these legacy LNPs is the potential for inflammatory reactions upon single and repeat administration that can impact tolerability and therapeutic index. Our later-developed, proprietary LNP systems are therefore designed to be highly tolerated and minimize any LNP vehicle-related toxicities with repeat administration in vivo. The changesTo overcome limitations of previous LNP formulations, we made have included engineeringengineered amino lipids to avoid the immune system and to be rapidly biodegradable relative to prior lipids. Administered intravenously in non-human primates, at 0.2 mg/kg, our proprietary LNPs demonstrate rapid clearance of the lipid from plasma and organs such as peripheral lymphoid organs and the liver.
Rapid clearance of lipid components of LNPs from plasma in non-human primate study
(y-axis in log-scale)
Even in the case of vaccines, where one might hypothesize that LNP-induced immune stimulation could potentially increase the effectiveness of the vaccine, we have demonstrated in preclinical studies that we can maintain the desired immune response to the vaccine while reducing undesired local immune reaction, or reactogenicity, to the LNP. The tolerability of our vaccine formulation has been demonstrated clinically and, to date, millions of individuals worldwide have received our vaccine against COVID-19.
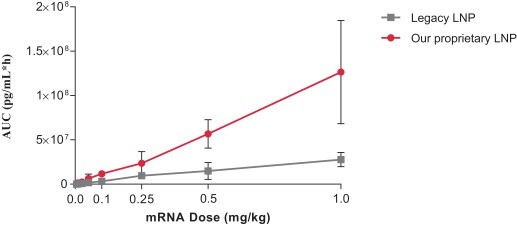
Composition: Proprietary LNPs enhance delivery efficiency
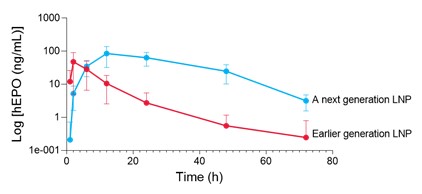
efficiency:
Our platform includes extensive in-house expertise in medicinal chemistry, which we have applied to design large libraries of novel lipids. Using these libraries in combination with our discovery biology capabilities, we have conducted high throughput screens for desired LNP properties and believe that we have made fundamental discoveries in preclinical studies about the relationships between structural motifs of lipids and LNP performance for protein expression. By screening for components and compositions that enhance the amount of mRNA delivered per cell and protein expression, we have demonstrated with intravenous administration up to a six-fold improvement in protein production over the prior state of the art for LNPs as shown in the figure below (n=3 rats, 95% CI shown).
Enhanced protein production with our proprietary LNP in rat study
Surface properties: Novel LNP design to avoid immune recognition
recognition:
We have designed our proprietary LNP systems for sustained pharmacology upon repeat dosing by eliminating or altering features that activate the immune system. These are based on insights into the surface properties of LNPs. Upon repeated dosing, surface features on traditional LNPs such as amino lipids, phospholipids, and PEG-lipids, can be recognized by the immune system, leading to rapid clearance from the bloodstream, a decrease in potency upon repeat dosing, and an increase in inflammation.
Based on our insights into these mechanisms, we have engineered our LNP systems to reduce or eliminate undesirable surface features. In preclinicalclinical studies in non-human primates for our systemic therapeutic developmentproduct candidates that use our novel LNP systems, we have been able to repeat dose with negligible or undetectable loss in potency, liver damage, and immune system activation.
Our platform: Manufacturing process science
We invest significantly in manufacturing process science to impart more potent features to our mRNA and LNPs, and to invent the technological capabilities necessary to manufacture our potential mRNA medicines at scales ranging from micrograms to kilograms, as well as achieve pharmaceutical properties such as solubility and shelf life. We view developing these goals of manufacturing and pharmaceutical properties as stage appropriate for each program. In some cases, this includes inventing novel analytical technologies that make it possible to connect analytical characterizationprogram, based on its stage of mRNA and LNPs to biological performance.development.
mRNA manufacturing process: Improving pharmacology
Our platform creates mRNA using a cell-free approach called in vitro transcription in which an RNA polymerase enzyme binds to and transcribes a DNA template, adding the nucleotides encoded by the DNA to the growing RNA strand. Following transcription, we employ proprietary purification techniques to ensure that our mRNA is free from undesired synthesis components and impurities that could activate the immune system in an indiscriminate manner. Applying our understanding of the basic science underlying each step in the manufacturing process, we have designed proprietary manufacturing processes to impart desirable pharmacologic features, for example increasing potency in a vaccine. Using a model antigen injected intramuscularly in mice at a 3 microgram (µg) mRNA dose, the figure below shows the significant improvement in CD8 T cell response we have achieved through mRNA manufacturing process science and engineering as evidenced by Process B.
Manufacturing process changes to tune immune response in mouse study
LNP manufacturing process: Improving pharmacology
pharmacology:
Our platform technology includes synthetic processes to produce LNPs. Traditionally LNPs are assembled by dissolving the four molecular components, amino lipid, phospholipid, cholesterol, and PEG-lipid, in ethanol and then mixing this with mRNA in an aqueous buffer. The resulting mixture is then purified to isolate LNPs from impurities. Such impurities include molecular components that have not been incorporated into particles, un-encapsulated mRNA that could activate the immune system, and particles outside of the desired size range.
Going beyond optimization of traditional manufacturing processes, we have invested in understanding and measuring the various biochemical and physical interactions during LNP assembly and purification. We have additionally developed state-of-the-art analytical techniques necessary to characterize our LNPs and biological systems to analyze their in vitro and in vivo performance. With these insights, we have identified manufacturing process parameters that drive LNP performance, for example, the potency in a secreted therapeutic setting. These insights have allowed us to make significant improvements in the efficiency of our processing and the potency of our LNPs, as exemplified in the figure below. For example, expression of a secreted protein in our Relaxin program demonstrates an approximate eight-fold increase in AUC and approximate six-fold increase in maximum concentration for manufacturing process Y versus manufacturing process X in rats dosed intravenously with 0.5 mg/kg mRNA.LNPs.
Manufacturing process changes to enhance relaxin protein production by mRNA in rat study
Our platform progress to date
Since our inception, we have solved numerous interdependent problems related tovision for harnessing the pharmacologic features of our potential mRNA medicines. These features are detailed and exemplified below. Please also see the section of this Annual Report on Form 10-K titled “Business—Program Descriptions” for recent clinical results for our investigational medicines, including our COVID-19 vaccine (mRNA-1273), CMV vaccine (mRNA-1647), hMPV/PIV3 vaccine (mRNA-1653), antibody against Chikungunya virus (mRNA-1944), and PCV (mRNA-4157) utilizing Moderna proprietary technology.
Dose-dependent protein expression in the clinic
We have demonstrated in the clinic the ability to generate consistent dose dependent levels of protein (antibody) as well as the ability to safely repeat dose. Interim data from our Phase 1 study evaluating escalating doses of mRNA-1944 in the 0.6 mg/kg dose with steroid premedication cohort and two doses of 0.3 mg/kg (without steroid premedication) given one week apart demonstrated dose-dependent increases in levels of antibody against chikungunya. Safety and increased CHKV-IgG production in the two-dose regimen shows the platform’s ability for repeat dosing.
Expression of antibody against Chikungunya virus in the Phase 1 study of mRNA-1944
Reproducible pharmacology, including upon repeated dosing
By combining advances in mRNA, delivery, and manufacturing process science, we have demonstrated in preclinical studies sustained and reproducible pharmacology. The figure below shows a recent example in a mouse model that recapitulates metabolic defects in propionic acidemia, or PA. In this rare disease, a defect in one or both of two different subunits (PCCA and PCCB) of the mitochondrial enzyme propionyl-CoA carboxylase results in accumulation of toxic metabolites such as 2-methylcitrate, or 2MC.In mice hypomorphic for the PCCA subunit, monthly intravenous, or IV, administration of mRNAs encoding PCCA and PCCB formulated in our proprietary LNP (mRNA-3927) resulted in a significant and sustained lowering of 2MC throughout the duration of the 6-month study compared to control (luciferase) mRNA (1 mg/kg, n=6/group). We are currently recruiting PA patients for our Phase 1 trial of mRNA-3927. As noted above, we have also seen success in repeat dosing of our antibody against Chikungunya virus.
Plasma 2-methylcitrate levels with repeat dosing of PCCA+PCCB mRNA in PA mouse study
Decreased immune activation upon repeat dosing in non-human primates
We have observed decreased immune activation which enables repeat dosing in non-human primates, as shown in the figure below. The data below indicates serum concentration of human erythropoietin, or hEPO, with repeat dosingpower of mRNA encoding hEPO in our proprietary LNPs with weekly IV administration at 0.2 mg/kg in non-human primates.
Repeat dosing with mRNA encoding for hEPO in our proprietary LNP in non-human primate study
We believe that by combining proprietary mRNA technologies, delivery technologies, and manufacturing process technologies we have significantly advanced the potential therapeutic index of our potential mRNA-based therapeutics.
Pharmacologic activity in the target tissue and cell
While some of our modalities, such as systemic secreted therapeutics, can leverage many different cell types to make therapeutic proteins, others such as systemic intracellular therapeutics, may require delivery of our mRNA into specific tissues and cell types, for instance hepatocytes in certain liver metabolic diseases. Combining our proprietary mRNA, delivery, and manufacturing process technologies we have observed on-target pharmacologic activity in hepatocytes in non-human primates. The on-target potency of this approach contrasts with traditional delivery technologies. In the figure below, one of our proprietary LNPs with increased hepatocyte transfecting properties result in protein expression in liver hepatocytes in non-human primates as demonstrated with a reporter protein detected by immunohistochemistry at 6 hours after IV infusion at 2 mg/kg.
Protein expression in hepatocytes with a proprietary LNP in non-human primate study
Additionally, this LNP results in extended expression of a secreted reporter protein in non-human primates as compared to one of our other proprietary LNPs after IV delivery at 0.1mg/kg.
Our platform’s future: Improving and expanding ourthrough modalities
We are committed to sustaining investment in our platform, both in basic science to elucidate new mechanistic insights, and in applied science to discover new technologies that harness these insights. Our platform investments have enabled six modalities to date, most of which have already led to multiple development candidates and investigational medicines in our pipeline. We believe that sustaining our investment in platform research and development will enable further improvements in the current modalities and will lead to the creation of new modalities, both of which will benefit our clinical pipeline in the years ahead.
CREATING MODALITIES WITH SHARED PRODUCT FEATURES
Our approach to developing modalities
Within our platform, we develop technologies that enable the development ofinvest in science to invent novel ways to deliver mRNA medicines for diverse applications. When we identify technologies that we believe could enableinto various cell types. Each novel delivery system is a new group of potential mRNA medicines with shared product features,application, which we call that group a “modality.“modality.” While the programs within a modality may target diverse diseases, they share similar mRNA technologies, delivery technologies,characteristics and manufacturing processes to achieve shared product features. The programs within a modality will also generally share similar pharmacology profiles, including the desired dose response, the expected dosing regimen, the target tissue for protein expression, safety and tolerability goals, as well as pharmaceutical properties. Programs within a modality often have correlated technology risk, but because they pursue diverse diseases, they often have uncorrelated biology risk. We have created six modalities to date:
•prophylactic vaccines;
•cancer vaccines;
•intratumoral immuno-oncology;
•localized regenerative therapeutics;
•systemic secreted and cell surface therapeutics; and
•systemic intracellular therapeutics.
When entering into a new modality, our approach is consistent with our strategic principles and perspectives on risk management discussed previously. The tenets of our approach are summarized below.
•We identify a first program (or programs) through which we seek to discover and develop solutions for any modality-specific technological challenges. We then leverage the learnings from this first program to the benefit of all subsequent programs in the modality.
•We seek to diversify biology risks within the modality by advancing multiple programs in parallel, against multiple diseases, following the first program.
•When we believe a strategic collaborator could significantly de-risk our early efforts in a new modality, we often seek a strategic collaborator to share the risks and benefits on a specific set of early programs.
•After experience with the first program (or programs) in a modality, we seek to rapidly expand our pipeline within that modality to take full advantage of the opportunity.
Illustrating our approach: From our first modality to today
We started with prophylactic vaccines as our first modality because we believed this modality faced lower technical hurdles, relative to other areas. Our early formulations of mRNA tended to stimulate the immune system, which would present a challenge to therapeutics but was a desired feature for vaccines. In addition, many potential prophylactic vaccine antigens are well-characterized, allowing us to reduce biology risk. Lastly, the dosing regimens for vaccines require as few as one or two administrations, and generally involve relatively low doses.
For our first programs in this modality we chose our H10N8 and H7N9 pandemic influenza vaccines, each requiring expression of a single membrane protein.
We chose to pursue two programs in separate, but parallel, clinical trials to establish the flexibility of our platform.
When both programs met our goals for safety, tolerability, and pharmacology, we accelerated and expanded our vaccine pipeline to include multiple commercially meaningful and increasingly complex vaccines. These included a combination vaccine, designed to protect against two unrelated respiratory viruses, human metapneumovirus, or hMPV, and human parainfluenza 3, or PIV3, and a vaccinebelieve that combines six different mRNAs, our cytomegalovirus, or CMV, vaccine, to express a complex pentameric antigen. We also sought strategic alliances with the Defense Advanced Research Projects Agency (DARPA), the Biomedical Advanced Research Development Authority (BARDA) and Merck & Co. (Merck), to allow us to rapidly expand our pipeline and complement our capabilities with their expertise. This early work in the prophylactic vaccines modality led to the ability to introduce our COVID-19 vaccine during 2020 in response to the ongoing pandemic.
Over time, we have taken on more challenging applications and technological hurdles with each successive modality, but we have also tried to build upon our prior experiences to manage risk. For example, in our cancer vaccines modality, we are now applying our technology to elicit T cell responses to potentially recognize and eradicate cancer as a logical extension of our prophylactic vaccines modality. Having demonstrated local expression of protein in our vaccines, we expanded into local therapeutic applications. For example, in our intra-tumoral immuno-oncology modality, we are seeking to use local expression to drive anti-cancer T cell responses by transforming tumor microenvironments. We can also use local expression to drive regenerative processes as in our Vascular Endothelial Growth Factor A, or VEGF-A program. Most recently, we have expanded into two new modalities that use systemic delivery of mRNA to encode secreted and cell surface or intracellular proteins. We have moved multiple programs in these areas into development for the treatment of diseases as varied as rare genetic disorders, preventing viral infections, or treating heart failure.
Expanding within our designated core modalities
In 2020 we designated the prophylactic vaccines and systemic secreted and cell surface therapeutics modalities as “core modalities” following positive Phase 1 data from our CMV vaccine and chikungunya antibody program, respectively. We believed that this data reduced the risk of these modalities, and our strategy is to invest in additional development candidates within these modalities.
Since January 2020, we have nominated six new development candidates within our prophylactic vaccines modality, including our COVID-19 vaccine (which is now authorized for emergency use or conditionally approved in over 30 countries), as well as vaccine
candidates for seasonal flu, HIV, Nipah virus, and EBV. We also regained rights to develop an RSV vaccine for adults, following the conclusion of our collaboration with Merck in October 2020, which consolidated our global commercial rights to all development candidates within our prophylactic vaccines modality.
Within our systemic secreted and cell surface therapeutics modality, we announced two new development candidates in 2020: Interleukin-2, or IL-2, and programmed death-ligand-1, or PD-L1. In 2021, we also announced that we have regained the rights to our Relaxin program from AstraZeneca, and we are currently evaluating how to proceed with the program. Our exploratory modalities continue to be a critical part of advancing our strategy to maximize the application of our potential mRNA medicines.
How modalities continue to build our pipeline
We believe our portfolio of modalities—each with distinct technological and biological risk profiles—allows us to maximize long-term value for patients and investors. We see our six current modalities as six distinct multi-product pipelines that represent different risk profiles and benefit from common infrastructure and a shared platform technology. We believe the high technologytechnological correlation within a modality allows us to rapidly accelerate the expansion of the pipeline inprograms within that modality based on learnings from the initial programs. We believeearlier programs, while the lower technology correlation between modalities allows us to compartmentalize the technology risks. Additionally, because programs within a modality pursue diverse diseases, they often have uncorrelated biology risk. Each time we add a modality and a new product candidate to our portfolio, we create a network effect because each incremental program can help us gain additional insight into the other programs in our pipeline.
Although developing a new modality is difficult, time-consuming and expensive, we believe our experience and technology provide us with unique advantages in the development of mRNA medicines. Over the last decade, we have developed a number of modalities, each with one or many product candidates in the clinic. We believe that our ongoing investments in our platform will lead to the identification of additional new modalities inand expand the future,utility of our existing modalities and will expand the diversity of our pipeline.
Our current modalities
Our current modalities are described below. More detail regarding our current programs in each modality is provided below under “—Our Pipeline.”
•Infectious disease vaccines: The goal of our infectious disease vaccines is to safely pre-expose the immune system to a small quantity of a protein from a pathogen, called an antigen, so that the immune system is prepared to fight the pathogen if exposed in the future, and prevent infection or disease. Our infectious disease vaccines include those targeting respiratory viruses, latent viruses and enteric viruses, as well as bacterial vaccines and public health vaccines. We believe mRNA vaccines have several advantages, including the ability to mimic many aspects of various infections, the ability to combine antigens for compelling product profiles, the rapid discovery and advancement of programs into the clinic and the capital efficiency and speed from shared manufacturing processes and infrastructure.
•Cancer vaccines and therapeutics: The goal of a cancer therapy is to safely expose the patient’s immune system to tumor-related antigens, known as neoantigens, to enable the immune system to elicit a more effective antitumor response. Our cancer therapies modality is focused on the use of mRNA to express neoantigens found in a particular tumor in order to elicit an immune response via T cells that recognize those neoantigens, and therefore the tumor. These neoantigens can either be unique to a patient or can be related to a driver oncogene found across subsets of patients. Recent breakthroughs in cancer immunotherapy, such as checkpoint inhibitors and chimeric antigen receptor T cell therapies, have demonstrated that powerful antitumor responses can be achieved by activating antigen specific T cells. We believe one approach to improve the efficacy of checkpoint inhibitors is to develop vaccines that increase both the number and antitumor activity of a patient’s T cells that recognize tumor neoantigens. We believe that mRNA technology is an attractive approach for cancer therapies for several reasons, including the ability to deliver multiple personalized neoantigens in a single mRNA molecule, and that mRNA encoding for neoantigens is translated and processed by patients’ endogenous cellular mechanisms for presentation to the immune system.
•Rare disease intracellular therapeutics: The goal of this modality is to provide intracellular proteins, such as intracellular enzymes and organelle-specific proteins, as safe, tolerable, and efficacious therapies. Our mRNA medicines aim to increase levels of intracellular proteins to achieve a therapeutic effect in one or more tissues or cell types and our initial focus is on rare genetic diseases. Intracellular therapeutics are not currently addressable with recombinant proteins, which are typically
administered systematically and cannot reach inside of the cell. Our potential advantages in these areas include encoding for intracellular and organelle-specific proteins and the production of hard-to-make or complex proteins with native post-translational modifications.
•Intratumoral immuno-oncology: The goal of this modality is to treat or cure cancer by transforming the tumor microenvironment to drive anti-cancer T cell responses against tumors. The outlook for any patients with advanced cancer remains poor, especially in patients with tumors that have little immune system engagement, sometimes termed immunologically “cold.” In conjunction with a checkpoint inhibitor, we aim to activate the immune system against these otherwise immunologically cold tumors. Intratumoral administration allows for localized effect of these therapeutics that could be toxic if administered systemically. We believe our approach to intratumoral immuno-oncology using mRNA medicines could complement checkpoint inhibitors and has several advantages over recombinant protein-based drugs, including production of membrane-associated immune stimulatory proteins, multiplexing of mRNA to access multiple immune stimulatory pathways and creation of engineered mRNA sequences to reduce off-target effects.
•Inhaled pulmonary therapeutics: The goal of this modality is to develop mRNA medicines that can be delivered to the lung as safe, tolerable and efficacious therapies. We are developing nebulized LNP formulations that can transfect airway epithelial cells to deliver mRNA into the lungs of patients in order to express proteins coded in the mRNA. We aim to leverage our technology for pulmonary diseases in patients for whom there are no existing effective therapies. Our potential advantages in these areas include lung-associated production of secreted, membrane-associated or intracellular hard-to-make or complex proteins.
•Systemic secreted and cell surface therapeutics: The goal of this modality is to provide secreted proteins, such as antibodies or enzyme replacement therapies across a wide range of diseases, such as heart failure, infectious diseases, and rare genetic diseases. Our mRNA medicines instruct various cells of the human body to secrete proteins for therapeutic effect. Systemically delivered, secreted and cell surface therapeutics, we believe, would allow us to target areas of biology that cannot be addressed using recombinant proteins. Our potential advantages in this area include encoding for hard-to-make or complex secreted or membrane-associated proteins, multiplexing of mRNA to encode for multiple proteins with complementary activity, native post-translational modifications and sustained production of proteins, which can increase exposure to proteins with short half-lives.
OUR PIPELINE
SinceOver the last decade, we nominated our first program in late 2014, we and our strategic collaborators have advanced in parallel a diverse development pipeline whichthat currently consists of 27 development candidates across our 2445 therapeutic and vaccine programs, with 13 having entered the clinic and an additional development candidate subject to an open investigational new drug application (IND). Aspectsnine of which are in late-stage development. The scope of our pipeline have been supported through strategic alliances, including with AstraZeneca, Merck, and Vertex Pharmaceuticals, or Vertex, and government-sponsored organizations and private foundations focused on global health initiatives, including BARDA, DARPA,reflects the National Institutebreadth of Allergy and Infectious Diseases (NIAID), the National Institutes of Health (NIH), the Coalition for Epidemic Preparedness Innovations (CEPI), and the Bill & Melinda Gates Foundation.
biology addressable using mRNA technology. Our selection process for advancing new developmentproduct candidates reflects both program-specific considerations as well asand portfolio-wide considerations. Program-specific criteria include, among other relevant factors, the severity of the unmet medical need, the biology risk of our chosen target or disease, the feasibility of clinical development, the costs of development and the commercial opportunity. Portfolio-wide considerations include the ability to demonstrate technical success for our platform components within a modality, thereby increasing the probability of success and learnings for subsequent programs in the modality and in some cases in other modalities.
The breadth of biology addressable using mRNA technology is reflected in our current development pipeline of 24 programs. The diversity of proteins made from mRNA within our development pipeline is shown in the figure below.
The following chart shows our current pipeline of 24 development programs, grouped into modalities—first our two core modalities where we believe we have reduced the technology risk, followed by our four exploratory modalities in which we are continuing to investigate the clinical use of mRNA medicines.
PROPHYLACTICOur full pipeline, grouped by modalities, is shown in the figure below:
INFECTIOUS DISEASE VACCINES MODALITY
We designed our prophylactichave 31 different infectious disease vaccine programs, of which 30 have entered the clinic. Our infectious disease vaccines modality to prevent or control infectious diseases. This modality includes our COVID-19 vaccine, which was first authorized by the U.S. FDA for emergency use in December 2020 for vaccination of adults age 18 and older against SARS-CoV-2. Our prophylactic vaccines modality currently includes ten development candidates in addition to our commercial COVID-19 vaccine, all of which are vaccines against viruses. The goal of any vaccine is to safely pre-expose the immune system to a small quantity of a protein from a pathogen, called an antigen, so that the immune system is prepared to fight the pathogen if exposed in the future,respiratory viruses, latent viruses and prevent infection or disease.
Prophylactic vaccines: Opportunity
Vaccines to prevent infectious diseases are one of the great innovations of modern medicine. In the United States alone, the Centers for Disease Controlenteric viruses, as well as bacterial vaccines and Prevention (CDC) estimates that childhood vaccinations given in the past two decades will in total prevent 322 million Americans from falling ill, 21 million hospitalizations, 732,000 deaths, $295 billion of direct costs, and $1.3 trillion in social costs. The commercial opportunity for vaccines is significant; the worldwide vaccine market in 2019 was $35 billion. More innovative vaccines have been able to achieve pricing per regimen generally ranging from 5 to 20 times that of seasonal flupublic health vaccines.
Prophylactic vaccines: Product features
We believe mRNA-based vaccines offer several advantages, including:
•Ability to mimic many aspects of natural viral infections. mRNA enters cells and is used to produce viral antigen proteins from within thecell that include natural, post-translational modifications. This mimics the process by which natural viral infections occur, where information from viral genomes is used to produce viral proteins from within a cell. This can potentially enhance the immune response, including improved B and T cell responses.
•Multiplexing of mRNA for more compelling product profiles. Multiple mRNAs encoding for multiple viral proteins can be included in asingle vaccine, either permitting production of complex multimeric antigens that are much more difficult to achieve with traditional technologies, or producing antigens from multiple viruses at once. As an example, our CMV vaccine (mRNA-1647) contains six mRNAs, five of which encode five different proteins that combine to form a pentameric protein complex that is a potentially critical antigen for immune protection against CMV.
•Rapid discovery and advancement of mRNA programs into the clinic. Many viral antigens are known. However, with traditional vaccines,the target pathogens or antigens have to be produced in dedicated cell-cultures and/or fermentation-based manufacturing production processes in order to initiate testing of potential vaccine constructs. Our ability to design our antigens in silico allows us to rapidly produce and test antigens in preclinical models, which can dramatically accelerate our vaccine selection.
•Capital efficiency and speed from shared manufacturing processes and infrastructure. Traditional vaccines require product-dedicated production processes, facilities, and operators. Our mRNA vaccines are produced in a manufacturing process that is sufficiently consistent across our pipeline to allow any one of our facilities the flexibility to produce any of our mRNA vaccines.
ProphylacticInfectious disease vaccines: Vaccines against respiratory viruses
COVID-19 vaccine (mRNA-1273)vaccines (mRNA-1273/Spikevax®, next-generation mRNA-1283)
Moderna’sOur COVID-19 vaccine has already been administered in millions of patients and is approved or authorized for use in more than 30 countries70 countries. COVID-19 is caused by the SARS-CoV-2 virus that was first identified in humans in 2019, driving a global pandemic resulting in millions of deaths. The risk of mortality increases with age and the risk of severe disease and mortality increases for persons with certain pre-existing diseases or comorbid conditions, such as cardiovascular disease, diabetes, chronic lung disease and obesity.
Our COVID-19 vaccine (mRNA-1273) was designed, subject to Phase 1, Phase 2 and Phase 3 clinical trials, delivered clinical trial results, and received emergency use and other conditional authorizations in less than a year, and has been and continues to be a key tool in fighting the global SARS-CoV-2 pandemic. The vaccine is referred toCOVID-19 as the ModernaSARS-CoV-2 virus evolves. As part of our strategy to combat the virus, we have continued to develop and assess variant-specific versions of our COVID-19 Vaccinevaccine. In September 2023, we received regulatory approvals in major markets for our updated COVID-19 vaccine (mRNA-1273.815), which targets the U.S.XBB.1.5 subvariant of SARS-CoV-2. We developed mRNA-1273.815 in accordance with regulatory guidance, with the goal of broadening vaccine-induced immunity and Canadian markets,providing protection against circulating SARS-CoV-2 XBB lineage variants. We have also observed preliminary clinical trial data showing that mRNA-1273.815 generates a robust immune response against other variants of SARS-CoV-2.
The FDA has approved mRNA-1273.815 for individuals ages 12 years and is generally referred to as the COVID-19 Vaccine Modernaolder, and granted Emergency Use Authorization for individuals six months through 11 years of age. mRNA-1273.815 has also been authorized for individuals six months and older in other markets. key markets, including the EU, Canada and Japan.
Forward-looking references to our COVID-19 vaccine in this Annual Report on Form 10-K may include future modifications to mRNA-1273 or other developmentproduct candidates that are designed to provide protection against variants of the SARS-CoV-2 virus.
We worked throughout 2020In addition to expand our manufacturing capacity and partnered withapproved or authorized COVID-19 vaccines, we have advanced other companies to supplement that production, and currently anticipate that we will be able to produce between 700 million and 1 billion dosesCOVID-19 vaccine candidates into the clinic as part of our effort to fight the evolving SARS-CoV-2 virus. Our next-generation COVID-19 vaccine, in 2021. Our COVID-19 vaccinewhich is a two-dose course, meaning this production will be sufficient to vaccinate between 350 million and 500 million people against COVID-19 at the current 100 microgram (µg) dose. As of December 31, 2020, we had committed orders for approximately 520 million doses of the COVID-19 vaccinedesigned to be deliveredrefrigerator-stable (mRNA-1283), is in 2021, for total contract consideration of $11.7 billion. We are conducting a pivotal Phase 2/3 study, of mRNA-1273 in adolescents 12 to 17 years of age. Additional studies are planned to evaluate our COVID-19 vaccine in children younger than 12 years and immunocompromised populations. We are also conducting a field effectivenesswe anticipate data from the trial in the United Statesfirst half of 2024. Our goal with mRNA-1283 is to gather additional data in other populations, including older adults and individuals with co-morbidities that place them at increased risk for the complications of COVID-19.
On February 25, 2021, we announced that the Company is developing a next-generation vaccine against COVID-19, mRNA-1283.This vaccine candidate encodes for the portions of the SARS-CoV-2 spike protein that are critical for neutralization, specifically the Receptor Binding Domain (RBD) and N-terminal Domain (NTD). The encoded mRNA-1283 antigen is shorter than mRNA-1273, and is being developed as a potential refrigerator stable mRNA vaccine that we anticipate will facilitate easier distribution and administration by healthcare providers. mRNA-1283 is intendedFurther, as SARS-CoV-2 evolves, we continue to perform continuous epidemiological monitoring, genomic surveillance and risk assessments of variants of concern to determine which new variants may have the ability to circumvent immunity provided by currently approved COVID-19 vaccines. For variants that appear to be evaluatedgrowing in circulation and have evolved the ability to evade immunity, we proactively develop new product candidates. We have taken several of these candidates to clinical trials, and our monitoring activities allow for use asexpedited delivery of new vaccines in the event that regulatory agencies request specific vaccine composition updates to address public health needs.
COVID-19 Commercial, Manufacturing and Supply Updates
Net product sales of our COVID-19 vaccine were $6.7 billion in 2023, which accounted for all of our product revenues.We anticipate that sales of our COVID-19 vaccine in 2024 will provide a booster dosesignificant portion of our commercial revenues for previously vaccinated or infected individuals as well asthe coming year. Prior to the third quarter of 2023, we sold our COVID-19 vaccine to the U.S. Government, foreign governments and international organizations. In the third quarter of 2023, we commenced sales of our latest COVID-19 vaccine to the U.S. commercial market, in a primary series for seronegative individuals.addition to continuing sales to foreign governments and international organizations.
COVID-19: Disease overview
SARS-CoV-2 is the virus that causesWith COVID-19 disease, which has led to over 2.4 million global deaths
Coronaviruses are a large family of viruses that can cause illnessentering an endemic phase in animals or humans. In humans there are several known coronaviruses that cause respiratory infections. These coronaviruses range from the common cold to more severe diseases such as severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and COVID-19. SARS-CoV-2 is the novel coronavirus first identified in humans in December 2019 and is the cause of COVID-19.
COVID-19 is the most severe global pandemic since the influenza pandemic of 1918. As of February 18, 2021, there have been over 110 million confirmed cases and over 2.4 million global deaths from COVID-19. The risk of mortality increases2023, we saw increased seasonality for sales, with age (estimated to be ~0.1% for individuals aged 0-19, ~6% for individuals over age 60). Risk of severe disease and mortality increase for persons with pre-existing diseases or comorbid conditions (e.g. cardiovascular disease, diabetes, chronic lung disease, obesity).
As the pandemic has continued variants of the original SARS-CoV-2 virus first detected in Wuhan, China, have continued to evolve. Certain of these variant strains of SARS-CoV-2 have already proven to lead to increased rates of transmission of the virus, and as the virus continues to evolve, future strains or those already in circulation could cause more severe forms of disease. Some variant strains have also demonstrated resistance to existing vaccines and therapeutics for COVID-19. Data from the primary efficacy analysis announced November 30, 2020 suggested that mRNA-1273—which was designed based upon the genetic sequence of the virus first detected in Wuhan, China—is 94.1% efficacious against symptomatic COVID-19 disease with a clinically-acceptable safety profile. However, while the neutralization titers are comparable for most variant strains tested, including the B.1.1.7 strain first identifiedgreater demand in the United Kingdom, there are datafall/winter season in each hemisphere as countries sought to suggest a 6.4-fold reductionprovide vaccinations to their populations.We expect this seasonality to continue in neutralization titers againstfuture years in the B.1.351 strain which was first identifiedendemic market. As such, we resized our global manufacturing footprint in South Africa. While2023 to account for demand in the titers against B.1.351 remain higher than those found to be protective against viral challenge in animal models, we have nonetheless announced a proactive strategy to address variantsendemic market. For further information on the sales and manufacturing of the virus. We are continuing to collaborate with the NIH to study the evolution of the SARS-CoV-2 virus and the effectiveness of mRNA-1273 against new strains. As one part of this strategy, we are testing additional boosters of mRNA-1273 to further increase protection against emerging strains. Additionally, we are advancing an emerging strain booster, referred to as mRNA-1273.351 (named for the B.1.351 strain) to assess protection against that strain. On February 24, 2021, doses of mRNA-1273.351 were shipped to the NIH for a Phase 1 clinical trial that will be led and funded by NIAID.
our COVID-19 vaccine, (mRNA-1273): Product concept
see “
Our vaccine is an mRNA vaccine against COVID-19 encoding for a prefusion stabilized formManufacturing” and “Management’s Discussion and Analysis of the Spike (S) protein, which was co-developed by ModernaFinancial Condition and investigators from NIAID’s Vaccine Research Center.
The Spike protein complex is necessary for membrane fusion and host cell infection and has been the target for the majorityResults of vaccines against COVID-19. Our COVID-19 vaccine encodes a stabilized version of the SARS-CoV-2 full-length spike glycoprotein trimer, S-2P, which has been modified to include two proline substitutions at the top of the central helix in the S2 subunit (S-2P).Operations” below.
COVID-19 vaccine (mRNA-1273): Preclinical data
mRNA-1273 led to protection against SARS-CoV-2 infection in the lungs and nose of non-human primates
On July 29, 2020, we announced the publication in The New England Journal of Medicine of data from a preclinical study of mRNA-1273, demonstrating that two doses of mRNA-1273 provided protection against lung inflammation following viral challenge with SARS-CoV-2 in non-human primates at both the 10 µg and 100 µg dose levels. In addition, both the 10 µg and 100 µg dose groups demonstrated protection against viral replication in the lungs, with the 100 µg dose also protecting against viral replication in the nose of the animals. Of note, none of the eight animals in the 100 µg group showed detectable viral replication in the nose compared to six out of eight in the placebo group on day 2.
COVID-19 vaccine (mRNA-1273): Clinical data
Phase 1 trial. A Phase 1 open-label study of mRNA-1273 is being conducted by the NIH. This study, which began on March 16, 2020, originally enrolled 45 healthy adult volunteers ages 18 to 55 years and is evaluating three dose cohorts (25 µg, 100 µg and 250 µg). An additional seven cohorts were subsequently added in the Phase 1 study: a 50 µg cohort in adults 18-55 (n=15), three cohorts of older adults (n=30, ages 56-70, 25 µg, 50 µg, and 100 µg) and three cohorts of elderly adults (n=30, ages 71 and above, 25 µg, 50 µg, and 100 µg).
On July 14, 2020, we announced the publication in The New England Journal of Medicine of an interim analysis of data from the original cohorts obtained through Day 57 in the Phase 1 study. This interim analysis demonstrated that mRNA-1273 induced binding antibodies to the full-length SARS-CoV-2 Spike protein (S) in all participants after the first vaccination, with all participants seroconverting by Day 15. Dose dependent increases in binding titers were seen across the three dose levels, and between prime and boost vaccinations within the dose cohorts. After two vaccinations, at Day 57, geometric mean titers exceeded those seen in convalescent sera obtained from 38 individuals with confirmed COVID-19 diagnosis. Of the 38 individuals in the convalescent sera group, 15% were classified as having severe symptoms (hospitalization requiring intensive care and/or ventilation), 22% had moderate symptoms and 63% had mild symptoms. Convalescent sera samples were tested using the same assays as the study samples. mRNA-1273 was generally well-tolerated, with no serious adverse events reported through Day 57. Adverse events were generally transient and mild to moderate in severity.
Evaluation of the durability of immune responses is ongoing, and participants will be followed for one year after the second vaccination, with scheduled blood collections throughout that period. On December 2, 2020, a letter to the editor was published in TheNew England Journal of Medicine reporting that participants in the Phase 1 study of mRNA-1273 retained high levels of neutralizing antibodies through 119 days following first vaccination (90 days following second vaccination). The study was led by NIAID.
Phase 1 older adult cohort. On September 29, 2020, we announced the publication in The New England Journal of Medicine of the second interim analysis of data from 40 healthy adult participants across two dose levels (25 µg and 100 µg) in two age cohorts (ages 56-70 and ages 71+), and reports results through Day 57 (1 month after the second dose). Both the 25 µg and 100 µg dose levels of mRNA-1273 were generally well-tolerated, with no serious adverse events reported through Day 57.
At both the 25 µg and 100 µg dose levels, after two vaccinations, mRNA-1273 induced dose-dependent binding antibody responses reaching the upper quartile of the distribution of convalescent sera. At Day 57 (1 month post-dose 2), geometric mean titers (GMT) exceeded the median of those seen in convalescent sera from 41 individuals with confirmed COVID-19 diagnosis.
Phase 3 trial. The Phase 3 COVE trial for mRNA-1273 is a randomized, 1:1 placebo-controlled study testing the vaccine at the 100 µg dose level in 30,000 participants in the U.S., ages 18 and older. The primary endpoint of the COVE study, which is ongoing, is the prevention of symptomatic COVID-19 disease. Key secondary endpoints include prevention of severe COVID-19 disease and prevention of infection by SARS-CoV-2. The Phase 3 COVE study was designed in collaboration with the FDA and NIH to evaluate vaccine efficacy in Americans at risk of severe COVID-19 disease and completed enrollment of more than 30,000 participants ages 18 and older in the U.S. on October 22, 2020, including those at high risk of severe complications of COVID-19 disease. In early September 2020, we announced that we were slowing enrollment in the trial to ensure representation of communities of color in the COVE study. Final enrollment in the study included more than 11,000 participants from communities of color, representing 37% of the study population.
On December 30, 2020, interim safety and primary efficacy results from the Phase 3 trial of mRNA-1273 were published in The New England Journal of Medicine. The primary endpoint of the Phase 3 COVE study was based on the analysis of COVID-19 cases confirmed and adjudicated starting two weeks following the second dose of vaccine. This final analysis was based on 196 cases, of which 185 cases of COVID-19 were observed in the placebo group versus 11 cases observed in the Moderna COVID-19 Vaccine group, corresponding to a 94% vaccine efficacy (95% CI 89.3-96.8%; p<0.0001). This efficacy is highlighted in the chart below. The most common solicited adverse reactions (ARs) after the two-dose series was injection site pain (86.0%). Solicited systemic adverse events occurred more often in the Moderna COVID-19 vaccine group (54.9% and 79.4%) than in the placebo (42.2% and 36.5%) group after both the first dose and the second dose respectively and were most commonly headache, fatigue and myalgia. While the majority of these ARs were mild (grade 1) or moderate (grade 2), there was a higher occurrence of severe (grade 3) reactions in the Moderna COVID-19 Vaccine group after the first (2.9%) and second (15.8%) injections. The majority of local solicited ARs occurred within the first one to two days after injection and generally persisted for a median of one to two days. Safety data continues to accrue, and the study continues to be monitored by an independent Data Safety Monitoring Board (DSMB) appointed by the NIH. All participants in the COVE study will be monitored for two years after their second dose to assess long-term protection and safety. Additional data to be collected will include longer term safety follow-up, duration of protection against COVID-19, and efficacy against asymptomatic SARS-CoV-2 infection. All placebo recipients in the Phase 2 and Phase 3 trials have been offered our COVID-19 vaccine. As of February 15, 2020, over 80% of placebo recipients have received at least one dose of our COVID-19 vaccine.
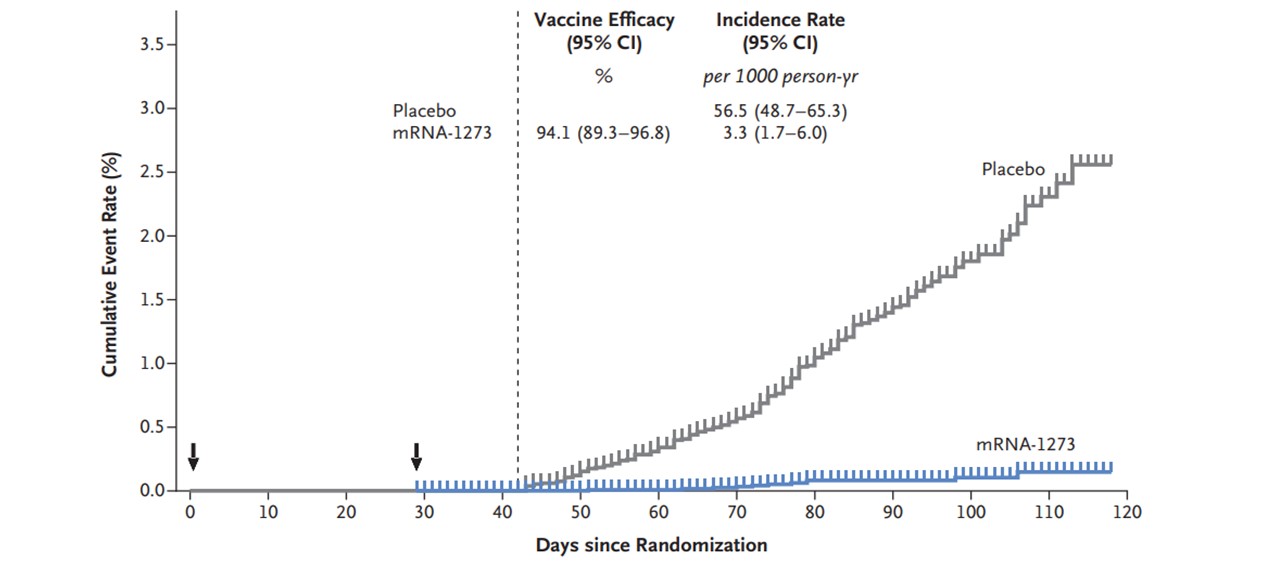
We are also conducting a Phase 2/3 study of mRNA-1273 in adolescents 12 to less than 18 years of age and a Phase 2/3 pediatric study in children less than 12 years old is expected to begin in April 2021. Finally, to assess the ability of a third vaccination with mRNA-1273 to further boost antibody titers, participants in the active arms (100 and 50ug) of our Phase 2 adult study will be offered a 50ug booster dose. Additional studies are planned to evaluate mRNA-1273 in special risk groups, such as the immunocompromised.
COVID-19 vaccine (mRNA-1273): Regulatory updates
On December 18, 2020, the U.S. Food and Drug Administration authorized the emergency use of mRNA-1273, Moderna’s vaccine against COVID-19, in individuals 18 years of age or older. Our COVID-19 vaccine has also been authorized for emergency or conditional use by regulatory authorities in Canada, Israel, the United Kingdom, the European Union, Switzerland, Singapore and Qatar. Additional authorizations are currently under review in additional markets and by the World Health Organization (WHO).We expect to submit a biologics license application, or BLA, to the FDA for approval of mRNA-1273, and to submit applications with other regulatory authorities for similar approvals, which will allow for the commercial sale and distribution of the vaccine after the pandemic subsides and conditions are no longer met for emergency or conditional use authorizations.
COVID-19 vaccine (mRNA-1273): Commercial, manufacturing and supply updates
As of December 31, 2020, we have signed supply agreements to deliver approximately 520 million doses of mRNA-1273 in 2021, for total contract consideration of $11.7 billion. We have confirmed the following supply agreements as of that date: United States: 200 million doses, with option for an additional 300 million doses; European Union: 160 million doses; Japan: 50 million doses; Canada: 40 million doses, with option for an additional 16 million doses; South Korea: 40 million doses; United Kingdom: 17 million doses; Switzerland: 7.5 million doses; Israel: 6 million doses. We also signed agreements with Qatar, Singapore, and other countries for which order amounts were not disclosed.
During 2021, and through the date of this Annual Report on Form 10-K, we have agreed to additional supply agreements, or received option exercises, for additional doses as follows: United States: 100 million doses, with an option remaining for 200 million doses; European Union: 150 million doses, with an option remaining for 150 million doses in 2022 (subject to final execution of the Purchase Agreement following the expiration of the opt out period for EU Member States); Canada: 4 million doses; Colombia: 10 million doses; and Taiwan: 5 million doses.
Our base case global production estimate is 700 million to 1 billion doses for 2021. We are continuing to invest and add staff as we meet production at the high end of this range. We are also working on increasing our potential supply to up to 1.4 billion doses for 2022 based upon the current 100 µg dose. Much of the production for the supply of the U.S. market will be completed at our MTC facility in Norwood, Massachusetts, with additional production by Lonza Ltd. for the U.S. market. We have also partnered with Lonza to complete all production of mRNA-1273 for markets other than the U.S. in Switzerland. Fill-finish services for mRNA-1273 are provided by Catalent Inc. in the U.S., and by ROVI and Recipharm outside the U.S.
We believe that the conditions under which mRNA-1273 can be shipped and stored are a key feature of our vaccine as such conditions provide for greater flexibility in distributing the vaccine to markets where special handling may be a barrier to distribution. mRNA-1273 does not require dilution prior to use and remains stable at 2° to 8°C (36° to 46°F), the temperature of a standard home or medical refrigerator, for 30 days. mRNA-1273 remains stable at -20° C (-4°F) for up to six months, at refrigerated conditions for up to 30 days and at room temperature for up to 12 hours.
hMPV/PIV3 vaccine (mRNA-1653)
We are developing a combination vaccine to address two viruses that are leading causes of respiratory infection
Human metapneumovirus, or hMPV, and human parainfluenza virus 3, or PIV3, are significant causes of respiratory tract infections in children. Despite the substantial impact hMPV and PIV3 have on human health, attention and research on these viruses have lagged relative to other respiratory infections, like respiratory syncytial virus (RSV). To date, no vaccine to prevent hMPV or PIV3 infections has been approved. Our platform allows us to combine mRNAs encoding antigens for the two pathogens in one combination vaccine, enabling a single vaccine that could protect against both respiratory infections. In our approach, we utilize mRNA sequences encoding for the membrane fusion (F) glycoproteins, or F proteins, for each of the viruses. We have generated safety, tolerability, and immunogenicity data from the Phase 1 trial for mRNA-1653 in the United States, which has been completed. Based on these data, we have a Phase 1b trial for mRNA-1653 ongoing in the United States in healthy adults and children aged 12-59 months.
hMPV/PIV3 vaccine (mRNA-1653): Disease overview
hMPV and PIV3 have a substantial impact on human health yet have lagged in research and attention relative to RSV
There is no approved vaccine for hMPV although this RNA virus has been determined to be one of the more frequent causes of upper and lower respiratory tract infections. hMPV has been detected in 4% to 15% of patients with acute respiratory infections. hMPV causes disease primarily in young children but can also infect adults, the elderly, and immunocompromised individuals. Clinical signs of infection range from a mild upper respiratory tract infection to life-threatening severe bronchiolitis and pneumonia. hMPV was discovered in 2001 and identified as a leading cause of respiratory infection.
There is no approved vaccine for PIV3 although this RNA virus is recognized as an important cause of respiratory tract infections in children. Infections from parainfluenza virus, or PIV, account for up to 7% of acute respiratory infections among children younger than 5 years. Of the four PIV types identified, PIV3 most frequently results in infections and leads to the more serious lower respiratory tract infections compared to the other three PIV types. Though PIV3 related infections were identified in the past, awareness of their burden to patients and hospitals has risen over the past several years.
Awareness of hospitalizations due to hMPV or PIV3 infections have risen, and we believe that a single, combined vaccine intended for active immunization of infants against both hMPV and PIV3 would be valuable. Previous attempts at developing a vaccine have focused on only hMPV or PIV alone with no known attempts at a combination vaccine.
hMPV/PIV3 vaccine (mRNA-1653): Our product concept
Our approach is to develop a combination vaccine for all infants
mRNA-1653 is a single investigational vaccine consisting of two distinct mRNA sequences that encode the membrane F proteins of hMPV and PIV3, co-formulated in our proprietary LNP.
hMPV/PIV3 vaccine (mRNA-1653): Preclinical information
Our mRNA vaccine is immunogenic and protective in preclinical multiple species
We have evaluated multiple combinations for hMPV/PIV3 mRNA vaccines encoding full-length F proteins for hMPV and PIV3 viruses in mice, Sprague Dawley rats, cotton rats, and African green monkeys, each following intramuscular injection. These studies demonstrate that mRNA encoding for F proteins from these viruses induce robust hMPV and PIV3 neutralizing antibody titers in all species tested. Further, we have demonstrated that our hMPV and PIV3 mRNA combination vaccine does not lead to vaccine-enhanced respiratory disease (evaluated in cotton rats) and is protective against hMPV or PIV3 viral challenge (evaluated in cotton rats and African green monkeys).
hMPV/PIV3 vaccine (mRNA-1653): Clinical data
We have generated safety, tolerability, and immunogenicity data from a Phase 1 trial in the U.S., which has been completed; based on the data, we have a Phase 1b trial in healthy adults and children aged 12-59 months ongoing in the U.S.
A first-in-human dose-ranging study, mRNA-1653-P101, in healthy adults (N = 124) was completed in January 2020. This study evaluated the safety, reactogenicity, and immunogenicity of a range of dose levels (25, 75, 150 or 300 µg) administered on a 1-dose or 2-dose vaccination schedule (approximately 28 days apart) compared with a placebo control in healthy adult subjects (18 through 49 years of age) with a 13 month follow-up period. The mRNA-1653 vaccine was generally well-tolerated at all dose levels. There were no deaths or adverse events of special interest reported during the study. No subject was withdrawn from the study due to an unsolicited adverse event. Injection site pain was the most commonly reported solicited adverse event and the most common grade 3 adverse event. Neutralizing antibodies against hMPV and PIV3 were present at baseline in all subjects, consistent with prior exposure
to both viruses. A single dose of mRNA-1653 boosted serum neutralization titers against hMPV and PIV3, and the magnitude of the boost was similar at all dose levels. The Month 1 to baseline geometric mean ratio (GMR) for the pooled mRNA-1653 treatment groups was approximately 6 for hMPV and 3 for PIV3. A second vaccination did not impact the magnitude of hMPV or PIV3 neutralization titers measured at Month 2. The hMPV neutralizing antibody titers remained above baseline at all dose levels through Month 13, and the PIV3 neutralizing antibody titers remained above baseline at all dose levels through Month 7.
We are conducting a Phase 1b trial to evaluate mRNA-1653 in healthy adults and children aged 12-59 months. The Phase 1b trial is a randomized, observer-blinded, placebo-controlled, dose-ranging trial to evaluate the safety and immunogenicity of two dose levels of mRNA-1653 in healthy adults (18-49 years of age) and three dose levels in children (12-59 months of age) with serologic evidence of prior hMPV and PIV3 exposure. The adult cohort includes 24 adults randomized in the ratio 1:1:1 to receive two doses of 30 μg of mRNA-1653, 150 μg of mRNA-1653, or placebo two months apart. As of January 2021, the adult cohort has been completed and the pediatric portion is ongoing.
RSV vaccine (mRNA-1345)
We are developing an RSV vaccine for children and older adults. We intend to explore a combination vaccine with mRNA-1653, our hMPV/PIV3 vaccine, and/or other respiratory viruses to address a wide array of viral respiratory illness in these populations.
Respiratory syncytial virus or RSV,(RSV) is one of the most common causes of lower respiratory disease in children under the age of five and also in older adults. ThePopulations that are especially vulnerable to developing severe RSV vaccine (mRNA-1345) is our first RSV vaccine to enter the clinic with the same proprietary LNP as our COVID-19 vaccine. We previously had two different RSV candidates in partnership with Merck (mRNA-1777 and mRNA-1172) enter the clinic. mRNA-1345 was initially nominated as a pediatric vaccine for RSV. In October 2020, we announced that we regained the rights to adult RSV vaccines from Merck and will be moving mRNA-1345 into the adult population.
RSV vaccine (mRNA-1345): Disease overview
RSV is the leading cause of unaddressed severe lower respiratory tract disease and hospitalization ininfections include infants, and young children, worldwidechildren and causes a substantial burden of illness inadults with chronic medical conditions and older adults
RSV causes upper and lower respiratory tract illness worldwide and is transmitted primarily via aerosolized droplets from an infected person, or via contamination of environmental surfaces with infectious secretions. Upper respiratory symptoms typically begin within several days of exposure. In healthy adults, the infection may remain confined to the upper respiratory tract. However, in those with compromised immune systems, such as premature infants, the elderly, or individuals with underlying respiratory disease, lower respiratory tract infections commonly occur and may manifest as wheezing, bronchiolitis, pneumonia, hospitalization or even death. Infections with RSV follow a seasonal pattern, occurring primarily in the Northern hemisphere between the months of November and April, and in the Southern hemisphere primarily between March and October.
RSV is a common cause of respiratory tract illness inadults. Most children with mostare infected at least once by two years of age.age two. In the United States, it is estimated that over two million children younger than five years of age receive medical attention and more than 86,000up to 80,000 are hospitalized due to RSV infection annually. Globally, it is estimated that RSV is responsible for over approximately 33 million episodes of acute lower-respiratory tract infection, 3.2 million hospitalizations and as many as 118,000 deaths per year in children younger than five years of age. RSV also causes a substantial burden of respiratory illness in older adults. RSV infection causes an estimated 177,00up to 160,000 hospitalizations and 14,000up to 10,000 deaths per year in adults aged >6565 years or older in the United States. To date, no effective vaccine to prevent RSV has been approved, and the only approved prophylactic treatment is the monoclonal antibody (mAb) palivizumab, marketed as SYNAGIS in the United States for the prevention of serious lower respiratory disease caused by RSV in pediatric patients at high risk for RSV infection
We are developing an RSV vaccine (mRNA-1345): Product concept
Prevent RSV disease in young for children, pregnant women and older adults with an improved RSV antigen and our proprietary LNP formulation
adults. mRNA-1345 encodes an engineered form of the RSV F proteinsprotein stabilized in the prefusion conformation and is formulated in our proprietary LNP. In January 2023, we announced that mRNA-1345 had met primary efficacy endpoints in the pivotal Phase 3 trial in older adults, ages 60 and older. mRNA-1345 demonstrated vaccine efficacy (VE) of 83.7% (95.88% CI: 66.1%, 92.2%; p<0.0001) against RSV-associated lower respiratory tract disease (RSV-LRTD) as defined by two or more symptoms. The F protein is present asother primary efficacy endpoint against RSV-LRTD defined by three or more symptoms was also met, with a homotrimerVE of 82.4% (96.36% CI: 34.8%, 95.3%; p=0.0078). mRNA-1345 was generally well-tolerated with no safety concerns identified. Based on the surfacepositive topline data from the pivotal Phase 3 efficacy trial, the FDA granted mRNA-1345 Breakthrough Therapy Designation for the prevention of RSV. The prefusion conformation of the F protein interacts with a host cell membrane, and the conformational change from prefusion to postfusion drives virus fusion with a host cell. The majority of RSV-specific neutralizing antibodiesRSV-LRTD in convalescent people are directed to epitopes present only on the prefusion conformation of the F protein. The prefusion state of the F protein elicits a superior neutralizing antibody response compared to the postfusion state in animal studies conducted by others. We believe that neutralizing antibodies elicited by mRNA-1345 may lead to an efficacious RSV vaccine.
RSV vaccine (mRNA-1345): Preclinical informationadults 60 years or older.
We have demonstrated thatsubmitted marketing authorization applications in several major markets for the RSV vaccine mRNA-1345 induces robust RSV neutralizing antibody titersprevention of RSV-associated lower respiratory tract disease (RSV-LRTD) and acute respiratory disease (ARD) in mice. For example, the left panel below shows the results of a study in which mice were immunized with different dose levels of mRNA-1345 intramuscularly on study days 1 and 21 and RSV neutralizing antibody titers were measured in serum collected on day 33. When compared to the results of a similar mouse study conducted with mRNA from mRNA-1777 formulatedadults 60 years or older. We expect regulatory approvals beginning in the same proprietary LNP as mRNA-1345, mRNA-1345 was shown to be significantly more immunogenic. We have leveraged our bodyfirst half of non-clinical, clinical and CMC experience from our vaccine portfolio to expedite preclinical development of our RSV mRNA-1345 vaccine.
Serum neutralizing titers in mice for mRNA-1345 and for mRNA-1777 mRNA formulated in our same proprietary LNP
Clinical trials of a formalin-inactivated RSV vaccine conducted in the 1960s resulted in higher rates of severe RSV disease in vaccinated infants than in control infants, a finding referred to as vaccine enhanced respiratory disease (ERD). It is thought that nucleic acid-based vaccines, including mRNA, present a lower risk of ERD because of their biologic similarities with live virus. Given that the RSV vaccine mRNA-1345 is designed to enable intracellular production of prefusion F protein by a person’s own cells, we believe that it likely recapitulates the antigenic presentation and immune cell stimulation as seen with natural infection. Further, the mRNA-1777 RSV vaccine did not predispose for ERD in a cotton rat RSV model (Espeseth et al, npj Vaccines (2020)5:16). To provide further confirmation that the pediatric RSV vaccine mRNA-1345 does not present a risk for ERD, additional preclinical studies will be conducted prior to clinical development of mRNA-1345 in RSV seronegative children or infants.
RSV vaccine (mRNA-1345): Clinical plan2024.
We have additional Phase 3 studies ongoing in adults to explore co-administration with licensed flu or COVID-19 vaccines, revaccination with mRNA-1345 and expansion to adults aged 18 and older who are at high risk for severe RSV disease. In pediatrics, mRNA-1345 is ongoing in Phase 1 and Phase 2 studies, and we are conducting a Phase 1 trial for mRNA-13452 study in healthy younger adults, healthy older adults and RSV-seropositive children. The Phase 1 trial is a randomized, observer-blind, placebo-controlled, dose escalation study to evaluate the safety, reactogenicity and immunogenicity of three dose levels of mRNA-1345 in healthy younger adults aged 18 to 49 years and healthy older adults aged 65-79 years and two dose levels in RSV-seropositive children aged 12-59 months. As of January 2021, 3 of the 4 younger adult cohorts are fully enrolled, and we began enrollment for the older adult cohort in February 2021.
Seasonal influenza vaccine (mRNA-1010, mRNA-1020 and mRNA-1030)
Three development candidates will be assessed in a phase 1 study planned to start in 2021. Mouse immunization studies have shown strong immune responses against the vaccine antigens.maternal populations.
Seasonal influenza vaccines (mRNA-1010, mRNA-1011, mRNA-1012, mRNA-1020 and mRNA-1030)
The World Health Organization (WHO) estimates that seasonal influenza viruses are estimated by the WHOcause three to cause 3 to 5five million cases of severe illness and up290,000 to 650,000 deaths each year, resulting in a severe challenge to public health. Currently licensed seasonal influenza virus vaccines rarely exceed 60% overall effectiveness and are poorly effectivecan provide low effectiveness during years when the circulating viruses do not match the strains selected for the vaccine antigens. Particularly in older adults, the population most affected by severe influenza outcomes, vaccine effectiveness remains low. mRNA influenza vaccines could provide several benefits compared to current vaccines, including the ability to more quickly respond to strain changes, avoidance of mutations that may be acquired during vaccine production in eggs or cell culture and stronger immune responses and improved protection in older adults. We are planning to test three development candidates (mRNA-1010, mRNA-1020 and mRNA-1030) in a Phase 1 study starting in 2021, which will allow us to identify a candidate to take forward into pivotal efficacy studies. The vaccine will be administered as a single dose and will aim to elicit protection from all seasonal influenza viruses. In the future we also plan to evaluate the combination of a seasonal influenza vaccine with vaccines against other respiratory viruses with similar epidemiology.
Seasonal influenza vaccine (mRNA-1010, mRNA-1020 and mRNA-1030): Disease overview
Influenza most substantially impacts young children and older adults, and current vaccines show low effectiveness
Influenza epidemics occur each year and manifest in mild to severe forms. Common symptoms of influenza infection include fever, runny nose, sore throat, muscle pain and coughing. The symptoms onset generally occurs within two days after infection and in uncomplicated cases the disease is self-limiting and resolves within a week. However, influenza virus infection can lead to severe disease, especially in high risk groups (including older adults and individuals with comorbidities), and even death (290,000-650,000 respiratory deaths worldwide annually).
Influenza viruses follow a seasonal circulation pattern with increased cases during the winter months in the Northern and Southern hemispheres, respectively. Since influenza viruses continuously change through a process termed antigenic drift, the circulating viruses are actively monitored by a worldwide monitoring network coordinated by the WHO. Based on the observed circulation patterns and antigenic changes, an expert panel recommends influenza virus strains to be used for vaccine manufacturing twice a year (once for the Northern and once for the Southern hemisphere). Influenza A and influenza B viruses are the most relevant influenza viruses for human infection. Therefore, current recommendations include one influenza A H1N1 strain, one influenza A H3N2 strain and two influenza B strains (covering the B/Victoria and B/Yamagata lineages).
Seasonal influenza vaccine (mRNA-1010, mRNA-1020 and mRNA-1030): Product concept
Prevent influenza virus infections by annual vaccination against circulating seasonal influenza viruses
Our investigational products willmRNA seasonal influenza vaccine program is taking an iterative approach to development. Our first-generation seasonal influenza vaccine candidate (mRNA-1010) encodes for the hemagglutinin (HA) proteins of the strains recommended by the WHO. We are intending to subsequently improve upon the first-generation candidate through inclusion of additional HA antigens that could provide expanded coverage of co-circulating strains, as well as broadening protection through the addition of another influenza protein, the neuraminidase (NA). We also aim to elicit protective antibodies against circulating seasonal influenza viruses. Three products will be tested in the clinic in 2021work with the goal of selecting one productWHO, regulators and public health authorities to move forward into pivotal studies. The final product is expectedenable strain selection closer to be updated twicethe influenza season to provide a year (forbetter match to the Northern and Southern hemispheres, respectively) based on the recommendations by WHO and will include antigens of the four strains recommended for seasonal vaccines. An mRNA-based influenza vaccine has a number of potential advantages over vaccines produced using traditional manufacturing processes, such as avoiding mutations to proteins acquired by passage during egg- or cell-propagation of viruses, presentation of antigens in their natural conformation, better immunogenicity in older adults and more rapid manufacturing timelines.circulating viruses.
Seasonal influenza vaccine (mRNA-1010, mRNA-1020The inclusion of additional HA antigens is being tested in our mRNA-1011/1012 programs, and mRNA-1030): Preclinical informationthe addition of NA antigens is being tested in our mRNA-1020/1030 programs. We aim to ultimately combine both approaches into a single next-generation vaccine.
Preclinical studies showIn February 2023, we announced interim results from the P301 study of mRNA-1010. The results indicated that strong serum antibody responsesmRNA-1010 achieved higher seroconversion rates for A/H3N2 and A/H1N1, as well as superiority on geometric mean titer ratios for A/H3N2 and non-inferiority on geometric mean titer rations for A/H1N1. Non-inferiority was not met for either endpoints for the influenza B/Victoria- or B/Yamagata-lineage strains. mRNA-1010 showed an acceptable safety and tolerability profile. In April 2023, we announced the P302 study of mRNA-1010 did not accrue sufficient cases at the interim efficacy analysis to declare early success in the Phase 3 Northern Hemisphere efficacy trial. In September 2023, we announced the P303 immunogenicity and safety study of mRNA-1010 met all 8 co-primary endpoints with an updated formulation that was able to generate an improved immune response to influenza B strains. mRNA-1010 also elicited higher titers than the licensed comparator against all antigensstrains in this study. An additional study to compare immunogenicity of mRNA-1010 against an enhanced influenza vaccine comparator is currently ongoing. Conversations with regulators regarding filing for approval of mRNA-1010 are elicited after a single administration of the mRNA vaccinescurrently ongoing and we intend to file for regulatory approval in mice, even at low mRNA dose levels.2024.
Phase 1/2 studies for our mRNA-1011/1012 and mRNA-1020/1030 programs have been initiated and interim results have been presented at scientific meetings.
Combination vaccines (mRNA-1083, mRNA-1230, mRNA-1045 and mRNA-1365)
We are developing combination vaccine candidates to protect against a range of respiratory diseases.
ProphylacticmRNA-1083, our next-generation COVID-19 and seasonal influenza combination vaccine, encodes the same antigens as our first-generation seasonal influenza vaccine (mRNA-1010) and our next-generation COVID-19 vaccine (mRNA-1283). In a Phase 1/2 trial, mRNA-1083 showed strong immunogenicity against influenza and COVID-19 compared to licensed standalone vaccines, with an acceptable reactogenicity and safety profile. In October 2023, we initiated a Phase 3 trial in adults 50 years and older. The trial is fully enrolled and data is anticipated in 2024.
We are also conducting Phase 1 studies for mRNA-1230, our COVID-19, seasonal flu and RSV combination vaccine, and mRNA-1045, our seasonal flu and RSV combination vaccine.
Enrollment is ongoing in a Phase 1 trial of mRNA-1365, our RSV and hMPV combination vaccine, in children five to under 24 months of age. In February 2024, the FDA granted Fast Track Designation for mRNA-1365.
Infectious disease vaccines: Vaccines requiring complex antigens and against highly prevalent infectionslatent viruses
CMV vaccine (mRNA-1647)
Our CMV program targets congenital CMV infections to reduce or prevent birth defects
CongenitalHuman cytomegalovirus or CMV, infection is the leading cause of birth defects in the United States. Despite several attempts, to date there is no vaccine approved to prevent congenital transmission of CMV. We believe that in addition to the glycoprotein B, or gB, protein antigen, a successful CMV vaccine would need to include the Pentamer, a 5-protein membrane-bound antigen complex required for epithelial, endothelial, and myeloid cell infection by the virus. A CMV vaccine containing the Pentamer as a recombinant protein or a replication defective virus is complex to make and scale. We used our platform to generate an mRNA vaccine designed to make the Pentamer in its natural membrane-bound conformation. This investigational medicine is designed to prevent or control CMV infection and includes five mRNAs encoding for the Pentamer, as well as one mRNA encoding for CMV gB that has previously demonstrated partial clinical efficacy. The Phase 1 and 2 trials for mRNA-1647 has generated safety and tolerability data, and demonstrated immunogenicity. Based on recent data from the 3-month interim analysis data from the Phase 2 trial, we have selected a dose and initiated planning for Phase 3 trials for mRNA-1647 in the United States, Canada, Europe, Australia, and Israel.
CMV (mRNA-1647): Disease overview
CMV is a major cause of birth defects with no approved vaccine
Human CMV(CMV) is a common human pathogen and member of the herpes virus family. Seropositivity, demonstrating prior exposure to virus, increases with age and is approximately 40-60% in women of child-bearing potential in the United States. However, general awareness of CMV is not high. Less than 10-20% of adults are aware of CMV and most healthy adults after initial (primary) CMV infection do not have symptoms. However, approximately 0.6-0.7% of newborns are congenitally infected by CMV annually in industrialized countries. Congenital CMV results from infected mothers transmitting the virus to their unborn child and it is the leading infectious cause of birth defects in the United States, with approximately 25,000 newborns per year in the United States infected. Birth defects occur in approximately 20% ofU.S. infected babies and include permanent neurodevelopmental disabilities, which can include hearing loss (often permanent), vision impairment, varying degrees of learning disability, decreased muscle strength and coordination, and even death. Some studies report approximately one-third of infants with severe congenital disease will die within the first year of life, and the survivors, their caregivers, and health systems bear significant long-term burdens.
annually. There is currently no available vaccine for CMV and many previous attempts at developing a vaccine to reduce or prevent congenital transmission have been missing a key antigen, the Pentamer. We believe the Pentamer is critical for the infection of epithelial, endothelial, and myeloid cells by the virus. We believe the Pentamer was not included in certain prior recombinant protein vaccine attempts due to the complexity of producing it as a multi-unit antigen complex. Prior vaccine studies demonstrated insufficient efficacy against CMV infection and limited durability of immune response. A vaccine that leads to durable immunity in women of child-bearing age would address a critical unmet need in the prevention of congenital CMV infection.
Our CMV vaccine (mRNA-1647): Our product conceptcandidate, mRNA-1647, combines six mRNAs in one vaccine, which encode for two proteins located on the surface of CMV: five mRNAs encode the subunits that form the membrane-bound pentamer complex and one mRNA encodes the full-length membrane-bound glycoprotein B (gB). Both pentamer and gB are essential for CMV to infect barrier epithelial surfaces and gain access to the body, which is the first step in CMV infection. mRNA-1647 is designed to produce an immune response against both pentamer and gB for the prevention of CMV infection, which could reduce the risk of birth defects and post-transplant infections.
We are developing a single vaccine with complex antigensconducting an ongoing Phase 3 study for mRNA-1647, known as CMVictory, to prevent or controlevaluate the safety and efficacy of mRNA-1647 against primary CMV infection
Our ability in female participants 16 to generate a multi-antigen vaccine enables us40 years of age. The study is fully enrolled, both for the adult and adolescent (ages 16 to combine a traditional target antigen (gB) with<18) cohorts. Timing of the Pentamerreadout will depend upon the number of CMV cases accrued in order to specifically focus the immune system on these important antigens.study. We believe this gives us greateranticipate potential to produce neutralizing antibodies that can block CMV transmissionefficacy data from the mother to the fetus. Our approach to block transmission could either be:
•direct, by vaccinating adolescents or adults of child-bearing potential (female and male); or
•indirect, by vaccinating toddlers who could spread CMV to each other, their mothers, and their childcare workers.
Unlike a protein-based or live-attenuated vaccine, our mRNA instructs cells to specifically make predetermined antigens with a structure that mimics the one presented to the immune system by the virus, thus focusing the immune system on these important antigens.
mRNA-1647 comprises six mRNAs that encode for these known hard-to-make CMV antigensstudy in a proprietary LNP:
•In CMV seropositive individuals, the majority of neutralizing antibodies target the Pentamer. The CMV Pentamer is made by five CMV glycoproteins that form a membrane-bound complex. The Pentamer is required for CMV entry into epithelial, endothelial, and myeloid cells. The mRNA-expressed Pentamer is displayed on the surface of the cell and stimulates the production of neutralizing antibodies that prevent the virus from entering the cells.
•gB is a trimeric CMV membrane glycoprotein that abundantly resides on the surface of the viral particles. Fusion between virus and host cells, and hence infection, requires gB. Antibodies to gB can prevent CMV infection. gB has been utilized in some earlier attempts at a CMV vaccine as the sole antigen which had resulted in partial efficacy but not at levels sufficient for approval.
An illustration of our proposed approach for CMV is shown in the figure below.
CMV vaccine (mRNA-1647): Preclinical information
We have published preclinical data for our CMV vaccine2024.
We have demonstrated that the Pentamer and gB mRNAs can elicit potent and durable antibody titers against the antigens in mice and non-human primates, and have published these results in the journal Vaccine in 2018. In one study, mice were immunized with the Pentamer and gB mRNAs encapsulated in our proprietary LNP. Serum samples were taken from the mice at specific timepoints post-vaccination. Post-vaccination neutralizing titers were measured by admixing serial dilutions of each sample with CMV virus, incubating the mixture in a human primary epithelial cell culture, and counting the number of infected cells. We used CytoGam, an approved product for prevention of CMV in transplant patients, as a control in our experiment. CytoGam is cytomegalovirus immune globulin from pooled plasma of CMV seropositive donors. The table below shows the neutralization antibody titers in epithelial cells for escalating vaccine doses in mice, demonstrating our ability to generate neutralizing antibodies. Weare also observed that at the highest dose, our mRNA vaccine generated a response more than 75-fold higher than CytoGam at estimated clinical levels. In addition, we have also observed that the Pentamer and gB mRNAs can elicit strong T cell responses.
CMV vaccine (mRNA-1647): Clinical data
We have demonstrated tolerability and generated immunogenicity data in our Phase 1 and 2 trials. Based on interim Phase 2 data, we have initiated planningconducting a Phase 3 trial1/2a study of mRNA-1647 vaccine efficacy.
Phase 1 12-Month Interim Analysis
in participants 9 to 15 years of age. We announced positive data fromenrolled the 12-month interim analysis offirst participant in November 2022, and the Phase 1 clinical trial of mRNA-1647, which has completed the final study visit of its last participant and is evaluating the safety and immunogenicity of mRNA-1647 in 181 healthy adult volunteers.
Tableto inform the selection of Contents
The clinical trial population includes those who are naïve to CMV infection (CMV-seronegative) and those who had previously been infected by CMV (CMV-seropositive). Participants were randomized to receive either placebo, or 30, 90, 180 or 300 µg of mRNA-1647 on a dosing schedule of 0, 2 and 6 months. This 12-month planned interim analysis assessed immunogenicity of the first three dose levels (30, 90, and 180 µg) at 12 months (six months after the third vaccination), and the highest dose level (300 µg) at seven months (one month after the third vaccination). The analysis also assessed safety of the highest dose level at seven months. Neutralizing antibody titers (levels of circulating antibodies that block infection) were assessedfor subsequent development in two assays utilizing epithelial cells and fibroblasts, which measure immune response to the pentamer and gB vaccine antigens, respectively. gB antigen-specific T cell responses after the second and third vaccinations were measured in a subset of CMV-seronegative participants in the 30, 90 and 180 µg dose levels utilizing an ELISpot assay. Pentamer-specific T cell assays remain in development. Vaccine-induced neutralizing antibody responses in the CMV-seronegative group were compared to the baseline neutralizing antibody titers in the CMV-seropositive group, noting that prior maternal CMV infection is associated with an approximately 30-fold lower risk of congenital CMV infection compared to the risk in the setting of maternal primary CMV infection.
In CMV-seronegative participants at seven months (one month after the third vaccination) in the 30, 90 and 180 µg dose levels:
•this age group.A dose-related increase in neutralizing antibody titers was observed in both epithelial cell and fibroblast assays.
•After the third vaccination, neutralizing antibody titers against epithelial cell infection were greater than 10 times higher in the 90 and 180 µg dose levels than CMV-seropositive baseline titers at the 90 and 180 µg dose levels.
•After the third vaccination, neutralizing antibody titers against fibroblast infection were 1.3 to 1.4 times higher than CMV-seropositive baseline titers at the 90 and 180 µg dose levels.
In CMV-seronegative participants at twelve months (six months after the third vaccination)Additionally, in the 30, 90, and 180 µg dose levels:April 2023 we announced that a Phase 2 proof-of-concept study for mRNA-1647 in allogeneic hematopoietic cell transplant (HCT) patients had started enrollment.
•Neutralizing antibody titers remained at least 3.5-fold higher than the natural infection benchmark in epithelial cell assays.
•Titers in fibroblast assays approximated that of the natural infection benchmark in the in the 90 μg and 180 μg treatment groups
In CMV-seropositive participants at seven months (one month after the third vaccination) in the 30, 90 and 180 µg dose levels:
•A dose-related increase in neutralizing antibody titers was observed in both epithelial cell and fibroblast assays.
•The third vaccination boosted neutralizing antibody titers against epithelial cell infection to levels of 22-fold to 40-fold over baseline titers in all dose levels.
•The third vaccination boosted neutralizing antibody titers against fibroblast infection to levels of approximately 4-fold to 6-fold over baseline titers in all dose levels.
In CMV-seropositive participants at twelve months (six months after the third vaccination) in the 30, 90, and 180 µg dose levels:
•Neutralizing antibody titers were 14-fold to 31-fold over baseline titers in epithelial cell assays.
•Titers in fibroblast assays were 6-fold to 8-fold over baseline titers.
Participants receiving 300 µg of mRNA-1647 followed through seven months (one month after the third vaccination) continued to show consistent dose-dependent increases in neutralizing antibodies against epithelial cell infection and against fibroblast infection in both CMV-seronegative and CMV-seropositive groups. In CMV-seronegative participants at seven months, the neutralizing antibody titers in epithelial cell assays increased further to 17-fold higher than the natural infection benchmark, and neutralizing antibody titers in fibroblast assays increased further to 5-fold higher than natural infection benchmark. In CMV-seropositive participants at seven months, the neutralizing antibody titers in epithelial cell assays was 13.4-fold over baseline titers and against fibroblast infection was 7.1-fold over baseline titer. In a subset of CMV-seronegative participants in the 30, 90 and 180 µg dose levels, gB antigen-specific T cell activation was observed at all dose levels after the second and third vaccinations.
A safety analysis indicated that the vaccine was generally well-tolerated. There were no vaccine-related serious adverse events. Across all mRNA-1647 treatment groups, the most common solicited local adverse reaction, or AR, was injection site pain and the most common solicited systemic ARs were headache, fatigue, myalgia, arthralgia, and chills. Fever was reported in 0-55% of CMV-seronegative treatment groups and in 8-75% of CMV-seropositive treatment groups. In general, solicited systemic ARs occurred less frequently after the third vaccination compared to the second, and were more common in the CMV-seropositive treatment groups compared to the CMV-seronegative treatment groups. Grade 3 solicited ARs were more common in CMV-seropositive participants, and were fatigue (0-27% of a given dose cohort), chills (0-38% of a given dose cohort) and fever (0-33% of a given dose cohort). As reported in the previous interim analysis, there was a single Grade 4 AR of an isolated lab finding of elevated partial thromboplastin time, which was elevated at baseline (Grade 1) and self-resolved on the next lab test with no associated clinical findings. Safety and tolerability in participants receiving 300 µg of mRNA-1647 was comparable to that observed at the 180 µg dose level in CMV-seropositive participants and generally higher in 300 µg dose level in CMV-seronegative participants.
Although the small sample size limits the conclusions that can be drawn from the data, the findings from this interim analysis build on an earlier interim analysis of safety and immunogenicity data through one month after the third vaccination in the 30, 90 and 180 µg dose levels and one month after the second vaccination in the 300 µg dose level.
Phase 2 Part 1 3-Month Interim Analysis
In Part 1 of the Phase 2 study, participants were randomized to receive either placebo, or 50, 100, or 150 µg of mRNA-1647 on a dosing schedule of 0, 2, and 6 months. The planned 3-month interim analysis of safety and immunogenicity through 3 months (1 month after the second vaccination) in September 2020 informed the selection of the 100 µg dose level for the Phase 3 pivotal efficacy study. In addition, we have initiated enrollment in Part 2 of the Phase 2 study, which is evaluating safety and immunogenicity of the 100 µg dose level to expand the safety dataset in the target population planned for the Phase 3 study.
In CMV-seronegative participants at three months (one month after the second vaccination) in the 50, 100 and 150 µg dose levels:
•Neutralizing antibody titers against epithelial cell infection increased to at least 12-fold over the CMV-seropositive baseline titers after the second vaccination and were generally numerically similar in the 50, 100, and 150 μg treatment groups.
•Neutralizing antibody titers against fibroblast infection increased to titers equivalent to the CMV-seropositive baseline titers after the second vaccination and were generally numerically similar in the 50, 100, and 150 μg treatment groups.
In CMV-seropositive participants at three months (one month after the second vaccination) in the 50, 100 and 150 µg dose levels:
•Neutralizing antibody titers against epithelial cell infection increased 12 to 51-fold over the CMV-seropositive baseline titers after the second vaccination.
•Neutralizing antibody titers against fibroblast infection increased to levels at least 2-fold over CMV-seropositive baseline titers after the first and second vaccination.
Phase 2 Continuation and Phase 3 Planning
Our randomized, observer-blind, placebo-controlled, dose-confirmation Phase 2 study is investigating the safety and immunogenicity of mRNA-1647 in approximately 252 healthy CMV-seronegative and CMV-seropositive adult volunteers in Part 1 of the study, and an additional approximately 250 healthy CMV-seronegative and CMV-seropositive female volunteers in Part 2 of the study in the U.S.
We are actively preparing for a global, randomized, observer-blind, placebo-controlled Phase 3 pivotal study to evaluate the efficacy of mRNA-1647 against primary CMV infection in females 16-40 years of age. We solicited and received Type C meeting feedback from the FDA on the preliminary design of the pivotal trial, requested an end-of-phase 2 (EoP2) meeting with FDA for mid-March 2021, and submitted our Phase 3 development plans to FDA in a briefing document. We believe this can be achieved with a trial with no more than 8,000 participants and study site selection and/or site feasibility has already begun across North America, Europe, Australia, and Israel. The pivotal Phase 3 trial design will be finalized after discussion with the FDA and other global health authorities. Additional adolescent bridging and concomitant vaccination trials are being planned.
Epstein-Barr Virus vaccine (mRNA-1189)
Our EBV vaccine seeks to prevent the development of infectious mononucleosis(mRNA-1189 and potentially prevent EBV infection.mRNA-1195)
Epstein-Barr virus or EBV,(EBV) is a member of the herpesvirus family that includesis related to CMV and infects approximately 90% of people in the U.S. by adulthood, with primary infection typically occurring during childhood andor late adolescence (approximately 50% and 89% seropositivity, respectively) in the U.S.. EBV is the major cause of infectious mononucleosis, in the U.S., accounting for over 90% of the approximately 1-2one to two million cases of infectious mononucleosis in the U.S. each year. Infectious mononucleosis can debilitate patients for weeks to months and, in some cases, can lead to hospitalization anddue to complications such as splenic rupture. EBV infection is also associated with the development and progression of certain lymphoproliferative disorders, cancers and autoimmune diseases. In particular, EBV infection and infectious mononucleosis are associated with increased risk of developing multiple sclerosis, an autoimmune disease of the central nervous system. There is no approved vaccine or effective treatment for EBV. Similar to CMV, EBV has lytic and latent stages in its lifecycle and contains on its surface (envelope) multiple glycoproteins and glycoprotein complexes (gp350, gH/gL, gH/gL/gp42 and gB) that mediate virus entry and infection in different cell types. EBV gp350 mediates attachment to B cells through binding to the complement receptor 2 (CR2), followed by binding of the viral gH/gL/gp42 complex to human leukocyte antigen (HLA) class II. Infection of epithelial cells instead requires binding of gH/gL to a different set of receptors. For both B cell and epithelial cell entry, binding of an EBV gH/gL complex to a cell-specific receptor leads to activation of gB, which in turn facilitates virus-cell-membrane fusion and infection. gH/gL and gB comprise the core viral-fusion machinery conserved across all herpesviruses.
Similar to our CMV vaccine (mRNA-1647) product concept, we used our platform to generate an mRNA vaccine containing five mRNAs encoding for gp350, gB, gH, gL, and gp42, which are expressed in their native membrane-bound conformation for recognition by the immune system. We have observed preclinical immunogenicity in the form of high and durable levels of antigen-specific antibodies against both B cell and epithelial cell infection in mice and in non-human primates (NHPs). We intend to conduct a Phase 1 trial to test the safety and immunogenicity of the vaccine to understand its potential to prevent primary infection, and prevent infectious mononucleosis following EBV infection, in seronegative adults.
Epstein-Barr Virus: Disease overview
EBV is the major cause (approximately 90%) of infectious mononucleosis and has been associated with the development of a range of malignancies and autoimmune disorders.
EBV is a common herpesvirus that is spread through bodily fluids, most commonly saliva, and is contracted primarily by young children and adolescents. Adolescents and young adults seroconvert at high rates, particularly in college-aged populations (approximately 10-25% per year) resulting a seroprevalence of approximately 90% by the age of 20. After primary infection, the virus establishes latency and persists in that state for life in most infected individuals. The virus can reactivate intermittently over time even in immunocompetent hosts. The virus usually infects resting B cells in the oropharynx or epithelial cells, which line the mucosal surfaces of the body and in turn infect B cells. B cells disseminate systemically and act as a reservoir for latent virus. Primary infection can cause infectious mononucleosis in 35% to 75% of instances, depending on age, and is characterized by symptoms requiring physician visits, including sore throat, lymph node swelling, fever, body aches and fatigue, often resulting in months of missed work and school for patients and caregivers.
There is currently no approved vaccine against EBV, but the potential of gp350 alone to reduce the rate of infectious mononucleosis has already been clinically demonstrated. An experimental vaccine, developed by others, consisting of adjuvanted recombinant gp350 protein led to a reduction in the incidence of infectious mononucleosis in 78% of the participants in a Phase 2 study of 181 healthy volunteers between the ages of 16-25. However, there was no significant difference between groups in protection against asymptomatic EBV infection. We believe that the addition of gH/gL and gB has the potential to provide protection against epithelial cell infection. We believe the immune response against gp350, gH/gL or gB has the potential to provide B cell protection, which may be further enhanced by the inclusion of gp42. By preventing infection in epithelial cells and B cells, this mRNA vaccine has the potential not only to significantly reduce the rate of infectious mononucleosis, but also to prevent EBV infection.
EBV infection is associated with increased risk of developing certain cancers and multiple sclerosis. In Western industrialized countries, EBV is implicated in the development of post-transplant lymphoproliferative disorder conditions as well as multiple cancers, including Hodgkin’s lymphoma. Additionally, in those seropositive for EBV, development of infectious mononucleosis is associated with a greater than 2-fold increased relative lifetime risk of developing multiple sclerosis. In East Asia, EBV is associated with 80-99% of nasopharyngeal carcinomas that arise. In Africa, EBV is implicated in the development of approximately 95% of cases of endemic Burkitt’s lymphoma. Together, approximately 1.5% of worldwide cancer deaths are attributable to EBV-associated malignancies.
EBV vaccine (mRNA-1189): Our product concept
We are developing two EBV vaccine candidates—a vaccine with multiple antigens designed to prevent development of infectious mononucleosis (mRNA-1189) and a vaccine to prevent the longer-term sequelae of EBV infections.
infection (mRNA-1195). Similar to our CMV vaccine (mRNA-1647) product concept, we believe that an effective EBV vaccine must generate an immune response to antigens that are required for viral entry in most of the susceptible cell types. We have thusmRNA-1189 is designed our EBV vaccine, mRNA-1189, to elicit an immune response to EBV envelope glycoproteins, gp350 as well as gB, gp42, and the gH/gL complex, which are required for infection of both epithelial and B cells. mRNA-1189 contains fivefour mRNAs encoding the viralfor these proteins gp350, gB, gp42, gH, and gL encapsulated in our proprietary LNPs. Proteins translated from our mRNAmRNA-1195 encodes for entry glycoproteins and latent antigens and will be displayed on the cell surface in their native conformation, stimulating the production of neutralizing antibodies. By training the immune system to recognize and neutralize the machinery used to infect B and epithelial cells, we believe that our vaccine has the potential to prevent EBV primary infection and therefore the development of infectious mononucleosis. Further,investigated in the long-run, should our EBV vaccine be approved, we may pursue post-marketingcontext of post-transplant lymphoproliferative disorders and population studies to potentially evaluate its impact on other EBV-associated diseases. Our EBV vaccine utilizes the same proprietary platform technology as our CMV vaccine (mRNA-1647), which was generally well-tolerated and demonstrated durable neutralizing antibody titers higher than those measured in CMV-seropositive patients following up to three doses of mRNA-1647 in our Phase 1 trial.multiple sclerosis.
We are conducting Phase 1, randomized, observer-blind, placebo-controlled studies of mRNA-1189 and mRNA-1195. The primary purpose of these studies is to assess the safety, tolerability and immunogenicity of these vaccine candidates.
EBV
HSV vaccine (mRNA-1189): Preclinical information(mRNA-1608)
Herpes simplex viruses (HSV), commonly known as herpes, are categorized into two types: HSV-1 primarily spreads by oral contact and is most commonly associated with cold sores, and HSV-2 spreads through sexual contact and is the major cause of recurrent genital herpes. Both viruses establish life-long latent infections within nearby sensory neurons from which they can reactivate and re-infect the skin. There is a significant burden of disease from HSV genital infections. Diagnosed, symptomatic genital herpes causes a reduction in quality of life, which antivirals (current standard of care) only partially restore. In the United States, approximately 18.6 million adults ages 18 to 49 years are living with HSV-2. Globally, an estimated 492 million persons have HSV-2 infection, representing 13% of the world’s population aged 15 to 49 years. We have demonstrated the abilitybelieve that an HSV vaccine could deliver similar efficacy as suppressive antiviral treatments and could improve compliance and quality of life. We aim to induce antibodies against EBV antigens required for viral entry into B cellsa strong antibody response with neutralizing and epithelial cells.
Naïve Balb/c mice were given two doses of a vaccine against EBV antigens in combination approximately four weeks apart. Antibody titers against viral proteins involved in epithelial cell entry (gH/gL and gB) or B cell entry (gp350, gH/gL and gB) were measured in peripheral blood at day 57. Results shown here represent five animals per group and demonstrate high levels of antigen-specific immunoglobulin G (IgG) as compared to negative controls.
EBV vaccine (mRNA-1189): Clinical plan
We are planning a Phase 1 clinical trial to test the safety and immunogenicity of mRNA-1189 in seronegative adults.
We intend to conduct a Phase 1 trial to test the safety and immunogenicity of the vaccine to understand its potential to prevent primary infection, and prevent infectious mononucleosis following EBV infection, in seronegative adults.
PROPHYLACTIC VACCINES: GLOBAL HEALTH PROGRAMS
Our global health portfolio for prophylactic vaccines seeks to leverage our mRNA technology to address epidemic and pandemic diseases. We are currently workingeffector functionality combined with strategic collaborators such as BARDA, DARPA, NIH, the Bill and Melinda Gates Foundation and the International AIDS Vaccine Initiative (IAVI) to fund and support our programs within this area.
Zika vaccine (mRNA-1893)
In collaboration with BARDA, we have advanced a second generation Zika vaccine candidate to a Phase 1 clinical trial
Zika is an infectious disease caused by the Zika virus; infection during pregnancy has been linked to severe brain damage in infants with congenital infection and Guillain-Barré Syndrome in adults. To date, no vaccine to prevent Zika infection has been approved. In September 2016, we were awarded a contract with BARDA to be reimbursed up to approximately $125.0 million for the development of a Zika mRNA vaccine. Preclinical and clinical studies were carried out on an initial candidate, mRNA-1325. When a second-generation Zika vaccine, mRNA-1893, demonstrated better protection than mRNA-1325 in non-human primates at 1/20 of the dose, we decided to move forward with mRNA-1893 and discontinue development of mRNA-1325. mRNA-1893 is currently under evaluation in a Phase 1 trial in the United States. Enrollment and dosing are complete, and the interim analysis is available.
Zika vaccine (mRNA-1893): Disease overview
We faced a Zika epidemic in 2015 for which there were no vaccines or treatments
The Zika virus is a single stranded RNA virus of the flaviviridae family. It was first isolated in a rhesus macaque in the Zika Forest, Uganda in 1947 and the first human case was documented in 1952. Seroepidemiology data suggest that it is endemic to regions of Africa and Asia where the Aedes mosquito vectors are found. Zika virus is predominantly spread by mosquitos from the Aedes genus, but it can also be transmitted congenitally, sexually, and through blood donation.
Zika infection is usually asymptomatic or mild in adults, leading to fever, rash, and conjunctivitis. However, infection of women during pregnancy can result in devastating microcephaly in newborns. Microcephaly is a birth defect characterized by an abnormally small head and brain, associated with lifelong neurodevelopmental delay, seizures, intellectual disability, balance problems, and dwarfism / short stature, resulting in significant disability and requiring lifelong support.
In 2007, a Zika infection outbreak progressed across the Pacific islands. An outbreak observed in Brazil in 2015 soon spread across the Americas. This led to the WHO declaring it a public health emergency of international concern in 2016. During the period, there were tens of thousands of cases of microcephaly and congenital Zika syndrome reported in infants and of resulting neurological sequelae such as Guillain-Barré syndrome reported in adults.
While the number of cases has declined in the past couple of years, there is currently no treatment or vaccine available for the Zika virus to prevent and respond to potential future epidemics.
Zika vaccine (mRNA-1893): Our product concept
A more immunogenic second-generation vaccine was rapidly advanced to a Phase 1 clinical trial
Continued efforts at identifying different mRNA sequences with improved immunogenicity led to mRNA-1893, a sequence distinct from the first Zika vaccine candidate, mRNA-1325, that increases production of Zika viral-like particles (VLPs) and generates enhanced immunogenicity and protection in preclinical animal models compared with mRNA-1325, as depicted in the image below.
Zika vaccine (mRNA-1893): Clinical datacell-mediated immunity.
We are conducting a Phase 1 trial for mRNA-18931/2, randomized, observer-blind, controlled, dose-ranging study of mRNA-1608, our HSV vaccine candidate against recurrent HSV-2 disease, in the United States.health adults 18 to 55 years of age with recurrent HSV-2 genital herpes. The resultsprimary purpose of the Phase 1 study support continued clinical development of mRNA-1893 and a Phase 2this study is planned for initiation in 2021to assess safety and immunogenicity data, and establish a proof-of-concept of clinical benefit.
Dosing has been completedVZV vaccine (mRNA-1468)
Herpes zoster, also known as shingles, is caused by the varicella zoster virus (VZV) and occurs in approximately one in three adults in their lifetime and incidence significantly increases at approximately 50 years of age. Protective immunity against VZV wanes as the immune system ages, allowing reactivation of the virus from latently infected neurons, causing painful and itchy lesions. Serious herpes zoster complications include postherpetic neuralgia (10-13% of herpes zoster cases), bacterial coinfections and cranial and peripheral palsies; 1-4% of individuals with herpes zoster cases are hospitalized for complications. Severity of disease and likelihood of complications, including postherpetic neuralgia (PHN) also increases with age. People with immunocompromising conditions, people with autoimmune disease using immunosuppressive therapies, people living with HIV and hematopoietic stem cell (HSCT) and organ transplant recipients have an increased risk of developing herpes zoster. The current standard of care is Shingrix, an FDA-approved vaccine for the prevention of shingles in adults 50 years and older. It is more than 90% effective against herpes zoster in adults aged 50-70 with only a slight reduction in efficacy for adults over age 70.
We are conducting an ongoing Phase 11/2, randomized, observer-blind, placebo-controlled,active-controlled, dose-ranging study to evaluate the safety, tolerability,reactogenicity and immunogenicity of mRNA-1893mRNA-1468, compared head-to-head with Shingrix, in healthy adults, (18 to 49aged 50 years and older. The first participant was dosed in February 2023 and enrollment of age, inclusive)500 participants was completed in endemic and non-endemic Zika regions. Key objectives of the study include:
•Assessing the safety, tolerability, and reactogenicity of a 2‑dose vaccination schedule of mRNA-1893 Zika vaccine, given 28 days apart, across a range of dose levels in flavivirus-seronegative and flavivirus-seropositive participants compared with placebo;
•Assessing the immunogenicity of a range of doses of mRNA-1893 Zika vaccine.
Subjects were randomly assigned in a blinded fashion in an approximate 4:1 ratio to receive mRNA-1893 or placebo at one of four dose levels (10 µg, 30 µg, 100 µg, and 250 µg), with each subject receiving two vaccinations separated by 28 days. Twenty-five of the enrolled participants at each dose level were flavivirus seronegative and five were flavivirus seropositive.
mRNA-1893 was well tolerated at all dose levels, and safety and tolerability did not appear to be influenced by the serostatus of the participants at baseline. ZIKV-specific neutralizing antibodies were measured by Plaque Reduction Neutralization Test (PRNT50) and Microneutralization (MN). All dose levels of mRNA-1893 induced a strong neutralizing ZIKV-specific antibody response in baseline flavivirus seronegative participants. GMTs post-dose two were comparable to those in a small panel of Zika convalescent sera collected during the epidemic. In participants with pre-existing flavivirus antibodies, neutralizing antibody titers were boosted with a single dose of the vaccine as shown by the GMTs and the seroconversion rates. The participants will continue to be followed through 12 months to measure antibody persistence with time and to monitor safety.
HIV vaccine program (mRNA-1644 & mRNA-1574)
We are advancing two development candidates against HIV – Phase 1 innovation is needed to accelerate human validation of novel strategies.
mRNA-1644, a collaboration with the IAVI and the Bill and Melinda Gates Foundation, is a novel strategy and approach to vaccination against human immunodeficiency virus (HIV) in humans designed to elicit broadly Neutralizing HIV-1 Antibodies (bNAbs). A Phase 1 study for mRNA-1644 will be the first trial in a series of human studies to iteratively test and improve HIV vaccine antigens that elicit bNAbs using a germline-targeting and immuno-focusing. A second trial, mRNA-1574, is being evaluated in collaboration with NIH and includes multiple native-like trimer antigens.
We expect to begin Phase 1 clinical trials for both mRNA-1644 and mRNA-1574 in 2021. These programs have the potential to inform the future of the HIV vaccine field, and provide an opportunity for our mRNA technology to play a key role in accelerating the discovery of a protective HIV vaccine.June 2023.
HIV vaccine program (mRNA-1644 &and mRNA-1574): Disease overview
HIV is the virus responsible for acquired immunodeficiency syndrome (AIDS), a lifelong, progressive illness with no effective cure. Approximately 38 million people worldwide are currently living with HIV, with 1.2 million in the U.S. Approximately 21.5 million new infections of HIV are acquired worldwide every year and approximately 690,000680,000 people die annually due to complications from HIV/AIDS. The primary routes of transmission are sexual intercourse and IV drug use, putting young adults at the highest risk of infection. From 2000 to 2015, a total of $562.6 billion globally was spent on care, treatment and prevention of HIV, representing a significant economic burden.
We are developing two HIV vaccine candidates—mRNA-1644 and mRNA-1574—both of which are in ongoing Phase 1 clinical trials. In collaboration with the International AIDS Vaccine Initiative (IAVI) and the Bill & Melinda Gates Foundation, mRNA-1644 is testing a novel HIV vaccine strategy in humans as delivered by mRNA to elicit broadly neutralizing HIV-1 antibodies (bnAbs) through sequential vaccination of novel prime and boost antigens that induce specific B-cell responses. In collaboration with IAVI, Scripps, NIH and the HIV Vaccine Trials Network, mRNA-1574 is testing multiple native-like HIV trimer mRNAs in humans to improve our understanding of how to make stable and immunogenic native-HIV trimers.
Infectious disease vaccines: Vaccines against enteric viruses
Norovirus vaccines (mRNA-1403 and mRNA-1405)
Among enteric viruses, norovirus is a leading cause of diarrheal disease, also referred to as acute gastroenteritis (AGE) globally, resulting in substantial health care burden. Norovirus is estimated to be the causative agent in 18% of all AGE cases worldwide. The highest incidence globally is in young children, and in low-income countries, there are approximately 70,000 deaths annually caused by norovirus in children under 5 years of age. In high-income countries, incidence is also highest among young children, but deaths and other severe outcomes are concentrated primarily among older adults and immunocompromised patients. Norovirus is also associated with large, costly and difficult to control outbreaks in closed and semi-closed settings, such as daycares, cruise ships, long term care facilities and other healthcare settings. The burden of norovirus among older adults is expected to rise along with societal aging and an increased need for institutionalized care. In the U.S., norovirus causes an estimated 20 million infections, 100,000 hospitalizations and 900 deaths per year. Globally, there are approximately 650 million infections annually with approximately 200,000 deaths across all ages.
Norovirus has broad genetic diversity; the virus is classified into 10 genogroups and 49 genotypes, 30 of which are known to infect humans. Vaccine development has been challenging to date for many reasons, including the lack of a robust cell culture system, no reliable immune markers of norovirus protection and the broad and shifting diversity of genotypes. A multivalent vaccine with broad genotype coverage is needed to maximize protection against the genotypes most frequently associated with AGE in young children and older adults.
We are currently developing pentavalent (mRNA-1405) and trivalent (mRNA-1403) vaccine candidates for norovirus. Both candidates are in a Phase 1 study to evaluate safety, reactogenicity and immunogenicity in healthy adult participants 18 to 49 years of age and 60 to 80 years of age. Approximately 660 participants were enrolled in the Phase 1 study, and dosing was completed in December 2023.
Infectious disease vaccines: Bacterial vaccines
Lyme vaccines (mRNA-1975 and mRNA-1982)
Approximately 120,000 Lyme disease cases are reported per year in the U.S. and Europe. With rising atmospheric temperatures, Lyme territory continues to increase in the U.S. Lyme disease burden follows a bimodal age distribution, affecting mainly children under 15 and older adults. Patients can develop rash, fever, headaches, fatigue, joint pain, swelling, stiffness and headaches. Older adults appear more likely to have an unfavorable treatment response and more common neurologic manifestations as compared with younger patients. There are no approved HIV vaccines and no effective cure.to prevent Lyme disease in humans currently on the market.
To address Lyme's biological complexity, we are advancing a seven-valent and single-valent approach with two Lyme disease vaccine candidates being developed in parallel. mRNA-1982 is designed to elicit antibodies specific for Borrelia burgdorferi, which causes almost all Lyme disease in the U.S. mRNA-1975 is designed to elicit antibodies specific for the four major Borrelia species causing disease in the U.S. and Europe.
Both the seven-valent (mRNA-1975) and single-valent (mRNA-1982) vaccines are in a Phase 1/2, randomized, observer-blind, placebo-controlled, dose-ranging study to evaluate safety and immunogenicity in healthy participants 18 through 70 years of age. We have completed enrollment of approximately 800 participants in this study. In January 2024, the FDA granted Fast Track Designation to our Lyme disease program.
Infectious disease vaccines: Public health vaccines
Zika vaccine (mRNA-1893)
The Zika virus is a single stranded RNA virus of the Flaviviridae family. Seroepidemiology data suggest that it is endemic to regions of Africa and Asia, where the Aedes mosquito vectors are found. Zika virus is predominantly spread by mosquitos from the Aedes genus, but it can also be transmitted congenitally, sexually and through blood donation. Zika infection is usually asymptomatic or mild in adults, leading to fever, rash and conjunctivitis. However, infection of women during pregnancy can result in devastating microcephaly in newborns. Microcephaly is a birth defect characterized by an abnormally small head and brain, associated with lifelong neurodevelopmental delay, seizures, intellectual disability, balance problems and dwarfism/short stature, resulting in significant disability and requiring lifelong support. In 2007, a Zika infection outbreak progressed across the Pacific islands. An outbreak observed in Brazil in 2015 soon spread across the Americas, which led the WHO to declare Zika a public health emergency of international concern in 2016. During the period, tens of thousands of cases of microcephaly and congenital Zika syndrome were reported in infants. Zika has also been associated with certain neurological sequelae, such as Guillain-Barré syndrome, reported in adults.
Our Zika vaccine candidate, mRNA-1893, encodes for the prME structural protein encapsulated in our proprietary LNP. In partnership with BARDA, we are conducting a Phase 2 study in the United States and Puerto Rico to evaluate mRNA-1893 in approximately 800
participants. The randomized, placebo-controlled study aims to evaluate the safety, tolerability and reactogenicity of mRNA-1893, as well as evaluate the immunogenicity of two dose levels of mRNA-1893 (one-dose or two-dose schedule) compared to placebo. The study is fully enrolled. We do not anticipate advancing the Zika program into further studies in the absence of further outside funding.
Nipah vaccine (mRNA-1215)
We are collaborating with the NIH-VRC for pandemic preparedness
mRNA-1215, our vaccine candidate against the Nipah virus (NiV), was co-developed along with the NIH-VRC. The Phase 1 clinical testing will be focused on pandemic preparedness.
Nipah vaccine (mRNA-1215): Disease overview
NiV is a zoonotic virus transmitted to humans from animals, contaminated food or through direct human-to-human transmission and causes a range of illnesses including fatal encephalitis. Severe respiratory and neurologic complications from NiV have no treatment other than intensive supportive care. The case fatality rate among those infected is estimated at 40-75%. NiV outbreaks cause significant economic burden to impacted regions due to loss of human life and interventions to prevent further spread, such as the slaughter of infected animals. NiV has been identified as the cause of isolated outbreaks in India, Bangladesh, Malaysia and Singapore since 2000 and is included on the WHO R&D Blueprint list of epidemic threats needing urgent R&D action.
H7N9 influenzaIn collaboration with the NIH-Vaccine Research Center (VRC), we are conducting an ongoing Phase 1 clinical trial of mRNA-1215, our vaccine (mRNA-1851)candidate against NiV, and testing will be focused on pandemic preparedness. This Phase 1 dose-escalation, open-label clinical trial is the first study of mRNA-1215 in healthy adults to evaluate the safety, tolerability and immunogenicity of a NiV mRNA vaccine candidate. The trial is sponsored and funded by the National Institute of Allergy and Infectious Diseases (NIAID).
H7N9 investigationalMpox vaccine demonstrates the potential of our platform to respond to an influenza pandemic
As a proof of concept and as part of our earlier efforts to develop a vaccine against influenza, we developed vaccines for H10N8 and H7N9 avian influenza strains, where there is a quantitative correlate for protection in humans (hemagglutinin inhibition, or HAI, titer of > 1:40). The results of the Phase 1 trial for H7N9 vaccine were reported by us in Vaccine in 2019. The trial has met its objectives of assessing the safety and tolerability profile of mRNA-1851 versus placebo, including capturing solicited and unsolicited local and systemic adverse events. The Phase 1 trial for H7N9 vaccine has also demonstrated immunogenicity and we have observed 96% of the subjects demonstrating HAI titers > 1:40 at day 43 (21 days post-second vaccination) for the 25 µg dose where HAI > 1:40 is regarded as a quantitative measure for protection from influenza. We believe the data provides support to advance the program in clinical development if we choose to with additional government or other funding. However, we do not intend to progress the H7N9 vaccine through clinical development on our own.(mRNA-1769)
TableMpox is an infectious viral disease that can occur in humans and some other animals. It is caused by the monkeypox virus, a zoonotic virus in the genus Orthopoxvirus. Transmission of Contents
SYSTEMIC SECRETED AND CELL SURFACE THERAPEUTICS MODALITY
We designed our systemic secreted therapeutics modality to increase levels of desired secreted proteins in circulation or inmpox has been associated with direct contact with the extracellular environment, in order to achieve a therapeutic effect in onebodily fluid or more tissues or cell types. The goal of this modality is to provide secreted proteins, such as antibodies or enzyme replacement therapies across a wide range of diseases, such as heart failure, infectious diseases, and rare genetic diseases. This modality has benefited from our strategic alliancessores. Mpox may also be transmitted via respiratory secretion with AstraZeneca, DARPA, and the Bill and Melinda Gates Foundation. We have accumulated several innovations in technology, have gained process insights, and have built a set of preclinical and clinical experiences in our systemic secreted and cell surface therapeutics modality. Based on these, we believe this modality is core to our portfolio and we have expanded this portfolio with two new development candidates in a new autoimmune therapeutic area in 2020. Our systemic secreted and cell surface therapeutics modality has four development candidates.
Systemic secreted and cell surface therapeutics: Opportunityclose face-to-face contact.
The ability to systemically deliver mRNA for a therapeutic effect would allow us to address a number of diseases of high unmet medical need. Systemically delivered, secreted and cell surface therapeutics address conditions often treated with recombinant proteins that are typically administered to the blood stream. These current therapies include, for example:
•Enzyme replacement therapies, or ERTs, for rare diseases;
•Antibodies for membrane and extracellular soluble targets; and
•Circulating modulation factors for common and rare diseases such as growth factors and insulin.
Systemic secreted and cell surface therapeutics: Product features
Systemically delivered, secreted and cell surface therapeutics, we believe, would allow us to target areas of biology that cannot be addressed using recombinant proteins. Our potential advantages in these areas include:
•mRNA can produce hard-to-make or complex secreted proteins. Some proteins, due to their folding requirements or complexity, arechallenging to make using recombinant technologies, but can potentially be produced by human cells using administered mRNA.
•mRNA can produce membrane associated proteins. In contrast to recombinant proteins, mRNA can lead to the production of membraneassociated proteins on the cell surface, allowing the expression of native forms of signaling receptors or other cell surface complexes.
•Native post-translational modifications are possible through intracellular protein production using mRNA. mRNA administered to a humancell uses natural secretory pathways inside the cell to make and process the encoded protein. The resulting post-translational modifications, such as glycosylation, are human. With recombinant proteins, these post-translational modifications are native to the non-human cells used for manufacture. These non-human post-translational modifications in recombinant proteins may lead to sub-optimal therapeutic outcomes, side effects, and increased immunogenicity.
•mRNA can sustain production of proteins, which can increase exposure to proteins with short half-lives. mRNA can lead to proteinproduction by cells that can last from hours to days depending on design. This feature could increase the levels of short half-life proteins for therapeutic benefit.
•mRNA allows for desirable pharmacology in rare genetic diseases currently addressed by enzyme replacement therapies. Our mRNAtechnology potentially permits several differentiated pharmacologic features for treating rare genetic diseases currently addressed by enzyme replacement therapies, including the ability to repeat dose as needed, lower immunogenicitymost widely known member of the replacement protein,Orthopoxvirus genus is Variola virus, which causes smallpox. Although smallpox was eradicated in 1977, continued protection from smallpox is of great interest given the ability to adjust dose levels in real-time based on individual patient needs, and the ability to stop dosing. Gene therapies may also prove to be useful for treating rare genetic diseases; however, mRNA is not limited by pre-existing immunity that may exist for certain gene therapies using viral vectors, and does not localize to the nucleus or require persistent changes to cellular DNA to have the desired effect.
Our approach
Our systemic secreted and cell surface therapeutics modality comprises programs where mRNAs instruct various cellslethality of the human body to secrete proteinsinfection and potential for therapeutic effect. For systemic therapeutic programs that utilize cells inuse as an agent of bioterrorism. Other diseases associated with the liver, the liver is a highly productive tissue for secreted protein production. The human liver can make tens of grams of proteins per day, well above the amounts necessary for the pharmacologic effect for virtually all protein therapeutics. We have demonstrated that mRNA can makeOrthopoxvirus genus include cowpox, horsepox and secrete monoclonal antibodies and soluble modulating factors in non-human primates. These proteins made in non-human primates can exert their pharmacological activity by binding to targets with biological effect. Recombinant protein therapeutics, which focus on secreted proteins, generate over $200 billion in annual worldwide sales.
Antibody against Chikungunya virus (mRNA-1944)
Systemic mRNA administration to instruct cells to secrete antibodies, in this case for passive immunization to prevent Chikungunya infection
We are using this program to help understand how mRNA can be used to make complex secreted proteins in the human body and to address the potential health threat of Chikungunya virus, particularly for the military and others exposed to this virus. This program highlights a potentially important advancement of our platform and expansion of our modalities.
Chikungunya is a serious health problem and is estimated to have caused at least 3 million cases during the 2005-2015 epidemic. There are no available vaccines or prophylactic treatments for this disease. This virus can cause severe and chronic arthritic-like conditions in up to 50% of infected people. This program offers a passive immunization approach using antibodies to prevent infection, to complement our vaccine approach. In this program, we utilize two mRNAs encoding for light chain and heavy chain of an antibody against the envelope glycoprotein E. We administered these mRNAs encapsulated in our proprietary LNPs intravenously to people to prevent infection by the Chikungunya virus. We are being financially supported for specific activities by DARPA.
Antibody against Chikungunya virus (mRNA-1944): Disease overview
Addressing a significant global health need
Chikungunya virus is a mosquito-borne alphavirus posing a significant public health problem in tropical and subtropical regions. While Chikungunya has been present in Africa for centuries, recent outbreaks and epidemics in new regions have arisen due to the expanding distribution of the Aedes mosquito in which it resides. A Chikungunya epidemic beginning in 2004 in Kenya spread to India and was exported to nearly all regions of the world and brought Chikungunya to the attention of the western world. As of April 2016, Chikungunya cases had been reported in 103 countries and territories around the world, including 46 countries and territories throughout the Americas.Chikungunya virus infection is characterized by an acute onset of fever, rash, myalgia, and sometimes debilitating polyarthralgia, giving the virus its name, which means “that which bends up” when translated from Makonde. While generally non-fatal, neurological sequelae such as Guillain-Barre syndrome and chronic arthritis have been recognized.
Chikungunya virus is an alphavirus of the Togaviridae family with a positive-strand RNA genome. The viral structural proteins are naturally expressed as a single polyprotein followed by subsequent cleavage by viral and cellular proteases into capsid (C) and envelope (E) glycoproteins E3, E2, 6k, and E1. The E proteins are major targets of protective neutralizing antibody responses that can be assessed in assays. In animal models, passive immunotherapy with convalescent sera from Chikungunya infected patients and human Chikungunya virus-specific neutralizing monoclonal antibodies that mainly recognize E2, have been shown to prevent CHKV infection, providing an approach for therapy.camelpox.
There are currently no effective therapies or approved vaccinestwo subtypes of the monkeypox virus—Clade I and Clade II. In 2022, an outbreak of the Clade II mpox virus was declared a Public Health Emergency of International Concern by the WHO. The outbreak resulted in approximately 93,000 confirmed cases across 117 countries. More recently, the Clade I monkeypox virus, which is associated with greater mortality, has been reported to treat or prevent Chikungunya infection or disease,be sexual transmitted and effective mosquito control has proven challenging, even in higher income countries. Currently, infected individuals are treated with non-steroidal anti-inflammatory drugs to relieve some symptoms. Therefore, in addition to an effective prophylactic vaccine, we believe there is a need for systemic secreted antibody with the potentialconcern that it may lead to provide passive immunity both prophylactically in susceptible populations and for treatment of active Chikungunya virus infections.
Antibody against Chikungunya virus (mRNA-1944): Our product concept
A systemically delivered mRNA instructing cells to secrete an antibody to glycoprotein E to neutralize Chikungunyafurther global outbreaks.
The mRNA-1944 development candidate contains two mRNAs that encodecurrent standard of care for mpox is Jynneos, which is approved by the heavyFDA for the prevention of mpox and light chainssmallpox disease. Our mpox vaccine (mRNA-1769) is designed to express four antigens from the monkeypox virus to provide a range of the Chikungunya antibody and utilizes our proprietary LNPs. The mRNA-1944 development candidate encodes a fully human IgG antibody isolated from B cells of a patient with a prior history of Chikungunya infection that targets the E2 protein. The systemic antibody against Chikungunya virus titers can be evaluated in clinical trials by enzyme-linked immunosorbent assay, or ELISA, to quantify the amount of expressed IgG. A neutralization assay can be used to ensure that the mRNA expressed antibody was properly folded and functional.
Antibody against Chikungunya virus (mRNA-1944): Preclinical information
Systemic mRNA administration results in antibody production and protection from Chikungunya infection in animals
In preclinical studies, mRNA-1944 administered by infusion, elicited high levels of neutralizing antibodies in a dose-dependent manner that were protective against arthritis, musculoskeletal tissue infection and lethal challenge in mouse models. After treatment with 0.5 mg/kg of mRNA-1944, complete survival of mice was observed when challenged 24 hours post-prophylaxis with Chikungunya virus. In cynomolgus macaques following infusion of a single-dose of mRNA-1944 at 0.5 mg/kg, mean maximum concentrations of human Chikungunya antibodies (10.1-35.9 µg/ml) were reached at 24 hours that exceeded the levels needed for protection in mice with a half-life of 23 day.
In addition, mRNA-1944 was tested in rats and non-human primates in a repeat-dose study via IV infusion at up to 5 and 3 mg/kg, respectively. No dose-limiting toxicities related to mRNA-1944 were observed and all other observations were generally reversible.
Antibody against Chikungunya virus (mRNA-1944): Clinical data
Two-dose regimen of Chikungunya antibody demonstrates the platform’s ability for safe repeat doingprotection.
We are conducting a Phase 1, randomized, placebo-controlled, dose-escalationdose-ranging, observer-blind Phase 1/2 study of mRNA-1944 in healthy adults. The objective of this first-in-human study is to evaluate the safety and tolerability of escalating single doses of mRNA-1944 at 0.1 and 0.3 mg/kg, and 0.6 mg/kg with and without dexamethasone included in the premedication regimen with 8 subjects per cohort, and 2 weekly doses of 0.3 mg/kg administered via intravenous infusion. No further dose escalation beyond 0.6 mg/kg is planned. Other objectives are to determine the pharmacokinetics of all dose levels of mRNA-1944, to determine if the antibodies produced are sufficiently active to neutralize viral infection in assays and to determine the pharmacodynamics of anti-Chikungunya virus IgG levels. The safety monitoring committee, or SMC, reviews the safety data for the dose level and recommends escalation to the next dose level.
In September 2019 we announced that single doses of mRNA-1944 at 0.1, 0.3 and 0.6 mg/kg resulted in levels of antibody that exceeded those expected to be protective against chikungunya infection (>1 μg/mL), and the 0.3 mg/kg and 0.6 mg/kg doses were projected to maintain antibody levels above protective levels for at least 16 weeks as shown in the panel below. The average serum antibody level was quantified at various time points to demonstrate a half-life of 62 days. No significant adverse events were observed at the low and middle doses; infusion-related adverse events were observed at the high dose, which resolved spontaneously without treatment.
In September 2020, we announced positive interim data from the Phase 1 study evaluating escalating doses of mRNA-1944 at 0.1, 0.3 and 0.6 mg/kg dose with and without steroid premedication and two doses of 0.3 mg/kg given one week apart. Administration of mRNA-1944 at all doses resulted in dose-dependent increases in levels of antibody against Chikungunya virus with a mean half-life of ~69 days. Post-single doses of 0.3 and 0.6 mg/kg therapeutically-relevant concentrations of antibody (>1 µg/mL) were observed for 16 weeks. Neutralizing antibodies were observed at all dose levels, indicating functional antibody production by mRNA-1944. Across doses of mRNA-1944, adverse effects were mild-to-moderate in severity and transient, and none were serious. The most common adverse events were headache, nausea, myalgia, dizziness and chills. Following a second dose of mRNA-1944 at the 0.3 mg/kg level, a 1.8-fold increase in the concentration of chikungunya virus was observed, with no worsening or exacerbation of side effects, and no significant accumulation of mRNA, LNP components or acute phase reactants.
The Phase 1 trial is ongoing in follow-up period during 2021.
Phase 1 interim analysis with two-dose regimen
Relaxin (mRNA-0184): Summary
We have regained rights to develop relaxin from AstraZeneca and are evaluating the program
Until December 2020, we collaborated with AstraZeneca on mRNA encoding for a relaxin protein designed for a long duration of action. Relaxin is a well-studied natural protein hormone that is known to have cardiovascular protective effects. Earlier attempts at developing relaxin as a protein therapeutic have not been successful. We believe that engineering the relaxin protein for a longer duration, utilizing repeat dosing, and pursuing an alternate indication might overcome the shortcomings of earlier attempts. We use mRNA encoding for an engineered relaxin protein designed for a longer duration of action. It is also designed to be produced by the body with human post-translational modifications.
In December 2020, we regained the rights to the relaxin program from AstraZeneca (previously AZD7970). We are currently evaluating our options for advancing the program.
Autoimmune Therapeutic Area (mRNA-6981 and mRNA-6231) Introduction
Our company strategy continues to be to invest in our platform technology and scalable infrastructure to pursue a pipeline of potential medicines that reflect the breadth of the mRNA opportunity. In January 2020, we announced the entry into a fifth therapeutic area, autoimmune diseases, building on the clinical validation of the systemic delivery of mRNA provided by the Phase 1 clinical proof of concept of the chikungunya antibody program. Autoimmune diseases are characterized by immune activation in response to antigens normally present in the body, reflecting a loss of tolerance. Within this therapeutic area, we are developing two potential medicines, mRNA-6981 and mRNA-6231, designed to engage peripheral tolerance pathways to dampen autoimmune activation and help restore immune homeostasis, thereby reducing autoimmune pathology. In this modality, mRNA is delivered systemically to create proteins that are either secreted or expressed on the cell surface.
Our approach to the treatment of autoimmune diseases is to leverage mechanisms of peripheral tolerance to modulate the immune system’s reaction to self-antigens.
Scientific and technical advances enable our expansion into new therapeutic areas, the latest of which is autoimmune disease. Autoimmune diseases are defined by pathology resulting from an adaptive immune response against an antigen or antigens normally present within the body. Pathology is present in a variety of organs across autoimmune diseases such as rheumatoid arthritis, systemic lupus erythematosus, inflammatory bowel disease, psoriasis, type 1 diabetes, multiple sclerosis, autoimmune hepatitis and related disorders such as graft vs host disease. Autoimmune diseases affect millions of patients worldwide, many of whose disease is not well-controlled by existing treatment options, and represent billions of dollars in healthcare costs.
In healthy people, autoimmune reactions are prevented or controlled by mechanisms of tolerance. Lymphocytes (e.g., T and B cells) that are reactive against self-antigens are deleted during development, thus establishing central tolerance. Central tolerance is not completely protective, and so other mechanisms, collectively known as peripheral tolerance, act on any self-reactive lymphocytes that escape central tolerance to control potential autoimmune pathology. These mechanisms of peripheral tolerance include induction of a reversible state of cellular non-responsiveness in self-reactive cells called anergy, and expression of inhibitory receptors or cytokines by other cells, such as dendritic cells, macrophages, and regulatory T cells (Tregs). The immune system works constantly to maintain balance between a state of immune activation and immune tolerance, sometimes called homeostasis. We are developing two potential medicines we believe have the potential to engage peripheral tolerance mechanisms to dampen autoimmune activation and help restore immune homeostasis. PD-L1 (mRNA-6981) aims to induce the expression of this inhibitory receptor on myeloid cells, and IL-2 (mRNA-6231) aims to preferentially increase the number of Tregs.
PD-L1 (mRNA-6981)
PD-L1 is a co-inhibitory receptor that can induce anergy in programmed cell death protein 1 (PD-1)-expressing T cells.
Antigen presenting cells, such as dendritic cells, form stable cell-cell junctions with T and B cells, called immune synapses, to communicate in three ways: Signal 1 (antigen presentation and recognition), Signal 2 (co-stimulatory signals to activate the cell) and Signal 3 (cytokines, chemokines, and certain metabolites to activate, repress, or modulate the immune response). When immune synapses occur in the context of high levels of co-inhibition, such as high levels of PD-L1 expressed on antigen presenting cells, this may result in the induction of peripheral regulatory T cells, induction of a reversible non-responsive state called anergy, or death of autoreactive lymphocytes due to removal of critical survival signals. Given their role in adaptive immune responses and their involvement in autoimmune disorders, dendritic cells and other myeloid populations have become a target of recent immunotherapies.
The PD-L1/PD-1 pathway has a critical function in immune regulation and promotes development and function of Tregs. PD-L1 is a transmembrane protein expressed on antigen presenting cells, such as dendritic cells and macrophages, activated T cells, B cells, and monocytes as well as peripheral tissues. Its cognate receptor, PD-1, is a co-inhibitory transmembrane protein expressed on T cells, B cells, natural killer cells and thymocytes. Engagement of PD-1 to PD-L1 results in decreased IL-2 production and glucose metabolism, with continued engagement leading to induction of T cell anergy or conversion of naïve cells into peripheral regulatory T cells. Engagement of PD-L1 with PD-1 also inhibits T cell proliferation, cytotoxic activity and cytokine production, and suppresses the reactivation of previously activated T effector cells.
Preclinical mouse models deficient in PD-1 spontaneously develop a variety of autoimmune diseases such as arthritis, myocarditis, lupus-like glomerulonephritis and type 1 diabetes, demonstrating the critical role of the PD-L1/PD-1 interaction in maintaining tolerance to self-antigens. Additionally, treatment of cancer patients with PD-1 or PD-L1 inhibitors sometimes results in immune-related adverse events, including the development of hepatitis, dermatitis and colitis, demonstrating the role of PD-1/PD-L1 in human autoimmune reactions.
We believe our PD-L1 therapy may augment PD-L1 expression on cell types similar to those that endogenously express it, and by reducing immune activation, potentially reduce the clinical manifestations of a variety of autoimmune diseases.
PD-L1 (mRNA-6981): Our product concept
We intend to induce expression of PD-L1 on myeloid cells to send a tolerizing signal to immune cells in their environment in order to treat autoimmune diseases.
Our intent is to use our platform to influence myeloid cells, including dendritic cells, to provide additional co-inhibitory signals by augmenting endogenous expression of PD-L1. We believe that this tolerizing signal to lymphocytes may limit autoreactivity in the context of ongoing autoimmune pathology without severe and global suppression of the immune system. Given that our platform allows us to modify myeloid cells in situ, our approach to the creation of a tolerogenic environment may provide unique benefits in treating autoimmune diseases by seeking to restore immune homeostasis. We believe the platform technologies used have already been substantially validated in humans; mRNA-6981 employs the same delivery technology used in clinical trials for our chikungunya antibody therapeutic, mRNA-1944. Results with mRNA-1944 demonstrate predictable dose-dependent pharmacology that translated effectively from preclinical species into humans.
PD-L1 (mRNA-6981): Preclinical information
We have observed disease modification in a range of preclinical models.
We have investigated the effect of mRNA-6981 in multiple disease models. In one example, we evaluated mRNA-6981 in a rat model of arthritis. Animals were given a single injection of chicken collagen type II in incomplete Freund’s adjuvant in order to induce chronic arthritis-like symptoms. mRNA-6981 was dosed subcutaneously at four times per week and compared to a negative PBS control and a positive control of daily high dose dexamethasone (Dex). Arthritis-like symptoms included paw swelling and joint rigidity, which were scored as a proxy for disease severity. Compared to animals treated with PBS, animals treated with PD-L1 mRNA presented with consistently less severe disease similar to animals treated daily with dexamethasone for at least three weeks.
Collagen-Induced Arthritis model
We have investigated mRNA-6981 in a range of other preclinical models of autoimmune and related diseases, including type 1 diabetes, colitis and graft-versus-host disease, and observed disease-modifying activity.
PD-L1 (mRNA-6981): Clinical plan
We are planning a Phase 1 clinical trial for patients with type 1 autoimmune hepatitis (AIH).
AIH is an autoimmune condition involving inflammation in the liver, which over time can lead to cirrhosis and liver failure. Type 1 AIH is characterized by a specific autoantibody profile and afflicts more than 75,000 patients in the U.S. Type 1 AIH is typically treated with steroids and azathioprine but some patients either do not respond to these treatments or are unable to tolerate them and are therefore in need of alternatives. A specific role for PD-L1 therapy in treating type 1 AIH is supported by clinical observations in cancer patients receiving PD-1/PD-L1 checkpoint inhibitor treatment: a noted adverse event is the development of AIH, which responds to discontinuation of checkpoint inhibitor therapy and treatment with corticosteroids. Checkpoint inhibitor-induced AIH has an identical histological and clinical manifestation compared to non-drug induced type 1 AIH. We believe that mRNA-6981 may provide benefit to type 1 AIH patients by increasing PD-L1 expression and plan to pursue proof-of-concept in type 1 AIH as a first step to addressing a range of autoimmune indications. We are planning a clinical trial to evaluate the safety, tolerability pharmacology, and durationimmunogenicity of the effect of mRNA-6981mRNA-1769 in type 1 AIH patients refractory or intolerant to the standard of care.healthy participants.
IL-2 Mutein (mRNA-6231)
IL-2 is a critical cytokine for Treg activation and expansion.
Cytokines are potent modulators of the immune system, directing function and homeostasis. IL-2 is critically important to T cell survival and function. IL-2 acts through a receptor complex that can be dimeric, IL-2Rß (CD122) plus the common γ chain (CD132), or trimeric, which is formed through the addition of IL-2Rα (CD25) to the dimeric form. The trimeric form has 10-fold to 100-fold greater affinity for IL-2. Under low or homeostatic IL-2 conditions, those cells which preferentially express the trimeric receptor, or IL-2R, such as Tregs and very recently activated effector T cells, are activated. Conversely, those cells that express the dimeric form, such as naïve or antigen-experienced cytotoxic T cells and natural killer cells (NK cells), are only activated by much higher concentrations of IL-2. Tregs play an obligate role in maintaining peripheral tolerance through the control of effector T cell responses, and several strategies are being developed to exploit IL-2 to treat autoimmune disease by selectively enhancing Treg function. These include recombinant protein forms of IL-2/mAb complexes, IL-2 Muteins and low-dose IL-2.
IL-2 Mutein (mRNA-6231): Our product concept
We intend to utilize subcutaneous mRNA administration to produce a version of IL-2 that is potentially longer acting and more selective for the trimeric IL-2 receptor (IL-2R) in order to treat autoimmune diseases.
IL-2-based therapeutics are being clinically evaluated for a wide range of immune-mediated disorders, including rheumatoid arthritis, systemic lupus erythematosus, graft versus host disease, inflammatory bowel diseases, and autoimmune hepatitis. We believe that our platform can be exploited to produce a modified IL-2 for the treatment of autoimmune conditions. Our modified IL-2 is engineered with mutations that selectively decrease binding to the dimeric IL-2 receptor present on CD4+ and CD8+ T effector cells and NK cells, and increase reliance upon CD25 of the trimeric IL-2 receptor complex to trigger the signaling cascade in regulatory T cells. Our modified IL-2 is also expressed as a fusion protein to extend its half-life in the serum. This will be the first demonstration of subcutaneous administration of the delivery technology that is also used in clinical trials for our chikungunya antibody therapeutic, mRNA-1944.
IL-2 Mutein (mRNA-6231): Preclinical information
We have observed preferential expansion of Tregs in non-human primates.
Preclinical studies have been conducted using mouse homologs as well as cynomolgus monkeys. In one example, monkeys were dosed subcutaneously with a single dose of mRNA-6231 and T cells in the peripheral blood were monitored on days 1, 3, 5, 8, and 14. The percentage of Tregs (CD4+ T cells that were also FoxP3+) increased about 12-fold (average across N=4 animals) at their maximum (day 8 post-dosing). Conversely, the percentage of activated CD8+ conventional T cells (that co-express CD25) did not significantly increase over baseline at any time during the monitoring period, illustrating the preferential expansion of Tregs by the IL-2 Mutein.
IL-2 Mutein (mRNA-6231): Clinical plan
We are planning a Phase 1 clinical trial in normal healthy volunteers to assess safety, tolerability, pharmacokinetics and pharmacodynamics.
We plan to conduct a Phase 1 dose escalation study of mRNA-6231 in adult healthy volunteers. The objectives of this study are to evaluate the safety and tolerability of mRNA-6231, to assess the pharmacodynamic response through Treg selective expansion, activation and duration, and to characterize the pharmacokinetic profile of mRNA-6231 in expressing IL-2 in the serum following subcutaneous administration.
CANCER VACCINES AND THERAPEUTICS MODALITY
We designed our cancer vaccines modality to treat or cure cancer by enhancing immune responses to tumor neoantigens, defined below. This modality has two programs currently for neoantigen vaccines, a personalized cancer vaccine, or PCV, program, and a vaccine against neoantigens related to a common oncogene called KRAS, both conducted in collaboration with Merck. The goal of a cancer vaccine is to safely expose the patient’s immune system to tumor related antigens, known as neoantigens, to enable the immune system to elicit a more effective antitumor response. Our cancer vaccines and therapeutics modality is focused oncurrently has three development programs, all of which have entered the use of mRNA to express neoantigens found in a particular tumor in order to elicit an immune response via T cells that recognize those neoantigens, and therefore the tumor. These neoantigens can either be unique to a patient, as in the case of our personalized cancer vaccine program, or can be related to a driver oncogene found across subsets of patients, as in the case of our KRAS vaccine program.clinic.
Disease overviewIndividualized Neoantigen Therapy (INT) (mRNA-4157)
More than 1.8 million new cancer cases and approximately 600,000 deaths due to cancer were predicted in the United States for 2020, according to the NIH. Despite the recent success of checkpoint inhibitors, the majority of patients with the most common types of epithelial cancer still do not benefit from checkpoint inhibitors, as many patients still have incomplete or no response to currently available therapies. In addition, treatment resistance is thought to arise from a number of mechanisms, principally the local immunosuppressive effects of cancer cells, which prevent either access to or recognition by T cells.
Recent breakthroughs in cancer immunotherapy, such as checkpoint inhibitors and chimeric antigen receptor T cell therapies, have demonstrated that powerful antitumor responses can be achieved by activating antigen specific T cells. We believe one approach to improve the efficacy of checkpoint inhibitors is to develop vaccines that increase both the number and antitumor activity of a patient’s T cells that recognize tumor neoantigens.
Cancer vaccines: Product features
We believe that mRNA technology is an attractive approach for cancer vaccines for many reasons, including:
•mRNA vaccines can deliver multiple neoantigens concatenated in a single mRNA molecule. We currently encode up to 34 neoantigens in one ofour personalized cancer vaccines (mRNA-4157), and four KRAS mutations in our KRAS vaccine (mRNA-5671). Given that a T cell response against a single antigen has the potential to eradicate cancer cells, we believe that delivering multiple neoantigens could increase the probability of a successful treatment outcome for a patient.
•mRNA encoding for neoantigens is translated and processed by patients’ endogenous cellular mechanisms for presentation to the immune system. Neoantigen peptides are then potentially processed in multiple ways to give rise to different, smaller peptides for presentation by theimmune system. We believe this endogenous antigen production and presentation has the potential to drive a more effective immune response.
•mRNA vaccines can be efficiently personalized. The shared features of mRNA, combined with our investments in automated manufacturingtechnology, enable us to manufacture individual cGMP batches of personalized cancer vaccines rapidly, in parallel. For example, we have demonstrated the ability to manufacture and release a “custom-designed” vaccine for an individual patient within 60 days of sequencing the patient’s tumor for the personalized cancer vaccine program (mRNA-4157).
Personalized Cancer Vaccines (PCV) (mRNA-4157)
Our personalized cancer vaccine, or PCV, is currently being evaluated in a Phase 1 and a Phase 2 study. The Phase 1 trial is assessing mRNA-4157 alone and in combination with KEYTRUDA®. The Phase 2 trial is assessing the mRNA-4157 in combination with KEYTRUDA vs. KEYTRUDA alone. (KEYTRUDA is a registered trademark of Merck Sharp & Dohme Corp.)
PCV (mRNA-4157): Our product concept
As tumors grow, they acquire mutations, some of which create new protein sequences,segments, or neoantigens, that can be presented on HLAhuman leukocyte antigen (HLA) molecules in the tumor and recognized as non-self by T cells. TheseWhile some of these neoantigens cancould be shared as in mRNA-5671, oracross tumors, the majority are completely unique to an individual patient’s tumor. Intumor, in addition to the neoantigens being unique and patient specific, the presentation of those neoantigens is also dependent on a patient’s specific HLA type. Identification of patient-specific HLA type and tumor neoantigens through next generation sequencing paired with our proprietary, in silico design of each patient’s mRNA
vaccine and rapid manufacturing for a specific patient allows us to rapidly deliver a completely unique and personalized medicine to patients.
Our personalized cancer vaccine program,INT, mRNA-4157, consists ofuses next generation sequencing and our proprietary algorithm to design an mRNA that encodes up to 34 neoantigens against each individual patient’s tumor mutations with specificity to their HLA type, and is predicted to elicit both class I (CD8) and class II (CD4) responses, designed against each individual patient’s tumor mutations and specific to their HLA type.responses. The neoantigens are encoded in a single mRNA sequence and formulated in our proprietary LNPs designed for intramuscular injection. The mRNA sequenceINT is then manufactured using an automated workflow to enable a rapid turnaround time.
We are developing mRNA-4157 in collaboration with Merck. In September 2022, Merck exercised its option for personalized cancer vaccines, including mRNA-4157, pursuant to the terms of our existing PCV (mRNA-4157): Clinical dataCollaboration and License Agreement with Merck, which was amended and restated in 2018 (PCV Agreement). Pursuant to the PCV Agreement, we and Merck will collaborate on further development and commercialization of mRNA-4157, and we will share costs and any profits and losses worldwide related to mRNA-4157 equally.
The Phase 1 trial is an open-label, multicenter study to assessIn December 2022, we announced that the safety, tolerability, and immunogenicity of mRNA-4157 alone in subjects with resected solid tumors and in combination with the checkpoint inhibitor, pembrolizumab (marketed in the United States as KEYTRUDA), in subjects with resected and unresected solid tumors. mRNA-4157 is administered on the first day of each 21-day cycle for a maximum of nine doses. mRNA-4157 is administered as monotherapy (Part A) or in combination with pembrolizumab (Parts, B, C, and D) in the United States. Studies have shown mRNA-4157 to be well tolerated at all dose levels. The majority of adverse events from mRNA-4157 have been low grade and reversible. Encouraging data emerging from an expansion arm in patients with head and neck cancer has recently caused us to increase the size of that cohort, which continues to recruit trial participants.
The randomized Phase 2 trial will assess whether post-operative adjuvant therapy withof mRNA-4157 had met its primary endpoint. The open-label Phase 2 study is investigating a 1 mg dose of mRNA-4157 in combination with Merck’s pembrolizumab improves relapse-free survival(KEYTRUDA®), compared
to pembrolizumab alone, for the adjuvant treatment of high-risk resected melanoma. The study showed that mRNA-4157 in combination with KEYTRUDA reduced the risk of recurrence or death by 44% (HR=0.56 [95% CI, 0.31-1.02]; one-sided p value=0.0266) compared with KEYTRUDA alone. The results are the first demonstration of efficacy for an investigational mRNA cancer treatment in a randomized clinical trial in melanoma. Adverse events observed were consistent with those previously reported in a Phase 1 clinical trial, which showed mRNA-4157 to be well-tolerated at all dose levels.
In February 2023, mRNA-4157 received a Breakthrough Therapy Designation from the FDA, and in April 2023 mRNA-4157 received PRIME Scheme Designation from the EMA.
In December 2023, we announced that at a planned median follow-up of approximately three years, mRNA-4157 in combination with KEYTRUDA showed sustained benefit, reducing the risk of recurrence or death by 49% (HR=0.510 [95% CI, 0.288-0.906]; one-sided nominal p=0.0095) and the risk of distant metastasis or death by 62% (HR=0.384 [95% CI, 0.172-0.858]; one-sided nominal p= 0.0077) compared to pembrolizumab alone.
KRAS vaccineVaccine (mRNA-5671)
Finding oncogenic driver mutations that encode targetable T cell epitopes has considerable therapeutic implications. Point mutationsWe are evaluating next steps for our KRAS vaccine program (mRNA-5671), having retained all rights to the program from Merck. Enrollment closed in the KRAS gene occur in about 22% of human cancers, such as colorectal, non-small cell lung and pancreatic cancers. It has been reported that KRAS-mutant neoantigens can be presented on certain human HLAs. We have designed an mRNA to generate and present KRAS neoantigens to the immune system from the four most common KRAS mutations. The Phase 1 trial is being conductedstudy led by Merck and is currently ongoing in the United States.
KRAS vaccine (mRNA-5671): Our product concept
Oncogenic driver mutations that encode targetable T cell neoantigens have considerable potential therapeutic implications: (1) driver mutations are subject to positive selection, as they confer survival advantages for the tumor, and (2) such neoantigens could be shared between patients, enabling an easier approach to developing and manufacturing such therapeutic or curative interventions.
early 2022. KRAS is a frequently mutated oncogene in epithelial cancers, primarily lung, colorectal cancer or CRC,(CRC) and pancreatic cancers. ThemRNA-5671 is designed to present neoantigens for the four most prevalent KRAS mutations associated with these malignancies are G12D, G12V, G13D, and G12C, which constitute 80% to 90% of KRAS mutations.
KRASCheckpoint cancer vaccine (mRNA-5671): Clinical plan(mRNA-4359)
Merck is conducting an open-label, multi-center, dose-escalationWe are developing a checkpoint cancer vaccine (mRNA-4359) that express Indoleamine 2,3-dioxygenase (IDO) and dose expansionprogrammed death-ligand 1 (PD-L1) antigens. We designed mRNA-4359 with the goal of stimulating effector T cells that target and kill suppressive immune and tumor cells that express target antigens. Our initial indications for mRNA-4359 are advanced or metastatic cutaneous melanoma and NSCLC. Our Phase 1 study to evaluate the safety and tolerability of mRNA-5671 administered as an intramuscular injection both as a monotherapy and in combination with pembrolizumab.mRNA-4359 is ongoing.
INTRATUMORAL IMMUNO-ONCOLOGY MODALITY
We designed ourOur intratumoral immuno-oncology modality to treat or cure cancer by transforming the tumor microenvironment to drive anti-cancer T cell responses against tumors. Our mRNA technology within this modality allows for the combination of multiple therapeutics that can be directly injected into a tumor with the goal of activating immune cells to kill cancer cellscurrently has one development program, which is in the injected tumor as well as in distal tumors, known as the abscopal effect. Intratumoral administration allows for localized effect of these therapeutics that could be toxic if administered systemically. This exploratory modality has three development candidates.
Disease overview
As noted above, more than 1.8 million new cancer cases and approximately 600,000 deaths due to cancer were predicted in the United States for 2020, according to the NIH. There have been several advances in the treatment of cancer through immune-mediated therapies in recent years. However, the outlook for many patients with advanced cancer remains poor, especially in tumors that have little immune system engagement and are sometimes termed immunologically “cold.” We aim to activate the tumor microenvironment with our mRNA therapeutic candidates, in conjunction with a checkpoint inhibitor, to activate the immune system against these otherwise immunologically cold tumors.
Intratumoral immuno-oncology: Our approach
We believe our approach to immuno-oncology using mRNA medicines could complement checkpoint inhibitors and has several advantages over recombinant protein-based drugs, including:
•mRNA focuses and limits exposure of immune stimulatory proteins. One of the intrinsic properties of mRNA is its transient nature. This allowsfor short exposure of the proteins encoded by the mRNA in the target tissue, thereby potentially enhancing tolerability.
•mRNA can produce membrane associated immune stimulatory proteins. In contrast to recombinant proteins, mRNA administered to a tumorsite can lead to the production of either secreted or membrane proteins, depending on the mRNA sequence.
•Multiplexing of mRNA allows access to multiple immune stimulatory pathways. The ability to combine multiple mRNAs to express multipleproteins allows for activation of several immune pathways simultaneously. For example, OX40L/IL-23/IL-36γ (Triplet) (mRNA-2752) encodes for two secreted cytokines (IL-23 and IL-36γ) and one membrane protein (OX40L).
•mRNA sequences can be engineered to reduce off-target effects. mRNA sequences can be designed to minimize translation in off-target tissues. Forimmune-stimulatory proteins this can potentially prevent toxicities.
•Local administration of mRNA can create a concentration gradient for encoded proteins. mRNA administered intratumorally allows for thelocal production of encoded immune-stimulatory proteins, such as cytokines. The mRNA and encoded protein are expected to form a concentration gradient that decreases as a function of the distance from the tumor, thereby potentially lowering undesirable systemic effects and increasing immune-stimulatory effects close to the tumor.
OX40L (mRNA-2416)
There have been several recent advances in the treatment of cancer through activation of the immune system. However, many patients with advanced stages of cancer respond to few therapies and continue to face a poor outlook. Alternative strategies to activate an immunologic anti-tumor response, while at the same time reducing systemic toxicities, are required. To this end, we have developed an investigational mRNA therapeutic coding for wildtype OX40 ligand, or OX40L, protein, a membrane protein normally expressed on antigen presenting cells upon immune stimulation that augments an activated immune response. mRNA-2416 encodes for wild-type OX40L which is a membrane protein, a class of proteins that we believe cannot be manufactured for administration to tumor cells by recombinant technologies. mRNA-2416 is being developed for the treatment of solid tumors following local intratumoral injection. We are currently sponsoring a Phase 1/2 trial that is ongoing in the United States, which includes Phase 1 dose escalation cohorts of mRNA-2416 as monotherapy and in combination with durvalumab, followed by a Phase 2 expansion cohort in patients with advanced ovarian carcinoma in combination with durvalumab.
OX40L (mRNA-2416): Our product concept
Our product consists of mRNA coding for the human sequence of OX40L formulated in our proprietary LNP. mRNA-2416 was designed to decrease the amount of protein that could be made in hepatocytes through incorporation of a microRNA binding site, thus potentially reducing off-target effects and resulting in better tolerability. Following intratumoral injection, a specific anti-tumor immune response is expected to be induced via proliferation and migration of T cell clones with specificity for the cancer that may also result in systemic anti-tumor responses.
OX40L (mRNA-2416): Clinical data
The Phase 1/2 trial for mRNA-2416 is an open-label, multicenter study of repeated intratumoral injections of mRNA-2416 as a monotherapy and in combination with durvalumab in patients with advanced relapsed/refractory solid tumor malignancies and lymphomas in the United States and Israel. The objectives of this Phase 1/2 study include evaluating safety and tolerability of mRNA-2416 administered intratumorally, and to define the maximum tolerated dose and recommended dose for expansion alone and in combination with durvalumab. Other endpoints include pharmacokinetic analyses as well as assessment of biomarkers of immunological response in tumor. The dose levels being tested in the monotherapy arm of the trial were 1 µg, 2 µg, 4 µg, and 8 µg. The monotherapy arm of the study has been completed and we are not planning an expansion cohort of mRNA-2416 as a monotherapy. We have completed a dose-finding cohort at 4 µg mRNA-2416 given in combination with durvalumab (IMFINZI®) and are currently enrolling a Phase 2 expansion cohort in ovarian cancer.
As of January 1, 2021, 60 patients were dosed with mRNA-2416 (39 patients in monotherapy and 21 patients in combination with durvalumab). As of February 12, 2020, safety was reported on 39 patients treated with monotherapy mRNA-2416. mRNA-2416 has been tolerable at all dose levels with no dose-limiting toxicities reported and the majority of treatment related adverse events being grade 1 or 2. We have observed systemic injection related reactions in seven patients, all of which have been reversible.
Of the evaluable cases for patients dosed with mRNA-2416 as of November 13, 2019, 14 achieved a best overall response rate (BOR) of stable disease, of these patients 6 had stable disease for ≥14 weeks; 15 patients had progressive disease per RECIST 1.1. Clinical observations include a reduction in injected tumor size in two patients with ovarian cancer, one patient with breast cancer and one patient with pseudomyxoma peritonei.
OX40L/IL-23/IL-36γ (Triplet) (mRNA-2752): Clinical update
Despite recent advances in immune-mediated therapies for cancer, the outlook for many patients with advanced cancer is poor. We are developing Triplet (mRNA-2752) and other programs to drive anti-cancer T cell responses by transforming cold tumor microenvironments into productive, “hotter” immune landscapes with local intratumoral therapies. Triplet (mRNA-2752)mRNA-2752 utilizes the intrinsic advantage of mRNA to multiplex and to produce membrane and secreted proteins with mRNA in a single investigational medicine.product candidate. Triplet (mRNA-2752) includes three mRNAs encoding human OX40L, interleukin 23, or IL-23 and interleukin 36 gamma, or IL-36γ, that are encapsulated in our proprietary LNP and administered intratumorally. OX40L is a membrane protein, whereas IL-23 and IL-36γ are secreted cytokines. We believe our approach has the advantage of localized high concentration gradients of IL-23 and IL-36γ compared to recombinant proteins administered systemically or intratumorally. Additionally, the mRNA for OX40L encodes for the wild type membrane protein, which we believe recombinant protein technologies cannot enable. The combination of OX40L, IL-23, and IL-36γ has shown robust activity in preclinical cancer models and is synergistic with checkpoint inhibitors. In addition, this combination elicits an anti-tumor response on distal tumors (via the “abscopal effect”), as well as treated tumors in preclinical studies. A Phase 1 trial of Triplet (mRNA-2752) is ongoing.
OX40L/IL-23/IL-36γ (Triplet) (mRNA-2752): Our product concept
We are developing Triplet (mRNA-2752)In December 2023, we completed enrollment in our ongoing Phase 1 dose-escalation study of mRNA-2752 for the treatment of advanced or metastatic solid tumor malignancies or lymphoma as(as a single agent or in combination with checkpoint inhibitors. Triplet (mRNA-2752) includesinhibitors).
RARE DISEASE INTRACELLULAR THERAPEUTICS
Our rare disease intracellular therapeutics modality currently has six development programs, three mRNAs encoding OX40L, IL-23,of which are in the clinic.
Propionic acidemia (PA) (mRNA-3927)
PA is a rare, inherited metabolic disorder with significant morbidity and IL-36γmortality, affecting one in 100,000-150,000 individuals worldwide. PA is caused by pathogenic variants in the propionyl-coenzyme A carboxylase (PCC) α or β subunits (PCCA and PCCB genes, respectively), encapsulatedleading to PCC deficiency and subsequent accumulation of toxic metabolites. PA is characterized by recurrent life-threatening metabolic decompensation events (MDEs) and multisystemic complications. Multisystemic complications include neurological manifestations, cardiomyopathy, arrythmias, growth retardation, recurrent pancreatitis, bone marrow suppression and predisposition to infection. Long-term, insults by toxic metabolites cause complications in our proprietary LNP. Triplet (mRNA-2752)various organs, and cognitive outcome is designed to make these proteins in cells of the local tumor environment or lymph node. Our approach potentially has the advantage of localized gradients of two important cytokines IL-23 and IL-36γ, rather than a systemic administration or intratumoral injection of cytokine proteins that would lead to quick diffusion away from the tumor. Additionally, the mRNA for OX40L encodes for the wild type membrane protein, which would be challenging to administer to either a tumor or systemically as a recombinant membrane protein capable of co-stimulation of T cells. mRNA for IL-23 produces a single-chain fusion protein of the IL-12B and IL-23A subunits, with a linker between the subunits. mRNA for IL-36γ produces a protein with introduced signal peptide to bypass a need for upstream processing for release and activity. In addition, all three mRNA were designed to decrease the amount of protein that could be made in hepatocytes through incorporation of microRNA binding sites, thus potentially reducing off-target effects and resulting in better tolerability.
OX40L/IL-23/IL-36γ (Triplet) (mRNA-2752): Clinical update
We have an ongoing Phase 1 study that is designed as an open-label, multicenter study of intratumoral injections of Triplet (mRNA-2752) alone or in combination with durvalumab (anti-PD-L1) with sites in the United States and Israel. The objectives of this study include:
•assessment of safety and tolerability of Triplet (mRNA-2752) administered alone and in combination with durvalumab;
•define the maximum tolerated dose, or MTD, and recommended dose for expansion, or RDE, for intratumoral injections of Triplet (mRNA-2752) alone and in combination with durvalumab; and
•assessment of anti-tumor activity, protein expression in tumors, and pharmacokinetics, and exploratory endpoints that include assessment of immunological responses.
The study consists of two arms: Arm A, Triplet monotherapy and Arm B, Triplet in combination with durvalumab. There are dose escalation and dose confirmation parts for each study arm followed by a dose expansions for Arm B. mRNA-2752 will be evaluated at 0.25, 0.5, 1, 2, 4, and 8 µg. mRNA-2752 is administered once every two weeks for cycle 1, followed by once every four weeks for cycles 2 through 6. Durvalumab is administered every four weeks. Biopsy and blood samples to be collected pre- and post-treatment with mRNA in both dose escalation and dose expansion to assess protein expression and changes in tumor immune landscape.
As of January 1, 2021, 44 patients have been dosed with mRNA-2752, including 19 patients in monotherapy and 25 patients in combination with durvalumab. Safety in this study was reported as of April 8, 2020, on 29 cases, including 17 patients treated with mRNA-2752 monotherapy and 12 patients in combination with durvalumab. Across dose levels tested, study treatment has been well tolerated with no dose-limiting toxicities, one patient with Grade 3 toxicity (at 4 µg dose level) and no Grade 4/5 toxicities related to treatment. One partial response with an 81% decrease in target lesions has been observed in a squamous cell bladder cancer patient on the combination arm; stable disease has been observed in nine patients (five on mRNA-2752 monotherapy and four treated with durvalumab combination). Tumor shrinkage was observed in seven patients in injected and/or un-injected target lesions (three on monotherapy and four on combination).
As of April 8, 2020, biomarker data from the first eight cohorts (monotherapy at dose levels between 0.25 µg– 4 µg; combination at 0.25 µg and 0.5 µg) demonstrated increased IL-23 and IL-36g protein expression, evidence of a pro-inflammatory effect of treatment including elevated interferon gamma, tumor necrosis factor alpha and PD-L1. Data consistent with demonstration of proof of mechanism. All post-treatment plasma cytokine levels evaluated were well below what has been suggested as clinically toxic levels for these cytokines in cytokine release syndromes. Significant increases in PD-L1 protein levels predominantly in immune cells in the tumor microenvironment were observed at days 2, 15 and 29 post-treatment demonstrating the sustained immunomodulatory effect of treatment. In the patientnegatively correlated with the partial response, increased T cells, particularlynumber of proliferating CD8+ cytotoxic T cells were observed post treatment.
IL-12 (MEDI1191)
Another strategy for cancer patients with immunologically cold tumors is to transform the tumor microenvironment by introducing pro-inflammatory cytokines directly into tumors or draining lymph nodes. In collaboration with AstraZeneca, we are developing MEDI1191, which is an mRNA for IL-12 encapsulated in our proprietary LNP to be delivered intratumorally. Systemic administration of recombinant IL-12 protein was poorly tolerated in early clinical trials and exhibited generally low response rates. MEDI1191 can enhance the immune response by positively impacting both antigen presenting cells and T cells, and local, intratumoral expression of IL-12 can potentially improve tolerability compared to systemic protein treatments. AstraZeneca is conducting a Phase 1 clinical trial for MEDI1191, which is to be co-administered with a checkpoint inhibitor.
IL-12 (MEDI1191): Our product concept
Intratumoral delivery of IL-12 has been observed to be a feasible approach to overcome the toxicity associated with systemic IL-12 administration. For example, intratumoral delivery of an IL-12 containing DNA plasmid by injection followed by electroporation has shown promising activity in combination with pembrolizumab in patients with metastatic melanoma. Such an approach may be limited to accessible lesions amenable to electroporation. In contrast, it may be more feasible to inject our mRNA delivered by our proprietary LNP into both accessible and visceral tumors.
MEDI1191 is being developed for the treatment of advanced or metastatic solid tumors in combination with a checkpoint inhibitor. MEDI1191 consists of our proprietary LNP encapsulating an mRNA for human IL-12B (p40) and IL-12A (p35) subunits. The mRNA produces a single-chain fusion protein of the IL-12B and IL-12A subunits, with a linker between the subunits. The mRNA sequence has been engineered to enhance protein production and is designed to decrease the amount of protein that might be made in hepatocytes for better tolerability.
IL-12 (MEDI1191): Preclinical information
We have conducted several preclinical studies in which we observed activity with our approach
Our preclinical studies were conducted with a mouse homolog of IL-12. In a tumor model that we have characterized as completely refractory to checkpoint therapy and associated with an immunosuppressive tumor microenvironment, treatment with IL-12 transformed the tumor microenvironment, with notable activation of natural killer and dendritic cells, and an increase in cytotoxic lymphocytes. In this checkpoint inhibitor refractory mouse model of cancer, a single dose of IL-12 mRNA yielded around 30% complete response rates as an mRNA monotherapy as shown in panel A below and was synergistically active with systemically administered anti-PD-L1 antibody, or α PD-L1, demonstrating complete response rates of > 70%, as shown in panel B of the figure below. The x-axis represents days after subcutaneous implantation of MC38-R tumor cells. Test articles were administered on Day 11 for mRNA treatments and on Days 11, 14, 18, and 21 for antibody treatments. All antibody treatments were administered at 20 mg/kg. There were 15 mice per group in this study. Survival curves were plotted by considering any reason a mouse was removed from study, including the predetermined tumor burden endpoint of 2,000 mm3, as a survival event. NTC is a non-translating control mRNA. Synergy of locally administered IL-12 mRNA with systemic α PD-L1 treatment was also observed on distal tumors that were not directly administered mRNA.
Panel APanel B
IL-12 (MEDI1191): Clinical plan
We are responsible for generating a preclinical data package to support the filing of an IND and clinical trial authorization (CTA) and clinical supply for early clinical development. AstraZeneca is leading the early clinical development. AstraZeneca is currently enrolling an open-label multicenter Phase 1 clinical trial of intratumoral injections of MEDI1191 alone and in combination with the checkpoint inhibitor, durvalumab.
LOCALIZED REGENERATIVE THERAPEUTICS MODALITY
We designed our localized regenerative therapeutics modality to develop mRNA medicines to address injured or diseased tissues. Our mRNA technology in this modality allows for the local production of proteins that provide a therapeutic benefit in the targeted tissue. The development of our program in this modality, AZD8601, for the local production of VEGF-A, is being led by our strategic collaborator, AstraZeneca. This program completed a Phase 1a/b clinical trial in which we observed both a dose-dependent protein production and a pharmacologic effect, as measured by changes in local blood flow in patients. We believe this data provides clinical proof of mechanism for our mRNA technology outside of the vaccine setting.
Localized regenerative therapeutics: Opportunity
There are multiple applications for tissue regeneration. With AstraZeneca, we have focused on ischemic heart failure for the first program. Coronary artery disease, the primary cause of ischemic heart failure, affects the arteries providing blood supply to the cardiac muscle. In 2015, coronary artery disease resulted in 366,000 deaths in the United States, and 8.9 million deaths globally.
Localized regenerative therapeutics: Product features
We believe our approach to localized regenerative therapeutics using mRNA has several advantages over alternative approaches, including:
•mRNA can be administered locally to produce the desired protein for an extended duration. Local exposure to the therapeutic protein encodedby our mRNA is sustained by the ongoing translation of the mRNA into protein, often from hours to days. This pharmacokinetic profile closely mimics the optimal tissue exposure profile for regenerative applications and cannot be achieved by injections of recombinant proteins that rapidly diffuse out of the tissue after injection.
•Local administration of mRNA allows for focused activity. mRNA administered to a specific tissue or organ should allow for local production ofthe encoded protein, which could lead to lower levels of encoded protein in distant or systemic locations. This could help to prevent potential toxicity from production of the encoded protein outside of the targeted tissue.
•mRNA allows for dose-dependent and repeated production of the encoded protein. mRNA therapies should also allow for dose titration andrepeat dosing. This provides several advantages over gene therapy. Gene therapy typically results in a permanent change to cellular DNA that may result in uncontrolled or constant production of the desired protein in local tissue or in distant sites, which could cause local or systemic side effects. Further, some gene therapy delivery vehicles are associated with immune responses that limit the ability to repeat dose, preventing dose titration.
VEGF-A (AZD8601)
Addressing ischemic heart failure—VEGF-A as a localized therapeutic in collaboration with AstraZeneca
Heart disease is the leading cause of death in the United States, accounting for one in every four deaths, and is often due to the inability of adults to regenerate heart tissue. Current approved therapies do not specifically address heart regeneration. Previous attempts at cardiac regeneration have included stem cell grafting and gene therapy, but have faced challenges with safety or efficacy. In collaboration with AstraZeneca, we are pioneering a unique approach to treating ischemic heart failure, a condition where the cardiac muscle does not get enough blood supply to perform its contractile function. Vascular Endothelial Growth Factor A, or VEGF-A, can promote cardiac tissue revascularization. The goal of this program is to promote recovery of cardiac function through partial tissue regeneration. The mRNA in this program is in a saline formulation without LNPs and is expected to act locally. Our strategic collaborator AstraZeneca has conducted a Phase 1a/b clinical study in diabetic patients in Europe. The study has met its primary objectives of describing safety and tolerability and secondary objectives of dose-dependent protein production and changes in blood flow. AstraZeneca has moved this program to a Phase 2a trial that is being conducted in Europe and is designed to test safety and tolerability of epicardial injections for patients undergoing coronary artery bypass grafting surgery.
VEGF-A (AZD8601): Disease overview
VEGF-A can promote blood vessel growth to potentially address ischemic heart failure
Several treatments are available for patients with ischemic heart failure. Current treatments include revascularization of the coronary arteries to relieve symptoms and improve cardiac function and therapies that reduce blood pressure or potentially help eliminate excess fluids in congested tissues, including: beta-blockers, angiotensin-converting enzyme inhibitors, angiotensin II inhibitors, and aldosterone receptor blockers as diuretics. However, adult humans are unable to regenerate myocardium tissue following injury and the treatment options described above cannot compensate for this.
VEGF-A is a potent angiogenic factor that promotes growth of blood vessels. Preclinical data suggests that expression of this growth factor in the ischemic heart could increase blood flow and partially restore cardiac function.
VEGF-A (AZD8601): Our product concept
Local delivery of VEGF-A mRNA to increase local concentration of VEGF-A protein while reducing systemic distribution of therapeutic VEGF-A protein
VEGF-A protein acts as a powerful promoter of blood vessel growth. Systemic injection of VEGF-A protein increases VEGF-A exposure throughout the body, which can lead to side effects, but is very short-lived in circulation. Therefore, any therapy involving VEGF-A needs to be localized to elevate local protein concentration and drive revascularization while minimizing systemic side effects. AstraZeneca has opted to pursue the localized application of VEGF-A mRNA in a simple saline formulation in the heart muscle to elevate local protein concentration for longer periods due to increased local protein production. This potentially allows for an extended pharmacodynamic effect at the specific site of injection compared to systemic or local administration of a recombinant protein version of VEGF-A. Some of the early animal work for mRNA VEGF-A was published by our academic co-founder Dr. Kenneth Chien in NatureBiotechnology in 2013, showing improved cardiac function with increased survival with treatment.
VEGF-A (AZD8601): Preclinical information
AstraZeneca has observed the activity of VEGF-A for ischemic heart failure in several preclinical animal models
Preclinical studies have been conducted at AstraZeneca in models of ischemic heart failure. In mouse, rat, and pig models of myocardial infarction, direct injection in the heart muscle (myocardium) of VEGF-A mRNA led to elevated cardiac VEGF-A protein levels and improved cardiac function. The data have been published by AstraZeneca in Molecular Therapy in 2018.
VEGF-A (AZD8601): Clinical data
AstraZeneca has completed a Phase 1a/b trial in Germany; A Phase 2a trial is currently ongoing in Finland and Germany
The Phase1a/b clinical trial for the AZD8601 program has met its primary objectives of describing safety and tolerability and secondary objectives of protein production and changes in blood flow post AZD8601 administration. AstraZeneca has moved this program to a Phase 2a trial.
The Phase 1a/b study was a randomized, double-blind, placebo-controlled study in men with type 2 diabetes mellitus conducted in Europe. VEGF-A mRNA was administered by intradermal injection into the forearm skin in single ascending doses. The primary objective was to evaluate the safety and tolerability of the drug product into the forearm skin, with safety follow-up for six months.
The study was divided into Part A (single ascending-dose cohorts) and Part B (pharmacodynamic cohort). There were three treatment regimens in Part A. Regimens were either AZD8601 at site 1 and placebo at site 2, placebo at site 1 and AZD8601 at site 2, or placebo at both sites. Each regimen comprised six 50 µL injections at one site and six 50 µL injections at a second site on the forearm. In part B, the regimen comprised one 50 µL intradermal injection of either AZD8601 or placebo at each of four sites on the forearm.
There were 27 patients in Part A with 18 receiving AZD8601 in at least one site of the forearm and 9 patients receiving placebo. There were three dose cohorts in Part A, each with 9 patients. In the first cohort, AZD8601 dose was at 24 µg per patient (4 µg per injection). The AZD8601 dose was increased to 72 µg and 360 µg in the next two dose cohorts. There were 15 patients in Part B receiving AZD8601 in at least two sites on the forearm per patient. In Part B, each patient received 200 µg of AZD8601 or placebo.
VEGF-A protein post injection of mRNA was produced at a high level, above the set expected threshold, as shown in the figure below. Expression was measured by skin microdialysis. At each sampling time, mean VEGF-A protein levels across all mRNA treated sites from patients across all cohorts were higher than that of placebo up to the 24-26 hour time point. Data are means with error bars showing standard error of the mean, or SEM. Asterisk indicates p-value <0.05.
VEGF-A protein levels in patients in Part A of the Phase 1a/b trial
The bioactivity of the VEGF-A protein post injection of mRNA was observed by an increase in blood flow at injection sites up to 7 days following a single injection, as shown in the figure below. Measurements were made using laser doppler imaging 7 and 14 days after administration (study part A, n = 27). Data shown are means with error bars showing SEM. Asterisk indicates p-value <0.05.
VEGF-A led to increase in blood flow at day 7 and day 14 in patients in the Phase 1a/b trial
As shown above, administration of AZD8601 demonstrated protein production and changes in local blood flow in diabetic patients. Tolerability of our mRNA injected intradermally was demonstrated for all dose levels. The only causally treatment-related adverse events were mild injection-site reactions, occurring in 32 of 33 participants receiving VEGF-A mRNA across both parts of the study design. All adverse events of injection-site reaction were of mild intensity. No deaths, serious adverse events, or adverse events leading to discontinuation occurred. The program is currently in a Phase 2a clinical trial. It is a randomized, double-blind, placebo-controlled, multi-center, Phase 2a study to evaluate safety and tolerability of epicardial injections of AZD8601 during coronary artery bypass grafting surgery. Some of the outcomes to be monitored in the Phase 2a study include adverse and serious adverse events, electrocardiogram, or ECG, and LVEF. The study is being conducted in Europe. The study is intentionally designed to provide initial safety and tolerability data in about 24 coronary artery bypass patients.
SYSTEMIC INTRACELLULAR THERAPEUTICS MODALITY
We designed our systemic intracellular therapeutics modality to increase levels of intracellular proteins, using cells in the human body to produce proteins located in the cytosol or specific organelles of the cell to achieve a therapeutic effect in one or more tissues or cell types. The goal of this modality is to provide intracellular proteins, such as intracellular enzymes and organelle-specific proteins, as safe, tolerable, and efficacious therapies. Our initial focus within this exploratory modality is on rare genetic diseases. This modality currently has four programs: PA, MMA, PKU and GSD1a.
Systemic intracellular therapeutics: Opportunity
Systemically delivered, intracellular therapeutics focus on areas currently not addressable with recombinant proteins, which are typically administered systemically and cannot reach the inside of the cell. Objectives for potential new therapies in this area include, for example, increasing the levels of:
•intracellular pathway proteins;
•soluble organelle-specific proteins; and
•organelle-specific membrane proteins.
Systemic intracellular therapeutics: Product features
Systemically delivered, intracellular therapeutics, we believe, would allow us to target areas of biology that cannot be addressed using recombinant proteins.
Our potential advantages in these areas include:
•Using mRNA to encode for intracellular and organelle-specific proteins. Our modality permits the expression of intracellular proteins,including those that must be directly translated and moved into organelles such as mitochondria. The ability of mRNA to produce protein inside of the cell enables production of these protein types that we believe are beyond the reach of recombinant proteins.
•mRNA can produce hard-to-make or complex proteins. For example, some proteins, due to their folding requirements or complexity, arechallenging to make using recombinant technologies, but can potentially be produced by human cells using administered mRNA.
•Native post-translational modifications are possible through intracellular protein production using mRNA. mRNA administered to a humancell uses natural secretory pathways inside the cell to make and process the encoded protein. The resulting post-translational modifications, such as glycosylation, are human as opposed to recombinant proteins where these post-translational modifications are native to the non-human cells used for manufacture. These non-human post-translational modifications in recombinant proteins may lead to sub-optimal therapeutic outcomes, side effects and increased immunogenicity.
•mRNA can sustain production of proteins, which can increase exposure to proteins with short half-lives. mRNA can lead to proteinproduction by cells that can last from hours to days depending on design. This feature could increase the levels of short half-life proteins for therapeutic benefit.
•mRNA allows for desirable pharmacology in complex metabolic diseases. Our mRNA technology potentially permits several differentiatedpharmacologic features for treating complex metabolic diseases, including the ability to repeat dose as needed, a rapid onset of action, the ability to adjust dose levels real-time based on individual patient needs, and the ability to stop dosing. Gene therapies may also prove to be useful for treating rare genetic diseases; however, mRNA is not limited by pre-existing immunity that may exist for certain gene therapies using viral vectors, and does not localize to the nucleus or require persistent changes to cellular DNA to have the desired effect.
Propionic acidemia (mRNA-3927)
We aim to produce an intracellular, mitochondrial enzyme complex to treat a pediatric metabolic disorder
Propionic acidemia, or PA, is a rare, life-threatening, inherited metabolic disorder due to a defect in the mitochondrial enzyme propionyl-CoA carboxylase, or PCC. It primarily affects the pediatric population. ThereMDEs. Currently, there is no approved therapy for PA including no approved enzyme replacement therapy, due tothat targets the complexityunderlying root cause of the enzyme, which comprises six copies each of two different subunits (PCCA and PCCB), and its mitochondrial localization. The only effective treatmentdisease.
Our PA therapy candidate, mRNA-3927, is a novel, IV-administered, LNP-encapsulated dual mRNA therapy that encodes for severely affected individuals is liver transplant, aimed at increasing enzyme activity to reduce the occurrence of life-threatening acute metabolic crises. Our platform is uniquely positioned to potentially address this disease by enabling synthesis of this complex enzyme that is localized in the mitochondria of the cell. We are developing an IV-administered mRNA therapeutic comprising two different mRNAs encoding PCCA and PCCB in our proprietary LNPsubunit proteins to replace the defectiverestore functional PCC enzyme activity in the liver. By encoding for intracellular proteins, mRNA therapy has a potential role in preventing and treating acute metabolic decompensations.
The global Phase 1/2 clinical trial for mRNA-3927, the Paramount Study, is ongoing and we have fully enrolled all five dose optimization cohorts, as well as a dose confirmation cohort. The objective of the study is to evaluate the safety and pharmacology of mRNA-3927 in patients 1 year of age and older with functional enzymePA. The primary endpoints are safety and pharmacokinetics and pharmacodynamics. Secondary endpoints include incidence and severity of adverse events (AEs) and change in liverplasma biomarkers: methylcitric acid (2-MC) and other cells. 3-Hydroxypropionic acid (3-HP). We have received Rare Pediatric Disease Designation, and Orphan Drug Designation and Fast Track Designation from the FDA and Orphan Drug Designation from the European Commission for the PA program. The FDA has also granted Fast Track designation to mRNA-3927. We plan to initiate a Phase 1/2 clinical trial in PA patients in 2021.
Propionic acidemia (mRNA-3927): Disease overview
PA is an inherited metabolism disorder with significant morbidity and mortality and no approved therapy
PA is a serious inborn error of metabolism disorder, closely related to MMA, with significant morbidity and mortality. There are approximately 325-2,000 PA patientsSeveral critical milestones have been reached in the United States basedtrial. mRNA-3927 has been generally well-tolerated to date with no drug-related serious adverse events, no discontinuations due to safety and only mild-to-moderate infusion related reactions (<10% of doses).Due to the objective and disease-defining nature of MDEs, regulators have provided initial support for MDE as a clinically meaningful, preferred primary clinical endpoint for development. Based on estimated birth prevalence (0.2-1.2:100,000 newborns) and mortality rates. The vast majority of patients present with life-threatening metabolic crises during the first days or weeks of life, with mortality rates ranging from 13-53% during the neonatal period. Similar to MMA, the cardinal feature of the disorder is the occurrence of life-threatening acute metabolic decompensations that are more frequentpreliminary data, there was a decrease in the first few yearsnumber of life. Longer term sequelae include cardiac complications (cardiomyopathy, arrhythmias) and severe neurologic complications.
The disorder is caused byMDEs post-mRNA-3927 treatment. We expect to advance mRNA-3927 into a defect or deficiency in PCC, an enzyme that is one step upstream in the same metabolic pathway as the MUT enzyme that is deficient in MMA. PCC is a complex hetero-dodecamer enzyme composed of six alpha subunits (PCCA) and six beta subunits (PCCB). The disorder is autosomal recessive, with PA patients generally having loss-of-function mutations in either PCCA or PCCB (and in rare instances, mutations in both PCCA and PCCB). To date, over 100 mutations have been identified for both PCCA and PCCB genes and, similar to MMA, most of the mutations are private. Also similar to MMA, due to this enzyme deficiency resulting in a metabolic block, the disorder is biochemically characterized by the accumulation of toxic metabolites such as 3-hydroxypropionic acid and 2-methylcitrate, among others, and these metabolites may be used as biomarkers of disease.
There is no approved therapy for PA to treat the underlying defect, including no enzyme replacement therapy, due to the complexity of PCC and mitochondrial localization. Carglumic acid (marketed as Carbaglu) is approved in the EU for the acute treatment of hyperammonemia due to various organic acidemias, including PA. Management of the disorder is otherwise limited to strict dietary restrictions and other supportive measures similar to MMA. Liver transplant is a radical yet effective treatment, with the aim of increasing PCC enzyme activity in liver for severely affected individuals.
Propionic acidemia (mRNA-3927): Our product concept
We are utilizing the strength of our platform to produce a complex enzyme comprising two different proteins that localize to the mitochondria
The ability of our platform to encode for large, multimeric complexes such as PCC and enable production of intracellular, mitochondrial proteins makes mRNA especially suited to potentially address PA. We are developing an IV-administered combination mRNA approach, which contains two mRNAs, one for each of the subunits of PCC (PCCA and PCCB) encapsulated in our proprietary LNP. The intent is to potentially treat the entire PA population, regardless of whether an individual has a defect or deficiency in the PCC alpha or beta subunit. The mRNA sequences have been engineered to improve protein translation and encode enzymatically-active PCC with the proper subcellular localization in the mitochondria.
Propionic acidemia (mRNA-3927): Preclinical information
We have demonstrated activity in a PA mouse model in a long-term repeat dose study
A series of in vitro and in vivo pharmacology studies have been performed to demonstrate preclinical proof-of-concept for the combined PCCA and PCCB mRNA therapy. PCCA and PCCB mRNAs administered in PA patient fibroblasts (both PCCA and PCCB-deficient) showed production of active PCC enzyme with the proper subcellular localization in mitochondria at concentrations above wild-type levels. In vivo studies in PA (PCCA -/- [A138T]) mice have resulted in a dose-dependent increase in hepatic PCC activity with a concomitant decrease in disease biomarkers. Notably, a reduction in plasma ammonia levels was observed 3-4 weeks after a single IV administration (1 mg/kg) of PCCA and PCCB mRNA encapsulated in our proprietary LNP in PA mice (n=4-5/group). The data are shown in panel A of the figure below. Additionally, a 6-month repeat-dosepivotal study in PA mice showed decreased heart weight (normalized to body weight) in mice treated with monthly IV administration of PCCA and PCCB mRNA (1 mg/kg) compared to control mRNA (n=6/group). This is shown in panel B of the figure below. Data in both panels is presented as mean ± standard deviation.
Reduction in plasma ammonia with PCCA+PCCB mRNA in PA mouse model study
Panel (A)
Decrease in heart weight with PCCA+PCCB mRNA in 6 month repeat dose study in PA mouse model study
Panel (B)
In the 6-month repeat dose study in PA mice, a significant and sustained lowering of additional disease biomarkers (e.g., 2-methylcitrate, or 2MC) was observed throughout the duration of the 6-month study. A comparison of 2-methylcitrate levels as a result of monthly IV administration of PCCA and PCCB mRNAs (0.5-1 mg/kg) compared to control mice injected with a control (luciferase) mRNA is shown in the figure below (n=6/group). Data are presented as mean ± standard deviation. The IND-enabling GLP toxicology program for PA (mRNA-3927) has been completed.
Plasma 2-methylcitrate levels with repeat dosing of PCCA+PCCB
mRNA in PA mouse model study
Propionic acidemia (mRNA-3927): Clinical plan
We are conducting a global natural history study and are planning a Phase 1/2 clinical trial
The clinical development plan for mRNA-3927 includes a global, natural history study in MMA and PA that was initiated in 2018 and a Phase 1/2 study in pediatric patients diagnosed with PA planned for early 2021.
We plan to conduct an open-label, multi-center, dose optimization Phase 1/2 study of multiple doses of mRNA-3927 in pediatric patients with PA in North America and Europe. The objectives of this study are to evaluate the safety and tolerability of mRNA-3927 administered via IV infusion, to assess the pharmacodynamic response from changes in plasma biomarkers, and to characterize the pharmacokinetic profile of mRNA-3927.
Methylmalonic acidemia (MMA) (mRNA-3705)
Program aims to produce an intracellular, mitochondrial enzyme to treat a pediatric, genetic, metabolic disorder
Isolated methylmalonic academia, or MMA, is a rare, life-threatening, inherited metabolic disorder that is primarily caused by a defect in the mitochondrial enzyme methylmalonyl-coenzyme A mutase, or MUT. It primarily affects the pediatric population. There is no approved therapy that addresses the underlying disorder, including no approved enzyme replacement therapy, due to the complexity of the protein and its mitochondrial localization. Liver or combined liver-kidney transplant is one option for severely affected individuals. Our platform may allow the cells in the human body to produce these and other complex mitochondrial enzymes. Therefore, we are developing an IV-administered mRNA encoding MUT in our proprietary LNP, in order to restore this deficient or defective mitochondrial enzyme in the liver and other cells. We have observed preclinical proof-of-concept in two different MMA mouse models, notably with a marked improvement in survival and reduction of biochemical abnormalities in a severe MMA mouse model. We recently ceased development of mRNA-3704 and will return to the clinic with the next generation formulation, mRNA-3705. To date, the new drug product mRNA-3705 has demonstrated greater potency and prolonged lowering of methylmalonic acid in mutase deficient mice when compared to mRNA-3704. mRNA-3705 has received
Rare Pediatric Disease Designation from the FDA. A Phase 1/2 clinical study with mRNA-3705 is in planning and will be initiated in 2021.
Methylmalonic acidemia (mRNA-3705): Disease overview
MMA is a rare, life-threatening pediatricinherited metabolic disorder with no approved therapies that address the underlying defect
significant morbidity and mortality caused by a deficiency in an enzyme called methylmalonyl-CoA mutase (MUT). There are an estimated 500-2,000 people with MMA MUT deficiency patients in the United States based on estimated birth prevalence (0.3-1.2:100,000 newborns) and mortality rates. Mortality is significant, with mortality rates of 50% for MMA patientsthose with complete MUT deficiency (mut 0)0) (median age of death 2 years) and 40% for MMA patients with partial MUT deficiency (mut -)-) (median age of death 4.5 years) reported in a large European study.
MMA mainly affects the pediatric population and usually presents in the first few days or weeks of life. The occurrence of acute metabolic decompensations is the hallmark of the disorder and decompensations are typically more frequent in the first few years of life. Each decompensation is life-threatening and often requires hospitalization and management at an intensive care unit. Surviving patientsSurvivors often suffer from numerous complications including chronic renal failure and neurologic complications such as movement disorders, developmental delays, and seizures. Consequently, the health-related quality of life for MMA patients and their families is significantly impaired.
The disorder is autosomal recessive and primarily caused by loss-of-function mutations in the gene encoding MUT, a mitochondrial enzyme that metabolizes certain proteins and fats, resulting in complete (mut 0) or partial (mut -) enzyme deficiency. There are currently no approved therapies that address the underlying defect for MMA. Carglumic acid (marketed as Carbaglu) is approved in the EU for the acute treatment of hyperammonemia due to various organic acidemias including MMA. Liver transplant and combined liver-kidney transplant have emerged as effective treatment options for severely affected individuals, resulting in substantial reductions in metabolic decompensations and circulating methylmalonic acid concentrations.
Methylmalonic acidemia (mRNA-3705): Our product concept
We are utilizing our ability to produce a complex intracellular enzyme (MUT) that is localized to the mitochondria
MUT is a complex intracellular enzyme that exists as a homodimer, and requires mitochondrial localization and engagement with its cofactor (a derivative of vitamin B12) to be enzymatically active. mRNA has the capability to encode any type of protein, including a functional, intracellular protein that is trafficked to the proper subcellular localization within target cells.
We are developing an mRNA encoding human MUT encapsulated in our proprietary LNPs for IV administration for the treatment of isolated MMA associated with MUT deficiency. The sequence has been engineered to improve protein translation. To function, the mRNA-encoded MUT protein is translocated to its site of action in the mitochondria.
Methylmalonic acidemia (mRNA-3705): Preclinical information
mRNA-3705 shows greater potency and more prolonged lowering of plasma methylmalonic acid compared to mRNA-3704 in animal studies.
We previously demonstrated, in a series of in vitro and in vivo pharmacology studies, that human MUT mRNA effectively directs the biosynthesis of active MUT protein with physiologically correct mitochondrial localization in vitro, and improves survival and corrects biochemical abnormalities in two different mouse models of MMA representing the spectrum of MUT deficiency (mut0 and mut-), as published by us in Cell Reports in 2017 and EBioMedicine in 2019. Technology and process improvements enabled the development of an updated drug product, mRNA-3705, which shows greater potency and better pharmacology compared to mRNA-3704. As shown in the figure below, mRNA-3705 produced approximately 3-times more MUT protein than mRNA-3704 in livers of rats 24 hours after a single intravenous injection.
mRNA-3705mRNA-3705 produced higher levels of MUT protein in the livers of rats compared to mRNA-3704
In MMA mice with partial MUT deficiency (Mut-/-;TgINS-CBA-G715V, mut-), mRNA-3705 showed greater potency and prolonged the reduction in plasma methylmalonic acid levels compared to mRNA-3704 in a 4-week study, as shown in the figure below. This data suggests that the minimal efficacious dose may be lower for mRNA-3705 than for mRNA-3704.
mRNA-3705 treatment produced a greater and more sustained reduction in plasma methylmalonic acid compared to mRNA-3704 in a 4-week study in mut- mice
Furthermore, mRNA-3705 treatment of a more severe MMA mouse model (Mut-/-;TgINS-MCK-Mut, mut0) improved survival, increased body weight, and produced a sustained reduction of plasma methylmalonic acid levels compared to PBS treatment in a 12-week study.
Methylmalonic acidemia (mRNA-3705): Clinical plan
We are conducting a global natural history study and a Phase 1/2 clinical trial in North America and Europe
We are conducting a global natural history study in methylmalonic acidemia, or MMA, and propionic acidemia, or PA, that was initiated in 2018. Our natural history study aims to identify and correlate clinical and biomarker endpoints for both MMA and PA. The natural history study is a global, multi-center, non-interventional study for patients with confirmed diagnosis of MMA due to
MUT deficiency or PA. Up to 60 MMA patients and up to 60 PA patients in the United States and Europe will be followed prospectively for 1-3 years. Enrollment in the study has been completed. Retrospective data are also being collected as available.
We recently ceased development of mRNA-3704 and will return to the clinic with the next generation formulation, mRNA-3705. The new drug product mRNA-3705 has demonstrated greater potency and prolonged lowering of methylmalonic acid in mutase deficient mice when compared to mRNA-3704. An open-label, multi-center, dose finding Phase 1/2 study of mRNA-3705 in patients with isolated MMA due to MUT deficiency between 1 to 18 years of age is in planning and will enter clinic in 2021. The objectives of this study are to evaluate the safety, pharmacodynamics (as assessed by changes in plasma methylmalonic acid), and pharmacokinetic profile of mRNA-3705 in patients affected by MMA.
Phenylketonuria (PKU) (mRNA-3283)
Our approachMMA therapy candidate, mRNA-3705, encodes for a missing or deficient hepatic enzyme. In an ongoing Phase 1/2 study, fifteen participants have been dosed. Thus far, all eligible participants have opted to Phenylketonuria with an mRNA encoding for an intracellular protein
Phenylketonuria, or PKU, is a rare inherited metabolic disease resulting from a deficiencyparticipate in the metabolism of phenylalanine, or PHE,Open-Label Extension study. To date, mRNA-3705 has generally been well-tolerated with no discontinuations due to mutations within the enzyme phenylalanine hydroxylase,safety or PAH. The most effective treatment is a restrictive diet of low protein, which controls PHE intake. Approximately 20-56% of PKU patients respond to sapropterin dihydrochloride (marketed as Kuvanmeeting protocol defined dose limiting toxicity criteria. Interim results demonstrated encouraging initial pharmacodynamic data, with dose-dependent reductions in the United States), a synthetic BH4 cofactor for PAH which improves PHE metabolism, but does not fully cure patients. In addition,methylmalonic acid in May 2018, Biomarin received approval for pegylated phenylalanine lyase, or PAL, marketed as Palynziq. Palynziq is a pegylated recombinant bacterial enzyme which metabolizes PHEcohorts 2 and 3. Early results suggest potential promising changes in the blood. We believe the immune risk is, at least in part, driven by bacterial PAL. With our mRNA technology, cells in the human body can be instructed to produce functional PAH, decreasing PHE levels in the blood and restoring production of tyrosine.clinical endpoints. We are developingcurrently dosing our fifth cohort as we prepare to select an intravenously administered mRNA which encodesoptimal dose for the PAH enzyme and is encapsulatedexpansion. We expect to advance mRNA-3705 into a pivotal study in our proprietary LNP. We plan to conduct a Phase 1 clinical trial for mRNA-3283.2024.
Phenylketonuria (mRNA-3283): Disease overview
There are options to treat PKU which are not widely applicable, and efforts by other companies are likely to face hurdles
PKU occurs in approximately 1:10,000-15,000 live births in the United States. Based on current population estimates that would translate into approximately 21,000-32,000 PKU patients in the United States. Affected individuals have a deficiency in the enzyme PAH, resulting in a reduced or complete inability to metabolize the essential amino acid phenylalanine into tyrosine. Thus, PKU patients suffer from a phenylalanine intoxication and a subsequent deprivation of tyrosine, leading to severe mental disability if left untreated.
PAH is expressed as a monomer, but functions as a tetramer and requires tetrahydrobiopterin (BH4) as a cofactor to complete the conversion of PHE to tyrosine, thereby maintaining adequate PHE:TYR ratios within circulation. To date, greater than 950 gene variants have been identified in the PAH gene, resulting in PKU.
Diagnosis of PKU occurs primarily through newborn screening in available countries, followed by genetic confirmation. Newly diagnosed patients receive medical formulas containing protein with low PHE content to control blood PHE and provide adequate nutrition for growing infants. As patients age, they are tested for sensitivity to synthetic BH4 and may transition to Kuvan. Approximately 20% of patients respond favorably to Kuvan, which can aid in PHE control. Nonresponsive patients are treated mainly with restricted diet; however, adherence to the diet is challenging, resulting in poor compliance. When PHE levels are not adequately controlled, patients begin to show multiple signs of disease, including depression, anxiety, poor executive function, and attention deficit hyperactivity disorder, or ADHD.
One option for PKU patients may be treatment with gene therapy. We believe there are potential advantages for mRNA therapeutics for this disorder over gene therapy as described in the systemic intracellular therapeutics modality section.
Phenylketonuria (mRNA-3283): Our product concept
We intend to utilize the cells in the human body to produce PAH intracellularly
We believe mRNA therapy is a viable therapeutic modality for PKU patients due to its ability to instruct cells in the human body to produce complex functional intracellular proteins such as PAH. Our program mRNA-3283 consists of an mRNA encoding human PAH encapsulated in our proprietary LNPs. The mRNA sequence is optimized for protein synthesis and contains a microRNA
binding site to reduce or potentially eliminate synthesis of protein outside of the target tissues. mRNA-3283 is designed to be administered intravenously to encode enzymatically-active PAH protein in liver to restore this deficient or defective enzyme.
Phenylketonuria (mRNA-3283): Preclinical information
We have demonstrated the ability to impact PHE levels by repeat dosing of our mRNA in preclinical studies
We have conducted several in vitro and in vivo pharmacology studies to demonstrate preclinical proof-of-concept for PAH therapy. A PKU mouse model demonstrated a significant reduction of blood PHE levels post dose as shown in the figure below. The study included IV administration of PAH mRNA every 7 days at 0.5 mg/kg in a PAH-/- mouse model. PHE level was measured using liquid chromatography with a combination of two mass analyzers (LC-MS/MS). The IND-enabling GLP toxicology program for PKU (mRNA-3283) is ongoing.
PHE reduction with repeat dosing of PAH mRNA in PKU mouse model study
Phenylketonuria (mRNA-3283): Clinical plan
We plan to conduct a Phase 1 open label clinical trial with single ascending dose to evaluate the safety, tolerability, and activity of our development candidate in patients.
Glycogen storage disease type 1a (GSD1a) (mRNA-3745)
Our approach to glycogen storage disease type 1a using an mRNA encoding for intracellular human glucose 6-phosphatase
Glycogen storage disease type 1a (GSD1a) is an inherited metabolic disease caused by the deficiency in the catalytic activity of glucose 6-phosphatase (G6Pase), which is encoded by the glucose 6-phosphatase gene (G6PC). The G6Pase enzyme is involved in the metabolic pathways of glycogenolysis and gluconeogenesis which allow the liver and kidney to release glucose into the blood. Those affected by GSD1a present with life-threatening hypoglycemia and a wide range of severe metabolic derangements and long-term complications such as hyperlipidemia, lactic acidemia, hepatomegaly, hepatocellular adenomas, and end-stage renal disease. The standard of care consists of strict diet control. Enzyme replacement therapy (ERT) is not an option for these patients due to challenges associated with delivering an enzyme inside the cell. Strict diet control via the frequent consumption of uncooked cornstarch is effective in improving hypoglycemia. However, the underlying pathologies continue and its efficacy in preventing the long-term metabolic complications has yet to be established. With our mRNA platform, cells in the liver may be instructed to produce functional G6Pase, with the goal of restoring the homeostasis of glycogenolysis and gluconeogenesis pathways and correcting the underlying pathologies. We are developing an intravenously administered mRNA which encodes for G6Pase and is encapsulated in our proprietary LNP. We have demonstrated activity in mouse models in the form of reduction in both liver and serum biomarkers and improvements in liver morphology. We plan to conduct a Phase 1 clinical trial for mRNA-3745.
Glycogen storage disease type 1a (mRNA-3745): Disease overview
There are no approved therapies for GSD1a that address the enzymatic deficiency
GSD1a is ana rare, inherited metabolic disorder caused by a deficiency in the catalytic activity of G6Pase. G6Pase catalyzes the hydrolysis of glucose-6-phosphate tointracellular protein glucose and inorganic phosphate, the final step of glycogenolysis and gluconeogenesis that mainly takes place in liver and kidney.6-phostphatase (G6Pase). GSD1a patients suffer from severe fasting hypoglycemia, hepatomegaly, nephromegaly, lactic acidemia, hypertriglyceridemia, hyperuricemia, hypercholesterolemia, hepatic steatosis and growth retardation. In addition, hepatocellular adenomas occur in 70% to 80% of GSD1a patients by their third decade of life and carries risk of transformation into hepatocellular carcinomas. Proteinuria has been observed in over half of patients above 25 years of age.
GSD1a occurs in approximately 1:100,000 live births in the United States and European Union but is more common in Ashkenazi Jews where the incidence is reported to be 1:20,000 live births. There are an estimated 2,500 people in the United States and over 4,000 people in the European Union with GSD1a. Although strict diet therapy, including frequent feeding with uncooked cornstarch, allows GSD1a patients to live into adulthood by preventing hypoglycemia, the underlying pathological processes remain uncorrected resulting in the development of many long-term complications including liver adenomas and hepatocellular carcinoma. While gene therapy is being investigated for treatment of GSD1a, we believe there are potential advantages for mRNA therapeutics for this disorder over gene therapy.
Glycogen storage disease type 1a (mRNA-3745): Our product concept
We intend to utilize the cells in the human body to produce G6Pase intracellularly
We believe that our platform can address GSD1a with its ability to instruct cells in the human body to produce complex functional intracellular membrane proteins such as G6Pase. Our program,therapy candidate, mRNA-3745, consists of an mRNA encoding for modified human G6Pase, and has been granted Orphan Drug Designation by the FDA and the European Medicines Agency (EMA). A Phase 1/2 study to evaluate the safety and pharmacology of mRNA-3745 in GSD1a patients 18 years of age and older is ongoing. We have observed encouraging signs of clinical benefit with mRNA-3745.
Ornithine transcarbamylase (OTC) deficiency (mRNA-3139)
Ornithine transcarbamylase (OTC) deficiency (OTCD) is an X-linked recessive disorder that is the most common urea cycle disorders (UCDs) in humans. OTCD prevents the breakdown and excretion of ammonia, allowing ammonia to accumulate, rising to toxic levels where it affects the central nervous system. With an incidence of approximately 1:57,000 live births, OTCD accounts for nearly half of all UCDs. OTCD causes high mortality and morbidity, particularly in males.
Our OTCD therapy candidate, mRNA-3139, is in preclinical development. mRNA-3139 is a chronic intravenous, mRNA, enzyme replacement therapy for OTCD, which may act as a bridge to liver transplant or a standalone therapeutic depending on efficacy. mRNA-3139 uses the same LNP as in our GSD1a program.
Phenylketonuria (PKU) (mRNA-3210)
PKU is a rare inherited metabolic disease, affecting approximately 40,000 patients in the United States, France, Germany, Italy, Spain and the UK. Mutations in the phenylalanine hydroxylase (PAH) gene encoding the PAH enzyme result in the inability to metabolize the essential amino acid Phe to Tyr in the liver. There is a high unmet medical need for patients with PKU with early and continuous treatment throughout life being fundamental to prevent the development of irreversible neuropsychiatric outcomes.
Our PKU therapy candidate, mRNA-3210, which is in preclinical development, is an mRNA encoding the PAH enzyme encapsulated in the same LNP as that used in our MMA and PA product candidates, with the potential to address the unmet need in PKU patients.
Crigler-Najjar Syndrome Type 1 (CN-1) (mRNA-3351)
CN-1 is a severe condition caused by the mutations in the UGT1A1 gene. CN-1 is characterized by high levels of a toxic substance called bilirubin in the blood (hyperbilirubinemia). It is caused by mutations in the UGT1A1 gene, which results in an inability to break down bilirubin, a substance made by the liver. Without the UGT1A1 enzyme, bilirubin can build up in the body and lead to jaundice and damage to the brain, muscles and nerves. The symptoms become apparent shortly after birth and can be life-threatening. It is estimated that there are only approximately 70-100 known cases of CN-1 in the world. Affected individuals rely on current standard of care, phototherapy treatments of up to 12 hours a day, throughout life. The only definitive treatment is liver transplant, which is associated with its own set of side effects and risk of death.
Our CN-1 therapy candidate, mRNA-3351, consists of an mRNA encoding human UGT1A1 encapsulated in our proprietary LNPs. The human G6Pase sequence is modified for improved protein production and G6Pase activity. mRNA-3745It is designed to restore the missing or dysfunctional protein that causes CN-1. We have licensed mRNA-3351 to Institute for Life Changing Medicines (ILCM) with no upfront fees and without any downstream payments. The goal of the collaboration is to make an mRNA therapy for the treatment of CN-1 available at no cost to patients. We are collaborating with the ILCM in developing a preclinical package for Investigational New Drug application and Clinical Trial Application filings. ILCM will be administered intravenously and encodes G6Pase protein to restore this deficient or defective enzyme.responsible for the clinical development of mRNA-3351.
Glycogen storageINHALED PULMONARY THERAPEUTICS
Our inhaled pulmonary therapeutics modality currently has one product candidate.
Cystic Fibrosis (CF) (mRNA-3692/VX-522)
CF is a rare genetic disease, type 1a (mRNA-3745): Preclinical informationwhich is progressive from birth and leads to multi-organ damage and early death due to lung dysfunction. It is caused by the mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, which results in the loss of CFTR chloride ion channel function. This decreased function of CFTR at the cell surface leads to thick, sticky mucus in multiple organ systems but most pathologically the lungs. There are approximately 92,000 patients living with cystic fibrosis in the United States, Europe, Australia and Canada, with over 5,000 of these patients not being able to benefit from the approved CFTR modulators.
We are collaborating with Vertex on our CF candidate, mRNA-3692/VX-522, which is designed to treat the underlying cause of CF by enabling cells in the lungs to produce functional CFTR protein for the treatment of the 10% of patients who do not produce any modulator-responsive CFTR protein. This would be our first demonstration of a nebulized mRNA therapy.
Vertex has initiated a Phase 1, single ascending dose clinical trial in CF patients who cannot benefit from CFTR modulators, and the FDA has granted VX-522 Fast Track designation. The trial is active and enrolling patients. Vertex expects to complete the single ascending dose study and to initiate the multiple ascending dose study.
SYSTEMIC SECRETED AND CELL SURFACE THERAPEUTICS MODALITY
Our systemic secreted and cell surface therapeutics modality currently has two active development programs, of which one has entered the clinic.
Relaxin (mRNA-0184)
Relaxin is a naturally occurring hormone that has been shown to promote vasodilation and angiogenesis, regulate extracellular matrix turnover and suppress arrhythmias post myocardial infarction. Relaxin plays an important role in women in pregnancy, but in addition, studies have demonstrated the abilitypointed to improve hypoglycemiaits vasodilatory, antifibrotic, anti-inflammatory and other metabolic abnormalities associatedprotective effects on multiple organs. There is a large body of evidence to support relaxin’s clinical potential in several therapeutic areas, with GSD1aits impact on cardiovascular diseases having been studied in both preclinical and clinical settings. Though prior studies have failed to demonstrate long-term benefit in clinical studies, we believe a mouse modelnovel approach can overcome potential flaws of previous approaches.
We have conducted severalare developing mRNA-0184, which encodes for a relaxin fusion protein, to treat decompensated heart failure. Acute heart failure is defined as the new onset or worsening of symptoms and signs of heart failure. In developed countries, heart failure has become a substantial public health problem, affecting 2% of the adult population and acute heart failure is the most frequent cause of unplanned hospital admission in vitropatients over 65 years of age. mRNA-0184 encodes for the relaxin fusion protein. The mRNA sequence of mRNA-0184 is engineered to increase protein expression and in vivo pharmacology studies to demonstrate preclinical proof-of-concept for GSD1a therapy. mRNA encoding for G6Pase introduced in human cells resulted in robust production of active G6Pase with subcellular localization into endoplasmic reticulum. We have examined the activity of mRNA encoding for human G6Pase in a liver-specific G6Pase -/- mouse model (G6PC.LKO). Like GSD1a patients, the G6PC.LKO mice are unable to produce endogenous glucose, leading to severe hypoglycemia during the fasting state.prolong half-life.
In December 2022, we initiated dosing in a dose-responsePhase 1 trial for mRNA-0184 and the trial is ongoing. Our Phase 1 trial is an adaptive, open-label, single ascending dose to single-blind, placebo-controlled, multiple ascending dose study performedto evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of mRNA-0184 in G6PC.LKO mice, we treatedparticipants with chronic heart failure.
PD-L1 (mRNA-6981)
PD-L1 is a co-inhibitory receptor that can induce anergy in programmed cell death protein 1 (PD-1)-expressing T cells. We intend to induce expression of PD-L1 on myeloid cells to send a tolerizing signal to immune cells in their environment in order to treat autoimmune diseases.
The PD-L1/PD-1 pathway has a critical function in immune regulation and promotes development and function of regulatory T (Treg) cells. PD-L1 is a transmembrane protein expressed on antigen presenting cells, such as dendritic cells and macrophages, activated T cells, B cells and monocytes, as well as peripheral tissues. Its cognate receptor, PD-1, is a co-inhibitory transmembrane protein expressed on T cells, B cells, natural killer cells and thymocytes. Preclinical mouse models deficient in PD-1 spontaneously develop a variety of autoimmune diseases such as arthritis, myocarditis, lupus-like glomerulonephritis and type 1 diabetes, demonstrating the micecritical role of the PD-L1/PD-1 interaction in maintaining tolerance to self-antigens. Additionally, treatment of cancer patients with three different dosesPD-1 or PD-L1 inhibitors sometimes results in immune-related adverse events, including the development of 0.2, 0.5,hepatitis, dermatitis and 1 mg/kgcolitis, demonstrating the role of G6Pase mRNA encapsulatedPD-1/PD-L1 in human autoimmune reactions.
We believe our proprietary LNPPD-L1 therapy may augment PD-L1 expression on cell types similar to those that endogenously express it, and examined fasting glucose, serum triglycerides, and liver enzymes (n=5-8). Of note, mice treated with G6Pase mRNA showedby reducing immune activation, potentially reduce the clinical manifestations of a dose-dependent improvement in fasting glycemia and a reduction in serum triglycerides, without a significant increase in liver enzymes (e.g. alanine transaminase - ALT). Fasting blood glucose and triglycerides are shownvariety of autoimmune diseases. Our intent is to use our platform to influence myeloid cells, including dendritic cells, to provide additional co-inhibitory signals by augmenting endogenous expression of PD-L1. We believe that this tolerizing signal to lymphocytes may limit autoreactivity in the figure below. Each bar representscontext of ongoing autoimmune pathology without severe and global suppression of the mean ± standard deviation. Singleimmune system. Given that our platform allows us to modify myeloid cells in situ, our approach to the creation of a tolerogenic environment may provide unique benefits in treating autoimmune diseases by seeking to restore immune homeostasis.
After undertaking preclinical development, we determined that the current design of mRNA-6981 does not meet our criteria for advancement to the clinic. PD-L1 continues to be an area of interest, and double asterisk denotes p < 0.05 and p < 0.0001, respectively, by one-way ANOVA, followed by Dunnett’s post-hoc test for multiple comparisons.we are currently evaluating other preclinical mRNA candidates.
Serum biomarkers after single dose of G6Pase mRNA in G6PC.LKO mice
In the same study, a reduction in liver weight compared to the control-treated group was observed after 24 hours of administration of G6Pase encoded mRNA in LNP. The reduction in liver weight was associated with significant improvement in liver morphology presumably due to reduction in liver glucose 6-phosphate, glycogen, and triglycerides.
Reduction in liver weight 24 hours after IV administration of G6Pase mRNA in G6PC.LKO mice
In addition, data for a 7-week repeat-dose study in G6PC.LKO mice receiving G6Pase mRNA in LNP at 0.25 mg/kg IV every other week have shown a pronounced improvement in fasting glycemia, in comparison with the G6PC.LKO mice receiving a control mRNA treatment as shown below (n=7-9).
Restoration of blood glucose above therapeutic threshold with repeat dosing of G6Pase mRNA in G6PC.LKO mice
Glycogen storage disease type 1a (mRNA-3745): Clinical plan
We are planning a Phase 1 clinical trial
We plan to conduct an open-label, dose escalation Phase 1 study of mRNA-3745 in adolescent and adult patients with GSD1a in the United States. The objectives of this study are to evaluate the safety and tolerability of mRNA-3745, to assess the pharmacodynamic response through changes in maintenance of euglycemia, and to characterize the pharmacokinetic profile of mRNA-3745.
MANUFACTURING (PRODUCT SUPPLY AND TECHNICAL DEVELOPMENT)
Manufacturing plays a critical role in our value chain and our ability to develop a new class ofour medicines. Our manufacturing capabilities support every stage of the development of our Research and Early Development Engines, in additionproducts, from discovery to our Late Stage Development and Commercial Engines. These capabilities have rapidly accelerated as a resultcommercialization. During the research stage of COVID-19 vaccine production.
Within the Research Engine,product development, manufacturing provides mRNA drug substance and drug product for platform research and therapeutic area drug discovery. For the Early Development Engine,During early development of our product candidates, we manufacture mRNA and drug product for IND-enabling GLP toxicology studies and initial human clinical studies. For the Late Stage Development Engine,late clinical development, we produce mRNA and drug product for phasePhase 3 studies. In addition, withintrials. At the Commercial Engine,commercial stage, we manufacture drug substance and drug product in collaboration with our contract manufacturing organizations or CMOs,(CMOs), both in the U.S.United States and internationally. Our approach
In 2023, as the COVID-19 vaccine market moved from a pandemic to date has beenan endemic market, we significantly resized our manufacturing infrastructure and reshaped our supply capabilities to proactively invest and build manufacturing capacity internally and externally with our network of strategic partners in anticipation of demand. This capacity planning was immediately leveraged and expanded duringhelp position our COVID-19 vaccine ramp-up into a commercial product in response to the ongoing pandemic.franchise for future profitability.
Overview of our manufacturing operating model
Our manufacturing activities generally focus on the following:on:
•Commercial Production: Our manufacturing expertise includescapabilities include state-of-the-art technologies for mRNA and drug productsubstance manufacturing, as well as quality control testing to attain a robust and consistent supply that matches target product profiles. Our manufacturing technology is built to scale-up and support industrializationproduction of products for commercial approval.
•Research and Development Support:The product supply enables platform research and drug discovery in our therapeutic and vaccine areas, in addition to activities related to clinical studies of our investigational medicines.product candidates.
Given our expectations for significant ongoing pipeline expansion and the long lead time required to build manufacturing infrastructure, weWe have built a dedicated in-house, multi-building manufacturing facilitycampus in Norwood, MA,Massachusetts, the Moderna Technology Center (MTC). The campus is comprised of two distinct buildings (MTC South and MTC North). MTC South is approximately 200,000 square feet, with a production capacity of over 100 cGMP lots per year. MTC North is approximately 240,000 square feet, directly supporting improvement inprovides supply for our manufacturing capabilities. MTC supports our Research Engine supply,preclinical research, IND-enabling GLP toxicology study supplies, our Phase 1 and Phase 2 pipeline activities, later-stage clinical development activities (e.g., Phase 3 CMV vaccine clinical trials), as well as COVID-19drug substance commercial production for vaccines. Our vaccine drug substance production.
production for the U.S. market is completed at our MTC campus. The MTC has been designed to allow us to continue to optimize our mRNA products as we explore new pharmaceutical delivery forms in our manufacturing network such as prefilled syringes. The MTC campus has been designed with a high level of automation and state-of-the-art digital integration to handle manufacturing execution, product testing and release, and regulatory filings.
In the second quarter of 2023, we acquired a newly constructed, 140,000 square feet biomanufacturing facility in Marlborough, Massachusetts. The facility is currently undergoing enhancements, including the addition of 60,000 square feet. We expect the facility to be operational in 2025. This new site is strategically intended to support our INT program.
We have also announced agreements with the governments of Australia, Canada and the United Kingdom to establish state-of-the-art mRNA manufacturing facilities in those countries, pursuant to which each government has entered into a multi-year commitment to purchase mRNA products from us, once approved. We expect that these local manufacturing facilities will provide direct access to rapid pandemic response capabilities and our respiratory virus vaccine candidates. We may seek to enter into future agreements with other governments to provide similar manufacturing capabilities in other geographies.
In addition substantialto our internal manufacturing capabilities are realized via CMOfacilities, we also maintain relationships with CMOs in the U.S.United States and abroad, providing drug substance and fill finishfill-finish capacity for our vaccines. As noted above, during the third quarter of 2023, we resized our manufacturing footprint to reduce our commitments to certain CMOs as a result of lower anticipated demand for COVID-19 vaccine.vaccines in the endemic market.
Manufacturing Technology Developmenttechnology development
To support our broad pipeline of products, which span multiple therapeutic areas and routes of administration, (e.g., intramuscular, intratumoral,our platform research and intravenous), there is close collaboration between our Platform Research and Technical Developmenttechnical development teams closely collaborate to facilitate rapid and seamless clinical translation of scientific breakthroughs. This in turn, enables us to develop potential vaccines and therapiesmedicines to serve a wideningbroad patient population.
Technical Developmentdevelopment encompasses the design and optimization of robust and consistent manufacturing processes, product characterization, fit-for-purpose formulations and product presentations. For instance, our novel hardware platforms’ automation and robotics, coupled with the flexibility of our in-house digital development systems, allows for thousands of experiments and process parameters across our projects, thus supporting our drug product pharmaceutical readiness. Moreover, our recent technical manufacturing advances have enabled internalization of new key capabilities, including DNA plasmids and small molecules.
In parallel, we have refined existing processes, resulting in increased manufacturing scale and more robust stability of our mRNA and drug product.profiles. These improvements allow us significant control over our supply chain, resulting in larger production yields and longer shelf life of our products. Furthermore, formulation development advancements have added new drug product images, including lyophilization, giving us a path from frozen to refrigerated storage conditions.
Our substantial investments in recent years in Technical Developmenttechnical development has enabled the breadth and depth of our pipeline, and laid the foundation to help meet the needs and requirements associated with late stagelate-stage development and the commercialization of the COVID-19 vaccine.our products.
Supply of mRNA for the ResearchAll Stages of Product Development and Early Development EnginesCommercialization
Supply for the Research Engine
High-throughput automation and custom engineered equipment allow us to produce and deliver high quality mRNA and formulated constructs in a short period of time: our proprietary platform is capable of producing up to 1,000 lots of mRNA sequences and formulations per month with a turnaround time of a few weeks from sequence to final product. The typical scale of mRNA manufactured by this team is 1–1,0001-1,000 mg. Since 2014, we have produced over 27,000 batches of research-grade mRNA. This has been possible, in part, thanksdue to the ability of researchers in the Moderna ecosystem to order constructs through an integrated digital portal that tracks materials end-to-end in less than 45 days. In addition, multiple integrated algorithms that leverage artificial intelligence and machine learning optimize manufacturability, reduce failures and increase quality of mRNA sequences.
Supply for the EarlyClinical Development Engine
Analogous to the Research Engine, weWe have established manufacturing capabilities that support the Early Development Engineearly development stage of product development in three key areas: GLP Tox, Clinical Studies and Personalized Cancer Vaccines.INTs. We supply mRNA and formulated product to conduct IND-enabling GLP toxicology studies. In addition, human clinical studies rely on supply to meet required cGMP standards. This is achieved via internal manufacturing at the MTC and external manufacturing at well-established contract manufacturing organizations (CMOs). We selected specialized CMOs to support a total of five programs in late 2015. We will continue to selectively partner with CMOs to complement our capacity and provide supply contingency where needed.campus. Our MTC facilitycampus is also suited to enable rapid technology development and scale-up for future needs.
Our manufacturing also produces cGMP Personalized Cancer Vaccines (PCVs).INTs. Due to the specialized nature of personalized medicine (i.e., where a batch is specifically designed and manufactured for a single patient), the manufacturing Personalized Vaccine Unit (PVU)process for INTs has unique requirements. We digitally integrate patient-specific data from sequencing tumor samples to automatically design PCVsINTs for patients. We have developed proprietary bioinformatics designed algorithms linked to an automated manufacturing process for rapid production of formulated mRNA, with a typical turnaround time of a few weeks. We have operationalized PCVINT manufacturing at the MTC campus to meet our Phase 1 and 2 pipeline supply needs by using single-use systems with fast “needle-to-needle” turnaround times. Unlike traditional process development, each PCVINT batch is manufactured for a single patient and thus scaled-out (in parallel) with extensive use of automation and robotics to account for the larger number of patients involved in later phases of development and commercialization. We have shown consistent quality in our production of over 160many patient batches, each with unique mRNA sequences. In the second quarter of 2023, we acquired a new manufacturing facility in Marlborough, Massachusetts, which we expect to support our INT program.
TheseOur manufacturing capabilities have allowed us to build our broad pipeline of 24 development candidates,programs, including the output required to supply related toxicological and human clinical studies. While the technology that underpins these 24 programs is the same, each program typically requires customization based on target product profiles. These custom features range from varying molecular architecture to different routes of administration, often requiring multivalent products. For example, our CMV vaccine (mRNA-1647) requires six different mRNA sequences to be manufactured for inclusion in an intramuscular mRNA medicine, whereas OX40L (mRNA-2416) requires a single mRNA sequence for inclusion in an intratumoral mRNA medicine. All programs, with the exception of PCV,INT, require that we progressively scale up supply to meet clinical demand requirements across development phases, in addition to the necessary preparation for regulatory approval and commercial production, which demand larger batch sizes. In contrast, the PCVINT program seeks to develop a cancer vaccinetherapeutic that is designed and manufactured for a specific patient, thus increasing the number of unique batches. As we scale manufacturing output for each program, we plan to continuously improve yield, purity and the pharmaceutical properties of our developmentproduct candidates.
Supply for the Late-Stage Development and Commercialization Engines
As we continue to manufacture theour COVID-19 vaccine,vaccines, our development pipeline continues to advance to later-stage development and towards commercialization. Our platform approach allows us to continue to evolve our manufacturing suites and other capabilities at our MTC campus. Building expansionsmanufacturing facilities. mRNA manufacturing is flexible and enhancements have continued throughout scale-up of our COVID-19 vaccineone plant can manufacture multiple vaccines and therapeutics. Our manufacturing capabilities. The modular nature of the MTC suites permitsfacilities also permit us to manufacture multiple products in parallel. For instance, we can produce drug substance and drug product for our phasePhase 3 CMV clinical trial while manufacturing COVID-19 drug substance.substance in the same facilities.
Quality Unit
Quality is core to the way we operate. We seek to ensure quality at Moderna through a combination of a robust Quality Management System (QMS), our quality culture and our people. In accordance with applicable regulations, we have established, documented and implemented a QMS to assure continued compliance with the requirements therein. The QMS facilitates cGMP compliance by implementing practices that identify the various required processes, their application throughout the organization and the sequence of interaction of these processes.
The primary mode of documenting these key practices is through policies, standard operating procedures (SOPs), forms and other quality records, which include an overarching Quality Policy and Quality Manual. We have implemented measurement tools and metrics to monitor, measure, and analyze these practices to support cGMP operations, achieve planned results, and support continuous improvement. We monitor these quality metrics through formal governance processes, including Quality Management Review (QMR), to enable continuous improvement. We have also established an independent Quality Unit that fulfills quality assurance and quality control responsibilities.
Our Quality Unit grew into an international organization with the introduction of COVID-19 vaccine manufacturing. Quality drives our quality cultureEnvironment, Health, and ensures it is applied consistently and thoughtfully across the globe.
While the Quality Unit is ultimately accountable and responsible for quality, this is everyone’s responsibility. All cGMP personnel are empowered to ensure quality systems are appropriately maintained and executed.Safety
We have established a cultureglobal Environment, Health, and Safety (EHS) organization to foster a safe and healthy work environment with a focus on compliance and sustainability. We achieve this through a combination of training, procedures, digital data collection and reporting tools, and corporate programs that encourages transparency, accountability, and ownership of quality at all levels in the organization. As we scale the quality organization, we have focused on hiring the best talent with the required experience, training, and education.drive towards continuous improvement.
Supply Chain Unit
We have established an internationala global supply chain to enable supply of the raw materials and components used to produce our mRNAs and the components of our formulations, securing supply for COVID-19 vaccine alongsideproducts, consistent with clinical and preclinical demands. We have worked with our supply chainexternal vendors to characterize critical raw materials and to understand their impact on the quality of mRNA drug substance and formulated drug product. We also assess the quality system and performance of our supply chainexternal vendors and work with them to comply with regulatory requirements.
DIGITAL INFRASTRUCTUREAND AI STRATEGY
We believeSince our founding, we have been a digital-first company, seeking to use the power of digital information to maximize our impact on patients. mRNA is an information molecule, and our company was built on the premise that the natural flow of information in life can be used to develop medicines. Leveraging over a decade of experience developing mRNA medicines, we have built a large library of data that, combined with our platform approach and cloud-native infrastructure, positions us well to scale a digital technologies, such as robotics, automation,operating model using artificial intelligence or AI, and cloud computing, are critical to operationalize our strategy, accelerate our pace of learning and execute at scale. Since our inception, we have invested over $100 million in our digital technologies, robotics/automation, analytics, data science and AI. Given our growth, we expect we will invest more than an additional $100 million in digital over the next 5 years. Our approach to bring these digital technologies into our workflows and processes has involved the following:(AI).
•utilizationLed by our Chief Information Officer, our digital organization partners across all Moderna functions to create an integrated AI ecosystem intended to drive performance and, ultimately, greater patient and business impact. To that end, in 2021, we launched our AI Academy, which offers cross-organization training to all of our employees on topics such as data visualization, machine learning algorithms and AI ethics. Through the AI Academy, our employees learn how to leverage AI in their specific job functions. By embedding AI across our organization, we are able to scale the value of our people and progress toward our goal of becoming a consistent set of digital building blocks;real-time AI company.
•applicationAI helps optimize each aspect of our value chain, from drug design to commercial manufacturing and beyond. At the research stage, our digital technologies in multiple business processes; and
•rapid iterations for maximum optimization.
We have seen several benefits AI infrastructure allows our scientists to design novel mRNA constructs, use AI algorithms to optimize them and order them from our investments in digitization, most importantly throughhigh throughput preclinical scale production line. Our capabilities allow us to design mRNA, protein and LNP components with desired properties, such as reduced toxicity or increased stability. At the depthproduct development stage, AI helps to improve the efficiency of our platform technologyclinical trial operations by, for example, forecasting participant enrollment and breadth of our pipeline. Other benefits include:
•Quality: Reduction in human errors by enabling automation, repeatability, and seamless integration;
•Scalability: Growth in our pipeline to 24 programs;
•Speed: Rapid manufacture of research-grade mRNA from the Research Engine; and
•Cost efficiencies: Digital infrastructure utilized across our platform, drug discovery,automating clinical development, and manufacturing to maximize efficiencies.trial data processing.
Our digital building blocksmanufacturing processes likewise utilize the power of AI. For example, we leverage a series of fully autonomous, integrated AI algorithms in connection with manufacturing mRNA-4157, our INT candidate. Our proprietary algorithms design the specific therapy for each individual patient and optimize the timely manufacture and delivery of INT to each patient.
We utilize six building blocks forAt the commercial stage, our digital infrastructure:and commercial organizations came together in 2023 to drive performance and prepare for product launches. Digital and AI are key components of our commercialization strategy, and are vital to our ability to increase our speed to market, enhance our commercial capabilities and continuously improve the quality of our products. We believe that our ability to move with both scale and speed positions us well to pursue our goals related to future product launches.
•Cloud enablement isFurther, as generative AI (GenAI) emerged as a critical componenttransformative technology in 2023, we moved quickly to develop and launch our own internal GenAI product. Recognizing the unique value of Large Language Models (LLMs) to streamline tasks across departments, we launched our internal product in May 2023 after two weeks of development and improved it sequentially thanks to the capabilities of our digital infrastructure. We are at the forefront of mRNA technology. We generate complexupdated machine learning platform Compute 4.0 for rigorous datasets, and our scientists need computational power and agility to operate without being limited by traditional computing technology. Maintaining digital infrastructure in the cloud provides the benefits of lower costs by simplifying provisioning and administration, flexibility, scalability, ease of maintenance, disaster recovery, and information security.
•Integration of business processes enables us to streamline processes and bring data together in a consistent manner, avoiding caches ofinformation and manual intervention. This efficient flow of data between systems enables the automation management. Approximately 75% of our business processes.
•Internet of things allows for smart interconnected devices that provide real-time synchronization of operations. The data from equipmentprovides real-time guidance to our scientists and engineers and helps us in supply chain and manufacturing with compliance and traceability, including tracking material, controlling inventory and optimizing instrument usage.
•Automation allows us to scale our operations reliably and reproducibly. With the help of custom hardware solutions and state-of-the-art robotics,we can continue to increase our operating efficiency, reduce errors, and improve our quality and compliance.
•Advanced analytics enable us to draw insights from our data. Weemployees are constantly generating large data sets that can provide important insights ifmined appropriately and regularly.
•Artificial intelligence, or AI, is enabling key breakthroughs in predictive modeling. It will allow us to improve our mRNA design algorithmsbased on machine learning, and will provide us with critical insights into research, supply chain, manufacturing, and other processes.
Digital technologiescurrently active users, embedding the tool into their specific functions for customized support and meaningful improvements to enabletheir everyday workflows. This rapid adoption across our Research Enginecompany demonstrates the power of our AI-centric culture.
We have deployed multiple digital technologies acrossbelieve that the integrated AI ecosystem we are building at Moderna will accelerate our Research Enginemission to drive a rapid pace of learning, enable efficient workflows and business processes, and draw insights from vast amounts of data. Our aim isdeliver the greatest possible impact to provide our platform and discovery scientists with access to an environment that helps thempeople through each step of their research cycle.mRNA medicines.
Drug Design Studio: Our proprietary in-house digital application suite contains a Sequence Designer module to tailor an entire mRNA, with ever-improvingrule sets that contain our accumulated learning about mRNA design. Drug Design Studio utilizes cloud-based computational capacity to run various algorithms we have developed to design each mRNA sequence. The utility of cloud-based capacity allows us to provide flexible computational capacity on demand, allowing the Research Engine to power parallel intake and design of multiple mRNA sequences. Once a sequence is designed, it can be ordered digitally using an internal order form application within Drug Design Studio.
Manufacture of research grade mRNA: Once an order is optimized, the mRNA production process is triggered. We have developed proprietary interfacesthat allow the manufacturing team to track production orders at every stage. We have automated several manufacturing steps using both off-the-shelf and custom automation. The equipment used in the manufacture of research-grade mRNA is integrated with the digital interfaces to capture, extract, and interpret the data generated at each step of the manufacturing process, building digital traceability on each mRNA order. We have also embedded real-time algorithms and analytics tools to allow for automated decision-making at some stages, accelerate the quality control workflows, and provide for continuous improvement of manufacturing processes.
Dispatching and shipping mRNA: Because we produce large quantities of research-grade mRNA, we require digital tools to track their shipment to ourscientists and to external contract research organizations, or CROs, conducting in vivo studies. Our dispatching and shipping application automatically generates bar-coded labels, allowing for traceability of product.
Inventory and registry: Material used in research and created in production, including mRNA, cell lines, chemicals, and reagents, is tracked in our Inventoryapplication. This application supports numerous workflow tools such as consumption, aliquoting, material transfer, and stock alerts. Critical material types are assigned unique registry identification by our Registry application.
Study design: Using our Drug Design Studio, our scientists can design theirin vivostudies using our proprietary Study Design application. This applicationcaptures in vivo study protocol design parameters, including dose amount, number of doses, frequency, samples, and assays for each sample. This application serves two purposes. It allows our scientists to maintain and track their in vivo study designs and associated research grade mRNA. Our Study Design application also allows our in vivo pharmacology teams to track the various ongoing studies and leverage external CROs to manage the in vivo demand as needed.
Experiment management: We have deployed Electronic Lab Notebooks for experiment management, allowing our scientists to streamline documentation oftheir experiments and track it in a standardized, searchable repository. We have also integrated Electronic Lab Notebooks further with our other research tools to connect inventory, in vivo studies, and instrument data.
Advanced analytics and AI to accelerate the pace of learning: We utilize AI to enable various parts of our platform and drug discovery. Examples include:
•Neural networks for protein engineering: One way to optimize the efficacy of the proteins encoded by our mRNA is to engineer the sequence ofthe protein itself. We use neural networks to analyze and model protein sequences. We train these models by inputting orthologous sequences from thousands of organisms, from which we can generate potential protein sequences optimized for specific attributes.
•Neural networks for mRNA engineering: The redundancy in the genetic code allows for a large number of mRNA sequences that encode thesame protein. mRNA sequence may impact translation, thereby impacting the amount of protein produced in circulation. We are developing AI tools to predict mRNA sequences that can enhance protein expression.
•Automated Sanger sequencing analysis: Sanger sequencing is us used repeatedly to quality check, or QC, our DNA templates and final mRNA; while the data contain every nucleotide in a sequence, it is very complex to analyze. A fully automated data pipeline starts processing raw data the moment it is saved to the cloud by the sequencers. The pipeline spawns numerous AWS computer servers to run an analysis algorithm and then shuts the servers down, resulting in minimal costs. The results are viewable in a powerful, dynamic visualization tool. We have run over 3 million Sanger data files through this system. We have further improved our Sanger analysis with a convolutional neural network, or CNN, to better analyze the tail sections of mRNA as well.
Digital technologies to enable our Early Development EngineCOMMERCIAL
We are building a differentiated commercial model, with active commercial subsidiaries in key markets across North America, Europe and the Asia-Pacific region. Our growing commercial footprint provides us with local commercial teams in major markets where respiratory vaccines have deployed multiple digital technologies across our Early Development Engine to drivehigh utilization rates and sales. To support the rapid pace of advancement, in parallel,build out of our development candidates intocommercial activities in markets around the clinic.globe, we have hired talent with extensive pharmaceutical company experience. Our commercial teams also work with third-party distributors and other partners in countries where we do not have a direct presence. Our commercial activities are dependent on regulatory approvals and on agreements that we have made or may make in the future with strategic collaborators.
Before 2023, we sold our COVID-19 vaccine to the U.S. Government, foreign governments and health ministries, Gavi, on behalf of the COVAX Facility, and other international organizations. During the pandemic, these sales were characterized by a relatively limited number of customers that purchased multi-dose vials for distribution through mass vaccination campaigns. As we have transitioned to an endemic market, we have witnessed a shift in demand to single-dose presentations and pre-filled syringes. In 2023, the COVID-19 vaccine market transitioned to an endemic, seasonal commercial market, characterized particularly in the U.S. (our largest market) by a fragmented customer base, less predictability in orders, seasonality of deliveries, significantly lower demand than during the pandemic and the assumption by us of full distribution costs. The private vaccine market is also characterized by market practices regarding rebates, discounts and returns. The COVID-19 vaccine market continues to depend on many evolving factors such as medical need, viral evolution, public health authority recommendations and consumer motivation to vaccinate.
Digital systems for cGMP manufacture: We are committed to having integrated systems connected with robotics to drivealso advancing a broader seasonal respiratory vaccine franchise, consisting of single-agent and combination vaccines against RSV, seasonal influenza and COVID-19, which have the highest medical burden among respiratory diseases. We expect regulatory approvals beginning in the first half of 2024 for our manufacturing in a paperlessRSV vaccine, wenvironment, and have designed and deployed automation to drive efficient manufacturing operations. We have also deployed digital tools within manufacturing process development that give usith multiple respiratory vaccine commercial launches globally over the ability to track, analyze, and rapidly deploy manufacturing process improvements. Additionally, we have implementednext several digital systems across manufacturing process development, quality, supply chain, and operations, including:years.
•enterprise Quality Management System, or QMS,As we advance our portfolio beyond respiratory diseases toward approval, we are also investing in building out our commercial capabilities for other franchises. These franchises include our latent vaccine, rare disease and oncology products, which we expect to electronically manage deviations, investigation, and correction and preventive actions;
•Laboratory Information Management System, or LIMS, to manage our analytical development data and automate our manufacturing quality control;
•computerized maintenance management system to manage equipment maintenance and calibration; and
•SAP/S4 Hana system for enterprise resource planning, or ERP, manufacturing execution system, and manufacturing control system to manage inventories, track raw material consumption, digitally integrate equipment with manufacturing recipes in batch records, and control automated equipment.
Digital systems for clinical development and clinical operations: In order to track the timelines of various development candidates through the EarlyDevelopment Engine, we have created a set of integrated applications. Workflows include timelines for regulatory filings, planning for IND-enabling GLP toxicology studies, scheduling for cGMP manufacturing, and clinical operations management. Below is a summary of our applications:
•Our portfolio application is a digital interface that maintains and tracks the timelines across multiple workstreams for each of our development candidates.
•The supply application manages the manufacturing schedule of IND-enabling GLP toxicology supplies and cGMP manufacture of clinical supplies to support our programs. This application helps us see how the manufacturing schedule changes over time, identifies supply/demand mismatches, and enables resource planning with real-time alerts should we have any issues.
•The GLP toxicology application tracks the planned and ongoing IND-enabling GLP toxicology studies and allows us to manage timelines with our external vendors.
•The regulatory application tracks timelines related to regulatory affairs including, pre-IND meetings, IND/CTA submission dates, and other planned regulatory interactions.
•Our clinical operations application allows us to track our ongoing trials by accessing clinical operations information in real-time from our CROs. It also has multiple tools and analytics to draw key insights, including, for example, enrollment by trial and enrollment by site to maintain our program timelines.
Digital systems for PCV: The PCV program aims to design, manufacture, and deliver a drug product that includes an mRNA sequence encoding for eachpatient’s specific neoantigens. The personalized nature of the PCV program adds additional steps and complexitylaunch in the overall patient treatment process. We have addressed those additional steps and complexity by digitizing and automating steps within the process, as described below.
•Each patient is provided a unique identifier. We track the entire workflow using a single integrated tracker based on this unique identifier. This is one of many ways we ensure that each patient receives the specific drug product lot manufactured for them.
•We use neural networks to design the mRNA sequences for the PCV program. Our proprietary vaccine design algorithm selects the top twenty neoantigens to be used and determines their amino acid sequences to trigger the desired immune response.
•We utilize Monte Carlo simulations of PCV supply/demand to manage our capacity. Since each drug product lot is personalized to a patient, there is a need to manage supply and demand to avoid bottlenecks at any stage of the workflow.
Digital systems for the Commercial Engine: We are building our commercial engine to establish medical affairs engagements with doctors, support our sales and marketing capabilities and deliver a world-class patient experience. In addition to a patient- and doctor-centric view, our commercial engine will strengthen our supply chain demand forecasting and our compliance. We are looking at building a robust serialization process for regulatory requirements as well as anti-counterfeiting technologies to ensure safe, efficacious medicines to patients.
Digital technologies to support our business processesnext several years.
We have deployed several digital systems across finance,are also working to establish state-of-the-art mRNA manufacturing facilities in Australia, Canada and human resourcesthe United Kingdom, and the government in each of these countries has entered into a multi-year commitment to automate our business processes and drive efficiencies. We have implemented the SAP S4/Hana systempurchase mRNA products from us, once approved. See “—Manufacturing” above for ERP. We have implemented various cloud-based solutions to improve business processes and drive efficiencies. For example, we have implemented the Workday system for human resource planning and management and integrated various applications across payroll, 401k services, equity plan management and expense reporting. Our class-leading integration platform, Dell Boomi, allows us to have a highly interconnected environment, moving us from simple cloud-to-cloud integrations to an evolving use of the integration platform for master data management, systems account management, and ultimately for cost savings and improved user experience.
THIRD-PARTY STRATEGIC ALLIANCES
Strategic alliances
To accelerate the discovery and advancement of potential mRNA medicines across therapeutic areas, weWe have entered into and intend to seek other opportunities to form,strategic alliances with a diverse group of strategic collaborators. We have forged productive strategic alliances withcollaborators, including pharmaceutical and biotechnology companies, government agencies, academic laboratories, foundations and research institutes with therapeutic area expertise and resources in an effortresources. Through our collaborations, we seek to advance our discovery and development programs, while leveraging our platform and our Researchresearch and Early Development Engines.
One key principleearly development capabilities. We also seek to partner with and invest in companies developing other types of therapeutics, such as gene editing and cell-therapy, where we believe we can leverage our core mRNA and LNP capabilities to expand the reach of our approach totechnology.
Through certain of our strategic alliances, is towe share the rewards and risks of developing a new mRNA modality or program, where we may have early research data and desire a strategic collaborator to join us in advancing early development candidates within such modality into the clinic. Representative relationships and associated programs include the following:
•AstraZeneca for the localized regenerative therapeutics modality, such as the VEGF-A program (AZD8601) currently in Phase 2a;
•AstraZeneca for the intratumoral immuno-oncology modality, such as the IL-12 program (MEDI1191) currently in Phase 1;
•those with Merck, for the cancer vaccines modality, such as the personalized cancer vaccine programour INT programs (mRNA-4157) currently in Phase 2 using a workflow that enables a rapid turnaround time to bring personalized vaccines to patients,, and the KRAS vaccine program (mRNA-5671) currently in Phase 1;
•DARPA for the systemic secreted therapeutics modality, such as the antibody against Chikungunya virus program (mRNA-1944) currently in Phase 1;
•Vertex, for the lung delivery modality, such as the cystic fibrosis, orour CF and cystic fibrosis transmembrane conductance regulator, or CFTR program currently in research;
•Vertex for gene editing, aimed at the discovery and development of LNPs and mRNAs for the delivery of gene-editing therapies for the treatment of CF; and
•Chiesi Farmaceutici S.P.A. for the discovery and development of potential mRNA medicines for the treatment of pulmonary arterial hypertension.(mRNA-3692).
We view strategic alliances as important drivers for accelerating execution of our goal of rapidly developing mRNA medicines to treat patients across a wide range of medical and disease challenges. To maintain the integrity of our platform, the terms of our agreements with our strategic collaboratorscollaboration agreements generally provide that either we receive rights to develop and commercialize potential mRNA medicines that we design and manufacture or our strategic collaborators receive rights to develop and commercialize potential mRNA medicines that we design and manufacture, as opposed to granting rights to our strategic collaborators to use our platform to generate new mRNA technologies, and that we generally own mRNA-related intellectual property related to our platform arising from research activities performed under
the strategic alliance. We plan to continue to identify potential strategic collaborators who can contribute meaningful resourcestechnology and insights to our programs and allow us to more rapidly expand our impact to broader patient populations.
AstraZeneca (NASDAQ: AZN)—Strategic Alliances in Cardiovascular and Oncology
We have had three alliances with AstraZeneca, two of whichBelow are ongoing. Our first strategic alliance established in 2013 and amended and restated in 2018, was to discover, develop, and commercialize potential mRNA medicines for the treatment of cardiovascular and cardiometabolic diseases, as well as selected targets for cancer. The relationship with AstraZeneca was expanded in 2016 by entering into a new immuno-oncology strategic alliance which is now focused on the joint development of an mRNA investigational medicine to make the IL-12 protein. It was further expanded in 2017 by entering into a third strategic alliance focused on the joint development of a potential mRNA medicine to make the relaxin protein, following discovery and preclinical development of the relevant development candidate internally. This last strategic alliance related to relaxin was terminated in December 2020. Additionally, AstraZeneca made several equity investments in Moderna, totaling approximately $290.0 million through December 31, 2020, although AstraZeneca has disclosed it is no longer a Moderna shareholder as of that date.
2013 Agreements with AstraZeneca, amended and restated in 2018
In March 2013, we entered into an Option Agreement and a related Services and Collaboration Agreement with AstraZeneca, which were amended and restated in June 2018 (2018 A&R Agreements). Under the 2018 A&R Agreements, we granted AstraZeneca certain exclusive rights and licenses to research, develop and commercialize potential therapeutic mRNA medicines directed at certain targets for the treatment of cardiovascular and cardiometabolic diseases and cancer, and agreed to provide related services to AstraZeneca. The activities to be performed by the parties under the 2018 A&R Agreements are limited to defined biological targets in the cardiovascular and cardiometabolic fields and one defined target in the cancer field.
As of the effective date of the original Option Agreement and Services and Collaboration Agreement in 2013, AstraZeneca made upfront cash payments to us totaling $240.0 million in exchange for the acquired options and our performancebrief descriptions of certain research-related services, each as described above. AstraZeneca will pay us a $10.0 million option exercise payment with respect to each development candidate (and related back-up candidates) for which it exercises an option. We are also eligible to receive, on a product-by-product basis, up to $400.0 million in aggregate contingent option exercise payments upon the achievement of certain development, regulatory and commercial milestone events. Additionally, we are entitled to receive, on a product-by-product basis, earn-out payments on worldwide net sales of products ranging from a high-single digit percentage to 12%, subject to certain reductions, with an aggregate minimum floor. Through December 31, 2020, we have received from AstraZeneca an option exercise payment of $10.0 million and a clinical milestone payment of $30.0 million with respect to AstraZeneca’s VEGF-A product (AZD8601) that is currently being developed in a Phase 2a clinical trial in the cardiovascular and cardiometabolic fields. Additionally, through December 31, 2020, we have received $120.0 million from AstraZeneca under the 2018 A&R Agreements for the achievement of specified technical milestones. Further detail on the terms of the 2018 A&R Agreements, including the financial arrangements are included in Note 5 to our Financial Statements (“Collaboration Agreements”).
2016 Strategic Alliance with AstraZeneca—IL-12
In January 2016, we entered into a new Strategic Drug Development Collaboration and License Agreement (2016 AZ Agreement) with AstraZeneca to discover, develop and commercialize potential mRNA medicines for the treatment of a range of cancers.
Under the terms of the 2016 AZ Agreement, we and AstraZeneca have agreed to work together on an immuno-oncology program focused on the intratumoral delivery of a potential mRNA medicine to make the IL-12 protein. The 2016 AZ Agreement initially included research activities with respect to a second discovery program. During a limited period of time, each party had an opportunity to propose additional discovery programs to be conducted under the 2016 AZ Agreement. We are responsible for conducting and funding all discovery and preclinical development activities under the 2016 AZ Agreement in accordance with an agreed upon discovery program plan for the IL-12 program and any other discovery program the parties agree to conduct under the 2016 AZ Agreement. For the IL-12 program and any other discovery program the parties agree to conduct under the 2016 AZ Agreement, during a defined election period that commenced as of the effective date of the 2016 AZ Agreement (for the IL-12 program) and otherwise will commence on initiation of any such new discovery program, AstraZeneca may elect to participate in the clinical development of a development candidate arising under the 2016 AZ Agreement from such program. If AstraZeneca so elects (as it has for the IL-12 program), AstraZeneca will lead clinical development activities worldwide and we will be responsible for certain activities, including being solely responsible for manufacturing activities, all in accordance with an agreed upon development plan. AstraZeneca will be responsible for funding all Phase 1 clinical development activities (including costs associated with our manufacture of clinical materials in accordance with the development plan), and Phase 2 clinical development activities (including costs associated with our manufacture of clinical materials in accordance with the development plan) up to a defined dollar threshold. We and AstraZeneca will equally share the costs of Phase 2 clinical development activities in excess of such dollar threshold, all Phase 3 clinical development activities and certain other costs of late-stage clinical development activities, unless we elect not to participate in further development and commercialization activities and instead receive tiered royalties, as described below. Further detail on the terms of the 2016 AZ Agreement, including the financial arrangements are included in Note 5 to our Financial Statements (“Collaboration Agreements”).
2017 Strategic Alliance with AstraZeneca—Relaxin
In October 2017, we entered a new Collaboration and License Agreement (2017 AZ License Agreement) under which AstraZeneca had the right to clinically develop and commercialize a development candidate, known as AZD7970, which is comprised of an mRNA construct for the relaxin protein designed by us and encapsulated in one of our proprietary LNPs. We discovered and performed preclinical development activities for AZD7970 prior toongoing collaborations. In the initiationfourth quarter of the strategic alliance with AstraZeneca under the 2017 AZ License Agreement. On December 16, 2020, the 2017 AZ License Agreement was terminated by mutual agreement, and we received the right to further develop relaxin on2023, our own without any further obligation to AstraZeneca.
Merck (NYSE: MRK)—Strategic Alliances in Infectious Diseases and Cancer Vaccines
We have established a multi-faceted relationship with Merck Sharp & Dohme Corp. (Merck) that includes distinct strategic alliances directed to the research, development, and commercialization of mRNA medicines for the prevention and treatment of viral infections and for the treatment of cancer. In connection with these alliances, Merck also made several equity investments in Moderna totaling approximately $182.0 million, although Merck has disclosed the sale of its direct equity interest.
2015 Strategic Alliance with Merck—Infectious Disease
In January 2015, we entered into a Master Collaboration and License Agreement with
Merck (2015 MerckVertex (the Vertex 2020 Agreement)
, to research, develop, was concluded and
commercialize potential mRNA medicines for the prevention and treatment of infections by RSV. As a part of the May 2019 amendment of the 2015 Merck Agreement, we and Merck agreed to conclude the collaboration as it relates to development of potential mRNA medicines for other viruses, including mRNA-1278 for the prevention of varicella zoster virus (VZV) infection. Pursuant to the 2015 Merck Agreement, Merck was primarily responsible for research, development and commercialization activities and associated costs. We were responsible for designing and, at Merck's cost, manufacturing all mRNA constructs for preclinical and Phase 1 and Phase 2 clinical development purposes. On October 7, 2020, the 2015 Merck Agreement between us and Merck related to our collaboration on RSV was terminated
by mutual agreement, and the right to pursue the continued development and commercialization of product candidates and products, including RSV, reverted to us. Further detail onin accordance with the terms of the
2015 Merckagreement. The Vertex 2020 Agreement
includinghad been aimed at the
discovery and development of potential medicines to treat cystic fibrosis (CF) with gene-editing therapies. For additional information on this collaboration, please see Note 5. Collaboration Agreements, to our consolidated financial arrangements arestatements included in Note 5 to our Financial Statements (“Collaboration Agreements”).2016 Expansion of the Infectious Disease Strategic Alliance with Merckthis Annual Report on Form 10-K.
In January 2016, we expanded our infectious disease strategic alliance with Merck. Specifically, we and Merck agreed to amend the original 2015 Merck Agreement to include the research, development, and commercialization of mRNA medicinesMerck—Strategic Alliance for the prevention and treatment of infection by the VZV in place of one of the viruses initially included under the 2015 Merck Agreement. Under the terms of the amended 2015 Merck Agreement, we received an upfront payment of $10.0 million from Merck for the inclusion of the new program and we agreed with Merck to increase the tiered royalty rates ranging from the mid-single digits to low-teens for net sales of products directed to this virus. In 2020, Merck elected not to continue with this program, and it was terminated.
2016 Cancer Vaccine Strategic Alliance—Personalized mRNA Cancer Vaccines with MerckIndividualized Neoantigen Therapies
In June 2016, we entered into a personalized mRNA cancer vaccines (PCV) Collaboration and License Agreement with Merck (PCVfor the development and commercialization of personalized mRNA cancer vaccines (also known as INTs), which was subsequently amended and restated in 2018 (the INT Agreement), to develop and commercialize PCVsINTs for individual patients using our mRNA vaccine and formulation technology. Under the strategic alliance, we identify genetic mutations present in a particular patient’s tumor cells, synthesize mRNA for these mutations, encapsulate the mRNA in one of our proprietary LNPs and administer to each patient a unique mRNA cancer vaccineINT designed to specifically activate the patient’s immune system against her or his own cancer cells.
Pursuant to the PCVINT Agreement, we arereceived an upfront payment of $200 million from Merck and we were responsible for designing and researching PCVs,INTs, providing manufacturing capacity and manufacturing PCVs,INTs and conducting Phase 1 and Phase 2 clinical trials for PCVs,INTs, alone and in combination with KEYTRUDA (pembrolizumab), Merck’s anti-PD-1 therapy, all in accordance with an agreed upon development plan and budget. We received an upfront payment of $200.0 million from
In September 2022, Merck which we will useexercised its option for INTs, including mRNA-4157, pursuant to fund the performance of our activities set forth in the agreed upon development plan and budget. In November 2017, we and Merck announced the achievement of a key milestone for the first-in-human dosing of a PCV (mRNA-4157) as a part of the alliance. Further detail on the terms of the PCVINT Agreement includingand in October 2022 paid us an option exercise fee of $250 million. Pursuant to the financial arrangements are included in Note 5 to our Financial Statements (“Collaboration Agreements”).
2018 Expansion of the Cancer Vaccine Strategic Alliance with Merck—Shared Neoepitope Cancer Vaccines
In April 2018,INT Agreement, we and Merck have agreed to expand our cancer vaccine strategic alliance to include thecollaborate on further development and commercialization of our KRAS vaccine development candidate, mRNA-5671,INTs, and potentially other shared neoantigen mRNA cancer vaccines (SAVs). We preclinically developed mRNA-5671 prior to its inclusion in the cancer vaccine strategic alliancewe will share costs and it is comprised of a novel mRNA construct designed by us and encapsulated in one of our proprietary LNPs. The PCV Agreement was amended and restated to include the new SAV strategic alliance (PCV/SAV Agreement). Further detail on the terms of the PCV/SAV Agreement, including the financial arrangements are included in Note 5 to our Financial Statements (“Collaboration Agreements”).any profits or losses worldwide equally.
Vertex (Nasdaq: VRTX)—
Vertex—2016 Strategic Alliance in Cystic Fibrosis
In July 2016, we entered into a Strategic Collaboration and License Agreement (Vertex Agreement) with Vertex Pharmaceuticals Incorporated, and Vertex Pharmaceuticals (Europe) Limited (together, Vertex). The Vertex Agreement is aimed at the discovery and development of potential mRNA medicines for the treatment of cystic fibrosis (CF)CF by enabling cells in the lungs of people with CF to produce functional cystic fibrosis transmembrane conductance regulator (CFTR) proteins. Further detail on the terms of the Vertex Agreement, including the financial arrangements are included in Note 5 to our Financial Statements (“Collaboration Agreements”).
Vertex —2020 Strategic Alliance in Cystic Fibrosis
Other Collaborations
In September 2020, we entered into a new Strategic Collaboration and License Agreementcollaboration with Vertex (Vertex 2020 Agreement). The Vertex 2020 Agreement isChiesi Farmaceutici S.p.A. (Chiesi), an international research-focused healthcare group, aimed at the discovery and development of potential medicines to treat CF by delivering gene-editing therapies to lung cells to facilitate production of functional CFTR proteins.
The three-year research period of the Vertex 2020 Agreement will initially focus on the identification and optimization of novel LNPs and mRNAs that can deliver gene-editing therapies to cells in the lungs. Following the initial three-year period, Vertex is responsible for conducting development and commercialization activities for candidates and products that arise from the strategic alliance, including the costs associated with such activities. Vertex is also obligated to pay us for research services in connection with our performance of certain activities in accordance with a jointly agreed research plan. Subject to customary “back-up” supply rights granted to Vertex, under the agreement, we are the exclusive manufacturer of related mRNA and LNPs for preclinical, clinical, and commercialization purposes. Further detail on the terms of the Vertex 2020 Agreement, including the financial arrangements are included in Note 5 to our Financial Statements (“Collaboration Agreements”).
Chiesi—2020 Collaboration and License Agreement with Chiesi
In September 2020, we entered into a Collaboration and License Agreement (Chiesi Agreement) with Chiesi Farmaceutici S.P.A. (Chiesi). The Chiesi Agreement is aimed at the discovery and development of potential mRNA medicines for the treatment of pulmonary arterial hypertension (PAH), a rare disease characterized by high blood pressure in the arteries of the lungs. Further detail onUnder the terms of the Chiesi Agreement, includingagreement, we will lead discovery efforts, and Chiesi will lead development and worldwide commercialization activities and will fund all expenses related to the financial arrangements are includedcollaboration.
We have entered into additional collaborations where we have agreed to provide funding in Note 5 toareas where we believe we can leverage our Financial Statements (“Collaboration Agreements”).mRNA technology. These collaborations include those with:
•Carisma Therapeutics, to discover, develop and commercialize in vivo engineered chimeric antigen receptor monocyte (CAR-M) therapeutics for the treatment of cancer, including solid tumors.
•CytomX Therapeutics, to create investigational mRNA-based conditionally activated therapies utilizing our mRNA technologies and CytomX’s Probody platform.
•Generation Bio Co., to combine our biological and technical expertise with core technologies of Generation Bio's non-viral genetic platform.
•Immatics N.V., to pioneer novel and transformative therapies for cancer patients with high unmet medical need.
•Life Edit Therapeutics, to discover and develop in vivo mRNA gene editing therapies.
•Metagenomi, focused on discovering and advancing new gene editing systems for in vivo human therapeutic applications.
We have made equity investments in Carisma, Generation Bio and Metagenomi pursuant to those collaborations.
Strategic alliances with government organizations and foundations
Defense Advanced Research Projects Agency (DARPA)
In October 2013, DARPA awarded Moderna up to approximately $24.6 million under Agreement No. W911NF-13-1-0417 to research and develop potential mRNA medicines as a part of DARPA’s Autonomous Diagnostics to Enable Prevention and Therapeutics, or ADEPT, program, which is focused on assisting with the development of technologies to rapidly identify and respond to threats posed by natural and engineered diseases and toxins. As of December 31, 2020, $19.7 million of the award amount has been funded. This award followed an initial award from DARPA of approximately $1.4 million given in March 2013 under Agreement No. W31P4Q-13-1-0007. The DARPA awards have been deployed primarily in support of our vaccine and antibody programs to protect against Chikungunya infection.
In September 2020, we entered into an agreement with DARPA for an award of up to $56.4 million to fund development of a mobile manufacturing prototype leveraging our existing manufacturing technology that is capable of rapidly producing vaccines and therapeutics. As of September 30, 2020, the committed funding was $5.0 million, with an additional $51.4 million available under Agreement No. HR0011-20-9-0118 if DARPA exercises additional contract options.
Biomedical Advanced Research and Development Authority (BARDA)
In September 2016, we received an award of up to approximately $125.8$126 million under Agreement No. HHSO100201600029C, subsequently adjusted to $117 million in 2021, from BARDA, a component of the Office of the Assistant Secretary for Preparedness and Response (ASPR), within the U.S. Department of Health and Human Services (HHS), to help fund our Zika vaccine program. UnderIn September 2022, the termsperformance period of the agreement withgrant expired, and BARDA an initial base awardwas released of approximately $8.2the obligation to fund the remaining $36 million supported toxicology studies, a Phase 1 clinical trial, and associated manufacturing activities. Additionally, four contract options were awarded under of the agreement with BARDA. Three out of four of these options have been exercised, bringing the total current award to approximately $117.3 million to support an additional Phase 1 study of an improved Zika vaccine candidate, Phase 2 and Phase 3 clinical studies, as well as large-scale manufacturing for the Zika vaccine.award.
In April 2020, we entered into an agreement with BARDA for an award of up to $483.3$483 million to accelerate development of mRNA-1273, our original COVID-19 vaccine. In July 2020, weThe agreement has been subsequently amended our agreement with BARDA to provide for an additional commitment of up to $471.6 millioncommitments to support various late-stage clinical development efforts of mRNA-1273, including the execution of a 30,000 participant Phase 3 study, in the U.S.pediatric clinical trials, adolescent clinical trials and pharmacovigilance studies. The amendment increased the maximum award from BARDA, from $483.3 million to $954.9 million. Under the termsinclusive of the agreement, BARDA will fund the advancement of mRNA-1273 to FDA licensure.all amendments, was approximately $1.8 billion. All contract options have been exercised. As of December 31, 2020,2023, the remaining available funding, net of revenue earned was $444.3 million.$97 million.
Institute for Life Changing Medicines (ILCM)
In September 2021, we entered into a collaboration agreement with the ILCM to develop a new mRNA therapeutic (mRNA-3351) for type 1 Crigler-Najjar syndrome (CN-1). Under the terms of the agreement, we agreed to license mRNA-3351 to ILCM with no upfront fees, and without any downstream payments. ILCM will be responsible for the clinical development of mRNA-3351.
The Bill & Melinda Gates Foundation
In January 2016, we entered a global health project framework agreement with the Bill & Melinda Gates Foundation to advance mRNA-basedmRNA development projects for various infectious diseases. The Bill & Melinda Gates Foundation has committed up to $20.0$20 million in grant funding to support our initial project related to the evaluation of antibody combinations in a preclinical setting as well as the conduct of a first-in-human Phase 1 clinical trial of a potential mRNA medicine to help prevent human immunodeficiency virus, or HIV infections. Follow-on projects, which could bring total potential funding under the framework agreement up to $100.0$100 million (including the HIV antibody project) to support the development of additional mRNA-basedmRNA projects for various infectious diseases, can be proposed and approved until the sixth anniversary of the framework agreement, subject to the terms of the framework agreement, including our obligation to grant to the Bill & Melinda Gates Foundation certain non-exclusive licenses.
INTELLECTUAL PROPERTY
Our
We rely on a combination of intellectual property laws, including patent, estatetrademark, copyright and approach, a strategic asset
Since our inception, we have considered the creationtrade secret, as well as confidentiality and building oflicense agreements, to protect our intellectual property or IP, portfolio as a critical part of our mission. In a relatively short amount of time, we have built a significant patent estate that includes over 600 world-wide pending patent applications and over 270 issued or allowed U.S. and foreign patents covering key components of our proprietary platform technology, investigational medicines, and development candidates. The figure below shows our internally developed estate and indicates the number of patents approved since 2010.
We regularly identify inventions and trade secrets as we surmount various challenges with our platform to create modalities. We seek to protect our proprietary position by, among other means, filing U.S. and certain foreign patent applications related to our platform, modality, and program inventions. Our company trade secrets and know-how are appropriately guarded to maintain our business
advantage. We also seek to identify and obtain third party licenses where useful to maintain our advantageous IP position in the mRNA medicines field. We seek to obtain and maintain, and intend to strategically enforce, patents in appropriate jurisdictions for our platform technologies, modalities, and programs.
Protecting our platform, modality and program investments: Building an expansive, multi-layered IP estate
We have built a substantial IP estate that includes numerous patents and patent applications related to the development and commercialization of mRNA vaccine and therapeutic development candidates, including related platform technologies. Our platform IP protects advances in mRNA design and engineering, proprietary LNP components, delivery systems, processes for the manufacture and purification of drug substances and products and analytical methods. A significant portion of our platform IP estate further provides multi-layered protection for our modalities and programs.
With respect to our IP estate, our solely-owned patent portfolio consists of more than 145230 issued or allowed U.S. patents or patent applications and more than 125170 granted or allowed patents in jurisdictions outside of the U.S. (including granted European patents that have been validated in numerous European countries) covering certain of our proprietary platform technology, inventions and improvements, and covering key aspects of our clinical and most advanced development candidates. AdditionalWe have over 400 additional pending patent applications are also pending that, in many cases, are counterparts to the foregoing U.S. and foreign patents.
Most of the patents and applications (if issued) in our portfolio have or will have expiry dates extending out tonot expire until 2033 at the earliest and at least 2041-2042 for patents ultimately granting based onearliest. Any patent that may issue from our moremost recently filed patent applications.applications is projected to expire between 2042 and 2043, at the earliest. We file additional U.S. and foreign patent applications as necessary to protect our evolving intellectual property positions.
We also rely on trademarks, copyright, trade secrets and know-how relating to our proprietary technology and programs, continuing innovation, and in-licensing opportunities to develop, strengthen and maintain our proprietary position in the field of mRNA therapeutic and vaccine technologies. We take additional steps, such as entering into confidentiality and license agreements, to protect our intellectually property and proprietary rights. We additionally plan to rely on data exclusivity, market exclusivity and patent term extensions when and where available, and plan to seek and rely on regulatory protection afforded through orphan drug designations. We also possess substantial proprietary know-how associated with related manufacturing processes and expertise.
IP protecting our platform
We have a broad IP estate covering key aspects of our platform. This estate provides multiple layers of protection covering the making and use of the mRNA drug substance and delivery technologies.
With respect to our platform, we have a portfolio that includes approximately 100 issued or allowed U.S. and foreign patents or patent applications, and approximately 220 granted foreign patents and pending foreign patent applications covering platform innovations that are directly related to the design, formulationmanufacturing and manufacturingformulating of mRNA medicines.
For example, these patents and patent applications include claims directed to:
•mRNA chemistry imparting improved properties for vaccine and therapeutic uses;
•methods for mRNA sequence optimization to enhance the levels and fidelity of proteins expressed from our mRNA medicines;
•methods for identifying epitopes having superior suitability in cancer vaccine contexts;
•engineering elements tailored to enhance stability and the in vivo performance of mRNA medicines;
•proprietary lipid nanoparticle, or LNP delivery systems, including novel lipid components designed for optimal delivery and expression of both therapeutic and vaccine mRNAs,nucleic acids, in particular, prophylactic infectious disease and cancer vaccine mRNAs,nucleic acids, intratumoral immuno-oncology therapeutics, local regenerative therapeutics, systemic secreted therapeutics, and systemic intracellularinhaled pulmonary therapeutics; and
•innovative processes for the manufacture and analysis of mRNA drug substance and formulated drug product.
IP protection for modalities
Our IP estate provides protection for the multiple programs within our modalities both at the product-specific level and at various broader levels. For example, we have patent coverage for LNP-encapsulated mRNAs having specific chemical modification suited for vaccine and therapeutic mRNA use. Our estate also includes IP covering certain LNP-encapsulated mRNAs coding for infectious disease antigens for use in prophylactic vaccination.preventing or treating infectious diseases, including those caused by respiratory and latent viruses, as well as bacterial, viral and parasitic diseases known to threaten public health. Our mRNA chemistry, formulation and manufacturing patent applications and related know-how, andalong with trade secrets, may also provide us with additional IP protection relating to our development candidates.
Our patent portfolio for our investigational medicines and development candidates features approximately 50 issued or scheduled-to-issue patents, with many additional pending applications in the U.S. and foreign jurisdictions directed to our development candidates.
ProphylacticRespiratory vaccines
For our respiratory vaccines programs, within our prophylactic vaccines modality, we typically pursuehave pursued patent protection featuring composition of matter and method of use claims. Our globalWhere we may pursue patent protection strategy may vary based on the unique geographic prevalence of various infectious diseases.
We have filed several patent applications covering our COVID-19 vaccine. Claimsbetacoronavirus vaccine program. We are pursuing patent protection for both our existing and next generation betacoronavirus vaccines. A non-exhaustive list of granted patents covering our COVID-19 vaccine compositions and methods of vaccinating subjects against SARS-CoV-2 infection with lipid nanoparticle-encapsulated mRNA encoding prefusion-stabilized Spike protein antigen are featured in a patent family that includes a pending PCT application, two pending U.S. patent applications, 7 pending U.S. provisional patent applications, and foreign patent applications filed in Argentina and Taiwan. Priority dates for these applications span a period from late January through late May 2020. The U.S. government has rights in certain of the foregoing patent applications. Issued U.S. Patent No. 10,702,600 includes claims to lipid nanoparticle-encapsulated mRNA encoding betacoronavirus spike protein. Claims to methods of using such compositions to elicit an immune response in subjects are featured an allowed and soon to be issued U.S. patent application featured. Corresponding vaccine composition and method of use claims are also featured in a pending European patent application. These patents and applications enjoy an October 2015 priority date.
Further coverage for our COVID-19 vaccine and many of our other prophylactic vaccines is found in a broad, infectious disease vaccine patent family featuring claims to lipid nanoparticle-encapsulated mRNAs encoding infectious disease antigens and methods using such compositions for vaccination. This patent family includes two issued U.S. patents, two pending U.S. patent applications and pending patent applications in Europe, Canada, Australia, Brazil, China, Hong Kong, India, Japan, Russia, and Singapore. Issued U.S. Patent Nos. 10,022,435 and 10,709,779 feature claims directed to methods of vaccinating subjects against infection with lipid nanoparticle-encapsulated mRNAs encoding infectious disease antigens.
Patent coverage for our human CMV vaccine, which includes mRNAs encoding several surface glycoproteins of the CMV virus, can be found in pending applications in Australia, Canada, Europe and Japan. In the United States, our CMV vaccine is covered in a pending U.S. patent application and in issued U.S. Patent Nos. 10,064,935, 10,383,937 and 10,716,846. Three provisional patent applications and a pending PCT application feature claims to clinical formulations of our CMV vaccine and methods of use.
Patent applications directed to our hMPV/PIV3 vaccine are pending in the United States, Europe and Hong Kong. Four U.S. patents have issued featuring hMPV/PIV3 vaccines with U.S. Patent No. 10,064,934 having claims covering LNP-encapsulated mRNA vaccines that encode the PIV3 and hMPV fusion proteins, U.S. Patent No. 10,272,150 having claims covering administration methods for these LNP-encapsulated mRNA vaccines, U.S. Patent No. 10,543,269 having claims covering vaccines that include HMPV-encoding mRNA formulated in LNPs, and U.S. Patent No. 10,702,599 having claims covering vaccines that include PIV3-encoding mRNA formulated in LNPs. A pending provisional patent application features claims to clinical aspects of our hMPV/PIV3 vaccine.
Our Zika mRNA vaccine is covered in a series of patent families directed to mosquito-borne viruses. These patent families include three issued U.S. Patents that cover our Zika vaccines, U.S. Patent Nos. 10,124,055, 10,449,244 and 10,653,767, several pending U.S. patent applications, one of which is recently allowed and soon to be issued as a U.S. patent, and several pending European and Hong Kong patent applications.
We filed patent applications in several jurisdictions covering RSV vaccines. At least two U.S. and two European patent applications are pending, as are applications in several African, Asian, European, Middle Eastern, South American, and other jurisdictions. Also pending is a provisional application featuring our pediatric RSV vaccine.
A pending provisional patent application includes claims covering our vaccine program for the prevention of human infection with seasonal influenza virus. The program also is protected by the broad, infectious disease vaccine patent family described above, in particular, issued U.S. Patent No. 9,872,900 having claims to HA-encoding mRNA vaccine compositions.
One of our earliest investigational medicines in the infectious disease pipeline, a vaccine containing mRNA encoding the H7 HA antigen for the prevention of infection with the influenza H7N9 avian influenza A virus is also protected by the broad, infectious disease vaccine patent family described above. In particular, issued U.S. Patent No. 9,872,900 includes claims to H7 mRNA vaccine compositions.
Pending patent applications in the United States, Australia, Canada, Europe, and Japan include claims covering our EBV vaccine and methods of use.
Pending patent applications in the United States and Europe include claims covering our Nipah vaccine and methods of use.
Cancer
| | | | | | | | | | | |
| Patent Number | Country/Region* | Patent Type | Expiration Date** |
| 11,524,023 | United States | Composition of Matter | October 22, 2041 |
| 11,485,972 | United States | Composition of Matter | May 18, 2038 |
| 10,898,574 | United States | Composition of Matter and Method of Use | April 2, 2032 |
| 10,703,789 | United States | Composition of Matter | March 9, 2033 |
| 10,702,600 | United States | Composition of Matter | October 21, 2036 |
| 10,577,403 | United States | Composition of Matter | March 9, 2033 |
| 10,442,756 | United States | Composition of Matter | September 16, 2036 |
| 10,266,485 | United States | Composition of Matter | September 16, 2036 |
| 10,064,959 | United States | Composition of Matter | October 3, 2031 |
| 9,868,692 | United States | Composition of Matter | September 16, 2036 |
| 3 590 949 | Europe | Composition of Matter and Method of Use | October 3, 2031 |
| 3 718 565 | Europe | Composition of Matter | October 21, 2036 |
* Selected granted patents in the U.S. and Europe only. Additional granted and pending patents in the U.S., Europe and other countries may be available.
** Expiration dates listed here include any granted or anticipated patent term adjustment (PTA), but not any patent term extension (PTE) or supplementary protection certificates (SPC).
RSV
We have filed multiple patent families directed to RSV vaccines, including a U.S. patent that issued on October 11, 2022.Our RSV patent portfolio includes multiple families of differing patent breadth. At least four U.S. and European patent applications are pending.
Influenza
We have multiple patent families spanning different levels of breadth, design and antigen valency pending in the U.S., Europe and around the world, including several granted patents.
hMPV
Human metapneumovirus (hMPV) is a single-stranded RNA virus that is used in a combination program. We have patent applications covering our hMPV vaccine pending in the U.S. and Europe, with a granted patent in the U.S.
Latent vaccines
We have vaccine programs and patent applications directed to both the acute and latent forms of diseases caused by various viruses, including CMV, EBV, HSV, VZV and HIV, using both preventative vaccines targeting the acute phase and therapeutic vaccines for treating the latent diseases in those who do become infected.
CMV
The patent coverage for our human CMV vaccine candidate is extensive and is based on a vaccine with six mRNAs encoding a pentamer surface glycoprotein complex and the gB surface glycoprotein. Both pentamer and gB facilitate entry of the virus into different cell types and therefore immune responses targeting these proteins can block virus entry, spread and reactivation. The current patent portfolio contains both compositions of matter and methods of treating subjects using the vaccine. In the U.S., our CMV vaccine is covered by multiple issued U.S. patents of differing breadth. Each family has counterparts consisting of pending applications and issued patents in non-U.S. jurisdictions, including Europe and Japan. A separate family of CMV patents, which includes mRNA-1647 plus mRNA-1443 for use in CMV vaccines for transplant indications, is also yielding patents and applications in foreign jurisdictions are pending.
EBV, HSV and VZV
Similar to CMV, we have multiple patent families pending for each of EBV, HSV and VZV, covering both prophylactic and therapeutic indications. These patent families have also been filed in Europe and Japan.
Public health vaccines
We maintain a multi-program effort at developing vaccines for potential future pandemics and for use in parts of the world with less well-established health care systems. This group of programs include infectious diseases such as flaviviruses such as Zika and dengue
viruses, HIV, Nipah virus, and the Mpox virus. In addition, programs are ongoing in many bacterial diseases. Specific patent families are being filed on most potential public health programs where possible, but in some scenarios, platform patents may be used to augment patent protection for public health target vaccines.
Individualized neoantigen therapy (INT)
Composition of matter and method claims alsoare being pursued to protect programs within our cancer vaccines modality. Proprietary methods around the making and therapeutic use of our PCVsINTs and resulting vaccine compositions are described and claimed in fourseven pending U.S. patent applications, a pending PCT application, foursix pending European patent applications, five pending patent applications in Japan, three pending patent applications in each of Australia, Canada, and Japan, and one or twogranted patent in China, and several pending patent applications in each of India, South Korea, New Zealand, Russia, SingaporeSouth Africa, Asian and South Africa.American countries, as well as one PCT application. These applications also relate to various vaccine design formats, in particular, polyepitopic vaccine formats, and methods of treating cancer with such personalized cancer vaccines.INTs. We also possess substantial know-how and trade secrets relating to the development and commercialization of our cancer vaccine programs, including related manufacturing process and technology.
Likewise, our KRAS antigen cancer vaccine and methods of treating cancer featuring such vaccines are covered in an issued U.S. Patent No. 10,881,730,patent, which includes claims to lipid nanoparticle-encapsulatedLNP-encapsulated mRNA encoding mutant KRAS antigens, and in a pending U.S. patent application and pending applications in Australia, Canada, Europe, and Japan, as well as in several other European, South American, Asian and Middle Eastern jurisdictions.antigens.
Intratumoral immuno-oncology
To protect programs within our intratumoral immuno-oncology modality, weWe have filed numerous patent applications featuring claims to mRNAs encoding immune-stimulatory proteins and methods of treating cancer using such compositions.
Three of ourOur immuno-oncology programs are designed to be administered intratumorally to alter the tumor microenvironment in favor of mounting an immune response against tumors. Our OX40L mRNA program and our mRNA program that includes mRNAs that encode OX40L, IL-23 and IL-36γ are covered by eighttwo granted European patents, by more than 10 issued U.S. patents, U.S. Patent Nos. 10,143,723, 10,172,808, 10,285,950, 10,322,090, 10,322,191, 10,379,767, 10,383,951 and 10,406,113, by several pending U.S. and European patent applications two of which are allowed and soon to issue, and by several pending patent applications in other foreign jurisdictions including European, Asian, South American and other jurisdictions. These applications feature claims to the mRNA therapeutics as compositions of matter, formulations that include such mRNAs and methods of reducing tumors and treating cancer featuringusing these development candidates. Similar claims cover our IL-12 development candidate which can be found in issued U.S. Patent No. 10,646,549, and in pending patent applications in the United States, Australia, Canada, China, Europe and Japan, as well as several other jurisdictions in Asia, South America and the Middle East.
Localized regenerative therapeuticsRare diseases
Our localized regenerative therapeutics modality is focused on regenerative therapeutics. Our sole program, VEGF-A, is being developed in collaboration with AstraZeneca and is covered by a pending U.S. patent application and by several national phase patent applications filed in South American, Asian and Middle Eastern jurisdictions. The VEGF patent applications are solely-owned by Moderna.
Systemic intracellular therapeutics
Within our systemic intracellular therapeutics modality, weWe have four programs featuring expression of therapeutic proteins, e.g., intracellular enzymes for the treatment of rare diseases. For our rare disease programs, we generally pursue patent protection featuring composition of matter and method of use claims, for example, pharmaceutical composition and method of treatment claims. Our most advanced rare disease development candidate MMA, is covered by a patent family that includes two issued U.S. Patents, U.S. Patent No. 10,406,112 and U.S. Patent No. 10,426,738, two pending U.S. patent applications, a pending PCT patent application, and foreign patent applications filed in Australia, Canada, Japan, Europe and the Middle East.
for PA. For our PA developmentthis candidate, we have patent applications pending in the United States, Canada, Europe, and Japan which cover mRNA encoding the alpha and beta subunits of the enzyme propionyl-CoA carboxylase (PCCA and PCCB, respectively), for the treatment of PA.
For MMA, we have patent applications issued and pending in the U.S. and foreign applications filed in Japan and Europe.
For our PKU development candidate, we have a pending PCT and a pending provisional patent applicationapplications in the U.S., Europe and Japan covering mRNA encoding phenylalanine hydroxylase or PAH,(PAH) for the treatment of PKU.
For our Glycogen Storage Disorder, Type 1a (GSD1a) development candidate, we have 2filed several patent families, including pending PCTU.S. and 2European patent applications, as well as applications pending provisional patent applicationsin China and Japan covering mRNA encoding glucose 6-phosphatase (G6Pase) for the treatment of this disorder.
For our Crigler-Najjar Syndrome Type 1 (CN-1) development candidate, we have patent applications pending in the U.S., Europe and Japan.
83Our ornithine transcarbamylase deficiency (OTC) development candidate is covered by a pending PCT application.
Any U.S. and foreign patents that may issue from these four patent families would be expected to expire in 2036 for the earliest of the MMA patents and 2038-20412038 to 2042 for the remaining MMA, PA, PKU, GSD1a and GSD1aCN-1 patents, excluding any patent term adjustments, and any patent term extensions.extensions and any terminal disclaimers.
As further described below, we have filed or intend to file patent applications on these and other aspects of our technology and development candidates, and as we continue the development of our intended products, we plan to identify additional means of obtaining patent protection that would potentially enhance commercial success, including protection for additional methods of use, formulation, or manufacture.
Systemic secreted and cell-surface therapeutics
Our systemic secreted and cell-surface therapeutics modality features programs directed to expression of secreted or cell-surface proteins including antibodies, circulating immune modulation factors, secreted enzymes and transmembrane proteins. Our mRNA-encoded antibody against Chikungunya virus reported positive interim Phase 1 results in clinical trials and utilizes the same lipid nanoparticle (LNP) formulation being advanced for our MMA program and other rare disease programs. Patent protection for mRNA-encoded antibody against Chikungunya virus is being sought by way of a pending U.S. and European patent applications, in which we share joint ownership rights.
Our Relaxin development candidate is covered by several pendingpatent families, including granted patents in Japan, China and the U.S., and by additional applications in the U.S. and additional foreign patent applications outside the United States, for example, in several Asian, European, Middle Eastern, South American and other jurisdictions and by a pending U.S. application and by issued U.S. Patent No. 10,730,924.PCT application.
Our PD-L1 development candidate is covered in pending patent applications filed in the U.S., Japan and IL-2Europe.
Inhaled pulmonary therapeutics
Our inhaled pulmonary therapeutics modality currently has one development candidate directed to expression of therapeutic protein in the lungs. This Cystic Fibrosis (CF) development candidate is covered by pending U.S., European and PCT patent applications.
Gene editing
Our gene editing program currently has one filed patent family that includes issued patents in the U.S., Europe and Japan, and also pending applications in these jurisdictions. We plan to file patent applications on development candidates are covered in three recently filed U.S. provisional patent applications.and other aspects of gene editing technology as we continue to innovate both internally and through strategic collaborations.
Trademarks
Our registered trademark portfolio currently contains at least 125 registered trademarks, consisting of1,000 trademark registrations, including at least 1022 registrations in the United States and approximately 115 registrationsthe remaining in Canada, the European Union, the United Kingdom, Israel, China, Japan, Australia, Canada, China, the EU, Japan, Singapore, Sweden, and under the Madrid Protocol.elsewhere. In addition, we have otherat least 600 pending trademark applications consisting of trademark applicationsin more than 95 jurisdictions, including in the United States, Australia, Canada, China, the EU, Japan, Singapore,aforementioned locations and under the Madrid Protocol.additional countries throughout Africa, Asia, and South America.
In-licensed intellectual property
While we develop and manufacture our potential mRNA medicines using our internally created mRNA technology platform, we also seek out and evaluate third party technologies and IP that may be complementary to our platform.
Patent sublicense agreements with Cellscript and mRNA RiboTherapeutics
The Trustees of the University of Pennsylvania or Penn, owns several issued U.S. patents, granted European patents and pending U.S. patent applications directed, in part, to nucleoside-modified mRNAs and their uses or the(the Penn Modified mRNA Patents.Patents). mRNA RiboTherapeutics, Inc., or MRT, (MRT) obtained an exclusive license to the Penn Modified mRNA Patents and granted its affiliate, Cellscript, LLC or Cellscript,(Cellscript), a sublicense to the Penn Modified mRNA Patents in certain fields of use.
In June 2017, we entered into two sublicense agreements, one with Cellscript, and one with MRT, which agreements we collectively refer to as the Cellscript-MRT Agreements. Together, the Cellscript-MRT Agreements grant us a worldwide, sublicensable sublicense to the Penn Modified mRNA Patents to research, develop, make, and commercialize products covered by the Penn Modified mRNA Patents or licensed products,(licensed products), for all in vivo uses in humans and animals, including therapeutic, prophylactic, and diagnostic applications. The Cellscript-MRT Agreements are non-exclusive, although Cellscript and MRT are subject to certain time restrictions on granting additional sublicenses for in vivo uses in humans under the Penn Modified mRNA Patents.
We paid Cellscript and MRT aggregate sublicense grant fees of $28 million upon entering into the Cellscript-MRT Agreements, $25 million in early 2018, and $22 million in early 2019. Cellscript and MRT are collectively eligible to receive, on a licensed product-by-licensed product basis, milestone payments totaling up to $0.5 million upon the achievement of certain regulatory-based events for diagnostic products, and milestone payments totaling up to $1.5 million upon the achievement of certain development and regulatory-based events for either therapeutic or prophylactic products, and up to $24 million upon the achievement of certain commercial-based events for either therapeutic or prophylactic products. The Cellscript-MRT Agreements require us to pay royalties based on annual net sales of licensed products at rates in the low single digits for therapeutic, prophylactic, and diagnostic uses, and royalties based on annual net sales of licensed products sold for research uses at rates in the mid-single digits, subject to certain reductions, with an aggregate minimum floor. Following the first commercial sale of licensed products under a Cellscript-MRT Agreement, we are required to pay Cellscript or MRT, as applicable, minimum annual royalties ranging from $10,000—$400,000 depending on the use of such licensed product, with all such payments creditable against earned royalties on net sales.
The Cellscript-MRT Agreements will expireterminate upon the expiration or abandonment of the last to expire or become abandoned of the Penn Modified mRNA Patents. Cellscript or MRT, as applicable, may terminate its respective Cellscript-MRT Agreement if we fail to make required payments or otherwise materially breach the applicable agreement, subject to specified notice and cure provisions. Cellscript or MRT, as applicable, may also terminate the applicable Cellscript-MRT Agreement upon written notice in the event of our bankruptcy or insolvency or if we challenge the validity or enforceability of the Penn Modified mRNA Patents. We have the right to terminate each Cellscript-MRT Agreement at will upon 60 days’ prior notice to Cellscript or MRT, as applicable, provided that we cease all development and commercialization of licensed products upon such termination. If rights to MRT or Cellscript under the Penn Modified mRNA Patents are terminated (e.g., due to bankruptcy of MRT or Cellscript), the terminated party will assign its interest in the respective Cellscript-MRT Agreement to the licensor from which it received rights under the Penn Modified mRNA Patents and our rights will continue under the new licensor.
Patent license agreement with NIAID
In December 2022, we entered into a non-exclusive patent license agreement with the National Institute of Allergy and Infectious Diseases (NIAID), an Institute or Center of the National Institutes of Health (NIH) to license certain patent rights concerning stabilizing prefusion coronavirus spike proteins and the resulting stabilized proteins for use in COVID-19 vaccine products. Pursuant to the agreement, we have agreed to pay low single-digit royalties on future net sales, a minimum annual royalty payment and certain contingent development, regulatory and commercial milestone payments on a licensed product-by-licensed product basis.
Formulation technology in-licenses
Our development candidates use internally developed formulation technology that we own. We do, however, have rights to use and exploit multiple issued and pending patents covering formulation technologies under licenses from other entities. If in the future we elect to use or to grant our strategic collaborators sublicenses to use these in-licensed formulation technologies, we or our strategic collaborators may be liable for milestone and royalty payment obligations arising from such use. We consider the commercial terms of these licenses and their provisions regarding diligence, insurance, indemnification and other similar matters, to be reasonable and customary for our industry.
In addition, we have entered into material transfer agreements that have provided us with opportunities to evaluate third party delivery systems.
HUMAN CAPITAL
EMPLOYEES
We had approximately 1,3005,600 full-time employees in 19 countries around the world as of December 31, 2020, representing a 59% increase over our 830 full-time employees as of the end of the prior year. Of our total full-time employees as of December 31, 2020, nearly 600 joined us during the course of the year. This growth in our employee base has largely been as a result of developments related to our COVID-19 vaccine. We have undertaken significant hiring of employees to facilitate manufacturing of the vaccine, in addition to building out our commercial and regulatory organizations, as well as other functions, to support this roll-out. As part of this expansion, we have also increased our hiring outside the United States during 2020, and at year-end we had employees in Switzerland, the United Kingdom, Canada and Spain. Much of this hiring has been of talent with experience at other pharmaceutical companies as we seek to build out our commercial and regulatory capabilities, particularly at more senior levels of the company. We have also continued to hire talent to support our research and clinical capabilities across the rest of our pipeline, unrelated to our COVID-19 vaccine.
2023.
We operate in a highly competitive environment for human capital,talent, particularly as we seek to attract and retain talent with experience in the biotechnology and pharmaceutical sectors. Our workforce is highly educated, and as of December 31, 2020, 51%2023, 41% of our employees hold Ph.D., Doctorate, M.D., J.D., or Master’s degrees. Among our employees, 46%as of December 31, 2023, 49% are female and 54% are male.female. Among our leadership (which we define as employees at the vice president level and above), as of December 31, 2020,2023, approximately 37%39% are female, an increase from 35% in the prior year. 35%female. 45% of our U.S. employees identify as racially or ethnically diverse as of December 31, 2020,2023, an increase from 32% in the prior year. In 2020, we expanded our emphasis2023, for the second year in a row, an outside statistical pay equity analysis confirmed zero statistically significant differences in pay across gender globally and across gender, race and ethnicity in the United States.
Our focus on belonging, inclusion &and diversity creating a Conscious Inclusion education series for senior leaders, conducting several internal seminars, panels, and discussions on inclusion, launching a new employee resource group in support of our Black and African American employees, and adding a full-time senior-level role focused on belonging, equity, inclusion and diversity.
To help promote alignment betweenWe are committed to building a culture of inclusion and belonging for all. In 2023, we continued to act on our employeescommitment to belonging, inclusion and our shareholders, all employees participate in our equity programs through the receipt of new hire and annual equity grants, and the percentage of equity as a component of overall pay mix increases with seniority. We believe that in addition to incentivizing growth that leads to shareholder value, broad eligibility for our equity programs helps promote employee retention as these awards generally vest over a four-year period.
In response to the COVID-19 pandemic, we undertook several initiatives to ensure the safety of our workforce and continuity of our operations. We created a Response Team that was responsible for implementing safety measures testing at our Cambridge and Norwood sites. This included regular COVID-19 testing, temperature and health screening as well as implementing digital tools to facilitate contact tracing and providing personal protective equipment (PPE). Throughout the pandemic, much of our workforce has worked remotely, wherever possible. We also implemented remote hiring and onboarding programs to facilitate significant hiring during 2020 in a remote work environment. In December 2020, following the receipt of an Emergency Use Authorization from the FDA for our COVID-19 vaccine, we made the vaccine available to our employees and adult members of their households to help ensure continuity of our operations due to the critical nature of our production of the vaccine.
None of our employees is representeddiversity by, a labor union, and none of our employees has entered into a collective bargaining agreement with us,among other than a small number of employees in Spain who are covered by collective bargaining agreements governing certain benefits. We consider our employee relations to be good.things:
We believe•continuing our monitoring and reporting of company-wide gender and ethnicity data;
•including a belonging, inclusion and diversity focus in every employee engagement survey;
•continuing to invest in our Employee Resource Groups, which are voluntary, employee-led groups that harness the power of belonging in service to our people, our company and the community at large, including by introducing mCARE, a group dedicated to supporting and empowering our employees who are highly engaged,caregivers and weparents;
•receiving recognition from Disability:IN Inclusion and our employees have been recognized by surveys conducted by external groups. Science magazine ranked usthe American Association of People with Disabilities as a top employerbest place to work for each ofDisability Inclusion; and
•conducting diversity-related events, celebrations and learning opportunities for all employees throughout the last six years. We measure employee engagement through a vendor-supplied engagement software, using validated external benchmarks to track quarter-over-quarter employee engagement factors.year.
Our approach to attracting and retaining talent
We are committed to ensuring that our employees find that their careers at Moderna are filled with purpose, growth and fulfillment. We believe that a career at Moderna provides opportunity for:
•Impact: Our people will have the opportunity to do work that is unparalleled in terms of its innovation and scope of impact on people’s lives.
•Growth: For the intellectually curious, weWe provide incredible opportunities for growth.growth and we obsess over learning (as demonstrated, in part, by our Mindsets (see below). We invest substantially in the development of our people as scientists and as leaders.people.
•WellnessWell-being: We are committed to the health and wellbeingwell-being of our employees and their families by providing family friendlyand provide numerous family-friendly benefits and opportunities to be healthy.healthy, including monthly contributions to employee lifestyle spending accounts.
•Inclusive environmentInclusion: We believe in the benefits of bringing together a diverse set of perspectives and backgrounds, and creating an environment where differences are celebrated and leveraged. We measure and hold management accountable to creating an environment of psychological safety.
•Compelling rewards: To attract and retain the best talent, we provide competitive rewards that help to drive groundbreaking work and allow employees to share in the value we will create together, including through our equity programs.
•Giving and volunteering: Our people have the opportunity to give back to their communities and directly support causes that they are passionate about through volunteer and employee matching donation programs.
To help promote alignment between our employees and our shareholders, all employees participate in our corporate equity programs through the receipt of equity awards, and the percentage of equity as a component of overall pay mix increases with seniority. We also allow our employees to select how they want the value of their award to be split between stock options and restricted stock units (RSUs). We believe that in addition to incentivizing growth that leads to shareholder value, broad eligibility for our equity programs further embeds our "We behave like owners" mindset and helps promote employee retention as these awards generally vest over a four-year period.
None of our employees are represented by a labor union or works councils, and none of our employees have entered into a collective bargaining agreement with us. A small number of employees in France, Italy and Spain are covered by statutory collective bargaining agreements governing certain benefits and working conditions. We consider our employee relations to be good.
We believe that our employees are highly engaged, and our company and team have been publicly recognized for our leadership, innovation and good corporate citizenship. Science magazine ranked us as a top employer for each of the last nine years. Additionally, in 2023, Biospace ranked us the number one large employer in its 2024 Best Places to Work in Biopharma report for the third consecutive year. We also received a perfect score from the Human Rights Campaign's Equality Index for 2023-2024. We measure employee engagement through a vendor-supplied engagement software, using validated external benchmarks to track employee engagement factors.
We continually monitor employee turnover rates, as our success depends upon retaining our highly trained personnel. We believe that the competitive compensation we offer, along with the combination of the factors listed above, among other factors, have helped reduce voluntary turnover. In 2023, our voluntary turnover rate was approximately 6%.
Our approach to training our employees
We
To further invest in our teams, we have established a structured training curriculum for our employees called Moderna University and have a full-time team dedicated to developing the curriculum and conducting activities for Moderna University. The objective of Moderna University is forso that every employee to bebecomes deeply familiar with our core technology and able to learn about technologies that might further enable our science.innovation. In addition, Moderna University is alsowe are focused on creating strong leaders through various management and leadership training. There are four core areas within Moderna University including:
•Professional development: Includes on-site training programs for our employees including for example, leadership, tools to improve interpersonal communication, and project management.
•Digital learning library:trainings. We have also built an online library of videos of a variety of scientific material that our employees can access flexibly. This content includes:
◦Presentationsincludes presentations by external speakers toat in-house scientific seminars, conducted in-house;
◦Scientificscientific courses at external universities;universities and
◦Peer-to-peer peer-to-peer video series in which in-house experts provide an introductory view of complex topics they tackle within their teams.
•Learning management system: We have deployedNew employees participate in our Moderna ONE onboarding program, which is an interactive learning experience designed to immerse our people in our culture and Mindsets from day one. Following onboarding, our employees continue to learn throughout their careers at Moderna and we deploy a digital learning management system to track and administer training programs for each of employee.
In December 2021, we launched our employees. Training contentArtificial Intelligence (AI) Academy in partnership with Carnegie Mellon University. The AI Academy is developed digitallyintended to educate and offeredempower our employees to identify and integrate AI and machine learning solutions into every Moderna system and process to bring mRNA medicines to patients.
Our culture
As an organization, we are bold, collaborative, curious and relentless. These values are underpinned by a core set of what we call “basecamp” values— integrity, quality, respect. Additionally, with the continued rapid growth of our company, we articulated the Moderna Mindsets, which define how we behave, lead and make decisions. We believe our Mindsets will be integral to our employees.
•New hire orientation: This program is designed tofuture success, and we integrate them into every facet of how we identify, onboard, all new employees. During this training program, new employees meet with the management teamgrow and senior functional leaders to learn about the Company and functional activities.manage our talent.
To further develop and retain our workforce, we conduct periodic talent reviews that identify key talent within the organization. We use that data to inform specific development opportunities for key current and potential future leaders, and to support our periodic succession planning activities for key roles. These steps together ensure we have a robust understanding of our workforce and a talent pipeline to grow future leaders.leaders, and provide our employees an opportunity to continuously grow and advance in a way that meets their aspirations and talents.
CORPORATE SOCIAL RESPONSIBILITY
In pursuit of
As we pursue our mission to deliver on the promisegreatest possible impact to people through mRNA medicines, we have developed a corporate social responsibility (CSR) program that demonstrates our commitment to patients, employees, the environment and local communities. Our CSR framework consists of mRNA science to create a new generation of transformativefive key focus areas: medicines for patients, we have scaled our operations, invested in research,community, governance and hired top-tier talent. As we continue to mature, we believe it is important to develop long-term programs that underscore our commitment to corporate social responsibility.ethics, employees and environment. Please refer to our 2022 ESG Report under the “Citizenship”“Responsibility—Corporate policies” section of our website, which can be found at www.modernatx.com,www.modernatx.com/responsibility/corporate-policies, as well as our proxy statement related to our 2024 Annual Meeting of Stockholders that we will file with the SEC, for a description of some of the measures we have taken to supportprogress our commitment to corporate social responsibility.
COMPETITION
The biotechnology and pharmaceutical industries utilize rapidly advancing technologies and are characterized by intense competition. There is also a strong emphasis on defense of intellectual property and proprietary products.
mRNA Medicines
We believe that mRNA as a medicine coupled with our capabilities across mRNA technology, drug discovery, development and manufacturing provide us with a competitive advantage. However, we will continue to face competition from differentothers developing mRNA vaccines and therapeutics, as well as other medicines that compete or could compete with our products. We face competition from various sources, including majorlarge pharmaceutical companies, biotechnology companies, academic institutions, government agencies and public and private research institutions. For any products that we eventually commercialize, we will not only compete with existing therapies butThe continued growth of the mRNA field is leading to increased competitive pressure, including from large and more established pharmaceutical companies. We also face competition when entering into strategic alliances to advance and grow our pipeline.
Our COVID-19 vaccine largely competes against Pfizer/BioNTech’s COVID-19 vaccine, which is also based on mRNA technology. We also compete with new therapies thatagainst other approved or authorized products, including Novavax’s COVID-19 vaccine. Additionally, some competitors have developed COVID-19 treatments, including Pfizer’s antiviral pill, and the existence of such treatments may become available in the future.reduce demand for vaccines.
We face significant competition in the market for our COVID-19 vaccine, particularly from established pharmaceutical companies with longer operating histories and significant experience in producing and marketing pharmaceutical products.
Competition for the sale of our COVID-19 vaccine can beis impacted by a number ofmany factors, including: theincluding, among others, actual and perceived vaccine efficacy of our vaccine in preventing COVID-19 (particularly in the prevention of severe cases of COVID-19); the ability of our vaccine, or future iterations of the vaccine, to protect effectively(including against variants of theemerging SARS-CoV-2 virus;variants), safety and tolerability, perceptions of the efficacy of our vaccine; concerns about potential side effects from the vaccine, its safety or tolerability; the novelty of mRNA-based technology;mRNA technology, storage and handling conditions for our vaccine and the relative ease or difficulty with which it can be distributed;of distribution and administration, the fact that our COVID-19 vaccine requires two doses, whereas certain competitors’ vaccines may only require a single dose.timing and scope of regulatory approvals, reimbursement coverage and production and distribution costs. The competitiveness of our COVID-19 vaccinevaccines in the future may also depend uponon whether we are successfulsuccessfully produce combination respiratory vaccines, which would combine in efforts to combine this with other vaccines, likea single shot protection against various respiratory diseases, such as seasonal flu, RSV and whether our competitors are successful in these efforts. Our competitive positioning may also be affected by the fact that we do not have as long a history of producing pharmaceutical products or existing commercial relationships compared to certain of our competitors.COVID-19.
Our RSV vaccine, which we expect to launch in 2024, will face competition from existing RSV vaccines, including those produced by Pfizer and GlaxoSmithKline. We are also developing a seasonal flu vaccine, for which there is an existing, well-developed market.
In markets that we enter after competitors have already introduced a competing product, we may have difficulty achieving market share. See “Risk Factors—The pharmaceutical market is intensely competitive, and we may not compete effectively in the segment of pharmaceuticalmarket for existing products, new treatment methods and biotechnology industries. new technologies.”
There are additional companies that are working on mRNA medicines, some of which have reached commercialization. Companies with mRNA programsThese companies include BioNTech (which has partneredand Pfizer (alone and in partnership with Pfizer for the production and commercialization of a COVID-19 vaccine) and CureVac (which also is developing a COVID-19 vaccine). Other competitors include eTheRNA Immunotherapies and Translate Bio and those with preclinical programs include Arcturus Therapeutics, Ethris, Genevant Sciences, StemirnaBioNTech, Beam Therapeutics and GlaxoSmithKline. others). Others include Sanofi, GlaxoSmithKline and CureVac.
Our Collaborations
We alsoand our strategic collaborators face competition from companies developing therapies in various areas, other than the development of mRNA medicines, related to our collaborations. For example, there are a growing number of pharmaceutical, biotechnology and academic institutions researching and developing autologous and allogeneic CAR-T therapies in both the solid and liquid tumor setting. These CAR-T cell therapies are at various stages of development and approval and could compete against any CAR-M therapeutics we discover, develop and commercialize in collaboration with Carisma Therapeutics.
Similarly, there are many companies and institutions researching and developing other nucleic acid and genetic medicines, which could compete against any therapies for genetic diseases we develop and commercialize in collaboration with Metagenomi or other collaborators.
Our Growing Commercial Footprint
Our commercial organization was established in 2020 and we have less commercial experience than many of our competitors. We are still growing our commercial footprint, and compete against other pharmaceutical companies in the market for COVID-19 vaccines that do not utilize mRNA-based technologies, including AstraZenecahave certain competitive advantages over us. See “Risk Factors—Risks related to commercialization and Johnson & Johnson, among others.our products.”
GOVERNMENT REGULATION
Government authorities in the United States at the federal, state and local level and in other countries and regions, such as in the European Union (EU) regulate, among other things, the research, development, testing, manufacture quality control, approval, labeling, packaging, storage, record-keeping, promotion, advertising, distribution, post-approval monitoring and reporting, marketing and export and import of drug and biological products, such as our investigational medicines and any future investigational medicines.products. Generally, before a new drug or biologicmedicine can be marketed, considerable data demonstrating its quality, safety and efficacy must be obtained organized into a format specific for each regulatory authority,and submitted for review and approved by the competent regulatory authority.
U.S. drug and biological product development
In the United States, the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act or FDCA,(FDCA) and its implementing regulations and biologics under the FDCA, the Public Health Service Act or PHSA,(PHSA), and their implementing regulations. Both drugs and biologics also are subject to other federal, state and local statutes and regulations. The process of obtaining regulatory approvals and the subsequent compliance with applicable federal, state and local statutes and regulations requires the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or following approval may subject an applicantus to administrative or judicial sanctions. These sanctions could include, among other actions, the FDA’s refusal to approve pending applications, withdrawal of an approval, license revocation, a clinical hold, untitled or warning letters, voluntary or mandatory product recalls, market withdrawals, product seizures, total or partial
suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement and civil or criminal penalties.
Any agency or judicial enforcement action could have a material adverse effect on us.
Our investigational medicines and any future investigational medicinesof our product candidates must be approved by the FDA through a biologics license application, or BLA or new drug application (NDA), or supplemental BLA or supplemental NDA, process before they may be legally marketed in the United States. The process generally involves the following:
•completion of extensive preclinical studies in accordance with applicable regulations, including studies conducted in accordance with GLP requirements;
•submission to the FDA of an IND application, which must become effective before human clinical trials may begin;
•approval by an institutional review board, or IRB, or independent ethics committee at each clinical trial site before each trial may be initiated;
•performance of adequate and well-controlled human clinical trials in accordance with applicable IND regulations, good clinical practice, or GCP, requirements and other clinical trial-related regulations to establish the safety and efficacy of the investigational product for each proposed indication;
•submission to the FDA of a BLA or an NDA;
•a determination by the FDA within 60 days of its receipt of a BLA or an NDA to accept the filing for review;
•satisfactory completion of one or more FDA pre-approval inspections of the manufacturing facility or facilities where the biologic or drug will be produced to assess compliance with cGMP requirements to assure that the facilities, methods and controls are adequate to preserve the biologic or drug’s identity, strength, quality and purity;
•potential FDA audit of the clinical trial sites that generated the data in support of the BLA or NDA;
•payment of user fees for FDA review of the BLA or NDA; and
•FDA review and approval of the BLA or NDA, including consideration of the views of any FDA advisory committee, prior to any commercial marketing or sale of the biologic or drug in the United States.
The preclinical and clinical testing and approval process requires substantial time, effort and financial resources, and we cannot be certain that any approvals for our investigational medicines and any future investigational medicines will be granted on a timely basis, or at all.
Preclinical studies
Before testing any biological or drug candidate, includingof our productdevelopment candidates may be tested in humans, the productdevelopment candidate must undergo rigorous preclinical testing. Preclinical studies include laboratory evaluation of product chemistry and formulation, as well as in vitro and animal studies to assess the potential for adverse events and in some cases to establish a rationale for therapeutic use. The conduct of preclinical studies is subject to federal regulations and requirements, including GLP regulations for safety/toxicology studies. An IND sponsor must submit the results of the preclinical tests, together with manufacturing information, analytical data, any available clinical data or literature and plans for clinical studies, among other things, to the FDA as part of an IND. An IND is a request for authorization from the FDA to administer an investigational product to humans and must become effective before human clinical trials may begin. Some long-term preclinical testing may continue afterUnless the IND is submitted. AnFDA raises concerns, an IND automatically becomes effective 30 days after receipt by the FDA, unless before that time the FDA raises concerns or questions related to one or more proposed clinical trials and places the trial on clinical hold.FDA. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. As a result, submission of an IND may not result in the FDA allowing clinical trials to commence.
Clinical trials
The clinical stage of development involves the administration of the investigational productmedicine to healthy volunteers or patients under the supervision of qualified investigators generally physicians not employed by or under the trial sponsor’s control,and in accordance with GCP requirements, which include the requirement that all patients provide their informed consent for their participation in any clinical trial.requirements. Clinical trials are conducted under protocols detailing, among other things, the objectives of the clinical trial, dosing procedures, subject selection and exclusion criteria and the parameters to be used to monitor subject safety and assess efficacy. Each protocol, and any subsequent amendments to the protocol, must be submitted to the FDA as part of the IND. Furthermore, each clinical trial must be reviewed and approved by an IRBInstitutional Review Board (IRB) for each institution at which the clinical trial will be conducted to ensure that the risks to individuals participating in the clinical trials are minimized and are reasonable in relation to anticipated benefits. The IRB also approves the informed consent form that must be provided to each clinical trial subject or his or her legal representativesubjects and must monitormonitors the clinical trial until completed. There also are requirements governingFurther, progress reports detailing the reportingresults of ongoingthe clinical trials, among other information, must be submitted at least annually to the FDA and completed clinical trial results to public registries.more frequently in other situations, including the occurrence of serious adverse events. Information about certain clinical trials including clinical trial results, must be submitted within specific timeframes for publication on the www.clinicaltrials.gov website.
In addition toUnder the submission of an IND to the FDA before initiation of a clinical trial in the United States, certain human clinical trials involving recombinant or synthetic nucleic acid molecules had historically been subject to review by the Recombinant DNA Advisory Committee, or RAC, of theU.S. National Institutes of Health or NIH, Office of Biotechnology Activities, or the OBA, pursuant to the NIH
Guidelines for Research Involving Recombinant DNA Molecules or NIH Guidelines. On August 17, 2018, the NIH issued a notice in the Federal Register and issued a public statement proposing changes to the oversight framework for gene therapy trials, including changes to the applicable NIH Guidelines to modify the roles and responsibilities of the RAC with respect to human clinical trials of gene therapy products, and requesting public comment on its proposed modifications. During the public comment period, which closed October 16, 2018, the NIH announced that it will no longer accept new human gene transfer protocols for review as a part of the protocol registration process or convene the RAC to review individual clinical protocols. In April 2019, NIH announced the updated guidelines, which reflect these proposed changes, and clarified that these trials will remain subject to the FDA’s oversight and other clinical trial regulations, and oversight at the local level will continue as set forth in the NIH Guidelines. Specifically, under the NIH Guidelines,(NIH Guidelines), supervision of human gene transfer trials includes evaluation and assessment by an institutional biosafety committee or IBC,(IBC), a local institutional committee that reviews and oversees research utilizing recombinant or synthetic nucleic acid molecules at that institution. The IBC assesses the safety of the research and identifies any potential risk to public health or the environment, and such review may result in some delay before initiation of a clinical trial. While the NIH Guidelines are notonly mandatory unless thefor research in question being conducted at or sponsored by institutions receiving NIH funding of recombinant or synthetic nucleic acid molecule research, many companies and other institutions not otherwise subject to the NIH Guidelines voluntarily follow them.
A sponsor who wishesForeign studies conducted under an IND must meet the same requirements that apply to conduct a clinical trial outside ofstudies being conducted in the United States may, but need not, obtain FDA authorization to conduct the clinical trial under an IND. IfStates. Data from a foreign clinical trial isstudy not conducted under an IND the sponsor may submit data from the clinical trial to the FDAbe submitted in support of a BLA or NDA. The FDA will accept a well-designed and well-conducted foreign clinical study not conducted under an IND if the study was conducted in accordance with GCP requirements, and the FDA is able to validate the data through an onsite inspection if deemed necessary.data.
Clinical trials generally are conducted in three sequential phases, known as Phase 1, Phase 2 and Phase 3, andwhich may overlap.overlap:
•Phase 1 clinical trials generally involve a small number of healthy volunteers or disease-affected patients who are initially exposed to a single dose and then multiple doses of the product candidate. The primary purpose of these clinical trials is to assess the metabolism, pharmacologic action, side effect tolerability, and safety of the product candidate.investigational medicine.
•Phase 2 clinical trials generally involve studies in disease-affected patients to evaluate proof of concept and/or determine the dosing regimen(s) for subsequent investigations. At the same time, safety and further pharmacokinetic and pharmacodynamic information is collected, possible adverse effects and safety risks are identified, and a preliminary evaluation of efficacy is conducted.
•Phase 3 clinical trials generally involve a large number of disease-affected patients at multiple sites and are designed to provide the data necessary to demonstrate the effectiveness of the productinvestigational medicine for its intended use, its safety in use and to establish the overall benefit/risk relationship of the product,investigational medicine, and provide an adequate basis for product labeling.
In August 2018, theThe FDA released a draft guidance entitled “Expansion Cohorts: Use in First-In-Human Clinical Trials to Expedite Development of Oncology Drugs and Biologics,” which outlines how drug developers can utilize an adaptive trial design commonly referred to as a seamless trial design in early stages of oncology drug development, i.e., the first-in-human clinical trial, to compress the traditional three phases of trials into one continuous trial called an expansion cohort trial. Information to support the design of individual expansion cohorts are included in IND applications and assessed by FDA. Expansion cohort trials can potentially bring efficiency to drug development and reduce developmental costs and time.
Post-approval trials, sometimes referred to asmay also require post-approval Phase 4 clinical trials, may be conducted after initial marketing approval. These trials are usednon-registrational studies to gain additional experience from the treatment of patients in the intended therapeutic indicationexplore scientific questions to further characterize safety and are commonly intended to generate additional safety data regardingefficacy during commercial use of the product in a clinical setting. In certain instances, the FDA may mandate the performance of Phase 4 clinical trials as a condition of approval of a BLA or NDA.drug.
Progress reports detailing the results of the clinical trials, among other information, must be submitted at least annually to the FDA and written IND safety reports must be submitted to the FDA and the investigators 15 calendar days after the trial sponsor determines the information qualifies for reporting for serious and unexpected suspected adverse events, findings from other studies or animal or in vitro testing that suggest a significant risk for human subjects and any clinically important increase in the rate of a serious suspected adverse reaction over that listed in the protocol or investigator brochure. The sponsor also must notify the FDA of any unexpected fatal or life-threatening suspected adverse reaction as soon as possible but in no case later than seven calendar days after the sponsor’s initial receipt of the information.
Phase 1, Phase 2, Phase 3, and other types of clinical trials may not be completed successfully within any specified period, if at all. The FDA or the sponsorclinical trial site may suspend or terminate a clinical trial at any time on various grounds, including a finding that the patients are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the drug or biologic has been associated with unexpected serious harm to patients. Additionally, some clinical trials are overseen by an independent group of qualified experts organized by the clinical trial sponsor, known as a data safety monitoring board or committee. This group provides
authorization for whether a clinical trial may move forward at designated check points based on access to certain data from the trial. Concurrent with clinical trials, companies usually complete additional animal studies and also must develop additional information about the chemistry and physical characteristics of the drug or biologic as well as finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the product and, among other things, companies must develop methods for testing the identity, strength, quality, and purity of the final product. Additionally, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the investigational medicines do not undergo unacceptable deterioration over their shelf life.trial.
FDA review process
Following completion of the clinical trials, data are analyzed to assess whether the investigational product is safe and effective for the proposed indicated use or uses. The results of preclinical studies and clinical trials are then submitted to the FDA as part of a BLA or NDA, along with proposed labeling, chemistry, and manufacturing information to ensure product quality and other relevant data. A BLA is a request for approval to market a biologic for one or more specified indications and must contain proof of the biologic’s safety, purity, and potency. An NDA for a new drug must contain proof of the drug’s safety and efficacy. The marketing application may include both negative and ambiguous results of preclinical studies and clinical trials, as well as positive findings. Data may come from company-sponsored clinical trials intended to test the safety and efficacy of a product’s use or from a number of alternative sources, including studies initiated by investigators. To support marketing approval, the data submitted must be sufficient in quality and quantity to establish the safety and efficacy of the investigational product to the satisfaction of the FDA. FDA approval of a BLA or NDA must be obtained before a biologic or drug may be marketed in the United States.
Under the Prescription Drug User Fee Act, or PDUFA, as amended, each BLA or NDA must be accompanied by a user fee. The FDA adjusts the PDUFA user fees on an annual basis. Fee waivers or reductions are available in certain circumstances, including a waiver of the application fee for the first application filed by a small business. Additionally, no user fees are assessed on BLAs or NDAs for products designated as orphan drugs, unless the product also includes a non-orphan indication.
The FDA reviews all submitted BLAs and NDAs before it accepts them for filing and may request additional information rather than accepting the BLA or NDA for filing. The FDA must make a decision on accepting a BLA or NDA for filing within 60 days of receipt, and such decision could include a refusal to file by the FDA. Once the submission is accepted for filing, the FDA begins an in-depth review of the BLA or NDA. Under the goals and policies agreed to by the FDA under PDUFA, the FDA has 10 months, from the filing date, in which to complete its initial review of an original BLA or NDA for a new molecular entity and respond to the applicant, and six months from the filing date of an original BLA or NDA designated for priority review. The FDA does not always meet its PDUFA goal dates for standard and priority BLAs and NDAs, and the review process is often extended by FDA requests for additional information or clarification.
Before approving a BLA or NDA, the FDA will conduct a pre-approval inspection of the manufacturing facilities for the new product to determine whether theythe facilities comply with cGMP requirements. The FDA will not approve the product unless it determines that the manufacturing processesrequirements and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications.
The FDA also may audit data from clinical trials to ensure compliance with GCP requirements. Additionally, the FDA may refer applications for novel products or products which present difficult questions of safety or efficacy to an advisory committee typically a panel that includes clinicians and other experts,of expert advisors for review, evaluation and a recommendation as to whether the application should be approved and under what conditions, if any. The committee makes a recommendation to the FDA that is not bound by recommendations of an advisory committee,binding but it considers such recommendations when making decisions on approval. The FDA likely will reanalyze the clinical trial data, which could result in extensive discussions between the FDA and the applicant during the review process. is generally followed.
After the FDA evaluates a BLA or NDA, it will grant marketing approval, request additional information or issue an approvala complete response letter or a Complete Response Letter. An approval letter authorizes commercial marketing of(CRL) outlining the biologic or drug with specific prescribing information for specific indications. A Complete Response Letter indicates that the review cycle of the application is complete, and the application will not be approved in its present form. A Complete Response Letter usually describes all of the specific deficiencies in the BLA or NDA identified by the FDA.submission. The Complete Response LetterCRL may require additional testing or information, including additional preclinical or clinical data, additional pivotal Phase 3 clinical trial(s), and/or other significant and time-consuming requirements relatedfor the FDA to clinical trials, preclinical studies, or manufacturing. If a Complete Response Letter is issued, the applicant may either resubmitreconsider the BLA or NDA, addressing all of the deficiencies identified in the letter, or withdraw the application or request an opportunity for a hearing.NDA. Even if such dataadditional information and informationdata are submitted, the FDA may decide that the BLA or NDA still does not satisfymeet the criteriastandards for approval. Data obtained from clinical trials are not always conclusive andIf the FDA may interpret data differently than we interpretgrants approval, it issues an approval letter that authorizes commercial marketing of the same data.product with specific prescribing information for specific indications.
Orphan drug designation
Under the Orphan Drug Act, the FDA may grant orphan designation to a drug or biologicalbiologic product intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States, or more than 200,000 individuals in the United States and for which there is no reasonable expectation that the cost of developing and making the product available in the United States for this type of disease or condition will be recovered from sales of the product.
Orphan drug designation must be requested before submitting a BLA or NDA. After the FDA grants orphan drug designation, the identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. Orphan drug designation on its own does not convey any advantage in or shorten the duration of the regulatory review and approval process.
If a product that has orphan designation subsequently receives the first FDA approval for the disease or condition for which it has such designation, the product is entitled to orphan drug exclusivity, which means that the FDA may not approve any other applications to market the same drug for the same indication for seven years from the date of such approval, except in very limited circumstances, such as a showing of clinical superiorityif the latter product is shown to be clinically superior to the product with orphan exclusivity by means of greater effectiveness, greater safety, or providing a major contribution to patient care, or in instances of drug supply issues. Competitors, however, may receive approval of either a different product for the same indication or the same product for a different indication; in the latter case, because health care professionals are free to prescribe products for off-label uses, the competitor’s product could be used for the orphan indication despite our orphan exclusivity. Orphan drug exclusivity also could block the approval of one of our products for seven years if a competitor obtains approval before we do for the same drug and same indication, as defined by the FDA, for which we are seeking approval, or if our product is determined to be contained within the scope of the competitor’s product for the same indication or disease. If we pursue marketing approval for an indication broader than the orphan drug designation we have received, we may not be entitled to orphan drug exclusivity. Orphan drug status in the European Union, or EU, has similar, but not identical, requirements and benefits.product.
Expedited development and review programs
The FDA hasmay employ one of several tools to facilitate and expedite the development and review of a fast track program that is intended to expedite or facilitate the process for reviewing new drugs and biologics that meet certain criteria. Specifically, new drugs and biologics are eligible formedicine, including fast track designation, if they are intended to treat a serious or life-threatening conditionbreakthrough therapy designation, accelerated approval and preclinical or clinical data demonstrate the potential to address unmet medical needs for the condition.priority review designation. Fast track designation appliesis designed to bothfacilitate the product and the specific indication for which it is being studied. The sponsor can request the FDA to designate the product for fast track status any time before receiving BLA or NDA approval, but ideally no later than the pre-BLA or pre-NDA meeting. Any product submitted to the FDA for marketing, including under a fast track program, may be eligible for other types of FDA programs intended to expedite development and review such as priority review and accelerated approval. Any product is eligible for priority review if itof a medicine that treats a serious or life-threatening condition and if approved,fills an unmet medical need. Breakthrough therapy designation is designed to expedite the development and review of a medicine that treats a serious condition and preliminary clinical evidence demonstrates substantial improvement over available therapies. Priority review designation means the FDA’s goal is to take action on an application within six months of filing. The FDA may grant priority review designation to a medicine that would provide a significant improvement in the safety andor effectiveness compared to available therapies. The FDA will attempt to direct additional resources to the evaluation of an application for a new drugtreatment, diagnosis or biologic designated for priority review in an effort to facilitate the review.prevention of a serious condition.
A product may also be eligible for accelerated approval if it treats a serious or life-threatening condition and generally provides a meaningful advantage over available therapies. In addition, itsuch product must demonstrate an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality or IMM,(IMM) that is reasonably likely to predict an effect on IMM or other clinical benefit. As a condition of approval, the FDA may require that a sponsor of a drug or biologic receiving accelerated approval perform adequate and well-controlled post-marketing clinical trials.trials with due diligence. If the FDA concludes that a drug or biologic shown to be effective can be safely used only if distribution or use is restricted, it will require such post-marketing restrictions, as it deems necessary to assure safe use of the product. IfUnder the Food and Drug Omnibus Reform Act of 2022 (FDORA), the FDA determinesis now permitted to require, as appropriate, that such trials be underway prior to approval or within a specific time period after the conditionsdate of approval are not being met,for a product granted accelerated approval. Additionally, under FDORA, the FDA canhas increased authority for expedited procedures to withdraw its accelerated approval for such drug or biologic.biologic if, for example, the confirmatory trial fails to verify the predicted clinical benefit of the product.
Additionally, a drug or biologic may be eligible for designation as a breakthrough therapy if the product is intended, alone or in combination with one or more other drugs or biologics, to treat a serious or life-threatening condition and preliminary clinical evidence indicates that the product may demonstrate substantial improvement over currently approved therapies on one or more clinically significant endpoints. The benefits of breakthrough therapy designation include the same benefits as fast track designation, plus intensive guidance from the FDA to ensure an efficient drug development program.
Even if a product qualifies for one or more of these programs, the FDA may later decide that the product no longer meets the conditions for qualification or that the time period for FDA review or approval may not be shortened. Furthermore, fast track designation, priority review, accelerated approval, and breakthrough therapy designation do not change the standards for approval, but may expedite the development or approval process.approval.
Emergency Use Authorization (EUA)
The Secretary of Health and Human Services (HHS) may authorize unapproved medical products including vaccines, to be marketed in the context of an actual or potential emergency that has been designated by the U.S. government. The COVID-19 pandemic has been designated as such a nationalan emergency. After an emergency has been announced, the Secretary of Health and Human ServicesHHS may authorize the issuance of and thereafter, the FDA Commissioner may issue EUAs for the use of specific products based on certain criteria, established by the FDCA, including that the product at issue may be effective in diagnosing, treating, or preventing serious or life-threatening diseases when there are no adequate, approved, and available alternatives. An EUA is subject to additional conditions and restrictions and is product-specific. An EUA terminates when the emergency determination underlying the EUA terminates. An EUA is not a long-term alternative to obtaining FDA approval, licensure, or clearance for a product. The FDA may revoke an EUA for a variety of
reasons, including where it is determined thatif the underlying health emergency no longer exists or warrants such authorization, so it is not possibleauthorization.
In the United States, the Public Readiness and Emergency Preparedness Act (PREP Act) provides immunity for manufacturers from all claims under state or federal law for “loss” arising out of the administration or use of a “covered countermeasure.” However, injured persons may still bring a suit for “willful misconduct” against the manufacturer under some circumstances. “Covered countermeasures” include “qualified pandemic or epidemic products,” including products intended to predict how long an EUA may remaindiagnose or treat pandemic or epidemic disease, such as pandemic vaccines. For these immunities to apply, the Secretary of HHS must issue a declaration in place.cases of public health emergency or “credible risk” of a future public health emergency. On March 17, 2020, the Secretary of HHS issued a declaration under the PREP Act and has issued subsequent amendments thereto to provide liability immunity for activities related to certain countermeasures against COVID-19. While we believe our products sold to the U.S. Government would be covered under the provisions of the PREP Act, this cannot be assured.
Pediatric information
Under the Pediatric Research Equity Act as amended, a BLAof 2003, all marketing applications for new active ingredients, indications, dosage forms, dosing regimens or NDA or supplement to a BLA or NDAroutes of administration must contain data to assessan assessment of the safety and efficacyeffectiveness of the drugproduct for the claimed indicationsindication in all relevant pediatric subpopulations and to support dosing and administration for each pediatric subpopulation for which the productpatients unless this requirement is safe and effective. The FDA may grant deferrals for submission of pediatric datawaived, deferred or full or partial waivers. A sponsor who is planning to submit a marketing application for a drug that includes a new active ingredient, new indication, new dosage form, new dosing regimen, or new route of administration must submit an initial Pediatric Study Plan, or PSP, within 60 days of an end-of-Phase 2 meeting or, if there is no such meeting, as early as practicable before the initiation of the Phase 3 or Phase 2/3 study. The initial PSP must include an outline of the pediatric study or studies that the sponsor plans to conduct, including study objectives and design, age groups, relevant endpoints, and statistical approach, or a justification for not including such detailed information, and any request for a deferral of pediatric assessments or a full or partial waiver of the requirement to provide data from pediatric studies along with supporting information. The FDA and the sponsor must reach an agreement on the PSP. A sponsor can submit amendments to an agreed-upon initial PSP at any time if changes to the pediatric plan need to be considered based on data collected from preclinical studies, early phase clinical trials, and/or other clinical development programs.inapplicable.
Under the Best Pharmaceuticals for Children Act, a product may be eligible for pediatric exclusivity, which adds six months to existing exclusivity periods and patent terms. This exclusivity may be granted based on the voluntary completion of a pediatric study in accordance with an FDA-issued written request for such a study.
Post-approval requirements
Following approval of a new product, the manufacturer and the approved product are subject to continuing regulation by the FDA, including, among other things, monitoring and record-keeping activities, reporting of adverse experiences, complying with promotion and advertising requirements, which include restrictions on promoting products for unapproved uses or patient populations (known as ‘‘off-label use’’), and limitations on industry-sponsored scientific and educational activities. Although physicians may prescribe legally available products for off-label uses, manufacturers may not market or promote such uses. Prescription drug and biologic promotional materials must be submitted to the FDA in conjunction with their first use. Further, if there are any modifications to the drug or biologic, including changes in indications, labeling or manufacturing processes or facilities, the applicant may be required to submit and obtain FDA approval of a new BLA or NDA or BLA or NDA supplement, which may require the development of additional data or preclinical studies and clinical trials.
The FDA may also place other conditions on approvals including the requirement for a Risk Evaluation and Mitigation Strategy or REMS,(REMS) to assure that the safe usebenefits of the product. Ifproduct outweigh the FDA concludes a REMS is needed, the sponsor of the BLA or NDA must submit a proposed REMS. The FDA will not approve the BLA or NDA without an approved REMS, if required.risks. A REMS could include medication guides, physician communication plans, or elements to assure safe use, such as restricted distribution methods, patient registries, and other risk minimization tools. Any of these limitations on approval or marketing could restrict the commercial promotion, distribution, prescription or dispensing of products. Newly discovered or developed safety or effectiveness data may require changes to a drug’sproduct’s approved labeling, including the addition of new warnings and contraindications, and also may require the implementation of other risk management measures, including a REMS or the conduct of post-marketing studies to assess a newly discovered safety issue. Product approvals may be withdrawn for non-compliance with regulatory standards, or if problems occur following initial marketing.
FDA regulations require that products be manufactured in specific approved facilities and in accordance with cGMP regulations. In addition to our own manufacturing facilities, we rely,Entities involved in the manufacture and expect to continue to rely, on third parties for the productiondistribution of certain clinicalapproved drugs or biologics and commercial quantities of our products in accordance with cGMP regulations. We, and thesetheir third-party manufacturers must comply with cGMP regulations that require, among other things, quality control and quality assurance, the maintenance of records and documentation, and the obligation to investigate and correct any deviations from cGMP. Manufacturers and otherSuch entities involved in the manufacture and distribution of approved drugs or biologics are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP requirements and other laws. Accordingly, manufacturers must continue to expend time, money, and effort in the area of production and quality control to maintain cGMP compliance. The discovery of violative conditions, including failure to conform to cGMP regulations,violations could result in enforcement actions, and the discovery of problems with a product after approval may result in restrictions on a product, manufacturer, or holder of an approved BLA or NDA, including recall.
U.S. patent term restoration and marketingregulatory data exclusivity
Depending upon the timing, duration, and specifics of FDA approval of our investigational medicines and any future investigational medicines,
In certain circumstances, some of our U.S. patents may be eligible for limited patent term extension under the Drug Price Competition and Patent
Term Restoration Act of 1984, commonly referred to as the Hatch Waxman Amendments. The Hatch Waxman Amendments permit restoration of the patent term of up to five years as compensation for patent term lost during product development and FDA regulatory review process. Patent term restoration, however, cannot extend the remaining term of a patent beyond a total of 14 years from the product’s approval date. The patent term restoration period is generally one half the time between the effective date of an IND and the submission date of a BLA or NDA, plus the time between the submission date of a BLA or NDA and the approval of that application, except that the review period is reduced by any time during which the applicant failed to exercise due diligence.application. Only one patent applicable to an approved drugproduct is eligible for such an extension and the application for the extension must be submitted prior to the expiration of the patent. The USPTO, in consultation with the FDA, reviews and approves the application for any patent term extension or restoration. In
If the future, weFDA approves a drug product that contains an active ingredient not previously approved, the product is typically entitled to five years of non-patent regulatory data exclusivity. Other products may apply for restoration of patent term for our currently owned or licensed patentsbe entitled to add patent life beyond the current expiration date, depending on the expected length of the clinical trials and other factors involved in the filing of the relevant BLA or NDA.
Marketing exclusivity provisions under the FDCA can also delay the submission or the approval of certain marketing applications. The FDCA provides three years of marketingregulatory data exclusivity for an NDA, or supplement to an existing NDA, if approval was based on the FDA’s reliance on new clinical investigations, other than bioavailability studies that were conducted or sponsoredessential to approval submitted by the NDA applicant. If the NDA applicant are deemedstudies the product for use by the FDA to be essential to the approval of the application, for example for new indications, dosages, or strengths of an existing drug. This three-year exclusivity covers only the modification for which the drug received approval on the basis of the new clinical investigations and does not prohibit the FDA from approving abbreviated new drug applications, or ANDAs, for drugs containing the active agent for the original indication or condition of use. The FDCA also provides a five-year period of non-patent marketing exclusivity within the United States to the first applicant to obtain approval of an NDA for a new chemical entity. A drug is a new chemical entity if the FDA has not previously approved any other new drug containing the same active moiety, which is the molecule or ion responsible for the action of the drug substance.
During the exclusivity period,children, the FDA may not accept for review an ANDA or a 505(b)(2) NDA submittedgrant pediatric exclusivity, which extends by another company for another drug based on180 days each existing exclusivity (patent and regulatory) related to the same active moiety, regardless of whether the drug is intended for the same indication as the original innovator drug or for another indication, where the applicant does not own or have a legal right of reference to all the data required for approval. However, an application may be submitted after four years if it contains a certification of patent invalidity or non-infringement to one of the patents listed with the FDA by the innovator NDA holder. Three-year and five-year exclusivity will not delay the submission or approval of a full NDA. However, an applicant submitting a full NDA would be required to conduct or obtain a right of reference to all of the nonclinical studies and adequate and well-controlled clinical trials necessary to demonstrate safety and efficacy.product.
An abbreviated approval pathway for biological products shown to be biosimilar to, or interchangeable with, an FDA-licensed reference biological product was created by the Biologics Price Competition and Innovation Act of 2009 or(the BPCI Act. This amendment to the PHSA, in part, attempts to minimize duplicative testing.Act). Biosimilarity which requires a showing that the biological product be highly similaris “highly similar” to the reference product notwithstanding minor differences in clinically inactive components and that there beare no clinically meaningful differences between the product and the reference product in terms of safety, purity, and potency, can be shown through analytical studies, animal studies, and a clinical trial or trials.potency. Interchangeability requires that a biological product be biosimilar to the reference product and that the product can be expected to produce the same clinical results as the reference product in any given patient and, for products administered multiple times to an individual, that the product and the reference product may be alternated or switched after one has been previously administered without increasing safety risks or risks of diminished efficacy relative to exclusive use of the reference biological product without such alternation or switch.
product. A reference biological product is granted 12 years of regulatory data exclusivity from the time of first licensure of the product
and the FDA will not accept an application for a biosimilar or interchangeable product based on the reference biological product until four years after the date of first licensure of the reference product. ‘‘First licensure’’ typically means the initial date the particular product at issue was licensed
Drug development in the United States. Date of first licensure does not include the date of licensure of (and a new period of exclusivity is not available for) a biological product if the licensure is for a supplement for the biological product or for a subsequent application by the same sponsor or manufacturer of the biological product (or licensor, predecessor in interest, or other related entity) for a change (not including a modification to the structure of the biological product) that results in a new indication, route of administration, dosing schedule, dosage form, delivery system, delivery device or strength, or for a modification to the structure of the biological product that does not result in a change in safety, purity, or potency.European Economic Area (EEA)
Pediatric exclusivity is another type of regulatory market exclusivity in the United States. Pediatric exclusivity, if granted, adds six months to existing regulatory exclusivity periods. This six month exclusivity may be granted based on the voluntary completion of a pediatric trial in accordance with an FDA issued ‘‘Written Request’’ for such a trial.
European Union drug development
In the EU, our future products also may be subject to extensive regulatory requirements. As in the United States, medicinalMedicinal products can be marketed only if a marketing authorization from the competent regulatory agencies has been obtained.
Similar to the United States, the various phases of preclinical and clinical research in the European Union are subject to significant regulatory controls. Although the EU Clinical Trials Directive 2001/20/EC has sought to harmonize the EU clinical trials regulatory framework, setting out common rules for the control and authorization of clinical trials in the EU, the EU Member States have transposed and applied the provisions of the Directive differently. This has led to significant variations in the Member State regimes. Under the current regime, before a clinical trial can be initiated it must be approved in each of the EU countries where the trial is to be conducted by two distinct bodies: the National Competent Authority, or NCA, and one or more Ethics Committees, or ECs. Under the current regime all suspected unexpected serious adverse reactions to the investigated drug that occur during the clinical trial have to be reported to the NCA and ECs of the Member State where they occurred.
The EU clinical trials legislation currently is undergoing a transition process mainly aimed at harmonizing and streamlining clinical trial authorization, simplifying adverse event reporting procedures, improving the supervision of clinical trials, and increasing their transparency. In April 2014, the EU adopted a new Clinical Trials Regulation (EU) No 536/2014, which is set to replace the current Clinical Trials Directive 2001/20/EC. It is expected that the new Clinical Trials Regulation (EU) No 536/2014 will apply following confirmation of full functionality of the Clinical Trials Information System (CTIS), the centralized EU portal and database for clinical trials foreseen by the Regulation, through an independent audit, currently expected to occur in December 2021. It will overhaul the current system of approvals for clinical studies in the EU. Specifically, the new Regulation, which will be directly applicable in all Member States (and so does not require national implementing legislation in each Member State), aims at simplifying and streamlining the approval of clinical studies in the EU. For instance, the new Regulation provides for a streamlined application procedure via a single point and strictly defined deadlines for the assessment of clinical study applications.
Pediatric investigation plan
An application for marketing authorization of a medicinal product for human use which is not yet authorized in the EU shall be considered valid only if it includes a Pediatric Investigational Plan, or PIP, according to Regulation (EC) No. 1901/2006 unless a waiver applies (e.g., because the relevant disease or condition occurs only in adults). The PIP or the application for waiver shall be submitted with a request for agreement, except in duly justified cases, early during the product development phase and not later than upon completion of the human pharmacokinetic studies in healthy subjects. The end of Phase 1 pharmacokinetic studies can coincide with the initial tolerability studies, or the initiation of the adult Phase 2 studies (proof-of-concept studies); in any case, submission of the PIP cannot be after initiation of pivotal trials or confirmatory (Phase 3) trials.
The Pediatric Committee, a scientific committee established at the Community level, shall assess the content of any PIP, waivers, and deferrals for a medicinal product submitted to it in accordance with the regulation on medicinal products for pediatric use and formulate an opinion thereon.
European drug review and approval
In the European Economic Area, or EEA, which is comprised of the 27 Member States of the EU and Norway, Iceland and Liechtenstein, only if a marketing authorization from the competent regulatory agency has been obtained. Similar to the United States, the various phases of preclinical and clinical research in the EU are subject to significant regulatory controls. Effective since January 2022, the Clinical Trials Regulation (No. 536/2014) aims to streamline and harmonize the procedures for assessment and governance of clinical trials throughout the EU and to require that information on the authorization, conduct and results of each clinical trial conducted in the EU be publicly available.
Pediatric investigation plan
An application for marketing authorization of a medicinal product for human use that is not yet authorized in the EU must include a Pediatric Investigational Plan (PIP) pursuant to the regulation on medicinal products can only be commercialized after obtainingfor pediatric use (known as the Paediatric Regulation), unless a Marketing Authorization, or MA. There are two types of marketing authorizations.
The Community MA is issued bywaiver applies. A scientific committee established at the European Commission throughMedicines Agency (EMA), the Centralized Procedure, based onPaediatric Committee (PDCO) assesses the content of any PIP, waivers, and deferrals for a medicinal product submitted to it and formulates an opinion ofthereon.
Review and approval process
In the CommitteeEEA, in order to obtain a marketing authorization from the applicable regulatory authority, a company may submit marketing authorization applications either under a centralized or decentralized procedure. The centralized procedure, which is compulsory for Medicinal Products for Human Use, or CHMP, of the EMA, and is valid throughout the entire territory of the EEA. The Centralized Procedure is mandatory formedicines produced by certain types of products, such as biotechnologybiotechnological processes, advanced therapy medicinal products (ATMPs), orphan medicinal products, advanced therapyor those medicines such as gene therapy, somatic cell therapy or tissue engineered medicines, and medicinal products containing a new active substance indicated for the treatment of HIV, and intended to treat specific diseases (HIV/AIDS, cancer, neurodegenerative disorders, diabetes, autoimmune and other immune dysfunctions and viral diseases. Fordiseases), and optional for those products for which the use of the Centralized Procedure is not mandatory, applicants may elect to use the Centralized Procedure where either the product containsmedicines that are highly innovative or contain a new active substance, not yet authorizedprovides for the grant of a single marketing authorization that is valid throughout the EEA. In addition to the centralized procedure, a marketing authorization can also be obtained in the EEA through a national procedure, which requires a separate application to and approval determination by each country; a decentralized procedure, whereby applicants submit identical applications to several countries and receive simultaneous approval; and a mutual recognition procedure, where applicants submit an application to one country for review and other countries may accept or wherereject the initial decision.
A conditional marketing authorization may be granted in the EU when comprehensive clinical data for the safety and efficacy of the medicinal product have not been supplied but all the following requirements are met: (i) the risk-benefit balance of the medicine is positive; (ii) it is likely that the applicant can show that the product constitutes a significant therapeutic, scientific, or technical innovation or for which the Centralized Procedure is in the interest of patients at a European level.
National Marketing Authorizations, which are issued by the competent authorities of the Member States of the EEA and only cover their respective territory, are available for products not falling within the mandatory scope of the Centralized Procedure. Where a product has already been authorized for marketingwill be in a Member State ofposition to provide the EEA, this National Marketing Authorization can be recognized in another Member State throughcomprehensive clinical data post-authorization; (iii) the Mutual Recognition Procedure. Ifmedicine fulfills an unmet medical need; and (iv) the product has not received a National Marketing Authorization in any Member State at the time of application, it can be approved simultaneously in various Member States through the
Decentralized Procedure. Under the Decentralized Procedure an identical dossier is submitted to the competent authorities of each of the Member States in which the marketing authorization is sought, one of which is selected by the applicant as the Reference Member State, or RMS. The competent authority of the RMS prepares a draft assessment report, a draft summary of the product characteristics, or SPC, and a draft of the labeling and package leaflet, which are sent to the other Member States (referred to as the Concerned Member States) for their approval. If the Concerned Member States raise no objections, based on a potential serious riskbenefit to public health in relation toof the assessment, SPC, labeling, or packaging proposed byimmediate availability on the RMS,market of the productmedicine outweighs the risk that additional data is subsequently grantedstill required. Conditional marketing authorizations are valid for one year, on a nationalrenewable basis. The marketing authorization in allholder will be required to fulfil specific obligations within certain timeframes, which may include completing ongoing trials or conducting new trials to confirm that the Member States (i.e., inbenefit-risk balance is positive. Once such obligations are fulfilled, provided the RMS and the Concerned Member States).
Under the above described procedures, before granting thebenefit-risk balance is still positive, a conditional marketing authorization the EMA or the competent authorities of the Member States of the EEA make an assessment of the risk benefit balance of the product on the basis of scientific criteria concerning its quality, safety, and efficacy.can be converted into a standard marketing authorization.
Now that the UK (which comprises Great Britain and Northern Ireland) has left the European Union, Great Britain will no longer be covered by centralized MAs (under the Northern Irish Protocol, centralized marketing authorizations will continue to be recognized in Northern Ireland). All medicinal products with a current centralized marketing authorization were automatically converted to Great Britain MAs on January 1, 2021. For a period of two years from January 1, 2021, the Medicines and Healthcare products Regulatory Agency, or MHRA, the UK medicines regulator, may rely on a decision taken by the European Commission on the approval of a new marketing authorization in the centralized procedure, in order to more quickly grant a new Great Britain MA. A separate application will, however, still be required.
European exclusivityregulatory data protection
In the EEA,EU, new innovative products authorized for marketing (i.e., reference products) qualify for regulatory data protection consisting of eight years of data exclusivity and an additional two years of market exclusivityprotection upon the grant of a marketing authorization. During the period of dataData exclusivity prevents generic or biosimilar applicants may not referencefrom referencing the innovator’s preclinical or clinical trial data contained in the dossier of the reference product when applying for a generic or biosimilar marketing authorization for a period of eight years from the date on which the reference product was first authorized in the EEA. authorization. During the additional two-year period of market exclusivity,protection, a generic or biosimilar marketing authorization application can be submitted, and the innovator’s data may be referenced, but no generic or biosimilar product can be marketed until the expiration of the market exclusivityprotection period. The overall 10-year periodThere is no guarantee that a product will be extendedconsidered by the EU’s regulatory authorities to a maximum of 11 years if, during the first eight years of those 10 years, the marketing authorization holder obtains an authorization for one or more new therapeutic indications which, during the scientific evaluation prior to their authorization, are determined to bring a significant clinical benefit in comparison with currently approved therapies. Even ifbe an innovative medicinal product, gains the prescribed period ofand products may not qualify for regulatory data exclusivity, another company may market another version of the product if such company obtained a marketing authorization based on an application with a completely independent data package of pharmaceutical tests, preclinical tests and clinical trials.protection.
European orphan designation and exclusivity
In
Orphan drug designation is available in the EEA, the EMA’s Committee for Orphan Medicinal Products grants orphan drug designationEU to promote the development of products that are intended for the diagnosis, prevention, or treatment of life threatening or chronically debilitating conditions affecting not more than five in 10,000 persons in the EU community, or where it is unlikely that the development of the medicine would generate sufficient return to justify the necessary investment in its development, and in each case for which no satisfactory method of diagnosis, prevention, or treatment has been
authorized (or, if a method exists, the product would be a significant benefit to those affected).
In the EEA, Medicinal products that receive and maintain orphan drug designation entitles a partyfollowing approval are entitled to financial incentives such as reduction of fees or fee waivers and 10 years of market exclusivity, is granted followingwhich protects against applications for and the grant of marketing authorizations for similar medicinal product approval.products in the same therapeutic indication. This period may be reduced to six years if, at the end of the fifth year, it is established that the orphan drug designation criteria are no longer met, including where it is shown that the product is sufficiently profitable not to justify maintenance of market exclusivity.met. During the period of market exclusivity, marketing authorization may only be granted to a “similarsimilar medicinal product”product for the same therapeutic indication if: (i) a second applicant can establish that its product, although similar to the authorized product, is safer, more effective or otherwise clinically superior; (ii) the marketing authorization holder for the authorized product consents to a second orphan medicinal product application; or (iii) the marketing authorization holder for the authorized product cannot supply enough orphan medicinal product. Orphan drug designation must be requested before submitting an application for marketing approval. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process.product
The aforementioned EU rules are generally applicable in the EEA.
European data collectionprotection regulations
The EU General Data Protection Regulation (GDPR) governs the collection and use of personal health data in the EU is governed by the provisions of the Data Protection Directive, and as of May 2018 the General Data Protection Regulation, or GDPR. This directiveEU. The GDPR imposes severalstrict requirements relating to the consent of the individuals to whom the personal data relates, the information provided to the individuals, notification of data processing obligations to the competent national data protection authorities, and the security and confidentiality of the personal data, data breach notification and the use of third-party processors in connection with the processing of the personal data. The Data Protection Directive and GDPR also imposeimposes strict rules on the transfer of personal data out of the EU, provides an enforcement authority and imposes large penalties for noncompliance, including the potential for fines of up to the United States. Failure to comply with the requirements€20.0 million or 4% of the Data Protection Directive,annual global revenues of the infringer, whichever is greater. The GDPR also confers a private right of action on data subjects and consumer associations to lodge complaints with competent national data protection authorities, seek judicial remedies and obtain compensation for damages resulting from violations of the GDPR. Non-compliance could also result in the imposition of orders to stop data processing activities.
The UK has incorporated the GDPR into UK law (the UK GDPR).The UK GDPR and the related nationalUK Data Protection Act 2018 set out the UK’s data protection laws ofregime, which is independent from but aligned to the EU’s data protection regime. Although the UK is regarded as a third country under the EU’s GDPR, the European Commission has issued a decision recognizing the UK as providing adequate protection under the EU Member StatesGDPR and, therefore, transfers of personal data originating in the EU to the UK remain unrestricted. Like the GDPR, the UK GDPR restricts personal data transfers outside the UK to countries not regarded by the UK as providing adequate protection. The UK government has confirmed that personal data transfers from the UK to the EEA remain free flowing. Non-compliance with the UK GDPR may result in fines and other administrative penalties. The GDPR introduces new data protection requirementsmonetary penalties of up to £17.5 million or 4% of worldwide revenue, whichever is higher.
Marketing of medicines in the EU and substantial fines for breaches of the data protection rules. The GDPR regulations may impose additional responsibility and liability in relation
Similar to personal data that we process and we may be required to put in place additional mechanisms ensuring compliance with the new data protection rules. This may be onerous and adversely affect our business, financial condition, results of operations, and prospects.
European Union drug marketing
Much like the Anti-Kickback Statute prohibition in the United States discussed below, the provision of benefits or advantages to physicians to induce or encourage the prescription, recommendation, endorsement, purchase, supply, order, or use of medicinal products is also prohibited in the EU. Infringement of relevant EU laws could result in substantial fines and imprisonment.
Payments may be made to physicians in limited circumstances, and in certain EU Member States such payments must be publicly disclosed. Moreover, agreements with physicians for the provision of services often must be the subject of prior notification and approval by the physician’s employer, his or her competent professional organization, and/or the regulatory authorities of the individual EU Member States. These requirements are provided in the national laws, industry codes, or professional codes of conduct, applicable in the EU Member States. Failure to comply with these requirements could result in reputational risk, public reprimands, administrative penalties, fines, or imprisonment.
Brexit and the Regulatory Framework in the United Kingdom
On June 23, 2016, the electorate in the UK voted in favor of leaving the EU (commonly referred to as Brexit). Thereafter, on March 29, 2017, the country formally notified the EU of its intention to withdraw pursuant to Article 50 of the Lisbon Treaty. The UK formally left the EU on January 31, 2020. A transition period began on February 1, 2020, during which EU pharmaceutical law remained applicable to the UK, however this ended on December 31, 2020. Since the regulatory framework in the UK covering quality, safety and efficacy of pharmaceutical products, clinical trials, marketing authorization, commercial sales and distribution of pharmaceutical products is derived from EU Directives and Regulations, Brexit could materially impact the future regulatory regime which applies to products and the approval of product candidates in the UK, as UK legislation now has the potential to diverge from EU legislation. It remains to be seen how Brexit will impact regulatory requirements for product candidates and products in the UK in the long-term. The MHRA has recently published detailed guidance for industry and organizations to follow from January 1, 2021 now the transition period is over, which will be updated as the UK’s regulatory position on medicinal products evolves over time.
Rest of the world regulation
For other countries outside
Outside of the EU and the United States such as countries in Eastern Europe, Latin America, Middle East, or Asia,and the EU, the requirements governing the conduct of clinical trials, product licensing, pricing, and reimbursement vary from country to country. Additionally, the clinical trials must be conducted in accordance with GCP requirements and the applicable regulatory requirements and the ethical principles that have their origin in the Declaration of Helsinki.
If we fail to comply with applicable foreign regulatorysuch requirements, we may be subject to, among other things, fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions, or criminal prosecution.
Coverage and reimbursement
Patients who are provided medical treatment for their conditions generally rely on third-party payors to reimburse all or part of the costs associated with their treatment. Coverage and adequate reimbursement from governmental healthcare programs, such as Medicare and Medicaid, and commercial payors is critical to new product acceptance. Government authorities and other third-party payors, such as private health insurers and health maintenance organizations, decide which drugs and treatments they will cover and the amount of reimbursement.
In the United States, no uniform policy of coverage and reimbursement for products exists among third-party payors. As a result, obtaining coverage and reimbursement approval of a product from a government or other third-party payor is a time-consuming and costly process that could require us to provide to each payor supporting scientific, clinical and cost-effectiveness data for the use of our products on a payor-by-payor basis, with no assurance that coverage and adequate reimbursement will be obtained. In the United States, the principal decisions about reimbursement for new medicines are typically made by the Centers for Medicare & Medicaid Services (CMS). CMS decides whether and to what extent a new medicine will be covered and reimbursed under Medicare and private payors tend to follow CMS to a substantial degree. Even if we obtain coverage for a given product, the resulting reimbursement payment rates might not be adequate for us to achieve or sustain profitability or may require co-payments that patients find unacceptably high. Additionally, third-party payors may not cover, or provide adequate reimbursement for, long-term follow-up evaluations required following the use of product candidates, once approved. Patients are unlikely to use our product candidates, once approved, unless coverage is provided and reimbursement is adequate to cover a significant portion of their cost. There is significant uncertainty related to insurance coverage and reimbursement of newly approved products. It is difficult to predict at this time what third-party payors will decide with respect to the coverage and reimbursement for our product candidates.
Net prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future relaxation of laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the United States. In addition, many pharmaceutical manufacturers must calculate and report certain price reporting metrics to the government, such as average sales price and best price. Penalties may apply in some cases when such metrics are not submitted accurately and timely.
We expect that healthcare reform measures that may be adopted in the future may result in more rigorous coverage criteria and in additional downward pressure on the price that we receive for any approved product. Additionally, legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products.
In addition, in some foreign countries, the proposed pricing for a drug must be approved before it may be lawfully marketed. The requirements governing drug pricing vary widely from country to country. For example, the European Union provides options for its Member States to restrict the range of medicinal products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. To obtain reimbursement or pricing approval, some of these countries may require the completion of clinical trials that compare the cost effectiveness of a particular product candidate to currently available therapies. A Member State may approve a specific price for the medicinal product or it may instead adopt a system of direct or indirect controls on the profitability of the company placing the medicinal product on the market. There can be no assurance that any country that has price controls or reimbursement limitations for pharmaceutical products will allow favorable reimbursement and pricing arrangements for any of our product candidates. Historically, products launched in the European Union do not follow price structures of the United States and generally prices tend to be significantly lower.
For further information on certain risks associated with the pricing and reimbursement of our products see “Risk Factors—Sales of pharmaceutical products depend on the availability and extent of reimbursement from third-party payors, and we may be adversely impacted by changes to such reimbursement policies or rules.”
Other healthcare laws
Healthcare providers, physicians, and third partythird-party payors, including governmental payors, such as Medicare and Medicaid in the United States, will play a primary role in the recommendation and prescription of any products for which we obtain marketing approval. Our futuremarketed products. Any arrangements with third party payors, healthcare providers, and physicians may expose us to broadly applicablethese parties implicate certain fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell, and distribute any drugs for which we obtain marketing approval.regulations. In the United States, these laws include, without limitation, state and federal anti-kickback, false claims, physician transparency, and patient data privacy and security laws and regulations, including but not limited to those described below.among others:
•The Anti-Kickback Statute, or AKS,which makes it illegal for any person including a prescription drug manufacturer (or a party acting on its behalf), to knowingly and willfully solicit, receive, offer, or pay any remuneration, directly or indirectly, in cash or in kind, that is intended to induce or reward referrals, including the purchase, recommendation, order or prescription of a particular drug or any other good or service, for which payment may be made under a federal healthcare program, such as Medicare or Medicaid. Violations of this law are punishable by up to five years in prison, criminal fines, administrative civil money penalties, and exclusion from participation in federal healthcare programs. In addition, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it.
•The federal False Claims Act, which imposes civil penalties, including through civil whistleblower or qui tam actions, against individuals or entities (including manufacturers) for, among other things, knowingly presenting, or causing to be presented, false or fraudulent claims for payment by a federal healthcare program or making a false statement or record material to payment of a false claim or avoiding, decreasing, or concealing an obligation to pay money to the federal government. The government may deem manufacturers to have “caused” the submission of false or fraudulent claims by, for example, providing inaccurate billing or coding information to customers or promoting a product off-label. Claims which include items or services resulting from a violation of the federal Anti-Kickback Statute are false or fraudulent claims for purposes of the False Claims Act. Our future marketing and activities relating to the reporting of wholesaler or estimated retail prices for our products, the reporting of prices used to calculate Medicaid rebate information, and other information affecting federal, state, and third-party reimbursement for our products, and the sale and marketing of our product and any future investigational medicines, are subject to scrutiny under this law.
•Health Insurance Portability and Accountability Act of 1996 or HIPAA,(HIPAA), which imposes criminal and civil liability for, among other things, knowingly and willfully executing a scheme, or attempting to execute a scheme, to defraud any healthcare benefit program, including private payors, or falsifying, concealing, or covering up a material fact or making any materially false statements in connection with the delivery of or payment for healthcare benefits, items or services. Similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
•HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009 or HITECH,(HITECH), and their respective implementing regulations, which impose, among other things, specified requirements on covered entities and their business associates relating to the privacy and security of individually identifiable health information, including mandatory contractual terms and required implementationinformation.
Table of technical safeguards of such information. HITECH also created new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties directly applicable to business associates, and gave state Attorneys General new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorneys’ fees and costs associated with pursuing federal civil actions.Contents
•The Physician Payments Sunshine Act, enacted as part of the Patient Protection and Affordable Care Act the (ACA), imposed new annual reporting requirements forwhich requires certain pharmaceutical manufacturers of drugs, devices, biologics, and medical supplies for which payment is availablewith products reimbursed under Medicare, Medicaid, orcertain government programs to disclose annually to the Children’s Health Insurance Program, forfederal government (for re-disclosure to the public) certain payments and “transfersother transfers of value”value provided to physicians, (defined to include doctors, dentists, optometrists, podiatrists and chiropractors) and teaching hospitals as well as ownership and investment interests held by physicianscertain other licensed health care practitioners.
•Federal government price reporting laws, which require us to calculate and their immediate family members. Effective January 1, 2022, these reporting obligations will extendreport complex pricing metrics in an accurate and timely manner to include transfers of value made to certain non-physician providers such as physician assistantsgovernment programs.
•Federal consumer protection and nurse practitioners.unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers.
•Analogous state and foreign fraud and abuse laws and regulations, such as state anti-kickback and false claims laws, which may be broader in scope and apply regardless of payor impose a variety of obligations on. Such laws are enforced by variouspayor.
Additionally, certain state agencies and through private actions. Some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant federal government compliance guidance, require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers, and restrict marketing practices or require disclosure of marketing expenditures. State and foreign laws also govern the privacy and security of health information in some circumstances.information. Such data privacy and security laws may differ from each other in significant ways and often are not pre-empted by HIPAA, thus complicating compliance efforts. For example, in California the California Consumer Protection Act (CCPA), which went into effect on January 1, 2020, establishes established a newcomprehensive privacy framework for covered businesses by creating an expanded definition of personal information, establishing new data privacy rights for consumers in the State of California, imposing special rules on the
collection of consumer data from minors, and creating a new and potentially severe statutory damages framework for violations of the CCPA and for businesses that fail to implement reasonable security procedures and practices to prevent data breaches. Further, a new California privacy law, the California Privacy Rights Act (CPRA), was passed by California voterswhich took effect on November 3, 2020. The CPRA will createJanuary 1, 2023, has established additional obligations with respect to processing and storing personal information that are scheduledinformation. The CPRA significantly modified the CCPA, including by expanding customers' rights with respect to take effect on January 1, 2023 (with certain provisions having retroactive effect to January 1, 2022).sensitive personal information. While clinical trial data and information governed by HIPAA are currently exempt from the current versions of the CCPA and CPRA, other personal information may beis applicable and possible changesdue to the CCPA and CPRA may broaden itsCPRA’s broader scope. Similar laws have been passed or proposed in numerous other states.
The scope and enforcement of each of these laws is uncertain and subject to rapid change in the current environment of healthcare reform, especially in light of the lack of applicable precedent and regulations. Federal and state enforcement bodies have recently increased their scrutiny of interactions between healthcare companies and healthcare providers, which has led to a number of investigations, prosecutions, convictions, and settlements in the healthcare industry. It is possible that governmental authorities will conclude that our business practices do not comply with current or future statutes, regulations, or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other related governmental regulations that may apply to us, we may be subject to significant civil, criminal, and administrative penalties, damages, fines, imprisonment, disgorgement, exclusion of drugs from government funded healthcare programs, such as Medicare and Medicaid, reputational harm, additional oversight, and reporting obligations if we become subject to a corporate integrity agreement or similar settlement to resolve allegations of non-compliance with these laws and the curtailment or restructuring of our operations. If any of the physicians or other healthcare providers or entities with whom we expect to do business is found to be not in compliance with applicable laws, they may be subject to similar actions, penalties, and sanctions. Ensuring business arrangements comply with applicable healthcare laws, as well as responding to possible investigations by government authorities, can be time- and resource-consuming and can divert a company’s attention from the business.reform.
Current and future healthcare reform legislation
In the United States and foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of our investigational medicines, restrict or regulate post-approval activities and affect our ability to profitably sell any investigational medicines for which we obtain marketing approval.approved products. The ACA, for example, contains provisions that subject biological products to potential competition by lower-cost biosimilars and may reduce the profitability of drug products through increased rebates for drugs reimbursed by Medicaid programs, extension of Medicaid rebates to Medicaid managed care plans, mandatory discounts for certain Medicare Part D beneficiaries and, annual fees based on pharmaceutical companies’ share of sales to federal health care programs. We expect that currentCurrent laws, as well as other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and in additional downward pressure on the price that we, or any strategic collaborators, may receive for any approved products.
Since its enactment, there have been numerous judicial, administrative, executive, and legislative challenges to certain aspects of the ACA, and we expect there will be additional challenges and amendments to the ACA in the future. For example, various portions of the ACA are currently undergoing legal and constitutional challenges inIn the United States, Supreme Court, and the Trump Administration issued various Executive Orders that eliminated cost sharing subsidies and various provisions that would impose a fiscal burden on states or a cost, fee, tax, penalty or regulatory burden on individuals, healthcare providers, health insurers, or manufacturers of pharmaceuticals or medical devices. Additionally, Congress has introduced several pieces of legislation aimed at significantly revising or repealing the ACA. Itit is unclear whether the ACA will be overturned or further amended, particularly given the new administration.amended. We cannot predict what effect further changes to the ACA would have on our business.
Additionally, other federal health reform measures have been proposed and adopted in the United States since the ACA was enacted:
•Theenacted, including the Budget Control Act of 2011, among other things, created measures for spendingwhich includes provisions to reduce the federal deficit. The Budget Control Act, as amended, resulted in the imposition of 2% reductions by Congress. A Joint Select Committee on Deficit Reduction, tasked with recommending a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, was unable to reach required goals, thereby triggering the legislation’s automatic reduction to several government programs. These changes included aggregate reductions toin Medicare payments to providers, of up to 2% per fiscal year, which went into effectbegan in April 2013 and will remain in effect through 20302031 unless additional Congressional action is taken. PursuantIn 2021, President Biden signed the American Rescue Plan Act of 2021 into law, which eliminated the statutory Medicaid drug rebate cap, previously set at 100% of a drug’s average manufacturer price, for single source and innovator multiple source drugs, beginning in 2024. Due to the Coronavirus Aid, Relief, and Economic Security Act, also known as the CARES Act, as well as subsequent legislation, these reductions have been suspended from May 1, 2020 through March 31, 2021 due to the COVID-19 pandemic. Proposed legislation, if passed, would extend this suspension until the end of the pandemic.
•The American Taxpayer ReliefStatutory Pay-As-You-Go Act of 2012, among other things, reduced2010, estimated budget deficit increases resulting from the American Rescue Plan Act of 2021, and subsequent legislation, Medicare payments to several providers will be further reduced starting in 2025 absent further legislation. These laws and increasedregulations may result in additional reductions in Medicare and other healthcare funding and otherwise affect the statuteprices we may obtain for any of limitations periodour product candidates for which we may obtain regulatory approval or the government to recover overpayments to providers from three to five years.frequency with which any such product candidate is prescribed or used.
TableIn August 2022, the Inflation Reduction Act of Contents2022 (IRA) was signed into law. The IRA includes several provisions that will impact our business to varying degrees, including provisions that create a $2,000 out-of-pocket cap for Medicare Part D beneficiaries, impose new manufacturer financial liability on all drugs in Medicare Part D, allow the U.S. government to negotiate Medicare Part B and Part D pricing for certain high-cost drugs and biologics without generic or biosimilar competition, require companies to pay rebates to Medicare for drug prices that increase faster than inflation and delay the rebate rule that would require pass through of pharmacy benefit manager rebates to beneficiaries. The effect of IRA on our business and the healthcare industry in general is not yet known.
Further, there has been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which have resulted in several recent Congressional inquiries and proposed bills designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs and reform government program reimbursement methodologies for products. In addition, the federal government, state legislatures, and foreign governments have shown significant interest in implementing cost containment programs, including price-controls and price transparency,
restrictions on reimbursement, and requirements for substitution of generic products for branded prescription drugs to limit the growth of government paid health care costs. For example, the federal government has passed legislation requiring pharmaceutical manufacturers to provide rebates and discounts to certain entities and governmental payors to participate in federal healthcare programs. Additionally, the previous administration released a “Blueprint” to lower drug prices and reduce out of pocket costs of drugs that contains additional proposals to increase manufacturer competition, increase the negotiating power of certain federal healthcare programs, incentivize manufacturers to lower the list price of their products and reduce the out of pocket costs of drug products paid by consumers. While any proposed measures will require authorization through additional legislation to become effective, Congress has indicated that it will continue to seek new legislative measures to control drug costs.
Individual states inEnvironment
We are subject to state and federal laws regarding environmental protection and hazardous substances, including the United States have also become increasingly aggressive in passing legislationOccupational Safety and implementing regulations designed to control pharmaceuticalHealth Act, the Resource Conservation and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product accessRecovery Act and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation, from other countries and bulk purchasing.
Packaging and distribution in the United States
If our products are made available to authorized users of the Federal Supply Schedule of the General Services Administration additional laws and requirements apply. Products must meet applicable child-resistant packaging requirements under the U.S. Poison Prevention PackagingToxic Substances Control Act. Manufacturing, sales, promotion,These and other activities also are potentially subject to federal and state consumer protection and unfair competition laws.
The distribution of pharmaceutical products is subject to additional requirements and regulations, including extensive record-keeping, licensing, storage, and security requirements intended to preventlaws govern the unauthorized sale of pharmaceutical products.
The failure to comply with any of these laws or regulatory requirements subjects firms to possible legal or regulatory action. Depending on the circumstances, failure to meet applicable regulatory requirements can result in criminal prosecution, fines or other penalties, injunctions, exclusion from federal healthcare programs, requests for recall, seizure of products, total or partial suspension of production, denial or withdrawal of product approvals, or refusal to allow a firm to enter into supply contracts, including government contracts. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. Prohibitions or restrictions on sales or withdrawal of future products marketed by us could materially affect our business in an adverse way.
Changes in regulations, statutes, or the interpretation of existing regulations could impact our business in the future by requiring, for example: (i) changes to our manufacturing arrangements; (ii) additions or modifications to product labeling; (iii) the recall or discontinuation of our products; or (iv) additional record-keeping requirements. If any such changes were to be imposed, they could adversely affect the operation of our business.
Other U.S. environmental, health, and safety laws and regulations
We may be subject to numerous environmental, health, and safety laws and regulations, including those governing laboratory procedures and theuse, handling use, storage, treatment, and disposal of hazardous materialsvarious biologic, chemical and wastes. From time to timeradioactive substances used in, and in the future,wastes generated by, operations. If our operations may involveresult in contamination of the use of hazardous and flammable materials, including chemicals and biological materials, and may also produce hazardous waste products. Even if we contract with third parties for the disposal of these materials and waste products, we cannot completely eliminate the risk of contamination or injury resulting from these materials. In the event of contamination or injury resulting from the use or disposalenvironment, breach of our hazardous materials,regulatory obligations or expose individuals to harm, we could be held liable for any resulting damages and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties for failure to comply with suchgovernmental fines. Equivalent laws and regulations.
We maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees, but this insurance may not provide adequate coverage against potential liabilities. However, we do not maintain insurance for environmental liability or toxic tort claimshave been adopted in foreign countries that may be asserted against us.
In addition, we may incur substantial costs in order to comply with current or future environmental, health, and safety laws and regulations. Current or future environmental laws and regulations may impair our research, development or production efforts. In addition, failure to comply with these laws and regulations may result in substantial fines, penalties or other sanctions.
Table of Contents
impose similar obligations.
CORPORATE INFORMATION
We were incorporated under the laws of the State of Delaware on July 22, 2016. We are the successor in interest to Moderna LLC, a limited liability company formed under the laws of the State of Delaware in 2013. Moderna LLC was the successor in interest to Moderna Therapeutics, Inc., a Delaware corporation incorporated in 2009 as Newco LS18, Inc. by Flagship Pioneering. In August 2018, we changed our name from Moderna Therapeutics, Inc. to Moderna, Inc. Our principal corporate office is located at 200 Technology Square, Cambridge, MA 02139, and our telephone number is (617) 714-6500.
Our website, www.modernatx.com, including the Investor Relations section, www.investors.modernatx.com; and corporate blog www.modernatx.com/moderna-blog;moderna-blog, and our Statements and Perspectives webpage, https://investors.modernatx.com/Statements--Perspectives/default.aspx; as well as our social media channels: Facebook, www.facebook.com/modernatx; Twitter,X, www.twitter.com/modernatx;moderna_tx; and LinkedIn, www.linkedin.com/company/modernatx; contain a significant amount of information about us, including financial and other information for investors. We encourage investors to visit these websites and social media channels as information is frequently updated and new information is shared. The information on our website and that we disclose through social media channels is not incorporated by reference in this Annual Report on Form 10-K or in any other filings we make with the Securities and Exchange Commission or SEC.(the SEC).
We make available free of charge on or through our website certain reports and amendments to those reports that we file with or furnish to the SEC in accordance withpursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended,soon as reasonably practicable after we electronically file such material with, or furnish it to, the Exchange Act.SEC. These include our Annual Reports on Form 10-K, our Quarterly Reports on Form 10-Q and our Current Reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act. We make this information available on or through our website free of charge as soon as reasonably practicable after we electronically file the information with, or furnish it to, the SEC.reports.
The SEC also maintains a websitean Internet site (http://www.sec.gov) that contains reports, proxy and information statements, and other information regarding us and other issuers that file electronically with the SEC. The SEC’s Internet website address is http://www.sec.gov.
Item 1A. Risk Factors
Investing in our common stock involves a high degree of risk. You should carefully consider the following risks and uncertainties, together with all other information in this Annual Report on Form 10-K, including our consolidated financial statements and related notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” before investing in our common stock.10-K. Any of the risk factors we describe below could adversely affect our business, financial condition or results of operations. Theoperations and the market price of our common stock could decline if one or more of these risks or uncertainties actually occur, causing you to lose all or part of your investment. Additional risks that we currently do not know about or that we currently believe to be immaterial may also impair our business. Certain statements in this Annual Report on Form 10-K are forward-looking statements. See the section of this Annual Report on Form 10-K titled “Special Note Regarding Forward-Looking Statements”.stock.
Risks related to COVID-19commercialization and our development of mRNA-1273, our vaccine against the SARS-CoV-2 virusproducts
WhileEvolving dynamics in the market for COVID-19 vaccines are likely to impact our financial results, which are likely to result in lower product revenues in 2024 than we have received Emergency Use Authorization fromexperienced in recent years.
Through 2023, our only approved product and sole source of product sales has been our COVID-19 vaccine. The global market is transitioning to an endemic, commercial market for COVID-19 vaccine sales, resulting in different market and production dynamics than during the pandemic, including a more fragmented customer base, less predictability in orders, greater seasonality of demand, increased distribution costs, and higher costs of goods sold. Furthermore, our assumptions regarding the product presentation that will be accepted or preferred by the market (e.g., single-dose presentation), which could vary by market, may prove incorrect. In addition, certain U.S. Foodprivate vaccine market practices, including regarding discounts, rebates and Drug Administration and other provisional, interim or conditional authorizations from regulatory authoritiesreturns, may cause us to realize significantly lower revenues than list prices. We may also be adversely affected by similar market practices outside of the United StatesStates.
We recognized $6.7 billion of sales for COVID-19 vaccines delivered in 2023, compared to $18.4 billion in 2022 and $17.7 billion in 2021, as demand for COVID-19 vaccines declined. We anticipate that sales of COVID-19 vaccines are likely to be lower in 2024 than 2023. We expect the market for COVID-19 vaccines to evolve based on a number of factors, including medical need, viral evolution, public health authority recommendations and consumer motivation to vaccinate. In the third quarter of 2023, we significantly resized our manufacturing infrastructure as we shifted to an endemic COVID-19 vaccine market, and we may in the future incur additional costs associated with exiting commitments with suppliers for raw materials and contract manufacturing organization (CMOs). As a result of lower demand, we have experienced, and may in the future experience, increased costs with respect to raw material suppliers as we seek to exit or modify our purchase commitments. Further, we have entered, and may in the future enter, into non-cancellable or take-or-pay purchase commitments for raw materials that require a long lead time to procure, increasing our commitment exposure. If we cannot effectively manage evolving demand dynamics, our business, financial condition, results of operations and prospects may suffer.
Additionally, we may not see demand materialize for our COVID-19 vaccine (mRNA-1273),products consistent with our projections. This may lead to lower than expected commercial sales or requests to defer, renegotiate or cancel existing contracts. Further, we may find that we need to dedicate greater resources to our commercial efforts than anticipated, and we may not realize a return on this investment.
We may encounter difficulties producing or successfully commercializing the vaccineshipping our products consistent with our existingprojections or potentialfuture contractual obligations.commitments.
In response to the global pandemic caused by SARS-CoV-2, we are pursuing the rapid manufacture and continued clinical testing of our COVID-19 vaccine (mRNA-1273). While we have received an Emergency Use Authorization (EUA), from the U.S. FDA and other authorizations for the distribution of our COVID-19 vaccine from regulatory authorities outside the United States (including Canada, the UK, the EU, Switzerland, Qatar, Israel and Singapore), weWe may encounter difficulties producing theor shipping our products, including our COVID-19 vaccine or other future products, consistent with our current expectations or on the timelines and in the quantitiesterms set forth in our existing supply agreements and those potential difficulties could also prevent us from successfully signing or performing under future supply agreements for the COVID-19 vaccine.sales contracts. Our ability to commercialize an effective vaccineour products depends on the success of our manufacturing capability, both at our own manufacturing facility,facilities and those of our manufacturing partners, which we rapidly scaled in response to the pandemic and in parallel with our development and clinical testing of our vaccine. We are also committing substantial financial resources and personnel to the development, manufacture and distribution ofparticularly for fill-finish capabilities. Further, adapting our COVID-19 vaccine to new variants requires significant coordination with our partners, including to supportfor the scale-upsourcing of manufacturing to enableraw materials and production. Any capacity or production issues or delays experienced by our pandemic response, whichpartners may cause delays in or otherwise negatively impactus to fail to meet obligations under our other development programs, despite uncertainties surrounding the longevity and extent of COVID-19 as a global health concern. Our business could be negatively impacted by our allocation of significant resources—including managerial and financial resources—to a global health threat against which our vaccine may not be fully effective, and may ultimately prove unsuccessful or unprofitable. Furthermore, notwithstanding our entry into supply agreements for the COVID-19 vaccine with the U.S. and foreign governments, there are no assurances that our vaccine will be approved for distribution and commercial use in all countries covered by such supply agreements.
Although we have a dedicated manufacturing facility, we do not have sufficient manufacturing infrastructure to support a global roll-out of the COVID-19 vaccine on our own. For example, we have entered into a strategic collaboration with Lonza Ltd. to enable increased production of our COVID-19 vaccine. We have also entered into a collaboration with Catalent, Inc. for large-scale, commercial fill-finish manufacturing of our COVID-19 vaccine in the United States,limited sales, distribution and a collaboration with Laboratorios Farmacéuticos Rovi, S.A., or ROVI,marketing experience and Recipharm for large-scale, commercial fill-finish manufacturing of mRNA-1273, for supply to markets outside of the U.S. from ROVI’s facilities in Spain, and Recipharm’s facilities in France. We have engaged other collaborators, and may need to engage others in the future, including contract manufacturing organizations, government and non-government organizations, and other funding and manufacturing sources, to assist in meeting our capacity needs in support of our global roll-out. Prior to 2020, we have not previously ramped our organization for a commercial launch of any product, and doing so in a pandemic environment with an urgent, critical global need creates additional challenges such as distribution channels, intellectual property disputes or challenges, and the need to establish teams of people with the relevant skills worldwide. We may also face challenges with sourcing a sufficient amount of raw materials to support the demand for our COVID-19 vaccine. We may be unable to effectively createestablish such capabilities or supplement our capabilities by entering into agreements with third parties.
We have limited experience as a supply chaincommercial organization and face risks and uncertainties as we shift to an endemic COVID-19 vaccine market and prepare for the commercial launch of other medicines, including our anticipated RSV vaccine launch in 2024. As a result of this limited commercial experience, we may be unsuccessful in accurately anticipating future rates of return for our products, which may adversely impact our accounting estimates. Additionally, we may seek to enter into agreements with others to utilize their marketing and distribution capabilities, but may be unable to enter into agreements on favorable terms, if at all. If we rely on others to commercialize our products, our revenues may be lower than if we commercialized these products ourselves. In addition, we may have little or no control over such third parties’ sales efforts. If our partners commit insufficient resources to commercialize our products, and we cannot independently develop necessary marketing capabilities, we may be unable to generate sufficient product revenue to sustain our business.
The pharmaceutical market is intensely competitive, and we may not compete effectively in the market for existing products, new treatment methods and new technologies.
The pharmaceutical market is intensely competitive and evolving. Many companies, academic institutions, governmental agencies and public and private research organizations are developing products for the same diseases that will adequately support demand as we are reliant ontargeting or expect to target and such other parties may have:
•greater resources and experience than us at every stage of drug discovery, development, testing, approval, manufacturing and commercialization;
•multiple products that have been approved or are in late stages of development; and
•arrangements in our third-party collaborators being able to fulfill demand. Any capacity issues or delays experienced by any of our third-party collaborators may result in us not being able to meet certain product volume or delivery timing obligations under our supply agreements for the COVID-19 vaccine. Furthermore, we will encounter significant additional capital requirements as we continue to expand our commercial launch efforts. While our collaborationtarget markets with BARDApurchasers, governments, leading companies and revenue from customer contracts will help us meet these capital requirements, additional investment, whether from our own capital resources or through collaborations with others, will be necessary. We cannot guarantee that any of these new challenges and requirements will be met in a timely manner or at all.research institutions.
In addition, another party may ultimately be successful in producing a more efficacious vaccine or other treatment for COVID-19. In particular, given the widespread media attention on the current pandemic, there are efforts by public and private entitiesWe face intense competition with respect to develop aour COVID-19 vaccine, as fast as possible, including by AstraZeneca, GlaxoSmithKline/Sanofi, Johnson & Johnson, Novavax and Pfizer/BioNTech. The Pfizer/BioNTech vaccine has also received an EUA in the United Statesit may not continue to compete favorably with existing or future vaccines and other territories (including the EU and UK), and othertreatments. Other vaccines including the AstraZeneca vaccine, have been authorized elsewhere.
Those other entities may develop COVID-19 vaccines that, as comparedor treatments could prove to mRNA-1273 or any other COVID-19 vaccine that we may develop, arebe safer, moreeffective, become the standard of care, have broader market acceptance, are safer ormore convenient, have fewer or less severe side effects, are more convenient, arebe easier to ship or distribute or able to be developed at a lower cost than our vaccine. Even if our products demonstrate superiority to those of competitors, consumers and the public may fail to appreciate that benefit, or existing purchase commitments for a competitor’s product may bediscourage them from purchasing from us. These factors, or the perception of these factors, could lead to a competitor’s vaccine or treatment being more successfully commercialized. Many of these other organizations are much larger than we areFurther, the mRNA medicines field is growing rapidly, with increased competitive pressure from large and have access to larger pools of capital and broader manufacturing infrastructure. Largermore established pharmaceutical and biotechnology companies have extensive experience in clinical testing and obtaining regulatory approval for their products, and may have the resources to heavily invest to accelerate development and commercialization of their vaccine candidates. Our business could be materially and adversely affected if competitors develop and commercialize onecompanies. The actual or more COVID-19 vaccines before we can fully expand our commercialization capabilities.
Theperceived success or failure of other entities, or perceived success or failure,others may adversely impact our ability to obtain any future funding forcommercialize our COVID-19 vaccine commercialization effort. In addition, we may not be able to compete effectively if our product candidates do not satisfy government procurement requirements with respect to biodefense products.
We are devoting significant resources to the scale-up, developmentalso will face competition from products that have already been approved and commercialization of our COVID-19 vaccine, including for useaccepted by the U.S. government medical community to treat certain conditions we target. For example, we are developing a seasonal flu vaccine, for which there is a well-developed market, and other global governmental and commercial partners.we may be unsuccessful in developing a product or achieving market share. Our RSV vaccine, which we expect to launch beginning in 2024, will also face competition from existing RSV vaccines. In markets that we enter after competitors have already introduced a competing product, we may have difficulty achieving market share. Further, we may need to offer more favorable terms to gain market share in an existing market or to compete in a new market, which may negatively impact our profitability.
We continue to work towardIf we successfully develop and obtain approval for other product candidates, we may compete with products under development by competitors based on many factors, including the large-scale technical development, manufacturing scale-up in several countriesrelative safety and larger scale deploymenteffectiveness of our COVID-19 vaccine. The number of doses of this vaccine that we are able to produce and distribute is dependent onproducts, the ease with which our ability,products can be administered and the abilityextent to which patients accept relatively new routes of our contract manufacturers, to successfullyadministration, the timing and rapidly scale upscope of regulatory approvals, the availability and cost of manufacturing, capacity. To support the scale-up, we have expendedmarketing and will need to continue to expend significant resourcessales capabilities, price, reimbursement coverage and capital. We may need to, or wepatent position. Our competitors may be required by the federal government to, divert resources and capital from our other programs. We may also seek and secure significant additional funding through contractual arrangements and collaborations with third parties. We may be unable to enter into such arrangements on favorable terms, or at all,more successful in commercializing their products, which would adversely affect our ability tobusiness. Competitive products may make any products we develop manufactureobsolete or noncompetitive before we can recover the expenses of developing and distribute the COVID-19 vaccine.
As part of this effort, we have a commitment from BARDA to fund up to $954.9 million to enable the initiation of and support the planning and execution of Phase 2 and Phase 3 clinical trials of mRNA-1273 undercommercializing our own IND, as well as the scale-up of mRNA-1273 manufacture in 2020 to enable our pandemic response. To the extent our funding collaborators have discretion over the distribution of funding commitments, we may not ultimately receive the full amount of committed funds and could be exposed to urgent needs for additional funding to support our manufacturing activities. Our funding collaborators may also impose restrictions on or mandate input as to our conduct of clinical trials, manufacturing activities or distribution activities, which may cause delays in the event of disagreement.
We have entered into, and plan to continue entering into, supply agreements for the COVID-19 vaccine that include cash deposits from the purchasers. In the event we are unable to successfully obtain regulatory authorization or approval for the commercialization of the vaccine in the relevant jurisdictions, or we fail to meet certain product volume or delivery timing obligations under our supply agreements, we may be required to refund significant portions of the deposits, which could have a material and adverse effect on our financial condition.
We have already incurred expenses in connection with the distribution of our COVID-19 vaccine, and these expenses could increase over time. Such expenses include the cost of implementing pharmacovigilance to collect, analyze and monitor safety data and to identify and evaluate adverse reactions to our COVID-19 vaccine as it is administered in jurisdictions around the globe.
In addition, since we have received an EUA for the COVID-19 vaccine and have commenced commercialization activities, we have a widely used vaccine in circulation in the United States and other countries prior to our receipt of full marketing approval. This is also the case in other countries, such as the UK, where a form of emergency or conditional approval has been granted, but not a full marketing approval. Unexpected safety issues, including any that we have not yet observed in our Phase 1, 2 or 3 clinical trials for the COVID-19 vaccine, could lead to significant reputational damage for Moderna and our technology platform going forward and other issues, including delays in our other programs, the need for re-design of our clinical trials and the need for significant additional financial resources.
The positive efficacy and safety data from the ongoing clinical studies of our COVID-19 vaccine and the EUA granted by the FDA may not be predictive of the final results of the clinical trials, which is one of a number of factors that may delay or prevent us from receiving full regulatory approval of our vaccine.
The positive efficacy and safety data we have announced from the ongoing studies of our COVID-19 vaccine are based on analyses of those studies to date, and while the efficacy assessment is now considered final, efficacy, effectiveness, safety, and immunogenicity data continue to accumulate. Further results from the ongoing studies for mRNA-1273, as well as the experience of the millions of individuals who have been vaccinated with mRNA-1273, could show diminished immunogenicity, or protection, as compared to the
results released to date as efficacy and antibody persistence may wane over time. Additionally, we may observe new, more frequent or adverse events of greater severity in subjects participating in these ongoing clinical studies or among those individuals who have been vaccinated with mRNA-1273. In addition, the interpretation of the data from our clinical trials of mRNA-1273 by the FDA and other regulatory agencies may differ from our interpretation of such data and the FDA or other regulatory agencies may require that we conduct additional studies or analyses.
The assays being used to estimate the effectiveness of vaccine candidates being developed to prevent COVID-19 have only recently been developed and are continuing to evolve. Validation reports for these assays have been submitted for review to regulatory agencies. Results obtained in clinical studies of mRNA-1273 with subsequent versions of these assays may be less positive than the results we have obtained to date. Moreover, the samples of convalescent sera, or blood samples from people who have recovered from COVID-19, used to benchmark the level of antibodies produced by subjects receiving mRNA-1273 in clinical studies, have been taken from a small number of people and may not be representative of the antibody levels in a broader population of people who have recovered from COVID-19, particularly as variant strains have begun to emerge. The future results in clinical studies of mRNA-1273 may not be as positive when compared to the antibody levels in other samples of convalescent sera. Various preclinical animal studies of mRNA-1273 are ongoing, including preclinical studies in non-human primates. If safety data observed in these preclinical studies are inconsistent with safety data from clinical studies, we may be required to conduct additional studies of mRNA-1273. Any of these factors could delay or prevent us from receiving regulatory approval of mRNA-1273, including a biologics license application, or BLA, apart from the EUA by the FDA in the United States and related authorizations in other jurisdictions, and there can be no assurance that mRNA-1273 will be otherwise approved in a timely manner,products, if at all.approved.
We may be unsuccessful or delayed in adapting our COVID-19 vaccine (mRNA-1273) or developing future versions ofupdating our COVID-19 vaccine to protect against future variants of the SARS-CoV-2 virus, and a market for vaccines against these variantsupdated versions of our COVID-19 vaccine may not develop.protect against such variants.
Our current COVID-19 vaccine (mRNA-1273) was developed based upon the genetic sequence of the SARS-CoV-2 virus that was first detected in Wuhan, China. As the pandemic has continued, the SARS-CoV-2 virus continues to evolve, and new strains of the virus or those that are already in circulation may prove more transmissible or cause more severe forms of COVID-19 disease than the predominant strains to date. There is a risk that mRNA-1273 will notearlier strains. Our current COVID-19 vaccines could be asineffective, or less effective than desired, in protecting against variant strainsthese new variants. Additionally, our decisions regarding vaccine development will be informed by guidance from the FDA and foreign regulatory authorities, which may impact the timing of the SARS-CoV-2 virus expressingdevelopment for our COVID-19 vaccines.
We may experience delays in producing variant-specific vaccines. Further, different regulators may issue differing guidance regarding vaccine composition or populations who should receive a vaccine. If our efforts to develop variant-specific vaccines against future variants of the spike protein, particularly strains with mutations in the receptor binding domain and N-terminal domain. Although we have shown comparable neutralization titers with the majority of variants that we have tested (including the B.1.1.7 strain, which was first detected in the United Kingdom), we did see a 6.4-fold reduction in neutralization titers in the B.1.351 strain (first detected in South Africa). The ultimate impact to protection against this variant remains unknown, butare unsuccessful, we are actively workingslower than competitors to mitigate this risk by beginning non-clinical investigations into the immunogenicity of a modification to mRNA-1273, designed to reflect the mutations of the B.1.351 variant. However, these efforts may be unsuccessful, and failure to adaptdevelop such vaccines or our vaccine to this or other variants of the SARS-CoV-2 virusvaccines prove less effective than competitors’ vaccines, we could lead to significantsuffer reputational harm, in addition to adversely affecting ourloss of market share and adverse financial results. It is also possible thatAdditionally, we may expend significant resources adapting our COVID-19 vaccinevaccines or conducting clinical trials to protect against variants, of the SARS-CoV-2 virus, but that a market for thisour adapted vaccine does notvaccines may fail to develop or demand doesmay not align with our projections or cost expenditures.
The regulatory pathway for mRNA-1273 is continually evolving, and may result in unexpected or unforeseen challenges.
To date, our COVID-19 vaccine has moved rapidly through the FDA regulatory review and EUA process, as well as the review and authorization process in a number of other jurisdictions, including the UK and EU. The speed at which all parties are acting to create and test many therapeutics and vaccines for COVID-19 is atypical, and evolving or changing plans or priorities within the FDA or the regulatory authorities in other jurisdictions, including changes based on new knowledge of COVID-19 and how the disease affects the human body, and new variants of the virus, may significantly affect the regulatory timeline for further authorizations or approvals for our COVID vaccine. We cannot anticipate or predict with certainty the timelines or regulatory processes that may be required for the authorization or approval of updated versionscommercial success of our COVID-19 vaccine, or vaccines that may be developed to fight against variantsproducts will depend on the degree of market acceptance by physicians, patients, third-party payors and others in the SARS-CoV-2 virus.medical community.
The FDA hascommercial success of our products will depend in part on the authoritymedical community, patients and third-party or governmental payors accepting mRNA medicines, and our products in particular, as medically useful, cost-effective and safe. The degree of market acceptance of our products will depend on numerous factors, including:
•the potential efficacy and advantages over alternative treatments;
•the ability to grant an EUAoffer products at competitive prices;
•the duration of protection provided by our products compared to allow unapproved medicalthose of competitors;
•acceptance of mRNA products togenerally and the availability of competing non-mRNA medicines that may be used in an emergency to diagnose, treat, or prevent serious or life-threatening diseases or conditions when there are no adequate, approved, and available alternatives. Although we have been granted an EUApreferred by the FDAmedical community or the public;
•the prevalence and severity of any side effects, including any limitations, restrictions (including for our COVID-19 vaccine (specifically mRNA-1273), the FDA may revoke an EUA where it is determined that the underlying health emergency no longer existsuse together with other medicines) or warrants such authorization, and we cannot predict how long the EUA will remain in place. Such revocation could adversely impact our businesswarnings contained in a varietyproduct’s approved labeling;
•the prevalence and severity of ways, including if mRNA-1273 is not yet approved by any side effects resulting from checkpoint inhibitors or other products or therapies with which our products are co-administered;
•relative convenience and ease of administration;
•the FDA pursuantwillingness of the target patient population to a BLA, as wetry, and physicians to prescribe, new therapies;
•the strength of marketing and distribution support and timing of market introduction of competitive products;
•whether our manufacturing partners have investedproduct presentation meets customer demand (e.g., for single-dose presentations, or combination vaccines);
•publicity concerning our products or competing products and treatments; and
•sufficient third-party insurance coverage or reimbursement, and patients’ willingness to pay out-of-pocket in the supply chain to provide mRNA-1273 under the EUA.absence of third-party coverage or adequate reimbursement.
Similarly,Even if a potential product displays a favorable efficacy and safety profile in clinical trials, market acceptance will be unknown until after it is launched. Our efforts to educate the European Medicines Agency (EMA) has granted a conditional marketing authorization for mRNA-1273. The EMA has the authority to grant a conditional marketing authorization in a public health emergency,medical community and third-party payors on the basis of less comprehensive clinical data than normally required, but where the benefit of immediate availability of the product outweighs the risk inherent in the fact that
additional data are still required. A conditional marketing authorization is a formal marketing authorization of the vaccine and covers all batches produced for the EU, however we are obliged to provide certain additional information and data by specified timelines, as conditions of the authorization and the EMA can take regulatory action if we do not comply with such conditions. Conditional marketing authorizations are valid for one year and can be renewed annually, however there is a risk that the EMA may decide not to renew our conditional authorization. If new data emerges that shows the benefits of our vaccine do not continueproducts may require significant resources, especially due to outweigh its risks, the EMA can suspend or revoke our authorization. Similar authorizations that we have received for mRNA-1273, including those in the UK and Canada, are temporary, emergency authorizations that could similarly be revoked if the conditions for granting the authorization no longer apply. Any such revocation of the temporary authorization to distribute mRNA-1273, without receiving final approval to distribute the vaccine, could adversely impact our ability to realize the full financial benefitcomplexity of our existingprograms, and may never be successful.
Sales of pharmaceutical products depend on the availability and extent of reimbursement from third-party payors, and we may be adversely impacted by changes to such reimbursement policies or future supply agreements.rules.
Sales of pharmaceutical products in general depends to a significant extent on adequate coverage, pricing and reimbursement from third-party payors. When a new product is approved, the availability and extent of government and private reimbursement, and the pricing, for that product may be uncertain. For example, it is uncertain whether any combination respiratory vaccine we develop, if approved, would qualify for coverage under Medicare Part B. Additionally, pricing and reimbursement for any product we develop may be adversely affected by a number of factors, including:
•changes in, and implementation of, federal, state or foreign government regulations or private third-party payors’ reimbursement policies;
•pressure by employers on private health insurance plans to reduce costs; and
•consolidation and increasing assertiveness of payors seeking price discounts or rebates in connection with the placement of our products on their formularies and, in some cases, the imposition of restrictions on access or coverage of particular drugs or pricing determined based on perceived value.
Our ability to produce a successful vaccine and deliver it to customers may be curtailedset the price for any product we develop will vary significantly by one or more government actions or interventions, which may be more likely during a global health crisis such as COVID-19.
Given the significant global impact of the COVID-19 pandemic, it is possible that one or more government entities may take actions that directly or indirectly have the effect of diminishing some of our rights or opportunities with respect to our COVID-19 vaccine, limiting our economic prospects. In the U.S., the Defense Production Act of 1950, as amended (the “Defense Production Act”), gives the U.S. government rights and authorities that may directly or indirectly diminish our own rights or economic opportunities with respect to our COVID-19 vaccine.country. Our current and potential third-party service providers may be impacted by government entities potentially invoking the Defense Production Act or other potential restrictions to all or a portion of services they might otherwise offer. As of January 21, 2021, President Biden has stated his intent to use the Defense Production Act to maximize the manufacture of vaccine and vaccine supplies, and that this effort will prioritize supplies that could cause bottlenecks including glass vials, stoppers, syringes, needles and the fill and finish capacity to package vaccine into vials.
Government entities imposing restrictions or limitations on our third-party service providers may require usinability to obtain alternative service sources forand maintain adequate prices in a particular country may limit the revenues from our COVID-19 vaccine or our vaccine candidates. If we are unable to timely enter into alternative arrangements, or if such alternative arrangements are not available on satisfactory terms, we will experience delays in the development or production of our COVID-19 vaccineproducts within that country and our vaccine candidates, increased expenses, and delays in distribution or commercialization of our COVID-19 vaccine, or, when and if approved, our vaccine candidates.
In addition, our supply contracts with the U.S. government for our COVID-19 vaccine contain certain restrictions onadversely affect our ability to export vaccine that is produced fromsecure acceptable prices in existing and potential new markets, which may limit market growth. This may create the opportunity for third-party cross-border trade or influence our U.S.-dedicated supply chaindecision whether to serve markets outside the U.S. prior to satisfyingsell a product, thus adversely affecting our delivery obligations to the U.S. government. Furthermore, governments have threatened to block or limit the export of COVID-19 vaccines manufactured in their territories in instances where the manufacturers have been delayed or have not fully satisfied their delivery obligations to those governments. Governments of the jurisdictions in which we or our contract manufacturing partners produce our COVID-19 vaccine may impose export restrictions, prohibiting us from delivering orders of our COVID-19 vaccine to customers in other jurisdictions. Such restrictions may also delay or prevent production of the vaccine. The imposition of export controls could severelygeographic expansion plans and adversely impact our manufacturing activities, commercial activities and financial results.revenues.
In addition, duringMany payors continue to adopt benefit plan changes that shift a globalgreater portion of prescription costs to patients, including more limited benefit plan designs, higher patient co-pay or co-insurance obligations and limitations on patients’ use of commercial manufacturer co-pay payment assistance programs. Significant consolidation in the health crisis,insurance industry has resulted in a few large insurers and pharmacy benefit managers exerting greater pressure in pricing and usage negotiations with drug manufacturers, significantly increasing discounts and rebates required of manufacturers and limiting patient access and usage. Further consolidation among insurers, pharmacy benefit managers and other payors would increase the negotiating leverage such entities have over us and other drug manufacturers. Additional discounts, rebates, coverage or plan changes, restrictions or exclusions as described above could have a material adverse effect on sales of our affected products, particularly our therapeutic products or those that are individualized for a particular patient. Coverage and reimbursement by a third-party payor may depend upon a number of factors, including the COVID-19 pandemic, where the spreadthird-party payor’s determination that use of a disease needs to be controlled, closed or heavily regulated national borders will create challengesproduct is a covered benefit under its health plan, safe, effective and potential delays in our developmentmedically necessary, appropriate for the specific patient, cost-effective and production activities and may necessitate that we pursue strategies to develop and produce our vaccines and vaccine candidates within self-contained national or international borders, at potentially much greater expense and with longer timeframes for public distribution.neither experimental nor investigational.
Certain aspectsAdditionally, target patient populations for some of our businessproduct candidates may be adversely affected by the ongoing coronavirus pandemic.
small (e.g., for rare genetic diseases) or require individual customization (e.g., for our INTs). The extent to which the COVID-19 pandemic impacts our businesspricing and operating results will depend in part on future developments that are highly uncertain and cannot be accurately predicted, including new information that may emerge concerning COVID-19, including potential variants of SARS-CoV-2, and the actions taken to contain COVID-19 or treat its impact, among others.
The spread of COVID-19 has resulted in the delay and interruption of certainreimbursement of our business operations. Manymedicines, if approved, must be adequate to support commercial infrastructure. If we cannot obtain adequate levels of our clinical trials have been affected by the pandemic. Site initiation, participant recruitment and enrollment, participant dosing, distribution of clinical trial materials, study monitoring and data analysis may be paused or delayed (or continue to be paused or delayed) due to changes in hospital or university policies, federal, state or local regulations or restrictions, prioritization of hospital resources toward pandemic efforts, travel restrictions, concerns for patient safety in a pandemic environment, or other reasons related to the pandemic. More specifically, as previously disclosed, certain of our clinical trials have been adversely affected, resulting in paused enrollment or delayed site initiations. As COVID-19 continues to spread, some participants and clinical investigators may not be able to comply with clinical trial protocols. For example, quarantines or other travel limitations (whether voluntary or required) have been implemented in many countries, and may impede participant movement, affect sponsor access to study sites, or interrupt healthcare services, andreimbursement, we may be unable to conductsuccessfully market and sell our clinical trials. Further, if the spread of the COVID-19 pandemic continuesproducts. The manner and level at which reimbursement is provided for services related to our operations areproducts (e.g., for administration to patients) is also important. Inadequate reimbursement for such services may discourage physicians from prescribing or recommending our products, adversely impacted, including dueaffecting our ability to facility access restrictionsmarket or from an outbreak in a facility, we risk manufacturing delays, or default and/or nonperformance under existing agreements.sell those products.
Infections and deaths related to the pandemic have disrupted and may continue to disrupt the United States’ healthcare and healthcare regulatory systems. Such disruptions could divert healthcare resources away from, or materially delay the review and/or approval by the FDA and other regulatory agencies with respect to, our clinical trials. Any elongation or de-prioritization of our clinical trials or delay in regulatory review resulting from such disruptions could materially affect the development and study of our development candidates.
We currently utilize third parties to, among other things, manufacture raw materials, components, parts, and consumables, perform quality testing and ship our products. For example, we rely on third-party manufacturers such as Lonza Ltd., Catalent Inc. and ROVI to enable larger scale manufacture and/or fill/finish capabilities for our mRNA vaccine (mRNA-1273) against the SARS-CoV-2 virus and others, such as McKesson, for shipping and distribution. We also manufacture our development candidates and investigational medicines and perform various services at our manufacturing facility. Certain of our third-party manufacturers and suppliers may pause their operations in response to the COVID-19 outbreak or otherwise encounter delays in providing their services. If either we or any third-party manufacturers or third parties in the supply chain for materials used in the production of our COVID-19 vaccine, development candidates or investigational medicines are adversely impacted by restrictions resulting from the COVID-19 outbreak, our supply chain may be disrupted, limiting our ability to manufacture our COVID-19 vaccine, as well as investigational medicines for our clinical trials, research and development operations and commercialization. In addition, delays and disruptions experienced by our strategic collaborators due to the COVID-19 outbreak could adversely impact the ability of such parties to fulfill their obligations, which could affect the clinical development or regulatory approvals of development candidates and investigational medicines under joint control.
The spread of COVID-19, which has caused a broad impact globally, including restrictions on travelmarket opportunities for our products and quarantine policies put into place by businesses and governments,product candidates may have a material economic effect on our business, including our abilitybe smaller than we believe, or we may be unable to successfully commercializeidentify clinical trial participants.
We focus certain of our COVID-19 vaccine. Dueresearch and product development activities on treatments for severe rare genetic diseases, where the patient populations are difficult to ascertain or small. Additionally, we expect to initially seek approval of our INT and intratumoral immuno-oncology product candidates for use by patients with relapsed or refractory advanced disease, i.e., the pandemic,populations the FDA often approves new therapies for initially. If any such medicines prove to be sufficiently beneficial, we would expect to seek approval in earlier lines of treatment and potentially as a first-line therapy. There is no guarantee that our products, if approved, would be approved for earlier lines of therapy and, prior to any such approvals, we may have difficulty meeting expectations with respect to commercial sales. While the potential economic impact brought by and the duration of the pandemic may be difficult to assess or predict, it has already caused, and may result in further, significant disruption of global financial markets, which may reduce our ability to access capital either at all or on favorable terms. In addition, a recession, depression or other sustained adverse market event resulting from the spread of COVID-19 could materially and adversely affect our business, prospects, operating results and financial condition, and the value of our common stock.conduct additional clinical trials.
The ultimate impactOur estimates of the current pandemic, or any other health epidemic, is highly uncertain and subject to change. We do not yet know the full extent of potential delays or impactsaddressable patient populations are based on our business,beliefs, and have been derived from a variety of sources, including scientific literature, surveys of clinics, patient foundations or market research, and may prove to be incorrect. Further, new studies may change the estimated incidence or prevalence of these diseases. The number of trial participants may be lower than expected and potential clinical trial participants or patients may not be otherwise amenable to treatment with our product candidates or products, or new clinical trials,trial participants or patients may become increasingly difficult to identify or gain access to. Even if we obtain significant market share for our research programs, healthcare systems orproducts, if approved, because the global economy as a whole. However,potential target populations are small, these effects could have a material impact on our operations, and we will continue to monitor the situation closely.
medicines may never be profitable.
Risks related to our businesspipeline, product development and creating a new class of medicinesregulatory review
Preclinical development is lengthy and uncertain, especially for mRNA medicines, and our preclinical programs or product candidates may be delayed or terminated.
Much of our pipeline is in preclinical development, and these programs could be delayed or not advanced into the clinic. Before we can initiate clinical trials for a product candidate, we must complete extensive preclinical studies, including IND-enabling good laboratory practice (GLP) toxicology testing. We attemptmust also complete extensive work on Chemistry, Manufacturing, and Controls (CMC) activities to distributebe included in an IND submission. CMC activities for mRNA medicines require extensive manufacturing processes and analytical development, which is uncertain and lengthy. We have had and may in the future have difficulty identifying appropriate buffers and storage conditions to enable sufficient shelf life of batches of our technology, biology, execution,product candidates. If we must produce new batches, our preclinical studies could be delayed. We cannot be certain of the timely completion of our preclinical testing and financing risks acrossstudies, whether the FDA or other regulators will accept the results or if the outcome of our preclinical testing, studies and CMC activities will ultimately support further development of our programs. As a wide varietyresult, we may be unable to submit INDs or similar applications for our preclinical programs on the timelines we expect, if at all, and such applications may not result in the FDA or other regulators allowing clinical trials to begin.
Clinical development is lengthy and uncertain, and our clinical programs may be delayed or terminated, or may be more costly to conduct than we anticipate.
Clinical testing is expensive, complex and lengthy, and its outcome is inherently uncertain. Most product candidates that commence clinical trials are never approved as products. We may be unable to initiate, may experience delays in or may have to discontinue clinical trials for our product candidates. We and our strategic collaborators also may experience unforeseen events during, or as a result of, therapeutic areas, disease states, programs,any clinical trials that we or they conduct that could delay or prevent us or them from successfully developing our product candidates and technologies. However,gaining approval from regulators. Events that might prevent us from proceeding with clinical trials could include:
•regulators, Institutional Review Boards (IRBs) or ethics committees may not authorize us or our assessmentinvestigators to commence a clinical trial or conduct a clinical trial at a prospective trial site;
•we may experience delays in reaching, or fail to reach, agreement on favorable terms with prospective trial sites and prospective contract research organizations (CROs);
•changes to the scale or site of our manufacturing could cause significant delays or changes in our clinical trial designs;
•the outcome of our preclinical studies and approach to, riskour early clinical trials may not be comprehensivepredictive of the success of later clinical trials, and interim results of a clinical trial do not necessarily predict final results;
•we may be unable to establish or effectively avoidachieve clinically meaningful endpoints for our studies;
•if we make changes to our product candidates after clinical trials have commenced (which we have done in the past), we may be required to repeat earlier stages or delay later stages of clinical testing;
•clinical trials of any product candidates may fail to show safety or efficacy, or may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional nonclinical studies or clinical trials, or we may decide to abandon product development programs;
•our product candidates, or other medicines in the same class as ours, may have undesirable side effects, such as the immunogenicity of the LNPs or their components, the immunogenicity of the protein made by the mRNA or degradation products, any of which could lead to serious adverse events, or other effects;
•administration of our LNPs could lead to systemic side effects related to the components of the LNPs and could contribute to immune reactions, infusion reactions, complement reactions, opsonization reactions, antibody reactions or reactions to PEG-lipids;
•significant adverse events or other side effects could be observed in our clinical trials;
•our third-party contractors may fail to comply with regulatory requirements or meet their contractual obligations to us in a timely manner, or at all, or may deviate from the applicable clinical trial protocol or withdraw from the applicable clinical trial, which may require that we add new clinical trial sites;
•regulators may impose a complete or partial clinical hold on a clinical trial, or we or our investigators, IRBs or ethics committees may suspend or terminate clinical research or trials for various reasons, including noncompliance with regulatory requirements or a finding that participants are being exposed to an unacceptable benefit-risk ratio;
•regulators may impose a complete or partial clinical hold on clinical trials of other companies working on mRNA medicines;
•the cost of preclinical or nonclinical testing and studies and clinical trials of product candidates may be greater than anticipated;
•the supply or quality of our product candidates or other materials necessary to conduct clinical trials may be insufficient or inadequate;
•safety and efficacy concerns regarding our product candidates will be considered by us and by the FDA and other regulators as we pursue clinical trials of new product candidates, develop effective informed consent documentation and work with IRBs and scientific review committees (SRCs);
•safety or efficacy concerns could arise from nonclinical or clinical testing of other therapies targeting a similar disease state or other therapies, such as gene therapy, that are perceived as similar to ours;
•adverse side effects could be observed in future clinical trials where our product candidates are administered in combination with other therapies (such as the co-administration of our INT product candidate, mRNA-4157); and
•a lack of adequate funding to continue a particular clinical trial.
Before commencing later-stage clinical trials for our programs, we must develop assays to measure and predict the potency of a given dose of our product candidates. Any delay in developing assays that are acceptable to the FDA or other regulators could delay the start of future clinical trials.
Additionally, we have conducted and may conduct in the future “open-label” clinical trials, where both the patient and investigator know whether the patient is receiving the investigational product candidate, an approved drug or a placebo. The results from an open-label clinical trial may not be predictive of future clinical trial results from a controlled environment with a placebo or active control. Further, the FDA or other regulators may change the requirements for approval even after they have reviewed and commented on the design for our clinical trials. Significant preclinical or nonclinical testing and studies or clinical trial delays or failures infor our product candidates could allow our competitors to bring products to market before we do and could harm our business, financial condition and prospects significantly.
There are risks that are unique to each of our programs and modalities and risks that are applicable across programs and modalities. These risks may impair our ability to advance one or more of our programs in clinical development, obtain regulatory approval or modalities. Failurescommercialize our products, or cause us to experience significant delays in onedoing so.
Certain features in our product candidates, including those related to mRNA, chemical modifications, surface chemistries, LNPs and their components, may result in risks that apply to some or moreall of our programs or modalities could adversely impact other programs or modalities in our pipeline and have a material adverse impact on our business, results of operations, and ability to fund our business.
We are creating a new class of medicines based on mRNA to improve the lives of patients. From the beginning, we designed our strategy and operations to realize the full potential value and impact of mRNA over a long time horizon across a broad array of human diseases. We have made investments in our platform, infrastructure, and clinical capabilities that have enabled us to establish a large pipeline of development candidates, of which many are in clinical trials or have an open IND.modalities. As our developmentproduct candidates and investigational medicines progress, we or others may determine that:that certain of our risk allocation decisions were incorrect or insufficient;insufficient, we made platform levelplatform-level technology mistakes;mistakes, individual programs or our mRNA science in general has technology or biology risks that were unknown or under-appreciated;under-appreciated, our choices on how to develop our infrastructure to support our scale will result in an inability to manufacture our investigational medicinesproduct candidates for clinical trials or otherwise impair our manufacturing;manufacturing or we have allocated resources in such a way that we cannot recover large investments are not recovered and capital allocation is not subject to rapid re-direction. All of these risks may relate to our current and future programs sharing similar science (including mRNA science) and infrastructure, and in the event material decisions in any of these areas turn out to have been incorrect or under-optimized, we may experience a material adverse impact on our business and ability to fund our operations and we may never realize what we believe is the potential of mRNA.
mRNA drugs have only been authorized for emergency, or other provisional or conditional use for COVID-19, and there is no guarantee that any other mRNA drug will be granted an EUA or will be granted full approval in the future as a result of efforts by others or us. mRNA drug development has substantial clinical development and regulatory risks due to the novel nature of this new class of medicines.
Other than the EUA and other similar authorizations for COVID-19 vaccines, no mRNA medicines have been granted EUA or have been granted full approval to date by the FDA or other regulatory agencies. Successful discovery and development of other mRNA medicines by us or our strategic collaborators is highly uncertain and depends on numerous factors, many of which are beyond our or their control. We have made and will continue to make a series of business decisions and take calculated risks to advance our development efforts and pipeline, including those related to mRNA technology, delivery technology, and manufacturing processes, which may be shown to be incorrect based on further work by us, our strategic collaborators, or others. Although we have received an EUA from the FDA for mRNA-1273, there may never be products in which mRNA is the primary active ingredient approved outside the context of authorization for emergency use. Our mRNA investigational medicines that appear promising in the early phases of development may fail to advance, experience delays in the clinic, experience clinical holds, or fail to reach the market for many reasons, including:rapidly re-direct capital.
•discovery efforts at identifying potential mRNA medicines may not be successful;
•nonclinical or preclinical study results may show potential mRNA medicines to be less effective than desired or to have harmful or problematic side effects;
•clinical trial results may show potential mRNA medicines to be less effective than expected (e.g., a clinical trial could fail to meet one or more endpoint(s)) or to have unacceptable side effects or toxicities;
•adverse effects in any one of our clinical programs or adverse effects relating to our mRNA, or our LNPs, may lead to delays in or termination of one or more of our programs;
•the insufficient ability of our translational models to reduce risk or predict outcomes in humans, particularly given that each component of our investigational medicines and development candidates may have a dependent or independent effect on safety, tolerability, and efficacy, which may, among other things, be species-dependent;
•manufacturing failures or insufficient supply of cGMP materials for clinical trials, or higher than expected cost could delay or set back clinical trials, or make mRNA-based medicines commercially unattractive;
•our improvements in the manufacturing processes for this new class of medicines and potential medicines may not be sufficient to satisfy the clinical or commercial demand of our investigational medicines or regulatory requirements for clinical trials;
•changes that we make to optimize our manufacturing, testing or formulating of cGMP materials could impact the safety, tolerability, and efficacy of our investigational medicines and development candidates;
•pricing or reimbursement issues or other factors that delay clinical trials or make any mRNA medicine uneconomical or noncompetitive with other therapies;
•failure to timely advance our programs or receive the necessary regulatory approvals or a delay in receiving such approvals, due to, among other reasons, slow or failure to complete enrollment in clinical trials, withdrawal by trial participants from trials, failure to achieve trial endpoints, additional time requirements for data analysis, data integrity issues, preparation of a BLA, or the equivalent application, discussions with the FDA or EMA, a regulatory request for additional nonclinical or clinical data, or safety formulation or manufacturing issues may lead to our inability to obtain sufficient funding; and
•the proprietary rights of others and their competing products and technologies that may prevent our mRNA medicines from being commercialized.
Currently, mRNA is considered a gene therapy product by the FDA. Unlike certain gene therapies that irreversibly alter cell DNA and could act as a source of side effects, mRNA-based medicines are designed to not irreversibly change cell DNA; however, side effects observed in gene therapy could negatively impact the perception of mRNA medicines despite the differences in mechanism. In addition, because no product in which mRNA is the primary active ingredient has been approved outside the context of authorization for emergency use, the regulatory pathway for approval is uncertain. The number and design of the clinical trials and preclinical studies required for the approval of these types of medicines have not been established, may be different from those required for gene therapy products, or may require safety testing like gene therapy products. Moreover, the length of time necessary to complete clinical trials and to submit an application for marketing approval for a final decision by a regulatory authority varies significantly from one pharmaceutical product to the next, and may be difficult to predict.
Our business is highly dependent on the clinical advancement of our programs and modalities. Delay or failure to advance programs or modalities could adversely impact our business.
Using our platform, we are developing product features for medicines based on mRNA. Over time, our platform work led to commonalities, where a specific combination of mRNA technologies, delivery technologies, and manufacturing processes generated a set of product features shared by multiple programs. This is what we call a “modality.” We have historically utilized, and expect to continue to utilize earlier programs in a modality to understand the technology risks within the modality, including the program’s manufacturing and pharmaceutical properties. Even if our earlier programs in a modality are successful in any phase of development, any of such earlier programsprogram may fail at a later phase of development, and other programs within the same modality may still fail at any phase of development, including at phases where earlier programs in that modality were successful. This may be a result of technical challenges or biology risk unique to that program or due to biology risk, which is unique to every program. As we progress our programs through clinical development, there may be new technical challenges that arise that cause an entire modality to fail.
While we aim to segregate risk using modalities, there may be foreseen and unforeseen risks across modalities in whole or in part. These include, but are not limited to, mRNA, chemical modifications, and LNPs and their components. In addition, if any one or more of our clinical programs encounter safety, tolerability, or efficacy problems, developmental delays, regulatory issues, or other problems, our platform approach and business could be significantly harmed. We may believe that a particular modality has been de-risked but later determine that new and different risks exist with respect to such modality.
In addition, theThe biology risk across the majority of our pipeline represents targets and pathways not clinically validated by one or more approved drugs. While we believe we have made progress in seeking to reduce biology risk in certain settings, such as for vaccine targets for which wedrugs, and others have shown the utility of neutralizing antibodies, the risk that the targets or pathways that we have selected may not be effective will continue to apply across the majority of our current and future programs.
As we attempt to diversify our risks by developing one or more programs in each modality, there are risks that are unique to each modality and risks that are applicable across modalities. These risks may impair our ability to advance one or more ofprogress our programs inthrough clinical development, obtain regulatory approval, or ultimately commercialize our programs, ornew technical challenges may arise that cause usan entire modality to experience significant delays in doing so,fail. Additionally, any of which may materially harm our business.
Certain features in our development candidates and investigational medicines, including those related to mRNA, chemical modifications, surface chemistries, LNPs, and their components, may result in foreseen and unforeseen risks that are active across some or all of our modalities. Any such portfolio spanningportfolio-spanning risks, whether known or unknown, such as an increased risk of a particular type of side effect, if realized in any one of our programs, would have a material and adverse effect on our other programs and on our business as a whole.
There are also specific additional risks to certain of our modalities and our programs as a whole.programs. For example, prophylactic vaccines typically require clinical testing in thousandsup to tens of thousands of healthy volunteers to define an approvable benefit-risk profile. The need to show a high degree of safety and tolerability when dosing healthy individuals could result in rare and even spurious safety findings, negatively impacting a program prior to or after commercial launch. WhileEven if we believe that certainobserve positive safety, tolerability and levels of immunogenicity we have observed in theearly clinical trials, in our prophylactic vaccine programs are sufficient to initiate additional trials, there can be no assurance that we willmay not observe acceptable safety or efficacy profiles in later-stage trials required for approval of these programs. For neoantigen cancer vaccines, to date, no molecular (non-cell-based) therapeutic protein vaccine has been shown to be effective against cancer and there
There are many clinical and manufacturing challenges specific to personalized medicines, including cell-based therapiesour INT product candidates and vaccines.any other neoantigen cancer vaccines we may develop. These risks include:include a rapid production turn-around time that is measured in weeks in order to supply patients in our clinical trials before further progression and mutation of their tumors, the significant costs incurred in making individualized vaccines,medicines and potential lack of immune responses potentially due to the biology of the tumor or immune status of the patient. These and other risks apply to our PCVINT product candidates and other neoepitope investigational medicine programs.
Additionally, there may be challenges in delivering an adequate quantity of active pharmaceutical ingredient (API) required to drive efficacy due to the limitation in volume of API that can be delivered to a specific location, like a tumor or injured tissue. Our investigational therapies for local injections often require specialized skills for conducting a clinical trial that could delay clinical trials or slow or impair commercialization of an approved investigational medicinea product due to the poor adoption of injected local therapeutics or intratumoral therapies. In addition, the uncertain translatability of target selection from preclinical animal models, including mouse and non-human primate models, to successful clinical trial results may be impossible, particularly for immuno-oncology and systemic therapies, and cancer vaccines. In general, several biological steps are required for delivery of mRNA to translate into therapeutically active medicines. These processing steps may differ between individuals or tissues, and this could leadpotentially leading to variable levels of therapeutic protein, variable activity, immunogenicity or variable distribution to tissues for a therapeutic effect. Gene therapies and mRNA-basedmRNA medicines may activate one or more immune responses against any and all components of the drug product (e.g., the mRNA or the delivery vehicle, such as an LNP) as well as against the encoded protein, giving rise to potential immune reaction related adverse events. Eliciting an immune response against the encoded protein may impede our ability to achieve a pharmacologic effect upon repeat administration or a side effect. These risks apply to all of our programs, including our systemic secreted therapeutics and systemic intracellular therapeutics modalities.
Risks related to our finances
We have incurred significant losses since our inception and we may incur significant losses again in the future.
We have incurred net losses in each year since our inception in 2009, including net losses of $747.1 million, $514.0 million and $384.7 million for the years ended December 31, 2020, 2019 and 2018, respectively. As of December 31, 2020, we had an accumulated deficit of $2.24 billion. Although we had entered into advance purchase agreements for the supply of our COVID-19 vaccine totaling $11.7 billion for deliveries scheduled for 2021 as of December 31, 2020, we may ultimately be unsuccessful in delivering under these agreements or those that we have subsequently enter into, and we may continue to recognize a loss in 2021 or in future years.
We have devoted most of our financial resources to research and development, including our clinical and preclinical development activities and the development of our platform. To date, we have financed our operations primarily through the sale of equity securities and proceeds from strategic alliances and through grants from governmental and private organizations, and more recently through upfront payments from the supply agreements that have been invested into the manufacture, development and commercialization of our COVID-19 vaccine. The amount of our future net losses, if any, will depend upon whether we can successfully commercialize our COVID-19 vaccine and deliver on existing and future supply agreements, as well as the rate of our future expenditures. To the extent we are unsuccessful in commercializing our products (including our COVID-19 vaccine), our access to financing and liquidity will likely depend upon our ability to obtain funding through equity or debt financings, sales of assets, strategic alliances, or additional grants. Other than with respect to our COVID-19 vaccine, we have not commenced or completed pivotal clinical trials for any of our programs in clinical trials, which means that for most of our investigational medicines it may be several years, if ever, before we or our strategic collaborators have a product ready for commercialization. Even if we obtain regulatory approval to market an investigational medicine, our future revenues will depend upon the size of any markets in which our investigational medicines have received approval, and our ability to achieve sufficient market acceptance, reimbursement from third-party payors, and adequate market share in those markets. In addition, under certain of our supply agreements for the COVID-19 vaccine, we rely on our customers to pay us portions of the purchase price upon the achievement of certain regulatory or performance milestones.
In the event that such milestones are never achieved, our customers could terminate their agreements with us, and we may not receive such regulatory or performance milestone payments. If we do not receive such milestone payments, our business may be materially harmed.
We expect to continue to incur significant expenses and could recognize significant operating losses in future periods. We anticipate that our expenses will increase substantially if and as we:
•continue or expand our research or development of our programs in preclinical development;
•continue or expand the scope of our mRNA clinical trials for our investigational medicines;
•initiate additional preclinical, clinical, or other studies for our development candidates and investigational medicines, including under our strategic alliance agreements;
•continue to invest in our platform to conduct research to identify novel mRNA technology improvements, including identifying novel methods of mRNA delivery, such as LNPs, that improve distribution and uptake of mRNA to specific tissues;
•change or add to internal manufacturing capacity or capability;
•change or add additional manufacturers or suppliers;
•add additional infrastructure to our quality control and quality assurance groups to support our operations as we progress our investigational medicines toward commercialization;
•attract and retain skilled personnel, particularly in Cambridge and Norwood, Massachusetts, Basel, Switzerland, and in other global regions where we have established and may continue to establish operations;
•create additional infrastructure to support our operations as a public company and our product development and planned future commercialization efforts, including new sites in the United States and abroad;
•seek marketing approvals and reimbursement for our investigational medicines;
•establish a sales, marketing, and distribution infrastructure to commercialize any products for which we may obtain marketing approval;
•seek to identify and validate additional development candidates and investigational medicines;
•acquire or in-license other development candidates, investigational medicines, and technologies;
•make milestone or other payments under any in-license agreements;
•maintain, protect, and expand our IP portfolio; and
•experience any delays or encounter issues with any of the above.
We have a limited history of recognizing revenue from product sales and may not be able to achieve or maintain long-term sustainable profitability.
Our ability to generate revenue and achieve profitability depends on our ability, alone or with strategic collaborators, to successfully complete the development of, and obtain the regulatory approvals necessary to commercialize our products and investigational medicines, including our commercialization of our COVID-19 vaccine. Our ability to recognize revenues from product sales depends heavily on our success in:
•manufacturing and delivering supply of our COVID-19 vaccine (and any boosters developed in connection with variants, if any) in accordance with contractual terms;
•completing research, preclinical, and clinical development of our development candidates and investigational medicines;
•seeking and obtaining U.S. and foreign marketing approvals for investigational medicines for which we complete clinical trials;
•developing a sustainable, stable, consistent, and transferable manufacturing process or processes for our development candidates and investigational medicines;
•developing a sustainable, scalable, consistent, time sensitive, and transferable manufacturing process for our personalized cancer vaccine investigational medicine;
•furthering the development of our own manufacturing capabilities and manufacturing relationships with third parties in order to provide adequate (in amount and quality) products and services to support clinical development and the market demand for our investigational medicines, if approved;
•obtaining market acceptance of our investigational medicines as a treatment option;
•launching and commercializing investigational medicines for which we obtain marketing approval and reimbursement, either by collaborating with a strategic collaborator or, if launched independently, by establishing a sales force, marketing, and distribution infrastructure;
•addressing any competing technological and market developments;
•implementing additional internal systems and infrastructure;
•negotiating favorable terms in any collaboration, licensing, or other arrangements into which we may enter;
•maintaining, defending, protecting, and expanding our portfolio of IP rights, including patents, trade secrets and know-how; and
•attracting, hiring, and retaining qualified personnel.
We anticipate incurring significant costs associated with the commercialization of our COVID-19 vaccine, and even if one or more of the other investigational medicines that we are developing is approved for commercial sale, we anticipate incurring significant costs associated the commercialization of any such approved investigational medicine. Our expenses could increase beyond expectations if we are required by the FDA, the EMA, or other regulatory agencies to perform clinical and other studies or make changes to our manufacturing or quality systems in addition to those that we currently anticipate. Even though we have begun to generate revenues from the sale of our COVID-19 vaccine, we may not be able to achieve or maintain long-term sustainable profitability and may need to obtain additional funding to continue operations.
Our quarterly and annual operating results may fluctuate. As a result, we may fail to meet or exceed the expectations of research analysts or investors, which could cause our stock price to decline and negatively impact our financing or funding ability as well as negatively impact our ability to exist as a standalone company.
Our financial condition and operating results have varied in the past and will continue to fluctuate from quarter-to-quarter and year-to-year in the future due to a variety of factors, many of which are beyond our control. Factors relating to our business that may contribute to these fluctuations include the following, as well as other factors described elsewhere in this Annual Report on Form 10-K:
•our ability to manufacture and deliver supply of our COVID-19 vaccine;
•delays or failures in advancement of existing or future development candidates into the clinic or investigational medicines in clinical trials;
•the feasibility of developing, manufacturing, and commercializing our programs;
•our ability to manage our growth;
•the outcomes of research programs, clinical trials, or other product development or approval processes conducted by us and our strategic collaborators;
•our ability to develop or successfully commercialize mRNA medicines;
•the ability of our strategic collaborators to develop and successfully commercialize mRNA medicines or other products developed from our IP;
•our relationships, and any associated exclusivity terms, with strategic collaborators;
•our contractual or other obligations to provide resources to fund our development candidates and investigational medicines, and to provide resources to our strategic collaborators or to the strategic alliances themselves;
•our operation in a net loss position for the foreseeable future;
•risks associated with the international aspects of our business including the conduct of clinical trials in multiple locations and potential commercialization in such locations;
•our ability to consistently manufacture our development candidates and investigational medicines;
•risks associated with committing financial resources and personnel to the commercialization of our COVID-19 vaccine, including to support a scale-up of manufacturing to enable a pandemic response;
•our ability to accurately report our financial results in a timely manner;
•our dependence on, and the need to attract and retain, key management and other personnel;
•our ability to obtain, protect, and enforce our IP rights;
•our ability to prevent the theft or misappropriation of our IP, know-how, or technologies;
•advantages that our competitors and potential competitors may have in securing funding, obtaining the rights to critical IP or developing competing technologies or products;
•our ability to obtain additional capital that may be necessary to expand our business;
•our strategic collaborators’ ability to obtain additional capital that may be necessary to develop and commercialize products under our strategic alliance agreements;
•business interruptions such as power outages, strikes, acts of terrorism, or natural disasters; and
•the ultimate impact of the COVID-19 pandemic, or any other health epidemic, on our business, our clinical trials, our research programs, healthcare systems or the global economy as a whole.
Due to the various factors mentioned herein, and others, the results of any of our prior quarterly or annual periods should not be relied upon as indications of our future operating performance.
Our financial results may fluctuate significantly from quarter-to-quarter and year-to-year, such that a period-to-period comparison of our results of operations may not be a good indication of our future performance. In any particular quarter or quarters, our operating results could be below the expectations of securities analysts or investors, which could cause our stock price to decline. We do not control the timing of disclosure of any such milestones related to any of our programs that are managed by our strategic collaborators. Any disclosure by our strategic collaborators or competitors of data or other events that are perceived as negative, whether or not such data are related to other data that we or others release, may have a material adverse impact on our stock price or overall valuation. Our stock price may decline as a result of unexpected clinical trial results in one or more of our programs, including adverse safety events reported for any of our programs.
If we are unsuccessful in commercializing our COVID-19 vaccine, we may need to seek and secure significant funding through financings or from other sources. Clinical data or trial execution that creates delays, setbacks, or failures in one or more of our programs or modalities or the entire pipeline could result in an impaired ability or inability to finance or fund the Company in the future.
We are currently advancing our pipeline of 24 development candidates with 13 having entered clinical studies. Discovering development candidates and developing investigational medicines is expensive, and we expect to continue to spend substantial amounts to (i) perform basic research, perform preclinical studies, and conduct clinical trials of our current and future programs, (ii) continue to develop and expand our platform and infrastructure and supply preclinical studies and clinical trials with appropriate grade materials (including cGMP materials), (iii) seek regulatory approvals for our investigational medicines, and (iv) launch and commercialize any products for which we receive regulatory approval, including building our own commercial sales, marketing, and distribution organization. Furthermore, our ongoing work on our COVID-19 vaccine, including the development of new formulations to respond to variants of the SARS-CoV-2 virus, will require significant additional investment during 2021 and beyond, some of which may not be reimbursed or otherwise paid for by our collaborators or through commercial sales.
As of December 31, 2020, we had approximately $5.25 billion in cash, cash equivalents, and investments. We expect that our existing cash, cash equivalents, and investments will be sufficient to fund our current operations through at least the next twelve months. However, our operating plan may change as a result of many factors currently unknown to us, including with respect to our development, manufacturing and commercialization of our COVID-19 vaccine, and we may need to seek additional funds sooner than planned, through public or private equity or debt financings, structured financings, government or other third-party funding, sales of assets, marketing and distribution arrangements, other collaborations and licensing arrangements, or a combination of these approaches. In any event, we will require additional capital to obtain regulatory approval for, and to commercialize, our investigational medicines. Even if we believe we have sufficient funds for our current or future operating plans, we may seek additional capital if market conditions are favorable or if we have specific strategic considerations. Our spending will vary based on new and ongoing development and corporate activities. Because the length of time and activities associated with discovery of development candidates and development of our investigational medicines are highly uncertain, we are unable to estimate the actual funds we will require for
development, marketing, and commercialization activities. Our future funding requirements, both near and long term, will depend on many factors, including, but not limited to:
•the initiation, progress, timing, costs, and results of preclinical or nonclinical studies and clinical trials for our development candidates and investigational medicines;
•the results of research and our other platform activities;
•the clinical development plans we establish for our investigational medicines;
•the terms of any agreements with our current or future strategic collaborators;
•the number and characteristics of development candidates and investigational medicines that we develop or may in-license;
•the outcome, timing, and cost of meeting regulatory requirements established by the FDA, the EMA, and other comparable foreign regulatory authorities;
•the cost of filing, prosecuting, defending, and enforcing our patent claims and other IP rights, including patent infringement actions brought by third parties against us regarding our investigational medicines or actions by us challenging the patent or IP rights of others;
•the effect of competing technological and market developments, including other products that may compete with one or more of our development candidates or investigational medicines;
•the cost and timing of completion and further expansion of clinical and commercial scale manufacturing activities sufficient to support all of our current and future programs, whether in-house or outsourced; and
•the cost of establishing sales, marketing, and distribution capabilities for any investigational medicines for which we may receive marketing approval and reimbursement in regions where we choose to commercialize our medicines on our own.
We expect to recognize significant revenue in 2021 upon the delivery of our COVID-19 vaccine pursuant to our supply agreements; however, at this time we cannot predict whether we will continue to recognize significant revenue in future years based solely on sales of our COVID-19 vaccine. Until we can generate sufficient product or royalty revenue to fully finance our operations and long-term strategic plan, which we may never do, we expect to finance our future cash needs through a combination of product sales, public or private equity or debt offerings, structured financings, debt financings, collaborations, strategic alliances, sales of assets, licensing arrangements, and other marketing or distribution arrangements. Any fundraising efforts may divert our management from their day-to-day activities, which may adversely affect our ability to develop and commercialize our investigational medicines. In addition, we cannot guarantee that future financing will be available in sufficient amounts, at the right time, on favorable terms, or at all. Negative clinical trial data or setbacks, or perceived setbacks, in our programs or with respect to our technology could impair our ability to raise additional financing on favorable terms, or at all. If our commercialization of our COVID-19 vaccine is unsuccessful, there can be no assurance that we will have the funds necessary to meet our existing payment obligations to third parties, or be able to raise such funds when needed, on terms acceptable to us, or at all. Moreover, the terms of any financing may adversely affect the holdings or the rights of our stockholders and the issuance of additional securities, whether equity or debt, by us, or the possibility of such issuance, may cause the market price of our shares to decline. If we raise additional funds through public or private equity offerings, the terms of these securities may include liquidation or other preferences that may adversely affect our stockholders’ rights.
Further, to the extent that we raise additional capital through the sale of common stock or securities convertible or exchangeable into common stock, your ownership interest will be diluted. If we raise additional capital through debt financings, we would be subject to fixed payment obligations and may be subject to covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures, or declaring dividends. If we raise additional capital through marketing and distribution arrangements, sales of assets or other collaborations, strategic alliances, or licensing arrangements with third parties, we may have to relinquish certain valuable rights to our development candidates and investigational medicines, technologies, future revenue streams, or research programs. We also could be required to seek strategic collaborators for one or more of our current or future investigational medicines at an earlier stage than otherwise would be desirable or relinquish our rights to development candidates, investigational medicines, or IP that we otherwise would seek to develop or commercialize ourselves. If we are unable to raise additional capital in sufficient amounts, at the right time, on favorable terms, or at all, we may have to significantly delay, scale back, or discontinue the development or commercialization of one or more of our products or investigational medicines, or one or more of our other research and development initiatives. Any of the above events could significantly harm our business, prospects, financial condition, and results of operations, cause the price of our common stock to decline, and negatively impact our ability to fund operations.
The investment of our cash, cash equivalents, and investments is subject to risks which may cause losses and affect the liquidity of these investments.
As of December 31, 2020, we had approximately $5.25 billion in cash, cash equivalents, and investments. These investments are subject to general credit, liquidity, market, and interest rate risks. We may realize losses in the fair value of these investments, which would have a negative effect on our consolidated financial statements. In addition, should our investments cease paying or reduce the amount of interest paid to us, our interest income would suffer. The market risks associated with our investment portfolio may have an adverse effect on our results of operations, liquidity, and financial condition.
The amount of and our ability to use net operating losses and research and development credits to offset future taxable income may be subject to certain limitations and uncertainty.
As of December 31, 2020, we had federal and state net operating loss carryforwards of $2.26 billion and $1.70 billion, respectively, a portion of which, if unused, will begin to expire in 2030 and 2032, respectively. As of December 31, 2020, we also had federal and state research and development tax credit carryforwards of $73.3 million and $26.1 million, respectively, which, if unused, begin to expire in 2030 and 2029, respectively. We expect to utilize all of these net operating loss and tax credit carryforwards to offset our future income tax liabilities in the taxable year ending December 31, 2021. Federal net operating losses generated in taxable years beginning after December 31, 2017 generally may not be carried back to prior taxable years, and while such federal net operating losses generated in taxable years beginning after December 31, 2017 will not be subject to expiration, the deduction for such net operating loss in any taxable year will be limited to 80% of our taxable income in such year, where taxable income is determined without regard to the net operating loss deduction itself. However, the Coronavirus Aid, Relief and Economic Security Act repeals the 80% limitation on the utilization of such federal net operating losses for taxable years beginning after December 31, 2017 and beginning before January 1, 2021 and allows for federal net operating losses generated in taxable years beginning after December 31, 2017 and before January 1, 2021 to be carried back to each of the five taxable years preceding the taxable year in which the loss arises. This change in law temporarily allowing for the carryback of federal net operating losses is not expected to produce any material benefit for the issuer. In general, under Sections 382 and 383 of the Internal Revenue Code, a corporation that undergoes an “ownership change” is subject to limitations on its ability to utilize its pre-change net operating losses, or NOLs or tax credits, or credits (including federal research and development tax credits), to offset future taxable income or taxes. For these purposes, an ownership change generally occurs where the aggregate stock ownership of one or more stockholders or groups of stockholders who owns at least 5% of a corporation’s stock increases its ownership by more than 50 percentage points over its lowest ownership percentage within a specified testing period. As of December 31, 2020, none of our NOLs or credits will expire due to Sections 382 and 383. However, future changes in our stock ownership, many of which are outside of our control, could result in an ownership change under Sections 382 and 383 of the Internal Revenue Code and limit our ability to utilize our NOLs and credits. Our NOLs or credits may also be impaired under state law. Accordingly, we may not be able to utilize a material portion of our NOLs or credits if we undergo an ownership change prior to the utilization of all of such NOLs or credits. In addition, the rules regarding timing of revenue and expense recognition for tax purposes in connection with various transactions we have undertaken are complex and uncertain in various respects and could be subject to challenge by taxing authorities. In the event any such challenge is sustained, our remaining net operating losses could be materially reduced and/or we could be determined to be a material cash taxpayer for one or more years.
Risks related to the research, development, regulatory review, and approval of our existing and future pipeline
Preclinical development is lengthy and uncertain, especially for a new class of medicines such as mRNA, and therefore our preclinical programs or development candidates may be delayed, terminated, or may never advance to the clinic, any of which may have a material adverse impact on our platform or our business.
Much of our pipeline is in preclinical development, and these programs could be delayed or not advance into the clinic. Before we can initiate clinical trials for a development candidate, we must complete extensive preclinical studies, including IND-enabling good laboratory practice (GLP), toxicology testing, that support our planned INDs in the United States, or similar applications in other jurisdictions. We must also complete extensive work on Chemistry, Manufacturing, and Controls (CMC) activities (including yield, purity and stability data) to be included in the IND submission. CMC activities for a new class of medicines such as mRNA require extensive manufacturing processes and analytical development, which is uncertain and lengthy. For instance, batch failures as we scale up our manufacturing have occurred and may continue to occur. In addition, we have in the past and may in the future have difficulty identifying appropriate buffers and storage conditions to enable sufficient shelf life of batches of our preclinical or clinical development candidates. If we are required to produce new batches of our development candidates due to insufficient shelf life, it may delay the commencement or completion of preclinical studies or clinical trials of such development candidates. For example, we cannot be certain of the timely completion or outcome of our preclinical testing and studies and cannot predict if the FDA or other regulatory authorities will accept the results of our preclinical testing or our proposed clinical programs or if the outcome of our preclinical testing, studies, and CMC activities will ultimately support the further development of our programs. As a result, we cannot be sure that we will be able to submit INDs or similar applications for our preclinical programs on the timelines we expect, if at all, and we cannot be sure that submission of INDs or similar applications will result in the FDA or other regulatory authorities allowing clinical trials to begin.
Clinical development is lengthy and uncertain, especially with a new class of medicines such as mRNA medicines. Clinical trials of our investigational medicines may be delayed, including as a result of the COVID-19 pandemic or other pandemics in the future, and certain programs may never advance in the clinic or may be more costly to conduct than we anticipate, any of which could have a material adverse impact on our platform or our business.
Clinical testing is expensive and complex and can take many years to complete, and its outcome is inherently uncertain. We may not be able to initiate, may experience delays in, or may have to discontinue clinical trials for our investigational medicines. We and our strategic collaborators also may experience numerous unforeseen events during, or as a result of, any clinical trials that we or our strategic collaborators conduct that could delay or prevent us or our strategic collaborators from successfully developing our investigational medicines, including:
•the FDA, other regulators, Institutional Review Boards (IRBs), or ethics committees may not authorize us or our investigators to commence a clinical trial or conduct a clinical trial at a prospective trial site for any number of reasons, including concerns regarding safety and aspects of the clinical trial design;
•we may experience delays in reaching, or fail to reach, agreement on favorable terms with prospective trial sites and prospective contract research organizations (CROs), the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites;
•we have in the past and intend to continue to optimize our manufacturing processes, including through changes to the scale and site of manufacturing, which may lead to potentially significant changes in our clinical trial designs, requiring additional cost and time, and, as a consequence, lead to a delay in plans for progressing one or more investigational medicines;
•the outcome of our preclinical studies and our early clinical trials may not be predictive of the success of later clinical trials, and interim results of a clinical trial do not necessarily predict final results;
•we may be unable to establish clinical endpoints that applicable regulatory authorities would consider clinically meaningful;
•in an effort to optimize product features, we have in the past and may continue to make changes to our investigational medicines after we commence clinical trials of an investigational medicine, which may require us to repeat earlier stages of clinical testing or delay later stage testing of the investigational medicine;
•clinical trials of any investigational medicines may fail to show safety or efficacy, or produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional nonclinical studies or clinical trials, or we may decide to abandon product development programs;
•differences in trial design between early-stage clinical trials and later-stage clinical trials make it difficult to extrapolate the results of earlier clinical trials to later clinical trials;
•preclinical and clinical data are often susceptible to varying interpretations and analyses, and many investigational medicines believed to have performed satisfactorily in preclinical studies and clinical trials have nonetheless failed to obtain marketing approval;
•our investigational medicines may have undesirable side effects, such as the immunogenicity of the LNPs or their components, the immunogenicity of the protein made by the mRNA, or degradation products, any of which could lead to serious adverse events, or other effects. One or more of such effects or events could cause regulators to impose a clinical hold on the applicable trial, or cause us or our IRBs or ethics committees to suspend or terminate the trial of that investigational medicine or any other of our investigational medicines for which a clinical trial may be ongoing;
•the number of trial participants required for clinical trials of any investigational medicines may be larger than we anticipate, identification of trial participants for such trials may be limited, enrollment in these clinical trials may be slower than we anticipate due to perceived adverse effects, competitive trials, size of the patient population, or other reasons, or participants may withdraw from clinical trials or fail to return for post-treatment follow-up at a higher rate than we anticipate;
•our third-party contractors may fail to comply with regulatory requirements or meet their contractual obligations to us in a timely manner, or at all, or may deviate from the clinical trial protocol or withdraw from the trial, which may require that we add new clinical trial sites;
•regulators may elect to impose a clinical hold, or we or our investigators, IRBs, or ethics committees may elect to suspend or terminate clinical research or trials for various reasons, including noncompliance with regulatory requirements or a finding that the participants are being exposed to unacceptable benefit risk ratio;
•the cost of preclinical or nonclinical testing and studies and clinical trials of any investigational medicines may be greater than we anticipate;
•the supply or quality of our investigational medicines or other materials necessary to conduct clinical trials may be insufficient or inadequate;
•safety and efficacy concerns regarding one or more of our investigational medicines will be considered by us and by the FDA and other global regulators as we pursue clinical trials of new investigational medicines, develop effective informed consent documentation and work with IRBs and scientific review committees (SRCs);
•safety or efficacy concerns regarding our investigational medicines may result from any safety or efficacy concerns arising from nonclinical or clinical testing of other therapies targeting a similar disease state or other therapies, such as gene therapy, that are perceived as similar to ours; and
•the FDA or other regulatory authorities may require us to submit additional data such as long-term toxicology studies, or impose other requirements before permitting us to initiate a clinical trial.
We could also encounter delays if a clinical trial is suspended or terminated by us, the FDA or other regulatory authorities, ethics committees, or the IRBs of the institutions in which such trials are being conducted, or if such trial is recommended for suspension or termination by the data safety monitoring board for such trial. We have in the past been, and may in the future be, delayed in gaining clearance from the FDA or other regulators to initiate clinical trials through the imposition of a clinical hold in order to address comments from such regulators on our clinical trial design or other elements of our clinical trials. The clinical trials of other companies working on mRNA medicines have been put on clinical hold by the FDA. A suspension or termination may be imposed due to a number of factors, including failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols, inspection of the clinical trial operations or trial site by the FDA or other regulatory authorities resulting in the imposition of a clinical hold, unforeseen safety issues, or adverse side effects, including those experienced by other investigational medicines in the same class as our investigational medicines, failure to demonstrate a benefit, or adequate benefit risk ratio, from using an investigational medicine, failure to establish or achieve clinically meaningful trial endpoints, changes in governmental regulations or administrative actions, or lack of adequate funding to continue the clinical trial. Many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our investigational medicines. We must also complete extensive CMC activities that require extensive manufacturing processes and analytical development, which is uncertain and lengthy. For instance, batch failures as we scale up our manufacturing have occurred and may continue to occur. In addition, we have in the past and may in the future have difficulty identifying appropriate buffers and storage conditions to enable sufficient shelf life of batches of our clinical development candidates or investigational medicines. If we are required to produce new batches of our development candidates or investigational medicines due to insufficient shelf life, it may delay the commencement or completion of clinical trials of such development candidates or investigational medicines.
Moreover, the FDA has indicated that prior to commencing later-stage clinical trials for our programs we will need to develop assays to measure and predict the potency of a given dose of our investigational medicines. Any delay in developing assays that are acceptable to the FDA or other regulators could delay the start of future clinical trials. Further, the FDA or other regulatory authorities may disagree with our clinical trial design and our interpretation of data for our clinical trials. Additionally, we have conducted and may conduct in the future clinical trials that utilize an “open-label” trial design. An “open-label” clinical trial is one where both the patient and investigator know whether the patient is receiving the investigational product candidate or either an existing approved drug or placebo. The results from an open-label trial may not be predictive of future clinical trial results when studied in a controlled environment with a placebo or active control. Further, the FDA or other regulators may change the requirements for approval even after they have reviewed and commented on the design for our clinical trials. Significant preclinical or nonclinical testing and studies or clinical trial delays for our investigational medicines also could allow our competitors to bring products to market before we do, potentially impairing our ability to successfully commercialize our investigational medicines and harming our business and results of operations. Any delays in the development of our investigational medicines may harm our business, financial condition, and prospects significantly.
We may experience delays in identifying and enrolling participants in our clinical trials which would delay the progress of our investigational medicines and result in increased expenses.trials.
We depend on enrollment ofEnrolling participants in our clinical trials for our investigational medicines. We may find it difficult to enroll trial participants in our clinical trials, which could delay or prevent clinical trials of our investigational medicines. Identifying and qualifying trial participants to participate in clinical trials of our investigational medicines is critical to our success. The timingDifficulties or delays in enrolling a sufficient number of our clinical trials depends on the speed at which we can recruit trial participants, to participate in testing our investigational medicines. Delays in enrollmentor those with required or desired characteristics, may result in increased costs or may affect the timing or outcome of theour planned clinical trials, which could prevent trial completion of these trials and adversely affect our ability to advance the development of our investigational medicines. If trial participants are unwilling to participate in our trials because of negative publicity from adverse events in our trials or other trials of similar products, or those related to specific therapeutic area, or for other reasons, including competitive clinical trials for similar patient populations, the timeline for recruiting trial participants, conducting studies, and obtainingobtain regulatory approval of potential products may be delayed. These delays could result in increased costs, delays in advancingfor our product development, delays in testing the effectiveness of our product, or termination of the clinical trials altogether.
candidates.
We may not be able to identify, recruit, and enroll a sufficient number of trial participants, or those with required or desired characteristics to achieve diversity in a trial to complete our clinical trials in a timely manner. In addition, as we did in our Phase 3 clinical study of mRNA-1273 in September 2020, we may slow enrollment in a trial to focus on achieving greater diversity in the subject population. Patient and subjectpopulation, as we did in the Phase 3 clinical trial of our original COVID-19 vaccine.
Participant enrollment is affected by many factors, including:
•severity of the disease under investigation;
•complexity and design of the studyclinical trial protocol;
•size of the patient population;
•eligibility criteria for the studyclinical trial in question, including age-based eligibility criteria limiting subject enrollment to adolescent or pediatric populations;
•proximity and availability of clinical studytrial sites for prospective trial participants;
•availability of competing therapies and clinical trials, including between our own clinical trials;
•efforts to facilitate timely enrollment in clinical trials;
•patient referral practices of physicians;
•ability to monitor trial participants adequately during and after treatment;
•ability to recruit qualified clinical trial investigators with the appropriate competencies and experience;investigators;
•clinicians’ and trial participants’ perceptions as to the potential advantages and side effects of the investigational medicineproduct candidate being studied in relation to other available therapies, including any new drugs or treatments that may be approved for the indications we are investigating;
•adverse results or other adverse safety signals in our trials or related to other product candidates, and the need,resulting negative publicity, could discourage potential clinical trial participants and their doctors from participating in the case of our personalized cancer vaccine, to wait for the manufacture of the personalized drug product;trials; and
•our ability to obtain and maintain participant informed consent.
In addition, our clinical trials will compete with other clinical trials for investigational medicines that are in the same therapeutic areas as our investigational medicines, and this competition will reduce the number and types of trial participants available to us, because some trial participants who might
Additionally, we may have opted to enroll in our trials may instead opt to enroll in a trial being conducted by a third party. Since the number of qualified clinical investigators is limited, we expect to conduct some of our clinical trials at the same clinical trial sites that some of our competitors use, which will reduce the number of trial participants who are available for our clinical trials at such clinical trial sites. Moreover, because in some cases our investigational medicines represent a departure from more traditional methods for disease treatment and prevention, potential trial participants and their doctors may be inclined to use conventional therapies or other new therapies rather than enroll trial participants in any future clinical trial involving mRNA investigational medicines. Additionally, if new investigational medicines, such as gene editing therapies, show encouraging results, potential trial participants and their doctors may be inclined to enroll trial participants in clinical trials using those investigational medicines. If such new investigational medicines show discouraging results or other adverse safety indications, potential trial participants and their doctors may be less inclined to enroll trial participants in our clinical trials. We also have entered into strategic alliances under which our strategic collaborators control the development of certain of our investigational medicines, which may provide us limited or no ability to influence the enrollment rate ofin clinical trials where we have entered into strategic alliances pursuant to which our collaborators control clinical trials.development. Even if we are able toor our strategic collaborators can enroll clinical trial participants, there is no guarantee that theysuch participants will ultimately be dosed as part of, or complete, a clinical trial.
mRNA drug development has substantial clinical development and regulatory risks due to the novel nature of this new class of medicines, are a novel approach, and the negative perception of the efficacy, safety or tolerability profile of any investigational medicinesproduct candidates that we or others develop could adversely affect our ability to conduct our business, advance our investigational medicines,product candidates or obtain regulatory approvals.
Other than the EUA and other similar authorizations for COVID-19 vaccines, including ours, noVery few mRNA medicines have been granted EUAauthorized or have been approved to date by the FDA or any other regulatory agency. Adverseregulators, and efficacy, safety and immunogenicity data and real-world evidence with respect to mRNA medicines continue to accumulate. We may observe new, more frequent or more severe adverse events in subjects participating in ongoing clinical trials or among individuals vaccinated with our COVID-19 vaccine. For example, some studies have suggested that our COVID-19 vaccine may be associated with higher rates of myocarditis and pericarditis in young males compared to other COVID-19 vaccines. If similar observations are made in recipients of our investigationalother products or product candidates, or if other unexpected safety issues arise, we could suffer significant damage to our reputation and that of our mRNA platform. Such events could lead to other issues, including delays in our other programs, the need to re-design our clinical trials and the need for significant additional financial resources. In addition, the FDA and other regulators may interpret data from our clinical trials differently than we do and such agencies may require us to conduct additional studies or analyses, which could delay or prevent us from obtaining full regulatory approvals in certain jurisdictions or for certain demographics. For example, in October 2021, the FDA requested that we explore a lower dosage for our COVID-19 vaccine in adolescents, which extended the length of clinical trials in this population prior to receiving regulatory authorization.
Successful discovery and development of mRNA medicines by us or our strategic collaborators is highly uncertain and depends on many factors beyond our or their control. We constantly make business decisions and take calculated risks to advance our development efforts and pipeline, including those related to mRNA technology, delivery technology and manufacturing processes, which ultimately may be unsuccessful.
Our product candidates that appear promising in the early phases of development may fail to advance, experience delays in the clinic, experience clinical holds or fail to reach the market for many reasons, including:
•nonclinical or preclinical study, or clinical trial, results may show potential mRNA medicines to be less effective than desired or to have harmful or problematic side effects or toxicities;
•adverse results in our clinical trials, or in clinical trialsthose of others developing similar products, and or adverse effects relating to mRNA, or our LNPs, may lead to negative publicity or delays in or termination of our programs;
•the resulting publicity, as well as any other efficacy or safety of a combination vaccine product candidate could be less than that seen with the administration of the vaccines separately, which could prevent the combination product from obtaining regulatory approval;
•adverse events in the field of mRNA medicine, or otherrelated to products that are perceived to be similar to mRNA medicines, such as those related to gene therapy or gene editing, could result in a decrease in the perceived benefit of one or more of our programs, increased regulatory scrutiny, decreased confidence by patients and clinical trial collaborators in our investigational medicines,product candidates and less demand for any product that we may develop. Ourdevelop;
•the insufficient ability of our translational models to reduce risk or predict outcomes in humans, particularly given that each component of our product candidates may have a dependent or independent effect on safety, tolerability and efficacy, which may be species-dependent;
•manufacturing failures or insufficient supply of cGMP materials for clinical trials, or higher than expected cost could delay or set back clinical trials, or make mRNA medicines commercially unattractive;
•changes that we make to optimize our manufacturing, testing or formulating of cGMP materials could impact the safety, tolerability and efficacy profile of our product candidates;
•pricing or reimbursement issues or other factors that delay clinical trials or make any mRNA medicine uneconomical or noncompetitive with other therapies;
•our large pipeline of developmentproduct candidates and investigational medicines could result in a greater quantity of reportable adverse events, including suspected unexpected serious adverse reactions, other reportable negative clinical outcomes, manufacturing reportable events or material clinical events that could lead to clinical delay or hold by the FDA or applicable regulatory authority or other clinical delays, any of which could negatively impact the perception of one or more of our programs, as well as our business as a whole. In addition, responseswhole;
•failure to timely advance our programs or a failure or delay in receiving necessary regulatory approvals due to, among other factors, slow or failure to complete enrollment in clinical trials, withdrawal by trial participants from trials, failure to achieve trial endpoints, additional time requirements for data analysis, data integrity issues, preparation of a BLA, or the equivalent application, discussions with the FDA or EMA, a regulatory request for additional nonclinical or clinical data or safety formulation or manufacturing issues may lead to our inability to obtain sufficient funding;
•new legislation or regulations passed by U.S., state or foreign governments in response to negative public perception of mRNA medicines; and
•the proprietary rights of others and their competing products and technologies that may result in new legislation or regulations that could limitprevent our ability to develop any investigationalmRNA medicines or commercialize any approved products, obtain or maintain regulatory approval, or otherwise achieve profitability. More restrictive statutory regimes, government regulations, or negative public opinion would have an adverse effect on our business, financial condition, results of operations, and prospects and may delay or impair the development of our investigational medicines and commercialization of any approved products or demand for any products we may develop.from being commercialized.
Because we are developing some of our developmentproduct candidates or investigational medicines for the treatment of diseases in which there is little clinical experience and, in some cases, using new endpoints or methodologies, the FDA or other regulatory authorities may not consider the endpoints of our clinical trials to provide clinically meaningful results.
There are no pharmacologic therapies approved to treat the underlying causes of many diseases that we are currently attemptattempting to address or may address in the future. For instance, for both MMA and PA,many of the rare diseases for which we are developing treatments, few clinical trials have been attempted. In addition,attempted, and there has been limited clinical trial experience for the development of pharmaceuticalsare no approved drugs to treat these rare diseases in general, and we are not aware of a registrational trial that led to approval of a drug to treat these diseases. There have been some historical trials with other agents to address organic acidemias which may have utilized clinical endpoints that are less applicable to our efforts with our MMA and PA
programs that address the underlying defect. As a result, the design and conduct of clinical trials of investigational medicinesproduct candidates for the treatment of these disorders and other disorders may take longer, be more costly or be less effective as part ofdue to the novelty of development in these diseases.
Even if the FDA does find our success criteria to be sufficiently validated and clinically meaningful, we may not achieve the pre-specified endpoint to a degree of statistical significance in any pivotal or other clinical trials we or our strategic collaborators may conduct for our programs.conduct. Further, even if we do achieve the pre-specified criteria, our clinical trials may produce results that are unpredictable or inconsistent with the results ofcompared against the more traditional efficacy endpoints in the trial. The FDA also could give overriding weight to other efficacy endpoints over a primary endpoint, even if we achieve statistically significant results on that endpoint, if we do not do so on our secondary efficacy endpoints. The FDA also weighs the benefits of a product against its risks and the FDA may view the efficacy results in the context of safety as not being supportive of licensure. Other regulatory authoritiesRegulators in Europe and other countries may make similar findings with respect to these endpoints.
Some of our investigational medicinesCertain mRNA therapies are classified as gene therapies by the FDA and the EMA, and the FDA has indicated that our investigational medicines will be reviewed within its Center for Biologics Evaluation and Research (CBER). Even though our mRNA investigational medicines are designed to have a different mechanism of action from gene therapies, theEMA. The association of our investigational medicines with gene therapies could result in increased regulatory burdens, impair the reputation of our investigational medicines or negatively impact our platform or our business.
There have beenare only a few approvals ofapproved gene therapy products in the United States or foreign jurisdictions, and there have been well-reported significant adverse events associated with their testing and use. Gene therapy products have the effect of introducing new DNA and potentially irreversibly changing the DNA in a cell. By contrast, mRNA is highly unlikely to localize to the nucleus, integrate into the DNA, or otherwise make any permanent changes to cell DNA. Consequently, we expect that our investigational medicines will have a different potential side effect profile from gene therapies.
Regulatory requirements governing gene and cell therapy products have evolved and may continue to change in the future, and the implications for mRNA-basedmRNA therapies are unknown. For example, the FDA has established an office, now called the Office of TissuesTherapeutics Products (OTP), within its Center for Biologics Evaluation and Advanced Therapies within CBERResearch (CBER) to consolidate the review of gene therapy and related products, and convenes the Cellular, Tissue and Gene Therapies Advisory Committee to advise CBER on its review. In the European Union,EU, certain mRNA hastherapies have been characterized as a gene therapy medicinal product,products, which falls within a broader category known as Advanced Therapy Medicinal Products or ATMPs,(ATMPs), which are subject to additional regulatory requirements. In certain countries, mRNA therapies have not yet been classified or any such classification is not known to us; specifically,for example, in Japan, the Pharmaceuticals and Medical Devices Agency has not taken a position on the regulatory classification.classification of mRNA therapies. Notwithstanding the differences between our mRNA investigational medicines and gene therapies, the classification of some of our mRNA investigational medicinesproduct candidates as gene therapies in the United States, the European Union,EU and potentially other countries could adversely impact our ability to develop our investigational medicines, and could negatively impact our platform and our business.product candidates. For instance, a clinical hold on gene therapy products across the field due to risks associated with altering cell DNA irreversibly may apply to our mRNA investigational medicinesproduct candidates irrespective of the mechanistic differences between gene therapies and mRNA.
Adverse events reported with respect to gene therapies or genome editing therapies could adversely impact one or more of our programs. Although our mRNA developmentproduct candidates and investigational medicines are designed not to make any permanent changes to cell DNA, regulatory agencies or others could believe that adverse effects of gene therapies products caused by introducing new DNA and irreversibly changing the DNA in a cell could also be a risk for our mRNA investigational therapies, and as a result may delay one or more of our clinical trials or impose additional testing for long-term side effects. Any new requirements and guidelines promulgated by regulatory review agencies may have a negative effect onnegatively affect our business by lengthening the regulatory review process, requiring us to perform additional or larger studies or increasing our development costs, any of which could lead to changes in regulatory positions and interpretations, delay or prevent advancement or approval and commercialization of our investigational medicines,product candidates, or lead to significant post-approval studies, limitations or restrictions. As we advance our investigational medicines,product candidates, we will be required to consult with these regulatory agencies and advisory committees and comply with applicable requirements and guidelines. If we fail to do so, we may be required to delay or discontinue development of some or all of our investigational medicines.product candidates.
Our work in genomic editing is subject to all risks associated with gene therapies. Although there have been significant advances in recent years in fields of gene therapy and genome editing, in vivo CRISPR-based genome editing technologies are relatively new and their therapeutic utility is largely unproven. Public perception and related media coverage of potential therapy-related efficacy or safety issues, as well as ethical concerns related specifically to genome editing, may adversely influence the willingness of subjects to participate in clinical trials. In addition, any review conducted by an institutional biosafety committee may result in delay or prevent initiation of a gene therapy clinical trial.
A breakthrough therapy designationAdditionally, if any such therapeutic is approved, physicians and patients may be slow or fast track designation by the FDA for a drugfail to accept these novel and personalized treatments. Physicians, health care providers and third-party payors often are slow to adopt new products, technologies and treatment practices, particularly those that may also require additional upfront costs and training. Physicians may not leadbe willing to a faster development or regulatory review or approval process,undergo training to adopt these novel and it would not increasepotentially personalized therapies, may decide the likelihood that the drug will receive marketing approval.
We may seek a breakthrough therapy designation for one or more of our investigational medicines. A breakthroughparticular therapy is defined as a drug that is intended, alonetoo complex or in combination with one or more other drugs,potentially risky to treat a serious or life-threatening disease or condition,adopt without appropriate training and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. For drugs that have been designated as breakthrough therapies, interaction and communication between the FDA and the sponsor of the trial can help to identify the most efficient path for clinical development while minimizing the number of patients placed in ineffective control regimens. Drugs designated as breakthrough therapies by the FDA are also eligible for priority review if supported by clinical data at the time of the submission of the BLA.
Designation as a breakthrough therapy is at the discretion of the FDA. Accordingly, even if we believe that one of our investigational medicines meets the criteria for designation as a breakthrough therapy, the FDA may disagree and instead determinechoose not to make such designation. In any event,administer the receipt of a breakthrough therapy designation for a drugtherapy. Further, due to health conditions, genetic profile or other reasons, certain patients may not be candidates for the therapies. In addition, responses by federal and state agencies, congressional committees and foreign governments to negative public perception, ethical concerns or financial considerations may result in a faster development process, review,new legislation, regulations or medical standards that could limit our ability to develop or commercialize any product candidates, obtain or maintain regulatory approval compared to drugs considered for approval under conventional FDA proceduresor otherwise achieve profitability. Based on these and it would not assure ultimate approval by the FDA. Even if we are successful in obtaining accelerated approval in the United States or under comparable pathways in other jurisdictions, we may face requirementsfactors, health care providers and limitations that will adversely affect our prospects. For example, we may be approved only for a very limited indication, we may not successfully complete required post-approval trials, such trials may not confirm the clinical benefit of our drug, or approval of the drug may be withdrawn. In addition, even if one or more of our investigational medicines qualify as breakthrough therapies, the FDA may later decide that the investigational medicine no longer meets the conditions for qualification or itpayors may decide that the time period for FDA reviewbenefits of these new therapies do not or approval will not be shortened.outweigh their costs.
We have received Fast Track Designation for some of our investigational medicines and may seek Fast Track Designation for others. If a therapy is intended for the treatment of a serious or life-threatening condition and the therapy demonstrates the potential to address significant unmet medical needs for this condition, the drug sponsor may apply for Fast Track Designation. The FDA has broad discretion whether or not to grant this designation, and even if we believe a particular investigational medicine is eligible for this designation, we cannot assure you that the FDA would decide to grant it. Even if we do receive Fast Track Designation, we may not experience a faster development process, review, or approval compared to conventional FDA procedures. The FDA may withdraw Fast Track Designation if it believes that the designation is no longer supported by data from our clinical development program. Fast Track Designation alone does not guarantee qualification for the FDA’s priority review procedures.
We may fail to obtain and maintain orphan drug designations from the FDA or EMA for our future investigational medicines, as applicable.
Our strategy includes filing for orphan drug designation where available for our investigational medicines, and we have received orphan drug designation from both the FDA and the European Commission for PA (mRNA-3927) and our prior MMA candidate (mRNA-3704). Under the Orphan Drug Act, the FDA may grant orphan drug designation to a drug or biologic intended to treat a rare disease or condition, which is defined as one occurring in a patient population of fewer than 200,000 in the United States, or a patient population greater than 200,000 in the United States where there is no reasonable expectation that the cost of developing the drug or biologic will be recovered from sales in the United States. In the United States, orphan drug designation entitles a party to financial incentives, such as opportunities for grant funding toward clinical trial costs, tax advantages, and user-fee waivers. However, orphan drug designation neither shortens the development time or regulatory review time of a drug, nor gives the drug any advantage in the regulatory review or approval process. If a product that has orphan drug designation subsequently receives the first FDA approval for the disease for which it has such designation, the product is entitled to orphan drug exclusivity, which means that the FDA may not approve any other applications, including a full new drug application or NDA, or biologics license application, or BLA, to market the same drug or biologic for the same indication for seven years, except in limited circumstances, such as a showing of clinical superiority to the product with orphan drug exclusivity or where the original manufacturer is unable to assure sufficient product quantity.
In addition, exclusive marketing rights in the United States may be limited if we seek approval for an indication broader than the orphan-designated indication or may be lost if the FDA later determines that the request for designation was materially defective. Further, even if we obtain orphan drug exclusivity for a product, that exclusivity may not effectively protect the product from competition because different drugs with different active moieties may receive and be approved for the same condition, and only the first applicant to receive approval will receive the benefits of marketing exclusivity. Even after an orphan-designated product is approved, the FDA can subsequently approve a later drug with the same active moiety for the same condition if the FDA concludes that the later drug is clinically superior if it is shown to be safer, more effective, or makes a major contribution to patient care. In addition, while we may seek additional orphan drug designation for our investigational medicines, we may never receive such further designations.
The criteria for designating an “orphan medicinal product” in the European Economic Area (consisting of Member States of the European Union, plus Iceland, Liechtenstein and Norway, or the EEA) are similar in principle to those in the United States. Under Article 3 of Regulation (EC) 141/2000, a medicinal product may be designated as orphan if it meets the following criteria: (i) it is intended for the diagnosis, prevention or treatment of a life-threatening or chronically debilitating condition; and (ii) either the prevalence of such condition must not be more than five in 10,000 persons in the EEA when the application is made; or without the benefits derived from orphan status, it must be unlikely that the marketing of the medicine would generate sufficient return in the EEA to justify the investment needed for its development; and (iii) there exists no satisfactory method of diagnosis, prevention or treatment of such condition, or if such a method exists, the product will be of significant benefit to those affected by the condition. Orphan medicinal products are eligible for financial incentives such as reduction of fees or fee waivers made available by the EEA and its Member States to support research into, and the development and availability of, orphan drugs, however orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process. If products with an orphan designation obtain a marketing authorization, they can receive ten years of market exclusivity, during which time no “similar medicinal product” for the same indication may be placed on the market. A “similar medicinal product” is defined as a medicinal product containing a similar active substance or substances as contained in an authorized orphan medicinal product, and which is intended for the same therapeutic indication. Market exclusivity may, however, be reduced to six years if, at the end of the fifth year, it is established that the product no longer meets the criteria for orphan designation, for example, if the product is sufficiently profitable not to justify maintenance of market exclusivity. Orphan medicine marketing exclusivity may also be revoked in certain cases, such as if it is established that a similar medicinal product is safer, more effective or otherwise clinically superior or if not enough orphan medicinal product can be supplied.
Our investigational medicines may face competition from biosimilars approved through an abbreviated regulatory pathway.
The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010 or ACA, includes a subtitle called the Biologics Price Competition and Innovation Act of 2009 (the BPCIA), which created an abbreviated approval pathway for biological products that are biosimilar to or interchangeable with an FDA-approved reference biological product. Under the BPCIA, an application for a biosimilar product may not be submitted to the FDA until four years following the date that the reference product was first approved by the FDA. In addition, the approval of a biosimilar product may not be made effective by the FDA until 12 years from the date on which the reference product was first approved. During this 12-year period of exclusivity, another company may still market a competing version of the reference product if the FDA approves a BLA for the competing product containing the sponsor’s own preclinical data and data from adequate and well-controlled clinical trials to demonstrate the safety, purity, and potency of the other company’s product. The law is complex and is still being interpreted and implemented by the FDA. As a result, its ultimate impact, implementation, and meaning are subject to uncertainty.
We believe that any of our investigational medicines approved as a biological product under a BLA should qualify for the 12-year period of exclusivity. However, there is a risk that this exclusivity could be shortened due to Congressional action or otherwise, or that the FDA will not consider our investigational medicines to be reference products for competing products, potentially creating the opportunity for generic competition sooner than anticipated. Other aspects of the BPCIA, some of which may impact the BPCIA exclusivity provisions, have also been the subject of recent litigation. Moreover, the extent to which a biosimilar, once approved, will be substituted for any one of our reference products in a way that is similar to traditional generic substitution for non-biological products is not yet clear, and will depend on a number of marketplace and regulatory factors that are still developing.
Any clinical trials of our oncology-related products that we conduct with a seamless trial design may not be acceptable to regulatory authorities in the form submitted, or at all, which may delay our clinical development and limit or change the type of information we may gather from our clinical trials.
We may pursue a development program for our oncology-related products that relies upon a seamless trial design, which presents additional risks compared to traditional three-phase development programs. A seamless trial design can be achieved through a first-in-human (FIH) multiple expansion cohort trial, which has a single protocol with an initial dose-escalation phase and also contains three or more additional patient cohorts with cohort-specific objectives. FIH multiple expansion cohort trials are intended to expedite development by seamlessly proceeding from initial determination of a potential effective dose to individual cohorts that have trial objectives typical of Phase 2 trials. Challenges and risks associated with such seamless trial designs include challenges in the timely dissemination of new safety information to investigators, IRBs, and regulators, exposing a large number of patients across cohorts to potentially suboptimal or toxic doses of an investigational drug, exposing more patients than is needed to achieve the cohort’s objectives, and missed interpretations of preliminary trial results and unplanned analyses which can lead to delays in clinical development. Regulatory authorities may find our seamless trial designs unacceptable based on these and other risks of utilizing such designs.
If we are not able to obtain, or if there are delaysdelayed in obtaining, required regulatory approvals, we will not be ableunable to commercialize, or will be delayed in commercializing, investigational medicinesproduct candidates we may develop, and our ability to generate revenue will be materially impaired.develop.
Even if we complete the necessary preclinical studies and clinical trials, the marketing approval process is expensive, time-consuming, and uncertain, and may prevent us from obtaining approvals for the commercialization of any development candidates and investigational medicines we may develop. Any mRNA medicine we may develop and the activities associated with its development and commercialization including design, testing, manufacture, record-keeping, labeling, storage, approval, advertising, promotion, sale, and distribution, are subject to comprehensive regulation by the FDA and by comparable global health authorities.foreign regulators. To obtain the requisiterequired regulatory approvals to commercialize any of our investigational medicines,product candidate, we and our strategic collaborators must demonstrate through extensive preclinical studies and clinical trials that our products are safe, pure and potent or effective in humans, including the target population. Successful completion of clinical trials is a prerequisite to submitting a BLA to the FDA, a marketing authorization application (MAA) to the EMA, and similar marketing applications to comparable global regulatory authorities,foreign regulators, for each investigational medicineproduct candidate and, consequently, the ultimate approval and commercial marketing of any investigational medicines.product.
Failure to obtain marketing approvalTo date, we have only received regulatory authorizations for an investigational medicine will prevent us from commercializing the investigational medicine in a given jurisdiction. We have not received approval to market any investigational medicines from regulatory authorities in any jurisdiction,our COVID-19 vaccine, and it is possible that none of our investigational medicinescurrent or any investigational medicines wefuture product candidates may seek to develop in the future will evernever obtain regulatory approval.approval. We have limited experience in filing and supporting the necessary applications necessary to gainfor marketing approvals and may need to rely on third-party CROs or regulatory consultantsthird parties to assist us in this process. To our knowledge, there is no current precedent for an mRNA-based medicine such as the types we are developing being approved for sale by the FDA or any other global regulatory agency. Although we expect to submit BLAs for our mRNA-based investigational medicinesmRNA product candidates in the United States, other jurisdictions may consider our mRNA-based investigational medicinesmRNA product candidates to be new drugs, not biologics, and require different marketing approval applications. Securing regulatory approval requires the submission of extensive preclinicalPreclinical studies and clinical data and supporting information to the various regulatory authorities for each therapeutic indication to establish the investigational medicine’s safety and efficacy. Securing regulatory approval also requires the submission of information about the product manufacturing process to, and inspection of manufacturing facilities by, the relevant regulatory authority. Any investigational medicines we developtrials conducted in one country may not be effective, may be only moderately effective, or may prove to have undesirable or unintended side effects, toxicities, or other characteristics that may preclude our obtaining marketingaccepted by regulatory authorities elsewhere, and approval or prevent or limit commercial use.in one country does not guarantee regulatory approval in another.
TheAdditionally, the process of obtaining marketing approvals, both in the United States and abroad, is expensive, may take many years if additional clinical trials are required, if approval is obtained at all,time-consuming and uncertain, and can vary substantially based upon a variety of factors, including the type, complexity and novelty of the investigational medicinesproduct candidates involved. Changes in marketing approval policies during the development period, changes in or the enactment of additional statutes or regulations,law or changes in regulatory review for eachmay delay the review of a submitted product application, may cause delays in the approval or rejection of an application. The FDA and comparable authorities in other countriesforeign regulators have substantial discretion in the approval process and may refuse to accept any marketing approval application or may decide that our data are insufficient for marketing approval and require additional preclinical, clinical or other studies. In addition, varying interpretations of the data obtained from preclinical and clinical testing could delay, limit or prevent marketing approval of an investigational medicine. Any marketing approval we ultimately obtain may be limited or subject to restrictions or post-approval commitments that render the approveda product not commercially viable.candidate. Additional delays or non-approval may result if an FDA Advisory Committee or other regulatory authority recommends non-approval or restrictions on approval. In addition, we may experience delays or rejections based upon additional government regulation from future legislation or administrative action, or changes in regulatory agency policy during the period of product development, clinical trials, and the review process.
Regulatory agencies also may approve an mRNA medicine for fewer or more limited indications than requested or may grant approval subject to the performance of post-marketing studies. In addition, regulatory agencies may not approve the labeling claims that are necessary or desirable for the successful commercialization of our investigational medicines.
The FDA and other regulatory agenciesforeign regulators review the CMC section of regulatory filings. Any aspects found unsatisfactory by regulatory agencies may result in delays in clinical trials and commercialization. In addition, the regulatory agencies conduct pre-approval inspections at the time of a BLA. Any negative findings by regulatory agencies and failure to comply with requirements may lead to delay in approval and failure to commercialize the potential mRNA investigational medicine.product candidate.
If we experience delays in obtaining, approval or if we fail to obtain, regulatory approval of any investigational medicinesproduct candidate we may develop, the commercial prospects for those investigational medicines willsuch product candidate would be harmed, and our ability to generate revenues will be materially impaired.
We may never obtain EMA or other foreign regulatory body approvalEmergency authorizations that we have received for any of our investigational medicines,COVID-19 vaccine for certain demographics, including pediatrics, are temporary and even if we do, we may nevercould be able to commercialize any of our investigational medicines in any other jurisdiction, which would limit our ability to realize their full market potential.revoked.
To date, our COVID-19 vaccine (mRNA-1273) is our only product that has obtainedWe currently operate under an EUA from the FDA in the United States and similar authorizations in other jurisdictions. Approval of an investigational medicineprovided by the FDA for our COVID-19 vaccines that are administered as a primary series in pediatric populations. The FDA may revoke this authorization for a variety of reasons, including if obtained, does not ensure approval by regulatory authorities in other countriesit determines that the underlying health emergency no longer exists or jurisdictions. In order to eventually market anywarrants such authorization. If new data emerges that shows the benefits of our investigational medicines in any particular foreign jurisdiction, we must establish and comply with numerous and varying regulatory requirements on a jurisdiction-by-jurisdiction basis regarding safety and efficacy. In addition, clinical trials conducted in one country may not be accepted by regulatory authorities in other countries, and regulatory approval in one country does not guarantee regulatory approval in any other country. Approval processes vary among countries and can involve additional product testing and validation and additional administrative review periods.
Seeking foreign regulatory approval could result in difficulties and costs for us and require additional preclinical studies or clinical trials which could be costly and time consuming. Regulatory requirements can vary widely from country-to-country and could delay or prevent the introduction of our products in those countries. The foreign regulatory approval process involves all of the risks associated with FDA approval. Wevaccine do not have any investigational medicines approved for sale in any jurisdiction, including international markets, and we do not have experience in obtaining regulatory approval in international markets. If we failcontinue to comply with regulatory requirements in international markets or to obtain and maintain required approvals, or if regulatory approvals in international markets are delayed, our target market will be reduced and our ability to realize the full market potential of our products will be unrealized.
Our planned clinical trials or those of our strategic collaborators may reveal significant adverse events not seen in our preclinical or nonclinical studies and may result in a safety profileoutweigh its risks, emergency authorizations that could delay or terminate clinical trials, or delay or prevent regulatory approval or market acceptance of any of our investigational medicines.
There is typically an extremely high rate of attrition for product candidates across categories of medicines proceeding through clinical trials. These product candidates may fail to show the desired safety and efficacy profile in later stages of clinical trials despite having progressed through nonclinical studies and initial clinical trials. A number of companies in the biopharmaceutical industry have suffered significant setbacks in later-stage clinical trials due to lack of efficacy or unacceptable safety profiles, notwithstanding promising results in earlier trials. Most investigational medicines that commence clinical trials are never approved as products and there can be no assurance that any of our current or future clinical trials will ultimately be successful or support further clinical development of any of our investigational medicines.
Some of our investigational medicines are developed or intended to be co-administered with other developmental therapies or approved medicines. For example, our PCV investigational medicine (mRNA-4157) and our KRAS investigational medicine (mRNA-5671) in collaboration with Merck may be co-administered with Merck’s anti-PD-1 therapy, pembrolizumab. Our IL-12 investigational medicine (MEDI1191) in collaboration with AstraZeneca is being developed to be co-administered with checkpoint inhibitors (e.g., anti-PD-L1, anti-CTLA4). These combinations may have additional side effects. The uncertainty resulting from the use of our investigational medicines in combination with other therapies may make it difficult to accurately predict side effects in future clinical trials.
Some of our development candidates and investigational medicines are developed or intended for adolescent and/or pediatric patients under the age of eighteen, including our hMPV/PIV3 vaccine (mRNA-1653), pediatric RSV vaccine (mRNA-1345), PA development candidate (mRNA-3927) and MMA development candidate (mRNA-3705). The first pediatric subjects in the Phase 1b age de-escalation clinical trial of mRNA-1653 have been enrolled and dosed. During the COVID-19 related pause, the Safety Monitoring Committee reviewed a preliminary data set on these small initial group of pediatric patients and recommended continuation of the study with no modification in the planned trial execution. Our PA development candidate (mRNA-3927) for which we are conducting a first-in-human Phase 1/2 trial in patients between one and eighteen years of age has resumed study start up activities. If participants are enrolled in the trial and successfully dosed, they will be the first of our rare disease investigational medicines from our systemic intracellular therapeutics modality dosed in humans. The uncertainty resulting from the first dosing of young, human subjects with an investigational medicine makes it difficult to accurately predict if significant adverse events or other side effects will be observed.
Most of our investigational medicines are formulated and administered in an LNP which, when administered, may lead to systemic side effects related to the components of the LNP, some of which may not have been previously tested in humans. While we have continued to optimizereceived for our LNPs, there can be no assurance that our LNPs will not have undesired effects. Our LNPs could contribute, in whole or in part, to one or more of the following: immune reactions, infusion reactions, complement reactions, opsonization reactions, antibody reactions, or reactions to PEG. Certain aspects of our investigational medicines may induce immune reactionsCOVID-19 vaccines from either the mRNA or the lipid as well as adverse reactions within liver pathways or degradation of the mRNA or the LNP, any of which could lead to significant adverse events in one or more of our clinical trials. Many of these types of side effects have been seen for
previously developed LNPs. There may be resulting uncertainty as to the underlying cause of any such adverse event, which would make it difficult to accurately predict side effects in future clinical trials and would result in significant delays in our programs.
If significant adverse events or other side effects are observed in any of our current or future clinical trials, we may have difficulty recruiting trial participants to any of our clinical trials, trial participants may withdraw from trials, or we may be required to abandon the trials or our development efforts of one or more development candidates or investigational medicines altogether. We, the FDA or other applicable regulatory authorities, or an IRB, may impose a clinical hold or suspend or terminate clinical trials of an investigational medicine at any time for various reasons, including a belief that participants in such trials are being exposed to unacceptable health risks or adverse side effects. Some potential therapeutics developed in the biotechnology industry that initially showed therapeutic promise in early-stage trials have later been found to cause side effects that prevented their further development. Evenregulators could be revoked if the side effects do not preclude the drug from obtaining or maintaining marketingconditions for granting such authorizations no longer apply. If such authorizations were revoked, without receiving final approval unfavorable benefit risk ratio may inhibit market acceptance of the approved product due to its tolerability versus other therapies. Any of these developments could materially harmdistribute our COVID-19 vaccines, our business financial condition, and prospects.would be adversely impacted.
Even if we obtain regulatory approval for an investigational medicine,
Our products are, and even though we have obtained an EUA for our COVID-19 vaccine, ourany future products will remainbe, subject to regulatory scrutiny.
Even if we obtain regulatory approval in a jurisdiction,the applicable regulatory authority we may still imposeremain subject to significant restrictions on the indicated uses or marketing of our product or imposeand ongoing requirements for potentially costly post-approval studiesstudies-such as those required under an accelerated approval by the FDA or other similar type of approval-or post-market surveillance. For example, the holder of an approved BLA is obligated tomust monitor and report adverse events and monitor and report any failure of a product to meet the specifications in the BLA. The holder of an approved BLA, must alsoas well as submit new or supplemental applications and obtain FDA approval for certain changes to the approved product, product labeling, or manufacturing process. AdvertisingAdditionally, pharmacovigilance obligations under the regulatory regimes of the jurisdictions where our products are distributed require us to collect, process, analyze and monitor safety data and to identify and evaluate adverse reactions to our products as they are administered in those jurisdictions. If we or any of our partners assisting us in meeting these obligations cannot comply with relevant regulations, we may be subject to sanctions, increased costs and reputational harm, or our regulatory authorizations to distribute our vaccines in the relevant jurisdiction may be revoked or curtailed.
Furthermore, advertising and promotional materials must comply with FDA rules and regulations and are subject to FDA review, in addition to other potentially applicable federal and state laws. In addition, regulatory agencies may not approve the labeling claims that are necessary or desirable for the successful commercialization of our product candidates.
We are required by the FDA to conduct post-marketing studies for our COVID-19 vaccine (mRNA-1273) to further assess the risks of myocarditis and pericarditis following vaccination. Additionally, we have committed to conducting additional post-marketing safety studies, including conducting a study to evaluate pregnancy and infant outcomes after receipt of mRNA-1273 during pregnancy. We or others could identify previously unknown side effects, or known side effects could be observed as being more frequent or severe than in clinical trials or earlier post-marketing periods. If we, our contract manufacturers or other strategic collaborators fail to comply with applicable post-approval regulatory requirements, following approval of any of our investigational medicines, a regulatory agency may:
•may issue a warning letter asserting that we are in violation of the law;
•law, seek an injunction or impose civil or criminal penalties or monetary fines;
•fines, suspend or withdraw regulatory approval or revoke a license;
•license, suspend any ongoing clinical trials;
•trials, refuse to approve a pending BLA or supplements to a BLA submitted by us;
•us, seize product; or
• recall products or product candidates, or require field alerts to physicians, pharmacists and hospitals or refuse to allow us to enter into supply contracts, including government contracts.
Any We could also be required to conduct additional nonclinical studies or clinical trials, or implement changes in labeling or to our manufacturing processes, specifications or facilities. Initiation of any government investigation of alleged violations of law couldor lawsuit, including class-action lawsuits, would require us to expend significant time and resources in response and couldwould likely generate negative publicity. The occurrence of any event or penalty described above may inhibit our ability to commercialize our COVID-19 vaccine, or any future approved products, and generate revenues.
If any of our investigational medicines cause undesirable side effects, it could delay or prevent their regulatory approval, limit the commercial potential, or result in significant negative consequences following any potential marketing approval. Investigational medicines we may develop may be associated with an adverse immune response or other serious adverse events, undesirable side effects, or unexpected characteristics. In addition to serious adverse events or side effects caused by any of our investigational medicines, the administration process or related procedures also can cause undesirable side effects. If any such events occur, the clinical trials of any of our investigational medicines could be suspended or terminated.
If in the future we are unable to demonstrate that such adverse events were caused by factors other than our investigational medicine, the FDA, the EMA, or other regulatory authorities could order us to cease further development of, or deny approval of, any of our investigational medicines for any or all targeted indications. Even if we are able to demonstrate that all future serious adverse events are not product-related, such occurrences could affect patient recruitment or the ability of enrolled trial participants to complete the trial. Moreover, if we elect, or are required, to delay, suspend, or terminate any clinical trial of any of our investigational medicines, the commercial prospects of such investigational medicines may be harmed and our ability to generate product revenues from any of these investigational medicines may be delayed or eliminated. Any of these occurrences may harm our ability to identify and develop investigational medicines, and may harm our business, financial condition, result of operations, and prospects significantly.
Additionally, if we successfully obtain regulatory approval for an investigational medicine, the FDA or other regulatory authorityforeign regulators could require us to adopt a Risk Evaluation and Mitigation StrategyStrategies (REMS) for any product to ensure that the benefits of treatment with such investigational medicine outweigh the risks for each potential patient, which may include, among other things, a medication guide outlining the risks of the product for distribution to patients, a communication plan to health care practitioners, extensive patient monitoring or
distribution systems and processes that are highly controlled, restrictive and more costly than what is typical for the industry. Furthermore, if we or others later identify undesirable side effects caused by any product that we develop, several potentially significant negative consequences could result, including:
•regulatory authorities may suspendincluding the suspension or withdrawwithdrawal of approvals or revokeand licenses, the addition of such product;
•regulatory authorities may require additional warnings on the label;
•we may be requiredwarning labels, changes to change the way a product is administered, the requirement to conduct further clinical trials, lawsuits or conduct additional clinical trials;
•we could be sued and held liableincreased liability for harm caused to patients and their children;children and
•our reputation may suffer.
reputational harm to us. Any of these events could prevent us from achieving or maintaining market acceptance of any products we may identify and develop and could have a material adverse impact on our business, financial condition, results of operations and prospects.
If we are successful in gaining approval for any of our investigational medicines, we will continue to face significant regulatory oversight of the manufacturing and distribution of our products. Product manufacturers and their facilities are subject to payment of user fees and continual review and periodic inspections by the FDA and other regulatory authorities for compliance with cGMP and adherence to commitments made in the BLA. If we or a regulatory agency discovers previously unknown problems with a product such as adverse events of unanticipated severity or frequency, or problems with the facility where the product is manufactured, a regulatory agency may impose restrictions relative to that product or the manufacturing facility, including requiring recall or withdrawal of the product from the market or suspension of manufacturing.
Even though mRNA-1273 has been granted an EUA, mRNA-1273 will remain the subject of regulatory scrutiny. For example, the EUA issued by the FDA contains numerous conditions of authorization, including the requirement to conduct post-authorization observational studies to evaluate the association between mRNA-1273 and a pre-specified list of adverse events of special interest, along with deaths and hospitalizations, and severe COVID-19. We or others could identify previously unknown side effects, or known side effects could be observed as being more frequent or severe than in clinical studies or earlier post-marketing periods, in which case:
•prospectssales of mRNA-1273 may be more modest than originally anticipated;
•the FDA and other regulatory agencies may revoke an EUA or other authorizations for mRNA-1273;
•we may decide, or be required, to conduct recalls or send field alerts to physicians, pharmacists and hospitals;
•additional nonclinical or clinical studies, changes in labeling, or changes to manufacturing processes, specifications and/or facilities may be required; and
•government investigations or lawsuits, including class action suits, may be brought against us.
Further, should we be unable to follow the conditions of authorization included in the EUA, the FDA may revoke EUA for mRNA-1273. Any of the above occurrences could reduce or prevent sales of mRNA-1273, increase our expenses and impair our ability to successfully commercialize mRNA-1273.
Risks related to the manufacturing of our commercial products developmentand product candidates investigational medicines and our future pipeline
Our mRNA products developmentand product candidates and investigational medicines are based on novel technologies and any development candidates and investigational medicines we develop may beare complex and difficult to manufacture. We or our third-party manufacturers may encounter difficulties in manufacturing, product release, shelf life, testing, storage, supply chain management or shipping for any of our medicines, including our COVID-19 vaccine. If we or any of our third-party manufacturers encounter such difficulties, our ability to supply commercial product or material for clinical trials or any approved product could be delayed or stopped.products.
The manufacturing processes for our commercial products, development candidates and investigationalmRNA medicines are novel and complex. Other than vaccines for active immunization to prevent COVID-19 caused by SARS-CoV-2, including our COVID-19 vaccine, that have received EUAs from the FDA or similar authorizations by authorities in other jurisdictions, there are no mRNA medicines commercialized to date or manufactured at such scale. Due to the novel nature of this technologyWe and our limited experience at larger scale production, wecollaborators have experienced and may continue to encounter difficulties in manufacturing, product release, shelf life, testing, storage, supply chain management or shipping. These difficultiesshipping, including delays as our supply chain expands and grows more complex. We could be due to any number of reasons including, but not limited to,experience issues resulting from complexities of producing batches at larger scale, equipment failure, human error, choice and quality of raw materials and excipients, analytical testing technology and product instability. Further, mRNA medicines encapsulated in LNPs must be developed and manufactured under well-controlled conditions, or pharmacological activity can be adversely impacted.
In an effort to optimize product features, we have in the past and may in the future make changes to our developmentproduct candidates or investigational medicines in their manufacturing and stability formulation and conditions. This has in the past and may in the future result in our having to resupply
batches for preclinical or clinical activities when there is insufficient product stability during storage and insufficient supply. Insufficient stability or shelf life of our developmentproduct candidates
and investigational medicines could materially delay our or our strategic collaborators’ ability to continue the clinical trial for that development candidate or investigational medicinetrials or require us to begin a new clinical trial with a newly formulated drug product, due to the need to manufacture additional preclinical or clinical supply.
Our high rate of innovation is high, which has resulted in and will continue to causecauses a high degree of technology change that can negatively impact product comparability during and after clinical development. Furthermore, technology changes may drive the need for changes in, modification to, or the sourcing of new manufacturing infrastructure or may adversely affect third-party relationships.
The process to generate mRNA medicines encapsulated in LNPs is complex and, if not developed and manufactured under well-controlled conditions, can adversely impact pharmacological activity. Furthermore, prior to our recent scale-up for our COVID-19 vaccine, we had not manufactured mRNA medicines at commercial scale. We may encounter difficulties in continuing to scale up our manufacturing process, thereby potentially impacting clinical and commercial supply.
We are scaling up our batch size to accommodate the clinical supply requirements of some of our programs. However, inIn many cases, we may haveneed to utilize multiple batches of drug substance and drug product to meet the clinical supply requirement of a single preclinical study or clinical trial. Failure in our ability to scale up batch size or failure in any batch, which we have experienced in the past, may lead to a substantial delay in our clinical trials or in the commercialization of any approved product.
As For example, the changes we make as we continue developing new manufacturing processes for our drug substance and drug product the changes we implement to manufacturing process may in turn impact specification and stability of the drug product. Changes in our manufacturing processesproduct, and may lead to failure of batches, and this could lead toresulting in a substantial delay in delivery of commercial product or conduct of our clinical trials. Our mRNA investigational medicinesproduct candidates may prove to have a stability profile that leads to a lower than desired shelf life of the final approved mRNA medicine. This poses risk in supply requirements, wasted stock and higher cost of goods.
We are dependent on a number of equipment providers who are also implementing novel technology. Further, we have developed our own custom manufacturing equipment for certain of our medicines. If such equipment malfunctions or we encounter unexpected performance issues with such equipment, we could encounter delays or interruptions to clinical and commercial supply. Due to the number of different programs, we may have cross contamination of investigational medicinesproduct candidates inside of our factories, CROs, suppliers or in the clinic that affect the integrity of our investigational medicines.product candidates.
As we scale the manufacturing output for commercial production and particular programs, we plan to continuously improve yield, purity and the pharmaceutical properties of our commercial products developmentand product candidates and investigational medicines fromIND-enabling studies through commercial launch, including shelf lifeshelf-life stability, and solubility properties of drug product and drug substance. Because of continuous improvement in manufacturing processes, we may switch processes for a particular program during development. However, after thea change in process, more time iswill be required for pharmaceutical property testing, such as 6 or 12 month stability testing. That may require resupplying clinical material or making additional cGMP batches to keep up with clinical trial demand before such pharmaceutical property testing is completed.
We are utilizing a number of raw materials and excipients that have a single source of supply, are new to the pharmaceutical industry and are being employed in a novel manner. Some of these raw materials and excipients have not been scaled to a level to support commercial supply and could experience unexpected manufacturing or testing failures or supply shortages. Such issues with raw materials and excipients could cause delays or interruptions to clinical and commercial supply of our investigational medicines.products or product candidates.
We have established a number ofseveral analytical assays and may have to establish several more to assess the quality of our mRNA investigational medicines.product candidates. We may identify gaps in our analytical testing strategy that might prevent release of product or could require product withdrawal or recall. For example, we may discover new impurities that have an impact on product safety, efficacy or stability. This may lead to an inability to release mRNA investigational medicinesproduct candidates until the manufacturing or testing process is rectified.
Our productsAs we grow as a commercial company and product intermediates are extremely temperature sensitive, and we may learn that any or all of our investigational medicines are less stable than desired. We may also find that transportation conditions negatively impact product quality. This may require changes to the formulation or manufacturing process for one or more of our investigational medicines and result in delays or interruptions to clinical or commercial supply. In addition, the cost associated with such transportation services and the limited pool of vendors may also add additional risks of supply disruptions.
As our drug development pipeline increases and matures, the increased demand for clinical and commercial supplies from our facilities and third parties may impact our ability to operate. We will require increased capacity across our entire supply chain. Furthermore, we rely on manythird-party service providers, including those that provide manufacturing or testing services, all of whom have inherent risks in their operations that may adversely impact our operations.
We have limited experience at larger scale production necessary to support large-scale clinical trials and commercial sales. Completion of our clinical trials and commercialization of our vaccineproduct candidates require access to, or development of, facilities to manufacture our vaccine candidates at sufficient yields and at commercial-scale.commercial scale. We expect to continue to make significant investments in our manufacturing capacity and commercial network as we continue to expand our commercial launch efforts. We are building regional manufacturing capability globally and are subject to risks associated with building and operating in foreign jurisdictions.
To supplement our internal manufacturing infrastructure, we have limited experienceentered into agreements for the production, as well as for commercial fill-finish manufacturing, any of our vaccine candidatesproducts to supply markets globally. We may need to engage additional third parties in the volumes that are necessaryfuture to support large-scale clinical trialsmeet our capacity needs. If we cannot enter into such arrangements on favorable terms, or commercial sales. Effortsat all, our ability to develop, manufacture and distribute our products would be adversely affected. Further, efforts to establish these capabilities may not meet initial expectations as to scheduling, scale-up, reproducibility, yield, purity, cost, potency or quality. In addition, other companies, many with substantial resources, compete with us for accessIf we are unable to institute
necessary controls related to product development, manufacturing and quality, we may encounter difficulties producing our products on the materials neededtimelines and in the quantities set forth in our supply agreements or to manufacture our vaccines.meet potential future demand.
We currently utilize, and expect to continue to utilize, third parties to, among other things, manufacture raw materials, components, parts, and consumables and to perform quality testing. If the field of mRNA and other nucleic acid medicines continues to expand, we may encounter increasing competition for these materials and services. Demand for third-party manufacturing or testing facilities may grow at a faster rate than their existing capacity, which could disrupt our ability to find and retain third-party manufacturers capable of producing sufficient quantities of such raw materials, components, parts and consumables required to manufacture our mRNA investigational medicines.product candidates. The use of service providers and suppliers could expose us to risks, including, but not limited to:
•termination or non-renewal of supply and service agreements with third parties in a manner or at a time that is costly or damaging to us;
•disruptions to the operations of these suppliers and service providers caused by conditions unrelated to our business or operations, including the bankruptcy of the supplier or service provider; and
•inspections of third-party facilities by regulatory authorities that could have a negative outcome and result in delays to or termination of their ability to supply our requirements.
Our reliance on third-partythird-party manufacturers may adversely affect our operations or result in unforeseen delays or other problems beyond our control. Because of contractual restraints and the limited number of third-party manufacturers with the expertise, required regulatory approvals and facilities to manufacture our bulk vaccines on a commercial scale, replacement of a manufacturer may be expensive and time-consuming and may cause interruptions in the production of our vaccine. Regulatory authorities may also require us to register our facilities or those of another supplier if we terminate an existing third-party manufacturer relationship, which could lead to delays or our inability to supply a particular market. A third-party manufacturer may also encounter difficulties in production. These problems may include:
•difficulties with production costs, scale up and yields;
•availability of raw materials and supplies;
•quality control and assurance;
•shortages of qualified personnel;
•compliance with strictly enforced federal, state and foreign regulations that vary in each country where products might be sold; and
•lack of capital funding.
As a result, anyAny delay or interruption could have a material adverse effect onadversely affect our business, financial condition or results of operations.
The illegal distribution and sale by third parties of counterfeit versions of mRNA products, stolen products, or alternative third-party distribution and sale of mRNA products could have a negative impact on our financial performance or reputation.
Third parties could illegally distribute and sell counterfeit versions of mRNA products, especially on online marketplaces, which do not meet the rigorous manufacturing and testing standards under cGMP. Counterfeit products are frequently unsafe or ineffective, and may even be life-threatening. Counterfeit medicines may contain harmful substances or the wrong dose. However, to distributors and users, counterfeit products may be visually indistinguishable from the authentic version.
Reports of adverse reactions to counterfeit products, increased levels of counterfeiting, or unsafe mRNA products could materially affect patient confidence in our mRNA products. It is possible that adverse events caused by unsafe counterfeit or other non-mRNA products will mistakenly be attributed to our mRNA products. In addition, thefts of inventory at warehouses, plants or while in-transit, which are not properly stored and which are sold through unauthorized channels could adversely impact patient safety, our reputation, and our business. Public loss of confidence in the integrity in mRNA products as a result of counterfeiting, theft, or improper manufacturing processes could have a material adverse effect on our business, results of operations, and financial condition.
We are subject to regulatory and operational risks associated with the physical and digital infrastructure at both our internal manufacturing facilities and at those of our external service providers.
In 2018, we completed construction of a newOur manufacturing facility, Moderna Technology Center, or MTC, in Norwood, Massachusetts that, among other things, is intended for cGMP manufacture of drug substance and drug product. While the design of the facility is based on current standards for biotechnology facilities and it has been visited by the FDA, it has not been subject to formal review, inspection or pre-approved by any regulatory agency, such as the FDA. We only recently began producing drug product and drug substance at the MTC for commercial use.
We have designed the MTC to incorporate a significant level of automation of equipment with integration of several digital systems to improve efficiency of operations. We have attemptedThe digitization of our facilities exposes us to achieve a high level of digitization for a clinical and commercial manufacturing facility relative to industry standards. While this is meant to improve operational efficiency, this may pose additionalthe risk of process equipment malfunction and even overall manufacturingmalfunctions. These risks include potential system failurefailures or shutdownshutdowns due to internal or external factors including, but not limited to, design issues, system compatibility or potential cybersecurity compromises, incidents or breaches. Upgrades or changes to our systems, infrastructure or the software that we implement, use, or upon which our business relies, may result in the introduction of new cybersecurity vulnerabilities and risks.
Our facilities and infrastructure or those of our contract manufacturers or other third-party providers may also be subject to intentional attacks or acts of sabotage whether by outside actors, contractors or employees. These disruptions may lead to delay in supply or shutdown of our facility or facilities. Any disruption in our manufacturing capabilities at the MTC, or those of our contract manufacturers,manufacturers’ manufacturing capabilities could cause delays in our production capacity for our drug substances or drug products or a shutdown of facilities, could impose additional costs, cause us to fail to meet certain product volume or delivery timing obligations, or may require us to identify, qualify and establish an alternative manufacturing site, the occurrence of which could have a material adverse effect onadversely affect our business, financial condition, results of operations, and prospects.
As we expand our development and commercial capacity,capacities, we mayhave and expect that we will continue to establish additional manufacturing capabilities insidein the MTC footprint or expand toUnited States, as well as in other locations or geographies, whichcountries, such as Australia, Canada and the United Kingdom. This expansion may lead to regulatory delays or prove costly.more costly than anticipated. If we fail to select the correct location,suitable locations, complete the construction in an efficient manner, engage effectively with local regulators, recruit the required personnel and generallyor manage our growth effectively, the development and production of commercial products or our investigational medicinesproduct candidates could be delayed or curtailed. AdditionalWe will require significant additional investments may be needed if changes in our manufacturing process lead to required changes in the MTC’sprocesses as we expand our manufacturing infrastructure.
There are risks inherent in pharmaceutical manufacturing operations that could affect our ability and the ability of our third-party manufacturers or contract manufacturing organizations to meet our delivery requirements or provide adequate amounts of material.
The convergence of process and analytical technology, raw materials, consumables, equipment, physical infrastructure, including a clean room environment, and air handling and other utilities, results in complex procedures and systems that have to work effectively to manufacture our investigational medicines. Failure or process defects in any of the interrelated systems at either our manufacturing facilities or those of our third-party providers, could adversely impact our ability to manufacture and supply our COVID-19 vaccine or our investigational medicines.
Our products and investigational medicinesproduct candidates are inherently sensitive to shipping and storage conditions, which, in some cases, requires cold-chain logistics and could subject our investigational medicinessubjects them to risk of loss or damage.
Our COVID-19 vaccineproducts and our investigational medicinesproduct candidates are sensitive to temperature, storage and handling conditions. Loss in investigationalconditions, and we could lose medicines could occur if the product or product intermediates are not stored or handled properly. Shelf life for our products and investigational medicines may vary by product andcandidates is not fully quantified and is expected to be variable, and it is possible that our investigational medicines could be lost due to expirationthey may expire prior to use. Cold-chain logistics are required for certain of our investigational medicines, as well as for our COVID-19 vaccine.products and product candidates. If we or third-party distributors do not effectively maintain effective cold-chain supply logistics, then we may experience an unusual number of returned or out of date products and critical batches of productsproduct may be rendered unusable.
Failure to effectively maintain cold-chain supply logistics, by us or third parties, This has in the pastled and could in the future lead to additional manufacturing costs and delays in our ability to supply required quantities for clinical trials or commercial sale, or otherwise.sale. In addition, the cost associated with such transportation services and the limited pool of vendors could cause supply disruptions.
We are subject to significant regulatory oversight with respect toregarding manufacturing our COVID-19 vaccineproducts and our mRNA investigational medicines.product candidates. Our manufacturing facilities or the manufacturing facilitiesthose of our third-party manufacturers or suppliers may not meet regulatory requirements. Failure to meet cGMPcurrent Good Manufacturing Practice (cGMP) requirements set forth in regulations promulgated by the FDA, EMA, and other global health authorities could result in significant delays in any approval of and costs of our products.
The manufacturing of vaccines and therapeuticsmedicines for clinical trials or commercial sale is subject to extensive regulation. Componentsregulation, and components of a finished product approved for commercial use or used in clinical trialssuch products must be manufactured in accordance with cGMP requirements. Theserequirements, which are enforced, in the case of the FDA, in part through its facilities inspection program. The regulations govern manufacturing processes and procedures, including record keeping, and the implementation and operation of quality systems to control and assure the quality of products and materials used in clinical trials. Poor control of the cGMP production processes can lead to product quality failures that can impact our ability to supply product, resulting in cost overruns and delays to clinical timelines, which could be extensive. Such production process issues include but are not limited to:
•critical deviations in the manufacturing process;
•facility and equipment failures;
•contamination of the product due to an ineffective quality control strategy;
•facility contamination as assessed by the facility and utility environmental monitoring program;
•ineffective process, equipment, or analytical change management, resulting in failed lot release criteria;
•raw material failures due to ineffective supplier qualification or regulatory compliance issues at critical suppliers;
•ineffective product stability;
•failed lot release or facility and utility quality control testing;
•ineffective corrective actions or preventative actions taken to correct or avoid critical deviations due to our developing understanding of the manufacturing process as we scale; and
•failed or defective components or consumables.
We must supply all necessary documentation in support of a BLA or other marketing authorization application on a timely basis and must adhere to the FDA’s, EMA’s, and other countries’ cGMP requirements which are enforced, in the case of the FDA, in part through its facilities inspection program.
Regulatory authorities typically require representative manufacturing site inspections to assess adequate compliance with cGMP and manufacturing controls as described in the filing.controls. If either we or one of our third-party manufacturing sites fails to provide sufficient quality assurance or control, the product approval to commercialize may not be granted. Inspections by regulatory authorities may occur at any time during thedevelopment or commercialization phase of products. The inspections may be product specific or facility specific for broader cGMP inspections, or as a follow up to market or development issues that the regulatory agency may identify. Deficient inspection outcomes may influencenegatively impact the ability of our third-party manufacturers or suppliers to fulfill their supply obligations, impacting or delaying supply or delaying programs.
The manufacturing process for our COVID-19 vaccine, and for any other products that we may develop, is subject to the FDA and foreign regulatory authority approval process, and we may need to contract with manufacturers who we believe can meet applicable FDA and foreign regulatory authority requirements on an ongoing basis.process. If we or our third-party manufacturers are unable to reliably produce products or investigational medicinesproduct candidates to specifications acceptable to the FDA or other regulatory authorities, we or our strategic collaborators may not obtain or maintain the approvals we or they needneeded to commercialize such products. Even if we or our strategic collaborators obtain regulatory approval is obtained for any of our mRNA medicines, and even though we have received an EUA for our COVID-19 vaccine, there is no assurance that either we or our contract manufacturing organizationsCMOs will be able to manufacture the approved medicine to specifications acceptable to the FDA or other regulatory authorities, to produce it in sufficient quantities to meet the requirements for the potential launch of the product, or to meet potential future demand.authorities. Any of these challenges could delay completion of clinical trials, require bridging clinical trials or the repetition of one or more clinical trials, increase clinical trial costs, delay approval of our investigational medicines,product candidates, impair commercialization efforts or increase our cost of goods. The occurrence of any of the foregoinggoods, which, in turn, could have an adverse effect on our business, financial condition, results of operations and prospects.
In addition, we may not have direct control over the ability of our contract manufacturers to maintain adequate quality control, quality assurance and qualified personnel. Furthermore, all of ourOur contract manufacturers are engaged with other companies to supply or manufacture materials or products for suchother companies which exposes our contract manufacturers to regulatory risks for the production of such materials and products. As a result,their failure to meet the applicable regulatory requirements for the production of those materials and products may generally affect the regulatory status of our contract manufacturers’ facility.their facilities. In addition, to the extent that we rely on foreign contract manufacturers, including for our COVID-19 vaccine, we are or will be subject to additional risks, including the need to comply with import and export regulations. Our failure, or the failure of our third-party manufacturers or other strategic collaborators, to comply with applicable regulations could result in sanctions being imposed on us, including clinical holds, fines, injunctions, civil penalties, delays, suspension or withdrawal of approvals, license revocation, seizures or recalls of investigational medicines or products, operating restrictions, and criminal prosecutions, any of which could significantly and adversely affect supplies of our products and investigational medicines (including those of our strategic collaborators) and our overall business operations. Our
potential future dependence upon others for the manufacture of our investigational medicines and raw materials may adversely affect our future profit margins and our ability to commercialize any products that receive regulatory approval on a timely and competitive basis.
The FDA, the EMA and other foreign regulatory authorities may require us to submit product samples of any lot of any approved product, together with the protocols showing the results of applicable tests, at any time. UnderIn some circumstances, the FDA, the EMA, or other foreign regulatory authoritiescases, regulators may require that we do not distributeprohibit us from distributing a lot or lots until the relevant agencyit authorizes such release. Deviations in the manufacturing process, including those affecting quality attributes and stability, may result incause unacceptable changes in the product, that could resultresulting in lot failures or product recalls. Our third-party contract
manufacturers have in the past, experienced lot failures and some may have experiencedresulting in product recalls.recalls of our COVID-19 vaccine. Lot failures have in the past caused, and lot failures or product recalls in the future with respect to product produced by either our own facilities or those of our third-party manufacturersmanufacturers’ facilities could cause, us and our strategic collaborators to delay clinical trials or product launches, which could be costly to us and otherwise harm our business, financial condition, results of operations and prospects.
We and our manufacturing partners also may encounter problems hiring and retaining the experienced scientific, quality-control,quality control and manufacturing personnel needed to operate our manufacturing processes and operations or those of our manufacturing partners, which could result in delays in production or difficulties in maintaining compliance with applicable regulatory requirements. While we will train and qualify all personnel around the appropriate handling of our products and materials,Additionally, we may not be able to control for or ultimately detect intentional sabotage or negligence by any employee or contractor.
Risks specific to certain investigational medicines
Our personalized cancer vaccine, or PCV, investigational medicine isINT product candidates are uniquely manufactured for each patient using a novel, complex manufacturing process and we may encounter difficulties in production.
We custom design and manufacture PCVsINTs that are unique and tailored specifically for each patient. Manufacturing unique lots of PCVsINTs is susceptible to product loss or failure due to issues with:
•logistics associated with the collection of a patient’s tumor, blood or other tissue sample;
•shipping such samples to a facility for genetic sequencing;
•next generationnext-generation sequencing of the tumor mRNA;
•identification of appropriate tumor-specific mutations;
•the use of a software program, including proprietary and open source components, which is hosted in the cloud and a part of our investigational medicine,product candidate, to assist with the design of the patient-specific mRNA, which software must be maintained and secured;
•effective design of the patient-specific mRNA that encodes for the required neoantigens;
•batch specific manufacturing failures or issues that arise due to the uniqueness of each patient-specific batch that may not have been foreseen;batch;
•quality control testing failures;
•unexpected failures of batches placed on stability;
•shortages or quality control issues with single-use assemblies, consumables or critical parts sourced from third-party vendors that must be changed out for each patient-specific batch;
•significant costs associated with individualized manufacturing that may adversely affect our ability to continue development;
•successful and timely manufacture and release of the patient-specific batch;
•shipment issues encountered during transport of the batch to the patient site of care; and
•the ability to define a consistent safety profile at a given dose when each participant receives a unique vaccine.therapy.
We have built and installed custom manufacturing equipment for PCV that has beenINTs incorporated into a personalized vaccinededicated unit in the MTC. This unit is currently operational and we are producing batches of PCV from the MTC.at our Norwood, Massachusetts facility. This equipment may not function as designed, which may lead toresulting in deviations in the drug product being produced. This canproduced, which could lead to increased batch failure and the inability to supply patients enrolled in thea clinical trial. IfAdditionally, as we continue our clinical development plans are expanded, duecurrent Phase 3 INT trials, expand to the custom natureadditional tumor types and prepare for commercialization, we anticipate an increase in manufacturing demand for our INTs that will require ongoing investments. Some of the additional equipment that will be required will be custom-made for us, which will lead to long lead times and single-use assemblies, we may not be ableexpedited procurement to supply this expanded need reliably without significant investments.meet our timelines. In addition, there will be considerable timeit may take longer than anticipated to scale up our facilities or build new facilities beforeand to complete our Marlborough, Massachusetts facility (which we can beginexpect to dedicate to INTs) to meet any commercial demand, if our PCVINT product candidate is approved. This expansion orand addition of new facilities could also lead to product comparability issues, which cancould further delay introduction of new capacity.
Because our PCVsINTs are manufactured for each individual patient, we will beare required to maintain a chain of identity with respect to each patient’s tissue sample, sequence data derived from such tissue sample, results of analysis of such patient’s genomic analysis and the custom manufactured product for each patient. Maintaining such a chain of identity is difficult and complex, and failure to do so has in the pastresulted and may in the future result in product mix up, adverse patient outcomes, loss of product or regulatory action, including
withdrawal of any approved products from the market. Further, as our PCV investigational medicine isINTs are developed through early-stage clinical trials to later-stage clinical trials towards approval and commercialization, we expect that multiple aspects of the complicated collection, analysis, manufacture and delivery process will be modified in an effort to optimize processes and results. These changes may not achieve the intended objectives, and any of these changes could cause our PCVsINTs to perform differently than we expect, potentially affecting the results of clinical trials.
Risks related to our reliance on third parties
We are dependent on single-source suppliers for some of the components and materials used in, and the processes required to develop, our products and product candidates.
We depend on single-source suppliers for some of the components and materials used in, and manufacturing processes required to develop and commercialize, our products and product candidates. We cannot ensure that these suppliers will remain in business, have
sufficient capacity or supply to meet our needs or that they will not be purchased by one of our competitors or another company that will cease working with us. Our use of single-source suppliers exposes us to several risks, including disruptions in supply, price increases, late deliveries or business interruptions.
There are, in general, few alternative sources of supply for substitute components. If we have to switch suppliers, the manufacture and delivery of our products or product candidates could be interrupted for an extended period. Establishing additional or replacement suppliers for any of the components or processes used in our products or product candidates, if required, may not be accomplished quickly, if at all. Any replacement supplier (or us, if we produced directly) would need to be qualified and may require additional regulatory authority approval, resulting in further delay. Any interruption or delay in the pastsupply of components or materials, or our inability to obtain components or materials from alternate sources at acceptable prices in a timely manner, could impair our ability to meet the demand for our products or product candidates. Additionally, as part of the FDA’s approval of our product candidates, the FDA will review the manufacturing processes and facilities of our single-source suppliers.
We have entered into, and in the future may enter into, strategic alliances with third parties for the development and commercialization of our products development candidates and investigational medicines.product candidates. If these strategic alliances are not successful, our business could be adversely affected.
We have limited resources to conduct clinical operations and we are in the process of establishing infrastructure for sales, marketing, and distribution. Accordingly, we have entered into strategic alliances under which our strategicwith collaborators that have provided, and may in the future provide, funding, intellectual property licenses and other resources for developing, manufacturing and commercializing our investigational medicines. Weproduct candidates. Additionally, we have entered into, and expect to enter into additionalfuture, strategic alliances where we agree to access additionalprovide funding, capabilities,intellectual property licenses and expertise in the future.other resources to third parties. Our existing strategic alliances, and any future strategic alliances we enter into, may pose a number of risks, including the following:
•strategic collaborators may not perform their obligations as expected;
•the clinical trials conducted as part of such strategic alliance may not be successful;
•strategic collaborators may not pursue development and commercialization of any investigational medicinesproducts that achieve regulatory approval or may elect not to continue or renew development or commercialization of programs based on clinical trial results, changes in the strategic collaborators’ focus or available funding or external factors such as an acquisition, that divert resources or create competing priorities;
•strategic collaborators may delay clinical trials, provide insufficient funding or resources for clinical trials (whether as a result of a business decision or necessitated by financial difficulties of such collaborator), stop a clinical trial, abandon an investigational medicine,a product candidate or repeat or conduct new clinical trials, or require a new formulation of an investigational medicine for clinical testing;
•strategic collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with our products or investigational medicines if the strategic collaborators believe that competitive products are more likely to be successfully developed or can be commercialized under terms that are more economically attractive than ours;trials;
•products or investigational medicinesproduct candidates developed in strategic alliances with us may be viewed by our strategic collaborators as competitive with their own investigational medicinesproducts or products,product candidates, which may cause strategic collaboratorsthem to cease to devote resources to the development or commercialization of our investigational medicines;commercialization;
•a strategic collaborator with marketing and distribution rights to one or more of our products or investigational medicines that achieve regulatory approval may not commit sufficientinsufficient resources to the marketing and distribution of any such product;
•disagreements with strategic collaborators, including disagreements over proprietary rights, contract interpretation or the preferred course of development of any investigational medicines,product candidates, may cause delays or termination of the research, development or commercialization of such investigational medicines, mayproduct candidates, lead to additional responsibilities for us with respect to such investigational medicines,product candidates or may result in litigation or arbitration, any of which would be time-consuming and expensive;
•strategic collaborators may not properly maintain or defend our IP rights or may use our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our IP or proprietary information or expose us to potential litigation;information;
•disputes may arise with respect to the ownership of IP developed pursuant to our strategic alliances;
•strategic collaborators may infringe the IP rights of third parties, which may exposeexposing us to potential litigation and potential liability;
•strategic alliances may be materially amended, or terminated for the convenience of the strategic collaborator and, if materially amended, or terminated, the development of our investigational medicines may be delayed, and we could be required to raise additional capital to pursue further development or commercialization of the applicable investigational medicines;
•future relationships may require us to incur non-recurring and other charges, increase our near- and long-term expenditures, issue securities that dilute our existing stockholders or disrupt our management and business;
•any equity investments we make in collaborators could face significant competitiondecrease in seeking appropriate strategic collaborators and the negotiation process is time-consuming and complex; value or become worthless; and
•our international operations through any future collaborations, acquisitions or joint ventures may expose us to certain operating, legal and other risks not encountered in the United States.
If ourOur strategic alliances do not resultcollaborators generally may materially amend or terminate their agreements with us, which has happened in the successful development and commercialization of programs, or if one of our strategic collaborators materially amends, or terminates itspast. If any collaboration agreement with us,is terminated, we may not receive anyanticipated future research or development funding or milestone, earn-out royalty, profit share or other contingent payments underand the strategic alliances. If we do not receive the funding we expect under these agreements, ourresearch or development of investigational medicines couldour product candidates may be delayed and weor discontinued. It may need additional resources to develop our
investigational medicines. In addition, in general our strategic collaborators have the right to terminate their agreements with us for convenience. A strategic collaborator has in the past terminated its agreement with us. If one of our strategic collaborators terminates its agreement with us, we may find it morealso be difficult to attract new strategic collaborators to continue development or commercialization of the applicable product candidate, and the perception of us in the business and financial communitiesour reputation could be adversely affected. All of the risks relating to product development, regulatory approval and commercialization described elsewhere in this Annual Report on Form 10-Kthese Risk Factors apply to the activities of our strategic collaborators.
Our strategic collaborators control aspects of our clinical trials, regulatory activities, and other aspects of our strategic alliances, which could result in delays and other obstacles in the development and commercialization of our proposed products and materially harm our results of operations.
For some programs, we depend on strategic collaborators to design and conduct clinical trials for our investigational medicines. As a result, we may not control the manner or time schedule in which these clinical trials are conducted, which may negatively impact our business operations. In addition, if any of our strategic collaborators withdraws support for one or more of our programs or proposed products or otherwise impairs their development, our business could be negatively affected.
We may seek to establish additional strategic alliances and, if we are not ableunable to establish them on commercially reasonable terms, we may have to alter our development and commercialization plans. Certain of our strategic alliance agreements may restrict our ability to develop certain products.
Our development programs and the potential commercialization of our developmentproduct candidates and investigational medicines will require substantial additional cash to fund expenses. For some of our investigational medicines, weWe may decide to collaborate with pharmaceutical and biotechnology companies for the development and potential commercialization of those investigational medicines.
Wesome of our product candidates, and we face significant competition in seeking appropriate strategic collaborators. Whether we reach a definitive agreement for anyOur ability to establish additional strategic alliances will depend, among other things, uponon our assessment of the strategic collaborator’s resources and expertise, the terms and conditions of the proposed strategic alliance and the proposed strategic collaborator’s evaluation of a number of factors. Those factors may include the design or results of clinical trials, the likelihood of approval by the FDA or similar foreign regulatory authorities, outside the United States, the potential market for the subject investigational medicine,product candidate, the costs and complexities of manufacturing and delivering such investigational medicineproduct candidate to trial participants, the potential of competing drugs, the existence of uncertainty with respect to our or the proposed collaborator’s ownership of technology, which can exist if there is a challenge to such ownership without regard to the merits of the challenge, and industry and market conditions generally. The strategic collaborator may also consider alternative investigational medicines or technologies for similar indications that may be available to collaborate on and whether such a collaboration could be more attractive than the one with us for our investigational medicine. The terms of any additional strategic alliances or other arrangements that we may establish may not be favorable to us.
We are also restricted under some of our existing strategic alliance agreements from entering into certain future agreements on certain terms with potential strategic collaborators to pursue other targets on our own. These restrictions on working with targets, polypeptides, routes of administration and fields could limit our ability to enter into strategic collaborations with future strategicother collaborators or to pursue certain potentially valuable development candidates or investigational medicines.product candidates.
We may not be able to negotiate additional strategic alliances on a timely basis, on favorable terms, or at all. Strategic alliances are complex and time-consuming to negotiate and document. If we are unable to negotiate andcannot enter into new strategic alliances on a timely basis, on favorable terms or at all, we may haveneed to curtail the development of the investigational medicineproduct candidate for which we are seeking to collaborate, reduce or delay its development program or one or more of our other development programs, delay its potential commercialization or reduce the scope of any sales or marketing activities, or increase our expenditures andundertake development or commercialization activities at our own expense. If we elect to increase our expenditures to fund development or commercialization activities on our own, we may need to obtain additional capital, which may not be available to us on favorable terms or at all. If we do not have sufficient funds, we may not be able to further develop our investigational medicines or bring them to market and generate product revenue.
We are dependent on single-source suppliers for some of the components and materials used in, and the processes required to develop, our products, development candidates and investigational medicines.
We currently depend on single-source suppliers for some of the components and materials used in, and manufacturing processes required to develop and commercialize, our products, development candidates and investigational medicines. We cannot ensure that these suppliers or service providers will remain in business, have sufficient capacity or supply to meet our needs, or that they will not be purchased by one of our competitors or another company that is not interested in continuing to work with us. Our use of single-source suppliers of raw materials, components, key processes, and finished goods exposes us to several risks, including disruptions in supply, price increases, or late deliveries. There are, in general, relatively few alternative sources of supply for substitute components. These vendors may be unable or unwilling to meet our future demands for our clinical trials or commercial sale. Establishing additional or replacement suppliers for these components, materials, and processes could take a substantial amount of time and it may
be difficult to establish replacement suppliers who meet regulatory requirements. Any disruption in supply from any single-source supplier or service provider could lead to supply delays or interruptions which would damage our business, financial condition, results of operations, and prospects.
If we have to switch to a replacement supplier, the manufacture and delivery of our products, development candidates or investigational medicines could be interrupted for an extended period, which could adversely affect our business. Establishing additional or replacement suppliers for any of the components or processes used in our products or investigational medicines, if required, may not be accomplished quickly. If we are able to find a replacement supplier, the replacement supplier would need to be qualified and may require additional regulatory authority approval, which could result in further delay. While we seek to maintain adequate inventory of the single-source components and materials used in our products, any interruption or delay in the supply of components or materials, or our inability to obtain components or materials from alternate sources at acceptable prices in a timely manner, could impair our ability to meet the demand for our investigational medicines.
In addition, as part of the FDA’s approval of our investigational medicines, we will also require FDA review of the individual components of our process, which include the manufacturing processes and facilities of our single-source suppliers.
Our reliance on these suppliers, service providers, and manufacturers subjects us to a number of risks that could harm our reputation, business, and financial condition, including, among other things:
•delays to the development timelines for our development candidates or investigational medicines;
•interruption of supply resulting from modifications to or discontinuation of a supplier’s operations;
•delays in product shipments resulting from uncorrected defects, reliability issues, or a supplier’s variation in a component;
•a lack of long-term supply arrangements for key components with our suppliers;
•inability to obtain adequate supply in a timely manner, or to obtain adequate supply on commercially reasonable terms;
•difficulty and cost associated with locating and qualifying alternative suppliers for our components in a timely manner;
•production delays related to the evaluation and testing of components from alternative suppliers, and corresponding regulatory qualifications;
•delay in delivery due to our suppliers’ prioritizing other customer orders over ours;
•damage to our reputation caused by defective components produced by our suppliers; and
•fluctuation in delivery by our suppliers due to changes in demand from us or their other customers.
If any of these risks materialize, costs could significantly increase and our ability to meet demand for our products could be impacted.
We rely on and expect to continue to rely on third parties to conduct aspects of our research, preclinical studies, protocol development and clinical trials for our development candidates or investigational medicines.product candidates. If these third parties do not perform satisfactorily, comply with regulatory requirements or meet expected deadlines, we may not be able to obtain regulatory approval for or commercialize our investigational medicines and our business could be substantially harmed.product candidates.
We currently rely and expect to continue to rely on third parties such as CROs to help manage certain preclinical work and our clinical data management organizations,trials, and on medical institutions, and clinical investigators and CROs to assist in the design and review of, and to conduct, our clinical trials. We currently relytrials, including enrolling qualified patients. In addition, we engage third-party contractors and expectcollaborators to continue to rely on third parties to conduct certainsupport numerous other research, commercial and preclinical testing activities. In some cases, these third parties may terminate their engagements with us. If we need to enter into alternative arrangements, it would delay our discovery or product development activities.
Our reliance on these third parties for research and developmentadministrative activities, will reducewhich reduces our control over these activities but willdoes not relieve us of our regulatory or contractual responsibilities. We will be responsible for ensuring that each of our preclinical studies and clinical trials is conducted in accordance with the applicable protocol, legal and regulatory requirements, and scientific standards. For example, we will remain responsible forresponsibilities, such as ensuring that each of our clinical trials is conducted in accordance with theits general investigational plan and protocols for the trial.protocols. Moreover, the FDA requires us to comply with regulations, commonly referred to as GCPsGLPs and good clinical practices for conducting, recording and reporting the results of preclinical studies and clinical trials to assure that data and reported results are credible and accurate and that in the case of clinical trials the rights, integrity and confidentiality of trial participants are protected. We also are required to register ongoing clinical trialsSuch standards will evolve and post the results of completed clinical trials on a government-sponsored database, ClinicalTrials.gov, within certain timeframes. Failure to do so can result in fines, adverse publicity, and civil and criminal sanctions. For any violations of laws and regulations during the conduct of our preclinical studies and clinical trials, we could be subject to warning letters or enforcement action that may include civil penalties up to and including criminal prosecution.
We and our CROs will be required to comply with regulations, including GCPs, for conducting, monitoring, recording, and reporting the results of preclinical studies and clinical trials to ensure that the data and results are scientifically credible and accurate and that the trial participants are adequately informed, among other things, of the potential risks of participating in clinical trials. We also are responsible for ensuring that the rights of our clinical trial participants are protected. These regulations are enforced by the FDA, the Competent Authorities of the Member States of the European Economic Area, and comparable foreign regulatory authorities for any
investigational medicines in clinical development. The FDA enforces GCP regulations through periodic inspections of clinical trial sponsors, principal investigators, and trial sites. If we or our CROs fail to comply with applicable GCPs, the clinical data generated in our clinical trials may be deemed unreliable and the FDA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. We cannot assure you that, upon inspection, the FDA will determine that any of our future clinical trials will comply with GCPs. In addition, our clinical trials must be conducted with investigational medicines produced in accordance with the requirements in cGMP regulations. Our failure or the failure of our CROs to comply with these regulations may require us to repeat clinical trials, which would delay the regulatory approval process and could also subject us to enforcement action.
Although we intend to design the clinical trials for certain of our investigational medicines, our strategic collaborators will design the clinical trials that they are managing (in some cases, with our input) and in the case of clinical trials controlled by us, we expect that CROs will conduct all of the clinical trials. As a result, many important aspects of our development programs, including their conduct and timing, will be outside of our direct control. Our reliance on third parties to conduct future preclinical studies and clinical trials will also result in less direct control over the management of data developed through preclinical studies and clinical trials than would be the case if we were relying entirely upon our own staff. Communicating with outside parties can also potentially lead to mistakes as well as difficulties in coordinating activities. Outside parties may:
•have staffing difficulties;
•fail to comply with contractual obligations;
•experience regulatory compliance issues;
•undergo changes in prioritiesnew or become financially distressed;
•form relationships with other entities, some of which may be our competitors;
•have human errors; or
•be subject to cyber-attacks.changing requirements.
These factorsIf third parties do not successfully carry out their contractual duties or meet expected deadlines, we may materiallyneed to replace them, which could cause a delay of the affected clinical trial, drug development program or applicable activity. If clinical trials are not conducted in accordance with our contractual expectations or regulatory requirements, action by regulatory authorities may significantly and adversely affect the willingnessconduct or abilityprogress of third partiessuch trials or even require a clinical trial to conductbe redone. Accordingly, our preclinical studies and clinical trials and may subject us to unexpected cost increases that are beyond our control. If the CROs do not perform preclinical studies and clinical trials in a satisfactory manner, breach their obligations to us or fail to comply with regulatory requirements, the development, regulatory approval, and commercialization of our investigational medicines may be delayed, we may not be ableefforts to obtain regulatory approvalapprovals for and commercialize our investigational medicines, or our development programs may be materially and irreversibly harmed. If we are unable to rely on preclinical and clinical data collected by our CROs, wedrug candidates could be required to repeat, extend the duration of, or increase the sizedelayed. In addition, failure of any clinical trials wethird-party contractor to conduct and thisactivities in accordance with our expectations could significantly delay commercialization and require significantly greater expenditures.
We also expectadversely affect the relevant research, development, commercial or administrative activity. Failure of any third-party contractor to rely on other third partiestimely provide access to transport, store, and distribute the required materials for our clinical trials and for our manufacturing processes. In the past certain of our third-party vendors have mishandled our materials, resultingdata in loss of full or partial lots of material. Any further performance failure on the part of these third parties coulda format that is acceptable to us may result in damaged products and could delay clinicaldelays or impediments to our regulatory submissions or other development or marketing approval of any investigational medicines we may develop or commercialization of our medicines, if approved, producing additional losses and depriving us of potential product revenue, causing us to default on our contractual commitments, result in losses that are not covered by insurance, and damage our reputation and overall perception of our products in the marketplace.activities.
Risks related to our intellectual property
Other companiesWe may be unable to obtain and enforce patent protection for our discoveries and the intellectual property rights therein, or organizationsprotect the confidentiality of our trade secrets.
Our success depends, in part, on our ability to protect proprietary methods and technologies that we develop under the patent and other IP laws of the United States and other countries, so that we can prevent others from unlawfully using our inventions and proprietary information. Because certain U.S. patent applications are confidential until the patents issue, third parties may challengehave filed patent applications for technology covered by our pending patent applications without our being aware of those applications, and our patent rightsapplications may not have priority over those applications. In addition, publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing, if at all. Therefore, we cannot be certain that we were the first to make the inventions claimed in our patents or
pending patent applications or that we were the first to file for patent protection of such inventions. We therefore may assertbe unable to secure desired patent rights, thereby losing exclusivity. In the past, we have obtained licenses under third-party patents to market our products or conduct our research and development or other activities, and we may do so in the future if necessary. If licenses are not available to us on favorable terms, we may not be able to market the affected products or conduct the desired activities.
The process of obtaining and enforcing patent protection is expensive and time-consuming and our pending patent applications may not result in issued patents. Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent applications may fail to result in valid enforceable patents, or our patent protection could be reduced or eliminated, for non-compliance with these requirements. If we or our strategic collaborators fail to file and prosecute all necessary and desirable patent applications at a reasonable cost and in a timely manner, our business may be adversely affected.
Despite our and our strategic collaborators’ efforts to protect our proprietary rights, unauthorized parties may obtain and use information that we regard as proprietary. While issued patents are presumed valid, they may not survive a validity challenge and could be held unenforceable. Any patents we have obtained, or obtain in the future, may be challenged, invalidated, adjudged unenforceable or circumvented by parties seeking to design around our IP. Also, third parties or the USPTO may commence patent office proceedings involving our patents or patent applications. Any challenge to, finding of unenforceability or invalidation, or circumvention of, our patents or patent applications, would be costly, would require significant time and attention of our management, could reduce or eliminate royalty payments to us from third-party licensors and could have a material adverse impact on our business.
The standards that the USPTO and its foreign counterparts use to grant patents are not always applied predictably or uniformly and can change. Similarly, the ultimate degree of protection that will be afforded to biotechnology inventions, including ours, in the United States and foreign countries, remains uncertain and is dependent upon the scope of the protection decided upon by patent offices, courts and lawmakers. For example, the America Invents Act included a number of changes to the patent laws of the United States. If any of the enacted changes prevent us from developingadequately protecting our discoveries, including our ability to pursue infringers of our patents to obtain injunctive relief or for substantial damages, our business could be adversely affected. One major provision of the America Invents Act changed U.S. patent practice from a first-to-invent to a first-to-file system. If we fail to file an invention before a competitor files on the same invention, we no longer have the ability to provide proof that we were in possession of the invention prior to the competitor’s filing date, and commercializingthus would not be able to obtain patent protection for our products.invention. There is also no uniform, worldwide policy regarding the subject matter and scope of claims granted or allowable in pharmaceutical or biotechnology patents. In certain countries, for example, methods for the medical treatment of humans are not patentable.
Accordingly, we do not know the degree of future protection for our proprietary rights or the breadth of claims that will be allowed in any patents issued to us or to others. We also rely to a certain extent on trade secrets, know-how and technology, which are not protected by patents, to maintain our competitive position. We also rely on non-disclosure agreements and invention assignment agreements entered into with our employees, consultants and third parties. If any trade secret, know-how or other technology not protected by a patent were to be disclosed to or independently developed by a competitor, our business and financial condition could be materially adversely affected.
Failure to obtain and maintain all available regulatory exclusivities and broad patent scope and to maximize patent term restoration or extension on patents covering our products may lead to loss of exclusivity and early biosimilar entry resulting in a loss of market share or revenue.
In addition, we may choose not to enforce our IP rights in certain circumstances or for certain periods of time. For example, in March 2022, we announced that we will not enforce our patents for COVID-19 vaccines against companies manufacturing in or for the Gavi COVAX Advance Market Commitment countries, provided that the manufactured vaccines are solely for use in the AMC 92 countries. In addition, we are willing to license our IP for COVID-19 vaccines to manufacturers, but we may never enter into such licenses of our IP, and our business may be otherwise adversely impacted if we are unable to enforce our IP.
Uncertainty over IP in the pharmaceutical and biotechnology industry has been the source of litigation and other disputes, which is inherently costly and unpredictable and can have adverse financial and freedom-to-operate consequences.
mRNA medicines are a relatively new scientific field and, as the continued developmentfield continues to mature, patent applications are being processed by national patent offices globally. There is uncertainty about which patents will issue, and, potential use of which has resulted inif they do, as to when, to whom and with what claims. Litigation is ongoing over the underlying technology to mRNA medicines between many different patentsmRNA market participants. It is likely that there will continue to be significant litigation and patent applications from organizations and individuals seekingoffice proceedings in various patent offices relating to obtain IP protectionpatent rights in the mRNA field.
We have obtained grants and issuances of patents on mRNA medicines and our delivery technology. The issued patents and pending patent applications in the United States and in key markets around the world that we own, claim many different methods, compositions and processes relating to the discovery, development, manufacture and commercialization of mRNA medicines and our delivery technology, including LNPs.
As the field of mRNA therapeuticsOppositions and vaccines is maturing, patent applications are being processed by national patent offices around the world. There is uncertainty about which patents will issue, and, if they do, as to when, to whom, and with what claims. It is likely that there will be significant litigation and other proceedings, such as interference, reexamination, and opposition proceedings, as well asinter partes and post-grant review proceedings introduced by provisions of the America Invents Act, which became available to third-party challengers on September 16, 2012, in various patent offices relating to patent rights in the mRNA field. An opposition haspetitions have been filed against onesome of our European platform patents, covering uridine-modified mRNAs and we expect that further oppositionsproceedings will
be filed in the European Patent Office (EPO), USPTO and elsewhere relating to patents and patent applications in our portfolio. In many cases, the possibility of appeal exists for either us or our opponents,any party, and it may be years before final, unappealable rulings are made with respect to these patents in certain jurisdictions. The timing and outcome of these and other proceedings is uncertain and may adversely affect our business if we are not successful in defending the patentability and scope of our pending and issued patent claims. We cannot be certain that suchany patent will survive or that the claims will remain in the current form. In addition, third parties may attempt to invalidate our IP rights. Even if our rights are not directly challenged, disputes could lead to the weakening of our IP rights. Our defense
In certain instances, we have instituted and may in the future institute inter partes review proceedings against any attemptissued U.S. patents and opposition proceedings against European patents owned by third parties to circumvent or invalidate our IP rights could be costly to us, could require significant time and attention of our management, and could have a material adverse impact on our business and our ability to successfully compete in the field of mRNA therapeutics.medicines. We have a number of these proceedings ongoing against third-party patents. If we are unsuccessful in narrowing or invalidating such third-party patents, those third parties may attempt to assert those patents against our products. As the biotechnology and pharmaceutical industries expand and more patents are issued, the risk increases that our products or product candidates may be subject to claims of infringement of the patent rights of third parties.
There are many issued and pending third-party patents that claim aspectsWe have invested billions of oligonucleotide delivery technologies that we may need fordollars in creating our patented mRNA platform, which is integral to the development of our mRNA therapeutic and vaccine candidates or marketed products, including our COVID-19 vaccine, if approved. There are also many issued third-party patents that claim targeting genes or portions of genes that may be relevant for mRNA medicines, we wish to develop. For example, there may be issued and pending patent applications that may be asserted against us in a court proceeding or otherwise based upon the asserting party’s belief that we may need such patents for our mRNA therapeutic candidates. Thus, it is possible that one or more organizations will hold patent rights to which we may need a license, or hold patent rights which could be asserted against us. If those organizations refuse to grant us a license to such patent rights on reasonable terms or a court rules that we need such patent rights that have been asserted against us and we are not ableinvolved in various intellectual property litigation as described under Part I, Item 3, “Legal Proceedings.” We expect to obtain a license on reasonable terms, we may be unable to perform researchexpend substantial financial and development or other activities or market products, including our COVID-19 vaccine, covered by such patents.managerial resources in connection with these legal proceedings, and the ultimate outcome of each proceeding is uncertain.
If weWe are, and may in the future become, involved in patent litigation or other proceedings related to a determination of rights, we could incur substantial costs and expenses, substantial liability for damages or be required to stop our product development and commercialization efforts.
Our commercial success depends in part on our avoiding infringement of the patents and proprietary rights of third parties. There is a substantial amount of litigation, both within and outside the United States, involving patent and other IP rights in the biotechnology and pharmaceutical industries, including patent infringement lawsuits, interferences, oppositions, ex parte reexaminations, post-grant review, and inter partes review proceedings before the U.S. Patent and Trademark Office (USPTO) and corresponding foreign patent offices. Numerous U.S. and foreign issued patents and pending patent applications, which are owned by third parties, exist in the fields in which we are pursuing development candidates. In certain instances, we have instituted and may in the future institute inter partes review proceedings against issued U.S. patents and opposition proceedings against European patents owned by third parties in the field of mRNA medicines. We have a number of these proceedings ongoing against third-party patents related to RNA vaccinations and mRNA delivery. If we are unsuccessful in invalidating certain of the third-party patents that we are currently challenging, those third parties may attempt to assert those patents against us should certain of our investigational medicines obtain regulatory approval. As the biotechnology and pharmaceutical industries expand and more patents are issued, the risk increases that our development candidates may be subject to claims of infringement of the patent rights of third parties.
Third parties may assert that we are employing their proprietary technology without authorization. There may be third-party patents or patent applications with claims to materials, formulations, methods of manufacture or methods for treatment related to the use or manufacture of our investigational medicines. Because patent applications can take many years to issue, thereproducts and product candidates, and third parties may be currently pending patent applications which may later result in issued patentsassert that our investigational medicines may infringe.we are employing their proprietary technology without authorization. In addition, third parties may obtain patents in the future and claim that our technologies infringe upon these patents. If any third-party patents were held by a court of competent jurisdiction to cover the manufacturing process of any of our investigational medicines,products or product candidates, any molecules formed during the manufacturing process or any final product itself, the holders of any such patents may obtain injunctive or other equitable relief, which could effectively block our ability to commercialize such investigational medicineproduct unless we obtained a license under the applicable patents, or until such patents expire. Similarly, if any third-party patents were held by a court of competent jurisdiction to cover aspects of our formulations, processes for manufacture or methods of use, including combination therapy, the holders of any such patents may be able to block our ability to develop and commercialize the applicable investigational medicineproduct or product candidate unless we obtained a license or until such patent expires. In either case, such a license may not be available on commercially reasonable terms or at all.
Defense of infringement and other claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion ofdivert employee resources from our business. In the event of a successful claim of infringement against us, we may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement, pay royalties, redesign our infringing products or obtain one or more licenses from third parties, which may not be made available on commercially favorable terms, if at all, or may require substantial time and expense.
In addition, any such licenses are likely to be non-exclusive and, therefore, our competitors may have access to the same technology licensed to us. If we fail to obtain a required license and are unable to design around a patent, we may be unable to effectively market some of our technology and products, which could limit our ability to generate revenues or achieve profitability, and possibly prevent
us from generating revenue sufficientwhich could jeopardize our ability to sustain our operations. Moreover, we expect that a number of our collaborations will provide that royalties payable to us for licenses to our IP may be offset by amounts paid by our collaborators to third parties who have competing or superior IP positions in the relevant fields, which could result in significant reductions in our revenues from products developed through collaborations.
In addition, in connection with certain license and strategic alliance agreements, we have agreed to indemnify certain third parties for certain costs incurred in connection with litigation relating to IP rights or the subject matter of the agreements. The cost to us of any litigation or other proceeding relating to IP rights, even if resolved in our favor, could be substantial, and litigation would divert our management’s efforts. Some of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. Uncertainties resulting from the initiation and continuation of any litigation could delay our research, development and commercialization efforts and limit our ability to continue our operations.
We may not be successful in obtaining or maintaining necessary IP rights to product components and manufacturing processes for our development pipeline.
Presently we have rights to certain IP, through licenses from third parties and under patents that we own, to develop our development candidates and investigational medicines. Because our pipeline may involve additional development candidates that could require the useIf third-party owners of proprietary rights held by third parties, the growth of our business could depend in part on our ability to acquire, in-license, or use these proprietary rights. In addition, our development candidates and investigational medicines may require specific formulations to work effectively and efficiently and these rights may be held by others. We may be unable to acquire or in-license any compositions, methods of use, processes, or other third-party IP rights from third parties that we identify. The licensing and acquisition of third-party IP rights is a competitive area, and a number of more established companies are also pursuing strategies to license or acquire third-party IPpatent rights that we may consider attractive. These established companies may have a competitive advantage over us due to their size, cash resources, and greater clinical development and commercialization capabilities.
For example, we sometimes collaborate with U.S. and foreign academic institutions to accelerate our preclinical research or development under written agreements with these institutions. Typically, these institutions provide us with an option to negotiate a license to any of the institution’s rights in technology resulting from the collaboration. Regardless of such right of first negotiation for IP, we may be unable to negotiate a license within the specified time frame or under terms that are acceptable to us. If we are unable to do so, the institution may offer the IP rights to other parties, potentially blocking our ability to pursue our program.
In addition, companies that perceive us to be a competitor may be unwilling to assign or license rights to us. We also may be unable to license or acquire third-party IP rights on terms that would allow us to make an appropriate return on our investment. If we are unable to successfully obtain rights to required third-party IP rights, our business, financial condition, and prospects for growth could suffer.
If we are not able to obtain and enforce patent protection for our discoveries, our ability to effectively compete using our development candidates will be harmed.
Our success depends, in part, on our ability to protect proprietary methods and technologies that we develop under the patent and other IP laws of the United States and other countries, so that we can prevent others from unlawfully using our inventions and proprietary information. However, we may not hold proprietary rights to some patents required for us to develop, manufacture, and commercialize our proposed products.
Because certain U.S. patent applications are confidential until the patents issue, such as applications filed prior to November 29, 2000, or applications filed after such date which will not be filed in foreign countries, third parties may have filed patent applications for technology covered by our pending patent applications without our being aware of those applications, and our patent applications may not have priority over those applications. In addition, publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. Therefore, we cannot be certain that we were the first to make the inventions claimed in our patents or pending patent applications, or that we were the first to file for patent protection of such inventions, including our COVID-19 vaccine.
For this and other reasons, we may be unable to secure desired patent rights, thereby losing exclusivity. Further, we may be required to obtain licenses under third-party patents to market our proposed products or conduct our research and development or other activities. If licenses are not available to us on favorable terms, we may not be able to market the affected products or conduct the desired activities.
Our strategy depends on our ability to rapidly identify and seek patent protection for our discoveries. In addition, we may rely on third-party strategic collaborators to file patent applications relating to proprietary technology that we develop jointly as a part of certain strategic alliances. The process of obtaining patent protection is expensive and time-consuming. If our present or future strategic collaborators fail to file and prosecute all necessary and desirable patent applications at a reasonable cost and in a timely
manner, our business may be adversely affected. Despite our efforts and the efforts of our strategic collaborators to protect our proprietary rights, unauthorized parties may be able to obtain and use information that we regard as proprietary. While issued patents are presumed valid, this does not guarantee that the patent will survive a validity challenge or be held enforceable. Any patents we have obtained, or obtain in the future, may be challenged, invalidated, adjudged unenforceable, or circumvented by parties attempting to design around our IP. Moreover, third parties or the USPTO may commence interference proceedings involving our patents or patent applications. Any challenge to, finding of unenforceability or invalidation, or circumvention of, our patents or patent applications, would be costly, would require significant time and attention of our management, could reduce or eliminate royalty payments to us from third-party licensors, and could have a material adverse impact on our business.
Our pending patent applications may not result in issued patents. The patent position of pharmaceutical or biotechnology companies, including ours, is generally uncertain and involves complex legal and factual considerations. The standards that the USPTO and its foreign counterparts use to grant patents are not always applied predictably or uniformly and can change. Similarly, the ultimate degree of protection that will be afforded to biotechnology inventions, including ours, in the United States and foreign countries, remains uncertain and is dependent upon the scope of the protection decided upon by patent offices, courts, and lawmakers. Moreover, there are periodic discussions in the U.S. Congress and in international jurisdictions about modifying various aspects of patent law. For example, the America Invents Act, which took effect in March 2013, included a number of changes to the patent laws of the United States. If any of the enacted changes prevent us from adequately protecting our discoveries, including our ability to pursue infringers of our patents to obtain injunctive relieve or for substantial damages, our business could be adversely affected. One major provision of the America Invents Act changed U.S. patent practice from a first-to-invent to a first-to-file system. If we fail to file an invention before a competitor files on the same invention, we no longer have the ability to provide proof that we were in possession of the invention prior to the competitor’s filing date, and thus would not be able to obtain patent protection for our invention. There is also no uniform, worldwide policy regarding the subject matter and scope of claims granted or allowable in pharmaceutical or biotechnology patents. In certain countries, for example, methods for the medical treatment of humans are not patentable.
Accordingly, we do not know the degree of future protection for our proprietary rights or the breadth of claims that will be allowed in any patents issued to us or to others. We also rely to a certain extent on trade secrets, know-how, and technology, which are not protected by patents, to maintain our competitive position. If any trade secret, know-how, or other technology not protected by a patent were to be disclosed to or independently developed by a competitor, our business and financial condition could be materially adversely affected.
Failure to obtain and maintain all available regulatory exclusivities and broad patent scope and to maximize patent term restoration or extension on patents covering our products may lead to loss of exclusivity and early biosimilar entry resulting in a loss of market share and/or revenue.
In addition, we may choose not to enforce our intellectual property rights in certain circumstances or for certain periods of time. For example, in October 2020 we announced that while the COVID-19 pandemic continues, we will not enforce our COVID-19 related patents against those making vaccines intended to combat the pandemic. We also noted that to eliminate any perceived intellectual property barriers to vaccine development during the pandemic period, upon request we are also willing to license our intellectual property for COVID-19 vaccines to others for the post pandemic period. However, we may never enter into such licenses of our intellectual property for the post-pandemic period, and our business may be otherwise adversely impacted by our decision not to enforce this intellectual property during the pandemic.
We license patent rights from third-party owners. If such owners do not properly or successfully obtain, maintain or enforce the patents underlying such licenses, our competitive position and business prospects may be harmed.
We aremay become a party to licenses that give us rights to third-party IP that is necessary or useful for our business. In particular, we have obtained licenses from Cellscript, LLC and its affiliates to patent rights covering modified mRNA chemistries and from certain other parties for IP useful insuch a case, our formulation efforts. Wesuccess may enter into additional licenses to third-party IP in the future.
Our success will depend in part on the ability of our licensors to obtain, maintain and enforce patent protection for our licensed IP. Our licensors may not successfully prosecute the patent applications we license. Even if patents issue in respect of these patent applications, our licensors may fail to maintain these patents, may determine not to pursue litigation against other companies that are infringing these patents or may pursue such litigation less aggressively than we would. Without protection for the IP we license, other companies might be able to offer substantially identical products for sale, which could adversely affect our competitive business position and harm our business prospects. In addition, we sublicense our rights under various third-party licenses to our strategic collaborators. Any impairment of these sublicensed rights could result in reduced revenues under our strategic alliance agreements or result in termination of an agreement by one or more of our strategic collaborators.
If we fail to comply with our obligations in the agreements under which we license IP rights from third parties or otherwise experience disruptions to our business relationships with our licensors, we could lose license rights that are important to our business.
Licensing ofWe license IP, is important to our business andwhich involves complex legal, business and scientific issues and is complicated by the rapid pace of scientific discovery in our industry. We are a party to certain IP license agreements that are important to our business and expect to enter into additional license agreements in the future. Our existing license agreements impose, and we expect that future license agreements will impose, various diligence, milestone payment, royalty and other obligations on us. If we fail to comply with our obligations under these agreements, or we are subject to a bankruptcy, the licensor may have the right to terminate the license, in which event we would not be able to market products covered by the license.license and may be subject to additional liabilities.
In some cases, patent prosecution of our licensed technology is controlled solely by the licensor. If our licensors fail to obtain and maintain patent or other protection for the proprietary IP we license from them, we could lose our rights to the IP and our competitors could market competing products using the IP. In certain cases, we control the prosecution of patents resulting from licensed technology. In the event we breach any of our obligations related to such prosecution, we may incur significant liability to our strategic collaborators. Disputes may arise regarding IP subject to a licensing agreement, including:
•the scope of rights granted under the license agreement and other interpretation-related issues;
•the extent to whichwhether our technology and processes that are not subject to the licensing agreement infringe on IP of the licensor;
•the sublicensing of patent and other rights under our collaborative development relationships;
•our diligence obligations under the license agreement and what activities satisfy those diligence obligations;
•the ownership of inventions and know-how resulting from the joint creation or use of IP by our licensors and us and our strategic collaborators; and
•the priority of invention of patented technology.
If disputes over IP that we have licensed prevent or impair our ability to maintain our current licensing arrangements on favorable terms, we may be unable to successfully develop and commercialize the affected development candidates or investigational medicines.product candidates. We are generally also subject to all of the same risks with respect to protection of IP that we license as we are for IP that we own, which are described below.own. If we or our licensors fail to adequately protect this IP, our ability to commercialize products could suffer.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to patent protection, we rely heavily upon know-how and trade secret protection, as well as non-disclosure agreements and invention assignment agreements with our employees, consultants, and third parties, to protect our confidential and proprietary information, especially where we do not believe patent protection is appropriate or obtainable. In addition to contractual measures, we try to protect the confidential nature of our proprietary information using physical and technological security measures. Such measures may not, for example, in the case of misappropriation of a trade secret by an employee or third party with authorized access, provide adequate protection for our proprietary information. Our security measures may not prevent an employee or consultant from misappropriating our trade secrets and providing them to a competitor, and recourse we take against such misconduct may not provide an adequate remedy to protect our interests fully. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret can be difficult, expensive, and time-consuming, and the outcome is unpredictable. In addition, trade secrets may be independently developed by others in a manner that could prevent legal recourse by us. If any of our confidential or proprietary information, such as our trade secrets, were to be disclosed or misappropriated, or if any such information was independently developed by a competitor, our competitive position could be harmed.
Certain former employees have obtained employment with companies or academic institutions that could be considered competitive with us and are operating their business in areas that are similar to ours, including in their business model, product discovery efforts, mRNA-based product development, or formulation technology such as our LNPs. This competition may be limited by contractual provisions which may or may not be enforceable by us in the Commonwealth of Massachusetts or other jurisdictions. In addition, we may not be aware of such competitive employment arrangements until after our trade secrets have been disclosed to potentially competitive companies.
We may be subject to claims that our employees, consultants or independent contractors have wrongfully used or disclosed confidential information of third parties or that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
We employ individuals who were previously employed at universities or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we try to ensure that our employees, consultants, and independent contractors do not use the proprietary information or know-how of others in their work for us, fromFrom time to time, we are subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise used or disclosed IP, including trade secrets or
other proprietary information, of any ofthird parties, including our employees’ former employers or other third parties.employers. Litigation may be necessary to defend against these claims. If we fail in defending anyto defend such claims, in addition to paying monetary damages, we may lose valuable IP rights or personnel, which could adversely impact our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
We may be subject to claims challenging the inventorship or ownership of our patents and other IP.
We may be and have been subject to claims that former employees, collaborators or other third parties have an ownership interest in our patents or other IP. Ownership disputes may arise, for example, from conflicting obligations of consultants or others who are involved in developing our developmentproduct candidates. Litigation may be necessary to defend against these and other claims challenging inventorship or ownership. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable IP
rights, such asincluding exclusive ownership of, or right to use, valuable IP. Such an outcome could have a material adverse impact on our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction todistract management and other employees.employees, and could impact or patenting strategy.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment, and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
It is possible that defects of form in the preparation or filing of our patents or patent applications may exist, or may arise in the future, for example with respect to proper priority claims, inventorship, claim scope, or requests for patent term adjustments. If we fail to establish, maintain or protect such patents and other intellectual property rights, such rights may be reduced or eliminated. If there are material defects in the form, preparation, prosecution, or enforcement of our patents or patent applications, such patents may be invalid and/or unenforceable, and such applications may never result in valid, enforceable patents. Any of these outcomes could impair our ability to prevent competition from third parties, which may have an adverse impact on our business.
Periodic maintenance fees, renewal fees, annuity fees, and various other governmental fees on patents or applications will be due to be paid to the USPTO and various governmental patent agencies outside of the United States in several stages over the lifetime of the patents or applications. We have systems in place to remind us to pay these fees, and we employ an outside firm and rely on our outside counsel to pay these fees due to non-U.S. patent agencies. The USPTO and various non-U.S. governmental patent agencies require compliance with a number of procedural, documentary, fee payment, and other similar provisions during the patent application process. We employ reputable law firms and other professionals to help us comply, and in many cases, an inadvertent lapse can be cured by payment of a late fee or by other means in accordance with the applicable rules. However, there are situations in which non-compliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, our competitors might be able to enter the market and this circumstance could have a material adverse impact on our business.
Issued patents covering our development candidates and investigational medicines could be found invalid or unenforceable if challenged in court.
If we or one of our strategic collaborators initiated legal proceedings against a third party to enforce a patent covering one of our development candidates or investigational medicines, the defendant could counterclaim that the patent covering our development candidate or investigational medicine is invalid or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including patent eligible subject matter, lack of novelty, obviousness, or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO, or made a misleading statement, during prosecution. Third parties may also raise similar claims before administrative bodies in the United States or abroad, even outside the context of litigation. Such mechanisms include reexamination, post-grant review, and equivalent proceedings in foreign jurisdictions (e.g., opposition proceedings). Such proceedings could result in revocation of or amendment to our patents in such a way that they no longer cover our development candidates or investigational medicines. The outcome following legal assertions of invalidity and unenforceability is unpredictable. With respect to the validity question, for example, there may be invalidating prior art that we and the patent examiner were unaware of during prosecution. If a defendant were to prevail on a legal assertion of invalidity and/or unenforceability, we would lose at least part of the patent protection for our development candidates and investigational medicines. Such a loss of patent protection could have a material adverse impact on our business.
Changes in U.S. patent and regulatory law could impair our ability to protect our products.
As is the case with other biotechnology companies, ourOur success is heavily dependent on IP, particularly patents. ObtainingU.S. patent law continues to evolve, and enforcing patents in the biotechnology industry involve both technological and legal complexity, and therefore obtaining and enforcing biotechnology patents is costly, time-consuming and inherently uncertain. In addition, the United States has enacted and is implementing wide-ranging patent reform legislation. Recentcertain U.S. Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. In addition to increasingThese rulings have increased uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertaintyas well as with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the federal courts and the USPTO, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future. Furthermore, depending on the Supreme Court’s review of the ACA, or legislation to repeal or amend the ACA, the twelve years of regulatory exclusivity currently provided to certain biologic products in the United States may be reduced or eliminated, as further discussed above under the risk factor entitled “Our investigational medicines may face competition from biosimilars approved through an abbreviated regulatory pathway.” Any such reduction or elimination could impair the length of exclusivity against biosimilar products.
We may not be ableunable to protect our IP rights throughout the world.
Filing, prosecuting and defending patents on development candidates and investigational medicines in all countries throughout the worldevery country would be prohibitively expensive, and our foreign IP rights in some countries outside the United States can be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect IP rights to the same extent as U.S. federal and state laws in the United States.laws. Consequently, we may not be ableunable to prevent third parties from practicing our inventions in all countries outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Competitors may use our technologies to develop their own products in jurisdictions where we have not obtained patent protection to develop their own products and further,or may export otherwise infringing products to territories where we have patent protection, but enforcement is not as strong as that in the United States. These products may compete with our products and our patents or other IP rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending IP rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets and other IP protection, particularly those relating to biotechnology products, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our IP rights around the world may be inadequate to obtain a significant commercial advantage from the IP that we develop or license.
ManyAdditionally, many countries have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. As a result, in response toFor example, during the COVID-19 pandemic, it is possible that certain countries may takethreatened steps to facilitate compulsory licenses thatto permit the distribution of a COVID-19 vaccine in those countries. In addition, many countries limit the enforceability of patents against government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of the relevant patent rights. If we or any of our licensors is forced to grant a license to third parties with respect to any patents relevant to our business, our competitive position may be impaired, and our business, financial condition, results of operations and prospects may be adversely affected.
Our reliance on government funding and collaboration from governmental and quasi-governmental entities for certain of our programs adds uncertainty to our research and development efforts with respect to those programs and may impose requirements related to intellectual property rights and requirements that increase the costs of development, commercialization and production of any programs developed under those government-funded programs.
The development of each of our Zika vaccine (mRNA-1893), our antibody against Chikungunya virus (mRNA-1944), and our Chikungunya vaccine (mRNA-1388), are currently being funded through subcontracts with funding from either BARDA or DARPA. Our COVID-19 vaccine is being developed in collaboration with NIAID. BARDA has agreed to fund the advancement of our COVID-19 vaccine to FDA licensure. Contracts and grants funded by the U.S. government and its agencies, including our agreements funded by BARDA and DARPA and our collaboration with NIAID, include provisions that reflect the government’s substantial rights and remedies, many of which are not typically found in commercial contracts, including powers of the government to:
•terminate agreements, in whole or in part, for any reason or no reason;
•reduce or modify the government’s obligations under such agreements without the consent of the other party;
•claim rights, including IP rights, in products and data developed under such agreements;
•audit contract-related costs and fees, including allocated indirect costs;
•suspend the contractor or grantee from receiving new contracts pending resolution of alleged violations of procurement laws or regulations;
•impose U.S. manufacturing requirements for products that embody inventions conceived or first reduced to practice under such agreements;
•suspend or debar the contractor or grantee from doing future business with the government;
•control and potentially prohibit the export of products;
•pursue criminal or civil remedies under the False Claims Act, False Statements Act and similar remedy provisions specific to government agreements; and
•limit the government’s financial liability to amounts appropriated by the U.S. Congress on a fiscal-year basis, thereby leaving some uncertainty about the future availability of funding for a program even after it has been funded for an initial period.
We may not have the right to prohibit the U.S. government from using certain technologies developed by us and we may not be ableunable to prohibit third-partyother companies, including our competitors, from using those technologies in providing products and services to theU.S. government. The U.S. government generally takes the position that it has the right to royalty-free use of technologies that are developed under U.S. government contracts.
In addition, government contracts and grants, and subcontracts and subawards awarded in the performance of those contracts and grants, normally contain additional requirements that may increase our costs of doing business, reduce our profits and expose us to liability for failure to comply with these terms and conditions. These requirements include, for example:
•specialized accounting systems unique to government contracts and grants;
•mandatory financial audits and potential liability for price adjustments or recoupment of government funds after such funds have been spent;
•public disclosures of certain contract and grant information, which may enable competitors to gain insights into our research program; and
•mandatory socioeconomic compliance requirements, including labor standards, non-discrimination, and affirmative action programs and environmental compliance requirements.
Further, under these agreements we are subject to the obligations to and the rights of the U.S. government set forth in the Bayh-Dole Act of 1980 (Bayh-Dole Act). As a result, the U.S. government may have rights in certain inventions developed under these government-funded programs, including a non-exclusive, non-transferable, irrevocable worldwide license to use inventions for any governmental purpose. In addition, the U.S. government has the right to require us to grant exclusive, partially exclusive or nonexclusive licenses to any of these inventions to a third party if it determines that: (i) adequate steps have not been taken to commercialize the invention; (ii) government action is necessary to meet public health or safety needs; or (iii) government action is necessary to meet requirements for public use under federal regulations, also referred to as “march-in rights.” While the U.S. government has sparingly used, and to our knowledge never successfully exercised, such march-in rights, anyAny exercise of the march-in rights by the U.S. government could harm our competitive position, business, financial condition, results of operations and prospects.In December 2023, the Biden administration released a proposed framework specifying for the first time that price can be a factor in considering whether an invention is sufficiently available to the public. The proposed framework could potentially enable march-in rights to be used as a tool to regulate drug pricing. The potential inclusion of price as a factor in a march-in determination is expected to draw extensive criticism and challenge, and the ultimate impact is currently unknown. If the U.S. government exercises such march-in rights, we may receive compensation that is deemed reasonable by the U.S. government in its sole discretion, which may be less than what we might be able to obtain in the open market. Intellectual propertyIP generated under a government fundedgovernment-funded program is also subject to certain reporting requirements, compliance with which may require us to expend substantial resources.
In addition, the U.S. government requires that any products embodying any invention generated through the use of U.S. government funding be manufactured substantially in the United States. The manufacturing preference requirement can be waived if the owner of the intellectual property can show that reasonable but unsuccessful efforts have been made to grant licenses on similar terms to potential licensees that would be likely to manufacture substantially in the United States or that under the circumstances domestic manufacture is not commercially feasible. This preference for U.S. manufacturers may limit our ability to contract with non-U.S. manufacturers for products covered by such intellectual property.
As an organization, we are relatively new to government contracting and new to the related regulatory compliance obligations that such contracting entails.obligations. If we fail to maintain compliance with those obligations, we may be subject to potential liability and to termination of our contracts.
Risks related to our financial condition and results of operations
We incurred net losses in 2023 and we may incur losses again in the future; we have a limited history of recognizing revenue from product sales and may be unable to achieve long-term sustainable profitability.
We incurred a net loss of $4.7 billion in 2023, and other than in 2021 and 2022, we have incurred net losses in each year since our inception. Currently, our COVID-19 vaccine is our only commercial product. While preparations are underway for additional potential product launches, including our anticipated RSV launch in 2024, the ultimate occurrence and timing of these launches is uncertain. Our ability to generate revenue and maintain profitability depends on our ability to successfully develop and obtain the regulatory approvals necessary to commercialize our products.
We have incurred, and expect to continue to incur, significant costs associated with commercializing our COVID-19 vaccine and our clinical and preclinical development activities. In addition, we incurred significant costs in 2023 as we resized our manufacturing capacity. We may be unable to achieve long-term sustainable profitability and may need additional funding to continue operations.
We anticipate that our expenses may increase substantially if and as we:
•expand our research or development of our programs in preclinical development;
•initiate additional preclinical, clinical or other studies for our product candidates, particularly large pivotal trials;
•continue to invest in our platform to conduct research to identify novel mRNA technology improvements;
•change or add to internal manufacturing capabilities, or additional manufacturers or suppliers;
•add additional infrastructure to our quality control and quality assurance groups to support our operations as we progress our product candidates toward commercialization;
•attract and retain skilled personnel;
•create additional infrastructure to support our product development and commercialization efforts, including new sites globally;
•seek marketing approvals and reimbursement for our product candidates;
•build out a sales, marketing and distribution infrastructure to commercialize any products;
•acquire or in-license other product candidates and technologies;
•make milestone or other payments under any in-license agreements; and
•experience any delays or encounter issues with any of the above.
Our quarterly and annual operating results may fluctuate. As a U.S. government contractor,result, we are subjectmay fail to financial auditsmeet or exceed the expectations of research analysts or investors, which could cause our stock price to decline and other reviews by the U.S. government ofnegatively impact our costs and performance on their contracts,financing or funding ability, as well as our accountingability to exist as a standalone company.
Our financial condition and generaloperating results may fluctuate from quarter-to-quarter and year-to-year due to many factors, many of which are beyond our control. As such, a period-to-period comparison of our operating results may not be predictive of our future performance. In any particular quarter, our operating results could be below the expectations of securities analysts or investors, which could cause our stock price to decline. Our stock price could be affected by events not necessarily tied to our actual operating results, including recommendations by securities analysts, the timing of certain public disclosures by us, our collaborators or our competitors and our ability to accurately report our financial results in a timely manner. Other factors relating to our business practices relatedthat may contribute to these contracts. Basedfluctuations include those described in these Risk Factors and elsewhere in this Annual Report on the results of its audits, the government may adjust our contract-related costs and fees, including allocated indirect costs. Although adjustments arising from government audits and reviews have not had a material adverse impact on our financial condition or results of operations in the past, we cannot assure you that future audits and reviews will not have those effects.
Table of Contents
Form 10-K.
The Coalition for Epidemic Preparedness Innovations is a global organization that has publicly stated its intent to work with multiple global organizations on potential vaccines and therapies targeting the novel coronavirus, including other companies working on mRNA based approaches. There is a possibility that our confidential information may become exposed to others during this process, including the details and timinginvestment of our vaccine efforts.cash, cash equivalents and investments is subject to risks which may cause losses and affect the liquidity of these investments.
As of December 31, 2023, we had approximately $13.3 billion in cash, cash equivalents and investments, which are subject to general credit, liquidity, market, inflation and interest rate risks. We may realize losses in the fair value of these investments. In addition, if our investments cease paying or reduce the amount of interest paid to us, our interest income would suffer. These and other market risks associated with our investment portfolio may adversely affect our results of operations, liquidity and financial condition.
Risks related to the commercialization of our pipelinebusiness and operations
We have limited sales, distribution,may encounter difficulties in managing the development and marketing experience, and have only recently invested significant financial and management resources to establish these capabilities as a resultexpansion of our rapid development and commercialization of our COVID-19 vaccine. If we are unable to effectively establish such capabilities or enter into agreements with third parties to market and sell our products or to help ensure compliance with local regulatory requirements, our ability to generate revenues may be adversely affected.company.
As of December 31, 2023, we had approximately 5,600 full-time employees in 19 countries, and we may increase our number of employees and the scope of our operations. To manage this global expansion, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities and recruit and train qualified personnel. Our COVID-19 vaccine (mRNA-1273) represents the first product that we have commercialized for sales, distribution and marketing. To enable the successful commercialization of this vaccine and other products thatmanagement may resultneed to divert significant attention away from our day-to-day activities to manage these development programs,activities.
Successfully developing products for and fully understanding the regulatory and manufacturing pathways for the many therapeutic areas and diseases we are investing in the developmentseek to address requires significant depth of sales, marketing, distribution, managerialtalent, resources and other non-technical capabilities in the United States, Europe, and other regions, both on our own and with others.corporate processes to allow simultaneous execution across multiple areas. We may enter into strategic alliances with other entities to utilize their mature marketing and distribution capabilities, but we may be unable to enter into marketing agreements on favorable terms, if at all. Toeffectively manage this simultaneous execution and expansion of our operations or recruit and train qualified personnel, which could cause weaknesses in our infrastructure, give rise to operational mistakes, loss of business opportunities, loss of employees and reduced productivity among remaining employees. The physical expansion of our operations, including the extent that we rely on third partiesexpansion of our Norwood and Marlborough campuses in Massachusetts and the construction of manufacturing facilities overseas, may lead to significant costs and may divert financial resources from other projects, such as the development of our product candidates. If our management is unable to effectively manage our development and expansion, our financial performance and ability to commercialize our approved products if any, we will receive lower revenues than if we commercialized these products ourselves. In addition, we may have little or no control over the sales efforts of third parties involved in our commercialization efforts. If our future strategic collaborators do not commit sufficient resources to commercialize our future products, if any, and we are unable to develop the necessary marketing capabilities on our own, we may be unable to generate sufficient product revenue to sustain our business. We are and will be competing with many companies that currently have extensive and well-funded marketing and sales operations. In the event that we develop our own marketing or sales force, we will also have to compete with such companies to recruit, hire, train and retain marketing and sales personnel. Without a significant internal team or the support of a third party to perform marketing and sales functions, we may be unable to compete successfully against these more established companies.
The commercialization and distribution of our COVID-19 vaccine also subjects us to pharmacovigilance obligations under various regulatory regimes in the jurisdictions in which our vaccine is distributed. Under these regulations, we are generally required to collect, process, analyze and monitor safety data and to identify and evaluate adverse reactions to our COVID-19 vaccine as it is administered in those jurisdictions. We partner with third party organizations to assist us in collecting and processing this safety data as it is reported from healthcare providers, recipients of our vaccine and others. To the extent that we or the third parties with whom we contract to conduct these pharmacovigilance activities are unable to comply with relevant regulations, including with respect to the timely processing of safety data, we may be subject to sanctions, increased costs, reputational harm, or our authorizations to distribute the COVID-19 vaccine in the relevant jurisdictions may be revoked or curtailed. There are a limited number of third-party service providers who are qualified and capable of providing pharmacovigilance services on a global basis, and our inability to identify or contract with qualified service providers may impede our commercial activities.
We are and will be competing with many companies that currently have extensive and well-funded marketing, sales and pharmacovigilance operations. In the event that we develop our own marketing or sales force, we will also have to compete with such companies to recruit, hire, train and retain marketing and sales personnel. We have and will continue to incur expenses associated with hiring third-party contractors to assist in conducting local pharmacovigilance services. Without a significant internal team or the support of a third party to perform these functions, we may be unable to compete successfully against these more established companies.
Certain of our customers for our COVID-19 vaccine prepay us for a portion of the product payment for the vaccine doses that they expect to receive from us, and under the terms of certain of our supply agreements, we may be required to refund some or all of those prepayments if a customer reduces its purchase commitment or if we fail to deliver the purchased volume.
Certain of our customers for our COVID-19 vaccine prepay us for a portion of the product payment for the vaccine doses that they expect to receive from us. In some cases, this prepayment can be substantial. We are generally not required by our contracts to retain these prepayments in cash or otherwise and we have generally used these prepayments to make capital expenditures and fund the manufacturing scale-up and commercialization of our vaccine. Under certain supply agreements with customers for our COVID-19 vaccine, if we fail to deliver a portion or all of the committed number of doses by a certain date, a customer may reduce the volume of vaccine doses that it commits to purchase or the contract may be terminated. Upon termination by the customer, we would generally be required to refund a portion of that customer’s prepayment. We may not have the cash or other available resources to satisfy that repayment obligation. Even if we are able to satisfy the repayment obligation from available resources, we may need to seek additional sources of capital to fund our operations, which funding may not be available when needed or on acceptable terms. In either of those circumstances, our business, financial condition, results of operations, and reputation could be materially and adversely affected. Furthermore, in the future, customers may elect not to prepay us for our services in which case we would have to find other sources of funding for our capital expenditures and operations, which may not be available when needed or on acceptable terms.
The pharmaceutical market is intensely competitive. If we are unable to compete effectively with existing products, new treatment methods, and new technologies, we may be unable to commercialize successfully any products that we develop.
The pharmaceutical market is intensely competitive and rapidly changing. Many large pharmaceutical and biotechnology companies, academic institutions, governmental agencies, and other public and private research organizations are pursuing the development of novel products for the same diseases that we are targeting or expect to target. Many of our competitors have:
•greater financial, technical, and human resources than we have at every stage of the discovery, development, manufacture, and commercialization of products;
•more extensive experience in preclinical testing, conducting clinical trials, obtaining regulatory approvals, and in manufacturing, marketing, and selling products;
•investigational medicines that are based on previously tested or accepted technologies;
•products that have been approved or are in late stages of development; and
•collaborative arrangements in our target markets with leading companies and research institutions.
We will face intense competition from products that have already been approved and accepted by the medical community for the treatment of the conditions for which we may develop products. For example, we announced in September 2020 that we plan to develop a vaccine for the seasonal flu. Although we believe there is an unmet need for a highly effective seasonal flu vaccine, this is a well-developed marketaffected negatively, and we may not be successful in either developing a successful product or achieving a market share for our product that justifies our investment. We also expectable to face competition from new products that enter the market. There are a number of products currently under development, which may become commercially available in the future, for the treatment of conditions for which we are trying, or may in the future try, to develop products. These products may be more effective, safer, less expensive, or marketed and sold more effectively, than any products we develop. While we believe that our COVID-19 vaccine has and will continue to have a competitive profile, it is possible it will not compete favorably with these products and product candidates, or others, and as a result, we may not achieve commercial success. Moreover, positive data and/or the commercial success of competitive products could negatively impact our stock price.
We anticipate competing with the largest pharmaceutical companies in the world, many of which are all currently conducting research in the fields of infectious diseases, immuno-oncology, rare genetic diseases, and cancer vaccines. Some of these companies have greater financial and human resources than we currently have. In addition to these large pharmaceutical companies, we may directly compete with fully-integrated biopharmaceutical companies and other immunotherapy-focused oncology companies, as well as a number of companies focused on mRNA medicines or shared tumor antigen and neoantigen therapeutics, some of which have entered into collaboration and funding agreements with larger pharmaceutical or biotechnology companies.
If we successfully develop investigational medicines, and obtain approval for them, we will face competition based on many different factors, including:
•the safety and effectiveness of our products relative to alternative therapies, if any;
•the ease with which our products can be administered and the extent to which patients accept relatively new routes of administration;
•the timing and scope of regulatory approvals for these products;
•the availability and cost of manufacturing, marketing, and sales capabilities;
•the price of any approved mRNA medicine;
•reimbursement coverage; and
•patent position.
Our competitors may develop or commercialize products with significant advantages over any products we develop based on any of the factors listed above or on other factors. In addition, our competitors may develop strategic alliances with or receive funding from larger pharmaceutical or biotechnology companies, providing them with an advantage over us. Our competitors may therefore be more successful in commercializing their products than we are, which could adversely affect our competitive position and business. Competitive products may make any products we develop obsolete or noncompetitive before we can recover the expenses of developing and commercializing our products, if approved.
The commercial success of any current or future investigational medicine, if approved, will depend upon the degree of market acceptance by physicians, patients, third-party payors, and others in the medical community.
Ethical, social, and legal concerns about genetic research could result in additional regulations restricting or prohibiting the products and processes we may use. Even with the requisite approvals, the commercial success of our products will depend in part on the medical community, patients, and third-party or governmental payors accepting mRNA medicines in general, and our products in particular, as medically useful, cost-effective, and safe. Any product that we bring to the market may not gain market acceptance by physicians, trial participants, patients, third-party payors, and others in the medical community. If these products do not achieve an adequate level of acceptance, we may not generate significant product revenue and may not become profitable. The degree of market acceptance of our investigational medicines, if approved for commercial sale, will depend on a number of factors, including:
•the potential efficacy and potential advantages over alternative treatments;
•the ability to offer our products, if approved, at competitive prices;
•the prevalence and severity of any side effects, including any limitations or warnings contained in a product’s approved labeling;
•the prevalence and severity of any side effects resulting from checkpoint inhibitors or other products or therapies with which our products are co-administered;
•relative convenience and ease of administration;
•any restrictions on the use of our products, if approved, together with other medications;
•the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies;
•the strength of marketing and distribution support and timing of market introduction of competitive products;
•publicity concerning our products or competing products and treatments; and
•sufficient third-party insurance coverage or reimbursement, and patients’ willingness to pay out-of-pocket in the absence of third-party coverage or adequate reimbursement.
Even if a potential product displays a favorable efficacy and safety profile in preclinical studies and clinical trials, market acceptance of the product will not be known until after it is launched. Our efforts to educate the medical community and third-party payors on the benefits of the products may require significant resources and may never be successful. Our efforts to educate the marketplace may require more resources than are required by the conventional technologies marketed by our competitors due to the complexity and uniqueness of our programs.
Even if we are successful in obtaining marketing approval for any product, commercial success of any approved products will also depend in large part on the availability of coverage and adequate reimbursement from third-party payors, including government payors such as the Medicare and Medicaid programs, and entry into managed care organizations, which may be affected by existing and future healthcare reform measures designed to reduce the cost of healthcare. Third-party payors could require us to conduct additional studies, including post-marketing studies related to the cost effectiveness of a product, to qualify for reimbursement, which could be costly and divert our resources. If government and other healthcare payors do not provide adequate coverage and reimbursement levels for any of our products once approved, whether due to healthcare reform legislation or otherwise, market acceptance and commercial success would be reduced.
In addition, if any of our products are approved for marketing, we or a strategic collaborator will be subject to significant regulatory obligations regarding the submission of safety and other post-marketing information and reports for such product, and will need to continue to comply (or ensure that our third-party providers comply) with cGMP and current GCPs for any clinical trials that we or a strategic collaborator conduct post-approval. In addition, there is always the risk that we or a strategic collaborator or regulatory authority might identify previously unknown problems with a product post-approval, such as adverse events of unanticipated severity or frequency. Compliance with these requirements is costly, and any such failure to comply or other issues with our investigational medicines identified post-approval could have a material adverse impact onimplement our business financial condition, and results of operations.strategy.
We will need to obtain FDA approval of any proposed product names, and any failure or delay associated with such approval may adversely impact our business.
Any name we intend to use for our proposed products will require approval from the FDA regardless of whether we have secured a trademark registration from the USPTO. The FDA typically conducts a review of proposed product names, including an evaluation of potential for confusion with other product names. The FDA may object to any product name we submit if it believes the name inappropriately implies medical claims. If the FDA objects to any of our proposed product names, we may be required to adopt an alternative name for our proposed products. If we adopt an alternative name, we would lose the benefit of any existing trademark applications for such developmental candidate and may be required to expend significant additional resources in an effort to identify a suitable product name that would qualify under applicable trademark laws, not infringe the existing rights of third parties and be acceptable to the FDA. We may be unable to build a successful brand identity for a new trademark in a timely manner or at all, which would limit our ability to commercialize our products, if approved.
We market our products outside of the United States, and we are subject to the risks of doing business outside of the United States.
Because we market our COVID-19 vaccine, and we plan to market any other products, if approved, outside of the United States, ourOur business is subject to risks associated with doing business outside of the United States, including an increase in our expenses, diversion of our management’s attention from the acquisition or development of investigational medicines, or forgoing profitable licensing opportunities in these geographies.and we have limited experience operating internationally. We are not permitted to market or promote any of our developmentalproduct candidates or investigational medicines before we receive regulatory approval or other authorization from thean applicable regulatory authority, in that foreign market, and we may never receive such regulatory approval for any of our developmental candidates or investigational medicines.approval. To obtain separate regulatory approval in many other countriesvarious jurisdictions, we must comply with numerous and varying regulatory requirements of such countries regarding safety and efficacy and governing, among other things, clinical trials, manufacturing, commercial sales, pricing and distribution of our developmentalproduct candidates, and investigational medicines, and we cannot predict success in these jurisdictions.may fail to obtain approval. We arehave rapidly expandingexpanded our global operations, establishing commercial subsidiaries and third-partyentering into arrangements to support the worldwide manufacture and distribution of our COVID-19 vaccine,products, which is a complex task thattask. For example, we are undertaking on an accelerated timeline. Accordingly, ourbuilding regional manufacturing facilities and investing in research and development in several countries. Our business and financial results may be adversely affected due to a variety ofby many factors associated with our expanding global business, including:
•efforts to develop an international commercial sales, marketing and supply chain and distribution organization, including efforts to mitigate longer accounts receivable collection times, longer lead times for shipping and potential language barriers;
•our customers’ ability to obtain reimbursement for our products in foreign markets;
•our inability to directly control commercial activities because we are relyingrely on third parties;
•different medical practices and customs in foreign countries affecting acceptance in the marketplace;
•changes in a specific country’s or region’s political and cultural climate or economic condition, including as a result of the COVID-19 pandemic;condition;
•an increased legal and compliance burden associated with establishing, maintainingto establish, maintain and operatingoperate legal entities in foreign countries;
•the burden of complying with complex and changing foreign regulatory, tax, accounting, reporting and legal requirements, including the European General Data Protection Regulation 2016/679 or GDPR;(GDPR);
•the interpretation of contractual provisions governed by foreign laws in the event of a contract dispute, and the difficulty of effective enforcement of contractual provisions in local jurisdictions, and the existence of potentially relevant third-party IP rights;jurisdictions;
•inadequate IP protection in foreign countries and the existence of potentially relevant third-party IP rights;
•trade-protection measures including trade restrictions, import or export licensing requirements such as Export Administration Regulations promulgated by the U.S. Department of Commerce and fines, penalties, or suspension or revocation of export privileges, the imposition of government controls and changes in tariffs;
•the effects of applicable foreign tax structures and potentially adverse tax consequences; and
•significant adverse changes in foreign currency exchange rates.
In addition to FDA and related regulatory requirements in the United States and abroad, weWe are also subject to extensive additional federal, state and foreign anti-bribery regulations, which includeincluding the U.S. Foreign Corrupt Practices Act (FCPA), the U.K.UK Bribery Act and similar laws in other countries outside of the United States.
The FCPA prohibits any U.S. individual or business from paying, offering, authorizing payment or offering anything of value, directly or indirectly, to any foreign official, political party or candidate for the purpose of influencing any act or decision of the foreign entity in order to assist the individual or business in obtaining or retaining business. The FCPA also obligates companies whose securities are listed in the United States to comply with certain accounting provisions requiring the company to maintain books and records that accurately and fairly reflect all transactions of the corporation, including international subsidiaries, and to devise and maintain an adequate system of internal accounting controls for international operations.
countries. Compliance with the FCPA is expensive and difficult, particularly in countries in whichwhere corruption is a recognized problem. In addition,Additionally, the FCPA presents particular challenges into the pharmaceutical industry because, in many countries, hospitals are operated by the government, and doctors and other hospital employees are considered foreign officials. Certain payments to hospitals in connection with clinical trials and other work have been deemed to be improper payments to government officials and have led to FCPA enforcement actions. Various laws, regulations and executive orders also restrict the use and dissemination outside the United States, or the sharing with certain non-U.S. nationals, of information classified for national security purposes, as well as certain products and technical data relating to those products. As we expand our presence outside the United States, itwe will require usneed to dedicate additional resources to comply with these laws, and these laws may preclude us from developing, manufacturing or selling certain products and product candidates outside the United States, which could limit our growth potential and increase our development costs.
We are developing and implementing a corporate compliance program based on what we believe are current best practices in the pharmaceutical industry for companies similar to ours, but we cannot guarantee that we, or our employees, our consultants or our third-party contractors are or will be in compliance with all federal, state and foreign regulations regarding bribery and corruption. Moreover, our strategic collaborators and third-party contractors located outside the United States may have inadequate compliance programs or may fail to respect the laws and guidance of the territories inwhere they operate, which they operate. The failure to comply with laws governing international business practices may result in substantial civil and criminal penalties and suspension or debarment from government contracting. The SEC also may suspend or bar issuers from trading securities on U.S. exchanges for violations of the FCPA’s accounting provisions. Even if we are not determined to have violated these laws, government investigations into these issues typically require the expenditure of significant resources and generate negative publicity, which could also have an adverse effect onadversely affect our business, financial condition and results of operations.
The insurance coverage and reimbursement status of newly-approved products, particularly in a new class of medicines, is uncertain. Failure to obtain or maintain adequate coverage and reimbursement for new or current products could limit our ability to market those products and decrease our ability to generate revenue.
The availability and extent of reimbursement by governmental and private payors is essential for most patients to be able to afford expensive treatments such as the medicines that we hope to develop and sell. Adequate coverage and reimbursement from governmental healthcare programs, such as Medicare and Medicaid, and commercial payors is critical to new product acceptance. In addition, because our personalized cancer vaccine and intratumoral immuno-oncology investigational medicines represent new approaches to the treatment of cancer, we cannot accurately estimate how these products would be priced, whether reimbursement could be obtained, or any potential revenue. Sales of our investigational medicines will depend substantially, both domestically and abroad, on the extent to which the costs of our investigational medicines will be paid by health maintenance, managed care, pharmacy benefit, and similar healthcare management organizations, or reimbursed by government health administration authorities, private health coverage insurers, and other third-party payors. If reimbursement is not available, or is available only to limited levels, we may not be able to successfully commercialize our investigational medicines. Even if coverage is provided, the approved reimbursement amount may not be high enough to allow us to establish or maintain pricing sufficient to realize a sufficient return on our investment in any of our products. In the U.S., we may establish various programs to help patients afford our products, which may include patient assistance programs and co-pay coupon programs for eligible patients.
There is significant uncertainty related to the insurance coverage and reimbursement of newly approved products, including genetic medicines and coverage may be more limited than the purposes for which the medicine is approved by the FDA or comparable foreign regulatory authorities. Third-party payors decide which medications they will pay for and establish reimbursement levels. In the United States, the principal decisions about reimbursement for new medicines are typically made by the Centers for Medicare & Medicaid Services (CMS), an agency within the U.S. Department of Health and Human Services (HHS), as CMS decides whether and to what extent a new medicine will be covered and reimbursed under Medicare. Private payors tend to follow CMS to a substantial degree. It is difficult to predict what CMS will decide with respect to reimbursement for novel products such as ours. Factors payors consider in determining reimbursement are based on whether the product is:
•a covered benefit under its health plan;
•safe, effective and medically necessary;
•appropriate for the specific patient;
•cost-effective; and
•neither experimental nor investigational.
In the United States, no uniform policy for coverage and reimbursement for products exists among third-party payors. Therefore, coverage and reimbursement for our products can differ significantly from payor to payor. The process for determining whether a payor will provide coverage for a product may be separate from the process for setting the reimbursement rate that the payor will pay for the product. One payor’s determination to provide coverage for a product does not assure that other payors will also provide coverage and reimbursement for the product. Third-party payors may also limit coverage to specific products on an approved list, or
formulary, which might not include all of the FDA-approved products for a particular indication. Net prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors. Many third-party payors are also increasingly requiring that drug companies provide them with predetermined discounts from list prices and are challenging the prices charged for medical products. Furthermore, many pharmaceutical manufacturers must calculate and report certain price reporting metrics to the government, such as average sales price (ASP), and best price. Penalties may apply in some cases when such metrics are not submitted accurately and timely. We cannot be sure that coverage and reimbursement will be available for, or accurately estimate the potential revenue from, our product candidates or assure that coverage and reimbursement will be available for any product that we may develop.
A decision by a third-party payor notOur failure to cover or not to separately reimburse forupgrade and maintain our medical products or therapies using our productsenterprise resource planning (ERP) system could reduce physician utilization of our products once approved. Assuming there is coverage for our product candidates, or therapies using our product candidates by a third-party payor, the resulting reimbursement payment rates may not be adequate or may require co-payments that patients find unacceptably high. We cannot be sure that coverage and reimbursement in the United States will be available for our current or future product candidates, or for any procedures using such product candidates, and any reimbursement that may become available may not be adequate or may be decreased or eliminated in the future.
Coverage and reimbursement of our COVID-19 vaccine, which was recently granted EUA, may be subject to unique regulatory policies. Under the ACA preventive care mandate, non-grandfathered group health plans and health insurance coverage offered in the individual or group market typically have at least one year before it must provide first-dollar coverage for a newly issued preventive care requirement or guideline. However, pursuant to the CARES Act, non-grandfathered group health plans and health insurance coverage offered in the individual or group market must cover any qualifying coronavirus preventive service 15 business days after the United States Preventive Services Task Force, or Advisory Committee on Immunization Practices (ACIP) designates such service as preventive. On December 19, 2020, ACIP voted 11–0 in favor of the interim recommendation for use of the Moderna COVID-19 vaccine. Currently, third party payors do not offer coverage or reimbursement for the vaccine. However, third-party reimbursement for providers administering our COVID-19 vaccine may affect market acceptance of the product. Currently, the CARES Act and its implementing regulations state (i) providers that participate in the CDC COVID-19 Vaccination Program must administer a COVID-19 immunization regardless of an individual’s ability to pay or health insurance coverage status, (ii) providers may not seek any reimbursement, including through balance billing, from an immunization recipient, (iii) that coverage is required, without cost-sharing, for the administration of the immunization even if a third party, such as the federal government, pays for the cost of the immunization, and that (iv) private health insurance plans must cover COVID-19 immunizations and their administration even when provided by out-of-network providers for the duration of the public health emergency for COVID-19.
There is no guarantee payors will provide coverage and reimbursement for our COVID-19 vaccine during or after the termination of the public health emergency, nor can we guarantee that even if coverage is provided, the approved reimbursement amount will be high enough to allow us to establish or maintain pricing sufficient to realize a sufficient return on our investment. The FDA may revoke EUA of mRNA-1273 or may not approve the BLA, if submitted. Under the CARES Act and an accompanying interim final rule, Medicare beneficiaries are expected to have coverage for COVID-19 vaccines through Medicare Part B with no cost sharing. Coverage is further expected to apply whether the vaccine receives FDA authorization through an EUA or is licensed under a BLA and will likely extend to employer-sponsored and individual health plans subject to the ACA’s preventive services standards. The outcome of current and future clinical trials, as well as the market demand for COVID-19 vaccines mayadversely impact patient eligibility and current and future coverage determinations. It is unclear whether the FDA will approve the administration of our COVID-19 vaccine for patients under eighteen years of age or if the vaccine will be required to be administered once (i.e. one-time, double dose), annually, or if future booster shots will be required. We cannot predict continued prevalence of COVID-19, whether herd immunity will be achieved which would affect the need for future administration of the vaccine, or whether mRNA-1273 will be effective against future mutations or variants of the SARS-CoV-2 virus. Such factors may impact if mRNA-1273 shall be reimbursable under Medicare Part D rather than Part B and if mRNA-1273 will be excluded from participating in certain federal entitlement programs such as the Vaccines for Children Program.
Reimbursement agencies in Europe may be more conservative than CMS. For example, a number of cancer drugs have been approved for reimbursement in the United States and have not been approved for reimbursement in certain European countries. Outside the United States, certain countries, including a number of member states of the European Union, set prices and reimbursement for pharmaceutical products, or medicinal products, as they are commonly referred to in the European Union, with limited participation from the marketing authorization holders. We cannot be sure that such prices and reimbursement will be acceptable to us or our strategic collaborators. If the regulatory authorities in these foreign jurisdictions set prices or reimbursement levels that are not commercially attractive for us or our strategic collaborators, our revenues from sales by us or our strategic collaborators, and the potential profitability of our drug products, in those countries would be negatively affected. An increasing number of countries are taking initiatives to attempt to reduce large budget deficits by focusing cost-cutting efforts on pharmaceuticals for their state-run healthcare systems. These international price control efforts have impacted all regions of the world, but have been most drastic in the European Union. Additionally, some countries require approval of the sale price of a product before it can be marketed. In many countries, the pricing review period begins after marketing or product licensing approval is granted. As a result, we might obtain
marketing approval for a product in a particular country, but then may experience delays in the reimbursement approval of our product or be subject to price regulations that would delay our commercial launch of the product, possibly for lengthy time periods, which could negatively impact the revenues we are able to generate from the sale of the product in that particular country.
Moreover, payment methodologies may be subject to changes in healthcare legislation and regulatory initiatives. For example, CMS may develop new payment and delivery models, such as bundled payment models. Recently, there has been heightened governmental scrutiny over the manner in which manufacturers set prices for their products, resulting in several recent U.S. Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to drug pricing, reduce the cost of prescription drugs under Medicare, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drugs. For example, at the federal level, the U.S. government’s budget proposal for fiscal year 2021 includes a $135 billion allowance to support legislative proposals seeking to reduce drug prices, increase competition, lower out-of-pocket drug costs for patients, and increase patient access to lower-cost generic and biosimilar drugs. On March 10, 2020, the U.S. government sent “principles” for drug pricing to Congress, calling for legislation that would, among other things, cap Medicare Part D beneficiary out-of-pocket pharmacy expenses, provide an option to cap Medicare Part D beneficiary monthly out-of-pocket expenses, and place limits on pharmaceutical price increases.
Additionally, the U.S. government previously released a “Blueprint” to reduce the cost of drugs. This Blueprint contains certain measures that the HHS is already working to implement. For example, in May 2019, CMS issued a final rule that amends the Medicare Advantage and Medicare Part D prescription drug benefit regulations to reduce out of pocket costs for plan enrollees and allow Medicare plans to negotiate lower rates for certain drugs. Among other things, the final rule now allows Medicare Advantage plans the option to use step therapy, a type of pre-authorization, for Part B drugs beginning January 1, 2020. This final rule codified CMS’s policy change that was effective January 1, 2019. In addition, there have been several changes to the 340B drug pricing program, which imposes ceilings on prices that drug manufacturers can charge for medications sold to certain health care facilities. Some of these changes are undergoing legal challenges, and their status is currently in question. It is unclear how these developments could affect covered hospitals who might purchase our future products and affect the rates we may charge such facilities for our approved products in the future, if any.
On July 24, 2020 and September 13, 2020, former President Trump signed a series of Executive Orders aimed at lowering drug prices and at implementing several of the administration's proposals. In response, the FDA released a final rule on September 24, 2020, which went into effect on November 30, 2020, creating a process for states to build and submit to the FDA importation plans for drugs from Canada. Further, on November 20, 2020 CMS issued an Interim Final Rule implementing the Most Favored Nation, or MFN Model under which Medicare Part B reimbursement rates will be calculated for certain drugs and biologicals based on the lowest price drug manufacturers receive in Organization for Economic Cooperation and Development countries with a similar gross domestic product per capita. The MFN Model regulations mandate participation by identified Part B providers and was intended to apply in all U.S. states and territories for a seven-year period beginning January 1, 2021, and ending December 31, 2027. However, on December 28, 2020, a judge in the U.S. District Court for the Northern District of California granted a preliminary injunction prohibiting CMS from implementing the MFN Model. Additionally, on November 20, 2020, HHS finalized a regulation removing safe harbor protection for price reductions from pharmaceutical manufacturers to plan sponsors under Part D, either directly or through pharmacy benefit managers, unless the price reduction is required by law. The rule also creates a new safe harbor for price reductions reflected at the point-of-sale, as well as a safe harbor for certain fixed fee arrangements between pharmacy benefit managers and manufacturers. Pursuant to an order entered by the U.S. District Court for the District of Columbia, the portion of the rule eliminating safe harbor protection for certain rebates related to the sale or purchase of a pharmaceutical product from a manufacturer to a plan sponsor under Medicare Part D has been delayed to January 1, 2023. Implementation of the this change and new safe harbors for point-of-sale reductions in price for prescription pharmaceutical products and pharmacy benefit manager service fees are currently under review by the Biden administration and may be amended or repealed. Although a number of these, and other proposed measures will require authorization through additional legislation to become effective, and the Biden administration may reverse or otherwise change these measures, Congress has indicated that it will continue to seek new legislative measures to control drug costs.
At the state level, legislatures are increasingly passing legislation and implementing regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, or restrictions on certain product access, and marketing cost disclosure and transparency measures, which, in some cases, are designed to encourage importation from other countries and bulk purchasing. Legally mandated price controls on payment amounts by third-party payors or other restrictions on coverage or access could harm our business, results of operations, financial condition and prospects. In addition, regional healthcare authorities and individual hospitals are increasingly using bidding procedures to determine what pharmaceutical products and which suppliers will be included in their prescription drug and other healthcare programs. This could reduce the ultimate demand for our product candidates that we successfully commercialize or put pressure on our product pricing.
We expect to experience pricing pressures in connection with the sale of any of our investigational medicines, due to the trend toward managed healthcare, the increasing influence of health maintenance organizations and additional legislative changes. The downward
pressure on healthcare costs in general, particularly prescription drugs and surgical procedures and other treatments, has become very intense. As a result, increasingly high barriers are being erected to the entry of new products.
Recent federal legislation and actions by federal, state and local governments may permit reimportation of drugs from foreign countries into the United States, including foreign countries where the drugs are sold at lower prices than in the United States, which could materially adversely affect our operating results.
We may face competition in the United States for our development candidates and investigational medicines, if approved, from therapies sourced from foreign countries that have placed price controls on pharmaceutical products. In the United States, the Medicare Modernization Act contains provisions that call for the promulgation of regulations that expand pharmacists’ and wholesalers’ ability to import cheaper versions of an approved drug and competing products from Canada, where there are government price controls. Further, the Medicare Modernization Act provides that these changes to U.S. importation laws will not take effect unless and until the Secretary of HHS certifies that the changes will pose no additional risk to the public’s health and safety and will result in a significant reduction in the cost of products to consumers. On September 23, 2020, the Secretary of HHS made such certification to Congress, and on October 1, 2020, the FDA published a final rule that allows for the importation of certain prescription drugs from Canada. Under the final rule, States and Indian Tribes, and in certain future circumstances pharmacists and wholesalers, may submit importation program proposals to the FDA for review and authorization. Since the issuance of the final rule, several industry groups have filed federal lawsuits challenging multiple aspects of the final rule, and authorities in Canada have passed rules designed to safeguard the Canadian drug supply from shortages. On September 25, 2020, CMS stated drugs imported by States under this rule will not be eligible for federal rebates under Section 1927 of the Social Security Act and manufacturers would not report these drugs for “best price” or Average Manufacturer Price purposes. Since these drugs are not considered covered outpatient drugs, CMS further stated it will not publish a National Average Drug Acquisition Cost for these drugs. Separately, the FDA also issued a final guidance document outlining a pathway for manufacturers to obtain an additional National Drug Code (NDC) for an FDA-approved drug that was originally intended to be marketed in a foreign country and that was authorized for sale in that foreign country. Since the issuance of the final rule, on November 23, 2020, several industry groups filed federal lawsuits in the U.S. District Court for the District of Columbia, requesting injunctive relief to prevent certification from the Secretary of HHS from taking effect and challenging multiple aspects of the final rule. This litigation has not progressed. If implemented, drug importation may materially and adversely affect the price we receive for any of our products and product candidates. The market implications of these rules and guidance are unknown at this time. For example, proponents of drug reimportation may attempt to pass legislation that would directly allow reimportation under certain circumstances. Legislation or regulations allowing the reimportation of drugs, if enacted, could decrease the price we receive for any products that we may develop and adversely affect our future revenues and prospects for profitability.
Healthcare legislative reform discourse and potential or enacted measures may have a material adverse impact on our business and results of operations and legislative or political discussions surrounding the desire for and implementation of pricing reforms may adversely impact our business.operations.
In the United States, thereWe are upgrading our global ERP system to support our continued growth as a commercial operation. We have beenincurred substantial costs in implementing our ERP system, and continueany disruptions or difficulties in implementing or using our system could adversely affect our controls, resulting in harm to be a number of legislative initiatives to contain healthcare costs. For example, in March 2010, the ACA was passed, which substantially changes the way health care is financed by both governmental and private insurers, and significantly impacts the U.S. pharmaceutical industry. The ACA, among other things, increased the minimum Medicaid rebates owed by manufacturers under the Medicaid Drug Rebate Program and extended the rebate program to individuals enrolled in Medicaid managed care organizations, established annual fees and taxes on manufacturers of certain branded prescription drugs, and promoted a new Medicare Part D coverage gap discount program. Considerable uncertainty remains regarding the implementation and impact of the ACA.
Since its enactment, there have been numerous judicial, administrative, executive, and legislative challenges to certain aspects of the ACA, and we expect there will be additional challenges and amendments to the ACA in the future. Various portions of the ACA are currently undergoing legal and constitutional challenges in the United States Supreme Court; the Trump Administration issued various Executive Orders that eliminated cost sharing subsidies and various provisions that would impose a fiscal burden on states or a cost, fee, tax, penalty or regulatory burden on individuals, healthcare providers, health insurers, or manufacturers of pharmaceuticals or medical devices; and Congress has introduced several pieces of legislation aimed at significantly revising or repealing the ACA. The United States Supreme Court is expected to rule on a legal challenge to the constitutionality of the ACA in early 2021. The implementation of the ACA is ongoing, the law appears likely to continue the downward pressure on pharmaceutical pricing, especially under the Medicare program, and may also increase our regulatory burdens and operating costs. Litigation and legislation related to the ACA are likely to continue, with unpredictable and uncertain results. It is unclear whether the ACA will be overturned, repealed, replaced, or further amended. We cannot predict what affect further changes to the ACA would have on our business.
In addition, other legislative changes have been proposed and adopted in the United States since the ACA was enacted. The Budget Control Act of 2011, among other things, created measures for spending reductions by the U.S. Congress. A Joint Select Committee on Deficit Reduction, tasked with recommending a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, was unable to reach required goals, thereby triggering the legislation’s automatic reduction to several government programs. This
includes aggregate reductions of Medicare payments to providers up to 2% per fiscal year. These reductions will remain in effect through 2030 unless additional Congressional action is taken. However, pursuant to the CARES Act and subsequent legislation, these Medicare sequester reductions will be suspended from May 1, 2020 through March 31, 2021 due to the COVID-19 pandemic. Proposed legislation, if passed, would extend this suspension until the end of the COVID-19 pandemic. On January 2, 2013, the American Taxpayer Relief Act of 2012 was signed into law, which, among other things, further reduced Medicare payments to several types of providers,business, including hospitals and cancer treatment centers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. Additionally, the Bipartisan Budget Act of 2018, among other things, amended the ACA, effective January 1, 2019, by increasing the point-of-sale discount (from 50% under the ACA to 70%) that is owed by pharmaceutical manufacturers who participate in Medicare Part D and closing the coverage gap in most Medicare drug plans, commonly referred to as the “donut hole”.
Throughout the COVID-19 pandemic, there has been public concern over the availability and accessibility of critical medical products, and the CARES Act enhances FDA’s existing authority with respect to drug shortage measures. Under the CARES Act, we must have in place a risk management plan that identifies and evaluates the risks to the supply of approved drugs for certain serious diseases or conditions for each establishment where the drug or API is manufactured. The risk management plan will be subject to FDA review during an inspection. If we experience shortages in the supply of our marketed products, our results could be materially impacted.
Further, legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. We cannot be sure whether additional legislative changes will be enacted, or whether FDA regulations, guidance, or interpretations will be changed, or what the impact of such changes on the marketing approvals, if any, of our development candidates, may be. In addition, increased scrutiny by the U.S. Congress of the FDA’s approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent product labeling and post-marketing conditions and other requirements.
The delivery of healthcare in the European Union, including the establishment and operation of health services and the pricing and reimbursement of medicines, is almost exclusively a matter for national, rather than EU, law and policy. National governments and health service providers have different priorities and approaches to the delivery of health care and the pricing and reimbursement of products in that context. In general, however, the healthcare budgetary constraints in most EU member states have resulted in restrictions on the pricing and reimbursement of medicines by relevant health service providers. Coupled with ever-increasing EU and national regulatory burdens on those wishing to develop and market products, this could prevent or delay marketing approval of our investigational medicines, restrict or regulate post-approval activities, and affect our ability to commercialize any products for which we obtain marketing approval.
We expect that additional foreign, state,forecast or make sales and federal healthcare reform measurescollect our receivables. Significant delays in documenting, reviewing and testing our internal controls could cause our non-compliance with our SEC reporting obligations related to our management’s assessment of our internal control over financial reporting. Moreover, such disruptions or proposals will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, whichdifficulties could result in reduced demand for our investigational medicines or additional pricing pressures. In the event that the pricing structures for healthcare products, such as the investigational medicines we are developing, change materiallyunanticipated costs and limit payments for such investigational medicines, our business will be adversely impacted as our products may no longer be commercially viable based on their expected net present value, we may have invested significant resources in products that cannot be commercially developed, or we may determine that assets that have reached an early phasediversion of development cannot or will not be taken into further development, notwithstanding their clinical viability. In addition, development assets or clinical programs that are part of our strategic alliances may no longer be deemed commercially viable to pursue based on our strategic collaborators’ assessments of the impact of any proposed, announced, or legislated pricing reforms.
We cannot predict what healthcare reform initiatives may be adopted in the future. Further federal, state, and foreign legislative and regulatory developments are likely, and we expect ongoing initiatives to increase pressure on drug pricing. Such reforms could have an adverse effect on anticipated revenues from investigational medicines that we may successfully develop and for which we may obtain regulatory approval, and may affect our overall financial condition and ability to develop investigational medicines.
Due to the novel nature of our technology, we face uncertainty related to pricing and reimbursement for these investigational medicines.
Target patient populations for certain of our investigational medicines, such as those for rare genetic diseases, may be relatively small, and certain of our investigational medicines, like PCV, require customization on an individual scale. As a result, the pricing and reimbursement of our investigational medicines, if approved, must be adequate to support commercial infrastructure. If we are unable to obtain adequate levels of reimbursement, our ability to successfully market and sell our investigational medicines will be adversely affected. The manner and level at which reimbursement is provided for services related to our investigational medicines (e.g., for administration of our product to patients) is also important. Inadequate reimbursement for such services may lead to physician resistance and adversely affect our ability to market or sell our products.
In addition, third-party payor coverage for our COVID-19 vaccine is not currently available and there is no guarantee payors will provide coverage for the vaccine during or after the termination of the public health emergency. We cannot guarantee that even if coverage is provided, the approved reimbursement amount will be high enough to allow us to establish or maintain pricing sufficient to realize a sufficient return on our investment. While coverage will most likely be provided under Medicare Part B, it is unclear whether such coverage may be re-designated to Part B and it is further unclear to what extent other payors, including certain federal entitlement programs, such as the Vaccines for Children Program, will provide coverage for the product.
If the market opportunities for our development candidates or investigational medicines are smaller than we believe they are, our revenue may be adversely affected and our business may suffer. Because the target patient populations for some of our programs are difficult to ascertain or small, we must be able to successfully identify clinical trial participants and achieve a significant market share to maintain profitability and growth.
An important area of focus of our research and product development activities is the development of treatments for severe rare genetic diseases. Our projections of both the number of people who have these diseases, as well as the subset of people with these diseases who have the potential to benefit from treatment with our programs are based on estimates. These estimates have been derived from a variety of sources, including scientific literature, surveys of clinics, patient foundations, or market research, and may prove to be incorrect. Further, new studies may change the estimated incidence or prevalence of these diseases. The number of clinical trial participants or patients in the United States, Europe, and elsewhere may turn out to be lower than expected, potential clinical trial participants or patients may not be otherwise amenable to treatment with our products, or new clinical trial participants or patients may become increasingly difficult to identify or gain access to, all of which would adversely affect our results of operations and our business.
The market opportunities of some of our programs may be limited to those patients who are ineligible for or have failed prior treatments and for which the market opportunities may be small.
The FDA often approves new therapies initially only for use by patients with relapsed or refractory advanced disease. We expect to initially seek approval of our PCV and intratumoral immuno-oncology investigational medicines in this context. Subsequently, for those products that prove to be sufficiently beneficial, if any, we would expect to seek approval in earlier lines of treatment and potentially as a first line therapy but there is no guarantee that our investigational medicines, even if approved, would be approved for earlier lines of therapy, and, prior to any such approvals, we may have to conduct additional clinical trials.
Our projections of both the number of people who have the cancers we may be targeting, as well as the subset of people with these cancers in a position to receive second or third line therapy, and who have the potential to benefit from treatment with our investigational medicines, are based on our beliefs and estimates. These estimates have been derived from a variety of sources, including scientific literature, surveys of clinics, patient foundations, or market research, and may prove to be incorrect. Further, new studies may change the estimated incidence or prevalence of these cancers. The number of trial participants may turn out to be lower than expected. Additionally, the potentially addressable patient population for our investigational medicines may be limited or may not be amenable to treatment with our investigational medicines. Even if we obtain significant market share for our products, if approved, because the potential target populations are small, we may never achieve profitability without obtaining regulatory approval for additional indications.
Risks related to our business and operations
We will need to develop and expand our company, and we may encounter difficulties in managing this development and expansion, which could disrupt our operations.
As of December 31, 2020, we had approximately 1,300 full-time employees and, in connection with the growth and advancement of our pipeline and operating as a public company, we expect to increase the number of employees and the scope of our operations. To manage our anticipated development and expansion, including expansion outside of the United States, we must continue to implement and improve our managerial, operational, and financial systems, expand our facilities, and continue to recruit and train additional qualified personnel. Also, our management may need to divert a disproportionate amount of its attention away from its day-to-day activities and devote a substantial amount of time to managing these development activities.
As a growing biotechnology company, we are actively pursuing development candidates and investigational medicines in many therapeutic areas and across a wide range of diseases. Successfully developing products for and fully understanding the regulatory and manufacturing pathways to all of these therapeutic areas and disease states requires a significant depth of talent, resources, and corporate processes in order to allow simultaneous execution across multiple areas. Due to our limited resources and early stage of growth, we may not be able to effectively manage this simultaneous execution and the expansion of our operations or recruit and train additional qualified personnel. This may result in weaknesses in our infrastructure, give rise to operational mistakes, loss of business
opportunities, loss of employees, and reduced productivity among remaining employees. The physical expansion of our operations may lead to significant costs and may divert financial resources from other projects, such as the development of our investigational medicines. If our management is unable to effectively manage our expected development and expansion, our expenses may increase more than expected, our ability to generate or increase our revenue could be reduced, and we may not be able to implement our business strategy. Our future financial performance and our ability to commercialize our COVID-19 vaccine or our other investigational medicines, if approved, and compete effectively will depend, in part, on our ability to effectively manage the future development and expansion of our company.
Our future success depends on our ability to retain key employees, consultants and advisors and to attract, retain and motivate qualified personnel. We may not be able to retain employees or executives who have vested stock options.
Our ability to compete in the highly competitive biotechnology and pharmaceutical industries depends uponon our ability to attract and retain highly qualified managerial, scientific, technical, quality-control, manufacturing, medical, regulatory and medicalcommercial personnel. The turnover rate in our industry is high and we compete with other companies, as well as academic institutions, for individuals with certain skill sets. Failure to attract and retain personnel could result in delays in production or difficulties in maintaining compliance with regulatory requirements. In addition, adverse publicity, including as the result of failure to succeed in clinical trials or obtain marketing approvals, may make it difficult to recruit and retain qualified personnel.
We are highly dependent uponon members of our management and scientific teams. Each of our executive officers and all of our employees, including key scientists and clinicians, are employed “at will,” meaning we or each officer or employeethey may terminate the employment relationship at any time. The loss of any of these persons’ services may adversely impact the achievement of our research, development, financing and commercialization objectives. We currently do not have “key person” insurance on any of our employees. ManySeveral of our key employees, including members of our executive team,executives, have been with us for a long period of time, and have valuable, fully vested stock options or other long-term equity incentives. We may not be able to retain these employees due to the competitive environment in the biotechnology industry, particularly in Cambridge, Massachusetts.
In addition, we rely on consultants, contractors and advisors, including scientific and clinical advisors, to assisthelp us in formulatingformulate our research and development, regulatory approval, manufacturing and commercialization strategy. Our consultants and advisorsstrategies. These individuals may be employed by employers other than usemployers and may have commitments under consulting or advisory contracts with other entitiesothers that may limit their availability to us. The loss of the services of one or more of our current employees or advisorsthese individuals might impede the achievement of our research, development, regulatory approval, manufacturing and commercialization objectives. In addition, we have flexibly grown our workforce through the use of contractors and part time workers. We may not be able to retain the services of such personnel which might result in delays in the operation of our business.
RecruitingIf we cannot maintain our corporate culture, we could lose the innovation, teamwork and retaining other qualified employees, consultants, and advisors for our business, including scientific and technical personnel, also will be criticalpassion that we believe contribute to our success. Competition for skilled personnel, including
We invest substantial time and resources in mRNAbuilding and LNP research, clinical operations, regulatory affairs, therapeutic area management,maintaining our culture and manufacturing, is intensedeveloping our personnel; however, as we continue to expand, it may be increasingly difficult to maintain our culture. The dramatic growth of our workforce, coupled with recent shifts in workplace and workstyle, increase the turnover rate can be high. We may not be ablerisk of our ability to attract and retain personnel on favorable terms given the competition among numerous pharmaceutical and biotechnology companies and academic institutions for individuals with similar skill sets. In addition, adverse publicity,maintain culture. Any failure to succeed in preclinical or clinical trials or applications for marketing approval may make it more challenging to recruit and retain qualified personnel. Furthermore, we may not be successful commercializingpreserve our first product and as a result, we may be unable to attract and retain highly qualified sales and marketing professionals to support our COVID-19 vaccine andculture could negatively affect our future products, if approved. The inabilitysuccess, including our ability to retain and recruit or loss of services of certain executives, key employees, consultants, or advisors, may impede the progress ofpersonnel and to effectively pursue our research, development and global commercialization objectives and have a material adverse impact on our business, financial condition, results of operations, and prospects.
Our internal computer systems and physical premises, or those of our strategic collaborators, other contractors, consultants, or regulatory agenciesthird parties with which we share sensitive data or information, may fail or suffer security breaches, including from cybersecurity incidents, which could result in a material disruption ofmaterially disrupt our product development programs and our manufacturing operations.
Our internal computer systems and infrastructure and those of our current and any future strategic collaborators, vendors, contractors, consultants or regulatory authorities with whom we share confidential, protected or sensitive data or information, or upon which our business relies, are vulnerable to damage from computer viruses, unauthorized access, misuse, natural disasters, terrorism, cybersecurity threats, war and telecommunication and electrical failures.failures, as well as security compromises or breaches, which may compromise our systems, infrastructure, data or that of those with whom we share such data or information or upon which our business relies, or lead to data compromise, misuse, misappropriation or leakage. We have experienced, and may experience in the future,additional, cyber-attacks on our information technology systems and infrastructure by threat actors of all types (including but not limited to nation states, organized crime, other criminal enterprises, individual actors and/or advanced persistent threat groups). In addition, we may experience intrusions on our physical premises by any of these threat actors. In addition to extracting sensitive information, such attacks could include the deployment of harmful malware, ransomware, digital extortion, business email compromise and denial-of-service attacks, social engineering and other means to affect server reliability and threaten the confidentiality, integrity and availability of information, systems or infrastructure. If any such cyber-attack or physical intrusion against us or those with whom we share confidential, protected or sensitive data or information, or upon which our business
relies, were to cause interruptionsresult in a loss of or damage to our data, systems or infrastructure, or interrupt our operations, such as a material disruption of our development programs or our manufacturing operations, whetheror due to a loss of any of our trade secretsproprietary or other proprietaryconfidential information, it would have a material and adverse effect on us. For example, the loss of clinical trial data from one or more ongoing or completed or future clinical trials could result in delays indelay our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. In addition, because of our approach to runningwe run multiple clinical trials in parallel, any breach or compromise of our computer systems or infrastructure or physical premises may result in a loss of data or compromised data integrity across many of ourmultiple programs in many stages of development. AnyWhile we seek to take steps to address cybersecurity risks, our efforts may not wholly mitigate such risks. Further, our cybersecurity liability insurance may not cover all damages we would sustain based on any breach loss, or compromise of our computer security protocols or cybersecurity attack.
Any data breach, security incident or compromise of confidential, protected or personal information, including any clinical trial participant personal data, may also subject us to civil fines and penalties, litigation, regulatory investigations or enforcement actions, or claims for damages either under the GDPR and UK GDPR and relevant member state law in the EU, other foreign laws, and the federal Health Insurance
Portability and Accountability Act of 1996 (HIPAA), and other relevant state and federal privacy laws in the United States including the California Consumer Privacy Act, as amended by the California Privacy Rights Act (the CCPA). On May 13, 2020, the Federal Bureau of Investigation (FBI) and Cybersecurity and Infrastructure Security Agency (CISA), announcedWe have from time to time received information that the FBI was investigating the targeting and compromise of U.S. organizations conducting COVID-19-related research by People’s Republic of China, or PRC-affiliated cyber actors. Furthermore,companies working on July 16, 2020, the National Security Agency and other U.S. and foreign agencies released a joint cybersecurity advisory regarding the Russian Intelligence Services’ targeting of COVID-19vaccine research and vaccine development.development may be a particular focus for those planning cyberattacks, including by nation states and affiliated cyber actors. To the extent that any disruption or security compromise incident or breach were to result in a loss of, or damage to, our data, systems, infrastructure or applications, or inappropriate use or disclosure of confidential or proprietary information, including but not limited to information related to the research and manufacturing of our rapid manufacture of mRNA-1273,products, we could incur liability, our competitive and reputational position could be harmed and the further development and commercialization of our investigational medicinesproduct candidates could be delayed. The CCPA is of particular concern since it provides for a private right of actions for certain breaches of personal information.
We may use our financial and human resourcescapital to pursue a particular research program or investigational medicineproduct candidate and fail to capitalize on programs or investigational medicines that may be more profitable or for which there is a greater likelihood of success.
Because we have limited resources, we must choose toWe pursue and fund the development of selected research programs or investigational medicinesproduct candidates and may choose to forego or delay pursuit of opportunities with other programs or investigational medicinesopportunities that could later prove to have greater commercial potential. For example, we have focused a significant amount of resources on our COVID-19 vaccines and other respiratory programs, for which preparations are underway for multiple vaccine launches. Additionally, we are increasing our investments in our INT programs. Our resource allocation decisions, or our contractual commitments to provide resources to our strategic collaborators, under strategic alliance agreements, may cause us to fail to capitalize on viablecertain commercial products or profitable market opportunities. Our spending on current and future research and development programs for investigational medicinesproduct candidates may not yield any commercially viable products. If we do not accurately evaluate the commercial potentialWe may also seek to enter into strategic collaborations or target market for a particular investigational medicine,financing arrangements pursuant to which we may relinquish valuable rights to that investigational medicineproduct candidates, including the rights to a portion of future revenues, through a strategic alliance, licensing or other royalty arrangements in cases in whichwhere it would have been more advantageous for us to retain sole development and commercialization rights to such investigational medicine, orrights. Additionally, we may allocate internal resources to an investigational medicinea product candidate in a therapeutic area in which it would have been more advantageous to enter into a strategic alliance.
If we are not successful in discovering, developing and commercializing additional products, beyond our current portfolio, our ability to expand our business and achieve our strategic objectives would be impaired.
Although a substantial amount of our efforts will focus on the commercialization of our COVID-19 vaccine and the clinical trials and potential approval of our investigational medicines, aA key element of our strategy is to discover, develop and potentially commercialize additional products beyond our current portfolio to treat various conditions and in a variety of therapeutic areas. We intend to do so by investing in our own drug discovery efforts, exploring potential strategic alliances for the development of new products and in-licensing technologies. Identifying new investigational medicinesproduct candidates requires substantial technical, financial and human resources, whether or not any investigational medicines are ultimately identified. Even if we identify investigational medicines that initially show promise, weresources. We may fail to identify promising candidates or to successfully develop and commercialize such products for many reasons, including the following:which would impair our potential for growth.
•the research methodology used may notOur business could be successful in identifying potential investigational medicines;
•competitors may develop alternatives that renderharmed if we suffer damage to our investigational medicines obsolete;
•investigational medicines we develop may nevertheless be covered by third parties’ patents or other exclusive rights;
•an investigational medicine may, on further study, be shown to have harmful side effects or other characteristics that indicate it is unlikely to be effective or otherwise does not meet applicable regulatory criteria;
•an investigational medicine may not be capablereputation, including as a result of being produced in commercial quantities at an acceptable cost, or at all; and
•an approveda product may not be accepted as safe and effective by patients, the medical community or third-party payors.recall.
If we are unsuccessfulThe FDA or foreign regulators could require the recall of our products. The FDA has authority to recall a biologic product if it finds that a batch, lot or other quantity of the biologic product presents an imminent or substantial hazard to the public health. In addition, foreign governmental bodies may require the recall of any products in identifyingthe event of material deficiencies or defects in design or manufacture. Manufacturers may independently recall a product if a material deficiency in a product is found. A government-mandated or voluntary recall by us or our strategic collaborators could occur as a result of manufacturing errors, design or labeling defects or other deficiencies and developing additionalissues, as occurred with the recall in 2021 of certain batches of our COVID-19 vaccine shipped to Japan that were found to contain foreign particulate. Recalls of any of our products would divert managerial and financial resources and adversely affect our potential for growth mayfinancial condition and results of operations. A recall announcement could harm our reputation and negatively affect our sales. Our reputation could be impaired.further impacted by public discourse regarding our business and perception of our business strategy.
Product liability lawsuits against us could cause us to incur substantial liabilities and could limit commercialization of any product or investigational medicine that we may develop, such as our COVID-19 vaccine.products.
We face an inherent risk ofare exposed to product liability exposurerisk related to the development, testing, manufacturing and marketing of our COVID-19 vaccineproducts and our other current or future investigational medicinesproduct candidates in clinical trials. Product liability claims and related cross-claims and claims for indemnification may be brought against us by patients, healthcare providers or others using, prescribing, selling or otherwise coming into contact with our COVID-19 vaccineproducts or other investigational medicines.product candidates. For example, we may be sued if the COVID-19 vaccineany product or any other investigational medicineproduct candidate allegedly causes injury or is found to be otherwise unsuitable during clinical trials, manufacturing or, if approved, marketing, sale or commercial use. If we cannot successfully defend ourselves against such claims, that our medicines caused injuries, we could incur substantial liabilities.
•decreased demand for any investigational medicine that we may develop;
•loss of revenue;
•substantial monetary awards to patients, healthy volunteers, or their family members;
•payments to indemnify clinical trial sites and other clinical trial partners;
•significant time and costs to defend the related litigation;
•withdrawal of clinical trial participants;
•withdrawal of regulatory or marketing approval in a territory;
•the inability to commercialize any investigational medicine(s) that we may develop; and
•injury to our reputation and significant negative media attention.
Notwithstanding the risks undertaken by all persons who participate in clinical trials or who receive our COVID-19 vaccine, and the information on risks provided to study investigators and patients participating in our clinical trials or receiving our COVID-19 vaccine, it is possible thatWe could also face product liability claims will be asserted against us relating to the worsening of a patient’s condition, injury or death alleged to have been caused by one of our products or investigational medicines or our COVID-19 vaccine.product candidates. Any such product liability claims may include allegations of defects in manufacturing defects inor design, a failure to warn of dangers inherent in the product, including as a result of interactions with alcohol or other drugs, knowledge of risks, negligence, strict liability and a breach of warranties. Claims could also be asserted under state consumer protection acts. Such claims might not be fully covered by product liability insurance. If we succeed in marketingFor any marketed products, including our COVID-19 vaccine, product liability claims could result in an FDA investigation of the safety and effectiveness of our products, our manufacturing processes and facilities or our marketing programs, and potentially a recall of our products or more serious enforcement action, limitations on the approved indications for which they may be used, suspension or withdrawal of approvals or license revocation. Regardless of the merits or eventual outcome, liability claims may also result incause decreased demand for our products, injury to our reputation and significant negative media attention, costs to defend the related litigation, withdrawal of clinical trial participants, loss of revenue, a diversion of management’s time and our resources, substantial monetary awards to trial participants, patients or patientstheir family members, payments to indemnify clinical trial sites and other clinical trial partners and a decline in our stock price. On occasion, large judgments have been awarded in individual, mass tort and class-action lawsuits based on drugs or medical treatments that had unanticipated adverse effects.
We are also exposedWith respect to liabilities that are unique to developing and commercializing an mRNAour COVID-19 vaccine, inalthough the context of an ongoing global pandemic. Although the U.S. government and certain foreign governments have contractually agreed to indemnify us or make statutory immunity available to us, such indemnification or statutory immunity may not be availableunavailable to cover potential claims or liabilities resulting from the research, development, manufacture, distribution or commercialization of ourthe vaccine. Additionally, other foreign governments that we contract with may not provide us with similar contractual indemnity or statutory immunity, and we will not have the benefit of such indemnities or immunities in the future as the COVID-19 vaccine.vaccine market transitions to a commercial market in the United States and elsewhere. Substantial claims arising from the vaccine outside the scope of or in excess of U.S. government or foreign government indemnity or statutory immunity could harm our financial condition and operating results. Moreover, any adverse event or injury for which we are liable, even if fully covered under an indemnity or immunity, could negatively affect our reputation, thereby making it more difficult for us to compete effectively.reputation.
We carrymay be unable to maintain our product liability insurance which we believe to be sufficient in light of our current commercial and clinical programs; however, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses due to liability. If and when we obtain marketing approval for investigational medicines, we intend to expand our insurance coverage to include the sale of commercial products; however, we may be unable to obtain product liability insurance on commercially reasonable terms or in adequate amounts. On occasion, large judgments have been awarded in individual, mass tort and class action lawsuits based on drugs or medical treatments that had unanticipated adverse effects. A successful product liability claim or series of claims brought against us could cause our stock price to decline and, if judgments exceed our insurance coverage, could adversely affect our results of operations and business.
If the costs of maintaining adequate insurance coverage increase significantly in the future, our operating results could be materially adversely affected. Likewise, if insurance coverage should become unavailable to us or become economically impractical, we would be required to operate our business without indemnity from commercial insurance providers. Additionally, even if we maintain insurance coverage for a type of liability, a particular claim may not be covered if it is subject to a coverage exclusion or we do not otherwise meet the conditions for coverage. If we operate our business without insurance, or with inadequate insurance, we could be responsible for paying claims or judgments against us, which could adversely affect our results of operations or financial condition.
Federal legislation and actions by federal, state and local governments may permit reimportation into the United States of drugs from foreign countries where the drugs are sold at lower prices.
We may face competition in the United States for our products from therapies sourced from foreign countries with price controls on pharmaceutical products. For example, in October 2020, the FDA published a final rule that would allow for the importation of certain prescription drugs from Canada, where there are government price controls. In January 2024, the FDA approved Florida’s request to import certain lower-priced medications from Canada. While the full implications of the final rule are currently unknown, legislation or regulations allowing the reimportation of drugs could decrease the price we receive for any products we may develop and adversely affect our future revenues and potential profitability.
Healthcare legislative reform discourse and potential or enacted measures may have a material adverse impact on our business and results of operations and legislative or political discussions surrounding the desire for and implementation of pricing reforms may adversely impact our business.
In the United States, federal and state legislatures, health agencies and third-party payors continue to focus on containing the cost of health care. Legislative and regulatory proposals, enactments to reform health care insurance programs and increasing pressure from social sources could significantly influence the manner in which our products are prescribed and purchased. For example, provisions of the ACA have resulted in changes in the way health care is paid for by both governmental and private insurers, including increased rebates owed by manufacturers under the Medicaid Drug Rebate Program, annual fees and taxes on manufacturers of certain branded prescription drugs, the requirement that manufacturers participate in a discount program for certain outpatient drugs under Medicare Part D and the expansion of the number of hospitals eligible for discounts under Section 340B of the PHSA. Additionally, the Inflation Reduction Act of 2022 includes several provisions such as drug pricing controls and Medicare redesign that are likely to impact our business to varying degrees, but its ultimate effect on our business and the healthcare industry in general is not yet known. See “Business—Government Regulation-Current and future healthcare reform legislation.”
We may face uncertainties as a result of efforts to repeal, substantially modify or invalidate some or all of the provisions of the ACA. There is no assurance that the ACA, as currently enacted or as amended in the future, will not adversely affect our business and financial results, and we cannot predict how future federal or state legislative or administrative changes relating to healthcare reform will affect our business.
There is increasing public attention on the costs of prescription drugs and there have been, and are expected to continue to be, legislative proposals to address prescription drug pricing, which could have significant effects on our business. These actions and the uncertainty about the future of the ACA and healthcare laws may put downward pressure on pharmaceutical pricing and increase our regulatory burdens and operating costs.
There is also significant economic pressure on state budgets that may result in states increasingly seeking to achieve budget savings through mechanisms that limit coverage or payment for drugs or that would allow for importation of pharmaceutical products from lower cost jurisdictions outside the United States. State Medicaid programs are increasingly requesting manufacturers to pay supplemental rebates and requiring prior authorization by the state program for use of any drug for which supplemental rebates are not being paid. Government efforts to reduce Medicaid expenses may lead to increased use of managed care organizations by Medicaid programs. This may result in managed care organizations influencing prescription decisions for a larger segment of the population and a corresponding limitation on prices and reimbursement for our products, if approved.
In the EU and some other international markets, the government provides health care at low cost to consumers and regulates pharmaceutical prices, patient eligibility or reimbursement levels to control costs for the government-sponsored health care system. Many countries have announced or implemented measures, and may in the future implement new or additional measures, to reduce health care costs to limit the overall level of government expenditures. These measures vary by country and may include, among other things, patient access restrictions, suspensions on price increases, prospective and possible retroactive price reductions and other recoupments and increased mandatory discounts or rebates, recoveries of past price increases and greater importation of drugs from lower-cost countries. These measures may adversely affect our revenues and results of operations.
We are subject, directly or indirectly, to federal and state healthcare fraud and abuse laws and false claims laws, information privacy and security laws. If we are unable tocannot comply, or have not fully complied, with such laws, we could face substantial penalties.
If we obtain FDA approval for any of our investigational medicinesHealthcare providers, physicians and begin commercializing those productsthird-party payors in the United States our operations will be directly, or indirectly through our prescribers,and elsewhere play a primary role in the recommendation and prescription of pharmaceutical products. Arrangements with third-party payors and customers and purchasers, subjectcan expose pharmaceutical manufacturers to various federal and statebroadly applicable fraud and abuse and other healthcare laws and regulations, including, without limitation,which may constrain the federal Health Care Program Anti-Kickback Statute,business or financial arrangements and relationships through which such companies sell, market and distribute pharmaceutical products. In particular, the federal civilpromotion, sales and criminal False Claims Act,marketing of healthcare items and Physician Payments Sunshine Act and regulations. These laws will impact, among other things, our proposed sales, marketing, and educational programs. In addition, we may be subject to patient privacy laws enacted
by both the federal government and the states in which we conduct our business. The laws that will affect our operations include, but are not limited to the following:
•The federal Anti-Kickback Statute, which prohibits, among other things, persons or entities from knowingly and willfully soliciting, receiving, offering, or paying any remuneration (including any kickback, bribe, or rebate), directly or indirectly, overtly or covertly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the for the purchase, order or recommendation or arranging of, any good, leasing, or furnishing of an item or service reimbursable under a federal healthcare program, such as the Medicare and Medicaid programs. The government may assert that a claim including items or services, resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act or federal civil money penalties statute. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on the one hand, and prescribers, purchasers, and formulary managers on the other. Although there are several statutory exceptions and regulatory safe harbors protecting certain common activities from prosecution, they are drawn narrowly, and practices that involve remuneration intended to induce prescribing, purchasing or recommending may be subject to scrutiny if they do not qualify for an exception or safe harbor. The ACA amends the intent requirement of the federal Anti-Kickback Statute to provide that a person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it.
•The federal civil and criminal false claims laws and civil monetary penalty laws, including the federal False Claims Act, prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment or approval from Medicare, Medicaid, or other government payors that are false or fraudulent. In addition, the government may assert that a claim including items or services resulting from a violation of the Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal False Claims Act or federal civil money penalties statute. Manufacturers can be held liable under the False Claims Act even when they do not submit claims directly to government payors if they are deemed to “cause” the submission of false or fraudulent claims. Companies that submit claims directly to payors may also be liable under the False Claims Act for the direct submission of such claims. The False Claims Act also permits a private individual acting as a “whistleblower” to bring actions on behalf of the federal government alleging violations of the False Claims Act and to share in any monetary recovery. The ACA provides, and recent government cases against pharmaceutical and medical device manufacturers support, the view that federal Anti-Kickback Statute violations and certain marketing practices, including off-label promotion, may implicate the False Claims Act.
•The anti-inducement law prohibits, among other things, the offering or giving of remuneration, which includes, without limitation, any transfer of items or services for free or for less than fair market value (with limited exceptions), to a Medicare or Medicaid beneficiary that the person knows or should know is likely to influence the beneficiary’s selection of a particular supplier of items or services reimbursable by a federal or state governmental program.
•HIPAA and its implementing regulations, which create new federal criminal statutes that prohibit a person from knowingly and willfully executing, or attempting to execute, a scheme or making false or fraudulent statements to defraud any healthcare benefit program, regardless of the payor (e.g., public or private), or obtain, by means of false or fraudulent pretenses, representations, or promises, any of the money or property owned by, or under the custody or control of, any healthcare benefit program, regardless of the payor (e.g., public or private) and knowingly and willfully falsifying, concealing or covering up by any trick or device a material fact or making any materially false statements in connection with the delivery of, or payment for, healthcare benefits, items or services relating to healthcare matters. Similar to the federal Anti-Kickback Statute, a person or entity can be found guilty of violating HIPAA without actual knowledge of the statute or specific intent to violate it.
•HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH, and their implementing regulations, imposes certain requirements relating to the privacy, security, and transmission of individually identifiable health information without appropriate authorization by entities subject to the rule, such as health plans, health care clearinghouses, and health care providers as well as their respective “business associates,” those independent contractors or agentsa wide range of covered entities that create, receive, maintain, transmit or obtain protected health information in connection with providing a service on behalf of a covered entity. HITECH also created new tiers of civil monetary penalties, amended HIPAApricing, discounting, marketing and promotion, structuring and commission(s), certain customer incentive programs and other business arrangements, are subject to make civilextensive laws designed to prevent fraud, kickbacks, self-dealing and criminal penalties directly applicable to business associates, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAAother abusive practices. These laws and seek attorneys’ feesregulations may restrict or prohibit a wide range of pricing, discounting, marketing and costs associated with pursuing federal civil actions.
•The U.S. Federal Food, Drugpromotion, structuring and Cosmetic Act, which prohibits, among other things, the adulteration or misbranding of drugs, biologics, and medical devices.
•Federal transparency laws, including the federal Physician Payment Sunshine Act, which require disclosure of paymentscommissions, certain customer incentive programs and other transfersbusiness arrangements generally. Activities subject to these laws also involve the improper use of value provided by manufacturersinformation obtained in the course of drugs, devices, biologicals and medical supplies to physicians (currently defined to include doctors, dentists, optometrists, podiatrists and chiropractors) and teaching hospitals, and ownership and investment interests held by physicians and otherpatient recruitment for clinical trials. See “Business—Government Regulation—Other healthcare providers and their immediate family members and applicable group purchasing organizations. Effective January 1, 2022, these reporting obligations will extend to include transfers of value made to certain non-physician providers such as physician assistants and nurse practitioners.laws.”
•State, local and foreign law and their regulatory equivalents of each of the above federal laws, such as state anti-kickback and false claims laws, which may apply to sales or marketing arrangements and claims involving healthcare items or services
reimbursed by any third-party payor, including private insurers and may be broader in scope than their federal equivalents; state and foreign laws that require biopharmaceutical companies to comply with the biopharmaceutical industry’s voluntary compliance guidelines and other relevant compliance guidance promulgated by the federal government or otherwise restrict payments that may be made to healthcare providers and other potential referral sources; state laws that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers, marketing expenditures, or drug pricing and state laws governing the privacy and security of health information in certain circumstances are also applicable to us and many of them differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts in certain circumstances.
Additionally, on November 20, 2020, HHS finalized a regulation that, among other things, removed safe harbor protection for price reductions from pharmaceutical manufacturers and created new safe harbor for certain fixed fees. Pursuant to an order entered by the U.S. District Court for the District of Columbia, the portion of the rule eliminating safe harbor protection for certain rebates related to the sale or purchase of a pharmaceutical product from a manufacturer to a plan sponsor under Medicare Part D has been delayed to January 1, 2023. Implementation of the this change and new safe harbors for point-of-sale reductions in price for prescription pharmaceutical products and pharmacy benefit manager service fees are currently under review by the Biden administration and may be amended or repealed. It is not clear at this time what effect, if any, these and other changes to the Anti-Kickback Safe Harbors, will have on our business.
The scope and enforcement of each of these laws is uncertain and subject to rapid change in the current environment of healthcare reform, especially in light of the lack of applicable precedent and regulations. Ensuring business arrangements comply with applicable
healthcare laws, as well as responding to possible investigations by government authorities, can beis time- and resource-consuming and can divert a company’s attention from the business. If our operations are found to be in violation ofviolate any of thethese laws described above or any other government regulations, that apply to us, we may be subject to significant sanctions, including civil, criminal and administrative penalties, damages, fines, disgorgement, imprisonment, reputational harm, exclusion from participation in federal and state funded healthcare programs, contractual damages and the curtailment or restricting of our operations, as well as additional reporting obligations and oversight if we become subject to a corporate integrity agreement or other agreement to resolve allegations of non-compliance with these laws. Further,non-compliance. Furthermore, if any of the physiciansphysician or other healthcare providersprovider or entitiesentity with whom we expect to do business areis found to be not in compliance with applicable laws, they may be subject to similar penalties. Any action for violation of these laws, even if successfully defended, could cause a biopharmaceutical manufacturerus to incur significant legal expenses and divert management’s attention from the operation of the business. In addition, the approval and commercialization of any product candidate we develop outside the United States will also likely subject us to foreign equivalents of the healthcare laws mentioned above, among other foreign laws. All of these could harm our ability to operate our business and our financial results.
The provision of benefits or advantages to physicians to induce or encourage the prescription, recommendation, endorsement, purchase, supply, order or use of medicinal products is prohibited in the European UnionEU and the UK. The provision of benefits or advantages to induce or reward improper performance generally is also governed by the national anti-bribery laws of EU Member States, and the UK Bribery Act 2010 in the UK. Infringement of these laws could result in substantial fines and imprisonment. The EU Directive (2001/83/EC, which is the EU Directiveas amended) governing medicinal products for human use provides that, where medicinal products are being promoted to persons qualified to prescribe or supply them, no gifts, pecuniary advantages or benefits in kind may be supplied, offered or promised to such persons unless they are inexpensive and relevant to the practice of medicine or pharmacy. This provision has been transposed into the Human Medicines Regulations 2012 and so remains applicable in the UK despite its departure from the EU.
Payments made to physicians in certain European UnionEU Member States must be publicly disclosed. Moreover, agreements with physicians often must beare the subject of prior notification and approval by the physician’s employer, his or her competent professional organization, or the regulatory authorities of the individual European UnionEU Member States. These requirements are provided in the national laws, industry codes or professional codes of conduct, applicable in the EU Member States. Failure to comply with these requirements could result in reputational risk, public reprimands, administrative penalties, fines or imprisonment.
The collectionWe are subject to various and useevolving laws and regulations governing the privacy and security of personally identifiable data, including healthpersonal data, and medicalour failure to comply could result in fines or criminal penalties and damage our reputation.
Privacy and data (personal data)security are significant issues in the European Union is regulated byUnited States, Europe and many other jurisdictions where we operate or collect personal information. We are subject to data privacy and security laws and regulations in various jurisdictions that apply to the GDPR, which became effective on May 25, 2018. The GDPR applies to personal data processed in connection with clinical trial activities in EU Member States. The GDPR imposes strict requirements on controllerscollection, storage, use, sharing and processorssecurity of personal data, including special protections for “sensitive information” which includes health information, and geneticimpose significant compliance obligations. In addition, numerous other federal and state laws, including state security breach notification laws, state health information privacy laws and federal and state consumer protection and privacy laws, govern the collection, use, disclosure and security of data subjects residing in the EU. personal information.
The GDPR grants individualsand UK GDPR impose stringent obligations on us with respect to our processing and the opportunity to objectcross-border transfer of personal data, including higher standards of obtaining consent or ensuring another appropriate legal basis or condition applies to the processing of their personal data, allows themmore robust transparency requirements, data breach notification requirements, requirements for contractual language with our processors and stronger individual data rights. Different EEA Member States have interpreted the GDPR differently and many have imposed additional requirements, adding to exercise certain data subject requests, including to request deletionthe complexity of processing personal data in certain circumstances,the EEA. The GDPR and provides the individual with an express right to seek legal recourse in the event the individual believes his or her rights have been violated. Further, theUK GDPR imposesalso impose strict rules on the transfer of personal data out ofto countries outside the European UnionEEA that are not considered to provide “adequate” protection to personal data, including the United States, and permits data protection authorities to impose large penalties for violations. Compliance with the GDPR and UK GDPR is a rigorous and time-intensive process that may increase our cost of doing business or require us to change our business practices. We could be subject to fines and penalties, litigation and reputational harm in connection with any activities falling within the scope of the GDPR or UK GDPR.
In the United States, California has passed the CCPA and numerous other regionsstates have passed their own similar comprehensive consumer privacy laws. There are also states that are specifically regulating health information. Further, a small number of states have not been deemedpassed laws that regulate biometric data specifically. In addition, numerous states and the federal government are actively considering proposed legislation governing the protection of personal data. State laws are changing rapidly and there is discussion in the U.S. Congress of a new comprehensive federal data privacy law to offer “adequate”which we may likely become subject, if enacted.
Additionally, many foreign jurisdictions have passed data privacy protections. Failurelegislation and others are considering various proposals for new privacy and data protection laws. Data privacy remains an evolving landscape at the domestic and international levels, with new laws and regulations being considered and coming into effect and continued legal challenges. We must devote significant resources to understanding and complying with the changing landscape in this area. Each law is also subject to various interpretations by courts and regulatory agencies, creating additional uncertainty, and we may fail to comply with the requirementsevolving data protection laws, which may expose us to risk of the GDPR may resultenforcement actions taken by authorities, private rights of action in monetary penalties of up to €20 million or 4% of annual worldwide revenue, whichever is higher. In addition to risking such fines for any failuresome jurisdictions and potential significant
penalties if we are found to be non-compliant. Some of Contents
these laws and regulations also carry the possibility of criminal sanctions. For example, we could be subject to complypenalties, including criminal penalties, if we knowingly obtain or disclose individually identifiable health information from a HIPAA-covered health care provider or research institution that has not complied with HIPAA’s requirements for disclosing such information. Furthermore, the GDPR, we maynumber of government investigations related to data security incidents and privacy violations continues to increase and government investigations typically require substantial costs in connection withsignificant resources and generate negative publicity, which could harm our efforts to put in place additional mechanisms ensuring compliance with European data protection requirements.business and our reputation.
Further, the U.K.’s decision to leave the EU, often referred to as Brexit, has created uncertainty in data protection issues involving the U.K. For example, pursuant to a post-Brexit trade deal between the U.K.The Clinical Trials Regulation (EU) No. 536/2014 (the Clinical Trial Regulation) and the EU, transfersEMA policy on publication of personalclinical data for medicinal products for human use both permit the EMA to publish clinical information submitted in MAAs. The ability of third parties to review or analyze data from our clinical trials may increase the European Economic Arearisk of commercial confidentiality breaches and result in enhanced scrutiny of our clinical trial results. Such scrutiny could result in public misconceptions regarding our drugs and drug candidates. These publications could also result in the disclosure of information to our competitors that we might otherwise deem confidential, which could harm our business.
Our use of GenAI and other AI technologies presents certain risks and challenges given the emerging nature of AI technologies.
The development and use of GenAI and other AI technologies (collectively, AI Technologies), along with an uncertain regulatory landscape, pose risks that could harm our reputation, expose us to liability or adversely affect our business. The integration of AI Technologies into our and our vendors’ systems (potentially without the vendor disclosing such use to us) subjects us to the U.K. arerisk that the providers of AI Technologies may not considered restricted transfers under the GDPR for a period of up to six months from January 1, 2021. However, unless the European Commission makes an adequacy findingmeet existing or rapidly evolving regulatory or industry standards with respect to the U.K. before the endprivacy and data protection. This may lead to breaches of that period, the U.K. will be considered a “third country” under the GDPR and transferssecurity or privacy, reduced levels of European personal information to the U.K. will require an adequacy mechanism to render such transfers lawful under the GDPR.service or experience, loss of intellectual property or exposure of confidential or proprietary information. Sophisticated cyber-attacks, including those using AI, could exacerbate these risks. Additionally, although the U.K. enacted a Data Protection Act in May 2018GenAI’s potential for producing false or misleading outputs, reflecting biases or generating content that is designed to be consistent with the GDPR, uncertainty remains regarding how data transfers to and from the U.K. will be regulated notwithstanding Brexit. The full effects of Brexit are uncertain and will remain so until the U.K. and EU reach a definitive resolution with regards to outstanding trade and legal matters. Given these possibilities and others we may not anticipate, as well as the lackbe subject to intellectual property protection or that infringes proprietary rights of comparable precedent, the full extentothers, poses additional risks to which our business, resultsbusiness. Regulatory changes or reinterpretations could introduce new compliance risks, including potential government enforcement actions or civil lawsuits. Our competitors’ faster or more effective adoption of operations, and financial conditionAI could also disadvantage us.
We could be adversely affected by Brexit is uncertain.outbreaks of epidemic, pandemic or other contagious diseases.
There is significant uncertainty relatedIn the event of a future epidemic or pandemic, our clinical trials could be paused or delayed due to restrictions (such as quarantines or travel limitations) or reprioritization of resources. Travel limitations could also create challenges and potential delays in our development and production activities, increasing the manner in which data protection authorities will seek to enforce compliance with GDPR. For example, it is not clear if the authorities will conduct random audits of companies doing business in the EU, or if the authorities will waitexpense and timelines for complaints to be filed by individuals who claim their rights have been violated. Enforcement uncertaintyproducing our products and the costs associated with ensuring GDPR compliance may be onerous and adversely affect our business, financial condition, results of operations, and prospects.product candidates.
We or theutilize third parties upon whom we depend mayto, among other things, manufacture raw materials, components, parts and consumables, perform quality testing and ship our products. If these third parties were to experience delays or disruptions in providing their services in response to an epidemic or pandemic, our supply chain could be disrupted, limiting our ability to manufacture and sell our products and manufacture product candidates for our clinical trials, as well as negatively impacting our research and development operations. Such delays or disruptions could adversely affected by natural disasters, health epidemicsimpact our strategic collaborators’ ability to fulfill their obligations, which could affect the clinical development or other business interruptions such as cybersecurity attacks and our business continuity and disaster recovery plans may not adequately protect us from a serious disaster.regulatory approvals of product candidates under joint development.
Natural disastersIn addition, during a global health crisis, one or health epidemicsmore government entities could severely disrupttake actions (such as via the Defense Production Act in the U.S.) that diminish our operations, and have a material adverse impactrights or economic opportunities with respect to our products. Our third-party service providers could be impacted by government-imposed restrictions on our business, results of operations, financial condition, and prospects. If a natural disaster, power outage, cybersecurity attack, health epidemicservices they might otherwise offer. Any such action could cause us to experience delays in the development, production, distribution or other event occurred that prevented us from using all or a significant portionexport of our headquarters, damaged critical infrastructure, such as our manufacturing facilities or those of our third-party contract manufacturers, limited our ability to access or use our digital information systems or that otherwise disrupted operations, it may be difficult or, in certain cases, impossible for us to continue our business for a substantial period of time. The disaster recoveryproducts and business continuity plans we have in place currently are limitedproduct candidates and are unlikely to prove adequate in the event of a serious disaster or similar event. Cybersecurity liability insurance is difficult to obtain and may not cover any damages we would sustain based on any breach of our computer security protocols or other cybersecurity attack. We may incur substantial expenses as a result of the limited nature of our disaster recovery and business continuity plans, which could have a material adverse impact on our business.increased expenses.
If our products become subject to a product recall it could harm our reputation, business, and financial results.
The FDA and similar foreign governmental authorities have the authority to require the recall of certain commercialized products. In the case of the FDA, the authority to require a recall of a biologic product must be based on an FDA finding that a batch, lot of other quantity of the biologic product presents an imminent or substantial hazard to the public health. In addition, foreign governmental bodies have the authority to require the recall of any investigational medicineEngaging in the event of material deficiencies or defects in design or manufacture. Manufacturers may, under their own initiative, recall a product if any material deficiency in a product is found. A government-mandated or voluntary recall by us or our strategic collaborators could occur as a result of manufacturing errors, design or labeling defects or other deficiencies and issues. Recalls of any of our investigational medicines would divert managerial and financial resources and have an adverse effect on our financial condition and results of operations. A recall announcement could harm our reputation with customers and negatively affect our sales, if any.
If we engage in future acquisitions, joint ventures or strategic collaborations this may increase our capital requirements, dilute our stockholders and cause us to incur debt or assume contingent liabilities, and subject us to other risks.liabilities.
We may evaluate variousengage in acquisitions, joint ventures and collaborations, including licensing or acquiring complementary products, IP rights, technologies or businesses. Any potential acquisition, joint venture, or collaborationSuch transactions and relationships may entail numerous risks, including:
•increased operating expenses and cash requirements;
•the assumption of additional indebtedness or contingent liabilities;
•assimilation of operations, IP and products, of an acquired company, including difficulties associated with integrating new personnel;
•the diversion of our management’s attention from our existing product programs and initiatives in pursuing such a strategic merger or acquisition;initiatives;
•retention of key employees, the loss of key personnel and uncertainties in our ability to maintain key business relationships;
•risks and uncertainties associated with the other party to such a transaction, including the prospects of that party and their existing products or investigational medicinesproduct candidates and regulatory approvals; and
•our inability to generate revenue from acquired technology or products sufficient to meet our objectives in undertaking the acquisition or even to offset the associated acquisition and maintenance costs.
If we undertake acquisitions, we may utilize our cash, issue dilutive securities, assume or incur debt obligations, incur large one-time expenses and acquire intangible assets that could result in significant future amortization expense.
Moreover, if we may not be able tocannot locate suitable acquisition or strategic collaboration opportunities, and this inability could impair our ability to grow or obtain access to technology or products that may be important to the development of our business may be impaired.
The illegal distribution and sale by third parties of counterfeit or stolen versions of mRNA products, or the unauthorized donation or re-sale of mRNA products, could negatively impact our financial performance or reputation.
Third parties could illegally distribute and sell, especially online, counterfeit versions of mRNA products that do not meet the rigorous cGMP manufacturing and testing standards. Counterfeit medicines may contain harmful substances or the wrong dose, are frequently unsafe or ineffective and could be life-threatening. However, to distributors and users, counterfeit products may be visually indistinguishable from the authentic version.
Reports of adverse reactions to counterfeit products, increased levels of counterfeiting or unsafe mRNA products could materially affect patient confidence in our mRNA products. Adverse events caused by unsafe counterfeit or other non-mRNA products could mistakenly be attributed to our mRNA products. In addition, thefts of inventory at warehouses, plants or while in-transit, which are not properly stored and which are sold through unauthorized channels, could adversely impact patient safety, our reputation and our business. Public loss of confidence in the integrity in mRNA products as a result of counterfeiting, theft or improper manufacturing processes could have a material adverse effect on our business, results of operations and financial condition. Further, the unauthorized donation or resale of our product could adversely affect our ability to sell in a particular territory, and have other adverse effects on our business, results of operations and financial condition.
Climate change or legal, regulatory or market measures to address climate change may negatively affect our business, results of operations and financial condition.
We are exposed to physical risks (such as rising temperatures, flooding and severe storms), risks in transitioning to a low-carbon economy (such as additional legal or regulatory requirements, changes in customer behavior and cost and availability of raw materials) and social and human effects (such as population dislocations and harm to health and well-being) associated with climate change. These risks can be either acute (short-term) or chronic (long-term). Climate-related physical risks to our facilities and those of our suppliers could disrupt our operations and supply chain, which may result in increased costs.
New legal or regulatory requirements may be enacted to prevent, mitigate or adapt to the implications of a changing climate and its effects on the environment. These regulations, which may differ across jurisdictions, could subject us to new or expanded carbon pricing or taxes, increased compliance costs, restrictions on greenhouse gas emissions, investment in new technologies, increased carbon disclosure and transparency, investments in data gathering and reporting systems, upgrades of facilities to meet new building codes and the redesign of utility systems, which could increase our operating costs, including the cost of electricity and energy we use. Our supply chain would likely be subject to these same transitional risks and would likely pass along any increased costs to us, which may affect our ability to procure raw materials or other supplies required to operate our business at the quantities and levels we require.
Our aspirations, goals and disclosures related to environmental, social and governance (ESG) matters expose us to numerous risks.
Institutional and individual investors are increasingly using ESG screening criteria to determine whether we qualify for inclusion in their investment portfolios. We are frequently asked by investors and other stakeholders to set ambitious ESG goals and provide new and more robust disclosure on goals, progress toward goals and other matters of interest to ESG stakeholders. In response, we have adapted the tracking and reporting of our corporate responsibility program to various evolving ESG frameworks, and we have established and announced goals and other objectives related to ESG matters. Statements about these goals reflect our current plans and aspirations and are not guarantees that we will be able to achieve them. Our efforts to accomplish and accurately report on these goals and objectives, including with respect to environmental and diversity initiatives, are subject to numerous risks, many of which are outside of our control, which could have a material negative impact, including on our reputation and stock price.
Further, the standards for tracking and reporting on ESG matters are relatively new, have not been harmonized and continue to evolve. Our selection of disclosure frameworks that seek to align with various reporting standards may change from time to time and may result in a lack of consistent or meaningful comparative data from period to period. In addition, our processes and controls may not always comply with evolving standards for identifying, measuring and reporting ESG metrics, our interpretation of reporting standards may differ from those of others and such standards may change over time, any of which could result in significant revisions to our goals or reported progress in achieving such goals.
If our ESG practices do not meet evolving investor or other stakeholder expectations and standards, then our reputation, our ability to attract or retain employees and our attractiveness as an investment, business partner or acquiror could be negatively impacted. Similarly, our failure or perceived failure to pursue or fulfill our goals, targets and objectives or to satisfy various reporting standards within the timelines we announce, or at all, could also have similar negative impacts and expose us to government enforcement actions and private litigation.
If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could harm our business.
We are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous and flammable materials and wastes, including chemicals and biological materials. We generally contract with third parties for the disposal of these materials and waste products, and we cannot eliminate the risk of contamination or injury from these materials. In the event of contamination or injury resulting from any use by us of hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines, penalties or other sanctions for failure to comply with such laws and regulations. We may also incur substantial costs to comply with such laws and regulations, and these laws and regulations could impair our research, development or production efforts.
Our workers’ compensation insurance may not provide adequate coverage against potential liabilities due to injuries to our employees resulting from the use of hazardous materials. We do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of biological or hazardous materials.
Risks related to ownership of our common stock
The price of our common stock has been volatile, and fluctuates substantially, which could result in substantial losses for stockholders.
Our stock price has been, and in the future, mayis expected to continue to be, subject to substantial volatility. FromSince our IPO in December 7, 2018, our first day of trading on the Nasdaq Global Select Market, through December 31, 2020, ourour stock price has traded within a range ofranged from a high price of $178.50and$497.49 to a low price of $11.54 per share. In addition, sinceSince we began our COVID-19 vaccine development efforts with respect to our COVID-19 vaccine in early 2020, our stock has experienced pronounced and extended periods of volatility. As a result of the volatility, in our stock price,which could cause our stockholders couldto incur substantial losses.
Public statements by us, government agencies, the media, competing vaccine developers,competitors, financial analysts or others relating to the coronavirus outbreak (including regarding our and others’ efforts to develop a coronavirus vaccine)COVID-19 have in the past resulted, and may in the future result, in significant fluctuations in our stock price. Given the global focus on the coronavirus pandemic, informationInformation in the public arena on this topic, whether or not accurate, has had and will likely continue to have an outsized impact (positive or negative) on our stock price. Information related to our clinical trials, manufacturing, regulatory and commercialization efforts with respect to our COVID-19 vaccine, or information regarding such efforts by competitors with respect to their vaccines and potential vaccines, or the evolution of the pandemic, may meaningfully impact our stock price.
The stock market in general, and the market for biopharmaceutical companies in particular, has experienced extreme volatility that has often been unrelated to the operating performance of particular companies. As a result of this volatility,companies, and you may not be able to sell your common stockshares at or above your initial purchase price. The market price for our common stock may be influenced by many factors, including:
•the successcommercial launch of our COVID-19 vaccine sales;any additional products;
•timing and results of clinical trials or progress of our investigational medicinesproduct candidates or those of our competitors;
•the success of competitive products or technologies;
•commencementthe emergence or terminationdecline of strategic alliances;new or existing variants of the SARS-CoV-2 virus;
•developments regarding our manufacturing, regulatory and commercialization efforts, or information regarding such efforts by competitors;
•regulatory or legal developments in the United States and other countries;
•developments or disputes concerning patent applications, issued patents or other proprietary rights;
•the recruitment or departure of key personnel;
•product revenue;
•the level of expenses related to any of our products investigational medicines or clinical development programs;
•the results of our efforts to discover, develop, acquire or in-license additional investigational medicines;product candidates;
•actual or anticipated changes in estimates as toof financial results, development timelines or recommendations by securities analysts;
•variations in our financial results or those of companies that are perceived to be similar to us;
•changes in the structure of healthcare payment systems;
•market conditions in the pharmaceutical and biotechnology sectors;
•general economic, industry and market conditions;
•conditions generally, and in the numerous programs in our pipeline, the development of which could each generate news or significant adverse events that could impact financial results or recommendations by securities analysts;biopharmaceutical sector specifically; and
•public announcements by us or our strategic collaborators regarding the progress of our development candidates or investigational medicines or similar public announcements by our competitors.
If our quarterly or annual results fall below the expectations of investors or securities analysts, the price of our common stock could decline substantially. Furthermore, any quarterly or annual fluctuations in our results may, in turn, cause the price of our stock to fluctuate substantially. We believe that period-to-period comparisons of our results are not necessarily meaningful and should not be relied upon as an indication of our future performance.
Our stock price is likely to continue to be volatile and subject to significant price and volume fluctuations in response to market and other factors, including the other factors discussed in our filings incorporated by reference herein or in future periodic reports; variations in our quarterly operating results from our expectations or those of securities analysts or investors; downward revisions in securities analysts’ estimates; and announcement by us or our competitors of the commencement or termination of significant acquisitions, strategic partnerships, joint ventures or capital commitments.
In the past, following periods of volatility in the market price of a company’s securities, securitiesSecurities class-action litigation often has been instituted against that company. Suchcompanies following periods of volatility in their stock price. If such litigation ifwere instituted against us, we could cause us to incur substantial costs to defend such claimsin defense and divert management’s attention and resources could be diverted, which could seriously harmadversely affect our business, financial condition and results of operations and prospects.
We have broad discretion in the use of our cash, cash equivalents, and investments, and may not use them effectively.
Our management will have broad discretion in the application of our cash, cash equivalents, and investments, and could spend the proceeds in ways that do not improve our results of operations or enhance the value of our common stock. Furthermore, our operating expenses have significantly increased due to development and manufacturing activities for our COVID-19 vaccine program, and we may not deploy our expanded capital base effectively. The failure by our management to apply these funds effectively could result in financial losses that could have a material adverse impact on our business, cause the price of our common stock to decline, and delay the development of our investigational medicines. Pending their use, we may invest our cash, cash equivalents, and investments in a manner that does not produce income or that loses value.
We are in the early stages of developing our policies and practices regarding pre-approval access and any policy we develop and implement may result in a negative perception of our Company and have a material adverse impact on our business.
As we advance our pipeline, patients and their physicians have sought access to our investigational medicines outside of sponsored clinical trials and prior to regulatory approval. While we will continue to review and respond to these early access requests, at this stage in our development of a new class of medicines, we are not providing access to our investigational medicines outside of the clinical trial setting. As our development programs progress further, we will continue our dialogue with patients and their families, advocacy leaders, physicians, and others on this and other topics. We will post our pre-approval access policies in accordance with regulatory guidelines.
Sales of a substantial number of shares of our common stock by our existing stockholders in the public market could cause our stock price to fall.
Sales of a substantial number of shares of our common stock in the public market could occur at any time, subject to certain restrictions described below. These sales, or the perception in the market that holders of a large number of shares intend to sell shares, could reduce the market price of our common stock.
The holders of up to 61.1 million shares of our common stock are entitled to rights with respect to the registration of their shares under the Securities Act. Registration of these shares under the Securities Act would result in the shares becoming freely tradable without restriction under the Securities Act, except for shares held by affiliates, as defined in Rule 144 under the Securities Act. Any sales of securities by these stockholders could have a material adverse effect on the trading price of our common stock.
Additionally, the number of shares of our common stock reserved for issuance under our 2018 Stock Option and Incentive Plan automatically increased on January 1, 2020 and will automatically increase each January 1 thereafter by 4% of the number of shares of common stock outstanding on the immediately preceding December 31 or such lesser number of shares determined by our compensation committee. Our Compensation Committee elected not to increase the number of shares reserved for issuance under our 2018 Stock Plan on January 1, 2021. If our Compensation Committee elects to increase the number of shares available for future grant each year, our stockholders may experience additional dilution.
In addition, certain of our employees, executive officers, and directors have entered or may enter into Rule 10b5-1 trading plans providing for sales of shares of our common stock from time to time. Under a Rule 10b5-1 trading plan, a broker executes trades pursuant to parameters established by the employee, director, or officer when entering into the plan, without further direction from the employee, officer, or director. A Rule 10b5-1 trading plan may be amended or terminated in some circumstances. Our employees, executive officers, and directors also may buy or sell additional shares outside of a Rule 10b5-1 trading plan when they are not in
possession of material, nonpublic information. To the extent that sales or other distributions of Moderna stock by our executive officers or directors are reported publicly, whether or not conducted under a 10b5-1 plan, such sales or distributions could lead to a decline in our stock price.
Raising additional capital may cause dilution to our existing stockholders, restrict our operations, or require us to relinquish rights to our technologies or development candidates or investigational medicines.
We may seek additional capital through a combination of public and private equity offerings, debt financings, strategic alliances, and licensing arrangements. To the extent that we raise additional capital through the sale of equity or debt securities, your ownership interest will be diluted and the terms may include liquidation that adversely affect your rights as a stockholder. The incurrence of indebtedness would result in increased fixed payment obligations and could involve restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire or license IP rights, and other operating restrictions that could adversely impact our ability to conduct our business. If we raise additional funds through collaborations and alliances and licensing arrangements with third parties or through asset sales, we may have to relinquish valuable rights to our technologies or development candidates or investigational medicines, or grant licenses on terms unfavorable to us.
Our principal stockholders and management own a significant percentage of our stock and will be able to exert significant control over matters subject to stockholder approval.
OurAs of February 16, 2024, our executive officers, directors five percentand affiliated stockholders and their affiliates beneficially ownowned, directly or indirectly, approximately 21.1% 12% of our outstanding common stock, asstock. In addition, non-affiliated five percent or greater stockholders owned approximately 27% of February 16, 2021. Therefore, theseour outstanding common stock. These stockholders will have the ability to influence us through their ownership positions. For example, these stockholders, actingif they were to act together, may be able tothey could exert significant influence over matters such as elections of directors, amendments of our organizational documents or approval of any merger, sale of assets or other major corporate transaction. This may prevent or discourage unsolicited acquisition proposals or offers for our common stock that youour stockholders may believe areto be in yourtheir best interest as one of our stockholders.interests.
Provisions in our amended and restated certificate of incorporation and by-laws,organizational documents, as well as provisions of Delaware law, could make it more difficult or costly for a third party to acquire us or increase the cost of acquiring us,remove our current management, even if doing so would benefit our stockholders or remove our current management.stockholders.
Our amended and restated certificate of incorporation (charter), amended and restated by-laws (by-laws) and Delaware law contain provisions that may have the effect of delayingcould delay or preventingprevent a hostile takeover or change in control of us or changes in our management. Our amendedcharter and restated certificate of incorporation and amended and restated by-laws include provisions that:
•authorize “blank check” preferred stock, which could be issuedauthorized for issuance by our board of directors without stockholder approval and may contain voting, liquidation, dividend and other rights superior to our common stock;
•create a classified board of directors whose members serve staggered three-year terms;
•specify that special meetings of our stockholders can be called only by our board of directors, the chairperson of our board of directors, our chief executive officer, or our president;directors;
•prohibit stockholder action by written consent;
•establish an advance notice procedure for stockholder approvals to be brought before an annual meeting of our stockholders, including proposed nominations of persons for election to our board of directors;
•provide that our directors may be removed only for cause;
•provide that vacancies on our board of directors may be filled only by a majority of directors then in office, even though less than a quorum;
•specify that no stockholder is permitted to cumulate votes at any election of directors;
•expressly authorize our board of directors to modify, alter or repeal our amended and restated by-laws; and
•require supermajority votes of the holders of our common stock to amend specified provisions of our amendedcharter and restated certificate of incorporation and amended and restated by-laws.
These provisions, alone or together, could delay or prevent hostile takeovers and changes in control or changes in our management.
In addition, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which limits the ability of stockholders owning in excess of 15% of our outstanding voting stock to merge or combine with us.
Any provision of our amended and restated certificate of incorporation or amended and restatedcharter, by-laws or Delaware law that has the effect of delaying or deterring a change in control could limit the opportunity for our stockholders to receive a premium for their shares, of our common stock, and could also affect the price that some investors are willing to pay for our common stock.
Because we do not anticipate paying any cash dividends on our capital stock in the foreseeable future, capital appreciation, if any, will be your sole source of gain.
We do not currently intend to declare or pay cash dividends on our capital stock. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business.business or to return cash to shareholders through share repurchases. In addition, the terms of any future debt agreements may preclude us from paying dividends. As a result, capital appreciation, if any, of our common stock will be your sole source of gain for the foreseeable future.future.
Our amended and restated by-laws designate the Court of Chancery of the State of Delaware or the United StatesU.S. District Court for the District of Massachusetts as the exclusive forum for certain litigation that may be initiated by our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us.
Pursuant to our amended and restated by-laws, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware is the sole and exclusive forum for state law claims for (1) any derivative action or proceeding brought on our behalf, (2) any action asserting a claim of or based on a breach of a fiduciary duty owed by any of our current or former directors, officers, or other employees to us or our stockholders, (3) any action asserting a claim against us or any of our current or former directors, officers, employees or stockholders arising pursuant to any provision of the Delaware General Corporation Law or our amended and restated by-laws or (4) any action asserting a claim governed by the internal affairs doctrine (the Delaware Forum Provision). The Delaware Forum Provision will not apply to any causes of action arising under the Securities Act or the Exchange Act. Our amended and restated by-laws further provide that the United StatesU.S. District Court for the District of Massachusetts is the exclusive forum for resolving any complaint asserting a cause of action arising
under the Securities Act (the Federal Forum Provision). We have chosen the United States District Court for the District of Massachusetts as the exclusive forum for such causes of action because our principal executive offices are located in Cambridge, Massachusetts. In addition, our amended and restatedOur by-laws provide that any person or entity purchasing or otherwise acquiring any interest in shares of our common stock is deemed to have notice of and consented to the Delaware Forum Provision and the Federal Forum Provision; provided, however, that stockholders will not be deemed to have waived our compliance with the federal securities laws and the rules and regulations thereunder.Provision.
We recognize that theThe Delaware Forum Provision and the Federal Forum Provision may impose additional litigation costs on stockholders in pursuing any such claims, particularly if the stockholders do not reside in or near the State of Delaware or the Commonwealth of Massachusetts, as applicable. Additionally, the forum selection clauses in our amended and restated by-laws may limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers or employees, which may discourage the filing of lawsuits against us and our directors, officers and employees, even though an action, if successful, might benefit our stockholders. While the Delaware Supreme Court ruled in March 2020 that federal forum selection provisions purporting to require claims under the Securities Act be brought in federal court are “facially valid” under Delaware law, there is uncertainty as to whether other courts will enforce our Federal Forum Provision. The Federal Forum Provision may also impose additional litigation costs on stockholders who assert that the provision is unenforceable or invalid, and if the Federal Forum Provision is found to be unenforceable, we may incur additional costs in resolving such matters. The Court of Chancery of the State of Delaware and the United StatesU.S. District Court for the District of Massachusetts may also reach different judgments or results than would other courts, including courts where a stockholder considering an action may be located or would otherwise choose to bring the action, and such judgments may be more or less favorable to us than our stockholders.
General risk factors
Unfavorable U.S. or global economic conditions could adversely affect our business, financial condition, or results of operations.
Our results of operations could be adversely affected by general conditions in the global economy and financial markets, including by the current COVID-19 pandemic, or any other health epidemic. The most recent global financial crisis caused extreme volatility and disruptions in the capital and credit markets. A severe or prolonged economic downturn, such as the most recent global financial crisis, could result in a variety of risks to our business, including weakened demand for our investigational medicines and our ability to raise additional capital when needed on favorable terms, if at all. A weak or declining economy could strain our suppliers, possibly resulting in supply disruption, or cause delays in payments for our services by third-party payors or our collaborators. Any of the foregoing could harm our business and we cannot anticipate all of the ways in which the current economic climate and financial market conditions could adversely impact our business.
Our business and operations in the UK and EU may be negatively impacted by the United Kingdom’s withdrawal from the EU.
On June 23, 2016, the UK held a referendum in which a majority of voters approved an exit from the EU, or Brexit. After nearly three years of negotiation and political and economic uncertainty, the UK’s withdrawal from the EU became effective on January 31, 2020. There was a transitional period, during which EU laws, including pharmaceutical laws, continued to apply in the UK, however this
ended on December 31, 2020. The UK and EU have signed a EU-UK Trade and Cooperation Agreement, which became provisionally applicable on January 1, 2021 and will become formally applicable once ratified by both the UK and the EU. This agreement provides details on how some aspects of the UK and EU’s relationship regarding medicinal products will operate, particularly in relation to Good Manufacturing Practice, however there are still many uncertainties. The long-term effects of Brexit will depend in part on how the EU-UK Trade and Cooperation Agreement, and any future agreements signed by the UK and the EU, take effect in practice. Such a withdrawal from the EU is unprecedented, and it is unclear how the restrictions on the UK’s access to the European single market for goods, capital, services and labor within the EU and the wider commercial, legal and regulatory environment, could impact our current and future operations and clinical activities in the UK.
We expect that, now the transition period has expired, Brexit could lead to legal uncertainty and potentially divergent national laws and regulations as the UK determines which EU laws to replicate or replace, including those related to the regulation of medicinal products. Any of these effects of Brexit, and others we cannot anticipate, could negatively impact our business and results of operations in the UK.
The uncertainty concerning the UK’s legal, political and economic relationship with the EU following Brexit may also be a source of instability in the international markets, create significant currency fluctuations, and/or otherwise adversely affect trading agreements or similar cross-border co-operation arrangements (whether economic, tax, fiscal, legal, regulatory or otherwise).
Our employees, principal investigators and consultants may engage in misconduct or other improper activities, including non-compliance with regulatory standards and requirements and insider trading.
We are exposed to the risk of fraud or other misconduct by our employees, principal investigators leading our clinical trials and consultants. Misconduct by these partiesSuch misconduct could include intentional failures to comply with FDA regulations or thesimilar regulations applicable in the EU and other jurisdictions;jurisdictions, provide accurate information to the FDA, the EMA and other regulatory authorities;authorities, comply with healthcare fraud and abuse laws and regulations in the United States and abroad;abroad or report financial information or data accurately or disclose unauthorized activities to us. Such misconduct also could involve the improper use of information obtained in the course ofduring clinical trials or interactions with the FDA or other regulatory authorities, which could result in regulatory sanctions and cause serious harm to our reputation. Sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These laws and regulations restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. We have adopted a code of conduct applicable to all of our employees, but itIt is not always possible to identify and deter employee misconduct, and the precautions we takemay fail to detect and prevent this activity may not be effective in controllingcontrol unknown or unmanaged risks or losses or in protectingtake steps that protect us from government investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, financial condition, results of operations and prospects, including through the imposition of significant fines or other sanctions.
Unfavorable U.S. or global economic conditions, including as a result of disease outbreak, war, conflict or other political instability, or geopolitical risks, could adversely affect our business, financial condition or results of operations.
Our results of operations could be adversely affected by general conditions in the global economy and financial markets, including disruptions caused by pandemic, war, conflict or other political instability, including Russia’s invasion of Ukraine and resulting sanctions against Russia or conflict in the Middle East. Adverse macroeconomic conditions, and perceptions or expectations about current or future conditions, such as inflation, slowing growth, rising interest rates, rising unemployment and recession, could negatively affect our business and financial condition. Additionally, global events, including war, conflict, political instability or other adverse economic conditions have and may in the future cause governments to divert spending away from healthcare, negatively impacting the marketability of our products.
Any severe or prolonged economic downturn could create a variety of risks to our business, including weakened demand for our medicines, and negatively impacting our ability to raise additional capital or financing when needed on favorable terms, if at all. A weak or declining economy could strain our suppliers, possibly resulting in supply disruption, or cause delays in payments for our services by third-party payors or our collaborators. Any of the foregoing could harm our business and we cannot anticipate all the ways in which the current economic climate and financial market conditions could adversely impact our business.
Additionally, geopolitical tensions could adversely impact our business and our commercial plans. For instance, restrictions by the U.S. government on the export of mRNA technology or our products to certain markets, or restrictions on the establishment of manufacturing in certain foreign jurisdictions, may prevent us from seeking commercial growth opportunities in a manner that harms our competitive position.
Employee litigation and unfavorable publicity could negatively affect our future business.
Our employees may, from time to time, bring lawsuits against us regarding injury, creating a hostile work place,workplace, discrimination, wage and hour disputes, sexual harassment or other employment issues. In recent yearsRecently, there has been an increase in the number of discrimination and harassment claims generally. Coupled with the expansion of social media platforms and similar devices that allow individuals access to a broad audience, these claims have had a significant negative impact on some businesses. Certain companies that have faced employment- or harassment-related lawsuits have had to terminate management or other key personnel, and have suffered reputational harm that hasharm. Any employment-related claim could negatively impacted their business. If we were to face any employment-related claims, our business could be negatively affected.
If we fail to comply with environmental, health, and safety laws and regulations, we could become subject to fines or penalties or incur costs that could harmaffect our business.
We are subject to numerous environmental, health,Ineffective internal controls could adversely impact our business and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment, and disposal of hazardous materials and wastes. Our operations involve the use of hazardous and flammable materials, including chemicals and biological materials. Our operations also may produce hazardous waste products. We generally contract with third parties for the disposal of these materials and wastes. We will not be able to eliminate the risk of contamination or injury from these materials. In the event of contamination or injury resulting from any use by us of hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties for failure to comply with such laws and regulations.operating results.
Although we maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials, this insuranceOur internal control over financial reporting may not prevent or detect misstatements because of its inherent limitations, including the possibility of human error, failure or interruption of information technology systems, the circumvention or overriding of controls, or fraud. Even effective internal controls can provide adequate coverage against potential
Tableonly reasonable assurance with respect to the preparation and fair presentation of Contents
liabilities. We do notfinancial statements. If we fail to maintain insurance for environmental liability or toxic tort claims that may be asserted against us in connection withthe adequacy of our storage or disposal of biological or hazardous materials.
In addition, we may incur substantial costs in order to comply with current or future environmental, health, and safety laws and regulations. These current or future laws and regulations may impair our research, development, or production efforts. Ourinternal controls, including any failure to comply with these lawsimplement required new or improved controls, or if we experience difficulties in their implementation, our business and regulations also may result in substantial fines, penalties or other sanctions.operating results could be harmed and we could fail to meet our financial reporting obligations.
Changes in tax law could adversely affect our business and financial condition.
The Company isWe are subject to evolving and complex tax laws in the jurisdictions in which it operates.we operate. The rules dealing with U.S. federal, state, and local and non-U.S. income taxation are constantly under review by persons involved in the legislative process and by tax authorities. Changes to tax laws (which changes may have retroactive application)could apply retroactively) could adversely affect us or holders ofand our common stock.stockholders. In recent years, many such changes have been made and changes are likely to continue to occur in the future. Future changes in tax lawsfuture, which could have a material adverse effect on our business, cash flow, financial condition orand results of operations. We urge investors to consult with their legal and tax advisers regarding the implications of potential changes in tax laws on an investment in our common stock.
If the estimates we make, or the assumptions on which we rely, in preparing our consolidated financial statements prove inaccurate, our actual results may vary from those reflected in our projections and accruals.
Our consolidated financial statements have been prepared in accordance with U.S. generally accepted accounting principles. The preparation of these consolidated financial statements requires us to make estimates and judgments that affect the reported amounts of our assets, liabilities, revenues, and expenses, the amounts of charges accrued by us and related disclosure of contingent assets and liabilities. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances. We cannot assure you, however, that our estimates, or the assumptions underlying them, will be correct.
The increasing use of social media platforms presents new risks and challenges.
Social media is increasingly being used to communicate about our research, developmentproduct candidates, investigational medicines,commercial products and the diseases our developmentproducts and product candidates and investigational medicines are being developeddesigned to treat. Social media practices in the biopharmaceutical industry continue to evolve and regulations relating to such use are not always clear. This evolutionuncertainty creates uncertainty and risk of noncompliance with regulations applicable to our business, resulting in potential regulatory actions against us. For example, subjects may use social media channels to comment on their experience in an ongoing blinded clinical trial or to report an alleged adverse event. When such disclosures occur, there is a risk that we may fail to monitor and comply with applicable adverse event reporting obligations or we may not be ableunable to defend our business or the public’s legitimate interests in the face of the political and market pressures generated by social media due to restrictions on what we may say about our development candidates and investigational medicines.product candidates. There is also a risk of inappropriate disclosure of sensitive information or negative or inaccurate posts or comments about us on any social networking website. If any of these events were to occur or we otherwise fail to comply with applicable regulations, we could incur liability, face regulatory actions or incur other harm to our business.
We have incurred and will continue to incur increased costs as a result of operating as a public company, and our management is required to devote substantial time to new compliance initiatives. We are subject to financial reporting and other requirements for which our accounting and other management systems and resources may not be adequately prepared.
As a public company, we incur significant legal, accounting, and other expenses that we did not incur as a private company. In addition, the federal securities laws, including the Sarbanes-Oxley Act of 2002 and rules subsequently implemented by the SEC and Nasdaq have imposed various requirements on public companies, including requirements to file annual, quarterly, and event driven reports with respect to our business and financial condition, and to establish and maintain effective disclosure and financial controls and corporate governance practices. Our management and other personnel devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations increase our legal and financial compliance costs and make some activities more time consuming and costly. For example, these rules and regulations may make it more difficult and more expensive for us to obtain director and officer liability insurance. We may not be able to produce reliable financial statements or file these financial statements as part of a periodic report in a timely manner with the SEC or comply with the Nasdaq listing requirements. In addition, we could make errors in our financial statements that could require us to restate our financial statements.
Pursuant to Section 404 of the Sarbanes-Oxley Act of 2002, or Section 404, we are required to furnish a report by our management on our internal control over financial reporting, including an attestation report on internal control over financial reporting issued by our independent registered public accounting firm. However, while we were an “emerging growth company” as defined in the Jumpstart Our Business Startups Act, our auditors were not required to formally attest to the effectiveness of our internal control over financial reporting. As of the end of our fiscal year ended December 31, 2019, we qualified as a “large accelerated filer” as defined in the
Securities Exchange Act of 1934, as amended, or the Exchange Act and, as a result, ceased to qualify as an emerging growth company. Accordingly, commencing with our Annual Report on Form 10-K for the year ended December 31, 2019, we were required to have our auditors formally attest to the effectiveness of our internal control over financial reporting pursuant to Section 404. Our compliance with Section 404 necessitates that we incur substantial accounting expense and expend significant management efforts. We will continue to dedicate internal resources, potentially engage outside consultants, and adopt a detailed work plan to assess and document the adequacy of internal control over financial reporting, continue steps to improve control processes as appropriate, validate through testing that controls are functioning as documented, and implement a continuous reporting and improvement process for internal control over financial reporting. Despite our efforts, there is a risk that neither we nor our independent registered public accounting firm will be able to conclude within the prescribed timeframe that our internal control over financial reporting is effective as required by Section 404. This could result in an adverse reaction in the financial markets due to a loss of confidence in the reliability of our financial statements.
Item 1B -1B. Unresolved Staff Comments
None.
Item 1C. Cybersecurity
Cyber Risk Management and Strategy
Our cybersecurity organization’s mission is to provide a targeted set of services, support and capabilities to reduce the risk of cyberattacks, rapidly detect and contain threats, and mitigate risks to critical data.
Recognizing the threat of security breaches and cyberattacks globally, we have developed a cybersecurity program, overseen by our Chief Information Security Officer (CISO) and Chief Information Officer (CIO), that is designed to protect patient trust, defend the Moderna brand, and reduce the risk and impact of cyber-attacks. Our cybersecurity program is informed by industry standards and includes periodic risk assessments and security testing supported by cybersecurity technologies, including third-party security solutions, vulnerability management, and monitoring tools, designed to monitor, identify, and manage risks from cyber threats. In addition, we have implemented employee security and awareness training.
Management has established a cyber incident response plan (CIRP) designed to assess, identify and manage risks from cybersecurity threats and enable prompt response in the event that a cybersecurity incident is detected. We have a process in place for notification to our leadership response team in the event of a significant cyber incident, and for escalation of these events to our Audit Committee and Board, as appropriate. To date, we have not experienced a cybersecurity incident that has had a material impact on our business strategy, results of operations, or financial condition.
We undergo several annual internal compliance audits and external reviews to evaluate our controls, including cybersecurity controls. In an effort to minimize third-party risk, we have established a process to assess the security practices of third-party suppliers and related risks, including through review of relevant supplier certifications and security and responses to standardized information gathering (SIG) questionnaires, as applicable and appropriate.
Governance Related to Cybersecurity Risks
Our Board of Directors oversees Moderna’s overall risk management strategy. The Board exercises oversight of risks from cybersecurity threats primarily through its Audit Committee, which oversees our risk management processes for information security and technology risks. Our cybersecurity risk management processes are integrated into our overall risk management strategy, which is overseen by the Audit Committee. At least annually, the Audit Committee discusses our risk management program, including information security and technology risks and findings from any audits, with our internal audit staff.
The Audit Committee receives cyber-related updates from management, including our CISO at committee meetings. During meetings, our CISO updates the committee on Moderna’s cybersecurity posture, potential threats and risk mitigation strategies, and the progress of the Company’s cybersecurity initiatives, as appropriate. The Chair of the Audit Committee and management provide regular briefings on such matters to the full Board of Directors, as appropriate.
At the management level, our CISO is primarily responsible for leading our cybersecurity strategy for assessing and managing material risks from cybersecurity threats. Our current CISO has over 25 years of cybersecurity experience across a wide array of industries, most recently serving in leadership positions at two different public companies and previous roles of increasing responsibility at multinational technology companies. Our CISO reports directly to our CIO, who is a member of our Executive Committee and reports to our Chief Executive Officer.
We have built a cybersecurity leadership team designed to align with key services, with a separate lead overseeing each service offering, all reporting to the CISO. We also maintain relationships with law enforcement and industry groups to support our cybersecurity intelligence and risk management efforts.
Item 2. Properties
We have two main campuses in Massachusetts. During the third quarter of 2023, we commenced a lease for a property in Cambridge, Massachusetts. This building, spanning approximately 462,000 square feet, is designated as our new Moderna Science Center (MSC). The MSC will accommodate a combination of scientific and office spaces, including our principal executive offices. The lease has a term of 15 years, with options for us to extend the lease for up to two additional seven-year terms. We expect to begin a phased move-in process starting in early 2024. Additionally, we occupy a multi-building campus inat Technology Square near the Kendall Square area in Cambridge, MA withMassachusetts, consisting of a mix of offices and research laboratory space, totaling approximately 175,000292,000 square feet. Kendall SquareThe lease will expire in early 2025. The Cambridge campus is the location of our corporate headquarters, platform, drug discovery manufacturing process development, and clinical development. Our facilities in Kendall Square are leased with the majority of the space being leased through 2029, with the option to extend.
We have a manufacturing facility in Norwood, MA,The Moderna Technology Center (MTC). The campus is located in Norwood, Massachusetts and is primarily comprised of twothree buildings (MTC South, MTC North and MTC North)East). The MTC Southcampus is approximately 200,000686,000 square feet with a production capacity of over 100 cGMP lots per year. MTC North is approximately 240,000 square feet and provideswhich includes lab and office space, directly supporting improvement in our manufacturing capabilities.capabilities and commercial and clinical activities. The MTC Southcampus is leased through 20322042 and we have the option to extend it for two ten-year terms. MTC North is leased into early 2031 and we have the option to extend it for up to fourthree additional five-year terms.
In the second quarter of 2023, we acquired a newly constructed biomanufacturing facility, encompassing 140,000 square feet, in Marlborough, Massachusetts. This facility is undergoing enhancements, including the addition of 60,000 square feet to the existing structure. Upon completion, the facility will feature office and mRNA manufacturing areas, including a full manufacturing clean room, quality control laboratories, a just-in-time satellite warehouse, and additional office spaces. We expect the facility to be operational in 2025. This new site is strategically intended to support our INT program.
We also own and lease various parcels of land, office and lab spaces across the globe for our business operations.
Item 3. Legal Proceedings
We are involved in various claims and legal proceedings of a nature considered ordinary course in our business, including the
intellectual property litigation described below. Most of the issues raised by these claims are highly complex and subject to substantial uncertainties. For a description of risks relating to these and other legal proceedings we face, see Part I, Item 1A, “Risk Factors,” including the discussion under the headings entitled “Risks related to our intellectual property” and “Risks related to the manufacturing of our commercial products and product candidates.”
The outcome of any such proceedings, regardless of the merits, is inherently uncertain; therefore, assessing the likelihood of loss and any estimated damages is difficult and subject to considerable judgment. We describe below those legal matters for which a material loss is either (i) possible but not currentlyprobable, and/or (ii) not reasonably estimable at this time.
Pfizer/BioNTech Patent Litigation
In August 2022, we filed a partylawsuit in the U.S. District Court for the District of Massachusetts against Pfizer Inc. (Pfizer) and BioNTech SE, BioNTech Manufacturing GmbH and BioNTech US Inc. (collectively, BioNTech), asserting infringement of certain U.S. patents concerning our mRNA platform technology and disease-specific vaccine designs in Pfizer and BioNTech’s manufacture and sale of their mRNA COVID-19 vaccines. The complaint seeks a judgment of infringement of the asserted patents and monetary damages.
Also in August 2022, we initiated patent infringement proceedings in Germany (in the Dusseldorf Regional Court), the Netherlands (in the District Court of The Hague) and the UK (in the High Court of Justice of England & Wales) against Pfizer, BioNTech and related entities with respect to any material legal proceedings.certain European patents that also concern our mRNA platform technology and disease-specific vaccine designs, including coronaviruses. In May 2023, we initiated patent infringement proceedings in Ireland (in the High Court) and Belgium (Brussels Business Court) against Pfizer, BioNTech and related entities with respect to the same European patents. As in the U.S. action, we seek a judgment of infringement of the asserted patents and monetary damages.
Pfizer Inc. and BioNTech SE have also filed an action seeking revocation of certain Moderna patents in the UK. In addition, the Moderna patents being asserted in the European actions are subject to notices of opposition, including by Pfizer and BioNTech SE and others. These actions seek to revoke the patents, which have been filed at the European Patent Office.
Proceedings Related to Patents Owned by Arbutus
In February 2022, Arbutus Biopharma Corporation (Arbutus) and Genevant Sciences GmbH (Genevant) filed a complaint against us in the U.S. District Court for the District of Delaware asserting that our manufacture and sale of our COVID-19 vaccine willfully infringes certain U.S. patents concerning lipid nanoparticles. The complaint seeks a judgment of infringement of the asserted patents and monetary damages, but does not seek to prevent or stop the marketing or sales of our COVID-19 vaccines.
Proceedings Related to Patents Owned by Alnylam
In March 2022 and July 2022, Alnylam Pharmaceuticals, Inc. (Alnylam) filed two complaints against us in the U.S. District Court for the District of Delaware asserting that our manufacture and sale of our COVID-19 vaccine infringes certain U.S. patents concerning cationic lipids. On August 25, 2023, the Court entered a Final Judgment of non-infringement of all asserted patents in these lawsuits. Alnylam has appealed this judgment to the Federal Circuit Court of Appeals. In May 2023, Alnylam filed a third complaint against us in the U.S. District Court for the District of Delaware asserting three additional U.S. patents concerning cationic lipids. The complaints seek judgments of infringement of the asserted patents and monetary damages, but do not seek to prevent or stop the marketing or sales of our COVID-19 vaccines.
Item 4. Mine Safety Disclosures
Not applicable.
PART II
Item 5. Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market for Our Common Stock
Our common stock began tradingtrades on the Nasdaq Global Select Market under the symbol “MRNA” on December 7, 2018. Prior to that time, there was no public market for our common stock..
Stock Performance Graph
The following performance graph shall not be deemed “soliciting material” or to be “filed” with the Securities and Exchange CommissionSEC for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or the Exchange Act or otherwise subject to the liabilities under that Section,section, and shall not be deemed to be incorporated by reference into any filing of Moderna, Inc. under the Securities Act or the Exchange Act.
The following graph showsillustrates a comparison from December 7, 2018,for the date on which our common stock first began trading on the Nasdaq Global Select Market, throughfive years ended December 31, 20202023 of the cumulative total return for our common stock, the Nasdaq Composite Total ReturnBiotechnology Index, and the Nasdaq BiotechnologyStandard & Poor’s 500 Stock Index(the S&P 500) each of which assumes an initial investment of $100 and reinvestment of all dividends. Such returns are based on historical results and are not intended to suggest future performance.
The comparisons shown in the graph below are based upon historical data. We caution that the stock price performance shown in the graph below is not necessarily indicative of, nor is it intended to forecast, the potential future performance of our common stock.
Stockholders
We had approximately 7475 stockholders of record as of February 16, 2021; however, because2024. Because many of our outstanding shares are held in accounts with brokers and other institutions, we believe we have morethe number of beneficial owners.owners is significantly greater than the number of record holders. This number of holders of record also does not include stockholders whose shares may be held in trust by other entities.
Dividend Policy
We have never declared or paid cash dividends on our common stock and do not expect to pay dividends on our common stock for the foreseeable future. Instead, we anticipate that all
Securities Authorized for Issuance Under Equity Compensation Plans
Information about our equity compensation plans in Item 12 of our earnings in the foreseeable future will be used for the operation and growthPart III of our business.this Annual Report on Form 10-K is incorporated herein by reference. Any future determination to pay dividends will be made at the discretion of our board of directors and will depend on various factors, including applicable laws, our results of operations, financial condition, future prospects, then applicable contractual restrictions and any other factors deemed relevant by our board of directors. Investors should not purchase our common stock with the expectation of receiving cash dividends.
Securities Authorized for Issuance Under Equity Compensation Plans
Information about our equity compensation plans in Item 12 of Part III of this Annual Report on Form 10-K is incorporated herein by reference.
Recent Sales of Unregistered Securities
None.
Issuer Purchases of Equity Securities
Not applicable.
On August 1, 2022, our Board of Directors authorized a share repurchase program for our common stock of up to $3.0 billion, with no expiration date. During the three months ended December 31, 2023, there were no shares repurchased. As of December 31, 2023, $1.7 billion of our Board of Directors’ authorization for repurchases of our common stock remains outstanding, with no expiration date.
Refer to Note 12 to consolidated financial statements for information regarding our share repurchase programs.
Item 6. Reserved[Reserved]
Item 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations together with our consolidated financial statements and related notes and other financial information appearing elsewhere in this Annual Report on Form 10-K. Some of the information contained in this discussion and analysis or set forth elsewhere in this Annual Report on Form 10-K, including information with respect to our plans and strategy for our business, includes forward-looking statements that involve risks and uncertainties. As a result of many factors, including those factors set forth in “Part I, Item 1A - Risk Factors” section of this Annual Report on Form 10-K, our actual results could differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Overview
We are a biotechnology company pioneeringadvancing a new class of medicines made of messenger RNA (mRNA) therapeutics and vaccines to create a new generation of transformative medicines to improve the lives of patients.. mRNA medicines are designed to direct the body’s cells to produce intracellular, membrane or secreted proteins that have a therapeutic or preventive benefit with the potential to address a broad spectrum of diseases. Our platform builds on continuous advances in basic and applied mRNA science, delivery technology and manufacturing, providing us the capability to pursue in parallel a robust pipeline of new development candidates. We are developing therapeutics and vaccines for infectious diseases, immuno-oncology, rare diseases autoimmune diseases and cardiovascularautoimmune diseases, independently and with our strategic collaborators.
WithinSince our platform,founding in 2010, we develop technologies that enablehave transformed from a research-stage company advancing programs in the developmentfield of mRNA medicinesto a commercial enterprise with a diverse clinical portfolio of vaccines and therapeutics across six modalities, a broad intellectual property portfolio and integrated manufacturing capabilities that allow for diverse applications. When we identify technologies that we believe could enable a new group of potential mRNA medicines with shared product features, we call that group a “modality.” While the programs within a modality may target diverse diseases, they share similar mRNA technologies, delivery technologies,rapid clinical and manufacturing processes to achieve shared product features. The programs within a modality will also generally share similar pharmacology profiles, including the desired dose response, the expected dosing regimen, the target tissue for protein expression, safety and tolerability goals, and pharmaceutical properties. Programs within a modality often have correlated technology risk, but because they pursue diverse diseases they often have uncorrelated biology risk.commercial production at scale. We have created six modalitiesa diverse and extensive development pipeline of 42 development candidates across our 45 development programs, of which 40 are in clinical studies currently.
Our COVID-19 vaccine is our first commercial product and is marketed, where approved, under the name Spikevax®. Our original vaccine, mRNA-1273, targeted the SARS-CoV-2 ancestral strain, and we have leveraged our mRNA platform to date:rapidly adapt our vaccine to emerging SARS-CoV-2 strains to provide protection as the virus evolves and regulatory guidance is updated.
•2023 Business Highlightsprophylactic vaccines;
•systemic secreted and cell surface therapeutics;
•cancer vaccines;
•intratumoral immuno-oncology;
•localized regenerative therapeutics; and
•systemic intracellular therapeutics.
We have designated our prophylactic vaccines and systemic secreted and cell surface therapeutics modalities as our “core modalities”. In these core modalities, our strategy is to invest in additional development candidates using our accumulated innovations in technology, our process insights and our preclinical and clinical experience. Our exploratory modalities continue to be a critical part of advancing our strategy to maximize the application of our potential mRNA medicines.
2020 Business Highlights
We brought five new development candidates forward in 2020: a COVID-19 vaccine (mRNA-1273); interleukin-2 (IL-2); programmed death-ligand 1 (PD-L1); a pediatric Respiratory Syncytial Virus (RSV) vaccine; and an Epstein-Barr Virus (EBV) vaccine, as part of our mission to use our technology to advance global public health.
In response to the global coronavirus pandemic,On September 11, 2023, we are pursuing the rapid development and manufacture of our mRNA-1273 vaccine, for the treatment of SARS-CoV-2, the novel strain of coronavirus that causes COVID-19, in collaboration with the Vaccine Research Center and Division of Microbiology and Infectious Diseasesreceived approval of the National Institute of Allergy and Infectious Diseases, or NIAID, part of the National Institutes of Health, or NIH. The progress of mRNA-1273 during 2020 has resulted in the need for us to devote significant resources toward the development and manufacture of this product. Significant capital investment is necessary for ongoing clinical development, manufacturing and distribution of the COVID-19 vaccine at a scale necessary to meet demand in a global pandemic environment. This capital investment includes the ongoing clinical development for updated versions of the COVID-19 vaccine that may provide continued protection against variants of the SARS-CoV-2 virus. BARDA has committed to fund up to $954.9 million to accelerate the clinical development and manufacturing process scale-up of our COVID-19 vaccine. Under the terms of the agreement, BARDA will fund the advancement of mRNA-1273 to FDA licensure and the scale-up of manufacturing processes. The agreement does not contemplate any product stockpiling.
In February and May 2020, we completed two separate public equity offerings, resulting in aggregate net proceeds of $1.85 billion, net of underwriting discounts, commissions and offering expenses. This additional funding has enabled us to substantially expand our manufacturing network, purchase the required capital equipment, hire appropriate global staff and secure the raw materials and other consumables to manufacture substantial doses of our COVID-19 vaccine.
In December 2020, we received an Emergency Use Authorization (EUA)supplemental Biologics License Application from the U.S. Food and Drug Administration (FDA) and an Interim Order from Health Canada authorizingfor our updated COVID-19 vaccine, which targets the Omicron XBB.1.5 sublineage of SARS-CoV-2 (mRNA-1273.815), for useindividuals 12 years and older. The FDA also issued an Emergency Use Authorization for mRNA-1273.815 for children aged 6 months to 11 years old. We subsequently received authorization from regulatory authorities around the globe for mRNA-1273.815 and initiated the shipment of doses both in the U.S. and Canada.Since January 1, 2021, we have received additional provisional, interim or conditional approvals for the use and distribution of our COVID-19 vaccine from regulatory authorities in the European Union, the United Kingdom, Switzerland, Qatar, Israel and Singapore.
In August 2020, we entered into a supply agreement with the U.S. Government (the U.S. Supply Agreement) for the supply of 100 million doses of our COVID-19 vaccine. The agreement was subsequently amended in December 2020, in connection with the U.S. Government's exercise of an option for an additional 100 million doses, bringing the total to 200 million doses of our COVID-19 vaccine for a total consideration of up to $3.19 billion. The total consideration includes approximately $300.0 million of incentive payments which we earned as a result of securing an Emergency Use Authorization (EUA) before January 31, 2021. As a result of our satisfying the requirements for this incentive payment, the total price for the supply of the first 100 million doses, inclusive of the $300.0 million incentive, is $1.53 billion. We will receive such incentive payments as product is delivered to and accepted by the U.S. Government. Pursuant to the U.S. Supply Agreement, the U.S. Government made a $601.4 million upfront payment to us which represents approximately 50% of the fixed price per dose that we are entitled to receive for the committed first 100 million doses. We will receive the remainder of the fixed price per dose upon delivery and acceptance of contracted doses to the U.S. Government. We are required to secure, manage and maintain storage for any contracted doses of mRNA-1273 based on the specific requirements of the contract and deliver the product to designated government facilities. We will be reimbursed for such services at a negotiated price. Subsequent to December 31, 2020, the U.S. Government exercised a second option to purchase 100 million doses on February 11, 2021, bringing its total order to 300 million doses, with a remaining option for 200 million doses. The price for each option is fixed at a price of $1.65 billion per 100 million doses. The U.S. Supply Agreement contains terms and conditions that are customary for U.S. Government agreements of this nature, including provisions giving the U.S. Government the right to terminate the agreement if the applicable Contracting Officer determines that a termination is in the U.S. Government’s interest. Following any such termination, we and the U.S. Government may agree upon the amount to be paid or remaining to be paid to us because of the termination.
In addition, we have entered into supply agreements with several other governmental agencies outside the United States for the supply of our COVID-19 vaccine. The agreements are generally subject to receipt of authorization or approval for the use and distribution of the vaccine from the relevant regulatory authority in each jurisdiction. Under these agreements, we are entitled to upfront deposits for our COVID-19 vaccine supply, initially recorded as deferred revenue. As of December 31, 2020, we had approximately $3.80 billion in deferred revenue in connection with the supply agreements with the U.S. Government and other governmental agencies, which will be recognized as revenue when revenue recognition criteria have been met. Between the granting of the EUA by the FDA on December 18, 2020 and the end of 2020, we delivered 12.8 million doses of our COVID-19 vaccine and recognized product sales of $199.9 million. In addition, we delivered 4.0 million doses of our COVID-19 vaccine to the U.S. government under the BARDA agreement through December 31, 2020, for a total of nearly 17 million doses delivered to the U.S. government and Canada between receipt of our EUA authorization and the end of the year and satisfying the criteria for revenue recognition.internationally.
For more information on our strategic priorities for 2021, please see “Business—The mRNA Opportunity—Our strategic priorities,” above.
The early investmentthe year ended December 31, 2023, we made in our manufacturing and digital capabilities prepared us to rapidly scale our production to accommodate between 700 million doses and 1recognized net product sales of $6.7 billion dosesfrom sales of our COVID-19 vaccine in 2021. We are continuingvaccines, compared to invest$18.4 billion and add staff to build up to potentially 1$17.7 billion doses for 2021.
We have a diverse development pipeline, and the broad potential applications of mRNA medicines have led us to raise significant capital and adopt a long-term approach to capital allocation that balances near-term risks and long-term value creation. As of December 31, 2020, we had cash, cash equivalents and investments of approximately $5.25 billion, of which $82.1 million was related to product sales and $2.84 billion was related to customer deposits for the future supply of our COVID-19 vaccine. We use this capital to fund operations and investing activities for technology creation, drug discovery and clinical development programs, infrastructure and capabilities to enable our research engine and early development engine (which includes our Moderna Technology Center), our digital infrastructure, creation of our portfolio of intellectual property, acquisition of key raw materials and supplies to support our commercial production quantities, development of a commercial team, expansion into global markets and administrative support.
Since our inception, we have incurred significant operating losses. Our net losses were $747.1 million, $514.0 million and $384.7 million for the years ended December 31, 2020, 20192022 and 2018,2021, respectively. As
In January 2023, we announced positive data from the interim analysis of December 31, 2020, our accumulated deficitpivotal ConquerRSV study of our vaccine candidate against respiratory syncytial virus (RSV) (mRNA-1345). In the study, mRNA-1345 met primary efficacy endpoints, demonstrating vaccine efficacy of 83.7% against RSV lower respiratory tract disease in older adults. We have filed for a Biologics License Application to the FDA for our RSV vaccine for adults aged 60 years or older, and used a Priority Review Voucher to accelerate review. We have also submitted marketing authorization applications for the vaccine for adults aged 60 years or older to medical authorities in several countries beyond the U.S. We have initiated the manufacturing of mRNA-1345 and are preparing for a marketing launch in 2024, subject to approval.
During the third quarter of 2023, we embarked on a strategic initiative to optimize the cost structure of our COVID-19 business, with a focus on resizing our manufacturing cost structure. The launch of this initiative was $2.24 billion.prompted by the completion of our long-range planning within the third quarter of 2023, which incorporated revised forecasts of vaccination rates. These projections accounted for the market’s transition from COVID-19 pandemic conditions towards an endemic seasonal market. Consequently, this strategic shift resulted in charges of $1.4 billion for the quarter. In the fourth quarter of 2023, we incurred additional charges of $169 million related to this initiative. Despite the immediate impact to our financial statements, we believe this strategic initiative will enhance the efficiency of our manufacturing operations and equip us with the agility to better adjust our scale according to future market demands.
ForProgram Developments
As of December 31, 2023, nine of our 45 development programs are in late-stage development, including seven programs in Phase 3 and two rare disease programs that are expected to enter pivotal studies in 2024.
Respiratory Vaccines
•Respiratory syncytial virus (RSV) vaccine: We have filed for regulatory approvals for our vaccine for the foreseeable future, we may continue to incur significant expensesprevention of RSV-associated lower respiratory tract disease (RSV-LRTD) and acute respiratory disease (ARD) in connectionadults ages 60 years or older (mRNA-1345). We expect regulatory approvals beginning in the first half of 2024. We anticipate entering the RSV market with our ongoing activities, includinga strong competitive profile as we:the only pre-filled syringe product available at the time of launch, along with robust efficacy data, a well-established safety and tolerability profile, and widespread consumer awareness and demand established in 2023.
•continue our platform research and drug discovery and development efforts;
•Next-generation COVID-19 vaccine:conduct clinical trials for our investigational medicines;
•manufacture our Our next-generation COVID-19 vaccine;
•manufacture clinical trial materials and develop large-scale manufacturing capabilities;
•seek regulatory approval for our investigational medicines;
•maintain, expand, and protect our intellectual property;
•hire additional personnelvaccine candidate, which is designed to support our program development effort to obtain regulatory approval and secure additional facilities for operations;
•build up our commercial operations and organization; and
•continue to operate as a public company.be refrigerator-stable (mRNA-1283), is currently in its pivotal Phase 3 study. We anticipate data from the study in the first half of 2024.
If we seek•Seasonal flu vaccine: Our seasonal flu vaccine candidate (mRNA-1010) demonstrated consistently acceptable safety and tolerability across three Phase 3 trials. In the most recent Phase 3 trial, mRNA-1010 met all immunogenicity endpoints, demonstrating higher titers compared to obtain regulatory approval fora currently licensed vaccine. mRNA-1010 has also shown higher or comparable titers compared to a currently licensed enhanced vaccine (Fluzone HD®) in a separate Phase 1/2 study. We are in discussions with regulators and commercialize further of our investigational medicines, we expectintend to incur significant commercialization expenses, which include establishing a sales, marketing, manufacturing, and distribution infrastructure globally. As a result, we may need substantial additional funding to support our continued operations and pursue our growth strategyfile in addition to our product sales. If we fail to become profitable or are unable to sustain profitability on a continuing basis, we may be required to finance our operations through a combination of public or private equity offerings, structured financings and debt financings, government funding arrangements, strategic alliances and marketing, manufacturing, distribution, and licensing arrangements. We may not be able to raise additional funds or enter into such other agreements on favorable terms, or at all. If we fail to raise capital or enter into such agreements as, and when, needed, we may have to significantly delay, scale back, or discontinue the development and commercialization of one or more of our programs. Because of the numerous risks and uncertainties associated with pharmaceutical development, we are unable to predict the timing or amount of expenses or when or if we will be able to achieve or maintain profitability. If we fail to become profitable or are unable to sustain profitability on a continuing basis, then we may be unable to continue our operations at planned levels and be forced to reduce our operations.
The COVID-19 pandemic impacted certain of our business operations. For instance, we are experiencing disruptions in the conduct of certain of our clinical trials, including our ability to initiate and complete our clinical trials within the originally anticipated timelines. Site initiation, participant recruitment and enrollment, participant dosing, distribution of clinical trial materials, study monitoring and data analysis may or continue to be paused or delayed. While to date we have not experienced significant disruptions in our supply chain and distribution, an extended duration of this pandemic could result in such disruptions in the future.
Seasonal flu + COVID-19 vaccine: The Phase 3 trial of our combination vaccine candidate against seasonal flu and COVID-19 (mRNA-1083) is fully enrolled. We anticipate data from the study in 2024.
Latent and Other Vaccines
•Cytomegalovirus (CMV) vaccine: The pivotal Phase 3 study of our CMV vaccine candidate (mRNA-1647) is fully enrolled and accruing cases, evaluating its efficacy, safety and immunogenicity in the prevention of primary infection in women of childbearing age. We anticipate potential efficacy data from the study in 2024.
Oncology Therapeutics
•Individualized Neoantigen Therapy (INT): We continue to demonstrate the potential clinical benefit of our INT program (mRNA-4157), which we are developing in collaboration with Merck. Two separate Phase 3 trials continue to enroll patients with resected high-risk (stage III/IV) melanoma and completely resected stage II, IIIA or IIIB non-small cell lung cancer. We and Merck plan to expand their clinical studies to additional tumor types in 2024.
In December 2023, we announced results of a three-year analysis of our Phase 2b study evaluating INT in combination with KEYTRUDA®, Merck’s anti-PD-1 therapy, in patients with resected high-risk melanoma. Compared to KEYTRUDA alone, this combination continued to show an improvement in recurrence-free survival, reducing the risk of recurrence or death by 49%, as well as in distant metastasis-free survival, reducing the risk of developing distant metastasis or death by 62%.
Rare Diseases
•Propionic acidemia (PA) & methylmalonic acidemia (MMA): We expect to advance our rare disease therapeutic programs for PA (mRNA-3927) and MMA (mRNA-3705) into pivotal studies in 2024.
Other Business Updates
In January 2023, we acquired OriCiro Genomics K.K., a Japan-based, privately held biotech company primarily focused on cell-free DNA synthesis and amplification technologies, for $86 million. With this acquisition, we obtained tools for cell-free synthesis and amplification of plasmid DNA, a key building block in mRNA manufacturing. OriCiro’s technology strategically complements our manufacturing process, and we expect it will allow us to further accelerate our research and development efforts.
In February 2023, we entered into a strategic collaboration and license agreement with Life Edit Therapeutics Inc. (Life Edit) to collaborate on the discovery and development of in vivo mRNA gene editing therapies. The partnership will combine Life Edit’s suite of proprietary gene editing technologies, including base editing, with our mRNA platform to advance in vivo gene editing therapies against a select set of therapeutic targets.
In March 2023, we entered into a strategic collaboration and license agreement with Generation Bio Co. (GBIO). The collaboration aims to expand the application of each company’s platform by developing novel nucleic acid therapeutics, including those capable of reaching immune cells, to accelerate our respective pipelines of non-viral genetic medicines. Under the agreement, we have the option to license GBIO’s proprietary cell-targeted lipid nanoparticle (ctLNP) and closed-ended DNA (ceDNA) technology for two immune cell programs and two liver programs, with an additional option for either a third immune cell or liver program.
In September 2023, we announced a strategic research and development collaboration agreement with Immatics, a clinical-stage biopharmaceutical company active in the discovery and development of T cell-redirecting cancer immunotherapies. Upon effectiveness of the agreement in October 2023, we made an upfront payment of $120 million to Immatics. This collaboration between Moderna and Immatics centers on combining mRNA technology and T-cell receptor (TCR) platforms to develop cancer therapies, focusing on in vivo expression of TCR bispecifics (TCER®), creation of mRNA-based cancer vaccine utilizing extensive tumor data, and enhancing TCR-T cell therapy through combined preclinical and clinical studies.
Financial Operations Overview
Revenue
Net product sales
Net product sales by customer geographic location were as follows for the periods presented (in millions):
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Years Ended December 31, |
| | | | | | 2023 | | 2022 | | 2021 |
| United States | | | | | | $ | 1,720 | | | $ | 4,405 | | | $ | 5,393 | |
| Europe | | | | | | 1,353 | | | 6,732 | | | 6,834 | |
| Rest of world | | | | | | 3,598 | | | 7,298 | | | 5,448 | |
| Total | | | | | | $ | 6,671 | | | $ | 18,435 | | | $ | 17,675 | |
In the third quarter of 2023, we commenced sales of our COVID-19 vaccine to the U.S. commercial market, in addition to continuing sales to foreign governments and organizations. In the U.S., our COVID-19 vaccine is now sold primarily to wholesalers and distributors, and to a lesser extent, directly to retailers and healthcare providers. Net product sales are recognized net of estimated wholesaler chargebacks, invoice discounts for prompt payments and pre-orders, provisions for sales returns, and other related deductions. Please refer to Note 3 to our consolidated financial statements.
The following table summarizes revenueproduct sales provision for each periodthe periods presented (in thousands)millions):
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Years Ended December 31, |
| | | | | | 2023 | | 2022 | | 2021 |
| Gross product sales | | | | | | $ | 8,203 | | | $ | 18,435 | | | $ | 17,675 | |
| Product sales provision: | | | | | | | | | | |
| Wholesaler chargebacks, discounts and fees | | | | | | (976) | | | — | | | — | |
| Returns and other fees | | | | | | (556) | | | — | | | — | |
| Total product sales provision | | | | | | $ | (1,532) | | | $ | — | | | $ | — | |
| Net product sales | | | | | | $ | 6,671 | | | $ | 18,435 | | | $ | 17,675 | |
| | | | | | | | | | | | | | | | | |
| Years Ended December 31, |
| 2020 | | 2019 | | 2018 |
| Revenue: | | | | | |
| Grant revenue | $ | 528,905 | | | $ | 12,173 | | | $ | 12,556 | |
| Product sales | 199,872 | | | — | | | — | |
| Collaboration revenue | 74,618 | | | 48,036 | | | 122,512 | |
| Total revenue | $ | 803,395 | | | $ | 60,209 | | | $ | 135,068 | |
As of December 31, 2023, we had deferred revenue of $613 million associated with customer deposits received or billable under supply agreements for delivery of our COVID-19 vaccine primarily in 2024.
We beganOur net product sales for the full year 2023 declined significantly as compared to recordthe full year 2022, reflecting the ongoing shift of the COVID-19 vaccine market toward a seasonal commercial market. In addition, we experienced greater seasonality for sales, with greater demand in the fall/winter seasons in each hemisphere as countries seek to provide booster vaccinations to their populations. For 2024, we expect the progression toward a seasonal commercial market to persist, resulting in further projected reductions in net product sales for our COVID-19 vaccine subsequentrelative to its authorization for emergency use by the FDA and Health Canada in December 2020. For the year ended December 31, 2020, we recognized $199.9 million of product sales from sales of our COVID-19 vaccine.2023.
Other revenue
Other than net product sales, our revenue has been primarily derived from government-sponsored and private organizations including BARDA, DARPAthe Biomedical Advanced Research and Development Authority (BARDA), the Defense Advanced Research Projects Agency (DARPA) and the Bill & Melinda Gates Foundation and from strategic alliances with AstraZeneca, Merck & Co., Inc (Merck), Vertex Pharmaceuticals Incorporated and Vertex Pharmaceuticals (Europe) Limited (together, Vertex) and others to discover, develop, and commercialize potential mRNA medicines.
The following table summarizes collaborationother revenue for the periods presented (in thousands)millions):
| | | | | | | | | | | | | | | | | |
| Years Ended December 31, |
| 2020 | | 2019 | | 2018 |
| BARDA | $ | 521,652 | | | $ | 7,608 | | | $ | 6,736 | |
| Other grant revenue | 7,253 | | | 4,565 | | | 5,820 | |
| Total grant revenue | $ | 528,905 | | | $ | 12,173 | | | $ | 12,556 | |
Grant revenue from BARDA increased significantly in 2020, as a result of an award to accelerate development of our COVID-19 vaccine.
The following table summarizes collaboration revenue for the periods presented (in thousands):
| | | | | | | | | | | | | | | | | |
| Years Ended December 31, |
| 2020 | | 2019 | | 2018 |
| Collaboration revenue: | | | | | |
| AstraZeneca | $ | 32,624 | | | $ | 5,233 | | | $ | 45,993 | |
| Merck | 26,076 | | | 36,608 | | | 66,082 | |
| Vertex | 15,505 | | | 6,195 | | | 10,437 | |
| Other | 413 | | | — | | | — | |
| Total collaboration revenue | $ | 74,618 | | | $ | 48,036 | | | $ | 122,512 | |
We expect our product sales to significantly increase in 2021. As of December 31, 2020, we had signed supply agreements of approximately $11.65 billion for the future supply of our COVID-19 vaccine and had deferred revenue of $3.80 billion associated with customer deposits received or billable under these agreements. Additional supply agreements have been agreed upon since December 31, 2020, and others are under discussion for 2021 and 2022 deliveries. In addition, we expect to continue to receive funding from our contract with BARDA. As of December 31, 2020, the remaining available funding net of revenue earned was $444.3 million. To the extent that existing or potential future products generate revenue, our revenue may vary due to many uncertainties in the independent development of our mRNA medicines and pursuant to our strategic alliances and other factors.
We expect to continue to incur significant expenses as we continue our research and development and commercialization efforts. We expect our programs to mature and advance to later stage clinical development, and we expect expenses to increase as we seek regulatory approvals for our investigational medicines and commercialize any approved mRNA medicines. If we fail to become profitable or are unable to sustain profitability on a continuing basis, we may incur losses in the future. | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Years Ended December 31, |
| | | | | | 2023 | | 2022 | | 2021 |
| Grant revenue | | | | | | $ | 94 | | | $ | 388 | | | $ | 735 | |
| Collaboration revenue | | | | | | 83 | | | 440 | | | 61 | |
| Total other revenue | | | | | | $ | 177 | | | $ | 828 | | | $ | 796 | |
Cost of Salessales
Cost of Salessales includes raw materials, personnel and facility and other costs associated with manufacturing our commercial product.products. These costs include production materials, production costs at our manufacturing facilities, third-party manufacturing costs, and final formulation and packaging costs. Cost of Salessales also includes shipping costs, andindirect overhead costs associated with our product sales during the period, third-party royalties payable to third parties based on net sales of our products.products, and charges for inventory valuation, excess and obsolete inventory and losses on firm purchase commitments.
Research and development expenses
The nature of our business and primary focus of our activities generate a significant amount of research and development costs.
Research and development expenses represent costs incurred by us for the following:
•cost to develop our platform;
•discovery efforts leading to development candidates;
•preclinical, nonclinical, and clinical development costs for our programs;
•costs related to pre-launch inventory;
•cost to develop our manufacturing technology and infrastructure; and
•digital infrastructure costs.costs related to our drug discovery efforts and clinical trials.
The costs above comprise the following categories:
•personnel-related expenses, including salaries, benefits, and stock-based compensation expense;
•expenses incurred under agreements with third parties, such as consultants, investigative sites, contract research organizations or CROs,(CROs), that conduct our preclinical studies and clinical trials, and in-licensing arrangements;
•expenses associated with developing manufacturing, capabilitiesmodification of formulation or design of a product or process, advancing the design to meet specific functional and acquiringeconomic requirements for manufacture and obtaining materials for preclinical studies, clinical trials and pre-launch inventory including bothfrom internal manufacturing and third-party contract manufacturing organizations or CMOs;(CMOs);
•expenses incurred for the procurement of materials, laboratory supplies, and non-capital equipment used in the research and development process;
•upfront fees and milestones paid to third-parties for licenses and technologies that had not reached technological feasibility and did not have an alternative future use; and
•facilities, depreciation, and amortization, and other direct and allocated expenses incurred as a result of research and development activities.
We use our employee and infrastructure resources for the advancement of our platform, and for discovering and developing programs. Due to the number of ongoing programs and our ability to use resources across several projects, indirect or shared operating costs incurred for our research and development programs are generally not recorded or maintained on a program- or modality-specific basis.
The following table reflects our research and development expenses, including direct program specific expenses summarized by modality and indirect or shared operating costs summarized under other research and development expenses during the years ended December 31, 2020, 20192023, 2022, and 20182021 (in thousands)millions):
| | | | | | | | | | | | | | | | | |
| Years Ended December 31, |
| 2020 | | 2019 | | 2018 |
| Program expenses by modality: | | | | | |
| Prophylactic vaccines | $ | 707,150 | | | $ | 47,650 | | | $ | 25,404 | |
| Cancer vaccines | 29,440 | | | 44,003 | | | 35,891 | |
| Intratumoral immuno-oncology | 8,755 | | | 17,607 | | | 15,405 | |
| Systemic secreted and cell surface therapeutics | 1,503 | | | 11,240 | | | 18,207 | |
| Localized regenerative therapeutics | 4 | | | 3,326 | | | 91 | |
| Systemic intracellular therapeutics | 20,982 | | | 33,360 | | | 45,695 | |
Total program-specific expenses by modality (1) | 767,834 | | | 157,186 | | | 140,693 | |
| Other research and development expenses: | | | | | |
| Discovery programs | 55,601 | | | 55,376 | | | 34,643 | |
| Platform research | 93,501 | | | 91,097 | | | 91,720 | |
| Technical development and unallocated manufacturing expenses | 279,489 | | | 85,304 | | | 83,117 | |
| Shared discovery and development expenses | 117,759 | | | 59,087 | | | 44,250 | |
| Stock-based compensation | 56,155 | | | 48,259 | | | 37,659 | |
Other expenses(2) | — | | | — | | | 22,000 | |
| Total research and development expenses | $ | 1,370,339 | | | $ | 496,309 | | | $ | 454,082 | |
| | | | | | | | | | | | | | | | | |
| Years Ended December 31, |
| 2023 | | 2022 | | 2021 |
| Program expenses by modality: | | | | | |
| Infectious disease vaccines | $ | 2,344 | | | $ | 1,734 | | | $ | 1,099 | |
| Cancer vaccines & therapeutics | 70 | | | 14 | | | 47 | |
| Rare disease intracellular therapeutics | 75 | | | 42 | | | 26 | |
| Intratumoral immuno-oncology | 23 | | | 10 | | | 20 | |
| Inhaled pulmonary therapeutics | 17 | | | 18 | | | 1 | |
| | | | | |
| Systemic secreted and cell surface therapeutics | 33 | | | 23 | | | 3 | |
| | | | | |
Total program-specific expenses by modality (1) | $ | 2,562 | | | $ | 1,841 | | | $ | 1,196 | |
| Other research and development expenses: | | | | | |
| Discovery programs | $ | 106 | | | $ | 69 | | | $ | 85 | |
| Platform research | 254 | | | 169 | | | 125 | |
| Technical development and unallocated manufacturing expenses | 821 | | | 464 | | | 275 | |
| Shared discovery and development expenses | 945 | | | 658 | | | 242 | |
| Stock-based compensation | 157 | | | 94 | | | 68 | |
| | | | | |
| Total research and development expenses | $ | 4,845 | | | $ | 3,295 | | | $ | 1,991 | |
__________
(1)Includes a total of 2142 development candidates at each of December 31, 2020, 20192023, 45 development candidates at December 31, 2022, and 2018.37 development candidates at December 31, 2021. Program-specific expenses include external costs and allocated manufacturing costs of pre-launch inventory, mRNA supply and consumables, and are reflected as of the beginning of the period in which the program was internally advanced to development or removed if development was ceased.
(2)Relates to an in-licensing agreement entered into in June 2017 with Cellscript, LLC to sublicense certain patent rights.
A “modality” refers to a group of programs with common product features and the associated combination of enabling mRNA technologies, delivery technologies, and manufacturing processes. The program-specific expenses by modality summarized in the table above include expenses we directly attribute to our programs, which consist primarily of external costs, such as fees paid to outside consultants, central laboratories, investigative sites, and CROs in connection with our preclinical studies and clinical trials, CMOs, and allocated manufacturing costs of pre-launch inventory, mRNA supply and consumables. Costs to acquire and manufacture pre-launch inventory, mRNA supply for preclinical studies and clinical trials are recognized and included in unallocated manufacturing expenses when incurred, and subsequently allocated to program-specific manufacturing costs after completion of the program-specific production. The timing of allocating manufacturing costs to the specific program varies depending on the program
development and production schedule. We generally do not allocate personnel-related costs, including stock-based compensation, costs associated with our general platform research, technical development, and other shared costs on a program-specific basis. These costs were therefore excluded from the summary of program-specific expenses by modality.
Discovery program expenses are costs associated with research activities for our programs in the preclinical discovery stage, and primarily consist of external costs for CROs and lab services, and allocated manufacturing cost of preclinical mRNA supply and consumables.
Platform research expenses are mainly costs to develop technical advances in mRNA science, delivery science, and manufacturing process design. These costs include personnel-related costs, computer equipment, facilities, preclinical mRNA supply and consumables, and other administrative costs to support our platform research.
Technology development and unallocated manufacturing expenses are primarily related to non-program-specific manufacturing process development and manufacturing costs.
Shared discovery and development expenses are research and development costs such as personnel-related costs and other costs, which are not otherwise included in development programs, discovery programs, platform research, technical development, certain collaborative and licensing arrangements, and unallocated manufacturing expenses, stock-based compensation, and other expenses.
The largest component of our total operating expenses has historically been our investment
Historically, we have made substantial investments in research and development activities, including development of our platform, mRNA technologies, and manufacturing technologies. We expense research and development costs as incurred and cannot reasonably estimate the nature, timing, and estimated costs required to complete the development of the development candidates and investigational medicines we are currently developing or may develop in the future. There are numerous risks and uncertainties associated with the research and development of such development candidates and investigational medicines, including, but not limited to:
•scope, progress, and expense of developing ongoing and future development candidates and investigational medicines;
•entry in and completion of related preclinical studies;
•enrollment in and completion of subsequent clinical trials;
•safety and efficacy of investigational medicines resulting from these clinical trials;
•changes in laws or regulations relevant to the investigational medicines in development;
•receipt of the required regulatory approvals; and
•commercialization, including establishing manufacturing and marketing capabilities.
As we continue to progress mRNA-1273 through the development process toward a Biologics License Application approval and indication expansion of mRNA-1273 during the current pandemic, we expect to continue to incur significant additional expenses. At this time, the magnitude of these potential expenditures is not known. In connection with the BARDA agreement to accelerate development of mRNA-1273, significant grant revenue and expenses are expected in 2021. BARDA's funding is expected to offset those expenses that are covered under the BARDA agreement, subject to our obtaining reimbursement from BARDA.
Changes in expectations or outcomes of any of the known or unknown risks and uncertainties may materially impact our expected research and development expenditures. Continued research and development is central to the ongoing activities of our business. Investigational medicines in later stages of clinical development, such as our RSV vaccine, seasonal flu vaccine, CMV vaccine, (mRNA-1647)new generations of COVID-19 vaccines, combination vaccines, and our COVID-19 vaccine,INT program, generally have higher development costs than those in earlier stages of clinical development, primarily due to the increased size and duration of later-stage clinical trials. We expect our research and development costs to continue to increasebe substantial in the foreseeable futurenear term as our investigational medicines progressadvance through the development phases and as we identify and develop additional programs. We expect a modest reduction in research and development costs in 2024. There are numerous factors associated with the successful commercialization of any of our investigational medicines, including future trial design and various regulatory requirements, many of which cannot be determined with accuracy at this time due to the early stage of development of many of our investigational medicines. Moreover, future commercial and regulatory factors beyond our control will impact our clinical development programs and plans.
Selling, general and administrative expenses
We started to incur sales and marketing expenses in the fourth quarter of 2020 to prepare for commercial operations in connection with the sale of our COVID-19 vaccine. Selling, general and administrative expenses consist primarily of personnel-related costs, including stock-based compensation, for executives, finance, legal, human resources, business development, commercial, marketing, and other administrative and operational functions, professional fees, accounting and legal services, information technology and facility-related costs, and expenses associated with obtaining and maintaining intellectual property or IP.(IP). These costs relate to the operation of the business, unrelated to the research and development function, or any individual program. Selling, general and administrative expenses, including IP-related expenses, totaled $188.3 million, $109.6 million and $94.3 million for the years ended December 31, 2020, 2019 and 2018, respectively. IP-related expenses, including our internal personnel-related costs, were $13.5 million, $13.4 million and $11.9 million, for the years ended December 31, 2020, 2019 and 2018, respectively.
We anticipate selling, general and administrative expenses will increase as we continue to expanddecrease marginally in 2024 compared to 2023. This is due to the numbercontinued development of our programs in development and preparepreparations for the establishment of commercial activities both within and outside the United States. We haveOur global expansion has already incurred additionalled to increased expenses, related to building out aparticularly as we build our regulatory, sales and marketing teamteams to support the sale, marketing and distributioncommercialization of our COVID-19 vaccine.vaccinesand extend our global presence, with active subsidiaries in 19 countries as of December 31, 2023. Commercial and marketing efforts were significant contributors to our selling, general and administrative expenses in 2023, making up a substantial portion of these costs. If we obtain regulatory approval for any of ouradditional investigational medicines and do not enter into one or morewithout establishing third-party commercialization collaboration and manufacturing arrangements,collaborations, we willmay incur significant additionalfurther costs in developing these operational capacities. However, we anticipate selling, general and administrative expenses related to building out these functions.decrease slightly compared to 2024, reflecting efficiencies gained from our established infrastructure and operations.
We have a broad IP portfolio covering our development and commercialization of mRNA vaccine and therapeutic programs, including those related to mRNA design, formulation, and manufacturing platform technologies. We regularly file patent applications to protect innovations arising from our research and development. We also hold trademarks and trademark applications in the United States and foreign jurisdictions. Costs to secure and defend our IP are expensed as incurred, and are classified as selling, general and administrative expenses.
Interest income
Interest income consists of interest generated from our investments in cash and cash equivalents, money market funds, and high-quality fixed income securities.
Other expense, net
Other expense, net consists of interest expense, gains (losses) from the sale of investments in marketable securities, gains (losses) related to changes in fair value of investments in equity securities, and other income and expense unrelated to our core operations. Interest expense is primarily derived from our finance leases related to our Moderna Technology Center and certain contract manufacturing facility, or MTC South, and Moderna Technology Center North, or MTC North.service agreements.
Critical accounting policies and significant judgments and estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our consolidated financial statements, which have been prepared in accordance with U.S. generally accepted accounting principles. The preparation of these consolidated financial statements requires us to make judgments and estimates that affect the reported amounts of assets, liabilities, revenues, and expenses and the disclosure of contingent assets and liabilities in our consolidated financial statements. We base our estimates on historical experience, known trends and events, and various other factors that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions. On an ongoing basis, we evaluate our judgments and estimates in light of changes in circumstances, facts, and experience. The effects of material revisions in estimates, if any, are reflected in the consolidated financial statements prospectively from the date of change in estimates.
While our significant accounting policies are described in more detail in the notes to our consolidated financial statements appearing elsewhere in this Annual Report on Form 10-K, we believe the following accounting policies used in the preparation of our
consolidated financial statements require the most significant judgments and estimates.
Revenue recognitionNet product sales
Product Sales
Product sales are associated withPrior to the third quarter of 2023, we sold our COVID-19 vaccine supply agreements withto the U.S. Government, foreign governments and international government agencies. Theseorganizations. The agreements and related amendments with these entities generally do not provide provisions for various forms ofinclude variable consideration, such as discounts, rebates or returns. In the third quarter of 2023, we commenced sales of our latest COVID-19 vaccine to the U.S. commercial market, in addition to continuing sales to foreign governments and organizations. In the U.S., our COVID-19 vaccine is sold primarily to wholesalers and distributors, and to a lesser extent, directly to retailers and healthcare providers.
We recognize revenue fromnet product sales using the five-step model under ASC 606, based on the fixed price per dose according to the contracts when control of the product transfers to the customer, typically upon delivery. Net product sales in the U.S. are recognized net of estimated wholesaler chargebacks, invoice discounts for prompt payments and customer acceptance has occurred, unless such acceptancepre-orders, provisions for sales returns, and other related deductions. These provisions are deemed perfunctory.
Tablerecorded based on contractual terms and our estimate of Contents
Collaboration Revenue
Our alliances with strategic collaborators typically contain multiple elements, including research and other licenses, options to obtain development and commercialization rights, research and development services, obligations to develop and manufacture preclinical and clinical material, and options to obtain additional research and development services and preclinical and clinical material. Such arrangements providereturns for various types of payments to us, including upfront fees, funding of research and development services and preclinical and clinical material, technical, development, regulatory, and commercial milestone payments, licensing fees, option exercise fees, and royalty and earnout payments on product sales. Such payments are often not commensurate with the timing of revenue recognition and therefore result in deferral of revenue recognition.
We analyze our collaboration arrangements to assess whether they are within the scope of Accounting Standards Codification ASC Topic 808, Collaborative Arrangements (ASC 808), to determine whether such arrangements involve joint operating activities performed by parties that are both active participants in the activities and exposed to significant risks and rewards that are dependent on the commercial success of such activities. If we conclude that some or all aspects of the arrangement are within the scope of ASC 808 and do not represent a transaction with a customer, we recognize our allocation of the shared costs incurred with respect to the jointly conducted activities as a component of the related expense insold during the period, incurred. If we conclude that some or all aspects of the arrangement represent a transaction with a customer, we account for those aspects of the arrangement within the scope of ASC 606 (Revenue from Contracts with Customers).
To determine the appropriate amount of revenue to be recognized for arrangements that we determine are within the scope of ASC 606, we perform the following steps: (i) identify the contract(s) with the customer; (ii) identify the performance obligations in the contract; (iii) determine the transaction price; (iv) allocate the transaction price to the performance obligations in the contract; and (v) recognize revenue when (or as) each performance obligation is satisfied. ASC 606 requires significant judgment and estimates and results in changes to, but not limited to: (i) the determination of the transaction price, including estimates of variable consideration, (ii) the allocation of the transaction price, including the determination of estimated selling price, and (iii) the pattern of recognition, including the application of proportional performance as a measure of progress on service-related promises and application of point-in-time recognition for supply-related promises.
The transaction price is generally comprised of an upfront payment due at contract inception and variable consideration in the form of payments for our services and materials and milestone payments due upon the achievement of specified events. Other payments the Company could be entitled to include tiered royalties earned when customers recognize net sales of licensed products. We consider the existence of any significant financing component within our arrangements and have determined that a significant financing component does not exist in our arrangements as substantive business purposes exist to support the payment structure other than to provide a significant benefit of financing. We measure the transaction price based on the amount of consideration to which we expect to be entitled in exchange for transferring the promised goods and/or services to the customer. We utilize eitherusing the expected value method or the most likely amount
methodmethod. Estimates are assessed each period and adjusted as required to
estimaterevise information or actual experience. Please refer to Note 2 to our consolidated financial statements for further discussion and analysis of each significant category of product sales provisions and the accounting policy.
The application of our critical accounting policies necessitates substantial management judgment and estimation, particularly when determining the amount of variable consideration dependingto recognize. The subjectivity of this process is heightened when assessing factors outside our direct control such as lack of pertinent historical data and limited third-party information. Among all variables, estimating returns presents the most significant judgment due to the broad range of potential outcomes and the current lack of return history with our customers. As we receive more historical data on which method is expected to better predict the amount of consideration to whichour product returns, we will be entitled. Amountsintegrate this information to refine our estimates and enhance the precision of variable considerationour financial projections. The actual results could differ from our estimates, and such differences could have a material impact to our financial statements.
Inventory
Inventory is recorded at the lower of cost or net realizable value, with cost determined using first-in, first-out and average cost methods for different inventory components. We periodically review the composition of inventory in order to identify excess, obsolete, slow-moving or otherwise unsaleable items. If unsaleable items are included inobserved and there are no alternate uses for the transaction priceinventory, we will record a write-down to the extent that it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved. With respect to arrangements that include payments for a development or regulatory milestone payment, we evaluate whether the associated event is considered probable of achievement and estimate the amount to be included in the transaction price using the most likely amount method. Milestone payments that are not within our control or the licensee, such as those dependent upon receipt of regulatory approval, are not considered to be probable of achievement until the triggering event occurs. At the end of each reporting period, we re-evaluate the probability of achievement of each milestone and any related constraint, and if necessary, adjust our estimate of the overall transaction price. Any such adjustments are recorded on a cumulative catch-up basis, which would affect revenue and net lossrealizable value in the period that the decline in value is first recognized through a charge to cost of adjustment. For arrangements that include sales-based royalties,sales. The process of determining whether inventory cost will be realizable requires significant judgment and estimates by management. If actual market conditions prove less favorable than projected by management, additional write-downs of inventory may be required. On a quarterly basis, we also assess whether we have any excess firm, non-cancelable, purchase commitment liabilities, resulting from our supply agreements with third-party vendors. The determination of net realizable value and firm purchase commitment liabilities requires significant judgment, including milestone payments based uponconsideration of many factors, such as estimates of future product demand, current and future market conditions, potential product obsolescence, expiration and utilization of raw materials under firm purchase commitments and contractual minimums, among others. In 2023, the achievementsignificant judgment included the write-down of a certain level of product sales, wherein the license is deemedraw materials due to be the sole or predominant itemour revised long-range plan, reflecting lower expected future demand for our COVID-19 vaccine. In prior years, this significant judgement was related to which the payments relate, we recognize revenue upon the later of: (i) when the related sales occur or (ii) when the performance obligation to which some or all of the payment has been allocated has been satisfied (or partially satisfied). Consideration that would be received for optional goods and/or services is excludedexcess raw material purchase commitments from the transaction price at contract inception.firm, non-cancelable purchase contracts with third-party vendors.
We generally allocate the transaction price to each performance obligation based on a relative standalone selling price basis. We develop assumptions that require judgment to determine the standalone selling price for each performance obligationmaintain raw materials beyond our one-year forecasted production plan, which were classified as non-current and included in considerationother non-current assets in our consolidated balance sheets. The classification and valuation of applicable market conditions and relevant entity-specific factors, including factors that were contemplated in negotiating the agreement with the customer and estimated research and development costs. However, in certain instances, we allocate variable consideration entirely to one or more performance obligation if the terms of the variable consideration relate to the satisfaction of the respective performance obligation and the amount allocated is consistent with the amount we would expect to receive for the satisfaction of the respective performance obligation.
We recognize revenue based onjudgment and estimates. While we consider the amount of the transaction price that is allocated to each respective performance obligation when or as the performance obligation is satisfied by transferring a promised good or service to the customer. For performance obligations that are satisfied at a pointassumptions used in time, we recognize revenue when control of the goods and/or services is transferred to the customer. For performance obligations that are satisfied over time, we recognize revenue by measuring the progress toward complete satisfaction of the performance obligation using a single method of measuring progress which depicts the performance in transferring control of the associated goods and/or services to the customer. We generally use input methods to measure the progress toward the complete satisfaction of performance obligations satisfied over time. With respect to arrangements containing a license to our intellectual property that is determinedestimating inventory write-downs to be distinct from the other performance obligations identifiedreasonable, any significant future changes in the arrangement, we recognize revenue from amounts allocatedthese assumptions or shifts in future events and market conditions could lead to the license when the license is transferreddifferent estimates. Please refer to the licensee and the licensee is ableNote 7 to use and benefit from the license. For licenses that are bundled with other promises, we utilize judgment to assess the nature of the combined performance obligation to determine whether the combined performance obligation is satisfied over time or at a point in time and, if over time, the appropriate method of measuring progress for purposes of recognizing revenue. Significant management judgment is required in determining the level of effort required under an arrangement and the period over which we are expected to complete our performance obligations under an arrangement. We evaluate the measure of progress each reporting period and, if necessary, adjust the measure of performance and related revenue recognition. Any such adjustments are recorded on a cumulative catch-up basis, which would affect revenue and net loss in the period of adjustment.
Prelaunch Inventory
Prior to an initial regulatory approval for our investigational medicines, we expense costs relating to raw materials and production of inventory as research and development expense in our consolidated statements of operations, in the period incurred. When we believe regulatory approval and subsequent commercialization of our investigational medicines is probable, and we also expect future economic benefit from the sales of the investigational medicines to be realized, we will then capitalize the costs of production as inventory.
Upon the authorization of distribution and use of our COVID-19 vaccine under an EUA in December 2020, we began to capitalize inventory costs associated with our COVID-19 vaccine, as it was determined that inventory costs incurred subsequent to the EUA had a probable future economic benefit.
Research and development costs
As part of the process of preparing our financial statements, we are required to estimate our accrued research and development expenses, a significant portion of which are clinical study expenses conducted by third-party service providers. This process involves reviewing open contracts and purchase orders, communicating with our personnel to identify services that have been performed on our behalf, and estimating the level of service performed and the associated cost incurred for the service when we have not yet been invoiced or otherwise notified of the actual cost. The majority of our service providers invoice us in arrears for services performed or when contractual milestones are met. Examples of estimated accrued research and development expenses include fees paid to:
•CROs to conduct our clinical trials;
•investigative sites in connection with clinical trials;
•vendors for laboratory services, supplies, and distribution of materials in connection with clinical trials; and
•vendors in connection with preclinical development activities.
We base our expenses related to clinical trials on our estimates of the services received and efforts expended pursuant to contracts with CROs that conduct and manage clinical trials on our behalf. The financial terms of these contracts are subject to negotiation, vary from contract to contract and may result in uneven payment flows. There may be instances in which payments made to our vendors will exceed the level of services provided and result in a prepayment of the expense. Payments under some of these contracts depend on factors such as the successful enrollment of subjects and the completion of clinical trial milestones. In accruing service fees, we estimate the time period over which services will be performed and the level of effort to be expended in each period and adjust accordingly.
We make estimates of our research and development accrued expenses as of each balance sheet date in our financial statements based on facts and circumstances known to us at that time. We recognize costs for certain development activities based on an evaluation of the progress to completion of specific tasks using information and data provided to us by our vendors and our clinical sites, such as number of sites activated, number of patient enrollments and visits, and patient duration. We determine accrual estimates through financial models that take into account discussions with applicable personnel and service providers as to the progress or state of completion of trials. We periodically confirm the accuracy of these estimates with the service providers and make adjustments, if necessary. Upon settlement, these costs may differ materially from the amounts accrued in our consolidated financial statements. Our historical accrual estimates have not been materially different from our actual costs. However, due to the naturestatements for further discussion and analysis of estimates, we
cannot provide assurance that we will not make changes to our estimates in the future as we become aware of additional information about the status or conduct of our research activities and clinical trials.
Stock-based compensation
We issue stock-based awards to employees and non-employees, generally in the form of stock options and restricted stock units. We measure and recognize compensation expense for our stock-based awards granted to our employees and non-employee directors based on the estimated grant date fair value in accordance with ASC 718, Compensation—Stock Compensation.
Our stock-based awards are subject to either service or performance-based vesting conditions. We recognize compensation expensethese inventory related to awards to employees and non-employee directors with service-based vesting on a straight-line basis based on the grant date fair value over the requisite service period, which is generally the vesting period. Compensation expense related to awards to employees and non-employee directors with performance-based vesting conditions is recognized based on the grant date fair value over the requisite service period using an accelerated attribution method to the extent the achievement of the performance condition is probable. We made an accounting policy election to recognize forfeitures of stock-based awards as they occur. We classify stock-based compensation expense in our consolidated statements of operations in the same manner in which the award recipient’s salary and related costs are classified or in which the award recipient’s service payments are classified.
We determine the fair value of restricted stock and restricted stock units, based on the fair value of our common stock. We estimate the fair value of our stock options using the Black-Scholes option pricing model, which requires inputs of subjective assumptions, including: (i) the expected volatility of our stock; (ii) the expected term of the award; (iii) the risk-free interest rate; (iv) expected dividends; and (v) the fair value of common stock. Due to the lack of company specific historical and implied volatility data, we based our estimate of expected volatility on the estimate and expected volatilities of a guideline group of publicly traded companies. For these analyses, we select companies with comparable characteristics to ours including enterprise value, risk profiles, and with historical share price information sufficient to meet the expected life of the stock-based awards. We compute the historical volatility data using the daily closing prices for the selected companies’ shares during the equivalent period of the calculated expected term of our stock-based awards. We will continue to apply this process until a sufficient amount of historical information regarding the volatility of our own stock price becomes available. We estimate the expected term of our stock options granted to employees and non-employee directors using the simplified method, whereby, the expected term equals the average of the vesting term and the original contractual term of the option. We utilize this method as we do not have sufficient historical exercise data to provide a reasonable basis upon which to estimate the expected term. For stock options granted to non-employees, we utilize the contractual term of the option as the basis for the expected term assumption. For the determination of the risk-free interest rates we utilize the U.S. Treasury yield curve for instruments in effect at the time of measurement with a term commensurate with the expected term assumption. The expected dividend yield is assumed to be zero as we have never paid dividends and do not have current plans to pay any dividends on our common stock.
Income taxes
We account for income taxes based on an asset and liability approach. We recognize deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. We determine our deferred tax assets and liabilities based on differences between financial reporting and tax bases of assets and liabilities, which are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. RealizationSignificant management judgment is required in assessing the realizability of our deferred tax assets. In performing this assessment, we consider whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the amount and timingperiods in which those temporary differences become deductible. In making this determination, we consider the scheduled reversal of which are uncertain. Valuation allowances are provided, if, based upon the weight of available evidence, it is more likely than not that some or all of the deferred tax assets will not be realized. Asliabilities, uncertain tax positions, projected future taxable income and the effects of December 31, 2020, we continued to maintaintax planning strategies in assessing the need for a full valuation allowance against allallowance.
During 2023, our assessment included consideration of a full-year pre-tax loss and a projected three-year cumulative loss as determined by our long-range financial planning process. This process indicated a significant decrease in expected sales of our deferred tax assets based on management’s evaluationCOVID-19 vaccine as we transition to a seasonal market and substantial research and development expenses for ongoing Phase 3 clinical trials and the advancement of all availableproduct candidates into later-stage development. These factors were considered as negative evidence including our history of incurring significant losses from operations. We will continue to monitorin the realizability of our deferred tax assets as we may generate profitsassets. Furthermore, in 2021 if we are able to fulfill our obligations underassessing the supply agreementsneed for our COVID-19 vaccine. We may release all or a portion of the valuation allowance, we evaluated other sources of taxable income, such as taxable income in the near-term; however, the release of the valuation allowance, as well as the exact timingcarryback years, available tax planning strategies, and the amountfuture reversals of such release, continue to be subject to, among other things, our leveltaxable temporary differences. After a thorough evaluation of profitability, revenue growth, clinical program progression and expectations regarding future profitability. We may become subject to income tax audits and adjustments by local tax authorities. The nature of uncertain tax positions is subject to significant judgment by management and subject to change, which may be substantial. We develop our assessment of uncertain tax positions, and the associated cumulative probabilities, using internal expertise and assistance from third-party experts. As additional information becomesall available estimates are revised and refined. Differences between estimates and final settlement may occur resulting in additional tax expense. We record reserves for potential tax payments to various tax authorities related to uncertain tax positions. These reserves are based on a determination of whether and how much of a tax benefit taken by us in our tax filings or positions isevidence, we determined it was more likely than not that we would not realize the majority of our deferred tax assets, leading us to be realized following resolutionmaintain a valuation allowance of any potential contingencies present related$2.2 billion against our deferred tax assets as of December 31, 2023. In the event that actual results differ from our estimates, or if future changes in estimates occur, the adjustments to the valuation allowance could materially impact our financial results. Such changes may result in significant increases or decreases to our tax benefit. Potential interest and penalties associated withprovision in a period in which such uncertain tax positions is recorded as a component ofestimates are changed, which in turn would affect net income tax expense.
Table of Contents
or loss.
Recently issued accounting pronouncements
We have reviewed all recently issued standards and have determined that such standards will not have a material impact on ourSee Note 2 - Summary of Significant Accounting Policies, to the consolidated financial statements, or do not otherwise apply to our operations.under the caption “Recently Issued Accounting Standards Not Yet Adopted”.
Results of operations
A discussion regarding our results of operations for the year ended December 31, 2023 compared to 2022 is presented below. A discussion regarding our results of operations for the year ended December 31, 2022 compared to 2021 can be found under Part II -Item 7 of our Annual Report on Form 10-K for the year ended December 31, 2022, which was filed with the Securities and Exchange Commission (SEC) on February 24, 2023.
The following tables summarizetable summarizes our consolidated statements of operations for each periodthe periods presented (in thousands)millions):
| | | | | | | | | | | | | | | | | | | | | | | |
| Years Ended December 31, | | Change 2020 vs. 2019 |
| 2020 | | 2019 | | Change | | % |
| Revenue: | | | | | | | |
| Grant revenue | $ | 528,905 | | | $ | 12,173 | | | $ | 516,732 | | | 4,245 | % |
| Product sales | 199,872 | | | — | | | 199,872 | | | 100 | % |
| Collaboration revenue | 74,618 | | | 48,036 | | | 26,582 | | | 55 | % |
| Total revenue | 803,395 | | | 60,209 | | | 743,186 | | | 1,234 | % |
| Operating Expenses: | | | | | | | |
| Cost of sales | 7,933 | | | — | | | 7,933 | | | 100 | % |
| Research and development | 1,370,339 | | | 496,309 | | | 874,030 | | | 176 | % |
| Selling, general and administrative | 188,267 | | | 109,620 | | | 78,647 | | | 72 | % |
| Total operating expenses | 1,566,539 | | | 605,929 | | | 960,610 | | | 159 | % |
| Loss from operations | (763,144) | | | (545,720) | | | (217,424) | | | 40 | % |
| Interest income | 24,715 | | | 38,530 | | | (13,815) | | | (36) | % |
| Other expense, net | (6,084) | | | (7,526) | | | 1,442 | | | (19) | % |
| Loss before provision for (benefit from) income taxes | (744,513) | | | (514,716) | | | (229,797) | | | 45 | % |
| Provision for (benefit from) income taxes | 2,551 | | | (695) | | | 3,246 | | | (467) | % |
| Net loss | $ | (747,064) | | | $ | (514,021) | | | $ | (233,043) | | | 45 | % |
| | | | | | | | | | | | | | | | | | | | | | | |
| Years Ended December 31, | | Change 2019 vs. 2018 |
| 2019 | | 2018 | | Change | | % |
| Revenue: | | | | | | | |
| Grant revenue | $ | 12,173 | | | $ | 12,556 | | | $ | (383) | | | (3) | % |
| Collaboration revenue | 48,036 | | | 122,512 | | | (74,476) | | | (61) | % |
| Total revenue | 60,209 | | | 135,068 | | | (74,859) | | | (55) | % |
| Operating Expenses: | | | | | | | |
| Research and development | 496,309 | | | 454,082 | | | 42,227 | | | 9 | % |
| General and administrative | 109,620 | | | 94,252 | | | 15,368 | | | 16 | % |
| Total operating expenses | 605,929 | | | 548,334 | | | 57,595 | | | 11 | % |
| Loss from operations | (545,720) | | | (413,266) | | | (132,454) | | | 32 | % |
| Interest income | 38,530 | | | 27,023 | | | 11,507 | | | 43 | % |
| Other (expense) income, net | (7,526) | | | 1,835 | | | (9,361) | | | (510) | % |
| Loss before (benefit from) provision for income taxes | (514,716) | | | (384,408) | | | (130,308) | | | 34 | % |
| (Benefit from) provision for income taxes | (695) | | | 326 | | | (1,021) | | | (313) | % |
| Net loss | $ | (514,021) | | | $ | (384,734) | | | $ | (129,287) | | | 34 | % |
| | | | | | | | | | | | | | | | | | | | | | | |
| Years Ended December 31, | | Change 2023 vs. 2022 |
| 2023 | | 2022 | | Change | | % |
| Revenue: | | | | | | | |
| Net product sales | $ | 6,671 | | | $ | 18,435 | | | $ | (11,764) | | | (64) | % |
| Other revenue | 177 | | | 828 | | | (651) | | | (79) | % |
| | | | | | | |
| | | | | | | |
| Total revenue | 6,848 | | | 19,263 | | | (12,415) | | | (64) | % |
| Operating expenses: | | | | | | | |
| Cost of sales | 4,693 | | | 5,416 | | | (723) | | | (13) | % |
| Research and development | 4,845 | | | 3,295 | | | 1,550 | | | 47 | % |
| Selling, general and administrative | 1,549 | | | 1,132 | | | 417 | | | 37 | % |
| Total operating expenses | 11,087 | | | 9,843 | | | 1,244 | | | 13 | % |
| (Loss) income from operations | (4,239) | | | 9,420 | | | (13,659) | | | (145) | % |
| Interest income | 421 | | | 200 | | | 221 | | | 111 | % |
| Other expense, net | (124) | | | (45) | | | (79) | | | 176 | % |
| (Loss) income before income taxes | (3,942) | | | 9,575 | | | (13,517) | | | (141) | % |
| Provision for income taxes | 772 | | | 1,213 | | | (441) | | | (36) | % |
| Net (loss) income | $ | (4,714) | | | $ | 8,362 | | | $ | (13,076) | | | (156) | % |
Revenue
Total revenue increaseddecreased by $743.2 million,$12.4 billion, or 1,234%64%, in 2020, due2023, primarily attributable to increasesa significant reduction in grant revenue,net product sales and collaboration revenue. Grant revenue increased by $516.7 million, or 4,245% in 2020, mainly due to an increase in revenue from BARDA related to our COVID-19 vaccine development in 2020. Product revenue was $199.9 million in 2020 from sales of our COVID-19 vaccine
subsequent to the authorizationvaccine. Net product sales decreased by the FDA and Health Canada for emergency use$11.8 billion, or 64%, in December 2020. Collaboration revenue increased by $26.6 million, or 55% in 2020, primarily2023, mainly due to increased revenue from AstraZeneca and Vertex attributablelower sales volume across all regions in 2023, reflecting the market's transition to a change in estimated costsseasonal commercial market for our future performance obligations under the collaboration agreement with AstraZeneca and an increase in reimbursable costs under the collaboration agreement with Vertex,COVID-19 vaccine. The decline was partially offset by a decreasehigher average selling price, particularly in revenue from Merck.
Totalthe United States, where commercial market sales commenced in the third quarter of 2023. Other revenue decreased by $74.9$651 million, or 55%79%, in 2019, primarily due to decrease in collaboration revenue. Collaboration revenue decreased by $74.5 million, or 61% in 2019,2023, mainly due to decreased revenue across all our strategic alliances, particularly AstraZeneca and Merck, largely driven by our adoption of ASC 606 and the completion of the initial four-year research period under the 2016 Merck Agreement. Grant revenue remained relatively flat in 2019. There was a decrease in grant revenue from DARPA asunder our agreement with BARDA for the researchdevelopment of our COVID-19 vaccine, and development activities underlower collaboration revenue following the DARPA awards were substantially concluded at the endrecognition of 2018, offset by increasesMerck's $250 million participation payment related to our INT collaboration in revenue from the Gates Foundation and BARDA.2022.
Operating expenses
Cost of sales
We began capitalizing our COVID-19 vaccine inventory costs in December 2020, in connection with an Emergency Use Authorization fromIn the FDA and an Interim Order from Health Canada for usethird quarter 2023, we launched a strategic initiative aimed at optimizing the cost structure of our COVID-19 vaccine, and based uponbusiness, with an emphasis on resizing our expectation that these costs would be recoverable through commercialization of mRNA-1273. Prior tomanufacturing cost structure. This initiative was triggered by the capitalizationcompletion of our long-range planning within the third quarter of 2023, which incorporated revised forecasts of vaccination rates reflecting the market's transition from COVID-19 vaccinepandemic conditions to an endemic seasonal market. The initiative involved scaling down our capacity and commitments with our third-party CMOs, reevaluating our raw material inventory levels, and reducing our purchase commitments related to raw materials not anticipated to be consumed before expiration. As a result of this initiative, we incurred inventory write-downs of $903 million related to raw materials, and CMO wind down costs suchand cancellation fees of $513 million. Furthermore, as a continuation of this strategic initiative during the fourth quarter of 2023, we incurred $153 million in CMO wind down costs were recorded as research and development expenses$16 million in inventory write-downs. We believe that this strategic initiative will enhance the period incurred. We expensed $242.0 millionefficiency of pre-launch inventory costs in 2020. our manufacturing operations and provide us with the flexibility to better scale according to future market needs.
Our cost of sales were $7.9 million,was $4.7 billion, or 4.0%70% of our net product sales, in 2020, comprised of2023, including third-party royalties of $6.9$301 million, inventory write-downs of $2.2 billion, unutilized manufacturing capacity and wind down costs of $981 million, and shippinglosses on firm purchase
commitments and handling costscancellation fees of $1.0 million as the associated inventory costs were expensed previously. If inventory sold during 2020 was valued at cost, our$205 million. Our cost of sales was $5.4 billion, or 29% of our net product sales, in 2022, including third-party royalties of $1.1 billion, inventory write-downs of $1.3 billion, unutilized manufacturing capacity and wind down costs of $677 million, and losses on firm purchase commitments and cancellation fees of $725 million. These charges, other than royalties, were largely attributable to a shift in product demand to the latest variant-targeted COVID-19 vaccine and a decline in customer demand, which ultimately led to our strategic initiative implemented in the third quarter of 2023.
Cost of sales for 2020 would have been $62.42023 decreased by $723 million, or 31.2%13%, compared to 2022. The decrease in cost of sales was primarily driven by lower sales volume, partially offset by an increase in inventory write-downs. Cost of sales as a percentage of net product sales for 2023 increased by 41 percentage points to 70% from 29% in 2022. The increase in cost of sales as a percentage of net product sales was mainly due to the strategic initiative described above, and other aforementioned charges (excluding royalties) over lower net product sales, driven by a decline in product demand and increased product seasonality. In light of our product sales. At December 31, 2020, we had $187.5 millionrecent strategic initiative implemented in the third quarter of zero-cost COVID-19 vaccine inventory that2023, we expect to sell prior to March 31, 2021. We expecta reduction in our cost of sales as a percentage of ournet product sales will increase, reflectingfor the full cost of manufacturing, subsequentyear 2024, compared to the utilization of our zero-cost mRNA-1273 inventory.full year 2023.
Research and development expenses
Research and development expenses increased by $874.0 million,$1.6 billion, or 176%47%, in 2020.2023. The increase was primarily attributable to an increaseincreases in clinical trial expenses of $321.2$411 million, an increase inclinical manufacturing expenses of $301.7$335 million, an increase in personnel relatedpersonnel-related costs of $105.2$286 million, an increase in technology and facility related expenses of $74.2 million, and an increase in consulting and outside services of $60.7$267 million. The increase in 20202023 was largely attributable to mRNA-1273the late-stage clinical development associated with our RSV vaccine, CMV vaccine and pre-launch inventory buildup prior to the authorization from the FDA.combination vaccine against seasonal influenza and COVID-19, as well as our INT program. The increase in personnel relatedpersonnel-related costs was primarilymainly driven by an increase in the number of employees supporting our mRNA-1273 development activities as well as otherincreased research and development programs.
Researchprograms and development expenses increased by $42.2 million, or 9% in 2019. The increase was primarily attributable to an increase in personnel related costs of $37.4 million, an increase in clinical trial and manufacturing costs of $16.9 million, and an increase in stock-based compensation of $10.6 million. The increases were partially offset by a decrease in in-licensing costs of $22.0 million related to in-licensing agreements executed in 2017 with Cellscript, LLC and its affiliate mRNA RiboTherapeutics, Inc. (Cellscript) to sublicense certain patent rights, and a decrease in lab supplies and materials of $8.2 million. The increases in personnel related costs and stock-based compensation were largely driven by an increase in the number of employees supporting our research and development programs. The in-licensing costs decreased in 2019 as the sublicense grant fees were fully recognized at the end of 2018.activities.
We expect thatanticipate a modest reduction in research and development expenses will increasein 2024 compared to the current levels of 2023, as we continue to progress mRNA-1273 throughadvance the development process toward a BLA approvalof variant-specific and indication expansion during the current pandemic,next-generation COVID-19 vaccine candidates and continue to develop our pipeline and advance our product candidates into later-stage development. In addition, we also expect to incur significant costs related to the development, of new generations ofin particular those in ongoing Phase 3 studies, including our COVID-19RSV, CMV, seasonal flu and combination vaccine designed to address new variants of the virus.programs, as well as our INT program.
Selling, general and administrative expenses
Selling, general and administrative expenses increased by $78.6$417 million, or 72%37%, in 2020.2023. The increase was mainly due to an increaseincreases in personnel relatedpersonnel-related costs of $30.9$188 million, an increase in consulting and outside services of $26.9$148 million, and marketing expense of $118 million, partially offset by an endowment to the Moderna Charitable Foundation of $50 million contributed in 2022 that did not recur in 2023. The increase in legal and other licensing expenses of $14.2 million. The increases in personnel costs and consulting and outside services were2023 was primarily attributable todriven by increased headcount and mRNA-1273 vaccine candidate commercialization-related activities.
Generalspend in digital, medical affairs and administrative expenses increased by $15.4 million, or 16% in 2019. The increase was primarily duecommercial functions to an increase in insurance costs of $5.2 million, an increase in consultingsupport our digital initiatives, marketed product and outside services of $4.9 million, an increase in information technology and facility-related costs of $4.0 million and an increase in personnel related costs of $2.5 million. The increases in insurance costs,
consulting and outside services and personnel related costs were primarily driven by an increase in the number of employees and costs in support of being a publicly traded company.Company expansion.
We expectanticipate that selling, general and administrative expenses in 2024 will increasebe slightly lower than the levels experienced in 2021 as compared2023. This reflects our ongoing commitment to 2020efficiency as we continue to expand the number of programs and our business operations, and continue to build out our global commercial, regulatory, sales and marketing infrastructure to support the commercialization ofinfrastructure. Moreover, it aligns with our COVID-19 vaccine.strategic focus on advancing our program development and enhancing our overall business processes.
Interest income
Interest income generated from our investments in marketable securities decreasedincreased by $13.8$221 million or 36%, in 2020,2023, mainly attributable to an overall higher interest rate environment, partially offset by lower interest rate.
Interest income generated from our investments in marketable securities increased by $11.5 million, or 43%, in 2019, mainly driven by a higher weighted average balance of cash and investments, primarily from the net proceeds of our IPO.investment balances.
Other (expense) income,expense, net
The following tablestable summarizes other (expense) income, net for each period presented (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | |
| Years Ended December 31, | Change 2020 vs. 2019 |
| 2020 | | 2019 | | Change | | % |
| Gain on investments | $ | 1,301 | | | $ | 323 | | | $ | 978 | | | 303 | % |
| Interest expense | (9,886) | | | (6,612) | | | (3,274) | | | 50 | % |
| Other income (expense), net | 2,501 | | | (1,237) | | | 3,738 | | | (302) | % |
| Total other expense, net | $ | (6,084) | | | $ | (7,526) | | | $ | 1,442 | | | (19) | % |
| | | | | | | | | | | | | | | | | | | | | | | |
| Years Ended December 31, | | Change 2019 vs. 2018 |
| 2019 | | 2018 | | Change | | % |
| Gain on investments | $ | 323 | | | $ | 31 | | | $ | 292 | | | 942 | % |
| Interest expense | (6,612) | | | (3,096) | | | (3,516) | | | 114 | % |
| Other (expense) income, net | (1,237) | | | 4,900 | | | (6,137) | | | (125) | % |
| Total other (expense) income, net | $ | (7,526) | | | $ | 1,835 | | | $ | (9,361) | | | (510) | % |
Total other expense, net decreased by $1.4 million, or 19% in 2020. The decrease was primarily due to an increase in foreign currency gains and a reclassification of other income in 2020, offset by a higher interest expense driven by a new finance lease commenced in 2020 for a laboratory and office space, Moderna Technology Center North (MTC North). Please refer to Note 11 to our consolidated financial statements.the periods presented (in millions):
| | | | | | | | | | | | | | | | | | | | | | | |
| Years Ended December 31, | Change 2023 vs. 2022 |
| 2023 | | 2022 | | Change | | % |
| Loss on investments | $ | (72) | | | $ | (20) | | | $ | (52) | | | 260 | % |
| Interest expense | (38) | | | (29) | | | (9) | | | 31 | % |
| Other (expense) income, net | (14) | | | 4 | | | (18) | | | (450) | % |
| Total other expense, net | $ | (124) | | | $ | (45) | | | $ | (79) | | | 176 | % |
Total other expense, net increased by $9.4$79 million, or 176%, in 2019.2023. The increase was mainly attributableprimarily due to losses on equity investments and a one-time $7.0 million cash receiptnet realized loss on available-for-sale debt securities as well as an increase in 2018 as consideration for the waiver of a third party's previously negotiated commitment, and higherinterest expense. Our interest expense in 2019 of $3.5 million is primarily
related to our financing lease liabilities. We started recording thefinance leases. The increase in interest expense was driven by new finance leases that commenced in July 2018 upon the completion of our Moderna Technology Center manufacturing facility, or MTC South.2023. Please refer to Note 1110 to our consolidated financial statements.
Provision for (benefit from) income taxes
ThereProvision for income taxes decreased by $441 million, or 36%, in 2023, primarily due to a significant decrease in pre-tax income, partially offset by an increase in valuation allowance on deferred tax assets of $2.1 billion. Our effective tax rate for the year ended December 31, 2023 was a(19.6)%, which is higher than the statutory rate. It is mainly driven by an increase in valuation allowances on deferred tax provision of $2.5 million in 2020, primarily related to foreign income taxes. There were no significant incomeassets, partially offset by tax benefits or provisionsfrom research and development credits and stock-based compensation. As a result of the significant reduction in 2019pre-tax income and 2018.the valuation allowance, the 2023 effective tax rate is not comparable to the prior year. Please refer to Note 13 to our consolidated financial statements.
Liquidity and capital resources
We have historically fundedThe following table summarizes our operations primarily from the sale of equity instruments and from proceeds from certain strategic alliance arrangements and grant agreements. During 2020, we entered into supply agreements with the U.S. Government and several governmental agencies outside the United States for the supply of mRNA-1273, our COVID-19 vaccine, and received upfront deposits of $2.92 billion, of which $82.1 million was recognized as product sales. In addition, we raised $1.85 billion in total through two public equity offerings in 2020. As of December 31, 2020, we had cash, cash equivalents, investments and investments of $5.25 billion. Cash,working capital for each period presented (in millions):
| | | | | | | | | | | | | | |
| | December 31, |
| | 2023 | | 2022 |
| Financial assets: | | | | |
| Cash and cash equivalents | | $ | 2,907 | | | $ | 3,205 | |
| Investments | | 5,697 | | | 6,697 | |
| Investments, non-current | | 4,677 | | | 8,318 | |
| Total | | $ | 13,281 | | | $ | 18,220 | |
| | | | |
| Working capital: | | | | |
| Current assets | | $ | 10,325 | | | $ | 13,431 | |
| Current liabilities | | 3,015 | | | 4,923 | |
| Total | | $ | 7,310 | | | $ | 8,508 | |
Our cash, cash equivalents and investments are invested in accordance with our investment policy, primarily with a view to liquidity and capital preservation. Investments,Our investments, consisting primarily of government and corporate debt securities, are stated at fair value. AsCash, cash equivalents and investments as of December 31, 2020,2023 decreased by $4.9 billion, or 27%, compared to December 31, 2022. For the year ended December 31, 2023, we had current and non-current investmentsa net cash outflow from operations of approximately $1.98$3.1 billion, repurchases of our common stock of $1.2 billion, and $0.64 billion, respectively.purchases of property, plant and equipment of $707 million.
We began constructionWorking capital, which is current assets less current liabilities, as of December 31, 2023 decreased by $1.2 billion, or 14%, compared to December 31, 2022, primarily due to a decrease in cash, cash equivalents and short-term investments of $1.3 billion, primarily to fund our operating activities and repurchases of our MTC South facility,common stock, a decrease in the second halfinventory of 2016$747 million, a decrease in prepaid and completed construction in 2019. In the second quarterother current assets of 2019, we entered into an additional lease for office and laboratory space nearby for our MTC North facility and construction on this facility began in 2019, and is continuing. Our capital expenditures related to our MTC facilities were $46.6 million, $3.9$568 million, and $86.5 million for the years ended December 31, 2020, 2019 and 2018, respectively. Cash disbursements related to our MTC facilities were $41.7 million, $14.6 million and $94.5 million for the years ended December 31, 2020, 2019 and 2018, respectively.a decrease in accounts receivable, net of $493 million. This was partially offset by a decrease in short-term deferred revenue of $1.5 billion, mainly driven by revenue recognized from deferred revenue in excess of customer deposits received.
On January 30, 2018 and February 15, 2018, we issued Series G preferred stock for total gross proceeds of $560.0 million. On May 7, 2018, we issued Series H preferred stock for gross proceeds of $125.0 million of which $13.0 million was determined to be a premium and recorded to deferred revenue as part of the Merck PCV/SAV agreement executed contemporaneously with our Series H redeemable convertible preferred stock issuance.
On December 11, 2018, we closed our IPO, whereby we sold 26,275,993 shares of common stock at a price of $23.00 per share. The shares began trading on the NASDAQ Global Select Market on December 7, 2018. The aggregate net proceeds received by us from the IPO were $563.0 million, net of underwriting discounts, commissions and offering expenses.
In February 2020, we completed a public equity offering of 30,263,158 shares of common stock, including exercise of the underwriters' option over-allotment option, at a price of $19.00 per share. The aggregate net proceeds from the offering were $549.5 million, net of underwriting discounts, commissions and offering expenses.
In May 2020, we completed a public equity offering of 17,600,000 shares of common stock, at a price of $76.00 per share. The aggregate net proceeds from the offering were $1.30 billion, net of underwriting discounts, commission and offering expenses.
We have used and intend to use the net proceeds from these equity offerings: (i) to fund working capital needs (raw materials, labor and capital equipment purchases) related to the manufacturing of mRNA-1273 for distribution in the United States and outside the United States, assuming necessary regulatory approvals are obtained and the remainder, if any, (ii) to fund clinical development and drug discovery in existing and new therapeutic areas, (iii) to fund further development of our mRNA technology platform and the creation of new modalities, or (iv) to fund working capital and other general corporate purposes.
Cash flow
The following table summarizes the primary sources and uses of cash for each periodthe periods presented (in thousands)millions): | | | | | | | | | | | | | |
| Years Ended December 31, |
| 2023 | | 2022 | | |
| Net cash (used in) provided by: | | | | | |
| Operating activities | $ | (3,118) | | | $ | 4,981 | | | |
| Investing activities | 4,206 | | | (5,176) | | | |
| Financing activities | (1,377) | | | (3,448) | | | |
| Net decrease in cash and cash equivalents | $ | (289) | | | $ | (3,643) | | | |
| | | | | | | | | | | | | | | | | |
| Years Ended December 31, |
| 2020 | | 2019 | | 2018 |
| Net cash provided by (used in): | | | | | |
| Operating activities | $ | 2,026,971 | | | $ | (458,968) | | | $ | (330,865) | |
| Investing activities | (1,671,928) | | | (14,945) | | | (373,094) | |
| Financing activities | 2,033,193 | | | 51,121 | | | 1,226,842 | |
| Net increase (decrease) in cash and cash equivalents | $ | 2,388,236 | | | $ | (422,792) | | | $ | 522,883 | |
Operating activities
We derive cash flows from operations primarily from cash collected from customer deposits and accounts receivable, net related to our COVID-19 vaccine supply agreementsproduct sales, as well as certain government-sponsored and private organizations and strategic alliances. Our cash flows from operating activities are significantly affected by our use of cash for operating expenses and working capital to support the business. Prior toBeginning in the third quarter of 2020, we have historically experienced negative cash flows from operating activities asentered into supply agreements with the U.S. Government and foreign governments and organizations for the supply of our COVID-19 vaccine and received upfront deposits. In the third quarter of 2023, we have investedcommenced sales of our COVID-19 vaccine to the U.S. commercial market, in addition to continuing sales to foreign governments and organizations. In the U.S., our mRNA technologies, development pipeline, digital infrastructure, manufacturing technology,COVID-19 vaccine is sold primarily to wholesalers and infrastructure.distributors, and to a lesser extent, directly to retailers and healthcare providers. Wholesalers and distributors typically do not make upfront payments to us. As of December 31, 2023, we had $613 million in deferred revenue related to customer deposits received or billable.
Net cash providedused by operating activities in 20202023 was $2.03$3.1 billion and consisted of net loss of $747.1 million less$4.7 billion and non-cash adjustments of $197.0 million,$1.7 billion, plus a net change in assets and liabilities of $2.58 billion.$139 million. Non-cash items primarily included stock-based compensationdeferred income taxes of $93.0 million, leased assets expensed of $62.3$828 million, depreciation and amortization of $31.3$621 million, and amortization of investment premiums and discounts of $10.2 million. The net change in assets and liabilities was primarily due to an increase in deferred revenue of $3.84 billion, an increase in accrued liabilities of $388.4 million, an increase in accounts payable of $11.9 million, and an increase in operating lease liabilities of $11.8 million, partially offset by an increase in accounts receivable of $1.39 billion, an increase in prepaid expenses and other assets of $241.0 million, an increase of inventory of $46.5 million, and an increase in operating lease right-of-use assets of $10.5 million.
Net cash used in operating activities in 2019 was $459.0 million and consisted of net loss of $514.0 million less non-cash adjustments of $108.7 million, plus a net change in assets and liabilities of $53.7 million. Non-cash items primarily included stock-based compensation of $81.1 million, depreciation and amortization of $31.0 million, and amortization of investment premiums and discounts of $3.7$305 million. The net change in assets and liabilities was primarily due to a decrease in deferred revenue of $44.1 million, a decrease in accounts payable$2.1 billion due to revenue recognized upon shipments of $24.0 million, a decrease in other liabilities of $6.2 million, an increase in right-of-use assets, operating leases of $5.7 million and an decrease in accrued liabilities of $3.4 million,our COVID-19 vaccine, partially offset by an increase in operating lease liabilities, non-current of $12.6 million, a decrease in prepaid expenses and other assets of $9.8 million$1.0 billion, primarily driven by write-downs of long term inventory, decrease in income tax receivable due to receipt of tax refunds and decrease in pre-tax income, and reductions in vendor prepayments and down payments as well as a decrease in accounts receivableinventory of $6.7 million.$747 million due to inventory write-downs.
Net operating cash usedflows decreased by $8.1 billion, or 163%, in operating activities in 2018 was $330.9 million and consisted of net loss of $384.7 million less non-cash adjustments of $96.5 million, plus a net change in assets and liabilities of $42.6 million. Non-cash items2023 compared to 2022, primarily included stock-based compensation of $72.6 million and depreciation and amortization of $24.9 million. The net change in assets and liabilities was primarily dueattributable to a decrease in deferred revenuenet income of $65.3 million,$13.1 billion, a $1.3 billion change in accounts receivable due to lower product sales and an increasetiming of collection, and a $1.2 billion change in accounts payable and accrued liabilities driven by higher vendor spend and timing of payments. This was partially offset by a change in prepaid expenses and other assets of $5.3 million, partially offset$2.7 billion primarily driven by inventory write-downs and reductions in vendor down payments, a change in deferred revenue of $2.1 billion due to less revenue recognized from deferred revenue in excess of customer deposits received, and a decrease in deferred income tax assets of $1.4 billion due to an increase in accounts payable of $15.0 million and an increase in accrued liabilities of $8.8 million.valuation allowance.
Investing activities
Our primary investing activities consist of purchases, sales, and maturities of our investments, and capital expenditures for land, leasehold improvements, manufacturing, laboratory, computer equipment and software.software, and business development.
Net cash used inprovided by investing activities in 20202023 was $1.67$4.2 billion, which primarily included purchases of marketable securities of $2.96 billion and purchases of property and equipment of $67.4 million, partially offset by proceeds from maturities of marketable securities of $1.14$5.6 billion and proceeds from sales of marketable securities of $214.7$3.2 billion, partially offset by purchases of marketable securities of $3.8 billion, and purchases of property, plant and equipment of $707 million.
Net investing cash flows increased by $9.4 billion, or 181%, in 2023 compared to 2022, primarily due to a decrease in purchases of marketable securities of $7.7 billion and an increase in proceeds from maturities of marketable securities of $2.4 billion.
Financing activities
Our primary financing activities consist of repurchases of common stock, issuance of common stock related to our equity plans, and finance leases.
Net cash used in financing activities in 2023 was $1.4 billion, primarily from repurchases of common stock of $1.2 billion and changes in financing lease liabilities of $270 million, partially offset by net proceeds from the issuance of common stock in connection with the exercise of stock options and employee stock purchases under our equity plans of $46 million.
Net cash used in investing activities in 2019 was $14.9 million, which included purchases of marketable securities of $1.15 billion and purchases of property and equipment of $31.6 million, partially offset by proceeds from maturities of marketable securities of $993.2 million and proceeds from sales of marketable securities of $168.7 million.
Net cash used in investing activities in 2018 was $373.1 million, which included purchases of marketable securities of $1.23 billion and purchases of property and equipment of $105.8 million, partially offset by proceeds from maturities of marketable securities of $783.4 million and proceeds from sales of marketable securities of $177.0 million.
Financing activities
We generated cash from financing activities of $2.03decreased by $2.1 billion, or 60%, in 2020, primarily from net proceeds from equity offerings of $1.85 billion, net proceeds from the issuance2023 compared to 2022, mainly due to a decrease in repurchases of common stock through our equity plans of $179.6 million, and proceeds from the purchase of common stock under our employee stock purchase plan of $7.0 million.
We generated cash from financing activities of $51.1 million in 2019, primarily from net proceeds from the issuance of common stock under our equity plans of $47.3 million, and net proceeds from the purchase of common stock from our employee stock purchase plan of $2.9 million.
We generated cash from financing activities of $1.23 billion in 2018, primarily from net proceeds from the issuance of redeemable convertible preferred stock of $661.1 million and net proceeds from the issuance of common stock of $563.0 million in connection with our IPO.stock.
Operation and funding requirements
SinceOur principal sources of funding as of December 31, 2023 consisted of cash and cash equivalents, investments, and cash we may generate from operations. We generated net income of $8.4 billion and $12.2 billion for the years ended 2022 and 2021, respectively,
following the authorization of our inception, we havefirst commercial product in December 2020. We also incurred significant losses and negative cash flows from operations due to our significant research and development expenses.a net loss of $4.7 billion for the year ended December 31, 2023. We have an accumulated deficitretained earnings of $2.24 billion and $1.50$13.6 billion as of December 31, 2020 and 2019, respectively. 2023.
We expect ourhave significant future capital requirements including expected operating expenses to increase in connection withconduct research and development activities, operate our organization, and meet capital expenditure needs. We anticipate maintaining substantial expenses across all areas of our ongoing activities, particularly as we continue research and development of our development candidates and clinical activities for our investigational medicines. WeThis also expectextends to our expenses to increase associated with manufacturing costs, including our arrangements with our international supply and manufacturing partners. Our ongoing work on mRNA-1273our RSV, seasonal flu and CMV vaccine candidates, individualized neoantigen therapy, COVID-19 vaccines, including development of any new vaccines against variants of SARS-CoV-2, combination vaccines, late-stage clinical development, and buildout of global commercial, regulatory, sales and marketing infrastructure and manufacturing facilities will require significant cash outflows during 2021,in future periods, most of which may not be reimbursed or otherwise paid for by our partners or collaborators. In addition, we have substantial facility, lease and purchase obligations. We have also entered into certain collaboration agreements with third parties that include the funding of certain research and development activities and potential future milestone and royalty payments by us.
We believe that our cash, cash equivalents, and investments as of December 31, 2020,2023, together with cash expected to be generated from product sales, will be sufficient to enable us to fund our projected operations and capital expenditures through at least the next 12 months from the issuance of ourthe financial statements.statementsincluded in this Annual Report on Form 10-K. We are subject to all the risks related to the development and commercialization of novel medicines, and we may encounter unforeseen expenses, difficulties, complications, delays, and other unknown factors, including expenses related to the ongoing coronavirus pandemic, which may adversely affect our business. For example, we have experienced a decline in customer demand for our COVID-19 vaccine, reflecting the market's ongoing transition to a seasonal model in an endemic market in 2023. We foresee that our commitment to investing in our business for future product launches may lead to continued negative cash flows from operations in upcoming periods. Our forecast of the period of time through which our financial resources will be adequate to support our operations is a forward-looking statement and involves risks and uncertainties, and actual results could vary as a result of a number of factors. We have based this estimate on assumptions that may prove to be wrong, and we could utilize our available capital resources sooner than we currently expect.
If we fail to become profitable or are unable to sustain profitability on a continuing basis, we may be required to finance future cash needs through a combination of public or private equity offerings, structured financings and debt financings, government funding arrangements, potential future strategic alliances from which we receive upfront fees, milestone payments, and other forms of consideration, and marketing, manufacturing, distribution and licensing arrangements. If we are required to finance future cash needs, additional capital may not be available on reasonable terms, if at all. If we are unable to raise additional capital in sufficient amounts or on terms acceptable to us, we may have to significantly delay, scale back, or discontinue the development or commercialization of one or more of our investigational medicines, or slow down or cease work on one or more of our programs. If we raise additional funds through the issuance of additional equity or debt securities, it could result in dilution to our existing stockholders or increased fixed payment obligations, and any such securities may have rights senior to those of our common stock. If we incur indebtedness, we could become subject to covenants that would restrict our operations and potentially impair our competitiveness, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. If we raise funds through strategic alliances or marketing, distribution, or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs, or investigational medicines or grant licenses on terms that may not be favorable to us. Any of these events could significantly harm our business, financial condition, and prospects.
Off balance sheet arrangements
As of December 31, 2020, we did not have any off-balance sheet arrangements, as defined in Item 303(a)(4)(ii) of Regulation S-K.
Contractual obligations and commitments
The following table summarizes our contractual obligations as of December 31, 20202023 and the effects that such obligations are expected to have on our liquidity and cash flows in future periods (in thousands)millions):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Payments Due by Period |
| Total | | Less than 1 year | | 1 - 3 years | | 3 - 5 years | | More than 5 years |
Operating leases (1) | $ | 173,747 | | | $ | 15,492 | | | $ | 31,921 | | | $ | 32,735 | | | $ | 93,599 | |
Financing leases (1) | 513,312 | | | 36,579 | | | 23,902 | | | 24,772 | | | 428,059 | |
Purchase obligations (2) | 686,472 | | | 677,539 | | | 8,855 | | | 78 | | | — | |
Total contractual cash obligations (1) | $ | 1,373,531 | | | $ | 729,610 | | | $ | 64,678 | | | $ | 57,585 | | | $ | 521,658 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Payments Due by Period |
| Total | | Less than 1 year | | 1 - 3 years | | 3 - 5 years | | More than 5 years |
Operating leases | $ | 1,133 | | | $ | 72 | | | $ | 134 | | | $ | 142 | | | $ | 785 | |
Financing leases(1) | 1,184 | | | 20 | | | 44 | | | 46 | | | 1,074 | |
| | | | | | | | | |
Purchase obligations(2) | 2,103 | | | 1,002 | | | 685 | | | 272 | | | 144 | |
| Total contractual cash obligations | $ | 4,420 | | | $ | 1,094 | | | $ | 863 | | | $ | 460 | | | $ | 2,003 | |
_______
(1)The amounts in the table include a total payment of $338.9$668 million associated with our MTC South and MTC North leases for the optional lease extension periods. For accounting purpose,purposes, a lease term is the non-cancelable period of the lease and includes options to extend or terminate the lease when it is reasonably certain that an option will be exercised. Please refer to Note 1110 to our consolidated financial statements. (2)The amounts represent non-cancelable fixed payment obligations related to purchases of raw materials, contract manufacturing services, clinical services and other goods or services in the normal course of business.
Under our strategic collaboration agreements, we are committed to perform certain research, development, and manufacturing activities. As part of our personalized mRNA cancer vaccines, or PCV, collaboration and license agreement with Merck, we are committed to perform certain research, development, and manufacturing activities related to PCV products through an initial Phase 2 clinical trial up to a budgeted amount of $243.0 million as of December 31, 2020. Please refer2023, $79 million of the purchase commitments related to Note 5 to the consolidated financial statements. The expenses we expect to incurraw materials was recorded as part of our commitments under the PCV and other collaboration agreements were not included in the above table as we are not able to determine the timing and amounts of such expenses.an accrued liability for loss on future firm purchase commitments.
We have agreements with certain vendors for various services, including services related to clinical operations, and support and contract manufacturing, which we are not contractually able to terminate for convenience. Certain agreements provide for termination rights subject to termination fees or wind down costs. Under such agreements, we are contractually obligated to make certain payments to vendors, mainly to reimburse them for their unrecoverable outlays incurred prior to cancellation. The exact amounts of such obligations are dependent on the timing of termination, and the exact terms of the relevant agreement and cannot be reasonably estimated. At December 31, 2020,2023, we had cancelable open purchase orders of $896.9 million$3.0 billion in total under such agreements for our clinical operations and support and contract manufacturing. These amounts represent only our estimate of those items for which we
had a contractual commitment to pay at December 31, 2020,2023, assuming we would not cancel these agreements. The actual amounts we pay in the future to the vendors under such agreements may differ from the cancelable open purchase order amounts of $896.9 million.$3.0 billion.
In addition to the above obligations, we enter into a variety of agreements and financial commitments in the normal course of business. The terms generally allow us the option to cancel, reschedule, and adjust our requirements based on our business needs, prior to the delivery of goods or performance of services. It is not possible to predict the maximum potential amount of future payments under these agreements due to the conditional nature of our obligations and the unique facts and circumstances involved in each particular agreement.
As of December 31, 2023, we did not have any off-balance sheet arrangements that were material or reasonably likely to become material to our financial condition or results of operations.
Item 7A. Quantitative and Qualitative Disclosures about Market Risk
Interest Rate Risk
Our primary exposure to market risk relates to changes in interest rates.
As of December 31, 20202023 and 2019,2022, we had cash, cash equivalents, restricted cash, and investments in marketable securities of $5.25$13.3 billion and $1.26$18.2 billion, respectively. Our investment portfolio comprises money market funds and marketable debt securities (including U.S. Treasury securities, debt securities of U.S. government agencies and corporate entities, and commercial paper), which are classified as available-for-sale securities. Our primary investment objectives are the preservation of capital and the maintenance of liquidity, and our investment policy defines allowable investments based on quality of the institutions and financial instruments designed to have very lowminimize risk exposure. We generally hold investments in marketable debt securities to maturity to limit our exposure to interest rate risk. Our exposure to interest rate sensitivity is affected by changes in the general level of U.S. interest rates.
Our marketable securities are subject to interest rate risk and will fall in value if market interest rates increase. Due to the short-term maturities and low risk profiles of our investments, we do not anticipate a significant exposure to interest rate risk. If market interest rates were to increase immediately and uniformly by one percentage point from levels at December 31, 2020 and 2019,2023, the net fair value of our interest sensitive marketable securities would not experience a material change in fair market value.decrease by approximately $83 million.
Foreign Currency Risk
OurWe transact business in various foreign currencies and have international sales and expenses denominated in foreign currencies. Therefore, we are exposed to certain risks arising from both our business operations and economic conditions. For the year ended December 31, 2023, our revenue generating activities and operations have beencontinued to be primarily denominated in U.S. dollars. However, we maintained a significant exposure to foreign currency risk, particularly in the Euro, Japanese Yen and Swiss Franc markets, Our significant foreign currency revenue exposure was the equivalent of $1.4 billion in Japanese Yen and $518 million in Euros for 2023. As we expand internationallypursue our international expansion strategy, our results of operations and cash flows will become increasinglyremain subject to fluctuations due to changes in foreign currency exchange rates. To help manage the exposure to foreign currency exchange rate fluctuations, we have implemented acash flow hedging and balance sheet hedging program.programs.
Cash Flow Hedging Activities
We mitigate the foreign exchange risk arising from the fluctuations in foreign currency denominated product sales in Euro and Japanese Yen through a foreign currency cash flow hedging program, using forward contracts and foreign currency options that do not exceed 15 months in duration. We hedge these cash flow exposures to reduce the risk that our earnings and cash flows will be adversely affected by changes in exchange rates. To receive hedge accounting treatment, all hedging relationships are formally documented at the inception of the hedge, and the hedges must be highly effective in offsetting changes to future cash flows on hedged transactions. The derivative assets or liabilities associated with our hedging activities are recorded at fair value in prepaid expenses and other current assets or other current liabilities, respectively, in our consolidated balance sheets. The gains or losses resulting from changes in the fair value of these hedges are initially recorded as a component of accumulated other comprehensive (loss) income (AOCI) in stockholders’ equity and subsequently reclassified to product sales in the period during which the hedged transaction affects earnings. In the event the underlying forecasted transaction does not occur, or it becomes probable that it will not occur, within the defined hedge period, we reclassify the gains or losses on the related cash flow hedge from AOCI to other expense, net, in our consolidated statements of operations. We evaluate hedge effectiveness at the inception of the hedge prospectively, and on an on-going basis both retrospectively and prospectively. If we do not elect hedge accounting, or the contract does not qualify for hedge accounting treatment, the changes in fair value from period to period are recorded as a component of other expense, net, in our consolidated statements of operations. We had no outstanding foreign currency forward contracts or foreign currency options as of December 31, 2023. Foreign currency hedging activities were immaterial during 2023.
Balance Sheet Hedging Activities
We useenter into foreign currency forward contracts to mitigate foreign currency exchange risk associated with foreign currency-denominated monetary assets and liabilities. Notwithstanding our efforts to mitigate some foreign currency exchange risks, there can be no assurance that our hedging activities will adequately protect us against the riskshedge fluctuations associated with foreign currency fluctuations.denominated monetary assets and liabilities, primarily cash, accounts receivable, accounts payable and lease liabilities in Euro, Swiss Franc and Japanese Yen, that are not designated for hedge accounting treatment. Therefore, these forward contracts are accounted for as derivatives whereby the fair value of the contracts are reported as prepaid expenses and other current assets or other current liabilities in our consolidated balance sheets, and gains and losses resulting from changes in the fair value are recorded as a component of other expense, net, in our consolidated statements of operations. The gains and losses on these foreign currency forward contracts generally offset the gains and losses in the underlying foreign currency denominated assets and liabilities, which are also recorded to other expense, net, in our consolidated statements of operations. As of December 31, 2023, our outstanding balance sheet hedging derivatives, carried at fair value, had maturities of less than three months.
We enter into these foreign exchange contracts to hedge our forecasted revenue and monetary assets and liabilities denominated in foreign currency in the normal course of business and accordingly, they are not speculative in nature. We believe the counterparties to
our foreign currency forward contracts are creditworthy multinational commercial banks. While we believe the risk of counterparty nonperformance is not material, a sustained decline in the financial stability of financial institutions as a result of disruption in the financial markets could affect our ability to secure creditworthy counterparties for our foreign currency hedging programs.
Notwithstanding our efforts to mitigate some foreign currency exchange risks, there can be no assurance that our hedging activities will adequately protect us against the risks associated with foreign currency fluctuations. As of December 31, 2020,2023, a hypothetical adverse movement of 10 percent in foreign currency exchange rates compared to the U.S. dollars across all maturities would have resulted in potential declines in the fair value on our foreign currency forward contracts used in balance sheet hedging of approximately $36.5$23 million. We expect that any increase or decrease in the fair value of the portfolio would be substantially offset by increases or decreases in the underlying exposures being hedged. We did not have any foreign currency hedge activities prior to 2020.
Item 8. Financial Statements and Supplementary Data
MODERNA, INC.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
Report of Independent Registered Public Accounting Firm
To the Stockholders and the Board of Directors of Moderna, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Moderna, Inc. (the Company) as of December 31, 20202023 and 2019,2022, the related consolidated statements of operations, comprehensive loss, redeemable convertible preferred stock andincome (loss), stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2020,2023, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 20202023 and 2019,2022, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2020,2023, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company's internal control over financial reporting as of December 31, 2020,2023, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) and our report dated February 26, 202123, 2024, expressed an unqualified opinion thereon.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit MatterMatters
The critical audit mattermatters communicated below is a matterare matters arising from the current period audit of the financial statements that waswere communicated or required to be communicated to the audit committee and that: (1) relatesrelate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective or complex judgments. The communication of the critical audit mattermatters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit mattermatters below, providing separate opinions on the critical audit mattermatters or on the accounts or disclosures to which it relates.they relate.
| | | | | | | | |
| | |
| | Collaboration RevenueReserves for returns on product revenue |
| Description of the Matter | | As discussed in Note 2 to the consolidated financial statements, the Company recognizes revenue under Accounting Standards Codification (ASC) Topic 606, Revenue from Contracts with Customers (ASC 606). The Company recognized $74.6 million in collaboration revenue forDuring the year ended December 31, 2020.
The Company recognizes revenue based on2023, the amountCompany’s net product revenues for Spikevax were $6.7 billion. As explained in Note 2 of the transaction price that is allocated to each respective performance obligation when or as the performance obligation is satisfied by transferring a promised good or service to the customer. In determining the totalconsolidated financial statements, revenue to be recognized under the proportional performance models, the Company develops assumptions that require judgment to determine the total expected effortfrom product sales includes estimates of variable consideration for each performance obligation,which reserves are established, including both internal and external estimated research and development costs. For performance obligations that are satisfied at a point in time, the Company recognizes revenue when control of the goods and/or services is transferred to the customer. For performance obligations that are satisfied over time, the Company recognizes revenue by measuring the progress toward complete satisfaction of the performance obligation using a single method of measuring progress which depicts the performance in transferring control of the associated goods and/or services to the customer. The Company evaluates the measure of progress each reporting period and, if necessary, adjusts the measure of performance and related revenue recognition.reserves for product returns.
Auditing the Company’s proportional performance models ismeasurement of reserves for product returns under its contracts with wholesalers, distributors and retail customers (collectively, “Customers”) was especially challenging because (1) it involves management assumptions about inventory remaining in the assessmentdistribution channel as of progress required a high degree of audit judgment due to the subjectivity, including estimating the remaining research and development costs necessary to satisfy a performance obligation. The recognition of revenue pursuant to collaboration arrangements isbalance sheet date that could be subject to these estimatesreturn in future periods and judgments developed by management, asprojected market demand, and (2) the underlying proportional performance models are sensitiveCompany has limited returns history on which to changes in the assumptions, including the Company’s estimate in determining the remaining level of effort required under an arrangement.base its assumptions.
|
| | | | | | | | |
| How We Addressed the Matter in Our Audit | | We obtained an understanding, evaluated the design and tested the operating effectiveness of internal controls over the collaboration revenue recognition process. This included testingCompany’s process to determine reserves for returns on product revenue. For example, we tested controls over themanagement’s review of management's significant judgmentsthe completeness and estimates related to the inputs to the proportional performance model including (i) an estimateaccuracy of the totaldata used in the process and remaining pre-clinical and clinical activities, (ii) an estimate of the total and remainingassumptions about the amount of clinical materialinventory in the distribution channel that could be subject to be delivered and (iii) an estimate of the total and remaining costs toreturn in future periods.
be incurred related to each performance obligation.
To evaluatetest the Company’s proportional performance models utilizedreserves for the Company’s ongoing recognition ofreturns on product revenue, for its collaborative arrangements, our audit procedures included, among others, reading the agreements and all related schedules, andother procedures, testing the accuracy and completeness of the underlying data used in the calculations and evaluating the estimatesassumptions used by management to estimate its reserves. To test management’s assumptions, we inspected agreements with significant Customers to validate the rights of return, made inquiries of members of the commercial function regarding any changes to the terms and conditions of commercial contracts. We also examined credit memos issued during and after year end for unusual items or trends not consistent with the Company’s analysis of product returns and performed revenue cutoff testing at period end to assess whether there were unusual trends that should have been considered in the Company analysis of product returns. In addition, we reviewed inventory on hand-reporting from significant judgments, described above. ToCustomers at the balance sheet date and subsequent to the balance sheet date and inspected vaccination data from third-party sources through the report date. We also performed sensitivity analyses over the Company’s return rate to assess the reasonablenesseffect of changes in assumptions.
|
| | Raw Material Inventory Write-downs |
| Description of the Matter | | As of December 31, 2023, the Company had $0.4 billion of inventory. As disclosed in Note 2, inventory is recorded at the lower of cost or net realizable value. The Company periodically reviews the composition of inventory in order to identify excess, obsolete, slow-moving or otherwise unsaleable items. For the year ended December 31, 2023, inventory write-downs were $2.2 billion, which included $903 million in raw material inventory write-downs resulting from the Company’s long-range financial planning process in the third quarter of 2023.
Auditing management's estimates for raw material inventory write-downs involved especially subjective auditor judgment because the estimates rely on a number of factors that are affected by market and economic conditions outside the Company’s control. In particular, raw material inventory write-downs are sensitive to significant estimatesassumptions, including the expected demand for the Company’s products and judgments, we corroborated management estimatesthe expiration dates of the raw materials.
|
| How We Addressed the Matter in Our Audit | | We obtained an understanding, evaluated the design, and judgments through atested the operating effectiveness of internal controls over the Company's raw material inventory write-down process including management’s review of the agreementssignificant assumptions described above and evaluatedcontrols over the completeness and accuracy of the information used to develop the estimates.
To test the Company’s raw material inventory write-downs, our audit procedures included, among other procedures, testing the accuracy and completeness of the underlying data used in the calculations and evaluating the assumptions used by management in its estimates. Our testing of the data used in the Company’s estimates included the accuracy of the prior period estimatesraw material expiration dates used, materials required in the Company’s products, and judgments as a potential sourceany alternate uses. We evaluated the Company’s forecast of corroborating or contradictory evidence.future demand, including testing of the significant assumptions within its forecast, and evaluating the consistency of the forecast with that used by management for other purposes. We also discussedperformed sensitivity analyses to assess the judgments withimpact of changes in significant assumptions to the Company’s researchraw material inventory write-downs and development personnel that oversee the collaboration arrangements.
|
| | |
| | |
| | |
| | |
| | |
| | |
| | |
/s/ Ernst & Young LLP
We have served as the Company’s auditor since 2014.
Boston, Massachusetts
February 26, 2021
MODERNA, INC.
CONSOLIDATED BALANCE SHEETS
(In thousands,millions, except share and per share data)
| | | | | | | | | | | |
| December 31, |
| 2020 | | 2019 |
| Assets | | | |
| Current assets: | | | |
| Cash and cash equivalents | $ | 2,623,850 | | | $ | 235,876 | |
| Investments | 1,983,758 | | | 867,124 | |
| Accounts receivable | 1,390,560 | | | 5,369 | |
| Inventory | 46,527 | | | 0 |
| Prepaid expenses and other current assets | 252,152 | | | 19,403 | |
| Restricted cash | 1,032 | | | 1,032 | |
| Total current assets | 6,297,879 | | | 1,128,804 | |
| Investments, non-current | 638,848 | | | 159,987 | |
| Property and equipment, net | 296,889 | | | 201,495 | |
| Right-of-use assets, operating leases | 90,201 | | | 86,414 | |
| Restricted cash, non-current | 11,053 | | | 10,791 | |
| Other non-current assets | 1,880 | | | 1,931 | |
| Total assets | $ | 7,336,750 | | | $ | 1,589,422 | |
| Liabilities and Stockholders’ Equity | | | |
| Current liabilities: | | | |
| Accounts payable | $ | 18,359 | | | $ | 7,090 | |
| Accrued liabilities | 469,591 | | | 67,652 | |
| Deferred revenue | 3,867,193 | | | 63,310 | |
| Other current liabilities | 33,665 | | | 5,063 | |
| Total current liabilities | 4,388,808 | | | 143,115 | |
| Deferred revenue, non-current | 177,419 | | | 138,995 | |
| Operating lease liabilities, non-current | 97,421 | | | 93,675 | |
| Financing lease liabilities, non-current | 109,874 | | | 38,689 | |
| Other non-current liabilities | 1,853 | | | 138 | |
| Total liabilities | 4,775,375 | | | 414,612 | |
| Commitments and contingencies (Note 12) | 0 | | 0 |
| Stockholders’ equity: | | | |
Preferred stock, $0.0001 par value; 162,000,000 shares authorized at December 31, 2020 and 2019; 0 shares issued or outstanding at December 31, 2020 and 2019 | 0 | | | 0 | |
| Common stock, par value $0.0001; 1,600,000,000 shares authorized as of December 31, 2020 and 2019; 398,787,678 and 336,536,985 shares issued and outstanding as of December 31, 2020 and 2019, respectively | 40 | | | 34 | |
| Additional paid-in capital | 4,801,849 | | | 2,669,426 | |
| Accumulated other comprehensive gain | 3,004 | | | 1,804 | |
| Accumulated deficit | (2,243,518) | | | (1,496,454) | |
| Total stockholders’ equity | 2,561,375 | | | 1,174,810 | |
| Total liabilities and stockholders’ equity | $ | 7,336,750 | | | $ | 1,589,422 | |
| | | | | | | | | | | |
| December 31, |
| 2023 | | 2022 |
| Assets | | | |
| Current assets: | | | |
| Cash and cash equivalents | $ | 2,907 | | | $ | 3,205 | |
| Investments | 5,697 | | | 6,697 | |
| Accounts receivable, net | 892 | | | 1,385 | |
| Inventory | 202 | | | 949 | |
| Prepaid expenses and other current assets | 627 | | | 1,195 | |
| | | |
| Total current assets | 10,325 | | | 13,431 | |
| Investments, non-current | 4,677 | | | 8,318 | |
| Property, plant and equipment, net | 1,945 | | | 2,018 | |
| Right-of-use assets, operating leases | 713 | | | 121 | |
| | | |
| Deferred tax assets | 81 | | | 982 | |
| Other non-current assets | 685 | | | 988 | |
| Total assets | $ | 18,426 | | | $ | 25,858 | |
| Liabilities and Stockholders’ Equity | | | |
| Current liabilities: | | | |
| Accounts payable | $ | 520 | | | $ | 487 | |
| Accrued liabilities | 1,798 | | | 2,101 | |
| Deferred revenue | 568 | | | 2,038 | |
| Income taxes payable | 63 | | | 48 | |
| Other current liabilities | 66 | | | 249 | |
| Total current liabilities | 3,015 | | | 4,923 | |
| Deferred revenue, non-current | 83 | | | 673 | |
| Operating lease liabilities, non-current | 643 | | | 92 | |
| Financing lease liabilities, non-current | 575 | | | 912 | |
| Other non-current liabilities | 256 | | | 135 | |
| Total liabilities | 4,572 | | | 6,735 | |
Commitments and contingencies (Note 11) | | | |
| Stockholders’ equity: | | | |
| Preferred stock, $0.0001; 162 shares authorized as of December 31, 2023 and 2022; no shares issued or outstanding at December 31, 2023 and 2022 | — | | | — | |
| Common stock, par value $0.0001; 1,600 shares authorized as of December 31, 2023 and 2022; 382 and 385 shares issued and outstanding as of December 31, 2023 and 2022, respectively | — | | | — | |
| Additional paid-in capital | 371 | | | 1,173 | |
| Accumulated other comprehensive loss | (123) | | | (370) | |
| Retained earnings | 13,606 | | | 18,320 | |
| Total stockholders’ equity | 13,854 | | | 19,123 | |
| Total liabilities and stockholders’ equity | $ | 18,426 | | | $ | 25,858 | |
The accompanying notes are an integral part of these consolidated financial statements.
MODERNA, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands,millions, except share and per share data)
| | | | | | | | | | | | | | | | | |
| Years Ended December 31, |
| 2020 | | 2019 | | 2018 |
| Revenue: | | | | | |
| Grant revenue | $ | 528,905 | | | $ | 12,173 | | | $ | 12,556 | |
| Product sales | 199,872 | | | 0 | | | 0 | |
| Collaboration revenue | 74,618 | | | 48,036 | | | 122,512 | |
| Total revenue | 803,395 | | | 60,209 | | | 135,068 | |
| Operating expenses: | | | | | |
| Cost of sales | 7,933 | | | 0 | | | 0 | |
| Research and development | 1,370,339 | | | 496,309 | | | 454,082 | |
| Selling, general and administrative | 188,267 | | | 109,620 | | | 94,252 | |
| Total operating expenses | 1,566,539 | | | 605,929 | | | 548,334 | |
| Loss from operations | (763,144) | | | (545,720) | | | (413,266) | |
| Interest income | 24,715 | | | 38,530 | | | 27,023 | |
| Other (expense) income, net | (6,084) | | | (7,526) | | | 1,835 | |
| Loss before provision for (benefit from) income taxes | (744,513) | | | (514,716) | | | (384,408) | |
| Provision for (benefit from) income taxes | 2,551 | | | (695) | | | 326 | |
| Net loss | (747,064) | | | (514,021) | | | (384,734) | |
| Reconciliation of net loss to net loss attributable to common stockholders: | | | | | |
| Premium paid on repurchase of preferred stock | 0 | | | 0 | | | (4,127) | |
| Cumulative preferred stock dividends | 0 | | | 0 | | | (12,996) | |
| Net loss attributable to common stockholders | $ | (747,064) | | | $ | (514,021) | | | $ | (401,857) | |
| Net loss per share attributable to common stockholders, basic and diluted | $ | (1.96) | | | $ | (1.55) | | | $ | (4.95) | |
| Weighted average common shares used in net loss per share attributable to common stockholders, basic and diluted | 381,333,059 | | | 330,802,136 | | | 81,114,183 | |
| | | | | | | | | | | | | | | | | |
| Years Ended December 31, |
| 2023 | | 2022 | | 2021 |
| Revenue: | | | | | |
| Net product sales | $ | 6,671 | | | $ | 18,435 | | | $ | 17,675 | |
| Other revenue | 177 | | | 828 | | | 796 | |
| | | | | |
| | | | | |
| Total revenue | 6,848 | | | 19,263 | | | 18,471 | |
| Operating expenses: | | | | | |
| Cost of sales | 4,693 | | | 5,416 | | | 2,617 | |
| Research and development | 4,845 | | | 3,295 | | | 1,991 | |
| Selling, general and administrative | 1,549 | | | 1,132 | | | 567 | |
| Total operating expenses | 11,087 | | | 9,843 | | | 5,175 | |
| (Loss) income from operations | (4,239) | | | 9,420 | | | 13,296 | |
| Interest income | 421 | | | 200 | | | 18 | |
| Other expense, net | (124) | | | (45) | | | (29) | |
| (Loss) income before income taxes | (3,942) | | | 9,575 | | | 13,285 | |
| Provision for income taxes | 772 | | | 1,213 | | | 1,083 | |
| Net (loss) income | $ | (4,714) | | | $ | 8,362 | | | $ | 12,202 | |
| | | | | |
| (Loss) earnings per share: | | | | | |
| Basic | $ | (12.33) | | | $ | 21.26 | | | $ | 30.31 | |
| Diluted | $ | (12.33) | | | $ | 20.12 | | | $ | 28.29 | |
| | | | | |
| Weighted average common shares used in calculation of (loss) earnings per share: | | | | | |
| Basic | 382 | | | 394 | | | 403 | |
| Diluted | 382 | | | 416 | | | 431 | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
MODERNA, INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSSINCOME (LOSS)
(In thousands)
| | | | | | | | | | | | | | | | | |
| Years Ended December 31, |
| 2020 | | 2019 | | 2018 |
| Net loss | $ | (747,064) | | | $ | (514,021) | | | $ | (384,734) | |
| Other comprehensive income (loss): | | | | | |
| Unrealized gain (loss) on available-for-sale debt securities, net of tax of $0, $1,148 and $0, for the years ended December 31, 2020, 2019 and 2018, respectively | 2,501 | | | 3,447 | | | (132) | |
| Less: amounts recognized for net realized gain included in net loss | (1,301) | | | (323) | | | (31) | |
| Total other comprehensive income (loss) | 1,200 | | | 3,124 | | | (163) | |
| Comprehensive loss | $ | (745,864) | | | $ | (510,897) | | | $ | (384,897) | |
| | | | | | | | | | | | | | | | | |
| Years Ended December 31, |
| 2023 | | 2022 | | 2021 |
| Net (loss) income | $ | (4,714) | | | $ | 8,362 | | | $ | 12,202 | |
| Other comprehensive income (loss), net of tax: | | | | | |
| Available-for-sale securities: | | | | | |
| Unrealized gains (losses) on available-for-sale debt securities | 210 | | | (348) | | | (42) | |
| Less: net realized losses (gains) on available-for-sale securities reclassified in net (loss) income | 38 | | | 26 | | | (1) | |
| Net increase (decrease) from available-for-sale debt securities | 248 | | | (322) | | | (43) | |
| Cash flow hedges: | | | | | |
| Unrealized gains on derivative instruments | — | | | 130 | | | 74 | |
| Less: net realized losses (gains) on derivative instruments reclassified in net (loss) income | 8 | | | (154) | | | (58) | |
| Net increase (decrease) from derivatives designated as hedging instruments | 8 | | | (24) | | | 16 | |
| Pension and postretirement benefit plans: | | | | | |
| Pension and postretirement obligation adjustments | (9) | | | — | | | — | |
| Total other comprehensive income (loss) | 247 | | | (346) | | | (27) | |
| Comprehensive (loss) income | $ | (4,467) | | | $ | 8,016 | | | $ | 12,175 | |
The accompanying notes are an integral part of these consolidated financial statements.
MODERNA, INC.
CONSOLIDATED STATEMENTS OF REDEEMABLE CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS’STOCKHOLDERS' EQUITY
(In thousands, except unit and share data)millions)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | Common Stock | | Additional
Paid-In
Capital | | Accumulated
Other
Comprehensive
(Loss) Income | | Retained Earnings (Accumulated
Deficit) | | Total Stockholders’ Equity |
| | | | | | | Shares | | Amount | | | | |
| Balance at December 31, 2020 | | | | | | | 399 | | | $ | — | | | $ | 4,802 | | | $ | 3 | | | $ | (2,244) | | | $ | 2,561 | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Exercise of options to purchase common stock | | | | | | | 7 | | | — | | | 112 | | | — | | | — | | | 112 | |
| Issuance of common stock under employee stock purchase plan | | | | | | | — | | | — | | | 12 | | | — | | | — | | | 12 | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Stock-based compensation | | | | | | | — | | | — | | | 142 | | | — | | | — | | | 142 | |
| Other comprehensive loss, net of tax | | | | | | | — | | | — | | | — | | | (27) | | | — | | | (27) | |
| Repurchase of common stock | | | | | | | (3) | | | — | | | (857) | | | — | | | — | | | (857) | |
| Net income | | | | | | | — | | | — | | | — | | | — | | | 12,202 | | | 12,202 | |
| Balance at December 31, 2021 | | | | | | | 403 | | | $ | — | | | $ | 4,211 | | | $ | (24) | | | $ | 9,958 | | | $ | 14,145 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Redeemable Convertible Preferred Stock | | | Common Stock | | Additional Paid-In Capital | | Accumulated Other Comprehensive Loss | | Accumulated Deficit | | Total Stockholders’ Equity |
| | Shares | | Amount | | | Shares | | Amount | | | | |
| Balance at December 31, 2017 | | 448,686,791 | | | $ | 1,176,661 | | | | 65,206,999 | | | $ | 6 | | | $ | 71,679 | | | $ | (1,157) | | | $ | (621,893) | | | $ | (551,365) | |
| Vesting of restricted common stock | | — | | | — | | | | 856,135 | | | — | | | — | | | — | | | — | | | — | |
| Issuance of Series G redeemable convertible preferred stock, net of issuance costs of $10,517 | | 55,666,004 | | | 549,413 | | | — | | | — | | | 51 | | | — | | | — | | | 51 | |
| Issuance of Series H redeemable convertible preferred stock, net of issuance costs of $474 | | 5,000,000 | | | 111,546 | | | | — | | | — | | | — | | | — | | | — | | | — | |
| Repurchase of Series D redeemable convertible preferred stock | | (269,180) | | | (704) | | | | — | | | — | | | (2,009) | | | — | | | — | | | (2,009) | |
| Repurchase of Series E redeemable convertible preferred stock | | (544,100) | | | (3,355) | | | | — | | | — | | | (2,118) | | | — | | | — | | | (2,118) | |
| Exercise of options to purchase common stock | | — | | | — | | | | 446,864 | | | — | | | 1,427 | | | — | | | — | | | 1,427 | |
| Conversion of redeemable convertible preferred stock into common stock | | (508,539,515) | | | (1,833,561) | | | | 236,012,913 | | | 24 | | | 1,833,537 | | | — | | | — | | | 1,833,561 | |
| Proceeds of initial public offering, net of issuance costs of $41,322 | | — | | | — | | | | 26,275,993 | | | 3 | | | 563,023 | | | — | | | — | | | 563,026 | |
| Stock-based compensation | | — | | | — | | | | — | | | — | | | 72,565 | | | — | | | — | | | 72,565 | |
| Other comprehensive loss, net of tax | | — | | | — | | | | — | | | — | | | — | | | (163) | | | — | | | (163) | |
| Net loss | | — | | | — | | | | — | | | — | | | — | | | — | | | (384,734) | | | (384,734) | |
| Balance at December 31, 2018 | | 0 | | | $ | 0 | | | | 328,798,904 | | | $ | 33 | | | $ | 2,538,155 | | | $ | (1,320) | | | $ | (1,006,627) | | | $ | 1,530,241 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Redeemable Convertible Preferred Stock | | | Common Stock | | Additional Paid-In Capital | | Accumulated Other Comprehensive Gain (Loss) | | Accumulated Deficit | | Total Stockholders’ Equity |
| | Shares | | Amount | | | Shares | | Amount | | | | |
| Balance at December 31, 2018 | | 0 | | | $ | 0 | | | | 328,798,904 | | | $ | 33 | | | $ | 2,538,155 | | | $ | (1,320) | | | $ | (1,006,627) | | | $ | 1,530,241 | |
| Vesting of restricted common stock and restricted stock units | | — | | | — | | | | 621,432 | | | — | | | — | | | — | | | — | | | — | |
| Exercise of options to purchase common stock | | — | | | — | | | | 6,945,306 | | | 1 | | | 47,258 | | | — | | | — | | | 47,259 | |
| Purchase of common stock under employee stock purchase plan | | — | | | — | | | | 171,343 | | | — | | | 2,891 | | | — | | | — | | | 2,891 | |
| Transition adjustment from adoption of ASC 606 (Note 2) | | — | | | — | | | | — | | | — | | | — | | | — | | | 27,984 | | | 27,984 | |
| Transition adjustment from adoption of ASC 842 (Note 2) | | — | | | — | | | | — | | | — | | | — | | | — | | | (3,790) | | | (3,790) | |
| Stock-based compensation | | — | | | — | | | | — | | | — | | | 81,122 | | | — | | | — | | | 81,122 | |
| Other comprehensive income, net of tax | | — | | | — | | | | — | | | — | | | — | | | 3,124 | | | — | | | 3,124 | |
| Net loss | | — | | | — | | | | — | | | — | | | — | | | — | | | (514,021) | | | (514,021) | |
| Balance at December 31, 2019 | | 0 | | | $ | 0 | | | | 336,536,985 | | | $ | 34 | | | $ | 2,669,426 | | | $ | 1,804 | | | $ | (1,496,454) | | | $ | 1,174,810 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | Common Stock | | Additional
Paid-In
Capital | | Accumulated
Other
Comprehensive
Loss | | Retained Earnings | | Total Stockholders’ Equity |
| | | | | | | Shares | | Amount | | | | |
| Balance at December 31, 2021 | | | | | | | 403 | | | $ | — | | | $ | 4,211 | | | $ | (24) | | | $ | 9,958 | | | $ | 14,145 | |
| | | | | | | | | | | | | | | | | |
| Vesting of restricted common stock | | | | | | | 1 | | | — | | | — | | | — | | | — | | | — | |
| Exercise of options to purchase common stock | | | | | | | 4 | | | — | | | 50 | | | — | | | — | | | 50 | |
| Issuance of common stock under employee stock purchase plan | | | | | | | — | | | — | | | 15 | | | — | | | — | | | 15 | |
| Stock-based compensation | | | | | | | — | | | — | | | 226 | | | — | | | — | | | 226 | |
| Other comprehensive loss, net of tax | | | | | | | — | | | — | | | — | | | (346) | | | — | | | (346) | |
| Repurchase of common stock | | | | | | | (23) | | | — | | | (3,329) | | | — | | | — | | | (3,329) | |
| Net income | | | | | | | — | | | — | | | — | | | — | | | 8,362 | | | 8,362 | |
| Balance at December 31, 2022 | | | | | | | 385 | | | $ | — | | | $ | 1,173 | | | $ | (370) | | | $ | 18,320 | | | $ | 19,123 | |
| | | | | | Common Stock | |
| | | | | Common Stock | |
| | | | | Common Stock | | | Additional
Paid-In
Capital | | Accumulated
Other
Comprehensive
(Loss) Income | | Retained Earnings | | Total Stockholders’ Equity |
| | | | | | | Shares | |
| Balance at December 31, 2022 | |
| Balance at December 31, 2022 | |
| Balance at December 31, 2022 | |
| | Redeemable Convertible Preferred Stock | | | Common Stock | | Additional Paid-In Capital | | Accumulated Other Comprehensive Gain (Loss) | | Accumulated Deficit | | Total Stockholders’ Equity |
| Shares | | Amount | | | Shares | | Amount | |
| Balance at December 31, 2019 | | 0 | | | 0 | | | | 336,536,985 | | | $ | 34 | | | $ | 2,669,426 | | | $ | 1,804 | | | $ | (1,496,454) | | | $ | 1,174,810 | |
| Proceeds of public offerings, net of issuance costs of $2,120 | | — | | | — | | | | 47,863,158 | | | 5 | | | 1,852,720 | | | — | | | — | | | 1,852,725 | |
| Vesting of restricted common stock | |
| Vesting of restricted common stock | |
| Vesting of restricted common stock | Vesting of restricted common stock | | — | | | — | | | | 247,349 | | | — | | | — | | | — | | | — | | | — | |
| Exercise of options to purchase common stock | Exercise of options to purchase common stock | | — | | | — | | | | 13,888,434 | | | 1 | | | 179,642 | | | — | | | — | | | 179,643 | |
| Purchase of common stock under employee stock option purchase plan | | — | | | — | | | | 251,752 | | | — | | | 7,040 | | | — | | | — | | | 7,040 | |
| Issuance of common stock under employee stock purchase plan | |
| Stock-based compensation | Stock-based compensation | | — | | | — | | | | — | | | — | | | 93,021 | | | — | | | — | | | 93,021 | |
| Other comprehensive income, net of tax | Other comprehensive income, net of tax | | — | | | — | | | | — | | | — | | | — | | | 1,200 | | | — | | | 1,200 | |
| Repurchase of common stock, including excise tax | |
| Net loss | Net loss | | — | | | — | | | | — | | | — | | | — | | | — | | | (747,064) | | | (747,064) | |
| Balance at December 31, 2020 | | 0 | | | $ | 0 | | | | 398,787,678 | | | $ | 40 | | | $ | 4,801,849 | | | $ | 3,004 | | | $ | (2,243,518) | | | $ | 2,561,375 | |
| Balance at December 31, 2023 | |
The accompanying notes are an integral part of these consolidated financial statements.
MODERNA, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)millions) | | | | | | | | | | | | | | | | | |
| Years Ended December 31, |
| 2023 | | 2022 | | 2021 |
| Operating activities | | | | | |
| Net (loss) income | $ | (4,714) | | | $ | 8,362 | | | $ | 12,202 | |
| Adjustments to reconcile net (loss) income to net cash (used in) provided by operating activities: | | | | | |
| Stock-based compensation | 305 | | | 226 | | | 142 | |
| Depreciation and amortization | 621 | | | 348 | | | 232 | |
| | | | | |
| Amortization/accretion of investments | (61) | | | 31 | | | 54 | |
| | | | | |
| Loss on equity investments, net | 35 | | | — | | | — | |
| Deferred income taxes | 828 | | | (559) | | | (318) | |
| Other non-cash items | 7 | | | 28 | | | — | |
| Changes in assets and liabilities, net of acquisition of business: | | | | | |
| Accounts receivable, net | 493 | | | 1,790 | | | (1,784) | |
| Prepaid expenses and other assets | 974 | | | (1,699) | | | (489) | |
| Inventory | 747 | | | 492 | | | (1,394) | |
| Right-of-use assets, operating leases | (605) | | | 21 | | | (58) | |
| Accounts payable | 13 | | | 240 | | | 204 | |
| Accrued liabilities | (340) | | | 612 | | | 989 | |
| Deferred revenue | (2,060) | | | (4,157) | | | 2,824 | |
| Income taxes payable | 15 | | | (828) | | | 876 | |
| Operating lease liabilities | 551 | | | (14) | | | 17 | |
| Other liabilities | 73 | | | 88 | | | 123 | |
| Net cash (used in) provided by operating activities | (3,118) | | | 4,981 | | | 13,620 | |
| Investing activities | | | | | |
| Purchases of marketable securities | (3,760) | | | (11,435) | | | (12,652) | |
| Proceeds from maturities of marketable securities | 5,575 | | | 3,151 | | | 1,338 | |
| Proceeds from sales of marketable securities | 3,206 | | | 3,548 | | | 3,105 | |
| Purchases of property, plant and equipment | (707) | | | (400) | | | (284) | |
| Acquisition of business, net of cash acquired | (85) | | | — | | | — | |
| Investment in convertible notes and equity securities | (23) | | | (40) | | | (30) | |
| Net cash provided by (used in) investing activities | 4,206 | | | (5,176) | | | (8,523) | |
| Financing activities | | | | | |
| | | | | |
| | | | | |
| | | | | |
| Proceeds from issuance of common stock through equity plans | 46 | | | 65 | | | 124 | |
| | | | | |
| Repurchase of common stock, including excise tax | (1,153) | | | (3,329) | | | (857) | |
| Changes in financing lease liabilities | (270) | | | (184) | | | (140) | |
| | | | | |
| | | | | |
| Net cash used in financing activities | (1,377) | | | (3,448) | | | (873) | |
| Net (decrease) increase in cash, cash equivalents and restricted cash | (289) | | | (3,643) | | | 4,224 | |
| Cash, cash equivalents and restricted cash, beginning of year | 3,217 | | | 6,860 | | | 2,636 | |
| Cash, cash equivalents and restricted cash, end of year | $ | 2,928 | | | $ | 3,217 | | | $ | 6,860 | |
| Supplemental cash flow information | | | | | |
| Cash (received) paid for income taxes | $ | (357) | | | $ | 2,729 | | | $ | 480 | |
| Cash paid for interest | $ | 39 | | | $ | 25 | | | $ | 14 | |
| Non-cash investing and financing activities | | | | | |
| | | | | |
| Purchases of property, plant and equipment included in accounts payable and accrued liabilities | $ | 130 | | | $ | 72 | | | $ | 111 | |
| | | | | |
| | | | | |
| | | | | |
| | | | | | | | | | | | | | | | | |
| Years Ended December 31, |
| 2020 | | 2019 | | 2018 |
| Operating activities | | | | | |
| Net loss | $ | (747,064) | | | $ | (514,021) | | | $ | (384,734) | |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | |
| Stock-based compensation | 93,021 | | | 81,122 | | | 72,565 | |
| Depreciation and amortization | 31,251 | | | 31,021 | | | 24,862 | |
| Leased assets expensed | 62,286 | | | 0 | | | 0 | |
| Amortization/accretion of investments | 10,185 | | | (3,742) | | | (1,866) | |
| Loss on disposal of property and equipment | 238 | | | 316 | | | 891 | |
| Changes in assets and liabilities: | | | | | |
| Accounts receivable | (1,385,191) | | | 7,216 | | | 832 | |
| Inventory | (46,527) | | | 0 | | | 0 | |
| Prepaid expenses and other assets | (241,041) | | | 9,751 | | | (5,289) | |
| Right-of-use assets, operating leases | (10,542) | | | (5,664) | | | 0 | |
| Accounts payable | 11,875 | | | (23,964) | | | 15,017 | |
| Accrued liabilities | 388,392 | | | (3,362) | | | 8,787 | |
| Deferred revenue | 3,842,307 | | | (44,119) | | | (65,260) | |
| Deferred lease obligation | 0 | | | 0 | | | 2,420 | |
| Operating lease liabilities | 11,792 | | | 12,647 | | | 0 | |
| Other liabilities | 5,989 | | | (6,169) | | | 910 | |
| Net cash provided by (used in) operating activities | 2,026,971 | | | (458,968) | | | (330,865) | |
| Investing activities | | | | | |
| Purchases of marketable securities | (2,956,295) | | | (1,145,226) | | | (1,227,709) | |
| Proceeds from maturities of marketable securities | 1,137,129 | | | 993,181 | | | 783,373 | |
| Proceeds from sales of marketable securities | 214,686 | | | 168,654 | | | 177,008 | |
| Purchases of property and equipment | (67,448) | | | (31,554) | | | (105,766) | |
| Net cash used in investing activities | (1,671,928) | | | (14,945) | | | (373,094) | |
| Financing activities | | | | | |
| Proceeds from issuance of redeemable convertible preferred stock, net of issuance costs | 0 | | | 0 | | | 661,111 | |
| Proceeds from offerings of common stock, net of issuance costs | 1,852,725 | | | 0 | | | 563,026 | |
| Repurchases of redeemable convertible preferred stock | 0 | | | 0 | | | (8,182) | |
| Proceeds from issuance of common stock under equity plans | 179,643 | | | 47,259 | | | 1,427 | |
| Proceeds from purchase of common stock under employee stock purchase plan | 7,040 | | | 2,891 | | | 0 | |
| Charges to financing lease liabilities | (6,215) | | | 971 | | | 0 | |
| Reimbursement of assets under financing lease obligation | 0 | | | 0 | | | 11,635 | |
| Payments on financing lease obligation | 0 | | | 0 | | | (2,175) | |
| Net cash provided by financing activities | 2,033,193 | | | 51,121 | | | 1,226,842 | |
| Net increase (decrease) in cash, cash equivalents and restricted cash | 2,388,236 | | | (422,792) | | | 522,883 | |
| Cash, cash equivalents and restricted cash, beginning of year | 247,699 | | | 670,491 | | | 147,608 | |
| Cash, cash equivalents and restricted cash, end of year | $ | 2,635,935 | | | $ | 247,699 | | | $ | 670,491 | |
| Supplemental cash flow information | | | | | |
| Cash paid for income taxes | $ | 572 | | | $ | 416 | | | $ | 294 | |
| Cash paid for interest | $ | 8,528 | | | $ | 5,585 | | | $ | 2,998 | |
| Non-cash investing and financing activities | | | | | |
| Issuance costs included in accounts payable and accrued liabilities | $ | 0 | | | $ | 0 | | | $ | 2,638 | |
| Purchases of property and equipment included in accounts payable and accrued liabilities | $ | 17,617 | | | $ | 4,676 | | | $ | 12,892 | |
| Leasehold improvements included in prepaid and other current assets | $ | 0 | | | $ | 0 | | | $ | 10,089 | |
| Lease financing obligation | $ | 0 | | | $ | 0 | | | $ | 10,089 | |
The accompanying notes are an integral part of these consolidated financial statements.
MODERNA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Organization and Description of the Business
Moderna, Inc. (collectively, with its consolidated subsidiaries, any of Moderna, we, us, our or the Company) was incorporated in Delaware on July 22, 2016. We are the successor in interest to Moderna LLC, a limited liability company formed under the laws of the State of Delaware in 2013. Our principal executive office is located at 200 Technology Square, Cambridge, MA.
We are a biotechnology company creatingadvancing a new generationclass of transformative medicines based onmade of messenger RNA (mRNA), to improve the lives of patients.. mRNA medicines are designed to direct the body’s cells to produce intracellular, membrane or secreted proteins that have a therapeutic or preventive benefit with the potential to address a broad spectrum of diseases. Our platform builds on continuous advances in basic and applied mRNA science, delivery technology and manufacturing, providing us the capability to pursue in parallel a robust pipeline of new development candidates. We are developing vaccinestherapeutics and therapeuticsvaccines for infectious diseases, immuno-oncology, rare diseases autoimmune and cardiovascularautoimmune diseases, independently and with our strategic collaborators.
In December 2020, we received an Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) and an Interim Order from Health Canada authorizing the ModernaOur COVID-19 vaccine (also referred to as mRNA-1273) inis our first commercial product and is marketed, where approved, under the U.S.name Spikevax®. Our original vaccine, mRNA-1273, targeted the SARS-CoV-2 ancestral strain, and Canada.
Since inception, we have incurred significant net losses, which were $747.1 million, $514.0 million,leveraged our mRNA platform to rapidly adapt our vaccine to emerging SARS-CoV-2 strains to provide protection as the virus evolves and $384.7 million for the years ended December 31, 2020, 2019 and 2018, respectively. On February 14, 2020 and May 21, 2020, we sold 30,263,158 and 17,600,000 shares of common stock at a price of $19.00 and $76.00 per share through public equity offerings, with aggregate net proceeds from the offering of $549.5 million and $1.30 billion, respectively, net of underwriting discounts, commissions and offering expenses. As of December 31, 2020, we had an accumulated deficit of $2.24 billion.
As of December 31, 2020, we had 21 mRNA development programs in our portfolio with 13 having entered the clinic. We expect to continue to incur significant expenses for the foreseeable future. If we seek to obtain regulatory approval for any of our investigational medicines, we expect to incur significant commercialization expenses. In addition, we anticipate that our expenses will increase significantly in connection with our development and commercializing our mRNA-1273 vaccine and ongoing activities to support our platform research, drug discovery and clinical development, including development of any new generations of vaccines against variants of SARS-CoV-2, infrastructure and Research Engine and Early Development Engine (which includes our Moderna Technology Center), digital infrastructure, creation of a portfolio of intellectual property, and administrative support. We may finance our future cash needs that exceed our operating costs through a combination of public or private equity offerings, structured financings and debt financings, government funding arrangements, strategic alliances and marketing, manufacturing, distribution and licensing arrangements. We may be unable to raise additional funds or enter into such other agreements on favorable terms, or at all.guidance is updated.
We believe thathave a diverse and extensive development pipeline of 42 development candidates across our cash, cash equivalents, and investments as45 development programs, of December 31, 2020 will be sufficient to enable us to fund our projected operations through at least the next 12 months from the issuance of our financial statements. Wewhich 40 are subject to numerous risks and uncertainties associated with pharmaceutical development and commercialization, and we are unable to predict the timing or amount of expenses or when or if we will be able to achieve or maintain profitability. If we fail to become profitable or are unable to sustain profitability on a continuing basis, then we may be unable to continue our operations at planned levels and be forced to reduce our operations.
2. Summary of Significant Accounting Policies
Basis of Presentation and Principles of Consolidation
Our consolidated financial statements are prepared in accordance with U.S. generally accepted accounting principles (GAAP). Any reference in these notes to applicable guidance is meant to refer to the authoritative accounting principles generally accepted in the United States as found in the Accounting Standard Codification (ASC) and Accounting Standards Updates (ASU) of the Financial Accounting Standards Board (FASB).
The consolidated financial statements include the Company and its subsidiaries. All intercompany transactions and balances have been eliminated in consolidation.
Other revenue in the consolidated statements of operations comprises grant revenue and collaboration revenue that were previously presented as separate line items in our consolidated statements of operations in our 2022 Form 10-K. The associated prior period amounts in the consolidated financial statements, as well as in the notes thereto, have been reclassified to conform to the current presentation.
Use of Estimates
We have made estimates and judgments affecting the amounts reported in our consolidated financial statements and the accompanying notes. On an ongoing basis, we evaluate our estimates, including critical accounting policies or estimates related to revenue recognition, research and development expenses, income tax provisions, stock-based compensation, leases, derivative financial instruments, inventory, and useful lives of long-lived assets. We base our estimates on historical experience and various relevant assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting periods that are not readily apparent from other sources. Changes in our estimates are recorded in the financial results of the period in which the new information becomes available. The actual results that we experience may differ materially from our estimates. Significant estimates relied upon in preparing these financial statements include, among others, those related to fair value of equity awards, revenue recognition, research and development expenses, leases, fair value of financial instruments, useful lives of property and equipment, income taxes, and our valuation allowance on our deferred tax assets.
Segment Information
We have determined that our chief executive officer is the chief operating decision maker (CODM). The CODM reviews financial information presented on a consolidated basis. Resource allocation decisions are made by the CODM based on consolidated results. There are no segment managers who are held accountable by the CODM for operations, operating results, and planning for levels or components below the consolidated unit level. As such, we have concluded that we operate as 1one segment.
On January 1, 2019, we adopted ASC 606 (Revenue from Contracts with Customers) using the modified retrospective transition method applied to those contracts which were not completed as of January 1, 2019. We recognized the cumulative effect of the adoption as an adjustment to the opening balance of accumulated deficit in the current period consolidated balance sheet. Results for reporting periods beginning after January 1, 2019 are presented under ASC 606, while prior period amounts have not been adjusted and continue to be reported in accordance with our historic accounting (ASC 605). ASC 606 applies to all contracts with customers, except for contracts that are within the scope of other standards, such as leases, insurance, collaboration arrangements and financial instruments.Recognition
To determine the appropriate amount of revenue to be recognized for arrangements that we determine are within the scope of ASC 606, we perform the following five steps (the five-step model): (i) identify the contract(s) with our customer; (ii) identify the performance obligations in the contract; (iii) determine the transaction price; (iv) allocate the transaction price to the performance obligations in the contract; and (v) recognize revenue when or as each performance obligation is satisfied.
OurNet Product Sales
Prior to the third quarter of 2023, we sold our COVID-19 vaccine to the U.S. Government, foreign governments and organizations. The agreements and related amendments with these entities generally do not include variable consideration, such as discounts, rebates or returns. Certain of these agreements entitle us to upfront deposits for our COVID-19 vaccine supply, initially recorded as deferred revenue. In the third quarter of 2023, we commenced sales of our latest COVID-19 vaccine to the U.S. commercial market, in addition to continuing sales to international governments and organizations. In the U.S., our COVID-19 vaccine is sold primarily to wholesalers and distributors, and to a lesser extent, directly to retailers and healthcare providers. Wholesalers and distributors typically do not make upfront payments to us.
We recognize net product sales when control of the product transfers to the customer, typically upon delivery. Payment terms generally range from 30 to 60 days, in line with customary practices in each country. Net product sales are recognized net of estimated wholesaler chargebacks, invoice discounts for prompt payments and pre-orders, provisions for sales returns, and other related deductions. These provisions are recorded based on contractual terms and our estimate of returns for product sold during the period, using the expected value method or the most likely amount method. We update our estimates quarterly and record necessary adjustments in the period when we identify the adjustments. Product sales, net of provisions, are recorded only to the extent a significant reversal in the amount of cumulative revenue recognized is primarily generated through grantsnot probable when the uncertainty associated with the provisions is subsequently resolved. Shipping and handling activities are considered fulfillment activities and not a separate performance obligation. Taxes assessed by governmental authorities that are imposed on and collected from government-sponsoredour product sales are excluded from net product sales.
Wholesaler chargebacks, discounts and privatefees
We contract with retailers, healthcare providers, and group purchasing organizations (GPO) to broaden our customer reach and offer contractual discounts. The chargeback represents the difference between the invoice price billed to the wholesaler and the negotiated price charged to the retailers, healthcare providers and GPO members. For distribution and related services, such as stocking and cold chain storage, we provide compensation to our wholesalers and distributors. We typically offer our customers invoice discounts on product sales for prompt payments and pre-orders. The estimation of these discounts and fees is based on contractual terms and our expectations regarding future customer payment behaviors. Wholesaler fees and invoice discounts are deducted from our gross product sales and accounts receivable at the time such product sales are recognized.
Product returns
We typically offer customers in the U.S. the right to return products, up to a certain limit as stipulated in our contracts. Estimated returns for our COVID-19 vaccine are determined considering available return rates for similar products, estimated levels of inventory in the distribution channel, and projected market demand. The estimated amount for product returns is presented within accrued liabilities on our consolidated balance sheets and is deducted from our gross product sales in the period the related product sales are recognized.
Other fees
Fees payable to third party payers and healthcare providers, along with fees to our direct customers that are settled via cash payments, including certain patient assistance programs, are recorded as accrued liabilities on our consolidated balance sheets.
Determining the amount of variable consideration to recognize necessitates substantial judgment, especially when assessing factors outside our direct control such as lack of pertinent historical data and limited third-party information. Among all variables, estimating returns presents the most significant judgment due to the broad range of potential outcomes and the current lack of return history with our customers. As we receive more historical data on our product returns, we will integrate this information to refine our estimates and enhance the precision of our financial projections. The actual results could differ from our estimates, and such differences could have a material impact to our financial statements.
Other Revenue
Other revenue consists primarily of grant revenue and collaboration arrangements.revenue.
Grant Revenuerevenue
We have contracts with Biomedical Advanced Research and Development Authority (BARDA), a division of the Office of the Assistant Secretary for Preparedness and Response (ASPR) within the U.S. Department of Health and Human Services (HHS); the U.S. government’s Defense Advanced Research Projects Agency (DARPA); the Bill & Melinda Gates Foundation (Gates Foundation) and other government-sponsored and private organizations for research and development related activities that provide for payments for reimbursed costs, which may include overhead and general and administrative costs as well as a related profit margin. We recognize grant revenue from these contracts as we perform services under these arrangements when the funding is committed. Associated expenses are recognized when incurred as research and development expense. RevenuesGrant revenue and related expenses are presented gross in the consolidated statements of operations as we have determined we are the primary obligor under the arrangements relative to the research and development services we perform as lead technical expert.
Collaboration Revenue
We accounthave entered into strategic collaborations and other similar arrangements with third parties for a contract with a customer that is within the scoperesearch and other licenses, development and commercialization of ASC 606 using the five-step model. We accountcertain products and product candidates. Such arrangements provide for a contract with a customer that is within the scope of ASC 606 when all of the following criteria are met: (i) the arrangement has been approved by the parties and the parties are committed to perform their respective obligations; (ii) each party’s rights regarding the
goods and/or services to be transferred can be identified; (iii) the payment terms for the goods and/or services to be transferred can be identified; (iv) the arrangement has commercial substance; and (v) collection of substantially all of the consideration to which we will be entitled in exchange for the goods and/or services that will be transferred to the customer is probable. We also determine the term of the contract based on the period in which we and our customer have present and enforceable rights and obligations for purposes of identifying the performance obligations and determining the transaction price.
We evaluate contracts that contain multiple promises to determine which promises are distinct. Promises are considered to be distinct and therefore, accounted for as separate performance obligations, provided that: (i) the customer can benefit from the good or service either on its own or together with other resources that are readily available to the customer and (ii) the promise to transfer the good or service to the customer is separately identifiable from other promises in the contract.Individual goods or services (or bundles of goods and/or services) that meet both criteria for being distinct are accounted for as separate performance obligations. Promises that are not distinct at contract inception are combined and accounted for as a single performance obligation. Options to acquire additional goods and/or services are evaluated to determine if such option provides a material right to the customer that it would not have received without entering into the contract. If so, the option is accounted for as a separate performance obligation. If not, the option is considered a marketing offer which would be accounted for as a separate contract upon the customer’s election.
The transaction price is generally comprised of an upfront payment due at contract inception and variable consideration in the formvarious types of payments for our services and materials and milestone payments due upon the achievementto us, including upfront fees, funding of specified events. Other payments the Company could be entitled to include tiered royalties earned when customers recognize net sales of licensed products. We measure the transaction price based on the amount of consideration to which we expect to be entitled in exchange for transferring the promised goods and/or services to the customer. We utilize either the expected value method or the most likely amount method to estimate the amount of variable consideration, depending on which method is expected to better predict the amount of consideration to which we will be entitled. Amounts of variable consideration are included in the transaction price to the extent that it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved.With respect to arrangements that include payments for a development or regulatory milestone payment, we evaluate whether the associated event is considered probable of achievement and estimate the amount to be included in the transaction price using the most likely amount method. Milestone payments that are not within our control or the licensee, such as those dependent upon receipt of regulatory approval, are not considered to be probable of achievement until the triggering event occurs. At the end of each reporting period, we re-evaluate the probability of achievement of each milestone and any related constraint, and if necessary, adjust our estimate of the overall transaction price. Any such adjustments are recorded on a cumulative catch-up basis, which would affect revenue and net loss in the period of adjustment. For arrangements that include sales-based royalties, including milestone payments based upon the achievement of a certain level of product sales, wherein the license is deemed to be the sole or predominant item to which the payments relate, we recognize revenue upon the later of: (i) when the related sales occur or (ii) when the performance obligation to which some or all of the payment has been allocated has been satisfied (or partially satisfied). Consideration that would be received for optional goods and/or services is excluded from the transaction price at contract inception.
We generally allocate the transaction price to each performance obligation based on a relative standalone selling price basis. We develop assumptions that require judgment to determine the standalone selling price for each performance obligation in consideration of applicable market conditions and relevant entity-specific factors, including factors that were contemplated in negotiating the agreement with the customer and estimated research and development costs. However, in certain instances, we allocate variable consideration entirely to one or more performance obligation if the terms of the variable consideration relate to the satisfaction of the respective performance obligationservices and the amount allocated is consistentpreclinical and clinical material, technical, development, regulatory, and commercial milestone payments, licensing fees, option exercise fees, and royalty and earnout payments on product sales. Such payments are often not commensurate with the amount we would expect to receive for the satisfactiontiming of the respective performance obligation.
revenue recognition and therefore result in deferral of revenue recognition. We recognize revenue based on the amount of the transaction price that is allocated to each respective performance obligation when or as the performance obligation is satisfied by transferring a promised good or service to the customer. For performance obligations that are satisfied at a point in time, we recognize revenue when control of the goods and/or services is transferred to the customer. For performance obligations that are satisfied over time, we recognize revenue by measuring the progress toward complete satisfaction of the performance obligation using a single method of measuring progress which depicts the performance in transferring control of the associated goods and/or services to the customer. We generally use input methods to measure the progress toward the complete satisfaction of performance obligations satisfied over time. With respect to arrangements containing a license to our intellectual property that is determined to be distinct from the other performance obligations identified in the arrangement, we recognize revenue from amounts allocated to the license when the license is transferred to the licensee and the licensee is able to use and benefit from the license. For licenses that are bundled with other promises, we utilize judgment to assess the nature of the combined performance obligation to determine whether the combined performance obligation is satisfied over time or at a point in time and, if over time, the appropriate method of measuring progress for purposes of recognizing revenue. Significant management judgment is required in
determining the level of effort required under an arrangement and the period over which we are expected to complete our performance obligations under an arrangement. We evaluate the measure of progress each reporting period and, if necessary, adjust the measure of performance and related revenue recognition. Any such adjustments are recorded on a cumulative catch-up basis, which would affect revenue and net loss in the period of adjustment.
Product Sales
Product sales are associated with our COVID-19 vaccine supply agreements with the U.S. Government and international government agencies. These agreements generally do not provide provisions for various forms of variable consideration, such as discounts, rebates or returns. We recognize revenue from product sales, using the five-step model under ASC 606, based on the fixed price per dose according to the contracts when control of the product transfers to the customer and customer acceptance has occurred, unless such acceptance provisions are deemed perfunctory.
Cash and Cash Equivalents
We consider all highly liquid investments with an original maturity of 90 days or less from the date of purchase to be cash equivalents.
Restricted Cash
Restricted cash is composed of amounts held on deposit related to our lease arrangements.arrangements and funds held in an escrow account related to a business acquisition in 2023. The funds are maintained in money market accounts and are recorded at fair value. We classify our restrictedRestricted cash is classified as either current or non-current based on the terms of the underlying lease arrangement.arrangement and is included in either prepaid expenses and other current assets or other non-current assets in our consolidated balance sheets.
Cash, Cash Equivalents and Restricted Cash shown in the Consolidated Statements of Cash Flows
The following table provides a reconciliation of cash, cash equivalents and restricted cash in the consolidated balance sheets that sum to the total of the same such amounts shown in the consolidated statements of cash flows (in thousands)millions):
| | | | | | | | | | | | | | | | | | | | |
| | December 31, |
| | 2023 | | 2022 | | 2021 |
| Cash and cash equivalents | | $ | 2,907 | | | $ | 3,205 | | | $ | 6,848 | |
Restricted cash(1) | | 17 | | | — | | | — | |
Restricted cash, non-current(2) | | 4 | | | 12 | | | 12 | |
| Total cash, cash equivalents and restricted cash shown in the consolidated statements of cash flows | | $ | 2,928 | | | $ | 3,217 | | | $ | 6,860 | |
_______
| | | | | | | | | | | | | | |
| | December 31, |
| | 2020 | | 2019 |
| Cash and cash equivalents | | $ | 2,623,850 | | | $ | 235,876 | |
| Restricted cash | | 1,032 | | | 1,032 | |
| Restricted cash, non-current | | 11,053 | | | 10,791 | |
Total cash, cash equivalents and restricted cash shown in the consolidated
statements of cash flows | | $ | 2,635,935 | | | $ | 247,699 | |
Investments
We invest our excess cash balances in marketable debt securities. We classify our investments in marketable debt securities as available-for-sale. We report our available-for-sale investmentssecurities at fair value at each balance sheet date, and include any unrealized holding gains and losses (the adjustment to fair value) in accumulated other comprehensive gainincome (loss), a component of stockholders’ equity. Realized gains and losses are determined using the specific-identification method, and are included in other (expense) income,expense, net in our consolidated statements of operations. We classify our available-for-sale marketable securities as current or non-current based on each instrument’s underlying effective maturity date and for which we have the intent and ability to hold the investment for a
period of greater than 12 months. MarketableAvailable-for-sale securities with maturities of less than 12 months are classified as current and are included in investments in the consolidated balance sheets. MarketableAvailable-for-sale securities with maturities greater than 12 months for which we have the intent and ability to hold the investment for greater than 12 months are classified as non-current and are included in investments, non-current in the consolidated balance sheets.
We evaluate securities for impairment at the end of each reporting period. Impairment is evaluated considering numerous factors, and their relative significance varies depending on the situation. Factors considered include whether a decline in fair value below the amortized cost basis is due to credit-related factors or non-credit-related factors, the financial condition and near-term prospects of the issuer, and our intent and ability to hold the investment to allow for an anticipated recovery in fair value. A credit-related impairment is recognized as an allowance on the balance sheet with a corresponding adjustment to earnings. Any impairment that is not credit- related is recognized in other comprehensive income (loss) income,, net of applicable taxes.
TableInvestments in publicly traded equity securities with readily determinable fair values are recorded at quoted market prices for identical securities, with changes in fair value recorded in other expense, net, in our consolidated statements of Contents
operations. Investments in equity securities without readily determinable fair values are recorded at cost minus impairment, if any, adjusted for changes resulting from observable price changes in orderly transactions for identical or similar securities. Such adjustments are recorded in other expense, net, in our consolidated statements of operations.
Accounts Receivable, and Allowance for Doubtful Accountsnet
We have accountsAccounts receivable, net represent amounts due from customers less wholesalers chargebacks, discounts and fees (please refer to our “Revenue Recognition” policy within Note 2 for product sales provision) and related vaccine supply agreements and our grant agreements. We also have accounts receivable amounts due from strategic collaborators as a result of manufacturing and research and development services provided under collaboration arrangements, or milestones achieved, but not yet paid.allowance for expected credit losses. Amounts payable to us are recorded as accounts receivable when our right to consideration is unconditional. To estimate the allowance for doubtful accounts,credit losses, we make judgments aboutdetermine the creditworthiness of our customersallowance based on ongoing credit evaluation, historical experience and historical experience.the aging of such receivables, among other factors. There was 0no allowance for doubtful accounts at December 31, 2020, and 2019. There was 02023 or 2022. Additionally, bad debt expenseexpenses were immaterial for the years ended December 31, 2020, 2019 or 2018.2023, 2022 and 2021.
Concentrations of Credit Risk
Financial instruments that subject us to significant concentrations of credit risk consist primarily of cash, cash equivalents, restricted cash, marketable debt securities, and accounts receivable.receivable, net. Our investment portfolio comprises money market funds and marketable debt securities, including U.S. Treasury securities, debt securities of U.S. government agencies and corporate entities and commercial paper. Our cash management and investment policy limits investment instruments to investment-grade securities with the objective to preserve capital and to maintain liquidity until the funds can be used in business operations. We invest in a variety of financial instruments and limit the amount of credit exposure with any individual financial institution. Bank accounts in the United States are insured by the Federal Deposit Insurance Corporation (FDIC) up to $250,000. Our primary operating accounts significantly exceed the FDIC limits.
We are also subject to credit risk from our accounts receivable, net related to our net product sales and strategic alliances. We sell our products primarily to wholesalers and distributors and other governments and organizations. We do not require collateral or other security to support accounts receivable. To date, we have not experienced material losses with respect to the collection of our accounts receivable.
Significant Customers
Our accounts receivable, net are generally unsecured and are from customers in different countries. We generated revenue from product sales to wholesalers and distributors and other governments and organizations, and to a lesser extent, grants made by government-sponsored and private organizations, product sales to the U.S. Government and international government agencies, and to a lesser extent, strategic alliances in 2020. Historically, we generatedcollaboration revenue primarily from our strategic alliances.
A significant portion of our revenue to date has been generated from the following entities that accounted for more than 10% of total revenue and accounts receivable for the periods presented:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Percentage of Revenue Years Ended December 31, | | Percentage of Accounts Receivable December 31, |
| 2020 | | 2019 | | 2018 | | 2020 | | 2019 |
| Merck | * | | 61 | % | | 49 | % | | * | | 13 | % |
| BARDA | 65 | % | | 13 | % | | * | | 22 | % | | 54 | % |
| Vertex | * | | 10 | % | | * | | * | | 17 | % |
| AstraZeneca | * | | * | | 34 | % | | * | | * |
| U.S. Government (excluding BARDA) | 24 | % | | * | | * | | * | | * |
| European Commission | * | | * | | * | | 28 | % | | * |
| United Kingdom Government | * | | * | | * | | 11 | % | | * |
| South Korea Government | * | | * | | * | | 24 | % | | * |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Percentage of Revenue Years Ended December 31, | | Percentage of
Accounts Receivable
December 31, |
| 2023 | | 2022 | | 2021 | | 2023 | | 2022 |
| | | | | | | | | |
| FFF Enterprises | * | | * | | * | | 39 | % | | * |
| | | | | | | | | |
| European Commission | * | | 28 | % | | 32 | % | | * | | 29 | % |
| U.S. Government (excluding BARDA) | * | | 23 | % | | 29 | % | | * | | * |
| Takeda Pharmaceutical Company | * | | 10 | % | | * | | * | | * |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| Ministry of Health, Labor, and Welfare of Japan | 21 | % | | * | | * | | * | | 30 | % |
| UK Health Security Agency | * | | * | | * | | 35 | % | | 11 | % |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
________
* - Represents an amount of less than 10%
Derivative Instruments and Hedging Activities
We record all derivatives on our consolidated balance sheets at fair value. The accounting for changes in the fair value of a derivative depends on whether the derivative has been designated and qualifies for hedge accounting. Hedge accounting generally provides for the matching of the timing of gain or loss recognition on the hedging instrument with the recognition of the changes in the fair value of the hedged asset or liability that are attributable to the hedged risk in a fair value hedge or the earnings effect of the hedged forecasted transactions in a cash flow hedge. We may enter into derivative contracts that are intended to economically hedge certain risk, even though hedge accounting does not apply or we elect not to apply hedge accounting. Foreign currency gains or losses associated with derivatives that are not designated as hedging instruments for accounting purposes are recorded within other (expense) income, net, in our consolidated statements of operations.
Fair Value Measurements
Fair value is defined as the price that would be received from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. When determining the fair value measurements for assets and liabilities, which are required to be recorded at fair value, we consider the principal or most advantageous market in which we would transact and the market-based risk measurements or assumptions that market participants would use in pricing the asset or liability, such as risks inherent in valuation techniques, transfer restrictions and credit risk. FASB ASC Topic 820 Fair(Fair Value Measurement (ASC 820),) establishes a fair value hierarchy for instruments measured at fair value that distinguishes between assumptions based on market data (observable inputs) and our assumptions (unobservable inputs). Observable inputs are inputs that market participants would use in pricing the asset or liability based on market data obtained from our independent sources. Unobservable inputs are inputs that reflect our assumptions about the inputs that market participants would use in pricing the asset or liability, and are developed based on the best information available in the circumstances. The following fair value hierarchy is used to classify assets and liabilities based on the observable inputs and unobservable inputs used to value the assets and liabilities:
•Level 1: Unadjusted quoted prices in active markets that are accessible at the measurement date for identical, unrestricted assets or liabilities;
•Level 2: Quoted prices for similar assets and liabilities in active markets, quoted prices in markets that are not active, or inputs which are observable, either directly or indirectly, for substantially the full term of the asset or liability; or
•Level 3: Prices or valuation techniques that require inputs that are both significant to the fair value measurement and unobservable (i.e., supported by little or no market activity).
To the extent that the valuation is based on models or inputs that are less observable or unobservable in the market, the determination of fair value requires more judgment. A financial instrument’s level within the fair value hierarchy is based on the lowest level of any input that is significant to the fair value measurement.
Our cash equivalents and marketable debt securities are reported at fair value determined using Level 1 and Level 2 inputs (Note 6)(Note 6). The fair value of our foreign currency forward contracts is calculated using Level 2 inputs, which include currency spot rates, forward rates, interest rate curve and credit or non-performance risk (Note 7).risk. We do not have any non-financial assets or liabilities that should be recognized or disclosed at fair value on a recurring basis at December 31, 2020, 20192023 and 2018.2022.
As of December 31, 2020 and 2019, we maintain letters of credit of $12.1 million and $11.8 million, respectively, related to our lease arrangements, which are secured by money market accounts in accordance with certain of our lease agreements. The amounts are recorded at fair value using Level 1 inputs and included as restricted cash in our consolidated balance sheets.
Inventory
Inventory is recorded at the lower of cost or net realizable value, with cost determined on ausing first-in, first-out (FIFO) basis.and average cost methods for different components of inventory. We periodically review the composition of inventory in order to identify excess, obsolete, slow-moving or otherwise unsaleable items. If unsaleable items are observed and there are no alternate uses for the inventory, we will record a write-down to net realizable value in the period that the decline in value is first recognized through a charge to cost of sales. The determination of whether inventory costs will be realizable requires estimates by management. If actual market conditions are less favorable than projected by management, additional write-downs of inventory may be required. We also assess whether we have any excess firm, non-cancelable, purchase commitment liabilities, resulting from our supply agreements with third-party vendors, on a quarterly basis. The determination of net realizable value and firm purchase commitment liabilities requires judgment, including consideration of many factors, such as estimates of future product demand, product net selling prices, current and future market conditions, potential product obsolescence, expiration and utilization of raw materials under firm purchase commitments and contractual minimums, among others. We hold raw materials beyond our one year forecasted production plan, which were classified as non-current and included in other non-current assets in our consolidated balance sheets.
Prior to an initial regulatory approval for our investigational medicines, we expense costsPre-launch Inventory
Costs relating to raw materials and production of inventory as research and development expense in our consolidated statements of operations, in the period incurred. When we believepreparation for product launch prior to regulatory approval and subsequentare capitalized when future commercialization of our investigational medicines is considered probable, and we also expectthe future
economic benefit from the sales of the investigational medicinesis expected to be realized, and we will then capitalize the costs of production as inventory.
Upon the authorization of distribution and use of our COVID-19 vaccine under an EUA in December 2020, we began to capitalize inventory costs associated with our COVID-19 vaccine, as it was determinedbelieve that inventory costs incurred subsequent to the EUA had a probable future economic benefit.
Construction in Progress
Construction in progress includes direct costsmaterial uncertainties related to the constructionultimate regulatory approval have been significantly reduced. For pre-launch inventory that is capitalized, we consider a number of various propertyfactors based on the information available at the time, including the product candidate’s current status in the drug development and equipment, including leasehold improvements,regulatory approval process, results from the related clinical trials, results from meetings with relevant regulatory agencies prior to the filing of regulatory applications, potential impediments to the approval process such as product safety or efficacy, historical experience, viability of commercialization and is stated at original cost. Construction in progress includes costs incurred under construction contracts including project management services, engineering services, design services and development, construction services and other construction-related fees and services. Such costs aremarket trends. As of December 31, 2023, we did not depreciated until the asset is completed and placed into service. Once the asset is placed into service, thesehave any capitalized costs will be allocated to certain property and equipment categories and will be depreciated over the estimated useful life of the underlying assets.pre-launch inventory on our consolidated balance sheets.
Property, Plant and Equipment
Property, plant and equipment are stated at cost, net of accumulated depreciation. Depreciation is calculated using the straight-line method over the estimated useful lives of the assets. The estimated useful lives of property, plant and equipment are described below:
| | | | | | | | |
| | Estimated Useful Life |
LaboratoryLand and land improvements | | Not depreciated |
| Manufacturing and laboratory equipment | | 5 years |
| Leasehold improvements | | Lesser of estimated useful life of improvement
or remaining life of related lease
|
| Computer equipment and software | | 3 to 5 years |
| Internally developed software | | 3 years
Furniture fixtures and otherfixtures | | 5 years |
Right of useRight-of-use asset, financing | | Lease term |
Expenditures for maintenanceConstruction in progress includes direct costs related to the construction of various property, plant and repairs are chargedequipment, including leasehold improvements, and is stated at original cost. Once the asset is placed into service, these capitalized costs will be allocated to expense as incurred. Upon retirement or sale,certain property, plant and equipment categories and will be depreciated over the costestimated useful life of the underlying assets.
Acquisitions
We account for acquisitions either as business combinations or asset acquisitions, based on whether the set of assets disposedacquired meets the definition of a business. When an acquisition is determined to be a business combination, we apply the acquisition method of accounting. This method requires that the assets acquired and liabilities assumed be recorded at their fair values as of the related accumulated depreciation, are removedacquisition date on our consolidated balance sheets. Any excess of the consideration transferred over the fair value of the net identifiable assets acquired is recognized as goodwill. The process of determining the fair value of assets and liabilities involves significant estimates and assumptions. During the measurement period, which may extend up to one year from the accounts,acquisition date, we may record adjustments to these fair values. Any such adjustments are recorded with a corresponding change to goodwill. Transaction costs incurred in connection with business combinations are expensed as incurred.
On January 31, 2023, we completed a business combination by acquiring all outstanding shares of OriCiro Genomics K.K. for a cash consideration of $86 million. As a result of this acquisition, we recognized $52 million in goodwill and any resulting gain or lossan intangible asset related to acquired technology valued at $48 million, which are included within other non-current assets in our consolidated balance sheets. This acquisition provided us with tools for cell-free synthesis and amplification of plasmid DNA, a key building block in mRNA
manufacturing. OriCiro’s technology strategically complements our manufacturing process and further accelerates our research and development efforts.
Goodwill and Intangible Assets
Goodwill represents the excess of purchase price over the fair value of net assets acquired and is recordedcarried at cost. Goodwill is tested at least annually for impairment by assessing qualitative factors in determining whether it is more likely than not that the fair value of net assets is below their carrying amounts. To date, an impairment of goodwill has not been recorded.
The fair value of acquired intangible assets is determined by applying the income-based approach, which is a valuation technique that provides an estimate of the fair value of an asset based on market participant expectations of the cash flows an asset would generate over its remaining useful life. To estimate the expected cash flows attributable to other (expense) income, net.an intangible asset, it requires the use of Level 3 fair value measurements and inputs. Finite-lived intangible assets are amortized on a straight-line basis over their estimated useful lives.
Impairment of Long-Lived Assets, including Intangibles and Lease Right-of-Use Assets
We evaluate our long-lived assets, which consist of property, plant and equipment, intangibles and right-of-use assets to determine if facts and circumstances indicate that the carrying amount of assets may not be recoverable. If such facts and circumstances exist, we assess the recoverability of the long-lived assets by comparing the projected future undiscounted net cash flows associated with the related asset or group of assets over their remaining lives against their respective carrying amounts. If such review indicates that such cash flows are not expected to be sufficient to recover the recorded value of the assets, the assets are written down to their estimated fair values based on the expected discounted future cash flows attributable to the assets or based on appraisals. For the year ended December 31, 2020, we incurred $1.6 million of impairment expenses. We did 0t record any impairmentImpairment expenses for the years ended December 31,201931, 2023, 2022 and 2018.2021 were immaterial.
Leases
We adopted ASC 842 (Leases) on January 1, 2019 on a modified retrospective basis under which we recognized and measured leases existing at, or entered into after, the beginning of the period of adoption. We elected the optional transition approach of not adjusting our comparative period financial statements for the impacts of adoption. Therefore, we recognized the effects of applying ASC 842 as a cumulative-effect adjustment to accumulated deficit as of January 1, 2019, the effective date of this standard.
Leases are classified at their commencement date, which is defined as the date on which the lessor makes the underlying asset available for use by the lessee, as either operating or finance leases based on the economic substance of the agreement. We recognize lease right-of-use assets and related liabilities in our consolidated balance sheets for both operating and finance leases. Lease liabilities are measured at the lease commencement date as the present value of the future lease payments using the interest rate implicit in the
lease. If the rate implicit is not readily determinable, we will utilize our incremental borrowing rate as of the lease commencement date. Lease right-of-use assets are measured as the lease liability plus initial direct costs and prepaid lease payments less lease incentives. The lease term is the non-cancelable period of the lease and includes options to extend or terminate the lease when it is reasonably certain that an option will be exercised.
We recognize operating lease cost in operating expenseexpenses in our consolidated statements of operations, inclusive of rent escalation provisions and rent holidays, on a straight-line basis over the respective lease term. For our finance leases, we recognize depreciation expense associated with the leased asset acquired and recognize interest expense related to the portion of the financing in our consolidated statements of operations. Additionally, we
We do not separate non-lease components from lease components for all classes of underlying assets. We do not recognize tenant improvement allowances asright-of-use assets and lease liabilities for leases with a reduction to rent expenselease term of 12 months or less. Instead, these lease payments are recognized in the statements of operations on a straight-line basis over the respective lease term.
Cost of SalesCollaboration Arrangements
CostWe analyze our collaboration arrangements to assess whether they are within the scope of sales includes costASC 808 (Collaborative Arrangements) to determine whether such arrangements involve joint operating activities performed by parties that are both active participants in the activities and exposed to significant risks and rewards that are dependent on the commercial success of raw materials, production, transportation, freightsuch activities. To the extent the arrangement is within the scope of ASC 808, we assess whether aspects of the arrangement between us and indirect overheadour collaboration partner are within the scope of other accounting literature. If we conclude that some or all aspects of the arrangement represent a transaction with a customer, we account for those aspects of the arrangement within the scope of ASC 606 (Revenue from Contracts with Customers). Please refer to our "Revenue Recognition" policy within Note 2 for additional discussion of revenue recognition under these types of arrangements. If we conclude that some or all aspects of the arrangement are within the scope of ASC 808 and do not represent a transaction with a customer, we recognize our allocation of the shared costs associatedincurred with our product revenue duringrespect to the jointly conducted activities as a component of the related expense in the period and third-party royalties on net sales of our product. Cost of sales also includes adjustmentsincurred. Additionally, for excess and obsolete inventoryany payments related to capital expenditures that are partially reimbursed by a collaboration partner, to the extent management determines that the underlying capital costs qualify for capitalization, any reimbursements received will offset the capitalized cost cannot be recovered based on estimates about future demand.of the asset.
Research and Development Costs
Research and development costs are expensed as incurred. Research and development expenses consist of costs incurred in performing research and development activities, including salaries and benefits, facilities costs, overhead costs, prelaunch inventory costs, contract services, and other outside costs. The value of goods and services received from contract research organizations and contract manufacturing organizations in the reporting period are estimated based on the level of services performed, and progress in the period in cases when we have not received an invoice from the supplier. Research and development costs also include costs and shared cost associated with third-party collaboration arrangements, including upfront fees and milestones paid to third-parties in connection with technologies that had not reached technological feasibility and did not have an alternative future use.
Equipment or facilitiesAssets that are acquired or constructed for research and development activities and that have alternative future uses, in research and development projects or otherwise, should beare capitalized and depreciated as tangible assets.over their useful lives. However, the costs of equipment or facilities that are acquired or constructed and intangibles that are purchased from others for a particular research and development project, and that have no alternative future uses and therefore no separate economic values, are considered research and development costs and are expensed when incurred. Certain equipment and leased facilities purchased for our COVID-19 vaccine program prior to the EUA from the FDA were deemed to have no alternative use. The related acquisition costs of such equipment and lease facilities of $109.6 million were charged to research and development expense for the year ended December 31, 2020. We did 0t recognize such expense for the years ended December 31, 2019 or 2018.
PatentAdvertising Costs
Costs to secure, defend and maintain patentsassociated with advertising are expensed as incurred and are classified asincluded in selling, general and administrative expense in the consolidated statements of operations. Advertising expenses due to the uncertainty of future benefits.were $204 million in 2023, $121 million in 2022, and immaterial in 2021.
Stock-Based Compensation
We issue stock-based awards to employees and non-employees, generally in the form of stock options, and restricted stock units (RSUs), and performance stock units (PSUs). We account for our stock-based compensation awards in accordance with ASC 718 Compensation—(Compensation—Stock CompensationCompensation). Most of our stock-based awards have been made to employees. We measure compensation cost for equity awards at their grant-date fair value and recognize compensation expense over the requisite service period, which is generally the vesting period, on a straight-line basis. The grant date fair value of stock options is estimated using the Black-Scholes option pricing model, which requires management to make assumptions with respect to the fair value of our common stock on the grant date, including the expected term of the award, the expected volatility of our stock, calculated based on a period of time generally commensurate with the expected term of the award, risk-free interest rates and expected dividend yields of our stock. Historically, for periods priorWe estimate the expected term of our stock options granted to employees and non-employees using the simplified method, whereby, the expected term equals the average of the vesting term and the original contractual term of the option. We utilize this method as we do not have sufficient historical exercise data to provide a reasonable basis upon which to estimate the expected term, and because significant changes in our business over the past few years have rendered historical experience less relevant. The expected volatility is based on a blended average of average historical stock volatilities of selected guideline companies over the expected term of the stock options, historical volatility of our stock price, and implied stock price volatility derived from the price of exchange traded options on our stock. Given the transformative changes in our business, we believe that a blended volatility rate is more indicative of future volatility than our historical volatility alone. We will continue to apply this process until a sufficient amount of historical information regarding the expected term and historical volatility of our own stock price becomes available, and until we believe that historical experience is relevant to our IPO, the fair value of the shares of common stock and common units underlying our stock-based awards were determined on each grant date by our board of directors based on valuation estimates from management considering our most recently available independent third-party valuation of our common stock. Our board of directors also assessed and considered, with input from management, additional objective and subjective factors that we believed were relevant and which may have changed from the date of the most recent valuation through the grant date.expectations for current grants. The grant date fair value of RSUs is estimated based on the fair value of our underlying common stock. For performance-based stock awards, we recognize stock-based compensation expense over the requisite service period using the accelerated attribution method when achievement is probable. We classify stock-based compensation expense in our consolidated statementstatements of operations in the same manner in which the award recipient’s salary and related costs are classified or in which the award recipient’s service payments are classified. We made an accounting policy election to recognize forfeitures of stock-based awards as they occur.
Income Taxes
We useaccount for income taxes based on an asset and liability approach to account for income taxes.approach. We recognize deferred tax assets and liabilities for the expected future tax consequences of temporary differences between the financial reporting and tax bases of assets and liabilities. These differences are measured using the enacted statutory tax rates and laws that are expected towill be in effect forwhen the years in which differences are expected to reverse. Valuation allowances are provided when the expected realization of deferred tax assets does not meet a “more likely than not” criterion. We periodically reassess the need for valuation allowances on our deferred tax assets, considering both positive and negative evidence to evaluate whether it is more likely than not that all or a portion of such assets will not be realized. We make estimates and judgments about our future taxable income that are based on assumptions that are consistent with our plans and estimates. Should the actual amounts differ from our estimates, the amount of our valuation allowance could be materially impacted. Changes in these estimates may result in significant increases or decreases to our tax provision in a period in which such estimates are changed, which in turn would affect net income or loss. We recognize tax benefits from uncertain tax positions if we believe the position is more likely than not to be sustained on examination by the taxing authorities based on the technical merits of the position. We make adjustments
to these tax reserves when facts and circumstances change, such as the closing of a tax audit or the refinement of an estimate. The provision for income taxes includes the effects of any reserves for uncertain tax positions, that are not more likely than not to be sustained, as well as the related net interest and penalties.
Net LossEarnings (Loss) per Share Attributable to Common Stockholders
We calculate basicdiluted net lossearnings (loss) per share attributable to common stockholders by dividing net loss attributable to common stockholders by the weighted average number of common shares outstanding for the period, without consideration for common stock equivalents. Upon the closing of our IPO, all outstanding shares of our redeemable convertible preferred stock were converted into common stock.
We calculate diluted net loss per share attributable to common stockholders by dividing net loss attributable to common stockholdersearnings (loss) by the weighted average number of common shares outstanding after giving consideration to the dilutive effect of restricted common stock and stock options that are outstanding during the period. WeFor periods in which we have generated a net loss, in all periods presented, therefore the basic and diluted net loss per share attributable to common stockholders are the same, as the inclusion of the potentially dilutive securities would be anti-dilutive.
Comprehensive LossIncome (Loss)
Comprehensive lossincome (loss) includes net lossincome (loss) and other comprehensive income (loss) for the period. Other comprehensive income (loss) mainly consists of unrealized gains and losses on our investments.investments and derivatives designated as hedging instruments. Total comprehensive income (loss) for all periods presented havehas been disclosed in the consolidated statements of comprehensive loss.income (loss).
The components of accumulated other comprehensive gain (loss)loss for the years ended December 31, 20202023 and 2019 are2022 were as follows (in thousands)millions):
| | | | | |
| Unrealized Gain on Available-for-Sale Debt Securities |
Accumulated other comprehensive loss, balance at December 31, 2018 | $ | (1,320) | |
Other comprehensive income | 3,124 | |
Accumulated other comprehensive gain, balance at December 31, 2019 | 1,804 | |
Other comprehensive income | 1,200 | |
Accumulated other comprehensive gain, balance at December 31, 2020 | $ | 3,004 | |
| | | | | | | | | | | | | | | | | | | | | | | |
| Unrealized (Loss) Gain on Available-for-Sale Securities | | Net Unrealized Gain (Loss) on Derivatives Designated As Hedging Instruments | | Pension and Postretirement Benefits | | Total |
| Accumulated other comprehensive loss, balance at December 31, 2021 | $ | (40) | | | $ | 16 | | | $ | — | | | $ | (24) | |
| Other comprehensive loss | (322) | | | (24) | | | — | | | (346) | |
| Accumulated other comprehensive loss, balance at December 31, 2022 | (362) | | | (8) | | | — | | | (370) | |
| Other comprehensive income (loss) | 248 | | | 8 | | | (9) | | | 247 | |
| Accumulated other comprehensive loss, balance at December 31, 2023 | $ | (114) | | | $ | — | | | $ | (9) | | | $ | (123) | |
Recently Adopted Accounting StandardsShare Repurchases
In June 2016, the FASB issued ASU No. 2016-13, Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments. This standard changes how companies account for credit losses for most financial assets and certain other instruments. For trade receivables, loans and held-to-maturity debt securities, companies will be required to recognize an allowance for credit losses rather than reducing the carrying value of the asset. The amendments in this standard should be applied on a modified retrospective basis to all periods presented. We adopted this standard in the first quarter of 2020. Based on the compositionShares of our investment portfolio and investment policy,common stock repurchased pursuant to our repurchase programs are retired. The purchase price of such repurchased shares of common stock is recorded as a reduction to additional paid-in-capital. If the adoption of this standard did not havebalance in additional paid-in-capital is exhausted, the excess is recorded as a material impact on our consolidated financial statements and disclosures.
In August 2018, the FASB issued ASU 2018-15, Intangibles—Goodwill and Other—Internal-Use Software (Topic 350): Customer’s Accounting for Implementation Costs Incurred in a Cloud Computing Arrangement That Is a Service Contract. This standard requires capitalizing implementation costs incurredreduction to develop or obtain internal-use software (and hosting arrangements that include an internal-use software license). We adopted this standard in the first quarter of 2020 using the prospective method. The adoption of this standard did not have a material impact on our consolidated financial statements and disclosures.
In December 2019, the FASB issued ASU 2019-12, Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes. This standard removes certain exceptions for investments, intra-period allocations and interim calculations, and adds guidance to reduce complexity in accounting for income taxes. We early adopted this standard in the second quarter of 2020. The adoption of this standard did not have a material impact on our consolidated financial statements and disclosures.retained earnings.
Recently Issued Accounting Standards Not Yet Adopted
From time to time, new accounting pronouncements are issued by the FASB or other standard setting bodies and adopted by us as of the specified effective date. Unless otherwise discussed,Except as noted below, we believe that the impact of recently issued standards that are not yet effective will not have a material impact on our consolidated financial statements and disclosures.
In November 2023, the FASB issued ASU No. 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures. This ASU broadens the disclosure requirements by requiring disclosures of significant segment expenses that are regularly provided to the chief operating decision maker (CODM) and included within each reported measure of segment profit or loss. The standard also requires entities to disclose, on an interim and annual basis, the amount and description, including the nature and type, of the other segment items. Additionally, entities are required to disclose the title and position of the individual identified as the CODM and an explanation of how the CODM uses the reported measures of a segment’s profit or loss in assessing segment performance and deciding how to allocate resources. These enhanced disclosure obligations apply to entities that operate with one reportable segment as well. ASU 2023-07 is effective for fiscal years beginning after December 15, 2023, and interim periods within fiscal years beginning after December 15, 2024 on a retrospective basis. Early adoption is permitted. We are currently assessing the impact that this new accounting standard will have on our consolidated financial statements and disclosures.
In December 2023, the FASB issued ASU No. 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures. This ASU requires disaggregated information about a reporting entity’s effective tax rate reconciliation as well as additional information on income taxes paid. The standard requires entities to disclose federal, state, and foreign income taxes in their rate reconciliation tables
and elaborate on reconciling items that exceed a quantitative threshold. Additionally, it requires an annual disclosure of income taxes paid, net of refunds, categorized by jurisdiction based on a quantitative threshold. The ASU is effective on a prospective basis for annual periods beginning after December 15, 2024. Early adoption is permitted. This ASU will result in the required additional disclosures being included in our consolidated financial statements, once adopted.
3. Net Product Sales
Net product sales by customer geographic location were as follows for the periods presented (in millions): | | | | | | | | | | | | | | | | | | | | |
| | Years Ended December 31, |
| | 2023 | | 2022 | | 2021 |
| United States | | $ | 1,720 | | | $ | 4,405 | | | $ | 5,393 | |
| Europe | | 1,353 | | | 6,732 | | | 6,834 | |
| Rest of world | | 3,598 | | | 7,298 | | | 5,448 | |
| Total | | $ | 6,671 | | | $ | 18,435 | | | $ | 17,675 | |
As of December 31, 2023, 2022 and 2021, our COVID-19 vaccine was our only commercial product authorized for use.
Prior to the third quarter of 2023, we sold our COVID-19 vaccine to the U.S. Government, foreign governments and organizations. The agreements and related amendments with these entities generally do not include variable consideration, such as discounts, rebates or returns. Certain of these agreements entitle us to upfront deposits for our COVID-19 vaccine supply, initially recorded as deferred revenue.
As of December 31, 2023 and 2022, we had deferred revenue of $613 million and $2.6 billion, respectively, related to customer deposits. We expect $554 million of our deferred revenue related to customer deposits as of December 31, 2023 to be realized in less than one year. Timing of product delivery and manufacturing, and receipt of marketing approval for the applicable COVID-19 vaccine will determine the period in which net product sales are recognized.
In the third quarter of 2023, we commenced sales of our latest COVID-19 vaccine to the U.S. commercial market, in addition to continuing sales to foreign governments and organizations. In the U.S., our COVID-19 vaccine is sold primarily to wholesalers and distributors, and to a lesser extent, directly to retailers and healthcare providers. Wholesalers and distributors typically do not make upfront payments to us.
Net product sales are recognized net of estimated wholesaler chargebacks, invoice discounts for prompt payments and pre-orders, provisions for sales returns, and other related deductions.
The following table summarizes product sales provision for the periods presented (in millions):
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Years Ended December 31, |
| | | | | | 2023 | | 2022 | | 2021 |
| Gross product sales | | | | | | $ | 8,203 | | | $ | 18,435 | | | $ | 17,675 | |
| Product sales provision: | | | | | | | | | | |
| Wholesaler chargebacks, discounts and fees | | | | | | (976) | | | — | | | — | |
| Returns and other fees | | | | | | (556) | | | — | | | — | |
| Total product sales provision | | | | | | $ | (1,532) | | | $ | — | | | $ | — | |
| Net product sales | | | | | | $ | 6,671 | | | $ | 18,435 | | | $ | 17,675 | |
The following table summarizes the activities related to product sales provision recorded as accrued liabilities for the year ended December 31, 2023 (in millions):
| | | | | | | | |
| | Returns and other fees |
| Balance at December 31, 2022 | | $ | — | |
| Provision related to sales made in current period | | (556) | |
| | |
| | |
| | |
| Balance at December 31, 2023 | | $ | (556) | |
3. Grant4. Other Revenue
In September 2020, we entered into an agreement withThe following table summarizes other revenue for the Defense Advanced Research Projects Agency (DARPA) for an award of up to $56.4 million to fund development of a mobile manufacturing prototype leveraging our existing manufacturing technology that is capable of rapidly producing vaccines and therapeutics. As of December 31, 2020, the committed funding, net of revenue earned was $4.6 million, with an additional $51.4 million available if DARPA exercises additional contract options.periods presented (in millions):
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Years Ended December 31, |
| | | | | | 2023 | | 2022 | | 2021 |
| Grant revenue | | | | | | $ | 94 | | | $ | 388 | | | $ | 735 | |
| Collaboration revenue | | | | | | 83 | | | 440 | | | 61 | |
| Total other revenue | | | | | | $ | 177 | | | $ | 828 | | | $ | 796 | |
Grant Revenue
In April 2020, we entered into an agreement with BARDABiomedical Advanced Research and Development Authority (BARDA), a division of the Office of the Assistant Secretary for Preparedness and Response (ASPR) within the U.S. Department of Health and Human Services (HHS), for an award of up to $483.3$483 million to accelerate development of mRNA-1273, our original vaccine candidate against COVID-19. In July 2020, weThe agreement has been subsequently amended our agreement with BARDA to provide for an additional commitment of up to $471.6 millioncommitments to support various late-stage clinical development efforts of mRNA-1273, including the execution of a 30,000 participant Phase 3 study, in the U.S.pediatric clinical trials, adolescent clinical trials and pharmacovigilance studies. The amendment increased the maximum award from BARDA, from $483.3 million to $954.9 million. Under the termsinclusive of the agreement, BARDA will fund the advancement of mRNA-1273 to FDA licensure.all amendments, was approximately $1.8 billion. All contract options have been exercised. As of December 31, 2020,2023, the remaining available funding, net of revenue earned was $444.3$97 million.
The following table summarizes grant revenue for the periods presented (in millions):
| | | | | | | | | | | | | | | | | |
| Years Ended December 31, |
| 2023 | | 2022 | | 2021 |
| BARDA | $ | 88 | | | $ | 372 | | | $ | 713 | |
| Other grant revenue | 6 | | | 16 | | | 22 | |
| Total grant revenue | $ | 94 | | | $ | 388 | | | $ | 735 | |
| | | | | |
In September 2016, we received an awardCollaboration Revenue
We have entered into collaboration agreements with strategic collaborators to accelerate the discovery and advancement of up to $125.8 million from BARDA, to help fund our Zika vaccine program. NaN of the 4 contract options have been exercised.potential mRNA medicines across therapeutic areas. As of December 31, 2020, the remaining available funding net2023, 2022 and 2021, we had collaboration agreements with Merck & Co., Inc (Merck), Vertex Pharmaceuticals Incorporated and Vertex Pharmaceuticals (Europe) Limited (together, Vertex), AstraZeneca plc (AstraZeneca) and others. Please refer to Note 5 to for further description of revenue earned was $70.8 million, with an additional $8.4 million available if the final contract option is exercised.
In January 2016, we entered a global health project framework agreement with the Gates Foundation to advance mRNA-based development projects for various infectious diseases, including human immunodeficiency virus (HIV). As of December 31, 2020, the available funding net of revenue earned was $11.4 million, with up to an additional $80.0 million available if additional follow-on projects are approved.
The following tables summarize grant revenue and deferred grant revenue for and as of the periods presented (in thousands):
| | | | | | | | | | | | | | | | | |
| Years Ended |
| 2020 | | 2019 | | 2018 |
| BARDA | $ | 521,652 | | | $ | 7,608 | | | $ | 6,736 | |
| Other grant revenue | 7,253 | | | 4,565 | | | 5,820 | |
| Total grant revenue | $ | 528,905 | | | $ | 12,173 | | | $ | 12,556 | |
| | | | | |
| | | | | | | | | | | | | | |
| | December 31, |
| | 2020 | | 2019 |
| Deferred grant revenue | | $ | 5,261 | | | $ | 2,777 | |
4. Product Sales
In December 2020, we began selling our COVID-19 vaccine to the U.S. Government and international government agencies. Under the supply agreements with these government agencies, we received or billed for upfront deposits for our future vaccine supply, which are initially recorded as deferred revenue. We recognize revenue based on the fixed price per dose when control of the product has transferred and customer acceptance has occurred, unless such acceptance provisions are deemed perfunctory.
Product sales by customer geographic location was as follows (in thousands):
| | | | | | | | |
| | Year Ended December 31, 2020 |
United States | | $ | 193,630 | |
Rest of world | | 6,242 | |
Total | | $ | 199,872 | |
There were no product sales in 2019 and 2018. We had one commercial product, our COVID-19 vaccine, authorized for use as of December 31, 2020.
As of December 31, 2020, we had deferred revenue of $3.80 billion related to customer deposits, classified as current deferred revenue in our consolidated balance sheet. Timing of product manufacturing, delivery and receipt of marketing approval will determine the period in which revenue is recognized.
5. Collaboration Agreements
The following table summarizes our total consolidated net revenue from our strategic collaborators for the periods presented (in thousands)millions):
| | | | | | | | | | | | | | | | | | | | |
| | Years Ended December 31, |
| Collaboration Revenue by Strategic Collaborator: | | 2020 | | 2019 | | 2018 (1) |
| Merck | | $ | 26,076 | | | $ | 36,608 | | | $ | 66,082 | |
| AstraZeneca | | 32,624 | | | 5,233 | | | 45,993 | |
| Vertex | | 15,505 | | | 6,195 | | | 10,437 | |
| Other | | 413 | | | 0 | | | 0 | |
| Total collaboration revenue | | $ | 74,618 | | | $ | 48,036 | | | $ | 122,512 | |
________
(1) Under ASC 605
The following table presents changes in the balances of our receivables and contract liabilities related to our strategic collaboration agreements during the year ended December 31, 2020 (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | December 31, 2019 | | Additions | | Deductions | | December 31, 2020 |
| Contract Assets: | | | | | | | | |
| Accounts receivable | | $ | 1,972 | | | $ | 120,821 | | | $ | (117,249) | | | $ | 5,544 | |
| Contract Liabilities: | | | | | | | | |
| Deferred revenue | | $ | 199,528 | | | $ | 121,882 | | | $ | (81,228) | | | $ | 240,182 | |
During the year ended December 31, 2020, we recognized the following revenue as a result of the change in the contract liability balances related to our collaboration agreements (in thousands):
| | | | | | | | | | | |
Revenue recognized in the period from: | | | Year Ended
December 31, 2020 |
Amounts included in contract liabilities at the beginning of the period (1)
| | | $ | 72,863 | |
Performance obligations satisfied (or partially satisfied) in previous reporting periods (2)
| | | 8,366 | |
______________
(1) We first allocate revenue to the individual contract liability balance outstanding at the beginning of the period until the revenue exceeds that balance. If additional consideration is received on those contracts in subsequent periods, we assume all revenue recognized in the reporting period first applies to the beginning contract liability.
(2) Related to changes in estimated costs for our future performance obligations and estimated variable considerations.
As of December 31, 2020, the aggregated amount of the transaction price allocated to performance obligations under our collaboration agreements that are unsatisfied or partially unsatisfied was $327.7 million. | | | | | | | | | | | | | | | | | | | | |
| | Years Ended December 31, |
| Collaboration Revenue by Strategic Collaborator: | | 2023 | | 2022 | | 2021 |
| Vertex | | $ | 82 | | | $ | 48 | | | $ | 26 | |
| Merck | | — | | | 309 | | | 23 | |
| AstraZeneca | | — | | | 80 | | | 7 | |
| Other | | 1 | | | 3 | | | 5 | |
| Total collaboration revenue | | $ | 83 | | | $ | 440 | | | $ | 61 | |
AstraZeneca – Strategic Alliances in Cardiovascular and Oncology
2013 Option Agreement and Services and5. Collaboration Agreement
In March 2013, we entered into an Option Agreement, the AZ Option Agreement, and a related Services and Collaboration Agreement, the AZ Services Agreement, with AstraZeneca, which were amended and restated in June 2018. We refer to these agreements in the forms that existed prior to the 2018 amendment and restatement as the 2013 AZ Agreements. Under the 2013 AZ Agreements we granted AstraZeneca certain exclusive rights and licenses, and options to obtain exclusive rights to develop and commercialize potential therapeutic mRNA medicines directed at certain targets for the treatment of cardiovascular and cardiometabolic diseases and cancer, and agreed to provide related services to AstraZeneca. Pursuant to the 2013 AZ Agreements, AstraZeneca was responsible for all research, development and commercialization activities, while we provided specified research and manufacturing services during a research and evaluation period, as described below, to further AstraZeneca’s activities pursuant to an agreed upon services plan. Under the 2013 AZ Agreements, AstraZeneca could have requested we provide additional services, at AstraZeneca’s expense. Subject to customary “back-up” supply rights granted to AstraZeneca, we exclusively manufactured (or had manufactured) mRNA for all research, development and commercialization purposes under the 2013 AZ Agreements until, on a product-by-product basis, the expiration of the time period for which we are entitled to receive earn-out payments with respect to such product pursuant to the 2013 AZ Agreements.
As of the effective date of the 2013 AZ Agreements, AstraZeneca acquired 40 options that it may exercise to obtain exclusive rights to clinically develop and commercialize identified development candidates (and related back-up candidates) directed to specified targets that arise during the research and evaluation period. During the research and evaluation period for research candidates under the 2013 AZ Agreements, AstraZeneca could have elected to designate a limited number of research candidates as development candidates in order to continue preclinical development on such development candidates (and related back-up candidates). From such pool of development candidates designated by AstraZeneca, during a specified option exercise period, AstraZeneca could have then exercised one of its options to obtain exclusive rights to clinically develop and commercialize an identified development candidate (and related back-up candidates). If AstraZeneca did not exercise one of its options to acquire exclusive rights to clinically develop and commercialize a particular development candidate during the defined option exercise period for such development candidate, AstraZeneca’s rights to exercise an option and other rights granted under the 2013 AZ Agreements with respect to such development candidate (and related back-up candidates) would terminate, all rights to exploit such development candidate (and related back-up candidates) would be returned to us and all data and results generated by AstraZeneca with respect to such development candidate (and related back-up candidates) would be either assigned or licensed to us. Upon the earlier of termination of the 2013 AZ Agreements for any reason and a specified anniversary of the effective date of the 2013 AZ Agreements, all exercised options, and the right to exercise any and all options if not previously exercised by AstraZeneca, would automatically terminate. On a target-by-target basis, we and AstraZeneca agreed to certain defined exclusivity obligations under the 2013 AZ Agreements with respect to the research, development and commercialization of mRNA medicines for such target.
As of the effective date of the 2013 AZ Agreements, AstraZeneca made upfront cash payments to us totaling $240.0 million. Under the 2013 AZ Agreements, we were entitled to receive payments that are not related to any specific program of up to $180.0 million in the aggregate for the achievement of 3 technical milestones relating to toxicity, delivery, and competition criteria. We achieved the toxicity and competition milestones in the year ended December 31, 2015. The delivery milestone has expired. Under the 2013 AZ Agreements, AstraZeneca was obligated to pay us a $10.0 million option exercise fee with respect to each development candidate (and related back-up candidates) for which it exercised an option. In addition, upon AstraZeneca’s exercise of each option, we were eligible to receive certain payments contingent upon the achievement of specified clinical, regulatory, and commercial events. For any product candidate optioned by AstraZeneca, we were eligible to receive, per product candidate, up to $100.0 million in payments for achievement of development milestones, up to $100.0 million payments for achievement of regulatory milestones, and up to $200.0 million payments for achievement of commercial milestones. Additionally, under the 2013 AZ Agreements, we were entitled to receive, on a product-by-product basis, earn-out payments on worldwide net sales of products ranging from a high-single digit percentage to 12%, subject to certain reductions, with an aggregate minimum floor.
We received from AstraZeneca under the 2013 AZ Agreements an option exercise payment of $10.0 million (the 2016 VEGF Exercise) in the year ended December 31, 2016, and a clinical milestone payment of $30.0 million with respect to AstraZeneca’s VEGF-A product (AZD8601) during the year ended December 31, 2018, that is currently being developed in a Phase 2 clinical trial. Unless earlier terminated, the 2013 AZ Agreements would have continued until the expiration of AstraZeneca’s earn-out and contingent option exercise payment obligations for optioned product candidates. Either party had the right to terminate the 2013 AZ Agreements upon the other party’s material breach, either in its entirety or in certain circumstances, with respect to relevant candidates, subject to a defined materiality threshold and specified notice and cure provisions. If AstraZeneca had the right to terminate the 2013 AZ Agreements for our material breach, then AstraZeneca could have elected, in lieu of terminating the 2013 AZ Agreements, in their entirety or with respect to such candidates, to have the 2013 AZ Agreements remain in effect, subject to reductions in certain payments we were eligible to receive and certain adjustments to AstraZeneca’s obligations under the 2013 AZ Agreements. AstraZeneca had the right to terminate the 2013 AZ Agreements in full, without cause, upon 90-days’ prior notice to us.
2016 Strategic Alliance with AstraZeneca – IL-12
In January 2016, we entered into a new Strategic Drug Development Collaboration and License Agreement, which we refer to as the 2016 AZ Agreement, with AstraZeneca to discover, develop and commercialize potential mRNA medicines for the treatment of a range of cancers.
Under the terms of the 2016 AZ Agreement, we and AstraZeneca have agreed to work together on an immuno-oncology program focused on the intratumoral delivery of a potential mRNA medicine to make the IL-12 protein.The 2016 AZ Agreement initially included research activities with respect to a second discovery program. During a limited period of time, each party had an opportunity to propose additional discovery programs to be conducted under the 2016 AZ Agreement. We are responsible for conducting and funding all discovery and preclinical development activities under the 2016 AZ Agreement in accordance with an agreed upon discovery program plan for the IL-12 program and any other discovery program the parties agree to conduct under the 2016 AZ Agreement. For the IL-12 program and any other discovery program the parties agree to conduct under the 2016 AZ Agreement, during a defined election period that commenced as of the effective date of the 2016 AZ Agreement (for the IL-12 program) and otherwise will commence on initiation of any such new discovery program, AstraZeneca may elect to participate in the clinical development of a development candidate arising under the 2016 AZ Agreement from such program. If AstraZeneca so elects (as it has for the IL-12 program), AstraZeneca will lead clinical development activities worldwide and we will be responsible for certain activities, including being solely responsible for manufacturing activities, all in accordance with an agreed upon development plan. AstraZeneca will be responsible for funding all Phase 1 clinical development activities (including costs associated with our manufacture of clinical materials in accordance with the development plan), and Phase 2 clinical development activities (including costs associated with our manufacture of clinical materials in accordance with the development plan) up to a defined dollar threshold. We and AstraZeneca will equally share the costs of Phase 2 clinical development activities in excess of such dollar threshold, all Phase 3 clinical development activities and certain other costs of late-stage clinical development activities, unless we elect not to participate in further development and commercialization activities and instead receive tiered royalties, as described below.
We and AstraZeneca will co-commercialize products in the U.S. in accordance with an agreed upon commercialization plan and budget, and on a product-by-product basis will equally share the U.S. profits or losses arising from such commercialization. Notwithstanding, on a product-by-product basis, prior to a specified stage of development of a given product, we have the right to elect not to participate in the further development and commercialization activities for such product. If we make such election, instead of participating in the U.S. profits and losses share with respect to such product, we are obligated to discuss future financial terms with AstraZeneca. If we are unable to agree on future financial terms within a short, defined period of time, we are entitled to receive tiered royalties at default rates set forth in the 2016 AZ Agreement, ranging from percentages in the mid-single digits to 20% on worldwide net sales of products, subject to certain reductions with an aggregate minimum floor. AstraZeneca has sole and exclusive responsibility for all ex-U.S. commercialization efforts. Unless we have elected to not to participate in further development (in which case royalties on ex-U.S. net sales will be at the default rates as described above, unless otherwise agreed by the parties), we are entitled to tiered royalties at rates ranging from 10% to 30% on ex-U.S. net sales of the products, subject to certain reductions with an aggregate minimum floor. Subject to customary “back-up” supply rights granted to AstraZeneca, we exclusively manufacture (or have manufactured) products for all development and commercialization purposes. We and AstraZeneca have agreed to certain defined exclusivity obligations with each other under the 2016 AZ Agreement with respect to the development and commercialization of mRNA medicines for IL-12.
Unless earlier terminated, our strategic alliance under the 2016 AZ Agreement will continue on a product-by-product basis (i) until both parties cease developing and commercializing such product without the intention to resume, if we have not elected our right not to participate in further development and commercialization of such product or (ii) on a country-by-country basis, until the end of the applicable royalty term for such product in such country, if we have elected our right not to participate in further development and commercialization of such product.
Either party may terminate the 2016 AZ Agreement upon the other party’s material breach, subject to specified notice and cure provisions. Each party may also terminate the 2016 AZ Agreement in the event the other party challenges such party’s patent rights, subject to certain defined exceptions. AstraZeneca has the right to terminate the 2016 AZ Agreement in full or with respect to any program for scientific, technical, regulatory or commercial reasons at any time upon 90 days’ prior written notice to us. On a product-by-product basis, we have the right to terminate the 2016 AZ Agreement in certain cases if AstraZeneca has suspended or is no longer proceeding with the development or commercialization of such product for a period of twelve consecutive months, subject to specified exceptions, including tolling for events outside of AstraZeneca’s control. On a product-by-product basis, if the 2016 AZ Agreement is terminated with respect to a given product, AstraZeneca’s rights in such product will terminate and, to the extent we terminated for AstraZeneca’s breach, patent challenge or cessation of development or AstraZeneca terminated in its discretion, AstraZeneca will grant us reversion licenses and take certain other actions so as to enable us to continue developing and commercializing such product in the oncology field.
If we continue developing and commercializing a given product following termination of the 2016 AZ Agreement by AstraZeneca in its discretion with respect to such product, AstraZeneca is entitled to receive a mid-single digit royalty on our worldwide net sales of such product and a high-single digit percentage of the amounts received by us from a third party in consideration of a license to such third party to exploit such product, in each case, until AstraZeneca recovers an amount equal to specified development costs incurred by AstraZeneca under the 2016 AZ Agreement with respect to such product prior to such termination. Such percentages increase by a low to mid-single digit amount to the extent such termination occurs after such product achieves a specified stage of development.
2013 Agreements with AstraZeneca, amended and restated in 2018
In June 2018, we entered into an Amended and Restated Option Agreement and a related Amended and Restated Services and collaboration Agreement with AstraZeneca, or the 2018 A&R Agreements, which amended and restated the 2013 AZ Agreements. Under the 2018 A&R Agreements, we granted AstraZeneca certain exclusive rights and licenses to research, develop and commercialize potential therapeutic mRNA medicines directed at certain targets for the treatment of cardiovascular and cardiometabolic diseases and cancer, and agreed to provide related services to AstraZeneca. The activities to be performed by the parties under the 2018 A&R Agreements are limited to defined biological targets in the cardiovascular and cardiometabolic fields and one defined target in the cancer field.
Pursuant to the 2018 A&R Agreements, AstraZeneca is responsible for all research, development and commercialization activities and associated costs, while we provide specified research and manufacturing services during a research and evaluation period, as described below, to further AstraZeneca’s activities conducted pursuant to an agreed upon services plan. During this research and evaluation period, these research services, and manufacturing services in excess of a specified threshold, are provided at AstraZeneca’s expense, and manufacturing services below the specified threshold are provided at no additional expense to AstraZeneca. AstraZeneca may request we provide additional research and manufacturing services, at AstraZeneca’s expense, following the end of the research and evaluation period. Subject to customary “back-up” supply rights granted to AstraZeneca, we exclusively manufacture (or have manufactured) mRNA for all research, development and commercialization purposes under the 2018 A&R Agreements until, on a product-by-product basis, the expiration of the time period for which we are entitled to receive earn-out payments with respect to such product pursuant to the 2018 A&R Agreements.
As of the effective date of the 2013 AZ Agreements, and as further reflected in the 2018 A&R Agreements, AstraZeneca acquired
40 options that it may exercise to obtain exclusive rights to clinically develop and commercialize identified development candidates (and related back-up candidates) directed to specified targets that arise during the research and evaluation period. During the research and evaluation period for research candidates, AstraZeneca may elect to designate a limited number of research candidates as development candidates in order to continue preclinical development on such development candidates (and related back-up candidates). From such pool of development candidates designated by AstraZeneca, during a specified option exercise period, AstraZeneca may then exercise one of its options to obtain exclusive rights to clinically develop and commercialize an identified development candidate (and related back-up candidates) in certain fields. If AstraZeneca does not exercise one of its options to acquire exclusive rights to clinically develop and commercialize a particular development candidate during the defined option exercise period for such development candidate, AstraZeneca’s rights to exercise an option and other rights granted under the 2018 A&R Agreements with respect to such development candidate (and related back-up candidates) will terminate, all rights to exploit such development candidate (and related back-up candidates) will be returned to us and all data and results generated by AstraZeneca with respect to such development candidate (and related back-up candidates) will be either assigned or licensed to us. Upon the earlier of termination of the 2018 A&R Agreements for any reason and a specified anniversary of the effective date of the 2013 AZ Agreements, all unexercised options, and the right to exercise any and all options if not previously exercised by AstraZeneca, will automatically terminate.
On a target-by-target basis, we and AstraZeneca have agreed to certain defined exclusivity obligations under the 2018 A&R Agreements with respect to the research, development and commercialization of mRNA medicines for such target in certain fields. In addition, we and AstraZeneca have agreed to certain defined exclusivity obligations with respect to the research, development and commercialization of mRNA medicines coding for the same polypeptide as any development candidate being developed under the 2018 A&R Agreements.
Unless earlier terminated, the 2018 A&R Agreements will continue until the expiration of AstraZeneca’s earn-out and contingent option exercise payment obligations for optioned product candidates. Either party may terminate the 2018 A&R Agreements upon the other party’s material breach, either in its entirety or in certain circumstances, with respect to relevant candidates, subject to a defined materiality threshold and specified notice and cure provisions. If AstraZeneca has the right to terminate the 2018 A&R Agreements for our material breach, then AstraZeneca may elect, in lieu of terminating the 2018 A&R Agreements, in their entirety or with respect to such candidates, to have the 2018 A&R Agreements remain in effect, subject to reductions in certain payments we are eligible to receive and certain adjustments to AstraZeneca’s obligations under the 2018 A&R Agreements. AstraZeneca may terminate the 2018 A&R Agreements in full, without cause, upon 90 days’ prior notice to us.
Accounting Treatment
Combined 2018 AZ Agreements
The 2013 Agreements, as amended by the 2018 A&R Agreements, and the 2016 AZ Agreement, referred to as the Combined 2018 AZ Agreements, are treated as a single agreement for accounting purposes as these agreements were negotiated in contemplation of each other. The Combined 2018 AZ Agreements represent a transaction with a customer and therefore is accounted for in accordance with ASC 606. We identified the following performance obligations in the Combined 2018 AZ Agreements: (i) a combined performance obligation that includes a research license, research and development pool services, and manufacturing obligations related to the 2013 AZ Agreements, as amended by the 2018 A&R Agreements, collectively referred to as the Combined 2018 AZ Agreement Performance Obligation, (ii) preclinical development services for IL-12, (iii) preclinical development services for an oncology development target, (iv) a combined performance obligation for a development and commercialization license and manufacturing obligations for IL-12, and (v) a material right to receive development and commercialization rights and manufacturing services for an oncology development target.
We concluded that the research license is not distinct from the research and development pool services or the manufacturing obligations related to the 2018 A&R Agreements, as AstraZeneca cannot fully exploit the value of the research license without receipt of such services and supply. Our services and supply involve specialized expertise, particularly as it relates to mRNA technology that is not available in the marketplace. Any supply requested by AstraZeneca in excess of the minimum quantities specified in the agreement are considered customer options and treated as separate contracts for accounting purposes. Further, we concluded that AstraZeneca cannot exploit the value of the development and commercialization license for IL-12 without receipt of supply as the development and commercialization license does not convey to AstraZeneca the right to manufacture and therefore combined the development and commercialization license and the manufacturing obligations for IL-12 into one performance obligation.
The following table summarizes the composition of the total transaction price for the periods presented (in thousands):
| | | | | | | | | | | | | | |
| | Transaction Price |
| | December 31, |
| Combined 2018 AZ Agreements: | | 2020 | | 2019 |
| Upfront payments | | $ | 240,000 | | | $ | 240,000 | |
| Sublicense reimbursement | | 1,000 | | | 1,000 | |
| Toxicity milestone payment | | 60,000 | | | 60,000 | |
| Competition milestone payment | | 60,000 | | | 60,000 | |
| Estimated reimbursement for IL-12 manufacturing obligations | | 35,830 | | | 40,782 | |
| Total | | $ | 396,830 | | | $ | 401,782 | |
We utilize the most likely amount method to determine the amount of reimbursement for IL-12 manufacturing obligations to be received. We determined that any sales-based royalties related to IL-12 will be recognized when the related sales occur as they were determined to relate predominately to the license granted and therefore have been excluded from the transaction price. In addition, we are eligible to receive future milestones and royalties on future commercial sales for optioned product candidates under the 2018 A&R Agreements and future royalties under the 2016 Agreement; however, these amounts are not considered variable consideration under the Combined 2018 Agreements as we are only eligible to receive such amounts if AstraZeneca exercises its options (including certain options that are deemed to be material rights). We have concluded that the exercise of an optioned product candidate represents a separate transaction under ASC 606. We re-evaluate the transaction price at the end of each reporting period. There was a $5.0 million decrease to the transaction price during the year ended December 31, 2020, resulting from a change in estimate of variable consideration.
The transaction price was allocated to the performance obligations based on the relative estimated standalone selling prices of each performance obligation. We developed the estimated standalone selling price for the licenses included in the Combined 2018 AZ Agreement Performance Obligation and the combined performance obligation for a development and commercialization license and manufacturing obligations for IL-12 primarily based on the probability-weighted present value of expected future cash flows associated with each license related to each specific program. In developing such estimate, we also considered applicable market conditions and relevant entity-specific factors, including those factors contemplated in negotiating the agreement, probability of success and the time needed to commercialize a product candidate pursuant to the associated license. We developed the estimated standalone selling price for the services and/or manufacturing and supply included in each of the performance obligation, as applicable, primarily based on the nature of the services to be performed and/or goods to be manufactured and estimates of the
associated costs, adjusted for a reasonable profit margin that would be expected to be realized under similar contracts. The estimated standalone selling price of the material right to receive development and commercialization rights and manufacturing services for an oncology development target was developed by estimating the amount of discount that AstraZeneca would receive when exercising the option and adjusting such amount by the likelihood that the option will be exercised.
The following table summarizes the allocation of the total transaction price to the identified performance obligations under the arrangement, and the amount of the transaction price unsatisfied as of December 31, 2020 (in thousands):
| | | | | | | | |
| | Transaction Price |
Combined 2018 AZ Agreements: | | December 31, 2020 |
Combined 2018 AZ Agreement performance obligation | | $ | 293,223 | |
Preclinical development service - IL-12 | | 8,133 | |
Preclinical development service - oncology development target | | 8,133 | |
Development and commercialization license and manufacturing obligation | | 85,750 | |
Material right to receive development and commercialization rights | | 1,591 | |
Total | | $ | 396,830 | |
Remaining unsatisfied performance obligation | | $ | 87,225 | |
As of December 31, 2020, $77.5 million of the remaining performance obligations that are unsatisfied is expected to be recognized as revenue through December 31, 2029 and $9.7 million is expected to be recognized as revenue at the earlier of expiration or modification of the Combined 2018 AZ Agreement.
We measure proportional performance over time using an input method based on cost incurred relative to the total estimated costs for the Combined 2018 AZ Agreement Performance Obligation and the preclinical development services for IL-12 and the other oncology target performance obligations. We recognize revenue related to the amounts allocated to the combined performance obligation for a development and commercialization license and manufacturing obligations for IL-12 based on the point in time upon which control of supply is transferred to AstraZeneca for each delivery of the associated supply.
We recognize revenue for the Combined 2018 AZ Agreement Performance Obligation by determining the proportion of effort incurred as a percentage of total effort we expect to expend. This ratio is applied to the transaction price allocated to this combined performance obligation. We also estimate the development plan, including expected demand from AstraZeneca, and the associated costs for this combined performance obligation, as we will satisfy this combined performance obligation as the manufacturing services are performed. Management has applied significant judgment in the process of developing our budget estimates. Any changes to these estimates will be recognized in the period in which they change as a cumulative catch up.
The following table summarizes the revenue recognized for the periods presented (in thousands):
| | | | | | | | | | | | | | | | | | | | |
| | Years Ended December 31, |
| | 2020 | | 2019 | | 2018 |
| Combined AZ Agreements | | $ | 18,371 | | | $ | 4,650 | | | $ | 45,393 | |
The revenue recognized for the year ended December 31, 2020 includes the amortization of deferred revenue due to the satisfaction of our performance obligation during the period, including a cumulative catch-up adjustment of $12.5 million due to changes in estimated costs for our future performance obligations.
The following table summarizes the balances of deferred revenue at period end, which is classified as current or non-current in the consolidated balance sheets based on the period the services are expected to be performed or control of the supply is expected to be transferred (in thousands):
| | | | | | | | | | | | | | |
| | December 31, |
| | 2020 | | 2019 |
| Combined AZ Agreements | | $ | 57,192 | | | $ | 73,669 | |
2016 VEGF Exercise
We concluded that the 2016 VEGF Exercise should be treated as a separate transaction for accounting purposes, represents a transaction with a customer and therefore is accounted for in accordance with ASC 606. We identified one performance obligation in this arrangement which is comprised of the exclusive license to develop and commercialize VEGF and the manufacturing of clinical supply. We concluded that the VEGF license is not distinct from the manufacturing obligations because AstraZeneca cannot fully exploit the value of the license without receipt of such supply. This is due to limitations inherent in the licenses conveyed wherein AstraZeneca does not have the contractual right to manufacture during the term of the agreement.
The following table summarizes the composition of the total transaction price for the periods presented (in thousands):
| | | | | | | | | | | | | | |
| | Transaction Price |
| | December 31, |
| 2016 VEGF Exercise: | | 2020 | | 2019 |
| Option exercise fee | | $ | 10,000 | | | $ | 10,000 | |
| Milestone payment | | 30,000 | | | 30,000 | |
| Sublicense reimbursement | | 2,250 | | | 2,250 | |
| Estimated reimbursement for clinical supply | | 18,062 | | | 15,621 | |
| Total | | $ | 60,312 | | | $ | 57,871 | |
We are eligible to receive future milestones and royalties on future commercial sales under this arrangement. We utilize the most likely amount method to estimate any development and regulatory milestone payments to be received and the amount of estimated reimbursement for clinical supply. As of December 31, 2020, there were no milestones that had not been achieved included in the transaction price. We considered the stage of development and the risks associated with the remaining development required to achieve each milestone, as well as whether the achievement of the milestone is outside of our or AstraZeneca’s control. The outstanding milestone payments were fully constrained, as a result of the uncertainty whether any of the milestones would be achieved. We determined that any commercial milestones and sales-based royalties will be recognized when the related sales occur as they were determined to relate predominantly to the license granted and therefore have also been excluded from the transaction price. We re-evaluate the transaction price at the end of each reporting period and as uncertain events are resolved or other changes in circumstances occur. When a milestone payment is included in the transaction price in the future, it is recognized as revenue based on the relative completion of the underlying performance obligation. There was a $2.4 million increase to the transaction price during the year ended December 31, 2020, resulting from a change in estimate of variable consideration.
The following table summarizes the total transaction price allocated to the single performance obligation under the arrangement, and the amount of the transaction price unsatisfied as of December 31, 2020 (in thousands):
| | | | | | | | |
| | |
| | Transaction Price |
| | December 31, 2020 |
2016 VEGF Exercise combined performance obligation | | 60,312 | |
Remaining unsatisfied performance obligation | | 41,877 | |
As of December 31, 2020, the remaining performance obligations that are unsatisfied is expected to be recognized as revenue through December 31, 2025.
We recognize revenue related to the amount of the transaction price allocated to the VEGF Exercise performance obligation based on the point in time upon which control of supply is transferred to AstraZeneca for each delivery of the associated supply.
The following table summarizes the revenue recognized for the periods presented (in thousands):
| | | | | | | | | | | | | | | | | | | | |
| | Years Ended December 31, |
| | 2020 | | 2019 | | 2018 |
| 2016 VEGF Exercise | | $ | 14,253 | | | $ | 583 | | | $ | 0 | |
The revenue recognized for the year ended December 31, 2020 includes the amortization of deferred revenue due to the satisfaction of our performance obligation during the period, offset by a cumulative catch-up adjustment of $0.4 million due to changes in estimated costs for our future performance obligations.
The following table summarizes the balances of deferred revenue at period end, which is classified as current or non-current in the consolidated balance sheets based on the period the services are expected to be performed or control of the supply is expected to be transferred (in thousands):
| | | | | | | | | | | | | | |
| | December 31, |
| | 2020 | | 2019 |
| 2016 VEGF Exercise | | $ | 29,335 | | | $ | 41,266 | |
Merck – Strategic Alliances in Infectious Diseases and Cancer Vaccines
2015 Strategic Alliance with Merck – Infectious Disease
In January 2015, we entered into a Master Collaboration and License Agreement with Merck, which was amended in January 2016, June 2016, and May 2019, and which we refer to, as amended, as the 2015 Merck Agreement. Pursuant to the 2015 Merck Agreement, we and Merck agreed to research, develop, and commercialize potential mRNA medicines for the prevention of infections by RSV. The 2015 Merck Agreement was terminated on October 7, 2020, by mutual agreement, and the right to pursue the continued development and commercialization of product candidates and products, including RSV, reverted to us.
As a part of the May 2019 amendment of the 2015 Merck Agreement, we and Merck agreed to conclude the collaboration as it relates to development of potential mRNA medicines for other viruses, including mRNA-1278 for the prevention of varicella zoster virus (VZV) infection. Pursuant to the 2015 Merck Agreement, Merck was primarily responsible for research, development, and commercialization activities and associated costs of such research and commercialization. We were responsible for designing and, at Merck's cost, manufacturing all mRNA constructs for preclinical and Phase 1 and Phase 2 clinical development purposes, and we were responsible for certain costs associated with the conduct of a Phase 1 clinical trial for a RSV vaccine product candidate (mRNA-1172).
We and Merck agreed to certain defined exclusivity obligations during the term of the 2015 Merck Agreement with respect to mRNA investigational medicines against RSV. As part of the May 2019 amendment of the 2015 Merck Agreement, we and Merck agreed to certain exceptions to the existing exclusivity obligations, pursuant to which we will no longer be restricted from researching, developing, and commercializing an mRNA investigational medicine for the prevention of a specific set of respiratory infections, including RSV, for the pediatric population.
Under the terms of the 2015 Merck Agreement, we received a $50.0 million upfront payment. We were eligible to receive, on a product-by-product basis, additional milestone payments upon the achievement of certain development, regulatory, and commercial milestone events, as well as royalties on Merck’s net sales of products at rates ranging from the mid-single digits to low teens, subject to certain reductions, with an aggregate minimum floor. During the term of the agreement, we received from Merck a clinical milestone payment of $5.0 million with respect to the initiation of a Phase 1 clinical trial for a Merck RSV vaccine product candidate. Concurrent with entering into the 2015 Merck Agreement, Merck made a $50.0 million equity investment in us, and concurrent with amending the 2015 Merck Agreement in January 2016, we received an upfront payment of $10.0 million from Merck.
Accounting Treatment
We determined that all aspects of amended 2015 Merck Agreement represent a transaction with a customer and therefore the amended 2015 Merck Agreement is accounted for in accordance with ASC 606. The four-year research period was complete as of December 31, 2018 and we recognized the total transaction price of $65.0 million (the $60.0 million in aggregate upfront payments and a $5.0 million payment pertaining to achievement of a development milestone) in full as we concluded there were no unsatisfied performance obligations pertaining to the amended 2015 Merck Agreement. Additionally, we concluded the following customer options were marketing offers as such options did not provide any discounts or other rights that would be considered a material right in the arrangement: (i) research services during the three-year period following the initial four-year research period during which Merck could continue to preclinically and clinically develop product candidates and (ii) clinical mRNA supply for Phase 1 and Phase 2 and/or non-cGMP mRNA supply beyond the initial four-year research period. Therefore, such options would be accounted for as a separate contract if exercised by the customer.
After completion of the initial four-year research period, and as part of the May 2019 amendment of the 2015 Merck Agreement, Merck elected to establish a new RSV vaccine product candidate and elected to conduct a Phase 1 clinical trial. We are responsible for
certain costs associated with the conduct of the Phase 1 clinical trial. We determined that our obligation under the May 2019 amendment to reimburse Merck for certain costs associated with the RSV vaccine Phase 1 clinical trial represents consideration payable to a customer and is accounted for as a reduction of the transaction price. The consideration amount is determined based on the most likely method and recorded as contra-revenue as costs are incurred. The one-time payment upon election by Merck to continue developing RSV is fully constrained as it is contingent upon completion of the RSV Phase 1 clinical trial and upon decisions to be made by Merck to continue development thereafter.
The following table summarizes the composition of the total transaction price for the periods presented (in thousands):
| | | | | | | | | | | | | | |
| | Transaction Price |
| | December 31, |
| 2015 Merck Agreement: | | 2020 | | 2019 |
| Upfront payments | | $ | 60,000 | | | $ | 60,000 | |
| Development milestones | | 5,000 | | | 5,000 | |
| Reduction of reimbursements paid to Merck | | (12,353) | | | (5,265) | |
| Total | | $ | 52,647 | | | $ | 59,735 | |
We utilize the most likely amount method to estimate any development and regulatory milestone payments to be received. As of December 31, 2020, there were no milestones that had not been achieved included in the transaction price. We considered the stage of development and the risks associated with the remaining development required to achieve each milestone, as well as whether the achievement of the milestone is outside of our or Merck’s control. The outstanding milestone payments were fully constrained, as a result of the uncertainty whether any of the milestones would be achieved. We re-evaluate the transaction price at the end of each reporting period and as uncertain events are resolved or other changes in circumstances occur. For the year ended December 31, 2020, there was a$7.1 million deduction to the transaction price related to reimbursements paid to Merck for RSV vaccine Phase I clinical trial costs.
The following table summarizes the total transaction price allocated to the combined performance obligation under the arrangement, and the amount of the transaction price unsatisfied as of December 31, 2020 (in thousands):
| | | | | | | | |
| | Transaction Price |
| | December 31, 2020 |
2015 Merck Agreement | | $ | 52,647 | |
Remaining unsatisfied performance obligation | | $ | 0 | |
The following table summarizes the revenue and contra-revenue recognized for the periods presented (in thousands):
| | | | | | | | | | | | | | | | | | | | |
| | Years Ended December 31, |
| | 2020 | | 2019 | | 2018 |
| Contra-revenue under the May 2019 Amendment | | $ | (7,088) | | | $ | (5,265) | | | $ | 0 | |
| Collaboration revenue under the 2015 Merck Agreement | | 62 | | | 492 | | | 24,582 | |
| Total contra-revenue | | $ | (7,026) | | | $ | (4,773) | | | $ | 24,582 | |
Contra-revenue recognized was related to consideration payable to Merck under the May 2019 Amendment of the 2015 Merck Agreement. Collaboration revenue recognized was pursuant to separate agreements with Merck related to the exercise of customer options to purchase clinical mRNA supply to further develop a product candidate after the initial four-year research period. Clinical mRNA supply was recognized as collaboration revenue at a point in time upon which control of supply was transferred to Merck for each delivery of the associated supply. We had no deferred revenue as of December 31, 2020 or December 31, 2019 from the amended 2015 Merck Agreement as all performance obligations under the amended 2015 Merck Agreement were completed as of December 31, 2018.
On October 7, 2020, the 2015 Master Collaboration and License Agreement between us and Merck related to our collaboration on RSV was terminated by mutual agreement. Merck will complete the Phase 1 study and transition the program to Moderna. The termination did not have an impact to our consolidated financial statements as of and for the year ended December 31, 2020.
2016 Cancer Vaccine Strategic Alliance-PersonalizedPersonalized mRNA Cancer Vaccines (Individualized Neoantigen Therapy)
In June 2016, we entered into a Collaboration and License Agreement for the development and commercialization of personalized mRNA cancer vaccines (PCV) Collaboration and License Agreement(also known as individualized neoantigen therapy (INT)) with Merck, which we refer to as the PCV Agreement, to develop and commercialize PCVsINTs for individual patients using our mRNA vaccine and formulation technology. Under theThis agreement was subsequently amended and restated in 2018 (INT Agreement). Our role in this strategic alliance we identifyinvolves identifying genetic mutations present in a particular patient’s tumor cells, synthesizesynthesizing mRNA for these mutations, encapsulateencapsulating the mRNA in one of our proprietary LNPslipid nanoparticles (LNPs), and administer to each patientadministering a unique mRNA cancer vaccineINT to each patient. Each INT is designed to specifically activate the patient’s immune system against her or his own cancer cells.
Pursuant to the PCVINT Agreement, we arereceived an upfront payment of $200 million from Merck and we were responsible for designing and researching PCVs,INTs, providing manufacturing capacity and manufacturing PCVs,INTs and conducting Phase 1 and Phase 2 clinical trials for PCVs,INTs, alone and in combination with KEYTRUDA (pembrolizumab), Merck’s anti-PD-1 therapy, all in accordance with an agreed upon development plan and budget and underbudget. We concluded that the oversight of a committee comprised of equal representatives of each party. The parties have entered into a clinical quality agreement with respect to Moderna’s manufacture and supply activities. We received an upfront payment of $200.0 million from Merck. In November 2017, we and Merck announcedcollaboration arrangement was governed by the achievement of a key milestone for the first-in-human dosing of a PCV (mRNA-4157) as a part of the alliance. The Phase 1 open-label, dose escalation, multicenter clinical trial in the United States (KEYNOTE-603) is designed to assess the safety, tolerability and immunogenicity of mRNA-4157 alone in subjects with resected solid tumors and in combination with KEYTRUDA, in subjects with unresectable solid tumors.revenue recognition standard ASC 606.
Until the expiration of a defined period of time following our completion of Phase 1 and Phase 2 clinical trialsIn September 2022, Merck exercised its option for PCVs under the PCV Agreement and delivery of an associated data packageINT, including mRNA-4157, pursuant to Merck, Merck has the right to elect to participate in future development and commercialization of PCVs by making a $250.0 million participation payment to us. If Merck exercises its election and pays the participation payment, then the parties will equally co-fund subsequent clinical development of PCVs, with Merck primarily responsible for conducting clinical development activities under a jointly agreed development plan and budget. Each party may also conduct additional clinical trials for PCVs that are not included in the jointly agreed development plan and budget, in which case the non-conducting party will reimburse the conducting party for half of the total costs for such trials, plus interest, from its share of future profits resulting from sales of such PCVs, if any. Merck will lead worldwide commercialization of PCVs, subject to Moderna’s option to co-promote PCVs in the United States, and the parties will equally share the profits or losses arising from worldwide commercialization. Until a PCV becomes profitable, we may elect to defer payment of our share of the commercialization and related manufacturing costs and instead reimburse Merck for such costs, plus interest, from our share of future profits resulting from sales of such PCV, if any. Subject to customary “back-up” supply rights granted to Merck, we will manufacture (or have manufactured) PCVs for preclinical and clinical purposes. Manufacture of PCVs for commercial purposes will be determined by the parties in accordance with the terms of the PCV Agreement. Under the PCV Agreement, we grant certain licenses to Merck to perform its collaboration activities.
If Merck does notagreement and in October 2022 paid us an option exercise its right to participate in future development and commercializationfee of PCVs, then Moderna will retain the exclusive right to develop and commercialize PCVs developed during the strategic alliance, subject to Merck’s rights to receive a percentage in the high teens$250 million. Pursuant to the low 20s, subject to reductions of our net profits on sales of such PCVs. During a limited period following such non-exercise, Merck has the right to perform clinical studies of such PCVs in combination with KEYTRUDA, for which we agree to use reasonable efforts to supply such PCVs. During such limited period, we also have the right to perform clinical studies of PCVs in combination with KEYTRUDA, for which Merck agrees to use reasonable efforts to supply KEYTRUDA. In addition, following its non-exercise, Merck is also entitled to receive a percentage in the high teens to the low 20s, subject to reductions, of our net profits on sales of certain PCVs first developed by us following such non-exercise and reaching a specified development stage within a defined period of time.
We and Merck have agreed to certain defined, limited exclusivity obligations with respect to the development and commercialization of PCVs.
2018 Expansion of the Cancer Vaccine Strategic Alliance-Shared Neoepitope Cancer Vaccines
In April 2018, we and Merck agreed to expand our cancer vaccine strategic alliance to include the development and commercialization of our KRAS vaccine development candidate, mRNA-5671 or V941, and potentially other shared neoantigen mRNA cancer vaccines (SAVs). We preclinically developed mRNA-5671 prior to its inclusion in the cancer vaccine strategic alliance and it is comprised of a novel mRNA construct designed by us and encapsulated in one of our proprietary LNPs. The PCV Agreement was amended and restated to include the new SAV strategic alliance (PCV/SAV Agreement).
We have granted Merck certain licenses andagreement, we and Merck have agreed to certain exclusivity obligations with respect to SAVs and particular SAV programs, which obligations are subject to termination or expiration upon certain triggering events. Under the PCV/SAV Agreement, Merck will be responsible for conducting Phase 1 and Phase 2 clinical trials for mRNA-5671 and for all costs associated with such activities, in accordance with a jointly agreed development plan and budget, and we will be responsible for manufacturing and supplying all mRNA-5671 required to conduct such trials and for all costs and expenses associated with such
manufacture and supply. Under the PCV/SAV Agreement, our budgeted commitment for PCV increased to $243.0 million. Until the expiration of a defined period of time following the completion of Phase 1 and Phase 2 clinical trials for mRNA-5671 under the PCV/SAV Agreement and our delivery of an associated data package to Merck, Merck has the right to elect to participate in futurecollaborate on further development and commercialization of mRNA-5671 by making a participation payment to us. If Merck exercises its participation rights, then the parties will equally co-fund subsequent clinical development of mRNA-5671,INT, with Merck primarily responsible for conducting clinical development activities under a jointly agreed development plancosts and budget. If Merck declines to participate in future development and commercialization activities following the initial Phase 1 and Phase 2 clinical trials for mRNA-5671, then we will retain the rights to develop and commercialize mRNA-5671. If Merck elects to participate in future development and commercialization of mRNA-5671, Merck may also conduct additional clinical trials for mRNA-5671 that are not included in the jointly agreed development plan and budget, in which case we will reimburse Merck for half of the total development costs for such clinical trials, plus interest, from our share of future profits resulting from sales of mRNA-5671, if any. If Merck does conduct additional clinical trials for mRNA-5671, we will be responsible for manufacturing and supplying all mRNA-5671 required to conduct such trials. Merck will lead worldwide commercialization of mRNA-5671, subject to our option to co-promote mRNA-5671 in the United States, and the parties will equally share the operatingany profits or losses arising from worldwide commercialization. Until mRNA-5671 becomes profitable, we may elect to defer payment of our share of the commercialization and related manufacturing costs and instead reimburse Merck for such costs, plus interest, from our share of future profits resulting from sales of mRNA-5671, if any. Subject to “back-up” supply rights granted to Merck, we will manufacture (or have manufactured) mRNA-5671 and other SAVs for preclinical and clinical purposes. After Merck exercises its right to participate in future development and commercialization of mRNA-5671 and other SAVs, we will grant the applicable development and commercialization licenses and the parties are obligated to discuss responsibility for future manufacturing, giving consideration to applicable criteria.
Pursuant to the PCV/SAV Agreement, for a defined period of time, either party may propose that the parties conduct additional programs for the research and development of SAVs directed to differentbe shared neoantigens. If the parties agree to conduct any such programs, then we will be responsible for conducting and funding preclinical discovery and research activities for such SAVs, and otherwise the programs would be conducted on substantially the same terms as mRNA-5671 program. If we or Merck propose a new SAV program and the other party does not agree to conduct such program, then the PCV/SAV Agreement includes provisions allowing the proposing party to proceed with such development, at the proposing party’s expense. If Merck is the proposing party, we will be responsible for manufacturing and supplying material for such program at Merck’s expense. In such case, the non-proposing party will have the right to opt-in to such SAV program any time before the proposing party commits to performing Good Laboratory Practice (GLP)-toxicity studies. Until the expiration of a defined period of time following our completion of Phase 1 and Phase 2 clinical trials for any SAV program mutually agreed by the parties under the PCV/SAV Agreement and our delivery of an associated data package to Merck, Merck has the right to elect to participate in future development and commercialization of such SAV by making a participation payment to us.
Unless earlier terminated, the PCV/SAV Agreement will continueequally on a program-by-program basis until Merck terminates its participation in such program. Following any such termination, we will retain the exclusive right to develop and commercialize PCVs or SAVs developed as a part of such program, subject to restrictions and certain limited rights retained by Merck.
In connection with the amendment of the PCV Agreement to include the development and commercialization of mRNA-5671 and potentially other SAVs, Merck made a contemporaneous equity investment in our Series H redeemable convertible preferred stock, resulting in gross proceeds of $125.0 million, of which $13.0 million was determined to be a premium and recorded to deferred revenue.
Accounting Treatment
We determined that the PCV/SAV Agreement should be accounted for separately from the amended 2015 Merck Agreement, as the agreements were not negotiated in contemplation of one another and the elements within each of the agreements are not closely interrelated or interdependent on each other. The PCV/SAV Agreement represents a transaction with a customer and therefore is accounted for in accordance with ASC 606.
We identified the following performance obligations in the PCV/SAV Agreement: (i) a research license and research and development services, including manufacturing and supply of PCVs, during the proof of concept (POC) term for the PCV program, referred to as the PCV Performance Obligation, and (ii) research license and manufacturing and supply of mRNA-5671 during the POC term for the KRAS program, referred to as the KRAS Performance Obligation.worldwide basis. We concluded that the research licensecollaboration arrangement under the Merck Participation Term is not distinct fromwithin the research and development services, including manufacturing and supplyscope of PCVs, duringASC 808. For the POC term for the PCV program, as Merck cannot fully exploit the value of the license without receipt of such services and supply. Our services and supply involve specialized expertise, particularly as it relates to mRNA technology that is not available in the marketplace. Therefore, the research license has been combined with the research and development services, including manufacturing and supply of PCVs, during the POC term for the PCV program, into a single performance obligation. Similarly, we concluded that the research license is not distinct from the manufacturing and supply of mRNA-5671 during the POC term for the KRAS program, as Merck cannot fully
exploit the value of the license without receipt of such supply which must be provided by us. This is due to limitations inherent in the licenses conveyed wherein Merck does not have the contractual right to manufacture during the POC term. Therefore, the research license has been combined with the manufacturing and supply of mRNA-5671, during the POC term for the KRAS program, into a single performance obligation. Conversely, we concluded that the PCV Performance Obligation and the KRAS Performance Obligation are distinct from each other because Merck can fully exploit the value of each program for its intended purpose without the promises associated with the other program. Additionally, we concluded the following customer options are marketing offers as such options did not provide any discounts or other rights that would be considered a material right in the arrangement: (i) Merck participation election license related to future joint development and commercialization on a program-by-program basis, (ii) manufacturing and supply in support of certain SAV programs and/or the PCV program upon Merck election to not participate in future development and commercialization of that program and (iii) research and development services associated with certain SAV programs. Therefore, such options will be accounted for as a separate contract upon the customer’s election.
The following table summarizes the composition of the total transaction price for the periods presented (in thousands):
| | | | | | | | | | | | | | |
| | Transaction Price |
| | December 31, |
| PCV/SAV Agreement: | | 2020 | | 2019 |
| Upfront payment | | $ | 200,000 | | | $ | 200,000 | |
| Premium associated with the contemporaneous sale of Series H redeemable convertible preferred stock | | 13,050 | | | 13,050 | |
| Reimbursement for clinical supply | | 310 | | | 0 | |
| Total | | $ | 213,360 | | | $ | 213,050 | |
We re-evaluate the transaction price at the end of each reporting period. During the yearyears ended December 31, 2020, there was a $0.32023 and 2022, we recognized expense of $184 million increase to the transaction price from a reimbursement for clinical supply.
and $6 million
The transaction price was allocated to the performance obligations based on the relative estimated standalone selling price, net of each performance obligation. We developed the estimated standalone selling price for the license included in each of the PCV Performance Obligation and the KRAS Performance Obligation primarily based on the probability-weighted present value of expected future cash flows associated with each license related to each specific program. In developing such estimate, we also considered applicable market conditions and relevant entity-specific factors, including those factors contemplated in negotiating the agreement, probability of success and the time needed to commercialize a product candidate pursuant to the associated license. We developed the estimated standalone selling price for the services and/or manufacturing and supply included in each of the PCV Performance Obligation and the KRAS Performance Obligation, as applicable, primarily based on the nature of the services to be performed and/or goods to be manufactured and estimates of the associated cost, adjusted for a reasonable profit margin that would be expected to be realized under similar contracts.
The following tables summarize the allocation of the total transaction price to the identified performance obligations under the arrangement, and the amount of the transaction price unsatisfied as of December 31, 2020 (in thousands):
| | | | | | | | |
| | Transaction Price |
PCV/SAV Agreement: | | December 31, 2020 |
PCV performance obligation | | $ | 206,356 | |
KRAS performance obligation | | 7,004 | |
Total | | $ | 213,360 | |
Remaining unsatisfied performance obligation | | $ | 51,007 | |
As of December 31, 2020, the remaining performance obligations that are unsatisfied is expected to be recognized as revenue through December 31, 2024.
We will recognize revenue related to amounts allocated to the PCV Performance Obligation over time as the underlying services are performed using a proportional performance model. We measure proportional performance using an input method based on the costs incurred relative to the total estimated costs of research and development efforts. We recognize revenueMerck's reimbursements, related to the amounts allocatedINT collaboration under the Merck Participation Term. Additionally, the net cost recovery for capital expenditures during the same periods were $102 million and $3 million, respectively, which were applied to reduce the KRAS Performance Obligation based on the point in time upon which control of supply is transferred to Merck for each deliverycapitalized cost of the associated supply.
The following table summarizes the revenue recognized for the periods presented (in thousands):
| | | | | | | | | | | | | | | | | | | | |
| | Years Ended December 31, |
| | 2020 | | 2019 | | 2018 |
| PCV/SAV Agreement | | $ | 33,103 | | | $ | 41,382 | | | $ | 41,498 | |
The revenue recognized during the year ended December 31, 2020 includes the amortization of deferred revenue due to the satisfaction of our performance during the period, offset by a cumulative catch-up adjustment of $3.5 million due to changes in estimated costs for our future performance obligations.
The following table summarizes the balances of deferred revenue, which is classified as current or non-current in the condensed consolidated balance sheets based on the period the services are expected to be performed or control of the supply is expected to be transferred for the periods presented (in thousands):
| | | | | | | | | | | | | | |
| | December 31 |
| | 2020 | | 2019 |
| PCV/SAV Agreement | | $ | 51,007 | | | $ | 83,799 | |
Vertex – Strategic Alliance in Cystic Fibrosis
2016 Strategic Alliance in Cystic Fibrosis
In July 2016, we entered into a Strategic Collaboration and License Agreement (Vertex Agreement), with Vertex Pharmaceuticals Incorporated, and Vertex Pharmaceuticals (Europe) Limited, together, Vertex, which we refer to as the Vertex Agreement.Vertex. The Vertex Agreement, which was amended in July 2019 which we refer to as the 2019(2019 Vertex Amendment,Amendment), is aimed at the discovery and development of potential mRNA medicines for the treatment of cystic fibrosis (CF) by enabling cells in the lungs of people with CF to produce functional cystic fibrosis transmembrane conductance regulator (CFTR) proteins.
Pursuant to the Vertex Agreement, we lead discovery efforts during an initial research period, that currently extends until March 2020, leveraging our Platformplatform technology and mRNA delivery expertise along with Vertex’s scientific experience in CF biology and the functional understanding of CFTR. Vertex is responsible for conducting development and commercialization activities for candidates and products that arise from the strategic alliance, including the costs associated with such activities. Subject to customary “back-up” supply rights granted to Vertex, we exclusively manufacture (or have manufactured) mRNA for preclinical, clinical and commercialization purposes. The parties established a joint steering committee to overseeThis collaboration arrangement is accounted for under ASC 606 and coordinate activities under the Vertex Agreement. We and Vertex have granted each other certain licenses under the Vertex Agreement.currently ongoing.
Under the terms of the Vertex Agreement, we received a $20.0 million upfront payment from Vertex. In July 2019, Vertex elected to extend the initial research period by six months by making a $2.0 million payment to us pursuant to the 2019 Vertex Amendment. In March 2020 based on the promising preclinical data generated to date, Vertex extended the conduct of the initial Research Plan through the First Extended Research Term (an additional 18-month term) by making an additional payment to us. Vertex has rights to further extend the research period for 2 additional one-year periods by making an additional payment to us for each one-year extension. We are eligible to receive up to $55.0 million in payments for achievement of development milestones, up to $220.0 million in payments for achievement of regulatory milestones and potentially could receive an additional $3.0 million milestone payment for achievement of a regulatory milestone for second and each subsequent product under the Vertex Agreement. Vertex will also pay us tiered royalties at rates ranging from the low- to high-teens on worldwide net sales of products arising from the strategic alliance, subject to certain reductions, with an aggregate minimum floor. In connection with the strategic alliance, Vertex also made a $20.0 million equity investment in us.
During the term of the Vertex Agreement, we and Vertex have agreed to certain defined exclusivity obligations under the Vertex Agreement with respect to the development and commercialization of certain mRNA medicines. Unless earlier terminated, the Vertex Agreement will continue until the expiration of all royalty terms. Vertex may terminate the Vertex Agreement for convenience upon 90 days’ prior written notice, except if termination relates to a product in a country where Vertex has received marketing approval, which, in such case, Vertex must provide 180 days’ prior written notice. Either party may terminate the Vertex Agreement upon the other party’s material breach, subject to specified notice and cure provisions. Each party may also terminate the Vertex Agreement in
the event that the other party challenges the validity or enforceability of such party’s patent rights, subject to certain exceptions, or if the other party becomes insolvent.
Accounting Treatment
The total transaction price for the Vertex Agreement was determined to be $24.4 million, comprised of the $20.0 million upfront payment and $4.4 million in research and development funding related to the research and development services and supply of non-cGMP mRNA. As of December 31, 2019, all performance obligations under the Vertex Agreement were completed. The 2019 Vertex Amendment represents a contract modification and is accounted for as a separate contract. As of December 31, 2020, all performance obligations under the 2019 Vertex Amendment were completed and the total transaction price of $4.5 million, comprised of the $2.0 million upfront payment and $2.5 million in research and development funding related to the research and development services and supply of non-cGMP mRNA, was fully recognized.
The First Extended Research Term represents a contract modification and is accounted for as a separate contract. Pursuant to the 2019 Vertex Amendment, we identified one performance obligation comprised of: (i) a research, development and commercialization license and (ii) research and development services, including manufacturing and supply of non-cGMP mRNA, during the 18-month First Extended Research Term. We concluded that the license is not distinct from the research and development services, including manufacturing and supply of non-cGMP mRNA. Additionally, we concluded that the following customer options are marketing offers as such options did not provide any discounts or other rights that would be considered a material right in the arrangement: (i) Vertex’s rights to extend the extended initial research period and (ii) clinical mRNA supply and/or non-cGMP mRNA supply beyond the extended initial research period. Therefore, such options will be accounted for as a separate contract upon the customer’s election.
The following table summarizes the composition of the total transaction price for the First Extended Research Term at December 31, 2020 (in thousands):
| | | | | | | | |
| | Transaction Price |
Vertex Agreement - First Extended Research Term: | | December 31, 2020 |
Upfront payment | | $ | 4,000 | |
Research and development | | 39,798 | |
Total | | $ | 43,798 | |
We utilize the most likely amount method to determine the amount of research and development funding to be received. As of December 31, 2020, there were no milestones included in the transaction price. We considered the stage of development and the risks associated with the remaining development required to achieve each milestone, as well as whether the achievement of the milestone is outside of our or Vertex’s control. The outstanding milestone payments were fully constrained, as a result of the uncertainty whether any of the milestones would be achieved. We determined that any sales-based royalties will be recognized when the related sales occur as they were determined to relate predominantly to the license granted and therefore have also been excluded from the transaction price. We re-evaluate the transaction price at the end of each reporting period and as uncertain events are resolved or other changes in circumstances occur. There was a $8.6 million increase to the transaction price during the year ended December 31, 2020, resulting from a change in the estimate of variable consideration.
The following table summarizes the total transaction price allocated to the single performance obligation under the arrangement, and the amount of the transaction price unsatisfied as of December 31, 2020 (in thousands):
| | | | | | | | |
| | Transaction Price |
| | December 31, 2020 |
First Extended Research Term transaction price | | $ | 43,798 | |
Remaining unsatisfied performance obligation | | $ | 30,346 | |
As of December 31, 2020 the aggregate amount of transaction price allocated to the remaining performance obligations that are unsatisfied is expected to be recognized as revenue through the fourth quarter of 2021.
We recognize revenue related to amounts allocated to the single performance obligation over time as the underlying services are performed using a proportional performance model. We measure proportional performance using an input method based on the costs incurred relative to the total estimated costs of the research and development efforts.
The following table summarizes the revenue recognized for the periods presented (in thousands):
| | | | | | | | | | | | | | | | | | | | |
| | Years Ended December 31, |
| | 2020 | | 2019 | | 2018 |
| Vertex First Extended Research Term | | $ | 13,452 | | | $ | 0 | | | $ | 0 | |
| Vertex Agreement/2019 Amendment | | 2,053 | | | 6,197 | | | 10,400 | |
| Total | | $ | 15,505 | | | $ | 6,197 | | | $ | 10,400 | |
The revenue recognized during the year ended December 31, 2020, includes the amortization of the deferred revenue due to the satisfaction of our performance during the period, offset by a cumulative catch-up adjustment of $0.2 million due to changes in estimated costs for our future performance obligations.
The following table summarizes the balances of deferred revenue, classified as current and non-current in the condensed consolidated balance sheets based on the term of the research period for the periods presented (in thousands):
| | | | | | | | | | | | | | |
| | December 31, |
| | 2020 | | 2019 |
Vertex Agreement (1) | | $ | 2,648 | | | $ | 793 | |
____________
(1) Balance as of December 31, 2019 represents deferred revenue related to the Vertex 2019 Amendment
Vertex —2020 Strategic Alliance in Cystic Fibrosis
In September 2020, we entered into a new Strategic Collaboration and License Agreement with Vertex (Vertex 2020 Agreement). The Vertex 2020 Agreement iswas aimed at the discovery and development of potential medicines to treat CF by delivering gene-editing therapies to lung cells to facilitate production of functional CFTR proteins.
The three-year research period of the Vertex 2020 Agreement willwas initially focus on the identification and optimization of novel LNPs and mRNAs that can deliver gene-editing therapies to cells in the lungs. Following the initial three-year period, Vertex iswas responsible for conducting development and commercialization activities for candidates and products that arise from the strategic alliance, including the costs associated with such activities. Vertex iswas also obligated to pay us for research services in connection with our performance of certain activities in accordance with a jointly agreed research plan. Subject to customary “back-up” supply rights granted to Vertex, underThis collaboration was concluded and terminated in the agreement, we are the exclusive manufacturerfourth quarter of related mRNA and LNPs for preclinical, clinical, and commercialization purposes.
Under2023 in accordance with the terms of the Vertex 2020 Agreement,Agreement.
AstraZeneca – Strategic Alliances in Cardiovascular and Oncology
We entered into collaboration agreements with AstraZeneca in 2013 and 2016, aimed at developing mRNA medicines for treatments in cardiovascular, cardiometabolic diseases, and cancer. These agreements provided AstraZeneca with exclusive development and commercialization rights for specific programs, including the VEGF-A product AZD8601 and an immuno-oncology program for IL-12, with upfront payments to us totaling $240 million.
In the third quarter of 2022, AstraZeneca terminated our collaborations with them, including the development of VEGF-A and IL-12 programs, for which termination became effective on November 21, 2022. All rights to these two programs reverted to us. As a result of the termination, we receivedrecognized the remaining deferred revenue of $76 million as collaboration revenue in the period.
Immatics – Strategic Multi-Platform Collaboration to Develop Oncology Therapeutics
In September 2023, we entered into a $75.0 millionstrategic research and development collaboration agreement with Immatics, a clinical-stage biopharmaceutical company active in the discovery and development of T cell-redirecting cancer immunotherapies. We will lead the clinical development and commercialization of cancer vaccines and TCER® therapeutics resulting from the collaboration. Immatics will be responsible for conducting the preclinical studies and a potential Phase 1 clinical trial investigating IMA203 TCR-T in combination with the PRAME mRNA vaccine to further enhance IMA203 T cell responses. Each party will retain full ownership of its investigational PRAME compound, and the parties will fund the clinical study on a cost sharing basis.
Upon effectiveness of the agreement in October 2023, we made an upfront payment from Vertex. We areof $120 million to Immatics. The payment was recognized as research and development expense in the same period. Immatics will also receive research funding and is eligible to receive up to $380.0 million in milestone payments upon the achievement of certain development, regulatory, and commercial milestone events with respectpayments. Immatics is also eligible to any products that result from the strategic alliance, and Vertex will also pay us areceive tiered percentage of its gross profits derived from worldwideroyalties on global net sales of TCER® products arising fromand certain vaccine products that are commercialized under the strategic alliance.
Duringagreement. Under the term of the Vertex 2020 Agreement, we and Vertex have agreedagreement, Immatics has an option to certain defined exclusivity obligations with respect to the development and commercialization of certain mRNA medicines.
Unless earlier terminated, the Vertex 2020 Agreement will continue until the expiration of all payment obligations. Vertex may terminate the Vertex 2020 Agreement for convenience upon 90 days’ prior written notice, except if termination relates to a product in a country where Vertex has received marketing approval; in such case, Vertex must provide 180 days’ prior written notice. Either party may terminate the Vertex 2020 Agreement upon the other party’s material breach, subject to specified notice and cure provisions. Each party may also terminate the Vertex 2020 Agreement in the event that the other party challenges the validity or enforceability of such party’s patent rights, subject to certain exceptions, or if the other party becomes insolvent.
Accounting Treatment
We determined that all aspects of the 2020 Vertex Agreement represent a transaction with a customer and therefore should be accounted for in accordance with ASC 606. We also determined that the 2020 Vertex Agreement should be accounted for separately from other Vertex Agreements as the agreements contemplate research on separate research candidates. We identified one performance obligation comprised of: (i) a research, development and commercialization license; and (ii) research and development
services, including manufacturing and supply of non-cGMP mRNA, performed as part of the Initial Research Plan and the Additional Research Plan (collectively, the research period). We concluded that the license is not distinct from the research and development services, including manufacturing and supply of non-cGMP mRNA used in the performance of the research services, as Vertex cannot fully exploit the value of the license without receipt of such services and supply. Our services and supply involve specialized expertise, particularly as it relates to mRNA technology that is not available in the marketplace. Therefore, the license has been combined with the research and development services, including manufacturing and supply of non-cGMP supply,enter into a single performance obligation. Additionally, we concluded the provision of clinical mRNA supply and/or non-cGMP mRNA supply provided to Vertex at their option represents a customer optionglobal profit and is considered a marketing offer as such options did not provide any discounts or other rights that would be considered a material right in the arrangement. Such options will be accountedloss share arrangement for as a separate contract upon the customer’s election.
The total transaction price was determined to be $75.0 million, comprised of the upfront payment. We utilize the most likely amount method to determine the amount of research and development funding to be received associated with the Additional Research Plan. Our funding estimate is based on our experience bringing research candidates to the point of nomination by our collaboration partners, including our experience with Vertex under separate transactions. We have fully constrained such amounts and will not include any expected funding in the transaction price until the scope of such services have been agreed to amongst the parties. We also utilize the most likely amount method to estimate any development and regulatory milestone payments to be received. Further, there were no milestones included in the transaction price. We considered the stage of development and the risks associated with the remaining development required to achieve each milestone, as well as whether the achievement of the milestone is outside of our or Vertex’s control. The outstanding milestone payments were fully constrained, as a result of the uncertainty whether any of the milestones would be achieved. We determined that any sales-based royalties will be recognized when the related sales occur as they were determined to relate predominantly to the license granted, and therefore have also been excluded from the transaction price.
For the 2020 Vertex Agreement, the total transaction price was allocated entirely to a single performance obligation. We recognize revenue related to amounts allocated to the single performance obligation over time as the underlying services are performed using a proportional performance model. We measure proportional performance using an input method based on the costs incurred relative to the total estimated costs of the research and development efforts.
For the year ended December 31, 2020, we did not recognize any revenue associated with the 2020 Vertex Agreement. As of December 31, 2020, the aggregate amount of the transaction price allocated to the remaining performance obligations that are unsatisfied is $75.0 million, which is expected to be recognized as revenue through the third quarter of 2023. We had deferred revenue of $75.0 million as of December 31, 2020, which is classified as current and non-current in the consolidated balance sheets based on the term of the research period.
Chiesi—2020 Collaboration and License Agreement with Chiesiadvanced TCER®.
In September 2020,addition to the collaborative arrangements mentioned above, we entered into a Collaborationhave other collaborative and License Agreement with Chiesi Farmaceutici S.P.A. (Chiesi), whichlicensing arrangements that we referdo not consider to as the Chiesi Agreement. The Chiesi Agreement is aimedbe individually significant to our business at the discovery and development of potential mRNA medicines for the treatment of Pulmonary Arterial Hypertension (PAH), a rare disease characterized by high blood pressure in the arteries of the lungs.
this time. Pursuant to the Chiesi Agreement,these agreements, we lead discovery efforts during a four-year research period, leveraging our Platform technologymay be required to make upfront payments and mRNA delivery expertise along with Chiesi’s scientific experience in PAH biology. Chiesi is responsible for conducting development and commercialization activities for candidates and products that arise from the collaboration, including the costs associated with such activities. Chiesi is also obligated to pay us for our performance of research activities during the research period in accordance with a jointly agreed research plan. Under the agreement, we are the exclusive manufacturer of related candidates and products for preclinical, clinical, and commercialization purposes.
Under the terms of the Chiesi Agreement, we are entitled to receive a $25.0 million upfront payment from Chiesi. Chiesi has the right to extend the initial four-year research period by one additional year by making an additional payment to us. We are eligible to receive up to $405.0 million in aggregate milestone payments upon the achievement of certainvarious development, regulatory and commercial milestones, which in the aggregate could be significant. Future milestone payments, if any, will be reflected in our consolidated financial statements when the corresponding events and Chiesi will alsobecome probable. In addition, we may be required to pay us tiered double-digitsignificant royalties on worldwide netfuture sales ofif products arising from the collaboration, subjectrelated to certain reductions, with an aggregate minimum floor.
During the term of the Chiesi Agreement, we and Chiesi have agreed to certain defined exclusivity with respect to the development and commercialization of certain mRNA medicines.these arrangements are commercialized.
Accounting Treatment
We determined that all aspects of the Chiesi Agreement represent a transaction with a customer and therefore should be accounted for in accordance with ASC 606. We identified the following performance obligations in the Chiesi Agreement: (i) a research license and research and development services, including manufacturing and supply during the research period; (ii) a material right for the first product development and commercialization license; and (iii) a material right for the second product development and commercialization license. We concluded that the license is not distinct from the research and development services, including manufacturing and supply of non-cGMP mRNA, during the four-year research period, as Chiesi cannot fully exploit the value of the license without receipt of such services and supply. Our services and supply involve specialized expertise, particularly as it relates to mRNA technology that is not available in the marketplace. Therefore, the license has been combined with the research and development services, including manufacturing and supply of non-cGMP supply, into a single performance obligation. Additionally, we concluded the provision of clinical mRNA supply and/or non-cGMP mRNA supply beyond the four-year research period and the right to extend the research period for one additional year represent customer options and are considered marketing offers as such options did not provide any discounts or other rights that would be considered a material right in the arrangement. Such options will be accounted for as a separate contract upon the customer's election.
The following table summarizes the composition of the total transaction price for the periods presented (in thousands):
| | | | | | | | |
| | Transaction Price |
Chiesi Agreement: | | December 31, 2020 |
Upfront payment | | $ | 25,000 | |
Research and development | | 17,496 | |
Total | | $ | 42,496 | |
We utilize the most likely amount method to determine the amount of research and development funding to be received associated with the research period. We also utilize the most likely amount method to estimate any development and regulatory milestone payments to be received. Further, there were no milestones included in the transaction price. We considered the stage of development and the risks associated with the remaining development required to achieve each milestone, as well as whether the achievement of the milestone is outside of our or Chiesi’s control. The outstanding milestone payments were fully constrained, as a result of the uncertainty whether any of the milestones would be achieved. We determined that any sales-based commercial milestone payments and royalties will be recognized when the related sales occur as they were determined to relate predominantly to the license granted and therefore have also been excluded from the transaction price.
The transaction price was allocated to the performance obligations based on the relative estimated standalone selling prices of each performance obligation. We developed the estimated standalone selling price for the services and manufacturing and supply included in the performance obligation, as applicable, primarily based on the nature of the services to be performed and the goods to be manufactured and estimates of the associated costs, adjusted for a reasonable profit margin that would be expected to be realized under similar contracts. We developed the estimated standalone selling prices for the licenses included in the associated performance obligations based on an analysis of our other transactions with collaboration partners and third party comparable transactions. In developing such estimates, we also considered applicable market conditions and relevant entity-specific factors, including those factors contemplated in negotiating the agreement, probability of success and the time needed to commercialize a product candidate pursuant to the associated license. The estimated standalone selling price of the material rights to receive development and commercialization rights was developed by estimating the amount of discount that Chiesi would receive when exercising the option and adjusting such amount by the likelihood that the option will be exercised.
The following table summarizes the allocation of the transaction price to the identified performance obligations under the arrangement, and the amount of the transaction price unsatisfied as of December 31, 2020 (in thousands):
| | | | | | | | |
| | Transaction Price |
| | December 31, 2020 |
Chiesi Agreement transaction price | | $ | 42,496 | |
Remaining unsatisfied performance obligation | | $ | 42,293 | |
| | |
We recognize revenue related to amounts allocated to the combined research license and research and development services performance obligation over time as the underlying services are performed using a proportional performance model. We measure proportional performance using an input method based on the costs incurred relative to the total estimated costs of the research and development efforts. We will recognize revenue associated with the material rights as revenue at the earlier of the exercise or expiry of the material rights.
For the year ended December 31, 2020, we recognized collaboration revenue of $0.2 million from the Chiesi Agreement. We did not recognize any revenue associated with the Chiesi Agreement for the years ended December 31, 2019 and 2018. As of December 31, 2020, $17.3 million of the remaining performance obligations that are unsatisfied is expected to be recognized as revenue through the third quarter of 2024 and $25.0 million is expected to be recognized as revenue at the earlier of the exercise or expiry of the material rights.
We had deferred revenue of $25.0 million as of December 31, 2020, classified as non-current in the consolidated balance sheets based on the term of the research period.
6. Financial Instruments and Fair Value Measurements
Cash and Cash Equivalents and Investments
The following tables summarize our cash and available-for-sale securities by significant investment category at December 31, 20202023 and 20192022 (in thousands)millions):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | December 31, 2023 |
| | Amortized Cost | | Unrealized Gains | | Unrealized Losses | | Fair Value | | Cash and Cash Equivalents | | Current Marketable Securities | | Non- Current Marketable Securities |
| Cash and cash equivalents | | $ | 2,907 | | | $ | — | | | $ | — | | | $ | 2,907 | | | $ | 2,907 | | | $ | — | | | $ | — | |
| Available-for-sale: | | | | | | | | | | | | | | |
| Certificates of deposit | | 27 | | | — | | | — | | | 27 | | | — | | | 27 | | | — | |
| U.S. treasury bills | | 807 | | | — | | | — | | | 807 | | | — | | | 807 | | | — | |
| U.S. treasury notes | | 4,407 | | | 3 | | | (67) | | | 4,343 | | | — | | | 2,664 | | | 1,679 | |
| Corporate debt securities | | 5,067 | | | 3 | | | (81) | | | 4,989 | | | — | | | 2,082 | | | 2,907 | |
| Government debt securities | | 211 | | | — | | | (3) | | | 208 | | | — | | | 117 | | | 91 | |
| Total | | $ | 13,426 | | | $ | 6 | | | $ | (151) | | | $ | 13,281 | | | $ | 2,907 | | | $ | 5,697 | | | $ | 4,677 | |
|
| | December 31, 2022 |
| | Amortized Cost | | Unrealized Gains | | Unrealized Losses | | Fair Value | | Cash and Cash Equivalents | | Current Marketable Securities | | Non- Current Marketable Securities |
| Cash and cash equivalents | | $ | 3,205 | | | $ | — | | | $ | — | | | $ | 3,205 | | | $ | 3,205 | | | $ | — | | | $ | — | |
| Available-for-sale: | | | | | | | | | | | | | | |
| Certificates of deposit | | 188 | | | — | | | — | | | 188 | | | — | | | 188 | | | — | |
| U.S. treasury bills | | 767 | | | — | | | — | | | 767 | | | — | | | 767 | | | — | |
| U.S. treasury notes | | 7,781 | | | — | | | (229) | | | 7,552 | | | — | | | 4,182 | | | 3,370 | |
| Corporate debt securities | | 6,595 | | | — | | | (226) | | | 6,369 | | | — | | | 1,560 | | | 4,809 | |
| Government debt securities | | 148 | | | — | | | (9) | | | 139 | | | — | | | — | | | 139 | |
| Total | | $ | 18,684 | | | $ | — | | | $ | (464) | | | $ | 18,220 | | | $ | 3,205 | | | $ | 6,697 | | | $ | 8,318 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | December 31, 2020 |
| | Amortized Cost | | Unrealized Gains | | Unrealized Losses | | Fair Value | | Cash and Cash Equivalents | | Current Marketable Securities | | Non- Current Marketable Securities |
| Cash and cash equivalents | | $ | 2,623,849 | | | $ | 1 | | | $ | 0 | | | $ | 2,623,850 | | | $ | 2,623,850 | | | $ | 0 | | | $ | 0 | |
| Available-for-sale: | | | | | | | | | | | | | | |
| Certificates of deposit | | 238,774 | | | 49 | | | (4) | | | 238,819 | | | 0 | | | 215,389 | | | 23,430 | |
| U.S. treasury securities | | 491,549 | | | 69 | | | 0 | | | 491,618 | | | 0 | | | 491,618 | | | 0 | |
| Debt securities of U.S. government agencies and corporate entities | | 1,888,022 | | | 4,294 | | | (147) | | | 1,892,169 | | | 0 | | | 1,276,751 | | | 615,418 | |
| | $ | 5,242,194 | | | $ | 4,413 | | | $ | (151) | | | $ | 5,246,456 | | | $ | 2,623,850 | | | $ | 1,983,758 | | | $ | 638,848 | |
|
| | | | | | | | | | | | | | |
| | December 31, 2019 |
| | Amortized Cost | | Unrealized Gains | | Unrealized Losses | | Fair Value | | Cash and Cash Equivalents | | Current Marketable Securities | | Non- Current Marketable Securities |
| Cash and cash equivalents | | $ | 225,874 | | | $ | 0 | | | $ | 0 | | | $ | 225,874 | | | $ | 225,874 | | | $ | 0 | | | $ | 0 | |
| Available-for-sale: | | | | | | | | | | | | | | |
| Certificates of deposit | | 82,028 | | | 79 | | | (6) | | | 82,101 | | | 10,002 | | | 69,197 | | | 2,902 | |
| U.S. treasury securities | | 117,891 | | | 260 | | | (2) | | | 118,149 | | | 0 | | | 110,186 | | | 7,963 | |
| Debt securities of U.S. government agencies and corporate entities | | 834,187 | | | 2,708 | | | (32) | | | 836,863 | | | 0 | | | 687,741 | | | 149,122 | |
| | $ | 1,259,980 | | | $ | 3,047 | | | $ | (40) | | | $ | 1,262,987 | | | $ | 235,876 | | | $ | 867,124 | | | $ | 159,987 | |
The amortized cost and estimated fair value of marketableavailable-for-sale securities, by contractual maturity at December 31, 20202023 and 2019 are2022 were as follows (in thousands)millions):
| | | | | | | | | | | | | | |
| | December 31, 2020 |
| | Amortized Cost | | Estimated Fair Value |
| Due in one year or less | | $ | 1,980,963 | | | $ | 1,983,758 | |
| Due after one year through five years | | 637,382 | | | 638,848 | |
| Total | | $ | 2,618,345 | | | $ | 2,622,606 | |
| | December 31, 2019 |
| | Amortized Cost | | Estimated Fair Value |
| Due in one year or less | | $ | 864,958 | | | $ | 867,124 | |
| Due after one year through five years | | 159,148 | | | 159,987 | |
| Total | | $ | 1,024,106 | | | $ | 1,027,111 | |
| | | | | | | | | | | | | | |
| | December 31, 2023 |
| | Amortized Cost | | Estimated Fair Value |
| Due in one year or less | | $ | 5,751 | | | $ | 5,697 | |
| Due after one year through five years | | 4,768 | | | 4,677 | |
| Total | | $ | 10,519 | | | $ | 10,374 | |
| | December 31, 2022 |
| | Amortized Cost | | Estimated Fair Value |
| Due in one year or less | | $ | 6,792 | | | $ | 6,697 | |
| Due after one year through five years | | 8,687 | | | 8,318 | |
| Total | | $ | 15,479 | | | $ | 15,015 | |
In accordance with our investment policy, we place investments in investment grade securities with high credit quality issuers, and generally limit the amount of credit exposure to any one issuer. We evaluate securities for impairment at the end of each reporting period. We did 0tnot record any impairment charges related to our available-for-sale securities during the years ended December 31, 2020, 20192023, 2022, and 2018.2021. We did not recognize any credit losses relatedcredit-related allowance to available-for-sale securities as of the years ended December 31, 20202023 and 2019.2022.
The following table summarizes the amount of gross unrealized losses and the estimated fair value for our available-for-sale securities in an unrealized loss position by length of time the securities have been in an unrealized loss position at December 31, 20202023 and 20192022 (in thousands)millions):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Less than 12 Months | | 12 Months or More | | Total |
| | Gross Unrealized Losses | | Estimated Fair Value | | Gross Unrealized Losses | | Estimated Fair Value | | Gross Unrealized Losses | | Estimated Fair Value |
| As of December 31, 2020: | | | | | | | | | | | | |
| Certificates of deposit | | $ | (4) | | | $ | 81,524 | | | $ | 0 | | | $ | 0 | | | $ | (4) | | | $ | 81,524 | |
| Debt securities of U.S. government agencies and corporate entities | | (148) | | | 507,016 | | | 0 | | | 0 | | | (148) | | | 507,016 | |
| Total | | $ | (152) | | | $ | 588,540 | | | $ | 0 | | | $ | 0 | | | $ | (152) | | | $ | 588,540 | |
| | | | | | | | | | | | |
| As of December 31, 2019: | | | | | | | | | | | | |
| Certificates of deposit | | $ | (6) | | | $ | 12,822 | | | $ | 0 | | | $ | 0 | | | $ | (6) | | | $ | 12,822 | |
| U.S. treasury securities | | (2) | | | 9,979 | | | 0 | | | 0 | | | (2) | | | 9,979 | |
| Debt securities of U.S. government agencies and corporate entities | | (32) | | | 62,360 | | | 0 | | | 0 | | | (32) | | | 62,360 | |
| Total | | $ | (40) | | | $ | 85,161 | | | $ | 0 | | | $ | 0 | | | $ | (40) | | | $ | 85,161 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Less than 12 Months | | 12 Months or More | | Total |
| | Gross Unrealized Losses | | Estimated Fair Value | | Gross Unrealized Losses | | Estimated Fair Value | | Gross Unrealized Losses | | Estimated Fair Value |
| As of December 31, 2023: | | | | | | | | | | | | |
| | | | | | | | | | | | |
| U.S. treasury bills | | $ | — | | | $ | 25 | | | $ | — | | | $ | — | | | $ | — | | | $ | 25 | |
| U.S. treasury notes | | (3) | | | 774 | | | (64) | | | 2,983 | | | (67) | | | 3,757 | |
| Corporate debt securities | | (1) | | | 562 | | | (79) | | | 3,518 | | | (80) | | | 4,080 | |
| Government debt securities | | — | | | 8 | | | (4) | | | 143 | | | (4) | | | 151 | |
| Total | | $ | (4) | | | $ | 1,369 | | | $ | (147) | | | $ | 6,644 | | | $ | (151) | | | $ | 8,013 | |
| | | | | | | | | | | | |
| As of December 31, 2022: | | | | | | | | | | | | |
| | | | | | | | | | | | |
| U.S. treasury securities | | $ | — | | | $ | 128 | | | $ | — | | | $ | — | | | $ | — | | | $ | 128 | |
| U.S. treasury notes | | (101) | | | 3,956 | | | (128) | | | 3,541 | | | (229) | | | 7,497 | |
| Corporate debt securities | | (138) | | | 3,505 | | | (88) | | | 1,890 | | | (226) | | | 5,395 | |
| Government debt securities | | (2) | | | 46 | | | (7) | | | 93 | | | (9) | | | 139 | |
| Total | | $ | (241) | | | $ | 7,635 | | | $ | (223) | | | $ | 5,524 | | | $ | (464) | | | $ | 13,159 | |
At December 31, 20202023 and 2019,2022, we held 108392 and 19 individual582 available-for-sale securities, respectively, out of our total investment portfolio that were in a continuous unrealized loss position for less than 12 months.position. We neither intend to sell these investments nor conclude that we are more-likely-than-not that we will have to sell them before recovery of their carrying values. We also believe that we will be able to collect both principal and interest amounts due to us at maturity.
Assets and Liabilities Measured at Fair Value on a Recurring Basis
The following tables summarize our financial assets measured at fair value on a recurring basis as of December 31, 20202023 and 20192022 (in thousands)millions): | | | | | | | | | | | | | | | | | | | | |
| | Fair value at December 31, 2023 | | Fair Value Measurement Using |
| | | Level 1 | | Level 2 |
| Assets: | | | | | | |
| Money market funds | | $ | 1,572 | | | $ | 1,572 | | | $ | — | |
| Certificates of deposit | | 27 | | | — | | | 27 | |
| U.S. treasury bills | | 1,246 | | | — | | | 1,246 | |
| U.S. treasury notes | | 4,343 | | | — | | | 4,343 | |
| Corporate debt securities | | 5,480 | | | — | | | 5,480 | |
| Government debt securities | | 208 | | | — | | | 208 | |
Equity investments(1) | | 24 | | | 24 | | | — | |
| Derivative instruments | | 4 | | | — | | | 4 | |
| Total | | $ | 12,904 | | | $ | 1,596 | | | $ | 11,308 | |
| Liabilities: | | | | | | |
Derivative instruments | | $ | 9 | | | $ | — | | | $ | 9 | |
| | | | | | |
| | Fair Value at December 31, 2020 | | Fair Value Measurement Using |
| Level 1 | | Level 2 |
| | Fair value at December 31, 2022 | | | | Fair value at December 31, 2022 | | Fair Value Measurement Using |
| | | Level 1 | | | | | Level 1 | | Level 2 |
| Assets: | Assets: | | | | | | |
| Money market funds | Money market funds | | $ | 620,947 | | | $ | 620,947 | | | $ | 0 | |
| Money market funds | |
| Money market funds | |
| Certificates of deposit | Certificates of deposit | | 238,819 | | | 0 | | | 238,819 | |
| U.S. treasury securities | | 491,618 | | | 0 | | | 491,618 | |
| Debt securities of U.S. government agencies and corporate entities | | 1,892,169 | | | 0 | | | 1,892,169 | |
| Derivative instruments (Note 7) | | 293 | | | 0 | | | 293 | |
| U.S. treasury bills | |
| U.S. treasury notes | |
| Corporate debt securities | |
| Government debt securities | |
| Derivative instruments | |
| Total | Total | | $ | 3,243,846 | | | $ | 620,947 | | | $ | 2,622,899 | |
| Liabilities: | |
Derivative instruments | |
Derivative instruments | |
Derivative instruments | |
|
_______
Table(1)Investments in publicly traded equity securities with readily determinable fair values are recorded at quoted market prices for identical securities, with changes in fair value recorded in other expense net, in our consolidated statements of Contents
operations.
| | | | | | | | | | | | | | | | | | | | |
| | Fair Value at December 31, 2019 | | Fair Value Measurement Using |
| | | Level 1 | | Level 2 |
| Assets: | | | | | | |
| Certificates of deposit | | $ | 82,101 | | | $ | 0 | | | $ | 82,101 | |
| U.S. treasury securities | | 118,149 | | | 0 | | | 118,149 | |
| Debt securities of U.S. government agencies and corporate entities | | 836,863 | | | 0 | | | 836,863 | |
| Total | | $ | 1,037,113 | | | $ | 0 | | | $ | 1,037,113 | |
During the years endedAs of December 31, 20202023 and 2019,2022, we did not have any financial liabilities measured at fair value on a recurring basis and did not have non-financial assets or liabilities measured at fair value on a recurring basis.
TableFor the year ended December 31, 2023, we recognized net losses of Contents
7. Derivative Financial Instruments
We transact business$35 million on equity investments from changes in various foreign currencies and have international sales and expenses denominated in foreign currencies. Therefore, we are exposed to certain risks arising from both our business operations and economic conditions. Our risk management strategy includes the use of derivative financial instruments to hedge foreign currency exchange rate fluctuations on monetary assets or liabilities denominated in foreign currencies. We do not enter into derivative financial contracts for speculative or trading purposes. We do not believe that we are exposed to more than a nominal amount of credit risk in our foreign currency hedges, as counterparties are large, global and well-capitalized financial institutions. We classify cash flows from our derivative transactions as cash flows from operating activities in our consolidated statements of cash flows.
Balance Sheet Hedges
Our foreign currency forward contracts, primarily accounts receivable, are not designated for hedge accounting treatment. Therefore, these forward contracts are accounted for as derivatives whereby the fair value of the contractssecurities. We did not have equity investments in publicly traded securities with readily determinable fair values during 2022 and 2021.
In addition, as of December 31, 2023 and 2022, we had $42 million, at each balance sheet date, in equity investments without readily determinable fair values, which are reported asrecorded within other currentnon-current assets or other current liabilities onin our consolidated balance sheets and gains and losses resultingexcluded from changes in the fair value are recorded as a component of other (expense) income, net, in our consolidated statements of operations. The gains and losses on these foreign currency forward contracts generally offset the gains and losses in the underlying foreign currency denominated assets and liabilities, which are also recorded to other (expense) income, net, in our consolidated statements of operations.
Total gross notional amount and fair value for foreign currency derivatives that are not designated as hedging instruments are accounted for as follows (in thousands):
| | | | | | | | | | | | | | | | | | | | |
| | December 31, 2020 |
| | Notional Amount | | Fair Value |
| | | Asset (1) | | Liability |
| Derivatives not designated as hedging instruments | | | | | | |
| Foreign currency forward contracts | | $ | 368,448 | | | $ | 293 | | | $ | 0 | |
| Total | | $ | 368,448 | | | $ | 293 | | | $ | 0 | |
_________
(1) As presented in the consolidated balance sheet within other current assets.
We did not have any derivative instruments as of December 31, 2019.
The effect of foreign currency forward contracts not designated as hedging instruments in our consolidated statements of operations for the year ended December 31, 2020 was as follows (in thousand):
| | | | | | | | | | | | | | |
| | Statement of Operations Classification | | Year Ended
December 31, 2020 |
Derivatives not designated as hedging instruments | | | | |
Foreign currency forward contracts | | Other (expense) income, net | | $ | 293 | |
Total | | | | $ | 293 | |
There were no hedging activities for the years ended December 31, 2019 and 2018.measurement tables above.
8.7. Inventory
Inventory, as of December 31, 2020 consists2023 and 2022 consisted of the following (in thousands)millions):
| | | | | | | | | | | | | | | | |
| | December 31, | | |
| | 2023 | | 2022 | | |
| Raw materials | | $ | 163 | | | $ | 575 | | | |
| Work in progress | | 15 | | | 205 | | | |
| Finished goods | | 24 | | | 169 | | | |
| Total inventory | | $ | 202 | | | $ | 949 | | | |
| | | | | | |
Inventory, non-current(1) | | $ | 170 | | | $ | 910 | | | |
_______
(1)Consisted of raw materials with an anticipated consumption beyond one year. Inventory, non-current is included in other non-current assets in the consolidated balance sheets.
| | | | | | | | | | |
| | December 31, | | |
| | 2020 | | |
Raw materials | | $ | 36,222 | | | |
Work in progress | | 9,015 | | | |
Finished goods | | 1,290 | | | |
Total inventory | | $ | 46,527 | | | |
Inventory write-downs as a result of excess, obsolescence, scrap or other reasons, and losses on firm purchase commitments are recorded as a component of cost of sales in our consolidated statements of operations. For the years ended December 31, 2023 and 2022, inventory write-downs were $2.2 billion and $1.3 billion, respectively. Inventory write-downs were immaterial for the year ended December 31, 2021. For the years ended December 31, 2023 and 2022, losses on firm purchase commitments were $141 million and $617 million, respectively. There were no such charges in 2021. Inventory write-downs were mainly related to obsolete inventory due to shelf-life expiration and inventory in excess of expected demand. Losses on firm purchase commitments were primarily related to excess raw material purchase commitments that will expire before the anticipated consumption of those raw materials. These charges in 2023 were primarily driven by a continued shift in product demand to the latest variant-targeted COVID-19 vaccine and a decline in customer demand as the COVID-19 vaccine market continues to transition to an endemic seasonal market in 2023.
We did 0t have anyIn the third quarter of 2023, we completed our long-range financial planning process, incorporating revised forecasts of vaccination rates. This resulted in the reassessment of future demand for our COVID-19 vaccine, leading to a strategic initiative to resize our manufacturing cost structure. This initiative, launched in the same quarter, involved reassessing our inventory at December 31, 2019.levels and renegotiating with our suppliers to reduce our purchase commitments related to raw materials which were not expected to be consumed before expiration. This initiative resulted in a raw materials write-down of $903 million, included in the total inventory write-down amount for the quarter.
9. PropertyAs of December 31, 2023 and EquipmentDecember 31, 2022, the accrued liability for losses on firm future purchase commitments in our consolidated balance sheets was $79 million and $268 million, respectively. As of December 31, 2023 and December 31, 2022, we had inventory on hand of $372 million and $1.9 billion, respectively. Our raw materials and work-in-progress inventory had variable shelf lives. We expect that the majority of this inventory will be consumed over the next three years. The shelf life of our COVID-19 vaccine product ranges from nine to twelve months.
8. Property, Plant and Equipment, Net
Property, plant and equipment, net as of December 31, 20202023 and 2019 consists2022 consisted of the following (in thousands)millions):
| | | | | | | | | | | | | | | | |
| | December 31, |
| | 2020 | | 2019 | | |
| Laboratory equipment | | $ | 120,714 | | | $ | 108,257 | | | |
| Leasehold improvements | | 179,901 | | | 152,426 | | | |
| Furniture, fixtures and other | | 4,662 | | | 3,316 | | | |
| Computer equipment and software | | 13,398 | | | 11,985 | | | |
| Internally developed software | | 7,020 | | | 7,020 | | | |
| Right-of-use asset, financing | | 56,348 | | | 9,853 | | | |
| Construction in progress | | 34,507 | | | 3,222 | | | |
| | 416,550 | | | 296,079 | | | |
| Less: Accumulated depreciation | | (119,661) | | | (94,584) | | | |
| Property and equipment, net | | $ | 296,889 | | | $ | 201,495 | | | |
| | | | | | | | | | | | | | | | |
| | December 31, |
| | 2023 | | 2022 | | |
| | | | | | |
| Land and land improvements | | $ | 22 | | | $ | 11 | | | |
| Manufacturing and laboratory equipment | | 345 | | | 284 | | | |
| Leasehold improvements | | 522 | | | 460 | | | |
| Furniture and fixtures | | 26 | | | 21 | | | |
| Computer equipment and software | | 74 | | | 38 | | | |
| | | | | | |
| Construction in progress | | 860 | | | 281 | | | |
Right-of-use assets, financing (Note 10) | | 529 | | | 1,581 | | | |
| Total | | 2,378 | | | 2,676 | | | |
| Less: Accumulated depreciation | | (433) | | | (658) | | | |
| Property, plant and equipment, net | | $ | 1,945 | | | $ | 2,018 | | | |
Depreciation and amortization expense for the years ended December 31, 2020, 20192023, 2022, and 20182021 was $31.3$617 million, $31.0$348 million, $24.9and $232 million, respectively.
10.9. Other Balance Sheet Components
Accounts Receivable, Net
Accounts receivable, net, as of December 31, 2023 and December 31, 2022 consisted of the following (in millions):
| | | | | | | | | | | | | | |
| | December 31, |
| | 2023 | | 2022 |
| Accounts receivable | | $ | 1,584 | | | $ | 1,385 | |
| Less: Wholesalers chargebacks, discounts and fees | | (692) | | | — | |
| | | | |
| | | | |
| Accounts receivable, net | | $ | 892 | | | $ | 1,385 | |
Prepaid Expenses and Other Current Assets
Prepaid expenses and other current assets, as of December 31, 20202023 and 2019 consists2022 consisted of the following (in thousands)millions): | | | | | | | | | | | | | | |
| | December 31, |
| | 2023 | | 2022 |
| Prepaid services | | $ | 182 | | | $ | 216 | |
| Down payments to manufacturing vendors | | 100 | | | 229 | |
| Down payments for materials and supplies | | 68 | | | 219 | |
| Collaboration receivable | | 61 | | | 11 | |
| Interest receivable | | 59 | | | 61 | |
| | | | |
| Value added tax receivable | | 50 | | | 140 | |
| Income tax receivable | | 19 | | | 10 | |
| Tenant improvement allowance receivable | | — | | | 42 | |
| Prepaid income taxes | | — | | | 187 | |
| Convertible note receivable | | — | | | 36 | |
| | | | |
| | | | |
| Other current assets | | 88 | | | 44 | |
| Prepaid expenses and other current assets | | $ | 627 | | | $ | 1,195 | |
| | | | | | | | | | | | | | |
| | December 31, |
| | 2020 | | 2019 |
| Down payments to manufacturing vendors | | $ | 216,538 | | | $ | 0 | |
| Prepaid expenses | | 15,647 | | | 8,475 | |
| Tenant incentives receivables | | 10,242 | | | 4,093 | |
| Interest receivable on marketable securities | | 9,725 | | | 6,835 | |
| Prepaid expenses and other current assets | | $ | 252,152 | | | $ | 19,403 | |
Other Non-Current Assets
Other non-current assets, as of December 31, 2023 and 2022 consisted of the following (in millions): | | | | | | | | | | | | | | |
| | December 31, |
| | 2023 | | 2022 |
| Down payments and prepayments, non-current | | $ | 342 | | | $ | — | |
Inventory, non-current(1) | | 170 | | | 910 | |
| Equity investments | | 66 | | | 42 | |
| Goodwill | | 52 | | | — | |
| Finite-lived intangible asset | | 44 | | | — | |
| Restricted cash | | 4 | | | 12 | |
| Other | | 7 | | | 24 | |
| Other non-current assets | | $ | 685 | | | $ | 988 | |
_______ (1)Consisted of raw materials with an anticipated consumption beyond one year.
Accrued Liabilities
Accrued liabilities, as of December 31, 20202023 and 2019 consists2022 consisted of the following (in thousands)millions): | | | | | | | | | | | | | | |
| | December 31, |
| | 2023 | | 2022 |
Provisions related to product sales (Note 3) | | $ | 556 | | | $ | — | |
| Compensation-related | | 245 | | | 190 | |
| Clinical trials | | 175 | | | 319 | |
| Manufacturing | | 167 | | | 400 | |
| Development operations | | 140 | | | 88 | |
| Other external goods and services | | 137 | | | 264 | |
| Royalties | | 122 | | | 203 | |
| Property, plant and equipment | | 94 | | | 5 | |
Loss on future firm purchase commitments(1) | | 79 | | | 268 | |
| Commercial | | 56 | | | 48 | |
| Raw materials | | 27 | | | 316 | |
| | | | |
| Accrued liabilities | | $ | 1,798 | | | $ | 2,101 | |
| | | | |
| | | | |
______
| | | | | | | | | | | | | | |
| | December 31, |
| | 2020 | | 2019 |
| Clinical trials | | $ | 97,249 | | | $ | 6,291 | |
| Compensation-related | | 95,247 | | | 27,428 | |
| Raw materials | | 78,085 | | | 0 | |
| Manufacturing | | 53,412 | | | 5,872 | |
| Development operations | | 29,253 | | | 2,567 | |
| Property and equipment | | 17,576 | | | 4,029 | |
| Commercial | | 6,922 | | | 0 | |
| Other external goods and services | | 91,847 | | | 21,465 | |
| Accrued liabilities | | $ | 469,591 | | | $ | 67,652 | |
Other Current Liabilities
Other current liabilities, as of December 31, 20202023 and 2019 consists2022 consisted of the following (in thousands)millions):
| | | | | | | | | | | | | | |
| | December 31, |
| | 2023 | | 2022 |
Lease liabilities - financing (Note 10) | | $ | — | | | $ | 161 | |
Lease liabilities - operating (Note 10) | | 25 | | | 35 | |
| Other | | 41 | | | 53 | |
| Other current liabilities | | $ | 66 | | | $ | 249 | |
| | | | |
| | | | | | | | | | | | | | |
| | December 31, |
| | 2020 | | 2019 |
| Lease liabilities - financing | | $ | 24,328 | | | $ | 0 | |
| Lease liabilities - operating | | 5,972 | | | 3,583 | |
| Other | | 3,365 | | | 1,480 | |
| Other current liabilities | | $ | 33,665 | | | $ | 5,063 | |
The following table summarizes the activities in deferred revenue during the year ended December 31, 20202023 (in thousands)millions):
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | December 31, 2019 | | Additions | | Deductions | | December 31, 2020 |
| Product sales | | $ | 0 | | | $ | 3,881,303 | | | $ | (82,134) | | | $ | 3,799,169 | |
| Grant revenue | | 2,777 | | | 6,775 | | | (4,291) | | | 5,261 | |
| Collaboration revenue | | 199,528 | | | 121,882 | | | (81,228) | | | 240,182 | |
| Total deferred liabilities | | $ | 202,305 | | | $ | 4,009,960 | | | $ | (167,653) | | | $ | 4,044,612 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | December 31, 2022 | | Additions | | Deductions | | December 31, 2023 |
| Net product sales | | $ | 2,626 | | | $ | 515 | | | $ | (2,528) | | | $ | 613 | |
| Grant revenue | | 4 | | | 2 | | | (2) | | | 4 | |
| Collaboration revenue | | 81 | | | 31 | | | (78) | | | 34 | |
| Total deferred revenue | | $ | 2,711 | | | $ | 548 | | | $ | (2,608) | | | $ | 651 | |
11.10. Leases
We have entered into various long-term non-cancelable lease arrangements for our facilities and equipment expiring at various times through 2032.2042. Certain of these arrangements have free rent periods or escalating rent payment provisions, which weprovisions. We recognize lease cost under such arrangements on a straight-line basis over the life of the leases. We have 2two main campuses in Massachusetts, our Cambridge facilitycampus and our Moderna Technology Center (MTC), located in Norwood.
Table We also lease various parcels of Contents
Operating Leasesland, office and lab spaces across the globe for our business operations.
Cambridge facilityCampus
We occupy a multi-floor buildingmulti-building campus at Technology Square in Cambridge, Massachusetts with a mix of offices and research laboratory space totaling approximately 175,000292,000 square feet. Our Cambridge facility leases have expiry ranges from 2020 to 2029.
In August 2019, we entered into an amendment to our lease agreements to consolidate our Technology Square space in Cambridge, Massachusetts. This included entering into a forward-starting lease agreement starting in January 2020 to acquire approximately 50,000 square feet of additional space at 200 Technology Square. In addition, our current 200 Technology Square lease has been extended for two years to 2029. As part of the lease amendment, we completely exited our leased space of approximately 60,000 square feet at 500 Technology Square by May 2020.
We record operating lease cost for each of our operating leases on a straight-line basis from lease commencement date through the end of the lease term. Operating lease cost is recorded in operating expenses in our consolidated statements of operations
Finance Leases
Moderna Technology Center North (MTC North)
In February 2019, we entered into a new lease agreement for office and laboratory space of approximately 200,000 square feet, MTC North, located in Norwood, Massachusetts. The lease commenced in the second quarter of 2019 and had an initial expiration date of 2031. We have the option to extend the lease for up to 4 additional five-year terms. Contemporaneously, we entered into an agreement to sublease approximately 64 percent of the leased space to a third party. In May 2020, we entered into an amendment to the lease whereby we exercised an option available in the original lease to receive a tenant improvement allowance in the amount of $22.2 million to be paid back over the term of the lease with interest and extend the term of the lease to 2035. In May 2020, we also amended our MTC North sublease agreement. As the result of that amendment, effective June 1, 2020, we obtained an additional, approximately 28,000 square feet, or 12 percent of the leased space in MTC North and the remainder of the space in July 2020 when the sublease expired. The lease modifications to MTC North in the second quarter of 2020 resulted in a change in lease classification, from operating to finance.
Moderna Technology Center manufacturing facility (MTC South)
In August 2016,September 2021, we entered into a lease agreement for a building space, approximately 200,000462,000 square feet, in Cambridge, Massachusetts. This space is designated to be the Moderna Science Center (MSC), housing a blend of office laboratory,space and light manufacturing space,laboratories. Following an approximately two-year building project, the lease term is 15 years, with options for two additional seven-year extensions. During the third quarter of 2023, we commenced the lease and recognized the related right-of-use asset and lease liability on our consolidated balance sheets. Following the commencement of the MSC lease, we amended the expiration dates of our existing leases at Technology Square in the fourth quarter of 2023. Originally scheduled to expire ranging from 2024 to 2029, these leases have been adjusted to conclude in early 2025. All our Cambridge leases are classified as operating leases.
Moderna Technology Center
We have an industrial technology center in Norwood, Massachusetts, our Moderna Technology Center (MTC), comprised of three buildings, MTC South, in Norwood, Massachusetts. The lease willMTC North, and MTC East, totaling approximately 686,000 square feet. Our MTC leases expire in September 2032. We2042 and we have the option to extend the term for 2three extension periods of tenfive years each at market-based rents. The base rent is subject to increases over the termeach. All of the lease.our MTC leases are classified as finance leases.
Embedded Leases
We have entered into multiple contract manufacturing service agreements with third parties which contain embedded leases within the scope of ASC 842. AsIn the third quarter of December 31, 2020,2023, as part of our strategic initiative to optimize our manufacturing footprint, we hadamended a contract manufacturing service agreement, resulting in decreases of $262 million in each of the right-of-use assets and lease liabilitiesliabilities. Additionally, it resulted in accelerated depreciation of $24.3 million related to the embedded leases. Certain embedded leases dedicated to our COVID-19 vaccine program prior to the EUA from the FDA were deemed to have no alternative use. The related right-of-use assets of $62.3$323 million were charged to research and development expense for the year ended December 31, 2020.
Operating and financing lease right-of-use assets and lease liabilities as of December 31, 20202023 and 20192022 were as follows (in thousands)millions):
| | | | | | | | | | | | | | |
| | December 31, |
| | 2020 | | 2019 |
| Assets: | | | | |
Right-of-use assets, operating, net (1) (2) | | $ | 90,201 | | | $ | 86,414 | |
Right-of-use assets, financing, net (3) (4) | | 55,045 | | | 9,544 | |
| Total | | $ | 145,246 | | | $ | 95,958 | |
| | | | |
| Liabilities: | | | | |
| Current: | | | | |
Operating lease liabilities(5) | | $ | 5,972 | | | $ | 3,583 | |
Financing lease liabilities(5) | | 24,328 | | | 0 | |
| Total current lease liabilities | | 30,300 | | | 3,583 | |
| Non-current: | | | | |
| Operating lease liabilities, non-current | | 97,421 | | | 93,675 | |
| Financing lease liabilities, non-current | | 109,874 | | | 38,689 | |
| Total non-current lease liabilities | | 207,295 | | | 132,364 | |
| Total | | $ | 237,595 | | | $ | 135,947 | |
| | | | | | | | | | | | | | |
| | December 31, |
| | 2023 | | 2022 |
| Assets: | | | | |
Right-of-use assets, operating, net(1) (2) | | $ | 713 | | | $ | 121 | |
Right-of-use assets, financing, net(3) (4) | | 436 | | | 1,150 | |
| Total | | $ | 1,149 | | | $ | 1,271 | |
| | | | |
| Liabilities: | | | | |
| Current: | | | | |
Operating lease liabilities(5) | | $ | 25 | | | $ | 35 | |
Financing lease liabilities(5) | | — | | | 161 | |
| Total current lease liabilities | | 25 | | | 196 | |
| Non-current: | | | | |
| Operating lease liabilities, non-current | | 643 | | | 92 | |
| Financing lease liabilities, non-current | | 575 | | | 912 | |
| Total non-current lease liabilities | | 1,218 | | | 1,004 | |
| Total | | $ | 1,243 | | | $ | 1,200 | |
_______
(1) These assets are real estate related assets, which include land, office and laboratory spaces.
(2) Net of accumulated depreciation.amortization.
(3) These assets are real estate assets related to the MTC North and MTC South leases.leases as well as assets related to contract manufacturing service agreements.
(4) Included in property, plant and equipment in the consolidated balance sheets, net of accumulated depreciation.
(5) Included in other current liabilities in the consolidated balance sheets.
The components of the lease costs for the year ended December 31, 2020 and 2019 were as follows (in thousands):
| | | | | | | | | | | | | | |
| | December 31, |
| | 2020 | | 2019 |
| Operating lease costs | | $ | 16,939 | | | $ | 17,015 | |
| Financing lease costs: | | | | |
| Amortization of right-of-use assets, financing leases | | 1,011 | | | 309 | |
| Interest expense for financing lease liabilities | | 9,891 | | | 6,557 | |
| Total financing lease costs | | $ | 10,902 | | | $ | 6,866 | |
| Short term lease costs | | $ | 13,322 | | | $ | 0 | |
| Variable lease costs | | $ | 5,229 | | | $ | 4,399 | |
Total rent expense for the year ended December 31, 2018 was $19.1 million. periods presented (in millions):
| | | | | | | | | | | | | | | | | | | | |
| | Years ended December 31, |
| | 2023 | | 2022 | | 2021 |
| Operating lease costs | | $ | 88 | | | $ | 48 | | | $ | 24 | |
| Financing lease costs: | | | | | | |
| Amortization of right-of-use assets, financing leases | | 500 | | | 280 | | | 189 | |
| Interest expense for financing lease liabilities | | 38 | | | 29 | | | 17 | |
| Total financing lease costs | | $ | 538 | | | $ | 309 | | | $ | 206 | |
| Short term lease costs | | $ | 2 | | | $ | — | | | $ | 49 | |
| Variable lease costs | | $ | 113 | | | $ | 165 | | | $ | 100 | |
Supplemental cash flow information relating to our leases for the year ended December 31, 2020 and 2019 was as follows for the periods presented (in thousands)millions):
| | | | | | | | | | | | | | | | | | | | |
| | December 31, |
| | 2023 | | 2022 | | 2021 |
| Cash paid for amounts included in measurement of lease liabilities: | | | | | | |
| Operating cash flows used in operating leases | | $ | (93) | | | $ | (57) | | | $ | (19) | |
| Operating cash flows used in financing leases | | (39) | | | (25) | | | (14) | |
| Financing cash flows used in financing leases | | (292) | | | (184) | | | (140) | |
| | | | | | |
| Operating lease non-cash items: | | | | | | |
| Changes in right-of-use assets related to lease modifications and reassessments | | $ | 67 | | | $ | — | | | $ | (7) | |
| Right-of-use assets obtained in exchange for operating lease liabilities | | 714 | | | 20 | | | 72 | |
| | | | | | |
| Finance lease non-cash items: | | | | | | |
| Changes in right-of-use assets related to lease modifications and reassessments | | $ | 213 | | | $ | — | | | $ | 674 | |
| Right-of-use assets obtained in exchange for financing lease liabilities | | — | | | 777 | | | 126 | |
| Changes in financing lease liabilities | | 3 | | | 4 | | | 3 | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | | | | | | | | | |
| | December 31, |
| | 2020 | | 2019 |
| Cash paid for amounts included in measurement of lease liabilities: | | | | |
| Operating cash flows used in operating leases | | $ | (15,089) | | | $ | (16,121) | |
| Operating cash flows used in financing leases | | (8,523) | | | (5,585) | |
| Financing cash flows used in financing leases | | (7,519) | | | 0 | |
| | | | |
| Operating lease non-cash items: | | | | |
| Right-of-use assets reduced through lease modifications and reassessments | | 6,755 | | | 2,717 | |
| Right-of-use assets obtained in exchange for operating lease liabilities | | 17,107 | | | 34,014 | |
| | | | |
| Finance lease non-cash items: | | | | |
| Right-of-use assets obtained through lease modifications and reassessments | | 46,495 | | | 0 | |
| Charge to financing lease obligation | | 1,304 | | | 971 | |
| | | | |
| | | | |
| | | | |
| | | | |
Weighted average remaining lease terms and discount rates as of December 31, 20202023 and 2022 were as follows:
| | | | | | | | |
| | December 31, |
| | 2020 |
Remaining lease term: | | |
Operating leases | | 11 years |
Finance leases | | 25 years |
Discount rate: | | |
Operating leases | | 10.3 | % |
Finance leases | | 13.1 | % |
| | | | | | | | | | | | | | |
| | December 31, |
| | 2023 | | 2022 |
| Remaining lease term: | | | | |
| Operating leases | | 14 years | | 6 years |
| Finance leases | | 33 years | | 22 years |
| Discount rate: | | | | |
| Operating leases | | 7.5 | % | | 7.5 | % |
| Finance leases | | 4.2 | % | | 3.6 | % |
Future minimum lease payments under non-cancelable lease agreements as of December 31, 2020,2023, were as follows (in thousands)millions):
| | | | | | | | | | | | | | | | | |
| Fiscal Year | | Operating Leases (1) | | Financing Leases (1) | |
| 2021 | | $ | 15,492 | | | $ | 36,579 | | |
| 2022 | | 15,913 | | | 11,848 | | |
| 2023 | | 16,008 | | | 12,054 | | |
| 2024 | | 16,168 | | | 12,279 | | |
| 2025 | | 16,567 | | | 12,493 | | |
| Thereafter | | 93,599 | | | 428,059 | | |
| Total minimum lease payments | | 173,747 | | | 513,312 | | |
| Less amounts representing interest or imputed interest | | (70,354) | | | (379,110) | | (2) |
| Present value of lease liabilities | | $ | 103,393 | | | $ | 134,202 | | |
| | | | | | | | | | | | | | | |
| Fiscal Year | | Operating Leases | | Financing Leases(1) | |
| 2024 | | $ | 72 | | | $ | 20 | | |
| 2025 | | 67 | | | 22 | | |
| 2026 | | 67 | | | 22 | | |
| 2027 | | 70 | | | 23 | | |
| 2028 | | 72 | | | 23 | | |
| Thereafter | | 785 | | | 1,074 | | |
| Total minimum lease payments | | 1,133 | | | 1,184 | | |
| Less amounts representing interest | | (465) | | | (609) | | |
| Present value of lease liabilities | | $ | 668 | | | $ | 575 | | |
______
(1) Include thecertain optional lease term extensions, inpredominantly related to the MTC North and MTC South lease termsleases, which represent a total of $338.9$668 million un-discountedof undiscounted future lease payments.
(2) MTC South interest is based on an imputed interest rate of 17.2%. MTC North interest is based upon an incremental borrowing rate of 8.2%.
12.11. Commitments and Contingencies
Strategic Collaborations
Under our strategic collaboration agreements, we are committed to perform certain research, development, and manufacturing activities. As part of our PCV Agreement and PCV/SAV Agreement with Merck, we are committed to perform certain research, development and manufacturing activities related to PCV products through an initial Phase 2 clinical trial up to a budgeted amount of $243.0 million as of December 31, 2020 and 2019 (Note 5).
Legal Proceedings
We are involved in various claims and legal proceedings of a nature considered ordinary course in our business. The outcome of any such proceedings, regardless of the merits, is inherently uncertain; therefore, assessing the likelihood of loss and any estimated damages is difficult and subject to considerable judgment. We are not currently a party to any legal proceedings for which a material legal proceedings.loss is probable, or for which a loss is reasonably estimable at this time.
Indemnification Obligations
As permitted under Delaware law, we indemnify our officers, directors, and employees for certain events, occurrences while the officer, or director is, or was, serving at our request in such capacity. The term of the indemnification is for the officer’s or director’s lifetime.
We have standard indemnification arrangements in our leases for laboratory and office space that require us to indemnify the landlord against any liability for injury, loss, accident, or damage from any claims, actions, proceedings, or costs resulting from certain acts, breaches, violations, or non-performance under our leases.
We enter into indemnification provisions under our agreements with counterparties in the ordinary course of business, typically with business partners, contractors, clinical sites and customers. Under these provisions, we generally indemnify and hold harmless the indemnified party for losses suffered or incurred by the indemnified party as a result of our activities. These indemnification provisions generally survive termination of the underlying agreement. The maximum potential amount of future payments we could be required to make under these indemnification provisions is unlimited.
Through December 31, 20202023 and 2019,2022, we had 0tnot experienced any significant losses related to these indemnification obligations, and 0no material claims were outstanding. We do not expect significant claims related to these indemnification obligations and, consequently, concluded that the fair value of these obligations is negligible, and 0no related reserves were established.
Purchase Commitments and Purchase Orders
In May 2020, we entered into a 10-year strategic collaboration agreement with Lonza Ltd. to enable larger scale manufacture for our COVID-19 vaccine and additional Moderna products in the future. This agreement was reflected in the Global Long Term Agreement entered into between Moderna and Lonza on September 4, 2020. Under the terms of the agreement, we established dedicated manufacturing suites at Lonza’s facilities in the United States and Switzerland for the manufacture of mRNA-1273 at both sites. Certain arrangements under this strategic collaboration agreement are within the scope of lease accounting (see Note 11).
We enter into agreements in the normal course of business with vendors and contract manufacturing organizations (CMOs) for raw materials and manufacturing services and with vendors for preclinical research studies, clinical trials and other goods or services. As of December 31, 2020,2023, we had $659.5 million$1.8 billion of non-cancelable purchase commitments related to raw materials and manufacturing agreements, including the Lonza agreement, which are expected to be paid through 2021.2029. As of December 31, 2020,2023, $79 million of the purchase commitments related to raw materials was recorded as an accrued liability for loss on future firm purchase commitments. As of December 31, 2023, we had $27.0$261 million of non-cancelable purchase commitments forrelated to clinical services and other goods and services which are expected to be paid through 2024.2039. These amounts represent our minimum contractual obligations, including termination fees.
In addition to purchase commitments, we have agreements with third parties for various services, including services related to clinical operations and support and contract manufacturing, for which we are not contractually able to terminate for convenience and avoid any and all future obligations to the vendors. Certain agreements provide for termination rights subject to termination fees or wind down costs. Under such agreements, we are contractually obligated to make certain payments to vendors, mainly, to reimburse them for their unrecoverable outlays incurred prior to cancellation. At December 31, 2020,2023, we had cancelable open purchase orders of $896.9 million$3.0 billion in total under such agreements for our significant clinical operations and support and contract manufacturing. These amounts represent only our estimate of those items for which we had a contractual commitment to pay at December 31, 2020,2023, assuming we would not cancel these agreements. The actual amounts we pay in the future to the vendors under such agreements may differ from the purchase order amounts.
Licenses to Patented Technology
On June 26,In 2017, we entered into sublicense agreements with Cellscript, LLC and its affiliate, mRNA RiboTherapeutics, Inc. to sublicense certain patent rights. Pursuant to each agreement, we are required to pay certain license fees, annual maintenance fees, minimum royalties on future net sales and milestone payments contingent on achievement of certain development, regulatory and commercial milestones for specified products, on a product-by-product basis. The development and regulatoryCommercial milestone payments up to $1.5 million for therapeutic and prophylactic products and up to $0.5 million for diagnostic products will be recognized as a cost of the asset acquired upon resolution of the associated contingency and will be capitalized or expensed depending on the nature of the associated asset as of the date of recognition. Conversely, commercial milestone payments, up to $24.0 million, and royalties based on annual net sales of licensed products for therapeutic and prophylactic products will beare accounted for as additional expense of the related net product sales in the period in which the corresponding sales occur.
In connectionDecember 2022, we entered into a non-exclusive patent license agreement with these sublicense agreements,the National Institute of Allergy and Infectious Diseases (NIAID), an Institute or Center of the National Institutes of Health (NIH) to license certain patent rights concerning
stabilizing prefusion coronavirus spike proteins and the resulting stabilized proteins for use in COVID-19 vaccine products. Pursuant to the agreement, we have agreed to pay low single-digit royalties on future net sales, a minimum annual royalty payment, and certain contingent development, regulatory and commercial milestone payments on a licensed product-by-licensed product basis. In addition, in December 2022, we made a catch-up royalty payment of $400 million to NIAID, which was recorded to cost of sales in our consolidated statements of operations.
In 2023, 2022, and 2021 we recognized sublicense grant fees$301 million, $1.1 billion, and $641 million, respectively, of $22.0 million in 2018royalties and have no further obligation for additional sublicense grant fees. We recognized $6.9 million of royaltiescommercial milestone payments associated with our net product sales, which was recorded to cost of sales in 2020.our consolidated statements of operations.
Additionally, we have other in-license agreements with third parties which require us to make future development, regulatory and commercial milestone payments for specified products associated with the agreements. The achievement of these milestones washave not deemed probableyet occurred as of December 31, 2020.2023.
13. Redeemable Convertible Preferred Stock12. Stock-Based Compensation and Common Stock
On February 28, 2018 and May 7, 2018, the Board of Directors approved an amendment to our Certificate of Incorporation resulting in a total of 775,000,000 shares of common stock and a total of 509,352,795 shares of redeemable convertible preferred stock being authorized, respectively. Upon completion of our IPO, our authorized capital stock consists of 1,600,000,000 shares of common stock, par value $0.0001 per share, and 162,000,000 shares of preferred stock, par value $0.0001 per share, all of which shares of preferred stock are undesignated.
On December 11, 2018, we completed our IPO, whereby we sold 26,275,993 shares of common stock at a price of $23.00 per share. The aggregate net proceeds received by us from the IPO were $563.0 million, net of underwriting discounts and commissions of $33.2 million and offering expenses of $8.1 million payable by us. Upon the closing of the IPO, all of the outstanding shares of our redeemable convertible preferred stock were converted into 236,012,913 shares of the common stock. As of December 31, 2020 and 2019, we did 0t have any convertible preferred stock issued or outstanding.
On February 14, 2020, we sold 26,315,790 shares of common stock at a price of $19.00 per share through a public equity offering. The aggregate net proceeds from the offering were $477.7 million, net of underwriting discounts, commissions and offering expenses. In addition, the underwriters exercised their options to purchase an additional 3,947,368 shares of common stock at the public offering price less underwriting discounts, resulting in additional net proceeds of $71.8 million.
On May 21, 2020, we sold 17,600,000 shares of common stock at a price of $76.00 per share through a public equity offering. The aggregate net proceeds from the offering were $1.30 billion, net of underwriting discounts, commissions and offering expenses.
14. Stock-Based CompensationShare Repurchase Programs
Equity Plans
In October 2013, we adopted the 2013 Equity Incentive Plan (the 2013 Incentive Plan) and the 2013 Unit Option and Grant Plan (the 2013 Option Plan), which provided for the grant of incentive units, non-qualified unit options, and restricted and unrestricted unit awards to our employees, officers, directors, advisors, and outside consultants. Historically, we also granted restricted stock to founders, officers, directors, and advisors outside any of the Plans.
In August 2016, we adopted the 2016 Stock Option and Grant Plan (the 2016 Equity Plan), which replaced the 2013 Option Plan and the 2013 Incentive Plan. The 2016 Equity Plan and provided for the grant of incentive stock options, non-qualified stock options, restricted stock, unrestricted stock, and restricted stock units to our employees, officers, directors, consultants, and other key persons.
In connection with the IPO,our initial public offering (IPO), we adopted the 2018 Stock Option and Incentive Plan (the 2018 Equity Plan) in November 2018. The 2018 Equity Plan became effective on the date immediately prior to the effective date of the IPO and replaced our 2016 Plan.Stock Option and Incentive Plan (the 2016 Equity Plan). The 2018 Equity Plan provides flexibility to our compensation committee to use various equity-based incentive awards as compensation tools to motivate our workforce. We have initially reserved 13,000,000 shares of our common stock for the issuance of awards under the 2018 Equity Plan. The 2018 Equity Plan provides that the number of shares reserved and available for issuance under the plan will
automatically increase each January 1, beginning on January 1, 2019, by 4% of the outstanding number of shares of our common stock on the immediately preceding December 31, or such lesser number of shares as determined by the Compensation and Talent Committee of our Board of Directors. The shares of common stock underlying any awards that are forfeited, canceled, held back upon exercise or settlement of an award to satisfy the exercise price or tax withholding, reacquired by us prior to vesting, satisfied without any issuance of stock, expire or are otherwise terminated (other than by exercise) under the 2018 Equity Plan and the 2016 Equity Plan will be added back to the shares of common stock available for issuance under the 2018 Equity Plan.
The Board of Directors may grant to employees, nonemployee directors, consultants and independent advisors equity-based awards during their period of service, generally in the form of stock options, restricted stock units, and performance stock units. The terms and conditions of stock-based awards are defined at the sole discretion of our Board of Directors. We issue service-based awards, vesting over a defined period of service, and performance-based awards, vesting upon achievement of defined conditions. Service based awards generally vest over a four-year period, with the first 25% of such awards vesting following twelve months of continued employment or service. The remaining awards vestsvest in 12twelve quarterly installments over the following twelve quarters. Stock options granted under the 2018 Equity Plan and the 2016 Equity Plan expire ten years from the date of grant and the exercise price must be at least equal to the fair market value of common stock on the grant date.
As of December 31, 2020,2023, we had a total of 64.247 million shares reserved for future issuance under our Equity Plans, of which 36.331 million shares were reserved for equity awards previously granted, and 27.916 million shares were available for future grants under the 2018 Equity Plan. No additional awards will be granted under the 2016 Equity Plan as it was replaced by the 2018 Equity Plan.
Options
We have granted options generally through the 2018 Equity Plan and 2016 Equity Plan. The following table summarizes our option activity as ofduring the year ended December 31, 2020 and 2019:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Number of Options | | Weighted Average Exercise Price per Share | | Weighted Average Grant Date Fair Value per Share | | Weighted- Average Remaining Contractual Term | | Aggregate Intrinsic Value (1) (in thousands) |
| Outstanding at December 31, 2019 | | 45,536,915 | | | $ | 13.82 | | | $ | 7.35 | | | 7.2 years | | $ | 286,310 | |
| Granted | | 5,036,520 | | | 36.04 | | | 19.30 | | | | | |
| Exercised | | (13,888,434) | | | 12.93 | | | 6.68 | | | | | |
| Canceled/forfeited | | (2,627,468) | | | 18.14 | | | 10.56 | | | | | |
| Outstanding at December 31, 2020 | | 34,057,533 | | | 17.14 | | | 9.12 | | | 6.7 years | | 2,976,235 | |
| Exercisable at December 31, 2020 | | 17,461,374 | | | 10.99 | | | 5.67 | | | 5.4 years | | 1,632,365 | |
| Expected to vest at December 31, 2020 | | 16,596,159 | | | 23.61 | | | 12.75 | | | 8.2 years | | 1,343,870 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Number of
Options
(in millions) | | Weighted
Average
Exercise
Price per
Share | | | | Weighted-
Average
Remaining
Contractual
Term | | Aggregate Intrinsic Value(1) (in millions) |
| Outstanding at December 31, 2022 | | 24.93 | | | $ | 42.23 | | | | | 5.7 years | | $ | 3,478 | |
| Granted | | 4.36 | | | 113.03 | | | | | | | |
| Exercised | | (3.11) | | | 8.13 | | | | | | | |
| Canceled/forfeited | | (0.68) | | | 130.23 | | | | | | | |
| Outstanding at December 31, 2023 | | 25.50 | | | 56.14 | | | | | 5.6 years | | 1,437 | |
| Exercisable at December 31, 2023 | | 18.98 | | | 33.34 | | | | | 4.5 years | | 1,378 | |
| Expected to vest at December 31, 2023 | | 6.52 | | | 122.50 | | | | | 8.9 years | | 59 | |
_______
(1)Aggregate intrinsic value is calculated as the difference between the exercise price of the underlying options and the fair value of common stock for those options in the money as of December 31, 2020.2023.
The total intrinsic value of options exercised was $785.7$413 million, $75.6$714 million, and $5.3 million$1.6 billion for the years ended December 31, 2020, 20192023, 2022, and 2018,2021, respectively. The aggregate intrinsic value represents the difference between the exercise price and the selling price received by option holders upon the exercise of stock options during the period. The excess tax benefits realized from tax deductions from option exercises were $84 million, $144 million, and $325 million during the years ended December 31, 2023, 2022, and 2021 respectively. The total consideration recorded as a result of stock option exercises was approximately $179.6$25 million, $50 million, and $112 million for the yearyears ended December 31, 2020.
Table of Contents
2023, 2022, and 2021.
Restricted Common Stock Units (RSUs) and Performance Stock Units (PSUs)
We have granted restricted stock unit awardsRSUs and PSUs generally through the 2018 Equity Plan. The following table summarizes our restricted stock unitRSU and PSU activity during the year ended December 31, 2020:
| | | | | | | | | | | |
| Number of Units | | Weighted Average Grant Date Price per Unit |
| Outstanding, non-vested at December 31, 2019 | 1,177,249 | | | $ | 19.01 | |
| Issued | 1,478,093 | | | 37.37 | |
| Vested | (247,349) | | | 20.28 | |
| Canceled/forfeited | (215,033) | | | 23.64 | |
| Outstanding, non-vested at December 31, 2020 | 2,192,960 | | | 30.85 | |
| | | | | | | | | | | | |
| Number of Units
(in millions) | | Weighted Average Grant Date Fair Value per Unit | |
| Outstanding, non-vested at December 31, 2022 | 2.90 | | | $ | 132.25 | | |
| Issued | 3.71 | | | 107.23 | | |
| Vested | (1.01) | | | 98.68 | | |
| Canceled/forfeited | (0.42) | | | 130.01 | | |
| Outstanding, non-vested at December 31, 2023 | 5.18 | | | 121.02 | | |
The total grant date fair value of restricted stock unitsRSUs and PSUs vested during the years ended December 31, 2020, 20192023, 2022, and 2018, were $5.02021, was $99 million, $5.5$55 million, and 0,$18 million, respectively. The total intrinsic value of RSUs and PSUs vested during the years ended December 31, 2023, 2022, and 2021, was $120 million, $125 million and $141 million, respectively.
2018 Employee Stock Purchase Plan
In November 2018,During 2023, 2022 and 2021, we adoptedgranted an immaterial amount of PSUs, respectively, primarily to certain senior executives with vesting that is contingent upon the 2018 Employee Stock Purchase Plan (the ESPP), which became effective on December 5, 2018.achievement of specified preestablished goals over the performance period, generally three years. The ESPP initially reserves and authorizes the issuance of up to a total of 810,000 sharesactual number of common stockshares ultimately issued is calculated by multiplying the number of PSUs by a payout percentage ranging from 0% to participating employees. We will make 1 or more offerings, consisting of 1 or more purchase periods, each year to our employees to purchase shares under the ESPP. Offerings will usually begin every six months and will continue for six-month periods, referred to as offering periods.200%. The purchase price at which shares are sold under the ESPP will be equal to 85% of the lower of theestimated fair market value of the sharesPSUs is based on the first business day of the offering period or the last business day of the purchase period. Employees are generally eligible to participate through payroll deductions of between 1% to 50% of their compensation and may not purchase more than 3,000 shares of common stock during each purchase period or $25,000 worth of shares of common stock in any calendar year. We began our first ESPP offering on June 1, 2019. There were 251,752 shares of common stock sold at a weighted average price of $27.97 per share under the ESPP during the year ended December 31, 2020. As of December 31, 2020, 3.6 million shares were available for future issuance under the ESPP.grant date fair value.
Valuation and Stock-Based Compensation Expense
Stock-based compensation for options granted under our Equity Plans and share purchases under our ESPP is determined using the Black-Scholes option pricing model. The weighted-average assumptions used to estimate the fair value of options granted and ESPP for the years ended December 31, 2020, 20192023, 2022, and 2018 are2021 were as follows:
| | | | | | | | | | | | | | | | | | | | |
| | Weighted Average |
| | Years Ended December 31, |
| | 2020 | | 2019 | | 2018 |
| Options: | | | | | | |
| Risk-free interest rate | | 0.83 | % | | 2.29 | % | | 2.76 | % |
| Expected term | | 6.11 years | | 6.07 years | | 6.27 years |
| Expected volatility | | 58 | % | | 61 | % | | 63 | % |
| Expected dividends | | 0 | % | | 0 | % | | 0 | % |
| Weighted average fair value per share | | $ | 19.30 | | | $ | 11.35 | | | $ | 9.33 | |
| | | | | | |
| ESPP: | | | | | | |
| Risk-free interest rate | | 0.14 | % | | 1.95 | % | | * |
| Expected term | | 0.50 years | | 0.50 years | | * |
| Expected volatility | | 54 | % | | 53 | % | | * |
| Expected dividends | | 0 | % | | 0 | % | | * |
| Weighted average fair value per share | | $ | 32.18 | | | $ | 5.98 | | | * |
_________
* - Not applicable | | | | | | | | | | | | | | | | | | | | |
| | Weighted Average |
| | Years Ended December 31, |
| | 2023 | | 2022 | | 2021 |
| Options: | | | | | | |
| Risk-free interest rate | | 4.13 | % | | 2.46 | % | | 0.84 | % |
| Expected term | | 6.07 years | | 6.13 years | | 6.10 years |
| Expected volatility | | 48 | % | | 50 | % | | 46 | % |
| Expected dividends | | — | % | | — | % | | — | % |
| Weighted average fair value per share | | $ | 57.87 | | | $ | 76.02 | | | $ | 91.84 | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
Stock-Based Compensation Expense
The following table presents the components and classification of stock-based compensation expense for the years ended December 31, 2020, 20192023, 2022, and 2018 as follows2021 (in thousands)millions):
| | | | | | | | | | | | | | | | | |
| Years Ended December 31, |
| 2020 | | 2019 | | 2018 |
| Options | $ | 77,748 | | | $ | 74,780 | | | $ | 63,288 | |
| Restricted common stock and units | 12,401 | | | 5,125 | | | 9,277 | |
| Employee stock purchase plan | 2,872 | | | 1,217 | | | 0 | |
| Total | $ | 93,021 | | | $ | 81,122 | | | $ | 72,565 | |
| Research and development | $ | 56,155 | | | $ | 48,259 | | | $ | 37,659 | |
| Selling, general and administrative | 36,866 | | | 32,863 | | | 34,906 | |
| Total | $ | 93,021 | | | $ | 81,122 | | | $ | 72,565 | |
| | | | | | | | | | | | | | | | | |
| Years Ended December 31, |
| 2023 | | 2022 | | 2021 |
| Options | $ | 136 | | | $ | 123 | | | $ | 96 | |
| RSUs and PSUs | 161 | | | 97 | | | 42 | |
| Employee stock purchase plan | 8 | | | 6 | | | 4 | |
| Total | $ | 305 | | | $ | 226 | | | $ | 142 | |
| | | | | |
| Cost of sales | $ | 37 | | | $ | 45 | | | $ | 22 | |
| Research and development | 157 | | | 93 | | | 68 | |
| Selling, general and administrative | 111 | | | 88 | | | 52 | |
| Total | $ | 305 | | | $ | 226 | | | $ | 142 | |
| | | | | |
For the years ended December 31, 2020, 20192023, 2022, and 2018,2021, we recognized stock-based compensation expense of $9.6$17 million, $9.8$18 million, and $10.6$16 million, respectively, related to performance-based awards, including awards with vesting or commencement contingent upon the IPO, for which achievement of such performance-based condition was deemed probable.our IPO. Stock-based compensation expenses related to non-employee awards were immaterial for the years ended December 31, 2020, 20192023, 2022, and 2018.2021.
As of December 31, 2020,2023, there were $226.8$835 million of total unrecognized compensation cost related to non-vested stock-based compensation with respect to options, RSUs and restricted stockPSUs granted. That cost is expected to be recognized over a weighted-average period of 2.92.6 years at December 31, 2020.2023.
15. Employee Benefit PlanShare Repurchase Programs
We provideOn August 2, 2021, our Board of Directors authorized a retirement savings optionShare Repurchase Program (2021 Repurchase Program) of our common stock, with an expiration date no later than August 2, 2023. Pursuant to the 2021 Repurchase Program, we were authorized to repurchase up to $1.0 billion of our outstanding common stock. By the end of January 2022, we had repurchased the entire $1.0 billion of common stock that was authorized under the 2021 Repurchase Program. On February 22, 2022, our Board of Directors authorized an additional share repurchase program of our common stock, with no expiration date, for up to $3.0 billion. On August 1, 2022, our Board of Directors authorized an increase of $3.0 billion under the repurchase program for our common stock, with no expiration date (collectively with the February 22, 2022 authorization, the 2022 Repurchase Programs).
As of December 31, 2023, $1.7 billion of our Board of Directors’ authorization for repurchases of our common stock remains outstanding (the 2022 Repurchase Programs), with no expiration date. The timing and actual number of shares repurchased under the 2022 Repurchase Programs will depend on a variety of factors, including price, general business and market conditions, and other investment opportunities, and shares may be repurchased through open market purchases through the use of trading plans intended to qualify under Rule 10b5-1 under the Securities Exchange Act of 1934, as amended.
The following table summarizes activity related to our eligible U.S. employees through the Moderna, Inc. 401(k) Plan (the 401(k) Plan), subject to certain limitations. As allowed under Section 401(k) of the Internal Revenue Code, the 401(k) Plan allowsshare repurchase programs (in millions, except per share data):
| | | | | | | | | | | | | | | | | | | | |
| | Years Ended December 31, |
| | 2023 | | 2022 | | 2021 |
| Number of shares repurchased | | 8 | | 23 | | 3 |
Average price per share(1) | | $ | 143.26 | | | $ | 142.83 | | | $ | 245.76 | |
| Aggregate purchase price | | $ | 1,153 | | | $ | 3,329 | | | $ | 857 | |
| Remaining authorization at end of period | | $ | 1,667 | | | $ | 2,814 | | | $ | 143 | |
_______
(1)Average price paid per share includes related expenses and excise tax, deferred salary deductions for eligible employees. We match 50% up to the first 6% contributed by a participant. All matching contributions are immediately vested. Total matching contributions to the 401(k) Plan were $5.0 million, $4.2 million, $2.1 million for the years ended December 31, 2020, 2019 and 2018, respectively.applicable beginning January 1, 2023.
16.13. Income Taxes
Loss(Loss) income before provision for (benefit from) income taxes for the years ended December 31, 2020, 20192023, 2022, and 2018 consist2021 consisted of the following (in thousands)millions):
| | Years Ended December 31, |
| 2020 | | 2019 | | 2018 |
| Years Ended December 31, | | | Years Ended December 31, |
| 2023 | | | 2023 | | 2022 | | 2021 |
| United States | United States | $ | (745,445) | | | $ | (508,595) | | | $ | (380,473) | |
| Foreign | Foreign | 932 | | | (6,121) | | | (3,935) | |
| Loss before provision for (benefit from) income taxes | $ | (744,513) | | | $ | (514,716) | | | $ | (384,408) | |
| (Loss) income before income taxes | |
The provision for (benefit from) income taxes for the years ended December 31, 2020, 20192023, 2022, and 2018 consist2021 consisted of the following components (in thousands)millions):
| | Years Ended December 31, |
| 2020 | | 2019 | | 2018 |
| Years Ended December 31, | | | Years Ended December 31, |
| 2023 | | | 2023 | | 2022 | | 2021 |
| Current: | Current: | | | | | |
| Federal | |
| Federal | |
| Federal | Federal | $ | 0 | | | $ | 0 | | | $ | (26) | |
| State | State | 32 | | | 505 | | | 352 | |
| Foreign | Foreign | 2,519 | | | 0 | | | 0 | |
| Total current | Total current | 2,551 | | | 505 | | | 326 | |
| Deferred: | Deferred: | |
| Federal | Federal | 0 | | | (1,200) | | | 0 | |
| Federal | |
| Federal | |
| State | |
| Foreign | |
| Total deferred | Total deferred | 0 | | | (1,200) | | | 0 | |
| Total provision for (benefit from) income taxes | $ | 2,551 | | | $ | (695) | | | $ | 326 | |
| Total provision for income taxes | |
The reconciliation of the U.S.federal statutory income tax rate to our effective tax rate for the years ended December 31, 2020, 20192023, 2022, and 2018 are2021 was as follows:
| | | | | | | | | | | | | | | | | |
| Years Ended December 31, |
| 2023 | | 2022 | | 2021 |
| Federal statutory tax rate | 21.0 | % | | 21.0 | % | | 21.0 | % |
| Change in valuation allowance | (52.6) | % | | — | % | | (5.4) | % |
| Foreign-derived intangible income | 0.2 | % | | (7.4) | % | | (4.8) | % |
| Stock-based compensation windfall | 2.4 | % | | (1.6) | % | | (2.6) | % |
| Federal research and development credits | 4.6 | % | | (0.5) | % | | (0.7) | % |
| State taxes, net of federal benefits | 5.7 | % | | 0.4 | % | | 0.5 | % |
| Non-deductible items | (0.4) | % | | — | % | | — | % |
| Other | (0.5) | % | | 0.8 | % | | 0.1 | % |
| Effective tax rate | (19.6) | % | | 12.7 | % | | 8.1 | % |
| | | | | | | | | | | | | | | | | |
| Years Ended December 31, |
| 2020 | | 2019 | | 2018 |
| Tax effected at statutory rate | 21.0 | % | | 21.0 | % | | 21.0 | % |
| State taxes, net of federal benefit | 3.6 | % | | 7.9 | % | | 6.3 | % |
| Non-deductible items | (0.8) | % | | 1.6 | % | | 0 | % |
| Change in valuation allowance | (47.4) | % | | (33.0) | % | | (28.5) | % |
| Federal research and development credits | 3.8 | % | | 2.5 | % | | 1.5 | % |
| Foreign tax rate differential | 0.0 | % | | (0.2) | % | | (0.2) | % |
| Stock compensation windfall | 19.8 | % | | 0 | % | | 0 | % |
| Other | (0.3) | % | | 0.2 | % | | (0.2) | % |
| Effective tax rate | (0.3) | % | | 0.0 | % | | (0.1) | % |
Our effective tax rate for the year ended December 31, 2023 was (19.6)% and was higher than the federal statutory tax rate, primarily due to an increase in valuation allowance against deferred tax assets. The effective tax rates for the year ended December 31, 2023 also include tax benefits from research and development credits and stock-based compensation. Our effective tax rate for the year ended December 31, 2022 was lower than the federal statutory tax rate, primarily due to the tax benefit of Contentsthe foreign-derived intangible income deduction (FDII) and excess tax benefit related to stock-based compensation. Our effective tax rate for the year ended December 31, 2021 was lower than the federal statutory tax rate, primarily due to the tax benefits related to the release of the valuation allowance on most of our deferred tax assets, FDII and stock-based compensation.
As of January 1, 2022, pursuant to the Tax Cuts and Jobs Act of 2017 (“TCJA”), research and development costs in the current period are required to be amortized over five or fifteen years, depending on where the research is conducted. The new capitalization requirement significantly increased our deferred tax assets and cash tax liabilities, but also decreased our effective tax rate by increasing the foreign-derived intangible income deduction.
The significantPresident signed into law the Inflation Reduction Act (the “IRA”) on August 16, 2022. The Act includes a new 15% corporate minimum tax and a 1% excise tax on the value of corporate stock repurchases, net of new share issuances, after December 31, 2022. We do not expect these provisions to have a material impact on our consolidated financial position; however, we will continue to evaluate their impact as further information becomes available.
Deferred income taxes reflect the tax effect of temporary differences between the carrying amount of assets and liabilities for financial reporting and the amounts used for income tax purposes, tax credit carryforwards and the tax effect of net operating loss carryforwards. Significant components of our deferred tax assets and tax liabilities as of December 31, 20202023 and 2019 are2022 were as follows (in thousands)millions): | | | | | | | | | | | |
| December 31, |
| 2023 | | 2022 |
| Deferred tax assets: | | | |
| Net operating loss carryforwards | $ | 90 | | | $ | 59 | |
| Stock-based compensation | 111 | | | 68 | |
| Capitalized licenses, research and development and start-up costs | 1,449 | | | 704 | |
| Tax credit carryforwards | 140 | | | 97 | |
| | | |
| Operating lease liabilities | 154 | | | 26 | |
| Financing lease liabilities | 139 | | | 135 | |
| Other comprehensive income | 34 | | | 106 | |
| Inventory reserve and capitalization | 250 | | | 86 | |
| Wholesaler chargebacks, discounts and fees | 92 | | | — | |
| Returns and other fees | 129 | | | — | |
| Other | 90 | | | 85 | |
| Total deferred tax assets | 2,678 | | | 1,366 | |
| Less: valuation allowance | (2,224) | | | (155) | |
| Net deferred tax assets | $ | 454 | | | $ | 1,211 | |
| Deferred tax liabilities: | | | |
| Right-of-use assets, financing | $ | (106) | | | $ | (117) | |
| Right-of-use assets, operating | (160) | | | (26) | |
| Property, plant and equipment | (107) | | | (85) | |
| Other | (15) | | | (1) | |
| Total deferred tax liabilities | (388) | | | (229) | |
| Net deferred tax (liabilities) assets | $ | 66 | | | $ | 982 | |
| | | | | | | | | | | |
| December 31, |
| 2020 | | 2019 |
| Deferred tax assets: | | | |
| Net operating loss carry-forwards | $ | 587,211 | | | $ | 268,173 | |
| Stock-based compensation | 33,089 | | | 43,978 | |
| Capitalized licenses, research and development and start-up costs | 13,985 | | | 19,891 | |
| Tax credit carry-forwards | 99,024 | | | 77,222 | |
| Accrued expenses | 26,962 | | | 7,377 | |
| Deferred revenue | 29,785 | | | 53,475 | |
| Operating lease liabilities | 22,313 | | | 26,571 | |
| Lease financing obligation | 23,718 | | | 10,570 | |
| Capitalized inventory | 38,058 | | | 0 | |
| Other | 0 | | | 11 | |
| Total gross deferred tax assets | 874,145 | | | 507,268 | |
| Less: valuation allowance | (823,468) | | | (470,753) | |
| Total deferred tax assets, net of valuation allowance | 50,677 | | | 36,515 | |
| Deferred tax liabilities: | | | |
| Financing right-of-use assets | (11,539) | | | (2,612) | |
| Operating right-of-use assets | (20,349) | | | (24,944) | |
| Fixed assets | (18,276) | | | (8,959) | |
| Other | (513) | | | 0 | |
| Total deferred tax liabilities | (50,677) | | | (36,515) | |
| Net deferred tax assets | $ | 0 | | | $ | 0 | |
The table below summarizes changes in the valuation allowance for deferred tax assets for the periods presented (in millions): | | | | | | | | | | | | | | | | | |
| Years Ended December 31, |
| 2023 | | 2022 | | 2021 |
| Valuation allowance at beginning of the period | $ | 155 | | | $ | 149 | | | $ | 823 | |
| Decreases recorded as benefit to income tax provision | — | | | (12) | | | (722) | |
| Increases to valuation allowance | 2,069 | | | 18 | | | 48 | |
| Valuation allowance at December 31 | $ | 2,224 | | | $ | 155 | | | $ | 149 | |
We have evaluatedperiodically reassess the need for valuation allowances on our deferred tax assets, considering both positive and negative evidence bearingto evaluate whether it is more likely than not that all or a portion of such assets will not be realized. During 2023, following the completion of our long-range financial planning process, we reassessed the evidence and concluded that a valuation allowance was necessary due to the preponderance of negative evidence, including:
•A pre-tax loss for the full year 2023, serving as a significant source of objectively verifiable negative evidence in accordance with ASC 740 (Income Taxes).
•A projected three-year cumulative loss resulting from our long-range financial planning process. This projection is due to a significant decrease in expected sales of our COVID-19 vaccine as we transition to a seasonal market. Additionally, we anticipate substantial research and development expenses for our on-going Phase 3 clinical trials and to advance our product candidates into later-stage development. These factors contribute additional negative evidence with respect to the realizability of our deferred tax assets. The projections are based upon revenue from our currently approved drug product, which we believe can be reasonably estimated. In contrast, future taxable income projections from our investigational medicines are deemed inherently subjective and not objectively verifiable; they are insufficient to override negative evidence, and therefore, they are not assigned any weight in our valuation allowance analysis assessment.
Our evaluation also included whether there were other sources of taxable income that would allow us to realize our deferred tax assets, such as taxable income in carryback years, available tax planning strategies and the realizationfuture reversals of taxable temporary differences. After assessing these strategies and all evidence, we determined it was more likely than not that we will not realize all of our deferred tax assets including our history of significant losses in every year since our inception and in accordance withtherefore increased the applicable accounting standards, have fully reserved the net deferred tax asset. We concluded that realization of our net deferred tax assets is not more-likely-than-not to be realized as of December 31, 2020. The valuation allowance increased by $352.7 million in the year ended December 31, 2020, primarily due to the increase in net operating loss carry-forwards, research and development tax credits and capitalized inventory.$2.1 billion during 2023.
On a periodic basis, we reassessIn the valuation allowance on our deferred income tax assets, weighing positive and negative evidence to assess the recoverabilityfirst quarter of the deferred tax assets. In 2020,2021, we reassessed the valuation allowance and considered negative evidence, including our cumulative losses overnoting the three years ended December 31, 2020, andincrease in positive evidence, including our recent EUA for our COVID-19 vaccine.significant revenue growth, expectations regarding future profitability, and successful supply chain and manufacturing capabilities to meet global product demand. After assessing both the negative and positive evidence and negative evidence, we concludeddetermined it was more likely than not that we should maintainwould realize the majority of our deferred tax assets, and we released the valuation allowance on the majority of our net operating losses and our other deferred tax assets, as of December 31, 2020.accordingly. The release of the valuation allowance as well aswas based on the exact timing andthen-prevailing positive outlook, which has since shifted significantly by 2023 due to the amountfactors outlined above, leading to the reinstatement of such release, continue to be subject to, among other things,a full valuation allowance.
Significant management judgment is required in assessing the realizability of our level of profitability, revenue growth, clinical program progression and expectations regarding future profitability. Our total deferred tax asset balance subjectassets. In the event that actual results differ from our estimates, we adjust our estimates in future periods and we may need to themodify our valuation allowance, was approximately $874.1 million at December 31, 2020. We will continue to monitor the need for a full or partial valuation allowance each quarter in 2021which could materially impact our financial position and future periods.results of operations.
At December 31, 2020,2023, we had approximately $2.26$1.6 billion and $1.70 billion of federal and state net operating loss carry-forwards, respectively, ofcarryforwards, which $380.1 million of federal and $1.70 billion of state loss carry-forwards begin to expire in 2030. Additionally, $1.88 billion of federal net operating loss carry-forward will carry forward indefinitely. At December 31, 20202023, we also had federal and state research and development tax credit carry-forwardscarryforwards of approximately $73.3$178 million, and $26.1 million,the majority of which will begin to expire in 2030 and 2032, respectively. At December 31, 2020, we also had federal orphan drug and state investment tax credit carry-forwards of approximately $2.0 million and $3.9 million which begin to expire in 2039 and 2021, respectively.2030.
In March 2020, the Coronavirus Aid, Relief and Economic Security Act (the CARES Act) was signed into law. The CARES Act includes provisions relating to several aspects of corporate income taxes. We do not currently expect the CARES Act to have a significant impact on our provision for income taxes; however, we will continue to monitor the provisions of the CARES Act in relation to its operations.
Utilization of the net operating loss (NOL) and tax credit carry-forwards may be subject to a substantial annual limitation due to ownership change limitations that have occurred previously, or that could occur in the future, as provided by Section 382 of the Internal Revenue Code of 1986, as amended, or Section 382, as well as similar state provisions and other provisions of the Internal Revenue Code. Ownership changes may limit the amount of NOLs and tax credit carry-forwards that can be utilized annually to offset future taxable income and tax, respectively. In general, an ownership change, as defined by Section 382, results from transactions that increase the ownership of 5% shareholders in the stock of a corporation by more than 50% in the aggregate over a three-year period. We may experience ownership changes in the future as a result of subsequent shifts in our stock ownership, some of which may be outside our control.
We file U.S. federal income tax returns and income tax returns in various state, local and foreign jurisdictions. All tax years since the date of our incorporation remain open to examination by the major taxing jurisdictions (state and federal) to which we are subject, as carry-forward attributes generated in years past may still be adjusted upon examination by the Internal Revenue Service (IRS) or other authorities if they have or will be used in a future period. We are not currently under examination by the IRS, or any other jurisdictions, for any tax year.
We recognize, in our financial statements, the effect of a tax position when it is more likely than not, based on the technical merits, that the position will be sustained upon examination. A reconciliation of the beginning and ending amounts of unrecognized tax benefits during the years ended December 31, 20202023, 2022, and 2019 are2021 were as follows (in thousands)millions):
| | | | | | | | | | | | | | | | | |
| Years Ended December 31, |
| 2023 | | 2022 | | 2021 |
| Unrecognized tax benefits at beginning of the period | $ | 128 | | | $ | 68 | | | $ | — | |
| Decrease due to prior positions: | | | | | |
| Tax positions for prior years | — | | | (1) | | | — | |
| Expiration of statutes | — | | | — | | | — | |
| Settlements with tax authorities | (27) | | | — | | | — | |
| Increase due to current year tax positions: | | | | | |
| Additions based on tax positions for current year | 44 | | | 57 | | | 54 | |
| Additions based on tax positions for prior years | 86 | | | 4 | | | 14 | |
| Unrecognized tax benefits at end of the period | $ | 231 | | | $ | 128 | | | $ | 68 | |
| | | | | |
Balance as of December 31, 2018 | $ | 141 | |
Decrease due to prior positions | 0 | |
Increase due to current year tax position | 0 | |
Balance as of December 31, 2019 | 141 | |
Decrease due to prior positions | 0 | |
Increase due to current year tax positions | 0 | |
Balance as of December 31, 2020 | $ | 141 | |
As of December 31, 2023, we had $231 million of net unrecognized tax benefits, which would affect our tax rate if recognized. Unrecognized tax benefits may change during the next twelve months for items that arise in the ordinary course of business. We do not anticipate a material change to our unrecognized tax benefits over the next twelve months that would have an adverse effect on our consolidated operating results. We recognize interest and penalties, if applicable, related to uncertain tax positions as a component of income tax expense.
We file U.S. federal income tax returns and income tax returns in various state, local and foreign jurisdictions. All tax years since our date of incorporation remain open to examination by the major taxing jurisdictions, as carryforward attributes generated in past years may be adjusted upon examination by the Internal Revenue Service or the state authorities. There are no open tax examinations at this time.
17. Net Loss14. (Loss) Earnings per Share
Net LossThe computation of basic earnings (loss) per Share Attributable to Common Stockholdersshare (EPS) is based on the weighted-average number of our common shares outstanding. The computation of diluted EPS is based on the weighted-average number of our common shares outstanding and potential dilutive common shares outstanding during the period as determined by using the treasury stock method.
Basic and diluted net loss per share attributable to common stockholdersEPS for the years ended December 31, 2020, 20192023, 2022 and 2018 are2021 were calculated as follows (in thousands,millions, except share and per share data):
| | | | | | | | | | | | | | | | | |
| Years Ended December 31, |
| 2023 | | 2022 | | 2021 |
| Numerator: | | | | | |
| Net (loss) income | $ | (4,714) | | | $ | 8,362 | | | $ | 12,202 | |
| | | | | |
| | | | | |
| | | | | |
| Denominator: | | | | | |
| Basic weighted-average common shares outstanding | 382 | | | 394 | | | 403 | |
| Effect of dilutive securities | — | | | 22 | | | 28 | |
| Diluted weighted-average common shares outstanding | 382 | | | 416 | | | 431 | |
| | | | | |
| Basic EPS | $ | (12.33) | | | $ | 21.26 | | | $ | 30.31 | |
| Diluted EPS | $ | (12.33) | | | $ | 20.12 | | | $ | 28.29 | |
| | | | | | | | | | | | | | | | | |
| Years Ended December 31, |
| 2020 | | 2019 | | 2018 |
| Numerator: | | | | | |
| Net loss | $ | (747,064) | | | $ | (514,021) | | | $ | (384,734) | |
| Premium paid on repurchase of redeemable convertible preferred stock | 0 | | | 0 | | | (4,127) | |
| Cumulative dividends on redeemable convertible preferred stock | 0 | | | 0 | | | (12,996) | |
| Net loss attributable to common stockholders | $ | (747,064) | | | $ | (514,021) | | | $ | (401,857) | |
| Denominator: | | | | | |
| Weighted average common shares used in net loss per share attributable to common stockholders, basic and diluted | 381,333,059 | | | 330,802,136 | | | 81,114,183 | |
| Net loss per share attributable to common stockholders, basic and diluted | $ | (1.96) | | | $ | (1.55) | | | $ | (4.95) | |
The following common stock equivalents, presented based on amounts outstanding as of December 31, 2020, 20192023, 2022 and 20182021, were excluded from the calculation of diluted net loss(loss) income per share attributable to common stockholders for the periods indicated because their inclusion would have been anti-dilutive:anti-dilutive (in millions):
| | | | | | | | | | | | | | | | | |
| December 31, |
| 2020 | | 2019 | | 2018 |
| Stock options | 34,057,533 | | | 45,536,915 | | | 50,821,132 | |
| Restricted common stock | 0 | | | 0 | | | 198,597 | |
| Restricted common stock units | 2,192,960 | | | 1,177,249 | | | 458,715 | |
| 36,250,493 | | | 46,714,164 | | | 51,478,444 | |
| | | | | | | | | | | | | | | | | | | | |
| | December 31, |
| | 2023 | | 2022 | | 2021 |
| Options | | 26 | | | 3 | | | 1 | |
| | | | | | |
| RSUs and PSUs | | 5 | | | — | | | — | |
| Total | | 31 | | | 3 | | | 1 | |
18.15. Geographic Information
Geographic Revenue
We operate in 1one reporting segment that primarily focuses on the discovery, development and commercialization of mRNA medicines. Our chief executive officer manages our operations and evaluates our financial performance on a consolidated basis. Most of our principal operations, other than manufacturing, and our decision-making functions are located at our corporate headquarters in the United States.
Total revenue by geographic area of our customers and collaboration partners was as follows (in thousands)millions):
| | Years Ended December 31, |
| 2020 | | 2019 | | 2018 |
| | Years Ended December 31, | | | | Years Ended December 31, |
| | 2023 | | | | 2023 | | 2022 | | 2021 |
| United States | United States | | $ | 764,529 | | | $ | 54,976 | | | $ | 89,075 | |
| Europe | |
| Rest of world | Rest of world | | 38,866 | | | 5,233 | | | 45,993 | |
| Total | Total | | $ | 803,395 | | | $ | 60,209 | | | $ | 135,068 | |
Our property, plant and equipment, including financing right-of-use asset, were principally located within the United Statesassets, by geographic area was as of December 31, 2020 and 2019.follows (in millions):
| | | | | | | | | | | | | | |
| | December 31, |
| | 2023 | | 2022 |
| United States | | $ | 1,560 | | | $ | 1,267 | |
| Europe | | 126 | | | 714 | |
| Rest of world | | 259 | | | 37 | |
| Total | | $ | 1,945 | | | $ | 2,018 | |
19. Subsequent Events
Subsequent to December 31, 2020, we have entered into several supply agreements with customers to provide our COVID-19 vaccine, up to 125.1 million doses, and have received upfront deposits of $125.4 million, based on the initial confirmed volume, subject to modifications.
On February 18, 2021, the European Commission advised us that we had successfully tendered for the provision of 150 million doses of our COVID-19 vaccine in 2021. This tender is subject to execution of the Purchase Agreement, which is expected shortly following an opt out period for individual Member States that expired on February 25, 2021.
Subsequent to December 31, 2020, we have entered into additional binding purchase commitments with third-party contractual manufacturing organizations for our COVID-19 vaccine under existing agreements. We are currently committed to minimum non-cancelable purchase obligations of $314.8 million related to these agreements, which are expected to be paid through 2021.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer and our Chief Financial Officer, evaluated the effectiveness of our disclosure controls and procedures as of December 31, 2020.2023. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, or the Exchange Act, means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the company’s management, including its principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Based on the evaluation of our disclosure controls and procedures as of December 31, 2020,2023, our Chief Executive Officer and Chief Financial Officer concluded that, as of such date, our disclosure controls and procedures were effective at the reasonable assurance level.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as such term is defined in Exchange Act Rule 13a-15(f)) to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. Management assessed our internal control over financial reporting as of December 31, 2020.2023. Management based its assessment on criteria established in Internal Control - Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 Framework). Based on that evaluation, our management concluded that our internal control over financial reporting was effective as of December 31, 2020.2023.
The effectiveness of our internal control over financial reporting as of December 31, 20202023 has been audited by Ernst & Young LLP, an independent registered public accounting firm, as stated in their report included in this Annual Report on Form 10-K.
Changes in Internal Controls over Financial Reporting
During the three months ended December 31, 2020, we implemented certain internal controls in connection with our inventory capitalization, product sales and derivative financial instruments. There2023, there were no other changes in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) during the three months ended December 31, 2020,, which have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Inherent Limitations on the Effectiveness of Controls
Our management, including our Chief Executive Officer and Chief Financial Officer, believe that our disclosure controls and procedures and internal control over financial reporting are designed to provide reasonable assurance of achieving their objectives and are effective at the reasonable assurance level. However, our management does not expect that our disclosure controls and procedures or our internal control over financial reporting will prevent all errors and all fraud. A control system, no matter how well-conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, have been detected. These inherent limitations include the realities that judgments in decision making can be faulty, and that breakdowns can occur because of a simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by the collusion of two or more people or by a management override of the controls. The design of any system of controls also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions; over time, controls may become inadequate because of changes in conditions, or the degree of compliance with policies or procedures may deteriorate. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected.
Report of Independent Registered Public Accounting Firm
To the Stockholders and the Board of Directors of Moderna, Inc.
Opinion on the Internal Control Over Financial Reporting
We have audited Moderna, Inc.’s internal control over financial reporting as of December 31, 2020,2023, based on criteria established in Internal Control—IntegratedControl-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the COSO criteria). In our opinion, Moderna, Inc. (the Company) maintained, in all material respects, effective internal control over financial reporting as of December 31, 2020,2023, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated balance sheets of the Company as of December 31, 20202023 and December 31, 2019,2022, the related consolidated statements of operations, comprehensive loss, redeemable convertible preferred stock andincome (loss), stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2020,2023, and the related notes and our report dated February 26, 202123, 2024 expressed an unqualified opinion thereon.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects.
Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ Ernst & Young LLP
Boston, Massachusetts
February 26, 202123, 2024
Item 9B. Other Information
10b5-1 Plans
As previously disclosed inOn November 7, 2023, Noubar Afeyan, our Chairman, amended a Current Report trading arrangement intended to satisfy the affirmative defense of Rule 10b5-1(c) (the Afeyan 10b5-1 Plan). The Afeyan 10b5-1 Plan was amended to increase certain price triggers under the plan. The Afeyan 10b5-1 Plan, as amended, is scheduled to commence on Form 8-K filed with the SEC on March 30, 2020 (the “March 30thFebruary 28, 2024, 8-K”),and will run through August 20, 2025. The aggregate maximum number of shares of common stock that may be sold pursuant to the Afeyan 10b5-1 Plan is 745,000, which represents the shares remaining under the original plan that have not been sold to date.
On November 6, 2023, Arpa Garay, our former Chief Commercial Officer, terminated a trading arrangement that was intended to satisfy the affirmative defense of Rule 10b5-1(c) (the Garay 10b5-1 Plan). The Garay 10b5-1 Plan was entered into on March 29, 2020,August 24, 2023, and was scheduled to commence on November 27, 2023, with a termination date of August 30, 2024. The Garay 10b5-1 Plan provided for the Company’s Chief Medical Officer, Tal Zaks,potential sale of approximately 4,540 shares of common stock and for the potential exercise of vested stock options and the Company entered into an Executive Retention Agreement (the “Zaks Retention Agreement”), setting forthassociated sale of up to 24,897 shares. No shares of common stock were sold, and no options to purchase shares of common stock were exercised, under the terms of Dr. Zaks’ continued service as the Company’s Chief Medical Officer through at least September 30, 2021 (the “Retention Date”). Details of the Zaks Retention Agreement are included on the March 30Garay 10b5-1 Plan prior to its termination.
th 8-K.
Amended and Restated By-laws
On February 23, 2021,21, 2024, our Board of Directors approved and adopted the Company entered into anCompany’s Second Amended and Restated Executive Retention AgreementBy-laws (the “Amended Zaks Retention Agreement”“By-laws”), which modifiedbecame effective immediately. The By-laws supersede and replace in their entirety the original Zaks Retention Agreement. Under theCompany's Amended Zaks Retention Agreement,and Restated By-laws in addition to the benefits already conferred under the original agreement, if a successor Chief Medical Officer is appointed by the Companyeffect immediately prior to September 30, 2021, Dr. Zaks will continue to serve as a Special Advisor to our CEO through that date, and as additional consideration, a pro rata share of Dr. Zaks’ 2020 restricted stock unit equity grant, equivalent to 11,449 shares, which otherwise would not have vested until February 2022 will vest aseffectiveness of the Retention Date.By-laws.
The above summary isBy-laws, among other things:
•implement a majority voting standard in uncontested director elections;
•implement a proxy access provision, which permits a stockholder, or a group of up to 20 stockholders, owning 3% of our outstanding common stock continuously for at least three years to nominate and include in our proxy materials director candidates constituting up to the greater of two nominees or 20% of the Board of Directors, subject to the terms and conditions set forth in the By-laws;
•update our advance notice provisions to take into account recent rules adopted by the SEC regarding usage of universal proxies;
•reflect recent amendments and updates to the Delaware General Corporation Law; and
•incorporate certain other administrative, technical, clarifying and conforming changes.
The foregoing description does not purport to be complete and is qualified in its entirety by reference to the Amended Zaks Retention Agreement,full text of the By-laws, a copy of which is attached hereto as Exhibit 10.173.2 and is incorporated herein by reference.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections
None Applicable.
PART III
Item 10. Directors, Executive Officers and Corporate Governance
The information required by this Item is incorporated herein by reference to the information that will be contained in our proxy statement related to the 20212024 Annual Meeting of Stockholders, which we intend to file with the Securities and Exchange Commission within 120 days of the end of our fiscal year pursuant to General Instruction G(3) of Form 10-K.
Item 11. Executive Compensation
The information required by this Item is incorporated herein by reference to the information that will be contained in our proxy statement related to the 20212024 Annual Meeting of Stockholders, which we intend to file with the Securities and Exchange Commission within 120 days of the end of our fiscal year pursuant to General Instruction G(3) of Form 10-K.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The information required by this Item is incorporated herein by reference to the information that will be contained in our proxy statement related to the 20212024 Annual Meeting of Stockholders, which we intend to file with the Securities and Exchange Commission within 120 days of the end of our fiscal year pursuant to General Instruction G(3) of Form 10-K.
Item 13. Certain Relationships and Related Transactions, and Director Independence
The information required by this Item is incorporated herein by reference to the information that will be contained in our proxy statement related to the 20212024 Annual Meeting of Stockholders, which we intend to file with the Securities and Exchange Commission within 120 days of the end of our fiscal year pursuant to General Instruction G(3) of Form 10-K.
Item 14. Principal Accounting Fees and Services
Our independent public accounting firm is Ernst & Young LLP, Boston, Massachusetts, PCAOB Auditor ID 00042.
The information required by this Item is incorporated herein by reference to the information that will be contained in our proxy statement related to the 20212024 Annual Meeting of Stockholders, which we intend to file with the Securities and Exchange Commission within 120 days of the end of our fiscal year pursuant to General Instruction G(3) of Form 10-K.
PART IV
Item 15. Exhibits, Financial Statement Schedules
(a) Documents filed as part of this report.
(1) Financial statements.
For a list of the consolidated financial statements included herein, see “Index to Consolidated Financial Statements” under Part II, Item 8 of this Annual Report on Form 10-K.
(2) Schedules.
No financial statement schedules have been submitted because they are not required or are not applicable or because the information required is included in the consolidated financial statements or the notes thereto.
(3) Exhibits.
| | | | | | | | |
| Exhibit No. | | Exhibit Index |
| 3.1 | | |
3.23.2* | | |
| 4.1 | | |
| 4.2 | | |
4.3* | | |
| 10.1# | | |
| 10.2# | | |
| 10.3# | | |
| 10.4† | | Master Collaboration and License Agreement, by and between Moderna Therapeutics, Inc. and Merck Sharp & Dohme Corp., dated as of January 12, 2015, as amended by Amendment No. 1 dated as of January 8, 2016, Amendment No. 2 dated as of June 28, 2016, Amendment No. 3 dated as of June 28, 2016 and Amendment No. 4 dated as of June 28, 2016. (1) |
| 10.5† | | |
| | |
| | |
| 10.6† | | |
10.7† | | |
10.8† | | |
10.910.7 | | Lease Agreement, by and between Moderna Therapeutics, Inc. and ARE-Tech Square, LLC, dated as of May 26, 2016, as amended by Amendment No. 1 dated as of August 31, 2016, Amendment No. 2 dated as of December 31, 2016,
Amendment No. 3 dated as of April 24, 2017, Amendment No. 4 dated as of April 13, 2018. (1) |
10.10 | | |
10.11 | | |
10.12#10.8 | | Third Amendment, dated September 11, 2018, Fourth Amendment, dated March 28, 2019, and Omnibus Amendment, dated December 30, 2021, to Net Lease, dated as of August 29, 2016, as amended. (5) |
| 10.9# | | |
10.13#10.10# | | |
10.14#10.11# | | |
| 10.15# | |
| | |
| | |
| | |
| 10.12# | | |
10.16#10.13# | | |
10.17#*10.14# | | |
10.18#10.15#* | | |
| 10.16# | | |
| 10.17# | | |
| | |
| 10.18# | | |
| 10.19# | | |
| | | | | | | | |
| 10.20# | | |
10.21#10.21#* | | |
10.22#10.22#* | | |
10.23#10.23#* | | |
| | | | | | | | |
10.24† | | Agreement No. HHSO100201600029C, by and between the Company and the Biomedical Advanced Research and Development Authority, dated as of April 16, 2020, as amended on May 24, 2020, June 16, 2020, July 25, 2020, August 31, 2020 and September 15, 2020. (6) |
10.25†10.24# | | Global Long Term Agreement, by and among ModernaTX Inc., Lonza Sales Ltd., and Lonza Ltd., dated September 4, 2020. (6) |
10.26† | | Award Contract No. W911QY20C0100, by and between Moderna US Inc. and the Army Contracting Command of the U.S. Department of Defense, dated August 9, 2020, as amended September 8, 2020, and September 11, 2020. (6) |
10.27* | | |
| 10.25# | | |
| 10.26# | | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| 21.1* | | |
23.123.1* | | |
31.131.1* | | |
31.231.2* | | |
| 32.1+ | | |
| 32.2+ | | |
| 97* | | |
| 101.INS* | | XBRL Instance Document |
101.SCH101.SCH* | | XBRL Taxonomy Extension Schema Document |
101.CAL101.CAL* | | XBRL Taxonomy Extension Calculation Document |
101.DEF101.DEF* | | XBRL Taxonomy Extension Definition Linkbase Document |
101.LAB101.LAB* | | XBRL Taxonomy Extension Labels Linkbase Document |
101.PRE101.PRE* | | XBRL Taxonomy Extension Presentation Link Document |
| 104* | | Cover Page Interactive Data File (formatted as Inline XBRL with applicable taxonomy extension information contained in Exhibits 101101) |
____________
___________ | | | | | |
| * | Filed herewith. |
| † | Confidential treatment hasPursuant to 17 C.F.R. §§230.406 and 230.83, the confidential portions of this exhibit have been granted by the Securitiesomitted and Exchange Commission as to certain portions.are marked accordingly. |
| # | Indicates a management contract or any compensatory plan, contract or arrangement. |
+
| The certifications furnished in ExhibitExhibits 32.1 and 32.2 hereto are deemed to accompany this Annual Report on Form 10-K and will not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended. Such certifications will not be deemed to be incorporated by reference into any filings under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, except to the extent that the Registrant specifically incorporates it by reference. |
| (1) | Incorporated by reference to the Registration Statement on Form S-1 (File No. 333-228300) filed with the Securities and Exchange Commission on November 9, 2018. |
| (2) | Incorporated by reference to the Registration Statement on Form S-1 (File No. 333-228300) filed with the Securities and Exchange Commission on November 28, 2018.
(3)(2) | Incorporated by reference to the Current Report on Form 8-K (File No. 001-38753) filed with the Securities and Exchange Commission on December 14, 2018. |
(4)(3) | Incorporated by reference to the Quarterly Report on Form 10-Q (File No. 001-38753) filed with the Securities and Exchange Commission on November 6, 2019. |
(5) | Incorporated by reference to the Current Report on Form 8-K (File No. 001-38753) filed with the Securities and Exchange Commission on May 7, 2020. |
(6) | Incorporated by reference to the Quarterly Report on Form 10-Q (File No. 001-38753) filed with the Securities and Exchange Commission on August 6, 2020. |
(7) | Incorporated by reference to the Quarterly Report on Form 10-Q (File No. 001-38753) filed with the Securities and Exchange Commission on May 9, 2019.6, 2021. |
| |
| |
| |
| |
| |
| (4) | Incorporated by reference to the Annual Report on Form 10-K (File No. 001-38753) filed with the Securities and Exchange Commission on February 27, 2020. |
| (5) | Incorporated by reference to the Annual Report on Form 10-K (File No. 001-38753) filed with the Securities and Exchange Commission on February 25, 2022. |
| (6) | Incorporated by reference to the Current Report on Form 8-K/A (File No. 001-38753) filed with the Securities and Exchange Commission on May 13, 2022. |
| (7) | Incorporated by reference to the Current Report on Form 8-K/A (File No. 001-38753) filed with the Securities and Exchange Commission on June 1, 2022. |
| (8) | Incorporated by reference to the Quarterly Report on Form 10-Q (File No. 001-38753) filed with the Securities and Exchange Commission on October 30, 2020.November 3, 2022. |
| (9) | Incorporated by reference to the Annual Report on Form 10-K (File No. 001-38753) filed with the Securities and Exchange Commission on February 24, 2023. |
| |
Item 16. Form 10-K Summary
Not applicable.
SIGNATURES
Pursuant to the requirements of the Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | | | | | | | |
| | | MODERNA, INC. |
| | | |
| Date: | | By: | /s/ Stéphane Bancel |
February 26, 202123, 2024 | | | |
| | | Stéphane Bancel |
| | | Chief Executive Officer and Director |
| | | |
POWER OF ATTORNEY AND SIGNATURES
Each individual whose signature appears below hereby constitutes and appoints each of Stéphane Bancel and David Meline andJames M. Mock as such person’s true and lawful attorney-in-fact and agent with full power of substitution and resubstitution, for such person in such person’s name, place and stead, in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K, and to file the same, with all exhibits thereto, and all documents in connection therewith, with the Securities and Exchange Commission granting unto each said attorney-in-fact and agent full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as such person might or could do in person, hereby ratifying and confirming all that any said attorney-in-fact and agent, or any substitute or substitutes of any of them, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| | | | | | | | | | | | | | |
| | | | |
| Signature | | Title | | Date |
| | | | |
| /s/ Stéphane Bancel | | Chief Executive Officer and Director (Principal Executive Officer) | | February 26, 202123, 2024 |
| Stéphane Bancel | | |
| | | | |
/s/ David MelineJames M. Mock | | Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) | | February 26, 202123, 2024 |
David MelineJames M. Mock | | |
| | | | |
| | | | |
| /s/ Noubar B. Afeyan, Ph.D. | | Chairman and Director | | February 26, 202123, 2024 |
| Noubar B. Afeyan, Ph.D. | | |
| | | | |
| /s/ Stephen Berenson | | Director | | February 26, 202123, 2024 |
| Stephen Berenson | | |
| | | | |
| /s/ Sandra Horning, M.D. | | Director | | February 26, 202123, 2024 |
| Sandra Horning M.D. | | |
| | | | |
| /s/ Robert Langer, Sc.D. | | Director | | February 26, 202123, 2024 |
| Robert Langer, Sc.D. | | |
| | | | |
| /s/ Francois Nader, M.D. | | Director | | February 26, 202123, 2024 |
| Francois Nader M.D. | | |
| | | | |
| /s/ Elizabeth Nabel, M.D. | | Director | | February 23, 2024 |
| Elizabeth Nabel, M.D. | | |
| | | | |
| /s/ Paul Sagan | | Director | | February 26, 202123, 2024 |
| Paul Sagan | | |
| | | | |
| /s/ Elizabeth Tallett | | Director | | February 26, 202123, 2024 |
| Elizabeth Tallett | | |
| | | | |