UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K/A
(Amendment No. 1)10-K
|
| |
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 20172018
|
| |
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File No. 001-38038
Valeritas Holdings, Inc.
(Exact name of registrant as specified in its charter)
|
| | |
| Delaware | | 46-5648907 |
(State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification No.) |
|
| | |
750 Route 202 South, Suite 600 Bridgewater, NJ | | 08807 |
| (Address of principal executive offices) | | (Zip code) |
(908) 927-9920
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
|
| | |
Common Stock, Par Value $0.001 Per ShareTitle of each class | | Nasdaq Capital MarketName of each exchange on which registered |
(Title of Class)Common Stock, $0.001 par value | | (Name of Exchange on Which Registered)The Nasdaq Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. YES ☐ NO ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. YES ☐ NO ☒
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. YES ☒ NO ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). YES ☒ NO ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☐☒
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer”, “smaller reporting company”and "emerging growth company" in Rule 12b-2 of the Exchange Act.
|
| | | | | | |
| Large accelerated filer | | ☐ | | Accelerated filer | | ☐ |
| Non-accelerated filer | | ☐ (Do not check if a smaller reporting company)☒ | | Smaller reporting company | | ☒ |
| | | | | Emerging growth company | | ☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). YES ☐ NO ☒
The aggregate market value of the voting common equity held by non-affiliates as of June 30, 2017,29, 2018, the last business day of the registrant’s most recently completed second fiscal quarter, was approximately $10,999,881.$25,983,391. The registrant has no non-voting common equity.
The number of outstanding shares of common stock of the registrant as of April 25, 2018March 4, 2019 was 7,092,869.99,948,437.
Documents Incorporated By Reference
Portions of registrant’s proxy statement relating to registrant’s 2019 Annual Meeting of Stockholders (the “Proxy Statement”) to be filed with the Securities and Exchange Commission pursuant to Regulation 14A, not later than 120 days after the close of the registrant’s fiscal year, are incorporated by reference in Part III of this Annual Report on Form 10-K. Except with respect to information specifically incorporated by reference in this Annual Report on Form 10-K, the Proxy Statement is not deemed to be filed as part of this Annual Report on Form 10-K.
Explanatory Note
We are filing this Amendment No. 1 on Form 10-K/A (the “Amendment”) of Valeritas Holdings, Inc. to amend our Annual Report on Form 10-K for the fiscal year ended December 31, 2017, filed on February 28, 2018 (the “Original Form 10-K”, and together with the Amendment, the “Form 10-K”), to include the information required by Part III of the Form 10-K as we no longer anticipate filing our proxy statement for the 2018 Annual Meeting of Stockholders within 120 days of December 31, 2017. With the exception of the inclusion of the information required by Part III, no information contained in the Original Form 10-K has been changed. Accordingly, this Amendment should be read in conjunction with the Original Form 10-K.
VALERITAS HOLDINGS, INC.
ANNUAL REPORT ON FORM 10-K/A10-K
TABLE OF CONTENTS
|
| | |
| | |
| PART I | | |
| Item 1 | | |
| Item 1A. | | |
| Item 1B | | |
| Item 2. | | |
| Item 3. | | |
| Item 4. | | |
| | |
| PART II | | |
| Item 5. | | |
| Item 6. | | |
| Item 7. | | |
| Item 7A. | | |
| Item 8. | | |
| Item 9. | | |
| Item 9A. | | |
| Item 9B. | | |
| | | |
| PART III | | |
| Item 10. | | |
| Item 11. | | |
| Item 12. | | |
| Item 13. | | |
| Item 14. | | |
| | | |
| PART IV | | |
| Item 15. | | |
| | Signatures | |
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This report contains forward-looking statements, including, without limitation, in the sections captioned “Risk Factors”, “Management’s Discussion and Analysis of Financial Condition and Plan of Operations”, and “Business”. Any and all statements contained in this report that are not statements of historical fact may be deemed forward-looking statements. Terms such as “may,” “might,” “would,” “should,” “could,” “project,” “estimate,” “pro-forma,” “predict,” “potential,” “strategy,” “anticipate,” “attempt,” “develop,” “plan,” “help,” “believe,” “continue,” “intend,” “expect,” “future,” and terms of similar import (including the negative of any of the foregoing) may be intended to identify forward-looking statements. However, not all forward-looking statements may contain one or more of these identifying terms. Forward-looking statements in this report may include, without limitation, statements regarding (i) the plans and objectives of management for future operations, including plans or objectives relating to the development of commercially viable new products, (ii) a projection of income (including income/loss), earnings (including earnings/loss) per share, capital expenditures, dividends, capital structure or other financial items, (iii) our future financial performance, including any such statement contained in a discussion and analysis of financial condition by management or in the results of operations included pursuant to the rules and regulations of the Securities and Exchange Commission, or the SEC, and (iv) the assumptions underlying or relating to any statement described in points (i), (ii) or (iii) above.
The forward-looking statements are not meant to predict or guarantee actual results, performance, events or circumstances and may not be realized because they are based upon our current projections, plans, objectives, beliefs, expectations, estimates and assumptions and are subject to a number of risks and uncertainties and other influences, many of which we have no control over. Actual results and the timing of certain events and circumstances may differ materially from those described by the forward-looking statements as a result of these risks and uncertainties. Factors that may influence or contribute to the inaccuracy of the forward-looking statements or cause actual results to differ materially from expected or desired results may include, without limitation:
our history of operating losses and uncertainty regarding our ability to achieve profitability;
our reliance on V-Go to generate all of our revenue;
our inability to retain a high percentage of our patient customer base or our significant wholesale customers;
the failure of V-Go to achieve and maintain market acceptance;
competitive products and other technological breakthroughs that may render V-Go obsolete or less desirable;
our inability to maintain or expand our sales and marketing infrastructure;
our inability to operate in a highly competitive industry and to compete successfully against competitors with greater resources;
any inaccuracies in our assumptions about the insulin-dependent diabetes market;
manufacturing risks, including risks related to manufacturing in Southern China, damage to facilities or equipment and failure to efficiently increase production to meet demand;
our dependence on limited source suppliers and our inability to obtain components for our product;
our failure to secure or retain adequate coverage or reimbursement for V-Go by third-party payors;
our inability to enhance and broaden our product offering, including through the successful commercialization of the pre-fill V-Go;
our inability to protect our intellectual property and proprietary technology;
our failure to comply with the applicable governmental regulations to which our product and operations are subject; and
other risks and uncertainties, including those listed under the section entitled “Risk Factors.”
Readers are cautioned not to place undue reliance on forward-looking statements because of the risks and uncertainties related to them and to the risk factors. The forward-looking statements contained in this report reflect our views and assumptions only as of the date that this report is signed. We disclaim any obligation to update the forward-looking statements contained in this report to reflect any new information or future events or circumstances or otherwise, except as required by law.
We qualify all of our forward-looking statements by these cautionary statements. In addition, with respect to all of our forward-looking statements, we claim the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995.
Readers should read this report in conjunction with the discussion under the caption “Risk Factors”, our financial statements and the related notes thereto in this report.
PART IIII
Item 1. Business
About this Annual Report
On May 3, 2016, pursuant to an Agreement and Plan of Merger and Reorganization, or the Merger Agreement, by and among Valeritas Holdings, Inc., a Delaware Corporation, Valeritas Acquisition Corp., a Delaware corporation and a direct wholly-owned subsidiary of Valeritas Holdings, Inc., or the Acquisition Subsidiary, and Valeritas, Inc., a Delaware Corporation, Acquisition Subsidiary was merged with and into Valeritas, with Valeritas surviving as a direct wholly-owned subsidiary of Valeritas Holdings, Inc.
As used in this Annual Report on Form 10-K, unless otherwise stated or the context otherwise indicates, references to “Valeritas,” the “Company,” “we,” “our,” “us” or similar terms refer to Valeritas Holdings, Inc. and its subsidiary Valeritas, Inc.
Overview
We are a commercial-stage medical technology company, focused on improving health and simplifying life for people with diabetes by developing and commercializing innovative technologies. Valeritas’ flagship product, V-Go® Wearable Insulin Delivery device, is a simple, affordable, all-in-one basal-bolus insulin delivery option for patients with type 2 diabetes that is worn like a patch and can eliminate the need for taking multiple daily shots. V-Go administers a continuous preset basal rate of insulin over 24 hours and it provides discreet on-demand bolus dosing at mealtimes. It is the only basal-bolus insulin delivery device on the market today specifically designed keeping in mind the needs of type 2 diabetes patients.
V-Go enables patients to closely mimic the body’s normal physiologic pattern of insulin delivery throughout the day and to manage their diabetes with insulin without the need to plan a daily routine around multiple daily injections.
We currently focus on the treatment of patients with type 2 diabetes- a pervasive and costly disease that, according to the 2017 National Diabetes Statistics Report released by the U.S. Centers for Disease Control and Prevention, or CDC, currently affects 90% to 95% of the approximately 23 million U.S. adults diagnosed with diabetes. The American Diabetes Association estimates the total costs of diagnosed diabetes in the United States, which includes direct medical and drug costs and indirect lost productivity costs, have risen to $327 billion in 2017 from $245 billion in 2012. We believe the majority of the 12.6 million U.S. adults treating their type 2 diabetes with more than one daily oral anti-diabetic drug, or OAD, or an injectable diabetes medicine can benefit from the innovative approach of V-Go to manage type 2 diabetes.
Our primary market consists of approximately 5.6 million of these patients who currently take insulin, of which up to 4.5 million may not be achieving their target blood glucose goal. This patient population represents a $19.3 billion annual U.S. market when applying the annual wholesale acquisition cost, or WAC, of V-Go to the 4.5 million patients not achieving glycemic control. WAC is the gross price paid by wholesalers and does not take into account fees, discounts, and rebates from us.
Insulin therapies using syringes, pens and programmable insulin pumps are often burdensome to the daily routine of a patient with type 2 diabetes, which can lead to poor adherence to prescribed insulin regimens and, as a result, ineffective diabetes management. We developed V-Go utilizing our core technology, the h-Patch platform, as a patient-focused solution to address the challenges of traditional insulin therapies. Our h-Patch platform facilitates the simple and effective subcutaneous delivery of injectable medicines to patients across a broad range of therapeutic areas. V-Go enables patients to closely mimic the body’s normal physiologic pattern of insulin delivery by releasing a single type of insulin at a continuous preset background, or basal, rate over a 24-hour period and on demand mealtime dosing (bolus dosing). We believe V-Go is an attractive management tool for patients with type 2 diabetes requiring insulin because it only requires a single fill of insulin prior to use and provides comprehensive basal-bolus therapy without the burden and inconvenience associated with multiple daily injections. V-Go is available in three different dosages depending on the patient’s needs and is generally cost competitive for both patients and third-party payors when compared to insulin pens or programmable insulin pumps.
V-Go was one of the first insulin delivery devices cleared by the U.S. Food and Drug Administration, or FDA, under its Infusion Pump Improvement Initiative, which established additional device manufacturing requirements designed to foster the development of safer, more effective infusion pumps, and is the only FDA-cleared mechanical basal-bolus insulin delivery device on the market in the United States. All other FDA-cleared basal-bolus insulin delivery products currently available in the United States are electronic and are classified as Durable Medical Equipment and, although cleared for both type 1 and type 2 diabetes, were designed primarily for patients with type 1 diabetes. As V-Go is a mechanical device, it does not include any electronics, batteries or audible alarms and does not require any recharging or programming, which allows for simple and discreet use. Unlike electronic insulin delivery devices, V-Go is not classified as durable medical equipment by the Centers for Medicare and Medicaid Services, or
Item 10. Directors, Executive Officers
CMS, allowing for potential Medicare reimbursement under Medicare Part D. The Medicare Part D outpatient drug benefit defines V-Go and Corporate Governance.certain other supplies used for injecting insulin as “drugs,” which allows V-Go to be available for coverage by Part D Plans under Medicare Part D. In addition to Medicare, a majority of commercially insured patients are currently covered for V-Go under their insurance plans.
DirectorsWe commenced commercial sales of V-Go in the United States during 2012. During the first half of 2012, we initiated an Early Access Program to provide a limited number of physicians with free V-Go devices for patients and Executive Officersbegan shipments to major wholesalers in anticipation of commercial launch. In the second half of 2012, we began hiring sales representatives in selected U.S. markets. In February 2016, we optimized our sales force to implement a high-service, high-touch sales model. At the end of 2018 and 2017, our field-based sales team consisted of 52 and 50 sales representatives, respectively and covered 52 and 50 territories, respectively, primarily within the East, South, Midwest and Southwest regions of the United States.
BelowOur net loss was $45.9 million and $49.3 million for the years ended December 31, 2018 and 2017, respectively. Our accumulated deficit as of December 31, 2018 and 2017 was $519.9 million and $473.9 million, respectively. Based on prescription data, we estimate that there were approximately 99,000 and 88,000 prescriptions reported for V-Go filled during both the years ended December 31, 2018 and 2017. Refill prescriptions account for slightly more than two-thirds of our total prescriptions, and generally move in parallel with our patient retention rates, so can be used as a proxy to determine patient retention. We estimate that as of December 31, 2018, V-Go had been used for over 17 million cumulative patient days.
Market Opportunity
Diabetes is a chronic, life-threatening disease and was reported in 2017 to impact an estimated 425 million people worldwide and is characterized by the body’s inability to properly metabolize glucose. Management of glucose is regulated by insulin, a hormone that allows cells in the body to absorb glucose from blood and convert it into energy. In people without diabetes, the body releases small amounts of insulin regularly over 24 hours and additional amounts of insulin when eating meals. Diabetes is classified into two main types. Type 1 diabetes is caused by an autoimmune response in which the body attacks and destroys the insulin-producing cells of the pancreas. As a result, the pancreas can no longer produce insulin, requiring patients to administer daily insulin injections to survive. Type 2 diabetes, the more prevalent form of the disease, occurs when either the body does not produce enough insulin to regulate the amount of glucose in the blood or cells become resistant to insulin and are unable to use it effectively. Type 1 diabetes is frequently diagnosed during childhood or adolescence, and the onset of type 2 diabetes generally occurs in adulthood, but its incidence is growing among the younger population, primarily due to the increasing incidence of childhood obesity. In addition, other factors commonly thought to be contributing to the prevalence and growth of type 2 diabetes include aging populations, sedentary lifestyles, worsening diets and increased adult obesity.
In 2017, the CDC estimated that 30.3 million people or 9.4% of the population in the United States have diabetes, but 8.1 million may be undiagnosed and unaware of their condition. About 1.4 million new cases of diabetes are diagnosed in United States every year. More than one in every 10 adults who are 20 years or older has diabetes. For seniors (65 years and older), that figure rises to more than one in four.
The CDC estimated in 2018 that 86 million Americans have "pre-diabetes" where their blood sugar (glucose) levels are higher than it should be and are likely on the path to diabetes. The American Diabetes Association released new research on March 22, 2018 estimating the total costs of diagnosed diabetes have risen to $327 billion in 2017 from $245 billion in 2012, when the cost was last examined. This cost includes $237 billion in direct medical costs and $90 billion in reduced productivity. Current diabetes forecasts report the prevalence of diabetes will increase by 54% to more than 54.9 million Americans between 2015 and 2030; and total annual medical and societal costs related to diabetes will increase 53% to more than $622 billion by 2030.
Type 2 diabetes is a progressive disease. Data from the United Kingdom Prospective Diabetes Study suggest that individuals with type 2 diabetes lose on average approximately 50% of the function of their beta cells, the cells that produce insulin, prior to diagnosis. If not closely monitored and properly treated, diabetes can lead to serious medical complications. According to the CDC, diabetes is the leading cause of kidney failure, non-traumatic lower limb amputations and new cases of blindness in the United States. The prevalence of other chronic disorders commonly occurring in patients with type 2 diabetes, including high blood pressure and high cholesterol, can significantly impact a patient’s lifestyle given the various daily treatment regimens often used to treat these conditions. Diabetes has a significant impact on overall patient mortality; according to the CDC, the risk for death among people with diabetes is approximately one and a half that of similarly aged people without diabetes.
A hemoglobin A1C test, which measures a patient’s trailing three-month average blood glucose level, or A1C level, is a key indicator of how well a patient is controlling his or her diabetes. Specifically, the A1C test measures the percentage of a patient’s hemoglobin, a protein in red blood cells that carries oxygen that is coated with sugar. A higher A1C level correlates with poorer blood sugar control and an increased risk of diabetes complications. The American Diabetes Association, or ADA, recommends an A1C goal of no more than 7% for most patients.
Once type 2 diabetes has been diagnosed, physicians and patients often first seek to manage the disease through meal planning and physical activity before progressing to medications designed to manage A1C levels. Patients often begin medical treatment with a once-daily OAD. If OAD monotherapy does not achieve or maintain the A1C target, combination therapy is recommended, which can include additional oral agents, a glucagon-like peptide 1 receptor agonist, or once-daily basal insulin. If basal insulin has been appropriately titrated and glucose levels remain elevated, advancement to an insulin regimen including mealtime insulin, or bolus dosing, is a recommended option. Generally, the introduction of insulin occurs within 10 years of diagnosis.
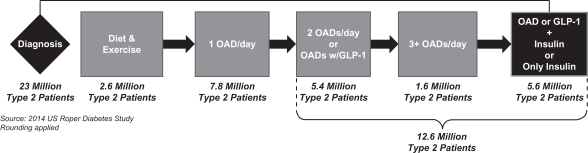
The following diagram depicts an illustrative treatment progression of a typical patient with type 2 diabetes, as well as the number of patients currently in each category according to the 2014 U.S. Roper Diabetes Patient Market Study.
Illustrative type 2 Diabetes Treatment Progression
As of December 31, 2017, there were approximately 12.6 million patients with type 2 diabetes in the United States using more than one OAD or some other injectable, such as insulin. Approximately 5.6 million patients were prescribed insulin. Our near-term target market consists of the up to 4.5 million patients in this group who may not be achieving the recommended target blood glucose goal (A1C <7%). Of these patients, nearly 3.0 million have an A1C greater than or equal to 8%. In addition, of the remaining approximately 7.0 million U.S. adults with type 2 diabetes who do not inject insulin, we believe those who are treating their diabetes with more than one OAD per day and/or an injectable GLP-1 diabetes medicine other than insulin can also benefit from the innovative approach of V-Go to manage type 2 diabetes.
We believe we compete primarily with insulin injections by either insulin pens or insulin syringes. We do not consider insulin pumps as competition because these are electronic devices that are replaced every two to four years based on medical necessity, which we consider common industry practice; have annual medical deductibles and require monthly medical co-insurance due to their classification as durable medical equipment; and, although cleared for type 1 and type 2 diabetes, are designed primarily for patients with type 1 diabetes.
Therapeutic Challenges and Limitations of Current Insulin Delivery Mechanisms
The goal of insulin therapy should be to closely mimic natural or non-diabetic insulin secretion as described below.
Insulin is secreted continuously (in the background) over 24 hours from the pancreas into the blood stream to keep blood glucose controlled overnight, during periods of fasting and between meals. Approximately 50% of the daily insulin requirement by the body is released in this continuous fashion; however, this nearly constant rate of insulin release is inadequate to treat post-mealtime glucose excursions (the change in blood glucose concentration from before to after a meal); thus
At mealtime a burst (bolus) of insulin is released by the body in response to food intake or a meal to control post-mealtime hyperglycemia- the exaggerated rise in blood glucose following a meal. This surge of insulin provides the remaining, approximate 50%, of the daily insulin requirement.
There are two components needed to mimic this insulin secretion, a background, or basal insulin replacement and a bolus insulin replacement. Multiple studies indicate that, when taken as prescribed, a basal-bolus insulin regimen is a very effective means for lowering blood glucose levels of patients with type 2 diabetes because it most closely mimics the body’s normal physiologic pattern of insulin delivery throughout the day. Unfortunately, despite the known advantages of basal-bolus therapy, patient adherence and compliance with basal-bolus insulin therapy using syringes and/or pens has proven difficult. Non-adherence has been associated with the inconvenience to daily living, pain and anxiety associated with the requirement to inject multiple times per day, and the complexity of the regimen as these therapies require the use of various forms of insulin and can require complicated dosing instructions.
The Diabetes Control and Complications Trial, a study of patients with type 1 diabetes conducted by the National Institute of Diabetes and Digestive and Kidney Diseases, or NIDDK, the results of which were published in TheNew England Journal of Medicine in 1993, indicated that conventional insulin therapy, defined as one or two insulin injections per day without routine changing of the insulin dose in response to blood glucose levels, is less effective in achieving recommended blood glucose levels over time than intensive insulin therapy in which a patient administers three or more insulin injections per day with varying doses depending upon blood glucose levels. Additionally, the Treating to Target in Type 2 Diabetes study of 708 men and women with suboptimal A1C levels published in The New England Journal of Medicine in 2009, found that by three years or study end, 81.6% of patients initiated on a basal insulin-based regimen required the addition of mealtime insulin three times daily. Sixty-three percent of patients in this basal insulin-based subgroup achieved the A1C goal of less than 7% by study end. We believe the outcomes of these studies confirm that an important factor of any insulin therapy is its ability to mimic the body’s normal physiologic pattern of insulin delivery.
Challenges Associated with Type 2 Diabetes Management
Regardless of the type of insulin therapy, many patients with type 2 diabetes on insulin fail to reach their A1C goal. Adding mealtime insulin to a basal-only regimen can help, but patient adherence to the prescribed treatment regimen is often a challenge. In a database analysis of 27,897 adult patients prescribed insulin in the United States, the results of which were published in the journal ClinicoEconomics and Outcomes Research in 2013, only 20.4% of patients had reached the ADA’s recommended A1C goal of less than 7%. Similarly poor results were observed across each patient group in the study regardless of whether they were prescribed basal-only insulin, basal-bolus insulin or a combination of both long-acting and fast-acting insulin.
Patient non-adherence to prescribed insulin therapy is often an important contributing factor in a patient’s failure to achieve target A1C goals. In a 2012 survey of 1,250 physicians who treat patients with diabetes and 1,530 insulin-treated patients (180 with type 1 diabetes and 1,350 with type 2 diabetes) published in Diabetic Medicine, patients reported insulin omission/non-adherence an average of 3.3 days per month. Additionally, 72.5% of physicians in the study reported that a typical patient did not take his or her insulin as prescribed, with an average of 4.3 days per month of non-compliance with a basal insulin regimen and 5.7 days per month of noncompliance with mealtime administration of insulin. Common reasons cited by patients for failing to comply with a prescribed treatment regimen include the burden of multiple daily injections, the potential embarrassment about injecting medication around family and friends or in public, and interference with the patient’s daily activities and resulting loss of freedom. Similarly, in the 2011 US Roper Diabetes Patient Market Study, or the 2011 Roper Study, of 2,104 patients with diabetes, of which 692 were on insulin, 72% of respondents who had been prescribed to take three or more insulin injections per day did not inject themselves when they were away from home. Failure to comply with prescribed insulin therapy, particularly mealtime insulin therapy, reduces the overall efficacy of insulin treatment in managing type 2 diabetes.
Limitations of Current Insulin Therapy
OADs are the namesfirst line of diabetic therapy for patients with type 2 diabetes, along with diet and certain information regarding our executive officerslifestyle changes. However, given that type 2 diabetes is progressive in nature and directorsthat the effectiveness of oral agents and other therapies may decline over time, patients are typically prescribed insulin therapy within 10 years of diagnosis. Depending on the progression of diabetes, there are four primary types of insulin therapy prescribed today for patients with type 2 diabetes that seek to control or manage their blood glucose levels:
a once-daily dose of basal insulin, typically a long-acting insulin such as Levemir® or Lantus®;
a twice-daily injection regimen comprised of either a daily injection of long-acting basal insulin in addition to a dose of insulin, typically a short- or fast-acting insulin, such as Humalog®, Apidra® or NovoLog®, with the datelargest meal or two injections of this report:premixed insulin, which combines long-acting and fast-acting formulations within a single insulin dose;
intensive therapy requiring multiple daily injections, or MDI, with syringes or preloaded insulin pens; and
continuous subcutaneous insulin infusion using programmable insulin pumps.
We believe conventional insulin therapy is among the least expensive insulin-based diabetes treatments and is typically initiated with a once-daily dose of a long-acting insulin. MDI intensive therapy with syringes can be effective and less costly than other therapies. MDI intensive therapy with insulin pens offers a more convenient alternative to syringes but can be more expensive. In addition, programmable insulin pumps offer an effective means of implementing intensive diabetes management with the goal of achieving near-normal blood glucose levels. However, we believe that patient concerns with lifestyle factors, ease of use, convenience and high costs have limited overall adherence to insulin regimens, resulting in a significant number of patients with type 2 diabetes failing to meet their A1C goals with MDI or the use of programmable insulin pumps.
We believe, based on customer feedback and experience, that the current insulin therapies described below present the following advantages and limitations for patients with type 2 diabetes.
|
| | |
| | |
| Basal Insulin |
|
Description: Once-daily dose of long-acting insulin (such as Lantus® and Levemir®) at bedtime or in the morning, although some patients require two injections (morning and bedtime). |
| |
| Advantages | | Limitations/Challenges |
| |
• Easiest to train, learn and correctly administer insulin as injections and can be performed at home • Least costly analog insulin therapy, which uses genetically altered (or chemically altered) human insulin designed to release injected insulin to more closely mimic human insulin, for patients with most favorable reimbursement coverage • Lowest risk for patient dosing error | | • Insulin absorption can have variability from day to day or between different patients such that insulin is not released over the entire intended delivery period • Basal insulin addresses fasting and pre-mealtime glucose levels only, no direct impact on mealtime glucose excursions • Most patients eventually need mealtime insulin to achieve their A1C goal |
|
Basal Insulin + 1 or Premixed Insulin |
|
Description: Considered a transition regimen towards MDI or intensive therapy typically consisting of a twice-daily injection regimen of either: (i) a daily injection of long-acting insulin (such as Lantus® and Levemir ®) at bedtime (basal) plus an injection of fast acting insulin (such as Humalog® and NovoLog®), before the day’s largest meal, or basal + 1; or (ii) premixed insulin injections before breakfast and dinner. |
| |
| Advantages | | Limitations/Challenges |
| |
• Basal +1 and Premix • Compared to basal only insulin regimens, provides insulin for at least one, or in the case of premix, two of the patient’s meals | | • Basal +1 and Premix • No insulin coverage for at least one meal each day, or in the case of Basal+1, two meals each day |
|
| | |
Name• Premixed Insulin • Injections can normally be performed at home • Single type of insulin used in a single device | | Age• Basal Insulin +1 • Additional patient co-pay for additional dose of mealtime insulin • Potential for dosing error increased with two types of insulin • Premix • Patients typically use more insulin, may be at increased risk of hypoglycemia and may gain more weight • Requires planning activities and eating around the timing of injections and absorption of insulin |
|
| Multiple Daily Injections—MDI (Intensive Therapy) |
|
Description: A once- or twice-daily injection of long-acting insulin at bedtime or in the morning (basal rate) plus an injection of fast-acting insulin before meals and, if appropriate, with snacks (bolus dose). |
| |
| Advantages | | Position Limitations/Challenges |
John E. Timberlake | |
• With strict adherence, can closely mimic the body’s normal physiologic pattern of insulin release • Allows insulin dosing based on the requirements of individual meals • Lower cost with favorable reimbursement coverage compared to programmable insulin pumps • Easier to teach, learn and correctly administer compared to programmable insulin pumps | | 53• Frequent injections (usually at least four per day) • Requires training around two different types of insulin and the need to carry two types of insulin or insulin pens • Potential for dosing error increased with two types of insulin • Requires careful planning of meals and other activities • Injections often administered outside the home creating adherence challenges especially around meals • Requires two patient co-pays |
|
|
| |
| | Chief Executive Officer, President and Director |
Erick J. LuceraProgrammable Insulin Pumps |
|
Description: A wearable electronic programmable insulin pump filled with a fast-acting insulin that delivers a continuous dose of insulin (basal rate) and the ability to deliver insulin with meals or snacks (bolus dose), based upon programmable settings and patient input. Most pumps require an infusion set to deliver the insulin in addition to the pump. |
| |
| Advantages | | 50 Limitations/Challenges |
|
|
• When used properly, can most closely mimic the body’s normal physiologic pattern of insulin release • Customized basal and bolus insulin doses • Eliminates the need for daily needle injections | | Chief Financial Officer |
Mark Conley | | 56 |
| | Vice President, Corporate Controller• Most complicated to teach, learn and Treasurer |
Geoffrey Jenkins | | 66 |
| | Executive Vice President, Manufacturing, Operationscorrectly administer and Research & Development |
Matthew Nguyen | | 48 | normally requires a proactive and adherent patient
| | Chief Commercial Officer |
Joseph Saldanha | | 53 |
| | Chief Business Officer |
Joe Mandato, D.M. | | 73 | • Bothersome to wear and least discreet alternative
| | Director |
Luke Düster | | 43 | • Most significant risk of dosing errors due to the wide range of programmable functions and features
| | Director |
Katherine D. Crothall, Ph.D. | | 68 |
| | Director |
Rodney Altman, M.D. | | 55 | • Highest up-front and maintenance cost
| | Director |
Peter Devlin | | 50 |
| | Director, Chair |
Brian K. Roberts | | 46 |
| | Director• Reimbursement coverage for patients with type 2 diabetes significantly less accessible than for injections |
Executive officers
Given the reasons cited by patients for non-adherence to and the limitations of currently prescribed insulin therapy, we believe simplicity of insulin delivery contributes to adherence with therapy. In turn, when patients more fully comply with their prescribed treatment regimen, we believe that insulin therapy will be more effective. While insulin syringes, insulin pens and programmable insulin pumps are appointedcapable of facilitating basal-bolus therapy, we believe these methods of administration generally lack the simplicity of operation and lifestyle adaptability desired by patients with type 2 diabetes. We believe that, in general, programmable insulin pump therapies tend to have more advantages for type 1 patients who may require varying basal rates over a 24-hour period or more complex bolus dosing regimens. These complexities are generally not encountered by patients with type 2 diabetes.
The following diagram demonstrates the benefits of V-Go as compared to other currently available insulin therapies in terms of simplicity of use and ability to mimic the body’s normal physiologic pattern of insulin delivery. We estimate that more than 95% of patients with type 2 diabetes are prescribed a regimen that relies on an insulin pen device or insulin syringe to deliver insulin including basal, basal + 1, premix and intensive therapy or multiple daily injection regimens.
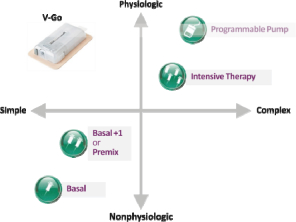
We believe V-Go is appealing to healthcare providers and patients because it combines the benefits of physiologic basal-bolus therapy with the convenience of a once-daily injection using just one type of insulin and lower insulin utilization compared to conventional injection (i.e. syringes or pens). Our internal studies indicate that these characteristics help support patient compliance with basal-bolus regimens, thereby improving glycemic control (lowers A1C). We also believe V-Go is an attractive option because it is discreet and simple to operate, yet mimics the body’s normal physiologic pattern of insulin delivery without the inconvenience associated with syringes and pens or the complexities associated with programmable pumps.
Our Solution
Simple, Discreet and Effective Type 2 Diabetes Management
V-Go fills a critical need of patients with type 2 diabetes who, we believe, desire and benefit from an easy-to-use, more discreet, basal-bolus insulin regimen. As depicted in the following image, V-Go is designed to be worn on the skin under clothing and measures just 2.4 inches wide by 1.3 inches long by 0.5 inches thick (excluding the adhesive component), weighing approximately less than two ounces when filled with insulin.
Specifically Designed for Patients with Type 2 Diabetes
Patients with type 2 diabetes who are prescribed intensive insulin therapy report the burden of multiple injections, embarrassment of injection and interference with daily activities as key factors for non-compliance with insulin therapy. Unlike programmable insulin pumps, V-Go is a 24 hour, disposable mechanical device that operates without electronics, batteries, infusion sets or programming and with fewer injections than using insulin syringes or pens. In the 2011 Roper Study, 72% of patients with type 2 diabetes prescribed basal-bolus injectable insulin regimens reported not taking injections away from home, making it difficult for many of them to remain in compliance with their prescribed therapy. V-Go was designed to facilitate basal-bolus therapy compliance by removing the complexity and stigma of insulin injections in patients with type 2 diabetes. In patients we surveyed prior to starting V-Go and again 30 days after being on V-Go, 53% of patients found V-Go very convenient compared to only 10% reporting their prior therapy was very convenient. In this same survey, 64% of patients felt their quality of life, based on how they felt physically or mentally, was generally good to excellent compared to only 35% prior to V-Go.
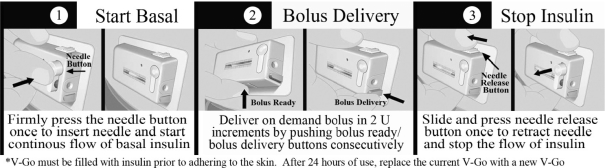
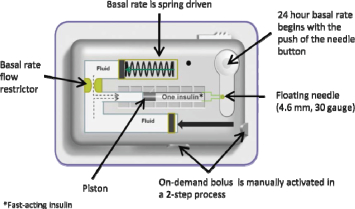
The following diagram demonstrates the basal and bolus functions of V-Go. The bolus operation can be completed through the patient’s clothing.
Simple, Effective and Innovative Approach to Insulin-Based Diabetes Management
V-Go utilizes our proprietary h-Patch drug delivery technology to enable patients to closely mimic the body’s normal physiologic pattern of insulin delivery by delivering a single type of insulin at a predictable and continuous preset basal rate over a 24-hour period and providing convenient and discreet on-demand bolus dosing at mealtimes. We believe V-Go is a simple and effective approach to insulin therapy and facilitates patient adherence to basal-bolus insulin regimens, which leads to better patient results. In a series of clinical studies examining patients with diabetes using V-Go, clinically relevant reductions in A1C levels were observed after switching to or initiating V-Go therapy, as well as reductions in the prescribed total daily insulin dose. These findings are summarized in the following figure and are described in more detail under “—Extensive Clinical Evidence Demonstrating Results.” As with all research, study design limitations should be considered when interpreting the results and whether or not the results can be applied to a general population. Refer to the full findings in the referenced public disclosure for additional information.
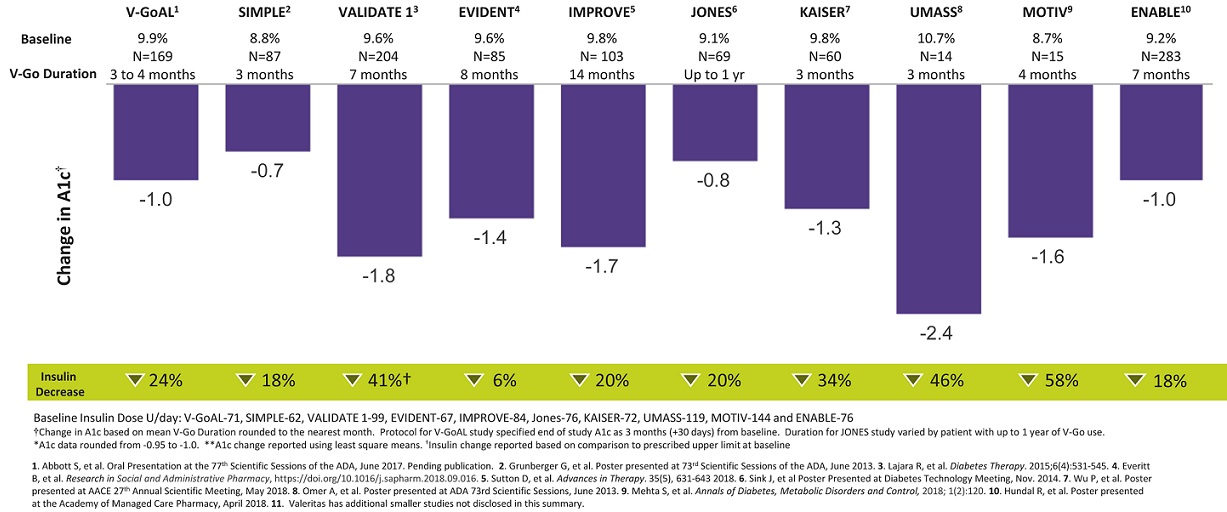
V‑GoAL=randomized controlled trial. SIMPLE=multicenter prospective observational study. VALIDATE 1= multicenter retrospective study. EVIDENT= retrospective study. IMPROVE= multicenter retrospective study. JONES= The Jones Center for Diabetes & Endocrine Wellness, retrospective study. KAISER= multicenter internal evaluation of V‑Go. UMASS=University of Massachusetts, retrospective study. MOTIV=retrospective pilot study. ENABLE=multicenter retrospective study.
User-Friendly Design
In addition to its small size and dosage versatility, V-Go offers many additional user-friendly features designed to treat and improve the quality of life of patients with type 2 diabetes requiring insulin, including:
using a single fast-acting insulin, such as Humalog® or NovoLog®, rather than a combination of multiple types or premixed insulin;
not requiring patients to carry syringes, pens or other supplies for mealtime bolus dosing;
offering the convenience of pressing buttons for on-demand bolus dosing through clothing;
allowing patients to easily maintain their daily routines and activities, including showering, exercising and sleeping;
only requiring application of a new V-Go every 24 hours, which offers patients the flexibility to selectively choose an application site that best suits the day’s activities; and
not burdening patients with the complexities associated with learning to use an electronic device or programming a pump.
Cost Effective for Payor and Patient Alike
V-Go is generally a cost competitive option for payors and patients when compared to insulin pens, which is the delivery method prescribed for a majority of all insulin therapies and approximately 66% of newly prescribed basal and mealtime insulin therapies. V-Go is available at retail and mail order pharmacies and is covered by Medicare as well as commercial insurance plans covering a majority of patients. As a result, out-of-pocket costs for covered patients using V-Go are generally equivalent to what they would pay if taking basal-bolus injections with insulin pens or syringes. We believe that, from a payor’s perspective, using V-Go for insulin delivery will generally be associated with an equal or lower cost, net of rebates and co-pays, to treat a patient compared to the cost of using multiple daily injections to deliver basal-bolus insulin therapy. This opinion is supported by a recent retrospective analysis by Raval, et al using medical and pharmacy claims from HealthCore Integrated Research Database to evaluate the clinical and economic outcomes for V‑Go compared to standard multiple daily injection insulin therapy for treating type 2 diabetes. With similar baseline number of insulin Rx fills and insulin total daily dose (TDD), V‑Go users had fewer insulin Rx fills (p<0.001), and a 21% decline in insulin TDD (p<0.001), as compared to MDI users, during the last 6 months of follow‑up. Also, with similar baseline diabetes‑related medication costs, V‑Go users experienced a lower increase in diabetes‑related medication costs ($1287, p=0.012), as compared to MDI users, during the last 6 months of follow‑up.
Moreover, insulin delivery with V-Go is significantly less expensive, especially in the first year, than treatment with programmable insulin pumps. This cost difference in the first year is attributed to the programmable durable medical equipment component associated with electronic insulin pumps that are not needed for V-Go. This durable medical equipment component can have initial costs for the pump and supplies of approximately $5,000 per device. In addition, daily consumables such as tubing and insertion sets are required for electronic pump therapy and not on V-Go.
Another consideration is out-of-pocket costs for patients with different diabetes regimens. Pharmacy formularies are separated into multiple tiers, of which Tier 2 and Tier 3 are most applicable to the V-Go. Tier 1 products are the lowest cost tier of prescription products, which mainly consists of generic drugs; Tier 2 products are generally preferred brand name products, for which co-pays are more than Tier 1; and Tier 3 consists mainly of non-preferred brand name products, which are more expensive than Tier 2. We believe that patient costs can be neutral when switching from basal-bolus insulin pen therapy to V-Go therapy. As every payor and every employer plan within a payor has their own co-pay structure, we reached this conclusion by utilizing national averages provided by a national employer health benefits survey conducted in 2014 by Kaiser Permanente to make comparisons. We estimated co-pays for insulin pens and pen needles to be $73 per month, which assumes national Tier 2 co-pay equal to $31 for each pen box and $11 for pen needles. The V-Go co-pay can be $31 for Tier 2 or $53 for Tier 3 for a month supply, depending on which formulary Tier it is assigned to. Insulin vials for V-Go would be similar to insulin pens at $31 per month. Therefore, expected monthly co-pay for V-Go can be $62 when in Tier 2 or $84 when in Tier 3, which is essentially cost neutral to patients.
Comprehensive Customer Support
The majority of patients using V-Go are trained to use the device by their healthcare provider or a Certified Diabetes Educator, or CDE, who has been trained by our sales force using a “train the trainer” approach. Our sales force has the ability to train patients directly or offer training through a contracted trainer and also trains physicians, physicians’ assistants, nurse practitioners, CDEs and any other staff in a healthcare provider’s office who are responsible for training their patients to properly use V-Go. Additionally, we provide a starter kit for new V-Go patients, which contains all the materials a patient needs to deliver basal-bolus insulin therapy with V-Go. We also offer supplemental training support and resources when healthcare providers or patients need additional V-Go training assistance, including online resources such as a learning management system and online videos.
Our V-Go Customer Care Center, or VCC, is a live customer care center operating 24 hours a day, seven days a week. The VCC provides broad-based V-Go operational assistance to healthcare providers, patients, caregivers and pharmacists. Every patient is encouraged to call the VCC in order to opt-in for support and, once a patient does opt- in, a VCC staff-member proactively contacts the patient at various times to provide additional patient support and promote proper use of V-Go. VCC representatives can also train patients on the operational aspects of V-Go either via phone or video. The VCC also offers a reimbursement support service to answer patients’ reimbursement-related questions.
Extensive Clinical Evidence Demonstrating Results
The V-Go solution to type 2 diabetes management is focused both on A1C management and on providing patients the requisite support to achieve their goal of improved health. We and others have conducted several studies, analyses and research surveys to evaluate the role of V-Go in A1C management. These studies include prospective studies, user preference studies, retrospective analyses of electronic medical records and patient and physician surveys. The results of these studies and analyses are described below.
V-GoAL: A Randomized Prospective Pragmatic Clinical Trial to Compare the Real-World Use of V-Go in Type 2 Diabetes Patients to Standard Treatment Optimization
In collaboration with HealthCore Inc., we conducted a randomized, observational, prospective trial to assess the effectiveness of V-Go compared with standard treatment optimization, or STO in adult patients with type 2 diabetes treated with insulin with or without concomitant anti-hyperglycemic medications in the United States in a real-world, community-based practice setting. Participating clinics were randomized to enroll patients to insulin delivery with V-Go or to optimize current insulin treatment based on standard of care for up to 4 months. Patients in the V-Go group discontinued baseline insulin therapy and patients in the STO group continued baseline insulin therapy and could add and/or change diabetes-related therapy to improve treatment. The primary endpoint was change in A1C from baseline to the end of study.
The analysis population consisted of 169 V-Go patients and 246 STO patients from 52 clinical sites. Baseline A1C ranged from 7.9% to 14.2% with a mean A1C of 9.9% in the V-Go group and 9.3% in the STO group. The mean insulin total daily dose or TDD at baseline was not significantly different between groups (V-Go 71 units/day vs STO of 72 units/day). Both groups demonstrated significant reductions in A1C from baseline with those in the V-Go group demonstrating significantly greater A1C reductions when compared to STO (V-Go=0.95% vs. STO=0.46%).
SIMPLE Study: A Prospective Clinical Trial to Evaluate Effectiveness of V-Go Across Multiple Centers
We conducted a multicenter prospective clinical trial of 89 patients to evaluate the effectiveness of V-Go for patients with diabetes in a real-world setting. Findings from this study have been presented in past years at the American Diabetes Association and American Association of Clinical Endocrinologists Annual Scientific Congresses. The primary objective was to compare changes of average glycemic control as measured by A1C from baseline to the end of V-Go use. Patients with type 2 diabetes who were administering one or more insulin injections per day and not meeting target A1C control of less than 7% were included in the study. The patient types included in this trial were:
Basal: Patients receiving once or twice daily injections of an intermediate- or long-acting insulin regardless of oral anti-diabetes medication (OAD) use (26 patients).
Premix: Patients receiving one to three daily injections of premix insulin regardless of OAD medication use (13 patients).
MDI: Patients receiving any insulin therapy with three or more insulin injections a day regardless of OAD medication use (47 patients).
Other: Patients receiving OADs only and patients receiving OADs plus non-insulin injectable (3 patients).
A1C levels in the overall population (n=89) decreased from 8.8% at baseline to 8.1% at month three, representing a statistically significant reduction of 0.7% with a p-value less than 0.0001. A1C levels were not available for two of the 89 patients at 3 months, therefore the statistical analysis is based on repeated measures for 87 patients.
When classifying the data by subgroups, both the basal and MDI subgroups demonstrated statistically significant A1C reductions from baseline (basal -0.76%, p=0.0003; Premix -0.66%, p=0.3006; MDI -0.66%, p=0.0002). In addition to the significant improvements in blood glucose, the average daily dose of insulin across all patients was also reduced by 18% (62.4 to 51.0 units, p=0.001) from baseline. A small but statistically significant decrease in body weight (-0.71 lbs., p<0.0001) was observed, although not clinically meaningful given the baseline weight of the patient population. Overall, the incidence of hypoglycemia after three months of V-Go use was low, with 90% of patients reporting no hypoglycemia.
VALIDATE 1: Use of the V-Go Insulin Delivery Device in Patients with Sub-optimally Controlled Diabetes Mellitus
A study was initiated by Lajara et al. in 2014 to evaluate the effect of switching patients with sub-optimally controlled (A1C > 7.0%) diabetes to V-Go. The study was conducted as a retrospective review of the EMR database for a specialized diabetes comprehensive care clinic setting which includes 13 centers located across major metropolitan areas of Texas. Patients were prescribed V-Go by health care providers as part of their standard clinical practice with the goal of improving A1C levels. The primary endpoint was change in A1C from baseline. Secondary endpoints included change in insulin dose, body weight, and hypoglycemic events.
The mean time from start of V-Go to the first follow-up visit was 13.87 ± 6.14 weeks and the mean time to the second follow-up visit was 26.86 ± 8.96 weeks. Results will therefore be presented for 14-week and 27-week visits. Of 245 eligible patients based on inclusion criteria, 204 were included in the analysis population.
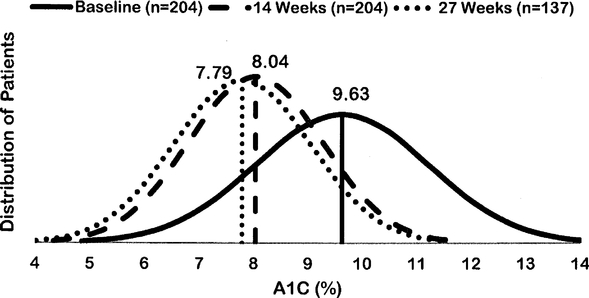
The distribution of A1C values for the study population was compared to baseline for each follow-up A1C time point in the figure below. On V-Go, the A1C distribution curve has narrowed and progressively shifted to the left, representing a reduction in variability and lower A1C values. We did not commission Diabetes Centers of America to conduct this study.
Change in A1C Distribution: Improved A1C Across the Entire Population
Change in A1C distribution. A1C data are arithmetic means at baseline (week 0) compared to first recorded A1C on V-Go (14 week mean) and second recorded A1C on V-Go (27 week mean). Curves represent the A1C distribution of patients for each time point based on available data. These results were published in a peer reviewed journal, Diabetes Therapy in 2015.
EVIDENT: Clinical and Economic Considerations based on Persistency with a Novel Insulin Delivery Device vs Conventional Insulin Delivery in Patients with Type 2 Diabetes
In 2016, Everitt et al. conducted a retrospective analysis in patients with type 2 diabetes to evaluate the clinical and economic impact of persistent use (>150 days) of V‑Go to resumed persistent use of conventional insulin delivery (CID) in patients discontinuing V‑Go after short‑term use. EMR were used to identify patients based on predetermined criteria. Insulin TDD, A1C, self‑monitored glucose or SMBG logs, all concomitant diabetes medications and weight were collected at baseline and at each follow‑up visit. Data analysis was conducted for all patients and was stratified based on persistent use of V‑Go or persistent use of CID in those that discontinued V‑Go. There were no significant differences in persistency time between groups, with V‑Go patients averaging a little over 8 months and CID patients nearly 7.5 months.
Persistent use of V‑Go resulted in a significantly greater reduction in A1C (−1.42 vs −0.20; p=0.003) with significantly less prescribed insulin (difference of 17.39 u/day; p=0.003) and resulted in a lower change in therapy cost. Persistency with V‑Go proved more cost‑effective compared to persistent CID use as the incremental cost impact to the pharmacy budget was $695.61 less per 1% change in A1C. These results were published in a peer reviewed journal, Research in Social and Administrative Pharmacy in September 2018. We provided an educational grant to the lead study author to conduct this retrospective analysis.
IMPROVE: Clinical Benefits Over Time Associated with V-Go
In 2015, we provided an educational grant to a large specialty clinical practice in Northeast Florida to conduct a retrospective analysis to evaluate the impact on glycemic control of switching to V-Go for patients with diabetes that were sub-optimally controlled. 103 patients were evaluated across four follow-up visits when an A1C result was recorded. Duration of V-Go use was approximately two, six, ten and fourteen months for the first, second, third and fourth follow-up visits. The baseline A1C was 9.8%. Statistically significant A1C reductions from baseline were seen at each of the four follow-up office visits. After six months of V-Go use, a reduction in A1C of 1.7% (p<0.001) was observed and sustained at 14 months. For the 80 patients previously administering insulin at baseline, a substantial reduction in total daily insulin from baseline was also observed at all follow-up visits. Insulin was reduced from 84 to 67 units/day (p<0.05) in these 80 patients after fourteen months of V-Go use. There was no reported change in the incidence of hypoglycemia compared to baseline. These results were published in a peer reviewed journal, Advances in Therapy in May 2018. We did not commission NEFEDA to conduct this study.
Jones: The Jones Center for Diabetes & Endocrine Wellness Clinical Evaluation
A retrospective clinical analysis was conducted to evaluate the clinical experience with V-Go in 91 patients treated at the Jones Center for Diabetes and Endocrine Wellness, a specialized diabetes care clinic. Using electronic medical records, clinical data was collected at V-Go initiation and up to one year of follow-up. Prior to V-Go initiation, 39.6% of patients were prescribed only insulin and 58.2% were prescribed combination therapy that included insulin. Of the 86 patients with type 2 diabetes, 69 patients, or 80%, had at least one follow-up visit. Mean baseline A1C in this group was 9.1% at baseline and 8.3% at follow-up for an average improvement in A1C of 0.8% (not tested for statistical significance). The mean total daily dose of insulin at baseline was 76 units and decreased to 61 units, a 20% (not tested for statistical significance) reduction, on V-Go. These results were also published and presented in the Journal of Diabetes Science and Technology. We did not commission the Jones Center to conduct this study.
Kaiser: Short‑Term Evaluation of Clinical Effectiveness.
A three month pragmatic evaluation was conducted across multiple clinics within the Southern California Kaiser Permanente Group. The purpose was to determine if switching to V‑Go insulin delivery device resulted in improved A1C control in patients with type 2 diabetes poorly controlled on prior regimens. Each participating clinician initiated and intensified therapy based on standard clinical practice. V‑Go devices were provided by the Boardmanufacturer for the 3 month study. Changes in A1C and insulin TDD as well as percentage of Directorspatients achieving A1C ≤ 9.0% were assessed.
Out of 85 patients initiated on V‑Go, 60 completed the evaluation with a baseline mean A1C of 9.8% and serveinsulin total daily dose of 72 units/day (range 30 to 200 units/day). Prior to initiating V‑Go, 60% of patients were poorly controlled defined by A1C values > 9.0%.. After 3 months of insulin delivery with V‑Go, significant mean reductions in A1C (−1.3%; p<0.0001) and TDD (−34 units/day; p<0.0001) were observed. Achievement of A1C ≤ 9.0% increased from 40% before V‑Go to 78% on V‑Go (p<0.0001).
UMASS: The University of Massachusetts Clinical Evaluation
In 2013, researchers at the University of Massachusetts examined 21 patients with type 2 diabetes who lacked glycemic control and switched from MDI therapy to V-Go. The clinical evaluation observed that, after 88 days of V-Go use, based on data from 14 of the 21 patients observed, A1C levels decreased from 10.7% to 8.3% (p<0.001) and total daily doses of insulin decreased by 46% from 119 units to 64 units (p=0.01). These results were also published and presented at the 73rd Scientific Sessions of the American Diabetes Association in June 2013. We did not commission nor sponsor this study.
MOTIV: Retrospective Proof‑of Concept Study Evaluating Optimization and Titration of Dosing with V‑Go
A retrospective proof‑of‑concept analysis based on data from EMR was conducted by Mehta et al. The objective was to evaluate the safety and efficacy of a physician‑directed insulin titration algorithm in patients prescribed V‑Go who were diagnosed with type 2 diabetes and were ≥ 21 years old. Primary endpoints were achievement of A1C targets (<7.5%) and prevalence of hypoglycemia. Of the 24 patients screened, 15 patients met eligibility requirements and were evaluated after 4 months of V‑Go use. Patients kept daily four‑point (fasting and 2 hour postprandial breakfast, lunch, and dinner) self-monitored blood glucose profiles which were used for weekly titration decisions. Based on the algorithm, bolus up‑titration was recommended weekly when 2 hour postprandial averages exceeded 170 mg/dl and down‑titrated when 2 hour postprandial averages were below 100 mg/dl. Basal rates were adjusted if needed following the optimization of bolus dosing for all meals.
A majority of the insulin titration occurred over the first three weeks with bolus up‑titration occurring in 73% of patients (from 18 to 33 total bolus units/day) at week one. V‑Go basal dose was increased in 5 patients and decreased in 1 patient by month one.
The A1C target of <7.5% was achieved in 67% of patients after switching to V‑Go for 4 months. A mean significant A1C reduction of −1.6% (8.7 to 7.1%; p<0.001) was observed despite a significant decrease in the mean insulin TDD (144 to 60 U/day; p=0.002). No change in weight from baseline was observed. Incidence of hypoglycemia decreased from 23% of patients at baseline to 7% of patients by month four. These results were published in a peer reviewed journal, Annals of Diabetes, Metabolic Disorders and Control in October 2018. We did not commission nor sponsor this study.
ENABLE: Multicenter Retrospective Study Evaluating Effectiveness of V‑Go
Hundal et al. conducted a retrospective multi‑state analysis to evaluate the clinical benefits of switching patients from insulin delivery via insulin pens/syringes to V‑Go. Electronic medical records across 9 specialty diabetes centers were used to identify patients and evaluate change in A1C and TDD. Patients (N=283) were evaluated after a mean of 3 and 7 months of V‑Go use.
A significant reduction in A1C of 1.0% (p<0.0001) from a baseline of 9.2% after 3 months of V‑Go use was observed and sustained at 7 months. Insulin total daily dose was reduced by 17 units/day and 14 units/day (p<0.0001) from a baseline of 76 units/day at 3 and 7 months, respectively
Clinical Evidence Summaries
Across these multiple peer-reviewed and published clinical trials, analyses and surveys using both prospective and retrospective study designs, switching patients who had suboptimal glycemic control to V-Go for insulin delivery resulted in statistically significant improvements in A1C, with A1C reductions ranging from 0.7% to 2.4%, depending on the patient population. Moreover, across this body of evidence, switching patients who had suboptimal glycemic control to V-Go for insulin delivery resulted in better glucose control with less insulin being used. Daily insulin dose reductions ranged from 7% to 46%, depending on the study and influenced by the amount of insulin patients were prescribed prior to using V-Go. Currently, clinical evidence in support of V-Go has been disclosed across 14 publications and 59 presentations and represents clinical experience in > 1500 patients with diabetes.
Our Current and Future Products
We believe our core technology represents a fundamentally different approach to basal-bolus insulin delivery. To facilitate therapy compliance, we have sought to eliminate the need for complex electronics and software by utilizing mechanical technology that delivers prescribed dosages of insulin and other injectable drugs with great accuracy without any electronics, batteries, recharging or programming.
For users and health care providers who desire additional data points, the Company is developing a V-Go accessory device which provides injectable drug delivery details through Bluetooth communication with smart devices utilizing a Valeritas developed application.
V-Go Wearable Insulin Delivery Device
V-Go is a wearable insulin delivery device for basal-bolus therapy that deploys our innovative proprietary h-Patch technology. Unlike programmable insulin pumps, V-Go is a small, discreet, daily-disposable insulin delivery device that operates without electronics, batteries, infusion sets or programming. V-Go measures just 2.4 inches wide by 1.3 inches long by 0.5 inches thick and weighs approximately less than two ounces when filled with insulin.
V-Go enables patients to closely mimic the body’s normal physiologic pattern of insulin delivery by delivering a single type of insulin at a continuous preset basal rate over a 24-hour period and also providing for on-demand bolus dosing at mealtimes, without the need for electronics or programming. A patient adheres V-Go to his or her skin and presses a button that inserts a small needle that commences a continuous preset basal rate of insulin. At mealtimes, a patient can discreetly press the bolus ready and bolus delivery buttons to deliver insulin on-demand at meals.
Each day prior to applying V-Go, a patient fills it with insulin using a filling accessory known as EZ Fill, which is included with each monthly supply of V-Go. V-Go uses a single type of fast-acting insulin, such as Humalog® or NovoLog®, and is available in a preset basal rate to continuously deliver 20, 30 or 40 units of insulin in one 24-hour period (0.83, 1.25 or 1.67 units per hour, respectively) and on-demand bolus dosing in two unit increments (up to 36 units per 24-hour time period). Our proprietary Floating Needle is deployed with the press of a button after V-Go is applied to the skin making the connection between the insulin reservoir and the patient’s tissue. The Floating Needle then pivots with the body’s natural movements, allowing for maximum comfort. After 24 hours of use, a patient presses a button that retracts the needle and then removes V-Go from the skin, discards V-Go in regular trash and replaces it with a new insulin-filled V-Go for the next 24 hours. The EZ fill device makes the filling process simple and does not require calculations, measuring or needles. The use of the EZ fill device can also prevent accidental needle sticks that occur with pen needles or syringes.
h-Patch Controlled Delivery Technology Platform
Our proprietary hydraulic h-Patch drug delivery core technology, which is a critical component of V-Go, facilitates the simple and effective delivery of injectable medicines to patients across a broad range of therapeutic areas. The deployment of our h-Patch technology results in a device specifically designed for patients with type 2 diabetes who, we believe, do not require complex and costly programmable insulin pumps generally designed to meet the needs of type 1 patients.
The hydraulic approach of our h-Patch technology can be used to deliver constant basal or on-demand bolus dosing of any drug than can be delivered subcutaneously. We believe it combines the user advantages of transdermal patches with the accuracy and flexibility of conventional electronic pumps. Once activated, our h-Patch device places a custom-formulated viscous fluid under pressure, which is separately compartmentalized and therefore designed not to come into contact with the active drug. Once pressurized, the fluid is forced through a flow restrictor that is designed to control the flow rate. After passing through the flow restrictor, the viscous fluid couples with and moves a piston in a cartridge that contains active drug. The viscous fluid continually pushes the piston, dispensing the drug at the prescribed preset basal rate through a needle into the patient’s subcutaneous tissue. Bolus delivery on demand is similarly driven by viscous fluid dispensed from a separate side chamber, which allows a patient to dispense active drug in two unit increments through a user-activated bolus button. Our h-Patch basal drug delivery technology results in a simple, yet innovative, device that operates without complex controls or an infusion set.
The operation of our h-Patch technology is depicted in the graphic below:
h-Patch Controlled Delivery Technology
We will continue to explore the use of our h-Patch technology in other drug delivery applications beyond the use of insulin to treat type 2 diabetes. We believe it has the potential to improve the utility of a variety of drugs that require frequent and cumbersome dosing regimens.
Next-Generation V-Go: V-Go Prefill & V-Go® SIMTM (Simple Insulin Management)
We are developing a next-generation, single-use disposable V-Go device that will feature a separate prefilled insulin cartridge that can be snapped by the patient into V-Go. While the current V-Go simplifies the use of insulin for patients with type 2 diabetes, we believe that a pre-filled V-Go will make insulin therapy even simpler by eliminating the device-filling process by the patient and the need for EZ fill refrigeration, which we expect could further promote adoption by patients with type 2 diabetes. Additionally, we believe V-Go Prefill could lower the number of co-pays because the insulin and V-Go would be packaged together, generate revenue from the sale of insulin and extend the patent life to 2032. A pre-filled V-Go would also enable V-Go usage for other injectable therapeutic drugs beyond insulin that are used by patients who could benefit from simple, convenient and continuous drug delivery. Currently, the V-Go Prefill is in the design-development stage, with a focus on ease of customer use and optimization of manufacturing efficiency.
We are in the later stages of developing V-Go SIMTM, which we intend to feature one-way communication to glucose meters and smart devices such as phones and tablets through RF/Bluetooth technology. V-Go SIMTM is a snap-on accessory for use with V-Go, is optional to use, and does not change the functionality of V-Go. We intend V-Go SIMTM to provide real-time tracking information of basal and bolus dosing utilization, allowing patients and their healthcare professionals to have a deeper understanding of their current dosing habits. We believe access to this technology could increase patient adherence and could be used as a diagnostic tool to make treatment adjustments.
Our Strategy
Our long term goal is to significantly expand and further penetrate the type 2 diabetes market and become a leading provider of simple-to-use insulin delivery devices designed for basal-bolus insulin therapy.
In 2016, we made a significant adjustment in our commercialization strategy by shifting from aggressively expanding sales representative headcount to focusing on fewer high—volume insulin prescribers and on maximizing our sales and marketing infrastructure’s frequency of interactions and contact points and methods with high-prescribing physicians. We estimate as of December 31, 2018 our sales force structure can directly focus on approximately 1,000 prescribers. This restructuring reduced our monthly cash burn rate significantly and resulted in a business plan that is more capital efficient.
We have learned that, in the competitive type 2 diabetes market, those prescribers of diabetes products who had more contacts with our sales representatives and marketing programs, both directly and indirectly, remember and prescribe V-Go to more patients, and more appropriate patients, and we believe that, under our prior sales and marketing model, we had diluted our resources across too many sales territories. By focusing on fewer and prioritized markets, while increasing the contacts each healthcare professional has through supplemental inside sales team, an inside peer to peer clinical sales team and through other marketing resources, we can be more competitive in those markets.
Our current business strategies include the following:
We intend to continue to focus the majority of our resources towards the prioritized markets through the use of sales professionals, inside sales, peer-to-peer programs, targeted direct-to-patient promotions, customer care and additional multi-channel promotional services. Some examples of multi-channel promotion include direct mail, search engine optimization, peer-to-peer email, and other forms of advertising. We intend to optimize our customer acquisition, conversion and retention programs by focusing all our resources. We believe this should allow us to significantly increase our promotional efforts on a per-territory basis, which will allow us to grow these markets. In addition, we plan to improve on and leverage our patient programs such as V-Go life, patient forums, enhanced starter kits and in-office material and promotion, such as context media to empower patients to ask for V-Go as an option for their diabetes treatment.
We intend to continue to drive V-Go sales growth by:
Leveraging the clinical and economic data that has been published in the last year, including several recent manuscripts, with healthcare professionals and payors. We believe this new data will help more prescribers see the value and understand the benefits of V-Go across a wider spectrum of patients.
Expanding third-party reimbursement for V-Go in the United States. We intend to expand patient coverage of V-Go by commercial insurance plans as a pharmacy benefit rather than a medical benefit. We believe that more than 70% of the approximate 158 million commercially insured lives in the United States and 60% of the approximate 43.5 million lives insured by Medicare Part D cover V-Go. In addition, TRICARE, a health care program of the United States Department of Defense Military Health System, covers V-Go as a pharmacy benefit and some State Medicaid plans cover V-Go as a pharmacy or medical benefit. We also offer reimbursement support services to assist patients in gaining access to V-Go throughout the reimbursement process.
Pursing our new business model. We intend to increase use and grow V-Go share by initially targeting approximately 20 to 30 doctors per sales representative and increasing to approximately 50 potential targets over time. We will either add new territories or split existing territories when adding new sales reps. We will expand our direct sales reach to approximately 300 additional prescribers so that we can provide the same higher level of inside sales and promotion as we provide our current prioritized markets. We believe we can grow these markets more quickly than our territories have grown in the past, since they will have significantly more support with our new strategy.
Our long-term business strategies include the following.
Continue to expand our U.S. sales force in a capital efficient and disciplined manner utilizing our new business model. We intend to eventually establish a national sales force, internally or through other means, such as
contract sales organization, co-promotion or other strategic relationships to ensure we can reach all the very high volume prescribers or explore other means to increase the number of prescribers we can reach.
Continue to explore additional international expansion opportunities. We intend to continue exploring international expansion through strategic collaborations, in-licensing arrangements or alliances outside the United States which not only would provide a revenue stream, but would also increase our production volume thereby improving our gross margins in the United States.
Capture Improved Economics Through the Commercialization of V-Go Prefill. We are developing and intend to commercialize our V-Go Prefill product, if it is approved by the FDA, which we believe would offer patients an even more simplified user experience, thereby increasing our target market to include patients with type 2 diabetes not currently on insulin. In addition, we expect to have additional opportunities to generate revenue through the sale of insulin in connection with V-Go Prefill. We believe a prefilled option would also lay the foundation for using our proprietary h-Patch technology with other injectable therapies where patients could benefit from simple, convenient and continuous drug delivery.
| |
| • | Advance our V-Go SIMTM next generation technology, which will feature one-way communication to smart devices such as phones and tablets through RF/Bluetooth technology. V-Go SIMTM is a snap-on accessory for use with V-Go, is optional to use, and does not change the functionality of V-Go. V-Go SIMTM will provide real-time tracking information of basal and bolus dosing utilization, allowing patients and their healthcare professionals to have a deeper understanding of their current dosing habits. |
Advance our Proprietary h-patch Drug Delivery Technologies into Other Therapeutic Areas. We have built a significant portfolio of proprietary technologies, designed to simply and effectively deliver injectable medicines to patients across a broad range of therapeutic areas. We intend to continue to advance these technologies, either by working with third parties to incorporate them into existing commercial products or by licensing the rights to them to third parties for further development and commercialization.
Leverage Our Scalable Manufacturing Operations to Increase Gross Margin. We intend to leverage our scalable and flexible manufacturing infrastructure and related operational efficiencies to increase our gross margin by reducing our product costs. We believe the existing production lines of our contract manufacturer, or CMO, will have the ability to meet our current and expected near-term V-Go demand. Our CMO also has the ability to replicate additional production lines within its pleasure.current facility footprint. In addition, we believe that due to shared product design features with V-Go, our production processes are readily adaptable to the manufacture of new products, including a prefilled V-Go.
Sales, Marketing and Distribution
As of December 31, 2018, our sales force covers 52 territories primarily within the East, South, and Midwest regions of the United States. We also have a team of inside sales representatives to take incoming calls from interested healthcare professionals as well as a targeted list of V-Go prescribers around the country. To date, we have focused our sales and marketing efforts in the regions where we have the greatest reimbursement coverage for patients. According to Symphony Health Solutions, there are approximately 16,000 high-volume insulin prescribers in the United States, generating 40% of all U.S. annual insulin prescriptions. Our sales representatives call on approximately 1,000 of these targeted, high-volume insulin prescribers, which include endocrinologists and primary care physicians. Our sales team has been supplemented by our V-Go Customer Care Center that provides support to customers and healthcare providers.
V-Go is distributed primarily through retail pharmacies and, to a lesser extent, medical supply companies. Similar to a pharmaceutical company, our overall distribution strategy focuses on making V-Go available at retail and mail-order pharmacies. We have adopted this strategy because patients with type 2 diabetes frequently visit their local retail pharmacies to fill other prescriptions prescribed for their other chronic conditions. We have distribution agreements with all of the national and many regional wholesalers, as well as with important medical supply companies. For the year ended December 31, 2018, the wholesale distributors McKesson Corporation, Cardinal Health and AmerisourceBergen Drug Corporation represented 37.3%, 24.6% and 29.4%, respectively, of our total product shipments. Our agreements with our distributors allow a patient whose insurance covers V-Go as either a pharmacy benefit or a medical benefit to be able to fill his or her V-Go prescription conveniently. Our agreements with each of Cardinal Health and AmerisourceBergen Drug Corporation are each on a one-year continuous renewal basis unless otherwise terminated by either party. Our agreement with McKesson Corporation has an indefinite term unless otherwise terminated by either party.
A patient using V-Go requires two separate prescriptions, one for V-Go itself and one for fast-acting insulin, such as Humalog® or NovoLog®, in vials. As V-Go is only available by prescription, we believe that educating physicians and other healthcare
providers regarding the benefits of V-Go is an important step in promoting its patient acceptance. In addition to calling on healthcare providers, our marketing initiatives include presentations and product demonstrations at local, regional and national tradeshows, including ADA Scientific Sessions and the American Association of Diabetes Educators Annual Meeting.
Reimbursement
In contrast to all other basal-bolus insulin delivery devices currently on the market in the United States, V-Go is not classified as a durable medical device, thereby allowing for potential Medicare reimbursement under Medicare Part D. As a result, a patient with Medicare, whose Medicare Part D Plan chooses to cover V-Go, can fill his or her V-Go prescription at a retail pharmacy that participates in the plan. Even for Medicare patients whose Medicare Part D Plan elects not to cover V-Go on formulary, those patients may still get V-Go at the pharmacy through a medical exceptions appeal process. In addition to the 60% of patients insured by Medicare Part D who have V-Go covered under their plans, a majority of commercially insured patients currently are covered for V-Go under their plans as either a pharmacy benefit or a medical benefit. For the year ended December 31, 2018, over 90% of our V-Go prescriptions were filled by retail or mail order pharmacies with the remainder filled by medical supply companies.
Manufacturing and Quality Assurance
We currently manufacture V-Go and EZ Fill in clean rooms at our CMO in Southern China in accordance with current good manufacturing practices, or cGMP. Our CMO uses Valeritas-owned custom-designed, semi-automated manufacturing equipment and production lines to meet our quality requirements. Separate CMOs in China perform release testing, sterilization, inspection and packaging functions.
V-Go is produced on flexible semi-automated production lines. In 2018, our CMO operated single-shifts in two manufacturing lines for the majority of the year. A second shift was added on one manufacturing line in December. Production across all lines in 2018 totaled approximately 4.2 million V-Go units. Each line has the capacity to run two shifts per day. We have two additional lines outfitted at our CMO on standby - with a combined theoretical annual capacity of 7.9 million V-Go Units - that can be quickly brought on-line as demand increases. We believe these production lines will have the ability to meet our current and expected near-term V-Go demand in the U.S. as well as for any distributor outside of the U.S. We also believe our CMO has the ability to scale production even further by replicating these production lines within its current facility. Furthermore, we believe that, due to shared product design features, our production processes are readily adaptable to new products, including a pre-fill V-Go.
V-Go is packaged with one EZ Fill accessory per 30 V-Go devices. Due to its lower-volume requirements, one manufacturing line is dedicated to EZ Fill production, with a second line on standby.
Both V-Go and its insulin filling accessory, EZ Fill, are assembled from components that are manufactured to our specifications. Each completed device is tested to ensure compliance with our engineering and quality assurance specifications. A series of automated inspection checks, including x-ray assessments and lot-released testing, are also conducted throughout the manufacturing process to verify proper assembly and functionality. When mechanical components are sourced from outside vendors, those vendors must meet our detailed qualification and process control requirements. We maintain a team of product and process engineers, supply chain and quality personnel who provide product and production line support for V-Go and EZ-Fill. We also employ a full-time dedicated contractor based in China.
We have received ISO 13485:2016 certification of our quality system from BSI Group, a Notified Body to the International Standards Organization, or ISO. The processes utilized in the manufacturing and testing of our devices have been verified and validated to the extent required by the FDA and other regulatory bodies. As a medical device manufacturer, our contract manufacturing facilities and the facilities of our sterilization and other critical suppliers are subject to periodic inspection by the FDA and corresponding state and foreign agencies. We believe that our manufacturing and quality systems are robust and ensure high product quality. To date, we have had no product recalls.
Some of the parts and components of V-Go and EZ Fill are purchased from sole-source vendors, and we manage any single-source components and suppliers through our global supply chain operation. We believe that, if necessary, alternative sources of supply would, in most cases, be available in a relatively short period of time and on commercially reasonable terms.
Research, Development and Engineering
Our research, development and engineering staff has significant experience in developing insulin-delivery systems and are focused on the continuous improvement and support of current product, as well as our products in development. We have a staff of experienced engineers specializing in mechanical engineering, material science and fluid mechanics. We utilize design and analysis tools to accelerate design times and reduce development risk. Through frequent usability testing, we seek to ensure that our product not only functions properly, but also meets patient needs and desires with respect to an insulin-delivery device, while at the same time reducing our development and commercialization risks.
We spent $7.4 million on research, development and engineering activities for the year ended December 31, 2018 and $7.1 million for the year ended December 31, 2017.
Intellectual Property
From our inception, we have understood that the strength of our competitive position will depend substantially upon our ability to obtain and enforce intellectual property rights protecting our technology, and we have developed what we consider to be a strong intellectual property portfolio, including patents, trademarks, copyrights, trade secrets and know-how. We continue to actively pursue a broad array of intellectual property protection in the United States, and in significant markets elsewhere in North America, as well as in Europe, Australia and Asia, including China. We believe our intellectual property portfolio effectively protects the products we currently market and we are actively building our intellectual property portfolio to protect our next-generation products, as well as additional drug delivery technologies for those products. Refer to "Risk Factors" for additional information on potential claims.
As more fully described below, our patents and patent applications are primarily directed to our h-Patch technology or aspects thereof including the commercialized V-Go, a hydraulically driven ambulatory insulin delivery device.
In addition to patent protection, we rely on materials and manufacturing trade secrets, and careful monitoring of our proprietary information to protect aspects of our business that are not amenable to, or that we do not consider appropriate for, patent protection.
We plan to continue to expand our intellectual property portfolio by filing patent applications directed to novel drug delivery systems and methods of their use.
Patents
As of January 25 2019, we owned 27 U.S. and 94 international issued or allowed patents and 11 U.S. and 39 international patents pending directed to various features of our commercial V-Go device and our proprietary h-Patch drug delivery technology. These patents are directed to the hydraulic drive for a basal-bolus delivery device as well as many of the other features of the h-Patch technology.
The principal occupationfollowing is a summary of our current and pending patents:
U.S. Patent Nos. 7,530,968, 8,070,726, 9,072,828, 9,125,983, and 9,511,187 are directed to the hydraulically driven pump system having basal and bolus fluid delivery. These patents are expected to expire in 2024. Foreign counterparts to these patents have been granted in Australia, Canada and Japan. We have applications pending in Australia, Japan and in Europe. One U.S. continuation application is pending.
U.S. Patent Nos. 6,939,324, 7,481,792, 8,858,511, 8,992,478, 9,636,451, and 9,981,083 are directed to the Floating Needle and bolus button configuration. These patents are expected to expire in 2022. Four Canadian counterparts to these patents have been granted and two European counterparts have been granted, one that has been validated in Germany, Spain, France, the United Kingdom, and Italy and another which is awaiting validation. One divisional application is allowed in Europe. One U.S. continuation application is pending.
U.S. Patent Nos. 9,101,706 and 9,968,731 are directed to an ambulatory fluid delivery device in which transitioning the needle from the storage position to the armed position transitions the piston from the locked position to the released position, thermally coupling the hydraulic chamber to the patient. These patents are expected to expire in 2031. The Australian (four), Canadian (two), Chinese (four), Japanese (two), Hong Kong, Israel, Korean (two), and Singapore counterparts to this patent application have been granted. Patent applications are pending in Australia, Europe, India, and Japan. One U.S. continuation application is pending.
U.S. Patent Nos. 8,667,996, 9,376,224 and 9,833,383 are directed to the closed looped filling configuration of the EZ Fill device. These patents are expected to expire in October 2032. The Canadian, Chinese (two), Japanese (two), Hong Kong, and Korean counterparts to this patent have been granted, and the European counterpart has been granted and validated in Germany, Spain, France, the United Kingdom and Italy. Patent applications are pending in Europe and India.
U.S. Design Patent Nos. D667946, D687948 and D706415 are directed to the ornamental appearance of the EZ Fill device and are expected to expire in September 2026, August 2027 and June 2029, respectively. A Chinese counterpart to these patents has been granted.
U.S. Patent Nos. 8,740,847 and 9,795,735 are directed to a fluid delivery device having a pre-filled cartridge. These patents are expected to expire in March 2032. Australian (four), Canadian, Chinese, Japanese (two),
Israel, Korean and Singapore counterparts to these patents have been granted. Patent applications are pending in Canada, China (two), Europe, and India, and Australian and Israel applications are allowed. One U.S. continuation application is pending.
U.S. Patent Nos. 7,914,499, 8,361,053, 8,821,443, and 9,687,599 are directed to fluid delivery devices having two or more fluid delivery reservoirs covering both composition and methods. These patents expire in March 2027. Foreign counterparts to these patents have been granted in Australia (four), Canada, China (two), Europe (with validations in Germany, Spain, France, the United Kingdom, and Italy), Israel, Korea, Japan, Russia and Singapore. A counterpart patent application is pending in India. One U.S. continuation application is pending.
Trademarks
We believe we have protected our trademarks, including our trademark of V-Go and V-Go SIMTM, through applications in all major markets worldwide as well as the United States. Our trademark portfolio consists of 16 registered trademarks, six of which are registered in the United States, including our V-Go logo. We also have nine trademark applications pending registration in several major markets outside the United States.
Trade Secrets and Know-How
We rely, in some circumstances, on trade secrets and know-how to protect our proprietary manufacturing processes and materials critical to our product. We seek to preserve the integrity and confidentiality of our trade secrets and know-how in part by limiting the employees and third parties who have access to certain information and requiring employees and third parties to execute confidentiality and invention assignment agreements, under which they are bound to assign to us inventions made during the term of their employment. These agreements further require employees to represent that they have no existing obligations and hold no interest that conflicts with any of their obligations under their agreements with us. We also generally require consultants, independent contractors and other third parties to sign agreements providing that any inventions that relate to our business experienceare owned by us, and prohibiting them from disclosing or using our proprietary information except as may be authorized by us.
Competition
The medical technology and biopharmaceutical industries are characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary products. Competition in the diabetes market is particularly intense, due largely to the fact that products designed to treat diabetes currently compete with both traditional and new products. We compete with these products based on efficacy, price, reimbursement, ease of use and healthcare provider education.
Within the diabetes market, V-Go is cleared by the FDA for adult patients who require insulin, with either type 1 or type 2 diabetes, although we position V-Go to compete primarily in the market for adult patients with type 2 diabetes requiring insulin, particularly as part of a basal-bolus insulin regimen. Our primary competitors in the basal-bolus insulin therapy market are manufacturers of insulin and insulin pens, such as Novo Nordisk, Sanofi S.A. and Eli Lilly and Company.
In addition to basal-bolus insulin therapy, glucagon-like peptide-1, or GLP-1, analog injection products are another potential competitor to V-Go. GLP-1 analog injection products are used in combination with OADs or basal insulin injection. Some physicians, when faced with a patient who is unable to reach or maintain glucose levels at his or her goal with OADs, will add a GLP-1 through twice-daily, once-daily or once-weekly injections. As a result, we also compete with pharmaceutical manufacturers of GLP-1 analog injection products, such as AstraZeneca, Novo Nordisk and GlaxoSmithKline plc. In addition, we may compete with inhaled insulin products for bolus therapy, which have been recently introduced to the market.
There is another mechanical bolus only insulin delivery device that has been approved by the FDA, ONETOUCH ViaTM. We do not consider this a direct competitor of V-Go, because this product only provides insulin at meal times and does not provide a continuous flow of insulin. Therefore, patients would still be required to take separate basal insulin injection or injections every day. This product has not yet been commercially launched in the U.S. and it is uncertain if and when it will be launched.
In the area of basal-bolus device competition, we do not consider programmable insulin pumps to be products that compete directly with V-Go, as those products, although cleared for both type 1 and type 2 diabetes, have been primarily designed and marketed for patients with type 1 diabetes. We believe that the simple and discreet design and interface of V-Go more directly addresses the needs of patients with type 2 diabetes. Patients with type 2 diabetes, for example, are often taking many drugs for multiple diseases, including medications to treat high blood pressure and elevated cholesterol, and, as a result, they desire a simple to use and discreet method to deliver their insulin. We are not aware of any other single-use disposable, mechanical (which means the device does not include any electronics, batteries or audible alarms and does not require any recharging or programming), basal-bolus insulin delivery devices currently marketed or in development at this time.
Government Regulation
V-Go, our first commercialized product, received 510(k) clearance by the FDA in December 2010. Our product and our operations are subject to extensive regulation by the FDA and other federal and state authorities in the United States, as well as comparable authorities in foreign jurisdictions. Our product is subject to regulation as a medical device in the United States under the Federal Food, Drug, and Cosmetic Act, or FDCA, and related regulations enforced by the FDA. The FDA regulates, among other things, the development, design, non-clinical and clinical research, manufacturing, safety, efficacy, labeling, packaging, storage, installation, servicing, recordkeeping, premarket clearance or approval, import, export, adverse event reporting, advertising, promotion, marketing and distribution of medical devices to ensure that medical devices distributed domestically are safe and effective for their intended uses and otherwise meet the requirements of the FDCA.
FDA Premarket Clearance and Approval Requirements
Unless an exemption applies, each medical device commercially distributed in the United States requires either FDA clearance of a 510(k) premarket notification submission, granting of a de novo classification request, or approval of a premarket approval application, or PMA. Under the FDCA, medical devices are classified into one of three classes - Class I, Class II or Class III - depending on the degree of risk associated with each medical device. Class I includes devices with the lowest risk to the patient and are subject to the FDA’s general controls for medical devices, which include compliance with the applicable portions of the Quality System Regulation, or QSR, facility registration and product listing, reporting of adverse medical events, and truthful and non-misleading labeling, advertising, and promotional materials. Class II devices are moderate risk devices and are subject to the FDA’s general controls, and special controls as deemed necessary by the FDA to ensure the safety and effectiveness of the device. These special controls can include performance standards, post-market surveillance, patient registries and FDA guidance documents. Devices deemed by the FDA to pose the greatest risks, such as life sustaining, life supporting, and some implantable devices, are placed in Class III. In addition, novel devices that have not been previously classified by FDA are considered Class III by default.
While most Class I devices are exempt from FDA premarket review, manufacturers of most Class II devices are required to submit to the FDA a premarket notification under Section 510(k) of the FDCA requesting permission to commercially distribute the device. The FDA’s permission to commercially distribute a device subject to a 510(k) premarket notification is generally known as 510(k) clearance. Devices that have been formally classified by FDA as Class III require approval of a PMA, which is the most burdensome type of medical device premarket submission. For a device that is Class III by default (because it is a novel device that was not previously classified and has no predicate), the device manufacturer may request that FDA reclassify the device to Class II or Class I via a de novo request.
Our currently marketed products are Class II devices subject to 510(k) clearance.
510(k) Marketing Clearance
To obtain 510(k) clearance, a premarket notification submission must be submitted to the FDA demonstrating that the proposed device is “substantially equivalent” to a predicate device. A predicate device is a legally marketed device that is not subject to premarket approval, i.e., a device that was legally marketed prior to May 28, 1976 (pre-amendments device) and for which a PMA is not required, a device that has been reclassified from Class III to Class II or I (e.g., via the de novo classification process), or a device that was previously cleared through the 510(k) process. The FDA’s 510(k) review process usually takes from three to six months, but may take longer. The FDA may require additional information, including clinical data, to make a determination regarding substantial equivalence.
If the FDA agrees that the device is substantially equivalent to a predicate device, it will grant 510(k) clearance to market the device.
After a device receives 510(k) marketing clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change or modification in its intended use, will require a new 510(k) marketing clearance or, depending on the modification, a de novo request or PMA approval. The FDA requires each manufacturer to determine whether the proposed change requires submission of a 510(k), de novo or a PMA in the first instance, but the FDA can review that decision and disagree with a manufacturer’s determination. If the FDA disagrees with a manufacturer’s determination, the FDA can require the manufacturer to cease marketing and/or request the recall of the modified device until FDA has cleared or approved a 510(k), de novo or PMA for the change. Also, in these circumstances, we may be subject to significant regulatory fines or penalties. We have made and plan to continue to make additional product enhancements to our 510(k)-cleared products. We cannot be assured that the FDA would agree with any of our decisions to not submit 510(k) premarket notifications or other premarket submissions for these modified devices.
V-Go is one of the first insulin delivery devices to be cleared under the FDA’s Infusion Pump Improvement Initiative, which established additional device manufacturing requirements designed to foster the development of safer, more effective infusion pumps. The FDA launched this initiative in 2010 to support the benefits of external infusion pumps while minimizing the risks associated with these devices. As part of the initiative, FDA issued guidance requesting the inclusion of additional information in premarket submissions for infusion pumps beyond what has traditionally been provided, including detailed engineering information, a comprehensive discussion of steps taken to mitigate risks and additional design validation testing specific to the environment in which the device is intended to be used.
De Novo Process
If a previously unclassified new medical device does not qualify for the 510(k) pre-market notification process because no predicate device to which it is substantially equivalent can be identified, the device is automatically classified into Class III. The Food and Drug Administration Modernization Act of 1997 established a new route to market for low to moderate risk medical devices that are automatically placed into Class III due to the absence of a predicate device, called the “Request for Evaluation of Automatic Class III Designation,” or the de novo classification procedure. This procedure allows a manufacturer whose novel device is automatically classified into Class III to request down-classification of its medical device into Class I or Class II on the basis that the device presents low or moderate risk, rather than requiring the submission and approval of a PMA. Prior to the enactment of the Food and Drug Administration Safety and Innovation Act, or FDASIA, in July 2012, a medical device could only be eligible for de novo classification if the manufacturer first submitted a 510(k) pre-market notification and received a determination from the FDA that the device was not substantially equivalent. FDASIA streamlined the de novo classification pathway by permitting (under Section 513(f)(2) of the FDCA) manufacturers to request de novo classification directly without first submitting a 510(k) pre-market notification to the FDA and receiving a not substantially equivalent determination. FDASIA sets a review time for FDA of 120 days following receipt of the de novo application, but FDA does not always meet this timeline and has publicly only committed to a review of 150 days for 50% of applications. If the manufacturer seeks reclassification into Class II, the manufacturer must include a draft proposal for special controls that are necessary to provide a reasonable assurance of the safety and effectiveness of the medical device. The FDA may reject the reclassification petition if it identifies a legally marketed predicate device that would be appropriate for a 510(k) or determines that the device is not low to moderate risk or that general controls would be inadequate to control the risks and special controls cannot be developed. If the FDA agrees with the down-classification, the de novo applicant will then receive authorization to market the device, and a classification regulation will be established for the device type. The device can then be used as a predicate device for future 510(k) submissions by the manufacturer or a competitor. In December 2018 FDA issued proposed regulations to govern the de novo classification process, which if finalized would further impact this path to market.
As an alternative to the de novo process, a company could also file a reclassification petition, or FDA could initiate such a process, seeking to change the automatic Class III designation of a novel postamendment device under Section 513(f)(3) of the
FDCA. FDA issued a final rule (to take effect March 17, 2019) to clarify the process where FDA initiates such reclassification (issuance of a proposed reclassification order; optional panel consultation; and final reclassification order published in the Federal Register).
Premarket Approval Process
Class III devices require submission through the Premarket Approval (PMA) process before they can be marketed. The PMA process is more demanding than the 510(k) premarket clearance process. In a PMA application, the manufacturer must demonstrate that the device is safe and effective, and the PMA application must be supported by extensive data, including data from preclinical studies and human clinical trials. The PMA must also contain, among other things, a full description of the device and its components, a full description of the methods, facilities and controls used for manufacturing, and proposed labeling. Following receipt of a PMA submission, the FDA determines whether the application is sufficiently complete to permit a substantive review. If FDA accepts the application for review, it has 180 days under the FDCA to complete its review of the PMA application, although in practice, the FDA’s review often takes significantly longer, and can take up to several years. An advisory panel of experts from outside the FDA may be convened to review and evaluate the application and provide recommendations to the FDA as to the approvability of the device. The FDA may or may not accept the panel’s recommendation. In addition, the FDA will generally conduct a pre-approval inspection of the applicant or its third-party manufacturers’ or suppliers’ manufacturing facility or facilities to ensure compliance with the QSR. The FDA will approve the new device for commercial distribution if it determines that the data and information in the PMA constitute valid scientific evidence and that there is reasonable assurance that the device is safe and effective for its intended use(s). The FDA may approve a PMA application with post-approval conditions intended to ensure the safety and effectiveness of the device, including, among other things, restrictions on labeling, promotion, sale and distribution, and collection of long-term follow-up data from patients in the clinical trial that supported premarket approval or requirements to conduct additional clinical trials post-approval. The FDA may condition premarket approval on some form of post-market surveillance when deemed necessary to protect the public health or to provide additional safety and efficacy data for the device in a larger population or for a longer period of use. In such cases, the manufacturer might be required to follow certain patient groups for a number of years and to make periodic reports to the FDA on the clinical status of those patients. Failure to comply with the conditions of approval can result in material adverse enforcement actions, including withdrawal of the approval.
Certain changes to an approved device, such as changes in manufacturing facilities, methods, or quality control procedures, or changes in the design performance specifications, that affect the safety or effectiveness of the device, require submission of a PMA supplement. PMA supplements often require submission of the same type of information as a PMA, except that the supplement is limited to information needed to support any changes from the device covered by the original PMA and may not require as extensive clinical data or the convening of an advisory panel. Certain other changes to an approved device require the submission of a new PMA, such as when the design change causes a different intended use, mode of operation, and technical basis of operation, or when the design change is so significant that a new generation of the device will be developed, and the data that were submitted with the original PMA are not applicable for the change in demonstrating a reasonable assurance of safety and effectiveness. Our product is not currently subject to PMA requirements. However, we may in the future develop devices that will require the submission of a PMA.
Clinical Trials
Clinical trials are almost always required to support a PMA and are sometimes required to support a de novo or 510(k) submission. All clinical investigations of devices to determine safety and effectiveness must be conducted in accordance with the FDA’s investigational device exemption, or IDE, regulations which govern investigational device labeling, prohibit promotion of the investigational device, and specify an array of recordkeeping, informed consent, reporting and monitoring responsibilities of study sponsors and study investigators. If the device presents a “significant risk,” to human health, as defined by the FDA, the FDA requires the device sponsor to submit an IDE application to the FDA, which must be approved prior to commencing human clinical trials. A significant risk device is one that presents a potential for serious risk to the health, safety or welfare of a patient and either is implanted, used in supporting or sustaining human life, substantially important in diagnosing, curing, mitigating or treating disease or otherwise preventing impairment of human health, or otherwise presents a potential for serious risk to a subject. An IDE application must be supported by appropriate data, such as animal and laboratory test results, showing that it is safe to test the device in humans and that the testing protocol is scientifically sound. The IDE will automatically become effective 30 days after receipt by the FDA, unless the FDA notifies us that the investigation may not begin or is subject to a clinical hold. If the FDA determines that there are deficiencies or other concerns with an IDE for which it requires modification, the FDA may permit a clinical trial to proceed under a conditional approval.
In addition, the clinical trial must be approved by, and conducted under the oversight of, an Institutional Review Board, or IRB, for each clinical site. The IRB is responsible for the initial and continuing review of the trial, and may pose additional requirements for the conduct of the trial. If an IDE application is approved by the FDA and one or more IRBs, human clinical trials may begin at a specific number of investigational sites with a specific number of patients, as approved by the FDA. If the device presents a non-significant risk to the patient, a sponsor may begin the clinical trial after obtaining approval for the trial by one or more IRBs without separate approval from the FDA, but must still certain IDE requirements, such as monitoring the investigation, ensuring that the investigators obtain informed consent, and labeling and record-keeping requirements. An IDE supplement must be submitted to, and approved by, the FDA before a sponsor or investigator may make a change to the investigational plan.
During a clinical trial, the sponsor and all clinical investigators are required to comply with applicable FDA regulations. Both must comply with good clinical practice requirements, or GCP, which among other things requires that informed consent be obtained from each research subject, that the investigational plan and study protocol be followed, that the disposition of the investigational device be controlled, and that reporting and recordkeeping requirements are followed. Additionally, after a trial begins, we, the FDA or the IRB could suspend or terminate a clinical trial at any time for various reasons, including a belief that the risks to study subjects outweigh the anticipated benefits. Even if a clinical trial is completed, there can be no assurance that the data generated during a clinical trial will meet the safety and effectiveness endpoints or otherwise produce results that will lead the FDA to grant marketing clearance or approval.
Post-Market Regulation
After a device is cleared or approved for marketing, numerous and pervasive regulatory requirements continue to apply. These include:
establishment registration and device listing with the FDA;
state licensure requirements for the manufacturing and distribution of medical devices;
Quality System Regulation, or QSR, requirements, which require manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all aspects of the design and manufacturing process;
labeling regulations and requirements related to promotional activities, including FDA prohibitions against the promotion of investigational products, or “off-label” uses of cleared or approved products;
clearance or approval of product modifications to 510(k)-cleared devices that could significantly affect safety or effectiveness or that would constitute a major change in intended use of one of our cleared devices;
medical device reporting requirements, which require that a manufacturer report to the FDA if a device it markets may have caused or contributed to a death or serious injury, or has malfunctioned and the device or a similar device that it markets would be likely to cause or contribute to a death or serious injury, if the malfunction were to recur;
correction, removal and recall reporting regulations, which require that manufacturers report to the FDA field corrections and product recalls or removals if undertaken to reduce a risk to health posed by the device or to remedy a violation of the FDCA that may present a risk to health;
the FDA’s mandatory recall authority, whereby the agency can order device manufacturers to recall from the market a product that is in violation of governing laws and regulations; and
post-market surveillance activities and regulations, which apply when deemed by the FDA to be necessary to protect the public health or to provide additional safety and effectiveness data for the device.
The Company's manufacturing processes are required to comply with the applicable portions of the QSR, which cover the methods and the facilities and controls for the design, manufacture, testing, production, processes, controls, quality assurance, labeling, packaging, distribution, installation and servicing of finished devices intended for human use. The QSR also requires, among other things, maintenance of a device master file, device history file, and complaint files. As a manufacturer, the Company and its third-party manufacturers are subject to periodic scheduled or unscheduled inspections by the FDA. The Company's failure to maintain compliance with the QSR requirements could result in the shut-down of, or restrictions on, its manufacturing operations and the recall or seizure of its product. The discovery of previously unknown problems with the Company's product, including unanticipated adverse events or adverse events of increasing severity or frequency, whether resulting from the use of the device within the scope of its clearance or off-label by a physician in the practice of medicine, could result in restrictions on the device, including the removal of the product from the market or voluntary or mandatory device recalls. The FDA has broad enforcement powers. If the FDA determines that the Company failed to comply with applicable regulatory requirements, it can take a variety of enforcement actions, which may result in any of the following sanctions:
warning letters, fines, injunctions, consent decrees and civil penalties;
recalls, withdrawals, or administrative detention or seizure of our product;
operating restrictions or partial suspension or total shutdown of production;
refusing or delaying requests for 510(k) marketing clearance or PMA approvals of new products or modified products;
withdrawing 510(k) clearances or PMA approvals that have already been granted;
refusal to grant export approvals for our product; or
criminal prosecution.
U.S. Anti-Kickback, False Claims and Other Healthcare Fraud and Abuse Laws
We are also subject to healthcare regulation and enforcement by the federal government and the states and foreign governments and authorities in the locations in which we conduct our business. These other agencies include, without limitation, the Centers for Medicare and Medicaid Services, or CMS, other divisions of the U.S. Department of Health and Human Services, the U.S. Department of Justice and individual U.S. Attorney offices within the Department of Justice, as well as state and local governments. Such agencies enforce a variety of laws which include, without limitation, state and federal anti-kickback, fraud and abuse, false claims, data privacy and security, and physician sunshine laws and regulations.
Healthcare providers, physicians and third-party payors often play a primary role in the recommendation and prescription of any currently marketed products and product candidates for which we may obtain marketing approval. Our current and future arrangements with healthcare providers, physicians, third-party payors and consumers, and our sales, marketing and education activities, may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations (at the federal and state level) that may constrain our business or financial arrangements and relationships through which we market, sell and distribute our products for which we obtain marketing approval.
The federal Anti-Kickback Statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving any remuneration (including any kickback, bribe or rebate), directly or indirectly, overtly or covertly, to induce or in return for purchasing, leasing, ordering or arranging for or recommending the purchase, lease or order of any good, facility, item or service reimbursable, in whole or part by Medicare, Medicaid or other federal healthcare programs. The term “remuneration” has been broadly interpreted to include anything of value, including cash, improper discounts, and free or reduced price items and services. Among other things, the Anti-Kickback Statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on one hand and prescribers, purchasers and formulary managers on the other. Although there are a number of statutory exceptions and regulatory safe harbors protecting some common activities from prosecution, the exceptions and safe harbors are drawn narrowly. Practices that involve remuneration that may be alleged to be intended to induce prescribing, purchases or recommendations may be subject to scrutiny if they do not qualify for an exception or safe harbor. Failure to meet all of the requirements of a particular applicable statutory exception or regulatory safe harbor does not make the conduct per se illegal under the Anti-Kickback Statute. Instead, the legality of the arrangement will be evaluated on a case-by-case basis based on a cumulative review of all of its facts and circumstances. Several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal healthcare covered business, the Anti-Kickback Statute has been violated. The Patient Protection and Affordable Care Act of 2010, as amended by the Health Care and Education Reconciliation Act, collectively the Affordable Care Act, among
other things, amended the intent requirement of the federal Anti-Kickback Statute such that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate, in order to have committed a violation.
The federal civil and criminal false claims laws and civil monetary penalty laws, including the False Claims Act which prohibits individuals or entities from, among other things, knowingly presenting, or causing to be presented, a false or fraudulent claim for payment to or approval by the federal government or knowingly making, using or causing to be made or used a false record or statement material to a false or fraudulent claim to the federal government. As a result of a modification made by the Fraud Enforcement and Recovery Act of 2009, a claim includes “any request or demand” for money or property presented to the U.S. government. In addition, the Affordable Care Act codified case law that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act. Several pharmaceutical and other healthcare companies have been prosecuted under the federal civil False Claims Act for, among other things, allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. Other companies have been prosecuted for causing false claims to be submitted because of the companies’ marketing of products for unapproved, and thus non-covered, uses. In addition, the federal civil monetary penalties statute imposes penalties against any person or entity that, among other things, is determined to have presented or caused to be presented a claim to a federal health program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent. The False Claims Act also permits a private individual acting as a “whistleblower” to bring actions on behalf of the federal government alleging violations of the False Claims Act and to share in any monetary recovery. In addition, federal Anti‑Kickback Statute violations and certain marketing practices, including off‑label promotion, may also implicate the False Claims Act. Although the False Claims Act is a civil statute, conduct that results in a False Claims Act violation may also implicate various federal criminal statutes.
The federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, created federal criminal statutes that prohibit knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private third-party payors and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Similar to its amendment of the Anti-Kickback Statute, the Affordable Care Act also broadened the reach of certain criminal healthcare fraud statutes created under HIPAA by amending the intent requirement such that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
HIPAA and its associated regulations also impose privacy and security standards on "covered entities" and "business associates." Even for entities that are not deemed "covered entities" or "business associates" under HIPAA, however, according to the U.S. Federal Trade Commission, or the FTC, failing to take appropriate steps to keep consumers’ personal information secure constitutes unfair acts or practices in or affecting commerce in violation of Section 5(a) of the Federal Trade Commission Act, or the FTCA, 15 U.S.C § 45(a). The FTC expects a company’s data security measures to be reasonable and appropriate in light of the sensitivity and volume of consumer information it holds, the size and complexity of its business, and the cost of available tools to improve security and reduce vulnerabilities. Medical data is considered sensitive data that merits stronger safeguards. The FTC’s guidance for appropriately securing consumers’ personal information is similar to what is required by the HIPAA Security Rule.
Analogous state laws and regulations, such as state anti‑kickback and false claims laws, may apply to items or services reimbursed by any third‑party payor, including commercial insurers and in some cases may apply regardless of payor (i.e. even for self-pay scenarios). Some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government in addition to requiring drug manufacturers to report pricing and marketing information, including, among other things, information related to payments to physicians and other healthcare providers or marketing expenditures, state and local laws that require the registration of pharmaceutical sales representatives, and state laws governing the privacy and security of health information and the use of prescriber‑identifiable data in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts.
There are numerous other laws and legislative and regulatory initiatives at the federal and state levels addressing privacy and security concerns, and some state privacy laws apply more broadly than HIPAA and its associated regulations. For example, California recently enacted legislation - the California Consumer Privacy Act, or CCPA - which goes into effect January 1, 2020. The CCPA, among other things, creates new data privacy obligations for covered companies and provides new privacy rights to California residents, including the right to opt out of certain disclosures of their information. The CCPA also creates a private right of action with statutory damages for certain data breaches, thereby potentially increasing risks associated with a data breach. Legislators have stated that they intend to propose amendments to the CCPA before it goes into effect, and the California Attorney General will issue clarifying regulations. Although the law includes limited exceptions, including for certain information collected as part of clinical trials as specified in the law, it may regulate or impact our processing of
personal information depending on the context. It remains unclear what, if any, modifications will be made to this legislation or how it will be interpreted.
The federal physician payment transparency requirements, sometimes referred to as the “Physician Payments Sunshine Act,” created under the Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010, imposed, among other things, tracking and annual reporting requirements for drug and device manufacturers subject to the law for certain payments and “transfers of value” provided to physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members. Failure to submit timely, accurately and completely the required information for all payments, transfers of value and ownership or investment interests may result in civil monetary penalties of up to an aggregate of $150,000 per year and up to an additional aggregate of $1 million per year for “knowing failures.” Manufacturers subject to the law must submit reports by the 90th day of each subsequent calendar year. In 2018 the Sunshine Act was extended to require tracking and reporting of payments and transfers of value to physician assistants, nurse practitioners, and other mid-level practitioners (with reporting requirements going into effect in 2022 for payments and transfers of value made in 2021). In addition, certain states require implementation of commercial compliance programs and compliance with the pharmaceutical and medical device industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, impose restrictions on marketing practices, and/or tracking and reporting of gifts, compensation and other remuneration or items of value provided to physicians and other healthcare professionals and entities.
Because of the breadth of these laws and the narrowness of the statutory exceptions and regulatory safe harbors available under such laws, it is possible that certain business activities could be subject to challenge under one or more of such laws. The scope and enforcement of each of these laws is uncertain and subject to rapid change in the current environment of healthcare reform, especially in light of the lack of applicable precedent and regulations. Federal and state enforcement bodies have recently increased their scrutiny of interactions between healthcare companies and healthcare providers, which has led to a number of investigations, prosecutions, convictions and settlements in the healthcare industry. Ensuring that business arrangements with third parties comply with applicable healthcare laws, as well as responding to possible investigations by government authorities, can be time‑ and resource‑consuming and can divert management’s attention from the business.
If our operations are found to be in violation of any of the health regulatory laws described above or any other laws that apply to us, we may be subject to penalties, including potentially significant criminal, civil and/or administrative penalties, damages, fines, individual imprisonment, disgorgement, exclusion from participation in government healthcare programs, contractual damages, reputational harm, administrative burdens, diminished profits and future earnings, and the curtailment or restructuring of our operations.
Healthcare Reform
A primary trend in the U.S. healthcare industry is cost containment. The federal government and state legislatures have attempted to control healthcare costs in part by limiting coverage and the amount of reimbursement for particular drug products, including implementing price controls, restrictions on coverage and reimbursement and requirements for substitution of generic products. By way of example, the Affordable Care Act contains provisions that may reduce the profitability of drug products.
On January 20, 2017, the new administration signed an Executive Order directing federal agencies with authorities and responsibilities under the ACA to waive, defer, grant exemptions from, or delay the implementation of any provision of the ACA that would impose a fiscal or regulatory burden on states, individuals, healthcare providers, health insurers, or manufacturers of pharmaceuticals or medical devices. Congress also could consider subsequent legislation to replace elements of the ACA that are repealed. More recently, the United States District Court for the Northern District of Texas struck down the ACA, deeming it unconstitutional given that Congress repealed the individual mandate in 2017. This decision has been stayed pending outcome of an appeal to the Fifth Circuit Court of Appeals. Although there is no immediate impact on the ACA, we will continue to evaluate the effect that the ACA and its possible repeal and replacement, or potential total revocation by the Supreme Court of the United States, has on our business.
Other legislative changes have been proposed and adopted since the Affordable Care Act was enacted. For example, on August 2, 2011, President Obama signed into law the Budget Control Act of 2011, which, among other things, created the Joint Select Committee on Deficit Reduction to recommend to Congress proposals in spending reductions. The Joint Select Committee did not recommend and Congress did not enact legislation to reduce the deficit by at least $1.2 trillion for the past five years 2013 through 2021, triggering the legislation’s automatic reduction to several government programs. This includes reductions to Medicare payments to providers of 2% per fiscal year, which went into effect on April 1, 2013 and will remain in effect through 2025 unless additional Congressional action is taken. Moreover, the U.S. Department of Health and Human Services Centers for Medicare & Medicaid has promulgated or amended a number of cost containment and value based reimbursement measures in the ordinary
course of business, and it is expected to continue revising its regulations and policies in response to market conditions and administrative directives.
We expect that additional state and federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, which could result in reduced demand for our executive officersproduct or additional pricing pressures.
Coverage and directorsReimbursement
Sales of our product depend, in significant part, on the extent to which our product is covered and reimbursed by third‑party payors, such as follows:government healthcare programs, including, without limitation, Medicare Part D plans, commercial insurance and managed care organizations (MCOs). Patients who use V‑Go generally rely on these third‑party payors to pay for all or part of the costs of our product. The containment of healthcare costs has become a priority of federal, state and foreign governments, and the prices of drug products have been a focus in this effort. Third‑party payors are increasingly scrutinizing and challenging the prices charged for drug products and medical services, volume and utilization, duration of use, medical necessity, cost effectiveness, and the safety and efficacy of such products and services, and more recently, the potential for lower-priced “equivalent” products. . If third‑party payors do not consider our product to be affordable and effective compared to other available therapies that may have varying delivery methods while achieving the same clinical result, they may not cover our product or, if they do, the level of payment may not be sufficient to allow us to sell our product at a reasonable profit. In addition, the U.S. government, state legislatures and foreign governments have continued implementing cost‑containment programs, including price controls, restrictions on reimbursement, bundled payments, evidence-based care, and requirements for substitution of generic products. Adoption of price controls and cost‑containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our net revenue and results.
John E. Timberlake has servedCurrently, a number of third‑party payors have coverage policies that permit coverage for V‑Go, either under the pharmacy or medical benefit. For example, a majority of Medicare Part D plans make coverage for our product available under the outpatient prescription drug benefit. A number of private payors and Medicaid programs also permit coverage for V‑Go under the pharmacy benefit. The process for determining whether a third‑party payor will provide coverage for a drug product typically is separate from the process for establishing the reimbursement rate that the payor will pay for the product once coverage is approved. Third‑party payors, including, without limitation, Medicare Part D plans, may limit coverage to specific drug products on an approved list, also known as a formulary, which might not include all of the FDA‑approved drugs for a particular indication. Continued placement on formularies is therefore critical for reimbursement. A decision by a third‑party payor not to cover our Chief Executive Officer, Presidentproduct could reduce physician utilization of our product and decrease overall use in the industry. Moreover, a memberthird‑party payor’s to provide coverage does not imply that an adequate reimbursement rate will be approved will not guarantee consistent payment. Adequate third‑party reimbursement may not be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development, sales and marketing. Additionally, coverage and reimbursement for drug products can differ significantly from payor to payor. One third‑party payor’s decision to cover a particular drug product or service does not ensure that other payors will also provide coverage for the medical product or service, or will provide coverage at an adequate reimbursement rate. As a result, the coverage determination process will require us to provide scientific and clinical support for the use of our product to each payor separately and will continue to be a time‑consuming process.
V‑Go is currently not covered under Medicare Part B because V‑Go is a disposable insulin dispensing device, which is not a Medicare Part B benefit. In addition, some private third‑party payors have determined there is insufficient data for coverage and concluded that V‑Go is investigational or experimental. Those payors may determine at a future date that our product, including V‑Go, will be covered. Because coverage and reimbursement varies significantly from payor to payor, the process to obtain favorable recognition is time‑consuming.
We currently have contracts establishing reimbursement for V‑Go with national and regional third‑party payors in the United States. While we anticipate entering into additional contracts with third‑party payors, we cannot guarantee that we will succeed in doing so or that the reimbursement contracts we are able to negotiate will enable us to sell our product on a profitable basis. In addition, contracts with third‑party payors generally can be modified or terminated by the third‑party payor without cause and with minimum notice. Moreover, compliance with the administrative procedures or requirements of third‑party payors may result in delays in processing approvals by those third‑party payors for customers to obtain coverage for V‑Go. Failure to secure or retain adequate coverage or reimbursement for V‑Go by third‑party payors, or delays in processing approvals by those payors, could result in the loss of sales, loss of customers, or reputational damage, which could have a material adverse effect on our business, financial condition and operating results.
Registration in China
We are seeking registration of V-Go in China. China's regulatory system is unique in many ways, and its registration requirements are not always consistent with U.S. or other international standards. In order to obtain registration approval for medical devices in China, a clinical trial authorization issued by the China State Drug Administration, or SDA, is required to conduct the clinical trial in China. Foreign applicants need to provide China-originated data in their clinical trial and meet the relevant technical review requirements. Registration will only be granted, following review, if the SDA determines that the clinical data confirm safety and effectiveness of the device.
More specifically, we will need to complete the following steps:
•We will first need to perform lab-based functional product testing in China at an approved testing facility for design verification;
•At the same time, we will prepare a clinical protocol submission package for technical review and approval by the SDA;
•After obtaining protocol approval, we may begin the clinical trial in China by first engaging in site selection, then subject enrollment and ultimately clinical trial execution; and
•Finally, upon completion of the proposed clinical trial, we will need to submit the clinical study for technical review by the SDA and registration approval.
Employees
As of December 31, 2018, we had 125 full-time employees including 25 in our manufacturing, quality, compliance and research organization, 89 in our commercial organization and 11 in general and administrative functions.
Available Information
We make available on our website (http://www.valeritas.com), or through a link posted on our website, free of charge, our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, reports filed pursuant to Section 16 and amendments to those reports filed pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934 as soon as reasonably practicable after we electronically file such material with, or furnish it to, the Securities and Exchange Commission (SEC). In addition, the SEC maintains an internet site that contains these reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC (http://www.sec.gov).
We also make available on our website, in a printable format, the charters for our Board of Directors since February 2016, prior to which he served as President and Chief Commercial Officer since August 2008. Before becoming Chief Executive Officer and President and Chief Commercial Officer, Mr. Timberlake was a General Manager with our company from September 2006 to August 2008. Prior to joining Valeritas, Mr. Timberlake held positions of increasing responsibility from 1991 to 2006 at Sanofi-Aventis (now Sanofi), with his last role as Vice President of Diabetes Marketing, where he was responsible for the diabetes franchise,committees, including the brands Lantus, ApidraAudit Committee, the Compensation Committee and Amaryl. Priorthe Nominating and Corporate Governance Committee, in addition to Sanofi, Mr. Timberlake wasour Corporate Governance Guidelines, Bylaws, Code of Business Conduct and Ethics Policy, Related Party Transactions Policy and Compensation Recovery Policy. Our website is not incorporated into or a manager with Deloitte & Touche LLP, from 1986 to 1991, and was both a Certified Management Accountant and a Certified Public Accountant. He earned a B.S. in Accounting at Northwest Missouri State University, an M.S. in Management from Purdue University and an M.B.A. from NEOMA Business School (f/k/a E.S.C. Rouen) in France. Mr. Timberlake is qualified to serve as a director becausepart of his role with us, and his extensive operational knowledge of, and executive level management experience in, the biopharmaceutical and medical technology industries.
Erick J. Lucera has served as our Chief Financial Officer since August 2016. Since August 2017 Mr. Lucera has served as a member of the board of directors as chairman of the audit committee of AIT Therapeutics. From April 2015 to August 2016, Mr. Lucera served as the Chief Financial Officer, Treasurer and Secretary of Viventia Bio Inc., a late-stage oncology company. From December 2012 to April 2015, he served as Vice President, Corporate Development at Aratana Therapeutics, Inc., a specialty pharmaceutical company focusedthis Annual Report on companion animals. He served as Vice President, Corporate Development at Sunshine Heart, Inc. a medical device company from March 2012 to December 2012. Mr. Lucera served as Vice President, Healthcare Analyst at Eaton Vance Management, a global asset manager, from February 2008 to November 2011. Mr. Lucera also held various positions at Intrepid Capital Partners, Independence Investment Associates, LLC and Price Waterhouse & Co. from 1990 to 2008. Mr. Lucera earned a C.P.H. from Harvard University in 2001, an M.S.F. from Boston College in 1999, an M.B.A. from Indiana University in 1995 and a Bachelor’s Degree in Accounting from The University of Delaware in 1990. Mr. Lucera currently holds a CFA designation. Mr. Lucera previously held CMA and CPA designations, both of which are expired.Form 10-K.
Mark Conley has served as our Vice President, Corporate Controller and Treasurer since February 2016, prior to which he served as our Director of Financial Planning & Analysis since joining Valeritas in August 2012. Mr. Conley was Global Finance Director of the radiation instrumentation business at Thermo Fisher Scientific from 2007 to 2012. In addition, he served at Iron Mountain, Inc. as Vice President, Financial Planning & Analysis from 2005 to June 2007 and Division Controller from 1998 to 2004, as
ChiefItem 1A. Risk Factors.
You should carefully consider the following risk factors, in addition to the other information contained in this Annual Report on Form 10-K, including the section of this report titled “Management’s Discussion and Analysis of Financial OfficerCondition and Controller at HoltraChem Group from 1996 to 1998Results of Operations” and in successiveour financial leadership roles including Operations Controller at Haemonetics Corporation from 1991 to 1996. Mr. Conley earned a B.S. in Accounting from Oklahoma State University, an M.B.A. from Bryant College,statements and is a Certified Public Accountant.
Geoffrey Jenkins has served as our Executive Vice President, Manufacturing, Operations and Research & Development since he joined Valeritas in April 2009. Mr. Jenkins was Vice Presidentrelated notes. If any of Worldwide Operations for Inverness Medical, a healthcare technology company, from 2005 to 2009. From 2000 to 2005, he was President and Founding Partner of UV-Solutions, LLC, a healthcare technology company, and from 1997 to 1999 he was Chief Operating Officer of MDI Instruments, Inc., a healthcare technology company. Mr. Jenkins was also Corporate Vice President of Operations of MediSense, Inc. from 1991 to 1997. Prior to becoming Corporate Vice President of Operations, he held various other positions in Operations and Engineering Management with MediSense from 1984 to 1991. Mr. Jenkins earned a B.A. and a B.S. from Clarkson University.
Matthew Nguyen has served as our Chief Commercial Officer since December 2016. Mr. Nguyen served as our Sr. Vice President, Commercial from February 2016 to December 2016 and as our Vice President for Integrated Healthcare Management since joining Valeritas in September 2006. Mr. Nguyen was a New Business Development Director for Janssen, LP, a division of Johnson & Johnson, from 2005 to 2006. He served as head of health economics research for metabolism, new product marketing, and head of analytics and commercial effectiveness for the CNS business unit at Sanofi from 2000 to 2005. Mr. Nguyen earned a B.S. in Pharmacy and a Doctor of Pharmacy from the Philadelphia College of Pharmacy and Science. He also completed a Fellowship in Health Economics and Outcomes Research in conjunction with Thomas Jefferson University Hospital and Janssen Pharmaceutical, Inc. and earned an M.B.A. from Rutgers University in New Jersey.
Joseph Saldanha has served as our Chief Business Officer since January 2018. From April 2016 to July 2017, Mr. Saldanha served as Vice President, Marketing and Business Development of MannKind Corporation. From January 2012 to May 2015, Mr. Saldanha was the General Manager, JULPHAR Diabetes for Gulf Pharmaceutical Industries. Mr. Saldanha has also worked on the Aventis-Pfizer partnership for Exubera and for Sanofi and its predecessors from 2001 to 2008, launching Actonel for osteoporosisevents described in the U.S.,following risk factors and for Lantusthe risks described elsewhere in international markets from Paris. Prior to that, from 2008 to 2010, Mr. Saldanha worked inthis Annual Report on Form 10-K occurs, our business, development at Johnsonoperating results and Johnson Diabetes, where he helped bring both LifeScan for self-monitoring blood glucosefinancial condition could be seriously harmed and Animas for insulin pumps to the market. Mr. Saldanha also worked in the United Arab Emirates in a general management role with responsibility for insulin API, diabetes orals and injectables, and distribution of Dexcom CGM for the Middle-Eastern markets from 2012 to 2015. Mr. Saldanha earned a Bachelor of Science degree from Drexel University and a Master of Science degree from the University of Pennsylvania, both in Philadelphia.
Joe Mandato, D.M. has served as a membertrading price of our board of directors since December 2016. Since March 2003, Dr. Mandato has served as a managing director of DeNovo Ventures, a venture capital firm focused on life sciences. Prior to DeNovo Ventures, Dr. Mandato held top leadership positions at Ioptex, Confer Software, Gynecare and Origin Medsystems. Dr. Mandato also served as a member of the Board of Directors of AxoGen Corporation from February 2006 until its merger with and into AxoGen, Inc. in September 2011, and then served on the Board of AxoGen, Inc. until September 2016. Dr. Mandato served as a member of the Board of Directors of Hansen Medical, Inc. from August 2006 until February 2012. Dr. Mandato received a doctorate in management from Case Western Reserve University, and now serves on its Board of Trustees. Dr. Mandato also holds the Carlo Rossi Chair in Entrepreneurship and Management at the University of San Francisco, is a Lecturer at Stanford University and has served as a Fellow in the Harvard University Advanced Leadership Initiative. Additionally, Dr. Mandato currently serves on the boards of both the Embrace Global and Save the Children organizations. Dr. Mandato is qualified to serve as a director because of his extensive work in the healthcare industry and his venture capital experience.
Luke Düster has served as a member of our board of directors since January 2016. Since 2009, Mr. Düster served as managing director at Capital Royalty Group, a healthcare-focused investment firm. Mr. Düster was at Harris Williams & Co., an investment firm, from 2004 to 2009, where he served as Vice President. Mr. Düster also held investment banking roles at the Wallach Company, a regional investment banking boutique, from 2000 to 2002, and at the Nord Companies, a healthcare advisory firm, from 1998 to 2000. Mr. Düster received his B.S. summa cum laude from the University of Colorado at Boulder and an M.B.A. with honors from the Wharton School at the University of Pennsylvania. Mr. Düster is qualified to serve as a director because of his significant experience working with companies backed by private equity investors, particularly in the healthcare industry, as well as his experience with healthcare investing.
Katherine D. Crothall, Ph.D. has served as a member of our board of directors since October 2016. Since 2010, Dr. Crothall has served as President, CEO and Chairman of the Board of Aspire Bariatrics, a company committed to providing safe and effective treatments for obesity to patients worldwide. Dr. Crothall was a Principal at Liberty Venture Partners, a venture capital firm, from 2006 until November 2010. Dr. Crothall was Founder, President and CEO of Animas Corporation, a manufacturer of insulin infusion pumps, from its inception to its acquisition by Johnson & Johnson Corporation in 2006. Dr. Crothall was also the Founder, President and CEO of two other medical device companies, Luxar Corporation, which was sold to ESC Medical, and Laakmann
Electro-Optics, which was sold to Johnson & Johnson. Dr. Crothall continued running Laakmann Electro-Optics for five years post-acquisition. Dr. Crothall received her B.S. from the University of Pennsylvania and her Ph.D. from the University of Southern California, both in Electrical Engineering. She holds over twenty patents and is the recipient of the Ernst & Young Entrepreneur of the Year Award and the Greater Philadelphia Raymond Rafferty Entrepreneurial Excellence Award. Dr. Crothall is a director of Adhezion BioMedical and Xanitos, Inc. She also sits on the Board of Overseers of the School of Engineering and Applied Sciences at the University of Pennsylvania. Dr. Crothall is qualified to serve as a director because of her extensive clinical and business experience, specifically in the healthcare industry.
Rodney Altman, M.D. has served as a member of our board of directors since April 2016. Since June 2016, Dr. Altman has been a member of the board of directors of Milestone Pharmaceuticals and Thrasos Pharmaceuticals in his capacity as an advisor to Business Development Bank of Canada. Since 2011, he has been an Advisor and beginning in March 2016 he has been a Managing Director at Spindletop Capital, a private equity and venture capital firm. Prior to joining Spindletop Capital, he was Regional Medical Director at TeamHealth, an American hospital staffing firm. Dr. Altman was a senior partner at a venture capital firm, CMEA Capital, LLC, from 2006 to 2011, where he built and managed the firm’s medical device practice. Dr. Altman has also held investing roles at other venture funds including Aphelion Capital, LLC, Piper Jaffray Ventures, and TVM Techno Venture Management. Dr. Altman received his medical degree from McGill University and an M.B.A. with honors from the University of Chicago, Booth School of Business. Dr. Altman is qualified to serve as a director because of his extensive clinical and venture capital experience.
Peter Devlin has served as a member of our board of directors since April 2016. Since September 2014, Mr. Devlin has served as a consultant for various life sciences and investment companies. From August 2009 to September 2014, Mr. Devlin was the Chief Commercial Officer at Insulet Corporation, a tubeless insulin pump technology company. Mr. Devlin held several leadership roles at Abbott Laboratories, Inc. From February 2008 to July 2009, he served as Divisional Vice President of Abbott’s Global Strategic Marketing in the diabetes care unit, prior to which he served as General Manager, Hospital & Government in the diabetes care unit from December 2006 to February 2008, and prior to which he served as Director of Abbott’s Canadian diabetes unit from September 2003 to December 2006. Mr. Devlin received his Bachelor of Science degree from the University of Massachusetts. Mr. Devlin is qualified to serve as a director because of his extensive business experience in the field of diabetes.
Brian K. Roberts has served as a member of our board of directors and as chairman of the audit committee since July 2016. Mr. Roberts currently serves as chief financial officer of Tarveda Therapeutics and serves as a member of the board of directors and audit chairman of ViewRay. Most recently he served as the chief operating and financial officer of Avedro, Inc., a privately held biotechnology company leading the organization through FDA approval, manufacturing readiness and commercial launch of its lead combination drug and medical device product. Prior to Avedro, he served as CFO for Insulet Corporation, a tubeless insulin pump technology company. Under his supervision, Insulet grew from approximately $30 million to nearly $300 million in revenue, achieved operating profitability and increased its market capitalization to over $2 billion. Previously, Mr. Roberts served as CFO for Jingle Networks, a leader in mobile voice-ad services that was acquired by Marchex, and as CFO for Digitas, which was sold for $1.3 billion to Publicis Groupe. He holds a Bachelor of Science in accounting and finance from Boston College, is a certified public accountant, and served as an auditor with Ernst & Young LLP. Mr. Roberts is qualified to serve as a director because of his extensive business experience and financial and accounting insight.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act requires our directors and executive officers, and persons who own more than ten percent of a registered class of our equity securities, to file with the SEC initial reports of ownership and reports of changes in ownershipshares of common stock could decline. This Annual Report on Form 10-K also contains forward-looking statements that involve risks and other equity securitiesuncertainties. Our actual results could differ materially from those anticipated in the forward-looking statements as a result of factors that are described below and elsewhere in this Annual Report on Form 10-K.
Risks Related to Our Business
We have incurred significant operating losses since our inception and anticipate that we will continue to incur significant losses for the foreseeable future. These factors raise substantial doubt about our ability to continue as a going concern.
Since our inception in 2006, we have incurred significant net losses. Our recurring losses from operations raise substantial doubt about our ability to continue as a going concern, and as a result our independent registered public accounting firm included an explanatory paragraph regarding the same in its report to this Annual Report on Form 10-K. Substantial doubt about our ability to continue as a going concern may create negative reactions to the price of our company. Officers, directorscommon stock and greater than ten percent stockholders are required by SEC regulation to furnish us with copies of all Section 16(a) forms they file.we may have a more difficult time obtaining financing in the future.
SEC regulations require us to identify in this report anyone who filed a required report late during our most recent fiscal year. Based on our review of forms we received or written representations from reporting persons, we believe that all reports of securities ownerships and changes in such ownership required to be filed duringOur net losses were $49.3 million for the year ended December 31, 2017 wereand $45.9 million for the year ended December 31, 2018. As of December 31, 2018, we had an accumulated deficit of $519.9 million. As of December 31, 2018, we had $47.8 million in cash and cash equivalents ($0.5 million of which is restricted cash), which we believe will be sufficient to finance our current operations into the fourth quarter of 2019. To date, we have financed our operations primarily through sales of our capital stock, debt financings and limited sales of V-Go. In April 2018, we completed an underwritten public offering, in which we sold 13,700,000 shares of common stock at a public offering price of $1.75 per share. We received gross proceeds of approximately $24 million and net proceeds of approximately $21.8 million after deducting underwriting discounts, commissions and estimated offering expenses. On May 2, 2018, we sold an additional 1,600,000 shares of common stock pursuant to the 30-day option we granted the underwriters in connection with the offering, for net proceeds of approximately $2.6 million. In January 2018, we entered into a common stock purchase agreement, or the First Purchase Agreement, with Aspire Capital Fund, LLC, or Aspire Capital, which provided that, upon the terms and subject to the conditions and limitations set forth therein, at our discretion, Aspire Capital would be committed to purchase up to an aggregate of $20.0 million of shares of our common stock over the 30-month term of the First Purchase Agreement. In June 2018, we terminated the First Purchase Agreement and entered into a second common stock purchase agreement, or the Second Purchase Agreement, with Aspire Capital, which provides that, upon the terms and subject to the conditions and limitations set forth therein, at our discretion, Aspire Capital is committed to purchase up to an aggregate of $21.0 million of shares of our common stock over the 30-month term of the Second Purchase Agreement. We sold 1,250,868 shares of common stock to Aspire Capital under the First Purchase Agreement for net proceeds of approximately $1.8 million, and as of December 31, 2018, have sold 1,205,409 shares of common stock to Aspire Capital under the Second Purchase Agreement for net proceeds of approximately $1.3 million. Additionally, in January 2018, we entered into an “at-the-market” sales agreement, or the Sales Agreement, with B. Riley FBR, Inc., or FBR, pursuant to which we may sell up a certain amount of shares of our common stock from time to time through FBR, acting as our sales agent, in one or more at-the-market offerings. FBR has the option to decline any sales orders at its discretion. Both the Second Purchase Agreement and the Sales Agreement are subject to limitations as described in this Annual Report on Form 10-K, and therefore there are no assurances that we will be able to raise funds from the Second Purchase Agreement or the Sales Agreement. We have devoted substantially all of our resources to the research, development and engineering of our products, the commercial launch of V-Go, the development of a sales and marketing team and the assembly of a management team to lead our business.
To implement our business strategy we need to, among other things, increase sales of our products with our existing sales and marketing infrastructure, fund ongoing research, development and engineering activities, expand our manufacturing capabilities, and obtain regulatory clearance in other markets outside the United States and European Union, or EU, or approval to commercialize our products currently under development. We expect our expenses to increase significantly as we pursue these objectives. The extent of our future operating losses and the timing of profitability are highly uncertain, especially given that we only recently commercialized V-Go, which makes predicting our sales more difficult. We will need to affect a financing or capital raise in the near term in order to sustain operations and implement our business strategy until we can achieve profitability from operations, if ever. Our inability to affect a financing or capital raise and continued losses from operations could have a material adverse effect on the Company. Any additional operating losses will have an adverse effect on our stockholders’ equity/(deficit), and we cannot assure you that we will ever be able to achieve or sustain profitability.
We currently rely on sales of V-Go to generate all of our revenue, and any factors that negatively impact our sales of V-Go would also negatively impact our financial condition and operating results.
V-Go is our only revenue-producing commercial product, which we introduced into the market in the first quarter of 2012. In the near term, we expect to continue to derive all of our revenue from the sale of V-Go. Accordingly, our ability to generate revenue is highly dependent on our ability to market and sell V-Go.
Sales of V-Go may be negatively impacted by many factors, including:
problems relating to our manufacturing capabilities, including, but not limited to, the destruction, loss, or temporary shutdown of our manufacturing facility;
failure to become or remain the preferred basal-bolus insulin therapy among patients with type 2 diabetes;
failure by patients to use V-Go as directed, which could limit its effectiveness and could have an adverse impact on repeat use;
inadequate coverage and reimbursement or changes in reimbursement rates or policies relating to V-Go or similar products or technologies by third-party payors;
our inability to enter into contracts with additional third-party payors on a timely filed, exceptbasis and on acceptable terms;
claims that V-Go, or any component thereof, infringes on patent rights or other intellectual property rights of third parties; and
adverse regulatory or legal actions relating to V-Go or similar products or technologies.
Because we currently rely on V-Go to generate all of our revenue, any factors that negatively impact our sales of V-Go, or that result in sales of V-Go increasing at a lower rate than expected, would also negatively impact our financial condition and operating results.
Our ability to maintain and grow our revenue depends both on retaining a high percentage of patients using V-Go and on preserving our relationships with a few significant wholesale customers that account for Mr. Devlin,nearly all of our sales.
A key to maintaining and growing our revenue is the retention of a high percentage of patients using V-Go, as a significant and increasing proportion of our business is generated through refill prescriptions. Refill prescriptions account for nearly two-thirds of our total prescriptions and since we do not have reliable data regarding retention rates, and because refill prescriptions generally move in parallel with our patient retention rates, we use these as a proxy to determine patient retention rates. During the year ended December 31, 2018, three wholesale customers accounted for approximately 91% of our total product shipments. If demand for V-Go fluctuates as a result of the introduction of competitive products, negative perceptions with respect to the effectiveness of V-Go, changes in reimbursement policies, manufacturing problems, perceived safety issues with our or our competitors’ products, the failure to secure regulatory clearance or approvals or for other reasons, our ability to attract and retain customers and ultimately patients could be harmed. The failure to retain a high percentage of patients using V-Go could negatively impact our revenue growth. Furthermore, the loss of any one of our significant wholesale customers or a sustained decrease in demand by any of these wholesale customers could result in a substantial loss of revenue or patients losing convenient access to V-Go, either of which was filed latewould hurt our business, financial condition and results of operations.
The failure of V-Go to achieve and maintain market acceptance could result in August 2017our achieving sales below our expectations.
Our current business strategy is highly dependent on V-Go achieving and maintaining market acceptance. In order for us to sell V-Go to people with type 2 diabetes who require insulin, we must convince them, their caregivers and healthcare providers that V-Go is an attractive alternative to other insulin delivery devices for the treatment of diabetes, including insulin pens and traditional syringes. Market acceptance and adoption of V-Go depends on educating people with diabetes, as well as their caregivers and healthcare providers, as to the distinct features, ease-of-use, positive lifestyle-impact and other perceived benefits of V-Go as compared to competitive products. If we are not successful in convincing existing and potential customers of the benefits of V-Go, or if we are not able to achieve the support of caregivers and healthcare providers for V-Go, our sales may decline or we may fail to increase our sales in line with our anticipated levels.
Achieving and maintaining market acceptance of V-Go could be negatively impacted by many factors, including:
the failure of V-Go to achieve wide acceptance among people with type 2 diabetes who require insulin, their caregivers, insulin-prescribing healthcare providers, third-party payors and key opinion leaders in the diabetes treatment community;
lack of availability of adequate coverage and reimbursement for patients and health care providers;
lack of evidence supporting the safety, ease-of-use or other perceived benefits of V-Go over competitive products or other currently available insulin treatment methods;
lack of long-term persistency of patients who do start V-Go, as future sales are heavily dependent on patient refills;
perceived risks associated with the use of V-Go or similar products or technologies generally;
the introduction of competitive products and the rate of acceptance of those products as compared to V-Go; and
any negative results of clinical studies relating to V-Go or similar competitive products.
In addition, V-Go may be perceived by people with type 2 diabetes requiring insulin, their caregivers or healthcare providers to be more complicated, only marginally more effective or even less effective than traditional insulin-delivery methods, and people may be unwilling to change their current treatment regimens. Moreover, we believe that healthcare providers tend to be slow to change their medical treatment practices because of perceived liability risks arising from the use of new products and the uncertainty of third-party payor reimbursement. Accordingly, healthcare providers may not recommend V-Go until there is sufficient evidence to convince them to alter the treatment methods they typically recommend, such as receiving recommendations from prominent healthcare providers or other key opinion leaders in the diabetes treatment community that our products are effective in providing insulin therapy.
If V-Go does not achieve and maintain widespread market acceptance, we may fail to achieve sales at or above our anticipated levels. If our sales do not meet anticipated levels, we may fail to meet our strategic objectives.
If we do not enhance our product offerings through our research, development and engineering efforts, including the successful commercialization of our pre-fill V-Go, we may fail to effectively compete in our market or become profitable.
In order to increase our sales and market share in the type 2 diabetes market, we must enhance and broaden our product offerings, including by commercializing our pre-fill V-Go, in response to the evolving demands of people with type 2 diabetes who require insulin and healthcare providers and competitive pressures from new technologies and market participants. We may not be successful in developing, obtaining regulatory approval for, or marketing our product candidates, including our pre-fill V-Go. In addition, notwithstanding our market research efforts, our future products may not be accepted by consumers, their caregivers, healthcare providers or third-party payors who reimburse consumers for our product. The success of any of our product candidates, including our pre-fill V-Go, will depend on numerous factors, including our ability to:
identify the product features that people with type 2 diabetes, their caregivers and healthcare providers are seeking in an insulin treatment and successfully incorporate those features into our product;
develop and introduce our product candidates in sufficient quantities and in a timely manner;
offer products at a price that is competitive with that of other products on the market;
adequately protect our intellectual property and avoid infringing upon the intellectual property rights of third parties;
demonstrate the safety and efficacy of our product candidates;
secure adequate financing to fund the research, development, engineering and marketing and sales efforts necessary to commercialize new product offerings; and
obtain the necessary regulatory approvals for our product candidates.
With respect to our pre-fill V-Go in particular, we anticipate that we will need to seek additional sources of capital to complete its development and commercialization, which we cannot assure you we will be able to procure at reasonable terms, if at all. Any delays in our anticipated product launches may significantly impede our ability to successfully compete in our markets. In particular, such delays could cause customers to delay or forego purchases of our product, or to purchase our competitors’ products. Even if
we are able to successfully develop proposed product candidates when anticipated, these product candidates, including our pre-fill V-Go, may not produce sales in excess of the costs of development, and they may be quickly rendered obsolete by changing consumer preferences or the introduction by our competitors of products embodying new technologies or features.
We depend on a limited number of third-party suppliers for some of the components of V-Go, and the loss of any of these suppliers, or their inability to provide us with an adequate supply of materials, could harm our business.
We rely on a limited number of third-party suppliers to supply components of V-Go. For our business strategy to be successful, our suppliers must be able to provide us with components and finished products in sufficient quantities, in compliance with regulatory requirements and quality control standards, in accordance with agreed upon specifications, at acceptable costs and on a timely basis. Increases in our product sales, whether forecasted or unanticipated, could strain the ability of our suppliers to deliver an increasingly large supply of components in a manner that meets these various requirements.
We do not have long-term supply agreements with most of our suppliers and, in many cases, we make our purchases on a purchase order basis. Under most of our supply agreements, we have no obligation to buy any given quantity of products, and our suppliers have no obligation to manufacture for us or sell to us any given quantity of products. As a result, our ability to purchase adequate quantities of the components for our products may be limited. Additionally, our suppliers may encounter problems that limit their ability to manufacture components for us, including financial difficulties or damage to their manufacturing equipment or facilities. If we fail to obtain sufficient quantities of high quality components to meet demand on a timely basis, we could lose customer orders, our reputation may be harmed and our business could suffer.
We generally use a small number of suppliers for our product, some parts and components of which are purchased from single-source vendors. Depending on a limited number of suppliers exposes us to risks, including limited control over pricing, availability, quality and delivery schedules. Moreover, due to administrative error.the recent commercialization of our products and the limited amount of our sales to date, we do not have long-standing relationships with our manufacturers and may not be able to convince suppliers to continue to make components available to us unless there is demand for such components from their other customers. If any one or more of our suppliers cease to provide us with sufficient quantities of components in a timely manner or on terms acceptable to us, we would have to seek alternative sources of supply. Because of factors such as the proprietary nature of our product, our quality control standards and regulatory requirements, we cannot quickly engage additional or replacement suppliers for some of our critical components. Failure of any of our suppliers to deliver products at the level our business requires would limit our ability to meet our sales commitments, which could harm our reputation and could have a material adverse effect on our business. We may also have difficulty obtaining similar components from other suppliers that meet the requirements of the FDA or other regulatory agencies, and the failure of our suppliers to comply with strictly enforced regulatory requirements could expose us to regulatory action including warning letters, product recalls, termination of distribution, product seizures or civil penalties. It could also require us to cease using the components, seek alternative components or technologies and modify our products to incorporate alternative components or technologies, which could result in a requirement to seek additional regulatory approvals. Any disruption of this nature or increased expenses could harm our commercialization efforts and adversely affect our operating results.
Board CompositionManufacturing risks, including risks related to manufacturing in China, may adversely affect our ability to manufacture our products and could reduce our gross margin and our profitability.
Our business strategy depends on our ability to manufacture our current and future products in sufficient quantities and on a timely basis so as to meet consumer demand, while adhering to product quality standards, complying with regulatory requirements and managing manufacturing costs. We are subject to numerous risks relating to our manufacturing capabilities, including:
quality or reliability defects in product components that we source from third-party suppliers;
our inability to secure product components in a timely manner, in sufficient quantities or on commercially reasonable terms;
our failure to increase production of products to meet demand;
our inability to modify production lines to enable us to efficiently produce future products or implement changes in current products in response to regulatory requirements;
difficulty identifying and qualifying alternative suppliers for components in a timely manner; and
potential damage to or destruction of our manufacturing equipment or manufacturing facility.
In addition, we rely on our contract manufacturer in Southern China to manufacture V-Go. As a result, our business is subject to risks associated with doing business in China, including:
adverse political and economic conditions, particularly those potentially negatively affecting the trade relationship between the United States and China;
trade protection measures, such as tariff increases, and import and export licensing and control requirements;
potentially negative consequences from changes in tax laws;
difficulties associated with the Chinese legal system, including increased costs and uncertainties associated with enforcing contractual obligations in China;
historically lower protection of intellectual property rights;
unexpected or unfavorable changes in regulatory requirements;
changes and volatility in currency exchange rates;
possible patient or physician preferences for more established pharmaceutical products and medical devices manufactured in the United States; and
difficulties in managing foreign relationships and operations generally.
These risks are likely to be exacerbated by our limited experience with our current products and manufacturing processes. As demand for our products increases, we will have to invest additional resources to purchase components, hire and train employees, and enhance our manufacturing processes. If we fail to increase our production capacity efficiently, our sales may not increase in line with our forecasts and our operating margins could fluctuate or decline. In addition, although we expect some of our product candidates in development to share product features and components with V-Go, manufacturing of these product candidates may require the modification of our production lines, the hiring of specialized employees, the identification of new suppliers for specific components, or the development of new manufacturing technologies. It may not be possible for us to manufacture these product candidates at a cost or in quantities sufficient to make these product candidates commercially viable. Any of these factors may affect our ability to manufacture our products and could reduce our gross margin and profitability.
We operate in a very competitive industry, and if we fail to compete successfully against our existing or potential competitors, many of whom have greater resources than we have, our revenue and operating results may be negatively affected.
The diabetes market, and especially the market for patients with type 2 diabetes, is intensely competitive, subject to change and highly sensitive to promotional effort, the number of sales force representatives, the introduction of new products or technologies, or other activities of industry and diabetes-related associations and participants. V-Go competes directly with a number of insulin-delivery devices, primarily insulin pens and syringes, but also indirectly with any other currently marketed or future marketed diabetes therapeutic intervention such as oral anti-diabetic medications, other injectable anti-diabetic medications such as glucagon- like peptide-1, or GLP-1, and analogs. We do not consider programmable insulin pumps or programmable insulin patch pumps to be products that compete directly with V-Go, as those products have been primarily designed and marketed for patients with type 1 diabetes. There are a significant number of very large global pharmaceutical companies that promote and sell anti-diabetic products that are aimed to be used either instead of insulin or to deliver insulin using insulin pens or syringes. Many of our existing and potential competitors are major global companies that are either publicly traded companies or divisions or subsidiaries of publicly traded companies that have significant resources available.
These competitors also enjoy several competitive advantages over us, including:
greater financial and human resources for sales and marketing, managed care and reimbursement, medical affairs and product development;
established relationships with healthcare providers and third-party payors;
established reputation and name recognition among healthcare providers and other key opinion leaders in the diabetes treatment community;
in some cases, an established base of repeat, long-time customers;
products supported by a large volume of short-term and long-term clinical data;
larger and more established distribution networks;
greater ability to cross-sell products or provide incentives to healthcare providers to use their products; and
more experience in conducting research, development and engineering activities, manufacturing, clinical trials, and obtaining regulatory approval or clearance.
For these and other reasons, we may not be able to compete successfully against our current or potential future competitors. If this occurs, we may fail to meet our strategic objectives, and our revenue and operating results could be negatively affected.
Competitive products or other technological breakthroughs for the treatment or prevention of diabetes may render our products obsolete or less desirable.
Our ability to achieve our strategic objectives will depend, among other things, on our ability to develop and commercialize products for the treatment of diabetes, in both specialist and primary care settings, which are organizedeasy-to- train and easy-to-use, provide clinical benefits as well as equivalent or improved patient adherence and persistency, receive adequate coverage and reimbursement from third-party payors with reasonable out-of-pocket costs to patients, and are more appealing than available alternatives. Our current competition is primarily with other non-electronic insulin delivery devices such as insulin pens and syringes. There are other U.S. Food and Drug Administration, or FDA,-cleared basal-bolus insulin delivery products, including one that includes a patch component, but these are electronic. These electronic basal-bolus insulin delivery devices and systems are cleared for use by both type 1 and type 2 patients. In addition, there is another mechanical bolus only insulin delivery device developed by Calibra Medical, Inc. that has been cleared by the FDA. This device, previously known as ONETOUCH ViaTM ,or Via. Via has not yet been commercially launched in the U.S., but the company who currently has rights to this technology has stated that they plan to make Via commercially available in the U.S. in 2019. We do not consider Via to be a direct competitor of V-Go by itself, because Via only provides insulin at meal times and does not provide a continuous flow of insulin. Therefore, patients would still be required to take separate basal insulin injection or injections every day. We do, however, believe that the commercial introduction of Via (although it only provides meal time dosing) will help to create additional awareness and acceptance of wearable insulin delivery devices in the U.S. Because V-Go is currently the only available commercial product in the U.S. that can deliver insulin at both a constant basal rate and on demand at meal time, we believe V-Go may benefit from the commercial introduction of Via by capturing a portion of the expanded market for wearable insulin delivery devices for patients with type 2 diabetes that will likely be created by the commercial introduction of Via. There is currently one inhaled insulin on the market, Afrezza®, manufactured by MannKind Corporation, which is also a meal time insulin delivery device. We do not consider Afrezza to be a direct competitor of V-Go for similar reasons as described above with respect to Via, namely because patients would still be required to take separate basal insulin injection or injections every day in order to match the basal-bolus regimen provided by V-Go. In the future, the insulin-delivery methods for patients with type 2 diabetes could change if other non-invasive formulations of insulin are approved and successfully commercialized, such as oral insulin in pill form, additional inhaled insulin or buccal insulin. If longer-acting and safer glucagon-like peptide-1analogs with fewer side effects are approved and successfully commercialized, they could reduce or delay the use of basal-bolus insulin in patients with type 2 diabetes. However, we do not anticipate that these types of medications, if approved and successfully commercialized, would reduce the number of patients with type 2 diabetes already on insulin or on a basal-bolus insulin regimen. In addition, a number of other companies are pursuing new electronic or mechanical insulin-delivery devices, delivery technologies, drugs and other therapies for the treatment and prevention of diabetes that are not currently available on the market. Therefore, we believe that V-Go remains in a class by itself because it is the only purely mechanical, disposable basal and bolus insulin delivery device currently on the market in the U.S., and that there will remain a large population of patients with type 2 diabetes on insulin who can benefit from V-Go both clinically and on a cost-effective basis. However, if we are incorrect about these assumptions, Via, Afrezza, and other bolus only insulin delivery devices currently on the market or in development, or other potential technological breakthroughs in diabetes treatment or prevention, could reduce the potential market for V-Go or render V-Go obsolete altogether, which would significantly reduce our sales and have an adverse impact on our business.
Because of the size of the type 2 diabetes market, we anticipate that companies will continue to dedicate significant resources to developing competitive products, including both drugs and devices. The frequent introduction of non-insulin drugs, for example, may delay the introduction of insulin to patients that would otherwise be introduced to insulin sooner, and could create market confusion, prevent us from successfully capturing the prescribers' or payors' attention or reduce our ability to capture a sufficient market share to realize our business objectives. In addition, the entry of multiple new products or the loss of market exclusivity on some diabetes drugs, including insulin delivered in pens, may lead some of our competitors to employ pricing strategies that could adversely affect the pricing of our products. If a competitor develops a product that is similar or is perceived to be superior to V-Go, or if a competitor employs strategies that place downward pressure on pricing within our industry, our sales may decline significantly or may not increase in accordance with our expectations.
If we are unable to leverage our current sales and marketing infrastructure, we may fail to increase our sales to meet our anticipated levels.
As of December 31, 2018, our field based sales team consisted of 52 sales representatives. Generally speaking, each sales professional covers one prioritized territory. We currently utilize sales professionals along with an inside sales team to focus on select healthcare providers with the most revenue potential. Our profitability will depend on the success of this new sales model.
Because we began commercialization of V-Go in 2012, and because our current sales force is not deployed in every state or major market in the United States, we have less experience marketing and selling our products, as well as training healthcare providers and new customers on the use of V-Go compared to other type 2 diabetes companies. We derive all of our revenue from the sale of V-Go and we expect that this will continue for the next several years. As a result, our financial condition and operating results are and will continue to be highly dependent on the ability of our sales representatives to adequately promote, market and sell V-Go and the ability of our sales force and other training personnel to successfully train healthcare providers and new customers on the use of V-Go. If our sales and marketing representatives or training personnel fail to achieve their objectives, our sales could decrease or may not increase at levels that are in line with our anticipated levels.
A key element of our business strategy is for our sales and marketing infrastructure to drive adoption of our products. The majority of patients using V-Go are trained to use the device by their healthcare provider who has been trained by our sales force using a “train the trainer” approach. Our sales force trains physicians, physicians’ assistants, nurse practitioners and any other staff in a healthcare provider’s office who interact with patients, on how V-Go works and how to train their patients to properly use V-Go. We can expect to face challenges in recruiting and hiring top personnel as we manage our sales and marketing infrastructure and work to retain the individuals who make up those networks due to the very competitive diabetes industry. If a sales and marketing representative were to depart and be retained by one of our competitors, we may fail to prevent them from helping competitors solicit business from our existing customers, which could further adversely affect our sales. In addition, if we are not able to maintain a sufficient network of training and customer care personnel, we may not be able to successfully train healthcare providers to train new patients on the use of V-Go, which could delay new sales and harm our reputation.
As we increase our sales and marketing expenditures with respect to existing or planned products, we will need to further expand the reach of our sales and marketing networks. Our future success will depend largely on our ability to continue to hire, train, retain and motivate skilled sales and marketing representatives with significant industry-specific knowledge in various areas, such as diabetes treatment techniques and technologies, as well as the competitive landscape for our products. Recently hired sales representatives require training and take time to achieve full productivity. If we fail to train recent hires adequately, or if we experience high turnover in our sales force in the future, we cannot be certain that new hires will become as productive as may be necessary to maintain or increase our sales. In addition, the expansion of our sales and marketing personnel will continue to place significant burdens on our management team.
If important assumptions about the potential market for our products are inaccurate, or if we have failed to understand what people with type 2 diabetes are seeking in a treatment, we may not be able to increase our revenue or achieve profitability.
Our business strategy was developed based on a number of important assumptions about the diabetes market in general, and the type 2 diabetes market in particular, any one or more of which may prove to be inaccurate. For example, we believe that the benefits of V-Go as compared to other common insulin delivery devices, such as traditional insulin injection pens, will continue to drive growth in the market for V-Go. In addition, we believe the incidence of diabetes in the United States and worldwide is increasing rapidly. However, each of these trends is uncertain and limited sources exist to obtain reliable market data. The actual incidence of diabetes, and the actual demand for our products or competitive products, could differ materially from our anticipated levels if our assumptions are incorrect. In addition, our strategy of focusing exclusively on patients with type 2 diabetes who require insulin may limit our ability to increase sales or achieve profitability, especially if there are any significant clinical breakthroughs or products or drug introductions that significantly delay or reduce the need for insulin therapy in patients with type 2 diabetes.
We operate at facilities in three locations, and any disruption at any of these facilities could harm our business.
Our principal offices are located in Bridgewater, New Jersey, and our only manufacturing operations are located at a contract manufacturing facility in Southern China. We also operate a facility in Marlborough, Massachusetts, which we primarily use for research and development, and contract with a domestic third party logistics provider to warehouse and distribute our finished product. Substantially all of our operations are conducted at these locations, including our manufacturing processes, research, development and engineering activities, customer and technical support and management and administrative functions. In addition, substantially all of our inventory of component supplies and finished goods is held at these locations.
Vandalism, terrorism or a natural or other disaster, such as an earthquake, fire or flood, at any of these facilities could damage or destroy our manufacturing equipment or our inventory of component supplies or finished goods, cause substantial delays in our operations, result in the loss of key information and cause us to incur additional expenses. Our contract manufacturing facility in
Southern China is our only manufacturing facility, and if damaged or rendered inoperable or inaccessible due to political, social, or economic upheaval or due to natural or other disasters, would make it difficult or impossible for us to manufacture our products for a period of time and may lead to a loss of customers and significant impairment of our financial condition and operating results.
We take precautions to safeguard these facilities, including acquiring insurance, employing back-up generators, adopting health and safety protocols and utilizing off-site storage of computer data. Our insurance may not cover our losses in any particular case. In addition, regardless of the level of insurance coverage, damage to our facilities may harm our business, financial condition and operating results.
The safety and efficacy of our products is not supported by long-term clinical data, which could limit sales, and our products could cause unforeseen negative effects.
V-Go, the only product we currently market in the United States, has received pre-market clearance under Section 510(k) of the U.S. Federal Food, Drug, and Cosmetic Act, or FDCA. This process is shorter and typically requires the submission of less supporting documentation than other FDA approval processes and does not always require long-term clinical studies. As a result, we currently lack significant published long-term clinical data supporting the safety and efficacy of our products and the benefits they offer that might have been generated in connection with other approval processes. For these reasons, people with type 2 diabetes who require insulin and their healthcare providers may be slower to adopt or recommend our product, we may not have comparative data that our competitors have or are generating and third-party payors may not be willing to provide coverage or reimbursement for our product. Further, future studies or clinical experience may indicate that treatment with our products is not superior to treatment with competitive products. Such results could slow the adoption of our products and significantly reduce our sales, which could prevent us from achieving our forecasted sales targets or achieving or sustaining profitability. Moreover, if future results and experience indicate that our products cause unexpected or serious complications or other unforeseen negative effects, we could be subject to mandatory product recalls, suspension or withdrawal of FDA clearance or approval, significant legal liability or harm to our business reputation.
Undetected errors or defects in V-Go or our future product candidates could harm our reputation, decrease market acceptance of our products or expose us to product liability claims.
V-Go or our future product candidates may contain undetected errors or defects. Disruptions or other performance problems with V-Go or these other product candidates may damage our customers’ businesses and could harm our reputation. If that occurs, we may incur significant costs, the attention of our key personnel could be diverted or other significant customer relations problems may arise. We may also be subject to warranty and liability claims for damages related to errors or defects in V-Go or our future product candidates. A material liability claim or other occurrence that harms our reputation or decreases market acceptance of V-Go or these other product candidates could harm our business and operating results.
The sale and use of V-Go or our other product candidates could lead to the filing of product liability claims if someone were to allege that V-Go or one of our product candidates contained a design or manufacturing defect. A product liability claim could result in substantial damages and be costly and time consuming to defend, either of which could materially harm our business or financial condition. While we currently maintain product liability insurance covering claims up to $5 million per incident, we cannot assure you that such insurance would adequately protect our assets from the financial impact of defending a product liability claim. Any product liability claim brought against us, with or without merit, could increase our product liability insurance rates or prevent us from securing insurance coverage in the future.
We may enter into strategic collaborations, in-licensing arrangements or alliances with third parties that may not result in the development of commercially viable products or the generation of significant future revenue.
In the ordinary course of our business, we may enter into strategic collaborations, in-licensing arrangements or alliances to develop product candidates and to pursue new markets. Proposing, negotiating and implementing strategic collaborations, in-licensing arrangements or alliances may be a lengthy and complex process. Other companies, including those with substantially greater financial, marketing, sales, technology or other business resources, may compete with us for these opportunities or arrangements. We may not identify, secure, or complete any such transactions or arrangements in a timely manner, on a cost-effective basis, on acceptable terms or at all. We have limited institutional knowledge and experience with respect to these business development activities, and we may also not realize the anticipated benefits of any such transaction or arrangement. In particular, these collaborations may not result in the development of products that achieve commercial success or result in significant revenue and could be terminated prior to developing any products.
Additionally, we may not be in a position to exercise sole decision making authority regarding the transaction or arrangement, which could create the potential risk of creating impasses on decisions, and our collaborators may have economic or business interests or goals that are, or that may become, inconsistent with our business interests or goals. We have limited control over the
amount and timing of resources that our current collaborators or any future collaborators devote to our collaborators’ or our future products. Disputes between us and our collaborators may result in litigation or arbitration that would increase our expenses and divert the attention of our management. Further, these transactions and arrangements are contractual in nature and may be terminated or dissolved under the directionterms of the applicable agreements and, in such event, we may not continue to have rights to the products relating to such transaction or arrangement or may need to purchase such rights at a premium.
We may seek to grow our business through acquisitions of complementary products or technologies, and the failure to manage acquisitions, or the failure to integrate them with our existing business, could impair our ability to execute our business strategies.
From time to time, we may consider opportunities to acquire other products or technologies that may enhance our product platform or technology, expand the breadth of our markets or customer base, or advance our business strategies. Potential acquisitions involve numerous risks, including:
problems assimilating the acquired products or technologies;
issues maintaining uniform standards, procedures, controls and policies;
unanticipated costs associated with acquisitions;
diversion of management’s attention from our existing business;
risks associated with entering new markets in which we have limited or no experience; and
increased legal and accounting costs relating to the acquisitions or compliance with regulatory matters.
We have no current commitments with respect to any acquisition. We do not know if we will be able to identify acquisitions we deem suitable, whether we will be able to successfully complete any such acquisitions on favorable terms or at all, or whether we will be able to successfully integrate any acquired products or technologies. Our inability to integrate any acquired products or technologies effectively could impair our ability to execute our business strategies.
If there are significant disruptions in our information technology systems, our reputation, financial condition and operating results could be harmed.
The efficient operation of our business depends on our information technology systems. We rely on our information technology systems to effectively manage sales and marketing data, accounting and financial functions, inventory management, product development tasks, research, development and engineering data, customer service and technical support functions. Our information technology systems are vulnerable to damage or interruption from earthquakes, fires, floods and other natural disasters, terrorist attacks, attacks by computer viruses or hackers, power losses, computer system or data network failures, cyber-attacks or cyber-intrusions over the internet, attachments to emails, persons inside our organization, or persons with access to systems inside our organization. The risk of a security breach or disruption, particularly through cyber-attacks or cyber intrusion, including by computer hackers, foreign governments, and cyber terrorists, has generally increased as the number, intensity and sophistication of attempted attacks and intrusions from around the world have increased.
The failure of our or our service providers’ information technology systems to perform as we anticipate or our failure to effectively implement new information technology systems, could disrupt our operations, which could have a negative impact on our reputation, financial condition and operating results.
Our failure to comply with data protection laws and regulations could lead to government enforcement actions and significant penalties against us, and adversely impact our operating results.
EU member states and other foreign jurisdictions, including Switzerland, have adopted data protection laws and regulations which impose significant compliance obligations. Moreover, the collection and use of personal health data in the EU, which was formerly governed by the provisions of the EU Data Protection Directive, was replaced with the EU General Data Protection Regulation, or the GDPR, in May 2018. The GDPR, which is wide-ranging in scope, imposes several requirements relating to the consent of the individuals to whom the personal data relates, the information provided to the individuals, the security and confidentiality of the personal data, data breach notification and the use of third-party processors in connection with the processing of personal data. The GDPR also imposes strict rules on the transfer of personal data out of the EU to the United States, provides an enforcement authority and imposes large penalties for noncompliance, including the potential for fines of up to €20 million or 4% of the annual global revenues of the noncompliant company, whichever is greater. The GDPR requirements apply not only to third-party transactions, but also to transfers of information between us and our subsidiaries, including employee information. The recent
implementation of the GDPR has increased our responsibility and liability in relation to personal data that we process, including in clinical trials, and we may in the future be required to put in place additional mechanisms to ensure compliance with the GDPR, which could divert management's attention and increase our cost of doing business. In addition, new regulation or legislative actions regarding data privacy and security (together with applicable industry standards) may increase our costs of doing business. In this regard, we expect that there will continue to be new proposed laws, regulations and industry standards relating to privacy and data protection in the United States, the EU and other jurisdictions, and we cannot determine the impact such future laws, regulations and standards may have on our business.
If we fail to properly manage our anticipated growth, our business could suffer.
We expect that any potential growth in our business will place a significant strain on our management team and on our financial resources. Failure to manage our growth effectively could cause us to misallocate management or financial resources, and result in losses or weaknesses in our infrastructure. Additionally, our anticipated growth will increase the demands placed on our suppliers, resulting in an increased need for us to manage our suppliers and monitor for quality assurance. Any failure by us to manage our growth effectively could impair our ability to achieve our business objectives.
We depend on the knowledge and skills of our senior management and other key employees, and if we are unable to retain and motivate them or recruit additional qualified personnel, our business may suffer.
We have benefited substantially from the leadership and performance of our senior management, as well as other key employees. Our success will depend on our ability to retain our current management and key employees, and to attract and retain qualified personnel in the future. Competition for senior management and key employees in our industry is intense and we cannot guarantee that we will be able to retain our personnel or attract new, qualified personnel. The loss of the services of members of our senior management or key employees could prevent or delay the implementation and completion of our strategic objectives, or divert management’s attention to seeking qualified replacements. We do not maintain key man life insurance on any of our senior management or key employees. Each of our executive officers may terminate employment without notice and without cause or good reason. Our executive officers are subject to non-competition agreements. Accordingly, the adverse effect resulting from the loss of our senior management could be compounded by our inability to prevent them from competing with us.
In addition, the sale of V-Go is logistically complex, requiring us to maintain a highly integrated, extensive sales, marketing and training infrastructure consisting of sales and marketing representatives, training personnel and customer care personnel. We face considerable challenges in recruiting, training, managing, motivating and retaining the members of these teams, including managing geographically dispersed efforts. These challenges are exacerbated by the fact that our strategic plan requires us to rapidly grow our sales, with limited marketing and training infrastructure growth, while generating increased demand for our product. If we fail to maintain and grow a dedicated team of sales representatives and are unable to retain our sales and marketing, managed care, medical and other personnel, we could fail to take advantage of an opportunity to enhance our brand recognition and grow our revenue.
Risks Related to Our Financial Condition and Capital Requirements
We will have broad discretion in how we use the net proceeds from our April 2018 public offering, November 2018 public offering, the First Purchase Agreement and past or future public offerings under the Second Purchase Agreement and the Sales Agreement, if any. We may not use these proceeds effectively, which could affect our results of operations and cause our stock price to decline.
We will have considerable discretion in the application of the net proceeds from our April 2018 public offering, November 2018 public offering, the First Purchase Agreement and past or future public offerings under the Sales Agreement or the Second Purchase Agreement, if any. We intend to use the majority of the net proceeds from our April 2018 public offering, the First Purchase Agreement and past or future public offerings under the Sales Agreement or the Second Purchase Agreement, if any, to continue implementing our sales strategy, and for working capital and other general corporate purposes, which may include funding for the hiring of additional personnel, validation of capital equipment and the costs of operating as a public company. As a result, investors will be relying upon management's judgment with only limited information about our specific intentions for the use of the balance of the net proceeds from our April 2018 public offering, November 2018 public offering, the First Purchase Agreement and past or future public offerings under the Sales Agreement or the Second Purchase Agreement, if any. We may use the net proceeds for purposes that do not yield a significant return or any return at all for our stockholders. In addition, pending their use, we may invest the net proceeds from our April 2018 public offering, November 2018 public offering, the First Purchase Agreement and past or future public offerings under the Sales Agreement or the Second Purchase Agreement, if any, in a manner that does not produce income or that loses value.
Our future capital needs are uncertain and we may need to raise additional funds in the future, and these funds may not be available on acceptable terms or at all.
At December 31, 2018, we had $47.8 million in cash and cash equivalents, of which $0.5 million is restricted cash. We do not believe that our cash on hand will be sufficient to satisfy our liquidity requirements through the next 12 months from the financial statement issuance date. The continued growth of our business, including the expansion of our research, development and engineering activities, and our efforts to commercialize our pre-fill V-Go will continue to significantly increase our expenses. In addition, the amount of our future product sales is difficult to predict and actual sales may not be in line with our forecasts. As a result, we will need to raise additional capital in addition to the amounts we could receive under the Second Purchase Agreement and the Sales Agreement, which may not be available on reasonable terms, if at all. Our future capital requirements will depend on many factors, including:
the revenue generated by sales of V-Go and any other future product candidates that we may develop and commercialize;
the costs associated with expanding our sales and marketing infrastructure;
the expenses we incur in maintaining our manufacturing facility and adding further manufacturing equipment and capacity;
the cost associated with developing and commercializing our proposed products or technologies, including our pre-fill V-Go;
the cost of obtaining and maintaining regulatory clearance or approval for our current or future products;
the cost of ongoing compliance and regulatory requirements;
expenses we incur in connection with potential litigation or governmental investigations;
anticipated or unanticipated capital expenditures; and
unanticipated general and administrative expenses.
If we issue equity or debt securities to raise additional funds, our existing stockholders may experience dilution, and the new equity or debt securities may have rights, preferences and privileges senior to those of our existing stockholders. In addition, if we raise additional funds through collaborations, licensing, joint ventures, strategic alliances, partnership arrangements or other similar arrangements, it may be necessary to relinquish valuable rights to our potential future products or proprietary technologies, or grant licenses on terms that are not favorable to us.
If we are unable to raise additional capital, we may not be able to expand our sales and marketing infrastructure, enhance our current products or develop new products, take advantage of future opportunities, or respond to competitive pressures, changes in supplier relationships, or unanticipated changes in customer demand. Any of these events could adversely affect our ability to achieve our strategic objectives and impact our ability to continue as a going concern.
Our operating results may fluctuate significantly from quarter to quarter.
We began commercial sales of V-Go in the first quarter of 2012. Due to our limited operating history, there has been and there may continue to be meaningful variability in our operating results among quarters, as well as within each quarter. Our operating results, and the variability of these operating results, will be affected by numerous factors, including:
our ability to increase sales of V-Go and to commercialize and sell our future products, if any, and the number of our products sold in each quarter;
acceptance of our products by people with type 2 diabetes who require insulin, their caregivers, healthcare providers and third-party payors;
the pricing of our products and competitive products, the effect of third-party coverage and reimbursement policies, and the amount and level of sales discounts or rebates required to obtain or retain effective third-party payor coverage and reimbursement;
our ability to establish and grow an effective sales and marketing infrastructure;
the amount of, and the timing of the payment for, insurance deductibles required to be paid by patients and potential patients under their existing insurance plans;
interruption in the manufacturing or distribution of our product;
seasonality and other factors affecting the timing of purchases of our product;
timing of new product offerings, acquisitions, licenses or other significant events by us or our competitors;
the ability of our suppliers to timely provide us with an adequate supply of components that meet our requirements;
regulatory clearance or approvals affecting our products or those of our competitors;
changes in healthcare rules, coverage and reimbursement under government healthcare programs, including Medicare and Medicaid; and
the timing of revenue recognition associated with our product sales pursuant to applicable accounting standards.
As a result of our limited operating history, and due to the complexities of the industry in which we operate, it will be difficult for us to forecast demand for our current or future products with any degree of certainty, which means it will be difficult for us to forecast our sales as well as production or inventory requirements. In addition, we will be significantly increasing our operating expenses as we expand our business. Accordingly, we may experience substantial variability in our operating results from quarter to quarter, including unanticipated quarterly losses. If our quarterly or annual operating results fall below the expectations of investors or securities analysts, the price of our common stock could decline substantially. Furthermore, any quarterly or annual fluctuations in our operating results may, in turn, cause the price of our common stock to fluctuate substantially. We believe that quarterly comparisons of our financial results are not necessarily meaningful and should not be relied upon as an indication of our future performance.
If we experience lower-than-anticipated revenue in any particular quarter, or if we announce that we expect lower revenue or earnings than previously forecasted, the market price of our common stock could decline.
Our revenue is difficult to forecast and is likely to fluctuate from quarter to quarter due to many factors, including many that are outside of our control. Any significant revenue shortfall, lowered revenue or earnings forecast, or failure to meet analysts' expectations could cause the market price of our common stock to decline substantially. Reliance should not be placed on the results of prior periods as an indication of our future performance. Our operating expense levels are based, in significant part, on our expectations of future revenue. If we have a shortfall in revenue or sales in any given quarter, we may not be able to reduce our operating expenses quickly in response. Therefore, any significant shortfall in our revenues could have an immediate material adverse effect on our business, financial position, and results of operations for that quarter. In addition, if we fail to manage our business effectively over the long term, we may experience high operating expenses, and our operating results may fall below the expectations of securities analysts or investors, which could result in a substantial decline in the market price of our common stock.
Forecasting future revenues is difficult, especially when the level of market acceptance of our products is changing rapidly. As a result, it is reasonably likely that our product sales will fluctuate to an extent that may not meet with market expectations and that also may adversely affect our stock price. There are a number of other factors that could cause our financial results to fluctuate unexpectedly, including:
cost of product sales;
achievement and timing of research and development milestones;
collaboration revenues;
cost and timing of clinical trials, regulatory approvals and product launches;
"at-risk" generic launches;
marketing and other expenses;
manufacturing or supply disruptions;
unanticipated conversion of our convertible notes; and
costs associated with the operations of recently-acquired businesses and technologies.
We may not be able to generate sufficient cash to service our credit facility with Capital Royalty Group. If we fail to comply with the obligations under our credit facility, the lender may be able to accelerate amounts owed under the facility and may foreclose upon the assets securing our obligations.
As of December 31, 2018, the aggregate principal amount of our term loan with Capital Royalty Group, or CRG, and certain of its affiliates, or our Term Loan, was $27.5 million. Borrowings under our credit facility are secured by substantially all of our assets, including our material intellectual property. Our ability to make scheduled payments or to refinance our debt obligations depends on numerous factors, including the amount of our cash reserves and our actual and projected financial and operating performance. These amounts and our performance are subject to numerous risks, including the risks in this section, some of which may be beyond our control. We cannot assure you that we will maintain a level of cash reserves or cash flows from operating activities sufficient to permit us to pay the principal, premium, if any, and interest on our existing or future indebtedness. If our cash flows and capital resources are insufficient to fund our debt service obligations, we may be forced to reduce or delay capital expenditures, sell assets or operations, seek additional capital or restructure or refinance our indebtedness. We cannot assure you that we would be able to take any of these actions, or that these actions would permit us to meet our scheduled debt service obligations. In addition, in the event of our breach of the Term Loan, we may be required to repay any outstanding amounts earlier than anticipated. If we fail to comply with our obligations under the Term Loan, the lender would be able to accelerate the required repayment of amounts due and, if they are not repaid, could foreclose upon our assets securing our obligations under the Term Loan. In addition, certain events of default have already occurred under the term loan with CRG in 2015 and we cannot assure you similar future events of default will not occur under the Term Loan. For more information on the Term Loan, see “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Indebtedness—Senior Secured Debt”.
Our Term Loan contains a financial covenant that may limit our operating flexibility.
Our Term Loan contains a restrictive covenant that requires us to maintain an end-of-day balance of $2.0 million in cash or cash equivalents, which may limit our ability to engage in new lines of business, make certain investments, pay dividends, or enter into various transactions. We therefore may not be able to engage in any of the foregoing transactions unless we obtain the consent of the lender or terminate our Term Loan. Furthermore, there is no guarantee that future working capital, borrowings or equity financing will be available to repay or refinance the amounts outstanding under the agreement. For additional information about the Term Loan, see “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Indebtedness—Senior Secured Debt.”
Prolonged negative economic conditions could adversely affect us, our customers and suppliers, which could harm our financial condition.
We are subject to the risks arising from adverse changes in general economic and market conditions. Economic turmoil and uncertainty about future economic conditions could adversely impact our existing and potential customers, the financial ability of health insurers to pay claims, patients’ ability or willingness to pay out-of- pocket costs, our ability to obtain financing for our operations on favorable terms, or at all, and our relationships with key suppliers.
The use of our net operating loss carryforwards and research tax credits may be limited.
Our net operating loss carryforwards and any future research and development tax credits may expire and not be used. As of December 31, 2018, we had U.S. net operating loss carryforwards of approximately $425.3 million. Our net operating loss carryforwards arising in taxable years ending on or prior to December 31, 2017 will begin expiring in 2028 if we have not used them prior to that time. Net operating loss carryforwards arising in taxable years ending after December 31, 2017 are no longer subject to expiration under the Internal Revenue Code of 1986, as amended, or the Code. Additionally, our ability to use any net operating loss and credit carryforwards to offset taxable income or tax, respectively, in the future will be limited under Sections 382 and 383 of the Code, respectively, if we have a cumulative change in ownership of more than 50% within a three-year period. The completion of our March 2017 public offering, together with private placements and other transactions that have occurred, may trigger, or may have already triggered, such an ownership change. In addition, since we will need to raise substantial additional funding to finance our operations, we may undergo further ownership changes in the future. We have never completed an analysis as to whether such a change of ownership has occurred, but in such an event, we will be limited regarding the amount of net operating loss carryforwards and research tax credits that could be utilized annually in the future to offset taxable income or tax, respectively. Any such annual limitation may significantly reduce the utilization of the net operating loss carryforwards and research tax credits before they expire. In addition, certain states have suspended use of net operating loss carryforwards for certain taxable years, and other states are considering similar measures. As a result, we may incur higher state income tax expense in the future. Depending on our future tax position, continued suspension of our ability to use net operating loss carryforwards in states in which we are subject to income tax could have an adverse impact on our results of operations and financial condition.
Risks Related to Intellectual Property
Intellectual property rights may not provide adequate protection, which may permit third parties to compete against us more effectively.
Our success depends significantly on our ability to maintain and protect our proprietary rights in the technologies and inventions used in or embodied by our product. To protect our proprietary technology, we rely on patent protection, as well as a combination of copyright, trade secret and trademark laws, as well as nondisclosure, confidentiality, and other contractual restrictions in our manufacturing, consulting, employment and other third party agreements. These legal means afford only limited protection, however, and may not adequately protect our rights or permit us to gain or keep any competitive advantage.
If we are unable to secure sufficient patent protection for our proprietary rights in our products and processes, and to adequately maintain and protect our existing and new rights, competitors will be able to compete against us more effectively, and our business will suffer.
The process of applying for patent protection itself is time consuming and expensive and we cannot assure you that we have prepared or will be able to prepare, file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of inventions made in the course of development and commercialization activities before it is too late to obtain patent protection on them. In addition, our patents and applications may not be prosecuted and enforced in a manner consistent with the best interests of our business. It is possible that defects of form in the preparation or filing of our patents or patent applications may exist, or may arise in the future, for example, with respect to proper priority claims, inventorship, claim scope or patent term adjustments. Moreover, we cannot assure you that all of our pending patent applications will issue as patents or that, if issued, they will issue in a form that will be advantageous to us. We own numerous issued patents and pending patent applications that relate to insulin- delivery methods and devices. The rights granted to us under our patents, however, including prospective rights sought in our pending patent applications, may not be of sufficient scope or strength to provide us with any meaningful exclusivity or commercial advantage, and competitors may be able to design around our patents or develop products that provide outcomes comparable to ours without infringing on our intellectual property rights. In addition, we may in the future be subject to claims by our former employees or consultants asserting an ownership right in our patents or patent applications, as a result of the work they performed on our behalf. If any of our patents are challenged, invalidated or legally circumvented by third parties, and if we do not exclusively own other enforceable patents protecting our product, competitors could market products and use processes that are substantially similar to, or superior to, ours, and our business will suffer.
The patent position of medical technology companies is generally highly uncertain. The degree of patent protection we require may be unavailable or severely limited in some cases and may not adequately protect our rights or permit us sufficient exclusivity, or to gain or keep our competitive advantage. For example:
we might not have been the first to invent or the first inventor to file patent applications on the inventions covered by each of our pending patent applications and issued patents;
others may independently develop similar or alternative technologies or duplicate any of our technologies;
other companies hold patents stating broad claims in the drug delivery device field which, if construed to cover our products and held to be valid and enforceable, could have a material adverse effect on our business;
any patents we obtain or license from others in the future may not encompass commercially viable products, may not provide us with any competitive advantages or may be challenged by third parties;
any patents we obtain or license from others in the future may not be valid or enforceable; and
we may not develop additional proprietary technologies that are patentable.
Patents have a limited lifespan. In the United States, the natural expiration of a utility patent typically is 20 years after it is filed. Various extensions may be available; however, the life of a patent, and the protection it affords, is limited. Without patent protection for our insulin-delivery methods and devices, we may be open to competition from generic versions of such methods and devices.
Changes in patent law could diminish the value of patents in general, thereby impairing our ability to protect our products and our technologies.
Patent reform legislation may increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents. On September 16, 2011, the Leahy- Smith America Invents Act, or the Leahy-Smith Act, was signed into law. The Leahy-Smith Act includes a number of significant changes to U.S. patent law. These include provisions that affect the way patent applications are prosecuted, redefine prior art, may affect patent litigation, and switch the U.S. patent system from a “first-to- invent” system to a “first-to-file” system. Under a “first-to-file” system, assuming the other requirements for patentability are met, the first inventor to file a patent application generally will be entitled to the patent on an invention regardless of whether another inventor had made the invention earlier. The Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents.
In addition, patent reform legislation may pass in the future that could lead to additional uncertainties and increased costs surrounding the prosecution, enforcement, and defense of our patents and applications.
Furthermore, the U.S. Supreme Court and the U.S. Court of Appeals for the Federal Circuit have made, and will likely continue to make, changes in how the patent laws of the United States are interpreted. Similarly, foreign courts have made, and will likely continue to make, changes in how the patent laws in their respective jurisdictions are interpreted. We cannot predict future changes in the interpretation of patent laws or changes to patent laws that might be enacted into law by United States and foreign legislative bodies. Those changes may materially affect our patents or patent applications and our ability to obtain and enforce or defend additional patent protection in the future.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Moreover, the USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. In addition, periodic maintenance fees on issued patents often must be paid to the USPTO and foreign patent agencies over the lifetime of the patent. While an unintentional lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Non-compliance events that could result in abandonment or lapse of a patent or patent application include, but are not limited to, failure to respond to official actions within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents. If we fail to maintain the patents and patent applications covering our products or procedures, we may not be able to stop a competitor from marketing products that are the same as or similar to our products and technologies.
We may not be able to adequately protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on our products and technologies in all countries throughout the world would be prohibitively expensive. The requirements for patentability may differ in certain countries, particularly developing countries, and the breadth of patent claims allowed can be inconsistent. In addition, the laws of some foreign countries may not protect our intellectual property rights to the same extent as laws in the United States. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where we have patent protection, but enforcement on infringing activities is inadequate.
We do not have patent rights in certain foreign countries in which a market may exist in the future. Moreover, in foreign jurisdictions where we do have patent rights, proceedings to enforce such rights could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly, and our patent applications at risk of not issuing, and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Thus, we may not be able to stop a competitor from marketing and selling in foreign countries products that are the same as or similar to our product.
We may in the future become involved in lawsuits to protect or enforce our intellectual property, which could be expensive, time consuming and unsuccessful.
The medical device industry has been characterized by frequent and extensive intellectual property litigation. Our competitors or other patent holders may assert that our products and the methods employed in our products are covered by their patents. For example, other companies hold patents stating broad claims in the drug delivery device field which, if construed to cover our products and held to be valid and enforceable, could have a material adverse effect on our business. Although we believe we have adequate defenses available if faced with any allegations that we infringe third-party patents, it is possible that V-Go could be found to infringe these patents. If our product or methods are found to infringe, we could be prevented from manufacturing or marketing our products.
We do not know whether our competitors or potential competitors have patents, or have applied for, will apply for, or will obtain patents that will prevent, limit or interfere with our ability to make, have made, use, sell, import or export our products. Competitors may infringe our patents or misappropriate or otherwise violate our intellectual property rights. To stop any such infringement or unauthorized use, litigation may be necessary. Our intellectual property has not been tested in litigation. A court may declare our patents invalid or unenforceable, may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question, or may interpret the claims of our patents narrowly, thereby substantially narrowing the scope of patent protection they afford.
In addition, third parties may initiate legal proceedings against us to challenge the validity or scope of our intellectual property rights, or may allege an ownership right in our patents, as a result of their past employment or consultancy with us. Many of our current and potential competitors have the ability to dedicate substantially greater resources to defend their intellectual property rights than we can. Accordingly, despite our efforts, we may not be able to prevent third parties from infringing upon or misappropriating our intellectual property.
Competing products may also be sold in other countries in which our patent coverage might not exist or be as strong. If we lose a foreign patent lawsuit, alleging our infringement of a competitor’s patents, we could be prevented from marketing our products in one or more foreign countries.
Litigation related to infringement and other intellectual property claims such as trade secrets, with or without merit, is unpredictable, can be expensive and time-consuming, and can divert management’s attention from our core business. If we lose this kind of litigation, a court could require us to pay substantial damages, treble damages, and attorneys’ fees, and could prohibit us from using technologies essential to our product, any of which would have a material adverse effect on our business, results of operations, and financial condition. If relevant patents are upheld as valid and enforceable and we are found to infringe, we could be prevented from selling our products unless we can obtain licenses to use technology or ideas covered by such patents. We do not know whether any necessary licenses would be available to us on satisfactory terms, if at all. If we cannot obtain these licenses, we could be forced to design around those patents at additional cost or abandon the products altogether. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. There could also be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could cause the price of our common stock to decline.
During 2018, we received an offer from Roche to license its US Patent No. 6,736,795 (the ‘795 patent), which expires on September 23, 2020 and that is alleged to cover our V-Go product. We do not believe the ‘795 patent to be valid, infringed, and/or enforceable and therefore we do not view Roche’s allegation as having merit. We also understand that Roche has never practiced the ‘795 Patent and Roche permitted the European equivalent patent to lapse. Accordingly, we declined Roche’s offer of a license to the ‘795 patent, and on January 24, 2019, we filed two Inter Partes Review petitions, which included three previously unconsidered prior art references, with the Patent Trial and Appeal Board, or PTAB. In response, in late February 2019, Roche filed a proceeding in federal court in Delaware seeking an unspecified amount of damages and potential injunctive relief. The PTAB is expected to issue its preliminary findings by July 24, 2019.Although we cannot guarantee the outcome of this matter, based on our review, we have determined that we have a number of defenses and we intend to defend ourselves vigorously against any allegations of patent infringement, which we believe are without merit. Further, given that the average time to trial in Delaware federal court is approximately three years, the ‘795 patent will expire before any potential trial, rendering Roche’s claim for injunctive relief moot.
We may be subject to damages resulting from claims that we or our employees have wrongfully used or disclosed alleged trade secrets of our competitors or are in breach of non-competition or non-solicitation agreements with our competitors.
We could in the future be subject to claims that we or our employees have inadvertently or otherwise used or disclosed alleged trade secrets or other proprietary information of former employers, competitors, or other third parties. Although we endeavor to ensure that our employees and consultants do not use the intellectual property, proprietary information, know-how or trade secrets of others in their work for us, we may in the future be subject to claims that we caused an employee to breach the terms of his or her non-competition or non-solicitation agreement, or that we or these individuals have, inadvertently or otherwise, used or disclosed the alleged trade secrets or other proprietary information of a former employer or competitor. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and could be a distraction to management. If our defense to those claims fails, in addition to paying monetary damages, a court could prohibit us from using technologies or features that are essential to our product, if such technologies or features are found to incorporate or be derived from the trade secrets or other proprietary information of the former employers or other third parties. An inability to incorporate technologies or features that are important or essential to our products may prevent us from selling our product. In addition, we may lose valuable intellectual property rights or personnel. Moreover, any such litigation or the threat thereof may adversely affect our ability to hire employees or contract with independent sales representatives. A loss of key personnel or their work product could hamper or prevent our ability to commercialize our product.
Our trademarks may be infringed or successfully challenged, resulting in harm to our business.
We rely on our trademarks as one means to distinguish our products from the products of our competitors, and we have registered or applied to register many of these trademarks. The USPTO or foreign trademark offices may deny our trademark applications, however, and even if published or registered, these trademarks may be ineffective in protecting our brand and goodwill and may be successfully opposed or challenged. Third parties may oppose our trademark applications, or otherwise challenge our use of our trademarks. In addition, third parties may use marks that are confusingly similar to our own, which could result in confusion among our customers, thereby weakening the strength of our brand or allowing such third parties to capitalize on our goodwill. In such an event, or if our trademarks are successfully challenged, we could be forced to rebrand our product, which could result in loss of brand recognition and could require us to devote resources to advertising and marketing new brands. Our competitors may infringe our trademarks and we may not have adequate resources to enforce our trademark rights in the face of any such infringement.
If we are unable to protect the confidentiality or use of our trade secrets, our competitive position may be harmed.
In addition to patent and trademark protection, we also rely on trade secrets, including unpatented know-how, technology and other proprietary information, to maintain our competitive position. We seek to protect our trade secrets, in part, by entering into non-disclosure and confidentiality agreements with parties who have access to them, such as our manufacturers, consultants and vendors, and our former or current employees. We also enter into invention or assignment agreements with our employees. Despite these efforts, any of these parties may breach the agreements and disclose our trade secrets and other unpatented or unregistered proprietary information. Monitoring unauthorized uses and disclosures of our intellectual property is difficult, and we do not know whether the steps we have taken to protect our intellectual property will be effective. In addition, we may not be able to obtain adequate remedies for any such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, some courts inside and outside the United States are less willing or unwilling to protect trade secrets.
Moreover, our competitors may independently develop equivalent knowledge, methods and know-how. Competitors could purchase our products and attempt to replicate some or all of the competitive advantages we derive from our development efforts,
willfully infringe our intellectual property rights, design around our protected technology or develop their own competitive technologies that fall outside of our intellectual property rights. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent them, or those to whom they communicate it, from using that technology or information to compete with us. If our intellectual property is not adequately protected so as to protect our market against competitors’ products and methods, our competitive position could be adversely affected.
Risks Related to Our Legal and Regulatory Environment
Our products and operations are subject to extensive governmental regulation, and failure to comply with applicable requirements could cause our business to suffer.
The medical technology industry is regulated extensively by governmental authorities, principally the FDA, and state regulatory agencies with oversight of various aspects of drug and device distribution, sale, and use. The regulations are very complex, have become more stringent over time, and are subject to rapid change and varying interpretations. Regulatory restrictions or changes could limit our ability to carry on or expand our operations or result in higher than anticipated costs or lower than anticipated sales. The FDA and other federal and state governmental agencies regulate numerous elements of our business, including:
• product design and development;
• pre‑clinical and clinical testing and trials;
• product safety;
• establishment registration and product listing;
• labeling and storage;
• marketing, manufacturing, sales and distribution;
• pre‑market clearance or approval;
servicing and post‑marketing surveillance, including reporting of deaths or serious injuries and malfunctions that, if they recurred, could lead to death or serious injury;
• advertising and promotion;
• post‑market approval studies;
• product import and export; and
recalls and field‑safety corrective actions.
Before we can market or sell a new regulated product or a significant modification to an existing product in the United States, we must obtain either clearance under Section 510(k) of the FDCA, grant of a de novo classification request, or approval of a pre‑market approval, or PMA, application from the FDA, unless an exemption from pre‑market review applies. In the 510(k) clearance process, the FDA must determine that a proposed device is “substantially equivalent” to a legally marketed “predicate” device (in most cases Class II devices, with a few exceptions), with respect to intended use, technology and safety and effectiveness, in order to clear the proposed device for marketing. Class III devices approved under the PMA process cannot serve as predicates. Clinical data are sometimes required to support substantial equivalence. In the de novo process, the FDA must determine that general and special controls are sufficient to provide reasonable assurance of the safety and effectiveness of a device, which is low to moderate risk and has no predicate (in other words, the applicant must justify the “down-classification” to Class I or II for a new product type that would otherwise automatically be placed into Class III, but is lower risk). The PMA process requires an applicant to demonstrate the safety and effectiveness of the device based on extensive data, including, but not limited to, technical, preclinical, clinical trial, manufacturing and labeling data. The PMA process is typically required for devices that are deemed to pose the greatest risk, such as life‑sustaining, life‑supporting or implantable devices. Products that are approved through a PMA application generally need FDA approval before they can be modified. Similarly, some modifications made to products cleared through a 510(k) may require a new 510(k). The 510(k), de novo, and PMA processes can be expensive and lengthy and require the payment of significant fees, unless an exemption applies. The FDA’s 510(k) clearance process usually takes from 3 to 12 months, but may take longer. The FDA’s stated goal is to review de novo classification requests within 150 days, 50% of the time (which is 30 days beyond the statutory timeline of 120 days), but in reality the process for many applicants generally takes even longer, up to a year or more. The process of obtaining a PMA is much more costly and uncertain than the 510(k) clearance process and generally takes
from one to three years, or longer, from the time the application is submitted to the FDA until an approval is obtained. The process of obtaining regulatory clearances or approvals to market a medical device can be costly and time‑consuming, and we may not be able to obtain these clearances or approvals on a timely basis, or at all for our proposed products.
We obtained initial pre‑market clearance for V‑Go under Section 510(k) of the FDCA in December 2010. If the FDA requires us to go through a lengthier, more rigorous examination for future products or modifications to our existing product than we had expected, our product introductions or modifications could be delayed or canceled, which could cause our sales to decline or to not increase in line with our forecasts. In addition, the FDA may determine that future products will require the more costly, lengthy and uncertain PMA process. Although we do not currently market any devices under PMA, the FDA may demand that we obtain a PMA prior to marketing certain of our future products. Further, even with respect to those future products where a PMA is not required, we cannot assure you that we will be able to obtain the 510(k) clearances with respect to those products.
The FDA can delay, limit or deny clearance or approval of a device for many reasons, including:
we may not be able to demonstrate that our products are safe and effective for their intended users;
the data from our clinical trials may be insufficient to support clearance or approval; and
the manufacturing process or facilities we use may not meet applicable requirements.
In addition, the FDA may change its clearance and approval policies, adopt additional regulations or revise existing regulations, or take other actions which may prevent or delay approval or clearance of our products under development or impact our ability to modify our currently cleared products on a timely basis. Any delay in, or failure to obtain or maintain, clearance or approval for our products under development could prevent us from generating revenue from these products and adversely affect our business operations and financial results. Additionally, the FDA and other regulatory authorities have broad enforcement powers.
Regulatory enforcement or inquiries, or other increased scrutiny on us, could dissuade some customers from using our products and adversely affect our reputation and the perceived safety and efficacy of our product.
Failure to comply with applicable regulations could jeopardize our ability to sell our products and result in enforcement actions such as fines, civil penalties, injunctions, warning letters, recalls of products, delays in the introduction of products into the market, refusal of the FDA or other regulators to grant future clearances or approvals, and the suspension or withdrawal of existing clearances or approvals by the FDA or other regulators. Any of these sanctions could result in higher than anticipated costs or lower than anticipated sales and negatively impact our reputation, business, financial condition and operating results.
Furthermore, we continue to evaluate international expansion opportunities. Any operations or product applications outside of the United States will subject us to various additional regulatory and legal requirements under the applicable laws and regulations of the international markets we enter. These additional regulatory requirements may involve significant costs and expenditures and, if we are not able to comply with any such requirements, our international expansion and business could be significantly harmed.
Modifications to our products may require new 510(k) clearances, de novo submissions, or pre‑market approvals, or may require us to cease marketing or recall the modified products until clearances are obtained.
Any modification to a 510(k)‑cleared device that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, design, or manufacture, requires a new 510(k) clearance or, possibly, a de novo or PMA. The FDA requires every manufacturer to make this determination in the first instance, and provides some guidance on decision making, but the FDA may review any manufacturer’s decision at any time. The FDA may not agree with our decisions regarding whether new clearances or approvals are necessary. We have made modifications to our 510(k) cleared product, and have determined based on our review of the applicable FDA guidance that in certain instances new 510(k) clearances, de novo or pre‑market approvals are not required. If the FDA disagrees with our determination and requires us to submit new 510(k) notifications, de novo submissions or PMAs for modifications to our previously cleared or approved products for which we have concluded that new clearances or approvals are unnecessary, we may be required to cease marketing or to recall the modified product until we obtain clearance or approval, and we may be subject to significant regulatory fines or penalties.
If we or our third‑party suppliers fail to comply with the FDA’s good manufacturing practice regulations or fail to adequately, timely, or sufficiently respond to an FDA Form 483 or subsequent Warning Letter, this could impair our ability to market our products in a cost‑effective and timely manner and could result in FDA enforcement action.
We and our third‑party suppliers are required to comply with the FDA’s Quality System Regulation, or QSR, and Current Good Manufacturing Practices (cGMP) which covers the methods and documentation of the design, testing, production, control, quality
assurance, labeling, packaging, sterilization, storage and shipping of our product. The FDA audits compliance with the QSR, cGMP and related regulations through periodic announced and unannounced inspections of manufacturing and other facilities. The FDA may conduct these inspections or audits at any time. If, during the inspection, FDA identifies issues which, in FDA’s judgment, may constitute violations of the Federal Food, Drug, and Cosmetic Act or FDA’s regulations, the FDA inspector may issue an FDA Form 483 listing these observations.
Note that if an entity does not address observations found in an FDA Form 483 to FDA’s satisfaction, the FDA could take enforcement action, including any of the following sanctions:
untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties;
customer notifications or repair, replacement, refunds, recall, detention or seizure of our product;
operating restrictions or partial suspension or total shutdown of production;
refusing or delaying our requests for 510(k) clearance or pre‑market approval of new products or modified products;
withdrawing 510(k) clearances or pre‑market approvals that have already been granted;
refusal to grant export approval for our product; or
criminal prosecution.
Any of the foregoing actions could have a material adverse effect on our reputation, business, financial condition and operating results.
Our latest FDA inspection occurred between August 21, 2018 and September 7, 2018 and resulted in an FDA Form 483 issued on September 7, 2018. The FDA Form 483 observations related to our medical device reporting, or MDR, complaint file, design risk analysis, and device history procedures and records. On September 21, 2018, we submitted a timely response to FDA describing how we intend to address the observations listed in the FDA Form 483. After submitting our corrective action plan to FDA, the agency sent us its establishment inspection report, or EIR, on October 3, 2018. As stated in the EIR, FDA concluded that the inspection is closed and the decision to not take regulatory enforcement action is closed. We are completing the corrective actions as communicated to FDA.
A recall of our product, or the discovery of serious safety issues with our product, could have a significant adverse impact on us.
The FDA has the authority to require the recall of commercialized products in the event of material deficiencies or defects in design or manufacture or in the event that a product poses an unacceptable risk to health.
Manufacturers may, under their own initiative, recall a product if any material deficiency in a device is found. A government‑mandated or voluntary recall by us or one of our distributors could occur as a result of an unacceptable risk to health, component failures, manufacturing errors, design or labeling defects or other deficiencies and issues. Recalls of our products would divert managerial and financial resources and have an adverse effect on our reputation, financial condition and operating results, which could impair our ability to produce our products in a cost‑effective and timely manner.
Further, under the FDA’s medical device reporting, or MDR regulations, we are required to report to the FDA any incident in which our products may have caused or contributed to a death or serious injury or in which our products malfunctioned and, if the malfunction were to recur, would likely cause or contribute to death or serious injury. Repeated product malfunctions may result in a voluntary or involuntary product recall, which could divert managerial and financial resources, impair our ability to manufacture our products in a cost‑effective and timely manner and have an adverse effect on our reputation, financial condition and operating results.
Depending on the corrective action we take to redress a product’s deficiencies or defects, the FDA may require, or we may decide, that we will need to obtain new approvals or clearances for the device before we may market or distribute the corrected device. Seeking such approvals or clearances may delay our ability to replace the recalled devices in a timely manner. Moreover, if we do not adequately address problems associated with our devices, we may face additional regulatory enforcement action, including FDA warning letters, product seizure, injunctions, administrative penalties, or civil or criminal fines. We may also be required to
bear other costs or take other actions that may have a negative impact on our sales as well as face significant adverse publicity or regulatory consequences, which could harm our business, including our ability to market our products in the future.
Any adverse event involving our products could result in future voluntary corrective actions, such as recalls or customer notifications, or regulatory agency action, which could include inspection, mandatory recall or other enforcement action. Any corrective action, whether voluntary or involuntary, will require the dedication of our time and capital, distract management from operating our business and may harm our reputation and financial results.
If we are unable to achieve and maintain adequate levels of coverage and reimbursement for V‑Go or any future products we may seek to commercialize, their commercial success may be severely hindered.
We have derived all of our revenue from the sale of V‑Go in the United States and expect to continue to do so for the next several years. Patients who use V‑Go generally rely on third‑party payors, including governmental healthcare programs, such as Medicare and Medicaid, and commercial health insurers, health maintenance organizations and other healthcare‑related organizations, to reimburse all or part of the costs associated with V‑Go. Successful sales of V‑Go and any future products depend, therefore, on the availability of adequate coverage and reimbursement from third‑party payors.
Securing coverage for new technologies is challenging and uncertain. Third‑party payors render coverage decisions based upon clinical and economic standards that disfavor new products when more established or lower cost therapeutic alternatives are already available or subsequently become available. Unless our products demonstrate superior affordability or efficacy, they may not qualify for coverage and reimbursement. Even if we obtain coverage for a given product, the resulting reimbursement payment rates might not be adequate or may require co‑payments, deductibles or co‑insurance payments that patients find unacceptably high.
Not only are third‑party payors, whether governmental or commercial, developing increasingly sophisticated methods of controlling healthcare costs, in addition, no uniform policy of coverage and reimbursement for medical products, including V‑Go, exists among third‑party payors. Therefore, coverage and reimbursement for our products can and do differ significantly from payor to payor. As a result, the coverage determination process is often a time‑consuming and costly process that requires us to provide economic, scientific and clinical support for the use of our products to each payor separately, with no assurance that coverage and adequate reimbursement will be obtained. Even where favorable coverage and reimbursement status has been attained for V‑Go, less favorable coverage policies and reimbursement rates may be implemented in the future. Moreover, a third‑party payor’s decision to provide coverage does not imply that an adequate reimbursement rate will be paid. There can be no assurance that our clinical data will allow for satisfactory pricing of V‑Go at current levels, and the failure to obtain and maintain coverage and adequate reimbursement for V‑Go would materially and adversely affect our business.
V‑Go currently is covered and reimbursed under the policies of a number of third‑party payors. The Medicare program recognizes V‑Go under the Medicare Part D prescription drug benefit, and a number of Part D drug plans have placed our products on their pharmacy formularies or otherwise allow for individual consideration. Although V‑Go is not covered under Medicare Part B, an outpatient medical benefit that does not recognize disposable insulin delivery devices, other third‑party payors may have adopted different coverage policies, classifying a disposable insulin delivery device as a coverable device. Some commercial payors, however, have declined to offer any coverage for V‑Go, whether on a pharmacy formulary or as a medical benefit, concluding that the delivery system is experimental or investigational, or that the current evidence is insufficient. In addition, coverage policies developed by third‑party payors generally can be modified or terminated by the third‑party payor without cause and with minimum notice to us.
We believe that future coverage and reimbursement will likely be subject to increased restrictions both in the United States and in international markets. Healthcare cost containment initiatives that limit or deny reimbursement for V‑Go would also materially and adversely affect our business. If reimbursement is not available or is available only to limited levels, we may not be able to commercialize our products profitably.
We are subject to additional federal, state and foreign laws and regulations relating to our healthcare business; our failure to comply with those laws could have a material adverse effect on our results of operations and financial condition.
Although we do not provide healthcare services, submit claims for third‑party reimbursement, or receive payments directly from Medicare, Medicaid or other third‑party payors for our product, we are subject to healthcare fraud and abuse regulation and enforcement by federal and state governments, which could significantly impact our business. Healthcare fraud and abuse laws potentially applicable to our operations include:
the federal Anti‑Kickback Statute, which applies to our marketing practices, educational programs, pricing policies and relationships with healthcare providers, by prohibiting, among other things, soliciting, receiving, offering or providing remuneration intended to induce or reward the purchase or recommendation of an item or service reimbursable under a federal healthcare program, such as the Medicare or Medicaid programs;
federal civil and criminal false claims laws and civil monetary penalty laws, including civil whistleblower or qui tam actions, that prohibit, among other things, knowingly presenting, or causing to be presented, claims for payment or approval to the federal government that are false or fraudulent, knowingly making, or causing to be made, a false statement material to an obligation to pay or transmit money or property to the federal government or knowingly concealing or knowingly and improperly avoiding or decreasing an obligation to pay or transmit money or property to the federal government;
| |
| �� | the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, and its implementing regulations, which created federal criminal laws that prohibit, among other things, executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters; |
Section 5(a) of the Federal Trade Commission Act, or the FTCA, 15 USC § 45(a), as even for entities that are not deemed “covered entities” or “business associates” under HIPAA, according to the United States Federal Trade Commission, or the FTC, failing to take appropriate steps to keep consumers' personal information secure constitutes unfair acts or practices in or affecting commerce, and the FTC expects a company's data security measures to be reasonable and appropriate in light of the sensitivity and volume of consumer information it holds, the size and complexity of its business, and the cost of available tools to improve security and reduce vulnerabilities (with medical data considered sensitive data that merits stronger safeguards); the FTC's guidance for appropriately securing consumers' personal information is similar to what is required by the HIPAA Security Rule;
federal “sunshine” requirements imposed by the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or collectively the ACA, on drug and device manufacturers subject to the law regarding any payments or “transfers of value” to physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members; failure to submit required information may result in civil monetary penalties of up to an aggregate of $150,000 per year, and up to an additional aggregate of $1 million per year for “knowing failures”; these reporting and transparency requirements were further extended by 2018 legislation to physicians assistants, nurse practitioners, and other mid-level practitioners (with reporting requirements going into effect in 2022 for payments and transfers of value made in 2021);
federal consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers;
state laws requiring licensure and registration for the manufacture and distribution of medical devices; and
state law equivalents of each of the above federal laws, such as anti‑kickback and false claims laws that may apply to items or services reimbursed by any third‑party payor, including commercial insurers; state laws that require device and pharmaceutical companies to comply with the industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government or otherwise restrict payments that may be made to healthcare providers; and state laws that require drug and device manufacturers to report information related to payments and other transfers of value to physicians, other healthcare providers and healthcare entities or marketing expenditures;
The risk of our being found in violation of these laws and regulations is increased by the fact that many of them have not been fully interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations. Moreover, recent health care reform legislation has strengthened these laws. For example, the ACA, among other things, amended the intent requirement of the federal anti‑kickback and criminal health care fraud statutes, such that a person or entity no longer needs to have actual knowledge of these statutes or specific intent to violate them. In addition, the ACA provided that the government may assert that a claim including items or services resulting from a violation of the federal anti‑kickback statute constitutes a false or fraudulent claim for purposes of the false claims statutes. We are unable to predict what additional federal or state legislation or regulatory initiatives may be enacted in the future regarding our business or the healthcare industry in general, or what effect such legislation or regulations may have on us. Financial arrangements and incentives that may impact healthcare decision-making continue to be a subject of attention for Congress and health regulators. For example, the federal Eliminating Kickbacks in Recovery Act of 2018 (EKRA) notably in some instances (relating to recovery centers, clinical treatment facilities, and clinical laboratories) applies to services payable by commercial insurers and self-pay patients, as opposed to only services for which
payment is available from government payors. Future laws of this nature could impact our business. Federal or state governments may impose additional restrictions or adopt interpretations of existing laws that could have a material adverse effect on us.
Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available under such laws, it is possible that some of our business activities, including without limitation certain of the marketing and distribution programs for V‑Go, as well as our relationships with physicians and other health care providers, some of whom recommend, purchase and/or prescribe our product, could be subject to challenge under one or more of such laws. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from governmental health care programs, individual imprisonment, disgorgement, contractual damages, reputational harm, diminished profits and future earnings, suspension or revocation of certifications or licenses that are required to operate our business, injunctions and other associated remedies, denial or withdrawal of product clearances, private “qui tam” actions brought by individual whistleblowers in the name of the government, and the curtailment or restructuring of our operations, any of which could impair our ability to operate our business and our financial results.
We may be liable if the FDA or other U.S. enforcement agencies determine we have engaged in the off‑label promotion of our products or have disseminated false or misleading labeling or promotional materials.
Our promotional materials and training methods must comply with FDA and other applicable laws and regulations, including laws and regulations prohibiting marketing claims that promote the off‑label use of our products or that make false or misleading statements. Healthcare providers may use our products off‑label, as the FDA does not restrict or regulate a physician’s choice of treatment within the practice of medicine. For example, although V‑Go is only cleared for insulin delivery in adult patients, a physician might independently choose to use it for insulin delivery in children. FDA also could conclude that a performance claim is misleading if it determines that there are inadequate non‑clinical and/or clinical data supporting the claim. If the FDA determines that our promotional materials or training promote of an off‑label use or make false or misleading claims, it could request that we modify our training or promotional materials or subject us to regulatory or enforcement actions, including the issuance of an untitled letter, a warning letter, injunction, seizure, civil fines and criminal penalties. It is also possible that other federal, state or foreign enforcement authorities might take action if they determine that our promotional or training materials promote an unapproved use or make false or misleading claims, which could result in significant fines or penalties. Although our policy is to refrain from statements that could be considered off‑label promotion of our products or false or misleading, the FDA or another regulatory agency could disagree. Violations of the FDCA may also lead to investigations alleging violations of federal and state health care fraud and abuse laws, as well as state consumer protection laws, which may lead to costly penalties and may adversely impact our business. Recent court decisions have impacted FDA’s enforcement activity regarding off-label promotion in light of First Amendment Considerations; however there are still significant risks in this area, in part due to the potential for False Claims Act exposure. In addition, the off‑label use of our products may increase the risk of product liability claims. Product liability claims are expensive to defend and could result in substantial damage awards against us and harm our reputation.
Legislative or regulatory healthcare reforms may make it more difficult and costly for us to obtain reimbursement for our products or regulatory clearance or approval of our future products, and to produce, market and distribute those products after clearance or approval is obtained.
Recent political, economic and regulatory influences are subjecting the healthcare industry to fundamental changes. Both the federal and state governments in the United States and foreign governments continue to propose and pass new legislation and regulations designed to contain or reduce the cost of healthcare. Such legislation and regulations may result in decreased reimbursement for our product, which may further exacerbate industry‑wide pressure to reduce the prices charged for our product. This could harm our ability to market our products and generate sales. In addition, FDA regulations and guidance are often revised or reinterpreted by the FDA in ways that may significantly affect our business and our current products and future products. Any new regulations or revisions or reinterpretations of existing regulations may impose additional costs or lengthen review times of our products. Delays in receipt of or failure to receive regulatory clearances or approvals for any future products would negatively impact our long‑term business strategy.
In the U.S., there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that restrict or regulate post‑approval activities, which may affect our ability to profitably sell V‑Go or any other product candidates for which we obtain marketing approval.
Such government‑adopted reform measures may adversely impact the pricing of healthcare products and services in the United States or internationally and the amount of reimbursement available from third‑party payors.
For example, in March 2010, the ACA was signed into law. While the goal of healthcare reform is to expand coverage to more individuals, it also involved increased government price controls, additional regulatory mandates and other measures designed to constrain medical costs. The ACA substantially changed the way healthcare is financed by both governmental and commercial insurers, encouraged improvements in the quality of healthcare items and services and significantly impacted the medical device industries. The ACA, among other things, established annual fees and taxes on manufacturers of certain branded prescription drugs and medical devices (discussed in more detail below), required manufacturers to participate in a discount program for certain outpatient drugs under Medicare Part D, and promoted programs that increase the federal government’s comparative effectiveness research.
In December 2017, President Trump signed into law the Tax Cuts and Jobs Act (Pub. Law 115‑97), repealing certain aspects of the ACA. Further, on January 20, 2017, the President signed an Executive Order directing federal agencies with authorities and responsibilities under the ACA to waive, defer, grant exemptions from, or delay the implementation of any provision of the ACA that would impose a fiscal or regulatory burden on states, individuals, healthcare providers, health insurers, or manufacturers of pharmaceuticals or medical devices. More recently, the United States District Court for the Northern District of Texas struck down the ACA, deeming it unconstitutional given that Congress repealed the individual mandate in 2017. This decision has been stayed pending outcome of an appeal to the Fifth Circuit Court of Appeals. Although there is no immediate impact on the ACA, we will continue to evaluate the effect that the ACA and its possible repeal and replacement, or potential total revocation by the Supreme Court of the United States, has on our business.
In the future there may continue to be additional proposals relating to the reform of the U.S. healthcare system generally, or operation of the Medicare Part D program specifically. Certain of these proposals could limit the prices we are able to charge for our product, or the amount of reimbursement available for our product, and could limit the acceptance and availability of our product. There have been numerous efforts by legislators and regulators to contain costs for medical products. For example, early in 2019 the U.S. Department of Health and Human Services (HHS) proposed a rule aimed at eliminating certain Anti-Kickback Statute safe harbor protections including those for drug rebates paid by manufacturers in relation to Medicare Part D. There is also increasing focus on the amount of money spent by companies on marketing and advertising, and the impact of these expenditures on the cost of their products. Moreover, the U.S. Department of Health and Human Services Centers for Medicare & Medicaid Services has promulgated or amended a number of cost containment and value based reimbursement measures, and it is expected to continue revising its regulations and policies in response to market conditions and administration directives.
Changes in tariffs or other government trade policies may materially adversely affect our business and results of operations, including by reducing demand for our products.
The imposition of tariffs and trade restrictions as a result of international trade disputes or changes in trade policies may adversely affect our sales and profitability. For example, in 2018, the U.S. government imposed and proposed, among other actions, new or higher tariffs on specified imported products originating from China in response to what it characterizes as unfair trade practices, and China has responded by imposing and proposing new or higher tariffs on specified products fabricated in the United States. There can be no assurance that a broader trade agreement will be successfully negotiated between the United States and China to reduce or eliminate these tariffs. These tariffs, and the related geopolitical uncertainty between the United States and China, may cause decreased end-market demand for our products from distributors and other customers, which could have a material adverse effect on our business and results of operations. Ongoing international trade disputes and changes in trade policies could also impact economic activity and lead to a general contraction of customer demand. In addition, tariffs on components that we import from China or other nations that have imposed, or may in the future impose, tariffs will adversely affect our profitability unless we are able to exclude such components from the tariffs or we raise prices for our products, which may result in our products becoming less attractive relative to products offered by our competitors. Future actions or escalations by either the United States or China that affect trade relations may also impact our business, or that of our suppliers or customers, and we cannot provide any assurances as to whether such actions will occur or the form that they may take. To the extent that our sales or profitability are negatively affected by any such tariffs or other trade actions, our business and results of operations may be materially adversely affected.
Our financial performance may be adversely affected by medical device tax provisions in the ACA.
Beginning in 2013 through the end of 2015, the ACA imposed, among other things, an annual excise tax of 2.3% on any entity that manufactures or imports medical devices offered for sale in the United States beginning in 2013. Congress first suspended this tax on December 18, 2015 for two years, for sales of devices during the period January 1, 2016 through December 31, 2017. Congress again suspended this tax in January 2018, for sales of devices during the period January 1, 2018 through December 31, 2019. We do not believe that V‑Go was subject to this tax based on the retail exemption under applicable Treasury Regulations. However, the guidance regarding this exemption as applied to V‑Go is not clear, and the availability of this exemption is subject to interpretation by the IRS, and the IRS may disagree with our analysis. We do not know if this provision will be repealed or if
there will be changes to the retail exemption when the suspension of the device tax ends at the end of 2017. The potential financial impact this tax may have on our business is unclear and there can be no assurance that our business and financial results will not be negatively impacted.
Failure to obtain any necessary clearance for the V‑Go SIM™(Simple Insulin Management), if required, or other delays in the development of V-Go SIM, would adversely affect our ability to grow our business.
Commercialization of the V‑Go SIM, formerly branded as the V‑Go Link, may require FDA clearance of a 510(k) premarket notification submission. The process for submitting and obtaining FDA clearance of a 510(k) can be expensive and lengthy. The FDA’s 510(k) clearance process can take several months or longer, and we may not be able to obtain 510(k) clearance for the V‑Go SIM on a timely basis, if at all. The FDA’s refusal of, or any significant delays in receiving 510(k) clearance of the V‑Go SIM, if required, would have an adverse effect on our ability to expand our business. In addition, any other delays in the development of V-Go SIM, for example, unforeseen issues during product validation, would have an adverse effect on our ability to expand our business.
In order to successfully develop the V‑Go Prefill, we will need to secure an insulin supply and/or insulin development partner and will need to obtain regulatory approval to package and sell the insulin in our prefill device, which may require additional nonclinical and/or clinical data to support the use of insulin in our prefill device. This process may be expensive and involves significant regulatory risks, and may also place additional regulatory compliance obligations on the company. If we are unable to secure an insulin supply or obtain an insulin development partner, or if we are unable to demonstrate the safety and/or effectiveness of the insulin in our prefill device, we will be unable to successfully develop or commercialize the V‑Go Prefill.
In order to successfully develop the V‑Go Pre‑fill, we will need to secure an insulin supply and/or insulin development partner. We cannot assure you that we will be able to secure an insulin supply for the US or any other market. We will also need to obtain FDA regulatory approval to package and sell the insulin in our prefill device. Although we hope to secure an insulin development partner with expertise in drug and biologic marketing authorization filings, there is a risk that we may not be able to identify or secure such a partner. In that case, we will face additional regulatory challenges, as we do not currently have filing expertise relative to drugs or biologics. If insulin that we gain access to is approved in the markets where we want to introduce V‑Go Pre‑fill, we, or our development partner, will still need to obtain approval to package and sell the insulin in our prefill device. If insulin supply that we obtain is not approved in our target market(s), the insulin must first be approved or it may be approved simultaneously for use in our prefill device. In either case, we, or our development partner, will be required to demonstrate the safety and if the insulin is not yet approved, the effectiveness of the insulin, which is regulated as a drug, when packaged and sold in our prefill device, which may require nonclinical and/or clinical data. Furthermore, we expect the V-Go Pre-fill to be considered a combination product for which regulatory approval would need to be obtained through coordinating centers within the FDA and, if we choose to pursue foreign marketing authorization, other comparable regulatory agencies. Obtaining regulatory approval of a drug or biological product in a prefill device can be a lengthy and expensive, and may face additional challenges, risks and delays in the product development and regulatory approval process. FDA will evaluate the characteristics of the drug biologic and the device constituent parts, and consider among other things the potential for undesirable interactions or unintended results of the combination. Even if we believe that we have sufficient nonclinical and/or clinical data to support the use of insulin in our prefill device, the FDA and other regulatory authorities may disagree that such data are adequate to support approval.
In order to successfully use our h‑Patch technology in other drug delivery applications beyond the use of insulin to treat type 2 diabetes, we will need to secure one or more collaboration partners and may need to obtain regulatory approval to sell any such resulting products, which may require additional nonclinical and/or clinical data to support the use of those products. This process may be expensive and involves significant regulatory risks. If we are unable to secure one or more collaboration partners, or if we are unable to demonstrate the safety and/or effectiveness of any such resulting products, we will be unable to successfully use our h‑Patch technology in other drug delivery applications beyond the use of insulin to treat type 2 diabetes, and will not realize the potential profits that could be derived from such potential future uses.
In order to successfully use our h‑Patch technology in other drug delivery applications beyond the use of insulin to treat type 2 diabetes, we will need to secure one or more collaboration partners. We cannot assure you that we will be able to successfully identify other potential drugs that could be combined with our h‑Patch technology, or if we are able to identify such drugs, that we will be able to reach an agreement with such third party or parties necessary in order to combine our h‑Patch technology with those drugs.
The drug or drugs that we and our collaboration partners combine with our h‑Patch technology may not be approved in the markets in which we and our collaboration partners want to introduce such products. If such drugs are not approved in our target market(s), those drugs must first be approved or they may be approved simultaneously for use in combination with our h‑Patch technology. In either case, we, or our collaboration partner, will be required to demonstrate the safety and if the drug is not yet approved, the
effectiveness of the drug when combined with our h‑Patch technology, which may require nonclinical and/or clinical data. Obtaining regulatory approval of a drug in combination with an injection technology
can be a lengthy, expensive, and uncertain process. Even if we believe and our collaboration partners agree that we have sufficient nonclinical and/or clinical data to support the use of such drugs in combination with our h‑Patch technology, the FDA and other regulatory authorities may disagree that such data are adequate to support approval. If we or our collaboration partners are unable to get such necessary approvals, then we will be unable to successfully expand the use of our h‑Patch technology to other potential drug delivery applications, and will not realize the potential profits that could be derived from any such potential future uses.
We may not be able to obtain regulatory approval from the China State Drug Administration (SDA) for V‑Go in a timely manner, if at all, which may delay or prevent our successful registration of V‑Go in China. Delays in or failure to obtain the necessary approvals from the SDA could harm our business.
We recently entered into an agreement with an international contract research organization to begin the medical device registration process for V‑Go in China. Before V‑Go can be marketed and sold in China, V‑Go must be approved by the SDA. Although we have determined our proposed regulatory pathway to obtain approval from the SDA, we have not yet received their regulatory approval regarding our clinical study protocol. The SDA will require successful completion of clinical trials in China and demonstrated manufacturing capability before it grants approval. Clinical trials are expensive and their results are unpredictable. It often takes a number of years before a product can be ultimately approved by the SDA. In addition, the SDA and other regulatory authorities may apply new standards for safety, manufacturing, packaging, and distribution of V‑Go. SDA may also conduct overseas inspection of the manufacturer for its compliance with the GSP requirements. Complying with such standards may be time‑consuming and expensive and could result in delays in obtaining SDA approval for V‑Go, or possibly preclude us from obtaining SDA approval altogether. The SDA and other regulatory authorities may not approve V‑Go and even if we do obtain the required regulatory approvals, such regulatory approvals may be subject to limitations on the indicated uses for which we may market V‑Go, which may limit the size of the market for V‑Go in China. If we do not obtain approval from the SDA in a timely manner or at all, it could delay or prevent the successful registration of V‑Go in China.
Further, we are subject to additional risks, including those listed below, which may delay or prevent the successful registration of V‑Go in China:
delays in design verification, which could take eight to 12 months or longer;
delays in obtaining clinical study protocol approval from the SDA;
delays in obtaining clinical trial sites or subjects in a timely manner to meet the SDA protocol requirements; which could take 18 to 24 months to complete the clinical trial from the point when we receive approval of our clinical study protocol;
delays in securing SDA approval for the clinical study report, which could add another 12 to 18 months to the process; and
insufficient working capital to support our registration efforts.
Risks Related to Ownership of Our Common Stock
The price of our common stock may be volatile and fluctuate substantially.
The quoted price of our common stock has been, and we expect it to continue to be, volatile. The stock market in general and the market for smaller medical device and pharmaceutical companies in particular have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. As a result of this volatility, you may not be able to sell your shares of common stock at or above your purchase price. The market price for our common stock may be influenced by many factors, including:
the success of competitive products or technologies;
developments related to our existing or any future collaborations;
regulatory or legal developments in the United States and other countries;
developments or disputes concerning patent applications, issued patents or other proprietary rights;
the recruitment or departure of key personnel;
the level of expenses related to any of our product candidates;
the results of our efforts to discover, develop, acquire or in-license additional product candidates or products;
actual or anticipated changes in estimates as to financial results, development timelines or recommendations by securities analysts;
variations in our financial results or those of companies that are perceived to be similar to us;
changes in the structure of healthcare payment systems;
market conditions in the pharmaceutical and biotechnology sectors;
general economic, industry and market conditions; and
the other factors described in this “Risk Factors” section.
Raising additional funds by issuing securities may cause dilution to existing stockholders and raising funds through lending and licensing arrangements may restrict our operations or require us to relinquish proprietary rights.
In the future, we may issue our authorized but previously unissued equity securities, resulting in the dilution of the ownership interests of our present stockholders and the purchasers of our common stock offered hereby. We are authorized to issue an aggregate of 300,000,000 shares of common stock and 50,000,000 shares of preferred stock. We may issue additional shares of our common stock or other securities that are convertible into or exercisable for our common stock in connection with hiring or retaining employees, future acquisitions, future sales of our securities for capital raising purposes, or for other business purposes. The future issuance of any such additional shares of our common stock may create downward pressure on the trading price of the common stock. We expect we will need to raise additional capital in the near future to meet our working capital needs, and there can be no assurance that we will not be required to issue additional shares, warrants or other convertible securities in the future in conjunction with these capital raising efforts, including at a price (or exercise prices) below the price you paid for your stock.
An active trading market for our common stock may not be sustained.
In March 2017, we closed our public offering of common stock, and prior to such time, there was no active, liquid and orderly trading market for shares of our common stock. Although shares of our common stock are listed and trading on The Nasdaq Capital Market, an active trading market for our shares may not be sustained. If an active market for our common stock does not continue, it may be difficult for our stockholders to sell their shares without depressing the market price for the shares or sell their shares at or above the prices at which they acquired their shares or sell their shares at the time they would like to sell. Any inactive trading market for our common stock may also impair our ability to raise capital to continue to fund our operations by selling shares.
If we are not able to comply with the applicable continued listing requirements or standards of the Nasdaq Capital Market, Nasdaq could delist our common stock.
Our common stock is currently listed on the Nasdaq Capital Market. In order to maintain that listing, we must satisfy minimum financial and other continued listing requirements and standards, including those regarding director independence and independent committee requirements, minimum stockholders' equity, minimum share price, and certain corporate governance requirements. There can be no assurances that we will be able to comply with the applicable listing standards.On December 31, 2018, we received a written notification from the Listing Qualifications Department of the Nasdaq Stock Market, or Nasdaq, indicating that we were not in compliance with Nasdaq Listing Rule 5550(a)(2) for continued listing on the Nasdaq Capital Market because our minimum bid price was less than $1.00 per share for 30 consecutive business days. The notification letter provided that the Company had 180 calendar days, or until July 1, 2019 to regain compliance with Nasdaq Listing Rule 5550(a)(2). To regain compliance, the bid price of the Company's common stock must have a closing bid price of at least $1.00 per share for a minimum of 10 consecutive business days. If the Company does not regain compliance by July 1, 2019, an additional 180 days may be granted to regain compliance, so long as the Company meets The Nasdaq Capital Market continued listing requirements (except for the bid price requirement) and notifies Nasdaq in writing of its intention to cure the deficiency during the second compliance period, by effecting a reverse stock split, if necessary. If the Company does not qualify for the second compliance period or fails to regain compliance during the second 180-day period, then Nasdaq will notify the Company of its determination to delist the Company's common stock, at which point the Company will have an opportunity to appeal the delisting determination to a Hearings Panel. In addition, in accordance with the Nasdaq Listing Rules, if we fail to maintain a minimum $2.5 million of stockholders' equity or otherwise do not meet the alternative compliance standards relating to the market value of listed securities of $35 million or net income from continuing operations of $500,000 in the most recently completed fiscal year or in two of the last three most recently completed
fiscal years, or if we fail to comply with any of the other Nasdaq Listing Rules, then we may receive additional notification letters from Nasdaq, which may subject us to delisting.
The ability of our Board of Directors to issue additional stock may prevent or make more difficult certain transactions, including a sale or merger.
Our Board of Directors is authorized to issue up to 50,000,000 shares of preferred stock with powers, rights and preferences designated by it. Shares of voting or convertible preferred stock could be issued, or rights to purchase such shares could be issued, to create voting impediments or to frustrate persons seeking to effect a takeover or otherwise gain control of us. The ability of our Board of Directors to issue such additional shares of preferred stock, with rights and preferences it deems advisable, could discourage an attempt by a party to acquire control of us by tender offer or other means. Such issuances could therefore deprive stockholders of benefits that could result from such an attempt, such as the realization of a premium over the market price for their shares in a tender offer or the temporary increase in market price that such an attempt could cause. Moreover, the issuance of such additional shares of preferred stock to persons friendly to our Board of Directors could make it more difficult to remove incumbent managers and directors from office even if such change were to be favorable to stockholders generally.
We are an “emerging growth company”, and we cannot be certain whether the reduced reporting requirements applicable to emerging growth companies will make our common stock less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act. For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002 or the Sarbanes-Oxley Act’s reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved.
We will remain an emerging growth company until the earlier of (1) the last day of the fiscal year (a) following the fifth anniversary of our first sale of common equity securities pursuant to an effective registration statement under the Securities Act, which occurred in May 2015; (b) in which we have total annual gross revenue of at least $1.07 billion; or (c) in which we are deemed to be a “large accelerated filer,” which means the market value of our common stock that is held by non-affiliates exceeds $700 million as of the prior June 30th and (2) the date on which we have issued more than $1.0 billion in non-convertible debt securities during the prior three-year period. We cannot predict if investors will find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may suffer or be more volatile.
Section 102 of the JOBS Act also provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. An “emerging growth company” can therefore delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. However, we are choosing to “opt out” of such extended transition period, and as a result, we will comply with new or revised accounting standards on the relevant dates on which adoption of such standards is required for non-emerging growth companies.
Section 107 of the JOBS Act provides that our decision to opt out of the extended transition period for complying with new or revised accounting standards is irrevocable.
We are a smaller reporting company, and we cannot be certain if the reduced disclosure requirements applicable to smaller reporting companies will make our common stock less attractive to investors.
We are currently a “smaller reporting company”, meaning that we are not an investment company, an asset- backed issuer, or a majority-owned subsidiary of a parent company that is not a smaller reporting company and have a non-affiliated public float of less than $75 million and annual revenues of less than $50.0 million during the most recently completed fiscal year. In the event that we are still considered a “smaller reporting company,” at such time as we cease being an “emerging growth company,” we will be required to provide additional disclosure in our SEC filings. However, similar to an “emerging growth companies”, “smaller reporting companies” are able to provide simplified executive compensation disclosures in their filings; are exempt from the provisions of Section 404(b) of the Sarbanes-Oxley Act requiring that independent registered public accounting firms provide an attestation report on the effectiveness of internal control over financial reporting; and have certain other decreased disclosure obligations in their SEC filings, including, among other things, only being required to provide two years of audited financial statements in annual reports and in a registration statement under the Exchange Act on Form 10. Decreased disclosures in our SEC filings due to our status as a “smaller reporting company” may make it harder for investors to analyze our results of operations and financial prospects.
If securities or industry analysts do not publish research or reports about our business, or if they issue an adverse or misleading opinion regarding our stock, our stock price and trading volume could decline.
The trading market for our common stock will be influenced by the research and reports that industry or securities analysts publish about us or our business. We do not currently have and may never obtain research coverage by securities and industry analysts. If no or few securities or industry analysts commence coverage of us, the trading price for our stock would be negatively impacted. In the event we obtain securities or industry analyst coverage, if any of the analysts who cover us issue an adverse or misleading opinion regarding us, our business model, our intellectual property or our stock performance, or if our operating results fail to meet the expectations of analysts, our stock price would likely decline. If one or more of these analysts cease coverage of us or fail to publish reports on us regularly, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline.
Our principal stockholders own a significant percentage of our stock and will be able to exert significant control over matters subject to stockholder approval.
As of March 4, 2019, our 5% stockholders and their affiliates beneficially owned an aggregate of 65,497,897 shares, or 65.5% of our outstanding common stock. As a result, these stockholders will have significant influence and may be able to determine all matters requiring stockholder approval. For example, these stockholders may be able to control elections of directors, amendments of our organizational documents, or approval of any merger, sale of assets, or other major corporate transactions. This concentration of ownership could delay or prevent any acquisition of our company on terms that other stockholders may desire, and may adversely affect the market price of our common stock.
We do not anticipate paying dividends on our common stock, and investors may lose the entire amount of their investment.
We have never declared or paid cash dividends on our common stock, and we do not anticipate such a declaration or payment for the foreseeable future.
We expect to use future earnings, if any, to fund business growth. Therefore, stockholders will not receive any funds absent a sale of their shares of common stock. We cannot assure stockholders of a positive return on their investment when they sell their shares, nor can we assure that stockholders will not lose the entire amount of their investment.
Provisions of our charter documents or Delaware law could delay or prevent an acquisition of the company, even if such an acquisition would be beneficial to our stockholders, which could make it more difficult for you to change management.
Provisions in our amended and restated certificate of incorporation and our amended and restated bylaws may discourage, delay or prevent a merger, acquisition or other change in control that stockholders may consider favorable, including transactions in which stockholders might otherwise receive a premium for their shares. In addition, these provisions may frustrate or prevent any attempt by our stockholders to replace or remove our current management by making it more difficult to replace or remove our board of directors. These provisions include:
a classified board of directors so that not all directors are elected at one time;
a prohibition on stockholder action through written consent;
no cumulative voting in the election of directors;
the exclusive right of our board of directors which currently consiststo elect a director to fill a vacancy created by the expansion of 7 members. The primary responsibilitythe board of directors or the resignation, death or removal of a director;
a requirement that special meetings of stockholders be called only by the board of directors, the chairperson of the board of directors, the chief executive officer or, in the absence of a chief executive officer, the president;
an advance notice requirement for stockholder proposals and nominations;
the authority of our board of directors is to provide oversight, strategic guidance, counseling, and direction to our management team. Our board of directors meets on a regular basis and additionallyissue Preferred Stock with such terms as required. One of our directors was elected to serve on our board of directors pursuantmay determine; and
a requirement of approval of not less than 66 2/3% of all outstanding shares of our capital stock entitled to the Third Amendedvote to amend any bylaws by stockholder action, or to amend specific provisions of our amended and Restated Voting Agreement, dated asrestated certificate of January 29, 2016,incorporation.
Any failure to maintain effective internal control over our financial reporting could materially adversely affect us.
Section 404 of the Sarbanes-Oxley Act of 2002 requires us to include in our annual reports on Form 10-K an assessment by management of the effectiveness of our internal control over financial reporting. In addition, at such time, if any, as we are an “accelerated filer” or a “large accelerated filer,” and among Valeritasno longer an “emerging growth company,” our independent registered public accounting firm will have to attest to and certainreport on management’s assessment of Valeritas’ stockholders. Pursuantthe effectiveness of such internal control over financial reporting. Our management assessed our internal control over financial reporting as of December 31, 2018 based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 Framework), or the 2013 Framework. Based on such assessment, we concluded that our internal control over financial reporting was effective as of December 31, 2018 to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external reporting purposes in accordance with U.S. generally accepted accounting principles.
Even though we have determined that our internal control over financial reporting was effective as of December 31, 2018, our internal control over financial reporting will not prevent or detect all error and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud will be detected. If we are not able to comply with the requirements of Section 404 in a timely manner, or if we identify one or more material weaknesses in our internal controls, investors could lose confidence in the reliability of our financial statements, the market price of our stock could decline and we could be subject to sanctions or investigations by the SEC, or other regulatory authorities.
Our disclosure controls and procedures may not prevent or detect all errors or acts of fraud.
We are subject to the voting agreement, Mr. Düster was selected to serve on our board of directors as designated by Capital Royalty Partners II L.P. or its affiliates.
A majorityperiodic reporting requirements of the authorized number of directors constitutes a quorum of the board of directors for the transaction of business. The directors must be present at the meeting to constitute a quorum. However, any action required or permitted to be taken by the board of directors may be taken without a meeting if all members of the board of directors individually or collectively consent in writing to the action.
Family Relationships
There are no family relationships among our directors or executive officers.
Director Independence
Under the listing requirementsSecurities and rules of the Nasdaq Capital Market, or Nasdaq, independent directors must compose a majority of a listed company’s board of directors within a one-year period following the completion of its initial public offering. In addition, applicable Nasdaq rules require that, subject to specified exceptions, each member of a listed company’s audit, compensation, and nominating and corporate governance committees must be independent within the meaning of applicable Nasdaq rules. Audit committee members must also satisfy the independence criteria set forth in Rule 10A-3 under the Securities Exchange Act of 1934, as amended, or the Exchange Act. Our disclosure controls and procedures are designed to reasonably assure that information required to be disclosed by us in reports we file or submit under the Exchange Act is accumulated and communicated to management, recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC. We believe that any disclosure controls and procedures or internal controls and procedures, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met.
These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because of simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people or by an unauthorized override of the controls. Accordingly, because of the inherent limitations in our control system, misstatements or insufficient disclosures due to error or fraud may occur and not be detected.
We could be subject to securities class action litigation.
In the past, securities class action litigation has often been brought against a company following a decline in the market price of its securities. This risk is especially relevant for us because biotechnology companies have experienced significant stock price volatility in recent years. If we face such litigation, it could result in substantial costs and a diversion of management’s attention and resources, which could harm our business.
Item 1B. Unresolved Staff Comments.
None.
Item 2. Properties.
Our boardcorporate headquarters are located in Bridgewater, New Jersey, where we currently lease approximately 7,146 square feet of directors undertookoffice space under a reviewlease that expires on June 30, 2023. We also occupy space in an office building in Marlborough, Massachusetts, where we currently lease approximately 15,171 square feet of space for offices, lab and pilot facilities and process and engineering under a lease that expires on February 15, 2024.
Item 3. Legal Proceedings.
We, and our subsidiary, are currently not a party to, and our property is not the subject of, any material legal proceedings; however, we may become involved in various claims and legal actions arising in the ordinary course of business.
Item 4. Mine Safety Disclosures.
Not applicable.
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Market Information
Our common stock has traded on the Nasdaq Capital Market since our public offering in March 2017 under the symbol "VLRX." From April 18, 2016 to March 23 2017, our common stock was quoted on the OTCQB Marketplace. Our common stock has, from time to time, traded on a limited, sporadic and volatile basis. On December 31, 2018, we received a letter (the “Notification Letter”) from the Listing Qualifications Department of the independence of each director and considered whether any director has a material relationship with usNasdaq Stock Market (“Nasdaq”) indicating that could compromise his or her ability to exercise independent judgment in carrying out his or her responsibilities. In making this determination, our board of directors considered the current and prior relationships that each non-employee director has with our company and all other facts and circumstances our board of directors deemed relevant in determining their independence, including the beneficial ownership of our capital stock by each non-employee director and the association of our directorsCompany no longer complies with the holdersminimum bid price requirements set forth in Nasdaq Listing Rule 5550(a)(2) for continued listing on the Nasdaq Capital Market. The Notification Letter provides that the Company has 180 calendar days, or until July 1, 2019, to regain compliance with Nasdaq Listing Rule 5550(a)(2). To regain compliance, the bid price of more than 5%the Company's common stock must have a closing bid price of at least $1.00 per share for a minimum of 10 consecutive business days. If the Company does not regain compliance by July 1, 2019, an additional 180 days may be granted to regain compliance, so long as the Company meets The Nasdaq Capital Market continued listing requirements (except for the bid price requirement) and notifies Nasdaq in writing of its intention to cure the deficiency during the second compliance period, by effecting a reverse stock split, if necessary. If the Company does not qualify for the second compliance period or fails to regain compliance during the second 180-day period, then Nasdaq will notify the Company of its determination to delist the Company's common stock, at which point the Company will have an opportunity to appeal the delisting determination to a Hearings Panel.
Holders
The closing price of our common stock on March 4, 2019 was $0.51 per share. As of March 4, 2019, we had approximately 46 record holders of our common stock. Because many of our shares are held by brokers and other institutions on behalf of stockholders, we are unable to estimate the total number of individual stockholders represented by these record holders.
As a result of this review,Dividends
We have never declared or paid any dividends on our board of directors determined thatcommon stock. We currently intend to retain all of our directors, other than Mr. Timberlakefuture earnings, if any, to finance the growth and Mr. Düster qualify as “independent” directors withindevelopment of our business. We do not intend to pay cash dividends to holders of our common stock in the meaningforeseeable future.
Our ability to pay cash dividends is restricted pursuant to the terms of the Nasdaq rules. Nasdaq rules require thatTerm Loan. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Indebtedness.”
Issuer Purchases of Equity Securities
None.
Recent Sales of Unregistered Securities
None
Use of Proceeds from Initial Public Offering of Common Stock
On March 22, 2017, our Registration Statement on Form S-1, as amended (File No. 333-215897) for our March 2017 public offering was declared effective by the Securities and Exchange Commission, or SEC. On March 28, 2018, we completed our March 2017 public offering whereby we sold 5,250,000 shares of common stock at a majoritypublic offering price of $10.00 per share, before underwriting discounts and expenses. The aggregate net proceeds received by us from the boardoffering were $48.8 million, after deducting the underwriting discounts and commissions and offering expenses paid by us.
As of directors and each memberDecember 31, 2018, we have used all of our audit, compensation, and nominating and corporate governance committees be independent. We believe we are compliantnet proceeds from our March 2017 initial public offering.
There was no material change in the planned use of proceeds from our March 2017 initial public offering as described in our final prospectus dated March 24, 2017, filed with these independence requirements. As required under applicable Nasdaq rules, we anticipate that our independent directors will meet in regularly scheduled executive sessions at which only independent directors are present. There are no family relationships among any of our directors or executive officers.
Audit committee members must also satisfy the independence criteria set forth in Rule 10A-3 under the Exchange Act. In order to be considered independent for purposes of Rule 10A-3, a member of an audit committee of a listed company may not, other than in his or her capacity as a member of the audit committee, the board of directors, or any other board committee: accept, directly or indirectly, any consulting, advisory, or other compensatory fee from the listed company or any of its subsidiaries; or be an affiliated person of the listed company or any of its subsidiaries. Each of Brian K. Roberts, Dr. Rodney D. Altman and Peter J. Devlin. qualify as an independent directorSEC pursuant to Rule 10A-3.424(b)(4) under the Securities Act of 1933, as amended.
Role
Item 6. Selected Financial Data.
The consolidated statements of operations data for the years ended December 31, 2018 and 2017 and the consolidated balance sheet data as of December 31, 2018 and 2017 are derived from our audited financial statements included elsewhere in this Annual Report on Form 10-K.
Our historical results are not necessarily indicative of the Boardresults to be expected in Risk Oversight
Onethe future. You should read the selected financial data below in conjunction with the section of the key functionsthis report entitled “Management’s Discussion and Analysis of our boardFinancial Condition and Results of directors is informed oversight of our risk management process. The board of directors does not have a standing risk management committee, but rather administers this oversight function directly through the board of directors as a whole, as well as through various standing committees of our board of directors that address risks inherent in their respective areas of oversight. In particular, our board of directors is responsible for monitoring and assessing strategic risk exposure,Operations” and our audit committee has the responsibility to consider and discuss our major financial risk exposuresstatements and the steps our management has taken to monitor and control these exposures, including guidelines and policies to govern the process by which risk assessment and management is undertaken. In December 2016, we established a nominating and corporate governance committee. Our nominating and corporate governance committee monitors the effectiveness of our corporate governance practices, including whether they are successfulrelated notes included elsewhere in preventing illegal or improper liability-creating conduct. While each committee is responsible for evaluating certain risks and overseeing the management of such risks, the entire board is regularly informed through committee reports about such risks.this Annual Report on Form 10-K.
Classified Board of DirectorsIn accordance with our amended and restated certificate of incorporation, our board of directors is divided into three classes with staggered, three-year terms. At each annual meeting of stockholders, the successors to directors whose terms then expire will be |
| | | | | | | |
| | Year Ended December 31, |
| | 2018 | | 2017 |
| Revenue, net | $ | 26,398 |
| | $ | 20,245 |
|
| Cost of goods sold | 13,974 |
| | 12,080 |
|
| Gross margin | 12,424 |
| | 8,165 |
|
| Operating expense: | | | |
| Research and development | 7,372 |
| | 7,126 |
|
| Selling, general and administrative | 47,142 |
| | 42,596 |
|
| Long-lived asset impairment costs | — |
| | 3,711 |
|
| Total operating expense | 54,514 |
|
| 53,433 |
|
| Operating loss | (42,090 | ) | | (45,268 | ) |
| Other income (expense), net: | | | |
| Interest expense, net | (3,812 | ) | | (4,263 | ) |
| Change in fair value of derivative liabilities | — |
| | 221 |
|
| Other income (expense) | (27 | ) | | 9 |
|
| Total other income (expense), net | (3,839 | ) |
| (4,033 | ) |
| Net loss | $ | (45,929 | ) | | $ | (49,301 | ) |
| Preferred Stock dividend | $ | (2,200 | ) | | $ | (1,711 | ) |
| Net loss attributable to common stockholders | $ | (48,129 | ) | | $ | (51,012 | ) |
| Net loss per share of common share outstanding — basic and diluted | $ | (1.71 | ) | | $ | (8.94 | ) |
| Weighted average common shares outstanding — basic and diluted | 28,131,836 |
| | 5,708,577 |
|
|
| | | | | | | |
| | December 31, |
| (Dollars in thousands) | 2018 | | 2017 |
| Cash and cash equivalents | $ | 47,758 |
| | $ | 25,961 |
|
| Total assets | $ | 68,620 |
| | $ | 45,089 |
|
| Total current liabilities | $ | 13,890 |
| | $ | 13,080 |
|
| Long-term debt, related parties | $ | 40,192 |
| | $ | 36,009 |
|
| Accumulated deficit | $ | (519,871 | ) | | $ | (473,921 | ) |
| Total stockholders’ equity (deficit) | $ | 14,289 |
| | $ | (4,058 | ) |
electedItem 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following management’s discussion and analysis should be read in conjunction with the historical financial statements and the related notes thereto contained elsewhere in this annual report. The following management’s discussion and analysis contains forward-looking statements, such as statements of our plans, objectives, expectations and intentions. Any statements that are not statements of historical fact are forward-looking statements. When used, the words “believe,” “plan,” “intend,” “anticipate,” “target,” “estimate,” “expect” and the like, and/or future tense or conditional constructions (“will,” “may,” “could,” “should,” etc.), or similar expressions, identify certain of these forward-looking statements. These forward-looking statements are subject to serverisks and uncertainties, including those under “Risk Factors” in this annual report that could cause actual results or events to differ materially from those expressed or implied by the forward-looking statements. See also the "Cautionary Note Regarding Forward-Looking Statements" set forth at the beginning of this annual report. Our actual results and the timing of events could differ materially from those anticipated in these forward-looking statements as a result of several factors. We do not undertake any obligation to update forward-looking statements to reflect events or circumstances occurring after the date of this annual report.
Overview
We are a commercial-stage medical technology company focused on improving health and simplifying life for people with diabetes by developing and commercializing innovative technologies. Valeritas’ flagship product, V-Go® Wearable Insulin Delivery device, is a simple, affordable, all-in-one basal-bolus insulin delivery option for patients with type 2 diabetes that is worn like a patch and can eliminate the need for taking multiple daily shots. V-Go administers a continuous preset basal rate of insulin over 24 hours and it provides discreet on-demand bolus dosing at mealtimes. It is the only basal-bolus insulin delivery device on the market today specifically designed keeping in mind the needs of type 2 diabetes patients.
V-Go enables patients to closely mimic the body’s normal physiologic pattern of insulin delivery throughout the day and to manage their diabetes with insulin without the need to plan a daily routine around multiple daily injections.
We currently focus on the treatment of patients with type 2 diabetes—a pervasive and costly disease that, according to the 2017 National Diabetes Statistics Report released by the U.S. Centers for Disease Control and Prevention, or CDC, currently affects 90% to 95% of the approximately 23 million U.S. adults diagnosed with diabetes. The American Diabetes Association estimates that the total costs of diagnosed diabetes in the United States, which includes direct medical and drug costs and indirect lost productivity costs have risen to $327 billion annually in 2017 from $245 billion annually in 2012. We believe the majority of the 12.6 million U.S. adults treating their type 2 diabetes with more than one daily oral anti-diabetic drug, or OAD, or an injectable diabetes medicine can benefit from the timeinnovative approach of electionV-Go to manage type 2 diabetes.
Our primary market consists of approximately 5.6 million of these patients who currently take insulin, of which up to 4.5 million may not be achieving their target blood glucose goal. This patient population represents a $19.3 billion annual U.S. market when applying the annual wholesale acquisition cost, or WAC, of V-Go to the 4.5 million patients not achieving glycemic control. WAC is the gross price paid by wholesalers and qualification untildoes not take into account fees, discounts, and rebates from us.
Insulin therapies using syringes, pens and programmable insulin pumps are often burdensome to a type 2 diabetes patient’s daily routine, which can lead to poor adherence to prescribed insulin regimens and, as a result, ineffective diabetes management. We developed V-Go utilizing our core technology, the third annual meeting following election.h-Patch platform, as a patient-focused solution to address the challenges of traditional insulin therapies. Our directorsh-Patch platform facilitates the simple and effective subcutaneous delivery of injectable medicines to patients across a broad range of therapeutic areas. V-Go enables patients to closely mimic the body’s normal physiologic pattern of insulin delivery by releasing a single type of insulin at a continuous preset background, or basal, rate over a 24-hour period and on demand around mealtime, or bolus dosing. We believe V-Go is an attractive management tool for patients with type 2 diabetes requiring insulin because it only requires a single fill of insulin prior to use and provides comprehensive basal-bolus therapy without the burden and inconvenience associated with multiple daily injections. V-Go is available in three different dosages depending on the patient’s needs and is generally cost competitive for both patients and third-party payors when compared to insulin pens or programmable insulin pumps.
V-Go was one of the first insulin delivery device cleared by the U.S. Food and Drug Administration, or FDA, under its Infusion Pump Improvement Initiative, which established additional device manufacturing requirements designed to foster the development of safer, more effective infusion pumps, and is the only FDA-cleared mechanical basal-bolus insulin delivery device on the market in the United States. All other FDA-cleared basal-bolus insulin delivery products currently available in the United States are divided amongelectronic and are classified as Durable Medical Equipment and, although cleared for both type 1 and type 2 diabetes, were designed primarily for patients with type 1 diabetes. As V-Go is a mechanical device, it does not include any electronics, batteries or audible
alarms and does not require any recharging or programming, which allows for simple and discreet use. Unlike electronic insulin delivery devices, V-Go is not classified as durable medical equipment by the three classesCenters for Medicare and Medicaid Services, or CMS, allowing for potential Medicare reimbursement under Medicare Part D. The Medicare Part D outpatient drug benefit defines V-Go and certain other supplies used for injecting insulin as follows:
the Class I directors are Brian K. Roberts, Joe Mandato, D.M. and Katherine Crothall, Ph.D., and their terms will expire at our annual meeting of stockholders“drugs,” which allows V-Go to be heldavailable for coverage by Part D Plans under Medicare Part D. In addition to Medicare, a majority of commercially insured patients are currently covered for V-Go under their insurance plans.
We commenced commercial sales of V-Go in the United States during 2012. During the year 2020;first half of 2012, we initiated an Early Access Program to provide a limited number of physicians with free V-Go devices for patients and began shipments to major wholesalers in anticipation of commercial launch. In the second half of 2012, we began hiring sales representatives in selected U.S. markets. In February 2016, we optimized our sales force to implement a high-service, high-touch sales model. At the end of 2018 and 2017, our field-based sales team consisted of 52 and 50 sales representatives, respectively and covered 52 and 50 territories, respectively, primarily within the East, South, Midwest and Southwest regions of the United States.
Our net loss was $45.9 million and $49.3 million for the Class II directorsyears ended December 31, 2018 and 2017, respectively. Our accumulated deficit as of December 31, 2018 and 2017 was $519.9 million and $473.9 million, respectively. Based on prescription data, we estimate that there were approximately 99,000 and 88,000 prescriptions reported for V-Go filled during both the years ended December 31, 2018 and 2017. Refill prescriptions account for slightly more than two-thirds of our total prescriptions, and generally move in parallel with our patient retention rates, so can be used as a proxy to determine patient retention. We estimate that as of December 31, 2018, V-Go had been used for over 17 million cumulative patient days with over 17 million V-Go’s dispensed to patients.
The following discussion highlights our audited results of operations and the principal factors that have affected our financial condition as well as our liquidity and capital resources for the periods described, and provides information that management believes is relevant for an assessment and understanding of the statements of financial condition and results of operations presented herein. The following discussion and analysis are Peter Devlinbased on our audited financial statements contained in this annual report, which we have prepared in accordance with United States generally accepted accounting principles. You should read the discussion and Rodney Altman, M.D.analysis together with such financial statements and the related notes thereto included elsewhere in this annual report.
Corporate Information
On May 3, 2016, pursuant to an Agreement and Plan of Merger and Reorganization, or the Merger Agreement, by and among Valeritas Holdings, Inc., a Delaware Corporation, Valeritas Acquisition Corp., a Delaware corporation and their terms will expire at our annual meetinga direct wholly-owned subsidiary of stockholders to be held duringValeritas Holdings, Inc., or the year 2018;Acquisition Subsidiary, and
Valeritas, Inc., a Delaware Corporation, Acquisition Subsidiary was merged with and into Valeritas, with Valeritas surviving as a direct wholly-owned subsidiary of Valeritas Holdings, Inc., or the Class III directors are Luke Düster and John E. Timberlake, and their terms will expire at the annual meeting of stockholders to be held during the year 2019.2016 Merger.
Results of Operations
Results of Operations for the Years Ended December 31, 2018 and 2017
The following is a comparison of revenue and expense categories for the years ended December 31, 2018 and 2017:
|
| | | | | | | | | | | | | | |
| | Year Ended December 31, | | Change |
| (Dollars in thousands) | 2018 | | 2017 | | $ | | % |
| Revenue, net | $ | 26,398 |
| | $ | 20,245 |
| | $ | 6,153 |
| | 30.4 | % |
| Costs of goods sold | 13,974 |
| | 12,080 |
| | 1,894 |
| | 15.7 | % |
| Gross margin | 12,424 |
| | 8,165 |
|
| 4,259 |
| | 52.2 | % |
| Operating expense: | | | | | | |
|
| Research and development | 7,372 |
| | 7,126 |
| | 246 |
| | 3.5 | % |
| Selling, general and administrative | 47,142 |
| | 42,596 |
| | 4,546 |
| | 10.7 | % |
| Long lived asset impairment costs | — |
| | 3,711 |
| | (3,711 | ) | | 100.0 | % |
| Total operating expense | 54,514 |
| | 53,433 |
|
| 1,081 |
| | 2.0 | % |
| Operating loss | (42,090 | ) | | (45,268 | ) | | 3,178 |
| | (7.0 | )% |
| Other income (expense), net: | | | | | | |
|
| Interest expense, net | (3,812 | ) | | (4,263 | ) | | 451 |
| | (10.6 | )% |
| Change in fair value of derivatives | — |
| | 221 |
| | (221 | ) | | (100.0 | )% |
| Other income (expense) | (27 | ) | | 9 |
| | (36 | ) | | (400.0 | )% |
| Total other income (expense), net | (3,839 | ) | | (4,033 | ) | | 194 |
| | (4.8 | )% |
| Net loss | $ | (45,929 | ) | | $ | (49,301 | ) | | $ | 3,372 |
| | (6.8 | )% |
Revenue, net
We generate revenue from sales of V-Go to third-party wholesalers and medical supply distributors that take delivery and ownership of V-Go and, in turn, sell it to retail pharmacies or directly to patients with type 2 diabetes. V-Go 30-day packages are sold to wholesalers and distributors at wholesale acquisition cost, or WAC, and we report net revenue after taking into consideration sales deductions as described in our financial statements included elsewhere in this annual report. Our amendedrevenue is primarily generated in the United States, and restated certificate of incorporation provides thatwe view our operations as one operating segment. Financial information is reviewed on a consolidated basis to allow management to make decisions regarding resource allocations and assess performance.
Within the authorized number of directors may be changedpast year, we have expanded our distribution network to include certain international distributors. Although we have entered into distribution agreements with distributors in several international countries, V-Go is currently only by resolutionavailable for distribution in Australia, New Zealand and Italy. We do not expect to see meaningful revenue from these distribution agreements until 2020 as each of the board of directors. Any additional directorships resulting frominternational distributors will need time to secure third party payor reimbursement in their local markets. Additionally, we recently entered into an increaseagreement with an international contract research organization to begin the medical device registration process for V-Go in China and expect the number of directors will be distributed among the three classes so that,countries in which V-Go is available to increase as nearly as possible, each class will consistwe continue to grow and expand our network of one-third of the directors. The division of our board of directors into three classes with staggered three-year terms may delay or prevent a change of our management or a change in control of our company. international agreements.
Our directors may be removed only for causerevenue increased by the affirmative vote of the holders of at least two-thirds of our outstanding voting stock entitled30.4% to vote in the election of directors.
Involvement in Certain Legal Proceedings
None of our directors or executive officers has been involved in any of the following events during the past ten years:
any bankruptcy petition filed by or against any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time;
any conviction in a criminal proceeding or being subject to a pending criminal proceeding (excluding traffic violations and other minor offences);
being subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining, barring, suspending or otherwise limiting his or her involvement in any type of business, securities or banking activities; or
being found by a court of competent jurisdiction (in a civil action), the SEC or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated.
Audit Committee
The members of our audit committee are Brian K. Roberts, Dr. Rodney D. Altman and Peter J. Devlin and Mr. Roberts serves as the chair. Our board of directors has determined that each of the members of our audit committee satisfies Nasdaq and SEC independence requirements and that Mr. Roberts qualifies as an audit committee financial expert within the meaning of SEC regulations. In making this determination, our board has considered the formal education and nature and scope of his previous experience. Our audit committee met seven times$26.4 million during the year ended December 31, 2017.
Among other matters,2018 from $20.2 million during the audit committee is responsible for:
appointing our independent registered public accounting firm;
evaluating our independent registered public accounting firm’s qualifications, independence and performance;
determining the engagementyear ended December 31, 2017. Because of our independent registered public accounting firm;
reviewinghigh touch and approvinghigher service sales and marketing model, we generated full year total prescription growth of 26% in our targeted accounts in 2018 vs. 2017. In addition, we realized year-over year total prescription growth in these accounts in every quarter of 2018. This growth was partially offset by an expected decline in 2018 of total prescriptions in our non-targeted accounts by 7% when compared to the scopesame period in 2017. We are seeing important signs of stabilization in these territories, particularly in the second half of 2018. The overall total volume of V-Go’s sold during the year ended December 31, 2018 grew by 22.1% as compared to the year ended December 31, 2017. This volume growth was accompanied by an increase of 6.8% in average net prices realized during the year ended December 31, 2018 as compared to the same period in 2017. This net price increase was primarily the result of an 8.0% WAC price increase implemented in both the third quarter of 2017 and the second quarter of 2018. During the second half of 2018, we implemented a V-Go initiation program in territories covered by our direct sales force. As part of this program, we allotted each of the annual auditphysicians interested in the program the ability to identify and the audit fee;
discussingprescribe V-Go for up to 10 of their patients with managementtype 2 diabetes. Under this program, new patients received V-Go units. The WAC price increase was partially offset by this program and our independent registered public accounting firm the results of the annual audit and the review of our quarterly financial statements;
approving the retention of our independent registered public accounting firm to perform any proposed permissible non-audit services;international distribution, which realized lower net pricing.
monitoringCost of Goods Sold and Gross Margins
Cost of goods sold includes raw materials, labor costs, manufacturing overhead expenses and reserves for anticipated scrap and inventory obsolescence.
Our cost of goods sold for the rotationyear ended December 31, 2018 was approximately $14.0 million on revenue of partnersapproximately $26.4 million, as compared to approximately $12.1 million in cost of goods sold on revenue of approximately $20.2 million during the year ended December 31, 2017. As a percentage of revenue, cost of goods sold decreased during the year ended December 31, 2018 to approximately 52.9% from approximately 59.7% during the year ended December 31, 2017. The $1.9 million or 15.7% increase in cost of goods sold was driven by an increase in sales volume. The manufacturing process improvements described below helped keep the cost of goods sold growth below the corresponding 22.1% volume growth.
We currently manufacture V-Go® and the EZ Fill accessory in cleanrooms at a contract manufacturing organization, or CMO, in Southern China. We also have a relationship with a separate CMO that performs our final inspection and packaging functions in Central China. Any single-source components and suppliers are managed through our global supply chain operation. Management reviews regularly the inventory levels and demand and will adjust production accordingly, which could give rise to under-absorbed fixed production costs. We continually work with our manufacturing CMO's to refine our manufacturing processes and production lines to improve efficiencies, reduce labor cost and leverage our overhead expenses. These improvements, combined with overhead cost reductions represent the primary drivers in the current year reduction in cost of goods sold per unit.
Our gross profit as a percentage of revenues, or gross margin, for the year ended December 31, 2018 was 47.1%, compared to 40.3% during the year ended December 31, 2017. The increase in our gross margin was due to manufacturing efficiencies, overhead cost reductions, implementation of more cost-effective processes in our product development and the impact of our net price increase.
Research and Development Expense
Our research and development activities primarily consist of activities associated with our core technologies and process engineering as well as research programs associated with products under development. These expenses are primarily related to employee compensation, including salary, fringe benefits, share-based compensation and contract employee expenses.
Total research and development expenses increased by $0.2 million, or 3.5%, during the year ended December 31, 2018 as compared to 2017. This increase is primarily due to increases in spending related to the initiation of our development projects, including the V-Go SIMTM, V-Go Prefill and studies to qualify the V-Go for regular insulin.
We expect our research, development and engineering expenses to increase from current levels as we advance our development projects, including both the V-Go SIM™ (Simple Insulin Management) and V-Go Prefill devices.
Selling, General and Administrative Expense
Selling, general and administrative expenses consist primarily of salary, fringe benefits and share-based compensation for our executive, financial, marketing, sales, business development, regulatory affairs and administrative functions. Other significant expenses include product demonstration samples, trade show expenses, professional fees for our contracted customer support center, external legal counsel, independent registeredauditors and other consultants, insurance, facilities and information technology expenses. We expect our selling, general and administrative expenses to increase as our business expands.
Additionally, we increased our investment in sales and marketing programs and materials used to drive our capital-efficient marketing initiatives, as well as the volume of sample products provided to patients. This overall growth in commercial spending is part of our strategic plan to expand our business operations.
Our selling, general and administrative expenses increased by $4.5 million or 10.7% in the year ended December 31, 2018 compared to 2017. This increase was driven primarily by the impact of a change in our sales and marketing strategy during the year ended December 31, 2018, which increased our field sales force and increased labor costs by $1.6 million or 14.1% compared to 2017. Additionally, our commercial external services increased by $3.0 million from 2017 to 2018. These cost included a $2.2 million increase in centralized marketing as well as $0.2 million in sales automation.
Other Income (Expense), Net
Other income (expense), net primarily consists of interest expense and amortization of debt discount associated with our loan agreements with Capital Royalty Group, or CRG, and WCAS Capital Partners IV, L.P., or WCAS. See “—Indebtedness” below for more information.
Interest expense, net decreased by 10.6% during the year ended December 31, 2018 as compared to the year ended December 31, 2017. The decrease was attributable to a restructuring of our Term Loan with CRG and the note payable with WCAS, in addition to an offset of interest expense with interest income of $0.4 million generated from cash and cash equivalent accounts. On March 28, 2017, $25.0 million and $2.5 million of the Term Loan and WCAS Note, respectively, were converted to preferred shares upon completion of our public accounting firmoffering in March 2017. The debt conversion resulted in a lower principal balance and lower interest rates in the year ended December 31, 2018 compared with the year ended December 31, 2017 and an interest expense decrease of $0.2 million or 5.6%.
The decrease in fair value of derivatives of a de minimis amount during the year ended December 31, 2018, as compared with the decrease in fair value of $0.2 million during the year ended December 31, 2017 is primarily caused by fluctuations in period end valuations of our derivative liabilities. Specifically, the decrease occurring in 2018 is primarily attributed to the decrease in the stock price. The warrants are accounted as a derivative liability at fair value as the warrant exercise price is subject to adjustment upon additional issuances of equity securities at a price per share lower than the exercise price of the warrants.
Liquidity and Capital Resources
We are subject to a number of risks similar to those of early stage commercial companies, including dependence on key individuals, the difficulties inherent in the development of a commercial market, the potential need to obtain additional capital necessary to fund the development of its products, and competition from larger companies. We expect that our sales performance and the level of selling and marketing efforts, as well as the status of each of our new product development programs, will significantly impact our cash requirements.
We have incurred losses each year since inception and have experienced negative cash flows from operations in each year since inception. As of December 31, 2018, we had $47.8 million in cash and cash equivalents, $0.5 million of which is restricted, and an accumulated deficit of $519.9 million. Our restructured Term Loan with CRG, see "Indebtedness - Senior Secured Debt," includes a liquidity covenant whereby we must maintain a cash balance greater than $2.0 million. We believe that our cash balance and liquidity will not be sufficient to satisfy our operating cash requirement for the next 12 months from the date of this report or to maintain this liquidity covenant, which raises substantial doubt about our ability to continue as a going concern. We expect our current cash and cash equivalents will be sufficient to finance our current operations into the fourth quarter of 2019. This estimate is based upon certain assumptions regarding volume growth in sales of V-Go and future expenses. Our ability to fund operations beyond the fourth quarter of 2019 is dependent on our engagement teamability to obtain cash from our common stock purchase agreement, or the Purchase Agreement, with Aspire Capital Fund, LLC, or Aspire Capital, that we entered into in June of 2018, our “at-the-market” sales agreement, or the Sales Agreement, with B. Riley FBR, Inc. or FBR, that we entered into on January 26, 2018, and warrant exercises from the November 2018 offering, all of which are described in further detail elsewhere in this annual report. FBR has the option to decline any sales orders at its discretion. We are not required to sell shares under with the Purchase Agreement or the Sales Agreement. No 2019 purchases were made under either the Purchase Agreement or the Sales Agreement as of March 5, 2019.
We intend to maintain compliance with the liquidity covenant and fund future operations by raising additional capital in addition to the Purchase Agreement and the Sales Agreement. There can be no assurances that these agreements will be fully available to us or that future financing will be available on terms acceptable to us, or at all. If we are unable to raise additional capital prior to achieving sustained profitability from operations, there could be a material adverse effect on our business, results of operations and financial condition.
Historically, our sources of cash have included private placement of equity securities, debt arrangements, and cash generated from operations, primarily from the collection of accounts receivable resulting from sales. In March 2017, we completed an underwritten public offering with net proceeds of $48.8 million, net of underwriting expenses and discounts. In 2018, we completed two public offerings and used the Purchase Agreement to generate an additional $60.0 million of capital, net of fees.
We are pursuing additional sources of financing to fund our operations. These sources may include the issuance of our equity to new or existing investors.
The following table shows a summary of our cash flows for the years ended December 31, 2018 and 2017: |
| | | | | | | |
| | Year Ended December 31, |
| (Dollars in thousands) | 2018 | | 2017 |
| Net cash provided by (used in): | | | |
| Operating activities | $ | (37,190 | ) | | $ | (31,970 | ) |
| Investing activities | (1,552 | ) | | (692 | ) |
| Financing activities | 60,539 |
| | 48,757 |
|
| Total | $ | 21,797 |
| | $ | 16,095 |
|
Operating Activities
The increase in net cash used in operating activities of 16.3% for the year ended December 31, 2018 as compared to the year ended December 31, 2017 was primarily associated with increased overhead and services related to both our research and development efforts and sales initiatives, partially offset by $4.3 million in higher gross margin as a result of improved product revenue and a manufacturing efficiencies, as well as working capital fluctuations.
Investing Activities
Net cash used in investing activities for the year ended December 31, 2018 increased $0.9 million versus 2017 primarily related to purchases of manufacturing automation. Additionally, we had $0.2 million lower proceeds from the sale of equipment in 2018 versus 2017. We do not expect to have significant investing activity in the next 12 months.
Financing Activities
Net cash provided by financing activities for the years ended December 31, 2018 and 2017 was the result of gross proceeds from our common stock financing rounds. The financing rounds in 2018 and 2017 raised gross proceeds of $60.4 million and $48.8 million, net of any offering costs, respectively.
Indebtedness
Senior Secured Debt
On May 23, 2013, we entered into the Term Loan of $50.0 million with Capital Royalty Group (“CRG”), structured as a senior secured loan with a six-year term (the “Term Loan” or the “Senior Secured Debt”). The Term Loan is secured by substantially all of our assets, including our material intellectual property. We later entered into a series of forbearance agreements to modify the Term Loan. The terms of the most recently executed amendment of the Term Loan agreement, dated February 9, 2017, bears interest at 11% per annum and allows the interest-only period of the Term Loan to extend to March 31, 2022, requires quarterly cash interest payments beginning June 30, 2019, extends the deadline for full payment under the Term Loan agreement to March 31, 2022 and brings our minimum covenant cash and cash equivalent requirements to $2.0 million. On March 28, 2017, $25.0 million of the Term Loan was converted to preferred shares upon completion of the public offering at a conversion rate of $10 per share. CRG received 2,500,000 preferred shares. As of December 31, 2018, we were in compliance with the financial covenant in the restructured Term Loan agreement, as we held cash and cash equivalents of $47.8 million, which includes $0.5 million of restricted cash. We may, at our discretion, repay the revised loan in whole or in part without any penalty or prepayment fees.
Warrants classified as Derivative liabilities
We issued 10,390 warrants to acquire shares of common stock to the placement agents in the private placement offering that was conducted as part of the 2016 Merger (the “PPO”). The warrants have a term of five years. The warrants are accounted for as a derivative liability at fair value as the warrant exercise price is subject to adjustment upon additional issuances of equity securities at a price per share lower than the exercise price of the warrants.
The fair value of the warrants at the date of issuance was $0.3 million. At December 31, 2018 and December 31, 2017, the fair value of the warrants was estimated to be a de minimis amount based on the Black Scholes option pricing model.
Other Note
In 2011, we issued a $5.0 million senior subordinated note, or the WCAS Note (the "Other Note Payable") to WCAS Capital Partners IV, L.P., or WCAS. We later entered into a series of forbearance agreements to modify the WCAS note. The terms of the most recently executed amendment of the WCAS note, dated May 23, 2013, bears interest at 12% per annum, and all interest accrues as compounded PIK interest and is added to the aggregate principal amount of the loan semi-annually. On March 28, 2017, $2.5 million of the WCAS Note was converted to preferred shares upon completion of the public offering at a conversion rate of $10 per share. WCAS received 250,000 preferred shares, respectively. Concurrent with the debt restructure due to the public offering, the remaining principal balance of $2.5 million of the WCAS note was amended to decrease the interest rate to 10% per annum, payable entirely as PIK interest. The outstanding principal amount of the note, including accrued PIK interest, is due in full in September 2021. No interest payments are required by law;during the term of the loan. We may pay off the WCAS Note at any time without penalty.
reviewingContractual Obligations
The following summarizes our significant contractual obligations as of December 31, 2018:
|
| | | | | | | | | | | | | | |
| | Payment Due by Period |
| (Dollars in thousands) | Total | | Less than 1 Year | | 1 to 3 Years | | 3 to 5 Years | | More than 5 Years |
| Purchase commitments(1) | 5,112 |
| | 5,112 |
| | — |
| | — |
| | — |
|
| Operating lease obligations(2) | 2,368 |
| | 568 |
| | 987 |
| | 791 |
| | 22 |
|
| Senior secured debt(3) | 36,758 |
| | 2,326 |
| | 5,614 |
| | 28,818 |
| | — |
|
| Other Note Payable(4) | 3,538 |
| | — |
| | 3,538 |
| | — |
| | — |
|
| Total | 47,776 |
| | 8,006 |
| | 10,139 |
| | 29,609 |
| | 22 |
|
| |
| (1) | Represents purchase commitments with suppliers for raw materials and finished goods. |
| |
| (2) | Represents operating lease commitments for office and research and development space in Marlborough, Massachusetts and Bridgewater, New Jersey and office equipment. |
| |
| (3) | Represents Term Loan agreement with CRG for $25.0 million, including accrued interest through December 31, 2018. |
| |
| (4) | Represents a $2.5 million Other Note Payable to WCAS, including accrued interest through December 31, 2018. |
Related Party Transactions
We transact business with certain parties related to us, primarily with key stakeholders with the intent of managing working capital through additional debt or equity financing. See “Certain Relationships and Related-Party Transactions, and Director Independence.”
Recent Accounting Pronouncements
See Note 3 to the consolidated financial statements for a discussion of recent accounting pronouncements.
Critical Accounting Policies and ourUse of Estimates
This management’s discussion and analysis of financial condition and results of operations is based on our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States, or U.S. GAAP. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the consolidated financial statements, as well as the reported revenue and expenses during the reporting periods. These items are monitored and analyzed by us for changes in facts and circumstances, and material changes in these estimates could occur in the future. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Changes in estimates are reflected in reported results for the period in which they become known. Actual results may differ materially from these estimates under different assumptions or conditions.
While our significant accounting policies are more fully described in the notes to our consolidated financial statements included elsewhere in this annual report, we believe that the following accounting policies are critical to the process of making significant judgments and estimates in the preparation of our consolidated financial statements and understanding and evaluating our reported financial results.
Revenue Recognition
The majority of our revenue is generated from V-Go sales in the United States to third-party wholesalers and medical supply distributors that, in turn, sell this product to retail pharmacies or directly to patients. A portion of our revenue is also generated from V-Go sales to international medical supply distributors. Under ASC 606, we recognizes revenue when our customer obtains control of promised goods or services, in an amount that reflects the consideration which we expect to receive in exchange for those goods or services. To perform revenue recognition for arrangements within the scope of ASC 606, we perform the following five steps:
(i) identification of the promised goods or services in the contract;
(ii) determination of whether the promised goods or services are performance obligations including whether they are distinct in the context of the contract;
(iii) measurement of the transaction price, including the constraint on variable consideration;
(iv) allocation of the transaction price to the performance obligations based on estimated selling prices; and
(v) recognition of revenue when (or as) we satisfy each performance obligation. A performance obligation is a promise in a contract to transfer a distinct good or service to the customer, and is the unit of account in ASC 606.
Revenue from product sales is recorded net of adjustments for managed care rebates, wholesale distributions fees, cash discounts, prompt pay discounts, and co-pay card redemptions, all of which are established at the time of sale. In order to prepare the consolidated financial statements, we are required to make estimates regarding the amounts earned or to be includedclaimed on the related product sales, including the following:
• managed care and Medicare rebates, which are based on the estimated end user payor mix and related contractual rebates;
• distribution fees, prompt pay discounts and other discounts, which are recorded based on specified payment terms, and which vary by customer and other incentive programs; and
• Co-pay card redemption charges which are based on the net transaction costs of prescriptions filled via a company-subsidized card program and other incentive programs.
We believe our estimates related to managed care rebates and Medicare rebates, distribution fees, prompt pay and other discounts, and co-pay card redemption do not have a high degree of estimation complexity or uncertainty as the related amounts are settled within a relatively short period of time.
Return Reserve
We record allowances for product returns as a reduction of revenue at the time product sales are recorded. Several factors are considered in determining whether an allowance for product returns is required, including the customers’ return rights and our historical experience with returns and the amount of product sales in the distribution channel not consumed by patients and subject to return. We rely on historical return rates to estimate returns. In the future, as any of these factors and/or the history of product returns change, adjustments to the allowance for product returns will be reflected.
Inventories
Inventories consist of raw materials, work in process and finished goods, which are valued at the lower of cost or net realizable value. Cost is determined on a first in, first out basis and includes material costs, labor and applicable overhead. We review our inventory for excess or obsolescence and write down inventory that has no alternative uses to its net realizable. Economic conditions, customer demand and changes in purchasing and distribution can affect the carrying value of inventory. As circumstances warrant, we record provisions for potentially obsolete or slow moving inventory and lower of cost or net realizable value inventory adjustments. In some instances, these adjustments can have a material effect on the financial results of an annual or interim period. In order to determine such adjustments, we evaluate the age, inventory turns, future sales forecasts and the estimated fair value of inventory.
Impairment of Long-Lived Assets
We assess the impairment of long-lived assets on an ongoing basis and whenever events or changes in circumstances indicate that the carrying value may not be recoverable. Our impairment review process is based upon an estimate of future undiscounted cash flow. Factors we consider that could trigger an impairment review include the following:
significant underperformance relative to expected historical or projected future operating results,
significant changes in the manner of our use of the acquired assets or the strategy for our overall business,
significant negative industry or economic trends, and
significant technological changes, which would render equipment and manufacturing processes obsolete.
Recoverability of assets that will continue to be used in our annual and quarterly reportsoperations is measured by comparing the carrying value to the future net undiscounted cash flows expected to be filedgenerated by the asset or asset group. Future undiscounted cash flows include estimates of future revenues, driven by market growth rates, and estimated future costs. During the fourth quarter of 2017, we performed further analyses of the year-to-date results of operations, compared them with earlier projections and determined that revenues and cash flows from operations were lower than the previously planned amounts. As a result, we revised our long term projections of future cash flows, which triggered an impairment review. The impairment review evaluated the estimated future undiscounted cash flows related to specific asset groups and determined that certain long lived assets would require impairment (See Note 5). No additional impairments to long-lived assets were required during 2018.
Share-Based Compensation
Effective January 1, 2017, we adopted ASU 2016-09, Improvements to Employee Share-Based Payment Accounting, which was applied retroactively effective December 31, 2016, to account for forfeitures as they occur. Under ASU 2016-09, all share-based awards will be recognized on a straight-line method, assuming all awards granted will vest. Forfeitures of share-based awards are recognized in the period in which they occur. Prior to the adoption of ASU 2016-09, share-based compensation cost was measured at grant date, based on the estimated fair value of the award, and was recognized as expense net of expected forfeitures, over the employee’s requisite service period on a straight-line basis. As of January 1, 2017, the cumulative effect adjustment of approximately $0.4 million was recognized to reflect the forfeiture rate that had been applied to unvested option and stock awards prior to 2017.
The fair value of stock options on the date of grant is calculated using the Black-Scholes option pricing model, based on key assumptions such as the fair value of common stock, expected volatility and expected term. Compensation cost for restricted stock
awards issued to employees is measured using the grant date fair value of the award, adjusted to reflect actual forfeitures. Our estimates of these important assumptions are primarily based on third-party valuations, historical data, peer company data and our judgment regarding future trends and other factors.
Off-Balance Sheet Arrangements
We did not engage in any “off-balance sheet arrangements” (as that term is defined in Item 303(a)(4)(ii) of Regulation S-K) as of December 31, 2018.
Subsequent Events
Management evaluates events that have occurred after the financial statement date but before the consolidated financial statements are issued. Based upon the evaluation, we identified the following subsequent event that would require adjustment or disclosure in the consolidated financial statements other than disclosed:
On February 12, 2019 the Board approved to have included at the annual shareholder meeting a resolution for the shareholders to approve an amendment to effect a reverse stock split ratio range, with the SEC;exact ratio for the Board to subsequently determine.
reviewing our critical accounting policies and estimates; and
annually reviewing the audit committee charter and the committee’s performance.
The audit committee operates pursuant to a charter adopted by our board of directors that satisfies the applicable standards of the SEC and Nasdaq.
Compensation Committee Interlocks and Insider Participation
None of our executive officers currently serves, or in the past year has served, as a member of the board of directors or compensation committee of any entity that has one or more executive officers on our board of directors or compensation committee.
Code of Business Conduct and Ethics
We have adopted a written code of business conduct and ethics that applies to our directors, officers and employees, including our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions. Our code of business conduct and ethics is available under the Corporate Governance section of our website at www.valeritas.com. In addition, we intend to post on our website all disclosures that are required by law or the listing standards of The Nasdaq Capital Market concerning any amendments to, or waivers from, any provision of the code. The reference to our website address does not constitute incorporation by reference of the information contained at or available through our website, and you should not consider it to be a part of this filing.
Item 11. Executive Compensation.7A. Quantitative and Qualitative Disclosures about Market Risk.
The following table sets forth information concerningMarket risk represents the compensationrisk of loss that may impact our financial position due to adverse changes in financial market prices and rates. Our market risk exposure is primarily the named executive officers forresult of fluctuations in interest rates and inflation.
Interest Rate Risk
We are exposed to financial market risks in the ordinary course of our business. Our cash and cash equivalents include cash in readily available checking and money market accounts, as well as certificates of deposit. These securities are not dependent on interest rate fluctuations that may cause the principal amount of these assets to fluctuate. Additionally, the interest rate on our outstanding indebtedness is fixed and is therefore not subject to changes in market interest rates.
Inflation Risk
Inflation generally affects us by increasing our cost of labor and pricing of contracts. We do not believe that inflation has had a material effect on our business, financial condition, or results of operations during the years ended December 31, 20172018 and 2016. All amounts reflect compensation received from Valeritas. No figures referenced in this section have been adjusted to reflect the exchange ratio after consummation of the 2016 Merger.2017.
|
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| Name and Principal Position | Year | | Salary ($) | | Option Awards(3) ($) | | Stock Awards(3) ($) | | Non-Equity Incentive Plan Compensation(4) ($) | | All Other Compensation(5) ($) | | | Total ($) |
| John E. Timberlake(2) | | 2017 | | 414,577 |
| | 1,335,000 |
| | — |
| | 174,900 |
| | 5,400 |
| | | 1,929,877 |
|
| Chief Executive Officer and President | | 2016 | | 389,268 |
| | 1,049,823 |
| | 441,500 |
| | 194,634 |
| | 5,200 |
| | | 2,080,425 |
|
| Erick Lucera(6) | | 2017 | | 276,250 |
| | 525,456 |
| | — |
| | 86,328 |
| | 5,400 |
| | | 893,434 |
|
| Chief Financial Officer | | 2016 | | 85,000 |
| | 368,430 |
| | — |
| | 29,750 |
| | 1,200 |
| | | 484,380 |
|
| Geoffrey Jenkins(7) | | 2017 | | 242,287 |
| | 961,200 |
| | — |
| | 90,858 |
| | 28,846 |
| | | 1,323,191 |
|
| Executive Vice President, Manufacturing, Operations and R&D | | 2016 | | 367,937 |
| | 546,061 |
| | 250,000 |
| | 128,778 |
| | 19,046 |
| | | 1,311,822 |
|
| Matthew Nguyen | | 2017 | | 302,269 |
| | 580,280 |
| | — |
| | 85,013 |
| | 5,400 |
| | | 972,962 |
|
| Chief Commercial Officer | | 2016 | | 281,976 |
| | 315,373 |
| | 130,000 |
| | 102,639 |
| | 48,200 |
| | | 878,188 |
|
| Former Executive Officer | | | | | | | | | | | | | | |
| Kristine Peterson(1) | | 2017 | | 277,315 |
| | — |
| | — |
| | — |
| | — |
| | | 277,315 |
|
| | | 2016 | | 491,090 |
| | — |
| | — |
| | — |
| | 1,387 |
| | | 492,477 |
|
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
For Fiscal Years Ended December 31, 2018 and 2017.
|
| |
(1) | Resigned as Chief Executive Officer on February 22, 2016. Received monthly salary through August 2017. No other compensation was paid during 2017. |
(2)Consolidated Financial Statements: | Appointed as Chief Executive Officer on February 22, 2016. Mr. Timberlake retained his title as President and Chief Commercial Officer until December 2016 when he was replaced by Mr. Nguyen as Chief Commercial Officer. |
(3) | Represents the aggregate grant-date fair value of stock options and restricted stock granted during the indicated year computed in accordance with ASC Topic 718, excluding the effect of estimated forfeitures. |
(4) | Represents amounts earned | |
(5) | Represents company matching contributions to 401(k) plan accounts and discretionary bonus payments. |
(6) | Appointed as Chief Financial Officer on August 29, 2016. |
(7) | Represents actual amounts paid. Base salary is presented on a FTE basis in the narrative disclosure below. |
Narrative Explanation
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Certain AspectsDirectors and
Stockholders of Valeritas Holdings, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Valeritas Holdings, Inc. and subsidiary (the “Company”) as of December 31, 2018 and 2017, and the related consolidated statements of operations, stockholders’ equity (deficit), and cash flows for each of the Summary Compensation Tableyears in the two-year period ended December 31, 2018, and the related notes (collectively referred to as the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2018 and 2017, and the results of its operations and its cash flows for each of the years in the two-year period ended December 31, 2018, in conformity with accounting principles generally accepted in the United States of America.
The primary elementsCompany’s Ability to Continue as a Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. The Company has recurring losses and negative cash flows from operations. As described in Note 2, these conditions, among others, raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of compensationthis uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the named executive officers are base salary, cash bonuses and long-term equity-based compensation awards. The named executive officers also participate in employee benefit plans and programs that are offered to other full-time employeespurpose of expressing an opinion on the same basis.effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Base SalariesOur audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Friedman LLP
We have served as the Company's auditor since 2016.
East Hanover, New Jersey
March 5, 2019
VALERITAS HOLDINGS, INC.
CONSOLIDATED BALANCE SHEETS
(Dollars in thousands, except share and per share data)
|
| | | | | | | | |
| | December 31, | |
| | 2018 | | 2017 | |
| Assets | | | | |
| Current assets: | | | | |
| Cash and cash equivalents | $ | 47,758 |
| | $ | 25,961 |
| |
| Accounts receivable, net | 6,294 |
| | 3,991 |
| |
| Inventories, net | 6,824 |
| | 8,105 |
| |
| Deferred cost of goods sold | — |
| | 539 |
| |
| Prepaid expense and other current assets | 1,200 |
| | 876 |
| |
| Total current assets | 62,076 |
| | 39,472 |
| |
| Property and equipment, net | 6,097 |
| | 5,469 |
| |
| Other assets | 447 |
| | 148 |
| |
| Total assets | $ | 68,620 |
| | $ | 45,089 |
| |
| Liabilities and stockholders’ equity (deficit) | | | | |
| Current liabilities: | | | | |
| Current portion of capital lease obligation | $ | 123 |
| | $ | — |
| |
| Accounts payable | 4,916 |
| | 5,644 |
| |
| Accrued expense and other current liabilities | 8,851 |
| | 5,798 |
| |
| Deferred revenue | — |
| | 1,638 |
| |
| Total current liabilities | 13,890 |
| | 13,080 |
| |
| Long-term debt, related parties (net of issuance costs of $104 and $125, respectively). | 40,192 |
| | 36,009 |
| |
| Other long-term liabilities | 109 |
| | 58 |
| |
| Capital lease obligation, less current portion | 140 |
| | — |
| |
| Total liabilities | 54,331 |
| | 49,147 |
| |
| Commitments and contingencies (note 15) |
| |
| |
| Stockholders’ equity (deficit) | | | | |
| Convertible preferred stock, $0.001 par value; 50,000,000 shares authorized at December 31, 2018; 2,750,000 shares issued and outstanding at December 31, 2018 and December 31, 2017. (aggregate liquidation value of $31,411 and $29,211 at December 31, 2018 and December 31, 2017, respectively). | 3 |
| | 3 |
| |
| Common stock, $0.001 par value, 300,000,000 shares authorized; 99,956,291 shares issued and 99,948,437 shares outstanding at December 31, 2018 and 7,015,636 shares issued and 7,007,782 shares outstanding at December 31, 2017 | 100 |
| | 7 |
| |
| Additional paid-in capital | 534,081 |
| | 469,877 |
| |
| Accumulated deficit | (519,871 | ) | | (473,921 | ) | |
| Treasury stock, at cost (7,854 shares) | (24 | ) | | (24 | ) | |
| Total stockholders’ equity (deficit) | 14,289 |
| | (4,058 | ) | |
| Total liabilities and stockholders’ equity (deficit) | $ | 68,620 |
| | $ | 45,089 |
| |
See accompanying notes to consolidated financial statements.
VALERITAS HOLDINGS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(Dollars in thousands, except share and per share data)
|
| | | | | | | | |
| | Year Ended December 31, | |
| | 2018 | | 2017 | |
| Revenue, net | $ | 26,398 |
| | $ | 20,245 |
| |
| Cost of goods sold | 13,974 |
| | 12,080 |
| |
| Gross margin | 12,424 |
| | 8,165 |
| |
| Operating expense: | | | | |
| Research and development | 7,372 |
| | 7,126 |
| |
| Selling, general and administrative | 47,142 |
| | 42,596 |
| |
| Long-lived asset impairment costs | — |
| | 3,711 |
| |
| Total operating expense | 54,514 |
| | 53,433 |
| |
| Operating loss | (42,090 | ) | | (45,268 | ) | |
| Other income (expense), net: | | | | |
| Interest expense, net | (3,812 | ) | | (4,263 | ) | |
| Change in fair value of derivative liabilities | — |
| | 221 |
| |
| Other income (expense) | (27 | ) | | 9 |
| |
| Total other income (expense), net | (3,839 | ) | | (4,033 | ) | |
| Loss before income taxes | (45,929 | ) | | (49,301 | ) | |
| Provision for income taxes | — |
| | — |
| |
| Net loss | $ | (45,929 | ) | | $ | (49,301 | ) | |
| Preferred stock dividend | $ | (2,200 | ) | | $ | (1,711 | ) | |
| Net loss attributable to common stockholders | $ | (48,129 | ) | | $ | (51,012 | ) | |
| Net loss per share of common share outstanding — basic and diluted | $ | (1.71 | ) | | $ | (8.94 | ) | |
| Weighted average common shares outstanding — basic and diluted | 28,131,836 |
| | 5,708,577 |
| |
See accompanying notes to consolidated financial statements.
VALERITAS HOLDINGS, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)
(Dollars in thousands, except share data) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Preferred Stock | Common Stock | Treasury Stock | Additional Paid-in capital | Accumulated Deficit | | Total stockholders’ equity (deficit) |
| | Shares | Amount | Shares | | Amount | Shares | Amount |
| Balance—December 31, 2016 | — |
| $ | — |
| 1,590,948 |
| | $ | 2 |
| $ | — |
| $ | — |
| $ | 387,737 |
| $ | (424,239 | ) | | $ | (36,500 | ) |
| Cumulative effect of change in accounting principle - stock compensation | — |
| — |
| — |
| | — |
| — |
| — |
| 381 |
| (381 | ) | | — |
|
| Share-based compensation expense | — |
| — |
| — |
| | — |
| — |
| — |
| 5,510 |
| — |
| | 5,510 |
|
| Issuance of common stock as a result of public offering, net of fees | — |
| — |
| 5,250,000 |
| | 5 |
| — |
| — |
| 48,781 |
| — |
| | 48,786 |
|
| Issuance of common stock for compensation | — |
| — |
| 2,030 |
| | — |
| — |
| — |
| 73 |
| — |
| | 73 |
|
| Issuance of preferred stock upon conversion of debt, net of discount | 2,750,000 |
| 3 |
| — |
| | — |
| — |
| — |
| 27,395 |
| — |
| | 27,398 |
|
| Commitment fee for Purchase Agreement | — |
| — |
| 125,000 |
| | — |
| — |
| — |
| — |
| — |
| | — |
|
| Restricted shares vested | — |
| — |
| 47,658 |
| | — |
| — |
| — |
| — |
| — |
| | — |
|
| Treasury Shares | — |
| — |
| (7,854 | ) | | — |
| 7,854 |
| (24 | ) | — |
| — |
| | (24 | ) |
| Net loss | — |
| — |
| — |
| | — |
| — |
| — |
| — |
| (49,301 | ) | | (49,301 | ) |
| Balance—December 31, 2017 | 2,750,000 |
| 3 |
| 7,007,782 |
| | 7 |
| 7,854 |
| (24 | ) | 469,877 |
| (473,921 | ) | | (4,058 | ) |
| Cumulative effect of change in accounting principle - revenue recognition | — |
| — |
| — |
| | — |
| — |
| — |
| — |
| (21 | ) | | (21 | ) |
| Share-based compensation expense | — |
| — |
| — |
| | — |
| — |
| — |
| 4,057 |
| — |
| | 4,057 |
|
| Restricted shares vested | — |
| — |
| 30,000 |
| | — |
| — |
| — |
| — |
| — |
| | — |
|
| Issuance of common stock through Employee Stock Purchase Program | — |
| — |
| 154,379 |
| | — |
| — |
| — |
| 236 |
| — |
| | 236 |
|
| Issuance of common stock as a result of April 2018 public offering, net of fees | — |
| — |
| 15,300,000 |
| | 16 |
| — |
| — |
| 24,343 |
| — |
| | 24,359 |
|
| Issuance of common stock as a result of November 2018 public offering, net of fees | — |
| — |
| 75,000,000 |
| | 75 |
| — |
| — |
| 32,464 |
| — |
| | 32,539 |
|
| Issuance of common stock from Purchase Agreements, net of fees | — |
| — |
| 2,192,424 |
| | 2 |
| — |
| — |
| 3,104 |
| — |
| | 3,106 |
|
| Commitment fee for Second Purchase Agreement | — |
| — |
| 263,852 |
| | — |
| — |
| — |
| — |
| — |
| | — |
|
| Net loss | — |
| — |
| — |
| | — |
| — |
| — |
| — |
| (45,929 | ) | | (45,929 | ) |
| Balance—December 31, 2018 | 2,750,000 |
| $ | 3 |
| 99,948,437 |
| | $ | 100 |
| 7,854 |
| $ | (24 | ) | $ | 534,081 |
| $ | (519,871 | ) | | $ | 14,289 |
|
See accompanying notes to consolidated financial statements.
VALERITAS HOLDINGS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
|
| | | | | | | | |
| | Year Ended
December 31, | |
| | 2018 | | 2017 | |
| Operating activities | | | | |
| Net loss | $ | (45,929 | ) | | $ | (49,301 | ) | |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | |
| Depreciation of property and equipment | 1,264 |
| | 1,852 |
| |
| Amortization of financing costs | 21 |
| | 20 |
| |
| Noncash interest expense | 4,162 |
| | 4,438 |
| |
| Share-based compensation expense | 4,057 |
| | 5,583 |
| |
| Change in fair value of derivative liabilities | — |
| | (221 | ) | |
| Bad debt expense | — |
| | 16 |
| |
| Impairment of property and equipment | — |
| | 3,711 |
| |
| Gain on sale of property and equipment | — |
| | (175 | ) | |
| Loss on disposal of property and equipment | 20 |
| | — |
| |
| Sales return provision | (158 | ) | | — |
| |
| Changes in: | | | | |
| Accounts receivable | (2,303 | ) | | (529 | ) | |
| Other receivables | — |
| | (69 | ) | |
| Inventories | 1,281 |
| | 1,279 |
| |
| Deferred cost of goods sold | — |
| | 151 |
| |
| Prepaid expense and other current assets | (324 | ) | | (65 | ) | |
| Other assets | (264 | ) | | 5 |
| |
| Accounts payable | (1,017 | ) | | 1,053 |
| |
| Accrued expense | 1,949 |
| | 279 |
| |
| Deferred revenue | — |
| | 15 |
| |
| Deferred rent liability | 51 |
| | (12 | ) | |
| Net cash used in operating activities | (37,190 | ) | | (31,970 | ) | |
| Investing activities | | | | |
| Proceeds from sales of property and equipment | — |
| | 185 |
| |
| Acquisition of property and equipment | (1,552 | ) | | (877 | ) | |
| Net cash used in investing activities | (1,552 | ) | | (692 | ) | |
| Financing activities | | | | |
| Repayment of capital lease | (97 | ) | | — |
| |
| Proceeds from issuance of common stock from public offerings ($7,200 and $40,000 from a related party in 2018 and 2017, respectively), net of issuance costs | 57,294 |
| | 48,792 |
| |
| Proceeds from issuance of common stock through purchase agreements, net of issuance costs | 3,106 |
| | — |
| |
| Proceeds from issuance of common stock through ESPP | 236 |
|
| — |
| |
| Costs associated with debt restructuring | — |
| | (35 | ) | |
| Net cash provided by financing activities | 60,539 |
| | 48,757 |
| |
| Net increase in cash, cash equivalents, and restricted cash | 21,797 |
| | 16,095 |
| |
| Cash, cash equivalents, and restricted cash—beginning of period | 25,961 |
| | 9,866 |
| |
| Cash, cash equivalents, and restricted cash—end of period | $ | 47,758 |
| | $ | 25,961 |
| |
| Supplemental disclosures of cash flow information | | | | |
| Commitment fee for Purchase Agreement | $ | 429 |
| | $ | 411 |
| |
| Deferred revenue reduction (Note 3) | $ | 1,638 |
| | $ | — |
| |
| Sales return reserve (Note 3) | $ | 778 |
| | $ | — |
| |
| Deferred cost of goods sold reduction (Note 3) | $ | 539 |
| | $ | — |
| |
| Accrued distribution fees and managed care costs (Note 3) | $ | 343 |
| | $ | — |
| |
| Capital lease obligation | $ | 360 |
| | $ | — |
| |
| Accounts payable and accrued offering costs | $ | 396 |
| | $ | — |
| |
| Write off of debt issuance costs | $ | — |
| | $ | 107 |
| |
| Related party conversion of debt to Series A preferred stock | $ | — |
| | $ | 27,500 |
| |
| Treasury shares acquired | $ | — |
| | $ | 24 |
| |
See accompanying notes to consolidated financial statements.
VALERITAS HOLDINGS, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. NATURE OF OPERATIONS AND ORGANIZATION
Organization and Nature of Operations
Valeritas Holdings, Inc. (the “Company”) was incorporated in the state of Delaware on May 3, 2016. Prior to its incorporation in Delaware, the Company was incorporated in Florida on May 9, 2014 under the name “Cleaner Yoga Mat, Inc.” Valeritas, Inc., the Company’s wholly-owned subsidiary, was incorporated in the state of Delaware on December 27, 2007, when it was converted into a Delaware corporation from a Delaware limited liability company, which was formed on August 2, 2006 under the name Valeritas LLC.
The named executive officers receiveCompany is a base salarycommercial-stage medical technology company focused on improving health and simplifying life for people with diabetes by developing and commercializing innovative technologies. The Company’s flagship product, V-Go® Wearable Insulin Delivery device, is a simple, wearable, basal-bolus insulin delivery device for adult patients with type 2 diabetes that enables patients to compensate themadminister a continuous preset basal rate of insulin over 24 hours. It also provides discreet on-demand bolus dosing at mealtimes. It is the only non-electronic basal-bolus insulin delivery device on the market today specifically designed keeping in mind the needs of adult patients with Type 2 diabetes. V-Go is a small, discreet, easy-to-use wearable and completely disposable insulin delivery device that a patient adheres to his or her skin every 24 hours. V-Go enables patients to closely mimic the body’s normal physiologic pattern of insulin delivery throughout the day and to manage their diabetes with insulin without the need to plan a daily routine around multiple daily injections.
2. LIQUIDITY, UNCERTAINTIES AND GOING CONCERN
The Company is subject to a number of risks similar to those of early stage companies, including dependence on key individuals and products, the difficulties inherent in the development of a commercial market, the potential need to obtain additional capital, competition from larger companies, other technology companies and other technologies.
In accordance with ASC 205-40, Going Concern, the Company has evaluated whether there are conditions or events, considered in the aggregate, that raise substantial doubt about the Company's ability to continue as a going concern within one year after the date that the consolidated financial statements are issued. As of December 31, 2018, the Company had $47.8 million in cash and cash equivalents ($0.5 million of which is restricted cash). The Company has incurred losses each year since inception and has experienced negative cash flows from operations in each year since inception. As of December 31, 2018 and 2017, the Company had an accumulated deficit of $519.9 million and $473.9 million, respectively. The Company's restructured Term Loan includes a liquidity covenant whereby the Company must maintain a cash balance greater than $2.0 million. Based on its current business plan assumptions and expected cash burn rate, excluding potential share sales associated with its financing agreements discussed below, the Company believes that it has sufficient cash and cash equivalents to fund its current operations into the fourth quarter of 2019. These factors raise substantial doubt regarding the Company’s ability to continue as a going concern.
On January 7, 2018, as a component of management's plan to pursue additional financing, the Company entered into a common stock purchase agreement (the "First Purchase Agreement") with Aspire Capital Fund, LLC ("Aspire Capital") for the satisfactory performancesale of services renderedup to our company.$20.0 million of its common stock, as described in further detail in Note 11. The base salary payableCompany sold 1,250,868 shares in 2018 and received net proceeds of approximately $1.8 million. The First Purchase Agreement was terminated in June 2018.
On January 26, 2018, the Company entered into an At Market Issuance Sales Agreement (the “ATM Agreement”) with B. Riley FBR, Inc. (“FBR”), under which the Company may, from time to each named executive officer is intendedtime in its sole discretion, issue and sell through FBR, acting as agent, shares of the Company’s common stock (the “Placement Shares”). FBR has the option to providedecline any sales orders at its discretion. The issuance and sale, if any, of the Placement Shares by the Company under the Agreement will be made pursuant to a fixed component of compensation reflectingprospectus supplement to the executive’s skill set, experience, roleCompany’s registration statement on Form S-3, originally filed with the Securities and responsibilities. Base salariesExchange Commission (the “SEC”) on October 4, 2017, and declared effective by the SEC on December 15, 2017, for the named executive officers have generally been set at levels deemed necessarysale of up to attract and retain individuals with superior talent and were originally established$2.8 million of shares of the Company's common stock. As of the date of this report the Company has not sold any shares pursuant to the Sales Agreement.
The Company completed a public offering on April 26, 2018, for net proceeds of approximately $21.8 million. On May 2, 2018, the underwriters in each named executive officer’s employment agreement.the public offering exercised a portion of their 30-day option to purchase additional shares of the Company's common stock for additional net proceeds of approximately $2.6 million.
In February 2016, Valeritas’ Compensation Committee reviewedOn June 11, 2018, the annual salariesCompany entered into a purchase agreement (the "Second Purchase Agreement") with Aspire Capital, pursuant to which, the Company has the right, in its sole discretion, to present Aspire Capital with a purchase notice, directing Aspire Capital (as principal) to purchase up to 150,000 shares of the named executive officers and approvedCompany’s common stock per business day, in an aggregate amount of up to $21.0 million of the Company's common stock (the "Purchase Shares") over the term of the Second Purchase Agreement at a 3% increase for Mr. Jenkins on his base salary and a 5% increase for Mr. Timberlake, effective February 24, 2016. Following the increases, the new base salary for Geoffrey Jenkins was $393,593 and for John E. Timberlake was $392,141.
In August 2016, Mr. Lucera was hired as our Chief Financial Officer with a starting base salary of $260,000. In December 2016, Mr. Nguyen was promotedper share price equal to the position of Chief Commercial Officer with a starting base salary of $287,000.
In February 2017, our compensation committee reviewed the annual salarieslesser of the named executive officers and approved a 3% increase for Mr. Jenkins on his base salary, a 6% increase for Mr. Lucera, a 5% increase for Mr. Nguyen and a 5% increase for Mr. Timberlake, effective February 27, 2017. Following the increases, the new base salary for Mr. Jenkins was $380,750, for Mr. Lucera was $275,000, for Mr. Nguyen was $300,000 and for Mr. Timberlake was $412,000.
In February 2018, our compensation committee reviewed the annual salarieslowest sale price of the named executive officers withCompany’s common stock on the advicepurchase date; or the arithmetic average of the compensation committee’s consultant and recommended tothree lowest closing sale prices for the boardCompany’s common stock during the ten consecutive trading days ending on the trading day immediately preceding the purchase date. The Company may sell an aggregate of directors, and the board4,726,383 shares of directors subsequently approved, a 3.0% increase for Mr. Jenkins on his base salary, a 12.7% increase for Mr. Lucera, a 4.7% increase for Mr. Nguyen and a 9.2% increase for Mr. Timberlake, effective February 19, 2018. Following the increases, the new base salary for Mr. Jenkins was $392,102, for Mr. Lucera was $310,000, for Mr. Nguyen was $314,000 and for Mr. Timberlake was $450,000.
The 2016, 2017 and 2018 increases in base salary were made in recognition of our named executive officers’ individual performance and contributions to company performance in those years and as a result of comparing the compensation paid to our named executive officers with the compensation paid to other executive officers within our company’s peer group who have similar titles and perform similar roles to our named executive officers.
Annual Performance Bonuses
We offer our named executive officers the opportunity to earn annual cash bonuses that are intended to compensate them for achieving short-term company and individual performance goals. Our compensation committee establishes the target bonuses of our named executive officers, which are evaluated from time to time.
Each named executive officer’s target annual bonus is typically expressed as a percentage of base salary. For 2016, Mr. Timberlake’s target bonus was 50% of his base salary and Mr. Jenkins’ target bonus was 35% of his base salary. No bonus amounts were paid to either Mr. Lucera or Mr. Nguyen in 2016. For 2017, Mr. Timberlake’s target bonus was 75% of his base salary, Mr. Lucera's target bonus was 50% of his base salary, Mr. Nguyen's target bonus was 50% of his base salary and Mr. Jenkins’ target bonus was 50% of his base salary. In February 2018, our compensation committee reviewed the achievementits common stock (which represented 19.99% of the foregoing target bonus percentages,Company’s outstanding shares of common stock on June 11, 2018) without stockholder approval. The Company may sell additional shares of its common stock above the 19.99% limit provided that (i) it obtains stockholder approval or (ii) shareholder approval has not been obtained at any time the 4,726,383 share limitation is reached and withat all times thereafter the advice ofaverage price paid for all shares issued under the compensation committee’s consultant, recommendedSecond Purchase Agreement, is equal to the board of directors, and the board of directors subsequently approved, bonus payments for the named executive officers as set forth in the table above.
In February 2018, our compensation committee reviewed the target annual bonus for each named executive officer with the advice of the compensation committee’s consultant and recommended to the board of directors, and the board of directors subsequently approved, a target bonus for Mr. Timberlake of 75% of his base salary, a target bonus for Mr. Lucera of 50% of his base salary, a target bonus for Mr. Nguyen of 50% of his base salary, and a target bonus for Mr. Jenkins of 50% of his base salary.
For 2016 and 2017, annual cash bonuses were based on achievement of a combination of individual and corporate objectives. The 2016 and 2017 corporate objectives related to revenue, manufacturing efficiency and quality, financial management and fundraising. The 2016 and 2017 individual objectives for each named executive officer related to each named executive officer’s areas of responsibility within our company and the named executive officer’s ability to influence the success of those areas.
Actual payouts of 2016 and 2017 cash bonuses were determined by multiplying each named executive officer’s respective target amount by his base pay earnings for the fiscal year, multiplied by an individual bonus multiplier (0-150%or greater than $1.62 (the “Minimum Price”), which was then multiplied by the company bonus multiplier (0-150%). The bonus multipliers represent our compensation committee's evaluation of company performance and each named executive officer’s individual performance against the established targets.
Notwithstanding the establishmentconsolidated closing bid price of the performance components andCompany’s common stock on June 11, 2018. In addition to these restrictions, the formula for determining the cash bonus payment amounts as described above, our compensation committee may exercise positive or negative discretion in determining the levels of achievement of performance goals or electCompany is prohibited from selling shares to award a greater or lesser amount to any named executive officer than the amount determined by the annual cash bonus formula if, in the exercise of its business judgment, our compensation committee determines that adjustments are warrantedAspire under the circumstances.
Equity Compensation
We offer stock options to our key employees, including our named executive officers, as the long-term incentive component of our compensation program, which we consider necessary to enable us and certain of our affiliates to obtain and retain services of these individuals, which we believe is essential to our long-term success. We typically grant stock options to key employees when they commence employment with us and may thereafter grant additional awards in the discretion of our board of directors. Our stock options generally allow key employees to purchase shares of our common stockPurchase Agreement at a price per share less than $1.00. Through December 31, 2018, the Company has issued 1,205,409 shares of common stock including the Initial Purchase Shares, for net proceeds of approximately $1.3 million.
The Company completed a public offering on November 16, 2018 for net proceeds of approximately $32.5 million, after deducting underwriting discounts and commissions of $2.7 million and offering expenses of $0.8 million. This offering also included 75,000,000 Series A warrants and 75,000,000 Series B warrants. The Series A warrants, if exercised at the exercise price of $0.60 per share prior to their expiration date of five years after issuance, could generate an additional $45.0 million before fees. The Series B warrants, if exercised at the exercise price of $0.48 per share prior to their expiration date of nine months after issuance, could generate $36.0 million before fees.
There is market uncertainty regarding the utilization of financing associated from the Sales Agreement and the Second Purchase Agreement. The uncertainty regarding these financings, in addition to potential proceeds that may be realized from the November 16, 2018 warrants, makes our ability to provide enough cash to fund operations through and beyond the fourth quarter of 2019 unknown. If the Company was able to fully utilize the potential funding from the above Purchase Agreement, ATM Agreement, and warrant exercises, it would have enough cash to support operating requirements beyond the first quarter of 2020.
The Company is actively pursuing additional sources of financing to fund its operations. The Company can provide no assurances that additional financings will be consummated on acceptable terms, or at all. If the Company is unable to effect a sufficient financing or capital raise, there could be a material adverse effect on the Company.
These consolidated financial statements have been prepared with the assumption that the Company will continue as a going concern and will be able to realize its assets and discharge its liabilities in the normal course of business and do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the inability of the Company to continue as a going concern.
3. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Principles of Consolidation
The consolidated financial statements reflect the operations of the Company and Valeritas, its wholly-owned subsidiary. All significant intercompany balances and transactions have been eliminated in consolidation.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America ("GAAP") generally requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenue and expense during the period. Due to the inherent uncertainty involved in making estimates, actual results reported in future periods may be based on amounts that differ from those estimates.
Basis of Presentation
Reclassification
Certain prior year amounts have been reclassified for consistency with the current year presentation. These reclassifications had no effect on the reported results of operations.
Change in Accounting Principle
On January 1, 2018, the Company adopted ASU 2014-09: Revenue from Contracts with Customers, which requires an entity to recognize the amount of revenue to which it expects to be entitled for the transfer of promised goods or services to customers. The Company elected to use the modified retrospective approach for adoption. The Company had previously deferred recognition of revenue and the related cost of goods sold on shipments of V-Go until a customer's right of return no longer existed, which was once the Company received evidence that the product had been distributed to patients based on analysis of third-party information. Management has determined that the variable consideration associated with rebates, chargebacks and other discounts can continue to be estimated and that there are no return estimate constraints for which it is considered probable that a subsequent change in the estimate would result in a significant revenue reversal. Accordingly, the Company is no longer using the “sell through” approach to recognition and has accelerated the recognition of revenue upon sale to the distributor. The variable consideration associated with sales returns was estimated based on the probability weighted expected value approach. In connection with its adoption of ASC 606, the Company recorded a cumulative-effect adjustment to increase the accumulated deficit by a de minimis amount. The reported results for 2018 reflect the application of ASC 606 guidance, while the reported results for 2017 were prepared under the guidance of ASC 605, Revenue Recognition, which is also referred to herein as "previous guidance."
The cumulative effect of applying the new guidance of ASC 606 to all contracts with customers that were not completed as of January 1, 2018 was recorded as an adjustment to accumulated deficit as of the adoption date. As a result of applying the modified retrospective method to adopt the new revenue guidance, the following adjustments were made to accounts on the Condensed Consolidated Balance Sheet as of January 1, 2018: |
| | | | | | | | | | | |
| | As Reported | | | | Adjusted |
| | December 31, 2017 | | New Revenue Standard Adjustment | | January 1, 2018 |
| Assets | | | | | |
| Current assets: | | | | | |
| Deferred cost of goods sold | $ | 539 |
| | $ | (539 | ) | | $ | — |
|
| | | | | | |
| Liabilities | | | | | |
| Current liabilities: | | | | | |
| Deferred revenue | 1,638 |
| | (1,638 | ) | | — |
|
| Sales return provision | — |
| | 778 |
| | 778 |
|
| Accrued distribution fees and managed care costs | 1,330 |
| | 342 |
| | 1,672 |
|
| | | | | | |
| Stockholders' deficit | | | | | |
| Accumulated deficit | $ | (473,921 | ) | | $ | (21 | ) | | $ | (473,942 | ) |
Under the modified retrospective method of adoption, the Company is required to disclose the impact on its statement of operations, had it continued to follow the accounting policies under the previous revenue guidance. The Company estimates that the impact to revenues for the year ended December 31, 2018 would have been a decrease of approximately $0.7 million, and the impact to gross profit for the year ended December 31, 2018 would have been a decrease of $0.3 million, had there been no adoption of ASC 606. The impact to net loss for the year ended December 31, 2018 would have been a decrease of $0.3 million, and a decrease in the net loss per share by $0.01.
On January 1, 2017, the Company adopted ASU 2016-09: Improvements to Employee Share-Based Payment Accounting, which was applied retroactively effective December 31, 2016, to account for forfeitures as they occur. Under ASU 2016-09, all share-based awards will be recognized on a straight-line method, assuming all awards granted will vest. Forfeitures of share-based awards will be recognized in the period in which they occur. Prior to the adoption of ASU 2016-09, share-based compensation cost was measured at grant date, based on the estimated fair value of the award, and was recognized as expense net of expected forfeitures, over the employee’s requisite service period on a straight-line basis. As of January 1, 2017, the cumulative effect adjustment of approximately $0.4 million was recognized to reflect the forfeiture rate that had been applied to unvested option and stock awards prior to 2017.
Significant Accounting Policies
Revenue Recognition
The majority of the Company’s revenue is generated from V-Go sales in the United States to third-party wholesalers and medical supply distributors that, in turn, sell this product to retail pharmacies or directly to patients. A portion of the Company's revenue is also generated from V-Go sales to international medical supply distributors. Under ASC 606, the Company recognizes revenue when its customer obtains control of promised goods or services, in an amount that reflects the consideration which the Company expects to receive in exchange for those goods or services. To perform revenue recognition for arrangements within the scope of ASC 606, the Company performs the following five steps:
(i) identification of the promised goods or services in the contract;
(ii) determination of whether the promised goods or services are performance obligations including whether they are distinct in the context of the contract;
(iii) measurement of the transaction price, including the constraint on variable consideration;
(iv) allocation of the transaction price to the performance obligations based on estimated selling prices; and
(v) recognition of revenue when (or as) we satisfy each performance obligation. A performance obligation is a promise in a contract to transfer a distinct good or service to the customer, and is the unit of account in ASC 606.
Revenue from product sales is recorded net of adjustments for managed care rebates, wholesale distributions fees, cash discounts, prompt pay discounts, and co-pay card redemptions, all of which are established at the time of sale. In order to prepare the consolidated financial statements, the company is required to make estimates regarding the amounts earned or to be claimed on the related product sales, including the following:
• managed care and Medicare rebates, which are based on the estimated end user payor mix and related contractual rebates;
• distribution fees, prompt pay discounts and other discounts, which are recorded based on specified payment terms, and which vary by customer and other incentive programs; and
• Co-pay card redemption charges which are based on the net transaction costs of prescriptions filled via a company-subsidized card program and other incentive programs.
The Company believes its estimates related to managed care rebates and Medicare rebates, distribution fees, prompt pay and other discounts, and co-pay card redemption do not have a high degree of estimation complexity or uncertainty as the related amounts are settled within a relatively short period of time.
Return Reserve
The Company records allowances for product returns as a reduction of revenue at the time product sales are recorded. Several factors are considered in determining whether an allowance for product returns is required, including the customers’ return rights and the Company's historical experience with returns and the amount of product sales in the distribution channel not consumed by patients and subject to return. The Company relies on historical return rates to estimate returns. In the future, as any of these factors and/or the history of product returns change, adjustments to the allowance for product returns will be reflected.
Segment and Geographic Information
Operating segments are defined as components of an enterprise about which separate discrete financial information is available for evaluation by the chief operating decision-maker ("CODM") or decision-making group in making decisions regarding resource allocation and assessing performance. The Company primarily generates its revenues in the United States, only has employees in the United States, and views its operations as one operating segment, as the CODM review financial information on a consolidated basis in making decisions regarding resource allocation and assessing performance. The Company also owns assets in China that are utilized by its contract manufacturer ("CMO") in the manufacture of the Company’s products.
Geographic information for property and equipment, net of accumulated depreciation at December 31, 2018 and 2017 is as follows:
|
| | | | | | | | |
| | December 31, | |
| (Dollars in thousands) | 2018 | | 2017 | |
| United States | $ | 1,079 |
| | $ | 751 |
| |
| China | 5,018 |
| | 4,718 |
| |
| Total property and equipment, net | $ | 6,097 |
| | $ | 5,469 |
| |
Cash and cash equivalents
The Company considers investments and interest-bearing deposits with original maturities of three months or less to be cash equivalents. At December 31, 2018 and 2017, there was $47.3 million and $25.7 million, respectively, on deposit at banks in excess of Federal Deposit Insurance Corporation ("FDIC") insured limits. No losses have been experienced on such bank deposits, money market fund or notes. The Company does not believe that it is subject to any unusual credit risk beyond the normal credit risk associated with commercial banking relationships. The company noted the following cash and restricted cash balances as of December 31, 2018 and December 31, 2017:
|
| | | | | | | | |
| | December 31, | |
| (Dollars in thousands) | 2018 | | 2017 | |
| Cash and cash equivalents | $ | 47,290 |
| | $ | 25,493 |
| |
| Restricted cash | 468 |
| | 468 |
| |
| Total cash, cash equivalents, and restricted cash | $ | 47,758 |
| | $ | 25,961 |
| |
Restricted Cash
The Company held restricted cash of $0.5 million as of December 31, 2018 and 2017, respectively as part of its lease agreements. The amounts are included within cash and cash equivalents balance.
Major Customers and Concentration of Credit Risk
As discussed above, the Company ships product to third-party wholesalers and medical supply distributors that, in turn, sell product to retail pharmacies or directly to patients with diabetes.
Estimated revenue from significant customers as a percentage of the Company’s consolidated gross revenue was as follows:
|
| | | | | | |
| | Year Ended December 31, | |
| | 2018 | | 2017 | |
| McKesson Corporation | 37.3 | % | | 38.3 | % | |
| AmerisourceBergen Corporation | 29.4 | % | | 28.0 | % | |
| Cardinal Health | 24.6 | % | | 24.6 | % | |
The Company’s three largest customers accounted for receivables in excess of ten percent of gross accounts receivable at December 31, 2018 and 2017:
|
| | | | | | |
| | December 31, | |
| | 2018 | | 2017 | |
| Amerisource Bergen Corporation | 21.4 | % | | 32.3 | % | |
| McKesson Corporation | 43.6 | % | | 35.4 | % | |
| Cardinal Health | 17.8 | % | | 22.6 | % | |
The Company believes that these customers are of high credit quality and that the Company is not subject to unusual risk with respect to such customers, and generally does not require collateral.
The Company maintains an allowance for doubtful accounts that was a de minimis amount as of December 31, 2018 and 2017, respectively.
Major Vendors
The Company maintains a single vendor for production of all finished goods inventory. During the years ended December 31, 2018 and 2017, the Company had aggregate purchases from this vendor of $9.0 million and $7.9 million, respectively.
Inventories
Inventories consists of raw materials, work in process and finished goods, which are valued at the lower of cost or net realizable value. Cost is determined on a first in, first out ("FIFO"), basis and includes material costs, labor and applicable overhead. The Company reviews its inventory for excess or obsolescence and writes down inventory that has no alternative uses to its net realizable value. Economic conditions, customer demand and changes in purchasing and distribution can affect the carrying value of inventory. As circumstances warrant, the Company records provisions for potentially obsolete or slow moving inventory and lower of cost or net realizable value inventory adjustments. In order to determine such adjustments, the Company evaluates the age, inventory turns, future sales forecasts and the estimated fair value of inventory.
Property and equipment
Property and equipment are carried at cost less accumulated depreciation. Depreciation is recorded on a straight-line basis over the estimated useful lives of the respective assets. Leasehold improvements are depreciated over the shorter of the lease term or the estimated useful life of the asset. Maintenance and repairs are expensed when incurred.
Construction-in-Progress
Assets under construction at manufacturing and other facilities are capitalized as construction-in-progress. The cost of construction-in-progress comprises its purchase price and any costs directly attributable to bringing it into working condition for its intended use. Construction-in-progress amounts incurred at manufacturing facilities are presented as a separate asset within property and equipment. Construction-in-progress is not depreciated. Once the asset is complete and available for use, depreciation is commenced.
Impairment of Long-Lived Assets
Long-lived tangible assets with finite lives are tested for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. The Company’s impairment review process is based upon an estimate of future undiscounted cash flow. Factors the Company considers that could trigger an impairment review include the following:
significant underperformance relative to expected historical or projected future operating results,
significant changes in the manner of our use of the acquired assets or the strategy for our overall business
significant negative industry or economic trends
significant technological changes, which would render equipment and manufacturing processes obsolete
Recoverability of assets that will continue to be used in the Company's operations is measured by comparing the carrying value to the future net undiscounted cash flows expected to be generated by the asset or asset group. Future undiscounted cash flows include estimates of future revenues, driven by market growth rates, and estimated future costs. During the fourth quarter of 2017, the Company performed further analyses of the year-to-date results of operations, compared them with earlier projections and determined that revenues and cash flows from operations were lower than the previously planned amounts. As a result, the Company revised its long term projections of future cash flows, which triggered an impairment review. The impairment review evaluated the estimated future undiscounted cash flows related to specific asset groups and determined that certain long lived assets would require impairment (See Note 5). No additional impairments to long-lived assets were required during 2018.
Liability and Equity Classified Warrants
The Company accounts for certain warrant instruments as a derivative liability at fair value as the warrant exercise price is subject to adjustment upon additional issuances of equity securities at a price per share lower than the exercise price of the warrants. These warrants are subject to revaluation at each balance sheet date, and any changes in fair value are recorded as a component of other income (expense), until the earlier of their exercise or expiration or the completion of a liquidation event.
The Company accounts for additional warrant instruments issued in conjunction with the Company’s common stock in permanent equity. These warrants are indexed to the Company’s stock and meet the requirements of equity classification as prescribed under ASC 815. The value ascribed to these warrants is recorded as additional-paid-in-capital. Warrants classified as equity are initially measured at fair value, and subsequent changes in fair value are not recognized so long as the warrants continue to be classified as equity.
Income taxes
The Company accounts for income taxes using the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax reporting purposes and for operating loss and tax credit carry forwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply in the years in which these temporary differences are expected to be recovered or settled. A valuation allowance is established to reduce net deferred tax assets to the amount expected to be realized. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in results of operations in the period that includes the enactment date (see 2017 amounts in Note 12 for impact of December 22, 2017 enacted "Tax Cuts and Jobs Act"). The Company recognizes the effect of income tax positions only if those positions are more likely than not of being sustained. Recognized income tax positions are measured at the largest amount that is greater than 50% likely of being recognized. Changes in recognition and measurement are reflected in the period in which the change in judgment occurs. Interest and penalties related to unrecognized tax benefits are included in income tax expense.
Research and development expenses
Research and development expenses are expensed as incurred and are primarily comprised of the following types of costs incurred in performing research and development activities:
contract services;
testing samples and supplies;
salaries and benefits; and
overhead and occupancy costs.
Advertising
Advertising costs, which include promotional expenses, are included in selling, general and administrative expenses in the consolidated statements of operations and are expensed as incurred. Advertising expenses were $10.6 million and $8.5 million for the years ended December 31, 2018 and 2017, respectively.
Share-based compensation
The Company measures the cost of awards of equity instruments based on the grant date fair value of the awards. That cost is recognized on a straight-line basis over the period during which the employee is required to provide service in exchange for the award.
The fair value of stock options on the date of grant is calculated using the Black Scholes option pricing model, based on key assumptions such as the fair value of common stock, expected volatility and expected term. These estimates require the input of subjective assumptions, including (i) the expected stock price volatility, (ii) the calculation of the expected term of the award, (iii) the risk-free interest rate and (iv) expected dividends. Due to the lack of substantial company-specific historical volatility data of its common stock, the Company previously based its estimate of expected volatility on the historical volatility of a group of similar companies that are publicly traded. When selecting these public companies on which it has based its expected stock price volatility, the Company selected companies with comparable characteristics to it, including enterprise value, risk profiles, position within the industry and with historical share price information sufficient to meet the expected term of the stock-based awards. The Company computes historical volatility data using the daily closing prices for the selected companies’ shares during the equivalent period of the calculated expected term of the stock-based awards. In 2018, the Company began to use a blended rate, which included the Company's historical volatility together with this peer group of companies to calculate volatility.
The Company has estimated the expected term of its employee stock options using the “simplified” method, whereby, the expected term equals the arithmetic average of the vesting term and the original contractual term of the option due to its lack of sufficient historical data. The risk-free interest rates for periods within the expected term of the option are based on the U.S. Treasury securities with a maturity date commensurate with the expected term of the associated award. The Company has never paid, and does not expect to pay dividends in the foreseeable future. The Company accounts for forfeitures when they occur. Stock-based compensation expense recognized in the financial statements is reduced by the actual awards forfeited.
Compensation cost for restricted stock awards issued to employees is measured using the grant date fair value of the award, and expense is recognized over the service period, adjusted to reflect actual forfeitures.
Fair Value Measurements
The Company’s financial instruments consist primarily of cash and cash equivalents, accounts receivable, accounts payable and accrued liabilities, and debt instruments.
Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. The fair value accounting guidance establishes a fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value:
Level 1—Quoted prices in active markets for identical assets or liabilities.
Level 2—Inputs other than quoted prices included within Level 1 that are observable for the asset or liability, either directly or indirectly. Level 2 inputs include quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets or liabilities in markets that are not active, inputs other than quoted prices that are observable for the asset or liability (i.e., interest rates, yield curves, etc.), and inputs that are derived principally from or corroborated by observable market data by correlation or other means (market corroborated inputs).
Level 3—Inputs are unobservable and reflect the Company’s assumptions as to what market participants would use in pricing the asset or liability. The Company develops these inputs based on the best information available.
It is the Company’s policy to maximize the use of observable inputs and minimize the use of unobservable inputs when developing fair value measurements. When available, the Company uses quoted market prices to measure fair value. If market prices are not available, the fair value measurement is based on models that use primarily market based parameters including interest rate yield curves, option volatilities and currency rates. In certain cases where market rate assumptions are not available, the Company is required to make judgments about assumptions market participants would use to estimate the fair value of a financial instrument. Changes in the underlying assumptions used, including discount rates and estimates of future cash flows could significantly affect the results of current or future values. The results may not be realized in an actual sale or immediate settlement of an asset or liability.
Please refer to Note 9 Fair Value Measurements for further discussion of the fair value of financial instruments.
Recently Issued Accounting Standards
In February 2016, the Financial Accounting Standards Board, or FASB issued ASU No. 2016-02, Leases. ASU 2016-2 sets out the principles for the recognition, measurement, presentation and disclosure of leases for both parties to a contract (i.e. lessees and lessors). The new standard requires lessees to apply a dual approach, classifying leases as either finance or operating leases based on the principle of whether or not the lease effectively finances a purchase by the lessee. This classification will determine whether lease expense is recognized based on an effective interest method (finance lease) or on a straight line basis over the term of the lease (operating lease). A lessee is also required to record a right-of-use asset and a lease liability for all leases with a term of greater than 12 months regardless of their classification. Leases with a term of 12 months or less will be accounted for similar to existing guidance for operating leases today. ASU 2016-2 supersedes the existing guidance on accounting for leases in "Leases (Topic 840)." Subsequent to ASU 2016-02, the FASB has issued ASU No. 2018-01 (“ASU 2018-01”) Leases (Topic 842): Land Easement Practical Expedient for Transition which clarifies the application of lease easements and eases adoption efforts for some land easements. The provisions of ASU 2016-2 are effective for fiscal years, and interim reporting periods within those fiscal years, beginning after December 15, 2018. In July 2018, the FASB issued ASU No. 2018-10 and No. 2018-11, Leases (ASC 842). ASU 2018-10 provides narrow amendments that clarify how to apply certain aspects of the guidance in ASU 2016-02. ASU 2018-11 provides entities with an option of an additional transition method, by allowing entities to initially apply the new leases standard at the adoption date and recognize a cumulative-effect adjustment to the opening balance of retained earnings in the period of adoption, if necessary. We elected the available practical expedients on adoption. In preparation for adoption of the standard, we have implemented internal controls to enable the preparation of financial information. The standard will have a material impact on our consolidated balance sheets, but will not have a material impact on our consolidated statements of operations. The most significant impact will be the recognition of right of use assets and lease liabilities for operating leases, while our accounting for capital leases remains substantially unchanged. The Company has evaluated the effect of the impact of the adoption
of this standard and has determined the adoption will result in the recognition of additional right of use assets and lease liabilities of approximately $1.7 million for operating leases.
In June 2016, the FASB issued ASU 2016-13 “Financial Instruments-Credit Losses-Measurement of Credit Losses on Financial Instruments”. This guidance replaces the current incurred loss impairment methodology with a methodology that reflects expected credit losses and requires consideration of a broader range of reasonable and supportable information to inform credit loss estimates. The guidance applies to loans, accounts receivable, trade receivables and other financial assets measured at amortized cost, loan commitments, debt securities and beneficial interests in securitized financial assets, but the effect on the Company is projected to be limited to accounts receivable. The guidance will be effective for the fiscal year beginning on January 1, 2020, including interim periods within that year. The Company is currently evaluating the potential effect of the guidance on its consolidated financial statements.
In July 2017, the FASB issued ASU 2017-11, Earnings Per Share (Topic 260) Distinguishing Liabilities from Equity (Topic 480) Derivatives and Hedging (Topic 815), which addresses the complexity of accounting for certain financial instruments with down round features. Down round features are features of certain equity-linked instruments (or embedded features) that result in the strike price being reduced on the basis of the pricing of future equity offerings. Current accounting guidance creates cost and complexity for entities that issue financial instruments, such as warrants and convertible instruments, with down round features that require fair value measurement of the entire instrument or conversion option. The amendments in Part I of this Update are effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2018 with early adoption permitted. The Company has determined the impact of adopting this guidance will not be material.
In June 2018, the FASB issued ASU No. 2018-07, Compensation-Stock Compensation (Topic 718): Improvements to Nonemployee Share-Based Payment Accounting. ASU 2018-07 amends the FASB Accounting Standards Codification or ASC to expand the scope of FASB ASC Topic 718, Compensation-Stock Compensation, to include accounting for share-based payment transactions for acquiring goods and services from non-employees. The amendments in ASU 2018-07 are effective for all entities for annual periods, and interim periods within those annual periods, beginning after December 15, 2018. Early adoption is permitted. The Company has determined the impact of adopting this guidance will not be material.
On August 28, 2018, the FASB issued ASU 2018-13, Fair Value Measurement: Disclosure Framework - Changes to the Disclosure Requirements for Fair Value Measurement (Topic 820), which changes the fair value measurement disclosure requirements of ASC 820. This ASU removes certain disclosure requirements regarding the amounts and reasons for transfers between Level 1 and Level 2 of the fair value hierarchy and the policy for timing of transfers between the levels. This ASU also adds disclosure requirements regarding unrealized gains and losses included in Other Comprehensive Income for recurring Level 3 fair value measurements and the range and weighted average of unobservable inputs used in Level 3 fair value measurements. ASU 2018-13 is effective for all entities with fiscal years beginning after December 15, 2019, including interim periods therein. Early adoption is permitted for any eliminated or modified disclosures upon issuance of ASU 2018-13. The Company is currently evaluating the impact of adopting this standard.
Recently Adopted Accounting Standards
On May 28, 2014, the Financial Accounting Standards Board ("FASB") issued ASU No. 2014-9, Revenue from Contracts with Customers, which requires an entity to recognize the amount of revenue to which it expects to be entitled for the transfer of promised goods or services to customers. The ASU replaced most existing revenue recognition guidance in U.S. GAAP when it became effective. In August 2015, the FASB issued ASU No. 2015-14, Revenue from Contracts with Customers (Topic 606): Deferral of the Effective Date, which delayed the effective date of the new standard from January 1, 2017 to January 1, 2018. The FASB also agreed to allow entities to choose to adopt the standard as of the original effective date. This ASU permits the use of either the retrospective or cumulative effect transition method. The Company elected the modified retrospective approach for adoption. The Company had previously deferred recognition of revenue and the related cost of goods sold on shipments of V-Go until a customer's right of return no longer existed, which was once the Company received evidence that the product had been distributed to patients based on analysis of third-party information. Upon adoption, management determined that the variable consideration associated with rebates, chargebacks and other discounts could be estimated and that there were no return estimate constraints for which it was considered probable that a subsequent change in the estimate would result in a significant revenue reversal under ASU 2014-9. Accordingly, the Company no longer utilized the “sell through” approach to recognition and accelerated the recognition of revenue upon sale to the distributor. The variable consideration associated with sales returns was estimated based on the probability weighted expected value approach. The Company adopted this accounting pronouncement on January 1, 2018. See table above in 'Change in Accounting Principle' for the impact on the Company's financial statements.
In January 2016, the FASB issued ASU No. 2016-01, Financial Instruments-Overall (Subtopic 825-10): Recognition and Measurement of Financial Assets and Financial Liabilities. This ASU addresses certain aspects of recognition, measurement, presentation and disclosure of financial instruments. This ASU is effective for fiscal years beginning after December 15, 2017,
including interim periods within those fiscal years. The adoption did not have a material impact on the Company's financial statements.
In August 2016, the FASB issued ASU No. 2016 -15, Statement of Cash Flows (Topic 230): Classification of Certain Cash Receipts and Cash Payments that addresses eight specific cash flow presentation and classification issues with the objective of reducing the existing diversity in practice. This amendment is effective for the Company in the fiscal year beginning after December 15, 2017, but early adoption is permissible. The Company's adoption of the standard did not have a material impact on the financial statements.
4. INVENTORY
Inventory, net consists of:
|
| | | | | | | | |
| | December 31, | |
| (Dollars in thousands) | 2018 | | 2017 | |
| Raw materials | $ | 1,355 |
| | $ | 1,368 |
| |
| Work in process | 3,110 |
| | 1,972 |
| |
| Finished goods | 2,359 |
| | 4,765 |
| |
| Total | $ | 6,824 |
| | $ | 8,105 |
| |
We state inventories at the lower of first-in, first-out cost, or net realizable value. Stated inventories include material costs, labor and applicable overhead. The Company reviews its inventory for excess or obsolescence and writes down inventory that has no alternative uses to its net realizable value. The inventory reserves for excess and obsolete inventory at December 31, 2018 and 2017 were $0.2 million and $0.3 million, respectively.
5. PROPERTY AND EQUIPMENT
Property and equipment consisted of the following:
|
| | | | | | | | | | |
| | | | December 31, | |
| (Dollars in thousands) | Useful lives (in years) | | 2018 | | 2017 | |
| Machinery and equipment | 5-10 | | $ | 10,712 |
| | $ | 10,552 |
| |
| Computers and software | 3 | | 2,017 |
| | 1,382 |
| |
| Leasehold improvements | 6-10 | | 408 |
| | 425 |
| |
| Office equipment | 5 | | 89 |
| | 89 |
| |
| Furniture and fixtures | 5 | | 187 |
| | 187 |
| |
| Construction in process | | | 1,248 |
| | 135 |
| |
| Total | | | 14,661 |
| | 12,770 |
| |
| Accumulated depreciation | | | (8,564 | ) | | (7,301 | ) | |
| Property and equipment, net | | | $ | 6,097 |
| | $ | 5,469 |
| |
Depreciation expense for the years ended December 31, 2018 and 2017 was $1.3 million and $1.9 million respectively. For the year ended December 31, 2018 the Company recognized a loss of a de minimis amount on the disposal of equipment. For the year ended December 31, 2017, the Company received proceeds and recognized a gain of $0.2 million on the sale of property and equipment, which was previously written off or fully depreciated.
In 2017, the Company evaluated long-lived assets and deemed certain manufacturing lines in China to be impaired. Impairment expense for the year ended December 31, 2017 was $3.7 million.
6. ACCRUED EXPENSE AND OTHER CURRENT LIABILITIES
The Company’s accrued expenses and other current liabilities consisted of the following:
|
| | | | | | | | |
| | December 31, | |
| (Dollars in thousands) | 2018 | | 2017 | |
| Compensation | $ | 3,766 |
| | $ | 2,830 |
| |
| Returns Reserve | 621 |
| | — |
| |
| Marketing services | 251 |
| | 109 |
| |
| Distribution agreements and managed care costs | 2,658 |
| | 1,330 |
| |
| Professional fees | 832 |
| | 926 |
| |
| Franchise taxes | 50 |
| | 53 |
| |
| Travel expenses | 114 |
| | 157 |
| |
| Manufacturing expenses | 132 |
| | 260 |
| |
| Other accruals | 427 |
| | 133 |
| |
| Total accrued expenses and other current liabilities | $ | 8,851 |
| | $ | 5,798 |
| |
7. DEBT
The Company had the following long-term debt, net of issuance costs outstanding:
|
| | | | | | | | |
| | December 31, | |
| (Dollars in thousands) | 2018 | | 2017 | |
| Senior secured debt | $ | 25,000 |
| | $ | 25,000 |
| |
| Payment-in-kind (PIK) interest | 11,758 |
| | 7,929 |
| |
| Issuance costs | (104 | ) | | (125 | ) | |
| Total senior secured debt, net | 36,654 |
| | 32,804 |
| |
| Other note payable | 2,500 |
| | 2,500 |
| |
| Payment-in-kind (PIK) interest | 1,038 |
| | 705 |
| |
| Total other note payable | 3,538 |
| | 3,205 |
| |
| Total debt | $ | 40,192 |
| | $ | 36,009 |
| |
Senior Secured Debt
On May 23, 2013, the Company entered into the Term Loan of $50.0 million with Capital Royalty Group (“CRG”), structured as a senior secured loan with a six-year term (the “Term Loan” or the “Senior Secured Debt”). The Term Loan is secured by substantially all of the Company’s assets, including its material intellectual property. The Company later entered into a series of forbearance agreements to modify the Term Loan. The terms of the most recently executed amendment of the Term Loan agreement, dated February 9, 2017, bears interest at 11% per annum and allows the interest-only period of the Term Loan to extend to March 31, 2022, requires quarterly cash interest payments beginning June 30, 2019, extends the deadline for full payment under the Term Loan agreement to March 31, 2022 and brings the Company’s minimum covenant cash and cash equivalent requirements to $2.0 million. As of December 31, 2018, the Company was in compliance with the financial covenant in the restructured Term Loan agreement, as the Company held cash and cash equivalents of $47.8 million, which includes $0.5 million of restricted cash.
Other Note Payable
In 2011, the Company issued a $5.0 million senior subordinated note, or the WCAS Note (the "Other Note Payable") to WCAS Capital Partners IV, L.P., or WCAS. The Company later entered into a series of forbearance agreements to modify the WCAS note. The terms of the most recently executed amendment of the WCAS note, dated May 23, 2013, bears interest at 12% per annum, and all interest accrues as compounded PIK interest and is added to the aggregate principal amount of the loan semi-annually. The outstanding principal amount of the note, including accrued PIK interest, is due in full in September 2021. No interest payments are required during the term of the loan. The Company may pay off the WCAS Note at any time without penalty.
On March 28, 2017, $25.0 million and $2.5 million of the Term Loan and WCAS Note, respectively, were converted to preferred shares upon completion of the public offering at a conversion rate of $10 per share. CRG and WCAS received 2,500,000 and 250,000 preferred shares, respectively. Concurrent with the debt restructure due to the public offering, the
remaining principal balance of $2.5 million of the WCAS note was amended to decrease the interest rate to 10% per annum, payable entirely as PIK interest.
During the years ended December 31, 2018 and 2017, the Company incurred non-cash interest expense of $4.2 million and $4.4 million, respectively.
8. WARRANTS
Liability Classified Warrants
The Company issued 10,390 warrants to acquire shares of its common stock to the agents in the private placement offering that was conducted as part of the 2016 Merger (the “PPO”). The warrants have a term of five years. The warrants are accounted for as a derivative liability at fair value, as the warrant exercise price is subject to adjustment upon additional issuances of equity securities at a price per share lower than the exercise price of the warrants.
The fair value of the warrants at the date of issuance was $0.3 million. As of December 31, 2018 and December 31, 2017, the fair value of the warrants was estimated to be a de minimis amount based on the Black Scholes option pricing model. Key assumptions used to apply this model were as follows:
|
| | | | | | | | | | |
| | May 3, 2016 | December 31, 2017 | December 31, 2018 | |
| Dividend yield | — |
| — |
| — |
| |
| Expected volatility | 80.0 | % | 69.0 | % | 92.5 | % | |
| Risk-free rate of return | 1.22 | % | 2.20 | % | 2.48 | % | |
| Expected term (years) | 5.0 |
| 3.3 |
| 2.3 |
| |
| Fair Value per share | $ | 25.60 |
| $ | 0.09 |
| $ | 0.02 |
| |
The activities of the liability classified common stock warrants are as follows:
|
| | | | | | | | | |
| | Number of shares | | Weighted average exercise price | | Weighted average remaining life | |
| Outstanding and exercisable—December 31, 2017 | 10,390 |
| | $ | 16.22 |
| | 3.3 years | |
| Warrants exercised | — |
| | — |
| | | |
| Outstanding and exercisable—December 31, 2018 | 10,390 |
| | $ | 0.44 |
| | 2.3 years | |
During the years ended December 31, 2018 and December 31, 2017, the Company sold shares of its common stock in underwritten public offerings. Pursuant to the terms of the warrants issued, the exercise price of the warrants was reduced as a result of the offerings.
Equity Classified Warrants
On November 16, 2018, the Company completed a public offering of 75,000,000 shares of common stock at a purchase price of $0.48 per share and received aggregate net proceeds of approximately $32.5 million. Included in the purchase of each share of common stock was one Series A warrant and one Series B warrant. Each Series A warrant has an exercise price of $0.60. The Series A warrants are exercisable commencing from the date of their issuance and will expire five years from the date of issuance. Each Series B warrant has an exercise price of $0.48. The Series B warrants are exercisable commencing from the date of their issuance and will expire nine months from the date of issuance. As of December 31, 2018, no warrants have been exercised.
The activities of the equity classified common stock warrants are as follows:
|
| | | | | | | | | | |
| | Number of shares | | Weighted average exercise price | | Weighted average remaining life | |
| Outstanding and exercisable—December 31, 2017 | — |
| | $ | — |
| | — |
| |
| Warrants issued in conjunction with public share offering | 150,000,000 |
| | 0.54 |
| | 2.9 years |
| |
| Warrants exercised | — |
| | — |
| | — |
| |
| Outstanding and exercisable—December 31, 2018 | 150,000,000 |
| | $ | 0.54 |
| | 2.8 years |
| |
9. FAIR VALUE MEASUREMENTS AND FAIR VALUE OF FINANCIAL INSTRUMENTS
Fair Value Measurements
The Company’s financial instruments consist primarily of cash and cash equivalents, accounts receivable, accounts payable and accrued liabilities, debt instruments and derivative liabilities. For accounts receivable, accounts payable and accrued liabilities, the carrying amounts of these financial instruments as ofDecember 31, 2018 and 2017 were considered representative of their fair values due to their short term to maturity. Cash equivalents are carried at cost which approximates their fair value. Debt is carried at its principal balance, plus accrued interest, which approximates its fair value. Debt would be considered a level 2 measurement. The Company’s derivative liabilities are classified within Level 3 because they are valued with an option pricing model, where certain inputs to the model are unobservable and reflect the Company’s assumptions as to what market participants would use.
10. RELATED PARTY TRANSACTIONS
On September 8, 2011, the Company issued the WCAS Note (see note 7). Certain affiliates of WCAS are also common stock shareholders as of December 31, 2018.
On May 23, 2013, the Company entered into the Term Loan with CRG (see note 7). CRG is also a common stock and preferred stock shareholder as of December 31, 2018.
On March 28, 2017, CRG participated in the Company's initial public offering of common stock, in which it acquired 4,000,000 shares for $40.0 million.
On November 16, 2018, CRG participated in the Company's public offering of common stock, and acquired 15,000,000 shares for $7.2 million. Concurrent with the acquisition of public stock, CRG acquired 15,000,000 Series A warrants and 15,000,000 Series B warrants.
CRG held an aggregate of 20,185,968 and 5,185,968 shares of the Company's common stock at December 31, 2018 and 2017, respectively.
During the year ended December 31, 2017, CRG and WCAS converted debt balances of $25.0 million and $2.5 million, respectively, into 2,500,000 and 250,000 shares of the Company's Series A Convertible Preferred Stock, respectively. At the time of the debt restructuring, $0.1 million of remaining debt issuance costs was extinguished and recorded against equity as the lender is also a shareholder of the Company.
11. STOCKHOLDERS’ EQUITY (DEFICIT)
The Company's capital structure consists of 300,000,000 shares of common stock, par value $0.001 per share and 50,000,000 shares of blank check preferred stock.
On March 28, 2017, the Company closed its initial public offering of 5,250,000 shares of common stock at a purchase price of $10.00 per share, for proceeds of approximately $48.8 million, net of financing costs. Existing investors of the Company invested $40.0 million in the public offering. On March 28, 2017, $25.0 million and $2.5 million of the Term Loan and WCAS Note, respectively, were converted to preferred shares, and as a result, CRG and WCAS received 2,500,000 and 250,000 of the Company's Series A Convertible Preferred Stock, respectively (see note 7).
On January 7, 2018, the Company entered into the First Purchase Agreement with Aspire Capital, pursuant to which, the Company has the right, in its sole discretion, to present Aspire Capital with a purchase notice, directing Aspire Capital (as principal) to purchase up to 50,000 shares of the Company’s common stock per business day, in an aggregate amount of up to $20.0 million of the Company’s common stock (the "Purchase Shares") over the 30-month term of the Purchase Agreement at a per share price equal to the fair market valuelesser of ourthe lowest sale price of the Company’s common stock on the purchase date; or the arithmetic average of the three lowest closing sale prices for the Company’s common stock during the ten consecutive trading days ending on the trading day immediately preceding the purchase date. The Company sold an aggregate of 1,250,868 shares of its common stock (which represents 19.99% of the Company’s outstanding shares of common stock on the date it entered into the First Purchase Agreement) and received net proceeds of grant,$1.8 million. In 2017, the Company issued 125,000 shares of common stock issued to Aspire Capital as determinedconsideration for entering into the First Purchase Agreement. The First Purchase Agreement was terminated in June 2018.
On January 26, 2018, the Company entered into the Sales Agreement with B. Riley FBR (FBR) with respect to an at the market offering program, under which the Company may, from time to time in its sole discretion, issue and sell through FBR, acting as agent, the Placement Shares. FBR has the option to decline any sales orders at its discretion. The issuance and sale, if any, of the Placement Shares by the plan administrator, and may be intended to qualify as incentive stock optionsCompany under the Internal Revenue CodeAgreement will be made pursuant to a prospectus supplement to the Company’s registration statement on Form S-3, originally filed with SEC on October 4, 2017, and declared effective by the SEC on December 15, 2017. As of 1986,December 31, 2018, the Company has not sold any shares under this Sales Agreement.
The Company completed a public offering on April 26, 2018 in which it sold 13,700,000 shares and received aggregate net proceeds of approximately $21.8 million, after deducting underwriting discounts and commissions of $1.8 million and offering expenses of $0.4 million. On May 2, 2018, the underwriters in the public offering exercised a portion of their 30-day option to purchase additional shares of the Company's common stock, as amended.a result of which the Company sold 1,600,000 shares and received additional net proceeds of approximately $2.6 million, after deducting underwriting discounts of $0.2 million.
In connectionJune 2018, the Company entered into a purchase agreement (the "Second Purchase Agreement") with our corporate mergerAspire Capital, pursuant to which, the Company has the right, in 2016,its sole discretion, to present Aspire Capital with a purchase notice, directing Aspire Capital (as principal) to purchase up to150,000 shares of the Company’s common stock per business day, in an aggregate amount of up to $21.0 million of the Company’s common stock (the "Purchase Shares") over the thirty month term of the Second Purchase Agreement at a per share price equal to the lesser of the lowest sale price of the Company’s common stock on the purchase date; or the 2016 Merger, we adoptedarithmetic average of the Valeritas Holdings, Inc. 2016 Equity Incentive Compensation Plan, orthree lowest closing sale prices for the 2016 Plan, and options held byCompany’s common stock during the named executive officers toten consecutive trading days ending on the trading day immediately preceding the purchase date. The Company may sell an aggregate of 4,726,383 shares of Valeritasits common stock (which represented 19.99% of the Company’s outstanding shares of common stock on June 11, 2018) without stockholder approval. The Company may sell additional shares of its common stock above the 19.99% limit provided that (i) it obtains stockholder approval or (ii) shareholder approval has not been obtained at any time the 4,726,383 share limitation is reached and at all times thereafter the average price paid for all shares issued under the Purchase Agreement, including the 263,852 shares of common stock issued to Aspire Capital as consideration for entering into the Purchase Agreement (the “Commitment Shares”) and the 791,557 shares purchased on the date of the agreement (the "Initial Purchase Shares"), is equal to or greater than $1.62 (the “Minimum Price”), which was the consolidated closing bid price of the Company’s common stock on the date it entered into the Second Purchase Agreement. In addition to these restrictions, the Company is prohibited from selling shares to Aspire under the Purchase Agreement at a price per share less than $1.00.
As of December 31, 2018, the Company has issued 1,205,409 shares of common stock under the 2014 Plan were canceled. For additional information aboutSecond Purchase Agreement, including the 2016 Plan, seeInitial Purchase Shares for net proceeds of approximately $1.3 million.
On November 16, 2018, the section entitled “2016 Incentive Compensation Plan” below.
From time to time, our boardCompany completed a public offering of directors may also construct alternate vesting schedules as it determines are appropriate to motivate particular employees. Stock options granted to our key employees may be subject to accelerated vesting in certain circumstances, including as described below for the named executive officers75,000,000 shares of common stock at a purchase price of $0.48 per share and received aggregate net proceeds of approximately $32.5 million, after deducting underwriting discounts and commissions of $2.7 million and offering expenses of $0.8 million. Included in the section entitled “Severance and Change in Control Benefits.”
In February 2018, our compensation committee recommended to our boardpurchase of directors, and our boardeach share of directors approved, an option grant to each named executive officer to purchase shares of our common stock under the 2016 Plan as compensation for such officer’s performance during calendar year 2017. The options were granted on February 13, 2018 withwas one Series A warrant and one Series B warrant. Each Series A warrant has an exercise price equal toof $0.60. The Series A warrants are exercisable commencing from the closingdate of their issuance and will expire five years from the date of issuance. Each Series B warrant has an exercise price of our common stock$0.48. The Series B warrants are exercisable commencing from the date of their issuance and will expire nine months from the date of issuance.
On December 31, 2018, we received a written notification from the Listing Qualifications Department of the Nasdaq Stock Market, or Nasdaq, indicating that we were not in compliance with Nasdaq Listing Rule 5550(a)(2) for continued listing on the Nasdaq Capital Market onbecause our minimum bid price was less than $1.00 per share for 30 consecutive business days. The notification letter provided that the Company had 180 calendar days, or until July 1, 2019 to regain compliance with Nasdaq Listing Rule 5550(a)(2). To regain compliance, the bid price of the Company's common stock must have a closing bid price of at least $1.00 per share for a minimum of 10 consecutive business days. If the Company does not regain compliance by July 1, 2019, an additional 180 days may be granted to regain compliance, so long as the Company meets The Nasdaq Capital Market continued listing requirements (except for the bid price requirement) and notifies Nasdaq in writing of its intention to cure the deficiency during the second compliance period, by effecting a reverse stock split, if necessary.
Preferred Stock
Shares of preferred stock may be issued from time to time in one or more series, each of which will have such distinctive designation or title as shall be determined by the Company's Board of Directors prior to the issuance of any shares thereof. The number of authorized shares of preferred stock may be increased or decreased (but not below the number of shares thereof then outstanding) by the affirmative vote of the holders of a majority of the voting power of all the then outstanding shares of the Company's capital stock entitled to vote.
On February 14, 2017, the Company entered into an agreement with CRG and WCAS to convert a total of $27.5 million of the outstanding principal amount of the Company's debt, including the Term Loan, into shares of Series A Convertible Preferred Stock
at the public offering price. The shares of Series A Preferred Stock are convertible at the option of the holder at any time into shares of the Company's common stock at a conversion rate determined by dividing the Series A Original Issue Price by the Series A Conversion Price (both as defined in the Certificate of Designation) in effect at the time of conversion. This formula initially results in a one-to-one conversion ratio, but may change in the future. The Series A Conversion Price is subject to adjustment for stock splits and the like subsequent to the date of grant, and vest as follows: 25%issuance of the Series A Preferred Stock. On or after January 1, 2021, at the Company's option, if the Company has achieved an average market capitalization of at least $300 million for the Company's most recent fiscal quarter, the Company may elect to automatically convert all of the outstanding shares underlying each option will vest one year after date of grant withSeries A Preferred Stock into shares of the remaining vesting in substantially equal quarterly installments for 10 quarters thereafter. Company's common stock.
The numberholders of shares underlyingof Series A Preferred Stock are entitled to receive cumulative annual dividends at a rate of $8 per every $100 of Series A Preferred Stock, payable either in cash or in shares of the Company's common stock, at each option is set forthholder’s election; provided, that to the extent any holder elects to receive cash dividends, such dividends shall accrue from day to day and be payable only upon a Deemed Liquidation Event (as defined in the table below.Certificate of Designation). The shares of Series A Preferred Stock will have no voting rights. The Company has the right to redeem all or less than all of the Series A Preferred Stock, at any time, at a price equal to the Series A Conversion Price, as adjusted, plus any accrued but unpaid dividends. In the event of a Deemed Liquidation Event the holders of Series A Preferred Stock are eligible to receive the greater of (i) $27.5 million, plus accrued but unpaid dividends or (ii) what they would have received as a holder of common stock had they converted their shares of Series A Preferred Stock into shares of the Company's common stock immediately prior to the Deemed Liquidation Event. To the extent permitted under Delaware law, the holders of shares of Series A Preferred Stock have the right to prevent the Company from liquidating, dissolving, amending the Company's governing documents in a manner that affects the rights of the Series A Preferred Stock, authorizing shares of capital stock on parity or senior to the Series A Preferred Stock, or issuing any shares of Series A Preferred Stock to any individual, entity or person other than CRG or WCAS.
Equity Compensation Plans
Total stock-based compensation expense related to stock options, restricted stock, and the employee stock purchase program was $4.1 million and $5.6 million for the year ended December 31, 2018 and 2017, respectively, and classified in the consolidated statements of operations as follows:
|
| | | |
Name | | Number of
Securities
Underlying
Options (#)
Unexercisable
| |
John E. Timberlake | 100,000 | |
Erick Lucera | 27,000 | |
Geoffrey Jenkins | 25,000 | |
Matt Nguyen | 26,000 | |
|
| | | | | | | | |
| | Year Ended December 31, | |
| (Dollars in thousands) | 2018 | | 2017 | |
| Cost of goods sold | $ | 108 |
| | $ | 203 |
| |
| Research and development expenses | 792 |
| | 1,425 |
| |
| General and administrative expenses | 3,157 |
| | 3,955 |
| |
| Total share-based compensation | $ | 4,057 |
| | $ | 5,583 |
| |
No stock options were grantedEmployee Stock Purchase Plan
Under the Employee Stock Purchase Plan (the “ESPP”), which was established in 2017, the Company is authorized to our named executive officers during 2016 or 2017 under the 2014 Plan.
Retirement, Health, Welfare and Additional Benefits
The named executive officers are eligible to participate in our employee benefit plans and programs, including medical and dental benefits, flexible spending accounts and short- and long-term disability and life insurance, to the same extent as other full-time employees, subject to the terms and eligibility requirements of those plans. The named executive officers are also eligible to participate in a tax qualified 401(k) defined contribution plan to the same extent as other full-time employees. Currently, we match contributions made by participants in the 401(k) planissue up to 2% of the employee contributions, and these matching contributions fully vest on the fifth anniversaryshares of the date on which the contribution is made.
In September 2017, we implemented an Employee Stock Purchase Program, enabling our employees to acquire equity ownership in our company through a payroll deduction program.
Outstanding Equity Awards as of December 31, 2017
The following tables summarize theits capital stock outstanding equity awards held by the named executive officers as of December 31, 2017 which consists of company options and restricted stock.
|
| | | | | | | | | | | | | | | |
| Name | Grant Date |
Number of Securities Underlying Options Exercisable (#) | Number of Securities Underlying Options Unexercisable (#) | | | Option Exercise Price ($) | | | Option Expiration Date |
| John E. Timberlake | 5/3/2016 | 23,921 |
| 20,834 | | (1) | | | 5.00 | | | | 5/3/2026 |
| John E. Timberlake | 3/23/2017 | 60,938 |
| 126,562 | | (2) | | | 7.12 | | | | 3/23/2027 |
| Erick Lucera | 9/7/2016 | 4,492 |
| 9,883 | | (3) | | | 5.36 | | | | 9/7/2026 |
| Erick Lucera | 3/23/2017 | — |
| 73,800 | | (6) | | | 7.12 | | | | 3/23/2027 |
| Geoffrey Jenkins | 5/3/2016 | 18,457 |
| 4,855 | | (4) | | | 5.00 | | | | 5/3/2026 |
| Geoffrey Jenkins | 3/23/2017 | 50,625 |
| 84,375 | | (5) | | | 7.12 | | | | 3/23/2027 |
| Matt Nguyen | 5/3/2016 | 6,732 |
| 6,018 | | (1) | | | 5.00 | | | | 5/3/2026 |
| Matt Nguyen | 3/23/2017 | 26,488 |
| 55,012 | | (2) | | | 7.12 | | | | 3/23/2027 |
| | |
| (1) | 33% of shares underlying option vested one year from the date of grant. The remaining shares vest in substantially equal monthly installments for 24 months thereafter. |
| (2) | 25% of shares underlying option vested six months from the date of grant. The remaining shares vest in substantially equal quarterly installments for 10 quarters thereafter. |
| (3) | 25% of shares underlying option vested one year from the date of grant. The remaining shares vest in substantially equal monthly installments for 36 months thereafter. |
| (4) | 50% of shares underlying option vested one year from the date of grant. The remaining shares vested in substantially equal monthly installments for 12 months thereafter. |
| (5) | 25% of shares underlying options vested six months from the date of grant. The remaining shares vest in substantially equal quarterly installments for 6 quarters thereafter. |
| (6) | 25% of shares underlying option vested six months from the date of grant. The remaining shares vest in substantially equal quarterly installments for 12 quarters thereafter. |
March 2015 Employment Agreements
Valeritas entered into a new employment agreement with each of Ms. Peterson, Mr. Timberlake, and Mr. Jenkins on March 4, 2015.
Ms. Peterson’s agreement was terminated upon her resignation, although Mr. Jenkins’ agreement is still effective after the 2016 Merger. Mr. Timberlake’s agreement was first amended in February 2016, whereby he was appointed as Interim Chief Executive Officer while retaining his titles of President and Chief Commercial Officer upon the resignation of Ms. Peterson, and this agreement was later amended to appoint him as Chief Executive Officer while retaining his titles of President and Chief Commercial Officer as of May 3, 2016. In December 2016, Mr. Nguyen replaced Mr. Timberlake2017. The purchase price of the stock will not be less than 85% of the lower of (i) the fair market value per share of the Company's common stock on the start date of the offering period or (ii) the fair market value on the purchase date. The fair market value per share of the Company’s common stock on any particular date under the ESPP will be the closing selling price per share on such date on the national stock exchange serving as Chief Commercial Officer. These agreements have, or had, in the case of Ms. Peterson, initial terms of three years and automatically renewprimary market for successive one-year periods following the initial term unless either party givesCompany’s common stock at least 30 days advance written notice of non-renewal prior tothat time (or if there is no closing price on such date, then the endclosing selling price per share on the last preceding date for which such quotation exists). Fair value is determined based on two factors: (i) the 15% discount amount on the underlying stock’s market value on the first day of the applicable term.offering period, and (ii) the fair value of the look-back feature determined by using the Black-Scholes model.
The agreements entitled Ms. Peterson priorCompany recognized $0.1 million of compensation expense for the years ended December 31, 2018 and December 31, 2017.
Shares of the Company's common stock will be offered for purchase under the ESPP through a series of successive offering periods. Each offering period will be comprised of one or more successive 6-month purchase intervals, unless determined otherwise by the plan administrator. On the start date of each offering period, each participant will be granted a purchase right to her resignation and entitle Mr. Timberlake and Mr. Jenkins to initial annual base salariesacquire shares of $450,639, $392,141 and $358,829, respectively, and annual target bonus opportunities of 50%, 50% and 35%, respectively, of their annual base salaries, with the amount of any such bonus basedCompany’s common stock on the levellast day of attainmenteach purchase interval during that offering period. As of performance goals established by our boardDecember 31, 2018 purchase rights of directors.154,379 shares have been requested and 154,379 shares have been granted under the ESPP. At December 31, 2017, no purchase rights had been requested and no shares had been granted under the ESPP.
In the event an NEO’s employment terminates on accountfourth quarter of death or disability,2018, the NEO (orCompany suspended the NEO’s estate in the case of death) is entitled to receive 3 months of base salary continuation.
Employment Agreements
Mr. Lucera
Employee Stock Purchase Plan. The employment agreement with Mr. Lucera was entered into on August 29, 2016 in connection with his appointment as our Chief Financial Officer. The agreement provides for an initial annual base salary of $260,000, subject to adjustment pursuant to Valeritas’Company will be refunding all remaining employee compensation policies in effect from time to time, and an annual target bonus opportunity of 35% of Mr. Lucera’s base salary based upon achievement of mutually agreed goals for each year.
If Mr. Lucera is terminated without cause or resigns for good reason, provided that he has timely executed a release of claims, he is entitled to receive, among other things, 9 months of base salary continuation and payment of a portion of COBRA premiums for up to 9 months of continued health insurance coverage, based on active employee cost-sharing rates.
Mr. Nguyen
The employment agreement with Mr. Nguyen was entered into on December 20, 2016 in connection with his appointment as our Chief Commercial Officer. The agreement provides for an initial annual base salary of $287,000, subject to adjustment pursuant to Valeritas’ employee compensation policies in effect from time to time, and an annual target bonus opportunity of 35% of Mr. Nguyen’s base salary based upon achievement of mutually agreed goals for each year.
If Mr. Nguyen is terminated without cause or resigns for good reason, provided that he has timely executed a release of claims, he is entitled to receive, among other things, 9 months of base salary continuation and payment of a portion of COBRA premiums for up to 9 months of continued health insurance coverage, based on active employee cost-sharing rates.
Severance and Change in Control Benefits
Unless otherwise set forth in the applicable employment agreement, in the event we terminate an NEO’s employment without cause or the NEO resigns for good reason (other than in connection with a change in control or due to death or disability), the NEO is entitled to receive (i) 9 months of base salary continuation; (ii) a pro-rated portion of the annual bonus the NEO would otherwise have earned for the year of termination, based on actual performance for the full year and payable when such bonus would have otherwise been paid; (iii) any annual bonus earned but not yet paid for the completed fiscal year immediately priorwithholding pertaining to the termination date; and (iv) reimbursement for the our cost of providing continued health coverage for a period of 9 months or until the NEO is offered benefits from a subsequent employer, if earlier.
In the event we terminate an NEO’s employment without cause or the NEO resigns for good reason (other than due to death or disability), in either case, within 3 months prior to a change in control (but within the “pre-closing period” described below) or within 12 months following a change in control, the NEO is entitled to receive (i) 12 months of base salary continuation; (ii) a pro-rated portion of the NEO’s target annual bonus for the year of termination; (iii) any annual bonus earned but not yet paid for the completed fiscal year immediately prior to the termination date; and (iv) reimbursement for the our cost of providing continued health coverage for a period of 12 months or until the NEO is offered benefits from a subsequent employer, if earlier. “Pre-closing period” means the period commencing with our execution of a definitive agreement for a change in control transaction and ending on the earlier to occur of the closing of the change in control or the termination of such definitive agreement without the consummation of the change in control.
The severance payments and benefits described above are subject to the NEO timely executing a release of claims in our favor (except in the event of the NEO’s death) and to reduction in the event that the payments and benefits received in connection with a change in control would result in the imposition of excise taxes under Section 4999 of the Code and such reduction results in the NEO retaining a greater after-tax amount.
“Cause” is generally defined in the employment agreements as the NEO’s (i) misappropriation of funds with respect to our company or affiliates; (ii) material violation of the employment agreement or the company’s employment policies; (iii) breach of any written confidentiality, nonsolicitation or noncompetition covenant with our company or affiliates; (iv) conviction of a felony; or (v) misconduct that has a material adverse effect on the business, operations, assets, properties, or financial condition of our company or affiliates.
“Good reason” is generally defined in the employment agreements as the occurrence of any of the following, subject to notice requirements and cure rights, without the NEO’s written consent (i) a material diminution in duties, authority or responsibilities; (ii) relocation of the NEO’s principal office location to a location more than 50 miles from the NEO’s principal
office location immediately before the change; (iii) a material diminution by the company of the NEO’s base salary or target annual bonus; or (iv) any material breach by the company of the employment agreement.
The employment agreements contain restrictive covenants pursuant to which each NEO has agreed to refrain from competing with us or soliciting our clients, customers or employees, in each case, while employed and following the NEO’s termination of employment for a period of 9 months or 12 months if such termination is in connection with a change in control.
Long-term Incentive Plans
The following summarizes the material terms of the long-term incentive plans in which our employees, including the NEOs, participate. The summaries below are not intendedESPP. Amounts to be a complete description of the long-term incentive plansrefunded to employees are included in accrued expense and are qualified in their entirety by the actual text of the plans to which reference is made.other current liabilities at December 31, 2018.
2016 IncentiveEmployee Equity Compensation Plan
We adoptedThe Company's 2016 Equity Incentive Compensation plan (the “2016 Plan”) was established concurrently with the 2016 Plan inMerger on May 2016 upon the closing of the 2016 Merger.3, 2016. The 2016 Plan permits usthe Company to grant cash, stock and stock-based awards to eligible service providers.its employees, consultants and directors. The 2016 Plan is intended to promote our interests by providing eligible service providers with the opportunity to participate in incentive compensation programs designed to encourage their continued service to us.
Eligibility and Administration
Our employees, consultants and directors, and employees, consultants and directors of our parent or subsidiaries are eligible to receive awards under the 2016 Plan. The 2016 Plan is administered by a committee of two or more non-employee directors who qualify as independent under applicable stock exchange rules, 162(m) of the Internal Revenue Code and the Securities Exchange Act of 1934, except that the board of directors may ratify or approve any awards as it deems appropriate and the board of directors administers the 2016 Plan with respect to awards made to non-employee directors. The committee may delegate authority to one or more subcommittees. The particular entity administering the plan is referred to in this summary as the plan administrator.
The plan administrator has the authority (subject to the provisions of the 2016 Plan) to establish such rules and regulations as it may deem appropriate for proper administration of the 2016 Plan and to make such determinations under, and issue such interpretations of, the provisions of the 2016 Plan and any outstanding awards thereunder as it may deem necessary or advisable. The plan administrator also sets the terms and conditions of all awards under the 2016 Plan, including any vesting and vesting acceleration conditions. Decisions of the plan administrator are final and binding on all parties having an interest under the 2016 Plan or any award thereunder.
Nothing in the 2016 Plan confers on the participant any right to continue in service for any period of time, or restricts our right, or the right of the participant, to terminate such person’s service at any time for any reason.
At December 31, 2017, an aggregate of 2,116,007 shares of common stock were allowed to be issued under the 2016 Plan, 47,658 of which were issued as voting and fully vested, restricted stock, 1,661,750 of which were issued as options, each to certain of our employees and officers, and 406,599 remained available for issuance under awards granted pursuant to the 2016 Plan. The number of shares available for issuance will be automatically increased on the first trading day in January of each calendar year during the term of the 2016 Plan by an amount equal to 4% of the shares of common stock outstanding on the final trading day of the immediately preceding calendar year, subject to an annual increase limit of 1,058,003 shares. As a result, the amount of shares available for issuance under the 2016 Plan automatically increased to 686,910 shares effective as of January 1, 2018. No more than 2,116,007 shares of common stock may be issued upon the exercise of incentive stock options, which amount will automatically be increased on the first trading day in January each calendar year by the number of shares of our common stock added to the share reserve on that day pursuant to automatic share increase feature of the 2016 Plan. Shares issued under the 2016 Plan may be authorized but unissued or reacquired shares, or shares purchased in the open market.
If an award under the 2016 Plan expires, terminates or is forfeited or cancelled, any shares subject to such award may, to the extent of such expiration, termination, forfeiture or cancellation, be used again for new grants under the 2016 Plan. If the exercise price of an option granted under the 2016 Plan is paid with shares of common stock, then the number of shares available for issuance under the 2016 Plan will be reduced by the net number of shares issued under the exercised option and not by the gross number of shares for which the option was exercised. Upon the exercise of a stock appreciation right under the 2016 Plan, the number of shares available for issuance under the 2016 Plan will be reduced by the net number of shares issued under the stock appreciation right and not by the gross number of shares for which the stock appreciation right was exercised. If shares of common stock are withheld in satisfaction of withholding taxes incurred in connection with the issuance, exercise or
vesting of an award, then the authorized number of shares available for issuance under the 2016 Plan will be reduced by the net number of shares issued, exercised or vested under such award.
The 2016 Plan imposes the following limitations on the size of the awards which may be made on a per participant basis:
No one person may receive stock options and stand-alone stock appreciation rights for more than 1,058,003 shares of our common stock in the aggregate per calendar year.
No one person may receive direct stock issuances or stock-based awards (other than stock options and stand-alone stock appreciation rights) for more than 1,058,003 shares of our common stock in the aggregate per calendar year.
The maximum dollar amount for which a participant may receive awards denominated in dollars and subject to one or more performance measures will be limited to $3,000,000 in the aggregate per calendar year within the applicable performance measurement period.
Awards
The 2016 Plan is divided into three incentive programs, which includeincludes (i) the discretionary grant program under which eligible persons may be granted options, including incentive stock options, or ISOs, and nonqualified stock options, or NSOs, or stock appreciation rights, or SARs; (ii) the stock issuance program under which eligible persons may be issued direct stock, restricted stock awards, restricted stock units, performance shares or other stock-based awards; and (iii) the incentive bonus program under which eligible persons may be issued performance unit awards, dividend equivalent rights or cash incentive awards.
Certain awards under the 2016 Plan may constitute or provide for a deferral of compensation, subject to Section 409A of the Internal Revenue Code, which may impose additional requirements on the terms and conditions of such awards. Awards under the 2016 Plan will be set forth in award agreements, which will detail the terms and conditions of the awards, including any applicable vesting and payment terms and post-termination exercise limitations. Awards other than cash awards generally will be settled in shares of our common stock, but the plan administrator may provide for cash settlement of any award. A brief description of each award type available for issuance under each of the discretionary grant program, stock issuance program and incentive bonus program follows.
Discretionary Grant Program
Stock Options. Stock options provide for the purchase of shares of our common stock in the future at an exercise price set on the grant date. ISOs, by contrast to NSOs, may provide tax deferral beyond exercise and favorable capital gains tax treatment to their holders if certain holding period and other requirements of the Internal Revenue Code are satisfied. ISOs may only be granted to our employees. Anyone eligible to participate in the 2016 Plan may receive an award of NSOs. The exercise price of a stock option may not be less than 100% of the fair market value of the underlying share on the date of grant (or 110% in the case of ISOs granted to certain significant stockholders), except with respect to certain substitute options granted in connection with a corporate transaction. The term of a stock option may not be longer than ten years (or five years in the case of ISOs granted to certain significant stockholders). To the extent that the aggregate fair market value of shares of our common stock, determined on the date of grant, with respect to which ISOs become exercisable for the first time by an option holder during any calendar year exceeds $100,000, such ISOs will be treated as NSOs. The plan administrator may determine the time or times when a stock option is to become exercisable, the vesting schedule (if any) applicable to a stock option and whether a granted stock option is an ISO or NSO. In general, an option may only be exercised while an option holder is employed by, or providing service to, us or our subsidiaries, unless provided otherwise in the option holder’s award agreement. An option holder may exercise an option by delivering notice of exercise to us. The option holder will pay the exercise price (in the form as provided in the 2016 Plan and the award agreement) and any withholding taxes for the option. The Plan Administrator will have the authority to effect, at any time and from time to time, with the consent of the affected option holders, the cancellation of any or all outstanding options under the 2016 Plan and to grant in substitution therefore new options covering the same or different number of shares with an exercise price per share based on the fair market value per share on the new option grant date.
SARs. Two types of SARs are authorized for issuance under the 2016 Plan, tandem SARs and stand-alone SARs. Tandem SARs entitle their holder to elect to exercise the underlying option in exchange for shares of common stock or, with the consent of the plan administrator, to surrender the option in exchange for an amount equal to the excess of the fair market value of the shares on the date of surrender over the aggregate exercise price of such shares. Stand-alone SARs entitle their holder, upon exercise, to receive from us an amount equal to the excess of the fair market value of the shares on the date of exercise over the aggregate base price of such shares. The base price of a stand-alone SAR will not be less than 100% of the fair market value of the underlying share on the date of grant and the term may not be longer than ten years. The plan administrator may determine the time
or times when a SAR is to become exercisable and the vesting schedule (if any) applicable to a SAR. SARs may be settled in cash, shares of common stock or a combination of the two, as determined by the plan administrator.
Rights as a Stockholder. Participants will not have any stockholder rights with respect to the shares subject to options or SARs until the award vests and the shares are actually issued.
Stock Issuance Program
Restricted Stock, RSUs, Performance Shares and Stock Payments. Restricted stock is an award of nontransferable shares of our common stock that remain forfeitable unless and until specified conditions are met, and which may be subject to a purchase price. RSUs are contractual promises to deliver shares of our common stock in the future, which may also remain forfeitable unless and until specified conditions are met. Delivery of the shares underlying RSUs may be deferred under the terms of the award or at the election of the participant, if the plan administrator permits such a deferral. Performance shares are contractual rights to receive a range of shares of our common stock in the future based on the attainment of specified performance goals, in addition to other conditions which may apply to these awards. Conditions applicable to restricted stock, RSUs and performance shares may be based on continuing service, the attainment of performance goals and/or such other conditions as the plan administrator may determine. Stock payments are awards of fully vested shares of our common stock that may be issued for any of the following items of consideration: cash or check, past services rendered to the company or any other valid consideration.
Rights as a Stockholder. Participants will have full stockholder rights with respect to any shares of stock issued under the stock issuance program, whether or not the participant’s interest in those shares is vested. Accordingly, participants will have the right to vote such shares and to receive any regular cash dividends paid on such shares, subject to any applicable vesting requirements, including (without limitation) the requirement that any dividends paid on shares subject to performance vesting conditions will be held in escrow by us and will not vest or be paid prior to the time those shares vest. Participants will not have any stockholder rights with respect to the shares subject to restricted stock units or share right awards until that award vests and the shares are actually issued. However, dividend equivalents (as described below) may be paid or credited, either in cash or in actual or phantom shares of stock, on outstanding restricted stock unit or share right awards, subject to terms and conditions the plan administrator deems appropriate. No dividend equivalents relating to restricted stock units or share right awards subject to performance vesting conditions will vest or otherwise become payable prior to the time the underlying award (or portion thereof to which the dividend equivalents units relate) vests.
Incentive Bonus Program
Cash Awards and Performance Unit Awards. Cash awards are cash incentive bonuses subject to vesting conditions or performance goals as determined by the plan administrator. Performance unit awards represent the holder’s right to receive cash or participate in a bonus pool, the value of which is tied to the attainment of pre-established corporate objectives and receipt of which may be based on continuing service as determined by the plan administrator.
Dividend Equivalents. Dividend equivalents represent the right to receive the equivalent value of dividends paid on shares of our common stock and may be granted alone or in tandem with awards. Dividend equivalents are credited as of dividend record dates during the period between the date an award is granted and the date such award vests, is exercised, is distributed or expires, as determined by the plan administrator.
Performance Awards
Performance awards include any of the foregoing awards that are granted subject to vesting and/or payment based on the attainment of specified performance objectives that may include but are not limited to: (i) revenue, organic revenue, net sales, or new-product revenue or net sales, (ii) achievement of specified milestones in the discovery and development of the company’s technology or of one or more of the company’s products, (iii) achievement of specified milestones in the commercialization of one or more of the company’s products, (iv) achievement of specified milestones in the manufacturing of one or more of the company’s products, (v) expense targets, (vi) share price, (vii) total shareholder return, (viii) earnings per share, (ix) operating margin, (x) gross margin, (xi) return measures (including, but not limited to, return on assets, capital, equity, or sales), (xii) productivity ratios, (xiii) operating income, (xiv) net operating profit, (xv) net earnings or net income (before or after taxes), (xvi) cash flow (including, but not limited to, operating cash flow, free cash flow and cash flow return on capital), (xvii) earnings before or after interest, taxes, depreciation, amortization and/or stock-based compensation expense, (xviii) economic value added, (xix) market share, (xx) working capital targets, (xxi) achievement of specified milestones relating to corporate partnerships, collaborations, license transactions, distribution arrangements, mergers, acquisitions, dispositions or similar business transactions, and (xxii) employee retention and recruiting and human resources management.
Performance goals may be based upon the attainment of specified levels of performance under one or more of these measures relative to the performance of other entities and may also be based on the performance of any of our business units or divisions or any of our affiliates. Each applicable performance goal may be structured at the time of grant to provide for appropriate adjustments or exclusions for unusual or infrequently occurring items or events, and such other events as set forth in the 2016 Plan.
The plan administrator has the authority, consistent with section 162(m) of the Internal Revenue Code, to structure awards under the stock issuance program so that the awards vest upon the achievement of certain pre-established corporate performance objectives based on one or more of the performance goals described above and measured over the performance period specified by the plan administrator.
Certain Transactions
The plan administrator has broad discretion to take action under the 2016 Plan, as well as to make adjustments to the terms and conditions of existing and future awards, to prevent the dilution or enlargement of intended benefits and facilitate necessary or desirable changes in the event of certain transactions and events affecting our common stock, such as stock dividends, stock splits, mergers, acquisitions, consolidations and other corporate transactions. In addition, in the event of certain non-reciprocal transactions with our stockholders, the plan administrator will make equitable adjustments to the 2016 Plan and outstanding awards. In the event of a change in control of our company (as defined in the 2016 Plan), each outstanding award under the discretionary grant program and stock issuance program may, as determined by the plan administrator, be continued or assumed by the successor entity, be replaced with a cash incentive program of the successor entity or be subject to other limitations as imposed by the plan administrator at the time of grant. However, if the plan administrator determines that any outstanding award is not to be continued, assumed or replaced, such award shall become fully vested and exercisable. Awards under the incentive bonus program may be structured by the plan administrator such that those awards automatically vest upon a change in control of our company or upon the holder’s subsequent termination within a specified period following a change in control and any performance-based vesting conditions may be converted into service-based vesting conditions that will vest upon the completion of a service period coterminous with the portion of the performance period remaining at the time of the change in control. Individual award agreements may provide for additional accelerated vesting and payment provisions as determined by the plan administrator.
No award under the 2016 Plan affects the right of the company to adjust, reclassify, reorganize or otherwise change its capital or business structure or to merge, consolidate, dissolve, liquidate or sell or transfer all or any part of its business or assets.
Foreign Participants, Clawback Provisions, Transferability, and Participant Payments
The plan administrator has authority to adopt and implement from time to time such addenda or subplans to the 2016 Plan as it deems necessary in order to bring the 2016 Plan into compliance with applicable laws and regulations of any foreign jurisdictions in which grants or awards are to be made under the Plan or to obtain favorable tax treatment in those foreign jurisdictions for the individuals to whom the grants or awards are made. All awards will be subject to the provisions of any clawback, recoupment or similar policy implemented by our company to the extent set forth in such policy and/or in the applicable award agreement. With limited exceptions for estate planning, domestic relations orders, certain beneficiary designations and the laws of descent and distribution, awards under the 2016 Plan are generally non-transferable prior to vesting, and are exercisable only by the participant.
With regard to tax withholding, exercise price and purchase price obligations arising in connection with awards under the 2016 Plan, the plan administrator may, in its discretion, accept cash or check, shares of our common stock that meet specified conditions, a market sell order or such other consideration as it deems suitable.
Plan Expiration, Amendment and Termination
The board of directors may amend or terminate the 2016 Plan at any time; however, stockholder approval will be required for any amendment that increases the number of shares available under the 2016 Plan or to the extent such approval is required under applicable law, regulation or stock exchange listing rule. No amendment of the 2016 Plan may adversely affect the rights and obligations of outstanding awards without the award holder’s consent. The 2016 Plan will expire on the first to occur of (i) May 2, 2026, (ii) the date on which allCompany initially had 2,116,004 shares available for issuance under the 2016 Plan shall have been issued as fully vestedPlan. In July, 2018 the Company's shareholders approved an amendment to the 2016 plan resulting in 8,000,000 shares being available for issuance. At December 31, 2018, an aggregate of 647,003 shares are available for issuance.
The options generally vest over a period of two to four years, and options that lapse or (iii) the terminationare forfeited are available to be granted again. The contractual life of all outstandingoptions is ten years from the date the option was granted. The restricted stock awards in connection withvest on the first, second and third anniversaries of the original grant date. The Company recognizes compensation expense on all of these awards on a change in control. Shouldstraight-line basis over the vesting period. The fair value of the award is determined based on the market value of the underlying stock price at the grant date.
Stock Options
2016 Employee Equity Compensation Plan stock option activity for the year ended December 31, 2018 and 2017 was as follows:
|
| | | | | | | | | | | | | | |
| Shares | | Weighted- average exercise price | | Weighted- average contractual life | | Aggregate intrinsic value (dollars in thousands) | |
| Options outstanding at December 31, 2016 | 252,850 |
| | $ | 40.16 |
| | 9.41 years |
| | $ | 37 |
| |
| Granted | 1,452,550 |
| | 7.11 |
| | — |
| | 91 |
| |
| Forfeited / Cancelled | (43,650 | ) | | — |
| | — |
| | — |
| |
| Options outstanding at December 31, 2017 | 1,661,750 |
| | $ | 11.96 |
| | 9.12 years |
| | $ | 75 |
| |
| Granted | 5,799,595 |
| | 0.65 |
| | — |
| | — |
| |
| Forfeited / Cancelled | (96,006 | ) | | 6.16 |
| | — |
| | — |
| |
| Options outstanding at December 31, 2018 | 7,365,339 |
| | $ | 3.13 |
| | 9.44 years |
| | $ | — |
| |
| Options vested and exercisable as of December 31, 2018 | 1,057,817 |
| | 13.33 |
| | 8.01 years |
| | — |
| |
Options vested and expected to vest as of
December 31, 2018 | 7,365,339 |
| | $ | 3.13 |
| | 9.44 years |
| | $ | — |
| |
Share based compensation related to options issued under 2016 Plan was $4.0 million and $3.9 million for the years ended December 31, 2018 and 2017, respectively. The weighted average grant date fair value of options granted under the 2016 Plan terminate on May 2, 2026, then all awards outstanding atduring the years ended December 31, 2018 and 2017 was $0.49 and $4.39, respectively. The total grant date fair value of options that time will continuevested during both the years ended December 31, 2018 and 2017 was $4.0 million and $4.7 million, respectively. There have been no option exercises under the 2016 Plan. As of December 31, 2018 there remained $5.6 million of unrecognized share-based compensation expense related to have force and effect in accordance withunvested stock options issued under the provisions2016 Plan to be recognized as expense over a weighted average period of 1.70 years.
The fair value of the documents evidencingoptions at the awards.
Director Compensation Table
Directors who are employeesdate of us or our principal stockholders have not historically received compensation for their servicesissuance was estimated based on our board of directors. During 2017, certain of our non-employee directors whothe Black Scholes option pricing model. Key assumptions used to apply this model upon issuance were not employees of our principalas follows:
stockholders received annual cash retainers |
| | | | | | | | |
| | Weighted Average on Grant Date | |
| | 2018 | | 2017 | |
| Dividend yield | — |
| | — |
| |
| Expected volatility | 92.54 | % | | 69.01 | % | |
| Risk-free rate of return | 2.87 | % | | 2.05 | % | |
| Expected term (years) | 5.75 |
| | 5.82 |
| |
| Fair Value per share | $ | 0.49 |
| | $ | 4.39 |
| |
Restricted Stock
No shares of $35,000restricted stock were granted in 2018. Shares of restricted stock were granted to third party consultants during 2017 as compensation for their services rendered to the Company. The shares vested upon completion of seven months in service to the Company.
Restricted stock award activity for the year ended December 31, 2018 is as follows:
|
| | | |
| Time-Based Restricted Stock Awards | |
| Non-vested awards outstanding at December 31, 2016 | 48,637 |
| |
| Awards granted | 30,000 |
| |
| Awards vested and issued | (47,658 | ) | |
| Awards forfeited | (979 | ) | |
| Non-vested awards outstanding at December 31, 2017 | 30,000 |
| |
| Awards granted | — |
| |
| Awards vested and issued | (30,000 | ) | |
| Awards forfeited | — |
| |
| Non-vested awards outstanding at December 31, 2018 | — |
| |
Share based compensation related to restricted stock issued under 2016 Plan was a de minimis amount and $1.7 million for the years ended December 31, 2018 and 2017, respectively. The fair value of the restricted stock on our boardthe date of issuance granted during 2017 was estimated to be $0.1 million, and there is no remaining unrecognized compensation related to these awards at December 31, 2018. The non-vested awards outstanding were not issued under the 2016 Plan.
12. INCOME TAXES
Income tax expense attributable to pretax loss from continuing operations differed from the amounts computed by applying the U.S. federal income tax rate of 21% to pretax loss from continuing operations as indicateda result of the following:
|
| | | | | | | | |
| | Year ended December 31, | |
| (Dollars in thousands) | 2018 | | 2017 | |
| Computed “expected” tax expense | $ | (9,645 | ) | | $ | (16,758 | ) | |
| Increase (reduction) in income taxes resulting from: | | | | |
| Change in the valuation allowance | 12,511 |
| | (40,114 | ) | |
| State taxes, net of federal benefit | (2,780 | ) | | 607 |
| |
| Federal research and development credits | (91 | ) | | (236 | ) | |
| Change in Federal Tax Rate |
|
| | 56,105 |
| |
| Other, net | 5 |
| | 396 |
| |
| Total income tax expense/(benefit) | $ | — |
| | $ | — |
| |
The tax effects of temporary differences that give rise to significant portions of the deferred tax assets and deferred tax liabilities at December 31, 2018 and 2017 are presented below:
|
| | | | | | | | |
| | December 31, | |
| (Dollars in thousands) | 2018 | | 2017 | |
| Deferred tax assets: | | | | |
| Intangible assets | $ | 744 |
| | $ | 916 |
| |
| Net operating loss carryforwards | 93,397 |
| | 82,069 |
| |
| Federal and state credit carryforwards | 2,860 |
| | 2,664 |
| |
| Plant and equipment, due to depreciation and impairment | 146 |
| | 182 |
| |
| Inventory reserves | 44 |
| | 219 |
| |
| Non-deductible interest | 2,182 |
| | 1,357 |
| |
| Capitalized R&D | 5,804 |
| | 5,361 |
| |
| Other deductible temporary differences | 1,925 |
| | 1,823 |
| |
| Total gross deferred tax assets | 107,102 |
| | 94,591 |
| |
| Less valuation allowance | (107,102 | ) | | (94,591 | ) | |
| Net deferred tax assets | $ | — |
| | $ | — |
| |
At December 31, 2018, the Company had net operating loss carryforwards for federal income tax purposes of $425.3 million which are available to offset future federal taxable income, if any. The federal net operating losses generated prior to January 1, 2018 begin to expire in 2028. Net operating losses generated after January 1,2018 will be limited when utilized but have an unlimited carryforward period. The Company had net operating loss carryforwards for state income tax purposes of $72.2 million which are available to offset future state taxable income, if any. The state net operating losses begin to expire in 2027.
Utilization of the net operating loss carryforwards and research and development tax credit carryforwards may be subject to substantial annual limitation under Section 382 of the Internal Revenue Code of 1986, and corresponding provisions of state law, due to ownership changes that have occurred previously or that could occur in the table below.future. These ownership changes may limit the amount of carryforwards that can be utilized annually to offset future taxable income. In addition, wegeneral, an ownership change, as defined by Section 382, results from transactions increasing the ownership of certain stockholders or public groups in the stock of a corporation by more than 50% over a three-year period. The Company is in process to complete a study in 2019 to determine whether a change in control has occurred or whether there have frombeen multiple changes in control since December 31, 2014. If the Company has experienced a change of control, as defined by Section 382, utilization of the net operating loss carryforwards or research and development tax credit carryforwards would be subject to an annual limitation under Section 382, which is determined by first multiplying the value of the Company’s stock at the time of the ownership change by the applicable long-term tax-exempt rate, and then could be subject to time granted stock option awards to certain non-employee directorsadditional adjustments, as compensation for their service on our board.required. Any limitation may result in expiration of a portion of the net operating loss carryforwards or research and development tax credit carryforwards before utilization. Further, until a study is completed and any limitation is known, no amounts are being presented as an uncertain tax position.
The table below shows information regarding compensation earnedvaluation allowance for deferred tax assets as of December 31, 2018 and 2017 was $107.1 million and $94.6 million, respectively. The net change in the total valuation allowance was an increase of $12.5 million in 2018 and a decrease of $40.0 million in 2017. The valuation allowance is primarily related to net operating loss carryforwards that, in the judgment of management, are not more likely than not to be realized. In assessing the realizability of deferred tax assets, management considers whether it is more likely than not that some portion of all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Based upon the level of historical taxable income and projections for future taxable income over the periods in which the deferred tax assets are deductible, management believes that a full valuation allowance is necessary at December 31, 2018.
The Company did not have any unrecognized tax benefits at December 31, 2018 and 2017.
The statute of limitations for assessment by the Internal Revenue Service, or the IRS, and state tax authorities is closed for tax years prior to December 31, 2015 for federal tax purposes and for years prior to December 31, 2015 or 2014 for state tax purposes, although carryforward attributes that were generated in years prior to 2015 may still be adjusted upon examination by the IRS or state tax authorities if they either have been or will be used in a future period. The Company files income tax returns in the U.S. federal and various state jurisdictions. There are currently no federal or state audits in progress.
In December 2015, U.S. legislation was enacted to permanently reinstate the Research & Development tax credit (R&D tax credit) which had expired on December 31, 2014. In 2018 and 2017, the Company recorded a benefit of approximately $91,000 and $236,000, respectively, for the 2018 R&D Credit.
The Tax Cuts and Jobs Act (the Act) was enacted on December 22, 2017. The Act reduces the US federal corporate tax rate from 35% to 21%, requires companies to pay a one-time transition tax on earnings of certain foreign subsidiaries that were previously tax deferred and creates new taxes on certain foreign sourced earnings. As of December 31, 2018 the Company did not have any repatriation of foreign income, therefore we recognized a provisional amount of $0.
As of December 31, 2018, the Company has completed its accounting for all of the tax effects of the enactment of the Act, including the effects on its existing deferred tax balances and the one-time transition tax. The Company has not recognized any material adjustment to the provisional tax expense estimate previously recorded related to the Act. As of December 31, 2017 the provisional amount recorded related to the re-measurement of the Company's deferred tax balance was a tax expense of $56 million which was fully offset with a valuation allowance against the Company's deferred taxes.
13. EMPLOYEE BENEFIT PLANS
The Company sponsors a defined contribution retirement plan for employees pursuant to Section 401(k) of the Internal Revenue Code under which eligible employees can defer a portion of their annual compensation. The Company provides an annual matching contribution based on a percentage of the employee’s contributions. The Company recorded an expense for the matching contributions to the plan for the years ended December 31, 2018 and 2017 of $0.4 million and $0.2 million, respectively.
14. NET LOSS PER SHARE
Basic net loss per share excludes the effect of dilution and is computed by dividing the net loss attributable to common stockholders by the weighted-average number of shares of common stock outstanding.
Diluted net loss per share is computed by giving effect to all potential shares of common stock, including convertible preferred stock, stock options and warrants to the extent dilutive. Basic net loss per share was the same as diluted net loss per share for the years ended December 31, 2018 and 2017 as the inclusion of all potential common shares outstanding would have an anti-dilutive effect.
During the year ended December 31, 2017, by our non-employee directors who served on our boardthe Company issued shares of directors during 2017.its Series A Convertible Preferred Stock. Holders of the Series A Convertible Preferred Stock do not have voting rights and receive cumulative annual dividends of $8 for every $100. Cumulative dividends are presented as a loss attributable to the common shareholders.
The following awards outstanding at December 31, 2018 and 2017 were not included in the computation of common shares for the years then ended.
|
| | | | | | | | | | | | | | |
| | | | | | | | | |
| Name | | | Fee Earned or Paid in Cash ($) | | Option Awards ($)(1) | | Total ($) | |
| Luke Düster | | | — |
| | — |
| | — |
| |
| Katherine D. Crothall, Ph.D. | | | $ | 37,000 |
| | $ | 50,589 |
| | $ | 87,589 |
| |
| Rodney Altman, M.D. | | | $ | 44,000 |
| | $ | 50,589 |
| | $ | 94,589 |
| |
| Peter Devlin | | | $ | 61,583 |
| | $ | 50,589 |
| | $ | 112,172 |
| |
| Brian K. Roberts | | | $ | 50,000 |
| | $ | 50,589 |
| | $ | 100,589 |
| |
| Joseph M. Mandato, D.M. | | | $ | 40,000 |
| | $ | 50,589 |
| | $ | 90,589 |
| |
| | | | | | | | | |
| (1) Represents the aggregate grant-date fair value of stock options granted during 2017 and still outstanding as at December 31, 2017, computed in accordance with ASC Topic 718, excluding the effect of estimated forfeitures. |
|
| | | | | |
| | Year Ended December 31, | Year Ended December 31, | |
| | 2018 | 2017 | |
| Stock options | 7,365,339 |
| 1,661,750 |
| |
| Derivative liability warrants | 10,390 |
| 10,390 |
| |
| Equity classified warrants | 150,000,000 |
| — |
| |
| Restricted stock | — |
| 30,000 |
| |
| Preferred stock | 2,750,000 |
| 2,750,000 |
| |
| Employee Stock Purchase Program | — |
| 36,738 |
| |
| Total | 160,125,729 |
| 4,488,878 |
| |
15. COMMITMENTS AND CONTINGENCIES
Litigation
The Company from time to time, is party to pending or threatened legal proceedings. The Company recognizes a liability for contingencies, including an estimate of legal costs to be incurred, when information available indicates both a loss is probable and the amount of the loss can be reasonably estimated. Based upon information presently available, and in light of available
legal and other defenses, contingent liabilities arising from threatened and pending litigation are not considered material in relation to the respective financial positions of the Company.
During 2018, we received an offer from Roche to license its US Patent No. 6,736,795 (the ‘795 patent), which expires on September 23, 2020 and that is alleged to cover our V-Go product. We do not believe the ‘795 patent to be valid, infringed, and/or enforceable and therefore we do not view Roche’s allegation as having merit. We also understand that Roche has never practiced the ‘795 Patent and Roche permitted the European equivalent patent to lapse. Accordingly, we declined Roche’s offer of a license to the ‘795 patent, and on January 24, 2019, we filed two Inter Partes Review petitions, which included three previously unconsidered prior art references, with the Patent Trial and Appeal Board, or PTAB. In response, in late February 2019, Roche filed a proceeding in federal court in Delaware seeking an unspecified amount of damages and potential injunctive relief. The following table provides information aboutPTAB is expected to issue its preliminary findings by July 24, 2019.Although we cannot guarantee the options held byoutcome of this matter, based on our non-employee directorsreview, we have determined that we have a number of defenses and we intend to defend ourselves vigorously against any allegations of patent infringement, which we believe are without merit.
Operating Leases
The Company leases buildings in Bridgewater, New Jersey and Marlborough, Massachusetts, expiring in June 2023 and February 2024, respectively. In addition to rent expense, the Company is obligated to pay costs of insurance, taxes, repairs and maintenance pursuant to the terms of the building leases. The rental payments include the minimum rentals plus common area maintenance charges. The leases include renewal options.
Rental expense under operating leases amounted to $0.6 million and $1.9 million for the years ended December 31, 2018 and 2017, respectively.
Capital Lease
In January and November 2018, the Company executed capital leases with Winthrop Resources Corporation ("Winthrop") for laptops and other electronic equipment. The initial term of the two leases expire in 2021, then continues year to year until terminated. At the end of the initial term, the Company has the option to purchase the leased equipment in whole for a mutually agreed upon price. The assets under this capital lease were recorded at the present value of the minimum lease payments, which amounted to $0.4 million upon commencement and is depreciated over the term of the lease. The Company is obligated to pay $0.1 million interest expense under the capital lease. Depreciation expense for assets under the capital leases amounted to less than $0.1 million as of December 31, 2018. There was no depreciation expense related to capital leases in 2017. The carrying value of the asset at December 31, 2018 was $0.3 million.
At December 31, 2018, the Company had the following minimum lease commitments:
|
| | | | | | | | |
| Name | | Aggregate Option Awards Outstanding (#) | | |
| Luke Düster | | | — |
| | | |
| Katherine D. Crothall, Ph.D. | | | 14,075 |
| | (1) | |
| Rodney Altman, M.D. | | | 14,075 |
| | (2) | |
| Peter Devlin | | | 14,075 |
| | (2) | |
| Brian K. Roberts | | | 14,075 |
| | (3) | |
| Joseph M. Mandato, D.M. | | | 14,075 |
| | (1) | |
| | |
| (1) | As of December 31, 2017, 5,575 shares underlying this option were vested. The shares underlying this option vest in equal quarterly installments over eight quarters. The shares underlying this option vest in full on June 23, 2019. |
| (2) | As of December 31, 2017, 6,168 shares underlying this option were vested and the remaining shares vest in equal quarterly installments over eight quarters. The shares underlying this option vest in full on June 23, 2019. |
| (3) | As of December 31, 2017, 5,871 shares underlying this option were vested and the remaining shares vest in equal quarterly installments over eight quarters. The shares underlying this option vest in full on June 23, 2019. |
|
| | | |
| (Dollars in thousands) | |
Year Ended December 31: | |
| 2019 | $ | 568 |
|
| 2020 | 554 |
|
| 2021 | 433 |
|
| 2022 | 436 |
|
| 2023 | 355 |
|
| 2024 | 22 |
|
| Total | $ | 2,368 |
|
Development Agreement
On April 16, 2018, the Company entered into an agreement with Glooko, a leader in diabetes data management. With this agreement, the Company will provide future V-Go SIM (Simple Insulin Management) users with Glooko’s cloud-based mobile and web diabetes data management platform to help track and analyze their diabetes care plan. Users can also share their data with their providers. Pursuant to the agreement, the Company was obligated to pay a one-time integration fee of $0.1 million, as well as an annual maintenance fee of $0.1 million as well as a monthly fee per user. The initial term of the agreement is for 3 years, with renewal options available in yearly increments thereafter.
Licensing Agreement
Pursuant to a formation agreement, dated as of August 22, 2006 (the "Formation Agreement"), BioValve Technologies, Inc. ("BioValve") and BTI Technologies Inc. ("BTI"), a wholly owned subsidiary of BioValve, contributed to Valeritas, Inc. (formerly Valeritas, LLC) all of their right, title and interest in and to all of the assets, properties and rights of BioValve and BTI to the extent related to BioValve’s drug delivery/medical device initiative, consisting of patents and equipment, hereafter referred to as the Device Assets.
On August 26, 2008, the Formation Agreement was amended and the Company agreed to pay BioValve an amount equal to 9% of any cash received from upfront license or signing fees and any cash development milestone payments received by the Company in connection with licenses or grants of third party rights to the use in development or commercialization of the Company's Rapid Infuser Technology. In certain circumstances the Company would owe 10% of such payments received. As of December 31, 2018 and 2017, no amounts were owed under this agreement. Although the Company believes the intellectual property rights around this technology have value, the technology licensed under this agreement is not used in the V-Go or any current products under development.
16. SUBSEQUENT EVENTS
The Company evaluates events that have occurred after the financial statement date but before the consolidated financial statements are issued. Based upon the evaluation, the Company identified the following subsequent event that would require adjustment or disclosure in the consolidated financial statements other than disclosed:
On February 12, 2019 the Board approved to have included at the annual shareholder meeting a resolution for the shareholders to approve an amendment to effect a reverse stock split ratio range, with the exact ratio for the Board to subsequently determine.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
None.
ITEM 9A. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
We maintain disclosure controls and procedures designed to provide reasonable assurance that information required to be disclosed in reports filed under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), is recorded, processed, summarized and reported within the specified time periods and accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate to allow timely decisions regarding disclosure.
Our Chief Executive Officer and Chief Financial Officer, evaluated the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) promulgated under the Exchange Act) as of December 31, 2018. In designing and evaluating disclosure controls and procedures, we recognize that any disclosure controls and procedures, no matter how well designed and operated, can only provide reasonable assurance of achieving the desired control objective. As of December 31, 2018, based on the evaluation of these disclosure controls and procedures, management concluded that our disclosure controls and procedures were effective.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining effective internal control over financial reporting as defined in Rule 13a-15(f) under the Exchange Act. Because of its inherent limitations, internal control over financial reporting is not intended to provide absolute assurance that a misstatement of our financial statements would be prevented or detected. Under the supervision of our CEO and CFO, we conducted an evaluation of the effectiveness of our internal control over financial reporting as of December 31, 2018 using the criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”) (2013 Framework), or the 2013 Framework. Based on management's assessment, the Company's management believes that, as of December 31, 2018, the Company's internal control over financial reporting was effective based on those criteria.
Changes in internal control over financial reporting.
During the quarter ended December 31, 2018, there was no change in our internal control over financial reporting or in other factors that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Limitations on the Effectiveness of Controls
Management does not expect that our disclosure controls or our internal controls over financial reporting will prevent or detect all errors and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. The design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Further, because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud, if any, within the Company have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty and that breakdowns can occur because of simple error or mistake. Controls can also be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of the controls. The design of any system of controls is based in part on certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Projections of any evaluation of controls effectiveness to future periods are subject to risks. Over time, controls may become inadequate because of changes in conditions or deterioration in the degree of compliance with policies or procedures.
Item 9B. Other Information.
None.
PART III
Item 10. Directors, Executive Officers and Corporate Governance.
The information required by this Item is incorporated herein by reference from our Proxy Statement for our 2019 Annual Meeting of Stockholders or will be included in an amendment to this Annual Report on Form 10-K.
Item 11. Executive Compensation.
The information required by this Item is incorporated herein by reference from our Proxy Statement for our 2019 Annual Meeting of Stockholders or will be included in an amendment to this Annual Report on Form 10-K.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
Beneficial ownershipThe information required by this Item is determinedincorporated herein by reference from our Proxy Statement for our 2019 Annual Meeting of Stockholders or will be included in accordance with the rules of the SEC and generally includes voting or investment power with respectan amendment to securities. In accordance with SEC rules, shares of our common stock which may be acquired upon exercise of stock options or warrants which are currently exercisable or which become exercisable within 60 days of the date of the applicable table below are deemed beneficially owned by the holders of such options and warrants and are deemed outstanding for the purpose of computing the percentage of ownership of such person, but are not treated as outstanding for the purpose of computing the percentage of ownership of any other person. Subject to community property laws, where applicable, the persons or entities named in the tables below have sole voting and investment power with respect to all shares of our common stock indicated as beneficially owned by them.this Annual Report on Form 10-K.
The following table sets forth information with respect to the beneficial ownership of our common stock as of April 25, 2018, or the Determination Date, by (i) each stockholder known by us to be the beneficial owner of more than 5% of our common stock (our only classes of voting securities), (ii) each of our directors and executive officers, and (iii) all of our directors and executive officers as a group. To the best of our knowledge, except as otherwise indicated, each of the persons named in the table has sole voting and investment power with respect to the shares of our common stock beneficially owned by such person, except to the extent such power may be shared with a spouse. To our knowledge, none of the shares listed below are held under a voting trust or similar agreement, except as noted.
Unless otherwise indicated in the following table, the address for each person named in the table is c/o Valeritas Holdings, Inc., 750 Route 202 South, Suite 600, Bridgewater, NJ 08807.
|
| | | | | | |
| | | | | |
| Name and address of beneficial owner | | Number of Shares Beneficially Owned | | Percentage of Shares Beneficially Owned (1) |
| 5% and Greater Stockholders | | | | |
Capital Royalty L.P.(2) 1000 Main St. Suite 2500 Houston, TX 77002 | | 7,685,968 |
| | 80.1 | % |
| Named Executive Officers and Directors | | | |
| John E. Timberlake (3) | | 160,742 |
| | 2.2 |
|
| Leisa Swanson | | — |
| | * |
|
| Kristine Peterson | | — |
| | * |
|
| Matt Nguyen(4) | | 61,378 |
| | * |
|
| Erick Lucera(5) | | 33,851 |
| | * |
|
| Geoffrey Jenkins(6) | | 114,537 |
| | 1.6 |
|
| Joseph Saldanha | | — |
| | * |
|
Luke Düster 1000 Main St. Suite 2500 Houston, TX 77002 | | — |
| | * |
|
| Rodney Altman, M.D.(7) | | 9,687 |
| | * |
|
| Peter Devlin(8) | | 12,687 |
| | * |
|
| Brian Roberts(9) | | 10,190 |
| | * |
|
| Joe Mandato, D.M.(10) | | 14,093 |
| | * |
|
| Katherine Crothall, Ph.D.(11) | | 9,093 |
| | * |
|
| All of our directors and executive officers as a group (13 Persons) | | 426,258 |
| | 5.7 | % |
* Less than 1%
|
| |
(1) | Percentage ownership is based on 7,092,869 shares of common stock outstanding as of the Determination Date, together with securities exercisable or convertible into shares of common stock within 60 days after the Determination Date, for each shareholder. Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. |
(2) | Includes (a) 576,686 shares of common stock and 273,648 shares of Series A Convertible Preferred Stock ("Preferred Stock") held by Capital Royalty Partners II L.P. (“Capital Royalty Partners II”), (b) 210,773 shares of common stock and 96,705 shares of Preferred Stock held by Capital Royalty Partners II (Cayman) L.P. (“Capital Royalty Partners Cayman”), (c) 645,058 shares of common stock and 306,397 shares of Preferred Stock held by Capital Royalty Partners II Parallel Fund “A” L.P. (“Capital Royalty Partners A”), (d) 2,687,061 shares of common stock and 500,250 shares of Preferred Stock held by Capital Royalty Partners II Parallel Fund “B” (Cayman) L.P. (“Capital Royalty Partners B”), and (e) 1,066,390 shares of common stock and 1,323,001 shares of Preferred Stock held by Parallel Investment Opportunities Partners II, L.P. (“Parallel Partners”). Capital Royalty Partners II, Capital Royalty Partners Cayman, Capital Royalty Partners A, Capital Royalty Partners B, and Parallel Partners are indirectly wholly owned by Capital Royalty L.P. (“Capital Royalty”). As the sole and managing member of Capital Royalty, Charles Tate may be deemed to beneficially own such shares of common stock held by Capital Royalty Partners II, Capital Royalty Partners Cayman, Capital Royalty Partners A, Capital Royalty Partners B, and Parallel Partners. |
(3) | Consists of 41,105 shares of unrestricted common stock and 119,637 shares of common stock underlying options that are vested and exercisable within 60 days of the Determination Date. |
(4) | Consists of 13,832 shares of unrestricted common stock, and 47,546 shares of common stock underlying options that are vested and exercisable within 60 days of the Determination Date. |
(5) | Consists of 4,500 shares of unrestricted common stock and 33,851 shares of common stock underlying options that are vested and exercisable within 60 days of the Determination Date. |
(6) | Consists of 6,850 shares of unrestricted common stock and 107,687 shares of common stock underlying options that are vested and exercisable within 60 days of the Determination Date. |
(7) | Represents 9,687 shares of common stock underlying options that are vested and exercisable within 60 days of the Determination Date. |
(8) | Consists of 3,000 shares of unrestricted common stock and 9,687 shares of common stock underlying options that are vested and exercisable with 60 days of the Determination Date. |
(9) | Consists of 800 shares of unrestricted common stock and 9,390 shares of common stock underlying options that are vested and exercisable within 60 days of the Determination Date. |
(10) | Consists of 5,000 shares of unrestricted common stock and 9,093 shares of common stock underlying options that are vested and exercisable within 60 days of the Determination Date. |
(11) | Represents 9,093 shares of common stock underlying options that are vested and exercisable within 60 days of the Determination Date. |
EQUITY COMPENSATION PLAN INFORMATION
The following table contains information about our equity compensation plans as of December 31, 2017.
|
| | | | | | | | | | |
| Equity Compensation Plan Information | |
| Plan Category | Number of securities to be issued upon exercise of outstanding options | | Weighted-average exercise price of outstanding options | | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) | |
| | | | | | | |
| Equity compensation plans approved by security holders | 1,661,750 |
| | $ | 11.96 |
| | 406,599 |
| |
| Equity compensation plans not approved by security holders | — |
| | | | — |
| |
| Total | 1,661,750 |
| | $ | 11.96 |
| | 406,599 |
| |
Item 13. Certain Relationships and Related-Party Transactions and Director Independence
SEC rules require us to disclose any transaction since January 1, 2017 or currently proposed transaction in which we are a participant in which the amount involved exceeded or will exceed $120,000 and in which any related person has or will have a direct or indirect material interest. A related person is any executive officer, director, nominee for director, or holder of 5% or more of our common stock, or an immediate family member of any of those persons.Independence.
The following is a description of transactions since January 1, 2017, in which we were a party and the amount involved exceeded or will exceed $120,000, and in which any of our executive officers, directors, or holders of more than 5% of any class of our voting securities, or an affiliate or immediate family member thereof, had or will have a direct or indirect material interest. We believe the terms obtained or consideration that we paid or received, as applicable, in connection with the transactions described below were comparable to terms available or amounts that would be paid or received, as applicable, in arm’s-length transactions with unrelated third parties.
Capital Royalty Group Term Loan
On May 23, 2013, we entered into a $50.0 million term loan with Capital Royalty, or the Term Loan, structured as a senior secured loan with a six-year term. The Term Loan is secured by substantially all of our assets, including our material intellectual property. The Term Loan bears interest at 11% per annum and compounds annually. Until the third anniversary of the Term Loan, we had the option to pay quarterly interest of 7.5% in cash and 3.5% payment-in-kind or PIK interest which is added to the aggregate principal amount of the Term Loan on the last day of each quarter. Thereafter, interest on the Term Loan was payable only in cash. The Term Loan contained a minimum revenue covenant, which was $50.0 million for 2015.
Certain events of default that occurred led us to enter into a series of forbearance agreements with Capital Royalty. The initial forbearance agreement was entered on May 18, 2015 and has subsequently been amended five times. The forbearance agreements, as amended in 2015, contained a number of terms and conditions in exchange for Capital Royalty’s agreement to forbear. The forbearance agreement imposed an interest rate at the default interest rate of 15% per annum and a prepayment premium of 4% on the aggregate outstanding balance on the date of the repayment. As of December 31, 2015, the parties deferred the forbearance expiration date again to January 22, 2016.
On January 22, 2016, we amended the forbearance agreement to extend the forbearance period to March 31, 2016. As part of the terms within the forbearance agreement, dated January 29, 2016, we issued warrants to Capital Royalty exercisable into 16,000,000 shares of private company Series AB Preferred Stock of the private company at $1.25 per share. On March 25, 2016, we amended the forbearance agreement to extend the expiration of the forbearance period to April 30, 2016 and included a number of events that could trigger an earlier expiration of the forbearance agreement.
Concurrently with the closing of the 2016 Merger on May 3, 2016, we restructured the Term Loan and executed a forbearance termination agreement whereby the forbearance agreement was terminated. Capital Royalty converted its outstanding accrued interest and prepayment premium of $16.5 million into 8,609,824 shares of private company Series AB preferred stock and 4,649,859 shares of private company common stock. The private company Series AB shares were then converted into 256,744 of our common stock upon the 2016 Merger and all private company common shares were canceled upon the 2016 Merger. The principal balance was restated as $50.0 million with interest rate charged at 11% per annum, which is PIK interest through June 30, 2018 and then both PIK and cash interest thereafter. The restructured Term Loan requires quarterly interest payments during the term of the loan, which are set to commence on June 30, 2018. The repayment of principal on amounts borrowed under the Term Loan is scheduled to be completed on March 31, 2021.
On February 9, 2017, we entered into an agreement with Capital Royalty to, among other things, reduce the amountinformation required by this liquidity covenant to $2.0 million. The minimum cash balance covenant would, however, revert back to $5.0 million if we were not able to consummate an underwritten public offering with gross proceedsItem is incorporated herein by reference from our Proxy Statement for our 2019 Annual Meeting of at least $40.0 million prior to December 31, 2017. This condition was satisfied upon the closing of our March 2017 public offering.
Debt Conversion
On February 14, 2017, we entered into an agreement with Capital Royalty and WCAS Capital Partners IV, L.P., or WCAS, whereby, upon completion of an underwritten public offering with gross proceeds of at least $40.0 million prior to December 31, 2017, approximately $27.5 million of the outstanding principal amount of our debt, including the Term Loan, would convert into shares of our to-be-designated Series A Convertible Preferred Stock, at a price set forth in the executed definitive documents. On March 22, 2017, the debt was converted into an aggregate of 2,750,000 shares of our Series A Convertible Preferred Stock.
WCAS Capital Partners Note Payable
In 2011, concurrently with the issuance of Series C Preferred Stock, we issued a $5.0 million senior subordinated note, or the WCAS Note, to WCAS. Amounts due under the WCAS Note originally bore interest at 10% per annum, payable semi-annually. On May 23, 2013, the WCAS Note was amended such that the note bore interest at 12% per annum, and all interest accrues as compounded PIK interest and is added to the aggregate principal amount of the loan semi-annually. The then outstanding principal amount of the WCAS Note, including accrued PIK interest, is due in full in September 2021.
Concurrently with the closing of the 2016 Merger, we restructured the WCAS Note. WCAS converted its outstanding accrued interest and fees of $2.1 million to 1,660,530 shares of private company Series AB preferred stock, which were then converted into 49,526 shares of our common stock upon closing of the 2016 Merger.
The principal balance was restated as $5.0 million with 10% per annum payable entirely as PIK interest and debt maturity date set at September 8, 2021. No interest payments are required during the term of the loan. The principal balance and any interest accrued during the term of the loan are due on the maturity date.
March 2017 Public Offering
Certain of our shareholders, including entities affiliated with CRG, purchased an aggregate of 4,000,000 shares of our common stock in our March 2017 public offering at the public offering price of $10.00 per share.
Indemnification Agreements
We have entered into indemnification agreements with each of our directors. These agreements, among other things, require us to indemnify each director (and in certain cases their related venture capital funds) to the fullest extent permitted by Delaware law, including indemnification of expenses such as attorneys’ fees, judgments, fines and settlement amounts incurred by the director in any action or proceeding, including any action or proceeding by or in right of us, arising out of the person’s services as a director.
Policies and Procedures for Related Person Transactions
Our board of directors has adopted a written related person transaction policy setting forth the policies and procedures for the review and approval or ratification of related person transactions. This policy covers, with certain exceptions set forth in Item 404 of Regulation S-K under the Securities Act, any transaction, arrangement or relationship, or any series of similar transactions, arrangements or relationships, in which we were or are to be a participant, where the amount involved exceeds $120,000 in any fiscal year and a related person had, hasStockholders or will have a direct or indirect material interest, including without limitation, purchases of goods or services by or from the related person or entities in which the related person has a material interest, indebtedness, guarantees of indebtedness and employment by us of a related person. In reviewing and approving any such transactions, our audit committee is tasked to consider all relevant facts and circumstances, including, but not limited to, whether the transaction is on terms comparable to those that could be obtainedincluded in an arm’s length transaction and the extent of the related person’s interest in the transaction. All of the transactions described inamendment to this section occurred prior to the adoption of this policy.Annual Report on Form 10-K.
Item 14. Principal Accounting Fees and Services.
The following table sets forth the aggregate fees incurredinformation required by usthis Item is incorporated herein by reference from our Proxy Statement for the fiscal years shown:
|
| | | | | |
| | 2017 | | 2016 |
| Audit Fees (1) | 397,000 |
| | 343,000 |
|
| Audit-Related Fees (2) | 15,500 |
| | — |
|
| Tax Fees (3) | — |
| | — |
|
| Total Fees | 412,500 |
| | 343,000 |
|
(1) Audit Fees consistour 2019 Annual Meeting of fees billed for professional services rendered for the audit of our consolidated annual financial statements and review of the interim consolidated financial statementsStockholders or will be included in quarterly reports and services that are normally provided by the Independent Registered Public Accounting Firm in connection with statutory and regulatory filings or engagements.an amendment to this Annual Report on Form 10-K.
(2) Audit-Related Fees consist of fees billed for assurance and related services that are reasonably related to the performance of the audit or review of our consolidated financial statements and are not reported under “Audit Fees”.
(3)Tax Fees consist of fees billed for professional services rendered for tax compliance, tax advice and tax planning (domestic and international). These services include assistance regarding federal, state and international tax compliance; tax planning and compliance work in connection with acquisitions and international tax planning.
Pre-Approval of Audit and Non-Audit Services
Consistent with requirements of the SEC and the Public Company Accounting Oversight Board regarding auditor independence, our audit committee is responsible for the appointment, compensation and oversight of the work of our independent registered public accounting firm. In recognition of this responsibility, our audit committee (or the chair if such approval is needed on a time urgent basis) generally pre-approves of all audit and permissible non-audit services provided by the independent registered public accounting firm. These services may include audit services, audit-related services, tax services and other services.
PART IV
Item 15. Exhibits and Financial Statement Schedules
|
| | |
| | |
Exhibit Number | | Description |
| |
| |
| 2.1 | | |
| |
| 3.1 | | |
| |
| 3.2 | | |
| |
| 3.3 | | |
| |
| 3.4 | | |
| 3.5 | | |
| 3.6 | | |
| 4.2 | | |
|
| | |
| 4.3 | | |
| 4.4 | | |
| 4.5 | | |
| |
| 10.1 | | |
| |
| 10.2 | | |
| |
| 10.3 | | |
| |
| 10.4 | | |
| |
| 10.5 | | |
| |
| 10.6 | | |
| |
| 10.7 | | |
| |
| 10.8† | | |
| |
| 10.8.1† | | |
| |
| 10.9† | | |
| |
| 10.10 | | Term Loan Agreement, dated May 24, 2013, by and between Valeritas, Inc., Capital Royalty Partners II L.P., Capital Royalty Partners II-Parallel Fund “A” L.P. and Parallel Investment Opportunities Partners II L.P., as lenders, and the guarantors party thereto (Incorporated by reference, Exhibit 10.9 to the Registrant’s Current Report on Form 8-K (File No. 333-198807), filed May 9, 2016) |
| |
| 10.11 | | Amended and Restated Term Loan Agreement, dated August 5, 2014, among Valeritas, Inc., as borrower, Capital Royalty Partners II L.P., Capital Royalty Partners II-Parallel Fund “A” L.P. and Parallel Investment Opportunities Partners II L.P., as lenders, and the guarantors party thereto (Incorporated by reference, Exhibit 10.10 to the Registrant’s Current Report on Form 8-K (File No. 333-198807), filed May 9, 2016) |
| |
| 10.12 | | Second Amended and Restated Term Loan Agreement, dated as of May 3, 2016, by and between Valeritas, Inc., a borrower, Valeritas Holdings, Inc., as Guarantor, Capital Royalty Partners II L.P., Capital Royalty Partners II-Parallel Fund “A” L.P., Parallel Investment Opportunities Partners II L.P., Capital Royalty Partners II-Parallel Fund “B” (Cayman) L.P., Capital Royalty Partners II (Cayman) L.P., as lenders, and the guarantors party thereto (Incorporated by reference, Exhibit 10.11 to the Registrant’s Current Report on Form 8-K (File No. 333-198807), filed May 9, 2016) |
| |
| 10.13 | | Joinder Agreement to Second Amended and Restated Term Loan Agreement, dated as of May 3, 2016, in favor of Capital Royalty Partners II L.P., Capital Royalty Partners II-Parallel Fund “A” L.P., Parallel Investment Opportunities Partners II L.P., Capital Royalty Partners II (Cayman) L.P., and Capital Royalty Partners II-Parallel Fund “B” (Cayman) L.P., as the Secured Parties, and Capital Royalty Partners II L.P., as Control Agent (Incorporated by reference, Exhibit 10.12 to the Registrant’s Current Report on Form 8-K (File No. 333-198807), filed May 9, 2016) |
| |
|
| | |
| 10.14 | | Limited Forbearance Agreement, dated May 18, 2015, by and between the Valeritas, Inc., Valeritas Holdings, LLC, Valeritas Security Corporation, Capital Royalty Partners II L.P., Parallel Investment Opportunities Partners II, L.P., Capital Royalty Partners II- Parallel Fund “A” L.P., Capital Royalty Partners II (Cayman) L.P. and Capital Royalty Partners II-Parallel Fund “B” (Cayman) L.P. (Incorporated by reference, Exhibit 10.13 to the Registrant’s Current Report on Form 8-K (File No. 333-198807), filed May 9, 2016) |
| |
| 10.15 | | Amendment No. 1 to Limited Forbearance Agreement, dated September 28, 2015, by and among Valeritas, Inc., Valeritas Holdings, LLC, Valeritas Security Corporation, Capital Royalty Partners II L.P., Parallel Investment Opportunities Partners II, L.P., Capital Royalty Partners II-Parallel Fund “A” L.P., Capital Royalty Partners II (Cayman) L.P. and Capital Royalty Partners II-Parallel Fund “B” (Cayman) L.P. (Incorporated by reference, Exhibit 10.14 to the Registrant’s Current Report on Form 8-K (File No. 333-198807), filed May 9, 2016) |
| |
| 10.16 | | Amendment No. 2 to Limited Forbearance Agreement, dated November 13, 2015, by and among Valeritas, Inc., Valeritas Holdings, LLC, Valeritas Security Corporation, Capital Royalty Partners II L.P., Parallel Investment Opportunities Partners II, L.P., Capital Royalty Partners II-Parallel Fund “A” L.P., Capital Royalty Partners II (Cayman) L.P. and Capital Royalty Partners II-Parallel Fund “B” (Cayman) L.P. (Incorporated by reference, Exhibit 10.15 to the Registrant’s Current Report on Form 8-K (File No. 333-198807), filed May 9, 2016) |
| |
| 10.17 | | Amendment No. 3 to Limited Forbearance Agreement, dated December 21, 2015, by and among Valeritas, Inc., Valeritas Holdings, LLC, Valeritas Security Corporation, Capital Royalty Partners II L.P., Parallel Investment Opportunities Partners II, L.P., Capital Royalty Partners II-Parallel Fund “A” L.P., Capital Royalty Partners II (Cayman) L.P. and Capital Royalty Partners II-Parallel Fund “B” (Cayman) L.P. (Incorporated by reference, Exhibit 10.16 to the Registrant’s Current Report on Form 8-K (File No. 333-198807), filed May 9, 2016) |
| |
| 10.18 | | Amendment No. 4 to Limited Forbearance Agreement, dated January 29, 2016, by and among Valeritas, Inc., Valeritas Holdings, LLC, Valeritas Security Corporation, Capital Royalty Partners II L.P., Parallel Investment Opportunities Partners II, L.P., Capital Royalty Partners II-Parallel Fund “A” L.P., Capital Royalty Partners II (Cayman) L.P. and Capital Royalty Partners II-Parallel Fund “B” (Cayman) L.P. (Incorporated by reference, Exhibit 10.17 to the Registrant’s Current Report on Form 8-K (File No. 333-198807), filed May 9, 2016) |
|
| | |
| 10.19 | | Amendment No. 5 to Limited Forbearance Agreement, dated March 25, 2016, by and among Valeritas, Inc., Valeritas Security Corporation, Capital Royalty Partners II L.P., Parallel Investment Opportunities Partners II, L.P., Capital Royalty Partners II-Parallel Fund “A” L.P., Capital Royalty Partners II (Cayman) L.P. and Capital Royalty Partners II-Parallel Fund “B” (Cayman) L.P. (Incorporated by reference, Exhibit 10.18 to the Registrant’s Current Report on Form 8-K (File No. 333-198807), filed May 9, 2016) |
| |
| 10.20 | | |
| |
| 10.21 | | Termination of Forbearance Agreement, dated May 3, 2016, by and among Valeritas, Inc., Valeritas Security Corporation, Capital Royalty Partners II L.P., Parallel Investment Opportunities Partners II, L.P., Capital Royalty Partners II-Parallel Fund “A” L.P., Capital Royalty Partners II (Cayman) L.P. and Capital Royalty Partners II-Parallel Fund “B” (Cayman) L.P. (Incorporated by reference, Exhibit 10.20 to the Registrant’s Current Report on Form 8-K (File No. 333-198807), filed May 9, 2016) |
| |
| 10.22 | | Lease, dated October 20, 2009, by and between Valeritas, Inc. and BTCT Associates, L.L.C., as amended on January 17, 2013, in respect of the building located at 750 Route 202, Bridgewater, New Jersey 08807 (Incorporated by reference, Exhibit 10.21 to the Registrant’s Current Report on Form 8-K (File No. 333-198807), filed May 9, 2016) |
| |
| 10.23 | | Lease, dated December 22, 2006, by and among Valeritas, LLC, The Taming of the Shrewsbury, LLC, O’Neill Partners, LLC and Chanski, LLC, as amended on April 24, 2009, in respect of the building located at 800 Boston Turnpike, Shrewsbury, Massachusetts 01545 (Incorporated by reference, Exhibit 10.22 to the Registrant’s Current Report on Form 8-K (File No. 333-198807), filed May 9, 2016) |
| |
| 10.24 | | Promissory Note, dated September 8, 2011, issued by Valeritas, Inc. to WCAS Capital Partners IV, L.P., in the original principal amount of $5,000,000, by Amendment No. 1 to Note, dated May 24, 2013. (Incorporated by reference, Exhibit 10.23 to the Registrant’s Current Report on Form 8-K (File No. 333-198807), filed May 9, 2016) |
| |
| 10.25 | | |
| |
| 10.26 | | |
| |
|
| | |
| 10.27† | | |
| |
| 10.28† | | |
| |
| 10.29† | | |
| |
| 10.30† | | |
| |
| 10.31† | | Employment Agreement, dated August 29, 2016, by and between Valeritas, Inc. and Erick J. Lucera Employment Agreement, dated December 20, 2016, by and between Valeritas, Inc. and Matthew Nguyen (Incorporated by reference, Exhibit 10.31 to the Registrant’s Annual Report on Form 10-K (File No. 333-198807), filed February 21, 2017) |
| |
| 10.32† | | |
| 10.33† | | |
| 10.34† | | Form of Indemnification Agreement (Incorporated by reference, Exhibit 10.34 to the Registrant's Registration Statement on Form S-1 (File No. 333-222454), filed January 8, 2018) |
| |
| 10.35 | | Series A Preferred Stock Purchase Agreement, dated as of February 14, 2017, by and among the Registrant, Capital Royalty Partners II L.P., Parallel Investment Opportunities Partners II, L.P., Capital Royalty Partners II-Parallel Fund “A” L.P., Capital Royalty Partners II (Cayman) L.P., Capital Royalty Partners II-Parallel Fund “B” (Cayman) L.P. and WCAS Capital Partners IV, LP (Incorporated by reference, Exhibit 10.1 to the Registrant’s Current Report on Form 8-K (File No. 333-198807), filed February 21, 2017) |
| |
| 10.36 | | Registration Rights Agreement, dated as of February 14, 2017, by and among the Registrant, Capital Royalty Partners II L.P., Parallel Investment Opportunities Partners II, L.P., Capital Royalty Partners II-Parallel Fund “A” L.P., Capital Royalty Partners II (Cayman) L.P., Capital Royalty Partners II-Parallel Fund “B” (Cayman) L.P. and WCAS Capital Partners IV, LP (Incorporated by reference, Exhibit 10.2 to the Registrant’s Current Report on Form 8-K (File No. 333-198807), filed February 21, 2017) |
| |
| 10.37 | | Amendment No. 1 to Second Amended and Restated Term Loan Agreement, dated as of February 9, 2017, by and among the Company, Capital Royalty Partners II L.P., Parallel Investment Opportunities Partners II, L.P., Capital Royalty Partners II-Parallel Fund “A” L.P., Capital Royalty Partners II (Cayman) L.P., Capital Royalty Partners II-Parallel Fund “B” (Cayman) L.P. and WCAS Capital Partners IV, LP (Incorporated by reference, Exhibit 10.3 to the Registrant’s Current Report on Form 8-K (File No. 333-198807), filed February 21, 2017) |
| 10.38 | | |
| 10.39 | | |
| 10.40 | |
|
| 10.41 | | |
| 10.42 | | |
| 10.43 † | | |
|
| | |
| 10.44 † | | |
| 10.45 † | | |
| |
| 21.1 | | |
| |
| 23.1** | | |
| |
| 31.1** | | |
| |
| 31.2** | | |
| | |
| 32.1** | | |
| | |
| 32.2** | | |
| | |
| 101.INS** | | XBRL Instance Document |
| |
| 101.SCH** | | XBRL Taxonomy Extension Schema Document |
| |
| 101.CAL** | | XBRL Taxonomy Extension Calculation Linkbase Document |
| |
| 101.DEF** | | XBRL Taxonomy Extension Definition Linkbase Document |
| |
| 101.LAB** | | XBRL Taxonomy Extension Label Linkbase Document |
| |
| 101.PRE** | | XBRL Taxonomy Extension Presentation Linkbase Document |
|
| | |
| | |
| |
| ** | Filed herewith. |
| † | Management contract or compensatory plan or arrangement. |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
| | | | | | |
| | | | | VALERITAS HOLDINGS INC. |
| | | | |
Date: April 30, 2018March 5, 2019 | | | | By: | | /s/ John E. Timberlake |
| | | | | John E. Timberlake |
| | | | | Chief Executive Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, this Report has been signed below by the following persons on behalf of the Registrant in the capacities and on the dates indicated.
|
| | | | |
| Signature | | Title | | Date |
| | | |
| /s/ John E. Timberlake | | President, Chief Executive Officer and Director | | April 30, 2018March 5, 2019 |
| John E. Timberlake | | (Principal Executive Officer) | | |
| | | |
| /s/ Erick Lucera | | Chief Financial Officer | | April 30, 2018March 5, 2019 |
| Erick Lucera | | (Principal Financial Officer) | | |
| | | |
| /s/ Mark Conley | | Vice President, Corporate Controller and Treasurer | | April 30, 2018March 5, 2019 |
| Mark Conley | | (Principal Accounting Officer) | | |
| | | |
| /s/ Katherine Crothall, Ph.D. | | Director | | April 30, 2018March 5, 2019 |
| Katherine Crothall, Ph.D. | | | | |
| | | |
| /s/ Luke Düster | | Director | | April 30, 2018March 5, 2019 |
| Luke Düster | | | | |
| | | |
| /s/ Joe Mandato, DM | | Director | | April 30, 2018March 5, 2019 |
| Joe Mandato, DM | | | | |
| | | |
| /s/ Rodney Altman, MD | | Director | | April 30, 2018March 5, 2019 |
| Rodney Altman, MD | | | | |
| | | |
| /s/ Peter Devlin | | Director | | April 30, 2018March 5, 2019 |
| Peter Devlin | | | | |
| | | |
| /s/ Brian K. Roberts | | Director | | April 30, 2018March 5, 2019 |
| Brian K. Roberts | | | | |
EXHIBIT INDEX
|
| | |
| | |
Exhibit
Number
| | Description |
| |
| |
10.41 | | |
| |
31.1* | |
|
| |
31.2* | |
|
| |
32.1* | | |
| |
32.2* | |
|