Aviragen Therapeutics, Inc.
CondensedConsolidated Statements of Operations
(unaudited)(in millions, except share and per share amounts)
Three Months Ended | Six Months Ended | |||||||||||||||
2017 | 2016 | 2017 | 2016 | |||||||||||||
Revenue: | ||||||||||||||||
Royalty revenue | $ | - | $ | 1.5 | $ | - | $ | 1.6 | ||||||||
Non-cash royalty revenue related to the sale of future royalties | 2.7 | 2.3 | 2.8 | 2.3 | ||||||||||||
Total revenue | 2.7 | 3.8 | 2.8 | 3.9 | ||||||||||||
Operating expense: | ||||||||||||||||
Research and development | 2.5 | 10.2 | 5.3 | 17.8 | ||||||||||||
General and administrative | 3.1 | 2.1 | 5.4 | 4.3 | ||||||||||||
Foreign exchange (gain) loss, net | - | 0.1 | - | - | ||||||||||||
Total operating expense | 5.6 | 12.4 | 10.7 | 22.1 | ||||||||||||
Loss from operations | (2.9 | ) | (8.6 | ) | (7.9 | ) | (18.2 | ) | ||||||||
Other (expense) income: | ||||||||||||||||
Non-cash interest expense on liability related to sale of future royalties | (0.4 | ) | (0.5 | ) | (0.8 | ) | (0.9 | ) | ||||||||
Interest income | - | 0.1 | 0.1 | 0.1 | ||||||||||||
Total other (expense) income | (0.4 | ) | (0.4 | ) | (0.7 | ) | (0.8 | ) | ||||||||
Loss before tax | (3.3 | ) | (9.0 | ) | (8.6 | ) | (19.0 | ) | ||||||||
Income tax expense | 0.1 | 0.1 | 0.1 | 0.1 | ||||||||||||
Net loss | $ | (3.4 | ) | $ | (9.1 | ) | $ | (8.7 | ) | $ | (19.1 | ) | ||||
Basic and diluted net loss per share | $ | (0.09 | ) | $ | (0.24 | ) | $ | (0.23 | ) | $ | (0.49 | ) | ||||
Basic and diluted weighted-average shares outstanding | 38,649,237 | 38,640,487 | 38,649,237 | 38,640,487 | ||||||||||||
The accompanying notes are an integral part of the condensed consolidated financial statements.
Aviragen Therapeutics, Inc.VAXART, INC.
CondensedConsolidatedStatement Statements of Stockholders’ Equity(unaudited)
Cash Flows
(in millions, except for share amounts)In thousands)
(Unaudited)
Common Stock | Accumulated | |||||||||||||||||||||||
Shares | Amount | Additional Paid-in Capital | Accumulated Deficit | Other Comprehensive Income | Total Stockholders’ Equity | |||||||||||||||||||
Balances at June 30, 2017 | 38,649,237 | $ | 3.9 | $ | 159.6 | $ | (163.8 | ) | $ | 19.0 | $ | 18.7 | ||||||||||||
Net loss | - | - | - | (8.7 | ) | - | (8.7 | ) | ||||||||||||||||
Share-based compensation | - | - | 0.8 | - | - | 0.8 | ||||||||||||||||||
Balances at December 31, 2017 | 38,649,237 | $ | 3.9 | $ | 160.4 | $ | (172.5 | ) | $ | 19.0 | $ | 10.8 | ||||||||||||
Nine Months Ended September 30, | ||||||||
2022 | 2021 | |||||||
Cash flows from operating activities: | ||||||||
Net loss | $ | (83,840 | ) | $ | (49,706 | ) | ||
Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
Depreciation and amortization | 3,915 | 3,010 | ||||||
Accretion of (discount) premium on investments | (13 | ) | 55 | |||||
Stock-based compensation | 10,194 | 5,954 | ||||||
Non-cash interest expense related to sale of future royalties | 988 | 1,137 | ||||||
Non-cash revenue related to sale of future royalties | (152 | ) | (908 | ) | ||||
Change in operating assets and liabilities: | ||||||||
Accounts receivable | 71 | 144 | ||||||
Prepaid expenses and other assets | (4,037 | ) | (3,570 | ) | ||||
Accounts payable | 2,528 | 1,483 | ||||||
Other accrued liabilities | 4,719 | (1,617 | ) | |||||
Net cash used in operating activities | (65,627 | ) | (44,018 | ) | ||||
Cash flows from investing activities: | ||||||||
Purchase of property and equipment | (5,700 | ) | (4,142 | ) | ||||
Cash paid for right-of-use assets | (5,038 | ) | — | |||||
Purchases of investments | (48,178 | ) | (41,278 | ) | ||||
Proceeds from maturities of investments | 22,700 | 4,500 | ||||||
Net cash used in investing activities | (36,216 | ) | (40,920 | ) | ||||
Cash flows from financing activities: | ||||||||
Net proceeds from issuance of common stock through ATM facilities | 8,650 | 122,210 | ||||||
Proceeds from issuance of common stock upon exercise of warrants | 2 | 1,849 | ||||||
Proceeds from issuance of common stock upon exercise of stock options | 214 | 1,240 | ||||||
Net cash provided by financing activities | 8,866 | 125,299 | ||||||
Net (decrease) increase in cash and cash equivalents | (92,977 | ) | 40,361 | |||||
Cash and cash equivalents at beginning of the period | 143,745 | 126,870 | ||||||
Cash and cash equivalents at end of the period | $ | 50,768 | $ | 167,231 | ||||
Supplemental disclosure of non-cash investing and financing activity: | ||||||||
Operating lease liabilities arising from obtaining right-of-use assets | $ | 9,997 | $ | 6,939 | ||||
Lease-related assets and liabilities derecognized on early termination and modification of leases | $ | — | $ | 235 | ||||
Acquisition of property and equipment included in accounts payable and accrued expenses | $ | 1,738 | $ | 289 |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
Notes to the Condensed Consolidated Financial Statements (Unaudited)
NOTE 1. Organization and Basis of Presentation
General
Vaxart Biosciences, Inc. was originally incorporated in California in March 2004, under the name West Coast Biologicals, Inc. The Company changed its name to Vaxart, Inc. (“Private Vaxart”) in July 2007, and reincorporated in the state of Delaware. On February 13, 2018, Private Vaxart completed a business combination with Aviragen Therapeutics, Inc.
Condensed Consolidated Statements (“Aviragen”), pursuant to which Aviragen merged with Private Vaxart, with Private Vaxart surviving as a wholly owned subsidiary of Cash Flows
(unaudited)(in millions)Aviragen (the “Merger”). Pursuant to the terms of the Merger, Aviragen changed its name to Vaxart, Inc. (together with its subsidiaries, the “Company” or “Vaxart”) and Private Vaxart changed its name to Vaxart Biosciences, Inc.
On October 13, 2020, the Company entered into the Open Market Sale Agreement (the “October 2020 ATM”), pursuant to which it could offer and sell, from time to time through sales agents, shares of its common stock having an aggregate offering price of up to $250 million. The Company incurred direct expenses of approximately $0.3 million in connection with filing a prospectus supplement, dated October 13, 2020, with the U.S. Securities and Exchange Commission (the “SEC”), and paid sales commissions of up to 4.5% of gross proceeds from the sale of shares. As of December 31, 2020, the Company had sold 692,651 shares for gross proceeds of $5.5 million which, after deducting sales commissions and expenses, resulted in net proceeds under the October 2020 ATM of $4.9 million in 2020.
Six Months Ended | ||||||||
2017 | 2016 | |||||||
Cash flows from operating activities: | ||||||||
Net loss | $ | (8.7 | ) | $ | (19.1 | ) | ||
Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
Share-based compensation | 0.8 | 0.9 | ||||||
Non-cash interest expense related to sale of future royalties | 0.8 | 0.9 | ||||||
Non-cash royalty revenue related to sale of future royalties, net of withholding tax | (2.7 | ) | (2.3 | ) | ||||
Change in operating assets and liabilities: | ||||||||
Accounts receivables | 0.3 | (1.4 | ) | |||||
Prepaid expenses and other current assets | 0.2 | (0.1 | ) | |||||
Accounts payable and accrued expenses | 0.1 | 1.4 | ||||||
Net cash used in operating activities | (9.2 | ) | (19.7 | ) | ||||
Cash flows from investing activities: | ||||||||
Purchases of short and long-term investments | (7.0 | ) | (8.4 | ) | ||||
Maturities of short-term investments | 27.9 | 16.6 | ||||||
Net cash provided by investing activities | 20.9 | 8.2 | ||||||
Cash flows from financing activities: | ||||||||
Payment on note payable | - | (0.1 | ) | |||||
Net cash used in financing activities | - | (0.1 | ) | |||||
Increase (decrease) in cash and cash equivalents | 11.7 | (11.6 | ) | |||||
Cash and cash equivalents at beginning of period | 17.7 | 49.7 | ||||||
Cash and cash equivalents at end of period | $ | 29.4 | $ | 38.1 | ||||
In the nine months ended September 30, 2021, the Company sold an additional 13,239,839 shares under the October 2020 ATM for gross proceeds of $127.9 million which, after deducting sales commissions and expenses, resulted in net proceeds of $122.2 million. A total of 13,932,490 shares were issued and sold under the October 2020 ATM for gross proceeds of $133.4 million which, after deducting sales commissions and expenses, resulted in net proceeds of $127.1 million.
On September 13, 2021, the October 2020 ATM was terminated, and on September 15, 2021, the Company entered into a Controlled Equity Offering Sales Agreement (the “September 2021 ATM”), pursuant to which it may offer and sell, from time to time through sales agents, shares of its common stock having an aggregate offering price of up to $100 million. The Company filed a prospectus supplement with the SEC on September 16, 2021, and will pay sales commissions of up to 3.0% of gross proceeds from the sale of shares. As of December 31, 2021, no shares had been issued under the September 2021 ATM. In the nine months ended September 30, 2022, 2,565,022 shares were issued and sold under the September 2021 ATM for gross proceeds of $9.2 million, which, after deducting sales commissions and expenses incurred to date, resulted in net proceeds of $8.6 million.
The accompanying notesCompany’s principal operations are an integral part of these condensed consolidated financial statements.
Aviragen Therapeutics, Inc.
Notes to the CondensedConsolidatedFinancial Statements (unaudited)(for the quarterly period ended December 31, 2017)
|
|
Aviragen Therapeutics, Inc., together with its wholly owned subsidiaries (“Aviragen”, or the “Company”)based in South San Francisco, California, and it operates in one reportable segment, which is a biopharmaceutical company focused on the discovery and development of direct-acting antivirals to treat infections that have limited therapeutic options and affect a significant numberoral recombinant protein vaccines, based on its proprietary oral vaccine platform.
NOTE 2. Summary of patients globally.Significant Accounting Policies
Basis of Presentation – The Company has three Phase 2 clinical stage compounds: BTA074 (teslexivir), an antiviral treatment for condyloma caused by human papillomavirus types 6 & 11; vapendavir, a capsid inhibitor forprepared the prevention or treatment of rhinovirus upper respiratory infections; and BTA585 (enzaplatovir), a fusion protein inhibitor in development for the treatment of respiratory syncytial virus (RSV) infections. The Company also has a preclinical RSV non-fusion inhibitor program. The Company is incorporated in the state of Delaware and its corporate headquarters are located in Alpharetta, Georgia.
Although several of the Company’s influenza product candidates have been successfully developed and commercialized to-date by other larger pharmaceutical companies under collaboration, license or commercialization agreements with the Company, it has not independently developed or received regulatory approval for any product candidate, and the Company does not currently have any sales, marketing or commercial capabilities. Therefore, it is possible that the Company may not successfully derive any significant product revenues from any product candidates that it is developing now, or may develop in the future. The Company expects to incur losses for the foreseeable future as it intends to support the clinical and preclinical development of its product candidates.
On October 30, 2017, the Company announced that it had entered into a definitive Agreement and Plan of Merger and Reorganization dated as of October 27, 2017, among the Company, Agora Merger Sub, Inc. and Vaxart, Inc. (the “Merger Agreement”) pursuant to which Vaxart, a privately-held clinical-stage company focused on developing oral recombinant vaccines from its proprietary delivery platform, would become a wholly-owned subsidiary of the Company (the “Merger”). This transaction marks the culmination of the Company’s Strategic Review process which was initiated in April. The Merger will result in a clinical-stage pharmaceutical company focused on developing Vaxart’s oral recombinant vaccines and Aviragen’s direct-acting antivirals to treat infections that have limited therapeutic options.
The exchange ratio in the merger agreement was determined by Vaxart assigning $60,000,000 in value to Aviragen for its financial and clinical assets, and $90,000,000 in value for its own assets. On a pro forma basis after giving effect to the number of shares of Aviragen common stock that will be issued to Vaxart security holders in the Merger and assuming no adjustments for cash balances as provided for in the Merger Agreement, current Vaxart security holders will own approximately 60% of the combined company and current Aviragen security holders will own approximately 40% of the combined company. The transaction has been approved by the boards of directors of both companies. The Merger is expected to close in February 2018, subject to the approval of the stockholders of each company as well as other customary conditions. Upon closing of the Merger, the name of the combined company will become Vaxart, Inc. and shares of the combined company are expected to continue trading on the NASDAQ Capital Market under the proposed ticker symbol VXRT. Wouter Latour, M.D., Chief Executive Officer of Vaxart, will serve as Chief Executive Officer of the combined company.
At the end of the quarter, a small group of dissident stockholders, who call themselves the Concerned Aviragen Shareholders (“CAS”) Group, launched a proxy contest against the proposed merger with Vaxart and are seeking an opportunity to nominate individuals for election to the Company’s Board at the upcoming Annual Meeting. The Company continues to believe the proposed merger with Vaxart is the best possible strategic alternative, and together, Aviragen and Vaxart will have the potential to create meaningful value for stockholders.
Prior to the completion of the proposed merger, the Company plans to continue to finance its operations with existing cash, cash equivalents and investments.
|
|
The accompanying unaudited condensed consolidated financial statements have beenpursuant to the rules and regulations of the SEC. Certain information and footnote disclosures normally included in consolidated financial statements prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) for interim financial information and with the instructions to Form 10-Q and Rule 10-01 of Regulation S-X. All material adjustments considered necessary for a fair presentation have been included. Certain information and footnote disclosure normally included in financial statements prepared in accordance with U.S. GAAP have been condensed or omitted pursuant to instructions,these rules and regulations prescribed by the U.S. Securities and Exchange Commission (“SEC”). Except as disclosed herein, there has been no material change in the information disclosed in the notes to theregulations. These condensed consolidated financial statements included in the Company’s Annual Report on Form 10-K that was filed with the SEC on September 1, 2017.
The unaudited interim condensed consolidated financial statements include the accounts of the Company and all of its wholly owned subsidiaries. All inter-company transactions and balances are eliminated in consolidation.
Operating results for the three and six months ended December 31, 2017 are not necessarily indicative of those in future quarters or the annual results that may be expected for the Company’s fiscal year ending June 30, 2018. For a more complete discussion of the Company’s significant accounting policies and other information, this report should be read in conjunction with the consolidatedCompany’s audited financial statements and footnotes related thereto for the fiscal year ended June 30, 2017December 31,2021,included in the Company’s Annual Report on Form 10-K.
The Company’s-K filed with the SEC on February 24, 2022 (the “Annual Report”). Unless noted below, there have been no material changes to the Company’s significant accounting policies havedescribed in Note 2 to the consolidated financial statements included in the Annual Report. In the opinion of management, the unaudited condensed consolidated financial statements include all adjustments (consisting only of normal recurring adjustments) necessary to present fairly the Company’s financial position and the results of its operations and cash flows. The results of operations for such interim periods are not changed since June 30, 2017.necessarily indicative of the results to be expected for the full year or any future periods.
Recently Issued Accounting StandardsBasis of Consolidation – The condensed consolidated financial statements include the financial statements of Vaxart, Inc. and its subsidiaries. All significant transactions and balances between Vaxart, Inc. and its subsidiaries have been eliminated in consolidation.
In May 2014, Use of Estimates – The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the FASB issued authoritative accounting guidance related to revenuereported amounts of assets, liabilities, revenues and expenses and disclosure of contingent assets and liabilities in the financial statements and accompanying notes. Actual results and outcomes could differ from contracts with customers. This guidance is a comprehensive new revenue recognition modelthese estimates and assumptions.
Concentration of Credit Risk – Financial instruments that requires a company to recognize revenue to depict the transfer of goods or services to a customer at an amount that reflects the consideration it expects to receive in exchange for those goods or services. This guidance is effective for annual reporting periods beginning after December 15, 2017. Accordingly,potentially subject the Company will adopt this guidance on July 1, 2018. Companies may use either a full retrospective or a modified retrospective approach to adopt this guidance.significant concentrations of credit risk consist principally of cash, cash equivalents and available-for-sale investments. The Company places its cash, cash equivalents and available-for-sale investments at financial institutions that management believes are of high credit quality. The Company is evaluating which transition approachexposed to usecredit risk in the event of default by the financial institutions holding the cash and its impact, ifcash equivalents to the extent such amounts are in excess of the federally insured limits. The Company has not experienced any losses on its consolidated financial statements.deposits since inception.
VAXART, INC.
In January 2016, the FASB issued guidance related to financial instruments - overall recognition and measurement of financial assets and financial liabilities. The guidance enhances the reporting model for financial instruments, which includes amendments to address aspects of recognition, measurement, presentation and disclosure. The updateNotes to the standard is effective for public companies for interim and annual periods beginning after December 15, 2017. Accordingly, the standard is effective for the Company on July 1, 2018. The Company is currently evaluating the impact that the standard will have on the consolidated financial statements.Condensed Consolidated Financial Statements (Unaudited)
In February 2016, the FASB issued new guidance on leases. This guidance replaces the prior lease accounting guidance in its entirety.
The underlying principleprimary focus of the new standardCompany’s investment strategy is to preserve capital and meet liquidity requirements. The Company’s investment policy addresses the recognitionlevel of lease assetscredit exposure by limiting the concentration in any one corporate issuer or sector and lease liabilities by lessees for substantiallyestablishing a minimum allowable credit rating.
Recent Accounting Pronouncements
The Company has reviewed all leases, with an exception for leases with terms of less thannewly-issued accounting pronouncements that are twelvenot months. The standard also requires additional quantitativeyet effective and qualitative disclosures. The guidance is effective for interim and annual reporting periods beginning afterconcluded that they are either December 15, 2018, notand early applicable to its operations or their adoption is permitted. The standard requires a modified retrospective approach, which includes several optional practical expedients. Accordingly, the standard is effective for the Company on July 1, 2019. The Company is currently evaluating the impact that this guidance will have on the consolidated financial statements.
In August 2016, the FASB issued new guidance on how certain cash receipts and cash payments are presented and classified in the statement of cash flows. The standard is effective for the Company beginning July 1, 2018. Early adoption is permitted. We do not expect the adoption of this guidanceexpected to have a material impact on the consolidatedits financial statements.position or results of operations.
NOTE 3. Fair Value of Financial Instruments |
|
AFair value accounting is applied for all financial assets and liabilities and nonfinancial assets and liabilities that are recognized or disclosed at fair value hierarchy has been establishedin the financial statements on a recurring basis (at least annually). Financial instruments include cash and cash equivalents, marketable securities, accounts receivable, accounts payable and accrued liabilities that requiresapproximate fair value due to their relatively short maturities.
Assets and liabilities recorded at fair value on a recurring basis in the Companybalance sheets are categorized based upon the level of judgment associated with inputs used to maximize the use of observable inputs, where available, and minimize the use of unobservable inputs whenmeasure their fair values. The accounting guidance for fair value provides a framework for measuring fair value. Thevalue and requires certain disclosures about how fair value hierarchy describesis determined. Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability (an exit price) in an orderly transaction between market participants at the reporting date. The accounting guidance also establishes a three levels of-level valuation hierarchy that prioritizes the inputs that may beto valuation techniques used to measure fair value:value based upon whether such inputs are observable or unobservable. Observable inputs reflect market data obtained from independent sources, while unobservable inputs reflect market assumptions made by the reporting entity.
The three-level hierarchy for the inputs to valuation techniques is briefly summarized as follows: |
|
the related assets or liabilities; and
Level 3 – Unobservable inputs that are significant to the measurement of the fair value of the assets or liabilities that are supported by little or no market data. |
| |
|
|
The following table sets forth the fair value of the Company’s financial assets and liabilities that wereare measured at fair value on a recurring basis at as of September 30, 2022 and December 31, 2017 2021and June 30, 2017, by level within the fair value hierarchy. The assets and liabilities measured at fair value are classified in their entirety based on the lowest level of input that is significant to the fair value measurement. (in thousands):
Level 1 | Level 2 | Level 3 | Total | |||||||||||||
September 30, 2022 | ||||||||||||||||
Financial assets: | ||||||||||||||||
Money market funds | $ | 30,873 | $ | — | $ | — | $ | 30,873 | ||||||||
U.S. Treasury securities | — | 49,389 | — | 49,389 | ||||||||||||
Commercial paper | — | 8,959 | — | 8,959 | ||||||||||||
Corporate debt securities | — | 5,651 | — | 5,651 | ||||||||||||
Total | $ | 30,873 | $ | 63,999 | $ | — | $ | 94,872 | ||||||||
Level 1 | Level 2 | Level 3 | Total | |||||||||||||
December 31, 2021 | ||||||||||||||||
Financial assets: | ||||||||||||||||
Money market funds | $ | 70,978 | $ | — | $ | — | $ | 70,978 | ||||||||
U.S. Treasury securities | — | 24,997 | — | 24,997 | ||||||||||||
Commercial paper | — | 7,491 | — | 7,491 | ||||||||||||
Corporate debt securities | — | 6,464 | — | 6,464 | ||||||||||||
Total | $ | 70,978 | $ | 38,952 | $ | — | $ | 109,930 | ||||||||
The Company’s short-term investments held no recurring financial liabilities as of JuneSeptember 30, 2017 have been classified as Level2022 or 2,December 31, 2021, or in the nine which have been initially valued at the transaction price and subsequently revalued, at the end of each reporting period, utilizing amonths ended thirdSeptember 30, party pricing service. The pricing service utilizes industry standard valuation models and observable market inputs to determine value that include surveying the bond dealer community, obtaining benchmark quotes, incorporating relevant trade data, and updating spreads daily. There have been no2022 transfers of assets or liabilities between the fair value measurement classifications.2021.
Quoted Prices in | Significant | |||||||||||||||
Active Markets | Other | Significant | ||||||||||||||
(in millions) | for Identical Assets | Observable Inputs | Unobservable Inputs | |||||||||||||
December 31, 2017 | Total | (Level 1) | (Level 2) | (Level 3) | ||||||||||||
Cash equivalents | $ | 18.7 | $ | 7.0 | $ | 11.7 | $ | — | ||||||||
Short-term investments available-for-sale | — | — | — | — | ||||||||||||
Total | $ | 18.7 | $ | 7.0 | $ | 11.7 | $ | — | ||||||||
| (in millions) | Quoted Prices in Active Markets for Identical Assets | Significant Other Observable Inputs | Significant Unobservable Inputs | |||||||||||||
June 30, 2017 | Total | (Level 1) | (Level 2) | (Level 3) | ||||||||||||
Cash equivalents | $ | 10.9 | $ | 5.9 | $ | 5.0 | $ | — | ||||||||
Short-term investments available-for-sale | 20.9 | — | 20.9 | — | ||||||||||||
Total | $ | 31.8 | $ | 5.9 | $ | 25.9 | $ | — | ||||||||
Cash equivalents consist primarily of money market funds, corporate notes and commercial paper with original maturities of 90 or fewer days when purchased. Short-term investments consist of certificates of deposit, corporate securities, U.S. Treasury securities andU.S. agency securities, classified as available-for-sale and have maturities less than 365 days from the date of acquisition.
The following table shows the unrealized gains and losses and fair values for those investments as of December 31, 2017 and June 30, 2017 aggregated by major security type:
(in millions) | Unrealized | Unrealized | ||||||||||||||
December 31, 2017 | At Cost | Gains | (Losses) | At Fair Value | ||||||||||||
Money market funds | $ | 7.0 | $ | - | $ | - | $ | 7.0 | ||||||||
Corporate notes | 6.5 | - | - | 6.5 | ||||||||||||
Commercial paper | 5.2 | - | - | 5.2 | ||||||||||||
Total | $ | 18.7 | $ | - | $ | - | $ | 18.7 | ||||||||
(in millions) | Unrealized | Unrealized | ||||||||||||||
June 30, 2017 | At Cost | Gains | (Losses) | At Fair Value | ||||||||||||
Money market funds | $ | 5.9 | $ | — | $ | — | $ | 5.9 | ||||||||
Commercial paper | 8.5 | — | — | 8.5 | ||||||||||||
Corporate notes | 17.4 | — | — | 17.4 | ||||||||||||
Total | $ | 31.8 | $ | — | $ | — | $ | 31.8 | ||||||||
AsVAXART, INC.
Notes to the Condensed Consolidated Financial Statements (Unaudited)
NOTE 4. Balance Sheet Components
(a) | Cash, Cash Equivalents and Investments |
Cash, cash equivalents and investments consisted of the following (in thousands):
Amortized | Gross Unrealized | Estimated | Cash and Cash | Short-Term | Long-Term | |||||||||||||||||||||||
Cost | Gains | Losses | Fair Value | Equivalents | Investments | Investments | ||||||||||||||||||||||
September 30, 2022 | ||||||||||||||||||||||||||||
Cash at banks | $ | 19,895 | $ | — | $ | — | $ | 19,895 | $ | 19,895 | $ | — | $ | — | ||||||||||||||
Money market funds | 30,873 | — | — | 30,873 | 30,873 | — | — | |||||||||||||||||||||
U.S. Treasury securities | 49,841 | — | (452 | ) | 49,389 | — | 49,389 | — | ||||||||||||||||||||
Commercial paper | 8,959 | — | — | 8,959 | — | 8,959 | — | |||||||||||||||||||||
Corporate debt securities | 5,716 | — | (65 | ) | 5,651 | — | 5,651 | — | ||||||||||||||||||||
Total | $ | 115,284 | $ | — | $ | (517 | ) | $ | 114,767 | $ | 50,768 | $ | 63,999 | $ | — | |||||||||||||
Amortized | Gross Unrealized | Estimated | Cash and Cash | Short-Term | Long-Term | |||||||||||||||||||||||
Cost | Gains | Losses | Fair Value | Equivalents | Investments | Investments | ||||||||||||||||||||||
December 31, 2021 | ||||||||||||||||||||||||||||
Cash at banks | $ | 72,767 | $ | — | $ | — | $ | 72,767 | $ | 72,767 | $ | — | $ | — | ||||||||||||||
Money market funds | 70,978 | — | — | 70,978 | 70,978 | — | — | |||||||||||||||||||||
U.S. Treasury securities | 25,055 | — | (58 | ) | 24,997 | — | 12,022 | 12,975 | ||||||||||||||||||||
Commercial paper | 7,491 | — | — | 7,491 | — | 7,491 | — | |||||||||||||||||||||
Corporate debt securities | 6,480 | — | (16 | ) | 6,464 | �� | — | 3,229 | 3,235 | |||||||||||||||||||
Total | $ | 182,771 | $ | — | $ | (74 | ) | $ | 182,697 | $ | 143,745 | $ | 22,742 | $ | 16,210 | |||||||||||||
(b) | Accounts Receivable |
Accounts receivable comprises royalties receivable of nil and $71,000 as of September 30, 2022 and December 31, 2017 2021and June 30, 2017, the Company had investments in an unrealized gain (loss) position below material disclosure thresholds in the table above., respectively. The Company determined that the unrealized gainshas provided no allowance for uncollectible accounts as of September 30, 2022 and losses on these investments were temporary in nature and expected the security to mature at its stated maturity principal. All available-for-sale securities held at December 31, 2017, 2021will mature in less than one year. The fair value of cash, accounts receivable, accounts payable.
(c) | Property and Equipment, Net |
Property and accrued liabilities approximate their carrying value becauseequipment, net consists of the short-term nature of these financial instruments at following (in thousands):
September 30, 2022 | December 31, 2021 | |||||||
Laboratory equipment | $ | 7,465 | $ | 5,057 | ||||
Office and computer equipment | 767 | 481 | ||||||
Leasehold improvements | 1,063 | 1,063 | ||||||
Construction in progress | 5,596 | 1,305 | ||||||
Total property and equipment | 14,891 | 7,906 | ||||||
Less: accumulated depreciation | (2,611 | ) | (1,305 | ) | ||||
Property and equipment, net | $ | 12,280 | $ | 6,601 | ||||
Depreciation expense was $489,000 and $211,000 for the December 31, 2017three months ended September 30,2022and June2021, respectively, and $1.3 million and $406,000 for the nine months ended September 30, 2017, respectively. The fair value2022 and 2021, respectively. There were no impairments of the Company’s short-term note payable, which is measured using Levelproperty and equipment recorded in the 2nine inputs, approximates book value, atmonths ended September 30,2022 or 2021.
(d) | Right-of-Use Assets, Net |
Right-of-use assets, net comprises facilities of $26.6 million and $13.2 million as of September 30, 2022 and December 31, 2021, respectively.
(4) VAXARTAccrued and Other Current Liabilities, INC.
Accrued expensesNotes to the Condensed Consolidated Financial Statements (Unaudited)
(e) | Intangible Assets, Net |
Intangible assets comprise developed technology and intellectual property. Intangible assets are carried at cost less accumulated amortization. Amortization is computed using the straight-line method over useful lives ranging from 1.3 to 11.75 years for developed technology and 20 years for intellectual property. As of September 30, 2022, developed technology and intellectual property had remaining lives of 7.1 and 5.25 years, respectively. Intangible assets consist of the following (in millions)thousands):
December 31, 2017 |
June 30, 2017 | |||||||
Professional fees | $ | 0.7 | $ | 0.4 | ||||
Salary and benefits | 1.3 | 0.4 | ||||||
Research and development expenses | 0.5 | 1.8 | ||||||
Other accrued expenses | - | 0.3 | ||||||
Total accrued expenses and other liabilities | $ | 2.5 | $ | 2.9 | ||||
September 30, 2022 | December 31, 2021 | |||||||
Developed technology | $ | 10,600 | $ | 10,600 | ||||
Intellectual property | 80 | 80 | ||||||
Total cost | 10,680 | 10,680 | ||||||
Less: accumulated amortization | (1,069 | ) | (56 | ) | ||||
Intangible assets, net | $ | 9,611 | $ | 10,624 | ||||
Total amortization expense for the three months ended September 30,2022 and 2021, was $338,000 and $433,000, respectively, and $1.0 million and $1.3 million for the nine months ended September 30,2022 and 2021, respectively. As of September 30, 2022, the estimated future amortization expense by year is as follows (in thousands):
Year Ending December 31, | Amount | |||
2022 (three months remaining) | $ | 338 | ||
2023 | 1,350 | |||
2024 | 1,350 | |||
2025 | 1,350 | |||
2026 | 1,350 | |||
Thereafter | 3,873 | |||
Total | $ | 9,611 | ||
(f) | Goodwill |
Goodwill, which represents the excess of the purchase price over the fair value of assets acquired, comprises $4.5 million as of September 30, 2022 and December 31, 2021. As of September 30, 2022, there have been no indicators of impairment.
(g) | Other Accrued Liabilities |
Other accrued liabilities consist of the following (in thousands):
September 30, 2022 | December 31, 2021 | |||||||
Accrued compensation | $ | 3,809 | $ | 2,786 | ||||
Accrued clinical and manufacturing expenses | 1,203 | 986 | ||||||
Accrued professional and consulting services | 715 | 556 | ||||||
Accrued litigation settlement | 2,000 | — | ||||||
Other liabilities, current portion | 2,617 | 736 | ||||||
Total | $ | 10,344 | $ | 5,064 | ||||
(NOTE 5) Liabilities Related to Sale of Future Royalties. Revenue
In April 2016, Royalty Agreement
The Company generates royalty revenue from the Company sold certain royalty rights relatedsale of Inavir in Japan, pursuant to the approved product Inavir®, sold bya collaboration and license agreement that Aviragen entered into with Daiichi Sankyo Company, Limited (“Daiichi Sankyo”) in 2009. In September 2010, laninamivir octanoate was approved for sale by the Japanese market,Ministry of Health and Welfare for the treatment of influenza in adults and children, which Daiichi Sankyo markets as Inavir. Under the agreement, the Company currently receives a 4% royalty on net sales of Inavir in Japan. The last patent related to Inavir is set to expire in $20December 2029, at which time royalty revenue will cease. No royalty revenue was recognized in the nine millionmonths ended September 30,2022 and 2021. The Company recognized non-cash royalty revenue related to the sale of future royalties (see Note 6) of nil and $200,000 in the three months ended September 30,2022 and 2021, respectively, and $85,000 and $805,000 in the nine months ended September 30,2022 and 2021, respectively. Both royalty revenue and the non-cash royalty revenue related to sale of future royalties are subject to a 5% withholding tax in Japan, for which nil and $10,000 was included in income tax expense in the three months ended September 30,2022 and 2021, respectively, and $4,000 and $40,000 in the nine months ended September 30,2022 and 2021.
The Company’s royalty revenue is seasonal, in line with the flu season, so the majority of the Company’s royalty revenue and non-cash royalty revenue related to the sale of future royalties are earned in the first and fourth fiscal quarters.
VAXART, INC.
Notes to the Condensed Consolidated Financial Statements (Unaudited)
NOTE 6.Liabilities Related to Sale of Future Royalties
In April 2016, Aviragen entered into a Royalty Interest Acquisition Agreement (the “RIAA”) with HealthCare Royalty Partners III, L.P. (“HCRP”). Under the RIAA, HCRP made a $20.0 million cash payment to Aviragen in consideration for acquiring certain royalty rights (“Royalty Rights”) related to the approved product Inavir in the Japanese market. The Royalty Rights were obtained pursuant to the collaboration and license agreements (the “License Agreement”) and a commercialization agreement that the Company entered into with Daiichi Sankyo. Per the terms of the RIAA, HCRP is entitled to the first $3.0 million plus 15% of the next $1.0 million in royalties earned in each year commencing on April 1, with any excess revenue being retained by the Company.
Under the relevant accounting guidance, due to a limit on the amount of royalties that HCRP can earn under the arrangement,RIAA, this transaction wasis accounted for as a liability that will beis being amortized using the interest method over the life of the arrangement. The Company has no obligation to pay any amounts to HCRP other than to pass through to HCRP its share of royalties as they are received from Daiichi Sankyo. In order to record the amortization of the liability, the Company is required to estimate the total amount of future royalty payments to be received under the License Agreement with Daiichi Sankyo and the payments that will be passed through to HCRP over the life of thethis agreement. The sum of the pass through amounts less the net proceeds received will be recorded as non-cash interest expense over the life of the liability. Consequently, the Company imputes interest on the unamortized portion of the liability and records non-cash interest expense using an estimated effective interest rate. The royalties earned in each period that will be passed through to HCRP are recorded as non-cash royalty revenue related to sale of future royalties, with any excess not subject to pass-through being recorded as royalty revenue. When the pass-through royalties are paid to HCRP in the following quarter, the imputed liability related to sale of future royalties is commensurately reduced. The Company will periodically assessassesses the expected royalty payments, and to the extent such payments are greater or less than the initial estimate, the Company will adjustadjusts the amortization of the liability and interest rate. As a result of this accounting, even though the Company does not retain HCRP’s share of the royalties, it will continue to record non-cash revenue related to those royalties until the amount of the associated liability, andincluding the related interest, is fully amortized.
The following table shows the activity within the liability account during the sixnine months ended December 31, 2017:September 30,2022 (in thousands):
Total liability related to sale of future royalties, start of period | $ | 11,522 | ||
Non-cash royalty revenue paid to HCRP | (152 | ) | ||
Non-cash interest expense recognized | 988 | |||
Total liability related to sale of future royalties, end of period | 12,358 | |||
Current portion | (1,331 | ) | ||
Long-term portion | $ | 11,027 |
in millions | ||||
Total Liability related to sale of future royalties, June 30, 2017 | $ | 16.7 | ||
Non-cash royalty revenue paid to HCRP | (0.3 | ) | ||
Non-cash interest expense recognized | 0.8 | |||
Total Liability related to sale of future royalties, December 31, 2017 | $ | 17.2 | ||
(6) Net Loss per shareVAXART, INC.
BasicNotes to the Condensed Consolidated Financial Statements (Unaudited)
The Company has obtained the right of use for office and manufacturing facilities under seven operating lease agreements with initial terms exceeding one year and has two operating lease agreements for facilities and one for equipment with initial terms of one year or less.
The Company obtained the right of use of real estate located in South San Francisco, California, in November 2020 under a lease that was scheduled to terminate on September 30, 2025, which has been extended until March 31, 2029, with no additional extension option. The Company also obtained the right of use of real estate located in South San Francisco, California, in June 2015 that was scheduled to terminate on April 30, 2020, with a five-year extension option that the Company exercised in July 2019, extending the lease until April 30, 2025, which has been further extended until March 31, 2029, with an option to extend for an additional eight years. In addition, in September 2021 the Company executed a lease for a facility in South San Francisco, California, with an initial term expiring on March 31, 2029, with an option to extend for an additional eight years. This lease includes a component for which tenant improvements that were substantially completed in the three months ended September 30, 2022, when the lease for this component was deemed to have commenced for accounting purposes. It also includes a component for which tenant improvements, estimated to cost approximately $2.8 million, are estimated to be completed in the first quarter of 2023. These will be recorded within right-of-use assets in the condensed consolidated balance sheet when they are substantially completed and this component of the lease is deemed to have commenced for accounting purposes. In addition, the Company has the right of use of a facility located in South San Francisco, California, under a lease that, following extensions, now terminates on December 31, 2022, with no extension option. Further, the Company has the right of use of a facility located in South San Francisco, California, under a lease that terminates on March 30, 2029, with a five-year renewal option. The Company also has the right of use of two facilities in Burlingame, California, under leases that terminate on May 31, 2025, both of which have two 30-month extension options. The Company has also identified two short-term embedded leases for the rental of facilities in South San Francisco, California and Lodi, Wisconsin and one short-term lease for equipment.
As of September 30, 2022, the weighted average discount rate for operating leases with initial terms of more than one year was 9.80% and the weighted average remaining term of these leases was 6.34 years. Discount rates were determined using the Company’s marginal rate of borrowing at the time each lease commenced or was extended.
The following table summarizes the Company’s undiscounted cash payment obligations for its operating lease liabilities with initial terms of more than twelve months as of September 30, 2022 (in thousands):
Year Ending December 31, | ||||
2022 (three months remaining) | $ | 994 | ||
2023 | 4,072 | |||
2024 | 4,213 | |||
2025 | 4,356 | |||
2026 | 4,964 | |||
Thereafter | 11,787 | |||
Undiscounted total | 30,386 | |||
Less: imputed interest | (8,218 | ) | ||
Present value of future minimum payments | 22,168 | |||
Current portion of operating lease liability | (2,149 | ) | ||
Operating lease liability, net of current portion | $ | 20,019 |
The Company presently has no finance leases and no future obligations under operating leases with initial terms of one year or less.
Certain operating lease agreements for facilities include non-lease costs, such as common area maintenance, which are recorded as variable lease costs. Operating lease expenses for the three and nine months ended September 30,2022 and 2021, including variable lease costs for one lease that has not yet commenced for accounting purposes (see below), are summarized as follows (in thousands):
Three Months Ended September 30, | Nine Months Ended September 30, | |||||||||||||||
2022 | 2021 | 2022 | 2021 | |||||||||||||
Lease cost | ||||||||||||||||
Operating lease cost | $ | 1,202 | $ | 651 | $ | 2,652 | $ | 1,824 | ||||||||
Short-term lease cost | 117 | 101 | 335 | 232 | ||||||||||||
Variable lease cost | 397 | 313 | 926 | 937 | ||||||||||||
Sublease income | — | — | — | (36 | ) | |||||||||||
Total lease cost | $ | 1,716 | $ | 1,065 | $ | 3,913 | $ | 2,957 | ||||||||
Net cash outflows associated with operating leases, which include expenditures on leasehold improvements, totaled $1.4 million and $970,000 in the three months ended September 30,2022 and 2021, respectively, and $7.8 million and $2.8 million in the nine months ended September 30,2022 and 2021, respectively.
VAXART, INC.
Notes to the Condensed Consolidated Financial Statements (Unaudited)
NOTE 8. Commitments and Contingencies
(a) | Purchase Commitments |
As of September 30, 2022, the Company had approximately $13.7 million of non-cancelable purchase commitments, principally for contract manufacturing and clinical services and leasehold improvements which are expected to be paid within the next year. In addition, the Company has operating lease commitments as detailed in Note 7 and a further commitment for an operating lease with unpaid rental payments totaling $0.4 million payable by March 31, 2029, which has been executed but has not yet commenced, for which we expect to spend a net total of approximately $2.8 million on leasehold improvements, of which $0.6 million has already been expended and $2.2 million is included within non-cancelable purchase commitments, which will be recorded as right-of-use assets when the lease commences.
(b) | Indemnifications |
In the ordinary course of business, the Company enters into agreements that may include indemnification provisions. Pursuant to such agreements, the Company may indemnify, hold harmless and defend indemnified parties for losses suffered or incurred by the indemnified party. Some of the provisions will limit losses to those arising from third-party actions. In some cases, the indemnification will continue after the termination of the agreement. The maximum potential amount of future payments the Company could be required to make under these provisions is not determinable. The Company has also entered into indemnification agreements with certain officers and directors which provide, among other things, that the Company will indemnify and advance expenses incurred in connection with certain actions, suits or proceedings to such officer or director, under the circumstances and to the extent provided for therein, for expenses, damages, judgments, fines and settlements he or she may be required to pay in actions or proceedings which he or she is or may be made a party by reason of his or her position as a director, officer or other agent of the Company, and otherwise to the fullest extent permitted under Delaware law and the Company’s Bylaws. The Company currently has directors’ and officers’ insurance.
(c) |
From time to time the Company may be involved in legal proceedings arising in connection with its business. Based on information currently available, the Company believes that the amount, or range, of reasonably possible losses in connection with any pending actions against it in excess of established reserves, in the aggregate, is not material to its consolidated financial condition or cash flows. However, any current or future dispute resolution or legal proceeding, regardless of the merits of any such proceeding, could result in substantial costs and a diversion of management’s attention and resources that are needed to run the Company successfully, and could have a material adverse impact on its business, financial condition and results of operations.
On August 4, 2020, a purported shareholder derivative complaint was filed in the Superior Court of California, San Mateo County, entitled Godfrey v. Latour, et al. An amended complaint was filed on September 4, 2020 and the case was re-named Ennis v. Latour, et al. A second amended complaint was filed on November 25, 2020. A third amended complaint was filed on June 11, 2021. The third amended complaint names certain current and former Vaxart directors as defendants, asserting claims against them for breach of fiduciary duty, unjust enrichment, and waste and seeking, among other things, an award of unspecified damages, certain equitable relief, and attorneys’ fees and costs. The complaint also asserts claims for breach of fiduciary duty and aiding and abetting breach of fiduciary duty against Armistice Capital, LLC (“Armistice”). The third amended complaint challenges certain stock options granted to certain of the Company’s officers and directors in June 2020; certain alleged statements and omissions made in the Company’s April 24, 2020, proxy statement; and certain amendments to two warrants held by Armistice, as disclosed on June 8, 2020. The third amended complaint purports to bring the lawsuit derivatively on behalf of and for the benefit of the Company and names the Company as a “nominal defendant” against which no damages are sought. On August 31, 2021, the Company and certain of its directors (the “Vaxart Defendants”), as well as all other defendants, filed demurrers to the third amended complaint. The demurrer filed by the Vaxart Defendants has not yet been decided. On July 14, 2022, the court held a hearing on the defendants’ pending demurrers and decided to defer ruling in favor of additional briefing on the effects of the dismissal of the In re Vaxart, Inc. Stockholder Litigation in the Delaware Court of Chancery on the viability of the third amended complaint.
VAXART, INC.
Notes to the Condensed Consolidated Financial Statements (Unaudited)
In August and September 2020, two substantially similar securities class actions were filed in the U.S. District Court for the Northern District of California. The first action, titled Himmelberg v. Vaxart, Inc. et al. was filed on August 24, 2020. The second action, titled Hovhannisyan v. Vaxart, Inc. et al. was filed on September 1, 2020 (together, the “Putative Class Action”). By Order dated September 17, 2020, the two actions were deemed related. On December 9, 2020, the court appointed lead plaintiffs and lead plaintiffs’ counsel. On January 29, 2021, lead plaintiffs filed their consolidated amended complaint. On July 8, 2021, all defendants moved to dismiss the consolidated amended complaint. On May 14, 2021, the court granted lead plaintiffs’ request to amend the consolidated amended complaint and denied defendants’ motions to dismiss as moot. On June 10, 2021, lead plaintiffs filed an amended consolidated complaint. On August 9, 2021, lead plaintiffs filed a corrected amended consolidated complaint. The amended consolidated complaint names certain of Vaxart’s current and former executive officers and directors, as well as Armistice, as defendants. It claims three violations of federal civil securities laws; violation of Section 10(b) of the Exchange Act and SEC Rule 10b-5, as against the Company and all individual defendants; violation of Section 20(a) of the Exchange Act, as against Armistice and all individual defendants; and violation of Section 20A of the Exchange Act against Armistice. The amended consolidated complaint alleges that the defendants violated securities laws by misstating and/or omitting information regarding the Company’s development of a norovirus vaccine, the vaccine manufacturing capabilities of a business counterparty, and the Company’s involvement with Operation Warp Speed (“OWS”); and by engaging in a scheme to inflate Vaxart’s stock price. The first amended consolidated complaint seeks to be certified as a class action for similarly situated shareholders and seeks, among other things, an unspecified amount of damages and attorneys’ fees and costs. As disclosed in a Form 8-K filed by the Company on July 28, 2022, a Stipulation of Settlement was filed with the court, announcing the terms of a partial settlement of the Putative Class action. On October 3, 2022, the parties' motion for preliminary approval of the partial class settlement was granted by the district court. Under the order preliminarily approving the partial class settlement, Vaxart shall cause $2,000,000 to be paid into an escrow account, with its insurers placing the remainder of the settlement funds into a designated escrow account pending final approval. A fairness hearing was scheduled for January 12, 2023, to determine whether final class settlement approval will be granted. If the settlement is not approved, the parties will revert back to their prior litigation positions and the defendants would vigorously contest the claims.
On October 23, 2020, a complaint was filed in the U.S. District Court for the Southern District of New York, entitled Roth v. Armistice Capital LLC, et al. The complaint names Armistice and certain Armistice-related parties as defendants, asserting a violation of Exchange Act Section 16(b) and seeking the disgorgement of short-swing profits. The complaint purports to bring the lawsuit on behalf of and for the benefit of the Company and names the Company as a “nominal defendant” for whose benefit damages are sought.
On January 8, 2021, a purported shareholder, Phillip Chan, commenced a pro se lawsuit in the U.S. District Court for the Northern District of California titled Chan v. Vaxart, Inc. et al. (the “Opt-Out Action”). Because this complaint is nearly identical to an earlier version of a complaint filed in the Putative Class Action, the Opt-Out Action has been stayed pending resolution of the Putative Class Action.
The Company has accrued $2.0 million with respect to the Putative Class Action pursuant to the terms of the settlement agreement reached in that case. No other amounts have been accrued because the Company’s management does not presently believe that any loss is probable and it is not possible to reasonably estimate the loss, or range of losses, if any, that may result from any of the ongoing litigation. The Company’s legal costs incurred in its defense against these claims are expensed as incurred.
VAXART, INC.
Notes to the Condensed Consolidated Financial Statements (Unaudited)
NOTE 9. Stockholders’ Equity
(a) | Preferred Stock |
The Company is authorized to issue 5,000,000 shares of preferred stock, $0.0001 par value per share. The Company’s board of directors may, without further action by the stockholders, fix the rights, preferences, privileges and restrictions of up to an aggregate of 5,000,000 shares of preferred stock in one or more series and authorize their issuance. These rights, preferences and privileges could include dividend rights, conversion rights, voting rights, terms of redemption, liquidation preferences, sinking fund terms and the number of shares constituting any series or the designation of such series, any or all of which may be greater than the rights of the Company’s common stock. The issuance of preferred stock could adversely affect the voting power of holders of common stock and the likelihood that such holders will receive dividend payments and payments upon liquidation. In addition, the issuance of preferred stock could have the effect of delaying, deterring or preventing a change of control or other corporate action. No shares of preferred stock are currently outstanding.
(b) |
As of September 30, 2022, the Company was authorized to issue 250,000,000 shares of common stock, $0.0001 par value per share, which includes an increase of 100,000,000 on August 4, 2022, when the Company’s stockholders approved an amendment to the Company’s certificate of incorporation to increase the number of authorized shares of common stock from 150,000,000. Except as otherwise required by law or as otherwise provided in any certificate of designation for any series of preferred stock, the holders of common stock possess all voting power for the election of the Company’s directors and all other matters requiring stockholder action. Holders of common stock are entitled to one vote per share on matters to be voted on by stockholders. Holders of common stock are entitled to receive such dividends, if any, as may be declared from time to time by the Company’s board of directors in its discretion out of funds legally available therefor. In no event will any stock dividends or stock splits or combinations of stock be declared or made on common stock unless the shares of common stock at the time outstanding are treated equally and identically. As of September 30, 2022, no dividends had been declared by the board of directors.
In the event of the Company’s voluntary or involuntary liquidation, dissolution, distribution of assets or winding-up, the holders of the common stock will be entitled to receive an equal amount per share of all of the Company’s assets of whatever kind available for distribution to stockholders, after the rights of the holders of the preferred stock have been satisfied. There are no sinking fund provisions applicable to the common stock.
The Company had shares of common stock reserved for issuance as follows:
September 30, 2022 | December 31, 2021 | |||||||
Options issued and outstanding | 14,965,813 | 10,216,106 | ||||||
RSUs issued and outstanding | 723,716 | — | ||||||
Available for future grants of equity awards | 11,932,077 | 5,582,742 | ||||||
Common stock warrants | 227,434 | 232,434 | ||||||
2022 Employee Stock Purchase Plan | 1,800,000 | — | ||||||
Total | 29,649,040 | 16,031,282 | ||||||
(c) | Warrants |
The following warrants were outstanding as of September 30, 2022, all of which contain standard anti-dilution protections in the event of subsequent rights offerings, stock splits, stock dividends or other extraordinary dividends, or other similar changes in the Company’s common stock or capital structure, and none of which have any participating rights for any losses:
Securities into which warrants are convertible | Warrants Outstanding | Exercise Price | Expiration Date | ||||||
Common Stock | 44,148 | $ | 1.10 | April 2024 | |||||
Common Stock | 26,515 | $ | 1.375 | April 2024 | |||||
Common Stock | 29,150 | $ | 2.50 | March 2025 | |||||
Common Stock | 100,532 | $ | 3.125 | February 2025 | |||||
Common Stock | 16,175 | $ | 3.125 | March 2024 | |||||
Common Stock | 10,914 | $ | 22.99 | December 2026 | |||||
Total | 227,434 | ||||||||
In the event of a Fundamental Transaction (a transfer of ownership of the Company as defined in the warrant) within the Company’s control, the holders of the unexercised common stock warrants exercisable for $1.10 and $2.50 and those exercisable for $3.125 expiring in February 2025 shall be entitled to receive cash consideration equal to a Black-Scholes valuation, as defined in the warrant. If such Fundamental Transaction is not within the Company’s control, the warrantholders would only be entitled to receive the same form of consideration (and in the same proportion) as the holders of the Company’s common stock, hence these warrants are classified as a component of permanent equity.
VAXART, INC.
Notes to the Condensed Consolidated Financial Statements (Unaudited)
NOTE 10. Equity Incentive Plans
On April 23, 2019, the Company’s stockholders approved the adoption of the 2019 Equity Incentive Plan (the “2019 Plan”), under which the Company is authorized to issue incentive stock options, nonqualified stock options, stock appreciation rights, restricted stock awards and restricted stock units, other stock awards and performance awards that may be settled in cash, stock, or other property. The 2019 Plan is designed to secure and retain the services of employees, directors and consultants, provide incentives for the Company’s employees, directors and consultants to exert maximum efforts for the success of the Company and its affiliates, and provide a means by which employees, directors and consultants may be given an opportunity to benefit from increases in the value of the Company’s common stock. Following adoption of the 2019 Plan, all previous plans were frozen, and on forfeiture, cancellation and expiration, awards under those plans are not assumed by the 2019 Plan.
The aggregate number of shares of common stock authorized for issuance under the 2019 Plan was initially 1,600,000 shares, which was increased through an amendment to the 2019 Plan adopted by the Company’s stockholders (a “Plan Amendment”) on June 8, 2020, to 8,000,000, by a Plan Amendment on June 16, 2021, to 16,900,000, and by a Plan Amendment on August 4, 2022, to 28,900,000. Further amendments to the 2019 Plan to increase the share reserve would require stockholder approval. Awards that are forfeited or canceled generally become available for issuance again under the 2019 Plan. Awards have a maximum term of ten years from the grant date and may vest over varying periods, as specified by the Company’s board of directors for each grant.
In the nine months ended September 30,2022, the Company granted 688,061 restricted stock unit (“RSU”) awards to employees which vest annually over four years, subject to each employee’s continued service relationship with the Company, and 29,500 RSUs to directors which vest annually over three years and 35,717 RSUs to directors which vest on the earlier of the day before the next annual meeting of stockholders and the anniversary of the grant date, subject to each director’s continued service on the Board. The related compensation cost, which is based on the grant date fair value of the Company’s common stock multiplied by the number of RSUs granted, is recognized, net of estimated forfeitures, as an expense ratably over the service period.
A summary of stock option and RSU transactions in the nine months ended September 30,2022, is as follows:
Weighted | Weighted | |||||||||||||||||||
Shares | Number of | Average | Number of | Average | ||||||||||||||||
Available | Options | Exercise | RSUs | Grant Date | ||||||||||||||||
For Grant | Outstanding | Price | Outstanding | Fair Value | ||||||||||||||||
Balance at January 1, 2022 | 5,582,742 | 10,216,106 | $ | 4.96 | — | $ | — | |||||||||||||
2019 Plan Amendment | 12,000,000 | — | $ | — | — | $ | — | |||||||||||||
Granted | (6,530,935 | ) | 5,777,657 | $ | 4.38 | 753,278 | $ | 3.98 | ||||||||||||
Exercised | — | (166,993 | ) | $ | 1.28 | — | $ | — | ||||||||||||
Forfeited | 818,749 | (789,252 | ) | $ | 6.40 | (29,562 | ) | $ | 3.79 | |||||||||||
Canceled | 61,521 | (71,705 | ) | $ | 8.82 | — | $ | — | ||||||||||||
Balance at September 30, 2022 | 11,932,077 | 14,965,813 | $ | 4.68 | 723,716 | $ | 3.99 | |||||||||||||
As of September 30, 2022, there were 14,965,813 options outstanding with a weighted average exercise price of $4.68, a weighted average remaining term of 8.45 years and an aggregate intrinsic value of $1.4 million. Of these options, 5,415,197 were vested, with a weighted average exercise price of $3.75, a weighted average remaining term of 7.21 years and an aggregate intrinsic value of $1.2 million. The Company received $214,000 for the 166,993 options exercised during the nine months ended September 30,2022, which had an intrinsic value of $474,000, and received $1.2 million for the 771,344 options exercised during the nine months ended September 30,2021, which had an intrinsic value of $4.6 million.
The weighted average grant date fair value of options awarded in the nine months ended September 30,2022 and 2021, was $3.88 and $6.67, respectively. Their fair values were estimated using the following assumptions:
Nine Months Ended September 30, | ||||||||
2022 | 2021 | |||||||
Risk-free interest rate | 1.62% - 3.20% | 0.91% - 1.19% | ||||||
Expected term (in years) | 5.42 - 6.08 | 5.44 - 6.08 | ||||||
Expected volatility | 125% - 131% | 122% - 131% | ||||||
Dividend yield | —% | —% | ||||||
VAXART, INC.
Notes to the Condensed Consolidated Financial Statements (Unaudited)
The Company measures the fair value of all stock-based awards on the grant date and records the fair value of these awards, net of estimated forfeitures, to compensation expense over the service period. Total stock-based compensation recognized for options and RSUs was as follows (in thousands):
Three Months Ended September 30, | Nine Months Ended September 30, | |||||||||||||||
2022 | 2021 | 2022 | 2021 | |||||||||||||
Research and development | $ | 2,567 | $ | 1,206 | $ | 6,977 | $ | 2,429 | ||||||||
General and administrative | 1,076 | 893 | 3,217 | 3,525 | ||||||||||||
Total stock-based compensation | $ | 3,643 | $ | 2,099 | $ | 10,194 | $ | 5,954 | ||||||||
As of September 30, 2022, the unrecognized stock-based compensation cost related to outstanding unvested stock options and RSUs expected to vest was $38.4 million, which the Company expects to recognize over an estimated weighted average period of 3.05 years.
On August 4, 2022, the 2022 Employee Stock Purchase Plan (the “2022 ESPP”) was approved by the Company’s stockholders. 1,800,000 shares of common stock are reserved for issuance under the 2022 ESPP.
NOTE 11. Net LossPer Share
The following table presents the calculation of basic and diluted net loss per share (in thousands, except share and per share amounts):
Three Months Ended September 30, | Nine Months Ended September 30, | |||||||||||||||
2022 | 2021 | 2022 | 2021 | |||||||||||||
Net loss | $ | (29,309 | ) | $ | (17,583 | ) | $ | (83,840 | ) | $ | (49,706 | ) | ||||
Shares used to compute net loss per share – basic and diluted | 126,889,718 | 123,984,141 | 126,374,424 | 120,110,780 | ||||||||||||
Net loss per share – basic and diluted | $ | (0.23 | ) | $ | (0.14 | ) | $ | (0.66 | ) | $ | (0.41 | ) | ||||
No adjustment has been computed based onmade to the net loss in the threeand nine months ended September 30,2022 or 2021, as the weighted-average number of common shares outstanding during the applicable period. For diluted net loss per share, common stock equivalents (shares of common stock issuable upon the exercise of stock options and unvested restricted stock units) are excluded from the calculation as their inclusioneffect would be anti-dilutive. The Company has excluded all anti-dilutive share-based awardsdue to purchase common stock in periods indicating a loss, as their effect is anti-dilutive.
the net loss.
The following tables set forthpotentially dilutive securities were excluded from the computation of historical basic and diluted net loss per share.weighted average shares outstanding because they would have been antidilutive:
Three Months Ended | ||||||||
2017 | 2016 | |||||||
Net loss (in millions) | $ | (3.4 | ) | $ | (9.1 | ) | ||
Weighted-average shares outstanding | 38,649,237 | 38,640,487 | ||||||
Dilutive effect of restricted stock and stock options | - | - | ||||||
Shares used to compute diluted earnings per share | 38,649,237 | 38,640,487 | ||||||
Basic net loss per share | $ | (0.09 | ) | $ | (0.24 | ) | ||
Diluted net loss per share | $ | (0.09 | ) | $ | (0.24 | ) | ||
Number of anti-dilutive share-based awards excluded from computation | 6,898,629 | 5,645,543 | ||||||
Six Months Ended | ||||||||
2017 | 2016 | |||||||
Net loss (in millions) | $ | (8.7 | ) | $ | (19.1 | ) | ||
Weighted-average shares outstanding | 38,649,237 | 38,640,487 | ||||||
Dilutive effect of restricted stock and stock options | - | - | ||||||
Shares used to compute diluted earnings per share | 38,649,237 | 38,640,487 | ||||||
Basic net loss per share | $ | (0.23 | ) | $ | (0.49 | ) | ||
Diluted net loss per share | $ | (0.23 | ) | $ | (0.49 | ) | ||
Number of anti-dilutive share-based awards excluded from computation | 6,898,629 | 5,645,543 | ||||||
Three Months Ended September 30, | Nine Months Ended September 30, | |||||||||||||||
2022 | 2021 | 2022 | 2021 | |||||||||||||
Options to purchase common stock | 14,735,710 | 8,464,753 | 13,170,959 | 7,683,510 | ||||||||||||
Restricted stock units | 660,660 | — | 353,738 | — | ||||||||||||
Warrants to purchase common stock | 227,434 | 232,434 | 229,156 | 413,121 | ||||||||||||
Total potentially dilutive securities excluded from denominator of the diluted earnings per share computation | 15,623,804 | 8,697,187 | 13,753,853 | 8,096,631 | ||||||||||||
NOTE 12. Subsequent Events |
|
Royalty agreements
The Company entered into a royalty-bearing research and license agreement with GlaxoSmithKline (“GSK”) inSince 1990September 30, 2022, for the development and commercialization of zanamivir, a neuraminidase inhibitor marketed by GSK as Relenza® to treat influenza. Under the terms of the agreement, the Company licensed zanamivir to GSK on an exclusive, worldwide basis. Mosthas issued 3,194,043 shares of common stock under the Company’s Relenza® patents have expired and the only substantial remaining intellectual property related to the Relenza® patent portfolio is scheduled to expire in July 2019September 2021 in Japan. Until that patent expires, the Company will receive aATM (see Note 7%1 royalty on GSK’s annual) for net sales of Relenza® in Japan.proceeds totaling $5.5 million.
The Company also generates royalty revenue from the sale of Inavir® (laninamivir octanoate or LANI) in Japan, pursuant to a collaboration and license agreement and a related commercialization agreement (collectively, the “Inavir® License Agreement”) with Daiichi Sankyo. Under the Inavir® License Agreement, the Company currently receives a 4% royalty on net sales of Inavir® in Japan and is eligible to earn sales milestone payments. Under the Inavir® License Agreement, the Company and Daiichi Sankyo have cross-licensed the world-wide rights to develop and commercialize the related intellectual property, and have agreed to share equally in any royalties, license fees, or milestone or other payments received from any third party licenses outside of Japan. The patent relating to hydrates and the crystalline form of LANI used in Inavir® expires in 2021 (not including extensions)Changes in the U.S.status of litigation since September 30,2022, are included in “Note 8. Commitments and EU and in 2024 in Japan. In February 2015, a patent containing claims relevant to the manufacture of Inavir® was issued in Japan and expires in December 2029.
In April 2016, the Company entered into a Royalty Interest Acquisition Agreement (“Agreement”) with HCRP. Under the Agreement, HCRP made a $20 million cash payment to the Company in consideration for acquiring from the Company certain royalty rights (“Royalty Rights”) related to Inavir® in the Japanese market.
The following tables summarize the key components of the Company’s revenues (in millions):
Three Months Ended December 31, | ||||||||
2017 | 2016 | |||||||
(in millions) | ||||||||
Royalty revenue - Relenza® | $ | - | $ | 1.5 | ||||
Non-cash royalty revenue related to the sale of future royalties | 2.7 | 2.3 | ||||||
Total revenue | $ | 2.7 | $ | 3.8 | ||||
Six Months Ended December 31, | ||||||||
2017 | 2016 | |||||||
(in millions) | ||||||||
Royalty revenue - Relenza® | $ | - | $ | 1.6 | ||||
Non-cash royalty revenue related to the sale of future royalties | 2.8 | 2.3 | ||||||
Total revenue | $ | 2.8 | $ | 3.9 | ||||
Relenza revenue declined to zero in the three and six months ended December 31, 2017 from $1.5 million in the same periods of the prior fiscal year due to the cessation of royalties on U.S. sales at the end of 2016 and the unfavorable impact of a returns adjustment in the current quarter.
Collaborative and contract arrangements
In July 2016, the Company entered into an exclusive, worldwide license for RSV replication inhibitors intellectual property with Georgia State University Research Foundation (“GSURF”) in exchange for an upfront fee, future milestone payments and royalties on future net sales of any products that utilize the underlying RSV intellectual property. The Company has an obligation to make a minimum payment of $10,000 to GSURF annually until the license agreement expires or is terminated. The Company also entered into a two year sponsored research agreement with GSURF for annual sponsored research payments.Contingencies—(c) Litigation”.
|
|
On December 22, 2017, the U.S. government enacted comprehensive tax legislation commonly referred to as the Tax Cuts and Jobs Act (the “Tax Act”). The Tax Act significantly revises the future ongoing U.S. corporate income tax by, among other things, lowering U. S. corporate income tax rates and implementing a territorial tax system. Since the Company is a calendar year tax filer, the lower corporate income tax rate will be effective beginning January 1, 2018.
Based upon the provisions of the Tax Act, the Company’s deferred tax assets and liabilities will be remeasured to incorporate the lower corporate tax rate of 21% into its tax provision; however, since the Company maintains a full valuation allowance, there is no net impact to income tax expense reported in the Company’s financial statements for the periods presented as the provisional valuation allowance will be adjusted accordingly. At this time, the Company is still evaluating the impact of this remeasurement.
There are also certain transitional impacts of the Tax Act. As part of the transition to the new territorial tax system, the Tax Act imposes a one-time repatriation tax on deemed repatriation of historical earnings and profits (“E&P”) of foreign subsidiaries. Due to the complexity of this calculation and the information required to complete such a calculation, the Company is still reviewing its E&P from our foreign subsidiaries in connection with the one-time transition tax.
The Company is also currently analyzing its global working capital and cash requirements and the potential tax liabilities attributable to a repatriation, including calculating any excess of the amount for financial reporting over the tax basis in its foreign subsidiaries, but has yet to determine whether it plans to change its prior assertion and repatriate earnings. Accordingly, the Company has not recorded any deferred taxes attributable to its investments in its foreign subsidiaries. The Company will record the tax effects of any change in its prior assertion in the period that it completes its analysis and are able to make a reasonable estimate, and disclose any unrecognized deferred tax liability for temporary differences related to its foreign investments, if practicable.
The changes included in the Tax Act are broad and complex. The final transition impacts of the Tax Act may affect our financial statements and/or disclosures, possibly materially, due to, among other things, changes in interpretations of the Tax Act, any legislative action to address questions that arise because of the Tax Act, any changes in accounting standards for income taxes or related interpretations in response to the Tax Act. The Securities Exchange Commission has issued rules that would allow for a measurement period of up to one year after the enactment date of the Tax Act to finalize the recording of the related tax impacts.
ITEM 2: Item 2. ManagementManagement’s’s Discussion and Analysis of Financial Condition and Results of Operations
FORWARD LOOKING STATEMENTS
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our condensed consolidated financial statements and related notes included elsewhere in this Quarterly Report on Form 10-Q and with our audited consolidated financial statements included in our Annual Report on Form 10-K filed with the SEC on February 24, 2022. This Quarterly Report on Form 10-Q contains forward-looking statements. These forward-looking statements involve knownwithin the meaning of Section 27A of the Securities Act of 1933, as amended, and unknown risks, uncertaintiesSection 21E of the Securities Exchange Act of 1934, as amended, which are subject to the “safe harbor” created by those sections. Forward-looking statements are based on our management’s beliefs and other factors that may cause actual results, performance or achievementsassumptions and on information currently available to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements.our management. In mostsome cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “could,” “goal,” “would,” “expect,” “plan,” “intend,” “anticipate,” “believe,” “estimate,” “project,” “predict,” “forecast,” “potential,” “likely” or “possible”, as well as the negative of such expressions,“potential” and similar expressions intended to identify forward-looking statements and reflect our beliefs and opinions on the relevant subject. Our actual results could differ materially from those discussed in the forward-looking statements. TheseFactors that could cause or contribute to these differences include those discussed below and in this Quarterly Report on Form 10-Q. The forward-looking statements include, without limitation,included in this Quarterly Report on Form 10-Q are made only as of the date hereof. These statements relating to:are based upon information available to us as of the filing date of this Quarterly Report on Form 10-Q, and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain, and we caution investors against unduly relying upon these statements. In all events, we undertake no obligation to revise or update any forward-looking statements, whether as a result of new information, change in circumstances, future events or otherwise, and you are advised to consult any additional disclosures that we may make directly to you or through reports that we, in the future, may file with the SEC, including annual reports on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K.
Company Overview and Background
We are a clinical-stage biotechnology company primarily focused on the development of oral recombinant vaccines based on our Vector-Adjuvant-Antigen Standardized Technology (“VAAST”) proprietary oral vaccine platform. Our oral vaccines are designed to generate broad and durable immune responses that may protect against a wide range of infectious diseases and may be useful for the treatment of chronic viral infections and cancer. Our investigational vaccines are administered using a room temperature-stable tablet, rather than by injection.
We are developing prophylactic vaccine candidates that target a range of infectious diseases, including SARS-CoV-2 (the virus that causes coronavirus disease 2019 (“COVID-19”)), norovirus (a widespread cause of acute gastro-intestinal enteritis) and seasonal influenza. We have completed a Phase 1 clinical trial for our first SARS CoV-2 vaccine candidate and reported that the study met its primary and secondary endpoints. A Phase 2 study with our second SARS CoV-2 vaccine candidate commenced in late 2021 has been completed. Vaxart has also initiated preclinical work on COVID-19 vaccine candidates that directly target the Omicron BA.4 and BA.5 subvariants. Several Phase 1 human studies with our norovirus vaccine candidate have been successfully completed. A Phase 2 challenge study evaluating safety and clinical efficacy of our GI.1 norovirus vaccine candidate is currently ongoing. Data indicating that our monovalent H1 influenza vaccine protected participants against H1 influenza infection in a Phase 2 challenge study was published in 2020 (Lancet ID). In addition, we are in early development of a prophylactic vaccine targeting respiratory syncytial virus (“RSV”) (a common cause of respiratory tract infection) and of our first therapeutic vaccine targeting cervical cancer and dysplasia caused by human papillomavirus (“HPV”).
Vaxart Biosciences, Inc. was originally incorporated in California in March 2004, under the name West Coast Biologicals, Inc. and changed its name to Vaxart, Inc. (“Private Vaxart”), in July 2007, and reincorporated in the state of Delaware. On February 13, 2018, Private Vaxart completed a reverse merger (the “Merger”), with Aviragen Therapeutics, Inc. (“Aviragen”), pursuant to which Private Vaxart survived as a wholly owned subsidiary of Aviragen. Under the terms of the Merger, Aviragen changed its name to Vaxart, Inc. and Private Vaxart changed its name to Vaxart Biosciences, Inc.
Business Update Regarding COVID-19
The COVID-19 outbreak has presented a substantial public health and economic challenge around the world and is affecting employers, employees, patients, communities and business operations, as well as the U.S. economy and financial markets. The full extent to which the COVID-19 outbreak will directly or indirectly impact our business, results of operations and financial condition will depend on future developments that are highly uncertain and cannot be accurately predicted, including new information that may emerge concerning COVID-19, the actions taken to contain it or treat its impact and the economic impact on local, regional, national and international markets.
To date, we have been able to continue our operations and do not anticipate any material interruptions in the foreseeable future. However, we are continuing to assess the potential impact of the COVID-19 pandemic and the development of other competing COVID-19 vaccines on our business and operations, including our expenses, supply chain and clinical trials. Our partners are currently operating their facilities at or near normal levels. While we currently do not anticipate any interruptions in our operations, it is possible that the COVID-19 pandemic and response efforts may have an impact in the future on our operations and/or the operations of our third-party suppliers and partners. Any recovery from negative impacts to our business and related economic impact due to the COVID-19 outbreak may also be slowed or reversed by a number of factors, including the emergence of new coronavirus strains.
Our Product Pipeline
The following table outlines the status of our oral vaccine development programs:
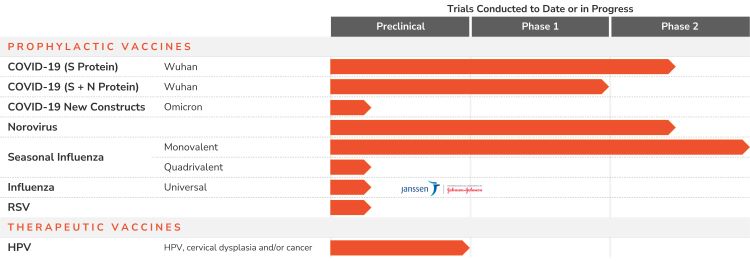
We are developing the following tablet vaccine candidates, which are based on our proprietary platform:
● |
|
|
|
|
|
|
|
|
|
Various important factorsWe are developing an oral tablet vaccine to protect against SARS-CoV-2 infection, the virus that causes COVID-19. We generated multiple vaccine candidates based on the published genome of SARS-CoV-2 and evaluated them in preclinical models for their ability to generate both mucosal and systemic immune responses. Of particular interest will be the mucosal immune responses, as coronavirus is primarily an infection of the respiratory tract. We believe the logistical advantages of an oral vaccine that is administered using a convenient room temperature-stable tablet could cause actual results, performance, events or achievementsbe of critical benefit when rolling out a major public health vaccination campaign. Given the recent emergence of coronavirus strains with mutated S proteins that are considered more contagious than the original strain, serum antibodies from injected vaccines may not adequately protect against these SARS-CoV-2 variants over time, whereas a vaccine that is able to materially differ from those expressed or implied by forward-looking statements, including create cross-reactive mucosal antibodies and T cells against conserved epitopes may have significant advantages.
On September 14, 2020, we announced that the U.S. Food and Drug Administration (“FDA”(the “FDA”) or a similar regulatory body in another country, a data safety monitoring board, or an institutional review board delaying, limiting, suspending or terminating anyhad cleared our Investigational New Drug (“IND”) application to allow initiation of human clinical testing of our first oral COVID-19 (S and N proteins) vaccine candidate VXA-CoV2-1. On October 13, 2020, we announced that Phase 1 clinical testing had commenced and on February 3, 2021, we announced the preliminary results of the Company’s clinical development programs at any time for a lacktrial. The study achieved both its primary and secondary endpoints of safety efficacy, tolerability, anti-viral activity, commercial viability, regulatory or manufacturing issues, or any other reason whatsoever; the Company's ability to secure, manage and retain qualified third-party clinical research, preclinical research,immunogenicity, respectively. Initial results showing cross-reactive mucosal antibody responses were published in Science Translational Medicine along with animal data management and contract manufacturing organizations upon which it relies to assist in the design, development, implementation and execution of the clinical and preclinical development of all its product candidates; and these third-party organizations fulfilling their contractual obligations on a timely and satisfactory basis; the safety or efficacy data from planned or ongoing future preclinical and clinical studies of any of its product candidates not supporting the clinical development of that product candidate; the successful enrollment of the requisite number ofdescribed below. Additional detailed study participants on a timely basis; the Company’s ability to comply with applicable government regulations in various countries and regions in which we are conducting, or expect to conduct, clinical trials; the Company’s ability to retain and recruit sufficient staff, including key executive management and employees, to manage our business; the Company’s ability to maintain, protect or defend its proprietary rights from unauthorized use by others, or not infringe on the intellectual property rights of others; our ability to successfully manage our expenses, operating results and financial positionmucosal durability data were reported in linemedRxiv in July 2022.
We announced in February 2021 that we would evaluate additional vaccine candidates that contain just the Spike protein, and different variant-specific vaccines. After preclinical evaluations (including in non-human primate studies) showed that an improved antibody response could be achieved with our plansa new vaccine candidate that expressed just the Spike protein, we decided to move this candidate forward for clinical evaluation. This new vaccine candidate, VXA-CoV2-1.1-S, was also able to elicit antibody responses against human coronavirus strain variants such as Beta (first identified in South Africa) and expectationsDelta (first identified in India) in animals (published in bioRxiv; the condition of the financial equity in February 2022). Further, this new vaccine candidate was tested in a vaccine breakthrough/transmission model led by Duke University and debt markets and our abilityfound to raise sufficient fundinginhibit aerosol transmission to vaccine-naïve animals better than an injected S-protein-based vaccine candidate. These results were published in such markets; changesbioRxiv in the general economic business or competitive conditions in the industry or with respect to our product candidates; potential employee resignations on short notice; provisions in certificate of incorporation, bylaws and laws of Delaware containing provisions that could delay or discourage a change in control of the Company; the Company’s obtaining the requisite stockholder approval and other conditions to the Merger being satisfied; and other cautionary statements contained elsewhere in this Quarterly Report on Form10-Q October 2021 and in the Company’s Annual Report on Form 10-Kpeer-reviewed journal Science Translational Medicine in May 2022. A press release issued in June 2022 described a hamster challenge study comparing an S specific vaccine expressing the original parental strain to a variant-specific S vaccine for protection against Omicron. Results showed that both constructs could provide protection, but the year ended June 30, 2017, as filed withOmicron-specific vaccine had slightly better immune responses against the U.S. Securities and Exchange Commission on September 1, 2017.Omicron variant.
There may be eventsA new IND was filed for this S-only vaccine candidate in June 2021 and was cleared by the FDA in July 2021. We initiated dosing with this candidate in a two-part Phase 2 clinical study in October 2021, with approximately 896 participants planned for enrollment utilizing a two-part study design. The first part of the study (“Part 1”) planned enrollment of 48 participants aged 18 to 55 and 48 participants aged 56 to 75, in order to further evaluate safety and immunogenicity and to assess optimal dosage. Further, half the subjects in the future that we are unabletrial would be prior vaccinated (have received two doses of an mRNA vaccine) to predict accurately, or over which we have no control. You should completely read this Form 10-Qtest the ability of the Vaxart COVID-19 vaccine candidate to boost immune responses and enhance variant-specific cross-reactivity, and half the documents that we reference herein that havesubjects would be naïve to prior vaccinations. The purpose of the study was to evaluate safety and immunogenicity and to assess optimal dosage. Upon dose selection from Part 1, the second part of the study (“Part 2”) planned enrollment of approximately 800 subjects aged 18 to 75. Part 2 is designed to test preliminary vaccine efficacy to protect against SARS-CoV-2 infection. The first part of the study (“Part 1”) has been filed or incorporated by reference as exhibits and withcompleted. The actual enrollment of participants for Part 1 was less than planned due to the understanding that our actual future results may be materially different from what we expect. Our business, financial condition, resultsinability to identify vaccine-naïve individuals. Approximately half of operations, and prospects may change. We may not update these forward-looking statements, even though our situation may changethe subjects in the future, unless we havetrial were prior vaccinated (have received two doses of an obligation undermRNA vaccine) to test the federal securities laws to update and disclose material developments related to previously disclosed information.We qualify allability of the information presentedVaxart COVID-19 vaccine candidate to boost immune responses and enhance variant-specific cross-reactivity. Top-line data from this portion of the trial was announced in September 2022, indicating that the primary and secondary end-points were both met. Vaxart’s oral vaccine candidate was able to boost the serum antibody responses for volunteers that previously received an mRNA vaccine (either Pfizer/BioNTech or Moderna). Serum neutralizing antibody responses to SARS-CoV-2 (Wuhan), a recognized correlate of protection, were boosted in this Form 10-Q,population from a geometric mean of 481 to 778, a fold rise of 1.6. Volunteers that had lower starting titers had larger increases than subjects that had higher titers. There were also substantial increases in the neutralizing antibody responses to the SARS-CoV-2 Omicron BA4/5 in these volunteers as measured by sVNT assay. Increases in the mucosal IgA antibody responses (antibodies in the nose and particularly our forward-looking statements, bymouth) were observed in approximately 50% of subjects. Subjects that had an increase in the mucosal IgA response to SARS-CoV-2 Wuhan S had an increase in IgA responses to other coronaviruses including SARS-CoV-2 Omicron BA4/5 and SARS-CoV-1, demonstrating the cross-reactive nature of these cautionary statements.immune readouts. We are currently evaluating the results from Part 1 to determine whether we will proceed to Part 2 of the study.
.
We have initiated work on vaccine candidates that directly target new Omicron BA.4 and BA.5 subvariants. These candidates are currently in preclinical testing. We are also developing bivalent candidates that contain Wuhan and Omicron antigens, and will also evaluate these new constructs as part of a potential bivalent vaccine for COVID-19. We will determine the best path forward in developing a vaccine that can hinder viral infection and transmission for current and emerging variants. Our expectation is that these vaccine candidates will be available to evaluate preclinically in the fourth quarter of 2022, and clinically in the first half of 2023. We have announced a partnership with hVivo to test a Vaxart vaccine candidate for efficacy in a SARS-CoV-2 human challenge study. This study is expected to begin in 2023 once hVivo has completed GMP manufacturing and testing of a viral challenge strain.
Additionally, international Phase 1b and Phase 2 COVID-19 trials, including a placebo-controlled efficacy trial in India, are under consideration.
● | Norovirus Vaccine. Norovirus is the leading cause of acute gastroenteritis symptoms, such as vomiting and diarrhea, among people of all ages in the United States. Each year, on average, norovirus causes up to 21 million cases of acute gastroenteritis and contributes up to 109,000 hospitalizations and 900 deaths, mostly among young children and older adults. Typical symptoms include dehydration, vomiting, diarrhea with abdominal cramps, and nausea. In a study by the CDC and Johns Hopkins University, published in 2016, the global economic impact of norovirus disease was estimated at $60 billion, $34 billion of which occurred in high income countries including the United States, Europe and Japan. An update by the lead authors estimated the burden in the U.S. alone to be $10.5 billion in 2018. Virtually all norovirus disease is caused by norovirus GI and GII genotypes, and we are developing a bivalent vaccine designed to protect against both. We anticipate that, if approved, the vaccine will be an annual, one-time administration ahead of the winter season when norovirus incidence is at its peak, similar to the influenza season. |
In 2019, we completed the active phase of a Phase 1b clinical trial with our bivalent oral tablet vaccines for the GI.1 and GII.4 norovirus strains. Both the oral norovirus GI.1 and GII.4 vaccines were well tolerated with no serious adverse events reported. Most solicited and unsolicited adverse events were mild in severity, and there were no significant differences observed between the vaccine and placebo treatment groups.
Vaxart’s bivalent vaccine (GI.1 and GII.4 co-administered) demonstrated robust immunogenicity, with an IgA ASC response rate of 78% for the GI.1 strain and 93% for the GII.4 strain for the bivalent cohort of the study, when compared to 86% and 90%, respectively, for the two monovalent cohorts of the study. These results indicate that co-administration of the two vaccines, the intended approach for proceeding into Phase 2 and 3 trials, shows no cross-interference, or reduction from the response observed with individual (monovalent) vaccine delivery.
We resumed clinical development of our norovirus vaccine candidate in late 2020 by planning the conduct of three clinical trials. In early 2021 we initiated dosing a subset of subjects (second dose after more than one year) in the Phase 1b bivalent study. In results announced on July 29, 2021, we reported that we were able to successfully boost immune responses with the G1.1 norovirus tablets in prior vaccinated subjects. These responses include IgA antibody secreting cells, as well as IgG and IgA serum antibody responses. In mid-2021 we started a placebo-controlled, dose ranging study in elderly adult subjects aged 55 to 80 to evaluate the safety and immunogenicity of the vaccine in the older population. The top-line results were disclosed in June of 2022. The immune response to the vaccine was similar in healthy older individuals (ages 55 to 80) as it was in younger individuals in a previous study as measured by the numbers of antibody secreting cells (IgA ASC) and serum antibodies. Lastly, we also conducted an open-label trial to evaluate the optimal timing of boost administration in young adults in which 3 cohorts of subjects received their second dose (boost) at varying timepoints between 1 and 3 months post initial vaccination. This study was performed as data from trials with adenovirus vaccines indicate that boost administration at a later timepoint (e.g., 12 weeks) may offer a more robust immune response. The top-line results from this study were disclosed in June of 2022. Data indicated that the vaccine candidate was able to successfully boost antibody responses, with antibody responses trending better with administration spread out over 3 months versus a shorter interval.
We are also conducting additional Phase 2 clinical trials with our norovirus vaccine candidates. The first trial, which was initiated in early 2022, is a Phase 2 norovirus challenge study which will evaluate safety, immunogenicity and clinical efficacy of a norovirus GI.1 vaccine compared to a placebo control post norovirus challenge. A futility analysis is expected in fourth quarter of 2022, and if positive, efficacy results are expected end of the first quarter of 2023. The second clinical trial will be a Phase 2 multi-center, placebo-controlled dose confirmation trial evaluating the safety and immunogenicity of Vaxart’s bivalent norovirus vaccine in subjects aged 18 years and older. This trial is expected to begin in late 2022 or early 2023. The data from these Phase 2 studies is expected to form the basis (safety, immunogenicity and preliminary efficacy data) for an End of Phase 2 Meeting with the FDA to gain concurrence on the scope and design of the Phase 3 pivotal efficacy study in adults over 18 years of age.
● | Seasonal Influenza Vaccine. Influenza is a major cause of morbidity and mortality in the U.S. and worldwide and, according to the CDC, only 49% of eligible U.S. citizens were vaccinated in 2018/2019, with particularly low vaccination rates among adults between ages 18 and 49. We believe our oral tablet vaccine has the potential to improve the protective efficacy of currently available influenza vaccines and increase flu vaccination rates. |
Influenza is one of the most common global infectious diseases, causing mild to life-threatening illness and even death. Approximately 350 million cases of seasonal influenza occur annually worldwide, of which three to five million cases are considered severe, causing 290,000 to 650,000 deaths per year. During the flu season of 2018/2019 there were 34,200 flu related deaths in the U.S. alone, according to the CDC. Very young children and the elderly are at the greatest risk. In the United States, between 5% and 20% of the population contracts influenza, 226,000 people are hospitalized with complications of influenza, and between 3,000 and 49,000 people die from influenza and its complications each year, with up to 90% of the influenza-related deaths occurring in adults older than 65. The total economic burden of seasonal influenza has been estimated to be $87.1 billion, including medical costs which average $10.4 billion annually, while lost earnings due to illness and loss of life amount to $16.3 billion annually.
We believe our tablet vaccine candidate may potentially address many of the limitations presented by injectable egg-based influenza vaccines for the following reasons: (i) our tablet vaccine candidates are designed to create broad and durable immune responses, which may provide more effective immunity and protect against additional strain variants; (ii) our vaccine is delivered as a room temperature-stable tablet, which we believe would provide a more convenient method of administration, enhancing patient acceptance and simplifying the distribution and administration process; (iii) we believe our tablet vaccine may be manufactured more rapidly than vaccines manufactured using egg-based methods by using recombinant methods; and (iv) using our tablet vaccine in lieu of egg-based vaccines would eliminate the risk of experiencing allergic reactions to egg protein.
In September 2018, we completed a $15.7 million contract with the U.S. Government through the Department of Health and Human Services, Office of Biomedical Advanced Research and Development Authority (“HHS BARDA”) under which a Phase 2 challenge study of our H1N1 flu vaccine candidate was conducted. We announced that, in healthy volunteers immunized and then experimentally infected with H1 influenza, our H1 influenza oral tablet vaccine reduced clinical disease by 39% relative to placebo. Fluzone, the market-leading injectable quadrivalent influenza vaccine, reduced clinical disease by only 27%. Our tablet vaccine also showed a favorable safety profile, indistinguishable from placebo.
On October 4, 2018, we presented data from the study demonstrating that our vaccine elicited a significant expansion of mucosal homing receptor plasmablasts to approximately 60% of all activated B cells. We believe these mucosal plasmablasts are a key indicator of a protective mucosal immune response and a unique feature of our vaccines. This data also indicates that our vaccines provide protection by inducing mucosal immunity (the first line of defense against mucosal infections such as flu, norovirus and RSV), marking what could be a key advantage over injectable vaccines.
In addition to our conventional seasonal flu vaccine, we entered into a research collaboration agreement with Janssen Vaccines & Prevention B.V. (“Janssen”) in July 2019 to evaluate our proprietary oral vaccine platform for the Janssen universal influenza vaccine program. Under the agreement, we produced a non-GMP oral vaccine candidate containing certain proprietary antigens from Janssen and tested the product in a preclinical challenge model. The preclinical study has been completed and we have submitted a report to Janssen.
● | RSV Vaccine. RSV is a major respiratory pathogen with a significant burden of disease in the very young and in the elderly. |
Based on the positive results of our preclinical cotton rat study, we believe our proprietary oral vaccine platform has the potential to be the optimal vaccine delivery system for RSV, offering significant advantages over injectable vaccines.
● | HPV Therapeutic Vaccine. Ourfirst therapeutic oral vaccine candidate targets HPV 16 and HPV 18, the two strains responsible for 70% of cervical cancers and precancerous cervical dysplasia. |
Cervical cancer is the fourth most common cancer in women worldwide and in the United States with about 13,000 new cases diagnosed annually in the United States according to the National Cervical Cancer Coalition.
We have tested our HPV 16 vaccine candidate in two different HPV 16 solid tumor models in mice. The vaccine elicited T cell responses and promoted migration of the activated T cells into the tumors, leading to tumor cell killing. Mice that received our HPV 16 vaccine showed a significant reduction in volume of their established tumors.
In October 2018, we filed a pre-IND meeting request with the FDA for our first therapeutic vaccine targeting HPV 16 and HPV 18 and we subsequently submitted our pre-IND briefing package. We received feedback from the FDA in January 2019 to support submission of an IND application to support initiation of clinical testing. Vaxart plans to initiate its clinical program of an oral HPV tableted vaccine with a clinical trial in young adult women with HPV 16 or HPV 18-associated High-grade Squamous Intraepithelial Lesion (HSIL), pending regulatory and IRB/EC approvals. The trial would evaluate the safety, immunogenicity and preliminary clinical efficacy with repeat dose vaccine administration against a placebo control group.
Antivirals
● | Through the Merger, we acquired two royalty earning products, Relenza and Inavir. We also acquired three Phase 2 clinical stage antiviral compounds, which we have discontinued independent clinical development of. However, for one of these, Vapendavir, we have entered into an exclusive worldwide license agreement with Altesa Biosciences, Inc. (“Altesa”) on July 6, 2021, permitting Altesa to develop and commercialize this capsid-binding broad spectrum antiviral. |
● | Relenza and Inavir are antivirals for the treatment of influenza, marketed by GlaxoSmithKline, plc (“GSK”) and Daiichi Sankyo Company, Limited (“Daiichi Sankyo”), respectively. We have earned royalties on the net sales of Relenza and Inavir in Japan. The last patent for Relenza expired in July 2019 and the last patent for Inavir expires in December 2029. Sales of these antivirals vary significantly by quarter, because influenza virus activity displays strong seasonal cycles, and by year depending on the intensity and duration of the flu season, the impact COVID-19 has had, and may continue to have, on seasonal influenza, and competition from other antivirals such as Tamiflu and Xofluza. |
Financial Operations Overview
Revenue
Aviragen isRevenue from Customer Service Contracts
We earned revenue from a registered trademarkfixed price service contract, as amended, for a total of Aviragen Therapeutics Inc., Relenza$617,000, which we completed in the first three months of 2021.
Royalty R®evenueis
We earn royalty revenue, net of amounts recognized as non-cash royalty revenue related to the sale of future royalties, based on a registered trademarkfixed percentage of GlaxoSmithKline plc, andnet sales of Inavir,®is a registered trademark oftreatment for influenza, from our licensee, Daiichi Sankyo, Company, Ltd.under a royalty agreement which will expire in December 2029.
Non-Cash Royalty Revenue Related to the Sale of Future Royalties
References
In April 2016, Aviragen sold certain royalty rights related to “we,” “us,”Inavir in the Japanese market for $20.0 million to HealthCare Royalty Partners III, L.P. (“HCRP”). We pay HCRP the first $3 million plus 15% of the next $1 million of royalties earned in annual periods ending on March 31. At the time of the Merger, the estimated future benefit to HCRP was remeasured at fair value and “our” referwas estimated to Aviragen Therapeutics, Inc.be $15.9 million, which we account for as a liability and its subsidiaries.amortize using the effective interest method over the remaining estimated life of the arrangement. The estimated future benefit was remeasured as of December 31, 2021, when the fair value was estimated to be $11.5 million, resulting in a revaluation gain of $3.8 million. Even though we do not retain the related royalties under the transaction, as the amounts are remitted to HCRP, we will continue to record revenue related to these royalties until the amount of the associated liability and related interest is fully amortized.
Research and Development Expenses
Research and development expenses represent costs incurred on conducting research, such as developing our tablet vaccine platform, and supporting preclinical and clinical development activities of our tablet vaccine candidates. We recognize all research and development costs as they are incurred. Research and development expenses consist primarily of the following:
● | employee-related expenses, which include salaries, benefits and stock-based compensation; |
● | expenses incurred under agreements with contract research organizations (“CROs”), that conduct clinical trials on our behalf; |
● | expenses incurred under agreements with contract manufacturing organizations (“CMOs”), that manufacture product used in the clinical trials; |
● | expenses incurred in procuring materials and for analytical and release testing services required to produce vaccine candidates used in clinical trials; |
● | process development expenses incurred internally and externally to improve the efficiency and yield of the bulk vaccine and tablet manufacturing activities; |
● | laboratory supplies and vendor expenses related to preclinical research activities; |
● | consultant expenses for services supporting our clinical, regulatory and manufacturing activities; and |
● | facilities, depreciation and allocated overhead expenses. |
We do not allocate our internal expenses to specific programs. Our employees and other internal resources are not directly tied to any one research program and are typically deployed across multiple projects. Internal research and development expenses are presented as one total.
We have incurred significant external costs for CROs that conduct clinical trials on our behalf, and for CMOs that manufacture our tablet vaccine candidates, although these costs have decreased in 2022 since we now perform the majority of our manufacturing activities in-house. We have captured these external costs for each vaccine program. We do not allocate external costs incurred on preclinical research or process development to specific programs.
The following table shows our period-over-period research and development expenses, identifying external costs that were incurred in each of our vaccine programs and, separately, on preclinical research and process development (in thousands):
Three Months Ended September 30, | Nine Months Ended September 30, | |||||||||||||||
2022 | 2021 | 2022 | 2021 | |||||||||||||
External program costs: | ||||||||||||||||
COVID-19 program | $ | 2,287 | $ | 1,860 | $ | 5,244 | $ | 8,020 | ||||||||
Norovirus program | 2,491 | 1,202 | 6,229 | 2,746 | ||||||||||||
All other programs | 52 | — | 171 | — | ||||||||||||
Preclinical research | 317 | 870 | 1,267 | 1,811 | ||||||||||||
Process development | 634 | 386 | 1,820 | 1,680 | ||||||||||||
Total external costs | 5,781 | 4,318 | 14,731 | 14,257 | ||||||||||||
Internal costs | 16,685 | 8,091 | 45,864 | 18,962 | ||||||||||||
Total research and development | $ | 22,466 | $ | 12,409 | $ | 60,595 | $ | 33,219 | ||||||||
We expect that research and development expenses will increase in 2022 and beyond as we advance our tablet vaccine candidates into and through clinical trials, pursue regulatory approval of our tablet vaccine candidates and prepare for a possible commercial launch, all of which will also require a significant investment in manufacturing and inventory related costs. To the extent that we enter into licensing, partnering or collaboration agreements, a significant portion of such costs may be borne by third parties.
The process of conducting clinical trials necessary to obtain regulatory approval is costly and time consuming. We may never succeed in achieving marketing approval for our tablet vaccine candidates. The probability of successful commercialization of our tablet vaccine candidates may be affected by numerous factors, including clinical data obtained in future trials, competition, manufacturing capability and commercial viability. As a result, we are unable to determine the duration and completion costs of our research and development projects or when and to what extent we will generate revenue from the commercialization and sale of any of our tablet vaccine candidates.
General and Administrative Expense
General and administrative expenses consist of personnel costs, allocated expenses and expenses for outside professional services, including legal, audit, accounting, public relations, market research and other consulting services. Personnel costs consist of salaries, benefits and stock-based compensation. Allocated expenses consist of rent, depreciation and other facilities-related expenses.
Results of Operations
The following table presents selected items in the condensed consolidated statements of operations and comprehensive loss for the three and nine months ended September 30, 2022 and 2021 (in thousands, except percentages):
Three Months Ended September 30, | Nine Months Ended September 30, | |||||||||||||||||||||||
2022 | 2021 | % Change | 2022 | 2021 | % Change | |||||||||||||||||||
Revenue | $ | — | $ | 200 | (100 | )% | $ | 85 | $ | 818 | (90 | )% | ||||||||||||
Operating expenses | 29,426 | 17,451 | 69 | % | 83,534 | 49,355 | 69 | % | ||||||||||||||||
Operating loss | (29,426 | ) | (17,251 | ) | 71 | % | (83,449 | ) | (48,537 | ) | 72 | % | ||||||||||||
Net non-operating income (expense) | 133 | (311 | ) | (143 | )% | (340 | ) | (1,080 | ) | (69 | )% | |||||||||||||
Loss before income taxes | (29,293 | ) | (17,562 | ) | 67 | % | (83,789 | ) | (49,617 | ) | 69 | % | ||||||||||||
Provision for income taxes | 16 | 21 | (24 | )% | 51 | 89 | (43 | )% | ||||||||||||||||
Net loss | $ | (29,309 | ) | $ | (17,583 | ) | 67 | % | $ | (83,840 | ) | $ | (49,706 | ) | 69 | % | ||||||||
Total Revenue
The following table summarizes our revenues for the three and nine months ended September 30, 2022 and 2021 (in thousands, except percentages):
Three Months Ended September 30, | Nine Months Ended September 30, | |||||||||||||||||||||||
2022 | 2021 | % Change | 2022 | 2021 | % Change | |||||||||||||||||||
Revenue from customer service contracts | $ | — | $ | — | N/A | $ | — | $ | 13 | (100 | )% | |||||||||||||
Non-cash royalty revenue related to sale of future royalties | — | 200 | (100 | )% | 85 | 805 | (89 | )% | ||||||||||||||||
Total revenue | $ | — | $ | 200 | (100 | )% | $ | 85 | $ | 818 | (90 | )% | ||||||||||||
Revenue from Customer Service Contracts
We earned revenue from customer service contracts of $13,000 in the nine months ended September 30, 2021, all in the first quarter. This revenue was recognized from a fixed price contract executed in July 2019, as amended, for a total of $617,000, which we have now completed.
Non-cash Royalty Revenue Related to Sale of Future Royalties
Total Operating Expenses
The following table presents our operating expenses for the three and three and nine months ended September 30, 2022 and 2021 (in thousands, except percentages):
Three Months Ended September 30, | Nine Months Ended September 30, | |||||||||||||||||||||||
2022 | 2021 | % Change | 2022 | 2021 | % Change | |||||||||||||||||||
Research and development | $ | 22,466 | $ | 12,409 | 81 | % | $ | 60,595 | $ | 33,219 | 82 | % | ||||||||||||
General and administrative | 6,960 | 5,042 | 38 | % | 22,939 | 16,136 | 42 | % | ||||||||||||||||
Total operating expenses | $ | 29,426 | $ | 17,451 | 69 | % | $ | 83,534 | $ | 49,355 | 69 | % | ||||||||||||
Research and Development
For the three months ended September 30,2022, research and development expenses increased by $10.1 million, or 81%, compared to the three months ended September 30,2021. The increase is primarily due to increased personnel costs, including stock-based compensation, facilities allocation and recruitment costs, higher in-house manufacturing costs, increased clinical trial expenses and higher depreciation, partially offset by a decrease in CMO costs.
For the nine months ended September 30, 2022, research and development expenses increased by $27.4 million, or 82%, compared to the nine months ended September 30, 2021. The increase is primarily due to increased personnel costs, including stock-based compensation and facilities allocation related to headcount increases, higher in-house manufacturing costs, increased clinical trial expenses related to our vaccine candidates and higher depreciation, partially offset by a decrease in CMO costs related to our vaccine candidate.
We expect that research and development expenses will be significantly higher in 2022 and beyond than in 2021 as we continue to increase our headcount and incur significant expenditures on manufacturing and clinical trials for our COVID-19 and norovirus vaccine candidates.
General and Administrative
For the three months ended September 30, 2022, general and administrative expenses increased by $1.9 million, or 38%, compared to the corresponding period in 2021, The principal reasons for the increase are increased personnel costs, including non-cash stock-based compensation expense, and increases in legal fees.
For the nine months ended September 30, 2022, general and administrative expenses increased by $6.8 million, or 42%, compared to the nine months ended September 30, 2021. The principal reasons for the increase are the cost of settling shareholder litigation, increased personnel costs, including non-cash stock-based compensation expense not related to award modifications, and increases in legal and other professional fees, partially offset by the absence of a one-off non-cash expense for modifying the terms of outstanding options awarded to our former Chairman of the Board.
Non-Operating Income (Expense)
The following table presents our non-operating income and expenses for the three and nine months ended September 30, 2022 and 2021, respectively (in thousands, except percentages):
Three Months Ended September 30, | Nine Months Ended September 30, | |||||||||||||||||||||||
2022 | 2021 | % Change | 2022 | 2021 | % Change | |||||||||||||||||||
Interest income, net | $ | 458 | $ | 26 | 1,662 | % | $ | 650 | $ | 58 | 1,021 | % | ||||||||||||
Non-cash interest expense related to sale of future royalties | (325 | ) | (337 | ) | (4 | )% | (988 | ) | (1,137 | ) | (13 | )% | ||||||||||||
Foreign exchange loss, net | — | — | N/A | (2 | ) | (1 | ) | 100 | % | |||||||||||||||
Net non-operating income (expense) | $ | 133 | $ | (311 | ) | (143 | )% | $ | (340 | ) | $ | (1,080 | ) | (69 | )% | |||||||||
For the three months ended September 30, 2022, we recorded net non-operating income of $133,000, a 143% decrease from the $311,000 net expense recorded in the three months ended September 30, 2021. For the nine months ended September 30, 2022, we recorded net non-operating expenses of $340,000, a 69% decrease from the $1,080,000 recorded in the nine months ended September 30, 2021.
Interest income increased in the nine months ended September 30, 2022, compared to the nine months ended September 30, 2021, due to higher interest rates. Non-cash interest expense related to sale of future royalties, which relates to accounting for amounts that will become payable to HCRP for royalty revenue earned from Inavir as debt, decreased in the three and nine months ended September 30, 2022, compared to the corresponding period in the prior year, as the outstanding balance due to HCRP was revalued as of December 31, 2021, resulting in a reduction of $3.8 million in the estimated liability.
Provision for Income Taxes
The following table presents our provision for income taxes for the three and nine months ended September 30, 2022 and 2021, respectively (in thousands, except percentages):
Three Months Ended September 30, | Nine Months Ended September 30, | |||||||||||||||||||||||
2022 | 2021 | % Change | 2022 | 2021 | % Change | |||||||||||||||||||
Foreign withholding tax on royalty revenue | $ | — | $ | 10 | (100 | )% | $ | 4 | $ | 40 | (90 | )% | ||||||||||||
Foreign taxes payable on intercompany interest | 16 | 15 | 7 | % | 45 | 48 | (6 | )% | ||||||||||||||||
State income taxes | — | (4 | ) | (100 | )% | 2 | 1 | 100 | % | |||||||||||||||
Provision for income taxes | $ | 16 | $ | 21 | (24 | )% | $ | 51 | $ | 89 | (43 | )% | ||||||||||||
The provision for income taxes comprises $16,000 and $21,000 in the three months ended September 30, 2022 and 2021, respectively, and $51,000 and $89,000 in the nine months ended September 30, 2022 and 2021, respectively. The tax charge relates primarily to interest on an intercompany loan from a foreign subsidiary and a 5% withholding tax on royalty revenue earned on sales of Inavir in Japan, which is potentially recoverable as a foreign tax credit but expensed because we record a 100% valuation allowance against our deferred tax assets.
Liquidity and Capital Resources
Our primary source of financing is from the sale and issuance of common stock and common stock warrants in public offerings, along with proceeds from the exercise of warrants. In the past, we have also obtained funds from the issuance of secured debt and preferred stock and from collaboration agreements. In September 2021 we entered into a Controlled Equity Offering Sales Agreement (the “September 2021 ATM”), under which we may offer and sell, from time to time through sales agents, shares of our common stock having an aggregate offering price of up to $100 million. As of September 30, 2022, we had received net proceeds of $8.6 million from the sale of common stock under the September 2021 ATM. We incur direct expenses and pay sales commissions of up to 2.0% of gross proceeds from the sale of shares under the September 2021 ATM.
As of September 30, 2022, we had approximately $114.8 million of cash, cash equivalents and marketable securities.
We believe our existing funds are sufficient to fund us for at least one year from the date of issuance of this Quarterly Report. To continue operations thereafter, we expect that we will need to raise further capital, through the sale of additional securities or otherwise. As of September 30, 2022, we had approximately $87 million in net proceeds still available to us under the September 2021 ATM. Our future capital requirements and the adequacy of our available funds will depend on many factors, most notably our ability to successfully commercialize our products and services.
We may fund a portion of our ongoing operations through partnering and collaboration agreements which, while reducing our risks and extending our cash runway, may also reduce our share of eventual revenues, if any, from our vaccine product candidates. We may be able to fund certain activities with assistance from government programs. The sale of additional equity would result in additional dilution to our stockholders. We may also fund our operations through debt financing, which would result in debt service obligations, and the instruments governing such debt could provide for operating and financing covenants that would restrict our operations. If we are unable to raise additional capital in sufficient amounts or on acceptable terms, we may be required to delay, limit, reduce, or terminate our product development or future commercialization efforts or grant rights to develop and market vaccine candidates that we would otherwise prefer to develop and market ourselves. Any of these actions could harm our business, results of operations and prospects.
Our future funding requirements will depend on many factors, including the following:
● | the timing and costs of our planned preclinical studies for our product candidates; |
● | the timing and costs of our planned clinical trials of our product candidates; |
● | our manufacturing capabilities, including the availability of contract manufacturing organizations to supply our product candidates at reasonable cost; |
● | the number and characteristics of product candidates that we pursue; |
● | the outcome, timing and costs of seeking regulatory approvals; |
● | revenue received from commercial sales of our future products, which would be subject to receipt of regulatory approval; |
● | the terms and timing of any future collaborations, licensing, consulting or other arrangements that we may enter into; |
● | the amount and timing of any payments that may be required in connection with the licensing, filing, prosecution, maintenance, defense and enforcement of any patents or patent applications or other intellectual property rights; and |
● | the extent to which we in-license or acquire other products and technologies. |
Cash Flows
The following table summarizes our cash flows for the periods indicated:
Nine Months Ended September 30, | ||||||||
2022 | 2021 | |||||||
Net cash used in operating activities | $ | (65,627 | ) | $ | (44,018 | ) | ||
Net cash used in investing activities | (36,216 | ) | (40,920 | ) | ||||
Net cash provided by financing activities | 8,866 | 125,299 | ||||||
Net (decrease) increase in cash and cash equivalents | $ | (92,977 | ) | $ | 40,361 | |||
Net Cash Used in Operating Activities
Vaxart experienced negative cash flow from operating activities for the nine months ended September 30, 2022 and 2021, in the amounts of $65.6 million and $44.0 million, respectively. The cash used in operating activities in the nine months ended September 30, 2022, was due to cash used to fund a net loss of $83.8 million and a decrease in working capital of $3.3 million, further decreased by adjustments for net non-cash income related to depreciation and amortization, accretion of premium on investments, stock-based compensation, non-cash interest expense related to sale of future royalties and non-cash revenue related to sale of future royalties totaling $14.9 million. The cash used in operating activities in the nine months ended September 30, 2021, was due to cash used to fund a net loss of $49.7 million and an increase in working capital of $3.6 million, partially offset by adjustments for net non-cash income related to depreciation and amortization, accretion of premium on investments, stock-based compensation, non-cash interest expense related to sale of future royalties and non-cash revenue related to sale of future royalties totaling $9.3 million.
Net Cash Used in Investing Activities
In the nine months ended September 30, 2022, we used $25.5 million to purchase marketable securities, net of maturities, $5.7 million to purchase property and equipment and $5.0 million to pay for right-of-use assets. In the nine months ended September 30, 2021, we used $36.8 million to purchase marketable securities, net of maturities, and $4.1 million to purchase property and equipment.
Net Cash Provided by Financing Activities
In the nine months ended September 30, 2022, we received net proceeds of $8.7 million from the sale of common stock under the September 2021 ATM and $216,000 from the exercise of stock options and warrants. In the nine months ended September 30, 2021, we received net proceeds of $122.2 million from the sale of common stock under the October 2020 ATM and $3.1 million from the exercise of common stock warrants and stock options.
Contractual Obligations and Commercial Commitments
We have the following contractual obligations and commercial commitments as of September 30, 2022 (in thousands):
Contractual Obligation | Total | < 1 Year | 1 - 3 Years | 3 - 5 Years | > 5 Years | |||||||||||||||
Long Term Debt, HCRP | $ | 17,027 | $ | 2,552 | $ | 5,816 | $ | 4,638 | $ | 4,021 | ||||||||||
Operating Leases | 30,814 | 4,098 | 8,513 | 10,150 | 8,053 | |||||||||||||||
Purchase Obligations | 13,696 | 13,696 | — | — | — | |||||||||||||||
Total | $ | 61,537 | $ | 20,346 | $ | 14,329 | $ | 14,788 | $ | 12,074 | ||||||||||
Long Term Debt, HCRP. Under an agreement executed in 2016, we are obligated to pay HCRP the first $3 million plus 15% of the next $1 million of royalty revenues that we earn for sales of Inavir in each year ending on March 31. See Note 6 to the Condensed Consolidated Financial Statements in Part I, Item 1 for further details.
Operating leases. Operating lease amounts include future minimum lease payments under all our non-cancellable operating leases with an initial term in excess of one year. See Note 7 to the Condensed Consolidated Financial Statements in Part I, Item 1 for further details of leases.
Purchase obligations. These amounts include an estimate of all open purchase orders and contractual obligations in the ordinary course of business, including commitments with contract manufacturers and suppliers for which we have not received the goods or services. We consider all open purchase orders, which are generally enforceable and legally binding, to be commitments, although the terms may afford us the option to cancel based on our business needs prior to the delivery of goods or performance of services.
Share based payment arrangements. Since we were a private company, we have issued stock options to all full-time new hires other than interns and have also issued options to these employees annually. Beginning in 2022, we have shifted from awarding options only to issuing a mixture of options and restricted stock units (“RSUs”). As of September 30, 2022, unrecognized stock-based compensation cost related to outstanding unvested stock options and RSUs expected to vest was $36.2 million and $2.2 million, respectively, which we expect to recognize over an estimated weighted average period of 3.03 and 3.46 years, respectively. In addition, we have adopted an Employee Stock Purchase Plan which we expect will become effective on December 15, 2022.
Critical Accounting Policies and Estimates
Our management’s discussion and analysis of the major factors contributing to ourfinancial condition and results of operations for the three and six months ended December 31, 2017, andis based on our financial condition at that date, and should be read in conjunction with thecondensed consolidated financial statements, and the notes thereto included inPart I,Item 1 of this Quarterly Report on Form 10-Q.
Company Overview
We are focused on the discovery and development of direct-acting antivirals to treat infections that have limited therapeutic options and affect a significant number of patients globally. The Company has three Phase 2 clinical stage compounds: BTA074 (teslexivir), an antiviral treatment for condyloma caused by human papillomavirus types 6 & 11; vapendavir, a capsid inhibitor for the prevention or treatment of rhinovirus (“RV”) upper respiratory infections; and BTA585 (enzaplatovir), a fusion protein inhibitor in development for the treatment of respiratory syncytial virus infections.
Although several of our influenza product candidateswhich have been successfully developed and commercialized to date by other larger pharmaceutical companies under license, collaboration or commercialization agreements with us, we have not independently developed or received regulatory approval for any product candidate, and we do not currently have any sales, marketing or commercial capabilities. Therefore, it is possible that we may not derive any significant product revenues from any product candidates that we are developing now, or may develop in the future. We expect to incur losses for the foreseeable future as we intend to support the clinical and preclinical development of our product candidates.
On October 30, 2017, the Company announced that it had entered into the Merger Agreement pursuant to which Vaxart, a privately-held clinical-stage company focused on developing oral recombinant vaccines from its proprietary delivery platform, would become a wholly-owned subsidiary of the Company. This transaction marks the culmination of the Company’s Strategic Review process which was initiated in April. The Merger will result in a clinical-stage pharmaceutical company focused on developing Vaxart’s oral recombinant vaccines and our direct-acting antivirals to treat infections that have limited therapeutic options. We believe Vaxart’s oral tablet vaccines have the potential to be major products in the worldwide vaccine market.
The exchange ratio in the merger agreement was determined by Vaxart assigning $60,000,000 in value to Aviragen for its financial and clinical assets, and $90,000,000 in value for its own assets. On a pro forma basis after giving effect to the number of shares of Aviragen common stock that will be issued to Vaxart security holders in the Merger and assuming no adjustments for cash balances as provided for in the Merger Agreement, current Vaxart security holders will own approximately 60% of the combined company and current Aviragen security holders will own approximately 40% of the combined company. The transaction has been approved by the boards of directors of both companies. The Merger is expected to close in February 2018, subject to the approval of the stockholders of each company as well as other customary conditions.
At the end of the quarter, a small group of dissident stockholders, who call themselves the Concerned Aviragen Shareholders (“CAS”) Group, launched a proxy contest against the proposed merger with Vaxart and are seeking an opportunity to nominate individuals for election to the Company’s Board at our upcoming Annual Meeting. We continue to believe the proposed merger with Vaxart is the best possible strategic alternative, and together, Aviragen and Vaxart will have the potential to create meaningful value for stockholders.
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
Management’s Discussion and Analysis of Results of Operations discusses our financial results, which (except to the extent described in the Notes thereto) have been presentedprepared in accordance with U.S. generally accepted accounting principles (“U.S. GAAP”).in the United States. The preparation of these financial statements requires us to make estimates and assumptionsjudgments that affect the reported amounts of assets, and liabilities and the disclosure of contingent assetsexpenses. On an ongoing basis, we evaluate these estimates and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period.
judgments. We base our estimates and judgments on historical experience current economic and industry conditions, andon various other factorsassumptions that we believe to be reasonable under the circumstances. This formsThese estimates and assumptions form the basis for making judgments about the carrying values of assets and liabilities and the recording of expenses that are not readily apparent from other sources. Actual results may differ materially from these estimates under different assumptions or conditions.estimates. We believe there have been no changes to our criticalthat the accounting policies that requirediscussed below are critical to understanding our historical and future performance, as these policies relate to the more significant judgmentareas involving management’s judgments and estimates as discussed in detail in our 2017 annual 10-K filing:estimates.
Accrued Research and Development Expenses |
| |
|
| |
|
| |
|
|
For a descriptionWe record accrued expenses for estimated costs of recent accounting policiesresearch and development activities conducted by third-party service providers, which include the impact on our financial statements, refer to Note 2conduct of preclinical studies, clinical trials and manufacturing activities. We record the estimated costs of research and development activities based upon the estimated amount of services provided and include the costs incurred but not yet invoiced within accrued liabilities in the condensed consolidated financial statements.
Results of Operations for the Three months ended December 31, 2017balance sheets and December 31, 2016
Summary. For the three months ended December 31, 2017, we reported a net loss of $3.4 million, as compared to a net loss of $9.1 million in the same period of the prior fiscal year. Basic and diluted net loss per share was $0.09 for the three month period ended December 31, 2017, as compared to a basic and diluted net loss per share of $0.24 in the same period of 2016. The following commentary provides details underlying changes from last year in the major line items of our statement of operations:
Revenue. Revenue decreased to $2.7 million for the three month periods ended December 31, 2017, as compared to $3.8 million in the same period in 2016, mostly due to a decrease in our Relenza royalties. Relenza revenue declined to zero in the three months ended December 31, 2017 from $1.5 million in the same period of the prior fiscal year due to the cessation of royalties on U.S. sales at the end of 2016 and the unfavorable impact of a returns adjustment in the current quarter. The following table summarizes the key components of our revenue for the three months ended December 31, 2017 and 2016:
Three Months Ended December 31, | ||||||||
2017 | 2016 | |||||||
(in millions) | ||||||||
Royalty revenue - Relenza® | $ | - | $ | 1.5 | ||||
Non-cash royalty revenue related to the sale of future royalties | 2.7 | 2.3 | ||||||
Total revenue | $ | 2.7 | $ | 3.8 | ||||
Research and Development Expense. Researchwithin research and development expense decreased to $2.5 million forin the three months ended December 31, 2017 from $10.2 million for the same period in 2016. The following table summarizes the componentscondensed consolidated statements of operations and comprehensive loss. These costs can be a significant component of our research and development expenseexpenses.
We estimate the amount of work completed through discussions with internal personnel and external service providers as to the progress or stage of completion of the services and the agreed-upon fee to be paid for such services. We make significant judgments and estimates in determining the three months ended December 31, 2017 and 2016.accrued balance in each reporting period. As actual costs become known, we adjust our accrued estimates.
Three Months Ended December 31, | ||||||||
(in millions) | ||||||||
2017 | 2016 | |||||||
Direct preclinical, clinical and product development expenses | $ | 1.4 | $ | 9.0 | ||||
Salaries, severance and share-based compensation expenses | 1.1 | 1.1 | ||||||
Depreciation and facility related expenses | - | 0.1 | ||||||
Total research and development expense | $ | 2.5 | $ | 10.2 | ||||
Direct preclinical, clinical and product development expense decreased largely due to reduced clinical trial activity and manufacturing costs, as two of our three Phase 2 clinical trials came to a close at the end of the prior fiscal year. Salaries, severance and share-based compensation expenses did not change as compared to the same period in 2016, as decreases due to reductions in personnel in the last quarter of the prior fiscal year were offset by severance expense for employees terminated in the current quarter.Intangible Assets
GeneralIntangible assets acquired in the Merger were initially recorded at their estimated fair values of $20.3 million for developed technology related to Inavir which was, until it was revalued, being amortized on a straight-line basis over the estimated period of future royalties of 11.75 years and Administrative Expense. General and administrative expense increased to $3.1$1.8 million for the threedeveloped technology related to Relenza which was fully amortized over the remaining royalty period of 1.3 years. The developed technology related to Inavir was revalued at $10.6 million as of December 31, 2021, resulting in an impairment loss of $3.0 million being recorded. The fair value is being amortized on a straight-line basis over 7.9 years, the estimated period of future royalties remaining as of December 31, 2021, when it was revalued. These valuations were prepared by an independent third party based on discounted cash flows of estimated future revenue streams, which are highly subjective, especially since the start of the COVID-19 pandemic due to the unpredictability of the duration of its impact on future flu seasons.
Stock-Based Compensation
We measure the fair value of all stock option awards to employees, non-executive directors and consultants on the grant date, and record the fair value of these awards, net of estimated forfeitures, as compensation expense over the service period. The fair value of options is estimated using the Black-Scholes valuation model and the expense recorded is affected by subjective assumptions regarding a number of variables, as follows:
Expected term – This represents the period that our stock-based awards granted are expected to be outstanding and is determined using the simplified method (the arithmetic average of its original contractual term and its average vesting term). We have very limited historical information to develop reasonable expectations about future exercise patterns and post-vesting employment termination behavior for our stock-based awards. Based on the weighted average applied to options awarded in nine months ended December 31, 2017 from $2.1 millionSeptember 30, 2022, a notional 10% decrease in expected term would have reduced the fair value and the related compensation expense by approximately 2.2%.
Expected volatility – This is a measure of the amount by which our common stock price has fluctuated or is expected to fluctuate. We measure volatility based on the historical volatility of our own stock over the retrospective period corresponding to the expected term of the options on the measurement date. Based on the weighted average applied to options awarded in nine months ended September 30, 2022, a notional 10% decrease in expected volatility (from 125% to 113%) would have reduced the fair value and the related compensation expense by approximately 4.4%.
Risk-Free Interest Rate – This is based on the U.S. Treasury yield curve on the measurement date corresponding with the expected term of the stock-based awards. Based on the weighted average applied to options awarded in nine months ended September 30, 2022, a notional doubling of the risk-free interest rate would have increased the fair value and the related compensation expense by approximately 0.9%.
Expected Dividend – We have not made any dividend payments and do not plan to pay dividends in the foreseeable future. Therefore, we use an expected dividend yield of zero.
Forfeiture Rate – This is a measure of the number of awards that are expected to not vest and is reassessed quarterly. An increase in the estimated forfeiture rate will cause a small decrease the related compensation expense early in the service period, but since the final expense recorded for each award is the same periodnumber of options vested times their grant date fair value, it has no impact on the total expense recorded.
Recent Accounting Pronouncements
See the “Recent Accounting Pronouncements” in 2016, largely dueNote 2 to legal and professional feesthe Condensed Consolidated Financial Statements in Part I, Item 1 for information related to the proposed merger with Vaxart announcedissuance of new accounting standards in October 2017 and severance due tothe first nine months of 2022, none of which had a reduction in personnel. The following table summarizes the components ofmaterial impact on our general and administrative expense for the three months ended December 31, 2017 and 2016. condensed consolidated financial statements.
Three Months Ended December 31, | ||||||||
(in millions) | ||||||||
2017 | 2016 | |||||||
Salaries, benefits and share-based compensation expenses | $ | 1.2 | $ | 1.1 | ||||
Professional and legal fees expenses | 1.4 | 0.4 | ||||||
Other expenses | 0.5 | 0.6 | ||||||
Total general and administrative expense | $ | 3.1 | $ | 2.1 | ||||
Foreign Exchange Loss (Gain),net. The impact of foreign exchange changed from a loss of $0.1 million in December 31, 2016 to no gain or loss for three months ended December 31, 2017. The positive impact on foreign exchange on our statement of operations was due to fluctuations in foreign currency exchange rates versus the U.S. dollar, largely related to the British Pound and Australian dollar. The vast majority of our cash holdings are held in the U.S. dollar. We re-measure all of our foreign assets and liabilities at the period-end exchange rate and the net effect of these translation adjustments is shown as a foreign currency loss or gain.
Results of Operations for the Six months ended December 31, 2017and December 31, 2016
Summary. For the six months ended December 31, 2017, we reported a net loss of $8.7 million, as compared to a net loss of $19.1 million in the same period of the prior fiscal year. Basic and diluted net loss per share was $0.23 for the six month period ended December 31, 2017, as compared to a basic and diluted net loss per share of $0.49 in the same period of 2016. The following commentary provides details underlying changes from last year in the major line items of our statement of operations:
Revenue. Revenue decreased to $2.8 million for the six month periods ended December 31, 2017, as compared to $3.9 million in the same period in 2016, mostly due to a decrease in our Relenza royalties. Relenza revenue declined to zero in the six months ended December 31, 2017 from $1.6 million in the same period of the prior fiscal year due to the cessation of royalties on U.S. sales at the end of 2016Item 3. Quantitative and the unfavorable impact of a returns adjustment in the current year. The following table summarizes the key components of our revenue for the six months ended December 31, 2017 and 2016:
Six Months Ended December 31, | ||||||||
2017 | 2016 | |||||||
(in millions) | ||||||||
Royalty revenue - Relenza® | $ | - | $ | 1.6 | ||||
Non-cash royalty revenue related to the sale of future royalties | 2.8 | 2.3 | ||||||
Total revenue | $ | 2.8 | $ | 3.9 | ||||
Research and Development Expense. Research and development expense decreased to $5.3 million for the six months ended December 31, 2017 from $17.8 million for the same period in 2016. The following table summarizes the components of our research and development expense for the six months ended December 31, 2017 and 2016.
Six Months Ended December 31, | ||||||||
(in millions) | ||||||||
2017 | 2016 | |||||||
Direct preclinical, clinical and product development expenses | $ | 3.3 | $ | 15.4 | ||||
Salaries, severance and share-based compensation expenses | 1.9 | 2.2 | ||||||
Depreciation and facility related expenses | 0.1 | 0.2 | ||||||
Total research and development expense | $ | 5.3 | $ | 17.8 | ||||
Direct preclinical, clinical and product development expense decreased largely due to reduced clinical trial activity and manufacturing costs, as two of our three Phase 2 clinical trials came to a close at the end of the prior fiscal year. Salaries, severance and share-based compensation expenses decreased compared to the same period in 2016 due to a reduction in personnel in the last quarter of the prior fiscal year, partially offset by severance expense for employees terminated in the current year.Qualitative Disclosures About Market Risk
General and Administrative Expense. General and administrative expense increasedInterest Rate Sensitivity
Our exposure to $5.4 millionmarket risk for the six months ended December 31, 2017 from $4.3 million for the same periodchanges in 2016, largely dueinterest rates relates primarily to legal and professional fees related to the proposed merger with Vaxart announcedour investments in October 2017 and severance due to a reduction in personnel.marketable debt securities. The following table summarizes the componentsprimary objective of our generalinvestment activities is to preserve principal, maintain liquidity that is sufficient to meet cash needs and administrative expense for the six months ended December 31, 2017 and 2016.
Six Months Ended December 31, | ||||||||
(in millions) | ||||||||
2017 | 2016 | |||||||
Salaries, benefits and share-based compensation expenses | $ | 2.3 | $ | 2.0 | ||||
Professional and legal fees expenses | 2.0 | 1.1 | ||||||
Other expenses | 1.1 | 1.2 | ||||||
Total general and administrative expense | $ | 5.4 | $ | 4.3 | ||||
LIQUIDITY AND CAPITAL RESOURCES
For the six months ended December 31, 2017,maximize total return without significantly increasing risk. To achieve this goal, we maintain our excess cash and cash equivalents increased by $11.7 million. This increase was primarily the resultin money market funds and debt securities. We do not enter into investments for trading or speculative purposes and we hold no equity securities. We presently have no borrowings or lines of credit.
Specifically, as of September 30, 2022, we had cash, cash equivalents and investments of approximately $114.8 million, which consist of bank deposits, money market funds, direct obligations of the maturitiesU.S. government or its agencies, commercial paper and corporate bonds. All of our investments must satisfy high credit rating requirements at the time of purchase. Such interest-earning instruments carry a degree of interest rate risk, however, because our investments are rated highly and mostly short-term, investments.we believe that our exposure to risk of loss due to interest rate changes is not significant.
Net cash used by operating activities was $9.2 million for the six months ended December 31, 2017, which reflected our net loss during the period of $8.7 million, a net decrease in a net operating assets of $0.5 million and a decrease in operating liabilities of $0.1 million, partially offset by net non-cash adjustments of $1.1 million. Non-cash adjustments consist of $2.7 million in non-cash royalty income, net of withholding taxes, partially offset by $0.8 million in non-cash interest expense and $0.8 million in share-based compensation expense.Exchange Rate Sensitivity
Our net loss resulted largely from our funding of research and development activities including conducting the CT4 clinical trial for BTA074 (teslexivir), as well as ongoing general and administrative expenses including legal and professional feesPresently, we are not retaining any cash related to the proposed merger with Vaxart. The net changes in operatingour income from royalties and substantially all of our expenses, assets and liabilities primarily reflectsare denominated in U.S. dollars. As a $0.1 million decrease in accounts payableresult, we have not experienced significant foreign exchange gains or losses recently and accrued expense dueconsider our exposure to reduced clinical trial activity, offset by a $0.2 million decrease in prepaid expenses, also dueexchange rate fluctuations to reduced clinical trial activity and a $0.3 million decrease in cash receivables primarily related to receipt of a research and development tax credit.be insignificant.
Net cash provided by investing activities during the six months ended December 31, 2017 consisted of the maturity of $27.9 million of investments, partially offset by the purchase of $7.0 million of investments.
At December 31, 2017, our cash and cash equivalents totaled $29.4 million. Our cash and cash equivalents are currently held in the form of short-term deposits with large U.S. banks, commercial paper and highly-rated corporate securities.
Based on our current strategy and operating plan, and considering the potential costs associated with advancing the preclinical and clinical development of our product candidates, we believe that our existing cash and cash equivalents of approximately $29.4 million as of December 31, 2017, along with the anticipated proceeds from existing royalty-bearing licenses will enable us to operate for a period of at least 12 months from the date of this report.
We have an ATM facility in place, which may allow us to quickly access the equity capital markets if we think it is prudent to do so and if market conditions allow. However, we currently do not have any commitments for future funding, nor do we anticipate that we will generate significant revenue, aside from revenue from existing royalty-bearing arrangements.
Contractual and Commercial Commitments
There have been no material changes from the information included in our Annual Report on Form 10-K for the fiscal year ended June 30, 2017.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements, as defined in Item 303(a)(4) (ii) of Regulation S-K under the Securities Exchange Act of 1934, as amended.
ITEM 3: Quantitative and Qualitative Disclosures about Market Risk
There has been no material change in our assessment of sensitivity to market risk since our presentation set forth in Item 7A “Quantitative and Qualitative Disclosures about Market Risk” in the our Annual Report on Form 10-K for the fiscal year ended June 30, 2017.
ITEM 4:4. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Our management, includingwith the participation of our Chief Executive Officerprincipal executive officer and Chief Financial Officer,principal accounting and financial officer, has evaluated the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended) as of the end of the period covered by this report.Quarterly Report on Form 10-Q. Based on thatsuch evaluation, our Chief Executive Officer and Chief Financial Officermanagement has concluded that our disclosure controls and procedures were effective at a reasonable assurance level as of the end of the period covered by this report.September 30, 2022.
Changes in Internal ControlsControl over Financial Reporting
Inherent Limitations on Effectiveness of Controls
Our management, including our principal executive officer and principal accounting and financial officer, does not expect that our disclosure controls and procedures or our internal controls will prevent all error and all fraud. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within Vaxart have been detected.
ITEMItem 1. LEGAL PROCEEDINGSLegal Proceedings
The Companyinformation included in “Note 8. Commitments and Contingencies—(c) Litigation” to the Condensed Consolidated Financial Statements in Part I, Item 1 is incorporated by reference into this Item.
We may also from time to time be involved in various legal proceedings arising in connection with our business. Based on information currently available, we believe that the amount, or range, of reasonably possible losses in connection with any pending actions against us in excess of established reserves, in the aggregate, is not material to our consolidated financial condition or cash flows. However, any current or future dispute resolution or legal proceeding, regardless of the merits of any such proceeding, could result in substantial costs and a diversion of management’s attention and resources that are incidentalneeded to the conduct of its business. The Company is not involved in any pending or threatened legal proceedings that it believesrun our business successfully, and could reasonably be expected to have a material adverse effectimpact on itsour business, financial condition orand results of operations.
ITEMItem 1A. RISK FACTORSRisk Factors
Any investment in our business involves a high degree of risk. Before making an investment decision, youYou should carefully consider the information we include in this Quarterly Report on Form 10-Q, including our condensed consolidated financial statementsrisks and accompanying notes, and the additional information in the other reports we file with the Securities and Exchange Commission along with the risksuncertainties described inunder Item 1A of Part I of our Annual Report on Form 10-K for the fiscal year ended June 30, 2017. The Company is also subject to the risk factors set forth under the captions “Risks Related to the Merger,” “Risks Related to Aviragen” and “Risks Related to the Combined Company” in the Prospectus that is part of the Registration Statement on Form S-4 (File No. 333-222009), as amended,December 31, 2021, which we filed with the Securities and Exchange Commission on February 24, 2022, together with all other information contained or incorporated by the Companyreference in this Quarterly Report on Form 10-Q, when evaluating our business and declared effective on December 29, 2017. We have also described below those risks that reflect substantiveour prospects. There are no material changes from, or additions to the risks describedrisk factors set forth in Part I, Item 1A, in our Annual Report on Form 10-K and Form S-4, as amended, referred to above. These risks may resultfor the year ended December 31, 2021, or in material harm to our business and our financial condition and results of operations. In this event, the market price of our common stock may decline and you could lose part or all of your investment.subsequently-issued Forms 10-Q.
We may be subject to the actions of activist shareholders.
We have been the subject of increased activity by activist shareholders, including Digirad Corporation, East Hill Management Company, LLC, Thomas M. Clay, and certain other investors (collectively, the “Activist Group”). Responding to shareholder activism can be costly and time-consuming, disrupt our operations and divert the attention of management and our employees. Activist campaigns, including the Activist Group’s ongoing campaign against our proposed transaction with Vaxart and the Activist Group’s notice of its intention to nominate a competing director slate for election at our annual stockholder meeting scheduled for April 11, 2018, can create uncertainties as to our future direction, strategy and leadership and may result in the loss of potential business opportunities and cause our stock price to experience periods of volatility. Moreover, if individuals are elected to our board of directors with a specific agenda, our ability to effectively and timely implement our current initiatives, retain and attract experienced executives and employees and execute on our current business strategy may be adversely affected.
ITEMItem 2. UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS
None.
ITEM 3. DEFAULTS UPON SENIOR SECURITIESUnregistered Sales of Equity Securities and Use of Proceeds
Not applicable.
ITEM 4. MINE SAFETY DISCLOSUREItem 3. Defaults Upon Senior Securities
Not applicable.
ITEM 5. OTHER INFORMATIONItem 4. Mine Safety Disclosures
None.Not applicable.
ITEM 6. EXHIBITSItem 5. Other Information
The exhibits to this report are listed in the Exhibit Index, which is incorporated into this Item 6 by reference.Not applicable.
| Incorporated by Reference | ||||||
Exhibit | Description of Document | Schedule/Form | File | Exhibit | Filing Date | ||
| 3.1 | Certificate of Amendment to Restated Certificate of Incorporation of Vaxart, Inc. | Form 8-K | 001-35285 | 3.1 | April 24, 2019 | ||
| 3.2 | Certificate of Amendment to Restated Certificate of Incorporation of Vaxart, Inc. | Form 8-K | 001-35285 | 3.1 | June 9, 2020 | ||
| 3.3 | Certificate of Amendment to Restated Certificate of Incorporation of Vaxart, Inc. | Form 10-Q | 001-35285 | 3.3 | August 8, 2022 | ||
| 3.4 | Amended and Restated Bylaws of Vaxart, Inc., effective as of April 7, 2021 | Form 8-K | 001-35285 | 3.1 | April 13, 2021 | ||
| 10.1 # | 2019 Equity Incentive Plan | Form 8-K/A | 001-35285 | 10.1 | August 8, 2022 | ||
| 10.2 # | 2022 Employee Stock Purchase Plan | Form 8-K/A | 001-35285 | 10.2 | August 8, 2022 | ||
31.1 * | |||||||
| 31.2 * | Certification of Principal Financial Officer pursuant to Exchange Act Rule, 13a-14(a) and 15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | ||||||
| 32.1 § | Certification of Principal Executive Officer and Principal Financial Officer pursuant to Rule 13a-14(b) of the Securities Exchange Act of 1934, as amended, and 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | ||||||
101.INS * | Inline XBRL Instance Document - the instance document does not appear in the Interactive Data File as its XBRL tags are embedded within the Inline XBRL document | ||||||
| 101.SCH * | Inline XBRL Taxonomy Extension Schema Document | ||||||
| 101.CAL * | Inline XBRL Taxonomy Extension Calculation Linkbase Document | ||||||
| 101.DEF * | Inline XBRL Taxonomy Extension Definition Linkbase Document | ||||||
| 101.LAB * | Inline XBRL Taxonomy Extension Label Linkbase Document | ||||||
| 101.PRE * | Inline XBRL Taxonomy Extension Presentation Linkbase Document | ||||||
| 104 | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) | ||||||
| * | Filed herewith. | ||||||
| # | Management contract or compensation plan or arrangement. | ||||||
§ | In accordance with Item 601(b)(32)(ii) of Regulation S-K and SEC Release Nos. 33-8238 and 34-47986, Final Rule: Management’s Reports on Internal Control Over Financial Reporting and Certification of Disclosure in Exchange Act Periodic Reports, the certification furnished in Exhibit 32.1 hereto is deemed to accompany this Quarterly Report on Form 10-Q and will not be deemed “filed” for purposes of Section 18 of the Exchange Act. Such certification will not be deemed to be incorporated by reference into any filing under the Securities Act or the Exchange Act, except to the extent that the registrant specifically incorporates it by reference. | ||||||
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| |||
|
|
| |
| |||
|
| ||
|
VAXART, INC. |
|
| ||||||||||
|
|
|
|
|
| |||||||
|
|
|
|
| ||||||||
|
|
|
| |||||||||
|
|
|
| |||||||||
(Principal Financial and Accounting Officer) | ||||||||||||
|
| |||||||||||
|
| |||||||||||
|
| |||||||||||
|
|
| ||||||||||
* This certification is being furnished solely to accompany this quarterly report pursuant to 18 U.S.C. Section 1350, and is not being filed for purposes of Section 18 of the Securities Exchange Act of 1934 and is not to be incorporated by reference into any filing of Aviragen Therapeutics, Inc., whether made before or after the date hereof, regardless of any general incorporation language in such filing.
23