UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-Q
☒QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the quarterly period ended March 31, 2021June 30, 2022
or
☐TRANSITION REPORT UNDERPURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from _____________ to _____________
Commission file number: 01-39834
Clene Inc.CLENE INC.
(Exact name of registrant as specified in its charter)
Delaware | | 85-2828339 |
Delaware |
| 85-2828339 |
(State or other jurisdiction of
incorporation or organization) |
| (I.R.S. Employer
Identification No.) |
| | |
6550 South Millrock Drive, Suite G50 Salt Lake City, Utah |
| 84121 |
(Address of principal executive offices) |
| (Zip Code) |
Registrant’s telephone number, including area code: (801) 676 9695(801) 676-9695
(Former name, former address, and former fiscal year, if changed since last report.): N/A
Securities registered pursuant to Section 12(b) of the Act:
| | | | |
Title of each class |
| Trading Symbol(s) |
| Name of each exchange on
which registered |
Common stock,Stock, $0.0001 par value |
| CLNN |
| The Nasdaq Capital Market |
Warrants, to acquire one-half of one share of Common Stock for $11.50 per share |
| CLNNW |
| The Nasdaq Capital Market |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| | | |
Large accelerated filer | ☐ | Accelerated filer | ☐ |
Non-accelerated filer | ☒ | Smaller reporting company | ☒ |
|
| Emerging Growth Companygrowth company | ☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
The number of shares outstanding of the Registrant’s Common Stockcommon stock as of May 7, 2021August 10, 2022 was 59,574,382.63,501,984.
CLENE INC.
Quarterly Report on Form 10-Q for the PeriodQuarter Ended March 31, 2021June 30, 2022
PART I – I—FINANCIAL INFORMATION
Throughout this Quarterly Report on Form 10-Q (the “Quarterly Report”), the “Company,” and references to “we,” “us,” or similar such references should be understood to be references to the combined company, Clene Inc. When this Quarterly Report references “Clene” and describes the business of Clene, it refers to the business of Clene Nanomedicine, Inc. and its subsidiaries, prior to the consummation of the business combination (referred to throughout as the “Reverse Recapitalization”). Following the date of the Reverse Recapitalization, references to “Clene” should be understood to reference Clene Inc. Given that the business combination is accounted for as a Reverse Recapitalization, as described in more detail below, and the accounting acquirer is Clene Nanomedicine, Inc., the post-Reverse Recapitalization financial statements included in this Quarterly Report show the condensed consolidated balances and transactions of the Company and Clene as well as comparative financial information of Clene (the acquirer for accounting purposes).
ITEMItem 1. FINANCIAL STATEMENTSFinancial Statements
CLENE INC.
CONDENSED CONSOLIDATED BALANCE SHEETS
(Amounts inIn thousands, except share and per share amounts)
(Unaudited)
| | | | | | | | |
| | June 30, | | | December 31, | |
| | 2022 | | | 2021 | |
ASSETS | | | | | | |
Current assets: | | | | | | |
Cash and cash equivalents | | $ | 7,253 | | | $ | 50,288 | |
Marketable securities | | | 19,033 | | | | 0 | |
Accounts receivable | | | 0 | | | | 49 | |
Inventory | | | 107 | | | | 41 | |
Prepaid expenses and other current assets | | | 5,194 | | | | 4,205 | |
Total current assets | | | 31,587 | | | | 54,583 | |
Restricted cash | | | 58 | | | | 58 | |
Right-of-use assets | | | 4,808 | | | | 3,250 | |
Property and equipment, net | | | 8,089 | | | | 5,172 | |
TOTAL ASSETS | | $ | 44,542 | | | $ | 63,063 | |
| | | | | | |
LIABILITIES AND STOCKHOLDERS’ EQUITY | | | | | | |
Current liabilities: | | | | | | |
Accounts payable | | $ | 4,526 | | | $ | 1,923 | |
Accrued liabilities | | | 2,566 | | | | 3,610 | |
Operating lease obligations, current portion | | | 440 | | | | 347 | |
Finance lease obligations, current portion | | | 123 | | | | 146 | |
Total current liabilities | | | 7,655 | | | | 6,026 | |
Operating lease obligations, net of current portion | | | 5,858 | | | | 4,370 | |
Finance lease obligations, net of current portion | | | 55 | | | | 97 | |
Notes payable | | | 15,551 | | | | 14,484 | |
Convertible notes payable | | | 4,709 | | | | 4,598 | |
Common stock warrant liability | | | 167 | | | | 474 | |
Clene Nanomedicine contingent earn-out | | | 9,847 | | | | 18,100 | |
Initial Stockholders contingent earn-out | | | 1,263 | | | | 2,317 | |
TOTAL LIABILITIES | | | 45,105 | | | | 50,466 | |
Commitments and contingencies (Note 11) | | | | | | |
Stockholders’ equity (deficit): | | | | | | |
Common stock, $0.0001 par value: 150,000,000 shares authorized; 63,421,908 and 62,312,097 shares issued and outstanding at June 30, 2022 and December 31, 2021, respectively | | | 6 | | | | 6 | |
Additional paid-in capital | | | 180,534 | | | | 175,659 | |
Accumulated deficit | | | (181,189 | ) | | | (163,301 | ) |
Accumulated other comprehensive income | | | 86 | | | | 233 | |
TOTAL STOCKHOLDERS’ EQUITY (DEFICIT) | | | (563 | ) | | | 12,597 | |
TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) | | $ | 44,542 | | | $ | 63,063 | |
| | | March 31, | | | December 31, | |
| | | 2021 | | | 2020 | |
| | | | | | | |
| ASSETS | | | | | | | | |
| Current assets: | | | | | | | | |
| Cash | | $ | 48,041 | | | $ | 59,275 | |
| Accounts receivable | | | 123 | | | | 21 | |
| Inventory | | | 355 | | | | 191 | |
| Prepaid expenses and other current assets | | | 4,824 | | | | 3,502 | |
| Total current assets | | | 53,343 | | | | 62,989 | |
| Right-of-use assets | | | 1,006 | | | | 1,029 | |
| Property and equipment, net | | | 4,182 | | | | 4,225 | |
| TOTAL ASSETS | | $ | 58,531 | | | $ | 68,243 | |
| | | | | | | | | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) | | | | | | | | |
| Current liabilities: | | | | | | | | |
| Accounts payable | | $ | 739 | | | $ | 1,124 | |
| Accrued liabilities | | | 2,730 | | | | 3,960 | |
| Income tax payable | | | 164 | | | | 164 | |
| Deferred revenue from related parties | | | 112 | | | | 112 | |
| Operating lease obligations, current portion | | | 202 | | | | 194 | |
| Finance lease obligations, current portion | | | 139 | | | | 190 | |
| Clene Nanomedicine contingent earn-out, current portion | | | - | | | | 5,924 | |
| Total current liabilities | | | 4,086 | | | | 11,668 | |
| Operating lease obligations, net of current portion | | | 1,723 | | | | 1,785 | |
| Finance lease obligations, net of current portion | | | 210 | | | | 205 | |
| Notes payable | | | 1,844 | | | | 1,949 | |
| Deferred income tax | | | 214 | | | | 260 | |
| Clene Nanomedicine contingent earn-out, net of current portion | | | 77,663 | | | | 46,129 | |
| Initial Shareholders contingent earn-out | | | 8,867 | | | | 5,906 | |
| TOTAL LIABILITIES | | | 94,607 | | | | 67,902 | |
| Stockholders’ equity (deficit): | | | | | | | | |
| Common stock, $0.0001 par value: 100,000,000 shares authorized; 59,574,382 and 59,526,171 shares issued and outstanding at March 31, 2021 and December 31, 2020, respectively | | | 6 | | | | 6 | |
| Additional paid-in capital | | | 156,886 | | | | 153,571 | |
| Accumulated deficit | | | (193,317 | ) | | | (153,561 | ) |
| Accumulated other comprehensive income | | | 349 | | | | 325 | |
| TOTAL STOCKHOLDERS’ EQUITY (DEFICIT) | | | (36,076 | ) | | | 341 | |
| TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) | | $ | 58,531 | | | $ | 68,243 | |
See accompanying notes to the condensed consolidated financial statements.
1
CLENE INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(Amounts inIn thousands, except share and per share amounts)
(Unaudited)
| | | | | | | | | | | | | | | | |
| | Three Months Ended June 30, | | | Six Months Ended June 30, | |
| | 2022 | | | 2021 | | | 2022 | | | 2021 | |
Revenue: | | | | | | | | | | | | |
Product revenue | | $ | 2 | | | $ | 138 | | | $ | 9 | | | $ | 337 | |
Royalty revenue | | | 33 | | | | 63 | | | | 56 | | | | 77 | |
Total revenue | | | 35 | | | | 201 | | | | 65 | | | | 414 | |
Operating expenses: | | | | | | | | | | | | |
Cost of revenue | | | 0 | | | | 555 | | | | 0 | | | | 798 | |
Research and development | | | 9,166 | | | | 6,472 | | | | 17,746 | | | | 12,747 | |
General and administrative | | | 4,464 | | | | 6,949 | | | | 9,250 | | | | 12,339 | |
Total operating expenses | | | 13,630 | | | | 13,976 | | | | 26,996 | | | | 25,884 | |
Loss from operations | | | (13,595 | ) | | | (13,775 | ) | | | (26,931 | ) | | | (25,470 | ) |
Other income (expense), net: | | | | | | | | | | | | |
Interest expense | | | (751 | ) | | | (26 | ) | | | (1,533 | ) | | | (577 | ) |
Gain on extinguishment of notes payable | | | 0 | | | | 0 | | | | 0 | | | | 647 | |
Gain on termination of lease | | | 0 | | | | 0 | | | | 420 | | | | 0 | |
Change in fair value of common stock warrant liability | | | 20 | | | | 133 | | | | 2 | | | | 133 | |
Change in fair value of Clene Nanomedicine contingent earn-out | | | 8,310 | | | | 8,640 | | | | 8,253 | | | | (16,970 | ) |
Change in fair value of Initial Stockholders contingent earn-out | | | 1,066 | | | | 1,232 | | | | 1,054 | | | | (1,729 | ) |
Australia research and development credit | | | 356 | | | | 375 | | | | 655 | | | | 714 | |
Other income (expense), net | | | 60 | | | | (2 | ) | | | 192 | | | | 1 | |
Total other income (expense), net | | | 9,061 | | | | 10,352 | | | | 9,043 | | | | (17,781 | ) |
Net loss before income taxes | | | (4,534 | ) | | | (3,423 | ) | | | (17,888 | ) | | | (43,251 | ) |
Income tax benefit | | | 0 | | | | 72 | | | | 0 | | | | 144 | |
Net loss | | | (4,534 | ) | | | (3,351 | ) | | | (17,888 | ) | | | (43,107 | ) |
| | | | | | | | | | | | |
Other comprehensive loss: | | | | | | | | | | | | |
Unrealized loss on available-for-sale securities | | | (37 | ) | | | 0 | | | | (87 | ) | | | 0 | |
Foreign currency translation adjustments | | | (110 | ) | | | (61 | ) | | | (60 | ) | | | (37 | ) |
Total other comprehensive loss | | | (147 | ) | | | (61 | ) | | | (147 | ) | | | (37 | ) |
Comprehensive loss | | $ | (4,681 | ) | | $ | (3,412 | ) | | $ | (18,035 | ) | | $ | (43,144 | ) |
| | | | | | | | | | | | |
Net loss per share-- basic and diluted (Note 16) | | $ | (0.07 | ) | | $ | (0.05 | ) | | $ | (0.28 | ) | | $ | (0.71 | ) |
Weighted average common shares used to compute basic and diluted net loss per share | | | 63,335,271 | | | | 61,165,018 | | | | 63,095,400 | | | | 60,919,340 | |
| | | Three Months Ended
March 31, | |
| | | | 2021 | | | | 2020 | |
| | | | | | | | | |
| Revenue: | | | | | | | | |
| Product revenue | | $ | 199 | | | $ | 70 | |
| Royalty revenue | | | 14 | | | | - | |
| Total revenue | | | 213 | | | | 70 | |
| Operating expenses: | | | | | | | | |
| Cost of revenue | | | 243 | | | | 58 | |
| Research and development | | | 6,275 | | | | 3,202 | |
| General and administrative | | | 5,390 | | | | 812 | |
| Total operating expenses | | | 11,908 | | | | 4,072 | |
| Loss from operations | | | (11,695 | ) | | | (4,002 | ) |
| Other income (expense), net: | | | | | | | | |
| Interest expense | | | (551 | ) | | | (51 | ) |
| Gain on extinguishment of notes payable | | | 647 | | | | - | |
| Change in fair value of preferred stock warrant liability | | | - | | | | 112 | |
| Change in fair value of derivative liability | | | - | | | | 4 | |
| Change in fair value of Clene Nanomedicine contingent earn-out | | | (25,610 | ) | | | - | |
| Change in fair value of Initial Shareholders contingent earn-out | | | (2,961 | ) | | | - | |
| Australia research and development credit | | | 339 | | | | - | |
| Other income (expense), net | | | 3 | | | | (4 | ) |
| Total other income (expense), net | | | (28,133 | ) | | | 61 | |
| Net loss before income taxes | | | (39,828 | ) | | | (3,941 | ) |
| Income tax benefit | | | 72 | | | | - | |
| Net loss | | | (39,756 | ) | | | (3,941 | ) |
| Other comprehensive income: | | | | | | | | |
| Foreign currency translation adjustments | | | 24 | | | | 6 | |
| Total other comprehensive income | | | 24 | | | | 6 | |
| Comprehensive loss | | $ | (39,732 | ) | | $ | (3,935 | ) |
| | | | | | | | | |
| Net loss per share-- basic and diluted (Note 19) (1) | | | (0.66 | ) | | | (0.23 | ) |
| Weighted average common shares used to compute basic and diluted net loss per share (1) | | | 60,670,932 | | | | 17,357,505 | |
(1) | | Retroactively restated for the three months ended March 31, 2020 for the Reverse Recapitalization as described in Note 1 |
See accompanying notes to the condensed consolidated financial statements.
2
CLENE INC.
CONDENSED CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)(1)
(Amounts inIn thousands, except share and per share amounts)
(Unaudited)
| | | | | | | | | | | | | | | | | | | | | | | | |
| | Common Stock | | | Additional
Paid-In | | | Accumulated | | | Accumulated
Other
Comprehensive | | | Total
Stockholders’
Equity | |
| | Shares | | | Amount | | | Capital | | | Deficit | | | Income (Loss) | | | (Deficit) | |
Balances at December 31, 2021 | | | 62,312,097 | | | $ | 6 | | | $ | 175,659 | | | $ | (163,301 | ) | | $ | 233 | | | $ | 12,597 | |
Reclassification of common stock warrant liability to equity | | | — | | | | — | | | | 305 | | | | 0 | | | | 0 | | | | 305 | |
Exercise of stock options | | | 934,448 | | | | 0 | | | | 267 | | | | 0 | | | | 0 | | | | 267 | |
Stock-based compensation expense | | | — | | | | 0 | | | | 2,202 | | | | 0 | | | | 0 | | | | 2,202 | |
Unrealized loss on available-for-sale securities | | | — | | | | 0 | | | | 0 | | | | 0 | | | | (50 | ) | | | (50 | ) |
Foreign currency translation adjustment | | | — | | | | 0 | | | | 0 | | | | 0 | | | | 50 | | | | 50 | |
Net loss | | | — | | | | 0 | | | | 0 | | | | (13,354 | ) | | | 0 | | | | (13,354 | ) |
Balances at March 31, 2022 | | | 63,246,545 | | | $ | 6 | | | $ | 178,433 | | | $ | (176,655 | ) | | $ | 233 | | | $ | 2,017 | |
Exercise of stock options | | | 110,000 | | | | 0 | | | | 17 | | | | 0 | | | | 0 | | | | 17 | |
Stock-based compensation expense | | | — | | | | 0 | | | | 2,184 | | | | 0 | | | | 0 | | | | 2,184 | |
Issuance of common stock upon vesting of restricted stock awards | | | 65,363 | | | | 0 | | | | 0 | | | | 0 | | | | 0 | | | | 0 | |
Unrealized loss on available-for-sale securities | | | — | | | | 0 | | | | 0 | | | | 0 | | | | (37 | ) | | | (37 | ) |
Offering costs | | | — | | | | 0 | | | | (100 | ) | | | 0 | | | | 0 | | | | (100 | ) |
Foreign currency translation adjustment | | | — | | | | 0 | | | | 0 | | | | 0 | | | | (110 | ) | | | (110 | ) |
Net loss | | | — | | | | 0 | | | | 0 | | | | (4,534 | ) | | | 0 | | | | (4,534 | ) |
Balances at June 30, 2022 | | | 63,421,908 | | | $ | 6 | | | $ | 180,534 | | | $ | (181,189 | ) | | $ | 86 | | | $ | (563 | ) |
| | | | | | | | | | | | | | | | | | |
Balances at December 31, 2020 | | | 59,526,171 | | | | 6 | | | | 153,571 | | | | (153,561 | ) | | | 325 | | | | 341 | |
Exercise of stock options | | | 48,211 | | | | 0 | | | | 50 | | | | 0 | | | | 0 | | | | 50 | |
Stock-based compensation expense | | | — | | | | 0 | | | | 3,265 | | | | 0 | | | | 0 | | | | 3,265 | |
Foreign currency translation adjustment | | | — | | | | 0 | | | | 0 | | | | 0 | | | | 24 | | | | 24 | |
Net loss | | | — | | | | 0 | | | | 0 | | | | (39,756 | ) | | | 0 | | | | (39,756 | ) |
Balances at March 31, 2021 | | | 59,574,382 | | | $ | 6 | | | $ | 156,886 | | | $ | (193,317 | ) | | $ | 349 | | | $ | (36,076 | ) |
Issuance of common stock upon the private offering | | | 960,540 | | | | 0 | | | | 9,250 | | | | 0 | | | | 0 | | | | 9,250 | |
Exercise of stock options | | | 124,680 | | | | 0 | | | | 58 | | | | 0 | | | | 0 | | | | 58 | |
Stock-based compensation expense | | | — | | | | 0 | | | | 4,255 | | | | 0 | | | | 0 | | | | 4,255 | |
Issuance of common stock upon vesting of restricted stock awards | | | 21,989 | | | | 0 | | | | 0 | | | | 0 | | | | 0 | | | | 0 | |
Foreign currency translation adjustment | | | — | | | | 0 | | | | 0 | | | | 0 | | | | (61 | ) | | | (61 | ) |
Net loss | | | — | | | | 0 | | | | 0 | | | | (3,351 | ) | | | 0 | | | | (3,351 | ) |
Balances at June 30, 2021 | | | 60,681,591 | | | $ | 6 | | | $ | 170,449 | | | $ | (196,668 | ) | | $ | 288 | | | $ | (25,925 | ) |
| | | Redeemable | | | | | | | | | | | | | | | Accumulated | | | Total | |
| | | Convertible | | | | | | Additional | | | | | | Other | | | Stockholders’ | |
| | | Preferred Stock | | | Common Stock | | | Paid-In | | | Accumulated | | | Comprehensive | | | Equity | |
| | | Shares | | | Amount | | | Shares | | | Amount | | | Capital | | | Deficit | | | Income | | | (Deficit) | |
| Balances at December 31, 2020 | | | - | | | $ | - | | | | 59,526,171 | | | $ | 6 | | | $ | 153,571 | | | $ | (153,561 | ) | | $ | 325 | | | $ | 341 | |
| Exercise of stock options | | | | | | | | | | | 48,211 | | | | - | | | | 50 | | | | - | | | | - | | | | 50 | |
| Stock-based compensation expense | | | - | | | | - | | | | - | | | | - | | | | 3,265 | | | | - | | | | - | | | | 3,265 | |
| Foreign currency translation adjustment | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 24 | | | | 24 | |
| Net loss | | | - | | | | - | | | | - | | | | - | | | | - | | | | (39,756 | ) | | | - | | | | (39,756 | ) |
| Balances at March 31, 2021 | | | - | | | $ | - | | | | 59,574,382 | | | $ | 6 | | | $ | 156,886 | | | $ | (193,317 | ) | | $ | 349 | | | $ | (36,076 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balances at December 31, 2019 | | | 27,499,837 | | | $ | 72,661 | | | | 17,357,505 | | | $ | 2 | | | $ | 1,754 | | | $ | (69,571 | ) | | $ | 41 | | | $ | (67,774 | ) |
| Stock-based compensation expense | | | - | | | | - | | | | - | | | | - | | | | 171 | | | | - | | | | - | | | | 171 | |
| Foreign currency translation adjustment | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 6 | | | | 6 | |
| Net loss | | | - | | | | - | | | | - | | | | - | | | | - | | | | (3,941 | ) | | | - | | | | (3,941 | ) |
| Balances at March 31, 2020 | | | 27,499,837 | | | $ | 72,661 | | | | 17,357,505 | | | $ | 2 | | | $ | 1,925 | | | $ | (73,512 | ) | | $ | 47 | | | $ | (71,538 | ) |
| (1) | Retroactively restated for the Reverse Recapitalization as described in Note 1 |
See accompanying notes to the condensed consolidated financial statements.
3
CLENE INC.
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(Amounts inIn thousands)
(Unaudited)
| | | | | | | | |
| | Six Months Ended June 30, | |
| | 2022 | | | 2021 | |
Cash flows from operating activities: | | | | | | |
Net loss | | $ | (17,888 | ) | | $ | (43,107 | ) |
Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | |
Depreciation | | | 482 | | | | 499 | |
Non-cash lease expense | | | 183 | | | | 46 | |
Change in fair value of common stock warrant liability | | | (2 | ) | | | (133 | ) |
Change in fair value of Clene Nanomedicine contingent earn-out | | | (8,253 | ) | | | 16,970 | |
Change in fair value of Initial Stockholders contingent earn-out | | | (1,054 | ) | | | 1,729 | |
Stock-based compensation expense | | | 4,386 | | | | 7,520 | |
Gain on extinguishment of notes payable | | | 0 | | | | (647 | ) |
Gain on termination of lease | | | (420 | ) | | | 0 | |
Accretion of debt discount | | | 442 | | | | 0 | |
Non-cash interest expense | | | 72 | | | | 389 | |
Changes in operating assets and liabilities: | | | | | | |
Accounts receivable | | | 49 | | | | (48 | ) |
Inventory | | | (66 | ) | | | 137 | |
Prepaid expenses and other current assets | | | (989 | ) | | | (1,528 | ) |
Accounts payable | | | 1,528 | | | | 565 | |
Accrued liabilities | | | (1,044 | ) | | | 125 | |
Deferred income tax | | | 0 | | | | (119 | ) |
Operating lease obligations | | | (240 | ) | | | (111 | ) |
Net cash used in operating activities | | | (22,814 | ) | | | (17,713 | ) |
Cash flows from investing activities: | | | | | | |
Purchases of marketable securities | | | (24,549 | ) | | | 0 | |
Proceeds from maturity of marketable securities | | | 2,500 | | | | 0 | |
Proceeds from sale of marketable securities | | | 3,016 | | | | 0 | |
Purchases of property and equipment | | | (1,824 | ) | | | (420 | ) |
Net cash used in investing activities | | | (20,857 | ) | | | (420 | ) |
Cash flows from financing activities: | | | | | | |
Proceeds from exercise of stock options | | | 284 | | | | 108 | |
Payments of finance lease obligations | | | (65 | ) | | | (83 | ) |
Proceeds from the issuance of notes payable | | | 694 | | | | 15,000 | |
Payments of debt issuance costs | | | (30 | ) | | | (471 | ) |
Payments of notes payable | | | 0 | | | | (5 | ) |
Proceeds from the private placement | | | 0 | | | | 9,250 | |
Payment of deferred offering costs | | | (100 | ) | | | (1,901 | ) |
Net cash provided by financing activities | | | 783 | | | | 21,898 | |
Effect of foreign exchange rate changes on cash and restricted cash | | | (147 | ) | | | (33 | ) |
Net increase (decrease) in cash, cash equivalents and restricted cash | | | (43,035 | ) | | | 3,732 | |
Cash, cash equivalents and restricted cash – beginning of period | | | 50,346 | | | | 59,275 | |
Cash, cash equivalents and restricted cash – end of period | | $ | 7,311 | | | $ | 63,007 | |
| | | | | | |
Reconciliation of cash, cash equivalents and restricted cash to the consolidated balance sheets | | | | | | |
Cash and cash equivalents | | | 7,253 | | | | 63,007 | |
Restricted cash | | | 58 | | | | 0 | |
Cash, cash equivalents and restricted cash | | $ | 7,311 | | | $ | 63,007 | |
| | | Three Months Ended | |
| | | March 31, | |
| | | 2021 | | | 2020 | |
| Cash flows from operating activities: | | | | | | | | |
| Net loss | | $ | (39,756 | ) | | $ | (3,941 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | |
| Depreciation | | | 244 | | | | 218 | |
| Non-cash lease expense | | | 22 | | | | 40 | |
| Change in fair value of preferred stock warrant liability | | | - | | | | (112 | ) |
| Change in fair value of Clene Nanomedicine contingent earn-out | | | 25,610 | | | | - | |
| Change in fair value of Initial Shareholders contingent earn-out | | | 2,961 | | | | - | |
| Stock-based compensation expense | | | 3,265 | | | | 171 | |
| Change in fair value of derivative | | | - | | | | (4 | ) |
| Gain on extinguishment of debt | | | (647 | ) | | | - | |
| Accretion of debt discount | | | - | | | | 20 | |
| Increase in interest accrued on notes payable | | | 543 | | | | 20 | |
| Changes in operating assets and liabilities: | | | | | | | | |
| Inventory | | | (164 | ) | | | - | |
| Accounts receivable | | | (103 | ) | | | (70 | ) |
| Prepaid expenses and other current assets | | | (1,321 | ) | | | (91 | ) |
| Accounts payable | | | 161 | | | | 604 | |
| Accrued liabilities | | | 125 | | | | (79 | ) |
| Deferred income tax | | | (46 | ) | | | - | |
| Operating lease obligations | | | (55 | ) | | | (27 | ) |
| Net cash used in operating activities | | | (9,161 | ) | | | (3,251 | ) |
| | | | | | | | | |
| Cash flows from investing activities: | | | | | | | | |
| Purchases of property and equipment | | | (203 | ) | | | (23 | ) |
| Net cash used in investing activities | | | (203 | ) | | | (23 | ) |
| | | | | | | | | |
| Cash flows from financing activities: | | | | | | | | |
| Proceeds from exercise of stock options | | | 50 | | | | - | |
| Payments of deferred offering costs | | | (1,901 | ) | | | - | |
| Payments of finance lease obligations | | | (45 | ) | | | (53 | ) |
| Proceeds from the issuance of note payable | | | - | | | | 1,600 | |
| Net cash provided by (used in) financing activities | | | (1,896 | ) | | | 1,547 | |
| | | | | | | | | |
| Effect of foreign exchange rate changes on cash | | | 26 | | | | 55 | |
| | | | | | | | | |
| Net decrease in cash | | | (11,234 | ) | | | (1,672 | ) |
| | | | | | | | | |
| Cash – beginning of period | | | 59,275 | | | | 8,788 | |
| | | | | | | | | |
| Cash – end of period | | $ | 48,041 | | | $ | 7,116 | |
| | | | | | | | | |
| Supplemental disclosure of non-cash investing and financing activities: | | | | | | | | |
| Issuance of derivative instrument related to convertible notes | | $ | - | | | $ | 197 | |
| | | | | | | | | |
| Supplemental disclosure: | | | | | | | | |
| Cash paid for interest expense | | $ | 8 | | | $ | 11 | |
See accompanying notes to the condensed consolidated financial statements.
4
CLENE INC.
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS (Continued)
(In thousands)
(Unaudited)
| | | | | | | | |
| | Six Months Ended June 30, | |
| | 2022 | | | 2021 | |
Supplemental disclosure of non-cash investing and financing activities: | | | | | | |
Lease liability arising from obtaining right-of-use assets, leasehold improvements, and lease incentives | | $ | 2,343 | | | $ | 0 | |
Lease incentive realized | | $ | 500 | | | $ | 0 | |
Lease liability settled through termination of lease | | $ | 602 | | | $ | 0 | |
Reclassification of common stock warrant liability to permanent equity | | $ | 305 | | | $ | 0 | |
Purchases of property and equipment in accounts payable | | $ | 1,075 | | | | |
Common stock warrant liability recorded at issuance of notes payable | | $ | — | | | $ | 1,457 | |
Supplemental disclosures: | | | | | | |
Cash paid for interest expense | | $ | 1,013 | | | $ | 188 | |
See accompanying notes to the condensed consolidated financial statements.
5
CLENE INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
Note 1. Nature of the Business
Clene Inc. (formerly Chelsea Worldwide, Inc.) (the “Company,” “we,” “us,” or similar such references) is a biopharmaceuticalclinical-stage pharmaceutical company focused onpioneering the discovery, development, and commercialization of novel clean-surfaced nanocrystal drugs.nanotechnology therapeutics. We have developed an electrocrystal chemistryelectro-crystal-chemistry drug development platform in which nanocrystals within a suspension are the therapeutic drug. Utilizing technology to createenables production of concentrated, stable, highly active, clean-surfaced nanocrystal drug suspensions, our platform has producedsuspensions. We have multiple drug assets of which our lead assets are currently in development for useapplications in neurologicalneurology, infectious disease, and oncology. Our efforts are currently focused on addressing the high unmet medical needs in two areas: first, those related to central nervous system disorders including Amyotrophic Lateral Sclerosis (“ALS”), Multiple Sclerosis (“MS”), and Parkinson’s Disease (“PD”); and second, those related to COVID-19, a highly infectious diseases, among others, such as a study for treatment of COVID-19 coronavirus pandemic. Secondaryviral respiratory disease with serious and sometimes fatal co-morbidities. Our patented electro-crystal-chemistry manufacturing platform further enables us to our drug development, as part of our identification of potential drug assets, we have also identified certain mineral solutions as dietary supplements. Ourdevelop very low concentration dietary supplements may also be commercialized byto advance the health and well-being of broad populations. These dietary supplements can vary greatly and include nanocrystals of varying composition, shapes and sizes as well as ionic solutions with diverse metallic constituents. Dietary supplements are marketed and distributed through our wholly owned subsidiary, dOrbital, Inc., or through an exclusive license with 4Life Research LLC (“4Life”), a related party as discussed in Note 20.
The accompanying condensed consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries, Clene Nanomedicine, Inc. (“Clene Nanomedicine”), a subsidiary incorporated in Delaware, Clene Australia Pty Ltd (“Clene Australia”), a subsidiary incorporated in Australia, and dOrbital, Inc., a subsidiary incorporated in Delaware, after elimination of all intercompany accounts and transactions. The wholly-owned subsidiary, Clene Netherlands B.V. (“Clene Netherlands”) was established subsequent to the quarter ended March 31, 2021 and has no financial positions or operations and therefore is not included in the condensed consolidated financial statements.
Reverse Recapitalization with Tottenham Acquisition 1 Limited
On December 30, 2020 (the “Closing Date”), Chelsea Worldwide, Inc., our predecessor company, consummated the previously announced business combination (referred to as the “Reverse Recapitalization”) pursuant to a merger agreement, dated as of September 1, 2020 (the “Merger Agreement”), by and among Clene Nanomedicine, Tottenham Acquisition I Limited (“Tottenham” or “TOTA”), Chelsea Worldwide Inc., a Delaware corporation and wholly-owned subsidiary of Tottenham (“PubCo”), Creative Worldwide Inc., a Delaware corporation and wholly owned subsidiary of PubCo (“Merger Sub”), and Fortis Advisors LLC, a Delaware limited liability company as the representative of the Company’s stockholders (“Stockholders’ Representative”). Prior to the Reincorporation Merger discussed below, Tottenham was incorporated in the British Virgin Islands as a blank check company for the purpose of entering into a merger, share exchange, asset acquisition, stock purchase, recapitalization, reorganization or other similar business combination with one or more businesses or entities.
The Reverse Recapitalization was effected in two steps: (i) Tottenham was reincorporated to the state of Delaware by merging with and into PubCo (the “Reincorporation Merger”); (ii) promptly following the Reincorporation Merger, Merger Sub was merged with and into Clene Nanomedicine, resulting in Clene Nanomedicine becoming a wholly-owned subsidiary of PubCo (the “Acquisition Merger”). On the Closing Date, PubCo changed its name from Chelsea Worldwide Inc. to Clene Inc. and listed its shares of common stock, par value $0.0001 per share (“Common Stock”) on the Nasdaq Stock Exchange (the “Nasdaq”) under the symbol “CLNN.”
Upon the consummation of the Reverse Recapitalization, each Tottenham ordinary share issued and outstanding immediately prior to the effective time of the Reincorporation Merger (excluding certain shares to be canceled pursuant to the Merger Agreement, any redeemed shares and any dissenting), was automatically cancelled and cease to exist and (i) for each Tottenham ordinary share, the Company issued to each shareholder one validly-issued share of the Company’s Common Stock; (ii) each warrant to purchase one half (1/2) of one Tottenham Ordinary Share converted into a warrant to purchase one-half of one share of the Company’s Common Stock; (iii) each right exchangeable into one-tenth (1/10) of one Tottenham ordinary share converted into a right exchangeable for one-tenth (1/10) of one share of the Company’s Common Stock; provided, however, that no fractional shares were issued and all fractional shares were rounded down to the nearest whole share.
On the Closing Date, each share of Clene Nanomedicine common stock was cancelled and the holders thereof in exchange received 0.1389 newly-issued shares of Clene Inc. Common Stock, which is the exchange ratio (the “Exchange Ratio”). Pursuant to the Merger Agreement, 5% of the aggregate amount of the closing payment shares, or 2,716,958 shares will be held in escrow to satisfy any indemnification obligation incurred and will be released six months after the closing of the Reverse Recapitalization. In addition, each share of Clene Nanomedicine’s preferred stock outstanding immediately prior to the closing of the Reverse Recapitalization was converted into the right to receive the Company’s Common Stock based on the same Exchange Ratio. All outstanding warrants exercisable for common stock in Clene Nanomedicine (other than warrants that expired, were exercised or were deemed automatically net exercised immediately prior to the Acquisition Merger) were exchanged for warrants exercisable for the Company Common Stock with the same terms and conditions except adjusted by the aforementioned Exchange Ratio. At the closing of the Reverse Recapitalization, each stock option of Clene Nanomedicine common stock was cancelled and the holders thereof in exchange received 0.1320 newly issued stock options of the Company’s Common Stock, which is 95% of the Exchange Ratio. Pursuant to the Merger Agreement, the Company issued 370,101 of restricted stock units (“RSUs”) to the option holders which complements the 5% closing payment shares held in escrow for Clene Nanomedicine common shareholders. The modification of the stock options did not result in a material incremental compensation expense upon closing of the Reverse Recapitalization.
In addition, the Company issued 1,136,961 RSUs to option holders to complement the earn-out payments that would contingently be issued to certain current Clene Nanomedicine’s shareholders upon the achievement of milestones. See Note 3 for the milestones detail.
The proceeds received from the Reverse Recapitalization is $3.7 million, net of offering costs of $5.9 million which excludes the fair value of common shares issued as a payment of related offering costs.
In connection with Tottenham’s initial public offering in August 2018, Tottenham issued to Chardan Capital Markets, LLC (“Chardan”), options to purchase 220,000 units at $10.00 per unit. Each of the units consists of one and one-tenth shares of Tottenham’s ordinary shares for $10.00 per share and one warrant to purchase one-half of one of Tottenham’s ordinary shares at an exercise price of $11.50 per share (the “Chardan Unit Purchase Option”). In connection with the Reverse Recapitalization, the Chardan Unit Purchase Option was converted into one Company unit purchase option. The warrants included in the Chardan Unit Purchase Option (the “Chardan Unit Purchase Option Warrants”) are exercisable upon the completion of the Reverse Recapitalization and will expire five years after the consummation of the Reverse Recapitalization (i.e., December 30, 2025) (see Note 10)17).
Going Concern
Also, in connection with the Reverse Recapitalization, 644,164 shares of the Company’s Common Stock were issued to LifeSci Capital LLC (“LifeSci”), as payment for advisory services rendered in connection with the Reverse Recapitalization (see Notes 3 and 18).
The transaction was accounted for asWe incurred a “reverse recapitalization” in accordance with GAAP. Under this method of accounting, Tottenham was treated as the “acquired” company for financial reporting purposes. This determination is primarily based on the fact that subsequent to the Reverse Recapitalization, Clene Nanomedicine’s stockholders have a majority of the voting power of the combined company, Clene Nanomedicine comprises all of the ongoingloss from operations of the combined entity, Clene Nanomedicine comprises a majority of the governing body of the combined company,$13.6 million and Clene Nanomedicine’s senior management comprises all of the senior management of the combined company. Accordingly,$13.8 million for accounting purposes, this transaction was treated as the equivalent of Clene Nanomedicine issuing shares for the net assets of Tottenham, accompanied by a recapitalization. The shares and net loss per common share, prior to the Reverse Recapitalization, have been retroactively restated as shares reflecting the Exchange Ratio established in the Reverse Recapitalization (0.1389 Clene Inc. shares for 1 Clene Nanomedicine share). The net assets of Tottenham were recorded at historical costs, with no goodwill or other intangible assets recorded. Operations prior to the Reverse Recapitalization are those of Clene Nanomedicine.
The PIPE Offering
Prior to the completion of the Reverse Recapitalization on December 30, 2020, the Company entered into a subscription agreement on December 28, 2020, with various investors. Pursuant to the subscription agreements, the Company issued 2,239,500 shares of the Company’s Common Stock (the “PIPE Shares”) at a price of $10.00 per share with net proceeds of $22.2 million. The purpose of the PIPE is to fund general corporate expenses. In addition, investors in the PIPE offering also received warrants to purchase a number of shares equal to one-half (1/2) of the number of PIPE Shares, totaling 1,119,750 shares of the Company’s Common Stock, at an exercise price of $0.01 per share for each of the PIPE Shares (the “PIPE Warrants”), subject to a 180-day holding period.
See Note 3 – Reverse Recapitalization with Tottenham and Clene Nanomedicine for additional details on Reverse Recapitalization.
Registration Statement
We filed a registration statement on Form S-1 (file number 333-253173) to register 4,541,481 shares of Common Stock underlying outstanding warrants that we have previously issued, among which 2,517,500 and 904,231 warrants were originally issued by Tottenham and Clene Nanomedicine, respectively, prior to the closing of the Reverse Recapitalization, and 1,119,750 warrants were issued as part of the PIPE offering in connection with the closing of the Reverse Recapitalization. We will receive aggregate proceeds of $30.7 million if all of these warrants are exercised. On April 19, 2021, the registration statement was declared effective by the Securities and Exchange Commission (the “SEC”). In connection with the registration statement on Form S-1, we incurred $27 thousand of certain offering costs during the three months ended MarchJune 30, 2022 and 2021, respectively; and $26.9 million and $25.5 million for the six months ended June 30, 2022 and 2021, respectively. Our accumulated deficit was $181.2 million and $163.3 million as of June 30, 2022 and December 31, 2021, recognizedrespectively. Our cash, cash equivalents, and marketable securities totaled $26.3 million and $50.3 million as expense within generalof June 30, 2022 and administrative expensesDecember 31, 2021, respectively, and net cash used in operating activities was $22.8 million and $17.7 million for the condensed consolidated statement of operations and comprehensive loss during the threesix months ended March 31, 2021.June 30, 2022 and 2021, respectively.
Accounting for Warrants Issued by SPACs
On April 12, 2021, the Staff of the SEC (the “Staff”) released the Staff Statement on Accounting and Reporting Considerations for Warrants Issued by Special Purpose Acquisition Companies (“SPACs”) (the “Statement”). The Statement provides additional information regarding the Staff’s views about equity treatment for SPAC-issued warrants, suggesting that certain nearly ubiquitous features in SPAC warrants require the warrants to be classified as liabilities on the SPAC’s balance sheet rather than as equity. It also highlights financial reporting considerations if a SPAC determines it has misclassified its warrants. As a result of the Statement, the Company re-evaluated the accounting for TOTA’s Public Warrants, Private Warrants, and Chardan Unit Purchase Option Warrants as of the date of their issuance in August 2018 and has concluded that they were appropriately classified as equity. The provisions highlighted in the Statement as potentially requiring liability classification are not featured in the Warrant Agreement and in the Chardan Unit Purchase Option Agreement, and the terms of the warrants do not preclude them from being considered indexed to the entity’s own stock and classified as equity.
Liquidity
We have incurred significant losses and negative cash flows from operations since our inception. We incurred net losses of $39.8 million and $3.9 million for the three months ended March 31, 2021 and 2020. As of March 31, 2021, our cash totaled $48.0 million, and our accumulated deficit was $193.3 million. As of December 31, 2020, our cash totaled $59.3 million, and our accumulated deficit was $153.6 million. We had net cash used in operating activities of $9.2 million and $3.3 million for the three months ended March 31, 2021 and 2020, respectively.
Prior to the Reverse Recapitalization, Clene Nanomedicine’s operations were financed through the issuance of equity instruments and the issuance of convertible promissory notes. We have not generated significant revenues to datesince our inception, and we do not anticipate generating any significant revenues unless we successfully complete development and obtain regulatory approval for our drugs or for our COVID-19 study.commercialization of a drug candidate. We expect our expenses to increase significantly and to incur additional losses in the future to fund our operations, and conduct productparticularly as we advance the development of our clinical-stage drug candidates, continue research and development of our preclinical drug candidates, and initiate additional clinical trials of, and seek regulatory approval for, these and other future drug candidates. We expect our expenses relating to regulatory compliance and sales and marketing personnel to increase significantly as we prepare to commence commercialization if we obtain regulatory approval for our drug candidates.
Our management performs strategic reviews of our operating plans and budgets, considering the status of our product development programs, human capital, capital needs and resources, and current capital market conditions. Based on these reviews, our Board of Directors (the “Board”) and management make adjustments to our operating plans and budgets to allocate our projected cash expenditures. Notwithstanding these ongoing adjustments, we project that within the next twelve months, we will not have sufficient cash and other resources on hand to sustain our current operations or meet our obligations as they become due, and we recognizemust obtain additional financing. Additionally, pursuant to our term loan with Avenue Venture Opportunities Fund, L.P. (“Avenue”), we must maintain unrestricted cash and cash equivalents of at least $5.0 million to avoid acceleration of the needfull balance of the loan. These conditions raise substantial doubt about the Company’s ability to continue as a going concern.
To mitigate our funding needs, we intend to implement plans to raise additional capitalfunding, including exploring equity financing and offerings, debt financing, licensing or collaboration arrangements with third parties, as well as potentially utilizing additional funds available under our term loan with Avenue, subject to fully implementcertain contingent conditions (see Note 9), as well as our business plan. Additionally,existing at-the-market facility. These plans are subject to market conditions and reliance on third parties, and there is no assurance that effective implementation of our plans will result in the necessary funding to continue current operations. Depending on the results of our plans, we may attemptneed to negotiate a collaboration agreement with a third party for development and commercialization of a drug candidate, which may provide upfront and milestone payments to reduce our spending going forward.
We expect to continue investing in product development, sales and marketing and customer support for our products. The long-term continuation of our business plan is dependent upon the generation of sufficient revenues from our products to offset expenses and capital expenditures. In the event that we do not generate sufficient revenues and are unable to obtain funding, we will be forced to delay, reduce,implement cost-saving initiatives, including potentially delaying or eliminate some or all of ourreducing research and development programs product portfolio expansion,and commercialization efforts or capital expenditures, which could adversely affectefforts. As a result, we have concluded that our business prospects,plans do not alleviate the substantial doubt about our ability to meet long-term liquidity needs or we may be unable to continue operations.
We expect that the cash on hand as of March 31, 2021 will be sufficient to fund our operations for a period extendinggoing concern beyond twelve monthsone year from the date thesethe condensed consolidated financial statements are issued.
The accompanying condensed consolidated financial statements have been prepared assuming we will continue as a going concern, which contemplates the realization of assets and satisfaction of liabilities in the normal course of business. As a result, the accompanying condensed consolidated financial statements do not include any adjustments relating to the recoverability and classification of assets and their carrying amounts, or the amounts and classification of liabilities that may result should we be unable to continue as a going concern.
6
Impact of the COVID-19 Coronavirus Pandemic
The COVID-19 pandemic, which began in December 2019 and has spread worldwide, has caused many governments to implement measures to slow the spread of the COVID-19 outbreak. The outbreakCOVID-19 pandemic and government measures taken in response have had a significant impact, both direct and indirect, on businesses and commerce, as worker shortages have occurred, supply chains have been disrupted, and facilities and production have been suspended. The future progression of the COVID-19 pandemic and its effects on our business and operations remainremains uncertain. The COVID-19 pandemic may affect our ability to initiate and complete preclinical studies and clinical trials, delay the initiation of future clinical trials, disrupt regulatory activities, or have other adverse effects on our business and operations. In particular, the Companywe and our clinicalthird-party contract research organizations (“CROs”CROs”) may facehave faced disruptions that may affecthave affected our ability to initiate and complete preclinical studies, caused manufacturing disruptions, and created delays at clinical trial sites.site initiation and clinical trial enrollment, leading to the early conclusion of an ongoing clinical trial. The COVID-19 pandemic has already caused significant disruptions in the financial markets, and may continue to cause such disruptions, which could impact our ability to raise additional funds to support our operations. Moreover, the COVID-19 pandemic has significantly impacted economies worldwide and could result in adverse effects on our business and operations.
We are monitoring the potential impact of the COVID-19 pandemic on our business, financial condition, results of operations, and financial statements.cash flows. While the COVID-19 pandemic has led to various research restrictions and pausedled to pauses and early conclusion of certain of our clinical trials, these impacts have been temporary and to date we have not experienced material business disruptions or incurred impairment losses in the carrying values of our assets as a result of the COVID-19 pandemic and we are not aware of any specific related event or circumstance that would require us to revise the estimates reflected in these condensed consolidated financial statements. The extent to which the COVID-19 pandemic will directly or indirectly impact our business, financial condition, results of operations, and cash flows, and financial condition, including planned and future clinical trials and research and development costs, will depend on future developments that are highly uncertain, including as a result of new information that may emerge concerning COVID-19, the actions taken to contain or treat it, and the duration and intensity of the related effects.
Note 2. Summary of Significant Accounting Policies
Basis of Presentation
The accompanying condensed consolidated financial statements include the accounts of Clene Inc. and our wholly-owned subsidiaries, Clene Nanomedicine, Inc. (“Clene Nanomedicine”), a subsidiary incorporated in Delaware, Clene Australia Pty Ltd (“Clene Australia”), a subsidiary incorporated in Australia, and dOrbital, Inc., a subsidiary incorporated in Delaware, after elimination of all intercompany accounts and transactions. Our wholly-owned subsidiary, Clene Netherlands B.V. was established on April 21, 2021 and has no financial positions or operations to date. We have prepared the accompanying condensed consolidated financial statements in accordance with accounting principles generally accepted in the United States of America (“GAAPU.S.”) Generally Accepted Accounting Principles (“GAAP”) for interim financial reporting and as required by Regulation S-X, Rule 10-01. The condensed consolidated financial statements have been prepared on the same basis as our audited annual consolidated financial statements and, in the opinion of management, reflect all adjustments, which include onlyare normal and recurring adjustments,in nature, necessary for a fair financial statement of our financial position as of March 31, 2021 and the results of our operations and our cash flows for the three months ended March 31, 2021 and 2020 and the condensed consolidated statement of stockholders’ equity (deficit) as of March 31, 2021 and 2020.presentation. The financial data and other information disclosed in thesethe condensed consolidated financial statements and related notes related tofor the three and six months ended March 31,June 30, 2022 and 2021 and 2020 are unaudited. The results
Results of operations for the three and six months ended March 31,June 30, 2022 and 2021 are not necessarily indicative of the results to be expected for the entire fiscal year ending December 31, 2021,or any other interim periods, or any future year or period.
Prior period balances The condensed consolidated financial statements for accounts receivable have been reclassified to conform to the current year presentation.three and six months ended June 30, 2022 and 2021 should be read in conjunction with the audited consolidated financial statements included in our Annual Report on Form 10-K.
Use of Estimates
The preparation of condensed consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, and disclosure of contingent assets and liabilities, at the date of the condensed consolidated financial statements, and the reported amounts of expenses during the reporting period. Significant estimates and assumptions made in the accompanying condensed consolidated financial statements include, but are not limited to the valuation of common stock, stock options, contingent earn-out liabilities, and Preferred Stock warrants.
expenses. We base our estimates on historical experience and on various other assumptions that are believedwe believe to be reasonable. Actual results may differ from those estimates or assumptions. Estimates are periodically reviewed in light of changes in circumstances, facts, and experience. Changesexperience, and any changes in estimates arewill be recorded in the period in whichfuture periods as they become known.develop.
Risks and Uncertainties
The product candidates we develop require approvals from the U.S. Food and Drug Administration (“FDA”) or foreign regulatory agencies prior to commercial sales. There can be no assurance that our current and future product candidates will receive the necessary approvals or be commercially successful. If we are denied approval or approval is delayed, it will have a material adverse impact on our business and our condensed consolidated financial statements.
7
We are subject to risks common to companies in the development stage including, but not limited to, dependency on the need for substantial additional financing to achieve our goals, uncertainty of broad adoption of our approved products, if any, by physicians and patients, significant competition, and untested manufacturing capabilities.
We are subject to certain risks and uncertainties and believe that changes in any of the following areas could have a material adverse effect on future financial position orcondition, results of operations:operations, or cash flows: ability to obtain futureadditional financing; regulatory approval and market acceptance of, and reimbursement for, product candidates; performance of third-party CROs and manufacturers upon which we rely; protection of our intellectual property; litigation or claims against us based on intellectual property, patent, product, regulatory, or other factors; and our ability to attract and retain employees necessary to support our growth.
Concentrations of Credit Risk
Financial instruments which potentially subject us to significant concentrations of credit risk consist primarily of cash. Our cash is mainly held in financial institutions. Amounts on deposit may at times exceed federally insured limits. We have not experienced any losses on our deposits of cash and do not believe that we are subject to unusual credit risk beyond the normal credit risk associated with commercial banking relationships.
Cash and Cash Equivalents
We consider all short-term investments with an original maturitymaturities of three months90 days or less when purchased to be cash equivalents. As
Restricted Cash
We classify cash as restricted when it is unavailable for withdrawal or use in our general operating activities. Restricted cash and investments are classified as current and noncurrent on the condensed consolidated balance sheets based on the nature of March 31, 2021 and December 31, 2020, we had no cash equivalents and nothe restriction. Our restricted cash balances.balance includes contractually restricted deposits related to our corporate credit card.
Marketable Securities
Derivative Instruments
The convertible promissory notes issuedMarketable securities are investments with original maturities of more than 90 days when purchased. We do not invest in February through July 2020 (“2020 Convertible Notes”) contained embedded features that provide the lenderssecurities with multiple settlement alternatives. Certainoriginal maturities of these settlement features provided the lenders with a right to a fixed number of our shares upon conversion of the notes. Other settlement features provided the lenders with the right or the obligation to receive cash or a variable number of shares upon the completion of a capital raising transaction, change of control or default of the Company (the “Redemption Features”).
The Redemption Features of the 2020 Convertible Notes met the requirements for separate accountingmore than one year. Marketable securities are considered available-for-sale, and were accounted for as a single derivative instrument (the “2020 Derivative Instrument”). The 2020 Derivative Instrument wasare recorded at fair value, with unrealized gains and losses included as a component of accumulated other comprehensive income (loss) until realized. Realized gains and losses are included in other income (expense), net, on the basis of specific identification. The cost of marketable securities is adjusted for amortization of premiums or accretion of discounts to maturity, and such amortization or accretion is included in other income (expense), net.
Inventory
Inventory is stated at inceptionhistoric cost on a first-in first-out basis. Our inventory consisted of $93,000 in raw materials and was subject$14,000 in finished goods as of June 30, 2022, and $26,000 in raw material and $15,000 in finished goods as of December 31, 2021. Inventory primarily relates to re-measurement to fair valueour Supplements segment.
Property and Equipment
Property and equipment are stated at each balance sheet datecost less accumulated depreciation. Property and immediately prior toequipment consist of laboratory and office equipment, computer software, and leasehold improvements. Depreciation is calculated using the extinguishmentstraight-line method over the estimated economic useful lives of derivative liability,the assets, which are 3-5 years for laboratory equipment, 3-7 years for furniture and fixtures, and 2-5 years for computer software. Leasehold improvements are amortized over the lesser of the estimated lease term or the estimated useful life of the assets. Costs for capital assets not yet placed into service are capitalized as construction in progress and depreciated or amortized in accordance with the above useful lives once placed into service. Upon retirement or sale, the related cost and accumulated depreciation and amortization are removed from the accounts and any changes in fair value recognizedresulting gain or loss is included in the condensed consolidated statements of operations and comprehensive loss. In August 2020, in connection with our issuanceMaintenance and sale of Series D Preferred Stock, allrepairs that do not improve or extend the lives of the outstanding principalrespective assets are expensed to operations as incurred.
We capitalize costs to obtain or develop computer software for internal use, including development costs incurred during the software development stage and accrued interest undercosts to obtain software for access and conversion of old data. We also capitalize costs to modify, upgrade, or enhance existing internal-use software that result in additional functionality. We expense costs incurred during the convertible promissory notes was automatically converted into shares of Series D Preferred Stockpreliminary project stage, training costs, data conversion costs, and the derivative liability was extinguished (see Notes 11 and 12). maintenance costs.
Contingent Earn-Out Liabilities
Contingent Earn-out
In connection with the Reverse Recapitalization, and pursuant to the Merger Agreement, Clene Nanomedicine’s common shareholders and Initial Shareholders of Tottenhamcertain stockholders are entitled to receive additional shares of our Clene Inc. common stock, par value $0.0001 (“Common StockStock”) (the “Contingent Earn-outs”“Contingent Earn-outs”) upon us achieving certain milestones described in(see Note 3 and 12. 3).
8
In accordance with ASCAccounting Standards Codification (“ASC”) 815, – Derivatives and hedgingHedging (“ASC 815”), the Contingent Earn-out sharesEarn-outs are not indexed to our own stock and therefore are accounted for as a liability at the Reverse Recapitalization date and subsequently remeasured at each reporting date with changes in fair value recorded as a component of other income (expense), net in the condensed consolidated statements of operations and comprehensive loss.net.
The estimated fair value of the Contingent Earn-out shares for Clene Nanomedicine’s common shareholders (the “Clene Nanomedicine Contingent Earn-out”) and the Contingent Earn-out shares for the Initial Shareholders of Tottenham (the “Initial Shareholders Contingent Earn-out”) were determined using a Monte Carlo simulation that simulated the future path of our Common Stock price over the earn-out periods. The assumptions utilized in the calculation are based on the achievement of certain stock price milestones including projected stock price, volatility, and risk-free rate. For potential payments related to a product development milestone, the fair value was determined based on our expectations of achieving such a milestone and the simulated estimated stock price on the expected date of achievement.
The Clene Nanomedicine Contingent Earn-out and Initial Shareholders Contingent Earn-out are categorized as Level 3 fair value measurements (see Fair Value of Financial Instruments accounting policy) because we estimate projections during the earn-out period utilizing unobservable inputs, including various potential pay-out scenarios. Contingent earn-out payments involve certain assumptions requiring significant judgment and actual results may differ from assumed and estimated amounts.
Preferred Stock Warrant Liability
Prior to the Reverse Recapitalization with Tottenham, we accounted for freestanding warrants to purchase shares of Preferred Stock as liabilities on the balance sheet at their estimated fair value as the underlying redeemable convertible Preferred Stock was considered contingently redeemable and may obligate us to transfer assets to the holders at a future date upon the occurrence of a deemed liquidation event. At the end of each reporting period, changes in the estimated fair value of the warrants to purchase shares of Preferred Stock were recorded in change in fair value of Preferred Stock warrant liability in the condensed consolidated statements of operations and comprehensive loss. The change in the estimated fair value of the Preferred Stock warrant liability was $0.1 million for the three months ended March 31, 2020. In connection with the Reverse Recapitalization, all Clene Nanomedicine Preferred Stock was converted to the Clene Inc. Common Stock and the Clene Nanomedicine Preferred Stock warrants were converted to warrants to purchase Clene Inc. Common Stock. We assessed the features of these warrants and determined that they qualify for classification as permanent equity. Accordingly, we remeasured the warrants to fair value upon the closing of the Reverse Recapitalization and reclassified the resulting warrant liability to additional paid-in capital (See Note 16).
Common Stock Warrants
We account for common stock warrants as either equity-classified instruments or liability-classified instruments based on an assessment of the warrant terms and applicable authoritative guidance in accordance with ASC 480, Distinguishing Liabilities from Equity (“ASC 480”) and ASC 815, Derivatives and Hedging (“ASC 815”).guidance. The assessment considers whether the warrants are freestanding financial instruments pursuant to ASC 480,Distinguishing Liabilities from Equity (“ASC 480”), meet the definition of a liability pursuant to ASC 480, and whether the warrants meet all of the requirements for equity classification under ASC 815, including whether the warrants are indexed to the Company’s ownour Common Stock, among other conditions for equity classification. This assessment, which requires the use of professional judgment, is conducted at the time of warrant issuance and, for liability-classified warrants, as of each subsequent quarterly period end date while the warrants are outstanding (See Note 10).outstanding.
Grant Funding
We may submit applications to receive grant funding from governmental and non-governmental entities. Grant funding received that involves no conditions or continuing performance obligations of the Company is recognized upon receipt. Grant funding with conditions or obligations of the Company is recognized as the conditions or obligations are fulfilled. We have made an accounting policy election to record such unconditional grants, such asincluding the Australian ResearchAustralia research and Development Credit,development credit, as other income in the condensed consolidated statements of operations and comprehensive loss. Income from grantsWe recognize the Australia research and development credit in an amount equal to the qualifying expenses incurred in each period multiplied by the applicable reimbursement percentage.
Grant funding with conditions or obligations is recognized in the period during which the related qualifying expenses are incurred, provided that the conditions under which the grants were provided have been met. We recognize the Australian Research and Development Credit in an amount equal to the qualifying expenses incurred in each period multiplied by the applicable reimbursement percentage. During the three months ended March 31, 2021 and 2020, we recognized $0.3 million and $0, respectively, of Australian Research and Development Credit within other income (expense), net in the condensed consolidated statements of operations and comprehensive loss. As of March 31, 2021, and December 31, 2020, we recorded $2.4 million and $2.1, respectively, of Australian Research and Development Credit receivable in prepaid expenses and other current assets on the condensed consolidated balance sheets.
Any amount received in advance of fulfilling such conditions or obligations is recorded in accrued liabilities inon the condensed consolidated balance sheets if the conditions or obligations are expected to be met within the next twelve months. As of March 31, 2021 and December 31, 2020, we recorded $0.6 million and $0.3 million, respectively, of deferred grant funds received in advance in accrued liabilities.
Grant funding recognized on conditional grants is included as a reduction in research and development expenses in the condensed consolidated statements of operations and comprehensive loss as the conditions are tied to our research and development efforts, and as the arrangement between us and the organizations are not part of our ongoing, major, or central operations. During the three months ended March 31, 2021, weWe recorded a grant of $0.5 million from the Michael J. Fox Foundationgrants as a reductionreductions of research and development expenses in the condensed consolidated statements of operations$0 and comprehensive loss. We did not record any grants$0.2 million for the three months ended March 31, 2020.June 30, 2022 and 2021, respectively; and $0 and $0.2 million for the six months ended June 30, 2022 and 2021, respectively.
Foreign Currency Translation and Transactions
Fair ValueOur functional currency is the U.S. dollar. Clene Australia determined its functional currency to be the Australian dollar and Clene Netherlands B.V. determined its functional currency to be the Euro. We use the U.S. dollar as our reporting currency for the condensed consolidated financial statements. The results of Financial Instruments
Certainour non-U.S. dollar based functional currency operations are translated to U.S. dollars at the average exchange rates during the period. Our assets and liabilities are carried at fair value under GAAP. Fair value is defined astranslated using the price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. Financial assets and liabilities carried at fair value are to be classified and disclosed in one of the following three levels of the fair value hierarchy:
Level 1 — Inputs based upon quoted market prices for identical assets or liabilities in active markets at the measurement date.
Level 2 — Observable inputs other than quoted market prices included in Level 1, such as quoted prices for similar assets and liabilities in active markets; quoted prices for identical or similar assets and liabilities in markets that are not active; or other inputs that are observable or can be corroborated by observable market data.
Level 3 — Inputs that are management’s best estimate of what market participants would use in pricing the asset or liability at the measurement date. The inputs are unobservable in the market and significant to the instrument’s valuation.
We review the fair value hierarchy classification of our applicable assets and liabilities on a quarterly basis. Changes in the observability of valuation inputs may result in a reclassification for certain financial assets or liabilities. Reclassifications impacting all levels of the fair value hierarchy are reported as transfers in or out of the Level 1, 2 or 3 categoriescurrent exchange rate as of the beginningbalance sheet date and stockholders’ equity is translated using historical rates.
Adjustments resulting from the translation of the quarter during whichcondensed consolidated financial statements of our foreign functional currency subsidiaries into U.S. dollars are excluded from the reclassifications occur. There were no transfers between the levelsdetermination of net loss and are accumulated in the fair value hierarchy during the three months ended March 31, 2021a separate component of stockholders’ equity.
We also incur foreign exchange transaction gains and 2020.
See Note 16losses for information on our liabilities measured at fair valuepurchases denominated in foreign currencies. Foreign exchange transaction gains and losses are included in other income (expense), net, as of March 31, 2021 and December 31, 2020.incurred.
Comprehensive Loss
Comprehensive loss includes net loss as well as other changes in stockholders’ equity (deficit) that result from transactions and economic events other than those with stockholders. The only elementelements of other comprehensive income (loss) in any periodperiods presented waswere translation of Australian dollar denominated balances of our Australian subsidiaryClene Australia to U.S. dollars for consolidation.consolidation and unrealized losses on available-for-sale securities.
Segment Information
We have determined that our chief executive officer is the chief operating decision maker (“CODM”). Operating segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation by the CODM
9
in making decisions regarding resource allocation and assessing performance. We view our operations and manage our business in 2 operating segments, which are our reportable segments: (1) the development and commercialization of novel clean-surfaced nanotechnology therapeutics (“Drugs”), and (2) the development and commercialization of dietary supplements (“Supplements”).
Income Taxes
We account for income taxes using the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in the condensed consolidated financial statements or in our tax returns. Deferred tax assets and liabilities are determined based on the differences between the financial statement and tax basis of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. Changes in deferred tax assets and liabilities are recorded in the provision for income taxes. We assess the likelihood that our deferred tax assets will be recovered from future taxable income and, to the extent we believe, based upon the weight of available evidence, that it is more likely than not that all or a portion of the deferred tax assets will not be realized, a valuation allowance is established through a charge to income tax expense. Potential for recovery of deferred tax assets is evaluated by estimating the future taxable profits expected and considering prudent and feasible tax planning strategies.
We account for uncertainty in income taxes recognized in the condensed consolidated financial statements by applying a two-step process to determine the amount of tax benefit to be recognized. First, the tax position must be evaluated to determine the likelihood that it will be sustained upon external examination by the taxing authorities. If the tax position is deemed more-likely-than-not to be sustained, the tax position is then assessed to determine the amount of benefit to recognize in the condensed consolidated financial statements. The amount of the benefit that may be recognized is the largest amount that has a greater than 50% likelihood of being realized upon ultimate settlement. The provision for income taxes includes the effects of any resulting tax reserves, or unrecognized tax benefits, which are considered appropriate as well as the related net interest and penalties.
Stock-Based Compensation
We account for stock-based compensation arrangements using a fair value-based method for costs related to all share-based payments including stock options and stock awards. Stock-based compensation expense is recorded in research and development and general and administrative expenses based on the classification of the work performed by the grantees.
The fair value is recognized over the period during which a grantee is required to provide services in exchange for the option award and service-based stock awards, known as the requisite service period (usually the vesting period), on a straight-line basis. For stock awards with market conditions, the fair value is recognized over the period based on the expected milestone achievement dates as the derived service period (usually the vesting period), on a straight-line basis. For stock awards with performance conditions, the grant-date fair value of these awards is the market price on the applicable grant date, and compensation expense will be recognized when the conditions become probable of being satisfied. We recognize a cumulative true-up adjustment once the conditions become probable of being satisfied as the related service period had been completed in a prior period.
Stock-based compensation expense is recognized at fair value. We elect to account for forfeitures as they occur, rather than estimating expected forfeitures.
We determine the fair value of each share of Common Stock underlying stock-based awards using a Black-Scholes option pricing model based on the closing price of our Common Stock as reported by the Nasdaq Capital Market (“Nasdaq”) on the date of grant. The fair value of stock awards with market conditions are determined using a Monte Carlo valuation model.
Recently Adopted Accounting Pronouncements
In March 2020,May 2021, the FASBFinancial Accounting Standards Board (“FASB”) issued ASU 2020-04, Reference Rate ReformAccounting Standards Update (“ASU”) 2021-04, Earnings Per Share (Topic 848):Facilitation260), Debt—Modifications and Extinguishments (Subtopic 470-50), Compensation—Stock Compensation (Topic 718), and Derivatives and Hedging—Contracts in Entity’s Own Equity (Subtopic 815-40). The amendments in this update relate to the recognition and measurement of the Effectsearnings per share for certain modifications or exchanges of Reference Rate Reform on Financial Reporting, which provides optional expedients and exceptions for applying GAAP to contracts, hedging relationships, and other transactions in which the reference LIBORequity-classified written call options or another reference rate is expected to be discontinued as a result of the Reference Rate Reform. This ASU is intended to ease the potential burden in accounting for (or recognizing the effects of) reference rate reform on financial reporting.warrants. The new guidance was effective immediately, and through December 31, 2022. As a result of our election to utilize the extended transition period for complying with new or revised accounting standards pursuant to Section 107(b) of the JOBS Act, ASU 2016-02 is effective for our fiscal yearsyear and interim periods within our fiscal year beginning after December 15, 2020, and all interim periods thereafter. Early adoption is permitted. We early adopted this guidance on March 1, 2020.2021. The adoption of this guidance did not have a materialan impact on our condensed consolidated financial statements.
In August 2018, the FASB issued ASU No. 2018-15, Intangibles – Goodwill and Other – Internal-Use Software (Subtopic 350-40): Customer’s Accounting for Implementation Costs Incurred in a Cloud Computing Arrangement That is a Service Contract. The new guidance provides for the deferral of implementation costs for cloud computing arrangements and expensing those costs over the term of the cloud services arrangement. The new guidance was effective for fiscal years beginning after December 15, 2020. The adoption of this guidance did not have a material impact on our condensed consolidated financial statements.
Recent Accounting Pronouncements Not Yet Adopted
In June 2016, the FASB issued ASU No. 2016-13, Financial Instruments – Instruments—Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments. The amendments in this ASU,update, among other things, require the measurement of all expected credit losses for financial assets held at the reporting date based on historical experience, current conditions, and reasonable and supportable forecasts.
10
Financial institutions and other organizations will now use forward-looking information to better inform their credit loss estimates. TheAs a smaller reporting company, the guidance is effective for our fiscal years beginning after December 15, 2022. We are currently evaluating the expected impact, if any, of the new guidance as a result of this extended deadline of implementation for smaller reporting companies.
In December 2019,November 2021, the FASB issued ASU No. 2019-12, Income Taxes(Topic 740), which amends2021-10, Government Assistance (Topic 832): Disclosures by Business Entities about Government Assistance. The amendments in this update add disclosure requirements for transactions with a government that are accounted for by applying a grant or contribution accounting model by analogy, including disclosure about the existing guidance relatingnature of the transactions, accounting policy, affected line items on the balance sheet and income statement, amounts applicable to each financial statement line item, and significant terms and conditions of the accounting for income taxes. This ASU is intended to simplify the accounting for income taxes by removing certain exceptions to the general principles of accounting for income taxestransactions including commitments and to improve the consistent application of GAAP for other areas of accounting for income taxes by clarifying and amending existing guidance.contingencies. The new guidance is effective for fiscal yearsall entities for financial statements issued for annual periods beginning after December 15, 2021.2021, with early adoption permitted. We do not expect thatare currently evaluating the adoptionexpected impact, if any, of thisthe new guidance will have a material impact on our condensed consolidated financial statements.guidance.
Note 3. Reverse Recapitalization with Tottenham and Clene Nanomedicine
On December 30, 2020 the Company(the “Closing Date”), Chelsea Worldwide Inc., our predecessor, consummated the Reverse Recapitalization pursuantby and among Clene Nanomedicine, Tottenham Acquisition I Limited (“Tottenham”), Chelsea Worldwide Inc. (“PubCo”), a Delaware corporation and wholly-owned subsidiary of Tottenham, Creative Worldwide Inc. (“Merger Sub”), a Delaware corporation and wholly-owned subsidiary of PubCo, and Fortis Advisors LLC, a Delaware limited liability company as the representative of our stockholders. The Reverse Recapitalization was effected in two steps: (i) Tottenham was reincorporated to which Tottenham mergedthe state of Delaware by merging with and into PubCo in connection with(the “Reincorporation Merger”); and (ii) promptly following the Reincorporation Merger, and PubCoMerger Sub was merged with and into Clene Nanomedicine, resulting in Clene Nanomedicine becoming a wholly-owned subsidiary of PubCo.PubCo (the “Acquisition Merger”). On the Closing Date, PubCo changed its name from Chelsea Worldwide Inc. to Clene Inc. (see Note 1). and listed its shares of Common Stock, par value $0.0001 per share on Nasdaq under the symbol “CLNN.”
Upon the consummationWe received gross proceeds of $9.4 million from the Reverse Recapitalization each Tottenham ordinary shareand incurred offering costs of $5.9 million, which excludes the fair value of Common Stock issued and outstanding immediately prioras payment of certain offering costs, resulting in net proceeds of $3.5 million. We paid LifeSci Capital LLC, an advisor to the effective time of the Reincorporation Merger was automatically cancelled and ceased to exist and (i) for each Tottenham ordinary share, the Company issued one validly-issued share of the Company’s Common Stock; (ii) each warrant to purchase one half of one Tottenham Ordinary Share was converted into a warrant to purchase one-half of one share of the Company’s Common Stock; and (iii) each Tottenham right exchangeable into one-tenth (1/10) of one Tottenham ordinary share was converted into a right exchangeable for one-tenth (1/10) of one share of the Company’s Common Stock. As a result of the Reverse Recapitalization, all outstandingClene Nanomedicine, 644,164 shares of Tottenham ordinary shares of 2,303,495 held by the Initial Shareholders and Tottenham public shareholders were converted into the same number of the Company’s Common Stock. In addition, pursuant to the Merger Agreement, the Initial Shareholders are entitled to receive up to 750,000 of the Company’s Common Stock as earn-out shares upon the achievement of certain milestones described below. consideration for its services.
The Initial Shareholders Contingent Earn-out istransaction was accounted for as a contingent liability“reverse recapitalization” in accordance with GAAP. Under this method of accounting, Tottenham was treated as the “acquired” company for financial reporting purposes. This determination was primarily based on the condensed consolidated balance sheets.
In accordance with the Merger Agreement, on the closing of the Reverse Recapitalization, each share of Clene Nanomedicine preferred stock and common stock then issued and outstanding was automatically cancelled, extinguished and exchanged for 0.1389 newly-issued shares of Clene Inc. Common Stock. At the closing offact that subsequent to the Reverse Recapitalization, Clene Inc. acquired 100%Nanomedicine’s stockholders have a majority of the issued and outstandingvoting power of the Company, Clene Nanomedicine common stock, in exchange for 54,339,012 sharescomprises all of Clene Inc. Common Stock issued to the ongoing operations of the Company, Clene Nanomedicine common shareholders,comprises a majority of which 2,716,958 sharesthe governing body of Clene Inc. Common Stock are to be issued and held in escrow to satisfy any indemnification obligations incurred under the Merger Agreement. In addition, all outstanding warrants (other than warrants that expired, were exercised or were deemed automatically net exercised immediately prior to the Acquisition Merger) exercisable for common stock in Clene Nanomedicine were assumed by the Company, with no changes to the terms and conditionsClene Nanomedicine’s senior management comprises all of the warrants. The warrants have been retroactively restated to reflectsenior management of the Exchange Ratio established inCompany. Accordingly, for accounting purposes, this transaction was treated as the Reverse Recapitalization.
In connection with the Reverse Recapitalization, a total of 53,286,115 stock optionsequivalent of Clene Nanomedicine common stock were cancelled and the holders thereof in exchange received 0.1320 newly-issued stock options of Clene Inc. Common Stock for a total of 7,032,591issuing shares which is 95% of the Exchange Ratio. Pursuant to the Merger Agreement, the Company issued RSUs to the option holders which complements the 5% closing payment shares held in escrow for Clene Nanomedicine common shareholders. In addition, the Company issued 1,136,961 RSUs to option holders to complement the earn-out payments that would be contingently issued to certain current Clene Nanomedicine shareholders upon the achievement of milestones described below.
Also, in connection with the Reverse Recapitalization, Clene Nanomedicine entered into a letter agreement with LifeSci on July 2, 2020, according to which LifeSci was engaged to act as Clene Nanomedicine’s financial advisor with respect to identifying and soliciting special purpose acquisition companies for the purposenet assets of entering intoTottenham, accompanied by a merger or similar transaction with Clene Nanomedicine and its shareholders. Under this agreement, Clene Nanomedicine agreed that if it consummated a merger with Tottenham, LifeSci would receive consideration of (i) 3% of the amount by which the total transaction consideration exceeded $350 million, plus (ii) 7% of cash and cash-equivalents received by Clene Nanomedicine from the Tottenham’s trust account. Clene Nanomedicine could elect to pay LifeSci either in cash, equity interests of the surviving company, or a combination of the two. Upon the consummation of the Reverse Recapitalization, 644,164 shares of the Company’s Common Stock were issued to LifeSci as consideration for its services as pursuant to the letter agreement (see Note 18). recapitalization.
Immediately after giving effect to the Reverse Recapitalization, there were 59,526,171 shares of Common Stock issued and outstanding and warrants to purchase 5,566,363 shares of Common Stock issued and outstanding (see Note 10). Earn-Out Shares
During Tottenham’s IPO, Tottenham incurred deferred underwriters’ fees which were payable to Chardan from the amounts held in the trust account upon completion of the Reverse Recapitalization. Upon the closing of the Reverse Recapitalization, the Company paid $2.1 million to Chardan as settlement of the deferred underwriting fees which amount was included in the total offering costs of the Reverse Recapitalization transaction.
During the year ended December 31, 2020, the Company recorded $5.9 million of offering costs related to third-party legal, accounting and other professional services to consummate the Reverse Recapitalization, excluding the fair value of common shares issued as a payment of related offering costs and Chardan underwriting fees discussed above. These offering costs are recorded as a reduction of additional paid-in capital upon the close of the Reverse Recapitalization in the Company’s condensed consolidated balance sheets.
On December 28, 2020 and prior to the close of the Reverse Recapitalization on December 30, 2020, various PIPE investors purchased 2,239,500 shares of the Company’s Common Stock at a price of $10.00 per share and 1,119,750 warrants to purchase one share of the Company’s Common Stock at an exercise price of $0.01 per share, for net proceeds of $22.2 million (see Notes 10 and 18).
Earn-out Shares
Certain of Clene Nanomedicine’s current stockholders are entitled to receive earn-out shares (the “Clene Nanomedicine Contingent Earn-out”) as follows (the “Clene Nanomedicine Contingent Earn-out”): (i) 3,333,333 shares of the Company’s Common Stock if (A)(a) the volume-weighted average price (“VWAP”VWAP”) of the shares of the Company’sour Common Stock equals or exceeds $15.00 (or any foreign currency equivalent)$15.00 (the “Milestone“Milestone 1 Price”Price”) in any twenty trading days within a thirty trading day period within the three years followingof the closing of the Reverse Recapitalization on any securities exchange or securities market on which the shares of the Company’s Common Stock are then traded or (B)(b) the change of control price equals or exceeds the Milestone 1 Price if a change of control transaction occurs within the three years followingof the closing of the Reverse Recapitalization (the requirements set forth in clause (A)(a) and (B), “Milestone 1”(b) collectively, “Milestone 1”); (ii) 2,500,000 shares of the Company’s Common Stock if (A)(a) the VWAP of the shares of the Company’sour Common Stock equals or exceeds $20.00 (or any foreign currency equivalent)$20.00 (the “Milestone“Milestone 2 Price”Price”) in any twenty trading days within a thirty trading day period within the five years followingof the closing of the Reverse Recapitalization on any securities exchange or securities market on which the shares of the Company’s Common Stock are then traded or (B)(b) the change of control price equals or exceeds the Milestone 2 Price if a change of control transaction occurs within the five years followingof the closing of the Reverse Recapitalization (the requirements set forth in clause (A) or (B), “Milestone 2”(a) and (b) collectively, “Milestone 2”); and (iii) 2,500,000 shares of the Company’s Common Stock if Clene Nanomedicine completescompleted a randomized placebo-controlled studyclinical trial for treatment of COVID-19 which results in a statistically significant finding of clinical efficacy within twelve months afterof the closing of the Reverse Recapitalization (“Milestone 3”3”)., which was not achieved. If Milestone 1 is not achieved but Milestone 2 is achieved, the Clene Nanomedicine stockholders will receive a catch-upan issuance equal to the shares issued upon satisfaction of Milestone 1. Upon the consummationAs of the Reverse Recapitalization,Closing Date, the Clene Nanomedicine Contingent Earn-out shares increased by 12,852 as a resultto 8,346,185 shares of the exerciseCommon Stock due to exercises of stock options during November 2020. Therefore, the total Clene Nanomedicine Contingent Earn-out shares has increased to 8,346,185 shares of the Company’s Common Stock.
The Initial Shareholders of TottenhamTottenham’s former officers and directors, sponsor, and public stockholders (the “Initial Stockholders”) may be entitled to receive earn-out shares as follows (the “Initial Shareholders“Initial Stockholders Contingent Earn-out”Earn-out”): (i) 375,000 shares of the Company’s Common Stock upon satisfaction of the requirements of Milestone 1; and (ii) another 375,000 shares of the Company’s Common Stock upon satisfaction of the requirements of Milestone 2. If
11
Milestone 1 is not achieved but Milestone 2 is achieved, the Initial Shareholders shallStockholders will receive a catch-upan issuance equal to the shares grantedissued upon satisfaction of the requirements of Milestone 1.
The Clene Nanomedicine Contingent Earn-out and Initial Shareholders Contingent Earn-out (collectively, the “Contingent Earn-out”)Earn-outs shares have been classified as liabilities in the condensed consolidated balance sheets and were initially measured at fair value on the date of the Reverse Recapitalization and will be subsequentlyare remeasured to fair value at each reporting date (see date. We did not achieve Milestone 3 and the 2,503,851 Milestone 3 Contingent Earn-out shares were cancelled as of December 31, 2021.
Note 16)4. Marketable Securities
Available-for-Sale Securities
Available-for-sale securities are recorded at fair value, with unrealized gains and losses included as a component of accumulated other comprehensive income (loss) until realized. Available-for-sale securities as of June 30, 2022 were as follows:
| | | | | | | | | | | | | | | | |
| | June 30, 2022 | |
(in thousands) | | Amortized Cost | | | Gross Unrealized Gains | | | Gross Unrealized Losses | | | Fair Value | |
Commercial paper | | $ | 6,967 | | | $ | 0 | | | $ | (51 | ) | | $ | 6,916 | |
Municipal debt securities | | | 1,526 | | | | 0 | | | | (2 | ) | | | 1,524 | |
Corporate debt securities | | | 10,627 | | | | 0 | | | | (34 | ) | | | 10,593 | |
Total | | $ | 19,120 | | | $ | 0 | | | $ | (87 | ) | | $ | 19,033 | |
All available-for-sale securities had a contractual maturity within one year.
AsFor the three and six months ended June 30, 2022, we received proceeds of $2.5 million from the maturity of available-for-sale securities, and $3.0 million from the sale of available-for-sale securities. Realized gains and losses included in earnings as a result of the Reverse Recapitalizationthose sales were insignificant. As of June 30, 2022, we did not have any allowance for credit losses or impairments of available-for-sale securities. As of December 31, 2021, there were 0 outstanding available-for-sale securities.
Note 5. Prepaid Expenses and the PIPE offering, Clene Nanomedicine’s stockholders own approximately 91% of the Common Stock of the Company, Tottenham public stockholders own approximately 4% of the Common Stock of the Company, and investors from the PIPE own approximately 4% of the Common Stock of the Company, based on the number of shares of Clene Inc. Common Stock outstanding on December 30, 2020 (in each case, not giving effect to any shares issuable upon exercise of Clene Inc. warrants, options, or earn-out shares). Other Current Assets
4. Prepaid expenses and other current assets
Prepaid expenses and other current assets consisted of the following as of March 31, 2021June 30, 2022 and December 31, 2020: 2021 were as follows:
| | | | | | | | |
| | June 30, | | | December 31, | |
(in thousands) | | 2022 | | | 2021 | |
Australia research and development credit receivable | | $ | 2,149 | | | $ | 1,564 | |
Metals to be used in research and development | | | 1,623 | | | | 2,237 | |
Other | | | 1,422 | | | | 404 | |
Total prepaid expenses and other current assets | | $ | 5,194 | | | $ | 4,205 | |
| | | March 31, | | December 31, |
| (in thousands) | | 2021 | | 2020 |
| Australia research and development credit receivable | | $ | 2,409 | | | $ | 2,148 | |
| CRO prepayments | | | 850 | | | | 1,211 | |
| Metals to be used in research and development | | | 486 | | | | 31 | |
| Directors & Officers Insurance | | | 882 | | | | - | |
| Other | | | 197 | | | | 112 | |
| | | $ | 4,824 | | | $ | 3,502 | |
5.Note 6. Property and Equipment, Net
Property and equipment, net, consisted of the following as of March 31, 2021June 30, 2022 and December 31, 2020:2021 were as follows:
| | | | | | | | |
| | June 30, | | | December 31, | |
(in thousands) | | 2022 | | | 2021 | |
Lab equipment | | $ | 3,410 | | | $ | 3,327 | |
Office equipment | | | 166 | | | | 147 | |
Computer software | | | 459 | | | | 0 | |
Leasehold improvements | | | 3,956 | | | | 3,943 | |
Construction in progress | | | 4,865 | | | | 2,052 | |
| | | 12,856 | | | | 9,469 | |
Less accumulated depreciation | | | (4,767 | ) | | | (4,297 | ) |
Total property and equipment, net | | $ | 8,089 | | | $ | 5,172 | |
12
| | | March 31, | | December 31, |
| (in thousands) | | 2021 | | 2020 |
| Lab equipment | | $ | 3,068 | | | $ | 3,077 | |
| Furniture and fixtures | | | 147 | | | | 147 | |
| Leasehold improvements | | | 3,927 | | | | 3,889 | |
| Construction in progress | | | 663 | | | | 490 | |
| | | | 7,805 | | | | 7,603 | |
| Less accumulated depreciation | | | (3,623 | ) | | | (3,378 | ) |
| Total property and equipment, net | | $ | 4,182 | | | $ | 4,225 | |
Depreciation expense related to property and equipment, net for the three months ended March 31, 2021 and 2020 was approximately $0.2 million and $0.2 million, respectively. Depreciation expense is reportedrecorded in research and development expense and in general and administrative expense for $0.2the three and six months ended June 30, 2022 and 2021 was as follows:
| | | | | | | | | | | | | | | | |
| | Three Months Ended June 30, | | | Six Months Ended June 30, | |
(in thousands) | | 2022 | | | 2021 | | | 2022 | | | 2021 | |
General and administrative | | $ | 67 | | | $ | 29 | | | $ | 96 | | | $ | 57 | |
Research and development | | | 220 | | | | 226 | | | | 386 | | | | 442 | |
Total depreciation expense | | $ | 287 | | | $ | 255 | | | $ | 482 | | | $ | 499 | |
Note 7. Accrued Liabilities
Accrued liabilities as of June 30, 2022 and December 31, 2021 were as follows:
| | | | | | | | |
| | June 30, | | | December 31, | |
(in thousands) | | 2022 | | | 2021 | |
Accrued compensation and benefits | | $ | 1,285 | | | $ | 2,049 | |
Accrued CRO fees | | | 694 | | | | 718 | |
Deferred grant funds | | | 520 | | | | 520 | |
Other | | | 67 | | | | 323 | |
Total accrued liabilities | | $ | 2,566 | | | $ | 3,610 | |
Note 8. Leases
We lease laboratory and office space and certain laboratory equipment under non-cancellable operating and finance leases. The carrying value of our right-of-use lease assets is substantially concentrated in our real estate leases, while the volume of lease agreements is primarily concentrated in equipment leases.
Operating Leases
In September 2021, we commenced an operating lease for laboratory space and recorded a right-of-use asset of $2.4 million and $1 thousand,lease liability of $2.4 million, net of a lease incentive of $1.0 million which represents an allowance from the lessor for facility alterations. As the lease incentive is payable based on events within our control and are deemed reasonably certain to occur, we recorded the lease incentive as a reduction of the right-of-use asset and lease liability at the lease commencement. As of June 30, 2022 and December 31, 2021, we incurred $1.0 million and $0.5 million, respectively, of costs related to the lease incentive which we recorded as construction in progress, with a corresponding increase to the lease liability, and the construction in progress will be capitalized as leasehold improvements when the facility is placed into service. The lease has an initial ten-year term and provides us the right and option to extend or renew for two periods of five years each. In accordance with ASC 842, Leases, the payments to be made in option periods have not been recognized as part of the right-of-use asset or lease liability because we do not assess the exercise of the option to be reasonably certain.
In February 2022, we commenced an operating lease for existing laboratory space and recorded a right-of-use asset of $2.3 million and lease liability of $2.3 million and terminated the previous right-of-use asset of $0.6 million and lease liability of $1.0 million. We recorded a gain on termination of lease of $0.4 million in the condensed consolidated statements of operations and comprehensive loss.loss for the six months ended June 30, 2022.
6. Accrued Liabilities
Accrued liabilities consistedOur right-of-use assets pertain to operating leases. As of the following as of March 31, 2021June 30, 2022 and December 31, 2020:
| | | March 31, | | December 31, |
| (in thousands) | | 2021 | | 2020 |
| Accrued professional fees | | $ | - | | | $ | 189 | |
| Accrued compensation and benefits | | | 1,316 | | | | 1,225 | |
| Accrued CRO fees | | | 818 | | | | 788 | |
| Deferred grant funds | | | 551 | | | | 301 | |
| Accrued expense reimbursements | | | 33 | | | | 33 | |
| Accrued transaction costs | | | - | | | | 1,354 | |
| Other | | | 12 | | | | 70 | |
| | | $ | 2,730 | | | $ | 3,960 | |
7. Leases
We adopted ASC 842 on January 1, 2019 using the modified retrospective approach.
We also made an accounting policy election not to recognize leases with an initial2021, our operating lease obligations had a weighted-average discount rate of 9.6% and 9.6%, respectively, and a weighted-average remaining term of 12 months or less within7.8 years and 8.1 years, respectively.
13
Finance Leases
Assets recorded under finance lease obligations and included with property and equipment as of June 30, 2022 and December 31, 2021 were as follows:
| | | | | | | | |
| | June 30, | | | December 31, | |
(in thousands) | | 2022 | | | 2021 | |
Lab equipment | | $ | 408 | | | $ | 408 | |
Work in process | | | 228 | | | | 228 | |
Total | | | 636 | | | | 636 | |
Less accumulated depreciation | | | (285 | ) | | | (244 | ) |
Net | | $ | 351 | | | $ | 392 | |
As of June 30, 2022 and December 31, 2021, our condensed consolidated balance sheetsfinance lease obligations had a weighted-average interest rate of 9.1% and to recognize those lease payments on a straight-line basis in our condensed consolidated statements of operations and comprehensive loss over the lease term.
At the lease commencement date, the discount rate implicit in the lease is used to discount the lease liability if readily determinable. If not readily determinable or leases do not contain an implicit rate, our incremental borrowing rate is used as the discount rate.
In April 2020, we terminated an existing operating lease for office space. At the time of termination, we removed the remaining right-of-use asset of $0.3 million, lease liability of $0.3 million, and recognized a gain of $51 thousand. Further, in April 2020, we commenced a new operating lease. At the time of commencement, we recorded the right-of-use asset value of $0.4 million, leasehold improvements of $0.4 million,8.8%, respectively, and a lease liabilityweighted-average remaining term of $0.8 million. The net effect1.5 years and 1.9 years, respectively.
Maturity Analysis of the change in leases being an increase in right-of-use assets of $56 thousand, an increase in leasehold improvements of $0.5 million, an increase in lease liability of $0.4 million, and a gain on termination of $51 thousand. Leases
We have noncancelable operating lease arrangements primarily for office and lab space. We also have noncancelable finance leases for certain lab equipment. The maturity analysis of our finance and operating lease liabilitiesleases as of March 31, 2021 areJune 30, 2022 was as follows:
| | | | | | | | |
(in thousands) | | Finance
Leases | | | Operating
Leases | |
2022 (remainder) | | $ | 77 | | | $ | 460 | |
2023 | | | 96 | | | | 1,145 | |
2024 | | | 27 | | | | 1,171 | |
2025 | | | 0 | | | | 1,202 | |
2026 | | | 0 | | | | 1,231 | |
2027 | | | 0 | | | | 1,129 | |
Thereafter | | | 0 | | | | 2,786 | |
Total undiscounted cash flows | | | 200 | | | | 9,124 | |
Less amount representing interest/discounting | | | (22 | ) | | | (2,826 | ) |
Present value of future lease payments | | | 178 | | | | 6,298 | |
Less lease obligations, current portion | | | (123 | ) | | | (440 | ) |
Lease obligations, long term portion | | $ | 55 | | | $ | 5,858 | |
| (in thousands) | | Finance
Leases | | Operating
Leases |
| 2022 | | $ | 118 | | | | 387 | |
| 2023 | | | 135 | | | | 433 | |
| 2024 | | | 82 | | | | 442 | |
| 2025 | | | 21 | | | | 454 | |
| 2026 | | | - | | | | 466 | |
| Thereafter | | | - | | | | 64 | |
| Total undiscounted cash flows | | | 356 | | | | 2,246 | |
| Less amount representing interest/discounting | | | (7 | ) | | | (321 | ) |
| Present value of future lease payments | | | 349 | | | | 1,925 | |
| Less lease obligations, current portion | | | (139 | ) | | | (202 | ) |
| Lease obligations – long term portion | | $ | 210 | | | $ | 1,723 | |
We expect that, in the normal course of business, the existing leases will be renewed or replaced by similar leases.
Finance Leases
Assets recorded under finance lease obligations and included with property and equipment as of March 31, 2021 and December 31, 2020 are summarized as follows:
| | | March 31, | | December 31, |
| (in thousands) | | 2021 | | 2020 |
| | | | | |
| Lab equipment | | $ | 920 | | | $ | 920 | |
| Furniture and fixtures | | | 46 | | | | 46 | |
| Work in process | | | 228 | | | | 228 | |
| Total | | | 1,194 | | | | 1,194 | |
| Less accumulated depreciation | | | (629 | ) | | | (593 | ) |
| | | | | | | | | |
| Net | | $ | 565 | | | $ | 601 | |
As of March 31, 2021, our finance lease obligations had a weighted-average interest rate of 8.4% and had a weighted-average remaining term of 2.5 years. As of December 31, 2020, our finance lease obligations had a weighted-average interest rate of 8.1% and had a weighted-average remaining term of 2.7 years.
Operating Leases
Our balance of right-of-use assets on the face of the balance sheet pertain to operating leases. As of March 31, 2021, our operating lease obligations had a weighted-average discount rate of 9.6% and had a weighted-average remaining term of 6.3 years. As of December 31, 2020, our operating lease obligations had a weighted-average discount rate of 9.6% and a weighted-average remaining term of 6.3 years.
Components of Lease Cost
The components of finance and operating lease costs for the three and six months ended March 31,June 30, 2022 and 2021 and 2020 were as follows:
| | | | | | | | | | | | | | | | |
| | Three Months Ended June 30, | | | Six Months Ended June 30, | |
(in thousands) | | 2022 | | | 2021 | | | 2022 | | | 2021 | |
Finance lease costs: | | | | | | | | | | | | |
Amortization | | $ | 21 | | | $ | 36 | | | $ | 41 | | | $ | 73 | |
Interest on lease liabilities | | | 1 | | | | 7 | | | | 6 | | | | 15 | |
Operating lease costs | | | 209 | | | | 70 | | | | 439 | | | | 140 | |
Short-term lease costs | | | 0 | | | | 54 | | | | 0 | | | | 116 | |
Variable lease costs | | | 108 | | | | 20 | | | | 173 | | | | 39 | |
Total lease costs | | $ | 339 | | | $ | 187 | | | $ | 659 | | | $ | 383 | |
| (in thousands) | | 2021 | | 2020 |
| Finance lease costs: | | | | |
| Amortization | | $ | 37 | | | $ | 48 | |
| Interest on lease liabilities | | | 8 | | | | 11 | |
| Operating lease costs | | | 70 | | | | 81 | |
| Short-term lease costs | | | 62 | | | | 88 | |
| Variable lease costs | | | 19 | | | | 34 | |
| | | | | | | | | |
| Total lease costs | | $ | 196 | | | $ | 262 | |
Supplemental Cash Flow Information
| | | | | | | | |
| | Six Months Ended June 30, | |
(in thousands) | | 2022 | | | 2021 | |
Operating cash flows from operating leases | | $ | (612 | ) | | $ | (295 | ) |
Operating cash flows from finance leases | | $ | (6 | ) | | $ | (15 | ) |
Financing cash flows from finance leases | | $ | (65 | ) | | $ | (83 | ) |
14
| (in thousands) | | 2021 | | 2020 |
| | | | | |
| Operating cash flows from operating leases | | $ | (151 | ) | | $ | (203 | ) |
| Operating cash flows from finance leases | | $ | (8 | ) | | $ | (11 | ) |
| Finance cash flows from finance leases | | $ | (45 | ) | | $ | (53 | ) |
8.Note 9. Notes Payable
Our long-term debt, net of original issue discount and unamortized debt issuance costs, as of June 30, 2022 and December 31, 2021 was as follows:
| | | | | | | | | | | | |
| | Stated | | | June 30, | | | December 31, | |
(in thousands, except interest rates) | | Interest Rate | | | 2022 | | | 2021 | |
Notes payable: | | | | | | | | | |
Maryland Department of Housing & Community Development | | | 8.00 | % | | $ | 634 | | | $ | 614 | |
Maryland Department of Housing & Community Development | | | 6.00 | % | | | 694 | | | | 0 | |
Advance Cecil, Inc. | | | 8.00 | % | | | 126 | | | | 122 | |
Avenue Venture Opportunities Fund, L.P. | | | 11.35 | % | | | 20,000 | | | | 20,000 | |
| | | | | | 21,454 | | | | 20,736 | |
Accrued and unpaid interest | | | | | | 2 | | | | 0 | |
Less unamortized debt issuance costs and original issue discounts | | | | | | (1,196 | ) | | | (1,654 | ) |
Less convertible notes payable, net of unamortized debt discount and issuance costs | | | | | | (4,709 | ) | | | (4,598 | ) |
Total notes payable | | | | | $ | 15,551 | | | $ | 14,484 | |
Maryland Loans
In February 2019, we entered into a loan agreement (the “2019“2019 MD Loan”Loan”) with the Department of Housing and Community Development (“DHCD”), a principal department of the State of Maryland (“Maryland”). Pursuant to the 2019 MD Loan, Maryland agreed to provideMaryland. The agreement provides for a $0.5term loan of $0.5 million term loan. Amounts outstanding under the 2019 MD Loan bearbearing simple interest at an annual rate of 8.00%8.00%. Under the 2019 MD Loan, we agreedWe are subject to affirmative and negative covenants to which we will remain subject until maturity. These covenants includematurity, including providing information about the Company and our operations; limitations on our ability to retire, repurchase, or redeem our common or preferred stock, options, and warrants other than per the terms of the securities; and limitations on our ability to pay dividends of cash or property. There are no financial covenants associated with the Loan Agreement. Events of default under the Loan Agreement include failure to make payments when due, insolvency events, failure to comply with covenants, and material adverse effects with respect to the Company.2019 MD Loan. We are not in violation of any affirmative or negative covenants.The 2019 MD Loan established “Phantom Shares” at issuance based on 119,907 shares of Common Stock. Repayment of the full balance outstanding is due on February 22, 2034. The 2019 MD Loan establishes “Phantom Shares,” based on 119,906 shares of our Common Stock (based on 863,110 Series C Preferred Shares prior to the Reverse Recapitalization), determined at issuance. The Loan Agreement states2034, with the repayment amount isand carrying value equal to be the greater of the balance of principal andplus accrued interest or the Phantom Share value. We determined that the note should be accounted for at fair value. We record the fair value of the debtPhantom Shares. The value of the Phantom Shares is based on the closing price of our Common Stock on Nasdaq at the end of each reporting period. In order to valueAs of June 30, 2022 and December 31, 2021, the note was recorded at principal plus accrued interest in the condensed consolidated balance sheets. We recognized interest expense of $10,000 and $20,000 for the three and six months ended June 30, 2022, respectively; and interest income (expense) of $0.2 million and $(0.3) million for the three and six months ended June 30, 2021, respectively.
In May 2022, we considerentered into a loan agreement (the “2022 MD Loan”) with DHCD which provides for a term loan of up to $3.0 million bearing simple interest at an annual rate of 6.00% for the purchase of certain personal property (the “Assets”) related to the production of pharmaceutical drugs. As of June 30, 2022, we had drawn $0.7 million under the term loan, with the remainder available upon our submission of disbursement requests to purchase the Assets. The 2022 MD Loan matures on July 1, 2027 (the “Maturity Date”). The first twelve payments, commencing on July 1, 2022, are deferred. Immediately thereafter, there shall be eighteen monthly installments of interest-only based on the actual amount advanced under the loan, each up to a maximum amount of $15,000; followed by thirty monthly installments of principal and interest, each in the amount of $33,306, which is due and payable even if the simpleentire loan has not been advanced prior to the date such monthly payment is due and payable, with a balloon payment of all accrued and unpaid interest and principal due on the Maturity Date. We recorded $31,000 of debt issuance costs that are being amortized over the contractual term using the effective interest method. Pursuant to the 2022 MD Loan, DHCD was granted a continuing security interest in the Assets as collateral. Under a priority of liens agreement by and between DHCD and Avenue, an existing secured creditor of the Company, DHCD’s continuing security interest in the Assets shall be a first priority lien. As of June 30, 2022, the note was recorded at amortized cost, which approximated fair value. We recognized interest expense that would be dueof $2,000 and the value of Phantom Shares. Upon the closing of the Reverse Recapitalization and as of December 31, 2020, the fair value of the 2019 MD Loan is determined based on the closing price of CLNN shares listed on the Nasdaq. Expense of $0.5 million and $10 thousand was recognized during$2,000 for the three and six months ended March 31, 2021 and 2020,June 30, 2022, respectively. The fair value of $1.5 million and $1.1 million of principal and accrued interest is included in long-term notes payable as of March 31, 2021 and December 31, 2020, respectively.
Cecil County Loan
In April 2019, we entered into a loan agreement (the “2019“2019 Cecil Loan”Loan”) with Advance Cecil County, Maryland (“Cecil”). Pursuant to the 2019 Cecil Loan, Cecil agreed to provideInc., a $0.1 million term loan. Amounts outstandingnon-stock corporation formed under the 2019 Cecil Loan bearlaws of the state of Maryland. The agreement provides for a term loan of $0.1 million bearing simple interest at an annual rate of 8.00%8.00%. UnderWe are subject to affirmative covenants until maturity, including providing information about the Company and our operations. There are no financial covenants associated with the 2019 Cecil Loan. We are not in violation of any covenants. The 2019 Cecil Loan established “Phantom Shares” at issuance based on 23,981 shares of Common Stock. Repayment of the full balance is due on April 30, 2034, with the repayment amount and carrying value equal to the greater of the balance of principal plus accrued interest or the value of the Phantom Shares. The value of the Phantom Shares is based on the closing price of our Common Stock on Nasdaq at
15
the end of each reporting period. As of June 30, 2022 and December 31, 2021, the note was recorded at principal plus accrued interest in the condensed consolidated balance sheets. We recognized interest expense of $2,000 and $4,000 for the three and six months ended June 30, 2022, respectively; and interest income (expense) of $36,000 and $(54,000) for the three and six months ended June 30, 2021, respectively.
PPP Loan
In May 2020, we agreedentered into a note payable in the amount of $0.6 million (the “PPP Loan”) under the Paycheck Protection Program of the CARES Act. The Paycheck Protection Program permits forgiveness of amounts loaned for payments of payroll and other qualifying expenses, subject to certain conditions. In January 2021, the full balance of the PPP Loan was forgiven and we recorded a gain on extinguishment of notes payable for the six months ended June 30, 2021.
Avenue Loan
In May 2021, we entered into a loan agreement (the “2021 Avenue Loan”) with Avenue. The agreement provides for a 42-month term loan of up to $30.0 million. The first tranche is $20.0 million (“Tranche 1”), of which $15.0 million was funded at close and $5.0 million was funded in September 2021. We incurred issuance costs of $0.6 million of which $46,951 was expensed immediately. The remaining unfunded tranche of $10.0 million (“Tranche 2”) is available until December 31, 2022. Funding of Tranche 2 is subject to (a) our receipt of $5.0 million financing through the state of Maryland; (b) our achievement of a statistically significant result in certain clinical trials (“Performance Milestone 1”); (c) our receipt of net proceeds of at least $30.0 million from the sale and issuance of our equity securities between May 2, 2021 and December 31, 2022; and (d) mutual agreement of us and Avenue. The 2021 Avenue Loan bears interest at a variable rate equal to the sum of (i) the greater of (a) the prime rate or (b) 3.25%, plus (ii) 6.60%. As of June 30, 2022 and December 31, 2021, the interest rate was 11.35% and 9.85%, respectively. Payments are interest-only for the first 12 months and have been extended an additional 12 months (the “First Interest-only Period Extension”) based on our achievement of Performance Milestone 1. Payments may be extended up to 36 months if we (i) achieve the First Interest-only Period Extension and (b) draw from Tranche 2. The loan principal will amortize equally from the end of the interest period to the expiration of the 42-month term on December 1, 2024. On the maturity date, an additional payment equal to 4.25% of the funded loans, currently equal to $0.9 million (the “Final Payment”), is due in addition to the remaining unpaid principal and accrued interest. The Final Payment was recorded as a debt premium and is being amortized over the contractual term using the effective interest method. The Final Payment is related to the loan host and is not bifurcated pursuant to ASC 815. We are subject to affirmative and negative covenants to which we will remain subject until maturity. These covenants includematurity in the absence of prepayments, including providing information about the Company and our operations; limitationslimitation on our ability to retire, repurchase, or redeem our common or preferred stock,Common Stock, options, and warrants other than per the terms of the securities; and limitations on our ability to pay dividends of cash or property. There are no financial covenants associated with the Loan Agreement. Events of default under the Loan Agreement include failure to make payments when due, insolvency events, failure to comply with covenants, and material adverse effects with respectAlso pursuant to the Company.2021 Avenue Loan, we are required to maintain unrestricted cash and cash equivalents of at least $5.0 million, provided that upon our (i) achievement of Performance Milestone 1, and (ii) receiving of net proceeds of at least $30.0 million from the sale and issuance of our equity securities, we shall no longer be subject to financial covenants. We are not in violation of any affirmativecovenants. Avenue also has the ability to make all obligations under the 2021 Avenue Loan immediately due and payable upon occurrence of certain events of default or negative covenants. Repaymentmaterial adverse effects, as outlined in the loan agreement. The 2021 Avenue Loan is collateralized by substantially all of our assets other than intellectual property, including our capital stock and the capital stock of our subsidiaries, in which Avenue is granted a continuing security interest.
Pursuant to the agreement, we granted Avenue a warrant to purchase 115,851 shares of Common Stock (the “Avenue Warrant”) at an exercise price of $8.63 per share. Upon the funding of Tranche 2, the Avenue Warrant shall be adjusted to include an additional estimated 245,832 shares of Common Stock, which is equal to 5% of the full balanceprincipal amount of Tranche 2, divided by the five (5)-day VWAP per share as of the end of trading on the last trading day before the issuance of Tranche 2. We accounted for the Tranche 2 contingently-issuable warrant at inception of the 2021 Avenue Loan in accordance with ASC 815 and the fair value and issuable shares are remeasured at each reporting period.
Avenue has the right, in its discretion, but not the obligation, at any time between May 21, 2022 through May 21, 2024, while the loan is outstanding, to convert up to $5.0 million of principal into Common Stock (the “Conversion Feature”) at a price per share equal to 120% of the Avenue Warrant exercise price. Exercise of the Conversion Feature is due on April 30, 2034. The 2019 Cecil Loan establishes “Phantom Shares,” based on 23,981 sharessubject to certain minimum price and volume conditions of our Common Stock (based on 172,622 Series C Preferred Shares priorNasdaq. The Conversion Feature did not meet the requirements for separate accounting and is not accounted for as a derivative instrument. The number of shares of Common Stock potentially issuable upon conversion is 482,703 shares. We classified $5.0 million of the 2021 Avenue Loan as convertible notes payable as of June 30, 2022 and December 31, 2021, with unamortized debt discount and issuance costs of $0.3 million and $0.4 million, respectively. For the three and six months ended June 30, 2022, we recognized (i) total interest expense of $0.2 million and $0.4 million, respectively; (ii) coupon interest expense of $0.1 million and $0.3 million, respectively; and (iii) amortization of debt discount and issuance costs of $0.1 million and $0.1 million, respectively; and the effective interest rate was 16.95%. For the three and six months ended June 30, 2021, we recognized (i) total interest expense of $0.1 million and $0.1 million, respectively; (ii) coupon interest expense of $0.1 million and $0.1 million, respectively;
16
and (iii) amortization of debt discount and issuance costs of $23,000 and $23,000, respectively; and the effective interest rate was 17.93%.
The net proceeds from the issuance of the loan were initially allocated to the Reverse Recapitalization), determinedwarrant at issuance.an amount equal to their fair value of $1.5 million and the remainder to the loan. The 2019 Cecil Loan states the repayment amount is to be the greaterallocation of the balanceincurred financing costs of principal and accrued interest or the Phantom Share value. We determined that the note should be accounted for at fair value. We record$0.5 million, which together with the fair value of the Avenue Warrant and the Final Payment, are recorded as a debt atdiscount and debt premium, respectively, and are being amortized over the end of each reporting period. In order to valuecontractual term using the note, we consider the amount of the simpleeffective interest method. We recognized interest expense that would be due and the value of Phantom Shares. Upon the closing of the Reverse Recapitalization and as of December 31, 2020, the fair value of the 2019 Cecil Loan is now determined based on the closing price of CLNN shares listed on the Nasdaq. Expense of $0.1$0.7 million and $2 thousand was recognized during$1.5 million for the three and six months ended March 31, 2021June 30, 2022, respectively; and 2020, respectively. The fair valueinterest expense of $0.3$0.2 million and $0.2$0.2 million for the three and six months ended June 30, 2021, respectively.
Future payments under the 2021 Avenue Loan, net of principalunamortized debt discounts, if Avenue does not exercise the Conversion Feature, and accrued interest is included in long-term notes payableunder the 2022 MD Loan, net of unamortized debt discounts, are as follows:
| | | | | | | | |
(in thousands) | | 2021 Avenue Loan | | | 2022 MD Loan | |
2022 (remainder) | | $ | 0 | | | $ | 0 | |
2023 | | | 6,667 | | | | 0 | |
2024 | | | 13,333 | | | | 0 | |
2025 | | | 0 | | | | 368 | |
2026 | | | 0 | | | | 326 | |
2027 | | | 0 | | | | 0 | |
Thereafter | | | 0 | | | | 0 | |
Subtotal of future principal payments | | | 20,000 | | | | 694 | |
Accrued and unpaid interest | | | 0 | | | | 2 | |
Less unamortized debt issuance costs and original issue discounts | | | (1,166 | ) | | | (30 | ) |
Total | | $ | 18,834 | | | $ | 666 | |
Note 10. Common Stock Warrants
As of March 31, 2021June 30, 2022 and December 31, 2020, respectively.
In May 2020, we entered into a note payable in the amount of $0.6 million (the “PPP Note”) under the Paycheck Protection Program of the CARES Act (the “PPP”). As amended, the PPP permits forgiveness of amounts loaned for payments of payroll and other qualifying expenses within 24 weeks of receipt of loaned funds, given that at least 60% of the total loan is used for payroll. Amounts not forgiven have a repayment period of five years. In January 2021, the full $0.6 million balance of the PPP Note was forgiven and has been recorded as a gain on extinguishment of debt during the three months ended March 31, 2021.
9. Preferred Stock Warrant Liability
Prior to the Reverse Recapitalization, we issued Series A Preferred Stock Warrants in 2013 in connection with certain note purchase agreements. The warrants expire 10 years from issuance. These warrants are exercisable at a fixed exercise price of $1.97, which is equal to the price per share of the Series A Preferred Stock. As of December 31, 2019, these warrants were exercisable into 1,608,672 shares of the Series A Preferred Stock.
Prior to the Reverse Recapitalization, on April 8, 2013, we issued 10-year warrants to purchase units of our most senior equity equal to 0.50% of our fully diluted equity at the time of exercise in connection with certain note purchase agreements. As of December 31, 2019, these warrants were exercisable into 271,439 shares of our most senior equity, Series C Preferred Stock, at a fixed exercise price of $1.97 per share. On August 11, 2020, in connection with our issuance of Series D Preferred Stock, these warrants became exercisable into 320,441 shares of our most senior equity, Series D Preferred Stock, at a fixed exercise price of $1.97 per share.
Prior to the Reverse Recapitalization, we classified Preferred Stock warrants as a liability on the condensed consolidated balance sheets because the warrants are freestanding financial instruments that may have required us to transfer assets upon exercise. The liability associated with each of these warrants was initially recorded at fair value upon the issuance date of each warrant and is subsequently remeasured to fair value as a component of other income (expense), net in the condensed consolidated statements of operations and comprehensive loss. Upon the closing of the Reverse Recapitalization (see Note 1), and pursuant to the Merger Agreement, all of the outstanding Clene Nanomedicine Preferred Stock was converted to the Clene Inc. Common Stock and the Clene Nanomedicine Preferred Stock warrants to purchase Clene Nanomedicine Preferred Stock were converted to warrants to purchase the Clene Inc. Common Stock (see Note 10). Upon conversion, we assessed the features of the warrants and determined that they qualify for classification as permanent equity upon the closing of the Reverse Recapitalization. Accordingly, we remeasured the warrants to fair value one final time upon the close of the Reverse Recapitalization, and recognized a loss of $14.6 million for the year ended December 31, 2020, within other income, (expense), net on the condensed consolidated statements of operations and comprehensive loss. Upon the closing of the Reverse Recapitalization, the warrant liability was reclassified to additional paid-in capital (see Notes 1 and 17).
As of March 31, 2021 and December 31, 2020, we do not have any Preferred Stock warrants outstanding.
We recognized a change in fair value of the outstanding warrants of $0.1 million during the three months ended March 31, 2020 in the condensed consolidated statements of operations and comprehensive loss.
10. Common Stock Warrants
As of March 31, 2021 and December 31, 2020, outstanding warrants to purchase shares of our Common Stock consistedwere as follows:
| | | | | | | | | | | | | | | | |
Date Exercisable | | Number of
Shares
Issuable | | | | | Exercise Price | | | Exercisable for | | Classification | | Expiration |
December 2020 | | | 2,407,500 | | | (1) | | $ | 11.50 | | | Common Stock | | Equity | | December 2025 |
December 2020 | | | 24,583 | | | (2) | | $ | 11.50 | | | Common Stock | | Equity | | December 2025 |
December 2020 | | | 1,929,111 | | | (3) | | $ | 1.97 | | | Common Stock | | Equity | | April 2023 |
May 2021 | | | 115,851 | | | (4) | | $ | 8.63 | | | Common Stock | | Equity | | May 2026 |
Total | | | 4,477,045 | | | | | | | | | | | | |
(1)Consists of the following:| Date Exercisable | | Number of Shares Issuable | | Exercise Price | | Exercisable for | | Classification | | Expiration |
| | | | | | | | | | | |
| June 2021 | | | 1,119,750 | | | $ | 0.01 | | | Common Stock | | Equity | | December 2021 |
| December 2020 | | | 2,407,500 | | | $ | 11.50 | | | Common Stock | | Equity | | December 2025 |
| December 2020 | | | 110,000 | | | $ | 11.50 | | | Common Stock | | Equity | | December 2025 |
| December 2020 | | | 1,929,113 | | | $ | 1.97 | | | Common Stock | | Equity | | April 2023 |
| Total | | | 5,566,363 | | | | | | | | | | | |
On December 28, 2020, the Company entered into a subscription agreement (the “Subscription Agreement”) with various investors for the private purchase of 2,239,500
2,407,500 shares of the Company’s Common Stock at a price of $10.00 per share with net proceeds of $22.2 million. Investors in the PIPE offering also received warrants (“PIPE Warrants”) to purchase a number of shares equal to one-half (1/2) of the number of PIPE Shares, totaling 1,119,750 shares of the Company’s Common Stock, at an exercise price of $0.01 per share. Also, pursuant to the Subscription Agreement, the 1,119,750 PIPE Warrants are subject to a 180-day holding period. A holder of the PIPE Warrants may not exercise the PIPE Warrant if the holder, together with its affiliates, would beneficially own more than 9.99% of the number of shares of the Company’s Common Stock outstanding immediately after giving effect to such exercise. As of March 31, 2021 and December 31, 2020, none of the warrants had been exercised.In connection with the Reverse Recapitalization, all of Tottenham’s issued and outstandingunderlying warrants to purchase one-half (1/2) of one share of Tottenham’s ordinary shares totaling 2,407,500 sharesCommon Stock, issued in connection with Tottenham’s initial public offering, were automatically converted into 4,815,000 warrants to purchase 2,407,500 shares of the Company’s Common Stock. The warrants became exercisable upon the completion of the Reverse Recapitalization and will expire five years after the consummation of the Reverse Recapitalization (i.e., December 2025). The Companyoffering.
We may redeem the outstanding warrants, in whole and not in part, at a price of $0.01$0.01 per warrant if, and only if the last sales price of the Company’sour Common Stock equals or exceeds $16.50$16.50 per share for any 20twenty trading days within a 30-tradingthirty-trading day period ending three business days before the Company sends the notice of redemption.period. As of March 31, 2021June 30, 2022 and December 31, 2020, none of the2021, no warrants had been exercised.
In(2)
Consists of 24,583 shares of Common Stock underlying warrants to purchase one-half (1/2) of one share of Common Stock, issued to Chardan Capital Markets, LLC (“Chardan”) upon exercise of their unit purchase option, which was issued in connection with Tottenham’s initial public offering in August 2018, Tottenham issued to Chardan options to purchase 220,000 units at $10.00 per unit. Each of Tottenham’s units consists of one and one-tenth shares of Tottenham’s ordinary shares for $10.00 per share and one warrant to purchase one-half of one Tottenham ordinary share at an exercise price of $11.50 per share. In connection with the Reverse Recapitalization, the Chardan Unit Purchase Option was converted into one Company unit purchase option. The Chardan Unit Purchase Option Warrants are exercisable upon the completion of the Reverse Recapitalization and will expire in December 2025.offering. As of March 31, 2021June 30, 2022 and December 31, 2020,2021, no Chardan Unit Purchase Options werewarrants had been exercised.In connection with the Reverse Recapitalization, all
(3)Consists of the 1,929,113 outstanding Series A and Series D Preferred1,929,111 shares of Common Stock Warrants were converted automatically into 1,929,113underlying warrants to purchase sharesone share of the Company Common Stock, at $1.97 per share (See Note 9).issued by Clene Nanomedicine as Series A preferred stock warrants and senior equity warrants in August 2013. As of March 31, 2021June 30, 2022 and December 31, 2020, none of the2021, no warrants had been exercised.11. Convertible Notes
In February through July 2020, we issued convertible promissory notes (the “2020 Convertible Notes”) in an aggregate principal amount
(4)Consists of $6.1 million, bearing interest at an annual rate of 5%. The 2020 Convertible Notes were convertible at the earlier of (i) one year, at which point the notes would be convertible into Series C preferred shares at the Series C preferred share issuance price, and (ii) next equity financing of no less than $10.0 million, at which point the notes would be convertible into shares issued in the next equity financing at 90% of the per share issuance price of the next equity financing. The 2020 Convertible Notes contained redemption features that met the requirements for separate accounting and were accounted for as a single derivative instrument. Accordingly, the 2020 derivative instrument of $0.7 million was recorded at fair value at inception as redeemable convertible preferred stock derivative liability in the condensed consolidated balance sheets (see Note 12).We recognized interest expense of $8 thousand, including amortization of debt discount of $20 thousand during the three months ended March 31, 2020, in connection with the 2020 Convertible Notes.
On August 11, 2020, in connection with our issuance and sale of Series D Preferred Stock, all of the outstanding principal and accrued interest under the 2020 Convertible Notes, totaling $6.9 million, was automatically converted into 1,497,135
115,851 shares of Series D PreferredCommon Stock at a price equal to 90% of $4.60 per share,underlying the per share price paid in cash by investors in the Series D preferred stock financing. Upon the closing of the Reverse Recapitalization (see Note 1), and pursuant to the Merger Agreement, all outstanding Clene Nanomedicine Series D Preferred Stock was converted to Clene Inc. Common Stock.We accounted for the conversion of the 2020 Convertible Notes as a debt extinguishment and recognized a loss on extinguishment of debt of $0.5 million within other income (expense), net in the consolidated statements of operations and comprehensive loss for the year ended December 31, 2020.Avenue Warrant. As of the date of conversion, the unamortized discount on the 2020 Convertible Notes was $0.5 million. The loss on extinguishment was calculated as the difference between (i) the fair value of the 1,497,135 shares of Series D Preferred Stock issued to settle the 2020 Convertible Notes of $6.9 millionJune 30, 2022 and (ii) the carrying value of the 2020 Convertible Notes, including the principal balance of the 2020 Convertible Notes of $6.1 million and accrued but unpaid interest of $76 thousand, net of the unamortized debt discount of $5.7 million, plus the then-current fair value of derivative liability associated with the 2020 Convertible Notes at the time of the extinguishment of $0.7 million.
12. Derivative Instruments
Derivative instrument in connection with the 2020 Convertible Notes
One of the redemption features of the 2020 Convertible Notes met the requirements for separate accounting and was accounted for as a derivative instrument. The 2020 Derivative Instrument was recorded at fair value, which was $0.7 million at issuance. In August 2020, in connection with our issuance and sale of Series D Preferred Stock, all of the outstanding principal and accrued interest under the 2020 Convertible Notes was automatically converted into shares of Series D Preferred Stock and the derivative liability was extinguished. Prior to the extinguishment of derivative liability, the 2020 Derivative Instrument was marked to fair value and we recorded the change in the 2020 Derivative Instrument of ($29) thousand in the consolidated statements of operations and comprehensive loss for the year ended December 31, 2020 (see Note 11). For the three months ended March 31, 2020, we recorded the change in the 2020 Derivative Instrument of $4 thousand in the condensed consolidated statements of operations and comprehensive loss. Upon the closing of the Reverse Recapitalization (see Note 1), and pursuant to the Merger Agreement, all outstanding Clene Nanomedicine Preferred Stock was converted to Clene Inc. Common Stock.
Derivative instruments in connection with the Contingent Earn-outs
The earn-out shares issued in connection with the Reverse Recapitalization met the requirements for separate accounting and are therefore accounted for as derivative instruments. Accordingly, upon the consummation of the Reverse Recapitalization, we recorded a liability in the condensed consolidated balance sheets and a debit to additional paid-in capital for the earn-out provision associated with the Initial Shareholders Contingent Earn-out and a debit to accumulated deficit for the earn-out provisions associated with the Clene Nanomedicine Contingent Earn-out. The contingent shares to be issued to the Clene Nanomedicine shareholders immediately prior to the Reverse Capitalization were treated as a deemed distribution. The contingent earn-out was subsequently remeasured to fair value at each reporting date as a component of other income (expense), net in the condensed consolidated statements of operations and comprehensive loss.
Upon the closing of the Reverse Recapitalization, we recognized the Clene Nanomedicine Contingent Earn-out and Initial Shareholders Contingent Earn-out liabilities at their fair value of $64.7 million and $7.4 million, respectively, in the condensed consolidated balance sheets. As of December 31, 2020, the carrying values of the Clene Nanomedicine Contingent Earn-out and Initial Shareholders Contingent Earn-out were $52.1 million and $5.9 million, respectively. As of March 31, 2021, the carrying values of the Clene Nanomedicine Contingent Earn-outwarrant had not been exercised.
Note 11. Commitments and Initial Shareholders Contingent Earn-out were $77.7 million and $8.9 million, respectively. For the three months ended March 31, 2021, we recognized losses of $25.6 million in change in fair value of the Clene Nanomedicine Contingent Earn-out and $3.0 million in change in fair value of the Initial Shareholders Contingent Earn-out as components of other income (expense), netContingencies
We enter into agreements in the condensed consolidated statementsnormal course of operationsbusiness with CROs for clinical trials and comprehensive loss. To date, nonewith vendors for preclinical studies and other services and products for operating purposes, which are cancelable at any time by us, subject to payment of the milestones have been achieved.our remaining
17
13. Commitmentsobligations under binding purchase orders and, Contingenciesin certain cases, nominal early termination fees. These commitments are not deemed significant.
Litigation
From time to time, we may have certain contingent legal liabilities that arise in the ordinary course of business activities. We accrue a liability for such matters when it is probable that future expenditures will be made, and such expenditures can be reasonably estimated. We are not aware of any current material pending legal matters or claims.
As of June 30, 2022 and December 31, 2021, we had commitments under various agreements for capital expenditures totaling $3.7 million and $0.6 million, respectively, related to the construction of our manufacturing facilities.
14.Note 12. Income Taxes
We have not recorded income tax benefits for the net operating losses incurred during the three and six months ended March 31,June 30, 2022 and 2021 and 2020 noror for research and development tax credits andor other deferred tax assets generated, due to its uncertainty of realizing a benefitbenefits from thosethese items.
The components of loss before income taxes for the three and six months ended March 31,June 30, 2022 and 2021 and 2020 werewas as follows (in thousands): follows:
| | | | | | | | | | | | | | | | |
| | Three Months Ended June 30, | | | Six Months Ended June 30, | |
(in thousands) | | 2022 | | | 2021 | | | 2022 | | | 2021 | |
United States | | $ | (3,398 | ) | | $ | (2,574 | ) | | $ | (16,355 | ) | | $ | (41,295 | ) |
Foreign | | | (1,136 | ) | | | (849 | ) | | | (1,533 | ) | | | (1,956 | ) |
Loss before provision for income taxes | | $ | (4,534 | ) | | $ | (3,423 | ) | | $ | (17,888 | ) | | $ | (43,251 | ) |
| | | Three Months Ended | | Three Months Ended |
| | | March 31, | | March 31, |
| | | 2021 | | 2020 |
| United States | | $ | (38,721 | ) | | $ | (3,386 | ) |
| Foreign | | | (1,107 | ) | | | (555 | ) |
| Total loss before income taxes | | $ | (39,828 | ) | | $ | (3,941 | ) |
The Company isWe are subject to taxation in the United States,U.S., Australia, Netherlands, and various state jurisdictions. The Company computes itsOur tax returns from 2015 to present are subject to examination by the U.S. and state authorities due to the carry forward of unutilized net operating losses and research and development credits. There are currently no pending examinations. We compute our quarterly income tax provision by using a forecasted annual effective tax rate and adjusts for any discrete items arising during the quarter. The primary difference between the effective tax rate and the federal statutory tax rate relates to the full valuation allowance on the Company's U.S.our net operating losses and other deferred tax assets.
Note 13. Benefit Plans
401(k) Plan
15. Stock-Based CompensationOur 401(k) plan is a deferred salary arrangement under Section 401(k) of the Internal Revenue Code. Under the 401(k) plan, participating U.S. employees may defer a portion of their pretax earnings, up to the U.S. Internal Revenue Service annual contribution limit. We match 100% of a participating employee’s deferral contributions up to 3% of annual compensation, limited to $4,500 of matching contributions. Our contributions to the 401(k) plan totaled $51,000 and $32,000 for the three months ended June 30, 2022 and 2021, respectively, and $0.1 million and $0.1 million for the six months ended June 30, 2022 and 2021, respectively.
2020 Stock Plan
In December 2020, in connection with the Reverse Recapitalization, the Company’s Board of Directors approved theThe 2020 Stock Plan (the “2020 Stock Plan”) and reserved reserves 12,000,000 shares of Common Stock for issuance thereunder, all of which may be issued pursuant to incentive stock options or any other type of award under the 2020 Stock Plan. The 2020 Stock Plan became effective immediately upon the closing of the Reverse Recapitalization. The maximum number of shares of Common Stock that may be issued pursuant to the exercise of incentive stock options under the 2020 Stock Plan is 12,000,000. Selected employees, officers, directors, and consultants of the Company are eligible to participate in the traditional stock option grants, stock appreciation rights, restricted stock awards, restricted stock unit awards, other stock awards, and performance awards under the 2020 Stock Plan. The purpose of thisthe 2020 Stock Plan is to enable us to offer competitive equity compensation packages in order to attract and retain talent and align the talent necessary for the combined company.
interests of management with those of stockholders. The 2020 Stock Plan is administered by the Company’s Board of Directors. Board. The exercise prices, vesting periods, and other restrictions are determined at the discretion of the Company’s Board, of Directors, except that the exercise price per share of stock options may not be less than 100% of the fair market value of the Common Stock on the date of grant. Stock options awarded under the 2020 Stock Plan expire ten years after the grant date unless the Company’s Board of Directors sets a shorter term. Stock options and restricted stock granted to employees, officers, members of the Company’s Board of Directorsdirectors, and consultants generally vest over a four-year period. If an option or other award granted under the 2020 Stock Plan expires, terminates or is terminated, forfeited, repurchased, or cancelled, the unissued shares subject to that option or award shall again be available under the 2020 Stock Plan. If shares awarded pursuantAs of June 30, 2022, the Board granted 7,659,218 stock options and rights to restricted stock awards under the 2020 Stock Plan, are forfeited to or repurchased at original cost by the Company, the number of shares forfeited or repurchased at original cost shall again be available under the 2020 Stock Plan.
As of March 31, 2021, the Company’s Board of Directors granted 1,634,804 restricted stocks units and stock options under the 2020 Stock Plan. As of March 31, 2021, 10,365,1964,340,782 shares remained available for future grant.
As of December 31, 2020, the Company’s Board of Directors granted 1,507,062 restricted stock units under the 2020 Stock Plan. As of December 31, 2020, 10,492,938 shares remained available for future grant.
2014 Stock Plan
Following the closing of the Reverse Recapitalization, theThe 2014 Stock Plan is administered by the Company’s Board of Directors.Board. Stock options awardedgranted under the 2014 Stock Plan expire ten years after the grant date. Stock options and restricted stock awards granted to employees, officers, members of the Company’s Board of Directorsdirectors, and consultants typicallygenerally vest over a four-year period.
As a result of the Reverse Recapitalization (as described in Note 1), stock options outstanding under the 2014 Stock Plan of 53,286,115 were converted into 7,032,591 of stock options of the Company based on the Exchange Ratio determined in accordance with the terms of the Merger Agreement. The exchange of Clene Nanomedicine’s stock options for Clene Inc. stock options was treated as a modification of the awards. The modification of the stock options did not result in a material incremental compensation expense to be recognized at the closing of the Reverse Recapitalization.
During the year ended December 31, 2020, the Company’s Board of Directors granted stock options for 270,555 shares under the 2014 Stock Plan. Effective as of the closing of the Reverse Recapitalization, on December 30, 2020, no additional awards may be madegranted under the 2014 Stock
18
Plan and as a result, if any award granted under the 2014 Stock Plan and as a result, (i) any shares in respect of stock options that are expiredexpires, or is terminated, under the 2014 Stock Plan without having been fully exercised will not be available for future awards; (ii) any shares in respect of restricted stock that are forfeited, to,repurchased, cancelled, or otherwise repurchased by the Company, will not be available for future awards; and (iii) any shares of Common Stock that are tendered to the Company by a participant to us to exercise an award, the unissued shares subject to that award will not be available for future awards.
Stock-Based Compensation Expense
Stock-based compensation for the three months ended March 31, 2021 and 2020 was approximately $3.3 million and $0.2 million, respectively. Stock-based compensation isexpense recorded in research and development expense and general and administrative expenses inexpense for the condensed consolidated statements of operationsthree and comprehensive losssix months ended June 30, 2022 and 2021 was as follows:
| | | | | | | | | | | | | | | | |
| | Three Months Ended June 30, | | | Six Months Ended June 30, | |
(in thousands) | | 2022 | | | 2021 | | | 2022 | | | 2021 | |
General and administrative | | $ | 1,351 | | | $ | 2,647 | | | $ | 2,809 | | | $ | 4,517 | |
Research and development | | | 833 | | | | 1,608 | | | | 1,577 | | | | 3,003 | |
Total stock-based compensation expense | | $ | 2,184 | | | $ | 4,255 | | | $ | 4,386 | | | $ | 7,520 | |
Stock-based compensation expense by award type for the three and six months ended June 30, 2022 and 2021 was as follows:
| | | Three months ended
March 31, | |
| (In thousands) | | 2021 | | | 2020 | |
| | | | | | | |
| General and administrative | | $ | 1,870 | | | $ | 71 | |
| Research and development | | | 1,395 | | | | 100 | |
| Total stock-based compensation | | $ | 3,265 | | | $ | 171 | |
| | | | | | | | | | | | | | | | |
| | Three Months Ended June 30, | | | Six Months Ended June 30, | |
(in thousands) | | 2022 | | | 2021 | | | 2022 | | | 2021 | |
Stock options | | $ | 2,184 | | | $ | 1,253 | | | $ | 4,386 | | | $ | 1,513 | |
Restricted stock awards | | | 0 | | | | 3,002 | | | | 0 | | | | 6,007 | |
Total stock-based compensation expense | | $ | 2,184 | | | $ | 4,255 | | | $ | 4,386 | | | $ | 7,520 | |
Stock Options
Outstanding stock options and related activity for the six months ended June 30, 2022 was as follows:
| | | | | | | | | | | | | | | | |
(in thousands, except share, per share, and term data) | | Number of
Options | | | Weighted
Average
Exercise
Price
Per Share | | | Weighted
Average
Remaining
Term
(Years) | | | Intrinsic
Value | |
Outstanding - December 31, 2021 | | | 10,395,027 | | | | 3.35 | | | | 6.32 | | | | 21,082 | |
Granted | | | 2,755,973 | | | | 3.01 | | | | 9.65 | | | | 0 | |
Exercised | | | (1,044,448 | ) | | | 0.27 | | | | — | | | | 2,348 | |
Forfeited | | | (35,276 | ) | | | 5.07 | | | | — | | | | 0 | |
Outstanding - June 30, 2022 | | | 12,071,276 | | | $ | 3.54 | | | | 6.97 | | | $ | 8,718 | |
Vested and exercisable - June 30, 2022 | | | 6,161,852 | | | $ | 2.10 | | | | 4.84 | | | $ | 8,711 | |
Vested, exercisable or expected to vest - June 30, 2022 | | | 12,071,276 | | | $ | 3.54 | | | | 6.97 | | | $ | 8,718 | |
As of MarchJune 30, 2022 and December 31, 2021, we had approximately $2.8$20.1 million and $18.3 million, respectively, of unrecognized stock-based compensation costs related to non-vested awardsstock options which is expected to be recognized over a weighted-average period of 2 years.2.82 years and 3.05 years, respectively.
The following sets forth the outstanding Common Stock options and related activity for the three months ended March 31, 2021 (in thousands, except share and per share data):
| Equity | | Number of Options | | | Weighted
Average
Exercise
Price
Per Share | | | Weighted
Average
Remaining
Term
(Years) | | | Intrinsic
Value | |
| Outstanding - December 31, 2020 | | | 7,032,591 | | | | 0.97 | | | | 5.34 | | | $ | 62,462 | |
| Granted | | | 130,000 | | | | 6.55 | | | | 9.85 | | | | - | |
| Exercised | | | (48,211 | ) | | | 1.04 | | | | - | | | | 384 | |
| Forfeited | | | (8,579 | ) | | | 4.32 | | | | - | | | | - | |
| Outstanding - March 31, 2021 | | | 7,105,801 | | | $ | 1.07 | | | | 5.17 | | | $ | 56,435 | |
Options vested and exercisable -
March 31, 2021 | | | 5,966,739 | | | $ | 0.60 | | | | 4.66 | | | $ | 50,173 | |
| Options vested and exercisable - Stock options vested and expected to vest March 31, 2021 | | | 7,105,801 | | | $ | 1.07 | | | | 5.17 | | | $ | 56,435 | |
Prior to the consummation of the Reverse Recapitalization, the exercise price of the stock options granted was based on the fair market value of the common shares of the Company as of the grant date as determined by the Board of Directors, with input from management. The Board of Directors determined the fair value of the common stock at the time of grant of the options by considering a number of objective and subjective factors, including third-party valuation reports, valuations of comparable companies, sales of redeemable convertible Preferred Stock, sales of common stock to unrelated third parties, operating and financial performance, the lack of liquidity of the Company’s capital stock, and general and industry-specific economic outlook.
Stock options are valued using thea Black-Scholes option pricingoption-pricing model. Since we haveDue to the limited trading history of our Common Stock, the expected volatility is derived from the average historical stock volatilities of several unrelated comparable public companies within our industry, that we consider to be comparable to its own business over a period equivalent to the expected term of the stock option grants. The risk-free interest rate for periods within the contractual life of the stock options is based on the U.S. Treasury yield curve in effect aton the time of the grant.grant date. The expected dividend is assumed to be zero as we have never paid dividendsa dividend and have no current plans to do so. The expected term represents the period that stock-based awardsthe stock options are expected to be outstanding. For option grantsstock options that are considered to be “plain vanilla,”in the ordinary course, we determine the expected term using the simplified method. The simplified method, deemswhich considers the term to be the average of the time-to-vesting and the contractual life of the stock options. For other stock option grants, we estimate the expected term using historical data on employee exercises and post-vesting employment termination behavior, taking into accountwhile also considering the contractual life of the award.
19
During the three months ended March 31, 2021, we granted stock options for 130,000 shares under the 2020 Stock Plan. The assumptions used to calculate the fair value of stock options granted during the stock option awards granted for the threesix months ended March 31,June 30, 2022 and 2021 are presentedwere as follows:
| | | | |
| | Six Months Ended June 30, |
| | 2022 | | 2021 |
Expected stock price volatility | | 89.57% – 93.38% | | 84.80% – 87.70% |
Risk-free interest rate | | 1.65% – 3.00% | | 0.59% – 1.90% |
Expected dividend yield | | 0.00% | | 0.00% |
Expected term of options | | 5.00 – 6.98 years | | 6.00 years |
| | Three months
ended
March 31, | |
| | 2021 | |
Expected stock price volatility | | | 84.80 | % |
Risk-free interest rate | | | 0.59 | % |
Expected dividend yield | | | 0 | % |
Expected term of options | | | 6 years | |
The weighted-average grant-date fair value of stock options granted during the threesix months ended March 31,June 30, 2022 and 2021 was $6.55.$2.24 and $6.44, respectively.
Restricted Stock UnitsAwards
In connection with the Reverse Recapitalization, the following outstanding and unvested rights to restricted stock awards were granted to various employees and non-employee directors:
On
•370,101 shares which vest on various dates between June 30, 2021 and July 15, 2022, subject to the holder’s continuous employment through such vesting date. The award represents 5% of the converted stock options under the 2014 Stock Plan as a result of the Reverse Recapitalization and complements the 5% closing payment shares held in escrow for Clene Nanomedicine common stockholders. The grant-date fair value of these awards was $4.0 million based on the closing price of our Common Stock on Nasdaq of $10.82 per share on December 30, 2020, we granted2020. As of June 30, 2022, there were 80,076 outstanding and unvested shares.
•454,781 shares which are eligible to vest based on certain market conditions, subject to the
following sharesholder’s continuous employment through such vesting date. The award complements the Milestone 1 earn-out share entitlement of
restricted common stock underClene Nanomedicine stockholders and vests based on the
2020 Stock Plan: | ● | 370,101 shares to various employees and non-employee directors, which vest on June 30, 2021, subject to the employee’s continuous employment through such vesting date. The award represents 5% of the converted stock options under 2014 Stock Plan as a result of the Reverse Recapitalization and complements the 5% closing payment shares held in escrow for Clene Nanomedicine common shareholders (as described in Note 1). The grant-date fair value of these awards was $4.0 million. No shares were vested as of March 31, 2021 and December 31, 2020. |
| ● | 454,781 shares to various employees and non-employee directors, which were eligible to vest based on certain market conditions, subject to the employee’s continuous employment through such vesting date. The award complements the Milestone 1 earn-out share entitlement of Clene Nanomedicine shareholders and vests based on the same market condition (as described insame market condition (see Note 3). The grant-date fair value of these awards, using a Monte Carlo simulation, was $4.3 million. Based on the outcome of the market condition as of the March 31, 2021 and December 31, 2020 measurement dates, no shares were vested. |
| ● | 341,090 shares to various employees and non-employee directors, which were eligible to vest based on certain market conditions, subject to the employee’s continuous employment through such vesting date. The award complements the Milestone 2 earn-out share entitlement of Clene Nanomedicine. shareholders and vests based on the same market condition (as described in Note 3). The grant-date fair value of these awards, using a Monte Carlo simulation, was $3.5 million. Based on the outcome of the market condition as of the March 31, 2021 and December 31, 2020 measurement dates, no shares were vested. |
| ● | 341,090 shares to various employees and non-employee directors, which were eligible to vest based on certain performance conditions tied to the completion of the COVID-19 coronavirus treatment study, subject to the employee’s continuous employment through such vesting date. The award complements the Milestone 3 earn-out share entitlement of Clene Nanomedicine shareholders and vests based on the same performance condition (as described in Note 3). The grant-date fair value of these awards was $3.7 million based on a weighted average grant date fair value of $10.82 per share. We did not recognize compensation expense because the occurrence of achieving this milestone was not probable. As of the March 31, 2021 and December 31, 2020 measurement dates, no shares were vested. |
The following table summarizes the restricted common stock activity during the three months ended March 31, 2021:
| | | Number of RSUs | | | Weighted-Average
Grant Date Fair Value | |
| Outstanding and unvested balance as of December 31, 2020 | | | 1,507,062 | | | $ | 10.30 | |
| Vested | | | - | | | | - | |
| Forfeited | | | (2,258 | ) | | | 10.82 | |
| Outstanding and unvested balance as of March 31, 2021 | | | 1,504,804 | | | $ | 7.59 | |
The assumptions used to calculate the value of the restricted stock units granted in 2020 in the Monte Carlo valuation model, include projected stock price, volatilitywas $4.3 million. Based on the outcome of the market condition as of the June 30, 2022 and risk-free rateDecember 31, 2021 measurement dates, no shares were vested.
•341,090 shares which are eligible to vest based on certain market conditions, subject to the holder’s continuous employment through such vesting date. The award complements the Milestone 2 earn-out share entitlement of Clene Nanomedicine stockholders and vests based on the
achievement of certain stock price milestones. Our significant unobservable inputs as of March 31, 2021 were as follows: (i) 85% of expected stock price volatility, (ii) risk-free interest rate of 0.6%, and (iii) expected term of 5 years. Our significant unobservable inputs as of December 31, 2020 were as follows: (i) 85% of expected stock price volatility, (ii) risk-free interest rate of 0.4%, and (iii) expected term of 5 years.same market condition (see Note 3). The
weighted average grant-date fair value of
RSUs granted duringthese awards, using a Monte Carlo valuation model, was $3.5 million. Based on the
three months ended March 31, 2021 andoutcome of the market condition as of
December 31, 2020 was $0 and $10.3034, respectively. The stock-based compensation expense associated with the RSUs was $3.0 million and $0 for the three months ended March 31, 2021 and 2020. As of March 31, 2021June 30, 2022 and December 31, 2020, total2021 measurement dates, no shares were vested.
Outstanding rights to restricted stock awards and related activity for the six months ended June 30, 2022 was as follows:
| | | | | | | | |
| | Number of
Restricted Stock Awards | | | Weighted Average Grant Date Fair Value | |
Unvested balance as of December 31, 2021 | | | 916,603 | | | $ | 10.00 | |
Converted to shares of Common Stock upon vesting | | | (65,363 | ) | | | 0 | |
Forfeited | | | (2,025 | ) | | | 9.84 | |
Unvested balance as of June 30, 2022 | | | 849,215 | | | $ | 9.93 | |
As of June 30, 2022 and December 31, 2021, there was 0 unrecognized compensation cost related to the unvested stock-based awards was $8.4 million and $15.5 million, which is expectedrights to be recognized over a weighted average period of 3 months and 6 months, respectively. We did not issue any RSUs during the three months ended March 31, 2020.restricted stock awards.
16.Note 14. Fair Value
The carrying amount ofCash and cash equivalents are carried at fair value. Financial instruments, including accounts receivable, accounts payable, and accrued expenses are carried at cost, which approximates fair value becausegiven their short-term nature. Marketable securities, the Avenue Warrant, and the Contingent Earn-outs are carried at fair value. The 2019 MD Loan and the 2019 Cecil Loan are carried at the greater of principal plus accrued interest or the immediate, short-term maturityvalue of these financial instruments.Phantom Shares. The 2021 Avenue Loan, including the convertible notes payable and Conversion Feature, and the 2022 MD Loan are carried at amortized cost, which approximate fair value due to our credit risk and market interest rates.
20
LiabilitiesFinancial Instruments with Fair Value Measurements on a Recurring Basis
The following tables present our fair value hierarchy for liabilitiesfinancial instruments measured at fair value on a recurring basis as of March 31, 2021 and December 31, 2020 (in thousands): June 30, 2022 is as follows:
| | | | | | | | | | | | | | | | |
| | June 30, 2022 | |
(in thousands) | | Level 1 | | | Level 2 | | | Level 3 | | | Total | |
Cash equivalents | | | | | | | | | | | | |
Money market funds | | $ | 3,568 | | | $ | 0 | | | $ | 0 | | | $ | 3,568 | |
Marketable securities | | | | | | | | | | | | |
Commercial paper | | | 0 | | | | 6,916 | | | | 0 | | | | 6,916 | |
Municipal debt securities | | | 0 | | | | 1,524 | | | | 0 | | | | 1,524 | |
Corporate debt securities | | | 0 | | | | 10,593 | | | | 0 | | | | 10,593 | |
Common stock warrant liability | | | 0 | | | | 0 | | | | 167 | | | | 167 | |
Clene Nanomedicine contingent earn-out | | | 0 | | | | 0 | | | | 9,847 | | | | 9,847 | |
Initial Stockholders contingent earn-out | | | 0 | | | | 0 | | | | 1,263 | | | | 1,263 | |
| | | Fair Value Measurements on a Recurring Basis
March 31, 2021 | |
| | | Level 1 | | | Level 2 | | | Level 3 | | | Total | |
| Notes payable | | $ | 1,839 | | | $ | - | | | $ | - | | | $ | 1,839 | |
| Clene Nanomedicine contingent earn-out | | | - | | | | - | | | | 77,663 | | | | 77,663 | |
| Initial Shareholders contingent earn-out | | | - | | | | - | | | | 8,867 | | | | 8,867 | |
| | | Fair Value Measurements on a Recurring Basis
December 31, 2020 | |
| | | Level 1 | | | Level 2 | | | Level 3 | | | Total | |
| Notes payable | | $ | 1,296 | | | $ | - | | | $ | - | | | $ | 1,296 | |
| Clene Nanomedicine contingent earn-out | | | - | | | | - | | | | 52,054 | | | | 52,054 | |
| Initial Shareholders contingent earn-out | | | - | | | | - | | | | 5,906 | | | | 5,906 | |
Valuation of Notes Payable
The carrying value of the notes payable includes certain notes remeasured at fair value on a recurring basis in the balance sheet as of March 31, 2021 and December 31, 2020. In order to value the note, we consider the amount of simple interest expense that would be due and the value of our Common Stock.
As of March 31, 2021, the fair value of our notes payable is determined based on the closing price of $12.78 per share as reported by the Nasdaq.
As of December 31, 2020, the fair value of our notes payable is determined based on the closing price of $9.01 per share as reported by the Nasdaq.
Valuation of Warrants to Purchase Preferred Stock
Our Preferred Stock warrant liabilities contain unobservable inputs that reflect our own assumptions. Accordingly, the Preferred Stock warrant liabilities werehierarchy for financial instruments measured at fair value on a recurring basis using unobservable inputs. Prior to the extinguishmentas of December 31, 2021 is as follows:
| | | | | | | | | | | | | | | | |
| | December 31, 2021 | |
(in thousands) | | Level 1 | | | Level 2 | | | Level 3 | | | Total | |
Notes payable | | $ | 736 | | | $ | 0 | | | $ | 0 | | | $ | 736 | |
Common stock warrant liability | | | 0 | | | | 0 | | | | 474 | | | | 474 | |
Clene Nanomedicine contingent earn-out | | | 0 | | | | 0 | | | | 18,100 | | | | 18,100 | |
Initial Stockholders contingent earn-out | | | 0 | | | | 0 | | | | 2,317 | | | | 2,317 | |
There were no transfers between Level 1, Level 2, or Level 3 during any of the Preferred Stock warrant liabilities on December 30, 2020, the Preferred Stock warrant liability was valued using a Black-Scholes valuation model. periods above.
The Board of Directors determinesChanges in the fair value of our Level 3 financial instruments for the Preferred Stock by considering a number of objective and subjective factors, including third-party valuations, valuations of comparable companies, sales of redeemable convertible Preferred Stock, sales of common stock to unrelated third parties, operating and financial performance, the lack of liquidity of our capital stock, and general and industry-specific economic outlook. We estimated the volatility of our Preferred Stock based on comparable peer companies’ historical volatility. The risk-free interest rate for periods within the contractual life of the warrants is based on the U.S. Treasury yield curvesix months ended June 30, 2022 were as follows:
| | | | | | | | | | | | |
(in thousands) | | Common Stock Warrant
Liability | | | Clene
Nanomedicine
Contingent
Earn-out | | | Initial
Stockholders
Contingent
Earn-out | |
Balance - December 31, 2021 | | $ | 474 | | | $ | 18,100 | | | $ | 2,317 | |
Change in fair value | | | (2 | ) | | | (8,253 | ) | | | (1,054 | ) |
Reclassification from liability to equity | | | (305 | ) | | | 0 | | | | 0 | |
Balance - June 30, 2022 | | $ | 167 | | | $ | 9,847 | | | $ | 1,263 | |
Changes in effect at the valuation date. We have no plans to declare any future dividends. The determination of the fair value of our Level 3 financial instruments for the Preferredsix months ended June 30, 2021 were as follows:
| | | | | | | | | | | | |
(in thousands) | | Common Stock Warrant
Liability | | | Clene
Nanomedicine
Contingent
Earn-out | | | Initial
Stockholders
Contingent
Earn-out | |
Balance - December 31, 2020 | | $ | 0 | | | $ | 52,053 | | | $ | 5,906 | |
Initial fair value of instrument | | | 1,457 | | | | 0 | | | | 0 | |
Change in fair value | | | (133 | ) | | | 16,970 | | | | 1,729 | |
Balance - June 30, 2021 | | $ | 1,324 | | | $ | 69,023 | | | $ | 7,635 | |
Valuation of Notes Payable and Convertible Notes Payable
As of June 30, 2022 and December 31, 2021, the carrying value of the 2019 MD Loan was $0.6 million and $0.6 million, respectively; and the carrying value of the 2019 Cecil Loan was $0.1 million and $0.1 million, respectively. In all periods presented, the loans were recorded at principal plus accrued interest in the condensed consolidated balance sheets.
As of June 30, 2022, the amortized cost of the 2021 Avenue Loan was $18.8 million, which included notes payable carried at $14.1 million; and convertible notes payable and embedded Conversion Feature, carried at $4.7 million. As of December 31, 2021, the amortized cost of the 2021 Avenue Loan was $18.3 million, which included notes payable carried at $13.7 million; and convertible notes payable and embedded Conversion Feature, carried at $4.6 million. The valuation of the Conversion Feature is discussed below. As of June 30, 2022, the amortized cost of the 2022 MD Loan was $0.7 million.
21
Valuation of Conversion Feature
The Conversion Feature of the convertible notes payable from the 2021 Avenue Loan is carried at amortized cost and did not meet the requirements for separate accounting as a derivative instrument. As of June 30, 2022 and December 31, 2021, the estimated fair value of the Conversion Feature was $0.3 million and $0.8 million, respectively, and was determined using a Black-Scholes option-pricing model. The unobservable inputs to the Black-Scholes option-pricing model were as follows:
| | | | | | | | |
| | June 30, | | | December 31, | |
| | 2022 | | | 2021 | |
Expected stock price volatility | | | 115.00 | % | | | 105.00 | % |
Risk-free interest rate | | | 3.00 | % | | | 0.70 | % |
Expected dividend yield | | | 0.00 | % | | | 0.00 | % |
Expected term | | 1.89 years | | | 2.39 years | |
Valuation of the Common Stock Warrant Liability
The common stock warrant liability could change in future periods based upon changes inassociated with the valueAvenue Warrant is comprised of our Preferredthe potentially issuable Tranche 2 warrant to purchase an estimated 245,832 shares of Common Stock, which was classified as a liability and other assumptions as presented above. We record any such change inrecorded at fair value at issuance. The fair value and number of underlying shares will be remeasured at each reporting period.
The estimated fair value was determined using a Black-Scholes option-pricing model. The carrying amount of the liability may fluctuate significantly and actual amounts may be materially different from the liabilities’ estimated value. The unobservable inputs to the Black-Scholes option-pricing model were as follows:
| | | | | | | | |
| | June 30, | | | December 31, | |
| | 2022 | | | 2021 | |
Expected stock price volatility | | | 115.00 | % | | | 105.00 | % |
Risk-free interest rate | | | 3.00 | % | | | 1.20 | % |
Expected dividend yield | | | 0.00 | % | | | 0.00 | % |
Expected term | | 3.70 years | | | 3.89 – 4.39 years | |
Probability of drawing Tranche 2 | | | 45.00 | % | | | 50.00 | % |
As we did not complete a bona fide round of equity financing by March 31, 2022, the exercise price and underlying shares of the Tranche 1 warrant became fixed and therefore qualified for equity classification. We remeasured the Tranche 1 warrant liability to fair value as of March 31, 2022 and recognized the change in fair value of Preferred Stock warrant liability expense line in the condensed consolidated statements of operations and comprehensive loss.
Upon the closing of the Reverse Recapitalization (see Note 1), all of the outstanding Clene Nanomedicine Preferred Stock was converted to Clene Inc. Common Stockloss and the Clene Nanomedicine Preferred Stock warrants were convertedTranche 1 warrant liability was reclassified to warrants for the purchase of Clene Inc. Common Stock. Accordingly, the Preferred Stock warrant liabilities were extinguished in connection with the conversion of Clene Nanomedicine Preferred Stock on December 30, 2020 (see Note 9).additional paid-in-capital.
Valuation of the Contingent Earn-outEarn-Outs
Pursuant to the Merger Agreement, Clene Nanomedicine’s common shareholders immediately prior to the Reverse Recapitalization and Initial Shareholders of Tottenham were entitled to receive additional shares of up to 8,333,333 shares and 750,000 shares of our Common Stock, respectively, upon us achieving certain milestones described in Note 3. Upon the consummation of the Reverse Recapitalization, The Clene Nanomedicine and the Initial Shareholders are entitled to receive up to 8,346,185 additional shares as a result of the exercise of the stock options in November 2020, and 750,000 shares of our Common Stock. TheStockholders Contingent Earn-outs were recorded at fair value onat the closing of the Reverse Recapitalization on December 30, 2020 and are remeasured at each reporting period. As of March 31, 2021June 30, 2022 and December 31, 2020, no milestone has been achieved. 2021, Clene Nanomedicine’s common stockholders were entitled to receive up to 5,842,334 shares of Common Stock and the Initial Stockholders were entitled to receive up to 750,000 shares of Common Stock. As of December 31, 2021, we did not achieve Milestone 3 and the 2,503,851 Milestone 3 Contingent Earn-out shares were cancelled (see Note 3).
The estimated fair value of the initial Contingent Earn-outs wasis determined using a Monte Carlo analysisvaluation model in order to simulate the future path of our stock price over the earn-out periods. The carrying amount of the liabilities may fluctuate significantly and actual amounts paid may be materially different from the liabilities’ estimated value. As of March 31, 2021 and December 31, 2020, the Contingent Earn-outs were revalued using a similar Monte Carlo analysis. The unobservable inputs to the modelsMonte Carlo valuation model were as follows:
| | | | | | | | |
| | June 30, | | | December 31, | |
| | 2022 | | | 2021 | |
Expected stock price volatility | | | 115.00 | % | | | 105.00 | % |
Risk-free interest rate | | | 3.00 | % | | | 1.10 | % |
Expected dividend yield | | | 0.00 | % | | | 0.00 | % |
Expected term | | 3.50 years | | | 4.00 years | |
22
| | | March 31, 2021 | | | December 31, 2020 | |
| Expected stock price volatility | | | 87.50 | % | | | 85.00 | % |
| Risk-free interest rate | | | 0.40 | % | | | 0.40 | % |
| Expected term | | | 5 years | | | | 5 years | |
The following is a summary of changes in the fair value of our financial liabilities related to the notes payable, the derivative instrument, the Preferred Stock warrants, and the Contingent Earn-outs measured at fair value for the three months ended March 31, 2021 and 2020 (in thousands):
| | | Notes
Payable | | | Clene Nanomedicine Contingent
Earn-out | | | Initial Shareholders Contingent
Earn-out | |
| Balance - December 31, 2020 | | $ | 1,296 | | | $ | 52,054 | | | $ | 5,906 | |
| Change in fair value | | | 543 | | | | 25,609 | | | | 2,961 | |
| Balance - March 31, 2021 | | $ | 1,839 | | | $ | 77,663 | | | $ | 8,867 | |
| | | Notes
Payable | | | Derivative Instrument | | | Preferred Stock Warrants | |
| Balance - December 31, 2019 | | $ | 640 | | | $ | - | | | $ | 3,213 | |
| Issuance of convertible promissory notes | | | - | | | | 189 | | | | - | |
| Change in fair value | | | 12 | | | | 4 | | | | (112 | ) |
| Balance - March 31, 2020 | | $ | 652 | | | $ | 193 | | | $ | 3,101 | |
17. Redeemable Convertible Preferred Stock
In connection with the closing of the Reverse Recapitalization, the Preferred Stock converted into 36,893,894 shares ofNote 15. Common Stock on a 1:0.1389 basis (see Note 1).
As of March 31, 2021June 30, 2022 and December 31, 2020, there were no Preferred Stock outstanding.
The redeemable convertible preferred stock is described in Note 17 “Redeemable Convertible Preferred Stock” in Part II, Item 8 of2021, our 2020 Annual Report on Form 10-K for the year ended December 31, 2020 (“2020 Annual Report”) which was filed with the SEC on March 29, 2021. There have been no changes since our 2020 Annual Report.
18. Common Stock
As of December 31, 2020, ouramended and restated certificate of incorporation as amended and restated, authorized us to issue 100,000,000150,000,000 shares of Common Stock, par value $0.0001$0.0001 per share, and 1,000,000 shares of Preferred Stock,preferred stock, par value $0.0001$0.0001 per share.
Our common shareholdersstockholders are entitled to one vote per share and to notice of any shareholders’stockholders’ meeting. Voting, dividend, and liquidation rights of the holders of Common Stock are subject to the prior rights of holders of all classes of stock and are qualified by the rights, powers, preferences, and privileges of the holders of Preferred Stock.preferred stock. No distributions shall be made with respect to Common Stock until all declared dividends to Preferred Sharespreferred stock have been paid or set aside for payment to the holders of Preferred Stock.payment. Common Stock is not redeemable at the option of the holder.
As of June 30, 2022 and December 31, 2021, our Common Stock issued and outstanding was 63,421,908 and 62,312,097 shares, respectively. As of June 30, 2022 and December 31, 2021, there was no preferred stock issued or outstanding.
OnPrivate Placements
Prior to the closingcompletion of the Reverse Recapitalization, we entered into subscription agreements with various investors (the “2020 PIPE”) for the total 2,303,495sale and issuance of the Tottenham ordinary shares held by the Initial Shareholders and public shareholders were converted into the same number our Common Stock (see Note 3).
On the closing of the Reverse Recapitalization, 644,1642,239,500 shares of our Common Stock were issued to LifeSci as financial advisor to the Reverse Recapitalization (see Note 3).
On December 28, 2020 and prior to the closing of the Reverse Recapitalization, various PIPE investors purchased 2,239,500 shares of our Common Stock at a price of $10.00$10.00 per share, generating net proceeds of $22.2 million. In addition, investors in the 2020 PIPE also received warrants (the “PIPE Warrants”) to purchase a number of shares equal to one-half (1/2) of the number of 2020 PIPE shares, totaling 1,119,750 shares of Common Stock, at $0.01 per share and 1,119,750 warrantssubject to purchase,a 180-day holding period. Between July 1, 2021 and December 20, 2021, the PIPE Warrants were exercised in full for 1,119,750 shares of Common Stock. We received cash proceeds of $11,198.
In May 2021, we entered into subscription agreements with various investors (the “2021 PIPE”) for the sale and issuance of 960,540 shares of Common Stock at an exercisea price of $0.01$9.63 per share, one share of our Common Stock forgenerating net proceeds of $22.2$9.3 million.
At-the-Market Offering
On April 14, 2022, we entered into an Equity Distribution Agreement (the “Distribution Agreement”) with Canaccord Genuity LLC and Oppenheimer & Co. Inc., as placement agents (the “Placement Agents”). In accordance with the terms of the Distribution Agreement, we may offer and sell shares of Common Stock having an aggregate offering price of up to $50.0 million (see Notes 1from time to time through the Placement Agents. The issuance and 3).sale of Common Stock, if any, by us under the Distribution Agreement will be made pursuant to our registration statement on Form S-3 (the “Registration Statement”), which was declared effective by the Securities and Exchange Commission on April 26, 2022 (file number 333-264299), and our prospectus supplement relating to the offering.
Subject to terms of the Distribution Agreement, the Placement Agents are not required to sell any specific number or dollar amount of Common Stock but will act as our placement agents, using commercially reasonable efforts to sell, on our behalf, all of the Common Stock requested by us to be sold, consistent with the Placement Agents’ normal trading and sales practices, on terms mutually agreed between the Placement Agents and us. The Placement Agents will be entitled to compensation under the terms of the Distribution Agreement at a fixed commission rate of 3.0% of the gross proceeds from each issuance and sale of Common Stock, if any.
AsDuring the three and six months ended June 30, 2022, 0 shares of March 31, 2021 and December 31, 2020, our common shares issued and outstandingCommon Stock were 59,574,382 and 59,526,171, respectively. As of March 31, 2021 and December 31, 2020, there were no preferred shares issued and outstanding (see sold under the Distribution Agreement.
Note 17).
19.16. Net Loss Per Share Attributable to Common Shareholders
The following table sets forth the computation of basic and diluted net loss per share attributable to common shareholders (in thousands, except share and per share data):
| | | Three months ended March 31, | |
| | | 2021 | | | 2020 | |
| Numerator: | | | | | | |
| Net loss attributable to common shareholders | | $ | (39,756 | ) | | $ | (3,941 | ) |
| | | | | | | | | |
| Denominator: | | | | | | | | |
| Weighted average shares outstanding | | | 60,670,932 | | | | 17,357,505 | |
| | | | | | | | | |
| Net loss per share attributable to common shareholders, basic and diluted | | $ | (0.66 | ) | | $ | (0.23 | ) |
Included within weighted average common shares outstanding as of March 31, 2021 are 1,119,750 common shares issuable upon the exercise of the PIPE warrants as the warrants are exercisable at any time for nominal consideration, and as such, the shares are considered outstandingstockholders for the purpose of calculating basicthree and diluted net loss per share attributable to common shareholders.six months ended June 30, 2022 and 2021 was as follows:
| | | | | | | | | | | | | | | | |
| | Three Months Ended June 30, | | | Six Months Ended June 30, | |
(in thousands, except share and per share data) | | 2022 | | | 2021 | | | 2022 | | | 2021 | |
Numerator: | | | | | | | | | | | | |
Net loss attributable to common stockholders | | $ | (4,534 | ) | | $ | (3,351 | ) | | $ | (17,888 | ) | | $ | (43,107 | ) |
Denominator: | | | | | | | | | | | | |
Weighted average shares outstanding | | | 63,335,271 | | | | 61,165,018 | | | | 63,095,400 | | | | 60,919,340 | |
Net loss per share attributable to common stockholders, basic and diluted | | $ | (0.07 | ) | | $ | (0.05 | ) | | $ | (0.28 | ) | | $ | (0.71 | ) |
23
We have not considered the effect of the Chardan Unit Purchase Option that would convert to 242,000 shares of our Common Stock and warrants to purchase 110,000 shares of our Commons Stock, in the calculation of diluted loss per share, since the conversion of the Chardan Unit Purchase Option and the exercise of the Chardan Unit Purchase Option Warrants into our Commons Stock would be anti-dilutive (see Notes 1 and 10).
The following outstanding shares of potentially dilutive securities were excluded from the computation of diluted net loss per share attributable to common shareholdersstockholders for the periods presentedthree and six months ended June 30, 2022 and 2021 because including them would have been antidilutive:they were antidilutive, out-of-the-money, or the issuance of such shares is contingent upon certain conditions which were not satisfied by the end of the period:
| | | | | | | | | | | | | | | | |
| | Three Months Ended June 30, | | | Six Months Ended June 30, | |
| | 2022 | | | 2021 | | | 2022 | | | 2021 | |
Convertible notes payable (see Note 9) | | | 482,703 | | | | 482,703 | | | | 482,703 | | | | 482,703 | |
Common stock warrants (see Note 10) | | | 4,477,045 | | | | 4,562,462 | | | | 4,477,045 | | | | 4,562,462 | |
Options to purchase common stock (see Note 13) | | | 12,071,276 | | | | 9,128,563 | | | | 12,071,276 | | | | 9,128,563 | |
Unvested restricted stock awards (see Note 13) | | | 849,215 | | | | 1,482,815 | | | | 849,215 | | | | 1,482,815 | |
Unit purchase option to purchase common stock | | | 0 | | | | 242,000 | | | | 0 | | | | 242,000 | |
Contingent earn-out shares (see Note 3) | | | 6,592,334 | | | | 9,096,185 | | | | 6,592,334 | | | | 9,096,185 | |
Total | | | 24,472,573 | | | | 24,994,728 | | | | 24,472,573 | | | | 24,994,728 | |
| | | Three months ended
March 31, | |
| | | 2021 | | | 2020 | |
| Series C redeemable convertible preferred stock | | | - | | | | 7,264,519 | |
| Series B redeemable convertible preferred stock | | | - | | | | 4,168,815 | |
| Series A redeemable convertible preferred stock | | | - | | | | 16,066,503 | |
| Series C redeemable convertible preferred stock warrants | | | - | | | | 271,439 | |
| Series A redeemable convertible preferred stock warrants | | | - | | | | 1,608,672 | |
| Common stock warrants (see Note 10) | | | 4,336,613 | | | | - | |
| Options to purchase common stock | | | 7,105,801 | | | | 7,240,181 | |
| Chardan Unit Purchase Option to purchase common stock (see Note 1) | | | 242,000 | | | | - | |
| Chardan Unit Purchase Option Warrants (see Notes 1 and 10) | | | 110,000 | | | | - | |
| Clene Nanomedicine contingent earn-out shares (see Note 3 and 12) | | | 8,346,185 | | | | - | |
| Initial Shareholders contingent earn-out shares (see Note 3 and 12) | | | 750,000 | | | | | |
| Total | | | 20,890,599 | | | | 36,620,130 | |
20.Note 17. Related Party Transactions
License and Supply Agreements
Supply Agreement
In August 2018, we entered into a license agreement and exclusive supply agreement (collectively, the “4Life Agreement”) in conjunction with an4Life’s investment made in our Series C Preferred Stockpreferred stock and Series C Preferred Stock Warrants bywarrants. Pursuant to the 4Life Research, LLC, an investor, we entered into a supply agreement with the investor. Under the terms of this agreement,Agreement, we granted the investor4Life an exclusive license to pursue development ofdevelop, make, manufacture, use, sell, and commercialize certain dietary supplements using certain of our intellectual property (“IP”).supplements. The exclusive rights to the IP will be for a term of 5the exclusive license is five years from the commencement of product sales under the 4Life Agreement, which was in April 2021, with options to renew for additional five-year terms. We provide non-pharmaceutical product to 4Life for development, and 4Life pays royalties of licensed product by the investor, with a deemed commencement date3% of January 1, 2023 if sales have not yet commenced, andincremental sales. 4Life is subject to annual minimum sales. The agreement may be renewed for additional 5-year terms. If the investor fails to meet thean annual minimum sales requirements,requirement. If the investorminimum sales are unmet, 4Life may pay us an additional fee to maintain exclusivity or have the investor’s license converted to non-exclusive rights. As part of this agreement, we will provide non-pharmaceutical product tonon-exclusive. Total revenue under the investor4Life Agreement for development efforts and potential future production, and the investor is to pay royalties of 3% of incremental sales, as defined in the agreement. For the three and six months ended March 31,June 30, 2022 and 2021 we sold $0.2 million of product under this agreement and received $0.1 million in advance to be applied against future sales of product under this agreement. We did not sell any products outside of this agreement. We recorded this advanced amountwas as deferred revenue as of March 31, 2021 within accrued liabilities, and we expect to fulfil the performance obligations to release the deferred revenue in the first half of 2021 as the investor purchases product. For the three months ended March 31, 2020, the investor has made commercial sales of their products under the agreement which we recognized as royalty revenues of $14 thousand. For the three months ended March 31, 2020, we did not sell any product under this agreement, and there were no balances outstanding due to or from the investor.
follows:
| | | | | | | | | | | | | | | | |
| | Three Months Ended June 30, | | | Six Months Ended June 30, | |
(in thousands) | | 2022 | | | 2021 | | | 2022 | | | 2021 | |
Product revenue from related parties | | $ | 0 | | | $ | 137 | | | $ | 0 | | | $ | 325 | |
Royalty revenue from related parties | | | 33 | | | | 63 | | | | 56 | | | | 77 | |
Total revenue from related parties | | $ | 33 | | | $ | 200 | | | $ | 56 | | | $ | 402 | |
21.
Note 18. Geographic and Segment Information
Geographic Information
Our long-livedLong-lived assets, which were composed of property and equipment, net by location, was as follows (in thousands):of June 30, 2022 and December 31, 2021, were as follows:
| | | | | | | | |
| | June 30, | | | December 31, | |
(in thousands) | | 2022 | | | 2021 | |
United States | | $ | 8,089 | | | $ | 5,142 | |
Australia | | | 0 | | | | 30 | |
Total property and equipment, net | | $ | 8,089 | | | $ | 5,172 | |
24
| | | As of
March 31, | | As of
December 31, |
| | | 2021 | | 2020 |
| United States | | $ | 4,018 | | | $ | 3,997 | |
| Australia | | | 164 | | | | 228 | |
| Total property and equipment, net | | $ | 4,182 | | | $ | 4,225 | |
Segment Information
We have twoOur operating segments: (i)segment profit measure is segment loss from operations, which is calculated as revenue less cost of revenue, research and development, and commercialization of proprietary nanotechnology drug suspensions (“Drugs”),general and (ii) developmentadministrative expenses. Profit and commercialization of proprietary dietary supplements (“Supplements”).
The operating results of the Drugs and Supplements segmentsloss information by reportable segment for the three and six months ended MarchJune 30, 2022 and 2021 was as follows:
| | | | | | | | | | | | | | | | |
| | Three Months Ended June 30, | | | Six Months Ended June 30, | |
(in thousands) | | 2022 | | | 2021 | | | 2022 | | | 2021 | |
Drugs: | | | | | | | | | | | | |
Revenue from external customers | | $ | 0 | | | $ | 0 | | | $ | 0 | | | $ | 0 | |
Depreciation expense | | | (275 | ) | | | (244 | ) | | | (459 | ) | | | (477 | ) |
Stock compensation expense | | | 2,184 | | | | 4,255 | | | | 4,386 | | | | 7,520 | |
Loss from operations | | | (13,596 | ) | | | (13,421 | ) | | | (26,939 | ) | | | (25,086 | ) |
Supplements: | | | | | | | | | | | | |
Revenue from external customers | | $ | 35 | | | $ | 201 | | | $ | 65 | | | $ | 414 | |
Depreciation expense | | | (12 | ) | | | (11 | ) | | | (23 | ) | | | (22 | ) |
Stock compensation expense | | | 0 | | | | 0 | | | | 0 | | | | 0 | |
Income (loss) from operations | | | 1 | | | | (354 | ) | | | 8 | | | | (384 | ) |
Consolidated: | | | | | | | | | | | | |
Revenue from external customers | | $ | 35 | | | $ | 201 | | | $ | 65 | | | $ | 414 | |
Depreciation expense | | | (287 | ) | | | (255 | ) | | | (482 | ) | | | (499 | ) |
Stock compensation expense | | | 2,184 | | | | 4,255 | | | | 4,386 | | | | 7,520 | |
Loss from operations | | | (13,595 | ) | | | (13,775 | ) | | | (26,931 | ) | | | (25,470 | ) |
A reconciliation of the total of the reportable segments’ loss from operations to consolidated net loss before income taxes for the three and six months ended June 30, 2022 and 2021 was as follows:
| | | | | | | | | | | | | | | | |
| | Three Months Ended June 30, | | | Six Months Ended June 30, | |
(in thousands) | | 2022 | | | 2021 | | | 2022 | | | 2021 | |
Segment loss from operations | | $ | (13,595 | ) | | $ | (13,775 | ) | | $ | (26,931 | ) | | $ | (25,470 | ) |
Total other income (expense), net | | | 9,061 | | | | 10,352 | | | | 9,043 | | | | (17,781 | ) |
Net loss before income taxes | | $ | (4,534 | ) | | $ | (3,423 | ) | | $ | (17,888 | ) | | $ | (43,251 | ) |
Segment assets exclude corporate assets, such as cash, restricted cash, and corporate facilities. Total assets by reportable segment as of June 30, 2022 and December 31, 2021, and 2020 were as follows (in thousands): follows:
| | | | | | | | |
| | June 30, | | | December 31, | |
(in thousands) | | 2022 | | | 2021 | |
Total assets: | | | | | | |
Drugs | | $ | 17,617 | | | $ | 12,052 | |
Supplements | | | 288 | | | | 337 | |
Corporate | | | 26,637 | | | | 50,674 | |
Consolidated | | $ | 44,542 | | | $ | 63,063 | |
| | | For the Three Months ended March 31, 2021 |
| | | Drugs | | Supplements | | Total |
| Revenue from external customers | | $ | - | | | $ | 213 | | | $ | 213 | |
| Loss from operations | | $ | (11,665 | ) | | $ | (30 | ) | | $ | (11,695 | ) |
| | | For the three months ended March 31, 2020 |
| | | Drugs | | Supplements | | Total |
| Revenue from external customers | | $ | - | | | $ | 70 | | | $ | 70 | |
| (Loss) Income from operations | | $ | (4,014 | ) | | $ | 12 | | | $ | (4,002 | ) |
OurAdditions to long-lived assets which were composed of propertythrough cash expenditure, accounts payable, and equipment, net by segment wasa lease incentive representing an allowance for facility alterations. For the six months ended June 30, 2022 and 2021, total additions were as follows (in thousands):follows:
| | | | | | | | |
| | Six Months Ended June 30, | |
(in thousands) | | 2022 | | | 2021 | |
Drugs | | $ | 3,399 | | | $ | 420 | |
Supplements | | | 0 | | | | 0 | |
Corporate | | | 0 | | | | 0 | |
Consolidated | | $ | 3,399 | | | $ | 420 | |
| | | As of
March 31, | | As of December 31, |
| | | 2021 | | 2020 |
| Drugs | | $ | 3,958 | | | $ | 3,990 | |
| Supplements | | | 224 | | | | 235 | |
| Total property and equipment, net | | $ | 4,182 | | | $ | 4,225 | |
22.Note 19. Subsequent Events
On August 5, 2022, we amended and restated a promissory note pursuant to the 2022 MD Loan with DHCD. The amended and restated promissory note clarified the repayment terms of the note (see Note 9 under the heading “Maryland Loans”).
On April 19,August 9, 2022, we entered into a second amendment to the 2021 the registration statementAvenue Loan which permits indebtedness secured by a lien of up to $3.0 million with any unrelated third-party providing equipment financing on Form S-1 referredterms reasonably acceptable to in Note 1 was declared effective by the SEC (file number 333-253173).Avenue.
25
ITEM
Item 2. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONSManagement’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our condensed consolidated financial statements and other parts ofrelated notes included elsewhere in this Quarterly Report containon Form 10-Q. This discussion contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 (the “Securities Act”), as amended, and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). The forward-looking statements include, but are not limited to,reflecting our or management team’s expectations, hopes, beliefs, intentions, or strategies estimates and assumptions concerning events and financial trends that may affectregarding our future results of operations or financial condition.operations. In addition, any statements that refer to projections, forecasts or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking statements. The words “anticipate,” “believe,” “contemplate,” “continue,” “could,” “estimate,” “expect,” “intends,” “may,” “might,” “plan,” “possible,” “potential,” “predict,” “project,” “should,” “will,” “would”“would,” and similar expressions may identify forward-looking statements, but the absence of these words does not mean that a statement is not forward-looking. TheseActual results and the timing of events may differ materially from those contained in these forward-looking statements are based on information available as of the date of this Quarterly Report and our management’s current expectations, forecasts and assumptions, and involvedue to a number of judgments, risks and uncertainties. Accordingly, forward-looking statements should not be relied upon as representingfactors, including those discussed in the section titled “Risk Factors” in our views as of any subsequent date.Annual Report on Form 10-K. We disclaim anyundertake no obligation to update forward-looking statements to reflect events or circumstances after the date they were made, whether as a result of new information, future events, or otherwise, except as specificallymay be required under applicable securities laws.
As a result of a number of known and unknown risks and uncertainties, our actual results and Unless the timing of events may differ materially from those expressed or implied by these forward-looking statements due to a number of factors, including those discussed in the section titled “Risk Factors” appearing elsewhere in this Quarterly Report.
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our condensed consolidated financial statements and the related notes appearing in Part I, Item Icontext otherwise requires, for purposes of this Quarterly Report on Form 10-Qsection, the terms “we,” “us,” the “Company” or “our” are intended to mean the business and with our Annual Report on Form 10-K for the year ended December 31, 2020 (the “2020 Annual Report”) which was filed with the SEC on March 29, 2021, pursuant to Rule 424(b)(4) under the Securities Act, as amended.operations of Clene Inc. and its consolidated subsidiaries.
Business Overview
We are a clinical-stage pharmaceutical company pioneering the discovery, development, and commercialization of novel clean-surfaced nano (CSN)nanotechnology (“CSN®”) therapeutics. CSN® therapeutics are comprised of atoms of transition elements that, when assembled in nanocrystallinenanocrystal form, possess unusually high, unique catalytic activities not present in those same elements in bulk form. These nanocatalyticcatalytic activities drive, support, and maintain beneficial metabolic and energetic intercellularcellular reactions within diseased, stressed, and damaged cells.
Our patent-protected, proprietary position affords us the potential to develop a broad and deep pipeline of novel CSN® therapeutics to address a range of diseases with high impact on human health. We began in 2013 by innovating an electrochemistryelectro-crystal-chemistry drug development platform that draws from advances in nanotechnology, plasma and quantum physics, material science, and biochemistry. Our platform process results in nanocrystals with faceted structures and surfaces that are free of the chemical surface modifications that accompany other production methods. Many traditional methods of nanoparticle synthesis involve the unavoidable deposition of potentially toxic organic residues and stabilizing surfactants on the particle surfaces. Synthesizing stable nanocrystals that are both nontoxic and highly catalytic has overcome this significant hurdle in harnessing transition metal catalytic activity for human therapeutic use.
Our clean-surfaced nanocrystals exhibit catalytic activities many foldmany-fold higher than multiple other commercially available nanoparticles, produced using various techniques, that we have comparatively evaluated. We now have multiple drug assets currently in development and/or clinical trials for applications in neurology, infectious disease, and oncology. Our development and clinical efforts are currently focused on addressing the high unmet medical needs in two areas: first, those related to central nervous system disorders including Multiple Sclerosis (“MS”), Parkinson’s Disease (“PD”) and Amyotrophic Lateral Sclerosis (“ALS”ALS”), Multiple Sclerosis (“MS”), and Parkinson’s Disease (“PD”); and second, those related to the pandemic caused by COVID-19, a highly infectious viral respiratory disease with serious and sometimes fatal co-morbidities.
On December 30, 2020, Chelsea Worldwide, Inc., our predecessor company, consummated the previously announced business combination (referred to as the “Reverse Recapitalization”) pursuant to a merger agreement, dated as of September 1, 2020 (the “Merger Agreement”), by and among Clene Nanomedicine, Inc. (“Clene Nanomedicine”), Tottenham Acquisition I Limited (“Tottenham” or “TOTA”), the public entity prior to the Reverse Recapitalization, Chelsea Worldwide Inc., a Delaware corporation and wholly-owned subsidiary of Tottenham (“PubCo”), Creative Worldwide Inc., a Delaware corporation and wholly-owned subsidiary of PubCo (“Merger Sub”), and Fortis Advisors LLC, a Delaware limited liability company as the representative of our shareholders (“Shareholders’ Representative”). Prior to the Reincorporation Merger discussed below, Tottenham was incorporated in the British Virgin Islands as a blank check company for the purpose of entering into a merger, share exchange, asset acquisition, stock purchase, recapitalization, reorganization or other similar business combination with one or more businesses or entities.
The Reverse Recapitalization was effected in two steps: (i) Tottenham was reincorporated to the state of Delaware by merging with and into PubCo (the “Reincorporation Merger”); (ii) promptly following the Reincorporation Merger, Merger Sub was merged with and into Clene Nanomedicine, resulting in Clene Nanomedicine being a wholly-owned subsidiary of PubCo (the “Acquisition Merger”). On the Closing Date, PubCo changed its name from Chelsea Worldwide Inc. to Clene Inc. and listed its shares of common stock, par value $0.0001 per share (“Common Stock”) on the Nasdaq Stock Exchange (the “Nasdaq”) under the symbol “CLNN.” As a result of the Reverse Recapitalization, Clene Nanomedicine became a wholly-owned direct subsidiary of Clene Inc. For periods prior to the closing of the Reverse Recapitalization on December 30, 2020, the disclosure in Management’s Discussion and Analysis of Financial Condition and Results of Operations has been updated to give effect to the Reverse Recapitalization.
We filed a registration statement on Form S-1 to register 4,541,481 shares of Common Stock underlying outstanding warrants that we had previously issued, which the SEC declared to be effective on April 19, 2021 (file number 333-253173). We will receive aggregate gross proceeds of $30.7 million if all of these warrants are exercised. In conjunction with the preparation of the registration statement on Form S-1, we incurred offering costs of $27 thousand, which were recognized as an expense within general and administrative expenses in the condensed consolidated statement of operations for the three months ended March 31, 2021.
We currently have no drugs approved by the US Food and Drug Administration (”FDA”) for commercial sale and have not generated any revenue from drug sales. We have never been profitable and have incurred operating losses in each year since inception. We began supplying low-dosegenerate revenue from sales of dietary supplements tothrough our wholly owned subsidiary, dOrbital, Inc., or through an exclusive license with 4Life Research LLC one of(“4Life”), a stockholder and related party. We anticipate these revenues to be small compared to our shareholders,operating expenses and had minimal directto the revenue we expect to generate from potential future sales of our rMetx™ ZnAg Immune Boost dietary supplement product. Our totaldrug candidates, for which we are currently conducting clinical trials. We incurred a loss from operations was $11.7of $13.6 million and $4.0$13.8 million for the three months ended March 31,June 30, 2022 and 2021, respectively; and 2020,$26.9 million and $25.5 million for the six months ended June 30, 2022 and 2021, respectively. Substantially all of our losses from operations resulted from research and development expenses and administrative expenses. As of March 31, 2021June 30, 2022 and December 31, 2020,2021, we had an accumulated deficit of $193.3$181.2 million and $153.6$163.3 million, respectively.
We expect to continue investing in product development and sales and marketing, and customer support for our products andwe expect to incur additional losses in the future to fund our operations and conduct product research and development. We also recognize the need to raise additional capital to fully implement our business plan. The long-term continuation of our business plan is dependent upon the generation of sufficient revenues from our products to offset expenses and capital expenditures. In the event that we do not generate sufficient revenues and are unable to obtain funding, we will be forced to delay, reduce, or eliminate some or all of our research and development programs, product portfolio expansion, commercialization efforts, or capital expenditures, which could adversely affect our business prospects, ability to meet long-term liquidity needs, or we may be unable to continue operations.
26
Recent Developments of Our Clinical Programs
CNM-Au8®: We have one Phase 2/3 registration clinical trial, the Healey ALS Platform Trial, which is currently ongoing to establish the safety and efficacy of CNM-Au8 in patients with ALS. Based on discussions with the sponsor and review of data from the RESCUE-ALS clinical trial, the secondary and exploratory endpoints for CNM-Au8 (Regimen C) were recently revised in the statistical analysis plan, which has been submitted to the U.S. Food and Drug Administration (“FDA”). Secondary endpoints now include a combined joint-rank score based on survival and change in ALSFRS-R score (combined assessment of function and survival, or CAFS), change in slow vital capacity, and overall survival. Exploratory endpoints include change in hand-held dynamometry measurements, voice pathology measurements, time to clinical events, and biofluid-based pharmacodynamic and metabolic assays. The primary endpoint, change of the slope of the ALSFRS-R score from baseline to week 24, incorporating survival into the statistical model, is unchanged. Topline results are anticipated in the third quarter of 2022, with full unblinded results anticipated in the fourth quarter of 2022. Should the Healey ALS Platform Trial result in a statistically significant treatment benefit that supports the submission of a New Drug Application (“NDA”), we plan to discuss the totality of CNM-Au8 clinical data with the FDA in anticipation of an NDA filing in 2023.
We recently reported positive topline data from our Phase 2 VISIONARY-MS clinical trial which evaluated the efficacy and safety of CNM-Au8 in stable relapsing remitting MS patients. The trial was stopped prematurely due to COVID-19 pandemic operational challenges, limiting enrollment to 73 out of the 150 planned participants. Due to the limited enrollment, the threshold for significance was pre-specified at p=0.10 prior to database lock. The primary analysis was conducted in a modified intent to treat (“mITT”) population, which censored invalid data. The mITT population excluded data from a single site (n=9) with Low Contrast Letter Acuity (“LCLA”) testing execution errors and the timed 25-foot walk data from one subject with a change in mobility assist device. The ITT results were directionally consistent with the mITT results, although the ITT results were not significant. The trial met the primary endpoint of change from baseline in LCLA at 48 weeks compared to placebo. The trial also met the secondary endpoints of mean standardized change from baseline in the modified MS Functional Composite (“mMSFC”) and mMSFC average rank score. CNM-Au8 was well-tolerated, and there were no significant safety findings reported. The open label extension of VISIONARY-MS is ongoing.
We also completed the first dosing cohort of REPAIR-MS, an open-label, investigator blinded Phase 2 clinical trial, and have initiated a second dosing cohort in non-active progressive MS patients which is expected to be complete by the end of 2022. We anticipate launching RESCUE-PD, a Phase 2 clinical trial for the treatment of patients with PD, in the fourth quarter of 2022.
CNM-ZnAg: We have one Phase 2 clinical trial that recently concluded the blinded treatment period. The objective of this study is to investigate the efficacy and safety of CNM-ZnAg for the treatment of COVID-19. As pre-specified in the protocol, due to insufficient hospitalization events in the randomized study population, the primary and secondary endpoints were interchanged. The primary endpoint is now time to substantial alleviation of COVID-19 symptoms up to 28-days, over a continuous period greater than or equal to 48 hours. The key secondary endpoints include (i) time to complete alleviation of COVID-19 symptoms up to 28-days, over a continuous period greater than or equal to 48 hours; and (ii) the proportion of participants who are hospitalized, require hospitalization, or are deceased from baseline to day 28 (the original primary endpoint). Topline results are anticipated in the third quarter of 2022.
27
The chart below reflects the respective stages of our main drug candidates.
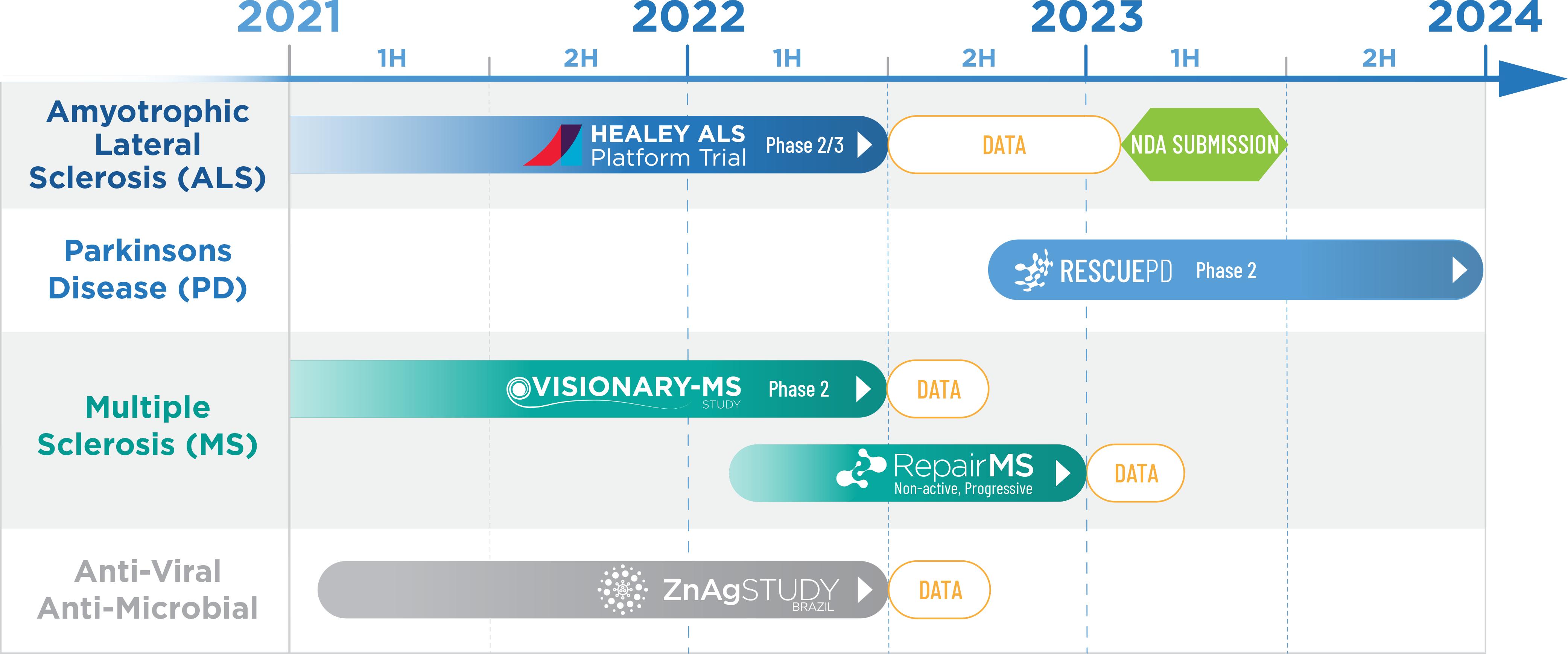
Recent Competition Update
Despite the great need for an effective disease-modifying treatment for ALS, and significant research efforts by the pharmaceutical industry to meet this need, there have been limited clinical successes and no curative therapies approved to date. In March 2022, the FDA Peripheral and Central Nervous System Drugs Advisory Committee (“PCNSDAC”) reviewed a New Drug Application (“NDA”) for AMX0035, an investigational drug from Amylyx Pharmaceuticals, Inc., for the treatment of ALS. The PCNSDAC voted 4 (yes) and 6 (no) as to whether the data from a single randomized, controlled trial and the open-label extension study established a conclusion that AMX0035 was effective in the treatment of patients with ALS. The PCNSDAC will reconvene on September 7, 2022 to discuss additional analyses of AMX0035 clinical trial data, and the FDA has extended the review timeline of the NDA, with a Prescription Drug User Fee Act (“PDUFA”) goal date of September 29, 2022. In June 2022, Health Canada conditionally approved AMX0035 for the treatment of ALS.
In May 2022, the FDA approved an orally administered version of edaravone, which has been available since 2017 as an intravenous infusion for the treatment of ALS. Additionally, in July 2022, the FDA accepted an NDA for tofersen, an investigational drug from Biogen Inc., for the treatment of superoxide dismutase 1 (“SOD1”) ALS. The NDA has been granted priority review with a PDUFA goal date of January 25, 2023.
Impact of the COVID-19 Coronavirus Pandemic
The COVID-19 pandemic, which began in December 2019 and has spread worldwide, has caused many governments to implement measures to slow the spread of the COVID-19 outbreak. The outbreakCOVID-19 pandemic and government measures taken in response have had a significant impact, both direct and indirect, on businesses and commerce, as worker shortages have occurred, supply chains have been disrupted, and facilities and production have been suspended. The future progression of the COVID-19 pandemic and its effects on our business and operations remain uncertain. The COVID-19 pandemic may affect our ability to initiate and complete preclinical studies and clinical trials, delay the initiation of future clinical trials, disrupt regulatory activities, or have other adverse effects on our business and operations. In particular, we and our clinicalthird-party contract research organizations (“CROs”CROs”) may facehave faced disruptions that may affecthave affected our ability to initiate and complete preclinical studies, causecaused manufacturing disruptions, or createand created delays at clinical trial sites.site initiation and clinical trial enrollment, leading to the early conclusion of an ongoing clinical trial. The COVID-19 pandemic has already caused significant disruptions in the financial markets, and may continue to cause such disruptions, which could impact our ability to raise additional funds to support our operations. Moreover, the COVID-19 pandemic has significantly impacted economies worldwide and could result in adverse effects on our business and operations.
We are monitoring the potential impact of the COVID-19 pandemic on our business, financial condition, results of operations, and financial statements.cash flows. While the COVID-19 pandemic has led to various research restrictions and pausedled to pauses and early conclusion of certain of our clinical trials, these impacts have been temporary and to date we have not experienced material business disruptions or incurred impairment losses in the carrying values of our assets as a result of the COVID-19 pandemic. We are not aware of any specific related
28
event or circumstance that would require us to revise the estimates reflected in our condensed consolidated financial statements. The extent to which the COVID-19 pandemic will directly or indirectly impact our business, financial condition, results of operations, and financial condition,cash flows, including planned and future clinical trials and research and development costs, will depend on future developments that are highly uncertain, including as a result of new information that may emerge concerning COVID-19, the actions taken to contain or treat it, and the duration and intensity of the related effects.
Reverse Recapitalization with Tottenham and Clene Nanomedicine
On December 30, 2020 we completed(the “Closing Date”), Chelsea Worldwide Inc., our predecessor, consummated a business combination (the “Reverse Recapitalization”) by and among Clene Nanomedicine, Inc. (“Clene Nanomedicine”), Tottenham Acquisition I Limited (“Tottenham”), Chelsea Worldwide Inc. (“PubCo”), a Delaware corporation and wholly-owned subsidiary of Tottenham, Creative Worldwide Inc. (“Merger Sub”), a Delaware corporation and wholly-owned subsidiary of PubCo, and Fortis Advisors LLC, a Delaware limited liability company as the previously announced Reverse Recapitalization (see “Business Overview” above).
At the closingrepresentative of our stockholders. Prior to the Reverse Recapitalization, Clene Inc. acquired 100%Tottenham was incorporated in the British Virgin Islands as a blank check company for the purpose of the issued and outstanding Clene Nanomedicine commonentering into a merger, share exchange, asset acquisition, stock in exchange for 54,339,012 shares of Clene Inc. Common Stock issuedpurchase, recapitalization, reorganization, or other similar business combination with one or more businesses or entities. Prior to the Clene Nanomedicine common shareholders, of which 2,716,958 shares of the Clene Inc. Common Stock are to be issued and held in escrow to satisfy any indemnification obligations incurred under the Merger Agreement.
At the closing of the Reverse Recapitalization, each stock optionthere was not a public market for the shares of Clene Nanomedicine common stock was cancelled and the holders thereof in exchange received 0.1320 newly-issued stock options of our Common Stock, which is 95% of the exchange ratio determined in the Merger Agreement. Pursuant to the Merger Agreement, we issued 370,101 of restricted stock units (“RSUs”) to the option holders which complements the 5% closing payment shares held in escrow for Clene Nanomedicine common shareholders discussed above. In addition, we issued 1,136,961 RSUs to option holders to complement the earn-out payments that would contingently be issued to certain current Clene Nanomedicine’s shareholders upon the achievement of milestones. See “Earn-out Shares” for the milestones detail. stock.
Immediately after giving effect to the Reverse Recapitalization and the PIPE offering discussed in below, there were 59,526,171 shares of Common Stock issued and outstanding and warrants to purchase 5,566,363 shares of Common Stock issued and outstanding.
The transaction was accounted for as a “reverse recapitalization” and Tottenham was treated as the “acquired” company for accounting purposes. Accordingly, for accounting purposes, the Reverse Recapitalization was treated aseffected in two steps: (i) Tottenham was reincorporated to the equivalentstate of Delaware by merging with and into PubCo; and (ii) Merger Sub was merged with and into Clene Nanomedicine, issuing shares forresulting in Clene Nanomedicine becoming a wholly-owned subsidiary of PubCo. On the net assets of Tottenham, accompanied by a recapitalization. The net assets of Tottenham were recorded at historical costs, with no goodwill or other intangible assets recorded. Reported amountsClosing Date, PubCo changed its name from operations included herein priorChelsea Worldwide Inc. to the Reverse Recapitalization are those of Clene Nanomedicine.
The PIPE Offering
Prior to the completion of the Reverse Recapitalization on December 30, 2020, we entered into subscription agreements on December 28, 2020, with various investors (the “PIPE”). Pursuant to the subscription agreements, we issued 2,239,500Inc. and listed its shares of our Common Stock (the “PIPE Shares”) at a price of $10.00common stock, par value $0.0001 per share with net proceeds of $22.2 million. The purpose of(“Common Stock”) on the PIPE is to fund general corporate expenses. In addition, investors inNasdaq Capital Market (“Nasdaq”) under the PIPE offering will also receive warrants to purchase a number of shares equal to one-half (1/2) of the number of PIPEsymbol “CLNN.”
Earn-Out Shares for an aggregate total of 1,119,750 shares of our Common Stock, at an exercise price of $0.01 per share (the “PIPE Warrants”), subject to a 180-day holding period.
Key Factors Affecting Our Results of Operations
Our results of operations, financial condition and the period-to-period comparability of our financial results are principally affected by the following factors:
Earn-out Shares
In connection with the Reverse Recapitalization, certain of Clene Nanomedicine’s current shareholderscommon stockholders are entitled to receive earn-out payments (the “Clene Nanomedicine Contingent Earn-out”), and Tottenham’s former officers and directors and the SponsorNorwich Investment Limited (collectively, the “Initial Shareholders”“Initial Stockholders”) are entitled to receive earn-out payments (the ““Initial Stockholders Contingent Earn-outsEarn-out,” and both collectively the “Contingent Earn-outs”) based on achieving milestones discussed below.certain milestones. The Contingent Earn-outs have been classified as liabilities in the condensed consolidated balance sheets and were initially measured at fair value on the date of the Reverse Recapitalization and will beare subsequently remeasured to fair value at each reporting date. The change in fair value of the Contingent Earn-outs has been recorded in the condensed consolidated statements of operations and comprehensive loss for the three and six months ended March 31,June 30, 2022 and 2021.
Financial Overview
The Contingent Earn-out provision for Clene Nanomedicine’s common shareholders (the “Clene Nanomedicine Contingent Earn-out”) includes (i) Milestone 1 that is based on achieving a certain volume-weighted average priceOur financial condition, results of operations, and the sharesperiod-to-period comparability of our Common Stock within three years afterfinancial results are principally affected by the closing of the Reverse Recapitalization or the change of control price equaling or exceeding a certain price if a change of control transaction occurs within the three years following the closing of the Reverse Recapitalization, (ii) Milestone 2 that is based on achieving a certain volume-weighted average price of the shares of our Common Stock within five years after the closing of the Reverse Recapitalization or the change of control price equaling or exceeding a certain price if a change of control transaction occurs within the five years following the closing of the Reverse Recapitalization, and (iii) Milestone 3 that is based on completing by December 30, 2021 a randomized placebo-controlled study for treatment of COVID-19 coronavirus.factors:
The Contingent Earn-out provision for the Initial Shareholders (the “Initial Shareholders Contingent Earn-out”) includes Milestone 1 and Milestone 2 listed above. Upon the consummation of the Reverse Recapitalization, Clene Nanomedicine and the Initial Shareholders are entitled to receive up to 8,346,185 and 750,000 shares of our Common Stock, respectively.
The estimated fair values of the contingent consideration were determined using Monte Carlo simulations that simulated the future path of our Common Stock price over the earn-out periods. The assumptions utilized in the calculations are based on the achievement of certain stock price milestones including projected stock price, volatility, and risk-free rate. For potential payments related to a product development milestone, the fair value was determined based on our expectations of achieving such a milestone and the simulated estimated stock price on the expected date of achievement.
Contingent Earn-out payments involve certain assumptions requiring significant judgment and actual results may differ from assumed and estimated amounts.
Research and Development ExpensesExpense
The discovery and development of novel drug candidates require a significant investment of resources over a prolonged period of time, and a core part of our strategy is to continue making sustained investments in this area. As a result of this commitment, our pipeline of drug candidates has been advancing and expanding, with two clinical-stage drug candidates currently being investigated.
We anticipate thatHistorically, substantially all of our research and development expenses will increase significantly duerelate to the increase in clinical trial expenses incurred to developCNM-Au8, our drug candidates, expenses incurred for payments to CROs, principal investigators and clinical trial sites, costs of materials to support our clinical trials and preclinical studies, costs associated with preclinical activities, share awards granted to our research and development personnel and salaries for our expanding research and development personnel headcount.lead asset. Our research and development expenses are affected by the timing and advancement of our existing product pipeline as well as the timing and quantity of new drug programs commenced.
Funding for Our Operations
Since our inception, we have dedicated substantially all of our resources to the development of our drug candidates. We have financed our operations principally through proceeds from the issuance of preferred stock, issuance of common stock upon exercise of common stock options, convertible promissory notes, issuances of notes payable, and the consummation of the Reverse Recapitalization.
Since our inception and through the date of this Quarterly Report, we have funded our operations primarily with proceeds from the following sources:
| ● | gross proceeds of $87.2 million from sales of our preferred stock and other equity financing; |
| ● | gross proceeds of $28.1 million from borrowings under convertible promissory notes; |
| ● | gross proceeds of $0.6 million through external lenders; |
| ● | gross cash proceeds of $31.7 million through the Reverse Recapitalization and the PIPE offering; |
| ● | gross cash proceeds of $0.6 million from a Program Paycheck Protection loan obtained through the U.S. Small Business Administration. This loan was forgiven in January 2021; and |
| ● | gross cash proceeds of $0.5 million from a grant from the Michael J. Fox Foundation obtained in January 2021 for our preclinical research funding. |
We have also been awarded grants from various other organizations, including the U.S. Congressionally Directed Medical Research Program administered by the Department of Defense, the National Multiple Sclerosis Society, and FightMND, a not-for-profit registered charity in Australia, who together have issued us grants totaling approximately $2.9 million. We also receive indirect financial support for one of the clinical studies in which we participate, the Healey ALS Platform Trial, administered by the Massachusetts General Hospital, which is conducting a study of our CNM-Au8 drug candidate along with other drugs in a platform trial, at significantly lower costs to us than we would otherwise incur if we were to conduct a comparably designed study on our own at reasonable market rates.
The net cash used in our operating activities was $9.2 million and $3.3 million for the three months ended March 31, 2021 and 2020, respectively. As of March 31, 2021, we had cash of $48.0 million. We expect that the cash on hand as of March 31, 2021 will be sufficient to fund our operations for a period extending beyond twelve months from the date the condensed consolidated financial statements are issued. We have based this estimate on assumptions that may prove to be wrong, and we may exhaust our available capital resources sooner than we anticipate. See “— Liquidity and Capital Resources.” We expect our expenses to increase significantly in connection with our ongoing activities, particularly as we advance the clinical development of our clinical-stage drug products and continue research and development of our preclinical drug products and initiate additional clinical trials of, and seek regulatory approval for, these and other future drug products. As we continue to grow and expand, we will incur more expenses relating to regulatory compliance and sales and marketing personnel as we prepare to commence commercialization once we obtain regulatory approval of our drug products.
General and Administrative Expenses
Our general and administrative expenses consist primarily of staff costs, agency and consulting fees, utilities, rent and general office expenses, share grants, and RSUs grants. We anticipate that our general and administrative expenses will increase in future periods to support increases in our research and development activities and as we continue to rapidly advance the clinical programs of our drug products and expect to commercialize our products once we receive regulatory approval. These increases will likely include increased headcount, increased share compensation charges, expanded infrastructure and increased insurance expenses. We also anticipate increasing legal, compliance, accounting and investor and public relations expenses associated with being a public company.
Grants and Government Tax Incentives
We received grants issued by non-government entities related to income which have future related costs expected to be incurred and require us to comply with conditions attached to the grants. These non-government grants related to income are recognized in profit or loss as an offset to research and development expenses when funding has been received and related costs have been incurred. We received tax incentives from the Australian government in the form of cash subsidies for research and development activities related to clinical trial activities conducted by our Australian subsidiary, which are recognized as other income upon compliance with certain conditions. We did not recognize grant funding against research and development expenses for the three months ended March 31, 2021. We recognized $0.2 million of grant funding against research and development expenses for the three months ended March 31, 2020. We recognized $0.3 million of other income for the three months ended March 31, 2021 that we classified as Australia research and development credit. We did not recognize other income for the three months ended March 31, 2020.
Commercialization of Our Drug Candidates
Our business and results of operations depend on our ability to commercialize our drug candidates, if approved for marketing. Our pipeline is comprised of four drug candidates ranging from pre-clinical to late-stage clinical programs, including two drug candidates at the clinical stage or IND stage. Although we currently do not have any drug candidates approved for commercial sale and have not generated any revenue from drug product sales, we expect to commercialize one or more of our drug products in the coming years as they move toward the final stages of development. While we began selling our ZnAg Immune Boost product online in May 2020, we anticipate revenue generated from sales of this dietary supplement will be small compared to our operating expenses as well as the revenue we expect to generate from future sales of our drug candidates for which we are currently conducting clinical trials.
Components of Results of Operations
Comparison of the three months ended March 31, 2021 and 2020
The following table summarizes our results of operations for the three months ended March 31, 2021 and 2020:
| | | Three Months ended | |
| | March 31, | |
| | | 2021 | | | 2020 | |
| | | (in thousands) | |
| Product revenue | | $ | 199 | | | $ | 70 | |
| Royalty revenue | | | 14 | | | | - | |
| Total revenue | | | 213 | | | | 70 | |
| Operating expenses: | | | | | | | | |
| Cost of revenue | | | 243 | | | | 58 | |
| Research and development | | | 6,275 | | | | 3,202 | |
| General and administrative | | | 5,390 | | | | 812 | |
| Total operating expenses | | | 11,908 | | | | 4,072 | |
| Loss from operations | | | (11,695 | ) | | | (4,002 | ) |
| Other income (expenses): | | | | | | | | |
| Interest expense | | | (551 | ) | | | (51 | ) |
| Gain on extinguishment of debt | | | 647 | | | | - | |
| Change in fair value of preferred stock warrant liability | | | - | | | | 112 | |
| Change in fair value of derivative liability | | | - | | | | 4 | |
| Change in fair value of Clene Nanomedicine contingent earn-out | | | (25,610 | ) | | | - | |
| Change in fair value of Initial Shareholders contingent earn-out | | | (2,961 | ) | | | - | |
| Australia research and development credit | | | 339 | | | | - | |
| Other income, net | | | 3 | | | | (4 | ) |
| Total other income (expense), net | | | (28,133 | ) | | | 61 | |
| Net loss before income taxes | | | (39,828 | ) | | | (3,941 | ) |
| Income tax benefit | | | 72 | | | | - | |
| Net loss | | | (39,756 | ) | | | (3,941 | ) |
| | | | | | | | | |
| Other comprehensive income (loss): | | | | | | | | |
| Foreign currency translation adjustments | | | 24 | | | | 6 | |
| Total other comprehensive income (loss) | | | 24 | | | | 6 | |
| Comprehensive loss | | $ | (39,732 | ) | | $ | (3,935 | ) |
Revenue
We generated revenue of $0.2 million and $70 thousand for the three months ended March 31, 2021 and 2020, respectively, which we separate as product revenue and royalty revenue. Product revenue of $0.2 million and $70 thousand was recognized in our dietary supplement segment under a supply agreement with 4Life Research, LLC, a related party, for KHC46 and a low dose zinc-silver solution, two dietary (mineral) supplements that we began supplying during those periods. We also generated minimal product revenue from sales of rMetx™ ZnAg Immune Boost during those periods. In addition, $14 thousand of our revenue during the three months ended March 31, 2021 was paid to us by 4Life Research, LLC under an exclusive and royalty-bearing license agreement relating to sales of KHC46. We did not generate royalty revenue during the three months ended March 31, 2020. For more details on the license agreement, see Note 20 to our condensed consolidated financial statements included in Part I, Item 1 of this Quarterly Report on Form 10-Q.
Operating Expenses
Cost of Sales
We incurred cost of sales of $0.2 million and $58 thousand for the three months ended March 31, 2021 and 2020, respectively, relating to production and distribution costs for the sales of our KHC46 and low dose zinc-silver solution dietary supplement products through supply agreements we have entered into with a related party.
Research and Development Expenses
Research and development expenses were $6.3 million and $3.2 million, representing 52.7%, and 78.6% of our total operating expenses for the three months ended March 31, 2021 and 2020, respectively. During these periods, substantially all of our research and development expenses were related to the development and clinical trials of our lead drug candidate, CNM-Au8. This increase of $3.1 million, or 96.0%, was primarily due to the progression of our drug candidates through the clinical development process, including increased enrollment into the REPAIR-PD and the REPAIR-MS studies, and calendar payments due for our participation in the Healey-ALS Platform Trial. These efforts resulted in greater associated costs and manufacturing expenses in support of these trials. Also, during the three months ended March 31, 2021, Research and Development expenses included $1.3 million of share-based compensation expense related to RSUs.
Historically, substantially all of our research and development expenses relate to CNM-Au8, our lead asset. Drug candidates in later stages of clinical development generally have higher development costs than those in earlier stages of clinical development, primarily due to per patient clinical trial site fees for larger studies,clinical trials, the costs of opening and monitoring clinical sites, CRO activity, and manufacturing expenses. We expectanticipate that our research and development expenses will increase significantly due to the increase in connection withclinical trial expenses incurred to develop our clinical development activities in the near term and in the future.drug candidates.
Research and development costs are charged to operations as incurred. Research and development costs include payroll and personnel expenses, including salaries and related benefits and stock-based compensation expense for employees engaged in research and development functions,functions; clinical trial supplies fees forand materials to support our clinical trials; payments to CROs, principal investigators, and clinical trial services,sites; costs associated with preclinical activities; consulting costs,costs; and allocated overhead, including rent, equipment, utilities, depreciation, insurance, and facilities maintenance costs. We account for nonrefundable advance payments for goods and services that will be used in future research and development activities initially as an asset and then as expenses when the goods have been received or when the service has been performed rather than when the payment is made.
29
Our clinical trial accrual process seeks to account for expenses resulting from obligations under contracts with CROs, consultants, and under clinical site agreements in connection with conducting clinical trials. The financial terms of these contracts are subject to negotiations, which vary from contract to contract and may result in payment flows that do not match the periods over which materials or services are provided to us under such contracts. We reflect the appropriate trial expenses in the condensed consolidated financial statements by matching the appropriate expenses with the period in which services and efforts are expended. In the event advance payments are made to a CRO, the payments will be recorded as a prepaid asset, which will be expensed over the period of time the contracted services are performed.
General and Administrative ExpensesExpense
General and administrative expenses consist primarily of employee salarypayroll and personnel expenses, including salaries and related benefits share-basedand stock-based compensation expenses,expense; professional fees for legal, consultingaccounting, tax, and audit servicesinformation technology services; fees for directors’ and officers’ insurance; expenses for business development activities,activities; utilities and facility expenses; travel expenses,expenses; rental feesfees; consulting fees; and other administrative expenses.
We expectanticipate that our general and administrative expenses will increase in future periods to increasesupport increases in our drug development activities and as we continuebuild out our commercial capabilities in advance of receiving regulatory approval for the rapidly advancing clinical trials of our drug candidates. These increases will likely include increased headcount, increased stock compensation expenses, expanded infrastructure including certain sales and marketing activities performed ahead of regulatory approval, and increased insurance expenses. We also anticipate increasing legal, compliance, accounting, and investor and public relations expenses associated with being a public company.
Total Other Income (Expense), Net
Total other income (expense), net, consists primarily of (i) changes in the fair value of our (a) common stock warrant liability and (b) Contingent Earn-outs, (ii) interest income and interest expense, (iii) interest income and expense resulting from changes in fair value of our notes payable, (iv) gains and losses on extinguishment of notes payable, (v) gains and losses on termination of leases, and (vi) the Australia research and development credit.
We also received grants issued by non-government entities which require us to growcomply with conditions attached to the grants. Income from grants is recognized in the period during which the related qualifying expenses are incurred, provided that the conditions under which the grants were provided have been met. We receive tax incentives from the Australian government in the form of cash subsidies for research and expand. Generaldevelopment activities related to clinical trial activities conducted by our Australian subsidiary, which are recognized as other income upon compliance with certain conditions.
Results of Operations
Our results of operations for the three and administrative expensessix months ended June 30, 2022 and 2021 were $5.4 millionas follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Three Months Ended June 30, | | | Change | | | Six Months Ended June 30, | | | Change | |
(in thousands) | | 2022 | | | 2021 | | | Dollars | | | % | | | 2022 | | | 2021 | | | Dollars | | | % | |
Product revenue | | $ | 2 | | | $ | 138 | | | $ | (136 | ) | | | (99 | )% | | $ | 9 | | | $ | 337 | | | $ | (328 | ) | | | (97 | )% |
Royalty revenue | | | 33 | | | | 63 | | | | (30 | ) | | | (48 | )% | | | 56 | | | | 77 | | | | (21 | ) | | | (27 | )% |
Total revenue | | | 35 | | | | 201 | | | | (166 | ) | | | (83 | )% | | | 65 | | | | 414 | | | | (349 | ) | | | (84 | )% |
Operating expenses: | | | | | | | | | | | | | | | | | | | | | | | | |
Cost of revenue | | | — | | | | 555 | | | | (555 | ) | | | (100 | )% | | | — | | | | 798 | | | | (798 | ) | | | (100 | )% |
Research and development | | | 9,166 | | | | 6,472 | | | | 2,694 | | | | 42 | % | | | 17,746 | | | | 12,747 | | | | 4,999 | | | | 39 | % |
General and administrative | | | 4,464 | | | | 6,949 | | | | (2,485 | ) | | | (36 | )% | | | 9,250 | | | | 12,339 | | | | (3,089 | ) | | | (25 | )% |
Total operating expenses | | | 13,630 | | | | 13,976 | | | | (346 | ) | | | (2 | )% | | | 26,996 | | | | 25,884 | | | | 1,112 | | | | 4 | % |
Loss from operations | | | (13,595 | ) | | | (13,775 | ) | | | 180 | | | | 1 | % | | | (26,931 | ) | | | (25,470 | ) | | | (1,461 | ) | | | (6 | )% |
Total other income (expense), net | | | 9,061 | | | | 10,352 | | | | (1,291 | ) | | | (12 | )% | | | 9,043 | | | | (17,781 | ) | | | 26,824 | | | | 151 | % |
Net loss before income taxes | | | (4,534 | ) | | | (3,423 | ) | | | (1,111 | ) | | | (32 | )% | | | (17,888 | ) | | | (43,251 | ) | | | 25,363 | | | | 59 | % |
Income tax benefit | | | — | | | | 72 | | | | (72 | ) | | | (100 | )% | | | — | | | | 144 | | | | (144 | ) | | | (100 | )% |
Net loss | | $ | (4,534 | ) | | $ | (3,351 | ) | | $ | (1,183 | ) | | | (35 | )% | | $ | (17,888 | ) | | $ | (43,107 | ) | | $ | 25,219 | | | | 59 | % |
30
Revenue
Product revenue totaled $2,000 and $0.8$0.1 million for the three months ended March 31,June 30, 2022 and 2021, respectively; and 2020, respectively. This increase of $4.6$9,000 and $0.3 million or 563.8% was primarily duefor the six months ended June 30, 2022 and 2021, respectively, in our Supplements segment related to (i) increased professional expenses, public company expenses, legal fees, accounting fees, tax fees, and director and officer insurance policies assales of an aqueous zinc-silver ion dietary (mineral) supplement sold by our wholly-owned subsidiary, dOrbital, Inc., under the trade name “rMetx™ ZnAg Immune Boost,” or under a result of becoming a public company on December 30, 2020 to support our efforts to complysupply agreement with SEC rules and regulations,4Life under the trade name “Zinc Factor,” and (ii) $1.9 millionsales of share-based compensation expense related to RSUs.
Other Income (Expenses)
Other income (expenses) consistsKHC46, an aqueous gold dietary (mineral) supplement of interest expenses, interest income, changes in fair value of preferred stock warrant liability, changes in fair value of derivative liability, change in fair value of contingent earn-out,very low-concentration, sold under a researchsupply agreement with 4Life under the trade name “Gold Factor.” Royalty revenue totaled $33,000 and development credit received from the Australian government, foreign exchange gain, gain on disposal of assets, and loss on extinguishment of notes payable. Other income (expenses), net$63,000 for the three months ended March 31,June 30, 2022 and 2021, respectively; and 2020 included the following:
(i) recognized interest expense of $0.6 million$56,000 and $51 thousand, respectively, due to an increase in the fair value of our notes payable. As of March 31, 2021, the fair value of our notes payable is determined based on the closing price of CLNN shares listed on the Nasdaq of $12.78 per share.
(ii) recognized gain on extinguishment of notes payable of $0.6 million, due to the forgiveness of the PPP Loan by the U.S. Small Business Administration. There was no gain on extinguishment of notes payable$77,000 for the threesix months ended March 31, 2020.
(iii) recognized expense of $112 thousandJune 30, 2022 and 2021, respectively, under an exclusive and royalty-bearing license agreement with 4Life relating to the changes in fair valuesale of preferred stock warrant liability forGold Factor. For more details on the three months ended March 31, 2020. There was no preferred stock warrant liability as a resultsupply and license agreements, see Note 17 to our condensed consolidated financial statements.
Cost of the Reverse Recapitalization on December 30, 2020. Upon the consummationRevenue
Cost of the Reverse Recapitalization, we determined that the warrants qualify for classification as permanent equityrevenue totaled $0 and we reclassified the resulting warrant liability to additional paid-in capital. No change in fair value of preferred stock warrant liability is recorded going forward.
(iv) recognized the change in fair value of our Clene Nanomedicine contingent earn-out liability of $25.6$0.6 million for the three months ended March 31, 2021. June 30, 2022 and 2021, respectively; and $0 and $0.8 million for the six months ended June 30, 2022 and 2021, respectively, relating to production and distribution costs for the sales of Gold Factor, Zinc Factor, and rMetx™ dietary supplements.
Research and Development Expense
Research and development expense for the three and six months ended June 30, 2022 and 2021 was as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Three Months Ended June 30, | | | Change | | | Six Months Ended June 30, | | | Change | |
(in thousands) | | 2022 | | | 2021 | | | Dollars | | | % | | | 2022 | | | 2021 | | | Dollars | | | % | |
CNM-Au8 | | $ | 3,028 | | | $ | 2,134 | | | $ | 894 | | | | 42 | % | | $ | 5,833 | | | $ | 4,506 | | | $ | 1,327 | | | | 29 | % |
CNM-ZnAg | | | 484 | | | | 384 | | | | 100 | | | | 26 | % | | | 1,893 | | | | 517 | | | | 1,376 | | | | 266 | % |
Unallocated | | | 2,179 | | | | 884 | | | | 1,295 | | | | 146 | % | | | 3,183 | | | | 1,287 | | | | 1,896 | | | | 147 | % |
Personnel | | | 2,642 | | | | 1,462 | | | | 1,180 | | | | 81 | % | | | 5,260 | | | | 3,434 | | | | 1,826 | | | | 53 | % |
Stock-based compensation | | | 833 | | | | 1,608 | | | | (775 | ) | | | (48 | )% | | | 1,577 | | | | 3,003 | | | | (1,426 | ) | | | (47 | )% |
Total research and development | | $ | 9,166 | | | $ | 6,472 | | | $ | 2,694 | | | | 42 | % | | $ | 17,746 | | | $ | 12,747 | | | $ | 4,999 | | | | 39 | % |
The change in fair valueresearch and development expenses was primarily a resultdue to the following:
(i)an increase in expenses related to our lead drug candidate, CNM-Au8, primarily due to the progression of the increaseclinical development process, including the timing of calendar payments for our participation in the Healey ALS Platform Trial which was partially offset by a decrease in expenses in the REPAIR-PD and RESCUE-ALS clinical trials due to completion of the closing pricetrials, and a decrease in expenses in the REPAIR-MS and VISIONARY-MS clinical trials as they near completion;
(ii)an increase in expenses related to CNM-ZnAg, primarily due to the progression of CLNN shares listedthe clinical development process, including full enrollment in the clinical trial for treatment of COVID-19;
(iii)an increase in unallocated expenses, primarily due to increased rent and utility expenses related to our newly-leased facility in Elkton, Maryland and our expanded facility in North East, Maryland; and increased research, manufacturing, and materials expenses; partially offset by decreased depreciation expense;
(iv)an increase in personnel expenses, primarily due to our increased headcount; and
(v)a decrease in stock-based compensation expense, primarily due to a decrease in stock-based compensation expense from restricted stock awards, partially offset by an increase in stock-based compensation expense from stock options.
31
General and Administrative Expense
General and administrative expense for the three and six months ended June 30, 2022 and 2021 was as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Three Months Ended June 30, | | | Change | | | Six Months Ended June 30, | | | Change | |
(in thousands) | | 2022 | | | 2021 | | | Dollars | | | % | | | 2022 | | | 2021 | | | Dollars | | | % | |
Directors’ and officersʼ insurance | | $ | 849 | | | $ | 929 | | | $ | (80 | ) | | | (9 | )% | | $ | 1,698 | | | $ | 1,903 | | | $ | (205 | ) | | | (11 | )% |
Legal | | | 149 | | | | 451 | | | | (302 | ) | | | (67 | )% | | | 327 | | | | 645 | | | | (318 | ) | | | (49 | )% |
Finance and accounting | | | 273 | | | | 1,115 | | | | (842 | ) | | | (76 | )% | | | 684 | | | | 2,341 | | | | (1,657 | ) | | | (71 | )% |
Public and investor relations | | | 139 | | | | 794 | | | | (655 | ) | | | (82 | )% | | | 469 | | | | 877 | | | | (408 | ) | | | (47 | )% |
Personnel | | | 1,260 | | | | 823 | | | | 437 | | | | 53 | % | | | 2,441 | | | | 1,629 | | | | 812 | | | | 50 | % |
Stock-based compensation | | | 1,351 | | | | 2,647 | | | | (1,296 | ) | | | (49 | )% | | | 2,809 | | | | 4,517 | | | | (1,708 | ) | | | (38 | )% |
Other | | | 443 | | | | 190 | | | | 253 | | | | 133 | % | | | 822 | | | | 427 | | | | 395 | | | | 93 | % |
Total general and administrative | | $ | 4,464 | | | $ | 6,949 | | | $ | (2,485 | ) | | | (36 | )% | | $ | 9,250 | | | $ | 12,339 | | | $ | (3,089 | ) | | | (25 | )% |
The change in general and administrative expense was primarily due to the following:
(i)a decrease in directors’ and officers’ insurance fees;
(ii)a decrease in legal fees after completing the Reverse Recapitalization and subsequent registration statement filings with the SEC, decreased patent and trademark expenses, and decreased corporate legal fees;
(iii)a decrease in finance and accounting fees after completing the Reverse Recapitalization and subsequent filings with the SEC, including decreased fees from consultants and other financial vendors; decreased fees for various institutions, investment bankers, advisors, and auditors; partially offset by an increase in tax fees;
(iv)a decrease in fees related to our public and investor relations efforts;
(v)an increase in personnel expenses, primarily due to our increased headcount;
(vi)a decrease in stock-based compensation expense, primarily due to a decrease in stock-based compensation expense from restricted stock awards, partially offset by an increase in stock-based compensation expense from stock options; and
(vii)an increase in other expenses, primarily due to an increase in expenses related to business development, travel, information technology, supplies and equipment, corporate and liability insurance, and depreciation; and increased rent and utility expenses related to our newly-leased facility in Elkton, Maryland and our expanded facility in North East, Maryland; partially offset by a decrease in office expenses.
Total Other Income (Expense), Net
Total other income (expense), net, for the three and six months ended June 30, 2022 and 2021 was as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Three Months Ended June 30, | | | Change | | | Six Months Ended June 30, | | | Change | |
(in thousands) | | 2022 | | | 2021 | | | Dollars | | | % | | | 2022 | | | 2021 | | | Dollars | | | % | |
Interest expense | | $ | (751 | ) | | $ | (26 | ) | | $ | (725 | ) | | | (2,788 | )% | | $ | (1,533 | ) | | $ | (577 | ) | | $ | (956 | ) | | | (166 | )% |
Gain on extinguishment of notes payable | | | — | | | | — | | | | — | | | | 0 | % | | | — | | | | 647 | | | | (647 | ) | | | (100 | )% |
Gain on termination of lease | | | — | | | | — | | | | — | | | | 0 | % | | | 420 | | | | — | | | | 420 | | | | 100 | % |
Change in fair value of common stock warrant liability | | | 20 | | | | 133 | | | | (113 | ) | | | (85 | )% | | | 2 | | | | 133 | | | | (131 | ) | | | (98 | )% |
Change in fair value of Clene Nanomedicine contingent earn-out | | | 8,310 | | | | 8,640 | | | | (330 | ) | | | (4 | )% | | | 8,253 | | | | (16,970 | ) | | | 25,223 | | | | 149 | % |
Change in fair value of Initial Stockholders contingent earn-out | | | 1,066 | | | | 1,232 | | | | (166 | ) | | | (13 | )% | | | 1,054 | | | | (1,729 | ) | | | 2,783 | | | | 161 | % |
Australia research and development credit | | | 356 | | | | 375 | | | | (19 | ) | | | (5 | )% | | | 655 | | | | 714 | | | | (59 | ) | | | (8 | )% |
Other income (expense), net | | | 60 | | | | (2 | ) | | | 62 | | | | 3,100 | % | | | 192 | | | | 1 | | | | 191 | | | | 19,100 | % |
Total other income (expense), net | | $ | 9,061 | | | $ | 10,352 | | | $ | (1,291 | ) | | | (12 | )% | | $ | 9,043 | | | $ | (17,781 | ) | | $ | 26,824 | | | | 151 | % |
The change in total other income (expense), net, was primarily due to the following:
32
(i)an increase in interest expense primarily due to increased interest expense on notes payable and increased amortization of debt discount and debt issuance costs from our term loan with Avenue Venture Opportunities Fund, L.P. (“Avenue”);
(ii)gain on extinguishment of debt due to forgiveness of a Paycheck Protection Program loan (the “PPP Loan”) by the NasdaqUnited States (“U.S.”) Small Business Administration during the six months ended June 30, 2021;
(iii)gain on termination of lease due to the termination of an operating lease for $12.78 per share on March 31, 2021office space for the six months ended June 30, 2022;
(iv)a gain from
$9.01 per share on December 31, 2020 when we remeasured the Clene Nanomedicine contingent earn-out liability at December 31, 2020. (v) recognized the changean increase in fair value of ourthe Clene Nanomedicine Contingent Earn-out liability and Initial Shareholders contingent earn-out liability of $3.0 million for the three months ended March 31, 2021.Stockholders Contingent Earn-out liability. The changechanges in fair value was primarily a result ofwere due to changes in the increase of the closing price of CLNN shares listedour Common Stock on Nasdaq and updates in the Nasdaq for $12.78 per share on March 31, 2021 from $9.01 per share on December 31, 2020 when we remeasuredvaluation model assumptions (see “Critical Accounting Policies and Estimates”);
(v)income due to the
Initial Shareholders contingent earn-out liability at December 31, 2020.(vi) recognized income of $0.3 million relating to aAustralia research and development credit received from the Australian government for the three months ended March 31, 2021. We did not recognize income relating to research and development credit received from the Australian government for the three months ended March 31, 2020.credit. We recognized AustralianAustralia research and development credit in an amount equal to the qualifying expenses incurred in each period multiplied by the applicable reimbursement percentage. The increase in researchpercentage; and development credit is the result of increased research
(vi)other income, primarily due to interest income on cash, cash equivalents, and development activities during the three months ended March 31, 2021.marketable securities.
Taxation
Comprehensive Loss
As a result of the foregoing, we incurred a comprehensive loss of $39.7 million and $3.9 million for the three months ended March 31, 2021 and 2020, respectively.
Taxation
United States
We are incorporated in Delaware in the U.S.state of Delaware and subject to statutory U.S. federal corporate income tax at a rate of 21% for the three and six months ended March 31, 2021June 30, 2022 and 2020.2021. We are also subject to state income tax in Utah and Maryland, at a rate of 4.95%4.85% and 8.25%, respectively, for the three and six months ended MarchJune 30, 2022 and 2021. As of June 30, 2022 and December 31, 2021, and 2020. Wewe recorded a full valuation allowance against our net deferred tax assets due to the uncertainty as to whether such assets will be realized resulting from our three-year cumulative loss position and the uncertainty surrounding our ability to generate pre-tax income in the foreseeable future.
Australia
Our wholly-owned subsidiary, Clene Australia Pty Ltd (“Clene Australia”), was established in Australia on March 5, 2018 and is subject to corporate income tax at a rate of 27.5%.25% for the three and six months ended June 30, 2022 and 2021, respectively. Clene Australia total income tax benefit was $72 thousandtotaled $0 and $0.1 million for the three months ended March 31, 2021. DuringJune 30, 2022 and 2021, respectively; and $0 and $0.1 million for the six months ended June 30, 2022 and 2021, respectively. We recorded other income of $0.4 million and $0.4 million for the three months ended March 31, 2020, Clene Australia had no taxable incomeJune 30, 2022 and therefore, no provision2021, respectively; and $0.7 million and $0.7 million for income taxes was required. We recorded $0.3 million as other income during the threesix months ended March 31,June 30, 2022 and 2021, respectively, for a refund of research and development credits pertaining to Clene Australia for the 2022 and 2021 tax year. We did not record any other income during the three months ended March 31, 2020 for a refund of research and development credits pertaining to Clene Australia for the 2020 tax year.years, respectively.
Netherlands
Netherlands
Our wholly-owned subsidiary, Clene Netherlands B.V., was established in the Netherlands on April 21, 2021 and will beis subject to corporate income tax at a rate of 15% up to €245,000€395,000 of taxable income and 25%25.8% for taxable income in excess of €245,000. As€395,000. Clene Netherlands was established subsequent to the quarter ended March 31, 2021, itB.V. had no taxable income and therefore, noor provision for income taxes was required.for the three and six months ended June 30, 2022 and 2021.
JOBS Act
The JOBS Act permits an emerging growth company (“EGC”) such as us to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies. We have elected to use the extended transition period under the JOBS Act until the earlier of the date we (1) are no longer an emerging growth company or (2) affirmatively and irrevocably opt out of the extended transition period provided in the JOBS Act. As a result, our condensed consolidated financial statements may not be comparable to companies that comply with new or revised accounting pronouncements as of public company effective dates.
We will remain an emerging growth company until the earliest to occur of: (1) the last day of the fiscal year in which we have more than $1.07 billion in annual revenue; (2) the date on which we are deemed to be a “large accelerated filer,” which would occur if the market value of our equity securities held by non-affiliates exceeds US$700 million as of the last business day of our most recently completed second fiscal quarter; (3) the date on which we have issued more than $1.0 billion in non-convertible debt securities during the prior three-year period; and (4) the last day of the fiscal year ending after the fifth anniversary of Tottenham’s initial public offering, or August 6, 2023.
We may choose to early adopt any new or revised accounting standards whenever such early adoption is permitted for public companies.
Smaller Reporting Company Status
We are a Smaller Reporting Company (“SRC”) as defined in Item 10(f)(1) of Regulation S-K. Smaller reporting companies may take advantage of certain reduced disclosure obligations, including, among other things, providing only two years of audited financial statements. We will remain a smaller reporting company until (1) the market value of our Common Stock held by non-affiliates exceeds $250 million as of the end of the second fiscal quarter and our annual revenues exceed $100 million during the previous fiscal year, or (2) the market value of our Common Stock held by non-affiliates exceeds $700 million as of the end of the second fiscal quarter.
Liquidity and Capital Resources
Sources of Capital
Since inception, weWe have incurred annual netsignificant losses and negative cash flows from operations since our inception. We expect to incur additional losses in the future to fund our operations and conduct product research and development. We recognize the need to raise additional capital to fully implement our business plan. The long-term continuation of our business plan is dependent upon the generation of sufficient revenues from our operations. Substantiallyproducts to offset expenses and capital expenditures. In the event that we do not generate sufficient revenues and are unable to obtain funding, we will be forced to delay, reduce, or eliminate some or all of our losses have resulted from the funding of our research and development programs, and general and administrative expenses associated withproduct portfolio expansion, commercialization efforts, or capital expenditures, which could adversely affect our business prospects, ability to meet long-term liquidity needs, or we may be unable to continue operations. We incurred net losses
Since our inception, we have dedicated substantially all of $39.8 million and $3.9 million forour resources to the three months ended March 31, 2021 and 2020, respectively. Our loss from operations was $11.7 million and $4.0 million for the three months ended March 31, 2021 and 2020, respectively.development of our drug candidates. We have financed our operations principally through the following sources:
•gross proceeds of $87.0 million from the salesales of preferred stock, the sale of preferred stock warrants and the sale of convertible notes that have converted into shares ofour preferred stock and other equity financing;
•gross proceeds of $32.3 million from borrowings under convertible promissory notes;
33
•gross proceeds of $0.9 million from stock option exercises;
•gross proceeds of $1.2 million through the funds we raisedgovernment lending;
•gross proceeds of $2.3 million from the consummationgrants from various organizations;
•gross cash proceeds of $31.8 million from the Reverse Recapitalization and the PIPE offering. During the three months ended March 31, 2021, we did not raise any significant funds. During the three months ended March 31,December 2020 we raised an aggregate of $1.6 million, consisting of net proceeds from issuance of notes payable. We filed a registration statement on Form S-1 to register 4,541,481 sharesprivate placement of Common Stock underlying outstanding warrants that we had previously issued, which the SEC declared to be effective on April 19, 2021 (file number 333-253173). We will receive aggregate Stock;
•gross cash proceeds of $30.7$0.6 million from a PPP Loan obtained through the U.S. Small Business Administration, which was forgiven in January 2021;
•gross cash proceeds of approximately $9.3 million from the May 2021 private placement of Common Stock; and
•gross cash proceeds of $20.0 million borrowings under notes payable.
We also receive indirect financial support for the Healey ALS Platform Trial, administered by Massachusetts General Hospital, which is conducting a platform trial of CNM-Au8 alongside other drugs at significantly lower costs than we would otherwise incur if allwe were to conduct a comparably designed clinical trial at reasonable market rates.
Going Concern
We incurred a loss from operations of these warrants are exercised.
The net cash used in our operating activities was $9.2$13.6 million and $3.3$13.8 million for the three months ended MarchJune 30, 2022 and 2021, respectively; and $26.9 million and $25.5 million for the six months ended June 30, 2022 and 2021, respectively. Our accumulated deficit was $181.2 million and $163.3 million as of June 30, 2022 and December 31, 2021, respectively. Our cash, cash equivalents, and 2020, respectively. Asmarketable securities totaled $26.3 million and $50.3 million as of MarchJune 30, 2022 and December 31, 2021, we hadrespectively, and net cash of $48.0 million. We expect thatused in operating activities was $22.8 million and $17.7 million for the cash on hand as of March 31,six months ended June 30, 2022 and 2021, will be sufficient to fund our operations for a period extending beyond twelve months from the date the condensed consolidated financial statements are issued. respectively.
We have based this estimate on assumptions that may prove to be wrong,incurred significant losses and negative cash flows from operations since our inception. We have not generated significant revenues since our inception, and we may exhaust our available capital resources sooner thando not anticipate generating significant revenues unless we anticipate.successfully complete development and obtain regulatory approval for commercialization of a drug candidate. We expect our expenses to increase significantly and to incur additional losses in connection withthe future to fund our ongoing activities,operations, particularly as we advance the clinical development of our clinical-stage drug products andcandidates, continue research and development of our preclinical drug productscandidates, and initiate additional clinical trials of, and seek regulatory approval for, these and other future drug products. As we continue to grow and expand, we will incur morecandidates. We expect our expenses relating to regulatory compliance and sales and marketing personnel to increase significantly as we prepare to commence commercialization onceif we obtain regulatory approval for our drug candidates.
Our management performs strategic reviews of our drug products. operating plans and budgets, considering the status of our product development programs, human capital, capital needs and resources, and current capital market conditions. Based on these reviews, our Board of Directors and management make adjustments to our operating plans and budgets to allocate our projected cash expenditures. Notwithstanding these ongoing adjustments, we project that within the next twelve months, we will not have sufficient cash and other resources on hand to sustain our current operations or meet our obligations as they become due, and we must obtain additional financing. Additionally, pursuant to our term loan with Avenue, we must maintain unrestricted cash and cash equivalents of at least $5.0 million to avoid acceleration of the full balance of the loan. These conditions raise substantial doubt about the Company’s ability to continue as a going concern.
OurTo mitigate our funding needs, we intend to implement plans to raise additional funding, including exploring equity financing and offerings, debt financing, licensing or collaboration arrangements with third parties, as well as potentially utilizing additional funds available under our term loan with Avenue, subject to certain contingent conditions (see Note 9), as well as our existing at-the-market facility. These plans are subject to market conditions and reliance on third parties, and there is no assurance that effective implementation of our plans will result in the necessary funding to continue current operations. Depending on the results of our plans, we may need to implement cost-saving initiatives, including potentially delaying or reducing research and development programs and commercialization efforts. As a result, we have concluded that our plans do not alleviate the substantial doubt about our ability to continue as a going concern may require obtaining additional funding to finance operations. As part of our ongoing business plans,beyond one year from the date the condensed consolidated financial statements are issued.
The accompanying condensed consolidated financial statements have been prepared assuming we will continue seeking fundingas a going concern, which contemplates the realization of assets and satisfaction of liabilities in the normal course of business. As a result, the accompanying condensed consolidated financial statements do not include any adjustments relating to the recoverability and classification of assets and their carrying amounts, or the amounts and classification of liabilities that may result should we be unable to continue as a going concern.
34
Short-Term Material Cash Requirements
For at least the next twelve months, our primary capital requirements are to fund our operations, including research and development, personnel, regulatory, and other clinical trial costs related to development of our lead drug candidate, CNM-Au8; and general and administrative costs to support our drug development and pre-commercial activities in advance of receiving regulatory approval for our drug candidates.
Firm commitments for funds include approximately $0.1 million and $1.0 million of payments under finance and operating lease obligations, respectively; payment of interest on notes payable totaling $2.3 million; and commitments under various agreements for capital expenditures totaling $3.7 million related to the construction of our manufacturing facilities. We expect to meet our short-term liquidity requirements primarily through cash on hand. Additional sources of funds include equity financing, and may seek debt financing, or other capital sources. We may not be able to obtain financing on acceptable terms, or at all. The terms of any financing may adversely affect the holdings or the rights of our shareholders. If we are unable to raise capital when needed or on acceptable terms, we would be forced to delay, reduce or eliminate research and development programs and commercialization efforts.
The following table provides information regarding our cash flows for relevant periods:
| | | Three months ended | |
| | | March 31, | |
| | | 2021 | | | 2020 | |
| | | (in thousands) | |
| Net cash used in operating activities | | $ | (9,161 | ) | | $ | (3,251 | ) |
| Net cash used in investing activities | | | (203 | ) | | | (23 | ) |
| Net cash provided by (used in) financing activities | | | (1,896 | ) | | | 1,547 | |
| Net effect of foreign exchange rate changes | | | 26 | | | | 55 | |
| Net decrease in cash | | | (11,234 | ) | | | (1,672 | ) |
Use of Funds
Our primary use of cash in all periods presented was to fund our research and development, regulatory and other clinical trial costs, and related supporting administration. Our prepaid expenses and other current assets, accounts payable and accrued expense balances in all periods presented were affected by the timing of vendor invoicing and payments, and impacted the cash provided by, or used in, operations. We have no commitments for capital expenditures as of the end of the latest fiscal period.
Operating Activities
Net cash used in operating activities was $9.2 million of cash for the three months ended March 31, 2021, which resulted from a net loss of $39.8 million, adjusted for (i) non-cash items of $32.0 million, which primarily consisted of depreciation expense of $0.2 million, stock-based compensation expenses of $3.3 million, changes in fair value of the Clene Nanomedicine contingent earn-out of $25.6 million, changes in fair value of the Initial Shareholders contingent earn-out of $3.0 million, gain on extinguishment of debt of $0.6 million and increase in interest accrued on notes payable and accretion of debt discount of $0.5 million, and (ii) a net decrease in operating assets and liabilities of $1. million. The net decrease in operating assets and liabilities was primarily attributable to an increase in inventory of $0.2 million, an increase in accounts receivable of $0.1 million, an increase in prepaid expenses and other current assets of $1.3 million due to the increase in Australia research and development credit receivable and prepayments to CROs and other vendors, $0.2 million increase in accounts payable, $0.1 million decrease in operating lease obligations, and $0.1 million increase in accrued liabilities due to the timing of vendor invoicing and payments.
Net cash used in operating activities was $3.3 million of cash for the three months ended March 31, 2020, which resulted from a net loss of $3.9 million, adjusted for (i) non-cash items of $0.3 million, which primarily consisted of depreciation expense of $0.2 million, stock-based compensation expenses of $0.2 million, and changes in the fair value of preferred stock warrant liability of $0.1 million , and (ii) a net decrease in operating assets and liabilities of $0.4 million. The net decrease in operating assets and liabilities was primarily attributable to an increase in prepaid expenses and other current assets of $0.1 million due to the increase in other vendors, $0.6 million increase in accounts payable, and $0.1 million decrease in accrued liabilities due to the timing of vendor invoicing and payments.
Investing Activities
Net cash used in investing activities was $0.2 million and $23 thousand for the three months ended March 31, 2021 and 2020, respectively, which in each instance was related to purchases of property and equipment.
Financing Activities
Net cash used in financing activities was $1.9 million for the three months ended March 31, 2021, which primarily resulted from payments of deferred offering costs of $1.9 million and payments on our finance lease obligations of $45 thousand, partially offset by proceeds from issuance of common stock upon exercise of common stock options of $50 thousand.
Net cash provided by financing activities was $1.5 million for the three months ended March 31, 2020, which primarily resulted from proceeds from the issuance of notes payable of $1.6 million partially offset by payments on our finance lease obligations of $0.1 million.
Debt Obligations
In February 2019, we entered into a loan agreement (the “2019 MD Loan”) with the Department of Housing and Community Development, a principal department of the State of Maryland (“Maryland”). Pursuant to the 2019 MD Loan, Maryland agreed to provide a $0.5 million term loan. Amounts outstanding under the 2019 MD Loan bear simple interest at an annual rate of 8.00%. Under the 2019 MD Loan, we agreed to affirmative and negative covenants to which we will remain subject until maturity. These covenants include providing information about the Company and our operations; limitations on our ability to retire, repurchase, or redeem our common or preferred stock, options, and warrants other than per the terms of the securities; and limitations on our ability to pay dividends of cash or property. There are no financial covenants associated with the Loan Agreement. Events of default under the Loan Agreement include failure to make payments when due, insolvency events, failure to comply with covenants, and material adverse effects with respect to the Company. We are not in violation of any affirmative or negative covenants. Repayment of the full balance outstanding is due on February 22, 2034. The 2019 MD Loan establishes “Phantom Shares,” based on 119,906 shares of our Common Stock (based on 863,110 Series C Preferred Shares prior to the Reverse Recapitalization), determined at issuance. The Loan Agreement states the repayment amount is to be the greater of the balance of principal and accrued interest or the Phantom Shares value. We determined that the note should be accounted for at fair value. We record the fair value of the debt at the end of each reporting period. In order to value the note, we consider the amount of the simple interest expense that would be due and the value of Phantom Shares. Upon the closing of the Reverse Recapitalization and as of December 31, 2020, the fair value of the 2019 MD Loan is determined based on the closing price of CLNN shares listed on the Nasdaq. Expense of $0.5 million and $10 thousand was recognized during the three months ended March 31, 2021 and 2020, respectively. The fair value of $1.5 million and $1.1 million of principal and accrued interest is included in long-term notes payable as of March 31, 2021 and December 31, 2020, respectively.
In April 2019, we entered into a loan agreement (the “2019 Cecil Loan”) with Cecil County, Maryland (“Cecil”). Pursuant to the 2019 Cecil Loan, Cecil agreed to provide a $0.1 million term loan. Amounts outstanding under the 2019 Cecil Loan bear simple interest at an annual rate of 8.00%. Under the 2019 Cecil Loan, we agreed to affirmative and negative covenants to which we will remain subject until maturity. These covenants include providing information about the Company and our operations; limitations on our ability to retire, repurchase, or redeem our common or preferred stock, options, and warrants other than per the terms of the securities; and limitations on our ability to pay dividends of cash or property. There are no financial covenants associated with the Loan Agreement. Events of default under the Loan Agreement include failure to make payments when due, insolvency events, failure to comply with covenants, and material adverse effects with respect to the Company. We are not in violation of any affirmative or negative covenants. Repayment of the full balance outstanding is due on April 30, 2034. The 2019 Cecil Loan establishes “Phantom Shares,” based on 23,981 shares of our Common Stock (based on 172,622 Series C Preferred Shares prior to the Reverse Recapitalization), determined at issuance. The 2019 Cecil Loan states the repayment amount is to be the greater of the balance of principal and accrued interest or the Phantom Share value. We determined that the note should be accounted for at fair value. We record the fair value of the debt at the end of each reporting period. In order to value the note, we consider the amount of the simple interest expense that would be due and the value of Phantom Shares. Upon the closing of the Reverse Recapitalization and as of December 31, 2020, the fair value of the 2019 Cecil Loan is determined based on the closing price of CLNN shares listed on the Nasdaq. Expense of $0.1 million and $2 thousand was recognized during the three months ended March 31, 2021 and 2020, respectively. The fair value of $0.3 million and $0.2 million of principal and accrued interest is included in long-term notes payable as of March 31, 2020 and December 31, 2020, respectively.
In February through July 2020, we issued convertible promissory notes (the “2020 Convertible Notes”) in an aggregate principal amount of $6.1 million, bearing interest at an annual rate of 5%. The 2020 Convertible Notes were convertible at the earlier of (i) one year, at which point the notes would be convertible into Series C preferred shares at the Series C preferred share issuance price, and (ii) next equity financing of no less than $10.0 million, at which point the notes would be convertible into shares issued in the next equity financing at 90% of the per share issuance price of the next equity financing. The redemption feature at the next equity financing met the requirements of an embedded derivative to be bifurcated and recorded at fair value. We bifurcated the embedded feature at issuance and recorded a derivative liability of $0.7 million at inception in conjunction with a discount on debt to be amortized over the life of the note. We recognized interest expense of $8 thousand, including amortization of debt discount of $20 thousand during the three months ended March 31, 2020, in connection with the 2020 Convertible Notes. We also identified two other embedded features in these convertible promissory notes that were not bifurcated, which were the conversion into Series C preferred shares upon maturity and the redemption upon a liquidation event. On August 11, 2020, in connection with our issuance and sale of Series D Preferred Stock prior to the Reverse Recapitalization, all of the outstanding principal and accrued interest under the 2020 Convertible Notes, totaling $6.9 million, was automatically converted into 1,497,135 shares of Series D Preferred Stock at a price equal to 90% of $4.60 per share, the per share price paid in cash by investors in the Series D preferred stock financing. We accounted for the conversion of the 2020 Convertible Notes as a debt extinguishment and recognized a loss on extinguishment of debt of $0.5 million within other income (expense), net in the consolidated statement of operations and comprehensive loss for the year ended December 31, 2020. As of the date of conversion, the unamortized discount on the 2020 Convertible Notes was $0.5 million. The loss on extinguishment was calculated as the difference between (i) the fair value of the 1,497,135 shares of Series D Preferred Stock issued to settle the 2020 Convertible Notes of $6.9 million and (ii) the carrying value of the 2020 Convertible Notes, including the principal balance of the 2020 Convertible Notes of $6.1 million and accrued but unpaid interest of $76 thousand, net of the unamortized debt discount of $5.7 million, plus the then-current fair value of derivative liability associated with the 2020 Convertible Notes at the time of the extinguishment of $0.7 million.
In May 2020, we entered into a note payable in the amount of $0.6 million (the “PPP Note”) under the Paycheck Protection Program of the CARES Act (the “PPP”). As amended, the PPP permits forgiveness of amounts loaned for payments of payroll and other qualifying expenses within 24 weeks of receipt of loaned funds, given that at least 60% of the total loan is used for payroll. Amounts not forgiven have a repayment period of five years. In January 2021, the full $0.6 million balance of the PPP Note was forgiven and has been recorded as a gain on extinguishment of debt during the three months ended March 31, 2021
Contractual Obligations and Commitments
There have been no material changes to our contractual obligations and other commitments as of March 31, 2021, as compared to those disclosed in Part II, Item 7 “Management’s Discussion and Analysis of Financial Condition and Results of Operations” of our 2020 Annual Report which was filed with the SEC on March 29, 2021.
We enter into agreements in the normal course of business with CROs for clinical trials and with vendors for preclinical studies and other services and products for operating purposes, which are cancelable at any time by us, subject to payment of our remaining obligations under binding purchase orders and, in certain cases, nominal early termination fees. These commitments are not deemed significant.
Long-Term Material Cash Requirements
Off-Balance Sheet ArrangementsBeyond the next twelve months, our primary capital requirements are to fund our operations, including research and development, personnel, regulatory, and other clinical trial costs related to development of our lead drug candidate, CNM-Au8; capital expenditures related to construction and expansion of our manufacturing facilities; and general and administrative costs to support our drug development and pre-commercial activities in advance of receiving regulatory approval for our drug candidates. Additional funds may be spent to initiate new clinical trials, at our discretion. Known obligations beyond the next twelve months include $0.1 million and $8.1 million of payments under finance and operating lease obligations, respectively; and interest and principal repayment of notes payable of $23.9 million.
Use of Funds
DuringOur cash flows for the six months ended June 30, 2022 and 2021 were as follows:
| | | | | | | | |
| | Six Months Ended June 30, | |
(in thousands) | | 2022 | | | 2021 | |
Net cash used in operating activities | | $ | (22,814 | ) | | $ | (17,713 | ) |
Net cash used in investing activities | | | (20,857 | ) | | | (420 | ) |
Net cash provided by financing activities | | | 783 | | | | 21,898 | |
Effect of foreign exchange rate changes on cash | | | (147 | ) | | | (33 | ) |
Net increase (decrease) in cash | | $ | (43,035 | ) | | $ | 3,732 | |
Our primary use of cash in all periods presented was to fund our research and development, regulatory and other clinical trial costs, and general corporate expenditures.
Operating Activities
Net cash used in operating activities was $22.8 million for the six months ended June 30, 2022, which resulted from a net loss of $17.9 million, adjusted for non-cash items totaling $4.2 million and a net change in operating assets and liabilities of $0.8 million. Significant non-cash items included (i) depreciation expense of $0.5 million relating to laboratory and office equipment and leasehold improvements; (ii) non-cash lease expense of $0.2 million; (iii) stock-based compensation expense of $4.4 million; (iv) gain on termination of lease of $0.4 million; (v) accretion of debt discount of $0.4 million; (vi) non-cash interest expense of $0.1 million; and (vii) the changes in fair value of the Clene Nanomedicine and Initial Stockholders Contingent Earn-outs of $8.3 million and $1.1 million, respectively. The changes in fair value of these instruments were primarily driven by the decrease of the closing price of our Common Stock on Nasdaq. The net change in operating assets and liabilities was primarily attributable to the following: (a) a decrease in accounts receivable of $49,000 and an increase in accounts payable of $1.5 million due to the timing of vendor invoicing and payments; (b) an increase in inventory of $0.1 million; (c) an increase in prepaid expenses and other current assets of $1.0 million due to the timing of vendor invoicing and payments, the timing of receipt of metals to be used in research and development, and an increase in Australia research and development credit receivable; (d) a decrease in accrued liabilities of $1.0 million primarily due to decreased accrued compensation and benefits; and (e) a decrease in operating lease obligations of $0.2 million.
35
Net cash used in operating activities was $17.7 million for the six months ended June 30, 2021, which resulted from a net loss of $43.1 million, adjusted for non-cash items totaling $26.4 million and a net change in operating assets and liabilities of $1.0 million. Non-cash items primarily consisted of the following: (i) depreciation expense of $0.5 million, (ii) stock-based compensation expense of $7.5 million, (iii) change in fair value of Clene Nanomedicine Contingent Earn-out of $17.0 million, (iv) change in fair value of Initial Stockholders Contingent Earn-out of $1.7 million, (iv) gain on extinguishment of debt of $0.6 million and increase in interest accrued on notes payable and accretion of debt discount of $0.4 million. The net change in operating assets and liabilities was primarily attributable to the following: (a) a decrease in inventory of $0.1 million, (b) an increase in accounts receivable of $48,000, (c) an increase in prepaid expenses and other current assets of $1.5 million due to the increase in Australia research and development credit receivable, metal to be used in research and development, and directors and officers insurance; partially offset by a decrease in CRO prepayments, (d) an increase in accounts payable of $0.6 million, (e) a decrease in operating lease obligations of $0.1 million, (f) an increase in accrued liabilities of $0.1 million due to the timing of vendor invoicing and payments, and (g) a decrease in deferred income tax of $0.1 million.
Investing Activities
Net cash used in investing activities was $20.9 million for the six months ended June 30, 2022, which consisted of (i) purchases of marketable securities of $24.5 million and (ii) purchases of property and equipment of $1.8 million, offset primarily by (iii) proceeds from maturity of marketable securities of $2.5 million and (iv) proceeds from sale of marketable securities of $3.0 million. Net cash used in investing activities was $0.4 million for the six months ended June 30, 2021, which consisted of purchases of property and equipment.
Financing Activities
Net cash provided by financing activities was $0.8 million for the six months ended June 30, 2022, which primarily consisted of (i) proceeds from exercise of stock options of $0.3 million and (ii) proceeds from the issuance of notes payable of $0.7 million, offset primarily by (iii) payments of finance lease obligations of $0.1 million, (iv) payment of debt issuance costs of $30,000, and (v) payment of deferred offering costs of $0.1 million. Net cash provided by financing activities was $21.9 million for the six months ended June 30, 2021, which primarily consisted of (i) proceeds from exercise of stock options of $0.1 million, (ii) proceeds from the issuance of notes payable of $15.0 million offset by payments of debt issuance costs of $0.5 million, and (iii) proceeds from the May 2021 private placement of common stock of $9.3 million, offset primarily by (iv) payments of finance lease obligations of $0.1 million and (v) payments of deferred offering costs of $1.9 million.
Maryland Loans
In February 2019, we entered into a loan agreement (the “2019 MD Loan”) with the Department of Housing and Community Development (“DHCD”), a principal department of the State of Maryland. The agreement provides for a term loan of $0.5 million bearing simple interest at an annual rate of 8.00%. We are subject to affirmative and negative covenants until maturity, including providing information about the Company and our operations; limitations on our ability to retire, repurchase, or redeem our common or preferred stock, options, and warrants other than per the terms of the securities; and limitations on our ability to pay dividends of cash or property. There are no financial covenants associated with the 2019 MD Loan. We are not in violation of any covenants. The 2019 MD Loan established “Phantom Shares” at issuance based on 119,907 shares of Common Stock. Repayment of the full balance is due on February 22, 2034, with the repayment amount and carrying value equal to the greater of the balance of principal plus accrued interest or the value of the Phantom Shares. The value of the Phantom Shares is based on the closing price of our Common Stock on Nasdaq at the end of each reporting period. As of June 30, 2022 and December 31, 2021, the note was recorded at principal plus accrued interest in the condensed consolidated balance sheets.
In May 2022, we entered into a loan agreement (the “2022 MD Loan”) with DHCD which provides for a term loan of up to $3.0 million bearing simple interest at an annual rate of 6.00% for the purchase of certain personal property (the “Assets”) related to the production of pharmaceutical drugs. As of June 30, 2022, we had drawn $0.7 million under the term loan, with the remainder available upon our submission of disbursement requests to purchase the Assets. The 2022 MD Loan matures on July 1, 2027 (the “Maturity Date”). The first twelve payments, commencing on July 1, 2022, are deferred. Immediately thereafter, there shall be eighteen monthly installments of interest-only based on the actual amount advanced under the loan, each up to a maximum amount of $15,000; followed by thirty monthly installments of principal and interest, each in the amount of $33,306, which is due and payable even if the entire loan has not been advanced prior to the date such monthly payment is due and payable, with a balloon payment of all accrued and unpaid interest and principal due on the Maturity Date. We recorded $31,000 of debt issuance costs that are being amortized over the contractual term using the effective interest method. Pursuant to the 2022 MD Loan, DHCD was granted a continuing security interest in the Assets as collateral. Under a priority of liens agreement by and between DHCD and Avenue Venture Opportunities Fund, L.P. (“Avenue”), an existing secured creditor of the Company, DHCD’s continuing security interest in the Assets shall be a first priority lien.
36
Cecil County Loan
In April 2019, we entered into a loan agreement (the “2019 Cecil Loan”) with Advance Cecil Inc., a non-stock corporation formed under the laws of the state of Maryland. The agreement provides for a term loan of $0.1 million bearing simple interest at an annual rate of 8.00%. We are subject to affirmative covenants until maturity, including providing information about the Company and our operations. There are no financial covenants associated with the 2019 Cecil Loan. We are not in violation of any covenants. The 2019 Cecil Loan established “Phantom Shares” at issuance based on 23,981 shares of Common Stock. Repayment of the full balance is due on April 30, 2034, with the repayment amount and carrying value equal to the greater of the balance of principal plus accrued interest or the value of the Phantom Shares. The value of the Phantom Shares is based on the closing price of our Common Stock on Nasdaq at the end of each reporting period. As of June 30, 2022 and December 31, 2021, the note was recorded at principal plus accrued interest in the condensed consolidated balance sheets.
Avenue Loan
In May 2021, we entered into a loan agreement (the “2021 Avenue Loan”) with Avenue. The agreement provides for a 42-month term loan of up to $30.0 million. The first tranche is $20.0 million (“Tranche 1”), of which $15.0 million was funded at close and $5.0 million was funded in September 2021. We incurred issuance costs of $0.6 million of which $46,951 was expensed immediately. The remaining unfunded tranche of $10.0 million (“Tranche 2”) is available until December 31, 2022. Funding of Tranche 2 is subject to (a) our receipt of $5.0 million financing through the state of Maryland; (b) our achievement of a statistically significant result in certain clinical trials (“Performance Milestone 1”); (c) our receipt of net proceeds of at least $30.0 million from the sale and issuance of our equity securities between May 2, 2021 and December 31, 2022; and (d) mutual agreement of us and Avenue. The 2021 Avenue Loan bears interest at a variable rate equal to the sum of (i) the greater of (a) the prime rate or (b) 3.25%, plus (ii) 6.60%. As of June 30, 2022 and December 31, 2021, the interest rate was 11.35% and 9.85%, respectively. Payments are interest-only for the first 12 months and have been extended an additional 12 months (the “First Interest-only Period Extension”) based on our achievement of Performance Milestone 1. Payments may be extended up to 36 months if we (i) achieve the First Interest-only Period Extension and (b) draw from Tranche 2. The loan principal will amortize equally from the end of the interest period presented,to the expiration of the 42-month term on December 1, 2024. On the maturity date, an additional payment equal to 4.25% of the funded loans, currently equal to $0.9 million (the “Final Payment”), is due in addition to the remaining unpaid principal and accrued interest. The Final Payment was recorded as a debt premium and is being amortized over the contractual term using the effective interest method. The Final Payment is related to the loan host and is not bifurcated pursuant to Accounting Standards Codification (“ASC”) 815, Derivatives and Hedging (“ASC 815”). We are subject to affirmative and negative covenants until maturity in the absence of prepayments, including providing information about the Company and our operations; limitation on our ability to retire, repurchase, or redeem our Common Stock, options, and warrants other than per the terms of the securities; and limitations on our ability to pay dividends of cash or property. Also pursuant to the 2021 Avenue Loan, we are required to maintain unrestricted cash and cash equivalents of at least $5.0 million, provided that upon our (i) achievement of Performance Milestone 1, and (ii) receiving of net proceeds of at least $30.0 million from the sale and issuance of our equity securities, we shall no longer be subject to financial covenants. We are not in violation of any covenants. Avenue also has the ability to make all obligations under the 2021 Avenue Loan immediately due and payable upon occurrence of certain events of default or material adverse effects, as outlined in the loan agreement. The 2021 Avenue Loan is collateralized by substantially all of our assets other than intellectual property, including our capital stock and the capital stock of our subsidiaries, in which Avenue is granted a continuing security interest.
Pursuant to the agreement, we granted Avenue a warrant to purchase 115,851 shares of Common Stock (the “Avenue Warrant”) at an exercise price of $8.63 per share. Upon the funding of Tranche 2, the Avenue Warrant shall be adjusted to include an additional estimated 245,832 shares of Common Stock, which is equal to 5% of the principal amount of Tranche 2, divided by the five (5)-day VWAP per share as of the end of trading on the last trading day before the issuance of Tranche 2. We accounted for the Tranche 2 contingently-issuable warrant at inception of the 2021 Avenue Loan in accordance with ASC 815 and the fair value and issuable shares are remeasured at each reporting period.
Avenue has the right, in its discretion, but not the obligation, at any time between May 21, 2022 through May 21, 2024, while the loan is outstanding, to convert up to $5.0 million of principal into Common Stock (the “Conversion Feature”) at a price per share equal to 120% of the Avenue Warrant exercise price. The Conversion Feature is subject to certain minimum price and volume conditions of our Common Stock on Nasdaq. The Conversion Feature did not have,meet the requirements for separate accounting and is not accounted for as a derivative instrument. The number of shares of Common Stock potentially issuable upon conversion is 482,703 shares. We classified $5.0 million of the 2021 Avenue Loan as convertible notes payable as of June 30, 2022 and December 31, 2021, with unamortized debt discount and issuance costs of $0.3 million and $0.4 million, respectively.
At-the-Market Offering
On April 14, 2022, we currently do not have,entered into an Equity Distribution Agreement (the “Distribution Agreement”) with Canaccord Genuity LLC and Oppenheimer & Co. Inc., as placement agents (the “Placement Agents”). In accordance with the terms of the Distribution
37
Agreement, we may offer and sell shares of Common Stock having an aggregate offering price of up to $50.0 million from time to time through the Placement Agents. The issuance and sale of Common Stock, if any, off-balance sheet arrangements, such as relationships with unconsolidated entities or financial partnerships,by us under the Distribution Agreement will be made pursuant to our registration statement on Form S-3 (the “Registration Statement”), which are often referredwas declared effective by the Securities and Exchange Commission on April 26, 2022 (file number 333-264299), and our prospectus supplement relating to as structured finance or special purpose entities, established for the purposeoffering.
Subject to terms of facilitating financing transactions thatthe Distribution Agreement, the Placement Agents are not required to be reflectedsell any specific number or dollar amount of Common Stock but will act as our placement agents, using commercially reasonable efforts to sell, on our balance sheets.behalf, all of the Common Stock requested by us to be sold, consistent with the Placement Agents’ normal trading and sales practices, on terms mutually agreed between the Placement Agents and us. The Placement Agents will be entitled to compensation under the terms of the Distribution Agreement at a fixed commission rate of 3.0% of the gross proceeds from each issuance and sale of Common Stock, if any.
During the three and six months ended June 30, 2022, no shares of Common Stock were sold under the Distribution Agreement.
Critical Accounting Policies and Estimates
Our discussion and analysis of our financial condition and results of operations is based on our condensed consolidated financial statements, which have been prepared in accordance with GAAP.U.S. Generally Accepted Accounting Principles. The preparation of these condensed consolidated financial statements requires us to make estimates, assumptions, and judgments that affect the reported amounts of assets, liabilities, revenues, costs, and expenses. We evaluate our estimates and judgments on an ongoing basis, and our actual results may differ from these estimates. We base our estimates on historical experience, known trends and events, contractual milestones, and other various factors that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources.
Our mostWe consider the following estimates to be critical accounting policiesas they involve a significant level of estimation uncertainty and have had or are described under the heading “Management’s Discussionreasonably likely to have a material impact on our financial condition and Analysisresults of Financial Condition and Results of Operations–Critical Accounting Policies” in Part II, Item 7 of our 2020 Annual Report which was filed with the SEC on March 29, 2021. There were no material changes to our critical accounting policies through March 31, 2021 from those discussed in our 2020 Annual Report.
Recent Accounting Pronouncements
operations. See Note 2 to our condensed consolidated financial statements included in Part I, Item 1, “Notes to Condensed Consolidated Financial Statements” of this Quarterly Report on Form 10-Q for a description of recentother significant accounting pronouncementspolicies.
Contingent Earn-Out Liabilities
In connection with the Reverse Recapitalization, certain stockholders are entitled to the Contingent Earn-outs payments based on achievement of certain milestones. In accordance with ASC 815, we classified the Contingent Earn-outs as liabilities and measured them at fair value on the date of the Reverse Recapitalization. We remeasure the liabilities at each reporting date and record the change in fair value as a component of other income (expense), net, in the condensed consolidated statements of operations and comprehensive loss. We estimate the fair value using a Monte Carlo valuation model, which requires significant judgment. The unobservable inputs include the expected stock price volatility, the risk-free interest rate, and the expected term.
As of June 30, 2022 and December 31, 2021, the unobservable inputs were as follows:
| | | | | | | | |
| | June 30, | | | December 31, | |
| | 2022 | | | 2021 | |
Expected stock price volatility | | | 115.00 | % | | | 105.00 | % |
Risk-free interest rate | | | 3.00 | % | | | 1.10 | % |
Expected dividend yield | | | 0.00 | % | | | 0.00 | % |
Expected term | | 3.50 years | | | 4.00 years | |
We recorded a gain due to the change in fair value of the Clene Nanomedicine Contingent Earn-out of $8.3 million and $8.6 million for the three months ended June 30, 2022 and 2021, respectively; and a gain of $8.3 million and loss of $17.0 million for the six months ended June 30, 2022 and 2021, respectively. We recorded a gain due to change in fair value of the Initial Stockholders Contingent Earn-out of $1.1 million and $1.2 million for the three months ended June 30, 2022 and 2021, respectively; and a gain of $1.1 million and loss of $1.7 million for the six months ended June 30, 2022 and 2021, respectively.
Convertible Notes
Pursuant to the 2021 Avenue Loan, $5.0 million of the outstanding principal is subject to the Conversion Feature. In accordance with Accounting Standards Update (“ASU”) 2020-06, Debt—Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging—Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity, we classified this portion as convertible notes payable in the condensed consolidated balance sheets and did not bifurcate the Conversion Feature from the host contract. Consequently, we account for the convertible note as a single liability
38
measured at its amortized cost. As of June 30, 2022 and December 31, 2021, the convertible note was carried at $4.7 million and $4.6 million, respectively.
Common Stock Warrant Liability
Pursuant to the 2021 Avenue Loan, we issued the Avenue Warrant. In accordance with ASC 815, we recognized both the issued Tranche 1 warrant and the Tranche 2 warrant issuable pursuant to the potential draw of Tranche 2 as liabilities measured at fair value. As we did not complete a bona fide round of equity financing by March 31, 2022, the exercise price and underlying shares of the Tranche 1 warrant became fixed and therefore qualified for equity classification. We remeasured the Tranche 1 warrant liability to fair value as of March 31, 2022 and recognized the change in fair value in the condensed consolidated statements of operations and comprehensive loss and the Tranche 1 warrant liability was reclassified to additional paid-in-capital. We remeasure the Tranche 2 warrant liability at each reporting date and record the change in fair value as a component of other income (expense), net, in the condensed consolidated statements of operations and comprehensive loss.
We estimate the fair value using a Black-Scholes option-pricing model, with a probability weight related to the potential draw of Tranche 2, which requires significant judgment. The unobservable inputs include the expected stock price volatility, risk-free interest rate, expected term, and the probability of drawing Tranche 2. As of June 30, 2022 and December 31, 2021, the unobservable inputs were as follows:
| | | | | | | | |
| | June 30, | | | December 31, | |
| | 2022 | | | 2021 | |
Expected stock price volatility | | | 115.00 | % | | | 105.00 | % |
Risk-free interest rate | | | 3.00 | % | | | 1.20 | % |
Expected dividend yield | | | 0.00 | % | | | 0.00 | % |
Expected term | | 3.70 years | | | 3.89 – 4.39 years | |
Probability of drawing Tranche 2 | | | 45.00 | % | | | 50.00 | % |
We recorded a change in fair value of the common stock warrant liability of $20,000 and $0.1 million for the three months ended June 30, 2022 and 2021, respectively; and $2,000 and $0.1 million for the six months ended June 30, 2022 and 2021, respectively.
Income Taxes
We account for uncertainty in income taxes by applying a two-step process to determine the amount of tax benefit to be recognized in the condensed consolidated financial statements. First, the tax position is evaluated to determine the likelihood that it will be sustained upon external examination by the taxing authorities. If the tax position is deemed more-likely-than-not to be sustained, the tax position is then assessed to determine the amount of benefit to recognize. The amount of the benefit that may be recognized is the largest amount that has a greater than 50% likelihood of being realized upon ultimate settlement. Additionally, we assess the likelihood that our deferred tax assets will be recovered from future taxable income and, to the extent we believe, based upon the weight of available evidence, that it is more likely than not that all or a portion of the deferred tax assets will not be realized, a valuation allowance is established through a charge to income tax expense. Potential for recovery of deferred tax assets is evaluated by estimating the future taxable profits expected and considering prudent and feasible tax planning strategies. The estimation of these factors requires significant judgment. Based on our evaluation of these factors, we have not recorded income tax benefits for the net operating losses or for research and development tax credits or other deferred tax assets due to uncertainty of realizing benefits from these items.
Stock-Based Compensation
We account for stock-based compensation arrangements using a fair value-based method for costs related to all share-based payments including stock options and stock awards. The fair value is recognized over the period during which a grantee was required to provide services in exchange for the option award and service-based stock awards, known as the requisite service period (usually the vesting period), on a straight-line basis. For stock awards with market conditions, the fair value is recognized over the period based on the expected milestone achievement dates as the derived service period (usually the vesting period), on a straight-line basis. For stock awards with performance conditions, the grant-date fair value of these awards is the market price on the applicable grant date, and compensation expense will be recognized when the conditions become probable of being satisfied. We will recognize a cumulative true-up adjustment once the conditions become probable of being satisfied as the related service period had been completed in a prior period. We elect to our business.account for forfeitures as they occur, rather than estimating expected forfeitures.
39
We estimate the fair value of stock options using a Black-Scholes option-pricing model, which requires significant judgment. The unobservable inputs include the expected price volatility, risk-free interest rate, expected dividend yield, and expected term. For the six months ended June 30, 2022 and 2021, the unobservable inputs were as follows:
| | | | |
| | Six Months Ended June 30, |
| | 2022 | | 2021 |
Expected stock price volatility | | 89.57% – 93.38% | | 84.80% – 87.70% |
Risk-free interest rate | | 1.65% – 3.00% | | 0.59% – 1.90% |
Expected dividend yield | | 0.00% | | 0.00% |
Expected term of options | | 5.00 – 6.98 years | | 6.00 years |
We estimate the fair value of restricted stock awards using a Monte Carlo valuation model to simulate the achievement of certain stock price milestones. The unobservable inputs include the expected stock price volatility, risk-free interest rate, and expected term. No restricted stock awards were granted during the six months ended June 30, 2022 and 2021.
ITEMItem 3. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISKQuantitative and Qualitative Disclosures About Market Risk
InformationAs a smaller reporting company, we are not required to provide information required by this Item is not applicable as we are electing scaled disclosure requirements available to Smaller Reporting Companies with respect to this Item.
Item 4. Controls and Procedures
ITEM 4. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
Under the supervision and with the participation of our management, including our Chief Executive Officerprincipal executive officer and Chief Financial Officer,principal financial officer, we conducted an evaluation of the effectiveness of our disclosure controls and procedures as of March 31, 2021,June 30, 2022, as such term is defined in Rules 13a-15(e) and 15d-15(e) underof the Securities Exchange Act. Based onAct of 1934 (the “Exchange Act”). As a result of this evaluation, our Chief Executive Officerprincipal executive officer and Chief Financial Officerprincipal financial officer have concluded that, as of March 31, 2021,June 30, 2022, our disclosure controls and procedures were not effective due to the material weaknesses in internal control over financial reporting described below. Notwithstanding the identified material weaknesses, management, including our Chief Executive Officerprincipal executive officer and Chief Financial Officer,principal financial officer, believes the condensed consolidated financial statements included in this Quarterly Report on Form 10-Q fairly represent, in all material respects, our financial condition, results of operations and cash flows atas of and for the periods presented in accordance with U.S. GAAP.United States Generally Accepted Accounting Principles.
Disclosure controls and procedures are designed to ensure that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in the SEC’sSecurities and Exchange Commission (the “SEC”) rules and forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officerprincipal executive officer and Chief Financial Officer,principal financial officer, as appropriate to allow timely decisions regarding required disclosure.
Material Weaknesses in Internal Control over Financial Reporting
In connection with the audit of our financial statements as of and for the years ended December 31, 20202021 and 2019,2020, our management identified material weaknesses in our internal control over financial reporting. A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis. The material weaknesses identified relate to the fact that we did not design or maintain an effective control environment commensurate with our financial reporting requirements, including (a) lack of a sufficient number of trained professionals with an appropriate level of accounting knowledge, training and experience to appropriately analyze, record and disclose accounting matters timely and accurately, and (b) lack of structures, reporting lines and appropriate authorities and responsibilities to achieve financial reporting objectives.requirements. This deficiency in our control environment contributed to the following additional deficiencies (each of which individually represents a material weakness) inweaknesses related to control activities and information and communication within our internal control over financial reporting:
•we did not design and maintain controls over the preparation and review of reconciliations and the review and segregation of duties over manual journal entries, including controls over the completeness and accuracy of information; and
•we did not design and maintain information technology (“IT”) general controls for IT systems that are relevant to the preparation of the financial statements. Specifically, we did not design and maintain: (a) user access controls to ensure appropriate segregation of duties and that adequately restrict user and privileged access to financial applications, programs, and data to our appropriate personnel; (b) program change management controls to ensure that IT program and data changes affecting financial IT applications and underlying accounting records are identified, tested, authorized, and implemented appropriately; (c) computer operations controls to ensure that data backups are authorized and monitored; and (d) testing and approval controls for program development to ensure that new software development is aligned with business and IT requirements.
40
| ● | we did not design and maintain formal accounting policies, procedures and controls to achieve complete, accurate and timely financial accounting, reporting and disclosures, including controls over the preparation and review of account reconciliations and journal entries; |
| ● | we did not design and maintain effective controls over segregation of duties related to manual journal entries. Specifically, certain personnel have the ability to both prepare and post manual journal entries without an independent review by someone without the ability to prepare and post manual journal entries; |
| ● | we did not design and maintain formal accounting policies, processes and controls to analyze, account for and disclose complex transactions. Specifically, we did not design and maintain controls to analyze, account for and disclose warrants to purchase preferred stock and convertible promissory notes with embedded derivatives, including ensuring complete and accurate data was used in the valuations; and |
| ● | we did not design and maintain effective controls over certain information technology (“IT”) general controls for IT systems that are relevant to the preparation of the financial statements. Specifically, we did not design and maintain: (a) user access controls to ensure appropriate segregation of duties and that adequately restrict user and privileged access to financial applications, programs, and data to appropriate personnel of Clene, (b) program change management controls to ensure that IT program and data changes affecting financial IT applications and underlying accounting records are identified, tested, authorized and implemented appropriately, (c) computer operations controls to ensure that data backups are authorized and monitored, and (d) testing and approval controls for program development to ensure that new software development is aligned with business and IT requirements. |
The control deficiencies described above resulted in the misstatement of our redeemable convertible preferred stock warrant liability, accrued liabilities, general and administrative expenses, Australian research and development credit, and amounts and classification within our statement of cash flows and related financial disclosures as of and for the year ended December 31, 2019 and in the misstatement of our prepaid expenses and other current assets, accrued liabilities, earn-out liabilities, redeemable convertible preferred stock warrant liability, general and administrative expenses, amounts and classification within our statement of equity, and amounts and classification within our statement of cash flows and related financial disclosures as of and for the year ended December 31, 2020. Additionally, eachEach of the control deficiencies described above could result in a misstatement of one or more account balances or disclosures that would result in a material misstatement to the annual or interim consolidated financial statements that would not be prevented or detected. Accordingly, our management has determined that each of the control deficiencies described above constitute material weaknesses.
Material Weakness Remediation
Management iscontinues to be actively engaged and committed to taking the steps necessary to remediate the control deficiencies that constituted the above material weakness. During 2020,2021, we made the following enhancements to our control environment:
•we have strengthened the experience of our internal accounting team, to provide oversight, structure and reporting lines, and to provide additional review over our disclosures, including hiring a new Vice President, Finance and Controller;
•we have engaged external consultants to assist with the evaluation of complex accounting and financial reporting related areas and to assist with the documentation around accounting and financial reporting policies and procedures;
•we engaged external consultants to assist in the design, implementation, and documentation of internal controls that address the relevant risks and provide for appropriate evidence of performance of the internal control; and
•we implemented a new Enterprise Resource Planning (“ERP”) system that will significantly enhance the information technology general controls environment.
| ● | we added finance personnel to the organization to strengthen our internal accounting team, to provide oversight, structure and reporting lines, and to provide additional review over our disclosures to include a Chief Financial Officer and a Manager of SEC Reporting; |
| ● | we engaged outside consultants to assist in the design, implementation, and documentation of internal controls that address the relevant risks, are properly designed, and provide for appropriate evidence of performance of the internal control; and |
| ● | we engaged outside consultants to assist us in the evaluation of a new Enterprise Resource Planning (“ ERP”) system in order to mitigate the internal control gaps and limitations that cannot be addressed by the current ERP around segregation of duties, and to enhance the information technology general controls environment. |
Our remediation activities are continuing during 2021.2022. In addition to the above actions, we expect to engage in additional activities, or have completed additional activities, including, but not limited to:
•adding more technical accounting resources to enhance our control environment;
•until we have sufficient technical accounting resources, engaging external consultants to provide support and to assist us in our evaluation of more complex applications of GAAP, and to assist us with documenting and assessing our accounting policies and procedures; and
•implemented a new ERP to enhance the accuracy of our financial records, enable the enforcement of systematic segregation of duties, and improve our information technology general controls environment.
| ● | adding more technical accounting resources to enhance our control environment; |
| ● | until we have sufficient technical accounting resources, engaging external consultants to provide support and to assist us in our evaluation of more complex applications of GAAP, and to assist us with documenting and assessing our accounting policies and procedures; |
| ● | implementing a new ERP to enhance the accuracy of our financial records, enable the enforcement of systematic segregation of duties, and to improve our information technology general controls environment. |
We continue to enhance corporate oversight over process-level controls and structures to ensure that there is appropriate assignment of authority, responsibility, and accountability to enable remediation of our material weaknesses. We believe that our remediation plan will be sufficient to remediate the identified material weakness and strengthen our internal control over financial reporting. As we continue to evaluate, and work to improve, our internal control over financial reporting, management may determine that additional measures to address control deficiencies or modifications to the remediation plan are necessary.
Changes in Internal Control over Financial Reporting
We are engagedOther than changes described under “—Material Weakness Remediation,” there were no changes in the process of the design and implementation of our internal control over financial reporting in a manner commensurate with the scale of our operations following the Reverse Recapitalization. Duringduring the quarter ended March 31, 2021, we began implementing a new ERPJune 30, 2022, that have materially affected, or are reasonably likely to enhance the accuracy ofmaterially affect, our internal control over financial records, enable the enforcement of systematic segregation of duties, and to improve our information technology general controls environment.reporting.
41
PART II – II—OTHER INFORMATION
Item 1. Legal Proceedings
ITEM 1. LEGAL PROCEEDINGS
We may be subject to legal proceedings, investigations and claims incidental to the conduct of our business from time to time. We are not currently a party to any material litigation or otherpending legal proceedings. From time to time, we may, however, be involved in legal proceedings brought against us.in the ordinary course of business. We are also not awarecannot predict the outcome of any such legal proceeding, investigation or claim, orproceedings, and despite the potential outcomes, the existence thereof may have an adverse impact on us because of defense and settlement costs, diversion of management resources and other legal exposure that has a more than remote possibility of having a material adverse effect on our business, financial condition or results of operations.factors.
Item 1A. Risk Factors
ITEM 1A. RISK FACTORS
Our business, financial condition, and results of operations can be affected by a number of factors, whether currently known or unknown, including but not limited to those described in Part I, Item 1A, “Risk Factors”Risk Factors of our 20202021 Annual Report on Form 10-K (“2020 Annual Report”) which was filed with the SEC on March 29, 2021.11, 2022. There have been no material changes to ourthe risk factors since previously disclosed in the 20202021 Annual Report.Report on Form 10-K. Any one or more of these factors could, directly or indirectly, cause our actual financial condition and results of operations to vary materially from past, or from anticipated future, financial condition and results of operations. Any of these factors, in whole or in part, could materially and adversely affect our business, financial condition, results of operations, and stock price.
ITEMItem 2. UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDSUnregistered Sales of Equity Securities and Use of Proceeds
(a)Recent Sales of Unregistered Securities
None.
None.
None.
We entered into subscription agreements with various investors for the private placement of Common Stock (the “Private Placement”), all of which closed shortly before the closing of the Reverse Recapitalization. Under the Private Placement, 2,239,500 shares of Common Stock (the “PIPE Shares”) were sold, resulting in net proceeds of $22.2 million. Pursuant to the subscription agreements, investors in the Private Placement also received warrants to purchase a number of shares equal to one-half (1/2) of the number of PIPE Shares, totaling 1,119,750 shares of PubCo Common Stock, at an exercise price of $0.01 per share for each of the PIPE Shares (the “PIPE Warrants”), subject to a 180-day holding period. We filed a registration statement on Form S-1 to register the PIPE Shares and the Common Stock underlying the PIPE Warrants, which the SEC declared to be effective on April 19, 2021 (file number 333-253173).
We have been using and will continue to use these proceeds primarily (1) to fund our VISIONARY-MS, REPAIR-MS, REPAIR-PD, and RESCUE-ALS studies, and for our participation in the Healey-ALS Platform Trial, and our other clinical research and development activities, and (2) for general and administrative purposes.
(c)Purchases of Equity Securities by the Issuer and Affiliated Purchasers
None.
None.
Item 3. Defaults Upon Senior Securities
ITEM 3. DEFAULTS UPON SENIOR SECURITIESNone.
None.
Item 4. Mine Safety Disclosures
ITEM 4. MINE SAFETY DISCLOSURES
Not Applicable.
Item 5. Other Information
ITEM 5. OTHER INFORMATIONOn August 5, 2022, we amended and restated a promissory note (the “Amended Promissory Note”) pursuant to a security agreement, dated May 17, 2022, with the Department of Housing and Community Development, a principal department of the State of Maryland (the “DHCD”). The Amended Promissory Note clarifies that borrowings mature on July 1, 2027 (the “Maturity Date”); the first twelve payments, commencing on July 1, 2022, are deferred; and immediately thereafter there shall be eighteen monthly installments of interest-only based on the actual amount advanced under the loan, each up to a maximum amount of $15,000; followed by thirty monthly installments of principal and interest, each in the amount of $33,306, which is due and payable even if the entire loan has not been advanced prior to the date such monthly payment is due and payable, with a balloon payment of all accrued and unpaid interest and principal due on the Maturity Date.
On August 9, 2022, we entered into a second amendment to loan and security agreement (the “Amended Loan Agreement”) with Avenue Venture Opportunities Fund, L.P. (“Avenue”), which permits indebtedness secured by a lien of up to $3.0 million with any unrelated third-party providing equipment financing on terms reasonably acceptable to Avenue.
None.The foregoing descriptions of the Amended Promissory Note and Amended Loan Agreement do not purport to be complete and are qualified in their entirety by reference to the full text of the Amended Promissory Note and Amended Loan Agreement, which are filed herewith as Exhibit 10.5 and Exhibit 10.6 to this Quarterly Report on Form 10-Q and are incorporated herein by reference.
42
ITEM
Item 6. EXHIBITS
Exhibits
Exhibit | | Description |
2.1Exhibit Number |
| Merger Agreement, dated as of September 1, 2020 (incorporated by reference to Annex A-1 to the Proxy Statement/Consent Solicitation Statement/Prospectus on Form S-4 filed by the Registrant on September 10, 2020).Exhibit Description |
3.1 |
| Third Amended and Restated Certificate of Incorporation of Clene Inc. (incorporated by reference to Exhibit 3.1 to the Current Report on Form 8-K filed by the Registrant on January 5,July 16, 2021). |
3.2 |
| Bylaws of Clene Inc. (incorporated by reference to Exhibit 3.2 to the Current Report on Form 8-K filed by the Registrant on January 5, 2021). |
4.110.1 |
| Specimen TOTA Warrant Certificate (incorporated by reference to Exhibit 4.4 to the Tottenham Registration Statement on Form S-1 filed with the Securities & Exchange Commission on July 5, 2018)
|
4.2 | | WarrantEquity Distribution Agreement, dated August 1, 2018, by and between Continental Stock Transfer & Trust Company and the Registrant (incorporated by reference to Exhibit 4.5 to the Current Report on Form 8-K filed with the Securities and Exchange Commission on August 7, 2018). |
10.1 | | Form of Registration Rights Agreement (incorporated by reference to Exhibit 10.10 to the Registration Statement on Form S-4 filed by Chelsea Worldwide Inc. with the Securities and Exchange Commission on December 15, 2020) |
10.2 | | Escrow Agreement,April 14, 2022, by and among Clene Inc., Fortis Advisors and Canaccord Genuity LLC and Continental Stock TransferOppenheimer & Trust Company, as the escrow agent (incorporated by reference to Exhibit 10.8 to the Registration Statement on Form S-4 filed by Chelsea Worldwide Inc. with the Securities and Exchange Commission on December 15, 2020) |
10.3 | | Form of Indemnification Agreement between the Registration and its directors and executive officers (incorporated by reference to Exhibit 10.18 to the Registration Statement on Form S-4 filed by Chelsea Worldwide Inc. with the Securities and Exchange Commission on December 15, 2020) |
10.4* | | License Agreement, effective August 31, 2018, between Clene Nanomedicine, Inc. and 4Life Research, LLC (incorporated by reference to Exhibit 10.14 to the Registration Statement on Form S-4 filed by Chelsea Worldwide Inc. with the Securities and Exchange Commission on December 15, 2020) |
10.5* | | Exclusive Supply Agreement, dated August 31, 2018, between Clene Nanomedicine, Inc. and 4Life Research, LLC (incorporated by reference to Exhibit 10.15 to the Registration Statement on Form S-4 filed by Chelsea Worldwide Inc. with the Securities and Exchange Commission on December 15, 2020) |
10.6* | | Lease Agreement, dated May 9, 2016, and First Amendment of Lease Agreement, dated January 6, 2017, between Upper Chesapeake Flex One, LLC and Clene Nanomedicine,Co. Inc. (incorporated by reference to Exhibit 10.16 to the Registration Statement on Form S-4 filed by Chelsea Worldwide Inc. with the Securities and Exchange Commission on December 15, 2020) |
10.7* | | Clinical Research Support Agreement, dated September 27, 2019, between Clene Nanomedicine, Inc. and The General Hospital Corporation (incorporated by reference to Exhibit 10.17 to the Registration Statement on Form S-4 filed by Chelsea Worldwide Inc. with the Securities and Exchange Commission on December 15, 2020) |
10.8** | | 2020 Equity Incentive Plan (Incorporated by reference to Exhibit 10.4 to the registrant’s Registration Statement on Form 8-K filed by the registrant on January 5, 2021) |
10.9 | | Clene Inc. Board of Directors Compensation Program (Incorporated by reference to Exhibit 10.1 to the registrant’s Current Report on Form 8-K filed by the registrantRegistrant on April 22, 2021)14, 2022). |
31.110.2 |
| Security Agreement, dated May 17, 2022, by Clene Nanomedicine, Inc. in favor of the Department of Housing and Community Development, a principal department of the State of Maryland (incorporated by reference to Exhibit 10.1 to Current Report on Form 8-K filed by the Registrant on May 19, 2022). |
10.3# |
| Disbursement Agreement, dated May 17, 2022, by and between Clene Nanomedicine, Inc. and the Department of Housing and Community Development, a principal department of the State of Maryland (incorporated by reference to Exhibit 10.2 to Current Report on Form 8-K filed by the Registrant on May 19, 2022). |
10.4 |
| Promissory Note, dated May 17, 2022, by Clene Nanomedicine, Inc. to the Department of Housing and Community Development, a principal department of the State of Maryland (incorporated by reference to Exhibit 10.3 to Current Report on Form 8-K filed by the Registrant on May 19, 2022). |
10.5* |
| Amended and Restated Promissory Note, dated July August 5, 2022, by Clene Nanomedicine, Inc. to the Department of Housing and Community Development, a principal department of the State of Maryland. |
10.6*## |
| Second Amendment to Loan and Security Agreement, dated August 9, 2022, by and between Clene Inc., Clene Nanomedicine, Inc. and Avenue Venture Opportunities Fund, L.P. |
31.1* |
| Certification of Chief Executive Officer pursuant to Rule 13a-14(a) and Rule 15d-14(a), promulgated under the Securities and Exchange Act of 1934, as amended. |
31.231.2* |
| Certification of Chief Financial Officer pursuant to Rule 13a-14(a) and Rule 15d-14(a), promulgated under the Securities and Exchange Act of 1934, as amended. |
32.132.1** |
| Certification of Chief Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
32.232.2** |
| Certification of Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
101.1101.INS |
| Inline XBRL Instance DocumentDocument. |
101.2101.SCH |
| Inline XBRL Taxonomy Extension Schema DocumentDocument. |
101.3101.CAL |
| Inline XBRL Taxonomy Extension Calculation Linkbase DocumentDocument. |
101.4101.DEF |
| Inline XBRL Taxonomy Extension Definition Linkbase DocumentDocument. |
101.5101.LAB |
| Inline XBRL Taxonomy Extension Label Linkbase DocumentDocument. |
101.6101.PRE |
| Inline XBRL Taxonomy Extension Presentation Linkbase DocumentDocument. |
104 |
| Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101). |
* | Portions |
* | Filed herewith. |
** | Furnished herewith. |
# | Certain information has been redacted from this Exhibit in accordance with Item 601(a)(6) of Regulation S-K. |
## | Schedules and similar attachments to this exhibit have been omitted pursuant to Rule 601(b)(10)Item 601(a)(5) of Regulation S-K. TheWe agree to furnish supplementally a copy of such omitted information is not material and would likely cause competitive harmmaterials to the registrant if publicly disclosed. |
** | Indicates a management contract or a compensatory plan or agreement.SEC upon request. |
SIGNATURES43
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| CLENE INC. | |
| | CLENE INC. |
|
|
|
Dated: May 10, 2021August 15, 2022 | By: | /s/ Robert Etherington |
| Name: | Robert Etherington |
| Title: | President, Chief Executive Officer and Director |
|
|
Dated: May 10, 2021August 15, 2022 | By: | /s/ Ted (Tae Heum) JeongMorgan R. Brown |
| Name: | Ted (Tae Heum) JeongMorgan R. Brown |
| Title: | Chief Financial Officer |
44
49
