UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-Q
(Mark One)
x | QUARTERLY REPORT PURSUANT TO SECTION 13 OR |
For the quarterly period ended |
or
o | TRANSITION REPORT UNDER SECTION 13 OR |
For the transition period from __________________ to __________________________ |
Commission file number: |
LOTUS PHARMACEUTICALS, INC. |
(Name of registrant as specified in its charter) |
NEVADA | 20-0507918 | |
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
16 Cheng Zhuang Road, Feng Tai District, Beijing100071 People’s Republic of China | N/A | |
(Address of principal executive offices) | (Zip Code) |
86-10-63899868 |
( |
N/A |
(Former name, former address and former fiscal year, if changed since last report) |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes xNo o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of "large“large accelerated filer," "accelerated filer"” “accelerated filer” and "smaller“smaller reporting company"company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer o | Accelerated filer o | |
Non-accelerated filer o (Do not check if smaller reporting company) | Smaller reporting company x |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act)
Yes oNo x
Indicated the number of shares outstanding of each of the issuer'sissuer’s classes of common stock, as of the latest practicable date, 42,420,239 shares of common stock are issued and outstanding as of AugustNovember 14, 2008.
TABLE OF CONTENTS
Page No. | ||||
PART I. - FINANCIAL INFORMATION | ||||
Item 1. | Financial | 4 | ||
Item 2. | Management’s Discussion and Analysis of Financial Condition and Results of Operations. | 28 | ||
Item 3. | Quantitative and Qualitative Disclosures About Market Risk. | 44 | ||
Item 4T | Controls and Procedures. | 44 | ||
PART II - OTHER INFORMATION | ||||
Item 1. | Legal Proceedings. | 45 | ||
Item 1A. | Risk Factors. | 45 | ||
Item 2. | Unregistered Sales of Equity Securities and Use of Proceeds. | 45 | ||
Item 3. | Defaults Upon Senior Securities. | 46 | ||
Item 4. | Submission of Matters to a Vote of Security Holders. | 46 | ||
Item 5. | Other Information. | 46 | ||
Item 6. | Exhibits. | 46 | ||
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING INFORMATION
Certain statements in this report contain or may contain forward-looking statements that are subject to known and unknown risks, uncertainties and other factors which may cause actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements. These forward-looking statements were based on various factors and were derived utilizing numerous assumptions and other factors that could cause our actual results to differ materially from those in the forward-looking statements. These factors include, but are not limited to, our ability to enforce the Contractual Arrangements, Lotus East'sEast’s strategic initiatives, economic, political and market conditions and fluctuations, U.S. and Chinese government and industry regulation, interest rate risk, U.S., Chinese and global competition, and other factors. Most of these factors are difficult to predict accurately and are generally beyond our control. You should consider the areas of risk described in connection with any forward-looking statements that may be made herein. Readers are cautioned not to place undue reliance on these forward-looking statements and readers should carefully review this report in its entirety together with our Annual Report on Form 10-K for the year ended December 31, 2007 as filed with the SEC, including the risks described in Item 1A. Risk Factors. Except for our ongoing obligations to disclose material information under the Federal securities laws, we undertake no obligation to release publicly any revisions to any forward-looking statements, to report events or to report the occurrence of unanticipated events. These forward-looking statements speak only as of the date of this report, and you should not rely on these statements without also considering the risks and uncertainties associated with these statements and our business.
We maintain a web site at www.lotuseast.com. Information on this web site is not a part of this report.
CERTAIN DEFINED TERMS USED IN THIS PROSPECTUS
Unless specifically set forth to the contrary, when used in this report the terms:
• |
|
• |
|
• |
|
• |
|
• |
|
• |
|
• |
|
• |
|
• |
|
• |
|
• |
|
• |
|
• |
|
• |
|
PART 1. - FINANCIAL INFORMATION
Item 1.
Financial Statements.LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
|
| September 30, |
| December 31, |
| ||
|
| 2008 |
| 2007 |
| ||
ASSETS |
| (Unaudited) |
|
|
| ||
|
|
|
|
|
|
|
|
CURRENT ASSETS: |
|
|
|
|
|
|
|
Cash |
| $ | 2,656,770 |
| $ | 4,557,957 |
|
Accounts receivable, net of allowance for doubtful accounts and sale returns |
|
| 13,882,298 |
|
| 20,430,827 |
|
Inventories, net of reserve for obsolete inventory |
|
| 8,621,880 |
|
| 3,410,739 |
|
Prepaid expenses |
|
| 77,440 |
|
| 1,009,382 |
|
Deferred debt costs |
|
| 563,928 |
|
| 29,340 |
|
|
|
|
|
|
|
|
|
Total Current Assets |
|
| 25,802,316 |
|
| 29,438,245 |
|
|
|
|
|
|
|
|
|
PROPERTY AND EQUIPMENT - Net |
|
| 7,679,960 |
|
| 6,169,966 |
|
|
|
|
|
|
|
|
|
OTHER ASSETS |
|
|
|
|
|
|
|
Deposit on patent license |
|
| 2,917,536 |
|
| — |
|
Deposit on land use right |
|
| 17,120,100 |
|
| — |
|
Intangible assets, net of accumulated amortization |
|
| 1,266,020 |
|
| 1,291,322 |
|
|
|
|
|
|
|
|
|
Total Assets |
| $ | 54,785,932 |
| $ | 36,899,533 |
|
|
|
|
|
|
|
|
|
LIABILITIES AND SHAREHOLDERS’ EQUITY |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CURRENT LIABILITIES: |
|
|
|
|
|
|
|
Convertible debt, net of debt discount |
| $ | — |
| $ | 2,561,645 |
|
Accounts payable and accrued expenses |
|
| 1,845,398 |
|
| 764,491 |
|
Value-added and service taxes payable |
|
| 3,303,152 |
|
| 572,200 |
|
Advances from customers |
|
| — |
|
| 34,531 |
|
Unearned revenue |
|
| 629,084 |
|
| 530,063 |
|
Due to related parties |
|
| 1,355,077 |
|
| 323,178 |
|
|
|
|
|
|
|
|
|
Total Current Liabilities |
|
| 7,132,711 |
|
| 4,786,108 |
|
|
|
|
|
|
|
|
|
LONG-TERM LIABILITIES: |
|
|
|
|
|
|
|
Due to related parties |
|
| 656,446 |
|
| 738,300 |
|
Notes payable - related parties |
|
| 5,055,787 |
|
| 4,738,508 |
|
Series A convertible redeemable preferred stock, $.001 par value; 10,000,000 shares authorized; 5,747,118 and -0- shares issued and outstanding at September 30, 2008 and December 31, 2007, respectively |
|
| 3,559,941 |
|
| — |
|
|
|
|
|
|
|
|
|
Total Liabilities |
|
| 16,404,885 |
|
| 10,262,916 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SHAREHOLDERS’ EQUITY: |
|
|
|
|
|
|
|
Common stock ($.001 par value; 200,000,000 shares authorized; 42,420,239 and 41,794,200 shares issued and outstanding at September 30, 2008 and December 31, 2007, respectively) |
|
| 42,420 |
|
| 41,794 |
|
Additional paid-in capital |
|
| 11,277,143 |
|
| 8,095,848 |
|
Statutory reserves |
|
| 3,022,992 |
|
| 2,161,505 |
|
Retained earnings |
|
| 19,883,460 |
|
| 14,355,913 |
|
Other comprehensive gain - cumulative foreign currency translation adjustment |
|
| 4,155,032 |
|
| 1,981,557 |
|
|
|
|
|
|
|
|
|
Total Shareholders’ Equity |
|
| 38,381,047 |
|
| 26,636,617 |
|
|
|
|
|
|
|
|
|
Total Liabilities and Shareholders’ Equity |
| $ | 54,785,932 |
| $ | 36,899,533 |
|
See notes to unaudited consolidated financial statements
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
(UNAUDITED)
|
| For the Three Months Ended |
| For the Nine Months Ended |
| ||||||||
|
| September 30, |
| September 30, |
| ||||||||
|
| 2008 |
| 2007 |
| 2008 |
| 2007 |
| ||||
|
|
|
| (As Restated) |
|
|
| (As Restated) |
| ||||
NET REVENUES: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Wholesale |
| $ | 13,818,369 |
| $ | 11,429,926 |
| $ | 40,749,696 |
| $ | 25,715,018 |
|
Retail |
|
| 1,203,698 |
|
| 1,643,501 |
|
| 2,606,091 |
|
| 3,244,739 |
|
Other revenues |
|
| 1,681,817 |
|
| 3,480,922 |
|
| 4,442,886 |
|
| 8,689,579 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Net Revenues |
|
| 16,703,884 |
|
| 16,554,349 |
|
| 47,798,673 |
|
| 37,649,336 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COST OF SALES |
|
| 8,219,358 |
|
| 9,856,382 |
|
| 25,684,501 |
|
| 22,738,456 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
GROSS PROFIT |
|
| 8,484,526 |
|
| 6,697,967 |
|
| 22,114,172 |
|
| 14,910,880 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OPERATING EXPENSES: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Selling expenses |
|
| 4,293,704 |
|
| 1,441,950 |
|
| 11,291,590 |
|
| 2,864,746 |
|
Research and development |
|
| 12,448 |
|
| 1,255,844 |
|
| 1,193,916 |
|
| 1,549,132 |
|
General and administrative |
|
| 420,716 |
|
| 912,546 |
|
| 1,589,176 |
|
| 2,631,356 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Operating Expenses |
|
| 4,726,868 |
|
| 3,610,340 |
|
| 14,074,682 |
|
| 7,045,234 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
INCOME FROM OPERATIONS |
|
| 3,757,658 |
|
| 3,087,627 |
|
| 8,039,490 |
|
| 7,865,646 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OTHER INCOME (EXPENSE): |
|
|
|
|
|
|
|
|
|
|
|
|
|
Debt issuance costs |
|
| (99,516 | ) |
| (58,679 | ) |
| (261,919 | ) |
| (146,699 | ) |
Registration rights penalty |
|
| — |
|
| — |
|
| (650 | ) |
| (110,000 | ) |
Interest income |
|
| 9,032 |
|
| — |
|
| 11,620 |
|
| — |
|
Interest expense |
|
| (453,498 | ) |
| (807,608 | ) |
| (1,399,507 | ) |
| (1,508,781 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Other Income (Expense) |
|
| (543,982 | ) |
| (866,287 | ) |
| (1,650,456 | ) |
| (1,765,480 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
INCOME BEFORE INCOME TAXES |
|
| 3,213,676 |
|
| 2,221,340 |
|
| 6,389,034 |
|
| 6,100,166 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
INCOME TAXES |
|
| — |
|
| — |
|
| — |
|
| — |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NET INCOME |
| $ | 3,213,676 |
| $ | 2,221,340 |
| $ | 6,389,034 |
| $ | 6,100,166 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COMPREHENSIVE INCOME: |
|
|
|
|
|
|
|
|
|
|
|
|
|
NET INCOME |
| $ | 3,213,676 |
| $ | 2,221,340 |
| $ | 6,389,034 |
|
| 6,100,166 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OTHER COMPREHENSIVE INCOME: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Unrealized foreign currency translation gain |
|
| 127,833 |
|
| 180,895 |
| $ | 2,173,475 |
|
| 552,815 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COMPREHENSIVE INCOME |
| $ | 3,341,509 |
| $ | 2,402,235 |
| $ | 8,562,509 |
| $ | 6,652,981 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NET INCOME PER COMMON SHARE: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic |
| $ | 0.08 |
| $ | 0.05 |
| $ | 0.15 |
| $ | 0.15 |
|
Diluted |
| $ | 0.07 |
| $ | 0.05 |
| $ | 0.13 |
| $ | 0.14 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
WEIGHTED AVERAGE COMMON SHARES OUTSTANDING: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic |
|
| 42,420,239 |
|
| 41,356,483 |
|
| 42,269,997 |
|
| 41,317,461 |
|
Diluted |
|
| 48,167,357 |
|
| 44,356,483 |
|
| 48,017,115 |
|
| 44,317,461 |
|
| June 30, | December 31 | ||||||
| 2008 | 2007 | ||||||
| ASSETS | (Unaudited) | ||||||
| CURRENT ASSETS: | |||||||
| Cash | $ | 3,401,444 | $ | 4,557,957 | |||
| Accounts receivable, net of allowance for doubtful accounts and sale returns | 20,786,631 | 20,430,827 | |||||
| Inventories, net of reserve for obsolete inventory | 7,086,895 | 3,410,739 | |||||
| Prepaid expenses | 284,819 | 1,009,382 | |||||
| Deferred debt costs | 398,067 | 29,340 | |||||
| Total Current Assets | 31,957,856 | 29,438,245 | |||||
| PROPERTY AND EQUIPMENT - Net | 6,551,138 | 6,169,966 | |||||
| OTHER ASSETS | |||||||
| Deposit on patent license | 2,910,446 | - | |||||
| Deposit on land use right | 6,033,353 | - | |||||
| Intangible assets, net of accumulated amortization | 1,298,038 | 1,291,322 | |||||
| Deferred debt costs | 265,377 | - | |||||
| Total Assets | $ | 49,016,208 | $ | 36,899,533 | |||
| LIABILITIES AND SHAREHOLDERS' EQUITY | |||||||
| CURRENT LIABILITIES: | |||||||
| Convertible debt, net of debt discount | $ | - | $ | 2,561,645 | |||
| Accounts payable and accrued expenses | 1,027,999 | 764,491 | |||||
| Value-added and service taxes payable | 2,030,543 | 572,200 | |||||
| Advances from customers | - | 34,531 | |||||
| Unearned revenue | 641,162 | 530,063 | |||||
| Due to related parties | 1,272,153 | 323,178 | |||||
| Total Current Liabilities | 4,971,857 | 4,786,108 | |||||
| LONG-TERM LIABILITIES: | |||||||
| Due to related parties | 654,850 | 738,300 | |||||
| Notes payable - related parties | 5,043,500 | 4,738,508 | |||||
| Series A convertible redeemable preferred stock, $.001 par value; 10,000,000 shares | |||||||
| authorized; 5,747,118 and -0- shares issued and outstanding at June 31, 2008 | |||||||
| and December 31, 2007, respectively | 3,305,813 | - | |||||
| Total Liabilities | 13,976,020 | 10,262,916 | |||||
| SHAREHOLDERS' EQUITY: | |||||||
| Common stock ($.001 par value; 200,000,000 shares authorized; | |||||||
| 42,420,239 and 41,794,200 shares issued and outstanding | |||||||
| at June 30, 2008 and December 31, 2007, respectively) | 42,420 | 41,794 | |||||
| Additional paid-in capital | 11,277,143 | 8,095,848 | |||||
| Statutory reserves | 2,616,019 | 2,161,505 | |||||
| Retained earnings | 16,081,308 | 14,355,913 | |||||
| Other comprehensive gain - cumulative foreign currency translation adjustment | 5,023,298 | 1,981,557 | |||||
| Total Shareholders' Equity | 35,040,188 | 26,636,617 | |||||
| Total Liabilities and Shareholders' Equity | $ | 49,016,208 | $ | 36,899,533 | |||
See notes to unaudited consolidated financial statements
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
(UNAUDITED)
|
| For the Nine Months Ended |
| ||||
|
| September 30, |
| ||||
|
| 2008 |
| 2007 |
| ||
|
|
|
| (As Restated) |
| ||
CASH FLOWS FROM OPERATING ACTIVITIES: |
|
|
|
|
|
|
|
Net income |
| $ | 6,389,034 |
| $ | 6,100,166 |
|
Adjustments to reconcile net income from operations to net cash provided by operating activities: |
|
|
|
|
|
|
|
Depreciation and amortization |
|
| 469,455 |
|
| 422,055 |
|
Amortization of deferred debt issuance costs |
|
| 261,545 |
|
| 146,699 |
|
Amortization of debt discount |
|
| 208,355 |
|
| 1,041,774 |
|
Amortization of discount on convertible redeemable preferred stock |
|
| 592,966 |
|
| — |
|
Stock based compensation |
|
| 392,341 |
|
| 239,200 |
|
Warrant repricing |
|
| 74,593 |
|
| — |
|
Decrease in allowance for doubtful accounts and sales returns |
|
| (490,310 | ) |
| (1,723,258 | ) |
Changes in assets and liabilities: |
|
|
|
|
|
|
|
Accounts receivable |
|
| 8,244,225 |
|
| (6,327,758 | ) |
Inventories |
|
| (4,880,418 | ) |
| 2,390,052 |
|
Prepaid expenses and other current assets |
|
| 1,080,576 |
|
| 251,225 |
|
Accounts payable and accrued expenses |
|
| 1,032,245 |
|
| (101,796 | ) |
Value-added and service taxes payable |
|
| 2,637,331 |
|
| 1,298,344 |
|
Unearned revenue |
|
| 62,225 |
|
| 122,924 |
|
Advances from customers |
|
| (36,086 | ) |
| (170,971 | ) |
|
|
|
|
|
|
|
|
NET CASH PROVIDED BY OPERATING ACTIVITIES |
|
| 16,038,077 |
|
| 3,688,656 |
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM INVESTING ACTIVITIES: |
|
|
|
|
|
|
|
Collections from related party advances |
|
| — |
|
| 801,238 |
|
Deposit on patent right |
|
| (2,857,608 | ) |
| — |
|
Deposit on land use right |
|
| (16,768,445 | ) |
| — |
|
Purchase of intangible asset |
|
| (3,429 | ) |
| — |
|
Purchase of property and equipment |
|
| (1,430,894 | ) |
| (384,198 | ) |
|
|
|
|
|
|
|
|
NET CASH (USED IN) PROVIDED BY INVESTING ACTIVITIES |
|
| (21,060,376 | ) |
| 417,040 |
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM FINANCING ACTIVITIES: |
|
|
|
|
|
|
|
Net proceeds (payments) from convertible debt |
|
| (2,520,000 | ) |
| 2,950,000 |
|
Proceeds from sale of convertible redeemable peferred stocks |
|
| 5,000,000 |
|
| — |
|
Payment of debt issuance costs |
|
| (468,568 | ) |
| (231,526 | ) |
Proceeds from related party advances |
|
| 860,916 |
|
| — |
|
Repayments of related party advances |
|
| — |
|
| (863,480 | ) |
Repayments of notes payable - related parties |
|
| — |
|
| (1,971,772 | ) |
|
|
|
|
|
|
|
|
NET CASH PROVIDED BY (USED IN) FINANCING ACTIVITIES |
|
| 2,872,348 |
|
| (116,778 | ) |
|
|
|
|
|
|
|
|
EFFECT OF EXCHANGE RATE ON CASH |
|
| 248,764 |
|
| 116,228 |
|
|
|
|
|
|
|
|
|
NET (DECREASE) INCREASE IN CASH |
|
| (1,901,187 | ) |
| 4,105,146 |
|
|
|
|
|
|
|
|
|
CASH - beginning of period |
|
| 4,557,957 |
|
| 2,089,156 |
|
|
|
|
|
|
|
|
|
CASH - end of period |
| $ | 2,656,770 |
| $ | 6,194,302 |
|
|
|
|
|
|
|
|
|
SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: |
|
|
|
|
|
|
|
Cash paid for: |
|
|
|
|
|
|
|
Interest |
| $ | 103,250 |
| $ | 1,582,393 |
|
Income taxes |
| $ | — |
| $ | — |
|
|
|
|
|
|
|
|
|
Non-cash investing and financing activities: |
|
|
|
|
|
|
|
Warrants issued for prepaid financing costs |
| $ | 327,565 |
| $ | — |
|
Warrants issued for consulting |
| $ | 178,187 |
| $ | — |
|
Common stock issued for compensation |
| $ | 318,551 |
| $ | — |
|
Common stock issued for conversion of convertible debt |
| $ | 250,000 |
| $ | — |
|
Debt discount for grant of warrants and beneficial conversion feature |
| $ | 2,033,025 |
| $ | — |
|
See notes to unaudited consolidated financial statements.
| For the Three Months Ended | For the Six Months Ended | ||||||||||||
| June 30, | June 30, | ||||||||||||
| 2008 | 2007 | 2008 | 2007 | ||||||||||
| (As Restated) | (As Restated) | ||||||||||||
| NET REVENUES: | |||||||||||||
| Wholesale | $ | 17,161,230 | $ | 9,819,373 | $ | 26,931,327 | $ | 14,993,316 | |||||
| Retail | 718,645 | 516,594 | 1,402,393 | 893,014 | |||||||||
| Other revenues | 1,505,737 | 2,469,485 | 2,761,069 | 5,208,657 | |||||||||
| Total Net Revenues | 19,385,612 | 12,805,452 | 31,094,789 | 21,094,987 | |||||||||
| COST OF SALES | 9,696,718 | 7,311,471 | 17,465,143 | 12,974,387 | |||||||||
| GROSS PROFIT | 9,688,894 | 5,493,981 | 13,629,646 | 8,120,600 | |||||||||
| OPERATING EXPENSES: | |||||||||||||
| Selling expenses | 5,875,549 | 791,784 | 6,997,886 | 1,422,796 | |||||||||
| Research and development | 471,243 | 109,153 | 1,181,468 | 200,975 | |||||||||
| General and administrative | 542,043 | 988,233 | 1,168,460 | 1,718,810 | |||||||||
| Total Operating Expenses | 6,888,835 | 1,889,170 | 9,347,814 | 3,342,581 | |||||||||
| INCOME FROM OPERATIONS | 2,800,059 | 3,604,811 | 4,281,832 | 4,778,019 | |||||||||
| OTHER INCOME (EXPENSE): | |||||||||||||
| Debt issuance costs | (99,517 | ) | (58,680 | ) | (162,403 | ) | (88,020 | ) | |||||
| Registration rights penalty | - | (56,000 | ) | (110,000 | ) | ||||||||
| Interest income | 2,027 | - | 2,588 | - | |||||||||
| Interest expense | (522,660 | ) | (448,606 | ) | (946,009 | ) | (701,173 | ) | |||||
| Total Other Income (Expense) | (620,150 | ) | (563,286 | ) | (1,105,824 | ) | (899,193 | ) | |||||
| INCOME BEFORE INCOME TAXES | 2,179,909 | 3,041,525 | 3,176,008 | 3,878,826 | |||||||||
| INCOME TAXES | - | - | - | - | |||||||||
| NET INCOME | $ | 2,179,909 | $ | 3,041,525 | $ | 3,176,008 | $ | 3,878,826 | |||||
| COMPREHENSIVE INCOME: | |||||||||||||
| NET INCOME | $ | 2,179,909 | $ | 3,041,525 | $ | 3,176,008 | 3,878,826 | ||||||
| OTHER COMPREHENSIVE INCOME: | |||||||||||||
| Unrealized foreign currency translation gain | 1,587,538 | 248,403 | $ | 3,041,741 | 371,920 | ||||||||
| COMPREHENSIVE INCOME | $ | 3,767,447 | $ | 3,289,928 | $ | 6,217,749 | $ | 4,250,746 | |||||
| NET INCOME PER COMMON SHARE: | |||||||||||||
| Basic | $ | 0.05 | $ | 0.07 | $ | 0.08 | $ | 0.09 | |||||
| Diluted | $ | 0.05 | $ | 0.07 | $ | 0.07 | $ | 0.09 | |||||
WEIGHTED AVERAGE COMMON SHARES OUTSTANDING: | |||||||||||||
| Basic | 42,352,139 | 41,389,362 | 42,194,048 | 41,297,531 | |||||||||
| Diluted | 48,099,257 | 44,389,262 | 47,941,166 | 44,297,531 | |||||||||
| For the Six Month Ended | |||||||
| June 30, | |||||||
| 2008 | 2007 | ||||||
| (As Restated) | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | |||||||
| Net income | $ | 3,176,008 | $ | 3,878,826 | |||
| Adjustments to reconcile net income from operations to net cash | |||||||
| provided by (used in) operating activities: | |||||||
| Depreciation and amortization | 307,713 | 275,197 | |||||
| Amortization of deferred debt issuance costs | 162,029 | 88,020 | |||||
| Amortization of debt discount | 208,355 | 433,735 | |||||
| Amortization of discount on convertible redeemable preferred stock | 338,838 | - | |||||
| Stock base compensation | 270,245 | 55,650 | |||||
| Warrant repricing | 74,593 | - | |||||
| Decrease in allowance for doubtful accounts and sales returns | (14,101 | ) | (1,567,166 | ) | |||
| Changes in assets and liabilities: | |||||||
| Accounts receivable | 946,083 | (524,467 | ) | ||||
| Inventories | (3,358,488 | ) | 1,201,912 | ||||
| Prepaid expenses and other current assets | 986,132 | 113,540 | |||||
| Other receivable | - | - | |||||
| Accounts payable and accrued expenses | 224,627 | 78,398 | |||||
| Value-added and service taxes payable | 1,381,155 | 921,996 | |||||
| Unearned revenue | 74,796 | 132,461 | |||||
| Advances from customers | (35,710 | ) | (145,138 | ) | |||
| NET CASH PROVIDED BY OPERATING ACTIVITIES | 4,742,275 | 4,942,964 | |||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | |||||||
| Collections from related party advances | - | 521,930 | |||||
| Deposit on patent right | (2,827,814 | ) | - | ||||
| Deposit on land use right | (5,862,059 | ) | - | ||||
| Purchase of property and equipment | (217,982 | ) | (376,201 | ) | |||
| NET CASH (USED IN) PROVIDED BY INVESTING ACTIVITIES | (8,907,855 | ) | 145,729 | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | |||||||
| Net proceeds (payments) from convertible debt | (2,520,000 | ) | 2,950,000 | ||||
| Proceeds from sale of convertible redeemable peferred stocks | 5,000,000 | - | |||||
| Payment of debt issuance costs | (468,568 | ) | (231,526 | ) | |||
| Proceeds from related party advances | 774,571 | 2,866 | |||||
| Repayments of related party advances | - | (719,302 | ) | ||||
| Repayments of notes payable - related parties | - | (846,781 | ) | ||||
| NET CASH PROVIDED BY FINANCING ACTIVITIES | 2,786,003 | 1,155,257 | |||||
| EFFECT OF EXCHANGE RATE ON CASH | 223,064 | 107,929 | |||||
| NET (DECREASE) INCREASE IN CASH | (1,156,513 | ) | 6,351,879 | ||||
| CASH - beginning of period | 4,557,957 | 2,089,156 | |||||
| CASH - end of period | $ | 3,401,444 | $ | 8,441,035 | |||
| SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: | |||||||
| Cash paid for: | |||||||
| Interest | $ | 56,557 | $ | 267,438 | |||
| Income taxes | $ | - | $ | - | |||
| Non-cash operting, investing and financing activities: | |||||||
| Warrants issued for prepaid financing costs | $ | 327,565 | $ | - | |||
| Warrants issued for prepaid consulting | $ | 178,187 | - | ||||
| Common stock issued for compensation | $ | 318,551 | - | ||||
| Common stock issued for conversion of convertible debt | $ | 250,000 | $ | - | |||
| Debt discount for grant of warrants and beneficial conversion feature | $ | 2,033,025 | $ | - | |||
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2008
NOTE 1 - ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Organization
Lotus Pharmaceuticals, Inc. (“Lotus” or the “Company”), formerly S.E. Asia Trading Company, Inc. (“SEAA”), was incorporated on January 28, 2004 under the laws of the State of Nevada. SEAA operated as a retailer of jewelry, framed art and home accessories. In December 2006, SEAA changed its name to Lotus Pharmaceuticals, Inc.
On September 6, 2006, the Company entered into a definitive Share Exchange Agreement with Lotus Pharmaceutical International, Inc. (“Lotus International”), whereby the Company acquired all of the outstanding common stock of Lotus International in exchange for newly-issued stock of the Company to Lotus International’s shareholders. On September 28, 2006 (the closing date), Lotus International became a wholly-owned subsidiary of the Company and Lotus International’s shareholders became the owners of the majority of the Company’s voting stock. The acquisition of Lotus International by the Company was accounted for as a reverse merger because on a post-merger basis, the former shareholders of Lotus International hold a majority of the outstanding common stock of the Company on a voting and fully-diluted basis. As a result, Lotus International is deemed to be the acquirer for accounting purposes.
Lotus International was incorporated under the laws the State of Nevada on August 28, 2006 to develop and market pharmaceutical products in the People’s Republic of China (“PRC” or “China”). PRC law currently has limits on foreign ownership of certain companies. To comply with these foreign ownership restrictions, Lotus operates its pharmaceutical business in China through Beijing Liang Fang Pharmaceutical Co., Ltd. (“Liang Fang”) and an affiliate of Liang Fang, Beijing En Zhe Jia Shi Pharmaceutical Co., Ltd. (“En Zhe Jia”), both of which are pharmaceutical companies headquartered in the PRC and organized under the laws of the PRC (hereinafter, referred to together as “Lotus East”). Lotus International has contractual arrangements with Lotus East and its shareholders pursuant to which Lotus International will provide technology consulting and other general business operation services to Lotus East. Through these contractual arrangements, Lotus International also has the ability to substantially influence Lotus East’s daily operations and financial affairs, appoint its senior executives and approve all matters requiring shareholder approval. As a result of these contractual arrangements, which enable Lotus International to control Lotus East, Lotus International is considered the primary beneficiary of Lotus East. Accordingly, the consolidated financial statements include the accounts of Lotus Pharmaceuticals, Inc. and its wholly-owned subsidiary, Lotus International and companies under its control (Lotus East).
On September 6, 2006, Lotus International entered into the following contractual arrangements:
Operating Agreement. Pursuant to the operating agreement among Lotus, Lotus East and the shareholders of Lotus East, (collectively “Lotus East’s Shareholders”), Lotus provides guidance and instructions on Lotus East’s daily operations, financial management and employment issues. The shareholders of Lotus East must designate the candidates recommended by Lotus as their representatives on Lotus East’s Board of Directors. Lotus has the right to appoint senior executives of Lotus East. In addition, Lotus agreed to guarantee Lotus East’s performance under any agreements or arrangements relating to Lotus East’s business arrangements with any third party. Lotus East, in return, agreed to pledge its accounts receivable and all of its assets to Lotus. Moreover, Lotus East agreed that without the prior consent of Lotus, Lotus East would not engage in any transaction that could materially affect the assets, liabilities, rights or operations of Lotus East, including, without limitation, incurrence or assumption of any indebtedness, sale or purchase of any assets or rights, incurrence of any encumbrance on any of its assets or intellectual property rights in favor of a third party or transfer of any agreements relating to its business operation to any third party. The term of this agreement is ten (10) years from September 6, 2006 and may be extended only upon Lotus’s written confirmation prior to the expiration of the this agreement, with the extended term to be mutually agreed upon by the parties.
NOTE 1 - ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2008
Equity Pledge Agreement. Under the equity pledge agreement between the shareholders of Lotus East and Lotus, the shareholders of Lotus East pledged all of their equity interests in Lotus East to Lotus to guarantee Lotus East’s performance of its obligations under the technology consulting agreement. If Lotus East or Lotus East’s Shareholders breaches its respective contractual obligations, Lotus, as pledgee, will be entitled to certain rights, including the right to sell the pledged equity interests. Lotus East’s Shareholders also agreed that upon occurrence of any event of default, Lotus shall be granted an exclusive, irrevocable power of attorney to take actions in the place and stead of Lotus East’s Shareholders to carry out the security provisions of the equity pledge agreement and take any action and execute any instrument that Lotus may deem necessary or advisable to accomplish the purposes of the equity pledge agreement. The shareholders of Lotus East agreed not to dispose of the pledged equity interests or take any actions that would prejudice Lotus’ interest. The equity pledge agreement will expire two (2) years after Lotus East’s obligations under the exclusive consulting services agreements have been fulfilled.
Option Agreement. Under the option agreement between the shareholders of Lotus East and Lotus, the shareholders of Lotus East irrevocably granted Lotus or its designated person an exclusive option to purchase, to the extent permitted under PRC law, all or part of the equity interests in Lotus East for the cost of the initial contributions to the registered capital or the minimum amount of consideration permitted by applicable PRC law. Lotus or its designated person has sole discretion to decide when to exercise the option, whether in part or in full. The term of this agreement is 10 years from September 6, 2006 and may be extended prior to its expiration by written agreement of the parties.
Proxy Agreement.. Pursuant to the proxy agreement among Lotus and Lotus East’s Shareholders, Lotus East’s Shareholders agreed to irrevocably grant a person to be designated by Lotus with the right to exercise Lotus East’s Shareholders’ voting rights and their other rights, including the attendance at and the voting of Lotus East’s Shareholders’ shares at the shareholders’ meetings (or by written consent in lieu of such meetings) in accordance with applicable laws and its Article of Association, including but not limited to the rights to sell or transfer all or any of his equity interests of Lotus East, and appoint and vote for the directors and Chairman as the authorized representative of the shareholders of Lotus East. The term of this Proxy Agreement is ten (10) years from September 6, 2006 and may be extended prior to its expiration by written agreement of the parties.
Liang Fang is a Chinese limited liability company and was formed under laws of the People’s Republic of China on June 21, 2000. Liang Fang is engaged in the production, trade and retailing of pharmaceuticals. Further, Liang Fang is focused on development of innovative medicines and investing strategic growth to address various medical needs for patients worldwide. Liang Fang’s operations are based in Beijing, China.
As of JuneSeptember 30, 2008, Liang Fang owns and operates 10 drug stores throughout Beijing, China. These drugstores sell Western and traditional Chinese medicines, and medical treatment accessories.
Liang Fang’s affiliate, En Zhe Jia is a Chinese limited liability company and was formed under laws of the People’s Republic of China on September 17, 1999. En Zhe Jia is the sole manufacturer for Liang Fang and maintains facilities for the production of medicines, patented Chinese medicine, as well as the research and production of other new medicines.
As a result of the management agreements between Lotus International and Lotus East, Lotus East was deemed to be the acquirer of Lotus International for accounting purposes. Accordingly, the financial statement data presented are those of Lotus East for all periods prior to the Company’s acquisition of Lotus International on September 28, 2006, and the financial statements of the consolidated companies from the acquisition date forward.
On May 29, 2007, the Company formed a new entity, Lotus Century Pharmaceutical (Beijing) Technology Co., Ltd. (‘‘Lotus Century’’), a wholly foreign-owned enterprise (“WFOE”) organized under the laws of the Peoples’ Republic of China. Lotus Century is a Chinese limited liability company and a wholly-owned subsidiary of Lotus Pharmaceutical International, Inc. Lotus Century intends to be engaged in development of innovative medicines, medical technology consulting and outsourcing services, and related training services.
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2008
Basis of presentation
The interim consolidated financial statements included herein have been prepared by the Company, pursuant to the rules and regulations of the Securities and Exchange Commission (the “SEC”). Certain information and footnote disclosures normally included in an annual financial statement prepared in accordance with generally accepted accounting principles in the United States (“GAAP”) have been condensed or omitted pursuant to such rules and regulations. In the opinion of management, the interim consolidated financial statements reflect all adjustments (consisting only of normal recurring adjustments) necessary for a fair presentation of the statement of the results for the interim periods presented. These interim consolidated financial statements should be read in conjunction with the audited consolidated financial statements and notes thereto, as well as the accompanying Management’s Discussion and Analysis of Financial Condition and Results of Operations for the year ended December 31, 2007 included in its Annual Report on Form 10-K. Interim financial results are not necessarily indicative of the results that may be expected for a full year.
The Company has adopted FASB Interpretation No. 46R “Consolidation of Variable Interest Entities” (“FIN 46R”), an Interpretation of Accounting Research Bulletin No. 51. FIN 46R requires a Variable Interest Entity (VIE) to be consolidated by a company if that company is subject to a majority of the risk of loss for the VIE or is entitled to receive a majority of the VIE’s residual returns. VIEs are those entities in which the Company, through contractual arrangements, bears the risks of, and enjoys the rewards normally associated with ownership of the entities, and therefore the Company is the primary beneficiary of these entities. As a VIE, Lotus East’s revenues are included in the Company’s total revenues, its income from operations is consolidated with the Company’s, and the Company’s net income includes all of Lotus East’s net income.
The accompanying unaudited consolidated financial statements are prepared in accordance with generally accepted accounting principles in the United States of America (“US GAAP”). The consolidated statements include the accounts of Lotus Pharmaceuticals, Inc. and its wholly-owned subsidiaries, Lotus and Lotus Century and variable interest entities under its control (Liang Fang and En Zhe Jia). All significant inter-company balances and transactions have been eliminated.
The Company has adopted FASB Interpretation No. 46R "Consolidation of Variable Interest Entities" ("FIN 46R"), an Interpretation of Accounting Research Bulletin No. 51. FIN 46R requires a Variable Interest Entity (VIE) to be consolidated by a company if that company is subject to a majority of the risk of loss for the VIE or is entitled to receive a majority of the VIE's residual returns. VIEs are those entities in which the Company, through contractual arrangements, bears the risks of, and enjoys the rewards normally associated with ownership of the entities, and therefore the Company is the primary beneficiary of these entities. As a VIE, Lotus East’s revenues are included in the Company’s total revenues, its income from operations is consolidated with the Company’s, and the Company’s net income includes all of Lotus East’s net income.
The preparation of financial statements in conformity with US GAAP requires management to make estimates and assumptions that affect certain reported amounts and disclosures. Accordingly, actual results could differ from those estimates. Significant estimates in 2008 and 2007 include the allowance for doubtful accounts, the allowance for obsolete inventory, the useful life of property and equipment and intangible assets, and accruals for taxes due.
Fair value of financial instruments
The carrying amounts reported in the balance sheet for cash, accounts receivable, accounts payable and accrued expenses, convertible debt, customer advances, and amounts due to related parties approximate their fair market value based on the short-term maturity of these instruments.
Cash and cash equivalents
For purposes of the consolidated statements of cash flows, the Company considers all highly liquid instruments purchased with a maturity of three months or less and money market accounts to be cash equivalents. The Company maintains cash and cash equivalents with various financial institutions mainly in the PRC and the United States. Balances in the United States are insured up to $100,000 at each bank.
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2008
Accounts receivable
The Company records accounts receivable, net of an allowance for doubtful accounts and sales returns. The Company maintains allowances for doubtful accounts for estimated losses. The Company reviews the accounts receivable on a periodic basis and makes general and specific allowances when there is doubt as to the collectability of individual balances. In evaluating the collectability of individual receivable balances, the Company considers many factors, including the age of the balance, customer’s historical payment history, its current credit-worthiness and current economic trends. The amount of the provision, if any is recognized in the consolidated statement of operations within “General and administrative expenses”. Accounts are written off after exhaustive efforts at collection. The Company policy regarding sales returns is discussed below. The activity in the allowance for doubtful accounts and sales returns accounts for accounts receivable for the periods ended JuneSeptember 30, 2008 and December 31, 2007 are as follows:
|
| Allowance for doubtful accounts |
| Allowance for sales returns |
| Total |
| |||
Balance - December 31, 2006 |
| $ | 539,627 |
| $ | 2,297,399 |
| $ | 2,837,026 |
|
Reductions |
|
| (26,617) |
|
| (2,354,720) |
|
| (2,381,337) |
|
Foreign currency translation adjustments |
|
| 35,073 |
|
| 57,321 |
|
| 92,394 |
|
Balance - December 31, 2007 |
|
| 548,083 |
|
| - |
|
| 548,083 |
|
Reductions (unaudited) |
|
| (490,310) |
|
| - |
|
| (490,310) |
|
Foreign currency translation adjustments (unaudited) |
|
| 26,416 |
|
| - |
|
| 26,416 |
|
Balance - September 30, 2008 (unaudited) |
| $ | 84,189 |
| $ | - |
| $ | 84,189 |
|
| Allowance for doubtful accounts | Allowance for sales returns | Total | ||||||||
| Balance - December 31, 2006 | $ | 539,627 | $ | 2,297,399 | $ | 2,837,026 | ||||
| Reductions | (26,617 | ) | (2,354,720 | ) | (2,381,337 | ) | ||||
| Foreign currency translation adjustments | 35,073 | 57,321 | 92,394 | |||||||
| Balance - December 31, 2007 | 548,083 | - | 548,083 | |||||||
| Reductions | (14,101 | ) | - | (14,101 | ) | |||||
| Foreign currency translation adjustments | 34,865 | - | 34,865 | |||||||
| Balance - June 30, 2008 | $ | 568,847 | $ | - | $ | 568,847 | ||||
NOTE 1 - InventoriesORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Inventories, consisting of raw materials and finished goods related to the Company’s products are stated at the lower of cost or market utilizing the weighted average method. An allowance is established when management determines that certain inventories may not be saleable. If inventory costs exceed expected market value due to obsolescence or quantities in excess of expected demand, the Company will record reserves for the difference between the cost and the market value. These reserves are recorded based on estimates and reflected in cost of sales.
Property and equipment
Property and equipment are carried at cost and are depreciated on a straight-line basis over the estimated useful lives of the assets. The cost of repairs and maintenance is expensed as incurred; major replacements and improvements are capitalized. When assets are retired or disposed of, the cost and accumulated depreciation are removed from the accounts, and any resulting gains or losses are included in income in the year of disposition. In accordance with Statement of Financial Accounting Standards (SFAS) No. 144, “Accounting for the Impairment or Disposal of Long-Lived Assets”, the Company examines the possibility of decreases in the value of fixed assets when events or changes in circumstances reflect the fact that their recorded value may not be recoverable.
ImpairmentImpairment of long-lived assets
In accordance with Statement of Financial Accounting Standards (SFAS) No. 144, “Accounting for the Impairment or Disposal of Long-Lived Assets”, the Company periodically reviews its long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be fully recoverable. The Company recognizes an impairment loss when the sum of expected undiscounted future cash flows is less than the carrying amount of the asset. The amount of impairment is measured as the difference between the asset’s estimated fair value and its book value. The Company did not consider it necessary to record any impairment charges during the year ended December 31, 2007 and during the sixnine months ended JuneSeptember 30, 2008.
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2008
Advances from customers
Income taxes
The Company is governed by the Income Tax Law of the People’s Republic of China and the United States. Income taxes are accounted for under Statement of Financial Accounting Standards No. 109, “Accounting for Income Taxes,” which is an asset and liability approach that requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in the Company’s financial statements or tax returns.
NOTE 1 - ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Basic earnings per share is computed by dividing net earnings by the weighted average number of shares of common stock outstanding during the period. Diluted income per share is computed by dividing net income by the weighted average number of shares of common stock, common stock equivalents and potentially dilutive securities outstanding during each period. Potentially dilutive common shares consist of the common shares issuable upon the conversion of convertible debt (using the if-converted method). The following table presents a reconciliation of basic and diluted earnings per share:
|
| For the Three Months Ended September 30, |
| ||||
|
| 2008 |
| 2007 |
| ||
Net income for basic and diluted earnings per share |
| $ | 3,213,676 |
| $ | 2,221,340 |
|
|
|
|
|
|
|
|
|
Weighted average shares outstanding - basic |
|
| 42,420,239 |
|
| 41,356,483 |
|
Effect of dilutive securities: |
|
|
|
|
|
|
|
Unexercised warrants |
|
| — |
|
| — |
|
Convertible debentures |
|
| — |
|
| 3,000,000 |
|
Convertible redeemable preferred shares |
|
| 5,747,118 |
|
| — |
|
Weighted average shares outstanding- diluted |
|
| 48,167,357 |
|
| 44,356,483 |
|
Earnings per share - basic |
| $ | 0.08 |
| $ | 0.05 |
|
Earnings per share - diluted |
| $ | 0.07 |
| $ | 0.05 |
|
|
| For the Nine Months Ended September 30, |
| ||||
|
| 2008 |
| 2007 |
| ||
Net income for basic and diluted earnings per share |
| $ | 6,389,034 |
| $ | 6,100,166 |
|
|
|
|
|
|
|
|
|
Weighted average shares outstanding - basic |
|
| 42,269,997 |
|
| 41,317,461 |
|
Effect of dilutive securities: |
|
|
|
|
|
|
|
Unexercised warrants |
|
| — |
|
| — |
|
Convertible debentures |
|
| — |
|
| 3,000,000 |
|
Convertible redeemable preferred shares |
|
| 5,747,118 |
|
| — |
|
Weighted average shares outstanding- diluted |
|
| 48,017,115 |
|
| 44,317,461 |
|
Earnings per share - basic |
| $ | 0.15 |
| $ | 0.15 |
|
Earnings per share - diluted |
| $ | 0.13 |
| $ | 0.14 |
|
For the Three Months Ended June 30, | |||||||
2008 | 2007 | ||||||
| Net income for basic and diluted earnings per share | $ | 2,179,909 | $ | 3,041,525 | |||
| Weighted average shares outstanding - basic | 42,352,139 | 41,389,362 | |||||
| Effect of dilutive securities: | |||||||
| Unexercised warrants | — | — | |||||
| Convertible debentures | — | 3,000,000 | |||||
| Convertible redeemable preferred shares | 5,747,118 | — | |||||
| Weighted average shares outstanding- diluted | 48,099,257 | 44,389,362 | |||||
| Earnings per share - basic | $ | 0.05 | $ | 0.07 | |||
| Earnings per share - diluted | $ | 0.05 | $ | 0.07 | |||
For the Six Months Ended June 30, | |||||||
2008 | 2007 | ||||||
| Net income for basic and diluted earnings per share | $ | 3,176,008 | $ | 3,878,826 | |||
| Weighted average shares outstanding - basic | 42,194,048 | 41,297,531 | |||||
| Effect of dilutive securities: | |||||||
| Unexercised warrants | — | — | |||||
| Convertible debentures | — | 3,000,000 | |||||
| Convertible redeemable preferred shares | 5,747,118 | — | |||||
| Weighted average shares outstanding- diluted | 47,941,166 | 44,297,531 | |||||
| Earnings per share - basic | $ | 0.08 | $ | 0.09 | |||
| Earnings per share - diluted | $ | 0.07 | $ | 0.09 | |||
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2008
At JuneSeptember 30, 2008 and 2007, a total of 5,166,999 and 1,500,000 outstanding warrants have not been included in the calculation of diluted earnings per shares as the effect would be anti-dilutive. The closing market price of the Company on JuneSeptember 30, 2008 and 2007 was lower than the exercise price of all outstanding warrants. Because of that, the Company assumes that none of the outstanding warrants at that date would have been exercised and therefore none were included in the computation of the diluted earnings per share for period ended JuneSeptember 30, 2008 and 2007. Accordingly, the Company has excluded any effect of outstanding warrants as their effect would be anti-dilutive.
NOTE 1 - ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Product sales
Product sales are generally recognized when title to the product has transferred to customers in accordance with the terms of the sale. The Company recognizes revenue in accordance with the Securities and Exchange Commission’s (SEC) Staff Accounting Bulletin (SAB) No. 101, “Revenue Recognition in Financial Statements”as amended by SAB No. 104 (together, “SAB 104”), and Statement of Financial Accounting Standards (SFAS) No. 48 “Revenue Recognition When Right of Return Exists.”SAB 104 states that revenue should not be recognized until it is realized or realizable and earned. In general, the Company records revenue when persuasive evidence of an arrangement exists, services have been rendered or product delivery has occurred, the sales price to the customer is fixed or determinable, and collectability is reasonably assured.
SFAS No. 48 states that revenue from sales transactions where the buyer has the right to return the product shall be recognized at the time of sale only if the seller’s price to the buyer is substantially fixed or determinable at the date of sale, the buyer has paid the seller, or the buyer is obligated to pay the seller and the obligation is not contingent on resale of the product, the buyer’s obligation to the seller would not be changed in the event of theft or physical destruction or damage of the product, the buyer acquiring the product for resale has economic substance apart from that provided by the seller, the seller does not have significant obligations for future performance to directly bring about resale of the product by the buyer, and the amount of future returns can be reasonably estimated.
The Company’s net product revenues represent total product revenues less allowances for returns.
Allowance for returns
The Company accounts for sales returns in accordance with Statements of Financial Accounting Standards (SFAS) No. 48, Revenue Recognition When Right of Return Exists, by establishing an accrual in an amount equal to its estimate of sales recorded for which the related products are expected to be returned. The Company determines the estimate of the sales return accrual primarily based on historical experience regarding sales returns, but also by considering other factors that could impact sales returns. These factors include levels of inventory in the distribution channel, estimated shelf life, product discontinuances, and price changes of competitive products, introductions of generic products and introductions of competitive new products. In general, for wholesale sales, the Company provides credit for product returns that are returned six months prior to and up to six months after the product expiration date. Upon sale, the Company estimates an allowance for future product returns. The Company provides additional reserves for contemporaneous events that were not known and knowable at the time of shipment. In order to reasonably estimate future returns, the Company analyzed both quantitative and qualitative information including, but not limited to, actual return rates, the level of product manufactured by the Company, the level of product in the distribution channel, expected shelf life of the product, current and projected product demand, the introduction of new or generic products that may erode current demand, and general economic and industry wide indicators. The Company also utilizes the guidance provided in SAB 104 in establishing its return estimates. At JuneThe Company’s allowance for sales return was zero both at September 30, 2008 and December 31, 2007, the Company’s allowance for returns was $0 and $0, respectively.2007.
LOTUS PHARMACEUTICALS, INC. AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2008
Other revenues
Other revenues consist of (i) leasing revenues received for the lease of retail space to various retail merchants; (ii) advertising revenues from the lease of counter space at the Company’s retail locations; (iii) leasing revenue from the lease of retail space to licensed medical practitioners; (iv) revenues received by the Company for research and development projects and lab testing jobs conducted on behalf of third party companies, and; (v) revenues received for performing third party contract manufacturing projects. In connection with third-party manufacturing, the customer supplies the raw materials and the Company is paid a fee for manufacturing their product and revenue is recognized at the completion of the manufacturing job. The Company recognizes revenues from leasing of space and advertising revenues as earned from contracting third parties. The Company recognizes revenues upon performance of any research or lab testing jobs. Revenues received in advance are reflected as deferred revenue on the accompanying balance sheet. Additionally, the Company receives income from the sale of developed drug formulas. Income from the sale of drug formulas are recognized upon performance of all of the Company’s obligations under the respective sales contract and are included in other income on the accompanying consolidated statement of operations.
Concentrations of credit risk
Financial instruments which potentially subject the Company to concentrations of credit risk consist principally of cash and trade accounts receivable. Substantially all of the Company’s cash is maintained with state-owned banks within the People’s Republic of China of which no deposits are covered by insurance. The Company has not experienced any losses in such accounts and believes it is not exposed to any risks on its cash in bank accounts. A significant portion of the Company’s sales are credit sales which are primarily to customers whose ability to pay is dependent upon the industry economics prevailing in these areas; however, concentrations of credit risk with respect to trade accounts receivables is limited due to generally short payment terms. The Company also performs ongoing credit evaluations of its customers to help further reduce credit risk.
Shipping costs
Shipping costs arerelated to costs of goods sold were included in costselling expenses with a total of sales$208,997 and totaled $206,818 and $505,697$590,000 for the sixnine months ended JuneSeptember 30, 2008 and 2007, respectively.
AAdvertisingdvertising
Advertising is expensed as incurred. Advertising expenses were included in general and administrative expenses amounted to $155,530$175,086 and $263,529$964,448 for the sixnine months ended JuneSeptember 30, 2008 and 2007, respectively.
Research and development
Research and development costs are expensed as incurred. These costs primarily consist of cost of material used and salaries paid for the development of the Company’s products and fees paid to third parties. For the sixnine months ended JuneSeptember 30, 2008 and 2007, the Company expensed $1,181,468$1,193,916 and $200,975$1,549,132 as research and development expense, respectively, and paid for future research and development services in the amount of $0 and $769,742 at JuneSeptember 30, 2008 and December 31, 2007, respectively, which has been included in prepaid expenses on the accompanying balance sheets. The December 31, 2007 prepaid research and development expense has been fully expensed during the period ended JuneSeptember 30, 2008.
NOTE 1 - ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
The reporting currency of the Company is the U.S. dollar. The functional currency of the Company is the local currency, the Chinese Renminbi (“RMB”). Results of operations and cash flows are translated at average exchange rates during the period, assets and liabilities are translated at the unified exchange rate at the end of the period, and equity is translated at historical exchange rates. Translation adjustments resulting from the process of translating the local currency financial statements into U.S. dollars are included in determining comprehensive income. The cumulative translation adjustment and effect of exchange rate changes on cash for the sixnine months ended JuneSeptember 30, 2008 and 2007 was $223,064$4,155,032 and $107,929,$1,981,557, respectively. Transaction gains and losses that arise from exchange rate fluctuations on transactions denominated in a currency other than the functional currency are included in the results of operations as incurred.
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2008
Asset and liability accounts at JuneSeptember 30, 2008 and December 31, 2007 were translated at 6.87186.8551 RMB to $1.00 USD and at 7.3141 RMB to $1.00 USD, respectively. Equity accounts were stated at their historical rate. The average translation rates applied to income statements for the sixnine months ended JuneSeptember 30, 2008 and 2007 were 7.07266.99886 RMB and 7.73007.67576 RMB to $1.00 USD, respectively. In accordance with Statement of Financial Accounting Standards No. 95, "Statement“Statement of Cash Flows,"” cash flows from the Company'sCompany’s operations is calculated based upon the local currencies using the average translation rate. As a result, amounts related to assets and liabilities reported on the statement of cash flows will not necessarily agree with changes in the corresponding balances on the balance sheet.
Accumulated other comprehensive income
Accumulated other comprehensive income consisted of unrealized gains on currency translation adjustments from the translation of financial statements from Chinese RMB to US dollars. As of JuneSeptember 30, 2008 and December 31, 2007, accumulated other comprehensive income was $5,023,298$4,155,032 and $1,981,557, respectively.
Reclassifications
Certain accounts and amounts in the period ended JuneSeptember 30, 2007 financial statements have been reclassified in order to conform withto the period ended JuneSeptember 30, 2008 presentation. These reclassifications have no effect on net income.
Recent Accounting Pronouncements
In February 2007, the FASB issued SFAS No. 159,“”The Fair Value Option for Financial Assets and Financial Liabilities, Including an Amendment of FASB Statement No. 115” , under which entities will now be permitted to measure many financial instruments and certain other assets and liabilities at fair value on an instrument-by-instrument basis. This Statement is effective as of the beginning of an entity’s first fiscal year that begins after November 15, 2007. Early adoption is permitted as of the beginning of a fiscal year that begins on or before November 15, 2007, provided the entity also elects to apply the provisions of SFAS 157. The adoption of this interpretation did not have an impact on the Company’s financial position, results of operations, or cash flows.
In December 2007, the FASB issued Statement of Financial Accounting Standards No. 141R, Business Combinations (SFAS 141R) and Statement of Financial Accounting Standards No. 160, Accounting and Reporting of Non-controlling Interests in Consolidated Financial Statements, an amendment of ARB No. 51 (SFAS 160). These two standards must be adopted in conjunction with each other on a prospective basis. The most significant changes to business combination accounting pursuant to SFAS 141R and SFAS 160 are the following: (a) recognize, with certain exceptions, 100 percent of the fair values of assets acquired, liabilities assumed and non-controlling interests in acquisitions of less than a 100 percent controlling interest when the acquisition constitutes a change in control of the acquired entity, (b) acquirers’ shares issued in consideration for a business combination will be measured at fair value on the closing date, not the announcement date, (c) recognize contingent consideration arrangements at their acquisition date fair values, with subsequent changes in fair value generally reflected in earnings, (d) the expensing of all transaction costs as incurred and most restructuring costs, (e) recognition of pre-acquisition loss and gain contingencies at their acquisition date fair values, with certain exceptions, (f) capitalization of acquired in-process research and development rather than expense recognition, (g) earn-out arrangements may be required to be re-measured at fair value and (h) recognize changes that result from a business combination transaction in an acquirer’s existing income tax valuation allowances and tax uncertainty accruals as adjustments to income tax expense. Should we decide to enter future business combinations, these new standards will significantly affect the Company’s accounting for future business combinations following adoption on January 1, 2009.
In March 2008, the FASB issued SFAS No. 161, Disclosures about Derivative Instruments and Hedging Activities (“SFAS 161”). This statement requires companies to provide enhanced disclosures about (a) how and why they use derivative instruments, (b) how derivative instruments and related hedged items are accounted for under Statement 133 and its related interpretations, and (c) how derivative instruments and related hedged items affect a company’s financial position, financial performance, and cash flows. SFAS 161 is effective for financial statements issued for fiscal years and interim periods beginning after November 15, 2008. The Company will adopt the new disclosure requirements on or before the required effective date and thus will provide additional disclosures in its consolidated financial statements when adopted.
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2008
In April 2008, FASB Staff Position No. 142-3, Determination of the Useful Life of Intangible Assets (FSP 142-3)(“FSP 142-3”) was issued. This standard amends the factors that should be considered in developing renewal or extension assumptions used to determine the useful life of a recognized intangible asset under FASB Statement No. 142, Goodwill and Other Intangible Assets. FSP 142-3 is effective for financial statements issued for fiscal years beginning after December 15, 2008, and interim periods within those fiscal years. Early adoption is prohibited. The Company has not determined the impact on its financial statements of this accounting standard.
In May 2008, the Financial Accounting Standards Board (FASB”(“FASB”) issued FASB Staff Position (FSP”(“FSP”) APB 14-1, Accounting for Convertible Debt Instruments That May Be Settled in Cash upon Conversion (Including Partial Cash Settlement) . FSP APB 14-1 clarifies that convertible debt instruments that may be settled in cash upon either mandatory or optional conversion (including partial cash settlement) are not addressed by paragraph 12 of APB Opinion No. 14, Accounting for Convertible Debt and Debt issued with Stock Purchase Warrants . Additionally, FSP APB 14-1 specifies that issuers of such instruments should separately account for the liability and equity components in a manner that will reflect the entity’s nonconvertible debt borrowing rate when interest cost is recognized in subsequent periods. FSP APB 14-1 is effective for financial statements issued for fiscal years beginning after December 15, 2008, and interim periods within those fiscal years. We will adopt FSP APB 14-1 beginning in the first quarter of fiscal 2010, and this standard must be applied on a retrospective basis. We are evaluating the impact the adoption of FSP APB 14-1 will have on our consolidated financial position and results of operations.
In May 2008, the FASB issued Statement of Financial Accounting Standards (SFAS”(“SFAS”) No. 162, The Hierarchy of Generally Accepted Accounting Principles . This standard is intended to improve financial reporting by identifying a consistent framework, or hierarchy, for selecting accounting principles to be used in preparing financial statements that are presented in conformity with generally accepted accounting principles in the United States for non-governmental entities. SFAS No. 162 is effective 60 days following approval by the U.S. Securities and Exchange Commission (SEC”(“SEC”) of the Public Company Accounting Oversight Board’s amendments to AU Section 411, The Meaning of Present Fairly in Conformity with Generally Accepted Accounting Principles . We do not expect SFAS No. 162 to have a material impact on the preparation of our consolidated financial statements.
On June 16, 2008, the FASB issued final Staff Position (FSP)(“FSP”) No. EITF 03-6-1, Determining Whether Instruments Granted in Share-Based Payment Transactions Are Participating Securities” to address the question of whether instruments granted in share-based payment transactions are participating securities prior to vesting. The FSP determines that unvested share-based payment awards that contain rights to dividend payments should be included in earnings per share calculations. The guidance will be effective for fiscal years beginning after December 15, 2008. We are currently evaluating the requirements of (FSP)FSP No. EITF 03-6-1.
NOTE 2 - ACCOUNTS RECEIVABLE
At JuneSeptember 30, 2008 and December 31, 2007, accounts receivable consisted of the following:
|
| September 30, 2008 |
| December 31, 2007 |
| ||
Accounts receivable |
| $ | 13,966,487 |
| $ | 20,978,910 |
|
Less: allowance for doubtful accounts |
|
| (84,189) |
|
| (548,083) |
|
|
| $ | 13,882,298 |
| $ | 20,430,827 |
|
June 30, 2008 | December 31, 2007 | ||||||
| Accounts receivable | $ | 21,355,478 | $ | 20,978,910 | |||
| Less: allowance for doubtful accounts | (568,847 | ) | (548,083 | ) | |||
| $ | 20,786,631 | $ | 20,430,827 | ||||
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2008
NOTE 3 - INVENTORIES
At JuneSeptember 30, 2008 and December 31, 2007, inventories consisted of the following:
|
| September 30, 2008 |
| December 31, 2007 |
| ||
Raw materials |
| $ | 2,163,247 |
| $ | 2,312,111 |
|
Work in process |
|
| 0 |
|
| 279,759 |
|
Packaging materials |
|
| 14,414 |
|
| 52,967 |
|
Finished goods |
|
| 6,489,622 |
|
| 808,456 |
|
|
|
| 8,667,283 |
|
| 3,453,293 |
|
Less: reserve for obsolete inventory |
|
| (45,403) |
|
| (42,554) |
|
|
| $ | 8,621,880 |
| $ | 3 ,410,739 |
|
June 30, 2008 | December 31, 2007 | ||||||
| Raw materials | $ | 2,231,592 | $ | 2,312,111 | |||
| Work in process | 310,768 | 279,759 | |||||
| Packaging materials | 24,721 | 52,967 | |||||
| Finished goods | 4,565,107 | 808,456 | |||||
| 7,132,188 | 3,453,293 | ||||||
| Less: reserve for obsolete inventory | (45,293 | ) | (42,554 | ) | |||
| $ | 7,086,895 | $ | 3 ,410,739 | ||||
NOTE 4 - PROPERTY AND EQUIPMENT
At JuneSeptember 30, 2008 and December 31, 2007, property and equipment consist of the following:
|
| Useful Life |
| September 30, 2008 |
| December 31, 2007 | |||
Office equipment and furniture |
|
| 5-8 Years |
| $ | 242,688 |
| $ | 154,488 |
Manufacturing equipment |
|
| 10 - 15 Years |
|
| 5,571,015 |
| 5,032,601 | |
Building and building improvements |
|
| 20 - 40 Years |
|
| 3,135,752 |
| 2,938,966 | |
Construction in progress |
|
|
|
|
| 1,181,602 |
| - | |
|
|
|
|
|
| 10,131,057 |
| 8,126,055 | |
Less: accumulated depreciation |
|
|
|
|
| (2,451,097) |
| (1,956,089) | |
|
|
|
|
| $ | 7,679,960 |
| $ 6,169,966 | |
| Useful Life | June 30, 2008 | December 31, 2007 | |||||||||||
| Office equipment and furniture | 5-8 Years | $ | 170,500 | $ | 154,488 | ||||||||
| Manufacturing equipment | 10 - 15 Years | 5,574,805 | 5,032,601 | ||||||||||
| Building and building improvements | 20 - 40 Years | 3,128,131 | 2,938,966 | ||||||||||
| 8,873,436 | 8,126,055 | ||||||||||||
| Less: accumulated depreciation | (2,322,298 | ) | (1,956,089) | ||||||||||
| $ | 6,551,138 | $ 6,169,966 | |||||||||||
For the sixnine months ended JuneSeptember 30, 2008, construction in progress amounted to $1,181,602, representing construction for a new manufacturing plant located in Cha Ha Er Industrial Garden District in Inner Mongolia, China. The amount included costs for road, electrical, sewage, heating and water pipes constructions.
For the nine months ended September 30, 2008 and 2007, depreciation expense amounted to $233,483$356,556 and $209,512,$315,000 of which $226,593$346,151 and $188,209$301,850 is included in cost of sales, respectively.
NOTE 5 - Deposit on patentDEPOSITED ON PATENT
The Company has made a deposit to acquire a Chinese Class I drug patent in accordance with a technoloytechnology transfer agreement the Company’ entered into in April 2008. The Company will need to make additional installment payments to obtain the patent. Accordingly, the Company recorded $2,910,446$2,917,536 as deposit on patent as of JuneSeptember 30, 2008.
NOTE 6 - Deposit on land use rightDEPOSIT ON LAND USE RIGHT
The Company has recorded as a deposit on land use rights for the amounts paid to a local government agency in Cha You in Inner Mongolia to acquire a long-term interest to utilize certain land to construct a new manufacture facility.plant. The Company will need to make additional payments to obtain the full land use right and construct the new facility. Accordingly, theThe Company has not received the full land use right title and has reflected amounts paid of $6,033,353approximately $17.1 million as of JuneSeptember 30, 2008 as a deposit on land use rights.
The deposit is non-refundable in the event the Company decides not to construct the manufacture facility.
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2008
NOTE 7 - INTANGIBLE ASSETSASSET
The Company’s intangible assets includedprimarily consisted of two approved drugs’ manufacturing rights.rights and a revenue right. The manufacturing rights issued are in connection with the Company’s products Valsartan and Brimonidine. The manufacturing rights for Valsartan became effective in November 2000 and had a life of 6.5 years which expired in 2007.and fully amortized. The manufacturing rights for Brimonidine became effective on August 27, 2005 and have a life of 5 years.
On October 9, 2006, the Company entered into a five-year loan agreement and a contract with Wu Lan Cha Bu Emergency Hospital (“Wu Lan”), whereby the Company agreed to lend to Wu Lan approximately $3,840,000$4,376,000 for the construction of a hospital ward in Inner Mongolia, China. In exchange for agreeing to lend to Wu Lan the funds, Wu Lan agreed that the Company will be the exclusive provider for all medicines and disposable medical treatment apparatus to Wu Lan for a period of twenty (20) years. In October 2006, the Company’s chief executive officer, Mr. Liu, lent these funds to Wu Lan on behalf of the Company. The Company entered into an assignment agreement whereby the Company assigned all of its rights, obligations, and receipts under the Loan Agreement to Mr. Liu, except for the rights to revenues from the sale of medical and disposable medical treatment apparatus which will remain with the Company. As compensation to Mr. Liu for accepting the assignment under the loan agreement including all of the risks and obligations and for Liu not accepting the rights to revenues from the sale of medical and disposable medical treatment apparatus which will remain with the Company, the Company agreed to pay Mr. Liu an aggregate of approximately $1,150,000$1,313,000 in 5 equal annual installments of approximately $230,000.$263,000. Accordingly, the Company recorded an intangible asset of $1,151,263approximately $1,313,000 related to exclusive rights to provide all medicines and disposable medical treatment apparatus to Wu Lan for a period of twenty (20) years. The Company will amortize this exclusive right over a term of 20 years.
At JuneSeptember 30, 2008 and December 31, 2007, intangible assets consist of the following:
|
| September 30, 2008 |
| December 31, 2007 |
| ||
Manufacturing rights |
| $ | 1,264,168 |
| $ | 1,187,569 |
|
Revenue right |
|
| 1,312,891 |
|
| 1,230,500 |
|
Other |
|
| 6,419 |
|
| 0 |
|
|
|
| 2,583,478 |
|
| 2,418,069 |
|
Less: accumulated amortization |
|
| (1,317,458) |
|
| (1,126,747) |
|
|
| $ | 1,266,020 |
| $ | 1,291,322 |
|
June 30, 2008 | December 31, 2007 | ||||||
| Manufacturing rights | $ | 1,263,969 | $ | 1,187,569 | |||
| Revenue rights | 1,309,738 | 1,230,500 | |||||
| 2,573,707 | 2,418,069 | ||||||
| Less: accumulated amortization | (1,275,669 | ) | (1,126,747 | ) | |||
| $ | 1,298,038 | $ | 1,291,322 | ||||
Amortization expense amounted to approximately $74,230$112,900 and $65,685$107,055 for sixthe nine months ended JuneSeptember 30, 2008 and 2007, respectively.
The projected amortization attributed for the next five calendar years and thereafter is:future periods is as follows:
Period ending September 30:
2009 |
| $ | 148,117 |
|
2010 |
|
| 66,812 |
|
2011 |
|
| 66,423 |
|
2012 |
|
| 65,644 |
|
thereafter |
|
| 919,024 |
|
Total |
|
| 1,266,020 |
|
| 2008 | $ | 76,399 | ||
| 2009 | 124,765 | |||
| 2010 | 65,485 | |||
| 2011 | 65,486 | |||
| 2012 and thereafter | 965,904 | |||
| Total | 1,298,038 |
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2008
NOTE 8 - RELATED PARTY TRANSACTIONS
Notes payable - related parties
June 30, 2008 | December 31, 2007 | ||||||
| Note to Song Guoan, father of Song Zheng Hong, director and spouse of Liu Zhong Yi, due on December 30, 2015 with variable annual interest at 80% of current bank rate (6.26% at June 30, 2008 and December 31, 2007), and unsecured | $ | 759,943 | $ | 1,895,424 | |||
| Note to Zheng Gui Xin, employee, due on December 30, 2015 with with variable annual interest at 80% of current bank rate (6.26% at June 30, 2008 and December 31, 2007), and unsecured | 1,645,129 | 1,545,645 | |||||
| Note to Ma Zhao Zhao, employee, due on December 30, 2015 with variable annual interest at 80% of current bank rate (6.26% at June 30, 2008 and December 31, 2007), and unsecured | 658,870 | 619,027 | |||||
| Note to Liu Zhong Yi, officer and director, due on December 30, 2015 with variable annual interest at 80% of current bank rate (6.26% at June 30, 2008 and December 31, 2007), and unsecured | 1,402,492 | 136,244 | |||||
| Note to Song Zheng Hong, director and spouse of the Company chief executive officer, Liu Zhong Yi, due on December 30, 2015 with variable annual interest at 80% of current bank rate (6.26% at June 30, 2008 and December 31, 2007), and unsecured | 577,066 | 542,168 | |||||
| Total notes payable - related parties, long term | $ | 5,043,500 | $ | 4,738,508 | |||
Several employees of the Company, from time to time, provided advances to the Company for operating expenses. During the sixnine months ended JuneSeptember 30, 2008 and 2007, the Company repaid approximately $0 and $118,800$119,000 of these advances, respectively. At JuneSeptember 30, 2008 and December 31, 2007, the Company had a payable to these employees amounting to $440,204$489,770 and $323,178, respectively. These advances are short-term in nature and non-interest bearing.
The chief executive officer of the Company and his spouse, from time to time, provided advances to the Company for operating expenses. During the sixnine months ended JuneSeptember 30, 2008 and 2007, the Company repaid approximately$0approximately $0 and $489,500$744,480 of these advances, respectively. At JuneSeptember 30, 2008 and December 31, 2007, the Company had a payable to the chief executive officer and his spouse amounting to $831,949$865,307 and $0, respectively. These advances are short-term in nature and non-interest bearing.
On October 9, 2006, the Company entered into a five-year loan agreement and a contract with Wu Lan Cha Bu Emergency Hospital (“Wu Lan”), whereby the Company agreed to lend to Wu Lan approximately $3,840,000$4,376,000 for the construction of a hospital ward in Inner Mongolia, China. In exchange for agreeing to lend to Wu Lan the funds, Wu Lan agreed that the Company will be the exclusive provider for all medicines and disposable medical treatment apparatus to Wu Lan for a period of twenty (20) years. In October 2006, the Company’s chief executive officer, Mr. Liu, lent these funds to Wu Lan on behalf of the Company. Accordingly, the Company entered into an assignment agreement whereby the Company assigned all of its rights, obligations, and receipts under the Loan Agreement to Mr. Liu, except for the rights to revenues from the sale of medical and disposable medical treatment apparatus which will remain with the Company. As compensation to Mr. Liu for accepting the assignment under the loan agreement including all of the risks and obligations and for Mr. Liu not accepting the rights to revenues from the sale of medical and disposable medical treatment apparatus which will remain with the Company, the Company agreed to pay Mr. Liu an aggregate of approximately $1,137,000$1,313,000 to be paid in 5 equal annual installments of approximately $230,000.$263,000. Accordingly, the Company recorded an intangible asset of approximately $1,137,000$1,313,000 related to exclusive rights to provide all medicines and disposable medical treatment apparatus to Wu Lan for a period of twenty (20) years and a corresponding related party liability. For the sixnine months ended JuneSeptember 30, 2008 and for the year ended December 31, 2007, the Company paid approximately $0 and $195,000 of this related party liability, respectively. At JuneSeptember 30, 2008 and December 31, 2007, amounts due under this assignment agreement amounted to $1,047,760$787,735 and $966,198 of which $654,850$656,446 and $ 738,300 is included in long-term liabilities and has been included in due to related parties on the accompanying balance sheet,sheets, respectively.
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2008
Notes payable - related parties
Notes payable - related parties consisted of the following at September 30, 2008 and December 31, 2007:
|
| September 30, 2008 |
| December 31, 2007 |
| ||
Note to Song Guoan, father of Song Zheng Hong, director and spouse of Liu Zhong Yi, due on December 30, 2015 with variable annual interest at 80% of current bank rate (6.26% at September 30, 2008 and December 31, 2007), and unsecured |
| $ | 761,794 |
| $ | 1,895,424 |
|
|
|
|
|
|
|
|
|
Note to Zheng Gui Xin, employee, due on December 30, 2015 with variable annual interest at 80% of current bank rate (6.26% at September 30, 2008 and December 31, 2007), and unsecured |
|
| 1,649,137 |
|
| 1,545,645 |
|
|
|
|
|
|
|
|
|
Note to Ma Zhao Zhao, employee, due on December 30, 2015 with variable annual interest at 80% of current bank rate (6.26% at September 30, 2008 and December 31, 2007), and unsecured |
|
| 660,475 |
|
| 619,027 |
|
|
|
|
|
|
|
|
|
Note to Liu Zhong Yi, officer and director, due on December 30, 2015 with variable annual interest at 80% of current bank rate (6.26% at September 30, 2008 and December 31, 2007), and unsecured |
|
| 1,405,909 |
|
| 136,244 |
|
|
|
|
|
|
|
|
|
Note to Song Zheng Hong, director and spouse of the Company chief executive officer, Liu Zhong Yi, due on December 30, 2015 with variable annual interest at 80% of current bank rate (6.26% at September 30, 2008 and December 31, 2007), and unsecured |
|
| 578,472 |
|
| 542,168 |
|
|
|
|
|
|
|
|
|
Total notes payable - related parties, long term |
| $ | 5,055,787 |
| $ | 4,738,508 |
|
For the nine months ended September 30, 2008 and 2007, the Company has recorded accrued interest relatingexpense related to notes payable -these related parties of $158,395loans amounted to $233,704 and $61,208, respectively which has been included in due to related parties on the accompanying balance sheets.
NOTE 9 - CONVERTIBLE DEBT
The convertible debenture liability is as follows at December 31, 2007:
Convertible debentures payable | $ | 2,770,000 | ||
Less: unamortized discount on debentures | (208,355) | |||
Convertible debentures, net | $ | 2,561,645 |
| $ | 2,770,000 | |||
| (208,355 | ) | |||
| Convertible debentures, net | $ | 2,561,645 |
In January 2008, the Company issued 250,000 shares of its common stock in connection with the conversion of $250,000 of convertible debt. In February 2008, in connection with a private placement (See Note 10), the remaining $2,520,000 of convertible debt and any unpaid interest was repaid in full. The Company amortized the remaining $208,355 in discount on debentures in the sixnine months ended JuneSeptember 30, 2008. As of JuneSeptember 30, 2008, the balance of the convertible debt is $0.
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2008
NOTE 10 - CONVERTIBLE REDEEMABLE PREFERRED STOCKS
On February 25, 2008, the Company entered into a Convertible Redeemable Preferred Share and Warrant Purchase Agreement (the "Purchase Agreement"“Purchase Agreement”) by and among the Company, Dr. Liu Zhong Yi and Mrs. Song Zhenghong (the "Founders"), and accredited investors (each a "Purchaser" and collectively, the "Purchasers"), pursuant to which the Company sold to the Purchasers 5,747,118 shares of the Company's series A convertible redeemable preferred stock and warrants to purchase 2,873,553 shares of the Company's common stock,“Founders”proxi matel y $46 9,000 in a private placement (the “February 2008 Private Placement”) pursuant to Regulation D under the Securities Act of 1933, for the aggregate purchase price of $5 million (the "Transaction"). The Transaction closed on February 25, 2008 (the "Closing Date"). Net proceeds, exclusive of expenses of the Transaction, from the sale to the Company were $4.6 million, after the Company paid fees of approximately $469,000c ash of which $400,000 was paid to Maxim Group, LLC, itsthe placement agent for the Transaction. The Company recorded the approximately $469,000$796,000 in fees, of which $327,565 was related to value of the warrants granted to placement agent, as a deferred debt cost and amortized approximately $99,500$232,205 of the deferred cost during the sixnine months ended JuneSeptember 30, 2008. The Company has presented the convertible redeembable preferred stocks as long term debt due to the mandatory redeemable feature of the preferred stocks.
The Company used $2,576,556 of the net proceeds of the Transaction to repay in full all of its outstanding principal obligations including accrued interest under the 14% Secured Convertible Notes due February 2008 and the Company will use the remainder of the net proceeds for working capital and general corporate purposes.
The Purchase Agreement includes customary representations and warranties by each party thereto. The following is a brief description of the terms and conditions of the Purchase Agreement and the transactions contemplated there under that are material to the Company:
Pursuant to the Purchase Agreement, the Company issued to the Purchasers an aggregate of 5,747,118 shares of the Company'sCompany’s series A convertible redeemable preferred stock, par value $0.001 per share, at a price equal to $0.87 per share (the "Preferred Shares"“Preferred Shares”). Each of these Preferred Shares may be converted into one share of the Company'sCompany’s common stock, par value $0.001 per share (as adjusted for stock splits, stock dividends, reclassification and the like). The Preferred Shares pay an 8% dividend annually, which is payable in additional Preferred Shares. Preferred Shares also pay any dividend paid on the common shares on an as converted basis. For a period of 90 days after February 25, 2010, these Preferred Shares may also be redeemed at the option of the Purchasers at the redemption price of $0.87 per share (as adjusted for stock splits, stock dividends, reclassification and the like), and no other capital stock of the Company may be redeemable prior to the Preferred Shares. Holders of Preferred Shares may not convert Preferred Shares to common shares if the conversion would result in the holder beneficially owning more than 4.99% of the Company'sCompany’s outstanding common shares. That limitation may be waived by a holder of Preferred Shares on not less than 61 days written notice to the Company. In addition, the Company issued to the Purchasers warrants to purchase up to 2,873,553 shares of the Company'sCompany’s common stock in the aggregate. The warrants have an initial exercise price of $1.20 (subject to adjustment pursuant to the terms of the warrants). The warrants are exercisable for a period of five (5) years from the Closing Date. Holders of the warrants may not exercise the warrants if the exercise would result in the holder beneficially owning more than 4.99% of the Company'sCompany’s outstanding common shares. That limitation may be waived by a holder of the warrants on not less than 61 days written notice to the Company.
Other key provisions of the February 2008 Private Placement:
The Preferred Shares vote with the common stock on an as converted basis, except that the Preferred Shares are not entitled to vote for directors. The Company is prohibited from taking certain corporate actions, including, without limitation, issuing securities senior to the Preferred Shares, selling substantially all assets, repurchasing securities and declaring or paying dividends, without the approval of the holders of a majority of the Preferred Shares outstanding.
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2008
The Company agreed to undertake to file a registration statement within 60 days following the Closing Date in order to register the maximum number of common stock issuable from conversion of the Preferred Shares and underlying the warrants that is allowable under applicable federal securities regulations. The Company is also obligated to have the registration statement declared effective within 120 days following the Closing Date. If the Company is informed by the SEC that there are no comments to the registration statement, then the registration statement must be effective within five (5) business days thereafter or on the 60th day after the filing date, whichever is sooner. The registration statement must be declared effective by the 120th day after its filing if the SEC has comments. Otherwise, the Company is subject to liquidated damages, equal to 1% of the total conversion price and exercise price for the common stock being registered under the registration statement, for every 30-day period following the date that the registration statement should have been effective, prorated for any period less than 30 days, until either all of common shares registered under the registration statement have been sold or all such common shares may be sold in any three (3) month period pursuant to Rule 144 promulgated under the Securities Act, whichever is earlier. The Company must also pay the liquidated damages if sales cannot be made pursuant to the registration statement for any reason (excepting market conditions). The maximum amount of liquidated damages is $500,000.
The Founders delivered |
In connection with the issuance of the series A convertible redeemable preferred stock and warrants to purchase 2,873,553 shares of the Company'sCompany’s common stock, the Company recorded a total debt discount of $2,033,025 to be amortized over the term of this Purchase Agreement. For the sixnine months ended JuneSeptember 30, 2008, amortization of debt discount amounted to $338,838$592,966 and has been included in interest expense.
For the sixnine months ended JuneSeptember 30, 2008, the Company evaluated whether or not the series A convertible redeemable preferred stock contain embedded conversion options, which meet the definition of derivatives under SFAS 133 “Accounting for Derivative Instruments and Hedging Activities” and related interpretations. The Company concluded that since the series A convertible redeemable preferred stock had a fixed redemption price of $0.87, the convertible redeemable preferred stock was not a derivative instrument. The Company analyzed this provision under EITF 05-04 and therefore it qualified as equity under EITF 00-19.
The series A convertible redeemable preferred stock is as follows at JuneSeptember 30, 2008:
Series A convertible redeemable preferred stock | $ | 5,000,000 | ||
Less: unamortized discount | (1,440,059) | |||
Series A convertible redeemable preferred stock, net | $ | 3,559,941 |
| Series A convertible redeemable preferred stock | $ | 5,000,000 | ||
| Less: unamortized discount | (1,694,187 | ) | ||
| Series A convertible redeemable preferred stock, net | $ | 3,305,813 |
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2008
NOTE 11 -SHAREHOLDERS’ EQUITY
In January 2008, the Company issued 250,000 shares of its common in connection with the conversion of $250,000 of convertible debt.
In April 2008, the Company issued in aggregate 331,668 shares of restricted common stock to the several directors, consultants and officers of the Company for services rendered. The Company valued these common shares at the fair market value on the date of grant at $0.87 per share or $288,551 in total. In connection with issuance of these shares, the Company recorded prepaid stock-based expenses of $288,551. As of JuneSeptember 30, 2008, the Company recorded amortization on prepaid stock-based expenses of approximately $166,000$243,550 related to those issuances.
In April 2008, in connection with a cashless exercise of 60,000 warrants, the Company issued 9,077 shares of common stock accordingly.
In June 2008, the Company issued 35,294 shares of restricted common stock to a consultant for services rendered to the Company. The Company valued these common shares at the fair market value on the date of the grant at $0.85 per share or $30,000 in total. The Company recorded stock-based compensation expenses of $30,000 for the sixnine months ended JuneSeptember 30, 2008 accordingly.
Stock warrants
In January, 2008, the Company granted 250,000 stock warrants to a consulting company at an exercise price of $1.50 per share in connection with a one year contract. The warrants are not vested until January 2009. The Company valued these warrants utilizing the Black-Scholes options pricing model at approximately $0.71 per warrant or $178,187 in total and recorded a prepaid consulting expense of $178,187 for the period ended JuneSeptember 30, 2008. Those warrants will expire in January 2012. For the sixnine months ended JuneSeptember 30, 2008, the Company recorded amortization expenses of $74,245$118,791 related to the consulting contract.
In connection with the preferred share financing (See Note 10), the Company granted 2,873,553 warrants to investors purchase up to 2,873,553 shares of common stock of the Company at an exercise price of $1.20 per share. The Warrants have a term of 5 years after the issue date of February 12, 2007. Additionally, the Company granted 603,446 stock warrants to an investment banking firm at an exercise price of $1.21 per share. The Company valued these warrants utilizing the Black-Scholes options pricing model at approximately $0.54 per warrant or $327,565 in total and recorded a deferred financing costs of $327,565. The Company amortized approximately $55,000 of this deferred cost during the six months ended June 30, 2008. Those warrants will expire in February 2013.
On February 25, 2008, pursuant to a Convertible Redeemable Preferred Share and Warrant Purchase Agreement (See Note 10), the exercise price of the 1,500,000 warrants granted in February 2007 were reduced and repriced to $0.87 per share. As a result of the repricing, the Company recorded an interest expense of $74,593 for the sixnine months ended JuneSeptember 30, 2008.
Stock warrants issued, terminated/forfeited and outstanding during the sixnine months ended JuneSeptember 30, 2008 (unaudited) are as follows:
|
| Shares |
| Average Exercise price per share |
| ||
Warrants outstanding December 31, 2007 |
|
| 1,500,000 |
|
| 0.87 |
|
Warrants issued |
|
| 3,726,999 |
|
| 1.22 |
|
Warrants terminated/forfeited |
|
| - |
|
| - |
|
Warrants exercised |
|
| (60,000) |
|
| 0.87 |
|
Warrants outstanding, September 30, 2008 |
|
| 5,166,999 |
|
| 1.13 |
|
Shares | Average Exercise price per share | ||||||
| Warrants outstanding December 31, 2007 | 1,500,000 | 0.87 | |||||
| Warrants issued | 3,726,999 | 1.22 | |||||
| Warrants terminated/forfeited | - | - | |||||
| Warrants exercised | (60,000 | ) | 0.87 | ||||
| Warrants outstanding, June 30, 2008 | 5,166,999 | 1.13 | |||||
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2008
NOTE 12 - SEGMENT INFORMATION
The following information is presented in accordance with SFAS No. 131, Disclosure about Segments of an Enterprise and Related Information. For the sixnine months ended JuneSeptember 30, 2008 and 2007, the Company operated in two reportable business segments - (1) the manufacture and distribution of pharmaceutical products and (2) the retailing of traditional and Chinese medicines and supplies through ten drug stores located in Beijing China and other ancillary revenues generated from retail location such as advertising income, rental income, and examination income. The Company'sCompany’s reportable segments are strategic business units that offer different products. They are managed separately based on the fundamental differences in their operations.
Information with respect to these reportable business segments for the three months ended JuneSeptember 30, 2008 and 2007 is as follows:
2008 |
| Wholesale and third-party manufacturing |
| Retail Operations |
| Unallocated |
| Total |
| ||||
Net Revenues |
| $ | 15,193,602 |
| $ | 1,420,670 |
| $ | 89,612 |
| $ | 16,703,884 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of Sales (excluding depreciation) |
|
| 7,428,195 |
|
| 667,774 |
|
| 3,831 |
|
| 8,099,800 |
|
Operating expenses (excluding depreciation and amortization) |
|
| - |
|
|
|
|
| 4,684,683 |
|
| 4,684,683 |
|
Depreciation and Amortization |
|
| 112,760 |
|
| 6,798 |
|
| 42,185 |
|
| 161,743 |
|
Other Expense |
|
| - |
|
|
|
|
| 90,484 |
|
| 90,484 |
|
Interest Expense |
|
| - |
|
|
|
|
| 453,498 |
|
| 453,498 |
|
Net Income (Loss) |
| $ | 7,652,647 |
| $ | 746,098 |
| $ | (5,185,069) |
| $ | 3,213,676 |
|
2007 |
| Wholesale and third-party manufacturing |
| Retail Operations |
| Unallocated |
| Total |
| ||||
Net Revenues |
| $ | 13,639,037 |
| $ | 2,688,918 |
| $ | 226,394 |
| $ | 16,554,349 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of Sales (excluding depreciation) |
|
| 8,982,872 |
|
| 663,984 |
|
| 8,239 |
|
| 9,655,095 |
|
Operating expenses (excluding depreciation and amortization) |
|
| - |
|
| - |
|
| 3,664,769 |
|
| 3,664,769 |
|
Depreciation and Amortization |
|
| 103,527 |
|
| 5,447 |
|
| 37,884 |
|
| 146,858 |
|
Other Expense |
|
| - |
|
| - |
|
| 58,679 |
|
| 58,679 |
|
Interest Expense |
|
| - |
|
| - |
|
| 807,608 |
|
| 807,608 |
|
Net Income (Loss) |
| $ | 4,552,638 |
| $ | 2,019,487 |
| $ | (4,350,785) |
| $ | 2,221,340 |
|
2008 | Wholesale and third-party manufacturing | Retail Operations | Unallocated | Total | |||||||||
Net Revenues | $ | 18,382,332 | $ | 903,404 | $ | 99,876 | $ | 19,385,612 | |||||
Cost of Sales (excluding depreciation) | 9,003,234 | 551,612 | 6,337 | 9,561,183 | |||||||||
Operating expenses (excluding depreciation and amortization) | - | - | 6,866,102 | 6,866,102 | |||||||||
Depreciation and Amortization | 126,492 | 9,043 | 23,294 | 158,829 | |||||||||
Other Expense | - | - | 96,929 | 96,929 | |||||||||
Interest Expense | - | - | 522,660 | 522,660 | |||||||||
Net Income | $ | 9,252,606 | $ | 342,749 | $ | (7,415,446 | ) | $ | 2,179,909 | ||||
2007 | Wholesale and third-party manufacturing | Retail Operations | Unallocated | Total | |||||||||
Net Revenues | $ | 11,845,544 | $ | 701,421 | $ | 258,487 | $ | 12,805,452 | |||||
Cost of Sales (excluding depreciation) | 6,702,701 | 498,391 | 7,173 | 7,208,265 | |||||||||
Operating expenses (excluding depreciation and amortization) | - | - | 1,841,813 | 1,841,813 | |||||||||
Depreciation and Amortization | 98,044 | 5,162 | 47,357 | 150,563 | |||||||||
Other Expense | - | - | 114,680 | 114,680 | |||||||||
Interest Expense | - | - | 448,606 | 448,606 | |||||||||
Net Income | $ | 5,044,799 | $ | 197,868 | ($ 2,201,142 | ) | $ | 3,041,525 | |||||
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2008
Information with respect to these reportable business segments for the sixnine months ended JuneSeptember 30, 2008 and 2007 is as follows:
2008 |
| Wholesale and third-party manufacturing |
| Retail Operations |
| Unallocated |
| Total | ||||
Net Revenues |
| $ | 44,254,921 |
| $ | 3,187,557 |
| $ | 356,195 |
| $ | 47,798,673 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of Sales (excluding depreciation) |
|
| 23,621,393 |
|
| 1,701,382 |
|
| 15,575 |
|
| 25,338,350 |
Operating expenses (excluding depreciation and amortization) |
|
| - |
|
| - |
|
| 13,951,377 |
|
| 13,951,377 |
Depreciation and Amortization |
|
| 325,757 |
|
| 20,394 |
|
| 123,305 |
|
| 469,456 |
Other Expense |
|
|
|
|
|
|
|
| 250,949 |
|
| 250,949 |
Interest Expense |
|
|
|
|
|
|
|
| 1,399,507 |
|
| 1,399,507 |
Net Income (Loss) |
| $ | 20,307,771 |
| $ | 1,465,781 |
| $ | (15,384,518) |
| $ | 6,389,034 |
2007 |
| Wholesale and third-party manufacturing |
| Retail Operations |
| Unallocated |
| Total |
| ||||
Net Revenues |
| $ | 32,769,631 |
| $ | 4,316,890 |
| $ | 562,815 |
| $ | 37,649,336 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
Cost of Sales (excluding depreciation) |
|
| 20,826,503 |
|
| 1,592,348 |
|
| 22,441 |
|
| 22,441,292 | |
Operating expenses (excluding depreciation and amortization) |
|
| - |
|
| - |
|
| 6,920,343 |
|
| 6,920,343 | |
Depreciation and Amortization |
|
| 282,306 |
|
| 14,858 |
|
| 124,891 |
|
| 422,055 | |
Other Expense |
|
| - |
|
| - |
|
| 256,699 |
|
| 256,699 | |
Interest Expense |
|
| - |
|
| - |
|
| 1,508,781 |
|
| 1,508,781 | |
Net Income (Loss) |
| $ | 11,660,822 |
| $ | 2,709,684 |
| $ | (8,270,340) |
| $ | 6,100,166 | |
2008 | Wholesale and third-party manufacturing | Retail Operations | Unallocated | Total | |||||||||
Net Revenues | $ | 29,061,319 | $ | 1,766,887 | $ | 266,583 | $ | 31,094,789 | |||||
Cost of Sales (excluding depreciation) | 16,193,198 | 1,033,608 | 11,744 | 17,238,550 | |||||||||
Operating expenses (excluding depreciation and amortization) | - | - | 9,266,694 | 9,266,694 | |||||||||
Depreciation and Amortization | 212,997 | 13,596 | 81,120 | 307,713 | |||||||||
Other Expense | - | - | 159,815 | 159,815 | |||||||||
Interest Expense | - | - | 946,009 | 946,009 | |||||||||
Net Income | $ | 12,655,124 | $ | 719,683 | $ | (10,198,799 | ) | $ | 3,176008 | ||||
2007 | Wholesale and third-party manufacturing | Retail Operations | Unallocated | Total | |||||||||
Net Revenues | $ | 19,130,594 | $ | 1,627,972 | $ | 336,421 | $ | 21,094,987 | |||||
Cost of Sales (excluding depreciation) | 11,843,631 | 928,364 | 14,202 | 12,786,197 | |||||||||
Operating expenses (excluding depreciation and amortization) | - | - | 3,255,574 | 3,255,574 | |||||||||
Depreciation and Amortization | 178,779 | 9,411 | 87,007 | 275,197 | |||||||||
Other Expense | - | - | 198,020 | 198,020 | |||||||||
Interest Expense | - | - | 701,173 | 701,173 | |||||||||
Net Income | $ | 7,108,184 | $ | 690,197 | ($ 3,919,555 | ) | $ | 3,878,826 | |||||
The Company does not allocate all of its operating expenses to its reportable segments, because these activities are managed at a corporate level.
Asset information by reportable segment is not reported to or reviewed by the chief operating decision maker and, therefore, the Company has not disclosed asset information for each reportable segment. Substantially all of the Company’s assets are located in China.
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2008
NOTE 13 - RESTATEMENT
For the six months ending June 30, 2007, the Company revised certain accounting treatment related to the recording of an intangible asset and a corresponding related party liability associated with an assignment agreement and exclusive revenue rights as described in Note 7. The Company initially did not record the value of the intangible asset and corresponding liability and treated payment due under the assignment agreement as a periodic payment of interest expense to the Company’s chief executive officer. Subsequently, the Company determined that an intangible asset and a corresponding related party liability should have been recorded. Accordingly, the adjustments to the financial statements for this adjustment were as follows:
1. Balance Sheet:
a) Intangible assets increased by $1,136,096 to $1,309,551 from $173,455 and total assets increased by $1,136,096. This increase reflects the addition of an intangible asset of approximately $1,165,000 offset by an increase in accumulated amortization of approximately $29,000.
b) Due to related parties increased by $1,003,304 which increased total current liabilities by $236,071 and long-term liabilities by $767,233. This increase reflects the addition of a related party payable of approximately $1,119,000 offset by the repayment of this payable of approximately $116,000. Stockholders’ equity increased $87,198 which reflect the changes made to the balance sheet and statement of operations as well as an increase in comprehensive income of $1,486.
2. Statement of Operations:
a) For the six months ended June 30, 2007, general and administrative expenses decreased by $87,323 which reflects an increase in depreciation and amortization expenses of $29,108 offset by a decrease in assignment fee expenses of $116,431 due to the reversal of the periodic assignment fee expense previously recorded.
3. Statement of Cash Flows:
a) For the six months ended June 30, 2007, net cash flows provided by operating activities increased by $116,431 and net cash provided by financing activities decreased by $116,431.
NOTE 14—14 - COMMITMENTS AND CONTINGENCIES
Technology Transfer Agreement
In April 2008, one of the Company’s affiliates, En Ze Jia, entered into a Technology Transfer Agreement with Dong Guan Kai Fa Biologicals Medicine LTD (“Dong Guan”) pursuant to which Dong Guan agreed to transfer the technology material, new medicine research and rights to the Chinese patent of the anti-asthma new medicine R-BM to En Ze Jia on an exclusive basis in exchange for a transfer technology fee of approximately $7 million (RMB 48 million) to be paid at various intervals. Under the terms of the agreement, En Ze Jia is obligated to:
• | complete the filing with the Chinese State Food and Drug Administration (SFDA) of the medicine’s clinical research ratification document, | |
• | complete the clinical research, | |
• | complete the medicine’s trial production, and | |
• | providing raw materials and formulation related documentation and apply for the new medicine certification and production approval. |
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2008
In addition to the payment of the technology transfer fee, En Ze Jia is responsible for paying all costs associated with its responsibility under the agreement which are presently estimated at $2.3 million (RMB 16 million). Lotus East intends to use its working capital to fund the project costs.
Dong Guan is responsible for preparing and transferring the clinical research and application documents as well as assisting En Ze Jia in the completing the clinical research and applying for the new medicine certification and production approval documents.
Under the terms of the agreement, the technology transfer fee is to be paid upon the following schedule:
• | Approximately $1.5 million is due by the 15th business day following the receipt of the processing notice of the receipt of the clinical application and all related material from SFDA is received, |
• | Approximately $1.2 million (RMB 8 million) is due by the 10th business day after the receipt of the medicine’s clinical ratification document, |
• | Approximately $1.5 million (RMB 10 million) is due by the 15th business day after the medicine’s Phase I clinical study is completed and ratification from the SFDA is obtained, and |
• | Approximately $2.9 million (RMB 20 million) is due by the 10th business day after the medicine’s Phase II clinical study is completed and ratification from the SFDA is obtained. |
En Ze Jia paid Dong Guan a deposit of approximately $2.9 million(RMBmillion (RMB 20 million) in April 2008 which is to be returned to En Ze Jia withwithin 10 days after the transfer technology fee is fully paid. In the event Dong Guan should be unable to timely return the deposit, it will pay En Ze Jia a late fee and En Ze Jia is entitled to damages for Dong Guan’s failure to timely return the deposit.
The intellectual property arising from the agreement will be jointly shared by the parties. In addition, En Ze Jia has guaranteed that both parties must jointly apply for related government grants prior to when the new medicine is marketed. Upon receipt of the government grants En Ze Jia guaranteed that the grant monies will be shared equally by both parties. As of September 30, 2008, the Company has not received any government grant. The agreement can be terminated by Dong Guan if En Ze Jia should fail to make any of the aforedescribed payments in which event the patent rights would revert to Dong Guan and it is entitled to transfer the project rights to a third party.
New Manufacturing Facility
In June 2008, one of the Company’s affiliates, Liang Fang, entered into an agreement with Cha You Qian Qi Economy Commission, a governmental agency (“Cha You”) related to the construction of a pharmaceutical plant in Cha You'sYou’s Cha Ha Er Industrial Garden District.District in Inner Mongolia. The new facility, which will be comprised approximately 40,000 square meters situated on 600 MU of land (approximately 400,200 square meters), will be used to expand Liang Fang'sFang’s current manufacturing capacity. The Company was subsequently granted the right to expand the land use right area to 1,000 MU (approximately 667,000 square meters). The new facility, which will manufacture medical injection products, including 0.9% physiological saline injection, hydroxyethyl starch 130/0.4 injection and hydroxyethyl starch 200/0.5 injection, Qiang Yi Ji starch, a medical corn starch commonly known as O-2-hydeoxyethyl starch, dextran and additional pharmaceuticals,ad ditio nal p harma ceuti cals, will require a total investment of RMB 500 million, or approximately $72.8$83.4 million. It anticipated thatThe construction will beginbegan on the project in SeptemberAugust 2008 and the Company anticipates that it will take between 12 to 30 months to complete the facility.
Included in the total cost of the project is land cost of approximately $15.7$26.3 million (RMB 108180 million) which is paid to Cha You. Other components of the project include construction costs of approximately $23.3 million (RMB 160 million) costs associated with the various production lines estimated at approximately $26.5 million (RMB 182 million) and working capital of approximately $7.3 million (RMB 50 million).
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2008
Liang Fang intends to use its present working capital together with bank loans and government grants to fund the project. The funds are required to be invested over the next 18 months under a specified schedule ending in December 2010. As of JuneSeptember 30, 2008, Liang Fang has paid approximately $6$17.3 million (approximately RMB 39118.9 million) of the total investment. Liang Fang, however, has not secured either the bank loans or government grants and does not have sufficient working capital to complete this project without securing substantial funds from those third party sources.
Under the terms of the agreement, Cha You agreed to abate fees associated with water resources, waste and other relative supplies for a period of 30 years and agreed to ensure that the land use tax to be paid by Liang Fang after it begins normal production will be at the lowest tax rate imposed for five years. Once the project is completed, for a period of eight years the local reserved portion of the imposed corporation income tax will be returned to Liang Fang. Liang Fang is required to commencehas already commenced construction by September 3, 2008. If for any reason Liang Fang should fail to begin construction within 90 days from the date of the agreement, Cha You has the right to restore the land use rights to it and Liang Fang will forfeit any funds invested to date. In addition, Liang Fang is prohibited from changing the land use designation.
NOTE 15 - OPERATING RISK
(a) Country risk
Currently, the Company’s revenues are primarily derived from the sale of pharmaceutical products to customers in the Peoples Republic of China (PRC). The Company hopes to expand its operations to countries outside the PRC, however, such expansion has not been commenced and there are no assurances that the Company will be able to achieve such an expansion successfully. Therefore, a downturn or stagnation in the economic environment of the PRC could have a material adverse effect on the Company’s financial condition.
(b) Products risk
In addition to competing with other manufacturers of pharmaceutical product offerings, the Company competes with larger Chinese and International companies who may have greater funds available for expansion, marketing, research and development and the ability to attract more qualified personnel. These Chinese companies may be able to offer products at a lower price. There can be no assurance that the Company will remain competitive.
(c) Political risk
Currently, PRC is in a period of growth and is openly promoting business development in order to bring more business into PRC. Additionally PRC allows a Chinese corporation to be owned by a United States corporation. If the laws or regulations are changed by the PRC government, the Company'sCompany’s ability to operate the PRC subsidiaries could be affected.
Overview
We operate, control and beneficially own the pharmaceutical businesses in China of Lotus East under the terms of the Contractual Arrangements. Other than the Contractual Arrangements with Lotus East, we do not have any business or operations. Pursuant to the Contractual Arrangements we provide business consulting and other general business operation services to Lotus East. Through these Contractual Arrangements, we have the ability to control the daily operations and financial affairs of Lotus East, appoint each of their senior executives and approve all matters requiring stockholder approval. As a result of these Contractual Arrangements, which enable us to control Lotus East, we are considered the primary beneficiary of Lotus East. Accordingly, we consolidate Lotus East'sEast’s results, assets and liabilities in our financial statements. The creditors of Lotus East, however, do not have recourse to any assets we may have.
PRC law currently places certain limitations on foreign ownership of Chinese companies. To comply with these foreign ownership restrictions, we operate our business in China through the Contractual Arrangements with Lotus East. The contractual relationship among the above companies as follows:

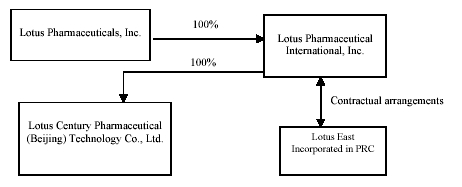
Based in Beijing, China, Lotus East is engaged in the production, trade and retailing of pharmaceuticals, focusing on the development of innovative medicines and investing in strategic growth to address various medical needs. Lotus East owns and operates 10 drug stores throughout Beijing, China that sell Western and traditional Chinese medications, lease medical treatment facilities to licensed physicians, and generate revenues from the leasing of retail space to third party vendors and the leasing of advertising locations at its retail stores.
When used in this section, and except as may be set forth otherwise, the terms "we," "us," "ours,"“we,” “us,” “ours,” and similar terms includes Lotus and its subsidiary Lotus International as well as Lotus East.
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
Our discussion and analysis of our financial condition and results of operations are based on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities and expenses. On an ongoing basis, we evaluate our estimates and judgments, including those related to accrued expenses, fair valuation of stock related to stock-based compensation and income taxes. We based our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
REVENUE RECOGNITION
Product sales are generally recognized when title to the product has transferred to customers in accordance with the terms of the sale. We recognizeThe Company recognizes revenue in accordance with the Securities and Exchange Commission'sCommission’s (SEC) Staff Accounting Bulletin (SAB) No. 101, " “Revenue Recognition in Financial Statements "” as amended by SAB No. 104 (together, "SAB 104"“SAB 104”), and Statement of Financial Accounting Standards (SFAS) No. 48 " “Revenue Recognition When Right of Return Exists. " ”SAB 104 states that revenue should not be recognized until it is realized or realizable and earned. In general, we recordthe Company records revenue when persuasive evidence of an arrangement exists, services have been rendered or product delivery has occurred, the sales price to the customer is fixed or determinable, and collectability is reasonably assured.
SFAS No. 48 states that revenue from sales transactions where the buyer has the right to return the product shall be recognized at the time of sale only if the seller'sseller’s price to the buyer is substantially fixed or determinable at the date of sale, the buyer has paid the seller, or the buyer is obligated to pay the seller and the obligation is not contingent on resale of the product, the buyer'sbuyer’s obligation to the seller would not be changed in the event of theft or physical destruction or damage of the product, the buyer acquiring the product for resale has economic substance apart from that provided by the seller, the seller does not have significant obligations for future performance to directly bring about resale of the product by the buyer, and the amount of future returns can be reasonably estimated.
The Company’s net product revenues represent total product revenues less allowances for the sale of pharmaceutical products and for payments received, if any, under reimbursement of development costs as follows:
PRODUCT RETURNS.
The Company accounts for sales returns in accordance with Statements of Financial Accounting Standards (SFAS) No. 48, Revenue Recognition When Right of Return Exists, by establishing an accrual in an amount equal to its estimate of sales recorded for which the related products are expected to be returned. We determineThe Company determines the estimate of the sales return accrual primarily based on historical experience regarding sales returns, but also by considering other factors that could impact sales returns. These factors include levels of inventory in the distribution channel, estimated shelf life, product discontinuances, and price changes of competitive products, introductions of generic products and introductions of competitive new products. In general, for wholesale sales, we providethe Company provides credit for product returns that are returned six months prior to and up to six months after the product expiration date. Upon sale, we estimatethe Company estimates an allowance for future product returns. We provideThe Company provides additional reserves for contemporaneous events that were not known and knowable at the time of shipment.
OTHER REVENUE
Other revenues consist of (i) rental income received for the lease of retail space to various retail merchants; (ii) advertising revenues from the lease of counter space at our retail locations; (iii) rental income from the lease of retail space to licensed medical practitioners; and (iv) revenues received by us for research and development projects. We recognize revenues upon performance of such funded research. We recognize revenues from leasing of space as earned from contracting third parties. Revenues received in advance are reflected as deferred revenue on the accompanying balance sheets. Additionally, we receive income from the sale of developed drug formulas. Income from the sale of drug formulas are recognized upon performance of all of our obligations under the respective sales contract and are included in other income on the accompanying consolidated statement of operations.
ACCOUNTS RECEIVABLE
Accounts receivable are carried at original invoice amount less an estimate made for doubtful receivables based on a review of all outstanding amounts on a monthly basis. Management judgment and estimates are made in connection with establishing the allowance for doubtful accounts. Specifically, we analyze the aging of accounts receivable balances, historical bad debts, customer concentrations, customer credit-worthiness, current economic trends and changes in our customer payment terms. Significant changes in customer concentration or payment terms, deterioration of customer credit-worthiness or weakening in economic trends could have a significant impact on the collectability of receivables and our operating results. If the financial condition of our customers were to deteriorate, resulting in an impairment of their ability to make payments, additional allowances may be required.
INVENTORIES
Inventories are stated at the lower of cost or market with cost determined under the weighted-average method. Inventory consists of finished capsules, liquids, finished oral suspension powder and other western and traditional Chinese medicines and medical equipment. At least on a quarterly basis, we review our inventory levels and write down inventory that has become obsolete or has a cost basis in excess of its expected net realizable value or is in excess of expected requirements. Inventory levels are evaluated by management relative to product demand, remaining shelf life, future marketing plans and other factors, and reserves for obsolete and slow-moving inventories are recorded for amounts which may not be realizable.
PROPERTY AND EQUIPMENT
Property and equipment are carried at cost. The cost of repairs and maintenance is expensed as incurred; major replacements and improvements are capitalized. When assets are retired or disposed of, the cost and accumulated depreciation are removed from the accounts, and any resulting gains or losses are included in income in the year of disposition. In accordance with Statement of Financial Accounting Standards (SFAS) No. 144, "Accounting“Accounting for the Impairment or Disposal of Long-Lived Assets"Assets”, we examine the possibility of decreases in the value of fixed assets when events or changes in circumstances reflect the fact that their recorded value may not be recoverable.
Depreciation is calculated on a straight-line basis over the estimated useful lives of the assets. The useful lives for property and equipment are as follows:
Buildings and leasehold improvement | 20 to 40 years | |||
Manufacturing equipment | 10 to 15 years | |||
Office equipment and furniture | 5 to 8 years |
INCOME TAXES
Taxes are calculated in accordance with taxation principles currently effective in the United States and PRC. We account for income taxes using the liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized as income in the period that includes the enactment date. A valuation allowance is provided for the amount of deferred tax assets that, based on available evidence, are not expected to be realized.
RECENT ACCOUNTING PRONOUNCEMENTS
In February 2007, the FASB issued SFAS No. 159, "The“The Fair Value Option for Financial Assets and Financial Liabilities, Including an Amendment of FASB Statement No.115"No.115”, under which entities will now be permitted to measure many financial instruments and certain other assets and liabilities at fair value on an instrument-by-instrument basis. This Statement is effective as of the beginning of an entity'sentity’s first fiscal year that begins after November 15, 2007. Early adoption is permitted as of the beginning of a fiscal year that begins on or before November 15, 2007, provided the entity also elects to apply the provisions of SFAS 157. The adoption of SFAS 159 did not have a material impact on our financial statements.
In December 2007, the FASB issued Statement of Financial Accounting Standards No. 141R, Business Combinations (SFAS 141R) and Statement of Financial Accounting Standards No. 160, Accounting and Reporting of Non-controlling Interests in Consolidated Financial Statements, an amendment of ARB No. 51 (SFAS 160). These two standards must be adopted in conjunction with each other on a prospective basis. The most significant changes to business combination accounting pursuant to SFAS 141R and SFAS 160 are the following: (a) recognize, with certain exceptions, 100 percent of the fair values of assets acquired, liabilities assumed and non-controlling interests in acquisitions of less than a 100 percent controlling interest when the acquisition constitutes a change in control of the acquired entity, (b) acquirers'acquirers’ shares issued in consideration for a business combination will be measured at fair value on the closing date, not the announcement date, (c) recognize contingent consideration arrangements at their acquisition date fair values, with subsequent changes in fair value generally reflected in earnings, (d) the expensing of all transaction costs as incurred and most restructuring costs, (e) recognition of pre-acquisition loss and gain contingencies at their acquisition date fair values, with certain exceptions, (f) capitalization of acquired in-process research and development rather than expense recognition, (g) earn-out arrangements may be required to be re-measured at fair value and (h) recognize changes that result from a business combination transaction in an acquirer'sacquirer’s existing income tax valuation allowances and tax uncertainty accruals as adjustments to income tax expense. Should we decide to enter into future business combinations, these new standards will significantly affect our accounting for future business combinations following adoption on January 1, 2009.
In March 2008, the FASB issued SFAS No. 161, "Disclosures“Disclosures about Derivative Instruments and Hedging Activities-- an amendment of FASB Statement No. 133" ("133” (“FAS 161"161”). FAS 161 changes the disclosure requirements for derivative instruments and hedging activities. Entities are required to provide enhanced disclosures about (a) how and why an entity uses derivative instruments, (b) how derivative instruments and related hedged items are accounted for under Statement 133 and its related interpretations, and (c) how derivative instruments and related hedged items affect an entity'sentity’s financial position, financial performance, and cash flows. The guidance in FAS 161 is effective for financial statements issued for fiscal years and interim periods beginning after November 15, 2008, with early application encouraged. This Statement encourages, but does not require, comparative disclosures for earlier periods at initial adoption. We are currently assessing the impact of FAS 161.
In May 2008, the Financial Accounting Standards Board (FASB”) issued FASB Staff Position (FSP”) APB 14-1, Accounting for Convertible Debt Instruments That May Be Settled in Cash upon Conversion (Including Partial Cash Settlement) . FSP APB 14-1 clarifies that convertible debt instruments that may be settled in cash upon either mandatory or optional conversion (including partial cash settlement) are not addressed by paragraph 12 of APB Opinion No. 14, Accounting for Convertible Debt and Debt issued with Stock Purchase Warrants . Additionally, FSP APB 14-1 specifies that issuers of such instruments should separately account for the liability and equity components in a manner that will reflect the entity’s nonconvertible debt borrowing rate when interest cost is recognized in subsequent periods. FSP APB 14-1 is effective for financial statements issued for fiscal years beginning after December 15, 2008, and interim periods within those fiscal years. We will adopt FSP APB 14-1 beginning in the first quarter of fiscal 2010, and this standard must be applied on a retrospective basis. We are evaluating the impact the adoption of FSP APB 14-1 will have on our consolidated financial position and results of operations.
In May 2008, the FASB issued Statement of Financial Accounting Standards (SFAS”) No. 162, The Hierarchy of Generally Accepted Accounting Principles . This standard is intended to improve financial reporting by identifying a consistent framework, or hierarchy, for selecting accounting principles to be used in preparing financial statements that are presented in conformity with generally accepted accounting principles in the United States for non-governmental entities. SFAS No. 162 is effective 60 days following approval by the U.S. Securities and Exchange Commission (SEC”) of the Public Company Accounting Oversight Board’s amendments to AU Section 411, The Meaning of Present Fairly in Conformity with Generally Accepted Accounting Principles . We do not expect SFAS No. 162 to have a material impact on the preparation of our consolidated financial statements.
On June 16, 2008, the FASB issued final Staff Position (FSP) No. EITF 03-6-1, Determining Whether Instruments Granted in Share-Based Payment Transactions Are Participating Securities” to address the question of whether instruments granted in share-based payment transactions are participating securities prior to vesting. The FSP determines that unvested share-based payment awards that contain rights to dividend payments should be included in earnings per share calculations. The guidance will be effective for fiscal years beginning after December 15, 2008. We are currently evaluating the requirements of (FSP) No. EITF 03-6-1.
Results of Operations
For the SixNine Months and Three Months Ended JuneSeptember 30, 2008 and Ended JuneSeptember 30, 2007
SixNine Months ended JuneSeptember 30, 2008 comparedCompared to six monthsNine Months ended JuneSeptember 30, 2007
Total Net Revenues
Total revenues for the sixnine months ended JuneSeptember 30, 2008 were $31,094,789$47,798,673 as compared to total revenues of $21,094,987$37,649,336 for the sixnine months ended JuneSeptember 30, 2007, an increase of $9,999,802$10,149,337 or approximately 47.40%.26.96%comparable period in 2007. For the sixnine months ended JuneSeptember 30, 2008 and 2007, net revenues consisted of the following:
|
| 2008 |
| 2007 |
| ||
Wholesale |
| $ | 40,749,696 |
| $ | 25,715,018 |
|
Retail |
|
| 2,606,091 |
|
| 3,244,739 |
|
Other revenues |
|
| 4,442,886 |
|
| 8,689,579 |
|
Total net revenues |
| $ | 47,798,673 |
| $ | 37,649,336 |
|
| 2008 | 2007 | ||||||
| Wholesale | $ | 26,931,327 | $ | 14,993,316 | |||
| Retail | 1,402,393 | 893,014 | |||||
| Other revenues | 2,761,069 | 5,208,657 | |||||
| Total net revenues | $ | 31,094,789 | $ | 21,094,987 | |||
• | For the | |
• | For the nine months ended September 30, 2008, retail revenues decreased by $638,648 or 20%. The decrease is primarily attributable to the slowdown in economy growth and the impact from 2008 Olympic held in Beijing. The slowdown in the economy growth resulted in less non-essential health supplements being purchases. The Company also experienced a reduction in the sale of higher priced drugs in 2008. As a result, the Company generated less per store revenue. Additionally, during the 2008 Olympics, the Beijing government restricted the city traffic and limited local roads used by locals. Consequentially, the Company’s retail stores received less visits from its customers. We expect our fourth quarter retail revenue will slightly increase from previous quarters in 2008 as we are approaching flu season. | |
• | For the nine months ended September 30, 2008, other revenues decreased by $4,246,693 or 49%. The decrease in other revenues is attributed to the following: |
|
| 2008 |
| 2007 |
| ||
Leasing revenues |
| $ | 581,238 |
| $ | 619,541 |
|
Third-party manufacturing |
|
| 3,505,225 |
|
| 7,054,613 |
|
Advertising revenues |
|
| 229 |
|
| 452,610 |
|
Research and development and lab testing services |
|
| 356,194 |
|
| 562,815 |
|
Total other revenues |
| $ | 4,442,886 |
| $ | 8,689,579 |
|
• | We sublease certain portion our retail stores and counter spaces to various other vendors and generate leasing revenue. The leasing revenue remained materially consistent with prior year same period. The slight decrease was primarily due to less counter space being leased to third parties due to less demand for our counter spaces during the three months ended September 30, 2008. We expect our leasing revenue to remain flat in the remaining part of the year. | |
• | For third-party manufacturing, customers supply the raw materials and we are paid a fee for manufacturing their products. We had less large manufacturing contracts during the nine months ended September 30, 2008 as the demand for the third-party manufacturing decreased as compared to the same period in 2007. Several of our historical, large third party manufacturing customers have built their own facilities and started manufacturing products on their own in 2008 and we were unable to find new customers to replace them. As a result, our third-party manufacturing revenue decreased. We anticipate the third-party manufacturing revenue will continue to decrease for the remainder of fiscal 2008 as we do not expect to have new customers with large third party manufacturing contracts. | |
• | A large advertisement contract whereby we receive approximately $50,000 per month for the lease of counter and other space at our retail locations ended in December 2007. Accordingly, our advertising revenues decreased during the nine months ended September 30, 2008 as compared to the same period in 2007. As the local government tightens the advertising rules and regulations in the pharmaceutical industry, we do not anticipate signing similar advertisement contracts in the near future. | |
• | We performed research and development and lab testing projects for various third parties and performed drug testing and analysis. We preformed less R&D testing services and drug testing and analysis work for third parties during the nine months ended September 30, 2008 as compared to the same period in 2007. Some of our historical customers for these services were able to find other companies that provide similar R&D and lab testing serves at a lower price and decided not to use our services in 2008. We expect the revenue from R&D and lab testing will maintain at its current level with minimal growth in the remaining of 2008. |
Cost of Sales
Cost of sales includes raw materials, packing materials, direct labor and manufacturing costs, which includes allocated portion of overhead expenses such as Labor fee, utilities and depreciation directly related to product production. For the nine months ended September 30, 2008, cost of sales amounted to $25,684,501 or approximately 53.73% of total net revenues as compared to cost of sales of $22,738,456 or approximately 60.40% of total net revenues for the nine months ended September 30, 2007. The collections on the previously reserved allowance for sales return of approximately $1.9 million in 2007 mentioned above had the effect of decreasing the Company’s cost of sales as a percentage of total revenue by 6% for the nine months ended September 30, 2007 as the Company recorded the costs associated with these products in its cost of sales during fiscal 2006 and the revenue was recognized in the second quarter of 2007. The decrease in cost of sales as a percentage of total net revenue was primarily due to better purchase pricing management and more efficient production cost controls.
Gross Profit
Gross profit for the nine months ended September 30, 2008 was $22,114,172 or 46.27% of total net revenues, as compared to $14,910,880 or 39.6% of total net revenues for the nine months ended September 30, 2007. The collections on the previously reserved allowance for sales return of approximately $1.9 million in 2007 mentioned above had the effect of increasing the Company’s profit margin by approximately $1.9 million for the nine months ended September 30, 2007, even though the sales of these products were actually made during the fourth quarter of fiscal 2006. As the costs associated with these products was recorded in our cost of sales during fiscal 2006, both the Company’s gross profit margin and income from operations for the nine months ended September 30, 2007 increased by approximately $1.9 million. The increase in gross profit was attributable to the decrease in cost of sales as a percentage of revenue. Although we recognized higher than average gross profits during the nine months ended September 30, 2008, there could be no assurance that we will continue to recognize similar gross profit margin in the future.
Operating Expenses
Total operating expenses for the nine months ended September 30, 2008 were $14,074,682, an increase of $7,029,448 or 99.78% from total operating expenses in the nine months ended September 30, 2007 of $7,045,234. This increase included the following:
For the nine months ended September 30, 2008, selling expenses amounted to $11,291,590 as compared to $2,864,746 for the nine months ended September 30, 2007, an increase of $8,426,844 or 294.16%from the comparable period in fiscal 2007. This increase is primarily attributable to an increase of approximately $5.9 million in commission paid to our sales representatives on sales generated to provide greater incentives; we have significantly increased the sales commission percentage in 2008. Additionally, we paid bonuses to our collection personnel of approximately $2.5 million to improve our collections on accounts receivables and cash flows. Because we expect to continue paying commission on sales to increase our sales and provide bonuses based on the collection personnel’s performance to generate sufficient cash to meet our near term capital commitments such as new manufacture plant construction project and acquiring Chinese Class I drug patent, we expect our selling expenses to increase as our revenues increase. As a result, the significant increase in the selling expenses negatively impacted of our net income.
For the nine months ended September 30, 2008, research and development costs amounted to $1,193,916 as compared to $1,549,132 for the nine months ended September 30, 2007, a decrease of $355,216 or 22.93%from the comparable period in 2007. In late 2007, we entered into several research and development agreements with third parties for the design, research and development of designated pharmaceutical projects. We fulfilled these research and development agreements on June 30, 2008. We did not enter into any new research and development agreement in the third quarter of 2008. As a result, expenses related to those research and development projects decreased during the nine months ended September 30, 2008.
For the nine months ended September 30, 2008, general and administrative expenses were $1,589,176 as compared to $2,631,356 for the nine months ended September 30, 2007, a decrease of $1,042,180 or 39.61% from the comparable period in fiscal 2007. These changes are summarized below:
|
| Nine months ended September 30, | ||||
|
| 2008 |
| 2007 | ||
Salaries and related benefits |
| $ | 965,682 |
| $ | 1,311,215 |
Amortization and depreciation expenses |
|
| 123,305 |
|
| 127,533 |
Rent |
|
| 133,336 |
|
| 227,997 |
Travel and entertainment |
|
| 49,609 |
|
| 273,074 |
Professional fees |
|
| 329,211 |
|
| 167,766 |
Bad debt recovery |
|
| (490,310) |
|
| - |
Other |
|
| 478,343 |
|
| 523,771 |
Total |
| $ | 1,589,176 |
| $ | 2,631,356 |
The changes in these expenses from the nine months ended September 30, 2008 as compared to the nine months ended September 30, 2007 included the following:
• | Salaries and related benefits decreased $345,533 or 26.35% primarily due to a decrease in bonuses paid to administrative personnel. The bonuses paid in 2007 were paid to several key employees and management and determined by meeting various individuals’ as well as overall Company’s operational goals. As we continue our efforts to control corporate spending, starting in 2008, we no longer provided certain benefits that were provided to employees in the 2007 including insurance, car allowances and other fringe benefits. As a result, the salaries and related benefits costs decreased accordingly. | |
• | Depreciation on our fixed assets and amortization of our intangible assets decreased by $4,228 or approximately 3.32% which is primarily attributable to the certain assets used in our office were fully depreciated in earlier part of 2008. | |
• | Bad debt recovery income increased $490,310 or 100% primarily due to our strong effort on accounts receivable collection. | |
Rent decreased by $94,661 or approximately 41.52% which primarily reflects our ability to renegotiate a portion of our leases at reduced rent rates on a short term basis. | ||
• | Travel and entertainment expenses decreased by $223,465 or 81.83% as a result of corporate spending control effort. | |
• | Professional fees increased $161,445 or 96.23% due to increase in investor relation professional fees, legal expenses related to February 2008 financing and a stock based compensations paid to a technology consultant for consulting services provided to the Company. | |
• | Other selling, general and administrative expenses, which includes utilities, office supplies and training and other office expenses decreased by $45,428 or approximately 8.67% reflecting efforts at reducing non-sales related corporate training and other activities as well as stricter controls on corporate spending. |
Income from Operations
We reported income from operations of $8,039,490 for the nine months ended September 30, 2008 as compared to income from operations of $7,865,646 for the nine months ended September 30, 2007, an increase of $173,844 or approximately 2.21%.
Other Expense
For the nine months ended September 30, 2008, total other expense amounted to $1,650,456 as compared to other expense of $1,765,480 for the nine months ended September 30, 2007, a decrease of $115,024 or 6.52% from the comparable period in 2007. This change is primarily attributable to:
• | For the nine months ended September 30, 2008, our debt issuance costs of $261,919 compared to $146,699 for the nine months ended September 30, 2007, an increased of $115,220 or approximately 78.54%, due to our recent funding during the nine months of 2008. | |
• | For the nine months ended September 30, 2008, we recorded registration rights penalties of $650 related to the late filing of our registration statement on Form SB-2 as compared to $110,000 for the nine months ended September 30, 2007, a decrease of $109,350 or 99.41%. | |
• | For the nine months ended September 30, 2008, interest expense was $1,399,507 as compared to $1,508,781 for the nine months ended September 30, 2007, a decrease of $109,274 or 7.24% which is primarily attributable to the amortization of discount on certain debt which was fully amortized earlier in 2008. |
NET INCOME, OTHER COMPREHENSIVE INCOME AND COMPREHENSIVE INCOME
As a result of these factors, we reported net income of $6,389,034 for the nine months ended September 30, 2008 as compared to net income of $6,100,166 for the nine months ended September 30, 2007. This translates to basic and diluted net income per common share of $0.15, $0.13 and $0.15, $0.14 for the nine months ended September 30, 2008 and 2007, respectively.
During the nine months ended September 30, 2008, our unrealized gain on foreign currency translation of $2,173,475, an increase of $1,620,660, or approximately 293.16%, from the same period in 2007. We report in U.S. dollars, but the functional currency of Lotus East is the RMB. Translation adjustments result from the process of translating the local currency financial statements into U.S. dollars, with the average translation rates applied to our income statement of 6.99886 RMB to $1.00 during the nine months ended September 30, 2008. As a result of this non-cash gain, we reported comprehensive income of $8,562,509 for the nine months ended September 30, 2008 as compared to $6,652,981 for the same period in 2007.
Three Months ended September 30, 2008 compared to three months ended September 30, 2007
Total Net Revenues
Total revenues for the three months ended September 30, 2008 were $16,703,884 as compared to total revenues of $16,554,349 for the three months ended September 30, 2007, an increase of $149,535 or approximately 0.9%. For the three months ended September 30, 2008 and 2007, net revenues consisted of the following:
|
| 2008 |
| 2007 |
| ||
Wholesale |
| $ | 13,818,369 |
| $ | 11,429,926 |
|
Retail |
|
| 1,203,698 |
|
| 1,643,501 |
|
Other revenues |
|
| 1,681,817 |
|
| 3,480,922 |
|
Total net revenues |
| $ | 16,703,884 |
| $ | 16,554,349 |
|
• | For the three months ended September 30, 2008, wholesale revenues increased by $2,388,443 or 21%. The significant increase in tangible product revenues is mainly attributed to strong sales of Brimonidine Tartrate Eye Drops, Valsartan Capsules and third party manufactured drugs included Recombinant Human Granulocyte Colony, Recombinant Human Interleukin-2 for Injection, Deproteinized Calfoblood Extractives Injection, and Cervus and Cucumis Polypeptide Injection. and Nicergoline offset by the decrease in sales in Nicergoline, Levofloxacin Lactate for Injection and third party manufactured drug Recombinant Human Erythropoietin Injection. The significant increased sales in Brimonidine Tartrate Eye Drops and Valsartan Capsules were primarily due to our strong marketing and sales effort on promoting those drugs. In late 2007, we obtained exclusive distribution rights from a third party Beijing based pharmaceutical company for four drugs: Octreotide Acetate Injection, Recombinant Human Erythropoietin Injection, Recombinant Human Granulocyte Colony-stimulating Factor Injection, and Recombinant Human Interleukin-2 for Injection. As a result, we experienced a significant increase in our third party manufactured drug sales during the | |
• | For the | |
For the three months ended September 30, 2008, other revenues decreased by $1,799,105 or 51.68%. The decrease in other revenues is attributed to the following:
| 2008 | 2007 |
| 2008 |
| 2007 |
| ||||||||
| Leasing revenues | $ | 364,279 | $ | 439,615 |
| $ | 216,959 |
| $ | 179,926 |
| |||
| Third-party manufacturing | 2,129,992 | 4,137,278 |
|
| 1,375,233 |
| 2,917,335 |
| ||||||
| Advertising revenues | 215 | 295,343 |
|
| - |
| 157,267 |
| ||||||
| Research and development and lab testing services | 266,583 | 336,421 |
|
| 89,625 |
|
| 226,394 |
| |||||
| Total other revenues | $ | 2,761,069 | $ | 5,208,657 |
| $ | 1,681,817 |
| $ | 3,480,922 |
| |||
• | We sublease certain portion our retail stores and counter spaces to various other vendors and generate leasing revenue. The |
• | For third-party manufacturing, customers supply the raw materials and we are paid a fee for manufacturing their products. We had less large manufacturing contracts during the | |
• | A large advertisement contract whereby we receive approximately $50,000 per month for the lease of counter and other space at our retail locations ended in December 2007. | |
• | We performed research and development and lab testing projects for various third parties and performed drug testing and analysis. We preformed less R&D testing services and drug testing and analysis work for third parties during the |
Cost of Sales
Cost of sales includes raw materials, packing materials, shipping, and manufacturing costs, which includes allocated portion of overhead expenses such as utilities and depreciation directly related to product production. For the sixthree months ended JuneSeptember 30, 2008, cost of sales amounted to $17,465,143$8,219,358 or approximately 56.17%49.21% of total net revenues as compared to cost of sales of $12,974,387$9,856,382 or approximately 61.5%59.54% of total net revenues for the sixthree months ended JuneSeptember 30, 2007. The collections on the previously reserved allowance for sales return of approximately $1.8$1.9 million in 2007 mentioned above had the effect of decreasing the Company’s cost of sales as a percentage of total revenue by 5.7%9% for the six months ended JuneSeptember 30, 2007as the Company recorded the costs associated with these products in its cost of sales during fiscal 2006 and the revenue was recognized in the second quarter of 2007. The decrease in cost of sales as a percentage of total revenue was primarily due to better purchase pricing management and more efficient production cost controls.
Gross Profit
Gross profit for the sixthree months ended JuneSeptember 30, 2008 was $13,629,646$8,484,526 or 43.83%50.79% of total revenues, as compared to $8,120,600$6,697,967 or 38.5%40.46% of revenues for the sixthree months ended JuneSeptember 30, 2007. The collections on the previously reserved allowance for sales return of approximately $1.8$1.9 million in 2007 mentioned above had the effect of increasing the Company’s profit margin by approximately $1.8 million$1.9million for the sixthree months ended JuneSeptember 30, 2007, even though the sales of these products were actually made during the fourth quarter of fiscal 2006. As the costs associated with these products was recorded in our cost of sales during fiscal 2006, both the Company’s gross profit margin and income from operations for the sixthree months ended JuneSeptember 30, 2007 increased by approximately $1.8$1.9 million. The increase in gross profit was attributable to the decrease in cost of sales as a percentage of revenue. Although we recognized higher than average gross profits during the sixthree months ended JuneSeptember 30, 2008, there could be no assurance that we will continue to recognize similar gross profit margin in the future.
Operating Expenses
Total operating expenses for the sixthree months ended JuneSeptember 30, 2008 were $9,347,814,$4,726,868, an increase of $6,005,233$1,116,528 or 179.66%30.93%, from total operating expenses in the sixthree months ended JuneSeptember 30, 2007 of $3,342,581.$3,610,340. This increase included the following:
For the sixthree months ended JuneSeptember 30, 2008, selling expenses amounted to $6,997,886$4,293,704 as compared to $1,422,796$1,441,950 for the sixthree months ended JuneSeptember 30, 2007, an increase of $5,575,090$2,851,754 or 391.84%197.77%. This increase is primarily attributable to an increase of approximately $3.7 million in commission paid on sales generated to our sales representatives to provide better incentives.
For the sixthree months ended JuneSeptember 30, 2008, research and development costs amounted to $1,181,468$12,448 as compared to $200,975$1,255,844 for the sixthree months ended JuneSeptember 30, 2007, an increasea decrease of $980,493$1,243,396 or 487.87%99.01%. In late 2007, we entered into several research and development agreements with third parties for the design, research and development of designated pharmaceutical projects and asthese agreements were fulfilled on June 30, 2008. We do not incur any research and development activities in third quarter 2008. There is an exchange rate effect in third quarter 2008 about research and development. As a result, expenses related to those research and development projects increaseddecreased during the sixthree months ended JuneSeptember 30, 2008.
For the sixthree months ended JuneSeptember 30, 2008, general and administrative expenses were $1,168,460$420,716 as compared to $1,718,810$912,546 for the sixthree months ended JuneSeptember 30, 2007, a decrease of $550,350$491,830 or 32%53.90% as summarized below:
|
| Three months ended June 30, | ||||
|
| 2008 |
| 2007 | ||
Salaries and related benefits |
| $ | 421,228 |
| $ | 484,252 |
Amortization and depreciation expenses |
|
| 42,185 |
|
| 77,416 |
Rent |
|
| 54,387 |
|
| 94,485 |
Travel and entertainment |
|
| 12,570 |
|
| 179,045 |
Professional fees |
|
| 143,593 |
|
| 19,506 |
Bad debt recovery |
|
| (490,310) |
|
| - |
Other |
|
| 237,063 |
|
| 57,842 |
Total |
| $ | 420,716 |
| $ | 912,546 |
Six months ended June 30, | |||||||
| 2008 | 2007 | ||||||
| Salaries and related benefits | $ | 544,454 | $ | 826,963 | |||
| Amortization and depreciation expenses | 81,120 | 50,117 | |||||
| Rent | 149,443 | 133,512 | |||||
| Travel and entertainment | 37,039 | 94,029 | |||||
| Professional fees | 185,618 | 148,260 | |||||
| Other | 170,786 | 465,929 | |||||
| Total | $ | 1,168,460 | $ | 1,718,810 | |||
| 2008 | 2007 | ||||||
| Wholesale | $ | 17,161,230 | $ | 9,819,373 | |||
| Retail | 718,645 | 516,594 | |||||
| Other revenues | 1,505,737 | 2,469,485 | |||||
| Total net revenues | $ | 19,385,612 | $ | 12,805,452 | |||
| 2008 | 2007 | ||||||
| Leasing revenues | $ | 184,756 | $ | 160,998 | |||
| Third-party manufacturing | 1,221,102 | 1,901,349 | |||||
| Advertising revenues | - | 148,651 | |||||
| Research and development and lab testing services | 99,879 | 258,487 | |||||
| Total other revenues | $ | 1,505,737 | $ | 2,469,485 | |||
Three months ended June 30, | |||||||
| 2008 | 2007 | ||||||
| Salaries and related benefits | $ | 274,986 | $ | 437,482 | |||
| Amortization and depreciation expenses | 23,294 | 6,301 | |||||
| Rent | 78,275 | 66,235 | |||||
| Travel and entertainment | 17,277 | 42,525 | |||||
| Professional fees | 130,638 | 95,160 | |||||
| Other | 17,573 | 340,530 | |||||
| Total | $ | 542,043 | $ | 988,233 | |||
The changes in these expenses from the three months ended JuneSeptember 30, 2008 as compared to the three months ended JuneSeptember 30, 2007 included the following:
• | Salaries and related benefits decreased |
• | Depreciation on fixed assets and amortization of our intangible assets | |
• | Bad debt recovery income increased by $490,310 or 100% which is primarily attributable to our effort on collection. | |
• | Rent | |
• | Travel and entertainment expenses decreased by | |
• | Professional fees increased | |
• | Other selling, general and administrative expenses, which includes utilities, office supplies and training and other office expenses |
Income from Operations
We reported income from operations of $2,800,059$3,757,658 for the three months ended JuneSeptember 30 2008 as compared to income from operations of $3,604,811$3,087,627 for the three months ended JuneSeptember 30, 2007, a decreasean increase of $804,752$670,031 or approximately 22.32%21.7%.
Other Expense
For the three months ended JuneSeptember 30, 2008, total other expense amounted to $620,150$543,982 as compared to other expense of $563,286$866,287 for the three months ended JuneSeptember 30, 2007, an increasea decrease of $56,864$322,305 or 10.10%37.21%. This change is primarily attributable to:
• | For the three months ended | |
• | For the three months ended | |
As a result of these factors, we reported net income of $2,179,909$3,213,676 for the three months ended JuneSeptember 30, 2008 as compared to net income of $3,041,525$2,221,340 for the three months ended JuneSeptember 30, 2007. This translates to basic and diluted net income per common share of $0.08, $0.07 and $0.05, $0.05 and $0.07, $0.07 for the three months ended JuneSeptember 30, 2008 and 2007, respectively.
During the three months ended JuneSeptember 30, 2008, we reported an unrealized gain on foreign currency translation of $1,587,538, an increase$127,833, a decrease of $1,339,135$53,062 or approximately 539.10%29.33%, from the same period in 2007. These gains are non-cash items. As described elsewhere herein, our functional currency is the Chinese Renminbi; however the accompanying consolidated financial statements have been translated and presented in U.S. dollars using period-end rates of exchange for assets and liabilities, and average rates of exchange for the period for revenues, costs, and expenses. Net gains and losses resulting from foreign exchange transactions are included in the consolidated statements of operations and can have a significant effect on our financial statements. As a result of this non-cash gain, we reported comprehensive income of $3,767,447$3,341,509 for the three months ended JuneSeptember 30, 2008 as compared to $3,289,928$2,402,235 for the same period in 2007.
Liquidity and Capital Resources
Liquidity is the ability of a company to generate funds to support its current and future operations, satisfy its obligations and otherwise operate on an ongoing basis.
At JuneSeptember 30, 2008, we had a cash balance of $3,401,444.$2,656,770. These funds are located in financial institutions located as follows:
China |
| $ | 2,649,898 |
|
USA |
|
| 6,872 |
|
Total |
| $ | 2,656,770 |
|
| China | $ | 3,285,478 | ||
| USA | 115,966 | |||
| Total | $ | 3,401,444 |
Our working capital position increased $2,333,862decreased $5,982,532 to $26,985,999$18,669,605 at JuneSeptember 30, 2008 from $24,652,137 at December 31, 2007. This increasedecrease in working capital is primarily attributable to an increasea decrease in accounts receivable net of allowance of approximately $355,804, and an increase of approximately $3,676,156 in inventories,$6,548,529, a decrease in convertible debtcash of $2,561,645,approximately $1.9 million, a decrease in prepaid expense of $931,942, an increase in accounts payable and accrued expenses of $263,508 offset by decrease$1,080,907, an increase in cashtax payable of approximately $1.2 million, a decrease in prepaid expense of $724,563,$2,730,952, an increase in unearned revenue of approximately $111,000$99,021 and an increase in due to related parties of approximately $949,000.
At JuneSeptember 30, 2008, our inventories of raw materials, work in progress, packaging materials and finished goods totaled $7,086,895,$8,621,880, an increaseincreased of approximately $3.7$5.2 million, or 107.78%152.79%, from December 31, 2007. Included in this change was an increase of $5,681,166 in finished goods offset by decreases in raw materials of $148,864 and work in progress of $279,759. We expect to maintain higher finished goods inventory levels to accommodate for anticipated future sales growth, new products development and productions as well as a wider variety of products.
At JuneSeptember 30, 2008, our accounts receivable, net of allowance for doubtful accounts, was $13,882,298 as compared to $20,430,827 at December 31, 2007, a decrease of $6,548,529. The decrease was primarily due to our strong collection effort which significantly improved our accounts receivable aging days.
At September 30, 2008, we maintainmaintained an allowance for doubtful accounts and sales return on accounts receivable balances of $568,847$84,189 as compared to $548,083 at December 31, 2007, an increasea decrease of $20,764 ($14,108 reflects as a reduction to allowance for doubtful accounts and sales return and $34,872 as foreign currency translation adjustment) and$463,894 reflects our best estimate of probable losses. In determining the allowance for doubtful accounts, our management reviews our accounts receivable aging as well as the facts and circumstances of specific customers which may indicate the collection of specific amounts are at risk. As is customary in the PRC, we extend relatively long payment terms to our customers. Our terms of sale generally require payment within four months to a year, which is considerably longer than customary terms offered in the United States, however, we believe that our terms of sale are customary among our competitors for a company in our size within our industry. As described elsewhere herein, in 2008 we began paying a bonus to certain employees who are involved in the collection of accounts receivable to generate sufficient cash to meet our near term capital commitments such as new manufacture plant construction project and acquiring Chinese Class I drug patent. At September 30, 2008, these bonuses totaled $2.5 million. We also occasionally offer established customers longer payment terms on new products as an incentive to purchase these products, which has served to further increase the average days outstanding for accounts receivable. As the market for these new products is established, we will discontinue offering this sales incentive. Occasionally we will request a customer to prepay an order prior to shipment. At JuneSeptember 30, 2008, our balance sheet reflected advances from customers of $0, a decrease of $34,531, or 100%, from December 31, 2007.
At JuneSeptember 30, 2008, we have a prepaid expenses of $77,440 as compared to $1,009,382 at December 31, 2007, a decrease of $ 931,942. The decrease was primarily due to the completion of our research and development contracts. The total amount of the prepaid expense for research and development contracts was approximately $ 1 million at December 31, 2007. Although we did not make similar research and development contract prepayments in 2008, we expect to incur approximately $14.5 million expenses related to our Laevo-Bambuterodrug that was recently accepted by the Chinese SFDA for clinical trial evaluations in the next 24 to 30 months.
At September 30, 2008, we have a deferred debt cost of $563,928 as compared to $29,340 at December 31, 2007, an increase of $ 534,588. The increase was primarily attributed to the issuance of the series A convertible redeemable preferred stock and warrants to purchase 2,873,553 shares of the company’s common stock. We recorded a total deferred debt cost of $796,133 to be amortized over the term of our purchase agreement.
At September 30, 2008, we have a deposit on patent of $2,910,446$2,917,536 to acquire a new Chinese Class I anti-asthma medicine drug patent in accordance with a technoloytechnology transfer agreement the Company entered into in April 2008. Additionally, in accordance with the agreement we entered into in June 2008, we also have a deposit on land use right of $6,033,353$17,120,100 for the amounts paid to a local government agency in Cha You to acquire a long-term interest to utilize certain land to construct a new manufacture facility. We did not have the two deposit amounts at December 31, 2007. We will need to make additional payments totaling approximately $73.7$62.6 million in the future.
At JuneSeptember 30, 2008, we have an accounts payable and accrued expenses of $1,845,398 as compared to $764,491 at December 31, 2007, an increase of $1,080,907. The increase was primarily attributed to the increase of construction in progress for our new facility in Inner Mongolia.
At September 30, 2008, we have a value-added and services taxes payable of $2,030,543$3,303,152 as compared to $572,200 at December 31, 2007. The amounts are related to accrued but unpaid value added and services taxes for the sixnine months ended JuneSeptember 30, 2008. The increase in the taxes payable is primarily due to increase in our revenues during the sixnine months ended JuneSeptember 30, 2008 and the tax payment timing differences.
Our balance sheet at JuneSeptember 30, 2008, also reflects a balance due to related parties of $1,272,153$1,355,077 which was a working capital advances made to us by our president, vice-president and an officer of the Company and a Board member as well as an amount payable of approximately $654,850$131,289 related to an assignment agreement as discussed elsewhere in this report. These advances are non-interest bearing and are due on demand. We are currently repaying these balances as operating cash become available.
Our balance sheet at JuneSeptember 30, 2008 also reflects notes payable to related parties of approximately $5 million due on December 30, 2015 which is a working capital loan made to us by the Company’s Chief Executive Officer, two employees of the Company and a Board member. These loans bear a variable annual interest at 80% of current bank rate and are unsecured. During the three months ended JuneSeptember 30, 2008, we did not repay any portion of these loan balances.
The changes in asset and liabilities discussed above is based on a comparison of amounts on our balance sheets at JuneSeptember 30, 2008 and December 31, 2007 and does necessarily reflect changes in assets and liabilities reflected on our cash flow statement, which we use the average foreign exchange rate during the period to calculate these changes.
Net cash provided by operating activities for the sixnine months ended JuneSeptember 30, 2008 was $4,742,275$16,038,077 as compared to net cash provided by operating activities of $4,942,964$3,688,656 for the sixnine months ended JuneSeptember 30, 2007. For the sixnine months ended JuneSeptember 30, 2008, net cash provided by operating activities was attributable primarily to decrease in accounts receivable of $946,083, increase in inventories of $3,358,488,$8,244,225, increase in value-added and service taxes payable of 1,381,155 offset by the net income2,637,331, an decrease in prepaid and other current assets of $3,176,008,$1,080,576, an increase in unearned revenue of $62,225, an increase in accounts payable and accrued expenses of $1,032,245 and the add back of net income of $6,389,034, depreciation and amortization of $307,713,$469,455, amortization of debt discount of $208,355, amortization of debt issuance costs of $162,029,$261,545, stock based compensation of $270,245 and$392,341, the amortization of discount on convertible redeemable preferred stock of $338,838,$592,966 and warrant repricing of $74,593 offset by an increase in inventories of $4,880,418, a decrease in allowance for doubtful accounts and sales return of $14,101,$490,310 and a decrease in prepaid and other current assetsadvances from customers of $986,132, and an increase in unearned revenue of $74,796.$36,086. Net cash provided by operating activities for the sixnine months ended JuneSeptember 30, 2007 was $4,942,964.$3,688,656. For the sixnine months ended JuneSeptember 30, 2007, net cash provided by operating activities was attributable primarily to our net income of $3,878,826,$6,100,166, the add back of depreciation and amortization of $275,197,$422,055, amortization of debt issuance costs of $88,020 and the$146,699, amortization of debt discount of $433,735, a$1,041,774, stock issued for compensation of 239,200 and decrease in allowance for doubtful accounts and sales returns of 1,723,258 and an increase in accounts receivable of $524,467, an increase in accounts payable and accrued expenses of $78,398,$6,327,758, an increase in value-added and service taxes payable of $921,996,$1,298,344, and an increase in unearned revenues of $132,461$122,924 offset by an decrease in inventory balance of $1,201,912,$2,390,052, an decrease in prepaid expenses and other current assets of $113,540,$251,225, a decrease in accounts payable and accrued expenses of $101,796 and a decrease in advances from customers of $145,138.
Net cash used in investing activities for the sixnine months ended JuneSeptember 30, 2008 amounted to $8,907,855.$21,060,376. For the sixnine months ended JuneSeptember 30, 2008, net cash used in investing activities was attributable to the deposit on patent right of $2,827,814,$2,857,608, the deposit on land use right of $5,862,059$16,768,445 and the purchase of property and equipment of $217,982.$1,430,894. Net cash provided by investing activities for the sixnine months ended JuneSeptember 30, 2007 was $145,729$417,040 attributable to the purchase of property and equipment of $376,201$384,198 offset by payment received on due from related parties of $521,930.
Net cash provided by financing activities was $2,786,003$2,872,348 for the sixnine months ended JuneSeptember 30, 2008 and was attributable to the receipt of net proceeds of $5,000,000 from our private financing and proceeds from related party advances of $774,571$860,916 offset by payments on convertible debt of $2,520,000 and debt issuance costs of $468,568. Net cash provided byused in financing activities was $1,155,257$116,778 for the sixnine months ended JuneSeptember 30, 2007 and was primarily attributable to the receipt of net proceeds of $2,950,000 from our debt financing offset by payments on related party advances and loansnotes of $1,566,083$2,835,252 and the payment of debt issuance costs of $231,526.
We reported a net decrease in cash for the sixnine months ended JuneSeptember 30, 2008 of $1,156,513$1,901,187 as compared to a net increase in cash of $6,351,879$4,105,146 for the sixnine months ended JuneSeptember 30, 2007.
In 2007, we lent approximately $2 million of the net proceeds received from the sale of $3 million principal amount convertible debt to Lotus East, which they used as working capital. These advances are unsecured and interest free. During the first quarter of 2008, we used a portion of the proceeds from the sale of equity to satisfy these notes, lent Lotus East an additional $1.6 million and retained the balance to fund our operating expenses. We have no operations other than the Contractual Arrangements with Lotus East and, accordingly, we are dependent upon the quarterly service fees due us to provide cash to pay our operating expenses. Such payments have not been tendered to us and those funds are being retained by Lotus East to fund their operations. At JuneSeptember 30, 2008, Lotus East owned us approximately $13.5million$17.6 million for such fees and we do not know when such funds will be paid to us. Our CEO is also the CEO and principal shareholder of Lotus East. Accordingly, we are solely reliant upon his judgment to ensure that the funds advanced to Lotus East are repaid to us. If these funds should not be repaid, or if Lotus East should continue to withhold payment of the quarterly service fee due us under the Contractual Arrangement, it is possible that we will not have sufficient funds to pay our operating expenses in future periods.
Other than our existing cash we presently have no other alternative source of working capital. We believe that our working capital may not be sufficient to fund our current operations for the next 12 months unless Lotus East pays us the amounts due to us. Lotus East has historically funded its capital expenditures from their working capital and has advised us that they believe this capital is sufficient for their current needs. Lotus East has contractual commitments for approximately $80.6$65.5 million related to a Technology Transfer Agreement and the construction of the new manufacturing facility. While it intends to fund the costs associated with the Technology Transfer Agreement and a portion of the construction of the new manufacturing facility with its existing working capital, it is dependent upon the continued growth of its operations and prompt payment of outstanding accounts receivables by its customers to ensure that it has sufficient cash for these commitments. In addition, its ability to fully fund the costs associated with the new manufacturing facility is materially dependent upon its ability to secured bank financing and/or government grants. As the banking industry is tightening the credit/lending policy, we expect the bank financing will become more challenging and the time to obtain the bank funding might be longer than expected. Although the Chinese government has recently announced an economic simulation plan, there is no guarantee that we will successfully be awarded the government grant. As it has no firm commitments for either, while its management believes the company will be successful in securing the necessary funding based uponthrough its preliminaryincreasing revenue, quicker collections on receivables, current discussions with various commercial banks there are no assurances the funding will be available in the amounts or at the time required to meet Liang Fang'sFang’s commitment.
However, the ability of Lotus East to raise any significant capital to expand their operations is very limited. We believe that it is in our best long term interests to assist Lotus East in their growth plans. Accordingly, it is likely that we will seek to raise working capital not only for our operating expense but to provide capital to Lotus East for these projects as well as providing working capital necessary for its ongoing operations and obligations. No assurances can be given that we will be successful in obtaining additional capital, or that such capital will be available in terms acceptable to our company.company, particularly given the current conditions in the capital markets. If we are unable to raise capital as necessary for our operations, and we were no longer able to timely file our reports with the Securities and Exchange Commission, our common stock would be removed from quotation on the OTC Bulletin Board. In this event, the ability of our stockholders to liquidate their investment in our company would be adversely impacted and it is possible that an event of default would occur under various agreements we are a party to with the purchases of our Series A Convertible Redeemable Preferred Stock and common stock purchase warrants.
Contractual Obligations and Off-Balance Sheet Arrangements
Contractual Obligations
We have certain fixed contractual obligations and commitments that include future estimated payments. Changes in our business needs, cancellation provisions, changing interest rates, and other factors may result in actual payments differing from the estimates. We cannot provide certainty regarding the timing and amounts of payments. We have presented below a summary of the most significant assumptions used in our determination of amounts presented in the tables, in order to assist in the review of this information within the context of our consolidated financial position, results of operations, and cash flows.
The following tables summarize our contractual obligations as of JuneSeptember 30, 2008, and the effect these obligations are expected to have on our liquidity and cash flows in future periods.
|
| Payments Due by Period | |||||||||||||
|
| Total |
| Less than 1 year |
| 1-3 Years |
| 3-5 Years |
| 5 Years + | |||||
Contractual Obligations: |
|
|
|
|
|
|
|
|
|
| |||||
Related Parties Indebtedness |
| $ | 5,843,522 |
| $ | 131,289 |
| $ | 656,446 |
| $ | - |
| $ | 5,055,787 |
Patent Purchase Obligations |
| $ | 7,002,086 |
| $ | 2,625,782 |
| $ | 4,376,304 |
| $ | - |
| $ | - |
Construction Obligations |
| $ | 58,517,017 |
| $ | 8,918,907 |
| $ | 49,598,110 |
| $ | - |
| $ | - |
Total Contractual Obligations: |
| $ | 71,362,625 |
| $ | 11,675,978 |
| $ | 54,630,860 |
| $ | 0 |
| $ | 5,055,787 |
Payments Due by Period | ||||||||||||||||
Total | Less than 1 year | 1-3 Years | 3-5 Years | 5 Years + | ||||||||||||
In Thousands | ||||||||||||||||
Contractual Obligations: | ||||||||||||||||
| Related Parties Indebtedness | 5,829,320 | 130,970 | $ | 654,850 | $ | 5,043,500 | ||||||||||
| Patent Purchase Obligations | $ | 6,985,069 | $ | 2,619,401 | $ | 4,365,668 | $ | - | ||||||||
| Construction Obligations | $ | 66,727,786 | $ | 16,483,309 | $ | 50,244,477 | $ | - | $ | - | ||||||
| Total Contractual Obligations: | $ | 79,542,175 | $ | 19,233,680 | $ | 55,264,995 | $ | 0 | $ | 5,043,500 | ||||||
Off-balance Sheet Arrangements
As of the date of this report, we do not have any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to investors, however we have agreed to guarantee loans for Lotus East, if required. As of the date of this report, we have not entered into any guarantee arrangements with Lotus East. The term "off-balance“off-balance sheet arrangement"arrangement” generally means any transaction, agreement or other contractual arrangement to which an entity unconsolidated with us is a party, under which we have: (i) any obligation arising under a guarantee contract, derivative instrument or variable interest; or (ii) a retained or contingent interest in assets transferred to such entity or similar arrangement that serves as credit, liquidity or market risk support for such assets.
Item 3.Quantitative and Qualitative Disclosures About Market Risk.
Not applicable for a smaller reporting company.
Item 4T.Controls and Procedures.
Evaluation of Disclosure Controls and Procedures. We maintain "disclosure“disclosure controls and procedures"procedures” as such term is defined in Rule 13a-15(e) under the Securities Exchange Act of 1934. In designing and evaluating our disclosure controls and procedures, our management recognized that disclosure controls and procedures, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of disclosure controls and procedures are met. Additionally, in designing disclosure controls and procedures, our management necessarily was required to apply its judgment in evaluating the cost-benefit relationship of possible disclosure controls and procedures. The design of any disclosure controls and procedures also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Our management, including our Chief Executive Officer and Chief Financial Officer, has evaluated the effectiveness of our disclosure controls and procedures as of the end of the period covered by this report. Based on such evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that, as of the end of the period covered by this report, our disclosure controls and procedures were not effective:
• | to give reasonable assurance that the information required to be disclosed by us in reports that we file under the Securities Exchange Act of 1934 is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange |
• | to ensure that information required to be disclosed in the reports that we file or submit under the Securities Exchange Act of 1934 is accumulated and communicated to our management, including our CEO and our CFO, to allow timely decisions regarding required disclosure. |
Our internal accounting staff was primarily engaged in ensuring compliance with PRC accounting and reporting requirements for our operating affiliates and their U.S. GAAP knowledge was limited. As a result, majority of our internal accounting staff, on a consolidated basis, is relatively inexperienced with U.S. GAAP and the related internal control procedures required of U.S. public companies. Although our accounting staff is professional and experienced in accounting requirements and procedures generally accepted in the PRC, management has determined that they require additional training and assistance in U.S. GAAP matters. Management has determined that our internal audit function is also significantly deficient due to insufficient qualified resources to perform internal audit functions. Finally, management determined that the lack of an Audit Committee of our Board of Directors also contributed to insufficient oversight of our accounting and audit functions.
In order to correct the foregoing weaknesses, we have taken the following remediation measures:
• | In first quarter of 2008, under our CEO and |
• | We have started searching for an independent director qualified to serve on an audit committee to be established by our Board of Directors and we anticipate that our Board of Directors will also establish a compensation committee to be headed by one of an independent director. As of the date of this report, we have not selected a suitable candidate for the independent director. |
Due to our sizeoperational needs and nature of the business, segregation of all conflicting duties may not always be possible and may not be economically feasible. However, to the extent possible, we will implement procedures to assure that the initiation of transactions, the custody of assets and the recording of transactions will be performed by separate individuals.
Changes in Internal Control over Financial Reporting. There have been no changes in our internal control over financial reporting during our last fiscal quarter that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
PART II - OTHER INFORMATION
Item 1. Legal Proceedings
Item 1A. Risk Factors.
Not applicable to smaller reporting company.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds
None.
Item 4. Submissions of Matters to a Vote of Security Holders
None.
Item 5. Other Information
None.
Item 6. Exhibits
No. | Description | |
Rule 13a-14(a)/ 15d-14(a) Certification of Chief Executive Officer | ||
Rule 13a-14(a)/ 15d-14(a) Certification of Chief Financial Officer | ||
Section 1350 Certification of Chief Executive Officer | ||
Section 1350 Certification of Chief Financial Officer |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
Lotus Pharmaceuticals, Inc.
Date: | By: | /s/ Liu Zhong Yi |
Liu Zhong Yi
Chief Executive Officer and President, principal executive officer
Date: | By: | /s/ Adam Wasserman |