ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 2023 TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 FOR THE TRANSITION PERIOD FROM TO British Columbia 84-2231905 (I.R.S. Employer 32351 (Address of principal executive offices) (Zip Code) Registrant’s telephone number, including area code: Title of each class Trading Symbol(s) Name of each exchange on which registered o o Large accelerated filer Accelerated filer Non-accelerated filer Smaller reporting company Emerging growth company $845,917,024. Part III incorporates certain information by reference from the definitive proxy statement to be filed by the registrant in connection with the Item 1B. Item CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS owned subsidiaries and indirectly owned subsidiaries in which we consolidate. Trulieve The modular nature of our standard designs enables quick and incremental additions to capacity where appropriate. In Our business operates year-round. Operations and sales trends in select markets where we operate do follow seasonal trends with various times of the year providing an opportunity for outdoor cultivation, seasonal impacts on sales in summer and winter months in markets in the Southwest and Northeast and promotional activity increases around specific industry and holiday In accordance with Staff Notice 51-352, the Company will evaluate, monitor and reassess this disclosure, and any related risks, on an ongoing basis and the same will be supplemented and amended to investors in public filings, including in the event of government policy changes or the introduction of new or amended guidance, laws or regulations regarding cannabis regulation. The Cole Memorandum offered guidance to federal agencies on how to prioritize civil enforcement, criminal investigations, and prosecutions regarding will be passed as presently proposed or at all. the Company's operations. employees. Only a pharmacist licensed as a dispensary may dispense Regulation of the Medical Cannabis Market in Florida We are authorized to sell a broad selection of products across various product categories. Georgia law requires eligible patients to obtain physician approval to be added to the Low THC Oil Registry unlikely. Nonetheless, DEA’s interference has had a chilling effect on pharmacy distribution. regulations. September 7, 2024. regions. Risks Related to Our Business and Industry Obstruct Terrorism Act of 2001, or the USA PATRIOT Act, and any related or similar rules, regulations or guidelines, issued, administered or enforced by governmental authorities in the U.S. . reorganization of Trulieve US or another of our subsidiaries, holders of indebtedness and trade creditors of that subsidiary may be entitled to payment of their claims from that subsidiary’s assets before we or our shareholders would be entitled to any payment or residual assets. management team. A failure in the demand for our products to materialize as a result of competition, technological change or other factors could have a material adverse effect on our business, results of operations, financial condition or prospects. by scientific research or findings, regulatory investigations, litigation, media attention and other publicity regarding the consumption of medical marijuana products. There can be no assurance that future scientific research, findings, regulatory proceedings, litigation, media attention or other research findings or publicity will be favorable to the medical marijuana market or any particular product, or consistent with earlier publicity. Future research reports, findings, regulatory proceedings, litigation, media attention or other publicity that are perceived as less favorable than, or that question, earlier research reports, findings or publicity could have a material adverse effect on the demand for our products and our business, results of operations, financial condition and cash flows. Furthermore, we cannot provide assurance that our internal controls and compliance systems will protect us from acts committed by our employees, agents or business partners in violation of U.S. federal or state or local laws. Any failure by third-party suppliers to fulfill our production requirements or any improper acts by employees or results of operations. civil claims under RICO. perception that such issuances could occur, may adversely affect prevailing market prices for the Subordinate Voting Shares. With any additional issuance of Subordinate Voting Shares, our investors will suffer dilution to their voting power and economic interest. to the reporting requirements of the United States Securities Exchange Act of 1934, as amended, and the regulations promulgated thereunder on February 4, 2021. Additional or new regulatory requirements may be adopted in the future. The requirements of existing and potential future rules and regulations will increase our legal, accounting, and financial compliance costs, make some activities more difficult, time-consuming or costly and may also place undue strain on our personnel, systems and resources, which could adversely affect our business, financial condition, and results of operations. Period Low High Volume Year Ended December 31, 2021 Fourth Quarter (through December 31, 2021) $ 29.05 $ 43.75 27,122,860 Third Quarter (September 30, 2021) $ 29.54 $ 48.34 21,068,170 Second Quarter (June 30, 2021) $ 43.20 $ 59.60 22,111,976 First Quarter (March 31, 2021) $ 39.26 $ 67.45 26,428,456 Year Ended December 31, 2020 Fourth Quarter (through December 31, 2020) $ 23.63 $ 42.30 30,428,249 Third Quarter (September 30, 2020) $ 16.81 $ 35.08 30,465,852 Second Quarter (June 30, 2020) $ 11.95 $ 18.92 14,113,517 First Quarter (March 31, 2020) $ 8.09 $ 15.34 18,658,398 Period Low High Volume Year Ended December 31, 2021 Fourth Quarter (through December 31, 2021) $ 23.10 $ 34.75 26,893,469 Third Quarter (September 30, 2021) $ 23.38 $ 39.01 23,772,558 Second Quarter (June 30, 2021) $ 34.52 $ 47.49 20,283,884 First Quarter (March 31, 2021) $ 31.62 $ 53.73 29,364,111 Year Ended December 31, 2020 Fourth Quarter (through December 31, 2020) $ 17.37 $ 33.45 27,585,871 Third Quarter (September 30, 2020) $ 12.38 $ 26.50 26,497,216 Second Quarter (June 30, 2020) $ 8.35 $ 14.03 14,145,962 First Quarter (March 31, 2020) $ 5.74 $ 11.81 14,971,972 will depend on the financial condition, business environment, operating results, capital requirements, any contractual restriction on the payment of dividends and any other factors the board deems relevant. Cresco Labs Inc. (Ticker: CRLBF) SEC, if applicable. following: continue to invest director fees. internally developed software. warrants and our interest rate swap. The Company has taken a position that it does not owe taxes attributable to the application of Section 280E of the Internal Revenue Code. to the consolidated financial statements included in Item 8 of this Annual Report on Form 10-K for additional financial information related to our discontinued operations. 2022 Year Ended Change December 31, Increase / (Decrease) 2021 2020 $ % (in thousands) Revenues, net of discounts $ 938,385 $ 521,533 $ 416,852 80 % Year Ended Change December 31, Increase / (Decrease) 2021 2020 $ % (in thousands) Cost of goods sold $ 372,255 $ 135,116 $ 237,139 176 % % of total revenues 40 % 26 % Year Ended Change December 31, Increase / (Decrease) 2021 2020 $ % (in thousands) Gross profit $ 566,130 $ 386,417 $ 179,713 47 % % of total revenues 60 % 74 % Year Ended Change December 31, Increase / (Decrease) 2021 2020 $ % (in thousands) Sales and marketing expenses $ 215,144 $ 119,395 $ 95,749 80 % % of total revenues 23 % 23 % share as well as expanding into new markets. Year Ended Change December 31, Increase / (Decrease) 2021 2020 $ % (in thousands) General and administrative expenses $ 100,573 $ 36,056 $ 64,517 179 % % of total revenues 11 % 7 % Expense amounts related to specific non-recurring items such as legal settlements. We also contributed $20.0 million to the Smart and Safe Florida campaign in 2022. Year Ended Change December 31, Increase / (Decrease) 2021 2020 $ % (in thousands) Loss on impairment and disposal of long-lived assets $ 5,371 $ 63 $ 5,308 8,425 % % of total revenues 1 % 0 % Net Year Ended Change December 31, Increase / (Decrease) 2021 2020 $ % (in thousands) Total other expense, net $ 33,440 $ 60,854 $ (27,414 ) (45 )% % of total revenues (4 )% (12 )% Provision for Income Taxes Year Ended Change December 31, Increase / (Decrease) 2021 2020 $ % (in thousands) Provision for income taxes $ 146,061 $ 94,451 $ 51,610 55 % Effective tax rate 89 % 60 % Year Ended Change December 31, Increase / (Decrease) 2021 2020 $ % (in thousands) Net income and comprehensive income $ 17,445 $ 62,998 $ (45,553 ) (72 )% Year Ended Change December 31, Increase / (Decrease) 2020 2019 $ % (dollars in thousands) Revenues, net of discounts $ 521,533 $ 252,819 $ 268,714 106 % Year Ended Change December 31, Increase / (Decrease) 2020 2019 $ % (dollars in thousands) Cost of goods sold $ 135,116 $ 60,982 $ 74,134 122 % % of total revenues 26 % 24 % Year Ended Change December 31, Increase / (Decrease) 2020 2019 $ % (dollars in thousands) Gross profit $ 386,417 $ 191,837 $ 194,580 101 % % of total revenues 74 % 76 % Year Ended Change December 31, Increase / (Decrease) 2020 2019 $ % (dollars in thousands) Sales and marketing expenses $ 119,395 $ 59,349 $ 60,046 101 % % of total revenues 23 % 23 % Year Ended Change December 31, Increase / (Decrease) 2020 2019 $ % (dollars in thousands) General and administrative expenses $ 36,056 $ 14,071 $ 21,985 156 % % of total revenues 7 % 6 % Year Ended Change December 31, Increase / (Decrease) 2020 2019 $ % (dollars in thousands) Loss on impairment and disposal of long-lived assets $ 63 $ 67 $ (4 ) (6 )% % of total revenues 0 % 0 % Year Ended Change December 31, Increase / (Decrease) 2020 2019 $ % (dollars in thousands) Depreciation and amortization expenses $ 12,600 $ 5,079 $ 7,521 148 % % of total revenues 2 % 2 % Year Ended Change December 31, Increase / (Decrease) 2020 2019 $ % (dollars in thousands) Total other expense $ 60,854 $ 9,590 $ 51,264 535 % % of total revenues 12 % 4 % Year Ended Change December 31, Increase / (Decrease) 2020 2019 $ % (dollars in thousands) Provision for income taxes $ 94,451 $ 50,586 $ 43,865 87 % Effective tax rate 60 % 49 % Year Ended Change December 31, Increase / (Decrease) 2020 2019 $ % (dollars in thousands) Net income and comprehensive income $ 62,998 $ 53,095 $ 9,903 19 % d December 31, 2022, an increase of $13.5 million, or 3%, from $384.8 million for the year ended December 31, 2021. The increase in Adjusted EBITDA in 2022 was primarily due to the acquisition of Harvest Health and Recreation in the fourth quarter of 2021 and the resulting growth in sales and synergies. cash from 2023. We believe our existing cash balances will be sufficient to meet our anticipated cash requirements from the date of this Annual Report on Form 10-K through at least the next 12 months. Year Ended Year Ended December 31, December 31, 2021 2020 (in thousands) Cash and cash equivalents $ 230,646 $ 146,713 Outstanding debt and warrant liabilities Notes payable 16,600 6,000 Notes payable - related party — 12,011 Private placement notes 462,929 117,165 Warrant liabilities 2,895 — Operating lease liabilities 131,970 31,397 Finance lease liabilities 71,429 38,935 Construction finance liabilities 176,189 82,047 Total debt and warrant liabilities $ 862,012 $ 287,555 discontinued operations. The table below highlights our cash flows for the periods Year Ended December 31, 2021 2020 2019 (in thousands) Net cash provided by operating activities $ 12,898 $ 99,643 $ 19,073 Net cash used in investing activities (215,184 ) (174,654 ) (94,672 ) Net cash provided by financing activities 289,232 129,911 142,982 Net increase in cash and cash equivalents 86,946 54,900 67,383 Cash, cash equivalents, and restricted cash, beginning of year 146,713 91,813 24,430 Cash, cash equivalents, and restricted cash, end of year $ 233,659 $ 146,713 $ 91,813 in 2023 compared to 2022. This was offset by reduced gross margins for 2023 as compared to 2022. increases in inventory. Watkins Cultivation Operation. Harvest acquisition. increase in cash used in financing activities in 2023 relative to 2022. Includes liabilities due in relation to our discontinued operations and excludes $180.4 million of uncertain tax position liabilities as we cannot make a reasonably reliable estimate of the period of potential cash settlement with the respective taxing authorities. <1 Year 1 to 3 Years 3 to 5 Years >5 Years Total (in thousands) Accounts payable and accrued liabilities $ 94,073 $ — $ — $ — $ 94,073 Notes payable 10,144 5,376 19 1,061 16,600 Private placement notes — 130,000 350,000 — 480,000 Operating lease liabilities 21,826 42,267 40,510 110,758 215,361 Finance lease liabilities 12,102 26,281 21,234 42,311 101,928 Construction finance liabilities 22,463 47,143 48,771 427,747 546,124 Total $ 160,608 $ 251,067 $ 460,534 $ 581,877 $ 1,454,086 without limitation, such considerations as liquidity and capital resources. to the consolidated financial statements included in Item 8 of this Annual Report on Form 10-K for further information. The evaluations are linked closely to the assumptions made by management regarding the future performance of these assets and any changes in the discount rate applied. The Company has taken a position that it does not owe taxes attributable to the application of Section 280E of the Internal Revenue Code. any events or changes in circumstances that would indicate the carrying amount of goodwill may be impaired. The Company did not identify any impairment of its goodwill during the years ended December 31, 2022 or 2021. Year Ended Change December 31, Increase / (Decrease) 2021 2020 $ % (in thousands) Adjusted EBITDA $ 384,581 $ 260,077 $ 124,504 48 % Year Ended December 31, 2021 2020 2019 (in thousands) Net income and comprehensive income attributable to common shareholders $ 18,032 $ 62,998 $ 53,095 Add (deduct) impact of: Interest expense 34,787 20,237 9,050 Provision for income taxes 146,061 94,451 50,586 Depreciation and amortization 48,096 12,600 5,079 Depreciation included in cost of goods sold 24,073 11,542 7,992 EBITDA 271,049 201,828 125,802 Inventory step up, fair value 41,189 955 — Integration and transition costs 25,601 — — Acquisition and transaction costs 15,831 4,724 — Share-based compensation and related premiums 13,444 2,765 — Other non-recurring expenses 6,797 — — COVID related expenses 6,188 9,125 — Loss on impairment and disposal of long-lived assets 5,371 63 67 Results of entities not legally controlled 458 — — Other expense (income), net (1,139 ) (2,062 ) (266 ) Change in fair value of derivative liabilities - warrants (208 ) 42,679 806 Total adjustments 113,532 58,249 607 Adjusted EBITDA $ 384,581 $ 260,077 $ 126,409 See Item 7, Liquidity and Capital Resources, and Item 8 of this Annual Report on Form 10-K for additional information. number of remediation actions during the year Changes in Internal Control Over Financial Reporting Cash and Cash Equivalents Restricted Cash contractual arrangements. Accounts Receivable and Notes Receivable Land Not depreciated Land improvements 20 to 30 years Buildings & improvements 7 to 40 years Furniture & equipment 3 to 10 years Vehicles 3 to 5 years Construction in progress Not depreciated Leasehold improvements The lessor of the life of the lease or the estimated useful life of the asset Year Ended December 31, 2021 2020 (in thousands) Trade accounts payable $ 14,781 $ 9,248 Accrued payroll 24,728 11,030 Accrued property and equipment 6,507 3,210 Accrued property and equipment - related party 11,353 10,403 Accrued inventory 8,373 1,415 Accrued insurance 6,620 86 Accrued interest 6,787 — Accrued utilities 990 202 Sales tax payable 5,352 — Other payables and accrued liabilities 8,582 5,605 Total accounts payable and accrued liabilities $ 94,073 $ 41,199 Year Ended December 31, 2021 2020 (in thousands) Prepaid insurance $ 10,175 $ 2,713 Prepaid expenses 17,644 7,332 Tenant improvement receivables 9,806 1,317 Held for sale assets, net 8,719 — Deposits 9,650 1,798 Other current assets 12,195 2,959 Total prepaids and other current assets $ 68,189 $ 16,119 The Company enters into leases in the normal course of business, primarily for retail space, production facilities, corporate offices, and equipment used in the production and sale of its products. Lease terms for real estate generally range from five to ten years. Most leases include options to renew for varying terms at the Company’s sole discretion. Other leased assets include passenger vehicles, trucks, and equipment. Lease terms for these assets generally range from three to five years. At the inception of a contract, the Company assesses whether a contract is, or contains, a lease. A contract is, or contains, a lease if the contract conveys the right to control the use of an identified asset for a period of time in exchange for consideration. The Company made an accounting policy election not to recognize right-of-use assets and lease liabilities for leases with a lease term of 12 months or less. Instead lease payments for these leases are recognized as lease expense on a straight-line basis over the lease term. operations. commencement. In the estimation of fair value, the Company uses assumptions and judgements for an asset retirement obligation. The costs associated with these liabilities are capitalized with the associated leasehold improvement and depreciated over the lease term with the liabilities accreted over the same period. Financial Instruments Warrants equivalents consist of the incremental common shares issuable upon the exercise of share options, warrants, and RSUs and the incremental shares issuable upon conversion of similar instruments. Revenue Recognition During the first quarter of 2023, the Company terminated the loyalty program associated with dispensaries acquired with the October 2021 acquisition of Harvest Health & Recreation, Inc. ("Harvest"). As a result of the termination of the loyalty program at certain dispensaries, the Company recorded a reduction in the accrual of $4.7 million in revenue on the consolidated statements of operations. The remaining reduction in deferred revenue during the year ended December 31, 2023 was primarily due to increased breakage in our remaining loyalty programs. Discontinued Operations Judgments Recently Issued Accounting Pronouncements In In Formula 420 Cannabis LLC The net assets were acquired for an aggregate purchase price of (in thousands) Consideration: Cash $ 15,000 Transaction costs 12 Fair value of consideration exchanged $ 15,012 Recognized amounts of identifiable assets acquired and liabilities assumed: Prepaid expenses and other current assets $ 531 Right of use asset - operating 271 Intangible asset 15,076 Other current liabilities (531 ) Deferred revenue (109 ) Lease liabilities (226 ) Total net assets acquired $ 15,012 cannabinoids. The following table summarizes the allocation of consideration exchanged for the estimated fair value of tangible and identifiable intangible assets acquired and liabilities assumed: (in thousands) Consideration: Trulieve Subordinated Voting Shares $ 1,369,024 Fair value of other equity instruments 18,394 Fair value of warrants classified as liabilities 3,103 Fair value of consideration exchanged $ 1,390,521 Recognized amounts of identifiable assets acquired and liabilities assumed: Cash and cash equivalents $ 85,318 Restricted cash 3,072 Accounts receivable 3,645 Inventories 92,537 Prepaid expenses and other current assets 100,129 Notes receivable 9,805 Property and equipment 191,801 Right of use assets - operating 73,476 Intangible assets: Dispensary license 946,000 Trademarks 27,430 Customer relationships 3,500 Other assets 5,289 Accounts payable and accrued liabilities (58,887 ) Income tax payable (24,863 ) Deferred revenue (4,523 ) Operating lease liabilities (76,558 ) Contingencies (26,599 ) Notes payable (285,238 ) Construction finance liabilities (79,683 ) Other long-term liabilities (1,085 ) Deferred tax liabilities (253,986 ) $ 730,580 Non-controlling interest $ (2,139 ) Goodwill 662,080 Total net assets acquired $ 1,390,521 acquired. The October 1, 2021. The which have subsequently been discontinued. The following table summarizes the allocation of consideration exchanged for the estimated fair value of tangible and identifiable intangible assets acquired and liabilities assumed: (in thousands) Consideration: Cash $ 20,251 Shares issued upon acquisition 35,385 Fair value of consideration exchanged $ 55,636 Recognized amounts of identifiable assets acquired and liabilities assumed: Cash $ 500 Prepaid expenses and other current assets 240 Inventories 1,766 Property and equipment 1,144 Right of use asset - finance 1,340 Intangible assets: Dispensary license 27,000 Tradename 100 Favorable leasehold interests 86 Goodwill 39,703 Other assets 40 Accounts payable and accrued liabilities (878 ) Income tax payable (2,892 ) Operating lease liabilities (1,340 ) Other long-term liabilities (2,179 ) Deferred tax liabilities (8,994 ) Total net assets acquired $ 55,636 The following table summarizes the allocation of consideration exchanged for the estimated fair value of tangible and identifiable intangible assets acquired and liabilities assumed: (in thousands) Consideration: Cash $ 7,000 Shares issued upon acquisition 9,139 Transaction costs 23 Fair value of consideration exchanged $ 16,162 Recognized amounts of identifiable assets acquired and liabilities assumed: Prepaid expenses and other current assets $ 12 Property and equipment 1,006 Right of use asset - finance 799 Intangible asset 15,274 Accounts payable and accrued liabilities (335 ) Finance lease liabilities (594 ) Total net assets acquired $ 16,162 (in thousands) Consideration: Shares issued upon acquisition $ 10,012 Transaction costs 18 Fair value of consideration exchanged $ 10,030 Recognized amounts of identifiable assets acquired and liabilities assumed: Right of use asset - finance $ 1,756 Intangible asset 10,594 Finance lease liabilities (2,320 ) Total net assets acquired $ 10,030 ASU 2017-01, Business Combinations (Topic 805): Clarifying the Definition of a Business, determining Solevo WV did not meet the definition of a business as substantially all of the fair value of the gross assets acquired are concentrated in a single identifiable asset. Therefore, the transaction has been accounted for as an asset acquisition. (in thousands) Consideration: Cash $ 19,000 Shares issued upon acquisition 29,711 Contingent consideration payable in shares 46,951 Fair value of consideration exchanged $ 95,662 Recognized amounts of identifiable assets acquired and liabilities assumed: Cash $ 563 Accounts receivable 1,300 Prepaid expenses and other current assets 376 Inventories 7,461 Property and equipment 26,233 Intangible assets: State license 45,310 Tradenames 3,540 Goodwill 45,431 Other assets 478 Accounts payable and accrued liabilities (2,189 ) Construction finance liabilities (17,413 ) Deferred tax liabilities (15,428 ) Total net assets acquired $ 95,662 (in thousands) Consideration: Cash $ 10,000 Shares issued upon acquisition 11,004 Contingent consideration payable in shares 15,249 Net working capital adjustment 624 Fair value of consideration exchanged $ 36,877 Recognized amounts of identifiable assets acquired and liabilities assumed: Cash $ 1,229 Accounts receivable 117 Prepaid expenses and other current assets 91 Inventories 2,337 Property and equipment 2,245 Right of use assets - operating 2,156 Intangible assets: Dispensary license 19,890 Tradename 930 Goodwill 10,828 Accounts payable and accrued liabilities (790 ) Lease liabilities (2,156 ) Total net assets acquired $ 36,877 Accounts receivable, net consisted of the following as of December 31: 2021 2020 (in thousands) Trade receivables $ 9,363 $ 313 Less: allowance for credit losses (509 ) (5 ) Accounts receivable, net $ 8,854 $ 308 2021 2020 (in thousands) Promissory note acquired from Harvest maturing in November 2025. Secured by certain assets. $ 8,827 $ — Promissory notes acquired from Harvest maturing in February 2022. Secured by certain assets. 850 — Convertible note receivable dated November 2021 maturing in November 2024. 4,124 — Notes receivable 13,801 — Less: discount on notes receivable (124 ) — Total notes receivable, net of discounts 13,677 — Less: current portion of notes receivable (1,530 ) — Notes receivable $ 12,147 $ — respectively. Stated maturities of the notes receivable, net are as follows as of December 31, Year Ending December 31, Expected principal payments (in thousands) 2022 $ 1,530 2023 733 2024 4,913 2025 6,625 2026 — Thereafter — Total 13,801 Less: discount on notes receivable (124 ) Total $ 13,677 Inventories are comprised of the following items as of December 31: 2021 2020 (in thousands) Raw material Cannabis plants $ 31,279 $ 10,661 Packaging and supplies 40,326 11,233 Total raw material 71,605 21,894 Work in process 94,249 54,780 Finished goods-unmedicated 4,824 3,908 Finished goods-medicated 41,510 17,730 Total inventories $ 212,188 $ 98,312 2021 2020 (in thousands) Land $ 32,904 $ 5,878 Buildings & improvements 435,185 156,372 Construction in progress 234,198 129,588 Furniture & equipment 140,281 51,714 Vehicles 959 351 Total 843,527 343,903 Less: accumulated depreciation (63,611 ) (29,858 ) Property and equipment, net $ 779,916 $ 314,045 December 31, (in thousands) Beginning balance Revisions to purchase price allocation Additions Disposals Amortization expense Ending balance Licenses $ 83,313 $ (5,659 ) $ 1,029,563 $ (3,168 ) $ (22,743 ) $ 1,081,306 Internal use software 3,656 — 3,716 — (1,117 ) 6,255 Tradenames 4,937 — 100 (403 ) (1,940 ) 2,694 Customer relationship 683 — 3,500 — (1,077 ) 3,106 Miscellaneous 7 — — — (7 ) — Trademarks — — 27,516 — (2,895 ) 24,621 $ 92,596 $ (5,659 ) $ 1,064,395 $ (3,571 ) $ (29,779 ) $ 1,117,982 December 31, (in thousands) Beginning balance Revisions to purchase price allocation Additions Disposals Amortization expense Ending balance Licenses $ 24,538 $ (1,204 ) $ 62,287 $ — $ (2,308 ) $ 83,313 Internal use software 3,656 — — — — 3,656 Tradenames 800 — 4,470 — (333 ) 4,937 Customer relationship 883 — — — (200 ) 683 Miscellaneous 25 — — — (18 ) 7 Trademarks 134 — — — (134 ) — $ 30,036 $ (1,204 ) $ 66,757 $ — $ (2,993 ) $ 92,596 2023. The following table outlines the estimated future annual amortization expense related to intangible assets as of December 31, Year Ending December 31, Estimated (in thousands) 2022 $ 85,655 2023 80,713 2024 78,971 2025 76,944 2026 75,447 Thereafter 720,252 $ 1,117,982 Goodwill (in thousands) As of December 31, 2019 $ 7,316 Acquisition of PurePenn, LLC and Pioneer Leasing & Consulting, LLC 47,311 Acquisition of Solevo Wellness 19,473 Revisions of purchase price allocations of PurePenn and Solevo Wellness (6,924 ) As of December 31, 2020 $ 67,176 Measurement period adjustments and revisions of purchase price allocation of Solevo Wellness (2,638 ) Measurement period adjustments and revisions of purchase price allocation of PurePenn (963 ) Acquisition of Keystone Shops 40,072 Revisions of purchase price allocation of Keystone Shops (369 ) Acquisition of Harvest Health & Recreation, Inc. 662,080 As of December 31, 2021 $ 765,358 2021 2020 (in thousands) Promissory notes dated April to July 2017, maturing between April and July 2022. Monthly interest payments due at 12% per annum. Principal balance due at maturity. The note was fully paid in the fourth quarter of 2021. $ — $ 4,000 Promissory note dated December 2017, maturing in December 2021. Monthly interest payments due at 12% per annum. Secured by property. Principal balance due at maturity. The note was fully paid in the fourth quarter of 2021. — 2,000 Promissory notes dated October 1, 2021, maturing in October 2022. Monthly interest payments due of 4.75%. Secured by mortgaged property with a $6 million book value. 6,156 — Promissory note dated October 2019, maturing in October 2024. Monthly interest payments due of 5.5%. Principal balance due at maturity. 829 — Promissory note acquired in Harvest acquisition dated August 2018, maturing in August 2024. Monthly interest payments due of 2%. Secured by certain assets. 1,022 — Promissory note acquired in Harvest acquisition dated January 2020, maturing in May 2023. Quarterly interest payments due of 2%. 425 — Promissory note acquired in Harvest acquisition dated January 2020, maturing in January 2023. Monthly interest payments due at 2%. 65 — Promissory note dated July 2018, maturing in July 2023. Monthly interest payments due at 4% per annum. Secured by certain assets. 1,113 — Promissory note acquired in Harvest acquisition dated February 2020, maturing in February 2023. Monthly interest payments due at 5.5%. 4,699 — Promissory note acquired in Harvest acquisition dated April 2021, maturing in April 2026. Principal due at maturity. Secured by equipment. 60 — Promissory notes of consolidated variable-interest entities acquired in Harvest Acquisition. Maturing December 2022 and 2029, interest ranging from 5.25% to 8.25%. Secured by real-estate. In the first quarter of 2022 these notes were fully paid. 2,231 — Total notes payable 16,600 6,000 Current portion (10,144 ) (2,000 ) Less: debt discount, current 92 — Less: Current portion, net (10,052 ) (2,000 ) Notes payable $ 6,456 $ 4,000 Year Ending December 31, (in thousands) 2022 $ 10,144 2023 4,711 2024 665 2025 14 2026 5 Thereafter 1,061 Total $ 16,600 "Public Warrants"). The extinguishment. Year Ending December 31, (in thousands) 2022 $ — 2023 — 2024 130,000 2025 — 2026 350,000 Thereafter — Total private placement notes 480,000 Less: Unamortized debt issuance costs (17,071 ) Private placement notes, net $ 462,929 The following table provides the components of lease cost Year Ended December 31, Statement of operations and comprehensive income location 2021 2020 2019 (in thousands) Operating lease cost Cost of goods sold, sales and marketing, general and administrative $ 11,574 $ 5,700 $ 5,542 Finance lease cost: Amortization of lease assets Cost of goods sold, sales and marketing, general and administrative 7,866 4,956 1,984 Interest on lease liabilities Interest expense 4,574 2,133 960 Finance lease cost 12,440 7,089 2,944 Variable lease cost Cost of goods sold, sales and marketing, general and administrative 6,098 222 192 Short term lease expense Cost of goods sold, sales and marketing, general and administrative 334 — — Total lease cost $ 30,446 $ 13,011 $ 8,678 operations. Other information related to operating and finance leases is as Year Ended December 31, 2021 2020 (in thousands) Cash paid for amounts included in the measurement of lease liabilities: Operating cash flows from operating leases 4,164 4,764 Operating cash flows from finance leases 4,574 2,485 Financing cash flows from finance leases 4,434 4,951 Lease assets obtained in exchange for new lease liabilities: Operating leases 102,922 12,206 Finance leases 37,821 23,220 Weighted average discount rate: Operating leases 9.69 % 8.64 % Finance leases 8.68 % 8.36 % Weighted average remaining lease term (in years): Operating leases 10.09 7.49 Finance leases 8.16 8.51 Future minimum lease payments under Year Ending December 31, Operating Finance leases (in thousands) 2022 $ 21,826 $ 12,102 2023 21,371 15,039 2024 20,896 11,242 2025 20,536 10,840 2026 19,974 10,394 Thereafter 110,758 42,311 Total undiscounted lease liabilities 215,361 101,928 Less: Interest (83,391 ) (30,499 ) Total present value of minimum lease payments 131,970 71,429 Lease liabilities- current portion 9,840 6,185 Lease liabilities $ 122,130 $ 65,244 Future minimum lease payments for the construction finance liabilities as of December 31, Year Ending December 31, (in thousands) 2022 $ 22,463 2023 23,406 2024 23,737 2025 24,176 2026 24,595 Thereafter 427,747 Total future payments 546,124 Less: Interest (369,935 ) Total present value of minimum payments 176,189 Construction finance liabilities - current portion 991 Construction finance liabilities $ 175,198 respect of each Subordinate Voting Share held. Holders of Subordinate Voting Shares are entitled to receive as and when declared by the directors, dividends in cash or property of the Company. No dividend will be declared or paid on the Subordinate Voting Shares unless the Company simultaneously declares or pays, as applicable, equivalent dividends (on an as-converted to Subordinate Voting Share basis) on the Multiple Voting Shares and Super Voting Shares. Number Weighted average exercise price Weighted average Outstanding and exercisable at December 31, 2018 214,178 6.00 1.66 Granted — — — Exercised (214,178 ) 6.00 — Outstanding and exercisable at December 31, 2019 — — — Granted — — — Exercised — — — Outstanding and exercisable at December 31, 2020 — — — Granted 1,679 1,125 1.31 Exercised — — — Outstanding and exercisable at December 31, 2021 1,679 1,125 1.31 On January 4, 2022 and February 24, 2022, under the 2021 Plan, the Board awarded options to purchase shares to board members, officers, and certain management employees of the Company. The options granted vest immediately for board members and all other options granted vest over a two-to three-year period. Year Ended Year Ended Fair value at grant date $1.44 - $14.13 $3.11 - $3.26 Stock price at grant date $25.80 - $33.42 $11.52 - $12.50 Exercise price at grant date $9.93 - $78.76 $11.52 - $12.50 Expected life in years 3.2 - 6.2 1.58 - 2.0 Expected volatility 49.64% - 56.04% 49.10% - 50.15% Expected annual rate of dividends 0% 0% Risk free annual interest rate 0.16% - 1.15% 1.40% - 1.58% Number Weighted Weighted average Aggregate intrinsic value Outstanding at January 1, 2020 — $ — — — Granted 1,252,403 11.70 Exercised (9,180 ) 11.52 Forfeited (113,444 ) 11.52 Outstanding, December 31, 2020 1,129,779 $ 11.72 4.01 $ 19.90 Exercisable, December 31, 2020 697,944 $ 11.70 4.01 $ 19.92 Outstanding at January 1, 2021 1,129,779 $ 11.72 Granted 2,168,528 36.86 Exercised (118,692 ) 12.46 Forfeited (205,720 ) 46.64 Outstanding, December 31, 2021 2,973,895 $ 27.61 6.26 $ — Exercisable, December 31, 2021 1,503,051 $ 17.27 3.77 $ 8.94 Restricted Stock Units period. Number of Weighted average Unvested balance as of January 1, 2021 — — Granted 3,255,424 25.45 Vested (2,920,336 ) 25.29 Forfeited (2,660 ) 26.88 Unvested balance as of December 31, 2021 332,428 26.86 31: Number of warrants Weighted average exercise price ($CAD) Weighted average remaining contractual life (Yrs) Outstanding as of December 31, 2018 8,784,872 6.00 2.72 Granted — — — Exercised — — — Exchanged for cashless exercise — — — Outstanding as of December 31, 2019 8,784,872 6.00 1.72 Granted — — — Exercised 2,723,311 — — Exchanged for cashless exercise — — — Cancelled — — — Outstanding as of December 31, 2020 6,061,561 6.00 0.72 Exercised 2,075,990 — — Exchanged for cashless exercise 413,057 — — Cancelled 3,572,514 — — Outstanding as of December 31, 2021 — — — The following is a reconciliation for the calculation of basic and diluted earnings per share 2021 2020 2019 (in thousands, except share and per share data) Net income and comprehensive income $ 17,445 $ 62,998 $ 53,095 Less: Net loss and comprehensive loss attributed to non-controlling interest (587 ) — — Net income and comprehensive income attributed to common shareholders $ 18,032 $ 62,998 $ 53,095 Weighted average number of common shares outstanding 139,366,940 113,572,379 110,206,103 Dilutive effect of options, warrants, and RSUs 7,390,346 4,753,345 5,111,839 Diluted weighted average number of common shares outstanding 146,757,286 118,325,724 115,317,942 Basic earnings per share $ 0.13 $ 0.55 $ 0.48 Diluted earnings per share $ 0.12 $ 0.53 $ 0.46 The components of the income tax provision Year Ended December 31, 2021 2020 2019 (in thousands) Current: Federal $ 142,203 $ 82,238 $ 41,847 State 29,924 17,100 9,647 Foreign — — — Total current tax expense 172,127 99,338 51,494 Deferred: Federal (21,755 ) (3,694 ) (738 ) State (4,507 ) (1,193 ) (170 ) Foreign — — — Total deferred tax expense (26,262 ) (4,887 ) (908 ) Change in valuation allowance 196 — — Total income tax expense $ 146,061 $ 94,451 $ 50,586 A reconciliation of the Federal statutory income tax rate percentage to the effective tax rate is as Year Ended December 31, 2021 2020 2019 (in thousands) Income before income taxes $ 163,506 $ 157,449 $ 103,681 Federal statutory rate 21.0 % 21.0 % 21.0 % Theoretical tax expense 34,336 33,064 21,773 State taxes 25,417 12,407 9,477 Other 681 (1,666 ) 1,310 Tax effect of non-deductible expenses: Section 280E permanent differences 85,627 50,646 18,026 111,725 61,387 28,813 Tax expense $ 146,061 $ 94,451 $ 50,586 Year Ended December 31, 2021 2020 2019 (in thousands) Deferred tax assets Lease liabilities $ 10,752 $ 1,219 $ 1,020 Finance liabilities 15,715 — — Net operating losses 7,845 — — Other deferred tax assets 1,567 7,025 969 Deferred tax liabilities Right of use assets (10,285 ) (1,210 ) (1,099 ) Intangible assets (261,673 ) (18,999 ) (6,144 ) Property and equipment (8,406 ) (3,482 ) (233 ) Lease payments — — — Valuation allowance (6,826 ) — — Net deferred tax liability $ (251,311 ) $ (15,447 ) $ (5,487 ) 2023. Year Ended December 31, 2021 2020 Beginning balance $ 3,770 $ 3,770 Additions based on tax positions related to the current year — — Additions for tax positions of prior years' recorded to goodwill 41,080 — Ending balance $ 44,850 $ 3,770 As of December 31, 2021 As of December 31, 2020 Operating Finance Operating Finance (in thousands) (in thousands) Right-of-use assets, net $ 2,082 $ 2,009 $ 12,003 $ 3,425 Lease liabilities: Lease liabilities - current portion $ 418 $ 215 $ 1,539 $ 281 Lease liabilities 1,862 2,127 11,083 3,500 Total related party lease liabilities $ 2,280 $ 2,342 $ 12,622 $ 3,781 Year Ended December 31, 2021 2020 (in thousands) Current assets: Cash $ 1,241 $ — Accounts receivable, net 905 — Inventories, net 2,451 — Other current assets 313 — Total current assets 4,910 — Property and equipment, net 8,335 — Intangible assets, net 17,735 — Other assets 544 — Total assets $ 31,524 $ — Current liabilities: Accounts payable and accrued liabilities $ 828 $ — Notes payable - current portion 1,170 — Income tax payable 522 — Total current liabilities 2,520 — Notes payable 1,061 — Deferred tax liabilities 4,479 — Total liabilities $ 8,060 $ — NOTE 22. REVENUE DISAGGREGATION 2021 2020 (in thousands) Retail $ 870,507 $ 520,217 Wholesale, licensing and other 67,878 1,316 Revenue, net $ 938,385 $ 521,533 Level 1 Level 2 Level 3 Total (in thousands) Financial Assets: Money market funds (1) $ 94,161 $ — $ — $ 94,161 Financial Liabilities: Warrant liabilities (2) $ — $ 2,895 $ — $ 2,895 Level 1 Level 2 Level 3 Total (in thousands) Financial Assets: Money market funds (1) $ 65,516 $ — $ — $ 65,516 ☒x2021☐o
Identification No.), FL(850) (850) 480-7955 YES ☐Yes No☒ ☒No YESYes ☐o No Nox ☒Yesx ☒No Noo ☐YES ☐Yes Nox ☒No x ☐o ☐o ☒o ☐o ☒☒o☐If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. oYES ☐Yes Noo No ☒x2021,2023, the last business day of the registrant’s most recently completed second fiscal quarter, held by non-affiliates was $2,845,823,537
As of March 23, 2022,February 22, 2024, there were 134,966,243160,052,094 Subordinate Voting Shares, 49,217,89926,226,386 Multiple Voting Shares (on an as converted basis) and zero Super Voting Shares (on an as converted basis) outstanding.20222024 Annual Meeting of Stockholders (the “2022“2024 Proxy Statement”). The 20222024 Proxy Statement will be filed by the registrant with the Securities and Exchange Commission not later than 120 days after December 31, 2021,2023, the end of the registrant’s fiscal year.Page PageBusiness215Item 1.4Item 1A.3853302.53315332533254335635573671527353735373547455745575567556755675567556755779611Explanatory ParagraphThis amendment No. 1 on Form 10-K/A (the “Amendment”) amends Trulieve Cannabis Corp.’s (the “Company’) Annual Report on Form 10-K for the year ended December 31, 2021, as originally filed with the Securities and Exchange Commission on March 30, 2022 (the ‘Original Filing’). The Company is filing this Amendment solely to include an additional paragraph in the opinion of its independent auditor, Marcum LLP, describing their procedures performed in auditing the adjustments related to the change in accounting principle and the revision of previously issued financial statements, as described in Note 2 to the consolidated financial statements and their opinion on the appropriateness and proper adjustments related to such. Under the new principle, tax basis is determined by applying the relevant tax laws, whereas previously, tax basis was determined by the future deductibility of the recovery or settlement. As required by Rule 12b-15 under the Securities Exchange Act of 1934, as amended, new certifications by the Company’s principal executive officer and principal financial officer are filed as exhibits to this Amendment under Item 15 of Part IV hereof, and included these files in Item 15 Exhibits 31. We have also updated our XBRL information to be based on the 2022 taxonomy. In addition, new consents of Marcum LLP and MNP LLP have been included in Item 15 Exhibits 23.1 and 23.2.No other changes were made to the Original Filing. Except as stated herein, this Amendment does not reflect events occurring after the date of the Original Filing, nor does it modify or update any of the financial or other disclosures as presented in the Original Filing. Information not affected by this Amendment remains unchanged and reflects the disclosures made at the time the Original Filing was filed.2wholly-owned subsidiaries.ten states and has received notice of intent to award a license in an eleventh state.nine states. Headquartered in Quincy, Florida, we are the market leader for quality medicallargest cannabis products and servicesretailer in Florida and we havethe United States with market leading retail operations in Arizona, Florida, Georgia, Pennsylvania, and Pennsylvania. By providing innovative,West Virginia. We are committed to delivering exceptional customer experiences through elevated service and high-quality products across our brand portfolio, webranded products. We aim to be the brand of choice for medical and adult-use customers in all of the major markets that we serve. We operateThe Company operates in highly regulated markets that require expertise in cultivation, manufacturing, retail and logistics.retail. We have developed proficiencies in each of these functionsfunctional areas and are committed topassionate about expanding access to high qualityregulated cannabis products through advocacy, education and delivering exceptional customer experiences.expansion of our distribution network.adopted legislationdeveloped programs to permit the use of cannabis products for medicinal purposes to treat specific conditions and diseases, which we refer to as medical cannabis. Recreational marijuana,cannabis, or adult-use cannabis, is legal marijuanacannabis sold in licensed dispensaries to adults ages 21 and older. Thus far, of the states in which we operate, only Arizona, California, Colorado, Connecticut, Massachusetts,Maryland, and NevadaOhio, have adopted legislationalready or are in the process of developing and launching programs permitting the commercialization of adult-use cannabis products. Trulieve operates its business through its directly and indirectly owned subsidiaries that hold licenses and have entered into managed service agreements in the states in which they operate. As of March 16, 2022, we operated 162 dispensaries, with 113 dispensaries in Florida, 19 affiliated dispensaries in Pennsylvania, 17 dispensaries in Arizona, five dispensaries in California, three dispensaries in Maryland, two dispensaries in Massachusetts, two dispensaries in West Virginia and one dispensary in Connecticut, and we operated cultivation and processing facilities in Arizona, Colorado, Florida, Maryland, Massachusetts, Nevada, Pennsylvania, and West Virginia. 2021,2023, the Company operated the following:State Number of Dispensaries Number of Cultivation and Processing Facilities Florida 131 6 Arizona 21 3 Pennsylvania 20 3 West Virginia 10 1 Georgia 5 1 Maryland 3 1 Ohio 1 — Connecticut 1 — Colorado — 1 Total 192 16 employed over 9,000 people and we are committed to providing patients and adult consumers, which we refer to herein as “customers,” a consistent and welcoming retail experience across Trulieve branded stores and affiliated retail locations.Our business and operations center around the Trulieve brand philosophyhave achieved varying percentages of “Customers First” which permeates our culture beginningvertical integration with high- quality and efficient cultivation and manufacturing practices, focus on the consumer experience at Trulieve brandedprocessing operations to support our retail and affiliated retail locations, at our in-house call center and where available at customer residences through a robust home delivery program.wholesale businesses. Our investments in vertically integrated operations in several of our markets afford us ownership of the entire supply chain, which mitigates third-party risks and allows us to completely control product quality and brand experience. We believe that this is contributive to high customer retention and brand loyalty. We successfully operate our core business functions of cultivation, production and distribution at scale, and are skilled at rapidly increasing capacity without any interruption to existing operations.has identified five regional geographic hubs in the U.S. and has established cannabis operations in three of the five hubs: Southeast, Northeast, and Southwest. In each of our three regional hubs we have market leading positions in cornerstone states and additional operations and assets in other state markets. Our hubs are managed by national and regional management teams supported by our corporate headquarters in Florida.Southeast HubOur southeast hub operations are anchored by our cornerstone market of Florida. Trulieve was the first licensed operator in the medical market in Florida with initial sales in 2016. Publicly available reports filed with the Florida Office of Medical Marijuana Use show Trulieve has the most dispensing locations and the greatest dispensing volume across product categories out of all licensed medical marijuana businesses in the state as of December 31, 2021. Trulieve cultivates and produces all of its productsemploys an in-house and distributes those products to customers in Trulieve branded stores (dispensaries) throughout Florida,quality team as well as via home delivery.In accordance with Florida law, Trulieve grows alltesting laboratories in select markets, both of its cannabis in secure enclosed indoor facilities and greenhouse structures. In furtherance of our customer-first focus, we have developed a suite of Trulieve branded products, including flower, edibles, vaporizer cartridges, concentrates, topicals, capsules, tinctures, dissolvable powders, and nasal sprays. This wide variety of products gives customers the abilitywhich allow us to select themore tightly control product that consistently delivers the desired effect and in their preferred method of delivery. These products are delivered to customers statewide in Trulieve-branded retail stores and by home delivery.quality.In Georgia, Trulieve received a Notice of Intent to award a Class 1 Production License from the Georgia Access to Medical Cannabis Commission in July 2021. The Notice of Intent to award is a notice of the Georgia Access to Medical Cannabis Commission’s expected contract award to Trulieve GA pending resolution of a protest process. If the contract is awarded, Trulieve GA will hold one4of two Class 1 Production Licenses in the state and will be permitted to cultivate cannabis for the manufacture of low tetrahydrocannabinol, or THC oil.Northeast HubOur northeast hub operations are anchored by our cornerstone market of Pennsylvania.We conduct cultivation, processing, and retail operations through its direct and indirect subsidiaries with permits for retail operations and grower/processor operations in Pennsylvania. These subsidiaries operate cultivation and processing facilities in McKeesport, Reading, and Carmichael, Pennsylvania to support our affiliated network of retail dispensaries and wholesale distribution network across the state.We operate three medical dispensaries and conducts wholesale sales supported by cultivation and processing in Hancock, Maryland.We operate two retail dispensaries in Massachusetts, serving medical patients and adult use customers in Northampton and adult use customers in Worcester. Our retail operations are supported by cultivation and manufacturing operations in Holyoke. We commenced wholesale sales in September 2021. Trulieve was the first to offer sales of clones supporting home grow for residents in the Massachusetts market in August 2021.We operate a medical cannabis dispensary located in Bristol, Connecticut. Under Connecticut’s adult-use cannabis legislation, which was enacted July 1, 2021, Trulieve can seek regulatory approval to expand sales at this dispensary to include adult use sales.We operate two medical dispensaries in Morgantown and Weston, West Virginia, supported by cultivation and processing operations in Huntington, West Virginia. Trulieve has been awarded and has acquired permits to operate up to a total of nine dispensaries in West Virginia.Southwest HubOur southwest hub operations are anchored by our cornerstone market of Arizona. In Arizona, Trulieve holds a market-leading position, offering medical and adult use customers a wide range of branded and third-party products, including brand partner products, as well as offering wholesale across the state. We also serve medical and adult use customers in California. Trulieve conducts wholesale operations in Nevada and Colorado, serving the medical and adult use markets in each state.Processing CapacityManufacturing of Cannabis Productsthis high-quality biomass into the extensive portfolio of products sold inthrough our storesretail and in our wholesale channels. Our prominence in our cornerstone market of Florida demonstrates the quality and affordability of the product we produce at scale.distribution network. With a focus on replicable, scalable operations, we have detaileddeveloped design standards, standard operating procedures, and training protocols that are employed across cultivation sites to achieve a high level of consistency and quality.our cornerstone market of Florida Trulieve cultivates, processes, and manufactures all cannabis products sold in our Florida dispensaries. In other markets including Arizona, Maryland, Massachusetts, Pennsylvania, and West Virginia, we have achieved varying percentages of vertical integration with cultivation and processing operationswhere demand is high enough to support larger scale production, our retailrecently ramped state-of-the-art 750,000 square foot indoor cultivation facility affords us greater flexibility for pricing, promotional cadence, and wholesale businesses. Trulieve investsassortment in a large numberthe Florida market by enabling production of in-househigh potency and high quality personnel as well as testing laboratories, both of which allow us to control quality in all aspects of our business while operatingproducts at scale.lower costs.super criticalsupercritical ethanol extraction, carbon dioxide extraction, hydrocarbon extraction, and mechanical separation. We have invested in light hydrocarbon extraction processes, allowing for concentrates that preserve the natural ratios of cannabinoids, terpenes, and other target compounds to better replicate the flower experience. Light hydrocarbon extraction also offers the benefit of greater extraction yields in many cases. In addition, we own CO2 extraction, distillation, purification and manufacturing technology used to produce a line of cannabis topicals and vapes featuring cannabinoidscannabinoids.hemp-derived product line soldcurated portfolio of our own branded retail products that we cultivate, manufacture and distribute throughout our branded retail locations. By providing customers with consistent high-quality products and outstanding experiences we aim to garner a large and loyal customer base across our distribution network.Colorado.Marketingselect markets and Community Outreachretail locations, providing our customers with access to greater variety and specialty brands. Brand partnerships include arrangements with Alien Labs, Bellamy Brothers, Binske, Black Tuna, Blue River, Connected, DeLisioso, Khalifa Kush, Love’s Oven, Miami Mango, Moxie, Seed Junky, SLANG, and Sunshine Cannabis.developedtailored to maximizeaddress the unique opportunitiesattributes of the markets in which we operate. InGenerally, in markets where we serve medical markets,patients, our marketing efforts are centeredmessaging centers around education and outreach for physicians and medical patients. Our educational materials are designed to help physicians understand cannabinoid science, the high standards pursuant to which our plants are cultivated, the processes required for regulatory compliance, and how our products provide relief for their patients. Our dedicated physician5education team delivers in-person outreach to hundreds of physicians each month as well as immediate phone support through a dedicated physician education team member in our call center. Patients primarily learn about us through their physicians, patient-centric community events, and digital marketing. We regularly participate in dozens of outreach and community events on a monthly basis.events. An engaged audience is captured through our digital content marketing and via multiple popular social media platforms.adult useadult-use markets, our marketing efforts driveaim to attract customers with varying levels of awareness of the Trulieve brandcannabis and Trulieve. We continue to delineate and refine our understanding of various customer personas, which factors such as well as our branded product portfolio directly to the end customer. Our marketing towardslocation, products and pricing attract and retain customers, whoand which incentives are more familiareffective in driving specific outcomes. Connecting with cannabis productsa broader audience requires different strategies that inspire, tap into relevant cultural moments in their lives, build community as well as educate customers on our products’ uniqueness versus our competitors.A core element of our strategy centers around the distribution of branded products through our retail locations and wholesale channels. We strive to set a high standard for excellence and deliver excellent customer experiences in our retail locations. We have developed and acquired a portfolio of our own branded retail products that we cultivate, manufacture and distribute in our regional hubs. Trulieve brands include premium tier brands Avenue, Cultivar Collection, and Muse; mid-tier brands Modern Flower, Alchemy, Momenta, and Sweet Talk, and value tier brands Co2lors, Loveli, and Roll One. We have a dedicated research and development team focused on product development and technological innovation. Our R&D team evaluates new technologies and performs rigorous testing prior to recommending new products for introduction into production. The team monitors developments in the fast-paced cannabis industry and adjacent industries to help us remain competitive.We have also developed relationships with brand partners that allow for the sale of partner branded products in select markets and retail locations. Brand partnerships include arrangements with Bellamy Brothers, Bhang, Binske, Black Tuna, Blue River, Connected, El Blunto, Love’s Oven, Moxie, SLANG, and Sunshine Cannabis.It is our goal to generate brand loyalty by providing customers with industry-leading branded products and superior service in an appealing, approachable setting. We accomplish this goal through several key strategies: training; branded store experiences; brand awareness; multiple channels of distribution; our loyalty program and 360 brand activation that builds community across platforms.Customer experience is an area of significant focus for Trulieve. We employ and continuously improve numerous training programs and methods to provide our front-line workers with the resources and information they need to provide customers with an excellent experience across all Trulieve locations. We offer specialized management training so there is daily reinforcement of customer experience best practices.As part of our customer centric approach, in select markets we offer various purchase options, including phone ordering, online ordering, home delivery, and in store.Our Truliever loyalty program provides customers the ability to earn points for dollars spent and receive discounts when their points exceed specified thresholds. Trulievers are also the first to be informed about special discounts or limited product releases and they are invited to exclusive promotions and events. engage with various communities who may benefit, such as veterans, seniors, organizations that serve qualifying populations,are able to collect and various healthanalyze data to discern customer preferences, patterns, and wellness groups. Search engine optimization oftrends which inform our website also captures potential customers researching the benefits of medical marijuana, which offers another pathway to informative materials about therapeutic uses of cannabis,production mix, product allocation, promotional strategies and targeted outreach. Investments in our productsenterprise grade platforms enable greater sophistication across production, retail, and how to legally access them.Recent DevelopmentsHarvest AcquisitionOn May 10, 2021, the Company entered intowholesale operations and numerous support functions including accounting and finance, human resources, legal and compliance. We believe infrastructure and data capabilities are prerequisites for long term success in an Arrangement Agreement (the “Arrangement Agreement”) with Harvest Health & Recreation Inc., a British Columbia corporation (“Harvest”), pursuant to which the Company agreed to acquire all of the issuedincreasingly competitive and outstanding equity securities of Harvest (the “Arrangement”). On October 1, 2021 (the “Closing Date”), the Company completed its acquisition of Harvest pursuant to the Arrangement Agreement. On the Closing Date, the Company acquired all of the issued and outstanding subordinate voting shares, multiple voting shares and super voting shares (collectively the “Harvest Shares”) of Harvest. Pursuant to the terms of the Arrangement Agreement, holders of Harvest Shares received 0.1170 of a Subordinate Voting Shareintegrated commerce environment. for each Harvest Share held. In total, the Company issued an aggregate of 50,921,236 Trulieve Shares, representing total consideration of approximately $1.4 billion based on the closing price of Trulieve’s Subordinate Voting Shares on September 30, 2021.Prior to the acquisition, Harvest was one of the largest multi-state vertically integrated operators in the cannabis industry in the United States operating from “seed to sale.” Harvest is one of the largest operators in the state of Arizona, which is one of the largest medical and recreational cannabis markets in the country and one of the oldest regulated cannabis markets in the world. Harvest operates6facilities or provides services to cannabis dispensaries in Arizona, California, Colorado, Florida, Maryland, Nevada and Pennsylvania. In addition, Harvest owns CO2 extraction, distillation, purification and manufacturing technology used to produce a line of cannabis topicals, vapes and gems featuring cannabinoids and a hemp-derived product line sold in Colorado. We expect the Harvest acquisition will have a significant impact on our results of operations and financial condition as of and for the fourth quarter ending December 31, 2021 and in future periods as we integrate Harvest’s business with our existing operations.Other DevelopmentsOn December 24, 2021, the Board of Directors of the Company appointed Steve White as President of the Company.On October 6, 2021, the Company closed its previously announced private placement of 8% Senior Secured Notes (the “2026 Notes”) for aggregate gross proceeds of $350.0 million and net proceeds of $342.6 million. The 2026 Notes were issued at 100% face value, bear an interest rate of 8% per annum payable semi-annually in equal installments until the maturity date, unless earlier redeemed or repurchased. The 2026 Notes will mature on October 6, 2026, and may be redeemed in whole or in part, at the Company's option, at any time, on or after October 6, 2023, at the application redemption price set forth in the indenture. The Company used a portion of the net proceeds to redeem certain outstanding indebtedness of Harvest, and intends to use the remaining portion of the net proceeds for capital expenditures and other general corporate purposes. On January 28, 2022, we closed a second tranche of our Notes totaling $75 million.On September 29, 2021, the Board of Directors of the Company appointed Rebecca Young as Vice President and Chief Accounting Officer (and principal accounting officer) of the Company.On July 8, 2021, we closed the acquisition of Keystone Shops, which holds a dispensary license and operates dispensaries in Philadelphia, Devon, and King of Prussia, Pennsylvania.On July 2 and June 28, 2021, we closed of the acquisition of certain assets of PCMV and Nature’s Remedy, respectively, including the rights to a Provisional Marijuana Retailer License from the Massachusetts Cannabis Control Commission for an adult-use marijuana retailer in Framingham and a Final Marijuana Retailer License from the Cannabis Control Commission for an adult-use marijuana retailer in Worcester, and accompanying leasehold interests, permits and entitlements.On June 9, 2021, we announced the closing of the acquisition of Solevo Wellness West Virginia LLC and its three West Virginia dispensary permits.On June 3, 2021, Life Essence opened its first dispensary in the Commonwealth of Massachusetts in the City of Northampton. The dispensary serves both adult-use and medical marijuana patients.On May 6, 2021, we announced the closing of the acquisition of Mountaineer Holding LLC, including its cultivation permit and two dispensary permits.On April 12, 2021, we announced the closing of an underwritten, marketed public offering of 5,750,000 Subordinate Voting Shares at a public offering price of C$50.00 per share ($39.63 per share after giving effect to the conversion rate published by Bloomberg at 4:30pm ET on April 7, 2021 to convert Canadian dollars to U.S. dollars). The gross proceeds from the offering, before deducting underwriting discounts and commissions and offering expenses payable by us, were C$287.5 million (or $228.5 million after giving effect to the conversion rate denoted above). The net proceeds from the offering were approximately $217.9 million.Corporate History7effectaffect a transaction, or the Transaction, whereby Trulieve US and Subco merged, and Trulieve US became a wholly-owned subsidiary of Trulieve Cannabis Corp.8adult useadult-use cannabis in our markets increases, the demand for products will increase and we expect that competition will become more intense, as current and future competitors begin to offer an increasing number of diversified products. We expect to continue to invest in several areas, including customer experience, product development and innovation, scaled production, marketing and branding, sales and distribution and customer service and retention.network expansion. Trulieve may not have sufficient resources to maintain investments on a competitive basis, which could materially and adversely affect our business, financial condition and results of operations. The management team monitors developments in the fast-paced cannabis industry and adjacent industries to help us remain competitive.Key Business ObjectivesTrulieve will continue to focus on achieving our vision and mission to be a leading customer-focused cannabis brand in the U.S., with depth in select markets with favorable attributes relative to our strategic vision. We aim to provide the highest level of cannabis products and customer experience through authentic and reciprocal relationships.Our strategic priorities include the following:• Developing exceptional customer experiences and building brand loyalty through:▪Providing superb service, expedient transactions, frictionless returns; and▪Innovating across product and consumer categories• Continuing to execute on our hub strategy by:▪Building additional scale and depth in our cornerstone markets of Arizona, Florida, and Pennsylvania;▪Expanding operations as appropriate in new and existing markets; and▪Pursuing organic license awards and strategic acquisition opportunities• Distributing branded products through branded retail locations and wholesale network by:▪Expanding distribution of branded products through branded retail locations;▪Converting acquired, affiliated, and/or operated retail locations to Trulieve branded retail; and▪Developing and expanding wholesale channels with initial emphasis on Arizona, Massachusetts, Maryland, and Pennsylvania markets• Focusing on profitable growth and prudent capital allocation.9Trulieve LeasesWe own or lease our facility locations including cultivation, processing, retail and corporate office locations. We do not have any one lease representing over 10% of our consolidated leasing costs and, as a result, do not consider any of our leases to be material. In Florida, we have 111 operational dispensaries, of which 109 are leased, and we have fourteen cultivation, manufacturing and distribution facilities, of which six are leased. In Arizona, we have seventeen operational dispensaries, of which thirteen are leased. In California, we have five operational dispensaries, of which three are leased. We have a single operational manufacturing facility in Colorado that is leased. We have one operational dispensary in Connecticut that is leased. In Georgia, we own land that is in the process of being developed into a cultivation facility. In Massachusetts, we operate two operational dispensaries, both of which are leased, and operate two separate cultivation properties, one of which is leased. We have three operational dispensaries in Maryland, of which two are leased, and we also operate a cultivation, manufacturing and distribution facility on adjacent leased properties. In Nevada, we lease a single cultivation, distribution and manufacturing facility. In Pennsylvania, there are eighteen operational affiliated dispensaries, seventeen of which are leased, and there are three affiliated cultivation and manufacturing facilities, of which a portion of one is leased. In West Virginia, we have two dispensaries and a cultivation facility that are leased and we own real property where additional cultivation operations are being be developed.Specialized SkillsWe recruit talented individuals to join the Trulieve team. Our employees have a wide range of skill sets, including employees with PhD and master’s degrees. Many of our employees are college graduates and have specific skills related to their job function. We intend to continue to build out our research and development team with scientists and other technical specialists. We use a variety of recruiting techniques, including online resources as well as recruiting professionals, to assist with filling specialized roles.Supply ChainWe have varying degrees of vertical operations in the markets in which we operate. In the Florida market, our operations are fully vertically integrated as required by state regulations. In other markets we may operate within one or more segment of the cannabis value chain. In the normal course of our business we purchase input materials and components that we utilize in the cultivation, processing, manufacture and distribution of our products. No individual suppliers represent a significant portion of our purchases or represent a material risk to our operations.Brand Recognition and Intellectual PropertyWe have built brand recognition throughout our markets. One of our key business objectives is to build brand equity in the Trulieve brand name through our branded retail stores and interactions with our customers. We intend to rebrand acquired and affiliated locations over time as part of our plan to expand our branded retail reach. Trulieve maintains a consistent approach to the design of its retail stores and endeavors to create a uniform experience for its customers.We regularly seek to protect our intellectual property rights in connection with our operating names and our brand names. The U.S. trademark statute, The Lanham Act, allows for the protection of trademarks and service marks on products and services used, or intended for use, lawfully. Because cannabis-related products and services remain illegal at the federal level under the Controlled Substances Act, we are not able to fully protect our intellectual property at the federal level; therefore, we currently seek trademark protections at the state level where commercially feasible. Nonetheless, our success depends upon other areas of our business such as product development and design, production and marketing and not exclusively upon trademarks and trade secrets.We have developed proprietary cultivation techniques. We have also developed certain proprietary intellectual property for production best practices, procedures and methods. This requires specialized skills in cultivation, extraction and refining.We rely on non-disclosure/confidentiality agreements to protect our intellectual property rights. Where commercially feasible, we will proactively seek intellectual property protection for newly developed brands as well as for expansion of existing brands across current markets and into new markets. We own several website domains numerous, social media accounts across all major platforms and various phone and web application platforms.Year-Round Businessevents.events including 4/20, 7/10, and Green Wednesday (the Wednesday before Thanksgiving).10Diversity, InclusionEquity (DE&I)We are committedaccounting experience serving middle-market, private-equity backed and public companies. Prior to contributing positivelyjoining the Company, Mr. Getman served as Partner, Advisory at WilliamsMarston, LLC. Mr. Getman previously served as the Vice President of Accounting & Finance at Blue Bird Corporation (Nasdaq: BLBD), a billion dollar North American manufacturer, from 2015-2020. In addition, Mr. Getman spent 16 years in public accounting at PwC, RSM, and Grant Thornton where he was a partner at the last two firms. Mr. Getman received his Bachelor of Science in Management from the State University of New York at Geneseo and his MBA from the Simon School at the University of Rochester.legal cannabis industry. AsCompany, from March 2020 through January 2024, Ms. Zhang served as the Chief Supply Chain Officer for Blaze Pizza, LLC and from July 2015 to March 2020, she served as the Global Vice President Supply Chain for FOCUS Brands LLC. Previously, Ms. Zhang served as Chief Supply Chain Officer for Yum! Brands (NYSE: YUM) from February 2013 to July 2015, Vice President Supply Chain at The Honey Baked Ham Company, LLC from February 2004 to February 2013 and as Director – Research & Development, Food Safety and Quality Assurance at Conagra Brands, Inc. (NYSE: CAG) from January 1997 to February 2004. Ms. Zhang received her Bachelor of Science in Chemistry from Jilin University and a business that producesMasters Degree in Food Science and distributes a product that many people– especially people of colorTechnology from Iowa State University.were arrested and incarcerated for in the past, we recognize the supreme importance of promoting diversity, equity, and inclusion in the cannabis industry. As such, we have hired a Director of DE&I and continue to support a DE&I Committee comprised of executives, senior management, and employees throughout the Company. The DE&I Committee is chargedIssuers with implementing and recording the efficacy of our efforts to recruit and develop diverse talent, implement company-wide diversity and cultural competency training, increase supplier diversity, engage in social justice initiatives and more.Environmental, Social, and Governance (ESG)As a purpose-driven company, ESG is integral to our growth and corporate achievements. Our commitment to ESG makes us better planners, cultivators, and retailers, supports transparency and strong governance, contributes to improved safety and environmental performance, and strengthens our connection with local communities. We have established governance policies and management systems, such as our SAP system implemented last year, to codify our responsibilities and help manage risk across the company. We also implemented other measures including environmental safeguards at our cultivation facilities, recycling initiatives, and safety training for security personnel at our dispensaries.Regulatory OverviewBelowU.S. Cannabis-Related Activities (“Staff Notice 51-352”), below is a discussion of the federal and state-level U.S.United States regulatory regimesbodies in those jurisdictions where we arethe Company is currently directly involved, through ourits subsidiaries, in the cannabis industry.Marijuana,Cannabis, which refers to certain parts and derivatives of the cannabis plant, is classified as a Schedule I controlled substance. As a Schedule I controlled substance, the federal Drug Enforcement Agency, or DEA, considers marijuanacannabis to have a high potential for abuse, no currently accepted medical use in treatment in the United States, and a lack of accepted safety for use of the drug under medical supervision. According to the U.S.Under federal government,law, cannabis and cannabis related products having a concentration of delta-9 tetrahydrocannabinol, or THC, greaterof more than 0.3% is marijuana.cannabis. Cannabis with a THC content below 0.3% or less is classified as hemp. The scheduling of marijuanacannabis as a Schedule I controlled substance is inconsistent with what we believethe US Department of Health and Human Services (HHS) recent recommendation to be widely acceptedreclassify cannabis to Schedule III based on its conclusion that it has medical usesuse in treatment in the United States and a lower potential for marijuana by physicians, researchers, customers,abuse than drugs in Schedule I and others.Schedule II. Moreover, as of December 31, 2021, and2023, despite the conflict with U.S. federal law, at least 36nearly all states and Puerto Rico have legalized cannabis for medical use. Cannabis is legal for adult-use in 24 states plus the District of Columbia, the Commonwealth of the Northern Mariana Islands, Guam, Puerto Rico, and the U.S. Virgin Islands have legalized marijuana for medical use. Eighteen of those states and the District of Columbia, the Commonwealth of the Northern Mariana Islands, and Guam have legalized the adult use of cannabis for recreational purposes. In November 2020, voters in Arizona, Montana, New Jersey, and South Dakota voted by referendum to legalize marijuana for adult use, and voters in Mississippi and South Dakota voted to legalize marijuana for medical use, although South Dakota’s adult-use measure has been declared unconstitutional by the State Supreme Court. In 2021, the states of Connecticut, New Mexico, New York, and Virginia enacted laws legalizing the adult use of cannabis.U.S. Virgin Islands.Marijuanalargelyprimarily regulated at the state level in the United States. State laws regulating marijuana conflict with the CSA, making marijuana use and possession federally illegal. Although certain states and territories of the United States authorize medical or adult-use marijuanacannabis production and distribution by licensed or registered entities, under United States federal law, the possession, use, cultivation, and transfer of marijuana and any related drug paraphernaliacannabis is illegal. Although our activities are compliant with the applicable state and local laws in those states where we maintain such licenses, strict compliance with state and local laws with respect to cannabis may neither absolve us of liability under United States federal law nor provide a defense to any federal criminal action that may be brought against us.frameworks. Until 2018, the federal government provided guidance to federal agencies and banking institutionsframeworks through a series of DOJ memoranda.Department of Justice (DOJ) memoranda stating it would not be a priority to prosecute cannabis activity compliant with state medical cannabis laws and that did not implicate certain federal enforcement priorities. The most notable of this guidance came in the form of a memorandum issued by former U.S. Deputy Attorney General James Cole on August 29, 2013, which we refercommonly referred to as the Cole Memorandum.marijuanacannabis in all states and quickly set a standard with which marijuana-relatedcannabis-related businesses would comply. TheIn sum, the Cole Memorandum put forth eightstated the DOJ’s prosecution priorities:1. Preventingpriorities would be aimed at preventing the distribution of marijuanacannabis to minors;2. Preventing preventing revenue from the sale of marijuana from going to criminal enterprises, gangs, and cartels;113. Preventing the diversion of marijuana from states where it is legal under state law in some form to other states;\4. Preventing the state-authorized marijuana activity from being used as a cover or pretext for the trafficking of other illegal drugs or other illegal activity;5. Preventingenterprises; preventing violence and the use of firearms in the cultivation and distribution of marijuana;6. Preventingcannabis; preventing drugged driving and the exacerbation of other adverse public health consequences associated with marijuana use;7. Preventing the growing of marijuana on public landscannabis; and the attendant public safety and environmental dangers posed by marijuana production on public lands; and8. Preventing marijuanapreventing cannabis cultivation, possession, or use on federal property.On 4, 2018 former United States Attorney General Sessions rescinded the Cole Memorandum by issuingissued a new memorandum to all United States Attorneys which we refer to as the Sessions Memo. Rather than establishing national enforcement priorities particular to marijuana-related crimes in jurisdictions where certain marijuana activity was legal under state law, the Sessions Memo simply(the “Sessions Memo”) that rescinded the Cole Memorandum and other Department of Justice memorandumsDOJ memoranda providing prosecutorial guidance on state and tribally authorized medical and adult-use cannabis activities and instructed that “[i]n deciding which marijuana activities to prosecute... with the [DOJ’s] finite resources, prosecutors should follow the well- established principles that govern all federal prosecutions.” Namely, these include the seriousness of the offense, history of criminal activity, deterrent effect of prosecution, the interests of victims, and other principles. Although rescinded, the tenets of the Cole Memorandum continue to be adhered to by state-legal cannabis businesses and those in compliance with adult-use and medical programs throughout the country operate without federal enforcement.was confirmed by the United States Senate on March 10, 2021. It is not yet known whether the Department of Justice, under President Biden and Attorney General Garland, will re-adopt the Cole Memorandum or announce a substantive marijuana enforcement policy. Duringmade comments to Senator Cory Booker (D-NJ) during his Senate confirmation Merrick Garland told Senator Cory Booker (D-NJ), “It doesindicating that he believed prosecution of state-legal cannabis businesses was not seem to me useful thea worthy use of limitedDOJ resources, that we have to be pursuing prosecutions in states that have legalized and are regulating the use of marijuana, either medically or otherwise.” Suchsuch statements are not official declarations or policies of the DOJ and are not binding on the DOJ or any United StatesU.S. Attorney or the United States federal courts.court. Substantial uncertainty regarding United States federal enforcement remains. To date, there havehas been no new federal cannabis memorandumsguidance issued by the Biden AdministrationDOJ or any published change in federal enforcement policy under the Biden administration. However, in October of 2022, the Biden Administration announced its intention to end the country’s “failed approach” to cannabis and directed the Secretary of Health and Human Services (“HHS”) and the Attorney General to expeditiously review cannabis’s Schedule I status. Concurrently, President Biden also announced a pardon of all prior federal simple possession of cannabis offenses and urged governors to do the same at the state level.there is no guaranteeeven if moved to Schedule III, the cultivation, manufacture, distribution, and sale of cannabis by state-regulated businesses that state laws legalizing and regulating the sale and use of marijuana willdo not be repealedproduce or overturned or that local government authorities will not limit the applicability of state laws within their respective jurisdictions.sell FDA regulated products remains illegal under federal law. Unless and until the United States Congress amends the CSA with respect to marijuana (and as to the timing or scope of any such potential amendments, there can be no assurance),cannabis, there is a risk that federal authorities may enforce current U.S. federal law. Currently, in the absence of uniform federal guidance, as had been established by the Cole Memorandum, enforcement priorities are determined by respective United States Attorneys.1. Continuously monitor our operations for compliance with all licensing requirements as established by the applicable state, county, municipality, town, township, borough, and other political/administrative divisions;2. Ensureensure that our cannabis-related activities adhere to the scope of the licensing obtained (for example: in the states where cannabis is permitted only for adult-use, the products are only sold to individuals who meet the requisite age requirements);3. Implement•implement policies and procedures to prevent the distribution of our cannabis products to minors;4. Implementimplement policies and procedures in place to avoid the distribution of the proceeds from our operations to criminal enterprises, gangs, or cartels;5. Implementimplement an inventory tracking system and necessary procedures to reliably track inventory and prevent the diversion of cannabis or cannabis products into those states where cannabis is not permitted by state law or across any state lines in general;6. Monitormonitor the operations at our facilities so that our state-authorized cannabis business activity is not used as a cover or pretense for trafficking of other illegal drugs or engaging in any other illegal activity; and127. Implementimplement quality controls so that our products comply with applicable regulations and contain necessary disclaimers about the contents of the products to avoid adverse public health consequences from cannabis use and discourage impaired driving. other illegal drugs, engaged in illegal activity or activities involving violence, or the use of firearms in the cultivation, manufacturing, or distribution of cannabis. We also conduct ongoing reviews of the activities of our cannabis businesses, the premises on which they operate, and the policies and procedures related to the possession of cannabis or cannabis products outside of the licensed premises.in recent years, certain temporary federal legislative enactments that protect the medical marijuana and hemp industries have also been in effect. For instance, certain marijuanacannabis businesses receive a measure of protection from federal prosecution by operation of temporary appropriations measures that have been enacted into law as amendments (or “riders”) to federal spending bills passed by Congress and signed by Presidents Obama, Trump, and most recently, President Biden. For instance, in the Appropriations Act ofEvery fiscal year since 2015, Congress included a budgethas passed an appropriations “rider” that prohibitsbarring the DOJ from expending anytaxpayer funds to enforce any law that interferes with a state’s implementation of its own medical marijuanacannabis laws. The rider, originally known as the “Rohrbacher-Farr” Amendment after its original lead sponsors is now known as the “Joyce” Amendment after its current sponsor. Originally, a Republican-controlled House and Democratic-controlled Senate passed the Rohrbacher-Farr Amendment. The bill was “a bipartisan appropriations measure that looks to prohibit the DEA from spending funds to arrest state-licensed medical marijuana patients and providers.” Subsequently, the rider tamendment, has been included in multiple budgets passed by successive Congresses controlled by both major political parties. Most recently, on February 18, 2022, the Amendment wasmedical cannabis appropriations rider is expected to be renewed through the signing of an additional stopgap spending bill, H.R.6617 - Further Additional Extending Government Fundingthe “Further Continuing Appropriations and Other Extensions Act, effective through March 11, 2022.2024.” While the Amendmentrider has been included in successive appropriations legislation or resolutionsbudget cycles since 2015, its inclusion or non-inclusion is subject to political change.Joycethe Rohrbacher-Farr Amendment has applied only to medical marijuanacannabis programs and has not provided the same protections to enforcement against adult-use activities. If the Amendmentrider is no longer in effect, the risk of federal enforcement and override of state marijuanacannabis laws would increase.United States Border EntryThe United States Customs and Border Protection, or CBP, enforces the laws of the United States as they pertain to lawful travel and trade into and out of the U.S. Crossing the border while in violation of the CSA and other related United States federal laws may result in denied admission, seizures, fines, and apprehension. CBP officers administer determine the admissibility of travelers who are non-U.S. citizens into the United States pursuant to the United States Immigration and Nationality Act. An investment in our Subordinate Voting Shares, if it became known to CBP, could have an impact on a non-U.S. citizen’s admissibility into the United States and could lead to a lifetime ban on admission.Because marijuana remains illegal under United States federal law, those investing in Canadian companies with operations in the United States cannabis industry could face detention, denial of entry, or lifetime bans from the United States for their business associations with United States marijuana businesses. Entry happens at the sole discretion of CBP officers on duty, and these officers have wide latitude to ask questions to determine the admissibility of a non-US citizen or foreign national. The government of Canada has started warning travelers that previous use of marijuana, or any substance prohibited by United States federal laws, could mean denial of entry to the United States. Business or financial involvement in the marijuana industry in the United States could also be reason enough for CBP to deny entry. On September 21, 2018, CBP released a statement outlining its current position with respect to enforcement of the laws of the United States. It stated that Canada’s legalization of cannabis will not change CBP enforcement of United States laws regarding controlled substances and because marijuana continues to be a controlled substance under United States law, working in or facilitating the proliferation of the legal marijuana industry in U.S. states where it is deemed legal may affect admissibility to the United States. As a result, CBP has affirmed that employees, directors, officers, managers, and investors of companies involved in business activities related to marijuana in the United States (such as Trulieve), who are not United States citizens, face the risk of being barred from entry into the United States.(USA("USA PATRIOT Act)Act"), and any related or similar rules, regulations or guidelines, issued, administered or enforced by governmental authorities in the United States.takeprovide services to the cannabis businesses, including taking any proceeds from the sale of any Schedule I controlled substance. For example, banks and other financial13institutions could potentially be prosecuted and convicted of aiding and abetting money laundering undersubstance or otherwise introducing them into the Bank Secrecy Act for providing services to cannabis businesses. Therefore, under the Bank Secrecy Act, banks or other financial institutions that provide a cannabis business with a checking account, debit or credit card, small business loan, or any other financial service could be charged with money laundering or conspiracy.United States banking system.marijuana, Thecannabis, in 2014 the U.S. Department of the Treasury Financial Crimes Enforcement Network “FinCEN”(“FinCEN”) issued guidance in 2014 and was not rescinded by the Sessions Memo. The FinCEN Guidance provided guidance to financial institutions on how to engage with state and tribally authorized cannabis entities in accordance with federal law. An additional Department of Justice memorandum, issued in parallel with the FinCEN Guidance and since rescinded as part of the Sessions Memo, provided guidance to prosecutors regarding money laundering and other financial crimes as it pertained to cannabis entities and financial institutions operating in accordance with the FinCEN Guidance. The FinCEN Guidance and now rescinded accompanying Department of Justice memorandum is often publicly viewedinterpreted as enablingsuggesting a way for financial institutions to work with cannabisprovide depository services to cannabis-related entities, in compliance with federal law so long asprovided that the cannabis-related business activities are legal in their state or territory and none of the federal enforcement priorities referenced in the Cole Memorandum are being violated (such as keeping marijuanacannabis out of the hands of organized crime). Importantly, the FinCEN Guidance also clarifies how financial institutions can provide financialdepository services to marijuana-relatedcannabis-related businesses consistent with their Bank Secrecy Act obligations, including enhancedexhaustive customer due diligence but makes it clear that they are doing so at their own risk. and reporting requirements.customer due diligence steps typically include:1. Verifying with the appropriate state authorities whether the business is duly licensed“Secure and registered;2. Reviewing the license application (and related documentation) submitted by the business for obtaining a state license to operate its marijuana-related business;3. Requesting available information about the business and related parties from state licensing and enforcement authorities;4. Developing an understanding of the normal and expected activity for the business, including the types of products to be sold and the type of customers to be served (e.g., medical versus adult-use customers);5. Ongoing monitoring of publicly available sources for adverse information about the business and related parties;6. Ongoing monitoring for suspicious activity, including for any of the red flags described in the FinCEN Guidance; and7. Refreshing information obtained as part of customer due diligence on a periodic basis and commensurate with the risk.With respect to information regarding state licensure obtained in connection with such customer due diligence, a financial institution may reasonably rely on the accuracy of information provided by state licensing authorities, where states make such information available.While the FinCEN Guidance decreased some risk for banks and financial institutions considering servicing the cannabis industry, in practice, challenges remain with financial institutions’ willingness to provide services to marijuana-related businesses. This is because current U.S. federal law does not guarantee banks immunity from prosecution. It also requiresFair Enforcement Regulation ("SAFER") Banking Act,” would grant banks and other financial institutions to undertake time-consuming and costly due diligence (i.e., enhanced due diligence) on each marijuana-relatedimmunity from federal criminal prosecution for servicing cannabis-related businesses if the underlying cannabis business they accept as a customer.Those commercial banks and/or credit unions thatfollows state law. While several iterations of the proposed legislation have agreed to work with marijuana businesses are typically limiting those accounts to small percentages of their total deposits to avoid creating liquidity and concentration risk. Since, theoretically, the federal government could change the banking laws as it relates to marijuana-related businesses at any time and without notice, these banks and credit unions must keep sufficient cash on hand to be able to return the full value of all deposits from marijuana-related businesses in a single day, while also keeping sufficient liquid capital on hand to service their other customers. Because many banks and credit unions that are providing banking services to marijuana-related businesses are smaller institutions, applicable concentration limits may also impose limits on the aggregate amounts of loans that might be provided to the industry. Those commercial banks and credit unions that do have customerspassed in the marijuana industryHouse, in September 2023 the Senate Banking Committee voted to pass the SAFER Banking Act by a bipartisan majority of 14-9. A Senate floor vote is now pending but is not guaranteed. While there is strong support in the public and within Congress for the SAFER Banking Act and similar legislation, there can charge marijuana businesses high fees to cover the added cost of ensuring compliance with the FinCEN Guidance.As mentioned previously, unlike the Cole Memorandum, the FinCEN Guidance has not been rescinded. FinCEN has statedbe no assurance that it views the FinCEN Guidance to include compliance with the requirements of the rescinded Cole Memorandum. Secretary of the Treasury, Janet Yellen, has not made any public statements with regards to how the Treasury Department plans to treat marijuana-related businesses.14 However, in the event that any of our operations, or any proceeds thereof, any dividends or distributions therefrom, or any profits or revenues accruing from such operations in the United States were found to be in violation of anti-money laundering legislation or otherwise, such transactions could be viewed as proceeds of crime under one or more of the statutes noted above or any other applicable legislation. This could restrict or otherwise jeopardize our ability to declare or pay dividends or affect other distributions.In the United States, the “SAFE Banking Act” was adopted by the U.S. House of Representatives, which would grant banks and other financial institutions immunity from federal criminal prosecution for servicing marijuana-related businesses if the underlying marijuana business follows state law. It is unclear whether the U.S. Senate will take up the SAFE Banking Act for a vote. Most recently, the SAFE Banking Act was included as an amendment to the House of Representatives’ versions of the National Defense Authorization Act, “NDAA,” and the America COMPETES Act. During the conference committee, the committee declined to include the SAFE Banking Act amendment in the version of the NDAA signed into law. The America COMPETES Act is still being considered by Congress. While there is strong support in the public and within Congress for the SAFE Banking Act and similar legislation, there can be no assurance that it will be passed as presently proposed or at all. In both Canada and the United States, transactions involving banks and other financial institutions are both difficult and unpredictable under the current legal and regulatory landscape. Legislative changes could help to reduce or eliminate these challenges for companies in the cannabis space and would improve the efficiency of both significant and minor financial transactions.Ability to Access Public and Private CapitalGiven the current laws regarding cannabis at the federal level in the United States, traditional bank financing is typically not available to United States marijuana companies (although there has been a recent increase in the availability of traditional loans from alternative lending providers). Specifically, since financial transactions involving proceeds generated by cannabis-related conduct can form the basis for prosecution under anti-money laundering statutes, the unlicensed money transmitter statute, and the Bank Secrecy Act, businesses involved in the cannabis industry often have difficulty finding a bank willing to accept their business. Banks that do accept deposits from cannabis-related businesses in the United States must do so in compliance with the FinCEN Guidance. We have banking relationships in the states where we operate with state-chartered banks for deposits and payroll; however, we have limited access to traditional bank financing.Tax ConcernsAn additional challenge for marijuana-related businesses is that the provisions of IRC Section 280E are being applied by the IRS to businesses operating in the medical and adult-use marijuana industry. IRC Section 280E prohibits marijuana businesses from deducting their ordinary and necessary business expenses, forcing them to pay higher effective federal tax rates than similar companies in other industries. The effective tax rate on a marijuana business depends on how large its ratio of non-deductible expenses is to its total revenues. Therefore, businesses in the legal cannabis industry may be less profitable than they would otherwise be. Furthermore, although the IRS issued a clarification allowing the deduction of the cost of goods sold, the scope of such items is interpreted very narrowly, and the bulk of operating costs and general administrative costs are not permitted to be deducted.The 2018 Farm BillCBD is a nonintoxicating chemical found in cannabis and is often derived from hemp, which contains, at most, only trace amounts of THC. On December 20, 2018, Former President Trump signed the Agriculture Improvement Act of 2018 (popularly known as the 2018 Farm Bill) into law. Until the 2018 Farm Bill became law, hemp fell within the definition of “marijuana” under the CSA, and the DEA classified hemp as a Schedule I controlled substance because hemp is part of the cannabis plant.The 2018 Farm Bill defines hemp as the plant Cannabis sativa L. and any part of the plant with a delta-9 THC concentration of not more than 0.3% by dry weight and removes hemp from the CSA. The 2018 Farm Bill requires the U.S. Department of Agriculture, or USDA, to, among other things: (1) evaluate and approve regulatory plans approved by individual states for the cultivation and production of industrial hemp, and (2) promulgate regulations and guidelines to establish and administer a program for the cultivation and production of hemp in the U.S. On October 29, 2019, the USDA published its interim rules for the regulation of hemp, and on January 15, 2021, published its final rule for hemp production (referred to herein as the “USDA Rule”). The USDA Rule became effective on March 22, 2021. The USDA Rule, among other things, sets minimum standards for the cultivation and production of hemp, as well as requirements for laboratory testing of hemp. The hemp production regulations promulgated by the USDA are in lieu of those states not adopting state-specific hemp regulations; however, state programs must meet certain requirements contained in the USDA Rule. Hemp and products derived from it, such as CBD, may be sold into commerce and transported across state lines provided that the hemp from which any product is derived was cultivated under a license issued by an authorized state program approved by the USDA or USDA hemp production license, meets the definition of hemp, and the products are compliant with other applicable federal laws including the U.S. Food, Drug, and Cosmetic Act. The 2018 Farm Bill explicitly preserved the authority of the FDA to regulate15hemp-derived products under the U.S. Food, Drug, and Cosmetic Act. The FDA has not yet promulgated its own rules for the regulation of hemp-derived products.We areThe Company is classified as having “direct” involvement in the United States cannabis industry and we believe that we are in compliance with applicable state laws, as well as related licensing requirements and the regulatory frameworks enacted in the states we operate in. We are not subject to any citations or notices of violation with applicable licensing requirements and the regulatory frameworks which may have an impact on our licenses, business activities, or operations. We use reasonable commercial efforts to ensure that our business remains compliant with applicable licensing requirements and the regulatory frameworks enacted by Arizona, California, Colorado, Connecticut, Florida, Georgia, Maryland, Massachusetts, Nevada,Ohio, Pennsylvania, and West Virginia through the advice of our DirectorCompany’s legal counsel and through ongoing review of Compliance, who monitors and reviews our business practices and changes to applicable state laws and regulations, as well as United States Federal enforcement priorities.regulations. Our Chief Legal Officer and General Counsel worklegal counsel works with external legal advisorsregulatory counsel in Arizona, California, Colorado, Connecticut, Florida, Nevada, Maryland, Massachusetts, Pennsylvania, and West Virginiathe states in which we operate to ensure that we are in ongoing compliance with applicable state laws. Although the Company no longer has licensed operations in California or Massachusetts, and is in the process of finalizing its exit from both states, we continue to work with local external regulatory counsel to maintain compliance during the exit process.United States,U.S., the regulation of cannabis varies significantly from state to state, with a key distinction being the authorization for medical use versus recreational use. These state regulations are characterized by differences in licensing regimes, allowable dosage forms, and possession limits. In states with a medical-only regulatory framework, cannabis is largely regulated atlegal exclusively for medical purposes only. Patients typically require a recommendation from a qualified healthcare provider to access medical cannabis. The distribution of cannabis is strictly controlled through licensed dispensaries. These states often limit the state level. Although each state in which we operate (and anticipate operating) authorizes, as applicable,types and forms of cannabis products available, with an emphasis on medicinal applications. Possession limits tend to be higher for registered patients, but recreational use is prohibited. In states that allow adult-use (recreational) cannabis, individuals who meet age requirements can purchase cannabis for both medical and/orand recreational purposes. While dosage forms and possession limits may vary, they are generally more permissive for recreational users. Some states regulate adult-use marijuana production and distribution by licensed or registered entities,medical cannabis under a single set of rules and numerouslicensing structures while other states have legalized marijuana in some form, under U.S. federal law, the possession, use, cultivation,maintain separate regulatory frameworks for medical and transfer of marijuana and any related drug paraphernalia remains illegal, and any such acts are criminal acts under U.S. federal law. Although we believe that our business activities are compliant with applicable state and local lawsadult-use cannabis.United States, strict compliancelicenses associated with state and local laws with respect to marijuana may neither absolve us of liability under U.S. federal law nor provide a defense to any federal proceeding which may be brought against us. Any such proceedings brought against us may result in a material adverse effect on our business.In DecemberArizona voters passedthrough Proposition 203, the Arizona Medical MarijuanaCannabis Act, (the “AMMA”), A.R.S. Section 36- 2801 et seq. The AMMA went into effect on April 14, 2011, making Arizona the fifteenth state to adopt a medical marijuana law. The AMMA designates the Arizona Department of Health Services (the “ADHS”) as the licensing and issuing authority for the Arizona Medical Marijuana Program. The ADHS has adopted rules and regulations for developing and implementing the Arizona Medical Marijuana Program. These rules and regulations are set forthadult-use in the Arizona Administrative Code Title 9, Chapter 17.The first medical marijuana licenses in Arizona were issued in 2012 and as of March 1, 2022, Arizona has 127 open dispensaries.Arizona2020 through Proposition 207, also known as the Smart and Safe Arizona Act, was a voter initiative to legalize the adult recreational use of marijuana that was approved by voters on November 3, 2020.Act. The Smart and Safe Arizona Act directed the Arizona State Department of Health Services is responsible for licensing and regulating medical and adult-use cannabis, cannabis retail sales, cannabis production, and testing facilities.establish rules for retail marijuana sales by June 1, 2021, allow marijuanaacquire, cultivate, process, manufacture, transfer, supply, and/or dispense medical and/or adult-use cannabis. All product categories are allowed to be subject to statesold as either adult-use or medical, except edibles for adult-use consumers, which cannot be more than 10mg per serving or 100mg per package.local sales taxes like other retail items, and would impose an additional 16% excise tax on marijuana products. Following the passage of Proposition 207, existing medical operators were permitted to apply for licensure to permit sales to adult use consumers. Recreational sales commenced in Arizona on January 22, 2021.Arizona Licenses and RegulationsArizona stateadult-use licenses are renewed once everyvalid for two years. Around the time of renewal, licensees are required to submit a renewal application per guidelines published by the ADHS. While renewals are annual, there is no ultimate expiry after which no renewals are permitted. Additionally, in respect of the renewal process, provided that the requisite renewal fees are paid, the renewal application is submitted in a timely manner (at least 30 days in advance of the expiration date of the license), and there are no material violations noted against the applicable licenses, we would expect to receive the applicable renewed license in the ordinary course of business. While our compliance controls have been developed to mitigate the risk of any material violations of a license arising, there is no assurance that our licenses will be renewed in the future in a timely manner. Any unexpected delays or costs associated with the licensing renewal process could impede our ongoing or planned operations and have a material adverse effect on our business, financial condition, results of operations, or prospects.Arizona is a vertically integrated system so that each license permitsholder to acquire, cultivate, process, distribute and/or dispense, deliver, manufacture, transfer,Medical and supply medical and/or adult-use marijuanaAdult-Use Markets in compliance with the AMMA and ADHS rules and regulations.Colorado16Dispensing Requirementsorder to dispense2000, Colorado legalized medical marijuana to a qualifying customer or designated caregiver, a licensed dispensary is required to (1) verify the qualifying customer’s or designated caregiver’s identity, (2) offer appropriate customer education or support materials, (3) make available testing results relatedvia Amendment 20 to the product sought, if requested by the qualifying customer or designated caregiver, (4) enter the qualifying customer’s or designated caregiver’s registry identification number on the identification card presented into the medical marijuana electronic verification system, (5) verify the validity of the identification card presented, (6) verify that the amount of marijuana product to be dispensed would not cause the qualifying customer to exceed the regulatory limit, and (7) enter information into the medical marijuana electronic verification system regarding the amount of medical marijuana dispensed, whether it was dispensed directly to the qualifying customer or to a caregiver, the date and time of dispensing, the registry identification number of the dispensary agent,Colorado Constitution, and the dispensary’s registry identification number.Requirements Relating to Recordkeeping and Inventory ControlLicensed dispensaries are required by regulation to utilize an inventory control system that documents (1) daily updated inventory amounts of marijuana products, (2) acquisitions of medical marijuana from qualifying customers or designated caregivers, (3) acquisitions of medical marijuana from other dispensaries, (4) information related to batches of marijuana cultivated by the licensee, (5) information regarding provision of medical marijuana to other dispensaries, (6) information relating to required testing of marijuana products, and (7) the disposition of marijuana products determined not to be dispensed to a customer or to be included in manufacturing a marijuana product. Licensed dispensaries are additionally required to keep records regarding qualifying customers that: (1) include dated entries from registered dispensary agents regarding dispensing, (2) are safeguarded against unauthorized access and tampering, (3) include documentation of requests by qualifying customers and caregivers regarding marijuana products and educational materials.Requirements Relating to Security and TransportationLicensed dispensaries are permitted to transport marijuana and marijuana products between dispensaries and cultivation sites, qualifying customers, other dispensaries, and licensed analytical laboratories. Dispensaries are required to complete a comprehensive trip plan prior to transport that describes the products being transported, the time of the trip, and the anticipated route. During transportation, dispensary agents are required to use unmarked vehicles, hide marijuana and marijuana products from view, and communicate with the licensed dispensary. Licensed dispensaries are required to keep records of trip plans.Licensed dispensaries are also required to implement a variety of security measures to protect dispensary and cultivation sites from unauthorized access: (1) intrusion detection devices, (2) exterior lighting, (3) video cameras covering the entrances and exits of limited access areas and facilities, as well as point of sale locations and grow rooms, (4) failure notification systems, (5) battery backup for cameras and other equipment, and (6) panic buttons on the interior of facilities. Licensed dispensaries are also required to implement policies and procedures that restrict access to the areas of the dispensary that contain marijuana, that provide for identification of authorized individuals, that prevent loitering, for the conduct of electronic monitoring, and the use of panic buttons.Arizona Reporting RequirementsThe State of Arizona uses the ADHS Medical Marijuana Verification System (“ADHS MMV”) to validate card holders, verify allotment amounts and track all retail transactions for Arizona qualified customers. The ADHS MMV system is also used annually by license holders to renew the dispensary registration certificate.We use Leaf Logix software as our computerized, seed-to-sale tracking and inventory system. Individual licensees whether directly or through third- party integration systems are required to capture and retain all information pertaining to the acquisition, possession, cultivation, manufacturing, delivery, transfer, transportation, supplying, selling, distributing, or dispensing of medical marijuana, to meet all reporting requirements for the State of Arizona.ADHS InspectionsIn December 2010, Arizona voters passed the Arizona Medical Marijuana Act (the “AMMA”), A.R.S. Section 36- 2801 et seq. The AMMA went into effect on April 14, 2011, making Arizona the fifteenth state to adopt a medical marijuana law. The AMMA designates the Arizona Department of Health Services (the “ADHS”) as the licensing and issuing authority for the Arizona Medical Marijuana Program. The ADHS has adopted rules and regulations for developing and implementing the Arizona Medical Marijuana Program. These rules and regulations are set forth in the Arizona Administrative Code Title 9, Chapter 17. Arizona Proposition 207, also known as the Smart and Safe Arizona Act, was a voter initiative to legalize the adult recreational use of marijuana that was approved by voters on November 3, 2020. Followinglegalized adult-use cannabis via the passage of Proposition 207, existing medical operators were permitted to apply for licensure to permit sales to adult use consumers. Recreational sales commenced in Arizona on January 22, 2021. In accordance with Proposition 207, the ADHS will issue up to 26 additional licenses for social equity applicants in the future.17The ADHS oversees licensing for medical marijuana and adult-use cannabis. The Arizona market is divided into two classes of licenses: medical and adult use. Each license grants the licensee the ability to have one dispensary, one processing site, and one cultivation site. License holders are not required to establish for vertically integrated operations and processing and cultivation sites can be used by third party companies. Arizona does not recognize third party operators and the license holder is responsible for compliance. As of December 31, 2021, there were 127 operating dispensaries. Dispensaries can hold dual licensure to serve both medical and adult use consumers.For medical card holders, qualifying conditions include Alzheimer’s disease, cachexia, cancer, chronic pain, Crohn’s disease, glaucoma, hepatitis C, human immunodeficiency virus (“HIV”) or acquired immune deficiency syndrome (“AIDS”), muscle spasms, nausea, post-traumatic stress disorder “(PTSD”), sclerosis, and seizures. All product categories are allowed to be sold as either adult use or medical, except edibles for adult use consumers, which cannot be more than 10mg per serving or 100mg per package.Regulation of the Marijuana Market in CaliforniaIn 1996, California was the first state to legalize medical marijuana through Proposition 215, the Compassionate Use Act of 1996. This provided an affirmative defense for defendants charged with the use, possession, and cultivation of medical marijuana by customers with a physician recommendation for treatment of cancer, anorexia, AIDS, chronic pain, spasticity, glaucoma, arthritis, migraine, or any other illness for which marijuana provides relief. In 2003, Senate Bill 420 was signed into law, decriminalizing the use, possession, and collective cultivation of medical marijuana and establishing an optional identification card system for medical marijuana customers.In September 2015, the California legislature passed three bills collectively known as the Medical Marijuana Regulation and Safety Act (“MMRSA”). The MMRSA established a licensing and regulatory framework for medical marijuana businesses in California. The system created testing laboratories and distributors. Edible infused product manufacturer licenses based on either volatile solvent or non-volatile solvent manufacturing-specific extraction methodology also became mandatory. Multiple agencies oversaw different aspects of the program, and businesses were required to obtain both a state license and local approval in order to operate.However, in November 2016, voters in California overwhelmingly passed Proposition 64, the Adult Use of Marijuana Act (“AUMA”), creating an adult-use marijuana program for consumers 21 years of age or older. In June 2017, the California State Legislature passed Senate Bill No. 94, known as Medicinal and Adult-Use Marijuana Regulation and Safety Act (“MAUCRSA”), which amalgamated MMRSA and AUMA into a set of regulations governing both medical and adult-use cannabis licensing in California. MAUCRSA went into effect on January 1, 2018.The three licensing agencies that used to regulate marijuana at the state level included the Bureau of Cannabis Control (“BCC”), California Department of Food and Agriculture (“CDFA”), and the California Department of Public Health (“CDPH”). However, California Governor Gavin Newsom signed Assembly Bill 141 (“AB 141”) on July 12, 2021. Effectively, AB 141 consolidated the three former state cannabis authorities into a single, new department now known as the Department of Cannabis Control (the “Department”). On or about September 15, 2021, the Department filed emergency regulations to consolidate, clarify, and make consistent cannabis regulations to the California Office of Administrative Law. After a limited comment period, these consolidated emergency regulations were approved and became effective on or about September 27, 2021. These regulations created consistent standards for cannabis licensees across all license types by aligning application requirements, unifying terminology, and clarifying ownership and financial interest requirements.One of the central features of MAUCRSA is known as “local control.” In order to legally operate a medical or adult-use marijuana business in California, an operator must have both a local and state license. This requires license-holders to operate in cities or counties with marijuana licensing programs. Cities and counties in California are also allowed to limit the number of licenses issued locally or even ban all cannabis licenses completely.Once an operator obtains local approval, the operator must obtain state licenses before conducting any commercial marijuana activity. There are multiple license categories that cover all commercial activity. Categories include: (1) cultivation/nurseries/processors, (2) testing laboratories, (3) distributors/transporters, (4) retailers (including delivery), (5) microbusinesses, (6) event organizers, and (7) manufacturers. Categories of licenses are further broken down into subtypes. For example, there are multiple types of cultivation licenses available depending upon the size of the cultivation operation and whether the operation is indoors/outdoors or uses mixed lighting. Different manufacturing licenses are available depending upon whether volatile or nonvolatile solvents are used. Retail licenses are available depending upon whether the retailer operates from a storefront or a non-storefront.The Department Regulating the Commercial Cannabis IndustryThe Department oversees and regulates all commercial cannabis activities. Operators must apply to the Department for their licenses.18The Marijuana Supply Chain in CaliforniaIn California, local law may determine whether plants must be cultivated outdoors, using mixed-light methods, or fully indoors. Cultivators must also obtain seeds, clones, teens, or other immature plants from licensed nurseries through the traceability system.The cultivation, processing, and movement of marijuana within the state are tracked by the California Cannabis Track and Trace System’s METRC software. All licensees are required to input their track and trace data (either manually or using another software that automatically uploads to METRC). Immature plants must be assigned a Unique Identifier number (“UID”) which then remains associated with that specific plant’s flowers and biomass as it moves through the supply chain to the consumer. Each licensee in the supply chain is required to meticulously log any processing, packaging, and sales associated with that UID.When marijuana plants mature and complete their life cycle, they are harvested, cured, and trimmed in preparation for being sold to processors, distributors, or manufacturers. Cultivators have two main products: flowers, or “buds,” and the biomass, or “trim,” which is typically removed from the mature flowers. Trim is commonly sold to manufacturers for further processing into cannabis extracts. Buds may also be sold to manufacturers or distributors for sale to retailers. The cultivator may package and label its marijuana flowers directly or sell flowers in bulk, allowing distributors to package and label the flower.Manufactured marijuana goods sold or transferred to a distributor for final retail sale by a manufacturer must be in final packaging. Distributors may not repackage manufactured marijuana goods. Certain tax rates apply to the marijuana flower and biomass, which are assessed per ounce of product sold. The California State cultivation tax is paid by the cultivator to the distributor, or, alternatively, from the cultivator to the manufacturer, after which the distributor bringing the marijuana goods to market bears responsibility for tendering the cultivation tax receipts to the California Department of Tax Fee Administration (“CDTFA”).California restricts the transportation of cannabis to licensed distributors. Some cultivators and manufacturers maintain their own distribution licenses in order to handle transportation directly, while others contract with third-party licensed distributors for transportation services. Distributors may or may not take possession of the marijuana and marijuana products, and like alcohol distribution, some retailers limit sales to a particular distributor’s brand portfolio. Brands may market products to retailers directly or only to distributors.Distributors are the point in the supply chain where final quality assurance testing is performed on products before they go to a retailer. Retailers may not accept or sell products without an accompanying and compliant Certificate of Analysis (“COA”). Distributors must clearly segregate the product awaiting testing from the product that has already passed testing at their licensed premises until an employee of a licensed testing laboratory comes to their premises and obtains samples from any and all goods proposed to be sold at retail. Marijuana and marijuana products are issued either a “pass” or “fail” by the testing laboratory. Under some circumstances, the Department’s regulations allow for failing product to be “remediated” or to be re-labeled to more accurately reflect the COA results.Retail Compliance in CaliforniaCalifornia requires specific warnings, images, and information regarding package contents to be printed on all marijuana packaging. The Department’s regulations also require packaging to be both tamper-evident and child-resistant. Distributors and retailers are independently responsible for confirming that products are properly labeled and packaged prior to the consumer sale.Consumers aged 21 and up may purchase marijuana in California from a dispensary with an “adult-use” license. Some localities still only allow medicinal dispensaries. Consumers aged 18 and up with a valid physician’s recommendation may purchase marijuana from a medicinal-only dispensary or an adult-use dispensary. Consumers without valid physician’s recommendations may not purchase marijuana from a medicinal-only dispensary. All marijuana businesses are prohibited from hiring employees under the age of 21.Security RequirementsIn California, local law may determine whether plants must be cultivated outdoors, using mixed-light methods, or fully indoors. Cultivators must also obtain seeds, clones, teens, or other immature plants from licensed nurseries through the traceability system.The cultivation, processing, and movement of marijuana within the state are tracked by the California Cannabis Track and Trace System’s METRC software. All licensees are required to input their track and trace data (either manually or using another software that automatically uploads to METRC). Immature plants must be assigned a Unique Identifier number (“UID”) which then remains associated with that specific plant’s flowers and biomass as it moves through the supply chain to the consumer. Each licensee in the supply chain is required to meticulously log any processing, packaging, and sales associated with that UID.When marijuana plants mature and complete their life cycle, they are harvested, cured, and trimmed in preparation for being sold to processors, distributors, or manufacturers. Cultivators have two main products: flowers, or “buds,” and the biomass, or “trim,” which is typically removed from the mature flowers. Trim is commonly sold to manufacturers for further processing into cannabis19extracts. Buds may also be sold to manufacturers or distributors for sale to retailers. The cultivator may package and label its marijuana flowers directly or sell flowers in bulk, allowing distributors to package and label the flower.Manufactured marijuana goods sold or transferred to a distributor for final retail sale by a manufacturer must be in final packaging. Distributors may not repackage manufactured marijuana goods. Certain tax rates apply to the marijuana flower and biomass, which are assessed per ounce of product sold. The California State cultivation tax is paid by the cultivator to the distributor, or, alternatively, from the cultivator to the manufacturer, after which the distributor bringing the marijuana goods to market bears responsibility for tendering the cultivation tax receipts to the California Department of Tax Fee Administration (“CDTFA”).California restricts the transportation of cannabis to licensed distributors. Some cultivators and manufacturers maintain their own distribution licenses in order to handle transportation directly, while others contract with third-party licensed distributors for transportation services. Distributors may or may not take possession of the marijuana and marijuana products, and like alcohol distribution, some retailers limit sales to a particular distributor’s brand portfolio. Brands may market products to retailers directly or only to distributors.Distributors are the point in the supply chain where final quality assurance testing is performed on products before they go to a retailer. Retailers may not accept or sell products without an accompanying and compliant Certificate of Analysis (“COA”). Distributors must clearly segregate the product awaiting testing from the product that has already passed testing at their licensed premises until an employee of a licensed testing laboratory comes to their premises and obtains samples from any and all goods proposed to be sold at retail. Marijuana and marijuana products are issued either a “pass” or “fail” by the testing laboratory. Under some circumstances, the Department’s regulations allow for failing product to be “remediated” or to be re-labeled to more accurately reflect the COA results.InspectionsAll licensees are subject to random inspections of their premises by the Department. If an inspection identifies any noncompliance of a licensee, the Department may issue notices to correct, or notices of violation, fines, or take other disciplinary action that may include cancellation of a license.Retail Taxes in CaliforniaRetailers generally pay excise tax amounts to final distributors upon making wholesale purchases. These distributors then remit receipts directly to the CDTFA. The tax liability is calculated based on the average market price of the cannabis goods sold at retail sale. The average market price is determined by the type of transaction that occurs between the seller (cultivator, manufacturer, or distributor) and the retailer. The CDTFA determines tax rates based upon estimated average ratios, including retail and wholesale prices during the tax period, and is commonly known as a “markup.” CDTFA’s current markup estimate (as of January 1, 2020) is 80%. Due to the 15% statutory tax rate and the 80% markup estimate, the current effective tax rate on wholesale gross receipts is 27%.In addition to the State taxes, cities and counties throughout California with licensed cannabis businesses apply their own approaches to taxing cannabis. These approaches fall into three broad categories. First, many local governments impose the same tax rate on all cannabis businesses regardless of type. Second, many local governments impose higher tax rates on retailers than other types of cannabis businesses. Third, a few local governments do not levy specific cannabis taxes. The California Legislative Analyst’s Office estimates that the average cumulative local tax rate over the whole supply chain is roughly equivalent to a 15% tax on retail sales.See Appendix A to this Annual Report on Form 10-K for a list of the licenses associated with our operations in California.Regulation of Cannabis Market in ColoradoIn 2000, Colorado voters passed Amendment 20, an amendment to the state constitution decriminalizing certain amounts of marijuana for medicinal purposes. The amendment created a regulatory system for granting patient registry cards authorizing individuals with debilitating medical conditions to have and use up to two ounces of a usable form of marijuana and no more than six marijuana plants. The amendment also created a regulatory system for granting patient registry cards authorizing individuals with debilitating medical conditions to have and use limited quantities of marijuana, and an affirmative defense under Colorado criminal law for any cardholder or their primary caregiver to charges of violating state drugs laws. In 2010, the Colorado legislature passed H.B. 10-1284, which enacted the Colorado Medical Marijuana Code, a framework regulating the growth, sale, and distribution of marijuana by licensed dispensaries, administered by the Colorado Department of Public Health and Environment.In 2012, Colorado voted passed Amendment 64 legalizing the recreational use of marijuana in Colorado. Among other provisions, the amendment legalized the cultivation, manufacturing, processing, and sale of marijuana to adults 21 years of age or older. Amendment 64 also directed the Colorado Department of Revenue (“DOR”) to establish a comprehensive framework of regulation and enforcement governing licensed marijuana businesses in the state.2012. The Colorado MarijuanaCannabis Enforcement Division (“MED”) is the licensing and regulatory agency overseeing all recreational and medical marijuanacannabis businesses in Colorado. In 2019, legislators passed20statutes updating, among other things, the state’s marijuana laws to allow for new sources of investment inColorado, with the Colorado marijuana industry, including by publicly traded companiesDepartment of Public Health and created accompanying disclosure requirements.Environment overseeing the medical patient registry and certifying the testing of licensed cannabis laboratories.In Colorado, cannabismarijuanacannabis cultivation facilities, product manufacturing facilities or retail dispensary facilities.There are multiple license categories that cover all commercial activity. Categories include: (1) medical/retail cultivation, (2) medical/retail stores, (3) medical/retail manufacturers, (4) medical/retail transporters, (5) retail hospitality and sales, (6) medical/retail marijuana business operator and (6) medical/retail testing facilities. Categories of licenses are further broken down into subtypes. For example, there are multiple types of cultivation licenses available depending upon the size of the cultivation operation. Licensees may also apply for permits that allow for the conduct of additional business functions such as centralized distribution, delivery, and off-premises storage.Each facility is authorized to engage only in the type of activity for which it is licensed. Licenses are valid for one year from the date of issuance. Before expiration, licensees are required to submit a renewal application.To acquire a license, the individuals and entities who own or control the licensee must be vetted by the State to determine whether they are suitable under Colorado law to operate a license. Generally, any natural person or entity that holds a 10% interest or greater (directly or indirectly) in the licensee must be disclosed and vetted for suitability. All board members and corporate officers of the licensee must be disclosed and vetted, including board members and corporate officers of the entity-owners with 10% or greater interest. Additionally, those who exercise an inordinate amount of control over a licensee—whether through financing, IP agreements, management agreements, or some combination thereof, can also be considered “controlling beneficial owners” by the MED and subsequently be required to undergo vetting for suitability. Colorado licensing requirements largely do not respect anonymity in ownership regularly pierce corporate veils to determine the natural people who ultimately control a given license.Local authorities also grant licenses to conduct marijuana businesses within their jurisdiction, and DOR licensing for a marijuana business is conditioned on approval from the local authorities. An applicant is prohibited from operating a marijuana business prior to obtaining all necessary licenses, registrations, permits, or approvals from both the State licensing authority and local licensing authorities.Security RequirementsColorado law requires that all licensees have a security alarm system and must ensure that their premises are continuously monitored. Licenses must also use commercial-grade non-residential door locks at all points of ingress and egress. All licensed marijuana businesses are required to utilize video surveillance and camera recording systems, which at a minimum, must consist of digital or network video recorders, video monitors, digital archiving devices, and a color printer capable of delivering still photos. Such equipment must be stored in a secure area that is only accessible to the licensee’s management staff. In addition to other requirements, all video surveillance equipment must be equipped with a failure notification system that provides prompt notification to the licensee of any prolonged surveillance interruption and/or complete failure of the surveillance system.InspectionsThe MED and local licensing authorities may conduct announced or unannounced inspections of licensees to determine compliance with applicable laws and regulations. Licensees may also be subject to inspection of the licensed premises by the local fire department, building inspector, or code enforcement officer to confirm that no health or safety concerns are present.Colorado Reporting RequirementsColorado uses METRC as the MED’s marijuana inventory tracking system for all medical and adult use licensees. Marijuana is required to be tracked and reported with specific data points from seed to sale through METRC for compliance purposes under Colorado marijuana laws and regulations. This tracking is conducted by using electronic tags on plants and shipments between licensees and facilities.See Appendix A to this Annual Report on Form 10-K for a list of the licenses associated with our operations in Colorado.MarketMarkets in ConnecticutThe State of has authorized cultivation, possession,legalized medical cannabis in 2012, and distribution of marijuana for medical purposes by certain licensed Connecticut marijuana businesses.adult-use cannabis in 2021. The Medical Marijuana Program or MMP,within the Department of Consumer Protection registers qualifying patients, primary caregivers,21Dispensary Facilities, or DFs, dispensary facilities, and Dispensary Facility Employees, or DFEs. The MMP was established by Connecticut General Statutes §§ 21a-408–21a429. DFs and production facilities are separately licensed.The MMP is administered by the Department of Consumer Protection, or DCP. Patients with qualifying debilitating medical conditions qualify to participate in the program, including patients with such conditions include but are not limited to cancer, glaucoma, positive status for human immunodeficiency virus (HIV) or acquired immune deficiency syndrome (AIDS), Parkinson’s disease, or multiple sclerosis (MS). A physician or advanced practice registered nurse must issue a written certification for an MMP patient, and the qualifying patient or caregiver must choose one designated DF where the patient’s marijuana will be obtained.In Connecticut, marijuana may not be produced or dispensed without the appropriate license. The DCP determines how manydispensary facility licenses to issue based on the size and location of the DFs in operation, the number of qualifying patients registered with the DCP, and the convenience and economic benefits to qualifying patients.When the DCP determines that additional licenses for DFs should be granted, it publishes a notice of open applications for DF licenses. This notice must include the maximum number of licenses to be granted, the deadline for receipt of applications, and the criteria that will be considered when awarding the licenses. Such criteria must include character and fitness of any person who may have control or influence over the operation of the proposed DF; the location for the proposed DF; the applicant’s ability to maintain adequate controls against the diversion, theft, or loss of marijuana; the applicant’s ability to maintain the knowledge, understanding, judgment, procedures, security controls, and ethics to ensure optimal safety and accuracy in the dispensing and sale of marijuana; and the extent to which the applicant or any of the applicant’s DF backers have a financial interest in another licensee, registrant, or applicant.Applicants for DF licenses must identify, among other things, the proposed DF location, financial statements, criminal background check applications for the applicant and applicant’s backers, a plan to prevent theft and diversion, and a blueprint of the proposed DF. An application for a DF license also requires the payment of a $5,000 fee. If approved, the licensee must pay an additional $5,000 before receiving its license. The decision of the DCP’s Commissioner, or Commissioner, not to award a DF license to an applicant is final.Connecticut Dispensary Facility RequirementsA DF may not dispense marijuana from, obtain marijuana from, or transfer marijuana to a location outside of the state of Connecticut. DFs are limited to the following modes of obtaining, delivering, transferring, transporting, and selling marijuana:•A DF may acquire marijuana from a producer;•A DF may dispense and sell marijuana to a qualifying patient or primary caregiver registered to their facility and who is registered with the DCP;•A DF may dispense or sell to a research program subject pursuant to the protocols of a research program approved by the Commissioner;•A DF may transfer, distribute, deliver, transport, or sell to a research program employee pursuant to the protocols of a research program approved by the Commissioner;•A DF may transfer, distribute, deliver or transport to a hospice or other inpatient care facility licensed by the Department of Public Health that has a protocol for handling and distributing marijuana that has been approved by the DCP; and•A DF may transfer, distribute, deliver or transport marijuana to an approved laboratory.marijuana,medical cannabis, and only a dispensary or dispensary technician may sell marijuanacannabis to qualifying customers, primary caregivers, or research program subjects who are registered with the DCP. A DF may not engagesubjects. Adult-use sales began in marijuana compounding, except that a dispensary may dilute a medical marijuana product with a USP grade substance with no active ingredient for the purposes of dose titration, tapering, for the addition of a flavoring agent, or creating a maintenance dose that is not available from any producer at the time of purchase. No person associated with a DF may enter into any agreement with a certifying health care provider or health care facility concerning the provision of services or equipment that may adversely affect any person’s freedom to choose the DF at which the qualifying customer or primary caregiver will purchase marijuana, except in the case of an approved research program. All DFEs must, at all times while at the DF, have their current dispensary license, dispensary technician registration, or DFE registration available for inspection by the Commissioner or the DCP. The DF shall establish, implement and adhere to a written alcohol-free, drug-free, and smoke-free workplace policy, which must be available to the DCP upon request. Marijuana may not be applied, ingested, or consumed inside a DF.Each DF must make publicly available the price of all its marijuana products to prospective qualifying patients and primary caregivers. As of February 18, 2022, the DCP increased the monthly allotments for qualifying patients from 3.0 ounces to 3.5 ounces. All marijuana must be sold in child-resistant, sealed containers except upon a written request from the qualifying patient or primary22caregiver. No marijuana may be sold without the producer label. All products sold to the qualifying patient or primary caregiver must be placed in an opaque package that shall not indicate the contents of the package, the originating facility, or in any other way cause another person to believe that the package may contain marijuana. Each DF must also provide information to qualifying patients and primary caregivers regarding the possession and use of marijuana. The DF manager must submit all informational material to the Commissioner for approval prior to such information being provided to qualifying patients and primary caregivers.Connecticut Security and Storage RequirementsAll facilities must have an adequate security system to prevent and detect the loss of marijuana. These systems must use commercial grade equipment, including perimeter alarms, motion detectors, video cameras with 24-hour recordings (which must be retained for at least 30 days), silent alarms, panic alarms, a failure notification system, and the ability to remain operational during a power outage. Each facility must also have a backup alarm system approved by the Commissioner. The outside perimeter of every facility must be well-lit. All equipment must be kept in good working order and tested at least twice per year.A DF must:•Not maintain marijuana in excess of the quantity required for normal, efficient operation;•Store all marijuana in an approved safe or approved vault and in such a manner as to prevent diversion, theft or loss;•Maintain all marijuana in a secure area or location accessible only to specifically authorized employees, which shall include only the minimum number of employees essential for efficient operation;•Keep all approved safes and approved vaults securely locked and protected from entry, except for the actual time required to remove or replace marijuana;•Keep all locks and security equipment in good working order;•Keep the dispensary department securely locked and protected from entry by unauthorized employees; and•Post a sign at all entry ways into any area of the DF containing marijuana stating, “Do Not Enter—Limited Access Area—Access Limited to Authorized Employees Only.” All deliveries must be carried out under the direct supervision of a pharmacist licensed as a dispensary, who must be present to accept the delivery. Upon delivery, the marijuana must immediately be placed in an approved safe or approved vault within the dispensary.No person may enter the area where marijuana is dispensed and sold unless such person is licensed or registered by the DCP; such person’s responsibilities necessitate access to the dispensary department and then for only as long as necessary to perform the person’s job duties; or such person has a patient or caregiver registration certificate, in which case such person must not be permitted behind the service counter or in other areas where marijuana is stored.Connecticut Transportation RequirementsPrior to transporting any marijuana or marijuana product, a DF must complete a shipping manifest using a form prescribed by the Commissioner and securely transmit a copy of the manifest to the laboratory, research program location, hospice, or other inpatient care facility that will receive the products and to the DCP at least 24 hours prior to transport. These manifests must be maintained and made available to the DCP. Marijuana may only be transported in a locked, secure storage compartment that is part of the vehicle transporting the marijuana. This compartment may not be visible from outside the vehicle. Routes must be randomized.All transport vehicles must be staffed with a minimum of two employees. At least one delivery team member is required to remain with the vehicle at all times that the vehicle contains marijuana. A delivery team member must have access to a secure form of communication with employees at the originating facility at all times that the vehicle contains marijuana. A delivery team member must physically possess a department-issued identification card at all times when transporting or delivering marijuana and must produce it to the Commissioner or law enforcement official upon request.No marijuana may be sold, dispensed, or distributed via a delivery service or any other manner outside of a DF without the approval of DCP, except that a primary caregiver may deliver marijuana to the caregiver’s qualified patient and a DFE may deliver to a hospice or other inpatient care facility licensed by the Department of Public Health that has a protocol for handling and distributing marijuana that has been approved by the DCP. As of September 27, 2012, a DF may register with DCP to provide delivery of cannabis products and paraphernalia to qualifying patients and caregivers.23Inspections by the CommissionerAll documents required to be kept by a facility must be maintained in an auditable format for no less than three years. These records must be provided to the Commissioner or an authorized delegate immediately upon request. Additionally, the Commissioner and authorized delegates may enter any place, including a vehicle, where marijuana is held, produced, or otherwise handled, and inspect in a reasonable manner such place and all pertinent items and documents within it.Passage of Adult Legislation in ConnecticutOn June 22, 2021, Governor Ned Lamont signed into law Senate Bill 1201, which authorizes the legal possession of certain quantities of cannabis for adults over the age of 21 effective July 1, 2021, and creates a process for the regulation of adult-use cannabis cultivation, production, distribution, and sale to consumers over the age of 21 within the state. The Department of Consumer Protection (DCP) has issued operational policies and procedures and implemented the licensing framework for the cultivators, micro-cultivators, product manufacturers, food and beverage manufacturers, product packagers, retailers, hybrid retailers, transporters, and delivery services. The number of retail licenses permitted per town may not exceed one per 25,000 residents until June 30, 2024. Half of the licenses are to be granted to social equity applicants—defined as people who have lived in geographic areas disproportionately impacted by the war on drugs and who make no more than three times the state’s median income.The state’s existing medical marijuana dispensaries can convert to “hybrid retailers” serving both medical and adult-use consumers. In order to convert, applicants must have a Social Equity Council-approved workforce development plan and pay a fee of $1 million, or $500,000 if the dispensary has committed to creating at least one equity joint venture. Hybrid retailers must maintain a licensed pharmacist on premises at all times when the hybrid retail location is open to the public or to qualifying patients and caregivers. The hybrid retailer must also accommodate an expedited method of entry that allows for priority entrance into the premises for qualifying patients and caregivers. A dispensary facility may apply to the DCP to convert its license to a hybrid retail location at any time after February 3, 2022, without applying through the lottery process. The license conversion will require a DF to submit to, and obtain approval from the DCP for, a detailed medical marijuana preservation plan for how it will prioritize sales and access to medical marijuana products for qualifying patients, including, but not limited to, managing customer traffic flow, preventing supply shortages, providing delivery services and ensuring appropriate staffing safeguards. The DCP estimates that retail sales will likely not be available until at least the end of 2022.See Appendix A to this Annual Report on Form 10-K for a list of the licenses associated with our operations in Connecticut.In 2014, the Legislature passed the Compassionate Use Act, or CUA, which wasis currently a low-THC (CBD) law, allowing cannabis containing not more than 0. 8% THC to be sold to patients diagnosed with severe seizures or muscle spasms and cancer. The CUA created a competitive licensing structure and initially allowed for one vertically integrated license to be awarded in each of five regions. The CUA set forth the criteria for applicants as well as the minimum qualifying criteria, which included the requirement to hold a nursery certificate evidencing the capacity to cultivate a minimum of 400,000 plants, to be operated by a nurseryman, and to be a registered nursery for at least 30 continuous years. The CUA also created a state registry to track dispensations. In 2016, the Florida Legislature passed the Right to Try Act, or RTA, which expanded the State’s medical cannabis program to allow for full potency THC products to be sold as “medical marijuana” to qualified patients.In November of 2016, the Florida Medical Marijuana Legalization ballot initiative (referred to herein as the “Initiative”) to expand the medical cannabis program under the RTA was approved by 71.3% of voters, thereby amending the Florida constitution. The Initiative is now codified as Article X, Section 29 of the Florida Constitution. The Initiative expanded the list of qualifyingmedical-only market. Qualifying medical conditions to include cancer, epilepsy, glaucoma, HIV and AIDS, ALS, Crohn’s disease, Parkinson’s disease, PTSD, multiple sclerosis, and other debilitating medical conditions of the same kind or class or comparable to those other qualifying conditions and for which a physician believes the benefits outweigh the risks to the patient. The Initiative also provided for the implementation of State-issued medical cannabis identification cards. In 2017, the Florida Legislature passed legislation implementing the constitutional amendment and further codifying the changes outlined in the constitution into law. The 2017 law provides for the issuance of 10 licenses to specific entities and another four licenses to be issued for every 100,000 active qualified patients added to the registry. The 2017 law also initially limited license holders to a maximum of 25 dispensary locations with the ability to purchase additional dispensary locations from one another and for an additional five locations to be allowed by the State for every 100,000 active qualified patients added to the registry. The 2017 legislation’s cap on dispensing facilities expired in April 2020.Under Florida law, a licensee is required to cultivate, process, and dispense medical cannabis. Licenses are issued by the Florida Department of Health, Office of Medical MarijuanaCannabis Use, or OMMU, and may be renewed biennially. Trulieve US received its most recent license renewal on July 24, 2020, and is classified as a Medical Marijuana Treatment Center, or MMTC, under Florida law.holders can only own one license.24There is no state-imposed limitation on the permitted size of cultivation or processing facilities in Florida, nor is there a limit on the number of plants that may be grown. The is expected to issue anywhere from 18-22 new MMTC licenses, depending on the number of registered patients at the start of the application process, beginning in 2022 with the issuance of a single license to a recognized class member of Pigford v. Glickman, 185 F.R.D. 82 (D.D.C. 1999) or In re Black Farmers Litigation, 856 F. Supp. 2d 1 (D.D.C. 2011).marijuanacannabis use registry by a qualified physician and possess a state-issued medical marijuanacannabis identification card and a valid certification from the qualified physician. The physician determines customer eligibility as well as the routes of administration (e.g., topical, oral, inhalation sublingual, suppository, edible, and smoking marijuana) and the number of milligrams per day a customer is able to obtain under the program. The physician may order a certification for up to three 70-day supply limits of marijuana, following which the certification expires, and a physician must issue a new certification. The number of milligrams dispensed, the category of cannabis (either low-THC or medical marijuana), and whether a delivery device, such as a vaporizer, has been dispensed is all recorded in the registry for each customer transaction. In addition, smokable flower was approved by the legislature and signed into law in March 2019. Customer must obtain a specific recommendation from their physician to purchase smokable flower. The maximum amount a customer may obtain is 2.5 ounces (measured by weight) of smokable flower per 35-day supply.OMMU implemented rules regulating the production and saleAs of edible products in August of 2020, and the Company’s Florida licensee shortly thereafter became the first MMTC to dispense edibles in Florida. OMMU implemented rules regulating the use of hydrocarbon solvents (e.g., N-butane, isobutane, propane, pentane, and heptane) for the extraction of derivative products in August 2021. Trulieve announced the first sale in Florida of hydrocarbon extraction-derived concentrate products in September 2021.Dispensaries may be located in any location zoned as appropriate for a pharmacy throughout the State of Florida as long as the local government has not expressly prohibited MMTC dispensaries in their respective municipality. Additionally, dispensaries must be located more than 500 feet from a public or private elementary, middle, or secondary school. Following the adoption of the cap on total dispensaries by each MMTC, as discussed above, our Florida licensee filed a claim in the Court for the Second Judicial Circuit in Leon County challenging the dispensary cap and asking the Court to disregard the dispensary locationsDecember 31, 2023 we had open and/or applied for prior to the limitation becoming effective. On February 4, 2019, we announced that we won our lawsuit in the trial court, with the court ruling that we may open an additional 14 dispensary locations based on these locations having previously vested. Moreover, the Court ruled that in the alternative, the statutory caps placed on the number of dispensaries allowed across the State were not only unconstitutionally added after Amendment 2 had been approved by voters but were also adversely impacting customer access. We have since settled our challenge with the Florida Department of Health. Our 14 dispensaries that were established before the statewide cap was enacted are now excluded from the statutory cap. The statutory cap expired in April 2020; thus, neither Trulieve US nor its competitors in Florida are subject to restrictions on the number of dispensaries that may be opened. As of March 16, 2022, we had 113131 approved dispensaries in the State of Florida. In addition, our license allows us to deliver products directly to customers.Reporting RequirementsFlorida law called for the OMMU to establish, maintain, and control a computer software tracking system that traces cannabis from seed to sale and allows real-time, 24-hour access by the OMMU to such data. The tracking system must allow for integration of other seed-to-sale systems and, at a minimum, include notification of certain events, including when marijuana seeds are planted, when marijuana plants are harvested and destroyed, and when cannabis is transported, sold, stolen, diverted, or lost. Each medical marijuana treatment center shall use the seed-to-sale tracking system established by the OMMU or integrate its own seed-to-sale tracking system with the seed-to-sale tracking system established by the OMMU. At this time, the OMMU has not implemented a statewide seed-to-sale tracking system; thus, we use our own seed-to-sale system. Additionally, the OMMU maintains a patient and physician registry, and licensees must comply with all requirements and regulations regarding the provision of required data or proof of key events to said system to retain their license. Florida requires all MMTCs to abide by representations made in their original application to the State of Florida or any subsequent variances to the same. The OMMU must approve any changes or expansions of previous representations and disclosures to the OMMU viasponsored an amendment or variance process.Florida Licensing RequirementsLicenses issued by the OMMU may be renewed biennially so long as the licensee continues to meet the requirements ofadult-use legalization measure, the Florida Statute 381.986 and pays a renewal fee. Individuals or entities who directly or indirectly own, control, or hold with power to vote five percent or more ofMarijuana Legalization Initiative (Initiative #22-05), that may appear on the voting shares of a MMTC may not acquire direct or indirect ownership or control of any voting shares or other form of ownership of any other MMTC. Applicants must demonstrate (and licensed MMTC’s must maintain) that: (i) they have been registered to do businessballot in the State of Florida for the previous five years, (ii) they possess a valid certificate of registration issuedNovember 2024 if approved by the Florida Department of Agriculture & Consumer Services, (iii) they have the technical and technological ability to cultivate and produce cannabis, including, but not limited to, low-THC cannabis, (iv) they have the ability to secure the premises, resources, and personnel necessary to operate as an MMTC, (v) they have the ability to maintain accountability of all raw materials, finished products,25and any by-products to prevent diversion or unlawful access to or possession of these substances, (vi) they have an infrastructure reasonably located to dispense cannabis to registered qualified patients statewide or regionally as determined by the OMMU, (vii) they have the financial ability to maintain operations for the duration of the two-year approval cycle, including the provision of certified financial statements to the OMMU, (viii) all owners, officers, board members and managers have passed a Level II background screening, inclusive of fingerprinting, (ix) they ensure that a medical director is employed to supervise the activities of the MMTC, and (x) they have a diversity plan and veterans plan accompanied by a contractual process for establishing business relationships with veterans and minority contractors and/or employees. Upon approval of the application by the OMMU, the applicant must post a performance bond of up to US $5 million, which may be reduced to US $2 million once the licensee has served 1,000 patients (which Trulieve has accomplished).There was a lawsuit that challenged essential aspects of the 2017 Legislation and OMMU regulations. In December 2017, Florigrown, LLC and other plaintiffs challenged aspects of the 2017 Legislation and OMMU regulations as unconstitutional due to: (1) requiring MMTCs to be vertically integrated (e.g., cultivate and process the cannabis to be sold at the MMTC’s own licensed dispensaries); (2) the cap of total number of MMTC licenses in the State; and (3) the authorization of the OMMU to issue MMTC licenses to certain applicants that met criteria defined by the 2017 legislation. On October 18, 2019, a trial judge in the Circuit Court for Leon County ruled that Florigrown, LLC had a substantial likelihood of succeeding on its claims, holding that the vertical integration and licensing cap conflicted with the language in Article X, Section 29 and that the provisions in 2017 defining the criteria for eligibility for MMTC licensure constituted an impermissible “special law” under Article III, Section 11(a)(12) of the Florida Constitution. On July 10, 2019, an intermediate appellate court affirmed aspects of the Circuit Court for Leon County’s ruling. On May 27, 2021, the Florida Supreme Court issuedfollowing a 6-1 decision findinglegal challenge by the State Attorney General. In January 2024, Florida Governor Ron DeSantis announced that Florigrown, LLC andhe believes the other plaintiffs did not demonstrate a substantial likelihood of successSupreme Court will approve the adult-use cannabis initiative to appear on the merits of any of its constitutional claims. In effect, the Florida Supreme Court’s 6-1 decision upholds Florida’s regulatory scheme.Security and Storage Requirements for Cultivation, Processing and Dispensing Facilities in FloridaAdequate outdoor lighting is required from dusk to dawn for all MMTC facilities. 24-hour per day video surveillance is required, and all MMTCs must maintainNovember’s ballot. If at least a rolling 45-day period that is made available to law enforcement and the OMMU upon demand. Alarm systems must be active at all items for all entry points and windows. Interior spaces must also have motion detectors, and all cameras must have an unobstructed view60% of key areas. Panic alarms must also be available for employees to be able to signal authorities when needed.In dispensaries, the MMTC must provide a waiting area with a sufficient seating area. There must also be a minimum of one private consultation/education room for the privacyvoters vote in favor of the patient(s)measure, individuals aged 21 and their caregiver (if applicable). The MMTC may only dispenseolder will be allowed to possess, purchase, or use cannabis products between 7:00 am and 9:00 pm. All active products must be kept in a secure location within the dispensary, and only empty packaging may be kept in the dispensary’s general area, which is readily accessible to customers and visitors. No product or delivery devices may be on display in the waiting area.An MMTC must at all times provide secure and logged access for all cannabis materials. This includes approved vaults or locked rooms. There must be at least two employees of the MMTC, or an approved security provider on-site at all times. All employees must wear proper identification badges, and visitors must be logged in and wear a visitor badge while on the premises. The MMTC must report to local law enforcement any loss, diversion, or theft of cannabis materials within 24 hours of becoming aware of such an occurrence.Florida Transportation RequirementsWhen transporting cannabis to dispensaries or customers for delivery, a manifest must be prepared, and transportation must be done using an approved vehicle. The cannabis must be stored in a separate, locked area of the vehicle, and at all times while in transit, there must be two people in a delivery vehicle. During deliveries, one person must remain with the vehicle. The delivery employees must at all times have identification badges. The manifest must include the following information: (i) departure date and time; (ii) name, address,accessories and license numberholders will be authorized to serve the adult-use market.the originating MMTC; (iii) name and address of the receiving entity; (iv) the quantity and form of cannabis and delivery device; (v) arrival date and time; (vi) the make, model and license plate of the delivery vehicle; and (vii) the name and signatures of the MMTC delivery employees. The MMTC must keep these manifests for inspection for up to three years. During the delivery, a copy of the manifest is also provided to the recipient.OMMU Inspections in FloridaThe OMMU may conduct announced or unannounced inspections of MMTCs to determine compliance with applicable laws and regulations. The OMMU is to inspect an MMTC upon receiving a complaint or notice that the MMTC has dispensed cannabis containing mold, bacteria, or other contaminants that may cause an adverse effect to humans or the environment. The OMMU is to26conduct at least a biennial inspection of each MMTC to evaluate the MMTC’s records, personnel, equipment, security, sanitation practices, and quality assurance practices.See Appendix A to this Annual Report on Form 10-K for details regarding the license associated with our operations in Florida.Regulation ofMedical Cannabis in GeorgiaOn April 17, 2019, Governor Brian Kemp signed into law the Hope Act which createscreated a regulatory scheme to permit the cultivation, production, manufacturing, and sale of low-THC oil that is oil withnot more than 5% by weight of THC, THCa, or a combination of THC content of no greater than 5%,and THCa, for provision to patients for medical purposes. In specific, the Hope Act created a seven-member Georgia Access to Cannabis Commission (the “GA Commission”), charged with drafting regulations to implement the low-THC oil program. Under the Hope Act, the GA Commission is permitted to issue up to six production licenses – two Class 1 licenses allowing up to 100,000 square feet of cultivation canopy each and four Class 2 licenses allowing up to 50,000 square feet of cultivation canopy each – and is also required to issue licenses to the University of Georgia and Fort Valley State University, in the event that either University chooses to participate, for cultivation and research regarding low-THC oil. The Hope Act envisions that the Georgia Board of Pharmacy would develop and oversee licensing criteria by which pharmacies would be permitted to dispense low-THC oil; the GA Commission is also expected to issue rules to license free-standing dispensaries. On July 24, 2021, the GA Commission announced its intention to award the two Class 1 and four Class 2 production licenses, including the award of one Class 1 license to Trulieve GA Inc. The regulations for the licensure of dispensaries have not currently been promulgated, and so there are no currently licensed dispensaries in Georgia.whichif they have certain qualifying conditions. The registry is administered by the Georgia Department of Public Health. Qualifying conditions include: (1) end-stage cancer-producing wasting illness or recalcitrant nausea and vomiting,(2) severe or end-stage amyotrophic lateral sclerosis, (3) seizure disorders, (4) multiple sclerosis, (5) Crohn’s disease, (6) mitochondrial disease, (7) severe or end-stage Parkinson’s disease, (8) severe or end-stage sickle cell disease, (9) severe Tourette’s syndrome, (10) autism spectrum disorder, (11) epidermolysis bullosa, (12) severe or end-stage Alzheimer’s diseases, (13) severe or end-stage AIDS, (14) severe or end-stage peripheral neuropathy, (15) inpatient or outpatient hospice treatment, (16) intractable pain, and (17) post-traumatic stress disorder. At present, Georgia law prohibits registered patients from ingesting low-THCthe production or sale of low THC oil through any manneredibles and vaporizers.employs a heating element or similar means to produce vapors, including combustible products as well as vaporizers.The GA Commission is authorized to issue regulationsallows for the oversightsale of cannabis by traditional pharmacies, however the DEA intervened by issuing a warning letter on November 27, 2023, advising that neither cannabis nor THC can be lawfully dispensed by a DEA-registered pharmacy. At the most recent Board of Pharmacy meeting held on December 13, 2023, the Board voted to request legal guidance from the state Attorney General’s office. Note, the appropriation riders mentioned above that bar the DOJ, inclusive of the low-THC oil program. At present, the GA Commission has not promulgated such regulations. The Hope Act contemplates that the GA Commission will issue regulations in the following categories:•to establish requirements related to quality control, security, and oversight of production. In particular, licensees will be required to establish security plans that include 24/7 monitoring and intrusion detection, recording and video storage systems, and licensed security personnel.•to establish requirements related to secured transportation of low-THC oil products and tracking of deliveries.•to prohibit pesticides in production, except for pesticides certified as organic by the Organic Materials Review Institute or a similar standards organization.•to establish requirements related to testing for purity and dosage levels, and to ensure that product labels accurately reflect product content.•to establish requirements for a track and trace system. In specific, the track and trace system will be operated by a GA Commission-approved vendor that licensees will be required to use. The system will need to be capable of tracking plants, products, and registered patient purchase totals, as well as waste, transfers, conversions, sales, and returns. It will also need to be able to track plants, low-THC oil, and products throughout the entire chain of custody, as well as monitor inventory and prevent theft, loss, and diversion. The track and trace system will provide real-time data to the GA Commission related to total low-THC oil daily sales, the total number of marijuana plants currently in production, and the number of plants destroyed or disposed of.•to require licensees to collect and report data.•to regulate marketing and signage related to low-THC oil, including prohibiting the direct advertisement of low-THC oil to registered patients or the public.•to prohibit cultivation, processing, and dispensing facilities within 3,000 feet of schools, early care and education programs, and places of worship.27The GA Commission is also authorizedDEA, from spending taxpayer funds to enforce the various regulatory requirements noted above, such as through inspections and collaborationlaws that interfere with state medical cannabis laws, makes prosecution under federal law enforcement.MarketMarkets in MarylandRegulatory LandscapeIn 2012, a state law was enactedlegalized medical cannabis in Maryland to establish a2013, and its state-regulated medical marijuana program. Legislation was signed in May 2013, and thecannabis program became operational on December 1, 2017. The Maryland Medical Cannabis Commission (the “MMCC”) regulates the state program and awarded operationalinitial cannabis business licenses in a highly competitive application process. One hundred two dispensary licenses were awarded out of a pool of over 800 applicants, while an original 15 cultivation licenses were awarded out of a pool of nearly 150 applicants. In April 2018, Maryland lawmakers agreed to expand the state’s medical marijuana industry by authorizing an additional 20 licenses, seven for cultivation and 13 for processing. The state medical program allows access to medical marijuanacannabis for patients with qualifying chronic or debilitating diseases or medical conditions, including but not limited to:to chronic pain, nausea, seizures, glaucoma, PTSD, and other conditions which are severe and for which other treatments have been ineffective.There are three principal medicallicense categoriesfor adults 21 years or older, effective July 2023. The Cannabis Reform Act, signed into law in Maryland: (1) grower, (2) processor,May 2023, created the framework for adult-use cannabis and (3) dispensary. We have control and/or ownership over one grower license, one processor license, and three dispensaries. All licenses are, as of the date hereof, active with the State of Maryland. The licenses are independently issued for each approved activity for use at our facilities in Maryland.All grower, processor, and dispensary establishments must register with the MMCC under the provisions ofestablished the Maryland Medical Cannabis Law, Md. Code, Health-Gen § 13-3301 et seq.Administration (the “MCA”), known as the Natalie M. LaPrade Medical Cannabis Commission. If applications contain all required information, establishments are issued a medical marijuana license.Licenses are valid for a period of six years of initial licensure, and annual license fees must be paidsuccessor agency to maintain licensure. After the expiration of the initial six-year licensure period, the licensee is required to renew the license every four years thereafter. Licensees are required to submit a renewal application per the guidelines published by the MMCC. Ninety days prior to the expiration of a license, the MMCC notifies the licensee of the date on which the license expires and provides the instructions and fee required to renew the license along with the consequences of failure to renew. At least 30 calendar days before a license expires, the licensee must submit the renewal application as provided by the MMCC. The annual licensing feeMCA is responsible for a grower is $125,000, $40,000 for a processor,administering and $40,000 for a dispensary.The medical grower license permits us to cultivate, manufacture, package, and distribute medical cannabis to licensed processors, licensed dispensaries, or registered independent testing laboratories. The medical processor license permits us to transformenforcing the medical and adult-use cannabis into another product or extractlaws, including licensing, registration, testing, inspection, and package and label medical cannabis. The dispensary licenses permit us to acquire, possess, repackage, process, transfer, transport, sell, distribute, or dispense products containing medical cannabis, related supplies, related products including tinctures, aerosols, oils, or ointments, or educational materials for use by a qualifying patient or caregiver.Dispensing RequirementsIn order to dispense medical cannabis, a licensed dispensary is required to comply with various dispensing requirements: (1) require presentment of a written certification from a qualifying patient or caregiver, (2) query the MMCC’s data network to verify that the patient is currently registered and has a certification from a provider, as well as the amount of medical cannabis that has already been dispensed pursuant to the written certification,(3) dispense no more than a 30-day supply, and (4) decline to dispense medical cannabis if the patient or caregiver appears to be under the influence of drugs or alcohol. Registered patients and caregivers are required to provide attestations relating to their knowledge of the status of medical cannabis under Maryland and Federal law, as well as limitations on the use of medical cannabis, such as keeping away from children and refraining from transfer to any other person.Requirements Relating to Recordkeeping and Inventory ControlLicensed growers are required to use a perpetual inventory system that identifies and tracks each lot of medical cannabis from the time of propagation to the time it is delivered to a licensed dispensary and dispensed to a qualified patient. The perpetual inventory system is required to be capable of tracking cannabis back to its original source and to promptly identify discrepancies. Each plant within the perpetual inventory system is required to be assigned a unique identifier that is traceable through the entire production and dispensing lifecycle. Licensees are required to investigate discrepancies identified by the perpetual inventory system and to report such discrepancies to the MMCC and Maryland State Police where the licensee finds evidence of theft or diversion.Licensed growers, processors, and dispensaries are required to retain, independent of the required inventory control system, a searchable, secure, tamper-evident record of distribution regarding the names and addresses of recipients, the quantity provided,enforcement, and the28name, strength, batch number and lot number promulgation of the product provided. Such records must be available to certifying providers upon request. Licensees are also required to retain records of production and distribution of each batch and lot and of daily checklists to maintain uniformity from batch to batch, as well as a log of all visitors to licensed premises. Records must be kept in duplicate at a secure off-site location.Requirements Relating to SecurityLicensees are required to take measures to ensure the safety and security of licensed premises such as: (1) constructing the premises so as to prevent unauthorized entry, (2) constructing secure rooms for the storage of cannabis that are not along the exterior wall of a facility and that have access-controlled entry, (3) utilizing exterior lighting fixtures to ensure proper surveillance, (4) installing security alarms covering perimeter entry points, which shall be continuously monitored, (5) installing separate alarm systems to protect on-site and off-site record storage areas, as well as areas used for cannabis storage, and (6) utilizing motion-activated video surveillance that covers entrances and exits to a facility, as well as any area that cannabis is packaged, tested, processed, stored, or dispensed.Maryland Reporting RequirementsThe State of Maryland uses METRC as the state’s computerized seed-to-sale tracking system. Individual licensees, whether directly or through third-party integration systems, are required to push data to the state to meet all reporting requirements. We use a third-party application for our computerized seed to sale software, which integrates with the state’s METRC program and captures the required data points for growing, processing and, dispensing as required in the Maryland Medical Cannabis law.MMCC InspectionsLicensees are required to submit to announced and unannounced inspections by the MMCC, including but not limited to inspections based upon an allegation of noncompliance.See Appendix A to this Annual Report on Form 10-K for a list of the licenses associated with our operations in Maryland.Cannabis Marketand Adult-Use Markets in MassachusettsOhioThe Commonwealth of Massachusetts has authorized the cultivation, possession, and distribution of marijuana for medical purposes by certain licensed Massachusetts marijuana businesses. The Medical Use of Marijuana Program, or MUMP, registers qualifying patients, personal caregivers, Medical Marijuana Treatment Centers, or MTCs, and MTC agents. MTCs were formerly known as Registered Marijuana Dispensaries or RMDs. The MUMP was established by Chapter 369 of the Acts of 2012, “An Act for the Humanitarian Medical Use of Marijuana,” following the passage of the Massachusetts Medical Marijuana Initiative, Ballot Question 3, in the 2012 general election. Additional statutory requirements governing the MUMP were enacted by the Legislature in 2017 and codified at G.L. c. 94I, et. seq. (referred to herein as the “Massachusetts Medical Act”). MTC Licenses are vertically integrated licenses in that each MTC license holder must cultivate, process, and dispense medical marijuana. Under a single MTC License, a licensee may not operate more than two locations in Massachusetts at which medical marijuana is cultivated, marijuana-infused products are manufactured, and marijuana is dispensed. No Person or Entity Having Direct or Indirect Control shall be granted, or hold more than three MTC Licenses.The Commonwealth of Massachusetts Cannabis Control Commission, or CCC, regulations, 935 CMR 501.000 et seq. (referred to herein as the “Massachusetts Medical Regulations”), provide a regulatory framework that requires MTCs to cultivate, process, transport, and dispensea vertically integrated marketplace. Patients with debilitating medical conditions qualify to participate in the program, including conditions such as cancer, glaucoma, positive status for human immunodeficiency virus (HIV), acquired immune deficiency virus (AIDS), hepatitis C, amyotrophic lateral sclerosis (ALS), Crohn’s disease, Parkinson’s disease, and multiple sclerosis (MS) when such diseases are debilitating, and other debilitating conditions as determined in writing by a qualifying patient’s healthcare provider.The CCC assumed control of the MUMP from the Department of Public Health on December 23, 2018. The CCC approved revised regulations for the MUMP on November 30, 2020, and the revised regulations became effective on January 8, 2021.Massachusetts Licensing Requirements (Medical)The Massachusetts Medical Regulations delineate the licensing requirements for MTCs in Massachusetts. Licensed entities must demonstrate the following: (i) they are licensed and in good standing with the Secretary of the Commonwealth of Massachusetts; (ii) no person or entity has “Direct or Indirect Control” over more than three MTC licenses; (iii) under a single MTC License, a licensee may not operate more than two locations in Massachusetts at which medical marijuana is cultivated, marijuana infused products are manufactured, and marijuana is dispensed; (iv) MTC agents must be registered with the Massachusetts Cannabis Control Commission; (v) an MTC must have a program to provide reduced cost or free marijuana to patients with documented verifiable financial hardships;29(vi) one executive of an MTC must register with the Massachusetts Department of Criminal Justice Information Services on behalf of the entity as an organization user of the Criminal Offender Record Information (CORI) system; (vii) the MTC applicant has initial capital resources of at least $500,000 in its control as evidenced by bank statements, lines of credit or equivalent; and (viii) payment of the required application fee.In an MTC application, an applicant must also demonstrate or include: (i) disclosure of each Person or Entity Having Direct or Indirect Control over the MTC’s operations; (ii) a plan to obtain liability insurance coverage; (iii) detailed summary of the business plan and proposed timeline for the MTC; (iv) an operational plan for the cultivation of marijuana and manufacturing of marijuana products, including a detailed summary of policies and procedures; and (v) a detailed summary of the operating policies and procedures for the MTC including security, prevention of diversion, storage of marijuana, transportation of marijuana, inventory procedures, procedures for quality control and testing of the product for potential contaminants, personnel policies, dispensing procedures, record-keeping procedures, plans for agent qualifications and training, and any plans for patient or personal caregiver home delivery. An MTC applicant must also: (i) disclose marijuana business interests in Massachusetts and other jurisdictions; (ii) provide documentation of a property interest in the proposed address; (iii) provide documentation that the applicant has held a community outreach meeting and entered in a Host Community Agreement with the host municipality; (iv) describe compliance with local codes, ordinances, and bylaws; (v) provide a diversity plan and a plan to positively impact “Areas of Disproportionate Impact”; and (vi) disclosure of relevant criminal, civil and administrative background matters. Finally, an MTC applicant must specify a cultivation tier for their license, which establishes the minimum and maximum square footage of canopy for their cultivation operation.After receipt of a Provisional MTC license, the CCC shall review architectural plans for the building of the MTC’s facilities, and once approved, the MTC provisional license holder shall construct its facilities in conformance with the requirements of the Massachusetts Regulations. Once the CCC completes its inspections of the facilities, the CCC shall issue a Final MTC License to the MTC applicant. A licensee may not begin cultivating marijuana until it has been issued a Final MTC License by the CCC.MTC Licenses in Massachusetts are renewed annually. Licensees are required to submit a renewal application at least 60 days prior to the expiration date of the license. While renewals are granted annually, there is no ultimate expiry after which no renewals are permitted. Additionally, in respect of the renewal process, provided that the requisite renewal fees are paid, the renewal application is submitted in a timely manner, and there are no material violations noted against the applicable license, we would expect to receive the applicable renewed license in the ordinary course of business.Massachusetts Dispensary Requirements (Medical)An MTC shall follow its written and approved operating procedures in the operation of its dispensary locations. Operating procedures shall include (i) security measures in compliance with the Massachusetts Medical Regulations; (ii) employee security policies including personal safety and crime prevention techniques; (iii) hours of operation and after-hours contact information; (iv) a price list for marijuana products; (v) storage and waste disposal protocols in compliance with state law; (vi) a description of the various strains of marijuana that will be cultivated and dispensed, and the forms that will be dispensed; (vii) procedures to ensure accurate recordkeeping including inventory protocols; (viii) plans for quality control; (ix) a staffing plan and staffing records; (x) diversion identification and reporting protocols; (xi) policies and procedures for the handling of cash on MTC premises including storage, collection frequency and transport to financial institutions; (xii) emergency procedures and procedures to promote workplace safety; (xiii) alcohol, smoke and drug free workplace policies; (xiv) plan for maintaining confidential information and records; (xv) procedures for determining prices and provision of free or reduced costs marijuana and marijuana products to patients with verified financial hardships; (xvi) policies and procedures for energy efficiency and conversation; and (xvii) procedures for patient education activities. The siting of dispensary locations is expressly subject to local/municipal approvals pursuant to state2016 via House Bill 523 into law, and municipalities may impose local permitting and licensing requirements that an MTC must comply with. More specifically, an MTC must comply with all local requirements regarding siting. The Massachusetts Medical Regulations require an MTC’s entrance to be no closer than 500 feet from the nearest school entrance, but this buffer zone distance may be reduced by a local municipality’s ordinance or bylaw. The Massachusetts Medical Regulations require that MTCs limit their inventory of seeds, plants, and usable marijuana to reflect the projected needs of registered qualifying patients. An MTC may only dispense to a registered qualifying patient or caregiver who has a currently valid certification.Massachusetts Security and Storage Requirements (Medical)An MTC must implement sufficient security measures to deter and prevent unauthorized entrance into areas containing marijuana and theft of marijuana at the MTC. These measures must include: (i) allowing only registered qualifying patients, caregivers, dispensary agents, authorized persons, or approved outside contractors access to the MTC facility; (ii) preventing individuals from remaining on the premises of an MTC if they are not engaginglegalized adult-use cannabis in activities that are permitted; (iii) disposing of marijuana or by-products in compliance with the law; (iv) establishing limited access areas accessible only to authorized personnel; (v) storing finished marijuana in a secure locked safe or vault; (vi) keeping equipment, safes, vaults or secured areas securely locked; (vii) ensuring that the outside perimeter of the MTC is sufficiently lit to facilitate surveillance; and (viii) ensuring that landscaping or foliage outside of the MTC does not allow a person to conceal themselves. An MTC shall also utilize a security/alarm system that: (i) monitors entry and exit points and30perimeter windows, (ii) includes a panic, duress, or holdup alarm, (iii) includes system failure notifications, (iv) includes 24-hour video surveillance of safes, vaults, sales areas, areas where marijuana is cultivated, processed or dispensed, and (v) includes date and time stamping of all recordings and the ability to produce a clear, color still photo. The security system shall have the capacity to remain operational during a power outage. The MTC must also maintain a backup alarm system with the capabilities of the primary system, and both systems are to be maintained in good working order and are to be inspected and tested at regular intervals.Massachusetts Transportation Requirements (Medical)Marijuana and marijuana-infused products, or MIPs, may be transported between licensed MTCs by MTC agents or agents of a licensed Marijuana Transporter on behalf of an MTC. MTCs or licensed Marijuana Couriers may, with CCC approval, transport marijuana or MIPS directly to registered qualifying customers and Caregivers as part of a home delivery program. An MTC shall staff transport vehicles with a minimum of two MTC agents. At least one agent shall remain with the vehicle when the vehicle contains marijuana or MIPs. Prior to leaving the origination location, an MTC must weigh, inventory, and account for, on video, the marijuana to be transported.Marijuana products must be packaged in sealed, labeled, and tamper-proof or child-resistant packaging prior to and during transportation. In the case of an emergency stop, a log must be maintained describing the reason for the stop, the duration, the location, and any activities of personnel exiting the vehicle. An MTC shall ensure that transportation times and routes are randomized. Each MTC agent shall carry his or her CCC-issued Agent Registration Card when transporting marijuana or MIPs and shall produce it to CCC representatives or law enforcement officials upon request. Where videotaping is required when weighing, inventorying, and accounting of marijuana before transportation or after receipt, the video must show each product being weighed, the weight, and the manifest. An MTC must document and report any unusual discrepancy in weight or inventory to the CCC and local law enforcement within 24 hours.An MTC shall report to the CCC and local law enforcement any vehicle accidents, diversions, losses, or other reportable incidents that occur during transport within 24 hours. An MTC shall retain transportation manifests for no less than one year and make them available to the CCC upon request. Vehicles used in transportation must be owned or leased, be properly registered, and contain a GPS system that is monitored during transport of marijuana and said vehicle must be inspected and approved by the CCC prior to use.During transit, an MTC must ensure that: (i) marijuana or MIPs are transported in a secure, locked storage compartment that is part of the vehicle transporting the marijuana or MIPs; (ii) the storage compartment cannot be easily removed (for example, bolts, fittings, straps or other types of fasteners may not be easily accessible and not capable of being manipulated with commonly available tools); (iii) marijuana or MIPs are not visible from outside the vehicle; (iv) product is transported in a vehicle that bears no markings indicating that the vehicle is being used to transport marijuana or MIPs. Each MTC agent transporting marijuana or MIPs shall have access to a secure form of communication with personnel at the origination location when the vehicle contains marijuana or MIPs.CCC Inspections (Medical)The CCC or its agents may inspect an MTC and affiliated vehicles at any time without prior notice. An MTC shall immediately, upon request, make available to the CCC information that may be relevant to a CCC inspection, and the CCC may direct an MTC to test marijuana for contaminants. Any violations found may be noted in a deficiency statement that will be provided to the MTC, and the MTC shall thereafter submit a Plan of Correction to the CCC outlining with particularity each deficiency and the timetable and steps to remediate the same. The CCC shall have the authority to suspend or revoke an MTC License in accordance with the applicable regulations.Regulation of the Adult-Use Cannabis Market in MassachusettsAdult-use (recreational) marijuana has been legal in Massachusetts since December 15, 2016, following2023 via Issue 2, a ballot initiative in November of that year.measure to legalize, regulate, and tax adult-use cannabis. The CCC licenses various types of adult-use marijuana businesses, including cultivators, product manufacturers, and retailers (referred to herein collectively as “Marijuana Establishments”) pursuant to 935 CMR 500.000 et seq. The first adult-use marijuana facilities in Massachusetts began operating in November 2018. The CCC approved revised regulations for the adult-use program effective November 1, 2019, and January 8, 2021.Massachusetts Licensing Requirements (Adult-Use)Many of the same application requirements exist for an adult-use Marijuana Establishment license application as to those for a medical MTC application, and each person having “Direct or Indirect Control” must undergo background checks and fingerprinting with the CCC. Applicants must submit the location and identification of each site and must establish a property interest in the same, and the applicant and the local municipality must have entered into a host community agreement authorizing the location of the adult-use Marijuana Establishment within the municipality and a host community agreement certification form must be included in the application. Applicants must include disclosure of any regulatory actions against it by the Commonwealth of Massachusetts, as well as the civil, criminal, and administrative history of the applicant and alllaw allows persons and entities having “Direct or Indirect Control.” The application31must include, amongst other information, the proposed timeline for achieving operations, liability insurance, business plan, and a detailed summary describing the Marijuana Establishment’s proposed operating policies, including security, prevention of diversion, storage, transportation, inventory procedures, quality control, dispensing procedures, personnel policies, record keeping, maintenance of financial records, diversity plans, and employee training protocols.Massachusetts Dispensary Requirements (Adult-Use)Marijuana retailerswho are subject to certain operational requirements in addition to those imposed on Marijuana Establishments generally. Dispensaries must immediately inspect consumers’ identification to ensure that everyone who enters is at least 21 years of age. Dispensaries may not sell more than one ounceage to purchase and possess up to 2.5 oz of marijuana or fiveflower and 15 grams of marijuana concentrate per transaction. Point-of-sale systems must be approved byextract. Issue 2 established the CCC, and retailers must record sales data. Records must be retained and available for auditing byDivision of Cannabis Control (“Division”) within the CCC and Department of Revenue. Retailers are required to conduct monthly analyses of equipment and sales data to determine that such systems have not been altered or interfered with to manipulate sales data, and to report any such discrepancies toCommerce, which is now the CCC.Dispensaries must also make educational materials available to consumers in commonly spoken languages designated by the CCC, with analogous materialsregulatory authority for visually- and hearing-impaired persons. Such materials must include:•A warning that marijuana has not been analyzed or approved by the FDA, that there is limited information on side effects, that there may be health risks associated with using marijuana, and that it should be kept away from children;•A warning that when under the influence of marijuana, driving is prohibited, and machinery should not be operated;•Information to assist in the selection of marijuana, describing the potential differing effects of various strains of marijuana, as well as various forms and routes of administration;•Materials offered to consumers to enable them to track the strains used and their associated effects;•Information describing proper dosage and titration for different routes of administration, with an emphasis on using the smallest amount possible to achieve the desired effect and the impact of potency;•A discussion of tolerance, dependence, and withdrawal;•Facts regarding substance use disorder signs and symptoms, as well as referral information for substance use disorder treatment programs;•A statement that consumers may not sell marijuana to any other individual;•Information regarding penalties for possession or distribution of marijuana in violation of Massachusetts law; and•Any other information required by the CCC.Massachusetts Security and Storage Requirements (Adult-Use)Each Marijuana Establishment must implement sufficient security measures to deter and prevent unauthorized entrance into areas containing marijuana and theft of marijuana at the establishment. Security measures taken by the establishments to protect the premises, employees, consumers, and the general public shall include, but not be limited to, the following:•Positively identifying and limiting access to individuals 21 years of age or older who are seeking access to the Marijuana•Establishment or to whom marijuana products are being transported;•Adopting procedures to prevent loitering and ensure that only individuals engaging in activity expressly or by necessary implication permitted by state law are allowed to remain on the premises;•Proper disposal of marijuana in accordance with applicable regulations;•Securing all entrances to the Marijuana Establishment to prevent unauthorized access;•Establishing limited access areas which shall be accessible only to specifically authorized personnel limited to include only the minimum number of employees essential for efficient operation;•Storing all finished marijuana products in a secure, locked safe or vault in such a manner as to prevent diversion, theft, or loss;•Keeping all safes, vaults, and any other equipment or areas used for the production, cultivation, harvesting, processing or storage, including prior to disposal, of marijuana or marijuana products securely locked and protected from entry, except for the actual time required to remove or replace marijuana;32•Keeping all locks and security equipment in good working order;•Prohibiting keys, if any, from being left in the locks or stored or placed in a location accessible to persons other than specifically authorized personnel;•Prohibiting accessibility of security measures, such as combination numbers, passwords, or electronic or biometric security systems, to persons other than specifically authorized personnel;•Ensuring that the outside perimeter of the marijuana establishment is sufficiently lit to facilitate surveillance, where applicable;•Ensuring that all marijuana products are kept out of plain sight and are not visible from a public place, outside of the marijuana establishment, without the use of binoculars, optical aids, or aircraft;•Developing emergency policies and procedures for securing all product following any instance of diversion, theft, or loss of marijuana, and conduct an assessment to determine whether additional safeguards are necessary;•Establishing procedures for safe cash handling and cash transportation to financial institutions to prevent theft, loss, and associated risks to the safety of employees, customers, and the general public;•Sharing the Marijuana Establishment’s floor plan or layout of the facility with law enforcement authorities, and in a manner and scope as required by the municipality and identifying when the use of flammable or combustible solvents, chemicals, or other materials are in use at the Marijuana Establishment;•Sharing the Marijuana Establishment’s security plan and procedures with law enforcement authorities, including police and fire services departments, in the municipality where the Marijuana Establishment is located and periodically updating law enforcement authorities, police and fire services departments, if the plans or procedures are modified in a material way; and•Marijuana must be stored in special limited access areas, and alarm systems must meet certain technical requirements, including the ability to record footage to be retained for at least 90 days.Massachusetts Transportation Requirements (Adult-Use)Marijuana products may only be transported between licensed Marijuana Establishments by registered Marijuana Establishment agents. A licensed marijuana transporter may contract with a Marijuana Establishment to transport that licensee’s marijuana products to other licensed establishments. All transported marijuana products are linked to the seed-to-sale tracking program. Any marijuana product that is undeliverable or is refused by the destination Marijuana Establishment shall be transported back to the originating establishment. All vehicles transporting marijuana products shall be staffed with a minimum of two Marijuana Establishment agents. At least one agent shall remain with the vehicle at all times that the vehicle contains marijuana or marijuana products. Prior to the products leaving a Marijuana Establishment, the originating Marijuana Establishment must weigh, inventory, and account for, on video, all marijuana products to be transported. Within eight hours after arrival at the receiving Marijuana Establishment, the receiving establishment must re-weigh, re-inventory, and account for, on video, all marijuana products transported. Marijuana products must be packaged in sealed, labeled, and tamper or child-resistant packaging prior to and during transportation. In the case of an emergency stop during the transportation of marijuana products, a log must be maintained describing the reason for the stop, the duration, the location, and any activities of personnel exiting the vehicle. A Marijuana Establishment or a marijuana transporter transporting marijuana products is required to ensure that all transportation times and routes are randomized and remain within Massachusetts.Vehicles must additionally be equipped with a video system that includes one or more cameras in the storage area of the vehicle and one or more cameras in the driver area of the vehicle. The video cameras must remain operational at all times during the transportation process and have the ability to produce a clear color still photo, whether live or recorded, with a date and time stamp embedded, and that does not significantly obscure the picture.Vehicles used for transport must be owned or leased by the Marijuana Establishment or transporter, and they must be properly registered, inspected, and insured in Massachusetts. Marijuana may not be visible from outside the vehicle, and it must be transported in a secure, locked storage compartment. Each vehicle must have a global positioning system, and any agent transporting marijuana must have access to a secure form of communication with the originating location.CCC InspectionsThe CCC or its agents may inspect a Marijuana Establishment and affiliated vehicles at any time without prior notice in order to determine compliance with all applicable laws and regulations. All areas of a Marijuana Establishment, all Marijuana Establishment agents and activities, and all records are subject to such inspection. During an inspection, the CCC may direct a marijuana establishment to test marijuana for contaminants as specified by the CCC, including but not limited to mold, mildew, heavy metals, plant-growth33regulators, and the presence of pesticides not approved for use on marijuana by the Massachusetts Department of Agricultural Resources. Moreover, the CCC is authorized to conduct a secret shopper program to ensure compliance with all applicable laws and regulations.Regulatory Changes for Medical and Adult Use Marijuana in MassachusettsThe CCC voted to adopt significant amendments of both the medical and adult-use cannabis regulations at its meeting on November 30, 2020.programs. The new regulations became effective on January 8, 2021. Significant changes included:•permitting Marijuana “Courier” Licensees to deliver directly to consumers fromDivision must complete the premises of licensed marijuana retailer establishments and Marijuana Delivery Operators to purchase wholesale marijuana products directly from marijuana cultivation and product manufacturer establishments and deliver the products directly to consumers from the Delivery Operator’s warehouse location. Both Marijuana Courier and Marijuana Delivery Operator Licensees are reserved for at least 36 months from the date the first Delivery Operator Licensee receives a notice to commence operations from the CCC for companies majority-owned and controlled by certified Economic Empowerment Priority Applicants or Social Equity Program Participant Applicants, for which Trulieve does not qualify;•permitting Personal Caregivers to be registered to care for more than one – and up to five – Registered Qualifying Patients at one time; and•permitting non-Massachusetts residents receiving end-of-life or palliative care or cancer treatment in Massachusetts to become Registered Qualifying Patients.See Appendix A to this Annual Report on Form 10-K for a list of the licenses associated with our operations in Massachusetts.Regulation of the Cannabis Market in NevadaNevada became a medical marijuana state in 2001. In 2013, the Nevada legislature passed SB374, providing for state licensing of medical marijuana establishments. On November 8, 2016, Nevada voters passed the Nevada Marijuana Legalization Initiative, also known as Question 2, allowingrulemaking process for the sale of marijuana for adult use starting on July 1, 2017. In 2018, the Nevada Department of Taxation (the “DOT”) opened up applications for additionalnew adult-use marijuana dispensary licenses. In December 2018, 61 additional marijuana dispensaryprogram and issue adult-use licenses were issuedto existing medical operators by the DOT. Effective July 1, 2020, the Cannabis Compliance Board obtained regulatory oversight authority from the DOT.Nevada Licenses and RegulationsThere are four principal license categories in Nevada: (1) cultivation, (2) processing, (3) testing laboratory, and (4) sales facility. GreenMart is licensed to operate a medical and adult-use cultivation facility. These licenses are active within the State of Nevada. The licenses are independently issued for each approved activity for use at GreenMart’s facility in Nevada.Under applicable laws, the licenses permit GreenMart to cultivate, process, package, and sell marijuana pursuant to the terms of the licenses, which are issued by the Cannabis Compliance Board under the provisions of Nevada Revised Statutes Title 56. If applications contain all required information, establishments are issued a medical and/or adult-use cannabis establishment license. In a local governmental jurisdiction that issues business licenses, the issuance by the Cannabis Compliance Board of a medical or adult-use cannabis establishment license is considered provisional until the local government has issued a business license for operation and an establishment is in compliance with all applicable local governmental ordinances. Licenses are valid for a period of one year, and the Nevada Cannabis Compliance Board shall issue a renewal license within ten days after the receipt of a renewal application and applicable fee if the license is not then under suspension or has not been revoked.The cultivation license permits GreenMart to cultivate, process, test, package, and sell marijuana to retail marijuana stores, marijuana product manufacturing facilities, and other cultivation facilities.The processing license permits GreenMart to acquire, possess, manufacture, deliver, transfer, transport, supply or sell edible marijuana products or marijuana-infused products to other marijuana production facilities or marijuana sales facilities. A distributor licensee must transport adult-use marijuana to sales facilities.In connection with our management of GreenMart, we were issued a Temporary Marijuana Support Business License by the Department of Business License in Clark County, Nevada, on August 4, 2020.Security RequirementsLicensees are required to ensure that licensed premises are enclosed and locked and that access is limited to officers, board members, and authorized agents. All other visitors must be escorted by an authorized agent. Cultivation facilities may not be visible34from a public place by normal, unaided vision. Licensees are required to maintain security equipment to detect and deter unauthorized intrusion: (1) intrusion detection devices covering the entirety of the cultivation area and the perimeter and exterior of the facility, (2) exterior lighting sufficient to permit surveillance, (3) video cameras covering the entirety of the cultivation area and the perimeter and exterior of the facility, (4) immediate automatic notification to local law enforcement in the event of a breach of security. All malfunctions of security equipment must be logged and reported to local law enforcement and regulators.Recordkeeping and Inventory RequirementsLicensees are required to maintain an inventory control system that tracks cannabis plants from seed to sale. The system must track key inventory figures on an ongoing basis: (1) each day’s beginning inventory, acquisitions, harvests, sales, disbursements, and disposal, (2) acquisitions from other cannabis establishments, (3) batches of cannabis cultivated, and (4) manufactured cannabis products and materials throughout the manufacturing process. The inventory control system must adequately document plant materials from seed to sale and reconcile raw materials and finished products on a per-job basis. The system must also provide for quarterly physical inventory counts.Nevada Reporting RequirementsThe State of Nevada uses METRC (Marijuana Enforcement Tracking Reporting & Compliance) as the State’s computerized T&T system for seed-to-sale. Effective November 1, 2017, all medical and adult-use marijuana establishments in Nevada must report their establishment data to the state of Nevada via METRC. Individual licensees, whether directly or through third-party integration systems, are required to push data to the State to meet all reporting requirements. Upon completion of its acquisition of GreenMart, we plan to use an in-house computerized seed-to-sale software that will integrate with METRC via API (GreenBits), which captures the required data points for cultivation and manufacturing as required in Nevada Revised Statutes section 678B. Nevada also requires licensees to submit quarterly physical inventory counts and monthly reports every quarter.See Appendix A to this Annual Report on Form 10-K for a list of the licenses associated with our operations in Nevada.marijuanacannabis program was signed into law on April 17, 2016, under Act 16, otherwise known as the Medical MarijuanaCannabis Act, and provided access to state residents with one or more qualifying conditions. Pennsylvania has promulgated regulations to implement Act 16, which are primarily found in Chapters1131 through 1210 of Title 28 of the Pennsylvania Code.Under Act 16, medical marijuana refers to marijuana obtained for certified medical use by a Pennsylvania resident with at least 1 of 23 qualifying medical conditions as set by theThe Pennsylvania Department of Health (“PA DOH”). Act 16 initially authorized 17 qualifying conditions; However, through regulatory approval, that list has expanded and now also includes any terminal illness, dyskinetic and spastic movement disorders, and opioid use disorder.Under Act 16 and regulates medical cannabis businesses in the DOH’s implementing regulations, patients who are residents of Pennsylvania and have a qualifying serious medical condition as certified by a physician are able to obtain medical marijuana at approved dispensariesCommonwealth. For licensing purposes, the PA DOH split the Commonwealth into six regions. For each dispensary permit, the locations must be within the Pennsylvania. A registered caregiver of an approved patient may also obtainregion where the permit was awarded. For medical marijuana from an approved dispensary. As of March 30, 2022, Pennsylvania does not permit home delivery of medical marijuana other than through a registered caregiver.On June 30, 2021, Pennsylvania Governor Tom Wolf signed into law H.B. 1024 (also known as Act 44), which made revisions to Pennsylvania’s medical marijuana program, including codification of certain practices permitted under emergency orders issued in responsecannabis grower/processors, the location is limited to the COVID-19 pandemic. The changesregion where the permit a wider range of individuals to serve as caregivers, including employees of long-term care and nursing facilities. The changes also permit dispensaries some additional operational flexibilities, including providing limited, on-site outdoor order pickups, providing remote patient consultations, and providing medical dosages up to a 90 days' supply as opposed to a 30 days' supply.Pennsylvania Permits and RegulationsAct 16 authorized two principal categories of permits for medical marijuana organizations (“MMO”): (1) a grower/ processor permit and (2) a dispensary permit. Act 16 authorized the DOH to issue up to 25 grower/processor permits and up to 50 dispensary permits. A dispensary permit holder may have up to three dispensary locations within the primary region in which the primary facilitywas awarded, but distribution is located. Pennsylvania is divided into six regions, with permits being awarded based on the patient population.Pennsylvania also allows for a clinical registrant permit, which allows clinical registrant permit holders to operate both a grower/processor operation and multiple dispensary locations. Additionally, clinical registrants must partner with an approved medical research institution within Pennsylvania to conduct marijuana-based clinical research programs. All permit holders are required to use35the DOH-approved seed-to-sale tracking software forpermissible across all inventory management and tracking. Pennsylvania currently utilizes the MJFreeway platform.All grower/processor and dispensary facilities must register with the DOH. Registration certificates are valid for a period of one year and are subject to continuing reporting and annual renewal requirements. A grower/processor permit allows a permit holder to acquire wholesale from another grower/processor, possess, cultivate, and manufacture/process into medical marijuana products and/or medical marijuana-infused products, deliver, transfer, have tested, transport, supply, or sell marijuana and related supplies to medical marijuana dispensaries. A grower/processor may transport products itself or may contract with an approved transporter. A grower/processor is not limited to distributing in the region in which it is located and may distribute medical marijuana products to any approved dispensary facility within the Commonwealth.Approved dispensaries may only purchase approved medical marijuana products from a permitted grower/processor and may only dispense to certified patients or caregivers who present valid identification cards. Prior to dispensing medical marijuana products to a patient or caregiver, the dispensary shall: (1) verify the validity of the patient or caregiver identification card using the electronic tracking system; and (2) review the information on the patient’s most recent certification by using the electronic tracking system to access the DOH’s database. The following requirements apply: (i) if a practitioner sets forth recommendations, requirements or limitations as to the form and/or dosage of a medical marijuana product on the patient certification, the medical marijuana product dispensed to a patient or caregiver by a dispensary must conform to those recommendations, requirements or limitations; (ii) if a practitioner does not set forth recommendations, requirements or limitations as to the form or dosage of a medical marijuana product on the patient certification, the physician, pharmacist, physician assistant or certified registered nurse practitioner employed by the dispensary and working at the facility shall consult with the patient or the caregiver regarding the appropriate form and dosage of the medical marijuana product to be dispensed; and (iii) the dispensary shall update the patient certification in the electronic tracking system by entering any recommendation as to the form or dosage of medical marijuana product that is dispensed to the patient.On March 5, 2021, the DOH proposed permanent regulations relating to medical marijuana, replacing the temporary regulations that have governed the program throughout its history. These proposed regulations are in substantially the same form as the temporary regulations, with only a few distinctions, including: (1) an MMO’s change in ownership without the DOH’s knowledge and written approval of all individuals affiliated with the MMO would be a violation of the act and proposed rules; (2) dispensaries and grower/processors must supplement ongoing reports to the DOH with information related to the average price per unit of medical marijuana products sold, as opposed to the per-dose price; (3) the list of reasons for which the DOH may suspend or revoke an MMO’s permit is augmented by adding falsification of information on any applications submitted to the DOH; and (4) principals, as well as employees, who have direct contact with patients or caregivers or who physically handle medical marijuana plants, seeds and products must also complete training. It is anticipated that the regulations will be finalized in 2022.Pennsylvania Permit Categories/TypesTrulieve has various interests in entities with permits for retail operations and grower/processor operations in Pennsylvania.Pennsylvania Department of Health InspectionsThe Pennsylvania Department of Health may conduct announced or unannounced inspections or investigations to determine the MMO’s compliance with its permit. An investigation or inspection may include an inspection of an MMO’s site, facility, vehicles, books, records, papers, documents, data, and other physical or electronic information.See Appendix A to this Annual Report on Form 10-K for a list of the licenses associated with our operations in Pennsylvania.On April 19, 2017,Governor Jim JusticeMedical Cannabis Act, was signed into law in 2017 with the passage of Senate Bill 386 which created aand allows cannabis to be used for certified medical cannabis program foruse by West Virginia residents with serious medical conditions, and permits medical cannabis to be cultivated, processed, and dispensed to registered patients in essentially non-combustible forms. The program is administered by the West Virginia Department of Health and Human Resources, Bureau for Public Health, Office of Medical Cannabis.Cannabis (“OMC”). The OfficeOMC has authority to (1) issue and oversee permits that authorize businesses to grow, process, or dispense medical cannabis in compliance with state law and regulations, (2) register medical practitioners who certify patients as having qualifying serious medical conditions, and (3) register and oversee patients with qualifying conditions.The statute establishes a list OMC has also promulgated regulations governing the activities of qualifying conditions, including (1) cancer, (2) positive status for HIV/AIDS, (3) amyotrophic lateral sclerosis, (4) Parkinson’s disease, (5) multiple sclerosis, (6) spinal cord injury, (7) epilepsy, (8) neuropathy, (9) Huntington’s disease, (10) Crohn’s disease, (11) post-traumatic stress disorder, (12) intractable seizures, (13) sickle cell anemia, (14) severe chronic pain,growers, processors, laboratories, and (15) illness with a prognosis of less than one year of life expectancy.dispensaries, as well as establishing general requirements related to West Virginia’s medical cannabis program.36initially allowed werefor use are pills, oils, gels, creams, ointments, tinctures, liquid, and non-wholedry leaf or plant forms for administration through vaporization. In 2020, the legislature passed SB 339, adding “dry leafvaporization or plant form.”nebulization, and dermal patch. Dispensaries cannot sell edibles, but medical cannabis products could be mixed into food or drinks by patients themselves. Vaporization (or oils) is allowed, but smoking is prohibited.In additionSenate Bill 386, codified in Chapter 16A of the West Virginia Code, the Office of Medical Cannabis has also promulgated regulations governing the activities of growers, processors, laboratories, dispensaries,legalize personal use and general provisions of West Virginia’s medical marijuana program.The West Virginia statute creates three categories of licenses that a cannabis business may obtain: (1) grower, (2) processor, and (3) dispensary, corresponding to the growing of medical cannabis, the processingpossession of cannabis plants into the products permitted under West Virginia law,by adults was introduced. HB-4873 would, if passed, allow counties to ban production and sales in their jurisdictions. Current medical dispensaries would have to registered patients, respectively. The statute provides that the Office may issue upfile for an additional license to ten grower permits, ten processor permits, and one hundred dispensary permits and that it may not (1) issue more than one grower permit to one person, (2) issue more than one processor permit to one person, and (3) issue more than ten dispensary permits to one person.The Office of Medical Cannabis awarded ten grower permits on October 3, 2020. It awarded ten processor permits on November 13, 2020. It awarded one hundred dispensary permits on January 29, 2021, and announced that beginning February 3, 2021, West Virginia residents with qualifyingexpand their operations beyond medical conditions would be able to begin to submit applications to become registered patients.cannabis.Permits issued by the Office of Medical Cannabis are effective for one year from the date of issuance and may be renewed by applicants in good standing with the terms of a currently effective permit. Permits may be suspended or revoked on the basis of failure to prevent diversion of medical cannabis or violation of laws and rules applicable to medical cannabis businesses.All permittees are required to make use of a state-mandated electronic tracking system that is accessible to the Office of Medical Cannabis. Permittees are also subject to requirements related to security and surveillance, recordkeeping and record retention, the acquisition, growth, and processing of medical cannabis, delivery and transportation, and controls on dispensing, including amounts and prices permitted. Growers and processors are required to contract with independent laboratories to test their products according to Office of Medical Cannabis rules.Dispensaries are prohibited from dispensing cannabis products to anyone other than a registered patient or caregiver who presents a valid identification card from the Office of Medical Cannabis. Dispensing amounts are limited to those indicated in a registered patient’s certification by his/her medical practitioner, and in any event, a dispensary may not dispense more than a 30-day supply at a given time.The Office of Medical Cannabis is permitted to conduct announced or unannounced inspections of permittees to determine their compliance with West Virginia law and regulations, and may inspect a permittee’s site, records, and other data, and may interview employees, principals, operators, and financial backers of the permittee. The Office of Medical Cannabis will have free access to review and, if necessary, make copies of books, records, papers, documents, data, or other physical or electronic information that relates to the business of the dispensary, including financial data, sales data, shipping data, pricing data, and employee data. The Office of Medical Cannabis will have free access to any area within a site or facility that is being used to store medical cannabis for testing purposes and are permitted to collect test samples for testing at an approved laboratory.Permittees must have security and surveillance systems utilizing commercial-grade equipment to prevent unauthorized entry and to prevent and detect an adverse loss. The security systems must incorporate a professionally monitored security alarm system that is operational 24 hours a day, seven days a week, and records all activity in images capable of clearly revealing facial detail; have the ability to clearly and accurately display the date and time; record all images captured by each surveillance camera for a minimum of two years in a format that may be easily accessed for investigative purposes; and utilize a security alarm system separate from the facility’s primary security system covering the limited access area or other room where the recordings are stored. Permittees must install commercial-grade, nonresidential doors and door locks on each external door of the facility, with keys or key codes for all doors remaining in possession of designated authorized individuals. During all nonworking hours, all entrances to and exits from the site and facility must be securely locked. Permittees must install lighting to ensure proper surveillance both inside and outside of the facility. Access to rooms containing security and surveillance monitoring equipment must be limited to persons who are essential to maintaining security and surveillance operations; federal, state, and local law enforcement; security and surveillance system service employees; the bureau or its authorized agents; and other persons with the prior written approval of the Office of Medical Cannabis.A permittee is permitted to transport and deliver medical cannabis to a medical cannabis organization or an approved laboratory. A grower/processor may contract with a third-party contractor for delivery so long as the contractor complies with the Office of Medical Cannabis’ rules and regulations. A grower/processor must use a global positioning system to ensure safe, efficient delivery of the medical cannabis to a medical cannabis organization or an approved laboratory. Vehicles permitted to transport medical cannabis must be equipped with a secure lockbox or locking cargo area, have no markings that would either identify or indicate that the vehicle is being37used to transport medical cannabis, be capable of being temperature-controlled for perishable medical cannabis, as appropriate, display current state inspection stickers and maintain a current state vehicle registration, and be insured in an amount that is commercially reasonable and appropriate. Medical cannabis stored inside the transport vehicle may not be visible from the outside of the transport vehicle. A transport vehicle is subject to inspection by the bureau or its authorized agents, law enforcement, or other federal or state officials if necessary to perform the government officials’ functions and duties.See Appendix A to this Annual Report on Form 10-K for a list of the licenses associated with our operations in West Virginia.OtherFTC,Federal Trade Commission ("FTC"), into allegedly anticompetitive, unfair, deceptive, or other business practices by us, could cause us to incur additional expenses and, if adversely concluded, could result in substantial civil or criminal penalties and significant legal liability.2021,2023, we had over 9,0005,400 employees. We are committed to hiring talented individuals and maximizing individual potential, while fostering growth and career advancement. Since the opening of our first store in 2016, our workforce has grown dramatically, including personnel in our cultivation, production, transportation and retail divisions, along with our executive and support services teams. Our goal is to use the highest standards in attracting the best talent, offering competitive compensation, as well as implementing best practices in evaluating, recruiting and onboarding itsour human capital. Please refer above to our discussion captioned “Diversity, Inclusion & Equity (DE&I)” for additional information regarding our programs to develop diversity, inclusion and equity in our workforce.Annual Reportannual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and any amendments to those reports, as well as proxy statements, and, from time to time, other documents as soon as reasonably practicable after we electronically file such material with, or furnish it to, the U.S. Securities and Exchange Commission or SEC.("SEC"). These SEC reports can be accessed through the “Investors” section of our website. The information found on our website is not part of this or any other report we file with or furnish to the SEC.38the effect of outbreaks of pandemic diseases, fear of such outbreaks or economic disturbances due to such outbreaks, particularly the impact of the COVID-19 pandemic;•the risks of a greater likelihood of an IRS audit of cannabis-related businesses;•potential criminal prosecution or civil liabilities under RICO;•the effect of risks related to our products;potential criminal prosecution or civil liabilities under RICO;39•the effect of being an “emerging growth company.”40adult useadult-use cannabis industries whose business models we can follow or build upon. Similarly, there is no or limited information about comparable companies available for potential investors to review in making a decision about whether to invest in us.acquisitionacquisitions include:41diversionDiversion of management time and focus from operating our business to addressing acquisition integration challenges;coordinationCoordination of research and development and sales and marketing functions;retentionRetention of employees from the acquired company;culturalCultural challenges associated with integrating employees from the acquired company into our organization;integrationIntegration of the acquired company's accounting, management information, human resources, and other administrative systems;theThe need to implement or improve controls, procedures, and policies at a business that prior to the acquisition may have lacked effective controls, procedures, and policies;potentialPotential write-offs of intangible assets or other assets acquired in transactions that may have an adverse effect on our operating results in a given period;liabilityLiability for activities of the acquired company before the acquisition, including patent and trademark infringement claims, violations of laws, commercial disputes, tax liabilities, and other known and unknown liabilities; andlitigationLitigation or other claims in connection with the acquired company, including claims from terminated employees, consumers, former stockholders, or other third parties.42oneone or more of the statutes noted above or any other applicable legislation. This could restrict or otherwise jeopardize our ability to declare or pay dividends or effect other distributions.
The re-classification of cannabis or changes in U.S. controlled substance laws and regulations could have a material adverse effect on our business, financial condition, and results of operations.43The COVID-19 pandemic could adversely affect our business, financial condition and results of operations.The global outbreak of the novel strain of the coronavirus known as COVID-19 has resulted in governments worldwide enacting emergency measures to combat the spread of the virus. These measures, which include the implementation of travel bans, self-imposed quarantine periods and social distancing, have caused material disruption to businesses globally, resulting in an economic slowdown. Global equity markets have experienced significant volatility and weakness. Governments and central banks have reacted with significant monetary and fiscal interventions designed to stabilize economic conditions. The duration and impact of the COVID-19 outbreak is unknown at this time, as is the efficacy of the government and central bank interventions. It is not possible to reliably estimate the length and severity of these developments or their impact on our financial results and condition. Thus far, the COVID-19 pandemic has not had a material adverse effect on our business, financial condition and results of operations.Nonetheless, our business could be materially and adversely affected by the risks, or the public perception of the risks, related to the continuing COVID-19 pandemic. The risk of a pandemic, or public perception of such a risk, could cause customers to avoid public places, including retail properties, and could cause temporary or long-term disruptions in our supply chains and/or delays in the delivery of our products. These risks could also adversely affect our customers' financial condition, resulting in reduced spending for the products we sell. Moreover, any epidemic, pandemic, outbreak or other public health crisis, including COVID-19, could cause our employees to avoid our properties, which could adversely affect our ability to adequately staff and manage our businesses. “Shelter-in-place” or other such orders by governmental entities could also disrupt our operations if employees who cannot perform their responsibilities from home are not able to report to work. Risks related to an epidemic, pandemic or other health crisis, such as COVID-19, could also lead to the complete or partial closure of one or more of our stores or other facilities.The ultimate extent of the impact of any epidemic, pandemic or other health crisis on our business, financial condition and results of operations will depend on future developments, which are highly uncertain and cannot be predicted, including new information that may emerge concerning the severity of such epidemic, pandemic or other health crisis and actions taken to contain or prevent its44further spread, among others. These and other potential impacts of an epidemic, pandemic or other health crisis, such as COVID-19, could therefore materially and adversely affect our business, financial condition, growth strategies and results of operations.establishmentestablishment will be located. These authorized areas are frequently removed from other retail operations. Because thethe cannabis industry remains illegal under U.S. federal law, the disadvantaged tax status of businesses derivingderiving their income from cannabis, and the reluctance of the banking industry to support cannabis businesses, it maymay be difficult for us to locate and obtain the rights to operate at various preferred locations. Property ownersowners may violate their mortgages by leasing to us, and those property owners that are willing to allow useuse of their facilities may require payment of above fair market value rents to reflect the scarcity of such locations andand the risks and costs of providing such facilities.4546mayhave not behistorically been effective, and our independent auditors may not be able to certify as to their effectiveness, which could have a significantadversely affect our business results and adverse effect on our business.operations.We are subject to various SEC reporting and other regulatory requirements. Effective internal controls over financial reporting are necessary for us to provide reliable financial reports and, together with adequate disclosure controls and procedures, are designed to prevent fraud. Any failure to implement required new or improved controls, or difficulties encountered in their implementation could cause us to fail to meet our reporting obligations. In addition, any testing by us conducted in connection with Section 404 of the Sarbanes-Oxley Act of 2002, or the subsequent testing by our independent registered public accounting firm when required, may reveal deficiencies in our internal controls over financial reporting that are deemed to be material weaknesses or that may require prospective or retrospective changes to our consolidated financial statements or identify other areas for further attention or improvement. Inferior internal controls could also cause investors to lose confidence in our reported financial information, which could have a negative effect on the trading price of our Subordinate Voting Shares.We haveacertain material weaknessweaknesses in our internal control over financial reporting, and our management has concluded that our disclosure controls and procedures are not effective. While we are working to remediate any material weaknessweaknesses or significant deficiencies in our internal controls over financial reporting, we cannot assure you that additional material weaknesses or significant deficiencies will not occur in the future. IfWe are in the process of remediating the material weaknesses, as described in Part II, Item 9A “Controls and Procedures”. While progress has been made to enhance our internal control over financial reporting, or our disclosure controls and procedureswe are not effective, we may not be able to accurately report our financial results or prevent fraud, which may cause investors to lose confidence in our reported financial information and may lead to a decline in our stock price.We have historically had a small internal accounting and finance staff. This lack of adequate accounting resources has resultedstill in the identificationprocess of a material weakness inbuilding and enhancing our processes, procedures, and controls, as well as evaluating and enhancing our internal controls over financial reporting. A “material weakness”policies and information technology. Additional time is a deficiency, or a combinationrequired to complete the remediation of deficiencies,certain material weaknesses and to ensure the sustainability of these remediation actions. We believe the above actions as well as those being implemented currently, when complete, will be effective in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of our financial statements will not be prevented or detected on a timely basis.47As disclosed in the Company’s Form 10-Q for the quarter ended September 30, 2021 and Item 9A of this Annual Report on Form 10-K, our management team identified errors in the accounting for leases, asset acquisitions, and classification of assets. In addition, during the development of the annual tax provision an error in the tax basis of assets acquired was identified. Management reviewed these errors identifying the root cause due to the control environment component of internal control as the Company did not maintain a sufficient complement of personnel with the appropriate level of knowledge, experience, and training in certain areas important to financial reporting. During 2021, even though a material misstatement was not identified in the Company’s financial statements, it was determined that there was a reasonable possibility that a material misstatement in the Company’s financial statements would not have been prevented or detected on a timely basis.We have taken actions toward the remediation of the material weaknessweaknesses described in our internal control over financial reporting including: adding additional positions including Chief Accounting Officer, Director Part II, Item 9A. Financial Reporting, Assistant Controller, Director of Shared Services, Northeast Controller, and Tax Director to provide enhanced oversight and technical experience in certain areas important to financial reporting; engaging third party experts to assist management in assessing current processes and designing improved processes and controls for the consolidated Company; adding a Chief Technology Officer to enhance the information technology environment including automation of processes and controls and finalization of an ongoing SAP implementation; and reviewing business processes surrounding leases, acquisitions, and other complex financial reporting areas to identify and being the implementation of enhanced procedures related to internal controls. Nonetheless, the material weaknesses in the Company’s internal control over financial reporting will not be considered remediated until the controls operate for a sufficient period of time and management has concluded, through testing that these controls operate effectively. As of the date of this Annual Report on Form 10-K, management is in the process of testing and evaluating these additional controls to determine whether they are operating effectively. We plan to continue to take additional steps to remediate the material weakness and improve our financial reporting systems and implement new policies, procedures and controls. If we do not successfully remediate the material weakness described above, or if other material weaknesses or other deficiencies arise in the future, we may be unable to accurately report our financial results, which could cause our financial results to be materially misstated and require restatement. ContentsWe may be at a higher risk of IRS audit.Based on anecdotal information, we believe there is a greater likelihood that the Internal Revenue Service will audit the tax returns of cannabis-related businesses. Any such audit of our tax returns could result in our being required to pay additional tax, interest and penalties, as well as incremental accounting and legal expenses, which could be material.48We could be subject to criminal prosecution or civil liabilities under RICO.The Racketeer Influenced Corrupt Organizations Act (“RICO”) criminalizes the use of any profits from certain defined “racketeering” activities in interstate commerce. While intended to provide an additional cause of action against organized crime, due to the fact that cannabis is illegal under U.S. federal law, the production and sale of cannabis qualifies cannabis related businesses as “racketeering” as defined by RICO. As such, all officers, managers and owners in a cannabis related business could be subject to criminal prosecution under RICO, which carries substantial criminal penalties.RICO can create civil liability as well: persons harmed in their business or property by actions which would constitute racketeering under RICO often have a civil cause of action against such “racketeers,” and can claim triple their amount of estimated damages in attendant court proceedings. Trulieve or its subsidiaries, as well as its officers, managers and owners could all be subject to civil claims under RICO.identifiableidentifiable information and other confidential information of our customerscustomers on our systems and applications. Though we maintain robust,robust, proprietary security protocols, we may experience attempts by third parties to obtainobtain unauthorized access to the personally identifiable information and other confidentialconfidential information of our customers. This information could also be otherwise exposed through humanhuman error or malfeasance. The unauthorized access or compromise of this personally identifiable information and other confidential information could have a material adverse impactimpact on our business, financial condition and results of operations.49allegationsthird-parties acting on our behalf could, damagedepending on the nature of any such failure, adversely impact our reputation and subject us to civil or criminal investigations and related stockholder lawsuits, could lead to substantial civil and criminal monetary and non-monetary penalties and could cause us to incur significant legal and investigatory fees.there is no labor organization that hasorganizations have been recognized as a representative of our employees with the exception of the employees atin both our affiliated Reading, Pennsylvania cultivation and processing facility. However, itfacility and our affiliated Harvest of Southwest PA, LLC d/b/a Trulieve Pittsburgh (Federal Street), dispensary location. It is possible that additional certain retail and/or manufacturing locations will be organized in the future, which could lead to work stoppages or increased labor costs and adversely affect our business, profitability and our ability to reinvest into the growth of our business. We cannot predict how stable our relationships with U.S. labor organizations would be or whether we would be able to meet any unions’ requirements without impacting our financial condition. Labor unions may also limit our flexibility in dealing with our workforce. Work stoppages and instability in our union relationships could delay the production and sale of our products, which could strain relationships with customers and cause a loss of revenues which would adversely affect our operations.face risks related to our products.We have committed and expect to continue committing significant resources and capital to develop and market existing products and new products and services. These products are relatively untested in the marketplace, and we cannot assure shareholders and investors that we will achieve market acceptance for these products, or other new products and services that we may offer in the future. Moreover, these and other new products and services maycould be subject to significant competition with offeringscriminal prosecution or civil liabilities under RICO.newRICO. As such, all officers, managers and existing competitorsowners in the industry. In addition, new productsa cannabis related business could be subject to criminal prosecution under RICO, which carries substantial criminal penalties.services may pose a varietycan claim triple their amount of challengesestimated damages in attendant court proceedings. Trulieve or its subsidiaries, as well as its officers, managers and require usowners could all be subject to attract additional qualified employees. The failure to successfully develop, manage and market these new products and services could seriously harm our business, prospects, revenue, results of operation and financial condition.50indebtedness, including the $70.0 million in aggregate principal amount of notes we issued on June 18, 2019, the $60.0 million in aggregate principal amount of notes we issued on November 7, 2019 and the $350.0 million aggregate principal amount of senior secured notes that we issued on October 6, 2021.indebtedness. The contractual restrictions in the instruments governing such notesindebtedness include restrictive covenants that limit our discretion with respect to certain business matters. These covenants place restrictions on, among other things, our ability to create liens or other encumbrances, to pay distributions or make certain other payments, and to sell or otherwise dispose of certain assets. A failure to comply with such obligations could result in a default, which, if not cured or waived, could permit acceleration of the relevant indebtedness. Our significant indebtedness could have important consequences, including: (i) our ability to obtain additional financing for working capital, capital expenditures, or acquisitions may be limited; and (ii) all or part of our cash flow from operations may be dedicated to the payment of the principal of and interest on our indebtedness, thereby reducing funds available for operations. These factors may adversely affect our cash flow. Our inability to generate sufficient cash flow to satisfy our debt obligations, or to refinance our indebtedness on commercially reasonable terms or at all, may materially and adversely affect our business, results of operations, and financial condition.pre-emptivepreemptive rights in connection with such further issuances. Our board of directors has discretion to determine the price and the terms of further issuances. The ability of our board of directors to issue additional Multiple Voting shares and/or Subordinate Voting Shares could also have anti-takeover effects. Moreover, we will issue additional Subordinate Voting Shares on the conversion of the Multiple Voting Shares in accordance with their terms. To the extent holders of our options, warrants or other convertible securities convert or exercise their securities and sell Subordinate Voting Shares they receive, the trading price of the Subordinate Voting Shares may decrease due to the additional amount of Subordinate Voting Shares available in the market. We cannot predict the size or nature of future issuances or the effect that future issuances and sales of Subordinate Voting Shares will have on the market price of the Subordinate Voting Shares. Issuances of a substantial number of additional Subordinate Voting Shares, or the51 As of December 31, 2021, we had an aggregate of 519,169.999 Multiple Voting Shares outstanding, which were convertible into an aggregate of 51,916,999 Subordinate Voting Shares. If all or a substantial portion of our Multiple Voting Shares are converted into Subordinate Voting Shares, the potential for sales of substantial numbers of Subordinate Voting Shares may increase. A decline in the market prices of the Subordinate Voting Shares could impair our ability to raise additional capital through the sale of securities should itwe desire to do so.Russia)Russia or Israel and Hamas); significant acquisitions or business combinations, strategic partnerships, joint ventures or capital commitments by or involving us or our competitors; (xi) operating and share price performance of other companies that investors deem comparable to us or from a lack of market comparable companies; (xii) false or negative reports issued by individuals or companies who have taken aggressive short sale positions; and (xiii) news reports relating to trends, concerns, technological or competitive developments, regulatory changes and other related issues in our industry or target markets.theThe number of shareholders;52We are an “emerging growth company” and will be able take advantage of reduced disclosure requirements applicable to emerging growth companies, which could make our Subordinate Voting Shares less attractive to investors.We are an “emerging growth company,” as defined in the JOBS Act and, for as long as we continue to be an emerging growth company, we intend to take advantage of certain exemptions from various reporting requirements applicable to other public companies but not to emerging growth companies, including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. We could be an emerging growth company for up to five years, or until the earliest of (i) the last day of the first fiscal year in which our annual gross revenues exceed $1.07 billion, (ii) the date that we become a “large accelerated filer” as defined in Rule 12b-2 under the Exchange Act, which would occur if the market value of our Subordinate Voting Shares that are held by non-affiliates exceeds $700 million as of the last business day of our most recently completed second fiscal quarter, or (iii) the date on which we have issued more than $1 billion in non-convertible debt during the preceding three year period.We intend to take advantage of these reporting exemptions described above until we are no longer an emerging growth company. Under the JOBS Act, emerging growth companies can also delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, we will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.We cannot predict if investors will find our Subordinate Voting Shares less attractive if we choose to rely on these exemptions. If some investors find our Subordinate Voting Shares less attractive as a result of any choices to reduce future disclosure, there may be a less active trading market for our Subordinate Voting Shares and the price of our Subordinate Voting Shares may be more volatile.None.We have no material properties.indicated for the periods indicated.
Trading
Price
(C$)
Trading
Price
(C$)
(#)Period Low Trading Price
(C$)High Trading Price
(C$)Volume
(#)Year Ended December 31, 2023 Fourth Quarter (December 31, 2023) $ 5.22 $ 8.43 20,003,628 Third Quarter (September 30, 2023) 4.68 10.12 30,922,434 Second Quarter (June 30, 2023) 5.20 7.63 26,217,104 First Quarter (March 31, 2023) 7.48 10.04 19,669,724 Year Ended December 31, 2022 Fourth Quarter (December 31, 2022) $ 8.72 $ 21.60 31,338,044 Third Quarter (September 30, 2022) 11.53 19.77 17,985,615 Second Quarter (June 30, 2022) 14.51 27.42 24,446,760 First Quarter (March 31, 2022) 21.71 34.61 26,884,845
Trading
Price
($)
Trading
Price
($)
(#)Period Low Trading Price
($)High Trading Price
($)Volume
(#)Year Ended December 31, 2023 Fourth Quarter (December 31, 2023) $ 3.85 $ 6.17 23,042,292 Third Quarter (September 30, 2023) 3.45 7.45 26,647,373 Second Quarter (June 30, 2023) 3.87 5.78 19,621,301 First Quarter (March 31, 2023) 5.59 7.50 25,890,697 Year Ended December 31, 2022 Fourth Quarter (December 31, 2022) $ 6.39 $ 16.11 27,927,795 Third Quarter (September 30, 2022) 8.29 15.20 15,699,686 Second Quarter (June 30, 2022) 11.28 21.99 17,861,958 First Quarter (March 31, 2022) 16.99 27.44 26,432,828 March 23, 2022,December 31, 2023, there were approximately 371350 shareholders of record of our Subordinate Voting Shares 34and 14 holders of record of our Multiple Voting Shares, and no holders Shares.54 Price Performance Graphcompares the cumulative total shareholder return on our Subordinate Voting Shares from September 25, 2018, when the Company began trading on the CSE, through December 31, 2021, with the comparable cumulative return of the Russell 2000 Index and a selected peer group of companies. The comparison assumes all dividends have been reinvested (if any) and an initial investment of $100 on September 25, 2018. The returns of each company in the peer group have been weighted to reflect their market capitalizations. All amounts below are disclosed in US Dollars. The stock price performance on the following graphrelated information is not necessarily indicative of future stock price performance.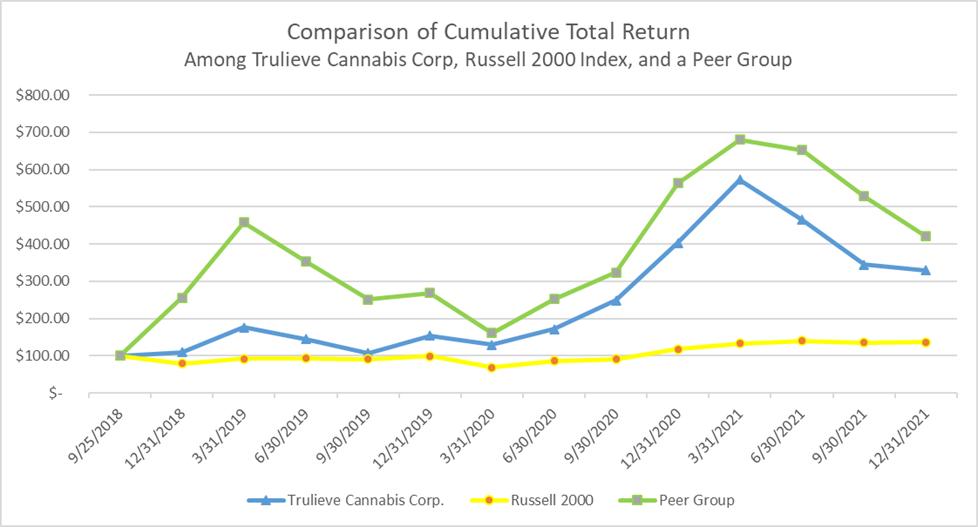
Below are the specific companies included in the peer group.•Columbia Care Inc.•Cresco Labs Inc.•Curaleaf Holdings Inc.•Green Thumb Industries Inc.•TerrAscend Corp.•Verano Holdings Corp.During the year ended December 31, 2021, the Company determined the above identified peer group was more in line with the Company's operations and market capitalization than those identified in the prior year.55Historical IndexThe following graph compares, for the period from September 25, 2018 (the day we commenced trading on the OTCQX Best Market) through December 31, 2021, the cumulative total return on our Subordinate Voting Shares, the CSE Composite Index and the Horizons Marijuana Index that consists of the North American publicly listed life sciences companies with significant business activities in the marijuana industry. The graph assumes $100 was invested on September 25, 2018, in the Subordinate Voting Shares of Trulieve, Inc., the CSE Composite Index and the Horizons Marijuana Index, and assumes the reinvestment of any dividends. The stock price performance on the following graph is not necessarily indicative of future stock price performance.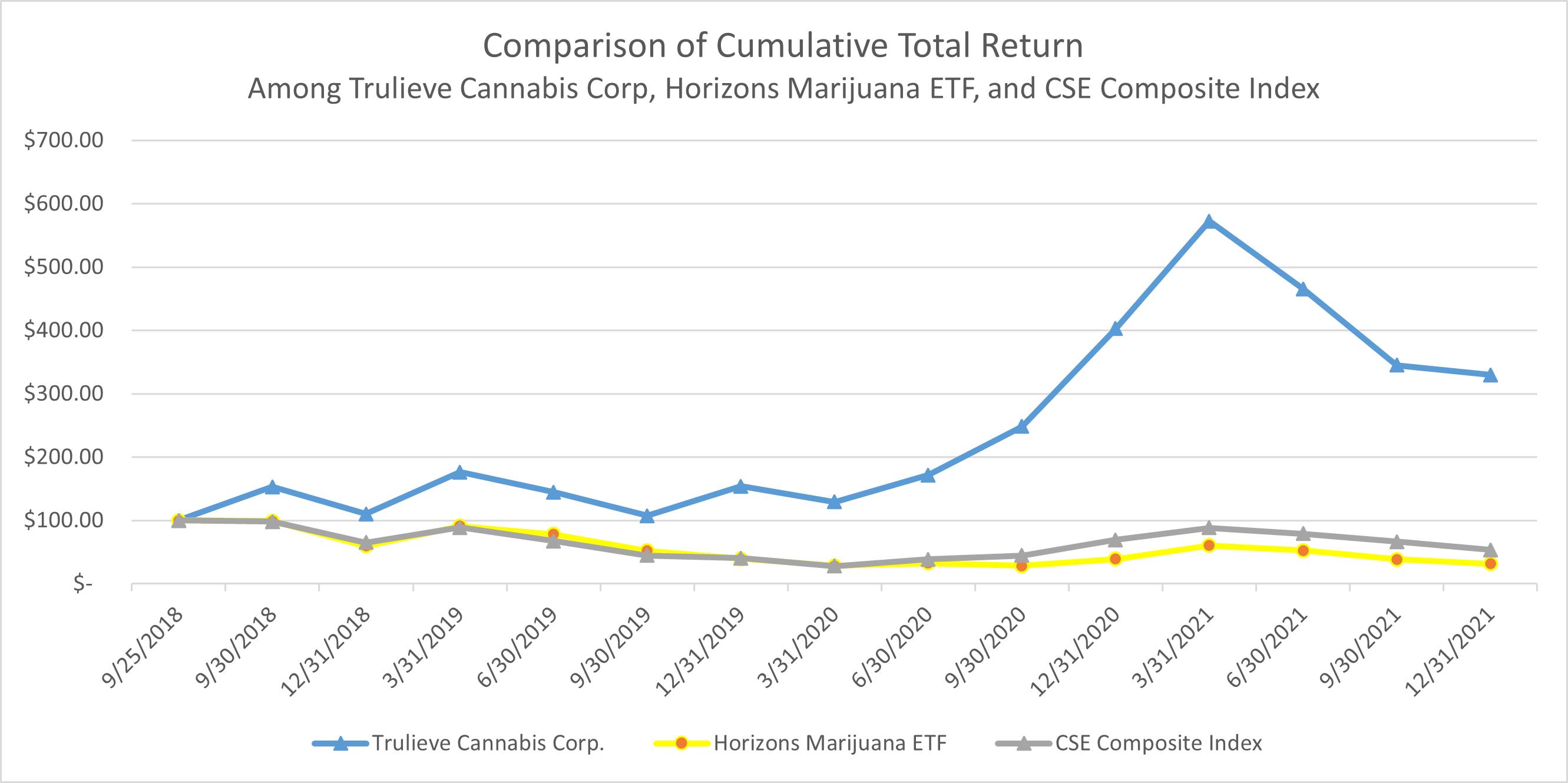
These performance graphs and other information furnished under this Part II Item 5 of this Form 10-K shall not be deemed to be “soliciting material” or to be “filed” with the SEC or subject to Regulation 14A or 14C under the Securities Exchange Act of 1934 or to the liabilities of Section 18 of the Securities Exchange Act.Act of 1934, and will not be deemed to be incorporated by reference into any filing under the Securities Act of 1933 or the Securities Exchange Act of 1934, except to the extent the Company specifically incorporates it by reference into such a filing: the SEC requires the Company to include a line graph presentation comparing cumulative five year stock returns with a broad-based stock index and either a nationally recognized industry index or an index of peer companies selected by the Company. The Company has chosen to use the Russell 2000 Index as the broad-based index.
20212023 were reported in a Form 8-K or Form 10-Q filed with the SEC.OverviewTrulieveieve is a vertically integrated cannabis company and multi-state operator which currently holds licenses to operate in ten states and has received notice of intent to award a license in an eleventh state.nine states. Headquartered in Quincy, Florida, we are the market leader for quality medicallargest cannabis products and servicesretailer in Florida and we havethe United States with market leading retail operations in Arizona, Florida, Georgia, Pennsylvania, and Pennsylvania. By providing innovative,West Virginia. We are committed to delivering exceptional customer experiences through elevated service and high-quality products across our brand portfolio, webranded products. We aim to be the brand of choice for medical and adult-use customers in all of the markets that we serve. We operateThe Company operates in highly regulated markets that require expertise in cultivation, manufacturing, retail and logistics.retail. We have developed proficiencies in each of these functionsfunctional areas and are committed topassionate about expanding access to high qualityregulated cannabis products through advocacy, education, and delivering exceptional customer experiences.expansion of our distribution network.adopted legislationdeveloped programs to permit the use of cannabis products for medicinal purposes to treat specific conditions and diseases, which we refer to as medical cannabis. Recreational marijuana,cannabis, or adult-use cannabis, is legal marijuanacannabis sold in licensed dispensaries to adults ages 21 and older. Thus far, of the states in which we operate, only Arizona, California, Colorado, Connecticut, Massachusetts,Maryland, and NevadaOhio have adopted legislationalready or are in the process of developing and launching programs permitting the commercialization of adult-use cannabis products. Trulieve operates its business through its directly and indirectly owned subsidiaries that hold licenses and have entered into managed service agreements in the states in which they operate. As of March 16, 2022, we operated 162 dispensaries, with 113 dispensaries in Florida, 19 affiliated dispensaries in Pennsylvania, 17 dispensaries in Arizona, five dispensaries in California, three dispensaries in Maryland, two dispensaries in Massachusetts, two dispensaries in West Virginia and one dispensary in Connecticut, and we operated cultivation and processing facilities in Arizona, Colorado, Florida, Maryland, Massachusetts, Nevada, Pennsylvania, and West Virginia. 2021,2023, we employed over 9,000 people and we are committed to providing patients and adult consumers, which we refer to herein as “customers,” a consistent and welcoming retail experience across Trulieve branded stores and affiliated retail locations.Our business and operations center aroundoperated the Trulieve brand philosophy of “Customers First” which permeates our culture beginning with high- quality and efficient cultivation and manufacturing practices, focus on the consumer experience at Trulieve branded and affiliated retail locations, at our in-house call center and where available at customer residences through a robust home delivery program. Our investments in vertically integrated operations in several of our markets afford us ownership of the entire supply chain which mitigates third-party risks and allows us to completely control product quality and brand experience. We believe that this contributes to high customer retention and brand loyalty. We successfully operate our core business functions of cultivation, production and distribution at scale, and are skilled at rapidly increasing capacity without any interruption to existing operations.Trulieve has identified five regional geographic hubs in the U.S. and has established cannabis operations in three of the five hubs: Southeast, Northeast, and Southwest. In each of our three regional hubs we have market leading positions in cornerstone states and additional operations and assets in other state markets. Our hubs are managed by national and regional management teams supported by our corporate headquarters in Florida.Southeast HubOur southeast hub operations are anchored by our cornerstone market of Florida. Trulieve was the first licensed operator in the medical market in Florida with initial sales in 2016. Publicly available reports filed with the Florida Office of Medical Marijuana Use show Trulieve has the most dispensing locations and the greatest dispensing volume across product categories out of all licensed medical marijuana businesses in the state as of December 31, 2021. Trulieve cultivates and produces all of its products in-house and distributes those products to customers in Trulieve branded stores (dispensaries) throughout Florida, as well as via home delivery.As of December 31, 2021, Trulieve operated cultivation and processing facilities across thirteen sites and 111 retail dispensaries throughout the state. In accordance with Florida law, Trulieve grows all of its cannabis in secure enclosed indoor facilities and greenhouse structures. In furtherance of our customer-first focus, we have developed a suite of Trulieve branded products, including flower, edibles, vaporizer cartridges, concentrates, topicals, capsules, tinctures, dissolvable powders, and nasal sprays. This wide variety of products gives customers the ability to select the product that consistently delivers the desired effect and in their preferred method of delivery.57In Georgia, Trulieve received a Notice of Intent to award a Class 1 Production License from the Georgia Access to Medical Cannabis Commission in July 2021. The Notice of Intent to award is a notice of the Georgia Access to Medical Cannabis Commission’s expected contract award to Trulieve GA pending resolution of a protest process. If the contract is awarded, Trulieve GA will hold one of two Class 1 Production Licenses in the state and will be permitted to cultivate cannabis for the manufacture of low tetrahydrocannabinol, or THC oil.Northeast HubOur northeast hub operations are anchored by our cornerstone market of Pennsylvania.We conduct cultivation, processing, and retail operations through its direct and indirect subsidiaries with permits for retail operations and grower/processor operations in Pennsylvania. These subsidiaries operate cultivation and processing facilities in McKeesport, Reading, and Carmichael, Pennsylvania to support our affiliated network of retail dispensaries and wholesale distribution network across the state.We operate three medical dispensaries and conducts wholesale sales supported by cultivation and processing in Hancock, Maryland.We operate two retail dispensaries in Massachusetts, serving medical adult use customers in Northampton and adult use customers in Worcester. Our retail operations are supported by cultivation and manufacturing operations in Holyoke. We commenced wholesale sales in September 2021. Trulieve was the first to offer sales of clones supporting home grow for residents in the Massachusetts market in August 2021.We operate a medical cannabis dispensary located in Bristol, Connecticut. Under Connecticut’s adult-use cannabis legislation, which was enacted July 1, 2021, Trulieve can seek regulatory approval to expand sales at this dispensary to include adult use sales.We operate two medical dispensaries in Morgantown and Weston, West Virginia, supported by cultivation and processing operations in Huntington, West Virginia. Trulieve has been awarded and has acquired permits to operate up to a total of nine dispensaries in West Virginia.Southwest HubOur southwest hub operations are anchored by our cornerstone market of Arizona. In Arizona, Trulieve holds a market-leading position, offering medical and adult use customers a wide range of branded and third-party products, including brand partner products. We also serve medical and adult use customers in California. Trulieve conducts wholesale operations in Nevada and Colorado, serving the medical and adult use markets in each state.State Number of Dispensaries Number of Cultivation and Processing Facilities Florida 131 6 Arizona 21 3 Pennsylvania 20 3 West Virginia 10 1 Georgia 5 1 Maryland 3 1 Ohio 1 — Connecticut 1 — Colorado — 1 Total 192 16 We derive our revenuewhich we manufacture,cultivate, process, distribute, and sell and distribute to our customers by home delivery and inthrough our dispensaries.wholesale distribution channels.product salesthe cultivation and includesproduction of cannabis and from wholesale purchases made from other licensed producers within the markets in which the Company operates. Costs of goods sold include the costs directly attributable to the production of inventory and amounts paid to produceincurred in the cultivation and manufacturing process of finished goods, such as flower, concentrates, and concentrates,edibles, as well as packaging and other supplies, fees for services and processing, and allocated overhead which includes depreciation and amortization of property and equipment associated with cultivation and production, allocations of rent, administrative salaries, utilities, and related costs. Cannabis costs are affected by various state regulations that limit the sourcing and procurement of cannabis product, which may create fluctuations in margins over comparative periods as the regulatory environment changes. marketing expenses related to advertising costs and marketing programs for our products. Personnelproducts and personnel related costs related to additional dispensaries are the primary costs of salesmanage and marketing.staff our dispensaries. As we continue to expand and open additional dispensaries, and gain additional customers, we expect our sales and marketing expenses to continue to increase.represent costs incurred at our corporate offices,are primarily related to personnel costs, including salaries, incentive compensation, benefits, and other professional service costs, including legal, accounting and accounting.acquisition related costs. We expect to58 considerably in this area to support our expansion plans, as we are able to access additional medical and adult-use markets, to further support the increasing complexitygrowth of the cannabis business. Furthermore, we expect to continue to incur acquisitionindustry. Other general and transaction costs related to our expansion plans,administrative expenses consist of travel, general office supplies and we anticipate a significant increase in compensation expenses related to recruitingmonthly services, facilities and hiring talent, accounting,occupancy, insurance, and legal and professional fees associated with becoming compliant with the Sarbanes-Oxley Act and other public company corporate expenses.expense is calculated on a straight-line basis using the estimated useful lifeand amortization consists of each asset. Estimated useful life is determined by asset classdepreciation of property and is reviewed on an annual basisequipment and revised if necessary. Amortization expense is amortized using the straight-line method over the estimated useful liferight-of-use assets, and amortization of the intangible assets. Useful lives for intangible assets, are determined by type of asset with the initial determination of useful life determined during the valuation of the business combination. On an annual basis, the useful lives of each intangible class of assets are evaluated for appropriatenessincluding cannabis licenses and adjusted if appropriate., NetInterest and other, net consist consists primarily of interest expense, interest income interest expense,on money market accounts and notes receivable, gain on debt extinguishment, and the impact of the revaluation of the liability classified warrants.As we operate in The IRS has taken the position that cannabis industry, wecompanies are subject to the limits of IRC Section 280E under which wethey are only allowed to deduct expenses directly related to costs of goods sold.20212023 Compared to Year Ended December 31, 20202023 2022 2023 vs. 2022 (in thousands) Statement of operations data: Amount Percentage of Revenue Amount Percentage of Revenue Amount Change Revenue $ 1,129,193 100.0 % $ 1,218,229 100.0 % $ (89,036) Cost of goods sold 540,565 47.9 % 529,102 43.4 % 11,463 Gross profit 588,628 52.1 % 689,127 56.6 % (100,499) Expenses: Sales and marketing 240,165 21.3 % 277,563 22.8 % (37,398) General and administrative 145,997 12.9 % 169,471 13.9 % (23,474) Depreciation and amortization 109,825 9.7 % 116,381 9.6 % (6,556) Impairments and disposals of long-lived assets, net 6,664 0.6 % 75,547 6.2 % (68,883) Impairment of goodwill 307,590 27.2 % — 0.0 % 307,590 Total expenses 810,241 71.8 % 638,962 52.5 % 171,279 (Loss) income from operations (221,613) (19.6 %) 50,165 4.1 % (271,778) Other income (expense): Interest expense, net (81,569) (7.2 %) (73,422) (6.0 %) (8,147) Interest income 6,164 0.5 % 1,631 0.1 % 4,533 Gain on debt extinguishment, net 5,937 0.5 % — — % 5,937 Other income, net 6,544 0.6 % 2,388 0.2 % 4,156 Total other expense, net (62,924) (5.6 %) (69,403) (5.7 %) 6,479 Loss before provision for income taxes (284,537) (25.2 %) (19,238) (1.6 %) (265,299) Provision for income taxes 151,358 13.4 % 163,380 13.4 % (12,022) Net loss from continuing operations (435,895) (38.6 %) (182,618) (15.0 %) (253,277) Net loss from discontinued operations, net of tax benefit of $4,101 and $12,223, respectively (97,241) (8.6 %) (70,109) (5.8 %) (27,132) Net loss (533,136) (47.2 %) (252,727) (20.7 %) (280,409) Less: net loss attributable to non-controlling interest from continuing operations (5,147) (0.5 %) (3,994) (0.3 %) (1,153) Less: net loss attributable to non-controlling interest from discontinued operations (1,193) (0.1 %) (2,669) -0.2 % 1,476 Net loss attributable to common shareholders $ (526,796) (46.7 %) $ (246,064) (20.2 %) $ (280,732) 20212023 was $938.4$1.13 billion, a decrease of $89.0 million an increase of $416.9 million,or 7.3%, from $521.5 million$1.22 billion for the year ended December 31, 2020. Increase2022. The decrease in revenue is the result of increased locations, increased or new wholesale operations in specific markets, organic growthdue to a $74.9 million decrease in retail salesrevenue and a $11.7 million decrease in wholesale revenue. The Company operated 192 and 178 dispensaries as of December 31, 2023 and December 31, 2022, respectively. We experienced increased competition and promotional activity in certain retail markets and also shed underperforming retail assets. The reduction in wholesale revenues is primarily due to an increasea focus on higher margin retail sales in products available for purchase and overall customer count and acquisitions, most notably the acquisition certain markets.20212023 was $372.3$540.6 million, an increase of $237.1$11.5 million or 2.2%, from $135.1$529.1 million for the year ended December 31, 2020,2022. Cost of goods sold as a percentage of revenue was 47.9% for the year ended December 31, 2023 as compared to 43.4% for the year ended December 31, 2022. The increase was primarily due to increased depreciation related to capital expenditures to support business growth totaling $10.3 million. Additional factors impacting the change include inventory reduction efforts to right-size inventory levels, the continued ramping of new production facilities in existing markets where additional economies of scale are anticipated in the future, and expansion into new markets which are not fully vertical, resulting in the sale of third-party products, which yield lower margin than our vertical markets. The Company also incurred additional costs related to excess capacity in certain temporarily idled facilities.Year Ended December 31, 2022 2021 2022 vs. 2021 (in thousands) Statement of operations data: Amount Percentage of revenue Amount Percentage of revenue Amount Change Revenue $ 1,218,229 100.0 % $ 931,934 100.0 % 286,295 Cost of goods sold 529,102 43.4 % 365,360 39.2 % 163,742 Gross profit 689,127 56.6 % 566,574 60.8 % 122,553 Expenses: Sales and marketing 277,563 22.8 % 211,905 22.7 % 65,658 General and administrative 169,471 13.9 % 99,456 10.7 % 70,015 Depreciation and amortization 116,381 9.6 % 45,791 4.9 % 70,590 Impairment and disposal of long-lived assets, net 75,547 6.2 % 5,371 0.6 % 70,176 Total expenses 638,962 52.5 % 362,523 38.9 % 276,439 Income from operations 50,165 4.1 % 204,051 21.9 % (153,886) Other income (expense): Interest expense, net (73,422) (6.0) % (29,121) (3.1 %) (44,301) Interest income 1,631 0.1 % 205 — % 1,426 Other income, net 2,388 0.2 % 1,112 0.1 % 1,276 Total other expense, net (69,403) (5.7) % (27,804) (3.0 %) (41,599) (Loss) income before provision for income taxes (19,238) (1.6) % 176,247 18.9 % (195,485) Provision for income taxes 163,380 13.4 % 145,722 15.6 % 17,658 Net (loss) income from continuing operations (182,618) (15.0) % 30,525 3.3 % (213,143) Net loss from discontinued operations, net of tax benefit (provision) of $12,223 and $(339), respectively (70,109) (5.8) % (13,080) (1.4 %) (57,029) Net (loss) income (252,727) (20.7) % 17,445 1.9 % (270,172) Less: net loss attributable to non-controlling interest from continuing operations (3,994) (0.3) % (587) (0.1 %) (3,407) Less: net loss attributable to non-controlling interest from discontinued operations (2,669) (0.2) % — — % (2,669) Net (loss) income attributable to common shareholders $ (246,064) (20.2) % $ 18,032 1.9 % $ (264,096) increased from 26%was 43.4% for the year ended December 31, 20202022 as compared to 40%39.2% for the year ended December 31, 2021, primarily due to increased depreciation related to capital expenditures to support business growth, new production facilities in existing markets where economies of scale are anticipated in the future, and expansion into new markets which are not fully vertical, resulting in sale of third-party products, and therefore yield lower margin than our vertical markets.our inventory step-up related to acquisitions,higher revenue offset by many factors including, increased wholesale business, which is generally lower margin than retail sales, increased depreciation related to capital expenditures in cultivation and processing to support business growth, new production facilities where economies of scale are anticipated in the future, and expansion into new markets which are not fully vertical, resulting in the sale of third partythird-party products, and therefore yield lower margin than the Floridaour vertical market.59Gross ProfitGross profitmarkets.20212022, was $566.1$277.6 million, up $179.7an increase of $65.7 million, or 47%31.0%, from $386.4 million for the year ended December 31, 2020, as a result of an increase in retail sales due to an increase in the number of dispensaries and customer count. Gross profit as a percentage of revenue decreased from 74% for the year ended December 31, 2020 to 60%, for the year ended December 31, 2021. This decrease is caused by inventory step-up related to acquisitions, increased wholesale business, which is generally lower margin than retail sales, increased depreciation related to capital expenditures in cultivation and processing to support business growth, expansion into new markets which are not fully vertical and therefore yield lower margin than the Florida vertical market and macro-economic factors centered around prices and labor.Sales and Marketing ExpensesSales and marketing expenses increased from $119.4 million for the year ended December 31, 2020, to $215.1$211.9 million for the year ended December 31, 2021, an increasebut remained consistent as a percentage of $95.7 million.revenue. The increase in sales and marketing is the result of a higher headcount for the year, as we continue to add additional dispensaries in efforts to maintain and further drive higher growth in sales and market share. This increased headcount resulted in higher personnel costs, which is the primary driver for the increase year over year.Expensesexpensesexpense for the year ended December 31, 2021 increased to $100.62022, was $169.5 million, an increase of $70.0 million, or 70.4%, from $36.1$99.5 million for the year ended December 31, 2020, an increase2021. General and administrative expense as a percentage of $64.5 million.revenue increased from 10.7% to 13.9%. The increase in general and administrative expense is the result of significant expenses incurredentering new markets, ramping our infrastructure to acquiresupport growth initiatives, repositioning of facilities which have been temporarily idled, and integrate new subsidiaries, most notably Harvest.2021 increased2022, was $75.5 million, an increase of $70.2 million as compared to 5.4$5.4 million from $63 for the year ended December 31, 2020, an increase of 5.3 million.2021. The increase is primarily due to exited facilities and the write offrepositioning of assets, primarily in our Southeast hub. The activity in 2021 primarily consisted of the write-off of certain licenses in our Southwest hub due to market changes and the disposal of certain long-lived assets.20212022 was $48.1$73.4 million, up $35.5an increase of $44.3 million or 282%, from $12.6$29.1 million for the year ended December 31, 2020.2021. The overall increase in depreciationis related to additional interest on private placement notes of $31.5 million, construction finance liabilities of $8.1 million, and amortization expensesfinance leases of $2.2 million, to support business growth.
Interest income for the year ended December 31, 2022 was due to amortization$1.6 million, an increase of intangibles acquired and fair valued in acquisitions, investment in infrastructure that resulted in more capitalized assets$1.4 million from $0.2 million for the additional dispensaries. Furthermore, depreciation expense increasedyear ended December 31, 2021. The increase was primarily due to additional finance leases added.interest earned on notes receivable acquired and entered into during the fourth quarter of 2021.Total (Expense), NetTotal otherOther income, (expense), net for the year ended December 31, 20212022 was $33.4$2.4 million, a decreasean increase of $27.4$1.3 million or (45)%, from $60.9$1.1 million for the year ended December 31, 2020.2021. The overall decreaseincrease is primarily driven by $42.7 million of other expense due to a $2.6 million revaluation of warrants partially offset by increased interest expense.On December 10, 2020, the Company entered into a Supplemental Warrant Indenture with Odyssey Trust Company pursuant to which it amended the terms of the issued and outstanding subordinate voting share purchase warrants of the Company (the “Public Warrants”) to convert the exercise price of the Public Warrants to $13.47 per share, the U.S. dollar equivalent of the Canadian dollar exercise price of the Public Warrants of C$17.25. As a result of this, the Public Warrants converted to equity and eliminated the necessity of revaluation expense on these warrants. The Company did acquire Canadian dollar warrants in the acquisition of Harvest and recorded income related to the revaluation of these warrants in the fourth quarter of the year ended December 31, 2021. Additionally, interest expense increased as a result of new debt to support business growth, additional finance leases and additional construction finance liabilities acquired in the Harvest acquisition.one time sales tax contingency.Income tax expense2021 increased to $146.12022 was $163.4 million, an increase of $17.7 million from $94.5$145.7 million for the year ended December 31, 2020, an increase of $51.6 million2021. Provision for income taxes as a resultpercentage of a $179.7 million increase in gross profit for the same periods. Under IRC Section 280E, cannabis companies are only allowedrevenue decreased from 15.6% to deduct expenses that are directly related to production of the products.13.4%. The increase in income tax expense is primarily due to the significant increase in gross profit as well as an increase in expenses with are not tax deductible under 280ENet Income61Net income for the year ended December 31, 2021 was $17.4 million, a decrease of $45.6 million or 72%, from $63.0 million for the year ended December 31, 2020. The decrease in net income was driven primarily by an increase in revenue due to increased dispensary locations, expansion of wholesale business, and acquisitions. This increase in revenue was offset by increased cost of goods sold driven by inventory step-up, expansion into new markets which are not fully vertical and therefore yield lower margin than Florida, and increased depreciation related to capital expenditures in cultivation and processing. In addition, increases in sales and marketing and general and administrative expenses such significant expenses incurred to acquire and integrate new subsidiaries, most notably Harvest, increases in personnel costs, dispensary expenses, depreciation and amortization, interest expense, ramping infrastructure and go-forward compliance, all contributed to the offset in net income. Income taxes also significantly increased period over period due to higher gross profit.Year Ended December 31, 2020 Compared to Year Ended December 31, 2019Revenue, Net of DiscountsRevenue for the year ended December 31, 2020 was $521.5 million, an increase of $268.7 million, from $252.8 million for the year ended December 31, 2019. Increase in revenue is primarily the result of an increase in our organic growth in retail sales due to the increase in products available for purchase and overall customer count. In addition, we opened 28 additional dispensaries for the year ended December 31, 2020, which increased retail sales year over year.Cost of Goods SoldCost of goods sold for the year ended December 31, 2020 was $135.1 million, an increase of $74.1 million, from $61.0 million for the year ended December 31, 2019 due to increased retail sales as a result of our increase in dispensaries and customer count. Our cost of goods sold as a percentage of revenue increased from 24% for the year ended December 31, 2019 to 26% for the year ended December 31, 2020 due to the change in product mix, expansion into new markets, one-time costs associated with the implementation of SAP, and inventory flow-through.Gross ProfitGross profit for the year ended December 31, 2020 was $386.4 million, an increase of $194.6 million, from $191.8 million for the year ended December 31, 2019. Gross profit as a percentage of revenue decreased from December 31, 2019 compared to December 31, 2020 from 76% to 74%, respectively. This decrease is caused by an increase in depreciation related to capital expenditures in cultivation and processing to support business growth, expansion into new markets, one-time costs associated with the SAP implementation, inventory flow-through and product mix.62Sales and Marketing ExpensesSales and marketing expenses increased from $59.3 million for the year ended December 31, 2019, to $119.4 million for the year ended December 31, 2020, an increase of $60.0 million. The increase in sales and marketing is the result of a higher headcount for the year, as we continue to add additional dispensaries in efforts to maintain and further drive higher growth in sales and market share. This increased headcount resulted in higher personnel costs, which is the primary driver for the increase year over year.General and Administrative ExpensesGeneral and administrative expenses for the year ended December 31, 2020 increased to $36.1 million from $14.1 million for the year ended December 31, 2019 an increase of $22.0 million. The increase in general and administrative expense is primarily the result of entering new markets and ramping up our infrastructure to support growth initiatives and go-forward compliance.Impairment and Disposal of Long-lived AssetsDuring the years ended December 31, 2020 and December 31, 2019 the Company had a nominal amount of disposals on property and equipment.Depreciation and Amortization ExpensesDepreciation and amortization expenses for the year ended December 31, 2020 were $12.6 million, up $7.5 million, or 148% from $5.1 million for the year ended December 31, 2019. The overall increase in depreciation and amortization expenses was due to investment in infrastructure that resulted in more capitalized assets from the additional dispensaries. Furthermore, depreciation expense increased due to additional finance leases added.63Total Other Income (Expense), NetTotal other expense for the year ended December 31, 2020 was $60.9 million, an increase of $51.3 million or 535%, from $9.6 million for the year ended December 31, 2019. The overall increase is the result of our revaluation of debt warrants impacted by the increases in our stock value which were originally denominated in Canadian dollars. The expense for the year ended December 31, 2020 was $42.7 million compared to $0.8 million for the year ended December 31, 2019.On December 10, 2020, the Company entered into a Supplemental Warrant Indenture with Odyssey Trust Company pursuant to which it amended the terms of the issued and outstanding subordinate voting share purchase warrants of the Company (the “Public Warrants”) to convert the exercise price of the Public Warrants to $13.47 per share, the U.S. dollar equivalent of the Canadian dollar exercise price of the Public Warrants of C$17.25. As a result of this, the Public Warrants converted to equity and eliminated the necessity of revaluation expense in future periods. Additionally, interest expense increased as a result of the addition of finance leases to support business growth, for the year ended December 31, 2020.Provision for Income TaxesIncome tax expense for the year ended December 31, 2020 increased to $94.5 million from $50.6 million for the year ended December 31, 2019, an increase of $43.9 million as a result of a $194.6 million increase in gross profit for the same periods. Under IRC Section 280E, cannabis companies are only allowed to deduct expenses that are directly related to production of the products. The increase in income tax expense is due to the significant increase in gross profit as a result of the increase in retail salesincreased revenue, partially offset by increasea more favorable tax position on intercompany management fees. In the third quarter of 2022, the Company adopted a more favorable tax position with respect to intercompany management fees based on an IRS position taken in productionaudit of similar businesses.percentagenormalized metric in making comparisons more meaningful across the cannabis industry, as well as to remove non-recurring, irregular and one-time items that may otherwise distort the GAAP net income measure.revenue.Net Incomeor as an alternative to, measures prepared in accordance with GAAP. There are a number of limitations related to the use of Adjusted EBITDA rather than net income, which is the most directly comparable financial measure calculated and Comprehensive IncomeNetpresented in accordance with GAAP. Because of these limitations, we consider, and you should consider, Adjusted EBITDA together with other operating and financial performance measures presented in accordance with GAAP. A reconciliation of net income, for the year ended December 31, 2020most directly comparable financial measure calculated in accordance with GAAP, to Adjusted EBITDA, has been included herein immediately following our discussion of “Adjusted EBITDA”.$63.0 million, an increase of $9.9 million, or 19%, from $53.1$322.3 million for the year ended December 31, 2019. The increase in net income was driven primarily by an increase in retail sales as2023, a resultdecrease of opening twenty-eight additional dispensaries in Florida during the year ended December 31, 2020. This net increase to net income was offset by gross profit which was driven by increased depreciation related to capital expenditures in cultivation and processing, expansion into new markets, one-time costs associated with the SAP implementation, inventory flow-through and product mix. In addition, increases in sales and marketing and general and administrative expenses such as personnel costs, dispensary expenses, depreciation, interest expense, costs of entering new markets, ramping up infrastructure and go-forward compliance, all contributed to the offset in net income. Income taxes also significantly increased period over period due to higher profit. Lastly, other expense increased as a result of the revaluation of our debt warrants$75.9 million, or 19%, from $398.2 million for the year ended December 31, 2020.2022. The decrease in Adjusted EBITDA in 2023 was primarily due to increased competition and margin pressure which was partially offset by efficiencies in payroll costs primarily in our retail locations and streamlining efforts. 2023 2022 2021 Net (loss) income attributable to common shareholders $ (526,796) $ (246,064) $ 18,032 Add (deduct) impact of: Interest expense, net 81,569 73,422 29,121 (6,164) (1,631) (205) Provision for income taxes 151,358 163,380 145,722 Depreciation and amortization 109,825 116,381 45,791 Depreciation included in cost of goods sold 57,195 46,933 21,232 EBITDA (Non-GAAP) $ (133,013) $ 152,421 $ 259,693 Impairment of goodwill $ 307,590 $ — $ — Impairments and disposals of long-lived assets, net 6,664 75,547 5,371 Legislative campaign contributions 20,062 20,000 — Integration and transition costs 26,889 21,042 25,601 Share-based compensation 10,575 18,124 13,444 Gain on debt extinguishment, net (5,937) — — Other income, net (6,544) (2,388) (1,112) Discontinued operations, net of tax, attributable to common shareholders 96,048 67,440 13,080 Acquisition and transaction costs — 24,757 15,831 Other non-recurring costs — 19,494 5,053 Inventory step up, fair value — 1,048 41,189 COVID related expenses — 783 6,188 Results of entities not legally controlled — (19) 458 Total adjustments 455,347 245,828 125,103 Adjusted EBITDA (Non-GAAP) $ 322,334 $ 398,249 $ 384,796
Liquidity and Capital Resourcesaffiliates, third-party debt and proceeds from the sale of our capital stock. affiliates. We are generating64salesoperations and are deploying our capital reserves to acquire and develop assets capable of producing additional revenues and earnings over both the immediate and near term to support our business growth and expansion.Our current principal sources of liquidity are our cash and cash equivalents provided by our operations and debt and equity offerings. The Company has and expects to retain additional cash from operations, starting in the second half of 2023, due in part to the Company's position that it does not owe taxes attributable to the application of Section 280E of the Internal Revenue Code. Cash and cash equivalents consist primarily of cash on deposit with banks and money market funds.$230.6 million and $146.7$201.4 million as of December 31, 2021 and 2020, respectively.Our primary uses of cash are for working capital Any additional future requirements capital expenditures and debt service payments. Additionally, from time to time, we may use capital for acquisitions and other investing and financing activities. Working capital is used principally for our personnel as well as costs related to the growth, manufacture and production of our products. Our capital expenditures consist primarily of additional facilities and dispensaries, improvements in existing facilities and product development.To the extent additional funds are necessary to meet our long-term liquidity needs as we continue to execute our business strategy, we anticipate that they will be obtainedfunded through incurrence of additional indebtedness, additional equity financings or a combination of these potentialthe following sources of funds. There can be no assurance that we will be ablecapital:funds on terms acceptabledebt from additional creditorsus, on a timely basis or at all. The failureoffer equity to obtain sufficient funds on acceptable terms when needed could haveadditional fundingmaterial adverse effect onpurchase price of $47.6 million, excluding fees and accrued interest. In December 2023, we completed the resultsearly redemption of our 9.75% senior secured notes due June 11, 2024 for a purchase price of $130.0 million, excluding accrued interest. These are collectively referred to as the private placement notes. In December 2023, we also completed a $25.0 million mortgage note with an interest rate of 8.31%. We expect to realize net interest savings of approximately $10.0 million as a result of these three transactions.financial condition.The following table presents our cash and outstanding debt as of the dates indicated:Cash Flowsindicated.ended December 31: 2023 2022 2021 Net cash provided by operating activities $ 201,841 $ 23,096 $ 12,898 Net cash used in investing activities (37,470) (215,057) (215,184) Net cash (used in) provided by financing activities (175,585) 177,796 289,232 Net (decrease) increase in cash and cash equivalents $ (11,214) $ (14,165) $ 86,946 $12.9$201.8 million for the year ended December 31, 2021, a decrease2023, an increase of $86.7$178.7 million, compared to $99.6$23.1 million in net cash provided by operating activities during the year ended December 31, 2020. This2022. The improvement is primarily due to acquisitions, most notably Harvest, and the resulting integration and transaction costsexecution of the Company's inventory wind-down strategy, as well as the inventory step-upimpacts of income tax accruals and increased depreciationthe impact of lower sales & marketing and amortizationgeneral & administrative expenses65$99.6$23.1 million for the year ended December 31, 2020,2022, an increase of $80.6$10.2 million, compared to $19.1$12.9 million in net cash provided by operating activities duringfor the year ended December 31, 2019.2021. This is primarily due to organic growththe timing of our business partiallyincome tax payments that were offset by net working capital including inventory, as we ramp the business to support the growth.$215.2$37.5 million for the year ended December 31, 2021, an increase2023, a decrease of $40.5$177.6 million, compared to the $174.7$215.1 million in net cash used in investing activities for the year ended December 31, 2020.2022. The increase is due toprimary use of cash in both periods was the increasepurchase of property and equipment, with the prior period having significantly more purchases forof property and equipment due to the constructionCompany's build out of additional dispensaries and continued expansion offacilities primarily at our cultivation sites in Florida and processing facilities. This increase is offset byother markets including Pennsylvania and West Virginia. Additionally, the saleprior period included the cash payment of the Florida license that occurred simultaneously with$27.8 million related to the acquisition of Harvest as well as the cash acquired through the Harvest acquisition.$174.7$215.1 million for the year ended December 31, 2020, an increase2022, a decrease of $80.0$0.1 million, compared to the $94.7$215.2 million in net cash used in investing activities for the year ended December 31, 2019.2021. The increasedecrease is mainly due to an acquisition in 2020 and the increasedecrease of property and equipment purchases foroffset by cash provided by the construction of additional dispensaries and continued expansion of our cultivation and processing facilities during the year ended December 31, 2020.provided byused in financing activities was $289.2$175.6 million for the year ended December 31, 2021,2023, an increase of $159.3$353.4 million, compared to the $129.9$177.8 million in net cash provided by financing activities for the year ended December 31, 2020. The increase was2022. This change is primarily related to proceeds from private placement$177.6 million in payments on our senior secured notes, due October 6, 2026 and proceeds fromsenior secured notes due June 11, 2024, collectively referred to as the private placement issuancenotes. These notes were extinguished early in an effort to save on interest and take advantage of shares offset by payments on notes that occurred forfavorable market conditions. Additionally, a decrease in proceeds from borrowings of approximately $141.5 million further contributed to the year ended December 31, 2021.$129.9$177.8 million for the year ended December 31, 2020,2022, a decrease of $13.1$111.4 million, compared to the $143.0$289.2 million in net cash provided by financing activities for the year ended December 31, 2019.2021. The increase wasdecrease is primarily related to $83.2 million ofa decrease in proceeds forfrom borrowings compared to the issuance of shares offering that occurred for the year ended December 31, 2020. The increase was partially offset by the $122.2 million in net proceeds received from the debt issuance in 2019.Funding SourcesFinance Liability, “June Warrants” and “November Warrants”On June 18, 2019, we completed an offering using our Canadian prospectus of 70,000 units (the “June Units”), comprised of an aggregate principal amount of US$70.0 million of 9.75% senior secured notes maturing in 2024 (the “June Notes”) and an aggregate amount of 1,470,000 subordinate voting share warrants (each individual warrant being a “June Warrant”) at a price of US$980 per June Unit for gross proceeds of US$68.6 million. Each June Unit was comprised of one June Note issued in denominations of $1,000 and 21 June Warrants.On November 7, 2019, we completed an offering using our Canadian prospectus of 60,000 units (the “November Units”), comprised of an aggregate principal amount of US$60.0 million of 9.75% senior secured notes maturing in 2024 (the “November Notes”) and an aggregate amount of 1,560,000 subordinate voting share warrants (each individual warrant being a “November Warrant”) at a price of US$980 per November Unit for a gross proceeds of US$61.1 million. Each November Unit was comprised of one November Note issued in denominations of $1,000 and 26 November Warrants.Secured Promissory NotesOn October 6, 2021, the Company closed on a private placement of 8% Senior Secured Notes (the "“Notes") for aggregate gross proceeds of $350.0 million and net proceeds of $342.6 million. The Notes were issued at 100% face value, bear an interest rate of 8% per annum payable semi-annually in equal installments until the maturity date, unless earlier redeemed or repurchased. The Notes mature on October 6, 2026 and may be redeemed in whole or in part, at any time from time to time, on or after October 6, 2023 at the applicable redemption price set forth in the trust indenture dated as of June 18, 2019 (the “Base Indenture”), as supplemented by a supplemental trust indenture dated as of October 6, 2021 (the “Supplemental Indenture” and, the Base Indenture as supplemented by the Supplemental Indenture, the “Indenture”), by and between the Company and Odyssey Trust Company, as trustee. The Company used a portion of the net proceeds to redeem certain outstanding indebtedness of Harvest, and intends to use the remaining net proceeds for capital expenditures and other general corporate purposes. The Indenture governing the Notes contains covenants that, among other things, limit the ability of the Company and its restricted subsidiaries to, among other things, declare or pay dividends or make certain other payments; purchase, redeem or otherwise acquire or retire for value any equity interests or otherwise make any restricted payments; conduct certain asset sales or consolidate, merge or transfer all or substantially all of the assets of the Company and its subsidiaries on a consolidated basis; make certain restricted investments, incur certain indebtedness or grant certain liens, or enter into certain affiliate transactions. These covenants are subject to a number of other limitations and exceptions as set forth in the Indenture.66Unsecured Promissory NotesOn April 10, 2017, we entered into an unsecured promissory note with a 12% annual interest rate, which was amended in January 2019 to extend the maturity by three years to 2022, with a balance asprior year.2019 of $4.0 million. On December 17, 2017, we entered into a promissory note dated December 7, 2017, with a 12% annual interest rate2023 and a balance as of December 31, 2019 of $2.0 million. Each promissory note is due in 2022. The promissory notes were repaid in full during the year ended December 31, 2021.Related Party Promissory NotesIn May 2018, the Company entered into two separate unsecured promissory notes (the “Traunch Four Note” and the “Rivers Note”) for a total of $12.0 million. The Traunch Four Note was held by Traunch Four, LLC, an entity whose direct and indirect owners included Kim Rivers, the Chief Executive Officer and Chair of the Board, as well as Thad Beshears, Richard May, George Hackney, all of whom are directors of Trulieve, and certain of Richard May’s family members. The Rivers Note was held by Kim Rivers. Each promissory note had a 24-month maturity and 12% annual interest rate. The two unsecured promissory notes were repaid in November 2021.In February 2019, the Company entered into a 24-month unsecured loan with an 8% annual interest rate with Benjamin Atkins, a former director and shareholder for $0.3 million. The loan was issued in March 2019. The Company determined that the stated interest rate was below market rates and recorded an insignificant debt discount using an annual discount interest rate of 12%.Balance Sheet ExposureAt December 31, 2021 and 2020,2022, 100% of our balance sheet is exposed to U.S. cannabis-related activities.activities, and substantially all our revenue is derived from U.S. cannabis operations. We believe our operations are in material compliance with all applicable state and local laws, regulations and licensing requirements in the states in which we operate. However, cannabis remains illegal under U.S. federal law. Substantially all our revenue is derived from U.S. cannabis operations. For information about risks related to U.S. cannabis operations, please refer to the “Risk Factors” section of this Annual Report on Form 10-K.At2021,2023, we had the following contractual obligations to make future payments, representing material contracts and other commitments that are known and committed: <1 Year 1 to 3 Years 3 to 5 Years >5 Years Total (in thousands) Notes payable $ 3,751 $ 8,986 $ 93,233 $ 15,783 $ 121,753 Private placement notes — 368,000 — — 368,000 Operating lease liabilities 21,452 42,180 39,532 81,820 184,984 Finance lease liabilities 14,129 27,392 24,252 34,247 100,020 Construction finance liabilities 22,498 46,941 49,659 295,672 414,770 Lease settlements 1,008 864 857 2,226 4,955 $ 62,838 $ 494,363 $ 207,533 $ 429,748 $ 1,194,482 10. 11. Notes Payable, Note 12. Private Placement Notes, Note 13. Leases, Note 14. Construction Finance Liabilities, Note 18. Income Taxes, Note 19. Discontinued Operations, and Note 20. 23. Commitments And Contingencies.Contingencies, for additional information.GAAPGenerally Accepted Accounting Principles ("GAAP") requires management to make judgments, estimates, and assumptions about the carrying amounts of assets and liabilities that are not readily apparent from other sources. The estimates and underlying assumptions are reviewed on an ongoing basis. The estimates and associated assumptions are based on historical experience and other factors that are considered to be relevant. Actual results may differ from these estimates, and revisions to accounting estimates are recognized in the period in which the estimate is revised.67judgementjudgment in estimating the probability and timing of when earn-outs are expected to be achieved, which is used as the basis for estimating fair value. For any intangible asset identified, depending on the type of intangible asset and the complexity of determining its fair value, an independent valuation expert or management may develop the fair value, using appropriate valuation techniques, which are generally based on a forecast of the total expected future net cash flows.
Cannabis licenses are the primary intangible asset acquired in business combinations as they provide the Company the ability to operate in each market. However, some cannabis licenses are subject to renewal and therefore there is some risk of non-renewal for several reasons, including operational, regulatory, legal or economic. To appropriately consider the risk of non-renewal, the Company applies probability weighting to the expected future net cash flows in calculating the fair value of these intangible assets. The key assumptions used in these cash flow projections include discount rates and terminal growth rates. Of the key assumptions used, the impact of the estimated fair value of the intangible assets havehas the greatest sensitivity to the estimated discount rate used in the valuation. The terminal growth rate represents the rate at which these businesses will continue to grow into perpetuity. Other significant assumptions include revenue, gross profit, operating expenses and anticipated capital expenditures which are based upon the Corporation’s historical operations along with management projections.AsThe IRS has taken the Company operates in theposition that cannabis industry, it iscompanies are subject to the limits of IRC Section 280E under which the Company isthey are only allowed to deduct expenses directly related to the cost of producing the products or cost of production.AssessmentsImpairment assessment estimates applies to goodwill,Assessmentand right-of-use assets.68Goodwill is allocated at the date the goodwill is initially recorded. We evaluate goodwill for impairment annually or more frequently when an event occurs or circumstances change that indicates the carrying value may not be recoverable. We review our long-lived assets, such asincluding property and equipment, definite life intangible assets, and right-of-use assets for impairment whenever events or changes in circumstances indicate that the carrying amountvalue of an asset or asset group may not be recoverable. Significant judgmentFactors which could trigger an impairment review include significant underperformance relative to historical or projected future operating results, significant changes in the manner of use of the assets or the strategy of the business, a significant decrease in the market value of the assets or significant negative industry or economic trends. In accordance with ASC 360-10, when evaluating long-lived assets with impairment indicators for potential impairment, we first compare the carrying value of the asset to its estimated undiscounted cash flows. If the sum of the estimated undiscounted cash flows is less than the carrying value of the asset, we calculate an impairment loss. The impairment loss calculation compares the carrying value of the asset to its estimated fair value, which is typically based on estimated discounted future cash flows. We recognize an impairment loss if the amount of the asset’s carrying value exceeds the asset’s estimated fair value.estimatesreporting unit evaluating goodwill for impairment as one singular reporting unit. We evaluate our goodwill for impairment annually at the beginning of the fourth quarter or earlier upon the occurrence of substantive unfavorable changes in economic conditions, industry trends, costs, cash flows, or ongoing declines in market capitalization. The Company applies the guidance in Financial Accounting Standards Board (“FASB”) Accounting Standards Update (“ASU”) 2011-08 “Intangibles-Goodwill and Other-Testing Goodwill for Impairment,” which provides entities with an option to perform a qualitative assessment (commonly referred to as “Step Zero”) to determine whether further quantitative analysis for impairment of goodwill is necessary. In performing Step Zero for the Company’s goodwill impairment test, the Company is required to make assumptions and judgments including but not limited to the following: the evaluation of macroeconomic conditions as related to the Company’s business, industry and market trends, and the overall future financial performance of its reporting units and future opportunities in the markets in which they operate. If impairment indicators are required when determiningpresent after performing Step Zero, the Company would perform a quantitative impairment analysis to estimate the fair value of thesegoodwill.forliabilities, and goodwill to reporting units, and the determination of fair value of each reporting unit. The impairment tests.Share-Based Payment ArrangementsWe usetest requires the Black-Scholes pricing model to determinecomparison of the fair value of options and warrants granted to employees and directors under share-based payment arrangements, where appropriate. In estimatinga reporting unit with the carrying amount, including goodwill. If the Company would conclude a quantitative impairment test is required, the Company would review fair value management is requiredtechniques for the most appropriate technique generally applying the income approach by using discounted cash flow (“DCF”) analyses. Determining fair value requires the Company to make certain assumptionsjudgments about appropriate forecasted revenue and estimates such asrelated revenue growth rate, the expected lifeearnings before interest, taxes, depreciation, and amortization ("EBITDA") margins rate and the weighted average cost of units, volatility of future share price, risk free rates, and future dividend yields at the initial grant date. Changes in assumptions used to estimate fair value could result in materially different results.Commitments and ContingenciesFrom time to time, the Company may be involved in litigation relating to claims arising out of operationscapital. The cash flows employed in the normal course of business. Periodically,DCF analysis are based on the Company reviews the status of each significant matter and assesses the potential financial exposure. If the potential loss from any claim or legal proceeding is considered probable, and the amount can be reliably estimated, such amount is recognized in contingencies. Contingent liabilities are measured at management’s best estimate of the expenditure required to settle the obligation at the endforecast of the reporting periodunit, long-term business plan and recent operating performance. Discount rate assumptions are discounted to presentbased on an assessment of the risk inherent in the future cash flows of the reporting unit and market conditions. Given the inherent uncertainty in determining the assumptions underlying a DCF analysis, actual results may differ from those used in our valuations. The reporting unit may be at risk of failing the quantitative impairment test if it has a fair value wherethat is not substantially in excess of the effect is material.carrying amount at the assessment date.performs evaluationsconcluded the decline in stock price was a triggering event to perform an interim quantitative goodwill impairment test, as of June 30, 2023, specific to the stock price decline and resulting market capitalization of the Company. As the sole risk to the value of goodwill was the stock price, the Company concluded it most appropriate to apply a market approach. The results of the Company’s interim test for impairment as of June 30, 2023, utilizing a market approach, indicated that the reporting unit's fair value fell below the carrying value. Based on the results of the goodwill impairment procedures, the Company recorded a $307.6 million goodwill impairment for the single reporting unit in the second quarter of 2023.onerous contracts and, where applicable, records contingent liabilities for such contracts.internally-produced work in process, and finished goods and packaging materials.goods. Costs incurred during the growing and production process are capitalized as incurred to the extent that cost is less than net realizable value. The costs include materials, labor and manufacturing overhead used in the growing and production processes. Pre-harvest costs are capitalized. Our inventory of purchased finished goods and packing materials are initially valued at cost and subsequently at the lower of cost and net realizable value.organizationorganizations by requiring the recognition of Right of Use Assets “ROU” assets and lease liabilities on the balance sheet. The requirements of this standard include a significant increase in required disclosures to meet the objectives of enabling users of financial statements to assess the amount, timing, and uncertainty of cash flows arising from leases. The new standard was effective beginning January 1, 2019 and the standard was adopted using the modified retrospective transition approach, which allows us to recognize a cumulative effect adjustment to the opening balance of accumulated deficit in the period of adoption rather than restate comparative prior year periods.We contract with our customers for the saleRevenues consist of driedretail and wholesale sales of cannabis cannabis oil and other cannabis related products, that consist of multiple performance obligations. Revenue from the direct sale of cannabis to customers for a fixed price iswhich are recognized when we transfer control of the goods has transferred to the customer atand collectability is reasonably assured. This is generally when goods have been delivered, which is also when the pointperformance obligation has been fulfilled under the terms of sale and the customer has paid for the goods.related sales contract.69stock basedstock-based payment awards made to employees. We measure the share-based payment awards based on its estimated fair value of the awards using the Black-Scholes option pricing model, and the fair value of the Company’s common stock on the date of grant, for the warrants and options. We measure the share-based payment awards based on its estimated fair value of the awards using the Black-Scholes option pricing model for warrants and options, and the fair value of the Company’s common stock on the date of grant for restricted stock awardsunits ("RSUs").Off-Balance Sheet ArrangementsWe account for business combinations using the acquisition method in accordance with Accounting Standards Codification ASC 805, Business CombinationsAs, which requires recognition of assets acquired and liabilities assumed, including contingent assets and liabilities, at their respective fair values on the date of this Annual Reportacquisition.Form 10-K, we doacquisition date. Acquisition-related costs are recognized as expenses in the periods in which the costs are incurred and the services are received.have any off-balance-sheet arrangementsto exceed one year from the date of acquisition, changes in assumptions and estimates that have, orresult in adjustments to the fair value of assets acquired and liabilities assumed are reasonably likely to have, a current or future effect onrecorded in the results period the adjustments are determined.condition of, including,assets and without limitation, such considerations as liquidity and capital resources.Management’s Use of Non-GAAP MeasuresOur management uses financial measuresliabilities that are not in accordance with generally accepted accounting principlesrecognized or disclosed at fair value in the U.S.,financial statements on a recurring basis. Fair value is defined as the price that would be received from selling an asset or GAAP,paid to transfer a liability in additionan orderly transaction between market participants at the measurement date. When determining the fair value measurements for assets and liabilities that are required to financial measures in accordance with GAAP to evaluate our operating results. These non-GAAP financial measures should be considered supplemental to, and not a substitute for, our reported financial results prepared in accordance with GAAP. Adjusted EBITDA is a financial measure that is not defined under GAAP. Our management uses this non-GAAP financial measure and believes it enhances an investor’s understanding of our financial and operating performance from period to period because it excludes certain material non-cash items and certain other adjustments management believes are not reflective of our ongoing operations and performance. Adjusted EBITDA excludes from net income as reported interest, provision for income taxes, depreciation and amortization to arriverecorded at EBITDA. This is then adjusted for items that do not represent the operationsfair value, we consider all related factors of the core businessasset by market participants in which the Company would transact and the market-based risk measurements or assumptions that market participants would use in pricing the asset or liability, such as inventory step-up for fair value adjustments in purchaseinherent risk, transfer restrictions, and credit risk.integration and transition costs, acquisition and transaction costs, other non-recurring costs, expenses relatedstandards applicable to the COVID-19 pandemic, impairments and disposals of long-lived assets,Company is described in Note 3. Summary Of Significant Accounting Policies, in the results of entities consolidated as VIEs but not legally controlled and operated by the Company, and other income and expense items. Integration and transition costs include those costs related to integration of acquired entities and to transition major systems or processes. Acquisition and transaction costs relate to specific transactions such as acquisitions whether contemplated or completed and regulatory filings and costs related to equity and debt issuances. Other non-recurring costs includes miscellaneous items which are not expected to reoccur frequently such as inventory adjustments related to specific issues and unusual litigation. During the year ended December 31, 2021, the Company adjusted the definition of Adjusted EBITDA to include expenses incurred as a result of the COVID-19 pandemic. Adjusted EBITDA for the year ended December 31, 2020 has been adjusted to reflect this current definition. Additionally, certain reclassifications have been made to Adjusted EBITDA for prior periods to conformnotes to the current period presentation.Trulieve reports Adjusted EBITDA to help investors assess the operating performance of the Company’s business. Theconsolidated financial measures noted above are metrics that have been adjusted from the GAAP net income measurestatements contained elsewhere in an effort to provide readers with a normalized metric in making comparisons more meaningful across the cannabis industry, as well as to remove non-recurring, irregularthis Report, and one-time items that may otherwise distort the GAAP net income measure.As noted above, our Adjusted EBITDA is not prepared in accordance with GAAP, and should not be considered in isolation of, or as an alternative to, measures prepared in accordance with GAAP. There are a number of limitations related to the use of Adjusted EBITDA rather than net income, which is the most directly comparable financial measure calculated and presented in accordance with GAAP. Because of these limitations, we consider, and you should consider, Adjusted EBITDA together with other operating and financial performance measures presented in accordance with GAAP. A reconciliation of Adjusted EBITDA from net income, the most directly comparable financial measure calculated in accordance with GAAP, has been included herein immediately following ourincorporate such discussion of “Adjusted EBITDA”.by reference herein.Adjusted EBITDA70Adjusted EBITDA for the year ended December 31, 2021, was $384.6 million, an increase of $124.5 million or 48%, from $260.1 million for the year ended December 31, 2020. The following table presents a reconciliation of GAAP net income (loss) to non-GAAP Adjusted EBITDA, for each of the periods presented:Currency RiskOur operating results and financial position are reported in U.S. dollars. Some of our financial transactions are denominated in currencies other than the U.S. dollar. The results of operations are subject to currency transaction risks.We have no hedging agreements in place with respect to foreign exchange rates. We have not entered into any agreements or purchased any instruments to hedge possible currency risks at this time.2021. Concentrations2023. The Company reviews its trade receivable accounts and notes receivable regularly and reduces amounts to their expected realizable values by adjusting the allowance credit losses when management determines that the account may not be fully collectable. The Company applies ASC 326 Financial Instruments – Credit Losses for the measurement of expected credit risk with respectlosses, which uses an expected loss allowance model for all trade and notes receivables. The Company has adopted standardized credit policies and performs assessments in an effort to our cash and cash equivalents are limited primarily to amounts held with financial institutions.minimize those risks.itsour financial obligations associated with financial liabilities. We manage liquidity risk through the management of itsour capital structure. Our approach to managing liquidity is to ensure that we will have sufficient liquidity to settle obligations and liabilities when due.AsOur debt exposes us to risk of fluctuations in interest rates. Fixed rate debt, where the interest rate is fixed over the life of the instrument, exposes us to changes in market interest rates reflected in the fair value of the debt and to the risk that we may need to refinance maturing debt with new debt at higher rates. Floating rate debt, where the interest rate fluctuates periodically, exposes us to short-term changes in market interest rates. We manage our debt portfolio to achieve an overall desired proportion of fixed and floating rate debts and may employ interest rate swaps ("Swaps") as a tool from time to time to achieve that position. To manage our interest rate risk exposure, we entered into one Swap contract during the year ended December 31, 2022, to hedge the floating rate term loans. Changes in market interest rates impact the fair value of our Swap contract, which was a liability of $2.3 million as of December 31, 2021,2023. In addition to our cash balances consisted of bank deposits and a money market fund. Our private placement debt (aggregate amount of $480 million)notes payable and long-term debt, (aggregate amount of $16.6 million) bears interest at fixed rates. As of December 31, 2021, we also have lease obligations and construction finance liabilities of $203.4 million and $176.2 million, respectively.that bare interest. Interest rates on existing leases and construction finance liabilities typically do not change unless there is a modification to an underlying agreement. Changes in market interest rates impact the fair value of our derivative liabilities of which the balance is $2.9 million as of December 31, 2021, the term of which expires in April 2023, and as such there is no significant interest rate exposure for the derivative liabilities. Therefore exposure to market risk for changes in interest rates is limited.BankingNotwithstanding thatmajorityhigher rate than revenues. Our costs are subject to fluctuations, particularly due to changes in the prices of states have legalized medical marijuana, there has been no changeraw product and packaging materials and the costs of labor, transportation and energy. Inflation pressures could also result in U.S. federal banking laws relatedincreases in these input costs. Therefore, our business results depend, in part, on our continued ability to manage these fluctuations through pricing actions, cost saving projects and sourcing decisions, while maintaining and improving margins and market share. Failure to manage these fluctuations could adversely impact our results of operations or cash flows. In addition, unfavorable macroeconomic conditions, such as a recession or continued slowed economic growth, could negatively affect consumer demand for cannabis products, which consequently, may negatively affect the deposit and holdingresults of funds derived from activities relatedoperations. Under difficult economic conditions, consumers may seek to the marijuana industry. Given that U.S. federal law provides that the production and possessionreduce discretionary spending by forgoing purchases of cannabis is illegal, there is a strong argument that banks cannot acceptproducts, negatively impacting our net sales and margins. Softer consumer demand for deposit funds from businesses involved with the marijuana industry. Consequently, businesses involved in the marijuana industry often have difficulty accessing the U.S. banking systemcannabis products could reduce our profitability and traditional financing sources. The inability to open bank accounts with certain institutions may make it difficult to operate the businesses of Trulieve, its subsidiaries and investee companies, and leaves their cash holdings vulnerable. We have banking relationships in all jurisdictions in which we operate.Financial Instruments and Financial Risk ManagementWe are exposed in varying degrees to a variety ofcould negatively affect our overall financial instrument related risks. The board of directors of Trulieve mitigate these risks by assessing, monitoring and approving the risk management processes.The Company’s financial instruments that are measured at fair valued on a recurring basis consist of money market funds and a warrant liability. Our financial instruments where carrying value approximates the fair value include cash, accounts payable and accrued liabilities, notes payable, notes payable related party, operating lease liability, finance lease liability, other long-term liabilities and construction finance liability. Financial instruments recorded at fair value are classified using a fair value hierarchy that reflects the significance of the inputs to fair value measurements. The three levels of hierarchy are:Level 1:Observable inputs based on unadjusted quoted prices in active markets for identical assets or liabilities;Level 2:Inputs other than quoted prices in active markets, that are observable for the asset or liability, either directly or indirectly; andLevel 3:Unobservable inputs for which there is little or no market data requiring the Company to develop its own assumptions.72report.Annual Report.Withwith Accountants on Accounting and Financial Disclosure.2021,2023, an evaluation was performed under the supervision and with the participation of our management, including the CEO and CFO, of the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act). Based on that evaluation, our management concluded that as of December 31, 2021,2023, we did not maintain effective disclosure controls and procedures because of the material weaknessweaknesses in internal control over financial reporting described below under the caption “—Material Weakness in Internal Control Over Financial Reporting.”2021,2023, our management carried out an evaluation, under the supervision and with the participation of our CEO and CFO, of the effectiveness of the design and operation of our disclosure controls over financial reporting and procedures using the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”) in Internal Control-Integrated Framework (2013). Based on the foregoing evaluation, our management concluded that our internal controls over financial reporting were not effective because of the material weaknessweaknesses identified in our internal control over financial reporting discussed below, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis.Aweakness isweaknesses have been identified:deficiency, or a combinationmaterial misstatement to the Company’s consolidated financial statements for the year ending December 31, 2023, they did represent material weaknesses as of deficiencies, in internal control over financial reporting, such thatDecember 31, 2023, since there isexisted a reasonable possibility that a material misstatement of ourthe Company’s annual or interim financial statements willwould not be prevented or detected on a timely basis.As disclosed in our Quarterly Report on Form 10-Q as of September 30, 2021 and "Item 1A. Risk Factors" management identified errors in the accounting for leases, asset acquisitions, and classification of assets. Management reviewed these errors identifying the root cause due to the control environment component of internal control as the Company did not maintain a sufficient complement of personnel with the appropriate level of knowledge, experience, and training in certain areas important to financial reporting. During 2021, even though a material misstatement was not identified in the Company’s financial statements, it was determined that there was a reasonable possibility that a material misstatement in the Company’s financial statements would not have been prevented or detected on a timely basis.During the testing for the Company’s audit, it was determined that the primary user access controls and program change management controls over information technology systems were not effectively designed or implemented to ensure appropriate authorization and segregation of duties.weakness in the overall control environment,weaknesses, management, with the oversight of the Audit Committee of the Board of Directors, has taken a73endedending December 31, 2021,2023, and continues to address these deficiencies. The Company will not be able to conclude that the material weaknesses are continuing our actions.remediated until the applicable controls operate for a sufficient period of time and management has concluded, through formal testing, that the controls are operating effectively. Remediation actions taken duringinclude the year are outlined below.•Added additional positions including Chief Accounting Officer (“CAO”), Executive Director Engaged third party experts The Company continued to assist management in assessing current processesstrengthen the design and designing improved processes and controls for the consolidated Company.•Added a Chief Technology Officer (“CTO”) to enhance the information technology environment including automationimplementation of processes and controls and finalization of an ongoing SAP implementation. There have also been additional Directors positions added to the Information Technology organization.•Reviewed business processes surrounding leases, acquisitions, and other complex financial reporting areas to identify and implement enhanced procedures related to internal controls.•Additional programlogical access, change management, and IT operation controls over information technology systems implemented and are in the process of adding additional access andthrough proper segregation of duties, monitoring controls, over financial relevant systems.and the development of controls around critical jobs.While significant progressbeen madepromoted risk-based prioritization of remediating findings and a mechanism to enhancetrack continuous improvement.we are still in the processas of building and enhancing our processes, procedures, and controls. Additional time is required to complete the remediation of the material weaknesses and the assessment to ensure the sustainability of these remediation actions. We believe the above actions as well as those being implemented currently, when complete, will be effective in the remediation of the material weaknesses described above.December 31, 2023, which report follows below.Except formaterial weaknesses and theongoing remediation efforts describedmeasures discussed above, there have been no other changechanges in our internal control over financial reporting (as defined in Rules13a-15(f) and 15d-15(f) under the Exchange Act) which occurred during the quarter ended December 31, 2021,2023, that hashave materially affected, or isare reasonably likely to materially affect, the Company’s internal control over financial reporting.20222024 Annual Meeting of Stockholders to be filed with the SEC within 120 days after the end of the fiscal year ended December 31, 2021.2023.20222024 Annual Meeting of Stockholders to be filed with the SEC within 120 days after the end of the fiscal year ended December 31, 2021.2023.20222024 Annual Meeting of Stockholders to be filed with the SEC within 120 days after the end of the fiscal year ended December 31, 2021.2023.The significant accounting policies used by the Company are as follows:carriedrecorded at fair value, cashvalue. Cash held in financial institutions and cash held at retail locations have carrying values that approximate fair value.As of December 31, 2021 restricted cash was $3.0 million, which represented cash consideration set aside as a reserve in relationThey are held by or with financial institutions pursuant to an escrow held for a pending legal settlement. Restricted cash was released subsequent to year-end as the litigation was settled in December 2021 and final escrow was released in January 2022. See further information in Note 20. Commitment and Contingencies. Reconciliation of cash, cash equivalents and restricted cash reported within the consolidated balance sheets as of December 31, 2021 was $230.7 million and $3.0 million, respectively, which sums to the cash, cash equivalents and restricted cash reported on the consolidated statements of cash flows as of December 31, 2021 of $233.7 million. There was no restricted cash as of December 31, 2020.F-10TRULIEVE CANNABIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTSInventoryInventories are primarily comprised of raw materials, internally produced work in process, finished goods and packaging materials. Inventory is valued at the lower of cost and net realizable value. Net realizable value is determined as the estimated selling price in the ordinary course of business less the estimated costs of completion, disposal and transportation for inventories in process. Cost is determined using the weighted average cost method.Costs incurred during the growing and production process are capitalized as incurred to the extent that cost is less than net realizable value. These costs include materials, labor and manufacturing overhead used in the growing and production processes. The Company capitalizes pre-harvest costs.The Company periodically reviews its inventory and identifies that which is excess, slow moving and obsolete by considering factors such as inventory levels, expected product life and forecasted sales demand. Any identified excess, slow moving and obsolete inventory is written down to its net realizable value through a charge to cost of goods sold.uncollectabilitynon-collectability of accounts receivable and notes receivable based on historical collection data and specific risks identified among uncollected accounts, as well as management’s expectation of future economic conditions. The Company also considers relevant qualitative and quantitative factors to assess whether historical loss experience should be adjusted to better reflect the risk characteristics of the companies receivables and the expected future losses. If current or expected future economic trends, events, or changes in circumstances indicate that specific receivable balances may be impaired, further consideration is given to the collectability of those balances and the allowance is adjusted accordingly. Trade accountsAccounts receivable and notes receivable are written off after exhaustive collection efforts occur and the receivable is deemed uncollectible.The Company classifies assets as held for sale and ceases depreciation of the assets when there is a plan for disposal of the assets and those assets meet the held for sale criteria. Gains or losses on disposal of an item are determined by comparing the proceeds from disposal with the carrying amount of the item and recognized in the statements of operations and comprehensive income. Construction in progress is transferred when available for use and depreciation of the assets commences at that point.reviews propertiesclassifies long-lived assets or disposal groups and related liabilities as held-for-sale when management having the appropriate authority, generally the Company's Board of Directors ("the Board") or certain Executive Officers, commit to a plan of sale, the disposal group is ready for impairment whenever events or changes in circumstances indicate thatimmediate sale, an active program to locate a buyer has been initiated and the sale is probable and expected to be completed within one year. Once classified as held-for-sale, disposal groups are valued at the lower of their carrying amount of an asset group may not be recoverable.F-11TRULIEVE CANNABIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTSIntangible AssetsIntangible assets are recorded at cost, less accumulated amortization and impairment losses, if any. Intangible assets acquired in a business combination are measured ator fair value at the acquisition date. Intangible assets that have indefinite useful lives are not subject to amortization and are tested annually for impairment, or more frequently if events or changes in circumstances indicate that they might be impaired. Theless estimated useful lives, residual values and amortization methods are reviewed at each year-end, and any changes in estimates are accounted for prospectively.Intangible assets are amortized using the straight-line method over estimated useful lives as follows:Licenses15 yearsInternal use software3 to 5 yearsTradenames2 to 10 yearsCustomer relationship1 to 5 yearsNon-compete2 yearsTrademarks1 to 5 yearsImpairment of long-lived assetsThe Company reviews long-lived assets, including property and equipment and definite life intangible assets for impairment whenever events or changes in circumstances indicate that the carrying value of an asset may not be recoverable. An impairment loss is recognized when the sum of projected undiscounted cash flows is less than the carrying value of the asset group. The measurement of the impairment loss to be recognized is based on the difference between the fair value and the carrying value of the asset group.Business combinations and goodwillThe Company accounts for business combinations using the acquisition method in accordance with Accounting Standards Codification ASC 805, Business Combinations which requires recognition of assets acquired and liabilities assumed, including contingent assets and liabilities, at their respective fair values on the date of acquisition.Contingent consideration is measured at its acquisition-date fair value and included as part of the consideration transferred in a business combination. Contingent consideration that is classified as equity is not remeasured at subsequent reporting dates and its subsequent settlement is accounted for within equity. Contingent consideration that is classified as an asset or liability is remeasured at subsequent reporting dates,selling costs with the corresponding gain or loss on disposal recognized in the consolidated statements of operationsoperations. Depreciation on these properties is discontinued at the time they are classified as held for sale, but operating revenues, operating expenses, and comprehensive income.Non-controlling interests in the acquiree are measured at fair value on acquisition date. Acquisition-related costs are recognized as expenses in the periods in which the costs are incurred and the services are received.Loans acquired in business combinations are initially recorded at fair value, which includes an estimate of credit losses expectedinterest expense continues to be realized over the remaining lives of the loans and, therefore, no corresponding allowance for loan losses is recorded for such loans at acquisition.Purchase price allocations may be preliminary and, during the measurement period not to exceed one year fromrecognized until the date of acquisition, changes in assumptions and estimates that result in adjustments to the fair value of assets acquired and liabilities assumed are recorded in the period the adjustments are determined.Goodwill represents the excess of the consideration transferred for the acquisition of subsidiaries over the net of the acquisition-date amounts of the identifiable assets acquired and the liabilities assumed. Following initial recognition, goodwill is measured at cost less any accumulated impairment losses.Cannabis licenses are the primary intangible asset acquired in business combinations as they provide the Company the ability to operate in each market. However, some cannabis licenses are subject to renewal and therefore there is some risk of non-renewal for several reasons, including operational, regulatory, legal or economic. To appropriately consider the risk of non-renewal, the Company applies probability weighting to the expected future net cash flows in calculating the fair value of these intangible assets. The key assumptions used in these cash flow projections includeF-12TRULIEVE CANNABIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTSdiscount rates and terminal growth rates. Of the key assumptions used, the impact of the estimated fair value of the intangible assets have the greatest sensitivity to the estimated discount rate used in the valuation. The terminal growth rate represents the rate at which these businesses will continue to grow into perpetuity. Other significant assumptions include revenue, gross profit, operating expenses and anticipated capital expenditures which are based upon the Corporation’s historical operations along with management projections. The evaluations are linked closely to the assumptions made by management regarding the future performance of these assets and any changes in the discount rate applied.Impairment of goodwill and indefinite-lived intangible assetsGoodwill is tested for impairment annually and whenever events or changes in circumstances indicate that the carrying amount of goodwill may have been impaired. In order to determine that the value of goodwill may have been impaired, the Company performs a qualitative assessment to determine that it was more-likely-than-not if the reporting unit’s carrying value is less than the fair value, indicating the potential for goodwill impairment. A number of factors, including historical results, business plans, forecasts and market data are used to determine the fair value of the reporting unit. Changes in the conditions for these judgments and estimates can significantly affect the assessed value of goodwill.The Company operates as one operating segment and reporting unit and therefore, evaluates goodwill and other intangible assets with indefinite lives for impairment as one singular reporting unit annually during the fourth quarter or more often when an event occurs or circumstances indicate the carrying value may not be recoverable. The Company did not identify any impairment of its goodwill during the years ended December 31, 2021, 2020, or 2019.Accounts Payable and Accrued LiabilitiesAccounts payable and accrued liabilities consists of:F-13TRULIEVE CANNABIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTSPrepaid Expenses and Other Current AssetsPrepaid Expenses and Other Current Assets consists of:LeasesWe have, we have the Company elected to combine lease and non-lease components for all classes of assets.operations and comprehensive income.valuesvalue of these obligations are recorded as liabilities on a discounted basis, which occurs as of leaseF-14TRULIEVE CANNABIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS Asset retirement obligations related to our leases were $0.8 million and zero as of December 31, 2021, and 2020, respectively, and are included in other long-term liabilities in the consolidated balance sheets.Revenue RecognitionRevenue is recognized byWhen the Company in accordance with ASU 2014-09, Revenue from Contracts with Customers (Topic 606). Through applicationenters into sale-leaseback transactions, an assessment is performed to determine whether a contract exists and whether there is a performance obligation to transfer control of the standard, the Company recognizes revenue to depictasset when determining whether the transfer of promised goods or services toan asset shall be accounted for as a sale of the customer in an amount that reflectsasset. If control is not transferred based on the consideration to whichnature of the transaction, and therefore does not meet the requirements for a sale under the sale-leaseback accounting model, the Company expectsis deemed to be entitledown this real estate and reflects these properties on our consolidated balance sheets in exchange for those goods or services.In orderproperty and equipment, net and depreciates them over the assets' useful lives. The liabilities associated with these leases are recorded to recognize revenue under ASU 2014-09, the Company applies the following five (5) steps:•Identify a customer along with a corresponding contract;•Identify the performance obligation(s) in the contract to transfer goods or provide distinct services to a customer;•Determine the transaction price the Company expects to be entitled to in exchange for transferring promised goods or services to a customer;•Allocate the transaction price to the performance obligation(s) in the contract;construction finance liabilities - current portion and•Recognize revenue when or as the Company satisfies the performance obligation(s).The Company’s contracts with customers for the sale of dried cannabis, cannabis oil and other cannabis related products consist of multiple performance obligations. Revenue from the direct sale of cannabis to customers for a fixed price is recognized when the Company transfers control of the goods to the customer at the point of sale and the customer has paid for the goods. The Company has a loyalty rewards program that allows customers to earn reward credits to be used on future purchases. Loyalty reward credits issued as part of a sales transaction results in revenue being deferred until the loyalty reward is redeemed by the customer. The loyalty rewards are shown as reductions to ‘revenue, net of discounts’ line on the accompanying consolidated statements of operations and comprehensive income and included as deferred revenue construction finance liabilities on the consolidated balance sheet.Contractsheets.definedrecorded at cost, less accumulated amortization and impairment losses, if any. Intangible assets acquired in business combinations are measured at fair value at the standard to include amounts that represent the right to receive payment for goods and servicesacquisition date. Intangible assets that have been transferredindefinite useful lives are not subject to the customeramortization and are tested annually for impairment, or more frequently if events or changes in circumstances indicate that they might be impaired. The estimated useful lives, residual values, and amortization methods are reviewed at each year-end, and any changes in estimates are accounted for prospectively.rights conditional upon something other than the passage of time. Contract liabilities are defined in the standard to include amounts that reflect obligations to provide goods and servicesobtaining or developing software for which payment has been received. There are no contract assets on unsatisfied performance obligations as of December 31, 2021, and 2020. For some of its locations,internal use. Further, the Company offers a loyalty reward program tocapitalizes qualifying costs incurred for upgrades and enhancements that result in additional functionality or extend the assets useful life. Amortization of such costs commences when the project is substantially completed and ready for its dispensary customers. A portion of the revenue generated in a sale must be allocated to the loyalty points earned. The amount allocated to the points earned is deferred until the loyalty pointsintended use. Capitalized software development costs are redeemed or expire. As of December 31, 2021, and 2020, the loyalty liability totaled $6.7 million and $5.3 million, respectively, that is included in deferred revenueclassified as intangible assets, net on the consolidated balance sheets.Income TaxesThe Company uses the asset and liability method to account for income taxes. Deferred income tax assets and liabilities are determined based on enacted tax rates and laws for the years in which the differences are expected to reverse. Deferred taxIntangible assets are reduced by a valuation allowance when, inamortized using the opinionstraight-line method over estimated useful lives as follows:Licenses 15 years Internal use software 3 to 5 years Tradenames 2 to 10 years Customer relationship 1 to 5 years Non-compete 2 years Trademarks 1 to 5 years deferred tax assets willfollowing as of December 31: 2023 2022 (in thousands) Trade accounts payable $ 28,245 $ 15,857 23,829 34,662 Accrued compensation and benefits 18,113 19,451 Non income taxes payable 7,061 5,747 Other 5,914 6,306 Total accounts payable and accrued liabilities $ 83,162 $ 82,023 be realized.As the Company operates in the cannabis industry, it is subject to the limits of IRC Section 280E under which the Company is only allowed to deduct expenses directly related to costs of goods sold.F-15TRULIEVE CANNABIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTSThe Company recognizes uncertain income tax positions at the largest amount that is more-likely-than-not to be sustained upon examination by the relevant taxing authority. An uncertain income tax position will be recognized if it has less than a 50% likelihood of being sustained. Recognition or measurement is reflected in the period in which the likelihood changes. Anylimited to: interest, utilities, and penalties related to unrecognized tax liabilities are presented within income tax expense in the consolidated statements of operations and comprehensive income.insurance.Non-controlling InterestNon-controlling interests (“NCI”) represent equity interests owned by outside parties. NCI may be initially measured at fair value or at the NCI’s proportionate share of the recognized amounts of the acquiree's identifiable net assets. The choice of measurement is made on a transaction-by-transaction basis. The Company has elected to measure each NCI at its proportionate share of the recognized amounts of the acquiree’s identifiable net assets. The share of net assets attributable to NCI are presented as a component of equity. NCI's share of net income or loss is recognized directly in equity. Total income or loss of subsidiaries is attributed to the shareholders of the Company and to the NCI, even if this results in the NCI having a deficit balance. See Note 21. Variable Interest Entities.Classification of financial instrumentsappliesutilizes interest rate swaps for the followingsole purpose of mitigating interest rate fluctuation risk associated with floating rate debt instruments. The Company does not use any other derivative financial instruments for trading or speculative purposes. In accordance with ASC 815, Derivatives and Hedging, derivative financial instruments are recognized as assets or liabilities on the consolidated balance sheets at fair value. The Company has not designated its interest rate swap ("Swap") contracts as hedges for accounting treatment. Pursuant to U.S. GAAP, income or loss from fair value hierarchy, which prioritizeschanges for derivatives that are not designated as hedges are reflected as income or loss within interest expense on the inputs used to measure fair value into three levels,consolidated statements of operations and basesa corresponding asset or liability is recognized on the categorization within the hierarchy upon the lowest level of input that is available and significant toconsolidated balance sheets based on the fair value measurement:position as of each reporting date.Level 1 –Observable inputs based on unadjusted quoted prices in active markets for identical assets or liabilities;Level 2 –Inputs other than quoted prices in active markets, that are observable for the asset or liability, either directly or indirectly; andLevel 3 –Unobservable inputs for which there is little or no market data requiring the Company to develop its own assumptions.isare recognized as a component of other (expense) income, innet on the consolidated statements of operations and comprehensive income as change in fair value of derivative liabilities - warrants. Transaction costs allocated to warrants that are presented as a liability were immediately expensed inwithin the consolidated statements of operations and comprehensive income.operations. Warrants classified as equity instruments are initially recognized at fair value and are not subsequently remeasured.F-16TRULIEVE CANNABIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTSInternal Use Softwarecapitalizes certain costsgenerates revenue from the sale of cannabis and cannabis related products. Revenue is recognized in connectionaccordance with obtainingASU 2014-09, Revenue from Contracts with Customers (Topic 606). Revenue is recognized when control of the promised goods or developing software for internal use. Further,services is transferred to our customers, in an amount that reflects the transaction price consideration that the Company capitalizes qualifying costs incurredexpects to receive in exchange for upgradesthose goods or services. Our revenue excludes sales, use, and enhancements that result in additional functionality or extendexcise-based taxes collected and is reported net of sale discounts. Revenue associated with any unsatisfied performance obligation is deferred until the assets useful life. Amortizationobligation is satisfied (i.e., when control of such costs commencesa product is transferred to the customer).projectperformance obligation has been fulfilled under the terms of the related sales contract.substantially completedrecognized when the Company transfers control of the goods to the customer at the point of sale and readythe customer has accepted and paid for the goods. Revenue from the wholesale of cannabis to customers is recognized upon delivery to the customer. Payment is typically due upon transferring the goods to the customer or within a specified time period permitted under the Company’s credit policy. See Note 22. Revenue Disaggregation.intended use. Capitalized software development costslocations, the Company offers a loyalty reward program to its dispensary customers that allows customers to earn reward credits to be used on future purchases. Loyalty reward credits issued as part of a sales transaction results in revenue being deferred until the loyalty reward is redeemed by the customer. The loyalty rewards are classifiedrecorded as intangible assets, netreductions to revenue on the consolidated statements of operations and included as deferred revenue on the consolidated balance sheets and are amortized using the straight-line method over the estimated useful lifesheets. A portion of the applicable software.revenue generated in a sale must be allocated to the loyalty points earned. The amount allocated to the points earned is deferred until the loyalty points are redeemed or expire. The loyalty reward points expire at the end of a six month period.inon the accompanying consolidated statements of operations and comprehensive income and totaled $7.5$12.1 million, $2.1$8.2 million, and $1.9$7.5 million for the years ended December 31, 2023, 2022, and 2021, 2020, and 2019, respectively.Held for saleWe classify long-lived assets or disposal groups and related liabilities as held-for-sale when management havingShare Based Compensationappropriate authority, generally our Boardestimated fair value of Directors or certain of our Executive Officers, commits to a plan of sale, the disposal group is ready for immediate sale, an active program to locate a buyer has been initiatedawards using the Black-Scholes option pricing model and the salerelated expense is probablerecognized using the graded-vesting method over the award term. The Company estimates expected volatility using the historical volatility of the Company. In cases where there is insufficient trading history, the expected volatility is estimated using the historical volatility of other companies that the Company considers comparable that have trading and volatility history prior to the Company becoming public. The expected life in years represents the period of time that options granted are expected to be completed within one year. Once classifiedoutstanding and is computed using the simplified method as held-for-sale disposal groups are valued at the lower of their carrying amount or fair value less estimated selling costs. DepreciationCompany has insufficient historical information regarding its stock options to provide a basis for an estimate. The risk-free rate was based on these properties is discontinuedthe United States bond yield rate at the time of grant of the award. The expected annual rate of dividends is based on the fact that the Company has never paid cash dividends and does not expect to pay any cash dividends in the foreseeable future. The Company has elected to account for forfeitures as they occur.classified as held for sale, but operating revenues, operating expenses and interest expense continuesatisfied. RSUs generally vest ratably over a two-to-three-year period subject to be recognized untilcontinued employment through each anniversary. The fair value of the Company’s RSUs are determined based on the stock price on the date of sale. As of December 31, 2021, the Company had $8.7 million in net assets held for sale which is recorded in prepaid expenses and other current assets in the consolidated balance sheets. The net assets held primarily consist of property and equipment, leases and related liabilities, and a note payable. There were no assets or liabilities held for sale as of December 31, 2020.Coronavirus PandemicIn March 2020, the World Health Organization categorized coronavirus disease 2019 (together with its variants, “COVID-19”) as a global pandemic. COVID-19 continues to spread throughout the U.S. and other countries across the world,grant and the durationrelated expense is recognized using the graded-vesting method over the award term.severity of its effects are currently unknown. goodwillcontinues to implement and evaluate actions to strengthen its financial position and supportaccounts for business combinations using the continuity of its business and operations.acquisition method in accordance with Accounting Standards Codification ASC 805, The Company’s consolidated financial statements presented herein reflect estimates and assumptions made by management that affect the reported amountsBusiness Combinations, which requires recognition of assets acquired and liabilities and disclosure ofassumed, including contingent assets and liabilities, at their respective fair values on the date of acquisition.financial statements of operations.reportedthe services are received.revenuethe identifiable assets acquired and expenses during the periods presented. Whileliabilities assumed. Following initial recognition, goodwill is measured at cost less any accumulated impairment losses.Company’sprimary intangible asset acquired in business combinations as they provide the Company the ability to operate in each market. However, some cannabis licenses are subject to renewal and therefore there is some risk of non-renewal for several reasons, including operational, regulatory, legal, or economic. To appropriately consider the risk of non-renewal, the Company applies probability weighting to the expected future net cash flows in calculating the fair value of these intangible assets. The key assumptions used in these cash flow projections include discount rates and terminal growth rates. Of the key assumptions used, the impact of the estimated fair value of the intangible assets has the greatest sensitivity to the estimated discount rate used in the valuation. The terminal growth rate represents the rate at which these businesses will continue to grow into perpetuity. Other significant assumptions include revenue, gross profit, operating expenses, and operating income were not impacted during 2021, it remains uncertain of howanticipated capital expenditures which are based upon the Corporation’s historical operations along with management projections. The evaluations are linked closely to the assumptions made by management regarding the future spreadperformance of COVID-19these assets and applicable vaccine mandatesany changes in the discount rate applied.public healthat the NCI’s proportionate share of the recognized amounts of the acquiree's identifiable net assets. The choice of measurement is made on a transaction-by-transaction basis. The Company measures each NCI at its proportionate share of the recognized amounts of the acquiree’s identifiable net assets. The share of net assets attributable to NCI is presented as a component of equity. NCI's share of net income or loss is recognized directly in equity. Total income or loss of subsidiaries is attributed to the shareholders of the Company and to the NCI, even if this results in the NCI having a deficit balance.impactnot be recoverable. Factors which could trigger an impairment review include significant underperformance relative to historical or projected future operating results, significant changes in the Company’smanner of use of the assets or the strategy of the business, a significant decrease in the market value of the assets or significant negative industry or economic trends.reasons includingdiscontinued operations and recorded in net loss from discontinued operations, net of tax benefit, and $2.7 million was for continuing operations and recorded in impairment and disposal of long-lived assets, net. The cost approach is based on market comparable data for replacement, adjusted for local variations, inflation, and other factors.quarantinefor goodwill impairment. A number of factors, including historical results, business plans, forecasts, market data, and a reasonable control premium are used to determine the fair value of the reporting unit. Changes in the conditions for these judgments and estimates can significantly affect the assessed value of goodwill.employeesinterim test for impairment as of June 30, 2023, utilizing a market approach, indicated that the reporting unit's fair value fell below the carrying value. Based on the results of the goodwill impairment procedures, the Company recorded a $307.6 million goodwill impairment for the single reporting unit in the second quarter of 2023.
For the Company's 2023 annual impairment test, the Company performed a Step Zero assessment reviewing the factors listed above, including but not limited to historical results, business plans, forecasts, market data, and a reasonable control premium.thosechanges in circumstances that would indicate the carrying amount of goodwill may be impaired. The Company did not identify any impairment of its supply chain partners.goodwill during the years ended December 31, 2022 or 2021.accounting estimatesAccounting Estimates and judgmentslong livedlong-lived assets; leases; fair value of financial instruments, income taxes; inventory;instruments; share-based payment arrangements,arrangements; and commitmentcommitments and contingencies. Actual results may differ from these estimates. Estimates and underlying assumptions are reviewed on an ongoing basis.F-17TRULIEVE CANNABIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS the AICPA and the SEC did not or are not believed by management to have a material effect on the Company’s present or future financial statements.statements or disclosures.June 2016,November 2023, the FASBFinancial Accounting Standards Board (“FASB”) issued Accounting Standards Update ("ASU") 2023-07, Improvements to Reportable Segment Disclosures (Topic 280). This ASU 2016-13, Financial Instruments - Credit Losses (Topic 326): Measurementupdates reportable segment disclosure requirements by requiring disclosures of Credit Losses on Financial Instruments.significant reportable segment expenses that are regularly provided to the Chief Operating Decision Maker (“CODM”) and included within each reported measure of a segment's profit or loss. This ASU 2016-13also requires disclosure of the measurementtitle and position of current expected credit losses for financial assets held at the reporting date based on historical experience, current conditions,individual identified as the CODM and reasonablean explanation of how the CODM uses the reported measures of a segment’s profit or loss in assessing segment performance and supportable forecasts. Adoption ofdeciding how to allocate resources. The ASU 2016-13 will require financial institutions and other organizations to use forward-looking information to better formulate their credit loss estimates. In addition, the ASU amends the accounting for credit losses on available for sale debt securities and purchased financial assets with credit deterioration. This update is effective for annual periods beginning after December 15, 2023, and interim periods within fiscal years beginning after December 15, 2019, including interim periods within those fiscal years. The Company 2024. Early adoption is also permitted. This ASU will result in additional required disclosures when adopted, where applicable.2016-13 on 2023-09January 1, 2020, and adoption did not have a material impact on the Company’s consolidated financial statements.August 2018,December 2023, the FASB issued ASU 2018-13, Disclosure Framework - Changes2023-09, Improvements to the Disclosure Requirements for Fair Value MeasurementIncome Tax Disclosures (Topic 820)740). The ASU 2018-13 adds, modifies, and removes certain fair value measurement disclosure requirements.requires disaggregated information about a reporting entity’s effective tax rate reconciliation as well as additional information on income taxes paid. The ASU 2018-13 is effective on a prospective basis for annual and interim periods beginning after December 15, 2019.2024. Early adoption is permitted. The Companyalso permitted for annual financial statements that have not yet been issued or made available for issuance. Once adopted, this ASU 2018-13 on January 1, 2020, and the adoption did not have a material impact on the Company’s consolidated financial statements.In December 2019, the FASB issued ASU 2019-12, Income Taxes (Topic 740) - Simplifying the Accounting for Income Taxes, which is intended to simplify various aspects related to accounting for income taxes. ASU 2019-12 removes certain exceptions to the general principleswill result in Topic 740 and also clarifies and amends existing guidance to improve consistent application. ASU 2019-12 is effective for the Company beginning January 1, 2021. The Company additional disclosures.adopted ASU 2016-13 on January 1, 2021, and adoption did not have a material impact on the Company’s consolidated financial statements.(a) Purplemed(b)Greenhouse Wellness WV Dispensaries, LLCInBusiness Combinations (Topic 805): Clarifying the Definition of a Business, determining Greenhouse WV did not meet the definition of a business as Greenhouse WV did not have inputs, processes, and outputs in place that constituted a business under Topic 805. As a result, the transaction has been accounted for as an asset acquisition whereby all of the assets acquired and liabilities assumed are assigned a carrying amount based on relative fair values. Total consideration was $0.3 million consisting of cash.Consideration: (in thousands) Cash $ 281 Fair value of consideration exchanged $ 281 Recognized amounts of identifiable assets acquired and liabilities assumed: Right of use asset - operating $ 170 Intangible asset 270 Favorable lease interest 11 Operating lease liabilities (170) Total net assets acquired $ 281 Consideration (in thousands) Cash $ 27,500 Fair value of consideration exchanged $ 27,500 Recognized amounts of identifiable assets acquired and liabilities assumed: Inventories $ 2,266 Property and equipment 692 Right of use asset - operating 4,737 Goodwill 24,542 Operating lease liability (4,737) Total net assets acquired $ 27,500 100%100% of certain assets of Purplemed Healing Center ("Purplemed") including the Medical Marijuana Dispensary License issued by the Arizona Department of Health Services ("ADHS") and the Marijuana Establishment License issued by the ADHS which collectively serve as the Purplemed license providing the ability to operate a marijuana retail sales dispensary as well as the assumption of the associated lease. The Company also acquired the right to operate an additional offsite cultivation business under the Arizona Adult Use Marijuana Act, and the option to purchase full ownership and management of Greenmed, Inc., the Greenmed license, and the Greenmed dispensary. As part of the transaction, the Company assumed the Purplemed loyalty program.$15.0$15.0 million consisting of cash. The acquisition provided for indemnity for pre-closing liabilities. Accordingly, the Company recognized an indemnification asset of $0.5$0.5 million offset the by associated liabilities based on the information that was available at the date of the acquisition, which is included in the net assets acquired.$15.0$15.0 million.F-18TRULIEVE CANNABIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTSConsideration: (in thousands) Cash $ 15,000 Transaction costs 12 Fair value of consideration exchanged $ 15,012 Recognized amounts of identifiable assets acquired and liabilities assumed: Prepaid expenses and other current assets $ 531 Right of use asset - operating 271 Intangible asset 15,076 Other current liabilities (531) Deferred revenue (109) Operating lease liabilities (226) Total net assets acquired $ 15,012 15-year15-year useful life.(b)100%100% of the common shares of Harvest Health & Recreation, Inc. (“Harvest”) and its portion of variable interest entities in exchange for Subordinate Voting Shares of the Company (the “Transaction”“Harvest Transaction”).iswas one of the largest multi-state vertically integrated operators in the cannabis industry in the United States operating from “seed to sale". Harvest operatesoperated facilities or provides services to cannabis dispensaries in Arizona, California, Colorado, Florida, Maryland, Nevada, and Pennsylvania, with two provisional licenses in Massachusetts. In addition, Harvest ownsowned CO2 extraction, distillation, purification, and manufacturing technology used to produce a line of cannabis topicals, vapes, and gems featuring cannabinoids and a hemp-derived product line sold in Colorado.$1.4$1.4 billion consisting of Trulieve Subordinate Voting Shares (“Trulieve Shares”) with a fair value of $1.37$1.37 billion, stock options, equity classified warrants, restricted stock units, and other outstanding equity instruments with a fair value of $18.4$18.4 million, and warrant liabilities convertible into equity with a fair value of $3.1$3.1 million at the time of the Harvest Transaction. The Company incurred $13.0$13.0 million in transaction costs related to the acquisition of Harvest. These costs were expensed as incurred and are included in general and administrative expenses inwithin the consolidated statements of operations and comprehensive income for the year ended December 31, 2021. No additional transaction costs have been incurred.$662.1$663.7 million arising from the acquisition primarily consistconsists of the synergies and economies of scale expected from combining the operations of Trulieve and Harvest including growing the Company's customer base, acquiring assembled workforces, and expanding its presence in new and existing markets. These benefits were not recognized separately from goodwill because they do not meet the recognition criteria for identifiable intangible assets. Goodwill is subject tolimitsthird quarter of IRC Section 280E under which2022, the Company is only allowed to deduct expenses directly related tofinalized the costaccounting for non-controlling interests on the acquired entities, which resulted in a measurement period adjustment increasing non-controlling interests and goodwill by $1.6 million.F-19TRULIEVE CANNABIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTSConsideration: (in thousands) Trulieve Subordinated Voting Shares $ 1,369,024 Fair value of other equity instruments 18,394 Fair value of warrants classified as liabilities 3,103 Fair value of consideration exchanged $ 1,390,521 Recognized amounts of identifiable assets acquired and liabilities assumed: Cash and cash equivalents $ 85,318 Restricted cash 3,072 Accounts receivable 3,645 Inventories 92,537 Prepaid expenses and other current assets 100,129 Notes receivable 9,805 Property and equipment 191,801 Right of use assets - operating 73,476 Intangible assets: Dispensary license 946,000 Trademarks 27,430 Customer relationships 3,500 Other assets 5,289 Accounts payable and accrued liabilities (58,887) Income tax payable (24,863) Deferred revenue (4,523) Operating lease liabilities (76,558) Contingencies (26,599) Notes payable (285,238) Construction finance liabilities (79,683) Other long-term liabilities (1,085) Deferred tax liabilities (253,986) $ 730,580 Non-controlling interest $ (3,734) Goodwill 663,675 Total net assets acquired $ 1,390,521
The above table includes the discontinued operations of Nevada. to five year-to-five-year useful life, and customer relationships amortized over a one yearone-year period.$6.2$6.2 million. There was consideration in the form of 1,011,095 warrants (1,009,416(1,009,416 equity classified SVSSubordinate Voting Shares ("SVS") warrants and 1,679 liability classified MVSMultiple Voting Share ("MVS") warrants, as converted) that had been issued before the acquisition date to employees and non-employees of Harvest. The pre-combination fair value of these awards is $7.7$7.7 million with $4.6$4.6 million representing the equity classified warrants and $3.1$3.1 million representing the liability classified warrants. There was consideration in the form of restricted stock units that had been issued before the acquisition date to non-employees of Harvest which vested for services performed pre-combination representing 18,297 SVS. The pre-combination fair value of these awards is $0.5$0.5 million. There was additional consideration in the form of other outstanding equity instruments issued before the acquisition date to non-employees which had a pre-combination fair value of $7.1$7.1 million.F-20TRULIEVE CANNABIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS$55.0$55.0 million where Trulieve was legally prohibited from holding this license and the sale occurred simultaneously with the Harvest Transaction. Therefore, a $55.0$55.0 million receivable for the sale proceeds was deemed acquired and recorded.Company has not yet finalized their accounting for non-controlling interestsfunds were received subsequent to the closing of the Harvest Transaction on the acquired entities but has recorded preliminary entries in this area. Any subsequent adjustments would be expected to impact non-controlling interest and goodwill. This accounting will be finalized during the measurement period.&and Recreation, Inc. and Keystone ShopsInc, as if the acquisitionsacquisition had occurred on January 1, 2019 and PurePenn, LLC, Pioneer Leasing & Consulting, LLC and Keystone Relief Centers, LLC as if the acquisitions had occurred on January 1, 2020. PurePenn, LLC, Pioneer Leasing & Consulting, LLC and Keystone Relief Centers, LLC are not included for the year ended December 31, 2019 as the appropriate historical information was not available, making it impractical.2021. This pro forma information is presented for informational purposes only and is not necessarily indicative of the results of operations that would have been achieved had the transaction been consummated as of that time nor does it purport to be indicative of future financial operating results.revenuesloss attributable to common shareholders for the year ended December 31, 2021 2020, and 2019 are $1,232 million, $833.6were $1,232.2 million and $380.3$8.0 million, respectively. Proforma net loss and comprehensive loss attributable to common shareholders for the years ended December 31, 2021, 2020, and 2019 are $8.0 million, $21.1 million, and $135.9 million, respectively. two companies, and adjustments for alignment of significant differences in accounting principles and elections.consolidated statementsabove unaudited supplemental pro formas include the results of operations and comprehensive income includes revenue of $115.2 million and a net loss attributable to common shareholders of $50.7 million related to acquired operations for the year ended December 31, 2021.(c) Keystone Shops100%100% of the membership interests of Anna Holdings, LLC, the sole member of Chamounix Ventures, LLC which holds a permit to operate dispensaries under Keystone Shops (“Keystone Shops”) with locations in Philadelphia, Devon, and King of Prussia, Pennsylvania. Total consideration was $55.6$55.6 million consisting of $20.3$20.3 million in cash, inclusive of net working capital adjustments, and 1,009,336 in Trulieve Subordinate Voting Shares ("Trulieve Shares") with a fair value of $35.4$35.4 million. The agreement provides for an additional $5.0$5.0 million in consideration which is contingent on the enactment, adoption or approval of laws allowing for adult-use cannabis in Pennsylvania. No liability was recorded for this contingent consideration, as itthe estimated value of the liability was not estimated to be probablesignificant at the time of acquisition noror as of December 31, 2021.2023 based on the likelihood of approval of laws allowing for adult-use cannabis in Pennsylvania. The acquisition was accounted for as a business combination in accordance with the Accounting Standards Codification (ASC) 805, Business Combinations.Combinations. Goodwill arose because the consideration paid for the business acquisition reflected the benefit of expected revenue growth and future market development. In the fourth quarter0.4 million as a result of the change in accounting principle noted in Note 2. Basis of Presentation.F-21TRULIEVE CANNABIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTSConsideration: (in thousands) Cash $ 20,251 Shares issued upon acquisition 35,385 Fair value of consideration exchanged $ 55,636 Recognized amounts of identifiable assets acquired and liabilities assumed: Cash $ 500 Prepaid expenses and other current assets 240 Inventories 1,766 Property and equipment 1,144 Right of use asset - finance 1,340 Intangible assets: Dispensary license 27,000 Tradename 100 Favorable leasehold interests 86 Goodwill 39,703 Other assets 40 Accounts payable and accrued liabilities (878) Income tax payable (2,892) Operating lease liabilities (1,340) Other long-term liabilities (2,179) Deferred tax liabilities (8,994) Total net assets acquired $ 55,636
The acquired intangible assets include a dispensary license which is treated as a definite-lived intangible asset amortized over a 15-year useful life, as well as tradename and net favorable leasehold interests which were fully amortized in the period of acquisition due to useful life and materiality considerations.(d)During the third quarter of 2021, the Company recorded an adjustment of $2.6 million increasing the cost of the acquisition due to an adjustment to the fair value of the equityTotal consideration and updated the purchase price allocation accordingly, which updated equity consideration from contract value to fair value as of the closing date, and also updated the associated deferred tax liability. This adjustment resulted in an updated total consideration of $16.2was $16.2 million consisting of $7.0$7.0 million in cash and 237,881 in Trulieve Shares with an updateda fair value of $9.1$9.1 million and less than $0.1$0.1 million in transaction costs. In the fourth quarter4.4 million as a result of the change in accounting principle noted in Note 2. Basis of Presentation.F-22TRULIEVE CANNABIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTSConsideration: (in thousands) Cash $ 7,000 Shares issued upon acquisition 9,139 Transaction costs 23 Fair value of consideration exchanged $ 16,162 Recognized amounts of identifiable assets acquired and liabilities assumed: Prepaid expenses and other current assets $ 12 Property and equipment 1,006 Right of use asset - finance 799 Intangible asset 15,274 Accounts payable and accrued liabilities (335) Finance lease liabilities (594) Total net assets acquired $ 16,162 (e)$10.0$10.0 million. The Company analyzed the acquisition under ASU 2017-01, Business Combinations (Topic 805): Clarifying the Definition of a Business, determining PCMV did not meet the definition of a business as PCMV did not have inputs, processes, and outputs in place that constituted a business under Topic 805. As a result, the acquisition of PCMV has been accounted for as an asset acquisition, whereby all of the assets acquired and liabilities assumed are assigned a carrying amount based on relative fair values. In the fourth quarter of 2021, the Company recorded an adjustment reducing the deferred tax liability and associated intangible asset by $2.7 million as a result of the change in accounting principle noted in Note 2. Basis of Presentation.Consideration: (in thousands) Shares issued upon acquisition $ 10,012 Transaction costs 18 Fair value of consideration exchanged $ 10,030 Recognized amounts of identifiable assets acquired and liabilities assumed: Right of use asset - finance $ 1,756 Intangible asset 10,594 Finance lease liabilities (2,320) Total net assets acquired $ 10,030 100%100% of the membership interests of Solevo Wellness West Virginia, LLC (“Solevo WV”) which holds three West Virginia dispensary licenses. The Company analyzed the acquisition underF-23TRULIEVE CANNABIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTSDuring the third quarter of 2021, the Company recorded an adjustment of $0.1 million decreasing the cost of the acquisition due to an adjustment to the fair value of the equityTotal consideration which updated equity consideration originally recorded at contract value to fair value as of the closing date, and also updated the associated deferred tax liability. This adjustment resulted in an updated total consideration of $0.8was $0.8 million consisting of $0.2$0.2 million in cash, 11,658 in Trulieve Shares with an updateda fair value of $0.4$0.4 million, $0.1$0.1 million in debt forgiveness and less than $0.1$0.1 million in transaction costs. The consideration of $0.8$0.8 million was allocated to acquired assets of $0.8$0.8 million, which are treated as definite-lived intangible assets amortized over a 15-year useful life. In the fourth quarter of 2021, the Company recorded an adjustment reducing the deferred tax liability and associated intangible asset by $0.2 million as a result of the change in accounting principle noted in Note 2. Basis of Presentation.(g)100%100% of the membership interests of Mountaineer Holding LLC (“Mountaineer”) which holds a cultivation permit and two dispensary permits in West Virginia. The Company analyzed the acquisition under ASU 2017-01, Business Combinations (Topic 805): Clarifying the Definition of a Business, determining Mountaineer did not meet the definition of a business as substantially all of the fair value of the gross assets acquired are concentrated in a single identifiable asset. Therefore, the transaction has been accounted for as an asset acquisition. During the third quarter of 2021, the Company recorded an adjustment of $0.5 million decreasing the cost of the acquisition due to an adjustment to the fair value of the equityTotal consideration which updated equity consideration originally recorded at contract value to fair value as of the closing date, and also updated the associated deferred tax liability. This adjustment resulted in an updated total consideration of $5.5was $5.5 million, consisting of $3.0$3.0 million in cash and 60,342 in Trulieve Shares with a fair value of $2.5$2.5 million. The consideration of $5.5$5.5 million has been allocated to the $5.5$5.5 million of acquired assets which are treated as definite-lived intangible assets and amortized over a 15-year useful life. In the fourth quarter of 2021, the Company recorded an adjustment reducing the deferred tax liability and associated intangible asset by $1.5 million as a result of the change in accounting principle noted in Note 2. Basis of Presentation.(h) PurePenn, LLC and Pioneer Leasing & Consulting, LLCOn November 12, 2020, the Company acquired 100% of the membership interests of both PurePenn, LLC, which holds a permit to cultivate and process medical marijuana in Pennsylvania, and Pioneer Leasing & Consulting, LLC (collectively “PurePenn”). The purpose of this acquisition was to operate the cultivation and manufacturing facility located in McKeesport, Pennsylvania. Trulieve acquired PurePenn for an upfront payment valued at $48.7 million, comprised of 1,298,964 in Trulieve Shares with a fair value of $29.7 million and $19.0 million in cash, plus a potential earn-out payment including bonuses of up to 2,904,648 Trulieve Shares based on the achievement of certain agreed upon EBITDA milestones. The earn-out period is through the end of 2021. As of December 31, 2021, the milestones for the earn-out had been achieved and the full share amount was earned.The acquisition was accounted for as a business combination in accordance with the Accounting Standards Codification (ASC) 805, Business Combinations. As of December 31, 2021, total transaction costs related to the acquisition were approximately $1.8 million. Goodwill arose because the consideration paid for the business acquisition reflected the benefit of expected revenue growth and future market development. These benefits were not recognized separately from goodwill because they do not meet the recognition criteria for identifiable intangible assets. Goodwill is subject to the limits of IRC Section 280E under which the Company is only allowed to deduct expenses directly related to the costs of goods sold, therefore goodwill is not deductible.For the three months ended June 30, 2021, the Company recorded an adjustment to the initial valuation of shares issued upon acquisition, which increased the fair value of the consideration exchanged and the estimated purchase price by $2.7 million and increased goodwill by $2.7 million and we recorded an adjustment to the initial valuation of contingent consideration payable in shares, which reduced contingent consideration payable in shares and the estimated purchase price by $3.0 million and decreased goodwill by $3.0 million. For the three months ended September 30, 2021, the Company recorded an adjustment to the deferred tax liability decreasing goodwill and the associated deferred tax liability by $0.6 million. In the fourth quarter of 2021, the Company recorded an adjustmentF-24TRULIEVE CANNABIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTSreducing the deferred tax liability and goodwill by $0.9 million as a result of the change in accounting principle noted in Note 2. Basis of Presentation.The following table summarizes the allocation of consideration exchanged for the estimated fair value of tangible and identifiable intangible assets acquired and liabilities assumed:The acquired intangible assets represent include a dispensary license that is treated as a definite-lived intangible asset amortized over a 15-year useful life and two tradenames amortized over a two and three year useful life.(i) Keystone Relief Centers, LLCOn November 12, 2020, the Company acquired 100% of the membership interests of Keystone Relief Centers, LLC (referred to herein as “Solevo Wellness”). The purpose of this acquisition was to acquire the licenses to operate three medical marijuana dispensaries in the Pittsburgh, Pennsylvania area. Trulieve acquired Solevo for an upfront purchase price of $21.0 million, comprised of $10.0 million in cash and 481,097 in Trulieve Shares with a fair value of $11.0 million, plus a potential earn-out payment of up to 721,647 Trulieve Shares based on the achievement of certain agreed EBITDA milestones. The earn-out period is through the end of 2021. As of December 31, 2021, the milestones for the earn-out had been achieved and the full share amount was earned.The acquisition was accounted for as a business combination in accordance with the Accounting Standards Codification (ASC) 805, Business Combinations, and related operating results are included in the accompanying consolidated statements of operations and comprehensive income, changes in shareholders’ equity, and statement of cash flows for periods of subsequent to the acquisition date. Total transaction costs related to the acquisition were approximately $0.9 million. Goodwill arose because the consideration paid for the business acquisition reflected the benefit of expected revenue growth and future market development. These benefits were not recognized separately from goodwill because they do not meet the recognition criteria for identifiable intangible assets. Goodwill is subject to the limits of IRC Section 280E under which the Company is only allowed to deduct expenses directly related to the costs of goods sold, therefore goodwill is not deductible. In the fourth quarter of 2021, the Company recorded an adjustment reducing the deferred tax liability and goodwill by $7.2 million as a result of the change in accounting principle noted in Note 2. Basis of Presentation.F-25TRULIEVE CANNABIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTSThe following table summarizes the allocation of consideration exchanged for the estimated fair value of tangible and identifiable intangible assets acquired and liabilities assumed:The acquired intangible assets include a dispensary license which is treated as a definite-lived intangible asset amortized over a 15-year useful life, as well as tradename which was fully amortized in the period of acquisition due to useful life and materiality considerations. 2023 2022 (in thousands) Trade receivables $ 10,420 $ 8,482 Less: allowance for credit losses (3,717) (1,975) Accounts receivable, net $ 6,703 $ 6,507 The allowance for credit losses was established during the year ended December 31, 2020, and had a nominal balance asTable of December 31, 2020. During the year ended December 31, 2021, the Company adjusted the reserve by $Contents0.5 million.F-26TRULIEVE CANNABIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTSNotes
The Company's notes receivable, consisted of the followingnet totaled $13.7 million and $12.7 million as of December 31:In October 2021, the Company acquired a note receivable with the Harvest acquisition. The note receivable is originally dated November 2020 maturing in November 2025. The note had an original principal balance of $12.0 million31, 2023 and accrues interest at a rate of 7.5% per annum with monthly interest and principal payments of $0.1 million.In October 2021, the Company acquired2022, respectively, which are reported as notes receivable with- current portion and notes receivable, net on the Harvest acquisition.Company's consolidated balance sheets. The notes receivable are originally dated February 2021 maturing in Februarysecured by certain assets. The weighted average effective interest rates were 8.05% and 8.31% as of December 31, 2023 and 2022,. The notes had an original principal balance of $0.9 million and accrue interest at a rate of 10% per annum with interest only payments due monthly. This note was repaid in full subsequent to year end and the Company received proceeds of $0.9 million in February 2022.As part of the acquisition of Harvest, we acquired $9.8 million in notes receivable on October 1, 2021. There were no notes receivable outstanding prior to October 1, 2021.See Note 3. Acquisitions for further details of the Harvest acquisition.In November 2021, the Company entered into a convertible note receivable agreement for a principal amount of $4.1 million that matures in November 2024. The note accrues interest monthly at 9.75%, and accrued interest is added to the principal balance at each quarter end. The note is convertible to equity of the holder at our option at any time prior to maturity. Further, the note was issued at a discount of 3% or $0.1 million, which is accreted to the note receivable balance over the term of the note.
During the yearyears ended December 31, 2023, 2022, and 2021, the Company recorded interest income of $0.2$1.2 million, $1.3 million, and $0.2 million, respectively, in otherinterest income on the consolidated statements of operations and comprehensive income.operations. The Company had accrued interest receivable of $0.1$0.1 million and $0.1 million as of December 31, 20212023 and 2022, respectively, in prepaids and other current assets on the consolidated balance sheets. The Company had no notes receivable outstanding as of December 31, 2020.2021:F-27TRULIEVE CANNABIS CORP.2023:NOTES TO CONSOLIDATED FINANCIAL STATEMENTSYear Ending December 31, 2024 $ 6,233 2025 6,943 2026 75 2027 75 2028 75 Thereafter 300 Total notes receivable 13,701 Less: discount on notes receivable (45) Less: current portion of notes receivable (6,233) Notes receivable, net $ 7,423 2023 2022 Raw material Cannabis plants $ 21,429 $ 21,523 Packaging and supplies 36,472 49,650 Total raw material 57,901 71,173 Work in process 104,428 158,448 Finished goods - unmedicated 6,516 7,323 Finished goods - medicated 44,275 39,561 Total inventories $ 213,120 $ 276,505 AsProperty and equipment, net consisted of the following as of December 31, 2021, and 2020,31:2023 2022 Land $ 26,699 $ 38,485 Buildings and improvements 528,173 497,493 Furniture and equipment 292,128 277,164 Vehicles 814 839 Construction in progress 28,023 55,145 Total property and equipment, gross 875,837 869,126 Less: accumulated depreciation (199,485) (125,866) Total property and equipment, net $ 676,352 $ 743,260 consisted of the following:For the years ended December 31, 2021, 2020, and 2019, the Company capitalized interest of $9.2 million, $4.8 million, and $0.5 million, respectively. For the years ended December 31, 2021, 2020, and 2019, the Company incurred depreciation expense of $34.8 million, $21.1 million, and $9.3 million, respectively.Duringfor the year ended December 31, 2021, the Company recorded a loss on the disposal of property and equipment of $31:Location on the consolidated statements of operations 2023 2022 2021 (in thousands) Capitalized interest Interest expense $ (148) $ 4,728 $ 9,231 Depreciation expense Cost of goods sold 55,114 44,383 19,924 Depreciation expense Depreciation and amortization 21,004 26,216 11,944 Loss on impairment Impairments and disposals of long-lived assets, net $ 2,712 $ 1,294 $ — Loss on disposal Impairments and disposals of long-lived assets, net 5,320 54,509 1,732 Gain on sale Impairments and disposals of long-lived assets, net (251) (654) — 1.8 million consisting of $1.6 million related to leasehold improvements and $0.2 million related to equipment, recorded in general and administrative expenses in the consolidated statements of operations and comprehensive income. The Company had insignificant disposals of property and equipment during the years ended December 31, 2020, and 2019.F-28TRULIEVE CANNABIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTSAs of December 31, 2021, and 2020,The Company's definite-lived intangible assets consisted of the following:
2021
2020 2023 2022 Gross Carrying Amount Accumulated Amortization Net Book Value Gross Carrying Amount Accumulated Amortization Net Book Value (in thousands) Licenses $ 1,046,544 $ 159,084 $ 887,460 $ 1,044,161 $ 89,367 $ 954,794 Trademarks 27,430 17,609 9,821 27,430 12,530 14,900 Internal use software 26,947 7,520 19,427 16,528 3,065 13,463 Tradenames 4,861 4,461 400 4,862 3,506 1,356 Customer relationships 3,535 3,452 83 3,536 3,252 284 Total $ 1,109,317 $ 192,126 $ 917,191 $ 1,096,517 $ 111,720 $ 984,797 2020, and 2019 was $respectively.29.8 million, $3.0 million, and $1.8 million, respectively.During the year ended December 31, 2021, we impaired $3.6$3.0 million, in relationwhich is recorded to the write off of certain licenses of approximately $3.2 million due to market changesimpairment and $0.4 million related to the rebranding of a tradename, recorded in loss on impairmentdisposal of long-lived assets, net on the consolidated statements of operations and comprehensive income. The Company had no impairments of intangible assets duringfor the yearsyear ended December 31, 2020, or 2019.F-29TRULIEVE CANNABIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS2021:
amortizationGoodwillGoodwill arose fromYear Ending December 31, Estimated
amortization (in thousands) 2024 $ 81,597 2025 78,197 2026 75,213 2027 72,712 2028 69,056 Thereafter 540,416 Total $ 917,191 acquisition of Harvest Health & Recreations Inc., Keystone Shops, PurePenn LLC, Pioneer Leasing & Consulting LLC, and Solevo Wellness, see Note 4. Acquisitions.weighted average amortization period remaining on our intangible assets was 12.4 years.F-30TRULIEVE CANNABIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTSNOTE 10. NOTES PAYABLEAs of December 31, 2021, and 2020,notes payable consisted of the following:(in thousands) As of December 31, 2021 $ 765,358 Acquisition of Watkins Cultivation Operations 24,542 Measurement period adjustment of Harvest Health and Recreation, Inc. 1,595 As of December 31, 2022 $ 791,495 (307,590) As of December 31, 2023 $ 483,905
Held for sale assets primarily consist of property and equipment and are recorded in other current assets on the consolidated balance sheets. The following table shows the activity of the Company's assets held for sale:(in thousands) Held for sale assets, net as of December 31, 2022 $ 14,521 Assets moved to held for sale 18,694 Non-cash settlement (2,481) Impairments (2,810) Assets sold (12,344) Held for sale assets, net as of December 31, 2023 $ 15,580 Held for sale liabilities as of December 31, 2022 $ — Liabilities moved to held for sale (1,997) Liabilities settled associated with held for sale assets 1,997 Held for sale liabilities as of December 31, 2023 $ — 2023 2022 Stated Interest Rate Effective Interest Rate Maturity Date Net Book Value of
Collateral (in thousands) Mortgage Notes Payable $ 70,046 $ 71,500 7.53% (7) 7.86% 1/1/2028 $ 166,932 25,000 — 8.31% (7) 8.48% 12/31/2028 59,677 18,470 18,900 7.30% (7) 7.38% 12/22/2032 9,017 5,645 6,095 8.14% (7) 8.29% 10/1/2027 11,860 Total mortgage notes payable 119,161 96,495 Promissory Notes Payable — 5,500 10.00% (7) 10.00% 12/22/2023 1,707 5,338 (6) (7) (6) (6) Note of consolidated variable-interest entity dated February 1, 2022 885 1,200 8.00% (7) 8.00% 12/31/2025 Total promissory notes payable 2,592 12,038 Total notes payable 121,753 108,533 Less: debt discount (2,139) (1,833) Less: current portion of notes payable (3,759) (12,453) Notes payable, net $ 115,855 $ 94,247
The Company's notes payable described above are subordinated to the private placement notes. See Note 12. Private Placement Notes for further details.Year Ending December 31, (in thousands) 2024 $ 3,751 2025 4,331 2026 4,655 2027 70,034 2028 23,199 Thereafter 15,783 Total $ 121,753 NOTE 11. NOTES PAYABLE RELATED PARTYIn February 2019, the Company entered into a 24-month unsecured loan with an 8% annual interest rate with Benjamin Atkins, a former director and shareholder for $0.3 million. The loan was issued in March 2019. The CompanyF-31TRULIEVE CANNABIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTSdetermined that the stated interest rate was below market rates and recorded an insignificant debt discount using an annual discount interest rate of 12%.In May 2018, the Company entered into two separate unsecured promissory notes (the “Traunch Four Note” and the “Rivers Note”) for a total of $12.0 million. The Traunch Four Note is held by Traunch Four, LLC, an entity whose direct and indirect owners include Kim Rivers, the Chief Executive Officer and Chair of the Board, as well as Thad Beshears, Richard May, George Hackney, all of whom are directors or former directors of Trulieve, and certain of Richard May’s family members. The Rivers Note is held by Kim Rivers. Each promissory note has a 24-month maturity and 12% annual interest rate. The two unsecured promissory notes were amended in December 2019 to extend the maturity one year to May 2021. The two unsecured promissory notes were further amended in May 2021 to extend the maturity date to November 2021.As of December 31, 2020, the Company had outstanding current notes payable due to related parties of $12.0 million. During the year end December 31, 2021, all outstanding related party notes were fully paid with zero balance outstanding as of December 31, 2021. During the years ended December 31, 2021, 2020, and 2019, the Company incurred interest expense on related party debt of $1.1 million, $1.6 million, and $1.7 million, respectively.2024On June 18,atwo private placement financing comprising notes (the “June Notes" and the “November Notes”), each comprised of 5-year senior secured promissory notes (the “June Notes”) with a face value of $70.0 million.$70.0 million and $60.0 million, respectively. The June Notes accrue interest at an annual rate of 9.75%, payable semi-annually, in equal installments, in arrears in June and December of each year, commencing in December 2019. The holderspurchasers of the June Notes also received warrants to purchase 1,470,000 Subordinate Voting Shares at an exercisea price of $13.47 (the “June Warrants”$13.47 ("June Warrants") and the purchasers of the November Notes received warrants to purchase 1,560,000 Subordinate Voting Shares at a price of $980 per Unit, with each unit consisting of one Note issued in Denominations of $1,000 and 26 warrants ("November Warrants"), which can be exercised for approximately three years after closing (collectively the closing.fair valueremaining outstanding Public Warrants expired in June 2022.63.9 million using an effective interest rate of 13.32%, which the Company estimates would have been the coupon rate required to issue the notes had the financing not included the June Warrants. The fair value of the June Warrants was determined to be $4.7 million using the Black-Scholes option pricing model and the following assumptions: Share Price: C$14.48; Exercise Price: C$17.25; Expected Life: three years; Annualized Volatility: 49.96%; Dividend yield: 0%; Discount Rate: 1.92%; C$ Exchange Rate: 1.34. Issuance costs totaling $3.1 million were allocated between the June Notes and the June Warrants based on their relative fair values with $2.9 million allocated to the June Notes and $0.2 million expensed as incurred.The June Notes will accrete from their carrying value on June 18, 2019, of $61.0 million to $70.0 million at maturity in five years using an effective interest rate of 13.32%. For the years ended31, 2021, 2020, and 2019 accretion expense of $1.7 million, $1.5 million and $0.7 million respectively, was included in interest expense in the consolidated statements of operations and comprehensive income.On November 7, 2019,1, 2023, the Company completed a prospectus offeringan early redemption of our private placement of 60,000 unitsnotes, both of the "June Notes" and the "November Notes", with a cash payment of $130.0 million, excluding accrued interest, which represented a redemption price of 100% of the principal amounts outstanding for both "Notes". The Company (the “November Units”), comprisedrecorded a loss on extinguishment of an aggregate principal$2.4 million representing the difference between the reacquisition price and the net carrying amount of $60.0 millionthe debt as of 9.75% senior secured notes of the Company maturing in 2024 (the “November Notes”) and an aggregate amount of 1,560,000 Subordinate Voting Share warrants of the Company (each individual warrant being a “November Warrant”) at a price of $980 per Unit for gross proceeds of $61.1 million. Each Unit was comprised of one Note issued in denominations of $1,000 and 26 Warrants.The fair value of the November Notes was determined to be $54.5 million using an effective interest rate of 13.43%, which the Company estimates would have been the coupon rate required to issue the notes had the financing not included the November Warrants. The fair value of the November Warrants was determined to be $4.4 million using the Black-Scholes option pricing model and the following assumptions: Share Price: C$14.29; Exercise Price: C$17.25; Expected Life: 2.6 years; Annualized Volatility: 48.57%; Dividend yield: 0%; Discount Rate: 1.92%; C$ Exchange Rate: 1.32. Issuance costs totaling $2.1 million were allocated between the November Notes and the November Warrants based on their relative fair values with $2.0 million allocated to the November Notes and $0.2 million expensed in the consolidated statements of operations and comprehensive income.F-32TRULIEVE CANNABIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTSThe November Notes will accrete from their carrying value on November 7, 2019, of $52.5 million to $60.0 million at maturity in 4.6 years using an effective interest rate of 13.43%. For the years ended December 31, 2021, 2020 and 2019, the Company incurred accretion expense of $1.5 million, $1.3 million, and $0.1 million which is included in interest expense in the consolidated statements of operations and comprehensive income.Because of the Canadian denominated exercise price, the June Warrants and November Warrants did not qualify to be classified within equity and were therefore classified as derivative liabilities at fair value with changes in fair value charged or credited to earnings in the consolidated statements of operations and comprehensive income prior to December 10, 2020.On December 10, 2020, the Company entered into a Supplemental Warrant Indenture with Odyssey Trust Company pursuant to which it amended the terms of the June Warrants and November Warrants (the “Public Warrants”) to convert the exercise price of the Public Warrants to $13.47 per share, the U.S. dollar equivalent of the Canadian dollar exercise price of the Public Warrants of C$17.25 at the date of closing. As of December 10, 2020, the June Warrants converted to equity as per ASC 815-40, at a fair value of $25.5 million and the November Warrants converted at a fair value of $27.1 million, which is included in additional paid-in-capital on the consolidated statements of changes in shareholders' equity.Notes"Notes - Tranche One") for aggregate gross proceeds of $350.0$350.0 million and net proceeds of $342.6$342.6 million. The 2026Company used a portion of the net proceeds to repay certain outstanding acquired indebtedness and used the remaining net proceeds for capital expenditures and other general corporate purposes. On January 28, 2022, the Company closed on a second tranche private placement of 8% Senior Secured Notes were issued at 100% face value, bear an interest rate(the "2026 Notes - Tranche Two") for aggregate gross proceeds of 8% per annum payable semi-annually in equal installments until$76.9 million and net proceeds of $75.6 million. The Company used the maturity date, unless earlier redeemed or repurchased.net proceeds for capital expenditures and other general corporate purposes. The 2026 Notes mature on October 6, 2026, andnotes may be redeemed in whole or in part, at the Company's option, at any time, on or after October 6, 2023, at the applicationapplicable redemption price set forthprice. These notes are collectively referred to as the "2026 Notes".Indenture. Theextinguishment of $57.0 million in principal at a discount of 16.5%. Cash consideration paid to repurchase the principal amount outstanding, excluding accrued interest, totaled $47.6 million, and the Company usedrecognized a portiongain of $8.2 million on the extinguishment of debt.
Private placementnotes payable consisted of the following as of December 31: 2023 2022 Stated Interest
RateEffective Interest
RateMaturity Date (in thousands) 2026 Notes - Tranche One $ 293,000 $ 350,000 8.00% 8.52% 10/6/2026 2026 Notes - Tranche Two 75,000 75,000 8.00% 8.43% 10/6/2026 June Notes — 70,000 9.75% 13.32% 6/11/2024 November Notes — 60,000 9.75% 13.43% 6/11/2024 Total private placement notes 368,000 555,000 Less: Unamortized debt discount and issuance costs (4,785) (13,336) Less: current portion of private placement notes, net — — Private placement notes, net $ 363,215 $ 541,664 proceeds to redeem certain outstanding indebtednesson the consolidated statements of Harvestoperations. Interest expense was $49.7 million, $52.0 million, and intends to use$22.2 million for the remaining net proceeds for capital expenditures and other general corporate purposes. For the yearyears ended December 31, 2023, 2022, and 2021, the Company incurredrespectively, and included accretion expense of $0.3 million.Scheduled annual$5.3 million, $5.2 million, and $3.5 million for the years ended December 31, 2023, 2022, and 2021, respectively.
Stated maturities of the principal portion of private placement notes outstanding as of December 31, 2021,2023 are as follows:Year (in thousands) 2026 368,000 Total private placement notes $ 368,000 The Company leases real estate used for dispensaries, production plants, and corporate offices. Lease terms for real estate generally range from five to ten years. Most leases include options to renew for varying terms at the Company’s sole discretion. Other leased assets include passenger vehicles, trucks, and equipment. Lease terms for these assets generally range from three to five years. Lease right-of-use assets and liabilities are recognized based on the present value of future minimum lease payments over the lease term at commencement date.Leases with an initial term of 12 months or less are not recorded on the balance sheet. Lease agreements for some locations provide for rent escalations and renewal options. Certain real estate leases require payment for taxes, insurance and maintenance which are considered non-lease components. The Company accounts for real estate leases and the related fixed non-lease components together as a single component.F-33TRULIEVE CANNABIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTSrecognizedfor the year ended December 31: Location on the consolidated statements of operations 2023 2022 2021 (in thousands) Operating lease cost Cost of goods sold, sales and marketing, general and administrative $ 20,291 $ 20,428 $ 10,754 Finance lease cost: Amortization of lease assets Cost of goods sold, Deprecation and amortization 10,357 10,935 7,638 Interest on lease liabilities Interest expense 6,449 6,549 4,385 Finance lease cost 16,806 17,484 12,023 Variable lease cost Cost of goods sold, sales and marketing, general and administrative 9,766 7,887 6,013 Short term lease expense Cost of goods sold, sales and marketing, general and administrative 406 751 334 $ 47,269 $ 46,550 $ 29,124
(1) Total lease cost recorded in cost of goods sold on the consolidated statements of operations was $3.2 million, $4.0 million, and comprehensive income$3.6 million for the yearyears ended December 31, 2023, 2022, and 2021, 2020, and 2019.Short term lease expense foryearyears ended December 31, 2020,2023, 2022, and 2019, was nominal. During2021, the year ended December 31, 2021, weCompany earned a nominal amount of sublease income which is recorded in other income, net on the consolidated statements of operations and comprehensive income. During the year ended December 31, 2021, the Company terminated $1.0 million in leases.follows:follows as of December 31:2023 2022 Cash paid for amounts included in the measurement of lease liabilities: Operating cash flows from operating leases $ 19,283 $ 21,092 Operating cash flows from finance leases $ 6,483 $ 6,542 Financing cash flows from finance leases $ 7,213 $ 7,042 ASC 842 lease additions and modifications Operating leases $ 14,016 $ 19,920 Finance leases $ 1,021 $ 25,909 Weighted average discount rate: Operating leases 8.33 % 9.29 % Finance leases 8.99 % 8.66 % Weighted average remaining lease term (in years): Operating leases 6.95 8.32 Finance leases 7.22 7.79 F-34TRULIEVE CANNABIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS our non-cancellable leases as of December 31, 2021,2023 are as follows:Operating leases Finance leases (in thousands) 2024 $ 19,713 $ 13,635 2025 19,651 13,492 2026 18,872 12,915 2027 18,232 12,047 2028 17,399 11,439 Thereafter 58,317 33,412 Total undiscounted lease liabilities 152,184 96,940 Less: Interest (49,881) (27,627) Total present value of minimum lease payments 102,303 69,313 Lease liabilities - current portion (10,068) (7,637) Lease liabilities $ 92,235 $ 61,676
leasesHolyokeIn July 2019, the Company sold property it had recently acquired in Massachusetts for $3.5 million, which was the cost to the Company. In connection with the sale of this location, the Company agreed to lease the location back for cultivation. This transaction was determined to be a finance lease, and therefore did not meet the definition of a sale because control was never transferred to the buyer-lessor. The transaction was treated as a failed sale-leaseback financing arrangement.Included in the agreement, the Company completed the tenant improvements related to the property, for which the landlord has provided a tenant improvement allowance (“TI Allowance”) for $40.0 million. 2021,2023 and 2022, total construction finance liabilities were $138.1 million and $138.4 million, respectively. The contractual terms range from 10.0 years to 25.0 years with a weighted average remaining lease term of 16.8 years.2020, $40.02023, 2022, and 2021 of $16.4 million, $15.9 million, and $40.0$7.8 million, respectively, of the TI Allowance has been provided. The initial term of the agreementwhich is ten years, with twofive-year options to renew. The initial payments are equal to 11% of the sum of the purchase price for the property and will increase when a draw is madeincluded in interest expense, net on the TI Allowance. In addition, a 3% increase in payments will be applied annually after the first year. Asconsolidated statements of December 31, 2021, and 2020, the total finance liability associated with this transaction is $44.6 million and $43.9 million, respectively.Ben BosticIn October 2019, the Company sold property in Florida in exchange for cash of $17.0 million. Concurrent with the closing of the purchase, the buyer entered into a lease agreement with the Company, for continued operation as a licensed medical cannabis cultivation facility. Control was never transferred to the buyer-lessor because the transaction was determined to be a finance lease and did not meet the requirements of a sale. The transaction was treated as a failed sale-leaseback financing arrangement.The initial term of the agreement is ten years, with twofive-year options to renew. The initial annualized payments are equal to 11% of the purchase price for the property. A 3% increase in payments will be applied annually after the first year. As of December 31, 2021, and 2020, the total finance liability associated with this transaction is $17.4 million and $17.2 million, respectively.McKeesportIn October 2019, prior to acquisition by the Company, PurePenn, LLC (“PurePenn”) sold their cannabis cultivation facility in Pennsylvania for $5.0 million. Simultaneously with the closing of the sale, PurePenn agreed to lease the cultivation facility back. The transaction was treated as a failed sale-leaseback financing arrangement.The initial term of the lease is 15 years, with twofive-year options to renew. The landlord has agreed to provide a TI Allowance of $21.0 million as an additional component of base rent. Payments are made based on one twelfth (1/12)F-35TRULIEVE CANNABIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTSof the TI allowance dispersed with 12.75% due for the first $5.0 million, 13.25% for $5.0 million to $15.0 million and 13.50% for $15.0 to $21.0 million. In 2021, the Company entered into an amendment with the landlord to increase the tenant improvement allowance by an additional $15.5 million for a total of $36.5 million at a rate of 10.75% on the additional allowance in excess of $21.0 million. As of December 31, 2021, $29.5 million of the TI allowance has been provided.AlachuaIn October 2021, in connection with the acquisition of Harvest, the Company acquired a transaction in which Harvest sold a licensed cultivation and processing facility and simultaneously with the closing of the sale, agreed to lease the facility back. The transaction was treated as a failed sale-leaseback financing arrangement.The initial term of the lease is 20 years, with twofive-year options to renew. The landlord has agreed to provide a TI Allowance of $17.85 million as an additional component of base rent. As of December 31, 2021, $15.3 million of the TI allowance has been provided.In the first quarter of 2022, the Company made the decision to discontinue the use of this facility. The Company is evaluating the impacts of this decision and remains in compliance with the associated lease obligation.HancockIn October 2021, in connection with the acquisition of Harvest, the Company acquired a transaction in which Harvest sold a licensed cultivation and processing facility and simultaneously with the closing of the sale, agreed to lease the facility back. The transaction was treated as a failed sale-leaseback financing arrangement.The initial term of the lease is ten years with twooptions to extend the term the first providing a ten-year renewal option and the second providing a five year renewal option. The landlord has agreed to provide a TI Allowance of $12.9 million as an additional component of base rent. As of December 31, 2021, $5.7 million of the TI allowance has been provided.Under the failed-sale-leaseback accounting model, the Company is deemed to own this real estate and will reflect the properties on our consolidated balance sheet and depreciate over the assets' remaining useful life.2021,2023 are as follows:Year Ending December 31, (in thousands) 2024 $ 17,043 2025 17,521 2026 18,013 2027 18,519 2028 19,039 Thereafter 283,385 Total future payments 373,520 Less: Interest (235,395) Total present value of minimum payments 138,125 Construction finance liabilities - current portion (1,466) Construction finance liabilities $ 136,659 F-36TRULIEVE CANNABIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS(ii) Unlimited number of Multiple Voting Shares, subject to adjustment in certain event.events. Holders of Multiple Voting Shares have the right to receive dividends, out of any cash or other assets legally available therefor, pari passu (on an as converted basis, assuming conversion of all Multiple Voting Shares into Subordinate Voting Shares at the Conversion Ratio) as to dividends and any declaration or payment of any dividend on the Subordinate Voting Shares. at any meeting of the shareholders of the Company, except a meeting of which only holders of another particular class or series of shares of the Company shall have the right to vote. At each such meeting, holders of Super Voting Shares are be entitled to two votes in respect of each Subordinate Voting Share into which such Super Voting Share could ultimately then be converted (initially, 200 votes per Super Voting Share). Holders of Super Voting Shares have the right to receive dividends, out of any cash or other assets legally available therefor, pari passu (on an as converted to SubordinatedSubordinate Voting Share basis) as to dividends and any declaration or payment of any dividend on the Subordinate Voting Shares. No dividend is to be declared or paid on the Super Voting Shares unless the Company simultaneously declares or pays, as applicable, equivalent dividends (on an as-converted to Subordinate Voting Share basis) on the Subordinate Voting Shares and Multiple Voting Shares. The initial “Conversion Ratio” for the Super Voting Shares is one Multiple Voting Share for each Super Voting Share,, subject to adjustment in certain events.WarrantsLiability warrants
of warrants
($CAD)
remaining contractual
life (Yrs)F-37TRULIEVE CANNABIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTSIn October 2021 we acquired 1,679 warrants in connection with the acquisition of Harvest ("Harvest liability warrants"). See Note 4. Acquisitions for further details. Each acquired warrant is exercisable into one Multiple There were no Super Voting Share. Changes in fair value are recognizedShares outstanding as a component of other (expense) income in the consolidated statements of operations and comprehensive income as change in fair value of derivative liabilities - warrants.Equity warrantsIn connection with the Harvest acquisition, we acquired certain equity classified warrants ("equity warrants"). The warrants range in exercise price from $23.76 to $145.24 and expire at various dates from June 2022 through December 2025. The warrants are exercisable into one Subordinate Voting Share. As of December 31, 2021, there were 1,009,416 acquired equity warrants outstanding. Each acquired equity warrant is exercisable into one Subordinate Voting Share.As of December 31, 2021, and 2020, there were 2,460,367 and 3,029,900 Public Warrants outstanding. See Note 12. Private Placement Notes for further details on warrants issued in connection with private placement debt in 2019.April OfferingOn April 12, 2021, the Company concluded the underwritten offer and sale of 5,750,000 Subordinate Voting Shares in the United States and Canada at a public offering price of $39.63. After paying the underwriters a commission of approximately $9.1 million, fees of $0.2 million and issuance costs of $1.2 million, the Company received aggregate consideration of approximately $217.9 million.Prospectus OfferingOn September 21, 2020, the Company concluded the offer and sale of 4,715,000 Subordinate Voting Shares pursuant to an agreement with Canaccord Genuity Corp. (the “Underwriter”) at a price of $18.56 per share. After paying the Underwriter a commission of approximately $4.1 million and issuance costs of $0.1 million, the Company received aggregate consideration of approximately $83.2 million.Options4,000,00014,000,000 Subordinate Voting Shares for issuance thereunder and replaced the Schyan Exploration Inc. Stock Option Plan (the “Prior Plan”). Awards previously granted under the Prior Plan including equity awards granted in the first quarter of 2021 for performance in 2020, remain subject to the terms of the Prior Plan. No further grants of awards shall be made under the Prior Plan. The Prior Plan is administered by the Board of Directors of the Company and the 2021 Plan is administered by the Compensation Committee.Committee of the Board of Directors.
In determining the amount of share-based compensation related to options issued, during the year ended December 31, 2021, and 2020, the Company used the Black-Scholes pricing model to establish the fair value of the options granted with the following assumptions:assumptions for the year-to-date periods ended December 31:
December 31, 2021
December 31, 2020 2023 2022 2021 Fair value at grant date $1.83 - $2.00 $8.39 - $11.01 $1.44 - $14.13 Expected term (in years) Expected term (in years) 3.30 - 3.97 3.5 - 4.5 3.20 - 6.20 Expected volatility 60.07% - 60.85% 51.81% - 52.87% 49.64% - 56.04% Expected annual rate of dividends 0% 0% 0% Risk free annual interest rate 4.34% - 4.53% 1.20% - 1.79% 0.16% - 1.15% expected volatility was estimated by usingfollowing table summarizes the historical volatility of the Company. In cases where there is insufficient trading history, the expected volatility is estimated using the historical volatility of other companies that the Company considers comparable that have trading and volatility history prior to the Company becoming public.F-38TRULIEVE CANNABIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTSThe expected life in years represents the period of time that options granted are expected to be outstanding and is computed using the simplified method. The risk-free rate was based on the United States bond yield rate at the time of grant of the award. Expected annual rate of dividends is based on the fact that the Company has never paid cash dividends and does not expect to pay any cash dividends in the foreseeable future.On January 3, 2020, under the Prior Plan, the Board awarded options to purchase shares to directors, officers, and key employees of the Company. The January 3, 2020 options generally vest over a two to three year period. For founding members of the Board of Directors, the options were fully vested on the date of grant.On January 4, 2021, under the Prior Plan, the Board awarded options to purchase shares to directors, officers, and key employees of the Company. The January 4, 2021 options generally vest over a two to three year period.On September 29, 2021, under the 2021 Plan, the Board awarded options to purchase shares to officers and other select employees of the Company. The September 29, 2021 options vest over a three year period.On October 1, 2021, the Company acquired Harvest which included consideration in the form of 1,266,641Company's stock options (as converted) that had been issued before the acquisition date to employees and non-employees of Harvest. The post-combination options vest over a one to three year period.On October 26, 2021, under the 2021 Plan, the Board awarded options to purchase shares to officers and other select employees of the Company. The options generally vest over a two to three year period.Foroption activity for the year ended December 31, 2021, the2023: Number
of optionsWeighted
average
exercise priceWeighted average
remaining contractual
life (Yrs.)Aggregate intrinsic
valueOutstanding options, beginning of year 3,177,815 $ 25.96 Granted 1,754,817 3.99 Forfeited (735,574) 20.48 Outstanding options, end of year 4,197,058 $ 17.73 4.91 $ — Vested and Exercisable options, end of year 3,248,599 $ 21.34 3.87 $ — infor stock options as follows for the amount of $7.5year million related to stock options. This is recognized as $0.7ended December 31: million cost of goods sold, net, $5.7 million general and administrative and $1.1 million sales and marketing in the consolidated statements of operations and comprehensive income. Statements of operations 2023 2022 2021 (in thousands) Cost of goods sold $ 66 $ 172 $ 662 General and administrative 3,344 8,157 5,722 Sales and marketing 59 243 1,089 Total share-based compensation expense $ 3,469 $ 8,572 $ 7,473 2021,2023, there was approximately $8.3$2.0 million of total unrecognized compensation cost related to nonvestedunvested stock option arrangements. That costarrangements which is expected to be recognized over a weighted-averageweighted average service period of 0.910.78 years.For the year ended December 31, 2020, the Company recorded share-based compensation in the amount of $2.8 million related to stock options. This is recognized as $0.2 million cost of goods sold, net, $2.1 million general and administrative and $0.5 million sales and marketing in the consolidated statements of operations and comprehensive income.The number and weighted-average exercise prices and remaining contractual life of options at December 31, 2020, and December 31, 2021, were as follows:
of options
average
exercise price
remaining contractual
life (Yrs.)F-39TRULIEVE CANNABIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS to -to-three year-year period subject to continued employment through each anniversary.period on a straight-line basis.September 29, 2021, under the 2021 Plan,January 4, February 24, and March 31, 2022, the Board awarded RSUs to board members, officers, and other select employees of the Company, which vest over a two to three year period. On October 26, 2021, under the 2021 Plan, the Board awarded RSUs to certain management employees of the Company. The RSUs immediately vested for board members and all other RSUs granted vest over a two-year period.same terms as those issued on September 29, 2021.In September 2021, the Board of Directors approved grants of RSUsCompany's RSU activity fortwo executive officers as replacement awards for cancelled warrants, which vested immediately. The previously held 3,572,514 warrants were cancelled on September 15, 2021 with the new RSUs granted on September 15, 2021 as a replacement of the previously held warrants. The two officers were awarded a total premium of $3.1 million, allocated between the two officers, to incentivize the cancellation and replacement. This premium payment was recorded to general and administrative expenses in the consolidated statements of operations and comprehensive income. No share-based compensation expense was recorded related to the cancellation and replacement of the previous warrants with the new RSUs during the year ended December 31, 2021.2023:Restricted Stock Unit Activity Number of
restricted stock unitsUnvested balance, beginning of year 720,707 $ 22.36 Granted 2,955,177 3.99 Vested (415,115) 23.06 Forfeited (574,553) 6.03 Unvested balance, end of year 2,686,216 $ 5.47
restricted stock units
grant priceDuring31, 2021, the Company recorded share-based compensation in the amount of $1.8 million related to RSUs. This is recognized as $0.3 million cost of goods sold, net, $1.2 million general and administrative and $0.3 million sales and marketing in the statements of operations and comprehensive income.Statements of operations 2023 2022 2021 (in thousands) Cost of goods sold $ 744 $ 900 $ 306 General and administrative 5,754 8,190 1,239 Sales and marketing 608 462 236 Total share based compensation expense $ 7,106 $ 9,552 $ 1,781 2021,2023, there was approximately $7.6$9.0 million of total unrecognized compensation cost related to nonvestedunvested restricted stock units. That costunits, which is expected to be recognized over a weighted-average service period of 0.860.81 years.WarrantsDuring the year ended December 31, 2018, the Company issued 8,784,872 warrants to certain employees and directors of the Company for past services provided. The warrants had no vesting conditions and are exercisable at any time for three years after the issuance, subject to certain lock-up provisions: (i) the warrants may not be exercised for 18 months following the Issue Date; (ii) 50% of the warrants may be exercised between months 19-24 following the Issue Date; and (iii) the remaining 50% of the warrants may be exercised at any time thereafter until expiration. The warrants are exchangeable into Subordinate Voting Shares. For the years ended December 31, 2021, 2020, and 2019, no warrants related to employee compensation were issued.F-40TRULIEVE CANNABIS CORP.NOTES TO CONSOLIDATED FINANCIAL STATEMENTSThe following table summarizes the warrants issued and outstanding to certain employees and directors of the Company as of December 31, 2021, 2020, and 2019, and the activity for the years then ended.for the years endedas of December 31, 2021, 2020, and 2019:31: 2023 2022 2021 Numerator: (in thousands, except for per share data) Continuing Operations Net (loss) income from continuing operations $ (435,895) $ (182,618) $ 30,525 Less: net loss attributable to non-controlling interest (5,147) (3,994) (587) Net (loss) income from continuing operations available to common shareholders of Trulieve Cannabis Corp. $ (430,748) $ (178,624) $ 31,112 Discontinued Operations Net loss from discontinued operations $ (97,241) $ (70,109) $ (13,080) Less: net loss attributable to non-controlling interest (1,193) (2,669) — Net loss from discontinued operations excluding non-controlling interest $ (96,048) $ (67,440) $ (13,080) Denominator: Weighted average number of common shares outstanding 188,974,176 187,995,317 139,366,940 Dilutive effect of securities outstanding — — 7,390,346 Diluted weighted average number of common shares outstanding 188,974,176 187,995,317 146,757,286 Loss per share - Continuing Operations Basic loss per share $ (2.28) $ (0.95) $ 0.22 Diluted loss per share $ (2.28) $ (0.95) $ 0.21 Loss per share - Discontinued Operations Basic and diluted loss per share $ (0.51) $ (0.36) $ (0.09) For the year ended December 31, 2021, and 2020, the Company1,694,424 and zero options, respectively, from the dilutive calculation as theydiluted per share amounts because their effect would have been anti-dilutive. There were no options outstandinganti-dilutive are as follows as of December 31, 2019. For the year ended31:2023 2022 2021 Stock options 4,197,058 3,177,815 1,694,424 Restricted share units 2,686,216 720,707 — Warrants 9,496 177,391 409,811 2021, 2020,2023, there were approximately 186.2 million issued and 2019outstanding shares which excludes approximately 2.9 million fully vested RSUs which are not contractually issuable until 2024 and approximately 0.1 million fully vested RSUs which are not contractually issuable until the Company excluded 409,811, 544,998, and zero warrants, respectively,earlier of triggering event, as they would have been anti-dilutive.defined, or December 1, 2030.F-41TRULIEVE CANNABIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTSUSU.S. federal income tax purposes under IRC Section 7874 and is subject to USU.S. federal income tax on its worldwide income. However, for Canadian tax purposes, the Company, regardless of any application of IRC Section 7874, is treated as a Canadian resident company (as defined in the Income Tax Act (Canada) (the “ITA”) for Canadian income tax purposes. As a result, the Corporation is subject to taxation both in Canada and the United States.include:include the following for the year ended December 31:2023 2022 2021 (in thousands) Current: Federal $ 121,722 $ 141,582 $ 141,684 State 46,808 38,633 29,677 Total current tax expense $ 168,530 $ 180,215 $ 171,361 Deferred: Federal $ (17,855) $ (17,814) $ (21,414) State (1,088) 793 (4,421) Foreign (1,191) (1,461) — Total deferred tax expense $ (20,134) $ (18,482) $ (25,835) Change in valuation allowance 2,962 1,647 196 Total income tax expense $ 151,358 $ 163,380 $ 145,722 follows:F-42TRULIEVE CANNABIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS2023 2022 2021 (in thousands) (Loss) income before income taxes $ (284,537) $ (19,238) $ 176,247 Federal statutory rate 21.0 % 21.0 % 21.0 % Theoretical tax (benefit) provision $ (59,753) $ (4,040) $ 37,012 Effects of tax rates in foreign jurisdictions $ 15 $ 16 $ — State taxes 835 14,598 25,580 Changes in state tax rates 5,772 4,763 (912) Uncertain tax position, inclusive of interest and penalties 130,481 146,702 77,783 Change in valuation allowance 2,962 1,647 196 Other 881 (4,793) (1,000) Tax effect of non-deductible expenses: IRC Section 280E disallowance — — 6,798 Goodwill impairment 64,594 — — Stock compensation 1,170 169 95 Political contributions 4,401 4,318 170 Total non-deductible expenses $ 70,165 $ 4,487 $ 7,063 Total income tax provision $ 151,358 $ 163,380 $ 145,722 Effective tax rates (53.2) % (849.3) % 82.7 % 2023 2022 (in thousands) Deferred tax assets: Lease liabilities $ 2,623 $ 3,094 Finance liabilities 27,695 27,386 Net operating losses 10,098 6,596 Inventory reserves 4,667 1,665 Other deferred tax assets 759 1,421 Total deferred tax assets: $ 45,842 $ 40,162 Deferred tax liabilities: Right of use assets $ (2,356) $ (3,112) Intangible assets (221,743) (236,353) Property and equipment (19,150) (19,004) Total deferred tax liabilities: $ (243,249) $ (258,469) Valuation allowance (9,557) (6,596) Net deferred tax liability $ (206,964) $ (224,903) 2023 2022 (in thousands) Balance, January 1 $ 41,781 $ 44,850 Reductions based on lapse of statute of limitations (1,053) (4,228) Additions based on tax positions related to the current year 152,313 — Additions based on refunds requested but not received related to prior year 111,664 — Additions based on refunds received related to prior years 62,391 — Additions based on tax positions related to the prior year 175,666 1,159 Balance, December 31 $ 542,762 41,781
A reconciliation of the beginning and ending amount of uncertain tax liabilities:2023 2022 (in thousands) Balance, January 1 $ 19,459 $ 6,665 Reductions based on lapse of statute of limitations (1,053) (4,228) Additions based on tax positions related to the current year 139,914 2,017 Additions based on tax positions related to the prior year 113,815 12,887 Additions based on refunds received related to prior years 62,391 — Reclass tax payment on account (157,063) — 3,150 (247) (263) 2,365 $ 180,350 $ 19,459
(1) Amounts represent the interest and penalties recorded on uncertain tax positions during the respective years which are recorded to the income tax provision on the consolidated statements of operations. Interest and penalties for the year ended December 31, 2021 and 2020, and 2019:was nominal.atas of December 31, 2021.2021,2023, the Company has $1.4had $11.2 million of non-capital Canadian losses which expire in 2031, $252.8from 2039 to 2043, $342.4 million of state net operating losses which expire in 2023-2041, and $271.7from 2031 to 2043, $92.6 million of USstate net operating losses which have an indefinite carryforward period, and $219.0 million of U.S. federal net operating losses which have an indefinite carryforward period. The Company determined a valuation allowance was applicable to $1.4$11.2 million of non-Capitalnon-capital Canadian losses and $138.9$100.3 million of state net operating losses. The Company also determined that it is more likely than not that the benefit from $268.9$209.0 million of USU.S. federal net operating losses and $106.2$319.5 million of state net operating losses will not be realized and therefore this amount has not been recorded.As the Company operates in the cannabis industry, the Company is subject to the limits of Internal Revenue Code ("IRC") Section 280E for US federal income tax purposes as well as state income tax purposes for all states except for California and Colorado. Under IRC Section 280E, the Company is only allowed to deduct expenses directly related to costs of goods sold. This results in permanent differences between ordinary and necessary business expenses deemed non-allowable under IRC Section 280E.The Company recognizes benefits from uncertain tax positions based on the cumulative probability method whereby the largest benefit with a cumulative probability of greater than 50% is recorded. An uncertain tax position is not recognized if it has less than a 50% likelihood of being sustained. As of December 31, 2021, and December 31, 2020, the Company recorded an uncertain tax liability of $6.7 million and $3.9 million, respectively, inclusive of interest and penalties. Additionally, there are unrecognized deferred tax benefits of $44.9 million2021,2023, the Company is no longer subject to examination by tax authorities for years before 2020.$Nevada operations. The assets and liabilities associated with discontinued operations consisted of the following as of December 31:2023 2022 (in thousands) Assets associated with discontinued operations Cash $ 301 $ 5,702 Accounts receivable, net 841 2,936 Inventories — 21,310 Income tax receivable — 2,299 Prepaid expenses 816 1,475 Other current assets — 11 Deferred tax asset — 766 Property and equipment, net — 53,687 Right of use assets - operating, net — 2,453 Right of use assets - finance, net — 5,736 Intangible assets, net — 27,849 Other assets 2,010 2,638 Total assets associated with discontinued operations $ 3,968 $ 126,862 Liabilities associated with discontinued operations Accounts payable and accrued liabilities $ 530 $ 1,617 Deferred revenue — 109 Operating lease liabilities - current portion 165 146 Finance lease liabilities - current portion 291 456 Construction finance liability - current portion 2,003 — Operating lease liabilities 15,332 17,108 Finance lease liabilities 2,048 5,890 Construction finance liability 24,167 45,217 Other long-term liabilities 6 155 Total liabilities associated with discontinued operations $ 44,542 $ 70,698 2023 2022 2021 (in thousands) Revenue $ 10,590 $ 24,634 $ 6,451 Cost of goods sold 29,843 37,897 6,895 Gross margin (19,253) (13,263) (444) Expenses: Operating expenses 7,522 13,821 6,661 Impairment and disposal of long-lived assets, net 69,480 49,130 — Total Expenses 77,002 62,951 6,661 Loss from operations (96,255) (76,214) (7,105) Other (expense) income: Other expense, net (5,087) (6,118) (5,636) Total other expense, net (5,087) (6,118) (5,636) Loss before provision for income taxes (101,342) (82,332) (12,741) Income tax (benefit) provision (4,101) (12,223) 339 Net loss from discontinued operations, net of taxes (97,241) (70,109) (13,080) Less: net loss attributable to non-controlling interest from discontinued operations (1,193) (2,669) — Net loss from discontinued operations excluding non-controlling interest $ (96,048) $ (67,440) $ (13,080) 2023 2022 2021 (in thousands) Depreciation and amortization $ 3,798 $ 10,787 $ 5,228 Purchases of property and equipment 67 844 57,479 Loss on impairment and disposal of long-lived assets 69,480 49,130 69 Other noncash investing and financing activities Noncash partial extinguishment of construction finance liability $ 18,486 $ — $ —
As a result of our exit from the Massachusetts market during the second quarter of 2023, the Company performed a lease term reassessment for the Holyoke failed sale-leaseback financing arrangement due to lease renewals previously included in the lease term being excluded as of the Massachusetts exit. The Company concluded the failed sale-leaseback accounting conclusion is maintained. The Company recognized a gain on partial extinguishment of $18.5 million as a result of the lease term reassessment, which was recorded to net loss from discontinued operations, net of taxes.
Future minimum lease payments for the construction finance liability associated with discontinued operations as of December 31, 2020. 2023, are as follows:Year Ending December 31, (in thousands) 2024 $ 5,455 2025 5,619 2026 5,788 2027 5,961 2028 6,140 Thereafter 12,287 Total future payments 41,250 Less: Interest (15,080) Total present value of minimum payments 26,170 Construction finance liabilities - current portion (2,003) Construction finance liabilities $ 24,167 Operating leases Finance leases (in thousands) 2024 $ 1,739 $ 494 2025 1,798 509 2026 1,859 476 2027 1,922 377 2028 1,979 389 Thereafter 23,503 835 Total undiscounted lease liabilities 32,800 3,080 Less: Interest (17,303) (741) Total present value of minimum lease payments 15,497 2,339 Lease liabilities - current portion 165 291 Lease liabilities $ 15,332 $ 2,048 doeshas entered into operating agreements with various entities related to the purchase and operation of cannabis dispensary, cultivation, and production licenses, in several states in which it determined to be variable interest entities. The Company determined certain of these entities to be variable interest entities in which it is the primary beneficiary and holds ownership interests in these entities ranging from 46% to 95% either directly or through a proxy as of December 31, 2023. The Company's VIEs are not reasonably expectmaterial to the unrecognized tax benefits will materially increaseconsolidated financial position or decrease withinoperations as of or for the next 12 months.years ended December 31, 2023, 2022, or 2021.During 2021,recordedmeeting the power and economics criteria. In particular, the Company controls the management decisions and activities most significant to certain VIEs, has provided a significant portion of the subordinated financial support provided to date, and holds membership interests exposing the Company to the risk of reward and/or loss. The Company allocates income and cash flows of the VIEs based on the outstanding ownership percentage in accordance with the underlying operating agreements, as amended. The Company has consolidated all identified variable interest entities for which the Company is the primary beneficiary in the accompanying consolidated financial statements.$2023, the Company paid $0.4 million i0.3n cash and $1.7 million in subordinate voting shares earned but not yet issued, based on the completion of certain milestones required as part of the acquisition of one of the Company's consolidated variable interest entities. The Company previously paid $0.8 million in cash for certain milestones. As part of the Company's decision to exit the Massachusetts market in the second quarter of 2023, it ceased its relationship with this variable interest entity. This terminated the payment of the $1.7 million subordinate voting shares earned but not yet issued. Based on the changes in circumstances, the Company reevaluated the variable interest entity, concluding it was no longer the primary beneficiary and as such, deconsolidated the entity in the second quarter of 2023. The Company deconsolidated total assets of $10.6 million and penaltiesliabilities of $0.3$4.8 million on uncertain liability assumed in acquisitions and recorded $a loss of $10.0 million related to the termination of the acquisition and deconsolidation of the variable interest entity which is included in net loss from discontinued operations on the consolidated statements of operations for the year ended December 31, 20230.6.interestlong-lived assets, net, in the consolidated statements of operations and comprehensive income. As offor the year ended December 31, 2021, all tax filings remain open for assessment.2022.A reconciliationTable of Contentsbeginning and ending amount of unrecognized tax benefits is as follows:F-43TRULIEVE CANNABIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTSNOTE 19. RELATED PARTIESThe balance of related party notes was $12.0 millionCompany’s VIEs in which it does not hold a majority interest as of December 31, 2020.31. The assets and liabilities in the table below include third-party assets and liabilities of our VIEs only and exclude intercompany balances that are eliminated in consolidation as included on our consolidated balance sheets.2023 2022 (in thousands) Current assets: Cash $ 9,491 $ 7,349 Accounts receivable, net 1,308 597 Inventories 8,341 7,590 Prepaid expenses 423 46 Other current assets 7 — Total current assets 19,570 15,582 Property and equipment, net 28,068 25,994 Right of use asset - operating, net 2,744 — Right of use asset - finance, net 259 224 Intangible assets, net 17,162 17,947 Other assets 140 344 Total assets $ 67,943 $ 60,091 Current liabilities: Accounts payable and accrued liabilities $ 1,939 $ 3,713 Income tax payable 2,017 1,615 Deferred revenue 2 6 Operating lease liability - current portion 63 — Finance lease liability - current portion 60 41 Total current liabilities 4,081 5,375 Notes payable 885 1,200 Operating lease liability 2,926 — Finance lease liability 210 185 Deferred tax liabilities 3,638 4,101 Other long-term liabilities 671 625 Total liabilities $ 12,411 $ 11,486 balance ofCompany incurred interest expense on the related party notes was zero as ofdebt during the year ended December 31, 2021. See Note 11. Notes Payable Related Party2021 totaling $1.1 million.further details.J.T. Burnette, the spouse of Kim Rivers,year ended December 31, 2023.Company, is a minority owner of a company (the “Supplier”) that provided construction and related services to the Company. The Supplier was responsible for the construction of the Company’s cultivation and processing facilities, and provided labor, materials and equipment on a cost-plus basis. For the years ended December 31, 2021, and 2020, property and equipment purchases totaled $148.4 million and $96.7 million, respectively. As of December 31, 2021, and 2020, $11.4 million and $10.4 million was included in accounts payable in the consolidated balance sheets. The use of the Supplier was reviewed and approved by the independent members of the Company’sCompany's board of directors, and all invoicesa member of the Supplier were reviewed by the officeCompany's board of the Company’s Chief Legal Officer. Asdirectors. Company has many leases from various real estate holding companies that are managed by various related parties including Benjamin Atkins, a former director and current shareholder of the Company, and the Supplier. As of December 31, 2021, and 2020, under ASC 842, the Company had the following inrelated party operating leases on the consolidated balance sheets: sheets, under ASC 842, as of December 31:2023 2022 (in thousands) (in thousands) (in thousands) Lease liabilities: Lease liabilities: Lease liabilities - current portion Lease liabilities - current portion Lease liabilities Lease liabilities Total related party lease liabilities Total related party lease liabilities foron related party leases was $2.7$0.2 million, $3.5$0.2 million, and $3.1$2.7 million for the years ended December 31, 2023, 2022, and 2021, 2020, and 2019, respectively.NOTE 20. COMMITMENTS AND CONTINGENCIESOperating LicensesAlthough the possession, cultivation and distribution of cannabis for medical use is permitted in Florida, California, Connecticut, Pennsylvania and West Virginia cannabis is a Schedule-I controlled substance and its use remains a violation of federal law. Since federal law criminalizing the use of cannabis preempts state laws that legalize its use, strict enforcement of federal law regarding cannabis would likely result in the Company’s inability to proceed with our business plans. In addition, the Company’s assets, including real property, inventory, cash and cash equivalents, equipment and other goods, could be subject to asset forfeiture because cannabis is still federally illegal.Claims and LitigationFrom time to time, the Company may be involved in litigation relating to claims arising out of operations in the normal course of business. Except as disclosed below, as of December 31, 2021, there were no pending or threatened lawsuits that could reasonably be expected to have a material effect on the results of the Company’s consolidated statements of operations and comprehensive income. There are also no proceedings in which any of the Company’s directors, officers or affiliates is an adverse party or has a material interest adverse to the Company’s interest.On December 30, 2019, a securities class-action complaint, David McNear v. Trulieve Cannabis Corp. et al., Case No. 1:19-cv-07289, was filed against the Company in the United States District Court for the Eastern District of New York. On February 12, 2020, a second securities class-action complaint, Monica Acerra v. Trulieve Cannabis Corp. et al., Case No. 1:20-cv-00775, which is substantially similar to the complaint filed on December 30, 2019, was filed against the Company in the United States District Court for the Eastern District of New York. Both complaints nameF-44TRULIEVE CANNABIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTSthe Company, Kim Rivers, and Mohan Srinivasan as defendants for allegedly making materially false and misleading statements regarding the Company’s previously reported financial statements and public statements about its business, operations, and prospects. The complaint alleges violations of Sections 10(b) and 20(a) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and SEC Rule 10b-5 promulgated thereunder. The complaint sought unspecified damages, costs, attorneys’ fees, and equitable relief. On March 20, 2020, the Court consolidated the two related actions under In re Trulieve Cannabis Corp. Securities Litigation, No. 1:19-cv-07289, and appointed William Kurek, John Colomara, David McNear, and Monica Acerra as Lead Plaintiffs. The Company filed a motion to dismiss on September 11, 2020. The Court granted the motion to dismiss, and final judgement was entered on March 11, 2022, dismissing the Plaintiff's claims with prejudice.During the year ending December 31, 2021, the Company settled certain litigation matters assumed in acquisitions during the year for $14.8 million.ContingenciesThe Company records contingent liabilities with respect to litigation on various claims in which we believe a loss may be probable and the loss is estimable. As of December 31, 2021, and 2020, $8.8 million and $0.7 million was included in contingent liabilities in the consolidated balance sheets related to pending litigation. The Company also recorded accruals related to sales tax audits or inquiries which were acquired through the Harvest acquisition. As of December 31, 2021, $2.3 million and was included in contingent liabilities in the consolidated balance sheet for estimates related to various sales tax matters. The Company also has amounts related to vendor disputes and various other matters which are probable and estimable but not known included in contingent liabilities in the consolidated balance sheet.Regulatory complianceThe Company’s compliance with state and other rules and regulations may be reviewed by state and federal agencies. If the Company fails to comply with these regulations, the Company could be subject to loss of licenses, substantial fines or penalties and other sanctions.NOTE 21. VARIABLE INTEREST ENTITIESThe Company through its acquisition of Harvest and through the acquired Harvest subsidiaries has entered into operating agreements with various entities related to the purchase and operation of cannabis dispensary, cultivation, and production licenses, in several states. The Company determined these entities to be variable interest entities ("VIEs") due to the financial relationship and as the Company is the primary beneficiary as of December 31, 2021. The Company holds ownership interests in these entities ranging from 25% to 49% as of December 31, 2021. The Company's VIEs are not material to the consolidated financial position or operations as of or for the year ended December 31, 2021. There were no variable interest entities as of December 31, 2020, or 2019.We have determined these entities to be variable interest entities and that we are the primary beneficiary. We consolidate these entities due to the other holder’s equity investment being insufficient to finance its activities without additional subordinated financial support and the Company meeting the power and economics criteria. In particular, the Company controls the management decisions and activities most significant to certain VIEs, has provided a significant portion of the subordinated financial support provided to date, and holds membership interests exposing the Company to the risk of reward and/or loss. The Company allocates income and cash flows of the VIEs based on the outstanding ownership percentage in accordance with the underlying operating agreements, as amended. The Company has consolidated all identified variable interest entities for which the Company is the primary beneficiary in the accompanying consolidated financial statements.The following table presents the summarized assets and liabilities of the Company’s VIEs in which we do not hold a majority interest as of December 31, 2021. The assets and liabilities in the table below include third-party assets and liabilities of our VIEs only and exclude intercompany balances that eliminate in consolidation as included in our consolidated balance sheets. The Company did not have VIEs prior to the acquisition of Harvest.F-45TRULIEVE CANNABIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTSNet revenues areRevenue is comprised of the following for the year endingended December 31:2023 2022 2021 (in thousands) Retail $ 1,083,545 $ 1,158,475 $ 869,707 Wholesale 43,390 55,087 61,137 Licensing and other 2,258 4,667 1,090 Total Revenue $ 1,129,193 $ 1,218,229 $ 931,934 23.24. FINANCIAL INSTRUMENTSCompany’s financial instruments that are measured atCompany applies the following fair value on a recurring basis consisthierarchy, which prioritizes the inputs used to measure fair value into three levels, and bases the categorization within the hierarchy upon the lowest level of money market fundsinput that is available and a warrant liability. Our financial instruments where carrying value approximatessignificant to the fair value include cash, accounts payable and accrued liabilities, notes payable, notes payable related party, operating lease liabilities, finance lease liabilities, other long-term liabilities and construction finance liabilities. Excluding the money market funds and warrant liability classified atmeasurement:Level 1 – Observable inputs based on unadjusted quoted prices in active markets for identical assets or liabilities; Level 2 – Inputs other than quoted prices in active markets, which are observable for the asset or liability, either directly or indirectly; and Level 3 – Unobservable inputs for which there is little or no market data requiring the Company to develop its own assumptions. value, the carrying values of these financial instruments approximate their fair valuesby class are as follows as of December 31, 2021, and 2020, due to their short-term nature or because the effective interest rate applied to the balance approximates the market rate.31: 2023 2022 Level 1 Level 2 Level 3 Total Level 1 Level 2 Level 3 Total (in thousands) Financial Assets (1): Money market funds (2) $ 145,995 $ — $ — $ 145,995 $ 340 $ — $ — $ 340 Financial Liabilities: Interest rate swap (3) $ — $ 2,341 $ — $ 2,341 $ — $ 2,536 $ — $ 2,536 Warrant liabilities (4) — — — — — 252 — 252 Financial instruments recorded at fair value are classified using a fair value hierarchy that reflects the significance of the inputs to fair value measurements. There have been were no transfers between hierarchy levels during the years ended December 31, 2021,2023 and 2020, respectively.2022.F-46TRULIEVE CANNABIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTSThe following tables present information about the Company’s financial instruments and their classifications as of December 31, 2021, and 2020, and indicate the fair value hierarchy of the valuation inputs utilized to determine such fair value.Fair Value Measurements as of December 31, 2021, using:Fair Value Measurements as of December 31, 2020, using:(1)(2)inon the Company’s consolidated balance sheets. As a short-term, highly liquid investments readily convertible to known amounts of cash, the Company’s money market funds have carrying values that isapproximate fair value. Interest income is recorded to interest income on the consolidated statements of operations. The Company recorded interest income of $4.8 million in relation to these money market funds for the year ended December 31, 2023. Interest income for the year ended December 31, 2022 was nominal.(2)(3)Warrant liabilities represent liability classified warrants acquired from HarvestThe VNB Swap is carried at fair value which is based on a valuation model that utilizes interest rate yield curves and credit spreads observable in October 2021 ("Harvest liability warrants") and includedactive markets as partthe significant inputs to the model. The Company considers credit risk associated with its own standing as well as the credit standing of any counterparties involved in the consideration transferred. See Note 3. Acquisitions.valuation of its financial instruments. The fair value of the Harvest acquiredinterest rate swap liability is recorded in other long-term liabilities on the consolidated balance sheets.Black-Scholes options pricing model. Share Price: C$fair value hierarchy. See Note 12. Private Placement Notes for further details.3,291; Exercise Price: C$1,125; Remaining term: 1.31 years; Annualized Volatility: 49.57%; Dividend yield: 0%; Discount Rate: 0.56%; C$ Exchange Rate: 0.788.
In the fourth quarter of 2022, the Company entered into an interest rate swap contract ("VNB Swap") for the purpose of hedging the variability of interest expense and interest payments on the Company's long-term variable rate debt. The notional value was $70.0 million and $71.5 million as of December 31, 2023 and December 31, 2022, respectively. The VNB Swap was entered into in conjunction with four promissory term notes of a total corresponding amount. The four promissory term notes were priced at the Secured Overnight Financing Rate ("SOFR"), as defined in the agreement plus 3.00%, per annum. The VNB Swap fixed the four term loans at 7.53% per annum until maturity on January 1, 2028. The VNB Swap effectively fixes the floating SOFR-based interest of the SOFR-based debt.24.25. SUBSEQUENT EVENTSSenior Secured Notes Due 2026On January 28, 2022,closed on a second tranche private placement of 8% Senior Secured Notes (the "2026 Notes") for aggregate gross proceeds of $75.6 million. The Notes were issued at 101% face value, bear an interest rate of 8% per annum payable semi-annuallyreceived $50.3 million in equal installments until the maturity date, unless earlier redeemed or repurchased. The Notes will mature on October 6, 2026, and may be redeemed in whole or in part, at the Company's option, at any time, on or after October 6, 2023, at the application redemption price set forth in the Indenture. AcquisitionIn February 2022, we entered into a purchase agreement with Sweet 5, LLC and CP4 Group LLC ("Watkins")refunds related to acquire a cultivation operation in Arizona and the right to a lease of the facility. The purchase was completed for total cash consideration of $27.5 million with a potential earnouts of $22.5 million based on the completion of certain milestones. This acquisition will be accounted for as a business combination in accordance with the Accounting Standards Codification (ASC) 805, Business Combinations. The Company has begun the process to determine the purchase price allocation for assets acquired and liabilities assumed, including estimating the fair value of intangible and tangible assets. These estimates and initial accounting for the business combination have not been completed. As a result, the Company is unable to prove the amounts recognized as of the acquisition date for the major classes of assets acquired and liabilities assumed.F-47TRULIEVE CANNABIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTSDivestment of VIEIn the first quarter of 2022, the Company completed the divesture of one of its VIEs through the full repayment of the subordinated financial support provided to the VIE and the sale of its equity interest to the majority shareholder. The total proceeds from the sale were approximately $1.6 million.F-48