
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-Q
☒ | QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended MarchDecember 31, 2023
OR
☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission File Number 001-35839
ENANTA PHARMACEUTICALS, INC.
(Exact name of registrant as specified in its charter)
|
| 04-3205099 |
(State or other jurisdiction of incorporation or organization) |
| (I.R.S. Employer Identification Number) |
500 Arsenal Street Watertown, Massachusetts |
| 02472 |
(Address of principal executive offices) |
| (Zip Code) |
(Registrants telephone number, including area code:) (617) 607-0800
Securities registered pursuant to Section 12(b) of the Act:
Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
Common Stock, par value $0.01 per share | ENTA | NASDAQ |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer | ☒ | Accelerated filer | ☐ | ||||||
Non-accelerated filer | ☐ | Smaller reporting company | ☐ | ||||||
Emerging growth company |
| ☐ |
|
|
|
| |||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
As of April 28, 2023,January 31, 2024, the registrant had 21,055,39221,155,983 shares of common stock, $0.01 par value per share, outstanding.
Table of Contents
Page | ||
PART I. | ||
Item 1. | 3 | |
3 | ||
4 | ||
5 | ||
| 6 | |
7 | ||
Notes to Condensed Consolidated Financial Statements (unaudited) | 8 | |
Item 2. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
|
Item 3. |
| |
Item 4. |
| |
PART II. |
| |
Item 1A. |
| |
Item 5. | 27 | |
Item 6. |
| |
| ||
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Quarterly Report on Form 10-Q ("Quarterly Report") contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”) and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) about us and our industry that involve substantial risks and uncertainties. All statements other than statements of historical facts contained in this Quarterly Report, including statements regarding our future results of operations and financial condition, business strategy and plans and objectives of management for future operations, are forward-looking statements. In some cases, forward-looking statements may be identified by words such as “anticipate,” “believe,” “continue,” “could,” “design,” “estimate,” “expect,” “intend,” “may,” “plan,” “potentially,” “predict,” “project,” “should,” “will” or the negative of these terms or other similar expressions. We caution you that the foregoing list may not encompass all of the forward-looking statements made in this Quarterly Report.
Forward-looking statements are based on our management’s beliefs and assumptions and on information currently available. These forward-looking statements are subject to a number of known and unknown risks, uncertainties and assumptions, including risks described in the section titled “Risk Factors” in our Annual Report on Form 10-K for the fiscal year ended September 30, 2022.2023 and as updated in Item 1A herein.
2
PART I—UNAUDITED FINANCIAL INFORMATION
ITEM 1. CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
ENANTA PHARMACEUTICALS, INC.
CONDENSED CONSOLIDATED BALANCE SHEETS
(unaudited)
(in thousands, except per share amounts)data)
|
| March 31, |
| September 30, |
|
| December 31, |
| September 30, |
| ||||||
|
| 2023 |
|
| 2022 |
|
| 2023 |
|
| 2023 |
| ||||
Assets |
|
|
|
|
|
|
|
|
|
| ||||||
Current assets: |
|
|
|
|
|
|
|
|
|
| ||||||
Cash and cash equivalents |
| $ | 73,178 |
|
| $ | 43,994 |
|
| $ | 39,933 |
|
| $ | 85,388 |
|
Short-term marketable securities |
|
| 136,906 |
|
|
| 205,238 |
|
|
| 297,218 |
|
|
| 284,522 |
|
Accounts receivable |
|
| 17,795 |
|
|
| 20,318 |
|
|
| 8,173 |
|
|
| 8,614 |
|
Prepaid expenses and other current assets |
|
| 14,484 |
|
|
| 13,445 |
|
|
| 13,245 |
|
|
| 13,263 |
|
Income tax receivable |
|
| 28,774 |
|
|
| 28,718 |
|
|
| 31,734 |
|
|
| 31,004 |
|
Total current assets |
|
| 271,137 |
|
|
| 311,713 |
|
|
| 390,303 |
|
|
| 422,791 |
|
Long-term marketable securities |
|
| 15,040 |
|
|
| 29,285 |
| ||||||||
Property and equipment, net |
|
| 11,050 |
|
|
| 6,173 |
|
|
| 12,119 |
|
|
| 11,919 |
|
Operating lease, right-of-use assets |
|
| 24,554 |
|
|
| 23,575 |
|
|
| 21,344 |
|
|
| 22,794 |
|
Restricted cash |
|
| 3,968 |
|
|
| 3,968 |
|
|
| 3,968 |
|
|
| 3,968 |
|
Other long-term assets |
|
| 696 |
|
|
| 696 |
|
|
| 765 |
|
|
| 803 |
|
Total assets |
| $ | 326,445 |
|
| $ | 375,410 |
|
| $ | 428,499 |
|
| $ | 462,275 |
|
Liabilities and Stockholders' Equity |
|
|
|
|
|
|
|
|
|
| ||||||
Current liabilities: |
|
|
|
|
|
|
|
|
|
| ||||||
Accounts payable |
| $ | 11,761 |
|
| $ | 6,000 |
|
| $ | 9,326 |
|
| $ | 4,097 |
|
Accrued expenses and other current liabilities |
|
| 15,482 |
|
|
| 20,936 |
|
|
| 11,603 |
|
|
| 18,339 |
|
Liability related to the sale of future royalties |
|
| 36,512 |
|
|
| 35,076 |
| ||||||||
Operating lease liabilities |
|
| 4,923 |
|
|
| 2,891 |
|
|
| 4,966 |
|
|
| 5,275 |
|
Total current liabilities |
|
| 32,166 |
|
|
| 29,827 |
|
|
| 62,407 |
|
|
| 62,787 |
|
Liability related to the sale of future royalties, net of current portion |
|
| 151,612 |
|
|
| 159,429 |
| ||||||||
Operating lease liabilities, net of current portion |
|
| 23,073 |
|
|
| 22,372 |
|
|
| 20,524 |
|
|
| 21,238 |
|
Series 1 nonconvertible preferred stock |
|
| 1,423 |
|
|
| 1,423 |
|
|
| 1,423 |
|
|
| 1,423 |
|
Other long-term liabilities |
|
| 408 |
|
|
| 454 |
|
|
| 649 |
|
|
| 663 |
|
Total liabilities |
|
| 57,070 |
|
|
| 54,076 |
|
|
| 236,615 |
|
|
| 245,540 |
|
Commitments and contingencies (Note 9) |
|
|
|
|
| |||||||||||
Commitments and contingencies (Note 11) |
|
|
|
|
| |||||||||||
Stockholders' equity: |
|
|
|
|
|
|
|
|
|
|
|
| ||||
Common stock; $0.01 par value per share, 100,000 shares authorized; |
|
| 210 |
|
|
| 208 |
| ||||||||
Common stock; $0.01 par value per share, 100,000 shares authorized; |
|
| 212 |
|
|
| 211 |
| ||||||||
Additional paid-in capital |
|
| 410,803 |
|
|
| 398,029 |
|
|
| 432,608 |
|
|
| 424,693 |
|
Accumulated other comprehensive loss |
|
| (1,815 | ) |
|
| (3,724 | ) |
|
| (534 | ) |
|
| (1,174 | ) |
Accumulated deficit |
|
| (139,823 | ) |
|
| (73,179 | ) |
|
| (240,402 | ) |
|
| (206,995 | ) |
Total stockholders' equity |
|
| 269,375 |
|
|
| 321,334 |
|
|
| 191,884 |
|
|
| 216,735 |
|
Total liabilities and stockholders' equity |
| $ | 326,445 |
|
| $ | 375,410 |
|
| $ | 428,499 |
|
| $ | 462,275 |
|
The accompanying notes are an integral part of these condensed consolidated financial statements.
3
ENANTA PHARMACEUTICALS, INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(unaudited)
(in thousands, except per share amounts)data)
|
|
|
|
|
|
|
|
| ||||||||||||||||
|
| Three Months Ended March 31, |
|
| Six Months Ended March 31, |
|
| Three Months Ended December 31, |
| |||||||||||||||
|
| 2023 |
|
| 2022 |
|
| 2023 |
|
| 2022 |
|
| 2023 |
|
| 2022 |
| ||||||
Revenue |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||
Royalty revenue |
| $ | 17,795 |
|
| $ | 18,716 |
|
| $ | 40,380 |
|
| $ | 46,364 |
|
| $ | 18,003 |
|
| $ | 22,585 |
|
License revenue |
|
| — |
|
|
| — |
|
|
| 1,000 |
|
|
| — |
|
|
| — |
|
|
| 1,000 |
|
Total revenue |
|
| 17,795 |
|
|
| 18,716 |
|
|
| 41,380 |
|
|
| 46,364 |
|
|
| 18,003 |
|
|
| 23,585 |
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||
Research and development |
|
| 43,468 |
|
|
| 42,087 |
|
|
| 84,370 |
|
|
| 90,636 |
|
|
| 36,371 |
|
|
| 40,902 |
|
General and administrative |
|
| 13,778 |
|
|
| 10,476 |
|
|
| 26,474 |
|
|
| 19,984 |
|
|
| 16,518 |
|
|
| 12,696 |
|
Total operating expenses |
|
| 57,246 |
|
|
| 52,563 |
|
|
| 110,844 |
|
|
| 110,620 |
|
|
| 52,889 |
|
|
| 53,598 |
|
Loss from operations |
|
| (39,451 | ) |
|
| (33,847 | ) |
|
| (69,464 | ) |
|
| (64,256 | ) |
|
| (34,886 | ) |
|
| (30,013 | ) |
Other income (expense): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||
Interest expense |
|
| (3,441 | ) |
|
| — |
| ||||||||||||||||
Interest and investment income, net |
|
| 1,837 |
|
|
| 255 |
|
|
| 2,830 |
|
|
| 549 |
|
|
| 4,298 |
|
|
| 993 |
|
Total other income, net |
|
| 1,837 |
|
|
| 255 |
|
|
| 2,830 |
|
|
| 549 |
|
|
| 857 |
|
|
| 993 |
|
Loss before income taxes |
|
| (37,614 | ) |
|
| (33,592 | ) |
|
| (66,634 | ) |
|
| (63,707 | ) |
|
| (34,029 | ) |
|
| (29,020 | ) |
Income tax expense |
|
| (44 | ) |
|
| — |
|
|
| (10 | ) |
|
| — |
| ||||||||
Income tax benefit |
|
| 622 |
|
|
| 34 |
| ||||||||||||||||
Net loss |
| $ | (37,658 | ) |
| $ | (33,592 | ) |
| $ | (66,644 | ) |
| $ | (63,707 | ) |
| $ | (33,407 | ) |
| $ | (28,986 | ) |
Net loss per share, basic and diluted |
| $ | (1.79 | ) |
| $ | (1.63 | ) |
| $ | (3.19 | ) |
| $ | (3.11 | ) |
| $ | (1.58 | ) |
| $ | (1.39 | ) |
Weighted average common shares outstanding, basic and |
|
| 21,035 |
|
|
| 20,551 |
|
|
| 20,882 |
|
|
| 20,473 |
|
|
| 21,088 |
|
|
| 20,816 |
|
The accompanying notes are an integral part of these condensed consolidated financial statements.
4
ENANTA PHARMACEUTICALS, INC.
CONDENSED CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
(unaudited)
(in thousands)
|
|
|
|
|
|
| ||||||||||
|
| Three Months Ended March 31, |
|
| Six Months Ended March 31, |
| ||||||||||
|
| 2023 |
|
| 2022 |
|
| 2023 |
|
| 2022 |
| ||||
Net loss |
| $ | (37,658 | ) |
| $ | (33,592 | ) |
| $ | (66,644 | ) |
| $ | (63,707 | ) |
Other comprehensive income (loss): |
|
|
|
|
|
|
|
|
|
|
|
| ||||
Net unrealized gains (losses) on marketable securities |
|
| 860 |
|
|
| (2,031 | ) |
|
| 1,909 |
|
|
| (2,655 | ) |
Total other comprehensive income (loss) |
|
| 860 |
|
|
| (2,031 | ) |
|
| 1,909 |
|
|
| (2,655 | ) |
Comprehensive loss |
| $ | (36,798 | ) |
| $ | (35,623 | ) |
| $ | (64,735 | ) |
| $ | (66,362 | ) |
|
|
|
| |||||
|
| Three Months Ended December 31, |
| |||||
|
| 2023 |
|
| 2022 |
| ||
Net loss |
| $ | (33,407 | ) |
| $ | (28,986 | ) |
Other comprehensive income: |
|
|
|
|
|
| ||
Net unrealized gains on marketable securities |
|
| 640 |
|
|
| 1,049 |
|
Total other comprehensive income |
|
| 640 |
|
|
| 1,049 |
|
Comprehensive loss |
| $ | (32,767 | ) |
| $ | (27,937 | ) |
The accompanying notes are an integral part of these condensed consolidated financial statements.
5
ENANTA PHARMACEUTICALS, INC.
CONDENSED CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(unaudited)
(in thousands)
|
|
|
|
|
|
|
| Accumulated |
|
|
|
|
|
|
|
|
|
|
|
| Accumulated |
|
|
|
|
| ||||||||||||||||||||||
|
|
|
|
|
| Additional |
| Other |
|
|
| Total |
|
|
|
|
|
| Additional |
| Other |
|
|
| Total |
| ||||||||||||||||||||||
|
| Common Stock |
|
| Paid-In |
| Comprehensive |
| Accumulated |
| Stockholders' |
|
| Common Stock |
|
| Paid-In |
| Comprehensive |
| Accumulated |
| Stockholders' |
| ||||||||||||||||||||||||
|
| Shares |
|
| Amount |
|
| Capital |
|
| Loss |
|
| Deficit |
|
| Equity |
|
| Shares |
|
| Amount |
|
| Capital |
|
| Loss |
|
| Deficit |
|
| Equity |
| ||||||||||||
Balances at September 30, 2022 |
|
| 20,791 |
|
| $ | 208 |
|
| $ | 398,029 |
|
| $ | (3,724 | ) |
| $ | (73,179 | ) |
| $ | 321,334 |
| ||||||||||||||||||||||||
Exercise of stock options |
|
| 56 |
|
|
| 1 |
|
|
| 1,125 |
|
|
| — |
|
|
| — |
|
|
| 1,126 |
| ||||||||||||||||||||||||
Balances, September 30, 2023 |
|
| 21,059 |
|
| $ | 211 |
|
| $ | 424,693 |
|
| $ | (1,174 | ) |
| $ | (206,995 | ) |
| $ | 216,735 |
| ||||||||||||||||||||||||
Vesting of restricted stock units, net of |
|
| 37 |
|
|
| — |
|
|
| (825 | ) |
|
| — |
|
|
| — |
|
|
| (825 | ) |
|
| 97 |
|
|
| 1 |
|
|
| (184 | ) |
|
| — |
|
|
| — |
|
|
| (183 | ) |
Stock-based compensation expense |
|
| — |
|
|
| — |
|
|
| 7,139 |
|
|
| — |
|
|
| — |
|
|
| 7,139 |
|
|
| — |
|
|
| — |
|
|
| 8,099 |
|
|
| — |
|
|
| — |
|
|
| 8,099 |
|
Other comprehensive income |
|
| — |
|
|
| — |
|
|
| — |
|
|
| 1,049 |
|
|
| — |
|
|
| 1,049 |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| 640 |
|
|
| — |
|
|
| 640 |
|
Net loss |
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| (28,986 | ) |
|
| (28,986 | ) |
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| (33,407 | ) |
|
| (33,407 | ) |
Balances at December 31, 2022 |
|
| 20,884 |
|
|
| 209 |
|
|
| 405,468 |
|
|
| (2,675 | ) |
|
| (102,165 | ) |
|
| 300,837 |
| ||||||||||||||||||||||||
Exercise of stock options |
|
| 61 |
|
|
| 1 |
|
|
| 881 |
|
|
| — |
|
|
| — |
|
|
| 882 |
| ||||||||||||||||||||||||
Vesting of restricted stock units, net of |
|
| 104 |
|
|
| — |
|
|
| (2,909 | ) |
|
| — |
|
|
| — |
|
|
| (2,909 | ) | ||||||||||||||||||||||||
Stock-based compensation expense |
|
| — |
|
|
| — |
|
|
| 7,363 |
|
|
| — |
|
|
| — |
|
|
| 7,363 |
| ||||||||||||||||||||||||
Other comprehensive income |
|
| — |
|
|
| — |
|
|
| — |
|
|
| 860 |
|
|
| — |
|
|
| 860 |
| ||||||||||||||||||||||||
Net loss |
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| (37,658 | ) |
|
| (37,658 | ) | ||||||||||||||||||||||||
Balances at March 31, 2023 |
|
| 21,049 |
|
| $ | 210 |
|
| $ | 410,803 |
|
| $ | (1,815 | ) |
| $ | (139,823 | ) |
| $ | 269,375 |
| ||||||||||||||||||||||||
Balances, December 31, 2023 |
|
| 21,156 |
|
| $ | 212 |
|
| $ | 432,608 |
|
| $ | (534 | ) |
| $ | (240,402 | ) |
| $ | 191,884 |
| ||||||||||||||||||||||||
|
|
|
|
|
|
|
| Accumulated |
| Retained |
|
|
|
|
|
|
|
|
|
| Accumulated |
|
|
|
|
| ||||||||||||||||||||||
|
|
|
|
|
| Additional |
| Other |
| Earnings |
| Total |
|
|
|
|
|
| Additional |
| Other |
|
|
| Total |
| ||||||||||||||||||||||
|
| Common Stock |
|
| Paid-In |
| Comprehensive |
| (Accumulated |
|
| Stockholders' |
|
| Common Stock |
|
| Paid-In |
| Comprehensive |
| Accumulated |
|
| Stockholders' |
| ||||||||||||||||||||||
|
| Shares |
|
| Amount |
|
| Capital |
|
| Loss |
|
| Deficit) |
|
| Equity |
|
| Shares |
|
| Amount |
|
| Capital |
|
| Loss |
|
| Deficit |
|
| Equity |
| ||||||||||||
Balances at September 30, 2021 |
|
| 20,238 |
|
| $ | 202 |
|
| $ | 351,033 |
|
| $ | (382 | ) |
| $ | 48,576 |
|
| $ | 399,429 |
| ||||||||||||||||||||||||
Balances, September 30, 2022 |
|
| 20,791 |
|
| $ | 208 |
|
| $ | 398,029 |
|
| $ | (3,724 | ) |
| $ | (73,179 | ) |
| $ | 321,334 |
| ||||||||||||||||||||||||
Exercise of stock options |
|
| 248 |
|
|
| 2 |
|
|
| 10,407 |
|
|
| — |
|
|
| — |
|
|
| 10,409 |
|
|
| 56 |
|
|
| 1 |
|
|
| 1,125 |
|
|
| — |
|
|
| — |
|
|
| 1,126 |
|
Vesting of restricted stock units, net of |
|
| 20 |
|
|
| 1 |
|
|
| (778 | ) |
|
| — |
|
|
| — |
|
|
| (777 | ) |
|
| 37 |
|
|
| — |
|
|
| (825 | ) |
|
| — |
|
|
| — |
|
|
| (825 | ) |
Stock-based compensation expense |
|
| — |
|
|
| — |
|
|
| 6,062 |
|
|
| — |
|
|
| — |
|
|
| 6,062 |
|
|
| — |
|
|
| — |
|
|
| 7,139 |
|
|
| — |
|
|
| — |
|
|
| 7,139 |
|
Other comprehensive loss |
|
| — |
|
|
| — |
|
|
| — |
|
|
| (624 | ) |
|
| — |
|
|
| (624 | ) | ||||||||||||||||||||||||
Other comprehensive income |
|
| — |
|
|
| — |
|
|
| — |
|
|
| 1,049 |
|
|
| — |
|
|
| 1,049 |
| ||||||||||||||||||||||||
Net loss |
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| (30,115 | ) |
|
| (30,115 | ) |
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| (28,986 | ) |
|
| (28,986 | ) |
Balances at December 31, 2021 |
|
| 20,506 |
|
|
| 205 |
|
|
| 366,724 |
|
|
| (1,006 | ) |
|
| 18,461 |
|
|
| 384,384 |
| ||||||||||||||||||||||||
Exercise of stock options |
|
| 97 |
|
|
| 1 |
|
|
| 3,801 |
|
|
| — |
|
|
| — |
|
|
| 3,802 |
| ||||||||||||||||||||||||
Vesting of restricted stock units, net of |
|
| 15 |
|
|
| — |
|
|
| (451 | ) |
|
| — |
|
|
| — |
|
|
| (451 | ) | ||||||||||||||||||||||||
Stock-based compensation expense |
|
| — |
|
|
| — |
|
|
| 6,471 |
|
|
| — |
|
|
| — |
|
|
| 6,471 |
| ||||||||||||||||||||||||
Other comprehensive loss |
|
| — |
|
|
| — |
|
|
| — |
|
|
| (2,031 | ) |
|
| — |
|
|
| (2,031 | ) | ||||||||||||||||||||||||
Net loss |
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| (33,592 | ) |
|
| (33,592 | ) | ||||||||||||||||||||||||
Balances at March 31, 2022 |
|
| 20,618 |
|
| $ | 206 |
|
| $ | 376,545 |
|
| $ | (3,037 | ) |
| $ | (15,131 | ) |
| $ | 358,583 |
| ||||||||||||||||||||||||
Balances, December 31, 2022 |
|
| 20,884 |
|
| $ | 209 |
|
| $ | 405,468 |
|
| $ | (2,675 | ) |
| $ | (102,165 | ) |
| $ | 300,837 |
| ||||||||||||||||||||||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
6
ENANTA PHARMACEUTICALS, INC.
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(unaudited)
(in thousands)
|
| Six Months Ended March 31, |
|
| Three Months Ended December 31, |
| ||||||||||
|
| 2023 |
|
| 2022 |
|
| 2023 |
|
| 2022 |
| ||||
Cash flows from operating activities |
|
|
|
|
|
|
|
|
|
|
|
| ||||
Net loss |
| $ | (66,644 | ) |
| $ | (63,707 | ) |
| $ | (33,407 | ) |
| $ | (28,986 | ) |
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
|
|
|
|
| ||||
Stock-based compensation expense |
|
| 14,502 |
|
|
| 12,533 |
|
|
| 8,099 |
|
|
| 7,139 |
|
Depreciation and amortization expense |
|
| 1,051 |
|
|
| 1,602 |
|
|
| 642 |
|
|
| 511 |
|
Premium paid on marketable securities |
|
| (42 | ) |
|
| (802 | ) | ||||||||
Non-cash interest expense associated with the sale of future royalties |
|
| 299 |
|
|
| — |
| ||||||||
Non-cash royalty revenue |
|
| 487 |
|
|
| — |
| ||||||||
Amortization (accretion) of premiums (discounts) on marketable securities |
|
| (1,345 | ) |
|
| 928 |
|
|
| 274 |
|
|
| (339 | ) |
Loss on disposal of property and equipment |
|
| 7 |
|
|
| — |
| ||||||||
Change in operating assets and liabilities: |
|
|
|
|
|
|
|
|
|
|
|
| ||||
Accounts receivable |
|
| 2,523 |
|
|
| 4,860 |
|
|
| 441 |
|
|
| (2,267 | ) |
Prepaid expenses and other current assets |
|
| (1,039 | ) |
|
| 110 |
|
|
| 18 |
|
|
| (4,501 | ) |
Income tax receivable |
|
| (56 | ) |
|
| 8,507 |
|
|
| (730 | ) |
|
| 15 |
|
Operating lease, right-of-use assets |
|
| 1,891 |
|
|
| 2,784 |
|
|
| 1,450 |
|
|
| 834 |
|
Other long-term assets |
|
| 38 |
|
|
| (5 | ) | ||||||||
Accounts payable |
|
| 5,963 |
|
|
| (3,473 | ) |
|
| 5,174 |
|
|
| (686 | ) |
Accrued expenses |
|
| (6,052 | ) |
|
| (808 | ) |
|
| (6,736 | ) |
|
| (6,599 | ) |
Operating lease liabilities |
|
| (137 | ) |
|
| (2,498 | ) |
|
| (1,023 | ) |
|
| (717 | ) |
Other long-term liabilities |
|
| (46 | ) |
|
| 318 |
|
|
| (14 | ) |
|
| (40 | ) |
Net cash used in operating activities |
|
| (49,424 | ) |
|
| (39,646 | ) |
|
| (24,988 | ) |
|
| (35,641 | ) |
Cash flows from investing activities |
|
|
|
|
|
|
|
|
|
|
|
| ||||
Purchase of marketable securities |
|
| (90,627 | ) |
|
| (124,631 | ) |
|
| (146,845 | ) |
|
| (67,375 | ) |
Proceeds from maturities and sales of marketable securities |
|
| 176,500 |
|
|
| 135,514 |
| ||||||||
Proceeds from maturities and sale of marketable securities |
|
| 134,515 |
|
|
| 104,100 |
| ||||||||
Purchase of property and equipment |
|
| (5,539 | ) |
|
| (437 | ) |
|
| (787 | ) |
|
| (3,156 | ) |
Net cash provided by investing activities |
|
| 80,334 |
|
|
| 10,446 |
| ||||||||
Net cash provided by (used in) investing activities |
|
| (13,117 | ) |
|
| 33,569 |
| ||||||||
Cash flows from financing activities |
|
|
|
|
|
|
|
|
|
|
|
| ||||
Proceeds from exercise of stock options |
|
| 2,008 |
|
|
| 14,211 |
| ||||||||
Payments on royalty sale liability, net of imputed interest |
|
| (7,167 | ) |
|
| — |
| ||||||||
Payments for settlement of share-based awards |
|
| (3,734 | ) |
|
| (1,228 | ) |
|
| (183 | ) |
|
| (825 | ) |
Proceeds from the exercise of stock options |
|
| — |
|
|
| 1,126 |
| ||||||||
Net cash provided by (used in) financing activities |
|
| (1,726 | ) |
|
| 12,983 |
|
|
| (7,350 | ) |
|
| 301 |
|
Net increase (decrease) in cash, cash equivalents and restricted cash |
|
| 29,184 |
|
|
| (16,217 | ) | ||||||||
Net decrease in cash, cash equivalents and restricted cash |
|
| (45,455 | ) |
|
| (1,771 | ) | ||||||||
Cash, cash equivalents and restricted cash at beginning of period |
|
| 47,962 |
|
|
| 57,814 |
|
|
| 89,356 |
|
|
| 47,962 |
|
Cash, cash equivalents and restricted cash at end of period |
| $ | 77,146 |
|
| $ | 41,597 |
|
| $ | 43,901 |
|
| $ | 46,191 |
|
Supplemental disclosure of noncash information: |
|
|
|
|
|
|
|
|
|
|
|
| ||||
Purchases of fixed assets included in accounts payable and |
| $ | 1,611 |
|
| $ | 174 |
|
| $ | 479 |
|
| $ | 1,079 |
|
Operating lease liabilities arising from obtaining right-of-use assets |
| $ | 2,870 |
|
| $ | 15,559 |
|
| $ | — |
|
| $ | 799 |
|
Supplemental disclosure of cash flow information |
|
|
|
|
| |||||||||||
Cash paid for interest |
| $ | 3,143 |
|
| $ | — |
| ||||||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
7
ENANTA PHARMACEUTICALS, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(unaudited)
(amounts in thousands, except per share data)
1. Nature of the Business and Basis of Presentation
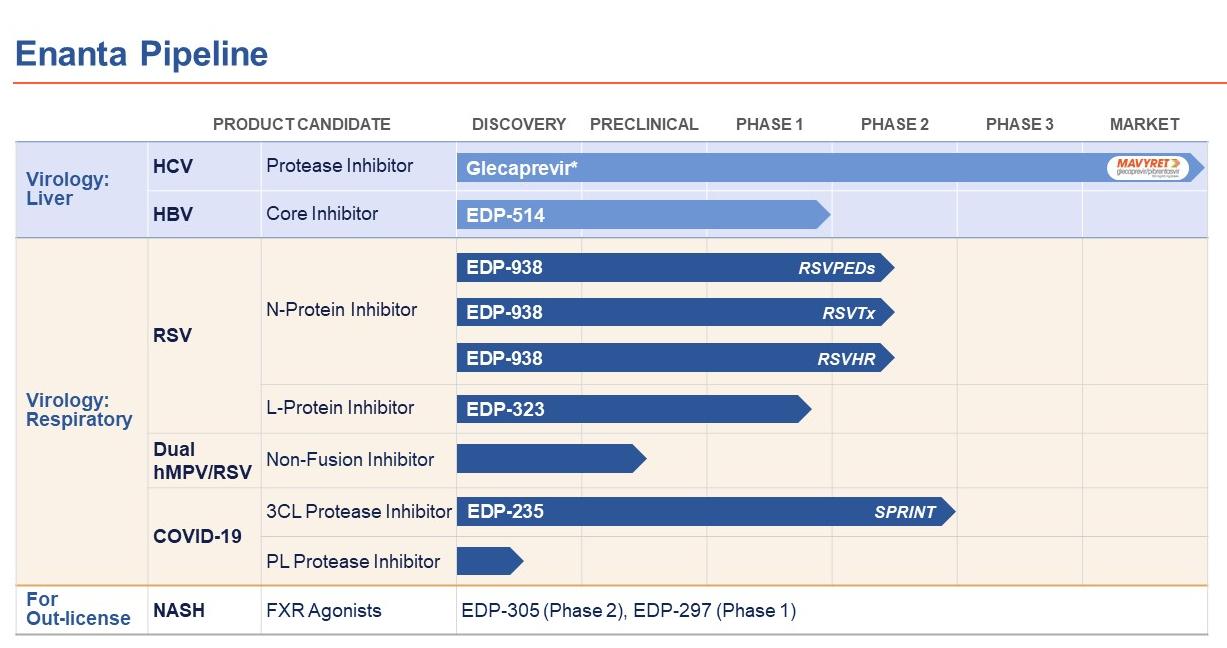
Enanta Pharmaceuticals, Inc. (the(collectively with its subsidiary, the “Company”), incorporated in Delaware in 1995, is a biotechnology company that uses its robust, chemistry-driven approach and drug discovery capabilities to become a leader in the discoverydiscover and development ofdevelop small molecule drugs with an emphasis on treatments for viral infections.virology and immunology indications. The Company discovered glecaprevir, the second of two protease inhibitors discovered and developed through its collaboration with AbbVie for the treatment of chronic infection with hepatitis C virus (“HCV”). Glecaprevir is co-formulated as part of AbbVie’s leading direct-acting antiviral (“DAA”) combination treatment for HCV, which is marketed under the tradenames MAVYRET® (U.S.) and MAVIRET®(ex-U.S.) (glecaprevir/pibrentasvir). Royalties from the Company’s AbbVie collaboration and its existing financial resources provide funding to support the Company’s wholly-owned research and development programs, which are primarily focused on the following disease targets: respiratory syncytial virus (“RSV”), SARS-CoV-2, human metapneumovirus (“hMPV”) and hepatitis B virus (“HBV”) and Chronic Spontaneous Urticaria (“CSU”).
The Company is subject to many of the risks common to companies in the biotechnology industry, including but not limited to, the uncertainties of research and development, competition from technological innovations of others, dependence on collaborative arrangements, protection of proprietary technology, dependence on key personnel and compliance with government regulation. Product candidates currently under development will require significant additional research and development efforts, including extensive preclinical and clinical testing and regulatory approvals, prior to commercialization. These efforts require significant amounts of capital, adequate personnel and infrastructure, and extensive compliance reporting capabilities.
COVID-19
In March 2020, the World Health Organization declared COVID-19 a global pandemic and countries worldwide implemented various measures to contain the spread of the SARS-CoV-2 virus. National, state and local governments in affected regions implemented varying safety precautions, including quarantines, border closures, increased border controls, travel restrictions, shelter-in-place orders and shutdowns, business closures, cancellations of public gatherings and other measures. The extent and severity of any continuing impact on the Company’s business and clinical trials will be determined largely by the extent to which there are disruptions in the supply chains for its research and product candidates, delays in the conduct of ongoing and future clinical trials, or reductions in the number of patients accessing AbbVie’s HCV regimens, or any combination of those events. In addition, AbbVie experienced a decline in HCV sales compared to periods prior to March 2020 as a result of reduced numbers of patients accessing AbbVie’s HCV regimens due to the COVID-19 pandemic.
The extent to which the COVID-19 pandemic will continue to impact, directly or indirectly, the Company’s business, results of operations and financial condition will depend on future developments that are highly uncertain and cannot be accurately predicted, including new variants and public health actions taken to contain their impact, as well as the cumulative economic impact of both of those factors and the public health impact of the announced ending of emergency public health measures.
Unaudited Interim Financial Information
The condensed consolidated balance sheet atas of September 30, 20222023 was derived from audited financial statements but does not include all disclosures required by accounting principles generally accepted in the United States of America (“GAAP”). The accompanying unaudited condensed consolidated financial statements as of MarchDecember 31, 2023 and for the three and six months ended MarchDecember 31, 2023 and 2022 have been prepared by the Company pursuant to the rules and regulations of the Securities and Exchange Commission (“SEC”) for interim financial statements. Certain information and footnote disclosures normally included in financial statements prepared in accordance with GAAP have been condensed or omitted pursuant to such rules and regulations. These condensed consolidated financial statements should be read in conjunction with the Company’s audited consolidated financial statements and the notes thereto included in the Company’s Annual Report on Form 10-K for the fiscal year ended September 30, 2022.2023.
In the opinion of management, all adjustments, consisting of normal recurring adjustments necessary for a fair statement of the Company’s financial position as of MarchDecember 31, 2023 and results of operations for the three and six months ended MarchDecember 31, 2023 and 2022 and cash flows for the sixthree months ended MarchDecember 31, 2023 and 2022 have been made. The results of operations for the three and six months ended MarchDecember 31, 2023 are not necessarily indicative of the results of operations that may be expected for subsequent quarters or the year ending September 30, 2023.
8
2024.
The accompanying condensed consolidated financial statements have been prepared in conformity with GAAP. All amounts in the condensed consolidated financial statements and in the notes to the condensed consolidated financial statements, except per share amounts, are in thousands unless otherwise indicated.
The accompanying condensed consolidated financial statements have been prepared based on continuity of operations, realization of assets and the satisfaction of liabilities and commitments in the ordinary course of business. The Company began reporting a net loss in fiscal 2020 and reported a net loss of $66,64433,407 for the sixthree months ended MarchDecember 31, 2023 and $121,755133,816 for the year ended September 30, 2022.2023. As of MarchDecember 31, 2023, the Company had an accumulated deficit of $139,823240,402. The Company expects to continue to generate operating losses for the foreseeable future as the Company continues to advance its wholly-owned programs. As of MarchDecember 31, 2023, the Company had $225,124337,151 in cash, cash equivalents and short-term and long-term marketable securities. The Company expects that its cash, cash equivalents short-term and long-termshort-term marketable securities as well as the $200,000 payment received in April 2023 from the sale of a portion of the MAVYRET/MAVIRET royalties and cash flows from the continuing portion of HCV royalties will be sufficient to fund its operating expenses and capital expenditure requirements for at least 12 months from the issuance date of the interim condensed consolidated financial statements. The Company may seek additional funding through equity offerings, non-dilutive financings, collaborations, strategic alliances or licensing agreements. The Company may not be able to obtain sufficient financing on acceptable terms, or at all, and the Company may not be able to enter into collaborations or other arrangements. The terms of any financing may adversely affect the holdings or the rights of the Company’s stockholders. If the Company is unable to obtain funding, the Company could be forced to delay, reduce or eliminate some or all of its research and development programs, product expansion or commercialization efforts, or the Company may be unable to continue operations.
8
2. Summary of Significant Accounting Policies
For the Company’s Significant Accounting Policies, please refer to its Annual Report on Form 10-K for the fiscal year ended September 30, 2022.2023. Any reference in these notes to applicable guidance is meant to refer to the authoritative GAAP as found in the Accounting Standards Codification and Accounting Standards Update (“ASU”) of the Financial Accounting Standards Board (“FASB”).
Use of Estimates
The preparation of condensed consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the condensed consolidated financial statements, and the reported amounts of revenues and expenses during the reporting period. Significant estimates and assumptions reflected in these condensed consolidated financial statements include, but are not limited to, management’s judgments with respect to its revenue arrangements; liability related to the sale of future royalties; valuation of stock-based awards and the accrual of research and development expenses. Estimates are periodically reviewed in light of changes in circumstances, facts and experience. The future developments of the COVID-19 pandemic also may directly or indirectly impact the Company’s business. The Company has made estimates of the impact of COVID-19 in the Company’s consolidated financial statements as of March 31, 2023. Actual results could differ from the Company’s estimates.
Net Income (Loss)Loss per Share
Basic net income (loss)loss per common share is computed by dividing the net income (loss)loss by the weighted average number of shares of common stock outstanding for the period. DilutedIn periods in which the Company has reported a net income (loss) per common share is computed by dividing net income (loss) by the weighted average number of common shares outstanding for the period, including potential dilutive common shares assuming the dilutive effect of outstanding stock options and unvested restricted stock units. For periods presented,loss, diluted net loss per common share is the same as basic net loss per common share since dilutive common shares are not assumed to have been issued if their effect is anti-dilutive.
The Company reported net losses for each of the three and six months ended March 31, 2023 and 2022. TheTherefore, the Company excluded the following potential common shares, presented based on amounts outstanding at each period end, from the computation of diluted net loss per share for the periods indicated because including themas its effect would have had an anti-dilutive effect:been anti-dilutive:
|
| As of March 31, |
| |||||
|
| 2023 |
|
| 2022 |
| ||
|
| (in thousands) |
| |||||
Options to purchase common stock |
|
| 4,522 |
|
|
| 4,124 |
|
Unvested rTSRUs |
|
| 81 |
|
|
| 112 |
|
Unvested PSUs |
|
| 81 |
|
|
| 112 |
|
Unvested restricted stock units |
|
| 430 |
|
|
| 217 |
|
9
|
| As of December 31, |
| |||||
|
| 2023 |
|
| 2022 |
| ||
|
| (in thousands) |
| |||||
Options to purchase common stock |
|
| 5,213 |
|
|
| 4,511 |
|
Unvested rTSRUs |
|
| 129 |
|
|
| 151 |
|
Unvested PSUs |
|
| 129 |
|
|
| 151 |
|
Unvested restricted stock units |
|
| 455 |
|
|
| 439 |
|
Recently Issued Accounting Pronouncements
Other accounting standards that have been issued or proposed byIn November 2023, the FASB orissued ASU 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures, which requires public entities to disclose information about their reportable segments’ significant expenses and other standards-setting bodiessegment items on an interim and annual basis. Public entities with a single reportable segment are required to apply the disclosure requirements in ASU 2023-07, as well as all existing segment disclosures and reconciliation requirements in ASC 280 on an interim and annual basis. This amendment is effective for the Company in the fiscal year beginning October 1, 2024, with early adoption permitted. The Company is currently evaluating the potential impact that do not requireASU 2023-07 may have on its financial statement disclosures.
In December 2023, the FASB issued ASU 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures, which requires public entities, on an annual basis, to provide disclosure of specific categories in the rate reconciliation, as well as disclosure of income taxes paid disaggregated by jurisdiction. ASU 2023-09 is effective for the Company in the fiscal year beginning October 1, 2025, with early adoption until a future date are not expected topermitted. The Company is currently evaluating the potential impact that ASU 2023-09 may have a material impact on the Company’s consolidatedits financial statements upon adoption.statement disclosures.
9
3. Fair Value of Financial Assets and Liabilities
The following tables present information about the Company’s financial assets and liabilities that were subject to fair value measurement on a recurring basis as of MarchDecember 31, 2023 and September 30, 2022,2023, and indicate the fair value hierarchy of the valuation inputs utilized to determine such fair value:
|
| Fair Value Measurements at March 31, 2023 Using: |
|
| Fair Value Measurements as of December 31, 2023 Using: |
| ||||||||||||||||||||||||||
|
| Level 1 |
|
| Level 2 |
|
| Level 3 |
|
| Total |
|
| Level 1 |
|
| Level 2 |
|
| Level 3 |
|
| Total |
| ||||||||
|
| (in thousands) |
|
| (in thousands) |
| ||||||||||||||||||||||||||
Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||
Cash equivalents: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||
Money market funds |
| $ | 70,562 |
|
| $ | — |
|
| $ | — |
|
| $ | 70,562 |
|
| $ | 38,905 |
|
| $ | — |
|
| $ | — |
|
| $ | 38,905 |
|
Marketable securities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||
U.S. Treasury notes |
|
| 22,003 |
|
|
| — |
|
|
| — |
|
|
| 22,003 |
|
|
| 264,537 |
|
|
| — |
|
|
| — |
|
|
| 264,537 |
|
Corporate bonds |
|
| — |
|
|
| 46,124 |
|
|
| — |
|
|
| 46,124 |
|
|
| — |
|
|
| 26,706 |
|
|
| — |
|
|
| 26,706 |
|
Commercial paper |
|
| — |
|
|
| 83,819 |
|
|
| — |
|
|
| 83,819 |
|
|
| — |
|
|
| 5,975 |
|
|
| — |
|
|
| 5,975 |
|
|
| $ | 92,565 |
|
| $ | 129,943 |
|
| $ | — |
|
| $ | 222,508 |
|
| $ | 303,442 |
|
| $ | 32,681 |
|
| $ | — |
|
| $ | 336,123 |
|
Liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||
Series 1 nonconvertible preferred stock |
| $ | — |
|
| $ | — |
|
| $ | 1,423 |
|
| $ | 1,423 |
|
| $ | — |
|
| $ | — |
|
| $ | 1,423 |
|
| $ | 1,423 |
|
|
| $ | — |
|
| $ | — |
|
| $ | 1,423 |
|
| $ | 1,423 |
|
| $ | — |
|
| $ | — |
|
| $ | 1,423 |
|
| $ | 1,423 |
|
|
| Fair Value Measurements at September 30, 2022 Using: |
|
| Fair Value Measurements as of September 30, 2023 Using: |
| ||||||||||||||||||||||||||
|
| Level 1 |
|
| Level 2 |
|
| Level 3 |
|
| Total |
|
| Level 1 |
|
| Level 2 |
|
| Level 3 |
|
| Total |
| ||||||||
|
| (in thousands) |
|
| (in thousands) |
| ||||||||||||||||||||||||||
Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||
Cash equivalents: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||
Money market funds |
| $ | 13,905 |
|
| $ | — |
|
| $ | — |
|
| $ | 13,905 |
|
| $ | 55,357 |
|
| $ | — |
|
| $ | — |
|
| $ | 55,357 |
|
U.S. Treasury notes |
|
| 29,755 |
|
|
| — |
|
|
| — |
|
|
| 29,755 |
| ||||||||||||||||
Marketable securities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||
U.S. Treasury notes |
|
| 91,328 |
|
|
| — |
|
|
| — |
|
|
| 91,328 |
|
|
| 236,782 |
|
|
| — |
|
|
| — |
|
|
| 236,782 |
|
Corporate bonds |
|
| — |
|
|
| 76,411 |
|
|
| — |
|
|
| 76,411 |
|
|
| — |
|
|
| 26,435 |
|
|
| — |
|
|
| 26,435 |
|
Commercial paper |
|
| — |
|
|
| 66,784 |
|
|
| — |
|
|
| 66,784 |
|
|
| — |
|
|
| 21,305 |
|
|
| — |
|
|
| 21,305 |
|
|
| $ | 105,233 |
|
| $ | 143,195 |
|
| $ | — |
|
| $ | 248,428 |
|
| $ | 321,894 |
|
| $ | 47,740 |
|
| $ | — |
|
| $ | 369,634 |
|
Liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||
Series 1 nonconvertible preferred stock |
| $ | — |
|
| $ | — |
|
| $ | 1,423 |
|
| $ | 1,423 |
|
| $ | — |
|
| $ | — |
|
| $ | 1,423 |
|
| $ | 1,423 |
|
|
| $ | — |
|
| $ | — |
|
| $ | 1,423 |
|
| $ | 1,423 |
|
| $ | — |
|
| $ | — |
|
| $ | 1,423 |
|
| $ | 1,423 |
|
During the three and six months ended MarchDecember 31, 2023 and 2022, there were no transfers between Level 1, Level 2 and Level 3.
The fair value of Level 2 instruments classified as marketable securities were determined through third-party pricing services. The pricing services use many observable market inputs to determine value, including reportable trades, benchmark yields, credit spreads, broker/dealer quotes, bids, offers, and current spot rates.
The outstanding shares of Series 1 nonconvertible preferred stock as of MarchDecember 31, 2023 and September 30, 20222023 are measured at fair value. These outstanding shares are financial instruments that might require a transfer of assets because of the liquidation features in the contract and are therefore recorded as liabilities and measured at fair value. The fair value of the outstanding shares is based on significant inputs not observable in the market, which represent a Level 3 measurement within the fair value hierarchy. The Company utilizes a probability-weighted valuation model which takes into consideration various outcomes that may require the Company to transfer assets upon liquidation. Changes in the fair values of the Series 1 nonconvertible preferred stock are recognized in other income (expense) in the condensed consolidated statements of operations.
The recurring Level 3 fair value measurements of the Company’s outstanding Series 1 nonconvertible preferred stock using probability-weighted discounted cash flow include the following significant unobservable inputs:
|
| Range |
|
| Range |
| ||||
|
| March 31, |
| September 30, |
|
| December 31, |
| September 30, |
|
Unobservable Input |
| 2023 |
| 2022 |
|
| 2023 |
| 2023 |
|
Probabilities of payout |
| 0%-65% |
| 0%-65% |
|
| 0%-65% |
| 0%-65% |
|
Discount rate |
| 7.25% |
| 7.25% |
|
| 7.25% |
| 7.25% |
|
10
There were no changes in the fair value of nonconvertible preferred stock during the three and six months ended MarchDecember 31, 2023 orand 2022.
In April 2023, the Company entered into a royalty sale agreement with an affiliate of OMERS, pursuant to which the Company was paid a $200,000 cash purchase price in exchange for 54.5% of future quarterly royalty payments on net sales of MAVYRET/MAVIRET, after June 30, 2023, through June 30, 2032, subject to a cap on aggregate payments equal to 1.42 times the purchase price. The Company accounted for the upfront payment as a liability related to the sale of future royalties. The carrying value of the liability related to the sale of future royalties approximates fair value as of December 31, 2023 and is based on current estimates of future royalties expected to be paid to OMERS over the next 10 years, which are considered Level 3 inputs. See Note 7 for a rollforward of the liability.
4. Marketable Securities
As of MarchDecember 31, 2023 and September 30, 2022,2023, the fair value of available-for-sale marketable securities, by type of security, was as follows:
|
| March 31, 2023 |
|
| December 31, 2023 |
| ||||||||||||||||||||||||||||||||||
|
| Amortized |
|
| Gross |
|
| Gross |
|
| Credit Losses |
|
| Fair Value |
|
| Amortized |
|
| Gross |
|
| Gross |
|
| Credit Losses |
|
| Fair Value |
| ||||||||||
|
| (in thousands) |
|
| (in thousands) |
| ||||||||||||||||||||||||||||||||||
Corporate bonds |
| $ | 47,329 |
|
| $ | 4 |
|
| $ | (1,209 | ) |
| $ | — |
|
| $ | 46,124 |
|
| $ | 27,043 |
|
| $ | — |
|
| $ | (337 | ) |
| $ | — |
|
| $ | 26,706 |
|
Commercial paper |
|
| 83,819 |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| 83,819 |
|
|
| 5,975 |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| 5,975 |
|
U.S. Treasury notes |
|
| 22,229 |
|
|
| 14 |
|
|
| (240 | ) |
|
| — |
|
|
| 22,003 |
|
|
| 264,350 |
|
|
| 207 |
|
|
| (20 | ) |
|
| — |
|
|
| 264,537 |
|
|
| $ | 153,377 |
|
| $ | 18 |
|
| $ | (1,449 | ) |
| $ | — |
|
| $ | 151,946 |
|
| $ | 297,368 |
|
| $ | 207 |
|
| $ | (357 | ) |
| $ | — |
|
| $ | 297,218 |
|
|
| September 30, 2022 |
|
| September 30, 2023 |
| ||||||||||||||||||||||||||||||||||
|
| Amortized |
|
| Gross |
|
| Gross |
|
| Credit Losses |
|
| Fair Value |
|
| Amortized |
|
| Gross |
|
| Gross |
|
| Credit Losses |
|
| Fair Value |
| ||||||||||
|
| (in thousands) |
|
| (in thousands) |
| ||||||||||||||||||||||||||||||||||
Corporate bonds |
| $ | 78,663 |
|
| $ | — |
|
| $ | (2,252 | ) |
| $ | — |
|
| $ | 76,411 |
|
| $ | 27,127 |
|
| $ | — |
|
| $ | (692 | ) |
| $ | — |
|
| $ | 26,435 |
|
Commercial paper |
|
| 66,784 |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| 66,784 |
|
|
| 21,305 |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| 21,305 |
|
U.S. Treasury notes |
|
| 92,416 |
|
|
| — |
|
|
| (1,088 | ) |
|
| — |
|
|
| 91,328 |
|
|
| 236,880 |
|
|
| 12 |
|
|
| (110 | ) |
|
| — |
|
|
| 236,782 |
|
|
| $ | 237,863 |
|
| $ | — |
|
| $ | (3,340 | ) |
| $ | — |
|
| $ | 234,523 |
|
| $ | 285,312 |
|
| $ | 12 |
|
| $ | (802 | ) |
| $ | — |
|
| $ | 284,522 |
|
As of MarchDecember 31, 2023 and September 30, 2022,2023, marketable securities consisted of investments that mature within one year, with the exception of certain corporate bonds and U.S. Treasury notes, which have maturities between one and two years and an aggregate fair value of $15,040 and $29,285, respectively..
5. Accrued Expenses
Accrued expenses and other current liabilities consisted of the following as of MarchDecember 31, 2023 and September 30, 2022:2023:
|
| March 31, |
|
| September 30, |
|
| December 31, |
|
| September 30, |
| ||||
|
| 2023 |
|
| 2022 |
|
| 2023 |
|
| 2023 |
| ||||
|
| (in thousands) |
|
| (in thousands) |
| ||||||||||
Accrued pharmaceutical drug manufacturing |
| $ | 4,521 |
|
| $ | 6,932 |
|
| $ | 1,099 |
|
| $ | 3,083 |
|
Accrued research and development expenses |
|
| 5,380 |
|
|
| 5,532 |
|
|
| 3,707 |
|
|
| 6,120 |
|
Accrued payroll and related expenses |
|
| 3,075 |
|
|
| 6,439 |
|
|
| 4,310 |
|
|
| 7,037 |
|
Accrued professional fees |
|
| 1,639 |
|
|
| 1,273 |
| ||||||||
Accrued other |
|
| 867 |
|
|
| 760 |
|
|
| 2,487 |
|
|
| 2,099 |
|
|
| $ | 15,482 |
|
| $ | 20,936 |
|
| $ | 11,603 |
|
| $ | 18,339 |
|
11
6. AbbVie Collaboration
The Company has a Collaborative Development and License Agreement (as amended, the “AbbVie Agreement”), with AbbVie to identify, develop and commercialize HCV NS3 and NS3/4A protease inhibitor compounds, including paritaprevir and glecaprevir, under which the Company has received license payments, proceeds from a sale of preferred stock, research funding payments, milestone payments and royalties totaling approximately $1,250,0001,296,000 through MarchDecember 31, 2023. Since the Company satisfied all of its performance obligations under the AbbVie Agreement by the end of fiscal 2011, all milestone payments received since then have been recognized as revenue when the milestones were achieved by AbbVie.
The Company is receiving annually tiered royalties per Company protease product ranging from ten percent up to twenty percent, or on a blended basis from ten percent up to the high teens, on the portion of AbbVie’s calendar year net sales of each HCV regimen that is allocated to the protease inhibitor in the regimen. Beginning with each January 1, the cumulative net sales of a given royalty-bearing protease inhibitor product start at zero for purposes of calculating the tiered royalties on a product-by-product basis.
117. Liability Related to the Sale of Future Royalties
In April 2023, the Company entered into a royalty sale agreement with an affiliate of OMERS, pursuant to which the Company was paid a $200,000 cash purchase price in exchange for 54.5% of future quarterly royalty payments on net sales of MAVYRET/MAVIRET, after June 30, 2023, through June 30, 2032, subject to a cap on aggregate payments equal to 1.42 times the purchase price.
Because the royalty sale agreement will be paid back to OMERS up to a capped amount as well as the Company’s significant continuing involvement in the generation of future cash flows under its AbbVie Agreement, the Company recorded the proceeds from the transaction as a liability on its condensed consolidated balance sheets which will be amortized as interest expense in the condensed consolidated statements of operations under the effective interest rate method over the life of the royalty sale agreement. The Company will continue to record the full amount of royalties earned on MAVYRET/MAVIRET sales as royalty revenue in the condensed consolidated statements of operations.
The Company’s liability related to the sale of future royalties is estimated based on forecasted worldwide MAVYRET/MAVYRET royalties to be paid to OMERS over the course of the royalty sale agreement. This estimate requires significant judgment, including the amount and timing of royalty payments up until the end of the royalty sale agreement, which is estimated to be the stated term of June 30, 2032. As royalties are earned by OMERS, the liability is reduced on the Company’s condensed consolidated balance sheets.
At December 31, 2023, the estimated future cash flows resulted in an effective annual imputed interest rate of approximately 7.05%.
7.The following table summarizes the activity of the liability related to the sale of future royalties:
|
| Liability related to the sale of future royalties |
| |
|
| (in thousands) |
| |
Balance - September 30, 2023 |
| $ | 194,505 |
|
Royalty payable to purchaser |
|
| (9,822 | ) |
Interest expense |
|
| 3,441 |
|
Balance - December 31, 2023 |
| $ | 188,124 |
|
8. Series 1 Nonconvertible Preferred Stock
As of MarchDecember 31, 2023, 1,930 shares of Series 1 nonconvertible preferred stock were issued and outstanding. Since theseThe outstanding shares qualify asare financial instruments that might require a derivative,transfer of assets because of the outstanding sharesliquidation features in the contract and are carried at fair value as a liability on the Company’s condensed consolidated balance sheets.sheets.
8.9. Stock-Based Awards
The Company grants stock-based awards, including stock options, restricted stock units and other unit awards under its 2019 Equity Incentive Plan (the “2019 Plan”), which was approved by its stockholders on February 28, 2019 and amended in March 2021, March 2022 and March 2023. The Company also has outstanding stock option awards under its 2012 Equity Incentive Plan (the “2012 Plan”) and its amended and restated 1995 Equity Incentive Plan (the “1995 Plan”), but is no longer granting awards under these plans.this plan.
12
The following table summarizes stock option activity, including performance-based options, for the year-to-date period ending MarchDecember 31, 2023:
|
| Shares |
|
| Weighted |
|
| Weighted |
|
| Aggregate |
| ||||
|
| (in thousands) |
|
|
|
|
| (in years) |
|
| (in thousands) |
| ||||
Outstanding as of September 30, 2022 |
|
| 3,993 |
|
| $ | 53.57 |
|
|
| 6.2 |
|
| $ | 28,778 |
|
Granted |
|
| 728 |
|
|
| 45.44 |
|
|
|
|
|
|
| ||
Exercised |
|
| (117 | ) |
|
| 17.14 |
|
|
|
|
|
|
| ||
Forfeited |
|
| (82 | ) |
|
| 65.95 |
|
|
|
|
|
|
| ||
Outstanding as of March 31, 2023 |
|
| 4,522 |
|
| $ | 52.98 |
|
|
| 6.5 |
|
| $ | 7,572 |
|
Options vested and expected to vest as of |
|
| 4,522 |
|
| $ | 52.98 |
|
|
| 6.5 |
|
| $ | 7,572 |
|
Options exercisable as of March 31, 2023 |
|
| 2,801 |
|
| $ | 51.94 |
|
|
| 5.0 |
|
| $ | 7,554 |
|
|
| Shares |
|
| Weighted |
|
| Weighted |
|
| Aggregate |
| ||||
|
| (in thousands) |
|
|
|
|
| (in years) |
|
| (in thousands) |
| ||||
Outstanding as of September 30, 2023 |
|
| 4,365 |
|
| $ | 52.68 |
|
|
| 5.9 |
|
| $ | — |
|
Granted |
|
| 1,092 |
|
|
| 8.99 |
|
|
|
|
|
|
| ||
Exercised |
|
| — |
|
|
| — |
|
|
|
|
|
|
| ||
Forfeited |
|
| (244 | ) |
|
| 38.99 |
|
|
|
|
|
|
| ||
Outstanding as of December 31, 2023 |
|
| 5,213 |
|
| $ | 44.17 |
|
|
| 6.7 |
|
| $ | 459 |
|
Options vested and expected to vest as of |
|
| 5,213 |
|
| $ | 44.17 |
|
|
| 6.7 |
|
| $ | 459 |
|
Options exercisable as of December 31, 2023 |
|
| 3,028 |
|
| $ | 53.37 |
|
|
| 5.1 |
|
| $ | — |
|
Market and Performance-Based Stock Unit Awards
The Company awards both performance share units, or PSUs, and relative total stockholder return units, or rTSRUs, to its executive officers. The number of units granted represents the target number of shares of common stock that may be earned; however, the actual number of shares that may be earned ranges from 0% to 150% of the target number. The number of shares cancelled represents the target number of shares, less any shares that vested. The following table summarizes PSU and rTSRU activity for the year-to-date period ending MarchDecember 31, 2023:
|
| PSUs |
|
| rTSRUs |
| ||||||||||
|
| Shares |
|
| Weighted |
|
| Shares |
|
| Weighted |
| ||||
|
| (in thousands, except per share data) |
| |||||||||||||
Unvested at September 30, 2022 |
|
| 101 |
|
| $ | 54.50 |
|
|
| 101 |
|
| $ | 36.14 |
|
Granted |
|
| 50 |
|
|
| 47.24 |
|
|
| 50 |
|
|
| 36.91 |
|
Vested |
|
| (70 | ) |
|
| 44.58 |
|
|
| (70 | ) |
|
| 23.89 |
|
Cancelled |
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
Unvested at March 31, 2023 |
|
| 81 |
|
| $ | 58.58 |
|
|
| 81 |
|
| $ | 45.82 |
|
During the six months ended March 31, 2023, a total of 100% of the target PSUs and 127% of the target rTSRUs granted in December 2020 vested, resulting in the issuance of an aggregate of 104 common shares, net of share withholding.
|
| PSUs |
|
| rTSRUs |
| ||||||||||
|
| Shares |
|
| Weighted |
|
| Shares |
|
| Weighted |
| ||||
|
| (in thousands) |
|
|
|
|
| (in thousands) |
|
|
|
| ||||
Unvested as of September 30, 2023 |
|
| 81 |
|
| $ | 58.58 |
|
|
| 81 |
|
| $ | 45.82 |
|
Granted |
|
| 48 |
|
|
| 9.21 |
|
|
| 48 |
|
|
| 9.89 |
|
Vested |
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
Unvested as of December 31, 2023 |
|
| 129 |
|
| $ | 40.23 |
|
|
| 129 |
|
| $ | 32.47 |
|
12
Restricted Stock Units
The following table summarizes the restricted stock unit activity for the year-to-date period ending MarchDecember 31, 2023:
|
| Restricted Stock |
|
| Weighted |
|
| Restricted Stock |
|
| Weighted |
| ||||
|
| (in thousands, except per share data) |
|
| (in thousands) |
|
|
| ||||||||
Unvested at September 30, 2022 |
|
| 219 |
|
| $ | 64.03 |
| ||||||||
Unvested as of September 30, 2023 |
|
| 411 |
|
| $ | 51.78 |
| ||||||||
Granted |
|
| 277 |
|
|
| 45.00 |
|
|
| 162 |
|
|
| 8.99 |
|
Vested |
|
| (56 | ) |
|
| 61.10 |
|
|
| (116 | ) |
|
| 52.00 |
|
Cancelled |
|
| (10 | ) |
|
| 54.51 |
|
|
| (2 | ) |
|
| 54.27 |
|
Unvested at March 31, 2023 |
|
| 430 |
|
| $ | 52.37 |
| ||||||||
Unvested as of December 31, 2023 |
|
| 455 |
|
| $ | 36.45 |
| ||||||||
Stock-Based Compensation Expense
During the three and six months ended MarchDecember 31, 2023 and 2022 the Company recognized the following stock-based compensation expense:
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
|
| Three Months Ended March 31, |
|
| Six Months Ended March 31, |
|
| Three Months Ended December 31, |
| |||||||||||||||
|
| 2023 |
|
| 2022 |
|
| 2023 |
|
| 2022 |
|
| 2023 |
|
| 2022 |
| ||||||
|
| (in thousands) |
|
| (in thousands) |
| ||||||||||||||||||
Research and development |
| $ | 2,520 |
|
| $ | 2,710 |
|
| $ | 5,052 |
|
| $ | 5,294 |
|
| $ | 2,055 |
|
| $ | 2,532 |
|
General and administrative |
|
| 4,843 |
|
|
| 3,761 |
|
|
| 9,450 |
|
|
| 7,239 |
|
|
| 6,044 |
|
|
| 4,607 |
|
| $ | 7,363 |
|
| $ | 6,471 |
|
| $ | 14,502 |
|
| $ | 12,533 |
|
| $ | 8,099 |
|
| $ | 7,139 |
| |
|
|
|
|
|
|
|
|
|
| |||||||
|
| Three Months Ended March 31, |
|
| Six Months Ended March 31, |
| ||||||||||
|
| 2023 |
|
| 2022 |
|
| 2023 |
|
| 2022 |
| ||||
|
| (in thousands) |
| |||||||||||||
Stock options |
| $ | 5,011 |
|
| $ | 4,962 |
|
| $ | 10,057 |
|
| $ | 9,710 |
|
rTSRUs |
|
| 546 |
|
|
| 531 |
|
|
| 1,007 |
|
|
| 823 |
|
Performance stock units |
|
| 175 |
|
|
| 79 |
|
|
| 542 |
|
|
| 512 |
|
Restricted stock units |
|
| 1,631 |
|
|
| 899 |
|
|
| 2,896 |
|
|
| 1,488 |
|
| $ | 7,363 |
|
| $ | 6,471 |
|
| $ | 14,502 |
|
| $ | 12,533 |
| |
13
|
|
|
| |||||
|
| Three Months Ended December 31, |
| |||||
|
| 2023 |
|
| 2022 |
| ||
|
| (in thousands) |
| |||||
Stock options |
| $ | 4,462 |
|
| $ | 5,046 |
|
rTSRUs |
|
| 445 |
|
|
| 461 |
|
Performance stock units |
|
| 1,579 |
|
|
| 367 |
|
Restricted stock units |
|
| 1,613 |
|
|
| 1,265 |
|
| $ | 8,099 |
|
| $ | 7,139 |
| |
During the three and six months ended MarchDecember 31, 2023 and 2022, the Company recognized stock-based compensation expense for performance-based stock units for which vesting became probable upon achievement of performance-based targets that occurred during the performance period.
As of MarchDecember 31, 2023, the Company had an aggregate of $70,25360,806 of unrecognized stock-based compensation cost, which is expected to be recognized over a weighted average period of 2.62.2 years.
9.10. Income Taxes
For the three months ended December 31, 2023, the Company recorded an income tax benefit of $622 due to interest recorded on a pending federal income tax refund. For the three months ended December 31, 2022, the Company recorded an income tax benefit of $34 due to the release of a state tax reserve during the period.
11. Commitments and Contingencies
Litigation and Contingencies Related to Use of Intellectual Property
From time to time, the Company may become subject to legal proceedings, claims and litigation arising in the ordinary course of business. For example,Except as described below, the Company currently is not a party to any material threatened or pending litigation. However, third parties might allege that the Company or its collaborators are infringing their patent rights or that the Company is otherwise violating their intellectual property rights. Such third parties may resort to litigation against the Company or its collaborators, which the Company has agreed to indemnify. With respect to some of these patents, the Company expects that it will be required to obtain licenses and could be required to pay license fees or royalties, or both. These licenses may not be available on acceptable terms, or at all. A costly license, or inability to obtain a necessary license, would have a material adverse effect on the Company’s financial condition, results of operations or cash flows. The Company accrues contingent liabilities when it is probable that future expenditures will be made and such expenditures can be reasonably estimated.
OnIn June 21, 2022, the Company announced that it filed suit in the United States District Court for the District of Massachusetts on June 21, 2022, against Pfizer, Inc. seeking damages for infringement of U.S. Patent No. 11,358,953 (the ’953 Patent) in the manufacture, use and sale of Pfizer’s COVID-19 antiviral, Paxlovid™ (nirmatrelvir tablets; ritonavir tablets). The United States Patent and Trademark Office awarded the '953 Patent to the Company in June 2022 based on the Company's July 2020 patent application describing coronavirus protease inhibitors invented by the Company. The Company is seeking fair compensation for Pfizer’s use of a coronavirus protease inhibitor
13
claimed in the ‘953 patent. The Company records all legal expenses associated with the patent infringement suit as incurred in the condensed consolidated statements of operations.
The Company currently is not a party to any other litigation.
Indemnification Agreements
In the ordinary course of business, the Company may provide indemnifications of varying scope and terms to customers, vendors, lessors, business partners, and other parties with respect to certain matters including, but not limited to, losses arising out of breach of such agreements or from services to be provided to the Company, or from intellectual property infringement claims made by third parties. In addition, the Company has entered into indemnification agreements with members of its board of directors and its executive officers that will require the Company, among other things, to indemnify them against certain liabilities that may arise by reason of their status or service as directors or officers. The maximum potential amount of future payments the Company could be required to make under these indemnification agreements is, in many cases, unlimited. To date, the Company has not incurred any material costs as a result of such indemnifications. In addition, the Company maintains directors’ and officers’ insurance coverage. The Company does not believe that the outcome of any claims under indemnification arrangements will have a material effect on its financial position, results of operations or cash flows, and has not accrued any liabilities related to such obligations in its condensed consolidated financial statements as of MarchDecember 31, 2023.
14
Leases
The Company leases laboratory and office space under various non-cancelable operating leases. There have been no material changes to the Company’s leases during the three and six months ended MarchDecember 31, 2023. For additional information, please read Note 11,12, Leases, to the consolidated financial statements in the Company's Annual Report on Form 10-K for the fiscal year ended September 30, 2022.2023.
10. Subsequent Events15
In April 2023, the Company entered into a royalty sale agreement with an affiliate of OMERS, pursuant to which the Company was paid a $200,000 cash purchase price in exchange for 54.5% of the Company’s future quarterly royalty payments on net sales of MAVYRET/MAVIRET, beginning with the payment for the quarter ending September 30, 2023, through June 30, 2032, subject to a cap on aggregate payments equal to 1.42 times the purchase price.
14
ITEM 2. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with the unaudited condensed consolidated financial statements and notes thereto included elsewhere in this Quarterly Report on Form 10-Q, or Form 10-Q, and the audited consolidated financial statements and notes thereto for our fiscal year ended September 30, 20222023 included in our Annual Report on Form 10-K for that fiscal year, which is referred to as our 20222023 Form 10-K. Please refer to our note regarding forward-looking statements on page 2 of this Form 10-Q, which is incorporated herein by this reference.
The Enanta name and logo are our trademarks. This Form 10-Q also includes trademarks, trade names and service marks of other persons. All other trademarks, trade names and service marks appearing in this Form 10-Q are the property of their respective owners.
Overview
We are a biotechnology company that uses our robust, chemistry-driven approach and drug discovery capabilities to become a leader in the discoverydiscover and development ofdevelop small molecule drugs with an emphasis on treatments for viral infections.virology and immunology indications. We discovered glecaprevir, the second of two protease inhibitors discovered and developed through our collaboration with AbbVie for the treatment of chronic infection with hepatitis C virus, or HCV. Glecaprevir is co-formulated as part of AbbVie’s leading brand of direct-acting antiviral, or DAA, combination treatment for HCV, which is marketed under the tradenames MAVYRET® (U.S.) and MAVIRET® (ex-U.S.) (glecaprevir/pibrentasvir). OurThe ongoing royalties from our AbbVie collaboration, providecombined with the proceeds from our April 2023 royalty sale transaction, have provided us funding to support our wholly-owned research and development programs which are primarily focused on the following disease targets:
Virology:
Since fiscal 2020, we have reportedImmunology:
As of MarchDecember 31, 2023, we had an accumulated deficit of $139.8 million. We expect to continue to incur net losses for the foreseeable future. As a result, we may need additional funding for expenses related to our operating activities, including general and administrative expenses and research and development expenses.
Because of the numerous risks and uncertainties associated with clinical development and commercialization, we are unable to accurately predict the timing or amount of increased expenses or when, or if, we will be able to achieve or maintain profitability. Until such time, if ever, as we can generate substantial revenue sufficient to achieve profitability, we expect to finance our operations through a combination of equity offerings, non-dilutive financings, collaborations, strategic alliances or licensing agreements. We may be unable to raise additional funds or enter into such other agreements or arrangements when needed on favorable terms, or at all. If we are unable to raise capital or enter into such agreements as, and when, needed, we may have to significantly delay, scale back or discontinue the further development and commercialization efforts of one or more of our products, or may be forced to reduce or terminate our operations.
15
As of March 31, 2023, we had $225.1$337.2 million in cash, cash equivalents and short-term and long-term marketable securities. In April 2023, we entered into a royalty sale agreement with an affiliate of OMERS, which paid us a $200.0 million cash purchase price in exchange for 54.5% of our future quarterly royalty payments on net sales of MAVYRET/MAVIRET, beginning with the payment for the quarter ending September 30, 2023, through June 30, 2032, subject to a cap on aggregate payments equal to 1.42 times the purchase price. We believeexpect that our existing cash, cash equivalents, and short-term and long-term marketable securities as well as the $200.0 million payment received in our April 2023 royalty sale transaction and our continuingretained portion of future HCV royalties, will enable us to fund our operating expenses and capital expenditure requirements into calendar 2026. We have based this estimate on assumptions that may prove to be wrong, and we could exhaust our available capital resources sooner than we expect. See “Liquidity and Capital Resources.”through fiscal 2027.
16
Our Wholly-Owned Programs
Our primary wholly-owned research and development programs are in virology namelyand immunology. Our virology programs have been focused on RSV, SARS-CoV-2 hMPV and HBV:HBV and our immunology program is focused on oral inhibitors of the receptor tyrosine kinase known as KIT for the treatment of CSU, and potentially other mast-cell-driven diseases.
Virology Programs:
16
17
17
Immunology Program:
We have utilized our internal chemistry and drug discovery capabilities to generate all of our development-stage programs. We continue to invest substantial resources in research programs to discover back-up compounds as well as new compounds targeting different mechanisms of action, both in ournew disease areas of focus as well as potentially in other disease areas.
18
The following table summarizes our product development pipeline in our virology program:programs:


*Fixed-dose antiviral combination contains glecaprevir and AbbVie’s NS5A inhibitor, pibrentasvir. Marketed by AbbVie as MAVYRET® (U.S.) and MAVIRET® (ex-U.S.).
Our Royalty Revenue Collaboration and Royalty Sale Agreement
Our royalty revenue is generated through our Collaborative Development and License Agreement with AbbVie, under which we have discovered and out-licensed to AbbVie two protease inhibitor compounds that have been clinically tested, manufactured, and commercialized by AbbVie as part of its combination regimens for HCV.
Glecaprevir is the HCV protease inhibitor we discovered that was developed by AbbVie in a fixed-dose combination with its NS5A inhibitor, pibrentasvir, for the treatment of chronic HCV. This patented combination, currently marketed under the brand names MAVYRET® (U.S.) and MAVIRET® (ex-U.S.), is referred to in this report as MAVYRET/MAVIRET. The first protease inhibitor
18
developed through this collaboration, paritaprevir, is part of Abbvie’s initial HCV regimens, which have been almost entirely replaced by MAVYRET/MAVIRET. Since August 2017, substantially all of our royalty revenue has been derived from AbbVie’s net sales of MAVYRET/MAVIRET. Our ongoing royalty revenues from this regimen consist of annually tiered, double-digit, per-product royalties on 50% of the calendar year net sales of the 2-DAA glecaprevir/pibrentasvir combination in MAVYRET/MAVIRET. The annual royalty tiers return to the lowest tier for sales on and after each January 1.
COVID-19 Update
The COVID-19 pandemic hadIn April 2023, we entered into a royalty sale agreement with an impactaffiliate of OMERS, a Canadian public employee pension fund, pursuant to which we were paid a $200.0 million cash purchase price in exchange for 54.5% of our future quarterly royalty payments on our royalty revenues received from AbbVie. We continued to report lower royalty revenue during fiscal 2022 and in the first half of fiscal 2023 as compared to periods ending before March 2020. The pandemic resulted in a decline in patient volumes, HCV diagnoses, HCV prescriptions andnet sales of MAVYRET/MAVIRET.MAVIRET, after June 30, 2023, through June 30, 2032, subject to a cap on aggregate payments to OMERS equal to 1.42 times the purchase price.
Please see Item 1A “Risk Factors”For accounting purposes, we continue to record 100% of HCV royalties earned under the AbbVie agreement as royalty revenue in our 2022 Form 10-K for additional discussioncondensed consolidated statements of risks and potential risksoperations. The $200.0 million received in April 2023 was recognized on our condensed consolidated balance sheets as a liability, which will be reduced by the payments made to OMERS over the term of the COVID-19 pandemicAgreement. We recognize imputed interest expense over the life of the royalty sale agreement based on our business, results of operations and financial condition.estimated future MAVYRET/MAVIRET royalties.
Financial Operations Overview
We are currently funding all research and development for our wholly-owned programs, which are targeted toward the discovery and development of novel compounds with an emphasis on treatments for viral infections.compounds. As of the date of this report, we are conducting threetwo Phase 2 studies for zelicapavir and one Phase 1 study for a different compound in our RSV program and one Phase 2 challenge study in our SARS-CoV-2 program.of EDP-323. We are also progressingconducting preclinical discovery research efforts in other compounds into preclinical development.disease areas.
19
As a result of the timing of our clinical and preclinical development programs, we expect our research and development expenses will fluctuate from period to period. However, in the coming years,next 12 months, we expect our external research and development expenses generally to increase asdecrease since we will not conduct any further development of EDP-235 into Phase 3 studies and we have made important adjustments to reduce our wholly-owned programs advance.spending significantly in 2024.
To date, we have funded our operations primarily through royalty payments received under our collaboration agreement with AbbVie, a $200.0 million payment from our royalty sale agreement, and our existing cash, cash equivalents, and short-term and long-term marketable securities. We believe that our existing cash, cash equivalents and short-term and long-term marketable securities, as well as the $200.0 million payment received in our April 2023 royalty sale agreement and our continuingretained portion of future HCV royalties, will enable us to fund our operating expenses and capital expenditure requirements into calendar 2026.through fiscal 2027.
Revenue
Our revenue is primarily derived from our collaboration agreement with AbbVie and AbbVie’s sales of MAVYRET/MAVIRET, an 8-week treatment regimen for chronic HCV. During the first halfquarter of fiscal 2023, we also generated $1.0 million of license revenue from an upfront payment related to a license agreement for one of the antibacterial compounds we are no longer developing.
The following table is a summary of revenue recognized for the three and six months ended MarchDecember 31, 2023 and 2022:
|
| Three Months Ended March 31, |
|
| Six Months Ended March 31, |
|
| Three Months Ended December 31, |
|
| |||||||||||||||
|
| 2023 |
|
| 2022 |
|
| 2023 |
|
| 2022 |
|
| 2023 |
|
| 2022 |
|
| ||||||
|
| (in thousands) |
|
| (in thousands) | ||||||||||||||||||||
Revenue |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||
Royalty revenue |
| $ | 17,795 |
|
| $ | 18,716 |
|
| $ | 40,380 |
|
| $ | 46,364 |
|
| $ | 18,003 |
|
| $ | 22,585 |
|
|
License revenue |
|
| — |
|
|
| — |
|
|
| 1,000 |
|
|
| — |
|
|
| — |
|
|
| 1,000 |
|
|
Total revenue |
| $ | 17,795 |
|
| $ | 18,716 |
|
| $ | 41,380 |
|
| $ | 46,364 |
|
| $ | 18,003 |
|
| $ | 23,585 |
|
|
AbbVie Agreement
To date, we have received annually tiered, double-digit royalties on our protease inhibitor product glecaprevir included in AbbVie’s net sales of MAVYRET/MAVIRET. Under the terms of our AbbVie agreement, as amended in October 2014,Agreement, 50% of AbbVie’s net sales of MAVYRET/MAVIRET are allocated to glecaprevir. Beginning with each January 1, the cumulative net sales of MAVYRET/MAVIRET start at zero for purposes of calculating the tiered royalties. As disclosed above regarding the OMERS royalty sale agreement, we will only retain 45.5% of the cash payments from royalties on net sales of MAVYRET/MAVIRET occurring after June 30, 2023 through June 30, 2032, subject to a cap on aggregate payments to OMERS equal to 1.42 times OMERS’ purchase price.
Internal Programs
As our internal product candidates are currently in Phase 1 or Phase 2 clinical development, we have not generated any revenue from our own product sales andsales. We do not expect to generate any revenue from product sales derived from these product candidates for at least the next several years.
19
Operating Expenses
Our operating expenses are comprised of research and development expenses and general and administrative expenses.
Research and Development Expenses
Research and development expenses consist of costs incurred to conduct basic research, such as the discovery and development of novel small molecules as therapeutics, as well as any external expenses of preclinical and clinical development activities. We expense all costs of research and development as incurred. These expenses consist primarily of:
Project-specific expenses reflect costs directly attributable to our clinical development candidates and preclinical candidates nominated and selected for further development. Our remaining research and development expenses are reflected in research and drug discovery, which represents early-stage drug discovery programs. At any given time, we typically have later stage programs in clinical development as well as several active early-stage research and drug discovery projects. Our internal resources, employees and infrastructure are not directly tied to any individual research or drug discovery project and are typically deployedutilized across multiple projects, including our early stage discovery projects. As such, we do not report information regarding costs incurred forbased on our early-stage researchprograms (i.e. disease area) rather than on a
20
project specific basis. All indirect costs are allocated to programs based on headcount and drug discovery programs on a project-specific basis.square footage of our facilities. We expect that our research and development expenses will increase in the futurefluctuate from period to period as we advance our research and development programs. However, in the next 12 months, we expect our external research and development expenses generally to decrease since we will not advance EDP-235 into Phase 3 studies on our own. To date, we have not identified any significant impact of inflation on spending in research and development, but it is uncertain whether there will be inflationary impacts in future periods.
Our research and drug discovery and development programs are atin early stages; therefore, the successful development of our product candidates is highly uncertain and may not result in approved products. Completion dates and completion costs can vary significantly for each product candidate and are difficult to predict. Given the uncertainty associated with clinical trial enrollments particularly in the context of the COVID-19 pandemic, and the risks inherent in the development process, we are unable to determine the duration and completion costs of the current or future clinical trials of our product candidates or if, or to what extent, we will generate revenue from the commercialization and sale of any of our product candidates. We anticipate that we will make determinations as to which development programs to pursue and how much funding to direct to each program on an ongoing basis in response to the preclinical and clinical success and prospects of each product candidate, as well as ongoing assessments of the commercial potential of each product candidate.
General and Administrative Expenses
General and administrative expenses consist primarily of personnel costs, which include salaries, related benefits and stock-based compensation, of our executive, finance, business and corporate development and other administrative functions. General and administrative expenses also include travel expenses, allocated facility-related costs not otherwise included in research and development expenses, directors’ and officers’ liability insurance premiums, professional fees for auditing, tax, and legal services, patent expenses and litigation expenses associated with prosecuting our patent expenses.infringement suit.
We expect that general and administrative expenses will continue tomay increase in the future primarily due to the ongoing expansion of our operating activities in support of our own research and development programs, as well as our patent litigation seeking damages for infringement of one of our COVID-19 patents.long term. To date we have not experienced a significant impact of inflation on spending in general and administrative expenses, but we anticipate inflation may impact future periods.
Other Income (Expense)
Other income (expense) consists of interest expense, interest and investment income, net and the change in fair value of our outstanding Series 1 nonconvertible preferred stock. Interest expense consists of the non-cash interest expense and amortization of debt issuance costs associated with the royalty sale agreement with an affiliate of OMERS. Interest income consists of interest earned on our cash equivalents and short-term and long-term marketable securities balances and interest earned for any refunds received from tax authorities.balances. Investment income consists of the amortization or accretion of any purchased premium or discount, respectively, on our short-term and long-term marketable securities. The change in fair value of our Series 1 nonconvertible preferred stock relates to the remeasurement of these financial instruments from period to period as these instruments may require a transfer of assets because of the liquidation preference features of the underlying instrument.
20
Income Tax Benefit
Income Tax Expense
Income tax expense is based on our best estimate of taxable net losses, applicableThe income tax rates, net research and developmentbenefit for the three months ended December 31, 2023 was driven by interest recorded on a pending federal income tax credits and carryforwards, net operating loss carrybacks, changes in valuation allowance estimates and deferredrefund. The income taxes.tax benefit for the three months ended December 31, 2022 is the result of the release of a state tax reserve.
Results of Operations
Comparison of the Three Months Ended MarchDecember 31, 2023 and 2022
|
| Three Months Ended March 31, |
|
| Three Months Ended December 31, |
| ||||||||||
|
| 2023 |
|
| 2022 |
|
| 2023 |
|
| 2022 |
| ||||
|
| (in thousands) |
|
| (in thousands) |
| ||||||||||
Royalty revenue |
| $ | 17,795 |
|
| $ | 18,716 |
| ||||||||
Revenue |
| $ | 18,003 |
|
| $ | 23,585 |
| ||||||||
Research and development |
|
| 43,468 |
|
|
| 42,087 |
|
|
| 36,371 |
|
|
| 40,902 |
|
General and administrative |
|
| 13,778 |
|
|
| 10,476 |
|
|
| 16,518 |
|
|
| 12,696 |
|
Interest expense |
|
| (3,441 | ) |
|
| — |
| ||||||||
Interest and investment income, net |
|
| 1,837 |
|
|
| 255 |
|
|
| 4,298 |
|
|
| 993 |
|
Income tax expense |
|
| (44 | ) |
|
| — |
| ||||||||
Income tax benefit |
|
| 622 |
|
|
| 34 |
| ||||||||
Net loss |
| $ | (37,658 | ) |
| $ | (33,592 | ) |
| $ | (33,407 | ) |
| $ | (28,986 | ) |
Royalty revenueRevenue
We recognized royalty revenue of $17.8$18.0 million during the three months ended MarchDecember 31, 2023 as compared to $18.7$23.6 million during the three months ended MarchDecember 31, 2022. The $0.9$5.6 million decrease in royalty revenue was primarily due to AbbVie’s lower reported HCV sales as compared to the comparable period in 2022.
Our royalty revenues eligible to be earned in the future will depend on AbbVie’s HCV market share, the pricing of the MAVYRET/MAVIRET regimen and the number of patients treated. In addition, at the beginning of each calendar year (the second quarter of our fiscal year), our royalty rate resets to the lowest tier for each of our royalty-bearing products licensed to AbbVie.
21
Beginning with the quarter ended September 30, 2023, 54.5% of our quarterly royalty payments on net sales of MAVYRET/MAVIRET that are included in our total revenue are paid to OMERS through June 30, 2032, subject to a cap on aggregate payments equal to 1.42 times the purchase price. The $200.0 million received in April 2023 was recognized on our condensed consolidated balance sheets as a liability which will be reduced by the payments made to OMERS over the term of the royalty sale agreement. We will continue to record 100% of HCV royalties earned under the AbbVie Agreement as royalty revenue in our condensed consolidated statements of operations since the AbbVie Agreement has not been amended and is independent of our agreement with OMERS.
Research and development expenses
|
| Three Months Ended March 31, |
|
| Three Months Ended December 31, |
| ||||||||||
|
| 2023 |
|
| 2022 |
|
| 2023 |
|
| 2022 |
| ||||
|
| (in thousands) |
|
| (in thousands) |
| ||||||||||
R&D programs: |
|
|
|
|
|
|
|
|
|
|
|
| ||||
Virology |
| $ | 41,384 |
|
| $ | 35,912 |
|
|
|
|
|
|
| ||
Liver disease (non-viral) |
|
| 515 |
|
|
| 5,547 |
| ||||||||
Other |
|
| 1,569 |
|
|
| 628 |
| ||||||||
RSV |
| $ | 24,294 |
|
| $ | 17,810 |
| ||||||||
COVID-19 |
|
| 3,251 |
|
|
| 18,951 |
| ||||||||
HBV |
|
| 66 |
|
|
| 1,997 |
| ||||||||
Total Virology |
| $ | 27,611 |
|
| $ | 38,758 |
| ||||||||
Immunology |
|
|
|
|
|
| ||||||||||
CSU |
|
| 3,931 |
|
|
| — |
| ||||||||
Total Immunology |
| $ | 3,931 |
|
| $ | — |
| ||||||||
Other Programs |
|
|
|
|
|
| ||||||||||
NASH |
|
| 368 |
|
|
| 851 |
| ||||||||
Early discovery |
|
| 4,461 |
|
|
| 1,293 |
| ||||||||
Total Other Programs |
| $ | 4,829 |
|
| $ | 2,144 |
| ||||||||
|
|
|
|
|
|
| ||||||||||
Total research and development expenses |
| $ | 43,468 |
|
| $ | 42,087 |
|
| $ | 36,371 |
|
| $ | 40,902 |
|
The level of research and development expenses for the three months ended MarchDecember 31, 2023 increaseddecreased by $1.4$4.5 million compared to the same period in 2022.
Virology
The increase in costs of $5.5 million in our virology program wasdecreased $11.1 million primarily due to a decrease in costs associated with our COVID-19 program as we stopped further internal development and will only progress the program in the context of one or more collaborations. This decrease was offset by an increase in costs for our RSV clinical trialprograms as we had two ongoing Phase 2 studies of zelicapavir and a challenge study initiated for EDP-323 in the three months ended December 31, 2023. Costs associated with HBV decreased as we continued to wind down this program.
Immunology
The costs and an increase in headcount, partiallyour immunology increased by $3.9 million as this is a new therapeutic area of focus for the company.
Other Programs
Other program costs increased by $2.7 million as we focused on early stage drug discovery programs, offset by a decrease in manufacturing costs due to timing offor our studies in our virology programs. The costs in our non-viral liver diseaseNASH program decreased by $5.0 million as we woundcontinued to wind down our non-alcoholic steatohepatitis, or NASH, program, which is now substantially complete.this program.
WeExpenses associated with our COVID-19, HBV and NASH programs will continue as we complete all the required procedures for concluded clinical trials.
In the next 12 months, we expect our external research and development expenses generally to decrease since we will increase in the future as we conduct more clinical development activities.not advance our COVID-19 candidate, EDP-235, into Phase 3 studies on our own.
General and administrative expenses
General and administrative expenses increased by $3.8 million for the three months ended December 31, 2023 compared to the same period in 2022. The change was due to an increase in stock compensation expense and an increase in legal expenses related to our patent infringement suit against Pfizer.
22
Other income (expense)
Changes in components of other income (expense) were as follows:
Interest expense
Interest expense increased $3.4 million for the three months ended December 31, 2023, as compared to the same period in 2022 due to interest expense and amortization of debt issuance costs associated with the royalty sale agreement entered into during April 2023 with an affiliate of OMERS.
Interest and investment income, net
Interest and investment income, net, increased $3.3 million for the three months ended MarchDecember 31, 2023, as compared to the same period in 2022. The increase was due to an increase in stock-based compensation expense and an increase in legal fees relatedour cash balance due to our patent infringement suit against Pfizer.
Interest and investment income, net
Interest and investment income, net, increased $1.6receipt of the $200.0 million for the three months ended March 31, 2023,from OMERS as compared to the same period in 2022. The increase was due towell as changes in interest rates year over year.
21
Income tax benefit
Comparison ofDuring the Six Months Ended Marchthree months ended December 31, 2023 and 2022,
|
| Six Months Ended March 31, |
| |||||
|
| 2023 |
|
| 2022 |
| ||
|
| (in thousands) |
| |||||
Royalty revenue |
| $ | 40,380 |
|
| $ | 46,364 |
|
License revenue |
|
| 1,000 |
|
|
| — |
|
Research and development |
|
| 84,370 |
|
|
| 90,636 |
|
General and administrative |
|
| 26,474 |
|
|
| 19,984 |
|
Interest and investment income, net |
|
| 2,830 |
|
|
| 549 |
|
Income tax expense |
|
| (10 | ) |
|
| — |
|
Net loss |
| $ | (66,644 | ) |
| $ | (63,707 | ) |
Royalty revenue
We recognized royalty revenue we recorded an income tax benefit of $40.4$0.6 million and less than $0.1 million, respectively. The income tax benefit in 2023 was driven by interest recorded on a pending federal tax refund. The income tax benefit for 2022 was driven by a release of a state tax reserve during the six months ended March 31, 2023 as compared to $46.4 million during the six months ended March 31, 2022. The $6.0 million decrease in royalty revenue was due to AbbVie’s lower reported HCV sales as compared to the comparable period in 2022.
Our royalty revenues eligible to be earned in the future will depend on AbbVie’s HCV market share, the pricing of the MAVYRET/MAVIRET regimen and the number of patients treated.
License revenue
We also recognized $1.0 million of license revenue during the six months ended March 31, 2023 related to an upfront payment received for a license agreement for one of the antibacterial compounds we are no longer developing.
Research and development expenses
|
| Six Months Ended March 31, |
| |||||
|
| 2023 |
|
| 2022 |
| ||
|
| (in thousands) |
| |||||
R&D programs: |
|
|
|
|
|
| ||
Virology |
| $ | 80,142 |
|
| $ | 78,434 |
|
Liver disease (non-viral) |
|
| 1,366 |
|
|
| 11,101 |
|
Other |
|
| 2,862 |
|
|
| 1,101 |
|
Total research and development expenses |
| $ | 84,370 |
|
| $ | 90,636 |
|
The level of research and development expenses for the six months ended March 31, 2023 decreased by $6.3 million compared to the same period in 2022. The increase in costs of $1.7 million in our virology program was primarily due to an increase in clinical trial costs and an increase headcount partially offset by a decrease in manufacturing costs due to the timing and scope of clinical trials. The costs in our non-viral liver disease program decreased by $9.7 million as we wound down our non-alcoholic steatohepatitis, or NASH, program, which is now substantially complete.
General and administrative expenses
General and administrative expenses increased by $6.5 million for the six months ended March 31, 2023 compared to the same period in 2022. The increase was due to an increase in stock-based compensation expense and an increase in legal fees related to our patent infringement suit against Pfizer.
Interest and investment income, net
Interest and investment income, net, increased $2.3 million for the six months ended March 31, 2023, as compared to the same period in 2022. The increase was due to changes in interest rates year over year.
22
period.
Liquidity and Capital Resources
We fund our operations with cash flows from our retained portion of our royalty revenue and our existing financial resources. At MarchDecember 31, 2023, our principal sources of liquidity were cash and cash equivalents and short-term and long-term marketable securities totaling $225.1of $337.2 million.
The following table shows a summary of our cash flows for the six months ended March 31, 2023 and 2022:flows:
|
|
|
|
|
|
| ||||||||||
|
| Six Months Ended March 31, |
|
| Three Months Ended December 31, |
| ||||||||||
|
| 2023 |
|
| 2022 |
|
| 2023 |
|
| 2022 |
| ||||
|
| (in thousands) |
|
| (in thousands) |
| ||||||||||
Cash provided by (used in): |
|
|
|
|
|
|
|
|
|
| ||||||
Operating activities |
| $ | (49,424 | ) |
| $ | (39,646 | ) |
| $ | (24,988 | ) |
| $ | (35,641 | ) |
Investing activities |
|
| 80,334 |
|
|
| 10,446 |
|
|
| (13,117 | ) |
|
| 33,569 |
|
Financing activities |
|
| (1,726 | ) |
|
| 12,983 |
|
|
| (7,350 | ) |
|
| 301 |
|
Net increase (decrease) in cash, cash equivalents and restricted cash |
| $ | 29,184 |
|
| $ | (16,217 | ) | ||||||||
Net decrease in cash, cash equivalents and restricted cash |
| $ | (45,455 | ) |
| $ | (1,771 | ) | ||||||||
Net cash used in operating activities
Cash used in operating activities was $49.4$25.0 million for the sixthree months ended MarchDecember 31, 2023 as compared to cash used in operating activities of $39.6$35.6 million for the same period in 2022. Our cash used in operating activities increased $9.8 million, drivendecreased primarily due to a significant decrease in spending in our clinical programs which was partially offset by timinglower cash receipts associated with our AbbVie agreement as we now only retain 45.5% of our research and development payments year-over-year as well as a federal tax refund of $8.5 million received in 2022.cash royalties following the royalty sale agreement with OMERS.
For the foreseeable future, we expect to continue to incur substantial costs associated with research and development for our internally developed programs.23
Net cash provided by (used in) investing activities
Cash provided byused in investing activities was $80.3$13.1 million for the sixthree months ended MarchDecember 31, 2023 as compared to cash provided by investing activities of $10.4$33.6 million for the same period in 2022. Our cash provided byused in investing activities increased $69.9$46.7 million, driven by the timing of purchases, sales and maturities of marketable securities in 2023 compared to 2022 offset by increasedand decreased capital expenditures in the first half of fiscal 2023 forcompared to fiscal 2022 due to the timing of our buildout of our leased premises at 400 Talcott Avenue expansion.Avenue.
Net cash provided by (used in) financing activities
Cash used in financing activities was $1.7$7.4 million for the sixthree months ended MarchDecember 31, 2023 as compared to cash provided by financing activities of $13.0$0.3 million for the same period in 2022. Our cash provided byused in financing activities decreased $14.7increased $7.7 million, driven by a decrease in proceeds from stock option exercises and an increase in paymentsour payment to OMERS for settlement of share-based awards.our royalty sale agreement.
Funding requirementsRequirements
As of MarchDecember 31, 2023, we had $225.1$337.2 million in cash, cash equivalents and short-term and long-term marketable securities. We believe that our existing cash, cash equivalents and short-term and long-term marketable securities as of MarchDecember 31, 2023, as well as the $200.0 million received in our April 2023 royalty sale transaction and cash flows from our continuingretained portion of future HCV royalties will be sufficient to meet our anticipated cash requirements into calendar 2026.through fiscal 2027. However, our forecastprojection of the period of time through which our financial resources will be adequate to support our operations is a forward-looking statement that involves risks and uncertainties, and actual results could vary materially.
On March 10, 2023, Silicon Valley Bank (“SVB”), which held less than 5% of our cash and marketable securities, was closed by the California Department of Financial Protection and Innovation and the Federal Deposit Insurance Corporation (“FDIC”) was appointed as SVB’s receiver. We have initiated the transfer of all of our cash, cash equivalents and short-term and long-term marketable securities previously held at SVB to a larger financial institution.
Our future capital requirements are difficult to forecast and will depend on many factors, including:
23
24
Off-Balance Sheet Arrangements
We do not engage in any off-balance sheet financing activities. We do not have any interest in entities referred to as variable interest entities, which include special purpose entities and other structured finance entities.
Contractual Obligations and Commitments
We currently lease space in Watertown, Massachusetts, under two separate lease agreements with one landlord. We have entered into a third lease agreement with the same landlord to lease additional laboratory and office space at a to-be-constructed facility located at Arsenal on the Charles in Watertown, Massachusetts.
Our first lease for office and laboratory space at 500 Arsenal Street expires on September 1, 2027. In May 2022, we entered into a new ten-year lease for laboratory and office space in Watertown, Massachusetts, adjacent to our 400 Talcott Avenue premises at Arsenal on the Charles, at 4 Kingsbury Avenue. The construction of the facility shell has been completed and we expect to gain access to the building to construct tenant improvements in February 2024. In connection with this lease, we amended our 500 Arsenal Street lease to shorten the expiration date from September 1, 2027, to the date the Kingsbury Avenue facility is ready for our occupancy, which is currently estimated to occur by the end of November 2024.
The second lease for office space located at 400 Talcott Avenue, commenced on September 24, 2018 for a term of six years. In May 2022, we amended this lease to expand the rented space and extend the lease term through June 1, 2034. We spent approximately $6.3 million in capital expenditures for the additional space, which primarily relate to tenant improvements. We received a tenant improvement allowance from the landlord of $2.5 million.
In April 2023, we entered into a royalty sale agreement with an affiliate of OMERS, pursuant to which we were paid a $200.0 million cash purchase price in exchange for 54.5% of our future quarterly royalty payments on net sales of MAVYRET/MAVIRET beginning with the payment for the quarter ending Septemberafter June 30, 2023, through June 30, 2032, subject to a cap on aggregate payments equal to 1.42 times the purchase price.
In our third fiscal quarter, we will record theThe $200.0 million up-front paymentreceived in April 2023 was recognized on our condensed consolidated balance sheets as a cash asset with offsetting short and long-term liabilities. Beginning in our third fiscal quarter, weliability which will account for all HCV royalties as royalty revenue in our consolidated statements of operations and we will recognize imputed interest expensebe reduced by the payments made to OMERS over the lifeterm of the Royalty Purchase Agreement based on the estimated future HCV royalties.Agreement.
Critical Accounting Policies
Our condensed consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States of America. The preparation of our condensed consolidated financial statements and related disclosures requires us to make estimates and assumptions that affect the reported amount of assets, liabilities, revenue, costs and expenses, and related disclosures. We evaluate our estimates and assumptions on an ongoing basis. Our actual results may differ from these estimates under different assumptions and conditions. See our 20222023 Form 10-K for information about our critical accounting policies as well as a description of our other significant accounting policies. There have been no significant changes to our critical accounting policies since the beginning of this fiscal year.
Recently Issued Accounting Pronouncements
A description of recently issued accounting pronouncements that may potentially impact our financial position and results of operations is set forth in Note 2 to the condensed consolidated financial statements included in this Form 10-Q.
2425
ITEM 3. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
During the three and six months ended MarchDecember 31, 2023, there were no material changes to our market risk disclosures as set forth in Part II, Item 7A “Quantitative and Qualitative Disclosures About Market Risk” in our Annual Report on Form 10-K for the fiscal year ended September 30, 2022.2023.
ITEM 4. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures and Internal Control over Financial Reporting
Evaluation of Disclosure Controls and Procedures.
Our management, with the participation of the principal executive officer and principal financial officer, has evaluated the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended, or the Exchange Act,Act), as of the end of the period covered by this quarterly report. Based on this evaluation, the principal executive officer and principal financial officer concluded that these disclosure controls and procedures are effective and designed to ensure that the information required to be disclosed in our reports filed or submitted under the Exchange Act is recorded, processed, summarized and reported within the requisite time periods.
Changes in Internal Control Over Financial Reporting.
There were no changes in our internal control over financial reporting that occurred during the quarter ended MarchDecember 31, 2023 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
PART II —OTHER INFORMATION
ITEM 1A. RISK FACTORS
RISK FACTORS
Our business faces significant risks and uncertainties. Certain factors may have a material adverse effect on our business prospects, financial condition and results of operations, and you should carefully consider them. Accordingly, in evaluating our business, we encourage you to consider the detailed discussion of risk factors included in our 20222023 Form 10-K. There
Except for the risk factor below, there have been no material changes to such risk factors during the quarter ended MarchDecember 31, 2023. Other events that we do not currently anticipate or that we currently deem immaterial may also affect our business, prospects, financial condition and results of operations.
We and AbbVie face substantial competition in the markets for HCV drugs, and there are many companies developing potential therapies for RSV, SARS-CoV-2, HBV and CSU which may result in others discovering, developing or commercializing products before we do or doing so more successfully than we do.
The pharmaceutical and biotechnology industries are intensely competitive and rapidly changing. Many large pharmaceutical and biotechnology companies, academic institutions, governmental agencies and other public and private research organizations are commercializing or pursuing the development of products that target HCV, RSV, SARS-CoV-2 HBV, CSU and other viral infections or immunology diseases that we may target in the future. Many of our competitors have substantially greater commercial infrastructure and greater financial, technical and personnel resources than we have, as well as drug candidates in late-stage clinical development.
In all the disease areas currently in the focus of our research and development efforts, there are other companies with product candidates that are more advanced than ours. Our competitors may succeed in developing these product candidates or others and obtaining regulatory approval before we can do so with any of our product candidates. If we are not “first to market” with one of our product candidates in one or more of these disease indications, our competitive position could be compromised because it may be more difficult for us to obtain marketing approval for that product candidate and market acceptance of that product candidate as a follow-on competitor. In addition, any new product that competes with an approved product typically must demonstrate compelling advantages in efficacy, convenience, tolerability or safety, or some combination of these factors, to gain regulatory approvals, overcome price competition and be commercially successful.
RSV, COVID-19, HBV and CSU represent competitive therapeutic areas.
26
For RSV, there are currently no safe and effective therapies for already established RSV infection. Several companies are seeking to develop antiviral treatments for RSV infection in adult and pediatric patients. Ark Biosciences, Pfizer/ReViral (acquired in June 2022) and Shionogi all have compounds in clinical development.
There are several prophylaxis options on the market or in development. AstraZeneca/Sanofi (Beyfortus – approved) and Merck (Clesrovimab – Phase 3) are developing long-acting monoclonal antibodies for prophylaxis use in infants, and Pfizer has an approved maternal vaccine (ABRYSVO™), all of which provide passive immunity to infants. There are also 2 approved RSV vaccines for adults over 60 years of age (Pfizer/ABRYSVO™ and GSK/AREXVY®).
For COVID, there are two oral antiviral treatments for non-hospitalized, high-risk patients with SARS-CoV-2 infection: PAXLOVID™, a 3CL protease inhibitor (nirmatrelvir) boosted with ritonavir (full approval), and LAGEVRIO™ (molnupiravir), a polymerase inhibitor (Emergency Use Authorization). Additionally, other companies are developing oral direct acting antivirals for SARS-CoV-2 that are currently in global Phase 3 studies include Shionogi, Atea, and Gilead (an oral formulation of remdesivir).
While there are antiviral medications prescribed for HBV that can suppress HBV DNA, they generally have low cure rates, resulting in the need for lifelong treatment. Many companies are seeking to develop new HBV drugs that alone or in combination with other mechanisms could lead to a functional cure for HBV. Vir, GSK, Arbutus, and Roche have multiple combination regimens under investigation in later stage clinical studies. In addition, a number of companies have Phase 1 or earlier stage HBV programs.
For CSU, there are a number of different mechanisms being explored, including inhibitors of IL-4R, IgE, BTK, SIGLEC-6, MRGPRX2 and SYK. Specifically for KIT inhibitors, there are companies with antibodies in development, including Celldex (barzolvolimab - Phase 2), Jasper (briquilimab - Phase 1), and Acelyrin (SLRN-517 - Phase 1/Phase 2), as well as companies with oral, small molecules in preclinical development, including ThirdHarmonic, Blueprint, Arcus, and Modulus.
In the chronic HCV market, we expect AbbVie’s MAVYRET/MAVIRET to continue to face intense competition due to existing approved HCV products. AbbVie’s MAVYRET/MAVIRET regimen currently faces competition in various world markets and subpopulations of HCV from Gilead’s Epclusa® (a fixed dose combination of sofosbuvir and velpatasvir), Vosevi® (a triple combination therapy of sofosbuvir, velpatasvir and voxilaprevir approved by the FDA for specified sofosbuvir -treatment failures and NS5A-inhibitor treatment failures) and Harvoni® (a fixed-dose combination of sofosbuvir and ledipasvir); and to a lesser extent - Merck’s Zepatier® (a fixed-dose combination of grazoprevir and elbasvir). Gilead launched authorized generic versions of Epclusa and Harvoni through its subsidiary, Asegua Therapeutics, LLC, which have had an impact on the competitive landscape. For example, the state of Louisiana selected Asegua as their HCV subscription model pharmaceutical partner to provide the state with unrestricted access to its direct-acting antiviral medication.
Other competitive products in the form of other treatment methods or a vaccine for HCV may render MAVYRET/MAVIRET obsolete or noncompetitive. MAVYRET/MAVIRET will face competition based on its safety and effectiveness, reimbursement coverage, price, patent position, AbbVie’s marketing and sales capabilities, and other factors. If MAVYRET/MAVIRET faces competition from generic products other than authorized generic versions by the manufacturer of the branded product (i.e. Gilead and Asegua Therapeutics), our collaboration agreement provides that the royalty rate applicable to our protease product contained in the regimen is reduced significantly by a specified percentage on a product-by-product, country-by-country basis. If AbbVie is not able to compete effectively against its competitors in HCV, our business will not grow and our financial condition, operations and stock price will suffer.
If we are not able to develop new products that can compete effectively against our current and future competitors, our business will not grow and our financial condition, operations and stock price will suffer.
ITEM 5. OTHER INFORMATION
ITEM 5.02 Departure of Directors of Certain Officers; Election of Directors; Appointment of Certain Directors; Compensatory Arrangements of Certain Officers
As reportedCompensatory Arrangement. On February 8, 2024, the Corporation entered into an Amended and Restated Employment Agreement (the “Agreement”) with Nathaniel S. Gardiner, who currently serves as the Corporation's Chief Legal Officer, in connection with his retirement on March 31, 2024. Pursuant to the Agreement, Mr. Gardiner will serve as Senior Counsel to the Corporation on an at-will basis until March 31, 2025 on a 50% schedule for the first three months, then reducing to a 25% schedule thereafter, for which he will receive 50% of his base salary and targeted variable compensation as in effect immediately before the effective date of the Agreement until June 30, 2024 (or earlier reduction to 25% time), which will reduce to 25% of base salary and target variable compensation thereafter. Each of Mr. Gardiner’s existing stock options and unvested restricted stock units will continue to vest during the term of his employment under the Agreement and, in the Company’s Current Report on Form 8-K filed withcase of stock options, shall continue to be exercisable until the SEC on March 7, 2023,earlier of the Company’s 2019 Equity Incentive Plan was amended atoriginal 10-year term of the annual meetingstock option or one year after termination of stockholders on March 2, 2023 to increasehis employment. In addition, the numberCorporation will accelerate the vesting of shares underlying stock options and restricted stock units that are outstanding and scheduled to vest within twelve months after termination of common stock reserved for issuance thereunder by 975,000 shares. We incorporate by reference hereinhis employment under the descriptionAgreement.
Rule 10b5-1 Trading Arrangements. During the three months ended December 31, 2023, no director or officer of the material termsCompany adopted or terminated a “Rule 10b5-1 trading arrangement” or a “non-Rule 10b5-1 trading arrangement,” as each term is defined in Item 408(a) of the Enanta Pharmaceuticals, Inc. 2019 Equity Incentive Plan, as amended, in our definitive proxy statement dated as of January 20, 2023 under the caption “Proposal 2 - Amendment to 2019 Equity Incentive Plan.” A copy of the plan as amended was filed with the Current Report on Form 8-K filed on March 7, 2023 and is filed as Exhibit 10.2 to the Quarterly Report on Form 10-Q.Regulation S-K.
2527
ITEM 6. EXHIBITS
|
|
|
| Incorporated by Reference |
|
| ||||||
Exhibit Number |
| Exhibit Description |
| Form |
| Date |
| Exhibit Number |
| File Number |
| Filed Herewith |
|
|
|
|
|
|
|
|
|
|
|
|
|
3.1 |
| Restated Certificate of Incorporation of Enanta Pharmaceuticals, Inc. |
| 8-K |
| 03/28/2013 |
| 3.1 |
| 001-35839 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.2 |
| Amended and Restated Bylaws of Enanta Pharmaceuticals, Inc. (as amended and restated in August 2015) |
| 8-K |
| 08/18/2015 |
| 3.2 |
| 001-35839 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.1 |
|
| 8-K |
| 04/27/2023 |
| 10.1 |
| 001-35839 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.2 |
| 2019 Equity Incentive Plan (as amended through March 2, 2023) |
| 8-K |
| 03/07/2023 |
| 10.1 |
| 001-35839 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31.1 |
|
| — |
| — |
| — |
| — |
| X | |
|
|
|
|
|
|
|
|
|
|
|
|
|
31.2 |
|
| — |
| — |
| — |
| — |
| X | |
|
|
|
|
|
|
|
|
|
|
|
|
|
32.1 |
|
| — |
| — |
| — |
| — |
| X | |
|
|
|
|
|
|
|
|
|
|
|
|
|
101 |
| The following financial statements from the Quarterly Report of Enanta Pharmaceuticals, Inc. on Form 10-Q for the quarter ended March 31, 2023 formatted in Inline XBRL: (i) Consolidated Balance Sheets, (ii) Consolidated Statements of Operations, (iii) Consolidated Statements of Comprehensive Income, (iv) Consolidated Statements of Stockholders’ Equity, (v) Consolidated Statements of Cash Flows, (vi) and Notes to Consolidated Financial Statements, tagged as blocks of text and including detailed tags |
|
|
|
|
| X | ||||
|
|
|
|
|
|
|
|
|
|
|
|
|
104
|
| Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101). |
|
|
|
|
|
|
|
|
| X |
|
|
|
| Incorporated by Reference |
|
| ||||||
Exhibit Number |
| Exhibit Description |
| Form |
| Date |
| Exhibit Number |
| File Number |
| Filed Herewith |
|
|
|
|
|
|
|
|
|
|
|
|
|
3.1 |
| Restated Certificate of Incorporation of Enanta Pharmaceuticals, Inc. |
| 8-K |
| 03/28/2013 |
| 3.1 |
| 001-35839 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.2 |
| Amended and Restated Bylaws of Enanta Pharmaceuticals, Inc. (as amended and restated in August 2015) |
| 8-K |
| 08/18/2015 |
| 3.2 |
| 001-35839 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31.1 |
|
| — |
| — |
| — |
| — |
| X | |
|
|
|
|
|
|
|
|
|
|
|
|
|
31.2 |
|
| — |
| — |
| — |
| — |
| X | |
|
|
|
|
|
|
|
|
|
|
|
|
|
32.1 |
|
| — |
| — |
| — |
| — |
| X | |
|
|
|
|
|
|
|
|
|
|
|
|
|
101.INS |
| XBRL Instance Document - The instance document does not appear in the interactive data file because its XBRL tags are embedded within the inline XBRL document |
|
|
|
|
| X | ||||
|
|
|
|
|
|
|
|
|
|
|
|
|
101.SCH |
| Inline XBRL Taxonomy Extension Schema with embedded Linkbases document |
|
|
|
|
|
|
|
|
| X |
|
|
|
|
|
|
|
|
|
|
|
|
|
104
|
| Cover Page Interactive Data File (formatted as Inline XBRL with applicable Taxonomy Extension information contained in Exhibit 101). |
|
|
|
|
|
|
|
|
| X |
2628
ENANTA PHARMACEUTICALS, INC.
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| ENANTA PHARMACEUTICALS, INC. | |
|
|
|
Date: |
| |
|
|
|
|
| /s/ Paul J. Mellett |
|
| Paul J. Mellett Chief Financial and Administrative Officer (Principal Financial and Accounting Officer) |
2729