SECURITIES AND EXCHANGE COMMISSION
WASHINGTON,
(
Amendment No. 2)
Mark One) | | | | | |
| ☒ | |
☒
| ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2023
OR
| | | | | |
| For the fiscal year ended December 31, 2020
|
OR
| |
☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| FOR THE TRANSITION PERIOD FROM __________ TO ________
|
COMMISSION FILE NUMBER
For the transition period from _____ to _____
Commission file number 001-39248
The Oncology Institute, Inc.
(Exact name of registrant as specified in its charter)
| | | | | | | | | | | |
| Delaware | | 84-3562323 |
Delaware
| | 84-3562323
|
(State or other jurisdiction of incorporation or organization) | |
(I.R.S. Employer Identification Number) No.) |
| incorporation or organization)
|
|
|
|
|
18000 Studebaker Rd,. |
|
Suite 800 |
Cerritos,, California |
| 90703 |
(Address of principal executive offices) |
| (Zip Code)
Principal Executive Offices) |
Registrant’s
(562) 735-3226
Registrant's telephone number, including area code: (213) 760-1328code
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | |
Title of each class | | Trading Symbol(s) | | Name of each exchange on which registered |
Common Stock, $0.0001 par value per share | | TOI | | The Nasdaq Stock Market LLC |
|
|
|
|
|
Redeemable warrants, each whole warrant exercisable for one share ofWarrants to purchase common stock each at an exercise price of $11.50 per share
| | TOIIW | | The Nasdaq Stock Market LLC |
Securities registered pursuant to
Sectionsection 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes
☐No☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes
☐No☒
Indicate by check mark whether the
registrantregistrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports)
; and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant
(1) has submitted electronically
and posted on its corporate web site, if any, every Interactive Data File required to be submitted
and posted pursuant to Rule 405 of Regulation S-T
(§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to
filesubmit and post such
reports) and has been subject to such filing requirements for the past 90 days.files). Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (Section 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer,
or a smaller reporting
company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated
filer,”filer” and “smaller reporting
company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one):
| | | | | | | | | | | |
Large accelerated filer ☐ | ☐ | Accelerated filer ☐ | ☐ |
Non-accelerated filer ☒ | ☒ | Smaller reporting company☒ | ☒ |
| | Emerging growth company☒ | |
| | | ☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐ Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements.☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to § 240.10D-1(b).☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☒☐ No ☐☒
The aggregate market value of
the commonvoting stock held by non-affiliates of the
registrant, computedRegistrant, based on the closing price of $0.55 per shares of the Registrant’s common stock as reported by the Nasdaq Capital Market as of June 30,
2020 (the last business day of the registrant’s most recently completed second fiscal quarter)2023, was approximately
$173,362,500.
As$41.6 million.
The registrant had outstanding 74,312,921 shares of common stock as of March
1, 2021, the Registrant had 23,000,000 shares of its Class A common stock, $0.0001 par value per share, and 5,750,000 shares of its Class B common stock, $0.0001 par value per share, outstanding.19, 2024.
DOCUMENTS INCORPORATED BY REFERENCE
None.
EXPLANATORY NOTE
References throughout this Amendment No. 2 toForm 10-K incorporates by reference information from the Annual Report on Form 10-K/A to “we,” “us,” the “Company” or “our company” are to The Oncology Institute, Inc. (formerly, DFP Healthcare Acquisitions Corp.), unless the context otherwise indicates.
This Amendment No. 2 ("Amendment No. 2") to the Annual Report on Form 10-K/A amends Amendment No. 1 to the Annual Report on Form 10-K/A of the Company as of andregistrant’s proxy statement for the fiscal period ended December 31, 2020, asannual meeting of stockholders expected to be held on June 13, 2024, which will be filed with the Securities and Exchange Commission ("SEC") on May 24, 2021 (the "2020 Form 10/A No. 1"). The Company has re-evaluatedpursuant to Regulation 14A within 120 days after the Company’s application of ASC 480-10-S99-3A to its accounting classificationend of the redeemable Class A common stock, par value $0.0001 per share (the “Public Shares”), issued as partRegistrant’s fiscal year ended December 31, 2023.
Table of Contents
| | | | | |
| Page |
| 6 |
| 6 |
| 16 |
| 45 |
| 45 |
| 46 |
| 46 |
| 46 |
| 47 |
| 47 |
| 47 |
| 48 |
| 60 |
| 61 |
| 110 |
| 110 |
| 111 |
| 111 |
| 112 |
| 112 |
| 112 |
| 112 |
| 112 |
| 112 |
| 113 |
| 113 |
| 115 |
| 116 |
INTRODUCTORY NOTE
Unless the
units sold in the Company’s initial public offering (the “IPO”) on March 13, 2020. Historically, a portion of the Public Shares was classified as permanent equity to maintain stockholders’ equity greater than $5 million on the basis that the Company will not redeem its Public Shares in an amount that would cause its net tangible assets to be less than $5,000,001, as described in the Company’s amended and restated certificate of incorporation (the “Charter”). Pursuant to such re-evaluation, the Company revised its interpretation to include temporary equity in net tangible assets. In addition, in connection with the change in presentation for the Public Shares, the Company determined it should restate its earnings per share calculation to allocate income and losses shared pro rata between the two classes of shares. This presentation contemplates a Business Combination as the most likely outcome, in which case, both classes of shares share pro rata in the income and losses of the Company.Therefore, on December 8, 2021, the Company’s management and the audit committee of the Company’s board of directors (the “Audit Committee”) concluded that the Company’s previously issued (i) balance sheet as of March 13, 2020 (the "Post IPO Balance Sheet"), as previously restated in the 2020 Form 10-K/A No. 1, (ii) financial statements included in the 2020 Form 10-K/A No. 1, as previously restated in the 2020 Form 10-K/A No.1 as previously restated (iii) unaudited interim financial statements included in the Form 10-Q for the quarterly period ended March 31, 2020 as previously restated in the 2020 Form 10-K/A No. 1; (iv) unaudited interim financial statements included in the Form 10-Q for the quarterly period ended June 30, 2020 as previously restated in the 2020 Form 10-K/A No. 1; (v) unaudited interim financial statements included in the Form 10-Q for the quarterly period ended September 30, 2020 as previously restated in the 2020 Form 10-K/A No. 1 (vi) unaudited interim financial statements included in the Company’s Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2021, filed with the SEC on May 24, 2021; (vii) unaudited interim financial statements included in the Company’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2021, filed with the SEC on August 12, 2021; and (viii) footnote 2 to the unaudited interim financial statements and Item 4 of Part 1 included in the Company’s Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2021, filed with the SEC on November 8, 2021 (collectively, the “Affected Periods”) , should be restated to report all Public Shares as temporary equity and should no longer be relied upon. As such, the Company will restate its financial statements for the Affected Periods in this Form 10-K/A for the Post IPO Balance Sheet, the Company's financial statements included in the 2020 Form 10-K/A No. 1, and the unaudited interim financial statements for the quarterly periods ended March 31, 2020, June 30, 2020, and September 30, 2020. The unaudited interim financial statements for the periods ended March 31, 2021, and June 30, 2021 will be restated in an amendment to the Company’s Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2021, to be filed with the SEC (the “Q3 2021 Form 10-Q/A”).
The restatement does not have an impact on its cash position and the cash formerly held in the trust account established in connection with the IPO (the “Trust Account”).
The Company’s management has concluded that a material weakness remains in the Company’s internal control over financial reporting and that the Company’s disclosure controls and procedures were not effective. As a result of that reassessment, we determined that our disclosure controls and procedures for such periods were not effective with respect to the proper accounting and classification of complex financial instruments. For more information, see Item 9A includedcontext dictates otherwise, references in this Annual Report on Form 10-K/A.
10-K to the “Company,” “we,” “us,” “our,” and similar words are references to The Oncology Institute, Inc., a Delaware corporation (“TOI”), and its consolidated subsidiaries and affiliated entities, as appropriate, including its consolidated variable interest entities (“VIEs”).
Trade names and trademarks of TOI referred to herein, and their respective logos, are our property. This Annual Report on Form 10-K may contain additional trade names and/or trademarks of other companies, which are the property of their respective owners. We do not intend our use or display of other companies’ trade names and/or trademarks, if any, to imply an endorsement or sponsorship of us by such companies, or any relationship with any of these companies.
We are filingThe Centers for Medicare & Medicaid Services (“CMS”) have not reviewed any statements contained in this Amendment No. 2 to amendAnnual Report on Form 10-K.
FORWARD-LOOKING STATEMENTS
In this Annual Report on Form 10-K, including "Business” in Item 1, “Risk Factors” in Item 1A, and restate the 2020 Form 10-K/A No. 1 with modification as necessary to reflect the restatements. The following items have been amended to reflect the restatements:Part I, Item 1A. Risk Factors
Part II, Item 7.
"Management’s Discussion and Analysis of Financial Condition and Results of OperationsPart II,
" in Item 8. Financial Statements7, and Supplementary DataPart II, Item 9A. Controls and Procedures
In addition, the Company’s Chief Executive Officer and Chief Financial Officer have provided new certifications dateddocuments incorporated by reference herein, we make forward-looking statements within the meaning of the Private Securities
Litigation Reform Act of 1995. These forward-looking statements relate to expectations for future financial performance, business strategies or expectations for our business. These statements may be preceded by, followed by or include the words "may," "might," "will," "will likely result," "should," "estimate," "plan," "project," "forecast," "intend," "expect," "anticipate," "believe," "seek," "continue," "target" or similar expressions. These forward-looking statements are based on information available to us as of the date of this filing in connection with this Form 10-K/A (Exhibits 31.1, 31.2, 32.1they were made, and 32.2).Except as described above, and including the closing of our Business Combination, no other information included in the 2020 Form 10-K/A No.1 is being amended or updated by this Amendment No. 2 and this Amendment No. 2 does not purport to reflect any information or events subsequent to the 2020 Form 10-K/A No.1. This Amendment No. 2 continues to describe the conditions as of the date of the 2020 Form 10-K/A No.1 and, except as expressly contained herein, we have not updated, modified or supplemented the disclosures contained in the 2020 Form 10-K/A No. 1 to give effect to any subsequent events, including the closing of the Company’s business combination. Accordingly, this Amendment No. 2 should be read in conjunction with the 2020 Form 10-K/A No.1 and with our filings with the SEC subsequent to the 2020 Form 10-K/A No. 1.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
The statements contained in this report that are not purely historical are forward-looking statements. Our forward-looking statements include, but are not limited to, statements regarding our or our management team’s expectations, hopes, beliefs, intentions or strategies regarding the future. In addition, any statements that refer to projections, forecasts or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking statements. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intends,” “may,” “might,” “plan,” “possible,” “potential,” “predict,” “project,” “should,” “would” and similar expressions may identify forward- looking statements, but the absence of these words does not mean that a statement is not forward-looking. Forward-looking statements in this Annual Report on Form 10-K/A may include, for example, statements about:
| ● | our ability to select an appropriate target business or businesses; |
| ● | our ability to complete our initial business combination; |
| ● | our expectations around the performance of any prospective target business or businesses; |
| ● | our success in retaining or recruiting, or changes required in, our officers, key employees or directors following our initial business combination; |
| ● | our officers and directors allocating their time to other businesses and potentially having conflicts of interest with our business or in approving our initial business combination; |
| ● | our potential ability to obtain additional financing to complete our initial business combination; |
| ● | our pool of prospective target businesses; |
| ● | the ability of our officers and directors to generate a number of potential investment opportunities; |
| ● | our public securities’ potential liquidity and trading; |
| ● | the lack of a market for our securities; |
| ● | the use of proceeds not held in the Trust Account (as described herein) or available to us from interest income on the Trust Account balance; or |
| ● | the Trust Account not being subject to claims of third parties; or |
| ● | our financial performance. |
The forward-looking statements contained in this report are based on our current expectations and beliefs concerning future developments and their potential effects on us. There can be no assurance that future developments affecting us will be those that we have anticipated. These forward-looking statements involve a number of risks and uncertainties (some of which are beyond our control) or other assumptions that may cause actual results or performancethem to turn out to be materially different from those expressed or implied by thesewrong. Accordingly, forward-looking statements. These risksstatements should not be relied upon as representing our views as of any subsequent date, and uncertainties include, but arewe do not limited to, those factors described under the heading “Risk Factors” in this Annual Report. Should one or more of these risks or uncertainties materialize, or shouldundertake any of our assumptions prove incorrect, actual results may vary in material respects from those projected in these forward-looking statements. We undertake no obligation to update or revise any forward-looking statements to reflect events or circumstances after the date they were made, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
As a result of a number of known and unknown risks and uncertainties, our actual results or performance may be materially different from those expressed or implied by these forward- looking statements. Some factors that could cause actual results to differ include:
•
expectations and assumptions about growth rate and market opportunity of TOI;•our public securities’ potential liquidity and trading;
•our ability to raise financing in the future;
•our success in retaining or recruiting, or changes required in, our officers, key employees or directors;
•the impact of the regulatory environment and complexities with compliance related to such environment;
•the outcome of judicial and administrative proceedings to which TOI may become a party or investigations to which TOI may become subject that could interrupt or limit TOI’s operations, result in adverse judgments, settlements or fines and create negative publicity;
•failure to continue to meet, or to cure any deficiency with respect to, stock exchange listing standards;
•factors relating to the business, operations and financial performance of TOI, including:
◦the potential short and long-term impact of a re-emergence of COVID-19 variants or any other pandemic, epidemic or similar broad health-related outbreaks;
◦the ability of TOI to maintain an effective system of internal controls over financial reporting;
◦the ability of TOI to grow market share in its existing markets or any new markets it may enter;
◦the ability of TOI to respond to general economic conditions;
◦the ability of TOI to manage its growth effectively;
◦the ability of TOI to achieve and maintain profitability in the future;
◦the ability of TOI to attract new patients;
◦the ability to recognize and react to changes in TOI’s clients’ preferences, prospects and the competitive conditions prevailing in the healthcare sector;
◦continued reimbursement from third-party payors; and
◦other factors detailed under the section titled “Risk Factors” within this Annual Report on Form 10-K.
Item 1. Business
Overview
The Oncology Institute, Inc. ("TOI", the "Company", "we", or "our) is a leading value-based oncology company that manages community-based oncology practices which serve patients at 83 clinic locations across 15 markets and five states throughout the United States. Our community-based oncology practices are staffed with 130 oncologists and advanced practice providers. 69 of these clinics are staffed with 119 providers employed by our affiliated physician-owned professional corporations, referred to as the "TOI PCs", which provided care for more than 72,000 patients in 2023 and managed a population of approximately 1.8 million patients under value-based agreements as of December 31, 2023. We also provide management services to 14 clinic locations owned by independent oncology practices. Our mission is to heal and empower cancer patients through compassion, innovation, and state-of-the-art medical care.

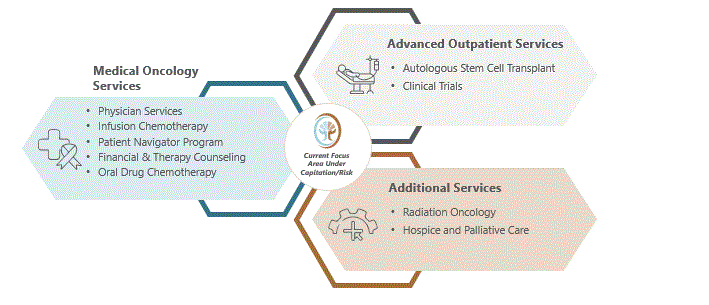
Our managed clinics provide a range of medical oncology services, including physician services, in-house infusion and dispensary, radiation, innovative programs like outpatient blood product transfusions, along with 24/7 patient support. Through TOI Clinical Research, LLC ("TCR"), the clinical research arm of the TOI PCs, we also provide and manage clinical trial services and research for the benefit of cancer patients. Many of our services, such as managing clinical trials and palliative care programs, are traditionally accessed through academic and tertiary care settings, while the TOI PCs bring these services to patients in a community setting, by which we mean, providing care in our convenient clinic locations rather than academic or tertiary care settings. As scientific research progresses and more treatment options become available, cancer care is shifting from acute care episodes to chronic disease management. With this shift, it is increasingly important for high-quality, high-value cancer care to be available in a local community setting to all patients in need.
As a value-based oncology company, we seek to deliver better quality care while managing costs for patients and payors that we serve. We define value-based care as care that focuses on providing optimal health outcomes and healthcare
affordability and a value-based contract as any contract that removes the incentive to drive up cost of care or overutilization, while rewarding better clinical outcomes, cost and quality. We work to accomplish this goal by reducing wasteful, inefficient or futile care that drives up costs but does not improve outcomes. We believe payors and employers are aligned with the value-based model due to its enhanced access, improved outcomes, and lower costs. Patients under our affiliated providers’ care can benefit from evidence-based and personalized care plans, gain access to sub-specialized care in convenient community locations, and lower out-of-pocket costs. We believe our affiliated providers enjoy the stability and predictability of a large multi-state practice, are not incentivized or pressured to over-treat when it may be inconsistent with a patient’s goals of care and can focus on practicing outstanding evidence-based oncology care.
In contrast to value-based care, we believe much of traditional fee-for-service, or FFS, oncology care is plagued by misaligned incentives that drive up costs and often lower the quality of care. In FFS care, oncologists are reimbursed on a “cost-plus” basis for drugs which in turn are often responsible for the majority of a practice’s revenue. This "cost-plus" model may incentivize oncologists to prescribe the most expensive treatments even if lower cost alternatives that are still medically appropriate are available, as well as to continue to utilize chemotherapy in advanced cancer patients who may no longer benefit from such treatment. In these cases, patients and payors not only bear the burden of higher cost of care, but patients may also suffer negative health outcomes including higher rates of emergency room visits and hospitalizations for supportive care needs due to the side effects associated with chemotherapy.
In 2023, we generated more than 47% of our revenue from patients who are covered by value-based contracts. Historically, our value-based contracts have predominately taken the form of capitated contracts. Our capitated contracts remove incentives to drive up costs, and they also have incentives for meeting or exceeding certain quality metrics. In some capitated contracts we are penalized if we fail to meet certain quality metrics. In other capitated contracts, we receive bonuses/rewards if we meet or exceed certain quality metrics. Our value-based contracts could also take on other forms, such as sharing with payors in the cost savings generated for specific medical oncology costs (which we refer to as 'gain-sharing' contract), along with incentives to meet certain quality metrics. These contracts, despite their modifications on how reimbursement is structured, still meet the definition of value-based care. We and our affiliated providers have contractual relationships with payors serving a variety of patients, including Medicare Advantage ("MA"), Medicaid, and commercial patients. These payors include affiliates of Anthem, CareMore Health, Heritage Provider Network and Optum Care.
In 2023, we continued to evolve the way we structure our value-based arrangements, particularly in areas of the country where there are limits on medical group delegation, or an increased desire from groups to incentivize TOI for cost of care that extends beyond medical oncology and includes elements such as management of acute care utilization and/or management of non-employed oncologists. We believe that these new contracting methodologies are furthering our mission to provide access to world-class oncology care in an affordable manner to underserved populations.
We believe that our position in the market and focus on elevating the state of oncology care with a value-based care model positions our affiliated providers well for future growth. Our technology platform supports this growth and enables the TOI PCs to standardize and deliver consistent value-based care at scale. We believe that our model will support growth into new markets, allow us to continue service more patients across the United States.
Our website is www.theoncologyinstitute.com. The information contained on our website is not a part of this annual report.
Affiliated Physician Practices
Some states have laws that prohibit business entities with non-physician owners from practicing medicine, which are generally referred to as the corporate practice of medicine. States that have corporate practice of medicine laws require only physicians to practice medicine, exercise control over medical decisions or engage in certain arrangements with other physicians, such as fee-splitting. For example, under California's corporate practice of medicine doctrine, physicians and certain licensed professionals cannot be employed by non-professional corporations, except under limited exceptions which do not apply to us. Additionally, all clinical decisions and certain business or management decisions that result in control over a physician's practice of medicine must be made by a licensed physician and not by an unlicensed person or entity. California also prohibits professional fee-splitting arrangements, but management fees based on a percentage of gross revenue or similar arrangement that is commensurate with fair market value of services provided by the management company are generally permissible.
We have entered into a management services agreement with each of the TOI PCs, which are entirely physician owned. Under our management services agreements, we have agreed to serve, on an exclusive basis, as manager and administrator of each TOI PC’s non-medical functions and services related to healthcare services and items provided to patients by physicians and other licensed healthcare providers employed by or under contract with a TOI PC. The non-medical functions and services we provide under the management services agreements include practice management services and non-clinical operational assistance for all TOI PC clinic locations, assistance with provider and payor contract negotiations and administration, billing
and collection services, financial and accounting services, electronic medical records and practice management technology solutions, assistance in maintaining licensure, permits and other credentialing requirements for the TOI PCs, risk management services, non-clinical personnel services, provider recruitment services and other administrative services required for the day-to-day operations of the clinics and TOI PCs. Our management services agreements with the TOI PCs have 20-year terms, unless terminated upon mutual agreement of the parties or unilaterally by a party following a material breach or commencement of bankruptcy or liquidation events by the other party, or a governmental or judicial termination order against a party. Under the management services agreement, we receive a monthly management fee that is structured as direct reimbursement of all costs incurred plus a percentage of the TOI PC’s gross revenue, which is defined as the TOI PC’s total revenues payable for all healthcare services and items rendered by the TOI PC, adjusted for bad debt, discounts and payor contract adjustments. In accordance with relevant accounting guidance, each of the TOI PCs is determined to be a variable interest entity, or VIE, of the Company as the Company has the ability, through the management services agreement, to direct the activities (excluding clinical decisions) that most significantly affect the TOI PC’s economic performance.
Market Overview
Our business is focused on caring for adult and senior populations with medical oncology and related care needs, including members of MA plans run by private insurance companies on behalf of the Centers for Medicare and Medicaid Services, or CMS, as well as traditional FFS Medicare, Medicaid, other government healthcare programs and commercial insurance populations. One of our primary focuses is on value-based contracts in which we manage the medical oncology care for a population of patients for a pre-determined, population-based capitated payment. Many of the patients that we manage under value-based arrangements are referred to as “capitated” populations, however our affiliated providers also provide care to patients outside of these arrangements under traditional FFS arrangements as well as other types of value-based contract.
As of December 31, 2023, we were active in 15 markets in five states. Across these states, there were approximately 81 million commercial, Medicaid, and MA lives. This population provides us with a substantial opportunity to capture a portion of those lives in both our legacy, existing markets, as well as in our new expansion geographies.
Our Care Model
Since our founding over 15 years ago, we have built a solid track record around our care model for value-based oncology care. Our care model is focused on delivering personalized, evidenced-based care, consistently, and at scale. We seek to deliver better patient outcomes for lower costs, and to care for more of our payors’ patient populations.
Our care model is designed to remove physicians’ incentives to over-prescribe or prescribe high-cost chemotherapy that is of limited clinical utility to patients, but rather focus on NCCN guideline adherence and, when possible, choose clinically equivalent but lower-cost therapeutics to benefit patients and our payor partners. We invest in nurse practitioners to help with advanced care planning and palliative care discussions with patients. We give patients the education and tools to make their own decisions about when the right time is to choose palliative care or hospice.
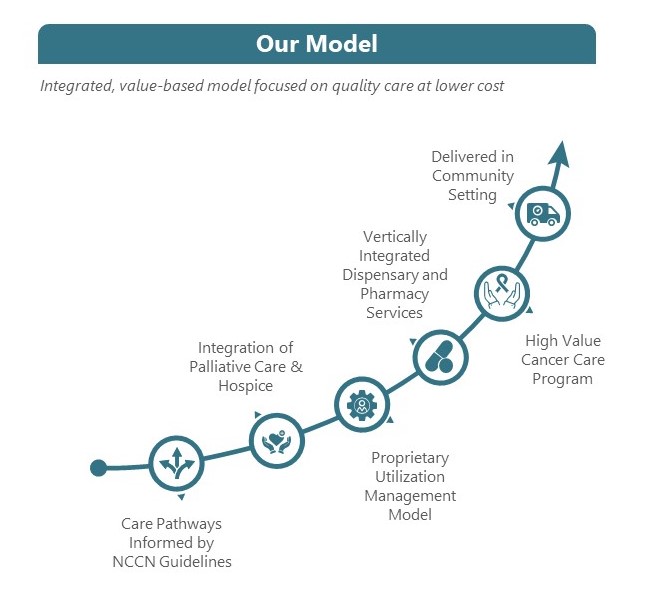
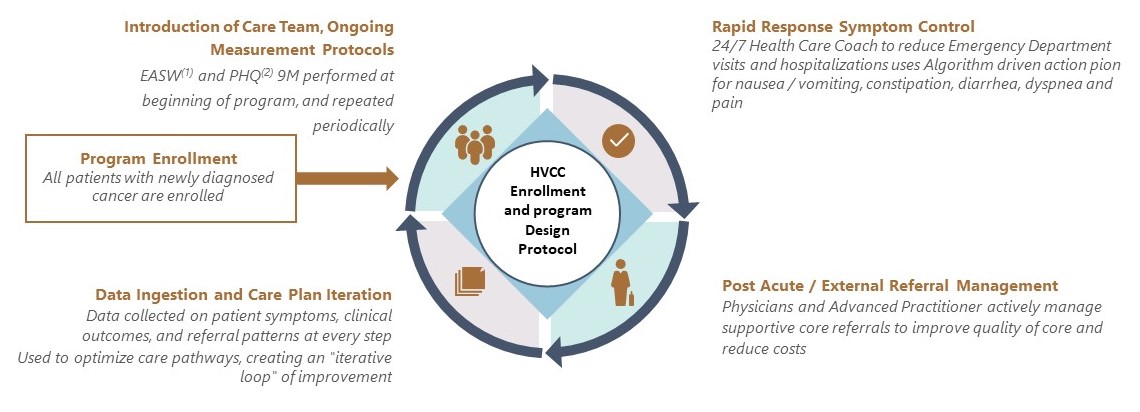
While the TOI PCs treat patients under both value-based and FFS contracts, our affiliated providers’ approach to care focuses on achieving the best outcomes at the lowest cost, regardless of the reimbursement methodology. We have developed a High Value Cancer Care, or HVCC, program, in which patients are able to access targeted care resources that augment and support their treatment. Our treatment regimens are based on algorithms established by the National Comprehensive Cancer Network (“NCCN”) and are evidence-based. NCCN is a not-for-profit alliance of 32 leading cancer centers devoted to patient care, research and education (not including TOI). NCCN focuses on improving cancer care through the input of clinical thought leaders at its member organizations. NCCN publishes guidelines developed from evidence- based medicine to ensure that all cancer patients receive preventative, diagnostic, treatment, and supportive services that are most likely to lead to optimal outcomes. The NCCN guidelines are widely recognized as the standard for clinical care in medical oncology, and the intent of the guidelines is to assist in clinical decision making. Our affiliated providers strive to ensure that clinical pathways in our electronic health records system, as well as recommendations on use of chemotherapy and supportive care medications are consistent with NCCN guidelines to ensure patients receive the best clinical care based on their individual disease and comorbidities. Moreover, the TOI PCs operate physician dispensaries that allow our affiliated providers to prescribe and dispense oral oncolytics and related medications to patients, alongside chemotherapy infusion and injections. This provides patients with holistic and convenient access to the most appropriate treatment pathways, all in a community setting. According to a study conducted by researchers at Stanford University on the TOI PCs’ patients in 2019 who were enrolled in our HVCC program, we saw improvements in several key metrics, including:

Overall, the study demonstrated greater than 25% lower median total healthcare costs from diagnosis to death. We are continuously improving and innovating our care model, using the clinical data from the HVCC program to develop evidence-based care and treatment protocols for all patients.
Our Value Proposition and Differentiated Care Model
Our managed clinics primarily serve adult and senior cancer patients in markets that have MA plans and primary care medical groups reimbursed on a capitated basis. Our affiliated providers provide these services primarily through employed providers who are responsible for patient care. We intend to leverage our long-established, strong relationships with payors to continue to build out our network and increase access to cancer patients in adjacent markets, while at the same time, decreasing oncology care costs for both patients and payors. Through the TOI PCs, we seek to provide high quality and lower cost care delivery through the following capabilities:
•recruiting process focused on selecting physicians that want to practice evidence-based medicine;
•technology-enabled care pathways ensuring adherence to evidence-based clinical protocols;
•strong clinical culture and physician oversight;
•care management to prevent unnecessary hospitalizations;
•care delivered in community clinics vs. hospital setting;
•clinically appropriate integration of palliative care and hospice aligned with patients’ goals of care;
•access to clinical trials providing cutting-edge treatment options at low or no cost to patients or payors; and
•appropriate provider training on clinical documentation to ensure proper risk adjustment and reimbursement for complex patients.
We strive to add value by consistently performing these activities effectively. The goal is a lower cost of care for the same or better clinical outcomes while providing a superior patient experience.
Patient Experience
We believe our patient-centric focus facilitates high levels of patient satisfaction and supports our care delivery model while strengthening payor relationships. We employ a continuous feedback mechanism to ensure superior patient experience and satisfaction among our affiliated physicians and advanced practice providers.
As of a recent patient survey in 2022, 88% of our oncologists and 90% of our locations were rated 4.0 or above as measured by our survey, which we distribute to patients via text or e-mail following their clinic visit.
Growth Strategy and Opportunities
To date, revenue has grown at a roughly 26% CAGR from 2016 to 2023, driven by robust growth in capitated lives under value-based contracts.
Our footprint as of December 31, 2023 spanned five states and is growing rapidly.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| California | | Arizona | | Nevada | | Florida | | Texas |
| Markets | 7 | | 1 | | 1 | | 5 | | 1 |
Managed and Affiliated Clinics(1) | 62 | | 4 | | 4 | | 11 | | 2 |
| Providers | 99 | | 5 | | 4 | | 21 | | 2 |
(1) 69 clinics operated under the TOI PCs, whereby we receive a percentage of revenues under our management services agreements, or MSAs and are consolidated; and 14 independent oncology practice locations that are under MSAs for limited management and administrative services but do not bear any direct operating costs.
We anticipate adding more TOI PC clinics and other managed practices in the future across our markets through acquisitions and through de novo clinic builds, and we are in constant discussion with payors and providers to enter new markets. We continually seek to evaluate our growth strategy and may continue to modify it in the future, and there can be no assurance that we will be able to successfully capitalize on growth strategies.
Our go-to-market strategy focuses on both payors and providers. This blend is important given the increasing penetration of non-traditional payors, such as Oscar and Bright HealthCare, and primary care risk models such as Agilon health and ChenMed LLC.
We believe that our existing payor relationships provide us leads on opportunities to enter new markets, and we often receive outreach from new management services organizations, health plans and risk bearing organizations. When evaluating a new market, we consider three primary factors:
1.the penetration and growth of Medicare Advantage and other value-based reimbursement models;
2.the presence of value-based primary care groups with whom we can partner to generate referrals and manage outcomes; and
3.how well oncology spend is currently managed in that market.
We believe that new markets we are focused on meet all of the above criteria and could provide us with significant opportunity to create value for patients, providers and payors.
We have multiple strategies we believe can achieve long term growth.
•Existing Market Contract Growth: Continue driving covered lives growth. Significant growth potential in existing markets can be achieved through expanding the scope of our services with existing partners and securing new contracts with new payors and independent practices. The addition of new de novo clinics and affiliated providers can drive additional growth. By continuing to build regional density in existing markets, we also have an opportunity to achieve efficiencies with increased scale.
•New Market Contract Growth: Our replicable operating model enables quick scaling in new markets. Oncology continues to be a key focus area for payors and providers, who are highly supportive of our entry into new markets. Our high priority markets have attractive market dynamics due to the high cost of oncology care in these geographies, the prevalence of risk-bearing organizations, and the presence of national payor partners with whom we collaborate in existing markets. Our initial approach to value-based contracting in new markets is likely to be in the form of gain share contracts These new contracts, which will enable us to work with payors and risk-taking providers as they continue their shift to value-based care, are likely to produce lower revenue and profitability in the initial period as compared to full capitation. Once payors and risk-bearing providers in these new markets become comfortable with our ability to generate savings and better outcomes, we believe these contracts are likely to shift to capitation.
•M&A Opportunities: Leveraging our existing pipeline and mergers and acquisition expertise can help us facilitate growth in both existing and new markets, allowing us to rapidly establish market presence. Once on-boarded, we can transition the affiliated practice to our value-based model, as well as expand and enhance the scope of services provided to patients by the affiliated practice, such as adding dispensary operations, managing clinical trials and access to our broad purchasing contracts. Independent oncologists continue to face multitude of challenges and our acquisition model offers a path for these oncologists to continue to practice in their community without the burdens of business building or administration, while at the same time working alongside a dynamic and growing organization at the forefront of value-based care. We look for acquisition targets where the practice is philosophically aligned with us in driving the shift to value-based care.
•Service Expansion: We can broaden scope and diversify service offerings, including ancillary and adjacent services focused on patient care and innovation and providing access to new oncology treatments being investigated in clinical trials that our affiliated practices manage. We have the potential to scale significantly faster with additional capital via
new oncologist on-boarding and training, further technology investments, investments in ancillaries, and strategic acquisitions. In Q4 2023, we opened our California-based pharmacy for specialty medication fills.
Contracting Overview
At a time when many FFS healthcare organizations have been struggling due to the decrease in service volumes, our value-based capitation payments have allowed us to maintain our level of member care and prioritize member safety by incentivizing the provision of care in the most appropriate setting.
We have focused our business on capitation arrangements and other types of value-based contracts, which we believe align provider incentives with both quality and efficiency of care. Under capitation arrangements, payors pay a fixed per member per month, or PMPM, amount for every plan member within a population assigned to us for oncology care.
Our affiliated providers are responsible for managing oncology care for this population based on a scope of medical services and drugs agreed upon by both parties. The PMPM rates for our capitation arrangements are determined based on our analysis of historical patient data and agreements with contractual partners. In new markets, this may require the TOI PCs to contract with both the health insurance company and their risk-bearing provider organization in order to service these members.
In addition to capitation-based arrangements, we continue to explore several other forms of value-based arrangements. Although many of these arrangements continue to be based on a FFS-based methodology, our affiliated providers are eligible to earn additional bonuses based on their ability to achieve oncology specific clinical and other quality of care based benchmarks. While these alternative value-based arrangements may not produce as much initial revenue on a PMPM basis as capitation, we believe this flexibility in contracting models will allow us to speed our expansion into new markets while preserving the value-based economics that are critical for our business’ growth and success.
Payor Relationships
Our ability to consistently attract patients across multiple geographic markets depends on our ability to contract with payors in each market. Depending on the market, payors can be delegated medical groups who are taking risk or insurance companies themselves. By opening clinics in locations where the TOI PCs currently manage the oncology care for a large number of insured Medicare, commercial and Medicaid members, we believe we are creating net benefits for payors, as our affiliated providers are able to reduce unnecessary costs and improve patient care and experience. This also allows us to benefit from the value-based offerings already established by payors in the market, therefore not requiring us to single-handedly drive patient growth. Some of the biggest and most respected names in healthcare contract with the TOI PCs to provide oncology care to their members, including Anthem, CareMore Health, Heritage Provider Network and Optum Care. More than half of our revenue in 2023 was generated from value-based contracts where payors have made our affiliated providers their preferred or exclusive oncology group.
While our relationships with payors are long-standing, we believe we have limited concentration risk as our largest customer by revenue in 2023 represented approximately 15% of our revenue.
Provider and Clinic Capacity Growth
Our primary driver for growth in provider and clinic capacity is to create network adequacy to service members from payors with whom we have capitated or other value-based arrangements. For each market we currently operate in or are considering entering, we do a detailed assessment of the existing market landscape and determine the optimal approach to create the capacity we need given our payor relationships and pipeline of contracts. We can achieve capacity growth through multiple avenues, including practice acquisitions and de novo clinics. Practice acquisitions offer an opportunity to gain scale and market presence rapidly, while de novo clinics allow us to build out our network in a highly capital efficient manner. We believe both approaches can work in tandem to achieve optimal scale, network presence and speed to market. In addition, we have an active recruitment pipeline for providers to join our network and help us both manage patient load and grow the patient base.
We believe we have built a robust and data-driven approach to acquisitions, with a dedicated team to identify, assess and integrate physician practices into our network, and a strong pipeline of targets in both existing and new markets. We have invested in resources to continually add to our pipeline.
Clinic Structure, Staffing and Network Design
We have a standard clinic design and approach to staffing that has been refined over many years. Managed clinics typically range from 2,000 to 3,000 square feet with 3-4 providers (physicians and advanced practice providers) per clinic. We have flexibility around clinic size to allow us to establish smaller clinics and part time staffing in areas where needed to ensure the
TOI PCs can meet network adequacy under existing payor contracts. We group our managed clinics in a similar geographic area into pods, with multiple pods in each market. We have operations teams managing our markets and pods allowing us to drive performance and scale efficiently.
Competition
The U.S. healthcare industry is generally highly competitive. We compete with large and medium-sized local and national providers of cancer care services, such as health system affiliated practices, for, among other things, contracts with payors, recruitment of physicians and other medical and non-medical personnel and patients. The closest competitors are traditional oncology physician practices, such as American Oncology Network, Inc., Florida Cancer Specialists & Research Institute, LLC, U.S. Oncology Network, Inc., and OneOncology, Inc. These organizations are predominantly reimbursed via FFS contracts, which we believe can often lead to over utilization of treatments that may be medically appropriate but often results in higher costs. Secondary competitors may include specialty benefit managers. These include companies such as AIM Specialty Health, eviCore Healthcare, Magellan Health, New Century Health, One Oncology, Thyme Care, and OncoHealth. These benefit managers seek to change provider behavior by reviewing and authorizing treatment requests. The benefit manager model can produce incremental improvement in utilization, but the benefit managers are often unable to achieve results comparable to managed healthcare practices like ours. Furthermore, the benefit manager model frequently results in an antagonistic relationship with physicians who are operating in a traditional FFS-based practice. We distinguish ourselves from other managed oncology practices and specialty benefit managers in our ability to align incentives across the care continuum, including physicians and payors in delivering high quality care at lower costs, and we believe there are currently no other value-based oncology management companies of meaningful scale in the U.S.
We believe the principal competitive factors for serving the healthcare market for Medicare beneficiaries include: patient experience, quality of care, health outcomes, total cost of care, brand identity and trust in that brand. We believe we compete favorably on all these factors.
Government Regulation
Regulatory Licensing, Accreditation and Certification
Many states, including California, require regulatory approval, including licensure, accreditation and certification before establishing certain types of clinics offering certain professional and ancillary services, including the services we offer. The operations of our managed clinics are subject to extensive federal, state and local regulation relating to, among other things, the adequacy of medical care, equipment, personnel, operating policies and procedures, dispensing of prescription drugs, fire prevention, rate-setting and compliance with building codes and environmental protection. Our ability to operate profitably will depend in part on the ability of our managed clinics and doctors to obtain and maintain all necessary licenses, accreditation and other approvals, and maintain updates to their enrollment in the Medicare and Medicaid programs, including the addition of new clinic locations, providers and other enrollment information. In addition, certain ancillary services such as the provision of diagnostic laboratory testing require additional state and federal licensure and regulatory oversight, including oversight by CMS, under Clinical Laboratory Improvement Amendments of 1988, which requires all clinical laboratories to meet certain quality assurance, quality control and personnel standards, and comparable state laboratory licensing authorities, including for example, the California Department of Public Health. Our dispensary operations must also comply with applicable laws. Sanctions for failure to comply with applicable state and federal licensing, accreditation, certification and other regulatory requirements include suspension, revocation or limitation of the applicable authorization, significant fines and penalties and/or an inability to receive reimbursement from government healthcare programs and other third-party payors.
State Corporate Practice of Medicine and Fee-Splitting Laws
Our arrangements with the TOI PCs are subject to various state laws, including California, commonly referred to as corporate practice of medicine and fee-splitting laws, which are intended to prevent unlicensed persons from interfering with or influencing the physician’s professional judgment, and prohibiting the sharing of professional service fees with non-professional or business interests. These laws vary from state to state and are subject to broad interpretation and enforcement by state regulators. A determination of non-compliance against us and/or the TOI PCs could lead to adverse judicial or administrative action, civil or criminal penalties, receipt of cease and desist orders from state regulators, loss of provider licenses, and/or restructuring of these arrangements.
Healthcare Fraud and Abuse Laws
We are subject to a number of federal and state healthcare regulatory laws that restrict certain business practices in the healthcare industry. These laws include, but are not limited to, federal and state anti- kickback, false claims, self-referral and other healthcare fraud and abuse laws.
The federal Anti-Kickback Statute, or AKS, prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving remuneration, directly or indirectly, in cash or kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under federal and state healthcare programs such as Medicare and Medicaid. A person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. The AKS includes statutory exceptions and regulatory safe harbors that protect certain arrangements. The AKS safe harbors for value-based arrangements require, among other things, that the arrangement does not induce a person or entity to reduce or limit medically necessary items or services furnished to any patient. Failure to meet the requirements of the safe harbor, however, does not render an arrangement illegal. Rather, the government may evaluate such arrangements on a case-by-case basis, taking into account all facts and circumstances, including the parties’ intent and the arrangement’s potential for abuse, and may be subject to greater scrutiny by enforcement agencies.
The Stark Law prohibits a physician who has a financial relationship, or who has an immediate family member who has a financial relationship, with entities providing Designated Health Services, or DHS, from referring Medicare and Medicaid patients to such entities for the furnishing of DHS, unless an exception applies. The Stark Law also prohibits the entity from billing for any such prohibited referral. Unlike the AKS, the Stark Law is violated if the financial arrangement does not meet an applicable exception, regardless of any intent by the parties to induce or reward referrals or the reasons for the financial relationship and the referral.
The Federal False Claims Act, or FCA, prohibits a person from knowingly presenting, or caused to be presented, a false or fraudulent request for payment from the federal government, or from making a false statement or using a false record to have a claim approved. The FCA further provides that a lawsuit thereunder may be initiated in the name of the United States by an individual, a “whistleblower,” who is an original source of the allegations. Moreover, the government may assert that a claim including items and services resulting from a violation of the AKS or the Stark Law constitutes a false or fraudulent claim for purposes of the civil False Claims Act. Penalties for a violation of the FCA include fines for each false claim, plus up to three times the amount of damages caused by each false claim.
Further, the Civil Monetary Penalties Statute authorizes the imposition of civil monetary penalties, assessments and exclusion against an individual or entity based on a variety of prohibited conduct, including, but not limited to offering remuneration to a federal health care program beneficiary that the individual or entity knows or should know is likely to influence the beneficiary to order or receive health care items or services from a particular provider.
Health Insurance Portability and Accountability Act of 1996 ("HIPAA") also established federal criminal statutes that prohibit, among other things, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private third-party payors, and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Similar to the AKS, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
Several states in which we operate have also adopted similar fraud and abuse laws as described above. The scope of these laws and the interpretations of them vary from state to state and are enforced by state courts and regulatory authorities, each with broad discretion. Some state fraud and abuse laws apply to items or services reimbursed by any payor, including patients and commercial insurers, not just those reimbursed by a federally funded healthcare program, including California's anti-kickback statutes and the Physician Ownership and Referral Act of 1993.
Violation of any of these laws or any other governmental regulations that apply may result in significant penalties, including, without limitation, administrative civil and criminal penalties, damages, disgorgement, fines, additional reporting requirements and compliance oversight obligations, contractual damages, the curtailment or restructuring of operations, exclusion from participation in governmental healthcare programs and/ or imprisonment.
Healthcare Reform
In the United States, there have been, and we expect there will continue to be, a number of legislative and regulatory changes to the healthcare system, many of which are intended to contain or reduce healthcare costs. By way of example, in the United States, the Affordable Care Act (“ACA”), substantially changed the way healthcare is financed by both governmental and private insurers. Since its enactment, there have been judicial, executive and Congressional challenges to certain aspects of the ACA. On June 17, 2021, the U.S. Supreme Court dismissed the most recent judicial challenge to the ACA without specifically ruling on the constitutionality of the ACA. Prior to the Supreme Court’s decision, President Biden issued an executive order initiating a special enrollment period from February 15, 2021 through August 15, 2021 for purposes of obtaining health insurance coverage through the ACA marketplace. The executive order also instructed certain governmental agencies to review and reconsider their existing policies and rules that limit access to healthcare. It is unclear how other
healthcare reform measures of the Biden administration or other efforts, if any, to challenge, repeal or replace the ACA will impact the ACA or our business.
In addition, other legislative changes have been proposed and adopted since the ACA was enacted. These changes included aggregate reductions to Medicare payments to providers of 2% per fiscal year, which went into effect on April 1, 2013 and, due to subsequent legislative amendments to the statute, will remain in effect through 2030, with the exception of a temporary suspension from May 1, 2020 through March 31, 2022 and a 1% payment reduction from April 1, 2022 to June 30, 2022, unless additional Congressional action is taken. In addition, on January 2, 2013, the American Taxpayer Relief Act of 2012 was signed into law, which, among other things, reduced Medicare payments to several providers, including hospitals, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years.
CMS, through the Centers for Medicare and Medicaid Innovation, or CMMI, has implemented or has announced plans to implement numerous demonstration models designed to test value-based reimbursement models, some of which are specifically focused on oncology services. For example, in 2016, CMS initiated the Oncology Care Model demonstration, which continued throughout 2023, and provides participating physician practices, including the TOI PCs that participate in this reportprogram, with performance-based financial incentives that aim to “we,” “us”manage or reduce Medicare costs without negatively affecting the efficacy of care. In late 2019, CMS issued a request for information on the Oncology Care First model, a new voluntary model that, if implemented, would build on the Oncology Care Model. More recently, CMMI has announced plans to implement the Radiation Oncology Model, which would require radiotherapy providers in certain regions to participate in a prospective, episode-based payments model where payment is based on a patient’s diagnosis as opposed to the traditional volume-based FFS payment model. Although the Radiation Oncology Model was originally intended to begin on January 1, 2022, recent legislation delayed its implementation until July 1, 2023 and therefore, the impact of this new model has yet to determined. There likely will continue to be regulatory proposals directed at containing or lowering the cost of healthcare. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue or attain growth, any of which could have a material impact on our business.
Further, healthcare providers and industry participants are also subject to a growing number of requirements intended to promote the interoperability and exchange of patient health information. For example, on April 5, 2021, healthcare providers and certain other entities became subject to information blocking restrictions pursuant to the Cures Act that prohibit practices that are likely to interfere with the access, exchange or use of electronic health information ("EHI"), except as required by law or specified by HHS as a reasonable and necessary activity.
Violations may result in penalties or other disincentives. It is unclear at this time what the costs of compliance with the new rules will be, and what additional risks there may be to our business.
Federal and State Insurance and Managed Care Laws
Regulation of downstream risk-sharing arrangements, including, but not limited to, global risk and other value-based arrangements, varies significantly from state to state. Some states require downstream entities and Risk Bearing Organization ("RBOs") to obtain an insurance license, a certificate of authority, or an equivalent authorization, in order to participate in downstream risk-sharing arrangements with payors. In some states, statutes, regulations and/or formal guidance explicitly address whether and in what manner the state regulates the transfer of risk by a payor to a downstream entity. However, the majority of states do not explicitly address the issue, and in such states, regulators may nonetheless interpret statutes and regulations to regulate such activity. If downstream risk-sharing arrangements are not regulated directly in a particular state, the state regulatory agency may nonetheless require oversight by the licensed payor as the party to such a downstream risk-sharing arrangement. Such oversight is accomplished via contract and may include the imposition of reserve requirements, as well as reporting obligations. Further, state regulatory stances regarding downstream risk-sharing arrangements can change rapidly and codified provisions may not keep pace with evolving risk-sharing mechanisms and other new value-based reimbursement models. Certain of the states where we currently operate or may choose to operate in the future regulate the operations and financial condition of RBOs like us and our affiliated providers. These regulations can include capital requirements, licensing or certification, governance controls and other similar matters. While these regulations have not had a material impact on our business to date, as we continue to expand, these rules may require additional resources and capitalization and add complexity to our business.
Privacy and Security
Numerous state, federal and foreign laws, including consumer protection laws and regulations, govern the collection, dissemination, use, access to, confidentiality and security of personal information, including health-related information. In the United States, numerous federal and state laws and regulations, including data breach notification laws, health information privacy and security laws, including HIPAA, and federal and state consumer protection laws and regulations (e.g., Section 5 of
the Federal Trade Commission ["FTC"] Act), that govern the collection, use, disclosure, and protection of health-related and other personal information could apply to our operations or the “Company” referoperations of the TOI PCs.
In addition, certain state laws, such as the California Consumer Privacy Act, or the CCPA and the California Privacy Rights Act of 2020, or the CPRA, govern the privacy and security of personal information, including health-related information in certain circumstances, some of which are more stringent than HIPAA and many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts. Failure to comply with these laws, where applicable, can result in the imposition of significant civil and/or criminal penalties and private litigation. Privacy and security laws, regulations, and other obligations are constantly evolving, may conflict with each other to complicate compliance efforts, and can result in investigations, proceedings, or actions that lead to significant civil and/or criminal penalties and restrictions on data processing.
Intellectual Property
At present, we own no material intellectual property.
Insurance
We maintain insurance, excess coverage, or reinsurance for property and general liability, professional liability, directors’ and officers’ liability, workers’ compensation, cybersecurity and other coverage in amounts and on terms deemed adequate by management, based on our actual claims experience and expectations for future claims. Future claims could, however, exceed our applicable insurance coverage.
Employees and Human Capital Resources
As of December 31, 2023, we and TOI PCs collectively had approximately 800 employees, including approximately 119 oncologists and advanced practice providers. We consider our relationship with our employees to be good. None of our employees are represented by a labor union or party to a collective bargaining agreement.
Our goal is to provide top quality oncology care to our patients, and we view our human capital-related initiatives as essential to continuing to reach that goal. Such initiatives include: (i) implementing a robust talent acquisition approach, including through competitive pay and benefits, (ii) implementing programs to promote diversity and foster a sense of connection and community throughout our company, (iii) offering an array of opportunities for learning and development opportunities, and (iv) conducting annual employee engagement surveys and developing action plans based on the survey outcomes.
Availability of Information
We were originally incorporated in Delaware on November 19, 2019 as a special purpose acquisition company (f/k/a DFP Healthcare Acquisition Corp.). In November 2021, we consummated our business combination with TOI Parent, Inc. (the “Business Combination”). In connection with the closing of the Business Combination, TOI Parent, Inc. became our wholly owned subsidiary and we changed our name to The Oncology Institute, Inc. (formerly, DFP Healthcare Acquisitions Corp.) References to our “management”We file or our “management team” refer to our executive officersfurnish annual, quarterly and directors,current reports, proxy statements and references to the “Sponsor” refer to DFP Sponsor LLC, a Delaware limited liability company. References to our “initial stockholders” refer to the Sponsor and the Company’s executive officers and independent directors.
ITEM 1: BUSINESS.
Introduction
We are a blank check company incorporated on November 1, 2019 as a Delaware corporation formed for the purpose of effecting a merger, share exchange, asset acquisition, share purchase, reorganization or similar business combination with one or more businesses. We have neither engaged in any operations nor generated any revenue to date. Based on our business activities, the Company is a “shell company” as defined under the Exchange Act of 1934 (the “Exchange Act”) because we have no operations and nominal assets consisting almost entirely of cash.
We were jointly founded by our management team and Deerfield Management Company, L.P. (“Deerfield Management”), a healthcare investment firm with over $10.5 billion in regulatory assets under management as of March 31, 2020. We believe that the experience of our management team and our relationship with Deerfield Management will allow us to source, identify and execute an attractive transaction for our stockholders.
On March 13, 2020, we consummated our initial public offering (the “initial public offering”) of 23,000,000 units, including the issuance of 3,000,000 units as a result of the underwriters’ exercise of their over-allotment option in full. Each unit consists of one share of Class A common stock and one-fourth of one warrant. Each whole warrant entitles the holder thereof to purchase one share of Class A common stock at a price of $11.50 per share. The units were sold at an offering price of $10.00 per unit, generating gross proceeds, before expenses, of $230,000,000.
Prior to the consummation of the initial public offering, on December 30, 2019, our sponsor, DFP Sponsor LLC (the “Sponsor”), received 4,312,500 shares of Class B common stock (the “founder shares”) in exchange for a capital contribution of $25,000, or $.006 per share. In January 2020, the Sponsor transferred 100,000 founder shares to each of Mr. Steven Hochberg, Mr. Christopher Wolfe, and Mr. Richard Barasch, our executive officers, and 30,000 founder shares to each of Dr. Jennifer Carter, Dr. Mohit Kaushal and Dr. Gregory Sorensen, our independent directors, for the same per-share price initially paid by our sponsor, resulting in our sponsor holding 3,922,500 founder shares. On February 19, 2020, we effected a 1:1 1/3 stock split of Class B common stock resulting in the Sponsor holding an aggregate of 5,360,000 founder shares, resulting in an increase in the total number of founder shares from 4,312,500 to 5,750,000. The number of founder shares outstanding was determined so that such founder shares would represent 20% of the outstanding shares after the initial public offering.
Simultaneouslyother documents with the consummation of the initial public offering, we consummated the private sale of an aggregate of 3,733,334 warrants, each exercisable to purchase one share of Class A common stock at $11.50 per share, to the Sponsor at the time of the initial public offering at a price of $1.50 per warrant, generating gross proceeds, before expenses, of approximately $5,600,000 (the “Private Placement”). The warrants sold in the Private Placement, or the private placement warrants, are identical to the warrants included in the units sold in the initial public offering, except that, so long as they are held by the Sponsor or its permitted transferees, (i) they will not be redeemable by the Company, (ii) they (including the shares of Class A common stock issuable upon exercise of these warrants) may not, subject to certain limited exceptions, be transferred, assigned or sold until 30 days after the Company completes its initial business combination; (iii) they may be exercised by the holders on a cashless basis and (iv) they will be entitled to registration rights.
Upon the closing of the initial public offering and the Private Placement, $230,000,000 was placed in a Trust account with Continental Stock Transfer & Trust Company acting as trustee (the “Trust Account”). Except with respect to interest earned on the funds held in the Trust Account that may be released to the Company to pay its taxes, the funds held
in Trust Account will not be released from the Trust Account until the earliest of (i) the completion of our initial business combination, the redemption of any shares of Class A common stock included in the units sold in the initial public offering (“public shares”) properly submitted in connection with a stockholder vote to approve an amendment to our second amended and restated certificate of incorporation to modify the substance or timing of the Company’s obligation to redeem 100% of the public shares if the Company does not complete its initial business combination by March 13, 2022 or with respect to any other material provisions relating to stockholders’ rights or pre-initial business combination activity and (ii) the redemption of the public shares if the Company is unable to complete an initial business combination by March 13, 2022, subject to applicable law. The proceeds held in the Trust Account may only be invested in United States “government securities” within the meaning of Section 2(a)(16) of the Investment Company Act of 1940, as amended (the “Investment Company Act”), having a maturity of 180 days or less or in money market funds meeting certain conditions under Rule 2a-7 promulgated under the Investment Company Act which invest only in direct U.S. government treasury obligations.
The net proceeds deposited into the Trust Account remain on deposit in the Trust Account earning interest. As of December 31, 2020, there was approximately $230.0 million remaining in the Trust Account and approximately $1.5 million of cash held outside the Trust Account available for working capital purposes. As of December 31, 2020, no funds had been withdrawn from the Trust Account to fund the Company’s working capital expenses. As of December 31, 2020, approximately $43,000 of interest income held within the Trust Account is available to pay for franchise and income taxes.
Effecting Our Initial Business Combination
General
We are not presently engaged in, and we will not engage in, any operations for an indefinite period of time. We intend to effectuate our initial business combination using cash from the proceeds of the initial public offering and the Private Placement, the proceeds of the sale of our shares in connection with our initial business combination (pursuant to forward purchase agreements or backstop agreements we may enter into following the consummation of the initial public offering or otherwise), shares issued to the owners of the targets, debt issued to bank or other lenders or the owners of the targets, or a combination of the foregoing. We may seek to complete our initial business combination with a company or business that may be financially unstable or in its early stages of development or growth, which would subject us to the numerous risks inherent in such companies and businesses.
If our initial business combination is paid for using equity or debt securities, or not all of the funds released from the Trust Account are used for payment of the consideration in connection with our initial business combination or used for redemptions of our Class A common stock, we may apply the balance of the cash released to us from the Trust Account for general corporate purposes, including for maintenance or expansion of operations of the post- transaction company, the payment of principal or interest due on indebtedness incurred in completing our initial business combination, to fund the purchase of other companies or for working capital.
Selection of Target Businesses
While we may pursue an initial business combination opportunity in any business, industry, sector or geographical location, we intend to focus on industries that complement our management team’s background, and to capitalize on the ability of our management team to identify and acquire a business, focusing on the healthcare or healthcare-related industries. Our strategy will be to identify, acquire and, after our initial business combination, build, a healthcare or healthcare-related business. We intend to focus our investment effort broadly across the entire healthcare industry, which encompasses services, therapeutics, devices, diagnostics and animal health. We have entered into an agreement with Deerfield Management pursuant to which we have agreed not to complete a business combination without their consent, which consent Deerfield Management has indicated it does not intend to provide if the proposed business combination is with a target that is not in the healthcare industry.
Our initial business combination must occur with one or more target businesses that together have an aggregate fair market value of at least 80% of the assets held in the Trust Account (excluding the deferred underwriting commissions and taxes payable on the interest earned on the trust account) at the time of our signing a definitive agreement in connection with our initial business combination. Our board of directors will make the determination as to the fair market value of our
initial business combination. If our board of directors is not able to independently determine the fair market value of our initial business combination, we will obtain an opinion from an independent investment banking firm which is a member of FINRA or a valuation or appraisal firm with respect to the satisfaction of such criteria. While we consider it unlikely that our board of directors will not be able to make an independent determination of the fair market value, If our board of directors is not able to independently determine the fair market value of our initial business combination, we will obtain an opinion from an independent investment banking firm which is a member of FINRA or a valuation or appraisal firm with respect to the satisfaction of such criteria. While we consider it unlikely that our board of directors will not be able to make an independent determination of the fair market value of our initial business combination, it may be unable to do so if it is less familiar or experienced with the business of a particular target or if there is a significant amount of uncertainty as to the value of the target’s assets or prospects.
We may pursue an initial business combination opportunity jointly with our sponsor, Deerfield Management or one or more of its affiliates, one or more of the domestic private pooled investment vehicles managed by Deerfield Management and its affiliates (the “Deerfield Funds”) and/or investors in the Deerfield Funds, which we refer to as an Affiliated Joint Acquisition. Any such parties may co-invest with us in the target business at the time of our initial business combination, or we could raise additional proceeds to complete the acquisition by issuing to such parties a class of equity or equity-linked securities. Any such issuance of equity or equity-linked securities would, on a fully diluted basis, reduce the percentage ownership of our then-existing stockholders. Notwithstanding the foregoing, pursuant to the anti-dilution provisions of our Class B common stock, issuances or deemed issuances of Class A common stock or equity-linked securities would result in an adjustment to the ratio at which shares of Class B common stock shall convert into shares of Class A common stock such that our initial stockholders and their permitted transferees, if any, would retain their aggregate percentage ownership at 20% of the sum of the total number of all shares of common stock outstanding upon completion of this offering plus all shares of Class A common stock and equity-linked securities issued or deemed issued in connection with the business combination (excluding any shares or equity-linked securities issued, or to be issued, to any seller in the business combination), unless the holders of a majority of the then-outstanding shares of Class B common stock agree to waive such adjustment with respect to such issuance or deemed issuance at the time thereof. Neither our sponsor, nor Deerfield Management, nor any of the Deerfield Funds, nor any of their respective affiliates, has an obligation to make any such investment, and may compete with us for potential business combinations.
We anticipate structuring our initial business combination so that the post-transaction company in which our public stockholders own shares will own or acquire 100% of the equity interests or assets of the target business or businesses. We may, however, structure our initial business combination such that the post-transaction company owns or acquires less than 100% of such interests or assets of the target business in order to meet certain objectives of the target management team or stockholders or for other reasons, including an Affiliated Joint Acquisition, as described above. However, we will only complete such business combination if the post-transaction company owns or acquires 50% or more of the outstanding voting securities of the target or otherwise acquires a controlling interest in the target sufficient for it not to be required to register as an investment company under the Investment Company Act of 1940, as amended, or the Investment Company Act. Even if the post-transaction company owns or acquires 50% or more of the voting securities of the target, our stockholders prior to the business combination may collectively own a minority interest in the post-transaction company, depending on valuations ascribed to the target and us in the business combination transaction. For example, we could pursue a transaction in which we issue a substantial number of new shares in exchange for all of the outstanding capital stock of a target. In this case, we would acquire a 100% controlling interest in the target. However, as a result of the issuance of a substantial number of new shares, our stockholders immediately prior to our initial business combination could own less than a majority of our outstanding shares subsequent to our initial business combination. If less than 100% of the equity interests or assets of a target business or businesses are owned or acquired by the post-transaction company, the portion of such business or businesses that is owned or acquired is what will be taken into account for purposes of the 80% of net assets test described above. If the business combination involves more than one target business, the 80% of net assets test will be based on the aggregate value of all of the target businesses even if the acquisitions of the target businesses are not closed simultaneously.
Redemption Rights for Holders of Public Shares Upon Consummation of Our Initial Business Combination
We will provide our public stockholders with the opportunity to redeem all or a portion of their public shares upon the completion of our initial business combination at a per-share price, payable in cash, equal to the aggregate amount
then on deposit in the trust account calculated as of two business days prior to the consummation of our initial business combination, including interest earned on the funds held in the trust account and not previously released to us to fund our working capital requirements (subject to an annual limit of $500,000) and/or to pay our taxes, divided by the number of then outstanding public shares, subject to the limitations and on the conditions described herein. There will be no redemption rights upon the completion of our initial business combination with respect to our warrants. Our initial stockholders, sponsor, officers and directors have entered into a letter agreement with us, pursuant to which they have agreed to waive their redemption rights with respect to any founder shares they hold and any public shares they may acquire during or after the initial public offering in connection with the completion of our initial business combination.
Conduct of Redemptions Pursuant to Tender Offer Rules
If we conduct redemptions pursuant to the tender offer rules of the U.S. Securities and Exchange Commission (the “SEC”), we will, pursuant to our second amended and restated certificate of incorporation: (a) conduct under the redemptions pursuant to Rule 13e-4 and Regulation 14E of theSecurities Exchange Act which regulate issuer tender offers; and (b) file tender offer documents withof 1934 (as amended, the SEC prior to completing our initial business combination which contain substantially the same financial and other information about the initial business combination and the redemption rights as is required under Regulation 14A of the Exchange Act, which regulates the solicitation of proxies.
Submission of Our Initial Business Combination to a Stockholder Vote
If we seek stockholder approval, we will complete our initial business combination only if a majority of the outstanding shares of common stock voted are voted in favor of the initial business combination. A quorum for such meeting will consist of the holders present in person or by proxy of shares of outstanding capital stock of the Company representing a majority of the voting power of all outstanding shares of capital stock of the Company entitled to vote at such meeting. Our initial stockholders will count towards this quorum and, pursuant to the letter agreement, our sponsor, officers and directors have agreed to vote any founder shares they hold and any public shares purchased during or after the initial public offering (including in open market and privately-negotiated transactions) in favor of our initial business combination. Additionally, the Deerfield Funds have agreed to vote the public shares underlying the units that they purchased in the initial public offering in favor of our initial business combination, subject to Deerfield Management’s consent right with respect to our initial business combination. These quorum and voting thresholds, and the voting agreements of our initial stockholders, may make it more likely that we will consummate our initial business combination. Each public stockholder may elect to redeem its public shares irrespective of whether they vote for or against the proposed transaction or whether they were a stockholder on the record date for the stockholder meeting held to approve the proposed transaction.
If we seek stockholder approval of our initial business combination and we do not conduct redemptions in connection with our initial business combination pursuant to the tender offer rules, our sponsor, initial stockholders, directors, executive officers, advisors or their affiliates may purchase shares or public warrants in privately negotiated transactions or in the open market either prior to or following the completion of our initial business combination. There is no limit on the number of shares our initial stockholders, directors, officers, advisors or their affiliates may purchase in such transactions, subject to compliance with applicable law and Nasdaq rules. However, other than as expressly stated herein, they have no current commitments, plans or intentions to engage in such transactions and have not formulated any terms or conditions for any such transactions. None of the funds held in the trust account will be used to purchase shares or public warrants in such transactions. If they engage in such transactions, they will be restricted from making any such purchases when they are in possession of any material nonpublic information not disclosed to the seller or if such purchases are prohibited by Regulation M under the Exchange Act. We do not currently anticipate that such purchases, if any, would constitute a tender offer subject to the tender offer rules under the Exchange Act or a going-private transaction subject to the going-private rules under the Exchange Act; however, if the purchasers determine at the time of any such purchases that the purchases are subject to such rules, the purchasers will comply with such rules.
The purpose of any such purchases of shares could be to vote such shares in favor of the business combination and thereby increase the likelihood of obtaining stockholder approval of the business combination or to satisfy a closing condition in an agreement with a target that requires us to have a minimum net worth or a certain amount of cash at the closing of our initial business combination, where it appears that such requirement would otherwise not be met. The
purpose of any such purchases of public warrants could be to reduce the number of public warrants outstanding or to vote such warrants on any matters submitted to the warrantholders for approval in connection with our initial business combination. Any such purchases of our securities may result in the completion of our initial business combination that may not otherwise have been possible. In addition, if such purchases are made, the public “float” of our Class A common stock or warrants may be reduced and the number of beneficial holders of our securities may be reduced, which may make it difficult to maintain or obtain the quotation, listing or trading of our securities on a national securities exchange.
Limitation on Redemption Rights Upon Completion of Our Initial Business Combination If We Seek Stockholder Approval
Notwithstanding the foregoing redemption rights, if we seek stockholder approval of our initial business combination and we do not conduct redemptions in connection with our initial business combination pursuant to the tender offer rules, our second amended and restated certificate of incorporation provides that a public stockholder, together with any affiliate of such stockholder or any other person with whom such stockholder is acting in concert or as a “group” (as defined under Section 13 of the Exchange Act), will be restricted from seeking redemption rights with respect to more than an aggregate of 20% of the shares sold in the initial public offering without our prior consent (the “Excess Shares”"Exchange Act"). We believe this restriction will discourage stockholders from accumulating large blocks of shares, and subsequent attempts by such holders to use their ability to exercise their redemption rights against a proposed business combination as a means to force us or our management to purchase their shares at a significant premium to the then-current market price or on other undesirable terms. Absent this provision, a public stockholder holding more than an aggregate of 20% of the shares sold in the initial public offering could threaten to exercise its redemption rights if such holder’s shares are not purchased by us, our sponsor or our management at a premium to the then-current market price or on other undesirable terms. By limiting our stockholders’ ability to redeem no more than 20% of the shares sold in the initial public offering without our prior consent, we believe we will limit the ability of a small group of stockholders to unreasonably attempt to block our ability to complete our initial business combination, particularly in connection with a business combination with a target that requires as a closing condition that we have a minimum net worth or a certain amount of cash. However, we would not be restricting our stockholders’ ability to vote all of their shares (including Excess Shares) for or against our initial business combination.
Redemption of Public Shares and Liquidation If No Initial Business Combination
Our second amended and restated certificate of incorporation provides that we will have only until March 13, 2022 to complete our initial business combination. If we do not complete our initial business combination within such 24-month period, we will: (i) cease all operations except for the purpose of winding up, (ii) as promptly as reasonably possible but not more than ten business days thereafter, redeem the public shares, at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the trust account, including interest earned on the funds held in the trust account and not previously released to us to fund our working capital requirements (subject to an annual limit of $500,000) (less taxes payable and up to $100,000 of interest to pay dissolution expenses), divided by the number of then outstanding public shares, which redemption will completely extinguish public stockholders’ rights as stockholders (including the right to receive further liquidating distributions, if any), and (iii) as promptly as reasonably possible following such redemption, subject to the approval of our remaining stockholders and our board of directors, liquidate and dissolve, subject in each case to our obligations under Delaware law to provide for claims of creditors and the requirements of other applicable law. There will be no redemption rights or liquidating distributions with respect to our warrants, which will expire worthless if we fail to complete our initial business combination by March 13, 2022.
Competition
In identifying, evaluating and selecting a target business for our initial business combination, we may encounter competition from other entities having a business objective similar to ours, including other special purpose acquisition companies, private equity groups and leveraged buyout funds, public companies and operating businesses seeking strategic acquisitions. Many of these entities are well established and have extensive experience identifying and effecting business combinations directly or through affiliates. Moreover, many of these competitors possess greater financial, technical, human and other resources than us. Our ability to acquire larger target businesses will be limited by our available financial resources. This inherent limitation gives others an advantage in pursuing the acquisition of a target business. Furthermore,
our obligation to pay cash in connection with our public stockholders who exercise their redemption rights may reduce the resources available to us for our initial business combination and our outstanding warrants, and the future dilution they potentially represent, may not be viewed favorably by certain target businesses. Either of these factors may place us at a competitive disadvantage in successfully negotiating an initial business combination.
Employees
We currently have three executive officers: Richard Barasch, Steven Hochberg and Christopher Wolfe. These individuals are not obligated to devote any specific number of hours to our matters but they intend to devote as much of their time as they deem necessary to our affairs until we have completed our initial business combination. The amount of time they will devote in any time period will vary based on whether a target business has been selected for our initial business combination and the stage of the business combination process we are in. We do not intend to have any full time employees prior to the completion of our initial business combination.
Available Information
We are required to file Annual Reports on Form 10-K and Quarterly Reports on Form 10-Q with the SEC on a regular basis, and are required to disclose certain material events (e.g., changes in corporate control, acquisitions or dispositions of a significant amount of assets other than in the ordinary course of business and bankruptcy) in a Current Report on Form 8-K. The SEC maintains an Internetinternet website at www.sec.gov that contains reports, proxy and information statements and other information regarding issuers, including us, that file electronically with the SEC. The SEC’s Internet
We also make available free of charge through our website, https://investors.theoncologyinstitute.com/, electronic copies of certain documents that we file with the SEC, including our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. Information on our website or any other website is located at http://www.sec.gov. In addition, the Company will provide copiesnot incorporated by reference into, and does not constitute a part of, these documents without charge upon request from usthis Annual Report.
Item 1A. Risk Factors
We operate in
writing at 345 Park Avenue South, New York, New York 10010 or by telephone at (212) 551-1600.ITEM 1A. RISK FACTORS.
An investment in our securitiesa rapidly changing environment that involves a high degreenumber of risk.risks. Our operations and financial results are subject to various risks and uncertainties including those described below. You should consider carefully all of the risks and uncertainties described below, together with thein addition to other information contained in this Annual Report on Form 10-K/A,10-K, including our consolidated financial statements and related notes, as well as our other public filings with the prospectus associated withSecurities and Exchange Commission. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties that we are unaware of, or that we currently believe are not material, may also become important factors that adversely affect our initial public offering and the registration statement of which such prospectus forms a part, before making a decision to invest in our securities.
business. If any of the following events occur,risks or others not specified below materialize, our business, financial condition and operating results mayof operations could be materially adversely affected. In that event,case, the trading price of our securitiescommon stock could decline,decline.
SUMMARY RISK FACTORS
The following is a summary of select risks and
youuncertainties that could
lose allmaterially adversely affect The Oncology Institute, Inc. ("TOI", "we", or
part"our") and its business, financial condition and results of
your investment.Risk Factor Summary
| ● | We have identified a material weakness in our internal control over financial reporting. This material weakness could continue to adversely affect our ability to report our results of operations and financial condition accurately and in a timely manner. |
| ● | We are a blank check company with no operating history and no revenues, and you have no basis on which to evaluate our ability to achieve our business objective. |
| ● | Our stockholders may not be afforded an opportunity to vote on our proposed initial business combination, and even if we hold a vote, holders of our founder shares will participate in such vote, which means we may complete our initial business combination even though a majority of our public stockholders do not support such a combination. |
| ● | Your only opportunity to affect the investment decision regarding a potential business combination may be limited to the exercise of your right to redeem your shares from us for cash. |
| ● | If we seek stockholder approval of our initial business combination, our initial stockholders and management team have agreed to vote in favor of such initial business combination, regardless of how our public stockholders vote. |
operations. You should read this summary together with the full and complete discussion of risk factors contained below: •Our growth strategy depends on our ability to build or acquire clinics to service our contracts and treat our patients.•We have experienced, and may continue to experience, rapid growth and organizational change, which has placed, and may continue to place, significant demands on our management and our operational and financial resources.
| ● | The ability of our public stockholders to redeem their shares for cash may make our financial condition unattractive to potential business combination targets, which may make it difficult for us to enter into a business combination with a target. |
| ● | The ability of our public stockholders to exercise redemption rights with respect to a large number of our shares may not allow us to complete the most desirable business combination or optimize our capital structure. |
| ● | The requirement that we complete our initial business combination by March 13, 2022, may give potential target businesses leverage over us in negotiating a business combination and may limit the time we have in which to conduct due diligence on potential business combination targets, in particular as we approach our dissolution deadline, which could undermine our ability to complete our initial business combination on terms that would produce value for our stockholders. |
| ● | Our search for a business combination, and any target business with which we ultimately consummate a business combination, may be materially adversely affected by the coronavirus (COVID-19) outbreak and the status of debt and equity markets. |
| ● | We may not be able to complete our initial business combination by March 13, 2022, in which case we would cease all operations except for the purpose of winding upin the future, and we would redeem our public shares and liquidate. |
| ● | If we seek stockholder approval of our initial business combination, the Sponsor, initial stockholders, directors, executive officers, advisors and their affiliates may elect to purchase shares or public warrants from public stockholders, which may influence a vote on a proposed business combination and reduce the public “float” of our Class A common stock. |
| ● | If a stockholder fails to receive notice of our offer to redeem our public shares in connection with our initial business combination or fails to comply with the procedures for submitting or tendering its shares, such shares may not be redeemed. |
| ● | You will not have any rights or interests in funds from the trust account, except under certain limited circumstances. Therefore, to liquidate your investment, you may be forced to sell your public shares or warrants, potentially at a loss. |
| ● | Nasdaq may delist our securities from trading on its exchange, which could limit investors’ ability to make transactions in our securities and subject us to additional trading restrictions. |
| ● | You will not be entitled to protections normally afforded to investors of many other blank check companies. |
| ● | Because of our limited resources and the significant competition for business combination opportunities, it may be more difficult for us to complete our initial business combination. If we are unable to complete our initial business combination, our public stockholders may receive only their pro rata portion of the funds in the trust account that are available for distribution to public stockholders, and our warrants will expire worthless. |
| ● | If the net proceeds of the initial public offering not being held in the trust account are insufficient to allow us to operate for at least until March 13, 2022, it could limit the amount available to fund our search for a target business or businesses and complete our initial business combination, and we will depend on loans from the Sponsor or management team to fund our search and to complete our initial business combination. |
| ● | Past performance by our management team and their affiliates may not be indicative of future performance of an investment in us. |
| ● | Unlike some other similarly structured special purpose acquisition companies, our initial stockholders will receive additional Class A common stock if we issue certain shares to consummate an initial business combination. |
Risks Relating to our Search for, Consummation of, or Inability to Consummate, a Business Combination and Post-Business Combination Risks
Our stockholders may not be affordedable to achieve or maintain profitability.
•A pandemic, epidemic or outbreak of an opportunityinfectious disease in the United States or worldwide, including the current COVID-19 epidemic, could adversely affect our business.
•Our services are concentrated in certain geographic areas and populations exposing us to voteunfavorable changes in local benefit costs, reimbursement rates, competition and economic conditions.
•If we are unable to attract new patients, our revenue growth will be adversely affected.
•We primarily depend on reimbursement from third-party payors, as well as payments by individuals, which could lead to delays, denials, or uncertainties in the reimbursement process.
•With many of our value-based agreements, our consolidating professional corporations ("TOI PCs") assume the risk that the cost of providing services will exceed our compensation. As oncology costs rise, if we do not accurately predict the cost to deliver care, some of the TOI PCs’ value-based agreements could become less profitable, or unprofitable.
•There are significant risks associated with estimating the amount of revenue that is recognized under TOI PCs’ risk agreements with health plans, and if our estimates of revenue are materially inaccurate, it could impact the timing and the amount of our revenue recognition or have a material adverse effect on our proposed initial business, combination,results of operations, financial condition and evencash flows.
•A significant portion of our consolidated Patient Services revenue is derived from a limited number of health insurance, Independent Practice Associations, or IPAs and medical group companies. Those payors could take action to remove, exclude, delay, or otherwise prevent the inclusion of the TOI PCs in their provider networks.
•A significant portion of sales are from prescription drug sales reimbursed by a number of pharmacy benefit management companies with which TOI PCs contract. Those pharmacy benefit management companies could take action to remove, exclude, delay or otherwise prevent the inclusion of the TOI PCs in their provider networks.
•Reductions in Medicare reimbursement rates or changes in the rules governing the Medicare program could have a material adverse effect on our financial condition and results of operations.
•We cannot predict the effect that health care reform and other changes in government programs may have on our business, financial condition or results of operations.
•Inflation can adversely affect us by increasing the costs of drugs, clinical trials and research, administration and other costs of doing business.
•The transition from volume to value-based reimbursement models may have a material adverse effect on our operations.
•Changes in the payor mix of patients and potential decreases in reimbursement rates as a result of consolidation among our customers could adversely affect our revenues and results of operations.
•We face significant competition from other healthcare services providers. Our failure to adequately compete could adversely affect our business.
•Competition for physicians and clinical personnel, including nurses, shortages of qualified personnel or other factors could increase our labor costs and adversely affect our revenue, growth rate, profitability and cash flows.
•Because competition for qualified personnel is intense, we may not be able to attract and retain the highly skilled employees we need to execute our business strategies and growth plans.
•If we are unable to provide consistently high quality of care, our business will be adversely impacted.
•If certain of our suppliers do not meet our needs, if there are material price increases on supplies, if we holdare not reimbursed or adequately reimbursed for drugs we purchase or if we are unable to effectively access new technology or superior products, it could negatively impact our ability to effectively provide the services we offer and could have a vote, holdersmaterial adverse effect on our business, results of operations, financial condition and cash flows.
•We depend on our information technology systems, and those of our founder sharesthird-party vendors, contractors and consultants, and any failure or significant disruptions of these systems, security breaches or loss of data could materially adversely affect our business, financial condition and results of operations.
•We may be subject to legal proceedings and litigation, including intellectual property and privacy disputes, which are costly to defend and could materially harm our business and results of operations.
•Some jurisdictions preclude the TOI PCs from entering into non-compete agreements with our physicians, and other non-compete agreements and restrictive covenants applicable to certain physicians and other clinical employees may not be enforceable.
•Current and future acquisitions may use significant resources, may be unsuccessful, and could expose us to unforeseen liabilities.
•We conduct some clinical trials in contract with TOI Clinical Research, LLC ("TCR"). If we fail to perform our clinical trial services in accordance with contractual requirements, government regulations and ethical considerations, we could be subject to significant costs or liability and our reputation could be adversely affected.
•We are dependent on our relationships with the TOI PCs, which are affiliated professional entities that we do not own, to provide healthcare services, and our business would be harmed if those relationships were disrupted or if our arrangements with the TOI PCs become subject to legal challenges.
•Our managed clinics and the TOI PCs providing professional services at such clinics may become subject to medical liability claims, which could have a material adverse impact on our business.
•If there is a change in accounting standards by the Financial Accounting Standards Board or the interpretation thereof affecting consolidation of entities, it could have a material adverse effect on our consolidation of total revenues derived from the TOI PCs.
•Our managed clinics and the TOI PCs may be subject to third-party payor audits, which, if adversely determined against us or the TOI PCs, may have a material effect on our results of operations and financial condition.
•We are subject to extensive fraud, waste, and abuse laws that may give rise to federal and state audits, investigations, lawsuits and claims against us, the outcome of which may have a material adverse effect on our business.
•If any of our managed clinics or TOI PCs lose their regulatory licenses, permits and/or accreditation status, or become ineligible to receive reimbursement under Medicare or Medicaid or other third-party payors, there may be a material adverse effect on our business, financial conditions, cash flows or results of operations.
•If we or the TOI PCs fail to comply with applicable data interoperability and information blocking rules, our consolidated results of operations could be adversely affected.
•Actual or perceived failures to comply with applicable data protection, privacy and security, advertising and consumer protection laws, regulations, standards and other requirements could adversely affect our business, financial condition and results of operations.
•We and our TOI PCs are subject to federal, state and local laws and regulations that govern our business.
•We may not be able to utilize a portion of our net operating loss carry forwards (“NOLs”) to offset future taxable income for U.S. federal income tax purposes, which could adversely affect our net income and cash flows.
•Future changes to applicable tax laws and regulations and/or their interpretations may have an adverse effect on our business, financial condition and results of operations. Tax rules and regulations are subject to interpretation and require judgment by us that may be successfully challenged by the applicable taxation authorities upon audit, which could result in additional tax liabilities.
Risks Related to Our Business
Our growth strategy depends on our ability to build or acquire new TOI PC clinics to service our contracts and treat our patients.
Our business strategy is to grow rapidly by expanding our network of oncology care clinics and is significantly dependent our ability to open new TOI PC clinics in our existing markets, expand into new geographical locations through existing TOI PCs or affiliating with new professional entities that would become a TOI PC, recruit new patients and partner or contract with payors, existing medical practices or other healthcare providers to provide oncology care services. We seek growth opportunities both organically and through TOI PCs’ agreements with payors or other oncology care providers. Our ability to grow organically depends upon a number of factors, including our affiliated providers obtaining referrals for cancer patient care services, the TOI PCs entering into contracts with additional payors, identifying appropriate facilities, obtaining leases, completing internal build-outs of new facilities within proposed timelines and budgets and hiring care teams and other employees. We cannot guarantee that we will participatebe successful in such vote, which meanspursuing our growth strategy. If we fail to evaluate and execute new business opportunities properly, we may complete our initial business combination even thoughnot achieve anticipated benefits and may incur increased costs.
Our growth strategy involves a majoritynumber of our public stockholders dorisks and uncertainties, including that:
•the TOI PCs may not support such a combination.We may choose notbe able to hold a stockholder vote to approve our initial business combination if the business combination would not require stockholder approval under applicable law or stock exchange listing requirement. Except for as required by applicable law or stock exchange requirement, the decision as to whether we will seek stockholder approval of a proposed business combination or will allow stockholders to sell their sharessuccessfully enter into contracts with local payors on terms favorable to us in a tender offer will be made by us, solely in our discretion, and will be based on a varietyor at all. In addition, the TOI PCs compete for payor relationships with other healthcare organizations, some of factors, such aswhom may have greater resources than we do. This competition may intensify due to the timing of the transaction and whether the terms of the transaction would otherwise require us to seek stockholder approval. Even if we seek stockholder approval, the holders of our founder shares will participateongoing consolidation in the vote onhealthcare industry, which may increase our costs to pursue such approval. Accordingly,opportunities;
•through the TOI PCs, we may completenot be able to recruit or retain a sufficient number of new patients to execute our initial business combination even if a majority of our public stockholders do not approve of the business combinationgrowth strategy, and we complete.Your only opportunitymay incur substantial costs to affect the investment decision regarding a potential business combinationrecruit new patients and we may be limitedunable to recruit a sufficient number of new patients to offset those costs;
•the exercise of your right to redeem your shares from us for cash.You willTOI PCs may not be provided with an opportunityable to evaluate the specific merits or riskshire sufficient numbers of one or more target businesses. Sincephysicians and other staff and may fail to integrate our board of directors may complete a business combination without seeking stockholder approval, public stockholdersemployees, particularly our medical personnel, into our care model;
•future value-based contracts may not have the right or opportunity to vote on thebe as favorable as current capitation contracts;
•when expanding our business combination, unlessinto new states, we seek such stockholder vote. Accordingly, your only opportunity to affect the investment decision regarding a potential business combination may be limitedrequired to exercising your redemption rights within the period of time (which will be at least 20 business days) set forth in our tender offer documents mailed to our public stockholderscomply with laws and regulations that may differ from states in which we describe our initial business combination.currently operate; and
If we seek stockholder approval of our initial business combination, our initial stockholders and management team have agreed to vote in favor of such initial business combination, regardless of how our public stockholders vote.•Our initial stockholders own shares representing approximately 20% of our outstanding common stock and have agreed to vote their shares in favor of an initial business combination. . In addition,depending upon the Deerfield Funds have indicated an interest in purchasing 5,000,000 units in this offering. Our initial stockholders and management team also may from time to time purchase Class A common stock prior to our initial business combination. Our second amended and restated certificate of incorporation provides that, if we seek stockholder approval of an initial business combination, such initial business combination will be approved if we receive the affirmative vote of a majoritynature of the shares voted at such meeting, including the founder shares. As a result,local market, we may not be able to implement our business model in addition toevery local market that we enter, which could negatively impact our initial stockholders’ founder sharesrevenues and the public shares included in the units the Deerfield Funds have indicated an interest in purchasing in this offering, we would need 2,875,001, or 12.50%, of the 23,000,000 public shares sold in this offering tofinancial condition.
There can be voted in favor of an initial business combination in order to have our initial business combination approved (assuming all outstanding shares are voted). Accordingly, if we seek stockholder approval of our initial business combination, the agreement by our initial stockholders and management team to vote in favor of our initial business combination will increase the likelihoodno assurance that we will receivebe able to successfully capitalize on growth opportunities, which may negatively impact our business model, revenues, results of operations and financial condition.
We have experienced, and may continue to experience, rapid growth and organizational change, which has placed, and may continue to place, significant demands on our management and our operational and financial resources.
Our organizational structure may become more complex as we improve our operational, financial and management controls, as well as our reporting systems and procedures. We may require significant capital expenditures and the requisite stockholder approval for such initialallocation of valuable management resources to grow and change in these areas. We must effectively increase our headcount and continue to effectively train and manage our employees. We will be unable to manage our business combination.
The abilityeffectively if we are unable to alleviate the strain on resources caused by growth in a timely and successful manner. If we fail to effectively manage our anticipated growth and change, the quality of our public stockholdersservices may suffer, which could negatively affect our brand and reputation and harm our ability to redeem their shares for cash may makeattract and retain patients and employees.
In addition, as we expand our business, it is important that we continue to maintain a high level of patient service and satisfaction. As our patient base continues to grow, through the TOI PCs, we will need to expand our medical, patient services and other personnel, and our network of partners, to provide personalized patient service. If we are not able to continue to provide high quality medical care with high levels of patient satisfaction, our reputation, as well as our business, results of operations and financial condition unattractivecould be adversely affected.
We have a history of net losses, we anticipate increasing expenses in the future, and we may not be able to potential business combination targets, whichachieve or maintain profitability.
We incurred a net loss of $83,068 in 2023, and a loss from operations in 2023. We expect our losses will continue as we expect to invest heavily in increasing our patient base, expanding our operations, and operating as a public company. These efforts may prove more expensive than we currently anticipate, and we may not succeed in increasing our revenue sufficiently to offset these higher expenses. To date, we have financed our operations principally from the sale of our equity, revenue from our patient services and the incurrence of indebtedness. We may not generate positive cash flow from operations or profitability in any given period, and our limited operating history may make it difficult for
usyou to
enter into aevaluate our current business
combination with a target.and our future prospects.
We
may seekhave encountered and will continue to
enter into a business combination transaction agreement with minimum cash requirement for (i) cash considerationencounter risks and difficulties frequently experienced by growing companies in rapidly changing industries, including increasing expenses as we continue to
be paidgrow our business. We expect our operating expenses to increase significantly over the next several years as we continue to hire additional personnel, expand our operations and infrastructure, and continue to expand to reach more patients. In addition to the
target or its owners, (ii) cash for working capital orexpected costs to grow our business, we also expect to incur additional legal, accounting and other
general corporate purposes or (iii) the retention of cash to satisfy other conditions. If too many public stockholders exercise their redemption rights, we would not be able to meet such closing condition and,expenses as a
result, would not be able to proceed with the business combination. Furthermore, in no event will we redeem our public
shares in an amount that would cause our net tangible assets to be less than $5,000,001. Consequently, if accepting all properly submitted redemption requests would cause our net tangible assets to be less than $5,000,001 or make us unable to satisfy a minimum cash condition as described above, we would not proceed with such redemption and the related business combination and may instead search for an alternate business combination. Prospective targets will be aware of these risks and, thus,company. These investments may be
reluctant to enter into a business combination transaction with us.The ability of our public stockholders to exercise redemption rights with respect to a large number of our shares may not allow us to complete the most desirable business combination or optimize our capital structure.
At the time we enter into an agreement for our initial business combination, we will not know how many stockholders may exercise their redemption rights, and therefore will need to structure the transaction based on our expectations as to the number of shares that will be submitted for redemption. If our initial business combination agreement requires us to use a portion of the cash in the Trust Account to pay the purchase price, or requires us to have a minimum amount of cash at closing, we will need to reserve a portion of the cash in the Trust Account to meet such requirements, or arrange for third party financing. In addition, if a larger number of shares is submitted for redemptionmore costly than we initially expected, we may need to restructure the transaction to reserve a greater portion of the cash in the Trust Account or arrange for third party financing. Raising additional third party financing may involve dilutive equity issuances or the incurrence of indebtedness at higher than desirable levels. Furthermore, this dilution would increase to the extent that the anti-dilution provision of the Class B common stock results in the issues of shares of Class A common stock on a greater than one-to-one basis upon conversion of the shares of Class B common stock at the time of our initial business combination. The above considerations may limit our ability to complete the most desirable business combination available to us or optimize our capital structure.
The ability of our public stockholders to exercise redemption rights with respect to a large number of our shares could increase the probability that our initial business combination would be unsuccessfulexpect, and that you would have to wait for liquidation in order to redeem your shares.
If our initial business combination agreement requires us to use a portion of the cash in the Trust Account to pay the purchase price, or requires us to have a minimum amount of cash at closing, the probability that our initial business combination would be unsuccessful is increased. If our initial business combination is unsuccessful, you would not receive your pro rata portion of the Trust Account until we liquidate the Trust Account. If you are in need of immediate liquidity, you could attempt to sell your shares in the open market; however, at such time our shares may trade at a discount to the pro rata amount per share in the Trust Account. In either situation, you may suffer a material loss on your investment or lose the benefit of funds expected in connection with your exercise of redemption rights until we liquidate or you are able to sell your shares in the open market.
The requirement that we complete our initial business combination by March 13, 2022 may give potential target businesses leverage over us in negotiating a business combination and may decrease our ability to conduct due diligence on potential business combination targets, in particular as we approach our dissolution deadline, which could undermine our ability to complete our initial business combination on terms that would produce value for our stockholders.
Any potential target business with which we enter into negotiations concerning a business combination will be aware that we must complete our initial business combination by March 13, 2022. Consequently, such target business may obtain leverage over us in negotiating a business combination, knowing that if we do not completeachieve the benefits anticipated from these investments, or if the realization of these benefits is delayed, they may not result in increased revenue or growth in our initial business
combination with that particular target business,operations. If we are not able to achieve or maintain positive cash flow in the long term, we may require additional financing, which may not be available on favorable terms or at all and/or which would be dilutive to our stockholders. If we are unable to complete our initial business combination with any target business. This risk will increasesuccessfully address these risks
and challenges as we
get closerencounter them, our business, results of operations and financial condition would be adversely affected. Our failure to
achieve or maintain profitability could negatively impact the
timeframe described above. In addition, we may have limited time to conduct due diligence and may enter intovalue of our
initial business combination on terms that we would have rejected upon a more comprehensive investigation.Our search for a business combination, and any target business with which we ultimately consummate a business combination, may be materially adversely affected by the recent coronavirus (COVID-19) outbreak and the status of debt and equity markets, as well as protectionist legislation in our target markets.
In December 2019, a novel strain of coronavirus was reported to have surfaced in Wuhan, China, which has and is continuing to spread throughout China and other parts of the world, including the United States. On January 30, 2020, the World Health Organization declared theCommon Stock.
A pandemic, epidemic or outbreak of the coronavirusan infectious disease (COVID-19) a “Public Health Emergency of International Concern.” On January 31, 2020, U.S. Health and Human Services Secretary Alex M. Azar II declared a public health emergency forin the United States to aidor worldwide, including the U.S. healthcare community in responding to COVID-19 and on March 11, 2020 the World Health Organization characterized the outbreak as a “pandemic”. Thispandemic, could adversely affect our business.
A pandemic, epidemic or outbreak of an infectious disease, including the current COVID-19 has resultedpandemic, that occurs in a widespread health crisis that has andthe United States or worldwide, may continue to adversely affect our business.
Adverse market conditions resulting from the economies and financial markets worldwide,spread of COVID-19 or other epidemic, pandemic, or infectious disease outbreak could materially adversely affect our business and the businessvalue of our Common Stock. Preventative measures taken to alleviate any public health crises, such as “shelter-in-place” orders, quarantines, executive orders and similar government orders may result in largely remote operations at our headquarters, work stoppages among some vendors and suppliers, slowdowns and delays, travel restrictions and cancellation of events, among other effects, thereby significantly and negatively impacting our operations. Other disruptions or potential target business with which we may consummate a business combination could be materially and adversely affected. Furthermore, we may be unable to complete a business combination if continued concerns relating to COVID-19 restrict travel, limitdisruptions include restrictions on the ability of our personnel to have meetingstravel; restrictions on our business development activities due to potential payors or other entities we and the TOI PCs engage with potential investorslimiting their corresponding business development efforts; inability of our suppliers to manufacture goods and to deliver these to us on a timely basis, or at all; inventory shortages or obsolescence; delays in actions of regulatory bodies; diversion of or limitations on employee resources that would otherwise be focused on the operations of our business, including because of sickness of employees or their families or the target company’s personnel, vendorsdesire of employees to avoid contact with groups of people; business adjustments or disruptions of certain third parties; and services providers are unavailable to negotiate and consummate a transaction in a timely manner. In addition, countriesadditional government requirements or supranational organizations in our target markets may develop and implement legislation that makes it more difficult or impossible for entities outside such countries or target markets to acquire or otherwise invest in companies or businesses deemed essential or otherwise vital.other incremental mitigation efforts. The extent to which COVID-19a pandemic, epidemic, or infectious disease outbreak impacts our search for and ability to consummate a business combination will depend on future developments whichthat are highly uncertain and cannot be predicted, including new information whichthat may emerge concerning the severity and spread of COVID-19the pandemic and the actions to contain COVID-19it or treat its impact, among others. If
Because of our business model, the disruptions posed byfull impact of the ongoing COVID-19 or other matters of global concern continue for an extensive period of time, and result in protectionist sentiments and legislationpandemic outbreaks may not be fully reflected in our target markets,results of operations and overall financial condition until future periods. It is not currently possible to reliably project the direct impact of the COVID-19 pandemic on our operating revenues and expenses. Key factors include the duration and extent of the outbreak in our service areas as well as societal and governmental responses. Patients may continue to be reluctant to seek necessary care given the risks of the COVID-19 pandemic. This could have the effect of deterring healthcare costs that we will need to incur to later periods and may also affect the health of patients who defer treatment, which may cause our costs to increase in the future. Further, as a result of the COVID-19 pandemic, we may experience slowed growth or a decline in new patient demand. We also may experience increased internal and third-party medical costs as the TOI PCs and our affiliated providers provide care for patients suffering from COVID-19. This increase in costs may be particularly significant given the number of patients who are under capitation agreements. Further, we may face increased competition due to changes to our competitors’ products and services, including modifications to their terms, conditions, and pricing that could materially adversely impact our business, results of operations, and overall financial condition in future periods.
The COVID-19 pandemic could also cause our third-party data center hosting facilities and cloud computing platform providers, which are critical to our infrastructure, to shut down their business, experience security incidents that impact our business, delay or disrupt performance or delivery of services, or experience interference with the supply chain of hardware required by their systems and services, any of which could materially adversely affect our business. Further, the COVID-19 pandemic has resulted in our employees and those of many of our vendors working from home and conducting work via the internet, and if the network and infrastructure of internet providers becomes overburdened by increased usage or is otherwise unreliable or unavailable, our employees’, and our customers’ and vendors’ employees’, access to the internet to conduct business could be negatively impacted. Limitations on access or disruptions to services or goods provided by or to some of our suppliers and vendors upon which our platform and business operations relies, could interrupt our ability to consummate aprovide our platform, decrease the productivity of our workforce, and significantly harm our business combination,operations, financial condition, and results of operations.
Our platform and the other systems or networks used in our business may experience an increase in attempted cyberattacks, targeted intrusion, ransomware, and phishing campaigns seeking to take advantage of shifts to employees working remotely using their household or personal internet networks and to leverage fears promulgated by the COVID-19 pandemic. The success of any of these unauthorized attempts could substantially impact our platform, the proprietary and other confidential data contained therein or otherwise stored or processed in our operations, and ultimately our business. Any actual or perceived security incident also may cause us to incur increased expenses to improve our security controls and to remediate security vulnerabilities.
To the extent the COVID-19 pandemic, or another pandemic, epidemic or outbreak of a targetan infectious disease occurs in the United States or worldwide, adversely affects our business with which we ultimately consummate a business combination,and financial results, it may be materially adversely affected. In addition,also have the effect of heightening many
of the other risks described in this “Risk Factors” section, including but not limited to those relating to cyberattacks and security vulnerabilities, interruptions or delays due to third-parties, or our ability to consummateraise additional capital or generate sufficient cash flows necessary to fulfill our obligations under our existing indebtedness or to expand our operations.
Our services are concentrated in certain geographic areas and populations exposing us to unfavorable changes in local benefit costs, reimbursement rates, competition and economic conditions.
The TOI PCs’ membership remains concentrated in certain geographic areas in the United States. We have clinic locations in five states. As of December 31, 2023, the vast majority of the TOI PC members under capitation agreements were residents of California. In addition, during 2023, approximately 85% of our revenues were generated in California. Unfavorable changes in health care or other benefit costs or reimbursement rates or increased competition in the states in which we operate or any other geographic area where the TOI PCs’ membership becomes concentrated in the future could therefore have a transactiondisproportionately adverse effect on our operating results. Additionally, the geographic concentration of a significant portion of the TOI PCs’ membership may make them more vulnerable to events such as the COVID-19 pandemic.
If we are unable to attract new patients, our revenue growth will be dependent onadversely affected.
To increase our revenue, our business strategy includes expanding the
abilitynumber of payor contracts entered into by the TOI PCs and clinic locations in our network. In order to
raise equitysupport such growth, the TOI PCs must continue to win new contracts and
debt financing which may be impacted by COVID-19retain or grow existing contracts with payors. We face competition from other oncology providers in the recruitment of potential patients. If the TOI PCs are unable to convince potential payors and
other events.Wepatients of the benefits of our value-based system, or if potential or existing payors and patients prefer the care provider model of one of our competitors, we may not be able to completeeffectively implement our initial business combination by March 13, 2022, ingrowth strategy, which case we would cease all operations exceptdepends on our ability to grow organically and attract new patient referrals and payors for the purposeTOI PCs. In addition, our growth strategy is dependent on payors electing to enter into capitation or other value-based arrangements and selecting the TOI PCs as their oncology provider. The TOI PCs’ inability to obtain new payor agreements and patient referrals and retain existing payors and patients, particularly those under capitation arrangements, would harm our ability to execute our growth strategy and may have a material adverse effect on our business operations and financial position.
We primarily depend on reimbursement by third-party payors, as well as payments by individuals, which could lead to delays and uncertainties in the reimbursement process.
The reimbursement process is complex and can involve lengthy delays. Although we recognize revenue when the TOI PCs and our affiliated providers provide services to patients, we may from time to time experience delays in receiving the associated capitation payments or, for patients on fee-for-service arrangements, the reimbursement for the service provided. In addition, third-party payors may disallow, in whole or in part, requests for reimbursement based on determinations that the patient is not eligible for coverage, certain amounts are not reimbursable under plan coverage or the services provided that were not medically necessary or additional supporting documentation is necessary. Retroactive adjustments may change amounts realized from third-party payors. As described below, the TOI PCs are subject to audits by such payors, including governmental audits of winding upour Medicare claims, and may be required to repay these payors if a finding is made that we would redeemwere incorrectly reimbursed. Delays and uncertainties in the reimbursement process may adversely affect accounts receivable, increase the overall costs of collection and cause us to incur additional costs associated with raising capital. Third-party payors are also increasingly focused on controlling healthcare costs, and such efforts, including any revisions to reimbursement policies, may further complicate and delay the TOI PCs’ reimbursement claims.
In addition, certain of our
public shares and liquidate.Wepatients are covered under health plans that require the patient to cover a portion of their own healthcare expenses through the payment of copayments or deductibles. The TOI PCs may not be able to findcollect the full amounts due with respect to these payments that are the patient’s financial responsibility, or in those instances where physicians provide services to uninsured individuals. To the extent permitted by law, amounts not covered by third-party payors are the obligations of individual patients for which the TOI PCs may not receive whole or partial payment. Any increase in cost shifting from third-party payors to individual patients, including as a suitable targetresult of high deductible plans for patients, increases our collection costs and reduces overall collections, which we may not be able to offset such additional costs with sufficient revenue.
In response to the COVID-19 pandemic, the Centers for Medicare and Medicaid Services, or CMS, the federal agency responsible for administering the Medicare program, made several changes in the manner in which Medicare will pay for telehealth visits, many of which relax previous requirements, including site requirements for both the providers and patients, telehealth modality requirements and others. The Consolidated Appropriations Act of 2023 extended many of the COVID-19 public health emergency provisions related to telehealth until December 31, 2024. State law applicable to telehealth, particularly licensure requirements, has also been relaxed in many jurisdictions as a result of the COVID-19 pandemic. It is
unclear which, if any, of these changes will remain in place permanently and which will be rolled-back. If regulations change to restrict the TOI PCs’ or our affiliated providers ability to deliver care through telehealth modalities, our financial condition and results of operations may be adversely affected.
With many of our value-based agreements, the TOI PCs assume some or all of the risk that the cost of providing services will exceed compensation. If we do not accurately predict the cost to deliver care, some of the TOI PCs’ value-based agreements could become less profitable, or unprofitable.
Approximately 22% of our revenue for 2023 was derived from fixed fees paid by payors under capitation agreements with the TOI PCs. While there are variations specific to each agreement, the TOI PCs generally contract with payors to receive a fixed fee per month for professional services and assume the financial responsibility for the specified medical oncology and related expenses of our patients. This type of contract is referred to as a “capitation” contract. To the extent that patients require more care than is anticipated and/or the cost of care increases, aggregate fixed compensation amounts, or capitation payments, may be insufficient to cover the costs associated with treatment. If medical costs and expenses exceed estimates, except in very limited circumstances, the TOI PCs will not be able to increase the fee received under these risk agreements during their then-current terms and we could suffer losses with respect to such agreements.
Changes in our anticipated ratio of medical expense to revenue can significantly impact our financial results. Accordingly, the failure to adequately predict and control medical costs and expenses could have a material adverse effect on our business, results of operations, financial condition and cash flows. Additionally, the Medicare expenses of our patients may be outside of the TOI PCs control in the event that patients take certain actions that increase such expenses, such as unnecessary hospital visits.
Historically, the TOI PCs’ medical costs and expenses as a percentage of revenue have fluctuated. Factors that may cause medical expenses to exceed estimates include:
•the health status of patients;
•changes to oncology treatment guidelines which our affiliated providers follow;
•higher than expected utilization of new or existing healthcare services, drugs or technologies;
•an increase in the cost of healthcare services and supplies, whether as a result of inflation or otherwise;
•changes to mandated benefits or other changes in healthcare laws, regulations and practices;
•increased costs attributable to provider and support staff compensation or providers with which the TOI PCs contract to provide care to patients;
•changes in the demographics of our patients and medical trends;
•contractual or claims disputes with providers, hospitals or other service providers within and outside a health plan’s network; and
•the occurrence of catastrophes, major epidemics or acts of terrorism.
In addition, we are reliant on our customers under value-based contracts to provide us with data related to the population of patients for which we are at risk. This data, in particular, which relates to membership eligibility, is subject to frequent changes, omissions and errors which we cannot control. We work closely with our customers to reconcile this data, but we cannot be certain of the accuracy of this data. If we underestimate or do not correctly predict the cost of the oncology care the TOI PCs provide to patients, the TOI PCs might be underpaid for the care that must be provided to our patients, which could have a negative impact on our results of operations and financial condition.
There are significant risks associated with estimating the amount of revenue that is recognized under TOI PCs’ risk agreements with health plans, and if our estimates of revenue are materially inaccurate, it could impact the timing and the amount of our revenue recognition or have a material adverse effect on our business, results of operations, financial condition and cash flows.
There are significant risks associated with estimating the amount of revenues that is recognize under the TOI PCs’ risk agreements with health plans in a reporting period. The billing and collection process is complex due to ongoing insurance coverage changes, geographic coverage differences, differing interpretations of contract coverage and other payor issues, such as ensuring appropriate documentation. Determining applicable primary and secondary coverage for our patients, together with the changes in patient coverage that occur each month, requires complex, resource-intensive processes. Errors in determining the correct coordination of benefits may result in refunds to payors. Revenues associated with Medicare and Medicaid programs are also subject to estimating risk related to the amounts not paid by the primary government payor that will ultimately be
collectible from other government programs paying secondary coverage, the patient’s commercial health plan secondary coverage or the patient. Collections, refunds and payor recoupments typically continue to occur for up to three years and longer after services are provided. If our estimates of revenues are materially inaccurate, it could impact the timing and the amount of our revenues recognition and have a material adverse impact on our business, results of operations, financial condition and cash flows.
A significant portion of our consolidated Patient Services revenue is derived from a limited number of health insurance, Independent Practice Associations ("IPAs"), and medical group companies. Those payors could take action to remove, exclude, delay, or otherwise prevent the inclusion of the TOI PCs in their provider networks.
Our operations are dependent on a concentrated number of payors with whom the TOI PCs contract to provide services to patients. We generally manage the TOI PCs’ payor contracts on a state by state basis, entering into a separate contract in each state with the local affiliate of the relevant payor such that no one local payor contract accounts for a majority of our collective revenue. Regal Medical Group accounted for a total of approximately 11% of the Patient Services revenue for the year ended December 31, 2023. No other non-government payor accounted for more than 10% of the Patient Services revenue in 2023. We believe that a majority of the TOI PCs’ revenues will continue to be derived from a limited number of key payors, which may terminate their contracts with the TOI PC or the individual TOI PC physicians credentialed by them upon the occurrence of certain events. The loss of any of the TOI PCs’ payor partners, or the renegotiation of any of the TOI PCs’ payor contracts, could adversely affect our operating results. In the ordinary course of business we engage in active discussions and renegotiations with payors in respect of the services the TOI PCs provide and the terms of the TOI PCs’ payor agreements. As the payors’ businesses respond to market dynamics and financial pressures, and as payors make strategic business decisions in respect of the lines of business they pursue and programs in which they participate, certain of the payors may seek to renegotiate or terminate their agreements with the TOI PCs. These discussions could result in reductions to the fees and changes to the scope of services contemplated by the original payor contracts and consequently could negatively impact our revenues, business and completeprospects.
Because we rely on a limited number of payors for a significant portion of the TOI PCs’ revenues, we depend on the creditworthiness of these payors. The payors are subject to a number of risks including reductions in payment rates from governmental programs, higher than expected health care costs and lack of predictability of financial results when entering new lines of business, particularly with high-risk populations. If the financial condition of the TOI PCs’ payor partners declines, our initialfinancial results could be impacted. Should one or more of the TOI PCs’ significant payor partners declare bankruptcy, be declared insolvent or otherwise be restricted by state or federal laws or regulation from continuing in some or all of their operations, this could adversely affect our ongoing revenues, the collectability of our accounts receivable, our bad debt reserves and our net income.
Although the TOI PCs have long-term contracts with many payors, these contracts may be terminated before their term expires for various reasons, such as changes in the regulatory landscape and poor performance by the TOI PCs and our affiliated providers, subject to certain conditions. Certain of the payor contracts are terminable immediately upon the occurrence of certain events. Certain of the payor contracts may be terminated immediately by the partner if the TOI PCs lose applicable licenses, go bankrupt, lose its liability insurance or receive an exclusion, suspension or debarment from state or federal government authorities. Additionally, if a payor were to lose applicable licenses, go bankrupt, lose liability insurance, become insolvent, file for bankruptcy or become subject to exclusion, suspension or debarment from state or federal government authorities, the TOI PC’s contract with such payor could in effect be terminated. In addition, certain of the payor contracts may be terminated immediately if a TOI PC becomes insolvent or file for bankruptcy. If any of the contracts with the TOI PCs’ payors is terminated, the TOI PCs may not be able to recover all fees due under the terminated contract, which may adversely affect our operating results.
A significant portion of sales are from prescription drug sales reimbursed by a limited number of pharmacy benefit management companies with which TOI PCs contract. Those pharmacy benefit management companies could take action to remove, exclude, delay or otherwise prevent the inclusion of the TOI PCs in their provider networks.
There is currently significant concentration in the U.S. healthcare industry, and in particular there are a limited number of pharmacy benefit managers, or PBMs, and a limited number of national pharmacy chains. CVS Caremark, OptumRx and Express Scripts together accounted for approximately 77% of our dispensary revenue in 2023. If the TOI PCs are unable to retain favorable contractual arrangements with PBMs, including any successor PBMs should there be further consolidation of PBMs, the negotiated rates provided by such PBMs may become less competitive, which could have an adverse impact on the TOI PCs’ ability to provide prescription drugs at the capitated rates negotiated with the payors with whom the TOI PCs contract to provide such drugs to patients. This could be exacerbated by further consolidation of PBMs or pharmacy chains. Specifically, PBMs have instituted Direct and Indirect Remuneration, or DIR, fees, which reduce the reimbursement for drugs dispensed by the TOI PCs. The impact of these fees in future is uncertain, and our ability to negotiate with PBMs on DIR fees is limited. In
addition, PBMs could at any time change their contracting and/or credentialing requirements, the effect of which could prohibit the TOI PCs from billing for prescription drugs dispensed by the TOI PCs. If such changes, individually or in the aggregate, are material, they would have an adverse effect on our business, combinationresults of operations and financial condition.
Reductions in government reimbursement rates or changes in the rules governing government healthcare programs could have a material adverse effect on our financial condition and results of operations.
The TOI PCs receive a significant portion of revenue directly from Medicare, which accounted for approximately 14% of our Patient Services revenue in 2023. In addition, many private payors base their reimbursement rates on the published Medicare rates or, in the case of Medicare Advantage, are themselves reimbursed by March 13, 2022. If we have not completed our initial business combination within such time period, we will: (i) cease all operations exceptMedicare for the purposeservices the TOI PCs provide. As a result, our results of winding up, (ii)operations are, in part, dependent on government funding levels for Medicare programs, particularly Medicare Advantage programs. Any changes that limit or reduce Medicare Advantage or general Medicare reimbursement levels, such as promptlyreductions in or limitations of reimbursement amounts or rates under programs, reductions in funding of programs, expansion of benefits without adequate funding, elimination of coverage for certain benefits, or elimination of coverage for certain individuals or treatments under programs, could have a material adverse effect on our business, results of operations, financial condition and cash flows.
The Medicare program and its reimbursement rates and rules are subject to frequent change. These include statutory and regulatory changes, rate adjustments (including retroactive adjustments), administrative or executive orders and government funding restrictions, all of which may materially adversely affect the rates at which Medicare reimburses the TOI PCs for patient care services. Budget pressures often lead the federal government to reduce or place limits on reimbursement rates under Medicare. Implementation of these and other types of measures has in the past and could in the future result in substantial reductions in our revenue and operating margins.
In addition, CMS often changes the rules governing the Medicare program, including those governing reimbursement. Changes that could adversely affect our business include:
•administrative or legislative changes to rates or the bases of payment;
•limits on the services or types of providers for which Medicare will provide reimbursement;
•changes in methodology for patient assessment and/or determination of payment levels;
•the reduction or elimination of annual rate increases; or
•an increase in co-payments or deductibles payable by beneficiaries.
There is also uncertainty regarding both Medicare Advantage payment rates and beneficiary enrollment, which, if reduced, would reduce our overall revenues and net income, as reasonably possible but notwell as future growth opportunities. For example, although the Congressional Budget Office (“CBO”) predicted in 2010 that Medicare Advantage participation would drop substantially by 2020, the CBO has more than ten business days thereafter, redeemrecently predicted, without taking into account potential future reforms, that enrollment in Medicare Advantage (and other contracts covering Medicare Parts A and B) could reach 36 million by 2027. Although Medicare Advantage enrollment has increased significantly over the public shares, atpast decade, there can be no assurance that this trend will continue. Further, fluctuation in Medicare Advantage payment rates are evidenced by CMS’s annual announcement of the expected average change in revenue from the prior year: for 2023, CMS announced an average increase of 4.88%; and for 2024, 2.28%. Uncertainty over Medicare Advantage enrollment and payment rates present a per-share price, payable in cash, equalcontinuing risk to our business.
According to the aggregate amount then on depositKaiser Family Foundation, or KFF, Medicare Advantage enrollment continues to be highly concentrated among a few payors, both nationally and in local regions. In 2023, the KFF reported that two payors together accounted for nearly half of Medicare Advantage enrollment and seven firms accounted for nearly 85% of covered lives. Consolidation among Medicare Advantage plans in certain regions, or the Medicare program’s failure to attract additional plans to participate in the Trust Account,Medicare Advantage program, could have a material adverse effect on our business, results of operations, financial condition and cash flows.
Moreover, the Medicaid program and its reimbursement rates and policies are subject to frequent change. By way of example, Medi-Cal recently implemented a new policy regarding reimbursement for pharmacy services. Although the policy was not intended to change the manner in which physician-administered drugs billed under the medical benefit are reimbursed, certain Medi-Cal managed care plans nevertheless began to transition these claims to be payable as a pharmacy benefit and exclude coverage of prescription drugs formerly available through the medical benefit or direct their subcontractors or network providers to no longer bill for prescription drugs through their medical claims. The California Department of Health Care Services, or DHCS, later issued clarifying guidance which instructed Medi-Cal managed care plans to ensure all medically necessary prescription drugs administered in an outpatient office or clinic setting by a health care professional continue to be available through the medical benefit, even though some may be available as a pharmacy benefit. In addition, during the
COVID-19 public health emergency, DHCS delayed the processing of Medi-Cal annual redeterminations and delayed discontinuances and negative actions for Medi-Cal and other state and county healthcare programs. As a result, the TOI PCs could experience a reduction in membership, which could have a material adverse effect on our business, results of operations, financial condition and cash flows.
Any reductions in reimbursement rates or the scope of services, including interest earnedpharmacy services, rendered by the TOI PCs being reimbursed could have a material, adverse effect on our financial condition and results of operations or even result in reimbursement rates that are insufficient to cover our operating expenses. Additionally, any delay or default by the government in making Medicare or Medicaid reimbursement payments to the TOI PCs or any reduction in patients eligible for such programs could materially and adversely affect our business, financial condition and results of operations.
We cannot predict the effect that health care reform and other changes in government programs may have on our business, financial condition or results of operations.
The impact of healthcare reform legislation and other changes in the healthcare industry and in healthcare spending is currently unknown, but may adversely affect our business, financial condition and results of operations. Our revenue is dependent on the funds heldhealthcare industry and could be affected by changes in healthcare spending, reimbursement and policy. The healthcare industry is subject to changing political, regulatory and other influences. By way of example, the ACA, which was enacted in 2010, made major changes in how healthcare is delivered and reimbursed, and it increased access to health insurance benefits to the uninsured and underinsured populations of the United States.
Since its enactment, there have been judicial, executive and Congressional challenges to certain aspects of the ACA. On June 17, 2021, the U.S. Supreme Court dismissed the most recent judicial challenge to the ACA brought by several states without specifically ruling on the constitutionality of the ACA. Prior to the Supreme Court’s decision, President Biden issued an executive order initiating a special enrollment period from February 15, 2021 through August 15, 2021 for purposes of obtaining health insurance coverage through the ACA marketplace. The executive order also instructed certain governmental agencies to review and reconsider their existing policies and rules that limit access to healthcare.
Other legislative changes have been proposed and adopted since the ACA was enacted. These changes include aggregate reductions to Medicare payments to providers of 2%, which began in 2013 and will remain in effect through 2030, with the exception of a temporary suspension from May 1, 2020 through March 31, 2022. Under current legislation, the actual reduction in Medicare payments varies from 1% from April 1, 2022 to June 30, 2022, up to 3% in the Trust Accountfinal fiscal year of this sequester, unless additional Congressional action is taken. In January 2013, the American Taxpayer Relief Act of 2012 was signed into law, which, among other things, further reduced Medicare payments to several types of providers, including hospitals, imaging centers and cancer treatment centers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. New laws may result in additional reductions in Medicare and other healthcare funding, which may materially adversely affect consumer demand and affordability for our products and services and, accordingly, the results of our financial operations. Additional changes that may affect our business include the expansion of new programs such as Medicare payment for performance initiatives for physicians under the Medicare Access and CHIP Reauthorization Act of 2015, or MACRA, which first affected physician payment in 2019. At this time, it is unclear how the introduction of the Medicare quality payment program will impact overall physician reimbursement. The Inflation Reduction Act of 2022, or IRA, signed into law on August 16, 2022, also contains a number of provisions designed to limit or reduce drug prices under the Medicare program, reduce beneficiary out-of-pocket spending under Medicare’s prescription drug benefit, and expand subsidies for individuals to obtain private health insurance under the ACA. While these provisions of the IRA do not previously releasedapply directly to healthcare providers like the TOI PCs, we are continuing to evaluate the potential impact, if any, that the IRA may have on our business.
Such changes in the regulatory environment may also result in changes to our payer mix that may affect our operations and revenue. In addition, certain provisions of the ACA authorize voluntary demonstration projects, which include the development of bundling payments for acute, inpatient hospital services, physician services and post-acute services for episodes of hospital care. Further, the ACA may adversely affect payers by increasing medical costs generally, which could have an effect on the industry and potentially impact our business and revenue as payers seek to offset these increases by reducing costs in other areas.
Uncertainty regarding future amendments to the ACA as well as new legislative proposals to reform healthcare and government insurance programs, along with the trend toward managed healthcare in the United States, could result in reduced demand and prices for our services. We expect that additional state and federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments and other third party payers will pay for healthcare products and services, which could adversely affect our business, financial condition and results of operations.
Inflation can adversely affect us by increasing the costs of drugs, clinical trials and research, administration and other costs of doing business
Recently, inflation has increased throughout the U.S. economy. Inflation can adversely affect us by increasing the costs of drugs, clinical trials and research, administration and other costs of doing business. We may experience increases in the prices of labor and other costs of doing business. In an inflationary environment, cost increases may outpace our expectations, causing us to use our cash and other liquid assets faster than forecasted. If this happens, we may need to raise additional capital to fund our working capital requirements (subjectoperations, which may not be available in sufficient amounts or on reasonable terms, if at all, sooner than expected.
The transition from volume to an annual limit of $500,000) (less taxes payablevalue-based reimbursement models may have a material adverse effect on our operations.
Healthcare reform is causing some payors to transition from volume to value-based reimbursement models, which can include risk-sharing, bundled payment and
upother innovative approaches. While these models may provide us with opportunities to
$100,000 of interestprovide new or additional services and to
pay dissolution expenses), dividedparticipate in incentive- based payment arrangements, there can be no assurance that such new models and approaches will be profitable to us or the TOI PCs. Further, new models and approaches may require investment by
the number of then outstanding public shares, which redemption will completely extinguish public stockholders’ rights as stockholders (including the rightus to
receive further liquidating distributions, if any),develop technology or expertise to offer necessary and
(iii) as promptly as reasonably possible following such redemption, subjectappropriate solutions or support to the
approval of our remaining stockholders and our board of directors, liquidate and dissolve, subject in each case, to our obligations under Delaware law to provide for claims of creditors and the requirements of other applicable law.If we seek stockholder approval of our initial business combination, our sponsor, initial stockholders, directors, executive officers, advisors and their affiliates may elect to purchase shares or public warrants from public stockholders, which may influence a vote on a proposed business combination and reduce the public “float” of our Class A common stock.
If we seek stockholder approval of our initial business combinationTOI PCs, and we do not conduct redemptionsfully know the amount and timing for return of such investment at this time. In addition, some of these new models are being offered as pilot programs and there is no assurance that they will continue or be renewed. Many states in connectionwhich these new value-based structures are being developed also lack regulatory guidance or a well-developed body of law for these new models and approaches, or may not have updated their laws or enacted legislation yet to reflect the new healthcare reform models. As a result, new and existing laws, regulations or guidance could have a material adverse effect on our operations and could subject us to the risk of restructuring or terminating our arrangements with the TOI PCs, as well as the risk of regulatory enforcement, penalties and sanctions, if state and federal enforcement agencies disagree with our initialinterpretation of these laws.
CMS, through the Centers for Medicare and Medicaid Innovation, or the CMMI, has implemented or has announced plans to implement numerous demonstration models designed to test value-based reimbursement models, some of which are specifically focused on oncology services. For example, in 2016, CMS initiated the Oncology Care Model, or OCM demonstration, which continued through June 30, 2022 and provided participating physician practices with performance-based financial incentives that aim to manage or reduce Medicare costs without negatively affecting the efficacy of care. In June 2022, CMS issued a request for applications for the Enhancing Oncology Model, a new 5-year voluntary model that builds on the OCM demonstration. While the extent to which these models may impact our business combination pursuant to the tender offer rules,is uncertain and will depend on future developments, such models may materially reduce Medicare reimbursement levels for our sponsor, initial stockholders,services or TOI PCs’ services and could have a material adverse effect on our results of operations and financial condition.
directors, executive officers, advisors or their affiliates may purchase shares or public warrants in privately negotiated transactions orChanges in the open market either priorpayor mix of patients and potential decreases in reimbursement rates as a result of consolidation among plans could adversely affect our revenues and results of operations.
The amounts the TOI PCs receive for services provided to or followingpatients are determined by a number of factors, including the completionpayor mix of patients and the reimbursement methodologies and rates utilized by our initial business combination, althoughpatients’ plans. Our Patient Services revenue consists of both capitation and fee-for-service agreements held by the TOI PCs. Reimbursement rates are generally higher for capitation agreements than they are under no obligationfee-for-service arrangements, and capitation agreements provide the TOI PCs with an opportunity to do so. There is no limit oncapture any additional surplus created by applying our care model. Under a capitation plan, the TOI PCs receive a fixed fee PMPM for services. Under a fee-for-service payor arrangement, the TOI PCs collect fees directly from the payor as services are provided. Our Patient Services revenue accounted for approximately 66% of total revenue for the year ended December 31, 2023. A significant decrease in the number of sharescapitation or FFS arrangements held by the TOI PCs could adversely affect our initial stockholders, directors, officers, advisorsrevenues and results of operation.
The healthcare industry has also experienced a trend of consolidation, resulting in fewer but larger payors that have significant bargaining power, given their market share. Payments from payors are the result of negotiated rates. These rates may decline based on renegotiations and larger payors have significant bargaining power to negotiate higher discounted fee arrangements with healthcare providers. As a result, payors increasingly are demanding discounted fee structures or the assumption by healthcare providers of all or a portion of the financial risk related to paying for care provided through capitation agreements.
We face significant competition from other healthcare services providers. Our failure to adequately compete could adversely affect our business.
We and the TOI PCs compete directly with national, regional and local providers of healthcare for patients and physicians. There are many other companies and individuals currently providing healthcare services, many of which have been in business
longer and/or have substantially more resources. Other companies could enter the healthcare industry in the future and divert some or all of our business. If we expand to other geographies, we expect competition may change based on a number of factors, including the number of competing oncology care facilities in the local market and the types of services available at those facilities, our local and the TOI PCs reputation for quality care of patients, the commitment and expertise of the TOI PCs medical staff, our local service offerings and community programs, the cost of care in each locality, and the physical appearance, location, age and condition of our facilities. If we are unable to attract patients to our managed clinics, our revenue and profitability will be adversely affected. Some of our competitors may have greater recognition and be more established in their affiliatesrespective communities than we are, and may purchasehave greater financial and other resources than we have. Competing oncology care providers may also offer larger facilities or different programs or services than we do, which, combined with the foregoing factors, may result in our competitors being more attractive to our current patients, potential patients and referral sources. Furthermore, while we budget for routine capital expenditures at our managed clinics to keep them competitive in their respective markets, to the extent that competitive forces cause those expenditures to increase in the future, our financial condition may be negatively affected. In addition, our relationships with governmental and private third-party payors are not exclusive and our competitors have established or could seek to establish relationships with such payors to serve their covered patients. Additionally, as we expand into new geographies, we may encounter competitors with stronger relationships or recognition in the community in such transactions, subject to compliancenew geography, which could give those competitors an advantage in obtaining new patients. Individual physicians, physician groups and companies in other healthcare industry segments, including those with applicable lawwhich the TOI PCs have contracts, and Nasdaq rules. However,some of which have greater financial, marketing and staffing resources, may become competitors in providing health care services, and this competition may have a material adverse effect on our business operations and financial position.
Competition for physicians and nurses, shortages of qualified personnel or other than as expressly stated herein, they have no current commitments, plans or intentions to engage in such transactionsfactors could increase our labor costs and have not formulated any terms or conditions for any such transactions. Noneadversely affect our revenue, profitability and cash flows.
Our operations are dependent on the efforts, abilities and experience of the
fundsTOI PCs’ physicians and clinical personnel. We compete with other healthcare providers, primarily hospitals and other oncology practices, in attracting physicians, nurses and medical staff to support our managed clinics, recruiting and retaining qualified management and support personnel responsible for the daily operations of each of our managed clinics and in the
Trust Account will be used to purchase shares or public warrantsTOI PCs contracting with payors in
such transactions. Such purchases may include a contractual acknowledgment that such stockholder, although still the record holdereach of our
shares, is no longermarkets. In some markets, the
beneficial owner thereoflack of availability of clinical personnel has become a significant operating issue facing all healthcare providers. This shortage may require us and
therefore agrees notthe TOI PCs to
exercise its redemption rights.Incontinue to enhance wages and benefits to recruit and retain qualified personnel or to contract for more expensive temporary personnel. We also depend on the event that our sponsor, initial stockholders, directors, executive officers, advisors or their affiliates purchase sharesavailable labor pool of semi-skilled and unskilled workers in privately negotiated transactions from public stockholders who have already elected to exercise their redemption rights, such selling stockholders would be required to revoke their prior elections to redeem their shares. The purpose of any such purchases of shares could be to vote such shares in favoreach of the business combinationmarkets in which we operate.
If our labor costs increase, we may not be able to raise rates to offset these increased costs. Because a significant percentage of our revenue consists of fixed, prospective payments, our ability to pass along increased labor costs is limited. In particular, if labor costs rise at an annual rate greater than our net annual consumer price index basket update from Medicare, our results of operations and therebycash flows will likely be adversely affected. Any union activity at our managed clinics that may occur in the future could contribute to increased labor costs. Certain proposed changes in federal labor laws and the National Labor Relations Board’s modification of its election procedures could increase the likelihood of obtaining stockholder approvalemployee unionization attempts. Although none of our employees or the employees of the business combinationTOI PCs are currently represented by a collective bargaining agreement, to the extent a significant portion of our employee base unionizes, it is possible our labor costs could increase materially. Our failure to recruit and retain qualified management and medical personnel for the TOI PCs, or to satisfy a closing condition in an agreement with a target that requires us tocontrol our collective labor costs, could have a minimum net worthmaterial adverse effect on our business, prospects, results of operations and financial condition.
Because competition for qualified personnel is intense, we may not be able to attract and retain the highly skilled employees we need to execute our business strategies and growth plans.
To execute on our growth plan, we and the TOI PCs must attract and retain highly qualified personnel. Competition for highly qualified personnel is intense, especially for physicians and other medical professionals who are experienced in providing oncology care services. We and the TOI PCs have, from time to time, experienced, and we expect to continue to experience, difficulty in hiring and retaining employees with appropriate qualifications. Many of the companies and healthcare providers with which we compete for experienced personnel have greater resources than we have. If we and the TOI PCs hire employees from competitors or other companies or healthcare providers, their former employers have attempted and may in the future attempt to assert that these employees or we have breached certain legal obligations, resulting in a diversion of our time and resources.
As we become a more mature company, we may find our recruiting efforts more challenging. The incentives to attract, retain, and motivate employees provided by our stock options and other equity awards, or by other compensation arrangements, may not be as effective as in the past. As such, we may not be successful in continuing to attract and retain qualified personnel.
Our recruiting efforts may also be limited by laws and regulations, such as restrictive immigration laws, and restrictions on travel or availability of visas. If we fail to attract new personnel or fail to retain and motivate our current personnel, our business and future growth prospects could be harmed.
Certain of our management has limited experience in operating a public company.
Certain of our executive officers and certain directors have limited experience in the management of a publicly traded company. Our management team may not successfully or effectively manage its transition to a public company that is subject to significant regulatory oversight and reporting obligations under federal securities laws. Their limited experience in dealing with the increasingly complex laws pertaining to public companies could be a significant disadvantage in that it is likely that an increasing amount of their time may be devoted to these activities which will result in less time being devoted to the management and growth of the company. It is possible that we will be required to expand our employee base and hire additional employees to support our operations as a public company, which will increase our operating costs in future periods.
If we are unable to provide consistently high quality of care, our business will be adversely impacted.
Our business is dependent upon the TOI PCs and our affiliated providers providing high-quality care to our patients. In particular, our ability to attract and retain patients and patient referrals dependent upon providing cost effective, quality patient care that meets or exceeds our patients’ and payors’ expectations. We depend on third parties for certain of our patient care needs. If we or the TOI PCs fail to provide service that meets our patients’ and payors’ expectations, we may have difficulty retaining or growing our patient base, which could adversely affect our business, financial condition and results of operations.
We expect the importance of high-quality patient experience to increase as we, through the TOI PCs, expand our business and pursue new lives served. Any failure to maintain high-quality patient experience, or a certain amountmarket perception that we do not maintain high-quality care, could harm the reputation of cash at the closing ofus and our initial business combination, where it appears that such requirement would otherwise not be met. The purpose of any such purchases of public warrants could beaffiliated providers and our ability to reducegrow the number of public warrants outstandinglives served, and our business, results of operations, and financial condition. Additionally, as the number of lives served by the TOI PCs in our managed clinics grows, we will need to hire additional personnel to provide quality care at scale. If we and the TOI PCs are unable to provide such care, our business, results of operations, financial condition, and reputation could be harmed.
If certain of our suppliers do not meet our needs, if there are material price increases on supplies, if we are not reimbursed or adequately reimbursed for drugs purchased or if we are unable to effectively access new technology or superior products, it could negatively impact the ability of the TOI PCs to effectively provide the services we offer and could have a material adverse effect on our business, results of operations, financial condition and cash flows.
The TOI PCs have significant drug suppliers that may be the sole or primary source of products critical to the services the TOI PCs provide, or to vote such warrantswhich we have committed obligations to make purchases, sometimes at particular prices. Approximately 57% of the TOI PCs’ total costs are related to drug purchases, including both oral and chemotherapy drugs, for the year ended December 31, 2023. If any of these suppliers do not meet the TOI PCs’ needs for the products they supply, including in the event of a product recall, shortage or dispute, and we are not able to find adequate alternative sources, if we experience material price increases from these suppliers that we are unable to mitigate, or if some of the drugs that the TOI PCs purchase are not reimbursed or not adequately reimbursed by commercial or government payors, it could have a material adverse impact on any matters submittedour business, results of operations, financial condition and cash flows. In addition, the technology related to the warrant holders for approval in connection with our initial business combination. Any such purchases of our securitiesproducts critical to the services we provide is subject to new developments which may result in superior products. If we are not able to access superior products on a cost-effective basis or if suppliers are not able to fulfill our requirements for such products, we and the completionTOI PCs could face patient attrition and other negative consequences which could have a material adverse effect on our business, results of operations, financial condition and cash flows.
We depend on our information technology systems, and those of our initialthird-party vendors, contractors and consultants, and any failure or significant disruptions of these systems, security breaches or loss of data could materially adversely affect our business, combinationfinancial condition and results of operations.
Our business is highly dependent on maintaining effective information systems as well as the integrity and timeliness of the data we use to serve our patients, support our care teams and operate our business. Because of the large amount of data that we collect and manage, it is possible that hardware failures or errors in our systems could result in data loss or corruption or cause the information that we collect to be incomplete or contain inaccuracies that our partners regard as significant. If our data were found to be inaccurate or unreliable due to fraud or other error, or if we, or any of the third-party service providers we engage, were to fail to maintain information systems and data integrity effectively, we could experience operational disruptions that may not otherwiseimpact our patients and care teams and hinder our ability to provide services, establish appropriate pricing for services,
retain and attract patients, manage our patient risk profiles, establish reserves, report financial results timely and accurately and maintain regulatory compliance, among other things.
Our information technology strategy and execution are critical to our continued success. We must continue to invest in long-term solutions that will enable us to anticipate patient needs and expectations, enhance the patient experience, act as a differentiator in the market and protect against cybersecurity risks and threats. We believe our success is dependent, in large part, on maintaining the effectiveness of existing technology systems and continuing to deliver and enhance technology systems that support our business processes in a cost-efficient and resource-efficient manner. Increasing regulatory and legislative changes will place additional demands on our information technology infrastructure that could have
been possible.a direct impact on resources available for other projects tied to our strategic initiatives. In addition, if such purchasesrecent trends toward greater patient engagement in health care require new and enhanced technologies, including more sophisticated applications for mobile devices. Connectivity among technologies is becoming increasingly important. We must also develop new systems to meet current market standards and keep pace with continuing changes in information processing technology, evolving industry and regulatory standards and patient needs. Failure to do so may present compliance challenges and impede our ability to deliver services in a competitive manner. Further, because system development projects are made,long-term in nature, they may be more costly than expected to complete and may not deliver the public “float”expected benefits upon completion.
Security incidents compromising the confidentiality, integrity, and availability of our
Class A common stockconfidential or
public warrantspersonal information and
the numberour and our third-party service providers’ information technology systems could result from cyber-attacks, computer malware, viruses, social engineering (including spear phishing and ransomware attacks), credential stuffing, supply chain attacks, efforts by individuals or groups of
beneficial holdershackers and sophisticated organizations, including state-sponsored organizations, errors or malfeasance of our
securities may be reduced, possibly making it difficult to obtainpersonnel, and security vulnerabilities in the software or
maintain the quotation, listingsystems on which we and our third party service providers rely. As techniques used by cyber criminals change frequently, a disruption, cyberattack or
tradingother security breach of our
securities on a national securities exchange.If a stockholder fails to receive noticeinformation technology systems or infrastructure, or those of our offerthird-party service providers, may go undetected for an extended period and could result in the theft, transfer, unauthorized access to, redeem our public shares in connection with our initial business combination,disclosure, modification, misuse, loss or fails to comply with the procedures for tendering its shares, such shares may not be redeemed.
We will comply with the proxy rules or tender offer rules, as applicable, when conducting redemptions in connection with our initial business combination. Despite our compliance with these rules, if a stockholder fails to receive our proxy materials or tender offer documents, as applicable, such stockholder may not become aware of the opportunity to redeem its shares. In addition, proxy materials or tender offer documents, as applicable, that we will furnish to holdersdestruction of our public shares in connection withemployee, representative, customer, vendor, consumer and/or other third-party data, including sensitive or confidential data, personal information and/or intellectual property. We and certain of our initial business combination will describe the various procedures that must be complied with in orderservice providers are from time to validly tender or submit public shares for redemption. For example, we intend to require our public stockholders seeking to exercise their redemption rights, whether they are record holders or hold their shares in “street name,” to, at the holder’s option, either deliver their stock certificates to our transfer agent, or to deliver their shares to our transfer agent electronically prior to the date set forth in the proxy materials or tender offer documents, as applicable. In the case of proxy materials, this date may be up to two business days prior to the vote on the proposal to approve the initial business combination. In addition, if we conduct redemptions in connection with a stockholder vote, we intend to require a public stockholder seeking redemption of its public shares to also submit a written request for redemption to our transfer agent two business days prior to the vote in which the name of the beneficial owner of such shares is included. In the event that a stockholder fails to comply with these or any other procedures disclosed in the proxy or tender offer materials, as applicable, its shares may not be redeemed. See the section of this prospectus entitled “Proposed Business - Submitting Stock Certificates in Connection with Redemption Rights.”
You will not have any rights or interests in funds from the Trust Account, except under certain limited circumstances. Therefore, to liquidate your investment, you may be forced to sell your public shares or warrants, potentially at a loss.
Our public stockholders will be entitled to receive funds from the Trust Account only upon the earlier to occur of: (i) our completion of an initial business combination, and then only in connection with those shares of Class A common stock that such stockholder properly elected to redeem,time, subject to the limitations described herein, (ii) the redemption of any public shares properly tendered in connection with a stockholder vote to amendcyberattacks and security incidents, and we cannot guarantee that our second amended and restated certificate of incorporation to modify the substancesecurity efforts will prevent breaches or timingbreakdowns of our obligation to redeem 100% ofor our public shares ifthird-party service providers’ information technology systems. While we do not complete our initial business combination by March 13, 2022 or with respect to any other material provisions
relating to stockholders’ rights or pre-initial business combination activity, and (iii) the redemption of our public shares if we do not complete an initial business combination by March 13, 2022, subject to applicable law and as further described herein. In addition, if our plan to redeem our public shares if we do not complete an initial business combination by March 13, 2022 is not completed for any reason, compliance with Delaware law may requirebelieve that we submit a plan of dissolutionhave experienced any significant system failure, accident or security breach to our then-existing stockholders for approval prior to the distribution of the proceeds held in our Trust Account. In that case, public stockholders may be forced to wait beyond 24 months from the closing of the initial public offering before they receive funds from our Trust Account. In no other circumstances will a public stockholder have any right or interest of any kind in the Trust Account. Accordingly, to liquidate your investment, you may be forced to sell your public shares or warrants, potentially at a loss.
You will not be entitled to protections normally afforded to investors of many other blank check companies.
Since the net proceeds of the initial public offering and the sale of the private placement warrants are intended to be used to complete an initial business combination with a target business that has not been selected,date, if we may be deemed to be a “blank check” company under the United States securities laws. However, because we have net tangible assets in excess of $5,000,000 and have filed a Current Report on Form 8-K, including an audited balance sheet demonstrating this fact, we are exempt from rules promulgated by the SEC to protect investors in blank check companies, such as Rule 419. Accordingly, investors will not be afforded the benefits or protections of those rules. Among other things, this means that we will have a longer period of time to complete our initial business combination than do companies subject to Rule 419. Moreover, if the initial public offering had been subject to Rule 419, that rule would have prohibited the release of any interest earned on funds held in the Trust Account to us unless and until the funds in the Trust Account were released to us in connection with our completion of an initial business combination.
If we seek stockholder approval of our initial business combination and we do not conduct redemptions pursuant to the tender offer rules, and if you or a “group” of stockholders are deemed to hold in excess of 15% of our Class A common stock, you will lose the ability to redeem all such shares in excess of 15% of our Class A common stock.
If we seek stockholder approval of our initial business combination and we do not conduct redemptions in connection with our initial business combination pursuant to the tender offer rules, our second amended and restated certificate of incorporation provides that a public stockholder, together with any affiliate of such stockholder or any other person with whom such stockholder is acting in concert or as a “group” (as defined under Section 13 of the Exchange Act), will be restricted from seeking redemption rights with respect to more than an aggregate of 15% of the shares sold in the initial public offering without our prior consent, which we refer to as the “Excess Shares.” However, we would not be restricting our stockholders’ ability to vote all of their shares (including Excess Shares) for or against our initial business combination. Your inability to redeem the Excess Shares will reduce your influence over our ability to complete our initial business combination and you could suffer a material loss on your investment in us if you sell Excess Shares in open market transactions. Additionally, you will not receive redemption distributions with respect to the Excess Shares if we complete our initial business combination. Andor disclosure of health-related or other personal or confidential information as a result you will continue to hold that number of shares exceeding 15% and, in order to dispose of such shares, would be required to sell your shares in open market transactions, potentially at a loss.
Becausebreach of our limited resourcesinformation technology systems, including those of our third-party service providers, we may suffer reputational, competitive and/or business harm, incur significant costs and the significant competition for business combination opportunities, it may be more difficult for ussubject to complete our initial business combination. If we do not complete our initial business combination, our public stockholders may receive only an estimated $10.00 per sharegovernment investigations, litigation, fines and/or damages, which could have a material adverse effect on our redemption, and our warrants will expire worthless.
We expect to encounter competition from other entities having a business, objective similar to ours, including private investors (whichprospects, results of operations, financial condition and/or cash flows. Moreover, while we maintain cyber insurance that may be individuals or investment partnerships), other blank check companies (including DFP) and other entities, domestic and international, competinghelp provide coverage for thethese types of businesses we intend to acquire. Many of these individuals and entities are well-established and have extensive experience in identifying and effecting, directly or indirectly, acquisitions of companies operating in or providing services to various industries. Many of these competitors possess similar or greater technical, human and other resources to ours or more local industry knowledge than we do and our financial resources will be relatively limited when contrasted with those of many of these competitors. While we believe there are numerous target businesses we could potentially acquire with the net proceeds of the initial public offering
and the sale of the private placement warrants, our ability to compete with respect to the acquisition of certain target businesses that are sizable will be limited by our available financial resources. This inherent competitive limitation gives others an advantage in pursuing the acquisition of certain target businesses. Furthermore, we are obligated to offer holders of our public shares the right to redeem their shares for cash at the time of our initial business combination in conjunction with a stockholder vote or via a tender offer. Target companies will be aware that this may reduce the resources available to us for our initial business combination. Any of these obligations may place us at a competitive disadvantage in successfully negotiating a business combination. If we do not complete our initial business combination, our public stockholders may receive only an estimated $10.00 per share on our redemption, and our warrants will expire worthless.
If the net proceeds of the initial public offering not being held in the Trust Account are insufficient, it could limit the amount available to fund our search for a target business or businesses and complete our initial business combination, and we will depend on loans from our sponsor or management team to fund our search and to complete our business combination.
We believe that the funds available to us outside of the Trust Account will be sufficient to allow us to operate until March 13, 2022; however,incidents, we cannot assure you that our estimate is accurate. Ofinsurance will be adequate to cover costs and liabilities related to these incidents. Further, our failure to effectively invest in, implement improvements to and properly maintain the funds availableuninterrupted operation and data integrity of our information technology and other business systems could adversely affect our results of operations, financial position and cash flow.
Finally, while we have been impacted by the recent Change Healthcare cyberattack which has caused disruptions to
us, we could use a portion ofhealthcare companies across the
funds available to us to pay fees to consultants to assist usUS, our team has been actively collaborating with our
searchpractice management vendor to swiftly establish alternative channels for
transmitting claims to payors. Significant progress has been made in successfully submitting claims to commercial payors and completed applications for Medicare and Medicaid agencies to accept our claims through a
target business. We could also use a portionnew intermediary, which is pending approval. It is anticipated that the delays in claim submissions will temporarily impact our cash flow in the first and second quarters of
the funds as a down payment or to fund a “no-shop” provision (a provision in letters of intent or merger agreements designed to keep target businesses from “shopping” around for transactions with other companies on terms more favorable to such target businesses) with respect to a particular proposed business combination, although we do not have any current intention to do so. If we entered into a letter of intent or merger agreement where we paid for the right to receive exclusivity from a target business and were subsequently required to forfeit such funds (whether as a result of our breach or otherwise), we might not have sufficient funds to continue searching for, or conduct due diligence with respect to, a target business.If we are required to seek additional capital, we would need to borrow funds from our sponsor, members of our management team or other third parties to operate or may be forced to liquidate. Neither our sponsor, members of our management team nor any of their affiliates is under any obligation to advance funds to us in such circumstances. Any such advances would be repaid only from funds held outside the Trust Account or from funds released to us upon completion of our initial business combination. Up to $1,500,000 of such loans may be convertible into warrants of the post-business combination entity at a price of $1.50 per warrant at the option of the lender. The warrants would be identical to the private placement warrants. Prior to the completion of our initial business combination, we do not expect to seek loans from parties other than our sponsor or affiliate or an affiliate of our sponsor and2024. Nevertheless, we do not believe third parties wouldthe impact to be willing to loan such fundsmaterial and provide a waiver against any and all rights to seek access to the fundsremain confident in our trust account. Ifability to resolve these challenges. Although, we do not complete our initial business combination because we doare confident that this recent cyberattack will not have sufficient funds available to us, wea material adverse impact, similar cybersecurity breaches could be successfully launched in the future, and there is no assurance that such attacks will be forced to ceasenot have material adverse effect on our results of operations and liquidatecash flows.
We may be subject to legal proceedings and litigation, including intellectual property and privacy disputes, which are costly to defend and could materially harm our business and results of operations.
We and the Trust Account. Consequently,TOI PCs may be party to lawsuits and legal proceedings in the normal course of business. These matters are often expensive and disruptive to normal business operations. We may face allegations, lawsuits and regulatory inquiries, audits and investigations regarding data privacy, security, labor and employment, consumer protection and intellectual property infringement, including claims related to privacy, patents, publicity, trademarks, copyrights and other rights. We may also face allegations or litigation related to our acquisitions, securities issuances or business practices, including public stockholdersdisclosures about our business. Litigation and regulatory proceedings may only receive an estimated $10.00 per share, onbe protracted and expensive, and the results are difficult to predict. Certain of these matters may include speculative claims for substantial or indeterminate amounts of damages and include claims
for injunctive relief. Additionally, our redemptionlitigation costs could be significant. Adverse outcomes with respect to litigation or any of these legal proceedings may result in significant settlement costs or judgments, penalties and fines, or require us to modify our public shares,services or require us to stop serving certain patients or geographies, all of which could negatively impact our geographical expansion and revenue growth. The TOI PCs may also become subject to periodic audits, which would likely increase our regulatory compliance costs and may require us to change our business practices, which could negatively impact our revenue growth. Managing legal proceedings, litigation and audits, even if we achieve favorable outcomes, is time-consuming and diverts the attention of management and our warrantsaffiliated providers from our business.
The results of regulatory proceedings, litigation, claims, and audits cannot be predicted with certainty, and determining reserves for pending litigation and other legal, regulatory and audit matters requires significant judgment. There can be no assurance that our expectations will
expire worthless.Subsequentprove correct, and even if these matters are resolved in our favor or without significant cash settlements, these matters, and the time and resources necessary to litigate or resolve them, could harm our completion of our initialreputation, business, combination, we may be required to take write-downs or write-offs, restructuring and impairment or other charges that could have a significant negative effect on our financial condition, results of operations and the market price of our securities, which could cause youcommon stock.
Furthermore, our business exposes the TOI PCs and our affiliated providers to
lose some or all of your investment.Even if we conduct extensive due diligence on a target business with which we combine, we cannot assure you that this diligence will identify all material issues that may be present with a particular target business, that it would be possible to uncover all material issues through a customary amount of due diligence, or that factors outside of the target business and outside of our control will not later arise. As a result of these factors, we may be forced to later write-down or write-off assets, restructure our operations, or incur impairmentpotential medical malpractice, professional negligence or other chargesrelated actions or claims that could resultare inherent in our reporting losses. Even if our due diligence successfully identifies certain risks, unexpected risks may arise and previously known risks may materialize in a manner not consistentthe provision of healthcare services. These claims, with our preliminary risk analysis. Even though these charges may be non-cash items and not have an immediate impact on our liquidity, the fact that we report charges of this natureor without merit, could contribute to negative market perceptions about us or our securities. In addition, charges of this nature may cause us to violate net worth or other covenants to which we may be subject asincur substantial costs, and could place a resultsignificant strain on our financial resources, divert the attention of assuming pre-existing debt held by a target business or by virtue of our obtaining debt financing to partially finance the initial business combination or thereafter. Accordingly, any
stockholders or warrantholders who choose to remain stockholders or warrantholders following the business combination could suffer a reduction in the value of their securities. Such stockholders or warrantholders are unlikely to have a remedy for such reduction in value unless they are able to successfully claim that the reduction was due to the breach by our officers or directors of a duty of care or other fiduciary duty owed to them, or if they are able to successfully bring a private claim under securities laws that the proxy materials or tender offer documents, as applicable, relating to the business combination contained an actionable material misstatement or material omission.
If third parties bring claims against us, the proceeds held in the Trust Account could be reduced and the per-share redemption amount received by stockholders may be less than $10.00 per share.
Our placing of funds in the Trust Account may not protect those funds from third party claims against us. Although we will seek to have all vendors, service providers, prospective target businesses and other entities with which we do business execute agreements with us waiving any right, title, interest or claim of any kind in or to any monies held in the Trust Account for the benefit of our public stockholders, such parties may not execute such agreements, or even if they execute such agreements they may not be prevented from bringing claims against the Trust Account, including, but not limited to, fraudulent inducement, breach of fiduciary responsibility or other similar claims, as well as claims challenging the enforceability of the waiver, in each case in order to gain advantage with respect to a claim against our assets, including the funds held in the Trust Account. If any third party refuses to execute an agreement waiving such claims to the monies held in the Trust Account, our management will consider whether competitive alternatives are reasonably available to us and will only enter into an agreement with such third party if management believes that such third party’s engagement would be in the best interests of the company under the circumstances.
Examples of possible instances where we may engage a third party that refuses to execute a waiver include the engagement of a third party consultant whose particular expertise or skills are believed by management to be significantly superior to those of other consultants that would agree to execute a waiver or in cases where management is unable to find a service provider willing to execute a waiver. In addition, there is no guarantee that such entities will agree to waive any claims they may have in the future as a result of, or arising out of, any negotiations, contracts or agreements with us and will not seek recourse against the Trust Account for any reason. Upon redemption of our public shares, if we do not complete our initial business combination within the prescribed timeframe, or upon the exercise of a redemption right in connection with our initial business combination, we will be required to provide for payment of claims of creditors that were not waived that may be brought against us within the 10 years following redemption. Accordingly, the per-share redemption amount received by public stockholders could be less than the $10.00 per public share initially held in the Trust Account, due to claims of such creditors. Pursuant to the letter agreement the form of which is filed as an exhibit to the registration statement of which this prospectus forms a part, our sponsor has agreed that it will be liable to us if and to the extent any claims by a third party for services rendered or products sold to us, or a prospective target business with which we have entered into a written letter of intent, confidentiality or other similar agreement or business combination agreement, reduce the amount of funds in the Trust Account to below the lesser of (i) $10.00 per public share and (ii) the actual amount per public share held in the Trust Account as of the date of the liquidation of the Trust Account, if less than $10.00 per public share due to reductions in the value of the trust assets, less taxes payable, provided that such liability will not apply to any claims by a third party or prospective target business who executed a waiver of any and all rights to the monies held in the Trust Account (whether or not such waiver is enforceable) nor will it apply to any claims under our indemnity of the underwriters of the initial public offering against certain liabilities, including liabilities under the Securities Act. However, we have not asked our sponsor to reserve for such indemnification obligations, nor have we independently verified whether our sponsor has sufficient funds to satisfy its indemnity obligations and we believe that our sponsor’s only assets are securities of our company. Therefore, we cannot assure you that our sponsor would be able to satisfy those obligations. As a result, if any such claims were successfully made against the Trust Account, the funds available for our initial business combination and redemptions could be reduced to less than $10.00 per public share. In such event, we may not be able to complete our initial business combination, and you would receive such lesser amount per share in connection with any redemption of your public shares. None of our officers or directors will indemnify us for claims by third parties including, without limitation, claims by vendors and prospective target businesses.
Our directors may decide not to enforce the indemnification obligations of our sponsor, resulting in a reduction in the amount of funds in the Trust Account available for distribution to our public stockholders.
In the event that the proceeds in the Trust Account are reduced below the lesser of (i) $10.00 per share and (ii) the actual amount per public share held in the Trust Account as of the date of the liquidation of the Trust Account, in each case less taxes payable, and our sponsor asserts that it is unable to satisfy its obligations or that it has no indemnification obligations related to a particular claim, our independent directors would determine whether to take legal action against our sponsor to enforce its indemnification obligations. While we currently expect that our independent directors would take legal action on our behalf against our sponsor to enforce its indemnification obligations to us, it is possible that our independent directors in exercising their business judgment and subject to their fiduciary duties may choose not to do so in any particular instance. If our independent directors choose not to enforce these indemnification obligations, the amount of funds in the Trust Account available for distribution to our public stockholders may be reduced below $10.00 per share.
If, after we distribute the proceeds in the Trust Account to our public stockholders, we file a bankruptcy petition or an involuntary bankruptcy petition is filed against us that is not dismissed, a bankruptcy court may seek to recover such proceeds, and the members of our board of directors may be viewed as having breached their fiduciary duties to our creditors, thereby exposing the members of our board of directors and us to claims of punitive damages.
If, after we distribute the proceeds in the Trust Account to our public stockholders, we file a bankruptcy petition or an involuntary bankruptcy petition is filed against us that is not dismissed, any distributions received by stockholders could be viewed under applicable debtor/creditor and/or bankruptcy laws as either a “preferential transfer” or a “fraudulent conveyance.” As a result, a bankruptcy court could seek to recover some or all amounts received by our stockholders. In addition, our board of directors may be viewed as having breached its fiduciary duty to our creditors and/or having acted in bad faith, by paying public stockholders from the Trust Account prior to addressing the claims of creditors, thereby exposing itself and us to claims of punitive damages.
If, before distributing the proceeds in the Trust Account to our public stockholders, we file a bankruptcy petition or an involuntary bankruptcy petition is filed against us that is not dismissed, the claims of creditors in such proceeding may have priority over the claims of our stockholders and the per-share amount that would otherwise be received by our stockholders in connection with our liquidation may be reduced.
If, before distributing the proceeds in the Trust Account to our public stockholders, we file a bankruptcy petition or an involuntary bankruptcy petition is filed against us that is not dismissed, the proceeds held in the Trust Account could be subject to applicable bankruptcy law, and may be included in our bankruptcy estate and subject to the claims of third parties with priority over the claims of our stockholders. To the extent any bankruptcy claims deplete the Trust Account, the per-share amount that would otherwise be received by our stockholders in connection with our liquidation may be reduced.
Our stockholders may be held liable for claims by third parties against us to the extent of distributions received by them upon redemption of their shares.
Under the DGCL, stockholders may be held liable for claims by third parties against a corporation to the extent of distributions received by them in a dissolution. The pro rata portion of our Trust Account distributed to our public stockholders upon the redemption of our public shares in the event we do not complete our initial business combination by March 13, 2022 may be considered a liquidating distribution under Delaware law. If a corporation complies with certain procedures set forth in Section 280 of the DGCL intended to ensure that it makes reasonable provision for all claims against it, including a 60- day notice period during which any third-party claims can be brought against the corporation, a 90-day period during which the corporation may reject any claims brought, and an additional 150-day waiting period before any liquidating distributions are made to stockholders, any liability of stockholders with respect to a liquidating distribution is limited to the lesser of such stockholder’s pro rata share of the claim or the amount distributed to the stockholder, and any liability of the stockholder would be barred after the third anniversary of the dissolution. However, it is our intention to redeem our public shares as soon as reasonably possible following the 24th month from the closing of the initial public offering in the event we do not complete our initial business combination and, therefore, we do not intend to comply with the foregoing procedures.
Because we will not be complying with Section 280, Section 281(b) of the DGCL requires us to adopt a plan, based on facts known to us at such time that will provide for our payment of all existing and pending claims or claims that may be potentially brought against us within the 10 years following our dissolution. However, because we are a blank check company, rather than an operating company, and our operations will be limited to searching for prospective target businesses to acquire, the only likely claims to arise would beaffiliated providers from our vendors (such as lawyers, investment bankers, etc.) or prospective target businesses. Ifcore business, harm our plan of distribution complies with Section 281(b) ofreputation and adversely affect the DGCL, any liability of stockholders with respect to a liquidating distribution is limited to the lesser of such stockholder’s pro rata share of the claim or the amount distributed to the stockholder, and any liability of the stockholder would likely be barred after the third anniversary of the dissolution. We cannot assure you that we will properly assess all claims that may be potentially brought against us. As such, our stockholders could potentially be liable for any claims to the extent of distributions received by them (but no more) and any liability of our stockholders may extend beyond the third anniversary of such date. Furthermore, if the pro rata portion of our Trust Account distributed to our public stockholders upon the redemption of our public shares in the event we do not complete our initial business combination by March 13, 2022 is not considered a liquidating distribution under Delaware law and such redemption distribution is deemed to be unlawful (potentially due to the imposition of legal proceedings that a party may bring or due to other circumstances that are currently unknown), then pursuant to Section 174 of the DGCL, the statute of limitations for claims of creditors could then be six years after the unlawful redemption distribution, instead of three years, as in the case of a liquidating distribution.
We may not hold an annual meeting of stockholders until after the consummation of our initial business combination, which could delay the opportunity for our stockholders to elect directors.
In accordance with Nasdaq corporate governance requirements, we are not required to hold an annual meeting until no later than one year after our first fiscal year end following our listing on Nasdaq. Under Section 211(b) of the DGCL, we are, however, required to hold an annual meeting of stockholders for the purposes of electing directors in accordance with our bylaws unless such election is made by written consent in lieu of such a meeting. We may not hold an annual meeting of stockholders to elect new directors prior to the consummation of our initial business combination, and thus we may not be in compliance with Section 211(b) of the DGCL, which requires an annual meeting. Therefore, if our stockholders want us to hold an annual meeting prior to the consummation of our initial business combination, they may attempt to force us to hold one by submitting an application to the Delaware Court of Chancery in accordance with Section 211(c) of the DGCL.
Because we are not limited to a target business in a particular industry sector or any specific target businesses with which to pursue our initial business combination, you will be unable to ascertain the merits or risks of any particular target business’ operations.
We will seek to complete a business combination target with an operating company in the healthcare or healthcare-related industries but may also pursue acquisition opportunities in other sectors, except that we will not, under our second amended and restated certificate of incorporation prohibits us from effectuating a business combination with another blank check company or similar company with nominal operations. Because we have not yet selected any specific target business with respect to a business combination, there is no basis to evaluate the possible merits or risks of any particular target business’s operations, results of operations, cash flows, liquidity, financial condition or prospects. To the extent we complete our initial business combination, we may be affected by numerous risks inherent in the business operations with which we combine. For example, if we combine with a financially unstable business or an entity lacking an established record of sales or earnings, we may be affected by the risks inherent in the business and operations of a financially unstable or a development stage entity. Although our officers and directors will endeavor to evaluate the risks inherent in a particular target business, we cannot assure you that we will properly ascertain or assess all of the significant risk factors or that we will have adequate time to complete due diligence. Furthermore, some of these risks may be outside of our control and leave us with noTOI PCs’ ability to control or reduce the chances that those risks will adversely impact a target business. We also cannot assure you that an investment in our units will ultimately prove to be more favorable to investors than a direct investment, if such opportunity were available, in a business combination target. Accordingly, any stockholders or warrantholders who choose to remain stockholders or warrantholders following the business combination could suffer a reduction in the value of their securities. Such stockholders or warrantholders are unlikely to have a remedy for such reduction in value unless they are able to successfully claim that the reduction was due to the breach by our officers or directors of a duty of care or other fiduciary duty owed to them, or if they are able to successfully bring a private
claim under securities laws that the proxy materials or tender offer documents, as applicable, relating to the business combination contained an actionable material misstatement or material omission.
We may seek business combination opportunities in industries outside of the healthcare industry (which industries may or may not be outside of our management’s area of expertise).
Although we intend to focus on identifying business combination candidates in the healthcare industry in the United States (including candidates based in the United States which may have operations or opportunities outside the United States) or other developed countries,attract and we will not initially actively seek to identify business combination candidates in other industries (which industries may be outside our management’s area of expertise), we will consider a business combination outside of the healthcare industry if a business combination candidate is presented to us and we determine that such candidate offers an attractive acquisition opportunity for our company or we are unable to identify a suitable candidate in the healthcare industry after having expended a reasonable amount of time and effort in an attempt to do so. Although our management will endeavor to evaluate the risks inherent in any particular business combination candidate, we may not adequately ascertain or assess all of the risks. An investment in our units may ultimately prove to be less favorable to investors in the initial public offering than a direct investment, if an opportunity were available, in a business combination candidate.
In the event we elect to pursue a business combination outside of the healthcare industry, our management’s expertise may not be directly applicable to its evaluation or operation, and the information contained in this prospectus regarding the healthcare industry would not be relevant to an understanding of the business that we elect to acquire.
Although we identified general criteria and guidelines that we believe are important in evaluating prospective target businesses, we may enter into our initial business combination with a target that does not meet such criteria and guidelines, and as a result, the target business with which we enter into our initial business combination may not have attributes entirely consistent with our general criteria and guidelines.
Although we have identified general criteria and guidelines for evaluating prospective target businesses, it is possible that a target business with which we enter into our initial business combination will not have all of these positive attributes. If we complete our initial business combination with a target that does not meet some or all of these guidelines, such combination may not be as successful as a combination with a business that does meet all of our general criteria and guidelines. In addition, if we announce a prospective business combination with a target that does not meet our general criteria and guidelines, a greater number of stockholders may exercise their redemption rights, which may make it difficult for us to meet any closing condition with a target business that requires us to have a minimum net worth or a certain amount of cash. In addition, if stockholder approval of the transaction is required by law, or we decide to obtain stockholder approval for business or other legal reasons, it may be more difficult for us to attain stockholder approval of our initial business combination if the target business does not meet our general criteria and guidelines. If we do not complete our initial business combination, our public stockholders may only receive their pro rata portion of the funds in the trust account that are available for distribution to public stockholders, and our warrants will expire worthless.
We are not required to obtain an opinion from an independent investment banking firm or from a valuation or appraisal firm, and consequently, you may have no assurance from an independent source that the price we are paying for the business is fair to our stockholders from a financial point of view.
Unless we complete our initial business combination with an affiliated entity or our board of directors cannot independently determine the fair market value of the target business or businesses (including with the assistance of financial advisors), we are not required to obtain an opinion from an independent investment banking firm which is a member of FINRA or from a valuation or appraisal firm that the price we are paying is fair to our stockholders from a financial point of view. If no opinion is obtained, our stockholders will be relying on the judgment of our board of directors, who will determine fair market value based on standards generally accepted by the financial community. Such standards used will be disclosed in our proxy materials or tender offer documents, as applicable, related to our initial business combination.
Because we must furnish our stockholders with target business financial statements, we may lose the ability to complete an otherwise advantageous initial business combination with some prospective target businesses.
The federal proxy rules require that the proxy statement with respect to the vote on an initial business combination include historical and pro forma financial statement disclosure. We will include the same financial statement disclosure in connection with our tender offer documents, whether or not they are required under the tender offer rules. These financial statements may be required to be prepared in accordance with, or be reconciled to, accounting principles generally accepted in the United States of America (“GAAP”), or international financial reporting standards as issued by the International Accounting Standards Board (“IFRS”), depending on the circumstances and the historical financial statements may be required to be audited in accordance with the standards of the Public Company Accounting Oversight Board (United States) (“PCAOB”). These financial statement requirements may limit the pool of potential target businesses we may acquire because some targets may be unable to provide such financial statements in time for us to disclose such statements in accordance with federal proxy rules and complete our initial business combination within the prescribed time frame.
Compliance obligations under the Sarbanes-Oxley Act may make it more difficult for us to effectuate our initial business combination, require substantial financial and management resources, and increase the time and costs of completing an initial business combination.
Section 404 of the Sarbanes-Oxley Act requires that we evaluate and report on our system of internal controls beginning with our Annual Report on Form 10-K/A for the year ending December 31, 2021. Only in the event we are deemed to be a large accelerated filer or an accelerated filer, and no longer qualify as an emerging growth company, will we be required to comply with the independent registered public accounting firm attestation requirement on our internal control over financial reporting. Further, for as long as we remain an emerging growth company, we will not be required to comply with the independent registered public accounting firm attestation requirement on our internal control over financial reporting. The fact that we are a blank check company makes compliance with the requirements of the Sarbanes-Oxley Act particularly burdensome on us as compared to other public companies because a target business with which we seek to complete our initial business combination may not be in compliance with the provisions of the Sarbanes-Oxley Act regarding adequacy of its internal controls. The development of the internal control of any such entity to achieve compliance with the Sarbanes-Oxley Act may increase the time and costs necessary to complete any such business combination.
We do not have a specified maximum redemption threshold. The absence of such a redemption threshold may make it possible for us to complete our initial business combination with which a substantial majority of our stockholders or warrantholders do not agree.
Our second amended and restated certificate of incorporation does not provide a specified maximum redemption threshold, except that in no event will we redeem our public shares in an amount that would cause our net tangible assets to be less than $5,000,001. In addition, our proposed initial business combination may impose a minimum cash requirement for: (i) cash consideration to be paid to the target or its owners, (ii) cash for working capital or other general corporate purposes or (iii) the retention of cash to satisfy other conditions. As a result, we may be able to complete our initial business combination even though a substantial majority of our public stockholders do not agree with the transaction and have redeemed their shares or, if we seek stockholder approval of our initial business combination and do not conduct redemptions in connection with our initial business combination pursuant to the tender offer rules, have entered into privately negotiated agreements to sell their shares to our sponsor, officers, directors, advisors orretain patients, any of their affiliates. In the event the aggregate cash consideration we would be required to pay for all shares of Class A common stock that are validly submitted for redemption plus any amount required to satisfy cash conditions pursuant to the terms of the proposed business combination exceed the aggregate amount of cash available to us, we will not complete the business combination or redeem any shares in connection with such initial business combination, all shares of Class A common stock submitted for redemption will be returned to the holders thereof, and we instead may search for an alternate business combination.
In order to effectuate an initial business combination, special purpose acquisition companies have, in the recent past, amended various provisions of their charters and other governing instruments, including their warrant agreements. We cannot assure you that we will not seek to amend our second amended and restated certificate of incorporation or governing instruments in a manner that will make it easier for us to complete our initial business combination that our stockholders may not support.
In order to effectuate a business combination, special purpose acquisition companies have, in the recent past, amended various provisions of their charters and governing instruments, including their warrant agreements. For example, special purpose acquisition companies have amended the definition of business combination, increased redemption thresholds, changed industry focus and, with respect to their warrants, amended their warrant agreements to require the warrants to be exchanged for cash and/or other securities. Amending our second amended and restated certificate of incorporation will require the approval of holders of 65% of our common stock, and amending our warrant agreement will require a vote of holders of at least 50% of the public warrants and, solely with respect to any amendment to the terms of the private placement warrants or any provision of the warrant agreement with respect to the private placement warrants, 50% of the number of the then outstanding private placement warrants. In addition, our second amended and restated certificate of incorporation requires us to provide our public stockholders with the opportunity to redeem their public shares for cash if we propose an amendment to our second amended and restated certificate of incorporation to modify the substance or timing of our obligation to redeem 100% of our public shares if we do not complete an initial business combination by March 13, 2022. We cannot assure you that we will not seek to amend our charter or governing instruments or extend the time to consummate an initial business combination in order to effectuate our initial business combination.
The provisions of our second amended and restated certificate of incorporation that relate to our pre-business combination activity (and corresponding provisions of the agreement governing the release of funds from our Trust Account) may be amended with the approval of holders of 65% of our common stock, which is a lower amendment threshold than that of some other special purpose acquisition companies. It may be easier for us, therefore, to amend our second amended and restated certificate of incorporation to facilitate the completion of an initial business combination that some of our stockholders may not support.
Our second amended and restated certificate of incorporation provides that any of its provisions related to pre-business combination activity (including the requirement to deposit proceeds of the initial public offering and the private placement of warrants into the Trust Account and not release such amounts except in specified circumstances, and to provide redemption rights to public stockholders as described herein) may be amended if approved by holders of 65% of our common stock entitled to vote thereon and corresponding provisions of the trust agreement governing the release of funds from our Trust Account may be amended if approved by holders of 65% of our common stock entitled to vote thereon. In all other instances, our second amended and restated certificate of incorporation may be amended by holders of a majority of our outstanding common stock entitled to vote thereon, subject to applicable provisions of the DGCL or applicable stock exchange rules. Our initial stockholders, who will collectively beneficially own 20% of our common stock upon the closing of the initial public offering (assuming they do not purchase any units in the initial public offering), may participate in any vote to amend our second amended and restated certificate of incorporation and/or trust agreement and will have the discretion to vote in any manner they choose. As a result, we may be able to amend the provisions of our second amended and restated certificate of incorporation which govern our pre-business combination behavior more easily than some other special purpose acquisition companies, and this may increase our ability to complete a business combination with which you do not agree. Our stockholders may pursue remedies against us for any breach of our second amended and restated certificate of incorporation.
Our sponsor, executive officers, directors and director nominees have agreed, pursuant to written agreements with us, that they will not propose any amendment to our second amended and restated certificate of incorporation to modify the substance or timing of our obligation to redeem 100% of our public shares if we do not complete our initial business combination by March 13, 2022 or with respect to any other material provisions relating to stockholders’ rights or pre-initial business combination activity, unless we provide our public stockholders with the opportunity to redeem their Class A common stock upon approval of any such amendment at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the Trust Account, including interest earned on the funds held in the Trust Account and not previously released to us to fund our working capital requirements (subject to an annual limit of $500,000) and/or to pay our taxes, divided by the number of then outstanding public shares. Our stockholders are not parties to, or third-party
beneficiaries of, these agreements and, as a result, will not have the ability to pursue remedies against our sponsor, executive officers, directors or director nominees for any breach of these agreements. As a result, in the event of a breach, our stockholders would need to pursue a stockholder derivative action, subject to applicable law.
We may be unable to obtain additional financing to complete our initial business combination or to fund the operations and growth of a target business, which could compel us to restructure or abandon a particular business combination.
We intend to target businesses combination targets with enterprise values that are greater than we could acquire with the net proceeds of the initial public offering and the sale of the private placement warrants. As a result, if the cash portion of the purchase price exceeds the amount available from the Trust Account, net of amounts needed to satisfy any redemption by public stockholders, we may be required to seek additional financing to complete such proposed initial business combination. Such financing may not be available on acceptable terms, if at all. To the extent that additional financing proves to be unavailable when needed to complete our initial business combination, we would be compelled to either restructure the transaction or abandon that particular business combination and seek an alternative target business candidate. Further, we may be required to obtain additional financing in connection with the closing of our initial business combination for general corporate purposes, including for maintenance or expansion of operations of the post-transaction businesses, the payment of principal or interest due on indebtedness incurred in completing our initial business combination, or to fund the purchase of other companies. If we do not complete our initial business combination, our public stockholders may only receive an estimated $10.00 per share on our redemption, and our warrants will expire worthless. In addition, even if we do not need additional financing to complete our initial business combination, we may require such financing to fund the operations or growth of the target business. The failure to secure additional financing could have a material adverse effect on the continued development or growth of the target business. None of our officers, directors or stockholders is required to provide any financing to us in connection with or after our initial business combination.
Our initial stockholders control a substantial interest in us and thus may exert a substantial influence on actions requiring a stockholder vote, potentially in a manner that you do not support.
Our initial stockholders own 20% of our issued and outstanding common stock. Accordingly, they may exert a substantial influence on actions requiring a stockholder vote, potentially in a manner that you do not support, including amendments to our second amended and restated certificate of incorporation. If our initial stockholders purchase any additional Class A common stock, this would increase their control. Neither our initial stockholders nor, to our knowledge, any of our officers or directors, have any current intention to purchase additional securities. Factors that would be considered in making such additional purchases would include consideration of the current trading price of our Class A common stock. In addition, our board of directors, whose members were elected by our sponsor, is and will be divided into three classes, each of which will generally serve for a terms for three years with only one class of directors being elected in each year. We may not hold an annual meeting of stockholders to elect new directors prior to the completion of our initial business combination, in which case all of the current directors will continue in office until at least the completion of the business combination. If there is an annual meeting, as a consequence of our “staggered” board of directors, only a minority of the board of directors will be considered for election and our initial stockholders, because of their ownership position, will have considerable influence regarding the outcome. Accordingly, our initial stockholders will continue to exert control at least until the completion of our initial business combination.
Resources could be wasted in researching business combinations that are not completed, which could materially adversely affect subsequent attempts to locate and acquire or merge with another business. If we have not completed our initial business combination within the required time period, our public stockholders may only receive their pro rata portion of the funds in the trust account that are available for distribution to public stockholders, and our warrants will expire worthless.
We anticipate that the investigation of each specific target business and the negotiation, drafting and execution of relevant agreements, disclosure documents and other instruments will require substantial management time and attention and substantial costs for accountants, attorneys and others. If we decide not to complete a specific initial business combination, the costs incurred up to that point for the proposed transaction likely would not be recoverable. Furthermore, if we reach an agreement relating to a specific target business, we may fail to complete our initial business combination
for any number of reasons including those beyond our control. Any such event will result in a loss to us of the related costs incurred which could materially adversely affect subsequent attempts to locate and acquire or merge with another business. If we have not completed our initial business combination within the required time period, our public stockholders may only receive their pro rata portion of the funds in the trust account that are available for distribution to public stockholders, and our warrants will expire worthless.
Our key personnel may negotiate employment or consulting agreements with a target business in connection with a particular business combination. These agreements may provide for them to receive compensation following our initial business combination and as a result, may cause them to have conflicts of interest in determining whether a particular business combination is the most advantageous.
Our key personnel may be able to remain with our company after the completion of our initial business combination only if they are able to negotiate employment or consulting agreements in connection with the business combination. Such negotiations would take place simultaneously with the negotiation of the business combination and could provide for such individuals to receive compensation in the form of cash payments and/or our securities for services they would render to us after the completion of the business combination. Such negotiations also could make such key personnel’s retention or resignation a condition to any such agreement. The personal and financial interests of such individuals may influence their motivation in identifying and selecting a target business, subject to their fiduciary duties under Delaware law.
We may have a limited ability to assess the management of a prospective target business and, as a result, may effect our initial business combination with a target business whose management may not have the skills, qualifications or abilities to manage a public company.
When evaluating the desirability of effecting our initial business combination with a prospective target business, our ability to assess the target business’s management may be limited due to a lack of time, resources or information. Our assessment of the capabilities of the targets’ management, therefore, may prove to be incorrect and such management may lack the skills, qualifications or abilities we suspected. Should the targets’ management not possess the skills, qualifications or abilities necessary to manage a public company, the operations and profitability of the post-combination business may be negatively impacted. Accordingly, any stockholders or warrantholders who choose to remain stockholders or warrantholders following the business combination could suffer a reduction in the value of their securities. Such stockholders or warrantholders are unlikely to have a remedy for such reduction in value unless they are able to successfully claim that the reduction was due to the breach by our officers or directors of a duty of care or other fiduciary duty owed to them, or if they are able to successfully bring a private claim under securities laws that the proxy solicitation or tender offer materials, as applicable, relating to the business combination contained an actionable material misstatement or material omission.
If we effect our initial business combination with a company located outside of the United States, we would be subject to a variety of additional risks that may adversely affect us.
If we pursue a target company with operations or opportunities outside of the United States for our initial business combination, we may face additional burdens in connection with investigating, agreeing to and completing such initial business combination, and if we effect such initial business combination, we would be subject to a variety of additional risks that may negatively impact our operations.
If we pursue a target a company with operations or opportunities outside of the United States for our initial business combination, we would be subject to risks associated with cross-border business combinations, including in connection with investigating, agreeing to and completing our initial business combination, conducting due diligence in a foreign jurisdiction, having such transaction approved by any local governments, regulators or agencies and changes in the purchase price based on fluctuations in foreign exchange rates.
If we effect our initial business combination with such a company, we would be subject to any special considerations or risks associated with companies operating in an international setting, including any of the following:
| ● | costs and difficulties inherent in managing cross-border business operations; |
| ● | rules and regulations regarding currency redemption; |
| ● | complex corporate withholding taxes on individuals; |
| ● | laws governing the manner in which future business combinations may be effected; |
| ● | exchange listing and/or delisting requirements; |
| ● | tariffs and trade barriers; |
| ● | regulations related to customs and import/export matters; |
| ● | local or regional economic policies and market conditions; |
| ● | unexpected changes in regulatory requirements; |
| ● | challenges in managing and staffing international operations; |
| ● | tax issues, such as tax law changes and variations in tax laws as compared to the United States; |
| ● | currency fluctuations and exchange controls; |
| ● | challenges in collecting accounts receivable; |
| ● | cultural and language differences; |
| ● | underdeveloped or unpredictable legal or regulatory systems; |
| ● | protection of intellectual property; |
| ● | social unrest, crime, strikes, riots and civil disturbances; |
| ● | regime changes and political upheaval; |
| ● | terrorist attacks and wars; and |
| ● | deterioration of political relations with the United States. |
We may not be able to adequately address these additional risks. If we were unable to do so, we may be unable to complete such initial business combination, or, if we complete such initial business combination, our operations might suffer, either of which may adversely impact our business, financial condition and results of operations.
We
Although the TOI PCs and our affiliated providers maintain third-party professional liability insurance coverage, it is possible that claims against them may
issue notes or other debt securities, or otherwise incurexceed the coverage limits of their insurance policies. Even if any professional liability loss is covered by an insurance policy, these policies typically have substantial
debt, to completedeductibles for which the TOI PCs and our affiliated providers are responsible. Professional liability claims in excess of applicable insurance coverage could have a
business combination, which may adversely affectmaterial adverse effect on our
leverage andcollective business, financial condition and
thus negatively impactresults of operations. In addition, any professional liability claim brought against the
valueTOI PCs or our affiliated providers, with or without merit, could result in an increase of
our stockholders’ investmenttheir professional liability insurance premiums. Insurance coverage varies in
us.Althoughcost and can be difficult to obtain, and we have no commitments as of the date of this prospectus to issue any notes or other debt securities, or to otherwise incur outstanding debt following the initial public offering, we may choose to incur substantial debt to complete our initial business combination. We and our officers have agreedcannot guarantee that we will not incur any indebtedness unless we have obtained frombe able to obtain insurance coverage on behalf of the lender a waiver of any right, title, interest or claim of any kind in or to the monies heldTOI PCs and our affiliated providers in the Trust Account. future on terms acceptable to us or at all. If costs of insurance and claims increase, then our collective earnings could decline.
Some jurisdictions preclude the TOI PCs from entering into non-compete agreements with physicians, and other non-compete agreements and restrictive covenants applicable to certain physicians and other clinical employees may not be enforceable.
The TOI PCs have employment contracts with physicians and other health professionals in many states. Some of these contracts include provisions preventing these physicians and other health professionals from competing with us both during and after the term of our contract with them. The law governing non- compete agreements and other forms of restrictive covenants varies from state to state. Some jurisdictions prohibit the TOI PCs from using non-competition covenants with our professional staff. Other states are reluctant to strictly enforce non-compete agreements and restrictive covenants applicable to physicians and other healthcare professionals. Additionally, the Federal Trade Commission recently proposed new rules which, if enacted, would ban non-compete agreements in employee contracts. There can be no assurance that the TOI PCs’ non-compete agreements related to physicians and other health professionals will be found enforceable if challenged in certain states. In such event, the TOI PCs would be unable to prevent physicians and other health professionals formerly employed by the TOI PCs from competing with us, potentially resulting in the loss of some of our patients.
Current and future acquisitions may use significant resources, may be unsuccessful, and could expose us to unforeseen liabilities.
As
such, no issuancepart of
our growth strategy, we may pursue acquisitions of oncology and other physician practices and services. These acquisitions may involve significant cash expenditures, debt
will affect the per share amount available for redemption from the Trust Account. Nevertheless, the incurrence,
of debtadditional operational losses and expenses, and compliance risks that could have a
varietymaterial adverse effect on our financial condition and results of
negative effects, including: | ● | default and foreclosure on our assets if our operating revenues after an initial business combination are insufficient to repay our debt obligations; |
| ● | acceleration of our obligations to repay the indebtedness even if we make all principal and interest payments when due if we breach certain covenants that require the maintenance of certain financial ratios or reserves without a waiver or renegotiation of that covenant; |
| ● | our immediate payment of all principal and accrued interest, if any, if the debt is payable on demand; |
| ● | our inability to obtain necessary additional financing if the debt contains covenants restricting our ability to obtain such financing while the debt is outstanding; |
| ● | our inability to pay dividends on our Class A common stock; |
| ● | using a substantial portion of our cash flow to pay principal and interest on our debt, which will reduce the funds available for dividends on our Class A common stock if declared, expenses, capital expenditures, acquisitions and other general corporate purposes; |
| ● | limitations on our flexibility in planning for and reacting to changes in our business and in the industry in which we operate; |
| ● | increased vulnerability to adverse changes in general economic, industry and competitive conditions and adverse changes in government regulation; and |
| ● | limitations on our ability to borrow additional amounts for expenses, capital expenditures, acquisitions, debt service requirements, execution of our strategy and other purposes and other disadvantages compared to our competitors who have less debt. |
In addition, Deerfield Management and its affiliates and certain of the Deerfield Funds invest in and may trade in loans and debt securities of corporate and other borrowers, and may provide or participate in any debt financing arrangement in connection with any acquisition of any target business that we may make. If Deerfield Management or any of its affiliates or the Deerfield Funds provides or participates in such debt financing arrangement, it may present a conflict of interest and will have to be approved under our related party transaction policy or by our independent directors.
operations. We may onlynot be able to complete one business combination withsuccessfully integrate the proceeds of the initial public offeringacquired businesses into ours and the sale of the private placement warrants, which will cause us to be solely dependent on a single business which may have a limited number of products or services. This lack of diversification may negatively impact our operationsTOI PCs, and profitability.
Approximately $230.0 million of the net proceeds of the initial public offering and certain of the proceeds of the private placement of warrants was placed in the Trust Account (including the $6.3 million deferred underwriting commissions).
We may effectuate our initial business combination with a single target business or multiple target businesses simultaneously or within a short period of time. However,therefore, we may not be able to effectuaterealize the intended benefits from an acquisition. These acquisitions could result in difficulties integrating acquired operations, technologies, and personnel into our initial business combination with more than one target business because of various factors, including the existence of complex accounting issuesbusiness. Such difficulties may divert significant financial, operational, and the requirement that we prepare and file pro forma financial statements with the SEC that present operating results and the financial condition of several target businesses as if they had been operated on a combined basis. By completingmanagerial resources from our initial business combination with only a single entity, our lack of diversification may subject us to numerous economic, competitive and regulatory developments. Further, we would not be able to diversify our operations or benefit from the possible spreading of risks or offsetting of losses, unlike other entities which may have the resources to complete several business combinations in different industries or different areas of a single industry. Accordingly, the prospects for our success may be:
| ● | solely dependent upon the performance of a single business, property or asset, or |
| ● | dependent upon the development or market acceptance of a single or limited number of products, processes or services. |
This lack of diversification may subject us to numerous economic, competitive and regulatory risks, any or all of which may have a substantial adverse impact upon the particular industry in which we may operate subsequent to our initial business combination.
We may attempt to simultaneously complete business combinations with multiple prospective targets, which may hinder our ability to complete our initial business combination and give rise to increased costs and risks that could negatively impact ourexisting operations and profitability.
If we determine to simultaneously acquire several businesses that are owned by different sellers, we will need for each of such sellers to agree that our purchase of its business is contingent on the simultaneous closings of the other business combinations, which may make it more difficult for us,to achieve our operating and delay our ability,strategic objectives. We and the TOI PCs may fail to complete our initial business combination. With multiple business combinations, weretain employees or patients acquired through these acquisitions, which may negatively impact the integration efforts. These acquisitions could also face additionalhave a negative impact on our results of operations if it is subsequently determined that goodwill or other acquired intangible assets are impaired, thus resulting in an impairment charge in a future period.
In addition, these acquisitions involve risks that the acquired businesses will not perform in accordance with expectations; that we may become liable for unforeseen financial or business liabilities of the acquires businesses, including additional burdens and costsliabilities for failure to comply with respect to possible multiple negotiations and due diligence investigations (if there are multiple sellers) andapplicable healthcare regulations; that the additional risksexpected synergies associated with acquisitions will not be achieved; and that business judgments concerning the subsequent assimilationvalue, strengths and weaknesses of the operationsbusinesses acquired will prove incorrect, which could have a material adverse effect on our financial condition and services or productsresults of the acquired companies in a single operating business. operations.
If we are unable to
adequately address these risks, itprotect the confidentiality of our trade secrets, know-how and other proprietary and internally developed information, the value of our technology could
negatively impact our profitability and results of operations.be adversely affected.
We may
attemptnot be able to
completeprotect our
initialtrade secrets, know-how and other internally developed information adequately. Although we use reasonable efforts to protect this internally developed information and technology, our employees, consultants and other parties (including independent contractors and companies with which we conduct business) may unintentionally or willfully disclose our information or technology to competitors. Enforcing a claim that a third party illegally disclosed or obtained and is using any of our internally developed information or technology is difficult, expensive and time-consuming, and the outcome is unpredictable. We rely, in part, on non-disclosure, confidentiality and assignment-of-invention agreements with our employees, independent contractors, consultants and companies with which we conduct business
combination with a private company about which little information is available, whichto protect our internally developed information. These agreements may
result in a business combination with a company that is not
as profitable as we suspected, if at all.In pursuing our business combination strategy,be self-executing, or they may be breached and we may seeknot have adequate remedies for such breach. Moreover, third parties may independently develop similar or equivalent proprietary information or otherwise gain access to effectuate our initial business combinationtrade secrets, know- how and other internally developed information.
We conduct some clinical trials in contract with a privately held company. Very little public information generally exists about private companies,the TCR. If we fail to perform our clinical trial services in accordance with contractual requirements, government regulations and ethical considerations, we could be requiredsubject to makesignificant costs or liability and our decision on whetherreputation could be adversely affected.
TCR contracts with biotechnology and pharmaceutical companies to pursueperform services to assist them in bringing new drugs and biologics to market. TCR’s services include monitoring clinical trials, laboratory analysis, electronic data capture, patient recruitment, data analytics, technology solutions, and other related services. Such services are complex and subject to contractual requirements, government regulations, and ethical considerations. TCR’s services are subject to various regulatory requirements designed to ensure the quality and integrity of the clinical trial process. In the United States, clinical development services must be performed in compliance with applicable laws, rules and regulations enforced by the United States Food and Drug Administration, or FDA, including Good Clinical Practice, or GCP, requirements, which govern, among other things, the design, conduct, performance, monitoring, auditing, recording, analysis, and reporting of clinical trials.
If TCR fails to perform services in accordance with these requirements, regulatory authorities may take action against TCR. Such actions may include injunctions or failure to grant marketing approval of products, imposition of clinical holds or delays, suspension or withdrawal of approvals, rejection of data collected in TCR’s studies, license revocation, product seizures or recalls, operational restrictions, civil or criminal penalties or prosecutions, damages, or fines. Additionally, there is a potential initial business combinationrisk that actions by regulatory authorities, if they result in significant inspectional observations or other measures, could harm TCR’s reputation and cause customers not to award TCR future contracts or to cancel existing contracts. Clients may also bring claims against TCR for breach of TCR’s contractual obligations and patients in the clinical trials and patients taking drugs approved on the basis of limited information, whichthose trials may result in a business combination with a company that is not as profitable as we suspected, if at all.
Because we intend to seek a business combination with a target business in the healthcare industry, we expect our future operations to be subject to risks associated with this industry.
Healthcare-related companies are generally subject to greater governmental regulation than most other industries at the U.S. state and federal levels, and internationally. In recent years, both local and national governmental budgets have come under pressure to reduce spending and control healthcare costs, which could both adversely affect regulatory processes and public funding available for healthcare products, services and facilities. In March 2010, comprehensive healthcare reform legislation was enacted in the United States through the Health Care Reform Act. These laws are intended to increase health insurance coverage through individual and employer mandates, subsidies offered to lower income individuals, tax credits available to smaller employers and broadening of Medicaid eligibility.
While one intent of healthcare reform is to expand health insurance coverage to more individuals, it may also involve additional regulatory mandates and other measures designed to constrain medical costs, including coverage and reimbursement for healthcare services. The Health Care Reform Act has had a significant impact on the healthcare sector in the U.S. and consequently has the ability to affect the companies within the healthcare industry. The ultimate effects of federal healthcare reform or any future legislation or regulation, or healthcare initiatives, if any, on the healthcare sector, whether implemented at the federal or state level, or internationally, cannot be predicted with certainty andbring personal injury claims against TCR. Any such reform, legislation, regulation or initiatives, including the Health Care Reform Act or any successor legislation, may adversely affect the performance of a potential business combination.
Changes in governmental policies may have a material effect on the demand for or costs of certain products and services. A healthcare-related company must receive government approval before introducing new drugs and medical devices or procedures. This process may delay the introduction of these products and services to the marketplace, resulting in increased development costs, delayed cost recovery and loss of competitive advantage to the extent that rival companies have developed competing products or procedures, adversely affecting the company’s revenues and profitability. Failure to obtain governmental approval of a key drug or device or other regulatory action could have a material adverse effect on our results of operations, financial condition, and reputation.
We may be subject to formal or informal inquiries or investigations, both internal or external, from time to time pertaining to clinical trials or studies with which we are involved. Regardless whether any such inquiry or investigation ultimately leads to enforcement action or litigation, the cost of such inquiries and investigations can be substantial and could require us to divert financial and human resources away from strategic initiatives we have planned for building the business, and the mere allegation of a portfolio company. Additionally, expansionmisconduct can severely harm our reputation.
Negative publicity regarding the managed healthcare industry generally could adversely affect our results of facilitiesoperations or business.
Negative publicity regarding the managed healthcare industry generally, or the MA program in particular, may result in increased regulation and legislative review of industry practices that further increase our costs of doing business and adversely affect our results of operations or business by:
•requiring us to change our products and services;
•increasing the regulatory, including compliance, burdens under which we operate, which, in turn, may negatively impact the manner in which the TOI PCs provide services and increase our costs of providing services;
•adversely affecting our ability to market the TOI PCs products or services through the imposition of further regulatory restrictions regarding the manner in which plans and providers market to MA enrollees; or
•adversely affecting our ability to attract and retain patients.
Our managed clinics may be negatively impacted by healthcare-related providers isweather and other factors beyond our control.
Our results of operations may be adversely impacted by adverse conditions affecting our managed clinics, including severe weather events such as hurricanes and flooding, natural disasters such as earthquakes and forest fires, public health concerns such as contagious disease outbreaks, violence or threats of violence or other factors beyond our control that cause disruption of patient scheduling, displacement of our patients, employees and care teams, or force certain of our managed clinics to close temporarily. Our future operating results may be adversely affected by these and other factors that disrupt the operation of our managed clinics.
Risks Related to Our Regulatory Environment
We are dependent on our relationships with the TOI PCs, which are affiliated professional entities that we do not own, to provide healthcare services, and our business would be harmed if those relationships were disrupted or if our arrangements with the TOI PCs become subject to “determinationslegal challenges.
Our contractual relationships with the TOI PCs may implicate certain state laws that generally prohibit non-professional entities from providing licensed medical services or exercising control over licensed physicians or other healthcare professionals (such activities generally referred to as the “corporate practice of
need” bymedicine”) or engaging in certain practices such as fee-splitting with such licensed professionals. The interpretation and enforcement of these laws vary significantly from state to state. There can be no assurance that these laws will be interpreted in a manner consistent with our practices or that other laws or regulations will not be enacted in the
appropriate government authorities. This process not only increasesfuture that could have a material and adverse effect on our business, financial condition and results of operations. Regulatory authorities, state boards of medicine, state attorneys general and other parties may assert that, despite the
time and cost involvedagreements through which we operate, we are engaged in
these expansions, but also makes expansion plans uncertain, limiting the
revenue and profitability growth potentialprovision of
healthcare-related facilities operators.Certain healthcare-related companies dependmedical services and/or that our arrangements with the TOI PCs constitute unlawful fee- splitting. If a jurisdiction’s prohibition on the exclusive rightscorporate practice of medicine or patents forfee-splitting is interpreted in a manner that is inconsistent with our practices, we would be required to restructure or terminate our arrangements with the products they develop and distribute. PatentsTOI PCs to bring our activities into compliance with such laws. A determination of non-compliance, or the termination of or failure to successfully restructure these relationships could result in disciplinary action, penalties, damages, fines, and/or a loss of revenue, any of which could have a limited durationmaterial and upon expiration,adverse effect on our business, financial condition and results of operations. State corporate practice and fee-splitting prohibitions also often impose penalties on healthcare professionals for aiding in the improper rendering of professional services, which could discourage physicians and other companies may market substantially similar “generic” products that are typically sold at a lower price than the patented product, causing the original developerhealthcare professionals from providing clinical services to members of the producthealth plans with whom we contract.
Our managed clinics and the TOI PCs providing professional services at such clinics may become subject to lose market share and/or reducemedical liability claims, which could have a material adverse impact on our business.
Our business entails the price charged forrisk of medical liability claims against us, the product, resultingTOI PCs and their clinicians. Although we, the TOI PCs and their clinicians carry insurance covering medical malpractice claims in lower profits foramounts that we believe are appropriate in light of the original developer.risks attendant to our business, successful medical liability claims could result in substantial damage awards that exceed the limits of our and our clinicians’ insurance coverage. In addition, professional liability insurance is expensive and insurance premiums may increase significantly in the future, particularly as we expand our services. As a result, adequate professional liability insurance may not be available to our clinicians, our affiliated practices or to us in the expirationfuture at acceptable costs or at all.
Any claims made against us or the TOI PCs that are not fully covered by insurance could be costly to defend, result in substantial damage awards against us and divert the attention of
patents may adversely affectour management and the
profitability of these companies. The profitability of healthcare-related companies may also be affected, among other factors, by restrictions on government reimbursement for medical expenses, rising or falling costs of medical products and services, pricing pressure, an increased emphasis on outpatient services, a limited product offering, industry innovation, changes in technologies and other market developments. Finally, because the products and services of healthcare-related companies affect the health and well-being of many individuals, these companies are especially susceptible to product liability lawsuits.The healthcare industry spends heavily on research and development. Research findings (e.g., regarding side effects or comparative benefits of one or more particular treatments, services or products) and technological innovation (together with patent expirations) may make any particular treatment, service or product less attractive if previously unknown or underappreciated risks are revealed, or if a more effective, less costly or less risky solution is or becomes available. Any such developmentTOI PCs from our operations, which could have a material adverse effect on our business, financial condition and results of operations. In addition, any claims may adversely affect our business or reputation.
If there is a change in accounting standards by the companiesFinancial Accounting Standards Board ("FASB") or the interpretation thereof affecting consolidation of entities, it could have a material adverse effect on our consolidation of total revenues derived from the TOI PCs.
Our financial statements are consolidated in accordance with applicable accounting standards and include the accounts of our subsidiaries and the TOI PCs, which we manage under long-term management services agreements but are not owned by us. Such consolidation for accounting and/or tax purposes does not, is not intended to, and should not be deemed to, imply or
provide us any control over the medical or clinical affairs of the TOI PCs. In the event a change in accounting standards promulgated by FASB or in interpretation of its standards, or if there is an adverse determination by a regulatory agency or a court, or a change in state or federal law relating to the ability to maintain present agreements or arrangements with the TOI PCs, we may not be permitted to continue to consolidate the total revenues of such practices.
Our managed clinics and the TOI PCs may be subject to third-party payor audits, which, if adversely determined against us or the TOI PCs, may have a material effect on our results of operations and financial condition.
As a result of the TOI PCs participation in the Medicare and Medicaid programs, our managed clinics and the TOI PCs are subject to various governmental inspections, reviews, audits and investigations to verify our compliance with these programs and applicable laws and regulations. Payors may also reserve the right to conduct audits. We also periodically conduct internal audits and reviews of our regulatory compliance. An adverse inspection, review, audit or investigation could result in:
•refunding amounts we have been paid pursuant to the Medicare or Medicaid programs or from payors;
•state or federal agencies imposing fines, penalties and other sanctions on us;
•temporary suspension of payment for new patients to the facility or agency;
•decertification or exclusion from participation in the Medicare or Medicaid programs or one or more payor networks;
•self-disclosure of violations to applicable regulatory authorities;
•damage to our reputation;
•the revocation of a facility’s or agency’s license; and
•loss of certain rights under, or termination of, our contracts with payors.
With respect to MA plans, the TOI PCs submit claims and encounter data applicable to MA plans that are target businessesused to establish the annual, average Medicare Risk Adjustment Factor, or RAF, scores attributable to each TOI PC’s MA population. These RAF scores determine, in part, the revenue to which the health plans and, in turn, the TOI PCs, are entitled for investment.
Risks Relatingmedical care to such population. The data submitted to CMS by each health plan is based, in part, on medical charts and diagnosis codes that the TOI PCs prepare and submit to the Sponsorhealth plans. CMS audits MA plans for documentation to support RAF-related payments for enrollees chosen at random. The MA plans then ask providers to submit the underlying documentation for members that they serve. It is possible that claims associated with members with higher RAF scores could be subject to more scrutiny in a CMS or plan audit. There is a possibility that a MA plan may seek repayment from the TOI PCs should CMS make any payment adjustments to the MA plan as a result of its audits. CMS has indicated that payment adjustments will not be limited to RAF scores for the specific MA enrollees for which errors are found but may also be extrapolated to the entire MA plan subject to a particular CMS contract. Based on a recent final rule issued by CMS in January 2023, although 2011 to 2017 plan years are still subject to audit, overpayments to MA plans that are identified as a result of a Risk Adjustment Data Validation, or RADV, audit will only be subject to extrapolation for plan year 2018 and Management Team
Our abilityany subsequent plan year. In addition, CMS will not apply an adjustment factor, known as a Fee-For-Service, or FFS, Adjuster, in RADV audits to successfullyaccount for potential differences in diagnostic coding between the Medicare Advantage program and Medicare FFS program. We are continuing to assess the potential impact this final rule may have on our business and operations.
We have in the past and will likely in the future be required to refund amounts we have been paid and/or pay fines and penalties as a result of these inspections, reviews, audits and investigations. If adverse inspections, reviews, audits or investigations occur and any of the results noted above occur, it could have a material adverse effect on our initial business combination and operating results. Furthermore, the legal, document production and other costs associated with complying with these inspections, reviews, audits or investigations could be significant.
We are subject to extensive fraud, waste, and abuse laws that may give rise to federal and state audits, investigations, lawsuits and claims against us, the outcome of which may have a material adverse effect on our business, financial condition, cash flows, or results of operations.
The U.S. healthcare industry is heavily regulated and closely scrutinized by federal, state and local governments. Comprehensive statutes and regulations govern the manner in which we provide and bill for services and collect reimbursement from governmental programs and private payors, our contractual relationships and arrangements with healthcare providers and vendors, our marketing activities and other aspects of our operations. Of particular importance are:
•the federal Anti-Kickback Statute, or AKS, which prohibits the knowing and willful offer, payment, solicitation or receipt of any bribe, kickback, rebate or other remuneration for referring an individual, in return for ordering, leasing, purchasing or recommending or arranging for or to induce the referral of an individual or the ordering, purchasing or
leasing of items or services covered, in whole or in part, by any federal healthcare program, such as Medicare and Medicaid. A person or entity does not need to have actual knowledge of the statute or specific intent to violate it to have committed a violation;
•the federal physician self-referral law, the Stark Law, which, subject to limited exceptions, prohibits physicians from referring Medicare or Medicaid patients to an entity for the provision of certain designated health services, or DHS if the physician or a member of such physician’s immediate family has a direct or indirect financial relationship (including an ownership interest or a compensation arrangement) with the entity, and prohibits the entity from billing Medicare or Medicaid for such DHS;
•the FCA, which imposes civil and criminal liability on individuals or entities that knowingly submit false or fraudulent claims for payment to the government or knowingly make, or cause to be successful thereafter willmade, a false statement in order to have a false claim paid, including qui tam or whistleblower suits. There are many potential bases for liability under the FCA. The government has used the FCA to prosecute Medicare and other government healthcare program fraud such as coding errors, billing for services not provided, and providing care that is not medically necessary or that is substandard in quality. In addition, the government may assert that a claim including items or services resulting from a violation of the AKS or Stark Law constitutes a false or fraudulent claim for purposes of the FCA;
•the Civil Monetary Penalties Law, which prohibits, among other things, an individual or entity from offering remuneration to a federal healthcare program beneficiary that the individual or entity knows or should know is likely to influence the beneficiary to order or receive healthcare items or services from a particular provider. We may also be dependent uponsubject to civil monetary penalties and other sanctions under the effortsstatute if we or the TOI PCs hire or contract with any individuals or entities that are or become excluded from government healthcare programs, for the provision of our key personnel,items or services for which payment may be made under such programs;
•the criminal healthcare fraud provisions of HIPAA and related rules that prohibit knowingly and willfully executing a scheme or artifice to defraud any healthcare benefit program or falsifying, concealing or covering up a material fact or making any material false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Similar to the AKS, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it to have committed a violation;
•reassignment of payment rules that prohibit certain types of billing and collection practices in connection with claims payable by the Medicare or Medicaid programs;
•similar state law provisions pertaining to anti-kickback, self-referral and false claims issues, some of whomwhich may join us following our initial business combination. The lossapply to items or services reimbursed by any payor, including patients and commercial insurers;
•laws that regulate debt collection practices;
•a provision of key personnel could negatively impact the operationsSocial Security Act that imposes criminal penalties on healthcare providers who fail to disclose, or refund known overpayments;
•federal and profitabilitystate laws that prohibit providers from billing and receiving payment from Medicare and Medicaid for services unless the services are medically necessary, adequately and accurately documented, and billed using codes that accurately reflect the type and level of our post-combination business.services rendered;Our ability
•federal and state laws and policies that require healthcare providers to successfully effect our initial business combination is dependent upon the efforts of our key personnel. The role of our key personnelmaintain licensure, certification or accreditation to enroll and participate in the target business, however, cannot presently be ascertained. AlthoughMedicare and Medicaid programs, to report certain changes in their operations to the agencies that administer these programs and, in some cases, to re-enroll in these programs when changes in direct or indirect ownership occur; and
•federal and state laws pertaining to the provision of services by nurse practitioners and physician assistants in certain settings, physician supervision of those services, and reimbursement requirements that depend on the types of services provided and documented and relationships between physician supervisors and nurse practitioners and physician assistants; and
•Medicare and Medicaid regulations, manual provisions, local coverage determinations, national coverage determinations and agency guidance imposing complex and extensive requirements upon healthcare providers.
The laws and regulations in these areas are complex, changing and often subject to varying interpretations. As a result, there is no guarantee that a government authority will find that we or the TOI PCs are in compliance with all such laws and regulations that apply to our business. Further, because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available, it is possible that some of our key personnel may remain with the target business in senior managementactivities undertaken by us or advisory positions following our initial business combination, it is likely that somethe TOI PCs could be subject to challenge under one or all of the management of the target business will remain in place. While we intend to closely scrutinize any individuals we engage after our initial business combination, we cannot assure you that our assessmentmore of these individuals will provelaws, including, without limitation, our patient assistance programs that waive or reduce the patient’s obligation to pay copayments, coinsurance or deductible amounts owed for the services we
provide to them if they meet certain financial need criteria. If our or the TOI PCs’ operations are found to be correct. These individualsin violation of any of such laws or any other governmental regulations that apply, we may be unfamiliar withsubject to significant penalties, including, without limitation, administrative, civil and criminal penalties, damages, fines, disgorgement, the requirementscurtailment or restructuring of operating a company regulated byoperations, integrity oversight and reporting obligations, exclusion from participation in federal and state healthcare programs and imprisonment. In addition, any action against us or the SEC, whichTOI PCs for violation of these laws or regulations, even if we successfully defend against it, could cause us to incur significant legal expenses, divert our management’s attention from the operation of our business and result in adverse publicity, or otherwise experience a material adverse impact on our business, results of operations, financial condition, cash flows, reputation as a result.
If any of our managed clinics or TOI PCs lose their regulatory licenses, permits and/or accreditation status, or become ineligible to receive reimbursement under Medicare or Medicaid or other third-party Payors, there may be a material adverse effect on our business, financial condition, cash flows, or results of operations.
The operations of our managed clinics through the TOI PCs are subject to extensive federal, state and local regulation relating to, among other things, the adequacy of medical care, equipment, personnel, operating policies and procedures, dispensing of prescription drugs, fire prevention, rate-setting and compliance with building codes and environmental protection. Our managed clinics and TOI PCs are also subject to extensive laws and regulation relating to facility and professional licensure, conduct of operations, including financial relationships among healthcare providers, Medicare and Medicaid fraud and abuse and physician self-referrals, and maintaining updates to the TOI PCs’ enrollment in the Medicare and Medicaid programs, including addition of new clinic locations, providers and other enrollment information. Our managed clinics and TOI PCs are subject to periodic inspection by licensing authorities and accreditation organizations to assure their continued compliance with these various standards. There can be no assurance that these regulatory authorities will determine that all applicable requirements are fully met at any given time. Should any of our managed clinics or TOI PCs be found to be noncompliant with these requirements, we could be assessed fines and penalties, could be required to refund reimbursement amounts or could lose our licensure or Medicare and/or Medicaid certification or accreditation so that we or the TOI PCs are unable to receive reimbursement from such programs and possibly from other third-party payors, any of which could materially adversely affect our business, financial condition, cash flows or results of operations.
If we or the TOI PCs fail to comply with applicable data interoperability and information blocking rules, our consolidated results of operations could be adversely affected.
The 21st Century Cures Act (the “Cures Act”), which was passed and signed into law in December 2016, includes provisions related to data interoperability, information blocking and patient access. In March 2020, the HHS Office of the National Coordinator for Health Information Technology, or ONC, and CMS finalized and issued complementary rules that are intended to clarify provisions of the Cures Act regarding interoperability and information blocking, and include, among other things, requirements surrounding information blocking, changes to ONC’s health IT certification program and requirements that CMS- regulated payors make relevant claims/care data and provider directory information available through standardized patient access and provider directory application programming interfaces, or APIs, that connect to provider electronic health record systems, or EHRs. The companion rules will transform the way in which healthcare providers, health IT developers, health information exchanges/health information networks, or HIEs/HINs, and health plans share patient information, and create significant new requirements for healthcare industry participants. For example, the ONC rule, which went into effect on April 5, 2021, prohibits healthcare providers, health IT developers of certified health IT, and HIEs/HINs from engaging in practices that are likely to interfere with, prevent, materially discourage, or otherwise inhibit the access, exchange or use of electronic health information, or EHI, also known as “information blocking.” To further support access and exchange of EHI, the ONC rule identifies eight “reasonable and necessary activities” as exceptions to information blocking activities, as long as specific conditions are met. Any failure to comply with these rules could have a material adverse effect on our business, results of operations and financial condition.
Actual or perceived failures to
expend timecomply with applicable data protection, privacy and
resources helping them become familiar with such requirements.We are dependent upon our executive officerssecurity, advertising and directorsconsumer protection laws, regulations, standards and their lossother requirements could adversely affect our abilitybusiness, financial condition and results of operations.
We and the TOI PCs collect, receive, generate, use, process, and store significant and increasing volumes of sensitive information, such as employee, individually identifiable health information and other personally identifiable information. We and the TOI PCs are subject to
operate.Our operationsa variety of federal and state laws and regulations, as well as contractual obligations, relating to the collection, use, storage, retention, security, disclosure, transfer, return, destruction and other processing of personal information, including health- related information. Enforcement actions and consequences for noncompliance with such laws, directives and regulations are dependent upon a relatively small grouprising, and the regulatory framework for privacy, data protection and data transfers is complex and rapidly evolving and is likely to remain uncertain for the foreseeable future.
In the United States, numerous such federal and state laws and regulations, including data breach notification laws, health information privacy laws, and consumer protection laws and regulations, including those that govern the collection, use, disclosure, and protection of
individualshealth-related and
in particular, our executive officers and directors. We believe that our success depends on the continued service of our officers and directors, at least until we have completed our initial business combination. In addition, our executive officers and directors are not required to commit any specified amount of timeother personal information, could apply to our
affairs and, accordingly, will have conflicts of interest in allocating their time among various business activities, including identifying potential business combinations and monitoringoperations or the
related due diligence. We do not have an employment agreement with, or key-man insurance on the life of, any of our directors or executive officers. The unexpected lossoperations of the
TOI PCs. For example, the Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, and regulations implemented thereunder, which we refer to collectively as HIPAA, imposes privacy, security and breach notification obligations on certain health care providers, health plans, and health care clearinghouses, known as covered entities, as well as business associates that perform certain services
that involve creating, receiving, maintaining or transmitting individually identifiable health information for or on behalf of
one or more of our directors or executive officers could have a detrimental effect on us.Since our sponsor, executive officerssuch covered entities. HIPAA requires covered entities, such as the TOI PCs, and directors will lose their entire investment inbusiness associates, such as us, if our initial business combination is not completed (other thanto develop and maintain policies with respect to public shares theythe protection of, use and disclosure of protected health information, or PHI, including the adoption of administrative, physical and technical safeguards to protect such information, and certain notification requirements in the event of a data breach.
Entities that are found to be in violation of HIPAA as the result of a breach of unsecured protected health information, or PHI, a complaint about privacy practices or an audit by HHS, may acquire during be subject to significant civil, criminal and administrative fines and penalties and/or afteradditional reporting and oversight obligations if required to enter into a resolution agreement and corrective action plan with HHS to settle allegations of HIPAA non-compliance. HIPAA also authorizes state Attorneys General to file suit on behalf of their residents. Courts may award damages, costs and attorneys’ fees related to violations of HIPAA in such cases. While HIPAA does not create a private right of action allowing individuals to sue us in civil court for violations of HIPAA, its standards have been used as the initial public offering), a conflictbasis for duty of interestcare in state civil suits such as those for negligence or recklessness in the misuse or breach of PHI.
Numerous other state and federal laws, including consumer protection laws and regulations, govern the collection, dissemination, use, access to, confidentiality, security and processing of personal information, including health-related information, some of which are more stringent than HIPAA and many of which differ from each other in significant ways and may
arise in determining whether a particular business combination target is appropriate for our initial business combination.The initial stockholders beneficially own approximately 20% ofnot have the Company’s 5,750,000 issued and outstanding shares, including 5,360,000 founder shares held by our sponsor, 100,000 founder shares held by each of our executive officers and 30,000 founder shares held by each of our directors. The founder shares will be worthless if we do not complete an initial business combination by March 13, 2022.same effect, thus complicating compliance efforts. In addition, the sponsor owns an aggregate of 3,733,334 private placement warrants that will also be worthless if we do not complete a business combination by March 13, 2022. The personalthese laws and financial interests of our executive officersregulations in many cases are more restrictive than, and directors may influence their motivation in identifying and selecting a target business combination, completing an initial business combination and influencing the operation of the business following the initial business combination. This risk may become more acute as the 24-month anniversary of the closing of the initial public offering nears, which is the deadline for our completion of an initial business combination.
Our executive officers and directors will allocate their time to other businesses thereby causing conflicts of interest in their determination as to how much time to devote to our affairs. This conflict of interest could have a negative impact on our ability to complete our initial business combination.
Our executive officers and directors are not required to, and will not, commit their full time to our affairs, which may result in a conflict of interest in allocating their time between our operations and our search for a business combination and their other businesses. We do not intend to have any full-time employees prior to the completion of our initial business combination. Each of our executive officers is engaged in several other business endeavors for which he may be entitled to substantial compensation, and our executive officers are not obligated to contribute any specific number of hours per week to our affairs. Our independent directors also serve as officers and board members for other entities. If our executive officers’ and directors’ other business affairs require them to devote substantial amounts of time to such affairs in excess of their current commitment levels, it could limit their ability to devote time to our affairs which may have a negative impact on our ability to complete our initial business combination.
Our officers and directors presently have, and any of them in the future may have additional, fiduciary or contractual obligations to other entities and, accordingly, may have conflicts of interest in determining to which entity a particular business opportunity should be presented.
Until we consummate our initial business combination, we intend to engage in the business of identifying and combining with one or more businesses. Each of our officers and directors presently has, and any of them in the future may have, additional fiduciary or contractual obligations to other entities, including DFP, private funds under the management of Deerfield Management and their respective portfolio companies, pursuant to which such officer or director is or will be required to present a business combination opportunity to such entity. In addition, DFP, existing and future funds managed by Deerfield Management and their respective portfolio companies may compete with us for business combination opportunities and, if such opportunities are pursued by such entities, we may be precluded from pursuing such opportunities. Accordingly, they may have conflicts of interest in determining to which entity a particular business opportunity should be presented. These conflicts may not be resolved in our favorpreempted by, HIPAA and a potential target business may be presentedsubject to another entity priorvarying interpretations by courts and government agencies. Laws in all 50 states and other United States territories require businesses to its presentationprovide notice to us. Our second amended and restated certificate of incorporation will provide that we renounce our interest in any corporate opportunity offered to any director or officer unless such opportunity is expressly offered to such person solely in his or her capacityindividuals whose personal information has been disclosed as a directorresult of a data breach. Such laws are not always consistent, and compliance in the event of a widespread data breach is costly and may be challenging.
States are also constantly amending existing laws, requiring attention to frequently changing requirements, and we expect these changes to continue. For example, in June 2018, California enacted the California Consumer Privacy Act, or officerthe CCPA, which became effective on January 1, 2020, and, among other things, requires covered companies to provide disclosures to California consumers, and affords such consumers certain data protection rights, including the ability to opt-out of certain sales of personal information. The CCPA provides for civil penalties for violations, as well as a private right of action for certain data breaches that result in the company and such opportunity is one we are legally and contractually permitted to undertake and would otherwise be reasonableloss of personal information that may increase data breach litigation. While the CCPA includes certain exceptions for health-related information, including PHI, it still may require us to pursue,modify our data practices and policies and to incur substantial costs and expenses in an effort to comply. Further, the extentCalifornia Privacy Rights Act, or CPRA, generally went into effect on January 1, 2023 and significantly amends the directorCCPA. The CPRA imposes additional data protection obligations on covered businesses, including additional consumer rights processes, limitations on data uses, new audit requirements for higher risk data, and opt outs for certain uses of sensitive data. It will also create a new California data protection agency authorized to issue substantive regulations and could result in increased privacy and information security enforcement. Additional compliance investment and potential business process changes may be required. Similar laws have passed in Virginia, Colorado, Connecticut and Utah, and have been proposed in other states and at the federal level, reflecting a trend toward more stringent privacy legislation in the United States. The enactment of such laws could have potentially conflicting requirements that would make compliance challenging.
As required by certain laws, we publicly post documentation regarding our privacy practices concerning the collection, processing, use and disclosure of certain data. The publication of our privacy policy and other documentation that provide promises and assurances about privacy and security can subject us to potential state and federal action if they are found to be deceptive, unfair, or
officer is permitted to refer that opportunity to us without violating another legal obligation.misrepresentative of our actual practices. In addition,
our sponsor and our officers and directors may sponsor or form other special purpose acquisition companies similaralthough we endeavor to
ours or may pursue other business or investment ventures during the period in which we are seeking an initial business combination. Any such companies, businesses or ventures may present additional conflicts of interest in pursuing an initial business combination. However, we do not believe that any such potential conflicts would materially affect our ability to complete our initial business combination.Our executive officers, directors, security holders and their respective affiliates may have competitive pecuniary interests that conflictcomply with our interests.
Wepublished policies and documentation, individuals could allege we have not adopted a policy that expressly prohibits our directors, executive officers, security holders or affiliates from having a direct or indirect pecuniary or financial interest in any investment to be acquired or disposed of by us or in any transaction to which we are a party or have an interest. In fact, we may enter into a business combination with a target business that is affiliated with our sponsor, our directors or executive officers, although we do not intendfailed to do so, or we may acquireat times actually fail to do so despite our efforts. Any failure by us, our third-party service providers or other parties with whom we do business to comply with this documentation or with laws or regulations applicable to our business could result in proceedings against us by governmental entities or others.
In addition, the Federal Trade Commission, or the FTC, expects a
target business through an Affiliated Joint Acquisition with one or more affiliates of Deerfield Management, one or more Deerfield Funds and/or one or more investorscompany’s data security measures to be reasonable and appropriate in
the Deerfield Funds. Nor do we have a policy that expressly prohibits any such persons from engaging for their own account in business activitieslight of the
types conducted by us. Accordingly, such persons or entities may have a conflict between their interestssensitivity and
ours.In particular, certainvolume of consumer information it holds, the Deerfield Funds are focused on investments in the healthcare industry. As a result, there may be substantial overlap between companies that would be a suitable business combination for ussize and companies that would make an attractive target for the Deerfield Funds.
The personal and financial interestscomplexity of our directors and officers may influence their motivation in timely identifying and selecting a targetits business, and completing a business combination. Consequently, our directors’the cost of available tools to improve security and officers’ discretion in identifying and selecting a suitable target businessreduce vulnerabilities. Our failure to take any steps perceived by the FTC as
appropriate to protect consumers’ personal information may result in a conflictclaims by the FTC that we have engaged in unfair or deceptive acts or practices in violation of interest when determining whetherSection 5(a) of the terms, conditionsFTC Act. State consumer protection laws provide similar causes of action for unfair or deceptive practices for alleged privacy, data protection and timing of a particular business combination are appropriatedata security violations.
In addition to government regulation, privacy advocates and in our stockholders’ best interest. If this were the case, it would be a breach of their fiduciary dutiesindustry groups may propose self- regulatory standards from time to time. These and other industry standards may legally or contractually apply to us, as a matter of Delaware lawor we may elect to comply with such standards or to facilitate our payors’ compliance with such standards. We expect that there will continue to be new proposed laws and regulations concerning privacy, data protection, and information security, and we cannot yet determine the impact such future laws, regulations, and standards may have on our business. New laws, amendments to or re-interpretations of existing laws and regulations, industry standards, contractual and other obligations may require us to incur additional costs and restrict our business operations. Because the interpretation and application of laws, standards, contractual and other obligations relating to privacy and data protection are still uncertain and changing, it is possible that these laws, standards, contractual and other obligations may be interpreted and applied in a manner that is inconsistent with our data management practices, our privacy, data protection or data security policies or procedures or the features of our technology. If so, in addition to the possibility of fines, lawsuits, regulatory investigations, imprisonment of company officials and public censure, other claims and penalties, significant costs for remediation and damage to our reputation, we could be required to fundamentally change our business activities and practices or modify our technology, any of which could adversely affect our business. We may be unable to make such changes or modifications in a commercially reasonable manner, or at all, and our ability to develop new software or provide new services could be limited. Any inability to adequately address privacy, data protection or information security-related concerns, even if such concerns are unfounded, or to successfully negotiate privacy, data protection or information security- related contractual terms with customers, or to comply with applicable laws and regulations, or our stockholders might have a claim against such individuals for infringing onpolicies relating to privacy, data protection, and information security, could result in additional cost and liability to us, harm our stockholders’ rights. However, we might not ultimately be successfulreputation and brand, and adversely affect our business, financial condition and results of operations.
We and our TOI PCs are subject to federal, state and local laws and regulations that govern our business. These include regulations of our employment practices, including minimum wage, living wage, and paid time-off requirements, permitting and licensing, employee health and safety and the storage, treatment and disposal of waste. Failure to comply with these laws and regulations, or changes to these laws and regulations that increase our expenses, could adversely impact our operations.
We and the TOI PCs are required to comply with all applicable federal, state and local laws and regulations related to the operation of our business. These regulations include regulations governing the TOI PCs’ dispensary services, the construction, the use of our managed clinics and the treatment of hazardous waste or drug products. Changes in
any claim we may make against them for such reason.We may engage in aregulations or new regulations could increase our costs, cause the TOI PCs to lose licenses or accreditations or otherwise harm our business combination with one or more target businesses that have relationships with entities that may be affiliated with our sponsor, executive officers, directors or existing holders which may raise potential conflicts of interest.
In lightthe business of the involvementTOI PCs.
We and the TOI PCs are required to comply with all applicable federal, state and local laws and regulations relating to employment, including occupational safety and health requirements, wage and hour and other compensation requirements, employee benefits, providing leave and sick pay, employment insurance, proper classification of our sponsor, executive officersworkers as employees or independent contractors, immigration and directors with other entities, weequal employment opportunity laws. These laws and regulations can vary significantly among jurisdictions and can be highly technical. Costs and expenses related to these requirements are a significant operating expense and may decide to acquire one or more businesses affiliated with our sponsor, executive officers, directors or existing holders. Our directors
also serve as officers and board members for other entities. Such entities may compete with us for business combination opportunities. Our sponsor, officers and directors are not currently aware of any specific opportunities for us to complete our initial business combination with any entities with which they are affiliated, and there have been no substantive discussions concerning a business combination with any such entity or entities. Although we will not be specifically focusing on, or targeting, any transaction with any affiliated entities, we would pursue such a transaction if we determined that such affiliated entity met our criteria for a business combination. Despite our agreement to obtain an opinion from an independent investment banking firm which is a member of FINRA or a valuation or appraisal firm regarding the fairness to our company from a financial point of view of a business combination with one or more domestic or international businesses affiliated with our sponsor, executive officers, directors or existing holders, potential conflicts of interest still may exist and,increase as a result of, among other things, changes in federal, state or local laws or regulations, or the terms of the business combination may not be as advantageousinterpretation thereof, requiring employers to our public stockholders as they would be absent any conflicts of interest.
Moreover, we may pursue an Affiliated Joint Acquisition opportunity with oneprovide specified benefits or more affiliates of Deerfield Management, one or more Deerfield Funds and/or one or more investorsrights to employees, increases in the Deerfield Funds. Any such parties may co-invest with usminimum wage and local living wage ordinances, increases in the target business atlevel of existing benefits or the timelengthening of our initial business combination, or we could raise additional proceeds to complete the business combination by issuing to such parties a class of equity or equity-linked securities. Accordingly, such persons or entities may have a conflict between their interests and ours.
Our managementperiods for which unemployment benefits are available. We may not be able to maintainoffset any increased costs and expenses. Furthermore, any failure to comply with these laws requirements, including even a seemingly minor infraction, can result in significant penalties which could harm our control ofreputation and have a target business aftermaterial adverse effect on our initial business combination. We cannot provide assurance that, upon loss of control of a target business, new management will possess the skills, qualifications or abilities necessary to profitably operate such business.
We may structurenot be able to utilize a portion of our initial business combination soNOLs to offset future taxable income for U.S. federal income tax purposes, which could adversely affect our net income and cash flows.
As of December 31, 2023, we had federal income tax NOLs of approximately $139,195 and state income tax NOLs of approximately $132,511 available to offset our future taxable income, if any, prior to consideration of annual limitations that the post-transaction company in which our public stockholders own shares will own less than 100%may be imposed under Section 382 of the equity interestsCode or assetsotherwise. The federal NOLs will be carried forward indefinitely and the state NOLs begin expiring after 2040. Utilization of a target business, but we will only complete such business combination if the post-transaction company owns or acquires 50% or morethese NOLs depends on many factors, including our future income, which cannot be assured. Some of these NOLs could expire unused and be unavailable to offset our future income tax liabilities. In addition, under Section 382 of the outstanding voting securitiesInternal Revenue Code of 1986, as amended, or the Code, and corresponding provisions of state law, if a corporation undergoes an “ownership change” (very generally defined as a greater than 50% change, by value, in the corporation’s equity ownership by certain stockholders or groups of stockholders over a rolling three-year period), the corporation’s ability to use is pre-ownership change NOLs to offset its post-ownership change income may be limited. In 2022
and 2023, we completed an ownership change analysis pursuant to IRC Section 382 of the target or otherwise acquires a controlling interest inCode for the target sufficient for us not to be required to register as an investment company under the Investment Company Act. We will not consider any transaction that does not meet such criteria. Even if the post-transaction company owns 50% or more of the voting securities of the target business, our stockholders prior to the business combination may collectively own a minority interest in the post business combination company, depending on valuations ascribed to the targetperiod from September 10, 2018 through taxable year ended December 31, 2021 and us in the business combination. For example, we could pursue a transactionfrom January 1, 2022 through taxable year ended December 31, 2022 in which we issue a substantial number of new shares of Class A common stock in exchange for all ofdetermined that the outstanding capital stock of a target. In this case, we would acquire a 100% interestCompany did not experience an ownership change. We are in the target. However,process of completing an analysis to determine whether there was a change in ownership during the year ended December 31, 2023. Additionally, we may experience ownership changes in the future as a result of the issuancesubsequent shifts in our stock ownership, some of a substantial number of new shares of Class A common stock, our stockholders immediately prior to such transaction could own less than a majoritywhich may be outside of our outstanding Class A common stock subsequentcontrol. The deferred tax asset associated with the Company’s federal and state net operating losses are fully offset by a valuation allowance. Due to such transaction.the existence of the valuation allowance, future changes in the Company’s unrecognized tax benefits will not impact its effective tax rate. To the extent we are not able to offset future taxable income with our NOLs, our net income and cash flows may be adversely affected.
Future changes to applicable tax laws and regulations and/or their interpretation may have an adverse effect on our business, financial condition and results of operations. Tax rules and regulations are subject to interpretation and require judgment by us that may be successfully challenged by the applicable taxation authorities upon audit, which could result in additional tax liabilities.
Changes in tax laws or their interpretation could decrease the amount of revenues we receive, the value of any tax loss carry-forwards and tax credits recorded on our balance sheet and the amount of our cash flow, and adversely affect our business, financial condition or results of operations. In addition, other minority stockholders may subsequently combine their holdingsfactors or events, including business combinations and investment transactions, changes in the valuation of our deferred tax assets and liabilities, adjustments to taxes upon finalization of various tax returns or as a result of deficiencies asserted by taxing authorities, increases in expenses not deductible for tax purposes, changes in available tax credits, other changes in the apportionment of our income, and changes in tax rates, could also increase our future effective tax rate.
In addition, our effective tax rate and tax liability are based on the application of current income tax laws, regulations and treaties. These laws, regulations and treaties are complex, and the manner which they apply to us and our diverse set of business arrangements is often open to interpretation, and can require us to take positions regarding the interpretation of applicable rules or the valuation of our assets that are subject to material uncertainty. Significant management judgment is required in determining our provision for taxes, our deferred tax assets and liabilities and any valuation allowance recorded against our net deferred tax assets. The proper tax treatment or characterization of many of the transactions we undertake, such as the transactions associated with our issuance of the Convertible Notes and DF Warrants, is often subject to significant uncertainty, and the resolution of any related issues could affect the withholding tax liabilities to which we are subject or the tax deductions that we are able to claim. The tax authorities could challenge our interpretation of laws, regulations and treaties or the positions that we have taken regarding the valuation of its assets, resulting in additional tax liability or adjustment to our income tax provision.
Our tax filings are subject to review or audit by various taxing authorities. As discussed above, we exercise significant judgment in determining our provision for taxes and, in the ordinary course of our business, there may be transactions and calculations where the proper tax treatment is uncertain. We may also be liable for taxes in connection with businesses we acquire. Our determinations are not binding on the IRS or any other taxing authorities, and accordingly the final determination in an audit or other proceeding may be materially different than the treatment reflected in our tax provisions, accruals and returns. An assessment of additional taxes because of an audit could have a single personmaterial adverse effect on our business, financial condition, results of operations and cash flows.
New income, sales, use or group obtainingother tax laws, statutes, rules, regulations or ordinances could be enacted at any time, or interpreted, changed, modified or applied adversely to us, any of which could adversely affect our business operations and financial performance. We are unable to predict what changes will occur and, if so, the ultimate impact on its business. To the extent that such changes have a larger sharenegative impact on us, they may materially and adversely impact its business, financial condition, results of operations and cash flows.
Risks Related to Our Financial Condition
Goodwill and other intangible assets represent a significant portion of our total assets. Goodwill is tested for impairment at least annually, which could result in a material, non-cash write-down of goodwill and could have a material adverse effect on our results of operations and stockholders’ equity.
Goodwill represents the excess of cost over the fair market value of net assets acquired in business combinations. For example, if our market capitalization drops significantly below the amount of the company’s shares thancarrying equity recorded on our balance sheet, it might indicate a decline in our fair value and would require us to further evaluate whether our goodwill has been impaired. If, as part of our annual review of goodwill, we initially acquired. Accordingly,are required to write down all or a significant part of our goodwill, our net earnings could be materially adversely affected, which could affect our flexibility to obtain additional financing. In addition, if our
assumptions used in preparing our valuations for purposes of impairment testing differ materially from actual future results, we may record impairment charges in the future and our financial results may be materially adversely affected. We had $7,230 and $21,418 of goodwill recorded on our Consolidated Balance Sheets at December 31, 2023 and 2022, respectively. Goodwill impairment charges of $16,867 and $9,944 were recorded during the years ended December 31, 2023 and 2022, respectively, based on management's evaluation of the value of goodwill. It is not possible at this time, under current market conditions, to determine if there will be any future impairment charge, or if there is, whether such charges would be material.
If the Company is required to record additional goodwill impairment, our financial condition and results could be negatively affected.
Goodwill represents the excess of the aggregate purchase price paid over the fair value of the net assets acquired in the Company’s Business Combinations. Goodwill is not amortized and is tested for impairment at least annually or whenever events or changes in circumstances indicate that the carrying value may makenot be recoverable. Based on a qualitative assessment factoring in our share price decrease, as well as factors related to macroeconomic conditions, industry and market considerations, cost factors, financial performance and market capitalization, we determined it morewas likely that our management willreporting unit fair value was less than the carrying value. After conducting a two-step quantitative assessment, we recorded an impairment of $16,867 of goodwill during the three months ended March 31, 2023 (there was no impairment recorded as of March 31, 2022). If our stock price remains low, or negative macroeconomic, industry or business factors worsen, we may be required to perform another goodwill impairment analysis, which could result in an impairment of up to the entire balance of the remaining goodwill. Additionally, significant impairment charges may negatively affect our compliance with the financial covenants of our Facility Agreement.
We may need additional capital to fund our operations and finance our growth, and we may not be able to
maintain our control of the target business.The officers and directors of an acquisition candidateobtain it on acceptable terms, or at all, which may resign upon completion of our initial business combination. The loss of a business combination target’s key personnel could negatively impact the operations and profitability of our post-combination business.
The role of an acquisition candidate’s key personnel upon the completion of our initial business combination cannot be ascertained at this time. Although we contemplate that certain members of an acquisition candidate’s management team will remain associated with the acquisition candidate following our initial business combination, it is possible that members of the management of an acquisition candidate will not wish to remain in place.
Deerfield Management and certain of its personnel have been the subject of SEC proceedings.
In September 2013, Deerfield Management voluntarily agreed to settle an SEC inquiry relating to six alleged violations of Rule 105 of Regulation M under the Exchange Act, without admitting or denying the SEC’s allegations. The violations allegedly occurred between December 2010 and January 2013. Rule 105 generally prohibits purchasing an equity security in a registered offering if the purchaser sold short the same security during a restricted period (generally defined as five business days before the pricing of the offering). Rule 105’s prohibition applies irrespective of any intent
to violate the rule. The settlement involved the payment by the Firm of disgorgement, prejudgment interest and a civil money penalty in the aggregate amount of $1,902,224.
On May 24, 2017, the United States Attorney’s office for the Southern District of New York arrested two then-partners of Deerfield Management and charged them with conspiracy to convert property of the United States, to commit securities fraud and to defraud the United States; conspiracy to commit wire and securities fraud; conversion of property of the United States; securities fraud; and wire fraud in connection with recommending trading in certain shares allegedly on the basis of material nonpublic information during 2012 and 2013. On the same day, the SEC filed a complaint against one of those individuals, alleging that he recommended trading in shares of certain securities during 2012 on the basis of material nonpublic information, in violation of Section 10(b) of the Exchange Act and Rule 10b-5 thereunder and Section 17(a)(1) of the Securities Act. Deerfield Management was not named in either proceeding. In May 2018, both individuals were convicted in the criminal proceeding on five counts of conversion of government property, conspiracy, wire fraud, and securities fraud. Both individuals were sentenced in September 2018 and appealed the verdict. On December 30, 2019, the United States Court of Appeals for the Second Circuit affirmed the judgments of the district court. A further appeal is pending. The subject individuals are no longer with Deerfield Management.
On August 21, 2017, Deerfield Management voluntarily agreed to settle an SEC administrative proceeding relating to alleged violations of Section 204A of the Investment Advisers Act of 1940 (the “Advisers Act”), without admitting or denying the SEC’s allegations, pursuant to an order under Section 203(e) and 203(k) of the Advisers Act (the “Order”).” The Order, which was entered on August 21, 2017, resolved the SEC’s allegations that Deerfield Management, from 2012 through 2014, failed to establish, maintain, and enforce policies and procedures reasonably designed to prevent the misuse of material, nonpublic information, particularly taking into consideration the nature of Deerfield Management’s business. The Order alleged that, as part of Deerfield Management’s research in the healthcare sector, the Firm engaged third party consultants and research firms, including firms that specialized in providing “political intelligence” regarding upcoming regulatory and legislative decisions, that Deerfield Management employees based trading recommendations on such information, and that hedge funds advised by Deerfield Management then made those trades. Based on the foregoing conduct, the SEC alleged that Deerfield Management violated Section 204A of the Advisers Act, which requires investment advisers to establish, maintain, and enforce written policies and procedures reasonably designed, taking into consideration the nature of the investment adviser’s business, to prevent the misuse of material, nonpublic information by such investment adviser or any person associated with such investment adviser. The Order requires Deerfield Management to cease and desist from committing or causing any violations and any future violations of Section 204A of the Advisers Act, censures Deerfield Management and provides that Deerfield Management will pay disgorgement of $714,110, prejudgment interest of $97,585 and a civil money penalty of $3,946,267.
These actions and/or any additional SEC or other governmental actions may harmlimit our ability to complete an initial business combination, including by making prospective target companies less likelygrow.
Our ability to
consummate a business combination with us.A conflict of interestmaintain our operations and grow in existing and new markets may arise from the needrequire additional capital, particularly if we were to accelerate our acquisition and expansion plans. Financing may not be available or may be available only on terms that are not favorable. If we are unable to obtain funds on acceptable terms, we may have to delay or abandon some or all of our growth strategies. Further, if additional funds are raised through the consentissuance of Deerfield Partners, which ownsadditional equity securities, the percentage ownership of our stockholders would be diluted. Any newly issued equity securities may have rights, preferences or privileges senior to those of the Common Stock.
Risks Related to Our Common Stock and Warrants
Our issuance of additional shares of Common Stock, Warrants or other convertible securities may dilute your ownership interest in us and could adversely affect our stock price.
From time to time in the future, we may issue additional shares of our Common Stock, Warrants or other securities convertible into Common Stock pursuant to a variety of transactions, including acquisitions. Additional shares of our Common Stock may also be issued upon exercise of outstanding stock options and Warrants. The issuance by us of additional shares of our Common Stock, Warrants or other securities convertible into our Common Stock would dilute your ownership interest in us and the sale of a significant interestamount of such shares in the public market could adversely affect prevailing market prices of our sponsorCommon Stock and managesWarrants. Shares issuable upon exercise of options will be available for resale immediately in the Deerfield Funds,public market without restriction.
In the future, we expect to
obtain financing or to further increase our
business combination.We will entercapital resources by issuing additional shares of our capital stock or offering debt or other equity securities, including senior or subordinated notes, debt securities convertible into an agreementequity, or shares of preferred stock. Issuing additional shares of our capital stock, other equity securities, or securities convertible into equity may dilute the economic and voting rights of our existing stockholders, reduce the market price of our Common Stock and Warrants, or both. Future Debt securities convertible into equity could be subject to adjustments in the conversion ratio pursuant to which wecertain events may increase the number of equity securities issuable upon conversion. Preferred stock, if issued, could have a preference with respect to liquidating distributions or a preference with respect to dividend payments that could limit our ability to pay dividends to the holders of our Common Stock. Our decision to issue securities in any future offering will agree not to completedepend on market conditions and other factors beyond our control, which may adversely affect the amount, timing or nature of our future offerings. As a business combination withoutresult, holders of our Common Stock and Warrants bear the consentrisk that our future offerings may reduce the market price of Deerfield Partners, which owns a significant interest in our sponsorCommon Stock and managesWarrants and dilute their percentage ownership.
Future sales, or the Deerfield Funds, which consent Deerfield Partners has indicated it does not intend to provide ifperception of future sales, of our proposed business combination is with a target that is notCommon Stock and Warrants by us or our existing securityholders in the healthcare industry. As a consequence, interestspublic market could cause the market price for our Common Stock and Warrants to decline.
Our Common Stock and Warrants are traded on The Nasdaq Capital Market. The sale of
affiliatessubstantial amounts of shares of our
sponsor may conflict with thoseCommon Stock or Warrants in the public market, or the perception that such sales could occur, could harm the prevailing market price of
the restshares of our
stockholders if Deerfield Partners does not wish to proceed with a business combination.A provision of our warrant agreementCommon Stock and Warrants. These sales, or the possibility that these sales may occur, also might make it more difficult for us to consummate an initial business combination.
If (x)sell equity securities in the future at a time and at a price that we issue additionaldeem appropriate.
In addition, the shares of Class A common stock or equity-linked securitiesour Common Stock reserved for capital raising purposes in connection with the closing of our initial business combination at a Newly Issued Price of less than $9.20 per share of Class A common stock, (y) the aggregate gross proceeds from such issuances represent more than 50% of the total equity proceeds, and interest thereon, availablefuture issuance under The Oncology Institute, Inc. 2021 Incentive Award Plan (the "2021 Plan") will become eligible for the funding of our initial business combination on the date of the consummation of our initial business combination (net of redemptions), and (z) the Market Value is below $9.20 per share,
the exercise price of the warrants will be adjusted (to the nearest cent) to be equal to 115% of the higher of the Market Value and the Newly Issued Price, and the $18.00 per share redemption trigger prices described below under “Description of Securities - Redeemable Warrants - Redemption of warrants when the price per share of Class A common stock equals or exceeds $18.00” and “Redemption of warrants when the price per share of Class A common stock equals or exceeds $10.00” will be adjusted (to the nearest cent) to be equal to 180% of the higher of the Market Value and the Newly Issued Price, and the $10.00 per share redemption trigger price described below under “Description of Securities - Redeemable Warrants - Redemption of warrants when the price per share of Class A common stock equals or exceeds $10.00” will be adjusted (to the nearest cent) to be equal to the higher of the Market Value and the Newly Issued Price. This may make it more difficult for us to consummate an initial business combination with a target business.
Risks Relating to our Securities
If we are deemed to be an investment company under the Investment Company Act, we may be required to institute burdensome compliance requirements and our activities may be restricted, which may make it difficult for us to complete our initial business combination.
If we are deemed to be an investment company under the Investment Company Act, our activities may be restricted, including:
| ● | restrictions on the nature of our investments; and |
| ● | restrictions on the issuance of securities, each of which may make it difficult for us to complete our initial business combination. In addition, we may have imposed upon us burdensome requirements, including: |
| ● | registration as an investment company with the SEC; |
| ● | adoption of a specific form of corporate structure; and |
| ● | reporting, record keeping, voting, proxy and disclosure requirements and other rules and regulations that we are not subject to. |
In order not to be regulated as an investment company under the Investment Company Act, unless we can qualify for an exclusion, we must ensure that we are engaged primarily in a business other than investing, reinvesting or trading of securities and that our activities do not include investing, reinvesting, owning, holding or trading “investment securities” constituting more than 40% of our assets (exclusive of U.S. government securities and cash items) on an unconsolidated basis. Our business is to identify and complete a business combination and thereafter to operate the post-transaction business or assets for the long term. We do not plan to buy businesses or assets with a view to resale or profit from their resale. We do not plan to buy unrelated businesses or assets or to be a passive investor.
We do not believe that our principal activities subject us to the Investment Company Act. To this end, the proceeds heldsale in the Trust Account may only be invested in United States “government securities” within the meaning of Section 2(a)(16) of the Investment Company Act having a maturity of 185 days or less or in moneypublic market funds meeting certain conditions under Rule 2a-7 promulgated under the Investment Company Act which invest only in direct U.S. government treasury obligations. Pursuantonce those shares are issued, subject to the trust agreement, the trustee is not permitted to invest in other securities or assets. By restricting the investment of the proceeds to these instruments, and by having a business plan targeted at acquiring and growing businesses for the long term (rather than on buying and selling businesses in the manner of a merchant bank or private equity fund), we intend to avoid being deemed an “investment company” within the meaning of the Investment Company Act. The Trust Account is intended as a holding place for funds pending the earliest to occur of either: (i) the completion of our initial business combination; (ii) the redemption of any public shares properly tendered in connection with a stockholder vote to amend our second amended and restated certificate of incorporation to modify the substance or timing of our obligation to redeem 100% of our public shares if we do not complete our initial business combination by March 13, 2022; and (iii) absent an initial business combination by March 13, 2022 or with respect to any other material provisions relating to stockholders’ rights or pre-initial business combination activity, our returnvarious vesting agreements, lock-up provisions and, in some cases, limitations on volume and manner of the funds held in the Trust Accountsale applicable to our public stockholdersaffiliates under Rule 144, as partapplicable. As of our redemption of the public shares. If we do not invest the proceeds
as discussed above, we may be deemed to be subject to the Investment Company Act. If we were deemed to be subject to the Investment Company Act, compliance with these additional regulatory burdens would require additional expenses for which 2023, we have not allotted funds and may hinder our ability to complete a business combination. If we do not complete our initial business combination, our public stockholders may only receive their pro rata portion of the funds in the Trust Account that are available for distribution to public stockholders, and our warrants will expire worthless.
Nasdaq may delist our securities from trading on its exchange, which could limit investors’ ability to make transactions in our securities and subject us to additional trading restrictions.
Our units, Class A common stock and warrants are listed on The Nasdaq Stock Market LLC (“Nasdaq”). We cannot assure you that our securities will be, or will continue to be, listed on Nasdaq in the future or prior to our initial business combination. In order to continue listing our securities on Nasdaq prior to our initial business combination, we must maintain certain financial, distribution and share price levels. Generally, we must hold an annual meeting of stockholders each year and maintain a minimum amount in stockholders’ equity (generally $2,500,000) and a minimum number of holders of our securities (generally 300 public holders). Additionally, in connection with our initial business combination, we will be required to demonstrate compliance with Nasdaq’s initial listing requirements, which are more rigorous than Nasdaq’s continued listing requirements, in order to continue to maintain the listing of our securities on Nasdaq. For instance, our share price would generally be required to be at least $4.00 per share and our stockholder’s equity would generally be required to be at least $5.0 million. We cannot assure you that we will be able to meet those initial listing requirements at that time.
If Nasdaq delists our securities from trading on its exchange and we are not able to list our securities on another national securities exchange, we expect our securities could be quoted on an over-the-counter market. If this were to occur, we could face significant material adverse consequences, including:
| ● | a limited availability of market quotations for our securities; |
| ● | reduced liquidity for our securities; |
| ● | a determination that our Class A common stock is a “penny stock” which will require brokers trading in our Class A common stock to adhere to more stringent rules and possibly result in a reduced level of trading activity in the secondary trading market for our securities; |
| ● | a limited amount of news and analyst coverage; and |
| ● | a decreased ability to issue additional securities or obtain additional financing in the future. |
The National Securities Markets Improvement Act of 1996, which is a federal statute, prevents or preempts the states from regulating the sale of certain securities, which are referred to as “covered securities.” Because we expect that our units and eventually our Class A common stock and warrants will be listed on Nasdaq, our units, Class A common stock and warrants will qualify as covered securities under the statute. Although the states are preempted from regulating the sale of our securities, the federal statute does allow the states to investigate companies if there is a suspicion of fraud, and, if there is a finding of fraudulent activity, then the states can regulate or bar the sale of covered securities in a particular case. While we are not aware of a state having used these powers to prohibit or restrict the sale of securities issued by blank check companies, other than the State of Idaho, certain state securities regulators view blank check companies unfavorably and might use these powers, or threaten to use these powers, to hinder the sale of securities of blank check companies in their states. Further, if we were no longer listed on Nasdaq, our securities would not qualify as covered securities under the statute and we would be subject to regulation in each state in which we offer our securities.
We may issue additional6,008,329 shares of Class A common stock or shares of preferred stock to complete our initial business combination or under an employee incentive plan after completion of our initial business combination. We may also issue shares of Class A common stock upon the conversion of the founder shares at a ratio greater than one-to-one at the time of our initial business combination as a result of the anti-dilution provisions contained in our second amended and restated certificate of incorporation. Any such issuances would dilute the interest of our stockholders and likely present other risks.
Our second amended and restated certificate of incorporation authorizes the issuance of up to 100,000,000 shares of Class A common stock, par value $0.0001 per share, 10,000,000 shares of Class B common stock, par value $0.0001 per share, and 1,000,000 shares of preferred stock, par value $0.0001 per share. There are 77,000,000 and 4,250,000 authorized but unissued shares of Class A common stock and Class B common stock, respectively, available for issuance under the 2021 Plan, which amount does not take into account shares reserved for issuance upon exercisewill automatically increase on January 1 of outstanding warrants or shares issuable upon conversioneach successive year through and including January 1, 2031 in amount equal to 4% of the Class B common stock. The Class B common stock is automatically convertible into Class A common stock at the time of our initial business combination, initially at a one-for-one ratio but subject to adjustmentfully diluted shares outstanding as set forth herein and in our second amended and restated certificate of incorporation. Immediately after the initial public offering, there will be no shares of preferred stock issued and outstanding.
We may issue a substantial number of additional shares of Class A common stock or shares of preferred stock to complete our initial business combination or under an employee incentive plan after completion of our initial business combination. We may also issue shares of Class A common stock upon conversion of the Class B common stock at a ratio greater than one-to-one atpreceding December 31 or such lesser amount as is determined by the time of our initial business combination as a result of the anti-dilution provisions as set forth therein. However, our second amended and restated certificate of incorporation provides, among other things, that prior to our initial business combination, we may not issue additional shares that would entitle the holders thereof to (i) receive funds from the Trust Account or (ii) vote as a class with our public shares (a) on any initial business combination or (b) to approve an amendment to our second amended and restated certificate of incorporation to (x) extend the time we have to consummate a business combination beyond 24 months from the closing of the initial public offering or (y) amend the foregoing provisions. These provisions of our second amended and restated certificate of incorporation, like all provisions of our second amended and restated certificate of incorporation, may be amended with a stockholder vote. The issuance of additional shares of common stock or shares of preferred stock:
| ● | may significantly dilute the equity interest of investors in the initial public offering; |
| ● | may subordinate the rights of holders of Class A common stock if shares of preferred stock are issued with rights senior to those afforded our Class A common stock; |
| ● | could cause a change in control if a substantial number of shares of Class A common stock is issued, which may affect, among other things, our ability to use our net operating loss carry forwards, if any, and could result in the resignation or removal of our present officers and directors; and |
| ● | may adversely affect prevailing market prices for our units, Class A common stock and/or warrants. |
Board. We have not registered the Class A common stock issuable upon exercise of the warrantsfiled multiple registration statements on Form S-8 under the Securities Act to register shares of our Common Stock or any state securities laws, andconvertible into or exchangeable for shares of our Common Stock issued pursuant to our equity incentive plans. Such Form S-8 registration statements automatically become effective upon filing. Accordingly, shares registered under such registration may not bestatements are available for sale in place when an investor desires to exercise warrants, thus precluding such investor from being able to exercise its warrantsthe open market.
Delaware law and
causing such warrants to expire worthless.We have not registered the Class A common stock issuable upon exerciseprovisions in our Charter and Bylaws could make a takeover proposal more difficult.
Our organizational documents are governed by Delaware law. Certain provisions of the warrants under the Securities Act or any state securities laws. However, under the terms of the warrant agreement, we have agreed that, as soon as practicable, but in no event later than 15 business days, after the closingDelaware law and of our initial business combination, we will use ourCharter and Bylaws could discourage, delay, defer or prevent a merger, tender offer, proxy contest or other change of control transaction that a stockholder might consider in its best efforts to file withinterest, including those attempts that might result in a premium over the SEC a registration statement covering the registration under the Securities Act of the Class A common stock issuable upon exercise of the warrants and thereafter will use our best efforts to cause the same to become effective within 60 business days following our initial business combination and to maintain a current prospectus relating to the Class A common stock issuable upon exercise of the warrants until the expiration of the warrants in accordance with the provisions of the warrant agreement. We cannot assure you that we will be able to do so if,market price for example, any facts or events arise which represent a fundamental change in the information set forth in the registration statement or prospectus,
the financial statements contained or incorporated by reference therein are not current or correct or the SEC issues a stop order.
If the shares of Class A common stock issuable upon exercise of the warrants are not registered under the Securities Act, under the terms of the warrant agreement, holders of warrants who seek to exercise their warrants will not be permitted to do so for cash and, instead, will be required to do so on a cashless basis in accordance with Section 3(a)(9) of the Securities Act or another exemption.
In no event will warrants be exercisable for cash or on a cashless basis, and we will not be obligated to issue any shares to holders seeking to exercise their warrants, unless the issuance of the shares upon such exercise is registered or qualified under the securities laws of the state of the exercising holder, or an exemption from registration or qualification is available.
If our shares of Class A common stock are at the time of any exercise of a warrant not listed on a national securities exchange such that they satisfy the definition of “covered securities” under Section 18(b)(1) of the Securities Act, we may, at our option, not permit holders of warrants who seek to exercise their warrants to do so for cash and, instead, require them to do so on a cashless basis in accordance with Section 3(a)(9) of the Securities Act; in the event we so elect, we will not be required to file or maintain in effect a registration statement or register or qualify the shares underlying the warrants under applicable state securities laws, and in the event we do not so elect, we will use our best efforts to register or qualify the shares underlying the warrants under applicable state securities laws to the extent an exemption is not available.
In no event will we be required to net cash settle any warrant, or issue securities (other than upon a cashless exercise as described above) or other compensation in exchange for the warrants in the event that we are unable to register or qualify the shares underlying the warrants under the Securities Act or applicable state securities laws.
If the issuance of the Class A common stock upon exercise of the warrants is not registered, qualified or exempt from registration or qualification under the Securities Act and applicable state securities laws, holders of warrants will not be entitled to exercise such warrants and such warrants may have no value and expire worthless. In such event, holders who acquired their warrants as part of a purchase of units will have paid the full unit purchase price solely for the Class A common stock included in the units.
You may only be able to exercise your public warrants on a “cashless basis” under certain circumstances, and if you do so, you will receive fewer shares of Class A common stock from such exercise than if you were to exercise such warrants for cash.
The warrant agreement provides that in the following circumstances holders of warrants who seek to exercise their warrants will not be permitted to do for cash and will, instead, be required to do so on a cashless basis in accordance with Section 3(a)(9) of the Securities Act: (i) if the shares of Class A common stock issuable upon exercise of the warrants are not registered under the Securities Act in accordance with the terms of the warrant agreement; (ii) if we have so elected and the shares of Class A common stock is at the time of any exercise of a warrant not listed on a national securities exchange such that they satisfy the definition of “covered securities” under Section 18(b)(1) of the Securities Act; and (iii) if we have so elected and we call the public warrants for redemption. If you exercise your public warrants on a cashless basis, you would pay the warrant exercise price by surrendering the warrants for that number of shares of Class A common stock equal to the quotient obtained by dividing (x) the product of the number of shares of Class A common stock underlying the warrants, multiplied by the excess of the “fair market value” of our shares of Class A common stock (as defined in the next sentence) over the exercise price of the warrants by (y) the fair market value. The “fair market value” is the average reported closing price of the shares of Class A common stock for the 10 trading days ending on the third trading day prior to the date on which the notice of exercise is received by the warrant agent or on which the notice of redemption is sent to the holders of warrants, as applicable. As a result, you would receive fewer shares of Class A common stock from such exercise than if you were to exercise such warrants for cash.
Unlike some other similarly structured special purpose acquisition companies, our initial stockholders will receive additional shares of Class A common stock if we issue certain shares to consummate an initial business combination.
The founder shares will automatically convert into shares of Class A common stock concurrently with or immediately following the consummation of our initial business combination on a one-for-one basis, subject to adjustment for stock splits, stock dividends, reorganizations, recapitalizations and the like, and subject to further adjustment as provided herein. In the case that additional shares of Class A common stock or equity-linked securities are issued or deemed issued in connection with our initial business combination, the number of shares of Class A common stock issuable upon conversion of all founder shares will equal, in the aggregate, on an as-converted basis, 20% of the total number of shares of Class A common stock outstanding after such conversion (after giving effect to any redemptions of shares of Class A common stock by public stockholders), including the total number of shares of Class A common stock issued, or deemed issued or issuable upon conversion or exercise of any equity-linked securities or rights issued or deemed issued, by the Company in connection with or in relation to the consummation of the initial business combination, excluding any shares of Class A common stock or equity-linked securities or rights exercisable for or convertible into shares of Class A common stock issued, or to be issued, to any seller in the initial business combination and any private placement warrants issued to our sponsor, officers or directors upon conversion of working capital loans, provided that such conversion of founder shares will never occur on a less than one-for-one basis. This is different than some other similarly structured special purpose acquisition companies in which the initial stockholders will only be issued an aggregate of 20% of the total number of shares to be outstanding prior to our initial business combination.
Certain agreements related to the initial public offering may be amended without stockholder approval.
Each of the agreements related to the initial public offering to which we are a party, other than the warrant agreement and the investment management trust agreement, may be amended without stockholder approval. Such agreements are: the underwriting agreement; the letter agreement among us and our initial stockholders, sponsor, officers and directors; the registration rights agreement among us and our initial stockholders; the private placement warrants purchase agreement between us and our sponsor; the strategic services agreement between us and our Chief Financial Officer; and the administrative services agreement among us, our sponsor and an affiliate of our sponsor. These agreements contain various provisions that our public stockholders might deem to be material. For example, our letter agreement and the underwriting agreement contain certain lock-up provisions with respect to the founder shares, private placement warrants and other securities held by our initial stockholders, sponsor, officers and directors. Amendments to such agreements would require the consent of the applicable parties thereto and would need to be approved by our board of directors, which may do so for a variety of reasons, including to facilitate our initial business combination. While we do not expect our board of directors to approve any amendment to any of these agreements prior to our initial business combination, it may be possible that our board of directors, in exercising its business judgment and subject to its fiduciary duties, chooses to approve one or more amendments to any such agreement. Any amendment entered into in connection with the consummation of our initial business combination will be disclosed in our proxy materials or tender offer documents, as applicable, related to such initial business combination, and any other material amendment to any of our material agreements will be disclosed in a filing with the SEC. Any such amendments would not require approval from our stockholders, may result in the completion of our initial business combination that may not otherwise have been possible, and may have an adverse effect on the value of an investment in our securities. For example, amendments to the lock-up provision discussed above may result in our initial stockholders selling their securities earlier than they would otherwise be permitted, which may have an adverse effect on the price of our securities.
We may amend the terms of the warrants in a manner that may be adverse to holders with the approval by the holders of at least 50% of the then outstanding public warrants. As a result, the exercise price of your warrants could be increased, the exercise period could be shortened and the number of shares of Class A common stock purchasable upon exercise of a warrant could be decreased, all without your approval.
Our warrants will be issued in registered form under a warrant agreement between Continental Stock Transfer & Trust Company, as warrant agent, and us. The warrant agreement provides that the terms of the warrants may be amended without the consent of any holder for the purpose of (i) curing any ambiguity or to correct any defective provision or mistake, including to conform the provisions of the warrant agreement to the description of the terms of the warrants and the warrant agreement set forth in this prospectus, (ii) adjusting the provisions relating to cash dividends on shares of
common stock as contemplated by and in accordance with the warrant agreement or (iii) adding or changing any provisions with respect to matters or questions arising under the warrant agreement as the parties to the warrant agreement may deem necessary or desirable and that the parties deem to not adversely affect the rights of the registered holders of the warrants, provided that the approval by the holders of at least 50% of the then-outstanding public warrants is required to make any change that adversely affects the interests of the registered holders of public warrants. Accordingly, we may amend the terms of the public warrants in a manner adverse to a holder if holders of at least 50% of the then outstanding public warrants approve of such amendment. Although our ability to amend the terms of the public warrants with the consent of at least 50% of the then outstanding public warrants is unlimited, examples of such amendments could be amendments to, among other things, increase the exercise price of the warrants, convert the warrants into cash or stock (at a ratio different than initially provided), shorten the exercise period or decrease the number of shares of Class A common stock purchasable upon exercise of a warrant.
We may redeem your unexpired warrants prior to their exercise at a time that is disadvantageous to you, thereby making your warrants worthless.
We have the ability to redeem outstanding warrants at any time after they become exercisable and prior to their expiration, at a price of $0.01 per warrant, provided that the closing price of our Class A common stock equals or exceeds $18.00 per share (as adjusted for stock splits, stock capitalizations, reorganizations, recapitalizations and the like) for any 20 trading days within a 30 trading-day period ending on the third trading day prior to proper notice of such redemption provided that on the date we give notice of redemption. We will not redeem the warrants unless an effective registration statement under the Securities Act covering the shares of Class A common stock issuable upon exercise of the warrants is effective and a current prospectus relating to those shares of Class A common stock is available throughout the 30-day redemption period, except if the warrants may be exercised on a cashless basis and such cashless exercise is exempt from registration under the Securities Act. If and when the warrants become redeemable by us, we may exercise our redemption right even if we are unable to register or qualify the underlying securities for sale under all applicable state securities laws. Redemption of the outstanding warrants could force you to (i) exercise your warrants and pay the exercise price therefor at a time when it may be disadvantageous for you to do so, (ii) sell your warrants at the then-current market price when you might otherwise wish to hold your warrants or (iii) accept the nominal redemption price which, at the time the outstanding warrants are called for redemption, is likely to be substantially less than the market value of your warrants.
In addition, we have the ability to redeem the outstanding public warrants at any time after they become exercisable and prior to their expiration, at a price of $0.10 per warrant upon a minimum of 30 days’ prior written notice of redemption provided that the closing price of our Class A common stock equals or exceeds $10.00 per share (as adjusted for adjustments to the number of shares issuable upon exercise or the exercise price of a warrant as described under the heading “Description of Securities - Redeemable Warrants - Anti-dilution Adjustments”) for any 20 trading days within a 30 trading-day period ending on the third trading day prior to proper notice of such redemption and provided that certain other conditions are met, including that holders will be able to exercise their warrants prior to redemption for a number of shares of Class A common stock determined based on the redemption date and the fair market value of our shares of Class A common stock. Please see “Description of Securities - Redeemable Warrants - Redemption of warrants when the price per share of Class A common stock equals or exceeds $10.00.” The value received upon exercise of the warrants (1) may be less than the value the holders would have received if they had been able to exercise their warrants at a later time where the underlying share price is higher and (2) may not compensate the holders for the value of the warrants, including because the number of common stock received is capped at 0.361 shares of Class A common stock per warrant (subject to adjustment) irrespective of the remaining life of the warrants.
None of the private placement warrants will be redeemable by us for cash so long as they are held by their initial purchasers or their permitted transferees.
Our warrants may have an adverse effect on the market price of our shares of Class A common stock and make it more difficult to effectuate our initial business combination.
We issued warrants to purchase 4,312,500 shares of our Class A common stock as part of our initial public offering and, simultaneously with the closing of the initial public offering, we issued 3,733,334 private placement warrants, each exercisable to purchase one share of Class A common stock at $11.50 per share. In addition, if our sponsor or an
affiliate of our sponsor or certain of our officers and directors makes any working capital loans, such lender may convert those loans into up to an additional 1,000,000 private placement warrants, at the price of $1.50 per warrant. To the extent we issue common stock to effectuate a business transaction, the potential for the issuance of a substantial number of additional shares of Class A common stock upon exercise of these warrants could make us a less attractive acquisition vehicle to a target business. Such warrants, when exercised, will increase the number of issued and outstanding shares of Class A common stock and reduce the value of the Class A common stock issued to complete the business transaction. Therefore, our warrants may make it more difficult to effectuate a business transaction or increase the cost of acquiring the target business.
Provisions in our second amended and restated certificate of incorporation and Delaware law may inhibit a takeover of us, which could limit the price investors might be willing to pay in the future for our shares of Class A common stock and could entrench management.
Our second amended and restated certificate of incorporation contains provisions that may discourage unsolicited takeover proposals that stockholders may consider to be in their best interests.Common Stock. These provisions include a staggered board of directors and the ability of the board of directorsour Board to designate the terms of and issue new series of preferred stock,preference shares, which may make more difficult the removal of management and may discourage transactions that otherwise could involve payment of a premium over prevailing market prices for our securities.
These anti-takeover provisions as well as certain provisions of Delaware law could make it more difficult for a third party to acquire us, even if the third party’s offer may be considered beneficial by many of our stockholders. As a result, stockholders of the Company may be limited in their ability to obtain a premium for their shares. If prospective takeovers are not consummated for any reason, we may experience negative reactions from the financial markets, including negative impacts on the price of our Common Stock and Warrants. These provisions could also discourage proxy contests and make it more difficult for stockholders of the Company to elect directors of their choosing and to cause us to take other corporate actions that stockholders of the Company desire.
We are alsoan “emerging growth company” and the reduced disclosure requirements applicable to emerging growth companies may make our Common Stock and Warrants less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act. As an emerging growth company, we may follow reduced disclosure requirements and do not have to make all of the disclosures that public companies that are not emerging growth companies do. We will remain an emerging growth company until the earlier of (a) the last day of the fiscal year in which we have total annual gross revenues of $1.235 billion or more; (b) the last day of the fiscal year following the fifth anniversary of the date of the completion of the initial public offering of DFP; (c) the date on which we have issued more than $1.0 billion in nonconvertible debt during the previous three years; or (d) the date on which we are deemed to be a large accelerated filer under the rules of the SEC, which means the market value of our Common Stock that is held by non-affiliates exceeds $700.0 million as of the prior June 30th. For so long as we remain an emerging growth company, we are permitted and intend to rely on exemptions from certain disclosure requirements that are applicable to other public companies that are not emerging growth companies. These exemptions include:
•not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act;
•not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements (i.e., an auditor discussion and analysis);
•reduced disclosure obligations regarding executive compensation in our periodic reports, proxy statements and registration statements; and
•exemptions from the requirements of holding a nonbinding advisory vote of stockholders on executive compensation, stockholder approval of any golden parachute payments not previously approved and having to disclose the ratio of the compensation of our chief executive officer to the median compensation of our employees.
In addition, the JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. This allows an emerging growth company to delay the adoption of
certain accounting standards until those standards would otherwise apply to private companies. We have elected to use the extended transition period for complying with new or revised accounting standards; and as a result of this election, our financial statements may not be comparable to companies that comply with public company effective dates.
We may choose to take advantage of some, but not all, of the available exemptions for emerging growth companies. We cannot predict whether investors will find our Common Stock or Warrants less attractive if we rely on these exemptions. If some investors find our Common Stock or Warrants less attractive as a result, there may be a less active trading market for our Common Stock and Warrants and our share and Warrant price may be more volatile.
Our Common Stock and Warrants may be delisted if we fail to comply with the requirements for continued listing on The Nasdaq Stock Market LLC (“Nasdaq”), and if our securities were delisted, the price of our Common Stock and Warrants, our ability to access the capital markets and our ability to comply with the covenants in our Facility Agreement could be negatively impacted.
Our Common Stock and Warrants are listed for trading on Nasdaq. To maintain this listing, we must satisfy Nasdaq’s continued listing requirements, including, among other things, a minimum closing bid price requirement of $1.00 per share, among others. Delisting could harm our ability to raise capital through alternative financing sources on terms acceptable to us, or at all, and may result in the potential loss of confidence by investors, employees, and business development opportunities. Such a delisting likely would impair investor’s ability to sell or purchase our common stock when investors wish to do so. Further, if we were to be delisted from Nasdaq, our common stock may no longer be recognized as a “covered security” and we would be subject to anti-takeover provisionsregulation in each state in which we offer our securities. Thus, delisting from Nasdaq could adversely affect our ability to raise additional financing through the public or private sale of equity securities, would significantly impact the ability of investors to trade our securities, and would negatively impact the value and liquidity of our common stock.
In addition, our Facility Agreement contains various covenants, including a requirement that the Company remain a reporting company and maintain the listing of our shares of common stock on an eligible market such as Nasdaq. Should our common stock be delisted, we would be in breach of the eligible market covenant in the Facility Agreement. If we breach this covenant and are unable to obtain a waiver or amendment under
Delaware law,the Facility Agreement, the lenders may, among other things, accelerate our outstanding indebtedness and exercise rights with respect to collateral securing our outstanding indebtedness, each of which could
delay or prevent a changehave an adverse effect on our business, financial condition and results of
control. Together these provisions may make the removal of management more difficult and may discourage transactions that otherwise could involve payment of a premium over prevailing market prices for our securities.operations.
Provisions in our second amended and restated
Our certificate of incorporation and our bylaws provide that the Court of Chancery of the State of Delaware law may haveis the effect of discouraging lawsuits againstsole and exclusive forum for substantially all disputes between us and our stockholders, which limits our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers, or employees.
Our Charter and
officers.Our second amended and restated certificate of incorporation will require,Bylaws provide that, unless we consent in writing to the selection of an alternative forum, the (a) Court of Chancery (the “Chancery Court”) of the State of Delaware (or, in the event that the Chancery Court does not have jurisdiction, the federal district court for the District of Delaware or other state courts of the State of Delaware) shall, to the fullest extent permitted by law, be the sole and exclusive forum for: (i) any derivative action, suit or proceeding brought on our behalf,behalf; (ii) any action, suit or proceeding asserting a claim of breach of a fiduciary duty owed by any current or former director, officer, stockholder or other employee of the company to usthe company or our stockholders,its stockholders; (iii) any action, suit or proceeding asserting a claim against us, our directors, officers or employeesthe Company arising pursuant to any provision ofunder the DGCL, or our second amended and restatedits certificate of incorporation or its bylaws or (iv) any action asserting a claim against us, our directors, officers or employees governed byas to which the internal affairs doctrine may be brought only inDGCL confers jurisdiction on the Court of Chancery inof the State of Delaware, exceptDelaware; (iv) any claim (A)action, suit or proceeding as to which the DGCL confers jurisdiction on the Court of Chancery of the State of Delaware determines that there is an indispensable party not subject to the jurisdiction of the Court of Chancery (and the indispensable party does not consent to the personal jurisdiction of the Court of Chancery within ten days following such determination), (B) which is vested in the exclusive jurisdiction of a court or forum other than the Court of Chancery, (C) for which the Court of Chancery does not have subject matter jurisdiction, or (D)(v) any action, arising undersuit or proceeding asserting a claim against the Securities Act, as to whichCompany or any current or former director, officer or stockholder governed by the Court of Chanceryinternal affairs doctrine, and, the federal district court for the District of Delaware shall have concurrent jurisdiction. If an action isif brought outside of Delaware, the stockholder bringing the suit will be deemed to have consented to (A) the personal jurisdiction of the state and federal courts within Delaware and (B) service of process on such stockholder’s counsel. Although we believe thisThe provision benefits us by providing increased consistencyof the Charter described in the application of Delaware law in the types of lawsuits to which it applies, a court may determine that this provision is unenforceable, and to the extent it is enforceable, the provision may have the effect of discouraging lawsuits against our directors and officers, although our stockholders will not be deemed to have waived our compliance with federal securities laws and the rules and regulations thereunder.
Notwithstanding the foregoing, our second amended and restated certificate of incorporation will provide that the exclusive forum provision willimmediately preceding sentence does not apply to (i) suits brought to enforce a duty or liability created by the Exchange Act or any other claim for which the federal courts have exclusive jurisdiction and (ii) any action arising under the Securities Act, as to which the federal district court for the United States of America shall have exclusive jurisdiction. The choice of forum provision may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers, or other employees, which may discourage such lawsuits against us and our directors, officers, and other employees. Alternatively, if a court were to find the choice of forum provision contained in our certificate of incorporation to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in other jurisdictions, which could harm our business, results of operations, and financial condition.
Additionally, Section 2722 of the ExchangeSecurities Act creates exclusiveconcurrent jurisdiction for federal jurisdictionand state courts over all suits brought to enforce any duty or liability created by the ExchangeSecurities Act or the rules and regulations thereunder. Although we believeAs noted above, our certificate of incorporation and our bylaws provide that the federal district courts of the United States shall have jurisdiction
over any action arising under the Securities Act. Accordingly, there is uncertainty as to whether a court would enforce such provision. Our stockholders will not be deemed to have waived our compliance with the federal securities laws and the rules and regulations thereunder.
The market price of our Common Stock may be volatile or may decline regardless of our operating performance. You may lose some or all of your investment.
The market price of our Common Stock is likely to be volatile. The stock market recently has experienced extreme volatility. This volatility often has been unrelated or disproportionate to the operating performance of particular companies. You may not be able to resell your shares at an attractive price due to a number of factors such as those listed in this provision benefits us by providing increased consistencysection and the following:
•the impact of a pandemic, epidemic, or outbreak of an infectious disease in the applicationUnited States or worldwide on our financial condition and the results of Delaware lawoperations;
•our operating and financial performance and prospects;
•our quarterly or annual earnings or those of other companies in our industry compared to market expectations;
•conditions that impact demand for our products;
•future announcements concerning our business, our customers’ businesses or our competitors’ businesses;
•the public’s reaction to our press releases, other public announcements and filings with the SEC;
•the size of our public float;
•coverage by or changes in financial estimates by securities analysts or failure to meet their expectations;
•market and industry perception of our success, or lack thereof, in pursuing our growth strategy;
•strategic actions by us or our competitors, such as acquisitions or restructurings;
•changes in laws or regulations that adversely affect our industry or us;
•changes in accounting standards, policies, guidance, interpretations or principles;
•changes in senior management or key personnel;
•issuances, exchanges or sales, or expected issuances, exchanges or sales, of our capital stock;
•changes in our dividend policy;
•adverse resolution of new or pending litigation against us; and
•changes in general market, economic and political conditions in the typesUnited States and global economies or financial markets, including those resulting from natural disasters, terrorist attacks, acts of lawsuitswar and responses to such events.
These broad market and industry factors may materially reduce the market price of our Common Stock and Warrants, regardless of our operating performance. In addition, price volatility may be greater if the public float and trading volume of our Common Stock is low. As a result, you may suffer a loss on your investment.
In the past, following periods of market volatility, stockholders have instituted securities class action litigation. If we were involved in securities litigation, it could have a substantial cost and divert resources and the attention of executive management from our business regardless of the outcome of such litigation.
If securities analysts cease publishing research or reports about us, or if they issue unfavorable commentary about us or our industry or downgrade our Common Stock, the price of our Common Stock could decline.
The trading market for our Common Stock depends, in part, on the research and reports that third-party securities analysts publish about us and the industries in which it applies,we operate. We may be unable or slow to attract research coverage, and if one or more analysts cease coverage of us, the provisionprice and trading volume of our securities would likely be negatively impacted. If any of the analysts that may havecover us change their recommendation regarding our Common Stock adversely, or provide more favorable relative recommendations about our competitors, the effectprice of discouraging lawsuits against our directorsCommon Stock would likely decline. If any analyst that may cover us ceases covering us or fails to regularly publish reports on us, we could lose visibility in the financial markets, which could cause the price or trading volume of our Common Stock to decline. Moreover, if one or more of the analysts who cover us downgrades our Common Stock, or if our reporting results do not meet their expectations, the market price of our Common Stock could decline.
The obligations associated with being a public company involve significant expenses and officers.require significant resources and management attention, which may divert from our business operations.
General Risk Factors
We have identified a material weakness inthe Exchange Act and the Sarbanes-Oxley Act. The Exchange Act requires that we file annual, quarterly and current reports with respect to our business and financial condition. The Sarbanes-Oxley Act requires, among other things, that we establish and maintain effective internal control over financial reporting. This material weakness couldAs a result, we have incurred and will continue to adversely affectincur increased legal, accounting and other expenses that Legacy TOI did not previously incur. Our entire management team and many of our abilityother employees have devoted and will continue to reportdevote substantial time to compliance and may not effectively or efficiently manage our transition into a public company.
In addition, the need to establish the corporate infrastructure demanded of a public company may also divert management’s attention from implementing our business strategy, which could prevent us from improving our business, results of operations and financial
condition accuratelycondition. We have made, and
in a timely manner.Following the issuance of the SEC Staff Statement on April 12, 2021, after consultation with our management and our audit committee concluded that, in light of the SEC Statement, it was appropriatewill continue to restate (i) previously issued audited balance sheet dated as of March 13, 2020 which was relatedmake, changes to its initial public offering, (ii) unaudited interim financial statements as of and for the quarterly periods ended March 31, 2020, June 30, 2020 and September 30, 2020, as reported in the Company’s Quarterly Reports on Form 10-Q filed with the SEC on May 8, 2020, August 13, 2020 and November 13, 2020, respectively, and (iii) audited financial statements as of December 31, 2020 and for the period from November 1, 2019 (inception) through December 31, 2020 as reported in the Company’s Annual Report on Form 10-K/A filed with the SEC on March 30, 2021 (collectively, the “Affected Periods Amendment No. 1”).
Our management is responsible for establishing and maintaining adequate internal control over financial reporting designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with GAAP. Our management is likewise required, on a quarterly basis, to evaluate the effectiveness of our internal controls and to disclose any changes and material weaknesses identified through such evaluation of those internal controls. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis.
As described in the 2020 Form 10-K/A No. 1, we identified a material weakness in our internal control over financial reporting, relatedincluding IT controls, and procedures for financial reporting and accounting systems to meet our reporting obligations as a public company. However, the accountingmeasures we take may not be sufficient to satisfy our obligations as a public company. If we do not continue to develop and implement the right processes and tools to manage our changing enterprise and maintain our culture, our ability to compete successfully and achieve our business objectives could be impaired, which could negatively impact our business, financial condition and results of operations. In addition, we cannot predict or estimate the amount of additional costs we may incur to comply with these requirements. We anticipate that these costs will materially increase our general and administrative expenses.
These rules and regulations result in our incurring legal and financial compliance costs and will make some activities more time-consuming and costly. For example, we expect these rules and regulations to make it more difficult and more expensive for a significantus to obtain director and unusual transaction relatedofficer liability insurance, and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the warrants we issued in connection with our initial public offering in March 2020.same or similar coverage. As a result, it may be more difficult for us to attract and retain qualified people to serve on our board of this material weakness,directors, on our management concludedboard committees or as executive officers.
We do not intend to pay dividends on our Common Stock for the foreseeable future.
We have never declared or paid any cash dividends on our capital stock. We currently intend to retain all available funds and future earnings, if any, to fund the development and growth of the business, and therefore, do not anticipate declaring or paying any cash dividends on Common Stock in the foreseeable future. Any future determination related to our dividend policy will be made at the discretion of our board of directors after considering our business prospects, results of operations, financial condition, cash requirements and availability, debt repayment obligations, capital expenditure needs, contractual restrictions, covenants in the agreements governing current and future indebtedness, industry trends, the provisions of Delaware law affecting the payment of dividends and distributions to stockholders and any other factors or considerations the board of directors deems relevant.
Our Warrants may have an adverse effect on the market price of our Common Stock.
Simultaneously with the closing of its IPO, DFP Healthcare Acquisitions Corp., issued in a private placement an aggregate of 4,333,333 private placement warrants, each exercisable to purchase one share of Common Stock at $11.50 per share through November 2026. As of December 31, 2023, there were 3,177,542 private placement warrants outstanding. To the extent such warrants are exercised, additional shares of our Common Stock will be issued, which will result in dilution to our stockholders and increase the number of shares of Common Stock eligible for resale in the public market. Sales of substantial numbers of such shares in the public market or the fact that such warrants may be exercised could adversely affect the market price of our internal control overCommon Stock.
Risks Related to Our Indebtedness
The Facility Agreement and the associated restrictive covenants thereunder could adversely affect our financial reporting was not effectivecondition and will restrict our ability to raise capital.
On August 9, 2022, we entered into a $110 million Facility Agreement with Deerfield Partners and certain of its affiliates, of which all is outstanding as of December 31, 2020. This2023. The Facility Agreement contains various covenants, including a requirement to retain $40,000,000 in unrestricted cash and cash equivalents, and maintain a minimum revenue of $50,000,000, $75,000,000, and $100,000,000 for each fiscal quarter ending during the fiscal year 2023, 2024, and 2025, respectively. In addition, the Facility Agreement restricts our and the guarantors’ ability to, among other things, (i) merge, consolidate, dissolve or liquidate into or convey, transfer, lease or dispose of all or substantially all of its assets (other than into another Loan Party or if the Company determines in good faith in the best interest of a subsidiary and not materially disadvantageous), (ii) create or incur any lien on our assets beyond those outstanding on the date of the Facility Agreement and certain other permitted liens, (iii) dispose of any assets or property or issue, transfer, or provide a controlling, management, or other interest in certain securities of the Company or its guarantors, (iv) incur any indebtedness not to exceed $1,000,000 or as otherwise permitted, (v)
make any investments other than as otherwise permitted, (vi) amend our organizational documents or any material weakness resultedagreements in a material misstatementmanner that would reasonably be expected to be materially adverse to the rights of the lenders or (vii) change our reporting practices or fiscal year, in each case, subject to exceptions set forth in the Facility Agreement. Furthermore, under the Facility Agreement, we are required to, among other things, (i) remain a reporting company and maintain the listing of our derivative warrant liabilities, changecommon shares on an eligible market, (ii) provide the lenders with information regarding any event of default or the occurrence of any material adverse event and (iii) publicly disclose material, nonpublic information that is provided to the lenders without their prior written consent. Subject to customary exceptions and exclusions, our obligations under the Facility Agreement are guaranteed by a perfected, first-priority security interest in fair valuesubstantially all of derivative warrant liabilities, Class A common stock subjectour personal property, including our intellectual property and the equity ownership interests directly and indirectly held by us in our wholly-owned subsidiaries. Compliance with such covenants and our indebtedness will result in the following, which could materially and adversely affect our business, financial condition and results of operations:
•require us to possible redemption, accumulated deficitdedicate a substantial portion of cash and related financial disclosures forcash equivalents to the Affected Periods Amendment No. 1. For a discussionpayment of management’s considerationinterest on, and principal of, the material weakness identified relatedindebtedness, which will reduce the amounts available to fund working capital, capital expenditures, product development efforts and other general corporate purposes;
•oblige us to comply with negative covenants restricting our activities, including limitations on dispositions, mergers or acquisitions, encumbering our intellectual property, incurring indebtedness or liens, paying dividends, making investments and engaging in certain other business transactions;
•limit our flexibility in planning for, or reacting to, changes in our business and our industry;
•place us at a competitive disadvantage compared to our accountingcompetitors who have less debt or competitors with comparable debt at more favorable interest rates; and
•limit our ability to borrow additional amounts for a significantworking capital, capital expenditures, research and unusual transaction related todevelopment efforts, acquisitions, debt service requirements, execution of our business strategy and other purposes and otherwise restrict our financing options.
Furthermore, because the
warrants we issued in connection with the July 2020 initial public offering, see “Note 2—Restatement of Previously Issued Financial Statements” to the 2020 Form 10-K/A No. 1, as well as “Part II, Item 9A. Controls and Procedures included in this Annual Report.”As described elsewhere in this Amendment No. 2, we have identified a material weakness in our internal control over financial reporting related to the Company’s application of ASC 480-10-S99-3A to its accounting classificationinterests of the Public Shares. As a resultlenders may potentially differ from ours and from those of this material weakness, our management has concluded that our internal control over financial reporting was not effective as of December 31, 2020. Historically, a portion of the Public Shares was classified as permanent equity to maintain stockholders’ equity greater than $5 million on the basis that the Company will not redeem its Public Shares in an amount that would cause its net tangible assets to be less than $5,000,001, as described in the Charter. Pursuant to the Company’s re-evaluation of the Company’s application of ASC 480-10-S99-3A to its accounting classification of the Public Shares, the Company’s management has determined that the Public Shares include certain provisions that require classification of all of the Public Shares as temporary equity regardless of the net tangible assets redemption limitation contained in the Charter. For a discussion of management’s consideration of the material weakness identified related to the Company’s application of ASC 480-10-S99-3A to its accounting classification of the Public Share, see “Note 2” to the accompanying financial statements, as well as Part II, Item 9A: Controls and Procedures included in this Amendment No. 2.
As described in “Part II, Item 9A. Controls and Procedures,” we have concluded that our internal control over financial reporting was ineffective as of December 31, 2020 because material weaknesses existed in our internal control over financial reporting. We have taken a number of measures to remediate the material weaknesses described therein; however, if we are unable to remediate our material weaknesses in a timely manner or we identify additional material weaknesses,stockholders, we may be unable to provide requiredengage in transactions or other activities that may be beneficial to our stockholders. The covenants under the Facility Agreement could materially and adversely affect our business, financial informationcondition and results of operations.
Upon the occurrence of a Major Transaction, as defined under the Senior Secured Convertible Note issued pursuant to the Facility Agreement, the holders of the convertible notes may elect to require us to redeem all or any portion of the notes for an amount equal to the principal amount thereof (in addition to accrued and unpaid interest, a make-whole amount and an exit fee, as applicable). There can be no assurance that we will have sufficient capital to redeem such notes upon the occurrence of a Major Transaction, under the Senior Secured Convertible Note.
Servicing our indebtedness requires a significant amount of cash. Our ability to repay the principal of, to pay interest on or to refinance our indebtedness depends on our future performance, which is subject to economic, financial, competitive and other factors beyond our control. Our business may not generate cash flow from operations in a timely and reliable manner andthe future sufficient to service our indebtedness. If we are unable to generate cash flow, we may incorrectly report financial information. Likewise, ifbe required to adopt one or more alternatives, such as restructuring debt or obtaining additional financing on terms that may be unfavorable to us or highly dilutive. Our ability to refinance our indebtedness will depend on the capital markets and our financial statements are not filed oncondition at the time we seek to refinance such indebtedness. Our inability to satisfy our debt obligations could materially and adversely affect our financial position and results of operations.
A failure to comply with the conditions of the Facility Agreement or the Senior Secured Convertible Note could result in an event of default. An event of default under the Facility Agreement includes, among other things, a timely basis, we could be
subjectfailure to sanctionspay any amount due under the Facility Agreement or investigations by the stock exchange on which our Class Ato issue common stock when required upon conversion of the Senior Secured Convertible Note as well as the occurrence of a criminal proceeding pursuant to which the remedy sought includes forfeiture of a material portion of property. If we fail to comply with any of the covenants under our indebtedness and are listed, the SEC or other regulatory authorities. Failure to timely file will cause us to be ineligible to utilize short form registration statements on Form S-3 or Form S-4, which may impair our abilityunable to obtain capital in a timely fashionwaiver or amendment, the lenders may, among other things, accelerate our outstanding indebtedness and exercise rights with respect to executecollateral securing our business strategies or issue shares to effectoutstanding indebtedness, each of which could have an acquisition. In either case, there could result a material adverse effect on our business. The existencebusiness, financial condition and results of material weaknesses or significant deficiencies in internal control over financial reportingoperations.
Any of these events could materially and adversely affect our reputation or investor perceptionsbusiness, financial condition and results of us, which couldoperations.
The terms of the Senior Secured Convertible Note may have a negative
effect on the trading price of our stock. In addition, we will incur additional costs to remediate material weaknesses in our internal control over financial reporting, as described in “Part II, Item 9A. Controls and Procedures.”We can give no assurance that the measures we have taken and plan to take in the future will remediate the material weakness identified or that any additional material weaknesses or restatements of financial results will not arise in the future due to a failure to implement and maintain adequate internal control over financial reporting or circumvention of these controls. In addition, even if we are successful in strengthening our controls and procedures, in the future those controls and procedures may not be adequate to prevent or identify irregularities or errors or to facilitate the fair presentation of our financial statements.
We are a blank check company with no operating history and no revenues, and you have no basis on which to evaluate our ability to achieve our business objective.
We are a blank check company incorporated under the laws of the State of Delaware with no operating results, and we will not commence operations until completing a business combination. Because we lack an operating history, you have no basis upon which to evaluate our ability to achieve our business objective of completing our initial business combination with one or more target businesses. We have no plans, arrangements or understandings with any prospective target business concerning a business combination and may be unable to complete our initial business combination. If we fail to complete our initial business combination, we will never generate any operating revenues.
Past performance by our management team, DFB, Deerfield Management or their affiliates may not be indicative of future performance of an investment in us.
Information regarding performance by, or businesses associated with, our management team, DFB, Deerfield Management and their affiliates, or businesses associated with them, is presented for informational purposes only. Past performance by such individuals and entities is not a guarantee either (i) of success with respect to any business combination we may consummate or (ii) that we will be able to locate a suitable candidate for our initial business combination. You should not rely on the historical record of the performance of our management team, DFB, Deerfield Management or their affiliates or businesses associated with them as indicative of our future performance of an investment in us or the returns we will, or is likely to, generate going forward.
Cyber incidents or attacks directed at us could result in information theft, data corruption, operational disruption and/or financial loss.
We depend on digital technologies, including information systems, infrastructure and cloud applications and services, including those of third parties with which we may deal. Sophisticated and deliberate attacks on, or security breaches in, our systems or infrastructure, or the systems or infrastructure of third parties or the cloud, could lead to corruption or misappropriation of our assets, proprietary information and sensitive or confidential data. As an early stage company without significant investments in data security protection, we may not be sufficiently protected against such occurrences. We may not have sufficient resources to adequately protect against, or to investigate and remediate any vulnerability to, cyber incidents. It is possible that any of these occurrences, or a combination of them, could have adverse consequencesimpact on our business and leadthe value of our securities and may result in substantial dilution to our other equity securityholders.
The Senior Secured Convertible Note provides for certain terms which may have a negative impact on our business. Obligations under such agreement mature on August 9, 2027 and carry the possibility of the issuance of Convertible Note Warrants upon prepayment.
The obligations under the Senior Secured Convertible Note are secured and the lenders thereunder will have a claim against the assets and equity interests securing the related debt obligations that will have priority to claims of the Company’s equity securityholders generally. Additionally, the Convertible Note is guaranteed by certain of our subsidiaries, effectively providing for claims against such subsidiaries which are structurally senior to our other equity securityholders generally.
The Senior Secured Convertible Note is convertible into common stock, subject to certain terms and conditions, which may result in dilution to our other equity securityholders.
Item 1B. Unresolved Staff Comments
None.
Item 1C. Cybersecurity
The Company prioritizes the protection of our critical systems and information through a robust cybersecurity risk management program. This program outlines our approach to identifying, assessing, and mitigating cybersecurity risks to ensure the confidentiality, integrity, and availability of our assets.
We adhere to the National Institute of Standards and Technology Cybersecurity Framework (NIST CSF) as the guiding framework for our cybersecurity risk management program. While we do not claim compliance with specific technical standards, the NIST CSF serves as a valuable tool for identifying, assessing, and managing cybersecurity risks relevant to our business. Our cybersecurity risk management program is integrated into our overall enterprise risk management program, and shares common methodologies, reporting channels and governance processes that apply across the enterprise risk management program to other legal, compliance, strategic, operational, and financial loss.risk areas.
Changes
Our cybersecurity risk management program includes:
a.risk assessment using industry-standard methodologies such as threat modeling, vulnerability scanning, and penetration testing. These assessments encompass a thorough examination of our critical systems, networks, and applications to identify and prioritize cybersecurity risks. Leveraging tools such as SIEM (Security Information and Event Management) and IDS/IPS (Intrusion Detection System/Intrusion Prevention System), we analyze network traffic patterns and behavior to detect potential threats and vulnerabilities. Additionally, we utilize vulnerability assessment tools to scan our infrastructure for known weaknesses and misconfigurations. These assessments inform our risk management strategies and resource allocation efforts, ensuring that we address the most critical vulnerabilities and threats effectively;
b.a security team principally responsible for managing cybersecurity risk assessment processes, implementing security controls, and orchestrating responses to cybersecurity incidents. They ensure alignment with organizational objectives and regulatory requirements.
c.The use of external service providers specializing in laws or regulations, orpenetration testing, security auditing, and incident response, selected based on their track record, certifications (e.g., CISSP), and adherence to standards (e.g., ISO/IEC 27001). We also enlist Managed Security Service Providers (MSSPs) for continuous monitoring and threat analysis. Cloud service providers with robust security measures host our critical infrastructure, ensuring encryption, multi-factor authentication, and regular audits. Through careful selection and oversight, these partners enhance our cybersecurity defenses and align with our security requirements efficiently.
d.cybersecurity awareness training covers phishing, social engineering, malware prevention, and secure password management. It includes hands-on exercises and simulations to teach employees to identify threats and adhere to secure coding practices. We also emphasize endpoint security measures like antivirus software and firewalls. Additionally, we educate on emerging threats like ransomware and zero-day exploits, fostering a failureculture of vigilance and proactive risk mitigation.
e.a cybersecurity incident response plan includes procedures for detecting, containing, and mitigating cybersecurity incidents promptly and effectively. We leverage Security Information and Event Management (SIEM) tools for real-time monitoring and alerting, enabling rapid response to complypotential threats. Additionally, we employ incident response playbooks with any lawspredefined actions for various scenarios, ensuring a coordinated and regulations, may adversely affectefficient response. Our plan also
incorporates post-incident reviews and lessons learned sessions to continuously improve our business,response capabilities and resilience against future threats.
There can be no assurance that our cybersecurity risk management program and processes, including our abilitypolicies, controls or procedures, will be fully implemented, complied with or effective in protecting our systems and information.
We have not identified risks from known cybersecurity threats, including as a result of any prior cybersecurity incidents, that have materially affected or are reasonably likely to negotiate and completematerially affect us, including our initialoperations, business combination, andstrategy, results of operations.operations, or financial condition
Our Board considers cybersecurity risk as part of its risk oversight function and has delegated to the Compliance Committee (the "Committee") oversight of cybersecurity and other information technology risks. The Committee oversees management’s implementation of our cybersecurity risk management program. The Committee receives quarterly reports from management on our cybersecurity risks. In addition, management updates the Committee, as necessary, regarding any material cybersecurity incidents, as well as any incidents with lesser impact potential.
Our management team, including the Vice President of Healthcare Information Services, is responsible for assessing and managing our material risks from cybersecurity threats. The team has primary responsibility for our overall cybersecurity risk management program and supervises both our internal cybersecurity personnel and our retained external cybersecurity consultants. Our management team’s experience includes over 25 years of experience in healthcare IT operations, infrastructure deployment, IT governance, and change management.
Our management team supervises efforts to prevent, detect, mitigate, and remediate cybersecurity risks and incidents through various means, which may include briefings from internal security personnel; threat intelligence and other information obtained from governmental, public or private sources, including external consultants engaged by us; and alerts and reports produced by security tools deployed in the IT environment.
Item 2. Properties
Our principal executive offices are located in Cerritos, California where we occupy a suite under a lease that expires in 2026. We use this facility for administration, billing and collections, technology and development and professional services.
We intend to procure additional space as we add team members and expand geographically. We believe that our facilities are subjectadequate to lawsmeet our needs for the immediate future, and regulations enacted by national, regional and local governments. In particular, wethat, should it be needed, suitable additional space will be requiredavailable to comply with certain SECaccommodate any such expansion of our operations. As of December 31, 2023, we have leases for 69 clinics located in California, Arizona, Nevada, Florida and other legal requirements. Compliance with,Texas. Generally, our leases are “net” leases, which require us to pay all of the cost of insurance, taxes, maintenance and monitoring of, applicable
utilities. We generally cannot cancel these leases at our option.laws and regulations may be difficult, time consuming and costly. Those laws and regulations and their interpretation and application may also change fromFrom time to time, we may be involved in various legal proceedings and those changes couldsubject to claims that arise in the ordinary course of business. Although the results of litigation and claims are inherently unpredictable and uncertain, we are not currently a party to any legal proceedings the outcome of which, if determined adversely to us, are believed to, either individually or taken together, have a material adverse effect on our business, investmentsoperating results, cash flows or financial condition.
Item 4. Mine Safety Disclosures
Not applicable.
PART II
Item 5. Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities Stock Price Information
Our common stock trades on the Nasdaq under the symbol “TOI.” Our publicly traded warrants trade on Nasdaq under the symbol “TOIIW.”
Holders
As of March 19, 2024, we had approximately 72 holders of record of our common stock.
Dividends
We have never declared or paid any cash dividends on our Common Stock or any other securities. Subject to applicable law and the rights and preferences of any holders of any outstanding series of preferred stock, under our third amended and restated certificate of incorporation, holders of our Common Stock will be entitled to the payment of dividends when, as and if declared by our board in accordance with applicable law.
Recent Sales of Unregistered Securities
None
Equity Compensation Plan Information
See Item 12 - “Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.”
Item 6. [ Reserved ]
Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations
The following discussion and analysis provides information that management believes is relevant to an assessment and understanding of the consolidated results of operations. In addition, a failure to complyoperations and financial condition of The Oncology Institute, Inc. ("TOI") along with applicable laws or regulations, as interpretedits consolidating subsidiaries (the "Company"). The discussion should be read together with the historical audited annual financial statements for the years ended December 31, 2023 and applied, could have a material adverse effect on our business, including our ability to negotiate 2022, and complete our initial business combination, and results of operations.Wethe related notes that are an emerging growth company and a smaller reporting companyincluded elsewhere in this Annual Report. The information in this discussion contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 (as amended, "Securities Act"), as amended, and ifSection 21E of the Securities Exchange Act of 1934 (as amended, the "Exchange Act"). Such statements are based upon current expectations, as well as management's beliefs and assumptions and involve a high degree of risk and uncertainty. Any statements contained herein that are not statements of historical fact may be deemed to be forward-looking statements. Statements that include the words "believes," "anticipates," "plans," "expects." "intends," and similar expressions that convey uncertainty of future events or outcomes are forward-looking statements. Our actual results could differ materially from those discussed or suggested in the forward-looking statements herein. Factors that could cause or contribute to such differences include those described under the heading "Risk Factors" section in this Annual Report on Form 10-K, and in subsequent filings we take advantagemake with the Securities and Exchange Commission, where we may discuss new risks that have not yet arisen at the time of certain exemptions from disclosure requirementsthis Annual Report. In addition, as a result of these and other factors, our past financial performance should not be relied on as an indication of future performance. All forward-looking statements in this document are based on information available to emergingus as of the filing date of this Annual Report on Form 10-K and we assume no obligation to update any forward-looking statements or the reasons why our actual results may differ. All dollar values are expressed in thousands, unless otherwise noted.
Unless the context dictates otherwise, references in this Annual Report on Form 10-K to the “Company,” “we,” “us,” “our,” and similar words are references to The Oncology Institute, Inc., a Delaware corporation (“TOI”), and its consolidated subsidiaries and affiliated entities, as appropriate, including its consolidated variable interest entities (“VIEs”).
Overview
The Company is a leading value-based oncology company that manages community-based oncology practices that serve patients at 83 clinic locations across 15 markets and five states throughout the United States. As of December 31, 2023, our community-based oncology practices are staffed with 130 oncologists and advanced practice providers. 69 of these clinics are staffed with 119 providers employed by our affiliated physician-owned professional corporations, referred to as the "TOI PCs," which provided care for more than 64,000 and 72,000 patients in 2022 and 2023, respectively, and managed a population of approximately 1.8 million patients under value-based agreements as of December 31, 2023. The Company also provides management services to 14 clinic locations owned by independent oncology practices. The Company's mission is to heal and empower cancer patients through compassion, innovation, and state-of-the-art medical care.
Operationally, the Company’s medical centers provide a complete suite of medical oncology services including: physician services, in-house infusion and pharmacy, clinical trials, radiation, educational seminars, support groups, counseling, and 24/7 patient assistance. Many of our services, such as managing clinical trials and palliative care programs, are traditionally accessed through academic and tertiary care settings, while the TOI PCs bring these services to patients in a community setting. As scientific research progresses and more treatment options become available, cancer care is shifting from acute care episodes to chronic disease management. With this shift, it is increasingly important for high-quality, high-value cancer care to be available in a local community setting to all patients in need.
As a value-based oncology company, the Company seeks to deliver both better quality care and lower cost of care for payors and patients. The Company works to accomplish this goal by reducing wasteful, inefficient or counterproductive care that drives up costs but does not improve outcomes. The Company believes payors and employers are aligned with the value-based model due to its enhanced access, improved outcomes, and lower costs. Patients under the Company's affiliated providers’ care can benefit from evidence-based and personalized care plans, gain access to sub-specialized care in convenient community locations, and lower out-of-pocket costs. The Company believes its affiliated providers enjoy the stability and predictability of a large multi-state practice, are not incentivized or pressured to overtreat when it may be inconsistent with a patient’s goals of care, and can focus on practicing outstanding evidence-based medicine, rather than business building.
2023 Highlights
•Consolidated revenue of $324.2 million, an increase of 28% compared to the prior year
•Gross profit of $59.6 million, an increase of 14% compared to the prior year, and gross margin of 18.4%, a decrease from 20.6% the prior year
•Adjusted EBITDA of $(25.8) million compared to $(23.5) million for the prior year
•Ended the fiscal year 2023 with $83 million in cash, cash equivalents, and investments
•Signed full-risk capitated contract in South Florida, effective January 1, 2024 and have now successful onboarded the membership and the IPA providers.
•Successfully acquired and launched our California-based pharmacy in November and have already completed over 1,300 specialty medication fills
Components of Results of Operations
Revenue
The Company receives payments from the following sources for services rendered: (i) commercial insurers; (ii) pharmacy benefit managers (“PBMs”), (iii) the federal government under the Medicare program administered by the Centers for Medicare and Medicaid Services (“CMS”); (iv) state governments under Medicaid and other programs; (v) other third-party payors and managed care organizations (e.g., risk bearing organizations and independent practice associations (“IPAs”)); and (vi) individual patients and clients.
Revenue primarily consists of capitation revenue, fee-for-service (“FFS”) revenue, dispensary revenue, and clinical trials revenue. Capitation and FFS revenue comprise the revenues within the Company’s patient services segment and are presented together in the results of operations. The following paragraphs provide a summary of the principal forms of our billing arrangements and how revenue is recognized for each type of revenue.
Capitation
Capitation revenues consist primarily of fees for medical services provided by the TOI PCs to the Company's patients under a capitated arrangement with various managed care organizations. Capitation revenue is paid monthly based on the number of enrollees by the contracted managed care organization (per member per month or “PMPM”). Capitation contracts generally have a legal term of one year or longer. Payments in capitation contracts are variable since they primarily include PMPM fees associated with unspecified membership that fluctuates throughout the term of the contract; however, based on our experience, our total underlying membership generally increases over time as penetration of Medicare Advantage products grows. Certain contracts include terms for a capitation deduction where the cost of out-of-network referrals of members are deducted from the future payment. Revenue is recognized in the month services are rendered on the basis of the transaction price established at that time.
Fee-for-service revenue
FFS revenue represents revenue earned under contracts in which we bill and collect for specific medical services rendered by the TOI PCs’ employed physicians. The terms for FFS contracts are short in duration and only last for the period over which services are rendered (typically, one day). FFS revenue consists of fees for medical services provided to patients. As specialist providers, our FFS revenue is dependent on referrals from other physicians, such as primary care physicians. The Company's affiliated providers build trusted, professional relationships with these physicians and their associated medical groups, which can lead to recurring FFS volume; however, this volume is subject to numerous factors the Company cannot control and can fluctuate over time. The Company also receives FFS revenue for capitated patients that receive medical services which are excluded from the Company's capitation contracts. Under the FFS arrangements, third-party payors and patients are billed for patient care services provided by the TOI PCs. Payments for services provided are generally less than billed charges. The Company records revenue net of an allowance for contractual adjustments, which represents the net revenue expected to be collected from third-party payors (including managed care, commercial, and governmental payors such as Medicare and Medicaid), and patients. These expected collections are based on fees and negotiated payment rates in the case of third-party payors, the specific benefits provided for under each patient’s healthcare plan, mandated payment rates in the case of Medicare and Medicaid programs, and historical cash collections (net of recoveries). The recognition of net revenue (gross charges less contractual allowances) from such services is dependent on certain factors, such as the proper completion of medical charts following a patient visit, the forwarding of such charts to our billing center for medical coding and entering into the Company's billing system, and the verification of each patient’s submission or representation at the time services are rendered as to the payor(s) responsible for payment of such services. Revenue is recorded on the date the services are rendered based on the information known at the time of entering of such information into the Company's billing systems as well as an estimate of the revenue associated with medical services.
Dispensary and pharmacy
Oral prescription drugs prescribed by doctors to their patients are sold directly through the TOI PCs’ dispensaries and our
newly-acquired pharmacy. Revenue for the prescriptions is based on fee schedules set by various PBMs and other third-party payors. The fee schedule is often subject to direct and indirect remuneration (“DIR”) fees, which are based primarily on pre-established metrics. DIR fees may be assessed in the periods after payments are received against future payments. The Company recognizes revenue, deducted by estimated DIR fees, at the time the patient takes possession of the oral drug.
Clinical trials & other revenue
The TOI PCs also enter into contracts to perform clinical research trials. The terms for clinical trial contracts last many months as the clinical research is performed. Each contract represents a single, integrated set of research activities that are satisfied over time as the output of results from the trial is captured for the trial sponsor to review. Under the clinical trial contracts, the TOI PCs receive a fixed payment for administrative, set-up, and close-down fees; a fixed amount for each patient site visit; and certain expense reimbursements. The Company recognizes revenue for these arrangements on the fees earned to date based on the state of the trial, as established under contract with the customer.
Operating Expenses
Direct costs - patient services
Direct costs - patient services primarily includes chemotherapy drug costs, clinician salaries and benefits, and medical supplies. Clinicians include oncologists, advanced practice providers such as physician assistants and nurse practitioners, and registered nurses employed by the TOI PCs.
Direct costs - dispensary
Direct costs - dispensary primarily includes the cost of oral medications dispensed in the TOI PCs’ clinic locations.
Direct costs - clinical trials & other
Direct costs - clinical trials & other primarily includes costs related to clinical trial contracts and medical supplies.
Selling, general and administrative expense
Selling, general and administrative expenses include employee-related expenses, including both clinic and field support staff as well as central administrative and corporate staff. These expenses include salaries and related costs and stock-based compensation for our executives and physicians. The Company's selling, general and administrative expenses also includes occupancy costs, technology infrastructure, operations, clinical and quality support, finance, legal, human resources, and business development. Following the consummation of the Business Combination, general and administrative expenses have increased, and the Company expects continued increases over time, due to the additional legal, accounting, insurance, investor relations and other costs that the Company incurs as a public company, as well as other costs associated with continuing to grow the business. While the Company expects its selling, general and administrative expenses to increase in absolute dollars in the foreseeable future. such expenses are on average expected to decrease as a percentage of revenue over the long term.
Results of Operations
The following table sets forth our Consolidated Statements of Operations data expressed as a percentage of total revenues for the periods indicated. The Company’s management is not aware of material events or uncertainties that would cause the financial information below to not be indicative of future operating results or results of future financial condition, although past results should not be relied upon as an indication of future performance or future financial condition.
| | | | | | | | | | | | | | |
| | Year Ended December 31, |
| | 2023 | | 2022 |
| Revenue | | | | |
| Patient services | | 65.9 | % | | 66.1 | % |
| Dispensary | | 32.0 | % | | 31.4 | % |
| Clinical trials & other | | 2.1 | % | | 2.5 | % |
| Total operating revenue | | 100.0 | % | | 100.0 | % |
| Operating expenses | | | | |
| Direct costs – patient services | | 55.8 | % | | 53.4 | % |
| Direct costs – dispensary | | 25.6 | % | | 25.8 | % |
| Direct costs – clinical trials & other | | 0.2 | % | | 0.2 | % |
| Goodwill impairment charges | | 5.2 | % | | 3.9 | % |
| | | | |
| Selling, general and administrative expense | | 35.1 | % | | 47.4 | % |
| Depreciation and amortization | | 1.8 | % | | 1.7 | % |
| Total operating expenses | | 123.7 | % | | 132.4 | % |
| Loss from operations | | (23.7) | % | | (32.4) | % |
| Other non-operating expense (income) | | | | |
| Interest expense, net | | 2.1 | % | | 1.6 | % |
| Change in fair value of derivative warrant liabilities | | 0.1 | % | | (0.7) | % |
| Change in fair value of earnout liabilities | | (0.2) | % | | (23.5) | % |
| Change in fair value of conversion option derivative liabilities | | (0.3) | % | | (9.6) | % |
| Gain on loan forgiveness | | — | % | | (0.1) | % |
| Other, net | | 0.3 | % | | (0.1) | % |
| Total other non-operating loss expense (income) | | 2.0 | % | | (32.4) | % |
| Loss before provision for income taxes | | (25.7) | % | | — | % |
| Income tax benefit (expense) | | — | % | | 0.1 | % |
| Net income (loss) | | (25.7) | % | | 0.1 | % |
Comparison of the Years Ended December 31, 2023 and 2022
Revenue
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, | | Change |
| (dollars in thousands) | | 2023 | | 2022 | | $ | | % |
| Patient services | | $ | 213,504 | | | $ | 166,785 | | | $ | 46,719 | | | 28.0 | % |
| Dispensary | | 103,835 | | | 79,343 | | | 24,492 | | | 30.9 | % |
| Clinical trials & other | | 6,900 | | | 6,355 | | | 545 | | | 8.6 | % |
| Total operating revenue | | $ | 324,239 | | | $ | 252,483 | | | $ | 71,756 | | | 28.4 | % |
Patient services
The increase in patient services revenue was primarily due to a 22.7% increase in FFS revenue as a result of practice acquisitions and an overall increase in clinic count as well as a 4.5% increase in capitation revenue due to new capitation contracts entered into during 2023 and in the latter half of 2022.
Dispensary
The increase in dispensary revenue was primarily due to a 31.5% increase in the number of fills offset by 0.5% decrease in the average revenue per fill.
Clinical trials & other
For the year ended December 31, 2023, the increase in clinical trials and other revenue was primarily due to an increase in other revenue compared to the prior year.
Operating Expenses
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, | | Change |
| (dollars in thousands) | | 2023 | | 2022 | | $ | | % |
| Direct costs – patient services | | $181,017 | | $ | 134,761 | | | $ | 46,256 | | | 34.3 | % |
| Direct costs – dispensary | | 83,071 | | 65,111 | | | 17,960 | | | 27.6 | % |
| Direct costs – clinical trials & other | | 578 | | 518 | | | 60 | | | 11.6 | % |
| Goodwill impairment charges | | 16,867 | | 9,944 | | | 6,923 | | | N/A |
| | | | | | | | |
| Selling, general and administrative expense | | 113,851 | | 119,689 | | | (5,838) | | | (4.9) | % |
| Depreciation and amortization | | 5,873 | | 4,411 | | | 1,462 | | | 33.1 | % |
| Total operating expenses | | $401,257 | | $ | 334,434 | | | $ | 66,823 | | | 20.0 | % |
Patient services cost
The increase in patient services cost was primarily due to a 26.1% increase in intravenous drug costs, primarily driven by the Company's patient mix and volume, as well as the rising rate of inflation during 2023. In addition, clinical payroll costs increased 8.1% from the prior year due to the growth in clinic count.
Dispensary cost
The increase in dispensary cost was primarily due to a 31.5% increase in the number of prescriptions filled offset by a 3.0% decrease in the average cost of the prescriptions filled.
Goodwill impairment charges
During the years ended December 31, 2023 and 2022, impairment charges of $16,867 and $9,944 were recorded related to goodwill, respectively. See Note 2 and Note 18 in Item. 8 Financial Statements and Supplementary Data for additional detail.
Selling, general and administrative expense
The decrease in selling, general and administrative expense was primarily driven by a 8.5% decrease in share-based compensation expense, a 2.6% decrease in transaction costs and a 1.7% decrease in professional fees offset by 1.9% increase in salaries and benefits due to the growth in the Company's management and corporate team, as well as a 3.1% increase in office expenses, and a 1.4% increase in real estate and equipment expenses.
Other Non-Operating Expenses (Income)
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, | | Change |
| (dollars in thousands) | | 2023 | | 2022 | | $ | | % |
| Interest expense, net | | $ | 6,777 | | | $ | 4,082 | | | $ | 2,695 | | | 66.0 | % |
| Change in fair value of derivative warrant liabilities | | 286 | | | (1,843) | | | 2,129 | | | (115.5) | % |
| Change in fair value of earnout liabilities | | (803) | | | (59,215) | | | 58,412 | | | (98.6) | % |
| Change in fair value of conversion option derivative liabilities | | (878) | | | (24,200) | | | 23,322 | | | N/A |
| Gain on loan forgiveness | | — | | | (183) | | | 183 | | | (100.0) | % |
| Other, net | | 704 | | | (501) | | | 1,205 | | | (240.5) | % |
| Total other non-operating expense (income) | | $ | 6,086 | | | $ | (81,860) | | | $ | 87,946 | | | (107.4) | % |
Interest expense
The increase in interest expense was primarily the result of interest and amortization related to the Senior Secured Convertible Notes issued during the year ended December 31, 2023.
Change in fair value of liabilities
The decrease in non-operating (income) expense was primarily due to the decrease in gains of $58,412 and $23,322, respectively during the year ended December 31, 2023, as a result of decreases in the fair value of earnout liabilities and conversion option derivative liabilities, which were created as part of the Business Combination and the issuance of the Senior Secured Convertible Note, respectively.
Gain on loan forgiveness
There was no gain on loan forgiveness during the year ended December 31, 2023. During the year ended December 31, 2022, gain on loan forgiveness of $183 was a result of forgiveness of all CARES Act loans, including those obtained through physician practice acquisition.
Other, net
The change in other, net was primarily due to Provider Relief Funding received under the CARES Act during the year ended December 31, 2022 that did not occur in 2023.
Key Business Metrics
In addition to our financial information, the Company's management reviews a number of operating and financial metrics, including the following key metrics, to evaluate our business, measure our performance, identify trends affecting our business, formulate business plans, and make strategic decisions.
| | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, | | |
| | 2023 | | 2022 | | | | |
Clinics (1) | | 83 | | | 76 | | | | | |
| Markets | | 15 | | | 15 | | | | | |
| Lives under value-based contracts (millions) | | 1.8 | | | 1.7 | | | | | |
| Net income (loss) | | $ | (83,068) | | | $ | 152 | | | | | |
Adjusted EBITDA (in thousands) (2) | | $ | (25,805) | | | $ | (23,543) | | | | | |
(1) Includes independent oncology practices to which we provide limited management services, but do not bear the operating costs.
(2) Adjusted EBITDA is a "non-GAAP" financial measure within the meaning of Item 10 of Regulation S-K promulgated by the SEC. The Company defines Adjusted EBITDA as net income (loss) adjusting for:
•Depreciation and amortization,
•Interest expense, net,
•Income tax expense,
•Non-cash addbacks,
•Share-based compensation,
•Goodwill impairment charges,
•Changes in fair value of liabilities,
•Unrealized (gains) losses on investments
•Practice acquisition-related costs,
•Post combination compensation expense,
•Consulting and legal fees,
•Infrastructure and workforce costs, and
•Transaction costs.
The Company includes Adjusted EBITDA because it is an important measure which our management uses to assess the results of operations, to evaluate factors and trends affecting the business, and to plan and forecast future periods.
Management believes that this measure provides an additional way of viewing aspects of the Company's operations that, when viewed with the GAAP results, provides a more complete understanding of the Company's results of operations and the factors and trends affecting the business. However, non-GAAP financial measures should be considered a supplement to, and not as a substitute for, or superior to, the corresponding measures calculated in accordance with U.S. GAAP. Non-GAAP financial measures used by management may differ from the non-GAAP measures used by other companies, or smaller reporting companies, this could make our securities less attractive toincluding the Company's competitors. Management encourages investors and others to review the Company's financial information in its entirety, not to rely on any single financial measure.
The following tables provide a reconciliation of net income (loss), the most closely comparable GAAP financial measure, to Adjusted EBITDA:
| | | | | | | | | | | | | | | | | | | | | | | |
| Year Ended December 31, | | Change |
| (dollars in thousands) | 2023 | | 2022 | | $ | | % |
| Net income (loss) | $ | (83,068) | | | $ | 152 | | | $ | (83,220) | | | (54,750.0) | % |
| Depreciation and amortization | 5,873 | | | 4,411 | | | 1,462 | | | 33.1 | % |
| Interest expense, net | 6,777 | | | 4,082 | | | 2,695 | | | 66.0 | % |
| Income tax benefit | (36) | | | (243) | | | 207 | | | (85.2) | % |
Non-cash addbacks(1) | 2,029 | | | 1,208 | | | 821 | | | 68.0 | % |
| Share-based compensation | 17,548 | | | 27,683 | | | (10,135) | | | (36.6) | % |
| Goodwill impairment charges | 16,867 | | | 9,944 | | | 6,923 | | | N/A |
| Change in fair value of liabilities | (1,395) | | | (85,258) | | | 83,863 | | | (98.4) | % |
| Unrealized (gains) losses on investments | (237) | | | (640) | | | 403 | | | N/A |
Practice acquisition-related costs(2) | 113 | | | 790 | | | (677) | | | (85.7) | % |
Post-combination compensation expense(3) | 2,048 | | | 2,243 | | | (195) | | | N/A |
Consulting and legal fees(4) | 1,570 | | | 3,797 | | | (2,227) | | | (58.7) | % |
Infrastructure and workforce costs(5) | 5,965 | | | 5,029 | | | 936 | | | 18.6 | % |
Transaction costs(6) | 141 | | | 3,259 | | | (3,118) | | | (95.7) | % |
| Adjusted EBITDA | $ | (25,805) | | | $ | (23,543) | | | $ | (2,262) | | | 9.6 | % |
(1) During the year ended December 31, 2023, non-cash addbacks were primarily comprised of net bad debt write-offs of $2,020. During the year ended December 31, 2022, non-cash addbacks were primarily comprised of a $476 of net bad debt write-off, deferred rent of $711, and other miscellaneous charges of $22.
(2) Practice acquisition-related costs were comprised of consulting and legal fees incurred to perform due diligence, execute, and integrate acquisitions of various oncology practices.
(3) Deferred consideration payments for practice acquisitions that are contingent upon the seller’s future employment at the Company.
(4) Consulting and legal fees were comprised of a subset of the Company’s total consulting and legal fees during the years ended December 31, 2023 and 2022, and related to certain advisory projects, software implementations, and legal fees for debt financing and predecessor litigation matters.
(5) Infrastructure and workforce costs were comprised of recruiting expenses to build out corporate infrastructure of $2,227 and $2,835, software implementation fees of $105 and $116, severance expenses resulting from cost rationalization programs of $979 and $248, temporary labor of $1,365 and $1,829, and lease terminations, settlements, and penalty addbacks of $1,289 and $0 during the years ended December 31, 2023 and 2022, respectively.
(6) Transaction costs were comprised of consulting, legal, administrative and regulatory fees associated with share repurchases and practice acquisitions during the year ended December 31, 2023, and related to the Senior Secured Convertible Note during the year ended December 31, 2022.
Liquidity and Capital Resources
General
To date, the Company has financed its operations principally through debt facilities, issuances of equity securities and payments received from various payors. As of December 31, 2023, the Company had $33,488 of cash and cash equivalents, none of which are restricted cash, as well as $49,367 of current marketable securities.
The Company expects to incur operating losses and generate negative cash flows from operations for the foreseeable future due to the investments; management intends to continue to make in expanding operations and sales and marketing and due to additional general and administrative expenses management expects to incur in connection with operating as a public company. As a result, the Company may makerequire additional capital resources to execute strategic initiatives to grow the business.
Management believes that the cash on hand and investments in marketable securities will be sufficient to fund the Company's operating and capital needs for at least the next 12 months. Management's assessment of the period of time through which our financial resources will be adequate to support our operations is a forward-looking statement and involves risks and uncertainties. The Company's actual results could vary because of, and its future capital requirements will depend on, many factors, including our growth rate, the timing and extent of spending to open or acquire new clinics and expand into new markets and the expansion of sales and marketing activities. The Company may in the future enter into arrangements to acquire or invest in complementary businesses, services and technologies, including intellectual property rights. The Company has based this estimate on assumptions that may prove to be wrong, and the Company could use its available capital resources sooner than management currently expects. The Company may be required to seek additional equity or debt financing. In the event that additional financing is required from outside sources, the Company may not be able to raise it more difficulton terms acceptable to compare our performance withmanagement or at all. If unable to raise additional capital when desired, or if the Company cannot expand operations or otherwise capitalize on business opportunities because the Company's lack of sufficient capital, the Company's business, results of operations, and financial condition would be adversely affected.
Cash Flows
The following table presents a summary of the Company's consolidated cash flows from operating, investing, and financing activities for the periods indicated.
| | | | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, | | Change |
| (dollars in thousands) | 2023 | | 2022 | | $ | | % |
| Net cash and cash equivalents used in operating activities | $ | (36,315) | | | $ | (61,756) | | | $ | 25,441 | | | (41.2) | % |
| Net cash and cash equivalents provided by (used in) investing activities | 62,640 | | | (131,614) | | | 194,254 | | | (147.6) | % |
| Net cash and cash equivalents provided by (used in) financing activities | (6,847) | | | 92,206 | | | (99,053) | | | (107.4) | % |
| Net (decrease) increase in cash and cash equivalents | $ | 19,478 | | | $ | (101,164) | | | $ | 120,642 | | | (119.3) | % |
| Cash and cash equivalents at beginning of period | 14,010 | | | 115,174 | | | (101,164) | | | (87.8) | % |
| Cash and cash equivalents at end of period | $ | 33,488 | | | $ | 14,010 | | | $ | 19,478 | | | 139.0 | % |
Operating Activities
Significant changes impacting net cash and cash equivalents used in operating activities for the year ended December 31, 2023 as compared to the year ended December 31, 2022 were as follows:
•Net loss increased to $83,220, primarily as a result of a decrease in the fair value of liabilities of $1,395 for the year ended December 31, 2023 as compared to a decrease in fair value of liabilities of $85,258 during the year ended December 31, 2022;
•Share based compensation for the year ended December 31, 2023 decreased by $9,873 as compared to the year ended December 31, 2022;
•Cash used by accounts receivable decreased $15,721 for the year ended December 31, 2023 as compared to the year ended December 31, 2022 due to the collection stabilization of the Company's receivables;
•Cash provided by accounts payable, accrued expenses and income taxes payable increased $11,874 for the year ended December 31, 2023 as compared to the year ended December 31, 2022 primarily due to an increase in vendor payables resulting from the growth in the Company's business;
•Cash used by purchasing inventory increased $2,653 for the year ended December 31, 2023 as compared to the year ended December 31, 2022 as a result of an increase in inventory acquired through practice acquisition; and
•Cash provided by prepaid and other public companies.current assets decreased $1,154 for the year ended December 31, 2023 as compared to the year ended December 31, 2022 primarily due to the financing of the Company's directors and officers insurance policy that occurred in 2022.We are
Investing Activities
Net cash used by investing activities decreased $194,254 for the year ended December 31, 2023 as compared to the year ended December 31, 2022 due to $107,913 reduction in purchases of marketable securities and a decrease in cash used for purchases of property and equipment of $962 for new clinic builds and clinic remodels, and a decrease in cash used for purchases of practice acquisitions and intangibles of $4,121.
Financing Activities
Net cash provided by financing activities decreased $99,053 for the year ended December 31, 2023 as compared to the year ended December 31, 2022 primarily due to $110,000 of proceeds from the issuance of the Senior Secured Convertible during the year ended December 31, 2022, offset by $7,981 decrease in common stock repurchase, and by deferred cash consideration and finance insurance premium payments of $2,584 and $3,269, respectively, during year ended December 31, 2023.
Material Cash Requirements
The Company's material cash requirements for the following five years consist of principal and interest due on the convertible note, operating leases and other miscellaneous administrative expenses. Additionally, the Company is subject to certain outside claims and litigation arising out of the ordinary course of business, however, no such litigation requires future cash expenditure as of December 31, 2023.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Material Cash Requirements Due by the Year Ended December 31, |
| (dollars in thousands) | | 2024 | | 2025-2026 | | 2027-2028 | | Thereafter | | Total |
Convertible note1 | | $ | 4,461 | | | $ | 8,922 | | | $ | 112,664 | | | $ | — | | | $ | 126,047 | |
| Operating leases | | 8,176 | | | 15,128 | | | 9,825 | | | 6,243 | | | 39,372 | |
| Deferred acquisition and contingent consideration | | 2,515 | | | 50 | | | — | | | — | | | 2,565 | |
Other2 | | 1,049 | | | 81 | | | 29 | | | — | | | 1,159 | |
| Total material cash requirements | | $ | 16,201 | | | $ | 24,181 | | | $ | 122,518 | | | $ | 6,243 | | | $ | 169,143 | |
| | | | | | | | | | |
(1) Includes principal and interest payments due.
(2) Other is comprised of finance leases and D&O financing
JOBS Act
The Company qualifies as an “emerging growth company” within the meaningcompany,” as defined in Section 2(a) of the Securities Act, as modified by the JOBSJumpstart Our Business Startups Act of 2012 (the “JOBS Act”), and we mayhas elected to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies. Section 102(b)(1) of the JOBS Act exempts emerging growth companies including, but not limited to, notfrom being required to comply with new or revised financial accounting standards until private companies (that is, those that have not had a Securities Act registration statement declared effective or do not have a class of securities registered under the auditor internal controls attestation requirements of Section 404Exchange Act) are required to comply with the new or revised financial accounting standards. The JOBS Act provides that a company can elect to opt out of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reportsextended transition period and proxy statements, and exemptions fromcomply with the requirements that apply to non-emerging growth companies, but any such election to opt out is irrevocable. The Company has elected not to opt out of holdingsuch extended transition period, which means that when a nonbinding advisory vote on executive compensationstandard is issued or revised and stockholder approval of any golden parachute payments not previously approved. As a result, our stockholders may not have access to certain information they may deem important. We could beit has different application dates for public or private companies, the Company, as an emerging growth company, for up to five years, although circumstances could cause us to lose that status earlier, including ifcan adopt the market valuenew or revised standard at the time private companies adopt the new or revised standard. This may make comparison of our Class A common stock held by non-affiliates exceeds $700 million as of any June 30 beforefinancial statements with another public company that time, in which case we would no longer beis neither an emerging growth company nor an emerging growth company which has opted out of using the extended transition period difficult or impossible because of the potential differences in accounting standards used.
Critical Accounting Policies
The Company prepares its financial statements in accordance with generally accepted accounting principles in the United States ("U.S. GAAP,"), which requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates under different assumptions or conditions.
Variable Interest Entities
The Company consolidates entities for which it has a variable interest and is determined to be the primary beneficiary. The Company holds variable interests in the TOI PCs, comprised of The Oncology Institute CA, a Professional Corporation (“TOI CA”) and The Oncology Institute FL, LLC (“TOI FL”) and The Oncology Institute TX, a Professional Association ("TOI TX"), all of which the Company cannot legally own due to jurisdictional laws governing the corporate practice of medicine. The TOI PCs employ physicians and other clinicians in order to provide professional services to patients of our managed clinics, and under substantially similar management services agreements, or MSAs, we serve as the exclusive manager and administrator of the TOI PCs’ non-medical functions and services. The TOI PCs are considered variable interest entities (“VIEs”) as they do not have sufficient equity to finance their activities without additional financial support from the Company. An enterprise having a controlling financial interest in a VIE must consolidate the VIE if it has both power and benefits — that is, it has (1) the power to direct the activities of a VIE that most significantly impacts the VIE’s economic performance (power), and (2) the obligation to absorb the losses of the VIE that potentially could be significant to the VIE or the right to receive benefits from the VIE that potentially could be significant to the VIE (benefits). The Company has the power to control all financial activities of the TOI PCs, the rights to receive substantially all benefits from the VIEs, and consequently consolidates the TOI PCs. Revenues,
expenses, and income from the TOI PCs are included in the consolidated amounts as presented on the Consolidated Statements of Operations.
Segment Reporting
The Company presents the financial statements by segment in accordance with the relevant accounting literature to provide investors with transparency into how the chief operating decision maker (“CODM”) manages the business. The Company's CODM is our Chief Executive Officer. The CODM reviews financial information and allocates resources across three operating segments: dispensary, patient services, and clinical trials & other.
Revenue Recognition
The Company recognizes consolidated revenue based upon the principle of the transfer of control of our goods and services to customers in an amount that reflects the consideration it expects to be entitled. This principle is achieved through applying the following five-step approach:
1.Identification of the contract, or contracts, with a customer.
2.Identification of the performance obligations in the contract.
3.Determination of the transaction price.
4.Allocation of the transaction price to the performance obligations in the contract.
5.Recognition of revenue when, or as, the entity satisfies a performance obligation.
Consolidated revenue primarily consists of capitation revenue, fee-for-service (FFS) revenue, dispensary revenue, and clinical trials revenue. Revenue is recognized in the period in which services are rendered or the period in which the TOI PCs are obligated to provide services. The form of billing and related risk of collection for such services may vary by type of revenue and the payor. The following paragraphs provide a summary of the principal forms of billing arrangements and how revenue is recognized for each.
Capitation
Capitation contracts have a single performance obligation that is a stand ready obligation to perform specified healthcare services to the population of enrolled members and constitutes a series for the provision of managed healthcare services for the term of the contract, which is deemed to be one month since the mix of patient-customers can and do change month over month. The transaction price for capitation contracts is variable as it primarily includes PMPM fees associated with unspecified membership that fluctuates throughout the term of the contract. Further, we adjust the transaction price for capitation deductions based on historical experience. Revenue is recognized in the month services are rendered on the basis of the transaction price established at that time. If subsequent information resolves uncertainties related to the transaction price, adjustments will be recognized in the period they are resolved. When payment has been received but services have not yet been rendered, the payment is recognized as a contract liability.
Fee For Service
FFS revenue consists of fees for medical services actually provided to patients. These medical services are distinct since the patient can benefit from the medical services on their own. Each service constitutes a single performance obligation for which the patient accepts and receives the benefit of the medical services as they are performed.
The transaction price from FFS arrangements is variable in nature because fees are based on patient encounters, credits due to patients, and reimbursement of provider costs, all of which can vary from period to period. The Company estimates the transaction price using the most likely methodology and amounts are only included in the net transaction price to the extent that it is probable that a significant reversal of cumulative revenue will not occur once any uncertainty is resolved. As a practical expedient, the Company adopted a portfolio approach to determine the transaction price for the medical services provided under FFS arrangements. Under this approach, the Company bifurcated the types of services provided and grouped health plans with similar fees and negotiated payment rates.
At these levels, portfolios share the characteristics conducive to ensuring that the results do not materially differ from the standard applied to individual patient contracts related to each medical service provided.
Revenue is recorded on the date the services are rendered based on the information known at the time of entering of such information into our billing systems as well as an estimate of the revenue associated with medical services. When the performance obligation is not satisfied, the billing is recognized as a contract liability.
Dispensary
Dispensed prescriptions that are filled and delivered to the patient are considered a distinct performance obligation. The transaction price for the prescriptions is based on fee schedules set by PBMs and other third-party payors. The fee schedule is often subject to DIR fees, which are based primarily on pre-established metrics. DIR fees may be assessed in periods after payments are received against future payments. The Company estimates DIR fees to arrive at the transaction price for prescriptions. Revenue is recognized based on the transaction at the time the patient takes possession of the oral drug.
Clinical Research & Other
Clinical research contracts represent a single, integrated set of research activities and thus are a single performance obligation. The performance obligation is satisfied over time as the output is captured in data and documentation that is available for the customer to consume over the course of arrangement and furthers progress of the clinical trial. The Company has elected to recognize revenue for clinical trials using the ‘as-invoiced’ practical expedient. The customer is invoiced periodically based on the progress of the trial such that each invoice captures the revenue earned to date based on the state of the trial as established under contract with the customer.
Leases
On January 1, 2022, the Company adopted ASU 2016-02, Leases, with various amendments issued in 2018 and 2019 (collectively, “ASC 842”) using the modified retrospective approach, for leases that existed on January 1, 2022. ASC 842 requires lessees to recognize assets and liabilities for most leases. The Company evaluates whether an arrangement is or contains a lease at contract inception. A lease exists when a contract conveys to the customer the right to control the use of an identified asset for a period of time in exchange for consideration. Upon lease commencement, the date on which a lessor makes the underlying asset available to the Company for use, the Company classifies the lease as either an operating or finance lease. The Company applied certain practical expedients permitted under the transition guidance, including the package of practical expedients, which permits the Company not to reassess its prior conclusions related to lease identification, lease classification, and initial direct costs capitalization. The Company solely acts as a lessee and its leases primarily consist of operating leases for its real estate in the states in which the Company operates. The Company has other operating or financing leases for various clinical and non-clinical equipment.
Generally, upon the commencement of a lease, the Company will record a right-of-use (“ROU”) asset and lease liability. An ROU asset represents the Company’s right to use an underlying asset for the lease term and lease liabilities represent the Company’s obligation to make lease payments arising from the lease. Lease liabilities are measured at the present value of the remaining, fixed lease payments at lease commencement. The Company uses its incremental borrowing rate, based on the information available at the later of adoption, inception, or modification in determining the present value of lease payments. ROU assets are measured at an amount equal to the initial lease liability, plus any prepaid lease payments (less any incentives received) and initial direct costs, at the lease commencement date. The Company has elected to account for lease and non-lease components as a single lease component for all underlying classes of assets. As a result, the fixed payments that would otherwise be allocable to the non-lease components are account for as lease payments and included in the measurement of the Company’s right-of-use asset and lease liability.
Lease arrangements with an initial term of 12 months or less are considered short-term leases and are not recorded on the balance sheet. The short-term lease payments are recognized as an expense on a straight-line basis over the lease term. The lease term includes any period covered by renewal options available that the Company is reasonably certain to exercise and any options to terminate the lease that the Company is not reasonably certain to exercise.
Direct Costs of Sales
Direct cost of sales primarily consists of wages paid to clinical personnel and other health professionals, oral and IV drug costs, and other medical supplies used to provide patient care. Costs for clinical personnel wages are expensed as incurred and costs for inventory and medical supplies are expensed when used, generally by applying the specific identification method.
Goodwill and Intangible Assets
The Company accounts for goodwill and intangible assets under Accounting Standards Codification Topic No. 350, Goodwill and Other (“ASC 350”). Goodwill represents the excess of the fair value of the consideration conveyed in acquisition over the fair value of net assets acquired.
Goodwill is not amortized but is required to be evaluated for impairment at the same time every year. The Company performs annual testing of impairment for goodwill in the fourth quarter of each year or earlier if potential impairment indicators exist. When impairment indicators are identified, the Company compares the reporting unit’s fair value to its carrying amount, including goodwill. An impairment loss is recognized as the difference, if any, between the reporting unit’s carrying amount and its fair value to the extent the difference does not exceed the total amount of goodwill allocated to the reporting unit.
Under ASC 350, finite-lived intangible assets are stated at acquisition-date fair value. Intangible assets are amortized using the straight-line method.
Finite-lived intangible assets are stated at acquisition-date fair value. Intangible assets are amortized using the straight-line method. Finite-lived intangible assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. When circumstances indicate that recoverability may be impaired, the Company assesses its ability to recover the carrying value of the asset group from the expected future pre-tax cash flows (undiscounted and without interest charges) of the related operations. If these cash flows are less than the carrying value of such asset, an impairment loss is recognized for the difference between estimated fair value and carrying value. Fair value is determined based on appropriate valuation techniques.
Recent Accounting Pronouncements
For information regarding recent accounting pronouncements, refer to Note 2 of our consolidated financial statements included in this Annual Report on Form 10-K.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
Market risk represents the risk of loss that may impact our financial position due to adverse changes in financial market prices and rates. Our market risk exposure is primarily a result of exposure due to potential changes in inflation or interest rates. We do not hold financial instruments for trading purposes.
Interest Rate Risk
We held cash and cash equivalents of $33,488, current marketable securities of $49,367 as of December 31, 2023, consisting of bank deposits and Treasury bills. Such interest-earning instruments carry a degree of interest rate risk. The goals of our investment policy are liquidity and capital preservation. We believe that we do not have any material exposure to changes in the fair value of these assets as a results of changes in interest rates due to the short-term nature of our cash and cash equivalents.
Inflation Risk
Recently, inflation has increased throughout the U.S. economy. Inflation can adversely affect us by increasing the costs of drugs, clinical trials and research, administration and other costs of doing business. We may experience increases in the prices of labor and other costs of doing business. In an inflationary environment, cost increases may outpace our expectations, causing us to use our cash and other liquid assets faster than forecasted. If this happens, we may need to raise additional capital to fund our operations, which may not be available in sufficient amounts or on reasonable terms, if at all, sooner than expected.
Impairment Risk
Impairment risk refers to the risk that the Company will write down a material amount of its goodwill or intangible assets. This risk is assessed at least annually in the fourth quarter each year when the Company performs its impairment testing. To the extent that, among other factors, (i) there is underperformance in one or more reporting units (ii) a potential recession further disrupts the economic environment or (iii) interest rates continue to rise in response to persistent inflation, the fair value of one or more of the reporting units could fall below their carrying value, resulting in a goodwill or intangible impairment charge.
For the year ended December 31, 2023, the Company recognized an impairment charge of $16,867 to write-down the carrying value of goodwill related to patient services in excess of the fair value.
Item 8. Financial Statements and Supplementary Data
Table of Contents
| | | | | |
| Page
Number(s) |
| 61 |
| 62 |
| 63 |
| 65 |
| |
| 66 |
| 67 |
| 69 |
Report of Independent Registered Public Accounting Firm
Shareholders and Board of Directors
The Oncology Institute, Inc.
Cerritos, California
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of The Oncology Institute, Inc. (the “Company”) as of December 31, 2023 and 2022, the related consolidated statements of operations, convertible preferred stock and changes in stockholders’ equity, and cash flows for each of the years then ended, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2023 and 2022, and the results of its operations and its cash flows for the years then ended, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ BDO USA, P.C.
We have served as the Company’s auditor since 2019.
Costa Mesa, California
March 28, 2024
| | | | | | | | | | | |
THE ONCOLOGY INSTITUTE, INC. CONSOLIDATED BALANCE SHEETS (US Dollars in thousands, except share data) |
| December 31, 2023 | | December 31, 2022 |
| Assets | | | |
| Current assets: | | | |
| Cash and cash equivalents | $ | 33,488 | | | $ | 14,010 | |
| Marketable securities | 49,367 | | | 59,796 | |
| Accounts receivable, net | 42,360 | | | 39,816 | |
| Other receivables | 551 | | | 617 | |
| Inventories | 13,678 | | | 9,261 | |
| Prepaid expenses and other current assets | 4,049 | | | 6,918 | |
| Total current assets | 143,493 | | | 130,418 | |
| Non-current investments | — | | | 58,354 | |
| Property and equipment, net | 10,883 | | | 8,547 | |
| Operating right of use assets | 29,169 | | | 24,494 | |
| Intangible assets, net | 17,904 | | | 17,957 | |
| Goodwill | 7,230 | | | 21,418 | |
| Other assets | 561 | | | 477 | |
| Total assets | $ | 209,240 | | | $ | 261,665 | |
| Liabilities and stockholders’ equity | | | |
| Current liabilities: | | | |
| Accounts payable | $ | 14,429 | | | $ | 9,372 | |
| Current portion of operating lease liabilities | 6,363 | | | 5,498 | |
| | | |
| Income taxes payable | — | | | 255 | |
| Accrued expenses and other current liabilities | 13,996 | | | 14,595 | |
| Total current liabilities | 34,788 | | | 29,720 | |
| Operating lease liabilities | 26,486 | | | 22,060 | |
| Derivative warrant liabilities | 636 | | | 350 | |
| Derivative earnout liabilities | — | | | 803 | |
| Conversion option derivative liabilities | 3,082 | | | 3,960 | |
| Long-term debt, net of unamortized debt issuance costs | 86,826 | | | 80,621 | |
| Other non-current liabilities | 365 | | | 868 | |
| Deferred income taxes liability | 32 | | | 108 | |
| Total liabilities | 152,215 | | | 138,490 | |
| Commitments and contingencies (Note 15) | — | | | — | |
| Stockholders’ equity: | | | |
Common Stock, 0.0001 par value, authorized 500,000,000 shares; 75,879,025 shares issued and 74,145,251 shares outstanding at December 31, 2023 and 73,265,621 shares issued and outstanding at December 31, 2022 | 8 | | | 7 | |
| Series A Convertible Preferred Stock, 0.0001 par value, authorized 10,000,000 shares; 165,045 shares issued and outstanding at December 31, 2023 and December 31, 2022 | — | | | — | |
| Additional paid-in capital | 204,186 | | | 186,250 | |
| Treasury Stock at cost, 1,733,774 and 0 shares at December 31, 2023 and December 31, 2022 | (1,019) | | | — | |
| Accumulated deficit | (146,150) | | | (63,082) | |
| Total stockholders’ equity | 57,025 | | | 123,175 | |
| Total liabilities and stockholders’ equity | $ | 209,240 | | | $ | 261,665 | |
Note: The Company’s consolidated balance sheets include the assets and liabilities of its consolidated variable interest entities (“VIEs”). The consolidated balance sheets include total assets that can be used only to settle obligations of the Company’s consolidated VIEs totaling $71,305 and $70,994 as of December 31, 2023 and December 31, 2022, respectively, and total liabilities of the Company’s consolidated VIEs for which creditors do not have recourse to the general credit of the Company totaling $210,422 and $154,572 as of December 31, 2023 and December 31, 2022, respectively. See Note 17 for further details.
See accompanying notes to the consolidated financial statements.
THE ONCOLOGY INSTITUTE, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(US Dollars in thousands, except share and per share data)
| | | | | | | | | | | | | |
| Year Ended December 31, |
| 2023 | | 2022 | | |
| Revenue | | | | | |
| Patient services | $ | 213,504 | | | $ | 166,785 | | | |
| Dispensary | 103,835 | | | 79,343 | | | |
| Clinical trials & other | 6,900 | | | 6,355 | | | |
| Total operating revenue | 324,239 | | | 252,483 | | | |
| Operating expenses | | | | | |
| Direct costs – patient services | 181,017 | | | 134,761 | | | |
| Direct costs – dispensary | 83,071 | | | 65,111 | | | |
| Direct costs – clinical trials & other | 578 | | | 518 | | | |
| Goodwill impairment charges | 16,867 | | | 9,944 | | | |
| Selling, general and administrative expense | 113,851 | | | 119,689 | | | |
| Depreciation and amortization | 5,873 | | | 4,411 | | | |
| Total operating expenses | 401,257 | | | 334,434 | | | |
| Loss from operations | (77,018) | | | (81,951) | | | |
| Other non-operating expense (income) | | | | | |
| Interest expense, net | 6,777 | | | 4,082 | | | |
| Change in fair value of derivative warrant liabilities | 286 | | | (1,843) | | | |
| Change in fair value of earnout liabilities | (803) | | | (59,215) | | | |
| Change in fair value of conversion option derivative liabilities | (878) | | | (24,200) | | | |
| Gain on loan forgiveness | — | | | (183) | | | |
| Other, net | 704 | | | (501) | | | |
| Total other non-operating loss expense (income) | 6,086 | | | (81,860) | | | |
| Loss before provision for income taxes | (83,104) | | | (91) | | | |
| Income tax benefit (expense) | 36 | | | 243 | | | |
| Net income (loss) | $ | (83,068) | | | $ | 152 | | | |
| Net income (loss) per share attributable to common stockholders: | | | | | |
| Net income (loss) attributable to common stockholders, basic | $ | (67,877) | | | $ | 68 | | | |
| Weighted-average number of shares outstanding, basic | 73,748,660 | | 72,793,497 | | |
| Net income (loss) per share attributable to common stockholders, basic | $ | (0.92) | | | $ | — | | | |
| | | | | |
| Net loss attributable to common stockholders, diluted | $ | (67,877) | | | $ | (16,980) | | | |
| Weighted-average number of shares outstanding, diluted | 73,748,660 | | 80,605,600 | | |
| Net loss per share attributable to common stockholders, diluted | $ | (0.92) | | | $ | (0.21) | | | |
See accompanying notes to the consolidated financial statements.
THE ONCOLOGY INSTITUTE, INC.
CONSOLIDATED STATEMENTS OF CONVERTIBLE PREFERRED STOCK AND CHANGES IN STOCKHOLDERS’ EQUITY
(US Dollars in thousands, except share data)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | Common Stock | | Series A Convertible Preferred Stock | | | | | | | | |
| | | | | | | | | Shares | | Amount | | Shares | | Amount | | Treasury stock | | Additional paid in capital | | Retained Earnings/ (Accumulated Deficit) | | Total Stockholders' Equity |
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| Balance at December, 31, 2021 | | | | | | | | | 73,249,042 | | | $ | 7 | | | 163,510 | | | $ | — | | | $ | — | | | $ | 167,386 | | | $ | (63,234) | | | $ | 104,159 | |
| Net income | | | | | | | | | — | | | — | | | — | | | — | | | — | | | — | | | 152 | | | 152 | |
| Issuance of common stock upon vesting of RSUs | | | | | | | | | 696,690 | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Issuance of common stock upon exercise of options | | | | | | | | | 973,389 | | | — | | | — | | | — | | | — | | | 858 | | | — | | | 858 | |
| Exchange of common stock for preferred stock | | | | | | | | | (153,500) | | | — | | | 1,535 | | | — | | | — | | | — | | | — | | | — | |
| Repurchase and retirement of common stock from related party | | | | | | | | | (1,500,000) | | | — | | | — | | | — | | | — | | | (9,000) | | | — | | | (9,000) | |
| Net settlement of taxes for equity awards | | | | | | | | | — | | | — | | | — | | | — | | | — | | | (413) | | | — | | | (413) | |
| Share-based compensation expense | | | | | | | | | — | | | — | | | — | | | — | | | — | | | 27,419 | | | — | | | 27,419 | |
| Balance at December, 31, 2022 | | | | | | | | | 73,265,621 | | | $ | 7 | | | 165,045 | | | $ | — | | | $ | — | | | $ | 186,250 | | | $ | (63,082) | | | $ | 123,175 | |
| Net loss | | | | | | | | | — | | | — | | | — | | | — | | | — | | | — | | | (83,068) | | | (83,068) | |
| Issuance of common stock upon vesting of RSUs | | | | | | | | | 2,475,089 | | | 1 | | | — | | | — | | | — | | | — | | | — | | | 1 | |
| Issuance of common stock upon exercise of options | | | | | | | | | 138,315 | | | — | | | — | | | — | | | — | | | 126 | | | — | | | 126 | |
| Share-based compensation expense | | | | | | | | | — | | | — | | | — | | | — | | | — | | | 17,810 | | | — | | | 17,810 | |
| Treasury stock purchase | | | | | | | | | — | | | — | | | — | | | — | | | (1,019) | | | — | | | — | | | (1,019) | |
| Balance at December, 31, 2023 | | | | | | | | | 75,879,025 | | | $ | 8 | | | 165,045 | | | $ | — | | | $ | (1,019) | | | $ | 204,186 | | | $ | (146,150) | | | $ | 57,025 | |
See accompanying notes to the consolidated financial statements.
| | | | | | | | | | | |
THE ONCOLOGY INSTITUTE, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(US Dollars in thousands) |
| Year Ended December 31, |
| 2023 | | 2022 |
| Cash flows from operating activities: | | | |
| Net income (loss) | $ | (83,068) | | | $ | 152 | |
| Adjustments to reconcile net income (loss) to cash and cash equivalents used in operating activities: |
| Depreciation and amortization | 5,873 | | | 4,411 | |
| Amortization of debt issuance costs and debt discount | 6,205 | | | 2,444 | |
| Goodwill impairment charges | 16,867 | | | 9,944 | |
| Share-based compensation | 17,810 | | | 27,683 | |
| Change in fair value of liability classified warrants | 286 | | | (1,843) | |
| Change in fair value of liability classified earnouts | (803) | | | (59,215) | |
| Change in fair value of liability classified conversion option derivatives | (878) | | | (24,200) | |
| | | |
| Unrealized (gain) loss on investments | (249) | | | 378 | |
| Accretion of discount on investment securities | (2,631) | | | (1,020) | |
| Deferred taxes | (76) | | | (263) | |
| Gain on loan forgiveness | — | | | (183) | |
| Bad debt expense | 2,020 | | | 476 | |
| (Gain) loss on disposal of property and equipment | (30) | | | 21 | |
| Changes in operating assets and liabilities, net of business combinations: | | | |
| Accounts receivable | (4,564) | | | (20,285) | |
| Inventories | (4,385) | | | (1,732) | |
| Other receivables | 66 | | | 620 | |
| Prepaid expenses | 3,128 | | | 4,282 | |
| | | |
| Operating lease right-of-use assets | 5,806 | | | 5,404 | |
| Other assets | (84) | | | (157) | |
| Accrued expenses and other current liabilities | 3,357 | | | 2,349 | |
| Income taxes payable | (255) | | | 123 | |
| Accounts payable | 5,057 | | | (6,187) | |
| Current and long-term operating lease liabilities | (5,324) | | | (3,801) | |
| Other non-current liabilities | (443) | | | (1,157) | |
| Net cash and cash equivalents used in operating activities | (36,315) | | | (61,756) | |
| Cash flows from investing activities: | | | |
| Purchases of property and equipment | (4,567) | | | (5,529) | |
| | | |
| Cash paid for practice acquisitions, net | (4,456) | | | (8,577) | |
| Purchases of marketable securities/investments | (9,595) | | | (117,508) | |
| Sales of marketable securities/investments | 81,258 | | | — | |
| Net cash and cash equivalents provided by (used in) investing activities | 62,640 | | | (131,614) | |
| Cash flows from financing activities: | | | |
| | | |
| | | |
| | | |
| | | |
| Proceeds from issuance of long-term debt | — | | | 110,000 | |
| Transactions costs related to issuance of long-term debt | — | | | (3,663) | |
| | | |
| Payments made for financing of insurance payments | (3,269) | | | (5,009) | |
| Payment of deferred consideration liability for acquisition | (2,584) | | | (509) | |
| | | |
| Principal payments on financing leases | (101) | | | (58) | |
| | | |
| Common stock repurchase | (1,019) | | | (9,000) | |
| Common stock issued for options exercised | 126 | | | 858 | |
| Taxes for common stock net settled | — | | | (413) | |
| | | |
| | | | | | | | | | | |
THE ONCOLOGY INSTITUTE, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(US Dollars in thousands) |
| Year Ended December 31, |
| 2023 | | 2022 |
| Net cash and cash equivalents provided by (used in) financing activities | (6,847) | | | 92,206 | |
| Net (decrease) increase in cash and cash equivalents | 19,478 | | | (101,164) | |
| Cash and cash equivalents at beginning of period | 14,010 | | | 115,174 | |
| Cash and cash equivalents at end of period | $ | 33,488 | | | $ | 14,010 | |
| Supplemental disclosure of cash flow information: | | | |
| Cash paid for: | | | |
| Income taxes | $ | 403 | | | $ | 150 | |
| Interest | $ | 4,506 | | | $ | 224 | |
| Supplemental disclosure of noncash investing and financing activities: | | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| Deferred consideration as part of practice acquisitions | $ | 1,813 | | | $ | — | |
| Discount on senior secured convertible note | $ | — | | | $ | 28,160 | |
| Financed insurance premiums | $ | 1,253 | | | $ | — | |
| Purchases of property and equipment included in accounts payable | $ | 182 | | | $ | — | |
See accompanying notes to the consolidated financial statements.
THE ONCOLOGY INSTITUTE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
As of December 31, 2023 and December 31, 2022 and for the years ended December 31, 2023 and 2022
(US Dollars in thousands, except share data)
Note 1. Description of the Business
Overview of the Business
The Oncology Institute, Inc. (“TOI”) was formerly known as DFP Healthcare Acquisitions Corp. ("DFPH"). The Company is a Delaware corporation originally formed in 2019 as a publicly-traded special purpose acquisition company for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase, reorganization, or similar business combination ("Business Combination"). TOI was originally founded in 2007 and is a community oncology practice that operates value-based oncology services platforms. TOI has various wholly-owned subsidiaries, including The Oncology Institute, LLC ("TOI LLC") (which was formerly known as TOI Parent, Inc.), The Oncology Institute of Hope and Innovation Patient Safety Organization, LLC, and TOI Management, LLC (“TOI Management”). Additionally, TOI Management holds master services agreements with affiliated physician-owned professional entities ("TOI PCs") that confer controlling financial interest over the professional entities and their wholly-owned subsidiaries (TOI PCs, together with TOI, the “Company”).
On November 12, 2021 ("Closing Date"), the Business Combination closed following December 31. Wea series of mergers, which resulted in DFPH emerging as the parent of the combined entity Orion Merger Sub II, LLC and the former TOI Parent (together, "Legacy TOI"). DFPH was renamed “The Oncology Institute, Inc.” and common stock and "Public Warrants" continued to be listed on Nasdaq under the ticker symbols “TOI” and “TOIIW,” respectively.
Operationally, the Company’s medical centers provide a complete suite of medical oncology services including: physician services, in-house infusion and pharmacy, clinical trials, radiation, educational seminars, support groups, counseling, and 24/7 patient assistance. TOI’s mission is to heal and empower cancer patients through compassion, innovation and state-of-the-art medical care. The Company brings comprehensive, integrated cancer care into the community setting, including clinical trials, palliative care programs, stem cell transplants, and other care delivery models traditionally associated with non-community-based academic and tertiary care settings. In addition, the Company, through it consolidating subsidiary TOI Clinical Research, LLC ("TCR"), performs cancer clinical trials through a network of cancer care specialists. TCR conducts clinical trials for a broad range of pharmaceutical and medical device companies from around the world.
The Company has 119 oncologists and mid-level professionals across 69 clinic locations located within five states: California, Florida, Arizona, Nevada, and Texas. The Oncology Institute CA, a Professional Corporation ("TOI CA"), one of the TOI PCs, is comprised of the clinic locations in California, Nevada, and Arizona. The Company has contractual relationships with multiple payors, serving Medicare, including Medicare Advantage, Medi-Cal, and commercial patients.
Note 2. Summary of Significant Accounting Policies
Basis of Presentation
The accompanying consolidated financial statements have been prepared in accordance with generally accepted accounting principles in the United States ("U.S. GAAP").
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of TOI, its subsidiaries, all of which are controlled by TOI through majority voting control, and variable interest entities (“VIE”) for which TOI (through TOI Management) is the primary beneficiary. The Company consolidates entities in which it has a controlling financial interest based on either the variable interest entity or voting interest model. All significant intercompany balances and transactions have been eliminated in consolidation.
Variable Interest Entities
The Company consolidates entities for which it has a variable interest and is determined to be the primary beneficiary. Noncontrolling interests in less-than-wholly-owned consolidated subsidiaries of the Company are presented as a component of total equity to distinguish between the interests of the Company and the interests of the noncontrolling owners. Revenues, expenses, and net income from these subsidiaries are included in the consolidated amounts as presented on the Consolidated Statements of Operations.
The Company holds variable interests in TOI PCs, which it cannot predict whether investors will find our securities less attractive because we will rely on these exemptions. If some investors find our securities less attractivelegally own, as a result of entering into master services agreements ("MSAs"). As of December 31, 2023, TOI held variable interest in TOI CA, The Oncology Institute FL, LLC, a Professional Corporation ("TOI FL"), and The Oncology Institute TX, a Professional Association ("TOI TX"), all of which are VIEs. The Company is the primary beneficiary of the TOI PCs, and thus, consolidates the TOI PCs in its financial statements. As discussed in Note 17, the shareholders of the Company's consolidating VIEs own a minority of the issued and outstanding common shares of the Company.
Business Combinations
The Company accounts for all transactions that represent business combinations using the acquisition method of accounting under Accounting Standards Codification Topic No. 805, Business Combinations (“ASC 805”). The Company first assesses whether an acquisition constitutes a business combination or asset acquisition by applying the screening test and analyzing whether the acquired entity has substantive inputs, processes, and the ability to produce outputs. Upon concluding an acquisition is a business combination, per ASC 805, the identifiable assets acquired, the liabilities assumed, and any noncontrolling interest in the acquired entity are recognized and measured at their fair values on the date an entity obtains control of the acquiree. Such fair values that are not finalized for reporting periods following the acquisition date are estimated and recorded as provisional amounts. Adjustments to these provisional amounts during the measurement period (defined as the date through which all information required to identify and measure the consideration transferred, the assets acquired, the liabilities assumed, and the noncontrolling interests obtained, limited to one year from the acquisition date) are recorded when identified. Goodwill is determined as the excess of the fair value of the consideration exchanged in the acquisition over the fair value of the net assets acquired.
The DFPH-Legacy TOI Business Combination was accounted for as a reverse recapitalization. Under this method of accounting, DFPH was treated as the “acquired” company for accounting purposes and the Business Combination was treated as the equivalent of Legacy TOI issuing stock for the net assets of DFPH, accompanied by a recapitalization. The net assets of DFPH are stated at historical cost, with no goodwill or other intangible assets recorded. Operations prior to the Business Combination were those of TOI Parent.
Segment Reporting
The Company presents the financial statements by segment in accordance with Accounting Standard Codification Topic No. 280, Segment Reporting (“ASC 280”) to provide investors with transparency into how the chief operating decision maker (“CODM”) manages the business. The Company determined the CODM is its Chief Executive Officer. The CODM reviews financial information and allocates resources across three operating segments: patient services, dispensary, and clinical trials & other. Each of the operating segments is also a reporting segment as described further in Note 20.
Use of Estimates
The preparation of the consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could materially differ from those estimates under different assumptions or conditions. Significant items subject to such estimates and assumptions include judgements related to revenue recognition, estimated accounts receivable and the allowance for credit losses, useful lives and recoverability of long-lived and intangible assets, recoverability of goodwill, fair values of acquired identifiable assets and assumed liabilities in business combinations, fair value of intangible assets and goodwill, fair value of share-based compensation, fair value of liability classified instruments, and judgements related to deferred income taxes.
Net Income (Loss) Per Share
Basic and diluted net income (loss) per share attributable to common stockholders is presented in conformity with the two-class method required for participating securities. The Company's Series A Convertible Preferred Stock is classified as a
participating security in accordance with ASC 260. Under the two-class method, basic and diluted net income (loss) per share attributable to common stockholders is computed by dividing the basic and diluted net income (loss) attributable to common stockholders by the basic and diluted weighted-average number of shares of common stock outstanding during the period. Diluted net loss per share attributable to common stockholders adjusts basic net loss per share for the potentially dilutive impact of stock options, restricted stock units, Medical RSUs (defined in Note 14), earnout shares (defined in Note 14), public warrants, private placement warrants, and Senior Secured Convertible Notes (defined in Note 11).
The treasury stock method is used to calculate the potentially dilutive effect of stock options, RSUs, public warrants, and private placement warrants. The if-converted method is used to calculate the potentially dilutive effect of the Senior Secured Convertible Notes. In both methods, diluted net income (loss) attributable to common stockholders and diluted weighted-average shares outstanding are adjusted to account for the impact of the assumed issuance of potential common shares that are dilutive, subject to dilution sequencing rules. The earnout shares are contingently issuable; therefore, the earnout shares are excluded from basic and diluted net income (loss) per share until the market conditions have been met (see more detail on the earnout shares in Note 14). The Medical RSUs (defined in Note 14) are also contingently issuable; therefore, they are excluded from basic net income (loss) per share until the performance and service conditions have been met (see more detail in Note 14). Further, the number of contingently issuable Medical RSUs included in diluted net income (loss) per share is based on the number of shares, if any, that would be issuable if the end of the reporting period were the end of the contingency period and if the result would be dilutive. For the periods presented, the public and private placement warrants are out of the money; therefore, the public and private placement warrants are antidilutive and excluded from diluted net loss per share.
Revenue Recognition
The Company follows the accounting requirements of Accounting Standard Codification Topic No. 606, Revenue from Contracts with Customers (“ASC 606”). The core principle of ASC 606 is to recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration the entity expects to be entitled in exchange for those goods or services. This principle is achieved through applying the following five-step approach:
1.Identification of the contract, or contracts, with a customer.
2.Identification of the performance obligations in the contract.
3.Determination of the transaction price.
4.Allocation of the transaction price to the performance obligations in the contract.
5.Recognition of revenue when, or as, an entity satisfies a performance obligation.
The Company receives payments from the following sources for services rendered: (i) commercial insurers; (ii) the federal government under the Medicare program administered by the Centers for Medicare and Medicaid Services (“CMS”); (iii) state governments under the Medicaid and other programs; (iv) other third-party payors (e.g., hospitals and independent practice associations (“IPAs”)); and (v) individual patients and clients.
Revenue primarily consists of capitation revenue, fee-for-service (“FFS”) revenue, dispensary revenue, and clinical trials revenue. Revenue is recognized in the period in which services are rendered or the period in which the Company is obligated to provide services. The form of billing and related risk of collection for such services may vary by type of revenue and the payor. The following paragraphs provide a summary of the principal forms of the Company’s billing arrangements and how revenue is recognized for each.
Capitation
Capitation revenues of the Company consist primarily of fees for medical services provided to patients by the Company under a capitated arrangement with various managed care organizations. Capitation revenue is paid monthly to the Company based on the number of enrollees assigned to the Company by the contracted managed care organization (per member, per month; or “PMPM”). Capitation contracts generally have a legal term of one year or longer. Capitation contracts have a single performance obligation that is a stand ready obligation to perform healthcare services to the population of enrolled members and constitutes a series for the provision of managed healthcare services for the term of the contract, which is deemed to be one month since the mix of patient-customers can and do change month over month. The transaction price for capitation contracts is variable as it primarily includes PMPM fees associated with unspecified membership that fluctuates throughout the contract. The Company generally estimates the transaction price using the most likely methodology and amounts are only included in the transaction price to the extent that it is probable that a significant reversal of cumulative revenue will not occur once any uncertainty is resolved. Certain contracts include terms for a capitation deduction where the cost of out-of-network referrals of members by the Company are deducted from the future payment. The deductions vary depending on the payor and are often not
known until a future period. As such, the Company adjusts the transaction price for capitation deductions based on historic experience such that the capitation revenue is recognized to the extent that it is not probable a significant reversal of revenue will occur in the future. Revenue is recognized in the month services are rendered on the basis of the transaction price established at that time. If subsequent information resolves uncertainties related to the transaction price, adjustments will be recognized in the period they are resolved. When payment has been received but services have not yet been rendered, the payment is recognized as a contract liability.
Fee-for-Service Revenue
FFS revenue represents revenue earned under contracts in which the Company bills and collects for medical services rendered by the Company’s employed or contracted physicians. The terms for FFS contracts are short in duration and only last for the period over which services are rendered (typically, one day). FFS revenue consists of fees for medical services provided to patients. These medical services are capable of being distinct since the patient can benefit from the medical services on their own. Each service constitutes a single performance obligation for which the patient accepts and receives the benefit of the medical services as they are performed.
Under the FFS arrangements, the Company bills third-party payors and patients for patient care services provided. Payments for services provided are generally less than billed charges. The Company records revenue net of an allowance for contractual adjustments, which represents the net revenue expected to be collected from third-party payors (including managed care, commercial, and governmental payors such as Medicare and Medicaid), and patients. These expected collections are based on fees and negotiated payment rates in the case of third-party payors, the specific benefits provided for under each patient’s healthcare plans, mandated payment rates in the case of Medicare and Medicaid programs, and historical cash collections (net of recoveries).
The transaction price from FFS arrangements is variable in nature because fees are based on patient encounters, credits due to patients, and reimbursement of provider costs, all of which can vary from period to period. The Company estimates the transaction price using the most likely methodology and amounts are only included in the net transaction price to the extent that it is probable that a significant reversal of cumulative revenue will not occur once any uncertainty is resolved. As a practical expedient, the Company uses a portfolio approach to determine the transaction price for the medical services provided under FFS arrangements. Under this approach, the Company bifurcates the types of services provided and grouped health plans with similar fees and negotiated payment rates. At these levels, portfolios share the characteristics conducive to ensuring that the results do not materially differ from the standard applied to individual patient contracts related to each medical service provided.
The recognition of net revenue (gross charges less contractual allowances) from such services is dependent on such factors as proper completion of medical charts following a patient visit, the forwarding of such charts to the Company’s billing center for medical coding and entering into the Company’s billing system, and the verification of each patient’s submission or representation at the time services are rendered as to the payor(s) responsible for payment of such services. Revenue is recorded on the date the services are rendered based on the information known at the time of entering of such information into the Company’s billing systems as well as an estimate of the revenue associated with medical services. When the performance obligation is not satisfied, the billing is recognized as a contract liability.
Dispensary
The Company sells oral prescription drugs directly through its dispensaries and pharmacy. Each prescription filled and delivered to the customer is a distinct performance obligation. The transaction price for the prescriptions is based on fee schedules set by various pharmacy benefit managers (“PBMs”) and other third party payors. The fee schedule is often subject to direct and indirect remuneration (“DIR”) fees, which are based primarily on pre-established metrics. DIR fees may be assessed in periods after payments are received against future payments. The Company estimates DIR fees to arrive at the transaction price for prescriptions. The Company recognizes revenue based on the transaction at the time the customer takes possession of the oral drug.
Clinical Trials & Other Revenue
The Company enters into contracts to perform clinical research trials. The terms for clinical trial contracts last many months as the clinical research is performed. Each contract represents a single, integrated set of research activities and thus is a single performance obligation. The performance obligation is satisfied over time as the output is captured in data and documentation that is available for the customer to consume over the course of arrangement and furthers progress of the clinical trial. Under the clinical trial contracts, the Company receives a fixed payment for administrative, set-up, and close-down fees; a fixed amount for each patient site visit; and certain expense reimbursements. Under ASC 606, the Company has elected to
recognize revenue for these arrangements using the ‘as-invoiced’ practical expedient. The Company invoices the customer periodically based on the progress of the trial such that each invoice captures the revenue earned to date based on the state of the trial as established between the Company and the customer.
Direct Costs of Sales
Direct cost of sales primarily consists of wages paid to clinical personnel and other health professionals, oral and IV drug costs, and other medical supplies used to provide patient care. The Company’s costs for clinical personnel wages are expensed as incurred and the Company’s costs for inventory and medical supplies are expensed when used, generally by applying the specific identification method.
Cash and Cash Equivalents
Cash primarily consists of deposits with banking institutions. The Company considers all highly liquid investments that are both readily convertible into cash and mature within three months from the date of purchase to be cash equivalents.
Accounts Receivable and Allowance for Credit Losses
The Company’s accounts receivables are recorded and stated at the amount expected to be collected determined by each payor, net of an allowance for credit losses, under ASC Topic No. 310, Receivables (“ASC 310”). In accordance with ASC Topic No. 326, Financial Instruments — Credit Losses (“ASC 326”), the Company recognizes credit losses based on a forward-looking current expected credit losses (“CECL”) model. The Company segregates accounts receivables into portfolio segments based on shared risk characteristics, such as line of business and customer type, for evaluation of expected credit losses. The Company makes estimates of expected credit losses based upon its assessment of various factors, including the age of accounts receivable balances, default-based statistics, current economic conditions, reasonable and supportable forecasts of future economic conditions, and other factors that may affect its ability to collect from customers. The allowance for credit losses is developed using a loss rate method and is recognized in the Consolidated Statement of Operations. The uncollectible accounts receivables are written off on a quarterly basis in the period when collection activities cease due to a final determination that all or a portion of the balance is no longer collectible and if there is no pending litigation activity related to the receivable. No allowance for credit losses was recorded as of December 31, 2023 and 2022.
Inventories
The Company accounts for inventory under Accounting Standard Codification Topic No. 330, Inventory (“ASC 330”). Inventories consist of intravenous chemotherapy drugs and oral prescription drugs. Inventories are stated at the lower of cost, determined using the weighted average cost method of inventory valuation, or net realizable value. Net realizable value is determined using the selling price, less costs to sell.
The Company receives purchase discounts on products purchased. Contractual arrangements with vendors, including manufacturers and wholesalers, normally provide for the Company to receive purchase discounts from established list prices in one, or a combination, of the following forms: (i) a direct discount at the time of purchase or (ii) a discount for the prompt payment of invoices. Additionally, in other circumstances, the Company may receive rebates when products are purchased indirectly from a manufacturer (e.g., through a wholesaler). These rebates are recognized when intravenous chemotherapy drugs and oral prescription drugs are dispensed and are generally calculated by manufacturers within 30 days after the end of each completed quarter. The Company also receives additional rebate under its wholesaler contracts if it exceeds contractually defined annual purchase volumes. Purchase rebates are recorded as reductions to cost of services.
Property and Equipment, net
The Company accounts for property and equipment under Accounting Standard Codification Topic No. 360, Property, Plant, and Equipment (“ASC 360”). As required under ASC 360, the Company states property and equipment at cost, net of accumulated depreciation. Property and equipment is depreciated using the straight-line method over the estimated useful lives of the related assets, as described further in Note 8. Maintenance and repairs are charged to expense as incurred. Significant renewals and improvements are capitalized. At the time of retirement or other disposition of property and equipment, the cost and accumulated depreciation are removed from the accounts and any resulting gain or loss is reflected in the Consolidated Statements of Operations.
When events or changes in circumstances indicate that the carrying amount of long-lived assets, including property and equipment, or other long-lived assets, may not be recoverable, an evaluation of the recoverability of currently recorded costs is performed. When an evaluation is performed, the estimated value of undiscounted future net cash flows associated with the asset groups is compared to the asset groups’ carrying value to determine if a write-down to fair value is required. If such assets
are considered to be impaired, the impairment to be recognized is measured as the amount by which the carrying amount of the asset group exceeds the fair value of the assets. There were no impairment adjustments recorded for long-lived assets during the years ended December 31, 2023 and 2022.
Accounts Payable, Accrued Expenses, and Other Current Liabilities
Accounts payable primarily consists of unpaid invoices related to routine operating expenses. Accrued expenses and other current liabilities primarily consist of accruals made for payroll expenses, and deferred capitation.
Leases
Effective January 1, 2022, the Company accounts for its leasing arrangements in accordance with Accounting Standards Codification, Topic No. 842, Leases ("ASC 842"), which requires lessees to recognize assets and liabilities for most leases. The Company evaluates whether an arrangement is or contains a lease at contract inception. A lease exists when a contract conveys to the customer the right to control the use of an identified asset for a period of time in exchange for consideration. Upon lease commencement, the date on which a lessor makes the underlying asset available to the Company for use, the Company classifies the lease as either an operating or finance lease. The Company applied certain practical expedients permitted under the transition guidance, including the package of practical expedients, which permits the Company not to reassess its prior conclusions related to lease identification, lease classification, and initial direct costs capitalization. The Company solely acts as a lessee and its leases primarily consist of operating leases for its real estate in the states in which the Company operates. The Company has other operating and financing leases for various clinical and non-clinical equipment.
Generally, upon the commencement of a lease, the Company will record a right-of-use (“ROU”) asset and lease liability. An ROU asset represents the Company’s right to use an underlying asset for the lease term and lease liabilities represent the Company’s obligation to make lease payments arising from the lease. Lease liabilities are measured at the present value of the remaining, fixed lease payments at lease commencement. The Company uses its incremental borrowing rate, based on the information available at the later of adoption, inception, or modification in determining the present value of lease payments. ROU assets are measured at an amount equal to the initial lease liability, plus any prepaid lease payments (less any incentives received) and initial direct costs, at the lease commencement date. The Company has elected to account for lease and non-lease components as a single lease component for all underlying classes of assets. As a result, the fixed payments that would otherwise be allocable to the non-lease components are accounted for as lease payments and included in the measurement of the Company’s right-of-use asset and lease liability.
Lease arrangements with an initial term of 12 months or less are considered short-term leases and are not recorded on the balance sheet. The operating lease payments are recognized as an expense on a straight-line basis over the lease term. The lease term includes any period covered by renewal options available that the Company is reasonably certain to exercise and any options to terminate the lease that the Company is not reasonably certain to exercise.
The Company displays ROU assets, current lease liabilities, and long term lease liabilities arising from operating leases as separate line items on the consolidated balance sheet. The Company includes ROU assets, current lease liabilities, and long term lease liabilities arising from finance leases within property and equipment, net; accrued expenses and other current liabilities; and other non-current liabilities.
Goodwill
The Company accounts for goodwill under Accounting Standards Codification Topic No. 350, Intangibles - Goodwill and Other (“ASC 350”). Goodwill represents the excess of the fair value of the consideration conveyed in and acquisition over the fair value of net assets acquired.
Goodwill is not amortized but is required to be evaluated for impairment annually or sooner if impairment indicators exist. The Company performs its annual testing of impairment for goodwill in the fourth quarter of each year. When impairment indicators are identified, the Company compares the reporting unit’s fair value to its carrying amount, including goodwill. An impairment loss is recognized as the difference, if any, between the reporting unit’s carrying amount and its fair value to the extent the difference does not exceed the total amount of goodwill allocated to the reporting unit.
For the years ended December 31, 2023 and 2022, the Company first performed a qualitative assessment to determine whether it was necessary to perform the quantitative analysis. Based on the qualitative assessment including our relianceshare price decrease as well as factors related to macroeconomic conditions, industry and market considerations, cost factors, financial performance and market capitalization, the Company determined it was likely that our reporting unit fair value was less than its carrying value and the quantitative impairment test was performed. Based on these exemptions, the trading pricesresults of our securitiesassessment performed the
Company recorded an impairment charge of $16,867 and $9,944 to goodwill for the years ended December 31, 2023 and 2022, respectively.
Intangible Assets
Under ASC 350, finite-lived intangible assets are stated at acquisition-date fair value. The Company's intangible assets are amortized using the straight-line method.
Finite-lived intangible assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. When circumstances indicate that recoverability may be lowerimpaired, the Company assesses its ability to recover the carrying value of the asset group from the expected future pre-tax cash flows (undiscounted and without interest charges) of the related operations. If these cash flows are less than they otherwisethe carrying value of such asset, an impairment loss is recognized for the difference between estimated fair value and carrying value. Fair value is determined based on appropriate valuation techniques.
For the years ended December 31, 2023 and 2022, the Company performed a qualitative analysis and determined that there were no indicators of impairment. Therefore, no impairment charge of its finite-lived intangible assets was recorded.
Investments in Marketable Securities
The Company's investments in marketable securities are classified as available-for-sale and are carried at fair value. The Company accounts for its investment securities available for sale using the fair value election pursuant to ASC 825, Financial Instruments ("ASC 825"), where changes in fair value are recorded in unrealized gains (losses), net on the Company's Consolidated Statements of Operations. The Company determines the appropriate classification of these investments at the time of purchase and reevaluates such designation at each balance sheet date. The Company’s marketable securities are classified as current assets if the maturity date is less than one year from the balance sheet date.
Interest income and accretion on marketable securities are included in interest income in the Consolidated Statements of Operations. Realized gains and losses on sales of securities, and other-than-temporary declines in the fair value of marketable securities, if any, are included as a component of other income (expense), net in the Consolidated Statements of Operations. The cost of securities sold is based on the First In, First Out method.
At each reporting period, the Company evaluates available-for-sale marketable securities, to the extent the fair value option is not elected, for any credit-related impairment when the fair value of the investment is less than its amortized cost. If the Company determines that the decline in fair value is below the carrying value and this decline is other-than-temporary, credit-related impairment is recognized in the Consolidated Statements of Operations in accordance with ASC 320, Debt Securities. As of December 31, 2023 and 2022, there were no available-for-sale instruments for which the fair value option was not elected.
Debt
The Company accounts for debt net of debt issuance costs and debt discount. Debt issuance costs and debt discount are capitalized, netted against the related debt for presentation purposes, and amortized to interest expense over the terms of the related debt using the effective interest method.
The Company accounts for bifurcated, debt-classified embedded features separately as derivative liabilities pursuant to Accounting Standards Codification Topic No. 815, Derivatives and Hedging ("ASC 815"). Bifurcated, debt-classified embedded features are recorded at fair value on the Company's balance sheet with subsequent changes in fair value recorded in the Consolidated Statement of Operations each reporting period.
Public Warrants and Private Placement Warrants
Upon completion of the Business Combination, the Company assumed public and private placement warrants that were issued by DFPH in connection with its initial public offering (declared effective by the Securities and Exchange Commission on March 10, 2020) whereby holders of the public and private placement warrants are entitled to acquire common stock of the Company.
Prior to the Business Combination, the public warrants were accounted for as liabilities per Accounting Standards Codification Subtopic No. 815-40 Contracts on an Entity's Own Equity ("ASC 815-40"). Following the Business Combination, the shares of common stock underlying the public warrants are not redeemable and the Company has one single class of voting stock; therefore, the public warrants are not precluded from being considered indexed to the Company’s common stock which
allows the public warrants to meet the criteria for equity classification per ASC 815-40. Warrants classified as equity are recorded at their issuance cost and are not subject to remeasurement at each subsequent balance sheet date.
Prior to the Business Combination, the private placement warrants were accounted for as liabilities per ASC 815-40. The private placement warrants are not considered indexed to the Company’s stock per ASC 815-40 and are therefore recorded as liabilities, given the settlement of the private placement warrants is dependent, in part, on who holds the warrants at the time of the settlement. Warrants classified as liabilities are recorded at their estimated fair value on the Closing Date and are revalued at each subsequent balance sheet date, with fair value changes recognized in other non-operating expense (income) in the accompanying Consolidated Statements of Operations. The Company estimates the value of these warrants using a Binomial Lattice valuation model in a risk-neutral framework.
Earnout Liability
As part of the Business Combination, DFPH issued to eligible Legacy TOI stockholders and Legacy TOI employees the contingent right to receive up to 12.5 million additional shares of common stock (“Legacy TOI Earnout Shares”), in two tranches of 5.0 million and 7.5 million, respectively, upon the Company common stock achieving a price per share of $12.50 during the two-year period following the Closing or a price per share of $15.00 during the three-year period following the Closing, in each case, as its last reported sales price per share for any 20 trading days within any 30 consecutive trading day period within the applicable period ("Earnout Terms"); provided, that (i) if one or both of the share price triggers has not been achieved prior to the end of the three-year period following the Closing, (ii) the Company enters into a definitive agreement that would result in a change of control and (iii) the price per share of the Company’s common stock in such transaction is equal to or greater than one or both of the share price triggers, then at the Closing of such transaction, the Company shall issue the applicable portion of the Legacy TOI Earnout Shares as if such share price trigger had been achieved.
In addition, certain DFPH common stockholders deposited 575,000 shares of DFPH common stock in an escrow account that will vest and be released to such holders in two tranches of 50%, each (“DFPH Earnout Shares”), upon the Company common stock achieving the Earnout Terms as described above; provided, that (i) if one or both of the share price triggers has not been achieved prior to the end of the three-year period following the closing, (ii) the Company enters into a definitive agreement that would result in a change of control and (iii) the price per share of common stock in such transaction is equal to or greater than one or both of the share price triggers, then at the closing of such transaction, the Company shall issue the applicable portion of the DFPH Earnout Shares as if such share price trigger had been achieved. To the extent any DFPH Earnout Shares remain unvested at the expiration of the three-year period following the closing, such DFPH Earnout Shares shall be forfeited and cancelled without any consideration.
Collectively, the Legacy TOI Earnout Shares and DFPH Earnout Shares constitute the “Earnout Shares”, the “Earnout”, and the “Earnout Liability”.
The Company determined that Earnout Shares issuable to Legacy TOI stockholders and DFPH stockholders fail to meet equity classification criteria under ASC 815-40 and therefore, represents a liability that meets the definition of a derivative and recognized it on the balance sheet at its fair value upon the Closing Date. The right to Earnout Shares issuable to Legacy TOI stockholders and DFPH stockholders are remeasured at fair value using a Monte Carlo simulation model each period through earnings. See Note 7 for further discussion.
Earnout Shares issuable to Legacy TOI employees is considered a share-based compensation award under Accounting Standards Codification Topic No. 718, Stock Based Compensation (“ASC 718”) due to the requirement that Legacy TOI employees must remain employed by the Company in order to not forfeit such unvested Earnout Shares. Such Earnout Shares are accounted for within equity over the service period. See Note 14 for further discussion.
Income Taxes
The Company accounts for income taxes under the asset and liability method under Accounting Standards Codification Topic No. 740, Income Taxes (“ASC 740”). Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. The Company recognizes the effect of income tax positions only if those positions are more likely than not of being sustained. Recognized income tax positions are measured at the largest amount that is greater than 50% likely of being realized. Changes in recognition or measurement are reflected in the period in which the change in judgment occurs. The Company records interest related to unrecognized tax benefits in interest expense and penalties in selling, general, and administrative expenses.
Retirement Plans
The Company provides a qualified 401(K) plan to all eligible employees which is administered through the John Hancock Life Insurance Company (U.S.A.). Employees are eligible to participate in the plan on the first day of the month subsequent to completing two months of service. Eligible employees may, subject to statutory limitations, contribute a portion of their salary to the plan through payroll deduction. In 2023 and 2022, the Company provided a matching contribution of 100% of the elective deferral that does not exceed 4% of compensation. Participants are always fully vested in their own contributions and the Company’s matching contributions vest immediately. The Company expensed to selling, general and administrative expenses $1,271 and $1,108 in matching contributions related to the 401(K) plan during the years ended December 31, 2023 and December 31, 2022, respectively.
Share-Based Compensation Plan
The Company accounts for share-based compensation under Accounting Standards Codification Topic No. 718, Compensation - Stock Compensation ("ASC 718"). As required under ASC 718, the Company accounts for employee and nonemployee share-based compensation as an expense in the consolidated financial statements. Equity-classified awards are measured at the grant date fair value of the award. Liability-classified awards are remeasured at fair value each reporting end date. For stock options, the Company estimates grant date fair value using the Black-Scholes-Merton option-pricing model. For restricted stock units (“RSU”), the fair value is based on the Company’s share price on the grant date. Liability-classified awards are settled in a variable number of the Company’s common stock on the vesting date based on a fixed monetary value. The Company accounts for forfeitures as incurred.
Excess tax benefits of awards related to stock option exercises are recognized as an income tax benefit in the Consolidated Statements of Operations and reflected in operating activities in the Consolidated Statements of Cash Flows.
Commitments and Contingencies
The Company accounts for contingent liabilities under Accounting Standards Codification Subtopic No. 450-20, Contingencies (“ASC 450-20”). As required by ASC 450-20, liabilities for loss contingencies arising from claims, assessments, litigation, fines, penalties, and other sources are recorded when it is probable that a liability has been incurred and the amount can be reasonably estimated. Legal costs incurred in connection with loss contingencies are expensed as incurred.
Comprehensive Loss
Comprehensive loss includes net loss to common stockholders as well as other changes in equity that result from transactions and economic events other than those with stockholders. There was no difference between comprehensive loss and net loss to common stockholders for the periods presented.
Fair Value Measurements
The Company accounts for fair value measurements under Accounting Standards Codification Topic No. 820, Fair Value Measurements (“ASC 820”). The Company uses valuation approaches that maximize the use of observable inputs and minimize the use of unobservable inputs to the extent possible. The Company determines fair value based on assumptions that market participants would use in pricing an asset or liability in the principal or most advantageous market. When considering market participant assumptions in fair value measurements, the following fair value hierarchy distinguishes between observable and unobservable inputs, which are categorized in one of the following levels (see Note 7 for further discussion):
Level 1inputs: Unadjusted quoted prices in active markets for identical assets or liabilities accessible to the reporting entity at the measurement date.
Level 2inputs: Other than quoted prices included in Level 1 inputs that are observable for the asset or liability, either directly or indirectly, for substantially the full term of the asset or liability.
Level 3inputs: Unobservable inputs for the asset or liability used to measure fair value to the extent that observable inputs are not available, thereby allowing for situations in which there mayis little, if any, market activity for the asset or liability at measurement date.
The Company's fair value measurement methodology for cash and cash equivalents, accounts receivable, other receivables, and accounts payable approximates fair value because of the short maturity and high liquidity of these instruments. Fair value measurement of investment securities available for sale is based upon quoted prices from active markets, if available (Level 1). If quoted prices are not available, fair values are measured using independent pricing models or other model-based valuation
methodologies. Level 2 investment securities include US Treasuries purchased in the secondary market that use pricing inputs other than quoted prices in active markets and fair value is determined using pricing models or other valuation methodologies such as broker price indications, which are based on quoted prices for identical or similar notes, which are Level 2 input measures. Fair value measurements used for the goodwill and intangible assets are based on the discounted cash flow method within the income approach and guideline public company method to value the reporting units, which is considered to be a less active tradingLevel 3 fair value measurement. The unobservable inputs utilized in determining the fair value of goodwill based on the income approach primarily include estimated future cash flows, discounted at a rate that approximates the cost of capital of a market participant. Inputs used to calculate the fair value based on the market approach include the revenue and EBITDA multiples based on guidelines for our securitiessimilar publicly traded companies and recent transactions. Fair value measurements of derivative warrants and earnout liabilities are based on Binomial Lattice and Monte-Carlo Simulation Models, respectively, which are considered to be Level 3 fair value measurements. The primary unobservable input utilized in determining the trading pricesfair value of our securities maythe derivative warrants and earnouts is the expected volatility of the common stock. Fair value measurements of the convertible note warrant and conversion option derivative liabilities are based on the Black-Derman-Toy model implemented in the Binomial Lattice and Black-Scholes Models, which are considered to be more volatile.Level 3 fair value measurements. The primary unobservable input utilized in determining the fair value of the convertible note warrant and conversion option derivative liabilities is the expected volatility of the common stock.
Emerging Growth Company
Pursuant to the Business Combination, the Company qualifies as an “emerging growth company,” as defined in Section 2(a) of the Securities Act of 1933 ("Securities Act"), as modified by the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”), and has elected to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies.
Further, Section 102(b)(1) of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies (that is, those that have not had a Securities Act registration statement declared effective or do not have a class of securities registered under the Exchange Act) are required to comply with the new or revised financial accounting standards. The JOBS Act provides that a company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging growth companies, but any such
an election to opt out is irrevocable.
We haveThe Company has elected not to opt out of such extended transition period which means that when a standard is issued or revised and it has different application dates for public or private companies,
we, as an emerging growth company, can adopt the new or revised standard at the time private companies adopt the new or revised standard. This may make comparison of our financial statements with another public company which is neither an emerging growth company nor an emerging growth company which has opted out of using the extended transition period difficult or impossible because of the potential differences in accounting standards used.Additionally, we are a “smaller reporting company” as defined in Item 10(f)(1) of Regulation S-K. Smaller reporting companies may take advantage of certain reduced disclosure obligations, including, among other things, providing only two years of audited financial statements. We will remain a smaller reporting company until the last day of the fiscal year in which (1) the market value of our common stock held by non-affiliates exceeds $250 million as of the prior June 30th, or (2) our annual revenues exceeded $100 million during such completed fiscal year and the market value of our common stock held by non-affiliates exceeds $700 million as of the prior June 30th. To the extent we take advantage of such reduced disclosure obligations, it may also make comparison of our financial statements with other public companies difficult or impossible.
We have not registered the Class A common stock issuable upon exercise of the warrants under the Securities Act or any state securities laws, and such registration may not be in place when an investor desires to exercise warrants, thus precluding such investor from being able to exercise its warrants and causing such warrants to expire worthless.
We have not registered the Class A common stock issuable upon exercise of the warrants under the Securities Act or any state securities laws. However, under the terms of the warrant agreement, we have agreed that, as soon as practicable, but in no event later than 15 business days, after the closing of our initial business combination, we will use
our best efforts to file with the SEC a registration statement covering the registration under the Securities Act of the Class A common stock issuable upon exercise of the warrants and thereafter will use our best efforts to cause the same to become effective within 60 business days following our initial business combination and to maintain a current prospectus relating to the Class A common stock issuable upon exercise of the warrants until the expiration of the warrants in accordance with the provisions of the warrant agreement. We cannot assure you that we will be able to do so if, for example, any facts or events arise which represent a fundamental change in the information set forth in the registration statement or prospectus, the financial statements contained or incorporated by reference therein are not current or correct or the SEC issues a stop order.
If the shares of Class A common stock issuable upon exercise of the warrants are not registered under the Securities Act, under the terms of the warrant agreement, holders of warrants who seek to exercise their warrants will not be permitted to do so for cash and, instead, will be required to do so on a cashless basis in accordance with Section 3(a)(9) of the Securities Act or another exemption.
In no event will warrants be exercisable for cash or on a cashless basis, and we will not be obligated to issue any shares to holders seeking to exercise their warrants, unless the issuance of the shares upon such exercise is registered or qualified under the securities laws of the state of the exercising holder, or an exemption from registration or qualification is available.
If our shares of Class A common stock are at the time of any exercise of a warrant not listed on a national securities exchange such that they satisfy the definition of “covered securities” under Section 18(b)(1) of the Securities Act, we may, at our option, not permit holders of warrants who seek to exercise their warrants to do so for cash and, instead, require them to do so on a cashless basis in accordance with Section 3(a)(9) of the Securities Act; in the event we so elect, we will not be required to file or maintain in effect a registration statement or register or qualify the shares underlying the warrants under applicable state securities laws, and in the event we do not so elect, we will use our best efforts to register or qualify the shares underlying the warrants under applicable state securities laws to the extent an exemption is not available.
In no event will we be required to net cash settle any warrant, or issue securities (other than upon a cashless exercise as described above) or other compensation in exchange for the warrants in the event that we are unable to register or qualify the shares underlying the warrants under the Securities Act or applicable state securities laws.
If the issuance of the Class A common stock upon exercise of the warrants is not registered, qualified or exempt from registration or qualification under the Securities Act and applicable state securities laws, holders of warrants will not be entitled to exercise such warrants and such warrants may have no value and expire worthless. In such event, holders who acquired their warrants as part of a purchase of units will have paid the full unit purchase price solely for the Class A common stock included in the units.
The grant of registration rights to our initial stockholders and holders of our private placement warrants may make it more difficult to complete our initial business combination, and the future exercise of such rights may adversely affect the market price of our shares of Class A common stock.
Pursuant to an agreement to be entered into on or prior to the closing of the initial public offering, our initial stockholders and their permitted transferees can demand that we register the shares of Class A common stock into which founder shares are convertible, holders of our private placement warrants and their permitted transferees can demand that we register the private placement warrants and the Class A common stock issuable upon exercise of the private placement warrants and holders of warrants that may be issued upon conversion of working capital loans may demand that we register such warrants or the Class A common stock issuable upon conversion of such warrants. The registration rights will be exercisable with respect to the founder shares and the private placement warrants and the Class A common stock issuable upon exercise of such private placement warrants. We will bear the cost of registering these securities. The registration and availability of such a significant number of securities for trading in the public market may have an adverse effect on the market price of our Class A common stock. In addition, the existence of the registration rights may make our initial business combination more costly or difficult to conclude. This is because the stockholders of the target business may increase the equity stake they seek in the combined entity or ask for more cash consideration to offset the negative impact on the market price of our Class A common stock that is expected when the shares of common stock owned by our initial
stockholders, holders of our private placement warrants or holders of our working capital loans or their respective permitted transferees are registered.
ITEM 1B. UNRESOLVED STAFF COMMENTS.
None.
ITEM 2: PROPERTIES.
We currently sub-lease executive offices at 345 Park Avenue South, New York, New York 10010 from our Sponsor. We consider our current office space adequate for our current operations.
ITEM 3: LEGAL PROCEEDINGS.
As of December 31, 2020, to the knowledge of our management, there was no material litigation, arbitration or governmental proceeding pending against us or any members of our management team in their capacity as such, and we and the members of our management team have not been subject to any such proceeding.
ITEM 4: MINE SAFETY DISCLOSURES.
Not applicable.
PART II
ITEM 5: MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES.
Market Information
Our units, Class A common stock and warrants listed on Nasdaq under the symbols “DFPHU,” “DFPH,” and “DFPHW,” respectively.
Holders
As of December 31, 2020, there was one holder of record of our units, one holder of record of our Class A common stock, seven holders of record of our Class B common stock and two holders of record of our warrants. The number of holders of record does not include a substantially greater number of “street name” holders or beneficial holders whose units, Class A common stock and warrants are held of record by banks, brokers and other financial institutions.
Dividend Policy
We have never declared or paid cash dividends on our common stock. We currently do not anticipate paying any cash dividends in the foreseeable future. Any future determination to declare cash dividends will be made at the discretion of our board of directors, subject to applicable laws, and will depend on our financial condition, results of operations, capital requirements, general business conditions and other factors that our board of directors may deem relevant.
Recent Sales of Unregistered Securities; Use of Proceeds from Registered Offerings
Unregistered Sales
The sales of the founder shares and private placement warrants to our Sponsor and our initial stockholders as described herein were deemed to be exempt from registration under the Securities Act, in reliance on Section 4(a)(2) of the Securities Act as transactions by an issuer not involving a public offering.
Use of Proceeds
On March 10, 2020, our registration statement on Form S-l (File No. 333-236578) was declared effective by the SEC for the initial public offering pursuant to which we sold an aggregate of 23,000,000 units at an offering price to the public of $10.00 per unit generating gross proceeds of approximately $230.0 million, with each unit consisting of one share of Class A common stock and one-fourth of one warrant. Each whole warrant entitles the holder thereof to purchase one share of Class A common stock at a price of $11.50 per share. Deutsche Bank Securities Inc., Jefferies LLC and UBS Securities LLC acted as representatives for the underwriters (the “Underwriters”). Our initial public offering did not terminate before all of the securities registered in our registration statement were sold. The initial public offering was consummated on March 13, 2020. Simultaneously with the closing of the initial public offering, the Company, consummated the private placement of 3,733,334 warrants to the sponsor at a purchase price of $1.50 per warrant, generating gross proceeds to the Company of approximately $5.6 million.
Net proceeds of $230,000,000 from the initial public offering and the sale of the private placement warrants, including deferred underwriting discounts of $6.3 million, are held in the Trust Account as of December 31, 2020. We paid $3.6 million in underwriting discounts and incurred offering costs of approximately $525,000 related to the initial public offering. In addition, the Underwriters agreed to defer $6.3 million in underwriting discounts, which amount will be payable when and if a business combination is consummated. No payments were made by us to directors, officers or persons owning ten percent or more of our common stock or to their associates, or to our affiliates. There has been no material change in the planned use of proceeds from the initial public offering as described in our final prospectus dated July 20, 2020 which was filed with the SEC.
ITEM 6. SELECTED FINANCIAL DATA
Not applicable.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS.
The following discussion and analysis of the Company’s financial condition and results of operations should be read in conjunction with the financial statements and the notes thereto contained elsewhere in this report. Certain information contained in the discussion and analysis set forth below includes forward-looking statements that involve risks and uncertainties.
We have re-evaluated our application of ASC 480-10-S99-3A to our accounting and classification of the Public Shares, issued as part of the units sold in the IPO on March 13, 2020. Historically, a portion of the Public Shares was classified as permanent equity to maintain stockholders’ equity greater than $5 million on the basis that we will not redeem our Public Shares in an amount that would cause our net tangible assets to be less than $5,000,001, as described in the Charter. Pursuant to such re-evaluation, our management has determined that the Public Shares include certain provisions that require classification of all of the Public Shares as temporary equity regardless of the net tangible assets redemption limitation contained in the Charter. In addition, in connection with the change in presentation for the Public Shares, management determined it should restate earnings per share calculation to allocate income and losses shared pro rata between the two classes of shares. This presentation contemplates a Business Combination as the most likely outcome, in which case, both classes of shares share pro rata in the income and losses of our Company.
Therefore, on December 8, 2021, our management and the Audit Committee concluded that our previously issued (i) balance sheet as of March 13, 2020, as previously revised in the 2020 Form 10-K/A No. 1, (ii) financial statements as previously revised in the 2020 Form 10-K/A No. 1, (iii) unaudited interim financial statements as previously revised in the 2020 Form 10-K/A No. 1; (iv) unaudited interim financial statements included in our Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2021, filed with the SEC on May 24, 2021 and (v) unaudited interim financial statements included in our Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2021, filed with the SEC on August 12, 2021, should be restated to report all Public Shares as temporary equity and should no longer be relied upon. As such, the Company is restating the 2020 periods herein and intends to restate its 2021 interim financial statements for the Affected Periods in its quarterly report on Form 10-Q for the period ended September 30, 2021.
The restatement does not have an impact on our cash position and cash held in the Trust Account.
Our management has concluded that a material weakness remains in the Company’s internal control over financial reporting and that the Company’s disclosure controls and procedures were not effective. As a result of that reassessment, we determined that our disclosure controls and procedures for such periods were not effective with respect to the proper accounting and classification of complex financial instruments. For more information, see Item 9A included in this Annual Report on Form 10-K/A.
We have not amended our previously filed Quarterly Report on Form 10-Qs for the periods affected by the restatement. The financial information that has been previously filed or otherwise reported for these periods is superseded by the information in this Amendment No. 2, and the financial statements and related financial information contained in such previously filed reports should no longer be relied upon.
The restatement is more fully described in Note 2 of the notes to the financial statements included herein.
Cautionary Note Regarding Forward-Looking Statements
This Annual Report on Form 10-K/A includes forward-looking statements within the meaning of Section 27A of the Securities Act and Section 21E of the Exchange Act. We have based these forward-looking statements on our current expectations and projections about future events. These forward-looking statements are subject to known and unknown risks, uncertainties and assumptions about us that may cause our actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by such forward- looking statements. In some cases, you can identify forward-looking statements by terminology such as “may,” “should,” “could,” “would,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “continue,” or the negative of such terms or other similar expressions. For information identifying important factors that could cause actual
results to differ materially from those anticipated in the forward-looking statements, please refer to the Risk Factors section of the Company’s final prospectus for its initial public offering filed with the SEC. The Company’s securities filings can be accessed on the EDGAR section of the SEC’s website at www.sec.gov. Except as expressly required by applicable securities law, the Company disclaims any intention or obligation to update or revise any forward-looking statements whether as a result of new information, future events or otherwise.
Overview
We are a blank check company incorporated on November 1, 2019 as a Delaware corporation and formed for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase, reorganization or similar business combination with one or more businesses (the “Business Combination”). We intend to focus our investment effort broadly across the entire healthcare industry, which encompasses services, therapeutics, devices, diagnostics and animal health. We intend to effectuate our initial Business Combination using cash from the proceeds of this offering and the private placement of the private placement warrants, the proceeds of the sale of our shares in connection with our initial Business Combination (pursuant to forward purchase agreements or backstop agreements we may enter into following the consummation of our initial public offering or otherwise), shares issued to the owners of the target, debt issued to bank or other lenders or the owners of the target, or a combination of the foregoing. Our sponsor is DFP Sponsor LLC, a Delaware limited liability company (the “Sponsor”).
Our registration statement for our initial public offering (the “Initial Public Offering”) was declared effective by the SEC on March 10, 2020. On March 13, 2020, we consummated our Initial Public Offering of 23,000,000 units (the “Units” and, with respect to the Class A common stock included in the Units being offered, the “Public Shares”), including 3,000,000 additional Units to cover over-allotments (the “Over-Allotment Units”), at $10.00 per Unit, generating gross proceeds of $230.0 million, and incurring offering costs of approximately $10.4 million, inclusive of approximately $6.3 million in deferred underwriting commissions.
Simultaneously with the closing of the Initial Public Offering, we consummated the private placement (“Private Placement”) of 3,733,334 warrants (each, a “Private Placement Warrant” and collectively, the “Private Placement Warrants”) at a price of $1.50 per Private Placement Warrant in a private placement to our Sponsor, generating proceeds of $5.6 million.
Upon the closing of the Initial Public Offering and the Private Placement, $230.0 million ($10.00 per Unit) of the net proceeds of the Initial Public Offering and certain of the proceeds of the Private Placement was placed in a trust account (the “Trust Account”) and was invested in permitted United States “government securities” within the meaning of Section 2(a)(16) of the Investment Company Act of 1940, as amended, which we refer to as the Investment Company Act, having a maturity of 185 days or less or in money market funds meeting certain conditions under Rule 2a-7 promulgated under the Investment Company Act that invest only in direct U.S. government treasury obligations.
Our management has broad discretion with respect to the specific application of the net proceeds of the Initial Public Offering and the sale of the Private Placement Warrants, although substantially all of the net proceeds are intended to be applied generally toward consummating a Business Combination.
We will only have 24 months from the closing of the Initial Public Offering, or March 13, 2022, to complete our initial Business Combination (the “Combination Period”). If we do not complete a Business Combination within this period of time, it will (i) cease all operations except for the purposes of winding up; (ii) as promptly as reasonably possible, but not more than ten business days thereafter, redeem the Public Shares for a per share pro rata portion of the Trust Account, including interest and not previously released to us to fund our working capital requirements (subject to an annual limit of $500,000) (less taxes payable and up to $100,000 of such net interest to pay dissolution expenses) and (iii) as promptly as possible following such redemption, liquidate and dissolve the balance of our net assets to our remaining stockholders, as part of our plan of dissolution and liquidation.
The issuance of additional shares in connection with a Business Combination to the owners of the target or other investors:
| ● | may significantly dilute the equity interest of investors in this offering, which dilution would increase if the anti-dilution provisions in the Class B common stock resulted in the issuance of Class A common stock on a greater than one-to-one basis upon conversion of the Class B common stock; |
| ● | may subordinate the rights of holders of Class A common stock if shares of preferred stock are issued with rights senior to those afforded our Class A common stock; |
| ● | could cause a change in control if a substantial number of shares of our Class A common stock are issued, which may affect, among other things, our ability to use our net operating loss carry forwards, if any, and could result in the resignation or removal of our present officers and directors; |
| ● | may have the effect of delaying or preventing a change of control of us by diluting the share ownership or voting rights of a person seeking to obtain control of us; and |
| ● | may adversely affect prevailing market prices for our units, Class A common stock and/or warrants. |
Similarly, if we issue debt securities or otherwise incur significant debt to bank or other lenders or the owners of a target, it could result in:
| ● | default and foreclosure on our assets if our operating revenues after an initial Business Combination are insufficient to repay our debt obligations; |
| ● | acceleration of our obligations to repay the indebtedness even if we make all principal and interest payments when due if we breach certain covenants that require the maintenance of certain financial ratios or reserves without a waiver or renegotiation of that covenant; |
| ● | our immediate payment of all principal and accrued interest, if any, if the debt is payable on demand; |
| ● | our inability to obtain necessary additional financing if the debt contains covenants restricting our ability to obtain such financing while the debt is outstanding; |
| ● | our inability to pay dividends on our Class A common stock; |
| ● | using a substantial portion of our cash flow to pay principal and interest on our debt, which will reduce the funds available for dividends on our Class A common stock if declared, expenses, capital expenditures, acquisitions and other general corporate purposes; |
| ● | limitations on our flexibility in planning for and reacting to changes in our business and in the industry in which we operate; |
| ● | increased vulnerability to adverse changes in general economic, industry and competitive conditions and adverse changes in government regulation; and |
| ● | limitations on our ability to borrow additional amounts for expenses, capital expenditures, acquisitions, debt service requirements, execution of our strategy and other purposes and other disadvantages compared to our competitors who have less debt. |
As indicated in the accompanying financial statements, as of December 31, 2020, we had approximately $0.9 million in our operating bank account. We expect to continue to incur significant costs in the pursuit of our acquisition plans. We cannot assure you that our plans to complete our initial Business Combination will be successful.
Results of Operations
Our entire activity from inception through December 31, 2020 related to our formation, the preparation for the Initial Public Offering, and, since the closing of the Initial Public Offering, the search for a prospective initial Business Combination. We have neither engaged in any operations nor generated any revenues to date. We will not generate any operating revenues until after completion of our initial Business Combination. We will generate non-operating income in the form of interest income on our investments held in the Trust Account. We expect to incur increased expenses as a result of being a public company (for legal, financial reporting, accounting and auditing compliance), as well as for due diligence expenses.
For the year ended December 31, 2020, we had a net loss of approximately $8.3 million, which consisted of approximately $0.3 million in general and administrative expenses, $0.2 million in administrative expenses – related party, approximately $0.2 million in franchise tax expense, approximately $11,000 in income tax expense, approximately $7.6 million in changes in fair value of derivative warrant liabilities, and approximately $0.3 million offering costs associated with derivative warrant liabilities, partly offset by approximately $0.3 million of interest income from investments in Trust Account.
For the period November 1, 2019 (inception) through December 31, 2019, our only activities had been organization activities and those necessary to prepare for the initial public offering.
As a result of the restatement described in Note 2 of the notes to the financial statements included herein, we classify the warrants issued in connection with our Initial Public Offering and Private Placement as liabilities at their fair value and adjust the warrant instruments to fair value at each reporting period. These liabilities are subject to re-measurement at each balance sheet date until exercised, and any change in fair value is recognized in our statement of operations. For the periods from January 1, 2020 through December 31, 2020, the change in fair value of warrants was an increase of approximately $7.6 million. We also recognized a charge of $0.3 million for the amount of offering costs ascribe to the derivative warrant liabilities that was offset by an increase in additional paid-in capital.
Contractual Obligations
We do not have any long-term debt obligations, capital lease obligations, operating lease obligations, purchase obligations or long-term liabilities, other than an agreement to pay our Sponsor a monthly fee of $10,000 for office space, secretarial and administrative services and an agreement to pay our Chief Financial Officer, Christopher Wolfe, $7,500 per month for his services prior to the initial Business Combination.
Registration Rights
The initial stockholders and holders of the Private Placement Warrants are entitled to registration rights pursuant to a registration rights agreement. The initial stockholders and holders of the Private Placement Warrants will be entitled to make up to three demands, excluding short form registration demands, that the Company register such securities for sale under the Securities Act. In addition, these holders will have “piggy-back” registration rights to include their securities in other registration statements filed by the Company. We will bear the expenses incurred in connection with the filing of any such registration statements.
Underwriting Agreement
We granted the underwriters a 45-day option to purchase up to 3,000,000 additional Units to cover any over-allotments, at the initial public offering price of $10.00 per Unit, less the underwriting discounts and commissions. The warrants that were be issued in connection with the 3,000,000 over-allotment Units are identical to the public warrants and have no net cash settlement provisions. The underwriters exercised the over-allotment option in full on March 13, 2020.
The underwriters did not receive any underwriting discounts or commission on the 5,000,000 Units purchased in the Initial Public Offering by certain domestic private pooled investment vehicles managed by Deerfield Management Company, L.P. We paid an underwriting discount of 2.0% of the per Unit offering price, or $3.6 million, at the closing of
the Initial Public Offering, with an additional fee (the “Deferred Underwriting Fees”) of 3.5% of the gross offering proceeds, or $6.3 million, payable upon the Company's completion of an Initial Business Combination. The Deferred Underwriting Fees will become payable to the underwriters from the amounts held in the Trust Account solely in the event we complete our initial Business Combination.
Critical Accounting Policies
Use of Estimates
This management’s discussion and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in accordance with GAAP. The preparation of our financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses and the disclosure of contingent assets and liabilities in our financial statements. On an ongoing basis, we evaluate our estimates and judgments, including those related to fair value of financial instruments and accrued expenses. We base our estimates on historical experience, known trends and events and various other factors that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
Investments Held in the Trust Account
Our portfolio of investments held in the Trust Account is comprised of U.S. government securities, within the meaning set forth in Section 2(a)(16) of the Investment Company Act, with a maturity of 185 days or less, or investments in money market funds that invest in U.S. government securities, or a combination thereof. The investments held in the Trust Account are classified as trading securities. Trading securities are presented on the balance sheets at fair value at the end of each reporting period. Gains and losses resulting from the change in fair value of these securities is included as interest income on investments in the Trust Account in the accompanying statements of operations. The estimated fair values of investments held in the Trust Account are determined using available market information.
Class A Common Stock Subject to Possible Redemption
The Company accounts for its Class A common stock subject to possible redemption in accordance with the guidance in ASC Topic 480 “Distinguishing Liabilities from Equity.” Class A common stock subject to mandatory redemption (if any) is classified as liability instruments and are measured at fair value. Conditionally redeemable Class A common stock (including Class A common stock that features redemption rights that are either within the control of the holder or subject to redemption upon the occurrence of uncertain events not solely within the Company’s control) are classified as temporary equity. At all other times, Class A common stock is classified as stockholders’ equity. The Company’s Class A common stock features certain redemption rights that are considered to be outside of the Company’s control and subject to the occurrence of uncertain future events. Accordingly, as of December 31, 2020, 23,000,000 shares of Class A common stock subject to possible redemption are presented as temporary equity, outside of the stockholders’ equity section of the Company’s balance sheets.
Under ASC 480-10-S99, we elected to recognize changes in the redemption value immediately as they occur and adjust the carrying value of the security to equal the redemption value at the end of each reporting period. This method would view the end of the reporting period as if it were also the redemption date for the security. As such, effective with the closing of the Initial Public Offering, we recognized the accretion from initial book value to redemption amount, which, resulted in charges against additional paid-in capital (to the extent available) and accumulated deficit.
Net Income (Loss) Per Share
We comply with accounting and disclosure requirements of FASB ASC Topic 260, “Earnings Per Share.” We have two classes of shares, which are referred to as Class A common stock and Class B common stock. Income and losses are shared pro rata between the two classes of shares. Net income (loss) per common share is calculated by dividing the net income (loss) by the weighted average shares of common stock outstanding for the respective period. We have not
considered the effect of the warrants sold in the Initial Public Offering and the Private Placement to purchase an aggregate of 9,483,334 of our Class A common stock in the calculation of diluted income per share, since their exercise is contingent upon future events and their inclusion would be anti-dilutive under the treasury stock method. Accretion associated with the redeemable Class A common stock is excluded from earnings per share as the redemption value approximates fair value.
Derivative Warrant Liabilities
We do not use derivative instruments to hedge exposures to cash flow, market or foreign currency risks. We evaluate all of our financial instruments, including issued stock purchase warrants, to determine if such instruments are derivatives or contain features that qualify as embedded derivatives, pursuant to ASC 480 and ASC 815-15. The classification of derivative instruments, including whether such instruments should be recorded as liabilities or as equity, is re-assessed at the end of each reporting period.
We issued 5,750,000 warrants to purchase Class A common stock to investors in our Initial Public Offering and issued 3,733,334 Private Placement Warrants. All of our outstanding warrants are recognized as derivative liabilities in accordance with ASC 815-40. Accordingly, we recognize the warrant instruments as liabilities at fair value and adjust the instruments to fair value at each reporting period. The liabilities are subject to re-measurement at each balance sheet date until exercised, and any change in fair value is recognized in our statement of operations. The fair value of warrants issued in connection with the Initial Public Offering and Private Placement were initially measured at fair value using a Monte Carlo simulation model and subsequently, the fair value of the Private Placement warrants have been estimated using a Monte Carlo simulation model each measurement date. The fair value of Warrants issued in connection with our Initial Public Offering have subsequently been measured based on the listed market price of such warrants.
Recent Accounting Pronouncements
Management does not believe that any recently issued, but not yet effective, accounting pronouncements, if currently adopted, would have a material effect on our financial statements.
Off-Balance Sheet Arrangements
As of December 31, 2020, we did not have any off-balance sheet arrangements as defined in Item 303(a)(4)(ii) of Regulation S-K.
JOBS Act
The JOBS Act contains provisions that, among other things, relax certain reporting requirements for qualifying public companies. We qualify as an “emerging growth company” and under the JOBS Act are allowed to comply with new or revised accounting pronouncements based on the effective date for private (not publicly traded) companies. We are electing to delay the adoption of new or revised accounting standards, and as a result, we may not comply with new or revised accounting standards on the relevant dates on which adoption of such standards is required for non-emerging growth companies. As a result, the financial statements may not be comparable to companies that comply with new or revised accounting pronouncements as of public company effective dates.
Additionally, we are in the process of evaluating the benefits of relying on the other reduced reporting requirements provided by the JOBS Act. Subject to certain conditions set forth in the JOBS Act, if, as an “emerging growth company,” we choose to rely on such exemptions we may not be required to, among other things, (i) provide an auditor’s attestation report on our system of internal controls over financial reporting pursuant to Section 404, (ii) provide all of the compensation disclosure that may be required of non-emerging growth public companies under the Dodd-Frank Wall Street Reform and Consumer Protection Act, (iii) comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements (auditor discussion and analysis) and (iv) disclose certain executive compensation related items such as the correlation between executive compensation and performance and comparisons of the CEO’s compensation to median employee compensation. These exemptions will
apply for a period of five years following the completion of our initial public offering or until we are no longer an “emerging growth company,” whichever is earlier.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We are a smaller reporting company as defined by Rule 12b-2 of the Exchange Act and are not required to provide the information otherwise required under this item.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA.
Please see our Financial Statements beginning on page F-1 of this Annual Report.
ITEM 9: CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE.
None.
ITEM 9A. CONTROLS AND PROCEDURES.
Evaluation of Disclosure Controls and Procedures
Disclosure controls and procedures are controls and other procedures that are designed to ensure that information required to be disclosed in our reports filed or submitted under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed in Company reports filed or submitted under the Exchange Act is accumulated and communicated to management, including our Chief Executive Officer and Chief Financial Officer, to allow timely decisions regarding required disclosure.
As required by Rules 13a-15 and 15d-15 under the Exchange Act, our Chief Executive Officer and Chief Financial Officer carried out an evaluation of the effectiveness of the design and operation of our disclosure controls and procedures as of December 31, 2020. Based upon their evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures (as defined in Rules 13a-15 (e) and 15d-15 (e) under the Exchange Act) were not effective as of December 31, 2020, because of a material weakness in our internal control over financial reporting. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the Company’s annual or interim financial statements will not be prevented or detected on a timely basis. Specifically, the Company’s management has concluded that its control around the interpretation and accounting for certain complex features of the Class A common stock and warrants issued by the Company was not effectively designed or maintained. This material weakness resulted in the restatement of the Company’s balance sheet as of March 13, 2020, its financial statements for the period ended December 31, 2020 and its interim financial statements and notes for the quarters ended March 31, 2020, June 30, 2020, September 30, 2020, March 31, 2021 June 30, 2021, and September 30, 2021. In light of this material weakness, we performed additional analysis as deemed necessary to ensure that our financial statements were prepared in accordance with U.S. generally accepted accounting principles. Accordingly, management believes that the financial statements included in this Annual Report on Form 10-K/A present fairly in all material respects our financial position, results of operations and cash flows for the period presented.
Changes in internal control over reporting
During the most recently completed fiscal quarter, there has been no change in our internal control over financial reporting that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting, as the circumstances that led to the restatement of our financial statements described in this Annual Report on Form 10-K/A had not yet been identified.
Our internal control over financial reporting did not result in the proper classification of our warrants. Since issuance on March 20, 2020, our warrants were accounted for as equity within our balance sheet. On April 12, 2021, the SEC Staff issued the SEC Staff Statement in which the SEC Staff expressed its view that certain terms and conditions common to SPAC warrants may require the warrants to be classified as liabilities on the SPAC’s balance sheet as opposed to equity. After discussion and evaluation, taking into consideration the SEC Staff Statement, we concluded that our Warrants should be presented as liabilities with subsequent fair value remeasurement as previously restated in our Amendment No. 1 to the Form 10-K/A as filed with the SEC on May 24, 2021. In addition, our management has concluded that our control around the interpretation and accounting for certain complex features of the Class A common stock issued by the Company was not effectively designed or maintained resulting in the misclassification of Class A common stock as permanent equity instead of temporary equity and changes to the Company's net income (loss) per share calculations that have been restated within this Form 10-K/A filing.
The Chief Executive Officer and Chief Financial Officer performed additional accounting and financial analyses and other post-closing procedures including consulting with subject matter experts related to the accounting for certain complex features of the Class A common stock and warrants. The Company’s management has expended, and will continue to expend, a substantial amount of effort and resources for the remediation and improvement of our internal control over financial reporting. While we have processes to properly identify and evaluate the appropriate accounting technical pronouncements and other literature for all significant or unusual transactions, we have expanded and will continue to improve these processes to ensure that the nuances of such transactions are effectively evaluated in the context of the increasingly complex accounting standards.
Management’s report on internal control over financial reporting
This annual report does not include a report of management’s assessment regarding internal control over financial reporting due to a transition period established by rules of the Securities and Exchange Commission for newly public companies. This annual report does not include an attestation report of the Company’s independent registered public accounting firm regarding internal control over financial reporting. As an emerging growth company, management’s report is not subject to attestation by our independent registered public accounting firm.
ITEM 9B. OTHER INFORMATION.
None.
PART III
ITEM 10: DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE.
Directors and Executive Officers
Our directors and executive officers are as follows:
| | | | |
Name
| | Age
| | Position
|
|
|
|
|
|
Richard Barasch
|
| 67
|
| Executive Chairman
|
Steven Hochberg
|
| 59
|
| President, Chief Executive Officer and Director
|
Christopher Wolfe
|
| 40
|
| Chief Financial Officer and Secretary
|
Dr. Jennifer Carter
|
| 56
|
| Director
|
Dr. Mohit Kaushal
|
| 40
|
| Director
|
Dr. Gregory Sorensen
|
| 57
|
| Director
|
Richard Barasch has served as the Company’s Chairman since May 2020 and served as the Chairman and Chief Executive Officer of DFB Healthcare Acquisitions Corp. (“DFB”) from its formation until the closing of its initial business combination with AdaptHealth Corp., which Mr. Barasch currently serves as Chairman. In addition, Mr. Barasch is Executive Chairman of Deerfield Healthcare Technology Acquisitions Corp. (“DFHT”). Mr. Barasch was Chief Executive
Officer of Universal American Corp., a publicly-traded health insurance and services company focused on the senior market and government programs, from 1995 until Universal American’s acquisition by WellCare Health Plans in May 2017. Mr. Barasch has developed an extensive network of contacts throughout the healthcare industry and speaks regularly at industry conferences as a healthcare services expert. He is currently founding partner of RAB Ventures, formed to invest in growth healthcare companies, Chairman of HouseWorks LLC and Co-Chairman of ELMC Risk Management Inc. He is on the Board of Advisors of the Health Policy and Management program at the Columbia University Mailman School of Public Health, where he is also an Assistant Adjunct Professor, and the Brown School of Public Health. He also serves on the Board of Trustees of the Maimonides Medical Center in Brooklyn, New York. Mr. Barasch graduated from Swarthmore College and Columbia University Law School. Mr. Barasch was selected to serve on the board of directors due to his significant experience managing and investing in healthcare companies.
Steven Hochberg has been the Company’s President and Chief Executive Officer since May 2020. A partner in the private transactions group at Deerfield Management, Deerfield Management Company, L.P., a Delaware series limited partnership (Series C) and its affiliates (“Deerfield Management”); Mr. Hochberg joined Deerfield Management in 2013 to work on structured transactions. Mr. Hochberg has been a co-founder and manager of many healthcare companies, including DFB and DFHT, and he led the merger of two New York City-based hospital systems, which created a healthcare delivery system in New York City with revenues in excess of $5 billion. Mr. Hochberg currently serves as the President and Chief Executive Officer of DFP. Mr. Hochberg has also led investments in more than 55 healthcare companies, including rollups of companies within the services and the medtech sectors. Since 2004, Mr. Hochberg has managed Ascent Biomedical Ventures, a leading venture capital firm he co-founded focused on early stage investment and development of biomedical companies. Since 2011, Mr. Hochberg had been the Chairman of the Board of Continuum Health Partners until its merger with Mount Sinai in 2013, where he is a Vice Chairman of the Icahn School of Medicine at Mt. Sinai and the Mount Sinai Health System, a non-profit healthcare integrated delivery system in New York City with over $7 billion in combined annual revenues. Mr. Hochberg serves on the board of Solar Capital and Solar Senior Capital, two publicly-traded business development companies and Solar’s private Business Development Corporation. Mr. Hochberg is also a member of the board of the Cardiovascular Research Foundation, a non-profit organization focused on advancing new technologies and education in the field of cardiovascular medicine. Mr. Hochberg graduated from the University of Michigan and earned his M.B.A. from Harvard Business School. Mr. Hochberg was selected to serve on the board of directors due to his significant experience managing and investing in healthcare companies.
Christopher Wolfe has served as the Company’s Chief Financial Officer and Secretary since May 2020. Mr. Wolfe currently serves as the Chief Financial Officer and Secretary of DFHT. Mr. Wolfe was a partner of Capital Z Partners, a middle market private equity firm, from June 2003 until December 2017. He was responsible for sourcing, structuring, execution and monitoring of private equity transactions across a variety of verticals. Mr. Wolfe served on the board of directors of Universal American Corp. from 2009 to 2014. Prior to joining Capital Z in 2003, Mr. Wolfe worked in the mergers and acquisitions group at Credit Suisse First Boston. Mr. Wolfe graduated magna cum laude from Harvard College.
Dr. Jennifer Carter has served as a director as of March 10, 2020. Dr. Carter is a board-certified internist and healthcare entrepreneur, with over 20 years of experience evaluating existing and emerging markets, new medical technologies and early-stage companies in the health care field. Dr. Carter currently serves as a managing director at Sandbox Industries ("Sandbox") and Blue Venture Fund ("Blue Venture"). Prior to Sandbox and Blue Venture, she was managing director of JLC Precision Health Strategies. Prior to this, Dr. Carter was vice president and head of Precision Health at Integral Health (now Valo Health). Dr. Carter has served on the board of directors of Houseworks, LLC, an elder-care provider, since 2019, on the board of directors of Target Cancer Foundation, a non-profit organization, since 2018 and has been a member of the Founding Strategic Board of Xsphera Biosciences, Inc., a biotechnology startup, since 2018. Dr. Carter has B.S. degrees in Biochemistry and Biophysics from Yale University. She received an M.D. from Harvard Medical School and an M.P.H. from The Harvard School of Public Health. Dr. Carter was selected to serve on the board of directors due to her significant experience in healthcare technology and strategic consulting.
Dr. Mohit Kaushal has served as a director as of March 10, 2020. He has had an extensive career within investing, clinical medicine and public policy. Dr. Kaushal has served as a special advisor to General Atlantic since 2015. He was a partner in Aberdare Ventures from 2013 to 2014. During his time in the Obama administration, he was a member of the White House Health IT task force; a cross agency team implementing the technology aspects of the ACA and
testified to Congress on the application of technology and payment reform to the Medicare population. He also built and led the first dedicated healthcare team at the Federal Communications Commission, where his team initiated collaboration with the Food and Drug Administration for the regulatory streamlining of converged telecommunications, data analytics and medical devices leading to the release of the mobile medical applications guidance by the FDA. In addition, his team reformed the Rural Healthcare fund to create the Healthcare Connect Fund, which aligned the funding mechanism with wider healthcare payment policy and technology reform. Dr. Kaushal is a lead investor, board member or advisor to numerous transformational healthcare companies. Dr. Kaushal is an emergency room physician, holds an MBA from Stanford and an MD with distinction from Imperial College of Science, Technology and Medicine, London. He is an Adjunct Professor at Stanford University with a joint position within the newly created Biomedical Data Science Department and the medical school's Clinical Excellence Research Center. Dr. Kaushal was selected to serve on the board of directors due to his significant management experience in the healthcare and technology industries.
Dr. Gregory Sorensen has served as a director as of March 10, 2020. He served as the president and CEO of Siemens Healthcare North America from June 2011 to September 2015. Prior to Siemens, he served as Professor of Radiology and Health Sciences & Technology at Harvard Medical School; a faculty member of the Harvard-MIT Division of Health Sciences and Technology; and co-director of the A.A. Martinos Center for Biomedical Imaging at Massachusetts General Hospital, as well as a visiting Professor of Neuroradiology at Oxford University. Leading up to his appointment with Siemens, Dr. Sorensen was a practicing Neuroradiologist and active researcher with significant experience in clinical care, clinical trials, and translational research. His research and techniques are utilized by numerous centers throughout the world in phase II and III trials in cancer, stroke, and other illnesses. He holds a B.S. in biology from California Institute of Technology, Pasadena, CA, a M.S. in computer science from Brigham Young University, Provo, Utah, and a medical degree from Harvard Medical School, Boston, Massachusetts. Dr. Sorensen has served as the Executive Chairman of the Board of Directors for IMRIS, Inc., the leader in image-guided therapy solutions, the President and Chief Executive Officer of DeepHealth, Inc. since April 2017, the Chairman of Fusion Healthcare Staffing, LLC, and a member of the board of directors of Inviero LLC since December 2017. Dr. Sorensen was selected to serve on the board of directors due to his significant management experience in the healthcare and technology industries.
Number and Terms of Office of Officers and Directors
Our Board consists of five members is divided into three classes with only one class of directors being elected in each year, and with each class (except for those directors appointed prior to its first annual meeting of stockholders) serving a three-year term. In accordance with Nasdaq corporate governance requirements, the Company is not required to hold an annual meeting until one year after its first fiscal year end following its listing on Nasdaq. The term of office of the first class of directors, consisting of Drs. Kaushal and Sorensen, will expire at the first annual meeting of stockholders. The term of office of the second class of directors, consisting of Messrs. Barasch and Hochberg, will expire at the second annual meeting of stockholders. The term of office of the third class of directors, consisting of Dr. Carter, will expire at the third annual meeting of stockholders.
The officers are appointed by the Board and serve at the discretion of the Board, rather than for specific terms of office. The Board is authorized to appoint officers as it deems appropriate pursuant to our second amended and restated certificate of incorporation.
Director Independence
Nasdaq listing standards require that a majority of our board of directors be independent. An “independent director” is defined generally as a person other than an officer or employee of the company or its subsidiaries or any other individual having a relationship which in the opinion of the company’s board of directors, would interfere with the director’s exercise of independent judgment in carrying out the responsibilities of a director. Our board of directors has determined that Drs. Carter, Kaushal and Sorensen are “independent directors” as defined in the Nasdaq listing standards and applicable SEC rules. Our independent directors will have regularly scheduled meetings at which only independent directors are present.
Board Committees
Audit Committee
We have an audit committee comprised of Drs. Sorensen, Kaushal and Carter, each of whom are independent under the Nasdaq listing standards and applicable SEC rules.
Dr. Sorensen serves as the Chairman of the audit committee. Each member of the audit committee is financially literate and our board of directors has determined that Dr. Sorensen qualifies as an “audit committee financial expert” as defined in applicable SEC rules.
The audit committee is responsible for:
| ● | meeting with our independent registered public accounting firm regarding, among other issues, audits, and adequacy of our accounting and control systems; |
| ● | monitoring the independence of the independent registered public accounting firm; |
| ● | verifying the rotation of the lead (or coordinating) audit partner having primary responsibility for the audit and the audit partner responsible for reviewing the audit as required by law; |
| ● | inquiring and discussing with management our compliance with applicable laws and regulations; |
| ● | pre-approving all audit services and permitted non-audit services to be performed by our independent registered public accounting firm, including the fees and terms of the services to be performed; |
| ● | appointing or replacing the independent registered public accounting firm; |
| ● | determining the compensation and oversight of the work of the independent registered public accounting firm (including resolution of disagreements between management and the independent registered public accounting firm regarding financial reporting) for the purpose of preparing or issuing an audit report or related work; |
| ● | establishing procedures for the receipt, retention and treatment of complaints received by us regarding accounting, internal accounting controls or reports which raise material issues regarding our financial statements or accounting policies; |
| ● | monitoring compliance on a quarterly basis with the terms of the initial public offering and, if any noncompliance is identified, immediately taking all action necessary to rectify such noncompliance or otherwise causing compliance with the terms of the initial public offering; and |
| ● | reviewing and approving all payments made to our existing holders, executive officers or directors and their respective affiliates. Any payments made to members of our audit committee will be reviewed and approved by our board of directors, with the interested director or directors abstaining from such review and approval. |
Compensation Committee
We have a compensation committee comprised of Drs. Kaushal, Sorensen and Carter, and Dr. Kaushal serves as chairman of the compensation committee. We have adopted a compensation committee charter, which details the principal functions of the compensation committee, including:
| ● | reviewing and approving on an annual basis the corporate goals and objectives relevant to our chief executive officer’s compensation, evaluating our chief executive officer’s performance in light of such goals and |
| | objectives and determining and approving the remuneration (if any) of our chief executive officer’s based on such evaluation; |
| ● | reviewing and approving the compensation of all of our other Section 16 executive officers; |
| ● | reviewing our executive compensation policies and plans; |
| ● | implementing and administering our incentive compensation equity-based remuneration plans; |
| ● | assisting management in complying with our proxy statement and annual report disclosure requirements; |
| ● | approving all special perquisites, special cash payments and other special compensation and benefit arrangements for our executive officers and employees; |
| ● | producing a report on executive compensation to be included in our annual proxy statement; and |
| ● | reviewing, evaluating and recommending changes, if appropriate, to the remuneration for directors. |
The charter also provides that the compensation committee may, in its sole discretion, retain or obtain the advice of a compensation consultant, legal counsel or other adviser and will be directly responsible for the appointment, compensation and oversight of the work of any such adviser. However, before engaging or receiving advice from a compensation consultant, external legal counsel or any other adviser, the compensation committee will consider the independence of each such adviser, including the factors required by Nasdaq and the SEC.
Director Nominations
We do not have a standing nominating committee though we intend to form a corporate governance and nominating committee as and when required to do so by law or Nasdaq rules. In accordance with Rule 5605(e)(2) of the Nasdaq Rules, a majority of the independent directors may recommend a director nominee for selection by our board of directors. Our board of directors believes that the independent directors can satisfactorily carry out the responsibility of properly selecting or approving director nominees without the formation of a standing nominating committee. The directors who shall participate in the consideration and recommendation of director nominees are Drs. Carter, Kaushal and Sorensen. In accordance with Rule 5605(e)(1)(A) of the Nasdaq Rules, all such directors are independent. As there is no standing nominating committee, we do not have a nominating committee charter in place.
The board of directors will also consider director candidates recommended for nomination by our stockholders during such times as they are seeking proposed nominees to stand for election at the next annual meeting of stockholders (or, if applicable, a special meeting of stockholders). Our stockholders that wish to nominate a director for election to our board of directors should follow the procedures set forth in our bylaws.
We have not formally established any specific, minimum qualifications that must be met or skills that are necessary for directors to possess. In general, in identifying and evaluating nominees for director, our board of directors considers educational background, diversity of professional experience, knowledge of our business, integrity, professional reputation, independence, wisdom, and the ability to represent the best interests of our stockholders.
Code of Ethics and Committee Charters
We have adopted a code of ethics that applies to our directors, officers and employees (“Code of Ethics”). We have filed a copy of our Code of Ethics and our audit committee and compensation committee charters as exhibits to our registration statement in connection with the initial public offering. You may review these documents by accessing our public filings at the SEC’s web site at www.sec.gov. In addition, a copy of the Code of Ethics will be provided without charge upon request from us in writing at 345 Park Avenue South, New York, New York 10010 or by telephone at (212)
551-1600. We intend to disclose any amendments to or waivers of certain provisions of our Code of Ethics in a Current Report on Form 8-K.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act requires our officers, directors and persons who own more than ten percent of a registered class of our equity securities to file reports of ownership and changes in ownership with the SEC. Officers, directors and ten percent stockholders are required by regulation to furnish us with copies of all Section 16(a) forms they file. Based solely on review of the copies of such forms furnished to us, or written representations that no Forms 5 were required, we believe that, during the fiscal year ended December 31, 2020, all Section 16(a) filing requirements applicable to our officers and directors were complied with.
ITEM 11: EXECUTIVE COMPENSATION.
None of the Company’s executive officers or directors have received any cash compensation for services rendered to the Company. From March 13, 2020 through the earlier of consummation of the Company’s initial business combination and its liquidation, the Company will pay (i) its Chief Financial Officer $7,500 per month for his services and (ii) the sponsor $10,000 per month for office space, secretarial and administrative services provided to members of its management team. In addition, the sponsor, the Company’s executive officers and directors, or any of their respective affiliates will be reimbursed for any out-of-pocket expenses incurred in connection with activities on the Company’s behalf such as identifying potential target businesses and performing due diligence on suitable business combinations. The Company’s audit committee will review on a quarterly basis all payments that were made to the sponsor, the Company’s executive officers or directors, or the Company’s or their affiliates. Any such payments prior to an initial business combination will be made from funds held outside the Trust Account. Other than quarterly audit committee review of such reimbursements, the Company does not expect to have any additional controls in place governing the Company’s reimbursement payments to its directors and executive officers for their out-of-pocket expenses incurred in connection with the Company’s activities on the Company’s behalf in connection with identifying and consummating an initial business combination. Other than these payments and reimbursements, no compensation of any kind, including finder’s and consulting fees, will be paid by the company to the sponsor, the Company’s executive officers and directors, or any of their respective affiliates, prior to completion of its initial business combination.
After the completion of our initial business combination, directors or members of our management team who remain with the combined company may be paid consulting or management fees from the combined company. All of these fees will be fully disclosed to stockholders, to the extent then known, in the proxy solicitation materials or tender offer materials furnished to our stockholders in connection with a proposed business combination. We have not established any limit on the amount of such fees that may be paid by the combined company to our directors or members of management. It is unlikely the amount of such compensation will be known at the time of the proposed business combination, because the directors of the post-combination business will be responsible for determining executive officer and director compensation. Any compensation to be paid to our executive officers will be determined, or recommended to the board of directors for determination, either by a compensation committee constituted solely by independent directors or by a majority of the independent directors on our board of directors.
We do not intend to take any action to ensure that members of our management team maintain their positions with us after the consummation of our initial business combination, although it is possible that some or all of our executive officers and directors may negotiate employment or consulting arrangements to remain with us after our initial business combination. The existence or terms of any such employment or consulting arrangements to retain their positions with us may influence our management’s motivation in identifying or selecting a target business but we do not believe that the ability of our management to remain with us after the consummation of our initial business combination will be a determining factor in our decision to proceed with any potential business combination. We are not party to any agreements with our executive officers and directors that provide for benefits upon termination of employment.
ITEM 12: SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS.
The following table sets forth information regarding the beneficial ownership of our common stock as of March 1, 2021, by:
| ● | each person known by us to be the beneficial owner of more than 5% of our outstanding shares of common stock; |
| ● | each of our executive officers, directors and director nominees; and |
| ● | all our executive officers and directors as a group. |
We have no compensation plans under which equity securities are authorized for issuance.
The beneficial ownership of our common stock is based on 29,500,000 shares of our common stock issued and outstanding as of March 1, 2021, of which 23,000,000 shares were Class A common stock and 5,750,000 were shares of Class B common stock.
Unless otherwise indicated, the Company believes that all persons named in the table have sole voting and investment power with respect to all shares of Class A common stock beneficially owned by them.
| | | | | |
| | Number of Shares | | % of | |
| | Beneficially | | Outstanding Shares | |
Name and Address of Beneficial Owner(1) | | Owned(2) | | of the Company | |
Directors and Executive Officers of the Company: | | | | | |
Richard Barasch(4) | | 100,000 | | * | |
Steven Hochberg(3) (4) | | 5,460,000 | | 18.8 | % |
Christopher Wolfe(4) | | 100,000 | | * | |
Dr. Jennifer Carter | | 30,000 | | * | |
Dr. Mohit Kaushal | | 30,000 | | * | |
Dr. Gregory Sorensen | | 30,000 | | * | |
Five Percent Holders: | | | | | |
Entities affiliated with Deerfield Management Company, L.P.(3) | | 10,460,000 | | 36.38 | % |
DFP Sponsor LLC(3)(4) | | 5,360,000 | | 18.64 | % |
Millennium Management LLC(5). | | 1,684,200 | | 7.3 | % |
Davidson Kempner Capital management LP(6) | | 1,440,048 | | 6.26 | % |
Park West Asset Management LLC(7) | | 1,317,263 | | 5.7 | % |
Park West Investors Master Fund, Limited(7) | | 1,199,515 | | 5.2 | % |
* Less than one percent
(1) | Unless otherwise indicated, the business address of each of the individuals and entities is 345 Park Avenue South, New York, New York 10010. |
(2) | Interests of our directors and executive officers consist solely of founder shares, classified as Class B common stock. Such shares will automatically convert into Class A common stock concurrently with or immediately following the closing of our initial business combination on a one-for-one basis, subject to adjustment, as described elsewhere in this Report. |
(3) | Shares held by Deerfield Management Company, L.P. consists of: (i) 2,500,000 units held by Deerfield Private Design Fund IV, (ii) 2,500,000 shares of Class A common stock included within 2,500,000 units held by Deerfield Partners; (iii) 5,360,000 shares of Class B common stock held by DFP Sponsor LLC that are convertible into shares of Class A common stock and (iv) 100,000 shares of Class B common stock held by Mr. Hochberg, for the benefit, and at the direction, of Deerfield Management. |
(4) | Shares held by DFP Sponsor LLC consists of: 5,360,000 shares of Class B common stock held by DFP Sponsor LLC that are convertible into shares of Class A common stock. Richard Barasch, through an investment vehicle, and Christopher Wolfe are among the members of DFP Sponsor LLC and may be entitled to distributions of securities held by DFP Sponsor LLC. Mr. Hochberg is among the managers of DFP Sponsor LLC and will be deemed to beneficially own the securities held by DFP Sponsor LLC. |
(5) | According to a Schedule 13G filed on March 16, 2020 and a Schedule 13G/A filed on February 1, 2021, Millennium Management LLC, Millennium Group Management LLC and Israel A. Englander have beneficial ownership of 1,684,200 shares of Class A common stock. Their business address is c/o Millennium Management LLC, 666 Fifth Avenue, New York, New York 10103. |
(6) | According to a Schedule 13G filed on February 11, 2021, Davidson Kempner Capital Management LP and Anthony A. Yoseloff have beneficial ownership of 1,440,048 shares of Class A common stock. Their business address is c/o Davidson Kempner Capital Management LP, 520 Madison Avenue, 30th Floor, New York, New York 10022. |
(7) | According to a Schedule 13G filed on March 12, 2021, Park West Asset Management LLC (“PWAM”) and Peter S. Park have beneficial ownership of 1,317,263 shares of Class A common stock. Park West Investors Master Fund, Limited (“PWIMF”) has beneficial ownership of 1,199,515 shares of Class A common stock. PWAM is the investment manager to PWIMF and Park West Partners International, Limited (“PWPI,” and collectively with PWIMF, the “PW Funds”). Mr. Park, through one or more affiliated entities, is the controlling manager of PWAM. Their business address is 780 Third Avenue, 37th Floor, New York, New York 10017. |
ITEM 13: CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE.
Founder Shares
On December 30, 2019, our sponsor purchased an aggregate of 4,312,500 shares of Class B common stock (the “founder shares”) in exchange for a capital contribution of $25,000, or approximately $0.006 per share. In January 2020, our sponsor transferred 100,000 founder shares to each of Mr. Hochberg, Mr. Wolfe, and Mr. Barasch, our executive officers, and 30,000 founder shares to each of Dr. Carter, Dr. Kaushal and Dr. Sorensen, our independent directors, for the same per-share price initially paid by our sponsor, resulting in our sponsor holding 3,922,500 founder shares. On February 19, 2020, we effected a 1:1 1/3 stock split of Class B common stock resulting in our sponsor holding an aggregate of 5,360,000 founder shares, resulting in an increase in the total number of founder shares from 4,312,500 to 5,750,000. The number of founder shares outstanding was determined so that such founder shares would represent 20% of the outstanding shares after our IPO.
Our initial stockholders agreed, subject to limited exceptions, not to transfer, assign or sell any of their founder shares until the earlier to occur of: (A) one year after the completion of the initial business combination or (B) subsequent to the initial business combination, (x) if the closing price of the our common stock equals or exceeds $12.00 per share (as adjusted for stock splits, stock dividends, reorganizations, recapitalizations and the like) for any 20 trading days within any 30-trading day period commencing at least 150 days after the initial business combination, or (y) the date on which we complete a liquidation, merger, capital stock exchange or other similar transaction that results in all of our stockholders having the right to exchange their shares of our common stock for cash, securities or other property.
Private Placement Warrants
Concurrently with the closing of our IPO, our sponsor purchased an aggregate of 3,733,334 private placement warrants at a price of $1.50 per private placement warrant, generating gross proceeds to the Company of $5,600,000. Each private placement warrant is exercisable for one share of our common stock at a price of $11.50 per share. The proceeds from the private placement warrants were added to the proceeds from our IPO held in the Trust Account. If we do not complete the Business Combination or another business combination by March 13, 2022, the private placement warrants will expire worthless. The private placement warrants are non-redeemable and exercisable on a cashless basis so long as they are held by our sponsor or its permitted transferees. Our initial stockholders have agreed, subject to limited exceptions, not to transfer, assign or sell any of their private placement warrants until 30 days after the completion of our initial business combination.
Administrative Services Agreement
Commencing on the date that our securities were first listed on Nasdaq, we agreed to pay the sponsor $10,000 per month for office space, secretarial and administrative services provided to members of our management team. Upon completion of the initial Business Combination or our liquidation, we will cease paying such monthly fees.
Wolfe Strategic Services Agreement
Commencing on the date that our securities were first listed on Nasdaq, we agreed to pay our Chief Financial Officer, Christopher Wolfe, $7,500 per month for his services prior to the initial Business Combination.
Registration Rights
The initial stockholders and holders of the private placement warrants are entitled registration rights pursuant to a registration rights agreement entered into on March 13, 2020. The initial stockholders and holders of the private placement warrants are entitled to make up to three demands, excluding short form registration demands, that we register such securities for sale under the Securities Act. In addition, these holders will have “piggy-back” registration rights to include their securities in other registration statements filed by us. We will bear the expenses incurred in connection with the filing of any such registration statements. In connection with the signing of the Business Combination Agreement, we entered into the Amended and Restated Registration Rights Agreement, which amended and restated in its entirety the existing registration rights agreement described above if the initial business combination is consummated.
Underwriting Agreement
We granted the underwriters a 45-day option to purchase up to 3,000,000 additional units to cover any over-allotments, at the initial public offering price less the underwriting discounts and commissions. The warrants that were issued in connection with the 3,000,000 over-allotment units are identical to the public warrants and have no net cash settlement provisions. The underwriters exercised the over-allotment option in full on March 13, 2020.
We paid an underwriting discount of 2.0% of the per unit offering price, or $3.6 million in the aggregate at the closing of the IPO, and agreed to pay an additional fee (the “Deferred Underwriting Fees”) of 3.5% of the gross offering proceeds, or $6.3 million in the aggregate upon the Company’s completion of an initial business combination. The Deferred Underwriting Fees will become payable to the underwriters from the amounts held in the Trust Account solely in the event the Company completes its initial business combination.
ITEM 14: PRINCIPAL ACCOUNTING FEES AND SERVICES.
The firm of WithumSmith+Brown, PC, or Withum, acts as our independent registered public accounting firm. The following is a summary of fees paid to Withum for services rendered.
Audit Fees. Audit fees consist of fees incurred for professional services rendered for the audit of our year-end financial statements, quarterly reviews and services that are normally provided by Withum in connection with regulatory
filings. The aggregate fees incurred by WithumSmith+Brown, PC for audit fees, inclusive of required filings with the SEC for the period from November 1, 2019 (inception) to December 31, 2020 and of services rendered in connection with our initial public offering, totaled $116,295.
Audit-Related Fees. Audit-related fees consist of fees billed for assurance and related services that are reasonably related to performance of the audit or review of our year-end financial statements and are not reported under “Audit Fees.” These services include attest services that are not required by statute or regulation and consultation concerning financial accounting and reporting standards. During the period from November 1, 2019 (inception) to December 31, 2020, WithumSmith+Brown, PC did not render any such services.
Tax Fees. Tax fees consist of fees billed for professional services relating to tax compliance, tax planning and tax advice. During the period from November 1, 2019 (inception) to December 31, 2020, an aggregate of $3,090 fees was billed to us by WithumSmith+Brown, PC for tax compliance.
All Other Fees. All other fees consist of fees billed for all other services. During the period from November 1, 2019 (inception) to December 31, 2020, WithumSmith+Brown, PC did not render any such services.
PART IV
ITEM 15: EXHIBITS, FINANCIAL STATEMENT SCHEDULES.
(a)The following documents are filed as part of this report:
(1)Financial Statements
Reference is made to the Index to Financial Statements of the Company under Item 8 of Part II above.
(2)Financial Statement Schedule
All financial statement schedules are omitted because they are not applicable or the amounts are immaterial, not required, or the required information is presented in the financial statements and notes thereto in Item 8 of Part II above.
(3)Exhibits
We hereby file as part of this report the exhibits listed in the attached Exhibit Index.
4.3
|
| Specimen Warrant Certificate (Incorporated by reference to the corresponding exhibit to the Company’s Registration Statement on Form S-1 (File No. 333-236578), filed with the SEC on March 5, 2020).
|
|
|
|
4.4
|
| Warrant Agreement, dated March 10, 2020, by and between the Company and Continental Stock Transfer & Trust Company, as warrant agent (Incorporated by reference to Exhibit 4.1 the Company’s Current Report on Form 8-K (File No. 001-39248), filed with the SEC on March 13, 2020).
|
|
|
|
4.5
|
| Description of Securities.
|
|
|
|
10.1
|
| Letter Agreement, dated March 10, 2020, by and among the Company, its executive officers, its directors and DFP Sponsor LLC (Incorporated by reference to the corresponding exhibit to the Company’s Current Report on Form 8-K (File No. 001-39248), filed with the SEC on March 13, 2020).
|
|
|
|
10.2
|
| Letter Agreement, dated August 7, 2020, by and between the Company and Deerfield Private Design Fund IV, L.P. (Incorporated by reference to the corresponding exhibit to the Company’s Current Report on Form 10-Q (File No. 001-39248), filed with the SEC on August 13, 2020).
|
|
|
|
10.3
|
| Investment Management Trust Agreement, dated March 10, 2020, by and between the Company and Continental Stock Transfer & Trust Company, as trustee (Incorporated by reference to the corresponding exhibit to the Company’s Current Report on Form 8-K (File No. 001-39248), filed with the SEC on March 13, 2020).
|
|
|
|
10.4
|
| Registration Rights Agreement, dated March 10, 2020, by and among the Company, DFP Sponsor LLC and the other holders party thereto (Incorporated by reference to the corresponding exhibit to the Company’s Current Report on Form 8-K (File No. 001- 39248), filed with the SEC on March 13, 2020).
|
|
|
|
10.5
|
| Private Placement Warrants Purchase Agreement, dated March 10, 2020, by and among the Company and DFP Sponsor LLC (Incorporated by reference to the corresponding exhibit to the Company’s Current Report on Form 8-K (File No. 001-39248), filed with the SEC on March 13, 2020).
|
|
|
|
10.6
|
| Administrative Services Agreement, dated March 10, 2020, by and between the Company and DFP Sponsor LLC (Incorporated by reference to the corresponding exhibit to the Company’s Current Report on Form 8-K (File No. 001-39248), filed with the SEC on March 13, 2020).
|
|
|
|
10.7
|
| Strategic Services Agreement, dated March 10, 2020, by and between the Company and Christopher Wolfe (Incorporated by reference to the corresponding exhibit to the Company’s Current Report on Form 8-K (File No. 001-39248), filed with the SEC on March 13, 2020).
|
|
|
|
10.8
|
| Promissory Note issued to DFP Sponsor LLC (Incorporated by reference to Exhibit 10.6 to the Company’s Registration Statement on Form S-1 (File No. 333-236578), filed with the SEC on March 5, 2020).
|
|
|
|
10.9
|
| Securities Subscription Agreement, dated as of December 30, 2019, by and between the Company and DFHTA Sponsor Inc. (Incorporated by reference to Exhibit 10.7 to the Company’s Registration Statement on Form S-1 (File No. 333-236578), filed with the SEC on March 5, 2020).
|
|
|
|
10.10
|
| Form of Indemnity Agreement (Incorporated by reference to Exhibit 10.5 to the Company’s Registration Statement on Form S-1 (File No. 333-236578), filed with the SEC on March 5, 2020).
|
|
|
|
31.1 *
|
| Certification of the Chief Executive Officer pursuant to Exchange Act Rules 13a-14(a) and 15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
|
|
|
|
THE ONCOLOGY INSTITUTE, INC
(FORMERLY KNOWN AS DFP HEALTHCARE ACQUISITIONS CORP.)
INDEX TO FINANCIAL STATEMENTS
| |
| Page
|
Financial Statements
| |
| |
Report of Independent Registered Public Accounting Firm
| F-1
|
| |
Balance Sheets as of December 31, 2020 (As Restated) and 2019
| F-2
|
| |
Statements of Operations for the year ended December 31, 2020 (As Restated) and for the period from November 1, 2019 (inception) through December 31, 2019
| F-3
|
| |
Statements of Changes in Stockholders’ Equity for the year ended December 31, 2020 (As Restated) and for the period from November 1, 2019 (inception) through December 31, 2019
| F-4
|
| |
Statements of Cash Flows for the year ended December 31, 2020 (As Restated) and for the period from November 1, 2019 (inception) through December 31, 2019
| F-5
|
| |
Notes to Financial Statements (As Restated)
| F-6
|
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and the Board of Directors of The Oncology Institute, Inc. (formerly known as DFP Healthcare Acquisitions Corp.)
Opinion on the Financial Statements
We have audited the accompanying balance sheets of The Oncology Institute, Inc. (the “Company”) as of December 31, 2020 and 2019, the related statements of operations, changes in stockholders’ equity and cash flows for the year ended December 31, 2020 and for the period from November 1, 2019 (inception) through December 31, 2019, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2020 and 2019, and the results of its operations and its cash flows for the year ended December 31, 2020 and for the period from November 1, 2019 (inception) through December 31, 2019, in conformity with accounting principles generally accepted in the United States of America.
Restatement of Financial Statements
As discussed in Note 2 to the financial statements, the 2020 financial statements have been restated to correct certain misstatements.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ WithumSmith+Brown, PC
We have served as the Company's auditor since 2020.
New York, New York
May 21, 2021, except for the effects of the restatement and subsequent event disclosed in Note 2 and 12, respectively, as to which the date is December 13, 2021
The Oncology Institute, Inc.
(formerly known as DFP Healthcare Acquisitions Corp.)
BALANCE SHEETS
| | | | | | |
| | December 31, 2020 | | December 31, 2019 |
| | (As Restated - See Note 2) | | | |
Assets: | | | | | | |
Current assets: | | | | | | |
Cash | | $ | 916,987 | | $ | 25,000 |
Prepaid expenses | | | 152,474 | | | — |
Total current assets | | | 1,069,461 | | | 25,000 |
Cash and investments held in Trust Account | | | 230,254,149 | | | — |
Deferred offering costs associated with initial public offering | | | 0 | | | 25,000 |
Total assets | | $ | 231,323,610 | | $ | 50,000 |
| | | | | | |
Liabilities, Class A Common Stock Subject to Possible Redemption and Stockholders’ Equity (Deficit): | | | | | | |
Current liabilities: | | | | | | |
Accrued expenses | | $ | 50,000 | | $ | 26,500 |
Accrued expenses - related parties | | | 17,500 | | | — |
Franchise tax payable | | | 200,050 | | | 800 |
Total current liabilities | | | 267,550 | | | 27,300 |
Deferred underwriting commissions | | | 6,300,000 | | | — |
Derivative warrant liabilities | | | 18,791,170 | | | — |
Total liabilities | | | 25,358,720 | | | 27,300 |
| | | | | | |
Commitments and Contingencies | | | | | | |
Class A common stock subject to possible redemption, $0.0001 par value; 23,000,000 and -0- shares at $10.00 per share redemption value as of December 31, 2020 and December 31, 2019, respectively | | | 230,000,000 | | | — |
| | | | | | |
Stockholders’ Equity (Deficit): | | | | | | |
Preferred stock, $0.0001 par value; 1,000,000 shares authorized; no shares issued or outstanding | | | — | | | — |
Class A common stock, $0.0001 par value; 100,000,000 shares authorized; 0 non-redeemable shares issued or outstanding | | | 0 | | | — |
Class B common stock, $0.0001 par value; 10,000,000 shares authorized; 5,750,000 shares issued and outstanding as of December 31, 2020 and December 31, 2019 (1) | | | 575 | | | 575 |
Additional paid-in capital | | | 0 | | | 24,425 |
Accumulated deficit | | | (24,035,685) | | | (2,300) |
Total stockholders’ equity (deficit) | | | (24,035,110) | | | 22,700 |
Total liabilities, Class A common stock subject to possible redemption and stockholders’ equity (deficit) | | $ | 231,323,610 | | $ | 50,000 |
(1) | As of December 31, 2019, this number includes up to 750,000 shares of Class B common stock subject to forfeiture if the over-allotment option is not exercised in full or in part by the underwriters. On March 13, 2020, the underwriter exercised its over-allotment option in full; thus, the Founder Shares were no longer subject to forfeiture. |
The accompanying notes are an integral part of these financial statements.
The Oncology Institute, Inc.
(formerly known as DFP Healthcare Acquisitions Corp.)
STATEMENTS OF OPERATIONS
| | | | | | |
| | | | | For the period from |
| | For the period from | | November 1, 2019 |
| | Year ended | | (inception) through |
| | December 31, 2020 | | December 31, 2019 |
| | (As Restated - See Note 2) | | | |
General and administrative expenses | | $ | 309,169 | | $ | 1,500 |
General and administrative expenses - related party | | | 175,000 | | | 0 |
Franchise tax expense | | | 199,700 | | | 800 |
Loss from operations | | | (683,869) | | | (2,300) |
Interest income from investments in Trust Account | | | 254,149 | | | 0 |
Change in fair value of derivative warrant liabilities | | | (7,583,670) | | | 0 |
Offering costs associated with derivative warrant liabilities | | | (315,080) | | | 0 |
Income/(loss) before income tax expense | | | (8,328,470) | | | (2,300) |
Income tax expense | | | 11,434 | | | 0 |
Net loss | | $ | (8,339,904) | | $ | (2,300) |
| | | | | | |
Weighted average shares outstanding of Class A common stock | | | 18,475,410 | | | 0 |
Basic and diluted net loss per share, Class A | | $ | (0.35) | | $ | 0 |
Weighted average shares outstanding of Class B common stock | | | 5,602,459 | | | 5,000,000 |
Basic and diluted net loss per share, Class B | | $ | (0.35) | | $ | (0.00) |
The accompanying notes are an integral part of these financial statements.
The Oncology Institute, Inc.
(formerly known as DFP Healthcare Acquisitions Corp.)
STATEMENTS OF CHANGE IN STOCKHOLDER’S EQUITY (DEFICIT)
| | | | | | | | | | | | | | | | | | | |
| | For the period from November 1, 2019 (inception) through December 31, 2019 |
| | Common Stock | | Additional | | | | | Total |
| | Class A | | Class B | | Paid-In | | Accumulated | | Stockholders' |
| | Shares | | Amount | | Shares | | Amount | | Capital | | Deficit | | Equity |
Balance - November 1, 2019 (inception) | | 0 | | $ | 0 | | 0 | | $ | 0 | | $ | 0 | | $ | 0 | | $ | 0 |
Issuance of Class B common stock to Sponsor (1)(2) | | — | | | — | | 5,750,000 | | | 575 | | | 24,425 | | | — | | | 25,000 |
Net loss | | — | | | — | | — | | | — | | | — | | | (2,300) | | | (2,300) |
Balance - December 31, 2019 | | — | | $ | — | | 5,750,000 | | $ | 575 | | $ | 24,425 | | $ | (2,300) | | $ | 22,700 |
(1)This number excludes an aggregate of up to 750,000 Class B common stock subject to forfeiture if the over-allotment option is not exercised in full or in part by the underwriters.
(2)The share amounts have been retroactively restated to reflect the split of Class B common stock on February 19, 2020, resulting in 5,750,000 shares outstanding (see Note 4).
| | | | | | | | | | | | | | | | | | | |
| | For the Year Ended December 31, 2020 (As Restated - See Note 2) |
| | Common Stock | | Additional | | | | | Total |
| | Class A | | Class B | | Paid-In | | Accumulated | | Stockholders’ |
| | Shares | | Amount | | Shares | | Amount | | Capital | | Deficit | | Equity (Deficit) |
Balance - December 31, 2019 | | — | | $ | — | | 5,750,000 | | $ | 575 | | $ | 24,425 | | $ | (2,300) | | $ | 22,700 |
Sale of private placement warrants to Sponsor in private placement, less allocation to derivative warrant liabilities | | — | | | — | | — | | | — | | | 1,120,000 | | | — | | | 1,120,000 |
Accretion of Class A common stock to redemption amount (restated – See Note 2) | | — | | | — | | — | | | — | | | (1,144,425) | | | (15,693,481) | | | (16,837,906) |
Net loss | | — | | | — | | — | | | — | | | — | | | (8,339,904) | | | (8,339,904) |
Balance - December 31, 2020 | | — | | $ | — | | 5,750,000 | | $ | 575 | | $ | — | | $ | (24,035,685) | | $ | (24,035,110) |
The accompanying notes are an integral part of these financial statements.
The Oncology Institute, Inc.
(formerly known as DFP Healthcare Acquisitions Corp.)
CONDENSED STATEMENTS OF CASH FLOWS
| | | | | | |
| | | | For the period from |
| | | | | November 1, 2019 |
| | For the Year Ended | | (inception) through |
| | December 31, 2020 | | December 31, 2019 |
| | (As Restated - See Note 2) | | | |
Cash Flows from Operating Activities: | | | | | | |
Net loss | | $ | (8,339,904) | | $ | (2,300) |
Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | |
Interest earned on investments held in Trust Account | | | (254,149) | | | 0 |
Offering costs associated with derivative warrant liabilities | | | 315,080 | | | 0 |
Change in fair value of derivative warrant liabilities | | | 7,583,670 | | | 0 |
Changes in operating assets and liabilities: | | | | | | |
Prepaid expenses | | | (152,474) | | | 0 |
Accrued expenses | | | 48,500 | | | 1,500 |
Accrued expenses - related parties | | | 17,500 | | | 0 |
Franchise tax payable | | | 199,250 | | | 800 |
Net cash used in operating activities | | | (582,527) | | | 0 |
| | | | | | |
Cash Flows from Investing Activities | | | | | | |
Cash deposited in Trust Account | | | (230,000,000) | | | 0 |
Net cash used in investing activities | | | (230,000,000) | | | 0 |
| | | | | | |
Cash Flows from Financing Activities: | | | | | | |
Proceeds from issuance of Class B common stock to Sponsor | | | — | | | 25,000 |
Proceeds received from note payable to related party | | | 200,000 | | | 0 |
Repayment of note payable to related party | | | (200,000) | | | 0 |
Proceeds received from initial public offering, gross | | | 230,000,000 | | | 0 |
Proceeds received from private placement | | | 5,600,000 | | | 0 |
Offering costs paid | | | (4,125,486) | | | 0 |
Net cash provided by financing activities | | | 231,474,514 | | | 25,000 |
| | | | | | |
Net increase in cash | | | 891,987 | | | 25,000 |
| | | | | | |
Cash - beginning of the period | | | 25,000 | | | 0 |
Cash - end of the period | | $ | 916,987 | | $ | 25,000 |
| | | | | | |
Supplemental disclosure of noncash activities: | | | | | | |
Offering costs included in accrued expenses | | $ | — | | $ | 25,000 |
Deferred underwriting commissions in connection with the initial public offering | | $ | 6,300,000 | | $ | 0 |
The accompanying notes are an integral part of these financial statements.
THE ONCOLOGY INSTITUTE, INC.
(formerly known as DFP Healthcare Acquisitions Corp.)
NOTES TO FINANCIAL STATEMENTS – AS RESTATED
Note 1 — Organization, Business Operations and Basis of Presentation
Incorporation
DFP Healthcare Acquisitions Corp., predecessor to The Oncology Institute, Inc. (the “Company”) was incorporated as a Delaware corporation on November 1, 2019.
Sponsor
The Company’s sponsor is DFP Sponsor LLC, a Delaware limited liability company (the “Sponsor”).
Business Purpose
The Company was formed for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase, reorganization or other similar business combination with one or more operating businesses that it has not yet selected (“Business Combination”). The Company has neither engaged in any operations nor generated revenue to date.
As of December 31, 2020, the Company had not commenced any operations. All activity for the period from November 1, 2019 (inception) through December 31, 2020 relates to the Company’s formation and the initial public offering (the “Initial Public Offering”) described below, and since the Initial Public Offering, identifying a target company for a Business Combination. The Company will not generate any operating revenues until after the completion of its initial Business Combination, at the earliest. The Company generates non-operating income in the form of interest income on cash and cash equivalents from the proceeds derived from the Initial Public Offering.
The Company’s management has broad discretion with respect to the specific application of the net proceeds of its Initial Public Offering, although substantially all of the net proceeds of the Initial Public Offering are intended to be generally applied toward completing a Business Combination. Furthermore, there is no assurance that the Company will be able to successfully complete a Business Combination.
Financing
The registration statement for the Company’s Initial Public Offering was declared effective by the Securities and Exchange Commission (the “SEC”) on March 10, 2020. On March 13, 2020, the Company consummated its Initial Public Offering of 23,000,000 units (the “Units” and, with respect to the Class A common stock included in the Units being offered, the “Public Shares”), including 3,000,000 additional Units to cover over-allotments (the “Over-Allotment Units”), at $10.00 per Unit, generating gross proceeds of $230.0 million, and incurring offering costs of approximately $10.4 million, inclusive of approximately $6.3 million in deferred underwriting commissions (Note 3).
Simultaneously with the closing of the Initial Public Offering, the Company consummated the private placement (“Private Placement”) of 3,733,334 warrants (each, a “Private Placement Warrant” and collectively, the “Private Placement Warrants”) at a price of $1.50 per Private Placement Warrant in a private placement to the Sponsor, generating proceeds of $5.6 million (Note 4).
Trust Account
Upon the closing of the Initial Public Offering and the Private Placement, $230.0 million ($10.00 per Unit) of the net proceeds of the Initial Public Offering and certain of the proceeds of the Private Placement was placed in a trust account (the “Trust Account”) and invested in permitted United States “government securities” within the meaning of Section 2(a)(16) of the Investment Company Act of 1940, as amended, which the Company refers to as the Investment
Company Act, having a maturity of 185 days or less or in money market funds meeting certain conditions under Rule 2a-7 promulgated under the Investment Company Act that invest only in direct U.S. government treasury obligations.
The Company’s second amended and restated certificate of incorporation provides that, other than the withdrawal of interest earned on the funds that may be released to the Company to pay taxes, none of the funds held in Trust Account will be released until the earlier of: (i) the completion of the Business Combination; (ii) the redemption of the Public Shares to its holders (the “Public Stockholders”) properly tendered in connection with a stockholder vote to amend the Company’s second amended and restated certificate of incorporation to modify the substance or timing of the Company’s obligation to redeem 100% of the Public Shares or with respect to any other material provision relating to stockholders’ rights or pre-initial Business Combination activity, or (iii) the redemption of 100% of the Public Shares if the Company does not complete a Business Combination within 24 months from the closing of the Initial Public Offering.
The Company, after signing a definitive agreement for a Business Combination, will either (i) seek stockholder approval of the Business Combination at a meeting called for such purpose in connection with which stockholders may seek to redeem their shares, regardless of whether they vote for or against the Business Combination, for cash equal to their pro rata share of the aggregate amount then on deposit in the Trust Account calculated as of two business days prior to the consummation of the initial Business Combination, including interest earned on the funds held in the Trust Account and not previously released to the Company to fund its working capital requirements (subject to an annual limit of $500,000) and/or to pay its taxes, or (ii) provide the Public Stockholders with the opportunity to sell their shares to the Company by means of a tender offer for an amount in cash equal to their pro rata share of the aggregate amount then on deposit in the Trust Account calculated as of two business days prior to commencement of the tender offer, including interest earned on the funds held in the Trust Account and not previously released to the Company to fund its working capital requirements (subject to an annual limit of $500,000) and/or to pay taxes. The decision as to whether the Company will seek stockholder approval of the Business Combination or will allow stockholders to sell their shares in a tender offer will be made by the Company, solely in its discretion, and will be based on a variety of factors such as the timing of the transaction and whether the terms of the transaction would otherwise require the Company to seek stockholder approval. If the Company seeks stockholder approval, it will complete its Business Combination only if a majority of the outstanding shares of common stock voted are voted in favor of the Business Combination. However, in no event will the Company redeem its Public Shares in an amount that would cause its net tangible assets to be less than $5,000,001.
If the Company holds a stockholder vote in connection with a Business Combination, a Public Stockholder will have the right to redeem its shares for an amount in cash equal to their pro rata share of the aggregate amount then on deposit in the Trust Account calculated as of two business days prior to the consummation of the initial Business Combination, including interest earned on the funds held in the Trust Account and not previously released to the Company to fund its working capital requirements (subject to an annual limit of $500,000) and/or to pay its taxes. As a result, such common stock is recorded at redemption amount and classified as temporary equity upon the completion of the Initial Public Offering, in accordance with FASB, ASC 480, “Distinguishing Liabilities from Equity.” The amount in the Trust Account is initially anticipated to be $10.00 per public share ($230.0 million held in the Trust Account divided by 23,000,000 public shares).
The Company will have 24 months from the closing of the Initial Public Offering, or until March 13, 2022, to complete its initial Business Combination (the “ Combination Period”). If the Company does not complete a Business Combination within this period of time, it will (i) cease all operations except for the purposes of winding up; (ii) as promptly as reasonably possible, but not more than ten business days thereafter, redeem the Public Shares for a per share pro rata portion of the Trust Account, including interest and not previously released to the Company to fund its working capital requirements (subject to an annual limit of $500,000) (less taxes payable and up to $100,000 of such net interest to pay dissolution expenses) and (iii) as promptly as possible following such redemption, liquidate and dissolve the balance of the Company’s net assets to its remaining stockholders, as part of its plan of dissolution and liquidation. The Sponsor and the Company’s officers and directors (the “initial stockholders”) have entered into a letter agreement with the Company, pursuant to which they have waived their rights to participate in any redemption with respect to their Founder Shares (as defined below); however, if the initial stockholders acquire shares of common stock in or after the Initial Public Offering, they will be entitled to a pro rata share of the Trust Account upon the Company’s redemption of common stock or liquidation in the event the Company does not complete a Business Combination within the required time period. In the event of such a liquidating distribution, it is possible that the per share value of the residual assets remaining available for distribution (including Trust Account assets) will be less than the initial price per Unit in the Initial Public Offering.
Basis of Presentation
The accompanying financial statements of the Company have been prepared in accordance with United States generally accepted accounting principles (“U.S. GAAP”) and pursuant to the rules and regulations of the Securities and Exchange Commission ("SEC").
Emerging Growth Company
The Company is an “emerging growth company,” as defined in Section 2(a) of the Securities Act of 1933, as amended (the “Securities Act”), as modified by the Jumpstart our Business Startups Act of 2012 (the “JOBS Act”), and it may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in its periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved.
Further, section 102(b)(1) of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies (that is, those that have not had a Securities Act registration statement declared effective or do not have a class of securities registered under the Securities Exchange Act of 1934, as amended) are required to comply with the new or revised financial accounting standards. The JOBS Act provides that a company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging growth companies but any such an election to opt out is irrevocable. We have elected not to opt out of such extended transition period which means that when a standard is issued or revised and it has different application dates for public or private companies, we, as an emerging growth company, can adopt the new or revised standard at the time private companies adopt the new or revised standard. This may make comparison of the Company’s financial statements with another public company which is neither an emerging growth company, nor an emerging growth company which has opted out of using the extended transition period, difficult or impossible because of the potential differences in accountantaccounting standards used.
Recently Adopted Accounting Standards
In June 2016, the FASB issued Accounting Standards Update 2016-13, Note 2Measurement of Credit Losses on Financial Instruments (“ASU 2016-13”), which changes the way entities recognize impairment of many financial assets by requiring immediate recognition of estimated credit losses expected to occur over their remaining life, instead of when incurred. In November 2018, the FASB issued Accounting Standard Update 2018-19, Codification Improvements to Topic 326, Financial Instruments — RestatementCredit Losses (“ASU 2018-19”), which amends Subtopic 326-20 (created by ASU 2016-13) to explicitly state that operating lease receivables are not in the scope of Previously IssuedSubtopic 326-20. Additionally, in April 2019, the FASB issued Accounting Standard Update 2019-04, Codification Improvements to Topic 326, Financial Statements
Instruments — Credit Losses, Topic 815, Derivatives and Hedging, and Topic 825, Financial Instruments (“ASU 2019-04”); in May 2019, the FASB issued Accounting Standards Update 2019-05, Financial Instruments — Credit Losses (Topic 326): Targeted Transition Relief (“ASU 2019-05”); and in November 2019, the FASB issued Accounting Standards Update 2019-10, Financial Instruments — Credit Losses (Topic 326), Derivatives and Hedging (Topic 815), and Leases (Topic 842): Effective Dates and ASU 2019-11, Codification Improvements to Topic 326, Financial Instruments — Credit Losses (“ASU 2019-10”), to provide further clarifications on certain aspects of ASU 2016-13 and to extend the nonpublic entity effective date of ASU 2016-13. The changes (as amended) are effective for the Company for annual and interim periods in fiscal years beginning January 1, 2023. The Company concluded it should restate its previously issuedadopted ASU 2016-13, as amended, effective January 1, 2023, which resulted in changes to the Company’s accounting policies for accounts receivables. Upon adoption of ASU 2016-13, the Company evaluated accounts receivables on a collective (i.e., portfolio) basis when similar risk characteristics were shared. The adoption of this standard did not have a
material impact on our consolidated financial statements by amending Amendment No. 1 to its Annual Report on Form 10-K/A, filedand there was no allowance for credit losses recorded as of December 31, 2023.
Recently Issued Accounting Standards
In August 2020, the FASB issued ASU 2020-06, Debt-Debt with the SEC on May 24, 2021, to classify all Class A common stock subject to possible redemptionConversion and Other Options (Subtopic 470-20) and Derivatives and Hedging-Contracts in temporary equity. In accordance with accounting guidance on redeemable equity instruments, ASC 480, paragraph 10-S99, redemption provisions not solely within the control of the Company require common stock subject to redemption to be classified outside of permanent equity. The Company had previously classified a portion of its Class A common stock in permanent equity, or total stockholders’ equity. Although the Company did not specify a maximum redemption threshold, its charter currently provides that, the Company will not redeem its public sharesEntity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an amountEntity’s Own Equity ("ASU 2020-06"), which simplifies accounting for convertible instruments by removing major separation models required under current U.S. GAAP. ASU 2020-06 also removes certain settlement conditions that would cause its net tangible assetsare required for equity-linked contracts to be less than $5,000,001. Previously, the Company did not consider redeemable stock classified as temporary equity as part of net tangible assets. Effective with these financial statements, the Company revised this interpretation to include temporary equity in net tangible assets. Also, in connection with the change in presentationqualify for the Class A common stock subject to possible redemption,derivative scope exception and it also simplifies the Company also restated itsdiluted earnings per share calculation to allocate income and losses shared pro rata between the two classes of shares. This presentation contemplates a Business Combination as the most likely outcome, in which case, both classes of shares share pro rata in the income and losses of the Company. As a result,certain areas. The new standard is effective for the Company restated its previously filedbeginning January 1, 2024. The Company is currently evaluating the effect of ASU 2020-06 on the Company’s consolidated financial statements to present all redeemable Class A common stock as temporary equity and torelated disclosures.
In October 2021, the FASB issued ASU 2021-08, Business Combinations (Topic 805): Accounting for Contract Assets and Contract Liabilities from Contracts with Customers ("ASU 2021-08"). Under ASU 2021-08, an acquirer must recognize, accretion from the initial book value to redemption value at the time of its Initial Public Offering and measure contract assets and contract liabilities acquired in a business combination in accordance with ASC 480.606, Revenue from Contract with Customers (“ASC 606”). The guidance is effective for interim and annual periods beginning after December 15, 2023, with early adoption permitted. The Company will adopt ASU 2021-08 on January 1, 2024 on a prospective basis. The Company is currently evaluating the effect of ASU 2021-08 on the Company’s previously filedconsolidated financial statements and related disclosures.
On October 9, 2023, the FASB issued ASU 2023-06: Disclosure Improvements: Codification Amendments in Response to the SEC's Disclosure Update and Simplification Initiative ("ASU 2023-06"), which amends the disclosure and presentation requirements related to various Codification subtopics. The ASU ("ASU 2023-06") was issued in response to the SEC’s August 2018 final rule that contained the error were initially reported in the Company’s Form 8-K filed withupdates and simplifies disclosure requirements the SEC on March 13, 2020 (the “Post-IPO Balance Sheet”),believed were “redundant, duplicative, overlapping, outdated, or superseded.” The new guidance is intended to align U.S. GAAP and SEC requirements while facilitating the Company’s Form 10-Qsapplication of U.S. GAAP for the quarterly periods ended March 31, 2020, June 30, 2020, and September 30, 2020, and the Company's Annual Report on 10-Kall entities. The effective date for the annual period ended December 31, 2020, which were all previously restated in the Company's Amendment No. 1 to its Form 10-K as filed with the SEC on May 12, 2021 (“the 2020 Affected Periods”), as well as the Company’s Form 10-Qs for the quarterly periods ended March 31, 2021, and June 30, 2021 (collectively, the “Affected Periods”). These financial statements restate the Company’s previously issued audited and unaudited financial statements covering the periods through December 31, 2020. The
quarterly periods ended March 31, 2021, and June 30, 2021,each amendment will be restatedthe date on which the SEC’s removal of that related disclosure requirement from Regulation S-X or Regulation S-K becomes effective, with an amendmentearly adoption prohibited. We are currently evaluating the Company’s Form 10-Q for the quarterly period ended September 30, 2021.
Impact of the Restatement
The impact of the restatementguidance on our consolidated financial statements.
In November 2023, the balance sheet, statementFASB issued ASU 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures ("ASU 2023-07"). The new standard requires a public entity to disclose significant segment expenses and other segment items on an annual and interim basis and provide, in interim periods, all disclosures about a reportable segment’s profit or loss and assets that are currently required annually. Additionally, it requires a public entity to disclose the title and position of the Chief Operating Decision Maker. The ASU ("ASU 2023-07") does not change how a public entity identifies its operating segments, aggregates them, or applies the quantitative thresholds to determine its reportable segments. The new standard is effective for fiscal years beginning after December 15, 2023, and interim periods within fiscal years beginning after December 15, 2024, with early adoption permitted. A public entity should apply the amendments in this ASU retrospectively to all prior periods presented in the financial statements. The Company expects this ASU to only impact our disclosures with no impacts to our results of operations, and statement of cash flows forand financial condition.
Moreover, in December 2023, the 2020 Affected Periods is presented below: | | | | | | | | | |
| | As Previously Restated on 10-K/A | | | | | | |
As of March 13, 2020 | | Amendment No. 1 | | Adjustment | | As Restated |
| | | | | | | | | |
Total assets | | $ | 231,769,236 | | $ | — | | $ | 231,769,236 |
Total liabilities | | $ | 17,812,302 | | $ | — | | $ | 17,812,302 |
Class A common stock subject to possible redemption | | | 208,956,930 | | | 21,043,070 | | | 230,000,000 |
Preferred stock | | | — | | | — | | | — |
Class A common stock | | | 210 | | | (210) | | | — |
Class B common stock | | | 575 | | | — | | | 575 |
Additional paid-in capital | | | 5,356,051 | | | (5,356,051) | | | — |
Accumulated deficit | | | (356,832) | | | (15,686,809) | | | (16,043,641) |
Total stockholders' equity (deficit) | | $ | 5,000,004 | | $ | (21,043,070) | | $ | (16,043,066) |
Total Liabilities, Class A Common Stock Subject to Possible Redemption and Stockholders' Equity (Deficit) | | $ | 231,769,236 | | $ | — | | $ | 231,769,236 |
Shares of Class A common stock subject to possible redemption | | | 20,895,693 | | | 2,104,307 | | | 23,000,000 |
Shares of Class A common stock | | | 2,104,307 | | | (2,104,307) | | | — |
FASB issued ASU 2023-09
, Income Taxes (Topic 740): Improvement to Income Tax Disclosures ("ASU 2023-09") | | | | | | | | | |
| | As Previously Restated on 10-K/A | | | | | | |
As of December 31, 2020 | | Amendment No. 1 | | Adjustment | | As Restated |
| | | | | | | | | |
Total assets | | $ | 231,323,610 | | $ | — | | $ | 231,323,610 |
Total liabilities | | $ | 25,358,720 | | $ | — | | $ | 25,358,720 |
Class A common stock subject to possible redemption | | | 200,964,880 | | | 29,035,120 | | | 230,000,000 |
Preferred stock | | | — | | | — | | | — |
Class A common stock | | | 290 | | | (290) | | | — |
Class B common stock | | | 575 | | | — | | | 575 |
Additional paid-in capital | | | 13,341,349 | | | (13,341,349) | | | — |
Accumulated deficit | | | (8,342,204) | | | (15,693,481) | | | (24,035,685) |
Total stockholders' equity (deficit) | | $ | 5,000,010 | | $ | (29,035,120) | | $ | (24,035,110) |
Total Liabilities, Class A Common Stock Subject to Possible Redemption and Stockholders' Equity (Deficit) | | $ | 231,323,610 | | $ | — | | $ | 231,323,610 |
Shares of Class A common stock subject to possible redemption | | | 20,096,488 | | | 2,903,512 | | | 23,000,000 |
Shares of Class A common stock | | | 2,903,512 | | | (2,903,512) | | | — |
. The impact of the restatementnew standard requires a public business entity (PBE) to disclose, on the statement of stockholders' equity is consistentan annual basis, a tabular rate reconciliation using both percentages and currency amounts, broken out into specified categories with the changescertain reconciling items further broken out by nature and jurisdiction to the impacted stockholders' equity accounts described above.
The impact of the restatement to the previously reported as restated statement of cash flows for the year ended December 31, 2020 is presented below:
| | | | | | | | | |
| | For the Year Ended December 31, 2020 |
| | As Reported | | Adjustment | | As Restated |
Supplemental Disclosure of Noncash Financing Activities: | | | | | | | | | |
Initial value of Class A common stock subject to possible redemption | | $ | 208,956,930 | | $ | (208,956,930) | | $ | — |
Change in value of Class A common stock subject to possible redemption | | $ | (7,992,050) | | $ | 7,992,050 | | $ | — |
The impact to the reported amounts of weighted average shares outstanding and basic and diluted earnings per common share for the year ended December 31, 2020 is presented below:
| | | | | | | | | |
| | Earnings (Loss) Per Share |
| | As Previously | | | | | | |
For the year ended December 31, 2020 | | Restated | | Adjustment | | Restated |
Net loss | | $ | (8,339,904) | | $ | — | | $ | (8,339,904) |
Weighted average shares outstanding - Class A common stock | | | 23,000,000 | | | (4,524,590) | | | 18,475,410 |
Basic and diluted loss per share - Class A common stock | | $ | — | | $ | (0.35) | | $ | (0.35) |
Weighted average shares outstanding - Class B common stock | | | 5,602,459 | | | — | | | 5,602,459 |
Basic and diluted loss per share - Class B common stock | | $ | (1.49) | | $ | 1.14 | | $ | (0.35) |
extent those items exceed a specified threshold. In addition, see Note 10 - Quarterly Financial Information where unaudited interimall entities are required to disclose income taxes paid, net of refunds received disaggregated by federal, state/local, and foreign and by jurisdiction if the amount is at least 5% of total income tax payments, net of refunds received. For PBEs, the new standard is effective for annual periods are presented as restated.
Note 3 — Summary of Significant Accounting Policies
Basis of presentation
The Company’s financial statements have been preparedbeginning after December 15, 2024, with early adoption permitted. An entity may apply the amendments in conformity with accounting principles generally accepted inthis ASU prospectively by providing the United States of America (“U.S. GAAP”) for financial information and pursuant to the rules and regulations of the Securities and Exchange Commission (“SEC”). The Company has no subsidiaries.
As described in Note 2—Restatement of Previously Issued Financial Statements, the Company’s financial statementsrevised disclosures for the period as ofending December 31, 2020,2025 and continuing to provide the year ended December 31, 2020, as of September 30, 2020 andpre-ASU disclosures for the threeprior periods, or may apply the amendments retrospectively by providing the revised disclosures for all period presented. The Company expects this ASU to only impact our disclosures with no impacts to our results of operations, cash flows, and nine months ended September 30, 2020, as of June 30, 2020 and for the three and six months ended June 30, 2020, and as of March 31, 2020 and for the three months ended March 31, 2020 (collectively, the “2020 Affected Periods”), are restated in this Annual Report on Form 10-K/A (Amendment No. 2) (this “Annual Report”) to correct the misapplication of accounting guidance related to the redeemable Class A common stock and earnings per share in the Company’s previously issued audited and unaudited condensed financial statements for such periods. The restated financial statements are indicated as “Restated” in the audited and unaudited condensed financial statements and accompanying notes, as applicable. See Note 2—Restatement of Previously Issued Financial Statements for further discussion.
Use of Estimatescondition.
The preparation of the financial statements in conformity with GAAP requires the Company's management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Making estimates requires management to exercise significant judgment. It is at least reasonably possible that the estimate of the effect of a condition, situation or set of circumstances that existed at the date of the financial statements, which management considered in formulating its estimate, could change in the near term due to one or more future conforming events. Accordingly, the actual results could differ from those estimates.
Note 3. Significant Risks and
Cash EquivalentsThe Company considers all short-term investments with an original maturity of three months or less when purchased to be cash equivalents. The Company had no cash equivalents as of December 31, 2020,Uncertainties Including Business and 2019.
Investments Held in the Trust Account
The Company’s portfolio of investments held in the Trust Account is comprised of U.S. government securities, within the meaning set forth in Section 2(a)(16) of the Investment Company Act, with a maturity of 185 days or less, or investments in money market funds that invest in U.S. government securities, or a combination thereof. The Company’s investments held in the Trust Account are classified as trading securities. Trading securities are presented on the balance sheet at fair value at the end of each reporting period. Gains and losses resulting from the change in fair value of these securities is included in net gain on investments, dividends and interest held in Trust Account in the accompanying statement of operations. The estimated fair values of investments held in the Trust Account were determined using available market information.
Concentration of Credit Concentrations
Financial instruments that potentially subject the Company to concentrations of credit risk consist primarily of cash, accounts receivable, and investment securities.
Cash accounts in a financial institution
which,may, at times,
may exceed the Federal Deposit Insurance Corporation
limitcoverage of
$250,000, and investments held in Trust Account.$250 per account ownership category. The Company has not experienced losses on these accounts, and management believes the Company is not exposed to significant risks on such accounts.
The Company’s accounts receivable has implicit collection risk. The Company grants credit without collateral to their patients, most of whom are local residents and are insured under third-party payor agreements. The Company believes this risk is partially mitigated by the Company’s establishment of long-term agreements and relationships with third-party payors that provide the Company with insight into historic collectability and improve the collections process.
The Company's investment securities portfolio is managed by a third party vendor to provide a relatively stable source of investment income from excess liquidity while satisfactorily managing risk, including credit risk, reinvestment risk, liquidity risk, and interest rate risk.
Revenue Concentration Risk
The concentration of net revenue on a percentage basis for major payors for the years ended December 31, 2023 and 2022 are as follows:
| | | | | | | | | | | | | | |
| | Year Ended December 31, |
| | 2023 | | 2022 |
| Percentage of Patient Services Net Revenue: | | | | |
| Payor A | | 11 | % | | 13 | % |
| Payor B | | 14 | % | | 16 | % |
The concentration of gross receivables on a percentage basis for major payors at December 31, 2023 and December 31, 2022 are as follows:
| | | | | | | | | | | |
| December 31, 2023 | | December 31, 2022 |
| Percentage of Gross Receivables of Patient Services Revenue: | | | |
| Payor B | N/A | | 13 | % |
| Payor C | N/A | | 10 | % |
| | | |
All of the Company’s revenue is generated from customers located in the United States.
Vendor Concentration Risk
The concentration of direct costs on a percentage basis for major vendors for the years ended December 31, 2023 and 2022 are as follows:
| | | | | | | | | | | | | | |
| | Year Ended December 31, |
| | 2023 | | 2022 |
| Percentage of Direct Costs: | | | | |
| Vendor A | | 99 | % | | 76 | % |
| Vendor B | | N/A | | 21 | % |
The concentration of gross payables on a percentage basis for major vendors at December 31, 2023 and December 31, 2022 are as follows:
| | | | | | | | | | | |
| December 31, 2023 | | December 31, 2022 |
| Percentage of Gross Payables: | | | |
| Vendor A | 70 | % | | 66 | % |
| | | |
Note 4. Accounts Receivable
The Company’s accounts receivable consists primarily of amounts due from third-party payors and patients. See Note 2 for a summary of the Company’s policies relating to accounts receivable and allowance for credit losses.
Accounts Receivable as of December 31, 2023 and December 31, 2022 consist of the following:
| | | | | | | | | | | |
| (in thousands) | December 31, 2023 | | December 31, 2022 |
| Oral drug accounts receivable (Dispensary) | $ | 2,914 | | | $ | 4,165 | |
| Capitated accounts receivable (Patient Services) | 1,757 | | | 1,623 |
| FFS accounts receivable (Patient Services) | 30,173 | | | 26,313 |
| Clinical trials accounts receivable | 2,595 | | | 2,443 |
| Other trade receivables | 4,921 | | | 5,272 |
| Total | $ | 42,360 | | | $ | 39,816 | |
The Company adopted ASU 2016-13, as amended, effective January 1, 2023, and determined no allowance for credit losses was required as of that date. No allowance for credit losses was recorded as of December 31, 2023.
No allowance for doubtful accounts was recorded as of December 31, 2022.
As of January 1, 2022, the accounts receivable balance amounted to $20,007.
During the years ended December 31, 2023 and 2022, the Company had net bad debt recoveries of $11, and bad debt recoveries of $169, respectively, and bad debt expense of $2,031 and $307, respectively. Bad debt write-offs were a result of accounts receivable on completed contracts that were deemed uncollectible during the period due to delayed collection efforts.
Note 5. Revenue
The Company recognizes revenue in accordance with ASC 606 on the basis of its satisfaction of outstanding performance obligations. The Company typically fulfills its performance obligations over time, either over the course of a single treatment (fee-for-service or "FFS"), a month (capitation), or a number of months (clinical research). The Company also has revenue that is satisfied at a point in time (dispensary). See Note 2 for summary of the Company’s policies and significant assumptions related to revenue recognition.
Disaggregation of Revenue
The Company categorizes revenue based on various factors such as the nature of contracts, payors, order to billing arrangements, and cash flows received by the Company, as follows:
| | | | | | | | | | | | | | |
| (in thousands) | | Year Ended December 31, |
| 2023 | | 2022 |
| Patient services | | | | |
| Capitated revenue | | $ | 70,370 | | | $ | 61,341 | |
| FFS revenue | | 143,134 | | 105,444 |
| Subtotal | | 213,504 | | | 166,785 | |
| Dispensary revenue | | 103,835 | | | 79,343 | |
| Clinical research trials and other revenue | | 6,900 | | | 6,355 | |
| Total | | $ | 324,239 | | | $ | 252,483 | |
Refer to Note 20 for Segment Reporting for disaggregation of revenue by reporting segment.
Contract Asset and Liabilities
Under ASC 606, contract assets represent rights to payment for performance contingent on something other than the passage of time and accounts receivable are rights to payment for performance without contingencies. The Company does not
have any contract assets as of December 31, 2023 and December 31, 2022. Refer to Note 4 for accounts receivable as of December 31, 2023 and December 31, 2022.
Contract liabilities represent cash that has been received for contracts, but for which performance is still unsatisfied. As of December 31, 2023 and December 31, 2022, contract liabilities amounted to $545 and $1,139, respectively. As of January 1, 2022, the contract liabilities amounted to $220. Contract liabilities are included within other current liabilities and presented in Note 9 along with refund liabilities due to amounts not being material. During the years ended December 31, 2023 and 2022, the Company recognized revenue of $594 and $220, respectively, related to deferred capitation revenue received (contract liability) as of the beginning of each respective year.
Remaining Unsatisfied Performance Obligations
The accounting terms for the Company’s patient services and dispensary contracts do not extend past a year in duration. Additionally, the Company applies the ‘as invoiced’ practical expedient to its clinical research contracts.
Note 6. Inventories
The Company purchases intravenous chemotherapy drugs and oral prescription drugs from various suppliers. See Note 2 for a summary of the Company’s policies relating to intravenous chemotherapy and oral prescription drugs inventory.
The Company’s inventories as of December 31, 2023 and December 31, 2022 were as follows:
| | | | | | | | | | | |
| (in thousands) | December 31, 2023 | | December 31, 2022 |
| Oral drug inventory | $ | 3,640 | | | $ | 2,130 | |
| IV drug inventory | 10,038 | | 7,131 |
| Total | $ | 13,678 | | | $ | 9,261 | |
Note 7. Marketable Securities and Fair Value
of Financial InstrumentsFair value is defined as the price that would be receivedMeasurements
Marketable Securities
The Company accounts for its investment securities available for sale using the fair value election pursuant to ASC 825, where changes in fair value are recorded in Other, net non-operating expense (income) on the Company's Consolidated Statements of Operations. The Company’s investments in cash equivalents and marketable securities at December 31, 2023 and December 31, 2022 is as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2023 |
| (in thousands) | Amortized Cost | | Gross Unrealized Gains | | Gross Unrealized Losses | | Fair Value |
| Cash equivalents: | | | | | | | |
| U.S. Treasury Bills | $ | 22,778 | | | $ | 5 | | | $ | — | | | $ | 22,783 | |
| Marketable securities: | | | | | | | |
| Short-term U.S. Treasuries | 49,501 | | | — | | | (134) | | | 49,367 | |
| | | | | | | |
| Total available for sale securities | $ | 72,279 | | | $ | 5 | | | $ | (134) | | | $ | 72,150 | |
| | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2022 |
| (in thousands) | Amortized Cost | | Gross Unrealized Gains | | Gross Unrealized Losses | | Fair Value |
| Cash equivalents: | | | | | | | |
| U.S. Treasury Bills | $ | 2,573 | | | $ | — | | | $ | — | | | $ | 2,573 | |
| Marketable securities: | | | | | | | |
| Short-term U.S. Treasuries | 59,876 | | | 6 | | | (86) | | | 59,796 | |
| Long-term U.S. Treasuries | 58,652 | | | — | | | (298) | | | 58,354 | |
| Total available for sale securities | $ | 121,101 | | | $ | 6 | | | $ | (384) | | | $ | 120,723 | |
The contractual maturities of the Company's investments in cash equivalents and marketable securities as of December 31, 2023 and December 31, 2022 is as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
December 31, 2023 (in thousands) | Due in One Year or less | | Due After One Year through Five Years | | Due After Five Years | | Total |
| Cash equivalents: | | | | | | | |
| U.S. Treasury Bills | $ | 22,783 | | | $ | — | | | $ | — | | | $ | 22,783 | |
| Marketable securities: | | | | | | | |
| Short-term U.S. Treasuries | 49,367 | | | — | | | — | | | 49,367 | |
| | | | | | | |
| Total available for sale securities | $ | 72,150 | | | $ | — | | | $ | — | | | $ | 72,150 | |
| | | | | | | | | | | | | | | | | | | | | | | |
December 31, 2022 (in thousands) | Due in One Year or less | | Due After One Year through Five Years | | Due After Five Years | | Total |
| Cash equivalents: | | | | | | | |
| U.S. Treasury Bills | $ | 2,573 | | | $ | — | | | $ | — | | | $ | 2,573 | |
| Marketable securities: | | | | | | | |
| Short-term U.S. Treasuries | 59,796 | | | — | | | — | | | 59,796 | |
| Long-term U.S. Treasuries | 10,523 | | | 47,831 | | | — | | | 58,354 | |
| Total available for sale securities | $ | 72,892 | | | $ | 47,831 | | | $ | — | | | $ | 120,723 | |
The Company recorded a net unrealized loss of $249 for the year ended December 31, 2023. At December 31, 2023, three securities were in an unrealized loss position. The decline in fair value of our securities was attributable to a combination of changes in interest rates and general volatility in the credit market conditions in response to the economic uncertainty caused by the risk of an asset, or paid for transferupcoming recession and monetary policy. The Company does not currently intend to sell any of a liability,the securities in an orderly transactionunrealized loss position and further believe, it is more likely than not, that we will not be required to sell these securities before their anticipated recovery.
Accrued interest receivable on cash equivalents and marketable securities was $242 and $274, respectively, at December 31, 2023 and December 31, 2022, and is included within other receivables in the Consolidated Balance Sheets.
Fair Value Measurements
The following tables present the carrying amounts of the Company’s financial instruments at December 31, 2023 and December 31, 2022:
| | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2023 |
| (in thousands) | Total | | Level 1 | | Level 2 | | Level 3 |
| Financial assets: | | | | | | | |
| Cash equivalents | $ | 22,783 | | | $ | — | | | $ | 22,783 | | | $ | — | |
| Marketable securities | 49,367 | | | — | | | 49,367 | | | — | |
| | | | | | | |
| Financial liabilities: | | | | | | | |
| Derivative warrant liabilities | $ | 636 | | | $ | — | | | $ | 636 | | | $ | — | |
| Earnout liabilities | — | | | — | | | — | | | — | |
| Conversion option derivative liabilities | 3,082 | | | — | | | — | | | 3,082 | |
| Contingent consideration liability | 1,944 | | | — | | | 1,944 | | | — | |
| Non-recurring fair value measurement | | | | | | | |
| Goodwill | $ | 7,230 | | | — | | | — | | | $ | 7,230 | |
As of December 31, 2023, derivative warrant liabilities of $636 were transferred from a Level 3 to a Level 2 financial instrument as a result of the valuation being based on the market price of our public warrants, which management considers to
be a similar and comparable instrument, as compared to the previous valuation which was based on the Binomial Lattice Model. There were no other transfers between fair value measurement levels during the years ended December 31, 2023 and 2022.
| | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2022 |
| (in thousands) | Total | | Level 1 | | Level 2 | | Level 3 |
| Financial assets: | | | | | | | |
| Cash equivalents | $ | 2,573 | | | $ | — | | | $ | 2,573 | | | — | |
| Marketable securities | 59,796 | | | — | | | 59,796 | | | — | |
| Non-current investments | 58,354 | | | — | | | 58,354 | | | — | |
| Financial liabilities: | | | | | | | |
| Derivative warrant liabilities | $ | 350 | | | $ | — | | | $ | — | | | $ | 350 | |
| Earnout liabilities | 803 | | | — | | | — | | | 803 | |
| Conversion option derivative liabilities | 3,960 | | | — | | | — | | | 3,960 | |
| Non-recurring fair value measurement | | | | | | | |
| Goodwill | $ | 21,418 | | | $ | — | | | $ | — | | | $ | 21,418 | |
The carrying amounts of cash, accounts receivable, other receivables, and accounts payable approximate fair value because of the short maturity and high liquidity of these instruments.
The Company measures its investments (including cash equivalents, marketable securities, and non-current investments) at fair value on a recurring basis and classifies those instruments within Level 2 of the fair value hierarchy. Investment securities, including U.S. Treasury Bills purchased in the secondary market
participants atand U.S. Treasury bonds, are classified within Level 2 of the
measurement date. U.S. GAAP establishes a three-tier fair value hierarchy
which prioritizes thebecause pricing inputs
used in measuring fair value.The hierarchy gives the highest priority to unadjustedare other than quoted prices in active markets, for identical assetswhich are either directly or liabilities (Level 1 measurements)indirectly observable as of the reporting date, and fair value is determined using models or other valuation methodologies.
The Company measures its private derivative warrants on a recurring basis and classifies those instruments within Level 2 of the
lowest priority tofair value hierarchy because the valuation is based on the observable input of a similar instrument. The Company measures its earnout, convertible note warrant derivative liability, optional redemption derivative liability and conversion option derivative liability, and contingent consideration liability on a recurring basis and classifies those instruments within level 3 of the fair value hierarchy because unobservable inputs
(Level 3 measurements). These tiers include: | ● | Level 1, defined as observable inputs such as quoted prices (unadjusted) for identical instruments in active markets; |
| ● | Level 2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable such as quoted prices for similar instruments in active markets or quoted prices for identical or similar instruments in markets that are not active; and |
| ● | Level 3, defined as unobservable inputs in which little or no market data exists, therefore requiring an entity to develop its own assumptions, such as valuations derived from valuation techniques in which one or more significant inputs or significant value drivers are unobservable. |
In some circumstances, the inputs used to measure fair value. See Note 2 for a summary of the Company’s policies relating to fair value might be categorizedmeasurements, and Note 11 for more detail on the convertible note warrant, optional redemption, and conversion option derivative liabilities.
The Company measures goodwill at fair value on a nonrecurring basis and classifies goodwill within different levelsLevel 3 of the fair value hierarchy. In those instances, the fair value measurement is categorized in its entiretyDue to significant declines in the fair value hierarchy based onCompany's share price during the lowest level input that is significant to the fair value measurement.As of
years ended December 31, 2020,2023 and 2019,2022, the carrying valuesCompany performed a quantitative analysis of cash, prepaid expenses, accounts payable, accrued expenses, accrued expenses - related party,impairment over goodwill and tax obligations approximate their fair values due todetermined goodwill was impaired. As a result, the short-term natureCompany recorded an impairment charge of $16,867 and $9,944 for the instruments.years ended December 31, 2023 and 2022, respectively. Goodwill was valued using an equally weighted income approach and market approach. The Company’s portfolio of marketable securities heldunobservable inputs utilized in the Trust Account is comprised mainly of investments in U.S. Treasury securities with an original maturity of 185 days or less. The fair value for trading securities is determined using quoted market prices in active markets. Offering Costs
The Company complied with the requirements of the ASC 340-10-S99-1. Offering costs consist of legal, accounting, underwriting fees and other costs incurred that were directly attributable to the Initial Public Offering.. Offering costs are allocated to the separable financial instruments issued in the Initial Public Offering based on a relative fair value basis, compared to total proceeds received. Offering costs associated with warrant liabilities are expensed as incurred, presented as non-operating expenses in the statement of operations. Offering costs associated with the Public Shares were charged to the carrying value of the Class A common stock subject to possible redemption upon the completion of the Initial Public Offering. The Company classifies deferred underwriting commissions as their liquidation is not reasonably expected to require the use of current assets or require the creation of current liabilities.
Derivative Warrant Liabilities
The Company does not use derivative instruments to hedge its exposures to cash flow, market or foreign currency risks. Management evaluates all of the Company’s financial instruments, including issued warrants to purchase its Class A common stock, to determine if such instruments are derivatives or contain features that qualify as embedded derivatives, pursuant to ASC 480 and ASC 815-15. The classification of derivative instruments, including whether such instruments should be recorded as liabilities or as equity, is re-assessed at the end of each reporting period.
The Company issued 5,750,000 warrants to purchase Class A common stock to investors in the Company’s Initial Public Offering, including the over-allotment, and simultaneously issued 3,733,334 Private Placement Warrants. All of the Company’s outstanding warrants are recognized as derivative liabilities in accordance with ASC 815-40. Accordingly, we recognize the warrant instruments as liabilities at fair value and adjust the instruments to fair value at each reporting period. The liabilities are subject to re-measurement at each balance sheet date until exercised, and any change in fair value is recognized in our statement of operations. The fair value of the Public Warrants issued in connection with the Public Offering and Private Placement Warrants were initially measured at fair value using a Monte Carlo simulation model and subsequently,determining the fair value of the Private Placement Warrants have been estimated usinggoodwill, which is categorized as a Monte Carlo simulation model each measurement date. The fair valueLevel 3 instrument, are the discount rate of Public Warrants issued25.0% and various revenue growth rates utilized in connection with the Initial Public Offering have subsequently been measured basedfinancial forecast of future cash flows. See Note 2 for further detail on the listed market price of such warrants.
Class A Common Stock Subject to Possible Redemption (Restated - Seeimpairment evaluation and Note 2)
The Company accounts18 for its Class A common stock subject to possible redemption in accordance with the guidance in ASC Topic 480 “Distinguishing Liabilities from Equity.” Class A common stock subject to mandatory redemption (if any) is classified as liability instruments and are measured at fair value. Conditionally redeemable Class A common stock (including Class A common stock that features redemption rights that are either within the control of the holder or subject to redemption upon the occurrence of uncertain events not solely within the Company’s control) are classified as temporary equity. At all other times, Class A common stock is classified as stockholders’ equity. The Company’s Class A common stock feature certain redemption rights that are considered to be outside of the Company’s control and subject to the occurrence of uncertain future events. Accordingly, as of December 31, 2020 , 23,000,000 shares of Class A common stock subject to possible redemption are presented as temporary equity, outside of the stockholders’ equity section of the Company’s balance sheets.
Under ASC 480-10-S99, the Company has elected to recognize changes in the redemption value immediately as they occur and adjust the carrying value of the security to equal the redemption value at the end of each reporting period.
This method would view the end of the reporting period as if it were also the redemption date for the security. Accordingly, effective with the closing of the Initial Public Offering, the Company recognized the accretion from the initial book value to redemption amount, which resulted in charges against paid-in -capital (to the extent available) and accumulated deficit
goodwill.The Class A common stock subject to possible redemption reflected on the balance sheet is reconciled on the following table:
| | | |
Gross proceeds | | $ | 230,000,000 |
Less: | | | |
Amount allocated to Public Warrants | | | (6,727,500) |
Class A common stock issuance costs | | | (10,110,406) |
Plus: | | | |
Accretion of carrying value to redemption value | | | 16,837,906 |
Class A common stock subject to possible redemption | | $ | 230,000,000 |
Net Income (Loss) Per Share of Common Stock
The Company complies with accounting and disclosure requirements of FASB ASC Topic 260, “Earnings Per Share.” The Company has two classes of shares, which are referred to as Class A common stock and Class B common stock. Income and losses are shared pro rata between the two classes of shares. Net income (loss) per share of common stock is calculated by dividing the net income (loss) by the weighted average number of common stock outstanding for the respective period.
The calculation of diluted net income (loss) per share of common stock does not consider the effect of the warrants underlying the Units sold in the Initial Public Offering and the Private Placement to purchase an aggregate of 9,483,334 of the Company’s Class A common stock in the calculation of diluted income per share, since their exercise is contingent upon future events and their inclusion would be anti-dilutive under the treasury stock method. Accretion associated with the redeemable Class A common stock is excluded from earnings per share as the redemption value approximates fair value.
The table below presents a reconciliation of the numerator and denominator used to compute basic and diluted net loss per share of common stock for each class of common stock:
| | | | | | | | | | | | |
| | For the Year Ended December 31, |
| | 2020 | | 2019 |
| | Class A | | Class B | | Class A | | Class B |
Basic and diluted net income (loss) per share of common stock: | | | | | | | | | | | | |
Numerator: | | | | | | | | | | | | |
Allocation of net income | | $ | (6,399,368) | | $ | (1,940,536) | | $ | — | | $ | (2,300) |
| | | | | | | | | | | | |
Denominator: | | | | | | | | | | | | |
Basic and diluted weighted average common shares outstanding | | | 18,475,410 | | | 5,602,459 | | | — | | | 5,000,000 |
| | | | | | | | | | | | |
Basic and diluted net income (loss) per share of common stock | | $ | (0.35) | | $ | (0.35) | | $ | — | | $ | — |
Income Taxes
The Company complies with the accounting and reporting requirements of Financial Accounting Standards Board Accounting Standard Codification, or FASB ASC, 740, “Income Taxes,” which requires an asset and liability approach to financial accounting and reporting for income taxes. Deferred income tax assets and liabilities are computed for differences between the financial statement and tax bases of assets and liabilities that will result in future taxable or deductible amounts, based on enacted tax laws and rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
There were no unrecognized tax benefits as of December 31, 2020 or 2019. FASB ASC 740 prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more-likely-than-not to be sustained upon examination by taxing authorities. The Company recognizes accrued interest and penalties related to
unrecognized tax benefits as income tax expense. No amounts were accrued for the payment of interest and penalties as of December 31, 2020 or 2019. The Company is currently not aware of any issues under review that could result in significant payments, accruals or material deviation from its position. The Company is subject to income tax examinations by major taxing authorities since inception.
Recent Accounting Pronouncements
Management does not believe that any recently issued, but not yet effective, accounting pronouncements, if currently adopted, would have a material effect on the Company’s financial statements.
Note 4 —Initial Public Offering
Public Units
On March 13, 2020, the Company consummated its Initial Public Offering of 23,000,000 Units, including 3,000,000 Over-Allotment Units, at $10.00 per Unit, generating gross proceeds of $230.0 million, and incurring offering costs of approximately $10.4 million, inclusive of approximately $6.3 million in deferred underwriting commissions. Of the Units sold in the Initial Public Offering, 5,000,000 Units were purchased by certain domestic private pooled investment vehicles managed by Deerfield Management Company, L.P. and its affiliates (the “Deerfield Funds”).
Each Unit consists of 1 of the Company’s shares of Class A common stock, $0.0001 par value, and one-fourth of one redeemable warrant (the “Warrants”). Each whole Warrant entitles the holder to purchase 1 share of Class A common stock at a price of $11.50 per share. The exercise price and number of shares of Class A common stock issuable upon exercise of the Warrants may be adjusted in certain circumstances including in the event of a stock dividend, or recapitalization, reorganization, merger or consolidation.
Note 5 — Related Party Transactions
Founder Shares
On December 30, 2019, the Sponsor received 4,312,500 shares of Class B common stock (the “Founder Shares”) in exchange for a capital contribution of $25,000, or approximately $0.004 per share. In January 2020, the Sponsor transferred 100,000 Founder Shares to each of Steven Hochberg, the Company’s President and Chief Executive Officer, Christopher Wolfe, the Company’s Chief Financial Officer and Secretary, and Richard Barasch, the Company’s Executive Chairman, and 30,000 Founder Shares to each of Dr. Jennifer Carter, Dr. Mohit Kaushal and Dr. Gregory Sorensen, the Company’s independent director nominees, for the same per-share price initially paid by the Sponsor, resulting in the Sponsor holding 3,922,500 Founder Shares. On February 19, 2020, the Company effected a split of its Class B common stock resulting in the Sponsor holding 5,360,000 Founder Shares, resulting in an increase in the total number of Founder Shares from 4,312,500 to 5,750,000. All shares and associated amounts have been retroactively restated. Of the 5,750,000 Founder Shares, up to 750,000 shares were subject to forfeiture by the initial stockholders depending on the exercise of the underwriters’ over-allotment option. The underwriters exercised their over-allotment option in full on March 13, 2020; thus, the Founder Shares are no longer subject to forfeiture.
The Founder Shares are identical to the shares of Class A common stock included in the Units being sold in the Initial Public Offering except that the Founder Shares are subject to certain transfer restrictions.
The initial stockholders have agreed not to transfer, assign or sell any of their Founder Shares until the earlier of (A) one year after the completion of the Company’s initial Business Combination, or earlier if, subsequent to the Company’s initial Business Combination, the closing price of the Company’s common stock equals or exceeds $12.00 per share (as adjusted for stock splits, stock capitalizations, reorganizations, recapitalizations and the like) for any 20 trading days within any 30-trading day period commencing at least 150 days after the Company’s initial Business Combination and (B) the date on which the Company completes a liquidation, merger, capital stock exchange or other similar transaction after the initial Business Combination that results in all of the Company’s stockholders having the right to exchange their common stock for cash, securities or other property.
Private Placement Warrants
Simultaneously with the closing of the Initial Public Offering, the Company sold 3,733,334 Private Placement Warrants to the Sponsor at a price of $1.50 per Private Placement Warrant in a Private Placement, generating proceeds of $5.6 million.
Each Private Placement Warrant entitles the holder to purchase 1 share of Class A common stock at $11.50 per share. Certain proceeds of the Private Placement Warrants were added to the proceeds from the Initial Public Offering held in the Trust Account. If the Company does not complete a Business Combination, the proceeds of the Private Placement will be part of the liquidating distribution to the Public Stockholders and the Warrants issued to the Sponsor will expire worthless.
Sponsor Loan
The Sponsor agreed to loan the Company up to an aggregate of $200,000 by the issuance of an unsecured promissory note (the “Note”) to cover expenses related to this Initial Public Offering. The Note was payable, without interest, upon the completion of the Initial Public Offering. The Company received the $200,000 proceeds under the Note and repaid this Note in full on March 13, 2020.
Administrative Services Agreement
Commencing on the date that the Company’s securities were first listed on Nasdaq, the Company has paid and will pay the Sponsor $10,000 per month for office space, secretarial and administrative services provided to members of the Company’s management team. Upon completion of the initial Business Combination or the Company’s liquidation, the Company will cease paying such monthly fees. The Company incurred $100,000 and $0, in expenses in connection with such services during the year ended December 31, 2020 and the period from November 1, 2019 (inception) through December 31, 2019, respectively, as included in general and administrative expenses - related party on the accompanying statements of operations. As of December 31, 2020, and 2019, the Company had $10,000 and $0 in connection with such services in accrued expenses to related parties, respectively, as included in the accompanying balance sheets.
Wolfe Strategic Services Agreement
Commencing on the date that the Company’s securities were first listed on Nasdaq, the Company will pay and has paid its Chief Financial Officer, Christopher Wolfe, $7,500 per month for his services prior to the initial Business Combination. The Company incurred $75,000 and $0 in expenses in connection with such services during the year ended December 31, 2020 and the period from November 1, 2019 (inception) through December 31, 2019, respectively, as included in general and administrative expenses - related party on the accompanying statement of operations. As of December 31, 2020, and 2019, the Company had $7,500 and $0 in connection with such services in accrued expenses to related parties, respectively, as included in the accompanying balance sheets.
Working Capital Loans
In order to finance transaction costs in connection with an intended initial Business Combination, the Sponsor or an affiliate of the Sponsor or certain of the Company’s officers and directors may, but are not obligated to, loan the Company funds as may be required for working capital (the “Working Capital Loans”). Up to $1.1 million of such Working Capital Loans may be convertible into warrants of the post-Business Combination entity at a price of $1.50 per warrant at the option of the lender. Such warrants would be identical to the Private Placement Warrants. As of December 31, 2020, except for the foregoing, the terms of such loans, if any, have not been determined, no written agreements exist with respect to such loans and no amounts have been borrowed under such loans to date.
Note 6 — Commitments and Contingencies
Registration Rights
The initial stockholders and holders of the Private Placement Warrants are entitled to registration rights pursuant to a registration rights agreement. The initial stockholders and holders of the Private Placement Warrants will be entitled to make up to three demands, excluding short form registration demands, that the Company register such securities for sale under the Securities Act. In addition, these holders will have “piggy-back” registration rights to include their securities in other registration statements filed by the Company. The Company will bear the expenses incurred in connection with the filing of any such registration statements.
Underwriting Agreement
The Company granted the underwriters a 45-day option to purchase up to 3,000,000 additional Units to cover any over-allotments, at the initial public offering price less the underwriting discounts and commissions. The warrants that were issued in connection with the 3,000,000 over-allotment Units are identical to the public warrants and have no net cash settlement provisions. The underwriters exercised the over-allotment option in full on March 13, 2020.
The underwriters did not receive any underwriting discounts or commission on the Units purchased by the Deerfield Funds. The Company paid an underwriting discount of 2.0% of the per Unit offering price, or $3.6 million, at the closing of the Initial Public Offering, with an additional fee (the “Deferred Underwriting Fees”) of 3.5% of the gross offering proceeds, or $6.3 million, payable upon the Company's completion of an Initial Business Combination. The Deferred Underwriting Fees will become payable to the underwriters from the amounts held in the Trust Account solely in the event the Company completes its initial Business Combination.
Risks and uncertainties
Management continues to evaluate the impact of the COVID-19 pandemic on its industry and has concluded that, while it is reasonably possible that the virus could have a negative effect on the Company’s financial position, results of its operations and/or search for a target company, the specific impact is not readily determinable as of the date of these financial statements. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Note 7 — Derivative Warrant Liabilities
As of December 31, 2020, the Company had 5,750,000 Public Warrants and 3,733,334 Private Warrants outstanding.
Public Warrants may only be exercised for a whole number of shares. No fractional Public Warrants will be issued upon separation of the Units and only whole Public Warrants will trade. The Public Warrants will become exercisable on the later of (a) 30 days after the completion of a Business Combination or (b) 12 months from the closing of the Initial Public Offering; provided in each case that the Company has an effective registration statement under the Securities Act covering the shares of Class A common stock issuable upon exercise of the Public Warrants and a current prospectus relating to them is available and such shares are registered, qualified or exempt from registration under the securities, or blue sky, laws of the state of residence of the holder (or the Company permits holders to exercise their Public Warrants on a cashless basis under certain circumstances). The Company has agreed that, as soon as practicable, but in no event later than 15 business days after the closing of a Business Combination, the Company will use its best efforts to file with the SEC and have declared effective a registration statement covering the shares of Class A common stock issuable upon exercise of the warrants and to maintain a current prospectus relating to those shares of Class A common stock until the warrants expire or are redeemed, as specified in the warrant agreement. If a registration statement covering the Class A common stock issuable upon exercise of the warrants is not effective by the 60th business day after the closing of the initial Business Combination, warrant holders may, until such time as there is an effective registration statement and during any period when the Company will have failed to maintain an effective registration statement, exercise warrants on a “cashless basis” in accordance with Section 3(a)(9) of the Securities Act or another exemption. Notwithstanding the above, if the Company’s shares of Class A common stock are at the time of any exercise of a warrant not listed on a national securities
exchange such that they satisfy the definition of a “covered security” under Section 18(b)(1) of the Securities Act, the Company may, at its option, require holders of Public Warrants who exercise their warrants to do so on a “cashless basis” in accordance with Section 3(a)(9) of the Securities Act and, in the event the Company so elects, the Company will not be required to file or maintain in effect a registration statement, and in the event the Company does not so elect, it will use its best efforts to register or qualify the shares under applicable blue sky laws to the extent an exemption is not available. The Public Warrants will expire five years after the completion of a Business Combination or earlier upon redemption or liquidation.
The Private Placement Warrants are identical to the Public Warrants, except that the Private Placement Warrants and the shares of Class A common stock issuable upon exercise of the Private Placement Warrants will not be transferable, assignable or salable until 30 days after the completion of the initial Business Combination and they will be non-redeemable for cash so long as they are held by the initial purchasers of the Private Placement Warrants or their permitted transferees. If the Private Placement Warrants are held by someone other than the initial purchasers of the Private Placement Warrants or their permitted transferees, the Private Placement Warrants will be redeemable by the Company and exercisable by such holders on the same basis as the warrants included in the Units being sold in the Initial Public Offering.
The Company may call the Public Warrants for redemption:
| ● | in whole and not in part; |
| ● | at a price of $0.01 per warrant; |
| ● | upon a minimum of 30 days’ prior written notice of redemption; and |
| ● | if, and only if, the last reported sales price of the Class A common stock equals or exceeds $18.00 per share (as adjusted) for any 20 trading days within the 30-trading day period ending on the third trading day prior to the date on which the Company sends the notice of redemption to the warrant holders. |
If the Company calls the Public Warrants for redemption, management will have the option to require all holders that wish to exercise the Public Warrants to do so on a “cashless basis,” as described in the warrant agreement.
In addition, commencing ninety days after the warrants become exercisable, the Company may redeem the outstanding warrants for shares of Class A common stock:
| ● | in whole and not in part; |
| ● | at a price of $0.10 per warrant upon a minimum of 30 days’ prior written notice of redemption provided that holders will be able to exercise their warrants on a cashless basis prior to redemption and receive that number of shares determined by reference to the table set forth under “Description of Securities—Warrants—Public Stockholders’ Warrants” based on the redemption date and the “fair market value” of our Class A common stock (as defined below) except as otherwise described in “Description of Securities—Warrants—Public Stockholders’ Warrants”; |
| ● | if, and only if, the last reported sale price of its Class A common stock equals or exceeds $10.00 per share (as adjusted per stock splits, stock dividends, reorganizations, recapitalizations and the like) on the trading day prior to the date on which it sends the notice of redemption to the warrant holders; |
| ● | if, and only if, the Private Placement Warrants are also concurrently exchanged at the same price (equal to a number of shares of Class A common stock) as the outstanding Public Warrants, as described above; and |
| ● | if, and only if, there is an effective registration statement covering the issuance of the shares of Class A common stock issuable upon exercise of the warrants and a current prospectus relating thereto available throughout the 30-day period after written notice of redemption is given. |
The “fair market value” of the Company’s Class A common stock shall mean the average last reported sale price of its Class A common stock for the 10 trading days ending on the third trading day prior to the date on which the notice of redemption is sent to the holders of warrants. This redemption feature differs from the typical warrant redemption features used in other blank check offerings.
No fractional shares of Class A common stock will be issued upon redemption. If, upon redemption, a holder would be entitled to receive a fractional interest in a share, the Company will round down to the nearest whole number of the number of shares of Class A common stock to be issued to the holder.
Pursuant to the warrant agreement, references above to Class A common stock shall include a security other than Class A common stock into which the Class A common stock has been converted or exchanged for in the event the Company is not the surviving company in its initial Business Combination.
In no event will the Company be required to net cash settle any warrant. If the Company does not complete a Business Combination within the Combination Period and the Company liquidates the funds held in the Trust Account, holders of warrants will not receive any of such funds with respect to their warrants, nor will they receive any distribution from the Company’s assets held outside of the Trust Account with the respect to such warrants. Accordingly, the warrants may expire worthless.
Note 8 — Stockholders’ Equity
Preferred Stock — The Company is authorized to issue 1,000,000 shares of preferred stock with a par value of $0.0001 per share. As of December 31, 2020, and 2019, there are 0 shares of preferred stock issued or outstanding.
Class A Common Stock — The Company is authorized to issue 100,000,000 shares of Class A common stock with a par value of $0.0001 per share. As of December 31, 2020, there were 23,000,000 shares of Class A common stock issued or outstanding, all were subject to possible redemption , and therefore classified outside of permanent equity. As of December 31, 2019, there were 0 shares of Class A common stock issued or outstanding.
Class B Common Stock — The Company is authorized to issue 10,000,000 shares of Class B common stock with a par value of $0.0001 per share. Holders of the Company’s Class B common stock are entitled to 1 vote for each share. On December 30, 2019, the Company initially issued 4,312,500 shares of Class B common stock. On February 19, 2020, in connection with the proposed increase of the size of the Initial Public Offering, the Company effected a split of its Class B common stock resulting in the Sponsor holding 5,360,000 Founder Shares and an increase in the total number of Class B common stock outstanding from 4,312,500 to 5,750,000. Of the 5,750,000 shares of Class B common stock outstanding, up to 750,000 shares were subject to forfeiture to the Company to the extent that the underwriters’ over-allotment option was not exercised in full or in part, so that the initial stockholders would collectively own 20% of the Company’s issued and outstanding common stock after the Initial Public Offering. All shares and associated amounts have been retroactively restated. The underwriters exercised their over-allotment option in full on March 13, 2020; thus, the 750,000 shares of Class B common stock were no longer subject to forfeiture. As of December 31, 2020, and 2019, there were 5,750,000 shares of Class B common stock issued outstanding.
The Class B common stock will automatically convert into Class A common stock at the time of the Initial Business Combination on a one-for-one basis, subject to adjustment. In the case that additional shares of Class A common stock or equity-linked securities are issued or deemed issued in connection with the initial Business Combination, the number of shares of Class A common stock issuable upon conversion of all Class B common stock will equal, in the aggregate, on an as-converted basis, 20% of the total number of shares of Class A common stock outstanding after such conversion (after giving effect to any redemptions of shares of Class A common stock by Public Stockholders), including the total number of shares of Class A common stock issued, or deemed issued or issuable upon conversion or exercise of any equity-linked securities or rights issued or deemed issued, by the Company in connection with or in relation to the consummation of the
initial Business Combination, excluding any shares of Class A common stock or equity-linked securities or rights exercisable for or convertible into shares of Class A common stock issued, or to be issued, to any seller in the initial Business Combination and any Private Placement Warrants issued to the Sponsor, officers or directors upon conversion of Working Capital Loans, provided that such conversion of Founder Shares will never occur on a less than one-for-one basis.
Note 9 — Fair Value Measurements
The following table presents information about the Company’s assets andLevel 3 liabilities that are measured at fair value on a recurring basis asat December 31, 2023:
| | | | | | | | | | | | | | | |
| (in thousands) | | | Earnout Liability | | Conversion Option Derivative Liability |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| Balance at December 31, 2021 | | | $ | 60,018 | | | $ | — | |
| Conversion option derivative liability acquired (See Note 11 for detail) | | | — | | | 28,160 | |
| Decrease in fair value included in other expense | | | (59,215) | | | (24,200) | |
| Balance at December 31, 2022 | | | $ | 803 | | | $ | 3,960 | |
| Decrease in fair value included in other expense | | | (803) | | | (878) | |
| Balance at December 31, 2023 | | | $ | — | | | $ | 3,082 | |
As of December 31, 20202023, the conversion option derivative and indicatesearnout liabilities were valued using a Binomial Lattice and Monte-Carlo Simulation Model, respectively, which is considered to be Level 3 fair value measurements. The derivative warrant liabilities were valued using the public warrant trading price, which is considered to be a Level 2 fair value measurement, and the contingent consideration liability was valued using a present value factor, which is considered to be a Level 2 fair value measurement. As of December 31, 2022, derivative warrant and earnout liabilities were valued using a Binomial Lattice and Monte-Carlo Simulation Model, respectively, which are considered to be Level 3 fair value measurements. A summary of the level 3 fair value measurements inputs used in the valuations is as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | December 31, 2023 |
| | | First Tranche Earnout | | Second Tranche Earnout | | Convertible Note Warrant Derivative Liability | | Conversion Option Derivative Liability |
| Unit price | | | $ | 2.04 | | $ | 2.04 | | $ | 2.04 | | $ | 2.04 |
| Term (in years) | | | 0.87 | | 0.87 | | 3.61 | | 3.61 |
| Volatility | | | 49.40 | % | | 49.40 | % | | 58.60 | % | | 58.60 | % |
| Risk-free rate | | | 4.90 | % | | 4.90 | % | | 3.90 | % | | 3.90 | % |
| Dividend yield | | | — | | | — | | | — | | | — | |
| Cost of equity | | | 16.90 | % | | 16.90 | % | | — | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2022 |
| Derivative Warrant Liability | | First Tranche Earnout | | Second Tranche Earnout | | Convertible Note Warrant Derivative Liability | | Conversion Option Derivative Liability |
| Unit price | $ | 1.65 | | $ | 1.65 | | $ | 1.65 | | $ | 1.65 | | $ | 1.65 |
| Term (in years) | 3.87 | | 1.54 | | 1.55 | | 4.61 | | 4.61 |
| Volatility | 71.80 | % | | 70.00 | % | | 70.00 | % | | 40.00 | % | | 40.00 | % |
| Risk-free rate | 4.08 | % | | 4.45 | % | | 4.45 | % | | 3.99 | % | | 3.99 | % |
| Dividend yield | — | | | — | | | — | | | — | | | — | |
| Cost of equity | — | | | 13.60 | % | | 13.60 | % | | — | | | — | |
On August 9, 2022, the Company issued a senior secured convertible note that contains embedded warrant, optional redemption, and conversion option features. Due to the economic disincentive to redeem and the make whole amount that would be required to be paid, it is highly unlikely that the optional redemption would occur, reducing the value during the period to a qualitatively immaterial amount. See Note 11 for additional detail. A summary of the inputs used in the initial measurement of the convertible note warrant and conversion option derivative liabilities is as follows:
| | | | | | | | | | | |
| August 9, 2022 |
| (Initial Measurement) |
| Convertible Note Warrant Derivative Liability | | Conversion Option Derivative Liability |
| Unit price | $ | 6.63 | | | $ | 6.63 | |
| Term (in years) | 5.00 | | | 5.00 | |
| Volatility | 42.5 | % | | 42.5 | % |
| Risk-free rate | 3.0 | % | | 3.0 | % |
| Dividend yield | — | | | — | |
| Cost of equity | — | | | — | |
Uncertainty of Fair Value Measurement from Use of Significant Unobservable Inputs
The inputs to estimate the fair value
hierarchy of the
valuation techniques thatCompany’s derivative warrant, earnout, convertible note warrant, and conversion option derivative liabilities were the
Company utilized to determine such fair value.
| | | | | | | | | |
| | Quoted Prices in | | Significant Other | | Significant Other |
| | Active Markets | | Observable Inputs | | Unobservable Inputs |
Description | | (Level 1) | | (Level 2) | | (Level 3) |
Assets | | | | | | | | | |
Assets held in Trust Account: | | | | | | | | | |
U.S. Treasury securities | | $ | 230,253,395 | | $ | 0 | | $ | 0 |
Cash equivalents - money market funds | | | 754 | | | 0 | | | 0 |
| | $ | 230,254,149 | | $ | 0 | | $ | 0 |
| | | | | | | | | |
Liabilities | | | | | | | | | |
Derivative warrant liabilities -Public Warrants | | $ | 11,212,500 | | $ | 0 | | $ | 0 |
Derivative warrant liabilities -Private Warrants | | $ | 0 | | $ | 0 | | $ | 7,578,670 |
Transfers to/from Levels 1, 2, and 3 are recognized at the endmarket price of the reporting period. There were no transfers between levels forCompany’s common stock, their remaining expected term, the year ended December 31, 2020. Thevolatility of the Company’s common stock price and the risk-free interest rate over the expected term. Significant changes in any of those inputs in isolation can result in a significant change in the fair value measurement.
Generally, an increase in the market price of the Company’s shares of common stock, an increase in the volatility of the Company’s shares of common stock, and an increase in the remaining term of the derivative liabilities would each result in a directionally similar change in the estimated fair value of the
Public Warrants transferred fromCompany’s derivative liabilities. Such changes would increase the associated liability while decreases in these assumptions would decrease the associated liability. An increase in the risk-free interest rate would result in a
Level 3 measurement to a Level 1decrease in the estimated fair value measurement
and thus a decrease in
June 2020, upon trading of the
Public Warrants in an active market.Level 1 instruments include investments in money market funds and U.S. Treasury securities.associated liability. The Company uses inputs such as actual trade data, benchmark yields, quoted market prices from dealers or brokers,has not, and other similar sourcesdoes not plan to, determine the fair value ofdeclare dividends on its investments.
The fair value of the Public Warrants issued in connection with the Public Offering and Private Placement Warrants were initially measured at fair value using a Monte Carlo simulation model and subsequently, the fair value of the Private Placement Warrants have been estimated using a Monte Carlo simulation model each measurement date. The fair value of Public Warrants issued in connection with the Initial Public Offering have been measured based on the listed market price of such warrants, a Level 1 measurement, beginning in June 2020.
The estimated fair value of the Private Placement Warrants, and the Public Warrants prior to being separately listed and traded, is determined using Level 3 inputs. Inherent in a Monte Carlo simulation are assumptions related to expected stock-price volatility, expected life, risk-free interest rate and dividend yield. The Company estimates the volatility of its Class A common stock warrants based on implied volatility from the Company’s traded warrants and, from historical volatility of select peer company’s Class A common stock that matches the expected remaining life of the warrants. The risk-free interest rateas such, there is based on the U.S. Treasury zero-coupon yield curve on the grant date for a maturity similar to the expected remaining life of the warrants. The expected life of the warrants is assumed to be equivalent to their remaining contractual term. The dividend rate is based on the historical rate, which the Company anticipates remaining at zero.
There were 0 assets or liabilities measured at fair value on a recurring basis as of December 31, 2019.
The following table provides quantitative information regarding Level 3 fair value measurements inputs at their measurement:
| | | | | | | |
| | As of March 13, 2020 | | As of December 31, 2020 | |
Stock Price | | $ | 9.71 | | $ | 10.80 | |
Volatility | | | 18.2 | % | | 24.0 | % |
Expected life of the options to convert | | | 6.55 | | | 5.75 | |
Risk-free rate | | | 0.85 | % | | 0.47 | % |
Dividend yield | | | 0.0 | % | | 0.0 | % |
Theno change in the estimated fair value of the derivative warrant liabilities measureddue to the dividend assumption.
Note 8. Property and Equipment, Net
The Company accounts for property and equipment at historical cost less accumulated depreciation. See Note 2 for a summary of the Company’s policies relating to property and equipment.
Property and equipment, net, consist of the following:
| | | | | | | | | | | | | | | | | |
| (in thousands) | Useful lives | | December 31, 2023 | | December 31, 2022 |
| Computers and software | 60 months | | $ | 3,035 | | | $ | 2,139 | |
| Office furniture | 84 months | | 724 | | | 606 | |
| Leasehold improvements | Shorter of lease term or estimated useful life | | 9,214 | | | 6,655 | |
| Medical equipment | 60 months | | 2,082 | | | 1,138 | |
| Construction in progress | | | 1,801 | | | 1,144 | |
| Finance lease ROU assets | Shorter of lease term or estimated useful life | | 207 | | | 371 | |
| Less: accumulated depreciation | | | (6,180) | | | (3,506) | |
| Total property and equipment, net | | | $ | 10,883 | | | $ | 8,547 | |
Depreciation expense for the years ended December 31, 2023 and 2022 was $2,864 and $1,526, respectively.
Note 9. Accrued Expenses and Other Current and Non-Current Liabilities
Accrued expenses and other current liabilities as of December 31, 2023 and December 31, 2022 consist of the following:
| | | | | | | | | | | |
| (in thousands) | December 31, 2023 | | December 31, 2022 |
| Compensation, including bonuses, fringe benefits, and payroll taxes | $ | 5,518 | | | $ | 5,310 | |
| Contract liabilities | 545 | | | 1,139 | |
| Directors and officers insurance premiums | 1,002 | | | 3,010 | |
| Deferred acquisition and contingent consideration (see Note 16) | 2,206 | | | 802 | |
| Accrued interest | 1,124 | | | 1,100 | |
| Other liabilities | 3,601 | | | 3,234 | |
| Total accrued expenses and other current liabilities | $ | 13,996 | | | $ | 14,595 | |
Contract liabilities as of December 31, 2023 and December 31, 2022 consist of cumulative adjustments made to capitated revenue recognized in prior periods.
Pursuant to the Business Combination, the Company has agreed to indemnify members of the Board and certain officers if they are named or threatened to be named as a party to any proceeding by reason of the fact that they acted in such capacity. The Company entered into a $1,250 financing arrangement in November 2023 with Level 3 inputsa maturity date of August 2024 at 8.75% annual interest rate to pay 10 monthly principal payments of approximately $122 in premiums for directors’ and officers’ (“D&O”) insurance coverage through November 2024 to protect against such losses on November 12, 2021. The principal outstanding balance was $1,002 as of December 31, 2023. As of February 2024, the remaining D&O principal balance was paid in full.
Note 10. Leases
The Company leases clinics, office buildings, and certain equipment under noncancellable financing and operating lease agreements that expire at various dates through June 2033. See Note 2 for a summary of the Company’s policies relating to leases.
The initial terms of operating leases range from 1 to 10 years and certain leases provide for free rent periods, periodic rent increases, and renewal options. Monthly payments for these leases range from $0 to $60. All lease agreements generally require the Company to pay maintenance, repairs, property taxes, and insurance costs, which are generally variable amounts based on actual costs incurred during each applicable period.
The Company has determined that periods covered by options to extend the Company's leases are excluded from the lease terms as it is not reasonably certain the Company will exercise such options. Operating lease expenses, including expenses related to short-term leases, were $7,596 and $6,364, respectively, for the years ended December 31, 2023 and 2022.
Lease Expense
The components of lease expense were as follows:
| | | | | | | | | | |
| (in thousands) | Year Ended December 31, 2023 | Year Ended December 31, 2022 | | |
| | | | |
| Operating lease costs: | $ | 7,556 | | $ | 6,002 | | | |
| Finance lease costs: | | | | |
| Amortization of ROU asset | $ | 59 | | $ | 62 | | | |
| Interest expense | $ | 11 | | $ | 8 | | | |
| Other lease costs: | | | | |
| Short-term lease costs | $ | 39 | | $ | 362 | | | |
| Variable lease costs | $ | 1,240 | | $ | 967 | | | |
| Total lease costs | $ | 8,905 | | $ | 7,401 | | | |
Operating and other lease costs are presented as part of selling, general, and administrative expenses. The components of finance lease costs appear in depreciation and amortization and interest expense.
Maturity of Lease Liabilities
The aggregate future lease payments for the Company's leases in years subsequent to December 31, 2023 are as follows:
| | | | | | | | |
| (in thousands) | Operating Leases | Finance Leases |
| 2024 | $ | 8,176 | | $ | 48 | |
| 2025 | 7,817 | | 42 | |
| 2026 | 7,311 | | 39 | |
| 2027 | 5,868 | | 29 | |
| 2028 | 3,957 | | — | |
| Thereafter | 6,243 | | — | |
| Total future lease payment | $ | 39,372 | | $ | 158 | |
| Less: amount representing interest | (6,523) | | (18) | |
| Present value of future lease payment (lease liability) | $ | 32,849 | | $ | 140 | |
| Reported as: | | |
| Lease liabilities, current | $ | 6,363 | | $ | 40 | |
| Lease liabilities, noncurrent | 26,486 | | 100 | |
| Total lease liabilities | $ | 32,849 | | $ | 140 | |
Lease Term and Discount Rate
The following table provides the weighted average remaining lease terms and weighted average discount rates for the Company's leases as of:
| | | | | | | | |
| December 31, 2023 | December 31, 2022 |
| Weighted-average remaining lease term (in years) | | |
| Operating | 5.31 | 5.32 |
| Finance | 3.50 | 3.75 |
| Weighted-average discount rate | | |
| Operating | 6.50 | % | 4.94 | % |
| Finance | 6.47 | % | 6.02 | % |
Supplemental Cash Flow Information
The following table provides certain cash flow and supplemental noncash information related to the Company's lease liabilities for the year ended December 31, 2020 is summarized2023.
| | | | | | | | | | |
| (in thousands) | Year Ended December 31, 2023 | Year Ended December 31, 2022 | | |
| Supplemental cash flow information | | | | |
| Cash paid for amounts included in the measurement of lease liabilities: | | | | |
| Operating cash payment from operating leases | $ | 7,513 | | $ | 5,342 | | | |
| Financing cash payments for finance leases | 63 | | 73 | | | |
| Lease liabilities arising from obtaining right-of-use assets: | | | | |
| Operating leases | $ | 11,096 | | $ | 30,800 | | | |
| Finance leases | 3 | | 203 | | | |
During the year ended December 31, 2023 and December 31, 2022, ROU assets of $11,096 and $11,668 were obtained in exchange for lease obligations, respectively.
Lease Modifications
During the year ended December 31, 2023, the Company extended its lease term for seven clinics in California and Florida. These extensions constitute lease modifications that qualify as follows:
| | | |
Level 3 derivative warrant liabilities as of January 1, 2020 | | $ | 0 |
Issuance of Public and Private Warrants | | | 11,207,500 |
Transfer of Public Warrants to Level 1 | | | (6,957,500) |
Change in fair value of derivative warrant liabilities | | | 3,328,670 |
Level 3 derivative warrant liabilities as of December 31, 2020 | | $ | 7,578,670 |
Note 10 — Quarterly Financial Information (unaudited) (restated)
The following tables contain unaudited quarterly financial informationa change of accounting for the three monthsoriginal leases and not separate contracts. Accordingly, in the year ended MarchDecember 31, 2020,2023, the Company recognized the difference of
$3,297 as an increase to the operating lease liability; $3,303, net of lease incentives, as an increase to operating lease right-of-use asset, and $67 as a net increase to rent expense. During the year ended December 31, 2022, the Company expanded its lease space and extended its lease term for two clinics and two corporate offices in California. These expansions and extensions constitute lease modifications that qualify as a change of accounting for the threeoriginal leases and six monthsnot separate contracts. Accordingly, in the year ended June 30, 2020,December 31, 2022, the Company recognized the difference of $2,186 as an increase to the operating lease liability; $2,052, net of lease incentives, as an increase to operating lease right-of-use asset, and $39 as a net increase to rent expense.
Note 11. Debt
Senior Secured Convertible Note
On August 9, 2022, TOIentered into a Facility Agreement (the “Facility Agreement”) with certain lenders (“Lenders”) and Deerfield Partners L.P. (“Agent”), pursuant to which, TOI borrowed cash loans from the Lenders in the amount of $110,000, in exchange for which, TOI issued to each Lender a secured convertible promissory note (“Senior Secured Convertible Note”), which is payable to such Lenders in an amount equal to the threeunpaid principal amount of loans held by such Lender.
The Senior Secured Convertible Note will mature on August 9, 2027 (the “Maturity Date”) and nine months ended September 30, 2020shall bear interest at the rate of 4.00% per annum from August 9, 2022, on the outstanding principal amount, any overdue interest and any other amounts and obligations. The interest shall be paid in cash quarterly in arrears commencing on October 1, 2022. In case of any
prepayment, repayment or redemption of the Senior Secured Convertible Note, the Company shall pay any accrued and unpaid interest on the principal, along with a make whole amount and an exit fee.
The Facility Agreement requires the Company to meet certain operational and reporting requirements, including, but not limited to, customary regulatory, financial reporting, and disclosure requirements. Additionally, limitations are placed on the Company's ability to merge with other companies and enter into other debt arrangements and permitted investments are limited to amounts specified in the Facility Agreement. The Facility Agreement also provides certain restrictions on dividend payments and other equity transactions and requires the Company to make prepayments under specified circumstances. Financial covenants in the Facility Agreement require the Company to maintain a minimum unrestricted cash and Cash Equivalent balance of $40,000 and a minimum net quarterly revenues of $50,000 during fiscal year 2023; $75,000 during fiscal year 2024; and $100,000 during fiscal year 2025. Cash Equivalents as defined by the Facility Agreement means (a) any readily-marketable securities (i) issued by, or directly, unconditionally and fully guaranteed or insured by the United States federal government or (ii) issued by any agency of the United States federal government the obligations of which are fully backed by the full faith and credit of the United States federal government, (b) any readily-marketable direct obligations issued by any other agency of the United States federal government, any state of the United States or any political subdivision of any such state or any public instrumentality thereof, in each case having a rating of at least “A-1” from S&P or at least “P-1” from Moody’s, (c) any commercial paper rated at least “A-1” by S&P or “P-1” by Moody’s and issued by any Person organized under the laws of any state of the United States, (d) any United States dollar-denominated time deposit, insured certificate of deposit, overnight bank deposit or bankers’ acceptance issued or accepted by any commercial bank that (A) is organized under the laws of the United States, any state thereof or the District of Columbia, (B) is “adequately capitalized” (as defined in the regulations of its primary federal banking regulators) and (C) has been updatedTier 1 capital (as defined in such regulations) in excess of $250,000 and (e) shares of any United States money market fund that (i) has substantially all of its assets invested continuously in the types of investments referred to reflectin clause (a), (b), (c) and/or (d) above with maturities as set forth in the restatementproviso below, (ii) has net assets in excess of $500,000 and (iii) has obtained from either S&P or Moody’s the highest rating obtainable for money market funds in the United States; provided, however, that the maturities of all obligations specified in any of clause (a), (b), (c) and (d) above shall not exceed one year. Additionally, the Registration Rights Agreement requires the Company to have an effective registration statement and calls for payment should the registration statement cease to remain effective. The Company was in compliance with the covenants of the Facility Agreement as of December 31, 2023.
Conversion Options
The Senior Secured Convertible Note contains several embedded conversion options (the “Conversion Options”) that grant the holders of the Senior Secured Convertible Note the ability to convert the Senior Secured Convertible Note at any time on or after date of issuance of the note. The Conversion Options are convertible into shares of the Company’s financial statementscommon stock (such converted shares, “Conversion Shares”) and, in certain circumstances, a combination of cash and shares of the Company’s common stock, or a combination of cash, other assets and securities or other property of any Company successor entity. The Conversion Shares or settlement amounts shall be computed on the basis of predefined formulae, with a set conversion price of $8.567 as describedone of the inputs and a conversion cap of 14,663,019 shares. The if-converted value did not exceed the principal amount as of December 31, 2023. No Conversion Shares were issued as of December 31, 2023.
The Company evaluated the Conversion Options of the Senior Secured Convertible Note under ASC 815 and concluded that they require bifurcation from the host contract as a separate unit of account. The Conversion Options do not meet the criteria to be classified in stockholders’ equity and hence, are accounted for as a derivative liability remeasured at fair value at each balance sheet date with changes in fair value reported in earnings.
The Conversion Options contain certain limits on exercise if, after giving effect to the exercise, the Lender would beneficially own a number of shares of common stock of the Company in excess of those permissible under the terms of the Senior Secured Convertible Note. The number of shares to be issued against these notes and conversion price are each subject to adjustments provided under the terms of Senior Secured Convertible Note.
The holder shall receive dividends on the Senior Secured Convertible Note 2—Restatementand distributions of Previously Issued Financial Statements. any kind made to the holders of common stock, other than dividends of, or distributions in, shares, to the same extent as if the holder had converted the Senior Secured Convertible Note into such shares and had held such shares on the record date for such dividends and distributions any limitations on conversion options.
Optional Redemption
The restatementFacility Agreement also provides the Company the right to redeem the outstanding principal amount of each note (“Optional Redemption”) for the principal amount, plus undiscounted interest. The Company shall not affect any Optional Redemption under this Senior Secured Convertible Note unless along with this, the Company effects an optional redemption
under all other notes in accordance with the terms thereof, on a pro rata basis, based upon the respective applicable original principal amount of each of the notes outstanding as of the date the notice for Optional Redemption is delivered to the holders.
The Company evaluated the Optional Redemption feature of the Senior Secured Convertible Note under ASC 815 and concluded that it requires bifurcation from the host contract as a separate unit of account. The Optional Redemption feature does not meet the criteria to be classified in stockholders’ equity and hence, is accounted for as a derivative liability remeasured at fair value at each balance sheet date with changes in fair value reported in earnings. The fair value of the Optional Redemption feature is de minimis.
If the principal redemption amount specified in an Optional Redemption notice is less than the entire principal amount then outstanding, the principal amount specified in each conversion notice shall be applied (i) first, to reduce, on a dollar-for-dollar basis, the principal amount of the note in excess of the principal redemption amount until such excess principal amount is reduced to zero and (ii) to reduce, on a dollar-for-dollar basis, the principal redemption amount until all of such principal redemption amount shall have been converted.
Convertible Note Warrants
The Facility Agreement also provides for the issuance of warrants (the “Convertible Note Warrants”) on each date any principal amount of any Senior Secured Convertible Note is paid, repaid, redeemed, or prepaid at any time prior to the Maturity Date. Convertible Note Warrants are exercisable from their original issue date to August 9, 2027, for purchase of an aggregate amount of Conversion Shares into which such principal amount of Senior Secured Convertible Note was convertible into, immediately prior to such payment, at an exercise price of $8.567.The holder of Convertible Note Warrants may pay the exercise price in cash or exercise the warrant on cashless basis or through a reduction of an amount of principal outstanding under any Senior Secured Convertible Note held by such holder. In the event that the Convertible Note Warrant has not been exercised in full as of the last business day during its term, the holder shall be deemed to have exercised the purchase rights represented by the Convertible Note Warrant in full as a cashless exercise, in which event the Company shall issue number of shares to the holder computed on the basis of a predefined formula.
The Company evaluated the Convertible Note Warrants of the Senior Secured Convertible Note under ASC 815 and concluded that they require bifurcation from the host contract as a separate unit of account. The Convertible Note Warrants do not meet the criteria to be classified in stockholders’ equity and hence, are accounted for as a derivative liability remeasured at fair value at each balance sheet date with changes in fair value reported in earnings.
The Convertible Note Warrant holder shall be entitled to receive any dividend or distribution made by the Company to the holders of common stock to the same extent as if the holder had exercised the Convertible Note Warrants in full in a cash exercise.
The number of shares to be issued against these warrants and exercise price are each subject to adjustments provided under the terms of Convertible Note Warrants. The Convertible Note Warrants contain certain limits on exercise if, after giving effect to the exercise, the Lender would beneficially own a number of shares of common stock of the Company in excess of those permissible under the terms of the Convertible Note Warrants. Further, the Convertible Note Warrants can be fully or partially settled in cash in certain cases in accordance with the terms of issuance such as when shares issuable upon exercise of the warrants exceed a predefined number, upon occurrence of predefined event of default and upon occurrence of predefined events that will bring a fundamental change in the Company such as merger, consolidation, business combination, recapitalization, reorganization, reclassification or other similar event.
As of December 31, 2023, there are no impact on net cash flows from operating, investing or financing activities. Convertible Note Warrants outstanding.
Allocation of Proceeds
The Company has not amended its previously filed Quarterly Reportsallocated total issuance proceeds of $110,000 among the Senior Secured Convertible Note and Convertible Note Warrants based on Form 10-Q forfair value. Upon issuance of the 2020 Affected Periods.Convertible Note Warrants, the Company recorded Convertible Note Warrants, Optional Redemption, and Conversion Options of $0, $0 and $28,160, which were recorded as a debt discount to the Senior Secured Convertible Note of $110,000. The financial information that has been previously filed or otherwise reported forCompany will amortize the 2020 Affected Periods is supersededdebt discount over a period of 5 years (of which 3.61 years remain).
The total issuance costs of $4,924 was allocated among the Senior Secured Convertible Note, Convertible Note Warrants, Optional Redemption, and Conversion Options, by
allocating costs of $0, $0, and $1,261 to the
information in this Annual Report,Convertible Note Warrants, Optional Redemption, and
Conversion Options with the
financial statements and related financial information forresidual cost of $3,663 being allocated to the
Affected Periods contained in such previously filed report should no longer be relied upon.
| | | | | | | | | |
| | As Previously Restated on 10-K/A | | | | | | |
As of March 31, 2020 (uaudited) | | Amendment No. 1 | | Adjustment | | As Restated |
| | | | | | | | | |
Total assets | | $ | 231,843,054 | | $ | — | | $ | 231,843,054 |
Total liabilities | | $ | 18,315,099 | | $ | — | | $ | 18,315,099 |
Class A common stock subject to possible redemption | | | 208,527,950 | | | 21,472,050 | | | 230,000,000 |
Preferred stock | | | — | | | — | | | — |
Class A common stock | | | 215 | | | (215) | | | — |
Class B common stock | | | 575 | | | — | | | 575 |
Additional paid-in capital | | | 5,778,354 | | | (5,778,354) | | | — |
Accumulated deficit | | | (779,139) | | | (15,693,481) | | | (16,472,620) |
Total stockholders' equity (deficit) | | $ | 5,000,005 | | $ | (21,472,050) | | $ | (16,472,045) |
Total Liabilities, Class A Common Stock Subject to Possible Redemption and Stockholders' Equity (Deficit) | | $ | 231,843,054 | | $ | — | | $ | 231,843,054 |
Shares of Class A common stock subject to possible redemption | | | 20,852,795 | | | 2,147,205 | | | 23,000,000 |
Shares of Class A common stock | | | 2,147,205 | | | (2,147,205) | | | — |
Senior Secured Convertible Note (in addition to the debt discount). The Company immediately expensed issuance costs allocated to Warrants,
Table of Contents
The Company's statement of stockholders' equity has been restated to reflect
Optional Redemption, and Conversion Options at inception and will amortize the
changescosts allocated to the
impacted stockholders' equity accounts described above.
| | | | | | | | | |
| | For the Three Months Ended March 31, 2020 (unaudited) |
| | As Reported | | Adjustment | | As Restated |
Supplemental Disclosure of Noncash Financing Activities: | | | | | | | | | |
Initial value of Class A common stock subject to possible redemption | | $ | 208,956,930 | | $ | (208,956,930) | | $ | — |
Change in value of Class A common stock subject to possible redemption | | $ | (428,980) | | $ | 428,980 | | $ | — |
| | | | | | | | | |
As of June 30, 2020 (unaudited) | | As Previously Restated | | Adjustment | | As Restated |
| | | | | | | | | |
Total assets | | $ | 231,466,990 | | $ | — | | $ | 231,466,990 |
Total liabilities | | $ | 20,557,770 | | $ | — | | $ | 20,557,770 |
Class A common stock subject to possible redemption | | | 205,909,210 | | | 24,090,790 | | | 230,000,000 |
Preferred stock | | | — | | | — | | | — |
Class A common stock | | | 241 | | | (241) | | | — |
Class B common stock | | | 575 | | | — | | | 575 |
Additional paid-in capital | | | 8,397,068 | | | (8,397,068) | | | — |
Accumulated deficit | | | (3,397,874) | | | (15,693,481) | | | (19,091,355) |
Total stockholders' equity (deficit) | | $ | 5,000,010 | | $ | (24,090,790) | | $ | (19,090,780) |
Total Liabilities, Class A Common Stock Subject to Possible Redemption and Stockholders' Equity (Deficit) | | $ | 231,466,990 | | $ | — | | $ | 231,466,990 |
Shares of Class A common stock subject to possible redemption | | | 20,590,921 | | | 2,409,079 | | | 23,000,000 |
Shares of Class A common stock | | | 2,409,079 | | | (2,409,079) | | | — |
Senior Secured Convertible Note over a period of 5 years (of which 3.61 years remain).
Amounts Outstanding and Recognized during the Periods Presented
The
impactSenior Secured Convertible Note as of December 31, 2023 consists of the
restatement on the statement of stockholders' equity is consistent with the changes to the impacted stockholders' equity accounts described above.
| | | | | | | | | |
| | For the Six Months Ended June 30, 2020 (unaudited) |
| | As Reported | | Adjustment | | As Restated |
Supplemental Disclosure of Noncash Financing Activities: | | | | | | | | | |
Initial value of Class A common stock subject to possible redemption | | $ | 208,956,930 | | $ | (208,956,930) | | $ | — |
Change in value of Class A common stock subject to possible redemption | | $ | (3,047,720) | | $ | 3,047,720 | | $ | — |
| | | | | | | | | |
As of September 30, 2020 (unaudited) | | As Previously Restated | | Adjustment | | As Restated |
| | | | | | | | | |
Total assets | | $ | 231,395,659 | | $ | — | | $ | 231,395,659 |
Total liabilities | | $ | 22,903,099 | | $ | — | | $ | 22,903,099 |
Class A common stock subject to possible redemption | | | 203,492,550 | | | 26,507,450 | | | 230,000,000 |
Preferred stock | | | — | | | — | | | — |
Class A common stock | | | 265 | | | (265) | | | — |
Class B common stock | | | 575 | | | — | | | 575 |
Additional paid-in capital | | | 10,813,704 | | | (10,813,704) | | | — |
Accumulated deficit | | | (5,814,534) | | | (15,693,481) | | | (21,508,015) |
Total stockholders' equity (deficit) | | $ | 5,000,010 | | $ | (26,507,450) | | $ | (21,507,440) |
Total Liabilities, Class A Common Stock Subject to Possible Redemption and Stockholders' Equity (Deficit) | | $ | 231,395,659 | | $ | — | | $ | 231,395,659 |
Shares of Class A common stock subject to possible redemption | | | 20,349,255 | | | 2,650,745 | | | 23,000,000 |
Shares of Class A common stock | | | 2,650,745 | | | (2,650,745) | | | — |
following: | | | | | | | | |
| December 31, 2023 | December 31, 2022 |
| Senior Secured Convertible Note, due August 9, 2027 | $ | 110,000 | | $ | 110,000 | |
| | |
| Less: Unamortized debt issuance costs | 2,875 | | 3,454 | |
| Less: Unamortized debt discount | 20,299 | | 25,925 | |
| Long-term debt, net of unamortized debt discount and issuance costs | $ | 86,826 | | $ | 80,621 | |
The impactamortization of the restatement ondebt issuance costs was charged to interest expense for all periods presented. For the statementyear ended December 31, 2023 and December 31, 2022, the effective yield was 13.38%. The amount of stockholders' equity is consistent with the changes to the impacted stockholders' equity accounts described above.
| | | | | | | | | |
| | For the Nine Months Ended September 30, 2020 (unaudited) |
| | As Reported | | Adjustment | | As Restated |
Supplemental Disclosure of Noncash Financing Activities: | | | | | | | | | |
Initial value of Class A common stock subject to possible redemption | | $ | 208,956,930 | | $ | (208,956,930) | | $ | — |
Change in value of Class A common stock subject to possible redemption | | $ | (5,464,380) | | $ | 5,464,380 | | $ | — |
| | | | | | | | | |
| | Earnings (Loss) Per Share |
| | As Previously |
| | As Reported | | Adjustment | | As Restated |
For the three months ended March 31, 2020 (unaudited) | | | | | | | | | |
Net loss | | $ | (776,839) | | $ | — | | $ | (776,839) |
Weighted average shares outstanding - Class A common stock | | | 23,000,000 | | | (18,197,802) | | | 4,802,198 |
Basic and diluted earnings (loss) per share - Class A common stock | | $ | — | | $ | (0.08) | | $ | (0.08) |
Weighted average shares outstanding - Class B common stock | | | 5,156,593 | | | — | | | 5,156,593 |
Basic and diluted earnings (loss) per share - Class B common stock | | $ | (0.15) | | $ | 0.07 | | $ | (0.08) |
For the three months ended June 30, 2020 (unaudited) | | | | | | | | | |
Net loss | | $ | (2,618,735) | | $ | — | | $ | (2,618,735) |
Weighted average shares outstanding - Class A common stock | | | 23,000,000 | | | — | | | 23,000,000 |
Basic and diluted earnings (loss) per share - Class A common stock | | $ | — | | $ | (0.09) | | $ | (0.09) |
Weighted average shares outstanding - Class B common stock | | | 5,750,000 | | | — | | | 5,750,000 |
Basic and diluted earnings (loss) per share - Class B common stock | | $ | (0.46) | | $ | 0.37 | | $ | (0.09) |
For the six months ended June 30, 2020 (unaudited) | | | | | | | | | |
Net loss | | $ | (3,395,574) | | $ | — | | $ | (3,395,574) |
Weighted average shares outstanding - Class A common stock | | | 23,000,000 | | | (9,098,901) | | | 13,901,099 |
Basic and diluted earnings (loss) per share - Class A common stock | | $ | — | | $ | (0.18) | | $ | (0.18) |
Weighted average shares outstanding - Class B common stock | | | 5,453,297 | | | — | | | 5,453,297 |
Basic and diluted earnings (loss) per share - Class B common stock | | $ | (0.62) | | $ | 0.44 | | $ | (0.18) |
For the three months ended September 30, 2020 (unaudited) | | | | | | | | | |
Net loss | | $ | (2,416,660) | | $ | — | | $ | (2,416,660) |
Weighted average shares outstanding - Class A common stock | | | 23,000,000 | | | — | | | 23,000,000 |
Basic and diluted earnings (loss) per share - Class A common stock | | $ | — | | $ | (0.08) | | $ | (0.08) |
Weighted average shares outstanding - Class B common stock | | | 5,750,000 | | | — | | | 5,750,000 |
Basic and diluted earnings (loss) per share - Class B common stock | | $ | (0.42) | | $ | 0.34 | | $ | (0.08) |
For the nine months ended September 30, 2020 (unaudited) | | | | | | | | | |
Net loss | | $ | (5,812,234) | | $ | — | | $ | (5,812,234) |
Weighted average shares outstanding - Class A common stock | | | 23,000,000 | | | (6,043,796) | | | 16,956,204 |
Basic and diluted earnings (loss) per share - Class A common stock | | $ | — | | $ | (0.26) | | $ | (0.26) |
Weighted average shares outstanding - Class B common stock | | | 5,552,920 | | | — | | | 5,552,920 |
Basic and diluted earnings (loss) per share - Class B common stock | | $ | (1.05) | | $ | 0.79 | | $ | (0.26) |
Note 11 — Income Taxes
The Company’s taxable income primarily consists ofdebt issuance costs included in interest income on the Trust Account, less any franchise taxes. The Company’s formation and operating costs are generally considered start-up costs and are not currently deductible.
The income tax provision (benefit)expense for the year ended December 31, 2020 consists2023 and December 31, 2022 was $6,205 and $2,444, respectively. The Company had interest expense of $4,473 and $1,772 on the Credit Agreement term loan for the years ended December 31, 2023 and December 31, 2022, respectively. The Company had $1,124 and $1,100 of accrued interest as of December 31, 2023 and December 31, 2022, respectively.
On August 9, 2022, the Company also entered into the Guarantee and Security Agreement (“Guarantee Agreement”) with the Agent for the purpose of providing a guarantee of all the obligations under the Facility Agreement (refer to Note 15. Commitments and Contingencies for detail).
Debt Maturities
The following table summarizes the stated debt maturity related to the Senior Secured Convertible Note as of December 31, 2023:
| | | | | | | | |
| (in thousands) | | |
| 2024 | | $ | — | |
| 2025 | | — | |
| 2026 | | — | |
| 2027 | | 110,000 | |
| Total debt | | $ | 110,000 | |
PPP Loan
The Company recorded a PPP loan as a result of the following:
| | | | | | |
| | December 31, 2020 | | December 31, 2019 |
Current | | | | | | |
Federal | | $ | 11,434 | | $ | — |
State | | | — | | | — |
Deferred | | | | | | |
Federal | | | (101,676) | | | (483) |
State | | | — | | | — |
Valuation allowance | | | 101,676 | | | 483 |
Income tax provision | | | 11,434 | | | — |
acquisition of the practice of Leo E. Orr, MD on November 12, 2021 with Pacific Western Bank in the amount of $183, with interest bearing at 1%. The maturity date of the loan is October 24, 2026.
The application for the PPP funds required an entity to, in good faith, certify that the current economic uncertainty made the loan request necessary to support the ongoing operations of the entity. This certification further required the entity to take into account its current business activity and its ability to access other sources of liquidity sufficient to support ongoing operations in a manner that is not significantly detrimental to the business. The receipt of these funds, and the forgiveness of the loan attendant to these funds, is dependent on the entity having initially qualified for the loan and qualifying for the forgiveness of such loan based on its future adherence to the forgiveness criteria. During the year ended December 31, 2022, the Company received notice of forgiveness of its PPP loan and accordingly has recognized the loan principal balance and accrued interest as a gain on loan forgiveness in the Consolidated Statement of Operations. Note 12. Income Taxes
The components of the provision (benefit) for income taxes was deemed to be de minimis for the period from November 1, 2019 (inception) through December 31, 2019.consists of:
| | | | | | | | | | | | | | | | | |
| (in thousands) | Current | | Deferred | | Total |
| Year ended December 31, 2023: | | | | | |
| U.S. federal | $ | 83 | | | $ | 2 | | | $ | 85 | |
| State and local | (43) | | (78) | | (121) |
| $ | 40 | | | $ | (76) | | | $ | (36) | |
| | | | | | | | | | | | | | | | | |
| (in thousands) | Current | | Deferred | | Total |
| Year ended December 31, 2022: | | | | | |
| U.S. federal | $ | — | | | $ | (135) | | | $ | (135) | |
| State and local | 20 | | (128) | | (108) |
| $ | 20 | | | $ | (263) | | | $ | (243) | |
The Company’s netincome tax expense differs from the amount that would have resulted from applying the federal statutory rate of 21% to pretax income from operations because of the effect of the following items:
| | | | | | | | | | | |
| (in thousands) | Year Ended December 31, |
| 2023 | | 2022 |
| Income tax at federal statutory rate | $ | (17,432) | | | $ | (19) | |
| State tax, net federal benefit | (62) | | | (101) | |
| Meals and entertainment | 20 | | | 14 | |
| Transaction costs | 30 | | | 684 | |
| | | |
| Stock based compensation | 1,970 | | | 1,411 | |
| Warrant expense | 60 | | | (387) | |
| Earnout expense | (169) | | | (12,435) | |
| | | |
| | | |
| 162(m) Analysis | 4 | | | — | |
| 162(m) Deferred haircut | 131 | | | 1,433 | |
| 163(l) Interest expense limitation | 2,242 | | | 885 | |
| DFP derivative expense | (184) | | | (5,082) | |
| Goodwill impairment | — | | | 569 | |
| Prior year deferred true-ups | (224) | | | (2,100) | |
| Other state items | — | | | 24 | |
| Amended return | 40 | | | — | |
| | | |
| Change in valuation allowance | 13,538 | | | 14,856 | |
| Other | — | | | 5 | |
| Income tax (benefit) expense | $ | (36) | | | $ | (243) | |
The tax effects of temporary differences that give rise to significant portions of the deferred tax assets and deferred tax liabilities at December 31, 2023 and 2022 are presented below.
| | | | | | | | | | | |
| (in thousands) | December 31, 2023 | | December 31, 2022 |
| Deferred tax assets: | | | |
| | | |
| Accrued Expenses | $ | 1,204 | | | $ | 1,293 | |
| Net operating loss carryforwards | 40,359 | | 26,357 |
| | | |
| Impaired assets | — | | | 1,313 |
| Deferred revenue | 160 | | 334 |
| Stock based compensation | 4,969 | | 3,497 |
| Interest expense limitation | — | | | 21 | |
| Charitable contributions | 1 | | | 1 | |
| Tenant improvement allowance | (21) | | | (43) | |
| ROU Lease liability | 9,446 | | | 7,913 | |
| Financing lease liability | 261 | | | 177 | |
| Unrealized gain/loss | 42 | | | 112 | |
| Intangibles | 7,736 | | | 2,530 | |
| Total gross deferred tax assets | 64,157 | | | 43,505 | |
| Valuation allowance | (53,979) | | | (34,915) |
| Net deferred tax assets | $ | 10,178 | | | $ | 8,590 | |
| Deferred tax liabilities: | | | |
| Property, plant, and equipment | $ | (1,595) | | | $ | (1,507) | |
| | | |
| ROU Asset | (8,363) | | | (7,013) |
| Financial lease asset | (261) | | | (176) |
| IRC 174 expenditures | 9 | | | (2) |
| | | |
| Total gross deferred liabilities | $ | (10,210) | | | $ | (8,698) | |
| Net deferred tax liabilities | $ | (32) | | | $ | (108) | |
The valuation allowance for deferred tax assets as follows:of
December 31, 2023 | | | | | | |
| | December 31, 2020 | | December 31, 2019 |
Deferred tax assets: | | | | | | |
Start-up/Organization costs | | $ | 101,676 | | $ | 315 |
Net operating loss carryforwards | | | — | | | 168 |
Total deferred tax assets | | | 101,676 | | | 483 |
Valuation allowance | | | (101,676) | | | (483) |
Deferred tax asset, net of allowance | | $ | — | | $ | — |
and 2022, was $53,979 and $34,915, respectively. The net change in the total
valuation allowance was an increase of $19,064 in 2023 and an increase of $20,196 in 2022.
The valuation allowance at December 31, 2023 was primarily related to net operating loss carryforwards of TOI, Inc., TOI CA, TOI FL, TOI TX, that, in the judgment of management, are not more likely than not to be realized. Similar to 2022, TOI Inc., TOI CA, TOI FL, and TOI TX will continue to file a consolidated 2023 federal return and state income tax return. Accordingly, net operating losses of TOI CA, TOI FL, and TOI TX can offset taxable income of TOI Parent for federal and state tax purposes. Deferred tax assets and deferred tax liabilities have been separately determined for all groups, as has the valuation allowance assessment for each. The table above reflects the combined deferred tax assets, deferred tax liabilities, and valuation allowance for TOI Inc., TOI CA, TOI FL, and TOI TX. Of the $53,979 total valuation allowance, $38,505 is attributable to the Federal Group, $4,695 is attributable to TOI Parent, $10,698 is attributable to TOI CA, $82 is attributable to TOI FL, and $1 to TOI TX.
In assessing the realizationrealizability of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences representing net future deductible amounts become deductible. Management considers the scheduled reversal of deferred tax assets,liabilities (including the effect of available carry back and carryforward periods), projected future taxable income, and tax planningtax-planning strategies in making this assessment. After considerationBased upon the level of allhistorical taxable income and projections for future taxable income over the periods in which the deferred tax assets are deductible, management believes it is more likely than not that the Company will realize the benefits of these deductible differences, net of the information available, management believes that significant uncertainty exists with respect to future realizationexisting valuation allowances at December 31, 2023. The amount of the deferred tax assetsasset considered realizable, however, could be reduced in the near term if estimates of future taxable income during the carryforward period are reduced.
At December 31, 2023, the Company has net operating loss carryforwards for Federal income tax purposes of $139,195, with $118,455 attributable to the Practice and $20,740 attributable to TOI Parent, which are available to offset future Federal taxable income of the Practice and Parent indefinitely. The Company has therefore establishednet operating loss carryforwards for state income tax purposes of $132,511, of which $107,592 is attributable to the Practice and will begin to expire after 2040, and $24,918 is attributable to Parent and will begin to expire after 2041.
Pursuant to Section 382 of the Internal Revenue Code of 1986, as amended, or the Code, and corresponding provisions of state law, if a full valuation allowance. Forcorporation undergoes an “ownership change” (very generally defined as a greater than 50% change, by value, in the corporation’s equity ownership by certain stockholders or groups of stockholders over a rolling three-year period), the corporation’s ability to use its pre-ownership change NOLs to offset its post-ownership change income may be limited. In 2022 and 2023, we completed an ownership change analysis pursuant to IRC Section 382 of the Code for the period from September 10, 2018 through taxable year ended December 31, 2021 and from January 1, 2022 through taxable year ended December 31, 2022 in which we determined that the Company did not experience an ownership change. We do not anticipate a change in ownership during the year ended December 31, 20202023. Additionally, we may experience ownership changes in the future as a result of subsequent shifts in our stock ownership, some of which may be outside of our control. If it is determined that an ownership change has occurred as a result of the Business Combination or we undergo an ownership change in the future, we may be prevented from fully utilizing our NOLs existing at the time of the ownership change prior to their expiration.
The deferred tax asset associated with the Company’s federal and forstate net operating losses are fully offset by a valuation allowance. Due to the period from November 1, 2019 (inception) to December 31, 2019,existence of the valuation allowance, was $101,676 and $483, respectively.future changes in the Company’s unrecognized tax benefits will not impact its effective tax rate.
A reconciliationsummary of the statutory federalchanges in the amount of unrecognized tax benefits (excluding interest and penalties) for 2023 and 2022 is as follows: | | | | | | | | | | | |
| (in thousands) | December 31, 2023 | | December 31, 2022 |
| Beginning balance of unrecognized tax benefits | $ | 99 | | | $ | 99 | |
| Additions based on tax positions related to the current year | — | | | — | |
| Reductions based on tax positions of prior years | — | | | — | |
| Reductions due to lapse of applicable statute of limitation | — | | | — | |
| Settlements | — | | | — | |
| Ending balance of unrecognized tax benefits | $ | 99 | | | $ | 99 | |
The Company does not anticipate a significant change in the amount of its unrecognized tax within the next 12 months. The Company recognizes interest and penalties related to unrecognized tax benefits in income tax
rate (benefit)expense. Due to the Company’s
effective tax rate is as follows:
| | | | | | |
| | December 31, 2020 | | December 31, 2019 |
Statutory federal income tax | | $ | (1,748,979) | | $ | (483) |
Change in fair value of derivative warrant liabilities | | | (1,450,150) | | | — |
Financing costs - derivative warrant liabilities | | | (2,503) | | | — |
Change in valuation allowance | | | 3,213,066 | | | 483 |
Income tax expense | | $ | 11,434 | | $ | — |
There were 0NOL position, no interest or penalties have been recognized with respect to unrecognized tax benefits, as of December 31, 2020, and 2019. NaNsuch amounts were accrued for the payment of interest and penalties as of December 31, 2020, and 2019.are considered immaterial. The Company is currently not aware of any issues under review that could result in significant payments, accruals or material deviation fromincludes unrecognized tax benefits within other non-current liabilities on its position. consolidated balance sheet.
The Company is subject to incometaxation in the U.S., California, Arizona, Florida, and Texas. As of December 31, 2023, the statute of limitations remains open for tax examinationsyear 2019 through the current year.
Note 13. Stockholders' Equity
Common Stock
As of December 31, 2023 there were 75,879,025 shares issued and 74,145,251 shares outstanding of common stock. As of December 31, 2022, there were 73,265,621 shares issued and outstanding of common stock.
Voting shares
The holders of the Company’s common stock are entitled to one vote for each share of common stock held at all meetings of stockholders (and written actions in lieu of meetings), and there is no cumulative voting.
Dividends
Common stockholders are entitled to receive dividends whenever funds are legally available and when declared by major taxing authorities since inception.the board of directors. No dividends have been declared as of December 31, 2023.
Preferred Stock
Upon the Closing Date of the Business Combination, pursuant to the terms of the Amended and Restated Certificate of Incorporation, the Company authorized 10,000,000 shares of Series A Common Equivalent Preferred Stock (“preferred stock”) with a par value and liquidation preference of $0.0001 per share. The Company’s
management does not expect thatboard of directors has the
total amountauthority, without further action by the stockholders to issue such shares of
unrecognized tax benefits will materially change over the next twelve months.
Note 12 — Subsequent Events
On November 12, 2021, (the “Closing Date”), DFP Healthcare Acquisitions Corp. (“DFP”) completed the business combination pursuantpreferred stock in one or more series, to that certain and Plan of Merger, dated June 28, 2021, by and among DFP, Orion Merger Sub I, Inc., a Delaware corporation and a direct, wholly owned subsidiary of DFP (“First Merger Sub”), Orion Merger Sub II,
LLC, a Delaware limited liability company and a direct, wholly owned subsidiary of DFP (“Second Merger Sub”) and TOI Parent, Inc., a Delaware corporation (“Old TOI”) (as it may be amended and/or restatedestablish, from time to time the “Merger Agreement”number of shares to be included in each such series, and to fix the dividend, voting, and other rights, preferences, and privileges of the shares. Immediately following the Closing Date and as of December 31, 2021, there were 163,510 shares of
preferred stock outstanding. As of December 31, 2023 and December 31, 2022, there were 165,045 shares of preferred stock outstanding.
Conversion
Each share of preferred stock is convertible, at any time on the part of the holder except with respect to the Beneficial Ownership Limitation (defined below), into 100 shares of common stock.
Blocker/Beneficial Ownership Limitation
The preferred stock is subject to a beneficial ownership limitation such that the preferred stock may not, at any time, be convertible into more than 4.9% of the total number of shares of common stock outstanding (“Beneficial Ownership Limitation”). As contemplated
Voting
The holders of preferred stock do not have voting rights in the Company.
Dividends
The holders of preferred stock are entitled to receive dividends whenever funds are legally available and when declared by the Merger Agreement,board of directors on an as-converted basis. No dividends have been declared as of December 31, 2023.
Assumed Public Warrants and Private Placement Warrants
Following the consummation of the Business Combination, holders of the public warrants and private placement warrants are entitled to acquire common stock of the Company. The warrants became exercisable 30 days from the completion of the Business Combination, on December 12, 2021, and will expire five years after the completion of the Business Combination or earlier upon redemption or liquidation. As of December 31, 2023, there are 5,749,986 public warrants outstanding and 3,177,542 private placement warrants outstanding.
Each warrant entitles the holder to purchase one share of common stock for $11.50 per share. Private warrants held by the initial purchaser or certain permitted transferees may be exercised on a cashless basis.
If the reported last sale price of the common stock equals or exceeds $18.00 per share for any 20 trading days within a 30-trading day period ending three business days before the Company sends the notice of redemption to the warrant holders, the Company may redeem all the public warrants at a price of $0.01 per warrant upon not less than 30 days’ prior written notice.
If the Company calls the public warrants for redemption, management will have the option to require all holders that wish to exercise the public warrants to do so on a cashless basis. The Company will not be required to net cash settle the warrants.
The private warrants are exercisable on a cashless basis and are non-redeemable so long as they are held by the initial purchasers or their permitted transferees. If the private warrants are held by someone other than the initial purchasers of their permitted transferees, the private warrants will be redeemable by the Company and exercisable by such holders on the same basis as the public warrants.
Share Repurchase Program
On May 10, 2022, the Company's Board consented to the adoption and approval of the Share Repurchase Program, authorizing up to $20,000 to be spent on the repurchase of the Company's common stock, expiring on December 31, 2022. The Company repurchased and immediately retired 1,500,000 shares of its common stock for $8,745 from a related party (see Note 21) during the year ended December 31, 2022.
On June 14, 2023, the Company's Board approved a share repurchase program with authorization to purchase up to 5 million shares of the Company's stock. The Company repurchased 1,593,128 shares of its common stock for $894 through one or more securities broker-dealers, in open market purchases and negotiated market purchases.
On August 28, 2023, the Company's Board approved a share repurchase program with authorization to purchase up to 2 million shares of the Company’s common stock. The Company repurchased 140,646 shares of its common stock for $125 through one or more securities broker-dealers, in open market purchases and negotiated market purchases.
The financial impact of the share buybacks, including the change in the number of outstanding shares and its effect on earnings per share (EPS), is disclosed in the earnings per share computation in accordance with ASC 260, Earnings Per Share.
Note 14. Share-Based Compensation
Non-Qualified Stock Option Plan
On January 2, 2019, the Company issued and adopted the 2019 Non-Qualified Stock Option Plan (the “2019 Plan”) to incentivize directors, consultants, advisors, and other key employees of the Company and its subsidiaries to continue their association by providing opportunities to participate in the ownership and further growth of the Company. The 2019 Plan provides for the grant of options (the “Stock Options”) to acquire common shares of the Company.
Stock Options are exercised from the pool of shares designated by the appropriate Committee of the Board of Directors. The grant-date fair value of each option award is estimated on the date of grant using the Black-Scholes-Merton option-pricing model. The grant date fair value of the service vesting and the performance vesting options is recognized as an expense over the requisite service period or vesting period and upon the achievement of the performance condition deemed probable of being achieved, respectively. The exercise price of each Stock Option shall be determined by the Committee and may not be less than the fair market value of the common stock on the date of grant. Stock Options have 10-year terms, after which they expire and are no longer exercisable.
The total number of shares of common stock for which Stock Options may be granted under the 2019 Plan shall not exceed 13,640,000. The 2019 Plan was amended on November 6, 2020, pursuant to which the total number of common shares for which Stock Options may be granted under the 2019 Plan shall not exceed 15,640,000.
Stock Options become vested upon fulfillment of either service vesting conditions, performance vesting conditions, or both, as determined by the award agreement entered into by the Company and optionee. The service vesting requirement states that: (i) 25% of the service vesting options shall vest on the first anniversary of the grant date and (ii) the remaining 75% shall vest on an equal monthly-basis, so long as the optionee has remained continuously employed by the Company from the date of the award through the fourth anniversary of the grant date. The performance vesting requirement states that Stock Options shall vest upon sale of the Company only if the optionee has been continuously employed by the Company or its subsidiaries from the grant date through the date of such sale of the Company. For the awards vesting based on service conditions only and that have a graded vesting schedule, the Company recognizes compensation expense for vested awards in earnings, net of actual forfeitures in the period they occur, on a straight-line basis over the requisite service period.
Conversion of the Stock Options
In conjunction with the Business Combination, the Company amended and fully restated the 2019 Plan through the establishment of the 2021 Incentive Plan (“2021 Plan”). Pursuant to the 2021 Plan, each remaining legacy Stock Option from the 2019 Plan that was outstanding immediately prior to the effective timeBusiness Combination, whether vested or unvested, was converted into an option to purchase a number of shares of common stock (each such option, an “Exchanged Option”) equal to the First Merger (the “Effective Time”),product (rounded down to the nearest whole number) of (i) the First Merger Sub merged with and into Oldnumber of shares of Legacy TOI (the “First Merger”), with Old TOI beingstockholders subject to such Stock Option immediately prior to the surviving corporationBusiness Combination, and (ii) an exercise price per share equal to (A) the exercise price per share of such Stock Option immediately followingprior to the First Merger, Old TOI merged with and into the Second Merger Sub (the “Second Merger”), with the Second Merger Sub being the surviving entity and a wholly owned subsidiary of DFP (the First Merger and Second Merger together, the “Business Combination”). Upon the closingconsummation of the Business Combination, DFP changeddivided by (B) the Common Stock Exchange Ratio ("Stock Option Exchange Ratio"). Following the Business Combination, each Exchanged Option that was previously subject to time vesting only, will continue to be governed by the same terms and conditions (including vesting and exercisability terms) as were applicable to the corresponding former old Stock Option immediately prior to the consummation of the Business Combination. Each Exchanged Option that was previously subject to performance vesting, will no longer be subject to the sale of the Company, and was modified to include service requirements only, under which, the Exchange Options will vest on a monthly-basis, so long as the optionee has remained continuously employed by the Company from the date of the Business Combination through the third anniversary of the Closing Date. The Company treated the Exchanged Options that were previously subject to performance conditions as a new award granted at the Closing Date. The Exchanged Options that were previously subject to service vesting only were not modified as a result of the Business Combination.
As of the Closing Date, the 11,850 Stock Options outstanding under the 2019 Plan were converted into 6,925,219 Exchanged Options after effect of the Common Stock Exchange Ratio.
As of December 31, 2023, the total number of shares of common stock remaining available for future awards (e.g., non-qualified stock options, incentive stock options, restricted stock units, restricted stock awards) under the 2021 Plan is 6,008,329.
The weighted average assumptions used in the Black-Scholes-Merton option-pricing model for the units granted during the years ended December 31, 2023 and 2022 Stock Options are provided in the following table:
| | | | | | | | | | | |
| December 31, 2023 | | December 31, 2022 |
| Valuation assumptions: | | | |
| Expected dividend yield | —% | | —% |
| Expected volatility | 56.2% to 64.0% | | 35.00% to 60.00% |
| Risk-free interest rate | 3.40% to 4.30% | | 2.33% to 3.87% |
| Expected term (years) | 6.25 | | 5.75 to 6.65 |
The Company used the simplified method to calculate the expected term of stock option grants because sufficient historical exercise data was not available to provide a reasonable basis for the expected term. Under the simplified method, the expected term is estimated to be the mid-point between the vesting date and the contractual term of the option.
Stock option activity during the years ended December 31, 2023 and 2022 is as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Stock options | | Number of shares | | Weighted average exercise price | | Weighted average remaining contractual term | | Aggregate intrinsic value (in thousands) |
| Balance at January 1, 2023 | | 8,049,474 | | | $ | 2.14 | | | | | |
| Granted | | 1,979,203 | | | 0.50 | | | | | |
| Exercised | | (138,315) | | | 0.91 | | | | | |
| Forfeited | | (1,225,337) | | | 2.36 | | | | | |
| Expired | | (139,763) | | | 2.18 | | | | |
Balance at December 31, 2023 | | 8,525,262 | | | $ | 1.74 | | | 7.04 | | $ | 8,220 | |
Vested Options Exercisable at December 31, 2023 | | 4,598,066 | | | $ | 1.60 | | | 6.05 | | $ | 4,550 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Stock options | | Number of shares | | Weighted average exercise price | | Weighted average remaining contractual term | | Aggregate intrinsic value (in thousands) |
| Balance at January 1, 2022 | | 6,921,180 | | $ | 0.88 | | | | | |
| Granted | | 2,940,064 | | | 4.67 | | | | | |
| Exercised | | (973,389) | | | 0.90 | | | | | |
| Forfeited | | (836,505) | | | 2.35 | | | | | |
| Expired | | (1,876) | | | 0.97 | | | | | |
| Balance at December 31, 2022 | | 8,049,474 | | | $ | 2.14 | | | 7.64 | | $ | 4,081 | |
| Vested Options Exercisable at December 31, 2022 | | 2,860,085 | | | $ | 1.34 | | | 6.90 | | $ | 2,061 | |
Total share-based compensation expense during the years ended December 31, 2023 and 2022 was $10,342 and $11,602, respectively, excluding costs associated with rolled over units and new units issued or replaced in connection with the Business Combination, respectively.
At December 31, 2023 there was $10,591 of total unrecognized compensation cost related to unvested service Stock Options granted under the 2021 Plan and 2019 Plan that are expected to vest. That cost is expected to be recognized over a weighted average period of 2.42 years as of December 31, 2023. During the year ended December 31, 2023, the Company received $126 in cash and $132 in tax benefit from the stock options exercised. The total fair value of common shares vested during the years ended December 31, 2023 and 2022 was $3,942 and $2,951, respectively.
Restricted Stock Awards (“RSAs”) and Restricted Stock Units (“RSUs”)
Agajanian Holdings (“Holdings”), a holder of Series A Preferred Stock of Legacy TOI, entered into arrangements with physicians employed by the TOI PCs to issue RSAs which represent Series A Preferred Stock of Legacy TOI. The Legacy TOI
RSAs only have performance vesting requirements linked to the sale of the Company so long as the grantee remains continuously and actively employed by the Company’s subsidiaries through the vesting date.
Conversion of the RSAs
Each of the Legacy TOI RSAs, from the Plan that was outstanding immediately prior to the Business Combination, whether vested or unvested, was converted into an RSU equal to the product (rounded down to the nearest whole number) of (i) the number of shares of RSAs immediately prior to the Business Combination, (ii) conversion rate of 1:10 of the Series A Preferred Stock of Legacy TOI, and (iii) the Common Stock Exchange Ratio. Following the Business Combination, each RSU is no longer subject to the sale of the Company event in order to vest, but was modified to include service requirements only. The service vesting requirement states that: (i) 16.67% of the RSUs shall vest on the sixth month anniversary of the Closing Date, and (ii) the remaining 83.33% shall vest on an equal quarterly-basis, so long as the grantee has remained continuously employed by the Company from the date of the award through the third anniversary of the grant date. The Company treated the RSUs that were previously subject to performance conditions as a new award granted at the Closing Date. All RSAs activity was retroactively restated to reflect the RSUs.
As of the Closing Date, the 2,210 RSAs outstanding under the Plan were converted into 1,291,492 RSUs upon the completion of the Business Combination after effect of the Common Stock Exchange Ratio.
The weighted-average grant date fair values of the RSUs granted during the year ended December 31, 2023 and 2022 were determined to be $0.83 and $5.74, respectively, based on the fair value of the Company’s common shares at the grant date.
A summary of the activity for the RSUs and RSAs for the years ended December 31, 2023 and 2022, respectively, are shown in the following tables: | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| | 2023 | | 2022 |
| | Number of Shares | | Weighted Average Grant Date Fair Value | | Number of Shares | | Weighted Average Grant Date Fair Value |
| Unvested at beginning of year | | 2,106,540 | | | $ | 7.25 | | | 1,291,492 | | | $ | 10.98 | |
| Granted | | 2,332,757 | | | 0.83 | | | 2,163,135 | | | 5.74 | |
| Vested | | (1,501,805) | | | 3.04 | | | (760,973) | | | 9.31 | |
| Forfeited | | (761,070) | | | 4.23 | | | (587,114) | | | 7.21 | |
| Unvested at end of year | | 2,176,422 | | | $ | 3.50 | | | 2,106,540 | | | $ | 7.25 | |
| | | | | | | | |
The total share-based compensation expense during the year ended December 31, 2023 was $5,959 related to the RSUs. The total share-based compensation expense during the year ended December 31, 2022 was $8,284 related to the RSUs.
As of December 31, 2023, there was $7,620 of unrecognized compensation expense related to the RSUs and RSAs that are expected to vest. That cost is expected to be recognized over a weighted average period of 2.00 years as of December 31, 2023.
RSUs granted to Medical Employees and Nonemployees
In 2022, the Company entered into arrangements with certain medical directors and supervisors of advanced practice providers employed by or engaged as independent contractors of TOI to issue RSUs of the Company (“Medical RSUs”). Vesting on each annual Medical RSU award is dependent on the participant performing a specified minimum number of service hours during the calendar year (“one-Year Term”) and further contingent upon the participant’s continued service to, or employment by, the Company through the grant date. The Company’s regular grant date for these Medical RSU awards is in the first quarter of the calendar year following the one-Year Term.
The number of Medical RSUs granted to each such participant is determined by dividing a fixed monetary value by the trailing five-day closing price per share of the Common Stock preceding the grant date. Due to the calculation, some Medical RSU awards are liability-classified whereas other Medical RSU awards have a fixed number of shares and are equity-classified.
In the fourth quarter of 2022, the Company amended the terms of Medical RSUs previously issued to approximately 21 participants during the first quarter of 2022. The amendment primarily updated the vesting period and conditions. The original terms of the Medical RSU awards were deemed improbable of vesting at the modification date whereas the amended Medical RSU awards were deemed probable of vesting at the modification date, and thus are a Type III modification under ASC 718.
The modification to the Medical RSUs resulted in $187 incremental share-based compensation expense before forfeitures, $(11) after accounting for forfeitures related to participants who did not perform the minimum number of service hours specified, recorded in the Company's Statement of Operations.
The total fair value of the liability-classified Medical RSU awards granted in 2022 and outstanding as of December 31, 2022 was approximately $264, which represents the fixed monetary value of the awards. There are no Medical RSU awards outstanding as of December 31, 2023. The weighted-average grant-date fair value, based on the Company’s share price on the modification date, was $3.56 for equity-classified Medical RSUs granted during 2022 and outstanding as of December 31, 2022.
A summary of the activity for the equity-classified Medical RSUs for the year ended December 31, 2023 is shown in the following table: | | | | | |
| Number of Shares |
| Balance at January 1, 2022 | — | |
| Granted | 208,881 | |
| Vested | — | |
| Forfeited | (61,411) | |
| Balance at December 31, 2022 | 147,470 | |
| Granted | 824,288 | |
| Vested | (971,758) | |
| Forfeited | — | |
| Balance at December 31, 2023 | — | |
Total compensation costs for Medical RSUs were $872 and $618 for the years ended December 31, 2023 and December 31, 2022, respectively. As of December 31, 2023, all Medical RSUs have vested.
Earnout Shares granted to Employees
As described in Note 2, the Company issued Earnout Shares to Legacy TOI option holders and Legacy RSU holders (“Option-holders Earnout” and “RSU-holders Earnout”, respectively, together “Employees Earnout Shares”).
The Option-holders Earnout vests upon the Company common stock achieving the price per share as provided above, so long as the optionee has remained continuously employed by the Company at that date. The RSU-holders Earnout vests upon (a) the Company common stock achieving the price per share as provided above, and (b) the underlying RSU vested, so long as the optionee has remained continuously employed by the Company at that date.
The grant date fair value of the First Earnout Tranche and Second Earnout Tranche as of Closing Date was determined to be $8.35 and $6.76, respectively.
A summary of the activity for the Employees Earnout Shares for the years ended December 31, 2023 and 2022 is shown in the following tables:
| | | | | | | | | | | | | | |
| | Year Ended December 31, |
| | 2023 | | 2022 |
| Outstanding at beginning of year | | 1,417,632 | | | 1,602,435 | |
| Granted | | — | | | — | |
| Forfeited | | (16,568) | | | (184,803) | |
| Outstanding at end of year | | 1,401,064 | | | 1,417,632 | |
The total share-based compensation expense related to the Employees Earnout Shares during the years ended December 31, 2023 and 2022 was $375 and $7,911, respectively.
As of December 31, 2023, there was $87 of unrecognized compensation expense related to the Employees Earnout Shares, that are expected to vest. That cost is expected to be recognized over a weighted average period of 0.29 years as of December 31, 2023. As of December 31, 2023, none of the Employee Earnout Shares have vested.
Note 15. Commitments and Contingencies
The Company evaluates contingencies based upon available evidence. In addition, allowances for losses are provided each year for disputed items which have continuing significance. The Company believes that allowances for losses have been provided to the extent necessary, and that its nameassessment of contingencies is reasonable. Due to “Thethe inherent uncertainties and subjectivity involved in accounting for contingencies, there is at least a reasonable possibility that recorded estimates will change by a material amount in the near term. To the extent that the resolution of contingencies results in amounts which vary from management’s estimates, future operating results will be charged or credited. The principal commitments and contingencies are described below.
Legal Matters
The Company is subject to certain outside claims and litigation arising in the ordinary course of business. In the opinion of Management, the outcome of such matters will not have a material effect on the Company’s consolidated financial statements. Loss contingencies entail uncertainty and a possibility of loss to an entity. If the loss is probable and the amount of loss can be reasonably estimated, the loss should be accrued according to Accounting Standards Codification No. 450-20, Disclosure of Certain Loss Contingencies.
The Company’s Articles of Incorporation and bylaws require it, among other things, to indemnify the director or officer against specified expenses and liabilities, such as attorneys’ fees, judgments, fines, and settlements, paid by the individual in connection with any action, suit, or proceeding arising out of the individual’s status or service as its director or officer, other than liabilities arising from willful misconduct or conduct that is knowingly fraudulent or deliberately dishonest, and to advance expenses incurred by the individual in connection with any proceeding against the individual with respect to which the individual may be entitled to indemnification by the Company. The Company also indemnifies its lessor in connection with its facility lease for certain claims arising from the use of the facilities. These indemnities do not provide for any limitation of the maximum potential future payments it could be obligated to make. Historically, the Company has not incurred any payments for these obligations and, therefore, no liabilities have been recorded for these indemnities in the accompanying consolidated balance sheets.
The Health Insurance Portability and Accountability Act
The Health Insurance Portability and Accountability Act (“HIPAA”) assures health insurance portability, reduces healthcare fraud and abuse, guarantees security and privacy of health information, and enforces standards for health information. Organizations are required to be in compliance with HIPAA provisions. The Health Information Technology for Economic and Clinical Health Act (“HITECH”) imposes notification requirements in the event of certain security breaches relating to protected health information. Organizations are subject to significant fines and penalties if found not to be compliant with the provisions outlined in the regulations. The Company believes it is in compliance with these laws.
Regulatory Matters
Laws and regulations governing the Medicare program and healthcare generally, are complex and subject to interpretation. The Company believes that it is in compliance with all applicable laws and regulations and is not aware of any pending or threatened investigations involving allegations of potential wrongdoing. While no regulatory inquiries have been made, compliance with such laws and regulations can be subject to future government review and interpretation as well as significant regulatory action including fines, penalties, and exclusion from the Medicare and Medi-Cal programs.
Many of the Company’s payor and provider contracts are complex in nature and may be subject to differing interpretations regarding amounts due for the provision of medical services. Such differing interpretations may not come to light until a substantial period of time has passed following contract implementation. Liabilities for claims disputes are recorded when the loss is probable and can be estimated. Any adjustments to reserves are reflected in current operations. The Company does not have any reserves for regulatory matters as of December 31, 2023 and December 31, 2022.
Liability Insurance
The Company believes that its insurance coverage is appropriate based upon the Company’s claims experience and the nature and risks of the Company’s business. In addition to the known incidents that have resulted in the assertion of claims, the Company cannot be certain that its insurance coverage will be adequate to cover liabilities, arising out of claims asserted against the Company or the Company’s affiliated professional organizations, in the future where the outcomes of such claims are unfavorable.
The Company believes that the ultimate resolution of all pending claims, including liabilities in excess of the Company’s insurance coverage, will not have a material adverse effect on the Company’s financial position, results of operations or cash flows; however, there can be no assurance that future claims will not have such a material adverse effect on the Company’s business. Contracted physicians are required to obtain their own insurance coverage.
Guarantees
The Company, along with certain of the Company's subsidiaries from time to time party to the Facility Agreement (“Guarantors”), has pledged a first priority perfected lien on substantially all of their respective personal and real property, as collateral security for the payment of outstanding obligations, under the Facility Agreement.
Note 16. Business Combinations
During the year ended December 31, 2022, the Company closed on five business combinations and one asset acquisition, consistent with the intent to strategically grow its existing markets and expand into new markets. During the year ended December 31, 2023, the Company closed on two business combinations and no asset acquisitions.
Practice Acquisitions
For the acquisition of various clinical practices, the Company applied the acquisition method of accounting, where the total purchase price was allocated, or preliminarily allocated, to the tangible and intangible assets acquired and liabilities assumed, based on their fair values as of the acquisition dates.
Perkins Practice Acquisition
On April 30, 2022 ("Perkins Acquisition Date"), the Company acquired certain non-clinical assets of California Oncology of the Central Valley Medical Group, Inc., (the “Perkins Practice”) from Christopher Perkins, M.D. (“Dr. Perkins”). Further, TOI CA acquired certain clinical assets of the Perkins Practice from Dr. Perkins. In conjunction with the acquisition, the Company also entered into a Professional Service Agreement with Oncology Associates of Fresno Medical Group, Inc. Intangible assets were recognized pursuant to the acquisition in the form of trade names of $2,480 and clinical contracts of $70, with weighted average amortization periods of 10 years and 5 years respectively. The Company transferred cash consideration of $8,920 and contingent consideration of $2,000 to Dr. Perkins for the purchase. The contingent cash consideration was to be paid in two equal installments on the first and second anniversary of the transaction closing date (April 29, 2023 and 2024, respectively), pending Dr. Perkins' continued employment at that time. Dr. Perkins terminated his employment with the Company before the first anniversary date, therefore no contingent consideration is payable as of December 31, 2023.
The Perkins Practice Acquisition was determined to constitute a business combination in accordance with ASC 805.
Parikh Practice Acquisition
On July 22, 2022 ("Parikh Acquisition Date"), the Company acquired certain non-clinical assets of Nutan K. Parikh, M.D., LTD., (the “Parikh Practice”) from Nutan K. Parikh, M.D. (“Dr. Parikh”). Further, TOI CA acquired certain clinical assets of the Parikh Practice from Dr. Parikh. Intangible assets of $20 were recognized pursuant to the acquisition in the form of clinical contracts with a weighted average amortization period of 3 years. The Company transferred cash consideration of $1,908 and contingent consideration of $400 to Dr. Parikh for the purchase. The contingent cash consideration is to be paid in two equal installments on the first and second anniversary of the transaction closing date (July 22, 2023 and 2024, respectively), pending Dr. Parikh's continued employment at that time. As of December 31, 2023, the Company paid its first installment of the contingent cash consideration. The contingent consideration is accounted for as post-combination compensation expense to Dr. Parikh.
The Parikh Practice Acquisition was determined to constitute a business combination in accordance with ASC 805.
Barreras Practice Acquisition
On August 30, 2022 ("Barreras Acquisition Date"), the Company acquired certain non-clinical assets of Broward Oncology Associates, P.A., (the “Barreras Practice”) from Luis Barreras, M.D. (“Dr. Barreras”). Further, TOI FL acquired certain clinical assets of the Barreras Practice from Dr. Barreras. Intangible assets of $3 were recognized pursuant to the acquisition in the form of clinical contracts with a weighted average amortization period of 5 years. The Company transferred cash consideration of $929 and contingent consideration of $250 to Dr. Barreras for the purchase. The contingent cash consideration is to be paid in two equal installments on the first and second anniversary of the transaction closing date (August 30, 2023 and 2024, respectively), pending Dr. Barreras's continued employment at that time. As of December 31, 2023, the Company paid its first
installment of the contingent cash consideration. The contingent consideration is accounted for as post-combination compensation expense to Dr. Barreras.
The Barreras Practice Acquisition was determined to constitute a business combination in accordance with ASC 805.
De La Rosa Costa Practice Acquisition
On October 7, 2022 ("De La Rosa Costa Acquisition Date"), the Company acquired certain non-clinical assets of Pedro De La Rosa Costa, M.D. PA, (the “De La Rosa Costa Practice”) from Pedro U De La Rosa Costa, M.D. (“Dr. De La Rosa Costa”). Further, TOI FL acquired certain clinical assets of the De La Rosa Costa Practice from Dr. De La Rosa Costa. The Company transferred cash consideration of $25 to Dr. De La Rosa Costa for the purchase.
The De La Rosa Costa Practice Acquisition was determined to constitute a business combination in accordance with ASC 805.
Hashimi Practice Acquisition
On November 21, 2022 ("Hashimi Acquisition Date"), the Company acquired certain non-clinical assets of Intercommunity Oncology of Chino Hills, A.P.C., Inc., (the “Hashimi Practice”) from Labib Hashimi, M.D. (“Dr. Hashimi”). Further, TOI CA acquired certain clinical assets of the Hashimi Practice from Dr. Hashimi. Intangible assets of $24 were recognized pursuant to the acquisition in the form of clinical contracts with a weighted average amortization period of 5 years. The Company transferred cash consideration of $445 and contingent consideration of $150 to Dr. Hashimi for the purchase. The contingent cash consideration is to be paid in three equal installments on the first, second, and third anniversary of the transaction closing date (November 21, 2023, 2024, and 2025, respectively), pending Dr. Hashimi's continued employment at that time. The contingent consideration is accounted for as post-combination compensation expense to Dr. Hashimi.
The Hashimi Practice Acquisition was determined to constitute a business combination in accordance with ASC 805.
Southland Practice Acquisition
On June 5, 2023 ("Southland Acquisition Date"), the Company acquired certain non-clinical assets of Covina Cancer Care Medical Center Inc. d/b/a Southland Radiation Oncology Network from Arvind Lapsiwala, M.D. (“Dr. Arvind”). Intangible assets of $2,844 were provisionally recognized pursuant to the acquisition in the form of payor contracts and non-compete agreements with a weighted average amortization period of 18 and 5 years, respectively. The Company transferred purchase considerations that consisted of $4,300 in cash paid upon closing and contingent consideration of $2,072. The deferred contingent cash consideration represents a fixed amount that is contingent upon the non-cancellation of the Transition Services Agreement by the seller. The fair value of the deferred cash consideration liability was determined to be $1,813 at the acquisition date. The contingent cash consideration is to be paid in full on the first anniversary of the transaction closing date (June 5, 2024), pending non-cancellation of the services agreement.
The Southland Practice Acquisition was determined to constitute a business combination in accordance with ASC 805. The deferred cash consideration liability will be remeasured at each reporting period until the contingent milestone is achieved or the liability is settled. Any changes in the fair value of the deferred cash consideration liability will be provisionally recognized in the Consolidated Statements of Operations. The Company recognized $131 for the period ended December 31, 2023 in the Consolidated Statements of Operations for the change in fair value for the deferred cash consideration liability. The fair value of the deferred cash consideration liability was $1,944 at December 31, 2023.
Bolsa Pharmacy Acquisition
On November 28, 2023 ("Bolsa Acquisition Date"), the Company acquired certain clinical and non-clinical assets of Bolsa Medical Pharmacy. Intangible assets of $113 were provisionally recognized pursuant to the acquisition in the form of clinical contracts and licenses with a weighted average amortization period of 10 and 2 years, respectively. The Company transferred purchase consideration of $157 in cash paid upon closing.
The Bolsa Practice Acquisition was determined to constitute a business combination in accordance with ASC 805.
Summary of Consideration Transferred
Goodwill is calculated as the excess of the consideration transferred over the net assets recognized and represents the estimated future economic benefits arising from other assets acquired that could not be individually identified and separately recognized. Such assets include synergies the Company expects to achieve, such as the use of the Company's existing infrastructure to support the added membership, and future economic benefits arising from the assembled workforce. The purchase consideration for the acquisitions has been allocated under the acquisition method of accounting to the estimated fair
market value of the net assets acquired including a residual amount to goodwill, which is expected to be deductible for tax purposes, as noted in the fair value table below.
Acquisition costs amounted to $114 and $790 for the years ended December 31, 2023 and 2022 respectively, and were recorded as “General and administrative expenses” in the accompanying Consolidated Statements of Operations.
The following table summarizes the fair values assigned to identifiable assets acquired and liabilities assumed.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| |
| (in thousands) | Perkins | Parikh | Barreras | De La Rosa Costa | Hashimi | Southland provisional | Bolsa provisional | Total |
| Consideration: | | | | | | | | |
| Cash | $ | 8,920 | | $ | 1,908 | | $ | 929 | | $ | 25 | | $ | 445 | | $ | 4,300 | | $ | 157 | | $ | 16,684 | |
| Deferred | — | | — | | — | | — | | — | | 1,813 | | — | | 1,813 | |
| Fair value of total consideration transferred | $ | 8,920 | | $ | 1,908 | | $ | 929 | | $ | 25 | | $ | 445 | | $ | 6,113 | | $ | 157 | | $ | 18,497 | |
| | | | | | | | |
| Estimated fair value of identifiable assets acquired and liabilities assumed: |
| | | | | | | | |
| | | | | | | | |
| Inventory | $ | 408 | | $ | 307 | | $ | 279 | | $ | — | | $ | 95 | | $ | — | | $ | 32 | | $ | 1,121 | |
| | | | | | | | |
| Property and equipment, net | 123 | | 15 | | 23 | | — | | 5 | | 590 | | 12 | | 768 | |
| Operating right of use assets | 447 | | 1,118 | | 83 | | 6 | | 88 | | 4,246 | | 44 | | 6,032 | |
| Clinical contracts and noncompetes | 70 | | 20 | | 3 | | — | | 24 | | 2,844 | | 113 | | 3,074 | |
| Trade names | 2,480 | | — | | — | | — | | — | | — | | — | | 2,480 | |
| Goodwill | 5,851 | | 1,566 | | 624 | | 25 | | 321 | | 2,679 | | — | | 11,066 | |
| Total assets acquired | 9,379 | | 3,026 | | 1,012 | | 31 | | 533 | | 10,359 | | 201 | | 24,541 | |
| | | | | | | | |
| Current portion of operating lease liabilities | 135 | | 169 | | 60 | | 6 | | 26 | | 378 | | 27 | | 801 | |
| Accrued liabilities | 12 | | — | | — | | — | | — | | — | | — | | 12 | |
| | | | | | | | |
| Operating lease liabilities | 312 | | 949 | | 23 | | — | | 62 | | 3,868 | | 17 | | 5,231 | |
| Total liabilities assumed | 459 | | 1,118 | | 83 | | 6 | | 88 | | 4,246 | | 44 | | 6,044 | |
| Net assets acquired | $ | 8,920 | | $ | 1,908 | | $ | 929 | | $ | 25 | | $ | 445 | | $ | 6,113 | | $ | 157 | | $ | 18,497 | |
The establishment of the allocation to goodwill requires the extensive use of accounting estimates and management judgement. The fair values assigned to the assets acquired are based on estimates and assumptions from data that is readily available.
Summary of UnauditedSupplementalProFormaInformation
The Company recognized $2,921 cumulative revenue and $1,723 cumulative net income in its Consolidated Statement of Operations for the year ended December 31, 2023, from the clinical practices acquired during the year ended December 31, 2023.
The Company recognized $12,981 cumulative revenue and $5 cumulative net loss in its Consolidated Statement of Operations for the year ended December 31, 2022, from the clinical practices acquired during the year ended December 31, 2022.
The pro forma results presented below include the effects of the Acquisitions which occurred during the year ended December 31, 2023, as if they had occurred on January 1, 2022. The pro forma results for the year ended December 31, 2023 and 2022 include the additional amortization resulting from the adjustments to the value of intangible assets resulting from purchase accounting. The pro forma results do not include any anticipated synergies or other expected benefits of the acquisitions. The pro forma information does not purport to be indicative of what the Company's results of operations would
have been if the acquisitions had in fact occurred at the beginning of the period presented and is not intended to be a projection of the Company's future results of operations. Transaction expenses are included within the pro forma results.
| | | | | | | | | | | | | | | | | |
| (in thousands) | | | Year Ended December 31, |
| | | | 2023 | | 2022 |
| Revenue | | | | | $ | 326,349 | | | $ | 256,756 | |
| Net income (loss) | | | | | $ | (83,177) | | | $ | 38 | |
Sapra Asset Acquisition
On July 1, 2022 ("Sapra Acquisition Date"), the Company acquired certain clinical assets of Ranjan K. Sapra, M.D. (the “Sapra Practice”) from Ranjan K. Sapra, M.D. (“Dr. Sapra”). The Company transferred cash consideration of $1 to Dr. Sapra for the purchase, which was assigned to property and equipment.
Note 17. Variable Interest Entities
The Company prepares its consolidated financial statements in accordance with Accounting Standards Codification Topic No. 810, Consolidations (“ASC 810”), which provides for the consolidation of VIEs of which an entity is the primary beneficiary.
Pursuant to the MSAs established with the TOI PCs, TOI Management is entitled to receive a management fee, which represents a variable interest in and the right to receive the benefits of the TOI PCs. Through the terms of the MSAs, TOI Management receives the right to direct the most significant activities of the TOI PCs. Therefore, the TOI PCs are variable interest entities and TOI Management is the primary beneficiary that consolidates the TOI PCs, and their subsidiaries.
The consolidated financial statements include the accounts of TOI and its subsidiaries and VIEs. All inter-company profits, transactions, and balances have been eliminated upon consolidation.
| | | | | | | | | | | |
| (in thousands) | December 31, 2023 | | December 31, 2022 |
| Assets | | | |
| Current assets: | | | |
| Cash | $ | 2,282 | | | $ | 1,070 | |
| Accounts receivable, net | 45,175 | | | 39,817 | |
| Other receivables | 129 | | | 220 | |
| Inventories | 13,646 | | | 9,262 | |
| Prepaid expenses and other current assets | 1,136 | | | 841 | |
| Total current assets | 62,368 | | | 51,210 | |
| Property and equipment, net | 105 | | | 168 | |
| Other assets | 525 | | | 441 | |
| Intangible assets, net | 5,628 | | | 3,343 | |
| Goodwill | 2,679 | | | 15,832 | |
| Total assets | $ | 71,305 | | | $ | 70,994 | |
| Liabilities | | | |
| Current liabilities: | | | |
| Accounts payable | $ | 12,729 | | | $ | 8,296 | |
| Income taxes payable | — | | | 132 | |
| Accrued expenses and other current liabilities | 8,413 | | | 5,129 | |
| | | |
| Amounts due to affiliates | 189,048 | | | 140,218 | |
| Total current liabilities | 210,190 | | | 153,775 | |
| Other non-current liabilities | 211 | | | 739 | |
| Deferred income taxes liability | 21 | | | 58 | |
| Total liabilities | $ | 210,422 | | | $ | 154,572 | |
Single physician holders, who are officers of the Company, retain equity ownership in TOI CA, TOI FL and TOI TX, which represents nominal noncontrolling interests. The noncontrolling interests do not participate in the profit or loss of TOI CA, TOI FL, or TOI TX, however.
Note 18. Goodwill and Intangible Assets
The Company accounts for goodwill at acquisition-date fair value, net of impairments recognized and other intangible assets at acquisition-date fair value less accumulated amortization. See Note 2 for a summary of the Company’s policies relating to goodwill and intangible assets, as well as a discussion of the goodwill impairment charges recorded for the years ended December 31, 2023 and December 31, 2022.
Intangible Assets
As of December 31, 2023, the Company’s intangible assets, net consists of the following:
| | | | | | | | | | | | | | | | | | | | | | | |
| (in thousands) | Weighted average amortization period | | Gross carrying amount | | Accumulated amortization | | Net carrying amount |
| Intangible assets | | | | | | | |
| Amortizing intangible assets: | | | | | | | |
| Payor contracts | 13 years | | $ | 22,191 | | | $ | (10,014) | | | $ | 12,177 | |
| Trade names | 10 years | | 6,650 | | | (2,594) | | | 4,056 | |
| Clinical contracts and noncompetes | 8 years | | 3,191 | | | (1,520) | | | 1,671 | |
| Total intangible assets | | | $ | 32,032 | | | $ | (14,128) | | | $ | 17,904 | |
As of December 31, 2022, the Company’s intangible assets, net consists of the following:
| | | | | | | | | | | | | | | | | | | | | | | |
| (in thousands) | Weighted average amortization period | | Gross carrying amount | | Accumulated amortization | | Net carrying amount |
| Intangible assets | | | | | | | |
| Amortizing intangible assets: | | | | | | | |
| Payor contracts | 10 years | | $ | 19,400 | | | $ | (8,038) | | | $ | 11,362 | |
| Trade names | 10 years | | 6,650 | | | (1,941) | | | 4,709 | |
| Clinical contracts and noncompetes | 8 years | | 3,025 | | | (1,139) | | | 1,886 | |
| Total intangible assets | | | $ | 29,075 | | | $ | (11,118) | | | $ | 17,957 | |
The estimated aggregate amortization expense for each of the five succeeding fiscal years as of December 31, 2023 is as follows:
| | | | | |
| (in thousands) | Amount |
| Year ending December 31: | |
| 2024 | $ | 3,078 | |
| 2025 | 3,075 | |
| 2026 | 3,050 | |
| 2027 | 2,923 | |
| 2028 | 2,818 | |
| Thereafter | 2,960 | |
| Total | $ | 17,904 | |
The aggregate amortization expense during the year ended December 31, 2023 and 2022 were $3,009 and $2,885, respectively.
Goodwill
The Company evaluates goodwill at the reporting unit level, which, for the Company, is at the level of the reportable segments of patient services, dispensary, and clinical trials & other. The goodwill allocated to each of the reporting units as of December 31, 2023 and December 31, 2022 is as follows:
| | | | | | | | | | | |
| (in thousands) | December 31, 2023 | | December 31, 2022 |
| Patient services | $ | 2,679 | | | $ | 16,235 | |
| Dispensary | 4,551 | | | 4,551 | |
| Clinical trials & other | — | | | 632 | |
| Total goodwill | $ | 7,230 | | | $ | 21,418 | |
The changes in the carrying amounts of goodwill for the year ended December 31, 2023 and December 31, 2022 are as follows:
| | | | | | | | | | | |
| (in thousands) | December 31, 2023 | | December 31, 2022 |
| Balance as of January 1: | $ | 21,418 | | | $ | 26,626 | |
| Goodwill acquired | 2,679 | | | 4,736 | |
| Goodwill impairment charges (see Note 2) | (16,867) | | | (9,944) | |
| Goodwill, net as of December 31 | $ | 7,230 | | | $ | 21,418 | |
The accumulated goodwill impairment for patient services was $26,179 and $9,944, and $0 as of December 31, 2023, December 31, 2022, and January 1, 2022, respectively. The accumulated goodwill impairment for clinical trails & others was $632,$0, and $0 as of December 31, 2023, December 31, 2022, and January 1, 2022, respectively. There was no accumulated goodwill impairment for dispensary as of December 31, 2023, December 31, 2022 and January 1, 2022, respectively.
Note 19. Net Income (Loss) Per Share
The following table sets forth the computation of the Company's basic net income (loss) per share to common stockholders for the years ended December 31, 2023 and 2022.
| | | | | | | | | | | | | | |
| (in thousands, except share data) | | Year Ended December 31, |
| 2023 | | 2022 |
| Net income (loss) attributable to TOI | | $ | (83,068) | | | $ | 152 | |
| Less: Deemed dividend | | — | | | 64 | |
| Net income (loss) attributable to TOI available for distribution | | (83,068) | | | 88 | |
| Net income (loss) attributable to participating securities, basic | | (15,191) | | | 20 | |
| Net income (loss) attributable to common stockholders, basic | | $ | (67,877) | | | $ | 68 | |
| Weighted average common shares outstanding, basic | | 73,748,660 | | | 72,793,497 | |
| Net income (loss) per share attributable to common stockholders, basic | | $ | (0.92) | | | $ | — | |
The following table sets forth the computation of the Company's diluted net loss per share to common stockholders for the years ended December 31, 2023 and 2022.
| | | | | | | | | | | | | | |
| (in thousands, except share data) | | Year Ended December 31, |
| 2023 | | 2022 |
| Net income (loss) attributable to TOI | | $ | (83,068) | | | $ | 152 | |
| Less: Deemed dividend | | — | | | 64 | |
Less: Change in fair value of convertible option derivative liabilities(1) | | — | | | 20,656 | |
| Net loss attributable to TOI available for distribution | | (83,068) | | | (20,568) | |
| Net loss attributable to participating securities, diluted | | (15,191) | | | (3,588) | |
| Net loss attributable to common stockholders, diluted | | $ | (67,877) | | | $ | (16,980) | |
| Weighted average common shares outstanding, basic | | 73,748,660 | | | 72,793,497 | |
| Dilutive effect of stock options | | — | | | 2,572,570 | |
| Dilutive effect of RSUs | | — | | | 77,717 | |
| Dilutive effect of Medical RSUs | | — | | | 61,007 | |
| Dilutive effect of convertible note | | — | | | 5,100,809 | |
| Weighted average shares outstanding, diluted | | 73,748,660 | | | 80,605,600 | |
| Net loss per share attributable to common stockholders, diluted | | $ | (0.92) | | | $ | (0.21) | |
(1) Inclusive of interest expense and amortization of debt issuance cost and debt discount related to the Senior Secured Convertible Note.
The following potentially dilutive outstanding securities were excluded from the computation of diluted net loss per share because their effect would have been anti-dilutive for the periods presented:
| | | | | | | | | | | | | | |
| | Year Ended December 31, |
| | 2023 | | 2022 |
| Convertible note | | 12,839,967 | | | — | |
| Stock options | | 8,525,262 | | | 4,461,592 | |
| RSUs | | 2,176,422 | | | 1,677,516 | |
| Medical RSUs | | — | | | 301,396 | |
| Earnout Shares | | 1,401,064 | | | 1,417,632 | |
| Public Warrants | | 5,749,986 | | | 5,749,986 | |
| Private Warrants | | 3,177,542 | | | 3,177,542 | |
Note 20. Segment Information
The Company operates its business and reports its results through three operating and reportable segments: dispensary, patient services, and clinical trials & other in accordance with ASC 280. See Note 2 for a summary of the Company’s policy on segment information.
Summarized financial information for the Company’s segments is shown in the following tables:
| | | | | | | | | | | | | | |
| (in thousands) | | Year Ended December 31, |
| 2023 | | 2022 |
| Revenue | | | | |
| Patient services | | $ | 213,504 | | | $ | 166,785 | |
| Dispensary | | 103,835 | | | 79,343 | |
| Clinical trials & other | | 6,900 | | | 6,355 | |
| Consolidated revenue | | 324,239 | | | 252,483 | |
| | | | |
| Direct costs | | | | |
| Patient services | | 181,017 | | | 134,761 | |
| Dispensary | | 83,071 | | | 65,111 | |
| | | | | | | | | | | | | | |
| (in thousands) | | Year Ended December 31, |
| 2023 | | 2022 |
| Clinical trials & other | | 578 | | | 518 | |
| Total segment direct costs | | 264,666 | | | 200,390 | |
| | | | |
| Depreciation expense | | | | |
| Patient services | | 2,156 | | | 1,202 | |
| Dispensary | | 106 | | | 4 | |
| Clinical trials & other | | 1 | | | 1 | |
| Total segment depreciation expense | | 2,263 | | | 1,207 | |
| | | | |
| Amortization of intangible assets | | | | |
| Patient services | | 2,799 | | | 2,675 | |
| | | | |
| Clinical trials & other | | 210 | | | 211 | |
| Total segment amortization | | 3,009 | | | 2,886 | |
| | | | |
| Operating income | | | | |
| Patient services | | 27,532 | | | 28,147 | |
| Dispensary | | 20,658 | | | 14,228 | |
| Clinical trials & other | | 6,111 | | | 5,625 | |
| Total segment operating income | | 54,301 | | | 48,000 | |
| | | | |
| Goodwill impairment charges | | | | |
| Patient services | | 16,235 | | | 9,944 | |
| | | | |
| Clinical trials & other | | 632 | | | — | |
| Total impairment charges | | 16,867 | | | 9,944 | |
| | | | |
| Selling, general and administrative expense | | 113,851 | | | 119,689 | |
| Non-segment depreciation and amortization | | 601 | | | 318 | |
| Total consolidated operating loss | | $ | (77,018) | | | $ | (81,951) | |
| | | | | | | | | | | |
| (in thousands) | December 31, 2023 | | December 31, 2022 |
| Assets | | | |
| Patient services | $ | 73,551 | | | $ | 64,869 | |
| Dispensary | 8,378 | | | 7,194 | |
| Clinical trials & other | 8,878 | | | 11,496 | |
| Non-segment assets | 118,433 | | | 178,106 | |
| Total assets | $ | 209,240 | | | $ | 261,665 | |
Note 21. Related Party Transactions
Related party transactions include payments for consulting services provided to the Company, clinical trials, board fees, and share repurchases. Related party payments for the years ended December 31, 2023 and 2022 were as follows:
| | | | | | | | | | | | | | | | | |
| (in thousands) | | | Year Ended December 31, |
| Type | | 2023 | | 2022 |
| American Institute of Research | Consulting | | $ | 38 | | | $ | 100 | |
| Karen M Johnson | Board Fees | | 63 | | | 56 | |
| Richard Barasch | Board Fees | | — | | | 12 | |
| Anne M. McGeorge | Board Fees | | 70 | | | 44 | |
| Mohit Kaushal | Board Fees | | 71 | | | 57 | |
| Ravi Sarin | Board Fees | | 63 | | | 57 | |
| Maeve O'Meara Duke | Board Fees | | 63 | | | 57 | |
| M33 Growth LLC (Gabe Ling) | Board Fees | | 63 | | | — | |
| Mark L. Pacala | Board Fees | | 69 | | | — | |
Richy Agajanian MD(1) | Share Repurchase | | — | | | 8,745 | |
| Richy Agajanian MD | Clinical Trials | | 17 | | | 22 | |
| Brad Hively | Board Fees/Other | | 46 | | | — | |
| Total | | | $ | 563 | | | $ | 9,150 | |
(1) Net of strike price.
Item 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosures
Notapplicable.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Our disclosure controls and procedures are designed to ensure that the information relating to our Company, including our consolidated subsidiaries, that are required to be disclosed in our Securities and Exchange Commission ("SEC") reports, is recorded, processed, summarized, and reported within the time periods specified in SEC rules and forms, and is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate to allow for timely decisions regarding required disclosure. We conducted an evaluation, under the supervision and with the participation of management, including our Chief Executive Officer and Chief Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of December 31, 2023. Based on this evaluation, our management, including our Chief Executive Officer and Chief Financial Officer, concluded that, as of December 31, 2023, our disclosure controls and procedures were effective.
Management's Annual Report on Internal Controls Over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Rules 13a-15(f) and 15d-15(f) of the Exchange Act. Management has evaluated the effectiveness of our internal controls over financial reporting based on criteria established in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. As a result of this assessment, management has concluded that, as of December 31, 2023, our internal controls over financial reporting was effective.
Remediation of Previously Reported Material Weaknesses in Internal Control over Financial Reporting
As previously reported in our Annual Report on Form 10-K for the year ended December 31, 2022, a material weakness in our internal controls existed relating to the review of complex accounting transactions. During the year ended December 31, 2023, we remediated this material weakness as follows:
•Hiring, training, and integrating additional qualified resources to the respective teams
•Implementing review controls specifically designed for non-routine complex transactions
•Hiring third-party specialist to prepare valuation reports for complex areas requiring a valuation
•Implementing monitoring activities over controls related to the review of complex accounting transactions
During the quarter ended December 31, 2023, we completed the testing of the controls described above and remediated the material weakness.
Attestation Report of the Independent Registered Public Accounting Firm
As an emerging growth company, we are not required to include an attestation report on internal control over financial reporting issued by our independent registered public accounting firm in this Annual Report on Form 10-K.
Changes in Internal Control over Financial Reporting
Except for the remediation of the material weakness noted above, there were no changes in the Company’s internal control over financial reporting that occurred during the quarter ended December 31, 2023 that have materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting.
Limitations on Effectiveness of Disclosure Controls and Procedures
In designing and evaluating our disclosure controls and procedures, management, including the Chief Executive Officer and Chief Financial Officer, recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within the company have been detected. The design of any system of controls also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Over time, controls may become inadequate because of changes in conditions, or the degree of compliance with the policies or procedures may deteriorate. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected.
Item 9B. Other Information
None.
Item 9C. Disclosure Regarding Foreign Jurisdiction that Prevent Inspections.
Not applicable.
PART III
Item 10. Directors, Executive Officers and Corporate Governance
The information required by this Item is incorporated herein by reference to our definitive proxy statement for our 2024 Annual Meeting of Stockholders, or Proxy Statement, to be filed with the SEC no later than 120 days after the end of our fiscal year ended December 31, 2023.
Item 11. Executive Compensation
The information required by this Item is incorporated herein by reference to our definitive proxy statement for our 2024 Annual Meeting of Stockholders, or Proxy Statement, to be filed with the SEC no later than 120 days after the end of our fiscal year ended December 31, 2023.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The information required by this Item is incorporated herein by reference to our definitive proxy statement for our 2024 Annual Meeting of Stockholders, or Proxy Statement, to be filed with the SEC no later than 120 days after the end of our fiscal year ended December 31, 2023.
Item 13. Certain Relationships and Related Transactions, and Director Independence
The information required by this Item is incorporated herein by reference to our definitive proxy statement for our 2024 Annual Meeting of Stockholders, or Proxy Statement, to be filed with the SEC no later than 120 days after the end of our fiscal year ended December 31, 2023.
Item 14. Principal Accounting Fees and Services
The information required by this Item is incorporated herein by reference to our definitive proxy statement for our 2024 Annual Meeting of Stockholders, or Proxy Statement, to be filed with the SEC no later than 120 days after the end of our fiscal year ended December 31, 2023.
PART IV
Item 15. Exhibits and Financial Statement Schedules
The following documents are filed as part of this Report:
(1) Financial Statements: All financial statement schedules are filed as part of this report under Item 8 - Financial Statements and Supplementary Data. See Index to Consolidated Financial Statements on Page 68 of this Annual Report.
(2) Financial Statement Schedules: No financial statement schedules are included in this Annual Report as such schedules are not required or the information that would be included in such schedules is not material or is otherwise furnished.
(3) Exhibits: See Index to Exhibits below.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | Incorporated by Reference | | | | Filed or Furnished Herewith | |
| Exhibit Number | | | | Description | | Form | | File Number | | | Exhibit | | | Filing Date | | | | |
| 2.1 | | | | | | S-4/A | | 333-258152 | | | 2.1 | | | October 20, 2021 | | | | | |
| 3.1 | | | | | | 8-K | | 001-39248 | | | 3.1 | | | November 18, 2021 | | | | | |
| 3.2 | | | | | | 8-K | | 001-39248 | | | 3.2 | | | November 18, 2021 | | | | | |
| 3.3 | | | | | | 8-K/A | | 001-39248 | | | 3.3 | | | November 22, 2021 | | | | | |
| 4.1 | | | | | | 8-K | | 001-39248 | | | 4.1 | | | March 13, 2020 | | | | | |
| 4.2 | | | | | | 8-K/A | | 001-39248 | | | 4.2 | | | November 22, 2021 | | | | | |
| 4.3 | | | | | | 8-K | | 001-39248 | | | 4.1 | | | August 10, 2022 | | | | | |
| 4.4 | | | | | | 8-K | | 001-39248 | | | 4.2 | | | August 10, 2022 | | | | | |
| 4.5 | | | | | | | | | | | | | | | | | | X | |
| 10.1 | | | | | | S-4/A | | 333-258152 | | | 10.1 | | | October 1, 2021 | | | | | |
| 10.2 | | | | | | S-4/A | | 333-258152 | | | 10.2 | | | October 1, 2021 | | | | | |
| 10.3 | | | | | | 8-K/A | | 001-39248 | | | 10.1 | | | November 22, 2021 | | | | | |
| 10.4* | | | | | | 8-K/A | | 001-39248 | | | 10.2 | | | November 22, 2021 | | | | | |
| 10.5* | | | | | | 8-K/A | | 001-39248 | | | 10.3 | | | November 22, 2021 | | | | | |
| 10.6 | | | | | | 8-K/A | | 001-39248 | | | 10.5 | | | November 22, 2021 | | | | | |
| 10.7 | | | | | | 8-K/A | | 001-39248 | | | 10.6 | | | November 22, 2021 | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | Incorporated by Reference | | | | Filed or Furnished Herewith | |
| Exhibit Number | | | | Description | | Form | | File Number | | | Exhibit | | | Filing Date | | | | |
| 10.8* | | | | | | 8-K/A | | 001-39248 | | | 10.7 | | | November 22, 2021 | | | | | |
| 10.9* | | | | | | 8-K/A | | 001-39248 | | | 10.1 | | | June 14, 2023 | | | | | |
| 10.10* | | | | | | 8-K | | 001-39248 | | | 10.1 | | | March 7, 2022 | | | | | |
| 10.11* | | | | | | 8-K | | 001-39248 | | | 10.2 | | | March 7, 2022 | | | | | |
| 10.12 | | | | | | 8-K | | 001-39248 | | | 10.1 | | | August 10, 2022 | | | | | |
| 10.13 | | | | | | 8-K | | 001-39248 | | | 10.2 | | | August 10, 2022 | | | | | |
| 10.14 | | | | Registration Rights Consent, Amendment, and Waiver, dated as of August 9, 2022, by and among Deerfield Private Design Fund IV, L.P., Deerfield Partners, L.P., M33 Growth I L.P., TOI M, LLC, and Oncology Care Partners, LLC | | 8-K | | 001-39248 | | | 10.3 | | | August 10, 2022 | | | | | |
| 10.15* | | | | | | 8-K/A | | 001-39248 | | | 10.3 | | | June 14, 2023 | | | | | |
| 10.16* | | | | | | 10-Q | | 001-39248 | | | 10.1 | | | May 10, 2023 | | | | | |
| 10.17* | | | | | | 10-Q | | 001-39248 | | | 10.2 | | | August 9, 2022 | | | | | |
| 10.18* | | | | | | 10-Q | | 001-39248 | | | 10.2 | | | May 10, 2023 | | | | | |
| 10.19* | | | | | | 10-Q | | 001-39248 | | | 10.1 | | | November 8, 2023 | | | | | |
| 21.1 | | | | | | S-1 | | 333-261740 | | | 21.1 | | | December 17, 2021 | | | | | |
| 23.1 | | | | | | | | | | | | | | | | | | X | |
| 31.1 | | | | | | | | | | | | | | | | | | X | |
| 31.2 | | | | | | | | | | | | | | | | | | X | |
| 32.1 | | | | | | | | | | | | | | | | | | X | |
| 32.2 | | | | | | | | | | | | | | | | | | X | |
| 97.1 | | | | | | | | | | | | | | | | | | X | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | Incorporated by Reference | | | | Filed or Furnished Herewith | |
| Exhibit Number | | | | Description | | Form | | File Number | | | Exhibit | | | Filing Date | | | | |
| 101 | | | | Interactive Data File — the following financial statements from The Oncology Institute's Annual Report on Form 10-K formatted in inline XBRL (Extensible Business Reporting Language) includes: (i) the Consolidated Balance Sheets, (ii) the Consolidated Statements of Operations, (iii) the Consolidated Statements of Convertible Preferred Stock and Changes in Stockholders’ Equity), (iv) the Consolidated Statements of Cash Flows, and (v) Notes to the Consolidated Financial Statements. | | | | | | | | | | | | | | | |
| 101.INS | | | | XBRL Instance Document | | | | | | | | | | | | | | | |
| 101.SCH | | | | XBRL Taxonomy Extension Schema Document | | | | | | | | | | | | | | | |
| 101.CAL | | | | XBRL Taxonomy Extension Calculation Linkbase Document | | | | | | | | | | | | | | | |
| 101.DEF | | | | XBRL Taxonomy Extension Definition Linkbase Document | | | | | | | | | | | | | | | |
| 101.LAB | | | | XBRL Taxonomy Extension Label Linkbase Document | | | | | | | | | | | | | | | |
| 104 | | | | Cover Page Interactive Data File - (formatted as Inline XBRL and contained in Exhibit 101) | | | | | | | | | | | | | | | |
* Management contract or compensatory plan or arrangement
† Furnished herewith. The certifications attached as Exhibit 32.1 that accompanies this Annual Report on Form 10-K is deemed furnished and not filed with the Securities and Exchange Commission and is not to be incorporated by reference into any filing of The Oncology Institute, Inc.” TOI continues under the existing business operationsSecurities Act of Old TOI1933, as a publicly traded company.Management has evaluated subsequent events and transactions that occurredamended, or the Securities Exchange Act of 1934, as amended, whether made before or after the balance sheet date up throughof this Annual Report on Form 10-K, irrespective of any general incorporation language contained in such filing.
– Certain of the date the financial statements were issued. Based uponexhibits and schedules to this review, other than described hereExhibit have been omitted in accordance with Regulation S-K Item 601(a)(5). The Registrant agrees to furnish a copy of all omitted exhibits and in Note 2 with respectschedules to the restatements, the Company did not identify any subsequent events that would have required adjustment or disclosure in the financial statements.SEC upon its request.
Item 16. Form 10-K Summary
None
Signatures
Pursuant to the requirements of
Section 13 or 15(d) of the Securities
and Exchange Act of 1934, the registrant has duly caused this
reportRegistration Statement to be signed on its behalf by the undersigned
thereuntohereunto duly
authorized.authorized, on this the day of March 28, 2024.
| | | | | |
| THE ONCOLOGY INSTITUTE, INC. |
| |
| |
| By: |
/s/ Mihir Shah |
| By:
| /s/ Brad Hively
Mihir Shah |
| | Name: Brad Hively
|
| | Title:
Chief ExecutiveFinancial Officer (Duly Authorized Officer) |
SIGNATURES AND POWER OF ATTORNEY
We, the undersigned officers and directors of The Oncology Institute, Inc., hereby severally constitute and appoint Daniel Virnich and Mihir Shah, and each of them singly (with full power to each of them to act alone), our true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution in each of them for him and in his name, place and stead, and in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement (or any other registration statement for the same offering that is to be effective upon filing pursuant to Rule 462(b) under the Securities Act of 1933), and to file the same, with all exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite or necessary to be done in and about the premises, as full to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents or any of them, or their or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities
and Exchange Act of
1934,1933, this
reportRegistration Statement has been signed
below by the following persons in the capacities
andheld on the dates
indicated below.indicated.
| Name
| | | | | | | | | | | | | |
| Signature | | Title | | Title
| Date |
/s/ Daniel Virnich | | | | |
| Daniel Virnich | | Chief Executive Officer
(Principal Executive Officer) | | March 28, 2024 |
| /s/ Mihir Shah | | | | |
| Mihir Shah | | Chief Financial Officer
(Principal Financial and Accounting Officer) | | March 28, 2024 |
| /s/ Richard Barasch | | | | |
| Richard Barasch | | Director | | March 28, 2024 |
| /s/ Karen Johnson | | | | |
| Karen Johnson | | Director | | March 28, 2024 |
| /s/ Mohit Kaushal | | | | |
| Mohit Kaushal | | Director | | March 28, 2024 |
| /s/ Gabriel Ling | | | | |
| Gabriel Ling | | Director | | March 28, 2024 |
| /s/ Anne McGeorge | | | | |
| Anne McGeorge | | Director | | March 28, 2024 |
| /s/ Maeve O'Meara | | | | |
| Maeve O’Meara | | Director | | March 28, 2024 |
| | | | | | | | | | | | | | |
| Signature | | Title | | Date |
| /s/ Mark Pacala | | | | |
| Mark Pacala | | Director | | March 28, 2024 |
| /s/ Brad Hively | | | Chief Executive Officer and Director (principal executive officer)
| | December 13, 2021
Brad Hively | | Director | | | |
| | | | |
/s/ Scott Dalgleish
| | Chief Financial Officer (principal financial and accounting officer)
| | December 13, 2021
|
Scott Dalgleish
| | | | |
| | | | |
/s/ Anne McGeorge
| | Director
| | December 13, 2021
|
Anne McGeorge
| | | | |
|
|
|
|
|
/s/ Maeve O’Meara
|
| Director
|
| December 13, 2021
|
Maeve O’Meara
|
|
|
|
|
|
|
|
|
|
/s/ Ravi Sarin
|
| Director
|
| December 13, 2021
|
Ravi Sarin
|
|
|
|
March 28, 2024 |