Form 10-K/A
(Amendment No. 1)
| |
| |
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| |
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
|
| ||
| ||||
| Delaware | 2834 | 45-4440364 | ||
f(State or Other Jurisdiction of Incorporation or Organization) | (Primary Standard Industrial Classification Code Number) | (I.R.S. Employer Identification No.) | ||
300 George Street, Suite 301
New Haven, CT 06511
(312) 767-0291
Title of Each Class | Name of Exchange on which Registered |
Common Stock, $0.001 Par Value | Nasdaq Global Market |
Large accelerated filer | ☐ | Accelerated filer | ☒ |
Non-accelerated filer | ☐ (Do not check if a smaller reporting company) | Smaller reporting company |
☒ |
Emerging growth company | ☐ | ||
The aggregate market value of the voting stock held by non-affiliates of the registrant, as of June 30, 2017,2018, was approximately $241.5 $180.3million. Such aggregate market value was computed by reference to the closing price of the common stock as reported on the Nasdaq Global Market on June 30, 2017.2018. For purposes of making this calculation only, the registrant has defined affiliates as including only directors and executive officers and shareholders holding greater than 10% of the voting stock of the registrant as of June 30, 2017.
2018.
Tableoutstanding
| |||
| Item 1. | |||
| Item 1A. | |||
| Item 1B. | |||
| Item 2. | |||
| Item 3. | |||
| Item 4. | |||
| Item 5. | |||
| Item 6. | |||
| Item 7. | |||
| Item 7A. | |||
| Item 8. | |||
| Item 9. | |||
| Item 9A. | |||
| Item 9B. | |||
| |||
| |||
| |||
| |||
| |||
| |||
| |||
| |||
| |||
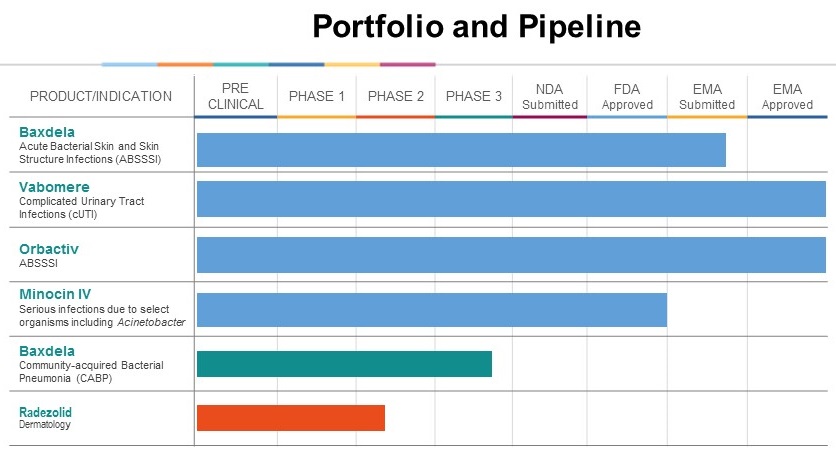
(delafloxacin), in June 2017. On March 16, 2018,November 3, 2017, we completed a reverse merger with Cempra, Inc., the result of which the registrant Cempra, Inc. was re-named Melinta Therapeutics, Inc. and we became a publicly-traded company. On January 5, 2018, we acquired the Infectious Disease Businesses (“IDB”) from The Medicines Company (“Medicines”), including the capital stock of certain subsidiaries of Medicines and certain assets related to its infectious disease business (the “Company”“IDB Transaction”), including the pharmaceutical products containing (i) meropenem and vaborbactam as the active pharmaceutical ingredient and distributed under the brand name Vabomere® (“Vabomere”), (ii) oritavancin as the active pharmaceutical ingredient and distributed under the brand name Orbactiv® (“Orbactiv”), and (iii) minocycline as the active pharmaceutical ingredient and distributed under the brand name Minocin® for injection and line extensions of such products (the “MDCO Products” and together with Baxdela, the "Products" or “Melinta”the “Product Portfolio”). See “Corporate History and information” below for further information.

| 1. | Commercial Product Sales Growth |
| a. | Commercialize Baxdela in the United States. We launched Baxdela oral and IV in the first quarter of 2018 for the treatment of ABSSSI and continue our commercialization of the product with an efficient, targeted sales force consisting of approximately 60 sales territories, prioritizing high-value retail and hospital accounts. In addition, sales representatives target other market channels, such as the emergency department and select community settings, in an attempt to realize the full market potential of Baxdela. We are also pursuing an additional indication, CABP, for which we expect to file an sNDA in the first half of 2019. |
| b. | Commercialize Vabomere for cUTI in the United States. We market Vabomere in the United States with our combined sales force consisting of approximately 70 sales representatives, prioritizing high-value hospital accounts, focusing on infectious disease and critical care physicians and pharmacists. |
| c. | Optimize commercialization of Orbactiv and Minocin for injection within the United States. Sales representatives target emergency department and community market channels to realize the full market potential of Orbactiv in the treatment of ABSSSI. In addition, we are leveraging our sales force presence within the hospital to appropriately position Minocin for injection for the treatment of serious infections due to Acinetobacter. |
| 2. | Stewardship of financial resources. We are focused on using the existing sources of cash for the Company, including cash on hand, revenue from product sales and licensing arrangements, and capital available under existing and permitted financing facilities, to achieve future profitability. In order to succeed with this strategy, we must successfully execute sales strategies and reduce operating spend from historical levels. We have an experienced management team and sales force to lead the Company's product sales effort. In addition, we have taken steps to reduce operating expenses, including (a) narrowing the scope of future research and development activities, which involved the natural reduction in costs for large clinical studies that are nearing completion and winding down our early-stage research programs, (b) rationalizing costs in the selling, general and administrative areas, many due to the integration of the legacy Cempra, IDB and Melinta businesses post-merger and acquisition, and (c) negotiation with various third-parties to reduce and/or extend payment for certain large commitments. |
| 3. | Optimize partnerships to maximize the value of the Product Portfolio. We have a number of partnerships, including established partnerships for our Product Portfolio in Europe and Asia-Pacific (excluding Japan) with Menarini, and for Baxdela in Central and South America with Eurofarma Laboratórios S.A. We have a development partnership with a contract research organization (“CRO”) for our pipeline asset, radezolid, which is focused on the topical dermatology space. We plan to evaluate the potential of existing and new business development opportunities to further generate non-dilutive capital and enhance shareholder value. |
| 4. | Leverage the enterprise’s commercial organization to promote complementary internally or externally developed products upon achievement of FDA regulatory approval. With an experienced team and commercial infrastructure in place, we believe we are well positioned to add either internally or externally developed products to our portfolio while adding minimal new costs. We may selectively pursue the addition of externally developed products to our existing marketed products and pipeline, leveraging our commercial infrastructure. |
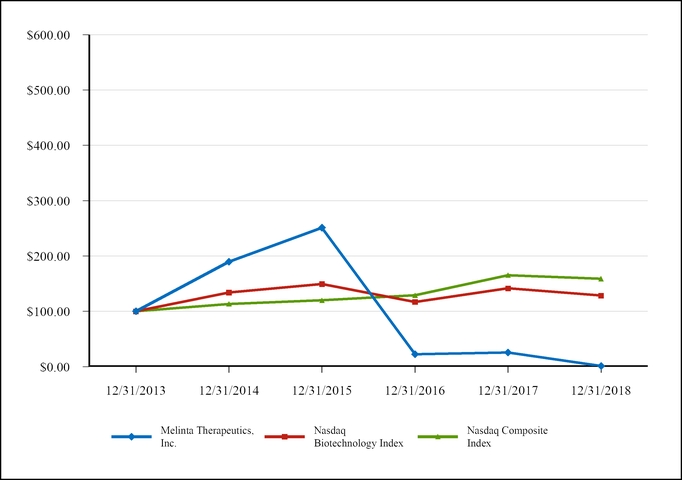
| $100 investment in stock or index | Ticker | 12/31/2013 | 12/31/2014 | 12/31/2015 | 12/31/2016 | 12/31/2017 | 12/31/2018 | |||||||||||||||||||
| Melinta Therapeutics, Inc. | MLNT | $ | 100.00 | $ | 189.75 | $ | 251.25 | $ | 22.60 | $ | 25.50 | $ | 1.28 | |||||||||||||
| Nasdaq Biotechnology Index | NBI | $ | 100.00 | $ | 134.10 | $ | 149.41 | $ | 117.01 | $ | 141.61 | $ | 128.45 | |||||||||||||
| Nasdaq Composite Index | IXIC | $ | 100.00 | $ | 113.40 | $ | 119.89 | $ | 128.89 | $ | 165.29 | $ | 158.87 | |||||||||||||
| Consolidated Statement of Operations Data | |||||||||||||||||||
| Year Ended December 31, | |||||||||||||||||||
| 2018 | 2017 | 2016 | 2015 | 2014 | |||||||||||||||
| (in thousands, except share and per share data) | |||||||||||||||||||
| Revenue: | |||||||||||||||||||
| Total revenue | $ | 96,430 | $ | 33,864 | $ | — | $ | — | $ | — | |||||||||
| Operating expenses: | |||||||||||||||||||
| Cost of goods sold | 41,057 | — | — | — | — | ||||||||||||||
| Research and development | 55,409 | 49,475 | 49,791 | 62,788 | 53,647 | ||||||||||||||
| Goodwill impairment | 25,088 | — | — | — | — | ||||||||||||||
| Selling, general and administrative | 133,312 | 63,325 | 19,410 | 14,159 | 13,562 | ||||||||||||||
| Total operating expenses | 254,866 | 112,800 | 69,201 | 76,947 | 67,209 | ||||||||||||||
| Loss from operations | (158,436 | ) | (78,936 | ) | (69,201 | ) | (76,947 | ) | (67,209 | ) | |||||||||
| Other income (expense), net | 1,244 | 20,020 | (4,731 | ) | (1,729 | ) | (575 | ) | |||||||||||
| Net loss | (157,192 | ) | (58,916 | ) | (73,932 | ) | (78,676 | ) | (67,784 | ) | |||||||||
| Accretion of redeemable convertible preferred stock dividends | — | (19,259 | ) | (21,117 | ) | (16,248 | ) | (9,859 | ) | ||||||||||
| Net loss attributable to common shareholders | (157,192 | ) | (78,175 | ) | (95,049 | ) | (94,924 | ) | (77,643 | ) | |||||||||
| Basic and diluted loss per share | $ | (17.12 | ) | $ | (109.28 | ) | $ | (20,600.13 | ) | $ | (130,929.66 | ) | $ | (246,485.71 | ) | ||||
| Weighted average shares used in computation of basic and diluted loss per share | 9,181,668 | 715,369 | 4,614 | 725 | 315 | ||||||||||||||
| Consolidated Balance Sheet Data | |||||||||||||||||||
| As of December 31, | |||||||||||||||||||
| 2018 | 2017 | 2016 | 2015 | 2014 | |||||||||||||||
| (in thousands) | |||||||||||||||||||
| Balance sheet data: | |||||||||||||||||||
| Cash and equivalents | $ | 81,808 | $ | 128,387 | $ | 11,409 | $ | 30,158 | $ | 10,541 | |||||||||
| Working capital | $ | 15,876 | $ | 118,034 | $ | (8,330 | ) | $ | 13,385 | $ | (10,800 | ) | |||||||
| Total assets | $ | 441,590 | $ | 160,273 | $ | 16,634 | $ | 36,228 | $ | 13,702 | |||||||||
| Total debt, net | $ | 110,476 | $ | 39,555 | $ | 68,849 | $ | 28,226 | $ | 17,552 | |||||||||
| Total shareholders' equity (deficit) | $ | 190,064 | $ | 72,336 | $ | (293,451 | ) | $ | (222,099 | ) | $ | (145,573 | ) | ||||||
| 2018 | 2017 | 2016 | ||||||
| Risk-free interest rate | 2.6% - 3.1% | 1.8% - 2.1% | 1.5 | % | ||||
| Weighted-average volatility | 84.8% - 88.9% | 87.5% - 108.1% | 67.1 | % | ||||
| Expected term - employee awards (in years) | 1.0 - 6.1 | 3.1 - 6.1 | 6.0 | |||||
| Forfeiture rate | — | — | — | |||||
| Dividend yield | 0 | % | 0 | % | 0 | % | ||
| Year Ended December 31, | Increase (Decrease) | |||||||||||||
| 2018 | 2017 | Dollars | Percent | |||||||||||
| Product sales, net | $ | 46,580 | $ | — | 46,580 | NA | ||||||||
| Contract research | 11,677 | 13,959 | (2,282 | ) | (16.3 | )% | ||||||||
| License revenue | 38,173 | 19,905 | 18,268 | 91.8 | % | |||||||||
| Total revenue | $ | 96,430 | $ | 33,864 | 62,566 | 184.8 | % | |||||||
| Cost of goods sold | $ | 41,057 | $ | — | $ | 41,057 | NA | |||||||
| Research and development | $ | 55,409 | $ | 49,475 | $ | 5,934 | 12.0 | % | ||||||
| Goodwill impairment | $ | 25,088 | $ | — | $ | 25,088 | NA | |||||||
| Selling, general and administrative | $ | 133,312 | $ | 63,325 | $ | 69,987 | 110.5 | % | ||||||
| Total other income (expense), net | $ | 1,244 | $ | 20,020 | $ | (18,776 | ) | (93.8 | )% | |||||
| ◦ | Principal accretion and debt cost amortization related to the Deerfield Facility of $6.3 million; |
| ◦ | Accretion of deferred purchase price and contingent royalty liabilities from the IDB acquisition of $15.4 million; and |
| ◦ | Accretion of a milestone liability from the IDB acquisition of $4.0 million. |
| Year Ended December 31, | Increase (Decrease) | |||||||||||||
| 2017 | 2016 | Dollars | Percent | |||||||||||
| Revenue | $ | 33,864 | $ | — | $ | 33,864 | NA | |||||||
| Research and development | $ | 49,475 | $ | 49,791 | $ | (316 | ) | (0.6 | )% | |||||
| Selling, general and administrative | $ | 63,325 | $ | 19,410 | $ | 43,915 | 226.2 | % | ||||||
| Total other expense, net | $ | 20,020 | $ | (4,731 | ) | $ | 24,751 | (523.2 | )% | |||||
| Year Ended December 31, | |||||||||||
| 2018 | 2017 | 2016 | |||||||||
| Cash, cash equivalents and restricted cash as of the end of the period | $ | 82,008 | $ | 128,587 | $ | 11,409 | |||||
| Net cash provided by (used in): | |||||||||||
| Operating activities | (171,545 | ) | (75,598 | ) | (70,580 | ) | |||||
| Investing activities | (170,072 | ) | 155,061 | (463 | ) | ||||||
| Financing activities | 295,038 | 37,715 | 52,294 | ||||||||
| Net increase in cash and equivalents | $ | (46,579 | ) | $ | 117,178 | $ | (18,749 | ) | |||
Contractual Obligations(1) | 2019 | 2020 | 2021 | 2022 | 2023 | 2024 & Thereafter | Total | ||||||||||||||||||||
Note payable(2) | $ | 17,508 | $ | 17,653 | $ | 17,605 | $ | 81,391 | $ | 78,756 | $ | 93,892 | $ | 306,805 | |||||||||||||
Purchase commitments(3) | 19,145 | — | — | — | — | — | 19,145 | ||||||||||||||||||||
Operating lease obligations(4) | 2,348 | 2,269 | 1,827 | 1,238 | 624 | 262 | 8,568 | ||||||||||||||||||||
Total contractual cash obligations | $ | 39,001 | $ | 19,922 | $ | 19,432 | $ | 82,629 | $ | 79,380 | $ | 94,154 | $ | 334,518 | |||||||||||||
| (1) | Table excludes milestones and other contingent payments that may become due under Melinta’s license and collaboration agreements because they are payable upon the occurrence of certain future events that are not currently probable. It also excludes any amounts that may become payable when we draw additional amounts under the Vatera Loan Agreement. |
| (2) | Represents amounts payable under the Deerfield Facility, as amended, and the Vatera Loan Agreement, to the extent we have drawn funds under either agreement (see Note 4 to the Consolidated Financial Statements). |
| (3) | Represents amounts that will be owed for firm commitments to purchase goods or services, principally inventory. |
| (4) | Includes the minimum lease rental payments for our corporate office building in Lincolnshire, Illinois, our research and administrative facility in New Haven, Connecticut, our facilities in Chapel Hill, North Carolina, office equipment leases and vehicle leases. |
In addition, as required by Rule 12b-15 under the Securities Exchange Act of 1934, as amended, new certifications by our principal executive officer and principal financial officer are filed as exhibits to this Amendment under Item 15 of Part IV hereof.
Except as stated herein, this Amendment does not reflect events occurring after the filingassets of the Original Form 10-KCompany; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. GAAP, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the financial statements.
|
|
| ||||||
| Name | Age | Position | ||||||
| Kevin T. Ferro |
| Chairman of the Board of Directors | ||||||
|
|
| ||||||
|
|
| ||||||
|
|
| ||||||
John H. Johnson |
| Chief Executive Officer and Director | ||||||
Bruce L. Downey | 71 | Director | ||||||
| Jay Galeota | 53 | Director | ||||||
| David Gill | 64 | Director | ||||||
| Thomas P. Koestler, Ph.D. |
| Director | ||||||
Garheng Kong, M.D., Ph.D. |
| Director | ||||||
|
|
| ||||||
David Zaccardelli, Pharm.D. |
| Director | ||||||
to 2009, he served on several public and private company boards of directors, including those of LeMaitre Vascular (NASDAQ: LMAT), and IsoTis, Inc. Earlier in his career, Mr. Gill served in a variety of senior executive leadership roles for several medical device and information technology companies, including NxStage Medical, CTIMolecular Imaging, Inc., InterlnadInterland Inc., Novoste Corporation and Dornier Medical. Mr. Gill holds a B.S. degree, cum laude, in Accounting from Wake Forest University and an M.B.A. degree, with honors, from Emory University, and was formerly a certified public accountant.
Cecilia Gonzalo
John H. Johnson—Mr. Johnson has served on the Company’s board of directors since June 2009. He served as president and chief executive officer of Dendreon Corp., a publicly traded biotechnology company (NASDAQ: DNDN), from February 2012, became chairman in July 2013, and served as chairman until June 2014 and president and chief executive officer until August 2014. He served as the chief executive officer and as a director of Savient Pharmaceuticals, Inc., a company that developed and commercialized specialty pharmaceuticals, from 2011 to January 2012. Mr. Johnson was senior vice president of Eli Lilly and Company (NYSE: LLY) and president of Lilly Oncology, Eli Lilly’s oncology business unit, from 2009 to 2011. From 2007 to 2009, Mr. Johnson was chief executive officer of ImClone Systems Incorporated, a biopharmaceutical development company, and was also a member of ImClone’s board of directors until it became a wholly owned subsidiary of Eli Lilly in 2008. From 2005 to 2007, Mr. Johnson served as company group chairman of Johnson & Johnson’s Worldwide Biopharmaceuticals unit. Mr. Johnson served as chairman of the board of Tranzyme, Inc. (NASDAQ: TZYM), a publicly traded biopharmaceutical company, from December 2010 until July 2013. Mr. Johnson serves as the chairman of the board of Strongbridge Biopharma PLC (NASDAQ: SBBP), a global biopharmaceutical company, and also serves as lead independent director of Portola Pharmaceuticals, Inc. (NASDAQ: PTLA), a biopharmaceutical company, and Histogenics Corporation (NASDAQ: HSGX), a regenerative medicine company. Mr. Johnson also serves on the board of directors of Aveo Pharmaceuticals, Inc. (NASDAQ: AVEO), a biopharmaceutical company. Mr. Johnson holds a B.S. in Education from East Stroudsburg University of Pennsylvania.
Thomas P. Koestler, Ph.D.—Dr. Koestler has served as a member of the Company’s board of directors since November 2017. Dr. KoestlerHe has served as an executive director of Vatera Holdings LLC, the manager ofa consultant to Vatera Healthcare Partners LLC since February 2010.January 2019. Dr. Koestler is also a member of the boards of directors of Momenta Pharmaceuticals Inc., ImmusanT, Inc. and was a member of the board of directors of Arisaph Pharmaceuticals, Inc. From March 2011 to April 2016, Dr. Koestler served as a member of the board of directors of Novo Nordisk A/S. Dr. Koestler served as an executive director of Vatera Holdings LLC, the former manager of Vatera Healthcare Partners LLC, from February 2010 through January 1, 2019. From 2006 to 2009, Dr. Koestler served as executive vice president of Schering Corporation and president of Schering-Plough Research Institute, the pharmaceutical research and development arm of Schering-Plough Corporation, and served as executive vice president, global development, of Schering-Plough Research Institute from 2005 to 2006, and executive vice president of Schering-Plough Research Institute from 2003 to 2005. Before joining Schering-Plough, Dr. Koestler served as senior vice president and head of global regulatory affairs for Pharmacia Corporation from 2001 to 2003. Before that, Dr. Koestler was senior vice president and global head, drug regulatory affairs, compliance assurance, clinical safety and epidemiology for Novartis. Dr. Koestler received his B.S. from Daemen College and his Ph.D. in Medicine and Pathology from SUNY Buffalo, Roswell Park Memorial Institute.
Garheng Kong, M.D., Ph.D.—Dr. Kong has served on the Company’s board of directors since September 2006. Dr. Kong has been the managing partner of Sofinnova HealthQuest, a healthcare investment firm, since July 2013. He was a general partner at Sofinnova Ventures, a venture firm focused on life sciences, from September 2010 to December 2013. From 2000 to September 2010, he was at Intersouth Partners, a venture capital firm, most recently as a general partner, where he was a founding investor or board member for various life sciences ventures, several of which were acquired by large pharmaceutical companies. Dr. Kong has also served on the board of directors of Histogenics Corporation (NASDAQ: HSGX)Avedro, Inc (Nasdaq: AVDR), a regenerative medicinecorneal health company, since July 2012,2018, Alimera Sciences, Inc. (NASDAQ:(Nasdaq: ALIM), a biopharmaceutical company, since October 2012, has served on the board of Laboratory Corporation of America Holdings (NYSE: LH), a healthcare company, since December 2013, and has served on the board of StrongBridge BioPharma plc (NASDAQ:(Nasdaq: SBBP) since September 2015. Dr. Kong holds a B.S. from Stanford University. Heholds an M.D., Ph.D. and M.B.A. from Duke University.
Daniel Wechsler
David Zaccardelli, Pharm.D.— Dr. Zaccardelli has served on the Company’s board of directors since August 2016 and was acting chief executive officer of Cempra from December 2016 until November 2017. From 2004 until 2016, Dr. Zaccardelli served in several senior management roles at United Therapeutics Corporation (NASDAQ:(Nasdaq: UTHR), including chief operating officer, chief manufacturing officer and executive vice president, pharmaceutical development and operations. Prior to joining United Therapeutics, Dr. Zaccardelli founded and led a startup company focused on contract pharmaceutical development services, from 1997 through 2003. From 1988 to 1996, Dr. Zaccardelli worked at Burroughs Wellcome & Co. and
| ||||
| Name | Age (as of 4/30/18) | Business Experience For Last Five Years | ||
|
|
| ||
Sue Cammarata, M.D. |
| Dr. Cammarata, current Executive Vice President and Chief Medical Officer of Melinta, has more than 20 years of clinical experience in the development, approval and launch of pharmaceuticals, including several anti-infective brands such as Cubicin (daptomycin) in the E.U., and Zyvox (linezolid) globally. Since joining Melinta in 2014, she has led the successful Phase 3 clinical development of Baxdela, which was approved by the FDA in June, and is also responsible for medical affairs. Prior to joining Melinta, Dr. Cammarata served as vice president of clinical research at Shire HGT, where she was responsible for clinical development and post approval commitments for novel therapies in rare and orphan diseases. Earlier, she held several senior positions at Novartis, most recently as vice president and global program head for the company’s immunology and infectious disease franchises. In this role, she managed the integration of Chiron’s infectious disease portfolio after its acquisition by Novartis in 2006. In addition, she managed the E.U. approval process for Cubicin’s endocarditis and bacteremia indications. Before joining Novartis, Dr. Cammarata held several positions at Pharmacia Upjohn (later Pfizer, Inc.) where she was an integral member of the team that led the Phase 3 and subsequent global regulatory programs for Zyvox, a first-in-class antibiotic for gram-positive infections, including those resistant to vancomycin. Dr. Cammarata received her M.D. from Michigan State University, completed her residency in internal medicine and her fellowship in pulmonary and critical care medicine at Henry Ford Health Systems and was a pulmonary and critical care medicine specialist for several years in private practice before entering the pharmaceutical industry. Dr. Cammarata earned her B.S. in pharmacy from Purdue University. | ||
| ||||
| Name | Age (as of 4/30/18) | Business Experience For Last Five Years | ||
|
|
Mr. Milligan joined Melinta in 2018 as Chief Financial | ||
Erin Duffy, Ph.D. |
| Dr. Duffy, current Executive Vice President and Chief Scientific Officer of Melinta, has more than 21 years of pharmaceutical research experience and has been responsible for translating Melinta’s Nobel Prize-winning ribosome technology platform into the discovery and early-stage development of novel antibiotic candidates. She joined the company in 2002 and has become one of the world’s leading experts on the structure and function of the bacterial ribosome and the interaction of antibiotics with their ribosomal targets. Dr. Duffy has led Melinta’s ESKAPE Pathogen Program from its infancy and has been instrumental in advancing the platform while also contributing to the development programs for other drug candidates. The ESKAPE Pathogen Program is Melinta’s most advanced preclinical initiative, focused on using a discrete, novel binding site within the bacterial ribosome to design and develop completely new classes of antibiotics to treat some of the deadliest multi-drug resistant gram-positive and gram-negative infections. Prior to joining Melinta, Dr. Duffy served as associate director of innovative discovery technologies at Achillion Pharmaceuticals, Inc. Dr. Duffy began her scientific career as a computational chemist with Pfizer Global Research and Development. Dr. Duffy trained at Yale University, where she received her Ph.D. in physical-organic chemistry and was a Howard Hughes postdoctoral fellow. She holds a B.S. in chemistry from Wheeling Jesuit University. | ||
NEOs 2018 ($) 2017 ($) Daniel Wechsler(1) 572,000 550,000 Paul D. Estrem 365,084 333,410 Sue K. Cammarata, M.D. 446,437 433,434 Erin Duffy, Ph.D. 340,458 330,542 John Temperato — 416,138 NEOs 2018 Target 2018 Maximum Daniel Wechsler 65.0 % 84.5 % Paul D. Estrem 40.0 % 52.0 % Sue K. Cammarata, M.D. 40.0 % 52.0 % Erin Duffy, Ph.D. 35.0 % 45.5 % John Temperato — — Name 2017 Bonus Achievement or Performance Cited Daniel Wechsler $ 100,000 For performance as CEO (partial year of service). Paul D. Estrem $ 100,000 Efforts in connection with the merger with Cempra. $ 50,000 Efforts in connection with The Medicines Company transaction. $ 105,024 For performance as CFO. Sue K. Cammarata, M.D. $ 200,000 Efforts in connection with the New Drug Application approval of Baxdela™. $ 50,000 Efforts in connection with the merger with Cempra. $ 130,030 For performance as Chief Medical Officer. Erin Duffy, Ph.D. $ 100,000 Efforts in connection with the merger with Cempra. $ 99,162 For performance as Chief Scientific Officer. John Temperato $ 100,000 Efforts in connection with the merger with Cempra. Name(1) Stock options (#) Grant date fair value ($) Restricted stock units (#) Grant date fair value ($) David Zaccardelli, Pharm.D. 0 0 0 0 Mark Hahn 30,000 319,497 15,000 115,250 John Bluth 19,999 116,500 10,000 115,250 David Oldach, M.D. 30,000 319,497 15,000 115,250 Name and principal position(1)(2)(3) Year Salary ($) Bonus ($)(4) Stock awards ($)(5) Option awards ($)(6) Non-equity incentive plan compensation ($) All other compensation ($)(7) Total ($) Daniel Wechsler President and Chief Executive Officer 2017 87,788 100,000 2,139,651 4,605,480 — 22,307 6,955,226 Paul D. Estrem Executive Vice President, Chief Financial Officer and Secretary 2017 333,410 255,024 — 73,185 — 10,791 672,410 Sue K. Cammarata, M.D. Executive Vice President and Chief Medical Officer 2017 433,434 380,030 — 54,680 — 2,376 870,520 Erin Duffy, Ph.D. Executive Vice President and Chief Scientific Officer 2017 330,542 199,162 — 65,867 — — 595,571 John Temperato(8) Former Executive Vice President and Chief Commercial Officer 2017 416,138 100,000 — 92,844 — 10,600 619,582 David Zaccardelli, Pharm.D. Former Acting Chief Executive Officer 2017 456,231 — — — — 1,871,992 2,328,223 2016 32,884 — 385,000 826,554 — — 1,244,438 Mark Hahn Former Executive Vice President and Chief Financial Officer 2017 350,000 — 174,750 234,300 — 1,045,850 1,804,900 2016 375,000 — — 1,170,299 109,500 29,727 1,684,526 2015 350,000 — — 699,955 97,633 27,832 1,175,420 John Bluth Former Executive Vice President, Investor Relations and Corporate Communications 2017 266,500 — 116,500 156,192 — 783,358 1,322,550 2016 108,330 — — 628,997 31,633 6,938 775,898 David Oldach, M.D. Former Chief Medical Officer 2017 350,000 — 174,750 234,300 — 1,074,401 1,833,451 2016 350,000 — — 900,243 96,600 25,304 1,372,147 2015 300,000 — — 347,237 84,210 23,090 754,537 Severance(A) Name Base salary Bonus COBRA premiums RSU acceleration(B) Outplacement assistance 401(k) matching contribution Reimbursement of advisor fees Total Daniel Wechsler — — — — — — 22,307 22,307 Paul D. Estrem — — — — — 10,791 — 10,791 Sue K. Cammarata, M.D. — — — — — 2,376 — 2,376 Erin Duffy, Ph.D. — — — — — — — — John Temperato — — — — — 10,600 — 10,600 David Zaccardelli, Pharm.D. 1,080,000 648,000 24,117 116,250 20,000 9,249 — 1,871,992 Mark Hahn 600,000 240,000 18,088 183,750 20,000 10,800 — 1,045,850 John Bluth 399,759 159,900 27,408 165,500 20,000 10,800 — 783,358 David Oldach, M.D. 600,000 240,000 27,408 183,750 20,000 10,800 — 1,074,401 Estimated future payouts under non-equity incentive plan awards Estimated future payouts under equity incentive plan awards Number of shares Number of securities Exercise or base price of Grant date fair value of stock and Name(1) Grant date Threshold ($) Target ($) Maximum ($) Threshold ($) Target ($) Maximum ($) of stock or units (#)(2) underlying options (#)(2) option awards ($/Sh)(2) option awards ($)(8) Daniel Wechsler 11/3/2017 — — — — — — 183,661 (3) — — 2,139,651 11/3/2017 — — — — — — — 550,981 (5) 11.65 4,605,480 Paul D. Estrem 8/8/2017 — — — — — — — 5,214 (6) 20.97 73,185 Sue K. Cammarata, M.D. 8/8/2017 — — — — — — — 3,895 (6) 20.97 54,680 Erin Duffy, Ph.D. 8/8/2017 — — — — — — — 4,692 (6) 20.97 65,867 John Temperato 8/8/2017 — — — — — — — 6,615 (6) 20.97 92,844 David Zaccardelli, Pharm.D. — — — — — — — — — — — Mark Hahn 1/3/2017 — — — — — — 10,000 (4) — — 116,500 1/3/2017 — — — — — — — 6,250 (7) 15.00 48,813 1/3/2017 — — — — — — — 18,750 (7) 15.00 146,438 2/23/2017 — — — — — — 5,000 (4) — — 58,250 2/23/2017 — — — — — — — 501 (7) 15.75 3,913 2/23/2017 — — — — — — — 4,499 (7) 15.75 35,137 John Bluth 1/3/2017 — — — — — — 5,000 (4) — — 58,250 1/3/2017 — — — — — — — 15,000 (7) 15.00 117,150 2/23/2017 — — — — — — 5,000 (4) — — 58,250 2/23/2017 — — — — — — — 104 (7) 15.75 812 2/23/2017 — — — — — — — 4,895 (7) 15.75 38,230 David Oldach, M.D. 1/3/2017 — — — — — — 10,000 (4) — — 116,500 1/3/2017 — — — — — — — 6,250 (7) 15.00 48,813 1/3/2017 — — — — — — — 18,750 (7) 15.00 146,438 2/23/2017 — — — — — — 5,000 (4) — — 58,250 2/23/2017 — — — — — — — 501 (7) 15.75 3,913 2/23/2017 — — — — — — — 4,499 (7) 15.75 35,137 Option awards Stock awards Name Grant date Number of securities underlying unexercised options (#) exercisable (1) Number of securities underlying unexercised options (#) unexercisable (1) Equity incentive plan awards: number of securities underlying unexercised unearned options (#) Option exercise price ($)(1) Option expiration date Number of shares or units of stock that have not vested (#)(1) Market value of shares or units of stock that have not vested ($)(1)(2) Equity incentive plan awards: number of unearned shares, units or other rights that have not vested (#) Equity incentive plan awards: market or payout value of unearned shares, units or other rights that have not vested ($) Daniel Wechsler 11/3/2017(3) — — — — — 183,661 2,901,844 — — 11/3/2017(4) — 550,981 — 11.65 11/2/2027 — — — — Paul D. Estrem 8/8/2017(5) — 5,214 — 20.97 8/7/2027 — — — — 9/1/2015(5) 5,630 3,379 — 36.25 8/31/2025 — — — — 4/8/2014(5) 20,220 1,839 — 27.52 4/7/2024 — — — — 12/17/2013(5) 25,793 — — 17.04 12/16/2023 — — — — Sue K. Cammarata, M.D. 8/8/2017(5) — 3,895 — 20.97 8/7/2027 — — — — 9/29/2015(5) 8,782 5,270 36.25 9/28/2025 9/1/2015(5) 2,815 1,689 — 36.25 8/31/2025 — — — — 4/8/2014(5) 10,109 920 — 27.52 4/7/2024 — — — — 12/17/2013(5) 12,896 — — 17.04 12/16/2023 — — — — Erin Duffy, Ph.D. 8/8/2017(5) — 4,692 — 20.97 8/7/2027 — — — — 9/1/2015(5) 5,067 3,041 — 36.25 8/31/2025 — — — — 4/8/2014(5) 18,198 1,655 — 27.52 4/7/2024 — — — — 12/17/2013(5) 23,213 — — 17.04 12/16/2023 — — — — John Temperato 8/8/2017(5) — 6,615 — 20.97 8/7/2027 — — — — 3/22/2016(5) 33,061 39,074 — 38.00 3/21/2026 — — — — David Zaccardelli, Pharm.D.(6) 12/9/2016 29,999 — — 38.50 12/8/2026 — — — — 8/10/2016 5,000 — — 113.85 8/9/2026 — — — — Mark Hahn(6) 2/23/2017 5,000 — — 15.75 2/22/2027 — — — — 1/3/2017 25,000 — — 15.00 1/2/2027 — — — — 1/1/2016 11,931 — — 155.65 12/31/2025 — — — — 1/1/2015 9,272 — — 117.55 12/31/2024 — — — — 1/8/2014 11,999 — — 65.50 1/7/2024 — — — — 1/18/2013 10,000 — — 33.15 1/17/2023 — — — — 3/20/2012 4,999 — — 38.10 3/19/2022 — — — — 12/8/2010 1,814 — — 10.45 12/7/2020 — — — — 7/28/2010 1,528 — — 10.45 7/28/2020 — — — — 2/2/2010 9,378 — — 10.45 2/1/2020 — — — — John Bluth(6)(7) 2/23/2017 — — — — — 5,000 79,000 — — 2/23/2017 4,999 — — 15.75 2/22/2027 — — — — 1/3/2017 — — — — — 5,000 79,000 — — 1/3/2017 15,000 — — 15.00 1/2/2027 — — — — 8/5/2016 7,999 — — 120.70 8/4/2026 — — — — David Oldach, M.D.(6) 2/23/2017 5,000 — — 15.75 2/22/2027 — — — — 1/3/2017 25,000 — — 15.00 1/2/2027 — — — — 1/1/2016 9,178 — — 155.65 12/31/2025 — — — — 1/1/2015 4,599 — — 117.55 12/31/2024 — — — — 1/8/2014 1,500 — — 65.50 1/7/2024 — — — — 3/7/2013 2,000 — — 33.20 3/6/2023 — — — — 1/18/2013 2,817 — — 33.15 1/17/2023 — — — — 3/21/2012 3,999 — — 37.35 3/20/2022 — — — — 3/1/2011 3,269 — — 11.40 3/2/2021 — — — — Option awards Stock awards Name Number of shares acquired on exercise (#) Value realized on exercise ($) Number of shares acquired on vesting (#) Value realized on vesting ($)(1) Daniel Wechsler — — — — Paul D. Estrem — — — — Sue K. Cammarata, M.D. — — — — Erin Duffy, Ph.D. — — — — John Temperato — — — — David Zaccardelli, Pharm.D.(2) — — 10,000 116,250 Mark Hahn(2) — — 15,000 183,750 John Bluth — — — — David Oldach, M.D.(2) — — 15,000 183,750 Name Benefit Termination without cause or resignation for good reason ($)(1) Termination without cause or resignation for good reason in connection with a change in control ($)(1) Death or disability ($)(1) Change in control (2) Daniel Wechsler Cash payment 1,320,000 1,760,000 — — President and Chief Executive Bonus 53,342 53,342 53,342 — Officer Continuation of benefits 15,746 15,746 — — Vesting of Options and RSUs — 5,188,414 — 5,188,414 Total 1,389,088 7,017,502 53,342 5,188,414 Paul D. Estrem Cash payment 333,410 333,410 — — Executive Vice President, Chief Bonus — 100,023 — — Financial Officer and Secretary Continuation of benefits 18,220 18,220 — — Total 351,630 451,653 — — Sue K. Cammarata, M.D. Cash payment 433,433 433,433 — — Executive Vice President and Bonus — 130,030 — — Chief Medical Officer Continuation of benefits 790 790 — — Total 433,512 563,542 — — Erin Duffy, Ph.D. Cash payment 330,541 330,541 — — Executive Vice President and Bonus — 99,162 — — Chief Scientific Officer Continuation of benefits 6,245 6,245 — — Total 336,786 435,948 — — Name(1) Fees earned or paid in cash ($)(2) Option awards (3)(4)($) All other compensation ($) Total ($) Michael R. Dougherty 46,292 31,699 — 77,991 Kevin T. Ferro — — — — Jay Galeota — — — — David Gill 50,500 31,699 — 82,199 Dov A. Goldstein, M.D. 42,083 31,699 — 73,782 Cecilia Gonzalo — — — — John H. Johnson 37,875 31,699 — 69,574 Richard Kent, M.D. 37,875 31,699 — 69,574 Thomas Koestler, Ph.D.(5) — — — — Garheng Kong, M.D., Ph.D. 82,063 38,038 — 120,101 P. Sherill Neff 37,875 31,699 — 69,574 David Zaccardelli, Pharm.D.(6) — — — — Item 12. Security Ownership of Certain Beneficial Plan Category Number of securities to be issued upon exercise of outstanding options, warrants and rights Weighted-average exercise price of outstanding options, warrants and rights(1) Number of securities remaining available for future issuance under equity compensation plans(2) Equity compensation plans approved by our shareholders(3) 1,647,078 $ 41.65 764,745 Equity compensation plans not approved by our shareholders(4) 734,642 $ 11.65 — Total 2,381,720 764,745 Each person known by us to beneficially own more than 5% of our outstanding common stock Each of our directors Each of our NEOs; and All of our directors and executive officers as a group Name of Beneficial Owner Total Beneficial Ownership Percentage of Common Stock Beneficially Owned 5% and Greater Stockholders Vatera Healthcare Partners LLC(1) 8,507,237 27.1 % The Medicines Company(2) 3,313,702 10.6 % Deerfield(3) 3,127,846 9.9 % Malin Life Sciences Holdings(4) 1,557,628 5.0 % Executive Officers and Directors Daniel Wechsler(5) — 0.0 % David Gill(6) 21,000 * Paul Estrem(6) 54,233 * John H. Johnson(6) 25,756 * Garheng Kong, M.D., Ph.D.(6) 27,922 * Sue Cammarata, M.D.(6) 37,068 * David Zaccardelli, Pharm.D.(7) 44,999 * Erin Duffy, Ph.D.(6) 48,809 * Kevin T. Ferro(1) 8,507,237 27.1 % Jay Galeota — 0.0 % Cecilia Gonzalo(1) — 0.0 % Thomas P. Koestler, Ph.D.(1)(6) 11,758 * All directors and current executive officers as a group (13 persons) 8,778,782 27.8 % Item 13. Certain Relationships and Item 14. Principal (3) Exhibits. The following exhibits are included herein or incorporated herein by reference: Exhibit No. Description Form Dated Exhibit Number Filed Herewith 1.1 Sales Agreement, dated May 6, 2016, between Cempra, Inc. and Cowen and Company, LLC 8-K 05/06/2016 1.1 2.1 S-1 10/12/2011 2.1 2.2 8-K 8/10/2017 2.1 2.3 8-K (Filed by the Medicines Company) 1/14/2009 2.1 2.4 8-K (Filed by the Medicines Company) 12/6/2014 2.1 2.5 8-K 12/1/2017 2.1 3.1 Certificate of Incorporation of Melinta Therapeutics, Inc. f/k/a Cempra, Inc. S-1/A 1/13/2012 3.1 3.2 8-K 11/3/2017 3.1 3.3 8-K 11/3/2017 3.2 3.4 Amended and Restated Bylaws of Melinta Therapeutics, Inc. f/k/a Cempra, Inc. 8-K 11/3/2017 3.3 4.1 Specimen of Common Stock Certificate of Melinta Therapeutics, Inc. (f/k/a Cempra, Inc.) S-1/A 11/22/2011 4.1 4.2 8-K 11/3/2017 10.1 Exhibit No. Description Form Dated Exhibit Number Filed Herewith 8-K 1/9/2018 4.1 4.4 8-K 1/9/2018 4.2 4.5 8-K 1/9/2018 4.3 4.6 8-K 1/9/2018 4.4 4.7 8-K 1/9/2018 4.5 4.8 8-K 3/14/2018 4.1 4.9 14A 10/5/2017 Annex D-2 10.1 * 8-K 3/14/2018 10.17 10.2 8-K 1/9/2018 10.1 10.3 8-K 1/9/2018 10.2 Exhibit No. Description Form Dated Exhibit Number Filed Herewith 8-K 1/9/2018 10.3 10.5 + Melinta Therapeutics, Inc. 2011 Equity Incentive Plan, as amended. S-8 11/13/2017 99.1 10.6 + Form of Stock Option Agreement Granted Under the 2011 Equity Incentive Plan, as amended. S-8 11/13/2017 99.2 10.7 + S-1/A 1/13/2012 10.2 10.8 + Cempra, Inc. 2011 Equity Incentive Plan, as amended May 23, 2013. 10-Q 7/31/2013 10.3 10.9 + S-1/A 1/13/2012 10.3 10.10 + Amendment No. 1 to the Cempra, Inc. 2011 Equity Incentive Plan, as amended May 21, 2015. 8-K 3/14/2018 10.1 10.11 + 8-K 6/28/2017 10.1 10.12 + 10-K 2/28/2017 10.40 10.13 + 8-K 11/03/2017 10.5 10.14 + Employment Inducement Stock Option Agreement with Daniel Mark Wechsler. S-8 11/13/2017 99.3 10.15 + Employment Inducement Restricted Stock Unit Agreement with Daniel Mark Wechsler. S-8 11/13/2017 99.4 10.16 + 8-K 3/14/2018 10.2 10.17 + 8-K 3/14/2018 10.3 Exhibit No. Description Form Dated Exhibit Number Filed Herewith + 8-K 3/14/2018 10.4 10.19 + 8-K 3/14/2018 10.5 10.20 + 8-K 3/14/2018 10.6 10.21 + 8-K 3/14/2018 10.7 10.22 + 8-K 3/14/2018 10.8 10.23 + 8-K 3/14/2018 10.9 10.24 + 8-K 3/14/2018 10.10 10.25 + 8-K 3/14/2018 10.11 10.26 + 8-K 3/14/2018 10.13 10.27 + 8-K 3/14/2018 10.12 10.28 + 8-K 3/14/2018 10.16 10.29 + Form of indemnification agreement by and between Melinta Therapeutics, Inc. and its directors. 8-K 11/3/2017 10.2 Exhibit No. Description Form Dated Exhibit Number Filed Herewith + 8-K 11/3/2017 10.3 10.31 + Form of indemnification agreement by and between Melinta Therapeutics, Inc. and its directors 8-K 11/3/217 10.4 10.32 S-1 (Filed by the Medicines Company) 3/11/2007 10.11 10.33 8-K 3/14/2018 10.18 10.34 10-Q 7/31/2013 10.13 10.35 10-Q 7/31/2013 10.14 10.36 10-Q 10/29/2013 10.19 10.37 10-K 02/25/2016 10.35 10.38 * 8-K 3/14/2018 10.14 10.39 8-K 3/14/2018 10.15 10.40 10-Q 7/31/2013 10.15 10.41 10-Q/A 11/8/2013 10.18 Exhibit No. Description Form Dated Exhibit Number Filed Herewith 10-K 2/29/2015 10.27 10.43 10-Q 05/02/2016 10.36 10.44 10-Q 10/27/2016 10.1 10.45 10-Q (Filed by the Medicines Company) 11/9/2011 10.1 10.46 8-K (filed by the Medicines Company) 3/2/2009 99.1 21.1 † 23.1 † 31.1 X 31.2 X 101 † + The exhibit contains a management contract, compensatory plan or arrangement which is required to be identified in this report. MELINTA THERAPEUTICS, INC. By: /s/ John H. JohnsonItem 11. ExecutiveExecutive CompensationCOMPENSATION DISCUSSION AND ANALYSISSection IOVERVIEWSection IIPAY FOR PERFORMANCE PHILOSOPHY; EXECUTIVE COMPENSATION PROGRAM OBJECTIVESSection IIICOMPENSATION DETERMINATION PROCESSSection IVCOMPONENTS OF EXECUTIVE COMPENSATIONSection VCOMPENSATION PRIOR TO, AND IN CONNECTION WITH, THE MERGER WITH CEMPRASection VIADDITIONAL COMPENSATION POLICIES AND PRACTICESI.OVERVIEWThis Compensation DiscussionThe information required by this Item concerning directors and Analysis describes: our executive compensation particularly following the November 2017 merger with Cempra; our early 2018 compensation decisions and actions; and, importantly, the contours of the philosophy, objectives, processes, components and additional aspects of our executive compensation program going forward. This Compensation Discussion and Analysis is intended to be read in conjunction with the tables that immediately follow this section, which provide further historical compensation information.BackgroundWe are a commercial-stage pharmaceutical company focused on discovering, developing and commercializing differentiated anti-infectives for the hospital and select non-hospital, or community, settings that address the need for effective treatments for infections due to resistant gram-negative and gram-positive bacteria. We currently market four antibiotics to treat a variety of infections caused by these resistant bacteria and other bacteria.On November 3, 2017, Cempra, Inc., a clinical-stage company developing anti-infectives, completed a merger with privately held Melinta Therapeutics Inc., a commercial-stage company developing novel antibiotics for serious bacterial infections, with the surviving company changing its name to Melinta Therapeutics, Inc., Melinta or the Company. The combination resulted in an entity with extensive core competencies in developing and commercializing novel antibiotics to treat serious, life-threatening infections. Following the merger, Melinta shareholders owned approximately 52% of the combined company, and Cempra shareholders owned approximately 48%.Prior to the merger, on June 19, 2017, we announced that the U.S. Food and Drug Administration had approved Baxdela™ (delafloxacin), indicated in adults for the treatment of acute bacterial skin and skin structure infections caused by susceptible bacteria. In the first quarter of 2018, we launched Baxdela in the United States and our European partner filed for marketing approval in Europe.On January 5, 2018, we acquired the infectious disease business of The Medicines Company, bolstering our position as a leading antibiotics-focused company, with a large portfolio of current and potential products and substantial expertise in antibiotic development and commercialization. With this acquisition, our combined portfolio added three additional marketed products: Vabomere™ (meropenem and vaborbactam), Orbactiv® (oritavancin), and Minocin® (minocycline) for Injection.In connection with the merger with Cempra, our board of directors recruited a new Chief Executive Officer (“CEO”) and established the rest of the management team by appointing the executive officers of the combined organization. This new CEO and certain other executive officers who were in office on December 31, 2017, the last day of our fiscal year, are included as 2017 Named Executive Officers, or NEOs, under SEC rules, and we refer to them as the Current NEOs. There are also other individuals who served as the principal executive officer or principal financial officer of Cempra during 2017 before the merger or who are otherwise included as NEOs under the SEC rules who we refer to as Former NEOs. Hence, the Company’s 2017 NEOs are as follows:Current NEOsDaniel WechslerPresident and CEOPaul D. EstremExecutive Vice President, Chief Financial Officer and SecretarySue K. Cammarata, M.D.Executive Vice President and Chief Medical OfficerErin Duffy, Ph.D.Executive Vice President and Chief Scientific OfficerJohn Temperato(1)Former Executive Vice President and Chief Commercial OfficerFormer NEOsDavid Zaccardelli, Pharm.D.Former Acting CEOMark HahnFormer Executive Vice President and Chief Financial OfficerJohn BluthFormer Executive Vice President, Investor Relations and Corporate CommunicationsDavid Oldach, M.D.Former Chief Medical Officer(1)Mr. Temperato’s employment with the Company ended on January 5, 2018.Leadership TransitionsEffective as of the closing of the Cempra merger on November 3, 2017, we appointed Daniel Wechsler as President and CEO and a member of the board of directors. Mr. Wechsler is a seasoned pharmaceutical executive with more than 25 years of healthcare experience at companies including Pfizer, Schering-Plough, Merck, Upjohn, Pharmacia and Bausch & Lomb.Also, effective upon the closing of the merger:We appointed Sue Cammarata, M.D., as Chief Medical Officer. Dr. Cammarata has served as chief medical officer of Melinta since 2014. She has more than 20 years of clinical experience in the development, approval and launch of pharmaceuticals, including several anti-infective brands.We appointed Erin Duffy, Ph.D., as Chief Scientific Officer. She joined Melinta in 2002 and has more than 21 years of pharmaceutical research experience. She has been responsible for translating Melinta’s ribosome technology platform into the discovery and early-stage development of novel antibiotic candidates.We appointed Paul Estrem as Chief Financial Officer, a position he held prior to the merger. Mr. Estrem has more than 27 years of financial leadership experience in the pharmaceutical industry. Prior to joining Melinta, Mr. Estrem held several senior positions at Baxter International.We appointed John Temperato as our Executive Vice President and Chief Commercial Officer, and effective as of January 5, 2018, Mr. Temperato’s employment with the Company ended.The employment of Dr. Zaccardelli terminated in connection with the completion of the merger, effective November 3, 2017. He continues to serve on our board of directors as a non-employee member. Each of Messrs. Hahn and Bluth and Dr. Oldach resigned as officers of the Company effective November 15, 2017.Our mission is to meet the continually evolving threat of bacterial infections by discovering, developing and commercializing a continuous stream of novel antibiotics. In order to accomplish our mission, we must attract, motivate and retain highly skilled and experienced people to execute our corporate strategy and lead our team. In addition, we have designed our pay-for-performance compensation programs to reward the achievement of key operational and strategic objectives intended to help achieve our mission, given our current stage of development, our commercial activities and our goal of progressing product development. In light of these two principles, our executive compensation programs and decisions have the following objectives:to link pay to performance over both the short- and long-term;to align our executives’ interests with the interests of shareholders through long-term incentives linked to specific performance, and with the creation of shareholder value;to attract, motivate and retain highly skilled and experienced executives;to provide incentives that reward the achievement of performance goals, tie into corporate strategic and operational goals, and that directly correlate to the enhancement of shareholder value; andto reduce incentives to expose Melinta to imprudent risks that might harm the Company or our shareholders.Key Compensation Decisions and Actions After 2017 MergerBetween June 2017 and early 2018, we undertook a significant strategic transformation. As described above in the “Background” section, we closed a merger with Cempra, secured U.S. Food and Drug Administration approval of Baxdela and began marketing it in the U.S., and acquired the infectious disease business from The Medicines Company, thereby bringing three additional marketed products to the Company. At the time of this filing, we have become a leading antibiotics-focused company, based on these combinations with other organizations or parts thereof. The compensation committee of the board of directors (“Compensation Committee”) is of the view that while the executive leadership team is initially managing the new, larger organization and implementing the integration of the companies and units, setting goals can be more challenging. As such, visibility into the future is inferior to visibility in time periods with less significant transitions taking place. Given this current situation, in early 2018 the Compensation Committee evaluated what it believes to be the best structure and component mix of the executive compensation program, as well as performance metrics and total and component target amounts.If we continue to achieve our corporate and operations goals and our research milestones, advance additional products toward commercialization and expand our executive team, we anticipate that our overall compensation philosophy and certain approaches, priorities and components of our executive compensation program will adapt and change.Corporate Governance PracticesAs part of the efforts of the Compensation Committee to ensure that our compensation program, which includes our policies and practices, aligns our executive officers’ interests with those of the Company and its shareholders, the Compensation Committee assesses the effectiveness of our compensation program periodically, including with respect to risk mitigation and governance of the program. The governance of our executive compensation program includes the following best practices:What We DO:What We DO NOT Do:√Align executive compensation with shareholderreturns by tying a significant portion of annualtarget compensation to corporate performanceXNo gross-ups of excise taxes that may be imposedas a result of severance or other payments madein connection with a change in control√Review compensation data from peers whose stageof development, revenues and number ofemployees share similarities with MelintaXNo special retirement plans for executive officers√Use equity for long-term incentive awards andprovide that awards have a vesting period of at leastone yearXNo compensation programs that encourage short-term risk-taking at the expense of long-termresults or that otherwise create risks reasonablylikely to have a material adverse effect on theCompany√Maintain an appropriate balance between short- andlong-term compensation, which discourages short-term risk-taking at the expense of long-term resultsXNo guaranteed performance bonuses√Require executive officers to comply with our stockownership guidelinesXNo repricing of stock options without shareholderapproval√Engage an independent compensation advisor to theCompensation Committee, who performs no otherservices for MelintaXNo discounted stock options√“Anti-Hedging policy” regarding our sharesapplicable to directors and executive officersXNo highly leveraged incentive plans thatencourage excessive risk taking√“Anti-Pledging policy” limiting the pledging ofshares applicable to directors and executive officersXNo single-trigger vesting of equity upon a changein control√Engage with shareholders to discuss andunderstand their perceptions or concernsregarding our executive compensation programsXNo perquisites2017 Say-on-Pay ResultsWe take very seriously and take into consideration the input and feedback that we receive from our shareholders about our executive compensation program. At the 2017 meeting of the legacy Cempra entity prior to the merger with Melinta, our say-on-pay proposal received support from 76% of the votes cast by our shareholders on the matter. Our Compensation Committee believes that the shareholders, through this advisory vote, generally endorsed the legacy Cempra compensation philosophy and principles. As described above, however, in early 2018 the Compensation Committee evaluated the nature of the executive compensation program following the Company’s strategic transformation. The Company is committed to regular, ongoing engagement with shareholders to ensure that we continue to understand shareholder feedback about our compensation programs and other key matters of interest to them, and to enable us to take that feedback into consideration for our compensation decisions.Compensation Data; Independent Compensation ConsultantThe Compensation Committee has the power to retain independent compensation consultants, legal counsel, or other advisors as it may deem appropriate to assist it in the performance of its duties and responsibilities, without consulting or obtaining the approval of management of the Company. The Compensation Committee recognizes the importance of objective, independent expertise and advice in carrying out its responsibilities. In connection with its need to review and modify our executive compensation program in connection with the Company’s merger with Cempra and acquisition from The Medicines Company, in December 2017, the Compensation Committee retained Radford, an Aon Company, as its independent compensation consultant. Radford reports directly to, and is directly accountable to, the Compensation Committee, and the Compensation Committee has the sole authority to retain, terminate and obtain the advice of Radford at the Company’s expense. The Compensation Committee selected Radford as consultants because of the firm’s expertise and reputation and the fact that Radford provides no services to the Company other than its services to the Compensation Committee, has no other ties to management that could jeopardize its fully independent status, and has strong internal governance policies that help ensure that it maintains its independence. The Compensation Committee, with the assistance of its independent compensation consultant, monitors market compensation practices and developments, as well as the appropriateness of the various components of the executive pay program, as our business progresses and evolves with anticipated growth and changing market conditions.In addition, in making determinations about executive compensation, our Compensation Committee believes that obtaining relevant market and benchmark data is very important. Collecting such information provides very helpful context and a solid reference point for making decisions, even though there are differences and unique aspects of the Company and its stage of development relative to other companies. Our Compensation Committee also considers corporate and individual performance, any shifts in the current or anticipated future domains and function of the executive officers and the input of other Company directors who are not members of the Compensation Committee.More specifically, when our Compensation Committee makes decisions about the structure and component mix of our executive compensation program, it takes into consideration the amounts paid under, and the structure and components of, the executive compensation programs of other, comparable U.S. biotechnology and pharmaceutical companies, as derived from public filings and other sources. The Compensation Committee has worked with Radford to: assess our executive compensation philosophy, objectives and components; develop a peer group of companies for compensation comparison purposes; review considerations and market practices related to short-term cash incentive plans and long-term equity and other incentive plans, and trends in the biopharmaceutical industry, based in part on the Radford Global Life Sciences Survey; collect comparative compensation levels for each of our executive positions; assess our executives’ base salaries, short-term cash incentives and long-term equity compensation levels; review our equity compensation strategy, including the development of award guidelines; and review director compensation market practices among biopharmaceutical companies of comparable size and/or stage.While the Compensation Committee takes into consideration the review and recommendations of Radford when making decisions about the Company’s executive compensation program, ultimately the Compensation Committee makes its own independent decisions about compensation matters.The Compensation Committee has assessed the independence of Radford pursuant to SEC and Nasdaq rules. In doing so, the Compensation Committee considered each of the factors set forth by the SEC and Nasdaq with respect to a compensation consultant’s independence. The Compensation Committee also considered the nature and amount of work performed for the Compensation Committee and the fees paid for those services in relation to the firm’s total revenues. Radford did not provide any services to the Company other than the services provided to the Compensation Committee as described herein and in the “Director Compensation” section.Radford has conducted a review of its performance, and has prepared an independence letter for the Compensation Committee that provides assurances and confirmation of the consultant’s independent status under the standards. None of the Compensation Committee members and none of our executive officers or directors have any personal relationship with Radford. The Compensation Committee also determined that there were no other factors that should be considered in connection with the assessment or that were otherwise relevant to the engagement of Radford. On the basis of its consideration of the foregoing, the Compensation Committee concluded that there were no conflicts of interest, and that Radford is independent.When making decisions related to executive compensation, the Compensation Committee considers peer group and other industry compensation data and the recommendations of Radford, as well as the competitiveness of our compensation program, viewed from a variety of perspectives, and individual circumstances.During the first part of 2018, our Compensation Committee, with input from our compensation consultant, established a peer group of companies that have similar characteristics to our Company, in order to make compensation decisions for 2018. This peer group consisted primarily of biopharmaceutical companies with marketed pharmaceutical products, as well as a couple of companies at the New Drug Application stage, and has been used as a frame of reference for compensation levels within our industry and for informing compensation decisions.The following companies located in North America constitute the peer group approved in January 2018. These companies were considered comparable to us in terms of their stage of development, revenues and number of employees.Achaogen, Inc.Omeros CorporationAdamas Pharmaceuticals, Inc.Osiris Therapeutics, Inc.Collegium Pharmaceutical, Inc.Pacira Pharmaceuticals, Inc.Corium International, Inc.PTC Therapeutics, Inc.Eagle Pharmaceuticals, Inc.Retrophin, Inc.Enzo Biochem, Inc.Spectrum Pharmaceuticals, Inc.Flexion Therapeutics, Inc.Supernus Pharmaceuticals, Inc.Keryx Biopharmaceuticals, Inc.Theravance Biopharma, Inc.Lexicon Pharmaceuticals, Inc.Vanda Pharmaceuticals Inc.Our peer group is subject to change, and we expect that our Compensation Committee will continue to periodically review and update the list, as appropriate, according to the criteria listed above.Determining Executive CompensationOur Compensation Committee has responsibility for establishing our compensation philosophy and objectives, determining the structure, components and other elements of our programs, and reviewing and approving, or recommending for approval by the board of directors, the compensation of our NEOs. The Compensation Committee exercises its judgment and experience when determining the particular component mix and amounts of compensation for each of our executive officers.Our Compensation Committee’s approach to setting compensation for our executive officers is to consider market forms and levels of compensation, an executive officer’s responsibilities and contributions, and the objectives and overall progress of the Company. With the assistance of Radford, the Compensation Committee determined to establish a philosophy to target total compensation for our NEOs at the 50th percentile of the market, based on our peer group. Total compensation for this purpose is comprised of base salary, annual cash incentives and long-term equity incentives.In addition to market benchmarking, as part of the process, our CEO will typically provide input and recommendations to the Compensation Committee on salary adjustments and target cash and equity incentive compensation levels for executive officers other than himself. The Compensation Committee will also consider the Company’s overall performance during the prior year, each executive’s individual contributions during the prior year, the individual’s annual performance reviews based on achievement of annual goals and other relevant market data. The Compensation Committee approves the overall compensation packages for each of our executive officers, including our CEO’s compensation package. When approving our CEO’s compensation package, the Compensation Committee meets with our independent compensation consultant, in an executive session, without management present. Our CEO does not have any control over setting the amount or mix of his compensation and is not present when the Compensation Committee discusses his compensation. With respect to new hires, our Compensation Committee considers an executive’s background, historical compensation and the role undertaken within the Company in lieu of prior year performance.2018 Comprehensive Review and Restart of Equity Compensation ProgramHaving secured U.S. Food and Drug Administration, (“FDA”) approval of Baxdela™, closed the merger with Cempra, and acquired the infectious disease business from The Medicines Company, we have become a leading antibiotics-focused company. In light of the new larger size and profile of the Company, in early 2018, we undertook a comprehensive review and restart of our executive and broad-based equity compensation strategies. As described above, we sought input from Radford regarding, among other things, executive compensation philosophy, equity compensation strategy, market practices related to long-term equity, and information regarding comparative equity awards received by, and stock ownership of, executives at companies in our peer group and our industry and at our current stage of development.As part of this comprehensive review, our Compensation Committee decided that the 2018 target total direct compensation for our CEO and other NEOs would be determined with reference to the 50th percentile of compensation for executives holding similar positions at the companies in our compensation peer group and companies that had recently completed initial public offerings. Consistent with this approach, in connection with the restart, the Compensation Committee made initial grants of equity to the CEO, the NEOs and the other executive officers in amounts representing the greater of an annual equity grant that approximated the market 50th percentile, or an amount that brought the recipient, considering their existing holdings, up to the market 50th percentile for a new hire equity grant for their position. The Compensation Committee also considered the resulting total ownership of the recipient relative to the market 50th percentile, and in some cases, sized the award so as not to exceed the range of the market 50th percentile of ownership. These initial restart grants were in the form of time-vested options and were made subject to shareholder approval of the 2018 Stock Incentive Plan at the 2018 Annual Meeting of Shareholders. The Compensation Committee has also taken note of the fact that as of the date of this filing, all of the stock options granted to the NEOs and other executive officers and employees are “underwater” (their exercise price is above the current trading price). After these 2018 restart grants, in 2019, the Compensation Committee intends to make annual grants to executive officers at the market 50th percentile.IV.COMPONENTS OF EXECUTIVE COMPENSATIONThe primary elements of our executive compensation program are:base salary;annual performance-based cash incentive compensation; andlong-term equity incentive awards.We also provide broad-based health and welfare benefits and severance and change in control benefits.Our intention is to structure these components of our executive compensation program in a way that achieves the objectives of the program of linking pay to performance over both the short- and long-term, aligning executives’ interests with the interests of shareholders and attracting, motivating and retaining highly skilled and experienced executives.ElementDescriptionStrategic RoleBase SalaryFixed cash compensation.Targeted generally within the range of the market median based on each NEO’s individual performance, skills, experience and internal equity.Base salaries provide stable compensation to executive officers, allow Melinta to attract and retain capable executive talent and maintain a stable management team.Short-Term Incentives: Annual Performance-Based Cash Incentive CompensationAnnual cash incentives are awarded based on the achievement of corporate goals.Performance objectives are determined annually and generally incorporate specific milestones or other metrics.Annual cash incentive amounts are designed to motivate executive officers to achieve key short-term milestones and measures.Long-Term Incentives: Stock OptionsSubject to time-based vesting conditions, stock options provide a right to purchase shares of the Company’s common stock at a specified price which is fixed at the time of grant during a specified period.Stock options align the executive’s interest with shareholder interests because recipients only realize value from stock options if the value of the Company’s common stock increases above its value on the date the option was granted (which benefits all shareholders) and the recipient continues to provide services to the Company through the vesting period.Discourages excessive risk taking.Long-Term Incentives: RSUsSubject to time-based vesting conditions, restricted stock units (RSUs) are contractual rights to receive a specified number of shares of the Company’s common stock at a future date.Time-vested RSUs are “full value grants,” meaning that, upon vesting, the recipient is awarded the full share. As a result, while the value executives realize in connection with an award of RSUs does depend on our stock price, time-vested restricted stock units generally have some value even if the Company’s stock price significantly decreases following their grant. As a result, time-vested RSUs help to secure and retain executives and instill an ownership mentality, regardless of whether the Company’s stock price increases or decreases.Discourages excessive risk taking.Base SalaryWe determine base salary amounts based on roles and responsibilities, skills, expertise, prior professional experience, achievements, educational background and seniority. For newly hired executive officers, we establish initial base salaries through arm’s-length negotiations at the time the executive is hired, considering the position, the executive’s experience, qualifications, and prior compensation salary. None of our executive officers is currently party to an employment agreement or offer letter that provides for automatic or scheduled increases in base salary.In addition, the Compensation Committee considers a variety of other factors in determining both initial base salary levels and increases, including the executive’s performance and contributions during the prior year, performance over a period of years, promotion, general labor market conditions, the relative ease or difficulty of replacing the executive with a well-qualified person, our overall growth and development as a Company, general salary trends in our industry and among our peer group, and where the executive’s salary falls within the salary range presented by those comparator groups. In making decisions regarding salary increases, we may also draw upon the experience of members of our board of directors with other companies. We do not provide for any formulaic base salary increases for our NEOs.(1)Effective November 3, 2017, in connection with the completion of the merger with Cempra, Mr. Wechsler was appointed President and CEO. The amount reflected in this table is annualized, and does not reflect the annual base salary actually earned by Mr. Wechsler with respect to 2017, which was prorated based on his period of employment with the Company.Annual Cash IncentiveFollowing the merger with Cempra, in contemplating variable, at-risk annual cash incentives for our executive officers, our Compensation Committee took into account the review of considerations and market practices related to short-term cash incentive plans provided by Radford. Going forward, each executive officer will be eligible to receive annual performance-based cash incentive compensation in an amount based on a percentage of his or her base salary. Such annual incentives will reward our NEOs for the achievement of annual corporate objectives. For 2018, target percentages for annual performance-based cash incentive compensation for our executives range from 35% to 65% of base salary. The 2018 target percentages for annual cash incentives for our Current NEOs are set forth in the following table.2017 Bonuses. In respect of 2017, a year during the majority of which Melinta was a private company, we paid annual bonuses to our NEOs based upon the achievement of significant milestones, performance or other factors. As described above in this “Compensation Discussion and Analysis,” between June 2017 and early 2018, the Company undertook a significant strategic transformation. We closed a merger with Cempra in November 2017, secured FDA approval of Baxdela in June 2017 and began marketing it in the U.S., and acquired the infectious disease business from The Medicines Company (announced in November 2017, closed in January 2018). At the time of this filing, the Company has become a leading antibiotics-focused Company. In light of the steps taken to effect this significant strategic transformation, the Compensation Committee evaluated the Current NEOs’ achievements, giving significant weight to the specific contributions of each Current NEO to Company-wide performance, as well as each Current NEO’s performance in their area of responsibility or function. In general, although each executive was eligible to earn an annual bonus equal to a certain percentage of his or her base salary, due to the unique nature of some of the elements of the strategic transformation that were executed in 2017, the Compensation Committee applied its judgement to determine bonus amounts based on a number of factors. By doing so, the Compensation Committee had the flexibility to take a holistic view of each Current NEO’s performance for the year, in contrast to an inflexible, formulaic approach that might not have accounted completely for certain intangible contributions, among other things. Based on the Compensation Committee’s evaluation of each Current NEO as described above, it awarded the following bonuses in 2017:Long-Term Equity IncentivesIn connection with the process currently being undertaken by the Compensation Committee of making decisions about the structure and components of our executive compensation program, and in connection with obtaining the compensation advisory services of Radford, the Compensation Committee determined that, in addition to base salary and annual cash incentives, the third component of our executive compensation program will continue to be long-term equity incentives. We believe that equity awards provide our executives with a strong link to our long-term performance, create an ownership culture and help to align the interests of our executives with those of our shareholders. In addition, we believe that equity awards that vest over time promote executive retention because the executives will only realize the value of the equity if they remain in our employment during the vesting period.On April 13, 2018, our board of directors approved the Melinta Therapeutics, Inc. 2018 Stock Incentive Plan, or the 2018 Incentive Plan, and we are seeking the approval of our shareholders of the 2018 Incentive Plan at the 2018 Annual Meeting of Shareholders. The 2018 Incentive Plan will authorize the grant of stock options, restricted stock awards, RSUs, stock appreciation rights, performance awards and other awards that may be settled in or based upon our common stock.We currently maintain the Melinta Therapeutics, Inc. 2011 Equity Incentive Plan, as amended, or the 2011 Equity Incentive Plan, and the Melinta Therapeutics, Inc. 2011 Equity Incentive Plan, as amended, or the Private Melinta 2011 Equity Incentive Plan. Under these plans, we have granted various forms of equity compensation to our service providers, including our key employees, non-employee directors and consultants. We have historically granted mostly options to service providers, except for a grant of restricted stock units upon the hiring of our CEO in 2017, and grants of restricted stock units to Cempra employees prior to the closing of the Cempra merger.Annual Equity Awards. Our 2011 Equity Incentive Plan allows the grant to officers and employees of stock options, as well as other forms of equity incentives, as part of our overall compensation program. Our practice has been to make annual stock option awards as part of our overall performance management program at the beginning of each calendar year. Typically, these grants have been made to ensure the executive officer’s average equity and option amounts were in line with similar positions at comparable companies. As with base salary and initial equity award determinations, a review of all components of the executive officer’s compensation was conducted when determining annual equity awards to ensure that an executive officer’s, including each NEO’s, total compensation conformed to our overall compensation philosophy and objectives.Going forward, we intend to grant equity awards in the first quarter of each year in conjunction with our annual performance and compensation review cycle covering the prior year.In determining the size of the annual equity awards granted to our NEOs, our Compensation Committee intends to consider recommendations developed by Radford, including information regarding comparative stock ownership of, and equity awards received by, executives at companies in our peer group and our industry and at our current stage of development. In addition, our Compensation Committee considers each executive’s individual performance, the amount of equity previously awarded to such executive and the future vesting of such awards, as well as our overall corporate performance and the executive’s potential for enhancing the creation of value for our shareholders. The Compensation Committee also plans to take into consideration the effects of the merger of Cempra with Melinta, as well as the acquisition of the infectious disease business from The Medicines Company, as a result of which the rights represented by stock options, RSUs and shares of stock held by NEOs and employees were diluted by more than half. The Compensation Committee has also taken note of the fact that as of the date of the filing of this amendment to our Annual Report on Form 10-K for the fiscal year ended December 31, 2017, all of the stock options granted to the NEOs and other executive officers and employees are “underwater” (their exercise price is above the current trading price). The grant date fair values of equity awards granted to our NEOs are included in the amounts set forth in the “Summary Compensation Table” which immediately follows this “Compensation Discussion and Analysis” section.Special Equity Awards. We might also make equity awards to certain of our NEOs and other employees other than the annual grants described above when specific circumstances warrant such grants. These situations might include grants upon promotion to a new position, or for purposes of recognizing the achievement of critical corporate objectives, or, if deemed necessary under the circumstances, for retention. In addition, we might make grants of equity to newly hired executive officers in connection with his or her commencement of employment on a case-by-case basis under the specific hiring circumstances. The size of each new hire grant is established through arm’s-length negotiations at the time the executive is hired, taking into account the position for which the executive is being considered and the executive’s qualifications, prior experience and equity holdings at prior companies, as well as external factors such as general labor market conditions and the relative ease or difficulty of replacing the executive with a well-qualified person. The new hire option award and RSUs granted to the CEO have the same vesting schedule as the annual awards.Stock Options and RSUs. To date, given the Company’s current profile as one that has become a commercial stage company relatively recently, the Compensation Committee determined to use primarily stock options for the long-term equity portion of its compensation program. Subject generally to their providing services throughout the applicable vesting period, recipients only realize value from stock options if the value of our common stock increases over the value of our common stock on the date the option is granted or the assigned exercise price, if higher. Because the value of the award to the recipient is solely tied to an increase in the value of our common stock, the interests of recipients are aligned with those of shareholders. Also, options promote the long view and motivate and reward the recipient for Company performance not only over the vesting period, but also over the whole term of the option. The Compensation Committee might also incorporate restricted stock units, or RSUs, over time, as these “full value” awards encourage ownership and retention and tend to be more reflective of a company that is maturing, with multiple marketed products and a broader pipeline. The Compensation Committee might also consider performance awards once the Company becomes larger. In determining individual grant amounts for the CEO and the other Current NEOs, the Compensation Committee took into account several factors, including the effects of stock price volatility, striving to maintain a reasonable annual burn rate, delivering competitive equity levels, and considerations specific to each individual.Typically, the stock options we have granted to our executive officers, including the RSUs we granted our Chief Executive Officer, vest with respect to 25% of the shares subject to the option or RSUs, as applicable, on the first anniversary of the grant date and with respect to the remaining shares in approximately equal monthly installments through the fourth anniversary of the grant date. Vesting ceases upon termination of employment, and exercise rights for options cease shortly after termination of employment. Prior to the exercise of a stock option or settlement of an RSU, the holder has no rights as a shareholder with respect to the shares subject to such option or RSU, including voting rights or the right to receive dividends or dividend equivalents; however, our CEO is entitled to dividend equivalents in connection with any dividends paid to shareholders of the Company during such vesting period; provided that any dividend equivalents paid with respect to unvested RSUs will be subject to the same vesting requirements as the underlying RSUs to which such dividend equivalents relate. To date, the Company has not paid dividends. We have historically granted stock options with exercise prices that are set at no less than the fair market value of shares of our common stock on the date of grant as determinedincorporated by reference to the closing market price of our common stock on the date of grant or, with respect to grants under the Private Melinta 2011 Equity Incentive Plan, based on a valuation prepared by a third-party valuation firm.Severance and Change in Control BenefitsPursuant to employment agreements or arrangements we have entered into with our executive officers, our executive officers are entitled to specified benefits in the event of the termination of their employment under specified circumstances, including termination following a change in control of Melinta. We have provided estimates of the value of the severance payments and other benefits that would have been made or provided to executive officers under various termination circumstances under the caption “Potential Payments on Termination and Change of Control” below.We believe that providing these benefits helps us compete for executive talent. After reviewing the practices of companies represented in the compensation peer group, we believe that our severance and change in control benefits are generally in line with severance packages offered to executives of our publicly traded peer companies.We have structured our change in control benefits as “double trigger” benefits. In other words, the change in control does not itself trigger benefits. Rather, benefits are paid only if the employment of the executive officer is terminated in connection with the change in control or our CEO’s equity awards are not assumed in connection with such transaction. We believe that a “double trigger” benefit maximizes shareholder value because it prevents an unintended windfall to executive officers in the event of a friendly change in control, while still providing them appropriate incentives to cooperate in negotiating any change in control in which they believe they may lose their jobs.V.COMPENSATION PRIOR TO, AND IN CONNECTION WITH, THE MERGER WITH CEMPRAEmployment Agreement with New President and Chief Executive Officer, Daniel WechslerIn connection with Mr. Wechsler’s employment, the Company entered into an employment agreement with Mr. Wechsler. For additional information please see “Narrative to Summary Compensation Table and Grant of Plan-Based Awards Table” below. Upon commencing employment on November 3, 2017, Mr. Wechsler received the following sign-on equity awards: (i) 183,661 time-vesting RSUs; and (ii) 550,981 options, with an exercise price of $11.65.Compensation Arrangements Prior to the Merger with CempraPrior to the closing of the merger with Cempra on November 3, 2017, the principal components of the executive compensation program for the Former NEOs were base salary, annual bonus, and long-term incentive. Annual base salary increases, annual bonuses, and long-term incentives, to the extent granted, were generally implemented during the first calendar quarter of the year.Annual Salaries. Cempra provided base salaries to the Former NEOs to compensate them for their services rendered during the fiscal year based on their position and scope of responsibilities, their prior experience and training, and competitive market compensation data Cempra reviewed for similar positions in Cempra’s industry.Annual Cash Bonus. Cempra had also implemented an annual cash incentive performance program, under which annual corporate goals were proposed by management and approved by the compensation committee of Cempra’s board of directors, or the Cempra Committee, at the start of each calendar year. These corporate goals include the achievement of qualitative operational goals and predefined research and development milestones. Each goal was weighted as to importance by the Cempra Committee. The Former NEOs did not receive any amounts in respect of the annual cash bonus component of the program for 2017.Long-Term Incentives. Cempra’s equity-based long-term incentive program was designed to align the Former NEOs’ long-term incentives with shareholder value creation. Cempra’s practice was to make annual stock option awards as part of Cempra’s overall performance management program at the beginning of each calendar year. In December 2016, Cempra also began granting restricted stock units as part of Cempra’s overall performance management program. Typically, these grants were made to ensure the Former NEO’s average equity and option amounts were in line with similar positions at comparable companies. As with base salary and initial equity award determinations, a review of all components of the Former NEO’s compensation was conducted when determining annual equity awards to ensure that total compensation conformed to Cempra’s overall philosophy and objectives. The Cempra Committee made 2017 grants to the Former NEOs as follows:(1)All amounts presented reflect the 5 for 1 stock split effected on November 3, 2017, in connection with the merger with Cempra.The stock options granted to each of Messrs. Hahn and Bluth and Dr. Oldach in January 2017 were initially scheduled to vest in equal monthly installments over a four-year period on each monthly anniversary of January 1, 2017, and the stock options granted to each of Messrs. Hahn and Bluth and Dr. Oldach in February 2017 were initially scheduled to vest in equal monthly installments over a four-year period on each monthly anniversary of February 23, 2017. The stock options granted to each of Messrs. Hahn and Bluth and Dr. Oldach were accelerated in connection with each such executive’s termination of employment pursuant to each executive’s separation agreement, as discussed in more detail below under “Potential Payments on Termination and Change of Control.”The RSUs granted to each of Messrs. Hahn and Bluth and Dr. Oldach in January 2017 were initially scheduled to vest and settle on January 1, 2019 and the RSUs granted to each of Messrs. Hahn and Bluth and Dr. Oldach in February 2017 were initially scheduled to vest and settle on January 9, 2019. The RSUs granted to each of Messrs. Hahn and Bluth and Dr. Oldach were accelerated in connection with each such executive’s termination of employment pursuant to each executive’s separation agreement, as discussed in more detail below under “Potential Payments on Termination and Change of Control.”David Zaccardelli Employment AgreementIn connection with the hiring of Dr. Zaccardelli as Acting Chief Executive Officer in December 2016, Cempra entered into an Executive Employment Agreement with him. For additional information, please see “Narrative to Summary Compensation Table and Grant of Plan-Based Awards Table” below.Severance and Change in Control Agreements with the Former NEOsIn connection with each Former NEO’s termination of employment, Cempra entered into severance agreements with such executive. For additional information, please see footnote (7) to the “Summary Compensation Table” below.Stock Ownership GuidelinesOn February 25, 2016, Cempra adopted, and we are maintaining, stock ownership guidelines for certain executive officers and directors, with the objective of more closely aligning the interests of certain executive officers with those of our shareholders. The stock ownership guidelines will require the CEO to hold shares of our common stock with a market value of five times his annual base salary and will require the Chief Financial Officer, Chief Scientific Officer and Chief Medical Officer to hold stock valued at two times their respective annual base salaries. The stock ownership guidelines will be applicable at the later of (i) five years after the date the executive officer assumes his or her position or (ii) five years from the date of adoption of the stock ownership guidelines.Current PositionRequired Annual Base Salary MultipleCEO5xAll other executive officers2xAnti-Hedging and Anti-Pledging PolicyOn September 25, 2015, Cempra’s board of directors adopted, and we are maintaining, an anti-hedging and anti-pledging policy, which we are maintaining. The anti-hedging and anti-pledging policy aligns the interests of our directors, executive officers and employees who are equity grant recipients with those of our shareholders. The policy prohibits our directors, executive officers and employees who are equity grant recipients from engaging in any transaction which could hedge or offset decreases in the market value of our common stock, including the use of financial instruments such as exchange funds, prepaid variable forwards, equity swaps, puts, calls, collars, forwards and other derivative instruments, or through the establishment of a short positionincluded in our securities. Additionally, directors and executive officers are prohibited from pledging our securities as collateral for a loan, including through the use of traditional margin accounts with a broker.Compensation Risk OversightOur Compensation Committee has responsibility for establishing our compensation philosophy and objectives, determining the structure, components and other elements of our programs, and reviewing and approving the compensation of our NEOs. In addition, our executive compensation programs and decisions have, as one of their objectives, to reduce incentives to expose Melinta to imprudent risks that might harm the Company Proxy Statement and/or our shareholders. Our Compensation Committee has overseen the establishment of a number of governors and controls that address compensation-related risk and serve to mitigate such risk, including stock ownership guidelines for executive officers and directors and maintains an anti-hedging and anti-pledging policy. As a result, the Compensation Committee has concluded that the Company’s compensation policies and practices are not reasonably likely to have a material adverse effect on our Company.Accounting ConsiderationsWe account for equity compensation paid to our employees in accordance with FASB Accounting Standards Codification Topic 718, Compensation—Stock Compensation, or ASC 718, which requires us to measure and recognize compensation expense in our financial statements for all stock-based payments based on an estimate of their fair value over the service period of the award. We record cash compensation as an expense at the time the obligation is accrued.Tax ConsiderationsSection 162(m) of the Internal Revenue Code, or Section 162(m), generally disallows public companies a tax deduction for compensation in excess of $1 million paid to their chief executive officers and three other highly compensated executive officers (other than the chief financial officer) unless an exemption applies. The Tax Cuts and Jobs Act, enacted on December 22, 2017, substantially modified Section 162(m) and, among other things, beginning in 2018, expanded the scope of the executive officers subject to Section 162(m), or the Covered Employees, to include any individual who served as the chief executive officer or the chief financial officer at any time during the taxable year and the three other most highly compensated officers (other than the chief executive officer and the chief financial officer) for the taxable year, and now provides that once an individual becomes a Covered Employee for any taxable year beginning after December 31, 2016, that individual will remain a Covered Employee for all future years, including following any termination of employment.The Compensation Committee periodically reviews the potential consequences of Section 162(m) with respect to the elements of our compensation program; however, given that the impact of Section 162(m) on taxes currently payable by us is mitigated by our net operating loss carryforwards and in order to maintain flexibility in compensating executive officers in a manner designed to promote varying corporate goals in the best interests of the company, the Compensation Committee has not limited compensation to those levels or types of compensation that would be deductible by us.Benefits and Other CompensationGeneral. We believe that establishing competitive benefit packages for our employees is an important factor in attracting and retaining highly qualified employees. We maintain broad-based benefits that are provided to all employees, including medical, dental and group life insurance, accidental death and dismemberment insurance, long- and short-term disability insurance, and a 401(k) plan. All of our executives are eligible to participate in all of our employee benefit plans, in each case on the same basis as other employees. Although we do not provide additional benefits or perquisites at this time, the Compensation Committee in its discretion may revise, amend or add to the NEOs’ benefits and perquisites if it deems it advisable.Pension Benefits. We do not maintain any qualified or non-qualified defined benefit plans. As a result, none of our NEOs participate in or have account balances in qualified or non-qualified defined benefit plans sponsored by us. Our Compensation Committee may elect to adopt qualified or non-qualified benefit plans in the future if it determines that doing so is in our best interests.Non-qualified Deferred Compensation. None of our NEOs participate in or have account balances in nonqualified defined contribution plans or other non-qualified deferred compensation plans maintained by us. Our Compensation Committee may elect to provide our officers and other employees with non-qualified defined contribution or other non-qualified deferred compensation benefits in the future if it determines that doing so is in our best interests.Compensation Committee ReportThe Compensation Committee of the board of directors of Melinta Therapeutics, Inc. has reviewed and discussed the Compensation Discussion and Analysis required by Item 402(b) of Regulation S-K with Melinta’s management. Based on such review and discussions, the Compensation Committee recommended to the board of directors that the Compensation Discussion and Analysis be included in Melinta’s Annual Report on Form 10-K/A for the year ended December 31, 2017.By the Compensation Committee of the board of directors of Melinta Corporation.
A.Cecilia Gonzalo, ChairJohn H. JohnsonThomas P. Koestler, Ph.D.Garheng Kong, M.D., Ph.D.This report shall not constitute “soliciting material,” shall not be deemed “filed” with the SEC and is not to be incorporated by reference into any of our other filings under the Securities Act of 1933, as amended, or the Securities Act, or the Exchange Act, except to the extent that we specifically incorporate this report by reference therein.The following table sets forth information concerning the compensation paid or accrued to the named executive officers in fiscal years 2017, 2016 and 2015.(1)This table includes compensation from the Company earned prior to and following the merger with Cempra.(2)Effective November 3, 2017, in connection with the completion of the merger with Cempra, Mr. Wechsler, Mr. Estrem, Dr. Cammarata, Dr. Duffy, and Mr. Temperato were appointed as our President and Chief Executive Officer, Executive Vice President, Chief Financial Officer and Secretary, Executive Vice President and Chief Medical Officer, Executive Vice President and Chief Scientific Officer, and Executive Vice President and Chief Commercial Officer, respectively. In accordance with SEC regulations, only compensation information for the fiscal year in which each executive became a named executive officer is included in the Summary Compensation Table.(3)Effective November 3, 2017, in connection with the completion of the merger with Cempra, Dr. Zaccardelli’s employment with the Company was terminated and each of Messrs. Hahn and Bluth and Dr. Oldach resigned as officers of the Company. Mr. Hahn’s and Dr. Oldach’s employment with the Company was terminated effective November 15, 2017. Mr. Bluth’s employment with the Company was terminated effective December 31, 2017. Dr. Zaccardelli continues to serve on our board of directors as a non-employee member.(4)The amounts reported in this column include the following payments with respect to 2017:Dan Wechsler – A bonus equal to $100,000 earned in respect of Mr. Wechsler’s service in 2017 and paid in March 2018, which is equal to 112% of his base salary prorated for his partial year of service.Sue K. Cammarata, M.D. – A one-time cash bonus equal to $200,000 paid in June 2017, in connection with the New Drug Application approval of Baxdela™, which occurred on June 19, 2017, a one-time cash bonus equal to $50,000, paid in November 2017, in recognition of Dr. Cammarata’s efforts in connection with the merger with Cempra, and an annual bonus equal to $130,030 earned in respect of Dr. Cammarata’s service in 2017 and paid in March 2018.Erin Duffy, Ph.D. – A one-time cash bonus equal to $100,000, paid in November 2017, in recognition of Dr. Duffy’s efforts in connection with the merger with Cempra, and an annual bonus equal to $99,162 earned in respect of Dr. Duffy’s service in 2017 and paid in March 2018.John Temperato – A one-time cash bonus equal to $100,000, paid in November 2017, in recognition of Mr. Temperato’s efforts in connection with the merger with Cempra.For additional information, please see “Compensation Discussion and Analysis—Components of Executive Compensation—Annual Cash Incentive” above.(5)The amounts reported in this column represent the aggregate grant date fair value of the restricted stock units granted to our named executive officers during our fiscal year ended December 31, 2017, calculated in accordance with FASB ASC Topic 718, disregarding for this purpose the estimate of forfeitures related to service-based vesting conditions. See note 12 to our consolidated financial statements for the fiscal year ended December 31, 2017, for a discussion of the assumptions used to calculate these values.(6)The amounts reported in this column represent the aggregate grant date fair value of the stock option awards granted to our named executive officers during our fiscal year ended December 31, 2017, calculated in accordance with FASB ASC Topic 718, disregarding for this purpose the estimate of forfeitures related to service-based vesting conditions. See note 12 to our consolidated financial statements for the fiscal year ended December 31, 2017, for a discussion of the assumptions used to calculate these values. In addition, in connection with the termination of each of Dr. Zaccardelli’s, Messrs. Hahn’s and Bluth’s and Dr. Oldach’s employment, the Company approved an extension of the exercise period for the vested stock options held by each executive as of the date of such termination until the earlier of (x) the end of the twelve-month period following such termination, and (y) the stated expiration date of the stock options; however, due to the then current per share price of our common stock relative to each applicable exercise price, in accordance with FASB ASC Topic 718, no incremental accounting charge was required to be taken by the Company as a result of such extension.(7)The amounts reported in this column as earned by each named executive officer include the estimated cost of the following perquisites and other benefits received by our named executive officers in 2017:(A)For additional information regarding the payments and benefits paid in connection with Dr. Zaccardelli’s and Messrs. Hahn’s and Bluth’s and Dr. Oldach’s departure from the Company, please see “— Potential Payments on Termination and Change of Control” below. We have included the full value of any COBRA reimbursements and outplacement assistance that were made available to each executive, although certain executives may elect not to utilize such benefits.(B)Amounts reported in this column were determined based on the closing sale price per share of Melinta common stock on the NASDAQ Global Market on the date each executive’s employment was terminated with respect to Dr. Zaccardelli, Mr. Hahn and Dr. Oldach, and as of January 2, 2018, with respect to Mr. Bluth.(8)Mr. Temperato’s employment with the Company ended on January 5, 2018. For additional information regarding the payments and benefits paid in connection with his departure from the Company, please see “Potential Payments on Termination and Change of Control” below.The following table provides information regarding grants of plan-based awards made to the named executive officers in 2017.(1)All stock options and restricted stock units granted to Dr. Zaccardelli and Messrs. Hahn and Bluth and Dr. Oldach were granted under the 2011 Equity Incentive Plan. All stock options granted to Mr. Estrem, Dr. Cammarata, Dr. Duffy and Mr. Temperato were granted under the Private Melinta 2011 Equity Incentive Plan. The stock option and restricted stock unit grants to Mr. Wechsler were made pursuant to the stock option and restricted stock unit inducement grant agreements entered into with Mr. Wechsler, in accordance with the provisions set forth in that certain employment agreement by and between the Company and Mr. Wechsler, dated October 30, 2017, and in accordance with the inducement grant exception under NASDAQ Listing Rule 5635(c)(4).(2)All amounts presented reflect the 5-for-1 stock split effected on November 3, 2017, in connection with the merger with Cempra.(3)The restricted stock units granted to Mr. Wechsler will become twenty-five percent vested on the first anniversary of Mr. Wechsler’s date of hire, with the remaining units vesting in equal monthly installments over the three-year period thereafter, subject to his continued service with the Company through such date. If the grant is not expressly assumed in a change in control, any then remaining unvested portion of such grant will become fully vested upon such change in control. The restricted stock unit grant entitles Mr. Wechsler to dividend equivalents in connection with any dividends paid to shareholders of the Company during such vesting period, provided that any dividend equivalents paid with respect to unvested restricted stock units will be subject to the same vesting requirements as the underlying restricted stock units to which such dividend equivalents relate, with the payment deferred and paid within ten days after such restricted stock units become vested.(4)The restricted stock units granted to each of Messrs. Hahn and Bluth and Dr. Oldach in January 2017 were initially scheduled to vest and settle on January 1, 2019, and the restricted stock units granted to each of Messrs. Hahn and Bluth and Dr. Oldach in February 2017 were initially scheduled to vest and settle on January 9, 2019. The restricted stock units granted to each of Messrs. Hahn and Bluth and Dr. Oldach were accelerated in connection with each such executive’s termination of employment pursuant to each executive’s separation agreement, as discussed in more detail below under “Potential Payments on Termination and Change of Control.”(5)The stock options granted to Mr. Wechsler will become twenty-five percent vested and exercisable on the first anniversary of Mr. Wechsler’s date of hire, with the remaining stock options vesting in equal monthly installments over the three-year period thereafter, subject to his continued service with the Company through such date. If the grant is not expressly assumed in a change in control, any then remaining unvested portion of such grant will become fully vested upon such change in control.(6)The stock options granted to each of Mr. Estrem, Dr. Cammarata, Dr. Duffy and Mr. Temperato will become twenty-five percent vested and exercisable on the first anniversary of the date of grant, with the remaining stock options vesting in equal monthly installments over the three-year period thereafter, subject to the executive’s continued service with the Company through such date.(7)The stock options granted to each of Messrs. Hahn and Bluth and Dr. Oldach in January 2017 were initially scheduled to vest in equal monthly installments over a four-year period on each monthly anniversary of January 1, 2017, and the stock options granted to each of Messrs. Hahn and Bluth and Dr. Oldach in February 2017 were initially scheduled to vest in equal monthly installments over a four-year period on each monthly anniversary of February 23, 2017. The stock options granted to each of Messrs. Hahn and Bluth and Dr. Oldach were accelerated in connection with each such executive’s termination of employment pursuant to each executive’s separation agreement, as discussed in more detail below under “Potential Payments on Termination and Change of Control.”Narrative to Summary Compensation Table and Grant of Plan-Based Awards TableExecutive Employment AgreementsCertain of the compensation paid to the named executive officers reflected in the Summary Compensation Table was provided pursuant to employment agreements or letter agreements with us, which are summarized below. In addition, with respect to our named executive officers, for a discussion of the potential payments and benefits that we would provide our currently employed named executive officers in connection with a termination of employment and/or a change in control of the Company and, with respect to our named executed officers who are no longer employed with us, for a discussion of the payments and benefits we have agreed to provide in connection with each executive’s respective termination of employment, please see “Potential Payments on Termination and Change of Control” below.Daniel Wechsler. In connection with Mr. Wechsler’s employment, the Company entered into an employment agreement pursuant to which Mr. Wechsler is entitled to an annual base salary, currently $572,000. In addition, pursuant to his employment agreement, Mr. Wechsler is eligible to earn an annual bonus, initially set at a target equal to 60% of his base salary, with a maximum annual bonus opportunity to be set at a value not be less than 150% of his base salary; effective January 1, 2018, it was mutually agreed that Mr. Wechsler’s annual bonus would be set at a target equal to 65% of his base salary, with the maximum bonus opportunity equal to 84.5% of his base salary, in each case subject to the achievement of applicable Company and specific individual performance objectives for each fiscal year. Mr. Wechsler’s employment agreement provides that his annual bonus for 2017 could not be less than target, but would be prorated for the period in 2017 based on the number of days in the year actually worked. The bonus for 2017 actually paid to Mr. Wechsler was equal to 112% of his base salary, and was prorated based on the number of days in the year actually worked.Paul D. Estrem; Sue K. Cammarata, M.D.; Erin Duffy, Ph.D.; John Temperato. Under the terms of each of Mr. Estrem’s, Dr. Cammarata’s, Dr. Duffy’s, and Mr. Temperato’s offer letters, each executive is or was to be employed on an “at-will” basis, as applicable. Currently, Mr. Estrem, Dr. Cammarata and Dr. Duffy earn a base salary of $365,084, $446,437 and $340,458, respectively, and each is eligible to earn an annual bonus equal to 30% of his or her base salary. During 2017, Mr. Temperato was eligible to earn a base salary equal to $416,138 and was eligible to earn an annual bonus equal to 50% of his base salary.David Zaccardelli. In connection with the hiring of Dr. Zaccardelli as Acting Chief Executive Officer in December 2016, the Company entered into an employment agreement pursuant to which Dr. Zaccardelli was paid a base salary at the annual rate of $540,000 during his employment and was eligible for an annual incentive bonus for calendar years during his term, beginning in 2017, with a target bonus equal to 60% of his base salary. This bonus was subject to achievement of objectives established by the Company’s board of directors, consistent with those established for the Company’s senior management team.The employment of Dr. Zaccardelli terminated in connection with the completion of the merger with Cempra, effective November 3, 2017. He continues to serve on our board of directors as a non-employee member, but did not receive any compensation for serving in that capacity while employed as the Company’s Acting Chief Executive Officer.Stock PlanDuring the fiscal year ended December 31, 2017, we granted stock options and restricted stock units to certain of our named executive officers. For a discussion of such stock option and restricted unit grants, please see “Components of Executive Compensation—Long-Term Equity Incentives” above.Outstanding Equity Awards at Fiscal Year-End 2017The following table contains certain information concerning outstanding equity awards to acquire shares of our common stock held by each of our named executive officers as of December 31, 2017.(1)All amounts presented reflect the 5-for-1 stock split effected on November 3, 2017, in connection with the merger with Cempra.(2)The market value was determined based on the closing sale price per share of Melinta common stock on the NASDAQ Global Market on December 29, 2017, the last trading day of fiscal year 2017, which was $15.80.portion of such grant will become fully vested upon such change in control. The restricted stock unit grant entitles Mr. Wechsler to dividend equivalents in connection with any dividends paid to shareholders of the Company during such vesting period, provided that any dividend equivalents paid with respect to unvested restricted stock units will be subject to the same vesting requirements as the underlying restricted stock units to which such dividend equivalents relate, with the payment deferred and paid within ten days after such restricted stock units become vested.(4)The stock options granted to Mr. Wechsler will become twenty-five percent vested and exercisable on the first anniversary of Mr. Wechsler’s date of hire, with the remaining stock options vesting in equal monthly installments over the three-year period thereafter, subject to his continued service with the Company through such date. If the grant is not expressly assumed in a change in control, any then remaining unvested portion of such grant will become fully vested upon such change in control.(5)The stock options granted to each of Mr. Estrem, Dr. Cammarata, Dr. Duffy and Mr. Temperato will become twenty-five percent vested and exercisable on the first anniversary of the date of grant, with the remaining stock options vesting in equal monthly installments over the three-year period thereafter, subject to the executive’s continued service with the Company through such date.(6)The stock options granted to each of Dr. Zaccardelli, Messrs. Hahn and Bluth, and Dr. Oldach became fully vested and exercisable, on November 3, 2017, with respect to Dr. Zaccardelli, and on November 15, 2017, with respect to Mr. Hahn and Dr. Oldach, and December 31, 2017, with respect to Mr. Bluth, in each case, in connection with the executive’s termination of employment.(7)The restricted stock units granted to Mr. Bluth became fully vested on December 31, 2017, in connection with Mr. Bluth’s termination of employment, which were settled on January 2, 2018.Option Exercises and Stock Vested TableThe following table contains information concerning the vesting of stock awards held by our named executive officers during the fiscal year ended December 31, 2017. There were no exercises of stock options by the named executive officers in 2017.(1)The value realized on vesting is calculated by multiplying the number of shares of common stock acquired on vesting by the closing price of our common stock on the vesting date.(2)Certain of Dr. Zaccardelli’s, Messrs. Hahn’s and Bluth’s, and Dr. Oldach’s restricted stock units vested in connection with each such executive’s termination of employment pursuant to each executive’s separation agreement, as discussed in more detail below under “Potential Payments on Termination and Change of Control.”Pension Benefits We do not currently sponsor or maintain any defined benefit pension plans or other benefit plans providing specified retirement payments and benefits for employees.Non-Qualified Deferred CompensationWe do not currently sponsor or maintain any non-qualified defined contribution or other non-qualified deferred compensation plans for employees.Potential Payments on Termination and Change of ControlThe following summaries describe, with respect to our named executive officers, the potential payments and benefits that we would provide our currently employed named executive officers in connection with a termination of employment and/or a change in control of the Company and, with respect to our named executive officers who are no longer employed with us, the payments and benefits we have agreed to provide in connection with each executive’s respective termination of employment.Daniel Wechsler. Under the terms of Mr. Wechsler’s employment agreement, if the Company terminates Mr. Wechsler’s employment without cause or if he terminates his employment with the Company for good reason in accordance with the terms of his employment agreement, subject to Mr. Wechsler’s execution of a release of claims, he will be entitled to receive severance equal to: (i) an amount equal to one and one-half times his then annual base salary and target annual bonus (provided that such multiple will be equal to two if the date of such termination occurs within three months prior to or twelve months following a change in control), payable in equal installments over eighteen months in accordance with the Company’s payroll practices; (ii) a prorated annual bonus for the year in which such termination occurs, based on actual performance; and (iii) reimbursement for the employer portion of the premium costs of his COBRA continuation coverage until the earlier of (x) the end of the eighteen-month period following such qualifying termination, and (y) the date Mr. Wechsler becomes eligible for coverage under another group health plan. In addition, if such termination of Mr. Wechsler’s employment by the Company without cause or by Mr. Wechsler with good reason occurs within three months prior to or twelve months following a change in control, all of his then outstanding equity awards subject to solely service-based vesting will become fully vested. In connection with any termination of Mr. Wechsler’s employment due to death or disability, he is entitled to a prorated bonus for the year in which such termination occurs, based on actual performance. In addition, if either of the inducement equity awards granted to Mr. Wechsler is not expressly assumed in a change in control, any then remaining unvested portion of such grants will become fully vested upon such change in control.Mr. Wechsler also entered into an Employee Noncompetition, Nondisclosure and Developments Agreement pursuant to which he is subject to customary confidentiality restrictions that apply during his employment and indefinitely thereafter, an invention assignment provision, and covenants not to compete or to solicit our employees or customers while employed with the Company and for eighteen months following any termination of employment.Paul D. Estrem; Sue K. Cammarata, M.D.; Erin Duffy, Ph.D. Each of Mr. Estrem, Dr. Cammarata, and Dr. Duffy has entered into a severance agreement, pursuant to which if the Company terminates the executive’s employment without cause or if the executive terminates his or her employment with the Company for good reason in accordance with the terms of the separation agreement, subject to his or her execution of a release of claims, the executive will be entitled to severance equal to: (i) continued payment of the executive’s base salary for a twelve-month period; (ii) reimbursement for the employer portion of the premium costs of the executive’s COBRA continuation coverage, until the earlier of (x) the end of the twelve-month period following such qualifying termination, and (y) the date the executive becomes eligible for coverage under another group health plan; and (iii) if such qualifying termination occurs within six months of a change in control, a prorated annual bonus for the year in which such termination occurs.Each of Mr. Estrem, Dr. Cammarata, and Dr. Duffy has also entered into an Employee Noncompetition, Nondisclosure and Developments Agreement pursuant to which the executive is subject to customary confidentiality restrictions that apply during the executive’s employment and indefinitely thereafter, an invention assignment provision, and covenants not to compete or to solicit our employees or customers while employed with the Company and for twelve months following any termination of employment.John Temperato. On December 28, 2017, we entered into a separation and release agreement with Mr. Temperato, pursuant to which his employment ended on January 5, 2018. Pursuant to the terms of the separation agreement, in consideration for his release of claims and continued compliance with his covenants not to compete or to solicit the Company’s employees or customers, in each case, for a twelve-month period following his termination of employment, the Company will pay Mr. Temperato an amount equal to: (i) continued payment of his base salary for a twelve-month period; (ii) reimbursement for the employer portion of the premium costs of his COBRA continuation coverage until the earlier to occur of (x) the end of the twelve month period following his termination of employment, and (y) the date he becomes eligible for coverage under another group health plan; and (iii) an additional payment equal to $208,068, representing the value of the annual bonus Mr. Temperato would have been eligible to earn with respect to performance in 2017. Consistent with the benefit provided to the other executive officers, notwithstanding the terms of his separation agreement, we have agreed to reimburse Mr. Temperato for the full cost of his COBRA continuation coverage.David Zaccardelli Pharm.D.; Mark Hahn; John Bluth; David Oldach, M.D. In connection with the termination of the employment of Dr. Zaccardelli, Messrs. Hahn and Bluth, and Dr. Oldach, upon the date of their respective termination, the Company and each of Dr. Zaccardelli, Messrs. Hahn and Bluth, and Dr. Oldach entered into a separation and release agreement. In consideration for the executive’s release of claims and continued compliance with his covenants not to compete or to solicit the Company’s employees or customers, in each case, for an eighteen-month period following his termination of employment, the Company will pay each executive an amount equal to (i) continued payment of his base salary for an eighteen-month period (twenty-four months in the case of Dr. Zaccardelli); (ii) a lump sum amount equal to one and one half times his target bonus for 2017 (two times in the case of Dr. Zaccardelli); and (iii) reimbursement for the entire cost of his COBRA continuation coverage until the earlier to occur of (x) the end of the eighteen-month period following his termination date, and (y) the date he becomes eligible for coverage under another group health plan. In addition, the Company will provide outplacement assistance through a service provider selected by the Company for a period of eighteen months. The vesting of all outstanding and unvested stock options and restricted stock units held by the executive was accelerated and, if applicable, became immediately and fully exercisable. The exercise period for any of the executive’s stock options that vested prior to his termination date, or pursuant to his separation agreement, was extended through the date that is twelve months following his termination date, but in no event beyond the original expiration date of the each affected stock option. At the time of this filing, the exercise prices of all of the stock options held by Dr. Zaccardelli, Messrs. Hahn and Bluth, and Dr. Oldach were above the fair market value of such shares. Dr. Zaccardelli continues to serve as a non-employee member of our board of directors.The table below reflects the amount of compensation and benefits payable to each named executive officer currently employed with us in the event of: (i) a termination of the executive’s employment by the Company without cause or by the executive for good reason; (ii) a termination of the executive’s employment by the Company without cause or by the executive for good reason, with respect to Mr. Wechsler within three months prior to or twelve months following a change in control, and with respect to Mr. Estrem, Dr. Cammarata and Dr. Duffy, within six months following a change in control; and (iii) with respect to Mr. Wechsler, a termination of his employment in connection with his death or disability.(1)The severance amounts reported are based on the base salaries in effect on December 31, 2017.(2)Amounts reported in this column assume that any equity awards are not assumed by an acquirer in connection with a change in control.CEO Pay Ratio – Annualized ApproachPursuant to Section 953(b) of the Dodd-Frank Wall Street Reform and Consumer Protection Act and Item 402(u) of Regulation S-K, we are required to disclose the ratio of our principal executive officer annual total compensation to the annual total compensation of our median employee.During fiscal year 2017, the principal executive officer (“PEO”), of Melinta as of the determination date, December 31, 2017, was our President and Chief Executive Officer, Mr. Dan Wechsler. For 2017, the annual total compensation for Mr. Wechsler, as reported in the Summary Compensation Table, was $6,955,227. We note that a substantial portion of our President and CEO’s total compensation for 2017 was the sign-on equity awards he received in accordance with his employment agreement, which had a grant date fair value of $6,745,131. However, because Mr. Wechsler became our President and Chief Executive Officer on November 3, 2017, and served as our PEO for fewer than two months of the year, for purposes of calculating the PEO pay ratio, we annualized Mr. Wechsler’s base salary to $550,000 in accordance with SEC rules to estimate the compensation that he likely would have received if he had served as CEO for the entire year.As a result, Mr. Wechsler’s compensation as CEO, for purposes of this pay ratio disclosure, was $7,417,439. The annual total compensation for our median employee was $215,327, annualized in a manner resulting in a pay ratio of approximately 34 to 1.To find the median of the annual total compensation of all our employees (other than our CEO), we used wages from our payroll records as reported to the Internal Revenue Service on Form W-2 for fiscal 2017. In making this determination, we annualized the compensation of 91 full-time and part-time permanent employees who were employed on December 31, 2017, but did not work for us the entire year. No full-time equivalent adjustments were made for part-time employees. We identified our median employee using this compensation measure and methodology, which was consistently applied to all our employees included in the calculation.The pay ratio reported above is a reasonable estimate calculated in a manner consistent with SEC rules based on our internal records and the methodology described above. The SEC rules for identifying the median compensated employee and calculating the pay ratio based on that employee’s annual total compensation allow companies to adopt a variety of methodologies, to apply certain exclusions, and to make reasonable estimates and assumptions that reflect their employee populations and compensation practices. Therefore, the pay ratio reported by other companies may not be comparable to the pay ratio reported above, as other companies have different employee populations and compensation practices and may utilize different methodologies, exclusions, estimates, and assumptions in calculating their own pay ratios.DIRECTOR COMPENSATIONDirector Compensation in Fiscal 2017The following table shows the compensation earned by each non-employee director of Melinta for the year ended December 31, 2017.(1)Effective November 3, 2017, in connection with the merger with Cempra, Michael Dougherty, Dov A. Goldstein, M.D., Richard Kent, M.D. and P. Sherrill Neff each resigned from our board of directors, and Kevin T. Ferro, Jay Galeota, Cecilia Gonzalo and Thomas P. Koestler, Ph.D. were appointed to our board of directors.(3)The reported amounts represent the aggregate grant date fair value of the stock option awards granted to non-employee directors during our fiscal year ended December 31, 2017, calculated in accordance with FASB ASC Topic 718, disregarding for this purpose the estimate of forfeitures related to service-based vesting conditions. See note 12 to our consolidated financial statements for the fiscal year ended December 31, 2017, for a discussion of the assumptions used to calculate these values. Our compensation committee approved an extension of the exercise period for stock options held by members of our board of directors who resigned in connection with the merger with Cempra to the earlier of (x) November 3, 2018, and (y) the stated expiration date of the stock options, however, due to the then current per share price of our common stock relative to each applicable exercise price, in accordance with FASB ASC Topic 718, no incremental accounting charge was required to be taken by the Company as a result of such extension.(4)The non-employee members of our board of directors held the following aggregate number of unexercised stock options as of December 31, 2017:NameAggregatenumberof optionsoutstandingas of December31, 2017Michael R. Dougherty17,000Kevin T. Ferro—Jay Galeota—David Gill20,000Dov. A Goldstein, M.D.7,832Cecilia Gonzalo—John H. Johnson23,756Richard Kent, M.D., Ph.D.15,000Thomas Koestler, Ph.D.19,876Garheng Kong, M.D., Ph.D.27,356P. Sherill Neff18,000David Zaccardelli, Pharm.D.34,999(5)Dr. Koestler served on the board of directors of Melinta Therapeutics, Inc. prior to the merger with Cempra, and earned a cash retainer equal to $84,167 during 2017 for such service. Dr. Koestler also earned an additional consulting fee equal to $42,083 in connection with his provision of consulting services from time to time. In addition, on August 8, 2017, Dr. Koestler received a grant of stock options with an aggregate grant date fair value equal to $23,434, calculated in accordance with FASB ASC Topic 718, disregarding for this purpose the estimate of forfeitures related to service-based vesting conditions. See note 12 to our consolidated financial statements for the fiscal year ended December 31, 2017, for a discussion of the assumptions used to calculate this value.Prior to the merger with Cempra, our board of directors had determined that compensation for our directors should be a mix of cash and equity-based compensation comprised of:An annual cash retainer for all members of our board of directors equal to $40,000;An additional annual cash retainer for the non-executive chair of our board of directors equal to $35,000;An additional annual cash retainer for the chair of each of the Audit Committee, Compensation Committee, and Nominating and Governance Committee equal to $20,000, $12,500, and $10,000, respectively;An additional annual retainer for members of the Audit Committee, Compensation Committee, and Nominating and Governance Committee (other than the chair of each such committee) equal to $10,000, $5,000, and $5,000, respectively;An initial grant of 25,000 stock options when first appointed to our board of directors and thereafter annual grants of 15,000 stock options; andAn additional annual grant of 3,000 stock options for the chair of our board, if not a named executive officer.Following the merger, we suspended our director compensation program in order to assess it in light of the significant strategic transformation. During the first quarter of 2018, with the assistance of Radford, an independent compensation consultant, the Compensation Committee reviewed our non-employee director compensation program against the peer group approved in January 2018 with the goal of aligning director compensation at the 50th percentile of the peer group. For a discussion of the peer group, see “Compensation Discussion and Analysis—Compensation Determination Process—Peer Group”. The Compensation Committee’s review confirmed that our director compensation program compared to our peers was below the 50th percentile in several respects. As a result, Radford proposed that the annual cash retainer be increased from $40,000 to $45,000, that the additional annual cash retainer for the chair of the board be reduced from $35,000 to $25,000, that the additional annual cash retainer for the chair of the Compensation Committee be increased from $12,500 to $14,000 and that the additional retainer for members of the Compensation Committee be increased from $5,000 to $7,000. In addition, Radford recommended that the additional annual grant for the chair of the board be eliminated.The Compensation Committee considered Radford’s recommendation and recommended to the board of directors, and our board of directors approved, that effective for fiscal 2018, each non-employee director will receive the following compensation for service on our board of directors:An annual cash retainer for all members of our board of directors equal to $45,000;An additional annual cash retainer for the chair of the board equal to $25,000;An additional annual cash retainer for the chair of each of the Audit Committee, Compensation Committee, and Nominating and Governance Committee equal to $20,000, $14,000, and $10,000, respectively;An additional retainer for members of the Audit Committee, Compensation Committee, and Nominating and Governance Committee (other than the chair of each such committee) equal to $10,000, $7,000, and $5,000, respectively; andAn initial grant of 25,000 stock options when first appointed to our board of directors that vests over a period of three years and thereafter annual grants of 15,000 stock options.Because most of the current members of the board of directors do not have any equity ownership and the remaining directors hold underwater stock options, Radford also recommended, and our board of directors approved, that all of the directors receive an initial grant of stock options in fiscal 2018 vesting over three years, and a pro-rata annual grant covering the period from the closing of the merger through the date of our 2018 Annual Meeting of Shareholders vesting on the first anniversary of the grant date.Director Stock Ownership GuidelinesWe have stock ownership guidelines for directors, which were updated by our compensation committee in March 2018 and further our goal of more closely aligning the interests of our directors and shareholders. The stock ownership guidelines require each of our directors to hold Melinta common stock (including unvested shares of time-based restricted stock and vested in-the-money stock options) with a market value equal to three times his or her then-current annual cash retainer (or such lesser amount as the director has been granted to date). The stock ownership guidelines will be applicable at the later of (i) five years after the date the director assumes his or her position or (ii) February 16, 2021, which is the date five years from the date the stock ownership guidelines were originally adopted. Directors generally are not permitted to sell any of the equity granted to them unless they have met their ownership requirements.OwnersOwners and Management and Related Shareholder MattersThe following table sets forth the indicated information as of December 31, 2017,2018, with respect to our equity compensation plans:Plan Category Number ofsecurities to beissued uponexercise ofoutstandingoptions, warrantsand rights Weighted-averageexercise priceof outstandingoptions, warrantsand rights(1) Number ofsecuritiesremaining availablefor futureissuance underequitycompensationplans(2)Equity compensation plans approved by our shareholders(3) 628,590 $ 63.18 813,526 Equity compensation plans not approved by our shareholders(4) 74,000 $ 22.50 — Total 702,590 813,526 (1)(1) Represents the weighted average exercise price of outstanding stock options and is calculated without taking into account the shares of common stock subject to outstanding restricted stock units that become issuable without any cash payment required for such shares. (2)(2) Includes only shares remaining available for future issuance under the 2011 Equity2018 Stock Incentive Plan. No shares are reserved for future issuance under the 2011 Equity Incentive Plan, the Private Melinta 2011 Equity Incentive Plan or the 2006 Stock Option and Incentive Plan, other than shares issuable upon exercise of equity awards outstanding under such plans. In addition, the 2011 Equity2018 Stock Incentive Plan contains an “evergreen” provision pursuant to which the number of shares reserved for issuance under that plan automatically increases on January 1 of each year, continuing through January 1, 2021, by 4% of the total number of shares of common stock outstanding on December 31 of the preceding calendar year (unless our board of directors determines to increase the number of shares subject to the plan by a lesser amount). The number of shares of common stock reported in this column does not include the 879,957175,991 shares of common stock that became available for issuance as of January 1, 2018, pursuant to the evergreen provision of our 2011 Equity2018 Stock Incentive Plan.(3)(3) Includes the 2018 Stock Incentive Plan, 2011 Equity Incentive Plan, the 2006 Plan and the Private Melinta 2011 Equity Incentive Plan. Of these 1,647,078628,590 securities outstanding, there were options to purchase 1,510,078611,230 shares of common stock and 137,00017,360 restricted stock units.(4)(4) Represents the inducement grant to Daniel Wechsler,Peter J. Milligan, our President and Chief ExecutiveFinancial Officer, of an optionoptions to purchase 550,981370,000 shares of common stock at a strike price of $11.65 per share, and 183,661 restricted stock units. These grants$22.50, which were not granted under any of the stock plans.Principal ShareholdersThe table below sets forth information as of April 27, 2018,February 28, 2019, regarding the beneficial ownership of our common stock by:Name of Beneficial Owner Total BeneficialOwnership Percentage ofCommon StockBeneficiallyOwned5% and Greater Stockholders Vatera Capital Management LLC(1) 12,696,590 61.6 % Executive Officers and Directors John H. Johnson(2) 23,637 * Peter J. Milligan — — David Gill(3) 8,686 * Garheng Kong, M.D., Ph.D.(4) 9,070 * Sue Cammarata, M.D.(5) 13,464 * David Zaccardelli, Pharm.D.(6) 19,785 * Erin Duffy, Ph.D.(7) 13,257 * Kevin T. Ferro(1)(8) 12,700,076 61.6 % Jay Galeota(9) 3,486 * Bruce Downey — — Thomas P. Koestler, Ph.D.(1)(10) 6,887 * Paul Estrem(11) 2,000 * Daniel Wechsler(12) 16,028 * All directors and current executive officers as a group (13 persons) 12798,348 61.8 % ** Indicates beneficial ownership of less than 1%. (1)(1) Based on the Schedule 13D/A filed with the SEC on February 22, 2019. Vatera Healthcare Partners LLC ("VHP") directly holds, and has voting and dispositive power over, 12,576,446 shares. VHPM Holdings LLC ("VHPM") directly holds, and has voting and dispositive power over, 120,144 shares. Vatera Capital Management LLC ("VCM"), as the manager of VHP and VHPM, has voting and dispositive power over all of the shares. The address for VateraVCM LLC is c/o Vatera Holdings LLC, 499 Park Avenue, 23rd Floor, New York, NY 10022. Each of Thomas Koestler, Ph.D.,400 Royal Palm Way, Suite 212, Palm Beach, FL 33480. Kevin Ferro and Cecilia Gonzalo presently serves on the Company’s board of directors. Kevin Ferrodirectors and is Chairman of the Board of the Company. Kevinboard. Mr. Ferro is the Chief Executive Officer Chief Investment Officer and the managing member of Vatera Holdings LLC,VCM, the manager of Vatera,VHP and VHPM, and by virtue of that position, may be deemed to have beneficial ownership of the shares held by VHP and VHPM. Other than for purposes of Vatera.Rule 13d-3 of the Exchange Act, Mr. Ferro disclaims beneficial ownership of the shares held by VateraVHP and VHPM except to the extent of his pecuniary interest therein. Dr.Mr. Koestler is an Executive Director,a consultant to VHP.(2) Consists of 400 shares owned by Mr. Johnson and Ms. Gonzalo is a Managing Director, of Vatera Holdings LLC.(2)Based on Schedule 13G filed with the SEC on January 11, 2018. The address of The Medicines Company is 8 Sylvan Way, Parsippany, NJ 07054.(3)Based on 13G filed with the SEC on January 9, 2018. The amount includes (i) 2,150,394 shares of common stock held by Deerfield Private Design Fund IV, L.P., (ii) 651,530 shares of common stock held by Deerfield Private Design Fund III, L.P., and (iii) 325,922 shares of common stock held by Deerfield Special Situations Fund, L.P. The address of Deerfield is c/o Deerfield Mgmt., L.P. 780 Third Avenue, 37th Floor, New York, NY 10017.(4)The address for Malin Life Sciences Holdings is 2 Harbour Square, Crofton Road, Dun Laoghaire, Co. Dublin Ireland.(5)Pursuant to the terms of Mr. Wechsler’s employment agreement with the Company, Mr. Wechsler has been granted an employee inducement award of an option to purchase 550,981 shares of Melinta common stock at an exercise price of $11.65, and a restricted stock unit award for 183,661 shares of Melinta common stock. The stock option and restricted stock unit grant will become 25% vested on the one year anniversary of Mr. Wechsler’s date of hire, with the remaining shares vesting in equal monthly installments thereafter over the next three years, subject to his continuing service with the Company.(6)Consists of23,237 shares issuable upon the exercise of options exercisable within 60 days of April 27, 2018.(7)(3) Consists of 1,200 shares owned by Mr. Gill and shares issuable upon the exercise of 7,486 options exercisable within 60 days of February 28, 2019. (4) Consists of 113 shares owned by Dr. Kong and shares issuable upon the exercise of 8,957 options exercisable within 60 days of February 28, 2019. (5) Consists of 800 shares owned by Dr. Cammarata and shares issuable upon the exercise of 12,664 options exercisable within 60 days of February 28, 2019. (6) Consists of 9,300 shares owned by Dr. Zaccardelli and shares issuable upon the exercise of 10,485 options exercisable within 60 days of February 28, 2019. (7) Consists of 800 shares owned by Dr. Duffy and shares issuable upon the exercise of 12,457 options exercisable within 60 days of February 28, 2019. (8) Includes shares issuable upon the exercise of 3,486 options exercisable within 60 days of February 28, 2019. (9) Consists of shares issuable upon the exercise of 34,9993,486 options exercisable within 60 days of April 27, 2018, and 10,000 restricted stock units.February 28, 2019.(10) Consists of shares issuable upon the exercise of 6,887 options exercisable within 60 days of February 28, 2019. (11) Consists of 2,000 shares owned by Mr. Estrem. (12) Consists of 16,028 shares owned by Mr. Estrem. RelatedRelated Transactions, and Director IndependenceRelated-Party TransactionsWith respectThe information required by this Item is incorporated by reference to reviewing and approving related-party transactions, the Audit Committee reviews related-party transactions for potential conflicts of interests or other improprieties. Under SEC rules, related-party transactions are those transactions to which we are or may be a party in which the amount involved exceeds $120,000 annually, and in which any of our directors or executive officers or any other related person had or will have a direct or indirect material interest, excluding, among other things, compensation arrangements with respect to employment and board membership. Our Audit Committee may approve a related-party transaction if it determines that the transaction isinformation included in our best interests. Our directors are required to disclose to this committee or the full board of directors any potential conflict of interest, or personal interest in a transaction that our board of directors is considering. Our executive officers are required to disclose any related-party transaction to the Audit Committee. We also poll our directors on an annual basis with respect to related-party transactions and their service as an officer or director of other entities. Any director involved in a related-party transaction that is being reviewed or approved must recuse himself or herself from participation in any related deliberation or decision. Whenever possible, we strive to ensure that the transaction is approved in advance and, if not approved in advance, it must be submitted for ratification as promptly as practical.Dr. Thomas Koestler was Melinta’s non-executive Chairman of the Board prior to the merger with Cempra, Inc. (“Cempra”) and is engaged by our largest investor. Dr. Koestler received compensation for his role as non-executive Chairman in the amount of $84,167 for the year ended December 31, 2017. In addition, during the year ended December 31, 2017, we granted additional options to Dr. Koestler to purchase 1,669 shares with an exercise price of $20.97. Dr. Koestler stepped down as non-executive Chairman of the Board in November 2017, in conjunction with the merger with Cempra, but remains on our board as a director.In August 2015, we entered into a supply and distribution agreement with Malin Life Sciences Holdings Limited (“Malin”), an investor of the Company. Pursuant to the terms and conditions of the supply and distribution agreement, Malin or its affiliates are entitled to exclusive rights to obtain product approval, procure supply from us and to commercialize Baxdela in Africa and the Middle East. In connection with the supply and distribution agreement, we are entitled to receive a royalty percentage of net sales of Baxdela. No upfront payments were received or paid in connection with this agreement.In 2016 and 2017, we issued convertible promissory notes to certain of our investors, none of which are outstanding as of December 31, 2017.Agreements with VateraRegistration RightsConcurrently with the closing of the merger of Melinta Subsidiary Corp. (f/k/a Melinta Therapeutics, Inc.) and Castle Acquisition Corp., a direct wholly owned subsidiary of the Company (the “Merger”), the Company entered into a Registration Rights Agreement with certain pre-closing Melinta stockholders (the “Vatera Registration Rights Agreement”). Under the Vatera Registration Rights Agreement, the Company was obligated to file, within 90 calendar days from the date of execution of the Vatera Registration Rights Agreement, a shelf registration statement on Form S-3 providing for the resale by such stockholders of the common stock of the Company, par value $0.001 (the “Company Common Stock”) issued or issuable to such stockholders in the Merger (the “Registrable Securities”). Stockholder Vatera Healthcare Partners LLC (the “Vatera Stockholder”) is entitled to two underwritten offerings under such shelf registration statement. Additionally, if the Company registers shares of Company Common Stock (subject to certain exceptions) for public sale, such stockholders will have the right to include their Registrable Securities in the registration statement. The registration rights under the Vatera Registration Rights Agreement will terminate upon the earliest of (i) termination of the agreement by the consent of the Vatera Stockholder, (ii) the date on which the Vatera Stockholder holds Company Common Stock equal to or less than 10% of the total Company Common Stock issued and outstanding (on a non-fully-diluted basis) and (iii) the dissolution, liquidation or winding up of the Company.Concurrently with the execution of the Agreement and Plan of Merger and Reorganization by and between the Company, Melinta Subsidiary Corp. and Castle Acquisition Corp., certain Melinta stockholders entered into voting and lock-up agreements with the Company (collectively, the “Voting and Lock-Up Agreements”). Pursuant to the Voting and Lock-Up Agreements, among other things, the stockholders party thereto are subject to a 180-day lock -up on the sale of shares of capital stock of the Company, which period began upon the consummation of the merger on November 3, 2017.Agreements with DeerfieldConcurrently with the execution of the Purchase and Sale Agreement, dated November 28, 2017 (the “Purchase Agreement”), by and between the Company and The Medicines Company (“Medicines”), Melinta entered into a Commitment Letter (the “Deerfield Commitment Letter”), dated November 28, 2017, with an affiliate of Deerfield Management Company, L.P. (together with certain funds managed by Deerfield Management Company, L.P., “Deerfield” or the “Deerfield Funds”), pursuant to which the Deerfield Funds committed, upon the satisfaction of certain conditions set forth therein, to provide $190.0 million (less the amount of the Deerfield equity investment described below) of senior secured loans, having an interest rate of 11.75% per annum, to finance the acquisition from Medicines of the capital stock of certain subsidiaries of Medicines (the “Transferred Subsidiaries”) and certain assets related to Medicines’ infectious disease business, including pharmaceutical products containing (i) meropenem and vaborbactam as the active pharmaceutical ingredient and distributed under the brand name VabomereTM (“Vabomere”), (ii) oritavancin as the active pharmaceutical ingredient and distributed under the brand name Orbactiv® (“Orbactiv”) and (iii) minocycline as the active pharmaceutical ingredient and distributed under the brand name Minocin® for injection (“Minocin” and, together with Vabomere and Orbactiv, the “Products”) and line extensions thereof (the “Purchase” or the “Acquisition”), together with, subject to certain conditions, up to $50.0 million in a senior secured delayed draw term loan facility, having an interest rate of 14.75% per annum. At the closing of the Acquisition, Melinta entered the Facility Agreement setting forth the definitive terms and conditions of the $147.8 million principal amount of initial senior secured loans and the $50.0 million senior secured delayed draw term loan facility, as further described below.Deerfield Facility AgreementOn January 5, 2018, Melinta became the borrower under that certain Facility Agreement, dated as of January 5, 2018 (the “Facility Agreement”) by and among Melinta (as borrower), certain of Melinta’s subsidiaries party thereto as guarantors, the Deerfield Funds (consisting of Deerfield Private Design Fund IV, L.P., Deerfield Private Design Fund III, L.P. and Deerfield Special Situations Fund, L.P.), and Cortland Capital Market Services LLC, as agent for the secured parties. Under the Facility Agreement, the lenders made available to Melinta an initial disbursement of $147.8 million in loan financing. The Facility Agreement includes provisions for additional subsequent disbursements in an amount of up to $50.0 million, which may be made available upon the satisfaction of certain conditions, such as Melinta having achieved annualized net sales of at least $75.0 million during the applicable period.The initial disbursement under the Facility Agreement bears interest at a rate of 11.75%, while funds distributed pursuant to any subsequent disbursement will bear interest at a rate of 14.75%. Melinta is also required to pay the lenders an end-of-term fee or exit fee of 2% of the amount of any loans on the payment, repayment, redemption or prepayment thereof upon the date of such payment, repayment, redemption or prepayment thereof. The principal of the initial disbursement must be paid by January 5, 2024. The loans are not permitted to be prepaid prior to January 6, 2021, and are subject to certain prepayment fees for prepayments occurring on or after such date.Obligations under the Facility Agreement are secured by liens on substantially all assets of Melinta and its subsidiaries. The proceeds of the initial loans under the Facility Agreement were used to pay part of the consideration for the Acquisition, pay certain fees and expenses associated with the Acquisition and the transactions contemplated by the Facility Agreement, pay amounts owed under a certain loan facility with Suchard SA LLC and certain other lenders and the remainder for general corporate purposes. The Facility Agreement has a financial maintenance covenant requiring Melinta and its subsidiaries to maintain a minimum cash balance of $25.0 million, and further requires the loan parties thereunder to maintain minimum net sales of at least (A) $45.0 million for the fiscal year ending December 31, 2018, (B) $75.0 million for the fiscal year ending December 31, 2019, and (C) $100.0 million for the fiscal year ending December 31, 2020, and each fiscal year ending thereafter. The Facility Agreement contains customary affirmative covenants, negative covenants (including, among others, limitations onindebtedness, liens, advances, equity and debt and other investments, dividends, asset sales, transactions with affiliates, changes in nature of business and mergers and acquisitions), representations and warranties and events of default (including, among others, a change of control event of default) that are customary for similar financings.Deerfield Equity PurchaseUnder the Deerfield Commitment Letter, subject to the conditions set forth therein, the Deerfield Funds committed to purchase the Deerfield equity investment, equal to 9.985% of the number of shares of Company Common Stock outstanding immediately following the Acquisition (inclusive of such shares) for a purchase price per share of $13.50, representing 90% of the closing price for the Company Common Stock on November 28, 2017, the date on which the Deerfield Commitment Letter was executed. At the closing of the Acquisition, Melinta and the Deerfield Funds entered into a Securities Purchase Agreement, dated January 5, 2018 (the “Securities Purchase Agreement”), setting forth the definitive terms of the Deerfield equity investment, pursuant to which the Deerfield Funds purchased 3,127,846 shares of Company Common Stock for an aggregate purchase price of $42.2 million. The Securities Purchase Agreement contains customary representations, covenants and indemnitees by each of the parties thereto.Deerfield WarrantsIn accordance with the Deerfield Commitment Letter and pursuant to the Facility Agreement, on the closing date of the Acquisition, Melinta issued to Deerfield Private Design Fund IV, L.P. a warrant to purchase 2,607,597 shares of Company Common Stock, to Deerfield Private Design Fund III, L.P. a warrant to purchase 790,054 shares of Company Common Stock, and to Deerfield Special Situations Fund, L.P. a warrant to purchase 395,217 shares of Company Common Stock (collectively, the “Deerfield Warrants”), in the aggregate representing a number of shares of Company Common Stock equal to 38.5% of the $147.8 million principal amount of the senior secured loans, divided by $15.00, representing the closing price for the Company Common Stock on November 28, 2017, the date on which the Deerfield Commitment Letter was executed. The Deerfield Warrants are exercisable for duration of 7 years at a strike price of $16.50. The number of shares with respect to which the Deerfield Warrants are exercisable and the strike price of the Deerfield Warrants are subject to adjustment to reflect certain events relating to the shares of Company Common Stock, such as any subdivision, combination, reclassification or similar transaction with respect to the shares of Company Common Stock. The Deerfield Warrants are subject to a restriction on the exercise thereof to the extent that, upon such exercise, a holder, its affiliates and any “group” of which such holder is a member would not beneficially own greater than 9.985% of the outstanding shares of Company Common Stock. In its capacity as the holder of a Deerfield Warrant, each of the Deerfield Funds is entitled to receive dividends paid, and distributions made, to holders of shares of Company Common Stock as if such Deerfield Warrant had been exercised in full on the applicable record date for such dividend or distribution. In addition, Melinta has reserved for issuance a number of authorized and unissued shares of Company Common Stock sufficient for the exercise of the Deerfield Warrants in full (assuming a cash exercise and disregarding any limitations on exercise). Other than with respect to dividends and distributions, the Deerfield Warrants do not entitle the Deerfield Funds, prior to the exercise of the Deerfield Warrants, to rights as a Melinta stockholder.The Deerfield Warrants also provide that, in the event of a major transaction as described in clause (i) or (ii) below where shares of Company Common Stock are converted into the right to receive cash or other assets or in which Melinta has announced its intention to liquidate and distribute its assets to its stockholders, each of the Deerfield Funds will be entitled to convert the Deerfield Warrants into an amount of the transaction consideration equal to the Black-Scholes value of the Deerfield Warrants following such major transaction. With respect to all other major transactions, each of the Deerfield Funds are entitled to exercise the Deerfield Warrants for a number of shares of Company Common Stock (calculated in accordance with the terms of the Deerfield Warrants) equal to the Black-Scholes value of its Deerfield Warrant. Alternatively, if the successor entity is publicly traded, each of the Deerfield Funds may require that its Deerfield Warrant be assumed by the successor entity (or its parent company) in a major transaction. The documentation reflecting any such assumption must provide that the Deerfield Warrants will be exercisable for the appropriate number of shares of the successor entity.The Deerfield Warrants also provide that in the case of certain transactions in which holders of Company Common Stock are entitled to receive stock, securities or assets in respect of their Company Common Stock (including certain major transactions in which the successor entity is not publicly traded and the holder of a Deerfield Warrant does not exercise its right to receive the amounts described above), the documentation reflecting the transaction will provide that the Deerfield Warrants will be exercisable for the kind and amount of securities, cashProxy Statement and/or other property which a holder of shares of Company Common Stock would have been entitled to receive in such transaction.The Black-Scholes value of the Deerfield Warrants for purposes of a major transaction will be determined based on, among other things, a risk-free interest rate corresponding to the US$ LIBOR/Swap rate (or, to the extent LIBOR ceases to be determined, a successor interest rate determined in accordance with the Deerfield Warrants) for a period equal to the remaining term of the Deerfield Warrants, zero borrowing cost, the arithmetic mean of the historical volatility of the shares of Company Common Stock for the 10, 30 and 50 trading day periods prior to the public announcement of such major transaction and a stock price equal to the greater of (1) the closing price of the Company Common Stock on the trading day immediately preceding the consummation of such major transaction, (2) the first closing market price following the first public announcement of such major transaction or (3) the closing market price of the date immediately prior to the first public announcement of such major transaction.A major transaction is defined in the Deerfield Warrants to include: (i) a consolidation, merger, business consolidation, reorganization, recapitalization or similar transaction that results in a change in control of Melinta (i.e., its current shareholders no longer holding at least 50% of the shares of Company Common Stock or no longer having the ability to elect a majority of its board of directors), (ii) a sale or transfer of assets for a purchase price of more than 50% of Melinta’s enterprise value or a sale of all or substantially all of its assets, (iii) a purchase, tender or exchange offer resulting in a change in control of Melinta, (iv) any issuance or series of issuances of shares of Company Common Stock equal to 50% or more of the shares of Company Common Stock outstanding as of such issuance, (v) any liquidation, bankruptcy, insolvency, dissolution or winding up affecting Melinta, (vi) any cessation of the Company Common Stock to be listed, traded or publicly quoted on the NASDAQ Global Market (without prompt re-listing or requoting on the New York Stock Exchange, the NYSE American, the NASDAQ Global Select Market or the NASDAQ Capital Market), or (vii) the Company Common Stock ceasing to be registered under Section 12 of the Exchange Act.Upon certain failures by Melinta to comply with its obligations under the Deerfield Warrants or the Deerfield registration rights agreement described below, Melinta may be required to pay to the holders of the Deerfield Warrants as partial liquidated damages an amount equal to 15% per annum (or the maximum rate permitted by law, whichever is less) of the Black-Scholes value of the Deerfield Warrants in cash or, at Melinta’s option (but subject to a 9.985% ownership limitation), in shares of Company Common Stock (valued based on the volume weighted average price of the Company Common Stock as of such time). If such failure remains uncured and the holders of the Deerfield Warrants deliver notice of a default under the Deerfield Warrants, Melinta will be entitled to redeem the Deerfield Warrants for cash in an amount equal to the Black-Scholes value of the Deerfield Warrants and, if following such notice of default Melinta does not redeem the Deerfield Warrants, the holders may elect to exercise the Deerfield Warrants for a number of shares (valued at the arithmetic average of the volume weighted average price of the Company Common Stock for the 5 trading days immediately prior to the date of the applicable default notice) equal to the greater of (x) the Black-Scholes value of the Deerfield Warrants as of the date of the notice of default and (y) the Black-Scholes value of the Deerfield Warrants on the trading day immediately prior to the date that such shares are issued to Deerfield.The Black-Scholes value of the Deerfield Warrants for purposes of such events of default or failures will be determined based on, among other things, a risk-free interest rate corresponding to the US$ LIBOR/Swap rate (or, to the extent LIBOR ceases to be determined, a successor interest rate determined in accordance with the Deerfield Warrants) for a period equal to the remaining term of the warrant, zero borrowing cost, the arithmetic mean of the historical volatility of the Company Common Stock for the 10, 30 and 50 trading day periods ending on the date of determination and a stock price equal to the volume weighted average price of the Company Common Stock on the date of such calculation.Pursuant to the Facility Agreement and Securities Purchase Agreement, Melinta and Deerfield Private Design Fund IV, L.P., Deerfield Private Design Fund III, L.P., and Deerfield Special Situations Fund, L.P. entered into a registration rights agreement on January 5, 2018 (the “Deerfield Registration Rights Agreement”), the date of the closing of the Acquisition, in respect of the shares of Company Common Stock issued pursuant to the Securities Purchase Agreement and issuable upon exercise of, or otherwise pursuant to, the Deerfield Warrants. Under the Deerfield Registration Rights Agreement, Melinta was obligated to file a shelf registration statement on Form S-3 providing for the resale by the Deerfield Funds of the 3,127,846 shares of Company Common Stock issued to them pursuant to the Securities Purchase Agreement and the shares of Company Common Stock issuable pursuant to the Deerfield Warrants. The Deerfield Registration Rights Agreement includes customary black-out and suspension periods related to the Deerfield Funds’ use of the Registration Statement. Under the Deerfield Registration Rights Agreement, the Deerfield Funds are also entitled to two demand registrations within a 12-month period (subject to a minimum proceeds requirement of $10.0 million) and customary piggy back registration rights, subject to customary grace periods and, in connection with an underwritten public offering in which the Deerfield Funds participate, lock-up periods.Subject to the Deerfield Funds’ compliance with certain covenants in the Deerfield Registration Rights Agreement, if a Registration Failure (for this paragraph as defined in the Deerfield Registration Rights Agreement) occurs then the Company is required to pay additional damages to Deerfield for each 30-day period (prorated for any partial period) after the date of such Registration Failure in a cash payment equal to one percent (1.00%) of the aggregate purchase price for all Registrable Securities (as set forth in the Deerfield Registration Rights Agreement) as of the date such Registration Failure occurs. Such payments shall accrue until the earlier of (i) such time as the Registration Failure has been cured and (ii) the date on which all of the Registrable Securities may be disposed of for the holder’s own account without restriction under Rule 144 of the Securities Act of 1933, as amended (the “Securities Act”).The Deerfield Funds are entitled to registration rights until the earlier of (1) such time as all of the registrable securities under the Deerfield Registration Rights Agreement have been sold and (2) assuming the Deerfield Warrants will be exercised for cash, the date on which all of the shares of Company Common Stock issuable under the Deerfield Warrants may be immediately sold to the public without limitation, restriction or condition (including any current public information requirement) pursuant to Rule 144 of the Securities Act.Deerfield Royalty AgreementOn the closing date of the Acquisition, Melinta entered into a Royalty Agreement (the “Royalty Agreement”) with Deerfield Private Design Fund IV, L.P., Deerfield Private Design Fund III, L.P., and Deerfield Special Situations Fund, L.P., pursuant to which, in respect of each fiscal year for a seven year period, the Company agreed to make royalty payments equal to 3% (or 2%, following the satisfaction of all of Melinta’s obligations under the Facility Agreement and other loan documents) of U.S. sales of VabomereTM and certain related products exceeding $75.0 million and less than or equal to $500.0 million, subject to conditions described therein.Agreements with MedicinesPurchase and Sale AgreementOn November 28, 2017, Melinta entered into the Purchase Agreement pursuant to which the Company completed the Acquisition. Certain businesses related to the Transferred Subsidiaries, including the research and development of Oravance, Omnivance, the pre-clinical polymyxin programs and the programs being conducted at Medicines’s San Diego facilities were specifically excluded from the assets purchased by the Company. The purchase price payable under the Purchase Agreement consisted of (i) a payment by the Company to Medicines of $165.0 million in cash and the issuance to the Medicines of a number of shares of the Company Common Stock equal to $50.0 million, divided by 90% of the volume weighted average price of the Company Common Stock for the trailing 10 trading day period ending 3 trading days prior to closing; (ii) a payment by the Company to Medicines of $25.0 million following each of the twelve and eighteen month anniversaries of the closing date, (iii) the assumption of certain liabilities related to the acquired business and (iv) royalties on annual net sales of the Products as follows:U.S. net sales of Vabomere:On net sales above $50.0 million and at or below $100.0 million = 5.0%On net sales above $100.0 million and at or below $200.0 million = 7.5%On net sales above $500.0 million = 25%U.S. combined net sales of Minocin and OrbactivOn net sales at or below $100.0 million = 5.0%On net sales above $100.0 million = 15.0%Ex-U.S. net sales of Vabomere, Orbactiv and MinocinOn all net sales, including all milestone and royalty payments or other consideration received from ex-U.S. transfers of rights with respect to the products = 15.0%The Purchase Agreement contains certain representations and warranties made by Medicines with respect to Medicines, the Transferred Subsidiaries and the purchased assets. The Purchase Agreement includes an indemnity from Medicines for breaches of representations, warranties and covenants and in respect of the excluded assets and the excluded liabilities. Medicines’s indemnity obligations are subject to specified limitations described in the Purchase Agreement.Pursuant to the terms of the Purchase Agreement and certain ancillary agreements that the parties intended to enter into upon the closing of the transaction, Medicines agreed to provide certain transition services to the Company following the closing to facilitate the transition of the supply, sale and distribution of the Products in exchange for agreed upon compensation.Medicines Registration RightsOn the closing date of the Acquisition, Melinta entered into a Registration Rights Agreement with Medicines (the “Medicines Registration Rights Agreement”). Under the Medicines Registration Rights Agreement, Melinta was obligated to file, by January 9, 2018, a shelf registration statement on Form S-3 (the “Medicines Shelf Registration Statement”) providing for the resale by Medicines of the 3,313,702 shares of Company Common Stock issued to Medicines pursuant to the Purchase Agreement. Under the Medicines Registration Rights Agreement Medicines is entitled to four underwritten offerings under the Medicines Shelf Registration Statement, subject to customary black-out and suspension periods related to Medicines’ use of the Medicines Shelf Registration Statement.In addition, if Melinta registers shares of Company Common Stock on a registration statement for public sale, Medicines will have the right, subject to certain limitations, to include its Registrable Securities (as defined in the Medicines Registration Rights Agreement) in such registration statement, subject to customary cutbacks as advised by the managing underwriter engaged in the public sale of Common Stock by Melinta.Further, the Medicines Registration Rights Agreement provides for a (i) 180-day lock-up expiring July 5, 2018, covering 50% of the shares of Company Common Stock (1,656,851 shares) issued to Medicines pursuant to the Purchase Agreement, subject to certain customary exceptions including, but not limited to hedging transactions, and (ii) covenant requiring Medicines to enter into underwriter lock-up agreements under certain circumstances in connection with an underwritten public offering of Company Common Stock by Melinta.Subject to Medicines compliance with certain covenants in the Medicines Registration Rights Agreement, if a Registration Failure (for this paragraph as defined in the Medicines Registration Rights Agreement) occurs then the Company is required to pay additional damages to Medicines for each 30-day period (prorated for any partial period) after the date of such Registration Failure in a cash payment equal to one percent (1.00%) of the aggregate purchase price for all Registrable Securities (as set forth in the Purchase Agreement) as of the date such Registration Failure occurs. Such payments shall accrue until the earlier of (i) such time as the Registration Failure has been cured and (ii) the date on which all of the Registrable Securities may be disposed of for the holder’s own account without restriction under Rule 144 of the Securities Act.The Medicines Registration Rights Agreement includes customary indemnification and expense reimbursement provisions. The registration rights provided in the Medicines Registration Rights Agreement terminate upon the date on which Medicines ceases to own any Registrable Securities (as defined in the Medicines Registration Rights Agreement), but in no event later than the fifth anniversary of the effective date of the Medicines Shelf Registration Statement.AccountantAccounting Fees and ServicesThe information required by this Item is incorporated by reference to information included in our Proxy Statement and/or Form 10-K/A.PART IVItem 15. Exhibits, Financial Statement SchedulesForPage (a) The following documents are filed as part of this report: (1) Financial Statements: (2) Financial Statement Schedules:All financial statement schedules have been omitted because they are not applicable, not required or the years ended December 31, 2016 and December 31, 2017, Deloitte & Touche LLP (“D&T”) performed professional services for us. The professional services provided by D&T andinformation required is shown in the fees billed for those services are set forth below.Audit FeesThe aggregate fees billed by D&T for the audit of our annual financial statements foror the fiscal years ended December 31, 2016 and 2017, were $433,000 and $2,236,000, respectively.Audit-Related FeesThe aggregate fees billed by D&T for assurance and related services that were reasonably related to the audit or review of our financial statements for the fiscal years ended December 31, 2016 and 2017, were $89,000 and $203,000, respectively.Tax FeesNo fees were billed by D&T for tax services for the fiscal years ended December 31, 2016 and 2017.All Other FeesNo fees were billed by D&T for services other than those reported above for each of the fiscal years ended December 31, 2016 and 2017.Audit Committee Pre-Approval Policies and ProceduresThe Audit Committee pre-approves the engagement of our independent auditor to render any audit services to our company. The Audit Committee also pre-approves any other engagement of our independent auditor, which is limited to specified permitted non-audit services on a case-by-case basis. The Audit Committee generally limits its pre-approval of non-audit services to a specified period of time and, for some services, to a maximum dollar amount.ExhibitNo. Description Form Dated ExhibitNumberFiledHerewith1.1 8-K 5/6/2016 1.1 2.1 S-1 10/12/2011 2.1 2.2 8-K 8/10/2017 2.1 2.3 8-K (Filed by the Medicines Company) 1/14/2009 2.1 2.4 8-K (Filed by the Medicines Company) 12/6/2014 2.1 2.5 8-K 12/1/2017 2.1 3.1 S-1/A 1/13/2012 3.1 3.2 8-K 11/3/2017 3.1 3.3 8-K 11/3/2017 3.2 ExhibitNo. Description Form Dated ExhibitNumberFiledHerewith3.4 8-K 2/21/2019 3.1 3.5 8-K 2/25/2019 3.1 3.6 8-K 11/3/2017 3.3 4.1 S-1/A 11/22/2011 4.1 4.2 8-K 11/3/2017 10.1 4.3 8-K 1/9/2018 4.1 4.4 8-K 1/9/2018 4.2 4.5 8-K 1/9/2018 4.3 4.6 8-K 1/9/2018 4.4 4.7 8-K 1/9/2018 4.5 4.8 8-K 3/14/2018 4.1 4.9 14A 10/5/2017 Annex D-2 10.1 * 8-K 6/8/2018 10.17 10.2 8-K 1/9/2018 10.1 ExhibitNo. Description Form Dated ExhibitNumberFiledHerewith10.3 8-K 1/9/2018 10.2 10.4 8-K 1/16/2019 10.2 10.5 8-K 2/25/2019 10.1 10.6 8-K 1/9/2018 10.3 10.7 + S-8 11/13/2017 99.1 10.8 + S-8 11/13/2017 99.2 10.9 + S-1/A 1/13/2012 10.2 10.10 + 10-Q 7/31/2013 10.3 10.11 + S-1/A 1/13/2012 10.3 10.12 + 8-K 3/14/2018 10.1 10.13 + 8-K 6/14/2018 10.1 10.14 + 8-K 6/14/2018 10.2 10.15 + 8-K 2/25/2019 10.3 10.16 + 8-K 10/24/2018 10.1 ExhibitNo. Description Form Dated ExhibitNumberFiledHerewith10.17 + 8-K 2/25/2019 10.2 10.18 + 8-K 11/3/2017 10.5 10.19 + S-8 11/13/2017 99.3 10.20 + S-8 11/13/2017 99.4 10.21 + 10-Q 8/9/2018 10.2 10.22 + 8-K 10/24/2018 10.2 10.23 + 10-Q 11/7/2018 10.1 10.24 + 10-Q 11/7/2018 10.2 10.25 + 10-Q 11/7/2018 10.3 10.26 8-K 3/14/2018 10.2 110.27 + 8-K 3/14/2018 10.3 10.28 + 8-K 3/14/2018 10.4 10.29 + 8-K 10/5/2018 10.1 10.30 + 8-K 3/14/2018 10.5 ExhibitNo. Description Form Dated ExhibitNumberFiledHerewith10.31 + 8-K 3/14/2018 10.6 10.32 + 8-K 3/14/2018 10.7 10.33 + 8-K 3/14/2018 10.8 10.34 + 8-K 3/14/2018 10.9 10.35 + 8-K 3/14/2018 10.10 10.36 + 8-K 11/3/2017 10.2 10.37 + 8-K 11/3/2017 10.3 10.38 + 8-K 11/3/2017 10.4 10.39 S-1 (Filed by the Medicines Company) 3/11/2007 10.11 10.40 8-K 3/14/2018 10.18 10.41 10-Q 7/31/2013 10.13 10.42 10-Q 7/31/2013 10.14 10.43 10-Q 10/29/2013 10.19 ExhibitNo. Description Form Dated ExhibitNumberFiledHerewith10.44 * 10-Q 11/7/2018 10.50 10.45 10-K 2/25/2016 10.35 10.46 * 8-K/A 6/8/2018 10.14 10.47 8-K 3/14/2018 10.15 10.48 10-Q 7/31/2013 10.15 10.49 10-Q/A 11/8/2013 10.18 10.50 10-K 2/29/2015 10.27 10.51 10-Q 5/2/2016 10.36 10.52 10-Q 10/27/2016 10.1 10.53 10-Q (Filed by the Medicines Company) 11/9/2011 10.1 10.54 8-K (filed by the Medicines Company) 3/2/2009 99.1 10.55 * 10-Q 11/7/2018 10.4 *ExhibitNo.Description Form Dated ExhibitNumberFiledHerewith21.1 X 23.1 X 31.1 X 31.2 X 32.1 X 32.2 X 101 The following financial statements and notes from the Melinta Therapeutics, Inc. Annual Report on Form 10-K for the year ended December 31, 2018 filed on March 13, 2019, formatted in XBRL: (i) Consolidated Balance Sheets; (ii) Consolidated Statements of Operations; (iii) Consolidated Statement of Changes in Shareholders Equity; (iv) Consolidated Statements of Cash Flows; and (v) the Notes to Consolidated Financial Statements. X * The Company has requestedreceived confidential treatment with respect to portions of this exhibit. Those portions have been omitted and filed separately with the SECSecurities and Exchange Commission pursuant to a confidential treatment request.†Filed with the Original Form 10-K.&These certifications are being furnished solely pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (subsections (a) and (b) of Section 1350, chapter 63 of title 18 United States Code) and are not being filed as part of this report.SIGNATURESPursuant to the requirements of the Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this Form 10-K to be signed on its behalf by the undersigned, thereunto duly authorized.Dated: April 30, 2018Daniel Mark WechslerDaniel Mark Wechsler
Chief Executive Officer48Pursuant to the requirements of the Securities Exchange Act of 1934, this Form 10-K has been signed by the following persons on behalf of the Registrant and on the dates indicated.Signature Title Date
/s/ John H. JohnsonChief Executive Officer and Director(Principal Executive Officer)March 13, 2019 John H. Johnson /s/ Peter J. MilliganChief Financial Officer(Principal Financial and Accounting Officer)March 13, 2019 Peter J. Milligan /s/ Kevin T. FerroChairman of the BoardMarch 13, 2019 Kevin T. Ferro /s/ James J. Galeota, Jr.DirectorMarch 13, 2019 James J. Galeota, Jr. /s/ Bruce Downey DirectorMarch 13, 2019 Bruce Downey /s/ David N. GillDirectorMarch 13, 2019 David N. Gill /s/ Thomas P. KoestlerDirectorMarch 13, 2019 Thomas P. Koestler /s/ Garheng KongDirectorMarch 13, 2019 Garheng Kong /s/ David ZaccardelliDirectorMarch 13, 2019 David Zaccardelli INDEX TOCONSOLIDATED FINANCIAL STATEMENTSMELINTA THERAPEUTICS, INC.PageREPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRMTo the shareholders and the Board of Directors of Melinta Therapeutics, Inc.Opinions on the Financial Statements and Internal Control over Financial ReportingWe have audited the accompanying consolidated balance sheets of Melinta Therapeutics, Inc. and subsidiaries (the "Company") as of December 31, 2018 and 2017, the related consolidated statements of operations, shareholders' equity, and cash flows, for each of the three years in the period ended December 31, 2018, and the related notes (collectively referred to as the "financial statements"). We also have audited the Company’s internal control over financial reporting as of December 31, 2018, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2018 and 2017, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2018, in conformity with accounting principles generally accepted in the United States of America. Also, in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2018, based on criteria established in Internal Control - Integrated Framework (2013) issued by COSO.Going ConcernThe accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company's recurring losses from operations and their need to obtain additional capital raise substantial doubt about its ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.Basis for OpinionsThe Company’s management is responsible for these financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on these financial statements and an opinion on the Company’s internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.Our audits of the financial statements included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures to respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.Definition and Limitations of Internal Control over Financial ReportingA company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate./s/ Deloitte & Touche LLPChicago, Illinois March 13, 2019 We have served as the Company's auditor since 2014.MELINTA THERAPEUTICS, INC.Consolidated Balance Sheets(In thousands, except share and per share data) December 31,
2018 December 31,
2017Assets Cash and equivalents $ 81,808 $ 128,387 Receivables (See Note 3) 22,485 7,564 Inventory 41,341 10,825 Prepaid expenses and other current assets 3,848 2,988 Total current assets 149,482 149,764 Property and equipment, net 1,586 1,596 Intangible assets, net 229,196 7,500 Other assets (including restricted cash of $200 in both periods) 61,326 1,413 Total assets $ 441,590 $ 160,273 Liabilities Current liabilities Accounts payable $ 16,765 $ 7,405 Accrued expenses (See Note 3) 33,924 24,041 Deferred purchase price and other liabilities (See Note 14) 78,394 — Accrued interest on notes payable 4,485 284 Warrant liability 38 — Total current liabilities 133,606 31,730 Long-term liabilities Notes payable, net of debt discount 110,476 39,555 Deferred revenue — 10,008 Other long-term liabilities 7,444 6,644 Total long-term liabilities 117,920 56,207 Total liabilities $ 251,526 $ 87,937 Commitments and Contingencies (Note 10) Shareholders' Equity Preferred stock; $.001 par value; 5,000,000 shares and none authorized; no shares issued or
outstanding at December 31, 2018 and December 31, 2017— — Common stock; $.001 par value; 80,000,000 shares authorized; 11,204,050 and 4,399,788
issued and outstanding at December 31, 2018 and December 31, 2017, respectively11 4 Additional paid-in capital 909,896 644,991 Accumulated deficit (719,843 ) (572,659 ) Total shareholders’ equity $ 190,064 $ 72,336 Total liabilities and shareholders’ equity $ 441,590 $ 160,273 The accompanying notes are an integral part of these consolidated financial statementsMELINTA THERAPEUTICS, INC.Consolidated Statements of Operations(In thousands, except share and per share data) Years Ended December 31, 2018 2017 2016 Revenue Product sales, net $ 46,580 $ — $ — Contract research 11,677 13,959 — License 38,173 19,905 — Total revenue 96,430 33,864 — Operating expenses Cost of goods sold 41,057 — — Research and development 55,409 49,475 49,791 Goodwill impairment 25,088 — — Selling, general and administrative 133,312 63,325 19,410 Total operating expenses 254,866 112,800 69,201 Loss from operations (158,436 ) (78,936 ) (69,201 ) Other income (expenses) Interest income 730 155 30 Interest expense (43,179 ) (3,608 ) (3,390 ) Interest expense on convertible promissory notes (See Note 4) — (4,016 ) (1,016 ) Change in fair value of tranche assets and liabilities — — (1,313 ) Change in fair value of warrant liability 33,226 335 781 Loss on extinguishment of debt (2,595 ) (607 ) — Grant income 5,828 — — Other income and expense 1,904 98 177 Gain on loss contract reversal 5,330 — — Bargain purchase gain — 27,663 — Total other income (expense), net 1,244 20,020 (4,731 ) Net loss $ (157,192 ) $ (58,916 ) $ (73,932 ) Accretion of convertible preferred stock dividends — (19,259 ) (21,117 ) Net loss available to common shareholders $ (157,192 ) $ (78,175 ) $ (95,049 ) Basic and diluted net loss per share $ (17.12 ) $ (109.28 ) $ (20,600.13 ) Basic and diluted weighted average shares outstanding 9,181,668 715,369 4,614 The accompanying notes are an integral part of these consolidated financial statementsMELINTA THERAPEUTICS, INC.Consolidated Statements of Shareholders’ Equity(In thousands, except share data) Ordinary Shares Common Stock AdditionalPaid-InCapital Accumulated Deficit TotalShareholders'Equity Shares Amount Shares Amount Balance as of December 31, 2015 4,595 $ — — $ — $ 217,712 $ (439,811 ) $ (222,099 ) Share-based compensation — — — — 2,515 — 2,515 Common stock forfeitures (1 ) — — — — — — Stock option exercises 725 — — — 65 — 65 Net loss — — — — — (73,932 ) (73,932 ) Balance as of December 31, 2016 5,319 $ — — $ — $ 220,292 $ (513,743 ) $ (293,451 ) Share-based compensation — — — — 6,450 — 6,450 Stock repurchase (8 ) — — — 1,123 — 1,123 Stock option exercises — — 172 — 88 — 88 Vesting of restricted stock grants — — 12,400 — — — — Conversion of convertible preferred stock, debt & warrants into common stock — — 2,286,722 2 291,226 — 291,228 Acquisition of Cempra, Inc. (5,311 ) — 2,100,494 2 125,812 — 125,814 Net loss (58,916 ) (58,916 ) Balance as of December 31, 2017 — $ — 4,399,788 $ 4 $ 644,991 $ (572,659 ) $ 72,336 Deferred revenue adjustment upon adoption of accounting standard (See Note 2) — — — — — 10,008 10,008 Share-based compensation, including amount capitalized as inventory — — — — 3,674 — 3,674 Issuance of common shares upon exercise of options — — 40 — 3 — 3 Vesting of restricted stock grants — — 10,920 — — — — Acquisition of IDB and related financing — — 1,865,301 2 145,961 — 145,963 Issuance of common shares — — 4,928,000 5 115,267 — 115,272 Net loss — — — — — (157,192 ) (157,192 ) Balance as of December 31, 2018 — $ — 11,204,049 $ 11 $ 909,896 $ (719,843 ) $ 190,064 The accompanying notes are an integral part of these consolidated financial statementsMELINTA THERAPEUTICS, INC.Consolidated Statements of Cash Flows(In thousands) Year ended December 31, 2018 2017 2016 Operating activities Net loss $ (157,192 ) $ (58,916 ) $ (73,932 ) Adjustments to reconcile net loss to net cash used in operating activities: Depreciation and amortization 16,901 451 497 Bargain purchase gain — (27,663 ) — Change in fair value of tranche assets and liabilities — — 1,313 Non-cash interest expense 25,673 5,091 2,010 Share-based compensation 3,465 6,450 2,515 Change in fair value of warrant liability (33,226 ) (335 ) (781 ) Change in fair value for IDB contingent liabilities (8,817 ) — — Loss on extinguishment of debt 2,595 607 — Reversal of loss contract (5,330 ) — — Provision for inventory obsolescence 8,042 — — Write off of deferred equity finance costs — — 969 Asset impairment 26,076 — — Other — 70 — Changes in operating assets and liabilities: Receivables (5,044 ) (3,140 ) 43 Inventory (17,541 ) (10,825 ) — Prepaid expenses and other current assets 2,180 600 (1,461 ) Accounts payable 8,285 (1,269 ) (21 ) Accrued expenses 1,445 12,014 (1,840 ) Accrued interest on notes payable 4,202 110 (14 ) Deferred revenues — 1,000 — Deposits on future inventory purchases (40,773 ) — — Other non-current assets and liabilities (2,486 ) 157 122 Net cash used in operating activities (171,545 ) (75,598 ) (70,580 ) Investing activities Cash acquired in merger with Cempra, Inc. — 161,410 — IDB acquisition (166,382 ) — — Purchases of intangible assets (2,000 ) (5,500 ) — Purchases of property and equipment (1,690 ) (849 ) (463 ) Net cash provided by (used in) investing activities (170,072 ) 155,061 (463 ) Financing activities Proceeds from financing (see Note 4): Proceeds from the issuance of notes payable 111,421 38,844 44,111 Costs associated with the issuance of notes payable (6,455 ) — — Proceeds from the issuance of warrants 33,264 — — Proceeds from the issuance of royalty agreement 1,472 — — Purchase of notes payable disbursement option (7,609 ) — — Proceeds from issuance of common stock, net, to lender 51,452 — — Other financing activities: Proceeds from issuance of common stock, net 155,273 — — Proceeds from the issuance of convertible promissory notes — 24,526 — The accompanying notes are an integral part of these consolidated financial statementsPayment of debt extinguishment fees (2,150 ) (1,240 ) — IDB acquisition deferred payments (1,633 ) — — Proceeds from the exercise of stock options, net of cancellations 3 88 65 Proceeds from the issuance of convertible preferred stock — — 13,625 Payment of preferred stock issuance costs — — (9 ) Repayment of notes payable (40,000 ) (24,503 ) (5,498 ) Net cash provided by financing activities 295,038 37,715 52,294 Net change in cash and equivalents (46,579 ) 117,178 (18,749 ) Cash, cash equivalents and restricted cash at beginning of the period 128,587 11,409 30,158 Cash, cash equivalents and restricted cash at end of the period (See Note 4) $ 82,008 $ 128,587 $ 11,409 Supplemental cash flow information Cash paid for interest $ 13,259 $ 2,326 $ 2,411 Cash received from exchange of state tax credits, net $ — $ 283 $ 207 Non-cash investing and financing activities Accrued purchases of fixed assets $ 10 $ 2,155 $ 12 Accrued notes payable issuance costs $ — $ — $ 475 MELINTA THERAPEUTICS, INC.December 31, 2018Notes to Consolidated Financial Statements(In thousands, except share and per share data)NOTE 1 – NATURE OF THE BUSINESSMelinta Therapeutics, Inc. (the “Company,” “we,” “us,” “our,” or “Melinta,” the registrant previously known as Cempra, Inc.) is a commercial-stage pharmaceutical company focused on discovering, developing and commercializing differentiated anti-infectives for the acute care and community settings to meet critical medical needs in the treatment of bacterial infectious diseases. Melinta is headquartered in New Haven, Connecticut, with locations in Lincolnshire, Illinois, Morristown, New Jersey and Chapel Hill, North Carolina. We have four products: (i) delafloxacin as the active pharmaceutical ingredient and distributed under the brand name Baxdela™, (ii) meropenem and vaborbactam as the active pharmaceutical ingredients and distributed under the brand name Vabomere™ (“Vabomere”), (iii) oritavancin as the active pharmaceutical ingredient and distributed under the brand name Orbactiv® (“Orbactiv”) and (iiii) minocycline as the active pharmaceutical ingredient and distributed under the brand name Minocin® for injection and line extensions of such products.Our stock is traded on the Nasdaq market under the symbol “MLNT.”The accompanying consolidated financial statements have been prepared assuming Melinta Therapeutics, Inc. (the “Company,” “we,” “us,” “our,” or “Melinta”) will continue as a going concern. We are not currently generating revenue from operations that is sufficient to cover our operating expenses and do not anticipate generating revenue sufficient to offset operating costs in the short-term. We have incurred losses from operations since our inception and had an accumulated deficit of $719,843 as of December 31, 2018, and we expect to incur substantial expenses and further losses in the short term for the development and commercialization of our product candidates and approved products. In addition, we have substantial commitments in connection with our acquisition of the infectious disease business of The Medicines Company ("Medicines") that we completed in January 2018, including payments related to deferred purchase price consideration, assumed contingent liabilities and the purchase of inventory. And, there are certain financial-related covenants under our Deerfield Facility, as amended in January 2019, including requirements that we (i) file an Annual Report on Form 10-K for the year ending December 31, 2019, with an audit opinion without a going concern qualification, (ii) maintain a minimum cash balance of $40,000 through March 2020, and thereafter, a balance of $25,000, and (iii) achieve net revenue from product sales of at least $63,750 for the year ending December 31, 2019. (See Note 4 to the consolidated financial statements for further details on the Deerfield Facility.)Our future cash flows are dependent on key variables such as our ability to access additional capital under our Vatera and Deerfield credit facilities, our ability to secure a working capital revolver, which is allowed under the Deerfield Facility and required under the Vatera facility, and most importantly, the level of sales achievement of our four marketed products. Our current operating plans include assumptions about our projected levels of product sales growth in the next 12 months in relation to our planned operating expenses. Revenue projections are inherently uncertain but have a higher degree of uncertainty in an early-stage commercial launch, which we have in Baxdela and Vabomere, where there is not yet a robust sales history. While we have a plan to achieve the current expected sales levels of our products, we are unable to conclude based on applying the requirements of FASB Accounting Standards Codification 205-40, Presentation of Financial Statements - Going Concern (“ASC 205”) that such revenue is “probable” (as defined under this accounting standard). In addition, given our forecasted product sales are not deemed probable under ASC 205, our ability to draw the additional $50,000 of capacity under the Deerfield Facility, which is conditional based on meeting certain sales-based milestones before the end of 2019, is also not considered to be probable under the accounting guidance. As such, we are not able to conclude under ASC 205 that the actions discussed below will be effectively implemented and, therefore, our current operating plans, existing cash and cash collections from existing revenue arrangements and product sales may not be sufficient to fund our operations for the next 12 months. As such, we believe there is substantial doubt about our ability to continue as a going concern. The financial statements do not include any adjustments relating to the recoverability and classification of liabilities that might be necessary should we be unable to continue as a going concern.In the fourth quarter of 2018, the Company took actions to reduce its operating spend, including a reduction to the workforce of approximately 20.0% and a decision to begin to wind down its research and discovery function. To provide additional operating capital, in December 2018, the Company entered into a Senior Subordinated Convertible Loan Agreement (the “Loan Agreement”) with Vatera Healthcare Partners LLC and Vatera Investment Partners LLC (together, “Vatera”) pursuant to which Vatera committed to provide $135,000 over a period of five months, subject to the satisfaction of certain conditions (see Note 4). We drew $75,000 under this facility in February 2019, and we plan to draw the remaining $60,000 by early July 2019. As of December 31, 2018, the Company had $81,808 in cash and cash equivalents. In addition to drawing the additional $60,000 available under the Vatera Facility, in the next several months, we plan to put a working capital revolver in place for up to $20,000, which is permitted under the Deerfield Facility (see Note 4) and required under the Vatera Loan Agreement (we must establish a working capital revolver of at least $10,000 in order to draw the final $35,000 tranche under the Vatera Loan Agreement in July 2019). We are also exploring options to modify the terms of certain liabilities to increase our liquidity over the next 12 to 18 months. Finally, if our cash collections from revenue arrangements, including product sales, and other financing sources are not sufficient, we plan to control spending and would take further actions to adjust the spending level for operations if required. However, there is no guarantee that we will be successful in executing any or all of these initiatives.NOTE 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIESPrinciples of Consolidation and Basis of Presentation—The accompanying consolidated financial statements include the accounts and results of operations of Melinta and its wholly-owned subsidiaries. The consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”). All intercompany balances and transactions have been eliminated in consolidation. We have changed the presentation of income from our contract with the Biomedical Advanced Research and Development Authority of the U.S. Department of Health and Human Services (“BARDA”) (see Grant Income discussion below) to conform to the 2018 presentation of revenue under Topic 606. Accordingly, grant income reported in 2018 has been moved from "Contract research" in Revenue to "Grant income" in Other income (expense). As we adopted Topic 606 using the modified retrospective method, we did not change the presentation of 2017 grant income. In addition, on February 20, 2019, the board approved a 1-for-5 reverse split of our common stock, which was effective February 22, 2019. All share, share price and per share amounts presented have been adjusted to reflect these values as if the reverse split had occurred at the beginning of the earliest period presented.Use of Estimates—The preparation of these consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.Cash and Cash Equivalents—We consider all highly liquid investments with original maturities of three months or less at time of purchase to be cash equivalents. We invest excess cash primarily in money market funds.Trade and Other Receivables—Trade receivables consist of amounts billed for product shipments. Receivables for product shipments are recorded as shipments are made and title to the product is transferred to the customer.Other receivables consist of amounts billed, and amounts earned but unbilled, under our licensing agreements and our contracts with BARDA. Receivables for license agreements are recorded as we achieve the requirements of the agreements, and receivables under the BARDA contracts are recorded as qualifying research activities are conducted and invoices from our vendors are paid. Unbilled receivables are also recorded based upon work estimated to have been completed for which we have not paid vendor invoices. We carry our receivables net of an allowance for doubtful accounts. On a periodic basis, we evaluate our receivables for collectability. We have recorded an allowance for doubtful accounts of $26 for specifically identified receivables that may not be fully collectible.Concentration of Credit Risk—Concentration of credit risk exists with respect to cash and cash equivalents and receivables. We maintain our cash andcash equivalents with federally insured financial institutions, and at times, the amounts may exceed the federally insured deposit limits. To date, we have not experienced any losses on our deposits of cash and cash equivalents. We believe that we are notexposed to significant credit risk due to the financial position of the depository institutions in which deposits are held.A significant portion of our trade receivables is due from three large wholesaler customers for our products, which constitute 46%, 32% and 15%, respectively, of our trade receivable balance at December 31, 2018.Inventory—Inventory is stated at the lower of cost or estimated net realizable value. Inventory is valued on a first-in, first-out basis and consists primarily of material costs, third-party manufacturing costs, overhead—principally the cost of managing our manufacturers—and related transportation costs. We capitalize inventory upon regulatory approval when, based on management’s judgment, future commercialization is considered probable and the future economic benefit is expected to be realized; otherwise, such costs are expensed. Costs of drug product to be consumed in any current or future clinical trials will continue to be recognized as research and development expense. Wereview inventories on hand at least quarterly and record provisions for estimated excess, slow-moving and obsolete inventory, as well as inventory with a carrying value in excess of net realizable value. As of December 31, 2018, we had recorded $7,070 reserves for our inventory. Activity in our inventory reserve account is summarized below:Beginning Balance as of January 1, 2018 Charges to Cost of Goods Sold Inventory Discards Ending Balance as of December 31, 218 $ — $ 8,042 $ (972 ) $ 7,070 Fair Value of Financial Instruments—The carrying amounts of our financial instruments, which include cash and cash equivalents, tax credit and other receivables, accounts payable, accrued expenses, notes payable, and preferred stock warrants, approximated their fair values at December 31, 2018 and 2017.Debt Issuance Costs—Debt issuance costs represent legal and other direct costs incurred in connection with our notes payable. These costs were recorded as a contra-liability included in the notes payable line item and amortized as a non-cash component of interest expense using the effective interest method over the term of the note.Property and Equipment—Property and equipment are recorded at cost less accumulated depreciation and are depreciated using the straight-line method over their estimated useful lives. Leasehold improvements are amortized over the shorter of their useful lives or the remaining term of the lease. Major improvements are capitalized, while repair and maintenance costs, which do not improve or extend the useful lives of the respective assets, are expensed as incurred. Upon retirement or sale, the cost of assets disposed of and the related accumulated depreciation are removed from the balance sheets and any resulting gain or loss is credited or charged to income.Depreciation periods for our property and equipment are as follows:Type Period Laboratory equipment 5 years Furniture and fixtures 5 years Office equipment 3 years Leasehold improvements Shorter of useful life or lease life Purchased software 3 years Goodwill and Intangible Assets—Intangible assets consist of capitalized milestone payments for the licenses we use to make our products and the fair value of identifiable intangible assets, including in-process research and development (“IPR&D”), acquired in the IDB transaction. Given the uncertainty of forecasts of future revenue for our products, we amortize the cost of intangible assets on a straight-line basis over the estimated economic life of each asset, generally the exclusivity period of each associated product. IPR&D is reclassified to intangible assets (developed product rights) once the associated product has received approval by the appropriate regulatory agency. Amortization for these developed product rights does not begin until both the associated product has received approval and sales have commenced. We will recognize amortization for our intangible assets over periods of approximately 10 to 17 years, commencing with the launch of the products. As of December 31, 2017, product right intangible assets were $7,500. Amortization had not yet commenced as product launch occurred in early 2018. As of December 31, 2018, gross product right intangible assets and the related accumulated amortization were $245,529 and $16,332. Amortization of intangible assets, which is included in cost of goods sold, was $16,332 for the year ended December 31, 2018. No intangible asset amortization was recorded in 2017. For each of the years ending December 31, 2019, through December 31, 2023, we expect to recognize amortization expense of $16,603, with approximately $125,113 to be recognized thereafter. In addition, there is $21,069 of developed product rights primarily related to Vabomere that will begin amortization over approximately 10 years when the launch of Vabomere in Europe has occurred.Goodwill and indefinite-lived assets, including IPR&D, are not amortized, but are subject to an impairment review annually and more frequently when indicators of impairment exist. An impairment of goodwill could occur if the carrying amount of a reporting unit exceeded the fair value of that reporting unit. An impairment of indefinite-lived intangible assets would occur if the fair value of the intangible asset is less than the carrying value. Prior to reclassifying an IPR&D asset to a definite-lived intangible asset, we perform an impairment analysis.We test goodwill, IPR&D and indefinite-lived assets for impairment by first assessing qualitative factors to determine whether it is more likely than not that the fair value is less than its carrying amount. If we conclude it is more likely than not that the fair value of the asset under review is less than its carrying amount, a quantitative impairment test is performed.In December 2018, we performed our annual impairment test for goodwill. As a result, we concluded that the implied value of goodwill was negative, and we recorded an impairment adjustment of $25,088 in December 2018 to reduce our goodwill value to zero. This was driven primarily by the sustained decrease in the Melinta's stock price over the course of theprevious year, and since we operate as one single operating unit, the market capitalization of the Company is a strong indicator of fair value.Impairment of Long-Lived Assets—Long-lived assets consist primarily of property and equipment and intangible assets with a definite life. We record impairment losses on long-lived assets used in operations when events and circumstances indicate that the carrying amount of an asset or group of assets may not be fully recoverable at the lowest level of identifiable cash flows, which is each product line. If impairment indicators are present, we assess whether the future estimated undiscounted cash flows attributable to the assets in question are greater than their carrying amounts. If these future estimated cash flows are less than carrying value, we then measure an impairment loss for the amount that carrying value exceeds fair value of the assets. In the year ended December 31, 2018, we recorded expense of $988 in research and development expenses related to the write-off of in-process validation costs for manufacturing projects that we abandoned during the year. We did not have a corresponding impairment expense in 2017 or 2016.During the fourth quarter of 2018, impairment indicators were present for our product rights intangible assets, so we prepared undiscounted cash flows attributable to each product to assess whether or not the intangible assets were impaired. The impairment indicators related to revised product sales forecasts, which were lower than those initially developed in connection of the acquisition of the assets. While we concluded that the intangible assets were not impaired because the undiscounted cash flows exceeded the carrying value, if revenue forecasts decrease again in the future, there is a risk that we may conclude that one or more of the intangible assets are impaired in a future period.Revenue Recognition—On January 1, 2018, we adopted Accounting Standards Update (“ASU”) 2014-9,Revenue from Contracts with Customers (“Topic 606”), and all related amendments. For further information regarding the adoption of Topic 606, see the “Adoption of Topic 606” section of this Note 2. Topic 606 outlines a single comprehensive model to use in accounting for revenue arising from contracts with customers and supersedes most current revenue recognition guidance. The core principle of this new revenue recognition guidance is that a company will recognize revenue when promised goods or services are transferred to customers in an amount that reflects the consideration to which the company expects to be entitled in exchange for those goods or services. Topic 606 defines the following five-step process to achieve this core principle, and in doing so, it is possible that significant judgment and estimates may be required within the revenue recognition process. 1. identify the contract(s) with a customer; 2. identify the performance obligations in the contract; 3. determine the transaction price; 4. allocate the transaction price to the performance obligations in the contract; and 5. recognize revenue when (or as) the entity satisfies a performance obligation. The new guidance only applies the five-step model to arrangements that meet the definition of a contract under Topic 606, including the consideration of whether it is probable that the entity will collect the consideration it is entitled to in exchange for the goods or services it transfers to the customer. At contract inception, once the contract is determined to be within the scope of Topic 606, we assess the goods or services promised within each contract and determine those that are performance obligations; the assessment includes the evaluation of whether each promised good or service is distinct within the context of the contract. Under Topic 606, we recognize revenue separately for performance obligations that are “distinct.” Performance obligations are considered to be distinct if (a) the customer can benefit from the license or services either on its own or together with other resources that are readily available to the customer, and (b) our promise to transfer the license or services is separately identifiable from other promises in the contract. If a license or service is not individually distinct, we combine the license or service with other promised licenses and/or services until we identify a bundle of licenses and/or services that together are distinct.We recognize, as revenue, the amount of the transaction price that is allocated to the respective performance obligation when (or as) the performance obligation is satisfied. In determining the transaction price, we consider all forms of variable consideration, which can take various forms, including, but not limited to, prompt-pay discounts, rebates, credits, and milestone payments. We estimate variable consideration using either the “expected value” or “most likely amount” method, depending on which method better predicts the amount of consideration to which we will be entitled. The expected value method is a probability-weighted approach that considers all possible outcomes while the most likely approach uses the single most likely amount in a range of possible outcomes. We apply a variable consideration constraint to the estimated transaction price if we conclude that it is probable that there is a risk of significant reversal of revenue once the uncertainty related to the variable consideration is resolved.Under the guidance of Topic 606, we recognize revenue for each performance obligation when the customer obtains control of the product and we have satisfied each of our respective obligations. Control is defined as the ability of the customer to direct the use of and obtain substantially all the benefits of the asset.In addition, as of December 31, 2018, we do not have any contract assets or liabilities and our contracts do not have any significant financing components. And, we have not capitalized contract origination costs. Licensing ArrangementsWe enter into license and collaboration agreements for the research and development and/or commercialization of therapeutic products. The terms of these agreements may include nonrefundable licensing fees, funding for research, development and manufacturing, milestone payments and royalties on any product sales derived from the collaborations in exchange for the delivery of licenses and rights to sell our products within specified territories outside the United States.In the determination of whether our license and collaboration agreements are accounted for under Topic 606 or Accounting Standards Classification (“ASC”) 808, Contract Accounting, we first assess whether or not the partner in the arrangement is a customer. If the partner in the arrangement is deemed a customer as it relates to some or all of our performance obligations, then the consideration associated with those performance obligations is accounted for as revenue under Topic 606.Our license agreements may include contingent or variable consideration based upon the achievement of regulatory- and sales-based milestones and future royalties based on a percentage of the partner’s net product sales. Our performance obligations to deliver distinct licenses are recognized at a point in time. Milestone payments from licensees that are contingent and/or variable upon future regulatory events and product sales are not considered probable of being achieved until the milestones are earned and, therefore, the contingent revenue is subject to significant risk of reversal. As such, we constrain this variable consideration and do not include it in the transaction price (or recognize the revenue related to these milestones) until such time that the contingencies are resolved and generally recognized at a point in time. In addition, under the sales- or usage- based royalty exception in Topic 606, we do not estimate, at the onset of the arrangement, the variable consideration from future royalties or sales-based milestones. Instead, we wait to recognize royalty revenue until the future sales occur.In September 2018, we entered into a license agreement with Menarini, which grants to Menarini the exclusive right to market Vabomere, Orbactiv and Minocin for injection in 68 countries in Europe, Asia-Pacific and the Commonwealth of Independent States. The agreement includes an upfront license fee of €17,000 (which we received in October 2018), a milestone payment of €15,000 upon European marketing approval for Vabomere, potential regulatory- and sales-based milestones, and sales-based royalties. Menarini received European marketing approval in November 2018, and we received the €15,000 milestone payment in the fourth quarter of 2018. We determined that Menarini is a customer and we should account for the agreement under Topic 606. We identified one performance obligation under the agreement, the delivery of the licensed rights. In addition, we agreed to supply the products to Menarini in the future at a cost that is consistent with our other similar licensing arrangements. We provided Menarini immediate access to the licensed rights and, as such, we allocated the upfront payment entirely to the license and recognized revenue at the point in time when the licensed rights were delivered, in September 2018. We will recognize any future contingent consideration, including regulatory and sales-based milestone payments and royalty revenue, at such time when the milestones or royalties have been achieved. Menarini received European marketing approval in November 2018, so we recognized the €15,000 milestone payment as revenue in the fourth quarter of 2018.Adoption of Topic 606We adopted Topic 606 on January 1, 2018, using the modified retrospective method applied to those contracts which were not complete as of January 1, 2018. Results for reporting periods beginning after January 1, 2018, are presented under Topic 606, while prior period amounts are not adjusted and continue to be reported in accordance with legacy U.S. GAAP under ASC 605. In our adoption of Topic 606, we did not use practical expedients. In addition, we have considered the nature, amount and timing of our different revenue sources. Accordingly, the disaggregation of revenue from contracts with customers is reflected in different captions within the consolidated statement of operations. For our Eurofarma distribution arrangements under which revenue was previously deferred, revenue is now recognized at the point in time when the license is granted and has benefit to Eurofarma. These deferred revenues were originally expected to be recognized in future periods over the period of time over which we supplied Baxdela under the supply arrangement, which could have lasted up to 10 years or longer. The cumulative effect of the adoption was recognized as a decrease to opening accumulated deficit and a decrease to deferred revenue of $10,008 on January 1, 2018. The effect of the adoption of Topic 606 on our consolidated balance sheet is as follows: Balance at December 31, 2017 Adjustments Due toTopic 606 Balance at January1, 2018Liabilities: Deferred revenue $ 10,008 $ (10,008 ) $ — Shareholders’ equity: Accumulated deficit $ (572,659 ) $ 10,008 $ (562,651 ) In connection with the adoption of Topic 606, we no longer recognize grant income as revenue (see Grant Income discussion below), but there was no change to the timing of historical recognition. Also, there was no change to the timing of recognition of contract revenue under our licensing agreements. For product sales, recognition of revenue under Topic 605 would have been the same as under Topic 606 for the year ended December 31, 2018, as we have established sufficient sales history to analyze trends in revenue constraints. In addition, the initial stocking of product in connection with product launches in the first quarter of 2018 was sold through to end customers before the end of 2018, establishing a fixed transaction price for these sales to wholesalers.We have no outstanding performance obligations as of December 31, 2018, related to our licensing arrangements. Although we have agreements in place to supply our drug products to our partners once they achieve regulatory approval in their respective territories, we concluded that the option to purchase our products from us is not a material right. All performance obligations under our licensing arrangements were satisfied historically at a point in time. Variable consideration in the form of regulatory and sales-based milestones, which are payable under the terms of our licensing arrangements, has been constrained because of the risk of significant revenue reversal as in our revenue recognition policy included in this Note 2. Further, we recognize contract research revenue from Menarini as we incur the reimbursable development costs.Product SalesProduct Year Ended December 31, 2018 Baxdela $ 6,485 Vabomere 7,416 Orbactiv 22,951 Minocin for injection 9,728 Total product sales, net $ 46,580 Beginning in January 2018, as a result of both the acquisition of IDB and the launch of Baxdela, we distribute Baxdela, Vabomere, Orbactiv, and Minocin for injection products commercially in the United States. While we sell some of our products directly to certain hospitals and clinics, the majority of our product sales are made to wholesale customers who subsequently resell our products to hospitals or certain medical centers, as well as specialty pharmacy providers and other retail pharmacies. The wholesaler places orders with us for sufficient quantities of our products to maintain an appropriate level of inventory based on their customers’ historical purchase volumes and demand. We recognize revenue once we have transferred physical possession of the goods and the wholesaler obtains legal title to the product and accepts responsibility for all credit and collection activities with the resale customer. In addition, we enter into arrangements with health care providers that purchase our products from wholesalers—as well as payers and certain other customers—that provide for government mandated and/or privately negotiated rebates, chargebacks and discounts with respect to the purchase of our products. The transaction price that we recognize as revenue reflects the amount we expect to be entitled to in connection with the sale and transfer of control of product to our customers. Variable consideration is only included in the transaction price, to the extent that it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved. At the time that our customers take control of the product, which is when our performance obligation under the sales contracts is complete, we record product revenues net of applicable reserves for various types of variable consideration based on our estimates of channel mix. The types of variable consideration in our product revenue are as follows:Prompt pay discountsProduct returnsChargebacks and rebatesFee-for-serviceGovernment rebatesCommercial payer and other rebatesGroup purchasing organization (“GPO”) administration feesMelintAssist voluntary patient assistance programsIn determining the amounts of certain allowances and accruals, we must make significant judgments and estimates. For example, in determining these amounts, we estimate hospital demand, buying patterns by hospitals, hospital systems and/or group purchasing organizations from wholesalers and the levels of inventory held by wholesalers and customers. Making these determinations involves analyzing third party industry data to determine whether trends in historical channel distribution patterns will predict future product sales. We receive data periodically from our wholesale customers on inventory levels and historical channel sales mix, and we consider this data when determining the amount of the allowances and accruals for variable consideration. The amount of variable consideration is estimated by using either of the following methods, depending on which method better predicts the amount of consideration to which we are entitled:a) The “expected value” is the sum of probability-weighted amounts in a range of possible consideration amounts. Under Topic 606, an expected value may be an appropriate estimate of the amount of variable consideration if we have many contracts with similar characteristics. b) The “most likely amount” is the single most likely amount in a range of possible consideration amounts (i.e., the single most likely outcome of the contract). Under Topic 606, the most likely amount may be an appropriate estimate of the amount of variable consideration if the contract has only two possible outcomes (i.e., either achieve or don’t achieve a threshold specified in a contract). The method selected is applied consistently throughout the contract when estimating the effect of an uncertainty on an amount of variable consideration. In addition, we consider all the information (historical, current, and forecasts) that is reasonably available to us and shall identify a reasonable number of possible consideration amounts. The relevant factors used in this determination include, but are not limited to, current contractual and statutory requirements, specific known market events and trends, industry data, and forecasted customer buying and payment patterns.In assessing whether a constraint is necessary, we consider both the likelihood and the magnitude of the revenue reversal. Actual amounts of consideration ultimately received may differ from our estimates. If actual results in the future vary from our estimates, we adjust these estimates, which would affect net product revenue and earnings in the period such variances become known. The specific considerations we use in estimating these amounts related to variable consideration associated with our products are as follows:Prompt Pay Discounts – We provide wholesale customers with certain discounts if the wholesaler pays within the payment term, which is generally between 30 and 60 days. Product returns – Generally, our customers have the right to return any unopened product during the 18-month period beginning six months prior to the labeled expiration date and ending 12 months after the labeled expiration date. Where historical rates of return exist, we use history as a basis to establish a returns reserve for product shipped to wholesalers. For our newly launched products, for which we currently do not have history of product returns, we estimate returns based on third-party industry data for comparable products in the market. As we distribute our products and establish historical sales over a longer period of time (i.e., two years), we will be able to place more reliance on historical purchasing and return patterns of our customers when evaluating our reserves for product return.At the end of each reporting period for any of our products, we may decide to constrain revenue for product returns based on information from various sources, including channel inventory levels and dating and sell-through data, the expiration dates of product currently being shipped, price changes of competitive products and introductions of generic products. In the year ended December 31, 2018, we increased our returns reserve by $1,700 due to risk factors that were present primarily in connection with the initial stocking of inventory for the launch of our new products. Chargebacks – Although we primarily sell products to wholesalers in the United States, we typically enter into agreements with medical centers, either directly or through GPOs acting on behalf of their hospital members, in connection with the hospitals’ purchases of products. Based on these agreements, most of our hospital customers have the right to receive a discounted price for products and volume-based rebates on product purchases. In the case of discounted pricing, we typically provide a credit to our wholesale customers (i.e., chargeback), representing the difference between the customer’s acquisition list price and the discounted price.Fees-for-service – We offer discounts and pay certain wholesalers service fees for sales order management, data, and distribution services which are explicitly stated at contractually determined rates in the customer’s contracts. In assessing if the consideration paid to the customer should be recorded as a reduction of the transaction price, we determine whether the payment is for a distinct good or service or a combination of both. Since our wholesaler fees are not specifically identifiable, we do not consider the fees separate from the wholesaler's purchase of the product. Additionally, wholesaler services generally cannot be provided by a third party. Because of these factors, the consideration paid is considered a reduction of revenue. We estimate our fee-for-service accruals and allowances based on wholesalers' purchases, demand pull-through, wholesaler inventory levels and the applicable discount rate. Government Rebates – We participate in three rebate programs under various government programs: Medicaid, TRICARE and Medicare Part D. At the time of the sale it is not known what the government rebate rate will be, but historical rates are used to estimate the current period accrual.Medicaid – The Medicaid Drug Rebate Program is a program that includes The Centers for Medicare and Medicaid Services, State Medicaid agencies, and participating drug manufacturers that helps to offset the federal and state costs of most outpatient prescription drugs dispensed to Medicaid patients. The Medicaid Drug RebateProgram is jointly funded by the states and the federal government. The program reimburses hospitals, physicians, and pharmacies for providing care to qualifying recipients who cannot finance their own medical expenses.TRICARE – TRICARE is a benefit established by law as the health care program for uniformed service members, retired service members, and their families. We must pay the Department of Defense (“DOD”) refunds for drugs entered into the normal commercial chain of transactions that end up as prescriptions given to TRICARE beneficiaries and paid for by the DOD. The refund amount is the portion of the price of the drug sold by us that exceeds the federal ceiling price. Refunds due to TRICARE are based solely on utilization of pharmaceutical agents dispensed through a TRICARE Retail Pharmacy to DOD beneficiaries.Medicare Part D – We maintain contracts with Managed Care Organizations (“MCOs”) that administer prescription benefits for Medicare Part D. MCOs either own pharmacy benefit managers (“PBMs”) or contract with several PBMs to fulfill prescriptions for patients enrolled under their plans. As patients obtain their prescriptions, utilization data are reported to the MCOs, which generally submit claims for rebates quarterly. Commercial Payer and Other Rebates – We contract with certain private payer organizations, primarily insurance companies and PBMs, for the payment of rebates with respect to utilization of Baxdela and contracted formulary status. We estimate these rebates and record reserves for such estimates in the same period the related revenue is recognized. Currently, the reserve for customer payer rebates considers future utilization based on third party studies of payer prescription data; the utilization is applied to product that remains in the distribution and retail pharmacy channel inventories at the end of each reporting period. As we distribute our products and establish historical sales over a longer period of time (i.e., two years), we will be able to place more reliance on historical data related to commercial payer rebates (i.e., actual utilization units) while continuing to rely on third party data related to payer prescriptions and utilization. In addition, we offer rebates to certain customers based on the volume of product purchased over an agreed period of time.GPO Administration Fees – We contract with GPOs and pay administration fees related to contracting and membership management services provided. In assessing if the consideration paid to the GPO should be recorded as a reduction in the transaction price, we determine whether the payment is for a distinct good or service or a combination of both. Since our GPO fees are not specifically identifiable, we do not consider the fees separate from the purchase of the product. Additionally, the GPO services generally cannot be provided by a third party. Because of these factors, the consideration paid is considered a reduction of revenue.MelintAssist – We offer certain voluntary patient assistance programs for prescriptions, such as savings/co-pay cards, which are intended to provide financial assistance to qualified patients with full or partial prescription drug co-payments required by payers. The calculation of the accrual for co-pay assistance is based on an estimate of claims and the cost per claim that we expect to receive associated with product that has been recognized as revenue but remains in the distribution and pharmacy channel inventories at the end of each reporting period.At the end of each reporting period, we adjust our allowances for cash discounts, product returns, chargebacks, fees-for-service and other rebates and discounts when believe actual experience may differ from current estimates. The following table provides a summary of activity with respect to our sales allowances and accruals during 2018: CashDiscounts ProductReturns Chargebacks Fees-for-Service MelintAssist GovernmentRebates CommercialRebates AdminFeeBalance as of January 1, 2018 $ — $ — $ — $ — $ — $ — $ — $ — Allowances for sales 1,184 3,664 5,792 3,240 1,224 864 1,491 476 Payments & credits issued (939 ) (694 ) (5,030 ) (2,422 ) (812 ) (171 ) (892 ) (338 ) Balance as of December 31, 2018 $ 245 $ 2,970 $ 762 $ 818 $ 412 $ 693 $ 599 $ 138 The allowances for cash discounts, bad debt, and chargebacks are recorded as contra-assets in trade receivables; the other balances are recorded in other accrued expenses.Grant IncomeWe have several agreements with BARDA related to certain development costs for solithromycin and Vabomere. We concluded that BARDA is not a customer under Topic 606 because it does not engage with us in reciprocal transactions but, rather, provides contributions to our development efforts to encourage the development of more antibiotics for the welfare of society. As such, we view the income as a contribution and classify it within other income and expense, net, rather than inrevenue. We recognize grant income under the BARDA contracts over time as qualifying research activities are conducted. In the first quarter of 2018, we and BARDA agreed to terminate the solithromycin BARDA contract and wind down the study, which was substantially complete as of December 31, 2018.In addition, in May 2018, we announced that we had entered into a partnership with the Combating Antibiotic Resistant Bacteria Biopharmaceutical Accelerator (“CARB-X”), under which Melinta was awarded up to $6,200 to support the development of the company’s investigational pyrrolocytosine compounds. CARB-X was established in 2016 by BARDA, the National Institute of Allergy and Infectious Diseases of the U.S. Department of Health and Human Services and the Wellcome Trust, a global charitable foundation dedicated to improving health, to accelerate pre-clinical product development in the area of antibiotic-resistant infections, one of the world’s greatest health threats.In November 2018, we decided to suspend the development of our pyrrolocytosine compounds and have accordingly suspended our partnership with CARB-X. In fiscal year 2018, we recognized $425 in grant income related to awards from CARB-X; we do not anticipate receiving any further award under this contract.Business Combinations—We account for acquired businesses using the acquisition method of accounting. This method requires that most assets acquired and liabilities assumed be recognized as of the acquisition date. On January 1, 2018, we adopted ASU 2017-1, Business Combinations (Topic 805) Clarifying the Definition of a Business, which narrows the definition of a business and requires an entity to evaluate if substantially all of the fair value of the gross assets acquired is concentrated in a single identifiable asset or a group of similar identifiable assets, which would not constitute the acquisition of a business. The guidance also requires a business to include at least one substantive process and narrows the definition of outputs. There is often judgment involved in assessing whether an acquisition transaction is a business combination under Topic 805 or anacquisition of assets. In our IDB acquisition, we evaluated the transaction and concluded that the IDB qualified as a “business” under Topic 805as it has both inputs and processes with the ability to create outputs. Among IDB’s inputs are developed product rights, in-process research and development and intellectual property across multiple classes of drugs and indications, third-party contract manufacturing agreements and tangible assets from which there is potential to create value and outputs.With respect to business combinations, we determine the purchase price, including contingent consideration, and allocate the purchase price of acquired businesses to the tangible and intangible assets acquired and liabilities assumed, based on estimated fair values. The excess of the purchase price over the identifiable assets acquired and liabilities assumed is recorded as goodwill. With respect to the purchase of assets that do not meet the definition of a business under Topic 805, goodwill is not recognized in connection with the transaction and the purchase price is allocated to the individual assets acquired or liabilities assumed based on their relative fair values.We engage a third-party professional service provider to assist us in determining the fair values of the purchase consideration, assets acquired, and liabilities assumed. Such valuations require management to make significant estimates and assumptions, especially with respect to contingent liabilities associated with the purchase price and intangible assets, such as developed product rights and in-process research and development programs. Critical estimates that we have used in valuing these elements include, but are not limited to, future expected cash flows using valuation techniques (i.e., Monte Carlo simulation models) and discount rates. Management's estimates of fair value are based upon assumptions believed to be reasonable, but which are inherently uncertain and unpredictable.We record contingent consideration resulting from a business combination at its fair value on the acquisition date. The purchase price of IDB included contingent consideration related to the achievement of future regulatory milestones, sales-based milestones associated with the products we acquired, and certain royalty payments based on tiered net sales of the acquired products. The sales-based milestones were assumed contingent liabilities from Medicines at the time of the acquisition.Changes to contingent consideration obligations can result from adjustments related, but not limited, to changes in discount rates and the number of remaining periods to which the discount rate is applied, updates in the assumed achievement or timing of any development or commercial milestone or changes in the probability of certain clinical events, changes in our forecasted sales of products acquired, the passage of time and changes in the assumed probability associated with regulatory approval. At the end of each reporting period, we revalue these obligations and record increases or decreases in their relative fair values in selling, general and administrative expenses within the accompanying consolidated statements of operations. Significant judgment is employed in determining the appropriateness of these assumptions as of the acquisition date and for each subsequent period. Accordingly, any change in the assumptions described above could have a material impact on the amount we may be obligated to pay as well as the results of our consolidated results of operations in any given reporting period. During the year ended December 31, 2018, we recorded adjustments to the liabilities discussed above, as discussed in Note 14.Advertising Expense—We record advertising expenses when they are incurred. We recognized advertising expense of $3,878, $1,234 and $1,066, in the years ended December 31, 2018, 2017, and 2016, respectively. Segment and Geographic Information—Operating segments are defined as components of an enterprise engaging in business activities for which discrete financial information is available and regularly reviewed by the chief operating decision maker in deciding how to allocate resources and in assessing performance. We operate and manage our business as one operating segment. Although substantially all of our license and contract research revenue is generated from agreements with companies that are domiciled outside of the U.S., we do not operate outside of the U.S., nor do we have any significant assets in any foreign country. See this Note 2 for further discussion of the license and contract research revenue.Research and Development Costs—Research and development costs are expensed as incurred and primarily include:employee-related expenses, which include salaries, benefits, travel and share-based compensation expense;fees paid to consultants and clinical research organizations (“CROs”) in connection with our pre-clinical and clinical trials, and other related clinical trial costs, such as investigator grants, patient screening, laboratory work and statistical compilation and analysis;costs related to acquiring and manufacturing clinical trial materials and costs for developing additional manufacturing sources for, and the manufacture of pre-approval inventory of, our drugs under development;costs related to compliance with regulatory requirements;consulting fees paid to third parties related to non-clinical research and development;research and laboratory supplies and facility costs; andlicense, research and milestone payments related to licensed technologies while the related drug is in development in the respective territory.Patent Costs—All patent-related costs incurred in connection with filing and prosecuting patent applications are expensed to selling, general and administrative expenses as incurred, as recoverability of such expenses is uncertain.Stock-Based Compensation— We account for share-based compensation following the provisions of ASC 718. We recognize stock-based compensation expense on a straight-line basis over the requisite service period of the individual grants, which is generally the vesting period, based on the estimated grant-date fair values. Stock options granted to employees typically vest over four years from the grant date and expire after 10 years. Stock options granted to non-employees are subject to periodic revaluation over their vesting terms. As a result, the charge to operations for non-employee options with vesting is affected each reporting period by changes in the fair value of the stock options.We have four active stock-based compensation plans, known as the Sixth Amended and Restated 2006 Stock Plan (the “2006 Plan”), the 2011 Equity Incentive Plan (the “2011 Incentive Plan”), the Melinta 2011 Equity Incentive Plan (the “Melinta 2011 Plan”) and the 2018 Stock Incentive Plan (the "2018 Plan"). Under these plans, restricted stock units, stock options and other stock-related awards may be granted to our directors, officers, employees and consultants. Stock options are granted at exercise prices not less than the estimated or actual fair market value of our common stock at the date of grant.We utilize the Black-Scholes option-pricing model for determining the estimated fair value of awards. Key inputs and assumptions include the expected term of the option, stock price volatility, risk-free interest rate, dividend yield, estimated fair value of our common stock, and exercise price. Many of the assumptions require significant judgment and any changes could have a material impact in the determination of stock-based compensation expense. We do not estimate forfeitures when recognizing compensation expense; instead, we recognize forfeitures as they occur.Comprehensive Loss—Comprehensive loss is equal to net loss as presented in the accompanying statements of operations.Income Taxes—We utilize the asset and liability method of accounting for income taxes, as set forth in ASC 740,Income Taxes. Under this method, deferred tax assets and liabilities are recognized based on the expected future tax consequences of temporary differences between the carrying amounts and the tax basis of assets and liabilities. A valuation allowance is established against net deferred tax assets if, based on the weight of available evidence, it is more likely than not that some or all of the net deferred tax assets will not be realized.We have recorded a full valuation allowance against deferred tax assets at each balance sheet date presented. Based on the available evidence, we do not believe that it is more likely than not that it will be able to utilize our deferred tax assets in the future.In accordance with the provisions of ASC 740, we would accrue for the estimated amount of taxes for uncertain tax positions if it is more likely than not that we would be required to pay such additional taxes. An uncertain tax position will not be recognized if it has a less than 50% likelihood of being sustained. Our policy is to recognize any interest and penalties related to income taxes in income tax expense. As of December 31, 2018 and 2017, we had no uncertain tax positions.Recently Issued and Adopted Accounting Pronouncements:In March 2016, the FASB issued ASU 2016-6, Contingent Put and Call Options in Debt Instruments, which clarifies that in assessing whether an embedded contingent put or call option is clearly and closely related to the debt host, an entity isrequired to perform only the four-step decision sequence in ASC 815-15-25-42 (as amended by ASU 2016-6), but it does not have to separately assess whether the event that triggers its ability to exercise the contingent option is itself indexed only to interest rates or credit risk. ASU 2016-6 is effective for financial statements issued for fiscal years beginning after December 15, 2017, and interim periods within fiscal years beginning after December 15, 2018 and requires the use of a modified retrospective transition approach. We adopted this guidance on January 1, 2018; the adoption had no material impact on our financial position, operations and cash flows.In January 2017, the FASB issued ASU 2017-1, Business Combinations (Topic 805) Clarifying the Definition of a Business, which narrows the definition of a business and requires an entity to evaluate if substantially all of the fair value of the gross assets acquired is concentrated in a single identifiable asset or a group of similar identifiable assets, which would not constitute the acquisition of a business. The guidance also requires a business to include at least one substantive process and narrows the definition of outputs. This guidance is effective for fiscal years beginning after December 15, 2017, and interim periods within those fiscal years, with early adoption permitted. We adopted this guidance as of January 1, 2018. The adoption did not have a material impact on our consolidated financial statements.In January 2017, the FASB issued ASU 2017-4, Intangibles - Goodwill and Other: Simplifying the Test for Goodwill Impairment, which removes step two from the goodwill impairment test. Step two measures a goodwill impairment loss by comparing the implied fair value of a reporting unit's goodwill with the carrying amount of that goodwill. The new guidance requires an entity to perform its annual, or interim, goodwill impairment test by comparing the fair value of a reporting unit with its carrying amount, including goodwill. An entity should recognize an impairment charge for the amount by which the carrying amount exceeds the reporting unit’s fair value, if any. The loss recognized should not exceed the total amount of goodwill allocated to that reporting unit. Additionally, an entity should consider income tax effects from any tax-deductible goodwill on the carrying amount of the reporting unit when measuring the goodwill impairment. The standard is effective for financial statements issued for fiscal years beginning after December 15, 2019. Early adoption is permitted for interim or annual goodwill impairment tests performed on testing dates after January 1, 2017. We early adopted this standard in connection with our annual goodwill impairment test on December 1, 2018.Recently Issued Accounting Pronouncements Not Yet AdoptedIn February 2016, the FASB issued ASU 2016-2, Leases, which requires lessees to recognize assets and liabilities for most leases with terms of more than 12 months on the balance sheet but recognize expense on the income statement in a manner similar to current accounting. The standard requires a modified retrospective approach for leases that exist or are entered into after the beginning of the earliest comparative period in the financial statements and is effective for us in the first quarter of 2019. In July 2018, the FASB issued ASU No. 2018-11, Targeted Improvements - Leases (Topic 842). This update provides an optional transition method that allows entities to elect to apply the standard at the adoption date and recognizes a cumulative-effect adjustment to the opening balance of retained earnings in the period of adoption. Consequently, the prior comparative period’s financials will remain the same as those previously presented. We have elected to apply the guidance at the beginning of the period of adoption and not restate comparative periods.We lease fleet vehicles as well as our research and administrative facility in New Haven, Connecticut, and our office facilities in Lincolnshire, Illinois, Chapel Hill, North Carolina and Morristown, New Jersey. We are evaluating the impact of ASU 2016-2, which we plan to adopt on January 1, 2019, on our consolidated financial statements. To date, we have identified all of our leases, we have evaluated practical expedients, and we are still evaluating our discount rates to apply to the leases. We anticipate that adoption of ASU 2016-2 will result in the recognition of additional right-to-use assets and lease liabilities of between $6,500 and $8,500.In August 2018, the FASB issued ASU 2018-13, Disclosure Framework-Changes to the Disclosure Requirements for Fair Value Measurement, which modifies or removes certain disclosure requirements in Topic 820, Fair Value Measurements. The update is effective for all entities for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2019. We are currently evaluating the impact of the adoption of this ASU on our financial statements.In August 2018, the FASB issued ASU 2018-15, Internal-Use Software (Subtopic 350-40): Customer's Accounting for Implementation Costs Incurred in a Cloud Computing Arrangement That Is a Service Contract, which provides guidance on implementation costs incurred in a cloud computing arrangement that is a service contract. The ASU amends Topic 350, Intangibles—Goodwill and Other, to include these implementation costs in its scope and clarifies that a customer should apply ASC 350-40 to determine which implementation costs should be capitalized. The update is effective for public entities for fiscal years beginning after December 15, 2019. we are currently evaluating the impact the adoption of this ASU will have on our financial statements.In November 2018, the FASB issued ASU 2018-18, Clarifying the Interaction between Topic 808 and Topic 606, which amends ASC 808 to clarify when transactions between participants in a collaborative arrangement under ASC 808 are within the scope of ASC 606, Revenue from Contracts with Customers. The ASU provides clarity in both ASC 606 and 808 regarding when transactions between collaborative arrangement participants should be accounted for as revenue under ASC 606. Theupdate is effective for public entities for fiscal years beginning after December 15, 2019. we are currently evaluating the impact the adoption of this ASU will have on our financial statements.NOTE 3 – BALANCE SHEET COMPONENTSCash, Cash Equivalents and Restricted Cash—Cash, cash equivalents and restricted cash, as presented on the Consolidated Statements of Cash Flows, consisted of the following: As of December 31, 2018 2017 Cash and cash equivalents $ 81,808 $ 128,387 Restricted cash (included in Other assets) 200 200 Total cash, cash equivalents and restricted cash shown in the Consolidated Statements of Cash Flows $ 82,008 $ 128,587 Accounts receivable—Accounts receivable consisted of the following: As of December 31, 2018 2017 Trade receivables $ 11,509 $ — Contracted services 10,293 7,202 Other receivables 683 362 Total receivables $ 22,485 $ 7,564 Inventory—Inventory consisted of the following: As of December 31, 2018 2017 Raw materials $ 24,507 $ 5,545 Work in process $ 11,700 $ 181 Finished goods 12,204 5,099 Gross value of inventory $ 48,411 $ 10,825 Less: valuation reserves $ (7,070 ) $ — Total inventory $ 41,341 $ 10,825 We review inventories on hand at least quarterly and record provisions for estimated excess, slow-moving and obsolete inventory, as well as inventory with a carrying value in excess of net realizable value. As of December 31, 2018, we had established reserves for our inventory totaling $7,070.Property and Equipment, Net—Property and equipment, net consisted of the following: As of December 31, 2018 2017 Laboratory equipment $ 3,387 $ 3,339 Manufacturing equipment 178 65 Office equipment 1,062 604 Purchased software 860 860 Furniture and fixtures 390 390 Leasehold improvements 4,943 4,869 Assets in development 301 437 Property and equipment, gross 11,121 10,564 Less-accumulated depreciation (9,535 ) (8,968 ) Property and equipment, net $ 1,586 $ 1,596 Depreciation expense relating to property and equipment was $570 and $451 in each of the years ended December 31, 2018 and 2017, respectively.Other Assets—Other assets consisted of the following: As of December 31, 2018 2017 Deerfield disbursement option (see Note 4) $ 7,608 $ — Long-term inventory deposits 51,127 — Other assets 2,391 1,213 Restricted cash 200 200 Total other assets $ 61,326 $ 1,413 The long-term inventory deposits relate primarily to prepayments we are required to make under long-term contracts for the manufacture and delivery of Vabomere inventory, which requires extended lead-times.Accrued Expenses—Accrued expenses consisted of the following: As of December 31, 2018 2017 Accrued contracted services $ 2,909 $ 5,596 Payroll-related expenses 15,585 9,885 Professional fees 3,598 3,621 Accrued royalty payments 2,052 2,040 Accrued sales allowances 5,630 — Accrued other 4,150 2,899 Total accrued expenses $ 33,924 $ 24,041 Accrued contracted services are primarily comprised of amounts owed to third-party clinical research organizations and contract manufacturers for research and development work performed on behalf of Melinta, and amounts owed to third-party marketing organizations for work performed to support the commercialization of our four products.Accrued payroll-related expenses are primarily comprised of accrued employee termination benefits, bonuses and vacation.NOTE 4 – FINANCING ARRANGEMENTSMelinta’s outstanding debt balances consisted of the following as of December 31, 2018 and 2017: As of December 31, 2018 2017 Principal balance under loan agreements $ 118,752 $ 40,000 Debt discount and deferred financing costs for loan agreements (8,276 ) (445 ) Total long-term debt, net of current maturities $ 110,476 $ 39,555 2014 Loan AgreementIn December 2014, we entered into an agreement with a lender pursuant to which we borrowed an initial term loan amount of $20,000 (the “2014 Loan Agreement”). In December 2015, pursuant to the achievement of certain milestones with respect to the terms in the 2014 Loan Agreement, we borrowed an additional term loan advance in the amount of $10,000.We were obligated to make monthly payments in arrears of interest only, at a rate of the greater of 8.25% or the sum of 8.25% plus the prime rate minus 4.5% per annum, commencing on January 1, 2015, and continuing on the first day of each successive month thereafter through and including June 1, 2016. Commencing on July 1, 2016, and continuing on the first day of each month through and including the maturity date of June 1, 2018, we were required to make consecutive equal monthly payments of principal and interest. In June 2017, we repaid the entire outstanding balance under the 2014 Loan Agreement (see discussion below under “2017 Loan Agreement”). In the years ended December 30, 2018 and 2017, we recognized $0 and $1,229 of interest expense, respectively, related to the 2014 Loan Agreement.2017 Loan AgreementOn May 2, 2017, we entered into a Loan and Security Agreement with a new lender (the “2017 Loan Agreement”). Under the 2017 Loan Agreement, the lender made available to us up to $80,000 in debt financing and up to $10,000 in equity financing.The 2017 Loan Agreement bore an annual interest rate equal to the greater of 8.25% or the sum of 8.25% plus the prime rate minus 4.5%. We were also required to pay the lender an end-of-term fee upon the termination of the arrangement. If the outstanding principal was at or below $40,000, the 2017 Loan Agreement required interest-only monthly payments for 18 months from the funding of the first tranche, at which time we would have had the option to pay the principal due or convert the outstanding loan to an interest plus royalty-bearing note.On June 28, 2017, we drew the first tranche of financing under the 2017 Loan Agreement, the gross proceeds of which were $30,000. We used the proceeds to retire amounts outstanding under the 2014 Loan Agreement. In August 2017, we drew the second tranche of financing, receiving $10,000. We retired the 2017 Loan Agreement in January 2018 (see discussion below).Deerfield FacilityOn January 5, 2018 (the “Agreement Date”), in connection with the IDB acquisition, we entered into the Deerfield Facility (the “Deerfield Facility”) with affiliates of Deerfield Management Company, L.P. (collectively, “Deerfield”). Pursuant to the terms of the Deerfield Facility, which was later amended in January 2019 (see discussion below), Deerfield agreed to loan us $147,774 as an initial disbursement (the “Term Loan”). The Deerfield Facility also provides us the right to draw from Deerfield additional disbursements up to $50,000 (the “Disbursement Option”), which may be made available upon the satisfaction of certain conditions, such as our having achieved annualized net sales of at least $75,000 before the end of 2019. The option to draw the additional $50 million expires on January 4, 2020. We agreed to pay Deerfield an upfront fee and a yield enhancement fee, both equal to 2.0% of the principal amount of the funds disbursed pursuant to the Deerfield Facility.The Term Loan bears interest at a rate of 11.75%, while funds distributed pursuant to the Disbursement Option will bear interest at a rate of 14.75%. We are also required to pay Deerfield an exit fee of 2.0% of the amount of any loans on the payment, repayment, redemption or prepayment thereof (later amended to 4%, as discussed below under Deerfield Facility Amendment). The principal of the Term Loan must be paid by January 5, 2024. The Deerfield Facility requires the outstanding principal amount of the Term Loan and any loans drawn pursuant to the Disbursement Option to be repaid in equal monthly cash amortization payments between the fourth and the sixth anniversary of the Agreement Date. The Term Loan and any loans drawn pursuant to the Disbursement Option are not permitted to be prepaid prior to January 6, 2021, under the terms of the Deerfield Facility and are subject to certain prepayment fees for prepayments occurring on or after such date. In addition, the Deerfield Facility permits us to secure a revolving credit line of up to $20,000 from a different lender, subject to working capital balances. Deerfield holds a first lien on all our assets, including our intellectual property, except for working capital accounts, for which they hold a second lien while any revolving credit line with a different lender is in place. The Deerfield Facility, while it is outstanding, limits our ability to raise debt financing in future periods outside of the $20,000 revolver permitted thereunder, to sell any assets, enter into partnerships, enter into any other debt, enter into capital leases, or pay dividends (however, as amended, it permits the execution of the Vatera Loan Agreement, discussed below). The Deerfield Facility, as amended, has a financial maintenance covenant requiring us to maintain a minimum cash balance of $40,000 through March 2020, and thereafter of $25,000, at all times, a requirement that we achieve net product sales of at least $63,750 during 2019, and other normal covenants, including periodic financial reporting and a restriction on the payment of dividends.In connection with the Deerfield Facility, we issued 625,569 shares of our common stock to Deerfield at a price of $67.50 on January 5, 2018, pursuant to a Securities Purchase Agreement. We received proceeds of $42,226 from this issuance of common stock. We received total proceeds of $190,000 from the Term Loan and the issuance of common stock together.We used these proceeds to fund the IDB acquisition, to retire the $40,000 of principal balance outstanding under the 2017 Loan Agreement and to fund ongoing working capital requirements and other general corporate expenses. As a result, we recognized a debt extinguishment loss of $2,595, comprised of prepayment penalties and exit fees related to retiring the 2017 Loan Agreement totaling $2,150 and unamortized debt issuance costs of $445.In connection with the Deerfield Facility and the Securities Purchase Agreement, we entered into the following freestanding instruments, with Deerfield as a counterparty, on January 5, 2018:Term Loan with stated principal of $147,774 with a 11.75% interest rate;Disbursement Option for additional draw of up to $50,000;625,569 shares of our common stock;Warrants to purchase 758,573 shares of our common stock with a purchase price of $82.50 and expiration date of January 5, 2025 (the “Warrants”); andRights to royalty payments equal to between 2.0% and 3.0% of certain U.S. sales of Vabomere for a period of 7 years, ending on December 31, 2024, as further described below (the “Royalty Agreement”).For accounting purposes, because there are multiple freestanding instruments within the arrangement to which we are required to assign value under U.S. GAAP, we performed a valuation to determine the allocation of the gross proceeds of $190,000 to the five financial instruments listed above. We first calculated the fair value of the warrants, and then we allocated the remaining proceeds across the other four instruments using the relative fair value approach. The relative fair values of these financial instruments, which approximated their respective fair values as of the Agreement Date, were as follows: Liabilities / (Assets) Term Loan $ 111,421 Warrants 33,263 Royalty Agreement 1,472 Disbursement Option (7,608 ) Common Stock Consideration 51,452 Total Consideration $ 190,000 The terms of these instruments and the methodology and assumptions used to value each of them are discussed below.Term LoanThe relative fair value of the term loan was estimated to be $111,421 using a discounted cash flow model (Level 3 inputs). We used a risk-adjusted discount rate of 19.8%. In connection with the Deerfield Facility, we paid $6,455 of upfront term loan fees and legal debt issuance costs. For accounting purposes, we elected to allocate these upfront fees and costs all to the term loan, leaving a net carrying value of $104,966. The upfront fees and costs were recorded as debt discount and are being amortized as additional interest expense over the term of the loan. In addition, a 2.0% exit fee of $2,956 is payable as the loan principal payments are made. Therefore, total required future cash payments are $150,730 (term loan principal of $147,774 plus exit fee of $2,956). The exit fee cost is also being amortized as additional interest expense over the life of the loan. The total cost of all items (cash-based interest payments, upfront fees and costs, and the 2.0% exit fee) is being expensed as interest expense using an effective interest rate of 21.4%. During the years ended December 31, 2018 and 2017, we recorded cash interest expense and term loan accretion expense of $17,460 and $5,510, and $0 and $0, respectively. All amounts were recorded as interest expense in our statement of operations.The accretion of the principal of the term loan and the future payments, including the 2.0% exit fee due at the end of the term, and excluding the 11.75% rate applied to the $147,774 note per the form of the Deerfield Facility, are as follows: BeginningBalance Accretion ofInterestExpense PrincipalPaymentsand Exit Fee Ending Balance January 5 - December 31, 2018 $ 104,966 $ 5,510 $ — $ 110,476 Year Ending December 31, 2019 110,476 7,135 — 117,611 Year Ending December 31, 2020 117,611 8,638 — 126,249 Year Ending December 31, 2021 126,249 10,799 — 137,048 Year Ending December 31, 2022 137,048 9,827 (69,084 ) 77,791 Year Ending December 31, 2023 77,791 3,846 (75,365 ) 6,272 Year Ending December 31, 2024 6,272 9 (6,281 ) — Total $ 45,764 $ (150,730 ) WarrantsUnder the terms of the Deerfield Facility, we issued Warrants to Deerfield to purchase 758,573 shares of common stock with an exercise price of $82.50 and a term of seven years. The holders of the Warrants may exercise the Warrants for cash, on a cashless basis or through a reduction of an amount of principal outstanding under the Term Loan or any subsequent disbursements pursuant to the Disbursement Option. In connection with certain major transactions (as defined therein), the holders may have the option to convert the Warrants, in whole or in part, into the right to receive the transaction consideration payable upon consummation of such major transaction in respect of a number of shares of common stock of the Company equal to the Black-Scholes value of the Warrants, as defined therein, and in the case of other major transactions, the holdersmay have the right to exercise the Warrants, in whole or in part, for a number of shares of common stock of the Company equal to the Black-Scholes value of the Warrants.We used the Black-Scholes option-pricing model to estimate the fair value of the Warrants (Level 3 inputs), which resulted in a fair value of $33,263 on the Agreement Date. To measure the Warrants at January 5, 2018, the assumptions used in the Black-Scholes option-pricing model were: the price of the common stock on January 5, 2018, an expected life of 7.0 years, a risk-free interest rate of 2.4% and an expected volatility of 50.0%.We classified the Warrants as a liability in our balance sheet and are required to remeasure the carrying value of these Warrants to fair value at each balance sheet date, with adjustments for changes in fair value recorded in Other income or expense in our statements of operations.To remeasure the Warrants at December 31, 2018, the assumptions used in the Black-Scholes option-pricing model were: the price of our common stock on December 31, 2018, an expected life of 6.0 years, a risk-free interest rate of 2.55% and an expected volatility of 50.0%. The fair value of the Warrants at December 31, 2018, was $38, resulting in a gain during the years ended December 31, 2018, of $33,226.Royalty Agreement In connection with the Deerfield Facility, we entered into a Royalty Agreement with Deerfield, pursuant to which we agreed to make royalty payments equal to 3.0% (or 2.0%, following the satisfaction of all our obligations under the Deerfield Facility and other loan documents) of annual U.S. sales of Vabomere exceeding $75,000 ($74,178 for 2018) and less than or equal to $500,000 for a seven-year period. To determine the fair value of the obligation under the Royalty Agreement, we applied a Monte Carlo simulation model to our revenue forecasts for Vabomere, which was discounted using an adjusted weighted average cost of capital (“WACC”). The WACC incorporated our estimated senior unsecured discount rate, our expected tax rate, and our estimated cost of equity, and then was adjusted for operational leverage.On January 5, 2018, we estimated the fair value of the royalty liability under the Royalty Agreement to be $1,472. Over the seven-year term, we will accrete the royalty liability using an effective interest rate of 42.9% and reduce the liability for any royalty payments made to Deerfield. During the year ended December 31, 2018 , we recorded accretion expense of $758. At the end of each quarter, we are required to prospectively revise the rate of accretion if there are any significant changes in our long-term sales forecasts. During the quarter ended December 31, 2018, we did not identify any significant changes in our sales forecasts and, accordingly, did not revise the rate of accretion. As we have not yet reached the $75,000 sales threshold for annual sales of Vabomere, we have not made any royalty payments to Deerfield.Disbursement OptionThe Disbursement Option allows us to draw additional funds up to $50,000 once we achieve annual net product sales of at least $75,000. The annual net sales target is measured by using the sales result for the preceding six months and multiplying by two. The disbursement must be drawn within two years from the effective date of the transaction and requires quarterly interest payments at a rate of 14.75% and requires the principal amount outstanding to be repaid in equal monthly cash payments between the fourth and the sixth anniversary of the effective date of the agreement.We calculated the fair value of the Disbursement Option using a discounted cash flow model (Level 3 inputs), under which estimated cash flows were discounted using a risk-adjusted rate that aligns with the lender’s estimated credit risk to disburse the $50,000. We estimated the relative fair value of the Disbursement Option to be $7,608 as of the effective date of the transaction, which we recorded as a long-term asset on our balance sheet to be carried at that value until settlement.Common Stock ConsiderationPursuant to the terms of the Securities Purchase Agreement, we issued 625,569 shares of our common stock to Deerfield at a price of $67.50 on January 5, 2018. Based on our closing stock price on January 5, 2018 , of $82.25 (Level 1 input), the fair value of this consideration was $51,452, which was recorded as additional paid-in capital in stockholders’ equity.Deerfield Facility AmendmentOn January 14, 2019, in conjunction with the Vatera Loan Agreement (discussed below),we entered into an amendment to the Deerfield Facility (the “Deerfield Facility Amendment”). The Deerfield Facility Amendment was a condition (among other conditions) to funding the Vatera Facility. The amendments set forth in the Deerfield Facility Amendment, except for specified amendments described below which were immediately effective, became effective upon the funding of the initial $75,000 disbursement under the Vatera Facility in February 2019.The Deerfield Facility Amendment(i) modifies the definition of “change of control” under the Deerfield Facility to permit Vatera Healthcare Partners LLC ("VHP"), Vatera Investment Partners LLC ("VIP" and, together with VHP, "Vatera") and their respective affiliates to own 50% or more of the equity interests in the Company on a fully diluted basis; (ii) modifies the definition of “Indebtedness” under the Deerfield Facility to exclude certain specific payments under (x) the Agreement andPlan of Merger, dated as of December 3, 2013, among the Medicines Company, Rempex Pharmaceuticals, Inc. and the other parties thereto and (y) the Purchase and Sale Agreement, dated as of November 28, 2017, between The Medicines Company and Melinta Therapeutics, Inc.; (iii) modifies the definition of “Permitted Indebtedness” under the Deerfield Facility to permit the payment of a certain amount of the interest on the Convertible Loans (described below) in cash; (iv) eliminates the requirement that the Company’s audited financial statements for the fiscal year ending December 31, 2018, be delivered without an explanatory paragraph expressing doubt as to the Company’s status as a going concern; (v) reduces the net sales covenant set forth in the Deerfield Facility for all periods after December 31, 2018, by 15%; (vi) requires the Company to hold a minimum cash balance of $40,000 through March 31, 2020, and thereafter $25,000 (the current requirement); (vii) increases the exit fee under the Deerfield Facility from 2% to 4%; and (viii) makes certain other technical modifications, including to accommodate the Vatera Facility and the Vatera Convertible Loans. Under the terms of the Deerfield Facility Amendment, $74,000 in principal amount of the loans under the Deerfield Facility (the “Deerfield Convertible Loan”) are convertible into shares of the Company’s common stock at Deerfield’s option at any time and evidenced by a convertible note (the “Deerfield Convertible Note”), subject to the 4.985% Ownership Cap as described below. The conversion price for the Deerfield Convertible Loan (the “Deerfield Convertible Loan Conversion Price”) is the greater of (i) $5.15, which is the minimum initial conversion price, subject to adjustment for stock splits (including a reverse split), stock combinations or similar transactions, and (ii) 95.0% of the lesser of (A) the closing price of the Company’s common stock on the trading day immediately preceding the conversion date and (B) the arithmetic average of the volume weighted average price of the Company’s common stock on each of the three trading days immediately preceding the conversion date. A lender’s ability to convert its portion of the Deerfield Convertible Loan is subject to a restriction that no lender will be permitted to convert such portion if it would result in such lender and its affiliates beneficially owning more than 4.985% of the total number of shares of the Company’s common stock outstanding (the “4.985% Ownership Cap”). However, that will not prevent a lender from periodically converting its portion of the loan up to the 4.985% Ownership Cap and then selling the shares such that up to $74,000 of the loan is converted over time. However, no lender may, without the approval of a majority of the Company’s board of directors, sell or dispose, in a pre-arranged single transaction or series of related transactions any shares of the Company’s common stock issued upon conversion of the Deerfield Convertible Loan to any person or group if such lender knows, in advance of effecting such transaction or series of related transactions, that such transferee holds, or after giving effect to such sale would hold, in excess of 15.0% of the issued and outstanding shares of the Company’s common stock. This 15.0% limitation does not apply if the sale is part of a tender offer or exchange offer made to all stockholders of the Company, or otherwise is in connection with a merger or other business combination transaction and also does not restrict the ability of any lender to transfer all or any portion of the Deerfield Convertible Note in accordance with its terms or to sell any shares of the Company’s common stock that have been issued upon conversion of the Deerfield Convertible Loan in open-market transactions.The Deerfield Convertible Loan continues to be secured by the collateral and the liens granted pursuant to the Deerfield Facility and related loan documents.The Deerfield Facility Amendment also amended the Deerfield Warrants to replace the 9.985% ownership cap set forth therein with a 4.985% Ownership Cap. As a result, the Company is subject to a restriction on its ability to satisfy interest due and payable through the issuance of the Company’s common stock to the extent that, upon such issuance, a holder, its affiliates and any “group” of which such holder is a member would beneficially own greater than 4.985% of the outstanding shares of the Company’s common stock.The Deerfield Facility Amendment also provides that the Deerfield Registration Rights Agreement will cover the shares of the Company’s common stock issuable upon conversion of the $5,000 of convertible loans that will be deemed funded by Deerfield upon the initial funding under the Vatera Loan Agreement (See Vatera Loan Agreement below).In addition to the funding of the initial $75,000 disbursement under the Vatera Facility, the effectiveness of the Deerfield Facility Amendment also was subject to the satisfaction (or waiver) of other customary conditions,In addition, the Company is required at all times after the effective date of the Deerfield Facility Amendment to reserve and keep available a sufficient number of shares of common stock for the purpose of enabling the Company to issue all of the underlying shares of common stock issuable pursuant to the Deerfield Convertible Note.Vatera Loan AgreementOn December 31, 2018, we entered into a Senior Subordinated Convertible Loan Agreement (the “Loan Agreement”) with Vatera for $135,000, and on January 14, 2019, we amended the Loan Agreement pursuant to which, among other things, Deerfield will be deemed to have funded an additional $5,000 of senior subordinated convertible loans (the "Convertible Loans") under the Vatera Loan Agreement as consideration for entering into the Deerfield Facility Amendment. No amount was drawn under the Loan Agreement as of December 31, 2018, as its effectiveness was contingent upon the satisfaction of several conditions.The proceeds of the Convertible Loans will be used for working capital and other general corporate purposes. The Convertible Loans are senior unsecured obligations of the Company and are contractually subordinated to the obligations under the Deerfield Facility. Interest on the Convertible Loans is 5% per year and will be paid in arrears at the end of each fiscal quarter, with 50% of such interest paid in cash and the remaining 50% of such interest paid in kind by increasing the principal balance of the outstanding Convertible Loans in an amount equal thereto (which increase will bear interest once added to such principal balance). The maturity date of the Convertible Loans is January 6, 2025.The Convertible Loans are convertible at Vatera's option into shares of convertible preferred stock of the Company at a conversion rate of 1.25 shares of preferred Stock per one thousand dollars. The preferred stock is further convertible at Vatera's option into shares of common stock of the Company at a rate of 100 shares of common stock per one share of preferred stock (the “Common Stock Conversion Rate”). At Vatera's option, the Convertible Loans are also directly convertible into common stock at an initial conversion rate equal to the Loan Conversion Rate multiplied by the Common Stock Conversion Rate. The preferred stock is non-participating, convertible preferred stock, with no dividend rights (other than to participate in common stock dividends on the Company’s common stock on an as-converted basis) or voting rights, and is senior to the common stock upon liquidation (with a liquidation preference equal to the Conversion Amount for the converted loans, as it may thereafter be adjusted pursuant to the Certificate of Designations (plus, if applicable, the amount of any declared but unpaid dividends on such shares of preferred stock)).An exit fee (the “Interim Exit Fee”) of 1% of the aggregate amount of Convertible Loans funded under the Loan Facility is payable upon repayment or conversion of such funded amount (payable in preferred stock in the case of conversion). In addition, an exit fee (the “Final Exit Fee” and, together with the Interim Exit Fee, the “Exit Fee”) of 3% on the portion of the aggregate committed amount of Convertible Loans not drawn by the Company under the Loan Facility is payable on any repayment in full or conversion in full of the Convertible Loans (payable in preferred stock in the case of conversion).Subject to the satisfaction (or waiver) of the conditions precedent set forth in the Loan Agreement, as amended, $75,000 of Convertible Loans may be drawn in a single draw on or prior to February 25, 2019, up to $25,000 of additional Convertible Loans may be drawn in a single draw after March 31, 2019, but on or prior to June 30, 2019, and up to $35,000 of additional Convertible Loans may be drawn in a single draw after June 30, 2019, but on or prior to July 10, 2019.Among the conditions precedent, the Loan Agreement required the approval of the shareholders of Melinta of measures to ensure the number of authorized shares of common stock was sufficient to accommodate the potential conversion of the Convertible Loans and approval of the issuance of the Convertible Loans, in accordance with Nasdaq rules. On January 19, 2019, at a Special Meeting of the shareholders, the shareholders approved both a reverse stock split and an increase of the authorized shares, as well as the issuance of the Convertible Notes. Melinta drew the first tranche of $75,000 on February 22, 2019.NOTE 5 – LICENSE AGREEMENTSEurofarmaIn December 2014, we entered into a Series 3-B Convertible Preferred Stock Purchase Agreement (the “Stock Purchase Agreement) and concurrent Distribution Agreement (the “Distribution Agreement”) and Supply Agreement (the “Supply Agreement”) with Eurofarma Laboratorios S.A. (“Eurofarma”). The Distribution Agreement and Supply Agreement, collectively, are referred to as the “Commercial Agreements.” Pursuant to the Stock Purchase Agreement, we agreed to issue to Eurofarma 1,052,474 shares of Series 3-B Convertible Preferred Stock at the purchase price of $13.301985 per share. Melinta received the proceeds of $14,000 from this stock sale on January 6, 2015, and thus, the purchaser’s right to the shares of Series 3-B Convertible Preferred Stock began accruing on that date.Under the Distribution Agreement, we appointed Eurofarma as our sole and exclusive distributor of Baxdela formulations in Brazil for use in various indications and Eurofarma agreed to commercialize Baxdela in Brazil for those indications. Eurofarma paid us $1,000 under the Distribution Agreement in January 2015. An additional $2,000 will be due upon the earlier of (i) approval of pricing to sell Baxdela in Brazil and (ii) Eurofarma’s first commercial sale of Baxdela in Brazil.We recorded approximately $6,000 of Series 3-B Convertible Preferred Stock and approximately $9,008 of deferred revenue in January 2015, the period when the funding provided by these agreements was received and the Series 3-B Convertible Preferred Stock was issued. Under ASC 605, the deferred revenue would be recognized as product is delivered under the Commercial Agreements.In August 2017, Melinta and Eurofarma entered into an amendment to the distribution and supply agreement to extend the licensed territory to substantially all of Central America and South America for upfront consideration of $1,000 (the “Eurofarma Amendment”) as well as other regulatory based milestones when and if they occur. Because the Eurofarma Amendment did not significantly change the nature of the deliverables under the arrangement, management concluded that it did not constitute a material modification of the original arrangement. As such, the $9,008 of deferred revenue recorded inconnection with the original agreement was appropriately deferred as of December 31, 2017, as we had not yet commenced commercial supply of Baxdela for the territory (Brazil).In addition, because the Eurofarma Amendment was negotiated at arms’ length, it was deemed to be a separate arrangement from the original contract. The Eurofarma Amendment has multiple elements, including the delivery of the license and the exclusive supply of Baxdela with respect to the licensed territories. We concluded that there was no standalone value for the delivered license in the Eurofarma Amendment; accordingly, the consideration of $1,000 was recorded as deferred revenue. Under ASC 605, we would commence recognition of the revenue when we begin to deliver under the supply agreement.We adopted Topic 606 on January 1, 2018, using the modified retrospective method applied to those contracts which were not complete as of January 1, 2018. In our adoption of Topic 606, we did not use practical expedients. In addition, we have considered the nature, amount and timing of our different revenue sources. Accordingly, the disaggregation of revenue from contracts with customers is reflected in different captions within the consolidated statement of operations. For our Eurofarma distribution arrangements under which revenue was previously deferred, revenue is now recognized at the point in time when the license is granted and has benefit to Eurofarma. These deferred revenues were originally expected to be recognized in future periods over the period of time over which we supplied Baxdela under the supply arrangement, which could have lasted up to 10 years or longer. The cumulative effect of the adoption was recognized as a decrease to opening accumulated deficit and a decrease to deferred revenue of $10,008 on January 1, 2018.In October 2018, Eurofarma received approval to sell Baxdela in Argentina, and, accordingly, we recognized milestone revenue of $500. Because the approval of Baxdela in Argentina was the first approval of Baxdela outside of the United States, we also recorded a milestone payable to Wakunaga of $1,200.MenariniIn February 2017, we executed a license agreement with A. Menarini Industrie Farmaceutiche Riunite S.r.l. (“Menarini”), a leading pharmaceutical company in Western Europe, under which we licensed rights to commercialize Baxdela in certain European, Asia-Pacific (except for Japan) and other rest-of-world territories (the “Agreement”). Pursuant to the terms and conditions of the Agreement, Menarini is entitled to exclusive rights to obtain product approval, procure supply from us and to commercialize Baxdela in the licensed territories, and we are entitled to receive regulatory, commercial and sales-based milestones as well as sales-based royalties on future net sales of Baxdela. In addition, we agreed with Menarini to share jointly in the future development cost of Baxdela. We received $19,905 upon the execution of the Agreement. On an ongoing basis, we receive reimbursement for 50% of the costs incurred for efforts to expand the applicable indications for Baxdela, and we may receive up to approximately €90,000 for regulatory, commercial and sales-based milestones as well as sales-based royalties on future sales of Baxdela.At the time the agreement was entered into, we identified two deliverables: the delivery of the Baxdela license to Menarini and the right to a related sublicense. While we are also providing development services in connection with the expansion of applicable indications for Baxdela, we are under no obligation to perform such services. In the event that we perform development services related to other indications of Baxdela, Menarini has the option to obtain the results of such services by reimbursing us for 50% of our related costs, and we have determined that Menarini’s option is not priced at a significant and incremental discount. To the extent that we are reimbursed for development services, such amounts will be recognized separately from the initial license.The agreement also states a separate Supply Agreement will be entered into at a future date under which Menarini will purchase Baxdela products from us until it can commence its own manufacturing. The pricing of Baxdela products under the Supply Agreement will not be at a significant, incremental discount. And, as noted above, we are entitled to receive up to approximately €90,000 for regulatory, commercial and sales-based milestones as well as royalties on future sales of Baxdela. We will recognize any future milestone payment received as revenue when Menarini achieves the milestone.For immediate use of the license and right to the sublicense, Menarini is able to leverage the information contained within the Baxdela NDAs, which were filed by us with the FDA in October 2016 for ABSSSI, to prepare the regulatory filings in the licensed territories. And, while the FDA approval was received in June 2017, regulatory approval in many of the licensed territories is not contingent upon U.S. FDA approval. We recognized $19,905, the consideration that was fixed and determinable at the inception of the agreement, upon delivery of the license and right to the sublicense in the first quarter of 2017, and we will recognize revenue associated with the development services as they are provided to Menarini. In the year ended December 31, 2018, we recognized revenue totaling $11,325 related to the development services. Of that amount, we have received $7,316 in cash payments; the balance of $4,009 is recorded in Receivables as of December 31, 2018.In connection with the Agreement, we paid Wakunaga Pharmaceutical Co. Ltd. (“Wakunaga”) $1,590, which was credited toward our future payment obligations under the license agreement we have with them for certain intellectual property underlying Baxdela. See Note 10 “Commitments and Contingencies” for further information on our license agreement withWakunaga. This expense was recorded in the year ended December 31, 2017, in selling, general and administrative expense, which is where we record all expenses related to intellectual property that are generated by events and activities outside our research and development activities. In this case, the payment was triggered by the receipt of upfront licensing fees from Menarini.In September 2018, we entered into a license agreement with Menarini, which grants to Menarini the exclusive right to market Vabomere, Orbactiv and Minocin for injection in 68 countries in Europe, Asia-Pacific and the Commonwealth of Independent States. The agreement includes an upfront license fee of €17,000 (which we received in October 2018), a milestone payment of €15,000 upon European marketing approval for Vabomere (which Menarini received in November 2018 and which we received in January 2019), potential regulatory- and sales-based milestones, and sales-based royalties. We determined that Menarini is a customer and we should account for the agreement under Topic 606. We identified one performance obligation under the agreement, the delivery of the licensed rights. In addition, we agreed to supply the products to Menarini at a cost that we concluded did not incorporate a significant incremental discount. We provided Menarini immediate access to the licensed rights and, as such, we allocated the upfront payment entirely to the license and recognized revenue at the point in time when the licensed rights were delivered, in September 2018. We will recognize any future contingent consideration, including milestone payments or royalty revenue, at such time when the milestones or royalties have been achieved.NOTE 6 – FAIR VALUE MEASUREMENTSThe provisions of the accounting standard for fair value define fair value as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. The transaction of selling an asset or transferring a liability is a hypothetical transaction at the measurement date, considered from the perspective of a market participant who holds the asset or owes the liability. Therefore, the objective of a fair value measurement is to determine the price that would be received when selling an asset or paid to transfer a liability (an exit price) at the measurement date. This standard classifies the inputs used to measure fair value into the following hierarchy:Level 1—Unadjusted quoted prices in active markets for identical assets or liabilities.Level 2—Unadjusted quoted prices in active markets for similar assets or liabilities, or unadjusted quoted prices for identical or similar assets or liabilities in markets that are not active, or inputs other than quoted prices that are observable for the asset or liability.Level 3—Unobservable inputs for the asset or liability.The following table lists our assets and liabilities that are measured at fair value and the level of inputs used to measure their fair value at December 31, 2018 and 2017. The money market fund is included in cash & cash equivalents on the balance sheet; the other items are in the captioned line of the balance sheet. As of December 31, 2018 Level 1 Level 2 Level 3 Total Assets: Money market fund $ 32,883 $ — $ — $ 32,883 Total assets at fair value $ 32,883 $ — $ — $ 32,883 Liabilities: Current royalty contingent consideration from IDB acquisition $ — $ — $ (1,006 ) $ (1,006 ) Long-term royalty contingent consideration from IDB acquisition — — (4,708 ) (4,708 ) Warrant liability — — (38 ) (38 ) Total liabilities at fair value $ — $ — $ (5,752 ) $ (5,752 ) As of December 31, 2017 Level 1 Level 2 Level 3 Total Assets: Money market fund $ 76,777 $ — $ — $ 76,777 Total assets at fair value $ 76,777 $ — $ — $ 76,777 The following tables summarize the changes in fair value of our Level 3 liabilities for the years ended December 31, 2018 and 2017:Level 3 Liabilities Fair Value at December 31,
2017 Accretion Recorded in interest Expense Change in Unrealized Gains(Losses) (Issuances) Settlements, Net Net Transfer (In) Out of Level 3 Fair Value at December 31,
2018Current royalty contingent consideration from IDB acquisition $ — $ (1,055 ) $ (1,151 ) $ 1,200 $ — $ (1,006 ) Long-term royalty contingent consideration from IDB acquisition $ — $ (5,489 ) $ 11,468 $ (10,687 ) $ — $ (4,708 ) Contingent milestone payment $ — (4,015 ) (1,500 ) (24,485 ) 30,000 $ — Warrant liability $ — $ — $ 33,225 $ (33,263 ) $ — $ (38 ) Total liabilities at fair value $ — $ (10,559 ) $ 42,042 $ (67,235 ) $ 30,000 $ (5,752 ) Level 3 Liabilities Fair Value at December 31,
2016 Accretion Recorded in interest Expense Change in Unrealized Gains(Losses) Reclassification to APIC Net Transfer (In) Out of Level 3 Fair Value at December 31,
2017Preferred stock warrants $ (674 ) $ — $ 335 $ 339 $ — $ — Total liabilities at fair value $ (674 ) $ — $ 335 $ 339 $ — $ — NOTE 7 – SHAREHOLDERS' EQUITYAt December 31, 2018, our certificate of incorporation, as amended, authorizes us to issue up to 80,000,000 shares of $0.001 par value common stock and 5,000,000 shares of $0.001 par value preferred stock. Each share of common stock entitles the holder to one vote on all matters submitted to a vote of our shareholders. Holders of our common stock are not entitled to receive dividends, unless declared by the board of directors. There have been no dividends declared to date. We have reserved and keep available out of our authorized but unissued common stock a sufficient number of shares of common stock to execute the conversion of all issued and outstanding warrants and stock options. On January 19, 2019, our shareholders voted to approve a reverse stock split and on February 20, 2019, our board authorized a one-for-five reverse split, which was effective on February 22, 2019. Earnings per share and all share information in this Annual Report on Form 10-K have been adjusted to reflect the split.On January 5, 2018, we issued 662,740 shares of common stock to Medicines as part of the purchase price of IDB (see Note 14 for further discussion). We also issued 625,569 shares of common stock and warrants to purchase 758,573 shares of common stock to Deerfield as part of the Deerfield Facility (see Note 4 for further discussion). In conjunction with the IDB transaction, we received $40,000 in additional equity financing from existing and new investors, in exchange for which we issued 576,992 shares of common stock. Further, in May 2018, we issued 4,928,000 shares of common stock to new and existing investors in a follow-on public offering for proceeds, net of issuance costs, of $115,273. And, during the year ended December 31, 2018, we issued 10,920 shares of common stock for restricted stock units that vested in the period.WarrantsWe have warrants to purchase our common stock outstanding at December 31, 2018, as follows: Issued WarrantsOutstanding Exercise Price Expiration February 2012 8 $ 86,670.35 February 2019 December 2014 6,757 $ 166.50 December 2024 December 2015 1,351 $ 166.50 December 2024 January 2018 758,573 $ 82.50 January 2025 Total 766,689 NOTE 8 – STOCK-BASED COMPENSATION2006 Stock Plan—Upon closing the merger with Cempra, Inc. (“Cempra”) on November 3, 2017, Melinta assumedthe 2006 Stock Plan, which hadbeenadopted by Cempra in January 2006 (the “2006 Plan”). The 2006 Plan provided for the granting of incentive share options, nonqualified share options and restricted shares to Company employees, representatives and consultants. As of December 31, 2018, there were options for an aggregate of 2,344 shares issued and outstanding under the 2006 Plan. During the period January 1, 2018, to December 31, 2018, 11,328 options were forfeited; there was no other activity during the period.2011 Equity Incentive Plan—Upon closing the merger with Cempra on November 3, 2017, Melinta assumed the 2011 Equity Incentive Plan, which had been adopted by Cempra in October 2011 (the “2011 Incentive Plan”). On January 1, 2018, under the evergreen feature of the 2011 Incentive Plan, the authorized shares under the 2011 Incentive Plan increased by 175,991 to 523,889. In April 2018, we awarded 321,193 shares to employees with an exercise price of $37.25 and a grant date fair value of $27.05. On June 12, 2018, the shareholders approved the adoption of the 2018 Stock Incentive Plan (the “2018 Plan”) (see below). With the adoption of the 2018 Plan, the 2011 Incentive Plan was frozen. At December 31, 2018, there were 304,883 shares outstanding under the 2011 Incentive Plan and no shares available to award as either options or restricted stock units.Private Melinta 2011 Equity Incentive Plan—In November 2011, the Melinta board of directors adopted the 2011 Equity Incentive Plan (“Melinta 2011 Plan”). The Melinta 2011 Plan provided for the granting of incentive stock options, nonqualified options, stock grants, and stock-based awards to employees, directors, and consultants of the Company. On November 3, 2017, in conjunction with the merger with Cempra, all outstanding options under the Melinta 2011 Plan converted to 146,499 options to purchase common shares of Cempra (re-named Melinta in the merger), the Melinta 2011 Plan was frozen and authorized shares under the Melinta 2011 Plan were reduced to 146,499. Any grants under the Melinta 2011 Plan that expire or are forfeited will reduce the authorized shares under the plan. As of December 31, 2018, we had 97,706 shares of common stock reserved under the Melinta 2011 Plan for issuance upon exercise of stock options.2018 Stock Incentive Plan—OnApril 20, 2018, we granted stock options to purchase 173,053 shares of common stock under the 2018 Plan at an exercise price of $37.25 and an average grant date fair value of $27.65, as measured on June 12, 2018, the date the grants received shareholder approval. The grants were subject to, and contingent upon, shareholder approval of the 2018 Plan at the annual meeting in June 2018. On June 12, 2018, the shareholders approved the 2018 Plan, which was initially authorized with 400,000 shares. Under the evergreen feature of the 2018 Plan, these authorized shares may be increased on January 1 of each of the first three years following the year in which the 2018 Plan was adopted by the lesser of (i) 4% of the outstanding shares of the Company on the last day of the immediately preceding fiscal year, or (ii) such number of shares determined by the compensation committee of the board of directors. Upon approval of the 2018 Plan in June 2018, the fair value of the stock options granted on April 20, 2018, was established at $28.71, which is the basis for the expense recognition. An additional 128,920 options were granted at various dates between May 2018 and December 2018, with a weighted-average exercise price of $26.80 and a weighted-average fair value of $18.35.The 2018 Plan replaces the 2011 Incentive Plan, and no further equity awards will be granted from the 2011 Incentive Plan, which has been frozen. Any shares that are undelivered as a result of outstanding awards under the 2011 Incentive Plan expiring or being canceled, forfeited or settled in cash without the delivery of the full number of shares to which the award related will become available for grant under the 2018 Plan. As of December 31, 2018, there were 235,323 shares available for awards under the 2018 Plan.In connection with his appointment as interim Chief Executive Officer in October 2018, Mr. Johnson received an option to purchase 30,000 common shares at a strike price of $14.00 and a grant date fair value of $4.75, and a grant of 10,000 restricted stock units. The options will vest ratably over 12 months, and the restricted stock units will cliff vest after 12 months.On February 19, 2019, the shareholders of Melinta approved a one-for-five reverse stock split, effective on February 22, 2019, which had the effect of reducing all existing awards by a factor of five and increasing all strike prices by a factor of five. All share and per share amounts in this Annual Report on Form 10-K have been adjusted to reflect this reverse stock split. Inaddition, they approved the addition of 400,000 shares to the authorized shares for the 2018 Plan for grants to our chief executive officer and the addition of 600,000 shares for awards for executive management and other employees.Inducement Grants—On November 3, 2017, Melinta granted Daniel Wechsler, our former President and Chief Executive Officer, an option to purchase 110,196 shares of common stock, at a strike price of $58.25 per share and a grant date fair value of $41.80, and 36,732 restricted stock units, pursuant to the option and restricted stock unit inducement agreements made with Mr. Wechsler. Both grants were to vest over four years, 25% after one year and then ratably monthly over the remaining 36 months. In October 2018, our board of directors appointed John H. Johnson as Interim Chief Executive Officer to succeed Daniel Wechsler, who stepped down from his role as President, CEO and director to pursue other opportunities. In connection therewith, Mr. Wechsler forfeited all of his inducement grant stock options and all but 9,183 of his restricted stock units, which vested on December 31, 2018.On September 21, 2018, Melinta granted Peter J. Milligan, our recently appointed Chief Financial Officer, an option to purchase 74,000 shares of common stock, at a strike price of $22.50 per share and a grant date fair value of $16.55, pursuant to the option inducement agreement made with Mr. Milligan. The grant will vest over four years, 25% after one year and then ratably monthly over the remaining 36 months.Stock Option Activity—The exercise price of each stock option issued under all of the stock plans is specified by the board of directors at the time of grant, but cannot be less than 100% of the fair market value of the stock on the grant date. In addition, the vesting period is determined by the board of directors at the time of the grant and specified in the applicable option agreement. Our practice is to issue new shares upon the exercise of options.All options granted under either the 2018 Plan, the 2011 Incentive Plan or the Melinta 2011 Plan during the years ended December 31, 2018 and 2017, were granted with exercise prices not less than the fair market value of our common stock on the grant date, as approved by our board of directors.A summary of the stock option activity under the 2006 Plan is presented in the table below: Number ofOptions WeightedAverageExercisePrice WeightedAverageContractualTerm (in years) AggregateIntrinsicValueOutstanding - November 3, 2017 13,672 $ 54.00 1.2 Outstanding - December 31, 2017 13,672 54.00 1.1 $ 342 Forfeited (11,328 ) $ 54.41 Outstanding - December 31, 2018 2,344 $ 52.25 1.4 $ — Exercisable - December 31, 2018 2,344 $ 52.25 1.4 $ — Vested and expected to vest at December 31, 2018 2,344 $ 52.25 1.4 $ — A summary of the stock-option activity under the 2011 Incentive Plan is presented in the table below: Number ofOptions WeightedAverageExercisePrice WeightedAverageContractualTerm (in years) AggregateIntrinsicValueOutstanding - November 3, 2017 149,060 $ 300.10 6.2 Exercised (172 ) 78.15 Forfeited (1,289 ) 140.00 Expired (3,056 ) 322.05 Outstanding - December 31, 2017 144,543 $ 301.30 3.9 $ 113 Granted 321,193 37.25 Exercised (40 ) 78.05 Forfeited (78,513 ) 46.80 Expired (89,660 ) 312.85 Outstanding - December 31, 2018 297,523 $ 79.95 8.8 $ — Exercisable - December 31, 2018 51,328 $ 278.30 6.2 $ — Vested and expected to vest at December 31, 2018 297,521 $ 79.95 8.8 $ — A summary of the stock-option activity under the Melinta 2011 Plan is presented in the table below: Number ofOptions WeightedAverageExercisePrice WeightedAverageContractualTerm (in years) AggregateIntrinsicValueOutstanding - December 31, 2015 4,362,836 $ 3.00 8.5 Granted 1,240,499 3.55 Exercised (31,685 ) 2.05 Canceled/expired (331,209 ) 3.85 Outstanding - December 31, 2016 5,240,441 3.10 7.9 Granted 1,830,199 2.40 Exercised (26,366 ) 3.60 Canceled/expired (797,571 ) 2.55 Conversion (6,102,901 ) (2 ) Outstanding - December 31, 2017 143,802 $ 129.40 7.9 Exercised (25,480 ) (1 ) 135.05 (1 ) Canceled/expired (20,616 ) (1 ) 151.90 (1 ) Outstanding - December 31, 2018 97,706 (1 ) $ 123.15 (1 ) 5.1 $ — Exercisable - December 31, 2018 81,419 (1 ) $ 124.15 (1 ) 4.5 $ — Vested and expected to vest at December 31, 2018 97,703 (1 ) $ 123.15 (1 ) 5.1 $ — (1) Amounts are after conversion(2) Options outstanding on November 3, 2017, were converted to options to purchase common shares of Cempra (re-named Melinta in the merger) at a 0.0229 conversion ratioThe weighted-average grant-date per share fair value of options granted under the Melinta 2011 Plan during the years ended December 31, 2018, 2017 and 2016 was $0.00, $1.30, and $2.15, respectively ($0.00, $37.00 and $31.60, respectively, after conversion and re-valuation on November 3, 2017). The total fair value of options that vested under the Melinta 2011 Plan during the years ended December 31, 2018, 2017 and 2016, was approximately $690, $2,900 and $2,300, respectively. The intrinsic value of options exercised in 2018 was not material.A summary of the stock-option activity under the 2018 Plan is presented in the table below: Number ofOptions WeightedAverageExercisePrice WeightedAverageContractualTerm (in years) AggregateIntrinsicValueOutstanding - December 31, 2017 — Granted 301,973 32.80 Canceled/expired (76,650 ) 37.41 Outstanding - December 31, 2018 225,323 $ 31.21 9.5 $ — Exercisable - December 31, 2018 14,402 $ 29.20 8.9 $ — Vested and expected to vest at December 31, 2018 225,323 $ 31.21 9.5 $ — The weighted-average grant-date per share fair value of options granted under the 2018 Plan during the years ended December 31, 2018, 2017 and 2016 was $27.65, $0.00 and $0.00, respectively. The total fair value of options that vested under the 2018 Plan during the years ended December 31, 2018, 2017 and 2016, was approximately $285, $0 and $0, respectively.The following table summarizes certain information about all options outstanding as of December 31, 2018: Options Outstanding Options Exercisable Exercise Price Number of Options Weighted Average Remaining Contractual Term (in years) Number of Options Weighted Average Remaining Contractual Term (in years) $10.60 - $25.00 147,720 9.8 5,000 9.8 $25.01 - $50.00 402,896 9.3 18,150 8.5 $50.01 - $75.00 8,004 5.7 7,504 5.6 $75.01 - $100.00 31,826 4.4 28,639 4.0 $100.01 - $125.00 31,569 6.8 17,697 5.6 $125.01 - $1,085.75 74,875 5.0 72,188 4.9 696,890 149,178 Stock-Based Compensation—We use a Black-Scholes option-pricing model for determining the estimated fair value for stock-based awards. The Black-Scholes option-pricing model requires the use of the subjective assumptions in order to determine the fair value of stock-based awards.On November 3, 2017, in conjunction with the merger with Cempra, all outstanding options under the Melinta 2011 Plan converted to 732,499 options to purchase common shares of Cempra (re-named Melinta in the merger). For accounting purposes, this was treated as a modification of the awards, generating a remeasurement of the estimated fair value of all vested and unvested Melinta 2011 Plan outstanding stock awards on the day of the stock conversion. After taking into consideration the terms of the Melinta 2011 Plan, the total remeasured estimated fair value of $2,362 on November 3, 2017, for the vested stock options was immediately recorded as additional stock-based compensation expense in the fourth quarter of 2017, since the options were fully vested and had no future service requirement. The remeasured fair value of the unvested options of $2,476 is being recognized as expense over the remaining service period of each grant. On November 3, 2017, all outstanding stock options issued under the assumed 2006 Plan and 2011 Incentive Plan were remeasured to estimated fair value on the day assumed by Melinta. The estimated fair value of $2,626 on the day of acquisition of the acquired vested stock options related to prior service periods was recorded as additional purchase price for the acquisition of Cempra (see Note 14). The remeasured fair values will be used to record stock-based compensation expense for the unvested shares over the remaining service period for each grant. Due to the different remaining contractual terms and remaining vesting periods of outstanding stock options on the day of merger from both the assumed 2011 Incentive Plan and Melinta 2011 Plan, a range of Black-Scholes assumptions were used to estimate the fair value on November 3, 2017.The range of assumptions used to value stock option grants for all plans—plus the vested stock option grants under the Melinta 2011 Plan which were remeasured on the merger date—were as follows: 2018 2017 2016 Risk-free interest rate 2.6% - 3.1% 1.8% - 2.1% 1.5 % Weighted-average volatility 84.8% - 85.6% 87.5% - 108.1% 67.1 % Expected term - employee awards (in years) 1.0 - 6.1 3.1 - 6.1 6.0 Forfeiture rate — % — % — % Dividend yield 0.00 % 0.00 % 0.00 % The range of assumptions used calculate the estimated value of the acquire vested stock options under the 2006 Plan and the 2011 Equity Plan that was recorded as additional purchase price for the acquisition of Cempra at the merger date were as follows (the wide range of volatility was due to the various remaining expected terms for the options, some of which were less than one year):November 3, 2017 Risk-free interest rate 1.2% - 2.0% Weighted-average volatility 51.0% - 147.4% Expected term - employee awards (in years) 0.2-5.6 Forfeiture rate 0.00 % Dividend yield 0.00 % Risk-Free Interest Rate—The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant for zero coupon U.S. Treasury notes with maturities approximately equal to the option’s expected term.Weighted-average Volatility—After the merger with Cempra, all outstanding options are to purchase common shares of Cempra (re-named Melinta in the merger). Since these shares are publicly traded, we primarily used the historical volatility information for the publicly traded shares. Prior to the merger, the Company had been privately held since inception. Therefore, there was no specific historical or implied volatility information available. Accordingly, prior to the merger, we determine volatility based on an average of reported volatility of selected peer companies in the pharmaceutical and biotechnology industry in a similar stage of development.Expected Term—Our historical exercise behavior for previous grants does not provide a reasonable estimate for future exercise activity for employees who have been awarded stock options in the past three years. Therefore, the average expected term was calculated using the simplified method, as defined by GAAP, for estimating the expected term.Forfeiture Rate—On January 1, 2016, Melinta adopted the guidance in ASU 2016-9, Improvements to Employee Share-Based Payment Accounting, and changed its accounting policy for stock-based compensation to recognize stock option forfeitures as they occur rather than estimating an expected amount of forfeitures. Expected Dividend Yield—We have never declared or paid any cash dividends and do not expect to pay any cash dividends in the foreseeable future.In 2016 and 2017, Cempra issued time-vested restricted stock units (RSUs) from the 2011 Incentive Plan to certain employees, subject to continuous service with us at the vesting time. When vested, the grants represented the right to be issued the number of shares of our common stock that is equal to the number of RSUs granted. On November 3, 2017, all outstanding RSUs issued under the acquired 2011 Incentive Plan were remeasured to fair value on the day acquired by Melinta. The estimated fair value of $834 on day of acquisition related to the prior service periods for unvested RSUs was recorded as additional purchase price for the acquisition of Cempra (see Note 3). After the merger, certain Cempra employees were terminated. Under their severance agreements and equity award clauses, the vesting of 16,400 RSUs was accelerated on the date of termination. In accordance with ASC 718, $573 of additional compensation expense was recognized in the fourth quarter of 2018, with the accelerated vesting. 12,400 common shares under the accelerated awards were issued in 2018, and 4,000 common shares will be issued in 2019 for the remaining accelerated awards.A summary of the activity related to our RSUs (except for the inducement grant discussed above) is as follows: Number ofRestrictedStock UnitsOutstanding WeightedAverageFair ValueBalance - November 3, 2017 40,400 $ 58.25 Vested and issued (12,400 ) 58.25 Forfeited (600 ) 58.25 Balance - December 31, 2017 27,400 58.25 Granted 10,000 14.00 Vested and issued (10,920 ) 58.25 Forfeited (5,120 ) 58.25 Balance - December 31, 2018 21,360 32.75 Vested at December 31, 2018 4,000 $ 58.25 Stock-based compensation reported in our statements of operations was as follows: Year Ended December 31, 2018 2017 2016 Cost of goods sold $ 48 $ — $ — Research and development 750 651 1,169 General and administrative 2,667 5,799 1,346 Total $ 3,465 $ 6,450 $ 2,515 Stock-based compensation expense for our manufacturing-related employees of $209 was capitalized in inventory as a component of overhead expense and recognized as cost of goods sold based on inventory turns. No related tax benefitsassociated with stock-based compensation expense have been recognized and no related tax benefits have been realized from the exercise of stock options due to our net operating loss carryforwards.Total aggregate unrecognized stock-based compensation cost under all the plans and inducement grants as of December 31, 2018 and the Melinta 2011 Plan on December 31, 2017, was $9,783 and $4,703, respectively. The unrecognized stock-based compensation as of December 31, 2018, will be recognized over a weighted-average period of 3.4 years.NOTE 9 – INCOME TAXESThe Company utilizes the liability method of accounting for income taxes and deferred taxes which are determined based on the differences between the financial statements and tax basis of assets and liabilities given the provisions of the enacted tax laws. In assessing the realizability of the deferred tax assets, the Company considered whether it is more likely than not that some portion or all of the deferred tax assets will not be realized through the generation of future taxable income. In making this determination, the Company assessed all of the evidence available at the time including recent earnings, forecasted income projections, and historical financial performance. The Company has fully reserved deferred tax assets as a result of this assessment.The income tax expenses (benefits) from continuing operations are summarized as follows: 2018 2017 Federal: Current $ — $ — Deferred — — — — State: Current (139 ) (103 ) Deferred — — (139 ) (103 ) Total $ (139 ) $ (103 ) The provision for income taxes differs from income taxes computed at the federal statutory tax rates for the years ended December 31, 2018 and 2017, due to the following items (presented as benefits/(expenses)): 2018 2017 Federal Statutory rate 21.0 % 34.0 % State income taxes, net of federal income tax benefit 17.7 0.2 Bargain purchase gain — 15.9 Transaction cost — (2.3 ) Interest expense — (2.3 ) Impact of change in fair value of tranche assets and liabilities 4.4 2.0 Other permanent differences (0.2 ) (2.0 ) Federal tax rate change — (75.3 ) Change in valuation allowance (14.1 ) 28.0 Research and development tax credits 1.2 3.3 Prior year adjustment (1.0 ) — Section 382 adjustment (28.9 ) — Other — (1.3 ) Effective income tax rate 0.1 % 0.2 % On November 3, 2017, we completed our tax-free merger with Cempra. To reflect the opening balance sheet deferred tax assets and liabilities of Cempra, we recorded a net deferred tax asset of $107,688 offset with valuation allowance of $107,688. (See Note 3 for further information regarding the merger.) During the fourth quarter, we completed a Section 382 study and reduced the opening balance sheet deferred tax assets and liabilities by $101,195 which was offset with a valuation allowance of $101,195 to record the reduction of the tax attributes from the section 382 analysis.On December 22, 2017, the Tax Cuts and Jobs Act of 2017 (the “Act”) was signed into law making significant changes to the Internal Revenue Code. Changes include, but not limited to, a corporate tax decrease from 34% to 21% effective for tax years beginning after December 31, 2017, limitation of the business interest deduction, modification of the net operating loss deduction, reduction of the business tax credit for qualified clinical testing expenses for certain drugs for rare diseases or conditions, and acceleration of depreciation for certain assets placed into service after September 27, 2017. On December 22, 2017, the SEC staff issued Staff Accounting Bulletin No. 118 (“SAB 118”), which provides guidance on accounting for the tax effects of the Tax Act. SAB 118 provides a measurement period that should not extend beyond one year from the Tax Act enactment date for companies to complete the accounting under ASC 740, Income Taxes. In accordance with SAB 118, a company must reflect the income tax effects of those aspects of the Tax Act for which the accounting under ASC 740 is complete. To the extent that a company’s accounting for certain income tax effects of the Tax Act is incomplete but it is able to determine a reasonable estimate, it must record and provisional estimate in the financial statement. During the fourth quarter, we completed accounting for the impact of the Act, and, as a result of purchase accounting adjustments, have recorded $83,007 as an additional income tax expense offset with $83,007 tax benefit from the change in the valuation allowance. The $83,007 of income tax expense included an adjustment of $82,013 related to the reduction of certain tax attributes as a result of the Section 382 analysis completed in 2018. The tax effects of the temporary differences and net operating losses that give rise to significant portions of deferred tax assets are as follows (in thousands): 2018 2017 Deferred tax assets: Net operating loss carryforwards $ 94,596 $ 172,481 Tax credit carryforwards 7,409 24,924 Deferred revenue — 3,364 Fixed assets 677 593 Stock compensation expense 2,195 2,503 Intangibles 12,026 3,978 Interest expense carryforward 7,617 — Others 4,273 2,151 Total deferred tax assets 128,793 209,994 Less valuation allowance (128,793 ) (209,994 ) Net deferred tax assets $ — $ — We have established a full valuation allowance because we do not believe that it is more likely than not that we will generate sufficient taxable income to realize the deferred tax assets nor recognize any benefits from our net operating losses, tax credits, and other deferred tax assets. For the year ended December 31, 2018, our valuation allowance increased by $22,443, excluding the deferred tax asset valuation allowance of $101,195 associated with the Cempra opening balance sheet adjustment and $2,449 from the adoption of Topic 606.We have determined that our ability to utilize our previously generated federal net operating losses and federal tax credits would be limited under Sections 382 and 383 of the Internal Revenue Code (“Section 382”). The limitations under Section 382 apply if an ownership change, as defined by Section 382, occurs. Generally, an ownership change occurs when certain shareholders increase their aggregated ownership by more than 50 percentage points over their lowest ownership percentage in a testing period (typically three years). We determined that certain deferred tax assets will be subject to Section 382 limitations due to previous ownership changes, specifically associated with our recapitalization in 2012 and from the November 2017 tax-free merger with Cempra. During the fourth quarter of 2018, we adjusted total Cempra deferred tax assets by $101,195 and recorded a corresponding valuation allowance of $101,195 as a result of Section 382 limitations pursuant to the study performed. Also during the fourth quarter of 2018, we adjusted total Melinta deferred tax assets by $26,126 and recorded a corresponding valuation allowance of $26,126 as a result of Section 382 limitations pursuant to the study performed.As of December 31, 2018, we had approximately $267,597 of gross Federal net operating loss carryforwards and $7,409 in tax credit carryforwards that are available to offset taxable income in the future. The tax credit carryforwards will begin to expire in 2037. The federal net operating loss carryforwards begin to expire in 2020. State net operating loss carryforwards and tax credit carryforwards, on a tax-effected basis and net of federal tax benefits, are $38,401 and $5,010, respectively. The state net operating loss carryforwards begin to expire in 2020.The Company files its tax returns as prescribed by the tax laws of the jurisdictions in which it operates. With few exceptions, the major jurisdictions subject to examination by the relevant tax authorities, and open tax years, stated as the Company's fiscal years, are as follows:Jurisdiction Open Tax Years U.S. Federal 2015 - 2017 U.S. State 2014 - 2017 ASC 740, Income Taxes, prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. Only tax positions that meet the more-likely-than-not recognition threshold at the effective date may be recognized.As of December 31, 2018, we did not have any unrecognized tax benefits. To the extent penalties and interest would be assessed on any underpayment of income tax, our policy is that such amounts would be accrued and classified as a component of income tax expense in the financial statements. To date, we have not recorded any such interest or penalties.NOTE 10 – COMMITMENTS AND CONTINGENCIESOperating LeasesWe are a lessee under lease agreements, as follows:a lease for our principal research facility at 300 George Street, New Haven, Connecticut, that expires in August 2021;a lease for our administrative facility at 300 Tri-State International, Lincolnshire, Illinois, that expires in March 2022;A lease for our administrative office at 44 Whippany Road, Morristown, New Jersey, that expires in February 2024; andthree leases for our office facilities at 6320 Quadrangle Drive, Chapel Hill, North Carolina, the last of which expires in March 2021. One of the spaces under lease in Chapel Hill has been sub-leased through the termination date of the lease, March 2021.The terms of the Connecticut lease provide for even rental payments over the life of the lease; the terms of the other leases provide for rental payments on a graduated scale. We are required to pay a proportionate share of building operating expenses for all locations. We recognize rent expense on a straight-line basis over the noncancelable lease term of each lease.We also lease vehicles for many of our field-based employees, principally sales representatives. At December 31, 2018, we had approximately 210 leased vehicles.Rent expense under operating leases for facilities, vehicles and equipment was $1,747, $907 and $690 for the years ended December 31, 2018, 2017 and 2016, respectively. As of December 31, 2018, minimum operating lease payments under noncancelable leases (as amended) were as follows: Amounts 2019 $ 2,796 2020 2,710 2021 2,327 2022 1,233 2023 and thereafter 886 Total future minimum payments * $ 9,952 *Minimum payments have not been reduced by minimum sublease rentals of $271 due in the future under noncancelable subleasesIn May 2017, we entered into a master fleet agreement with Automotive Rentals, Inc. (“ARI”) under which we lease vehicles for certain field-based employees. Under the master fleet agreement, each vehicle is leased under a separate lease agreement for a term of up to four years. All other terms of the individual leases are essentially identical. In connection with the fleet agreement, in June 2017, we issued to ARI a $200 letter of credit, which auto-renews annually. As of December 31, 2018, we had vehicles under lease with annual minimum lease payments totaling approximately $1,091. We have determined that these vehicle leases are operating leases under current U. S. GAAP because (i) we have no right to purchase the vehicles at any time, (ii) the future minimum lease payments are less than 90% of the fair value of the vehicles at the time of lease and (iii) ouruse of the vehicles does not exceed 75% of the vehicles useful lives. Accordingly, we are recognizing the lease payments as expense as incurred.License Agreements with Future PaymentsWe are parties to several license agreements, under which we will be required to make payments based on the achievement of agreed-upon milestones or circumstances. As of December 31, 2018, we were not obligated to make any of the future payments discussed below.Yale University. In December 2001, we entered into an exclusive license agreement with Yale University (“Yale”) under which we obtained an exclusive right to use certain technology related to the high-resolution X-ray crystal structure of a 50S ribosome through the term of Yale’s patent rights on such technology. In return, we issued 61 shares of our common stock. The fair value of the shares of $35 was charged to operations in 2001. In September 2004 and December 2009, the license agreement was amended to include additional 50S ribosome technology and 70S ribosome technology owned by Yale, and we paid Yale license fees of $15 upon each amendment. We use the licensed technology in our ESKAPE pathogen program. We are obligated to certain diligence requirements and have the right to grant sublicenses to third parties, although Yale is entitled to a portion of payments received from the sublicensees. Under the license agreement, we may be required to make payments to Yale of upto $900 upon achieving certain regulatory approval milestones for each of the first three products developed under the license. In accordance with the license agreement, Yale is also entitled to receive percentage royalty payments in the single digits based on net sales, if any, of products using the subject matter of the license. Upon the occurrence of certain events, Yale has the right to terminate the license agreement upon 60 days’ written notice to us, should we fail to make a material payment under the agreement, commit a material breach of the agreement, fail to carry insurance required by the agreement, cease to carry on our business, or become subject to bankruptcy or a similar insolvency event. We have the right to terminate the license agreement upon 90 days’ written notice to Yale. Unless earlier terminated, the agreement will continue in effect until the last of the licensed patents expires.Medical Research Council. In March 2005, we entered into an exclusive license agreement with the Medical Research Council (“MRC”) under which we acquired rights to certain patent applications and other intellectual property related to the high-resolution X-ray crystal structure of a 30S ribosome through the term of the MRC’s patent rights on such technology. Upon entering into the license agreement, we paid the MRC a license fee of $10. We use the licensed technology in our ESKAPE pathogen program. We are obligated to certain diligence requirements and have the right to grant sublicenses to third parties. Under the license agreement, we may be required to pay the MRC an aggregate of $610 upon the achievement of specified development and regulatory approval milestones for a pharmaceutical product and $100 for a diagnostic product. In accordance with the license agreement, the MRC is also entitled to receive percentage royalty payments in the single digits based on net sales, if any, of licensed pharmaceutical and diagnostic products. We and the MRC have the right to terminate the license agreement upon 30 days’ written notice if the other party commits a material breach of the agreement or an insolvency event occurs with respect to the other party, and the MRC may terminate the agreement if we challenge the protection of the licensed patent rights and know-how. Unless earlier terminated, the term of the agreement continues until the expiration of the last to expire claim of the licensed patent rights on a country-by-country basis.Wakunaga Pharmaceutical Co., Ltd. In May 2006, we and Wakunaga executed a license agreement under which we acquired rights to certain patents, patent applications, and other intellectual property related to Baxdela. To date, we have made $11,600 of nonrefundable payments to Wakunaga. Under the license, we have the right to grant sublicenses, although Wakunaga is entitled to a substantial portion of non-royalty income received from a sublicense of the Wakunaga technology. Pursuant to an amendment of the license agreement in November 2012, and later amended in May 2017, the future payments under the license agreement were adjusted. The license agreement, as amended, provided for potential additional future payments of up to $9,000 to Wakunaga upon the achievement of specified development and regulatory milestones, in addition to potential future sales milestone payments, and tiered royalty payments in the single digits on net sales, if any, of the licensed product. Of the $9,000, we paid Wakunaga $1,590 in March 2017 in connection with our license and collaboration agreement with Menarini (see Note 5) and we owed $6,000 in June 2017 in connection with the FDA approval of Baxdela, of which we paid $4,000 in 2017; the remaining $2,000 was paid in June 2018. In addition, we have paid Wakunaga $200 in connection with licensing and milestone payments we received from Eurofarma. Wakunaga has certain termination rights, should we fail to perform our obligations under the agreement, we become subject to bankruptcy or similar events, or our business is transferred or sold and the successor requires us to terminate a substantial part of our development activities under the agreement. We have the right to terminate the license for cause upon six months’ written notice to Wakunaga. Unless earlier terminated, the license agreement will continue in effect on a country-by-country and product‑by‑product basis until we is no longer required to pay any royalties, which is the later of the date the manufacture, use or sale of a licensed product in a country is no longer covered by a valid patent claim, or a specified number of years following the first commercial sale in such country. The remaining $1,210 of license payments became due when Eurofarma received approval to market Baxdela in Argentina. The payment has been recorded in accrued liabilities as of December 31, 2018, and paid in the first quarter of 2019.CyDex Pharmaceuticals, Inc. In November 2010, we entered into a license and supply agreement with CyDex Pharmaceuticals, Inc. (now a wholly-owned subsidiary of Ligand Pharmaceuticals Incorporated, both hereafter referred to as Ligand) under which we obtained an exclusive right, under certain patents and patent applications, to use Ligand’s beta sulfobutyl cyclodextrin, Captisol, in our development and commercialization of a Baxdela product. In addition, under the terms of the license agreement, we obtained a nonexclusive license to Ligand’s Captisol data package. Upon entering into the license agreement, we made a nonrefundable payment of $300 to Ligand. In January 2011, May 2013, October 2016 and July 2017, we made milestone payments to Ligand under the agreement of $150, $500, $1,500 and $1,500, respectively. We are obligated to certain diligence requirements and have the right to grant sublicenses to third parties. The license agreement provides for payments of up to $600 to Ligand upon the achievement of future development and commercial milestones, and obligations to make percentage royalty payments in the single digits based on net sales, if any, of the licensed product. Additionally, we have agreed to purchase our requirements of Captisol from Ligand for use in a Baxdela product, with pricing established pursuant to a tiered pricing schedule. Ligand has certain rights to terminate the agreement following a cure period, should we fail to perform our obligations under the agreement. In addition, Ligand may terminate the agreement immediately if we fail to pay milestones or royalties due under the agreement or if we become subject to bankruptcy or similar events. We have the right to terminate the license upon 90 days’ written notice to Ligand. Unless earlier terminated, the agreement will continue in effect until the expiration of our obligation to pay royalties. Such obligation expires, on a country-by-country basis, over a specified number of years following the expiration date of the last valid claim of a licensed product in the country of sale; if there has never been a valid claim of a licensed product in the country of sale, then such number of years after the first sale of the licensed product in such country.Radezolid. In December 2014, we entered into a license agreement with a contract research organization (“CRO”) for the development and commercialization of Radezolid in topical formulations for a variety of dermatological indications. Melinta retains the option to co-develop or fully regain rights to Radezolid upon completion of specific development milestones. In March 2016 and February 2017, we paid milestones totaling $900 and $450, respectively, to the CRO under this license agreement, which was recorded as an expense when paid. In addition, in 2017 we agreed to reimburse the CRO up to $250 of certain development expenses, of which we have paid $154 through December 31, 2018.Commitments Assumed in the Merger with Cempra, Inc.Optimer Pharmaceuticals, Inc. (now owned by Merck). In March 2006, Cempra entered into a Collaborative Research and Development and License Agreement with Optimer, a biotechnology company focused on discovering, developing and commercializing innovative anti-infective products. Under this agreement, we obtained access to a library of over 500 macrolide compounds, including solithromycin. Optimer was acquired by Cubist in October 2013, which in turn was acquired by Merck in 2015. Optimer granted us an exclusive license to these compounds in all countries of the world except the member-nations of the Association of Southeast Asian Nations (“ASEAN”)—which are Brunei, Cambodia, Indonesia, Laos, Malaysia, Myanmar (Burma), the Philippines, Singapore, Thailand and Vietnam—with the right to sublicense, under Optimer’s patents and know-how related to certain macrolide and ketolide antibiotics and related proprietary technology. The exclusivity of our license is potentially subject to the U.S. government’s right to obtain a non-exclusive, irrevocable, royalty-free, paid-up right to practice and have practiced certain patents worldwide. As partial consideration for granting us such license, we issued shares of our common stock to Optimer. We also have an obligation to make additional payments upon achievement of specified development, regulatory and commercialization milestones. The aggregate amount of such milestone payments we may need to pay is based in part on the number of products developed under the agreement. The aggregate amount would be $27,500 if four products are developed and gain FDA approval. Additional limited milestone payments would be due if we develop more than four products. In July 2010 and July 2012, Cempra made $500 and $1,000 milestone payments, respectively, to Optimer after our successful completion of the Phase 1 and Phase 2 trials for oral solithromycin. If we decide to pursue further development of solithromycin, we will owe a milestone payment of $9,500 upon FDA approval of solithromycin. We are also obligated to make tiered, mid-single-digit royalty payments to Optimer based on annual net sales of licensed products outside the ASEAN countries, which royalties are subject to reduction in the event additional licenses are obtained from third parties in order to practice our rights under the agreement and/or we are required to grant a compulsory license to a third party.The agreement also includes the grant of an exclusive license to Optimer and its affiliates, with rights of sublicense, under our patents and other intellectual property in any products covered by the agreement to permit Optimer to develop and/or commercialize such products in ASEAN countries. In consideration of such license, Optimer will pay us $1,000 in milestone payments for the first two products that receive regulatory approval or have a first commercial sale in any ASEAN country, as well as tiered, mid-single-digit royalty payments based on net sales of such products, which royalties are subject to reduction in the event additional licenses are obtained from third parties in order to practice Optimer’s rights under the agreement and/or Optimer is required to grant a compulsory license to a third party. The agreement also included a collaborative research program, to be performed by the parties, which was completed on March 31, 2008.Subject to certain exceptions, on a country-by-country and product-by-product basis, a party’s rights and obligations under the agreement continue until the later of: (i) the expiration of the last to expire patent rights of a covered product in theapplicable country or (ii) 10 years from the first commercial sale of a covered product in the applicable country. As a result, the final expiration date of the Optimer license is indeterminable until the last such patents issue and results of potential patent extensions are known, or each of the first commercial sales are made, as applicable. Upon expiration of the agreement with respect to a particular product and country, the licenses granted in the agreement with respect to such product and country will remain in effect and convert to a perpetual, unrestricted, fully-paid, royalty-free, worldwide license. Either party may terminate the agreement (i) in the event of a material breach by the other party, subject to prior notice and the opportunity to cure, (ii) in the event the other party fails to use diligent efforts to develop and commercialize products in its respective territory, or if the other party makes a determination not to develop and commercialize at least one product under the agreement, or (iii) in the event of the other party’s bankruptcy. In the case of these terminations, the terminating party can elect that all licenses granted by the other party survive, subject to continuing royalty, payment and other obligations. Additionally, either party may terminate the agreement for any reason upon 30 days’ prior written notice, in which case the non-terminating party can elect that all licenses granted by the other party survive, subject to continuing royalty, payment, and other obligations.The Scripps Research Institute. Effective June 12, 2012, Cempra entered into a license agreement with The Scripps Research Institute (“TSRI”), whereby TSRI licensed rights to us, with rights of sublicense, to make, use, sell, and import products for human or animal therapeutic use that use or incorporate one or more macrolides as an active pharmaceutical ingredient and is covered by certain patent rights owned by TSRI claiming technology related to copper-catalyzed ligation of azides and acetylenes. The rights licensed to us are exclusive as to the People’s Republic of China (excluding Hong Kong), South Korea and Australia, and are non-exclusive in all other countries worldwide, except ASEAN, which are not included in the territory of the license. Under the terms of the agreement with TSRI, Cempra paid a one-time only, non-refundable license issue fee in the amount of $350 which was charged to research and development expense in the second quarter of 2012. Our rights under the agreement are subject to certain customary rights of the U.S. government that arise or result from TSRI’s receipt of research support from the U.S. government.Cempra was also obligated to pay annual maintenance fees to TSRI in the amount of (i) $50 each year for the first three years (beginning on the first anniversary of the agreement), and (ii) $85 each year thereafter (beginning on the fourth anniversary of the agreement). Each calendar year’s annual maintenance fees will be credited against sales royalties due under the agreement for such calendar year. Under the terms of the agreement, we must pay TSRI low single-digit percentage royalties on the net sales of the products covered by the TSRI patents for the life of the TSRI patents, a low single-digit percentage of non-royalty sublicensing revenue received with respect to countries in the nonexclusive territory and a mid-single-digit percentage of sublicensing revenue received with respect to countries in the exclusive territory, with the sublicensing revenue royalty in the exclusive territory and the sales royalties subject to certain reductions under certain circumstances. TSRI is eligible to receive milestone payments of up to $1,100 with respect to regulatory approval in the exclusive territory and first commercial sale, in each of the exclusive territory and nonexclusive territory, of the first licensed product to achieve those milestones that is based upon each macrolide covered by the licensed patents. Each milestone is payable once per each macrolide. Each milestone payment made to TSRI with respect to a particular milestone will be creditable against any payment due to TSRI with respect to any sublicense revenues received in connection with the achievement of such milestone. Pursuant to the terms of the Optimer Agreement, any payments made to TSRI under this license for territories subject to the Optimer Agreement can be deducted from any sales-based royalty payments due under the Optimer Agreement up to a certain percentage reduction of the royalties due to Optimer.Under the terms of the agreement, we are also required to pay additional fees on royalties, sublicensing and milestone payments if we, an affiliate with TSRI, or a sublicensee challenges the validity or enforceability of any of the patents licensed under the agreement. Such increased payments would be required until all patent claims subject to challenge are invalidated in the particular country where such challenge was mounted. The term of the license agreement (and the period during which we must pay royalties to TSRI in a particular country for a particular product) will end, on a country-by-country and product-by-product basis, at such time as no patent rights licensed from TSRI cover a particular product in the particular country.TSRI may terminate the agreement in the event (i) we fail to cure any non-payment or default on our indemnity or insurance obligations, (ii) we declare insolvency or bankruptcy, (iii) if we are convicted of a felony relating to the development, manufacture, use, marketing, distribution or sale of any products licensed under the agreement, (iv) we fail to cure any underreporting or underpayment by a certain amount in any 12-month period, or (v) we fail to cure any default on any other obligation under the agreement. We may terminate the agreement with or without cause upon written notice. In the event of such termination, (i) all licenses granted to us will terminate except in the case of any sublicensee that was not the cause of the termination, is not in default on its obligations under its sublicense, and that pays any unpaid amounts owed by us under the agreement with respect to the sublicense, and (ii) we may complete any work in progress and sell any completed inventory on hand for a period of time after termination.BARDA. In May 2013, Cempra entered into an agreement with the BARDA for the evaluation and development of solithromycin for the treatment of bacterial infections in pediatric populations and infections caused by bioterror threat pathogens, specifically anthrax and tularemia.The agreement is a cost-plus fixed fee development contract, with a base performance segment valued at approximately $18,700, and four optional work segments that BARDA may request in its sole discretion. If all 4 option segments are requested, the cumulative value of the agreement would be approximately $68,200 and the estimated period of performance would be until approximately May 2018. Three of the options are cost plus fixed fee arrangements, and one option is a cost sharing arrangement for which we are responsible for a designated portion of the costs associated with that work segment. The period of performance for the base performance segment was May 2013 through February 2016.BARDA exercised the second option in November 2014. The value of the second option work segment is approximately $16,000 and the period of performance was November 2014 through April 2017. In February 2016, BARDA exercised the third option work segment of the agreement, which is intended to fund a Phase 2/3 study of intravenous, oral capsule and oral suspension formulations of solithromycin in pediatric patients from two months old to 17 years with CABP. This option is a cost sharing arrangement under which BARDA will contribute $25,500 and we will be responsible for an additional designated portion of the costs associated with the work segment. In September 2016, the contract was modified to increase the third option work segment by $8,000 for increased manufacturing work related to the development of a second supply source for solithromycin. The amendment raises the value of the third option work segment to approximately $33,500.In the first quarter of 2018, we agreed with BARDA to terminate the agreement and wind down the study of solithromycin. We continued to incur expenses--and receive BARDA grant income--into the fourth quarter of 2018, when the study and BARDA both terminated.Toyama Chemical Co., Ltd. Under our agreement with Optimer, we have global rights (other than ASEAN countries) to solithromycin. In May 2013, Cempra entered into a license agreement with Toyama whereby it licensed to Toyama the exclusive right, with the right to sublicense, to make, use and sell any product in Japan that incorporates solithromycin as its sole API for human therapeutic uses, other than for ophthalmic indications or any condition, disease or affliction of the ophthalmic tissues. Toyama also has a nonexclusive license in Japan and certain other countries, with the right to sublicense, to manufacture or have manufactured API for solithromycin for use in manufacturing such products, subject to limitations and obligations of the concurrently executed supply agreement discussed below. Toyama has granted us certain rights to intellectual property generated by Toyama under the license agreement with respect to solithromycin or licensed products for use with such products outside Japan or with other solithromycin-based products inside or outside Japan. Toyama has successfully completed a Phase 1 trial in healthy Japanese volunteers, a Phase 1 trial to measure solithromycin levels in the upper respiratory tract, and a Phase 2trial in CABP. In December 2016,Toyama initiated Phase 3 trials in patients infected with CABP and other respiratory infections. Toyama and we are sharing the results of our respective development activities.As consideration for the execution of the license agreement, Toyama paid us an upfront payment of $10,000. Toyama is also obligated to pay us up to an aggregate of $60,000 in milestone payments, depending on the achievement of various regulatory, patent, development and commercial milestones. The first of these milestones was achieved in the third quarter of 2014 for which Cempra received a payment of $10,000 from Toyama. In March 2015, Cempra recognized a $10,000 milestone from Toyama based on the Japan Patent Office issuing a Decision of Allowance for our patent covering certain crystal forms of solithromycin in Japan, which payment was received in April 2015. In October 2016, Cempra received a $10,000 milestone payment when Toyama decided to progress to Phase 3 studies. Under the terms of the license agreement, Toyama must also pay us a royalty equal to a low-to-high first double decimal digit percentage of net sales, subject to downward adjustment in certain circumstances.The term of the license agreement (and the period during which Toyama must pay royalties under the license agreement) will end, on a product-by-product basis, at the later of: (i) such time as no patent rights under the agreement cover a particular licensed product in Japan; (ii) 15 years after such product is first launched in Japan, or (iii) the first commercial sale in Japan by a third party of a generic equivalent of such licensed product.Toyama may terminate the license agreement (i) at any time, with or without cause, upon advance notice to us, (ii) upon the occurrence of any serious adverse effect in any human clinical trial of any licensed product that would significantly impact the long term commercial viability of a licensed product in Japan, or (iii) upon our failure to obtain the issuance of certain patents or file for U.S. regulatory approvals by certain dates, or to continue certain key clinical trials. We may terminate the license agreement if Toyama or any of its sublicensees is convicted of a felony relating to the development, manufacture, use, marketing, distribution or sale of a licensed product, or upon Toyama’s failure to (i) initiate certain clinical trials in Japan by certain dates, (ii) obtain regulatory approval in Japan within a certain period of completing certain clinical trials in Japan, (iii) launch and commercialize approved licensed products in Japan within a certain period of approval, (iv) use commercially reasonable efforts to market and sell licensed products, or (v) achieve expected benchmarks for net sales of licensed products. Either party may terminate the license agreement due to the other party’s insolvency or for uncured material breach.As part of the license agreement, Toyama and Cempra also entered into a supply agreement, whereby we will be the exclusive supplier (with certain limitations) to Toyama and its sublicensees of API for solithromycin for use in licensed products in Japan, as well as the exclusive supplier to Toyama and its sublicensees of finished forms of solithromycin to beused in clinical trials in Japan. Pursuant to the supply agreement, Toyama will pay us for such clinical supply of finished product and all supplies of API for solithromycin for any purpose, other than the manufacture of products for commercial sale in Japan, at prices equal to our costs. All API for solithromycin supplied by us to Toyama for use in the manufacture of finished product for commercial sale in Japan will be ordered from us at fixed prices determined by our agreement, which currently exceeds our expected cost to supply such API. Accordingly, we recorded $5,330 as part of the Cempra merger fair value adjustments upon assuming this agreement. The supply agreement will continue until the expiration or termination of the license agreement. Either party may terminate the supply agreement for an uncured material breach or in the event of insolvency of the other party, with Toyama’s right to terminate for our breach subject to certain further conditions in the case of our failure to supply API for solithromycin or clinical supply.On September 26, 2018, Cempra Pharmaceuticals, Inc. (“CPI”), a wholly-owned subsidiary of Melinta and Toyama entered into the following agreements related to the development of solithromycin : (i) Amendment No. 2 to the Exclusive License and Development Agreement, dated as of May 8, 2013 and amended as of September 26, 2013, by and between CPI and Toyama, (ii) Amendment to the Quality Agreement effective as of February 1, 2017 by and among CPI, Fujifilm Finechemicals Co., Ltd. (“FFFC,” now known as Fujifilm Wako Pure Chemical Corporation (“FFWK”), and Toyama, and (iii) Agreement to Assign the API Manufacturing and Supply Agreement, entered into as of December 16, 2015, by and between CPI and FFFC. In addition, the parties terminated the Supply Agreement between CPI and Toyama dated May 8, 2013, which related to supply of the active pharmaceutical ingredient used in the manufacture of solithromycin (“API”) and clinical supply. These agreements are referred to collectively as the "Amended Toyama Arrangement."Under the terms of the Amended Toyama Arrangement, CPI is legally relieved of all obligations related to the supply of API or clinical supply to Toyama, which resulted in the extinguishment of the $5,330 long-term liability, representing the fair value of the loss contract upon our merger with Cempra in November 2017. We recognized this gain in other income in the statement of operations in September 2018. In consideration for this relief, Cempra granted Toyama the right to manufacture and procure such API and clinical supply, and forfeited rights to all future milestone payments related to Toyama’s development of solithromycin in Japan. In addition, Melinta will be entitled to receive royalties on sales of solithromycin by Toyama if and when the product receives regulatory approval in Japan, at a rate generally between 4% and 6% of net sales. The amended terms have been treated as a contract modification, and under the sales- or usage- based royalty exception in Topic 606, we do not estimate variable consideration from future royalties. As such, we will not recognize royalty revenue under this arrangement until the future sales occur and royalties are earned.FUJIFILM Finechemicals Co., Ltd. In January 2016, Cempra entered into a supply agreement with FUJIFILM Finechemicals Co., Ltd., which is intended to provide us with solithromycin in sufficient quantities and at reasonable prices to ensure we meet our obligation to Toyama under the supply agreement. We may be subject to a minimum purchase obligation for a designated number of years in the event of the successful completion of a manufacturing facility to be built and validation studies to be conducted by FFFC that could run to $80,000 in the aggregate, which expense would be reduced by any supply sold to Toyama. The agreement’s initial term runs until December 16, 2025. After the end of the initial term, and at the end of each year thereafter, the term will automatically extend for an additional year unless either party gives written notice to the other of its intent to terminate within a designated period of time prior to the expiration of the term, in which case the agreement will terminate at the end of such term. The parties may at any time terminate the agreement by mutual written consent. Each party has the right to terminate the agreement immediately if there is a product failure, the other party becomes involved in bankruptcy, insolvency or similar proceedings or materially breaches the agreement and such breach remains uncured for a period of time following notice of the breach. A violation by us of the minimum purchase obligation is considered a material breach. We have the right to terminate the agreement upon written notice if there is a supply failure. We also may terminate in the event that FFFC cannot provide us with solithromycin for more than a designated period of time. Upon termination, any unfulfilled binding portion of the forecast must be delivered by FFFC and paid for by us. We also may elect to purchase the remaining inventory of FFFC’s solithromycin and any remaining raw materials. If FFFC terminates the agreement for a material breach by us and, prior to such termination, (i) FFFC has constructed a facility in Japan for the primary purpose of manufacturing API for us under the agreement and (ii) such facility is completed and fully operational and qualified for the manufacture of API for delivery thereunder, then, except to the extent otherwise agreed to by the parties, we may be subject to declining penalties that could aggregate as much as $17,500. As stated above, on September 26, 2018, we entered into a series of agreements, including one with FFFC, which assigned our rights and obligations under the supply agreement to Toyama. We have no further obligations or commercial relationship with FFFC.All payments made under these license agreements have been expensed as research and development expenses in our statements of operations.ContingenciesAs discussed in Note 14, on November 3, 2017, Melinta merged with Cempra, Inc. in a business combination. Prior to the merger, on November 4, 2016, a securities class action lawsuit was commenced in the United States District Court, Middle District of North Carolina, Durham Division, naming Cempra, Inc. (now known as Melinta Therapeutics, Inc.) (for purposes of this Contingencies section, “Cempra”) and certain of Cempra’s officers as defendants. Two substantially similar lawsuits were filed in the United States District Court, Middle District of North Carolina on November 22, 2016, and December 30, 2016, respectively. Pursuant to the Private Securities Litigation Reform Act, on July 6, 2017, the court consolidated the three lawsuits into a single action and appointed a lead plaintiff and co-lead counsel in the consolidated case. On August 16, 2017, the plaintiff filed a consolidated amended complaint. Plaintiff alleged violations of the Securities Exchange Act of 1934 (the “Exchange Act”) in connection with allegedly false and misleading statements made by the defendants between July 7, 2015 and November 4, 2016 (the “Class Period”). Plaintiff sought to represent a class comprised of purchasers of Cempra’s common stock during the Class Period and sought damages, costs and expenses and such other relief as determined by the court. On September 29, 2017, the defendants filed a motion to dismiss the consolidated amended complaint. After the motion to dismiss was fully briefed, the court heard oral arguments on July 24, 2018. On October 26, 2018, the court granted Defendants' motion to dismiss and dismissed the plaintiff's consolidated amended complaint in its entirety. On November 21, 2018, the plaintiff filed its notice of appeal, and on December 20, 2018, the Fourth Circuit entered its briefing schedule. The appellant filed its brief on January 28, 2019 and the appellee filed its response brief on February 27, 2019. The appellant's reply brief is due on March 20, 2019. Cempra believes it has meritorious defenses and intends to defend the lawsuit vigorously. It is possible that similar lawsuits may yet be filed in the same or other courts that name the same or additional defendants.On December 21, 2016, a shareholder derivative lawsuit was commenced in the North Carolina Durham County Superior Court, naming certain of Cempra’s former and current officers and directors as defendants and Cempra as a nominal defendant, and asserting claims for breach of fiduciary duty, unjust enrichment, abuse of control, gross mismanagement, and corporate waste (the “December 2016 Action”). A substantially similar lawsuit was filed in the North Carolina Durham County Superior Court on February 16, 2017 (the “February 2017 Action”). The complaints are based on similar allegations as asserted in the securities lawsuits described above, and seek unspecified damages and attorneys’ fees. Both cases were served and transferred to the North Carolina Business Court as mandatory complex business cases. The Business Court consolidated the February 2017 Action into the December 2016 Action and appointed counsel for the plaintiff in the December 2016 Action as lead counsel. On July 6, 2017, the court stayed the action pending resolution of the putative securities class action. That stay was then lifted. The plaintiff filed an amended complaint on December 29, 2017, and was required to file a further amended complaint by February 6, 2018. On February 6, 2018, the plaintiff filed his second amended complaint. On March 8, 2018, defendants filed their motion to dismiss or, in the alternative, stay plaintiff’s second amended complaint. On April 9, 2018, plaintiff filed his opposition to defendants’ motion. Defendants’ filed their reply on April 26, 2018. On June 27, 2018, the parties filed a joint stipulation and consent order to stay the case until (1) 30 days after a final order dismissing the putative securities class action appeal with prejudice is entered; or (2) the parties file a joint stipulation to terminate the stay in the event that a plaintiff in a subsequently filed derivative action makes similar allegations and does not agree to stay the proceedings on substantially the same terms. On June 29, 2018, the court entered an order staying the case pursuant to the joint stipulation, which expired by its term following entry of the court’s dismissal order in the above putative securities class action. On November 29, 2018, the parties filed a second joint stipulation to continue the stay until (1) 30 days after the putative securities class action appeal and any appeals therefrom have been resolved; or (2) the parties file a joint stipulation to terminate the stay in the event that a plaintiff in a subsequently filed derivative action makes similar allegations and does not agree to a stay of proceedings on substantially the same terms. On November 30, 2018, the court entered an order staying the case pursuant to the second joint stipulation. Cempra believes it has meritorious defenses and intends to defend the lawsuit vigorously. It is possible that similar lawsuits may yet be filed in the same or other courts that name the same or additional defendants.On January 3, 2018, the plaintiff who commenced the February 2017 Action, which was subsequently consolidated into the December 2016 Action, transmitted to the former Acting Chief Executive Officer of Cempra a litigation demand (the “Demand”). The Demand requested that Cempra’s Board of Directors (the “Board”) “commence an independent investigation into the matters raised” in the complaint filed in the February 2017 Action and the Demand, “take any and all appropriate steps for Cempra to recover, through litigation if necessary, the damages proximately caused by the directors' and officers' alleged breaches of fiduciary duty,” and “implement corporate governance enhancements to prevent recurrence of the alleged wrongdoing.” The Board has not yet formally responded to the Demand.On July 31, 2017, a shareholder derivative lawsuit was commenced in the Court of Chancery of the State of Delaware, naming certain of Cempra’s former and current officers and directors as defendants and Cempra as nominal defendant, and asserting claims for breach of fiduciary duty, unjust enrichment, and corporate waste. The complaint is based on similar allegations as asserted in the putative securities class action described above, and seeks unspecified damages and attorneys’ fees. On October 23, 2017, the defendants filed a motion to dismiss or, in the alternative, stay, the complaint, which was supported by an opening brief filed on November 9, 2017. On January 8, 2018, the plaintiff filed his answering brief in opposition to the defendants’ motion. The defendants filed their reply in support of their motion on February 7, 2018. On June 18, 2018, the parties filed a joint letter (1) indicating they have agreed to stay the case until the pending motion to dismiss inthe November 4, 2016 consolidated federal securities action pending in the United States District Court, Middle District of North Carolina, Durham Division is decided; and (2) requesting that the June 22, 2018 oral argument scheduled for defendants’ motion to dismiss be canceled. On June 27, 2018, the parties filed a stipulation and proposed order to stay the case until (1) 30 days after a final order dismissing the November 4, 2016, consolidated federal securities action pending in the United States District Court, Middle District of North Carolina, Durham Division with prejudice is entered; or (2) the parties file a joint stipulation to terminate the stay in the event that a plaintiff in a subsequently filed derivative action makes similar allegations and does not agree to stay the proceedings on substantially the same terms. On June 28, 2018, the court granted the proposed order and stayed the case on such terms. On November 28, 2018, the parties filed a joint stipulation agreeing to stay the case, including all discovery, until (1) 30 days after the appeal for the November 4, 2016 consolidated federal securities action pending in the United States District Court, Middle District of North Carolina, Durham Division, and any appeals therefrom, was resolved or (2) the parties file a joint stipulation to terminate the stay in the event that a plaintiff in a subsequently filed derivative action makes similar allegations and does not agree to a stay of proceedings on substantially the same terms. On November 30, 2018, the court stayed the case pursuant to the joint stipulation. Cempra believes it has meritorious defenses and intends to defend the lawsuit vigorously. It is possible that similar lawsuits may yet be filed in the same or other courts that name the same or additional defendants. On September 15, 2017, a shareholder derivative lawsuit was commenced in the United States District Court for the Middle District of North Carolina, Durham Division, naming certain of Cempra’s former and current officers and directors as defendants and Cempra as nominal defendant, and asserting claims for breach of fiduciary duty, unjust enrichment, abuse of control, gross mismanagement, corporate waste, and violation of Section 14(a) of the Exchange Act. The complaint is based on similar allegations as asserted in the putative securities class action described above, and seeks unspecified damages and attorneys’ fees. On December 1, 2017, the parties filed a joint motion seeking to stay the shareholder derivative lawsuit pending resolution of the putative securities class action, which stipulation was ordered by the court on December 11, 2017. On December 11, 2018, the parties filed a joint status report indicating that they believe the action remains stayed pending the outcome of the putative securities class action. Cempra believes it has meritorious defenses and intends to defend the lawsuit vigorously. It is possible that similar lawsuits may yet be filed in the same or other courts that name the same or additional defendants. On September 27, 2017, and October 6, 2017, putative class action complaints were filed against Cempra, members of its board of directors and Melinta Therapeutics, Inc. (now known as Melinta Subsidiary Corp.) (for the purposes of this Contingencies section, “Melinta”) on behalf of the public stockholders of Cempra in the United States District Court for the Middle District of North Carolina. The complaints allege that the preliminary proxy statement issued in connection with the proposed merger between Cempra and Melinta omitted material information in violation of Sections 14(a) and 20(a) of the Exchange Act, rendering the preliminary proxy statement false and misleading. On February 7, 2018, the plaintiffs and the defendants executed a settlement agreement that resolved plaintiffs’ claims with prejudice as to the named plaintiffs and without prejudice to any other member of the putative class. Defendants agreed to pay attorneys’ fees of $263 in connection with settlement of the actions, which was paid in early 2018.On December 3, 2018, James Naples, a purported Company shareholder, filed a putative class action suit against the Company and its Board of Directors in the Court of Chancery of the State of Delaware, alleging that the Board had breached its fiduciary duties related to a proposed, and subsequently abandoned, $75,000 common stock financing that was contemplated with affiliates of Vatera Holdings LLC. The suit alleged that the Board of Directors breached its fiduciary duties by, among other things, failing to disclose all material information to Company shareholders. The suit sought, among other things, to enjoin the shareholder vote on the financing proposal until additional disclosures were issued. On February 27, 2019, the suit was voluntarily dismissed with prejudice as moot, though the court has retained jurisdiction solely for the purpose of adjudicating a claim by the plaintiff for attorneys' fees and expenses, if one is submitted.On December 18, 2018, we filed a complaint in the Court of Chancery of the State of Delaware against Medicines for breach of contract claim and fraud arising from the Purchase and Sale Agreement (“Purchase Agreement”), dated November 28, 2017, pursuant to which we acquired the Infectious Disease Businesses from Medicines. In the complaint, we alleged claims for damages of at least $68.3 million. On December 28, 2018, we received a letter from Medicines demanding the payment of Milestone No. 4 under the Agreement and Plan of Merger, dated as of December 3, 2013, among Medicines, Rempex Pharmaceuticals, Inc. and the other parties thereto, in the amount of $30,000 (a milestone which the Company had assumed as an “Assumed Liability” under the Purchase Agreement). On January 7, 2019, we notified Medicines that we would not be making the Milestone No. 4 payment in the amount of $30,000, or the First Deferred Payment in the amount of $25,000 under the Purchase Agreement, because the Company had asserted claims in the litigation in excess of these amounts. On January 9, 2019, Medicines filed a motion to dismiss our claims.Other than as described above, we are not a party to any legal proceedings and we are not aware of any claims or actions pending or threatened against us. In the future, we might from time to time become involved in litigation relating to claims arising from our ordinary course of business.NOTE 11 – BENEFIT PLANWe have a 401(k) Plan in which all of our employees are eligible to participate. Each year, we may, but are not required to, make matching contributions to the 401(k) Plan. For the years ended December 31, 2018, 2017 and 2016, we made matching contributions to the 401(k) Plan of $1,961, $475 and $306, respectively.NOTE 12 – RELATED PARTY TRANSACTIONSWe use various software tools in the research and development discovery process. These tools are licensed at market rates from a company owned by Dr. William Jorgensen. Dr. Jorgensen is a founder of the Company and is the spouse of our Chief Scientific Officer. Total fees paid to Dr. Jorgensen’s company were $40, $40 and $43 for the years ended December 31, 2018 and 2017 and 2016, respectively.Dr. Thomas Koestler was our non-executive Chairman of the Board prior to the merger with Cempra, continues to serve on our board of directors and is a consultant to our largest investor, Vatera Healthcare Partners LLC. Dr. Koestler received compensation for his role in the amount of $58 and $126 for the years ended December 31, 2018 and 2017, respectively. In addition, during the years ended December 31, 2018 and 2017, we granted options to purchase 6,820 and 333 shares, respectively, with exercise prices of $37.25 and $104.85, respectively. In 2018 and 2017, we recorded $73 and $151, respectively, of share-based compensation related to these options. Dr. Koestler stepped down as Chairman of the Board in November 2017, in conjunction with the merger with Cempra, but remains on our board as a director.Vatera is a significant investor in the company. Kevin Ferro, one of our directors and chairman of the Board, serves as the chief executive officer and managing member of Vatera Capital Management LLC, the current manager of Vatera Healthcare Partners LLC, and Dr. Koestler, one of our directors, serves as a consultant to Vatera Healthcare Partners LLC. On December 31, 2018, we entered into a Senior Subordinated Convertible Loan Agreement (the “Loan Agreement”) with Vatera pursuant to which Vatera committed to provide $135,000 over a period of five months, subject to the satisfaction of certain conditions (see Note 4 to the consolidated financial statements). This agreement was approved by shareholders at a Special Meeting held on February 19, 2019. On February 22, 2019, we drew $75,000 in the first tranche of the Loan Agreement.NOTE 13 – SELECTED QUARTERLY DATA (Unaudited) Quarter Ended March 31,
2018 June 30,
2018 September 30,
2018 December 31,
2018Revenue $ 14,841 $ 12,022 $ 34,078 $ 35,489 Operating expenses: Cost of goods sold 7,686 10,989 13,393 8,989 Research and development 16,129 15,813 13,065 10,402 Goodwill impairment — — — 25,088 Selling, general and administrative 34,624 34,946 34,287 29,455 Loss from operations (43,598 ) (49,726 ) (26,667 ) (38,445 ) Total Other Income (Expense), net 14,166 (6,054 ) (1,193 ) (5,675 ) Net loss $ (29,432 ) $ (55,780 ) $ (27,860 ) $ (44,120 ) Accretion of preferred dividends — — — — Net loss available to shareholders $ (29,432 ) $ (55,780 ) $ (27,860 ) $ (44,120 ) Net loss per share - basic and diluted $ (4.76 ) $ (6.92 ) $ (2.49 ) $ (3.94 ) Weighted average shares used in calculating basic and diluted net loss per share 6,184 8,059 11,203 11,204 Quarter Ended March 31,
2017 June 30,
2017 September 30,
2017 December 31,
2017Revenue $ 22,463 $ 3,979 $ 3,191 $ 4,231 Operating expenses: Research and development 12,917 14,075 10,884 11,599 Selling, general and administrative 7,973 7,699 10,304 37,349 Loss from operations 1,573 (17,795 ) (17,997 ) (44,717 ) Total Other (Income) Expense, net 1,647 2,631 1,639 (25,937 ) Net loss $ (74 ) $ (20,426 ) $ (19,636 ) $ (18,780 ) Accretion of preferred dividends (5,720 ) (5,721 ) (5,720 ) (2,098 ) Net loss available to shareholders $ (5,794 ) $ (26,147 ) $ (25,356 ) $ (20,878 ) Net loss per share - basic and diluted $ (1,040.78 ) $ (4,420.46 ) $ (4,286.73 ) $ (7.40 ) Weighted average shares used in calculating basic and diluted net loss per share 6 6 6 2,821 NOTE 14 – BUSINESS COMBINATIONSAcquisition of the Infectious Disease BusinessOn January 5, 2018, we completed the acquisition of the IDB, in which we acquired a group of antibiotic drug products and certain other assets from Medicines, including 100% of the capital stock of certain subsidiaries and the pharmaceutical products containing (i) meropenem and vaborbactam as the active pharmaceutical ingredient and distributed under the brand name Vabomere, (ii) oritavancin as the active pharmaceutical ingredient and distributed under the brand name Orbactiv and (iii) minocycline as the active pharmaceutical ingredient and distributed under the brand name Minocin for injection and line extensions of such products. The integration of the acquired products within our existing portfolio further strengthens our ability to serve the needs of providers treating patients with serious bacterial infections across the healthcare delivery continuum. In addition to the products acquired in the IDB transaction, we hired approximately 135 individuals from Medicines to our team. The new team members bring with them significant experience specific to infectious diseases and better position us to effectively execute our commercial and other activities.The acquisition was financed using borrowings under the Deerfield Facility and additional equity financing from existing and new investors. See Note 4 for further information regarding these financing arrangements. Expenses related tolegal and other services in connection with the IDB acquisition were $2,339 and $2,617 in the years ended December 31, 2018 and 2017, respectively. The expenses incurred in the year ended December 31, 2018, included 1,320 related to certain transition services that Medicines agreed to provide to us to facilitate transition and integration of the IDB. The transition services included the temporary provision of facilities and equipment for newly hired personnel, assistance with finance functions and support in connection with the transition of the supply, sale and distribution of the products to our third-party logistics provider. The transition services were substantially complete at the end of 2018.The consideration paid to Medicines consisted of a cash payment of $166,383 and 662,740 shares of our common stock, which was calculated by dividing $50,000 by $75.45, representing 90% of the volume weighted average price of the common stock for the trailing ten trading day period ending three trading days prior to the closing date. In addition, subject to the terms and conditions of the IDB purchase agreement, we are required to make two additional payments of $25,000 on each of the twelve and eighteen-month anniversaries of the closing date (January and July 2019, respectively), and we will pay royalties to Medicines on certain net sales of the acquired antibiotic products.The purchase price, including non-cash consideration, for the acquisition of IDB is as follows (except for the initial cash payment, these items have been treated as non-cash investing activity in the condensed consolidated statement of cash flows, and will be treated as financing activity when the remaining items are paid in cash):Cash of $166,383, including a net working capital adjustment of $1,383;Common stock of $54,510;Deferred consideration of $38,541, representing the present value of two payments of $25,000 each due in January and July 2019; andContingent consideration of $12,271, representing the fair value of sales-based royalty payments.We recorded the contingent consideration related to the sales-based royalty payments at fair value, and, during 2018, we were accreting the amount to the estimated aggregate amounts payable to Medicines, $354,000, based on an effective interest of rate of 49%, which is in line with the effective interest rate, 43%, used to accrete the royalty liability associated with the Deerfield Facility. During the year ended December 31, 2018, we recorded $6,544, respectively, of non-cash interest expense related to the accretion of the fair value of sales-based royalty payments. As of December 31, 2018, $2,784 of the royalty liability is currently due and has been reclassified out of current deferred purchase price and recorded either as a credit offset to receivables (up to the amount due from Medicines) or in accrued liabilities. At the end of the fourth quarter of 2018, we updated our estimate of the remaining aggregate amounts of royalty payable to Medicines for future periods to approximately $60,000. We recorded the updated fair value of $5,714 at December 31, 2018 ($1,006 and $4,708, in short-term and long-term liabilities, respectively). We recorded $10,317 of favorable net fair value adjustments to operating expenses in the fourth quarter of 2018.As of December 31, 2018, we have finalized the valuation for the acquisition. The goodwill resulting from the acquisition largely consists of the estimated value of IDB’s assembled and trained workforce, our expected future product sales, synergies resulting from combining IDB products with our existing product offering and IDB’s going concern value.The following table sets forth our final of the purchase price allocation.Current assets $ 33,725 Goodwill 25,088 Intangible assets 236,819 Non-current assets 9,486 Current liabilities (32,837 ) Non-current liabilities (576 ) Total purchase price $ 271,705 We believe that the historical values of IDB’s current assets and current liabilities (except for certain inventory items, which we stepped up in value) approximate their fair values based on the short-term nature of such items. The current liabilities include a contingent liability of $24,485, representing the probability-weighted present value of a $30,000 milestone payment payable third parties upon the approval of Vabomere in Europe. During the year ended December 31, 2018, we recorded $4,015 of non-cash accretion, included within interest expense, of this contingent payment and a fair value adjustment of $1,500 in the fourth quarter of 2018 when Vabomere received approval in Europe. The accretion is based on an effective interest rate of 20.9% which is consistent with the interest rate associated with the Deerfield Facility.We recorded the two $25,000 deferred payments to Medicines at fair value, and we are accreting them to $50,000 based on an effective interest rate of 21.1%. During the year ended December 31, 2018, we recorded $8,847 of non-cash accretion, included within interest expense, of these deferred payments. At December 31, 2018, the carrying value of the short-term deferred payments was $47,388.The following table sets forth the components of identifiable intangible assets acquired and their estimated useful lives as of the date of the acquisition: Average useful life Fair value Developed product rights 13 to 17 years $ 216,960 In-process research and development Indefinite 19,859 Total intangible assets $ 236,819 During the year ended December 31, 2018, we recorded $16,332 of amortization expense related to the developed product rights. In the fourth quarter of 2018, we transferred our in-process research and development balance of $19,859 to developed product rights (intangible assets) because the related product, Vabomere, received regulatory approval in Europe. We will begin amortizing the intangible asset over its estimated useful life when sales of Vabomere commence in Europe, anticipated to be sometime in 2019 or 2020. At December 31, 2018, the carrying value of all our developed product rights was $221,276. For the year ended December 31, 2018, IDB added $40,096 of revenue and $1,938 of grant income, respectively, to our consolidated results. It is impracticable to measure the effect IDB had on our net loss for the year ended December 31, 2018, because IDB has been integrated into our existing operations and is not accounted for separately. Since the date of the acquisition, IDB’s results are reflected in our consolidated financial statements.Goodwill ImpairmentAs noted above, we initially recorded goodwill of $25,088 at the date of the acquisition. In December 2018, we performed our annual impairment assessment. Due to the decrease in the market price of the Company's stock during 2018, our market capitalization was significantly below the carrying value of our net assets. Given that we operate as one reporting unit, the market capitalization reflects the value that the public markets attribute to our equity. After further fair value analysis and testing, including considering control premiums, we concluded that goodwill was impaired and had a current fair value of $0. Accordingly, we recognized impairment of our goodwill totaling $25,088 in the fourth quarter of 2018. We determined that there is no impairment of the acquired IDB identified intangible assets based on the undiscounted forecasted cash flows associated with each of the acquired intangible assets.Merger with CempraCempra’s results have been reflected in our condensed consolidated financial statements since the date of the merger on November 3, 2017. As a result of the merger, Cempra's operations contributed $3,465 and $870 of grant income to our consolidated results of operations in the years ended December 31, 2018 and 2017, respectively. We incurred $256 and $9,119 of acquisition-related expense (excluding severance) in the years ended December 31, 2018 and 2017, respectively, related to the merger with Cempra.Pro Forma Financial InformationThe following table provides unaudited supplemental pro forma information as if the acquisition of IDB and the Cempra merger took place at the beginning of fiscal 2017. 2017 Pro forma revenue $ 67,617 Pro forma net loss $ (264,909 ) The unaudited supplemental pro forma consolidated results reflect the historical financial information of Melinta, Medicine’s IDB and Cempra, adjusted to give effect to the IDB acquisition and the Cempra merger as if they had occurred as of the beginning of fiscal 2017 and 2016, respectively, primarily for the following adjustments:Effects of Topic 606.Additional interest expense and accretion related to the financing of the business combinations.Additional accretion expense related to the IDB acquisition.Additional accretion on deferred and contingent payments and additional amortization of intangible assets recognized as part of the business combinations.Elimination of historical nonrecurring transaction costs and severance related to the business combinations.The remeasurement gain on the warrant liability (using $33,226 for the year ended December 31, 2018).Elimination of the bargain purchase gain in the Cempra merger of $27,663.Elimination of the tax valuation allowance reversals recorded by IDB.In addition, excluded from the supplemental pro forma information was $10,497 of nonrecurring IDB acquisition costs (debt extinguishment costs, inventory fair value step-up amortization, and transaction and severance costs) recorded during the year ended December 31, 2018.NOTE 15 –NET LOSS PER SHAREBasic net loss attributable to common shareholders per share is computed by dividing the net loss attributable to common shareholders by the weighted-average number of common shares outstanding for the period. During periods where we might earn net income, we would allocate to participating securities a proportional share of net income determined by dividing total weighted-average participating securities by the sum of the total weighted-average common shares and participating securities (the “two-class method”). Our preferred stock, if any, participates in any dividends declared by us and are therefore considered to be participating securities. Participating securities have the effect of diluting both basic and diluted earnings per share during periods of income. During periods where we incurred net losses, we allocate no loss to participating securities because they have no contractual obligation to share in our losses. We computed diluted loss per common share after giving consideration to the dilutive effect of stock options and warrants that are outstanding during the period, except where such nonparticipating securities would be antidilutive. Because we have reported net losses for the years ended December 31, 2018, 2017 and 2016, diluted net loss per common share is the same as basic net loss per common share for those periods. The weighted-average shares outstanding, reported loss per share and potential dilutive common share equivalents for the periods prior to November 3, 2017, the date of the Cempra merger, have been retrospectively adjusted to reflect historical weighted-average number of common shares outstanding multiplied by the exchange ratio established in the merger agreement. All shares have also been adjusted to reflect the one-for-five reverse stock split that was effective on February 22, 2018.During 2017 and 2016, we had shares of convertible preferred stock outstanding which earned dividends at a rate of 8% per year. Our net loss attributable to common shareholders for those years was adjusted to reflect the accretion of dividends on the convertible preferred stock before computing net loss per share.The following potentially dilutive securities (in common stock equivalent shares) have been excluded from the computation of diluted weighted-average shares outstanding because such securities have an antidilutive impact due to losses reported: December 31, 2018 2017 2016 Options to purchase common stock 696,890 412,211 120,007 Preferred stock warrants — — 6,331 Unvested restricted stock 17,360 64,132 — Common stock warrants 766,691 11,913 9 Convertible preferred stock — — 1,166,029 1,480,941 488,256 1,292,376 NOTE 16 – SEVERANCEIn connection with the merger with Cempra, several employees were terminated under established individual employment plans and a corporate-wide severance plan. The legacy Cempra entity had put in place a severance plan that provided severance benefits to employees who, in connection with a change-in-control event, either were terminated or resigned due to having a diminished role going forward with the combined company.Most of the affected employees were notified that they would be terminated in connection with the change-in-control event in advance of the merger, and the Company recognized the associated severance costs when the liability became probable, which was after the merger closed. The postemployment benefits for the individuals include continued salary and benefits for a period of time determined by historical length of service to, and role with, the Company (up to six months for non-executives, 18 months for executives, and 24 months for the CEO), outplacement services and contractual or prorated bonuses. While all the affected employees were notified before or immediately after the merger, some of the termination dates were extended into 2018.In addition to the severance costs incurred in connection with the Cempra merger, the Company incurs additional severance costs as part of its on-going operations. In October 2018, our Board of Directors appointed John H. Johnson as Interim Chief Executive Officer to succeed Daniel Wechsler, who stepped down from his role as President, CEO and director to pursue other opportunities. In connection with Mr. Wechsler's termination from the Company, we recorded total severance-related payments of approximately $1,469 (before the adjustment to net present value) in Q4 2018, which will be paid over 18 months. He will also receive a pro-rata bonus for 2018, to be paid in the first quarter of 2019.In November 2018, the Board of Directors approved a plan to reduce our operating expenses, principally through an approximately 20% reduction in headcount. In addition to the severance expense recorded in the fourth quarter of 2018 for Daniel Wechsler, we recorded a fourth quarter 2018 net charge of $5,894 post-employment benefit costs. The remainder of the total 2018 severance expense was incurred in the first three quarters of 2018 as part of the company’s on-going operations. In addition to severance payments, terminated employees will receive a pro-rated bonus for 2018 to be paid in the first quarter of 2019 (except some contractual bonus payments which were or will be paid out at the time of termination).A summary of merger and non-merger activity in our severance accrual (included in accrued expenses or long-term liabilities on the consolidated balance sheets) is below. Balance - December 31, 2016 $ — Severance accruals (recorded in SG&A) 6,383 Severance liability acquired in Cempra merger 769 Severance payments (431 ) Balance - December 31, 2017 $ 6,721 Severance accruals (recorded in SG&A) 12,371 Severance payments (9,325 ) Balance - December 31, 2018 $ 9,767 On December 31, 2018, $9,390 was included in accrued expenses and $377 was included in long-term liabilities. We also recognized $407 and $1,547 of additional stock-based compensation expense related to the acceleration of equity awards for terminated employees under ASC 718 as severance expense during the years ended December 31, 2018 and 2017, respectively. The 2018 stock-based compensation expense includes $174 for the acceleration of Mr. Wechsler's first-year vesting of his incentive RSU award. The acceleration of the first-year vesting was considered a modification and accounted for as a new award at a fair value of $174. The cumulative stock-based compensation expense for the original awards recorded through the third quarter of 2018 of $486 was reversed in the fourth quarter of 2018.In the second quarter of 2018, we vacated a significant portion of our office facilities in Chapel Hill, North Carolina. As a consequence, we recorded an estimated lease exit liability of $556. The lease exit liability was determined by computing the fair value of the remaining lease payments, net of any projected sub-lease rentals. A summary of activity in our lease liability is below. Balance - December 31, 2017 $ — Fair value of lease liability recognized 556 Lease termination adjustment (233 ) Termination fee 136 Less: payments (391 ) Balance - December 31, 2018 $ 68 F-50