We will need to obtain additional financing to fund our future operations, including completing the commercialization of our approved product and the development and commercialization of our other product candidates. Changing circumstances may cause us to increase our spending significantly faster than we currently anticipate and we may need to raise additional funds sooner than we presently anticipate. Moreover, research and development and our operating costs and fixed expenses such as rent and other contractual commitments, including those for our research collaborations, are substantial and are expected to increase in the future.
Our future funding requirements will depend on many factors, including, but not limited to:
Unless and until we can generate a sufficient amount of revenues, we may finance future cash needs through public or private equity offerings, license agreements, debt financings, collaborations, strategic alliances and marketing or distribution arrangements. However, we may be unable to raise additional funds or enter into such other arrangements when needed on favorable terms, or at all, including but not limited to the offering, issuance and sale by us of our common stock that may be issued and sold under the ATM.
To the extent that we raise additional capital through the sale of equity or equity-linked securities (including warrants), convertible debt or through the ATM, our shelf registration statement,statements, or other offerings, or if any of our current debt is converted into equity or if our existing warrants are exercised, your ownership interest will be diluted, and the terms may include liquidation or other preferences that adversely affect your rights as a stockholder. The incurrence of additional indebtedness would result in increased fixed payment obligations and could involve certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. If we raise additional funds through strategic partnerships and alliances and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies or product candidates, or grant licenses on terms unfavorable to us. We have no committed source of additional capital and if we are unable to raise additional capital in sufficient amounts or on terms acceptable to us, we may be required to delay or reduce the scope of or eliminate one or more of our research or development programs or our commercialization efforts. Our current license and collaboration agreements may also be terminated if we are unable to meet the payment obligations under those agreements. As a result, we may seek to access the public or private capital markets whenever conditions are favorable, even if we do not have an immediate need for additional capital at that time.
We have material cash requirements to pay related-party affiliates and third parties under various contractual obligations discussed below:
In Part II, Item 7. “Management’s Discussion and Analysis of Financial Condition and Results of Operations” of our Annual Report on Form 10-K filed with the SEC on March 1, 2023,19, 2024, we disclose those accounting policies that we consider to be significant in determining our results of operations and financial condition. There have been no material changes to those policies that we consider to be significant as of the date of this Quarterly Report on Form 10-Q.Report.
ITEM 3. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
Financial market risks related to interest rates, foreign currency exchange rates, market risk on the price and volatility of our common stock, and inflation are described in Part II, Item 7A. “Quantitative and Qualitative Disclosures About Market Risk” of our Annual Report on Form 10-K filed with the SEC on March 1, 2023.19, 2024. There have been no material changes to such financial market risks as of the date of this Quarterly Report on Form 10-Q.Report. We do not currently anticipate any other near-term changes in the nature of our financial market risk exposures or in management’s objectives and strategies with respect to managing such exposures.
ITEM 4. CONTROLS AND PROCEDURES.
Our disclosure controls and procedures are designed to provide reasonable assurance of achieving their objectives of ensuring that information we are required to disclose in the reports we file or submit under the Exchange Act is accumulated and communicated to our management, including our CEO and CFO, as appropriate, to allow timely decisions regarding required disclosures, and is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms. There is no assurance that our disclosure controls and procedures will operate effectively under all circumstances.
Management, with the participation of our CEO and CFO, evaluated the effectiveness of our disclosure controls and procedures as of June 30, 2023.March 31, 2024. The term “disclosure controls and procedures,” as defined in Rule 13a-15(e) of the Exchange Act means controls and other procedures of a company that are designed to provide reasonable assurance that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to provide reasonable assurance that information required to be disclosed is accumulated and communicated to our management, including our CEO and CFO, as appropriate, to allow timely decisions regarding required disclosure. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their desired control objectives, and management necessarily is required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Based on the evaluation of our disclosure controls and procedures as of June 30, 2023,March 31, 2024, our CEO and CFO have concluded that, as of June 30, 2023,March 31, 2024, our disclosure controls and procedures were effective at the reasonable assurance level.
There have been no changes in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) of the Exchange Act) during the fiscal quarter ended June 30, 2023,March 31, 2024, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Management recognizes that a control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud or error, if any, have been detected. These inherent limitations include the realities that judgments in decision making can be faulty, and that breakdowns can occur because of a simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people or by management override of the controls. The design of any system of controls also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions; over time, controls may become inadequate because of changes in conditions, or the degree of compliance with policies or procedures may deteriorate. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected.
ITEM 1. LEGAL PROCEEDINGS.
From time to time, we may be involved in various claims and legal proceedings relating to claims arising out of our operations. We are not currently a party to any legal proceedings that, in the opinion of our management, are likely to have a material adverse effect on our business. If we are served with any such complaints, we will assess at that time any contingencies for which we may need to reserve. Regardless of the outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources, and other factors.
Shenzhen Beike Biotechnology Co. Ltd. Arbitration
In 2020, we received a Request for Arbitration before the International Chamber of Commerce, International Court of Arbitration. The arbitration relates to a license, development, and commercialization agreement that Altor entered into with Beike in 2014, which agreement was amended and restated in 2017, pursuant to which Altor granted to Beike an exclusive license to use, research, develop and commercialize products based on N-803ANKTIVA in China for human therapeutic uses. In the arbitration, Beike is asserting a claim for breach of contract under the license agreement. Among other things, Beike alleges that we failed to use commercially reasonable efforts to deliver to Beike materials and data related to N-803.ANKTIVA. Beike is seeking specific performance and declaratory relief for the alleged breaches. On September 25, 2020, the parties entered into a standstill and tolling agreement (standstill agreement) under which, among other things, the parties affirmed they will perform certain of their obligations under the license agreement by specified dates and agreed that all deadlines in the arbitration are indefinitely extended. The standstill agreement could be terminated by any party on ten calendar days’ notice, and upon termination, the parties had the right to pursue claims arising from the license agreement in any appropriate tribunal. On March 20, 2023, we terminated the standstill agreement, and on April 11, 2023, Beike served an amended Request for Arbitration. We served an Answer and Counterclaims on May 19, 2023. Beike served a Reply to our counterclaims on June 21, 2023. Beike served its Statement of Claim on March 22, 2024, and the company’s Statement of Defense and Counterclaim is due on June 21, 2024. The hearing in the arbitration is scheduled to begin on June 9, 2025. Given that no discovery has occurred, it remains too early to evaluate the likely outcome of the case or to estimate any range of potential loss. We believe the claims asserted against the company lack merit and intend to defend the case, and to pursue our counterclaims, vigorously.
Altor BioScience, LLC, and NantCell, Inc. Matters Against Dr. Hing Wong and HCW Biologics, Inc. (HCW)
On December 23, 2022, Altor and NantCell filed an arbitration demand against Dr. Hing Wong, former CEO of Altor and NantCell. The demand asserts claims for breach of Dr. Wong’s contracts with the companies, breach of the covenant of good faith and fair dealing, conversion, fraudulent concealment, unjust enrichment, breach of fiduciary duty, and replevin. The same day, Dr. Wong filed an arbitration demand seeking a declaratory judgment finding that Dr. Wong is not liable to Altor or NantCell for any of their claims. The parties have agreed to consolidate the arbitration filings in one proceeding, and on January 23, 2023, Dr. Wong filed an Answering Statement denying the claims.
Also, on December 23, 2022 Altor and NantCell filed a complaint in the United States District Court for the Southern District of Florida against HCW, Biologics, Inc. (HCW), Dr. Wong’s new company. Altor’s and NantCell’s complaint asserts claims for misappropriation of trade secrets under both Florida and federal law, inducement of breach of contract, tortious interference with contractual relations, inducement of breach of fiduciary duty, conversion, unjust enrichment, replevin, request for assignment of patents and patent applications, and establishment of a constructive trust. On January 31, 2023, HCW filed motions to compel arbitration of Altor’s and NantCell’s claims, or in the alternative to stay or dismiss them. Altor and NantCell filed an opposition to the motions on February 14, 2023, and HCW filed reply papers on February 21, 2023. At a hearing on April 18, 2023, the court heard argument and requested supplemental briefing. After the hearing, the parties reached an agreement to consolidate all claims in a single arbitration proceeding. On May 1, 2023, we filed our arbitration demand asserting the same claims against HCW that were asserted in the federal court complaint. On May 15, 2023, HCW filed an Answering Statement denying the claims.
ITEM 1A. RISK FACTORS.
Investing in our common stock involves a high degree of risk. You should carefully consider the risks described below, any of which may be relevant to decisions regarding an investment in or ownership of our stock. The occurrence of any of these risks could have a significant adverse effect on our reputation, business, financial condition, results of operations, growth, and ability to accomplish our strategic objectives. We have organized the description of these risks into groupings in an effort to enhance readability, but many of the risks interrelate or could be grouped or ordered in other ways, so no special significance should be attributed to the groupings or order below.
Risks Related to Our Limited Operating History, Financial Condition and Capital Requirements
Risks Related to Our Limited Operating History, Financial Condition and Capital Requirements
The development of biopharmaceutical products, including conducting preclinical studies and clinical trials, is a very time-consuming, expensive and uncertain process that takes years to complete. Our operations have consumed substantial amounts of cash since inception. A significant portion of our funding had been in the form of promissory notes totaling $802.5$735.0 million in indebtedness (consisting of related-party promissory notes and accrued and unpaid interest) outstanding as of June 30, 2023March 31, 2024 held by entities affiliated with Dr. Soon-Shiong.
The occurrence of any of the foregoing factors could have a material adverse effect on our business, results of operations and financial condition.
On July 20, 2023, we completed a registered direct offering of 14,569,296 shares, generating gross proceeds of approximately $40.0 million before deducting placement agent fees and other estimated offering costs. In connection with the sale of our common stock, we entered into a warrant agreement that allows the investors to purchase up to 14,569,296 shares of our common stock at an exercise price of $3.2946 per share. The warrants became immediately exercisable on July 25, 2023 and expire three years after the issuance date on July 24, 2026.
We account for the warrants as a derivative instrument,instruments, and changes in the fair value of the warrants are included in other (expense) income,expense, net, on the condensed consolidated statementstatements of operations for each reporting period. At June 30, 2023,As of March 31, 2024, the fair value of warrant liabilities included in the company’s condensed consolidated balance sheet was $27.8$110.0 million. We useduse the Black-Scholes option pricing model to determine the fair value of the warrants. As a result, the valuation of thisthese derivative instrumentinstruments is subjective, and the Black-Scholes option pricing model requires the input of highly subjective assumptions, including the expected stock price volatility and probability of a fundamental transaction (a strategic merger or sale). Changes in these assumptions can materially affect the fair value estimate. We could, at any point in time, ultimately incur amounts different than the carrying value, which could have a significant impact on our results of operations and financial position.
We account for the revenue interest liability as a liability, net of a debt discount comprised of deferred issuance costs, the fair value of a freestanding option agreement related to the SPOA, and the fair value of embedded derivatives requiring bifurcation on the consolidated balance sheet. The fluctuationscompany imputes interest expense associated with this liability using the effective interest rate method. The effective interest rate is calculated based on the rate that would enable the debt to be repaid in full over the anticipated life of the arrangement. Interest expense is recognized over the estimated term on the consolidated statement of operations. The interest rate on the liability and the underlying value of the bifurcated embedded derivative may vary during the term of the agreement depending on a number of factors, including the level of actual and forecasted net sales, and in the case of the derivative, specific probabilities associated with RIPA Put/Call events or Test Date payments underlying our Monte Carlo analysis. The company evaluates the interest rate quarterly based on actual and forecasted net sales utilizing the prospective method. A significant increase or decrease in actual or forecasted net sales will materially impact the revenue interest liability and/or the bifurcated embedded derivative, interest expense, and the time period for repayment.
Fluctuations in warrant, valuerevenue interest liability, and derivative values, and changes in the assumptions and factors used in the model may impact our operating results, making it difficult to forecast our operating results and making period-to-period comparisons less predictive of future performance. In one or more future quarters, our results of operations may fall below the expectations of securities analysts and investors. In that event, the market price of our common stock could decline. In addition, the market price of our common stock may fluctuate or decline regardless of our operating performance.performance.
The accounting method for convertible debt securities could have a material effect on our reported financial results. WeIn accordance with ASC 470-50, we recorded amendments to our related-party promissory notes entered into on September 11, 2023 under the debt modification accounting model, as the amendments were not substantially different than the terms of the promissory notes prior to the amendment. Under this model, the unamortized debt discounts from the promissory notes are amortized as an adjustment of interest expense over the remaining term of modified promissory notes using the effective interest rate method. Also, the increase in fair value of the embedded conversion feature from the debt modification was accounted for as a clinical-stage biotechnology companydebt discount to the $200.0 million convertible note that is not recorded at fair value with a limited operating history and no products approved for commercial sale. We have a history of operating losses, and we expect to continue to incur losses and may never be profitable, which together with our limited operating history, makes it difficult to assess our future viability.
We are a clinical-stage biotechnology company with a limited operating history upon which you can evaluate our business and prospects, and we have a broad portfolio of product candidates at various stages of development. None of our products have been approved for commercial sale, and we have not generated any revenue from product sales, although we have generated revenues from non-exclusive license agreements related to our cell lines, the sale of our bioreactors and related consumables and grant programs.corresponding increase in additional paid-in capital. In addition, we have limited experience and have not yet demonstrated an abilityrecorded amendments to successfully overcome many ofour related-party promissory notes entered into on December 29, 2023 under the risks and uncertainties frequently encountered by companies in new and rapidly evolving fields, particularly in the biotechnology industry, including in connection with obtaining marketing approvals, manufacturing a commercial-scale product or arranging for a third party to do so on our behalf or conducting sales and marketingactivities necessary for successful product commercialization. Because of the numerous risks and uncertainties associated with our product development efforts, we are unable to predict when we may become profitable, if at all.
Since the commencement of our operations, we have incurred significant losses each year,debt extinguishment model, and as a result recognized a total net gain on extinguishment of June 30, 2023 we had an accumulated deficit of $2.6 billion. We expect to continue to incur significant expenses as we seek to expand our business, including$36.1 million, which was recorded in connection with conducting research and development across multiple therapeutic areas, participating in clinical trial activities, continuing to acquire or in-license technologies, maintaining, protecting and expanding our intellectual property, seeking regulatory approvals, increasing our manufacturing capabilities and, upon successful receipt of FDA approval, commercializing our products. Moreover, we do not expect to have significant product sales or revenue in the near term, if ever.
If we are required by the FDA or any equivalent foreign regulatory authority to perform clinical trials or studies in addition to those we currently expect to conduct, or if there are any delays in completing the clinical trials of our product candidates, our expenses could increase substantially. We have submitted a BLA for our product candidate, Anktiva in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease. On May 9, 2023, the FDA delivered a CRL to us regarding the BLA, indicating that the FDA had determined that it cannot approve the BLA in its present form, and the FDA made recommendations to address the issues raised. The company has submitted a formal meeting request and the associated briefing book to the FDA and plans to diligently address and resolve the issues identified in the CRL and seek approval as expeditiously as possible. It is unclear when the FDA will approve our BLA, if at all, for commercialization and even if approved, the resulting revenue may not enable us to achieve profitability. Even if we obtain regulatory approval to market a product candidate, our future revenues will depend upon the size of any markets in which our product candidates have received approval, and our ability to achieve sufficient market acceptance, reimbursement from third-party payors and adequate market share for our product candidates in those markets.
We expect our expenses and net losses to increase significantly as we prepare to potentially commercialize our product candidate, Anktiva in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease, if approved by the FDA, continue our development of, and seek regulatory approvals for, our other product candidates, and begin to commercialize other approved products, if any, as well as hire additional personnel, protect our intellectual property and incur additional costs associated with operating as a public company. Our net losses may fluctuate significantly from quarter to quarter and year to year, depending paid-in capital, on the timingconsolidated statement of our clinical studies and trials, associated manufacturing needs, commercialization activities if our product candidates are approved and our expenditures on other research and development activities.
If our research and development efforts are successful, we may also facestockholders’ deficit, as the risks associated with the shiftdebt was acquired from development to commercialization of new products based on innovative technologies. Our ability to achieve profitability, if ever, is dependent upon, among other things, obtaining regulatory approvals for our product candidates and successfully commercializing our product candidates alone or with third parties. However, our operations may not be profitable even if one or more of our product candidatesentities under development are successfully developed and produced and thereafter commercialized. Even if we do become profitable, we may not be able to sustain or increase our profitability on a quarterly or annual basis.common control. As a result it mayof the debt amendments, we will be more difficult for yourequired to assessrecord a greater amount of non-cash interest expense in current periods presented as a result of the amortization of the discount associated with certain promissory notes. We will either report lower net income or a higher net loss in our consolidated financial results because FASB ASC Topic 470-20, Debt with Conversion and Other Options, requires interest to include both the current period’s amortization of the debt discount and the instrument’s coupon interest, which could adversely affect our reported or future viability than it could be if we had a longer operating history.
We invest our cash on hand in various financial instruments which are subject to risks that could adversely affect our business, results of operations, liquidity and financial condition.
We investhave typically invested our cash in a variety of financial instruments, principally commercial paper,including investment-grade short- to intermediate-term corporate debt securities, government-sponsored securities and governmentEuropean bonds; however, after our entry into the RIPA, we can no longer invest our excess funds in corporate or European bonds. AllCertain of theseour investments are subject to credit, liquidity, market, and interest rate risk. Such risks, including the failure or severe financial distress of the financial institutions that hold our cash, cash equivalents and investments, may result in a loss of liquidity, impairment to our investments, realization of substantial future losses, or a complete loss of the investments in the long-term, which may have a material adverse effect on our business, results of operations, liquidity and financial condition. In order toTo manage the risk to our investments, we maintain
an investment policy that, among other things, limits the amount that we may invest in any one issue or any single issuer and requires us to only invest in high credit quality securities to preserve liquidity.
Our ability to use NOLs and research and development credits to offset future taxable income may be subject to certain limitations.
In general, under Sections 382 and 383 of the Internal Revenue Code, of 1986, as amended, a corporation that undergoes an “ownership change”ownership change is subject to limitations on its ability to utilize its pre-change NOLs or credits, to offset future taxable income or taxes. For these purposes, an ownership change generally occurs where the aggregate stock ownership of one or more stockholders or groups of stockholders who owns at least 5% of a corporation’s stock increases its ownership by more than 50 percentage points over its lowest ownership percentage within a specified testing period. We have not conducted a complete study to assess whether a change of control has occurred or whether there have been multiple changes of control since inception due to the significant complexity and cost associated with such a study. If we have experienced a change of control, as defined by Section 382, at any time since inception (including as a result of the March 2021 merger which pursuant to which NantKwest and NantCell combined their businesses), utilization of the NOL carryforwards or research and development tax credit carryforwards would be subject to an annual limitation under Section 382. Any limitation may result in expiration of a portion of the NOL carryforwards or research and development tax credit carryforwards before utilization. In addition, our NOLs or credits may also be impaired under state law. Accordingly, we may not be able to utilize a material portion of our NOLs or credits.
Since we will need to raise substantial additional funding to finance our operations, we may experience further ownership changes in the future, some of which may be outside of our control. Limits on our ability to use our pre-change NOLs or credits to offset U.S. federal taxable income could potentially result in increased future tax liability to us if we earn net taxable income in the future. In addition, under the legislation commonly referred to as the Tax Cuts and Jobs Act of 2017 (TCJA),TCJA, as modified by the Coronavirus Aid, Relief, and Economic Security Act, (CARES Act), the amount of NOLs generated in taxable periods beginning after December 31, 2017, that we are permitted to deduct in any taxable year beginning after December 31, 2020 is limited to 80% of our taxable income in such year, where taxable income is determined without regard to the NOL deduction itself. The TCJA allows post-2017 unused NOLs to be carried forward indefinitely. Similar rules may apply under state tax laws.
Our transfer pricing policies may be subject to challenge by the Internal Revenue ServiceIRS or other taxing authorities.
Our intercompany relationships are subject to complex transfer pricing regulations administered by taxing authorities in various jurisdictions. The relevant taxing authorities may disagree with our determinations as to the value of assets sold or acquired or income and expenses attributable to specific jurisdictions. If such a disagreement were to occur, and our position were not sustained, we could be required to pay additional taxes, interest and penalties, which could result in one-time tax charges, higher effective tax rates, reduced cash flows, and lower overall profitability of our operations. We believe that our consolidated financial statements reflect adequate reserves to cover such a contingency, but there can be no assurances in that regard.
Unanticipated changes in effective tax rates or adverse outcomes resulting from examination of our income or other tax returns could expose us to greater than anticipated tax liabilities.
The tax laws applicable to our business, including the laws of the U.S. and other jurisdictions, are subject to interpretation and certain jurisdictions may aggressively interpret their laws in an effort to raise additional tax revenue. It is possible that tax authorities may disagree with certain positions we have taken, are currently taking or will take, and any adverse outcome of such a review or audit could have a negative effect on our financial position and results of operations. Further, the determination of our provision for income taxes and other tax liabilities requires significant judgment by management, and there are transactions where the ultimate tax determination is uncertain. Although we believe that our estimates are reasonable, the ultimate tax outcome may differ from the amounts recorded on the consolidated financial statements and may materially affect our financial results in the period or periods for which such determination is made.
In addition, tax laws are dynamic and subject to change as new laws are passed and new interpretations of the law are issued or applied. For example, in August 2022, the U.S. enacted the IRA, which imposes a 15% minimum tax on the adjusted financial statement income of certain large corporations, as well as a 1% percent excise tax on corporate stock repurchases by publicly-traded companies. Additionally, for taxable years beginning on or after January 1, 2022, the Code eliminated the right to deduct research and development expenditures currently and requires taxpayers to capitalize and amortize U.S. and foreign research and development expenditures over 5 and 15 tax years, respectively. These updates, as well as any other changes to tax laws that are enacted, could adversely affect our tax liability.
Risks Related to the Discovery, Development and Commercialization of our Approved Product and our Other Product Candidates
We will beare substantially dependent on the successsuccessful commercialization of our approved product and the success and regulatory approval of our other product candidates. If we are unable to successfully commercialize our approved product or successfully complete clinical development of, obtain regulatory approval for, or commercialize, our other product candidates, and cannot guarantee that these product candidatesor if we experience delays in doing so, our business will successfully complete development, receive regulatory approval or be successfully commercialized.materially harmed.
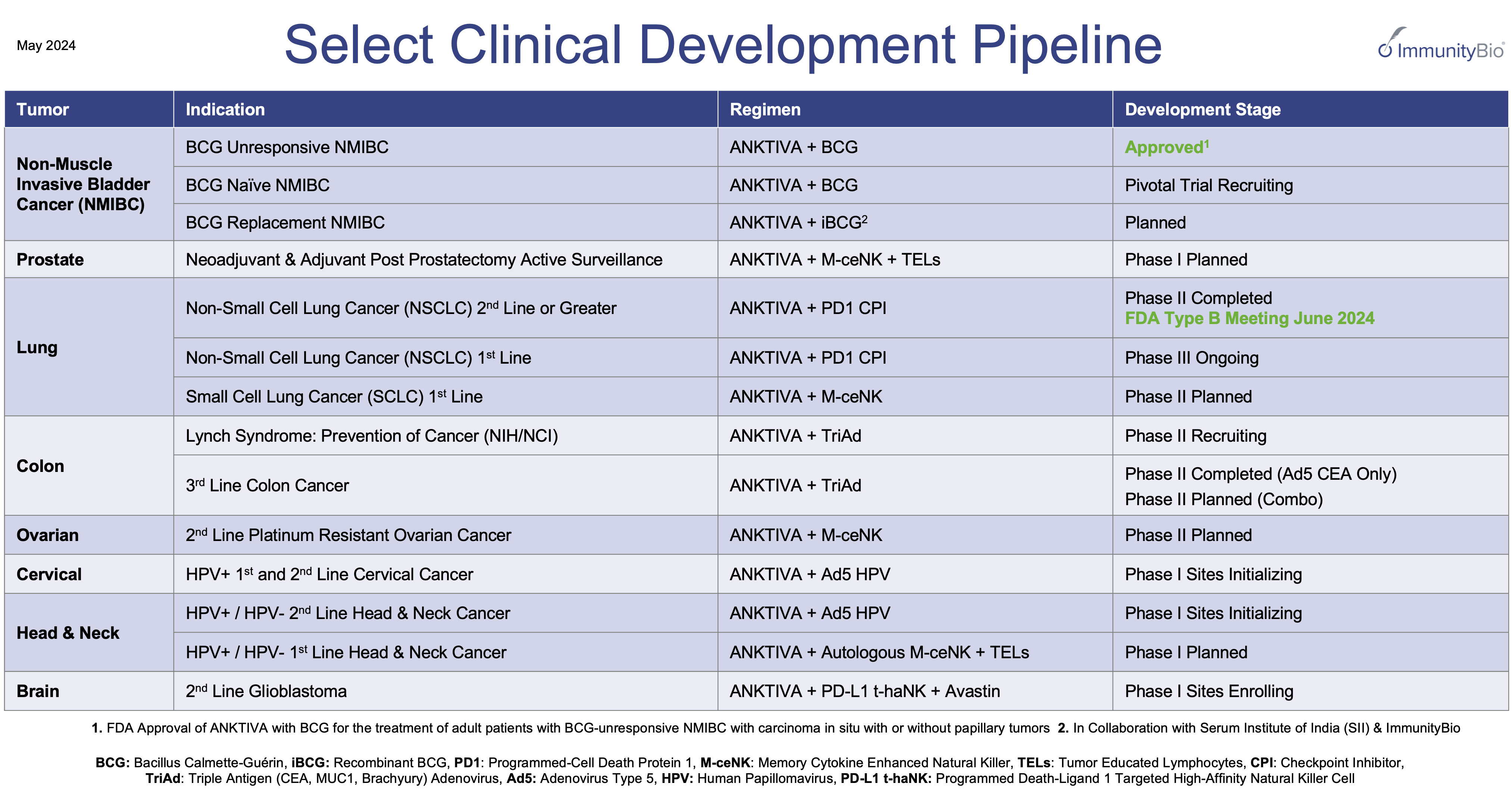
From inception through the date of this Quarterly Report, on Form 10-Q, we have generated minimal revenue from non-exclusive license agreements related to our cell lines, the sale of our bioreactors and related consumables, and grant programs. We have no clinical products approved for commercial sale and have not generated any revenue from therapeutic and vaccine product candidates that are under development. In May 2022, we announced the submission of a BLA toOn April 22, 2024, the FDA for our product candidate, Anktiva in combinationapproved ANKTIVA with BCG for the treatment of adult patients with BCG-unresponsive NMIBC with CIS, with or without Tapapillary tumors. We are required to comply with certain post-marketing commitments, including completion of our QUILT 3032 clinical trial and annual reporting for up to four years, with a final report submission to FDA by the end of 2029. Our business currently depends heavily on our ability to successfully commercialize our approved product in the U.S. and in other jurisdictions where we may obtain marketing approval. We may never be able to successfully commercialize our approved product or T1 disease. On May 9, 2023,meet our expectations with respect to revenues. We have never marketed, sold, or distributed for commercial use any pharmaceutical product other than our approved product, with respect to which we only recently began efforts to initiate commercial sales. There is no guarantee that the FDA delivered a CRLinfrastructure, systems, processes, policies, relationships, and materials we have built for the launch and commercialization of our approved product in the U.S. or elsewhere will be sufficient for us to achieve success at the levels we expect.
We may encounter issues and challenges in commercializing our approved product and generating substantial revenues. We may also encounter challenges related to reimbursement of our approved product, including potential limitations in the scope, breadth, availability, or amount of reimbursement covering our approved product. Similarly, healthcare settings or patients may determine that the financial burdens of treatment are not acceptable. We may face other limitations or issues related to the price of our approved product. Our results may also be negatively impacted if we have not adequately sized our field teams or our physician segmentation and our targeting strategy is inadequate or if we encounter deficiencies or inefficiencies in our infrastructure or processes. Other factors that may hinder our ability to successfully commercialize approved product, or any of our product candidates if or when approved and generate substantial revenues, include:
•the acceptance of our approved product by patients and the medical community;
•the ability of our third-party manufacturer(s) to manufacture commercial supplies of our approved product at acceptable costs, to remain in good standing with regulatory agencies, and to maintain commercially viable manufacturing processes that are, to the extent required, compliant with cGMP regulations;
•our ability to remain compliant with laws and regulations that apply to us and our commercial activities;
•FDA-mandated package-insert requirements and successful completion of FDA post-marketing requirements;
•the actual market size for our approved product, which may be different than expected;
•the length of time that patients who are prescribed our drug remain on treatment;
•our ability to obtain marketing approval for our approved product outside of the U.S.;
•the sufficiency of our drug supply to meet commercial and clinical demands which could be negatively impacted if our projections regarding the BLA, indicatingpotential number of patients are inaccurate, we are subject to unanticipated regulatory requirements, or our current drug supply is destroyed, or negatively impacted at our manufacturing sites, storage sites, or in transit;
•our ability to effectively compete with other therapies that may emerge for the FDA had determined that it cannot approve the BLAtreatment of bladder cancer; and
•our ability to maintain, enforce, and defend third party challenges to our intellectual property rights in its present form, and the FDA made recommendations to address theour approved product or any of our other product candidates.
Any of these issues raised. The company has submitted a formal meeting request and the associated briefing bookcould impair our ability to successfully commercialize our product or to generate substantial revenues or profits or to meet our expectations with respect to the FDAamount or timing of revenues or profits. Any issues or hurdles related to our commercialization efforts may materially adversely affect our business, results of operations, financial condition, and plansprospects. There is no guarantee that we will be successful in our launch or commercialization efforts with respect to diligently addressour approved product. We may also experience significant fluctuations in sales of our approved product from period to period and, resolve the issues identifiedultimately, we may never generate sufficient revenues from our approved product to reach or maintain profitability or sustain our anticipated levels of operations. Any inability on our part to successfully commercialize our approved product in the CRLU.S., and seek approval as expeditiously as possible. It is unclear when the FDA will approveany other international markets where it may subsequently be approved, or any significant delay, could have a material adverse impact on our BLA, if at all. ability to execute upon our business strategy.
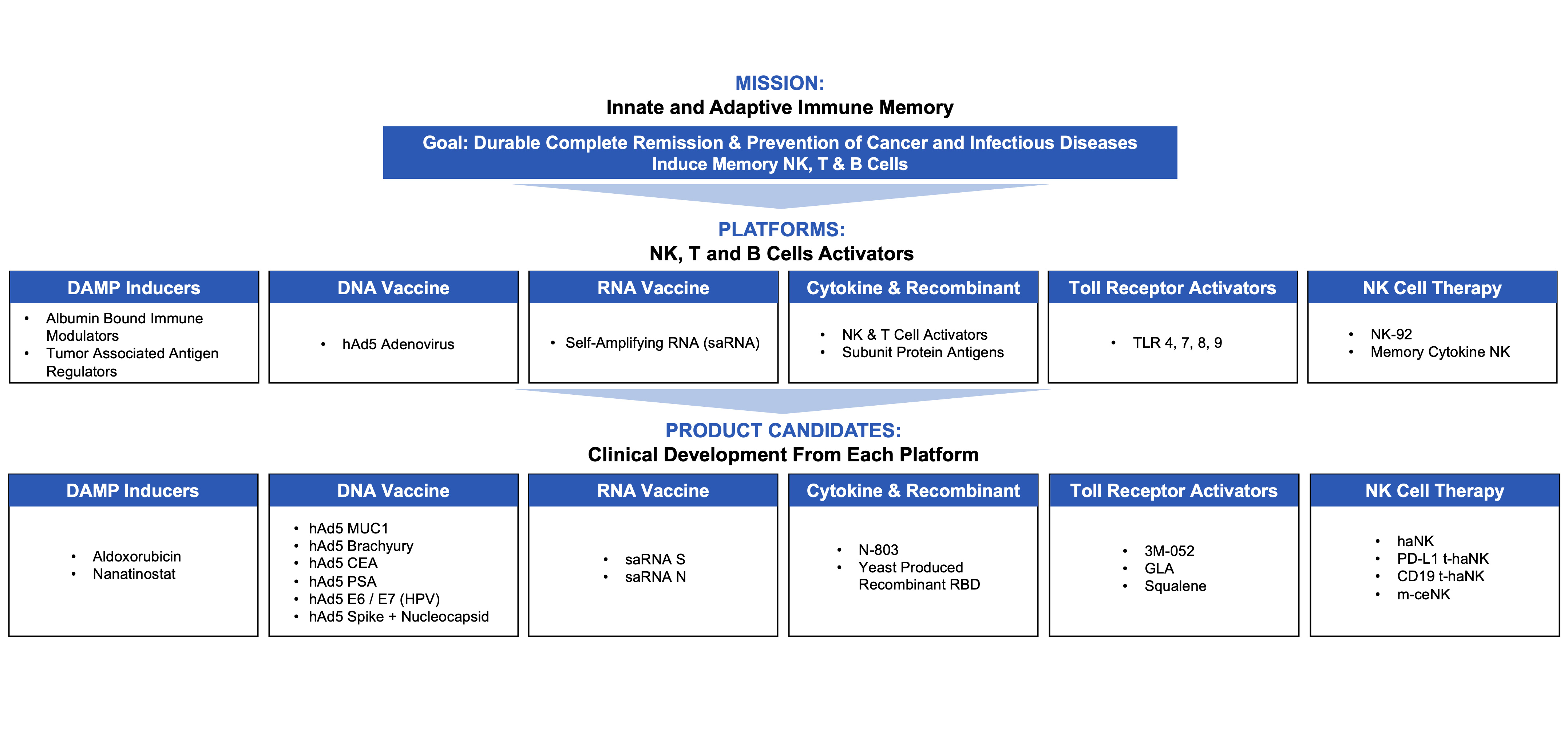
We have invested a significant portion of our efforts and financial resources in the development of our main product candidates, N-803,ANKTIVA, our novel antibody cytokineantibody-cytokine fusion protein, saRNA and our other product candidates, ssecond-generationecond-generation hAd5 and saRNA vaccine candidates, and aldoxorubicin, some of which are used in combination with our NK cell therapy candidates. Our other product candidates will require additional clinical and non-clinical development, regulatory approval, commercial manufacturing arrangements, establishmentenhancement of aour commercial organization and service providers, significant marketing efforts, and further investment before we can generate any revenues from product sales.the sale of these other potential products. We expect to invest heavily in these product candidates as well as in our other existingcurrent product candidates and in any future product candidates that we may develop. Our product candidates are susceptible to the risks of failure inherent at any stage of product development, including the appearance of unexpected adverse events or failure to achieve primary endpoints in clinical trials. Furthermore, we cannot assure you that we will meet our timelines for current or future clinical trials, including post-market study requirements for our approved product, which may be delayed or not completed for a number of reasons. Additionally, our ability to generate revenues from our approved product and any other combination therapy products will also depend on the availability of the other therapies used in combination therewith, including BCG, with which our products are intended to be used. WeIn particular, there has been a shortage of BCG in the U.S. According to the American Urological Association, Merck & Co., Inc. is the sole manufacturer and supplier of BCG in the U.S. and many other countries around the world. Although Merck has boosted its production of BCG, increasing demand for BCG has led to supply constraints for BCG, which can materially impact the demand for our approved product and our ability to commercialize our approved product. While we plan to begin shipping our approved product to customers in May 2024, we currently generate no meaningful revenues from the sale of anyour approved product or of our other product candidates, and we may never be able to develop or commercialize a product.
The CRL we received fromWe have limited experience as a commercial company and the FDA for the BLA has delayed,sales, marketing, and may decrease the likelihood of, ultimate approval and successful commercializationdistribution of our approved product candidate, Anktiva in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Taany future approved products may be unsuccessful or T1 diseaseless successful than anticipated.
We recently began commercializing our approved product in the U.S. As a company, we had no prior experience commercializing a product. The success of our commercialization efforts for our approved product and potentially other markets.
In May 2022, we announced the submission of a BLAany future approved products is difficult to predict and subject to the FDA for our product candidate, Anktiva in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease. In July 2022 we announced that the FDA had accepted our BLA for review and set a target PDUFA action date of May 23, 2023. On May 9, 2023, the FDA delivered a CRL to us regarding the BLA, indicating that it had determined that it cannot approve the BLA in its present form, and the FDA made recommendations to address the issues raised. The deficiencies relate to the FDA’s pre-license inspectioneffective execution of our third-party CMOs,business plan, including, among other items. Satisfactory resolutionthings, the continued development of our internal and external sales, marketing, and distribution capabilities and our ability to navigate the observations noted atsignificant expenses and risks involved with the pre-license inspection is required before the BLA may be approved. The FDA further provided recommendations specificdevelopment and management of such capabilities.
For example, we have completed hiring and contracting for service providers in areas to additional CMC issuessupport commercialization, including in sales management, sales representatives, marketing, access and assaysreimbursement, sales support, and distribution. There are significant expenses and risks involved with establishing our sales, marketing, and distribution capabilities, including our ability to be resolved. We have submitted a formal meeting requesthire, contract for, retain, and the associated briefing bookappropriately incentivize qualified individuals, provide adequate training to the FDA,sales and planmarketing personnel, and effectively manage geographically dispersed sales and marketing teams to diligently address and resolve the issues identified and seek approval as expeditiously as possible. Notwithstanding these efforts, we and/generate sufficient demand. Any failure or our third-party CMOs may be unable to adequately address and resolve the issues raised in the CRL. Further, there is uncertainty regarding the timing, contents, and outcome of the requested FDA meeting, the FDA may disagree with our proposed approach for the BLA resubmission, and the timeline of FDA review of any resubmission remains uncertain. The CRL and any subsequent resulting delay in the development and approval of such product candidate may prevent, decrease and/or delay expected revenue. Any of these riskscapabilities could delay or negatively affect the success of our commercialization efforts and our business. For example, the commercialization of our approved product may not develop as planned or anticipated, which may require us to, among others, adjust or amend our business plan and incur significant expenses.
Further, given our lack of experience commercializing products, we do not have a material impacttrack record of successfully executing on the commercialization of an approved product. If we are unsuccessful in accomplishing our objectives and executing on our business operating results,plan, or if the commercialization of our approved product or any future approved products does not develop as planned, we may require significant additional capital and financial condition.resources, we may not become profitable, and we may not be able to compete against more established companies in our industry.
There can be no assurance that we will complete a strategic partnership transaction on acceptable terms in accordance with our anticipated timeline, or at all.
As previously disclosed, weWe have been exploring partnering with a large biopharmaceutical company for commercialization of N-803 for intravesical administration with a view of completing such a transaction during 2023. While we have confirmed with the potential partner that the negotiations will continue notwithstanding the CRL that we received from the FDA, there is a risk that our ability to enter into such a strategic partnership may be impacted by the CRL. Further, theredeveloped an approved product and are developing other factors that may impact our ability, or decision, to enter into such a strategic partnership, and ultimately there can be no assurance that we will complete a transaction on acceptable terms in accordance with our anticipated timeline, or at all. If we do not execute a strategic partnership transaction in the near-term, it would eliminate a potential source of near-term funding, and may impact our ability to raise additional funds to meet our business needs. In addition, there are significant risks involved with building and managing a commercial infrastructure on a stand-alone basis, which could materialize in the event we do not execute a strategic partnership transaction.
We are developing product candidates in combination with other therapies, which exposes us to additional risks.
We have developed an approved product and are developing other product candidates in combination with one or more other therapies. We are studying N-803ANKTIVA therapy along with other products and product candidates, such as BCG, PD-L1 t-haNK, hAd5 and yeast tumor-associated antigens (TAAs),TAAs and aldoxorubicin. IfSince we have developed a product, or if we choose to develop a product candidateother products for use in combination with an approved therapy, we are subject to the risk that the FDA, EMA or comparable foreign regulatory authorities in other jurisdictions could revoke approval of, or that safety, efficacy, manufacturing or supply issues could arise with the therapy used in combination with our approved product candidate.or our other product candidates. In particular, supply chain issues or shortages of other products used in combination with our approved product or any other product candidates could impact our ability to obtain FDA regulatory approval, meet clinical trial timelines and commercialize our approved product or other product candidates. The FDA may require us to use more complex clinical trial designs in order to evaluate the contribution of each product and product candidate to any observed effects. To the extent that we do not have rights to already approved products, this may require us to work with another company to satisfy such a requirement or increase our cost of development. It is possible that the results of these trials could show that any positive results are attributable to the already approved product. Following product approval, the FDA may require that products used in conjunction with each other be cross labeled for combined use. If the therapies we use in combination with our product or our other product candidates are replaced as the standard of care for the indications we choose for our product or any of our other product candidates, the FDA or comparable foreign regulatory authorities may require us to conduct additional clinical trials. The occurrence of any of these risks could result in our own products, if approved, being removed from the market or being less successful commercially.
In addition, unapproved therapies face the same risks described with respect to our product candidates currently in development and clinical trials, including the potential for serious adverse effects, delays in clinical trials and lack of FDA approval. If the FDA or comparable foreign regulatory authorities do not approve or revoke their approval of these other therapies, or if safety, efficacy, quality, manufacturing or supply issues arise with, the therapies we choose to evaluate in combination with any of our product candidates, we may be unable to obtain approval of or market such other combination therapy.
We may choose to expend our limited resources on programs that do not yield successful product candidates as opposed to indications that may be more profitable or for which there is a greater likelihood of success.
We do not have sufficient resources to pursue development of all or even a substantial portion of the potential opportunities that we believe will be afforded to us by our product candidates. Because we have limited resources and access to capital to fund our operations, our management must make strategic decisions as to which product candidates and indications to pursue and how much of our resources to allocate to each. Our management must also evaluate the benefits of developing in‑licensed or jointly-owned technologies, which in some circumstances we may be contractually obligated to pursue, relative to developing other product candidates, indications or programs. Our management has broad discretion to suspend, scale down, or discontinue any or all of these development efforts, or to initiate new programs to treat other diseases. If we select and commit resources to opportunities that we are unable to successfully develop, or we forego more promising opportunities, our business, financial condition and results of operations will be adversely affected.
Our projections regarding the market opportunities for our product candidates may not be accurate, and the actual market for our products, if approved, may be smaller than we estimate.
Since our current product candidates and any future product candidates will represent novel approaches to treating various conditions, it may be difficult, in any event, to accurately estimate the potential revenues from these product candidates. Accordingly, we may spend significant capital trying to obtain approval for product candidates that have an uncertain commercial market. Our projections of addressable patient populations that may benefit from treatment with our product candidates are based on our beliefs and estimates. These estimates, which have been derived from a variety of sources, including scientific literature, surveys of clinics, patient foundations, or market research by third parties, may prove to be incorrect. Further, new studies or approvals of new therapeutics may change the estimated incidence or prevalence of these diseases. The number of patients may turn out to be lower than expected. Additionally, the potentially addressable patient population for our product candidates may be limited or may not be amenable to treatment with our product candidates and may also be limited by the cost of our treatments and the reimbursement of those treatment costs by third-party payors. Even if we obtain significant market share for our product candidates, because the potential target populations may be small, we may never achieve profitability without obtaining regulatory approval for additional indications.therapies.
Our clinical trials may fail to adequately demonstrate the safety and efficacy of our product candidates, which would prevent or delay regulatory approval and commercialization. If our trials are not successful, we will be unable to commercialize ourcommercialization of other product candidates.
Our research and development programs of our other non-FDA-approved product candidates are at various stages of development. The clinical trials of our product candidates as well as the manufacturing and marketing of our product candidates will be subject to extensive and rigorous review and regulation by numerous government authorities in the U.S. and in other countries where we intend to test and market our product candidates. Before obtaining regulatory approvals for the commercial sale of any of our other product candidates, we must demonstrate through lengthy, complex and expensive preclinical testing and clinical trials that our product candidates are safe, pure, and potent for use in their target indications. Each product candidate must demonstrate an adequate risk versus benefit profile in its intended patient population and for its intended use. The risk/benefit profile required for product licensure will vary depending on these factors and may include not only the ability to show tumor shrinkage, but also adequate duration of response, a delay in the progression of the disease, and/or an improvement in survival. For example, response rates from the use of our product candidates or their contribution of effect, may not be sufficient to obtain regulatory approval unless we can also show an adequate duration of response. The clinical trials for our product candidates under development may not be completed on schedule and regulatory authorities may ultimately disagree with our chosen endpoints or may find that our studies or study results do not support product approval and we cannot guarantee that the FDA or foreign regulatory authorities will interpret the results as we do or accept the therapeutic effects as valid endpoints in clinical trials necessary for market approval or they may find that our clinical trial design or conduct does not meet the applicable approval requirement and more trials could be required before we submit our product candidates for approval. Success in early clinical trials does not ensure that large-scale clinical trials will be successful, nor does it predict final results. Product candidates in later stages of clinical trials may fail to show the desired safety, tolerability and efficacy traits despite having progressed through preclinical studies and initial clinical trials and after reviewing test results, we or our collaborators may abandon projects that we might previously have believed to be promising.
In addition, we do not have data on possible harmful long-term effects of our product candidates and do not expect to have this data in the near future. As a result, our ability to generate clinical safety and effectiveness data sufficient to support submission of a marketing application or commercialization of our product candidates is uncertain and is subject to significant risk.
The ongoing shortage of BCG may adversely impact market uptake of our approved product, ANKTIVA, and it may also delay our ability to execute our clinical trials or seek new approvals.
There is an ongoing shortage of BCG, which may adversely impact market uptake of our approved product. The BCG shortage may impact the number of patients who are treated with BCG for NMIBC with CIS with or without papillary tumors, therefore limiting the pool of BCG-unresponsive patients who may be candidates for our product. In addition, the BCG shortage may also constrain the number of patients we can treat with our product since our product is administered along with BCG. In addition, ANKTIVA was awarded Fast Track designation by the FDA for the treatment of BCG-naïve NMIBC with CIS. We are currently enrolling patients in our Phase IIb blinded, randomized, two-cohort, open-label, multi-center trial of intravesical BCG with ANKTIVA versus BCG alone, in BCG-naïve patients with high-grade NMIBC with CIS (Cohort A) and NMIBC papillary (Cohort B), which is impacted by the availability of BGC. If we do not complete new trials timely, our ability to generate clinical safety and effectiveness data sufficient to support submission of a marketing application or commercialization of our product candidates in new indications could harm our business, operating results, prospects or financial condition.
We may choose to expend our limited resources on programs that do not yield successful product candidates as opposed to indications that may be more profitable or for which there is a greater likelihood of success.
We do not have sufficient resources to pursue development of all or even a substantial portion of the potential opportunities that we believe will be afforded to us by our product candidates. Because we have limited resources and access to capital to fund our operations, our management must make strategic decisions as to which product candidates and indications to pursue and how much of our resources to allocate to each. Our management must also evaluate the benefits of developing in‑licensed or jointly-owned technologies, which in some circumstances we may be contractually obligated to pursue, relative to developing other product candidates, indications or programs. Our management has broad discretion to suspend, scale down, or discontinue any or all of these development efforts, or to initiate new programs to treat other diseases. If we select and commit resources to opportunities that we are unable to successfully develop, or we forego more promising opportunities, our business, financial condition and results of operations will be adversely affected.
Our projections regarding the market opportunities for our approved product and our other product candidates may not be accurate, and the actual market for our other products, if approved, may be smaller than we estimate.
Since our approved product, current product candidates, and any future product candidates represent novel approaches to treating various conditions, it may be difficult, in any event, to accurately estimate the potential revenues from our approved product and these other product candidates. Accordingly, we may spend significant capital trying to successfully commercialize our product or obtain approval for our other product candidates that have an uncertain commercial market. Our projections of addressable patient populations that may benefit from treatment with our product or product candidates are based on our beliefs and estimates, and estimates of the therapeutic benefit and adverse event profile of our approved product and other product candidates. These estimates, which have been derived from a variety of sources, including scientific literature, preclinical and clinical studies, surveys of clinics, patient foundations, or market research by third parties, may prove to be incorrect. Further, new studies or approvals of new therapeutics may change the estimated incidence or prevalence of these diseases. The number of patients may turn out to be lower than expected. Additionally, the potentially addressable patient population for our product or product candidates may be limited or may not be amenable to treatment with our product or product candidates and may also be limited by the cost of our treatments and the reimbursement of those treatment costs by third-party payors. Even if we obtain significant market share for our product or product candidates, because the potential target populations may be small, we may never achieve profitability without obtaining regulatory approval for additional indications.
There can be no assurance that we will complete a strategic partnership transaction on acceptable terms in accordance with our anticipated timeline, or at all.
We continue to explore potential global strategic partnership transactions for commercialization of ANKTIVA for certain indications. Factors that may impact our ability, or decision, to enter into such a strategic partnership, include, without limitation, the put/call features of the RIPA that may be triggered by entry into a strategic partnership depending on its scope and terms, and ultimately there can be no assurance that we will complete a transaction on acceptable terms, or at all. If we do not execute a strategic partnership transaction in the near-term, it would eliminate a potential source of near-term funding, and may
impact our ability to raise additional funds to meet our business needs. In addition, there are significant risks involved with building and managing a commercial infrastructure on a stand-alone basis, which could materialize in the event we do not execute a strategic partnership transaction, or depending on the geographic scope of any executed transaction.
Interim, initial, “top-line”top-line and preliminary data from our clinical trials that we announce or publish from time to time may change as more patient data become available and are subject to audit and verification procedures that could result in material changes in the final data.
From time to time, we may publicly disclose preliminary, interim or top-line data from our preclinical studies and clinical trials, which are based on preliminary analyses of then-available data, and the results and related findings and conclusions are subject to change following a more comprehensive review of the data related to the particular study or trial. Interim data from clinical trials that we may complete are subject to the risk that one or more of the clinical outcomes may materially change as patient enrollment continues and more patient data become available or as patients from our clinical trials continue other treatments for their disease.disease, or as inclusion and exclusion criteria is discussed with regulators. We also may make assumptions, estimations, calculations and conclusions as part of our analyses of data, and we may not have received or had the opportunity to fully and carefully evaluate all data. As a result, the interim, top-line or preliminary results that we report may differ from future results of the same studies, or different conclusions or considerations may qualify such results, once additional data have been received and fully evaluated. Top-line data also remain subject to audit and verification procedures that may result in the final data being materially different from the preliminary data we previously published. As a result, top-line data should be viewed with caution until the final data are available. Adverse differences between preliminary or interim data and final data could significantly harm our business prospects. Further, disclosure of interim data by us or by our competitors could result in volatility in the price of our common stock.
In addition, the information we choose to publicly disclose regarding a particular study or clinical trial is typically selected from a more extensive amount of available information, and you or others may not agree with what we determine is material or otherwise appropriate information to include in our disclosure. If the interim, top-line or preliminary data that we report differ from actual results, or if others, including regulatory authorities, disagree with the conclusions reached, our ability to obtain approval for, and commercialize, our product candidates may be harmed, which could harm our business, operating results, prospects or financial condition.
Our clinical trials may not be initiated or completed when we expect, or at all, they may take longer and cost more to complete than we project, our clinical trial costs may be higher than for more conventional therapeutic technologies or drug products, and we may be required to conduct additional clinical trials or modify current or future clinical trials based on feedback we receive from the FDA.
We cannot guarantee that any current or future clinical trials will be conducted as planned or completed on schedule, if at all, or that any of our other product candidates will receive regulatory approval. A failure of one or more clinical trials can occur at any stage of the clinical trial process, other events may cause us to temporarily or permanently stop a clinical trial temporarily or permanently, and our future clinical trials may not be successful.
Because our product candidates include, and we expect our future product candidates to include, candidates based on advanced therapy technologies, we expect that they will require extensive research and development and have substantial manufacturing costs. In addition, costs to treat patients and to treat potential side effects that may result from our product candidates can be significant. Some clinical trial sites may not bill, or obtain coverage from Medicare, Medicaid, or other third-party payors for some or all of these costs for patients enrolled in our clinical trials, and clinical trial sites outside of the U.S. may not reimburse for costs typically covered by third-party payors in the U.S., and as a result we may be required by those trial sites to pay such costs. Accordingly, our clinical trial costs are likely to be significantly higher per patient than those of more conventional therapeutic technologies or drug products.
Collaborations with other entities may be subject to additional delays because of the management of the trials, contract negotiations, the need to obtain agreement from multiple parties and the necessity of obtaining additional approvals for therapeutics used in the combination trials. These combination therapies will require additional testing and clinical trials will require additional FDA regulatory approval and will increase our future costs.
Any inability to successfully complete preclinical and clinical development could result in additional costs to us, slow down our product development and approval process or impair our ability to commence product sales and generate revenues. In addition, if we make manufacturing changes to our product or product candidates, we may be required to, or we may elect to, conduct additional trials to bridge our modified product or product candidates to earlier versions. These changes may require FDA approval or notification and may not have their desired effect. The FDA may also not accept data from prior versions of the product to support an application, delaying our clinical trials or programs or necessitating additional clinical trials or preclinical studies. We may find that this change has unintended consequences that necessitates additional development and manufacturing work, additional clinical and preclinical studies, or that results in refusal to file or non-approval of a BLA and/or NDA.
Clinical trial delays could shorten any periods during which our product candidates have patent protection and may allow our competitors to bring products to market before we do, which could impair our ability to successfully commercialize our product candidates and may harm our business and results of operations. In addition, we have in the past experienced clinical holds imposed upon certain of our or investigator-led clinical trials for various reasons, and we may experience further clinical trial holds in the future. If we fail to commence or complete, or experience delays in, any of our planned clinical trials, our stock price and our ability to conduct our business as currently planned could be harmed.
Even if onemore of our product candidates isare approved and commercialized, we may not become profitable.
If approved for marketing by applicable regulatory authorities, our ability to generate revenues from our other product candidates will depend on our ability to:
•price our other product candidates competitively such that third-party and government reimbursement leads to broad product adoption;
•prepare a broad network of clinical sites for administration of our product;other product candidates;
•create market demand for our other product candidates through our own or our partner’s marketing and sales activities, and any other arrangements to promote these product candidates that we may otherwise establish;
•receive regulatory approval for the targeted patient population(s) and claims that are necessary or desirable for successful marketing;
•manufacture our other product candidates through third-party CMOs or in our own or our affiliates’, manufacturing facilities or facilities owned by entities affiliated with Dr. Soon-Shiong in sufficient quantities and at acceptable quality and manufacturing cost to meet regulatory requirements and commercial demand at launch and thereafter;
•establish and maintain agreements with wholesalers, distributors, pharmacies, and group purchasing organizations on commercially reasonable terms;
•obtain, maintain, protect and enforce patent and other intellectual property protection and regulatory exclusivity for our other product candidates;
•successfully commercialize any of our other product candidates that receive regulatory approval;
•maintain compliance with applicable laws, regulations, and guidance specific to commercialization including interactions with health care professionals, patient advocacy groups, and communication of health care economic information to payors and formularies;
•achieve market acceptance of our other product candidates by patients, the medical community, and third-party payors;
•achieve appropriate reimbursement for our product candidates;
•maintain a distribution and logistics network capable of product storage within our specifications and regulatory guidelines, and further capable of timely product delivery to commercial clinical sites;
•effectively compete with other therapies or competitors; and
•following launch, ensure that our approved product will be used as directed and that additional unexpected safety risks will not arise.
Even ifOn April 22, 2024, the FDA approves N-803approved ANKTIVA with BCG for the treatment of adult patients with BCG-unresponsive NMIBC with CIS, with or without papillary tumors. We are required to comply with certain indicationspost-marketing commitments, including completion of our QUILT 3032 clinical trial and annual reporting for up to four years, with a final report submission to FDA by the end of 2029. We can provide no assurance with respect to the profitability or in combination with other therapeutic products, and even ifthe market share that we might achieve for our approved product. The target patient population for which we obtain significant market share for it, because the potential target populationapproval may be small,narrower than we may never achieve profitability without obtaining regulatory approval for additional indications. The FDA often approves new therapies initially only for use in patients with r/r metastatic disease, which may limit our patient population.expect. Additionally, we may not be able to obtain the labeling claims necessary or desirable for the promotion of our approved product. Further, supply chain issues or shortages associates with combination products that may be used with our approved product, candidatessuch as ANKTIVA plus BCG, may limit the demand for our approved product.
In connection with our 2017 acquisition of Altor, we issued CVRs under which we agreed to pay the prior stockholders of Altor approximately $304.0 million of contingent consideration upon the successful regulatory approval of a BLA by the FDA, or foreign equivalent, for N-803 by December 31, 2022, and approximately $304.0 million of contingent consideration upon calendar-year worldwide net sales of N-803 exceeding $1.0ANKTIVA exceeding $1.0 billion prior to December 31, 2026 with amounts payable in cash or shares of our common stock or a combination thereof.
With respect to the regulatory milestone CVR agreement, in May 2022 we announced the submission As of a BLA to the FDA for our product candidate, Anktiva (N-803) in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease. On May 9, 2023, the FDA delivered a CRL to us regarding the BLA, indicating that the FDA had determined that it cannot approve the BLA in its present form, and the FDA made recommendations to address the issues raised. The company has submitted a formal meeting request and the associated briefing book to the FDA and plans to diligently address and resolve the issues identified in the CRL and seek approval as expeditiously as possible. It is unclear when the FDA will approve our BLA, if at all. The FDA did not approve our BLA on or before DecemberMarch 31, 2022, and therefore the regulatory milestone was not met, and the regulatory milestone CVR agreement terminated in accordance with its terms.
With respect to the net sales milestone CVR agreement, as of December 31, 2022,2024, Dr. Soon-Shiong and his related party hold approximately $139.8 million of net sales CVRs, and they have both irrevocably agreed to receive shares of the company’s common stock in satisfaction of their CVRs. We may be required to pay the other prior Altor stockholders up to $164.2 million for their net sales CVRs should they choose to have their CVRs paid in cash instead of common stock. If this were to occur, we may need to seek additional sources of capital and any such financing activities may be restricted by the covenants included in the terms of the RIPA. As such, we may face difficulties raising additional capital and may have to accept unfavorable terms and as a result, we may not be able to achieve profitability or positive cash flow.
We planIn connection with our financing in December 2023, we entered into the RIPA with Infinity and Oberland. Oberland has the right to collaborate with governmental, academicreceive quarterly Revenue Interest Payments from us based on, among other things, our worldwide net sales, excluding those in China, which are tiered payments ranging from 4.50% to 10.00% after funding of the Second Payment, subject to increase or decrease, following the Test Date depending on whether our aggregate payments made to Oberland as of the Test Date have met or exceeded the Cumulative Purchaser Payments. In addition, if our aggregate payments made as of the Test Date to Oberland do not equal or exceed the amount of the Cumulative Purchaser Payments as of such date, then we are obligated to make a one-time payment True-Up Payment as described above. In addition to other considerations of the RIPA and corporate partners, including affiliates,the associated impact to improveour profitability and develop N-803, hAd5cash flow, if we were required to make a True-Up Payment, we may need to seek additional sources of capital, and other therapies for new indications for use in combination with other therapies and to improve and develop other product candidates, which may expose us to additional risks, or we may not realize the benefits of such collaborations.be able to achieve profitability or positive cash flow.
If we encounter delays or difficulties enrolling and/or maintaining patients in our clinical trials, our clinical development activities and receipt of necessary marketing approvals could be delayed or otherwise adversely affected.
The timely completion of clinical trials in accordance with their protocols depends, among other things, on our ability to enroll a sufficient number of patients who remain in the trial until its conclusion. We may experience difficulties or delays in patient enrollment and retention in our clinical trials for a variety of reasons.
Because the number of qualified clinical investigators is limited, we may need to conduct some of our clinical trials at the same clinical trial sites that some of our competitors use, which will reduce the number of patients who are available for our clinical trials at such clinical trial sites. In addition, in the past we have engaged, and we intend to continue to engage, in clinical trial efforts outside of the U.S., which gives rise to additional potential complexity and challenges, and further reliance upon third parties in foreign jurisdictions. Moreover, because our product candidates represent a departure from more commonly used methods for cancer and/or viral disease treatment, potential patients and their doctors may be inclined to use conventional therapies, such as chemotherapy and approved immunotherapies that have established safety and efficacy profiles, rather than enroll patients in any future clinical trial.
Delays or failures in planned patient enrollment or retention may result in increased costs or may affect the timing or outcome of the planned clinical trials, which could prevent completion of these trials and adversely affect our ability to advance the development of our product candidates or could render further development impossible.
Our other product candidates may cause undesirable side effects or have other properties that could halt their clinical development, delay or prevent their regulatory approval, limit their commercial potential or result in significant negative consequences.
Results of our trials could reveal a high and unacceptable severity and prevalence of side effects, adverse events or unexpected characteristics. Combination immunotherapy that includes our current product candidates may be associated with more frequent adverse events or additional adverse events. Undesirable side effects or unacceptable toxicities caused by our other product candidates could cause us or regulatory authorities to interrupt, delay or halt clinical trials or order our clinical trials to be placed on clinical hold, and could result in a more restrictive label or the delay or denial of regulatory approval by the FDA or other regulatory authorities for any or all targeted indications. The FDA or comparable foreign regulatory authorities may also require additional data, clinical trials, or preclinical studies should unacceptable toxicities arise. We may need to abandon development or limit development of that product candidate to certain uses or subpopulations in which the undesirable side effects or other characteristics are less prevalent, less severe or more acceptable from a risk/benefit perspective. Toxicities associated with our clinical trials and product candidates may also negatively impact our ability to conduct clinical trials using tumor-infiltrating lymphocyte therapy in larger patient populations, such as in patients that have not yet been treated with other therapies or have not yet progressed on other therapies. Even if we were to receive product approval, such approval could be contingent on inclusion of unfavorable information in our product labeling, such as limitations on the indicated uses for which the products may be marketed or distributed, a label with significant safety warnings, including boxed warnings, contraindications, and precautions, a label without statements necessary or desirable for successful commercialization, or requirements for costly post marketing testing and surveillance, or other requirements, including a Risk Evaluation and Mitigation Strategy (REMS)REMS to monitor the safety or efficacy of the products, and in turn prevent us from commercializing and generating revenues from the sale of our current or future product candidates. In addition, these serious adverse effects may not be appropriately recognized or managed by the treating medical staff, as toxicities resulting from our other product candidates are not normally encountered in the general patient population and by medical personnel. They may have difficulty observing patients and treating toxicities, which may be more challenging due to personnel changes, shift changes, house staff coverage or related issues. This could lead to more severe or prolonged toxicities or even patient deaths, which could result in us or the FDA delaying, suspending or terminating one or more of our clinical trials and which could jeopardize regulatory approval. Any of these occurrences may materially harm our business, financial condition and prospects.
The manufacture of our approved product and other product candidates is complex, and we may encounter difficulties in production, particularly with respect to process development, quality control, or scaling-up of our manufacturing capabilities. If we or our related parties, or any of our third-party manufacturers, encounter such difficulties, our ability to gain approval, or to provide adequate supply of our product candidates for clinical trials or our products for patients, if approved, could be delayed or stopped, or we may be unable to maintain a commercially viable cost structure.
The manufacture of our approved product and other product candidates involves complex processes, especially for our biologics, vectors and cell therapy product candidates, which are complex, highly regulated and subject to multiple risks. As a result of the complexities, the cost to manufacture biologics, vectors and cell therapies is generally higher than traditional small molecule chemical compounds, and the manufacturing process is less reliable and is more difficult to reproduce. The manufacture of fusion proteins, DNA and RNA constructs, and cell therapy products requiresrequire significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls. Manufacturers of cell therapy products often encounter difficulties in production, particularly in scaling up initial production. These problems include difficulties with production costs and yields, quality control, including stability of the product candidateor other product candidates and quality assurance testing, shortages of qualified personnel and compliance with strictly enforced federal, state, local and foreign regulations. We may also find that the manufacture of our approved product or other product candidates is more difficult than anticipated, resulting in an inability to produce a sufficient amount of our other product candidates for our clinical trials or, if approved, commercial supply. Even minor deviations from normal manufacturing processes could result in reduced production yields, product defects, and other supply disruptions. Currently, ourOur approved product and other product candidates are manufactured using processes developed or modified by us, our affiliates or by our third-party collaborators that we may not utilize for more advanced clinical trials or commercialization.
Currently we manufacture our approved product and other product candidates in our own manufacturing facilities, facilities owned by entities affiliated with Dr. Soon-Shiong and/or we may usethrough third-party CMOs or some of our related parties to manufacture our product candidates.CMOs. Our clinical trials will need to be conducted with product candidates and materials that were produced under cGMP and/or Good Tissue Practice (GTP)GTP regulations, which are enforced by regulatory authorities. Our approved product and other product candidates may compete with other products and product candidates for access to manufacturing facilities. Moreover, because of the complexity and novelty of our manufacturing process, there are only a limited number of manufacturers that operate under cGMP regulations and that are both capable of manufacturing our approved product and other product candidates for us and willing to do so. If our third-party CMOs should cease manufacturing for us, we would experience delays in obtaining sufficient quantities of our approved product for commercial supply and other product candidates for clinical trials and, if approved, commercial supply.supply of such future products. Further, our third-party CMOs may breach, terminate, or not renew our agreements with them. If we were to need to find alternative manufacturing facilities or transfer between existing facilities it may take us significant time to find a replacement, if we are able to find a replacement at all and it would significantly impact our ability to develop, obtain regulatory approval for or market our approved product and/or other product candidates, if approved. The commercial terms of any new arrangement could be less favorable than our existing arrangements and the expenses relating to the transfer of necessary technology and processes could be significant.
Our failure to comply or our third-party CMOs’ failure to comply with these regulations may require us to cease sales of our approved product or repeat clinical trials, which would delay the regulatory review process.process of our other product candidates. We may not be able to demonstrate sufficient comparability between products manufactured in different runs at the same or at different facilities to allow for inclusion of the clinical results from patients treated with products from these different facilities,runs, in our product registrations.registrations or to assure a cGMP process to qualify our other product candidates. On May 9, 2023,April 22, 2024, the FDA delivered a CRL to us regardingapproved ANKTIVA with BCG for the BLA, indicating that the FDA had determined that it cannot approve the BLA in its present form,treatment of adult patients with BCG-unresponsive NMIBC with CIS, with or without papillary tumors. We and the FDA made recommendations to address the issues raised. The deficiencies relate to the FDA’s pre-license inspection of the company’s third-partyour suppliers and CMOs amongmust maintain compliance with cGMP requirements and other items. Satisfactory resolution of the observations noted at the pre-license inspection is required before the BLA may be approved.applicable regulatory requirements.
We also are required to register certain clinical trials and post the results of certain completed clinical trials on a government-sponsored database, ClinicalTrials.gov, within specified timeframes. Failure to do so could result in enforcement actions and adverse publicity.
Reliance on third-party manufacturers entails exposure to risks to which we would not be subject if we manufactured the product candidate ourselves, including:
•inability to negotiate manufacturing and quality agreements with third parties under commercially reasonable terms;
•reduced day-to-day control over the manufacturing process for our other product candidates as a result of using third-party manufacturers for all aspects of manufacturing activities;
•reduced control over the protection of our trade secrets, know-how and other proprietary information from misappropriation or inadvertent disclosure or from being used in such a way as to expose us to potential litigation;
•termination or nonrenewal of manufacturing agreements with third parties in a manner or at a time that may be costly or damaging to us or result in delays in the development or commercialization of our approved product or other product candidates; and
•disruptions to the operations of our third-party manufacturers or suppliers caused by conditions unrelated to our business or operations, including the bankruptcy ofor personnel turnover at the manufacturer or supplier.
Moreover, any problems or delays we or our third-party CMOs experience in preparing for commercial scale manufacturing of a product candidate may result in a delay in the FDA approval of the product candidate or may impair our ability to manufacture commercial quantities or such quantities at an acceptable cost, which could result in the delay, prevention, or impairment of clinical development and commercialization of our product candidates and could adversely affect our business. Furthermore, if we or our third-party CMOs fail to deliver the required commercial quantities of our product candidates on a timely basis and at reasonable costs, we would likely be unable to meet demand for our products and we would lose potential revenues. We may ultimately be unable to reduce the cost of goods for our other product candidates to levels that will allow for an attractive return on investment if and when those product candidates are commercialized.
In addition, the manufacturing process and facilities for our approved product and any other products that we may develop are subject to FDA and foreign regulatory authority approval processes, and we or our third-party CMOs will need to meet all applicable FDA and foreign regulatory authority requirements, including cGMP, on an ongoing basis. The cGMP requirements include quality control, quality assurance and the maintenance of records and documentation. The FDA and other regulatory authorities enforce these requirements through facility inspections. Manufacturing facilities must submit to pre-approval inspections by the FDA that will be conducted after we submit our marketing applications, including BLAs and NDAs, to the FDA. Manufacturers are also subject to continuing FDA and other regulatory authority inspections following marketing approval. Further, we and our third-party CMOs must supply all necessary CMC documentation in support of a BLA or NDA on a timely basis. Our or our third-party CMOs’ manufacturing facilities may be unable to comply with our specifications, cGMP, and with other FDA, state, and foreign regulatory requirements, and there is no guarantee that we or our third-party CMOs will be able to successfully pass all aspects of a pre-approval or continued inspection by the FDA or other foreign regulatory authorities.authorities On May 9, 2023, the FDA delivered a CRL to us regarding the BLA filed in May 2022, indicating that the FDA had determined that it cannotcould not approve the original BLA submission in its presentinitial form, and the FDA made recommendations to address the issues raised. The deficiencies relatein the CRL related to the FDA’s pre-license inspection of the company’s third-party CMOs, among other items. Satisfactory resolutionOn April 22, 2024, the FDA approved ANKTIVA with BCG for the treatment of the observations noted at the pre-license inspection is required before the BLA may be approved.adult patients with BCG-unresponsive NMIBC with CIS, with or without papillary tumors.
Poor control of production processes can lead to the introduction of adventitious agents or other contaminants, or to inadvertent changes in the properties or stability of our product or other product candidates that may not be detectable in final product testing. If microbial, viral, environmental or other contaminants are discovered in our product or other product candidates or in the manufacturing facilities in which our product or other product candidates are made, such manufacturing facilities may need to be closed for an extended period of time to investigate and remedy the contamination which could delay commercial sales and clinical trials and adversely harm our business. If we or our third-party CMOs are unable to reliably produce products to specifications acceptable to the FDA or other regulatory authorities, or in accordance with the strict regulatory requirements, we may not obtain or maintain the initial or continued approvals we need to commercialize such products. Even if we obtain regulatory approval for any of our product candidates, thereThere is no assurance that either we or our third-party CMOs will be able to manufacture theour approved product or any subsequently approved product candidate to specifications acceptable to the FDA or other regulatory authorities, to produce itour product or other product candidates in sufficient quantities to meet the requirements for the potential launch of the product,such products, or to meet potential future demand. Deviations from manufacturing requirements may further require remedial measures that may be costly and/or time-consuming for us or a third party to implement and may include the temporary or permanent suspension of a clinical trial or commercial sales or the temporary or permanent closure of a facility. Any such remedial measures imposed upon us or third parties with whom we contract could materially harm our business.
As product candidates progress through preclinical and clinical trials to marketing approval and commercialization, it is common that various aspects of the development program, such as manufacturing methods and formulation, are altered along the way in an effort to optimize yield and manufacturing batch size, minimize costs and achieve consistent quality and results. Such changes carry the risk that they will not achieve these intended objectives. Any of these changes could cause our product or other product candidates to perform differently and affect our commercial sales or the results of planned clinical trials or other future clinical trials conducted with the altered materials. This could cause commercial sales to cease, delay completion of clinical trials, require the conduct of bridging clinical trials or the repetition of one or more clinical trials, increase clinical trial costs, delay approval of our product candidates and jeopardize our ability to commercialize our other product candidates, if approved, and generate revenues.
To the extent we use third-party CMOs, we are ultimately responsible for the manufacture of our products, ifonce approved, and our other product candidates. A failure to comply with these requirements may result in regulatory enforcement actions against our manufacturers or us, including fines and civil and criminal penalties, which could result in imprisonment, suspension or restrictions of production, injunctions, delay or denial of product approval or supplements to approved products, clinical holds or termination of clinical trials, warning or untitled letters, regulatory authority communications warning the public about safety issues with the biologic, refusal to permit the import or export of the products, product seizure, detention, or recall, operating restrictions, suits under the federal civil False Claims Act (FCA),FCA, corporate integrity agreements, consent decrees, or withdrawal of product approval.
Any of these challenges could cause us to cease commercial sales, delay completion of clinical trials, require bridging clinical trials or the repetition of one or more clinical trials, increase clinical trial costs, delay approval of our product candidate, impair commercialization efforts, increase our cost of goods, and have a material adverse effect on our business, financial condition, results of operations and growth prospects.
We may not be successful in managing the build-out of our manufacturing facilities and associated costs or satisfying manufacturing-related regulatory requirements.
We have entered into facility leases for our planned manufacturing operations and related activities under which we are responsible for the build-out of the facility space and associated costs. The build-out of these facilities and related equipment purchases are complex and specialized and will involve substantial capital expenditure, and it could take longer, and cost more, than currently expected. Significant delays and/or cost overruns would result in higher expenditures and could be disruptive ofto operations, any of which could have a negative impact on our financial condition or results of operations. For example, during the first quarter of 2022 we acquired a leasehold interest in the 409,000 square foot Dunkirk Facility as described below. While we believe that governmental funding will assist in funding a small portion of the further build-out of the Dunkirk Facility, we will need to plan and fund most of the additional build-out of, and purchase additional equipment for, the Dunkirk Facility in connection with our planned full operations. In addition, it is possible that, once built, the leased facilities may prove to be less conducive to our operations than is currently anticipated, resulting in operational inefficiencies or similar difficulties that could prove difficult or impossible to remediate and result in an adverse impact on our financial condition or results of operations.
We also may not successfully realize the anticipated benefits from the capital expenditure at such facilities based on factors such as delays and uncertainties regarding development, regulatory approval and commercialization of our product candidates, as well as the potential to lose access to the leased facilities.
Further, in the future if we transition from our current third-party CMOs to our own manufacturing facilities, or to alternative third-party CMOs, for one or more of our products or other product candidates, including our approved product, candidate, Anktiva in combination with BCG for the treatment of patients with BCG‑unresponsive NMIBC with CIS with or without Ta or T1 disease, we maywill need to conduct additional preclinical, analytical and/or clinical trialstesting and obtain FDA approval before such manufacturing changes are implemented. If we are unsuccessful in demonstrating the comparability of supplies before and after a manufacturing change, such manufacturing change can result in a delay or disruption in our clinical development plan or our ability to commercialize any approved product. Any production shortfall that impairs the supply of our product or other product candidates could negatively impact our ability to sell our approved product, complete clinical trials, obtain regulatory approval and commercialize our other product candidates. If our product candidates receive approval, aA product shortfall could have a material adverse effect on our business, financial condition and results of operations and adversely affect our ability to satisfy demand for our product or other product candidates, which could materially and adversely affect our revenue and results of operations.
In addition, our planned operations, including our development, testing and future manufacturing activities, are subject to numerous environmental, health and safety laws and regulations. These laws and regulations govern, among other things, the controlled use, handling, release and disposal of and the maintenance of a registry for, hazardous materials and biological materials, such as chemical solvents, human cells, carcinogenic compounds, mutagenic compounds and compounds that may have a toxic effect on reproduction, laboratory procedures and exposure to blood-borne pathogens. If we fail to comply with such laws and regulations, we could be subject to fines or other sanctions. Failure to successfully complete our build-outs and successfully operate our planned manufacturing facilities and satisfy manufacturing-related regulatory requirements could adversely affect the commercial viability of our product candidates and our business.
Cell-based therapies and biologics rely on the availability of reagents, specialized equipment and other specialty materials, which may not be available to us on acceptable terms or at all. For some of these reagents, equipment and materials, we rely or may rely on sole source vendors or a limited number of vendors, which could impair our ability to manufacture and supply our approved product and any future products, if approved.
We currently depend on a small number of suppliers for some of the materials used in, and processes required to develop, our approved product and other product candidates. For some of these reagents, equipment and materials used in the manufacture of our approved product and other product candidates, we rely, and we may in the future rely, on sole source vendors or a limited number of vendors. Some of these suppliers may not have the capacity to support commercial scale or clinical trials and commercial products manufactured under cGMP by biopharmaceutical firms or may otherwise be ill-equipped to support our
needs. We also do not have supply contracts with many of these suppliers and may not be able to obtain supply contracts with them on acceptable terms or at all. Accordingly, we may experience delays in receiving key materials and equipment to support clinical or commercial manufacturing. An inability to continue to source product from any of these suppliers could adversely affect our ability to satisfy demand for our approved product and other product candidates, which could adversely and materially affect our product sales and operating results or our ability to conduct clinical trials, either of which could significantly harm our business.
As we seek to develop and scale our manufacturing process, we expect that we will need to obtain rights to and supplies of certain materials and equipment to be used as part of that process. We may not be able to obtain rights to such materials on commercially reasonable terms, or at all, and if we are unable to alter our process in a commercially viable manner to avoid the use of such materials or find a suitable substitute, it would have a material adverse effect on our business. Even if we are able to alter our process so as to use other materials or equipment, such a change may lead to a delay in our clinical development and/or commercialization plans. If such a change occurs for a product candidate that is already in clinical testing, the change may require us to perform both ex vivo comparability studies and to collect additional data from patients prior to undertaking more advanced clinical trials.
Because our currentapproved product and other product candidates represent, and our other potential product candidates will represent, novel approaches to the treatment of disease, there are many uncertainties regarding the development, market acceptance, public opinion, third-party reimbursement coverage and the commercial potential of our approved product and other product candidates, which may impact public perception of us and our approved product and other product candidates and which may adversely affect our ability to conduct our business and implement our business plans.
Human immunotherapy products are a new category of therapeutics. We use relatively novel technologies involving N-803,ANKTIVA, hAd5, saRNA hAd5 and yeast technologies, aldoxorubicin,constructs, and cell-based therapies, and our NK cell platform utilizes a relatively novel technology involving the genetic modification of human cells and utilization of those modified cells in other individuals. Because this is a relatively new and expanding area of novel therapeutic interventions, there are many uncertainties related to development, marketing, reimbursement and the commercial potential for our approved product and other product candidates. There can be no assurance as to the length of the trial period, the number of patients the FDA will require to be enrolled in the trials in order to establish the safety, efficacy, purity and potency of immunotherapy products, or that the data generated in these trials will be acceptable to the FDA to support marketing approval. Adverse public attitudes may adversely impact our ability to enroll patients in clinical trials. The FDA may take longer than usual to come to a decision on any BLA and/or NDA that we submit and may ultimately determine that there is not enough data, information, or experience with our product candidates to support an approval decision. The FDA may also require that we conduct additional post-marketing studies or implement risk management programs, such as REMS, until more experience with our other product candidates is obtained. Finally, after increased usage, we may find that our product or other product candidates do not have the intended effect, do not work with other combination therapies or have unanticipated side effects, potentially jeopardizing initial or continuing regulatory approval and commercial prospects. More restrictive government regulations or negative public opinion could have an adverse effect on our business or financial condition and may delay or impair the commercialization of our approved product and development and commercialization of our product candidates or demand for our product or any products we may develop. Adverse events in our clinical trials, even if not ultimately attributable to our product candidates, and the resulting publicity could result in increased governmental regulation, unfavorable public perception, potential regulatory delays in the testing or approval of our potential product candidates, stricter labeling requirements for those product candidates that are approved and a decrease in demand for any such product candidates.
There is no assurance that the approaches offered by our product or other product candidates will gain broad acceptance among doctors or patients or that governmental agencies or third-party medical insurers will be willing to provide reimbursement coverage for our proposed product candidates. Public perception may be influenced by claims, such as claims that our technologies are unsafe, unethical or immoral and, consequently, our approach may not gain the acceptance of the public or the medical community. Negative public reaction to cell-based immunotherapy in general could result in greater government regulation and stricter labeling requirements of immunotherapy products, including our other product candidates, and could cause a decrease in the demand for our approved product and any other products we may develop. Moreover, our success will depend upon physicians specializing in the treatment of those diseases that our product or other product candidates target prescribing, and
their patients being willing to receive treatments that involve the use of our product or other product candidates in lieu of, or in addition to, existing treatments they are already familiar with and for which greater clinical data may be available. The market for any products that we successfully develop will also depend on the cost of the product. We do not yet have sufficient information to reliably estimate what it will cost to commercially manufacture our current product candidates, and the actual cost to manufacture these products could materially and adversely affect the commercial viability of these products. Our goal is to reduce the cost of manufacturing and providing our therapies. However, unless we can reduce those costs to an acceptable amount, we may never be able to develop a commercially viable product. If we do not successfully develop and commercialize products based upon our approach or find suitable and economical sources for materials
used in the production of our product or potential products, we will not become profitable, which would materially and adversely affect the value of our common stock. Our N-803ANKTIVA therapies and our other therapies may be provided to patients in combination with other agents provided by third parties or our affiliates. The cost of such combination therapy may increase the overall cost of therapy and may result in issues regarding the allocation of reimbursements between our therapy and the other agents, all of which may affect our ability to obtain reimbursement coverage for the combination therapy from governmental or private third-party medical insurers.
If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit commercialization of our approved product or other product candidates.
We face an inherent risk of product liability as a result of the commercialization of our approved product and clinical development, testing and manufacturing of our other product candidates and will face an even greater risk if we commercialize any products.candidates. For example, we may be sued if our approved product or other product candidates cause or are perceived to cause injury or are found to be otherwise unsuitable during clinical testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, negligence, strict liability or a breach of warranties. Claims could also be asserted under state consumer protection acts. Large judgements have been awarded in class action lawsuits based on therapeutics that had unanticipated side effects. If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit commercialization of our approved product or other product candidates. Even successful defense would require significant financial and management resources. Regardless of the merits or eventual outcome, liability claims may result in a regulatory investigation of the safety and effectiveness of our products, our third-party manufacturer’s manufacturing processes and facilities or our marketing programs and potentially a recall of our products or more serious enforcement action, including limitations on the approved indications for which our product candidates may be used or suspension or withdrawal of approvals, decreased demand for our products, injury to our reputation, costs to defend the related litigation, a diversion of management’s time and our resources, substantial monetary awards to trial participants or patients and a decline in our stock price.
Our inability to obtain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims could prevent or inhibit the commercialization of our approved product or other products we may develop, alone or with corporate collaborators. Our insurance policies may also have various exclusions, and we may be subject to product liability claims for which we have no coverage. While we have obtained clinical trial insurance for our clinical trials, we may have to pay amounts awarded by a court or negotiated in a settlement that exceed our coverage limitations or that are not covered by our insurance, and we may not have, or be able to obtain, sufficient capital to pay such amounts. Even if our agreements with any future corporate collaborators entitle us to indemnification against losses, such indemnification may not be available or adequate should any claim arise.
We will face significant competition from other biotechnology and pharmaceutical companies and from non-profit institutions.
Competition in the field of cancer and viral infectious disease therapy is intense and is accentuated by the rapid pace of technological development. We compete with a variety of multi-national biopharmaceutical companies and specialized biotechnology companies, as well as technology being developed at universities and other research institutions. These competitors have developed, may develop and are developing product candidates and processes competitive with our approved product or other product candidates. Research and discoveries by others may result in breakthroughs which may render our approved product or other product candidates obsolete even before they generate any revenues. We believe that a significant number of products are currently under development, and may become commercially available in the future, for the treatment of conditions for which our product treats or for which we are developing product candidates. Many of our competitors have several therapeutic products that have already been developed, approved and successfully commercialized, or are in the process of obtaining regulatory approval for their therapeutic products in the U.S. and internationally. Many of our competitors, either alone or with their strategic partners,
have substantially greater financial, technical, and human resources than we do, as well as significantly greater experience in the discovery and development of product candidates, obtaining FDA and other regulatory approvals of treatments and commercializing those treatments. Accordingly, our competitors may be more successful in obtaining approval of treatments and achieving widespread market acceptance, rendering our treatments obsolete or non-competitive, possibly even before we are able to enter the market. Accelerated merger and acquisition activity in the biotechnology and biopharmaceutical industries may result in even more resources being concentrated among a smaller number of
our competitors. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These third parties compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs.
Even if we obtain regulatory approval for our product candidates, theThe availability and price of our competitors’ products could limit the demand and the price we are able to charge for our therapies.approved product or any of our other product candidates, if approved. The level of generic competition and the availability of reimbursement from government and other third-party payors will also significantly affect the pricing and competitiveness of our products.
A large number of companies, government agencies and academic centers around the world are developing COVID‑19 vaccines, and many of these entities are in more advanced stages of development than we are, including some that have started Phase 2 and/or 3 clinical trials or have already obtained emergency regulatory approval in the U.S. and internationally. Even if one of our COVID‑19 vaccine candidates is ultimately approved for marketing, the value of our opportunity will be adversely impacted by other COVID‑19 vaccines that have obtained emergency regulatory approval, obtain full regulatory approval, or demonstrate better efficacy or safety than our COVID‑19 vaccine candidate.
We may not be able to implement our business plan if the acceptance of our approved product or other product candidates is inhibited by price competition or the reluctance of physicians to switch from other methods of treatment to our approved product, or if physicians switch to other new therapies, drugs or biologic products or choose to reserve our product candidatesproducts for use in limited circumstances. We may be adversely impacted if any of these competitors gain market share as a result of new technologies, commercialization strategies or otherwise.
We may seek orphan drug status or Breakthrough Therapy or Fast Track or Breakthrough Therapy designations or other designation for one or more of our product candidates, but even if any such designation or status is granted, it may not lead to a faster development process or regulatory review and may not increase the likelihood that our product candidates will receive marketing approval, and we may be unable to maintain any benefits associated with such designations or status, including market exclusivity.
In 2012, the FDA established a Breakthrough Therapy designation, which is intended to expedite, although there is no guarantee, the development and review of products that treat serious or life-threatening conditions. We have been awarded, and may seek in the future, Fast TrackBreakthrough Therapy or Breakthrough TherapyFast Track designation for current or future product candidates. Receipt of a designation to facilitate product candidate development is within the discretion of the FDA. Accordingly, even if we believe one of our product candidates meets the criteria for a designation, the FDA may disagree. In any event, the receipt of such a designation for a product candidate may not result in a faster development process, review or approval compared to product candidates considered for approval under conventional FDA procedures and does not assure ultimate marketing approval by the FDA. In addition, the FDA may later decide that the product candidates no longer meet the designation conditions.
Under the Orphan Drug Act, the FDA may grant orphan designation to a drug or biologic intended to treat a rare disease or condition or for which there is no reasonable expectation that the cost of developing and making available the drug or biologic will be recovered from sales in the U.S. If a product that has orphan drug designation subsequently receives the first FDA approval for the disease for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications, including a full BLA to market the same drug or biologic for the same indication for seven years, except in limited circumstances. We may seek orphan drug status for one or more of our product candidates, but exclusive marketing rights in the U.S. may be lost if we seek approval for an indication broader than the orphan designated indication and may be lost if the FDA later determines that the request for designation was materially defective or if we are unable to assure sufficient quantities of the product to meet the needs of patients with the rare disease or condition. In response to Catalyst Pharms., Inc. v. Becerra, 14 F.4th 1299 (11th Cir. 2021), the FDA clarified in a January 2023 notice that it intends to continue to apply its longstanding interpretation of the regulations to matters outside of the scope of the Catalyst order – that is, the agency will continue tying the scope of orphan-drug exclusivity to the uses or indications for which a drug is approved, which permits other sponsors to obtain approval of a drug for new uses or indications within the same orphan designated disease or condition that have not yet been approved. It is unclear how future litigation, legislation, agency decisions, and administrative actions will impact the scope of the orphan drug exclusivity.
As a condition of approval, the FDA may require that we implement various post-marketing requirements and conduct post-marketing studies, any of which would require a substantial investment of time, effort, and money, and which may limit our commercial prospects.
As a condition of biologic licensing, the FDA is authorized to require that sponsors of approved BLAs implement various post-market requirements, including REMS and Phase 4 trials. For example, in connection with FDA approval of another company’s drug, the FDA required significant post-marketing commitments, including a Phase 4 trial, revalidation of a test method, and a substantial REMS program that included, among other requirements, the certification of hospitals and their associated clinics that dispensed the drug, including the implementation of a training program and limited distribution only to certified hospitals and their associated clinics. If we receive approval of our other product candidates, the FDA may determine that similar or additional or more burdensome post-approval requirements are necessary to ensure that our product candidates are safe, pure and potent. To the extent that we are required to establish and implement any post-approval requirements, we will likely need to invest a significant amount of time, effort and money. Such post-approval requirements may also limit the commercial prospects of our product candidates.
We have never commercialized a product candidate before, and we may lack the necessary expertise, personnel and resources to successfully commercialize any products on our own or together with suitable collaborators. We may be unable to establish effective marketing and sales capabilities or enter into agreements with third parties or related parties to market and sell our other product candidates, if they are approved, and as a result, we may be unable to generate product revenues.
We have little to no prior experience in, and currently have a limited commercial infrastructure for, the marketing, sale and distribution of biopharmaceutical products. To achieve commercial success for theour approved product and other product candidates, which we may license to others, we willmay rely on the assistance and guidance of those collaborators. For our approved product and our other product candidates for which we retain commercialization rights and marketing approval, if approved, in order to commercialize our approved product and such other product candidates, we must continue to build out our marketing, sales and distribution capabilities, including a comprehensive healthcare compliance program, and/or arrange with third parties to perform these services, which will continue to take time and require significant financial expenditures and could delay any product launch and we may not be successful in doing so. There are significant risks involved with building and managing a commercial infrastructure. We, or our collaborators and third-party contractors, will have to compete with other pharmaceutical and biotechnology companies to recruit, hire, train, manage and retain medical affairs, marketing, sales and commercial support personnel. Recruiting, training and retaining a sales force is expensive and time-consuming and could delay any product launch. If the commercial launch of aour approved product or any other product candidate for which we or our third-party contractors recruit a sales force and establish marketing capabilities is delayed or does not occur for any reason, we would have incurred these commercialization expenses prematurely or unnecessarily. These efforts may be costly, and our investment would be lost if we cannot retain or reposition our sales and marketing personnel. In the event we are unable to develop a commercial infrastructure, we may not be able to commercialize our current or future product candidates, which would limit our ability to generate product revenues. Even if we and/or our third-party contractors are able to effectively establish a sales force and develop a marketing and sales infrastructure, oursuch sales force and marketing teams may not be successful in commercializing our approved product or any other current or future product candidates. To the extent we rely on third parties to commercialize any products for which we obtain regulatory approval, which we wouldare doing to a certain extent in connection with our approved product launch, we may have less control over their sales efforts and could be held liable if they failed to comply with applicable legal or regulatory requirements.
If our approved product or any of our other product candidates do not achieve broad market acceptance, the revenues that we generate from their sales will be limited.
We have not commercialized aare in the process of commercializing our approved product. Our approved product candidate for any indication. Even if ourand other product candidates, areif approved by the appropriate regulatory authorities for marketing and sale, they may not gain acceptance among physicians, patients, third-party payors and others in the medical community. If our approved product or any other product candidate for which we obtain regulatory approval does not gain an adequate level of market acceptance, we may not generate significant product revenues or become profitable. Market acceptance of our approved product or any other product candidates by the medical community, patients and third-party payors will depend on a number of factors, some of which are beyond our control. For example, physicians are often reluctant to switch their patients, and patients may be reluctant to switch from existing therapies
switch from existing therapies even when new and potentially more effective or safer treatments enter the market. Efforts to educate the medical community and third-party payors on the benefits of our approved product and other product candidates may require significant resources and may not be successful. Even if the medical community accepts that our approved product and other product candidates are safe and effective for their approved indications, physicians and patients may not immediately be receptive to such approved product or other product candidates and may be slow to adopt them as an accepted treatment of the approved indications. If our product or any of our other product candidates is approved but does not achieve an adequate level of market acceptance, we may not generate significant revenues and we may not become profitable. The degree of market acceptance of our approved product or any of our other product candidates will depend on a number of factors, including:
•the continued safety and efficacy of our approved product or other product candidates;
•the prevalence and severity of adverse events associated with such approved product or other product candidates;
•the clinical indications for which the products are approved and the approved claims that we may make for the products;
•limitations or warnings contained in the product’s FDA-approved labeling, including potential limitations or warnings for such products that may be more restrictive than other competitive products or distribution and use restrictions imposed by the FDA with respect to such approved product or other product candidates or to which we agree as part of a mandatory REMS or voluntary risk management plan;
•changes in the standard of care for the targeted indications for such approved product or other product candidates;
•the relative difficulty of administration of such approved product or other product candidates;
•our ability to offer such product or other product candidates for sale at competitive prices, including the cost of treatment versus economic and clinical benefit in relation to alternative treatments or therapies;
•the availability of adequate coverage or reimbursement by third parties, such as insurance companies and other healthcare payors, and by government healthcare programs, including Medicare and Medicaid;
•the extent and strength of our marketing and distribution of such approved product or other product candidates;
•the safety, efficacy and other potential advantages over, and availability of, alternative treatments already used or that may later be approved for any of our intended indications;
•the timing of market introduction of such approved product or other product candidates, as well as competitive products;
•the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies;
•the extent and strength of our third-party manufacturer and supplier support;
•adverse publicity about the product or favorable publicity about competitive products; and
•potential product liability claims.
If our approved product or any other product candidate that we commercialize fails to achieve market acceptance, it could have a material and adverse effect on our business, financial condition, results of operations and prospects.
Our product candidates may face competition sooner than anticipated.
The enactment of the Biologics Price Competition and Innovation Act of 2009 (BPCIA)BPCIA created an abbreviated approval pathway for biological products that are biosimilar to or interchangeable with an FDA-licensed reference biological product. Under the BPCIA, the FDA cannot make an approval of an application for a biosimilar product effective until 12 years after the original branded product was approved under a BLA. Certain changes, however, and supplements to an approved BLA, and subsequent applications filed by the same sponsor, manufacturer, licensor, predecessor in interest or other related entity do not qualify for the 12-year exclusivity period.
Our product candidates may qualify for the BPCIA’s 12-year period of exclusivity. There is a risk that any product candidates we may develop that are approved as a biological product under a BLA would not qualify for the 12-year period of exclusivity or that this exclusivity could be shortened due to congressional action or otherwise, or that the FDA will not consider our approved product or any other product candidates we may develop to be reference products for competing products, potentially creating the opportunity for generic competition sooner than anticipated. Additionally, this period of regulatory exclusivity does not block companies pursuing regulatory approval via their own traditional BLA, rather than via the abbreviated pathway. Even if we receive a period of BPCIA exclusivity for our first licensedapproved product, if subsequent products do not include a modification to the structure of the product that impacts safety, purity, or potency, we may not receive additional periods of exclusivity for those products. Moreover, the extent to which a biosimilar, once approved, will be substituted for any one of our reference product candidates in a way that is similar to traditional generic substitution for non-biological products is not yet clear, and will depend on a number of marketplace and regulatory factors that are still developing. Medicare Part B encourages use of biosimilars by paying the provider the same percentage of the reference product average sale price as a mark-up, regardless of which product is reimbursed. It is also possible that payors will give reimbursement preference to biosimilars even over reference biologics absent a determination of interchangeability.
For our small molecular product candidates, if qualified, the regulatory exclusivity period is less than for our biologic product candidates. The Federal Food, Drug, and CosmeticFD&C Act (FFDCA) provides a five-year period of non-patent marketing exclusivity within the U.S. to the first applicant to gain approval of an NDA for a drug where the FDA has not previously approved any other new drug containing the same active molecule or ion responsible for the action of the drug substance. During the exclusivity period, the FDA may not accept for review an abbreviated NDA or a 505(b)(2) NDA submitted by another company for a generic version of such drug where the applicant does not own or have a legal right of reference to all the data required for approval. However, an application may be submitted after four years if it contains a certification of patent invalidity or non-infringement. The FDCAFD&C Act also provides three years of marketing exclusivity for an NDA, 505(b)(2) NDA or supplement to an existing NDA if new clinical investigations, other than bioavailability studies, thatwhich were conducted or sponsored by the applicant are deemed by the FDA to be essential to the approval of the application, for example, new indications, dosages or strengths of an existing drug. As such, we may face competition from generic versions of our small molecule product candidates, which will negatively impact our long-term business prospects and marketing opportunities.
We will need to obtain FDA approval of any proposed branded product names, and any failure or delay associated with such approval may adversely affect our business.
Any name we intend to use for our product candidates in the U.S. will require approval from the FDA regardless of whether we have secured a formal trademark registration from the U.S. Patent and Trademark Office (USPTO).USPTO. The FDA typically conducts a review of proposed product names, including an evaluation of the potential for confusion with other product names. The FDA may also object to a product name if it believes the name inappropriately implies medical claims or contributes to an overstatement of efficacy. If the FDA objects to any of our proposed product names, we may be required to adopt alternative names for our product candidates. If we adopt alternative names, we wouldwill lose the benefit of any existing trademark applications for such product candidate and may be required to expend significant additional resources in an effort to identify a suitable product name that would qualify under applicable trademark laws, not infringe or otherwise violate the existing rights of third parties, and be acceptable to the FDA. We may be unable to build a successful brand identity for a new product name in a timely manner or at all, which would limit our ability to commercialize our product candidates.
Our internal computer systems, infrastructure or data, or those used by our CROs, CMOs, clinical sites or other contractors or consultants, may or may be perceived to fail or suffer security breaches. A breakdown,a cyberattack, or information security breach couldor other incident, including a breakdown or compromise of the confidentiality, integrity and availability of our information technology systems, network-connected control systems and/networks or our data, interruptwhich could adversely affect the operation of our business and/or affect ourand reputation.
We are and will be dependent upon information technology systems, infrastructure and data. In the ordinary course of our business, we will directly or indirectly collect, store, transmit, and transmitotherwise process sensitive data,and confidential information, including intellectual property, confidential information, preclinical and clinical trial data, proprietary business information, personal data and personally identifiable healthpersonal information of our clinical trial subjectspatients and employees, including in our data centers and on our systems and networks or on those of third parties. The secure maintenance, transmission, and processing maintenance and transmission of this informationdata is critical to our operations. The multitude and
complexity of our computer systems and those of our CROs, CMOs, clinical sites or other contractors or consultants makemay subject them inherently vulnerable to servicevarious threats, including interruption, or destruction, malicious intrusion, and random attack. Data privacyPrivacy or security breaches or other incidents, including by third parties, employees, contractors or others, may pose a risk that sensitive data,or confidential information, including our intellectual property, trade secrets or personal information of our employees, patients, or other business partners may be exposed to unauthorized persons or to the public. Further, as many of our employees are workingwork remotely, our reliance on our and third-party information technology systems has increased substantially and is expected to continue to increase.
Despite the implementation of security measures, our internal computer systems, infrastructure and data, and those of our CROs, CMOs, clinical sites and other contractors and consultants, are vulnerablesubject to failurerisks relating to cyberattacks, security breaches, or damage from computerother incidents, including through viruses and other malware, employee error, unauthorized and authorized access, or other cybersecurity attacks, natural disasters, terrorism, war, fire, and telecommunication and electrical failures.failures, denial of service attacks, social engineering (including phishing attacks) and other means. As the cyberthreat landscape evolves, these cyberattacks are increasing in their frequency, sophistication and intensity and are becoming increasingly difficult to detect. The techniques used by cybercriminals change frequently, may not be recognized until launched and can originate from a wide variety of sources, including outside groups such as external service providers, organized crime affiliates, terrorist organizations or hostile foreign governments or agencies. Cyberattacks could include the deployment of harmful malware, denial-of-service, social engineering and other means to affect service reliability and threaten data confidentiality, integrity and availability. While we and our shared services partner, NantWorks, have invested, and continue to invest, in the protection of our data, systems, and information technology infrastructure, there can be no assurance that our efforts, or the efforts of our partners, vendors, CROs, CMOs, clinical sites and other contractors and consultants, will be successful. Any failure or perceived failure in our systems, infrastructure or data, or to identify or prevent cyberattacks, security breaches or other incidents, including service interruptions or identify breaches in our or their systems, that could adversely affect our business and operations, and/or result in the loss, unavailability, misuse, unauthorized use or acquisition, or other unauthorized processing of critical or sensitive information, which couldand result in financial, legal, business or reputational harm to us.harm. In addition, our liability insurance may not be sufficient in type or amount to cover us against claims related to any such failures, security breaches, cyberattacks andor other related breaches.incidents.
If any such event were to occur, andit could also cause interruptions in our operations, it could result inincluding a disruption of our product development programs. For example, the loss of clinical trial data from completed or ongoing clinical trials for a product candidate could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data or may limit our ability to effectively execute a product recall, if required. To the extent that any disruption or security breach were toAny such event could result in a loss of or damage to our data or applications, or inappropriate disclosure of personal, confidential or proprietary information, we could incur liability, anddelays in the further development and commercialization of any product candidates, could be delayed. Any such event could also result in legal claims, demands or proceedings liability underinitiated by regulatory authorities or private parties, violations of laws, including laws that protect the privacy or security of personal information, and significant liabilities, including regulatory penalties, and damage to our reputation and a loss of confidence in us and our ability to conduct clinical trials.
A pandemic, epidemic or outbreak of an infectious disease, such as COVID-19, or the perception of its effects, may materially and adversely affect our business, operations and financial condition.
Public health outbreaks, such as epidemics or pandemics may significantly disrupt our business. Such outbreaks pose the risk that we or our employees, contractors, suppliers, and other partners may be prevented from conducting business activities for an indefinite period of time due to the spread of the disease, due to shutdowns that may be requested or mandated by federal, state, and local governmental authorities or certain employers, or due to the economic consequences associated with the pandemic. Business disruptions could include disruptions or restrictions on our ability to travel, as well as temporary closures of our facilities and the facilities of our partners, clinical trial sites, service providers, suppliers, or contract manufacturers. For example, the COVID-19 pandemic caused a temporary disruption in our ability to recruit participants for our clinical trials in the calendar year 2020 and the first quarter of 2021. While it is not possible to predict whether another pandemic, epidemic, or infectious disease outbreak similar to COVID-19 will materialize, any measures taken by governments and local authorities in response to such future health crises have the potential to disrupt and delay the initiation of new clinical trials, the progress of our ongoing clinical trials and our preclinical activities, and potentially the manufacture or shipment of both drug substance and finished drug product of our approved product and our other product candidates for preclinical testing and clinical trials, may adversely impact our business, financial condition, or operating results.
Our business could be adversely affected by the effects of health epidemics, pandemics or contagious diseases, including the recent COVID‑19 pandemic, which may have a material adverse effect, on our clinical trials, operations, supply chains, distribution systems, product development, business and results of operations.
Outbreaks of epidemic, pandemic or contagious diseases, such as the ongoing COVID‑19 pandemic, and measures taken in response by governments and businesses worldwide to contain its spread have adversely impacted and may continue to significantly disrupt our operations and adversely affect our business, financial condition and results of operations. The continued spread of this pandemic has caused significant volatility and uncertainty in the U.S. and international markets and has resulted in increased risks to our operations. The COVID‑19 pandemic and any actions we have taken in response, are affecting and could materially affect our operations, including at our headquarters and at our manufacturing facilities, which have been and may in the future be subject to state executive orders and shelter-in-place orders, and at our clinical trial sites, as well as the business or operations of our CROs, CMOs, clinical sites or other third parties with whom we conduct business. Any such epidemic or pandemic may heighten the risk that a significant portion of our workforce could suffer illness or otherwise not be permitted or be unable to work, and may require that certain of our employees work remotely, which heightens certain risks, including but not limited to, those associated with an increased demand for information technology resources, increased risk of cybersecurity attacks (including social engineering attacks), risks related to internal controls and increased risk of unauthorized dissemination of sensitive personal information or our proprietary or confidential information.
The rapid development and fluidity of the pandemic preclude any prediction as to the ultimate effect of COVID-19 on us. While the U.S. and other countries have reopened their economies to varying degrees, the extent to which COVID-19 will impact our future operations will depend on many factors which cannot be predicted with confidence, including the duration of the outbreak. Any resurgence in COVID-19 infections could result in the imposition of new mandates and prolonged restrictive measures implemented in order to control the spread of the disease.
We are monitoring a number of risks related to this pandemic, including the following:
•Financial: We expect to continue spending on research and development during the year ending December 31, 2023 and beyond, and we could also have unexpected expenses related to the pandemic. The short-term continued expenses, as well as the overall uncertainty and disruption caused by the pandemic, will likely cause a delay in our ability to commercialize a product and adversely impact our financial results.
•Manufacturing: The pandemic has impacted, and may continue to impact, our manufacturing locations, including through the effects of facility closures, reductions in operating hours and other social distancing efforts.
•Supply Chain: As the pandemic continues to progress, it has resulted and could continue to result in significant disruptions in our respective supply chains and distribution channels in the future. In addition, there may be unfavorable changes in the availability or cost of raw materials, intermediates and other materials necessary for production, which may result in disruptions in our supply chain and adversely affect our ability to have manufactured certain product candidates for clinical supply.
•Clinical Trials: This pandemic may adversely affect certain of our clinical trials, including our ability to initiate and complete our clinical trials within the anticipated timelines. Due to site and participant availability during the pandemic, new subject enrollment has slowed and is expected to continue to slow, at least in the short-term, for most of our clinical trials. For ongoing trials, we have seen, and expect to continue to see an increasing number of clinical trial sites imposing restrictions on patient visits to limit risks of possible COVID‑19 exposure, and we may experience issues with participant compliance with clinical trial protocols as a result of quarantines, travel restrictions and interruptions to healthcare services. The current pressures on medical systems and the prioritization of healthcare resources toward the COVID‑19 pandemic have also resulted, and may continue to result, in interruptions in data collection and submissions for certain clinical trials and delayed starts for certain planned studies. As a result, our anticipated filing and marketing timelines may be adversely impacted.
•Overall Economic and Capital Markets Environment: The continued spread of COVID‑19 has led to and could continue to lead to severe disruption and volatility in the U.S. and global capital markets, which could result in a decline in stock price, high inflation, increase our cost of capital and adversely affect our ability to access the capital markets in the future even after local conditions improve. In addition, trading prices on the public stock market have been highly volatile as a result of the COVID‑19 pandemic.
•Regulatory Reviews: The operations of the FDA or other regulatory agencies may be adversely affected. The legislative and regulatory environment governing our businesses is dynamic and changes frequently in response to COVID‑19. In response to COVID‑19, federal, state and local governments are issuing new rules, regulations, orders and advisories on a regular basis. These government actions can impact us, our members and our suppliers. There is also the possibility that we may experience delays with obtaining approvals for our IND applications, BLAs, and/or NDAs. The pandemic may also result in greater regulatory uncertainty.
Risks Related to Reliance on Third Parties
We have limited experience conducting clinical trials and have relied and will continue to rely on third parties and related parties to conduct manysome of our preclinical studies and clinical trials, to manufacture productsour approved product and toother product candidates, and perform many essential services for any products that we commercialize, including services related to sales and marketing, distribution, government price reporting, customer service, accounts receivable management, cash collection and adverse event reporting. Any failure by a third party, related party, or us to perform as expected, to comply with legal and regulatory requirements or to conduct the clinical trials according to GCP guidelines, and in a timely manner, may delay or prevent our ability to commercialize our approved product, to seek or obtain regulatory approval for or commercialize our other product candidates or may subject us to regulatory sanctions.
We have relied and will continue to rely on third parties and related parties to conduct some of our preclinical studies and clinical trials, manufacture our approved product and other product candidates, and perform many essential services for any products that we commercialize. Any failure by a third party, related party, or by us to perform as expected, to comply with legal and regulatory requirements or to conduct the clinical trials according to GCP regulations,guidelines, and in a timely manner, may delay or prevent our ability to commercialize our approved product, to seek or obtain regulatory approval for or commercialization ofcommercialize our other product candidates, and our ability to commercialize our current or future product candidates will be significantly impacted and we may be subject us to regulatory sanctions.
Large-scale clinical trials require significant financial and management resources. We expect to be heavily reliant on third and related parties, including medical institutions, academic institutions, clinical investigators or CROs to conduct, supervise or monitor some or all aspects of our clinical trials, and in some cases, third-party CMOs to manufacture products,our approved product or other product candidates, which may force us to encounter delays and challenges that are outside of our control. Nevertheless, we are responsible for ensuring that each of our trials is conducted in accordance with the applicable trial protocol and legal, regulatory and scientific standards, and our reliance on CROs, clinical trial sites, and other third parties does not relieve us of these responsibilities. Our CROs and other third parties must communicate and coordinate with one another in order for our trials to be successful. We have a limited history of conducting clinical trials and have nolimited experience as a company in filingsubmitting and supporting the applications necessary to gain marketing approvals. Our relative lack of experience conducting clinical trials may contribute to our planned clinical trials not beginning or completing on time, if at all. Securing marketing approval also requires the submission of information about the product manufacturing process to, and inspection of manufacturing facilities and clinical trial sites by, applicable regulatory authorities.
For example, we will remain responsible for ensuring that each of our clinical trials is conducted in accordance with the general investigational plan and protocols for the trial and for ensuring that our preclinical studies are conducted in accordance with Good Laboratory Practice (GLP) regulations,GLP guidelines, as appropriate. Moreover, the FDA and comparable foreign regulatory authorities require us and the third parties upon which we intend to rely for conducting our clinical trials to comply with GCP guidelines for conducting, recording, and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity, and confidentiality of trial participants are protected. Regulatory authorities enforce these requirements through periodic inspections (including pre-approval inspections once a BLA or NDA is filed with the FDA) of trial sponsors, clinical investigators, trial sites and certain third parties including CMOs. If we, our CROs, clinical trial sites, or other third parties fail to comply with applicable GCP guidelines or other regulatory requirements, we or they may be subject to enforcement or other legal actions, the clinical data generated in our clinical trials may be deemed unreliable and have to be repeated, and our submission of marketing applications may be delayed or the FDA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. We cannot assure you that upon inspection by a given regulatory authority, such regulatory authority will determine that any of our clinical trials comply with GCP regulations.guidelines.
We rely on third parties to manufacture, package, label and ship our approved product and some of our other product candidates for the clinical trials that we conduct. Any performance failure on the part of these third parties could delay commercialization of our product or other product candidates or the clinical development or marketing approval of our product candidates or commercialization of our product candidates, if approved, producing additional losses and depriving us of potential product revenues.
Our CROs, clinical trial sites and other third parties may also have relationships with other entities, some of which may be our competitors, for whom they may also be conducting clinical trials or other therapeutic development activities that could harm our competitive position. In addition, these third parties are not our employees, and except for remedies available to us under our agreements with them, we cannot control whether or not they devote sufficient time and resources to our ongoing clinical and preclinical programs. If these third parties conducting our clinical trials (i) do not successfully carry out their contractual duties, (ii) do not meet expected deadlines, (iii) experience work stoppages, (iv) do not conduct our clinical trials in accordance with regulatory requirements or our stated protocols, (v) need to be replaced, (vi) experience financial hardships or (vii) terminate their agreements with us or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to our clinical trial protocols, GCP guidelines or other regulatory requirements or for other reasons, our trials may need to be repeated, extended, delayed or terminated, we may not be able to obtain, or may be delayed in obtaining, marketing approvals for our product candidates, we will not be able to, or may be delayed in our efforts to, successfully commercialize our product candidates or we or they may be subject to regulatory enforcement actions. Additionally, we may need to conduct additional clinical trials or enter into new arrangements with alternative CROs, clinical investigators or other third parties, which we may not be able to do on commercially reasonable terms, or at all and which may involve additional cost and time and require management time and focus. As a result, delays could occur, which could compromise our ability to meet our desired development timelines. Furthermore, if any of the third parties conducting our clinical trials experience any financial hardships due to difficulties relating to the operation of their business, it could damage our business, financial condition, results of operations and prospects. In addition, if an agreement with any of our collaborators terminates, our access to technology and intellectual property licensed to us by that collaborator may be restricted or terminate entirely, which may delay the continued development of our product candidates using the collaborator’s technology or intellectual property or require us to stop development of those product candidates completely. As a result, our results of operations and the commercial prospects for our product candidates would be harmed, our costs could increase and our ability to generate revenues could be delayed. To the extent we are unable to successfully identify and manage the performance of third-party service providers in the future, our business may be materially and adversely affected.
We have and we expect to continue to retain third-party service providers to perform a variety of functions related to the sale of our approved product and current or future product candidates, key aspects of which will be out of our direct control. These service providers may provide key services related to sales, market access, distribution, customer service, accounts receivable management, state reporting, compliance support, and cash collection. If we retain a service provider, we wouldwill substantially rely on it as well as other third-party providers that perform services for us, including entrusting our inventories of products to their care and handling. If these third-party service providers fail to comply with applicable laws and regulations, fail to meet expected deadlines or otherwise do not carry out their contractual duties to us, or encounter physical or natural damage at their facilities, our ability to deliver product to meet commercial demand would be significantly impaired and we may be subject to regulatory enforcement action.
In addition, we may engage in the future with third parties to perform various other services for us relating to adverse event reporting, safety database management, fulfillment of requests for medical information regarding our product candidates and related services. If the quality or accuracy of the data maintained by these service providers is insufficient, or these third parties otherwise fail to comply with regulatory requirements related to adverse event reporting, we could be subject to regulatory sanctions.
Additionally, we may contract in the future with a third party to calculate and report pricing information mandated by various government programs. If a third party fails to timely report or adjust prices as required or errs in calculating government pricing information from transactional data in our financial records, it could impact our discount and rebate liability, and potentially subject us to regulatory sanctions or FCA lawsuits.
Our reliance on third and related parties can also present intellectual property-related risks. For example, collaborators may not properly obtain, maintain, enforce or defend intellectual property or proprietary rights relating to our product candidates or technology or may use our proprietary information in such a way as to expose us to potential litigation or other intellectual property-related proceedings, including proceedings challenging the scope, ownership, validity and enforceability of our intellectual property. Collaborators may also own or co-own intellectual property covering our product candidates or technology
that results from our collaboration with them, and in such cases, we may not have the exclusive right to commercialize such intellectual property or such product candidates or technology. Collaborators may also gain access to our trade secrets or formulations and impact our ability to commercialize proprietary technology. We may also need the cooperation of our collaborators to enforce or defend any intellectual property we contribute to or that arises out of our collaborations, which may not be provided to us.
We also anticipate that part of our strategy for pursuing the wide range of indications potentially addressed by N-803ANKTIVA will involve further investigator-led clinical trials. While these trials generally provide us with valuable clinical data that can inform our future development strategy, we generally have less control over not only the conduct but also the design of these clinical trials. Third-party investigators may design clinical trials involving our product candidates with clinical endpoints that are more difficult to achieve or in other ways that increase the risk of negative clinical trial results compared to clinical trials we may design on our own. Negative results from investigator-led clinical trials, regardless of how the clinical trial was designed or conducted, could have a material adverse effect on our business and the perception of our product candidates.
Moreover, principal investigators for our clinical trials may serve as scientific advisors or consultants to us from time to time and receive compensation in connection with such services.
If third-party manufacturers, wholesalers and distributors fail to perform as expected, or fail to devote sufficient time and resources to our approved product or other product candidates, our clinical development may be delayed, our costs may be higher than expected or our other product candidates may fail to be approved, or we may fail to commercialize our approved product or any other product candidates, if approved.
Our reliance on third-party manufacturers, wholesalers and distributors exposes us to the following risks, any of which could delay FDA approval of our product candidates and commercialization of our approved product or any other product candidates, if approved, result in higher costs, or deprive us of potential product revenues:
•our CMOs, or other third parties we rely on, may encounter difficulties in achieving the volume of production needed to satisfy commercial demand, may experience technical issues that impact quality or compliance with applicable and strictly enforced regulations governing the manufacture of pharmaceutical products, and may experience shortages of qualified personnel to adequately staff production operations;
•our wholesalers and distributors could become unable to sell and deliver our approved product or other product candidates for regulatory, compliance and other reasons;
•our CMOs, wholesalers and distributors could breach or default on their agreements with us to meet our requirements for commercialization of our approved product or other product candidates;
•our CMOs, wholesalers and distributors may not perform as agreed or may not remain in business for the time required to successfully produce, store, sell and distribute our approved product or other product candidates and we may incur additional cost;
•our CMOs, wholesalers and distributors may misappropriate our proprietary information; and
•if our CMOs, wholesalers and distributors were to terminate our arrangements or fail to meet their contractual obligations, we may be forced to delay our commercial programs.
Our reliance on third parties reduces our control over our approved product and other product candidate development activities but does not relieve us of our responsibility to ensure compliance with all required legal, regulatory and industry standards. For example, the FDA and other regulatory authorities require that our approved product, other product candidates and any other products that we may eventually commercialize be manufactured according to cGMP requirements. Any failure by our third-party manufacturers to comply with cGMP or maintain a compliance status acceptable to the FDA or other regulatory authorities or failure to scale up manufacturing processes, including any failure to deliver sufficient quantities of product candidates in a timely manner, could lead to a delay in, or failure to obtain, regulatory approval of any of our product candidates. On May 9, 2023, the FDA delivered a CRL to us regarding the BLA filed in May 2022, indicating that the FDA had determined that it cannotcould not approve the original BLA submission in its presentinitial form, and the FDA made recommendations to address the issues raised. The company has submitted a formal meeting request and the associated briefing book to the FDA and plans to diligently address and resolve the issues identifieddeficiencies in the CRL and seek approval as expeditiously as possible. The deficiencies relaterelated to the FDA’s pre-license inspection of the company’s third-party CMOs, among other items. Satisfactory resolution
In October 2023, we announced the resubmission of the observations noted atBLA addressing the pre-license inspection is required beforeissues in the CRL, and that the FDA accepted our BLA may be approved. In addition, ourresubmission for review and considered it as a complete response to the CRL setting a new user fee goal date (PDUFA date) of April 23, 2024. On April 22, 2024, the FDA approved ANKTIVA with BCG for the treatment of adult patients with BCG-unresponsive NMIBC with CIS, with or without papillary tumors. Our third-party manufacturers will beare subject to periodic inspections by the FDA and other regulatory authorities, and failure to comply with cGMP could be the basis for the FDA to issue a warning or untitled letter, withdraw approvals for our approved product or our other product candidates previously granted to us, or take other regulatory or legal action, including a request to recall or seize our approved product or our other product candidates, total or partial suspension of production, suspension of clinical trials, refusal to approve pending applications or supplemental applications, detention of product, refusal to permit the import or export of other product candidates, injunction, imposing civil penalties or pursuing criminal prosecution.
Additionally, as we scale up manufacturing of our approved product or other product candidates and conduct required stability testing, we may encounter additional challenges or cGMP issues. These issues may require refinement or resolution in order to proceed with commercial marketing of our approved product or any of our other product candidates, if approved. In addition, quality issues may arise during scale-up and validation of commercial manufacturing processes. Any issues in our manufacturing process could result in increased scrutiny by regulatory authorities, delays in our regulatory review process, increases in our operating expenses, or failure to obtain or maintain approval for our approved product or other product candidates. If such issues relate to an approved product, we may not be able to commercialize the approved product as we planned or fail to meet commercial demand, any of which can materially and adversely affect our position in the market.
We use the Clinic, a related party, in some of our clinical trials which may expose us to significant regulatory risks. If our data for this site is not sufficiently robust or if there are any data integrity issues, we may be required to repeat such studies or required to contract with other clinical trial sites, andwhich could delay and/or increase the cost of our clinical development plans will be significantly delayed, and we will incur additional costs.plans.
The Clinic has conducted, is currently conducting, and in the future may conduct, clinical trials involving our product candidates. The Clinic is a related party as it is owned by an officer of the company and additionally, NantWorks manages the administrative operations of the Clinic. Prior to June 30, 2019, one of the company’s officers was an investigator or sub‑investigator for certain of the company’s trials conducted at the Clinic. NantWorks, which is wholly owned by our Executive Chairman and Global Chief Scientific and Medical Officer, Dr. Soon‑Shiong, provides certain administrative services (and has loaned money) to the Clinic. Under certain circumstances, we may be required to report some of these relationships to the FDA.
Relying on a related partyrelated-party clinical site to develop data that is used as the basis to support regulatory approval can expose us to significant regulatory risks. The FDA may conclude that a financial relationship between us, the Clinic and/or a principal investigator has created a conflict of interest or otherwise affected interpretation of the study. The FDA or comparable regulatory authorities may therefore question the integrity of the data generated at the applicable clinical trial site and the utility of the clinical trial itself may be jeopardized. If any data integrity, or regulatory non-compliance issues occur during the study, we may not be able to use the data for our regulatory approval. This could result in a delay in approval, or rejection, of our marketing applications by the FDA and may ultimately lead to the denial of regulatory approval of one or more of our product candidates.
We have formed, and may in the future form or seek, strategic alliances or enter into collaborations with third parties or additional licensing arrangements, in the future, and we may not realize the benefits of such alliances or licensing arrangements. If we fail to enter intoConflicts may arise between us and our collaborators or strategic partners, and such strategic alliances, collaborations or licensing arrangements or such strategic alliances, collaborations or licensing arrangements are not successful, we may not be able to capitalize on the market potential of our product candidates.successful.
We have formed, and may in the future form or seek, strategic alliances, create joint ventures or collaborations or enter into collaborations with third parties or additional licensing arrangements with third and related parties that we believe will complement or augment our development and commercialization efforts with respect to our approved product, other product candidates and any future product candidates that we may develop. We plan to collaborate with governmental, academic and corporate partners, including affiliates, to improve and develop N-803,ANKTIVA, hAd5, saRNA hAd5 and yeast technologies,constructs, and other cell therapies for new indications for use in combination with other therapies and to improve and develop other product candidates, which may expose us to additional risks, or we may not realize the benefits of such collaborations.
Because some of our collaborations are conducted at outside laboratories, and we do not have complete control over how the studies are conducted or reported, or over the manufacturing methods used to manufacture our product candidate, Anktiva in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease, the results of such studies, which we may use as the basis for our conclusions, projections or decisions with respect to our current or future product candidates, may be incorrect or unreliable, or may have a negative impact on us if the results of such studies are imputed to our product candidates or proposed indications, even if such imputation is improper. Additionally, we may use third‑party data to analyze, reach conclusions or make predictions or decisions with respect to our product candidates that may be incomplete, inaccurate or otherwise unreliable.
99We also plan to collaborate with governmental, academic and corporate partners, including affiliates, to improve and develop ANKTIVA, hAd5, saRNA and yeast constructs, cell therapies and other therapies for new indications for use in combination with other therapies and to improve and develop other product candidates, which may expose us to additional risks, or we may not realize the benefits of such collaborations.
TableFurthermore, conflicts may arise between us and our collaborators or strategic partners, and such strategic alliances, collaborations or licensing arrangements may result in litigation. For example, in 2019 Sorrento, with which we jointly established a new entity called NANTibody as a stand-alone biotechnology company, commenced litigation against us and certain of Contentsour officers and directors, alleging that we improperly caused NANTibody to acquire IgDraSol. Additionally, in 2020 we received a Request for Arbitration before the International Chamber of Commerce, International Court of Arbitration, served by Beike asserting breach of contract under our subsidiary Altor’s license agreement with them. See Item 1. “Legal ProceedingsFurther,In addition, collaborations involving our product candidates will be subject to numerous risks, which may include the following:
•collaborators, including their related or affiliated companies, may be entitled to receive exclusive rights for or involving our products;
•collaborators have significant discretion in determining the efforts and resources that they will apply to a collaboration;
•collaborators may not pursue development and commercialization of our product candidates or may elect not to continue or renew development or commercialization of our product candidates based on clinical trial results, changes in their strategic focus due to the acquisition of competitive products, availability of funding or other external factors, such as a business combination that diverts resources or creates competing priorities;
•collaborators may delay clinical trials, provide insufficient funding for a clinical trial, stop a clinical trial, abandon a product candidate, repeat or conduct new clinical trials or require a new formulation of a product candidate for clinical testing;
•collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with our product candidates;
•a collaborator with marketing and distribution rights to one or more products may not commit sufficient resources to their marketing and distribution;
•collaborators may not properly maintain, defend or enforce our intellectual property rights or may use our intellectual property or proprietary information in a way that gives rise to actual or threatened litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to potential liability;
•disputes may arise between us and a collaborator that cause the delay or termination of the research, development or commercialization of our product candidates, or that result in costly litigation or arbitration that diverts management attention and resources;
•collaborations may be terminated and, if terminated, may result in a need for additional capital to pursue further development or commercialization of the applicable product candidates;
•if an agreement with any collaborator terminates, our access to technology and intellectual property licensed to us by that collaborator may be restricted or terminate entirely, which may delay our continued development of our product candidates using the collaborator’s technology or intellectual property or require us to stop development of those product candidates completely; and
•collaborators may own or co-own intellectual property covering our product candidates or technology that results from our collaborating with them, and in such cases, we may not have the exclusive right to commercialize such intellectual property.
As a result, if we enter into collaboration agreements and strategic partnerships or license our product candidates, we may not be able to realize the benefit of such transactions if we are unable to successfully integrate them with our existing operations and company culture, which could delay our timelines or otherwise adversely affect our business. Additionally, exclusive rights that we may grant in connection with collaboration agreements may limit our ability to enter into new or additional collaboration agreements or strategic partnerships if we experience issues with existing collaborations. We also cannot be certain that, following a strategic transaction or license, we will achieve the revenues or specific net income that justifies such transaction. Any delays in entering into new collaborations or strategic partnership agreements related to our product candidates could delay the development and commercialization of our product candidates in certain geographies for certain indications, which would harm our business prospects, financial condition and results of operations.
Any of these relationships may require us to incur non-recurring and other charges, increase our near and long-term expenditures, issue securities that dilute our existing stockholders or disrupt our management and business. In addition, we face significant competition in seeking appropriate strategic partners and the negotiation process is time-consuming and complex. Moreover, we may not be successful in our efforts to establish a strategic partnership or other alternative arrangements for our product candidates because they may be deemed to be at too early of a stage of development for collaborative effort and third parties may not view our product candidates as having the requisite potential to demonstrate safety and efficacy.
If conflicts arise between us and our collaborators or strategic partners, these parties may act in a manner adverse to us and could limit our ability to implement our strategies.
If conflicts arise between our corporate or academic collaborators or strategic partners and us, the other party may act in a manner adverse to us and could limit our ability to implement our strategies. Some of our existing academic collaborators and strategic partners are conducting multiple product development efforts. Such current or future collaborators or strategic partners could become our competitors in the future and could develop competing products, preclude us from entering into collaborations with their competitors, fail to obtain timely regulatory approvals, terminate their agreements with us prematurely or fail to devote sufficient resources to the commercialization of our approved product and the development and commercialization of our other product candidates. Competing product candidates, either developed by the collaborators or strategic partners or to which the collaborators or strategic partners have rights, may result in the withdrawal of our collaborator’s or partner’s support for our product candidates.
For example, in 2019, Sorrento with which we jointly established a new entity called Immunotherapy NANTibody, LLC as a stand-alone biotechnology company, commenced litigation against us and certain of our officers and directors, alleging that we improperly caused NANTibody to acquire IgDraSol. Additionally, in 2020, we received a Request for Arbitration before the International Chamber of Commerce, International Court of Arbitration, served by Shenzhen Beike Biotechnology Co. Ltd. asserting breach of contract under our subsidiary Altor’s license agreement with them. For more information regarding these disputes, see Note 7, Commitments and Contingencies—Litigation, of the “Notes to Condensed Consolidated Financial Statements” that appears in Part I, Item 1. “Financial Statements” of this Quarterly Report on Form 10-Q. Any of these developments could harm ouror product development efforts.candidates.Our use of joint ventures, strategic partnerships and alliances may expose us to risks associated with jointly owned investments.
We may operate parts of our business through joint ventures, strategic partnerships and/or alliances with other companies. While such arrangements may, in some cases, give us access to technologies that we may not otherwise have or may give us access to capital, they involve risks not otherwise present in our own investments, including: (i) we may not control the venture, and it may divert management time and resources; (ii) the partner(s) may not agree to distributions that we believe are appropriate; (iii) we may experience impasses or disputes with such partner(s) on certain decisions, which could require us to expend additional resources to resolve such impasses or disputes, including litigation or arbitration; (iv) our partner(s) may become insolvent or bankrupt, fail to fund their share of required capital contributions or fail to fulfil their obligations as a venture partner; (v) the arrangements governing these relationships may contain certain conditions or milestone events that may never be satisfied or achieved; (vi) our partner(s) may have business or economic interests that are inconsistent with our interests and may take actions contrary to our interests; (vii) we may suffer losses as a result of actions taken by the partner(s); and (viii) it may be difficult for us to exit if an impasse arises or if we desire to sell our interest for any reason. For example, in December 2021 we have established a joint venture relationship with Amyris. However, in August 2023, Amyris and thereannounced that it had filed for Chapter 11 bankruptcy
protection. As of March 31, 2024, the carrying amount of our equity investment in the joint venture was zero. There can be no guarantee that itthe strategic partnerships that we currently have or may enter into will be successful. In addition,Furthermore, we may, in certain circumstances, be liable for the actions of our partners. Any of the foregoing risks could have a material adverse effect on our business, financial condition and results of operations.
We are heavily dependent on our senior management, particularly Dr. Soon-Shiong, our Executive Chairman and Global Chief Scientific and Medical Officer, and a loss of a member of our senior management team in the future, even if only temporary, could harm our business.
Our operations will be dependent upon the services of our executives and our employees who are engaged in research and development. If we lose the services of members of our senior management, particularly Dr. Soon-Shiong, our Executive Chairman and Global Chief Scientific and Medical Officer, for a short or an extended time, for any reason, we may not be able to find appropriate replacements on a timely basis, and our business, financial condition and results of operations could be materially adversely affected. Our existing operations and our future development depend to a significant extent upon the performance and active participation of certain key individuals, particularly Dr. Soon-Shiong, our Executive Chairman and Global Chief Scientific and Medical Officer.Soon-Shiong. Although Dr. Soon-Shiong focuses heavily on our matters and is highly active in our management, he does devote a significant amount of his time to a number of different endeavors and companies, including NantHealth, Inc., NantMedia Holdings, LLC (which operates the Los Angeles Times) and NantWorks, which is a collection of multiple companies in the healthcare and technology space. The risks related to our dependence upon Dr. Soon-Shiong are particularly acute given his ownership percentage, the commercial and other relationships that we have with entities affiliated with him, his role in our company and his public reputation. We may also be dependent on additional funding from Dr. Soon-Shiong and his affiliates, which may not be available when needed and which he is under no obligation to provide.
To induce valuable employees to remain at our company, in addition to salary and cash incentives, we have provided, and plan to continue providing, equity incentive awards that vest over time. The value to employees of equity incentive awards that vest over time may be significantly affected by movements in our stock price that are beyond our control and may at any time be insufficient to counteract more lucrative offers from other companies. Despite our efforts to retain valuable employees, members of our management, scientific and development teams may terminate their employment with us on short notice. We do not have employment agreements with our NEOs and do not maintain “key man” insurance policies on the lives of these individuals ormost of the lives of anymembers of our other employees.management.
We will need to grow the size and capabilities of our organization, and we may experience difficulties in managing this growth.
Our future financial performance and our ability to commercialize our approved product and other product candidates will depend, in part, on our ability to effectively manage any future growth, and our management may also have to divert a disproportionate amount of their attention away from day-to-day activities in order to devote a substantial amount of time to managing these growth activities. In order to develop our business in accordance with our business plan, we will have to hire additional qualified personnel, including in the areas of research, manufacturing, clinical trials management, regulatory affairs, and sales and marketing. We are continuing our efforts to recruit and hire the necessary employees to support our planned operations in the near term. However, competition for qualified personnel in the biotechnology and pharmaceuticals industry is intense due to the limited number of individuals who possess the skills and experience required, and no assurance can be given that we will be able attract, hire, retain and motivate the highly skilled employees that we need, on acceptable terms or at all. Future growth will impose significant added responsibilities on members of management, including:
•identifying, recruiting, integrating, maintaining, and motivating additional employees;
•managing our internal development efforts effectively, including the clinical and FDA review process for our product candidates, while complying with our contractual obligations to contractors and other third parties; and
•improving our operational, financial and management controls, reporting systems, and procedures.
We currently rely, and for the foreseeable future we expect to rely, in substantial part, on certain independent organizations, advisors and consultants to provide certain services. There can be no assurance that the services of these independent organizations, advisors and consultants will continue to be available to us on a timely basis when needed, or that we can find qualified replacements on economically reasonable terms, or at all. In addition, if we are unable to effectively manage
our outsourced activities or if the quality, compliance or accuracy of the services provided by consultants is compromised for any reason, our clinical trials may be extended, delayed, or terminated, and we may not be able to obtain regulatory approval of our product candidates or otherwise advance our business.
If we are not able to effectively expand our organization by hiring new employees and expanding our groups of consultants and contractors, we may not be able to successfully implement the tasks necessary to further commercialize our product or develop and commercialize our product candidates and, accordingly, may not achieve our research, development, and commercialization goals on a timely basis, or at all.
If we engage in future acquisitions or strategic partnerships, this may increase our capital requirements, dilute our stockholders, cause us to incur debt or assume contingent liabilities, and subject us to other risks.
We may evaluate various acquisitions and strategic partnerships, including licensing or acquiring complementary products, intellectual property rights, technologies, or businesses. Any potential acquisition or strategic partnership may entail numerous risks, including:
•assimilation of operations, intellectual property, and products of an acquired company or product, including difficulties associated with integrating new personnel;
•the diversion of our managements’ attention from our existing product programs and initiatives in pursuing such a strategic merger or acquisition;
•retention of key employees, the loss of key personnel, and uncertainties in our ability to maintain key business relationships;
•significant upfront milestone and/or royalty payments from which we may not realize the anticipated benefits;
•risks and uncertainties associated with the other party to such a transaction, including the prospects of that party and their existing products or product candidates and regulatory approvals; and
•our inability to generate revenues from acquired technology and/or products sufficient to meet our objectives in undertaking the acquisition or even to offset the associated acquisition and maintenance costs.
Depending on the size and nature of future strategic acquisitions, we may acquire assets or businesses that require us to raise additional capital or to operate or manage businesses in which we have limited experience. Making larger acquisitions that require us to raise additional capital to fund the acquisition will expose us to the risks associated with capital raising activities. Acquiring and thereafter operating larger new businesses will also increase our management, operating and reporting costs and burdens (including increased cash requirements). In addition, if we undertake acquisitions, we may issue dilutive equity securities, assume or incur additional debt obligations or contingent liabilities, incur large one-time expenses and acquire intangible assets that could result in significant future amortization expense. Moreover, we may not be able to locate suitable acquisition opportunities and this inability could impair our ability to grow or obtain access to technology or products that may be important to the development of our business.
A variety of risks associated with marketing our approved product and other product candidates internationally could materially adversely affect our business.
We plan to seek regulatory approval of our approved product and other product candidates outside of the U.S. and, accordingly, we expect that we will be subject to additional risks related to operating in foreign countries if we obtain the necessary approvals, including:
•differing regulatory requirements in foreign countries;
•unexpected changes in tariffs, trade barriers, price and exchange controls and other regulatory requirements;
•economic weakness, including inflation, or political instability in particular foreign economies and markets;
•compliance with tax, employment, immigration and labor laws for employees living or traveling abroad;
•foreign taxes, including withholding of payroll taxes;
•foreign currency fluctuations, which could result in increased operating expenses and reduced revenues, and other obligations incident to doing business in another country;
•difficulties staffing and managing foreign operations;
•workforce uncertainty in countries where labor unrest is more common than in the U.S.;
•differing payor reimbursement regimes, governmental payors or patient self-pay systems, and price controls;
•potential liability under the Foreign Corrupt Practices Act (FCPA)FCPA or comparable foreign regulations;
•challenges enforcing our contractual and intellectual property rights, especially in those foreign countries that do not respect and protect intellectual property rights to the same extent as the U.S.;
•production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad;
•the impact of public health epidemics on the global economy, such as the coronavirus pandemic currently having an impact throughout the world;pandemic; and
•business interruptions resulting from geopolitical actions, including war and terrorism.
These and other risks associated with international operations may materially adversely affect our ability to attain or maintain profitable operations.
In particular, there is currently significant uncertainty about the future relationship between the U.S. and various other countries, most significantly China, with respect to trade policies, treaties, tariffs, taxes, and other limitations on cross-border operations. The U.S. government has made and continues to make significant additional changes in U.S. trade policy and may continue to take future actions that could negatively impact U.S. trade. For example, legislation has been introduced in Congress to limit certain U.S. biotechnology companies from using equipment or services produced or provided by select Chinese biotechnology companies, and others in Congress have advocated for the use of existing executive branch authorities to limit those Chinese service providers’ ability to engage in business in the U.S. We cannot predict what actions may ultimately be taken with respect to trade relations between the U.S. and China or other countries, what products and services may be subject to such actions or what actions may be taken by the other countries in retaliation. If we are unable to obtain or use services from existing service providers or become unable to export or sell our products to any of our customers or service providers, our business, liquidity, financial condition, and/or results of operations would be materially and adversely affected.
We are party to a public-private partnership regarding our manufacturing facility in Dunkirk, New York, and if we or our counterparties fail to meet the obligations of those agreements, it could materially impact our development, operations and prospects.
On February 14, 2022, we acquired a leasehold interest in the Dunkirk Facility from Athenex. The facility is expected to beAthenex, which we believe will provide us with a state-of-the-art biotech production center that we believe will substantially expand and diversify our existing manufacturing capacity in the U.S. and the ability to scale production associated with certain of our product candidates.
We paid approximately $40.0 million to Athenex, and the leasehold interest in the Dunkirk Facility was transferred to us. Our annual lease payment will be $2.00 per year for an initial 10-year term, with an option to renew the lease under substantially the same terms and conditions for an additional 10-year term. As part of the transaction, we assumed obligations under various third-party agreements, and committed to spend $1.52 billion on operational expenses during the initial term, and an additional $1.50 billion on operational expenses if we elect to renew the lease for the additional 10-year term. We also committed to hiring 450 employees at the Dunkirk Facility within the first five years of operations,following the Commencement Date, with 300 such employees to be hired within the first 2.5 years of operation.following the Commencement Date. We are eligible for certain sales-tax exemption savings during the development of the Dunkirk Facility, and certain property tax savings over the next 20 years, subject to certain terms and conditions, including performance of certain of the obligations described above.
In addition, we believe that the Dunkirk Facility has construction needs that may require approximately 12 to 18 months to complete in order for it to be used as intended.intended, and which needs remain as a result of an ongoing dispute with the Dunkirk Facility’s general contractor and stay related to Athenex’s ongoing bankruptcy proceedings, as described below. Consequently, during the third quarter of 2022, we determined to conduct a reduction-in-force of a significant portion of the then-current employees at the Dunkirk Facility, which became effective in late December 2022. The construction period and reduction-in-force may
have adversely affectaffected our ability to satisfy certain operational obligations described above. Inabove, including the initial employee count requirement, which was not timely satisfied, and in addition, while we believe we are in compliancehave complied with all applicable lawsfederal and agreementsstate laws implicated by the reduction-in-force, we could become subject to litigation in connection with these measures.
Failure to satisfy the obligations over the lease term, including the milestones we have committed to achieve, may give rise to certain rights and remedies of the lessor and other governmental authorities including, for example, termination of the lease agreement and other related agreements and potential recoupment of a percentage of the grant funding received by the SellerAthenex for construction of the Dunkirk Facility and other benefits received, subject to the terms and conditions of the applicable agreements. If we lose access to the Dunkirk Facility and related leased equipment, it could disrupt our operations and planned manufacturing activities, cause us to divert resources to finding alternative facilities, which would not have any subsidies, and could have a significant impact on our operations and financial performance. We may also be subject to lawsuits or claims for damages against us if we are unable to comply with our obligations under these arrangements or in connection with other aspects of the Dunkirk Facility, which could materially and adversely affect our business, results of operations and financial condition. For example, we were named as a defendant in a lawsuit filed during the fourth quarter of 2022 by Exyte, U.S., Inc. (Exyte)the general contractor for the Dunkirk Facility, in New York state court arising from a construction agreement Exyte entered with Athenex pertaining to construction of the Dunkirk Facility. We believe
we are entitled to defense costs and indemnification and, accordingly, we have provided notice to Athenex. On May 14, 2023, Athenex, together with certain of its subsidiaries, filed voluntary petitions for relief under Chapter 11 of the United States Bankruptcy Court for the Southern District of Texas. The lawsuit with Exyte has remained stayed as a result of Athenex’s bankruptcy proceedings and the construction needs of the Dunkirk Facility remain. The extent of the impact of the Athenex Proceedings and its automatic stay will have on any continuing obligations Athenex may have under the purchase agreement remain unclear. We further believe Exyte’s claims against us are without merit, and we intend to defend the claims vigorously. Furthermore, there is no guarantee that the counterparties to our public-private partnerships will comply with the terms of the agreements, including that their ability to fund their capital commitments under the agreements may be subject to their ability to raise additional capital and that further construction or operational timetables may not be met. Public-private partnerships are also subject to risks associated with government and government agency counterparties, including risks related to government relations compliance, sovereign immunity, shifts in the political environment, changing economic and legal conditions and social dynamics.
Our contractors and subcontractors may place liens on our projects, and if they then successfully foreclose on such projects, we may not be able to use such assets for our business.
Under general property law, any contractor or subcontractor doing work on a project may attach a lien on the property with respect to which it does work to secure the dollar value of all labor and material furnished to the project. A valid lien holder could, after the lien is perfected, institute a collection suit, according to the lien, and if it were successful in obtaining a judgment, the real property and the equipment thereon could be foreclosed upon. If a contractor were to successfully foreclose on such liens, we may not then be able to use such assets to manufacture our products, and our business could be materially harmed. For example, certain contractors have recently recorded liens related to tenant improvements that we have contracted to have installed at one of our leased properties.
Risks Related to Healthcare and Other Government Regulations
We may be unable to obtain U.S. or foreign regulatory approval and, as a result, be unable to commercialize our approved product or other product candidates. We are, and if we receive regulatory approval of our product candidates, will continue to be, subject to ongoing extensive regulation, regulatory obligations and continued regulatory review, which may result in significant additional expense.
Our approved product and other product candidates are subject to extensive governmental regulations relating to, among other things, the research, development, testing, manufacture, quality control, import, export, safety, effectiveness, labeling, packaging, storage, distribution, record keeping, approval, advertising, promotion, marketing, post-approval monitoring and post-approval reporting of drugs and therapeutic biologics. Rigorous preclinical testing and clinical trials and an extensive regulatory review process are required to be successfully completed in the U.S. and in many foreign jurisdictions before a new drug or therapeutic biologic can be marketed. Satisfaction of these and other regulatory requirements is costly, lengthy, time-consuming, uncertain and subject to unanticipated delays and can vary substantially based upon the type, complexity and novelty of the products involved. In May 2022,In October 2023, we announced the submissionresubmission of the BLA addressing the issues in the CRL, and that the FDA accepted our BLA resubmission for review and considered it as a BLAcomplete response to the CRL setting a new user fee goal date (PDUFA date) of April 23, 2024. On April 22, 2024, the FDA for our product candidate, Anktiva in combinationapproved ANKTIVA with BCG for the treatment of adult
patients with BCG-unresponsive NMIBC with CIS, with or without Ta or T1 disease. On May 9, 2023, the FDA deliveredpapillary tumors. We are required to comply with certain post-marketing commitments, including completion of our QUILT 3032 clinical trial and annual reporting for up to four years, with a CRL to us regarding the BLA, indicating that the FDA had determined that it cannot approve the BLA in its present form, and the FDA made recommendations to address the issues raised. The company has submitted a formal meeting request and the associated briefing bookfinal report submission to the FDA and plans to diligently address and resolveby the issues identified in the CRL and seek approval as expeditiously as possible. It is unclear when the FDA will approveend of 2029.
Other than our BLA, if at all. If the FDA requires additional data, finds that the CMC information in the BLA is deficient, disagrees with our interpretation or analysis of clinical data, identifies any deficiency in our clinical data, or finds deficiencies in our pre-approval inspection,approved product, we may fail to obtain approval of the BLA for our product candidate, Anktiva in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease, or approval may be delayed. The letter referenced above noted deficiencies related to the FDA’s pre-license inspection of the company’s third-party CMOs, among other items. Satisfactory resolution of the observations noted at the pre-license inspection is required before the BLA may be approved. The FDA further provided recommendations specific to additional CMC issues and assays to be resolved. We have not submitted any other marketing or drug approval applications to the FDA or comparable foreign authorities for any other product candidate, and we may never receive such regulatory approval for any of our other product candidates or regulatory approval that will allow us to successfully commercialize oursuch other product candidates. In addition, regulatory agencies may lack experience with our technologies and products, which may lengthen the regulatory review process, increase our development costs and delay or prevent their commercialization.
Regulatory authorities have substantial discretion in the approval process and may refuse to accept any application or may decide that our data are insufficient for approval and require additional preclinical studies, clinical trials or other research. The number and types of preclinical studies and clinical trials that will be required for regulatory approval also vary depending on the product candidate, the disease or condition that the product candidate is designed to address and the regulations applicable to any particular product candidate. Approval policies, regulations or the type and amount of clinical data necessary to gain approval may change during the course of a product candidate’s clinical development and may vary among jurisdictions and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of our other product candidates.
Any delay in completing development or obtaining, or failing to obtain, required approvals would have a material and adverse effect on our ability to generate revenue from the particular product candidate for which we are developing and seeking approval. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained, be subject to other regulatory enforcement action, and we may not achieve or sustain profitability.
Obtaining and maintaining regulatory approval of our approved product or other product candidates in one jurisdiction does not mean that we will be successful in obtaining regulatory approval of our other product candidates in other jurisdictions.
Obtaining and maintaining regulatory approval of our approved product or other product candidates in one jurisdiction does not guarantee that we will be able to obtain or maintain regulatory approval in any other jurisdiction, however a failure or delay in obtaining regulatory approval in one jurisdiction may have a negative effect on the regulatory review process in others. Approval policies, procedures and requirements may vary among jurisdictions and can involve requirements and administrative review periods different from, and greater than, those in the U.S., including additional preclinical studies or clinical trials as clinical trials conducted in one jurisdiction may not be accepted by regulatory authorities in other jurisdictions. For example, even if the FDA grants marketing approval of a product candidate, comparable regulatory authorities in foreign jurisdictions must also approve the manufacturing, marketing and promotion of the product candidate in those countries. In many jurisdictions outside the U.S., a product candidate must be approved for reimbursement before it can be approved for sale in that jurisdiction. In some cases, the price that we intend to charge for our product candidates is also subject to approval.
Obtaining foreign regulatory approvals and establishing and maintaining compliance with foreign regulatory requirements could result in significant delays, difficulties and costs for us and could delay or prevent the introduction of our approved product or other product candidates in certain countries. If we fail to comply with the regulatory requirements in international markets and/or fail to receive applicable marketing approvals, our target market will be reduced and our ability to realize the full market potential of our approved product or other product candidates will be harmed.
Even ifthough we receivehave received regulatory approval for our approved product, candidate, Anktiva in combination with BCG for the treatment of patients with NMIBC with CIS with or without Ta or T1 disease, or any other product candidates, theywe will continue to be subject to ongoing regulatory requirements concerning it and our other product candidates, which may result in significant additional expenses. Additionally, our other product candidates, if approved, could be subject to labeling and other restrictions, and we may be subject to penalties if we fail to comply with regulatory requirements or experience unanticipated problems with our approved product or other product candidates.
Any regulatory approvals that we receive for our product candidates may also be subject to limitations on the approved indicated uses for which the product may be marketed, or to conditions of approval, or contain requirements for potentially costly post-marketing testing, including Phase 4 clinical trials, and surveillance to monitor safety and efficacy. In addition, the manufacturing processes, labeling, packaging, distribution, adverse event reporting, storage, advertising, promotion and
recordkeeping for any approved product, including our current product, will be subject to extensive and ongoing regulatory requirements. These requirements include submissions of safety and other post-marketing information and reports, including reporting of certain adverse events as well as continued compliance with cGMP for the drug products, and GCP guidelines for any clinical trials that we conduct post-approval.
Later discovery of previously unknown problems with an approved product, including adverse events of unanticipated severity or frequency, or with manufacturing operations or processes, or failure to comply with regulatory requirements, may result in, among other things:
•holds on clinical trials;
•restrictions on the marketing or manufacturing of the product, withdrawal of the product from the market, or voluntary or mandatory product recalls;
•imposition of a REMS, which may include distribution or use restrictions;
•requirements to conduct additional post-market clinical trials to assess the safety of the product;
•revisions to the labeling, including limitation on approved uses or the addition of additional warnings, contraindications or other safety information, including boxed warnings;
•manufacturing delays and supply disruptions where regulatory inspections identify observations of noncompliance requiring remediation;
•fines, warning or untitled letters;
•refusal by the FDA to approve pending applications or supplements to approved applications submitted by us, or withdrawal of product approvals;
•product seizure or detention, or refusal to permit the import or export of product candidates; and
•injunctions or the imposition of civil or criminal penalties.
The FDA’s policies may change, and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of our product candidates. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the U.S. or abroad. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or are not able to maintain regulatory compliance, we may lose any marketing approval that may have been obtained and we may not achieve or sustain profitability, which would adversely affect our business.
If we are unable to establish sales, marketing and distribution capabilities, we may not be successful commercializing our approved product or our other product candidates if and when they are approved.
We are in the process of implementing our sales and marketing personnel hiring plan and building out key commercialization infrastructure.infrastructure for the commercialization of our approved product. To achieve commercial success for any other product for which we have obtainedobtain marketing approval, we willmay need to establish ahire additional sales and marketing team.personnel.
We expecthave built, and are continuing to build, a focused sales and marketing infrastructure to market our approved product candidate, Anktiva in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease, and potentially other product candidates in the U.S., if and when they are approved.approved, including by partnering with experienced third party contractors. There are risks involved with establishing our own sales, marketing and distribution capabilities and entering into arrangements with third parties to perform these services. For example, recruiting and training a sales force is expensive and time consuming and could delay any product launch. If the commercial launch of a product candidate for which we recruit a sales force and establish marketing capabilities is delayed or does not occur for any reason, including failure to receive marketing approval from the FDA, we would have prematurely or unnecessarily incurred these commercialization expenses. For example, we had previously hired sales and marketing personnel for a launch of our now-approved product, but we received a CRL from the FDA in May 2023. We may also inaccurately estimate the number of representatives needed to build our sales force, which may result in unnecessary expense or the inability to scale as quickly as needed. This may be costly, and our investment would be lost if we cannot retain or reposition our sales and marketing personnel.
Factors that may inhibit our efforts to commercialize our approved product and other product candidates, if approved, on our own include:
•our inability to recruit, train and retain adequate numbers of effective sales, marketing, reimbursement, customer service, medical affairs, and other support personnel;
•the inability of sales personnel to obtain access to physicians or increase market acceptableacceptance of our approved product;product or any other product candidate, if approved;
•the inability of reimbursement professionals to negotiate arrangements for coverage or adequate reimbursement by payors for our approved products;product or any other product candidate, if approved;
•the inability to price our approved product or any other product candidates, if approved, at a sufficient price point to ensure an adequate and attractive level of profitability;
•restricted or closed distribution channels that make it difficult to distribute our approved product or any other product candidates, if approved, to segments of the patient population; and
•unforeseen costs and expenses associated with creating an independent commercialization organization.
If we do not establish sales, marketing and distribution capabilities successfully, either on our own or in collaboration with third parties, we will not be successful in commercializing our approved product candidates.or any other product candidates, if approved.
Problems related to large-scale commercial manufacturing could cause delays in product launches, an increase in product costs, product recalls or shortages of product candidates.shortages.
Manufacturing finished drug products, especially in large quantities, is complex. IfOur product, and if our other product candidates receive regulatory approval, they will require several manufacturing steps and may involve complex techniques to ensure quality and sufficient quantity, especially as the manufacturing scale increases. Our approved product and other product candidates will need to be made consistently and in compliance with a clearly defined manufacturing process pursuant to FDA regulations. Accordingly, it will be essential to be able to validate and control the manufacturing process to assure that it is reproducible. Slight deviations anywhere in the manufacturing process, including obtaining materials, filling, labeling, packaging, storage, shipping, quality control and testing, may result in lot failures, delay in the release of lots, product recalls or spoilage. Success rates can vary dramatically at different stages of the manufacturing process, which can lower yields and increase costs. We may experience deviations in the manufacturing process that may take significant time and resources to resolve and, if unresolved, may affect manufacturing output and cause us to fail to satisfy contractual commitments, cause recalls, lead to delays in our clinical trials or result in litigation or regulatory action. Such actions would hinder our ability to meet contractual obligations and could cause material adverse consequences for our business.
If we fail to comply with U.S. and foreign regulatory requirements, regulatory authorities could limit or withdraw any marketing or commercialization approvals we have or may receive and subject us to other penalties that could materially harm our business. For example, our GMP-in-a-Box willmay be regulated by the FDA as a medical device, and regulatory compliance for medical devices is expensive, complex and uncertain, and a failure to comply could lead to enforcement actions against us and other negative consequences for our business.
The FDA and similar agencies regulate medical devices. All of our potential medical device products and material modifications will be subject to extensive regulation and clearance or approval from the FDA and non-U.S. regulatory agencies prior to commercial sale and distribution as well as after clearance or approval. Complying with these regulations is costly, time‑consuming, complex and uncertain. For instance, before a new medical device, or a new intended use for an existing device, can be marketed in the U.S., a company must first submit a pre-market submission, such as a pre-market notification (510(k)), De Novo request, or PMA, and receive either 510(k) clearance, De Novo grant, or pre-marketing approval from the FDA, unless an exemption applies.
Any regulatory approvals that we receive for our approved product and other product candidates will require surveillance to monitor the safety and efficacy of the product candidate.product. The FDA and similar agencies have significant pre- and post-market authority, including requirements related to product design, development, testing, laboratory and preclinical studies, clinical trials and preclinical studies approval, manufacturing processes and quality (including suppliers), labeling, packaging, distribution, adverse event and deviation reporting, storage, shipping, pre-market clearance or approval, advertising, marketing, promotion, sale, import, export, product change, recalls, submissions of safety and effectiveness, post-market surveillance and reporting of deaths or serious injuries and certain malfunctions, and other post-marketing information and reports such as deviation reports, registration, product listing, annual user fees, and recordkeeping for our product candidates. The FDA may also require a REMS to approve our product candidates, which may impose further requirements or restrictions on the distribution or use of an approved drug or therapeutic biologic. The FDA may also require post-approval Phase 4 trials. Moreover, the FDA and comparable foreign regulatory authorities will continue to closely monitor the safety profile of any product even after approval.
Medical devices regulated by the FDA are subject to general controls which include: registration with the FDA; listing commercially distributed products with the FDA; complying with cGMP under Quality System Regulation (QSR);QSR; filing reports with the FDA of and keeping records relative to certain types of adverse events associated with devices under the medical device reporting regulation; assuring that device labeling complies with device labeling requirements; and reporting certain device field removals and corrections to the FDA; and obtaining pre-market notification 510(k) clearance for devices prior to marketing. Some devices known as 510(k)-exempt devices can be marketed without prior marketing-clearance or approval from the FDA. In addition to the general controls, some Class 2 medical devices are also subject to special controls,controls. Most medical devices that require pre-market review by the FDA, including adherence tomost Class 2 medical devices, require the submission of a particular guidance document510(k) or a De Novo request and compliance with the performance standard. Instead of obtaining 510(k) clearance mostor De Novo grant prior to marketing the device. Some devices known as 510(k)-exempt devices can be marketed without prior clearance or approval from the FDA. Most Class 3 devices are subject to premarket approval (PMA).the FDA’s PMA requirement. Further, in February 2024, the FDA issued a final rule replacing the QSR with the QMSR, which incorporates by reference the quality management system requirements of ISO 13485:2016. The FDA has stated that the standards contained in ISO 13485:216 are substantially similar to those set forth in the existing QSR. This final rule does not go into effect until February 2026.
The FDA can also refuse to clear or approve pre-market applicationssubmissions for any medical device we develop. We may not be able to obtain the necessary clearances or approvals or may be unduly delayed in doing so, for any medical device products we develop, which could harm our business. Furthermore, even if we are granted regulatory clearances or approvals for any medical device products, they may include significant limitations on the indicated uses for the product, which may limit the market for the product.
In addition, we, our contractors, and our collaborators are and will remain responsible for FDA compliance. We and any of our collaborators, including our contract manufacturers, could be subject to periodic unannounced inspections by the FDA to monitor and ensure compliance with regulatory requirements. Application holders must further notify the FDA, and depending on the nature of the change, obtain FDA pre-approval for product and manufacturing changes. The cost of compliance with post-approval regulations may have a negative effect on our operating results and financial condition.
If the FDA or comparable foreign regulatory authorities become aware of new safety information or previously unknown problems after approval of our approved product or any of our other product candidates, including: (i) adverse events of unanticipated severity or frequency, (ii) that the product is less effective than previously thought, (iii) problems with our third-party manufacturers or manufacturing processes or (iv) failure to comply with regulatory requirements, or if we violate regulatory requirements at any stage, whether before or after marketing approval is obtained, we may face a number of regulatory consequences, including fines, warnings or untitled letters, holds on clinical trials, delay of approval or refusal by the FDA to approve pending applications or supplements to approved applications, suspension or withdrawal of regulatory approval, product recalls and seizures, administrative detention of products, refusal to permit the import or export of products, operating restrictions or partial suspension or total shutdown of production, injunctions, consent decrees, civil penalties and criminal prosecution, among other consequences. Additionally, we may face unanticipated expenditures to address or defend such actions and customer notifications for repair, replacement or refunds. Any such restrictions could limit sales of the product. Any of these events could further have other material and adverse effects on our operations and business and could adversely impact our stock price and could significantly harm our business, financial condition, results of operations, and prospects.
The FDA also regulates the advertising and promotion of medical devices to ensure that the claims are consistent with their regulatory clearances or approvals, that there are adequate and reasonable data to substantiate the claims and that the promotional labeling and advertising is neither false nor misleading in any respect. If the FDA determines that any of our advertising or promotional claims are misleading, not substantiated or not permissible, we may be subject to enforcement actions, including warning letters, and we may be required to revise our promotional claims and make other corrections or restitutions. Failure to comply with applicable U.S. requirements regarding, for example, promoting, manufacturing, or labeling our medical device products, may subject us to a variety of administrative or judicial actions and sanctions, such as Form 483 observations, warning letters, untitled letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties and criminal prosecution. If any of our medical device products cause or contribute to a death or a serious injury or malfunction in certain ways, we will be required to report under applicable medical device reporting regulations, which can result in voluntary corrective actions or agency enforcement actions.
If any of these events were to occur, it would have a material and adverse effect on our business, financial condition and results of operations.
The FDA and other regulatory agencies actively enforce the laws and regulations prohibiting pre-approval promotion and the promotion of off-label uses.
The FDA prohibits the pre-approval promotion of drugs as safe and effective for the purposes for which they are under investigation. Similarly, the FDA prohibits the promotion of approved drugs for new or unapproved indications. If the FDA finds that we have engaged in pre-approval promotion of our future product candidates, or if our approved product or any of our other product candidates are approved and we are found to have improperly promoted off-label uses of those products, we may become subject to significant liability. The FDA and other regulatory agencies strictly regulate the promotional claims that may be made about prescription products, such as our approved product and other product candidates, if approved. In particular, an approved product may not be promoted for uses that are not approved by the FDA or such other regulatory agencies as reflected in the product’s approved labeling. However, physicians may nevertheless prescribe our product, or any future approved product, to their patients in a manner that is inconsistent with the approved label, which is within their purview as part of their practice of medicine. If we are found to have promoted such off-label uses, however, we may become subject to significant liability. The U.S. federal government has levied large civil and criminal fines against companies for alleged improper promotion of off-label use and has enjoined several companies from engaging in off-label promotion. The FDA has also requested that companies enter into consent decrees or permanent injunctions under which specified promotional conduct is changed or curtailed. The FDA may also issue a public warning letter or untitled letter to the company. If we cannot successfully manage the promotion of our product or any future approved products, we could become subject to significant liability, which would materially adversely affect our business and financial condition.
Results for any patient who receives compassionate use access to any of our non-approved product candidates should not be viewed as representative of how the product candidate will perform in a well-controlled clinical trial and cannot be used to establish safety or efficacy for regulatory approval.
We often receive requests for compassionate use access to our investigational drugs by patients that do not meet the entry criteria for enrollment into our clinical trials. Generally, patients requesting compassionate use have no other treatment alternatives for life threateninglife-threatening conditions. We evaluate each compassionate use request on an individual basis, and in some cases grant access to our investigational product candidates outside of our sponsored clinical trials if a physician certifies that the patient receiving treatment is critically ill and does not meet the entry criteria for one of our open clinical trials. Individual patient results from compassionate use access may not be used to support submission of a regulatory application, may not support approval of a product candidate, and should not be considered to be indicative of results from any on-goingongoing or future well-controlled clinical trial. Before we can seek regulatory approval for any of our product candidates, we must demonstrate in well-controlled clinical trials statistically significant evidence that the product candidate is both safe and effective for the indication for which we are seeking approval. The results of our compassionate use program may not be used to establish safety or efficacy or regulatory approval.
We are and will be subject to U.S. and certain foreign export and import controls, sanctions, embargoes, anti-corruption laws and anti-money laundering laws and regulations. Compliance with these legal standards could impair our ability to compete in domestic and international markets. We can face criminal and/or civil liability and other serious consequences for violations, which can harm our business.
Our approved product and our other product candidates will be subject to export control and import laws and regulations, including the U.S. Export Administration Regulations, U.S. Customs regulations and various economic and trade sanctions regulations administered by the U.S. Treasury Department’s Office of Foreign Assets Controls,OFAC, the FCPA, the U.S. domestic bribery statute contained in 18 U.S.C. §201, the U.S. Travel Act, the USA PATRIOT Act and possibly other state and national anti-bribery and anti-money laundering laws in countries in which we conduct activities. Anti-corruption laws are interpreted broadly and prohibit companies and their employees, agents, third-party intermediaries, joint venture partners and collaborators from authorizing, promising, offering or providing, directly or indirectly, improper payments or benefits to recipients in the public or private sector. We use CROs abroad for clinical trials. In addition, we may engage third-party intermediaries to sell our approved product or other product candidates and solutions abroad once we enter a commercialization phase for our approved product or such other product candidates and/or to obtain necessary permits, licenses, and other regulatory approvals. We or our third-party intermediaries may have direct or indirect interactions with officials and employees of government agencies or state-owned or affiliated entities. We can be held liable for the corrupt or other illegal activities of these third-party intermediaries, our employees, representatives, contractors, partners and agents, even if we do not explicitly authorize or have actual knowledge of such activities. ifIf we fail to comply with these laws and regulations, we and certain of our employees could be subject to substantial civil or criminal fines and penalties, imprisonment, the loss of export or import privileges, debarment, tax reassessments, breach of contract and fraud litigation, reputational harm and other consequences.
We have adopted an anti-corruption policy, which mandates compliance with the FCPA and other anti-corruption laws applicable to our business throughout the world. However, there can be no assurance that our employees and third-party intermediaries will comply with this policy or such anti-corruption laws. Non-compliance with anti-corruption and anti-money laundering laws could subject us to whistleblower complaints, investigations, sanctions, settlements, prosecution, other investigations, or other enforcement actions. If such actions are launched, or governmental or other sanctions are imposed, or if we do not prevail in any possible civil or criminal litigation, our business, results of operations and financial condition could be materially harmed. In addition, responding to any action will likely result in a materially significant diversion of management’s attention and resources and significant defense and compliance costs and other professional fees. In certain cases, enforcement authorities may even cause us to appoint an independent compliance monitor, which can result in added costs and administrative burdens.
There is currently significant uncertainty about the future relationship between the U.S. and various other countries, most significantly China, with respect to trade policies, treaties, tariffs, taxes, and other limitations on cross-border operations. The U.S. government has made and continues to make significant additional changes in U.S. trade policy and may continue to take future actions that could negatively impact U.S. trade. For example, legislation has been introduced in Congress to limit certain U.S. biotechnology companies from using equipment or services produced or provided by select Chinese biotechnology companies, and others in Congress have advocated for the use of existing executive branch authorities to limit those Chinese service providers’ ability to engage in business in the U.S. We cannot predict what actions may ultimately be taken with respect to trade relations between the U.S. and China or other countries, what products and services may be subject to such actions or what actions may be taken by the other countries in retaliation. If we are unable to obtain or use services from existing service providers or become unable to export or sell our products to any of our customers, our business, liquidity, financial condition, and/or results of operations would be materially and adversely affected.
Our failure to comply with state, national and/or international data protectionprivacy and security laws and regulations could lead to government enforcement actions and significant penalties against us, and adversely impact our operating results.
There are numerous laws and legislative and regulatory initiativesregulations at the federal and state levels addressing privacy and security concerns, and some state privacy laws apply more broadly than the Health Insurance Portability and Accountability Act of 1996 (HIPAA)HIPAA and associated regulations. For example, California recently enacted legislation—the California Consumer Privacy Act of 2018 (CCPA)—CCPA, which went into effect on January 1, 2020. The CCPA,2020, provides, among other things, creates new data privacy and security obligations for covered companies and provides new privacy rights to California consumers, including the right to opt out of certain disclosuressales of their personal information. The CCPA also provides for civil penalties as well as a private right of action with statutory damages for certain data breaches, thereby potentially increasing risks associated with a data breach. Although the lawCCPA includes limited exceptions, including for certain personal
information collected as part of certain clinical trials as specified in the law,or other biomedical research studies, it may regulate or impact our processing of personal information depending on the context. Additionally, a new privacy law, the California Privacy Rights Act (CPRA),CPRA was approved by California voters in November 2020 and went into effect in most material respects on January 1, 2023.2020. The CPRA significantly modifiedmodifies the CCPA, which may require us to modify our practices and policies and may further increase our compliance costs and potential liability. Certain other statestates have also enacted or proposed privacy laws impose similar privacy obligations,governing health information, including for example, Washington’s My Health, My Data Act and Nevada’s Senate Bill 370, and all 50 states have enacted laws includingimposing obligations to provide notification of certain security breaches of computer databases that contain personal informationinformation. Additionally, several states have enacted or proposed laws similar to affected individuals, state officers and others. For example, the CCPA, has prompted the enactment of several new state laws or amendments of existing state laws, such as in New York, Nevada, Virginia, Colorado, Utah, Connecticut, Iowa, Indiana, Montana, Tennessee, Oregon, Florida, Delaware, and Colorado.Texas. These laws could mark the beginning of a further trend toward more stringent privacy legislationlaws in otherthe U.S. states and have prompted a number of proposals for new federal and state-level privacy legislation. Tolaws. We cannot yet determine the extentimpact these state laws as well as other federal and state privacy laws, including new laws andor changes in existing laws, apply tomay have on our business and operations, but anticipate they could increase our compliance costs and potential liability, with respectimpair our ability to collect, use or otherwise process personal information, we collect could expose us to greatgreater liability and increase compliance costs.require us to modify our practices and policies in an effort to comply.
There are also various laws and regulations in other jurisdictions relating to privacy and security. For example, European Union (EU)EU member states and other foreign jurisdictions, including the UK and Switzerland, have adopted data protection laws and regulations which impose significant compliance obligations on us. The collection, use, and useother processing of personal data, including patient or health data, in the EU, ismay be governed by the EU General Data Protection Regulation (GDPR).GDPR. The GDPR, which is wide-ranging in scope and applies extraterritorially, imposes, severalamong other things, requirements relating to the consent of the individuals to whom the personal data relates, the informationnotices provided to such individuals, the security and confidentiality of the personal data, data breach notification, the adoption of appropriate privacy governance, including policies, procedures, training and audits, and the use of third-party processors in connection with the processing of personal data. The GDPR also imposes strict rules on the transfer of personal data out of the EU, including to the U.S., provides andata protection authorities with enforcement authority and imposes large penalties for noncompliance, including the potential for fines of up to €20 million or up to 4% of the total worldwide annual global revenues of the noncompliant entity, whichever is greater. The GDPR requirements apply not only to third-party transactions,personal data transfers, but also to transfers of informationpersonal data between us and our subsidiaries, including employee information. In addition, in January 2021, following its exit from the EU, the UK transposed the GDPR into its domestic law with its own version of the GDPR (combining the GDPR and the UK Data Protection Act of 2018) (UK GDPR), which currently imposes the same obligations as the GDPR in most material respects and provides for fines of up £17.5 million or up to 4% of the total worldwide annual global revenues of the noncompliant entity, whichever is greater.
Complying with these numerous, complex, and often changing laws and regulations is expensive and difficult, anddifficult. Any actual or alleged failure to comply with any privacy laws or data security lawslaw or anyregulation, or security breach or other incident, or breachincluding those involving the misappropriation, loss, or other unauthorized processing, use, disclosure or disclosureother processing of sensitive or confidential patient, consumer or other personal information, whether by us, one of our CROs or business associates or another third party, could adversely affect our business, financial condition, and results of operations, including but not limited to: investigation costs;and could subject us to investigations, litigation, and other proceedings, material fines and penalties;penalties, compensatory, special, punitive and statutory damages; litigation;damages, consent orders regarding our privacy and security practices;practices, requirements that we provide notices, credit monitoring services and/or credit restoration services or other relevant services to impacted individuals;individuals, adverse actions against our licenses to do business;business, reputational damage;damage, and injunctive relief. The recent implementationenactment of, the CCPA, GDPR and UK GDPR haschanges to, privacy and security laws and regulations have increased our responsibility and potential liability, including in relation to the personal data that we process including inand our clinical trials, and we may in the future be required to put in place additional mechanisms in an effort to ensure compliancecomply with the CCPA, GDPR, UK GDPR and other applicable laws and regulations, which could divert management’s attention and increase our cost of doing business. In addition, any new law or regulation or legislative actions regarding datarelating to privacy and security, (together withor any applicable industry standards)standard, may increase our costs of doing business. In this regard, we expect that there will continue to be new proposed laws, regulations and industry standards relating to privacy and data protectionsecurity in the U.S., the United Kingdom,UK, the EU, and other jurisdictions, and we cannot determine the impact such future laws, regulations and standards may have on our business.
We cannot assure you that our CROs or other third-party service providers with access to our or our customers’, suppliers’, trial patients’ and employees’ personally identifiable andpersonal information or other sensitive or confidential information in relation to which we are responsible will not breach applicable laws or regulations or contractual obligations imposed by us, or that they will not experience data security breaches or incidents, which could have a corresponding effect on our business, including putting us in breach of our obligations under privacy and security laws and regulations and/or which could in turn adversely affect our business, results of operations and financial condition. We cannot assure you that our contractualthe measures and our own privacy and security-related safeguards we have taken will protect us from the foregoing risks, associated with the third-party processing, use, storage and transmissionany of such information. Any of the foregoingwhich could have a material adverse effect on our business, financial condition, results of operations and prospects.
We and our third-party contractors must comply with environmental, health and safety laws and regulations. A failure to comply with these laws and regulations could expose us to significant costs or liabilities.
We and any of our third-party contract manufacturers or suppliers are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, generation, manufacture, storage, treatment and disposal of hazardous materials and wastes. Hazardous chemicals, including flammable and biological materials, are involved in certain aspects of our business, and we cannot eliminate the risk of injury or contamination from the use, generation, manufacture, distribution, storage, handling, treatment or disposal of hazardous materials and wastes. In the event of contamination or injury, or failure to comply with such environmental, health and safety laws and regulations, we could be held liable for any resulting damages, fines and penalties associated with such liability, which could exceed our assets and resources.
Although we will maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of biological or hazardous materials or wastes arising out of and in the course of employment, this insurance may not provide adequate coverage against potential liabilities. We do not maintain comprehensive insurance coverage for liabilities arising from medical or hazardous materials, environmental liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of biological or hazardous materials.
Environmental, health and safety laws and regulations are becoming increasingly more stringent. We may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations may impair our research, development or production efforts, which could harm our business, prospects, financial condition or results of operations. Our failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions.
Coverage and reimbursement may be limited or unavailable in certain market segments for our approved product or other product candidates, which could make it difficult for us to sell our approved product or other product candidates profitably.
In both domestic and foreign markets, sales of our approved product or other product candidates, if approved, depend on the availability of coverage and adequate reimbursement from third-party payors. Third-party payors, whether domestic or foreign, or governmental or commercial, are developing increasingly sophisticated methods of controlling healthcare costs. Regulatory authorities and third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications, which could affect our ability or that of our collaborators to sell our product candidates profitably. In addition, third‑party payors are requiring higher levels of evidence of the benefits and clinical outcomes of new technologies and are challenging the prices charged. Patients who are provided medical treatment for their conditions generally rely on third-party payors to reimburse all or part of the costs associated with their treatment. Patients are unlikely to use our approved product or other product candidates unless coverage is provided, and reimbursement is adequate to cover a significant portion of the cost of our approved product or other product candidates. Such third-party payors include government health programs such as Medicare and Medicaid, managed care providers, private health insurers and other organizations. Obtaining coverage and adequate reimbursement from governmental healthcare programs, such as Medicare and Medicaid, and commercial payors is critical to new product acceptance. In addition, because our approved product and other product candidates represent new approaches to the treatment of cancer, we cannot accurately estimate the potential revenues from our approved product or other product candidates.
Government authorities and third-party payors decide which drugs and treatments they will cover and the amount of reimbursement. Coverage decisions may depend upon clinical and economic standards that disfavor new drug products when more established or lower cost therapeutic alternatives are already available or subsequently become available. These payors may not view our approved product or other future products, if any, as cost-effective, and coverage and reimbursement may not be available to our customers, or those of our collaborators, or may not be sufficient to allow our approved product or other future approved products, if any, to be marketed on a competitive basis. If reimbursement is not available, or is available only to limited levels, our product and other product candidates may be competitively disadvantaged, and we, or our collaborators, may not be able to successfully commercialize our approved product or other product candidates. Alternatively, securing favorable reimbursement terms may require us to compromise pricing and prevent us from realizing an adequate margin over cost.
Reimbursement by a third-party payor may depend upon a number of factors, including, but not limited to, the third-party payor’s determination that use of a product is:
•a covered benefit under its health plan;
•safe, effective and medically necessary;
•appropriate for the specific patient;
•cost-effective; and
•neither experimental nor investigational.
In the U.S., no uniform policy of coverage and reimbursement for products exists among third-party payors. Therefore, coverage and reimbursement for products can differ significantly from payor to payor. As a result, the coverage determination process is often a time-consuming and costly process that will require us to provide scientific and clinical support for the use of our approved product and our other product candidates, if approved, to each payor separately, with no assurance that coverage and adequate reimbursement will be obtained. Moreover, the factors noted above have continued to be the focus of policy and regulatory debate that has, thus far, shown the potential for movement towards permanent policy changes; this trend is likely to continue, and may result in more or less favorable impacts on pricing. The recent and ongoing series of congressional hearings relating to drug pricing has presented heightened attention to the biopharmaceutical industry, creating the potential for political and public pressure, while the potential for resulting legislative or policy changes presents uncertainty. Congress is consideringhas considered and may continue to consider legislation that, if passed, could have significant impact on prices of prescription drugs covered by Medicare, including limitations on drug price increases. The impact of these regulations and any future healthcare measures and agency rules implemented by the Biden administration on us and the pharmaceutical industry as a whole is currently unknown. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability, or commercialize our approved product and other product candidates if approved. Complying with any new legislation and regulatory changes could be time-intensive and expensive, resulting in a material adverse effect on our business.
Prices paid for a drug also vary depending on the class of trade. Prices charged to government customers are subject to price controls, including ceilings, and private institutions obtain discounts through group purchasing organizations. Net prices for drugs may be further reduced by mandatory discounts or rebates required by government healthcare programs and demanded by private payors. It is also not uncommon for market conditions to warrant multiple discounts to different customers on the same unit, such as purchase discounts to institutional care providers and rebates to the health plans that pay them, which reduces the net realization on the original sale.
In addition, federal programs impose penalties on manufacturers of drugs marketed under a BLA or NDA, in the form of mandatory additional rebates and/or discounts if commercial prices increase at a rate greater than the Consumer Price Index-Urban, and these rebates and/or discounts, which can be substantial, may impact our ability to raise commercial prices. For example, under the American Rescue Plan Act of 2021, effective January 1, 2024, the statutory cap on Medicaid Drug Rebate Program rebates that manufacturers pay to state Medicaid programs will behas been eliminated. Elimination of this cap may require pharmaceutical manufacturers to pay more in rebates than it receives on the sale of products, which could have a material impact on our business. In August 2022, Congress passed the Inflation Reduction Act of 2022,IRA, which includes prescription drug provisions that have significant implications for the pharmaceutical industry and Medicare beneficiaries, including allowing the federal government to negotiate a maximum fair price for certain high-priced single source Medicare drugs, imposing penalties and excise tax for manufacturers that fail to comply with the drug price negotiation requirements, requiring inflation rebates for all Medicare Part B and Part D drugs, with limited exceptions, if their drug prices increase faster than inflation, and redesigning Medicare Part D to reduce out-of-pocket prescription drug costs for beneficiaries, among other changes. Various industry stakeholders, including certain pharmaceutical companies and the Pharmaceutical Research and Manufacturers of America, have initiated lawsuits against the federal government asserting that the price negotiation provisions of the IRA are unconstitutional. The impact of these judicial challenges, legislative, executive, and administrative actions and any future healthcare measures and agency rules implemented by the Biden administration on us and the pharmaceutical industry as a whole is unclear. Cost control initiatives could cause us, or our collaborators, to decrease, discount, or rebate a portion of the price we, or they, might establish for products, which could result in lower than anticipated product revenues. If the realized prices for our product candidates, if any, decrease or if governmental and other third-party payors do not provide adequate coverage or reimbursement, our prospects for revenues and profitability will suffer.
Even if we obtain coverage for a given product, the resulting approved reimbursement payment rates might not be high enough to allow us to establish or maintain a market share sufficient to realize a sufficient return on our or their investments or achieve or sustain profitability or may require co-payments that patients find unacceptably high. If payors subject our product or other product candidates to maximum payment amounts or impose limitations that make it difficult to obtain reimbursement, providers may choose to use therapies which are less expensive when compared to our approved product or other product candidates. Additionally, if payors require high co-payments, beneficiaries may decline prescriptionsour therapies and seek alternative therapies. We may need to conduct post-marketing studies in order to demonstrate the cost-effectiveness of any future products to the satisfaction of hospitalsphysicians and other target customers and their third-party payors. Such studies might require us to commit a significant amount of management time and financial and other resources. Our future products might not ultimately be considered cost-effective. Adequate third-party coverage and reimbursement might not be available to enable us to maintain price levels sufficient to realize an appropriate return on investment in product development.
We, and our collaborators, cannot be sure that coverage will be available for our approved product or any other product candidate that we, or they, commercialize and, if available, that the reimbursement rates will be adequate. Further, the net reimbursement for drug products may be subject to additional reductions if there are changes to laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the U.S. An inability to promptly obtain coverage and adequate payment rates from both government-funded and private payors for any of our product candidates for which we obtain marketing approval could have a material adverse effect on our operating results, our ability to raise capital needed to commercialize products, and our overall financial condition.
There have been, and likely will continue to be, legislative and regulatory proposals at the federal and state levels directed at broadening the availability of healthcare and containing or lowering the cost of healthcare. We cannot predict the initiatives that may be adopted in the future. The continuing efforts of the government, insurance companies, managed care organizations and other payors of healthcare services to contain or reduce costs of healthcare and/or impose price controls may adversely affect:
•the demand for our approved product or other product candidates if we obtain regulatory approval;
•our ability to set a price that we believe is fair for our approved product and other product candidates;
•our ability to generate revenues and achieve or maintain profitability;
•the level of taxes that we are required to pay; and
•the availability of capital.
Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors, which may adversely affect our future profitability. A particularpossible challenge for our approved product and other product candidates arises from the fact that they will primarilymay potentially be used in an inpatient setting. Inpatient reimbursement generally relies on stringent packaging rules that may mean that there is no separate payment for our approved product or other product candidates. Additionally, data used to set the payment rates for inpatient admissions is usually several years old and would not take into account all of the additional therapy costs associated with the administration of our other product candidates. If special rules are not created for reimbursement for immunotherapy treatments such as our approved product or other product candidates, hospitals might not receive enough reimbursement to cover their costs of treatment, which will have a negative effect on their adoption of our approved product or other product candidates.
We may face difficulties from changes to current regulations and future legislation.
In the U.S. and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of our approved product or other product candidates, restrict or regulate post-approval activities, and affect our ability, or the ability of our collaborators, to profitably sell any products for which we obtain marketing approval. We expect that current laws, as well as other federal and state healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria, increased regulatory burdens and operating costs, decreased revenues from our biopharmaceutical product candidates, decreased potential returns from our development efforts, and additional downward pressure on the price that we, or our collaborators, may receive for any approved products.
Since enactment of the Affordable Care Act (ACA)ACA in 2010, in both the U.S. and certain foreign jurisdictions, there have been a number of legislative and regulatory changes to the health care system that could impact our ability to sell our approved product or other product candidates profitably. These changes included aggregate reductions of Medicare payments to providers of up to 2% per fiscal year, effective April 1, 2013, which, due to subsequent legislative amendments, will stay in effect through 2031,2032, with the exception of a temporary suspension implemented under various COVID‑19 relief legislation. The Consolidated Appropriations Act, 2023, extends the 2% Medicare sequester for the first six months of the fiscal year ending December 31, 2032 and revises the sequester percentage up to 2% for the fiscal years ending December 31, 2030 and 2031. In January 2013, the American Taxpayer Relief Act of 2012 (ATRA)ATRA was approved which, among other things, reduced Medicare payments to several providers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. These laws may result in additional reductions in Medicare and other healthcare funding, which could have a material adverse effect on customers for our approved product or other product candidates, if approved, and accordingly, our financial operations.
Since its enactment, various portions of the ACA have been subject to judicial and constitutional challenges. In June 2021, the U.S. Supreme Court held that Texas and other challengers had no legal standing to challenge the ACA, dismissing the case without specifically ruling on the constitutionality of the ACA. Accordingly, the ACA remains in effect in its current form. It is unclear how this Supreme Court decision, future litigation, or healthcare measures promulgated by the Biden administration will impact our business, financial condition and results of operations. Complying with any new legislation or reversing changes implemented under the ACA could be time-intensive and expensive, resulting in a material adverse effect on our business.
Any reduction in reimbursement from Medicare or other government healthcare programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenues, attain profitability or commercialize our approved product or other product candidates.
Legislative and regulatory proposals may also be made to expand post-approval requirements and restrict sales and promotional activities for drugs. We cannot be sure whether additional legislative changes will be enacted, or whether the FDA regulations, guidance, or interpretations will be changed, or what the impact of such changes on the marketing approvals of our approved product or other product candidates, if any, may be. In addition, increased scrutiny by Congress of the FDA’s approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent product labeling and post-marketing testing and other requirements. Further, if the Supreme Court reverses or curtails the Chevron doctrine, which gives deference to regulatory agencies in litigation against the FDA and other agencies, more companies may bring lawsuits against the FDA to challenge longstanding decisions and policies of the FDA, which could undermine the FDA’s authority, lead to uncertainties in the industry, and disrupt FDA’s normal operations, which could delay the FDA’s review of our marketing applications.
In addition, there have been increasing legislative efforts and enforcement interest in the U.S. with respect to drug pricing practices, including Congressional inquiries and proposed federal and state legislation designed to, among other things, bring more transparency to drug pricing, reduce the cost of prescription drugs under Medicare, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drugs. As discussed above, in August 2022, Congress passed the Inflation Reduction Act of 2022,IRA, which includes prescription drug provisions that have significant implications for the pharmaceutical industry and Medicare beneficiaries, including allowing the federal government to negotiate a maximum fair price for certain high-priced single source Medicare drugs, imposing penalties and excise tax for manufacturers that fail to comply with the drug price negotiation requirements, requiring inflation rebates for all Medicare Part B and Part D drugs, with limited exceptions, if their drug prices increase faster than inflation, and redesigning Medicare Part D to reduce out-of-pocket prescription drug costs for beneficiaries, among other changes. Various stakeholders have initiated lawsuits against the federal government asserting that the price negotiation provisions of the IRA are unconstitutional. The impact of these judicial challenges, legislative, executive, and administrative actions and any future healthcare measures and agency rules implemented by the Biden administration on us and the pharmaceutical industry as a whole is unclear. At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. For example, the FDA recently authorized the state of Florida to import certain prescription drugs from Canada for a period of two years to help reduce drug costs, provided that Florida’s Agency for Health Care Administration meets the requirements set forth by the FDA. Other states may follow Florida.
We are unable to predict the future course of federal or state healthcare legislation in the U.S. directed at broadening the availability of healthcare and containing or lowering the cost of healthcare. The ACA and any further changes in the law or regulatory framework that reduce our revenues or increase our costs could also have a material and adverse effect on our business, financial condition and results of operations. We expect that additional state and federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, which could result in reduced demand for our current product candidates and any future product candidates or additional pricing pressures.
Governments outside the U.S. tend to impose strict price controls, which may adversely affect our revenues, if any.
In international markets, reimbursement and health care payment systems vary significantly by country, and many countries have instituted price ceilings on specific products and therapies. In some countries, particularly the countries of the EU, the pricing of prescription pharmaceuticals is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a product. To obtain coverage and reimbursement or pricing approval in some countries, we may be required to conduct a clinical trial that compares the cost‑effectiveness of our product candidate to other available therapies. There can be no assurance that our product candidates will be considered cost-effective by third-party payors, that an adequate level of reimbursement will be available, or that the third-party payors’ reimbursement policies will not adversely affect our ability to sell our product candidates profitably. If reimbursement of our product or other product candidates is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, our business could be harmed, possibly materially.
Our employees, independent contractors, consultants, commercial partners, principal investigators, CROs, suppliers and vendors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements.
We are exposed to the risk of employee fraud or other illegal activity by our employees, independent contractors, consultants, commercial partners, principal investigators, CROs, suppliers and vendors. Misconduct by these parties could include intentional, reckless and/or negligent conduct that fails to: comply with the laws of the FDA and other similar foreign regulatory bodies, provide true, complete and accurate information to the FDA and other similar foreign regulatory bodies, comply with manufacturing standards we have established, comply with healthcare fraud and abuse laws in the U.S. and similar foreign fraudulent misconduct laws, or report financial information or data accurately or to disclose unauthorized activities to us. IfAs we obtain FDA approval of any ofbegin commercializing our approved product and may in the future commercialize our other product candidates, and begin commercializing those product candidatesif any, in the U.S., our potential exposure under such laws will increase significantly, and our costs associated with compliance with such laws are also likely to increase. In particular, the promotion, sales and marketing of healthcare items and services, as well as certain business arrangements in the healthcare industry, are subject to extensive laws and regulations designed to prevent fraud, kickbacks, self‑dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, structuring and commission(s), certain customer incentive programs and other business arrangements. Activities subject to these laws also involve the improper use of information obtained in the course of patient recruitment for clinical trials, which could result in regulatory sanctions and serious harm to our reputation.
It is not always possible to identify and deter misconduct or other improper activities by our employees or third parties that we engage for our business operations and the precautions we take to detect and prevent inappropriate conduct may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with such laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a material adverse effect on our business, financial condition, results of operations and prospects, including the imposition of significant fines or other sanctions, including exclusion from government healthcare programs, and serious harm to our reputation. In addition, the approval and commercialization of any of our product candidates outside the U.S. will also likely subject us to foreign equivalents of the healthcare laws mentioned above, among other foreign laws. Efforts to ensure that our business arrangements will comply with applicable healthcare laws may involve substantial costs.
Our relationships with health care professionals, institutional providers, principal investigators, consultants, potential customers and third-party payors are, and will continue to be, subject, directly and indirectly, to federal and state health care fraud and abuse, false claims, marketing expenditure tracking and disclosure, government price reporting, and privacy and data security laws. If we are unable to comply, or have not fully complied, with such laws, we could face significant penalties and liabilities.
Our business operations and activities may be directly or indirectly subject to various federal and state fraud and abuse laws, including, without limitation, the federal Anti-Kickback Statute and the federal False Claims Act. IfFCA. As we obtain FDA approval for any ofbegin commercializing our approved product and may in the future commercialize our other product candidates, and begin commercializing those product candidatesif any, in the U.S., our potential exposure under such laws will increase significantly, and our costs associated with compliance with such laws are also likely to increase. Our current and future arrangements with healthcare professionals, clinical investigators, CROs, third-party payors and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute our products for which we obtain marketing approval. In addition, we may be subject to laws of the federal government and state governments in which we conduct our business relating to privacy and data security with respect to patient information.or health data. The laws that may affect our ability to operate include, but are not limited to:
•the U.S. federal Anti-Kickback Statute, which prohibits, among other things, persons and entities from soliciting, receiving or providing remuneration, directly or indirectly, to induce either the referral of an individual for a healthcare item or service, or the purchasing or ordering of an item or service, for which payment may be made under a federal healthcare program such as Medicare or Medicaid;
•the U.S. federal false claims and civil monetary penalties laws, including the federal civil False Claims Act,FCA, which prohibit, among other things, individuals or entities from knowingly presenting or causing to be presented, claims for payment by government funded programs such as Medicare or Medicaid that are false or fraudulent, and which may apply to us by virtue of statements and representations made to customers or third parties;
•HIPAA, which created additional federal criminal statutes that prohibit, among other things, knowingly and willfully executing or attempting to execute a scheme to defraud healthcare programs;programs, as well as;
•HIPAA, as amended by the Health Information Technology for Economic and Clinical Health (HITECH) Act,HITECH, which imposes requirements on certain types of people and entities relating to the privacy, security, and transmission of individually identifiable Protected Health Information (PHI),PHI, and requires notification to affected individuals and regulatory authorities of certain breaches of the privacy or security of PHI;PHI, and other U.S. laws and foreign laws that govern the privacy or security of health or patient data;
•the federal Physician Payment Sunshine Act, which requires certain manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid, or the Children’s Health Insurance Program, to report annually to the Centers for Medicare & Medicaid Services (CMS)CMS information related to payments and other transfers of value to covered recipients, including physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors), other healthcare providers (such as physician assistants and nurse practitioners) and teaching hospitals, and ownership and investment interests held by physicians and their immediate family members, which is published in a searchable form on an annual basis;
•federal criminal laws that prohibit executing a scheme to defraud any healthcare benefit program or making, or causing to be made, false statements relating to healthcare matters;
•the federal Civil Monetary Penalties Law, which prohibits, among other things, offering or transferring remuneration to a federal healthcare beneficiary that a person knows or should know is likely to influence the beneficiary’s decision to order or receive items or services reimbursable by the government from a particular provider or supplier;
•the FCPA, the U.K. Bribery Act of 2010, and other local anti-corruption laws that apply to our international activities; and
•state laws comparable to each of the above federal laws, such as, for example, anti-kickback and false claims laws that may be broader in scope and also apply to commercial insurers and other non-federal payors, requirements for mandatory corporate regulatory compliance programs, and laws relating to patient or health data, privacy andor security. Other state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government;government, and require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures; and state and foreign laws govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.expenditures.
We expect to incur increased costs of compliance with such laws and regulations as they continue to evolve. If we or our contractors are unable to comply, or have not fully complied, with such laws, we could face penalties, including, without limitation, civil, criminal, and administrative penalties, damages, monetary fines, disgorgement, possible exclusion from participation in Medicare, Medicaid and other federal and state health care programs, contractual damages, reputational harm, diminished profits and future earnings, and curtailment or restructuring of our operations. Any of these could adversely affect our business, financial condition, and results of operations.
As we grow our business and expand our sales organization or rely on distributors outside of the U.S., we would be at increased risk of violating these laws or our internal policies and procedures. The risk of us being found in violation of these or other laws and regulations is further increased by the fact that many have not been fully interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations. Any action brought against us for violation of these or other laws or regulations, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business.
Disruptions at the FDA, the SEC and other government agencies caused by funding shortages could hinder their ability to hire and retain key leadership and other personnel, prevent new products from being developed or commercialized in a timely manner, or otherwise prevent those agencies from performing normal business functions, which could negatively impact our business and the approval of our future BLA submissions, as well as adversely affect the U.S. and global economy and our liquidity, financial condition and earnings.
The ability of the FDA to review and approve new products can be affected by a variety of factors, including government budget and funding levels and related government shutdowns, the ability to hire and retain key personnel and accept the payment of user fees, and statutory, regulatory, and policy changes. Average review times at the agency have fluctuated in recent years as a result. In addition, government funding of the SEC and other government agencies on which our operations may rely is subject to the impacts of political events, which are inherently fluid and unpredictable.
Disruptions at the FDA and other agencies, including disruptions due to public health concerns, resurgence of COVID-19 cases, travel restrictions, or staffing shortages, may slow the time necessary for new drugs to be reviewed and/or approved by necessary government agencies, which could adversely affect our business. For example, over the last several years, the U.S. government has shut down several times and certain regulatory agencies, such as the FDA and the SEC, have had to furlough critical FDA, SEC and other government employees and stop critical activities. If a prolonged government shutdown occurs in the future, including as a result of any failure by the U.S. federal government to increase the debt ceiling, it could significantly impact the ability of the FDA and the SEC to timely review and process our submissions, as well as cause interest rates and borrowing costs to further increase, which may negatively impact our ability to access the debt markets, including the corporate bond markets, on favorable terms, which could have a material adverse effect on our business, financial condition and results of operations and/or our BLA submissions.
Risks Related to Intellectual Property
If we are unable to obtain, maintain, protect and enforce patent protection and other proprietary rights for our approved product and other product candidates and technologies, we may not be able to compete effectively or operate profitably and may lose our ability to prevent our competitors from commercializing similar or identical technology and our approved product and other product candidates would be adversely affected.
Our success is dependent in large part on our obtaining, maintaining, protecting and enforcing patents and other proprietary rights in the U.S. and other countries with respect to our approved product and other product candidates and technology and on our ability to avoid infringing the intellectual property and other proprietary rights of others. Certain of our intellectual property rights are licensed from other entities, and as such the preparation and prosecution of any such patents and patent applications was not performed by us or under our control. Furthermore, patent law relating to the scope of claims in the biotechnology field in which we operate is still evolving and, consequently, patent positions in our industry may not be as strong as in other more well-established fields. The patent positions of biotechnology and pharmaceutical companies can be highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved and has been the subject of much litigation in recent years. No consistent policy regarding the breadth of claims allowed in biotechnology patents has emerged to date. As a result, the issuance, scope, validity, enforceability, or commercial value of our patent rights remain highly uncertain.
Any future patents we obtain may not be sufficiently broad to prevent others from using our technology or from developing competing therapeutics and technology. There is no guarantee that any of our pending patent applications will result in issued or granted patents, any of our issued or granted patents will not later be found to be invalid or unenforceable, or any issued or granted patents will include claims sufficiently broad to cover our product candidates and technology, or to provide meaningful protection from our competitors. Our owned or in-licensed pending and future patent applications may not result in patents being issued that protect our N-803,ANKTIVA, hAd5, saRNA hAd5 and yeast technologies and constructs, cell-based therapies, aldoxorubicin or other product candidates and technologies or that effectively prevent others from commercializing competitive technologies and product candidates.
Moreover, the coverage claimed in a patent application can be significantly reduced before the patent is issued, and its scope can be reinterpreted after issuance. Even if patent applications we license or own currently or in the future issue as patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors or other third parties from competing with us, or otherwise provide us with any competitive advantage. Any patents that we own or in-license may be challenged, narrowed, circumvented, or invalidated by third parties. Consequently, we do not know whether our N-803,ANKTIVA, hAd5, saRNA hAd5 and yeast technologies and constructs, cell-based therapies or other product candidates and technologies will be protectable or remain protected by valid and enforceable patents. Our competitors or other third parties may be able to circumvent our patents by developing similar or alternative technologies or products in a non-infringing manner which could materially adversely affect our business, financial condition, results of operations and growth prospects.
The issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability and it is uncertain how much protection, if any, will be provided by our patents, including if they are challenged in the courts or patent offices or in other proceedings, such as re-examinations or oppositions, which may be brought in the U.S. or foreign jurisdictions to challenge the validity of a patent. A third party may challenge the validity or enforceability of a patent after its issuance. It is possible that a competitor may successfully challenge our patents or that a challenge will result in limiting their coverage. Moreover, it is possible that competitors may infringe our patents or successfully avoid the patented technology through design innovation. To counter infringement or other unauthorized use, we may be required to file infringement claims, which can be expensive and time‑consuming, even if we were successful in stopping the violation of our patent rights.
We or our licensors may be subject to a third-party preissuance submission of prior art to the USPTO, or become involved in opposition, derivation, revocation, reexamination, post-grant and inter partes review, or interference proceedings or other similar proceedings challenging our owned or licensed patent rights. Should third parties file patent applications, or be issued patents claiming technology also used or claimed by our licensor(s) or by us in any future patent application, we, or one of our licensors, may be required to participate in interference proceedings in the USPTO to determine priority of invention for those patents or patent applications that are subject to the first-to-invent law in the U.S., or may be required to participate in derivation proceedings in the USPTO for those patents or patent applications that are subject to the first-inventor-to-file law in the U.S. We may be required to participate in such interference or derivation proceedings involving our issued patents and pending applications. We may also be required to participate in post-grant challenge proceedings, such as oppositions in a foreign patent office, which challenge our or our licensor’s priority of invention or other features of patentability with respect to our owned or in‑licensed patents and patent applications. Such challenges may result in loss of patent rights, loss of exclusivity, or in patent claims being narrowed, invalidated, or held unenforceable, which could limit our ability to stop others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection of our N-803,ANKTIVA, hAd5, saRNA hAd5 and yeast technologies and constructs, cell-based therapies or other product candidates and technologies. For example, the validity of one of our European patents, EP Patent No. 3601363, has recently been opposed by a third party.is being challenged in an opposition proceeding. This patent is directed to methods of using ANKTIVA-based combination therapy with anti-CD38 antibodies to treat cancer, which does not directly relate to any of our current programs. We intend to defend our patent and believe we have meritorious defenses against this opposition. An adverse determination in any such submission,of the type of submissions described above, proceeding or litigation could reduce the scope of, or invalidate or render unenforceable, our owned or in‑licensed patent rights, allow third parties to commercialize our N-803,ANKTIVA, hAd5, saRNA hAd5 and yeast technologies and constructs, cell-based therapies or other product candidates or technologies and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize products without infringing third-party patent rights.
If we or our collaborators are unsuccessful in any such proceeding or other priority or inventorship dispute, we may be required to cease using the technology or to obtain and maintain license rights from prevailing third parties, including parties involved in any such interference proceedings or other priority or inventorship disputes. A prevailing party in that case may not offer us a license on commercially acceptable terms or at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors and other third parties access to the same technologies licensed to us, and it could require us to make substantial licensing and royalty payments. If we are unable to obtain and maintain such licenses, we may need to cease the development, manufacture, and commercialization of one or more of the product candidates we may develop. The loss of exclusivity or the narrowing of our owned and licensed patent claims could limit our ability to stop others from using or commercializing similar or identical technology and products. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations and prospects.
In addition, given the amount of time required for the development, testing, and regulatory review of new product candidates, patents protecting such product candidates might expire before or shortly after such product candidates are commercialized. As a result, our intellectual property may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
Some of our owned and in-licensed patents and patent applications are, and may in the future be, co-owned with third parties. In addition, certain of our licensors co-own the patents and patent applications we in-license with other third parties with whom we do not have a direct relationship. Our exclusive rights to certain of these patents and patent applications are dependent, in part, on inter-institutional or other operating agreements between the joint owners of such patents and patent applications, who are not parties to our license agreements. If our licensors do not have exclusive control of the grant of licenses under any such third-party co-owners’ interest in such patents or patent applications or we are otherwise unable to secure such exclusive rights, such co-owners may be able to license their rights to other third parties, including our competitors, and our competitors could market competing products and technology. In addition, we may need the cooperation of any such co-owners of our patents in order to enforce such patents against third parties, and such cooperation may not be provided to us. Any of the foregoing could have a material adverse effect on our competitive position, business, financial conditions, results of operations and growth prospects.
If any of our owned or in-licensed patent applications do not issue as patents in any jurisdiction, we may not be able to compete effectively.
Changes in either the patent laws or their interpretation in the U.S. and other countries may diminish our ability to protect our inventions, obtain, maintain, and enforce our intellectual property rights and, more generally, could affect the value of our intellectual property or narrow the scope of our owned and licensed patents. With respect to both in-licensed and owned intellectual property, we cannot predict whether the patent applications we and our licensors are currently pursuing will issue as patents in any particular jurisdiction or whether the claims of any issued patents will provide sufficient protection from competitors or other third parties. The patent prosecution process is expensive, time-consuming, and complex, and we may not be able to file, prosecute, maintain, enforce, or license all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of our research and development output in time to obtain patent protection. Although we enter into nondisclosure and confidentiality agreements with parties who have access to confidential or patentable aspects of our research and development output, such as our employees, corporate collaborators, outside scientific collaborators, CROs, CMOs, consultants, advisors, and other third parties, any of these parties may breach the agreements and disclose such output before a patent application is filed, thereby jeopardizing our ability to seek patent protection. In addition, our ability to obtain and maintain valid and enforceable patents depends on whether the differences between our inventions and the prior art allow our inventions to be patentable over the prior art. Furthermore, publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the U.S. and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. Therefore, we cannot be certain that we or our licensors were the first to make the inventions claimed in any of our owned or licensed patents or pending patent applications, or that we or our licensors were the first to file for patent protection of such inventions.
We or our licensors, collaborators, or any future strategic partners may become subject to third-party claims or litigation alleging infringement of patents or other proprietary rights or seeking to invalidate patents or other proprietary rights, and we may need to resort to litigation to protect or enforce our patents or other intellectual property or the patents or other intellectual property of our licensors, all of which could be expensive, time-consuming and unsuccessful, may delay or prevent the development and commercialization of our product candidates, or may put our patents and other proprietary rights at risk.
If we or one of our licensors initiate legal proceedings against a third party to enforce a patent covering one of our product candidates or other technologies, the defendant could counterclaim that the patent is invalid and/or unenforceable or that we infringe their patents. In patent litigation in the U.S., defendant counterclaims alleging invalidity and/or unenforceability are commonplace, and there are numerous grounds upon which a third party can assert invalidity or unenforceability of a patent. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness, or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO or other applicable body, or made a misleading statement, during prosecution. Third parties may also raise similar claims before administrative bodies in the U.S. or abroad, even outside the context of litigation. Such mechanisms include re-examination, post grant review, inter partes review, interference proceedings, derivation proceedings, and equivalent proceedings in foreign jurisdictions (e.g., opposition proceedings).
With respect to the validity question, for example, we cannot be certain that there is no invalidating prior art, of which we, our licensor, our or our licensor’s patent counsel and the patent examiner were unaware during prosecution. Moreover, even if our patents were to survive such a litigation challenge to their validity, the patents might still be held to be valid but unenforceable if a court were to decide that the patents are being enforced in a manner inconsistent with the antitrust laws, or that the patents were obtained through deceit during patent office examination or other such failure of sufficient candor to the patent office. If a third party were to prevail on a legal assertion of invalidity and/or unenforceability, we would lose at least part, and perhaps all, of the patent protection on our product candidates. Such a loss of patent protection could have a material adverse impact on our business, financial condition, results of operations and prospects. OneThe validity of one of our European patents, EP Patent No. 3601363, has recently been opposed by a third party.is being challenged in an opposition proceeding. We intend to defend our patent and believe we have meritorious defenses against this opposition.
The cost to us of any litigation or other proceeding relating to intellectual property rights, even if resolved in our favor, could be substantial. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources, including our scientists and management, from our business.
An adverse result in any litigation or defense proceeding could put one or more of our owned or licensed patents at risk of being invalidated, held unenforceable, or interpreted narrowly, and could put our patent applications at risk of not being issued. Such proceedings could result in revocation or cancellation of, or amendment to, our patents in such a way that they no longer cover our product candidates or technologies. If the outcome of litigation is adverse to us, third parties may be able to use our patented invention without payment to us. In addition, in an infringement proceeding, there is a risk that a court may decide that one or more of our patents is not valid or is unenforceable and that we do not have the right to stop the other party from using the inventions. There is also the risk that, even if the validity of our patents were upheld, a court would refuse to stop the other party on the grounds that its activities are not covered by, that is, do not infringe, our patents. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation.
In addition, there could be public announcements of the results of hearings, motions, or other interim proceedings or developments, and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock. Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development activities or any future sales, marketing, or distribution activities. We may not have sufficient financial or other resources to conduct such litigation or proceedings adequately. Some of our competitors may be better able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources and more mature and developed intellectual property portfolios. The outcome following legal assertions of invalidity and unenforceability is unpredictable. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace.
The use of our technology and product or our other product candidates could potentially conflict with the rights of others, and third-party claims of intellectual property infringement, misappropriation or other violation against us, our licensors or our collaborators may prevent or delay the development and commercialization of our product, product candidates and technologies.
Our commercial success depends in part on our, our licensors’ and our collaborators’ ability to avoid infringing, misappropriating and otherwise violating the patents and other intellectual property rights of third parties. There is a substantial amount of complex litigation involving patents and other intellectual property rights in the biopharmaceutical industry. Our potential competitors or other parties may have, develop or acquire patent or other intellectual property rights that they could assert against us. If they do so, then we may be required to alter our approved product or other product candidates, pay licensing fees or cease our development and commercialization activities with respect to the applicable approved product or product candidates or technologies. If our approved product or other product candidates conflict with patent or other intellectual property rights of others, such parties could bring legal actions against us or our collaborators, licensees, suppliers or customers, claiming damages and seeking to enjoin manufacturing, use and marketing of the affected products.
Although we have conducted freedom-to-operate (FTO)FTO analyses of the patent landscape with respect to our leadapproved product or other product candidates and continue to undertake FTO analyses of our manufacturing processes, our product candidate, Anktiva in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease, and contemplated future processes and products,no FTO analysis can be considered exhausted because patent applications do not publish for 18 months and because the claims of patent applications can change over time, no FTO analysis can be considered exhaustive.time. We may not be aware of patents that have already been issued and that a competitor or other third party might assert are infringed by our currentapproved product or futureother product candidates or technologies. It is also possible that we could be found to have infringed patents owned by third parties of which we are aware, but which we do not believe are relevant to our approved product or other product candidates or technologies. In addition, because patent applications can take many years to issue, there may be currently pending patent applications that may later result in issued patents that our approved product or other product candidates or technologies may infringe. Furthermore, patent and other intellectual property rights in biotechnology remains an evolving area with many risks and uncertainties. As such, we may not be able to ensure that we can market our approved product or other product candidates without conflict with the rights of others.
If intellectual property-related legal actions asserted against us are successful, in addition to any potential liability for damages (including treble damages and attorneys’ fees for willful infringement), we could be enjoined from, or required to obtain a license to continue, manufacturing, promoting the use of or marketing the affected products. We may not prevail in any legal action and a required license under the applicable patent or other intellectual property may not be available on acceptable terms or at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors and other third parties access to the same technologies licensed to us, and it could require us to make substantial licensing and royalty payments. We also could be required to redesign our infringing products, which may be impossible or require substantial time and monetary expenditure.
Defense of infringement claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of management and other employee resources from our business, and may impact our reputation. Some of our competitors may be able to sustain the costs of litigation or administrative proceedings more effectively than we can because of greater financial resources. In addition, there could be public announcements of the results of hearings, motions, or other interim proceedings or developments, and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock. The occurrence of any of the foregoing could have a material adverse effect on our business, financial condition, results of operations and prospects.
Changes in U.S. patent law could diminish the value of patents in general, thereby impairing our ability to protect our approved product and other product candidates.
As is the case with other immunotherapy and biopharmaceutical companies, our success is dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the biopharmaceutical industry involves both technological and legal complexity, and is therefore costly, time-consuming and inherently uncertain. In addition, the U.S. has recently enacted and is currently implementing wide-ranging patent reform legislation. Assuming that other requirements for patentability are met, prior to March 2013, in the U.S., the first to invent the claimed invention was entitled to the patent, while outside the U.S., the first to file a patent application was entitled to the patent. After March 2013, under the Leahy-Smith America Invents Act (America Invents Act) enacted in September 2011, the U.S. transitioned to a first-to-file system in which, assuming that other requirements for patentability are met, the first inventor to file a patent application will be entitled to the patent on an invention regardless of whether a third party was the first to invent the claimed invention. A third party that files a patent application in the USPTO after March 2013, but before us could therefore be awarded a patent covering an invention of ours even if we had made the invention before it was made by such third party.party made it. This will require us to be cognizant of the time from invention to filing of a patent application. Since patent applications in the U.S. and most other countries are confidential for a period of time after filing or until issuance, we cannot be certain that we or our licensors were the first to either file any patent application related to our product candidates or other technologies or invent any of the inventions claimed in our or our licensor’s patents or patent applications. The America Invents Act also includes a number of significant changes that affect the way patent applications will be prosecuted and also may affect patent litigation. These include allowing third-party submission of prior art to the USPTO during patent prosecution and additional procedures to attack the validity of a patent by USPTO-administered post-grant proceedings, including post-grant review, inter partes review, and derivation proceedings. Therefore, the America Invents Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our owned or in-licensed patent applications and the enforcement or defense of our owned or in-licensed issued patents, all of which could have a material adverse effect on our business, financial condition, results of operations, and prospects.
Additionally, U.S. Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future. While we do not believe that any of the patents owned or licensed by us will be found invalid based on the foregoing, we cannot predict how future decisions by Congress, the federal courts or the USPTO may impact the value of our patents.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment, and other requirements imposed by government patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees, and various other government fees on patents and patent applications are due to be paid to the USPTO and foreign patent agencies in several stages over the lifetime of a patent. The USPTO and various foreign governmental patent agencies require compliance with several procedural, documentary, fee payment and other similar provisions during the patent application process. In certain circumstances, we rely on our licensors to pay these fees and take the necessary actions to comply with these requirements. While an inadvertent lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Noncompliance events that could result in abandonment or lapse of a patent or patent application include, but are not limited to, failure to respond to official actions within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents. In such an event, our competitors might be able to enter the market with similar or identical products or technology, which would have a material adverse impact on our business, financial condition, results of operations and prospects.
Our rights to develop and commercialize our product candidates and technologies are subject, in part, to the terms and conditions of licenses granted to us by others.
We will rely on licenses to certain patent rights and proprietary technology from third parties that are important or necessary to the development of aldoxorubicin as well as products enabled by our adenoviral, saRNA and yeast including Tarmogen,(including Tarmogen), vaccine technologies, and saRNA technology.technologies.
License agreements may not provide exclusive rights to use certain licensed intellectual property and technology in all relevant fields of use and in all territories in which we may wish to develop or commercialize our technology and product candidates in the future. As a result, we may not be able to prevent competitors or other third parties from developing and commercializing competitive products that also utilize technology that we have in-licensed.
In addition, subject to the terms of any such license agreements, we do not have the right to control the preparation, filing, prosecution and maintenance, and we may not have the right to control the enforcement, and defense of patents and patent applications covering the technology that we license from third parties. We cannot be certain that our in-licensed or out-licensed patents and patent applications that are controlled by our licensors or licensees will be prepared, filed, prosecuted, maintained, enforced, and defended in a manner consistent with the best interests of our business. If our licensors or licensees fail to prosecute, maintain, enforce, and defend such patents, or lose rights to those patents or patent applications, the rights we have licensed may be reduced or eliminated, our right to develop and commercialize N-803our approved product and any of our product candidates that are subject of such licensed rights could be adversely affected, and we may not be able to prevent competitors from making, using and selling competing products. In addition, even where we have the right to control patent prosecution of patents and patent applications we have licensed to and from third parties, we may still be adversely affected or prejudiced by actions or inactions of our licensees, our licensors and their counsel that took place prior to the date upon which we assumed control over patent prosecution.
Furthermore, our owned and in-licensed patents may be subject to a reservation of rights by one or more third parties. For example, certain of our in-licensed intellectual property was funded in part by the U.S. government. As a result, the U.S. government may have certain rights to such intellectual property. When new technologies are developed with U.S. government funding, the U.S. government generally obtains certain rights in any resulting patents, including a non-exclusive license authorizing the U.S. government to use the invention or to have others use the invention on its behalf. The U.S. government’s rights may also permit it to disclose the funded inventions and technology to third parties and to exercise march-in rights to use or allow third parties to use the technology we have licensed that was developed using U.S. government funding. The U.S. government may exercise its march-in rights if it determines that action is necessary because we fail to achieve practical application of the government-funded technology, or because action is necessary to alleviate health or safety needs, to meet requirements of federal regulations, or to give preference to U.S. industry. In addition, our rights in such inventions may be
subject to certain requirements to manufacture products embodying such inventions in the U.S. in certain circumstances if this requirement is not waived. Any exercise by the U.S. government of such rights or by any third party of its reserved rights could have a material adverse effect on our competitive position, business, financial condition, results of operations and growth prospects.
If we fail to comply with our obligations in the agreements under which we license intellectual property rights from third parties or otherwise experience disruptions to our business relationships with our licensors, we may be required to pay damages and we could lose license rights that are important to our business.
We have entered into license agreements with third parties and may need to obtain additional licenses from others to advance our research or allow commercialization of our product candidates. We may be unable to obtain certain additional licenses at a reasonable cost or on reasonable terms, if at all. In that event, we may be required to expend significant time and resources to redesign our technology, product, product candidates, or the methods for manufacturing them or to develop or license replacement technology, all of which may not be feasible on a technical or commercial basis. If we are unable to do so, we may be unable to develop or commercialize the affected product or product candidates or continue to utilize our existing technology, which could harm our business, financial condition, results of operations and growth prospects significantly. We cannot provide any assurances that third-party patents do not exist which might be enforced against our current technology, manufacturing methods, product, product candidates, or future methods or products resulting in either an injunction prohibiting our manufacture or future sales, or, with respect to our future sales, an obligation on our part to pay royalties and/or other forms of compensation to third parties, which could be significant.
In addition, each of our license agreements, and we expect our future agreements, will impose various development, diligence, commercialization, and other obligations on us. Certain of our license agreements also require us to meet development timelines, or to exercise commercially reasonable efforts to develop and commercialize licensed products, in order to maintain the licenses. In spite of our efforts, our licensors might conclude that we have materially breached our obligations under such license agreements and might therefore terminate the license agreements, thereby removing or limiting our ability to develop and commercialize products and technology covered by these license agreements. If these in-licenses are terminated, or if the underlying patents fail to provide the intended exclusivity, competitors or other third parties would have the freedom to seek regulatory approval of, and to market, products identical to ours and we may be required to cease our commercialization of our approved product or the development and commercialization of certain of our other product candidates or of N-803.candidates. Any of the foregoing could have a material adverse effect on our competitive position, business, financial conditions, results of operations and growth prospects.
Moreover, disputes may arise regarding intellectual property subject to a licensing agreement, including:
•the scope of rights granted under the license agreement and other interpretation-related issues;
•the extent to which our technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement;
•the sublicensing of patent and other rights under our collaborative development relationships;
•our diligence obligations under the license agreement and what activities satisfy those diligence obligations;
•the inventorship and ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and us and our partners; and
•the priority of invention of patented technology.
In addition, the agreements under which we currently license intellectual property or technology from third parties are complex, and certain provisions in such agreements may be susceptible to multiple interpretations. The resolution of any contract interpretation disagreement that may arise could narrow what we believe to be the scope of our rights to the relevant intellectual property or technology, or increase what we believe to be our financial or other obligations under the relevant agreement, either of which could have a material adverse effect on our business, financial condition, results of operations and growth prospects. Moreover, if disputes over intellectual property that we have licensed prevent or impair our ability to maintain our current licensing arrangements on commercially acceptable terms, we may be unable to successfully develop and commercialize the affected product or product candidates, which could have a material adverse effect on our business, financial conditions, results of operations and growth prospects.
We have limited foreign intellectual property rights and may not be able to protect our intellectual property rights in various jurisdictions throughout the world.
We have limited intellectual property rights outside the U.S. Filing, prosecuting and defending patents on our approved product and other product candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the U.S. can be less extensive than those in the U.S. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the U.S. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the U.S., or from selling or importing products made using our inventions in and into the U.S. or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and further, may export otherwise infringing products to territories where we have patent protection, but enforcement is not as strong as that in the U.S. These products may compete with our product or our other product candidates and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets and other intellectual property protection, particularly those relating to biopharmaceutical products, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate, and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
Many countries have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In addition, many countries limit the enforceability of patents against government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of such patent. If we or any of our licensors is forced to grant a license to third parties with respect to any patents relevant to our business, our competitive position may be impaired, and our business, financial condition, results of operations and prospects may be adversely affected.
We may be subject to claims that our employees, consultants or independent contractors have wrongfully used or disclosed trade secrets or other confidential information of third parties or claims asserting ownership of what we regard as our own intellectual property.
We have received confidential and proprietary information from third parties and their employees and contractors. In addition, we plan to employ and contract with individuals who were previously employed at other biotechnology or pharmaceutical companies. We may be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise used or disclosed the trade secrets or other confidential information of these third parties or our employees’ former employers. Litigation may be necessary to defend against or pursue these claims. Even if we are successful in resolving these claims, litigation could result in substantial cost and be a distraction to our management and employees.
In addition, while it is our policy to require our employees, consultants and independent contractors who may be involved in the conception or development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who, in fact, conceives or develops intellectual property that we regard as our own. The assignment of intellectual property rights may not be self-executing, or the assignment agreements may be breached, and we may be forced to bring claims against third parties, or defend claims that they may bring against us, to determine the ownership of what we regard as our intellectual property. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations, and prospects.
We may not be able to license or acquire new or necessary intellectual property rights or technology from third parties.
An element of our intellectual property strategy is to license intellectual property rights and technologies from third parties and/or our affiliates. Other parties, including our competitors or our affiliates, may have patents relevant to our business, may have already filed patent applications relevant to our business, and are likely filing patent applications potentially relevant to our business. In order to avoid infringing these patents, we may find it necessary or prudent to obtain licenses to such patents from such parties. In addition, with respect to any patents we co-own with other parties, including our affiliates, we may require licenses to such co-owners’ interest to such patents. The licensing or acquisition of intellectual property rights is a competitive area, and other more established companies may pursue strategies to license or acquire third-party intellectual property rights that we may consider attractive or necessary. These established companies may have a competitive advantage over us due to their size, capital resources, and greater clinical development and commercialization capabilities. In addition, companies that perceive us to be a competitor may be unwilling to assign or license rights to us. We also may be unable to license or acquire third-party intellectual property rights on terms that would allow us to make an appropriate return on our investment or at all. No assurance can be given that we will be successful in licensing any additional rights or technologies from third parties and/or our affiliates. Our inability to license the rights and technologies that we have identified, or that we may in the future identify, could have a material adverse impact on our ability to complete the development of our product candidates or to develop additional product candidates. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors and other third parties access to the same technologies licensed to us, and it could require us to make substantial licensing and royalty payments. Failure to obtain any necessary rights or licenses may detrimentally affect our planned development of our current or future additional product candidates and could increase the cost, and extend the timelines associated with our development, of such other products, and we may have to abandon development of the relevant program or product candidate. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations and prospects.
If we do not obtain patent term extension and data exclusivity for our approved product or any other product candidates we may develop, our business may be materially harmed.
Depending upon the timing, duration and specifics of any FDA marketing approval of any product candidates we may develop, including our approved product, one or more of our owned or in-licensed U.S. patents may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984 (the Hatch-Waxman Act).Act. The Hatch-Waxman Act permits a patent term extension of up to five years as compensation for patent term lost during the FDA regulatory review process. A patent term extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval. Only one patent may be extended per new drug, and only those claims covering the approved drug, a method for using it, or a method for manufacturing it may be extended. Similar extensions as compensation for patent term lost during regulatory review processes are also available in certain foreign countries and territories, such as in Europe under a Supplementary Patent Certificate. However, we may not be granted an extension in the U.S. and/or foreign countries and territories because of, for example, failing to exercise due diligence during the testing phase or regulatory review process, failing to apply within applicable deadlines, failing to apply prior to expiration of relevant patents, or otherwise failing to satisfy applicable requirements. Moreover, the applicable time period or the scope of patent protection afforded could be less than we request. If we are unable to obtain patent term extension or the term of any such extension is shorter than what we request, our competitors may obtain approval of competing products following our patent expiration, and our business, financial condition, results of operations and growth prospects could be materially harmed.
We may be subject to claims challenging rights in our patents and other intellectual property.
We or our licensors may be subject to claims that former employees, collaborators or other third parties have an interest in our owned or in-licensed patents, trade secrets, or other intellectual property, including as an inventor or co-inventor. For example, we or our licensors may have disputes arising from conflicting obligations of employees, consultants or others who are involved in developing our product candidates. Litigation may be necessary to defend against these and other claims challenging inventorship, or our or our licensors’ ownership of our owned or in-licensed patents, trade secrets or other intellectual property. If we or our licensors fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of or right to use valuable intellectual property. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations and growth prospects.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to seeking patents for N-803,ANKTIVA, hAd5, saRNA hAd5 and yeast technologies and constructs, cell therapies, and other product candidates and technologies, we also rely on trade secrets and confidentiality agreements to protect our unpatented know-how, technology, and other proprietary information and to maintain our competitive position. Trade secrets and know-how can be difficult to protect. We expect our trade secrets and know-how will over time be disseminated within the industry through independent development, the publication of journal articles describing the methodology, and the movement of personnel from academic to industry scientific positions.
We seek to protect these trade secrets and other proprietary technology, in part, by entering into nondisclosure and confidentiality agreements with parties who have access to them, such as our employees, corporate collaborators, outside scientific collaborators, CROs, CMOs, consultants, advisors, and other third parties. We also enter into confidentiality and invention or patent assignment agreements with our employees and consultants as well as train our employees not to bring or use proprietary information or technology from former employers to us or in their work and remind former employees when they leave their employment of their confidentiality obligations. We cannot guarantee that we have entered into such agreements with each party that may have or have had access to our trade secrets or proprietary technology and processes. Despite our efforts, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive, and time-consuming, and the outcome is unpredictable. In addition, some courts inside and outside the U.S. are less willing or unwilling to protect trade secrets. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor or other third party, we would have no right to prevent them from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed by a competitor or other third party, our competitive position would be materially and adversely harmed.
If our trademarks and trade names are not adequately protected, then we may not be able to build name recognition in our markets of interest and our business may be adversely affected.
Our registered or unregistered trademarks or trade names may be challenged, infringed, circumvented or declared generic or determined to be infringing on other marks. We may not be able to protect our rights to these trademarks and trade names, which we need to build name recognition among potential partners or customers in our markets of interest. At times, competitors or other third parties may adopt trade names or trademarks similar to ours, thereby impeding our ability to build brand identity and possibly leading to market confusion. In addition, there could be potential trade name or trademark infringement claims brought by owners of other registered trademarks or trademarks that incorporate variations of our registered or unregistered trademarks or trade names. Over the long term, if we are unable to establish name recognition based on our trademarks and trade names, then we may not be able to compete effectively, and our business may be adversely affected. Our efforts to enforce or protect our proprietary rights related to trademarks, trade secrets, domain names, copyrights or other intellectual property may be ineffective and could result in substantial costs and diversion of resources and could adversely affect our business, financial condition, results of operations and growth prospects.
Intellectual property rights do not necessarily address all potential threats.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations and may not adequately protect our business or permit us to maintain our competitive advantage. For example:
•others may be able to make products that are similar to our approved product or other product candidates or utilize similar technology but that are not covered by the claims of the patents that we license or may own;
•we, or our current or future licensors or collaborators, might not have been the first to make the inventions covered by the issued patent or pending patent application that we license or own now or in the future;
•we, or our current or future licensors or collaborators, might not have been the first to file patent applications covering certain of our or their inventions;
•others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our owned or licensed intellectual property rights;
•it is possible that our current or future pending owned or licensed patent applications will not lead to issued patents;
•issued patents that we hold rights to may be held invalid or unenforceable, including as a result of legal challenges by our competitors or other third parties;
•our competitors or other third parties might conduct research and development activities in countries where we do not have patent rights and then use the information learned from such activities to develop competitive products for sale in our major commercial markets;
•we may not develop additional proprietary technologies that are patentable;
•the patents of others may harm our business; and
•we may choose not to file a patent in order to maintain certain trade secrets or know-how, and a third party may subsequently file a patent covering such intellectual property.
Should any of these events occur, they could have a material adverse effect on our business, financial condition, results of operations and growth prospects.
Risks Related to Our Common Stock and CVRs
Dr. Soon-Shiong, our Executive Chairman, Global Chief Scientific and Medical Officer and our principal stockholder, has significant interests in other companies which may conflict with our interests.
Our Executive Chairman, Global Chief Scientific and Medical Officer and our principal stockholder, Dr. Soon-Shiong, is the founder of NantWorks. The various NantWorks companies are currently exploring opportunities in the immunotherapy, oncology, infectious disease and inflammatory disease fields. In particular, we have agreements with a number of related parties that provide services, technology and equipment for use in their efforts to develop their product pipelines. Dr. Soon-Shiong holds a controlling interest, either directly or indirectly, in these entities. Consequently, Dr. Soon-Shiong’s interests may not be aligned with our other stockholders, and he may from time to time be incentivized to take certain actions that benefit his other interests and that our other stockholders do not view as being in their interest as investors in our company. In addition, other companies affiliated with Dr. Soon-Shiong may compete with us for business opportunities or, in the future, develop products that are competitive with ours (including products in other therapeutic fields which we may target in the future). Moreover, even if they do not directly relate to us, actions taken by Dr. Soon-Shiong and the companies with which he is involved could impact us.
We are also pursuing supply arrangements for various investigational agents controlled by affiliates to be used in their clinical trials. If Dr. Soon-Shiong were to cease his affiliation with us or NantWorks, these entities may be unwilling to continue these relationships with us on commercially reasonable terms, or at all, and as a result may impede our ability to control the supply chain for our combination therapies. These collaboration agreements do not typically specify how sales will be apportioned between the parties upon successful commercialization of the product. As a result, we cannot guarantee that we will receive a percentage of the revenues that is at least proportional to the costs that we will incur in commercializing the product candidate.
We have entered into shared services agreements with NantWorks, pursuant to which NantWorks and its affiliates provide corporate, general and administrative and other support services to us. If Dr. Soon-Shiong was to cease his affiliation with us or with NantWorks, we may be unable to establish or maintain this relationship with NantWorks on a commercially reasonable basis, if at all. As a result, we could experience a lack of business continuity due to loss of historical and institutional knowledge and a lack of familiarity of new employees and/or new service providers with business processes, operating requirements, policies and procedures, and we may incur additional costs as new employees and/or service providers gain necessary experience. In addition, the loss of the services of NantWorks might significantly delay or prevent the commercialization of our approved product or the development of our other product candidates or achievement of other business objectives by diverting management’s attention to transition matters and identification of suitable replacements, if any, and could have a material adverse effect on our business and results of operations.
Dr. Soon-Shiong, through his voting control of the company, has the ability to control actions that require stockholder approval.
Dr. Soon-Shiong, through his direct and indirect ownership of the company’s common stock, has voting control of the company. As of June 30, 2023,March 31, 2024, Dr. Soon-Shiong and his affiliates own approximately 73.4%78.7% of the company’s common stock outstanding.
As of June 30, 2023, the company has a $300.0 million promissory note with an entity affiliated with Dr. Soon-Shiong that is due and payable on December 31, 2023. In the event of a default on the loan (as defined in the promissory note), including if the company does not repay the loan at maturity, the company has the right, at its sole option, to convert the outstanding principal amount and accrued and unpaid interest due under this note into shares of the company’s common stock at price of $5.67 per share.
Additionally, the March 31, 2023 $30.0 million promissory note and accrued interest may be converted into shares of the company’s common stock at a conversion price of $2.28 per share (subject to appropriate adjustment from time to time for any stock dividend, stock split, combination of shares, reorganization, recapitalization, reclassification or other similar event), at the option of the note holder.
Furthermore, entities affiliated with Dr. Soon-Shiong hold fixed-rate promissory notes representing $267.5 million in indebtedness (including principal and accrued and unpaid interest) as of June 30, 2023. The terms of these fixed-rate promissory notes were amended and restated to include a conversion feature that gives each lender, at its sole option, at any time (other than when the lender is in receipt of a written notice of prepayment from the borrower), the right to convert the entire outstanding principal amount and accrued and unpaid interest due under each note at the time of conversion into shares of the company’s common stock at a price of $5.67 per share. Upon receipt of a written notice of prepayment from the borrower, the lender has the right, at its option, to convert the outstanding principal amount to be prepaid and the accrued and unpaid interest thereon (as specified in the notice of prepayment) into shares of the company’s common stock at a price of $5.67 per share.
Dr. Soon-Shiong also has a total of 1,626,064 stock options outstanding as of June 30, 2023, of which 1,159,398 are exercisable and 466,666 are unvested and unexercisable.stock” below.
Dr. Soon-Shiong is in a position to control the outcome of corporate actions that require, or may be accomplished by, stockholder approval, including amending the bylaws of the company, the election or removal of directors and transactions involving a change of control. Dr. Soon-Shiong’s controlling ownership could limit the ability of the remaining stockholders of the company to influence corporate matters, and the interests of Dr. Soon-Shiong may not coincide with the company’s interests or the interests of its remaining stockholders.
In addition, pursuant to the Nominating Agreement between us and Cambridge, Equities, LP (Cambridge), an entity that Dr. Soon-Shiong controls, Cambridge has the ability to designate one director to be nominated for election to the Board of Directors for as long as Cambridge continues to hold at least 20% of the issued and outstanding shares of our common stock. Dr. Soon-Shiong was selected by Cambridge to hold this board seat. Dr. Soon-Shiong and his affiliates will therefore have significant influence over management and significant control over matters requiring stockholder approval, including the annual election of directors and significant corporate transactions, such as a merger or other sale of our company or its assets, for the foreseeable future. This control will limit stockholders’ ability to influence corporate matters and, as a result, we may take actions that our stockholders do not view as beneficial. As a result, the market price of our common stock could be adversely affected.
Conversion of certain related-party promissory notes, exercise of outstanding warrants and options to purchase our common stock, the achievement of the milestone under our outstanding CVRs, and potential additional equity issuances may dilute the ownership interest of existing stockholders or may otherwise depress the price of our common stock.
As of March 31, 2024, the company had outstanding promissory notes representing an aggregate of $610.0 million principal amount held by entities affiliated with Dr. Soon-Shiong that are convertible into shares of our common stock under certain circumstances, including the following:
•a $380.0 million principal amount of Tranche 2 of our convertible promissory note due December 31, 2025 bearing interest at 3-month Term SOFR plus 7.5% per annum, which provides that the noteholder has the sole option to convert all (but not less than all) of the outstanding principal amount and accrued but unpaid interest into shares of the company’s common stock at a conversion price of $8.2690 per share (subject to appropriate adjustment from time to time for any stock dividend, stock split, combination of shares, reorganization, recapitalization, reclassification or other similar event);
•a $200.0 million principal amount convertible promissory note due September 11, 2026 bearing interest at 1-month Term SOFR plus 8.0% per annum provides that the noteholder has the sole option to convert all (but not less than all) of the outstanding principal amount and accrued but unpaid interest into shares of the company’s common stock at a conversion price of $1.9350 per share (subject to appropriate adjustment from time to time for any stock dividend, stock split, combination of shares, reorganization, recapitalization, reclassification or other similar event); and
•a $30.0 million principal amount convertible promissory note due December 31, 2025 bearing interest at 3-month Term SOFR plus 8.0% per annum, which provides that the noteholder has the sole option to convert all (but not less than all) of the outstanding principal amount and accrued but unpaid interest into shares of the company’s common stock at a conversion price of $2.28 per share (subject to appropriate adjustment from time to time for any stock dividend, stock split, combination of shares, reorganization, recapitalization, reclassification or other similar event).
In addition, as of March 31, 2024, we had outstanding warrants, stock options and unvested RSU awards covering the issuance of up to:
•9,090,909 shares of our common stock at an exercise price of $6.60 per share, which are currently exercisable with an expiration date of December 12, 2024 (these warrants were issued to certain institutional investors);
•24,357,263 shares of our common stock at an exercise price of $3.2946 per share, which are currently exercisable with an expiration date of July 24, 2026 (these warrants were issued to certain institutional investors);
•any shares of our common stock that may be issued upon the exercise of the $10.0 million option held by Oberland, for which the price per share shall be determined by the 30-day trailing volume weighted-average price of our common stock, calculated from the date of exercise, and which option is exercisable by Oberland until the earliest of (i) December 29, 2028, (ii) a change of control of the company, or (iii) a sale of substantially all of the company’s assets;
•3,162,648 stock options and RSU awards issued to Dr. Soon-Shiong that are outstanding as of March 31, 2024, of which 1,392,730 are vested and exercisable and 1,769,918 are unvested and unexercisable; and
•1,638,000 shares of our common stock at an exercise price of $3.24 per share exercisable from the 30th day following the achievement of a performance-based vesting condition pertaining to building manufacturing capacity to support supply requirements for one of our product candidates (which has not yet been satisfied) with an expiration date on the tenth anniversary of such initial exercise date (this warrant was issued to an affiliate of Dr. Soon-Shiong).
In addition, as of March 31, 2024, we had outstanding an aggregate of approximately $300.6 million of CVRs issued to the former stockholders of Altor, including Dr. Soon-Shiong and certain affiliates, which such stockholders may choose to receive either in cash or shares of our common stock based upon an average of closing prices on a 20-trading day trailing period, upon the first calendar year prior to December 31, 2026 in which worldwide net sales of ANKTIVA exceed $1.0 billion. ANKTIVA is now approved for commercial sale with BCG for the treatment of adult patients with BCG-unresponsive NMIBC with CIS, with or without papillary tumors, but there can be no assurance that such sales milestone will be achieved. Dr. Soon-Shiong and his related party hold approximately $139.8 million of such CVRs, and have irrevocably agreed to receive shares of the company’s common stock in satisfaction of their CVRs.
The conversion or exchange of some or all of our outstanding promissory notes into shares of our common stock, the exercise of any of our outstanding warrants and stock options, and the decision of the holders of our CVRs to receive shares of our common stock could dilute the ownership interests of existing stockholders. Any sales in the public market of our outstanding promissory notes or warrants, or our common stock issuable upon conversion of the promissory notes or exercise of the warrants or options, could adversely affect prevailing market prices of our common stock.
The market price of our common stock has been and may continue to be volatile, and investors may have difficulty selling their shares.
Although our common stock is listed on the Nasdaq Global Select Market, the market for our shares has demonstrated varying levels of trading activity. You may not be able to sell your shares quickly or at the market price if trading in shares of our common stock is not active. Further, an inactive market may also impair our ability to raise capital by selling shares of our common stock and may impair our ability to enter into strategic partnerships or acquire companies or products by using our shares of common stock as consideration.
The stock market in general and the market for biopharmaceutical companies in particular have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. The market price of our common stock has been and may continue to be highly volatile and could be subject to wide fluctuations in response to various factors, some of which are beyond our control, including:
•the commencement, enrollment or results of the planned clinical trials of our non-FDA-approved product candidates or any future clinical trials we may conduct, or changes in the development status of oursuch product candidates;
•any delay in our regulatory filingssubmissions for our product candidates and any adverse development or perceived adverse development with respect to the applicable regulatory authority’s review of such filings,submissions, including without limitation the FDA’s issuance of a CRL or a “refusal to file” letter or a request for additional information;
•adverse results or delays in clinical trials;
•our decision to initiate a clinical trial, not to initiate a clinical trial or to terminate an existing clinical trial;
•adverse regulatory decisions, including failure to receive regulatory approval of our product candidates;
•changes in laws or regulations applicable to our products,approved product or other product candidates, including but not limited to clinical trial requirements for approvals;
•our failure to commercialize our approved product or other product candidates;
•additions or departures of key scientific or management personnel;
•unanticipated serious safety concerns related to the use of our approved product or other product candidates;
•announcements by us or our competitors of significant contracts, acquisitions, strategic partnerships, joint ventures or capital commitments;
•our ability to effectively manage our growth;
•variations in our quarterly operating results;results, including those driven by liability accounting associated with embedded derivatives;
•our liquidity position, RIPA liability covenants and the amount and nature of any debt we may incur;
•announcements that our revenue or income are below or that costs or losses are greater than analysts’ expectations;
•publication of research reports about us or our industry, or immunotherapy in particular, or positive or negative recommendations or withdrawal of research coverage by securities analysts;
•changes in the market valuations of similar companies;
•sales of large blocks of our common stock;
•fluctuations in stock market prices and volumes;
•disputes or other developments relating to proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our technologies;
•significant lawsuits, including patent or stockholder litigation;
•the perception of our clinical trial results by retail investors, which investors may be subject to the influence of information provided by third party investor websites and independent authors distributing information on the internet;
•general economic slowdowns;
•government-imposed lockdowns, supply chain disruptions, and adverse economic effects from the ongoing COVID-19a potential pandemic, epidemic, or outbreak of an infectious disease, in the U.S. and abroad;
•geopolitical tensions and war, including the war in Ukraine;Ukraine and ongoing conflicts in Gaza and Yemen;
•coordinated actions by independent third-party actors to affect the price of certain stocks, coordinated via the Internetinternet and otherwise; and
•other factors described in this “Risk Factors” section.
In the past, securities class action litigation has often been instituted against companies following periods of volatility in the market price of a company’s securities. This type of litigation could result in substantial costs and a diversion of management’s attention and resources, which would harm our business, operating results or financial condition.
We are currently subject to securities class action litigation and may be subject to similar or other litigation in the future, all of which will require significant management time and attention, result in significant legal expenses and may result in unfavorable outcomes, which may have a material adverse effect on our business, operating results and financial condition, and negatively affect the price of our common stock.
We are, and may in the future become, subject to various legal proceedings and claims that arise in or outside the ordinary course of business. For example, on June 30, 2023, a putative securities class action complaint, captioned Salzman v. ImmunityBio, Inc. et al., No. 3:23-cv-01216-BEN-WVG, was filed in the U.S. District Court for the Southern District of California against the Companycompany and three of its officers and/or directors, asserting violations of Sections 10(b) and 20(a) of the Exchange Act. StemmingAct stemming from the company’s disclosure on May 11, 2023 that it had received an FDA CRL stating, among other things, that it could not approve the company’s original BLA for its product candidate, Anktiva in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease,submission in its presentinitial form due to deficiencies related to its pre-license inspection of the company’s third-party CMOs, theCMOs. The complaint alleges that the defendants had previously made materially false and misleading statements and/or omitted material adverse facts regarding its third-party clinical manufacturing organizations and the prospects for regulatory approval of the BLA. On September 27, 2023, the court appointed a lead plaintiff, approved their selection of lead counsel, and re-captioned the case In re. ImmunityBio, Inc. Securities Litigation, No. 3:23-cv-01216. On November 17, 2023, lead plaintiff filed an amended complaint, which named the same defendants and asserted the same claims as the previous complaint. On January 8, 2024, defendants filed a motion to dismiss the amended complaint. A hearing on the motion is currently scheduled for May 31, 2024.
The results of the securities class action lawsuit, and any future legal proceedings cannot be predicted with certainty. Also, our insurance coverage may be insufficient, our assets may be insufficient to cover any amounts that exceed our insurance coverage, and we may have to pay damage awards or otherwise may enter into a settlement arrangement in connection with such claim. Any such payments or settlement arrangements in current or future litigation could have a material adverse effect on our business, operating results or financial condition. Even if the plaintiffs’ claims are not successful, current or future litigation could result in substantial costs and significantly and adversely impact our reputation and divert management’s attention and resources, which could have a material adverse effect on our business, operating results and financial condition, and negatively affect the price of our common stock. In addition, such lawsuits may make it more difficult to finance our operations.
Future sales and issuances of our common stock or rights to purchase common stock, including pursuant to our equity incentive plan, could result in additional dilution of the percentage ownership of our stockholders and could cause our stock price to fall.
Sales of a substantial number of shares of our common stock in the public market could occur at any time. If our stockholders sell, or the market perceives that our stockholders intend to sell substantial amounts of our common stock in the public market, including shares obtained from the conversion or exchange of our convertible promissory notes, exercise of our warrants, satisfaction of our CVRs, or the exercise or settlement of our equity incentive awards, the market price of our common stock could decline significantly. In addition, our Executive Chairman and Global Chief Scientific and Medical Officer, Dr. Soon-Shiong, and his affiliates currently ownowned approximately 73.4%78.7% of our outstanding shares of common stock as of June 30, 2023.March 31, 2024. Sales of stock by Dr. Soon-Shiong and his affiliates could have an adverse effect on the trading price of our common stock.
Certain holders of our common stock are entitled to certain rights with respect to the registration of their shares under the Securities Act.Act, including the shares purchased by affiliates of Oberland in connection with our entry into the RIPA. Registration of these shares under the Securities Act would result in the shares becoming freely tradable without restriction under the Securities Act, except for shares held by our affiliates as defined in Rule 144 under the Securities Act. Any sales of securities by these stockholders could have an adverse effect on the market price of our common stock.
In addition, we expect that additional capital may be needed in the future to continue our planned operations, including conducting clinical trials, regulatory approval efforts, pre-commercialization and commercialization activities, expanded research and development activities and costs associated with operating as a public company. To raise capital, we may sell common stock, including as part of ourthe ATM, convertible securities or other equity securities (including warrants) in one or more transactions at prices and in a manner we determine from time to time. If we sell common stock, convertible securities or other equity securities, existing investors may be materially diluted, and new investors could gain rights, preferences and privileges senior to the holders of our common stock. The issuance of additional shares of common stock or warrants to purchase common stock, perception that such issuances may occur, or the exercise of outstanding warrants or other equity securities will have a material dilutive impact on existing stockholders and could have a material negative effect on the market price of our common stock.
We have incurred and will continue to incur costs as a result of operating as a public company and our management has been and will be required to devote substantial time to compliance initiatives and corporate governance practices, including maintaining an effective system of internal control over financial reporting.
As a public company listed in the U.S., we have incurred and will continue to incur significant additional legal, accounting and other expenses as a result of operating as a public company. In addition, changing laws, regulations and standards relating to corporate governance and public disclosure, including the Sarbanes-Oxley Act of 2002 (Sarbanes-Oxley) and regulations implemented by the SEC and Nasdaq, may increase legal and financial compliance costs and make some activities more time consuming. These laws, regulations and standards are subject to varying interpretations and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. We intend to invest resources to create a larger finance function with additional personnel to comply with evolving laws, regulations and standards, and this investment will result in increased general and administrative expenses and a diversion of management’s time and attention from revenue-generating activities to compliance activities. If, notwithstanding our efforts to comply with new laws, regulations and standards, we fail to comply, regulatory authorities may initiate legal proceedings against us, and our business may be harmed.
As a public company in the U.S., we are required, pursuant to Section 404 of Sarbanes-Oxley (Section 404) to furnish a report by management on, among other things, the effectiveness of our internal control over financial reporting. The controls and other procedures are designed to ensure that information required to be disclosed by us in the reports that we file with the SEC is disclosed accurately and is recorded, processed, summarized and reported within the time periods specified in SEC rules and forms.
In the normal course of business our controls and procedures may become inadequate because of changes in conditions or the degree of compliance with these policies or procedures may deteriorate and material weaknesses in our internal control over financial reporting may be discovered. We may err in the design or operation of our controls, and all internal control systems, no matter how well designed and operated, can provide only reasonable assurance that the objectives of the control system are met. Because there are inherent limitations in all control systems, there can be no absolute assurance that all control issues have been or will be detected. If we are unable, or are perceived as unable, to produce reliable financial reports due to internal control deficiencies, investors could lose confidence in our reported financial information and operating results, which could result in a negative market reaction.
To fully comply with Section 404, we will need to retain additional employees to supplement our current finance staff, and we may not be able to do so in a timely manner, or at all. In addition, in the process of evaluating our internal control over financial reporting, we expect that certain of our internal control practices will need to be updated to comply with the requirements of Section 404 and the regulations promulgated thereunder, and we may not be able to do so on a timely basis, or at all. In the event that we are not able to demonstrate compliance with Section 404 in a timely manner, or are unable to produce timely or accurate financial statements, we may be subject to sanctions or investigations by regulatory authorities, such as the SEC or Nasdaq, and investors may lose confidence in our operating results and the price of our common stock could decline. Furthermore, if we are unable to certify that our internal control over financial reporting is effective and in compliance with Section 404, we may be subject to sanctions or investigations by regulatory authorities, such as the SEC or stock exchanges, and investors could lose confidence in the accuracy and completeness of our financial reports, which could hurt our business, the price of our common stock and our ability to access the capital markets.
Operating as a public company makes it more expensive for us to obtain directors’ and officers’ liability insurance, and we may be required to accept reduced coverage or incur substantially higher costs to obtain coverage. These factors could also make it more difficult for us to attract and retain qualified persons to serve on the Board of Directors, on committees of the Board of Directors, or as members of senior management.
If a restatement of our consolidated financial statements were to occur, our stockholders’ confidence in the company’s financial reporting in the future may be affected, which could in turn have a material adverse effect on our business and stock price.
If any material weaknesses in our internal control over financial reporting are discovered or occur in the future, our consolidated financial statements may contain material misstatements, and we could be required to restate our financial results. In addition, if we are unable to successfully remediate any future material weaknesses in our internal controls or if we are unable to produce accurate and timely financial statements, our stock price may be adversely affected, and we may be unable to maintain compliance with applicable stock exchange listing requirements.
We have not paid cash dividends in the past and do not expect to pay dividends in the future. Any return on investment may be limited to the value of our common stock.
We have never paid cash dividends on our common stock and do not anticipate paying cash dividends for the foreseeable future. The payment of dividends on our common stock will depend on earnings, financial condition and other business and economic factors affecting us at such time as the Board of Directors may consider relevant. If we do not pay dividends, our common stock may be less valuable because a return on your investment will only occur if our stock price appreciates.
Because we are relying on the exemptions from corporate governance requirements as a result of being a “controlled company” within the meaning of the Nasdaq listing standards, you do not have the same protections afforded to stockholders of companies that are subject to such requirements.
Our Executive Chairman and Global Chief Scientific and Medical Officer, Dr. Soon-Shiong, and entities affiliated with him, control a majority of our common stock. As a result, we are a “controlled company”controlled company within the meaning of the Nasdaq listing standards. Under these rules, a company of which more than 50% of the voting power is held by an individual, a group or another company is a “controlled company”controlled company and may elect not to comply with certain Nasdaq corporate governance requirements, including (1) the requirement that a majority of the Board of Directors consist of independent directors, and (2) the requirement that we have a Nominating and Corporate Governance Committee that is composed entirely of independent directors with a written charter addressing the committee’s purpose and responsibilities. Accordingly, you do not have the same protections afforded to stockholders of companies that are subject to all of the Nasdaq corporate governance requirements. However, our Board of Directors is currently comprised of a majority of independent directors, and we currently have a Nominating and Corporate Governance Committee and the majority of the members of such committee are independent directors.
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our share price and trading volume could decline.
The trading market for our common stock and the value of our warrants will depend on the research and reports that securities or industry analysts publish about us or our business. We do not have any control over these analysts. There can be no assurance that analysts will cover us or provide favorable coverage. If one or more of the analysts who cover us downgrade our stock or change their opinion of our stock, our share price would likely decline. If one or more of these analysts’ cease coverage of our company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which could cause our share price or trading volume to decline.
Holders of our CVRs that are payable contingent upon us achieving certain milestones may not receive any further consideration.
In connection with our 2017 acquisition of Altor, we issued CVRs under which we agreed to pay the prior stockholders of Altor approximately $304.0 million of contingent consideration upon the successful regulatory approval of a BLA by the FDA, or foreign equivalent, for N-803 by December 31, 2022, and approximately $304.0 million of contingent consideration upon calendar-year worldwide sales of N-803ANKTIVA exceeding $1.0 billion prior to December 31, 2026.
With respect to the regulatory milestone CVR agreement, in May 2022 we announced the submission of a BLA to the FDA ANKTIVA with BCG is now approved for our product candidate, Anktiva (N-803) in combination with BCGcommercial sale for the treatment of adult patients with BCG-unresponsive NMIBC with CIS, with or without Ta or T1 disease. On May 9, 2023, the FDA delivered a CRL to us regarding the BLA, indicating that the FDA had determined that it cannot approve the BLA in its present form, and the FDA made recommendations to address the issues raised. The company has submitted a formal meeting request and the associated briefing book to the FDA and plans to diligently address and resolve the issues identified in the CRL and seek approval as expeditiously as possible. It is unclear when the FDA will approve our BLA, if at all. The FDA did not approve our BLA on or before December 31, 2022, and therefore the regulatory milestone was not met, and the regulatory milestone CVR agreement terminated in accordance with its terms. With respect to the sales milestone CVR agreement, N-803 is not currently approved for commercial sale, andpapillary tumors, but there can be no assurance that such sales milestone will be achieved. Accordingly, holders of our CVRs that are payable contingent upon us achieving the aforementioned milestonesmilestone may not receive any further consideration.
We are not subject to the provisions of Section 203 of the Delaware General Corporation Law (DGCL),DGCL, which could negatively affect your investment.
We elected in our amendedAmended and restated certificateRestated Certificate of incorporationIncorporation to not be subject to the provisions of Section 203 of the DGCL. In general, Section 203 prohibits a publicly held Delaware corporation from engaging in a “business combination”business combination with an “interested stockholder”interested stockholder for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the business combination is approved in a prescribed manner. A “business combination”business combination includes a merger, asset sale or other transaction resulting in a financial benefit to the interested stockholder. An “interested stockholder”interested stockholder is a person who, together with affiliates and associates, owns (or, in certain cases, within three years prior, did own) 15% or more of the corporation’s voting stock. Our decision not to be subject to Section 203 will allow, for example, our Executive Chairman and Global Chief Scientific and Medical Officer (who, with members of his immediate family and entities affiliated with him, currently own,owned, in the aggregate, approximately 73.4%78.7% of our common stock as of June 30, 2023)March 31, 2024) to transfer shares in excess of 15% of our voting stock to a third-party free of the restrictions imposed by Section 203. This may make us more vulnerable to takeovers that are completed without the approval of our Board of Directors and/or without giving us the ability to prohibit or delay such takeovers as effectively.
Some provisions of our charter documents and Delaware law may have anti-takeover effects that could discourage an acquisition of us by others, even if an acquisition would be beneficial to our stockholders and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our Amended and Restated Certificate of Incorporation and Amended and Restated Bylaws, as well as provisions of Delaware law, could make it more difficult for a third party to acquire us or increase the cost of acquiring us, even if doing so would benefit our stockholders. These provisions include:
•a requirement that special meetings of stockholders be called only by the board of directors, president or chief executive officer;
•advance notice requirements for stockholder proposals and nominations for election to the board of directors; and
•the authority of the board of directors to issue preferred stock on terms determined by the board of directors without stockholder approval and which preferred stock may include rights superior to the rights of the holders of common stock.
These anti-takeover provisions and other provisions in our Amended and Restated Certificate of Incorporation and Amended and Restated Bylaws could make it more difficult for stockholders or potential acquirers to obtain control of our Board of Directors or initiate actions that are opposed by the then-current Board of Directors and could also delay or impede a merger, tender offer or proxy contest involving our company. These provisions could also discourage proxy contests and make it more difficult for you and other stockholders to elect directors of your choosing or cause us to take other corporate actions you desire. Any delay or prevention of a change of control transaction or changes in our Board of Directors could cause the market price of our common stock to decline.
Claims for indemnification by our directors and officers may reduce our available funds to satisfy successful third-party claims against us and may reduce the amount of money available to us.
Our Amended and Restated Certificate of Incorporation and Amended and Restated Bylaws provide that we will indemnify our directors and officers, in each case to the fullest extent permitted by Delaware law. In addition, as permitted by Section 145 of the DGCL, our Amended and Restated Bylaws and our indemnification agreements that we have entered into with our directors and officers provide that:
•We will indemnify our directors and officers for serving us in those capacities or for serving other business enterprises at our request, to the fullest extent permitted by Delaware law. Delaware law provides that a corporation may indemnify such person if such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to the best interests of the registrant and, with respect to any criminal proceeding, had no reasonable cause to believe such person’s conduct was unlawful.
•We may, in our discretion, indemnify employees and agents in those circumstances where indemnification is permitted by applicable law.
•We are required to advance expenses, as incurred, to our directors and officers in connection with defending a proceeding, except that such directors or officers shall undertake to repay such advances if it is ultimately determined that such person is not entitled to indemnification.
•We are not obligated pursuant to our Amended and Restated Bylaws to indemnify a person with respect to proceedings initiated by that person against us or our other indemnitees except with respect to proceedings authorized by our Board of Directors or brought to enforce a right to indemnification.
•The rights conferred in our Amended and Restated Bylaws are not exclusive, and we are authorized to enter into indemnification agreements with our directors, officers, employees and agents and to obtain insurance to indemnify such persons.
•We may not retroactively amend our bylaw provisions to reduce our indemnification obligations to directors, officers, employees and agents.
To the extent that a claim for indemnification is brought by any of our directors or officers, it would reduce the amount of funds available for use in our business.
ITEM 2. UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS.
(a) Recent Sales of Unregistered Securities
None.
(b) Issuer Purchases of Equity Securities
None.
ITEM 3. DEFAULTS UPON SENIOR SECURITIES.
None.
ITEM 4. MINE SAFETY DISCLOSURES.
Not applicable.
ITEM 5. OTHER INFORMATION.
None.
ITEM 6. EXHIBITS.
The documents listed below are incorporated by reference or are filed or furnished with this Quarterly Report, in each case as indicated therein (numbered in accordance with Item 601 of Regulation S‑K).
| | | | | | | | |
Exhibit
Number | | Description of Exhibit |
| | |
| 3.1 | | |
| | |
| 3.2 | | |
| | |
| 3.3 | | |
| | |
| 3.4 | | |
| | |
| 3.5 | | |
| | |
4.110.1* | | |
| | |
10.1 | | |
| | |
10.2* | | |
| | |
| 31.1* | | |
| | |
| 31.2* | | |
| | |
| 32.1** | | |
| | |
| 32.2** | | |
| | |
| 101.INS | | Inline XBRL Instance Document (the instance document does not appear in the
Interactive Data File because its XBRL tags are embedded within the Inline XBRL document). |
| | |
| 101.SCH | | Inline XBRL Taxonomy Extension Schema Document. |
| | |
| 101.CAL | | Inline XBRL Taxonomy Extension Calculation Linkbase Document. |
| | |
| 101.DEF | | Inline XBRL Taxonomy Extension Definition Linkbase Document. |
| | |
| 101.LAB | | Inline XBRL Taxonomy Extension Label Linkbase Document. |
| | |
| 101.PRE | | Inline XBRL Taxonomy Extension Presentation Linkbase Document. |
| | |
| 104 | | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101). |
_______________
| | | | | |
| ** | The certifications attached as Exhibits 32.1 and 32.2 that accompany this Quarterly Report on Form 10-Q are deemed furnished and not filed with the SEC and are not to be incorporated by reference into any filing of ImmunityBio, Inc. under the Securities Act or the Exchange Act, whether made before or after the date of this Quarterly Report, irrespective of any general incorporation language contained in such filing. |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| | | | | | | | |
| IMMUNITYBIO, INC. |
| Registrant |
| | |
Date: August 8, 2023May 9, 2024 | By: | /s/ Richard Adcock |
| | Richard Adcock |
| | Chief Executive Officer |
| | (Principal Executive Officer) |
| | |
Date: August 8, 2023May 9, 2024 | By: | /s/ David C. Sachs |
| | David C. Sachs |
| | Chief Financial Officer |
| | (Principal Financial Officer) |